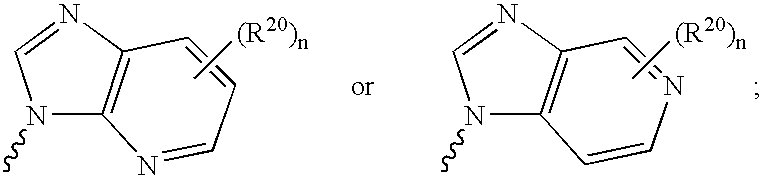Patents
Literature
11861results about "Carbohydrate active ingredients" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
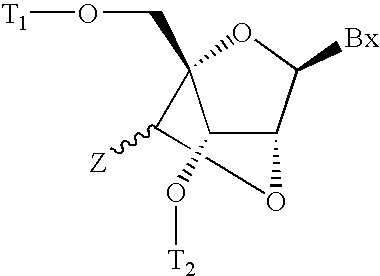
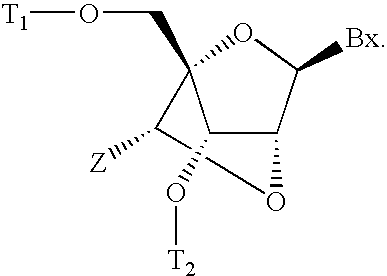
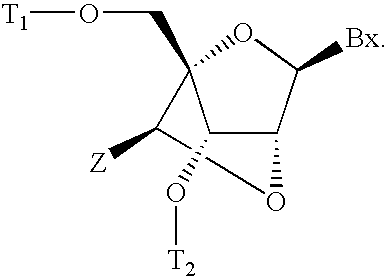
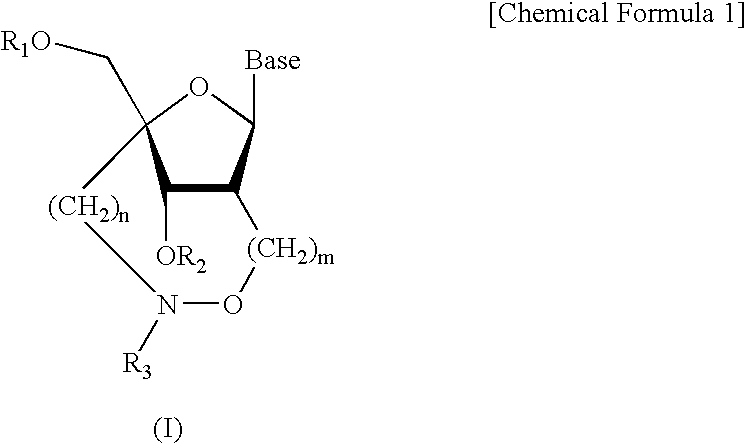
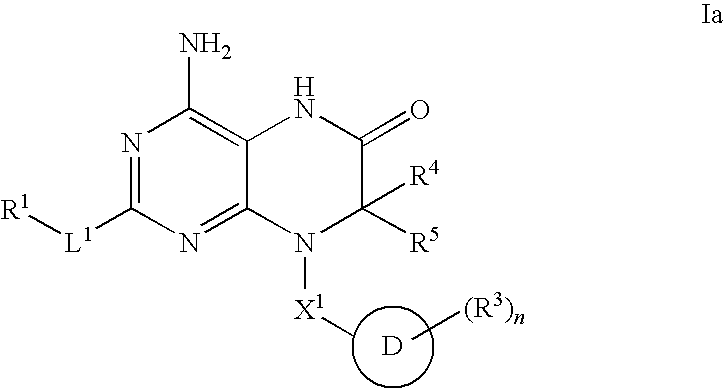
6-modified bicyclic nucleic acid analogs
The present invention provides 6-modified bicyclic nucleoside analogs and oligomeric compounds comprising these nucleoside analogs. In preferred embodiments the nucleoside analogs have either (R) or (S)-chirality at the 6-position. These bicyclicnucleoside analogs are useful for enhancing properties of oligomeric compounds including nuclease resistance.
Owner:IONIS PHARMA INC
Genetic constructs for delaying or repressing the expression of a target gene
The present invention relates generally to synthetic genes for modifying endogenous gene expression in a cell, tissue or organ of a transgenic organism, in particular a transgenic animal or plant. More particularly, the present invention provides novel synthetic genes and genetic constructs which are capable of repressing delaying or otherwise reducing the expression of an endogenous gene or a target gene in an organism when introduced thereto.
Owner:COMMONWEALTH SCI & IND RES ORG
Artificial nucleic acids of n-o bond crosslinkage type
ActiveUS7427672B2High sensitivity analysisConfirmed its usefulnessBiocideSugar derivativesSingle strandDouble strand
Owner:RIKEN GENESIS
Drug administration method
InactiveUS7427607B2Reduce weightPrevention of the adhesion of an organBiocidePowder deliveryBiopolymerDrug administration
A method of administering a drug whereby a fine drug powder can be accurately administered to a target site (in particular, a target site in the body cavity) via fluidization and spraying with a gas by using a micro tube. Concerning the administration mode, in particular, the drug alone or a biopolymer is administered or the biopolymer is employed as a carrier in the above method. More specifically speaking, a method of administering a fine drug powder which comprises finely milling one or more types fine particles of the drug and / or the biopolymer, blending them each other, fluidizing the blend with a gas, then transporting the fluidized matter in a micro tube by the gas stream and spraying the fine drug powder from the tip of the micro tube toward the target site. Further, an administration method which comprises concentrically providing a capillary tube in the micro tube, supplying an aqueous solution of the drug and / or the biopolymer from the capillary tube into the gas stream and then mixing it with other fine particles of the drug and / or the biopolymer under transportation by the gas.
Owner:NEXT21 KK
Wound closure material
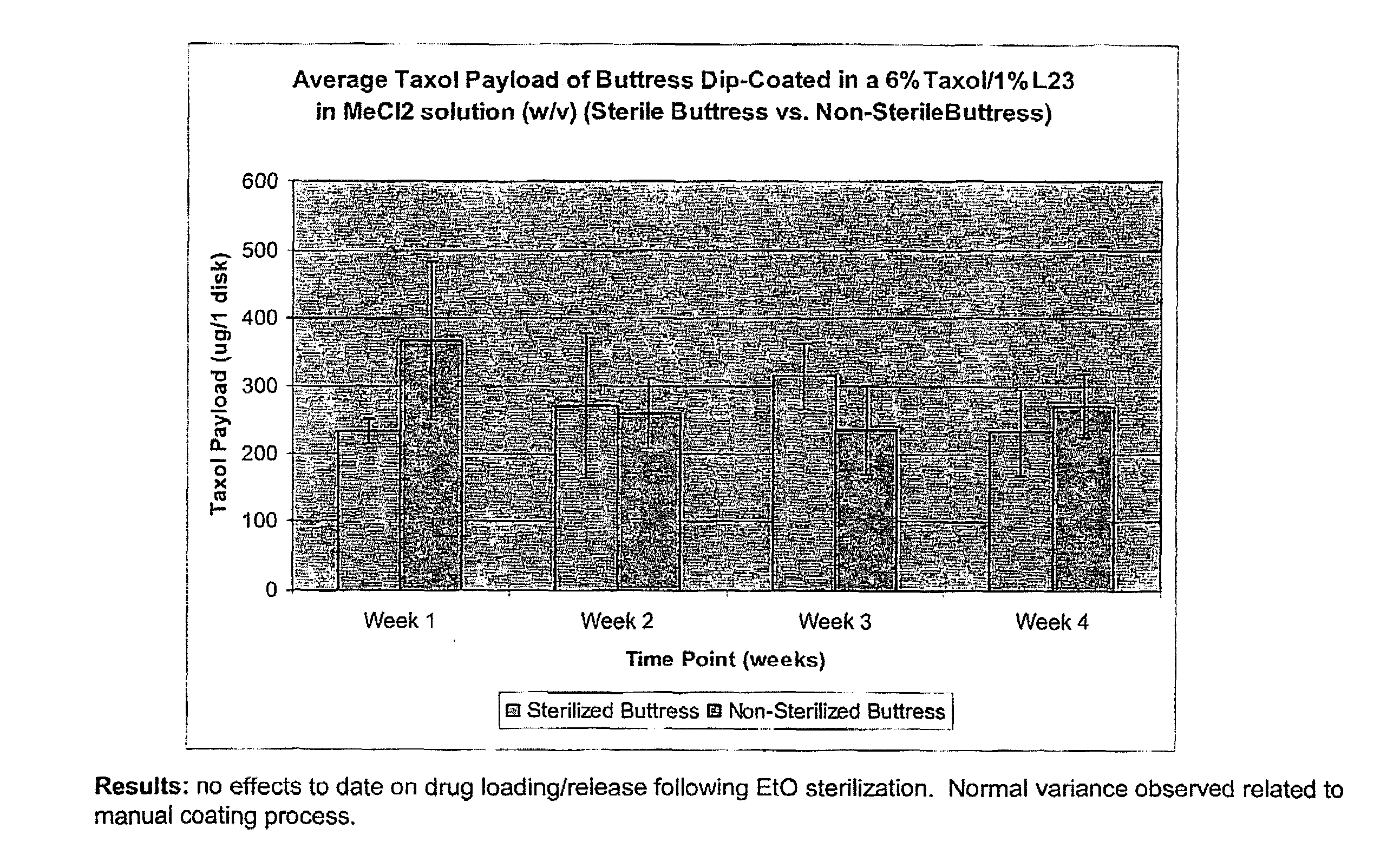
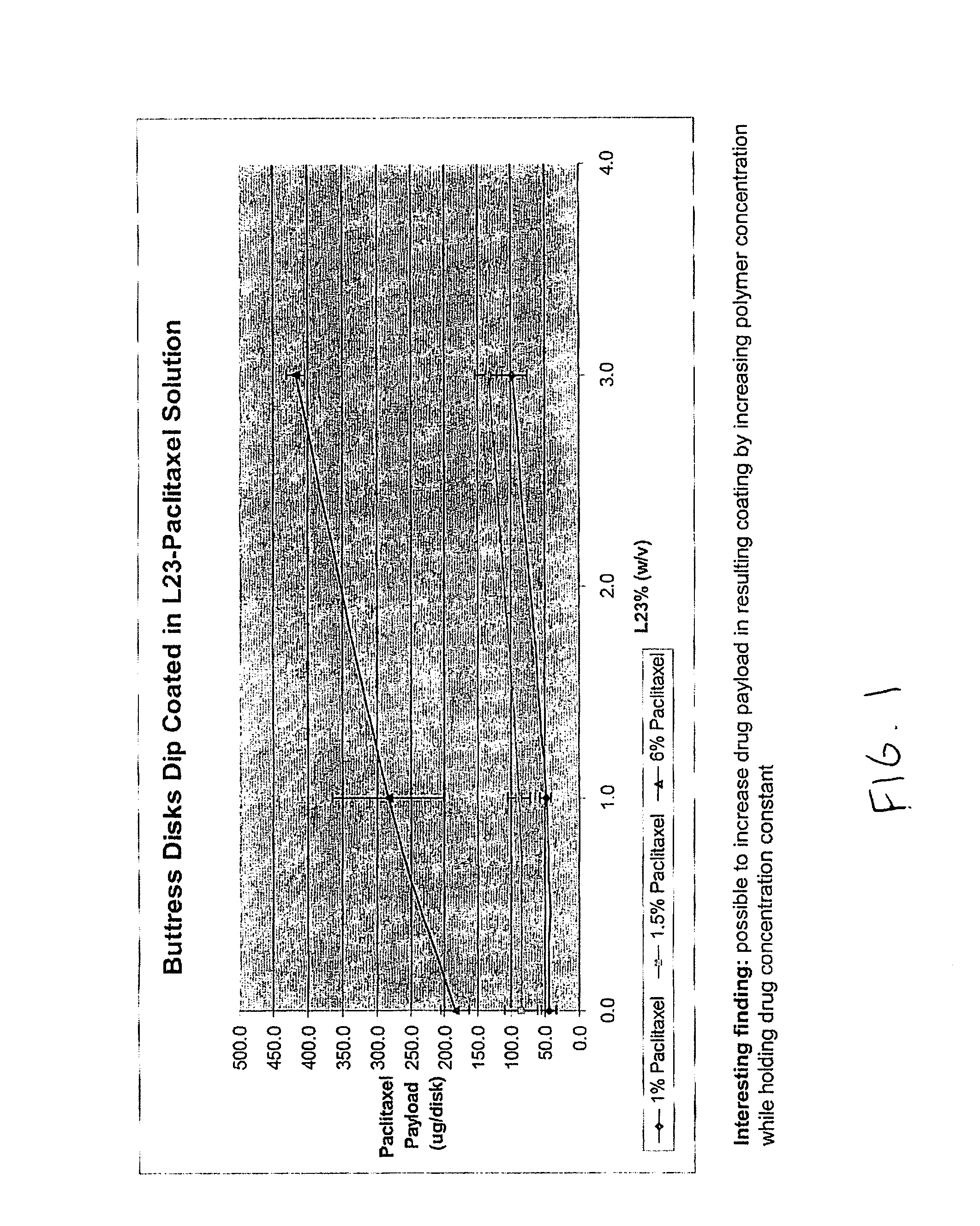
InactiveUS20100076489A1Prevent leakageSuture equipmentsHeavy metal active ingredientsButtressBiomedical engineering
Articles are provided having no orientation or a multi-directional orientation. Such articles may be in the form of films, ribbons, sheets, and / or tapes and may be utilized as buttresses with a surgical stapling apparatus or as reinforcing means for suture lines. The articles may be produced with a polymeric material having an agent, such as a chemotherapeutic agent or a radiotherapeutic agent, incorporated therein or applied as a coating thereon.
Owner:TYCO HEALTHCARE GRP LP
Method of treating androgen independent prostate cancer
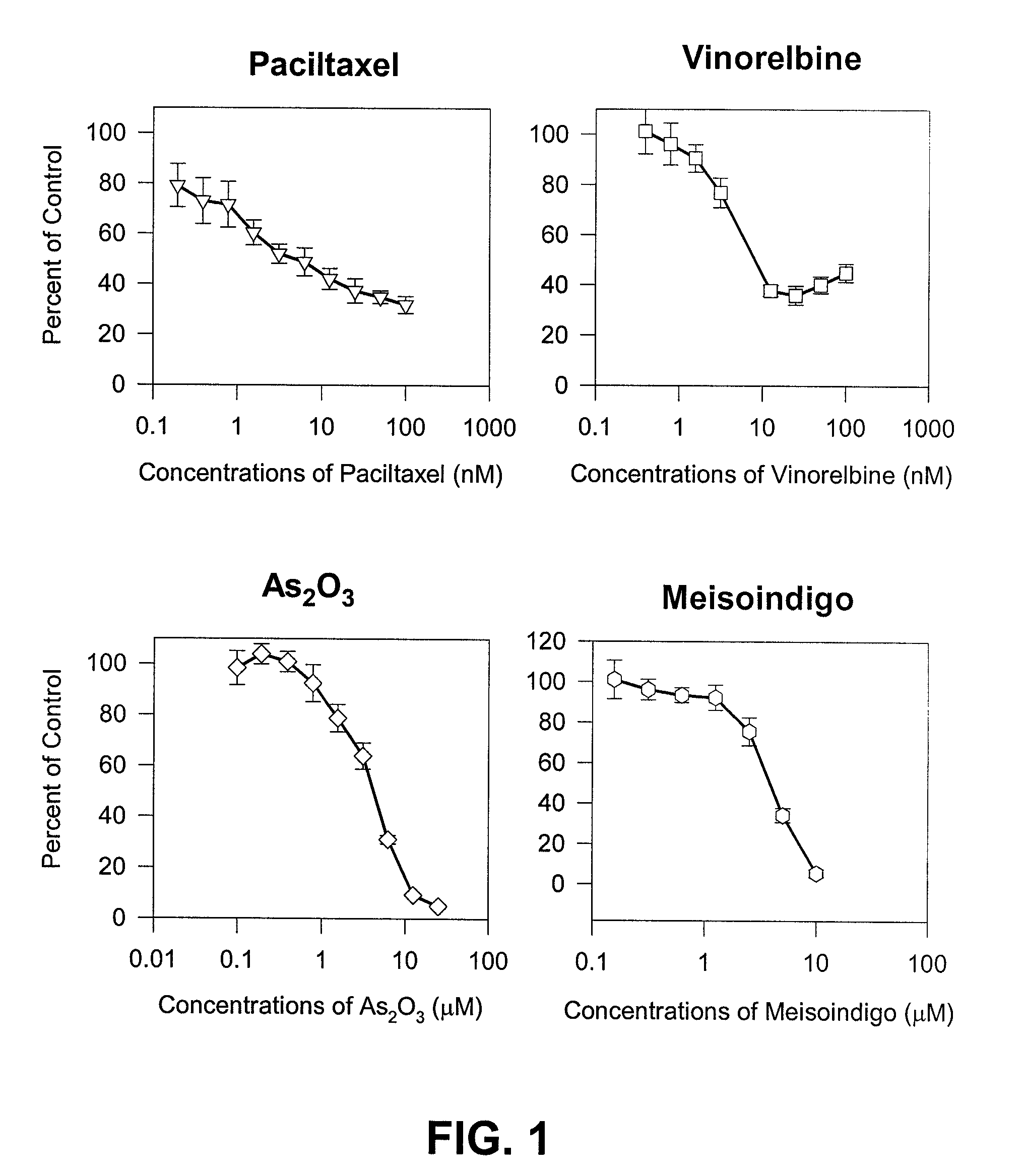
The present invention is directed to a method treating prostate cancer. The method comprises administering to a patient in need thereof at least one compound selected from N-methyl-Δ3,3′-dihydroindole-2,2′ diketone; N-1-(β-D-O-triacetyl-xylopranosyl)-Δ3,3′-dihydroindole-2,2′ diketone; and N-1-(β-D-O-triacetyl-xylopranosyl)-N′-methyl-Δ3,3′-dihydroindole-2,2′ diketone. Preferably the compound is in an amount sufficient to inhibit growth, invasion, and / or metastasis of prostate cancer cells.
Owner:NATROGEN THERAPEUTICS INT
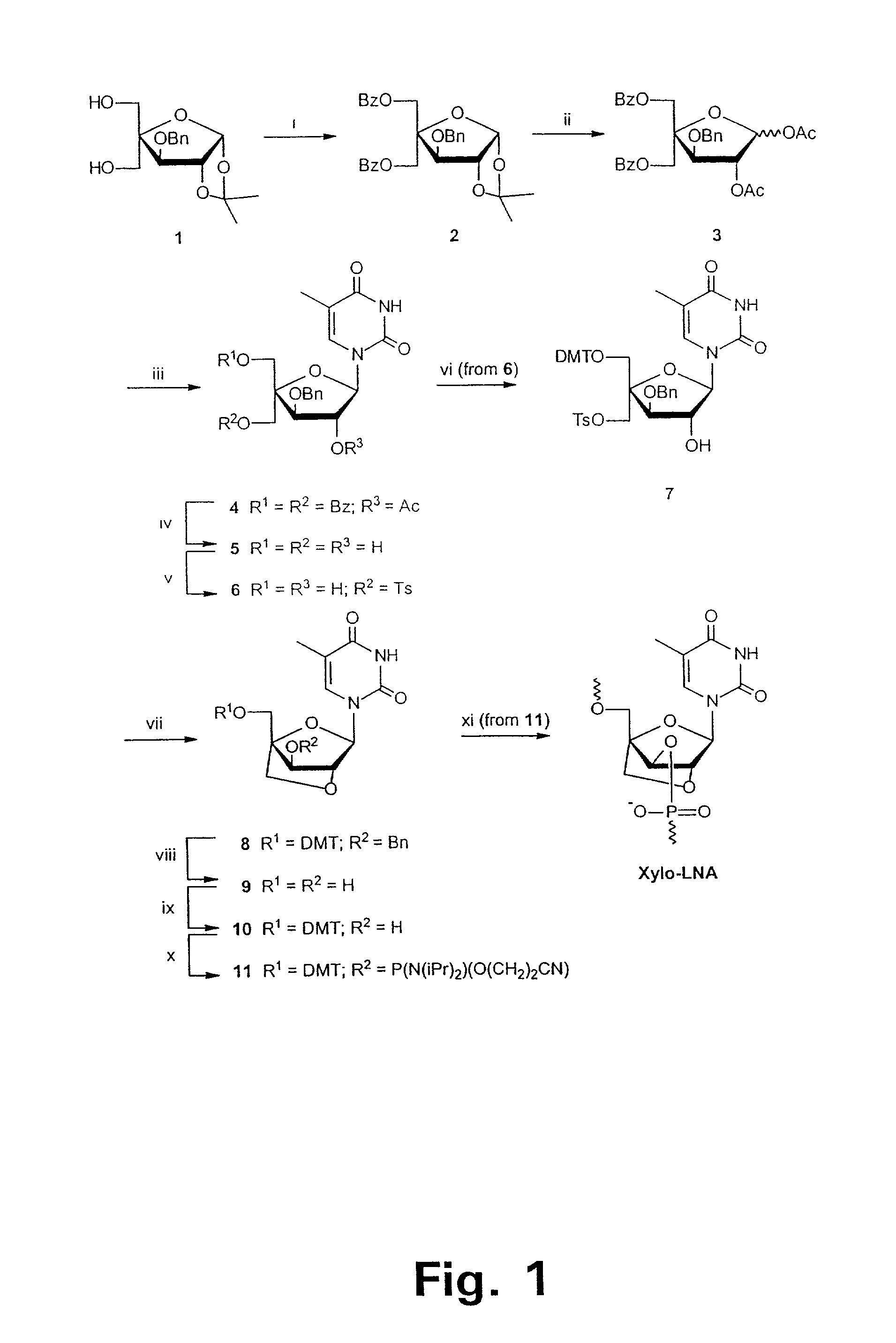
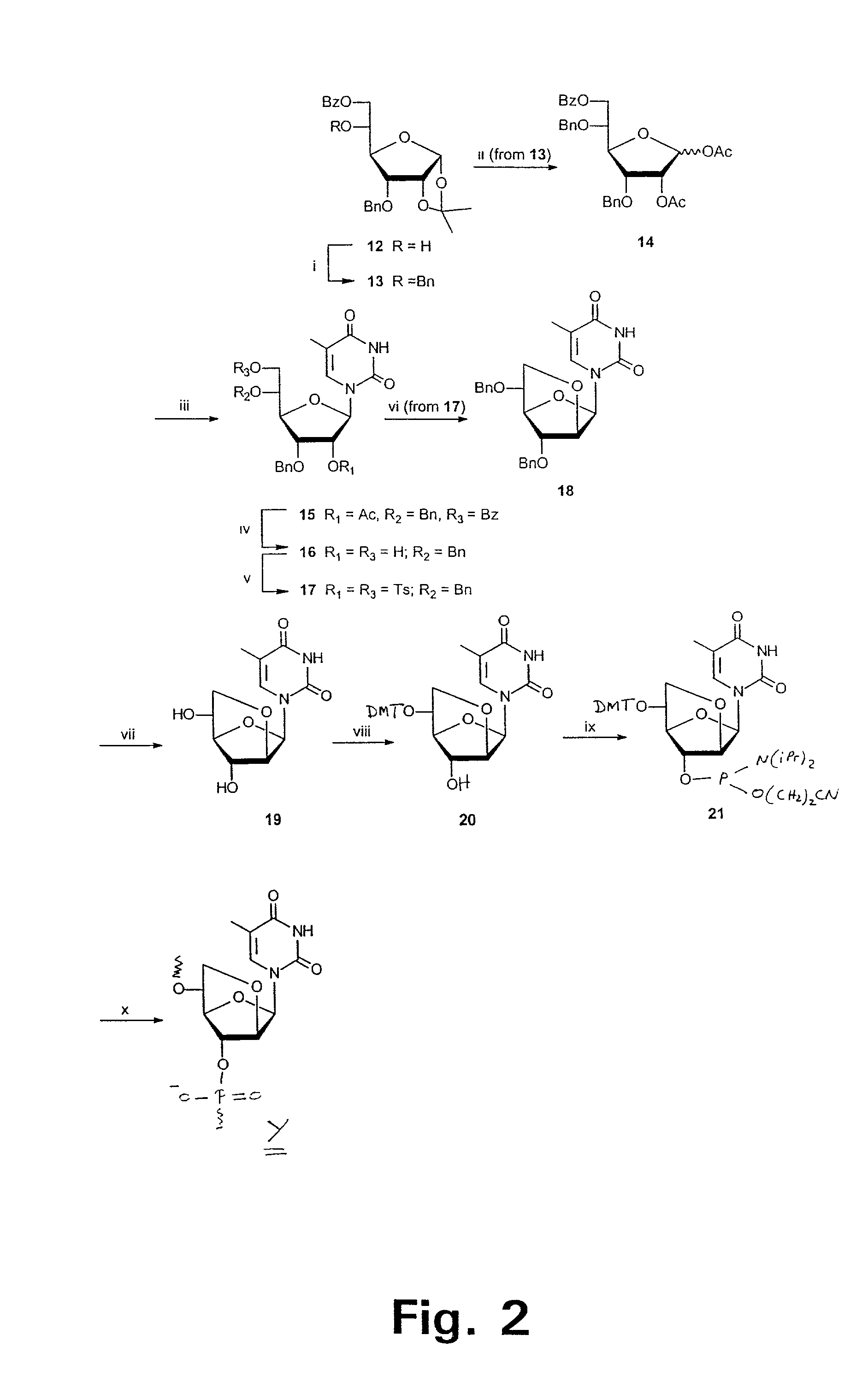
Xylo-LNA analogues
Based on the above and on the remarkable properties of the 2′-O,4′-C-methylene bridged LNA monomers it was decided to synthesise oligonucleotides comprising one or more 2′-O,4′-C-methylene-β-D-xylofuranosyl nucleotide monomer(s) as the first stereoisomer of LNA modified oligonucleotides. Modelling clearly indicated the xylo-LNA monomers to be locked in an N-type furanose conformation. Whereas the parent 2′-deoxy-β-D-xylofuranosyl nucleosides were shown to adopt mainly an N-type furanose conformation, the furanose ring of the 2′-deoxy-β-D-xylofuranosyl monomers present in xylo-DNA were shown by conformational analysis and computer modelling to prefer an S-type conformation thereby minimising steric repulsion between the nucleobase and the 3′-O-phopshate group (Seela, F.; Wömer, Rosemeyer, H. Helv. Chem. Acta 1994, 77, 883). As no report on the hybridisation properties and binding mode of xylo-configurated oligonucleotides in an RNA context was believed to exist, it was the aim to synthesise 2′-O,4′-C-methylene-β-D-xylofuranosyl nucleotide monomer and to study the thermal stability of oligonucleotides comprising this monomer. The results showed that fully modified or almost fully modified Xylo-LNA is useful for high-affinity targeting of complementary nucleic acids. When taking into consideration the inverted stereochemistry at C-3′ this is a surprising fact. It is likely that Xylo-LNA monomers, in a sequence context of Xylo-DNA monomers, should have an affinity-increasing effect.
Owner:QIAGEN GMBH
Drug-eluting medical devices
Owner:ZILBERMAN MEITAL
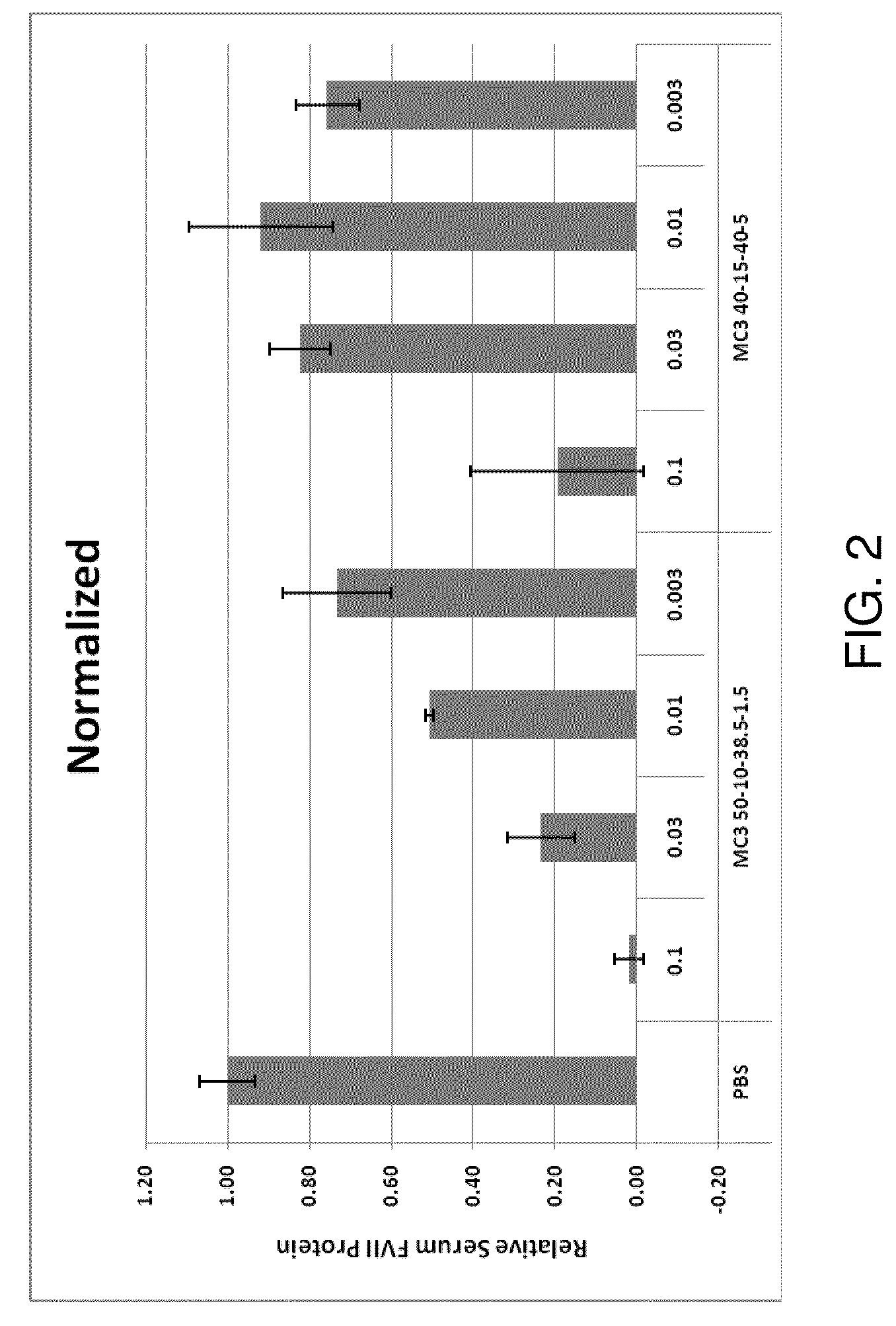
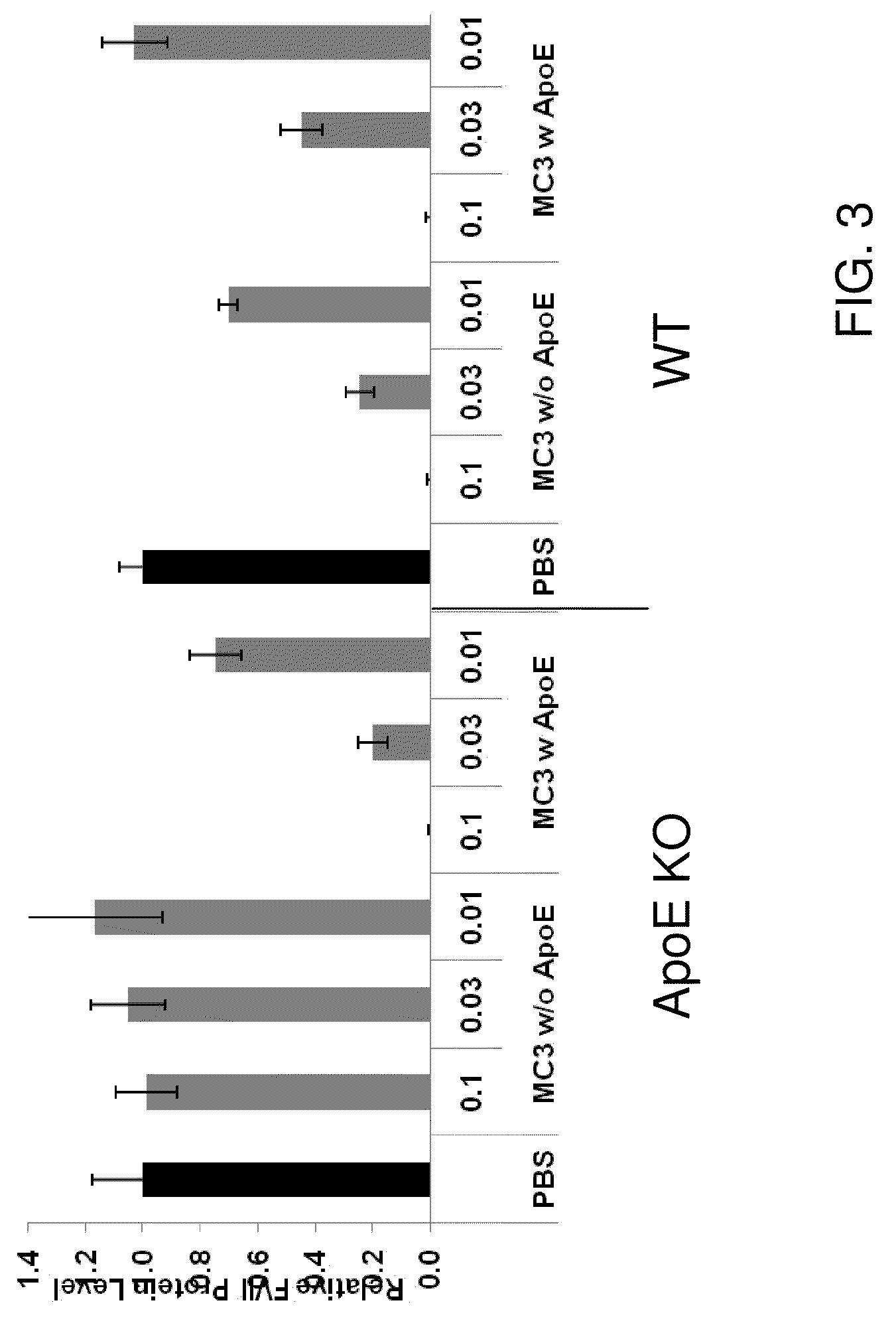
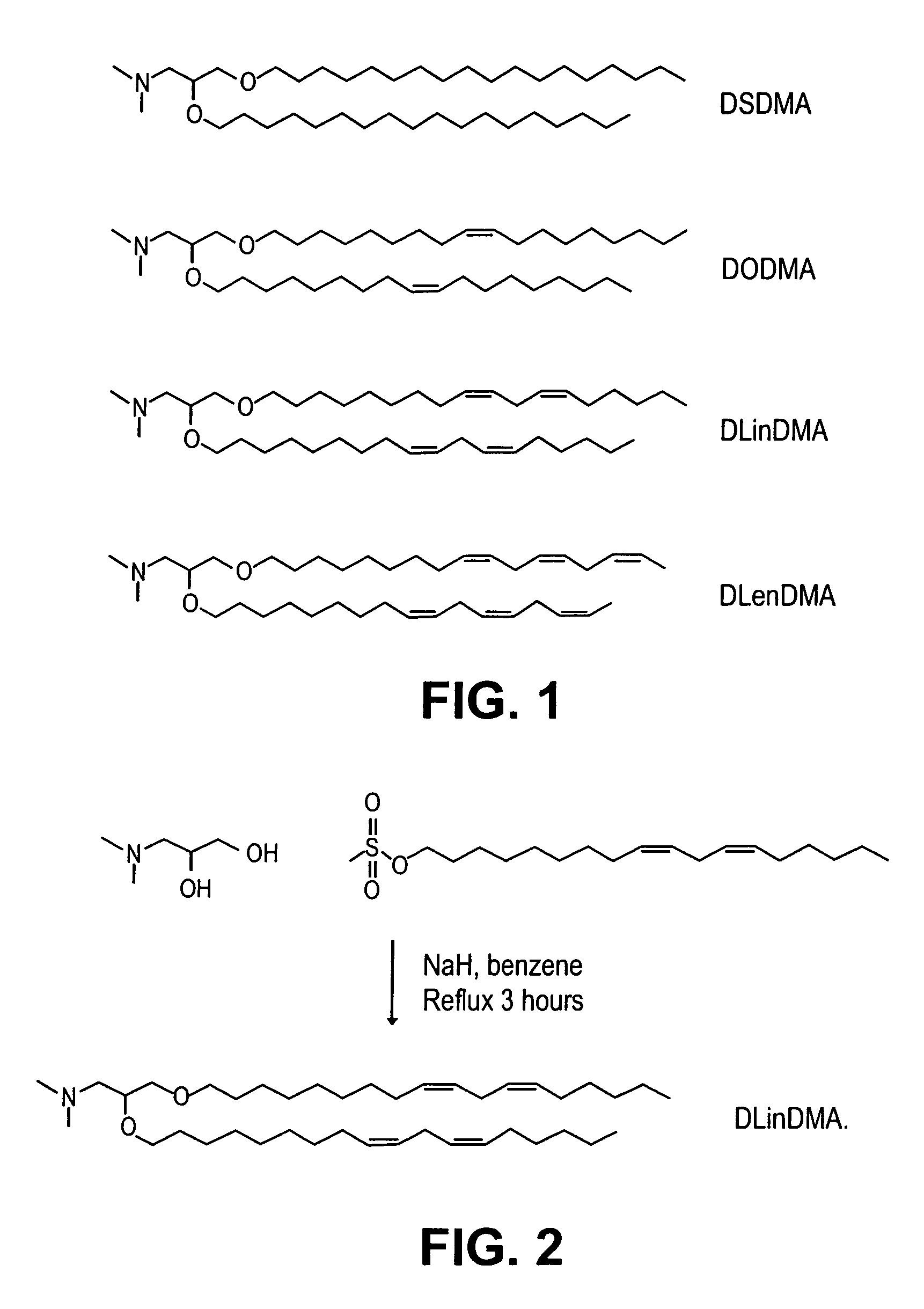
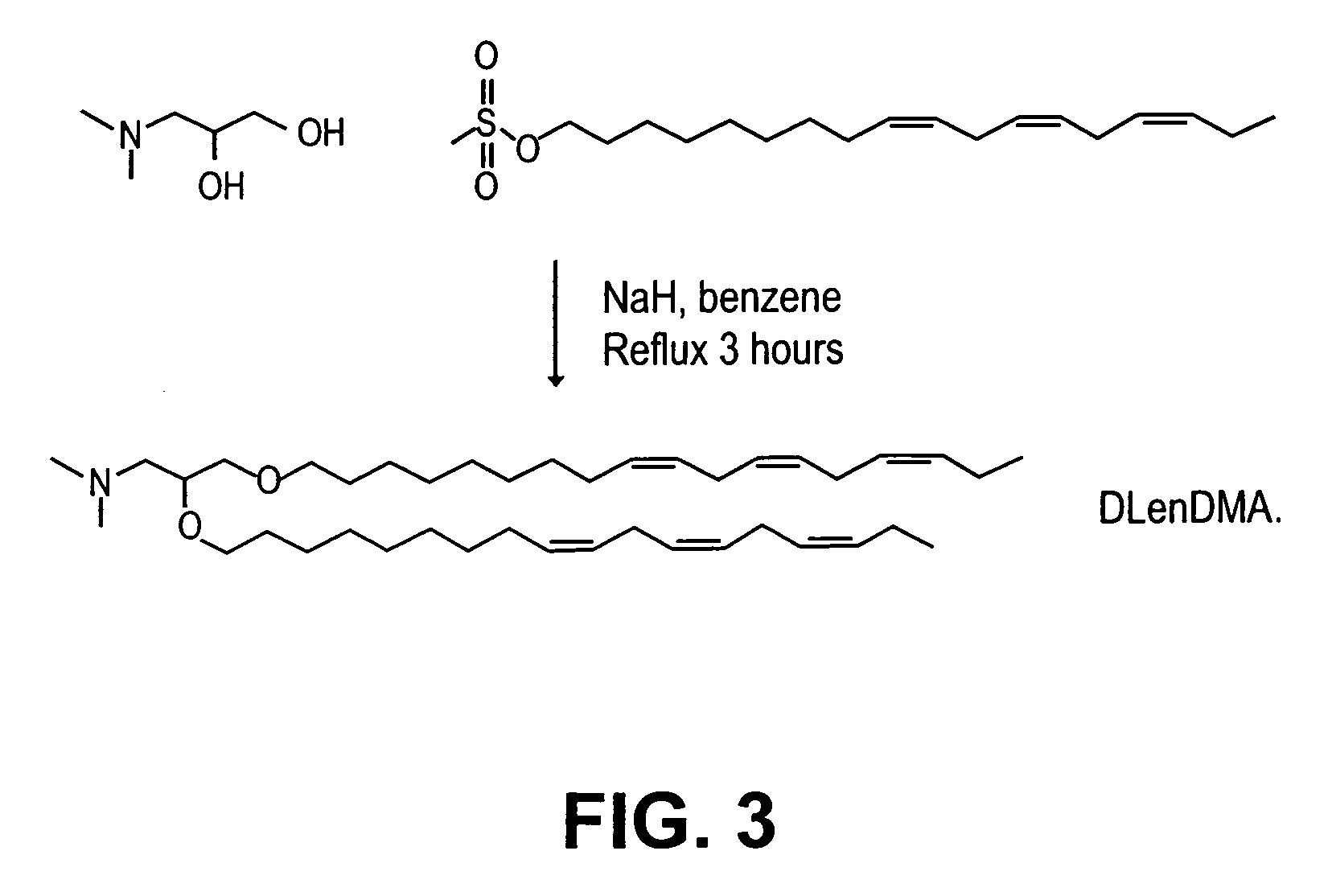
Lipid formulation
Owner:ARBUTUS BIOPHARMA CORPORAT ION
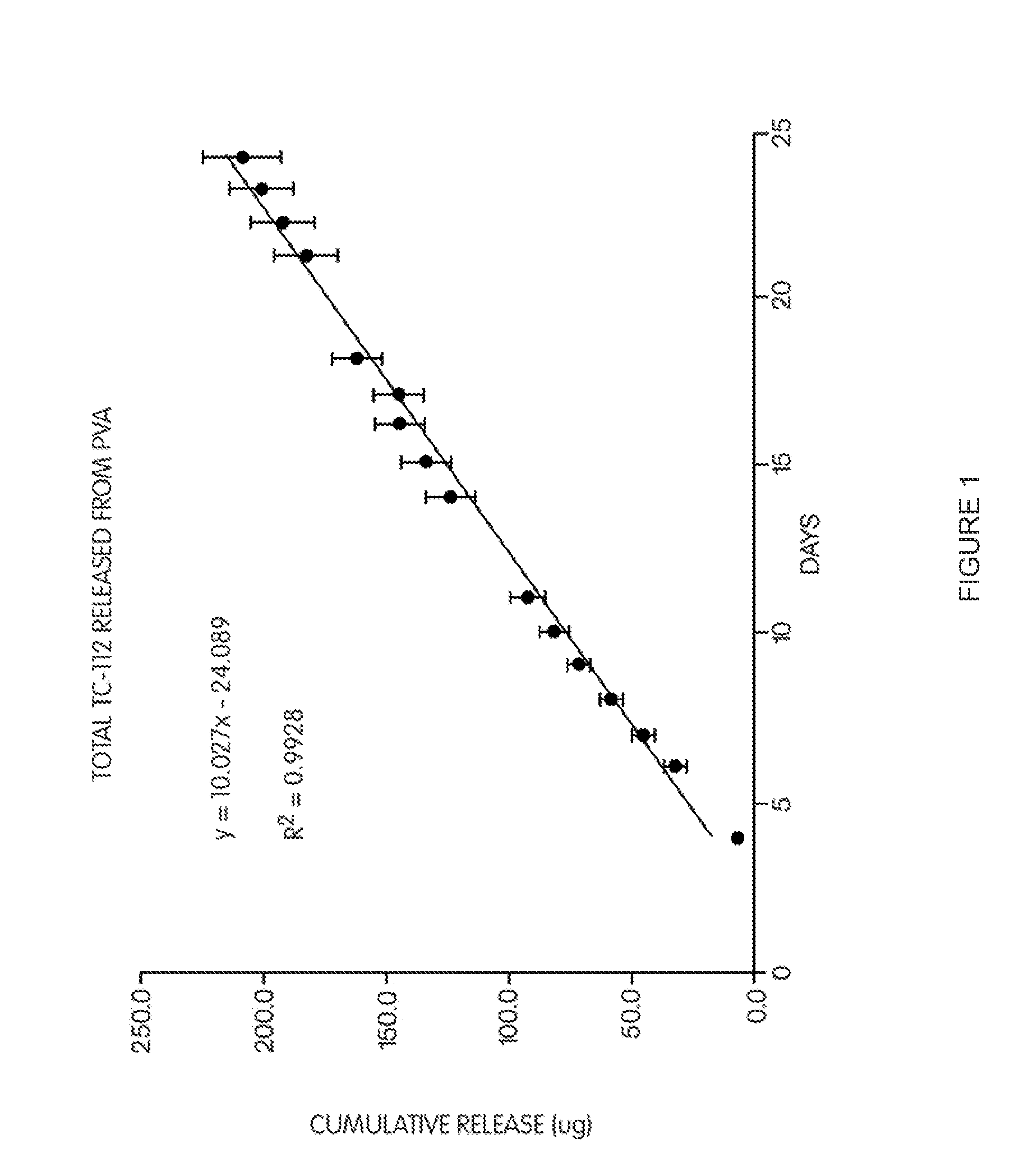
Polymer-based, sustained release drug delivery system
InactiveUS20120016467A1Reduce interactionSuture equipmentsAntibacterial agentsRate limitingSolubility
Disclosed is a sustained release system that includes a polymer and a prodrug having a solubility less than about 1 mg / ml dispersed in the polymer. Advantageously, the polymer is permeable to the prodrug and may be non-release rate limiting with respect to the rate of release of the prodrug from the polymer. This permits improved drug delivery within a body in the vicinity of a surgery via sustained release rate kinetics over a prolonged period of time, while not requiring complicated manufacturing processes.
Owner:PSIVIDA INC
Drug-eluting medical devices
Composite structures composed of a device as a core structure, being a medical device or article, and a porous polymeric coat and designed capable of encapsulating bioactive agents while retaining the activity of these agents are disclosed. Further disclosed are processes of preparing such composite structures.
Owner:RAMOT AT TEL AVIV UNIV LTD
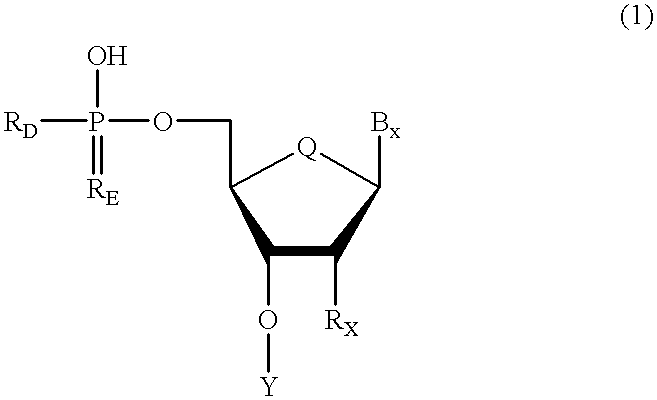
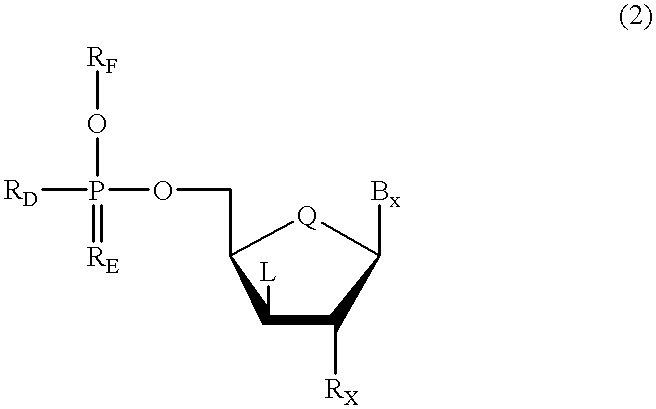
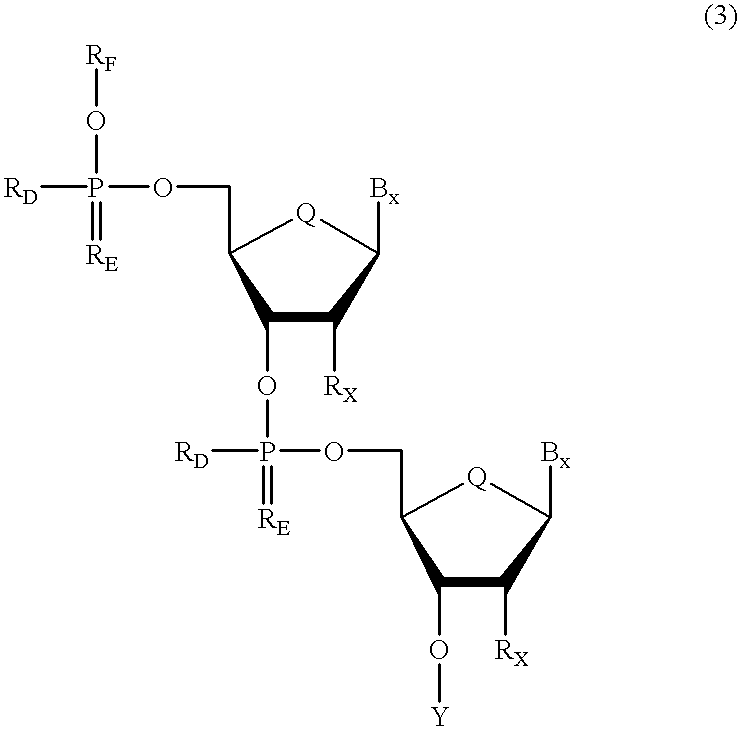
Oligonucleotides having chiral phosphorus linkages
InactiveUS6239265B1Improved pharmacokinetic propertiesImprove propertiesPeptide/protein ingredientsGenetic material ingredientsSugar moietyPhosphoramidate
Sequence-specific oligonucleotides are provided having substantially pure chiral Sp phosphorothioate, chiral Rp phosphorothioate, chiral Sp alkylphosphonate, chiral Rp alkylphosphonate, chiral Sp phosphoamidate, chiral Rp phosphoamidate, chiral Sp phosphotriester, and chiral Rp phosphotriester linkages. The novel oligonucleotides are prepared via a stereospecific SN2 nucleophilic attack of a phosphodiester, phosphorothioate, phosphoramidate, phosphotriester or alkylphosphonate anion on the 3' position of a xylonucleotide. The reaction proceeds via inversion at the 3' position of the xylo reactant species, resulting in the incorporation of phosphodiester, phosphorothioate, phosphoramidate, phosphotriester or alkylphosphonate linked ribofuranosyl sugar moieties into the oligonucleotide.
Owner:IONIS PHARMA INC
Modified hyaluronic acid for use in musculoskeletal tissue repair
The present invention includes hyaluronic acid complexes of a monovalent alkali metal salt of hyaluronic acid and a tetra alkyl ammonium halide that are suitable for incorporation with tissue scaffolds that are suitable for use in repair and / or regeneration of muscoloskeletal tissue and that include a biodegradable, porous substrate made from a biodegradable, hydrophobic polymer, where the hyaluronic acid complex is substantially insoluble in water at room temperature, yet soluble in mixtures of organic and aqueous solvents in which the selected hydrophobic polymer is soluble.
Owner:ADVANCED TECH & REGENERATIVE MEDICINE
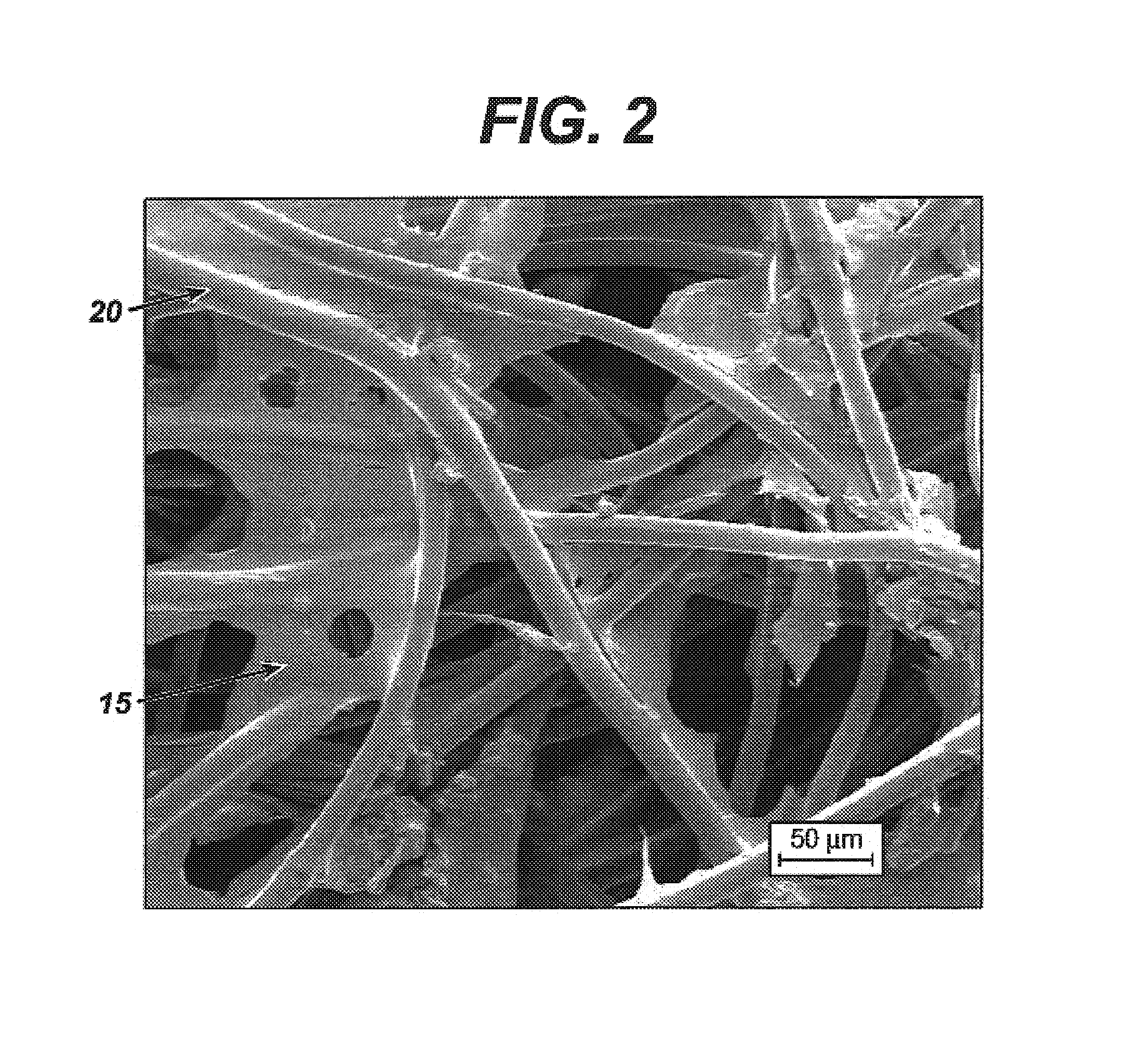
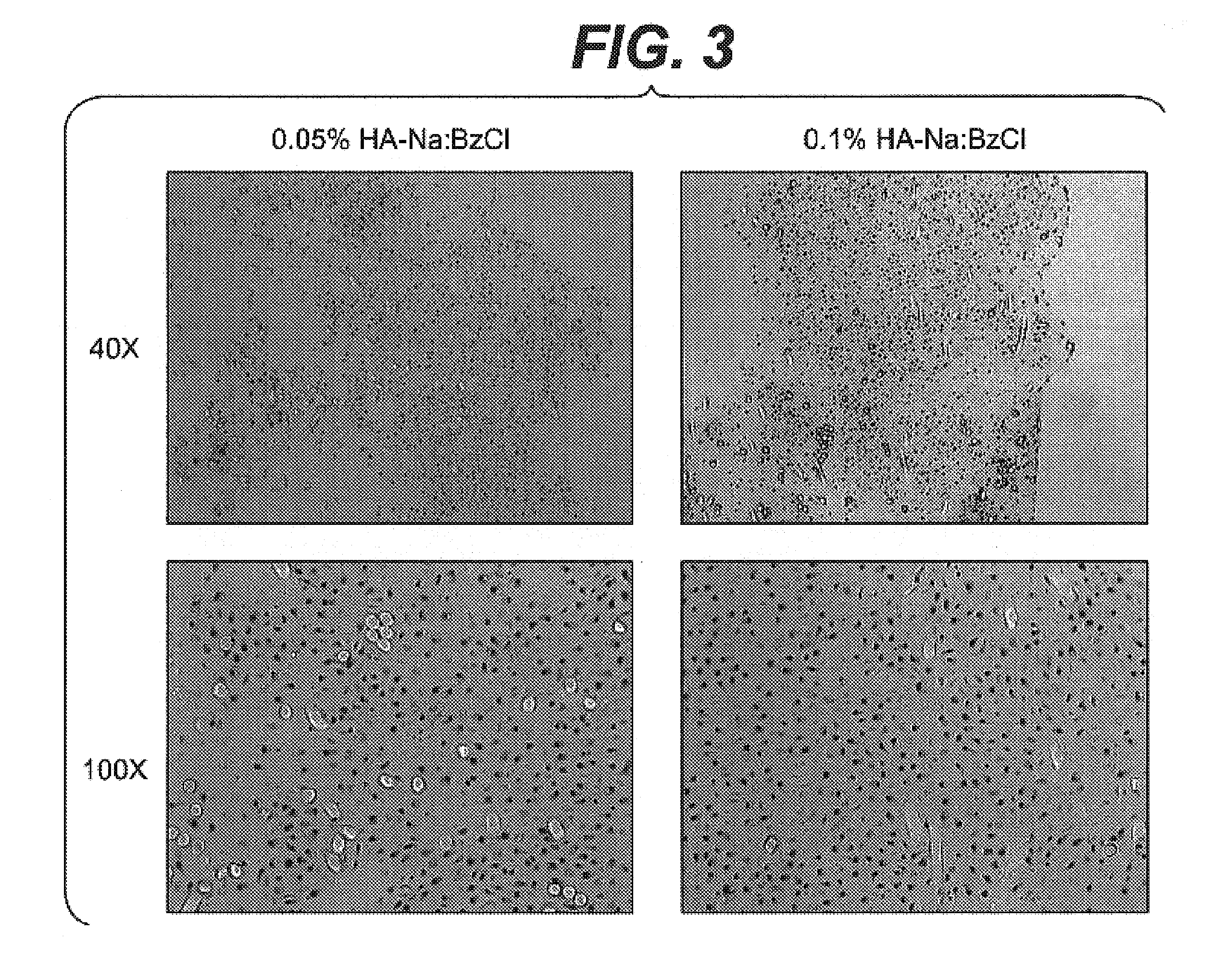
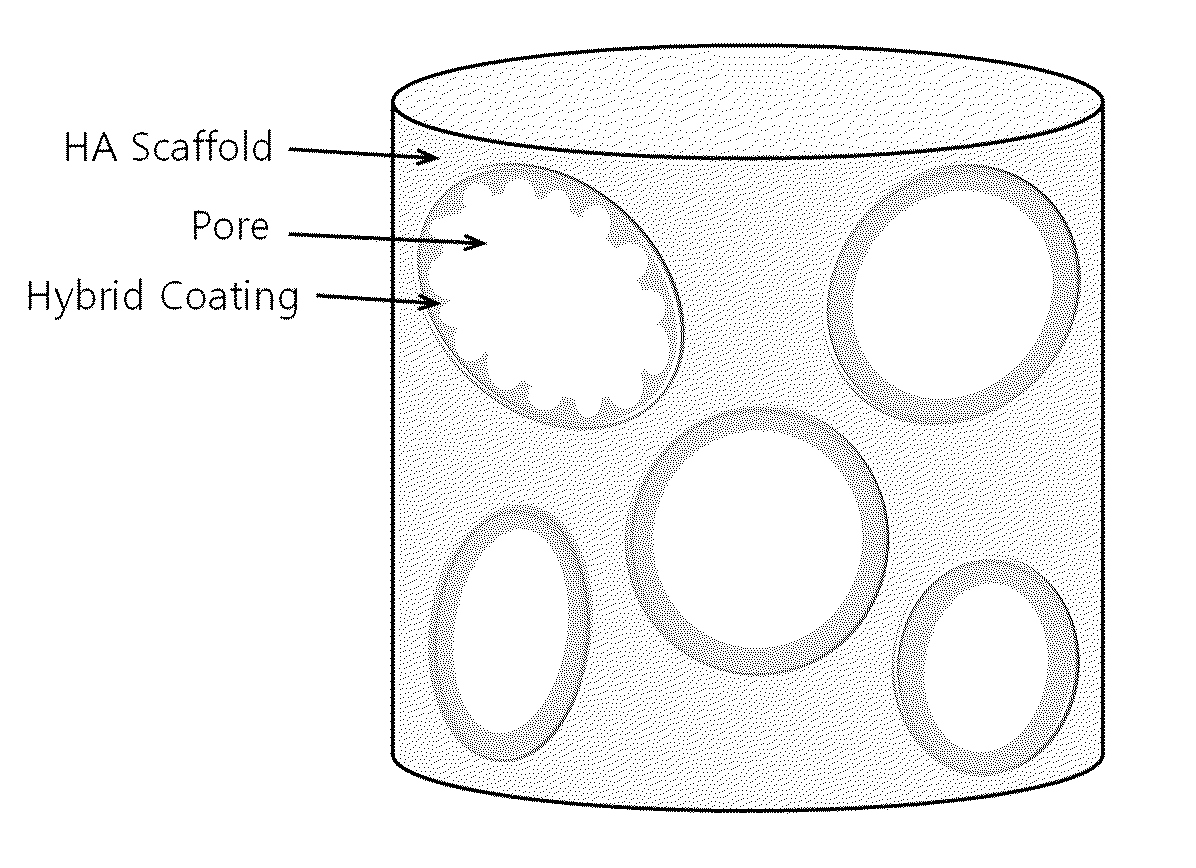
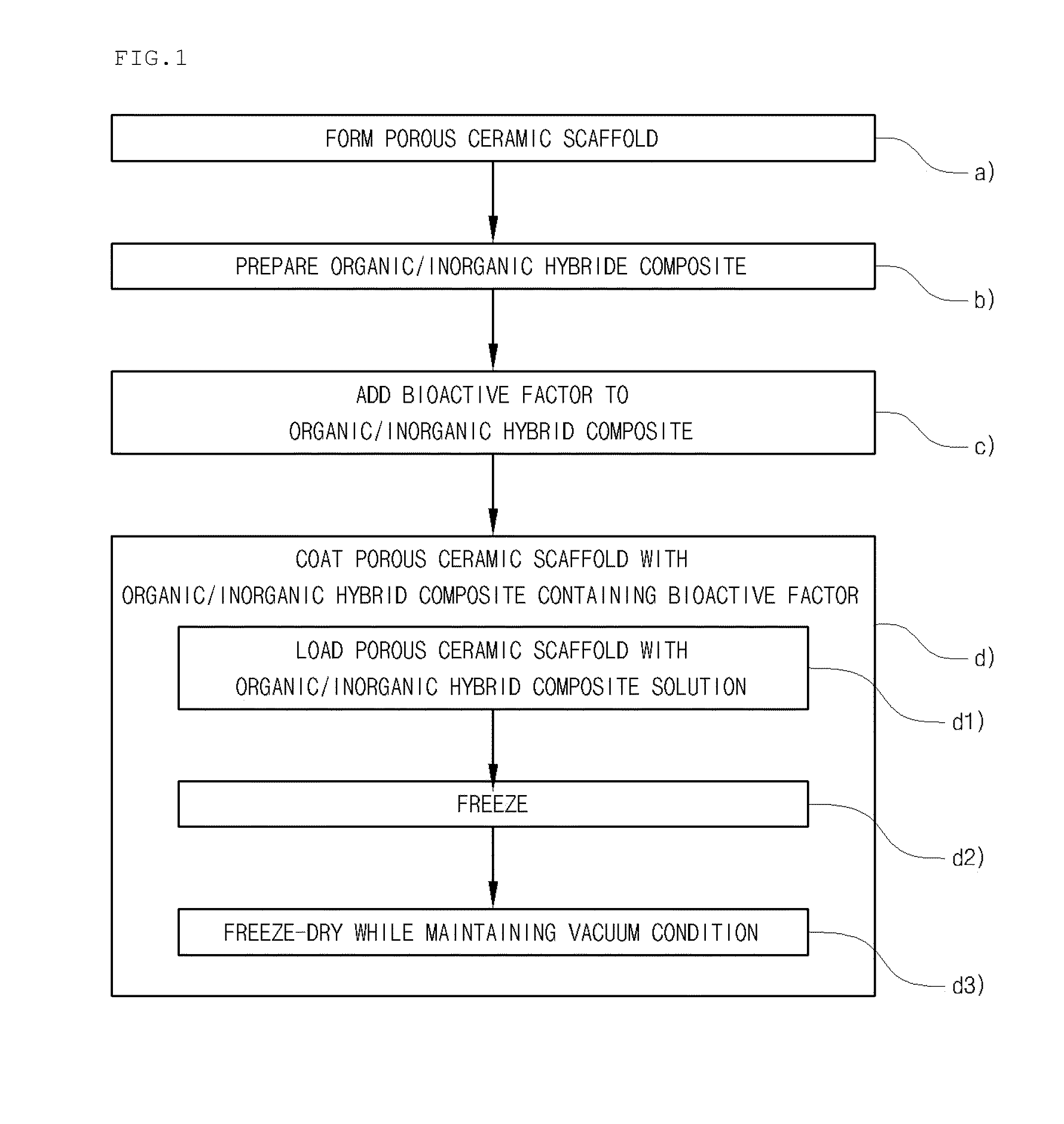
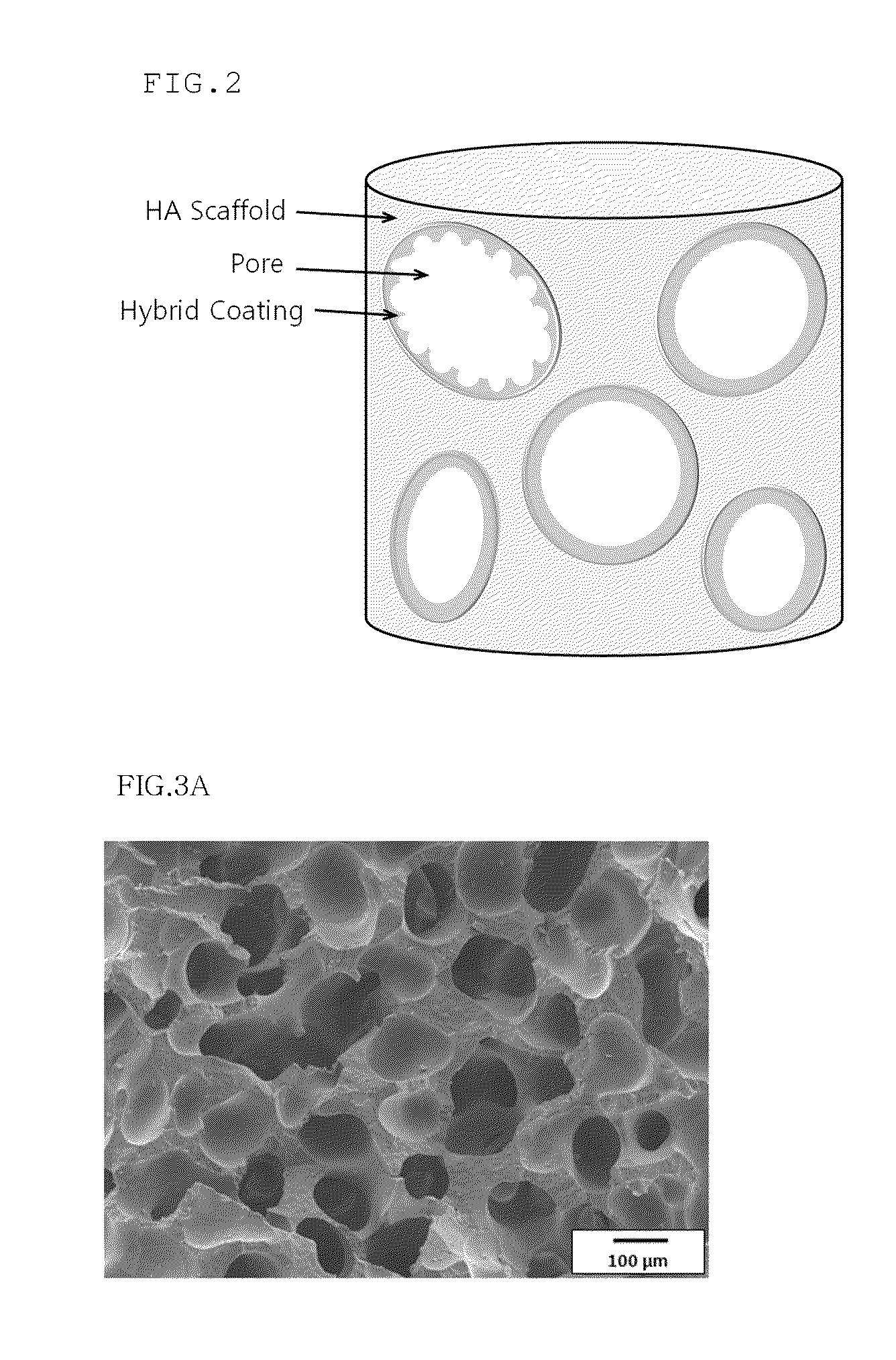
Method for manufacturing a porous ceramic scaffold having an organic/inorganic hybrid coating layer containing a bioactive factor
ActiveUS8734831B2Good biocompatibilityEasy to controlBiocideSurgical adhesivesOrganic matterPorous ceramics
A method for manufacturing a porous ceramic scaffold having an organic / inorganic hybrid coating layer containing a bioactive factor includes (a) forming a porous ceramic scaffold; (b) mixing a silica xerogel and a physiologically active organic substance in a volumetric ratio ranging from 30:70 to 90:10 and treating by a sol gel method to prepare an organic / inorganic hybrid composite solution; (c) adding a bioactive factor to the organic / inorganic hybrid composite solution and agitating until gelation occurs; and (d) coating the porous ceramic scaffold with the organic / inorganic composite containing the bioactive factor added thereto. In accordance with the method, the porous ceramic scaffold may be uniformly coated with the organic / inorganic hybrid composite while maintaining an open pore structure, and stably discharge the bioactive factor over a long period of time.
Owner:SEOUL NAT UNIV R&DB FOUND
Chimeric receptor genes and cells transformed therewith
ActiveUS7741465B1Limit acquisitionMicroorganismsGenetic material ingredientsAntibody typesLymphocyte
Chimeric receptor genes suitable for endowing lymphocytes with antibody-type specificity include a first gene segment encoding a single-chain Fv domain of a specific antibody and a second gene segment encoding all or part of the transmembrane and cytoplasmic domains, and optionally the extracellular domain, of an immune cell-triggering molecule. The chimeric receptor gene, when transfected to immune cells, expresses the antibody-recognition site and the immune cell-triggering moiety into one continuous chain. The transformed lymphocytes are useful in therapeutic treatment methods.
Owner:HEALTH & HUMAN SERVICES GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF +1
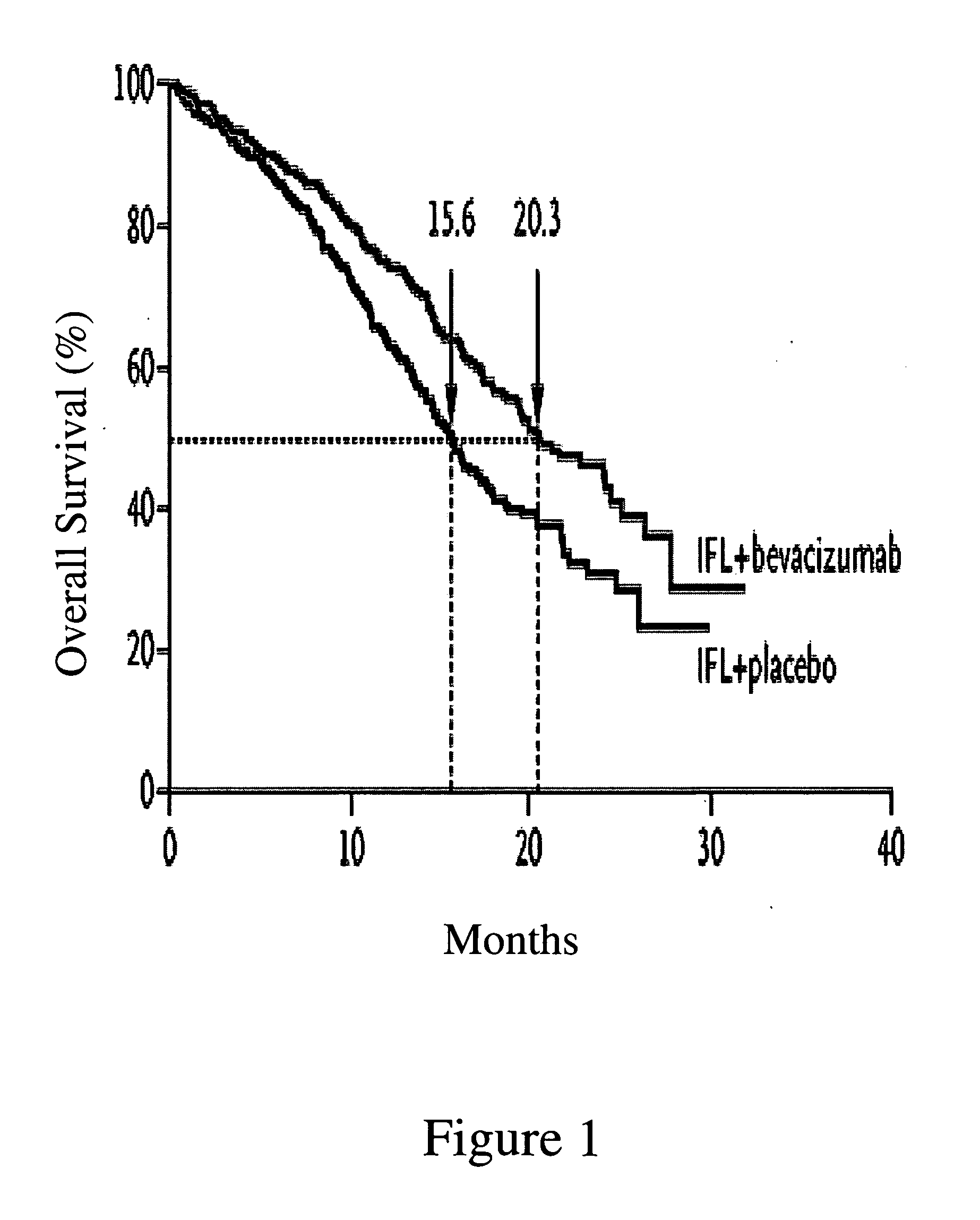
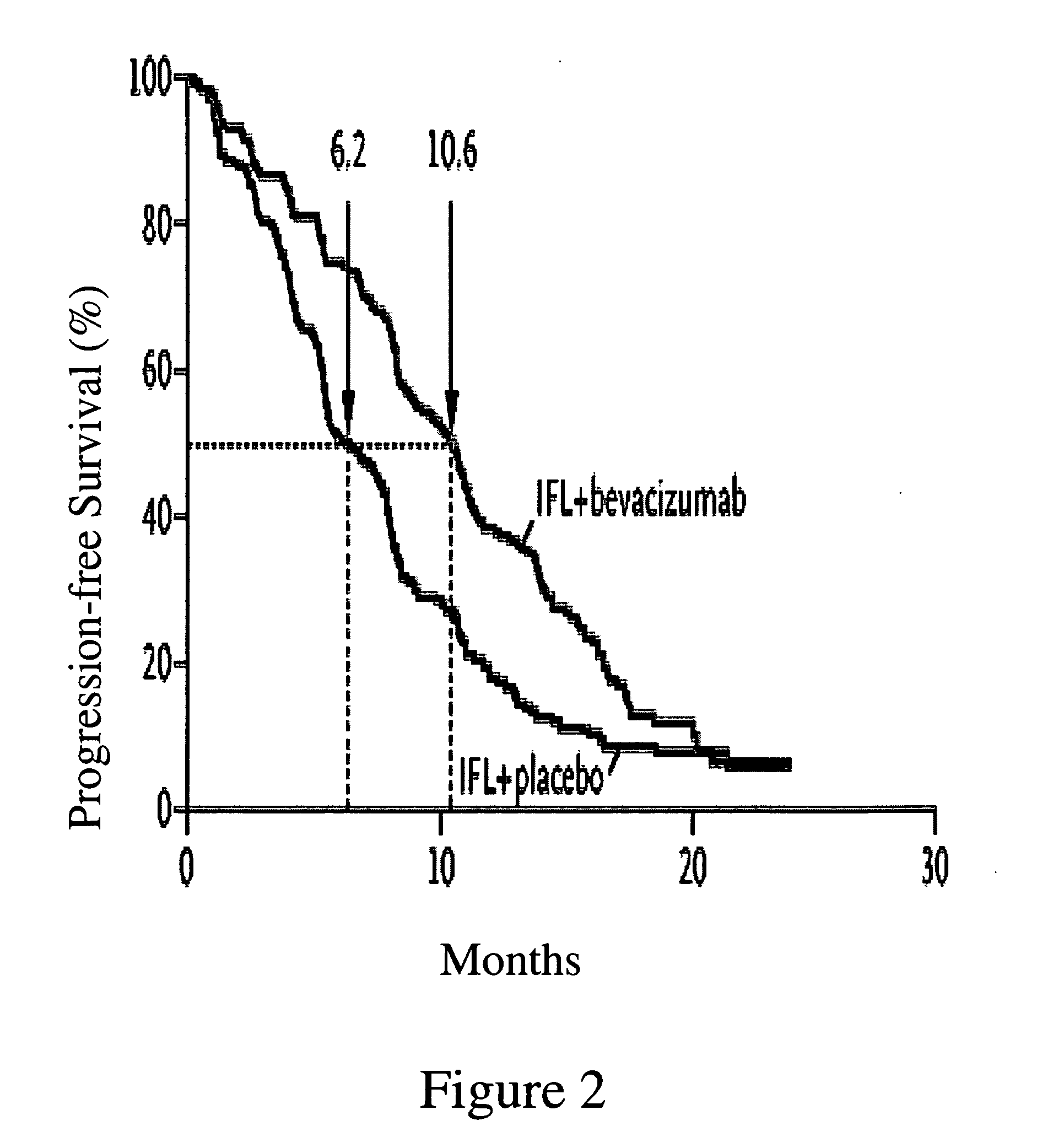
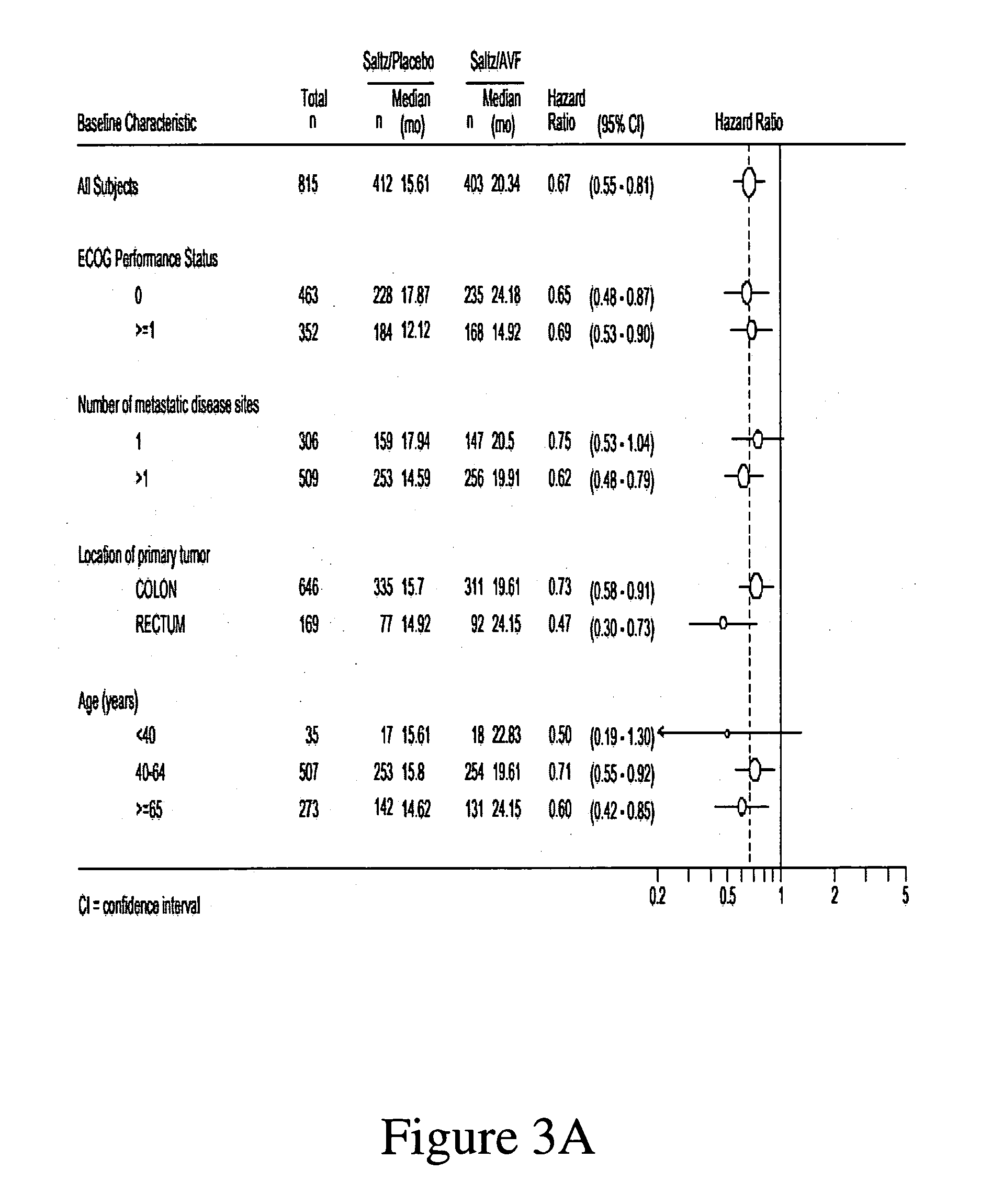
Treatment with anti-VEGF antibodies
InactiveUS20050186208A1Effective approachExtended durationBiocidePeptide/protein ingredientsAbnormal tissue growthAnti vegf antibody
This invention concerns in general treatment of diseases and pathological conditions with anti-VEGF antibodies. More specifically, the invention concerns the treatment of human patients susceptible to or diagnosed with cancer using an anti-VEGF antibody, preferably in combination with one or more additional anti-tumor therapeutic agents.
Owner:GENENTECH INC
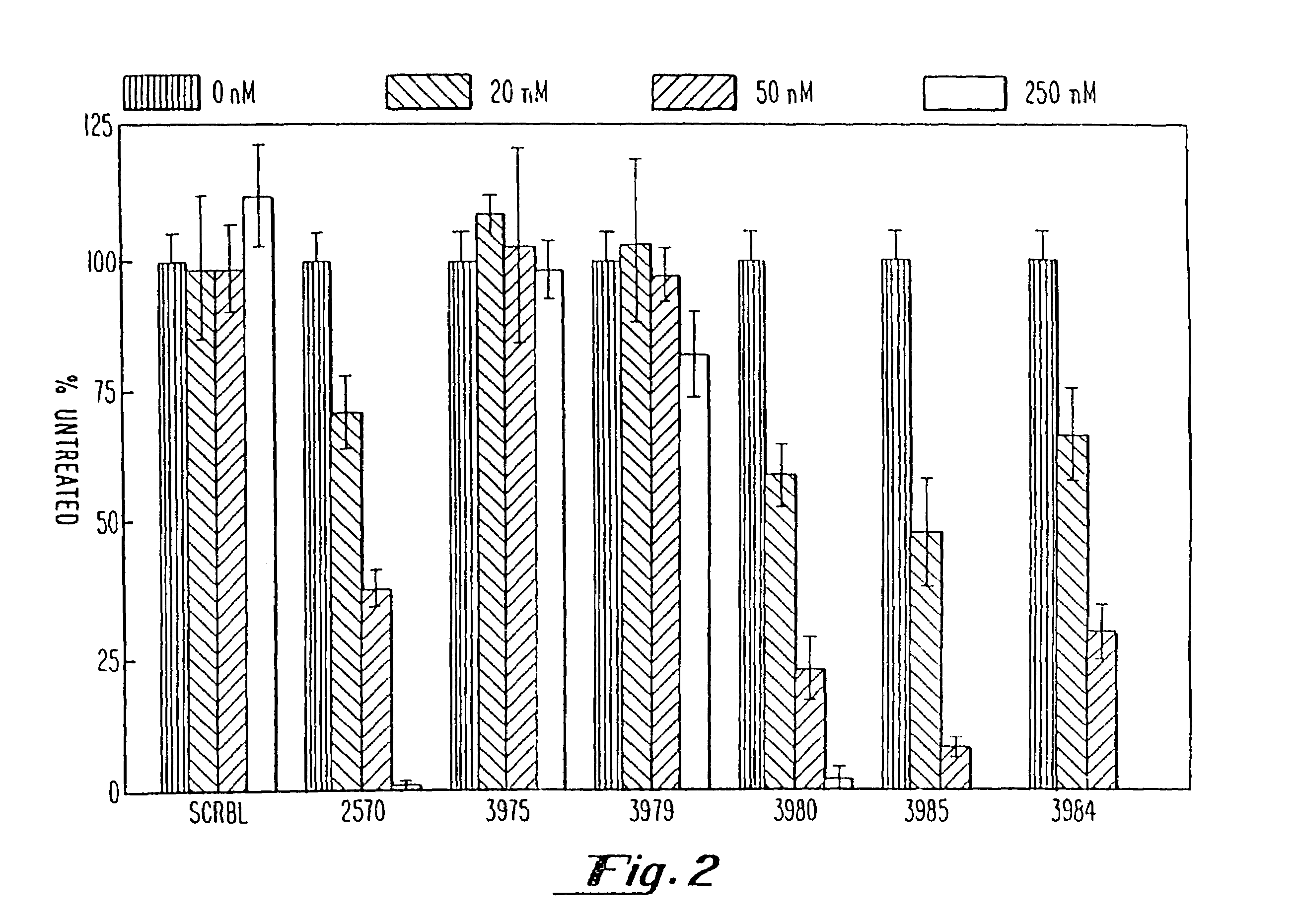
Gapped oligonucleotides
InactiveUS7015315B1Increased nuclease resistanceHigh binding affinityPeptide/protein ingredientsGenetic material ingredientsADAMTS ProteinsNuclease
Oligonucleotides and other macromolecules are provided which have increased nuclease resistance, substituent groups for increasing binding affinity to complementary strand, and subsequences of 2′-deoxy-erythro-pentofuranosyl nucleotides that activate RNase H. Such oligonucleotides and macromolecules are useful for diagnostics and other research purposes, for modulating the expression of a protein in organisms, and for the diagnosis, detection and treatment of other conditions susceptible to oligonucleotide therapeutics.
Owner:IONIS PHARMA INC
Modulators of toll-like receptors
Owner:GILEAD SCI INC
Nucleoside derivatives for treating hepatitis C virus infection
Owner:GENELABS TECH INC
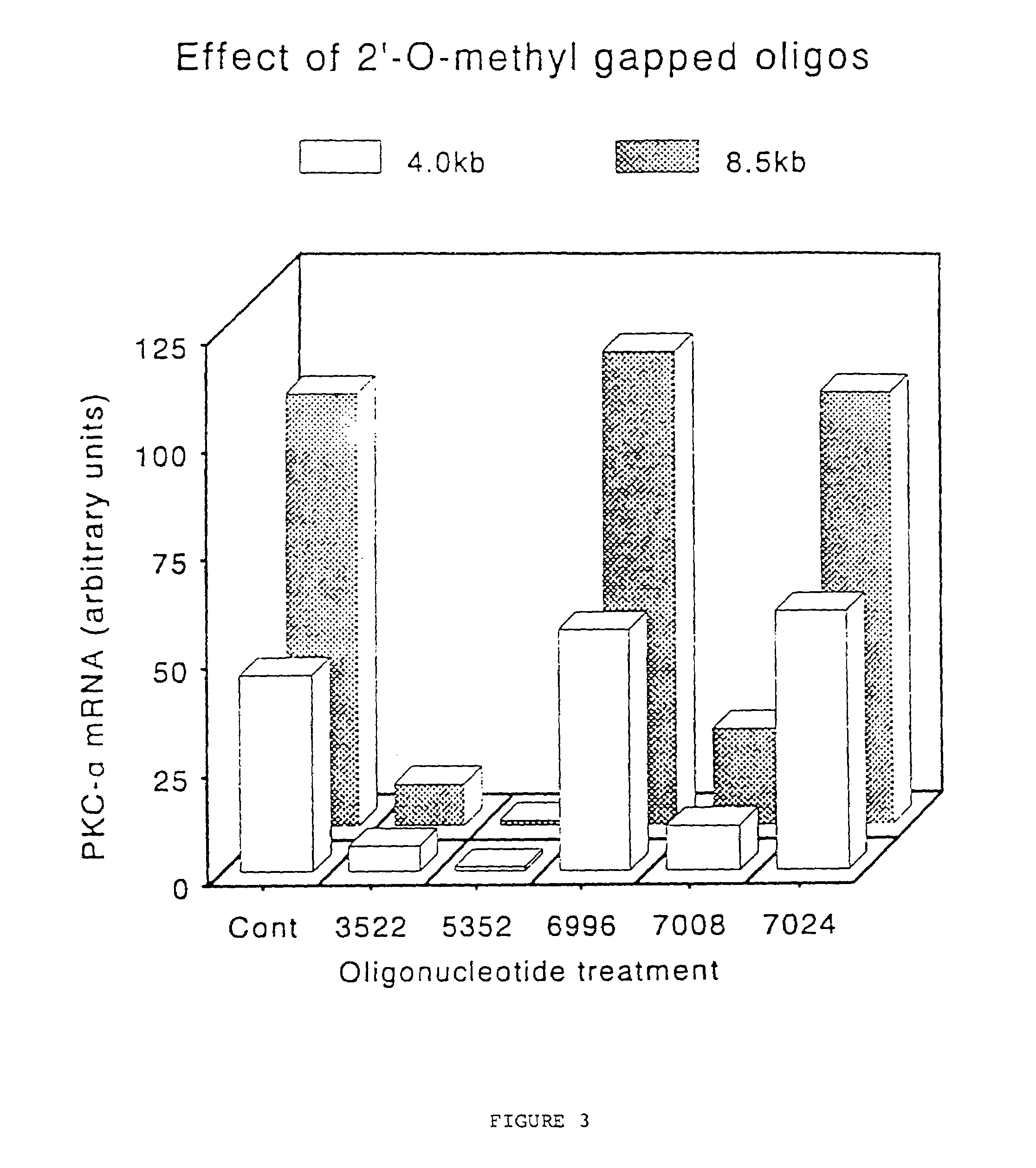
Gapped 2' modified oligonucleotides
InactiveUS6326199B1High affinityAvoid degradationHydrolasesPeptide/protein ingredientsNucleotideNuclease
Oligonucleotides and other macromolecules are provided that have increased nuclease resistance, substituent groups for increasing binding affinity to complementary strand, and sub-sequences of 2'-deoxy-erythro-pentofuranosyl nucleotides that activate RNase H enzyme. Such oligonucleotides and macromolecules are useful for diagnostics and other research purposes, for modulating protein in organisms, and for the diagnosis, detection and treatment of other conditions susceptible to antisense therapeutics.
Owner:IONIS PHARMA INC
Nucleic Acid-Lipopolymer Compositions
InactiveUS20090042829A1Increase efficiency and dosing flexibilityEfficiently be lyophilizedSpecial deliveryPeptide/protein ingredientsCholesterolFiller Excipient
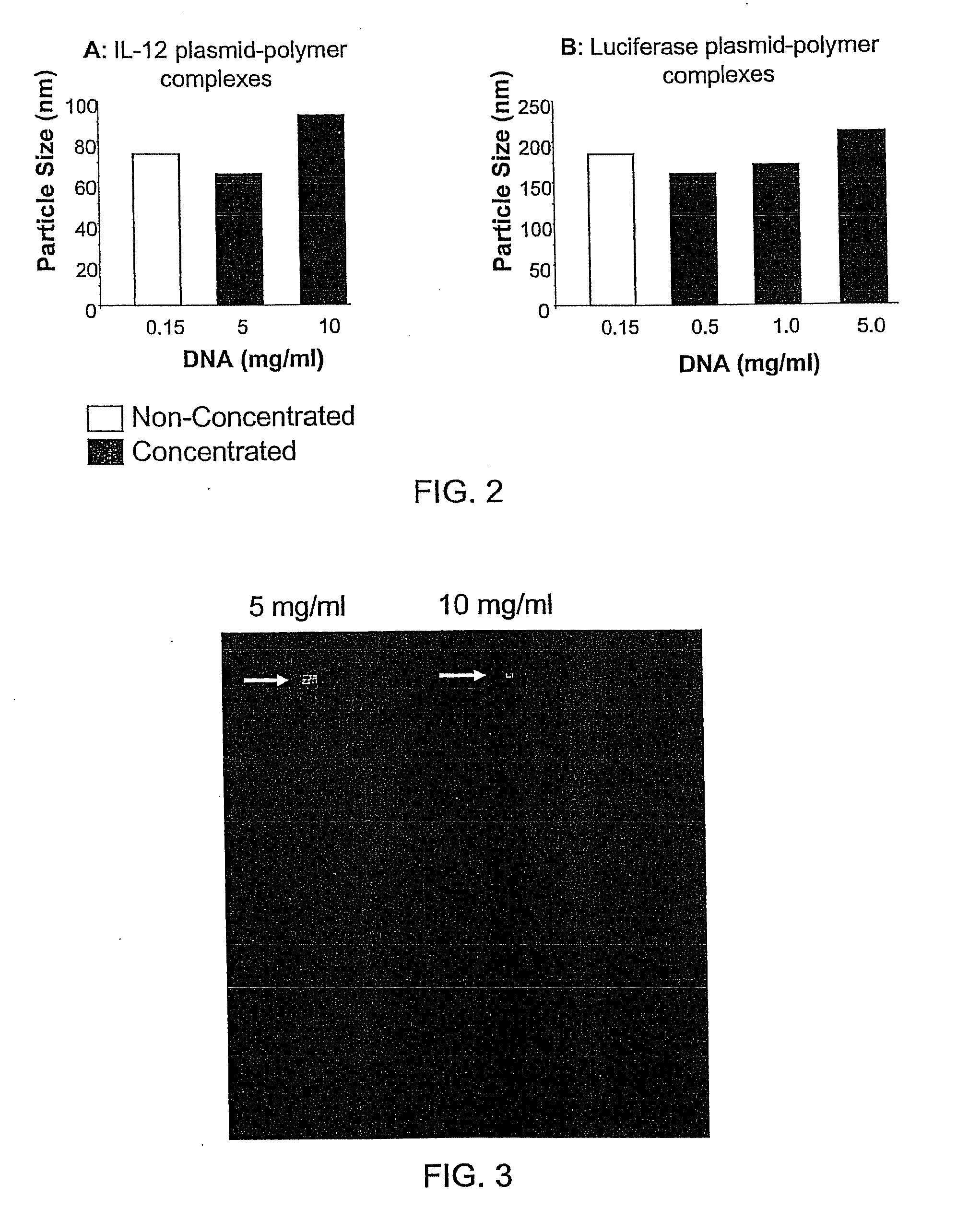
Compositions, methods, and applications that increase the efficiency of nucleic acid transfection are provided. In one aspect, a pharmaceutical composition may include at least about 0.5 mg / ml concentration of a nucleic acid condensed with a cationic lipopolymer suspended in an isotonic solution, where the cationic lipopolymer includes a cationic polymer backbone having cholesterol and polyethylene glycol covalently attached thereto, and wherein the molar ratio of cholesterol to cationic polymer backbone is within a range of from about 0.1 to about 10, and the molar ratio of polyethylene glycol to cationic polymer backbone is within a range of from about 0.1 to about 10. The composition further may include a filler excipient.
Owner:CLSN LAB
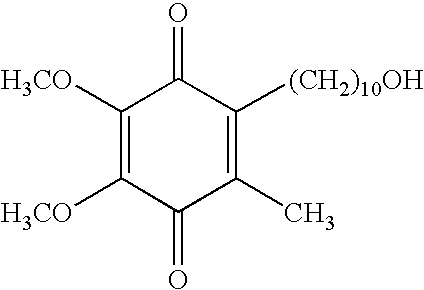
Skin care compositions containing idebenone
InactiveUS20060275237A1Effective supervisionMultiple skin care benefitBiocideCosmetic preparationsIdebenoneDermatology
Owner:THE PROCTER & GAMBLE COMPANY
Glucocorticoid blocking agents for increasing blood-brain barrier permeability stan-261con
InactiveUS20050124533A1Improve breathabilityIncrease volumeAntibacterial agentsBiocideBlood brain barrier permeabilityDisease cause
Glucocorticoid blockers, including glucocorticoid receptor antagonists, are effective to prevent glucocorticoid-induced decrease in permeability of the blood-brain barrier and to increase the permeability of the blood-brain barrier. Administration of glucocorticoid blockers, including glucocorticoid receptor antagonists, concomitant with administration of drugs for treating diseases of the central nervous system increases delivery of such drugs into the central nervous system.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Modified nucleotide compounds
ActiveUS7495088B1Increased nuclease resistanceSugar derivativesMicrobiological testing/measurementBiologyModified nucleosides
Disclosed is a nuclease resistant nucleotide compound capable of hybridizing with a complementary RNA in a manner which inhibits the function thereof, which modified nucleotide compound includes at least one component selected from the group consisting of MN3M, B(N)xM and M(N)xB wherein N is a phosphodiester-linked modified 2′-deoxynucleoside moiety; M is a moiety that confers endonuclease resistance on said component and that contains at least one modified or unmodified nucleic acid base; B is a moiety that confers exonuclease resistance to the terminus to which it is attached; and x is an integer of at least 2.
Owner:ENZO BIOCHEM
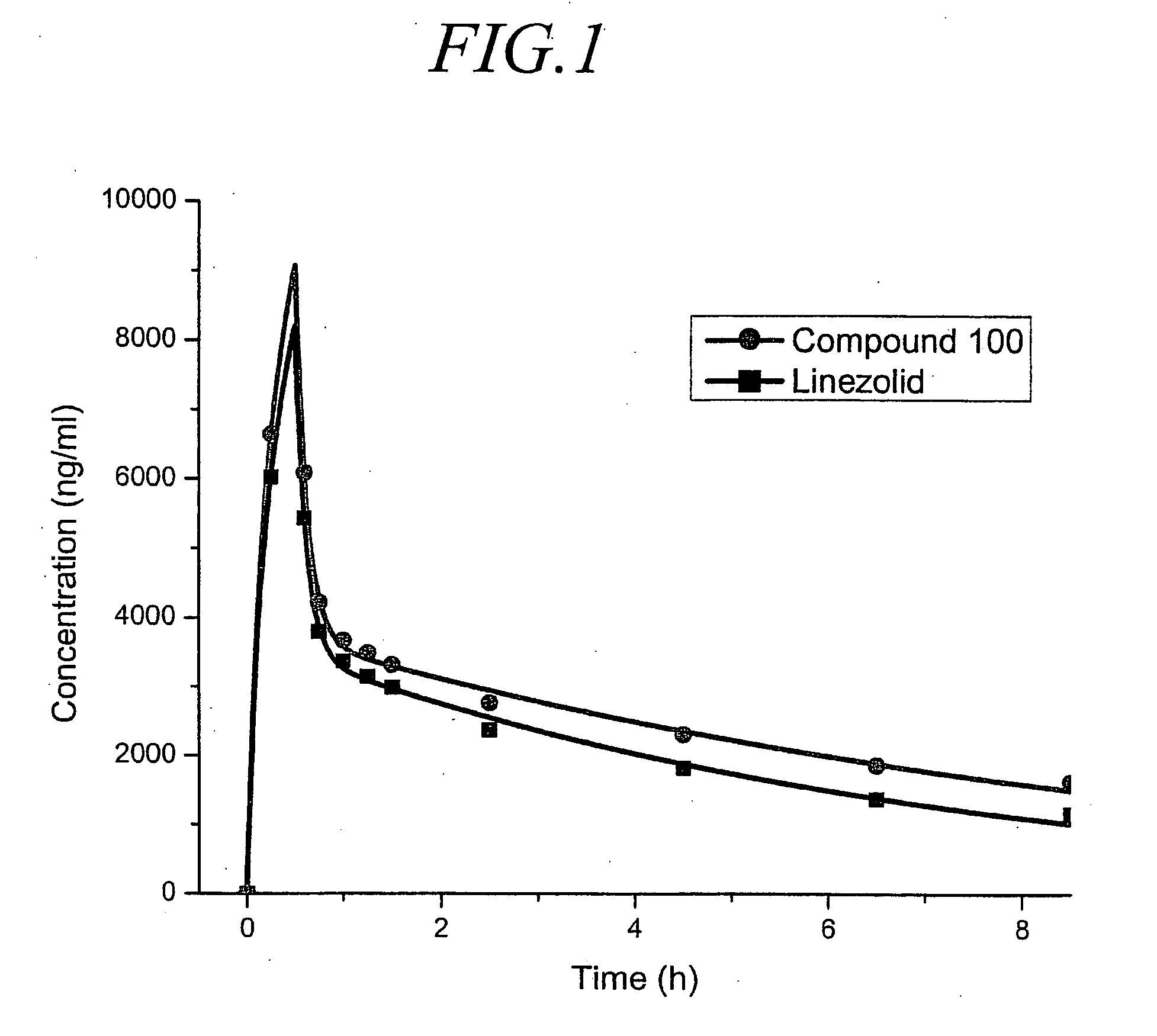
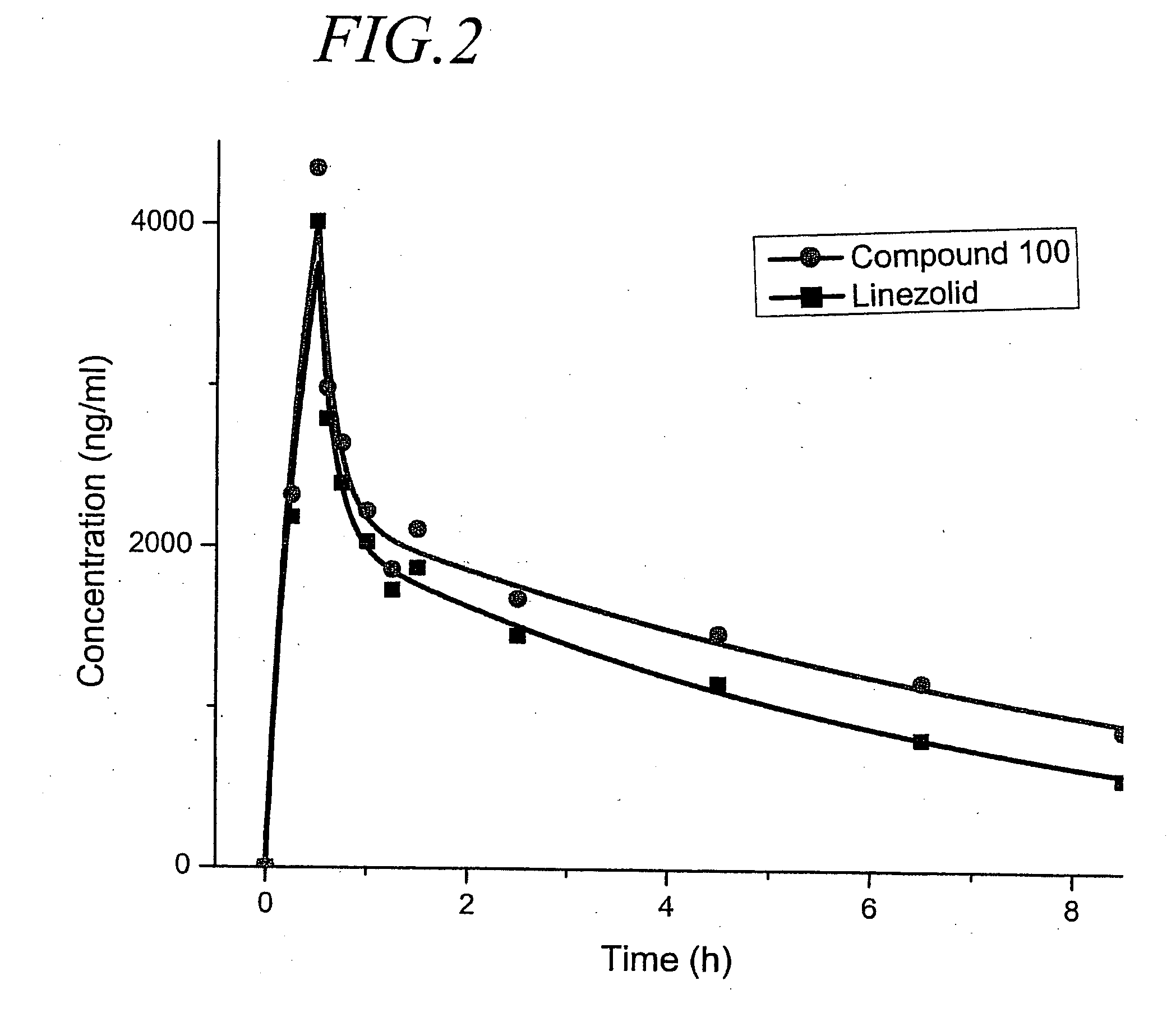
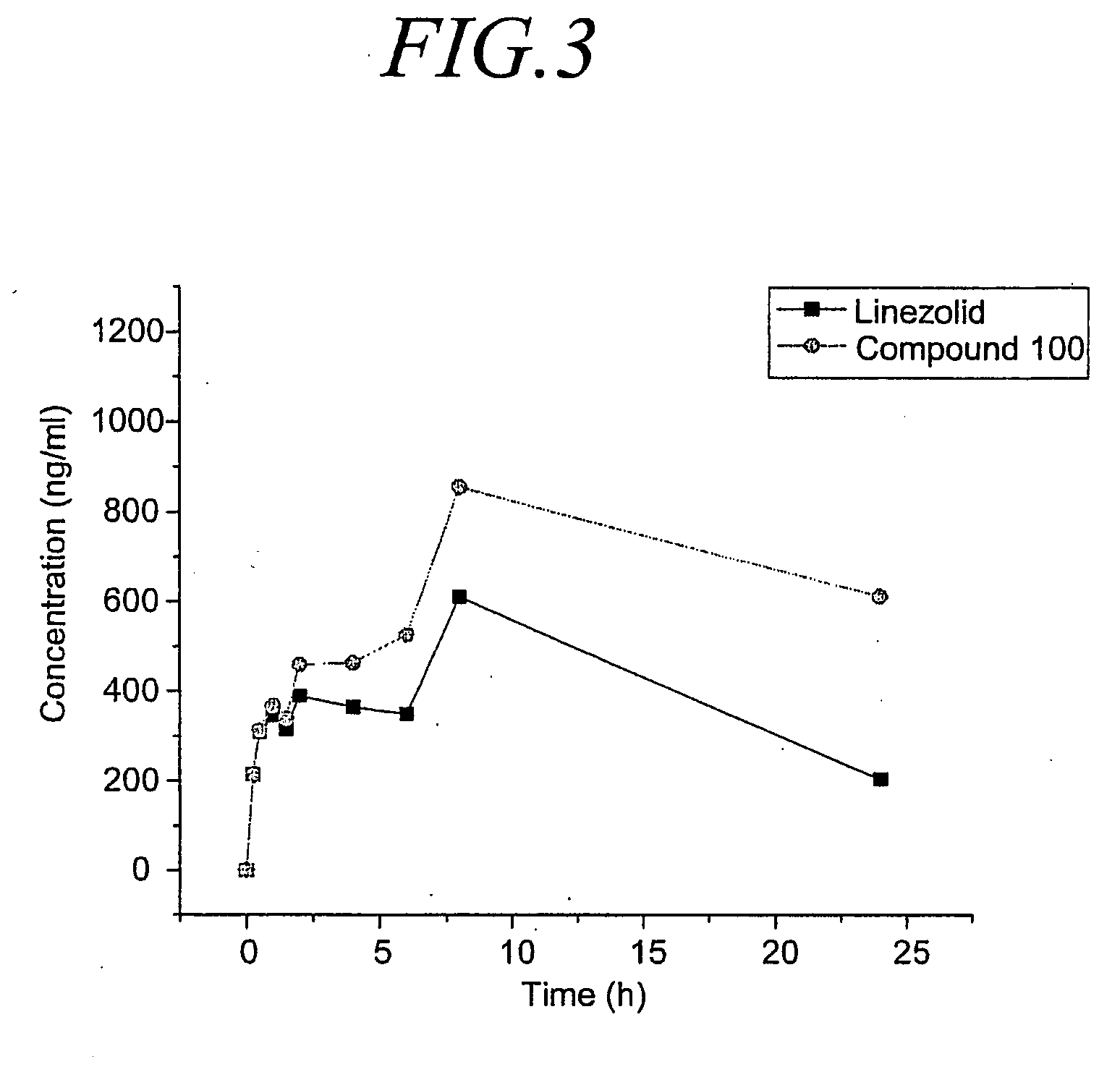
Oxazolidinone derivatives and methods of use
This invention relates to novel N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]-acetamide derivatives, their acceptable acid addition salts, solvates and hydrates. The invention also provides compositions comprising a compound of this invention and the use of such compositions in methods of treating diseases and conditions beneficially treated by antimicrobial agents.
Owner:CONCERT PHARMA INC
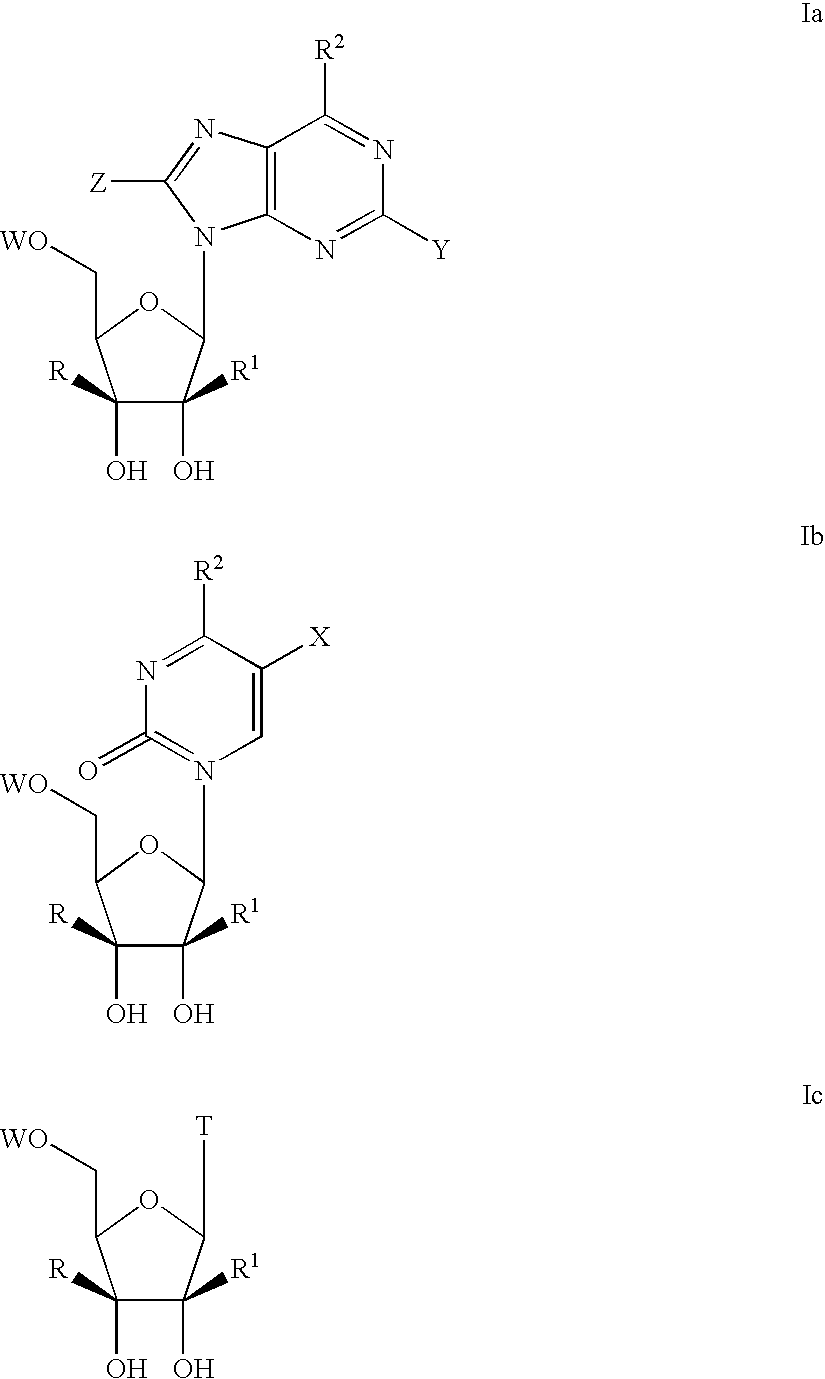
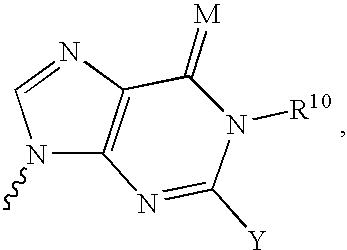
6-disubstituted or unsaturated bicyclic nucleic acid analogs
The present disclosure describes 6-disubstituted bicyclic nucleosides, oligomeric compounds prepared therefrom and methods of using the oligomeric compounds. More particularly, the 6-disubstituted bicyclic nucleosides each comprise a 2′-O—C(Ri)(R2)-4′ or 2′-O—C=(R3)(R.4)-4′ bridge wherein each R is, independently a substituent group and Ri and R2 include H. The 6-disubstituted bicyclic nucleosides are useful for enhancing properties of oligomeric compounds including nuclease resistance. In certain embodiments, the oligomeric compounds provided herein hybridize to a portion of a target RNA resulting in loss of normal function of the target RNA.
Owner:IONIS PHARMA INC
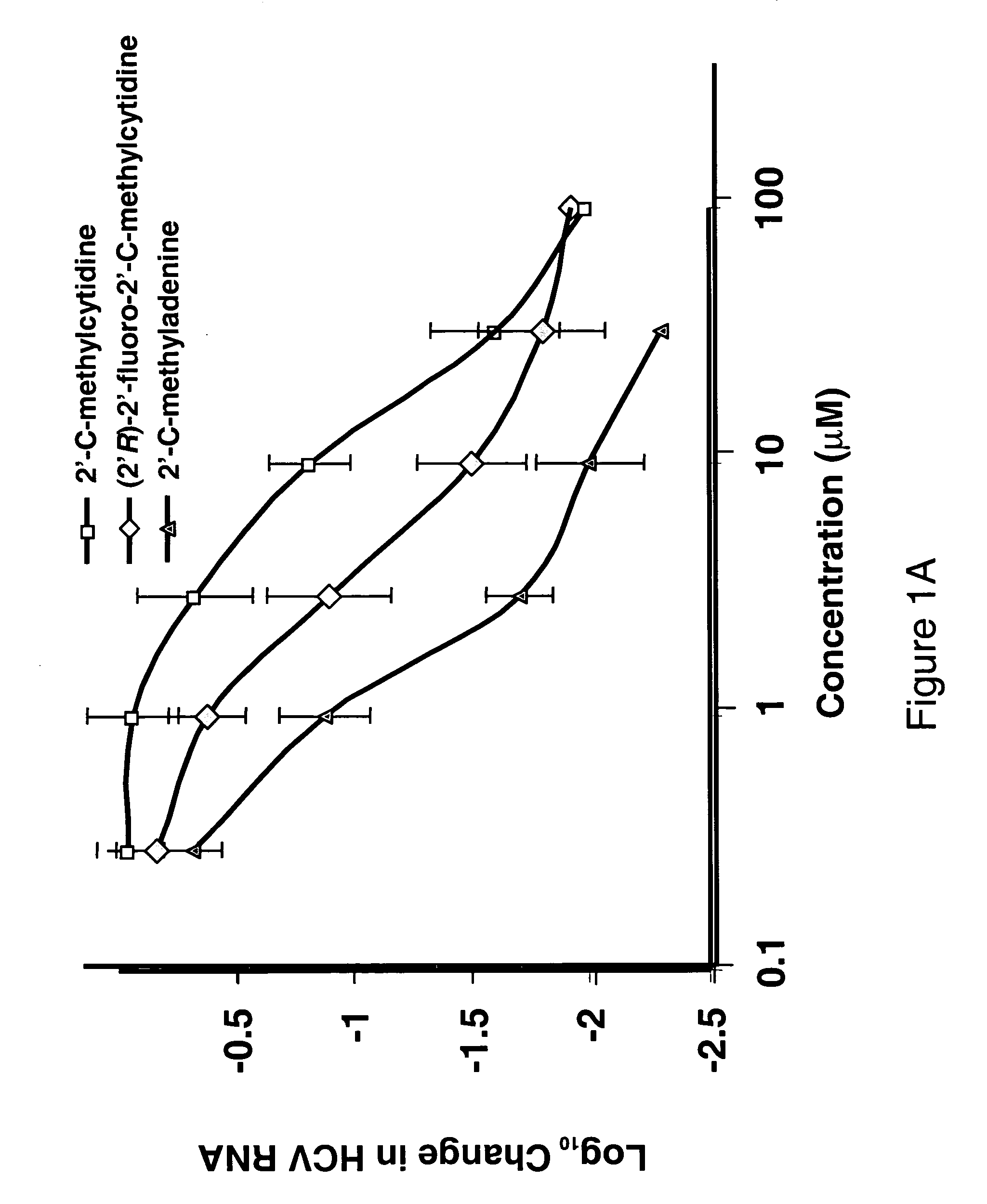
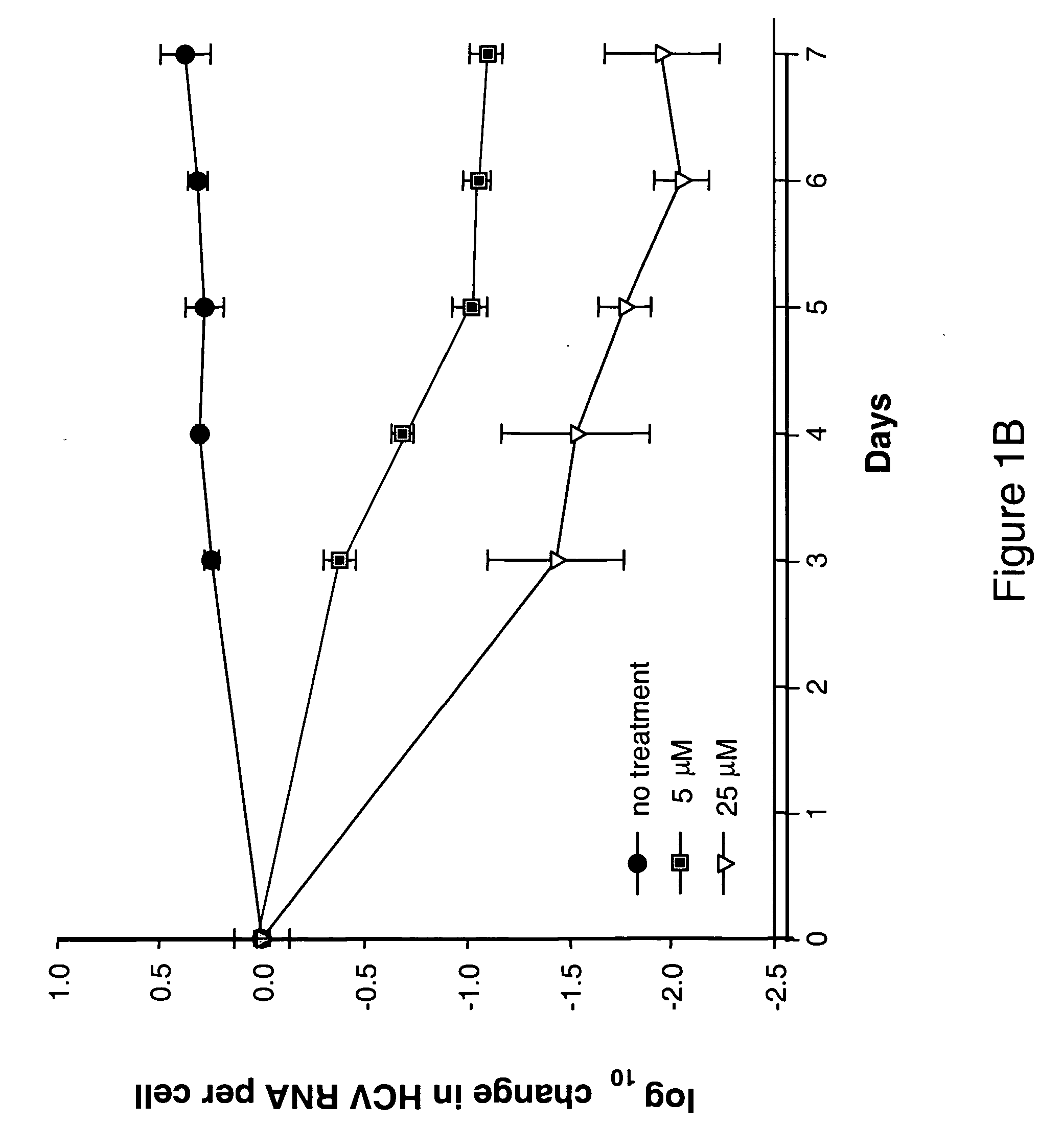
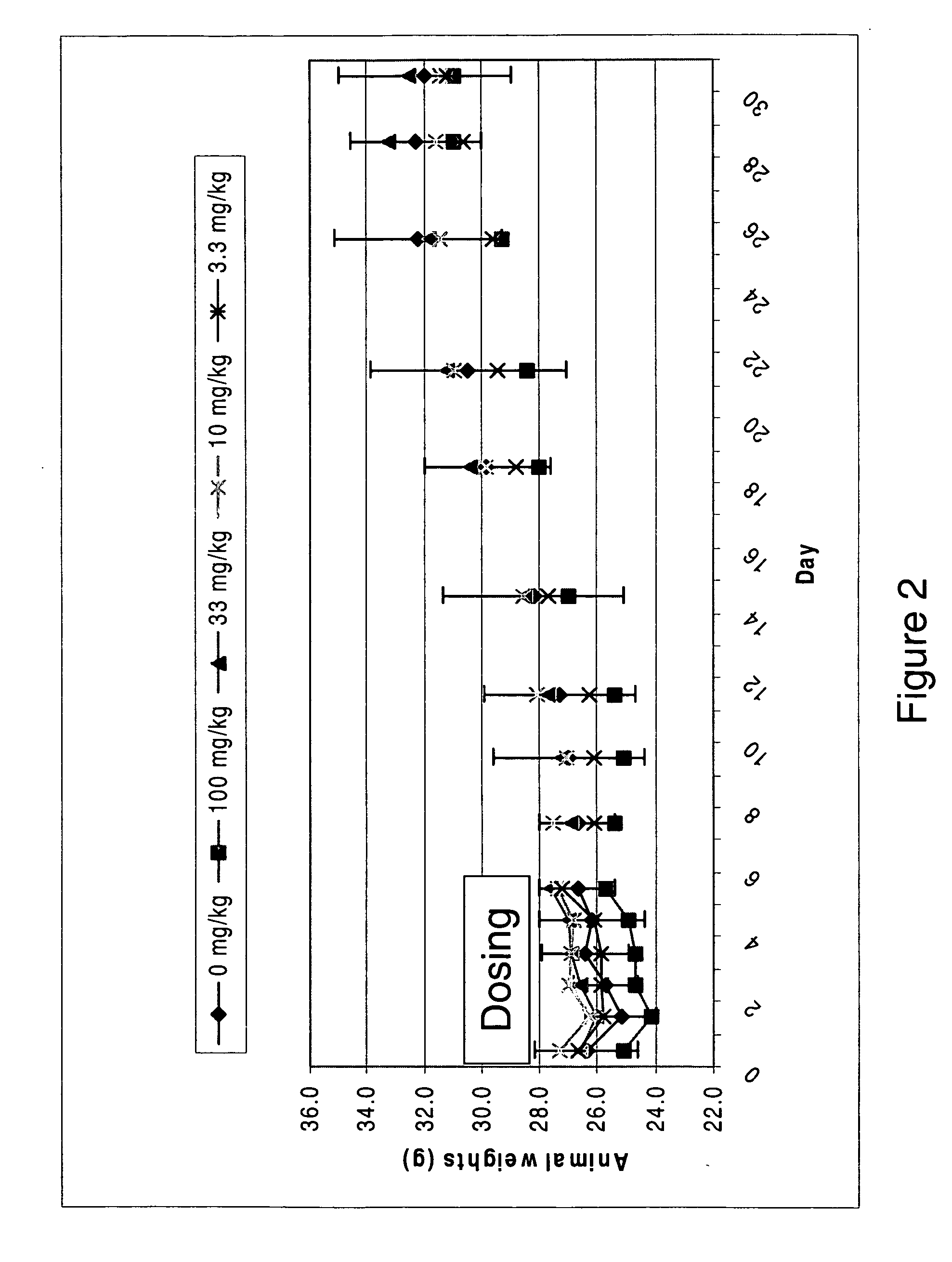
Modified fluorinated nucleoside analogues
ActiveUS20050009737A1Strong specificityLow toxicityAntibacterial agentsBiocideRhinovirus infectionWest Nile virus RNA
The disclosed invention provides compositions and methods of treating a Flaviviridae infection, including hepatitis C virus, West Nile Virus, yellow fever virus, and a rhinovirus infection in a host, including animals, and especially humans, using a (2′R)-2′-deoxy-2′-fluoro-2′-C-methyl nucleosides, or a pharmaceutically acceptable salt or prodrug thereof.
Owner:GILEAD SCI INC
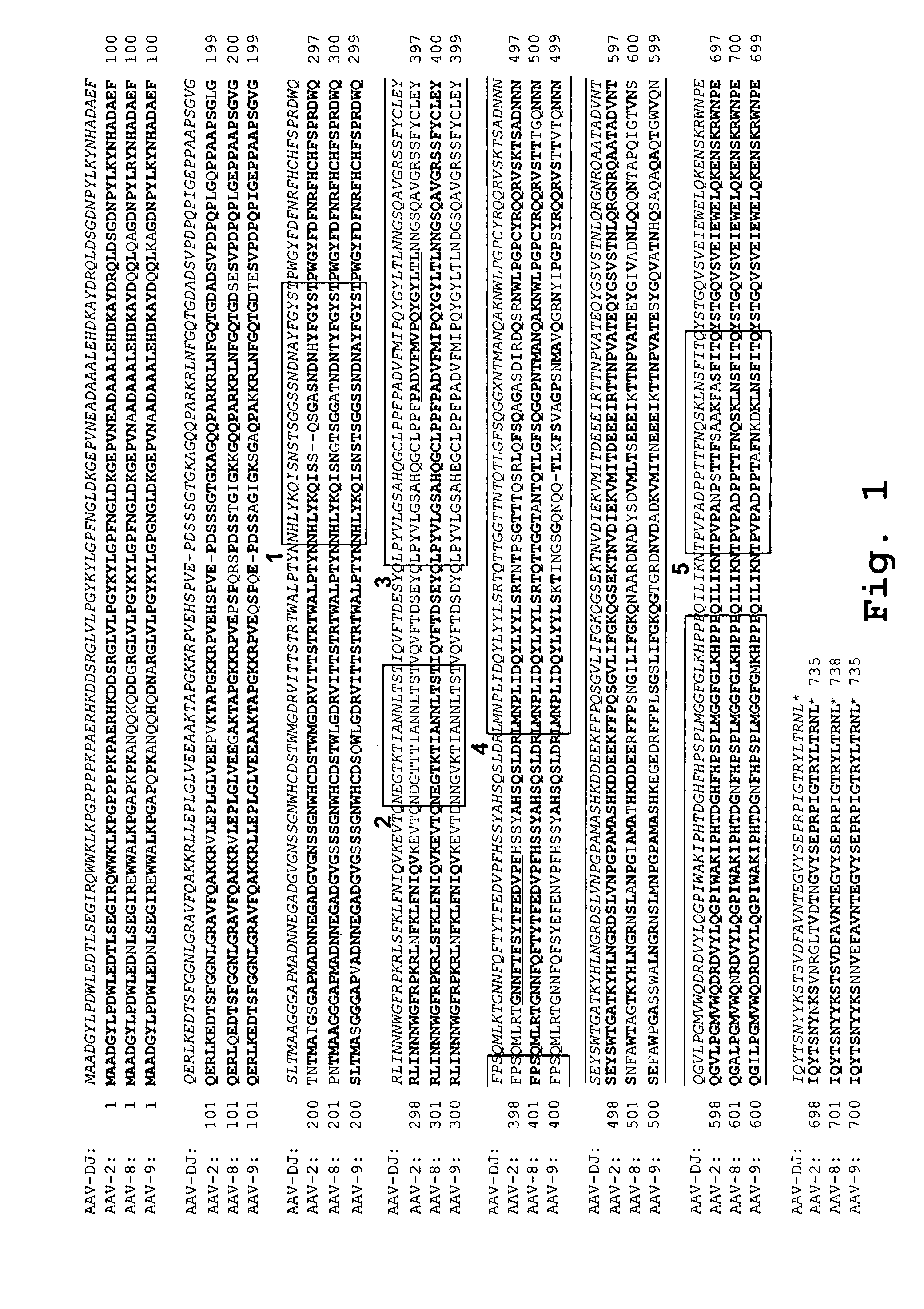
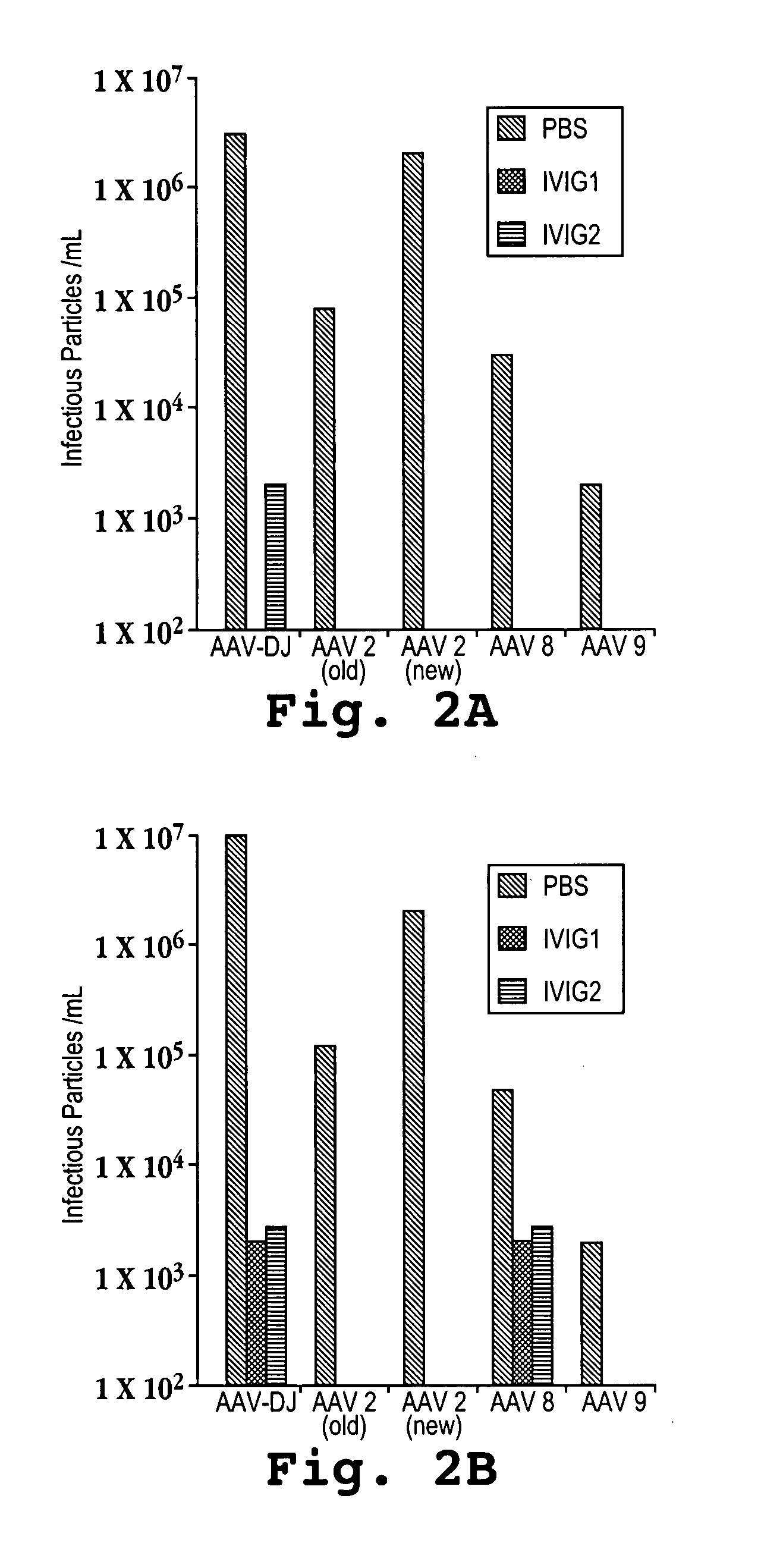
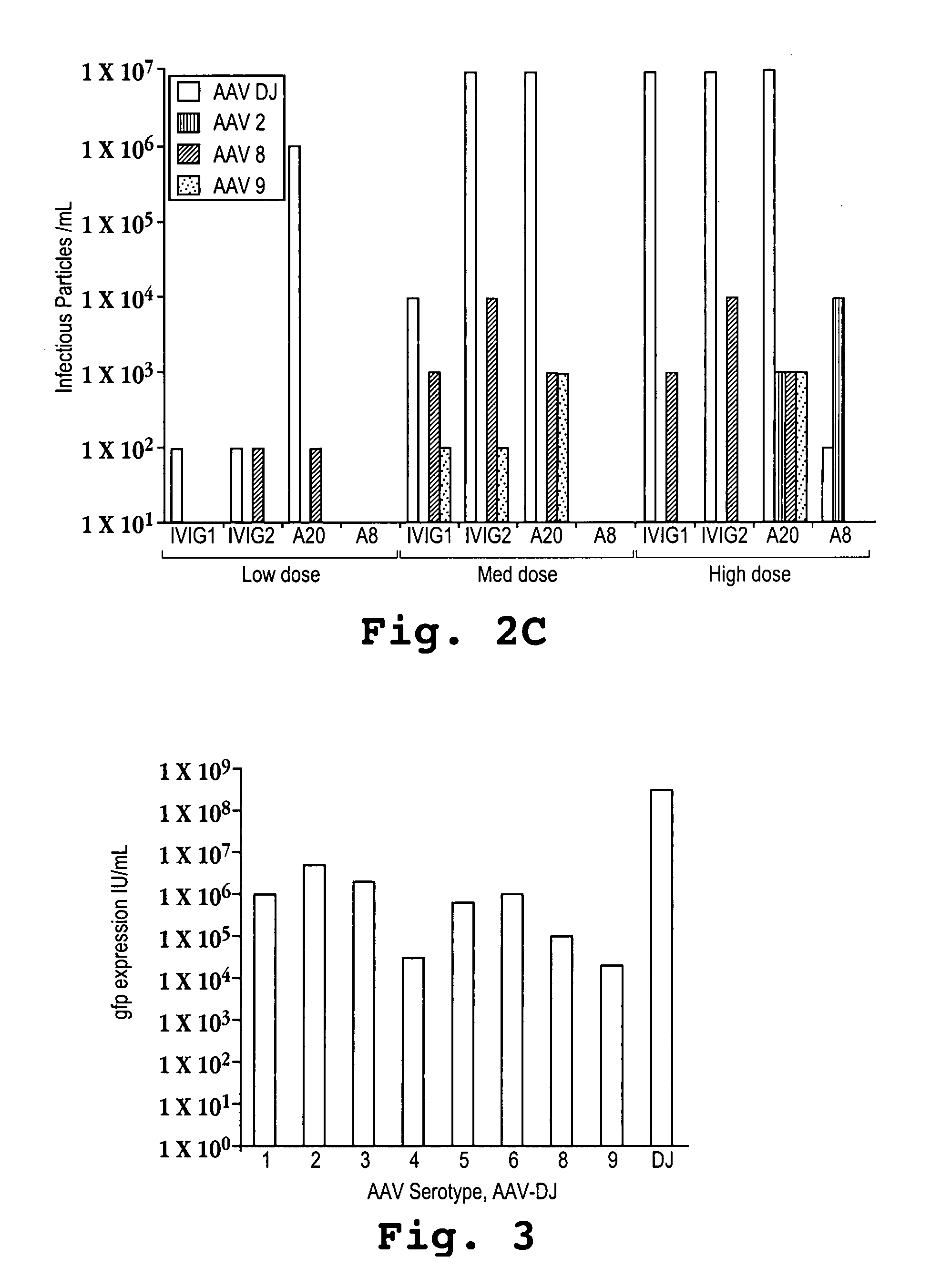
AAV capsid library and AAV capsid proteins
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
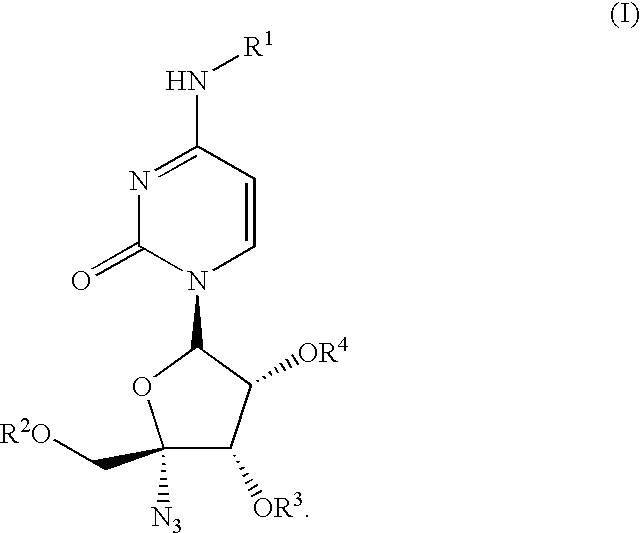
Antiviral nucleoside derivatives
ActiveUS6846810B2Improve the level ofImproved pharmacokinetic propertiesBiocideSugar derivativesMedicineCombination therapy
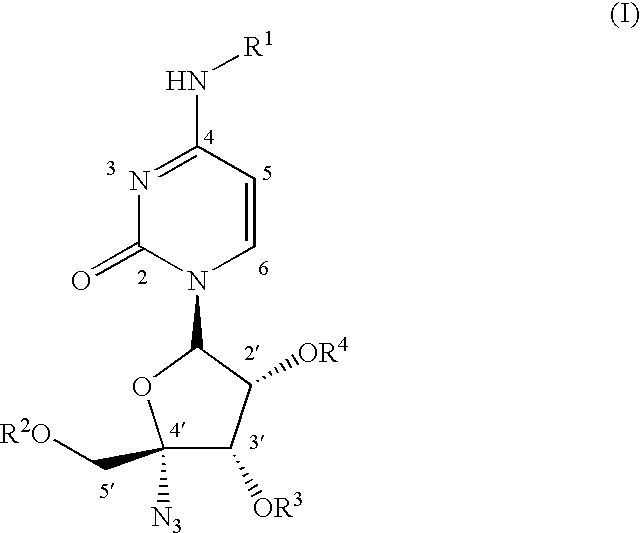
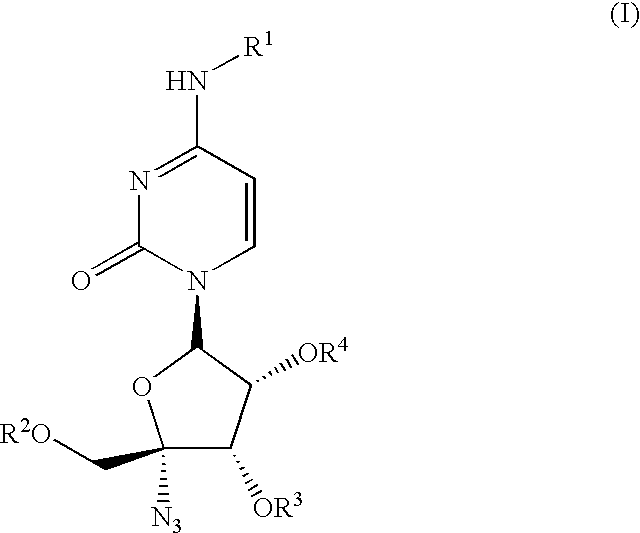
The present invention relates to nucleoside derivatives for the treatment of Hepatitis C viral infections including compounds of formula I, pharmaceutical compositions comprising these compounds and methods for treatment or prophylaxis of Hepatitis C Virus mediated diseases employing said compounds in monotherapy or in combination therapy. The present invention further provides a process for preparing 1′,3′,4′-triacyl pyrimidine nucleoside from a N, 1′,3′,4′-tetraacylpyrimidine nucleoside
Owner:RIBOSCI
Lipid encapsulated interfering RNA
The present invention provides lipid-based formulations for delivering, e.g., introducing, nucleic acid-lipid particles comprising an interference RNA molecule to a cell, and assays for optimizing the delivery efficiency of such lipid-based formulations.
Owner:ARBUTUS BIOPHARMA CORPORAT ION
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com