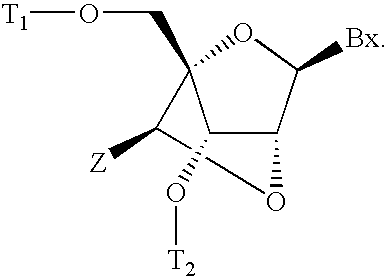
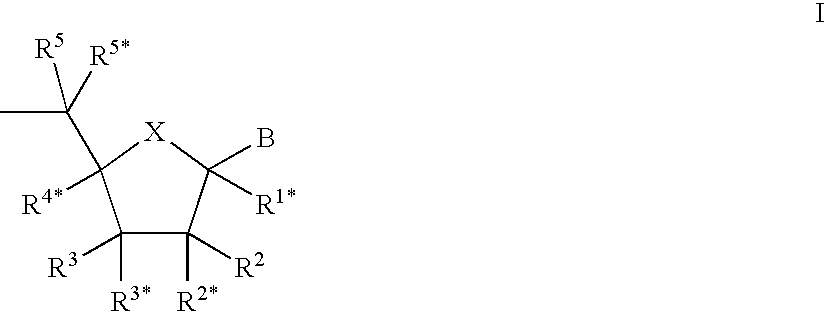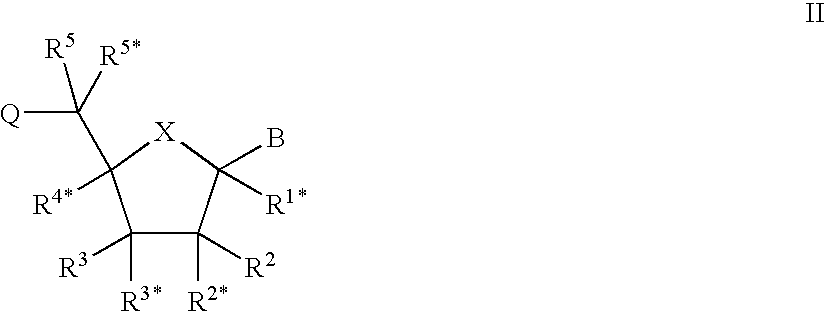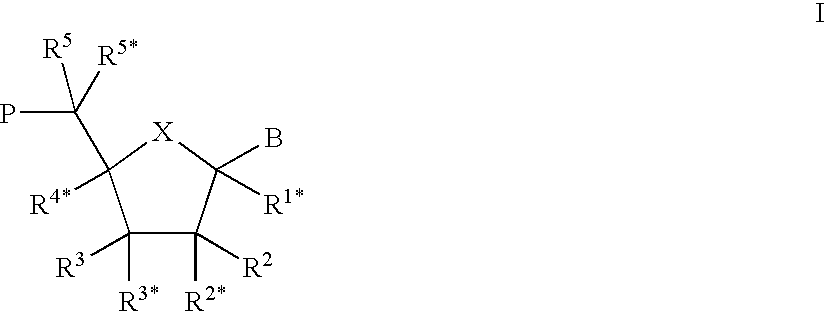Patents
Literature
203005results about "Animal repellants" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
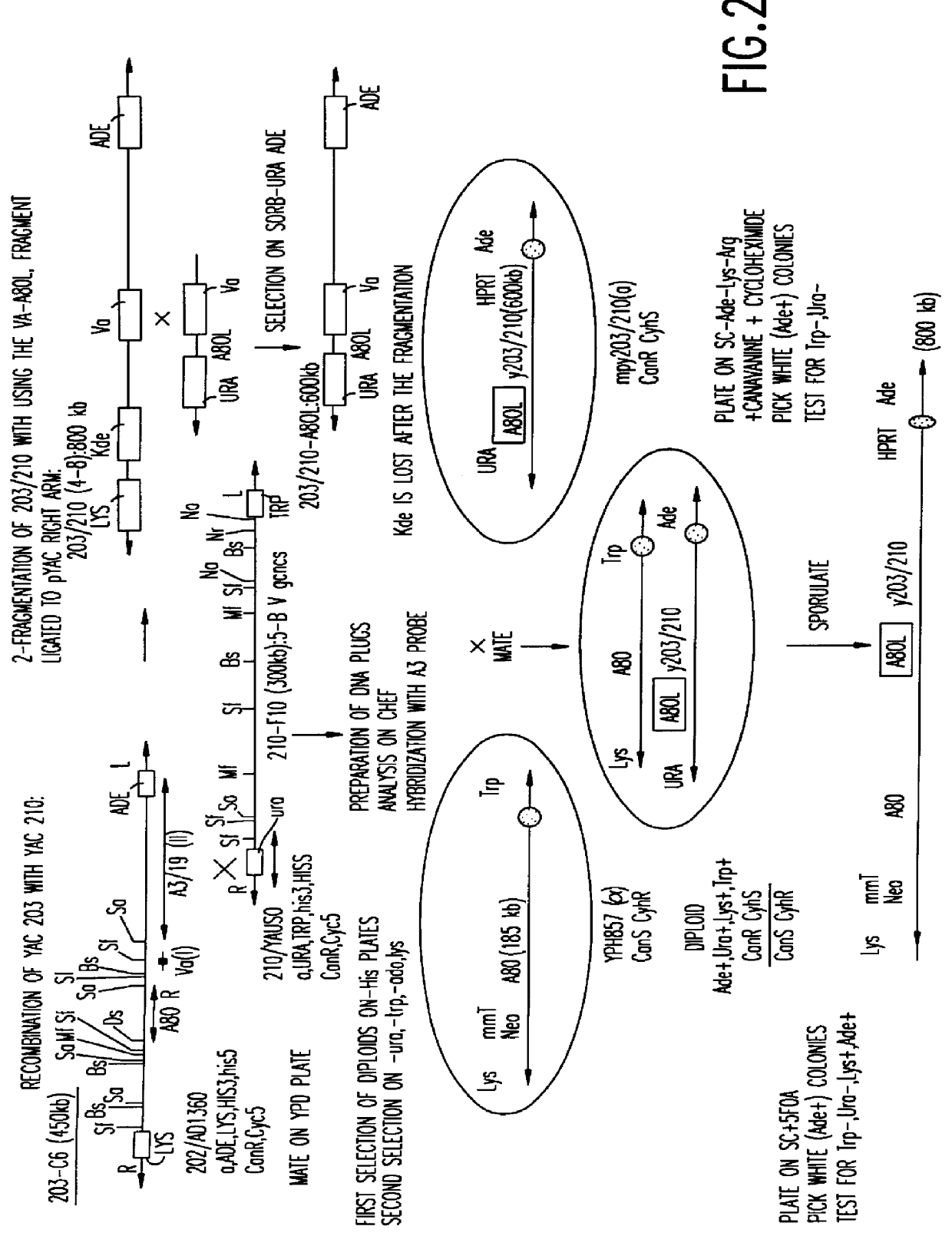
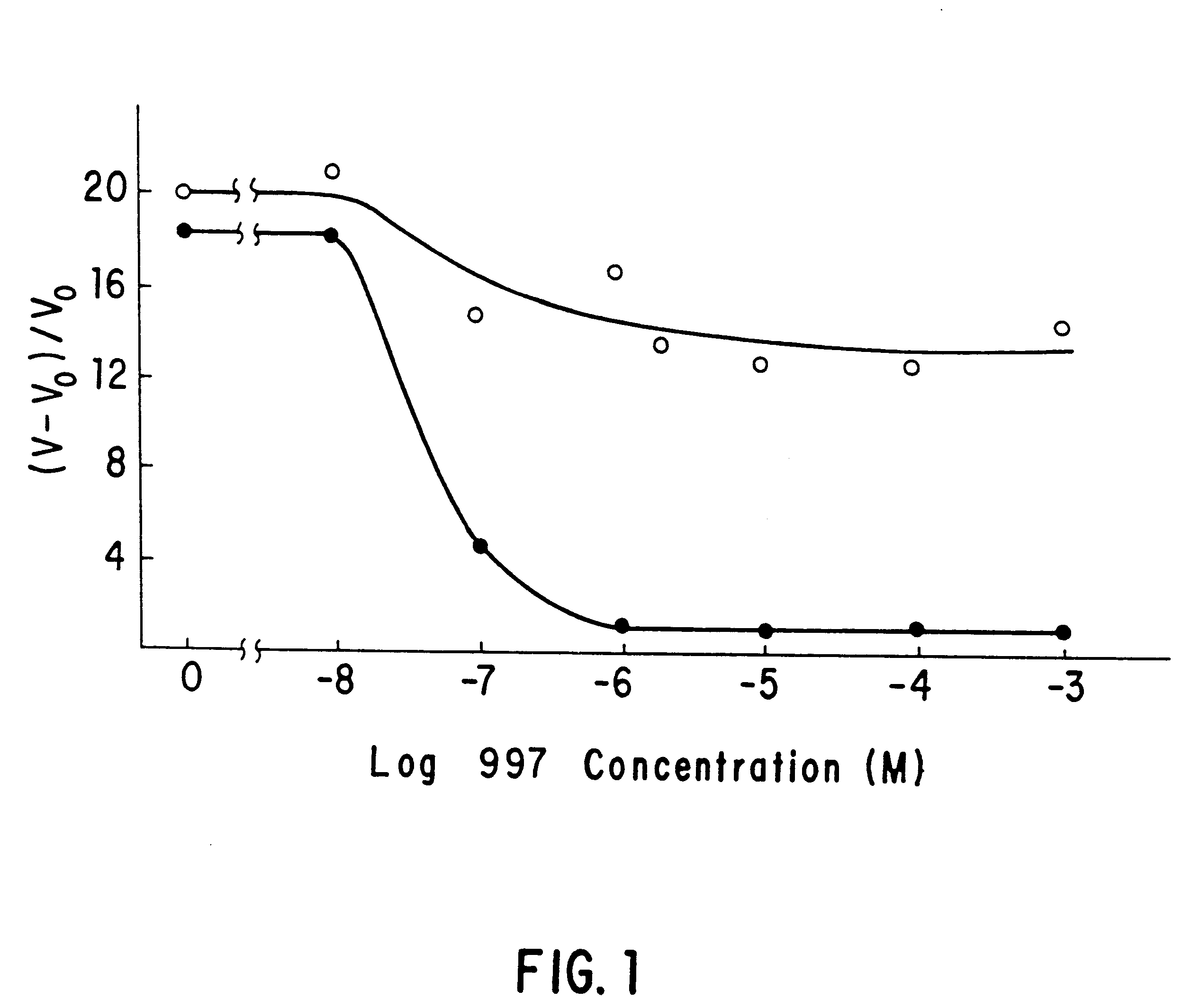
Human antibodies derived from immunized xenomice
Fully human antibodies against a specific antigen can be prepared by administering the antigen to a transgenic animal which has been modified to produce such antibodies in response to antigenic challenge, but whose endogenous loci have been disabled. Various subsequent manipulations can be performed to obtain either antibodies per se or analogs thereof.
Owner:AMGEN FREMONT INC
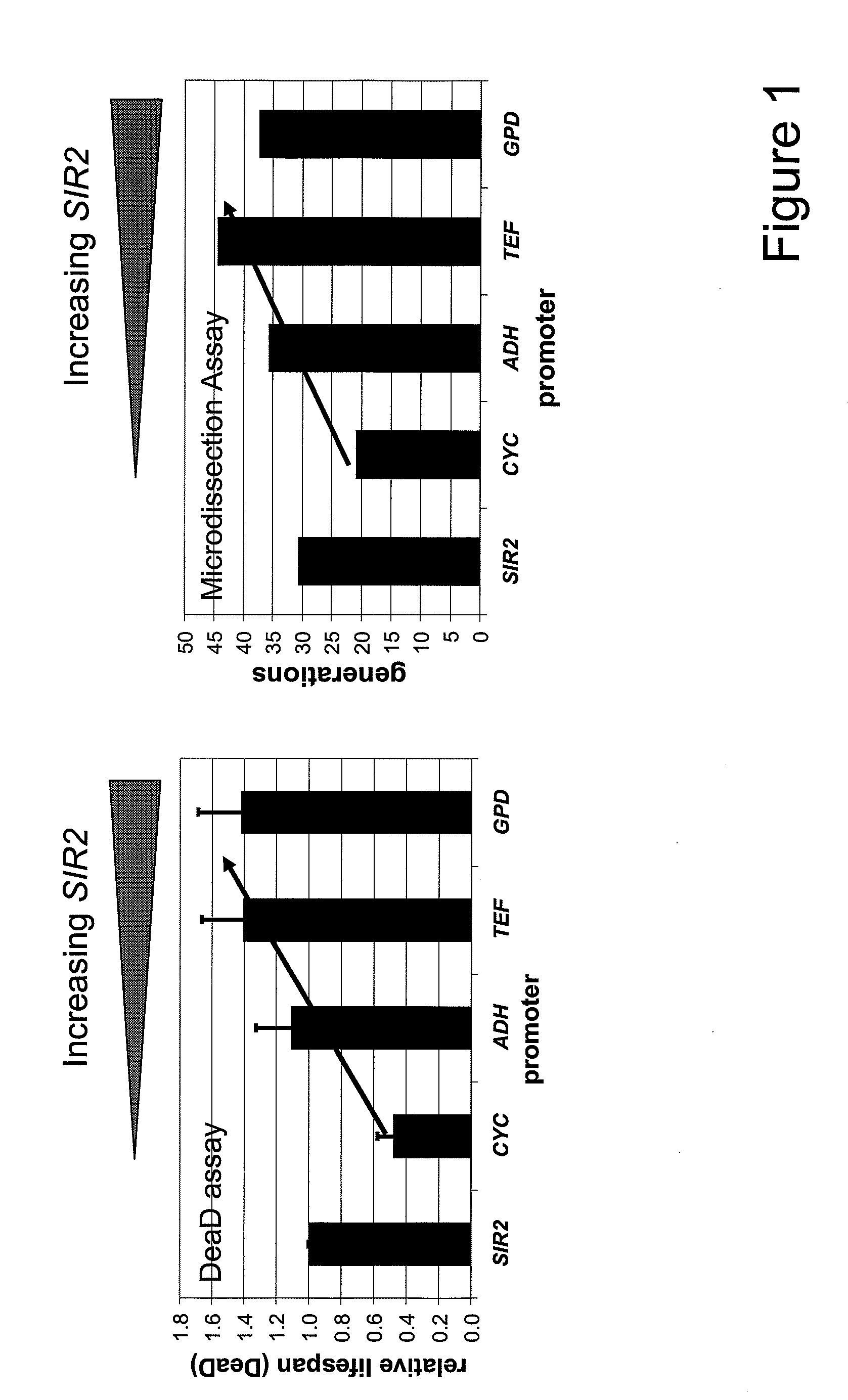
Method For Altering The Lifespan Of Eukaryotic Organisms
A method for altering the lifespan of a eukaryotic organism. The method comprises the steps of providing a lifespan altering compound, and administering an effective amount of the compound to a eukaryotic organism, such that the lifespan of the organism is altered. In one embodiment, the compound is identified using the DeaD assay.
Owner:UNIVERSITY OF ROCHESTER
6-modified bicyclic nucleic acid analogs
The present invention provides 6-modified bicyclic nucleoside analogs and oligomeric compounds comprising these nucleoside analogs. In preferred embodiments the nucleoside analogs have either (R) or (S)-chirality at the 6-position. These bicyclicnucleoside analogs are useful for enhancing properties of oligomeric compounds including nuclease resistance.
Owner:IONIS PHARMA INC
Oligonucleotide analogues
Novel oligomers, and synthesis thereof, comprising one or more bi-, tri, or polycyclic nucleoside analogues are disclosed herein. The nucleoside analogues have a “locked” structure, termed Locked Nucleoside Analogues (LNA). LNA's exhibit highly desirable and useful properties. LNA's are capable of forming nucleobase specific duplexes and triplexes with single and double stranded nucleic acids. These complexes exhibit higher thermostability than the corresponding complexes formed with normal nucleic acids. The properties of LNA's allow for a wide range of uses such as diagnostic agents and therapeutic agents in a mammal suffering from or susceptible to, various diseases.
Owner:EXIQON AS
Genetic constructs for delaying or repressing the expression of a target gene
The present invention relates generally to synthetic genes for modifying endogenous gene expression in a cell, tissue or organ of a transgenic organism, in particular a transgenic animal or plant. More particularly, the present invention provides novel synthetic genes and genetic constructs which are capable of repressing delaying or otherwise reducing the expression of an endogenous gene or a target gene in an organism when introduced thereto.
Owner:COMMONWEALTH SCI & IND RES ORG
Monomethylvaline compounds capable of conjugation to ligands
ActiveUS20050238649A1Improve bioavailabilityImprove compoundAntibacterial agentsBiocideD-norephedrineBiochemistry
Owner:SEAGEN INC
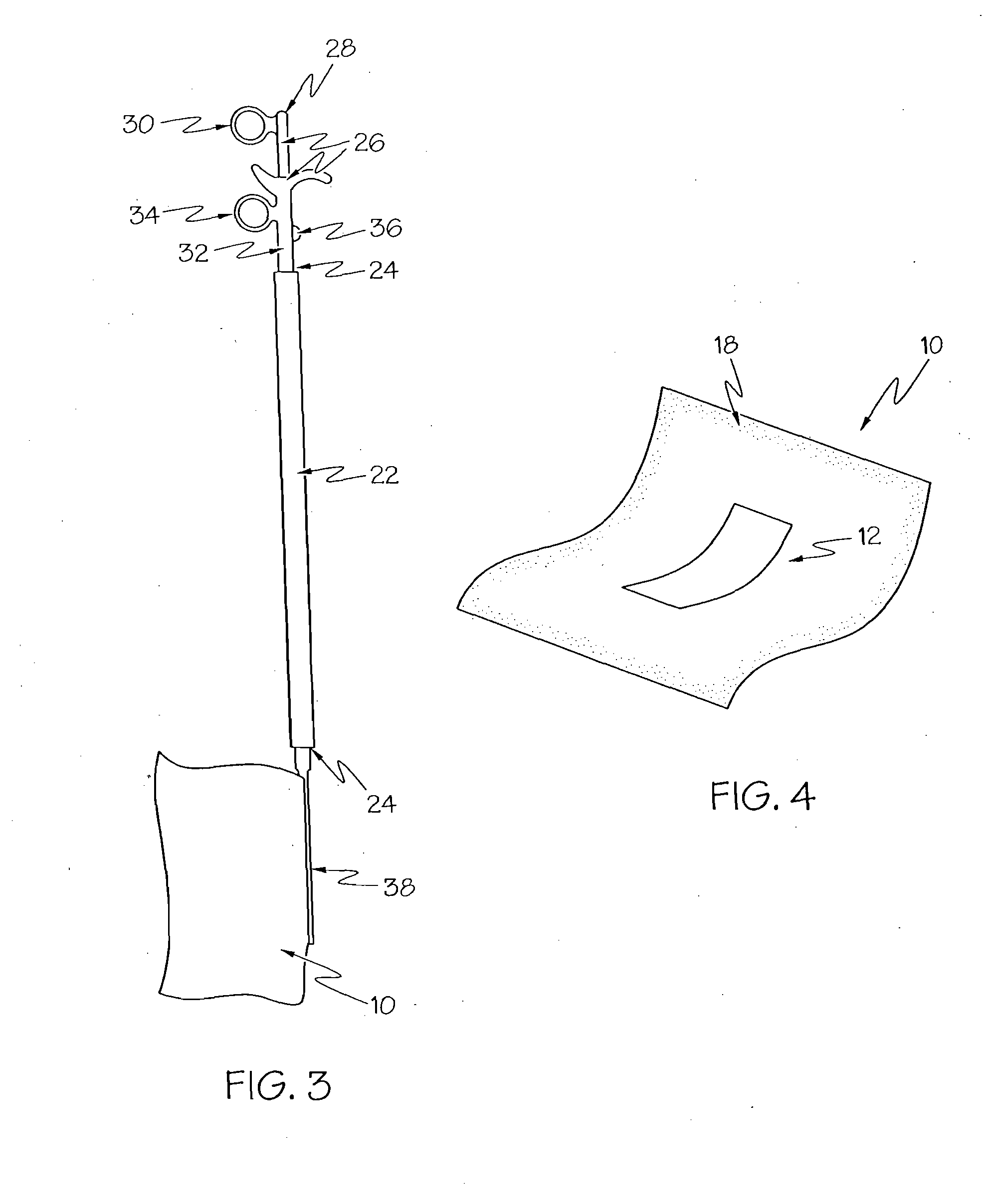
Medical film
InactiveUS7718556B2Good biocompatibilityHigh strengthSuture equipmentsBiocideGelatin filmThin membrane
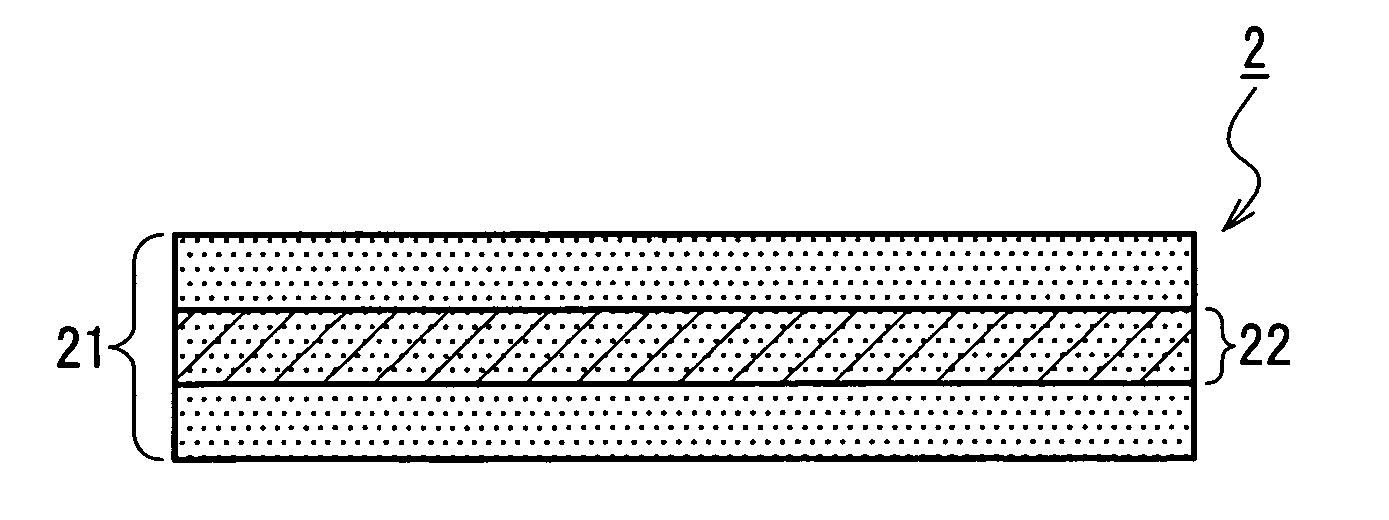
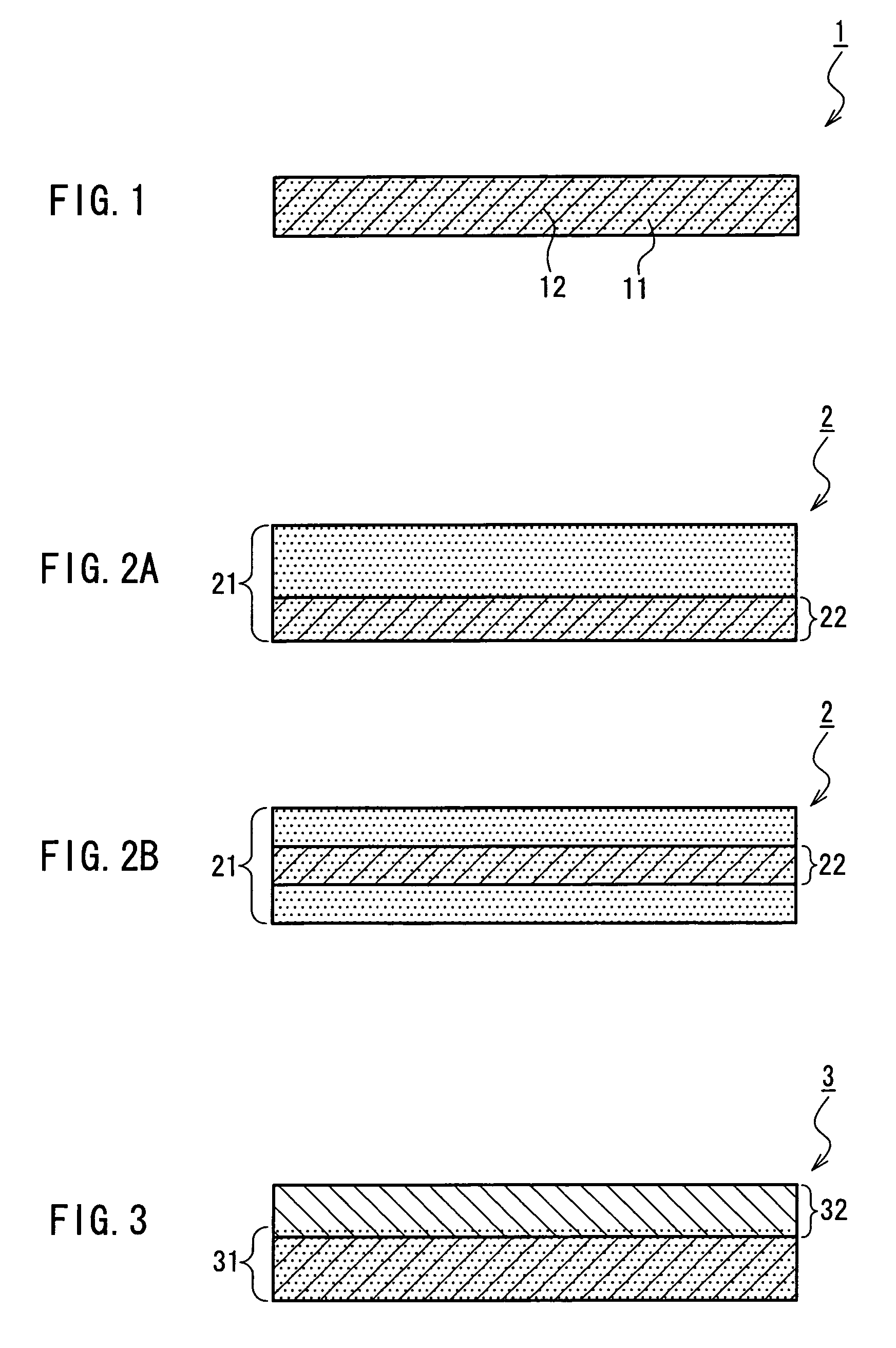
A medical film that is excellent in biocompatibility and bioabsorbability and has an excellent strength in suturing and bonding is provided. A reinforcing material 12 made of a biodegradable polymer is placed in a gelatin solution so as to allow the solution to infiltrate in the reinforcing material 12 and then the gelatin is dried. This allows the gelatin that has infiltrated entirely in an internal part of the reinforcing material 12 to gel, thereby forming a gelatin film 11. Thus, a medical film 1 in which the reinforcing material 12 and the gelatin film 11 are integrated is obtained. The gelatin film 11 preferably is a cross-linked gelatin film.
Owner:GUNZE LTD
Artificial nucleic acids of n-o bond crosslinkage type
ActiveUS7427672B2High sensitivity analysisConfirmed its usefulnessBiocideSugar derivativesSingle strandDouble strand
Owner:RIKEN GENESIS
Simultaneous stimulation and concentration of cells
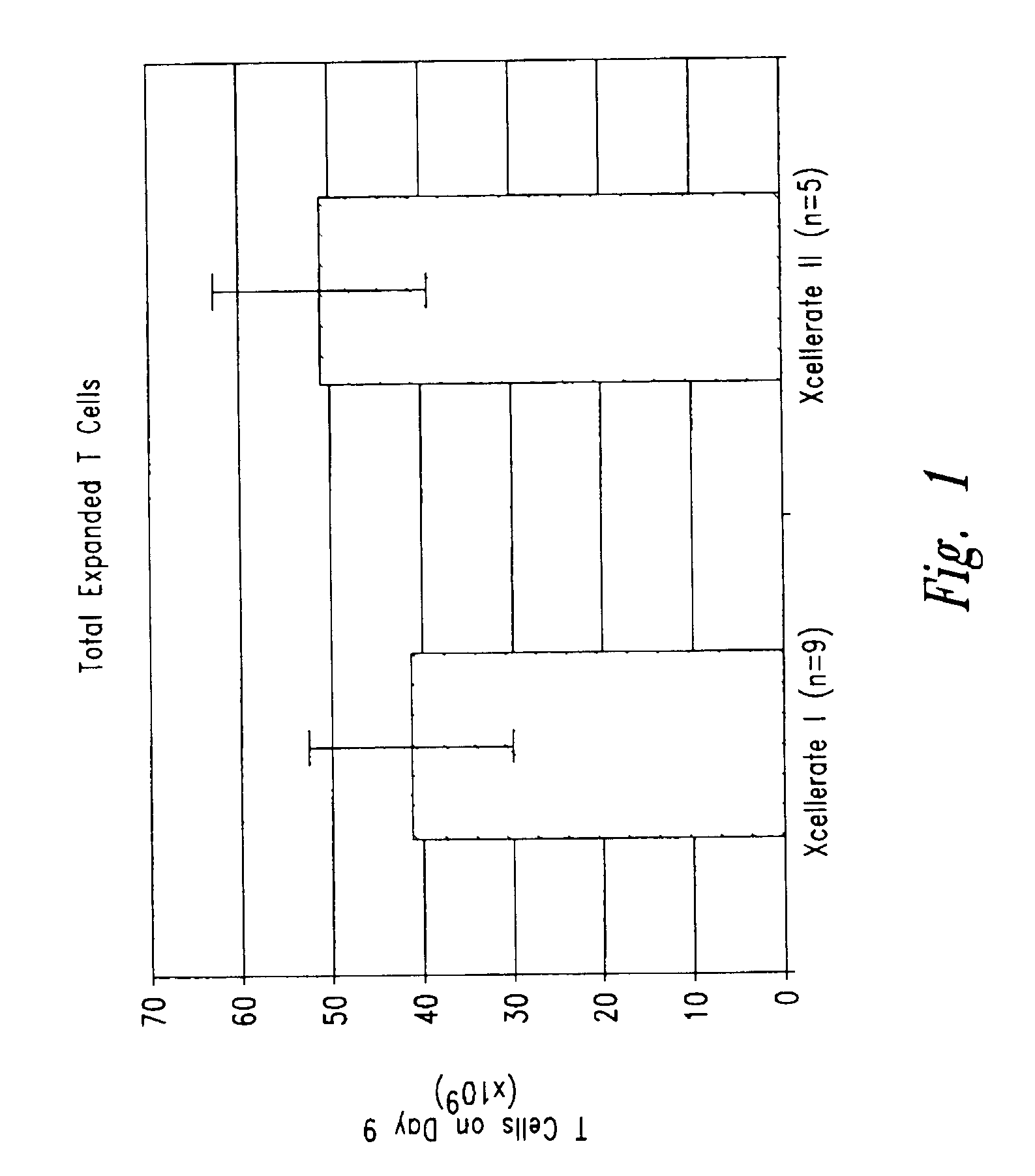
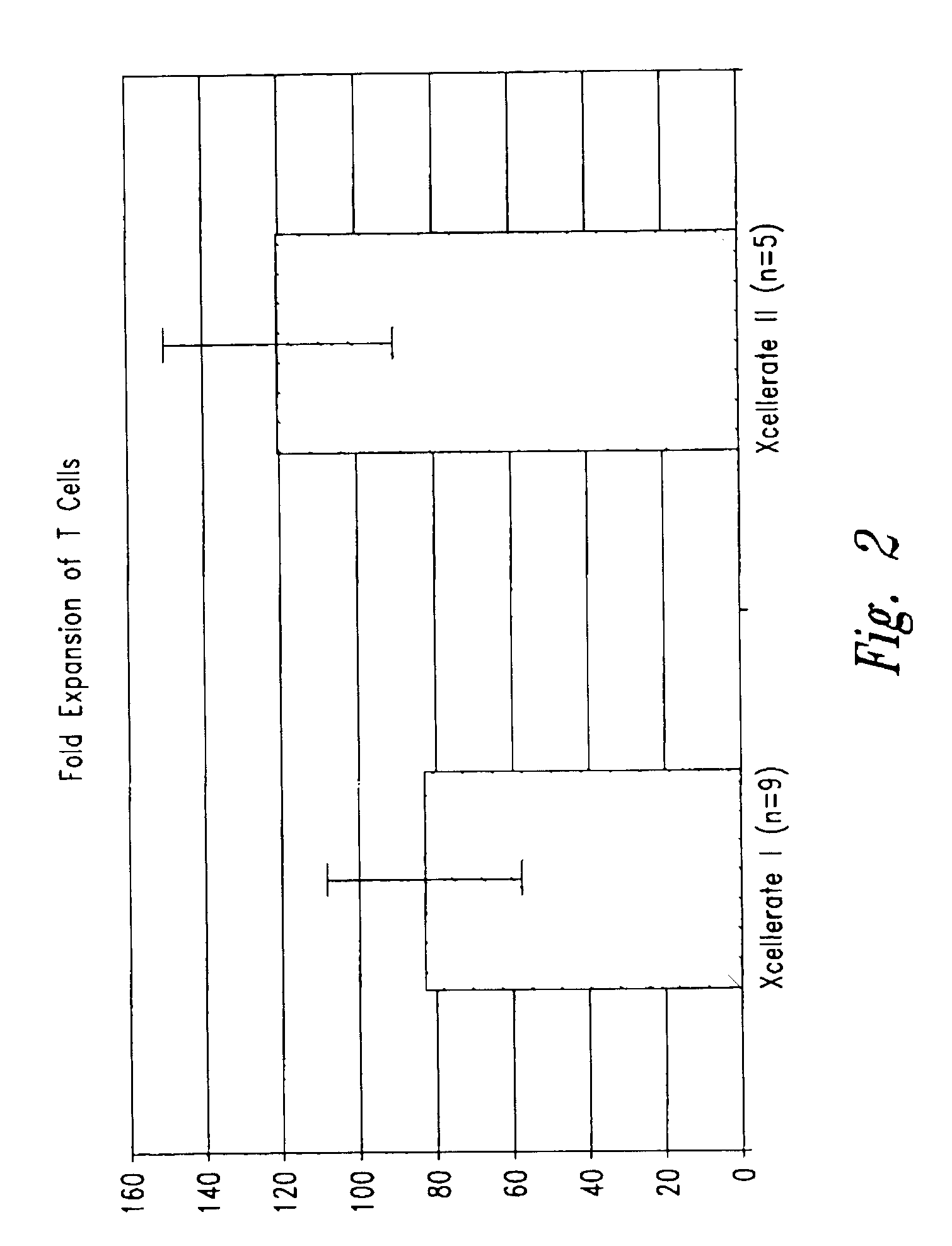
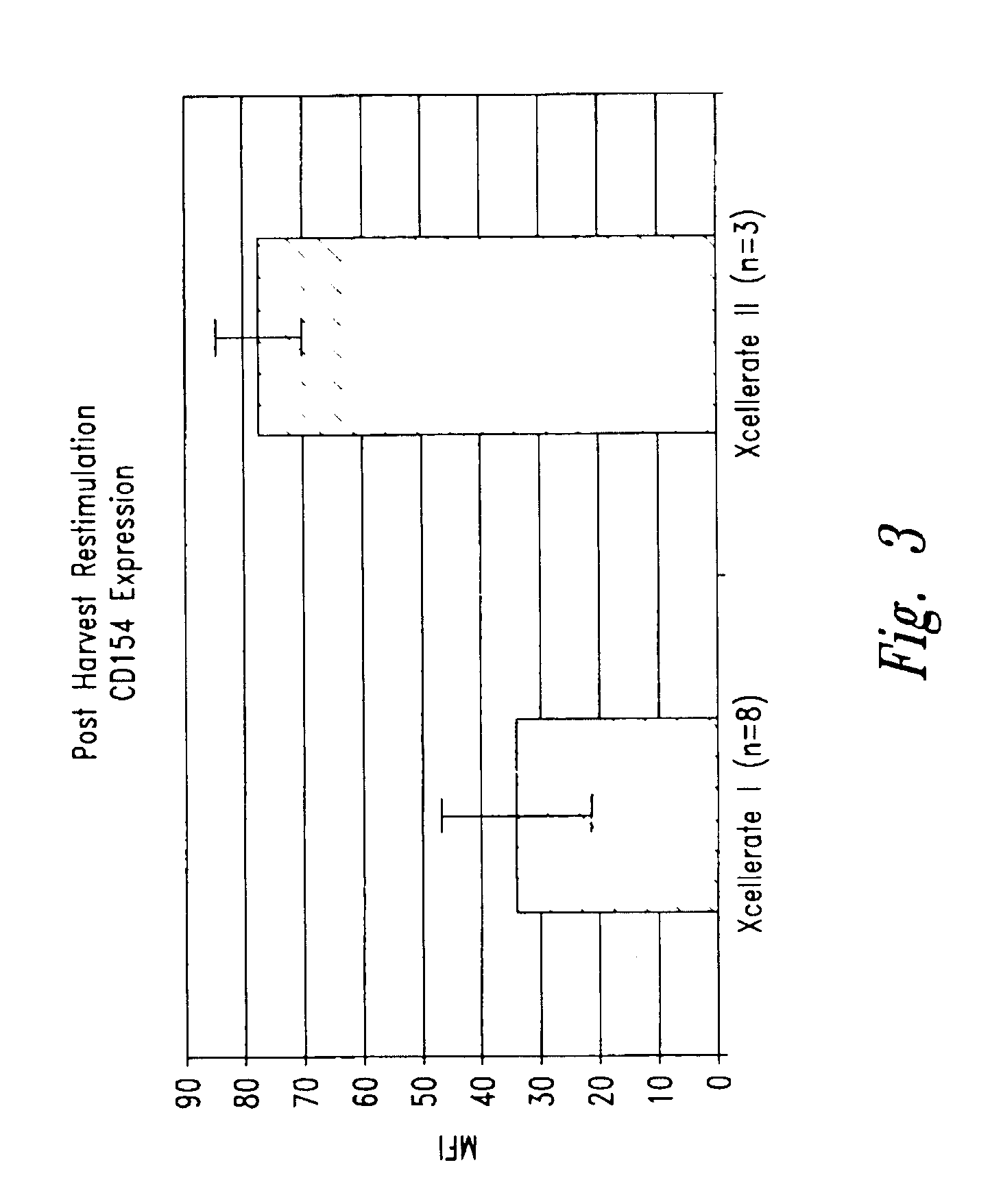
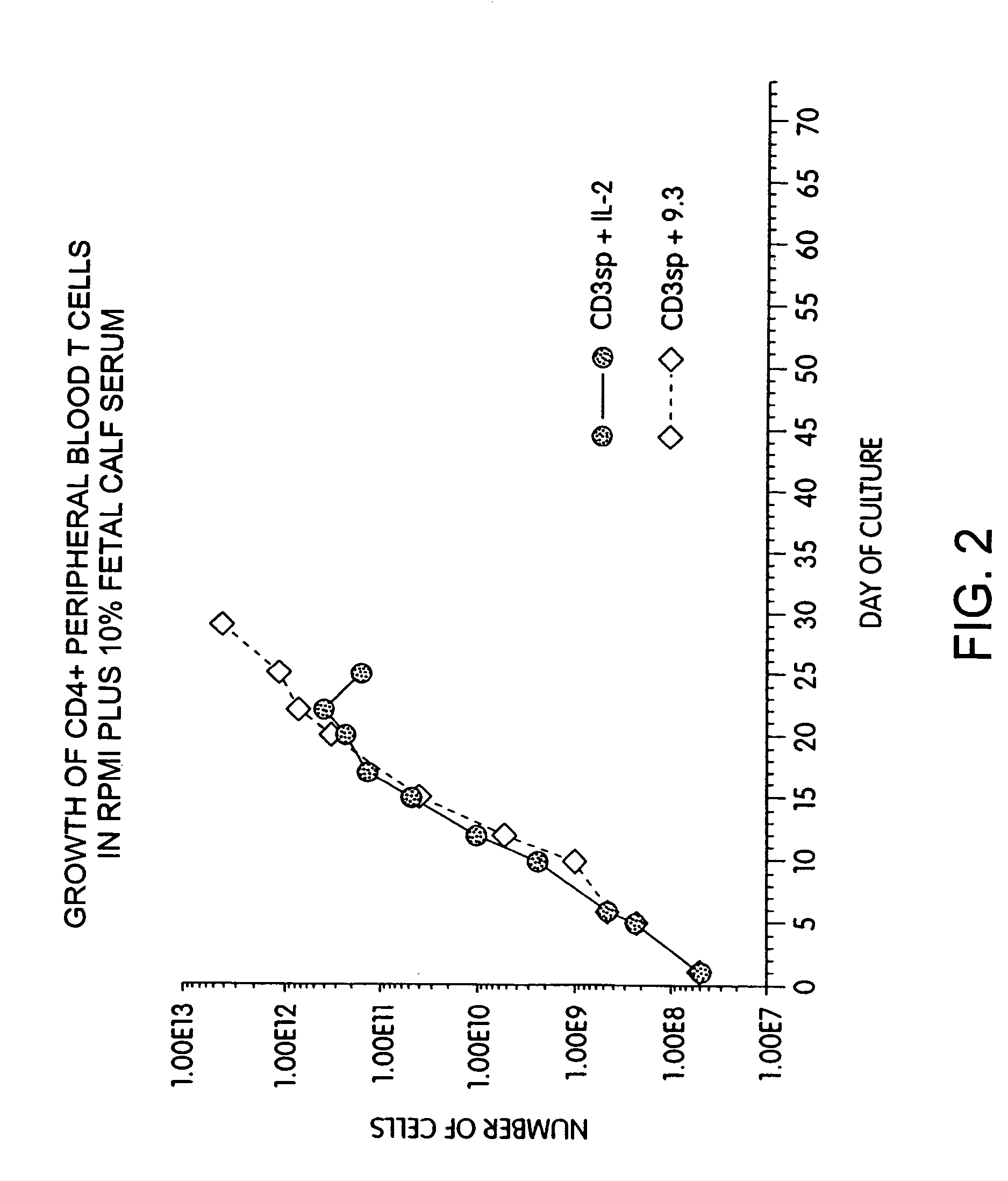
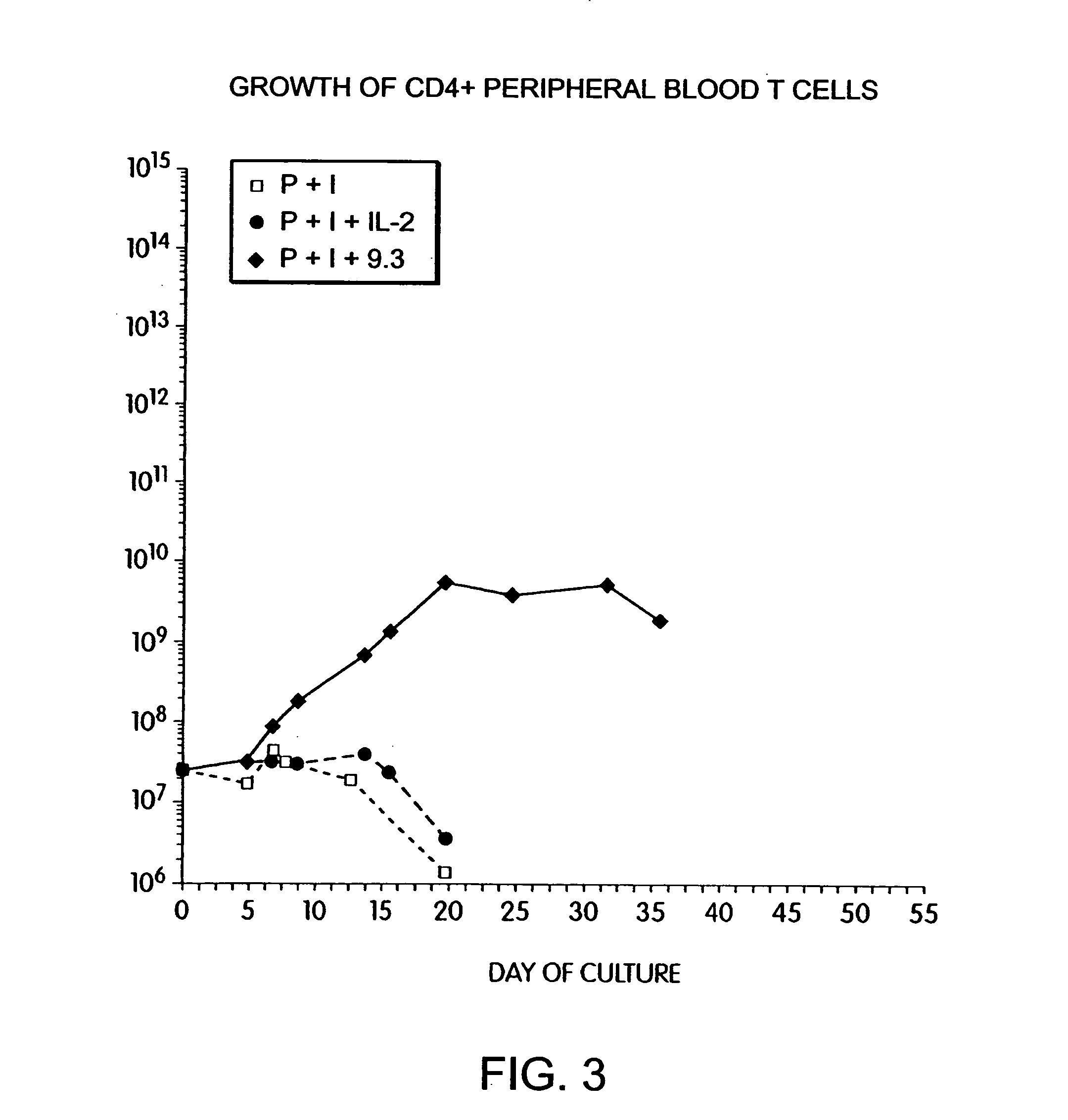
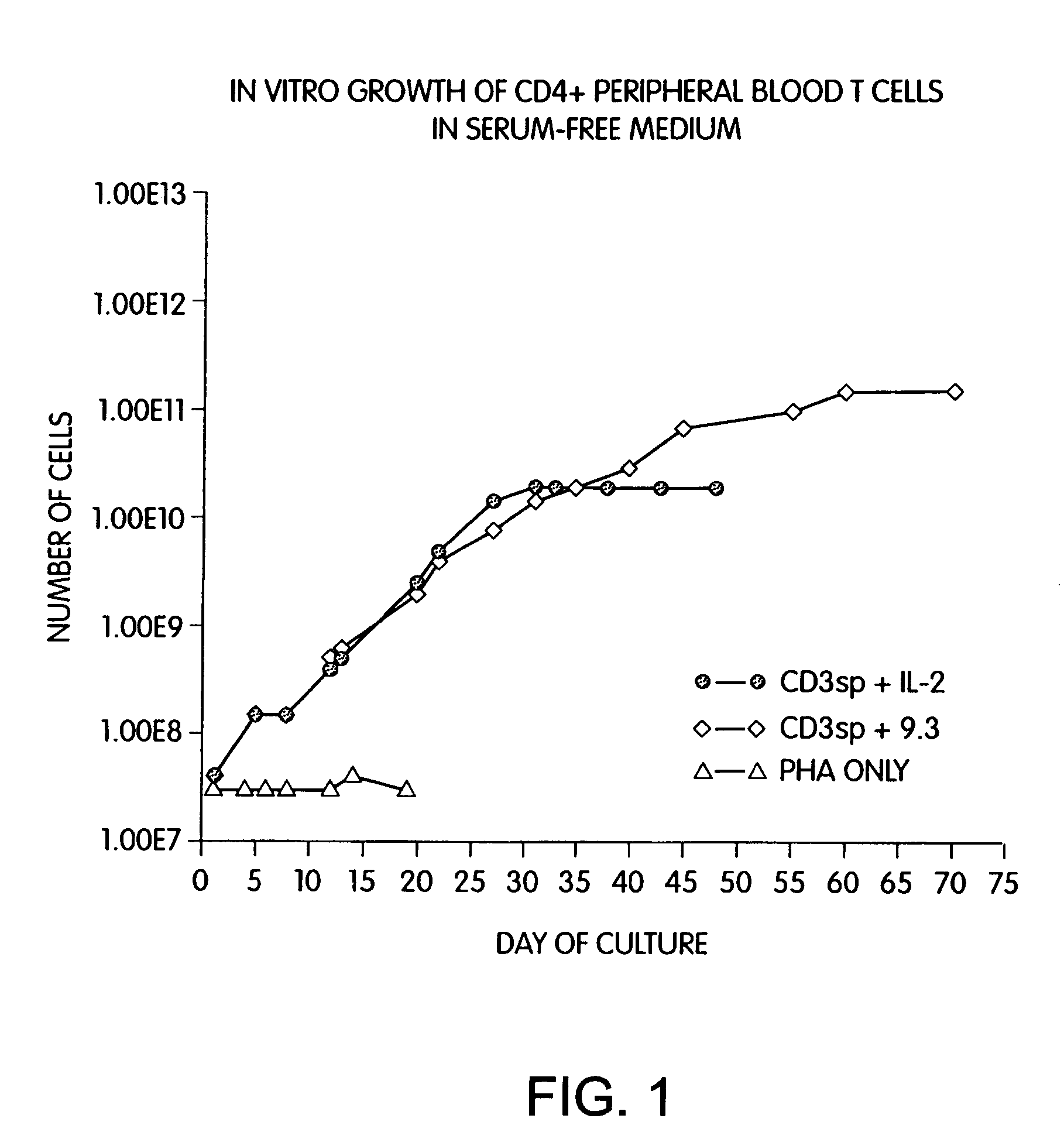
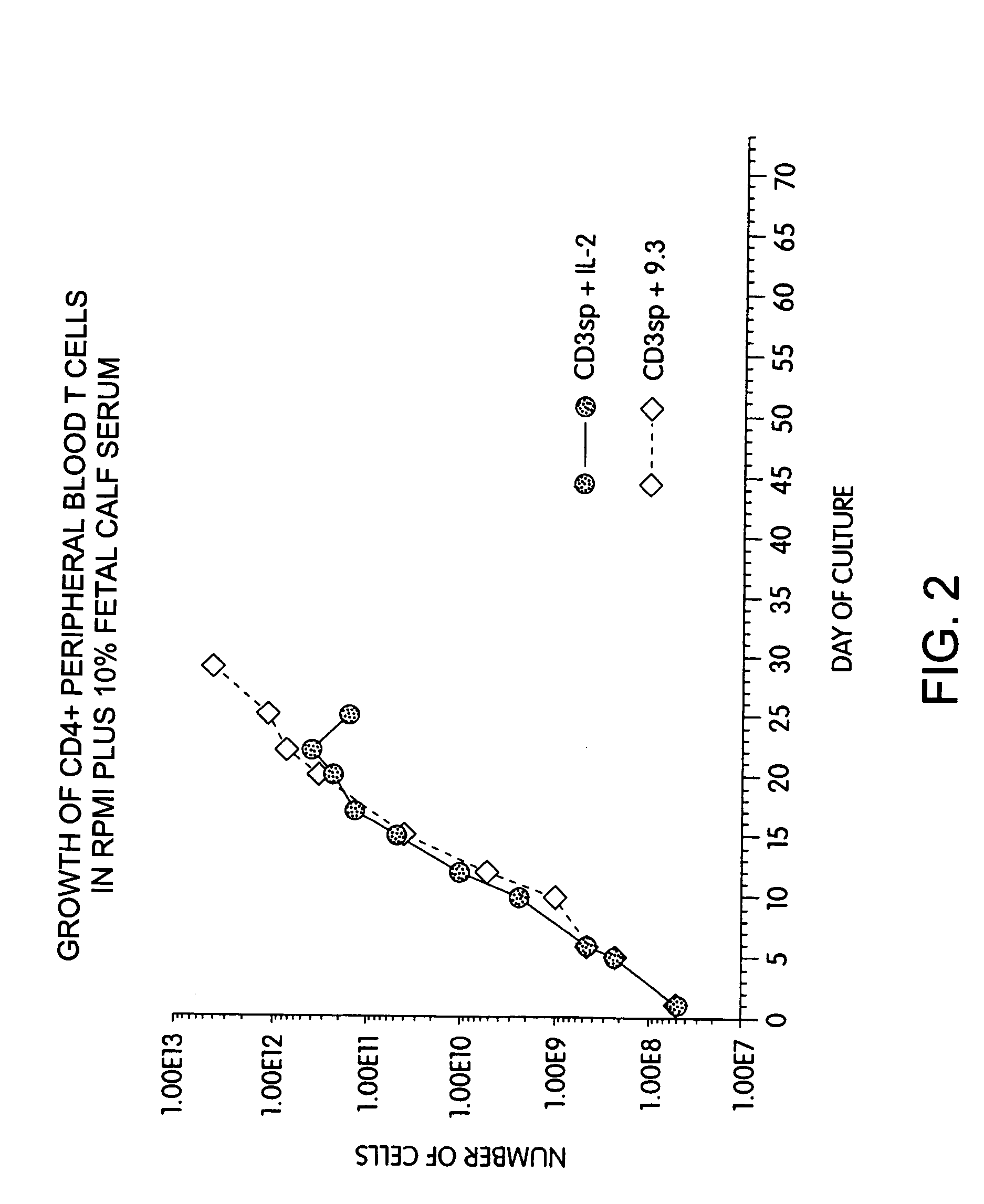
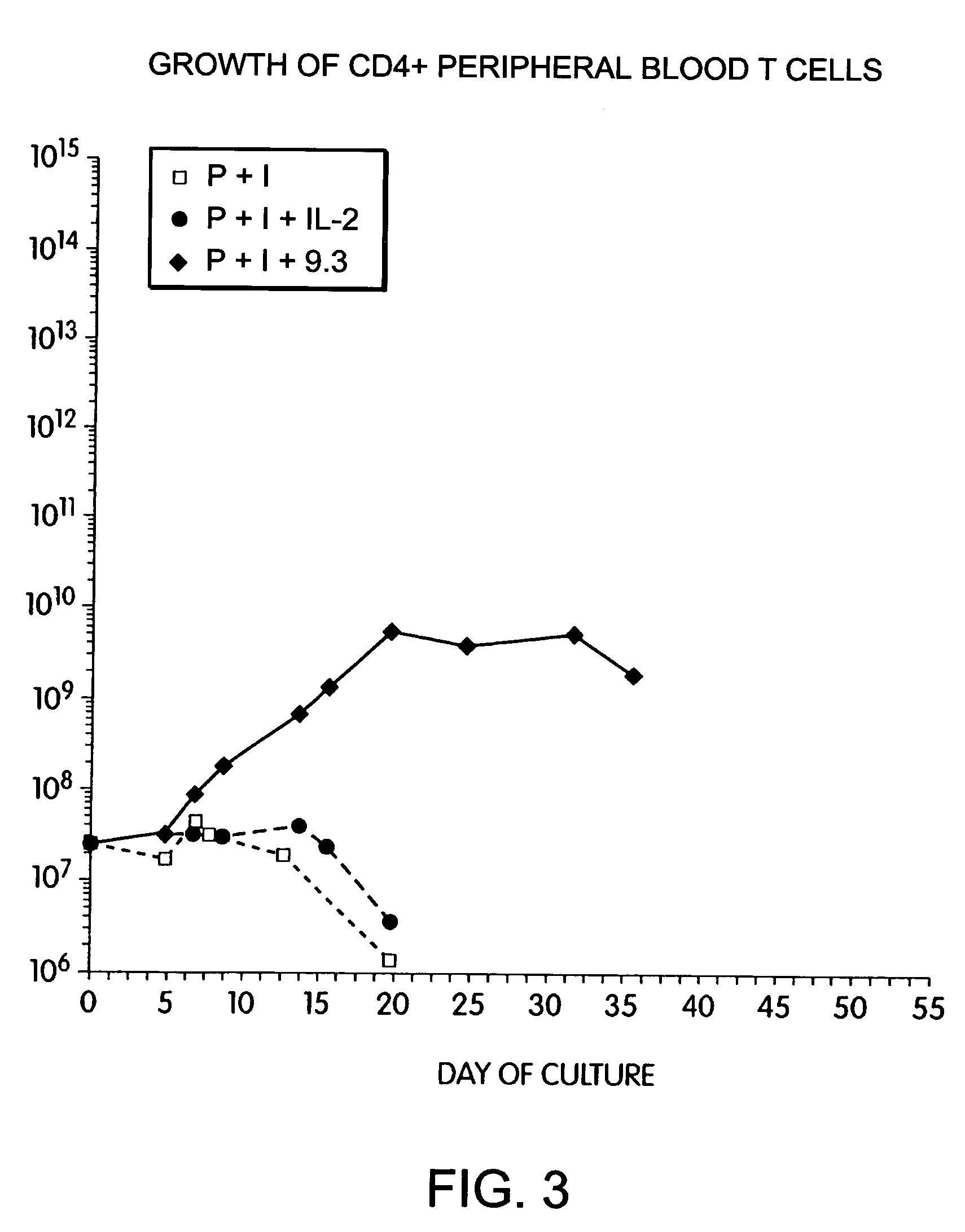
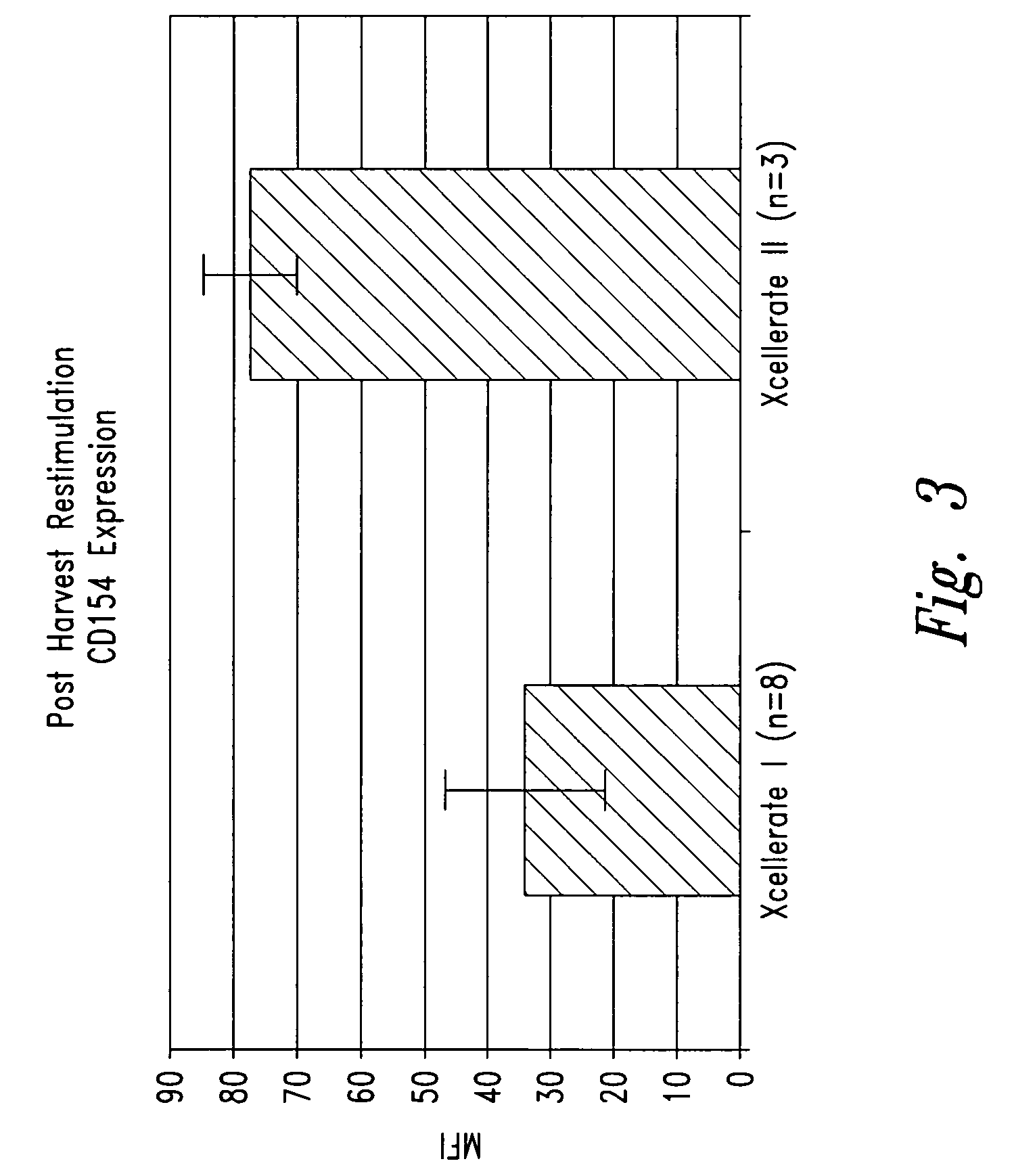
InactiveUS6905874B2Maximizes stimulationBiocideImmunoglobulins against cell receptors/antigens/surface-determinantsT cellCells signal
The present invention relates generally to methods for stimulating cells, and more particularly, to a novel method to concentrate and stimulate cells that maximizes stimulation and / or proliferation of such cells. In the various embodiments, cells are stimulated and concentrated with a surface yielding enhanced proliferation, cell signal transduction, and / or cell surface moiety aggregation. In certain aspects methods for stimulating a population of cells such as T-cells, by simultaneous concentration and cell surface moiety ligation are provided by contacting the population of cells with a surface, that has attached thereto one or more agents that ligate a cell surface moiety and applying a force that predominantly drives cell concentration and cell surface moiety ligation, thereby inducing cell stimulation, cell surface moiety aggregation, and / or receptor signaling enhancement. Also provided are methods for producing phenotypically tailored cells, including T-cells for the use in diagnostics, drug discovery, and the treatment of a variety of indications, including cancer, viral infection, and immune related disorders. Compositions of cells having specific phenotypic properties produced by these processes are further provided.
Owner:LIFE TECH CORP
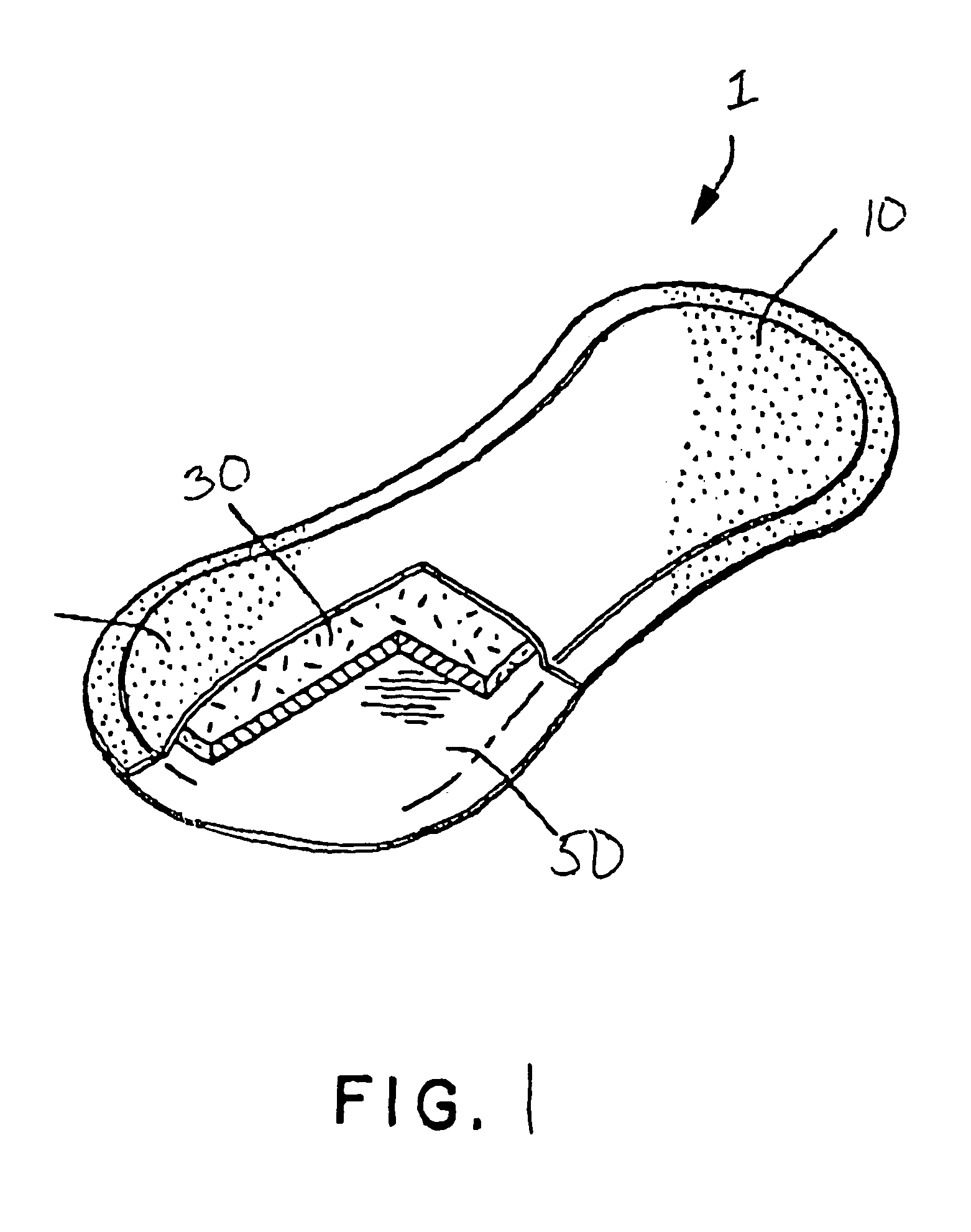
Use of reinforced foam implants with enhanced integrity for soft tissue repair and regeneration
A biocompatible tissue repair stimulating implant or "scaffold" device is used to repair tissue injuries, particularly injuries to ligaments, tendons, and nerves. Such implants are especially useful in methods that involve surgical procedures to repair injuries to ligament, tendon, and nerve tissue in the hand and foot. The repair procedures may be conducted with implants that contain a biological component that assists in healing or tissue repair.
Owner:DEPUY MITEK INC
Enzymatic nucleic acid synthesis: compositions and methods for altering monomer incorporation fidelity
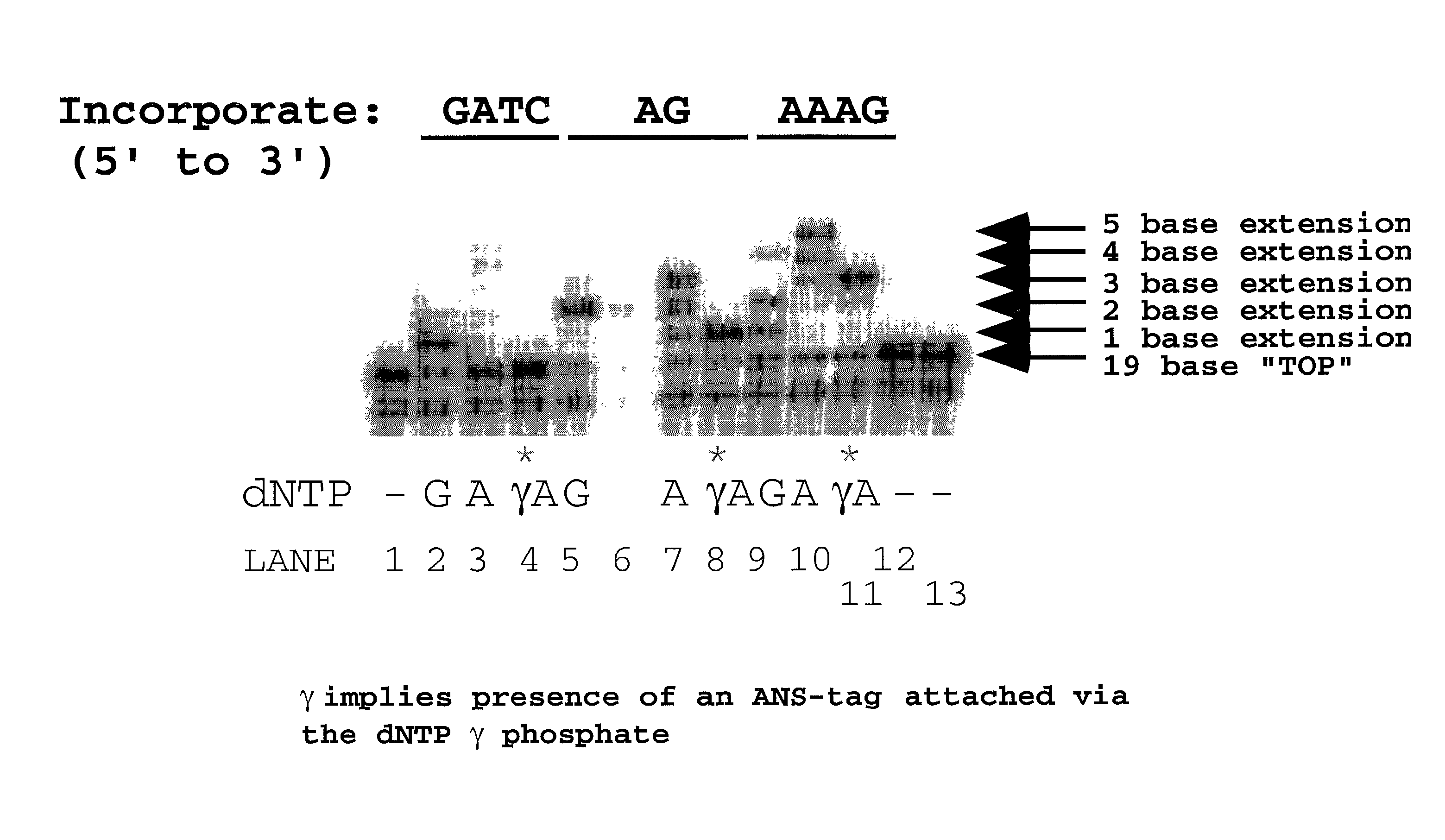
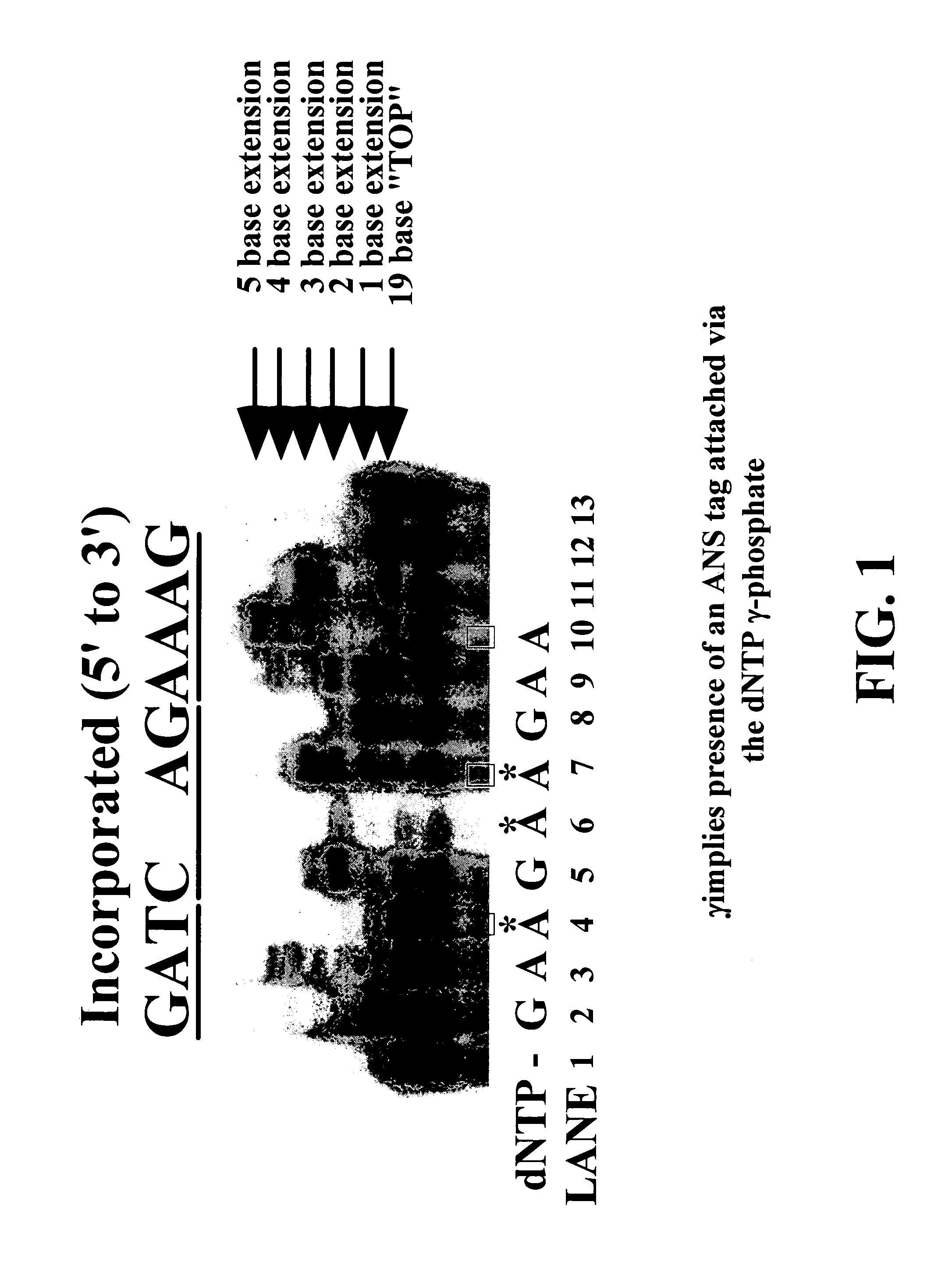
InactiveUS7211414B2Extent of pyrophosphorolysis of a primer extension product is reducedImprove fidelityBiocideSugar derivativesPhosphatePhosphoric acid
Nucleotide triphosphate probes containing a molecular and / or atomic tag on a a γ and / or β phosphate group and / or a base moiety having a detectable property are disclosed, and kits and method for using the tagged nucleotides in sequencing reactions and various assay. Also, phosphate and polyphosphate molecular fidelity altering agents are disclosed.
Owner:LIFE TECH CORP
Drug administration method
InactiveUS7427607B2Reduce weightPrevention of the adhesion of an organBiocidePowder deliveryBiopolymerDrug administration
A method of administering a drug whereby a fine drug powder can be accurately administered to a target site (in particular, a target site in the body cavity) via fluidization and spraying with a gas by using a micro tube. Concerning the administration mode, in particular, the drug alone or a biopolymer is administered or the biopolymer is employed as a carrier in the above method. More specifically speaking, a method of administering a fine drug powder which comprises finely milling one or more types fine particles of the drug and / or the biopolymer, blending them each other, fluidizing the blend with a gas, then transporting the fluidized matter in a micro tube by the gas stream and spraying the fine drug powder from the tip of the micro tube toward the target site. Further, an administration method which comprises concentrically providing a capillary tube in the micro tube, supplying an aqueous solution of the drug and / or the biopolymer from the capillary tube into the gas stream and then mixing it with other fine particles of the drug and / or the biopolymer under transportation by the gas.
Owner:NEXT21 KK
Porous membranes for use with implantable devices
A membrane for implantation in soft tissue comprising a first domain that supports tissue ingrowth, disrupts contractile forces typically found in a foreign body response, encourages vascularity, and interferes with barrier cell layer formation, and a second domain that is resistant to cellular attachment, is impermeable to cells and cell processes, and allows the passage of analytes. The membrane allows for long-term analyte transport in vivo and is suitable for use as a biointerface for implantable analyte sensors, cell transplantation devices, drug delivery devices, and / or electrical signal delivering or measuring devices. The membrane architecture, including cavity size, depth, and interconnectivity, provide long-term robust functionality of the membrane in vivo.
Owner:DEXCOM INC
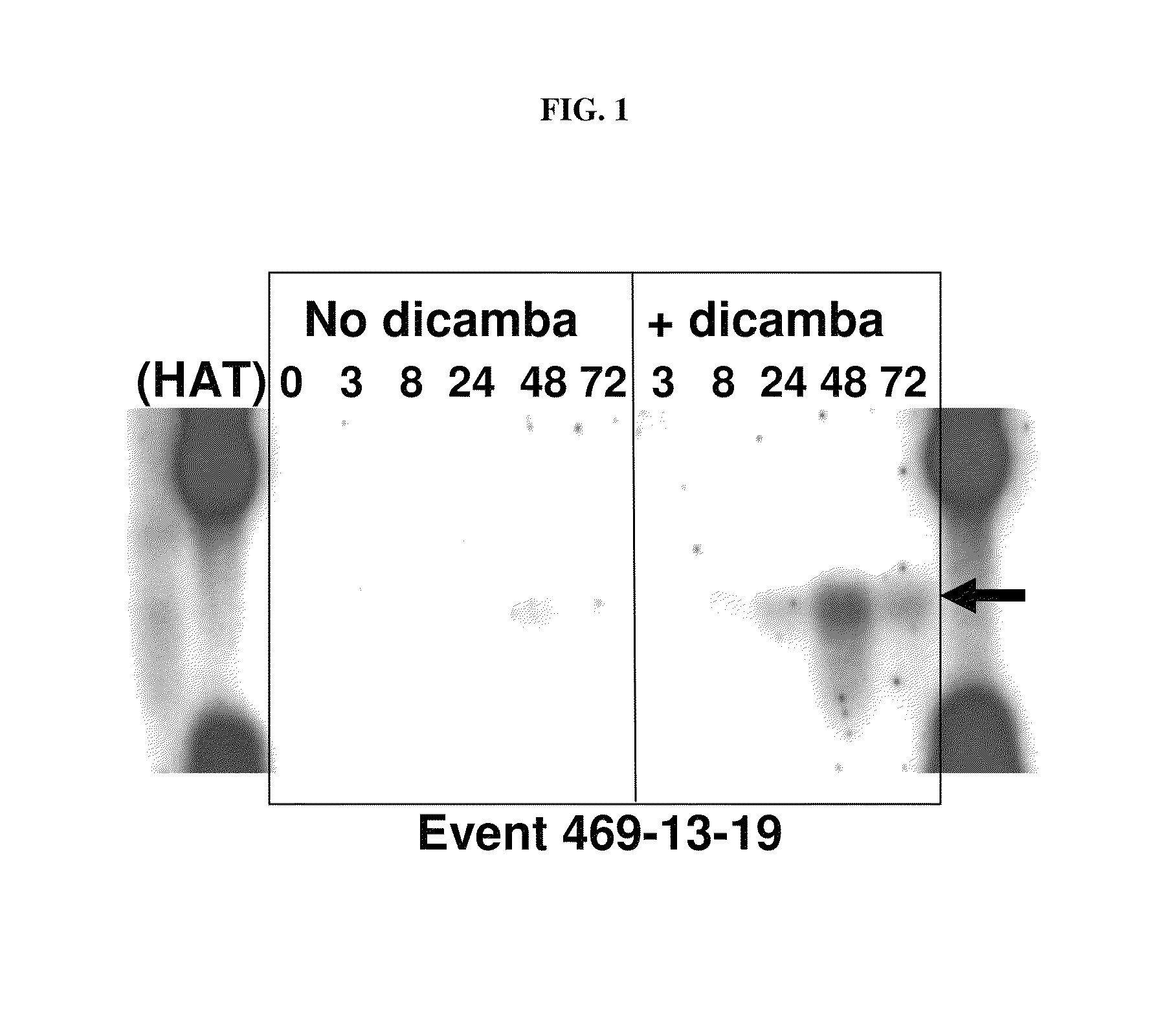
Method for disease control in MON89788 soybean
The present invention relates to a method to control diseases of MON89788 soybean by treatment with formulations and mixtures containing glyphosate. In particular, the formulations and mixtures are effective at controlling fungal diseases of MON89788. More specifically, the invention relates to a method to control the severity of leaf rust disease on MON89788.
Owner:MONSANTO TECH LLC
Nanostructure-enhanced platelet binding and hemostatic structures
InactiveUS8319002B2Enhancing overall rate and strengthInduce platelet binding and efficient hemostasisBiocideSurgical adhesivesPlateletNanofiber
Methods, systems, and apparatuses for nanomaterial-enhanced platelet binding and hemostatic medical devices are provided. Hemostatic materials and structures are provided that induce platelet binding, including platelet binding and the coagulation of blood at a wound / opening caused by trauma, a surgical procedure, ulceration, or other cause. Example embodiments include platelet binding devices, hemostatic bandages, hemostatic plugs, and hemostatic formulations. The hemostatic materials and structures may incorporate nanostructures and / or further hemostatic elements such as polymers, silicon nanofibers, silicon dioxide nanofibers, and / or glass beads into a highly absorbent, gelling scaffold. The hemostatic materials and structures may be resorbable.
Owner:NANOSYS INC
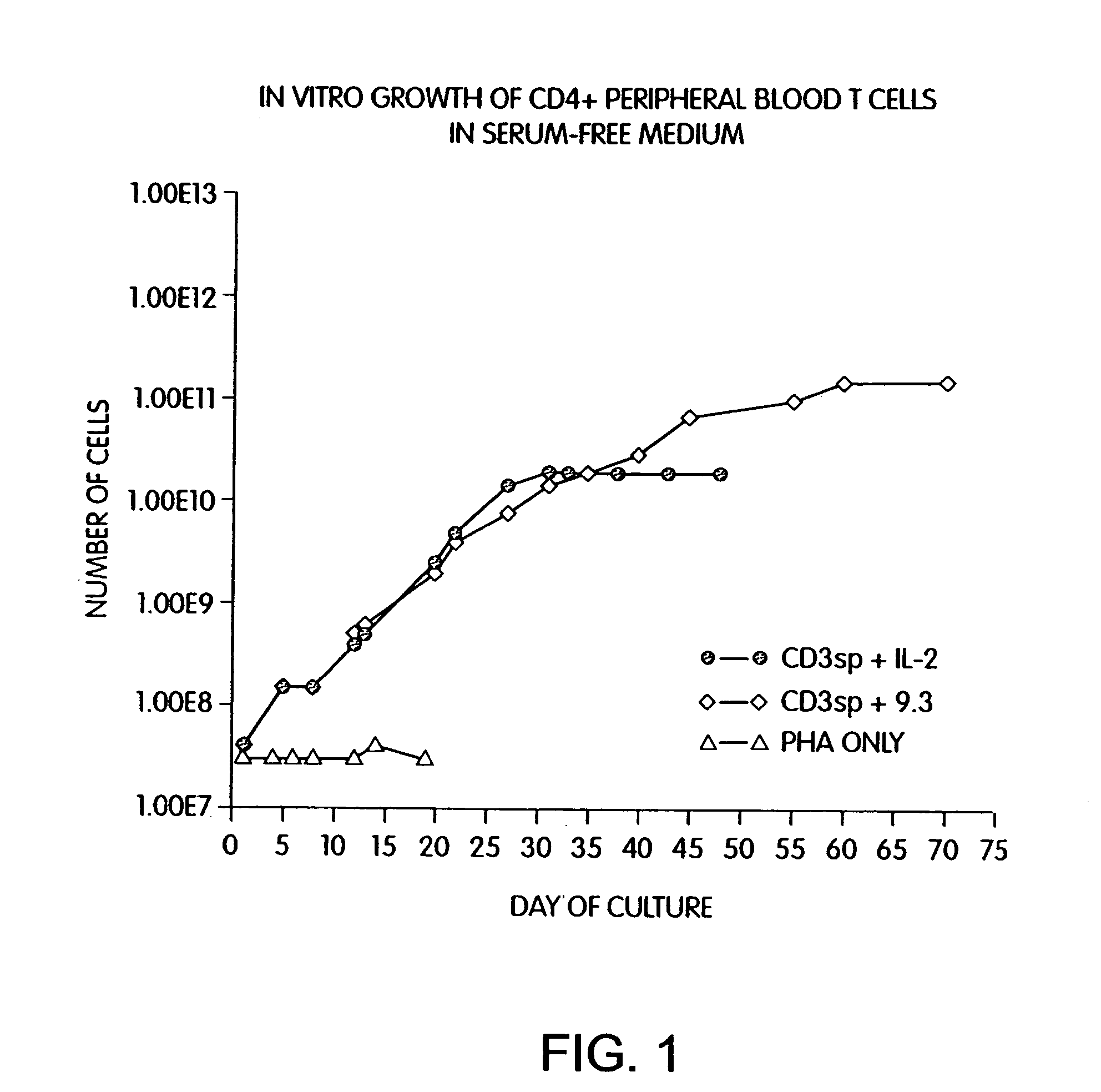
Methods for selectively stimulating proliferation of T cells
InactiveUS7175843B2Increase the number ofBiocideCell receptors/surface-antigens/surface-determinantsAccessory moleculeExogenous growth
Owner:GENETICS INST LLC +2
Methods for reduction of post operative ileus.
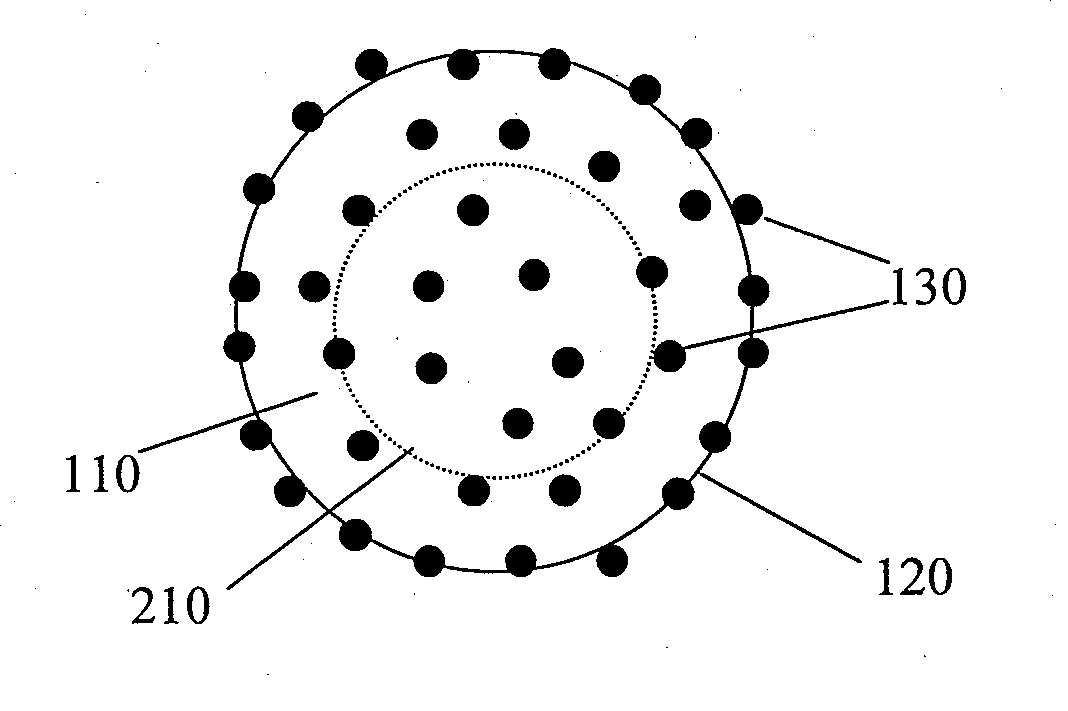
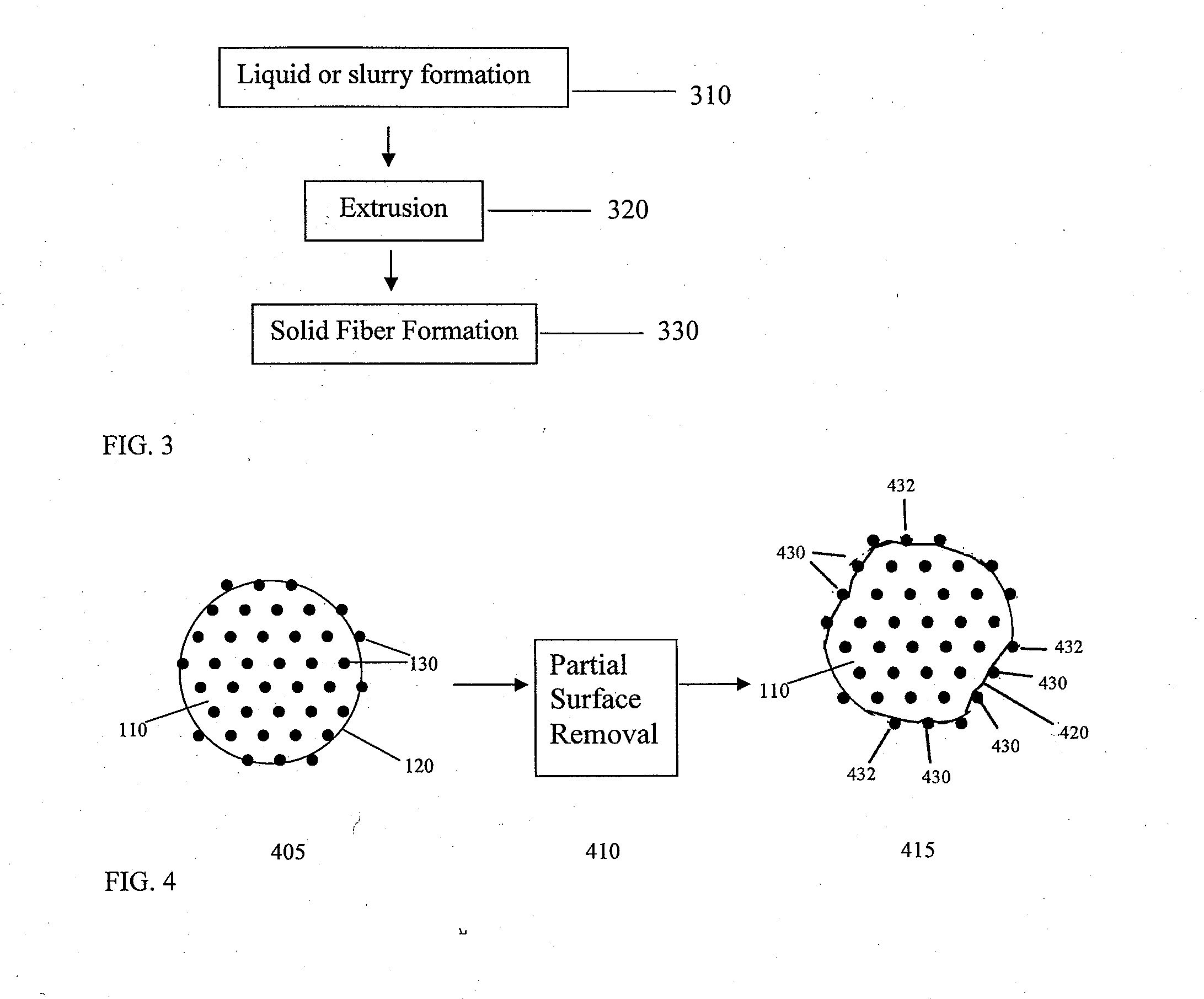
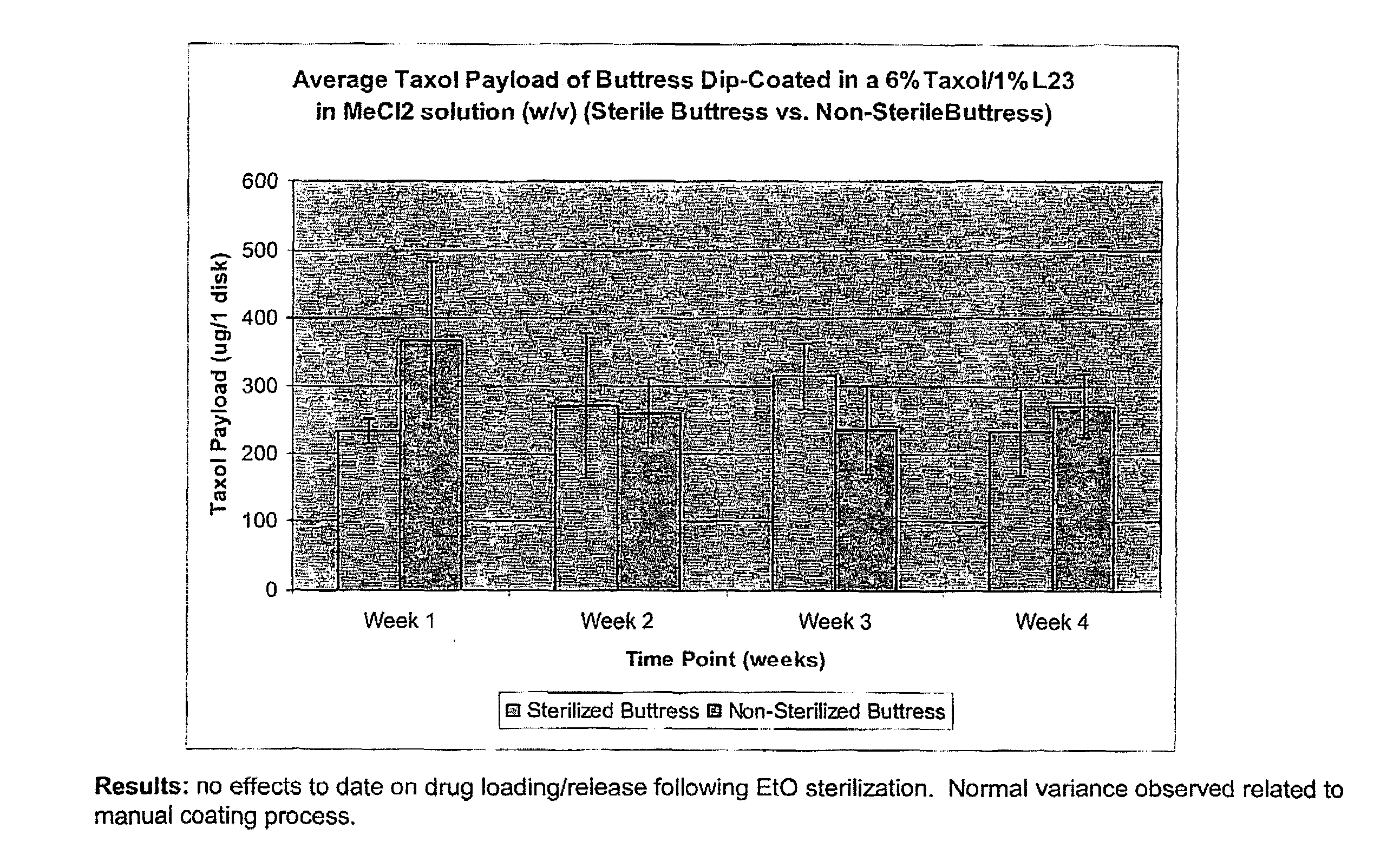
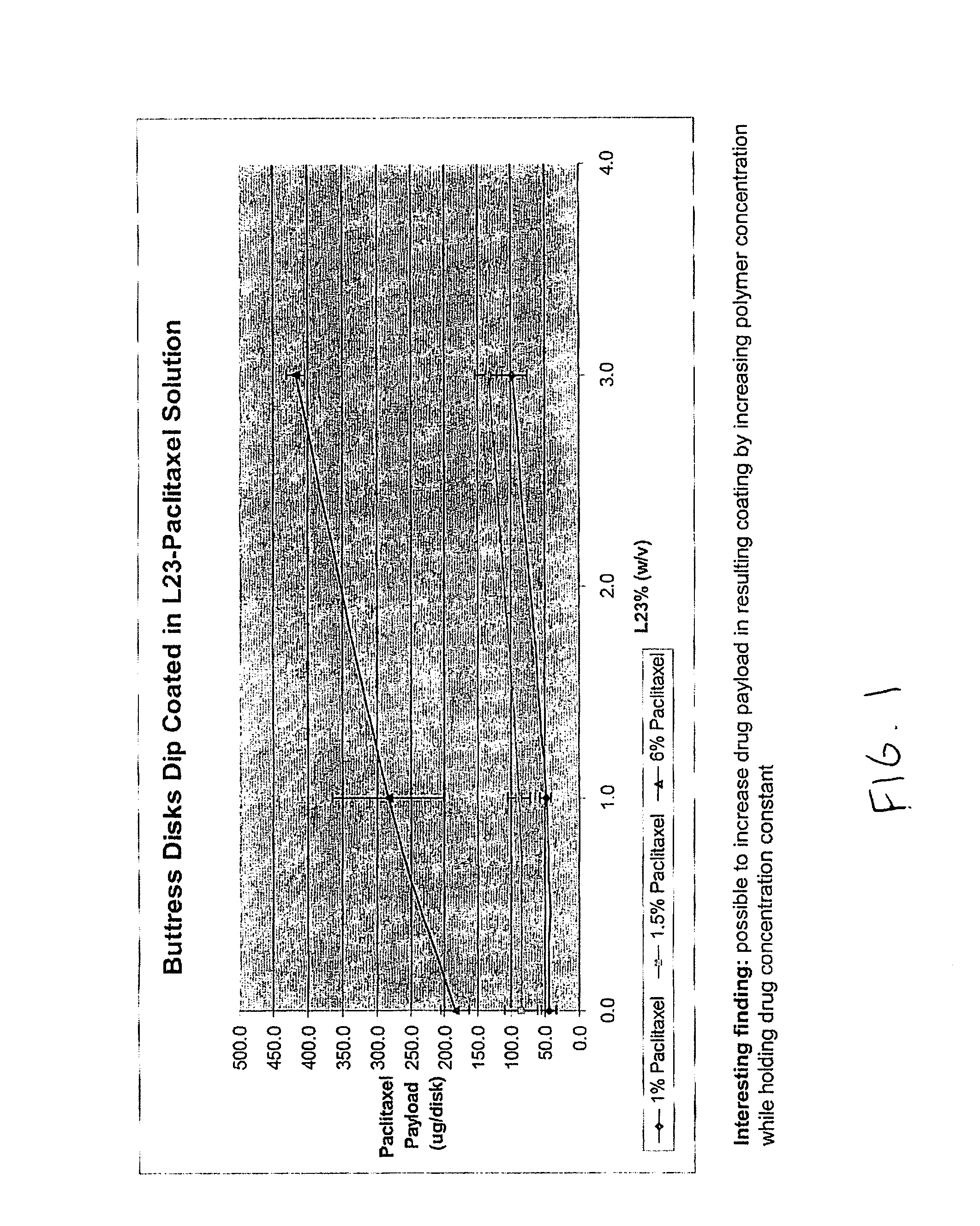
InactiveUS20080085296A1Reducing post-operative ileus and/or gastric stasisReducing post-operative ileusBiocideDigestive systemButtressSurgical department
An apparatus and method for reducing post-operative ileus and / or gastric stasis is described. The method can include applying to the intestine a therapeutically effective amount of a composition comprising a drug that is effective in reducing post-operative ileus and / or gastric stasis, such as by introducing the composition through a surgical access device, such as a trocar or endoscope. The apparatus can include a sheet of material, a surgical fastener, or a buttress comprising the composition.
Owner:ETHICON ENDO SURGERY INC
Plasma protein matrices and methods for their preparation
InactiveUS7009039B2Rapid cell growthRapid vascularizationBiocidePeptide/protein ingredientsBiological propertyFreeze-drying
A freeze dried biocompatible matrix comprising plasma proteins, useful as implants for tissue engineering as well as in biotechnology, and methods of producing the matrix are provided. Mechanical and physical parameters can be controlled by use of auxiliary components or additives which may be removed after the matrix is formed in order to improve the biological properties of the matrix. The matrices according to the present invention may be used clinically per se, or as a cell-bearing implant.
Owner:PROCHON BIOTECH
Hemostatic fibrous material
InactiveUS20120004636A1Promote blood clottingPromoting blood clottingBiocideDiagnosticsFiberMolecular materials
Owner:TELEFLEX LIFE SCI LTD
Wound closure material
InactiveUS20100076489A1Prevent leakageSuture equipmentsHeavy metal active ingredientsButtressBiomedical engineering
Articles are provided having no orientation or a multi-directional orientation. Such articles may be in the form of films, ribbons, sheets, and / or tapes and may be utilized as buttresses with a surgical stapling apparatus or as reinforcing means for suture lines. The articles may be produced with a polymeric material having an agent, such as a chemotherapeutic agent or a radiotherapeutic agent, incorporated therein or applied as a coating thereon.
Owner:TYCO HEALTHCARE GRP LP
Cyclodextrin polymers for carrying and releasing drugs
This invention discloses methods for preparing compositions of cyclodextrin polymers for carrying drugs and other active agents. Methods are also disclosed for preparing cyclodextrin polymer carriers that release drugs under controlled conditions. The invention also discloses methods for preparing compositions of cyclodextrin polymer carriers that are coupled to biorecognition molecules for targeting the delivery of drugs to their site of action. The advantages of the water soluble (or colloidal) cyclodextrin polymer carrier are: (1) Drugs can be used that are designed for efficacy without conjugation requirements. (2) It will allow the use of drugs designed solely for efficacy without regard for solubility. (3) Unmodified drugs can be delivered as macromolecules and released within the cell. (4) Drugs can be targeted by coupling the carrier to biorecognition molecules. (5) Synthesis methods are independent of the drug to facilitate multiple drug therapies.
Owner:KOSAK KENNETH M
Avenanthramide-containing compositions
Methods and compositions for treating or preventing a skin condition, an inflammation, an irritation or an allergy associated with an ectoparasitic infection or infestation on an animal. The methods involve applying to the skin of the animal a pharmaceutical composition that contains a therapeutically effective amount of one or more than one avenanthramide, an optional ecto and / or endo-parasiticidal agent, and a pharmaceutically acceptable diluent or carrier
Owner:CEAPRO
Methods for selectively stimulating proliferation of T cells
InactiveUS7144575B2Increase the number ofBiocidePeptide/protein ingredientsAccessory moleculeExogenous growth
Methods for inducing a population of T cells to proliferate by activating the population of T cells and stimulating an accessory molecule on the surface of the T cells with a ligand which binds the accessory molecule are described. T cell proliferation occurs in the absence of exogenous growth factors or accessory cells. T cell activation is accomplished by stimulating the T cell receptor (TCR) / CD3 complex or the CD2 surface protein. To induce proliferation of an activated population T cells, an accessory molecule on the surface of the T cells, such as CD28, is stimulated with a ligand which binds the accessory molecule. The T cell population expanded by the method of the invention can be genetically transduced and used for immunotherapy or can be used in methods of diagnosis.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY +2
Cropping systems for managing weeds
The invention provides cropping systems for managing weeds in crop environments. The cropping systems comprise, in one embodiment, transgenic plants that display tolerance to an auxin-like herbicide such as dicamba. Method for minimizing the development of herbicide resistant weeds are also provided.
Owner:MONSANTO TECH LLC
Absorbent articles comprising nanoparticles
Owner:NANO MET ZERO
Activation and expansion of cells
Owner:LIFE TECH CORP
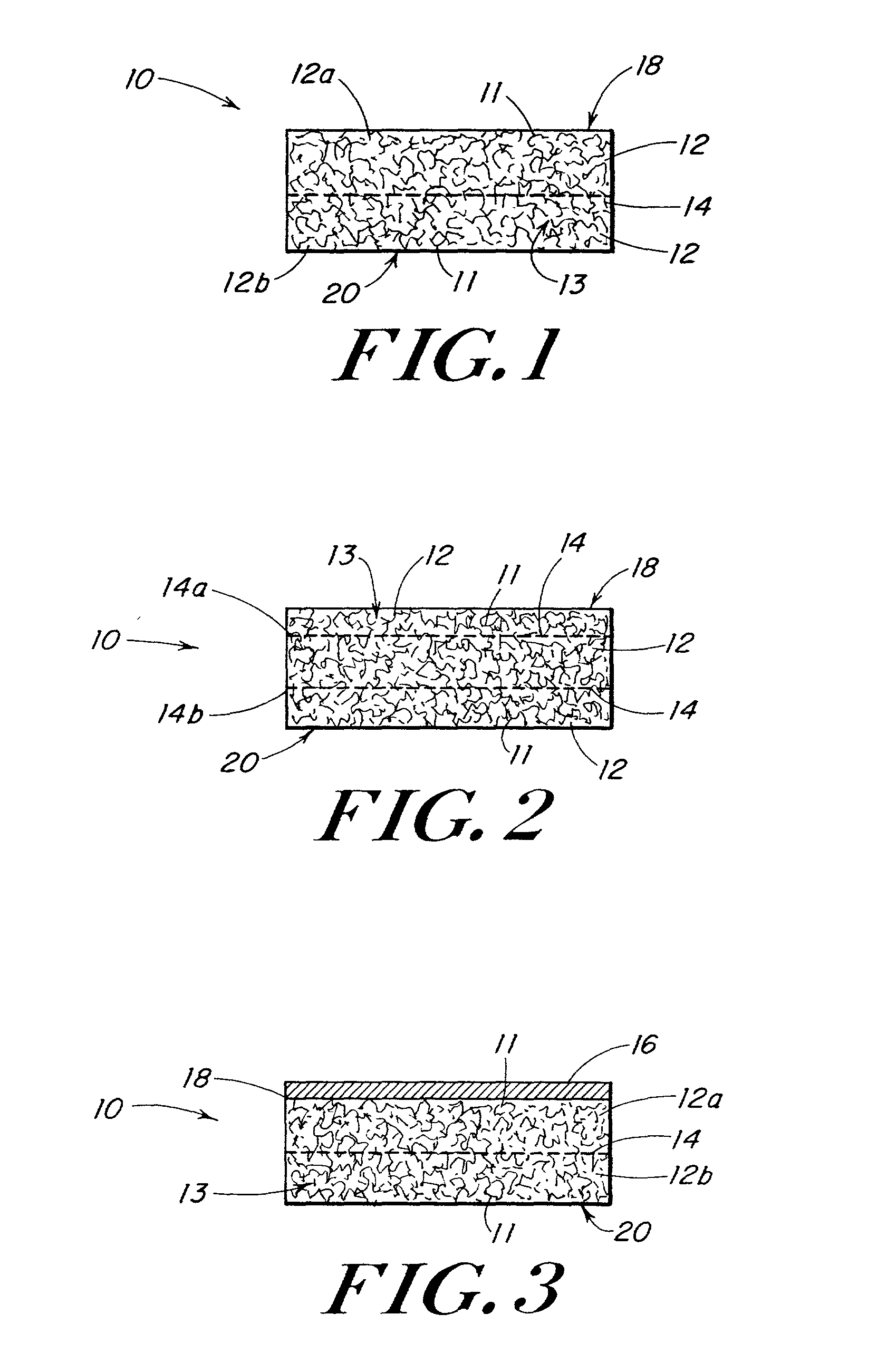
Porous medical device and method for its manufacture
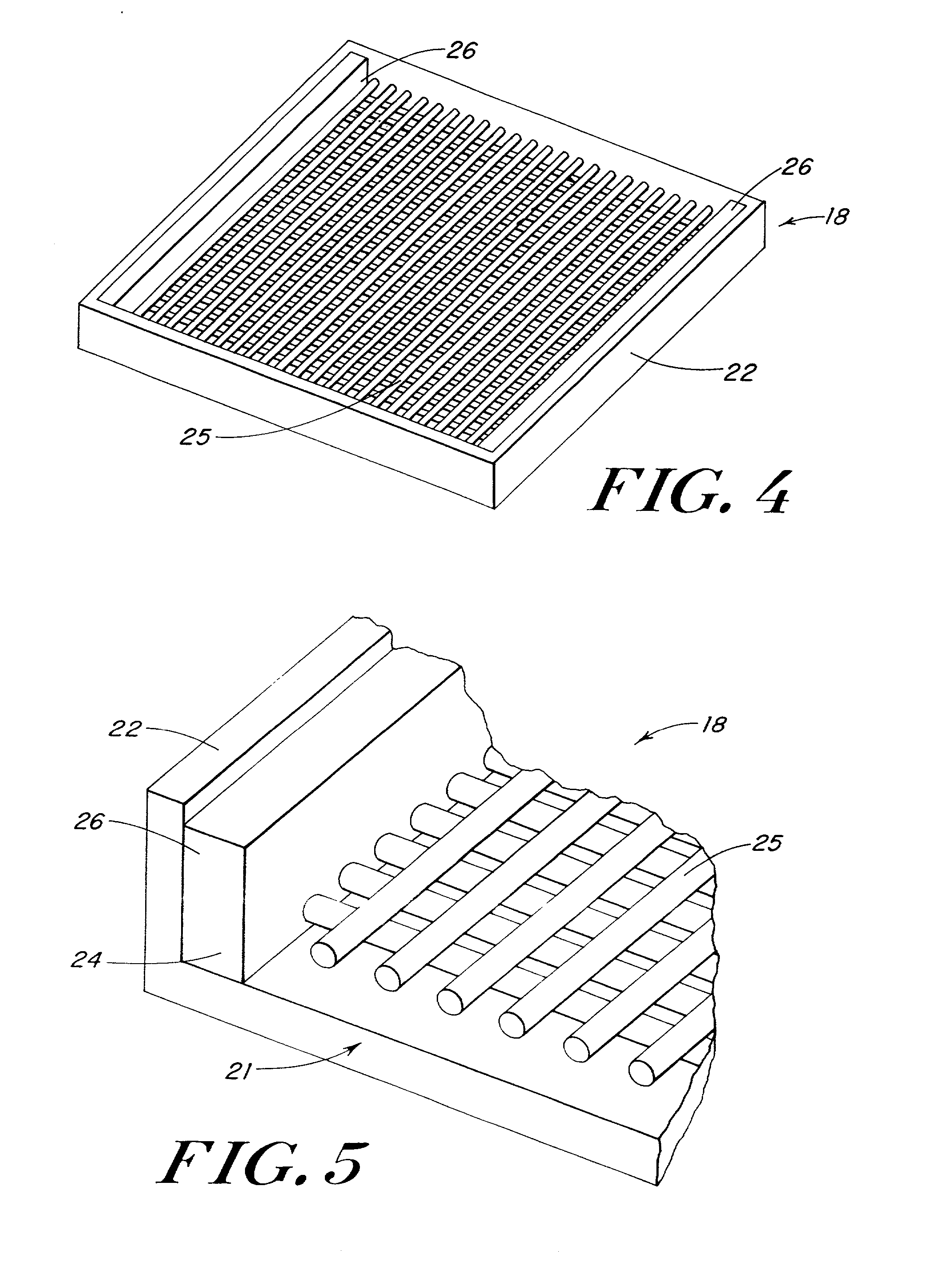
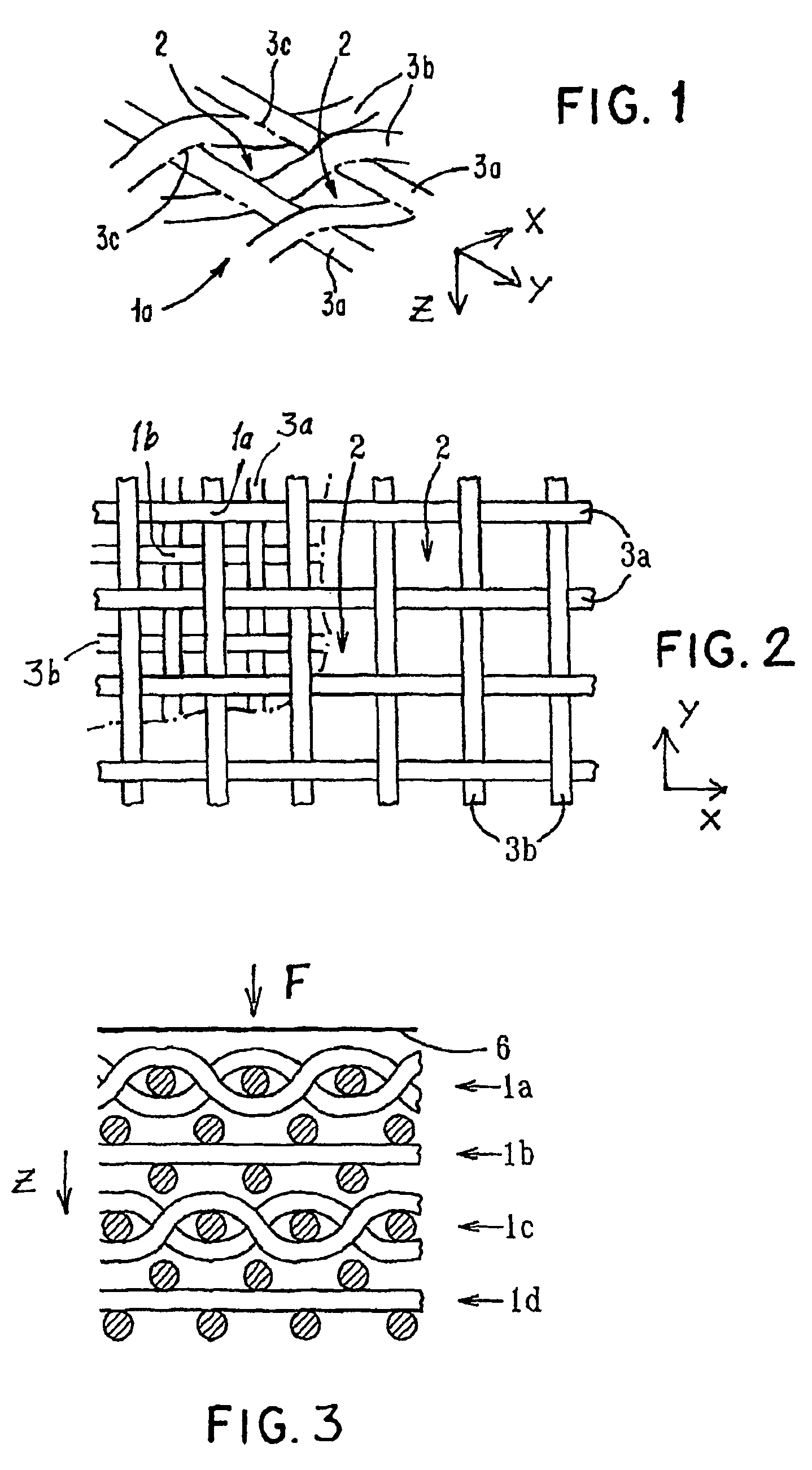
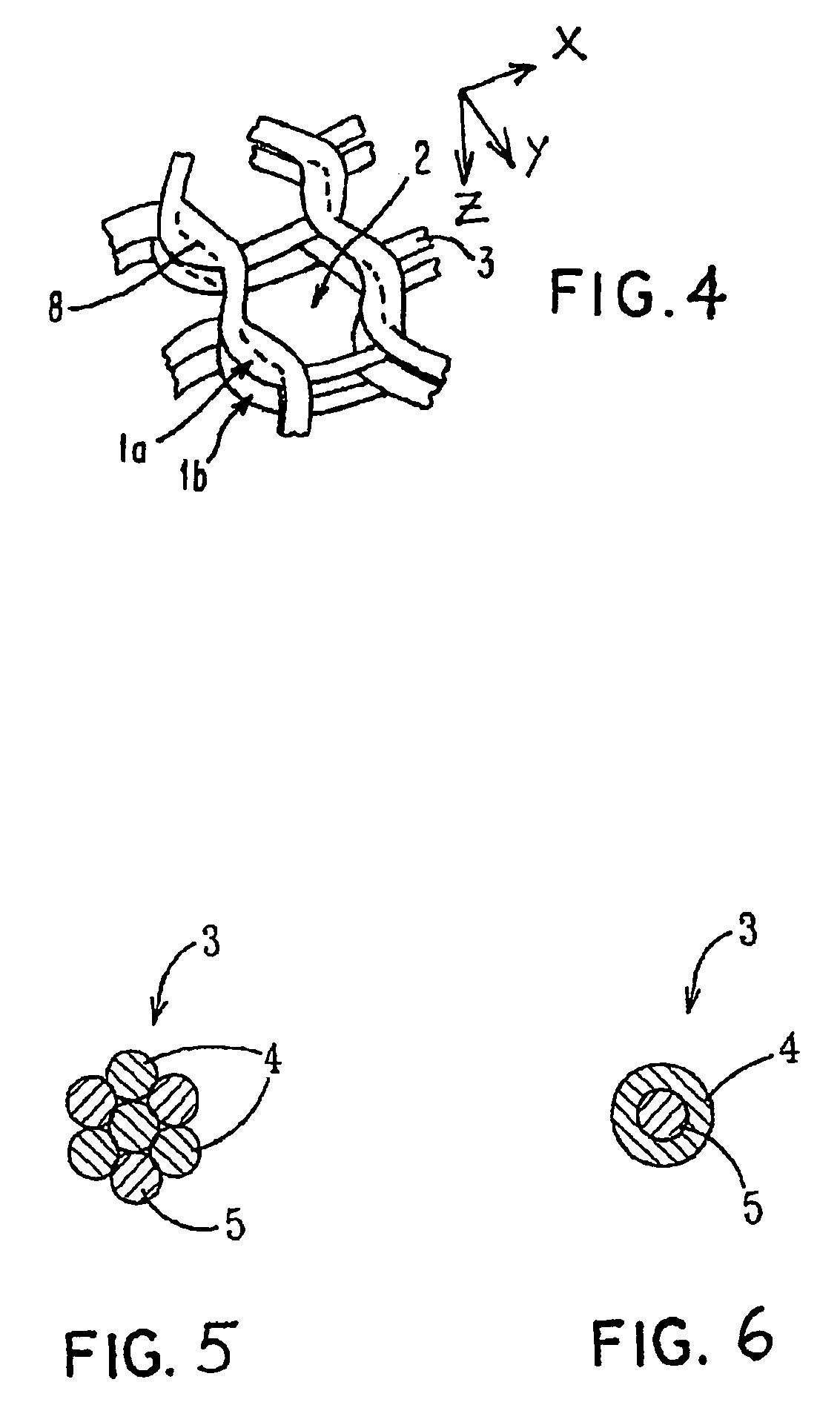
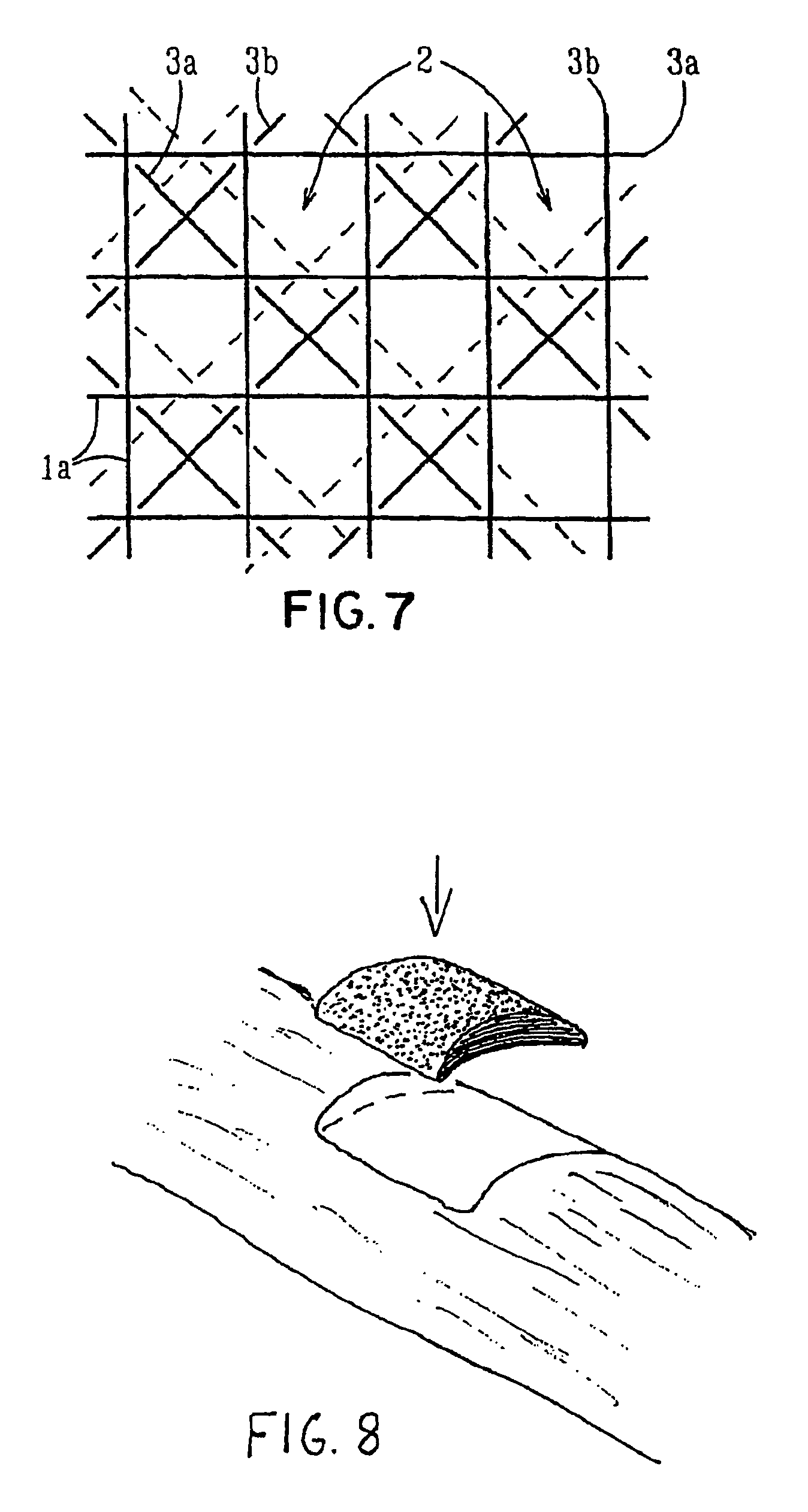
ActiveUS7964206B2Thickness of device can be variedControllable porosityBiocideGenetic material ingredientsFiberBioceramic
Porous bioabsorbable, bioactive and load-bearing composite medical device structure includes a plurality of regular textile planar layers (1a, 1b . . . ) formed of continuous bioabsorbable polymer matrix and bioceramic fibers acting as reinforcements, both included in continuous fibrous elements (3) forming the textile layers. The layers are placed on top of each other to form a structure having two dimensions (x, y) at right angles to each other according to the two dimensions of the textile layer and a third dimension (z) perpendicular to them and resulting from the piling of the layers. A plurality of passages extend through the layers as a result of the openings (2) defined by portions of the continuous fibrous elements (3) extending substantially in the direction of the plane. The continuous fibrous elements (3) comprise both bioactive ceramic reinforcing fibers which form a reinforcing structure and a bioabsorbable polymer matrix material which forms a matrix which binds the layers together and also binds the portions of continuous fibers defining the openings together, thereby forming the passages and stiffening the structure. This bioactive and bioabsorbable composite structure is suitable to be used as a basic structure in medical devices, especially in osteochondral applications where the load-bearing properties of implant are required.
Owner:BIORETEC
Methods and compositions for improving plant health
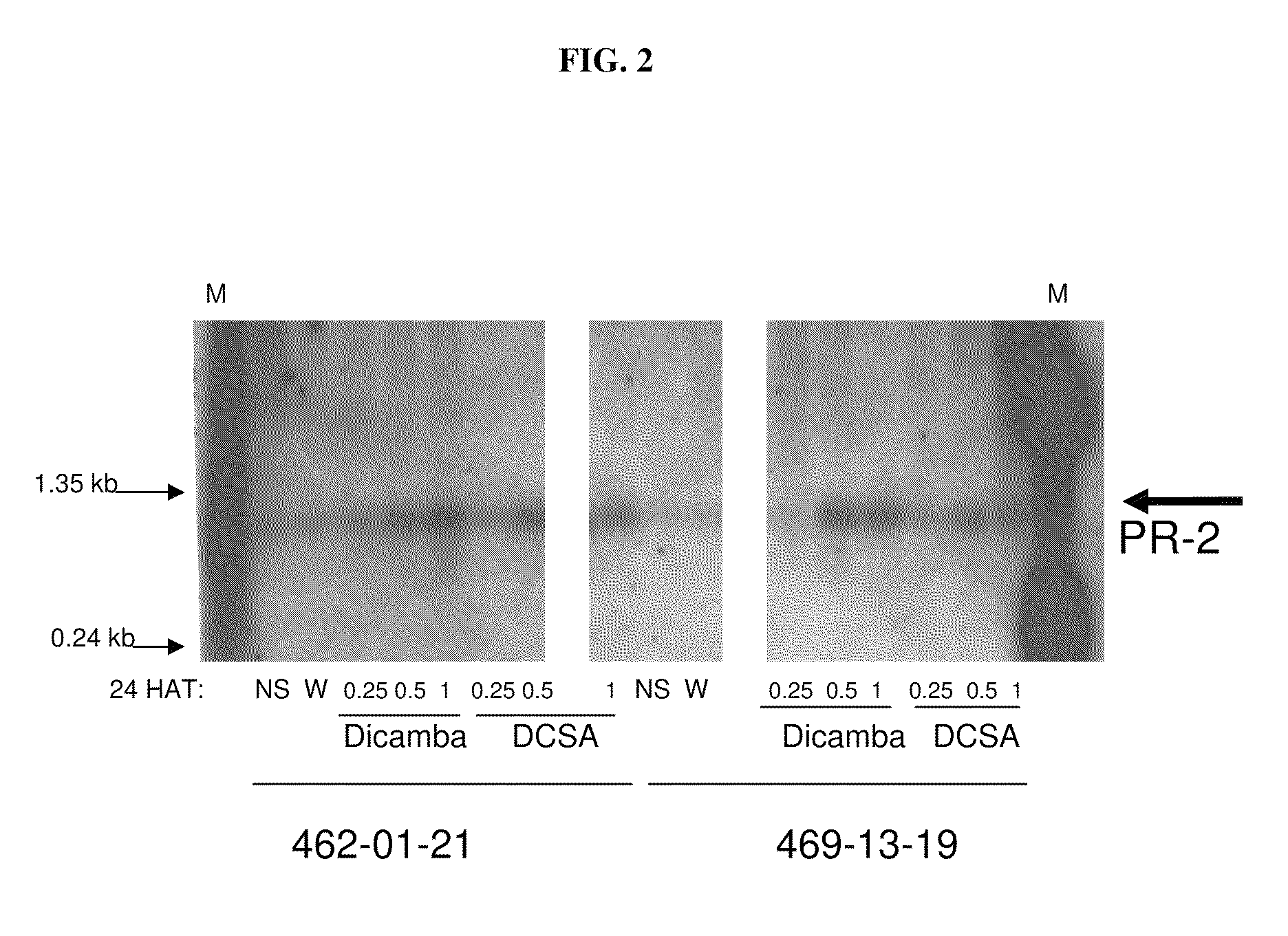
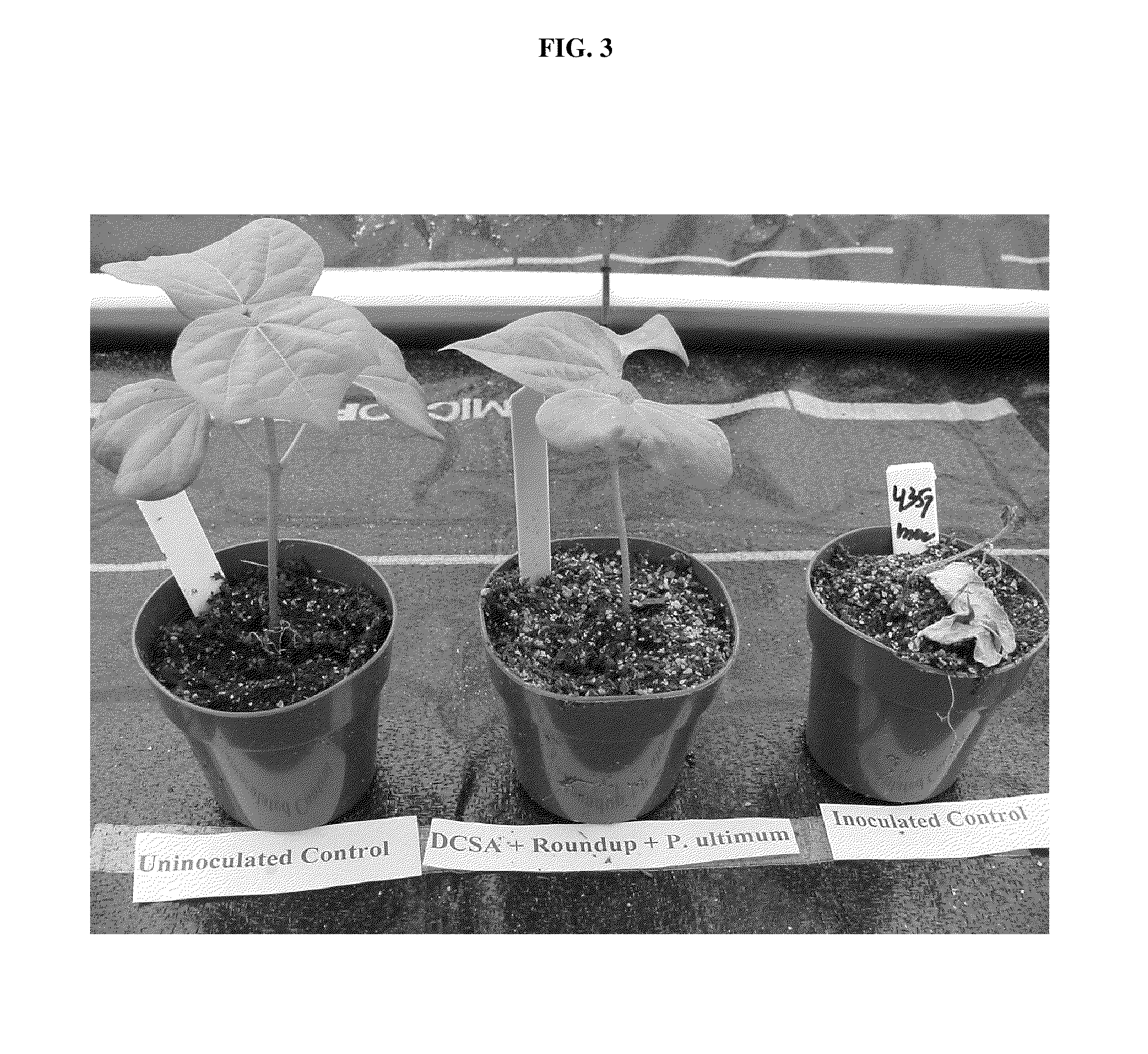
The present invention provides methods and compositions for improving plant health. In particular, application of dicamba or another substrate of DMO, or metabolites thereof including DCSA, to a plant confers tolerance to, or defense against, abiotic or biotic stresses such as oxidative stress including herbicide application, and plant disease, and enhances crop yield. Such application may be in combination with the application of another herbicide such as glyphosate.
Owner:MONSANTO TECH LLC
Methods for weed control using plants having dicamba-degrading enzymatic activity
Owner:MONSANTO TECH LLC
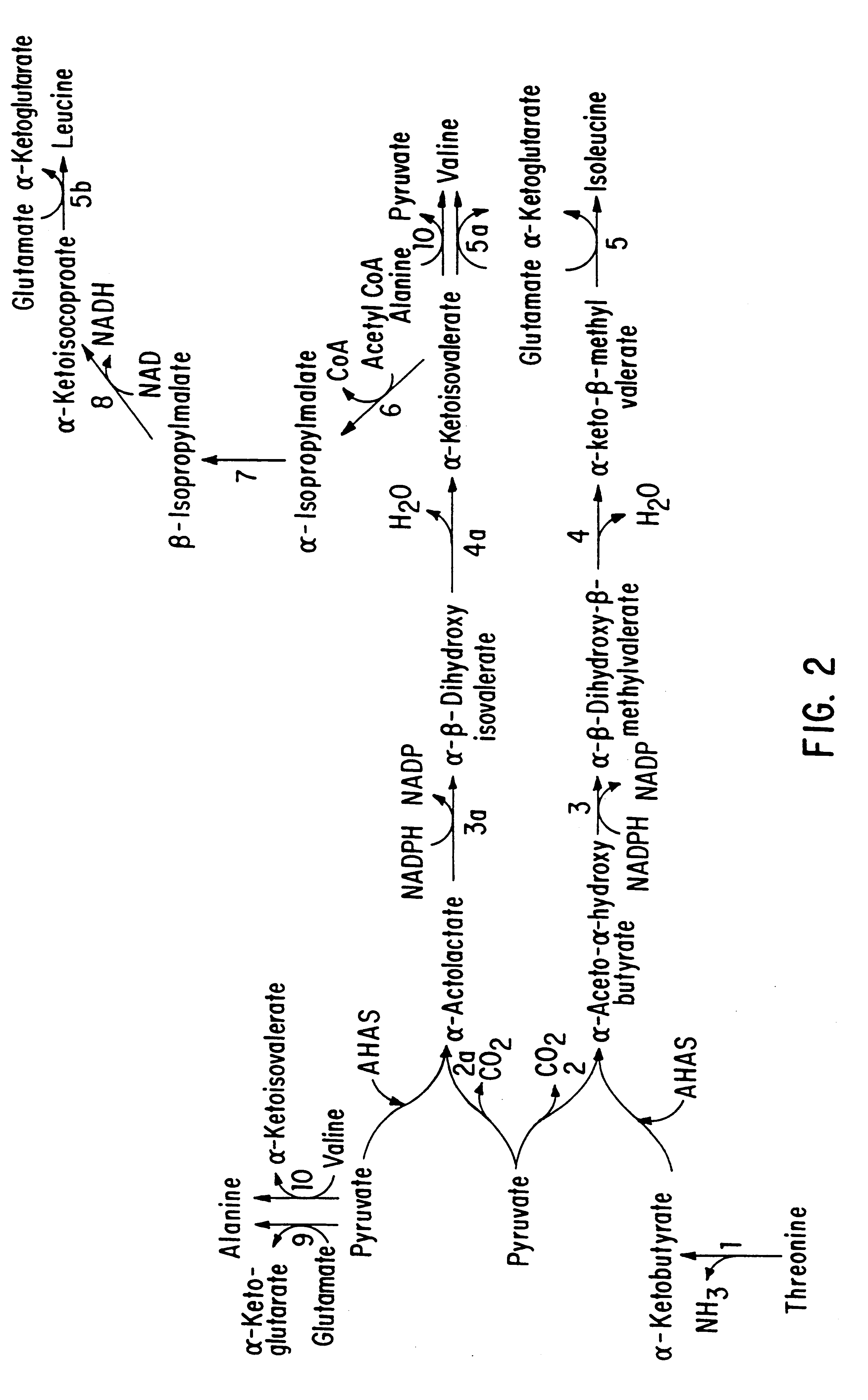
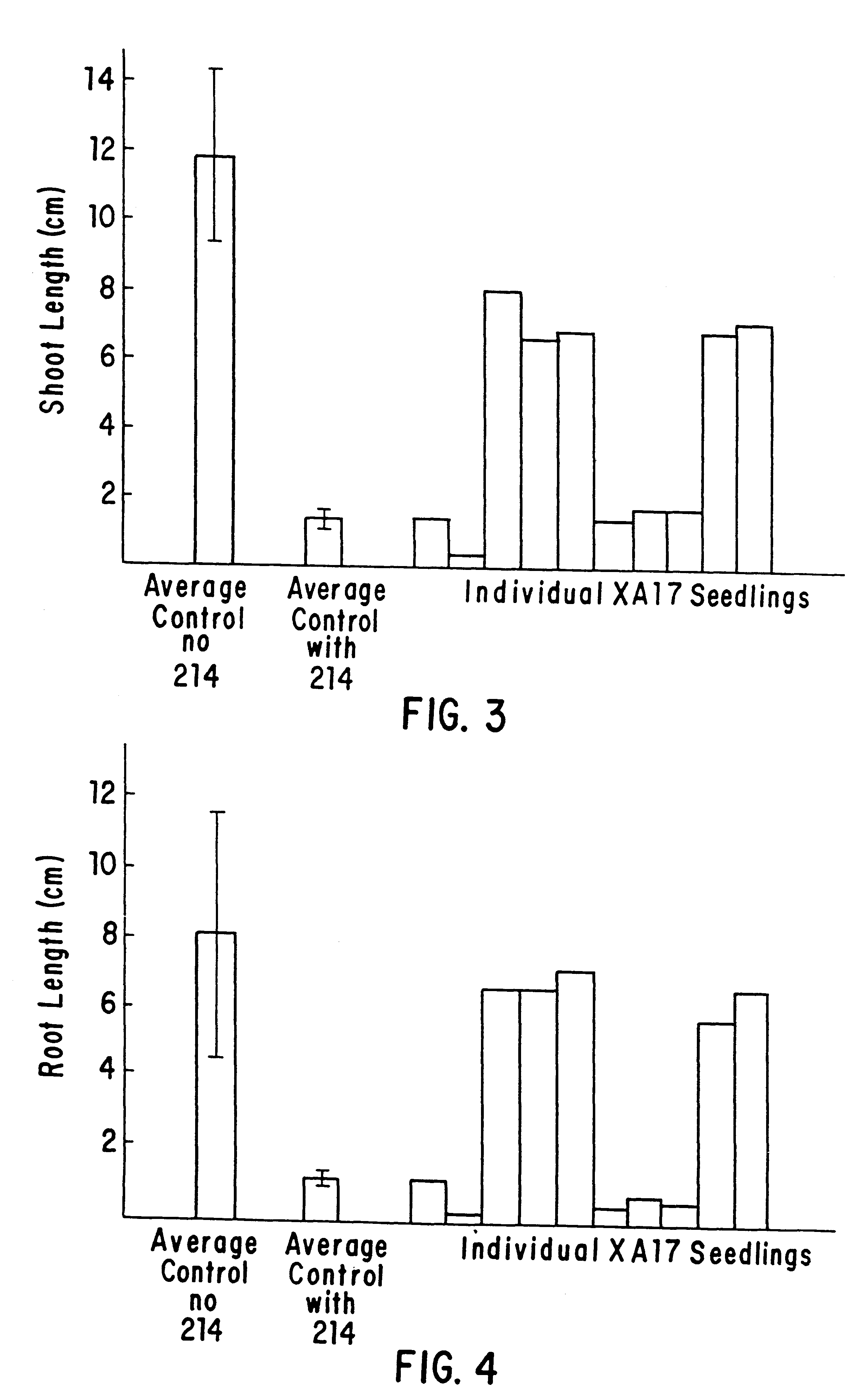
Herbicide resistance in plants
InactiveUS6222100B1Confers resistanceEffectively combat weed problemBiocideSeed and root treatmentPlant tissueNovel gene
This invention is directed to the production of plants, plant tissues and seeds which are resistant to inhibition by an herbicide which normally inhibits the growth and development of those plants, plant tissues and plant seeds. In particular this invention is directed to altered acetohydroxyacid synthase enzymes which are resistant to inhibition by herbicides which normally inhibit the activity of the synthase before such alteration. This invention further relates to genes encoding such enzymes, and to processes for utilizing these novel genes and enzymes. Further products of the invention include plants, plant tissues and seeds which exhibit resistance to such herbicides resulting from expression of genes encoding herbicide resistant acetohydroxyacid synthase enzyme.
Owner:MGI PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com