Patents
Literature
142results about "Urinary bladder" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
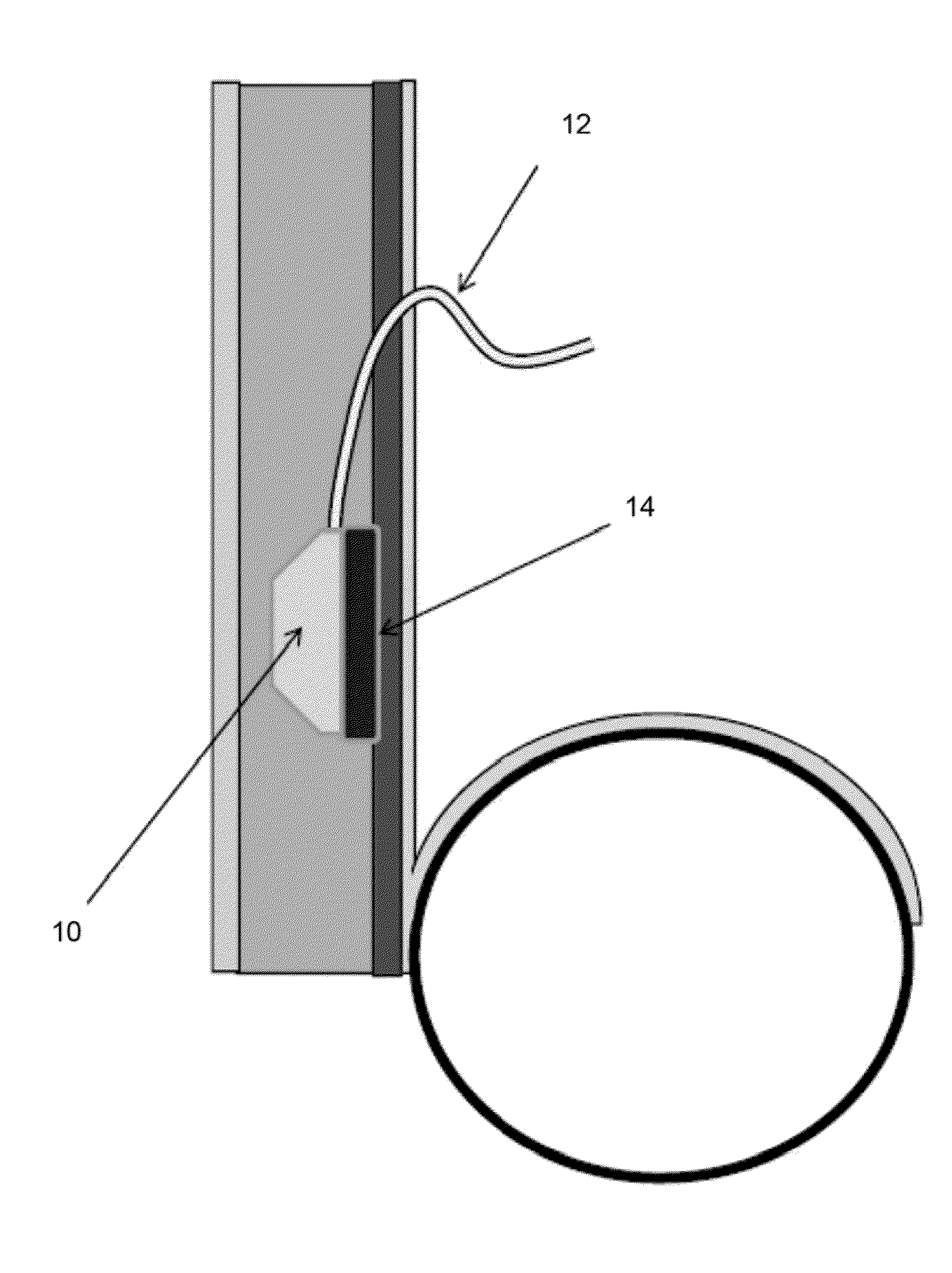
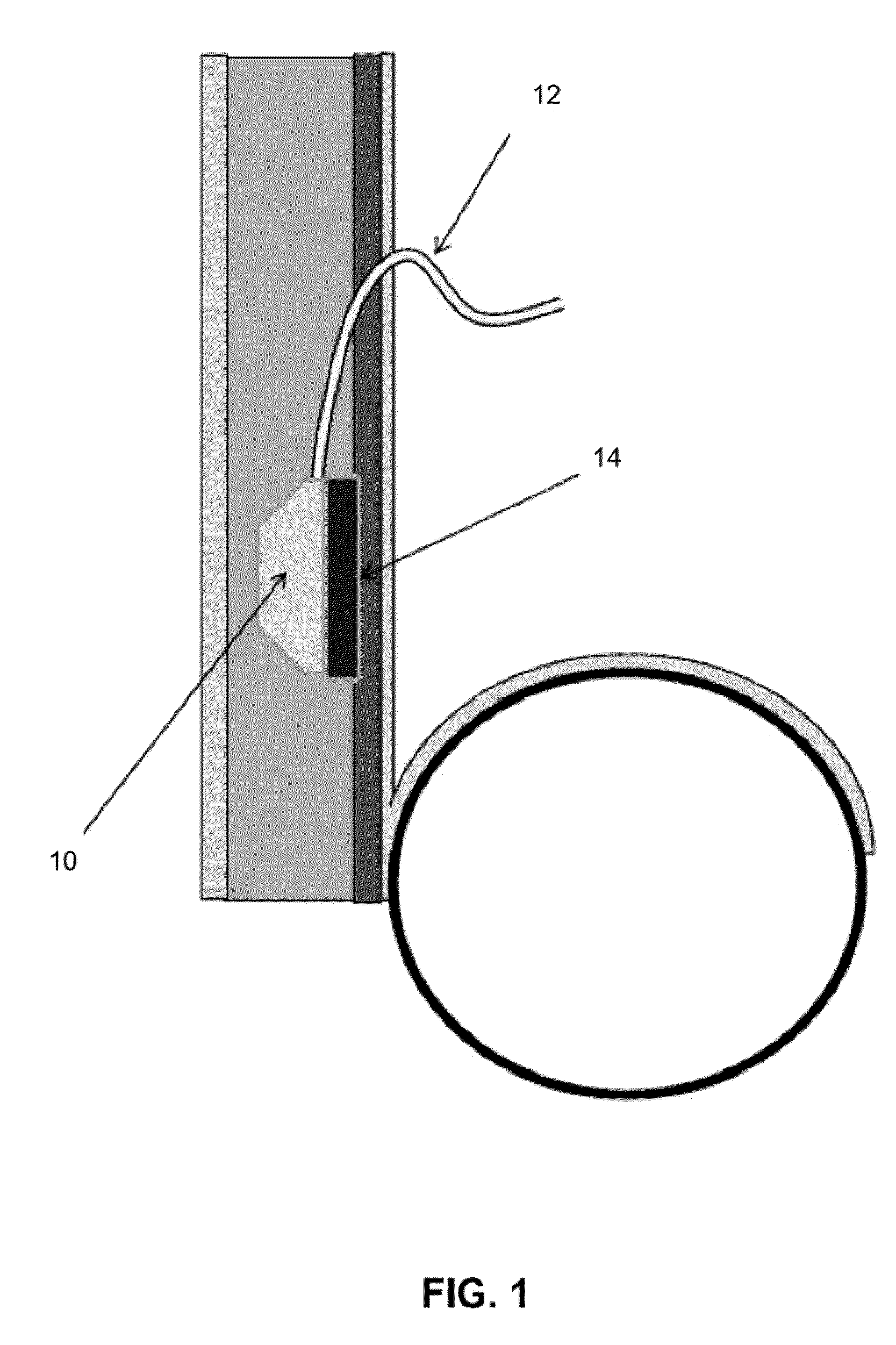
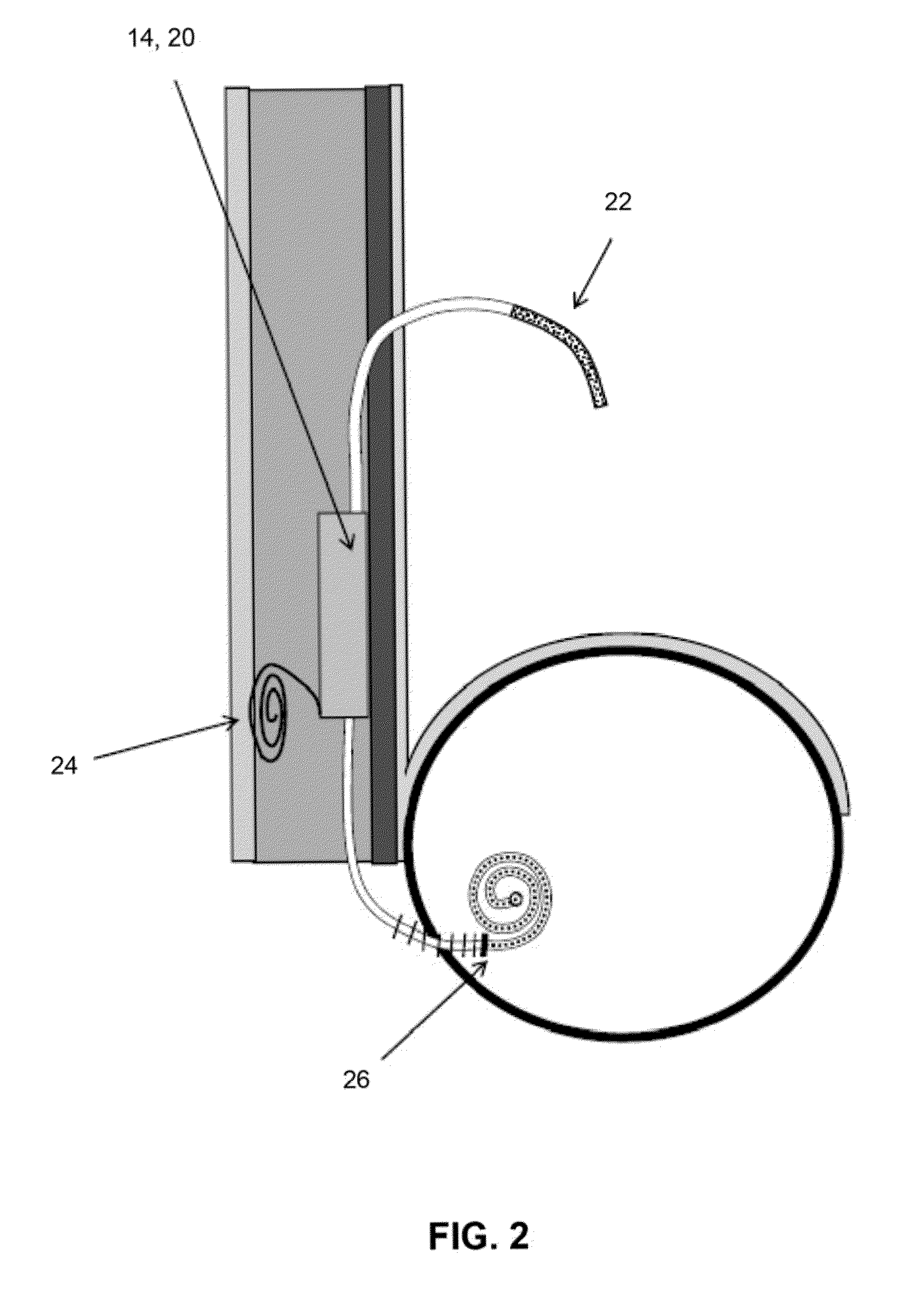
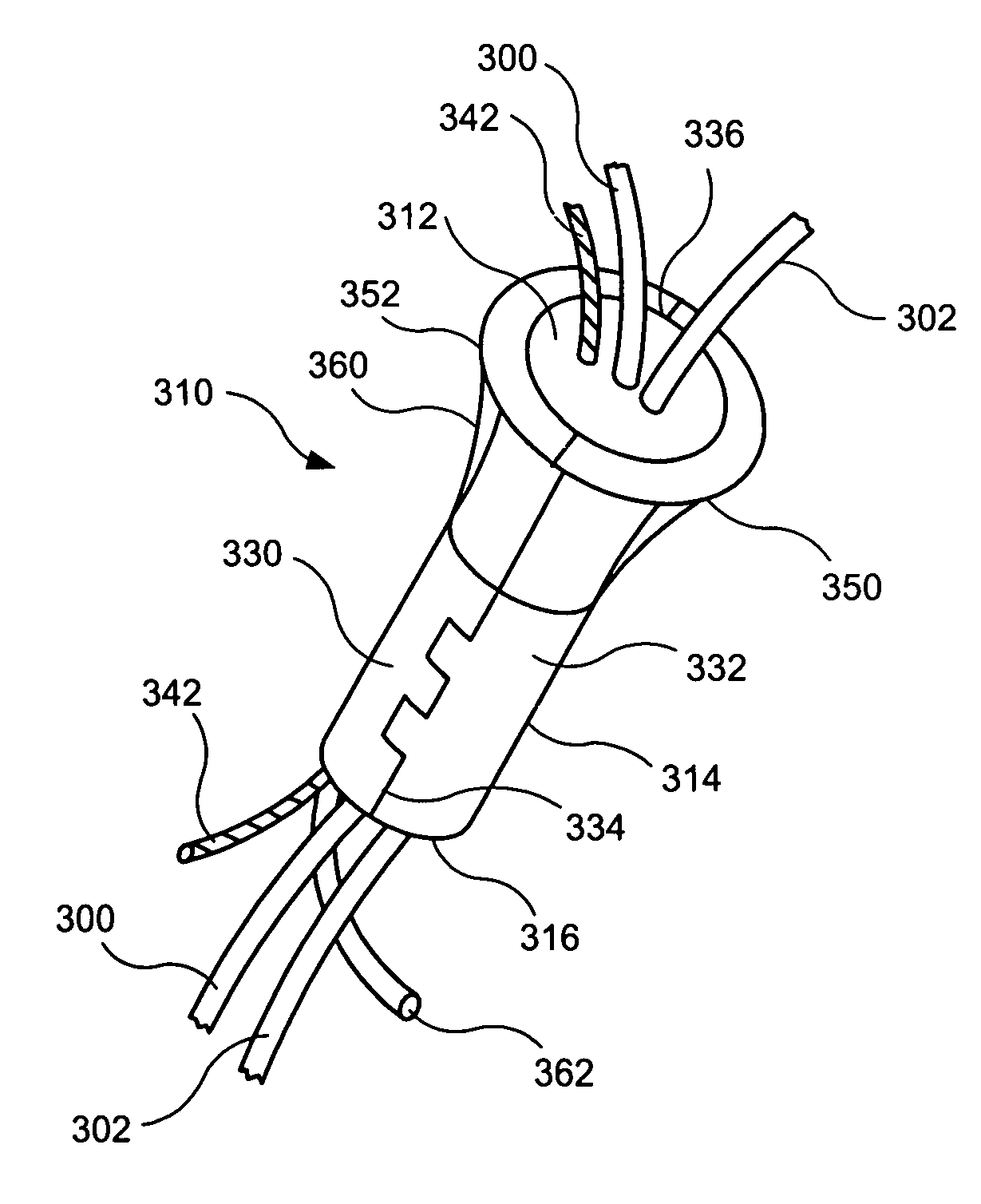
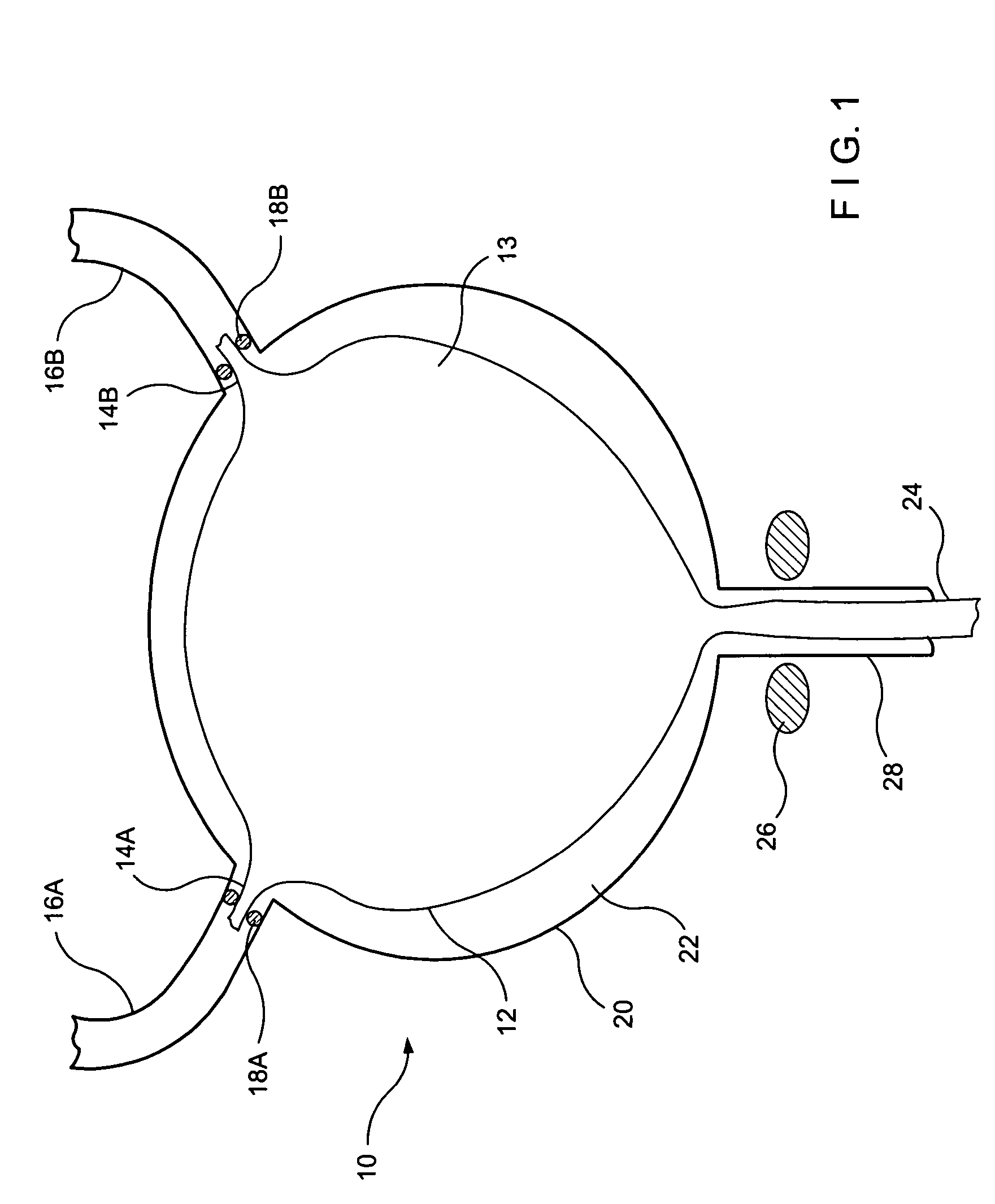
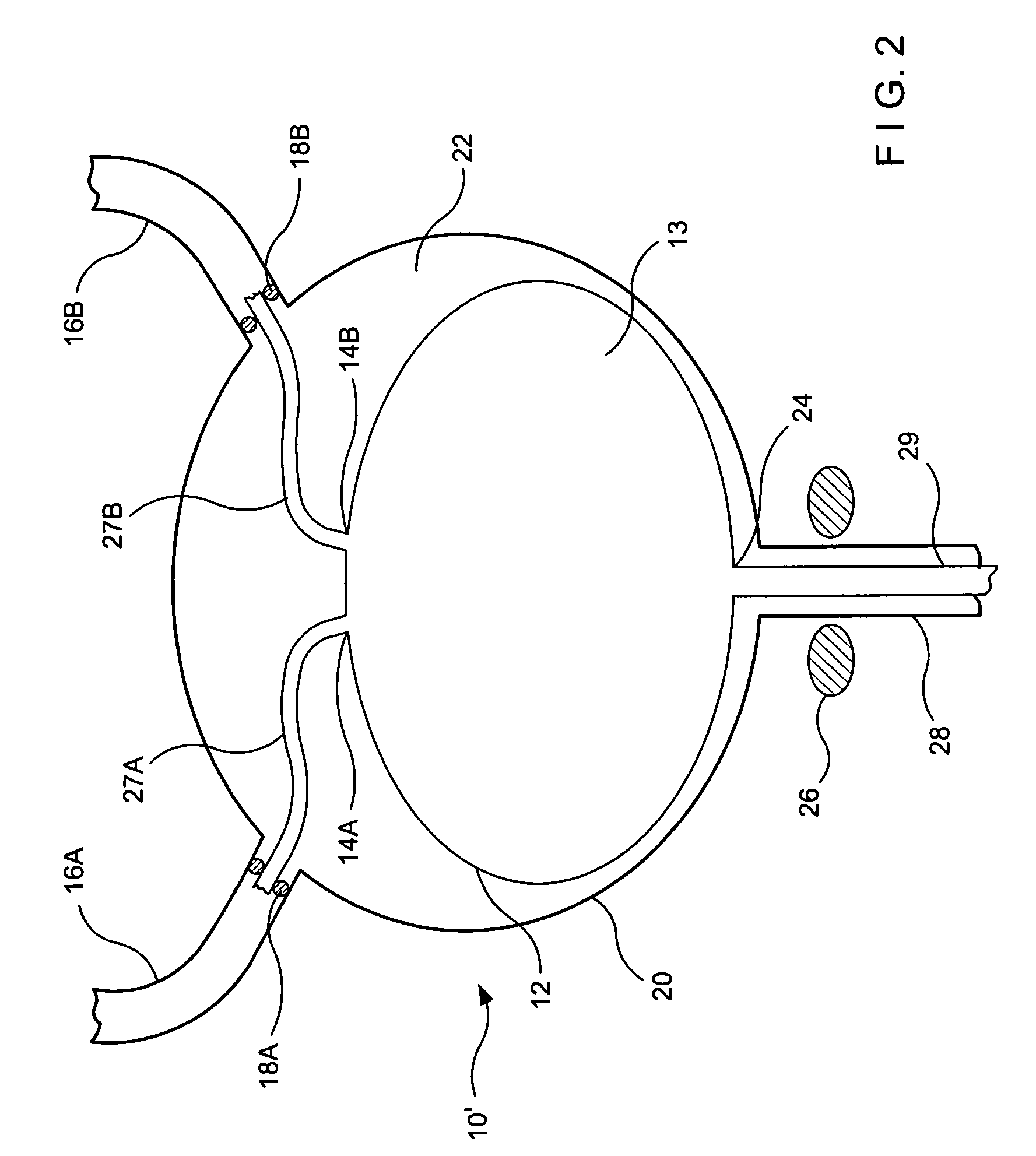
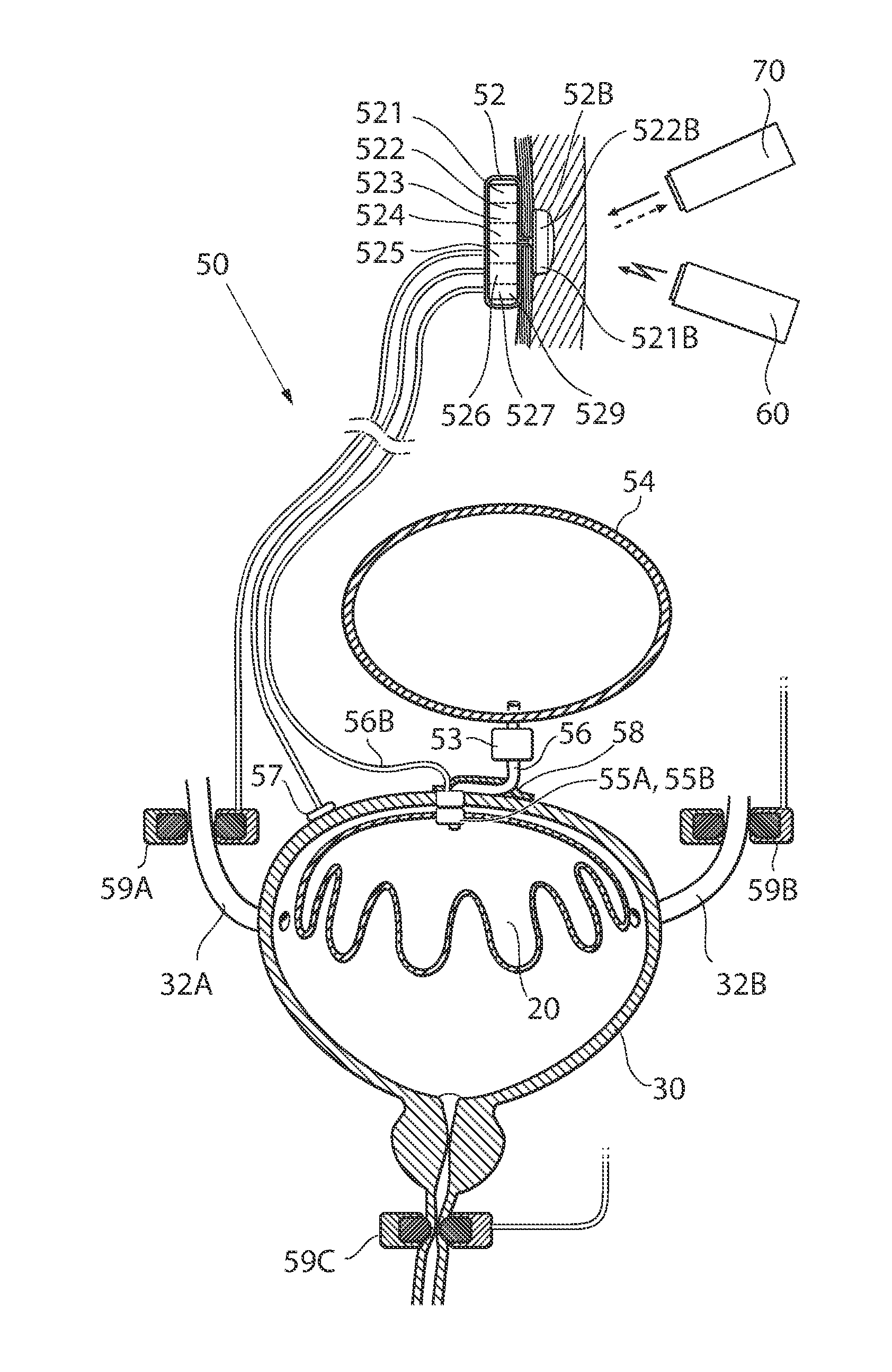
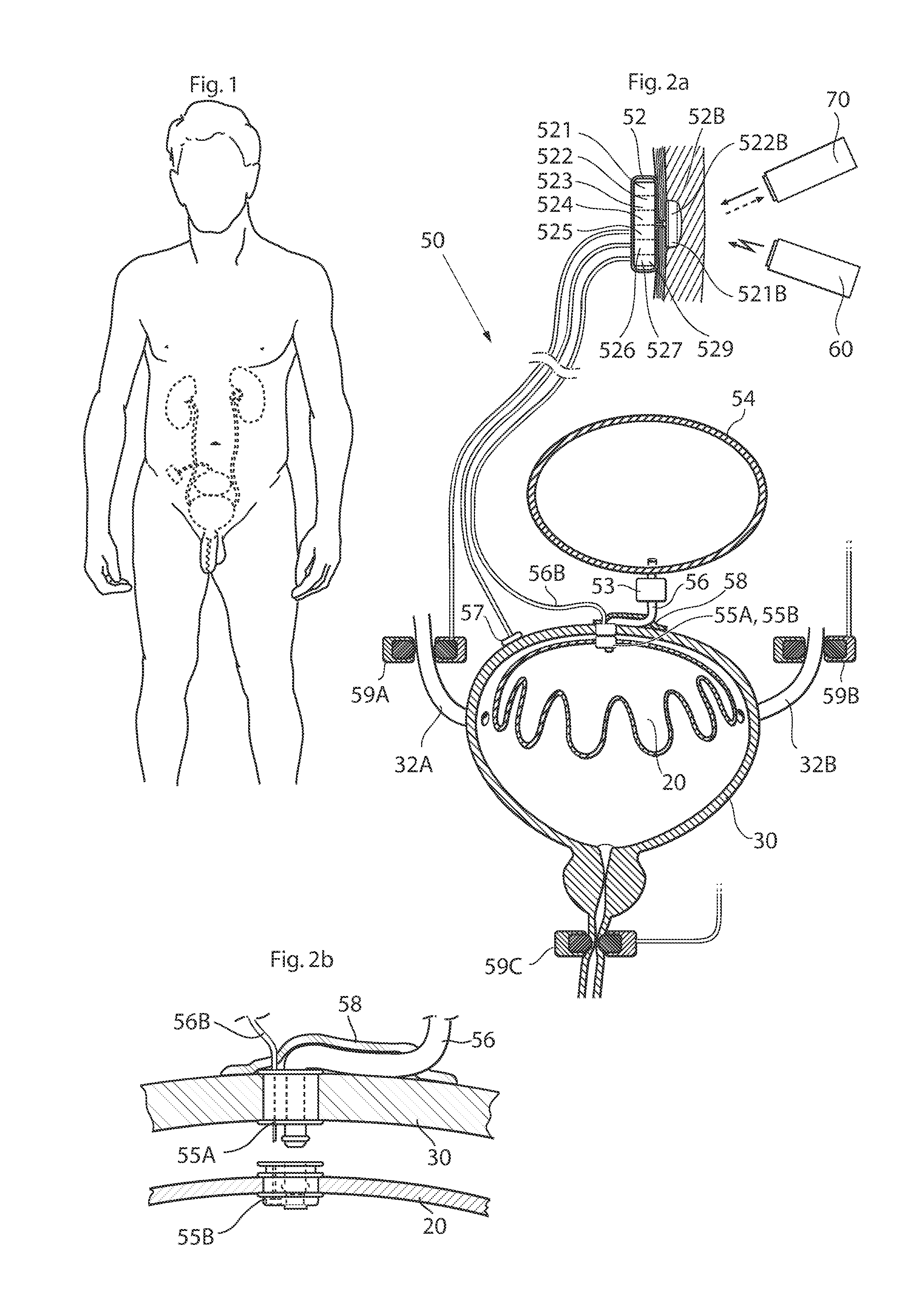
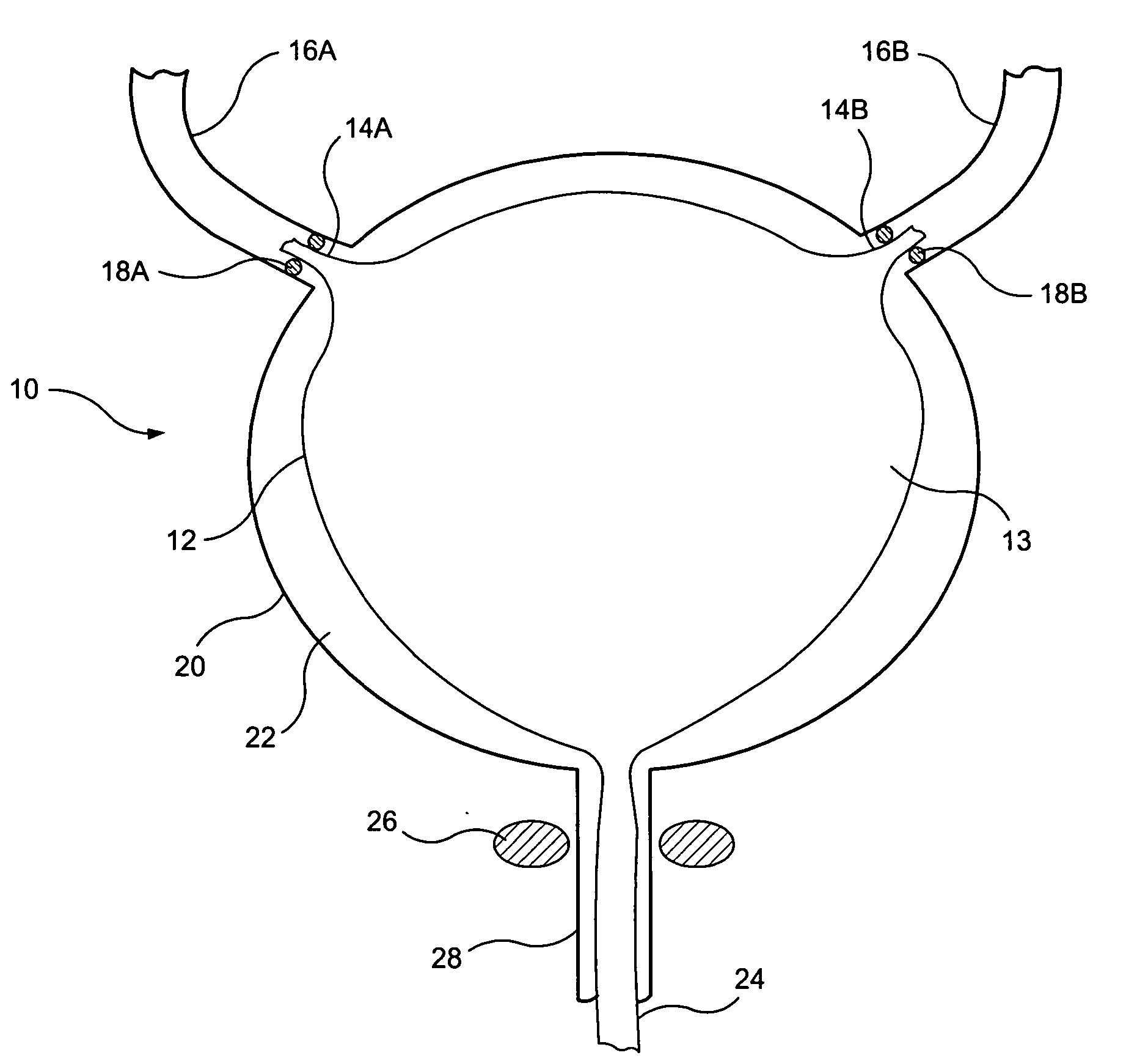
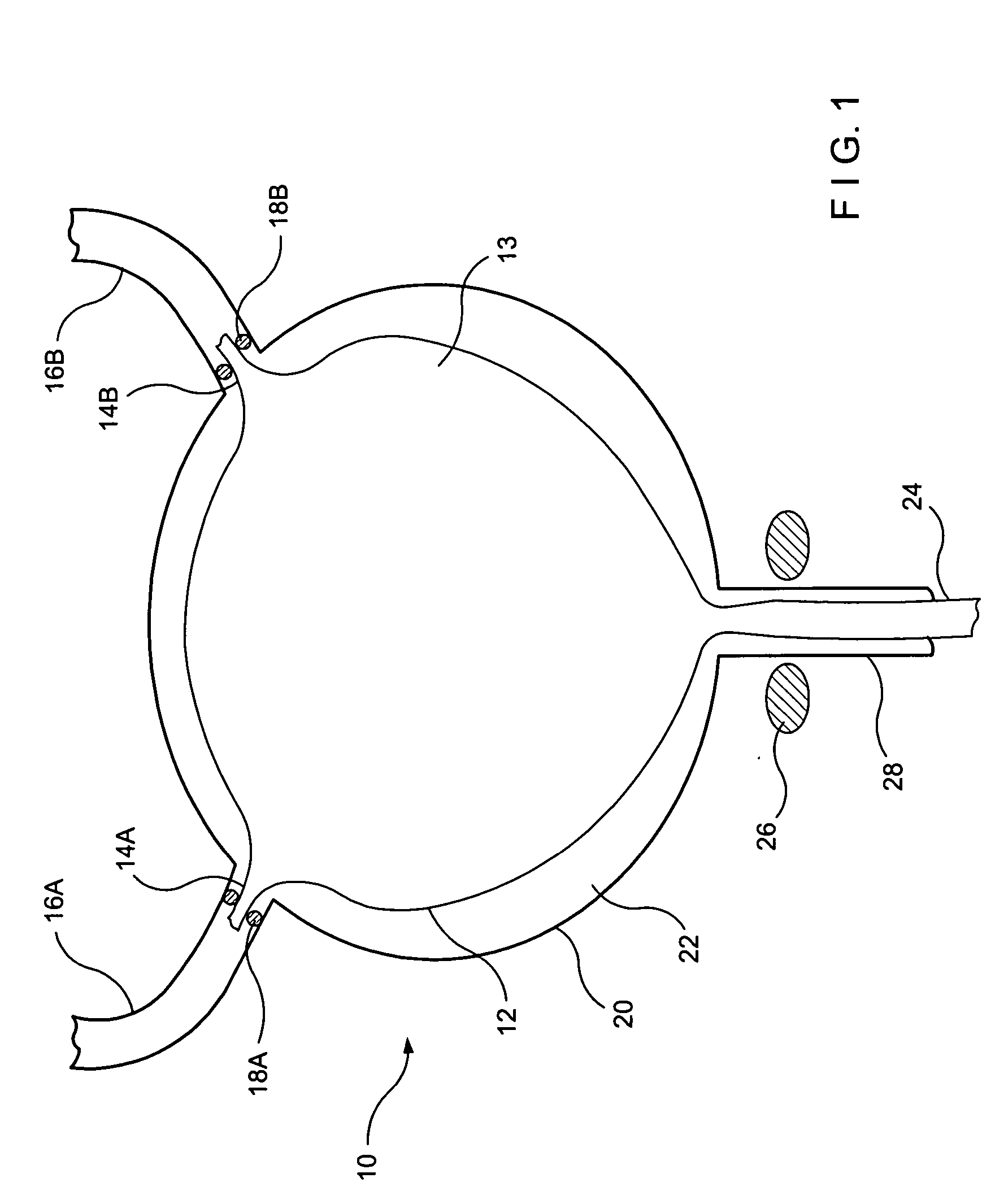
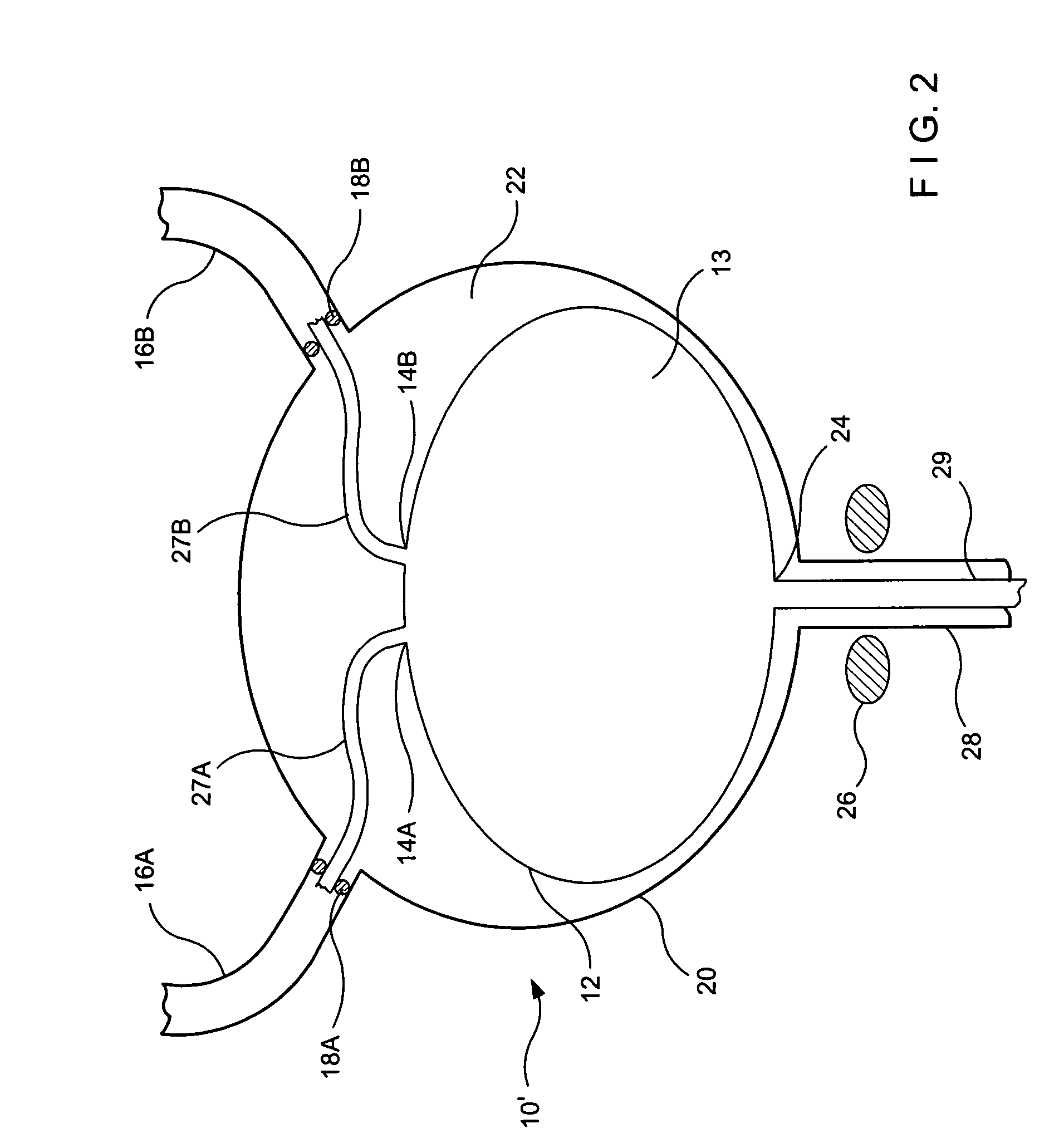
Implant with high vapor pressure medium
InactiveUS20100222802A1Promote recoveryImprove the immunitySuture equipmentsUrinary bladderHydrostatic pressureProduct gas
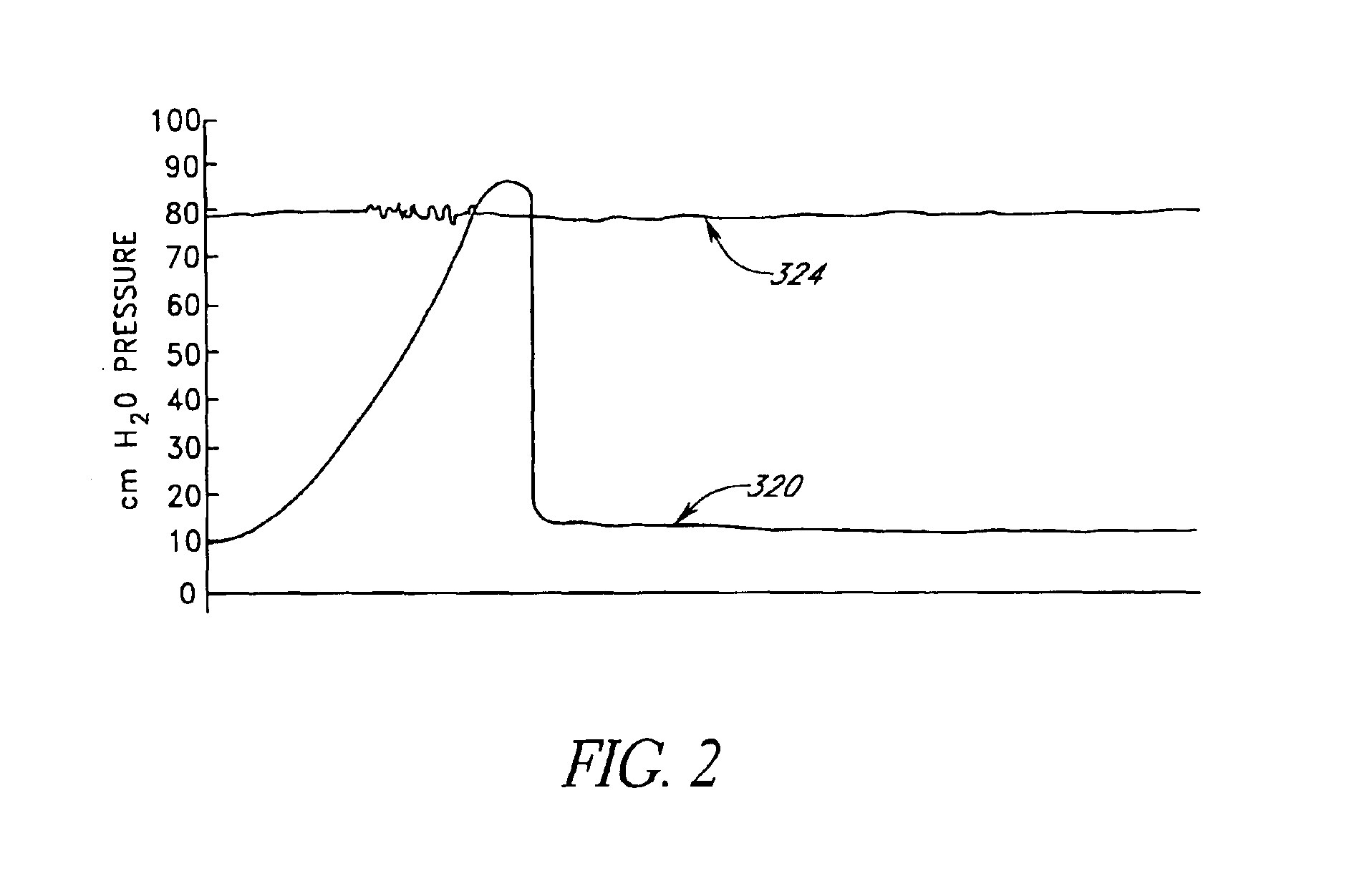
An implant for use in a human or animal body can include a flexible housing with an outer wall and having a chamber therein. The implant can have at least one high vapor pressure medium within the chamber. The at one high vapor pressure medium can have a combined vapor pressure equal to or greater than about the average value of the hydrostatic pressure of the implantation site plus the skin tension of the housing minus the gas tension of the dissolved gasses present at the implantation site.
Owner:SOLACE THERAPEUTICS
Ureteral stent system apparatus and method
A stent having an elongate tubular configuration is formed of a plurality of elongate elements interwoven or braided to form a tubular configuration. The elements may be relatively strong and rigid, but movable relative to each other within the weave or braid in order to provide the stent with generally soft characteristics. The elements may be formed of different materials, such as an absorbent material permitting the stent to be doped with materials such as drugs and chemicals. Even the absorbency can be controlled and varied to provide a predetermined time-release of the absorbent.
Owner:APPL MEDICAL RESOURCES CORP
Implantable valved pressure attenuation device
InactiveUS6976950B2Increase pressureLower the volumeUrinary bladderStentsUltrasound attenuationSurgery
Owner:SOLACE THERAPEUTICS
Scaffolds for organ reconstruction and augmentation
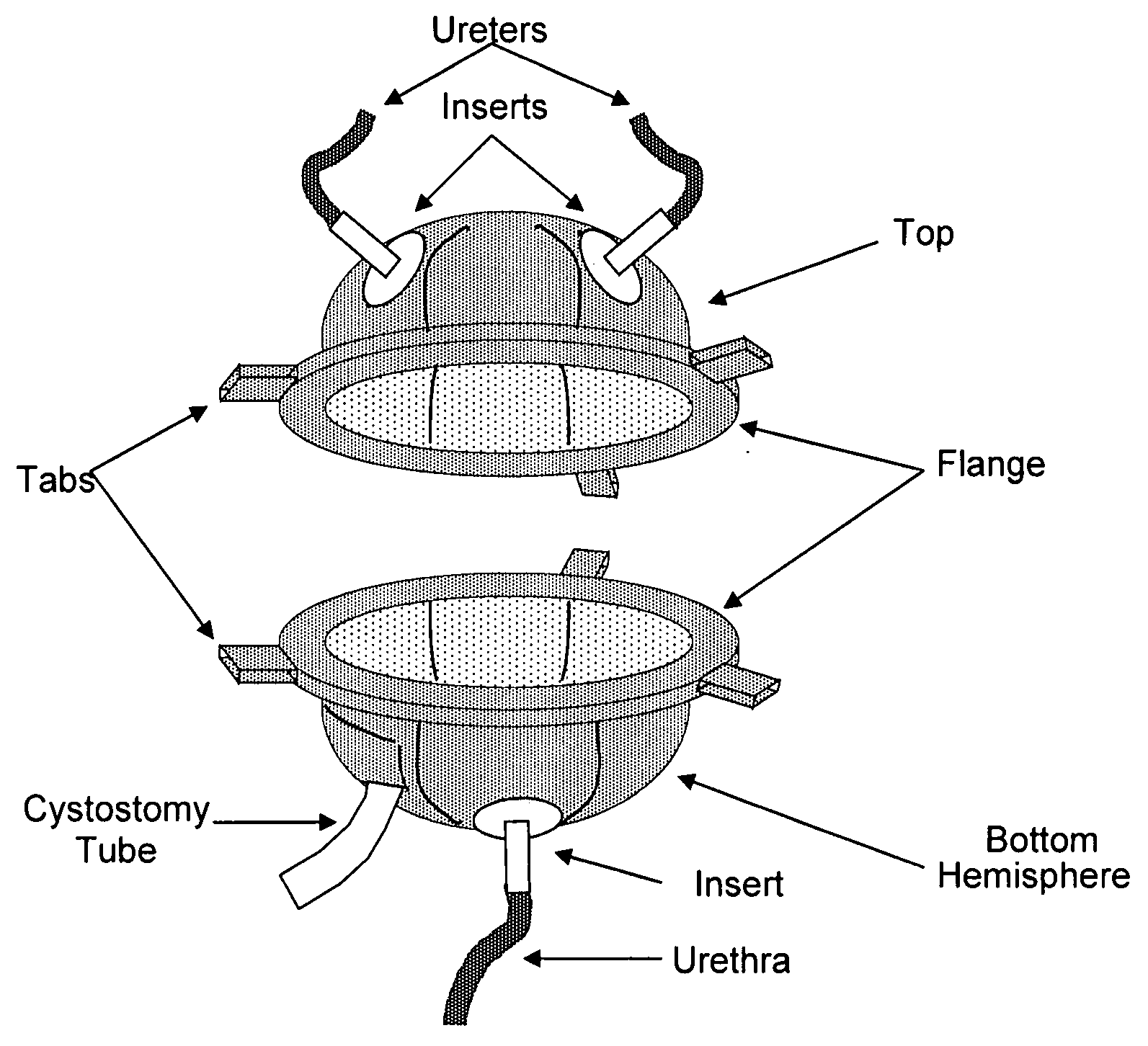
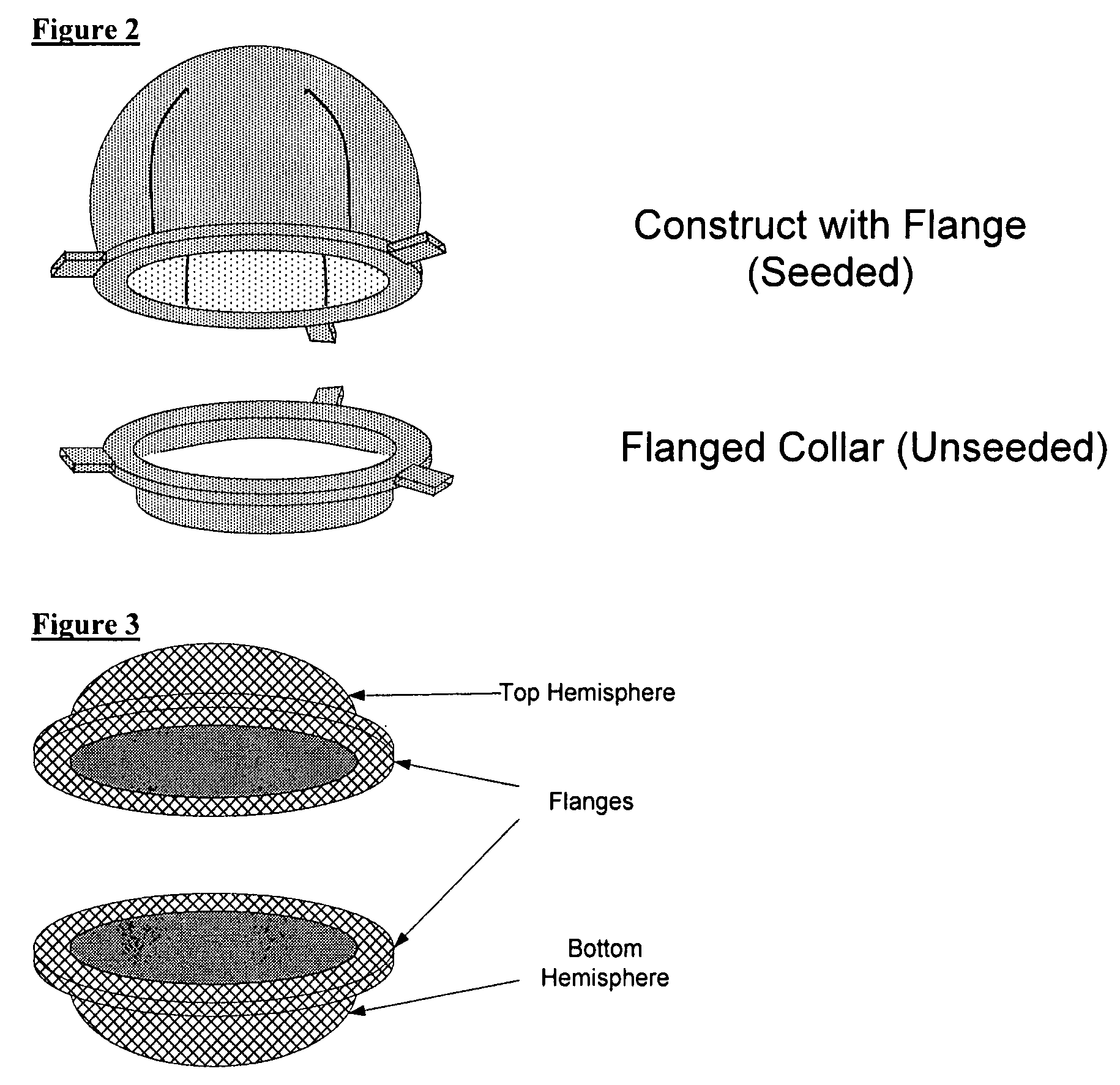
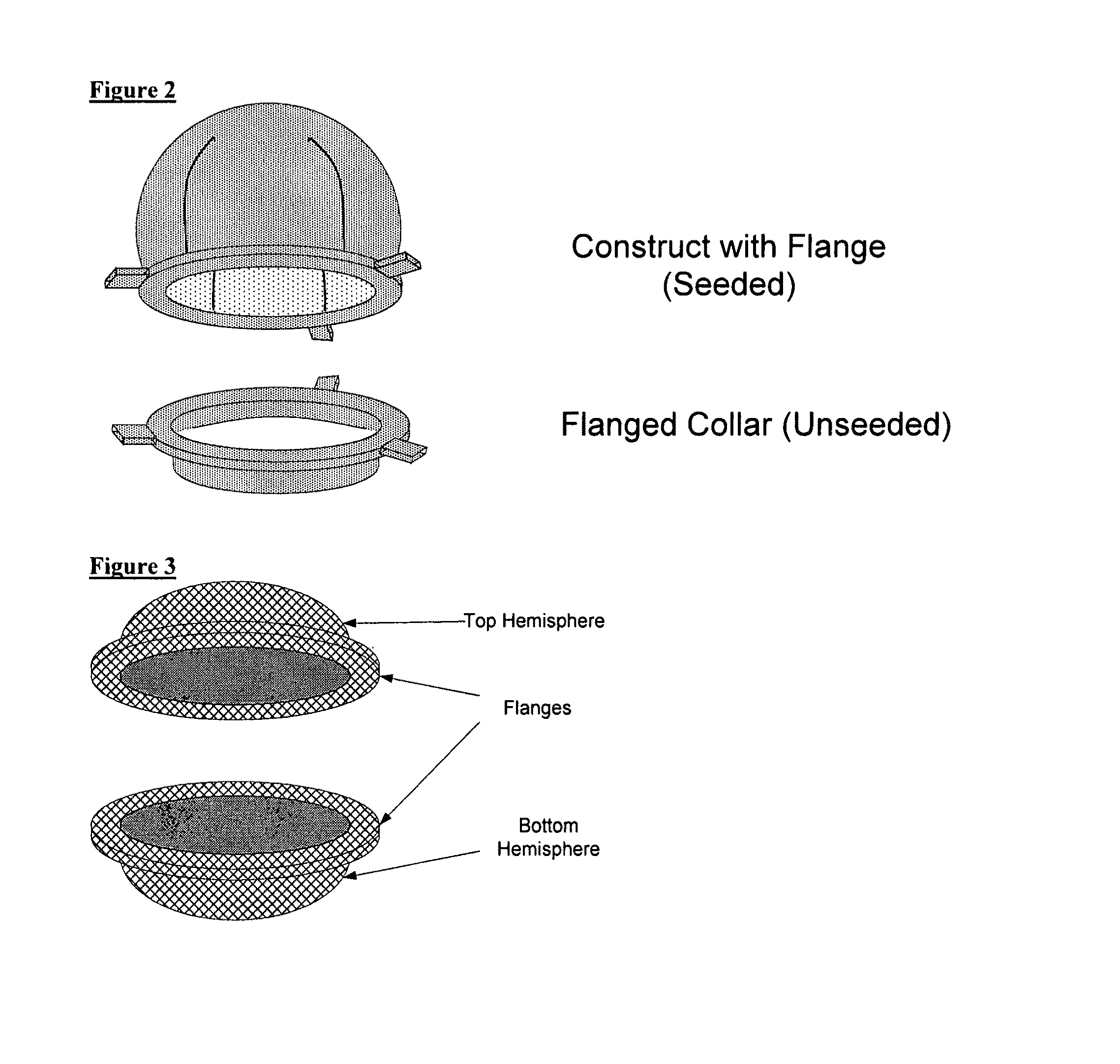
Biocompatible synthetic or natural scaffolds are provided for the reconstruction, repair, augmentation or replacement of organs or tissue structures in a patient in need of such treatment. The scaffolds are shaped to conform to at least a part of the organ or tissue structure and may be seeded with one or more cell populations. Inserts, receptacles and ports are also provided for the attachment of tubular vessels to the neo-organ scaffolds. The seeded scaffolds are implanted into the patient at the site in need of treatment to form an organized organ or tissue structure. The scaffolds may be used to form organs or tissues, such as bladders, urethras, valves, and blood vessels.
Owner:ORGAGEN INC
Method of producing fused biomaterials and tissue
InactiveUS6087552AReduce the possibilityGood biocompatibilitySuture equipmentsUrinary bladderVeinTissue repair
It is a general object of the invention to provide a method of effecting tissue repair or replacement using a biomaterial. It is a specific object of the invention to provide a biomaterial suitable for use as a stent, for example, a vascular stent, or as a conduit replacement, as an artery, vein or a ureter replacement. The biomaterial can also be used as a stent or conduit covering or lining. The present invention relates to a method of repairing, replacing or supporting a section of a body tissue. The method comprises positioning a biomaterial at the site of the section and bonding the biomaterial to the site or to the tissue surrounding the site. The bonding is effected by contacting the biomaterial and the site, or tissue surrounding the site, at the point at which said bonding is to be effected, with an energy absorbing agent. The agent is then exposed to an amount of energy absorbable by the agent sufficient to bond the biomaterial to the site or to the tissue surrounding the site.
Owner:GREGORY KENTON W +1
Elastin and elastin-based materials
It is a general object of the invention to provide a method of effecting repair or replacement or supporting a section of a body tissue. Specifically to provide an elastin or elastin-based biomaterial suitable for use as a stent, for example, a vascular stent, or as conduit replacement, as an artery, vein or a ureter replacement. The biomaterial can also be used as a stent or conduit covering or coating or lining. It is also an object of the invention to provide a method of securing an elastin or elastin-based biomaterial to an existing tissue without the use of sutures or staples.
Owner:PROVIDENCE HEALTH SYST OREGON AN OREGON NONPROFIT CORP +2
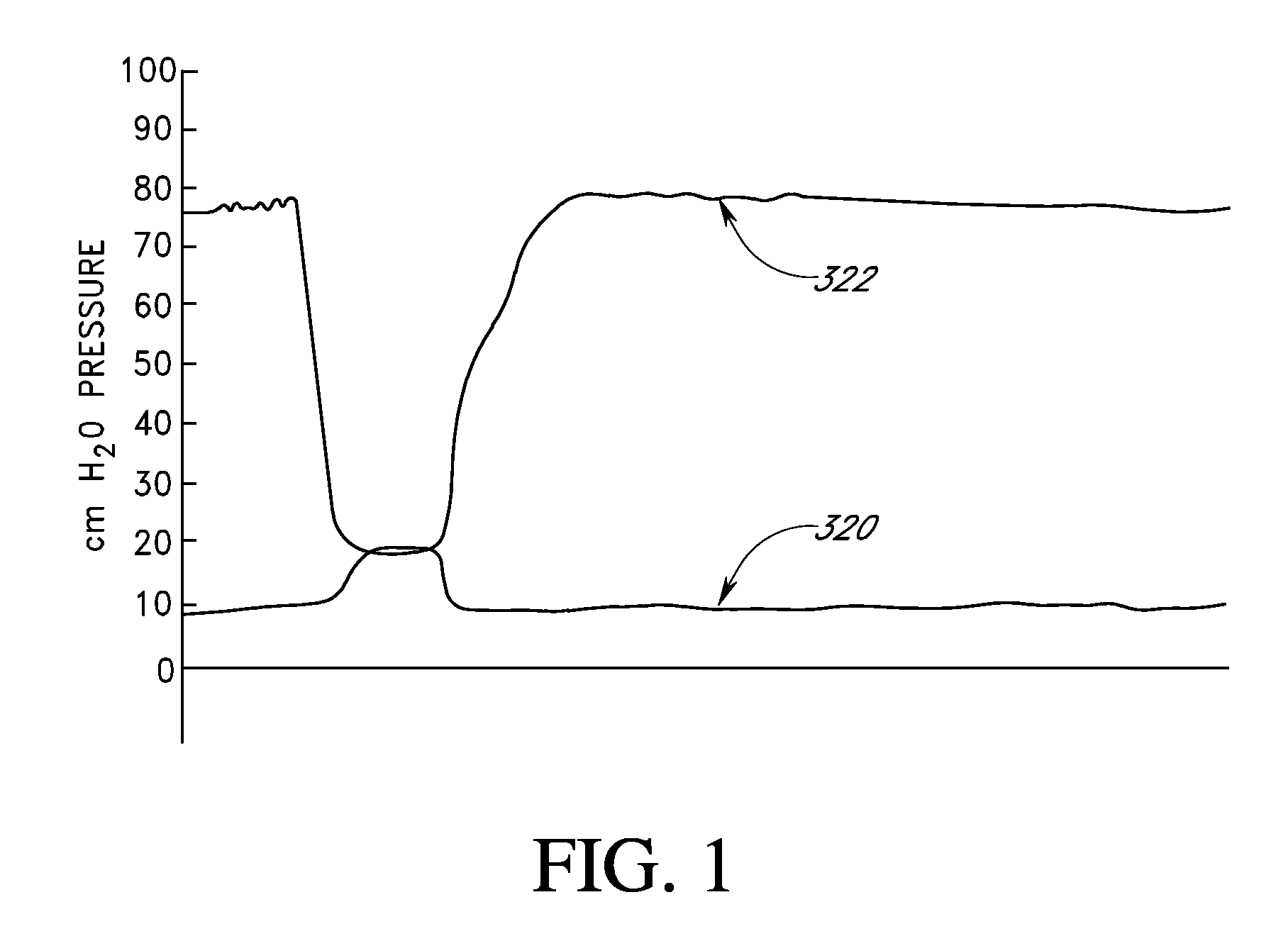
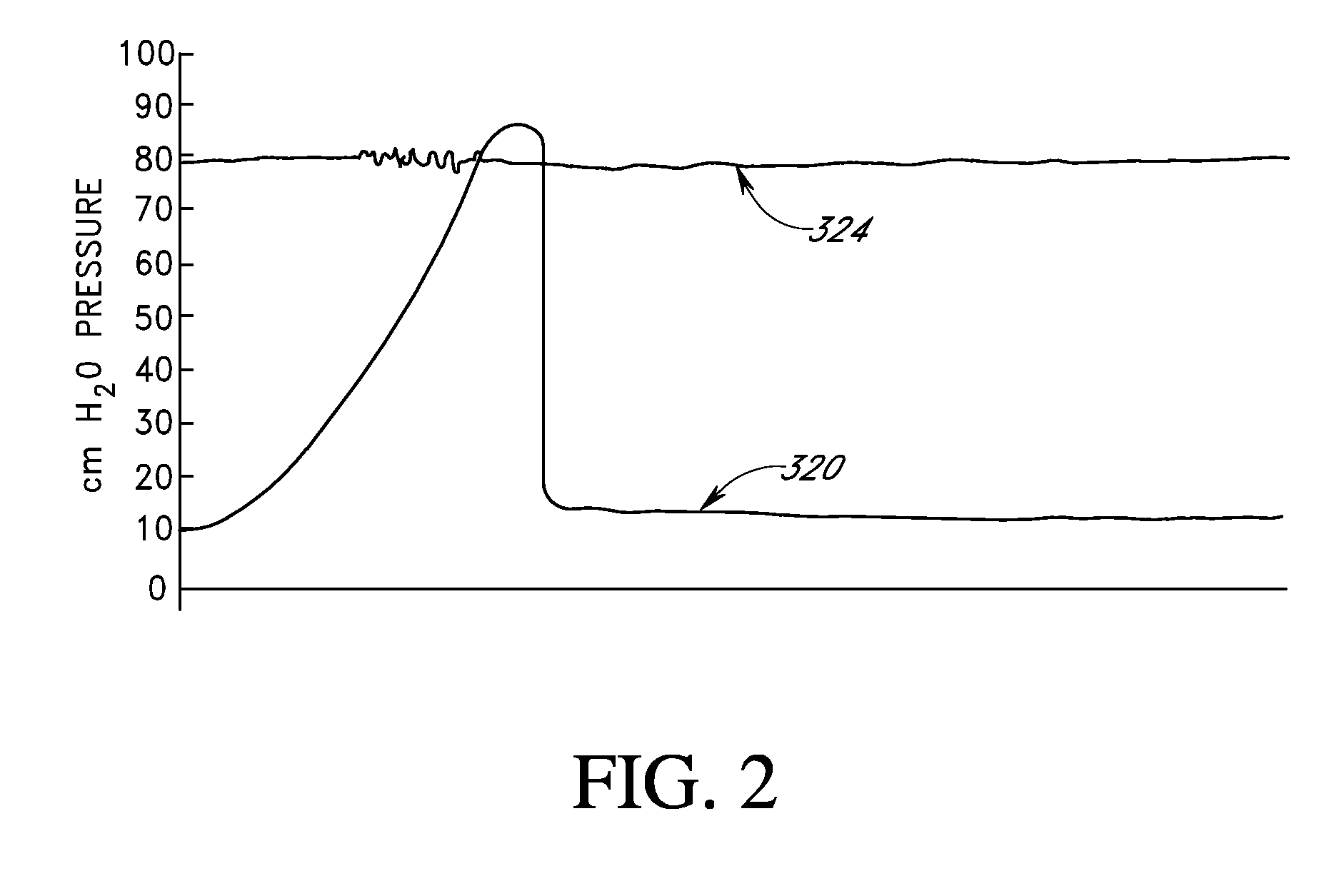
Treatment of patients with a compressible attenuation device
InactiveUS7074178B2Increase pressureLower the volumeUrinary bladderDiagnostic signal processingUltrasound attenuationAbdominal cavity
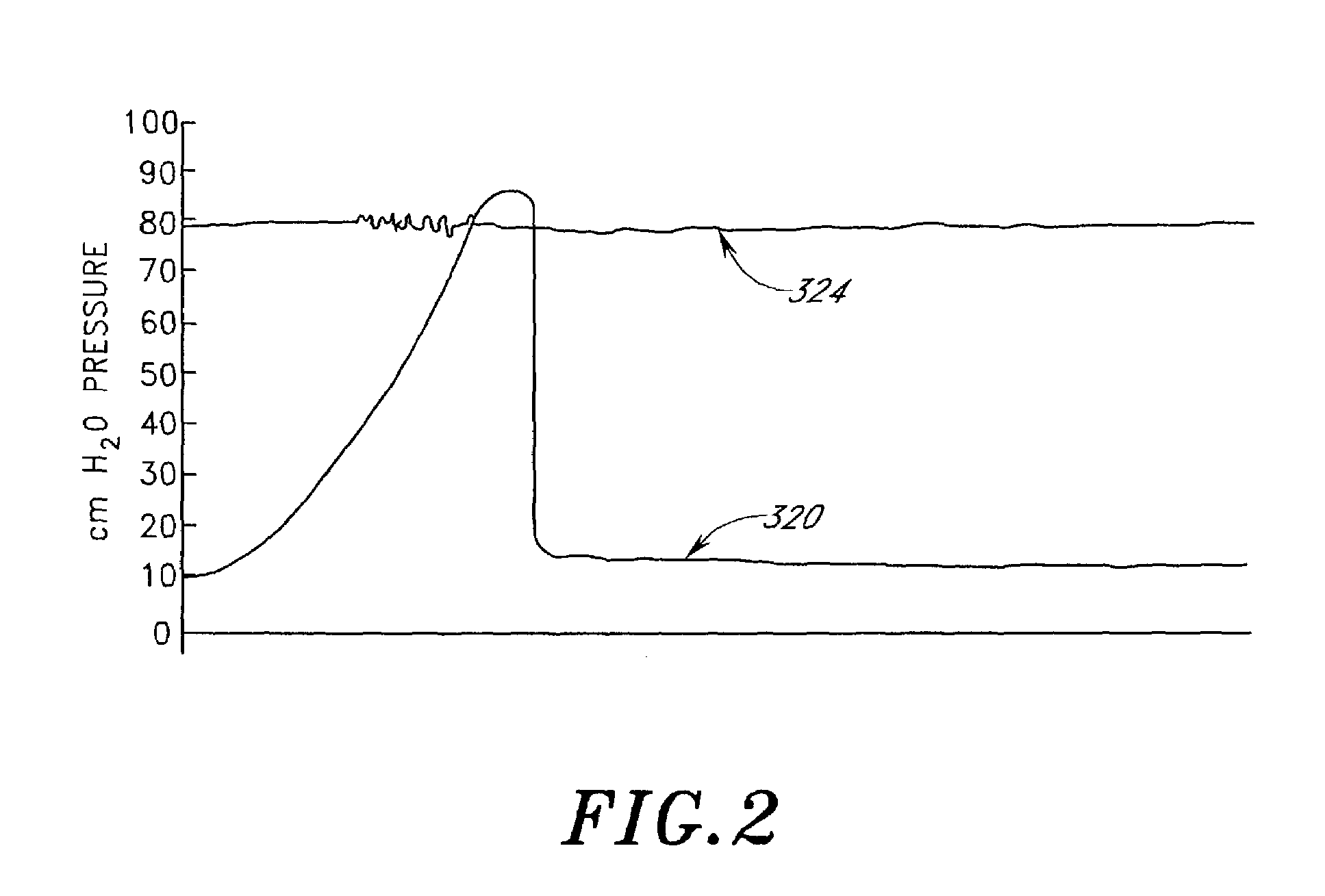
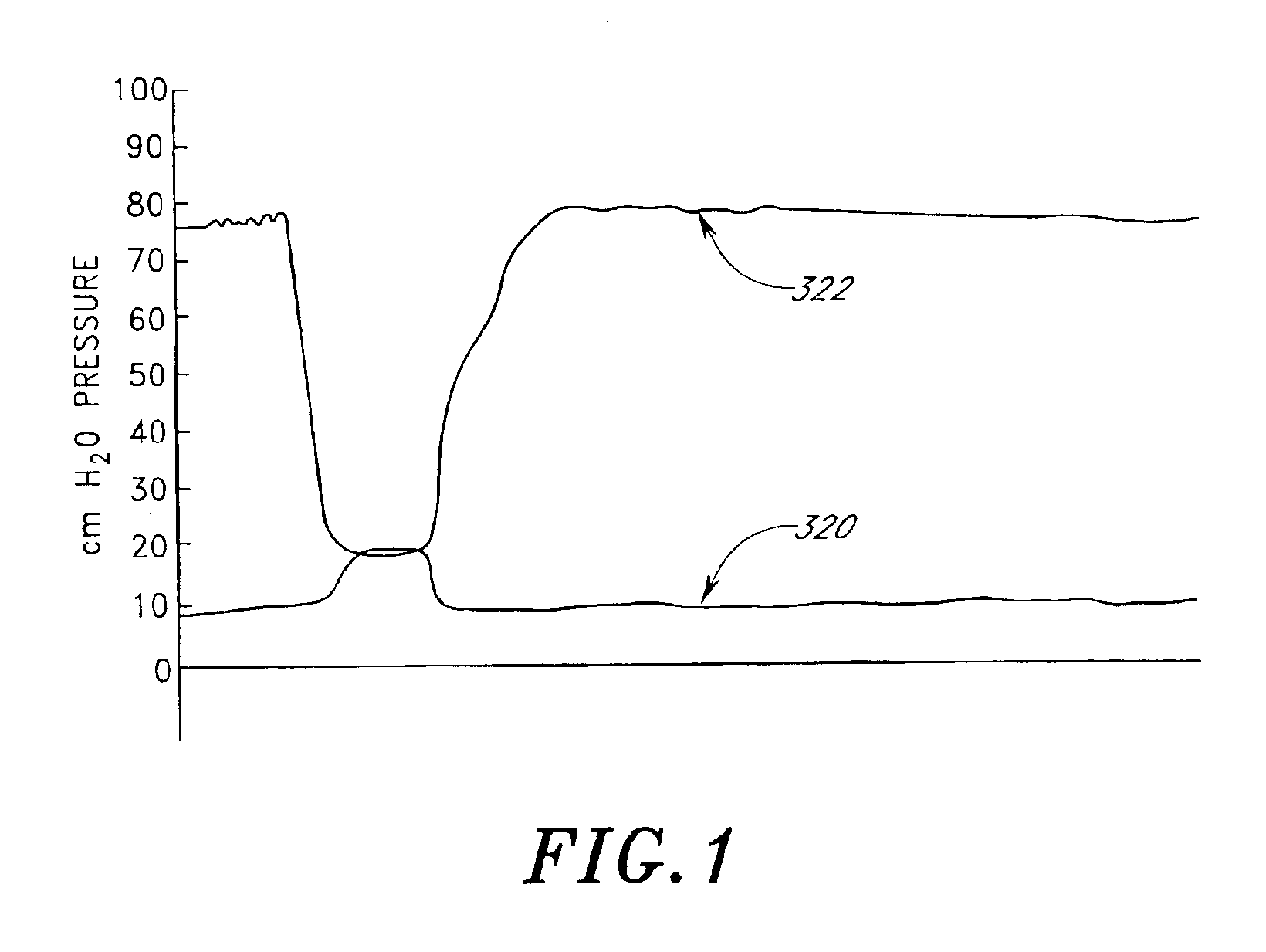
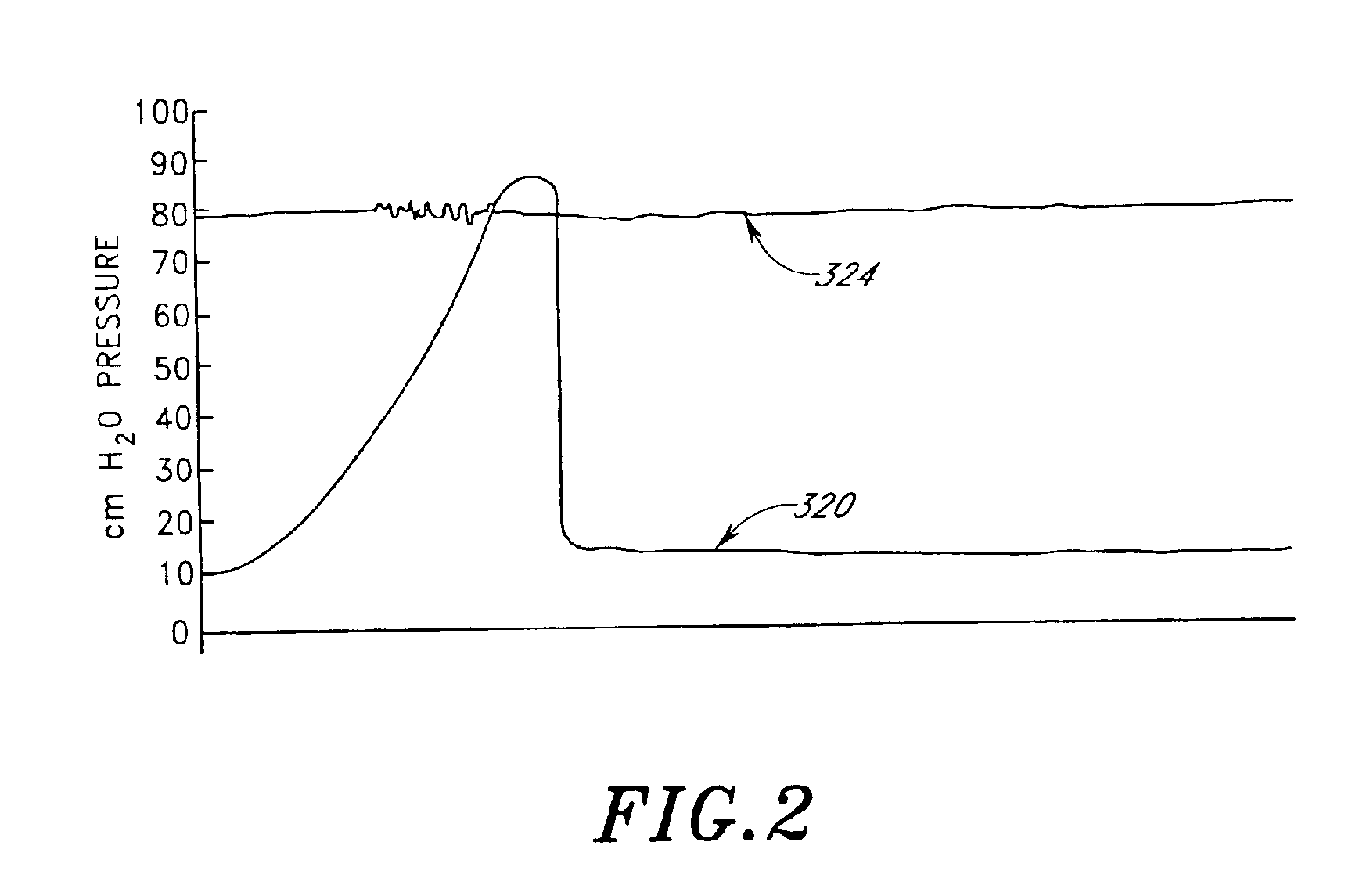
Disclosed herein are methods of treating a patient, comprising providing a compressible attenuation device that is moveable from a first, introduction configuration to a second, implanted configuration. The attenuation device is introduced into a treatment site. In one embodiment, the treatment site is the bladder. In another embodiment, the treatment site is the abdominal cavity. The volume of the attenuation devices changes in response to pressure changes in the treatment site and attenuates pressure changes in the bladder.
Owner:SOLACE THERAPEUTICS
High vapor pressure attenuation device
InactiveUS6976951B2Increase pressureLower the volumeStentsUrinary bladderUltrasound attenuationEngineering
Disclosed herein is an attenuation device, comprising a flexible housing and a high vapor pressure media having a vapor pressure approximately equal to the intravesical pressure of the bladder and a permeability of less than 1 ml / day at body temperature through the outer wall of the flexible housing. In one embodiment, the high vapor pressure media comprises perfluorooctylbromide. In another embodiment, the high vapor pressure media comprises perfluorohexane. In yet another embodiment, the high vapor pressure media comprises perfluorodecalin. Also disclosed herein is a method of treating a patient, comprising providing a compressible attenuation device and introducing within the attenuation device at least one high vapor pressure media.
Owner:SOLACE THERAPEUTICS
Cell-Scaffold Constructs
InactiveUS20100131075A1Increase capacityBladder volume capacityUrinary bladderWound drainsAutologous cellOrgan of Corti
Owner:ORGAGEN INC
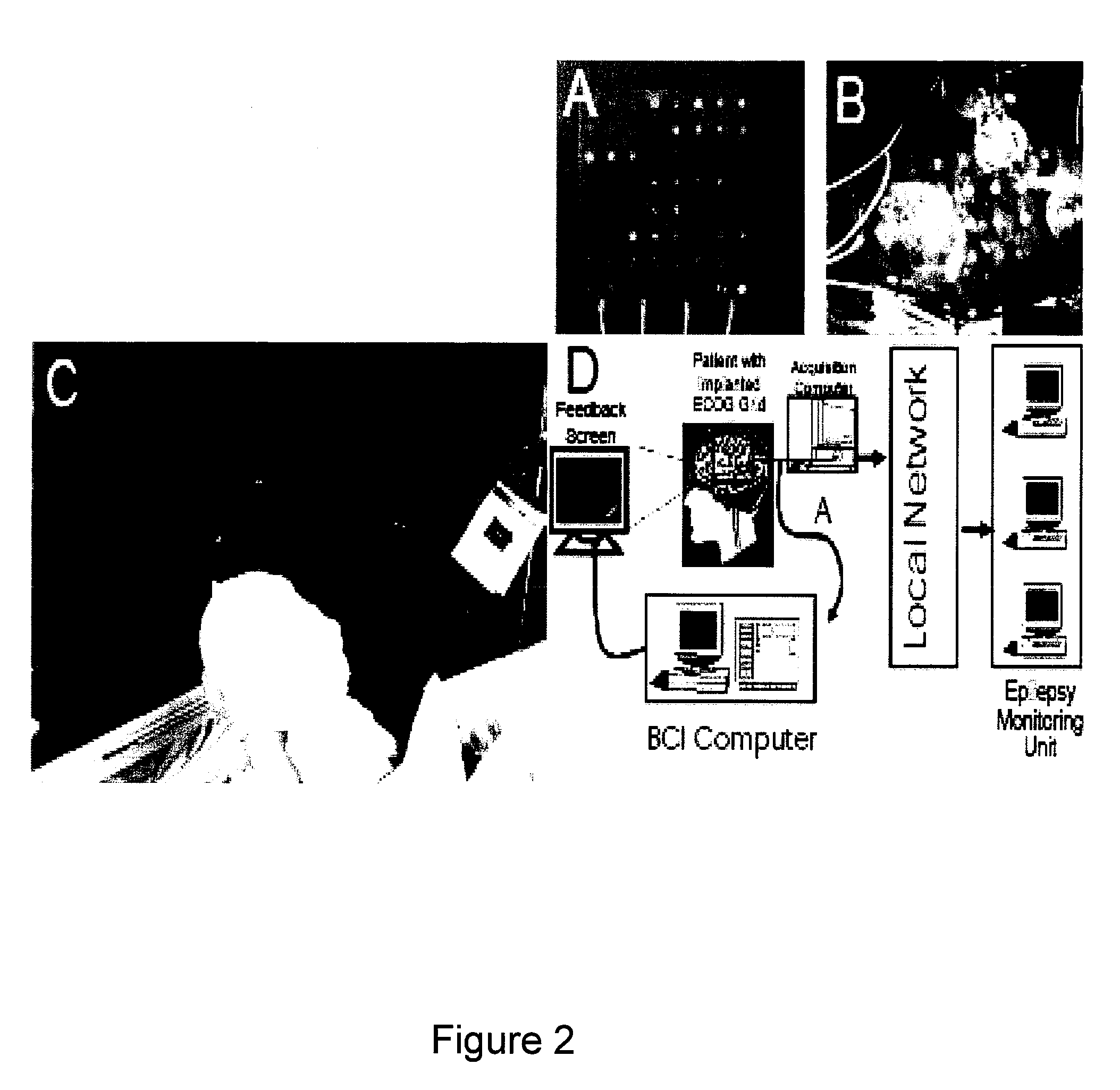
Methods and systems for controlling body parts and devices using ipsilateral motor cortex and motor related cortex
A system for controlling a body part includes a number of sensing devices that sense signals from a hemisphere of a brain. A signal translating unit translates the signals into a command signal for controlling the body part, which is on a same side of the body as the hemisphere of the brain. A prosthetic device receives the command signal from the signal translating unit and manipulates the body part in response to the command signal.
Owner:WASHINGTON UNIV IN SAINT LOUIS
High vapor pressure attenuation device
InactiveUS20060100478A1Increase pressureLower the volumeUrinary bladderEye surgeryUltrasound attenuationEngineering
Disclosed herein is an attenuation device, comprising a flexible housing and a high vapor pressure media having a vapor pressure approximately equal to the intravesical pressure of the bladder and a permeability of less than 1 ml / day at body temperature through the outer wall of the flexible housing. In one embodiment, the high vapor pressure media comprises perfluorooctylbromide. In another embodiment, the high vapor pressure media comprises perfluorohexane. In yet another embodiment, the high vapor pressure media comprises perfluorodecalin. Also disclosed herein is a method of treating a patient, comprising providing a compressible attenuation device and introducing within the attenuation device at least one high vapor pressure media.
Owner:SOLACE THERAPEUTICS
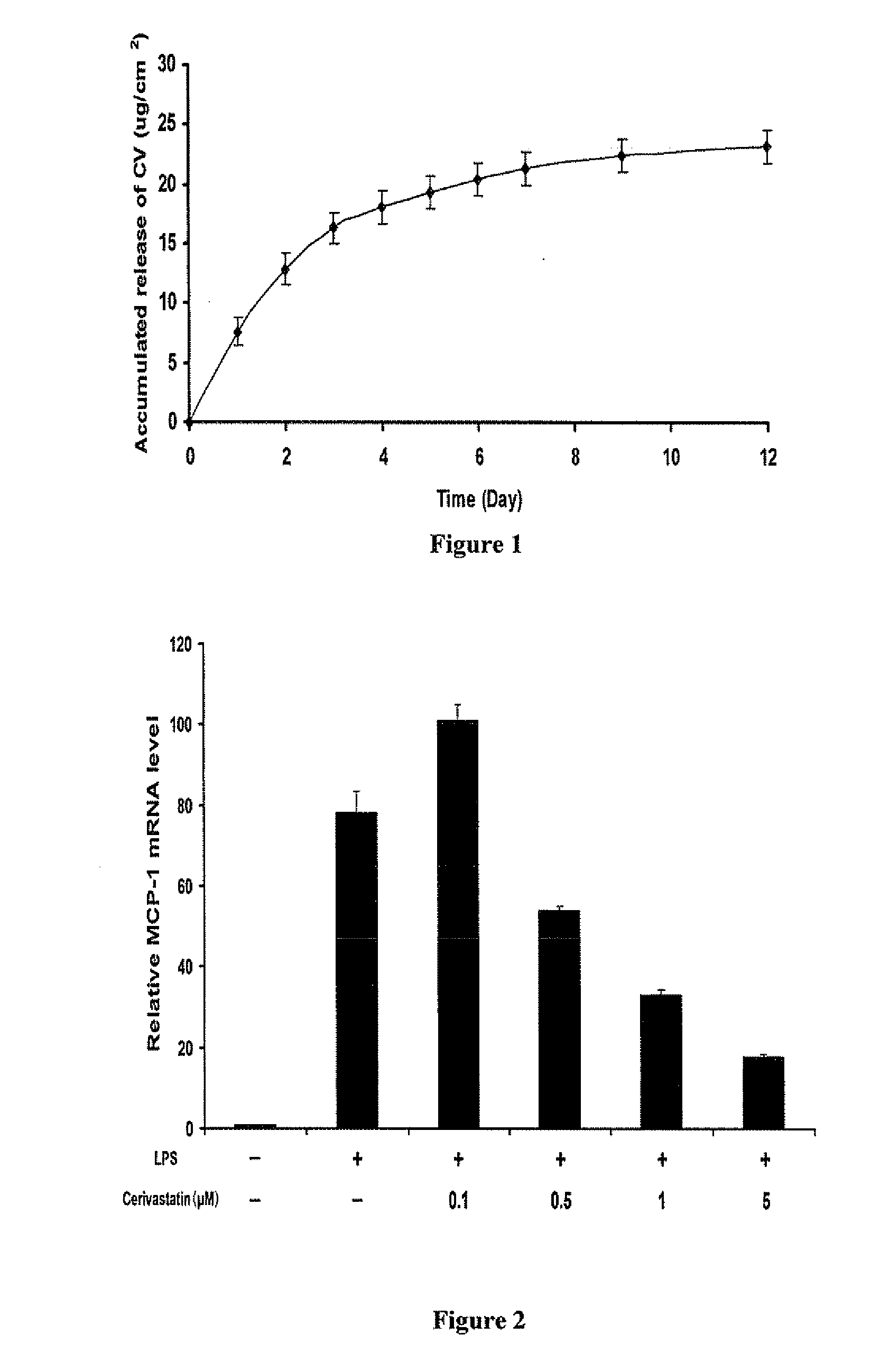
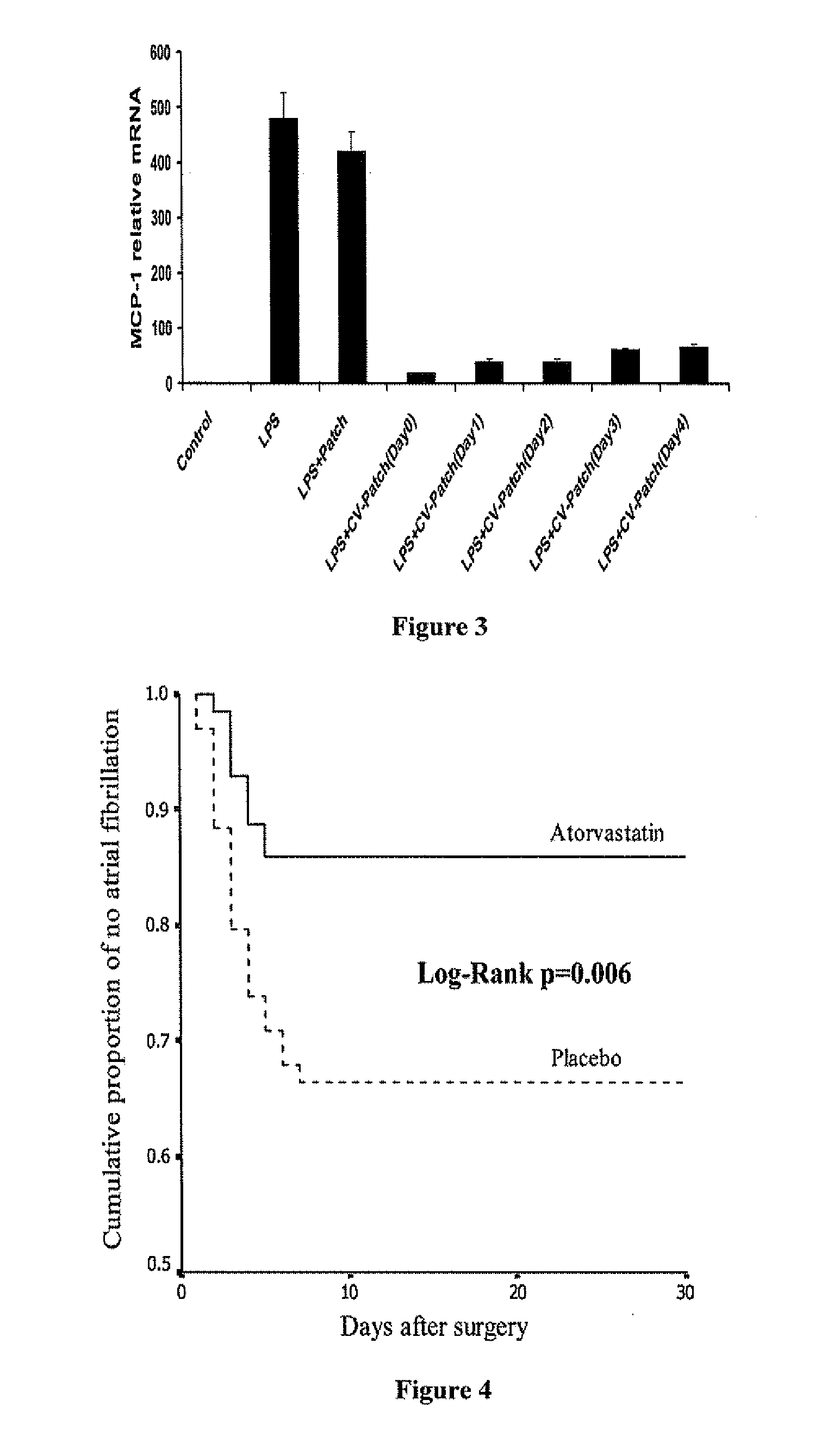
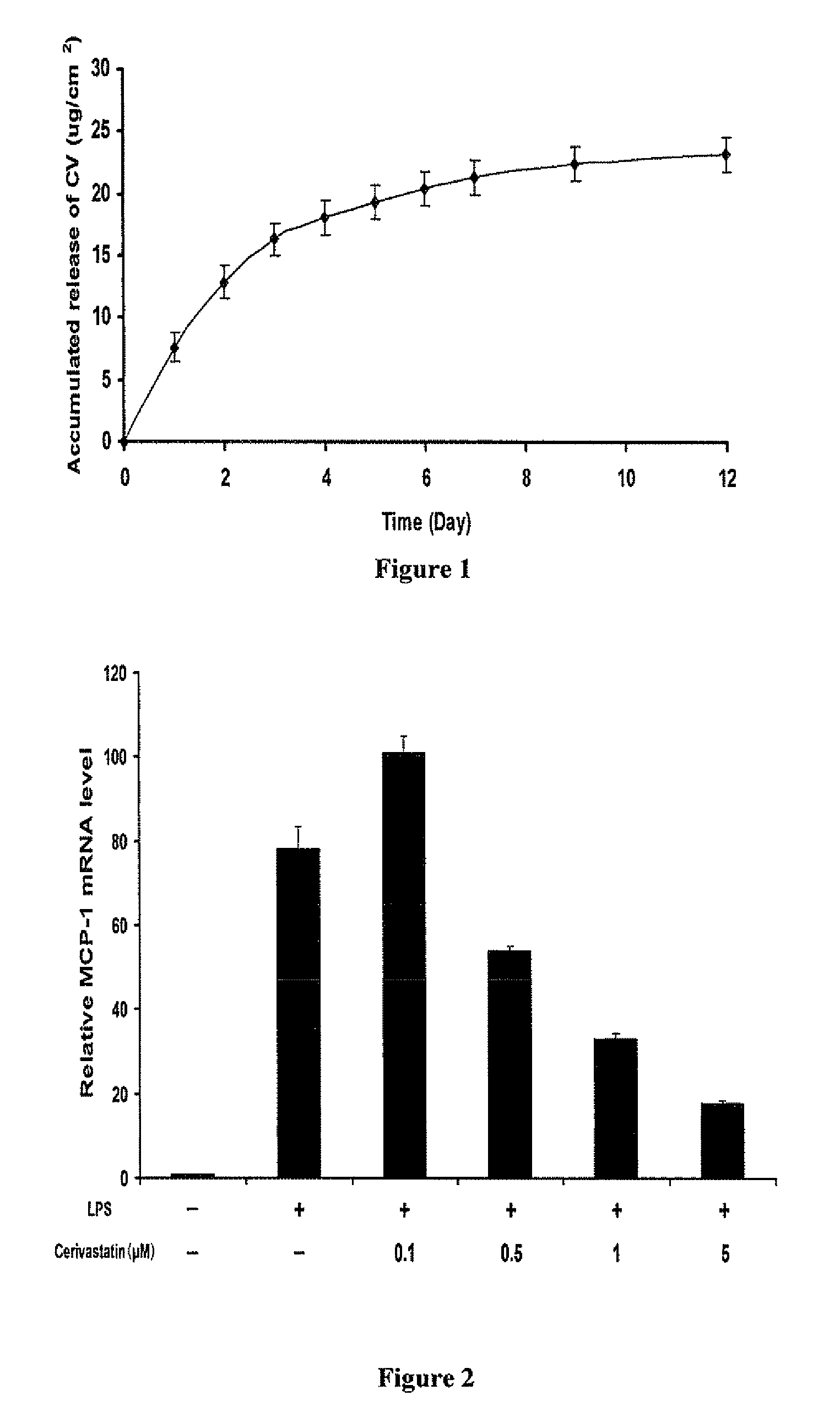
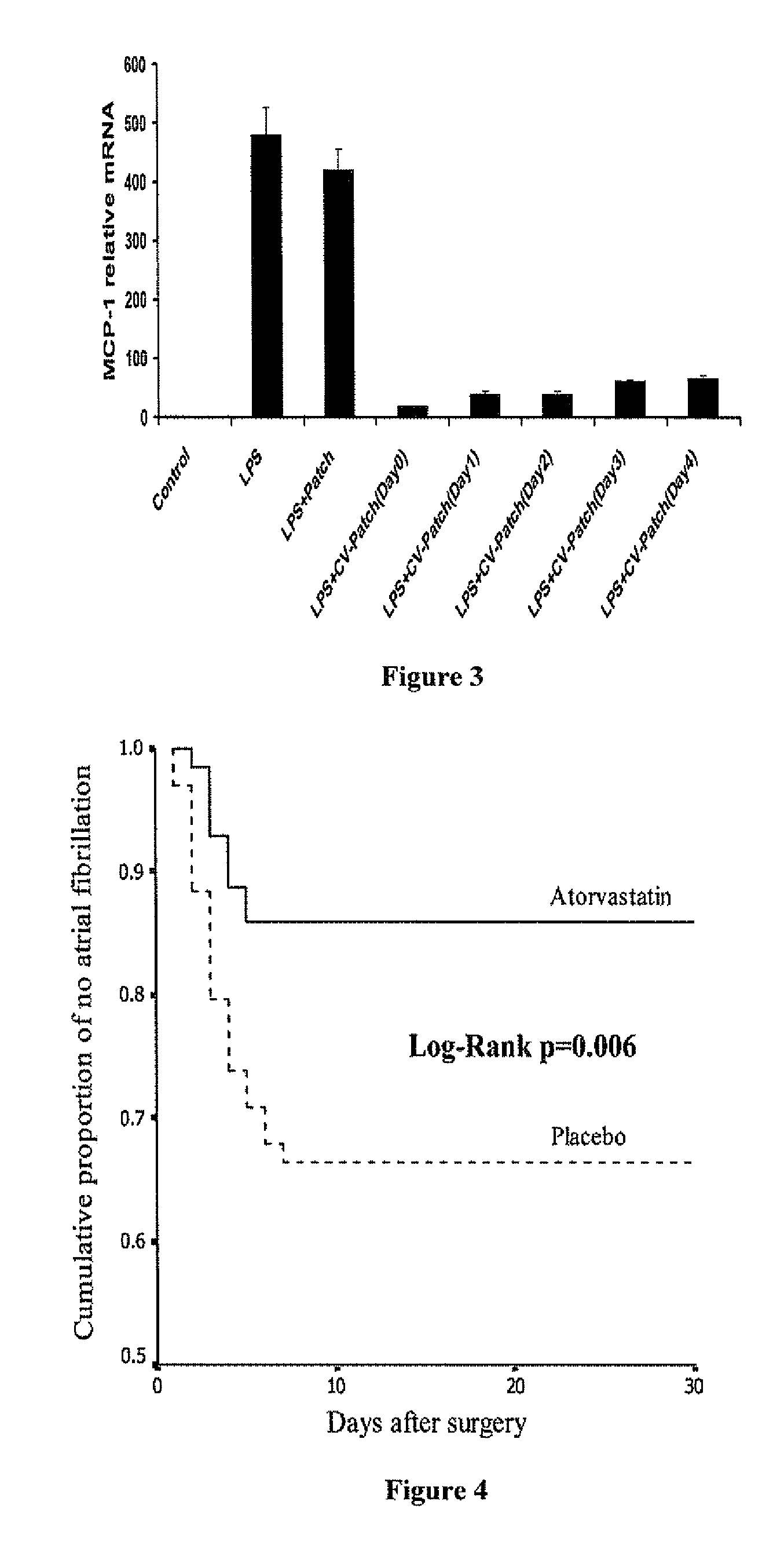
Drug eluting patch for the treatment of localized tissue disease or defect
A polymeric matrix for delivery of an HMG CoA reductase inhibitor such as a statin to tissue such as cardiac tissue in need thereof for the treatment or prevention of a disease or defect such as atrial fibrillation has been developed. In the preferred embodiment, a statin is delivered by means of a patch sutured to cardiac tissue at the time of cardiothoracic surgery. In the most preferred embodiment, the patch is a biodegradable material providing controlled or sustained release over a prolonged period of time, such as a week. Suitable materials include extracellular matrix, or other biodegradable hydrogels or polymeric materials providing sustained or controlled release of statin at the site of application.
Owner:MIDCAP FINANCIAL TRUST AS AGENT
Medical implant
InactiveUS20090192595A1Limited decomposition rateHigh strengthUrinary bladderStentsBiomedical engineeringBiodegradable polymer
Disclosed herein is a medical implant including an implant body of which at least a part is comprised of a biodegradable metal, wherein the part comprised of the biodegradable metal has a crystal grain diameter of not more than 10 μm.
Owner:TERUMO KK
Apparatuses for the treatment of urinary stress and urge incontinence
InactiveUS20120271098A1Improve comfortPrevent movementUrinary bladderAnti-incontinence devicesUrethraVagina
An apparatus for insertion into a human vagina for treating urinary incontinence, comprising: an intermediate section; an anchoring section located on one side of the intermediate section provided with a plurality of beveled anchoring arms adapted to prevent movement of the apparatus into the vagina; and, a support section located on the opposite side of the intermediate section from the anchoring section provided with a plurality of beveled supporting arms adapted to prevent movement of the apparatus out of the vagina and to provide sub-urethral support.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Method and apparatus for automated active sterilization of fully implanted devices
InactiveUS20150011928A1Effective sterilizationPromote resultsUrinary bladderStentsUltravioletProsthetic knee
The current invention provides this advance in infection control via its unique application of active sterilization to a catheter or implant. While most catheters, and many implants, are passive devices, the current invention will provide an active component as a integral part of the implanted catheter or device to continuously or intermittently sterilize the exposed surfaces / areas of the device. This active sterilization may be accomplished by a variety of mechanisms, including, application of heat, RF, microwave, ultrasound, ultraviolet radiation or other energy capable of sterilizing the device or dislodging any problematic Biofilm that may form. The active sterilization may also employ the pumping of a sterilizing chemical from all attached drug reservoir, the use of electricity or freezing temperatures or any other mechanism for either inhibiting, killing or dislodging any infectious material in contact with the implant. One major advantage of this design is that through the use of small, battery powered or inductively powered sterilization element, the implanted catheter or device can be effectively sterilized without requiring the standard removal surgery, waiting period, then replacement of the infected device. This is expected to translate into greatly improved outcomes (particularly for devices where infection may be catastrophic, ie a prosthetic knee or hip), greatly improved costs, and greatly improved longevity of susceptible devices (ie IV ports, etc.).
Owner:THERANOVA LLC
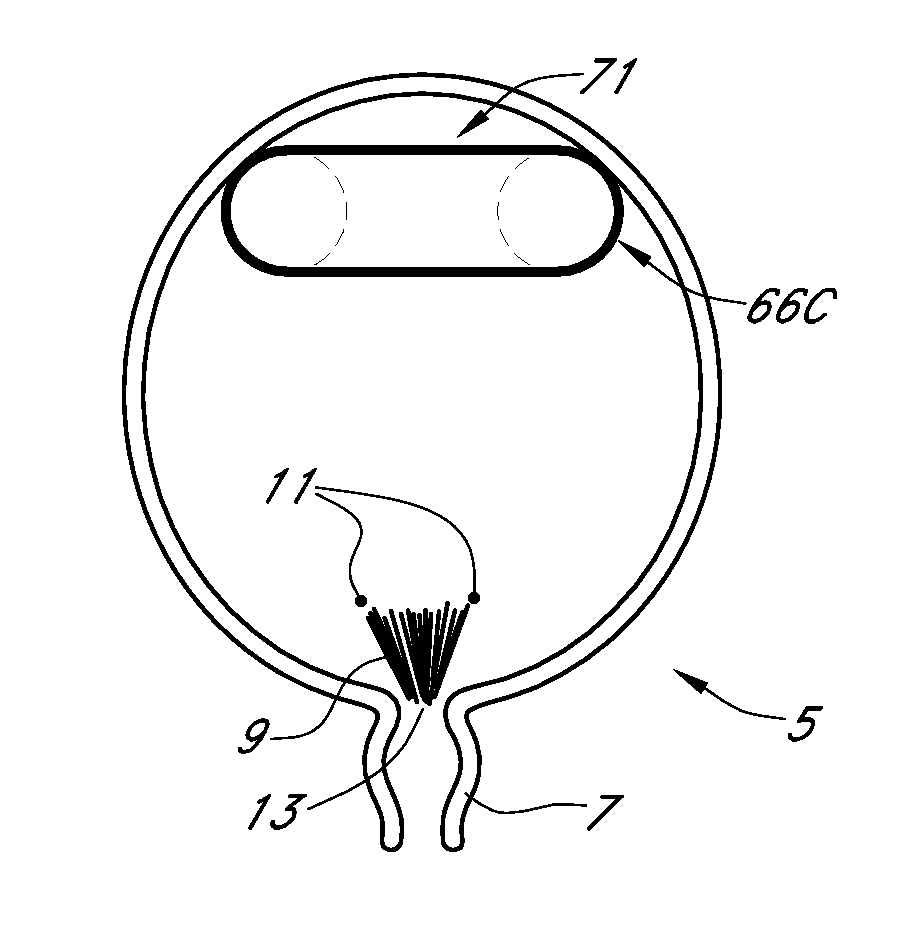
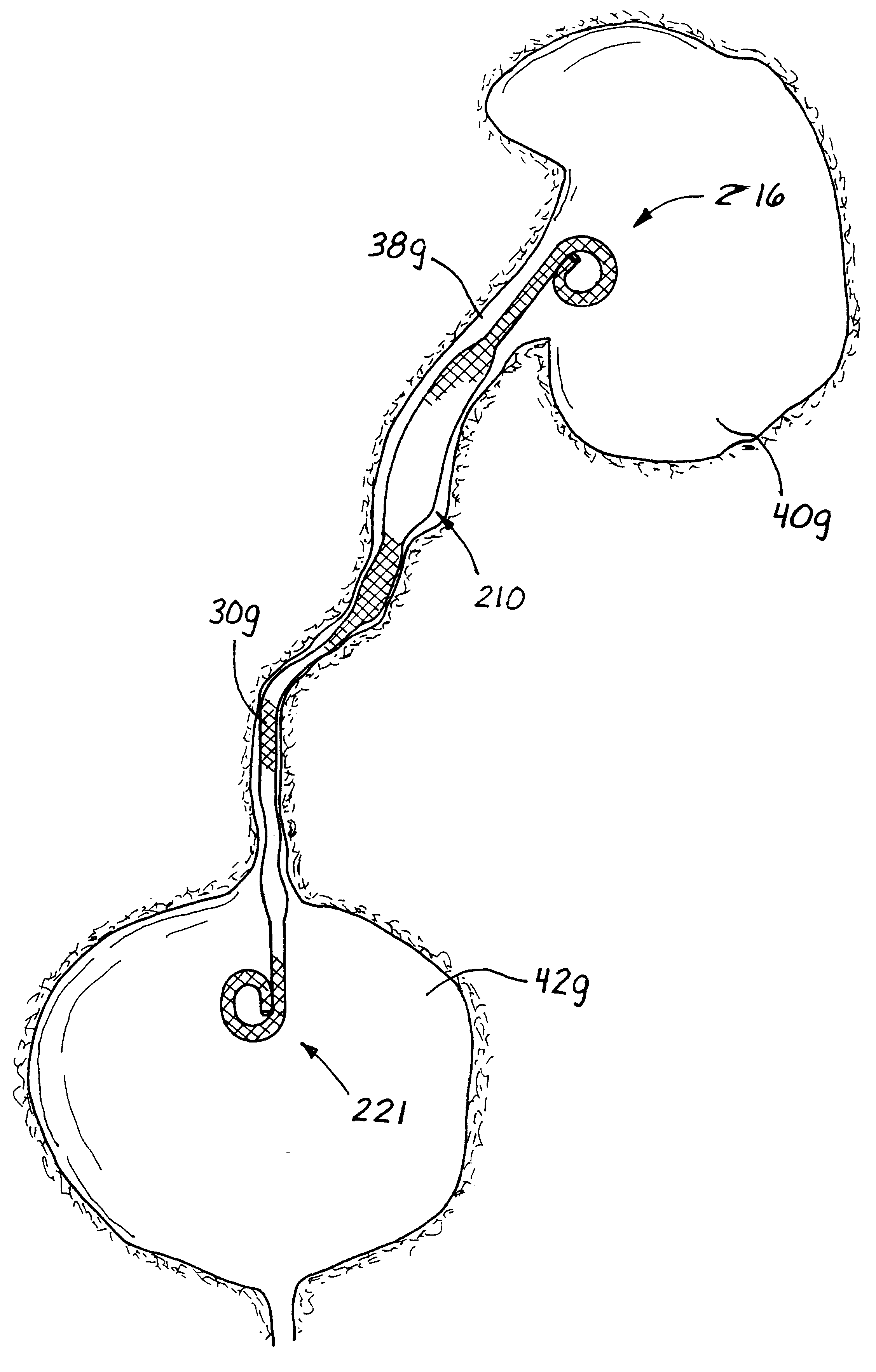
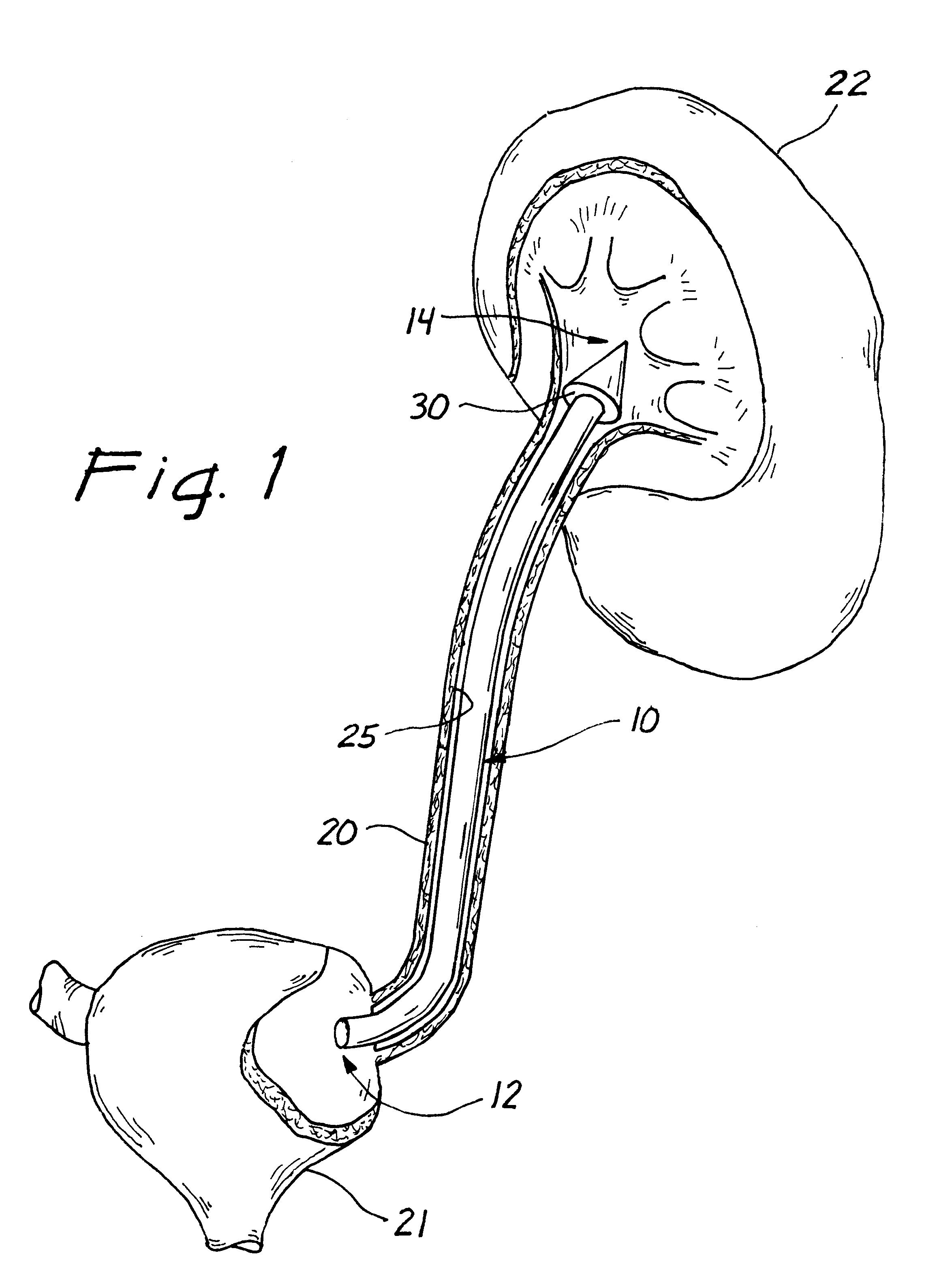
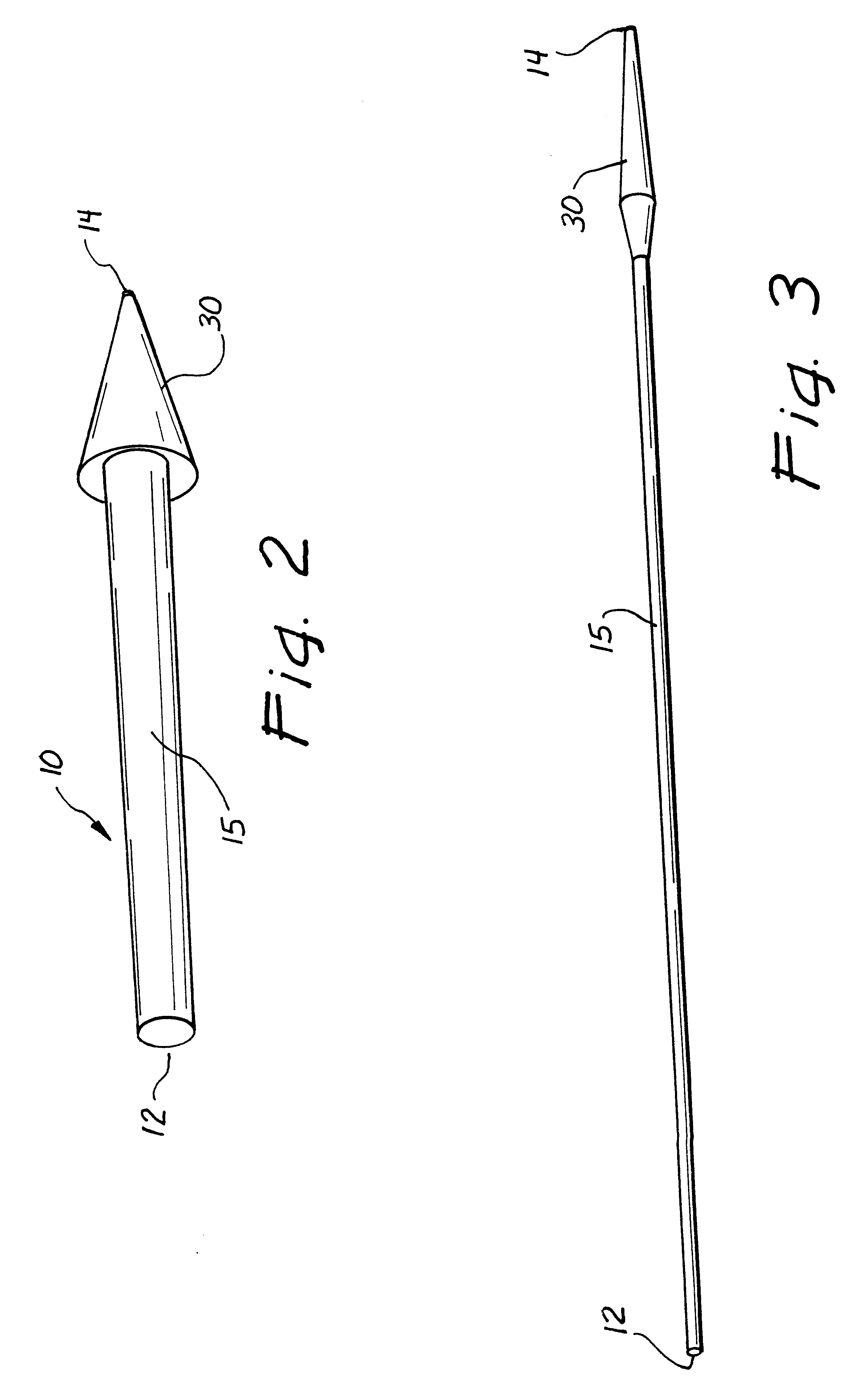
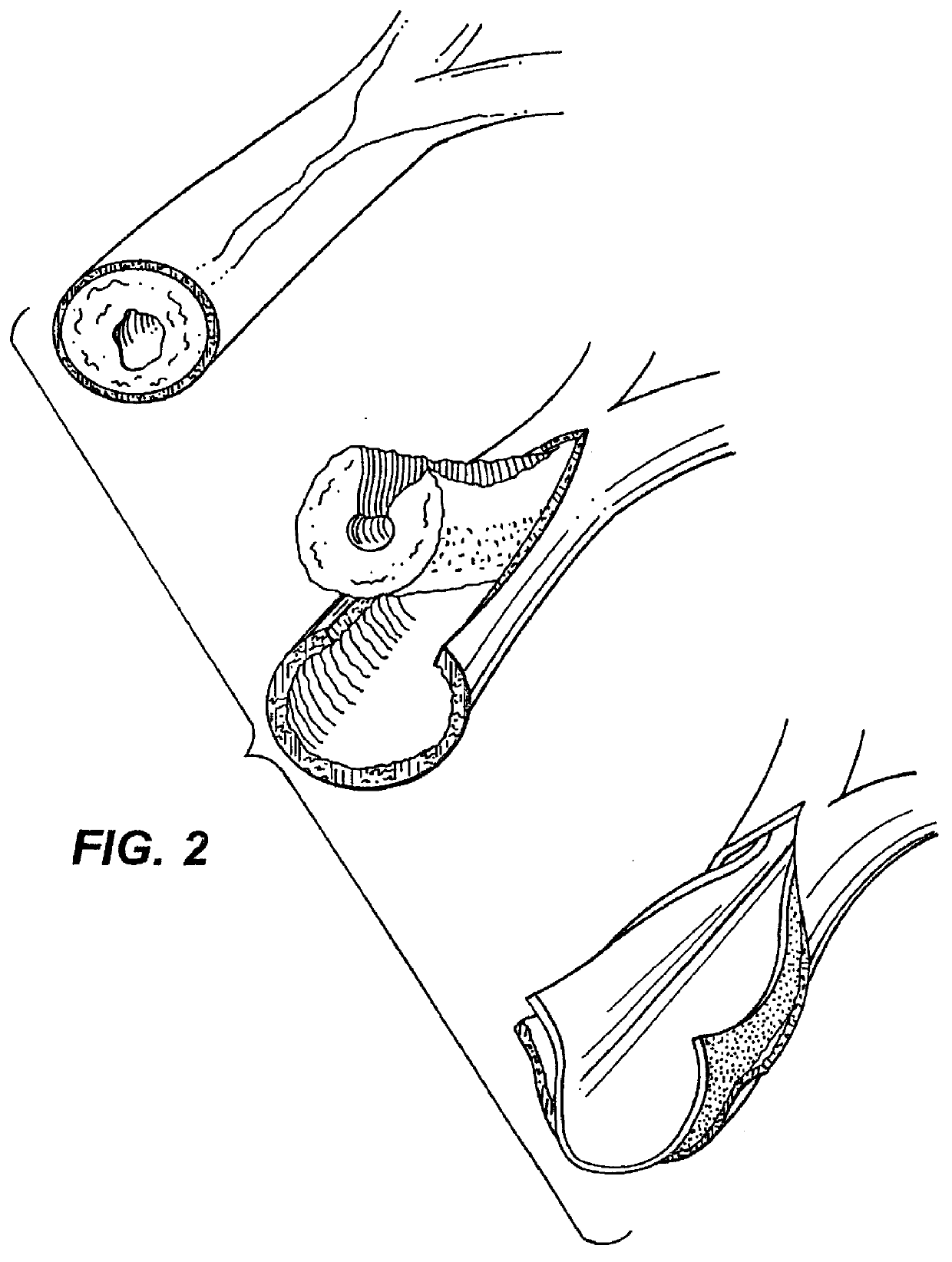
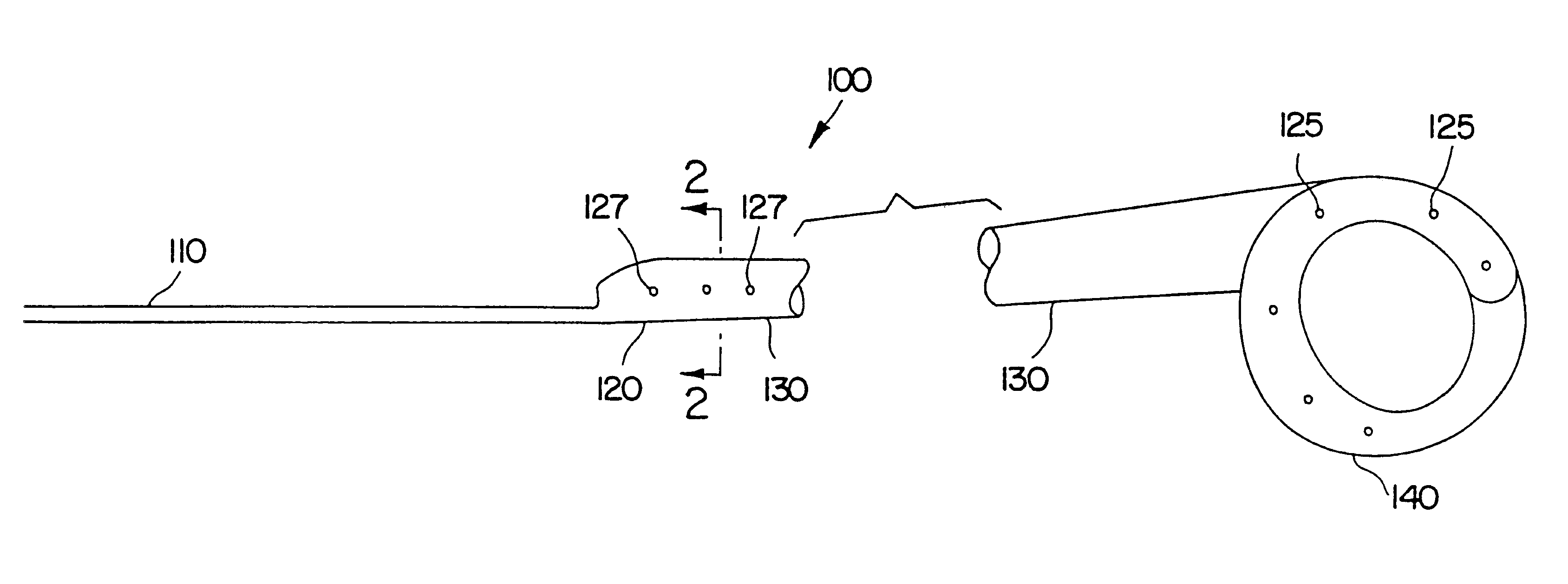
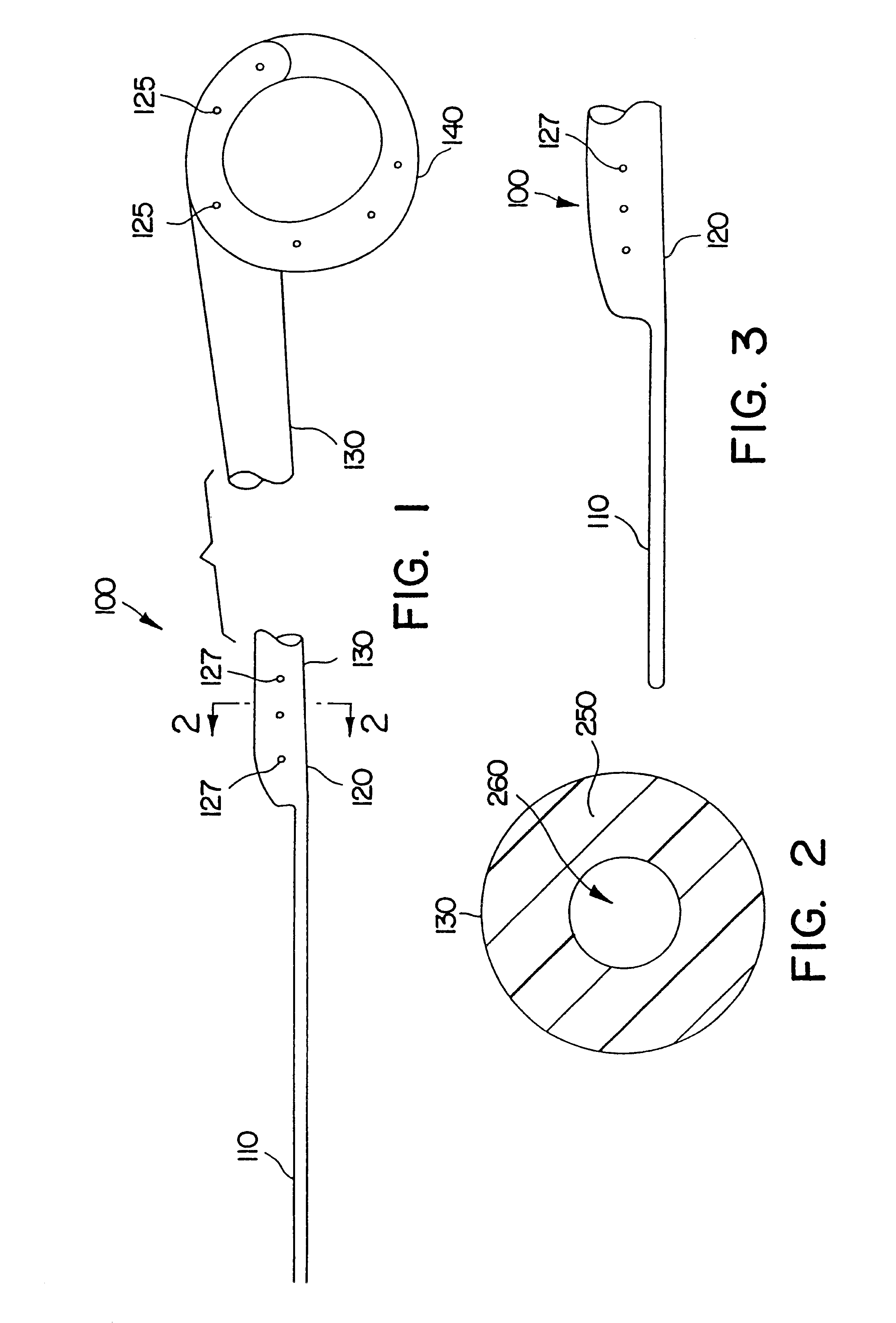
Ureteral stent with small bladder tail(s)
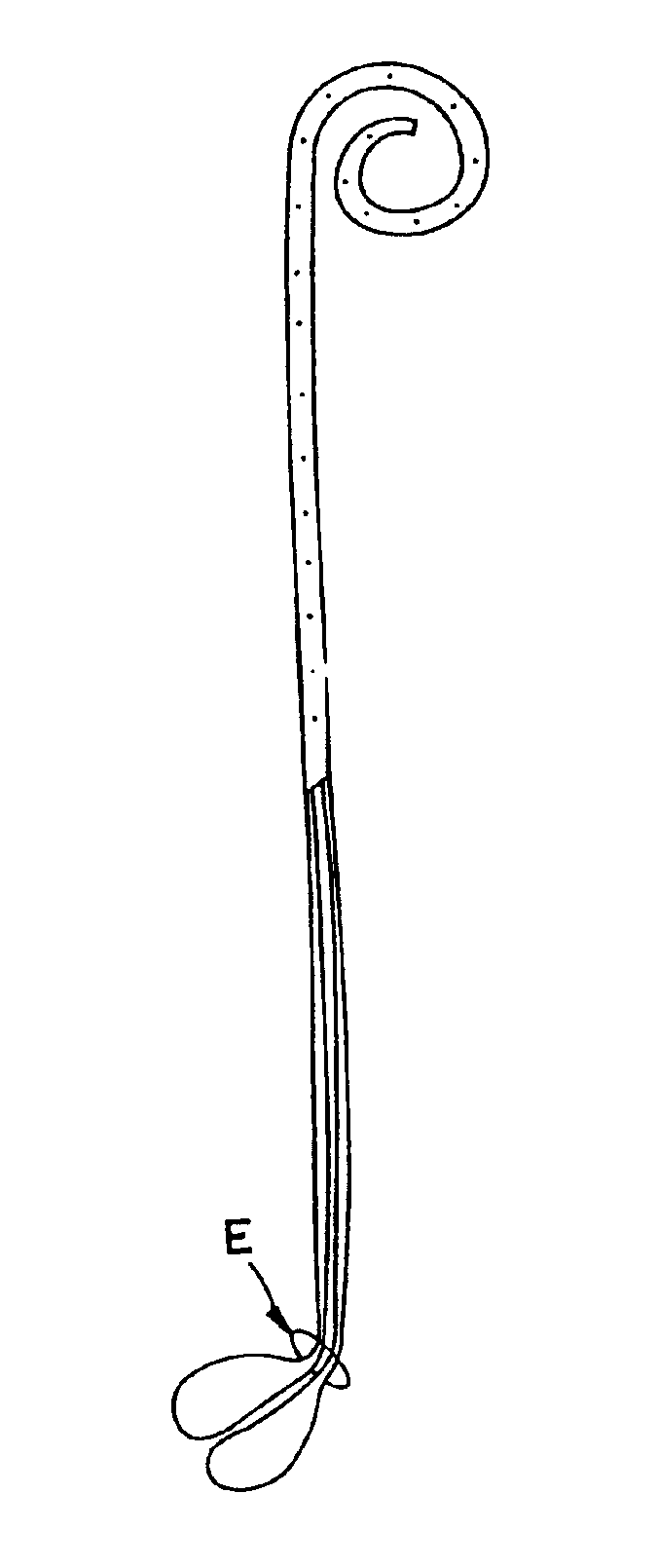
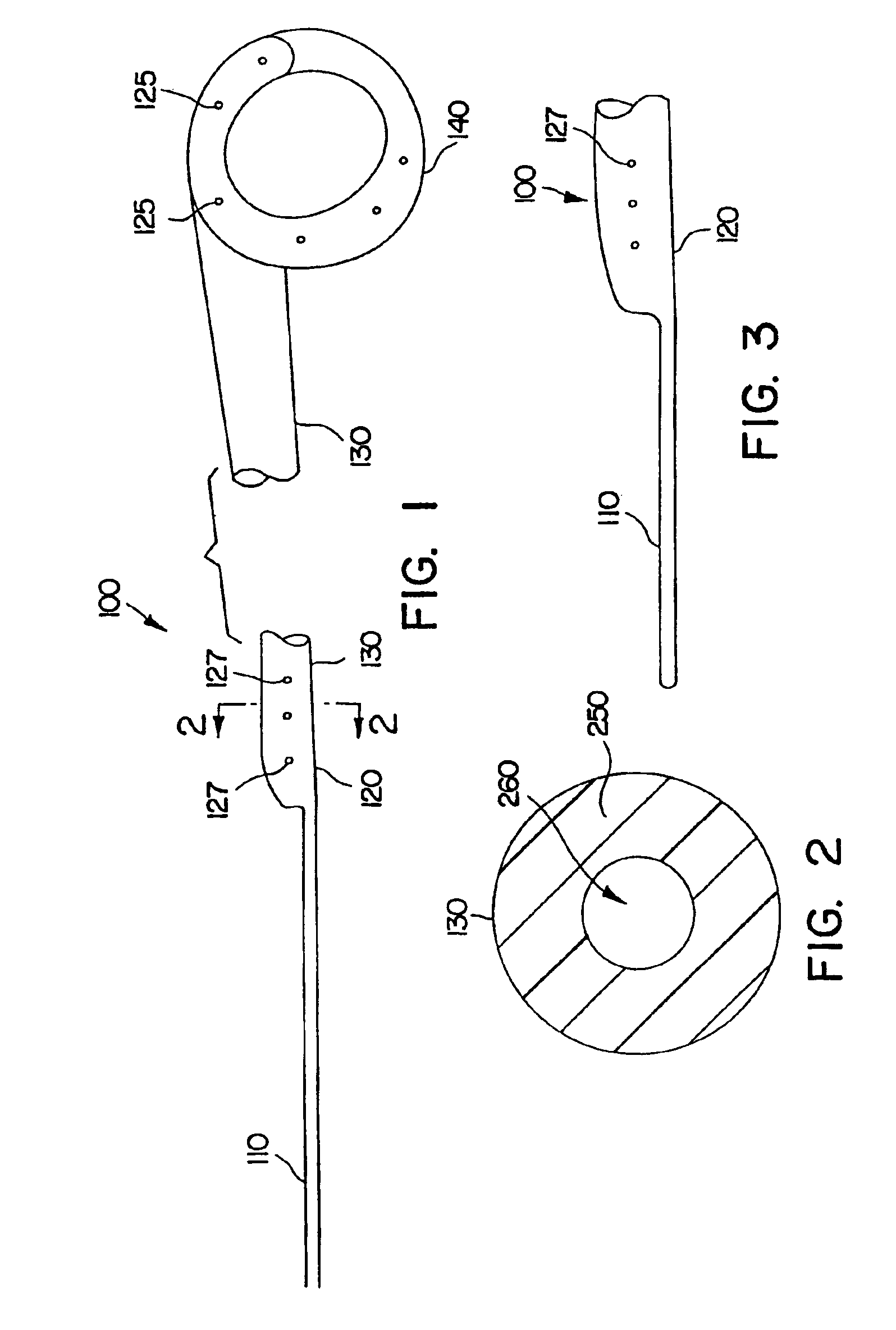
InactiveUS6945950B2Decreases patient discomfortInhibit migrationUrinary bladderStentsInsertion stentLeft ureter
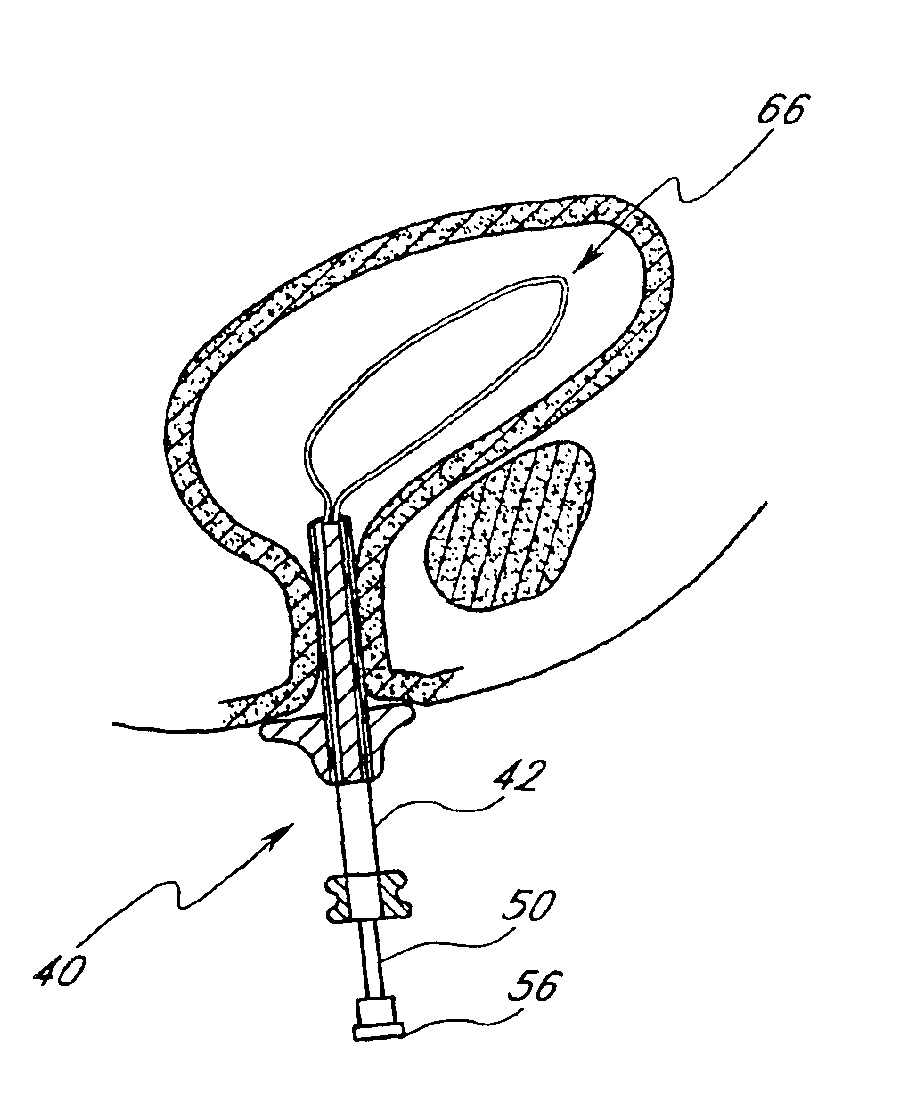
A ureteral stent for assisting movement of urine along a patient's ureter and into the patient's bladder. The stent includes an elongated tubular segment extending toward the bladder from a kidney end region for placement in the renal cavity to a bladder end region. A central lumen connects at least one opening at the first end region to at least one opening in the bladder end region. Thin flexible tail(s) are attached to the bladder end region of the tubular segment at a point outside the bladder so as to receive urine from the opening in the bladder end region and to transport urine from there across the ureter / bladder junction and into the bladder. The tails include an elongated external urine-transport surface configured to transport urine. The transporting surface(s) are configured to extend along at least part of the ureter, across the ureter / bladder junction, and into the bladder.
Owner:BOSTON SCI SCIMED INC +1
Method and apparatus for automated active sterilization of fully implanted devices
InactiveUS20100256607A1Avoid spreadingEffective sterilizationUrinary bladderStentsProsthetic kneeCatheter device
The current invention provides this advance in infection control via its unique application of active sterilization to a catheter or implant. While most catheters, and many implants, are passive devices, the current invention will provide an active component as a integral part of the implanted catheter or device to continuously or intermittently sterilize the exposed surfaces / areas of the device. This active sterilization may be accomplished by a variety of mechanisms, including, application of heat, RF, microwave, ultrasound, ultraviolet radiation or other energy capable of sterilizing the device or dislodging any problematic Biofilm that may form. The active sterilization may also employ the pumping of a sterilizing chemical from an attached drug reservoir, the use of electricity or freezing temperatures or any other mechanism for either inhibiting, killing or dislodging any infectious material in contact with the implant. One major advantage of this design is that through the use of a small, battery powered or inductively powered sterilization element, the implanted catheter or device can be effectively sterilized without requiring the standard removal surgery, waiting period, then replacement of the infected device. This is expected to translate into greatly improved outcomes (particularly for devices where infection may be catastrophic, ie a prosthetic knee or hip), greatly improved costs, and greatly improved longevity of susceptible devices (ie IV ports, etc.).
Owner:THERANOVA LLC
Implantable device for internal urinary control
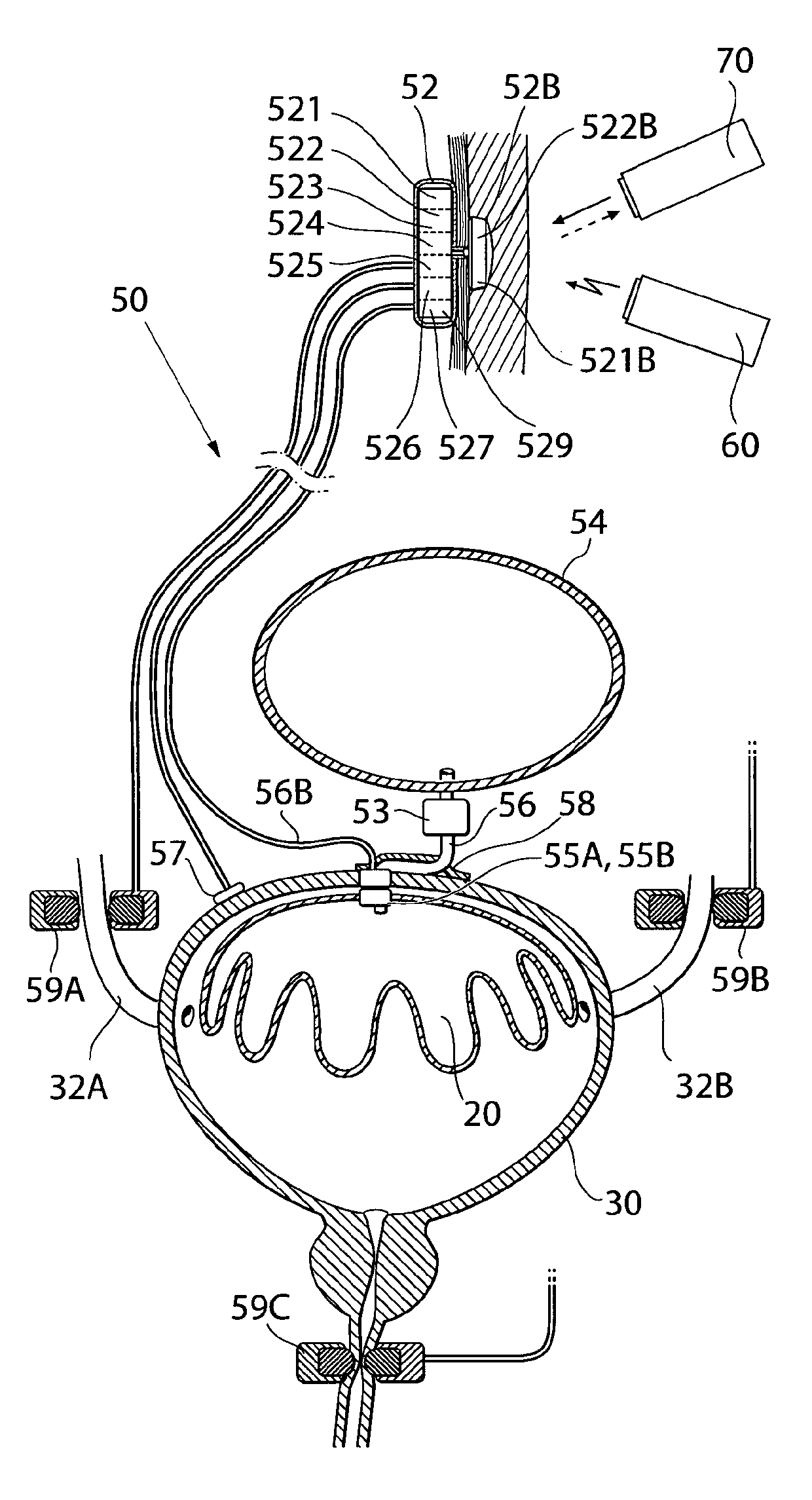
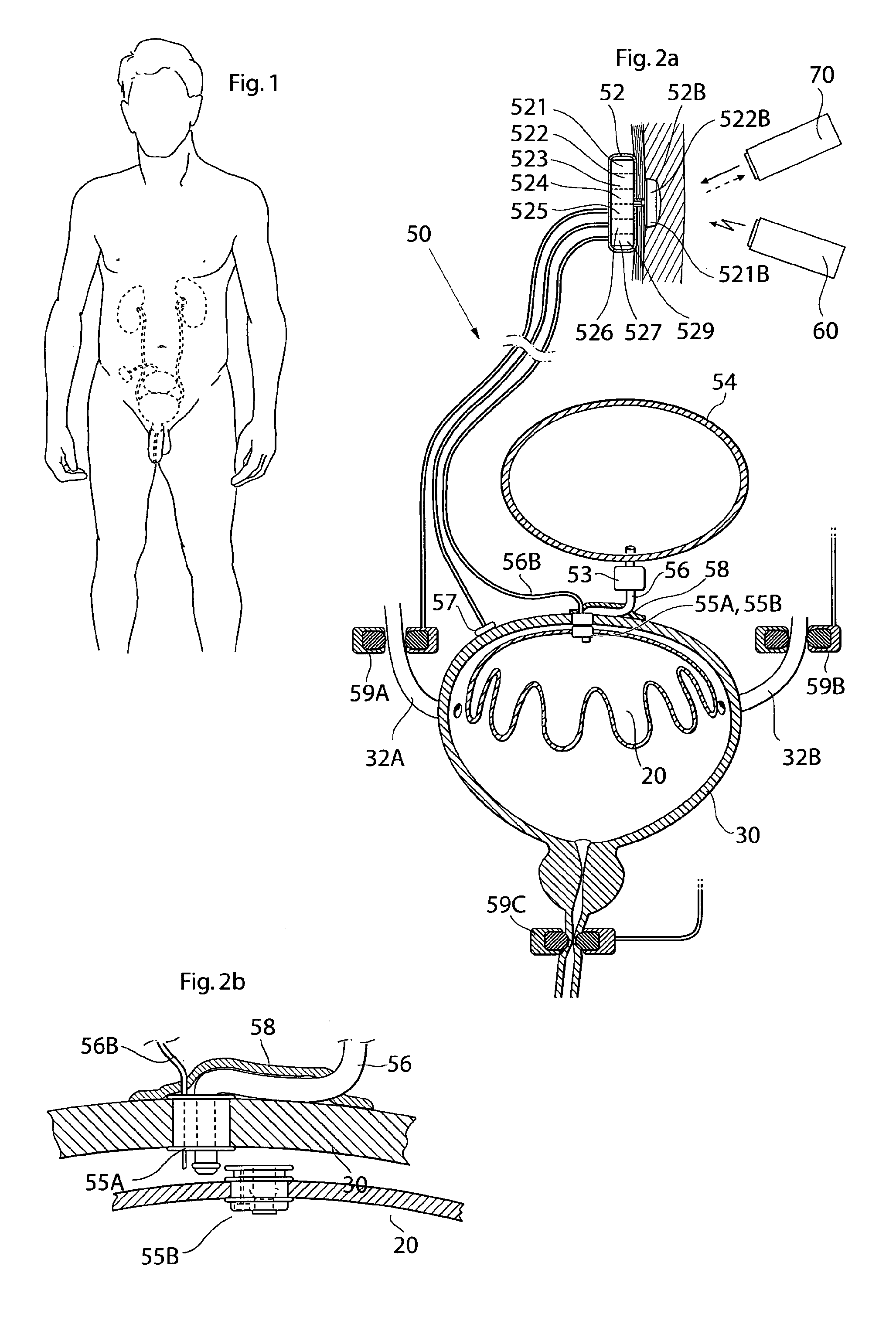
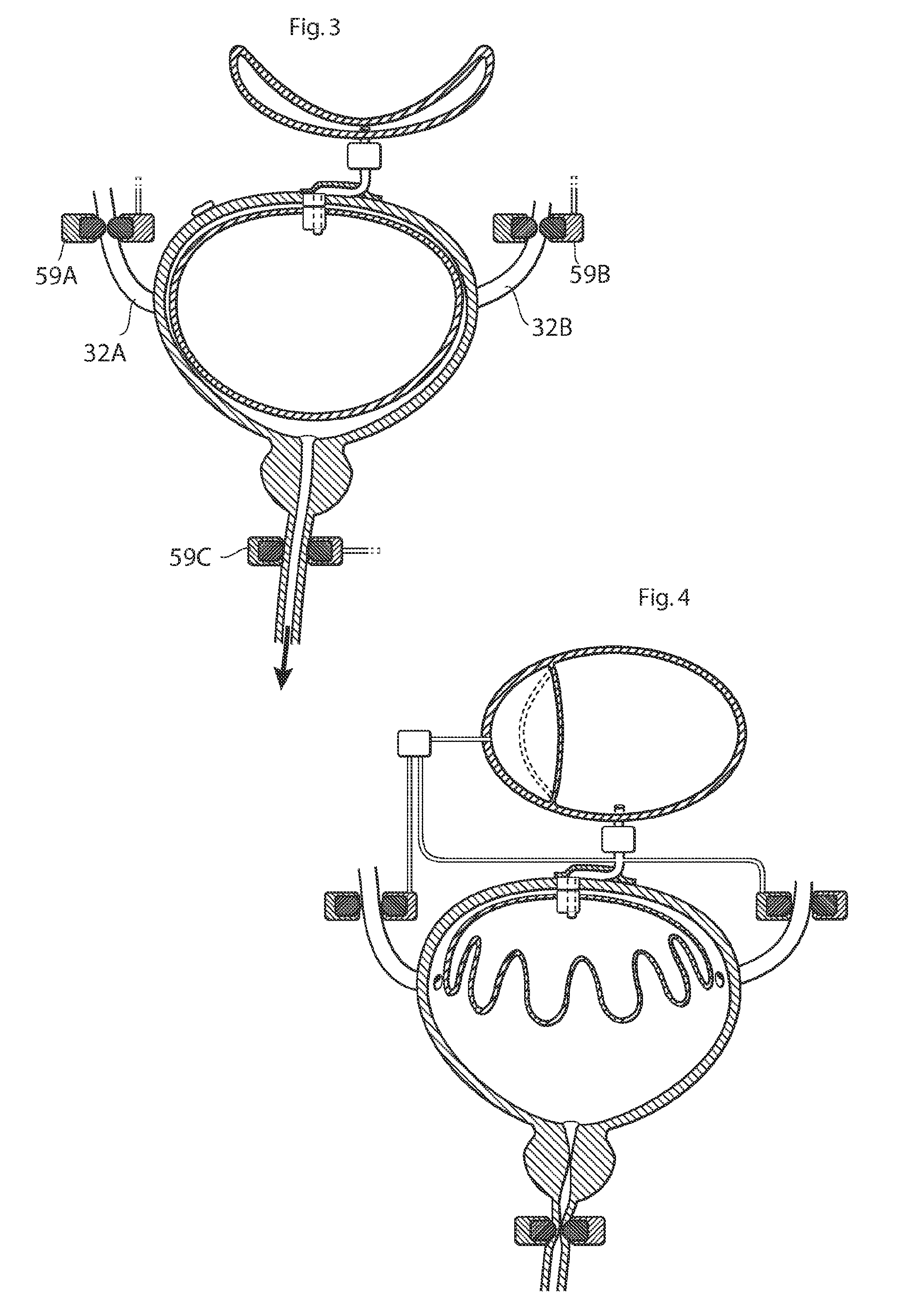
ActiveUS20110196194A1Easy to replaceReliable and goodUrinary bladderAnti-incontinence devicesImplanted deviceUrinary control
The present invention relates to an implantable apparatus for obtaining urinary control and emptying of the urinary bladder, thereby preventing from or treating involuntary urinary retention. In general terms, the apparatus comprises an expandable member adapted to be implanted inside the urinary bladder of the patient for discharging urine, and a control device for controlling the volume of the expandable member. The control device is adapted to be connected to the expandable member through the wall of the urinary bladder.
Owner:FORSELL PETER
Scaffolds for organ reconstruction and augmentation
Biocompatible synthetic or natural scaffolds are provided for the reconstruction, repair, augmentation or replacement of organs or tissue structures in a patient in need of such treatment. The scaffolds are shaped to conform to at least a part of the organ or tissue structure and may be seeded with one or more cell populations. Inserts, receptacles and ports are also provided for the attachment of tubular vessels to the neo-organ scaffolds. The seeded scaffolds are implanted into the patient at the site in need of treatment to form an organized organ or tissue structure. The scaffolds may be used to form organs or tissues, such as bladders, urethras, valves, and blood vessels.
Owner:ORGAGEN INC
Catheter assembly/package utilizing a hydrating/hydrogel sleeve and a foil outer layer and method of making and using the same
Owner:CR BARD INC
Method of treating benign hypertrophy of the prostate
InactiveUS20090105527A1Increase pressureLower the volumeUrinary bladderDiagnostic signal processingUltrasound attenuationEccentric hypertrophy
Disclosed herein are methods of treating a patient with benign hypertrophy of the prostate, comprising providing a compressible attenuation device that is moveable from a first, introduction configuration to a second, implanted configuration and attenuating a pressure change within the bladder by reversibly changing the volume of the attenuation device in response to the pressure change. In one embodiment, the attenuation device is advanced percutaneously into the bladder. In another embodiment, the attenuation device is positioned within the bladder to inhibit a decrease in compliance of the bladder wall as a consequence of the benign hypertrophy of the prostate.
Owner:SOLACE THERAPEUTICS
Method and apparatus for automated active sterilization of fully implanted devices
Owner:THERANOVA LLC
Urethral sealing method and device
An urethral sealing device comprising first and second body portions selectively attacheable to one another to form a unitary element including a first catheter lumen extending therethrough between proximal and distal ends of the unitary element wherein, when the sealing device is in an operative position, the distal end is positioned within a urinary bladder and a sealing element extending radially outward from the unitary element so that, when the sealing device is in the operative position, the sealing element engages one of a wall of the urethra and a wall of the urinary bladder around an orifice at which the urethra opens into the urinary bladder to seal the urethra.
Owner:BOSTON SCI SCIMED INC
Medical device with tail(s) for assisting flow of urine
InactiveUS6849069B1Decrease patient discomfortDecreases patient discomfortUrinary bladderStentsInsertion stentLeft ureter
A ureteral stent for assisting movement of urine along a patient's ureter and into the patient's bladder. The stent includes an elongated tubular segment extending toward the bladder from a kidney end region for placement in the renal cavity to a bladder end region. A central lumen connects at least one opening at the first end region to at least one opening in the bladder end region. Thin flexible tail(s) are attached to the bladder end region of the tubular segment at a point outside the bladder so as to receive urine from the opening in the bladder end region of the tubular segment and to transport urine from there across the ureter / bladder junction and into the bladder. The tails include an elongated external urine-transport surface sized and configured to transport urine along the ureter. The urine transporting surface(s) are sized and configured to extend along at least part of the ureter, across the ureter / bladder junction, and from there into the bladder.
Owner:BOSTON SCI CORP
Reconstruction of urological structures with polymeric matrices
A method for repairing defects and reconstructing urothelial structures in vivo has been developed using a fibrous, open synthetic, biodegradable polymeric matrix which is configured to provide the desired corrective structure. The matrix is shaped to correct the defect, then implanted surgically to form a scaffolding for the patient's own cells to grow onto and into. The implantation of the matrix initiates an inflammatory reaction, resulting in urothelial cells, endothelial cells and mesenchymal cells, to migrate into the matrix. The polymer forming the matrix is selected to be biocompatible and degradable in a controlled manner over a period of one to six months, in the preferred embodiment. A preferred material is a poly(lactic acid-glycolic acid) in a fibrous form, such as a woven or non-woven mesh. Examples demonstrate the repair of defects in bladder in rabbits.
Owner:CHILDRENS MEDICAL CENT CORP
Methods and systems for performing a medical procedure
Method and system for treating a patient using a compressible, pressure-attenuating device. According to one embodiment, the system is used to treat urinary tract disorders and comprises an access device, a delivery device, a pressure-attenuating device, and a removal device. The access device may be used to create a passageway to an anatomical structure, such as the patient's bladder. The delivery device may be inserted through the passageway created by the access device and may be used to deliver the pressure-attenuating device to the anatomical structure. The removal device may be inserted through the passageway created by the access device and may be used to view the bladder and / or to capture, to deflate and to remove the pressure-attenuating device.
Owner:SOLACE THERAPEUTICS
Drug eluting patch for the treatment of localized tissue disease or defect
A polymeric matrix for delivery of an HMG CoA reductase inhibitor such as a statin to tissue such as cardiac tissue in need thereof for the treatment or prevention of a disease or defect such as atrial fibrillation has been developed. In the preferred embodiment, a statin is delivered by means of a patch sutured to cardiac tissue at the time of cardiothoracic surgery. In the most preferred embodiment, the patch is a biodegradable material providing controlled or sustained release over a prolonged period of time, such as a week. Suitable materials include extracellular matrix, or other biodegradable hydrogels or polymeric materials providing sustained or controlled release of statin at the site of application.
Owner:MIDCAP FINANCIAL TRUST AS AGENT
Implantable device for internal urinary control
ActiveUS20110263928A1Easy to replaceGood adhesionUrinary bladderAnti-incontinence devicesImplanted deviceUrinary control
The present invention relates to an implantable apparatus for obtaining urinary control and emptying of the urinary bladder, thereby preventing from or treating involuntary urinary retention. In general terms, the apparatus comprises an expandable member adapted to be implanted inside the urinary bladder of the patient for discharging urine, and a control device for controlling the volume of the expandable member. The control device is adapted to be connected to the expandable member through the wall of the urinary bladder.
Owner:FORSELL PETER
Urological devices incorporating collagen inhibitors
InactiveUS20090028920A1Reduce and prevent formationSuture equipmentsBiocideKidneyBiomedical engineering
Owner:WAKE FOREST UNIV HEALTH SCI INC
Urethral sealing method and device
An urethral sealing device comprising first and second body portions selectively attacheable to one another to form a unitary element including a first catheter lumen extending therethrough between proximal and distal ends of the unitary element wherein, when the sealing device is in an operative position, the distal end is positioned within a urinary bladder and a sealing element extending radially outward from the unitary element so that, when the sealing device is in the operative position, the sealing element engages one of a wall of the urethra and a wall of the urinary bladder around an orifice at which the urethra opens into the urinary bladder to seal the urethra. A system for treating a urinary tract, comprising a bladder liner element which, when in an operative position, is inserted in the bladder, an outflow catheter extending from a proximal end of the bladder liner wherein, when in the operative position, the outflow catheter extends into the urethra, a first inflow catheter extending from a distal end of the bladder liner wherein, when in the operative position, the first inflow catheter extends into a first ureter and a first ureter sealing element which, when the first inflow catheter is in the operative position, prevents urine from flowing into the bladder so that urine flows from the first inflow catheter into the bladder liner and through the outflow catheter to pass out of the body.
Owner:BOSTON SCI SCIMED INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com