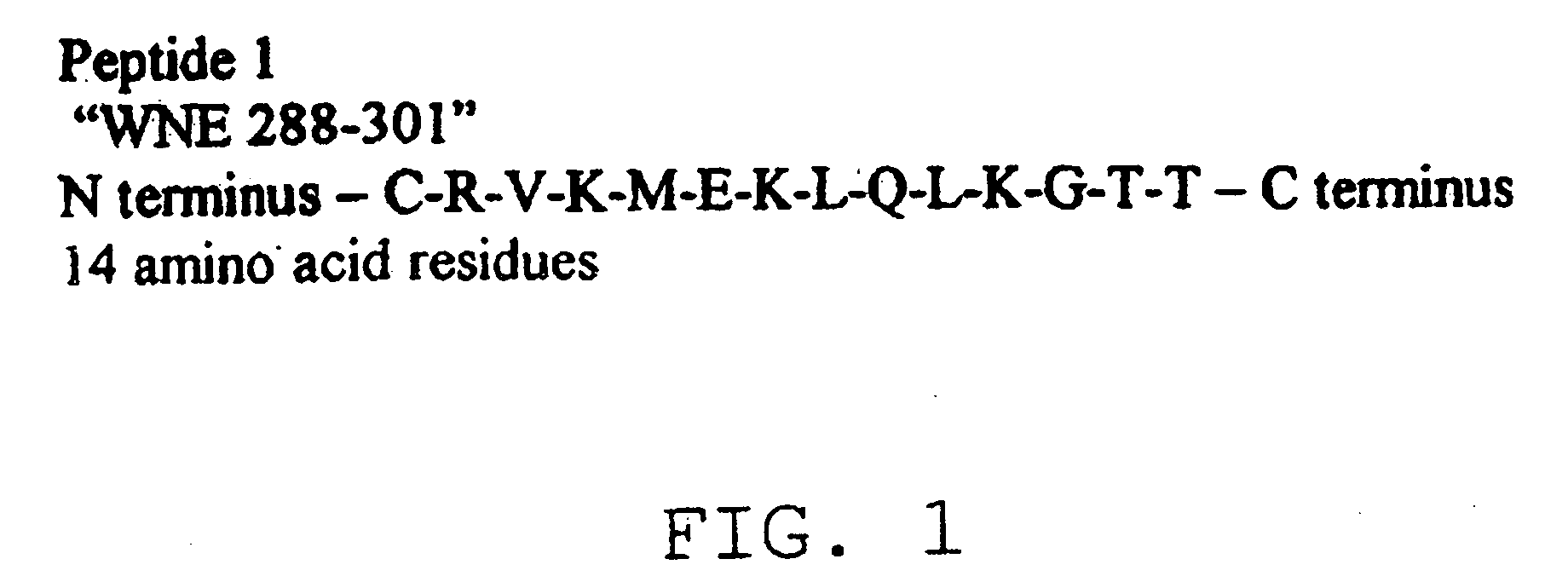
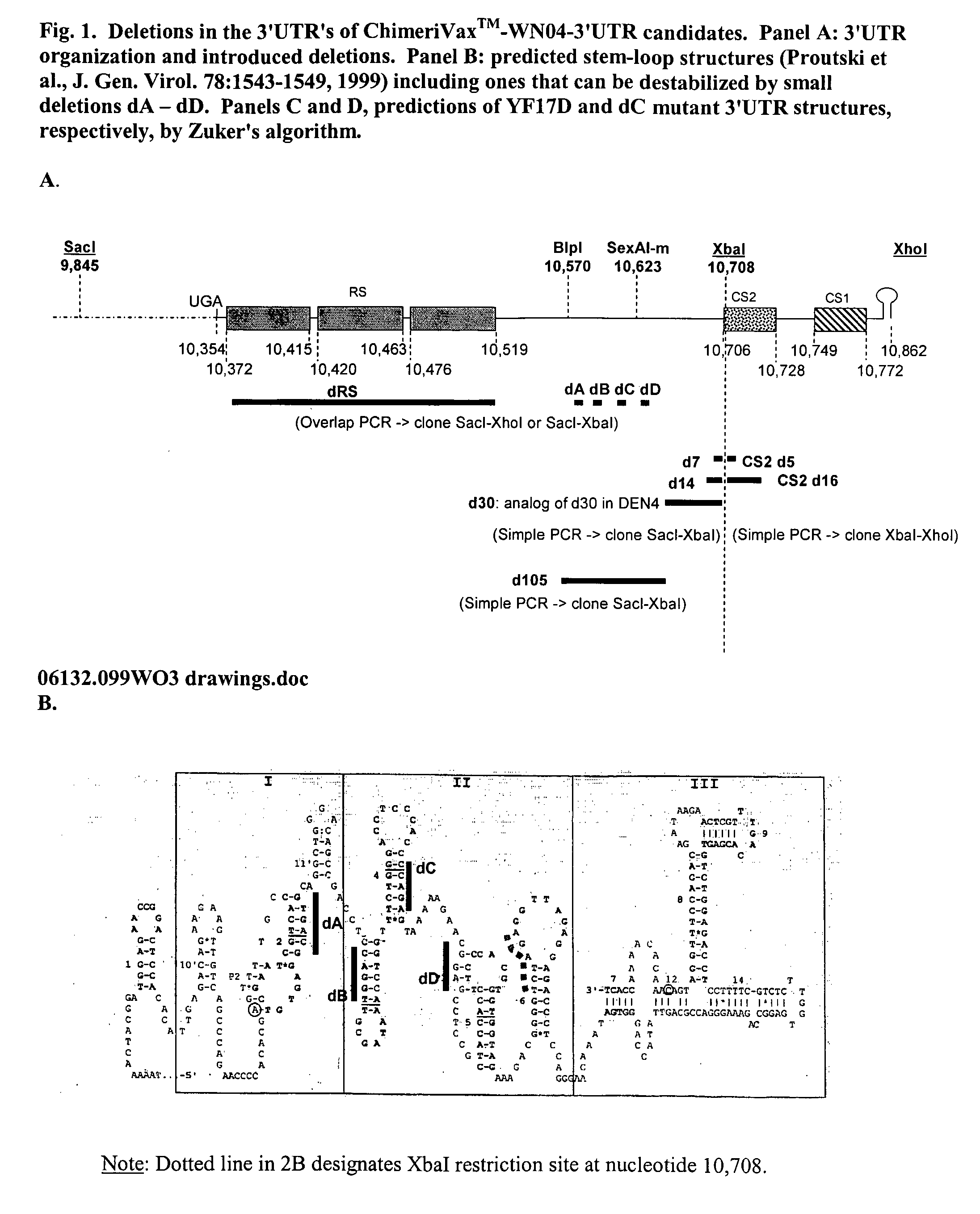
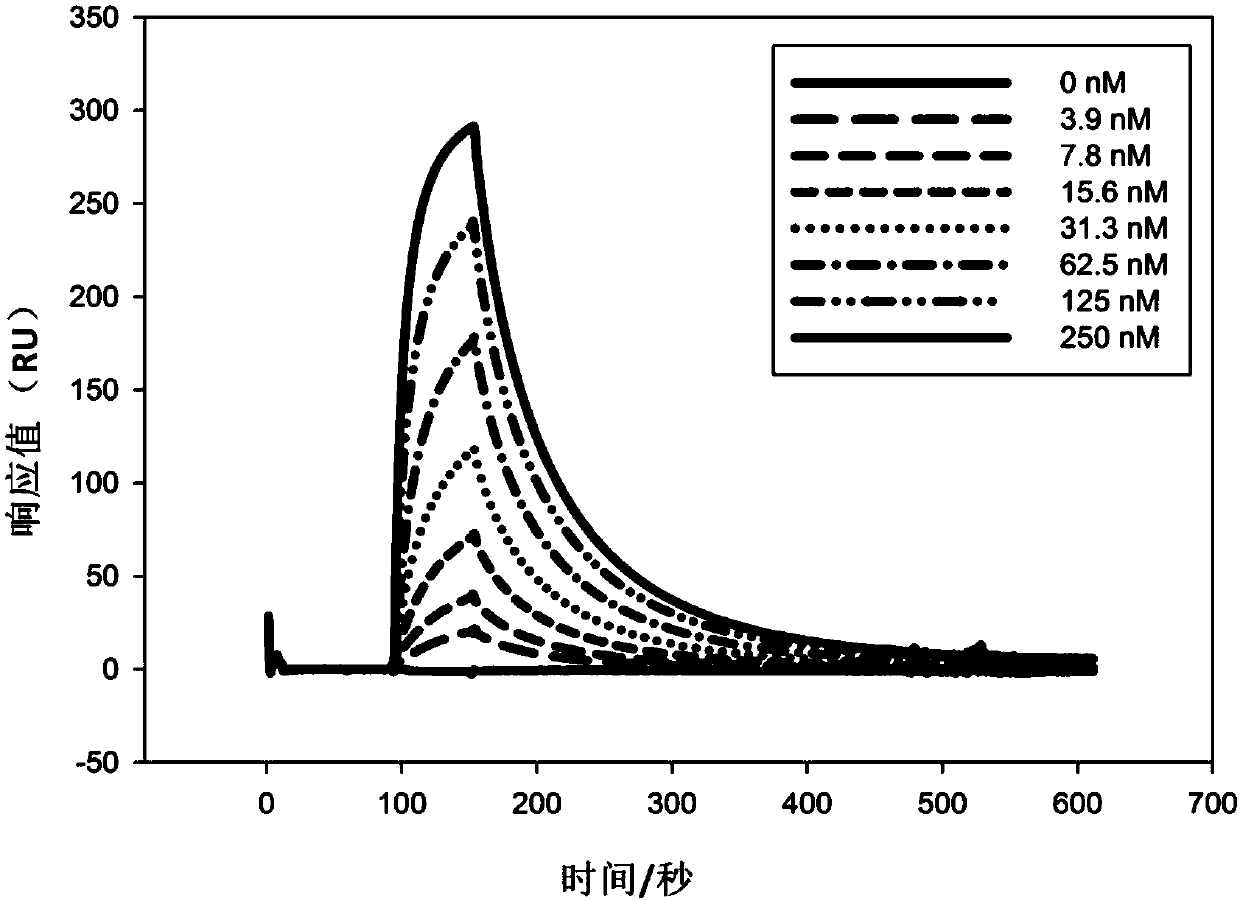
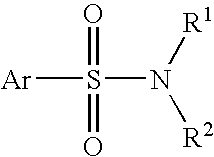
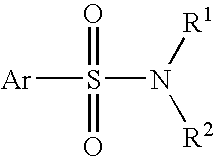
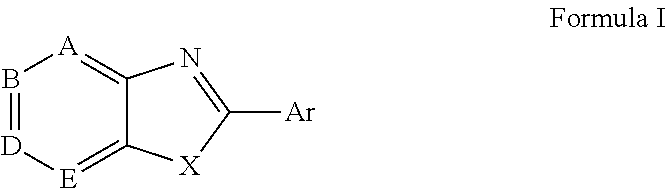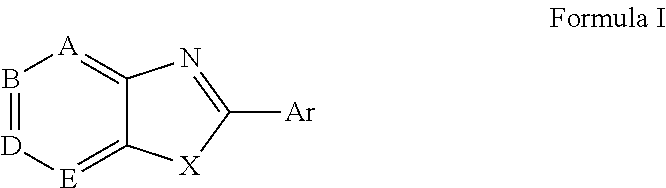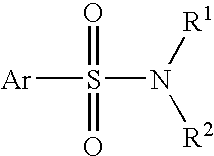Patents
Literature
182 results about "West Nile virus RNA" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
West Nile virus (WNV) is a single-stranded RNA virus that causes West Nile fever. It is a member of the family Flaviviridae, specifically from the genus Flavivirus, which also contains the Zika virus, dengue virus, and yellow fever virus.
Modified fluorinated nucleoside analogues
ActiveUS20050009737A1Strong specificityLow toxicityAntibacterial agentsBiocideRhinovirus infectionWest Nile virus RNA
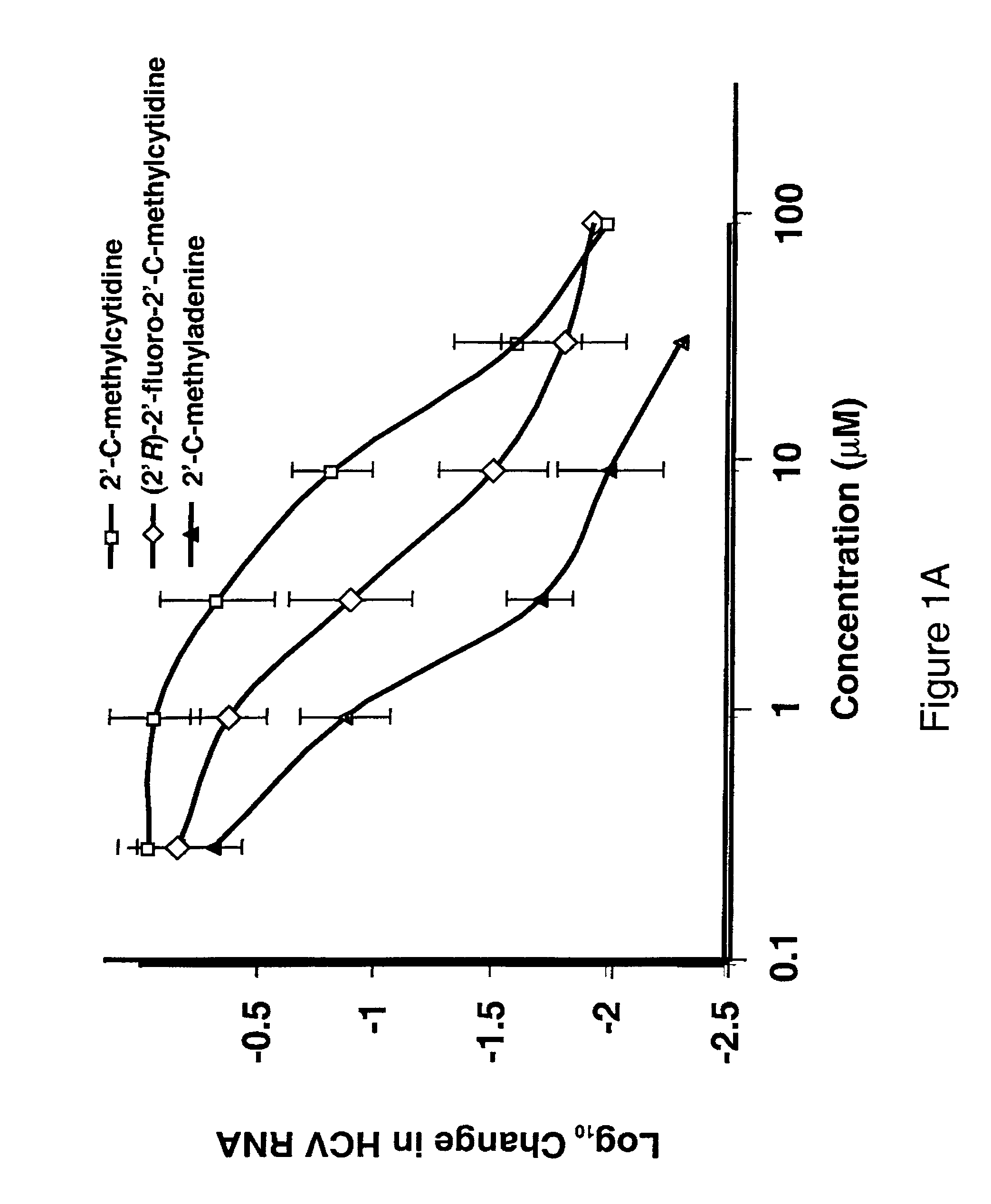
The disclosed invention provides compositions and methods of treating a Flaviviridae infection, including hepatitis C virus, West Nile Virus, yellow fever virus, and a rhinovirus infection in a host, including animals, and especially humans, using a (2′R)-2′-deoxy-2′-fluoro-2′-C-methyl nucleosides, or a pharmaceutically acceptable salt or prodrug thereof.
Owner:GILEAD SCI INC
Screening for west nile virus antiviral therapy
InactiveUS20050058987A1Improve efficiencySsRNA viruses positive-senseVectorsHigh-Throughput Screening MethodsImmunogenicity
The instant invention provides stable and novel lineage I WNV reverse genetics systems, and methods for making the reverse genetics systems, specifically, a fully-infectious lineage I WNV cDNA or replicon system engineered with one or more nucleotide sequences each encoding a reporter gene to be used in high throughput cell-based screening assays for the identification of novel antiflaviviral chemotherapeutics and / or vaccines effective to treat and / or immunize against infections by WNV and other emerging flaviviruses, such as, for example, JEV, SLEV, AV, KV, JV, CV, YV, TBEV, DENV-1, DENV-2, DENV-3, DENV-4, YFV and MVEV. The present invention further provides methods of high throughput screening of antiflaviviral compounds or improved derivatives thereof using novel lineage I WNV reverse genetics systems and / or cell lines stably containing the reverse genetics systems. Also, the invention provides novel pharmaceutical compositions comprising an attenuated lineage I WNV that is less virulent but similarly immunogenic as the parent WNV and is capable of providing a protective immune response in a host.
Owner:HEALTH RES INC
Modified fluorinated nucleoside analogues
ActiveUS20080070861A1Strong specificityLow toxicityAntibacterial agentsBiocideRhinovirus infectionWest Nile virus RNA
The disclosed invention provides compositions and methods of treating a Flaviviridae infection, including hepatitis C virus, West Nile Virus, yellow fever virus, and a rhinovirus infection in a host, including animals, and especially humans, using a (2′R)-2′-deoxy-2′-fluoro-2′-C-methyl nucleosides, or a pharmaceutically acceptable salt or prodrug thereof.
Owner:GILEAD SCI INC
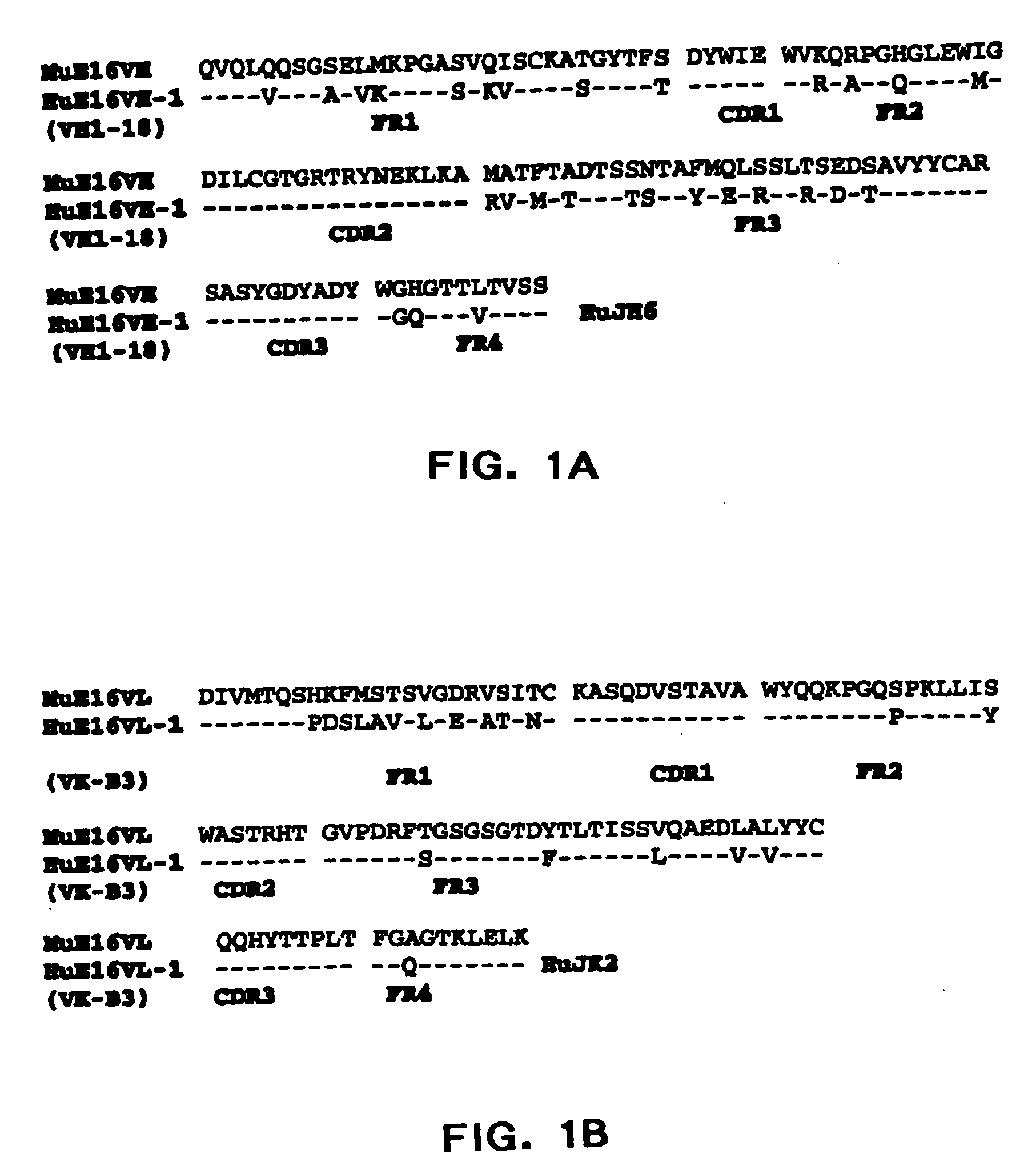
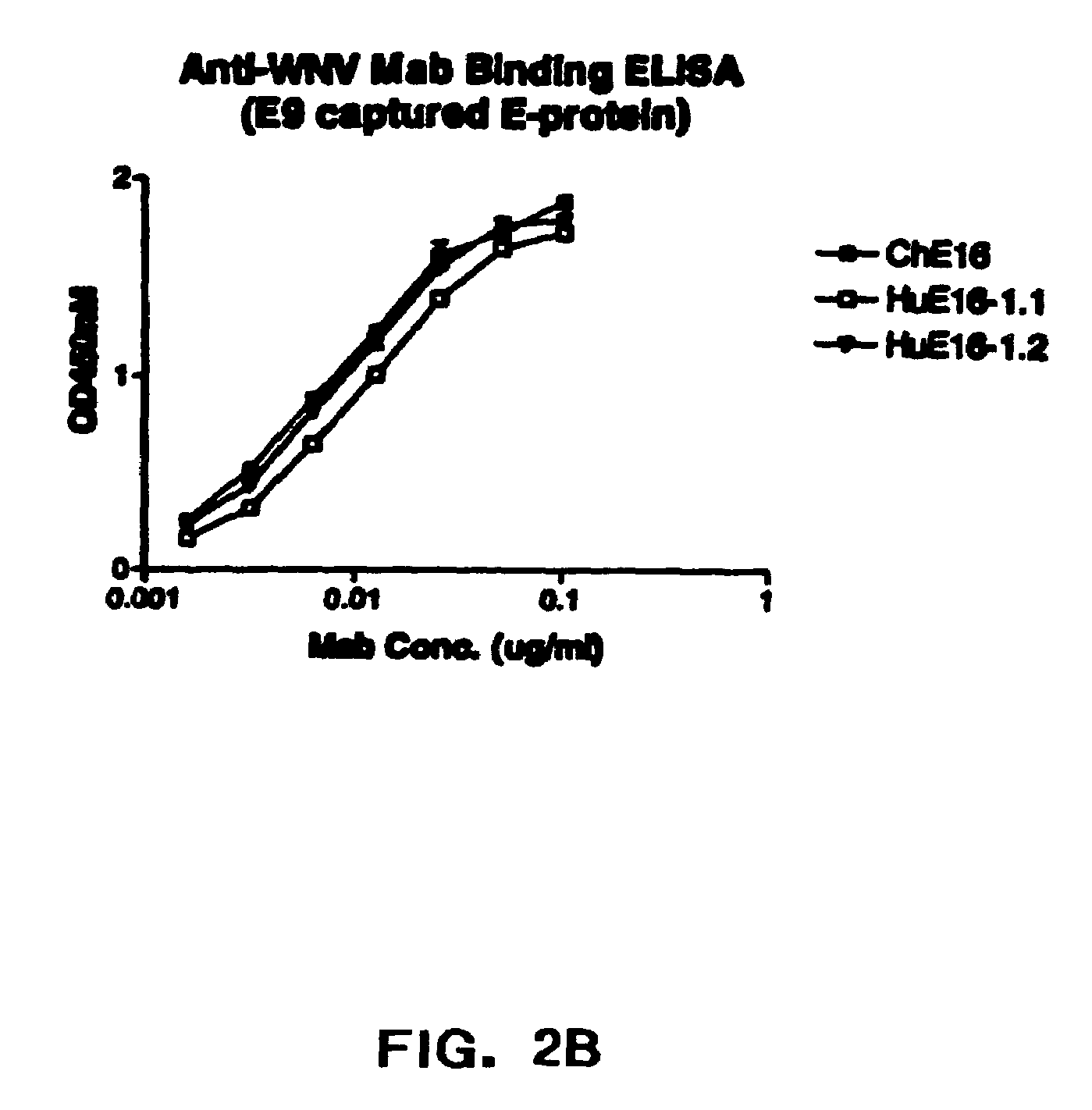
Humanized antibodies against West Nile Virus and therapeutic and prophylactic uses thereof
InactiveUS20060057149A1Easy to neutralizeEasy to removeCosmetic preparationsToilet preparationsHumanized antibodyViral infection
The present invention relates to compositions comprising humanized antibodies or fragments thereof that immunospecifically bind to one or more antigens of a flavivirus, particularly of West Nile Virus (WNV) and methods for preventing, treating or ameliorating symptoms associated with a flavivirus, particularly of West Nile Virus (WNV) infection utilizing said compositions. In particular, the present invention relates to methods for preventing, treating or ameliorating symptoms associated with WNV infection, said methods comprising administering to a human subject an effective amount of one or more humanized antibodies or fragments thereof that immunospecifically bind to a WNV antigen. The present invention also relates to detectable or diagnostic compositions comprising humanized antibodies or fragments thereof that immunospecifically bind to a WNV antigen and methods for detecting or diagnosing WNV infection utilizing said compositions.
Owner:MACROGENICS INC
Recombinant vaccine against west nile virus
InactiveUS20030104008A1Inhibition effectEasy to storeSsRNA viruses positive-senseViral antigen ingredientsImmunogenicityNucleotide
Disclosed and claimed are immunogenic compositions to induce an immune response against West Nile (WN) virus, recombinants, for instance recombinant avipox viruses containing and expressing exogenous polynucleotide(s) from WN virus, and methods for making and using the same.
Owner:MERIAL SAS
Novel Imino Sugar Derivatives Demonstrate Potent Antiviral Activity and Reducted Toxicity
InactiveUS20110189771A1Good effectImprove performanceOrganic chemistryTissue cultureBovine Viral Diarrhea VirusesSide chain
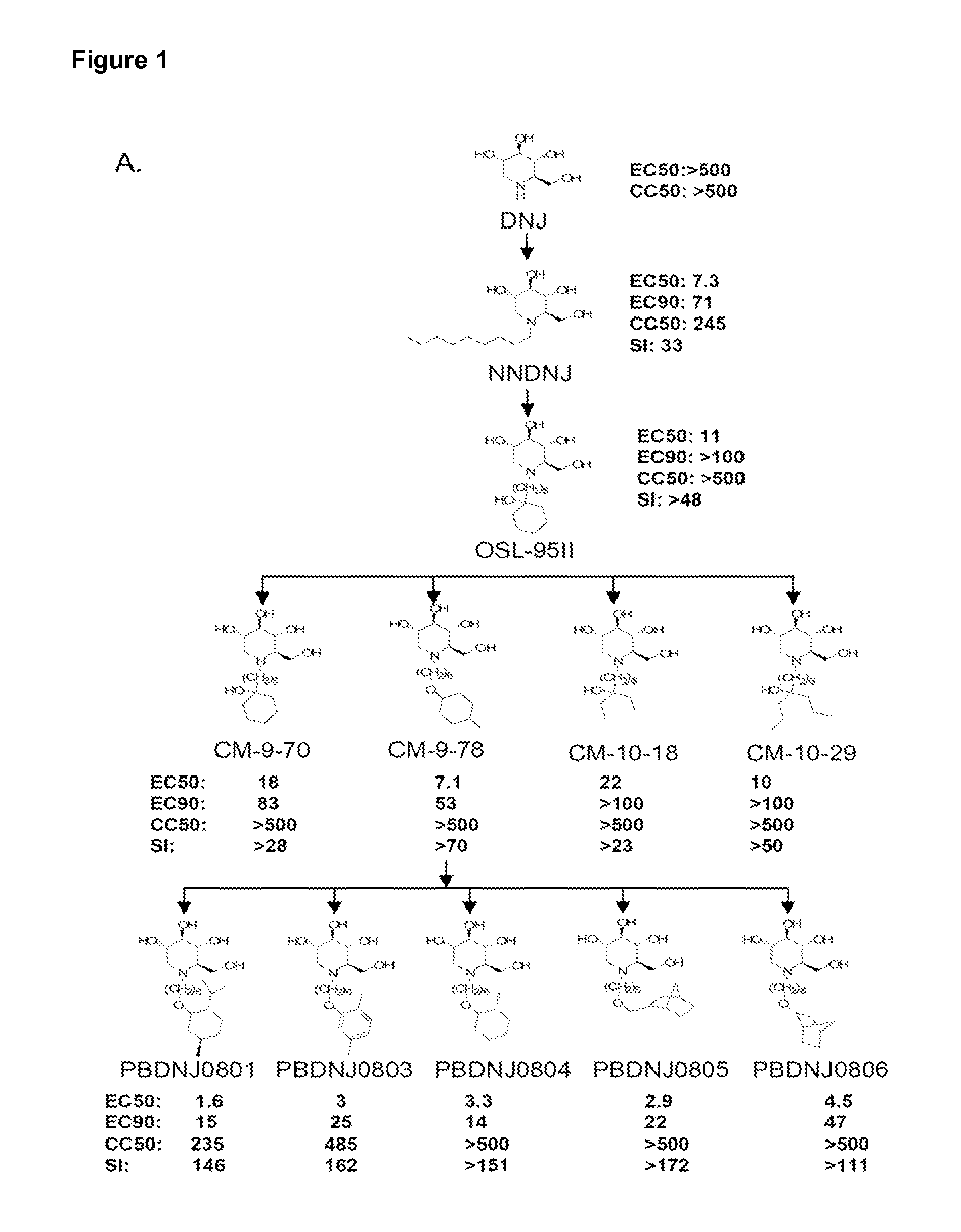
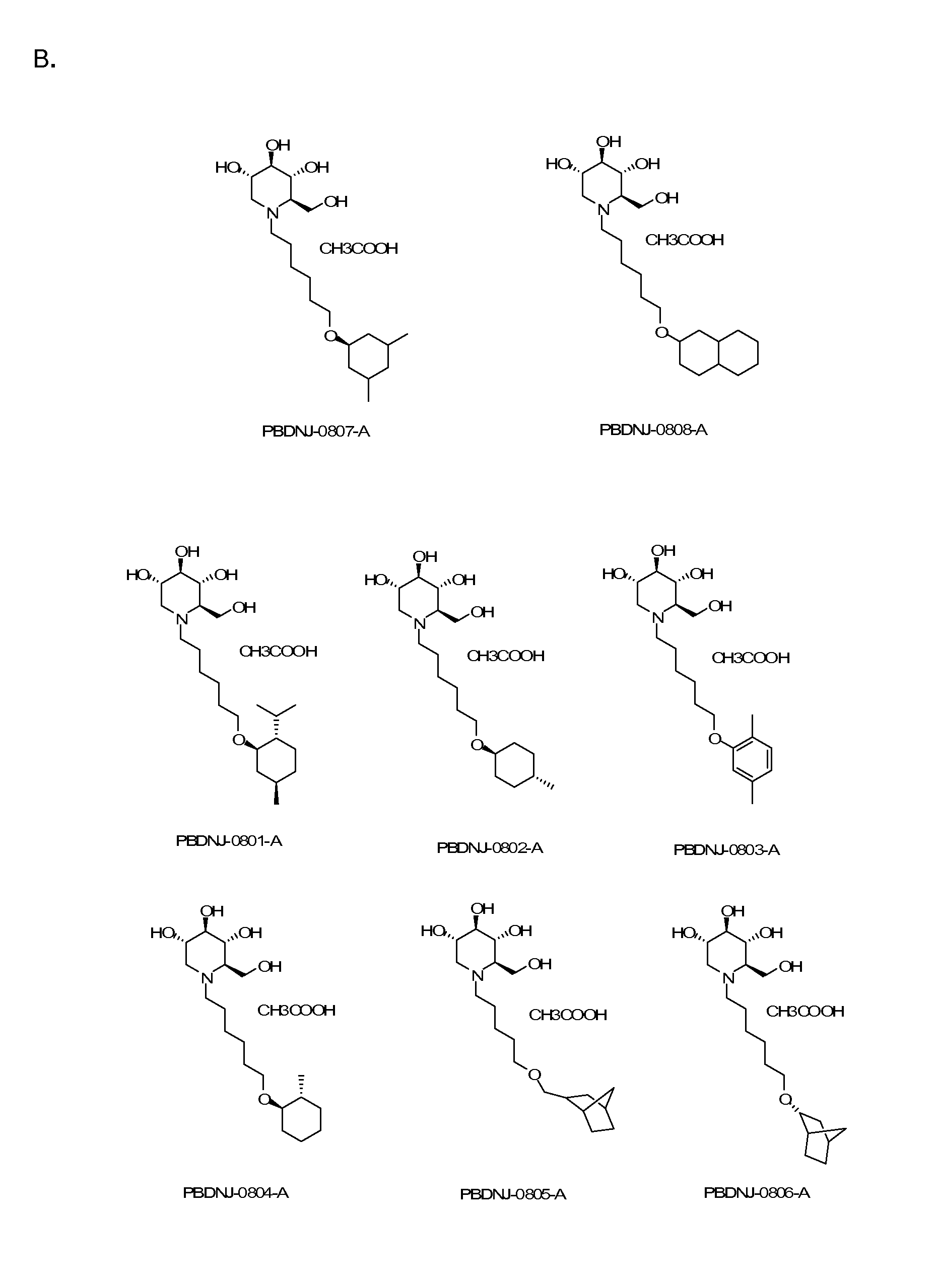
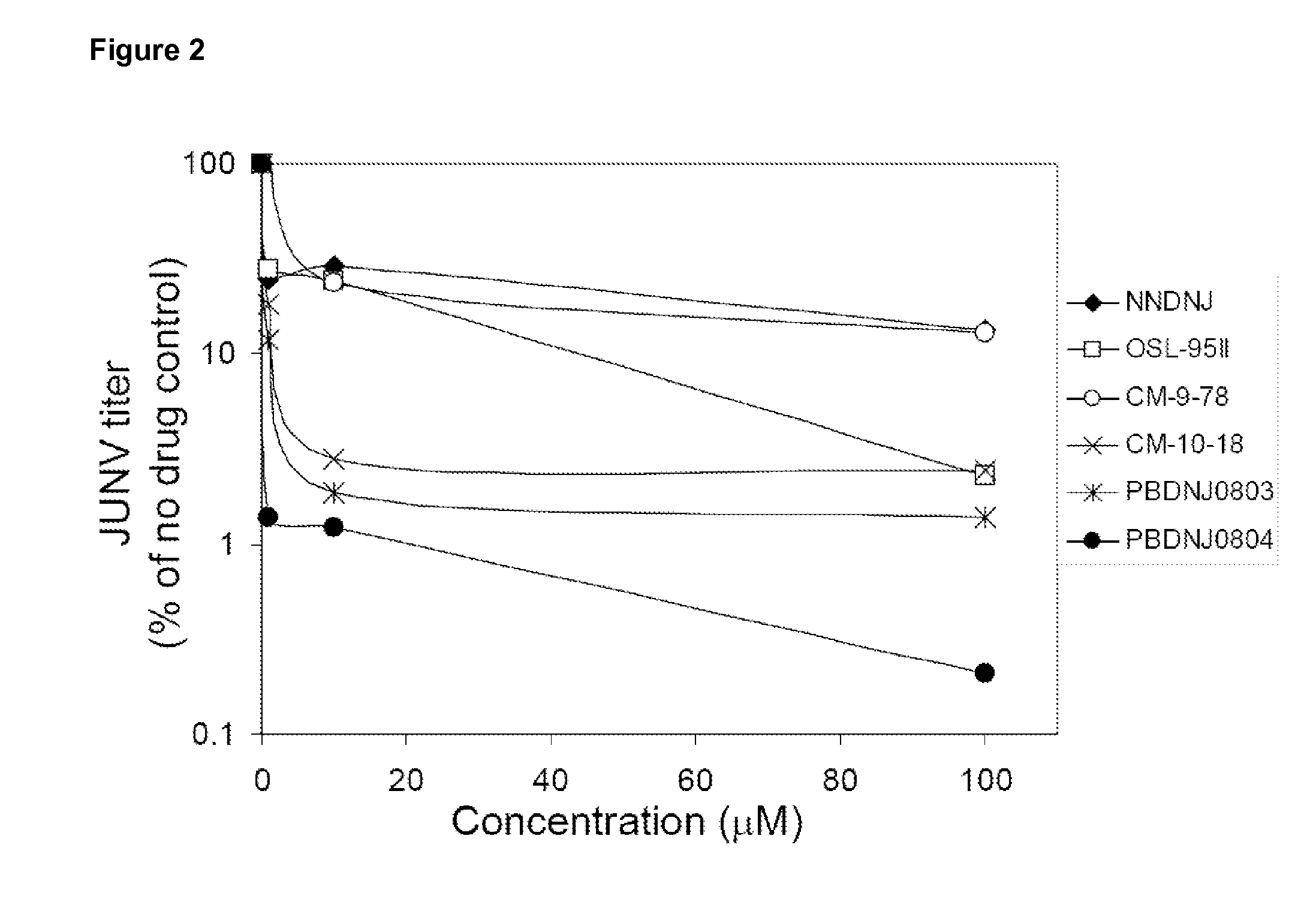
Imino sugars, such as deoxynojirimycin (DNJ), are glucose analogues that selectively inhibit cellular α-glucosidase I and II (enzymes that process N-linked glycans in glycoprotein) and exhibit broad spectrum antiviral activities against many enveloped viruses. Previously we have reported a novel DNJ derivative, OSL-95II, with antiviral activity and reduced cytotoxicity. In order to develop imino sugars with more potent antiviral activity as well as improved toxicity profile, OSL-95II was modified by diversifying the nitrogen linked alkylated side chain. The antiviral activities were initially tested in bovine viral diarrhea virus (BVDV) infected MDBK cells, yielding several imino sugar derivatives with novel structure and superior antiviral activity and toxicity profile. Furthermore, these new compounds were shown to be active against Dengue virus (DV) and West Nile virus (WNV) infection in BHK cells where potent anti-DV activity having submicromolar EC50 values and SI of greater than 900. These compounds represent a new generation of iminio sugars and their analogues, having application in the clinical treatment of infection of DV and other members of flaviviridae.
Owner:INST FOR HEPATITS & VIRUS RES +1
Diagnostic test for West Nile virus
InactiveUS20040197769A1More sensitiveEasy to useViral antigen ingredientsMicrobiological testing/measurementSt Louis encephalitis virusSerum ige
The present invention provides a rapid and sensitive method for the detection of a West Nile virus (WNV), Japanese encephalitis virus (JEV), St. Louis encephalitis virus (SLEV) and Dengue virus (DENV) and antibodies directed against thereof involving contacting a biological specimen suspected of being infected with WNV, JE, SLE or DEN with a substantially purified and isolated WNV E glycoprotein or subfragment thereof having a native conformation wherein the E glycoprotein or subfragment thereof has a reactivity with antibodies against WNV and a cross-reactivity with antibodies against JEV, SLEV and DENV. The instant invention further provides a rapid, sensitive, and consistent method for the specific detection of WNV by employing diagnostic assays having the antigen NS5 which is specifically reactive with anti-WNV antibodies but not cross-reactive with antibodies against other flaviviruses such as JEV, SLEV, or DENV. The present invention also provides a rapid, sensitive, and consistent method for the specific detection of DENV by employing diagnostic assays having the antigen NS5 which is specifically reactive with anti-DENV antibodies but do not cross-react with antibodies against other flaviviruses such as JEV, SLEV, or WNV. Further, the DENV NS5 antigens are serospecific and do not cross react with antibodies to other DENV strains. Thus, the method of the present invention provides a manner by which to discriminate infections by each DENV strain. Further, diagnostic kits for carrying out the methods are provided. The methods and kits for carrying out the methods of the invention are rapid and require as little as 10 minutes to detect a result.
Owner:HEALTH RES INC
Treatment and prevention of dengue virus infections
Methods and pharmaceutical compositions for treating viral infections, by administering certain 2-aryl-benzothiazole or 2-heteroaryl-benzothiazole derivative compounds in therapeutically effective amounts are disclosed. Methods of using the compounds and pharmaceutical compositions thereof are also disclosed. In particular, the treatment and prophylaxis of viral infections such as caused by flavivirus is disclosed, i.e., including but not limited to, Dengue virus, West Nile virus, yellow fever virus, Japanese encephalitis virus, and tick-borne encephalitis virus.
Owner:SIGA TECH INC
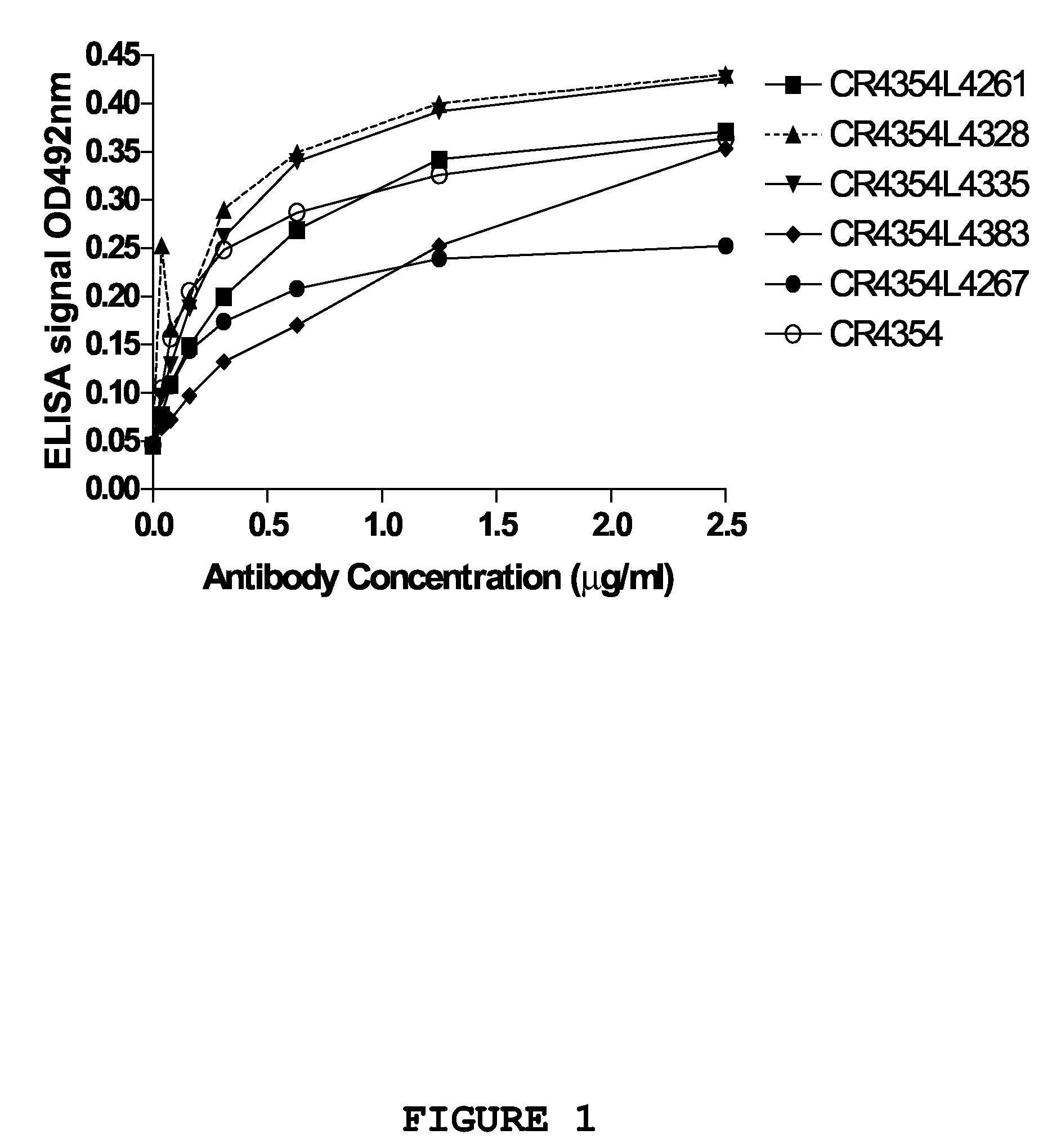
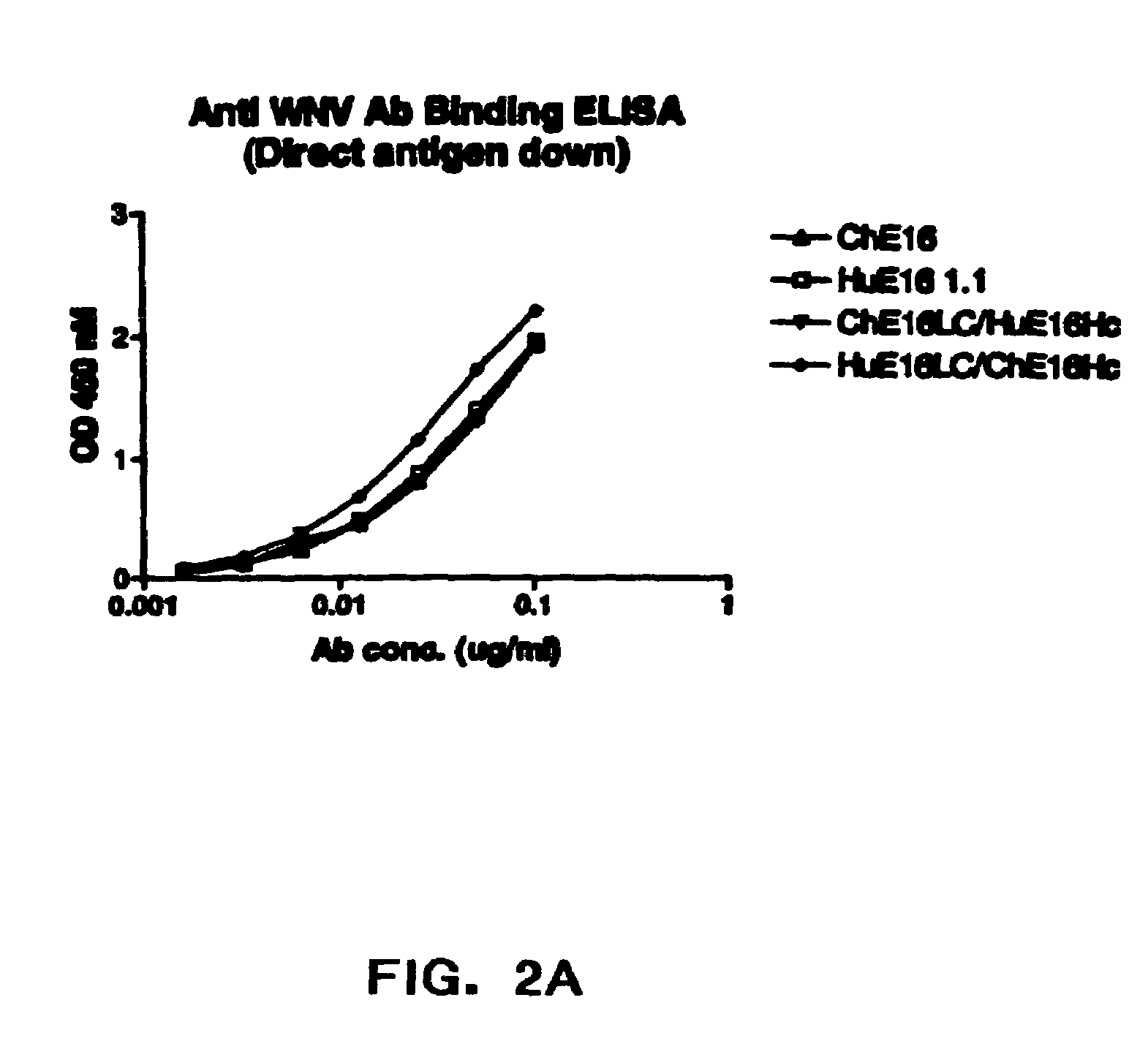
Optimization of West Nile Virus Antibodies
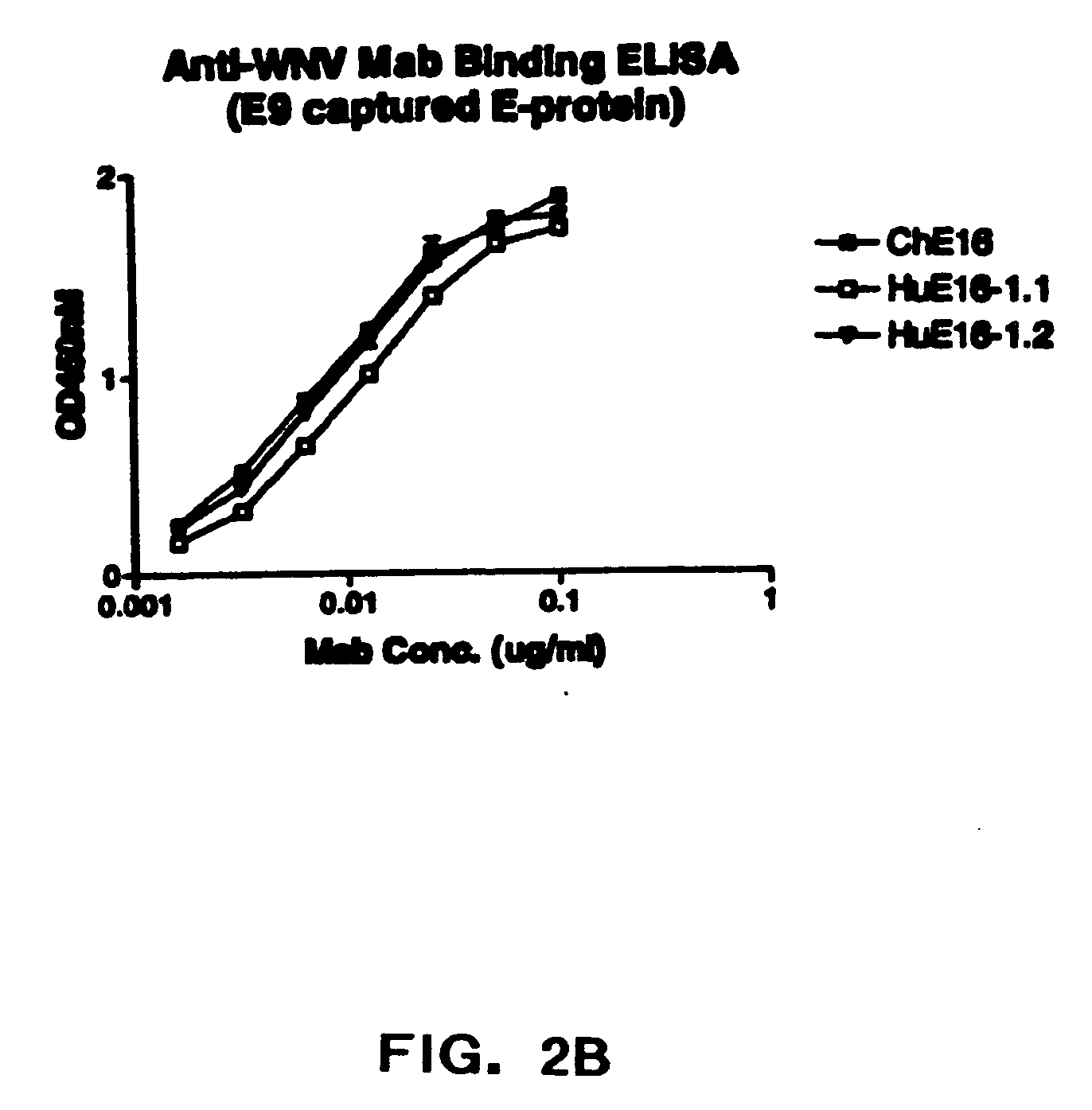
InactiveUS20090130652A1Function increaseEnhance interestMicrobiological testing/measurementAntibody ingredientsWest Nile virus RNAVirology
Owner:JANSSEN VACCINES & PREVENTION BV
Diagnostic test for west nile virus
ActiveUS20060115896A1More sensitiveEasy to useAnimal cellsMicrobiological testing/measurementDiagnostic testFlavivirus
The present invention provides a rapid and sensitive method for the detection of a West Nile virus (WNV), Japanese encephalitis virus (JEV), St. Louis encephalitis virus (SLEV) and Dengue virus (DENV) and antibodies directed against thereof involving contacting a biological specimen suspected of being infected with WNV, JE, SLE or DEN with a substantially purified and isolated WNV E glycoprotein or subfragment thereof having a native conformation wherein the E glycoprotein or subfragment thereof has a reactivity with antibodies against WNV and a cross-reactivity with antibodies against JEV, SLEV and DENV. The instant invention further provides a rapid, sensitive, and consistent method for the specific detection of WNV by employing diagnostic assays having the antigen NS5 which is specifically reactive with anti-WNV antibodies but not cross-reactive with antibodies against other flaviviruses such as JEV, SLEV, or DENV. The present invention also provides a rapid, sensitive, and consistent method for the specific detection of DENV by employing diagnostic assays having the antigen NS5 which is specifically reactive with anti-DENV antibodies but do not cross-react with antibodies against other flaviviruses such as JEV, SLEV, or WNV. Further, the DENV NS5 antigens are serospecific and do not cross react with antibodies to other DENV strains. Thus, the method of the present invention provides a manner by which to discriminate infections by each DENV strain. Further, diagnostic kits for carrying out the methods are provided. The methods and kits for carrying out the methods of the invention are rapid and require as little as 10 minutes to detect a result. The invention also provides monoclonal antibodies against WNV NS5 and DENV NS5 antigen and their use in detecting WNV and DENV infections in a biological sample.
Owner:HEALTH RES INC
West nile virus vaccine
InactiveUS20050053624A1Decreased neurovirulenceEffective and safe approachSsRNA viruses positive-senseSugar derivativesWest Nile Virus InfectionViral Vaccine
The invention provides chimeric flavivirus vaccines against West Nile virus and methods of using these vaccines to prevent or treat West Nile virus infection.
Owner:SANOFI PASTEUR BIOLOGICS CO
Recombinant vaccine against West Nile Virus
ActiveUS20050255127A1Provide securityPractical and convenientSsRNA viruses positive-senseViral antigen ingredientsAdjuvantRecombinant vaccines
An immunogenic or vaccine composition to induce an immune response or protective immune response against West Nile virus (WNV) in an animal susceptible to WNV. The composition includes a pharmaceutically or veterinarily acceptable vehicle or excipient, and a vector. The vector contains heterologous nucleic acid molecule(s), expresses in vivo in the animal WNV antigen, immunogen or epitope thereof, e.g., WNV E; WNV prM and E; WNV M and E; WNV prM, WNV M and E, WNV polyprotein prM-E, WNV polyprotein M-E, or WNV polyprotein prM-M-E. The composition can contain an adjuvant, such as carbomer. Methods for making and using such a composition, including prime-boost regimes and including as to differential diagnosis, are also contemplated.
Owner:MERIAL LTD
Vaccines Against Japanese Encephalitis Virus and West Nile Virus
ActiveUS20070269458A1Decrease viscerotropism/viremiaHigh genetic stabilitySsRNA viruses positive-senseSugar derivativesViral VaccineWest Nile virus RNA
The invention provides attenuated Flavivirus vaccines, such as vaccines against Japanese encephalitis virus and West Nile virus, as well as methods of making and using these vaccines.
Owner:SANOFI PASTEUR BIOLOGICS CO
Thienopyridine Derivatives for the Treatment and Prevention of Dengue Virus Infections
Methods and pharmaceutical compositions for treating viral infections, by administering certain thienopyridine derivative compounds in therapeutically effective amounts are disclosed. Methods of using the compounds and pharmaceutical compositions thereof are also disclosed. In particular, the treatment of viral infections such as caused by flavivirus is disclosed, i.e., including but not limited to, Dengue virus, West Nile virus, yellow fever virus, Japanese encephalitis virus, and tick-borne encephalitis virus.
Owner:SIGA TECH INC
Antibodies against West Nile Virus and therapeutic and prophylactic uses thereof
ActiveUS20060067940A1Reduce morbidityLimit mortalityImmunoglobulins against virusesAntiviralsAntigenAnti-West Nile virus IgG
The present invention relates to compositions comprising antibodies or fragments thereof that immunospecifically bind to one or more antigens of a flavivirus, particularly of West Nile Virus (WNV), and methods for preventing, treating or ameliorating symptoms associated with a flavivirus, particularly of West Nile Virus (WNV), infection utilizing said compositions. In particular, the present invention relates to methods for preventing, treating or ameliorating symptoms associated with WNV infection, said methods comprising administering to a human subject an effective amount of one or more antibodies or fragments thereof that immunospecifically bind to a WNV antigen. The present invention also relates to detectable or diagnostic compositions comprising antibodies or fragments thereof that immunospecifically bind to a WNV antigen and methods for detecting or diagnosing WNV infection utilizing said compositions.
Owner:WASHINGTON UNIV IN SAINT LOUIS
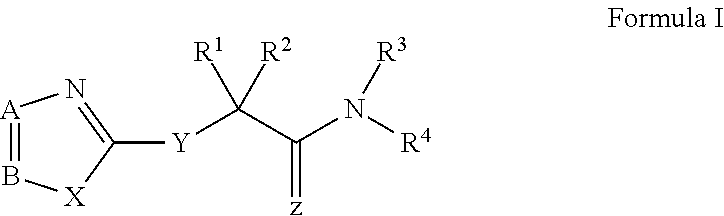
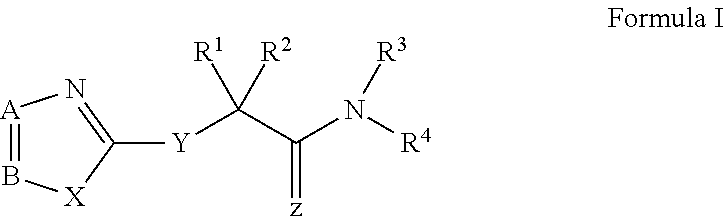
Modified nucleosides for the treatment of viral infections and abnormal cellular proliferation
The disclosed invention is a composition for and a method of treating a Flaviviridae (including BVDV and HCV), Orthomyxoviridae (including Influenza A and B) or Paramyxoviridae (including RSV) infection, or conditions related to abnormal cellular proliferation, in a host, including animals, and especially humans, using a nucleoside of general formula (I)-(XXIII) or its pharmaceutically acceptable salt or prodrug.This invention also provides an effective process to quantify the viral load, and in particular BVDV, HCV or West Nile Virus load, in a host, using real-time polymerase chain reaction (“RT-PCR”). Additionally, the invention discloses probe molecules that can fluoresce proportionally to the amount of virus present in a sample.
Owner:GILEAD SCI INC
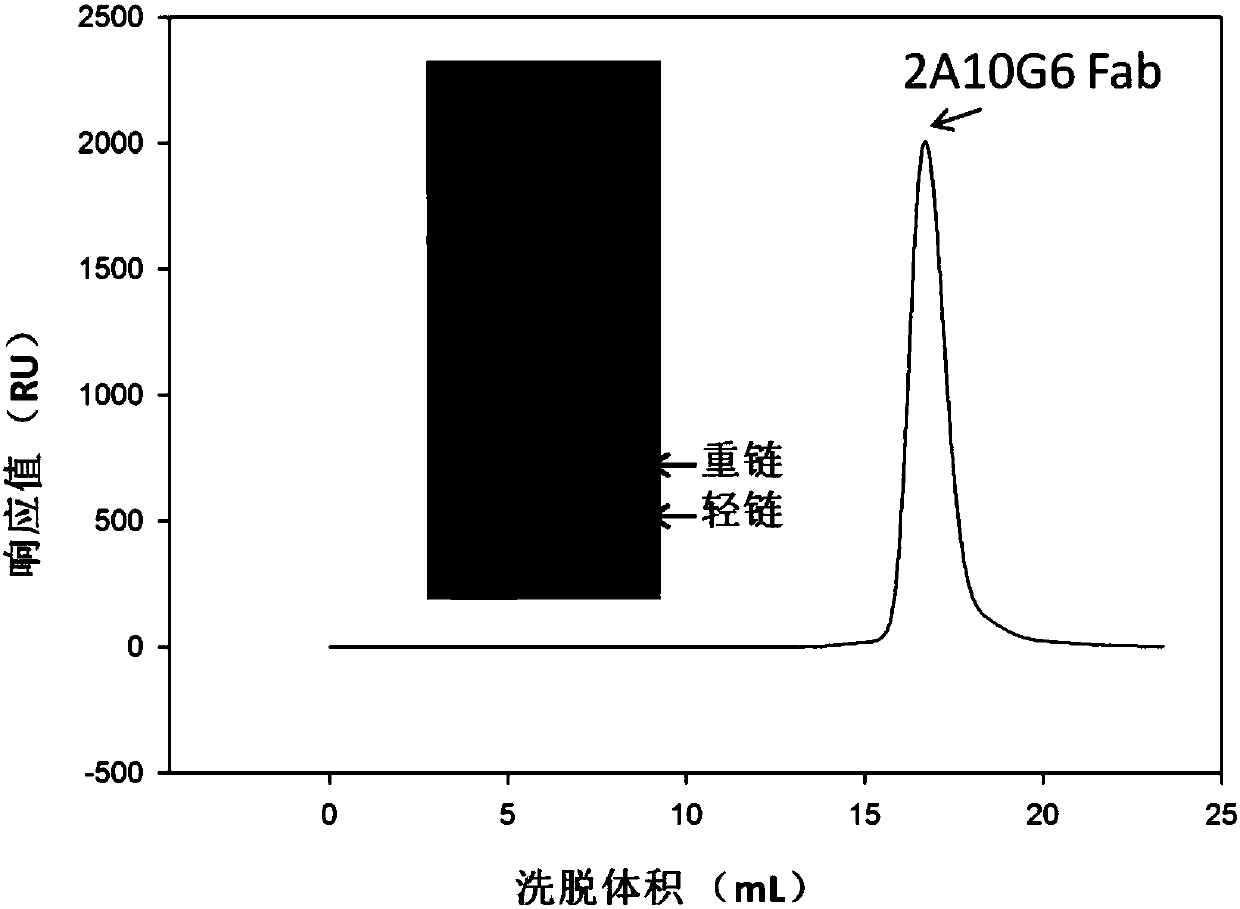
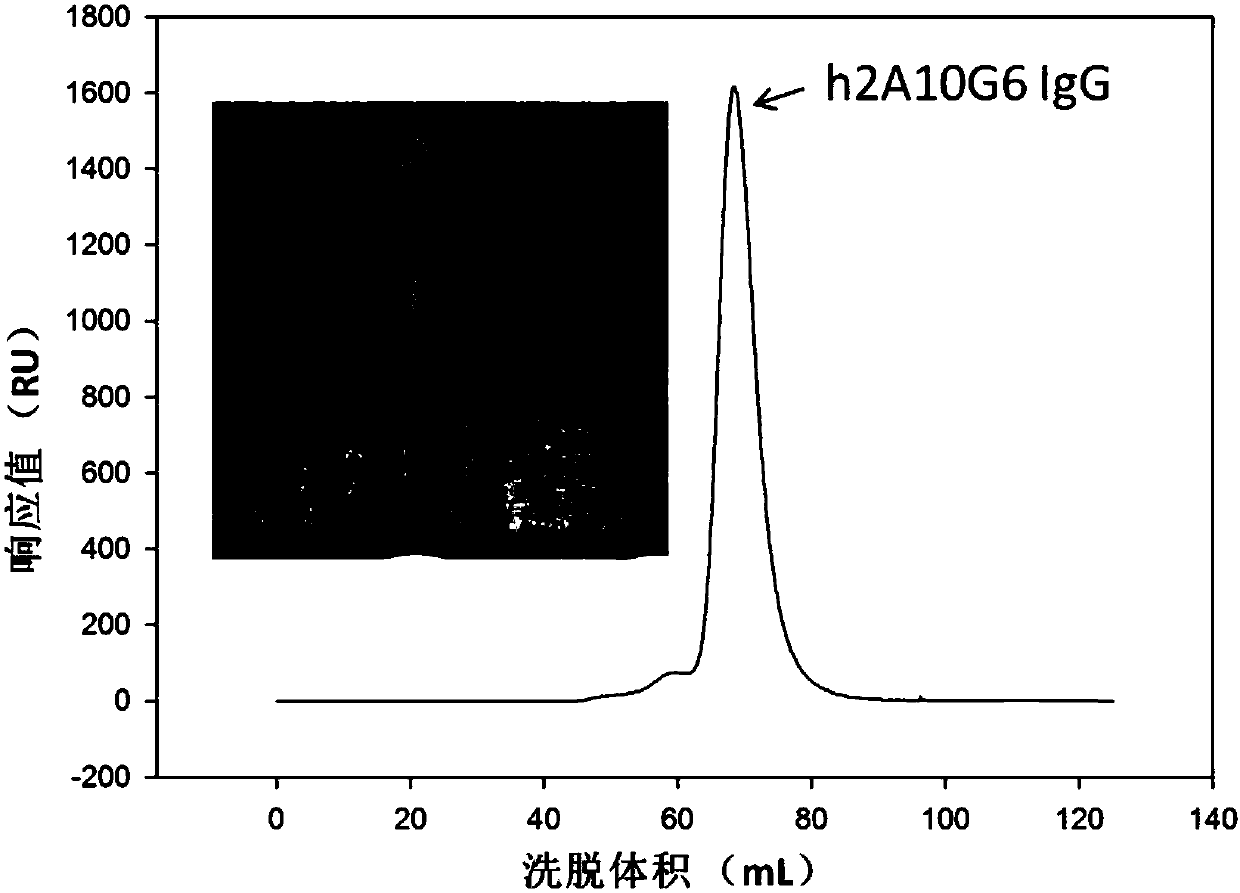
Humanized monoclonal antibody and application thereof
ActiveCN107586335AStrong neutralizing activityImmunoglobulins against virusesAntiviralsBaculovirus expressionHumanized antibody
The present invention discloses a humanized monoclonal antibody and an application thereof, belonging to the technical field of medicine. In the invention, the humanized transformation is carried outon a rat monoclonal antibody 2A10G6, the rat monoclonal antibody 2A10G6 is expressed by baculovirus, and the humanized antibody h2A10G6 is obtained. The h2A10G6 antibody of the present invention has high affinity and neutralization activity against yellow fever virus, dengue fever and West Nile virus, and can be applied to clinical treatment and prevention of yellow fever virus, dengue virus and West Nile virus.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI +1
West nile vaccine
InactiveUS7153513B2Safe and effectiveSuitable for useAntibacterial agentsSsRNA viruses negative-senseEquidaeDisease
The present invention provides a safe and effective vaccine composition against West Nile virus disease. An immunogenically active component of West Nile virus or plasmid DNA, an adjuvant such as a metabolizable oil, and a pharmacologically acceptable carrier are formulated into an immunizing vaccine. The invention also provides a method for the prevention or amelioration of West Nile disease, such as encephalitis, in equidae by administering the vaccine composition herein set forth.
Owner:ZOETIS SERVICE LLC
Antiviral Drugs for Treatment or Prevention of Dengue Infection
Compounds, methods and pharmaceutical compositions for treating viral infections, by administering certain compounds in therapeutically effective amounts are disclosed. Methods for preparing the compounds and methods of using the compounds and pharmaceutical compositions thereof are also disclosed. In particular, the treatment and prophylaxis of viral infections such as caused by flavivirus is disclosed, i.e., including but not limited to, Dengue virus, West Nile virus, yellow fever virus, Japanese encephalitis virus, and tick-borne encephalitis virus.
Owner:SIGA TECH INC
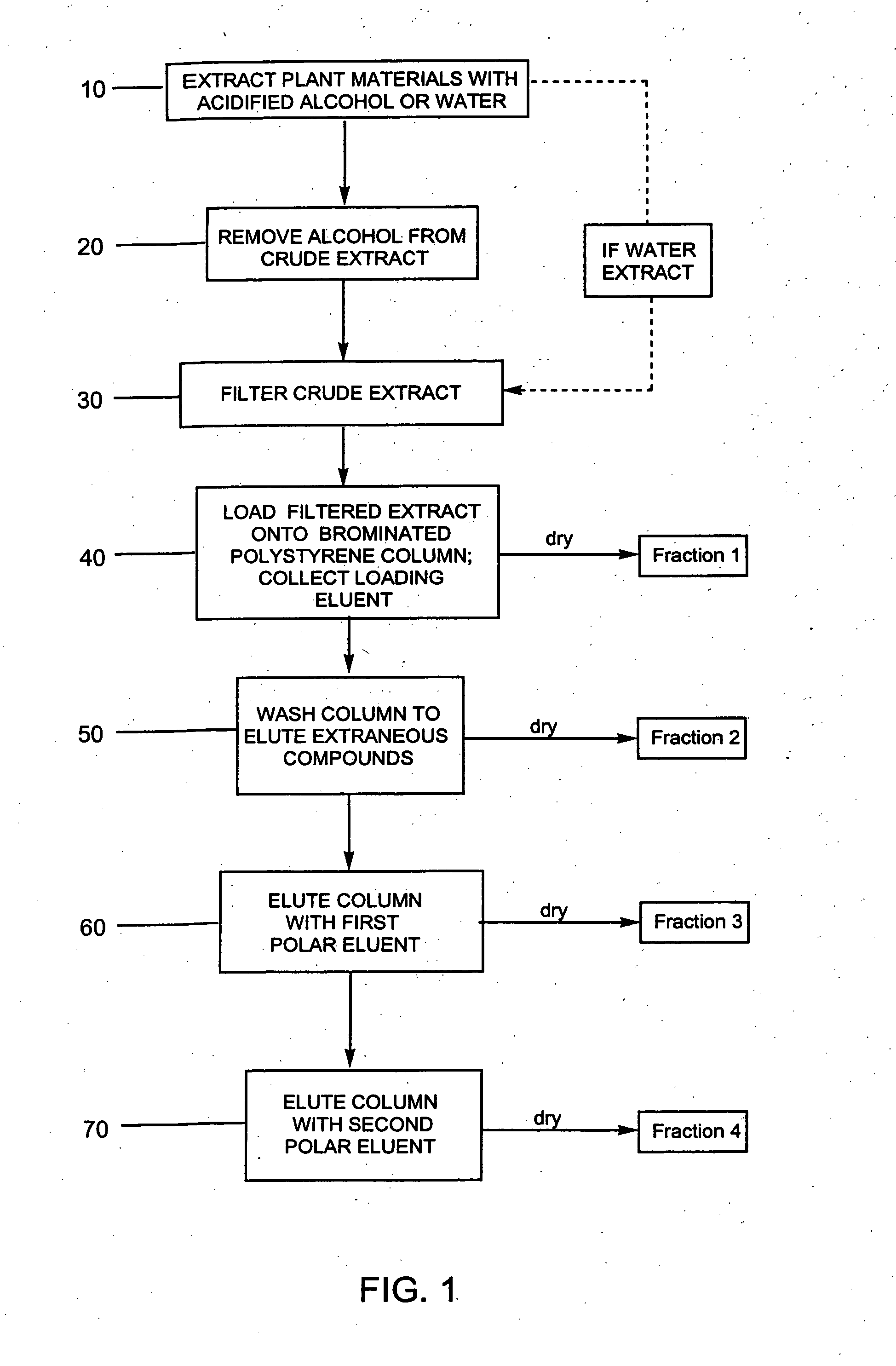
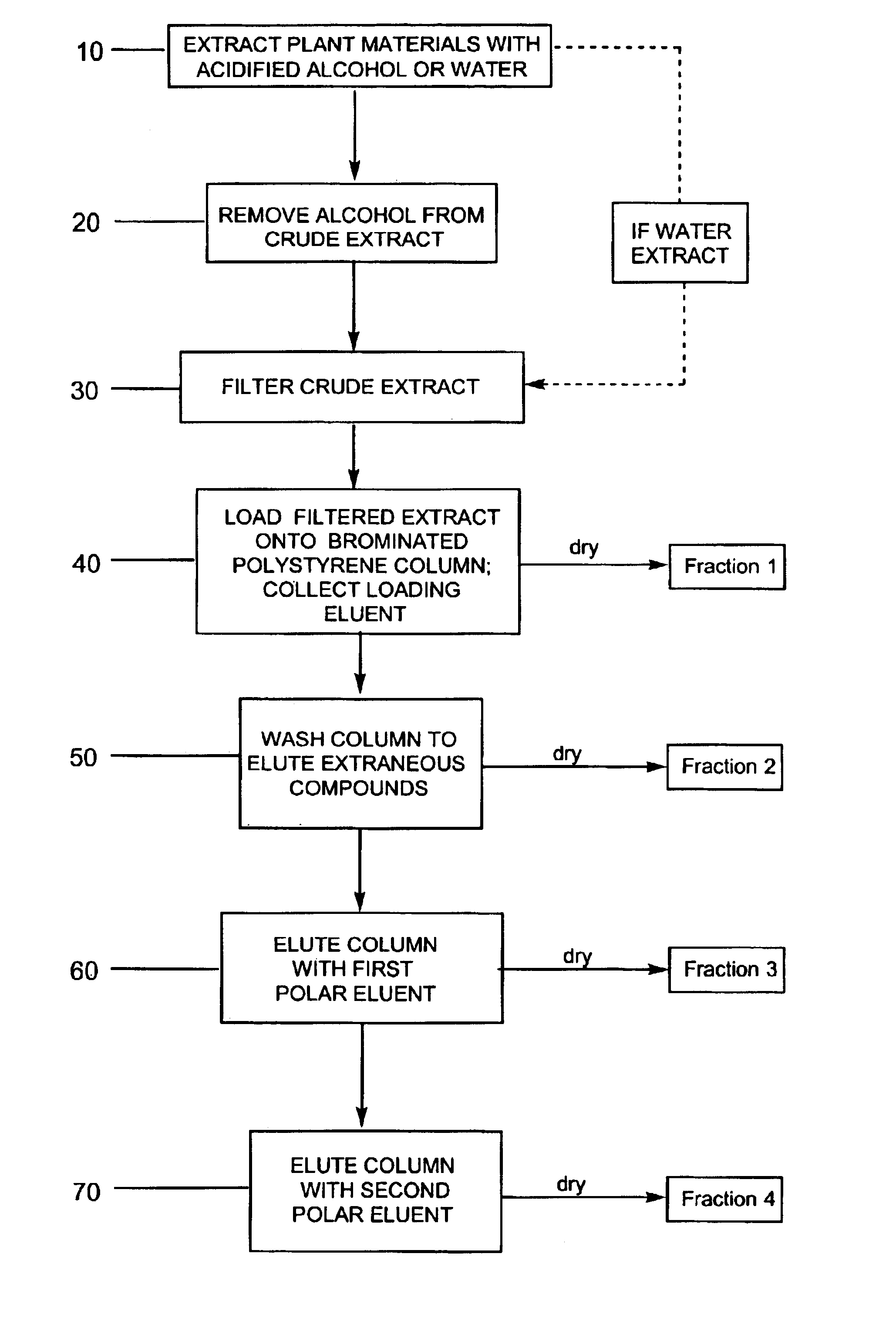
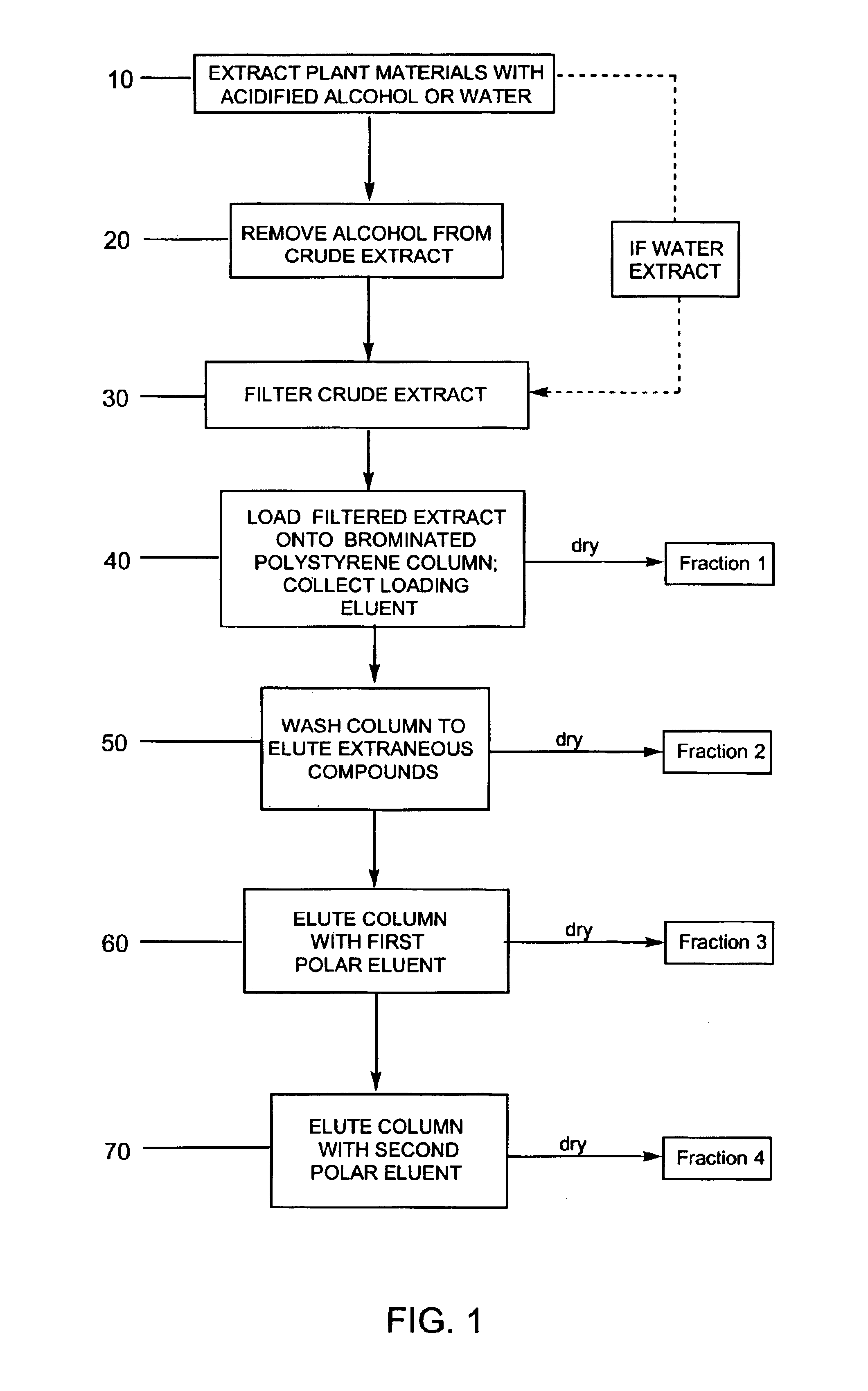
Compositions enriched in phenolic compounds and methods for producing the same
Provided are processes for the preparation of compositions enriched in phenolic compounds from a crude plant extract. One process includes a novel column purification step using a polymer resin that releasably adsorbs the phenolic compounds but does not retain polar non-phenolic compounds, wherein the resin comprises aromatic rings substituted with one or more electron-withdrawing groups. This invention also includes compositions enriched in phenolic compounds. This invention encompasses methods of using the phenolic-enriched compositions for treating warm-blooded animals, including humans, infected with paramyxovaridae such as respiratory syncytial virus, orthomyoxovaridae such as influenza A, B, and C, parainfluenza, Herpes viruses such as HSV-1 and HSV-2, and Flaviviruses such as West Nile Virus, and for treating inflammation such as caused by arthritis, stress and digestive disease. The compositions are also useful as meat additives to inhibit food-borne pathogens.
Owner:PHENOLICS LLC
Samll molecule inhibitors for the treatment or prevention of dengue virus infection
Methods and pharmaceutical compositions for treating viral infections, by administering certain compounds in therapeutically effective amounts are disclosed. Methods of using the compounds and pharmaceutical compositions thereof are also disclosed. In particular, the treatment and prophylaxis of viral infections such as caused by flavivirus is disclosed, i.e., including but not limited to, Dengue virus, West Nile virus, yellow fever virus, Japanese encephalitis virus, and tick-borne encephalitis virus.
Owner:SIGA TECH INC
Humanized antibodies against West Nile Virus and therapeutic and prophylactic uses thereof
InactiveUS7572456B2Easy to neutralizeEasy to removeCosmetic preparationsToilet preparationsHumanized antibodyViral infection
The present invention relates to compositions comprising humanized antibodies or fragments thereof that immunospecifically bind to one or more antigens of a flavivirus, particularly of West Nile Virus (WNV) and methods for preventing, treating or ameliorating symptoms associated with a flavivirus, particularly of West Nile Virus (WNV) infection utilizing said compositions. In particular, the present invention relates to methods for preventing, treating or ameliorating symptoms associated with WNV infection, said methods comprising administering to a human subject an effective amount of one or more humanized antibodies or fragments thereof that immunospecifically bind to a WNV antigen. The present invention also relates to detectable or diagnostic compositions comprising humanized antibodies or fragments thereof that immunospecifically bind to a WNV antigen and methods for detecting or diagnosing WNV infection utilizing said compositions.
Owner:MACROGENICS INC
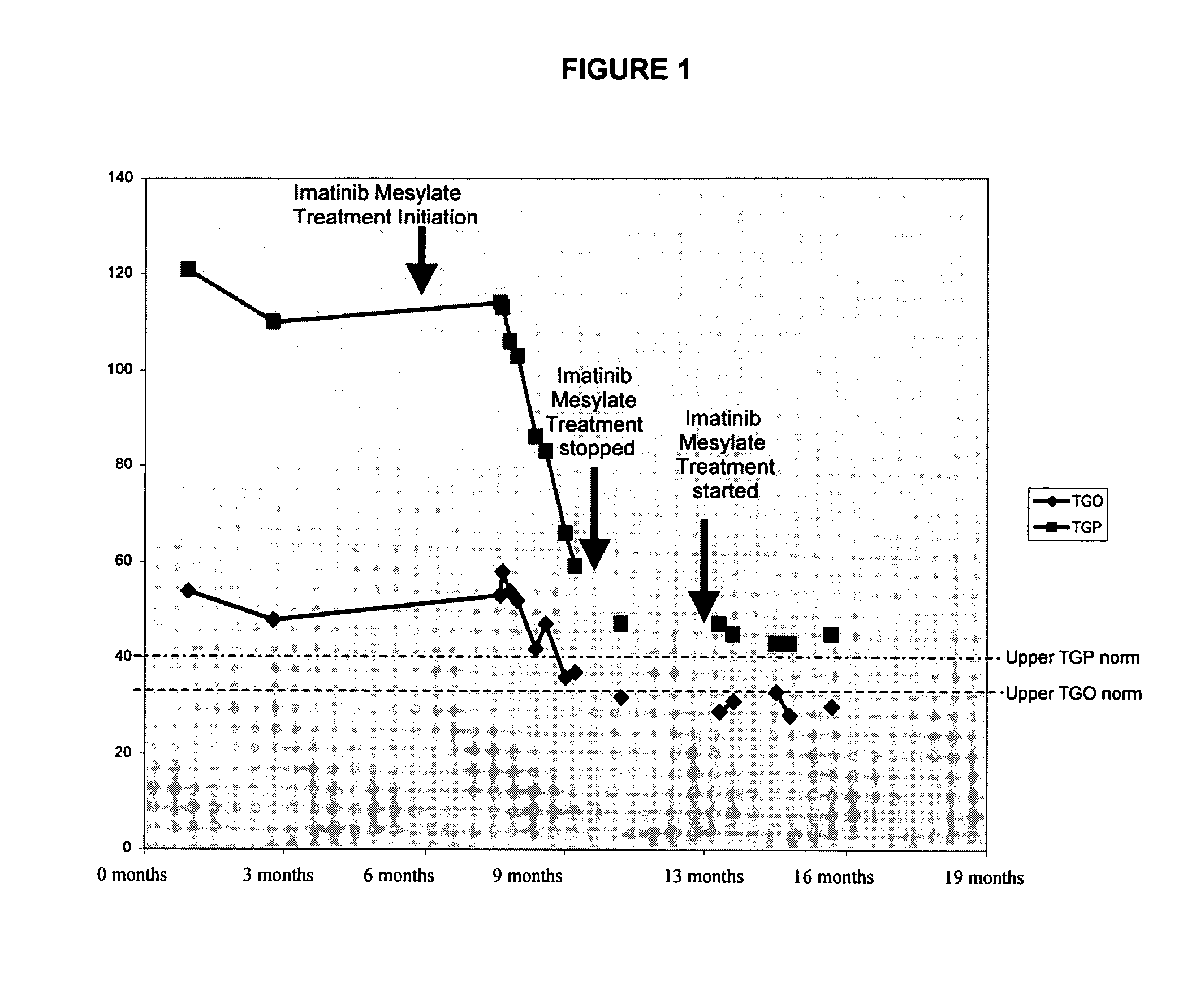
Use of imatinib to treat liver disorders and viral infections
The present invention relates to the use of imatinib for treating viral liver diseases and in particular for viral hepatitis. The invention provides the use of imatinib for inhibiting replication, transmission or both of hepatitis viruses. The invention further relates to the use of imatinib for inhibiting replication, transmission or both of other viruses including herpes virus, poxvirus, influenza virus, para influenza virus, respiratory syncytial virus, rhinovirus, yellow fever virus, west nile virus, and encephalitis virus.
Owner:BIONICHE LIFE SCI
Efficient method for producing compositions enriched in total phenols
This invention provides a process for the preparation of compositions enriched in total phenols from a crude plant extract. The process includes a novel column purification step using a brominated polystyrene resin. This invention also includes compositions enriched in total phenols. The enriched compositions are characterized as containing monomeric, oligomeric and polymeric phenols and having HPLC chromatograms substantially as set forth in FIGS. 10-13. This invention encompasses methods of using the total phenol-enriched compositions for treating warm-blooded animals, including humans, infected with paramyxovaridae such as respiratory syncytial virus, orthomyoxovaridae such as influenza A, B, and C, parainfluenza, Herpes viruses such as HSV-1 and HSV-2, and Flaviviruses such as West Nile Virus, and for treating inflammation such as caused by arthritis, stress and digestive disease.
Owner:PHENOLICS LLC
Kit for rapid joint detection of epidemic JEV, DEV and WNV and detection method thereof
InactiveCN101629215AStrong specificityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesDeoxyribonucleoside triphosphateDensovirus
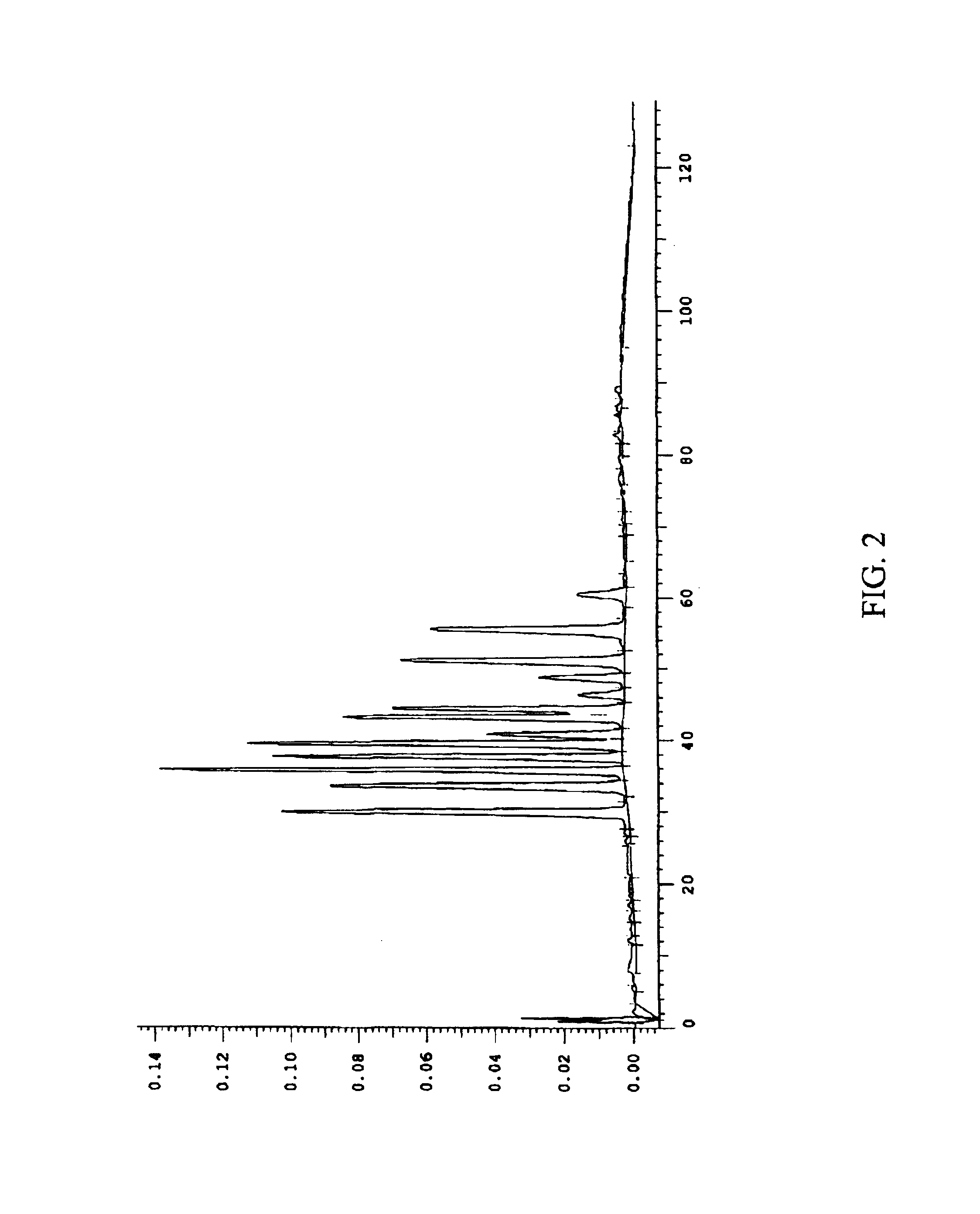
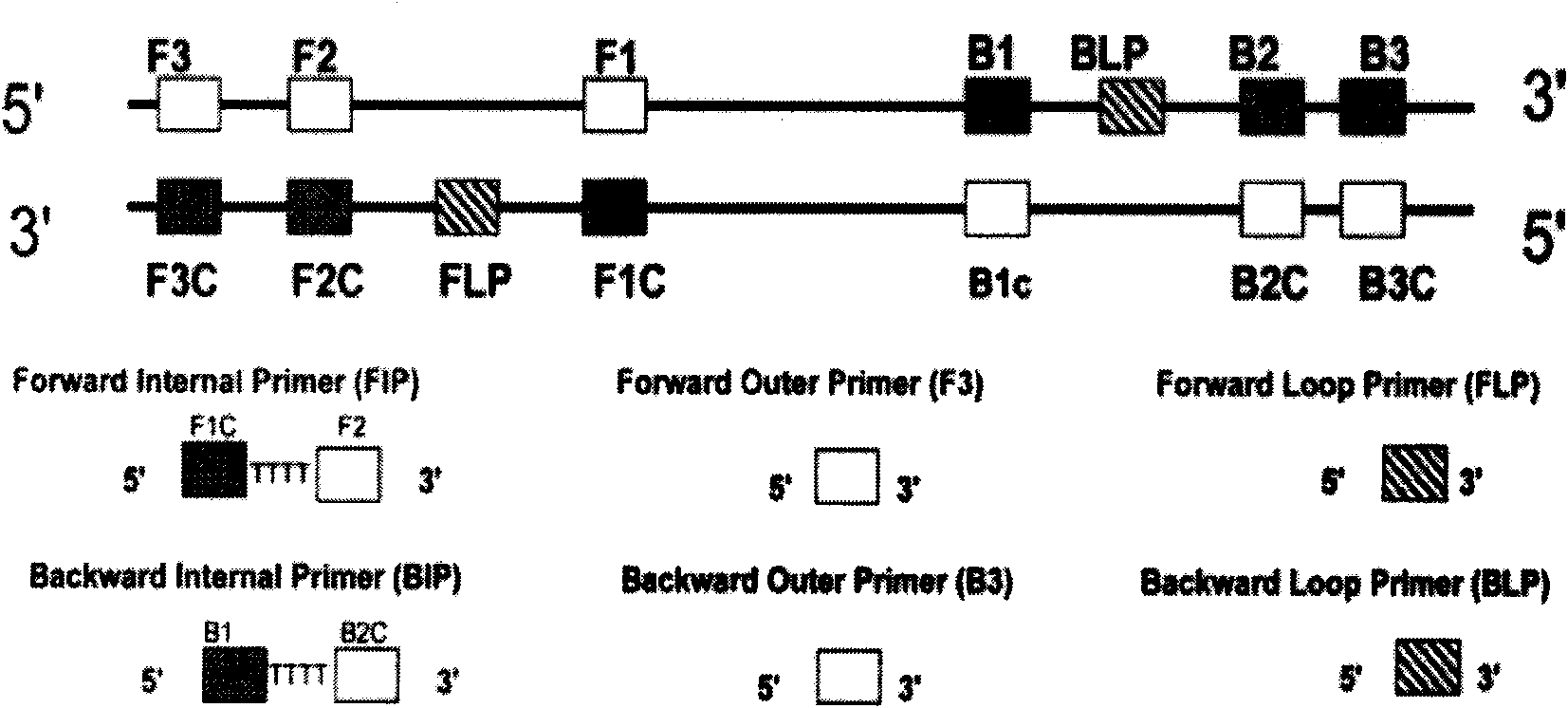
The invention relates to a kit for the rapid joint detection of epidemic JEV (Japanese Encephalitis Virus), DEV (Dengue Virus) and WNV (West Nile Virus). The kit consists of a main reaction solution and specific primers, wherein, the main reaction solution comprises a reaction buffer, AMV (Avian Myeloblastosis Virus) reverse transcriptase, a nuclease inhibitor, Bst (Bacillus stearothermophilus) DNA polymerase, dNTP (deoxyribonucleoside triphosphate), magnesium sulfate, betaine and DEPC (diethylpyrocarbonate) and conditioning water; and the specific primers comprise a specific amplification primer of JEV (PJEV), a specific amplification primer of DEV (PDV) and a specific amplification primer of WNV (PWNV). The invention is capable of rapidly detecting three viruses of flaviviridae by employing the LAMP (Loop-mediated Isothermal Amplification) technology and designing high-degree specific primers, thereby achieving the purpose of highly-efficient specific amplification. The invention further provides a detection method in which an ultraviolet-visible spectrophotometer is utilized for detecting the absorbance value of the reaction system and for indirectly reflecting the DNA amplification of target genes. Therefore, the invention is applicable to rapid detection at primary and field levels and is of greater application value.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Infectious DNA as a vaccine against west nile and other flavivirues
InactiveUS20050276816A1Easy to prepareStable and safe vaccineSsRNA viruses positive-senseViral antigen ingredientsDna encodingInfectious agent
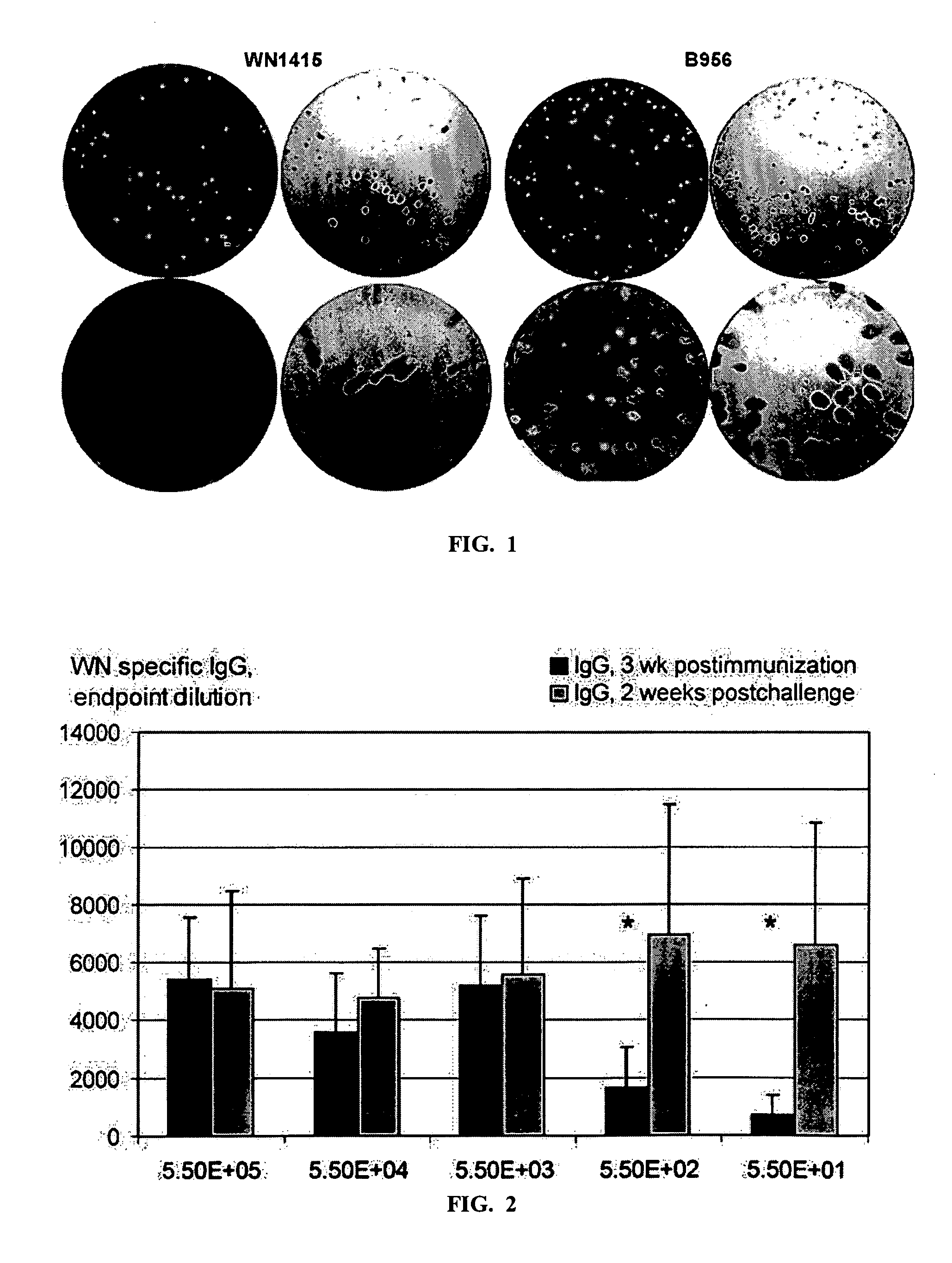
A vaccine for West Nile virus that protects a subject against West Nile infection comprising an a pharmaceutically acceptable carrier and a therapeutically effective does of an infectious agent selected from the group consisting of: a live attenuated infectious (+) RNA virus designated as WN1415, a vector comprising infectious DNA encoding an infectious (+) RNA molecule encoding the West Nile virus, and the West Nile (+) RNA virus designated as WN956D117B3 (GenBank #M12294).
Owner:UNIVERSITY OF KANSAS
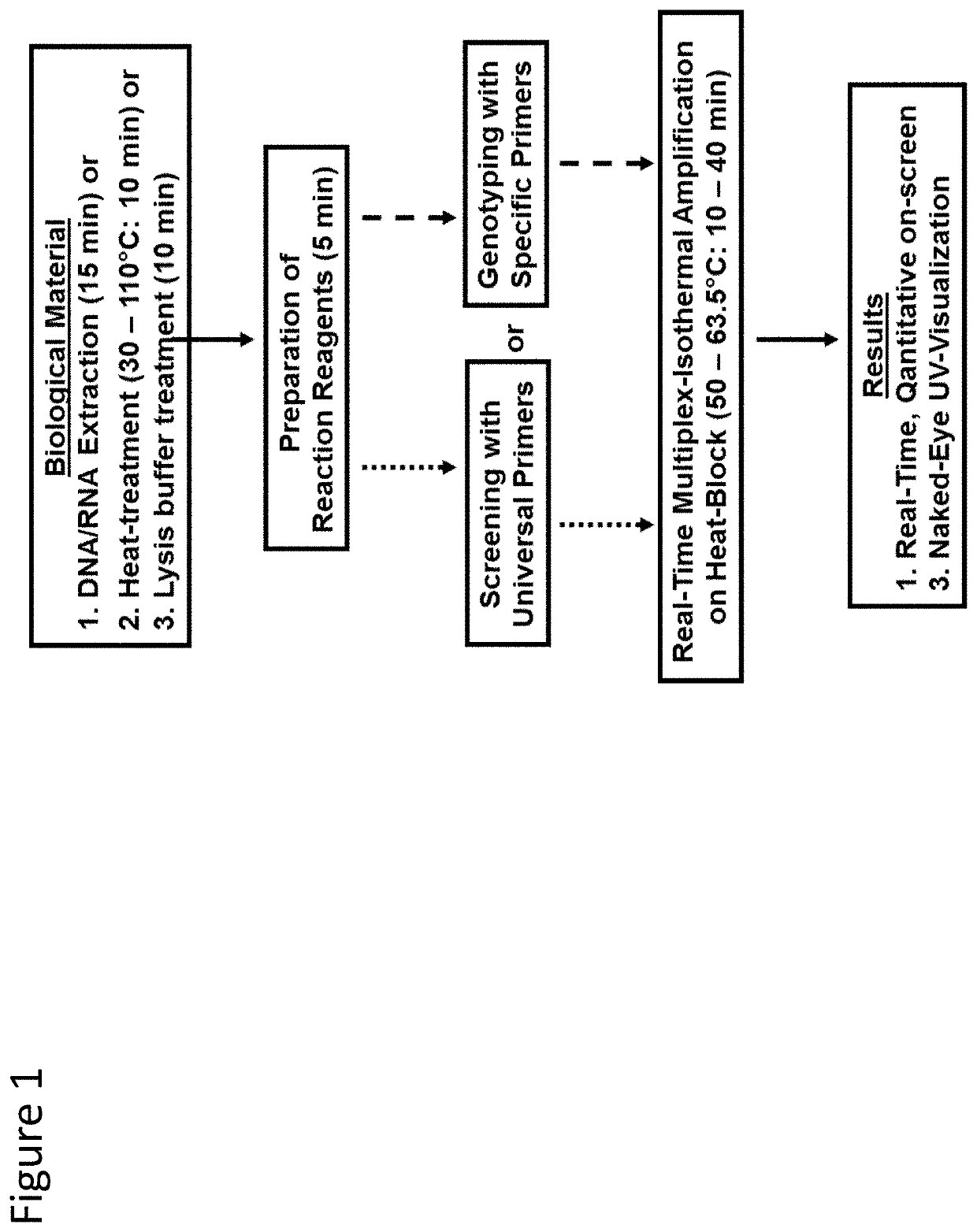
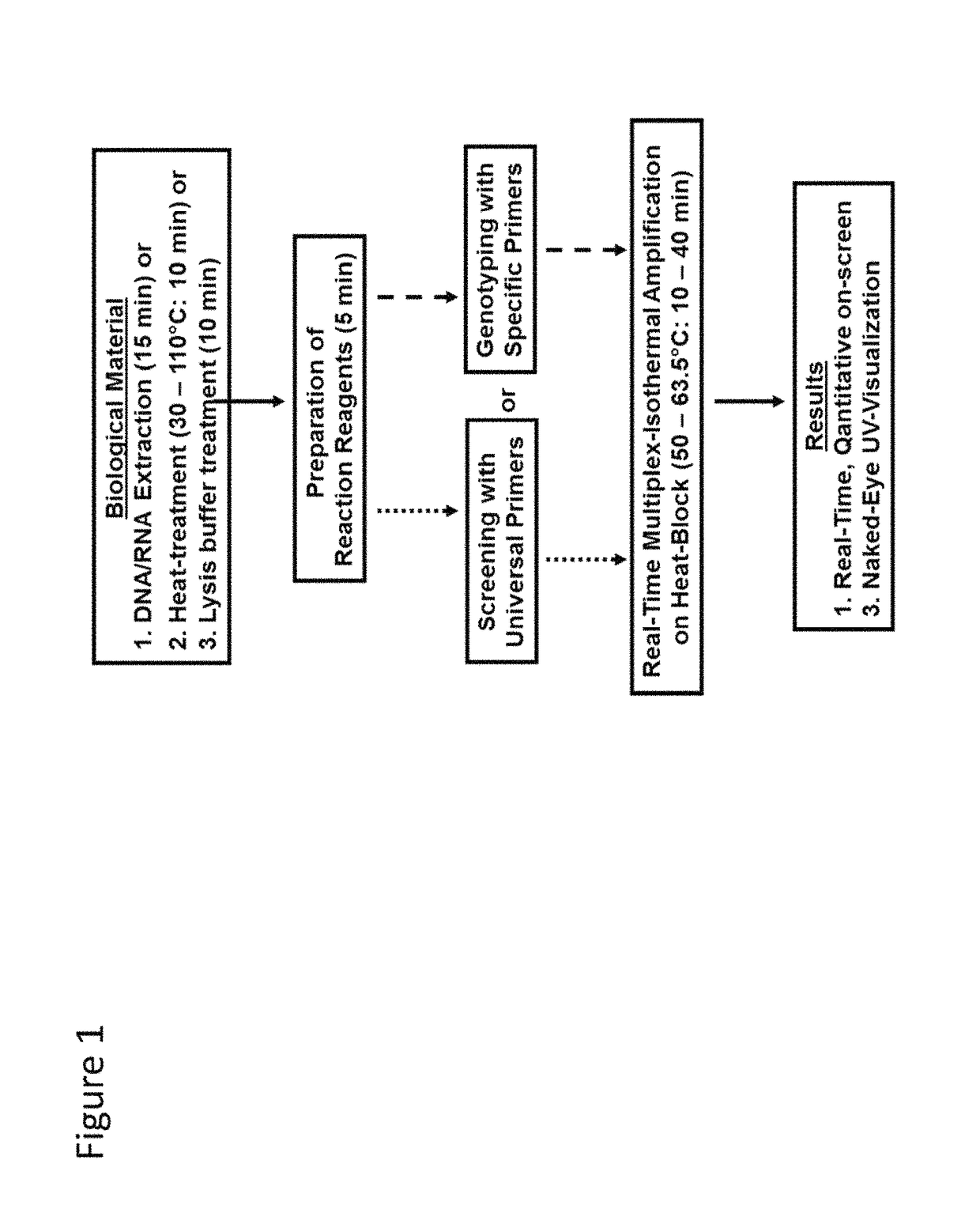
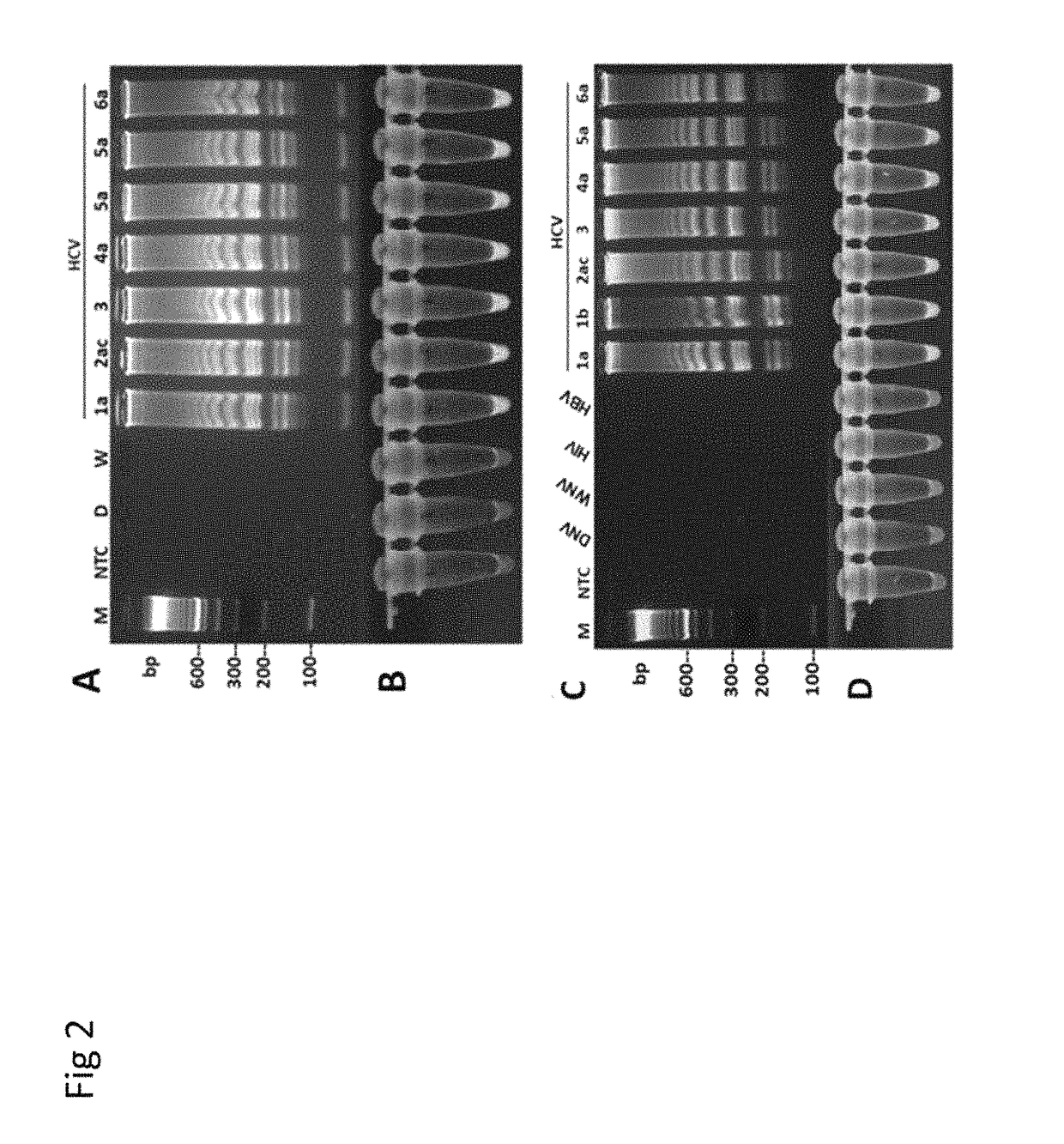
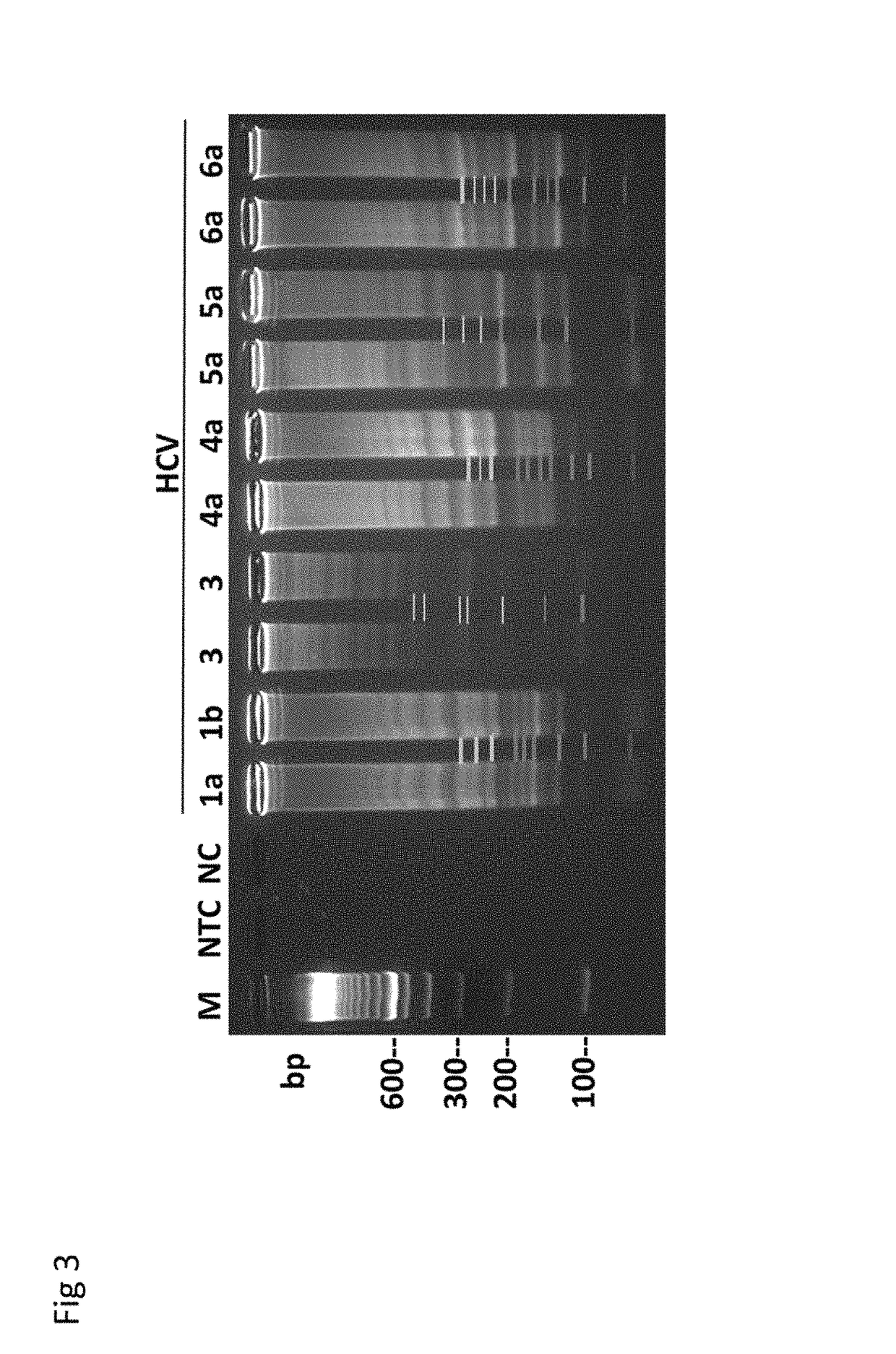
Methods for real-time multiplex isothermal detection and identification of bacterial, viral, and protozoan nucleic acids
ActiveUS20200048722A1Flexible processFacilitate strand displacementMicrobiological testing/measurementAgainst vector-borne diseasesProtozoaLoop-mediated isothermal amplification
Herein disclosed are rapid real-time isothermal multiplex methods of detecting, identifying and quantifying bacterial, viral, and protozoan nucleic acids in a sample. These include contacting the sample with two or more sets of pathogen-specific reverse transcription loop-mediated isothermal amplification primers and novel oligofluorophores specific for the target bacterial, viral, and parasitic nucleic acids of interest such as human immunodeficiency virus, Ebola virus, Marburg virus, Yellow fever virus, hepatitis-B virus, Lassa fever virus, Plasmodium, hepatitis-C virus, hepatitis-E virus, dengue virus, Chikungunya virus, Japanese Encephalitis virus, Middle Eastern Respiratory Syndrome Corona virus, Mycobacterium, West Nile virus, Cytomegalovirus, Parvovirus, Leishmania, Trypanosoma, and Zika virus nucleic acids, under conditions sufficient to produce detectable real-time amplification signals in about 10 to 40 minutes. The amplification signals are produced by pathogen-specific fluorogenic labels included in one or more of the primers. Also, novel reaction and sample lysis buffers, primers, and kits for rapid multiplex detection, quantification, and identification of bacterial, viral, and protozoan nucleic acids by real-time isothermal amplification are herein disclosed.
Owner:NYAN DOUGBEH CHRIS
Bispecific monoclonal antibody therapeutics against West Nile virus with improved CNS penetration
ActiveUS9499608B2Hybrid immunoglobulinsImmunoglobulins against virusesTherapeutic antibodyBispecific monoclonal antibody
Owner:ARIZONA STATE UNIVERSITY
Methods for real-time multiplex isothermal detection and identification of bacterial, viral, and protozoan nucleic acids
ActiveUS10072309B1Flexible processFacilitate strand displacementMicrobiological testing/measurementAgainst vector-borne diseasesProtozoaFluorescence
Herein disclosed are rapid real-time isothermal multiplex methods of detecting, identifying and quantifying bacterial, viral, and protozoan nucleic acids in a sample. These include contacting the sample with two or more sets of pathogen-specific reverse transcription loop-mediated isothermal amplification primers and novel oligofluorophores specific for the target bacterial, viral, and parasitic nucleic acids of interest such as human immunodeficiency virus, Ebola virus, Marburg virus, Yellow fever virus, hepatitis-B virus, Lassa fever virus, Plasmodium, hepatitis-C virus, hepatitis-E virus, dengue virus, Chikungunya virus, Japanese Encephalitis virus, Middle Eastern Respiratory Syndrome Corona virus, Mycobacterium, West Nile virus, Cytomegalovirus, Parvovirus, Leishmania, Trypanosoma, and Zika virus nucleic acids, under conditions sufficient to produce detectable real-time amplification signals in about 10 to 40 minutes. The amplification signals are produced by pathogen-specific fluorogenic labels included in one or more of the primers. Also, novel reaction and sample lysis buffers, primers, and kits for rapid multiplex detection, quantification, and identification of bacterial, viral, and protozoan nucleic acids by real-time isothermal amplification are herein disclosed.
Owner:NYAN DOUGBEH CHRIS
Monoclonal antibody of membrane protein E for resisting West Nile virus and application thereof
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com