Urological devices incorporating collagen inhibitors
a technology of collagen inhibitors and urological devices, applied in the field of medical devices, can solve the problems of vexing urologists urethral or ureteral strictures, and high failure rate of techniques, and achieve the effect of preventing or reducing the formation of capsules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Coated Ureteral and Urethral Catheter Material
[0118]A collagen inhibitor coated catheter could be inserted following stricture incision, preventing recurrence by delivering a small amount of collagen inhibitor to the specific area of interest.
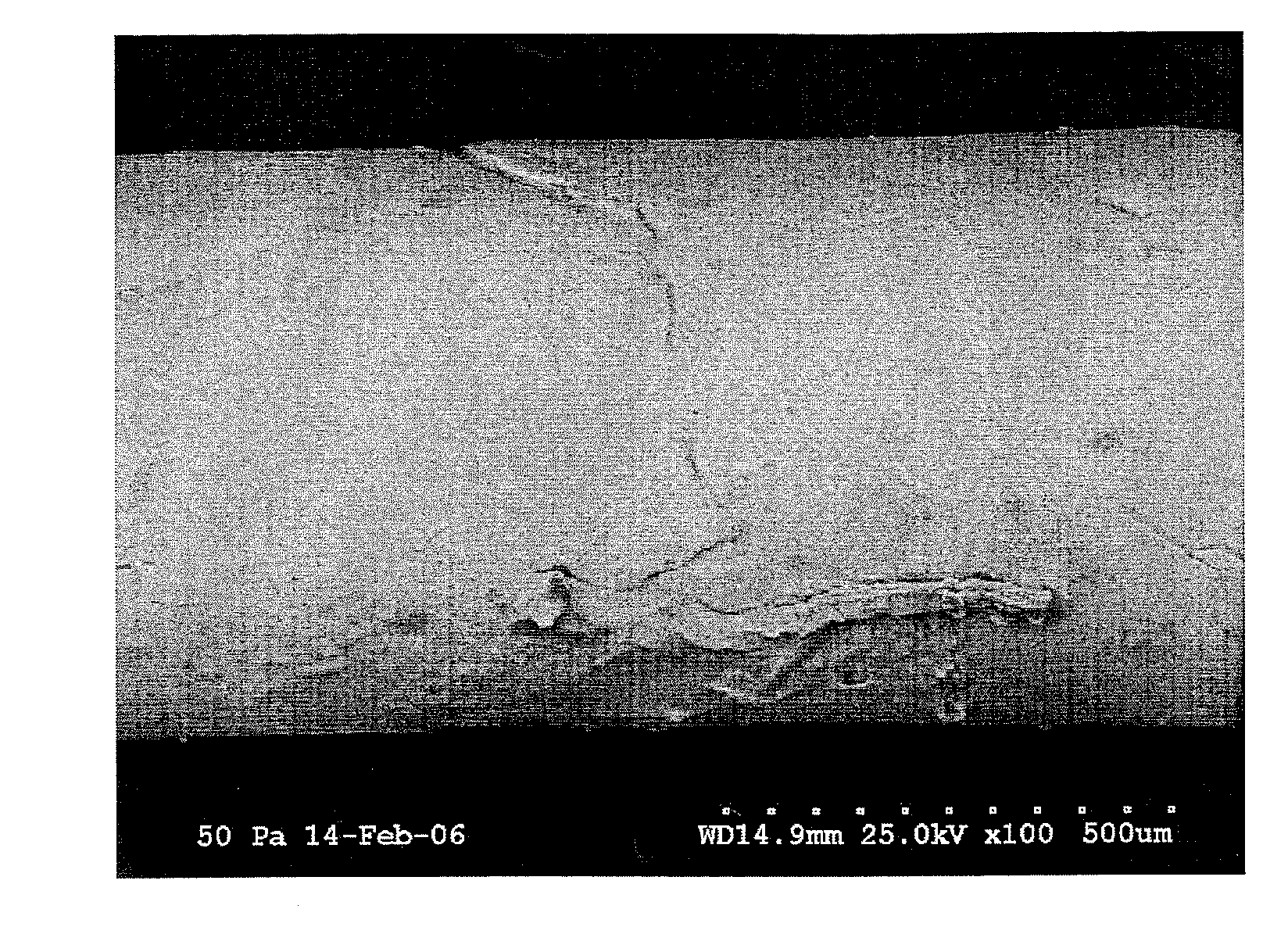
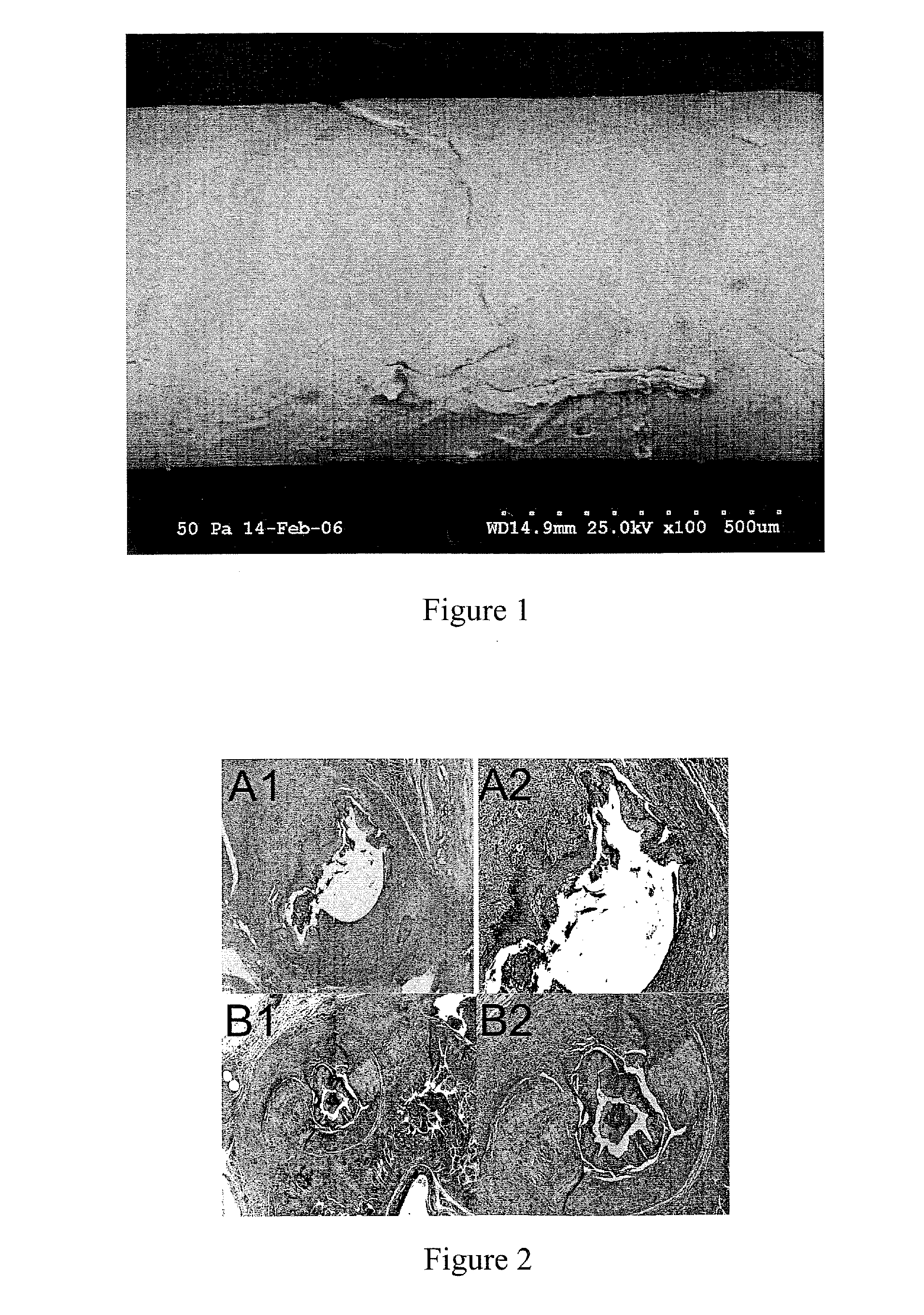
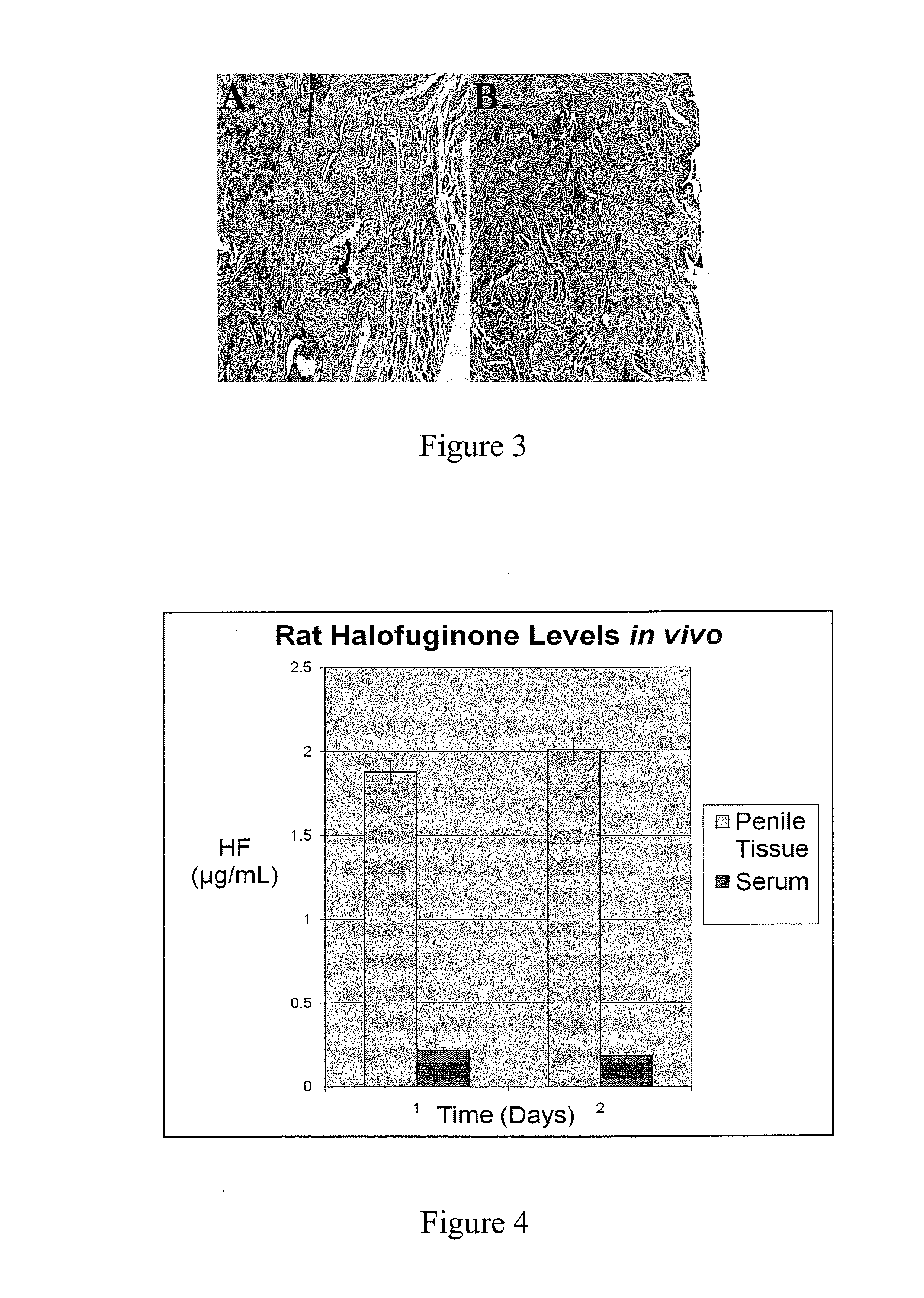
[0119]Halofuginone bromide (HF) is a substance known to be a potent Collagen Type I inhibitor, and previous studies have demonstrated that oral and local halofuginone administration can prevent luminal strictures, including urethral strictures. However, no previous studies have demonstrated the ability of HF coated stents to prevent urethral stricture formation. The objectives of this study were to successfully coat urethral stents with HF, and then to test whether HF coated stents could prevent spongiofibrosis in a small animal model of urethral stricture disease.
[0120]Halocur® (Oral Halofuginone. 0.5 mg / mL) was obtained from Intervet International BV of Norway. The rat stents were made of silicone tubing (0.30 mm×0.64 mm) from SMI, while the ...
example 2
Human Testing
[0129]Ten male patients with comparable urethral strictures amenable to treatment by DVIU therapy (<2 cm length) are recruited and divided into 2 treatment groups. Group A (5 men) are treated with DVIU and then stented for 4 days with a silicone urethral Foley catheter. Group B (5 men) are treated with DVIU and then stented for 4 days with a type-I collagen inhibitor coated silicone catheter.
[0130]The type-I collagen inhibitor coated silicone urethral catheter consists of a Bard All-Silicone 16 french Foley catheter (already in widespread use in humans), coated with the specific type-I collagen inhibitor halofuginone, approximately 0.375 mg of halofuginone in the form of the solution Halocur. The catheters used will be Bard 16 french 100% silicone Foley catheters, purchased for hospital use through the usual vendors, and therefore packaged sterilely. The catheter will then be removed from its packaging and coated with the drug Halocur (0.5 mg / ml halofuginone solution) a...
example 3
Catheter Coating
[0133]The following is a list of ureteral and urethral catheter material that we have demonstrated the ability to coat with halofuginone using imaging studies (microscopic and gross), weight changes, and elution data over 4 days:
[0134]General device material: Silicone, Silastic, Latex, Polyurethane, Nitinol, PLGA.
[0135]Boston Scientific products: Percuflex stents, Flexima stents, Pebax material.
[0136]Cook stents: Polyurethane, Sof-flex, AQ stents, Endo-sof stents.
[0137]Bard stents: Polyurethane, Latex, Woven stents, Lubricath Foley, Inlay stent, Elastomer coated catheters, Silver coated catheters.
[0138]The stents were coated as follows: 1. Wet stent with PBS and cover with PBS soaked gauze and microwave for 40 sec; 2. Dip stent in 2% PLGA-COOH to cool; 3. Dry under hood; 4. Cover with PBS soaked gauze and microwave (or plasma) for 30 sec; 5. Coat stent with halofuginone (immerse) and freeze in liquid nitrogen and lyophilize overnight; 6. Weight should be measured bef...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| period of time | aaaaa | aaaaa |
| period of time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com