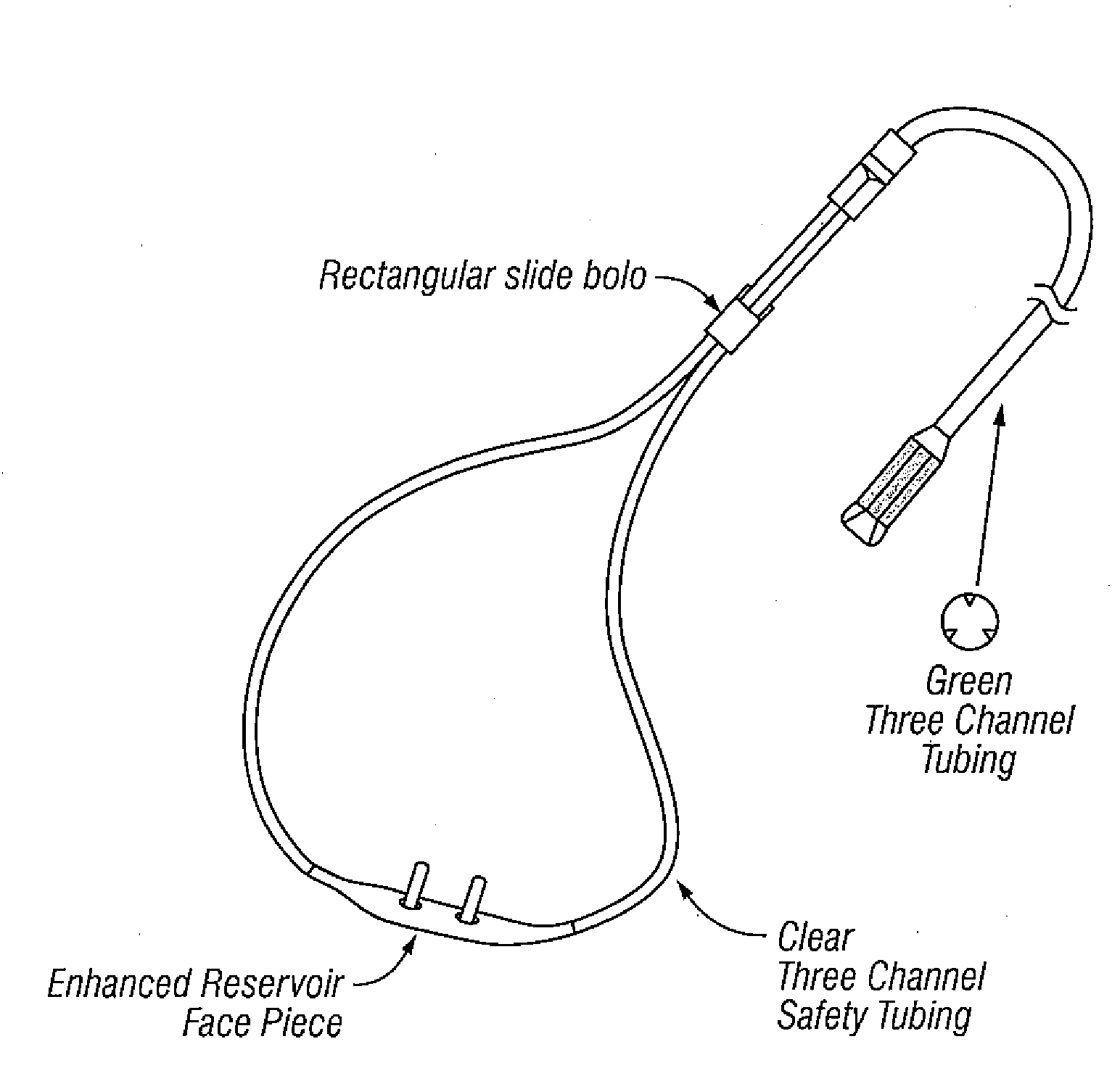
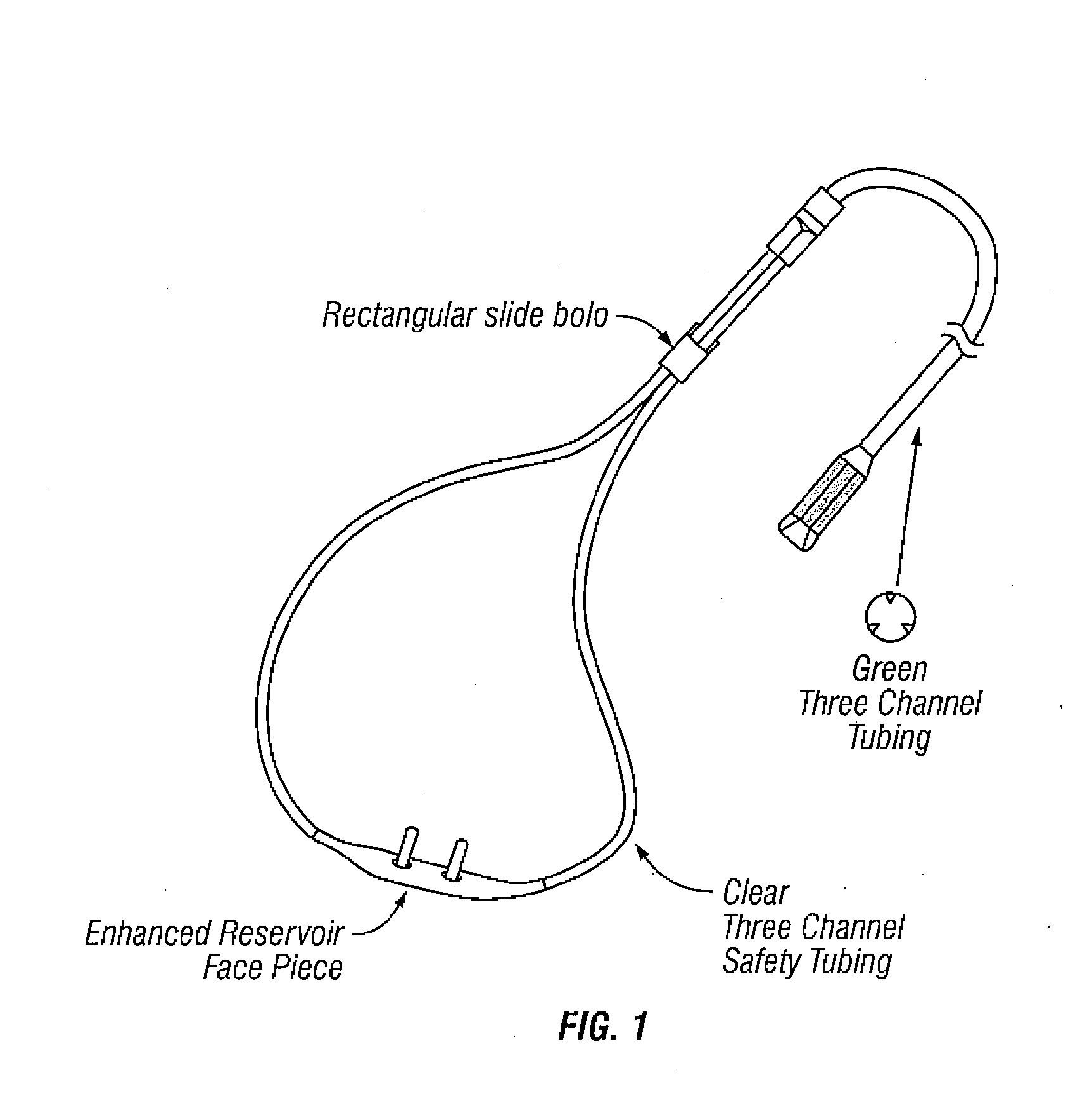
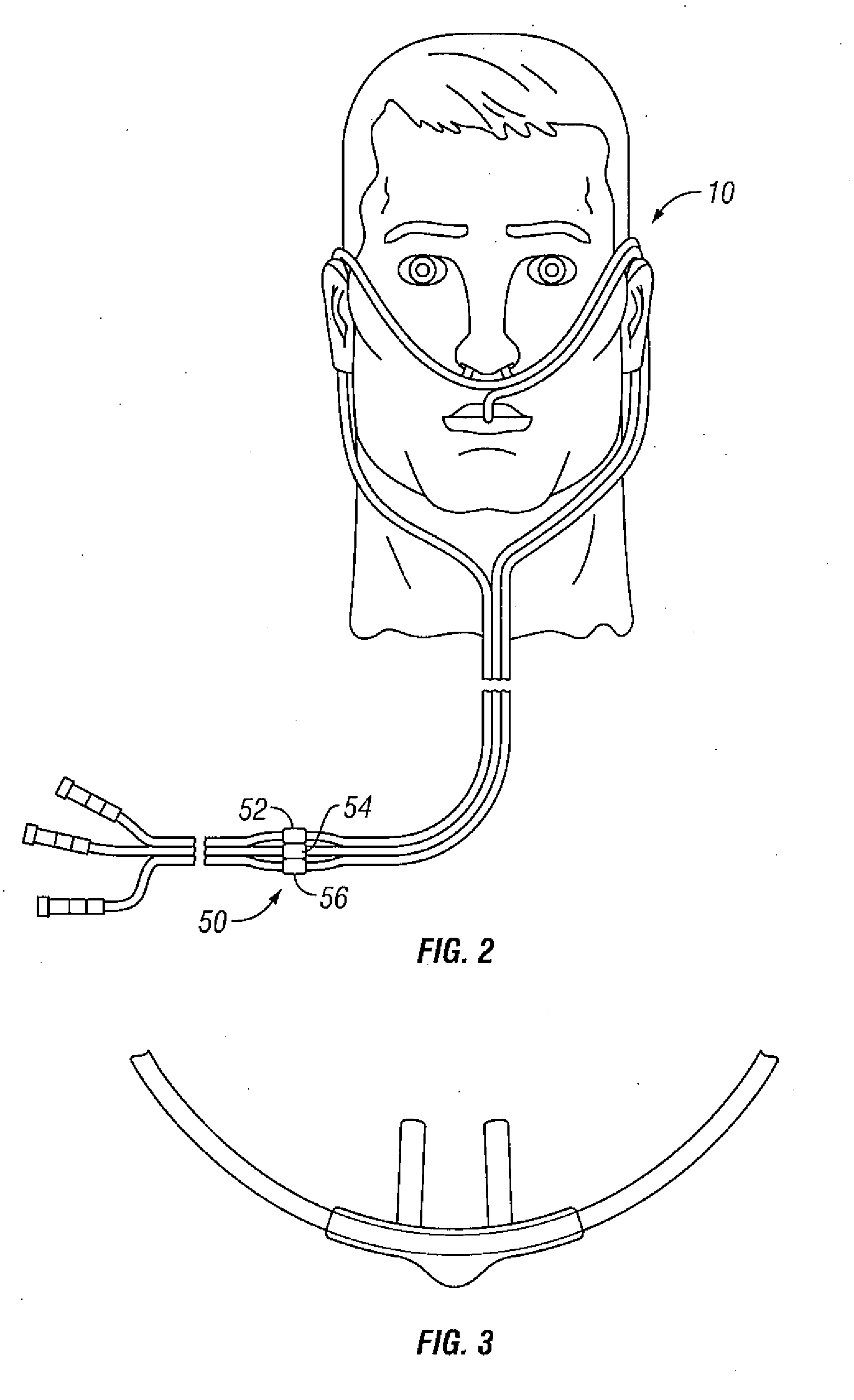
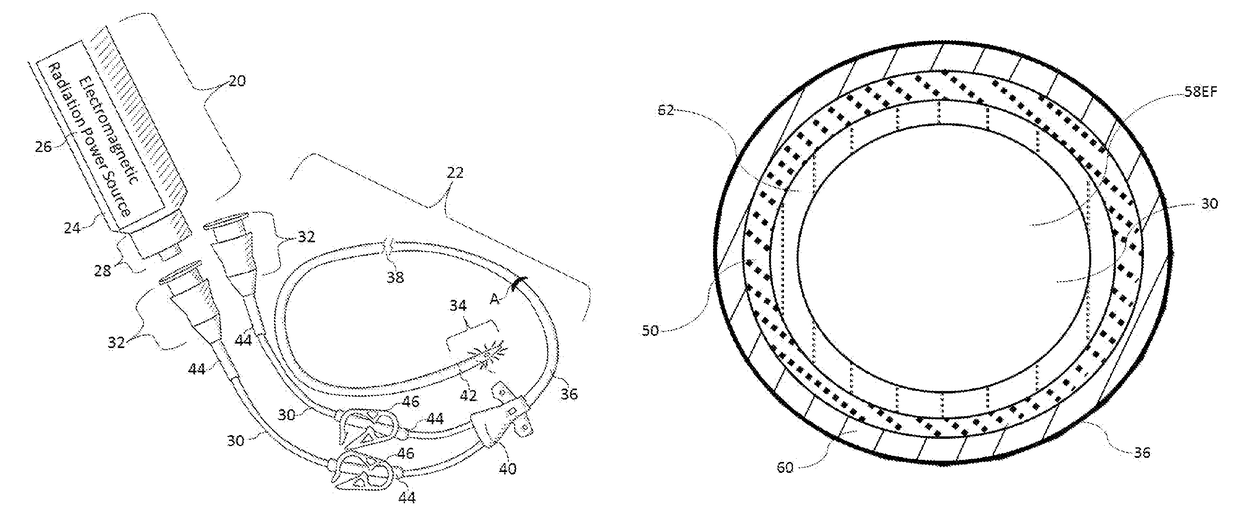
Patents
Literature
482 results about "Infectious agent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An infectious agent is something that infiltrates another living thing, like you. When an infectious agent hitches a ride, you have officially become an infected host. There are four main classes of infectious agents: bacteria, viruses, fungi, and parasites.
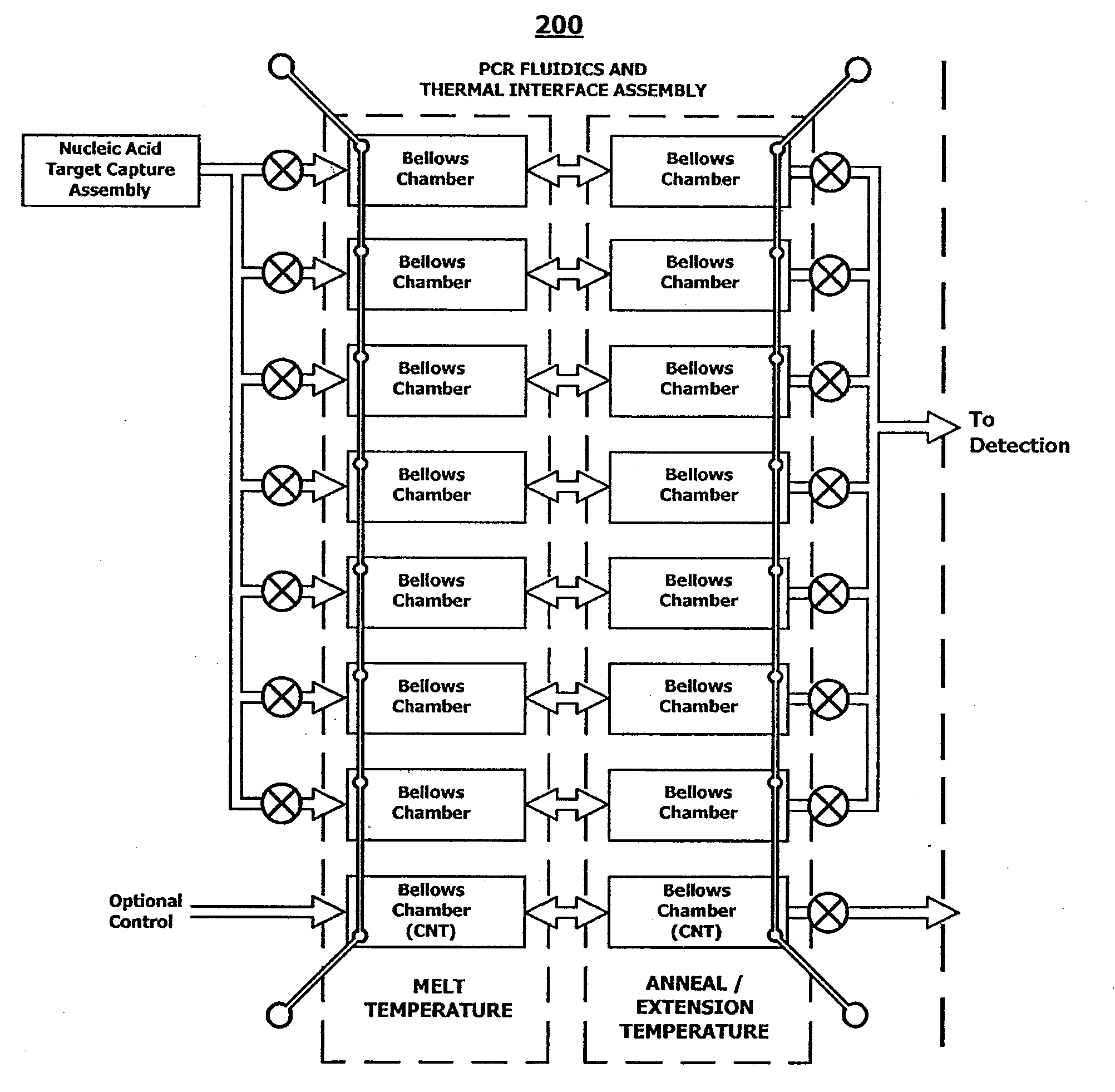
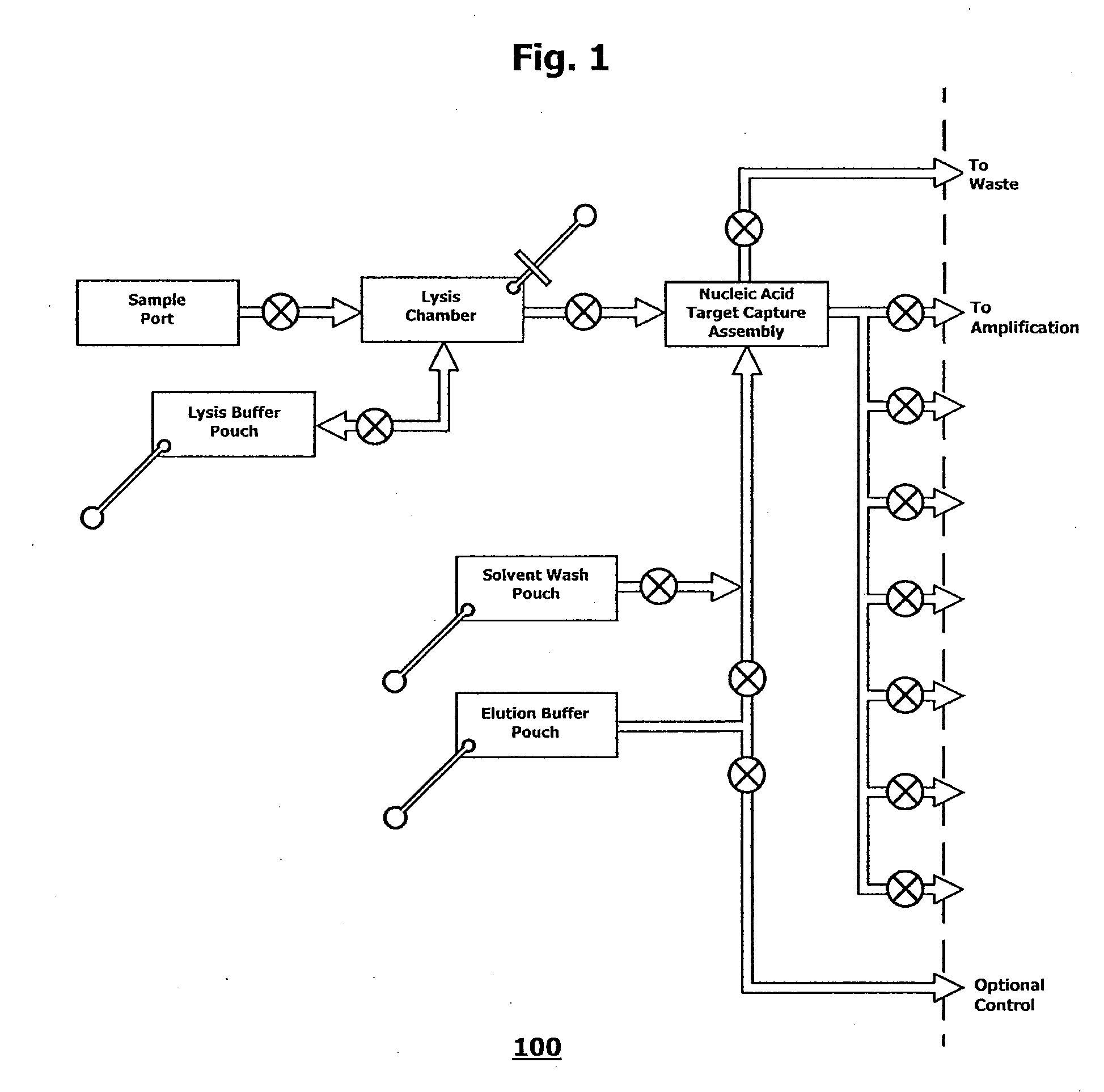
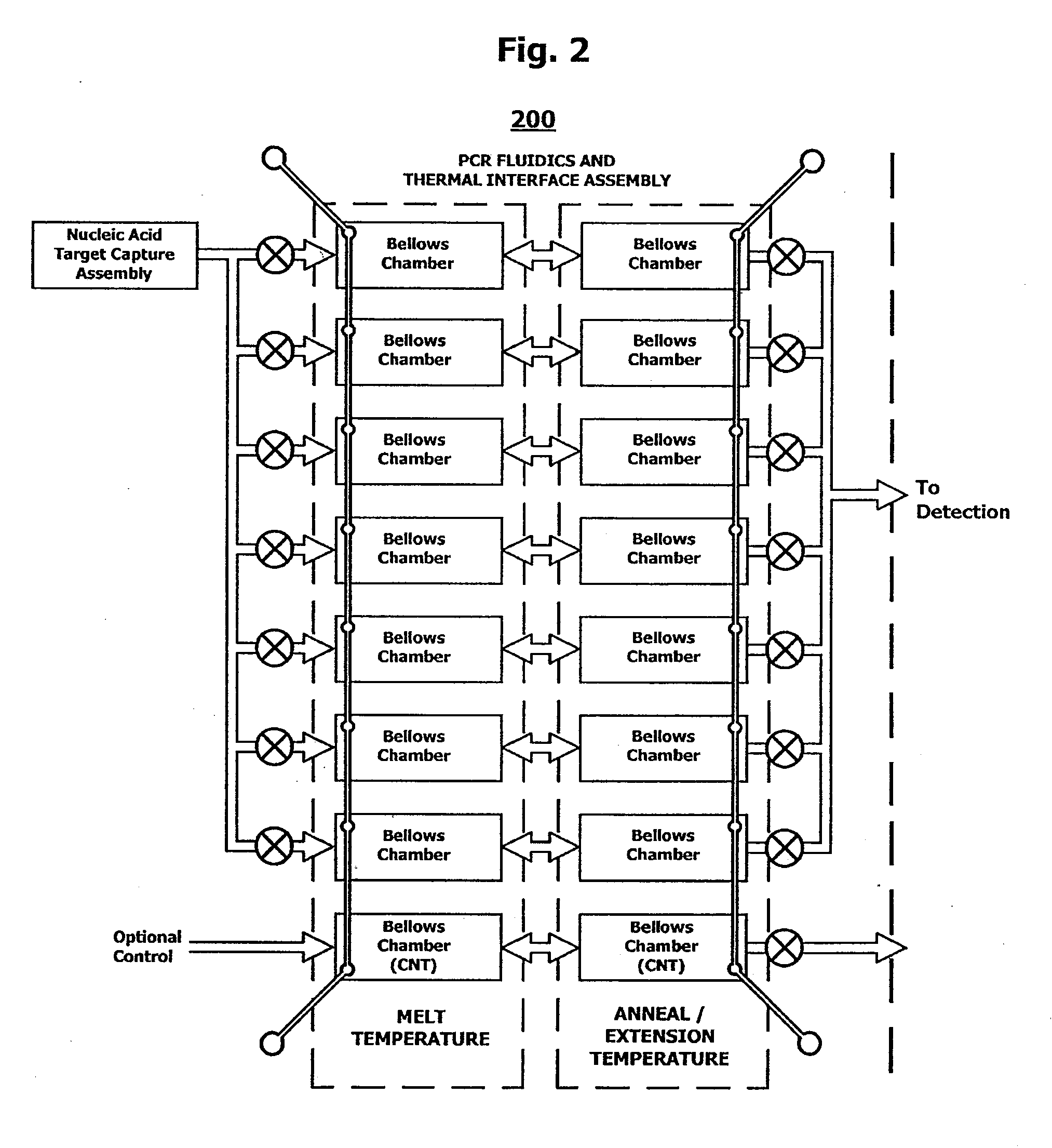
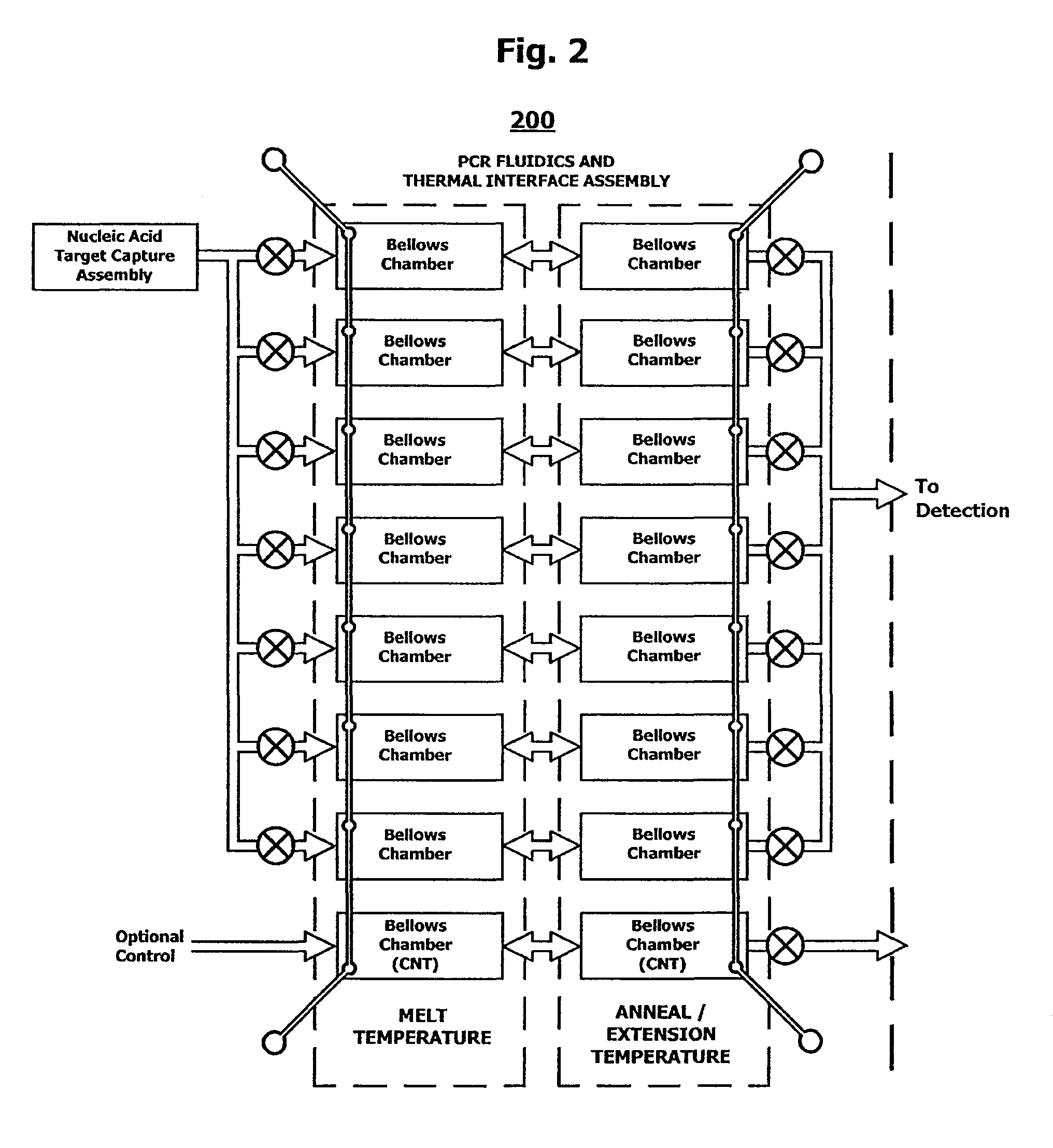
Integrated nucleic acid assays
ActiveUS20090148933A1Reduce pervasive dangerEasy to useBioreactor/fermenter combinationsHeating or cooling apparatusCancer cellAssay
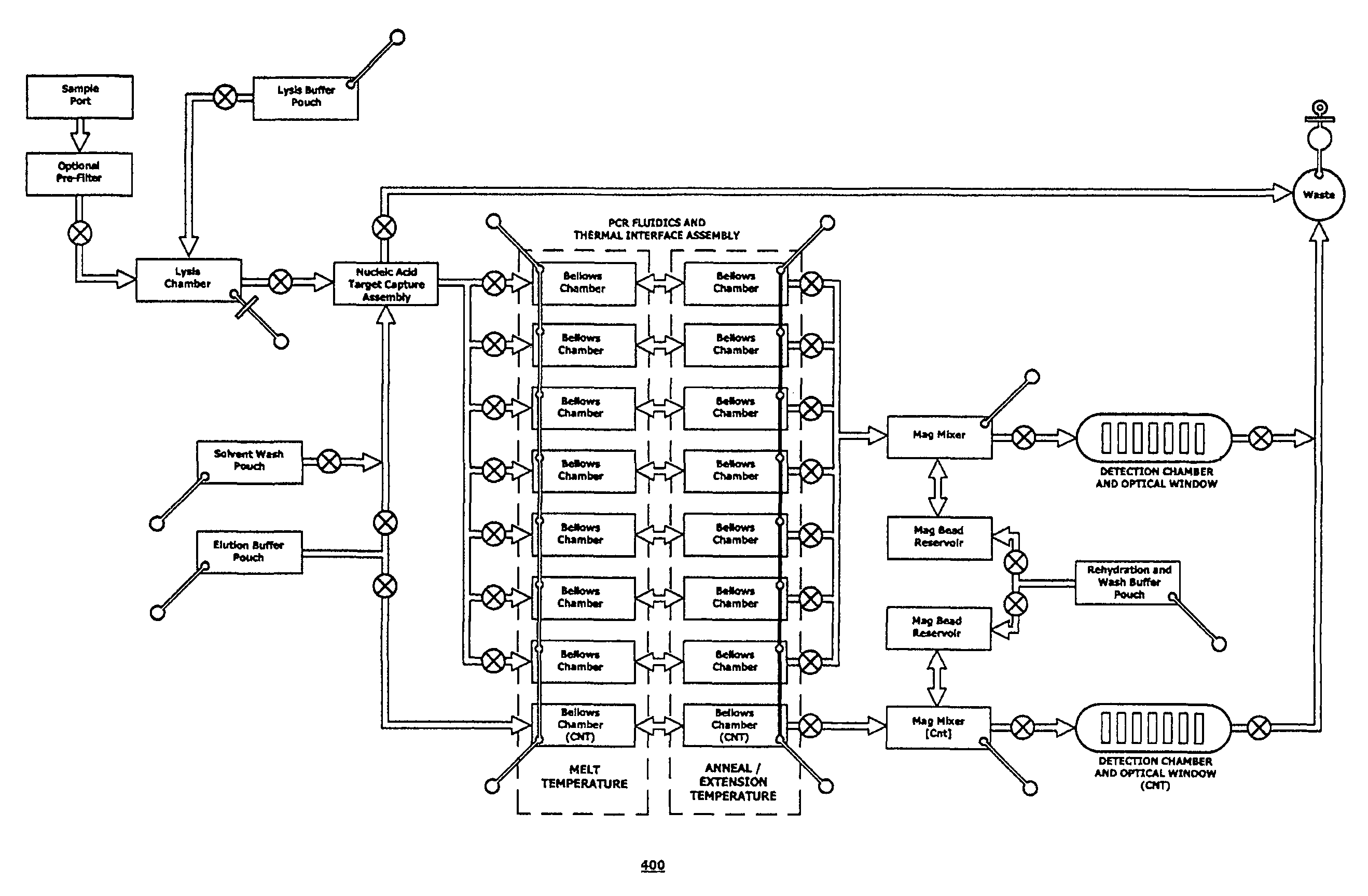
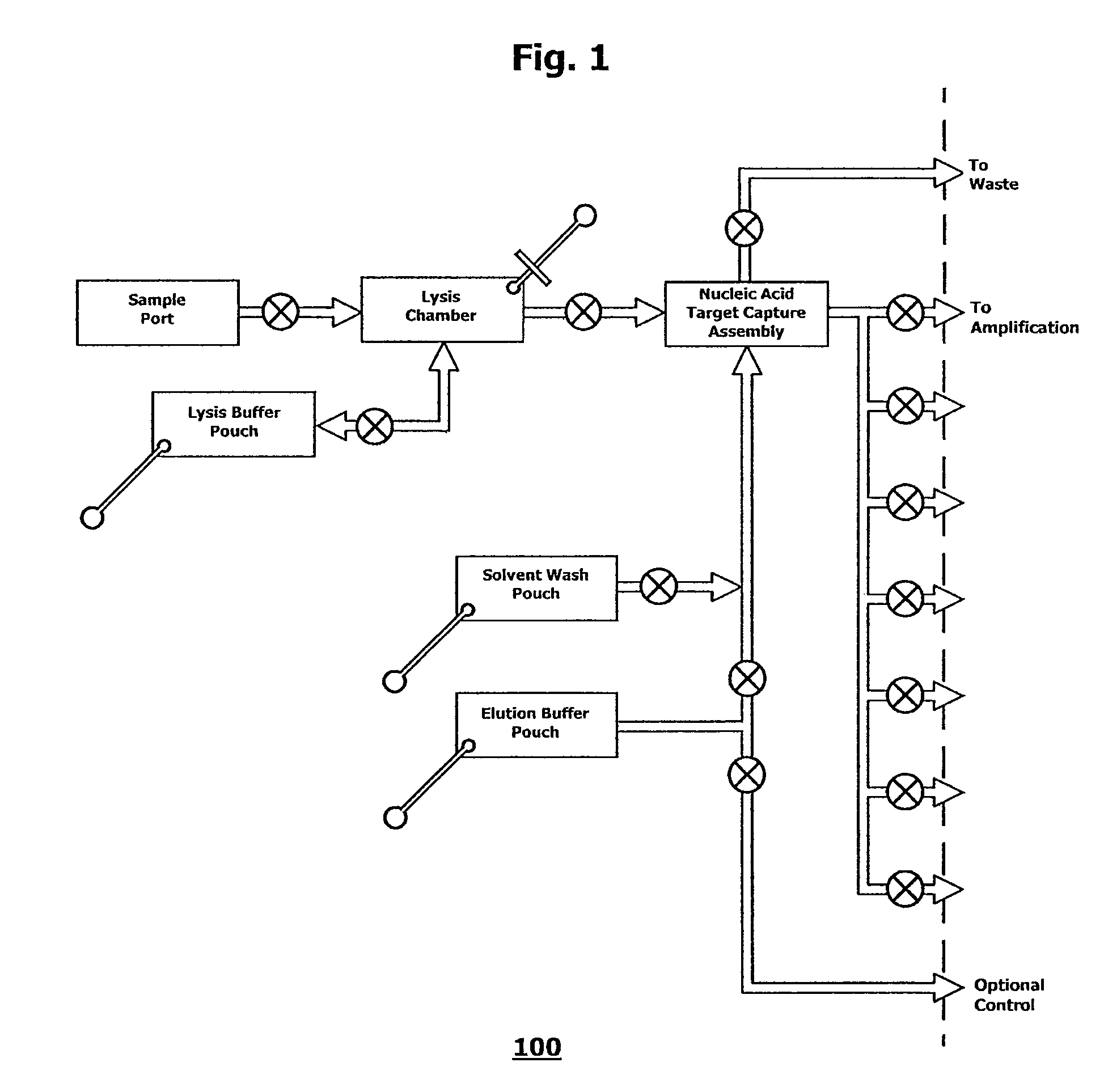
Integrated microfluidic cartridges for nucleic acid extraction, amplification, and detection from clinical samples are disclosed. The devices are single-entry, sanitary, and disposable. The devices enable simplex or multiplex nucleic acid target detection, as for example: assay panels for multiple infectious agents, or assay panels for cancerous cell types. Methods for use of microfluidic cartridges in a fully automated, pneumatically controlled apparatus are also disclosed.
Owner:PERKINELMER HEALTH SCIENCES INC
Communicable disease barrier method of use
InactiveUS6553243B2Easy to useEasy to transportFeet bandagesSurgical glovesPulse oximetersInfectious agent
A disposable digit cover structured to be applied to envelop a portion of a human finger or toe, i.e., digit, for aiding in prevention of the spread of communicable diseases and cross-patient contamination from pulse oximeter probes. The cover is a flexible, tubular structure of material impervious to the passage of infectious agents, and having an open end, a closed end and being sufficiently transparent to light to serve as a barrier between a human digit and a pulse oximeter probe. The barrier is first placed over the digit, the medical probe is then clamped on the barrier to pass light into the digit. The readings are taken, the probe is removed, then the barrier is removed and discarded.
Owner:GURLEY MARIRUTH D
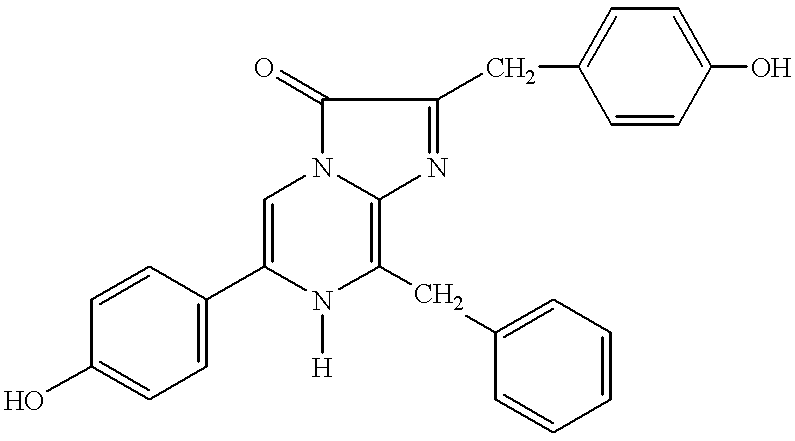
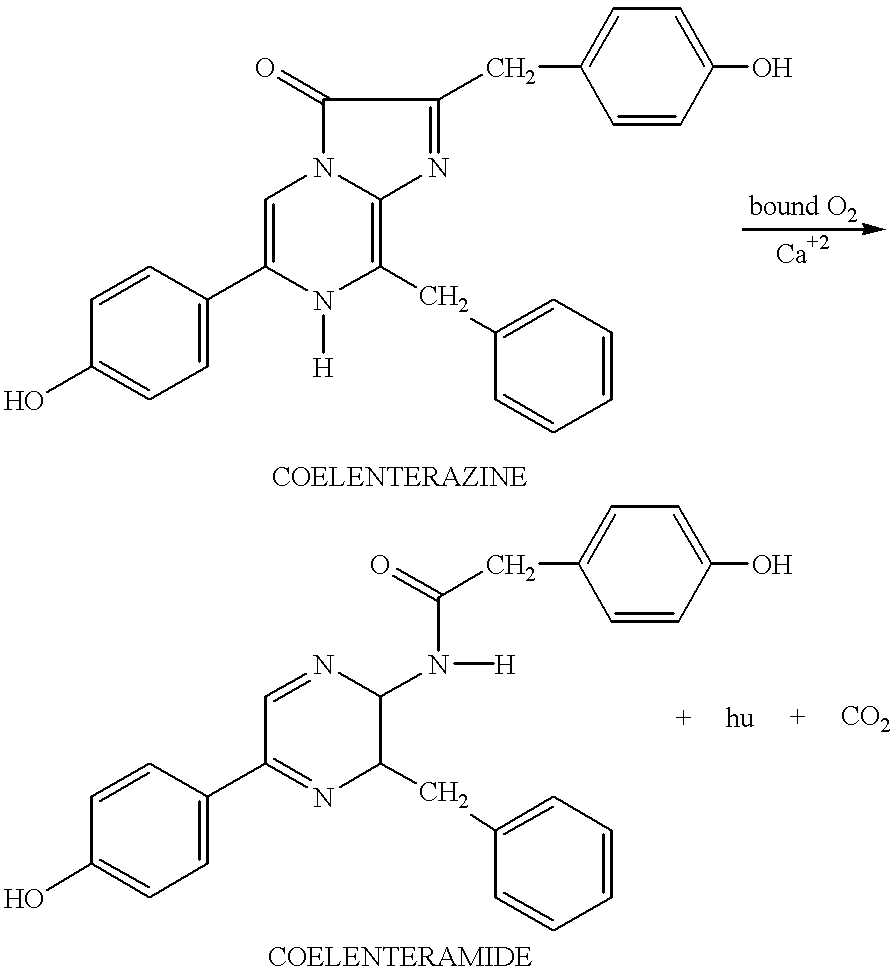
Apparatus and method for detecting and identifying infectious agents
Solid phase methods for the identification of an analyte in a biological medium, such as a body fluid, using bioluminescence are provided. A chip designed for performing the method and detecting the bioluminescence is also provided. Methods employing biomineralization for depositing silicon on a matrix support are also provided. A synthetic synapse is also provided.
Owner:PROLUME
Communicable disease barrier digit cover and dispensing package therefor
InactiveUS6179159B2Easy to useEasy to transportDiagnosticsSurgical needlesPulse oximetersEngineering
A disposable digit cover structured to be applied to envelop a portion of a human finger or toe, i.e., digit, for aiding in prevention of the spread of communicable diseases and cross-patient contamination from pulse oximeter probes. The cover is a flexible, tubular structure of material impervious to the passage of infectious agents, and having one open end and an oppositely disposed closed end, and is sufficiently thin and transparent to light to serve as infectious agent barrier between a human digit and a pulse oximeter probe. The covers are stored in a flattened state in a clean condition with a dispensing package containing a plurality of the covers stacked upon each other and secure to the package. The package includes a closeable opening allowing access to the digit covers. The dispensing package is small enough to be carried in a garment pocket.
Owner:GURLEY MARIRUTH D
Bioreactor wound dressing
The present invention is a bioreactor wound dressing which includes a first layer, being a transport layer, in direct contact with a wound. It includes at least one layer of a permeable polymeric media containing, in equilibrium with body fluids, at least 40% by weight of liquid, and is impermeable for infectious agents of any kind and being permeable to water soluble substances having molecular weight up to at least 1000 Daltons. There is a second layer, being a fluid reservoir layer that is adjacent to the transport layer and is capable of containing between 40% and 100% of its volume of an aqueous liquid, wherein the transport layer and reservoir layer are permeably interconnected for aqueous solutions and are in a substantial osmotic equilibrium. The invention also includes a method of wound treatment utilizing the bioreactor wound dressing.
Owner:REPLICATION MEDICAL
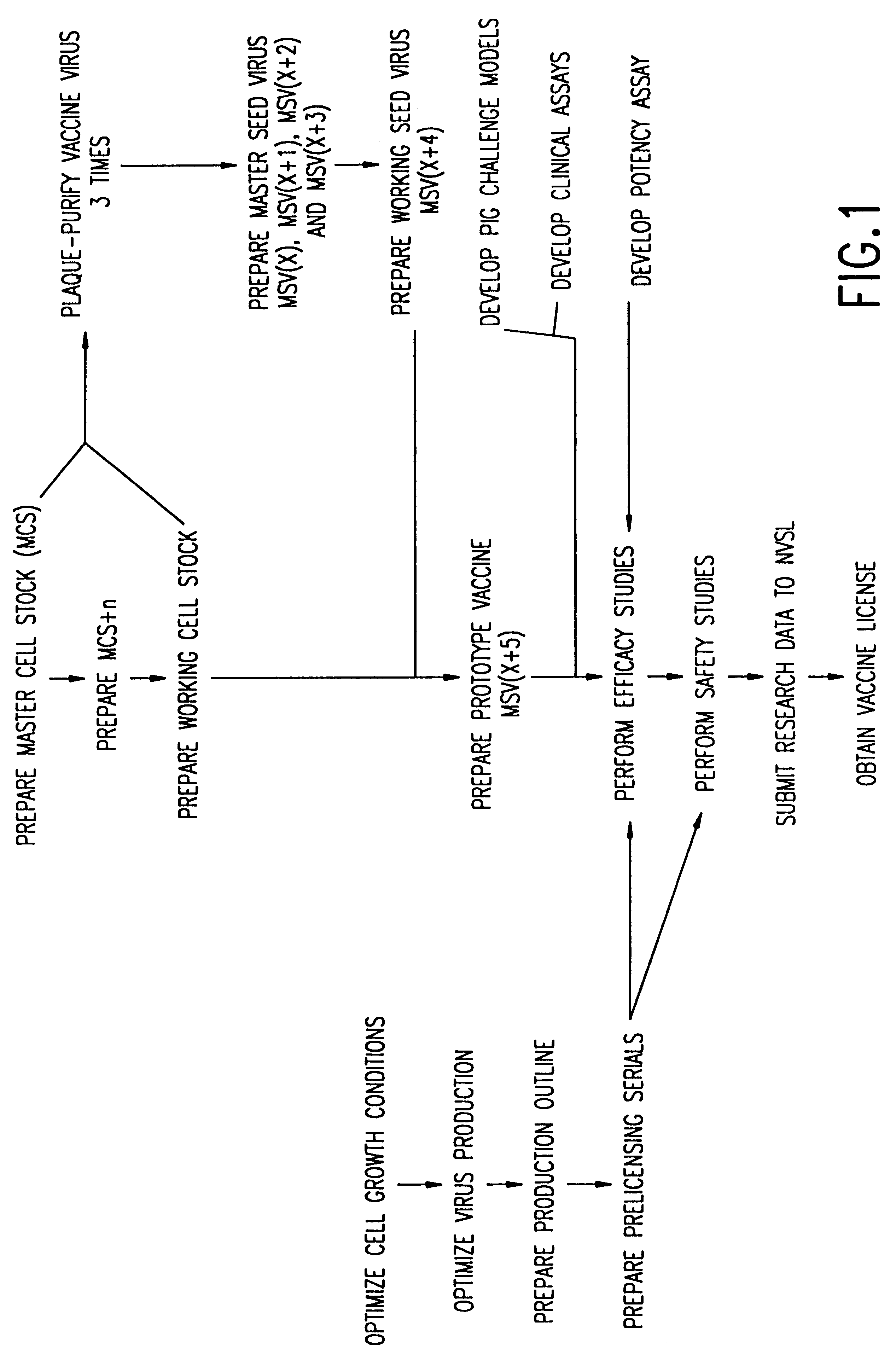
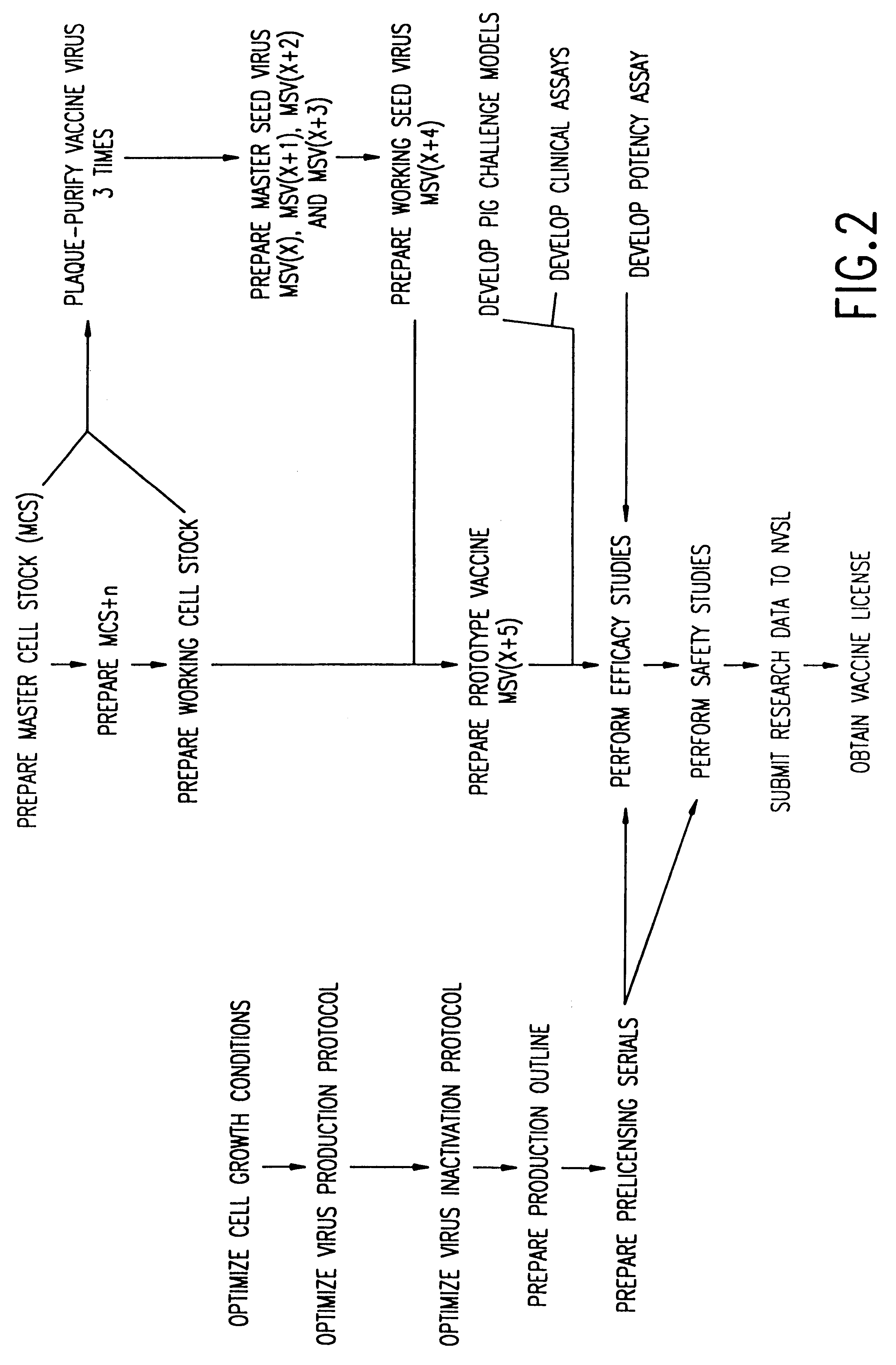
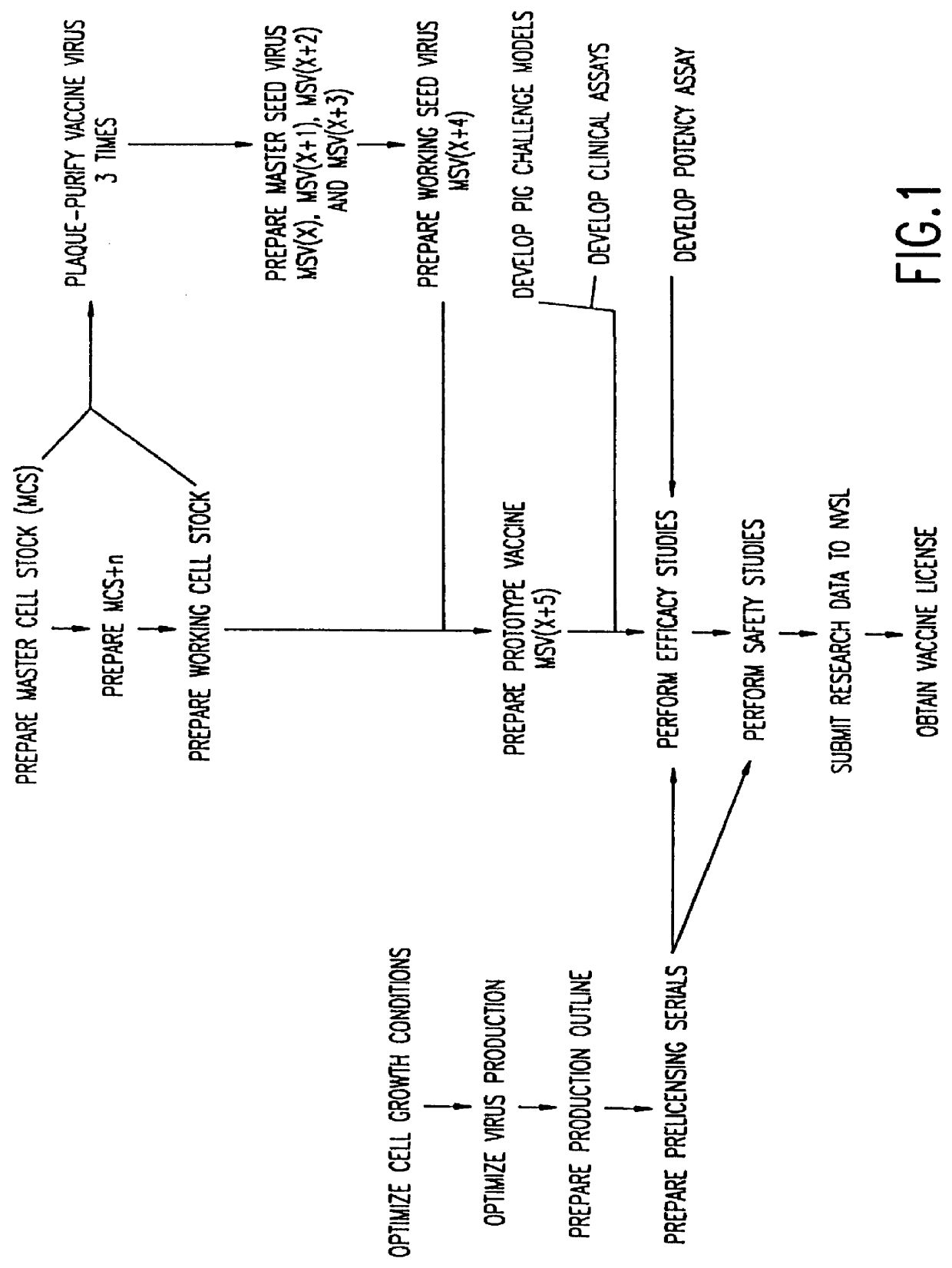
Method of producing a vaccine which raises an immunological response against a virus causing a porcine respiratory and reproductive disease
InactiveUS6251404B1Efficient responseAntibacterial agentsSsRNA viruses positive-senseInfectious agentRespiratory disease
The present invention provides a vaccine which protects pigs from a virus and / or an infectious agent causing a porcine respiratory and reproductive disease, a method of protecting a pig from a disease caused by a virus and / or an infectious agent which causes a respiratory and reproductive disease, a method of producing a vaccine against a virus and / or an infectious agent causing a porcine reproductive and respiratory disease, and a biologically pure sample of a virus and / or infectious agent associated with a porcine respiratory and reproductive disease, particularly the Iowa strain of porcine reproductive and respiratory syndrome virus (PRRSV), and an isolated polynucleotide which is at least 90% homologous with a polynucleotide obtained from the genome of a virus and / or infectious agent which causes a porcine respiratory and reproductive disease.
Owner:ZOETIS WHC 2
Methods of promoting immunopotentiation and preparing antibodies with anti-CD3 antibodies
Disclosed are immunopotentiating agents, and vaccines thereof, which enhance and / or otherwise modify immune responses, and method for their preparation and use in vivo. Immunopotentiating agents can be single agents that act directly, adjuvants added concurrently with the agents, or heteroconjugates wherein the immunopotentiating agent is chemically coupled to the compound against which an immune response is desired. Examples of immunopotentiating agents include monoclonal antibodies, such as anti-CD3, anti-CD2) and anti-CD5 antibodies, and proteins derived from microorganisms (e.g., enterotoxins) which activate T cells. The compounds against which an immune response can be generated, which may be the second component in a heteroconjugate, include compound from abnormal or diseased tissues such as tumors, or infectious agents, such as viruses, bacteria, fungi, protozoal or metozoal parasites, and can be obtained by natural or recombinant means. Methods of using the invention to prepare monoclonal antibodies are particularly disclosed.
Owner:MACROGENICS INC
Integrated nucleic acid assays
ActiveUS8222023B2Reduce pervasive dangerEasy to useBioreactor/fermenter combinationsHeating or cooling apparatusCancer cellInfectious agent
Integrated microfluidic cartridges for nucleic acid extraction, amplification, and detection from clinical samples are disclosed. The devices are single-entry, sanitary, and disposable. The devices enable simplex or multiplex nucleic acid target detection, as for example: assay panels for multiple infectious agents, or assay panels for cancerous cell types. Methods for use of microfluidic cartridges in a fully automated, pneumatically controlled apparatus are also disclosed.
Owner:PERKINELMER HEALTH SCIENCES INC
Isolated porcine respiratory and reproductive virus, vaccines and methods of protecting a pig against a disease caused by a porcine respiratory and reproductive virus
InactiveUS6110467AEfficient responseAntibacterial agentsSsRNA viruses positive-senseInfectious agentRespiratory disease
The present invention provides a vaccine which protects pigs from a virus and / or an infectious agent causing a porcine respiratory and reproductive disease, a method of protecting a pig from a disease caused by a virus and / or an infectious agent which causes a respiratory and reproductive disease, a method of producing a vaccine against a virus and / or an infectious agent causing a porcine reproductive and respiratory disease, and a biologically pure sample of a virus and / or infectious agent associated with a porcine respiratory and reproductive disease, particularly the Iowa strain of porcine reproductive and respiratory syndrome virus (PRRSV), and an isolated polynucleotide which is at least 90% homologous with a polynucleotide obtained from the genome of a virus and / or infectious agent which causes a porcine respiratory and reproductive disease.
Owner:ZOETIS WHC 2
Controlled Delivery of TLR Agonists in Structural Polymeric Devices
ActiveUS20130202707A1Increase successStimulate immune responsePowder deliveryOrganic active ingredientsTlr agonistsDendritic cell
The present invention comprises compositions, methods, and devices for creating an stimulating an antigen-specific dendritic cell immune response. Devices and methods provide prophylactic and therapeutic immunity to subjects against cancer and infectious agents.
Owner:DANA FARBER CANCER INST INC +1
Zinc salt compositions for the prevention of dermal and mucosal irritation
InactiveUS20050281762A1Minimize and prevent irritationReduce transmissionBiocideCosmetic preparationsMedicineIrritation
The present invention relates to methods and compositions which employ low concentrations of combinations of zinc salts to prevent the irritation of skin or mucous membranes that may be caused by therapeutic agents, by personal hygiene products, or by various physical, chemical, mechanical, or biological irritants, including infectious agents.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Vaccines against cancer and infectious diseases
InactiveUS6440416B1Promote antibody productionPromote productionOrganic active ingredientsImmunoglobulins against animals/humansProtozoaPrimate
A method of stimulating an immune response in a human against malignant cells or an infectious agent comprises the step of administering to the human an immunogenic amount of a primate anti-idiotype antibody or antibody fragment that acts as an immunogenic functional mimic of an antigen produced by or associated with a malignant cell or an infectious agent. Sub-human primate anti-idiotype antisera, especially from baboons, are preferred. Such anti-idiotype antibodies are used to make vaccines for inducing preventive immunity or a therapeutic immune response against tumors, viruses, bacteria, rickettsia, mycoplasma, protozoa, fungi and multicellular parasites.
Owner:IMMUNOMEDICS INC
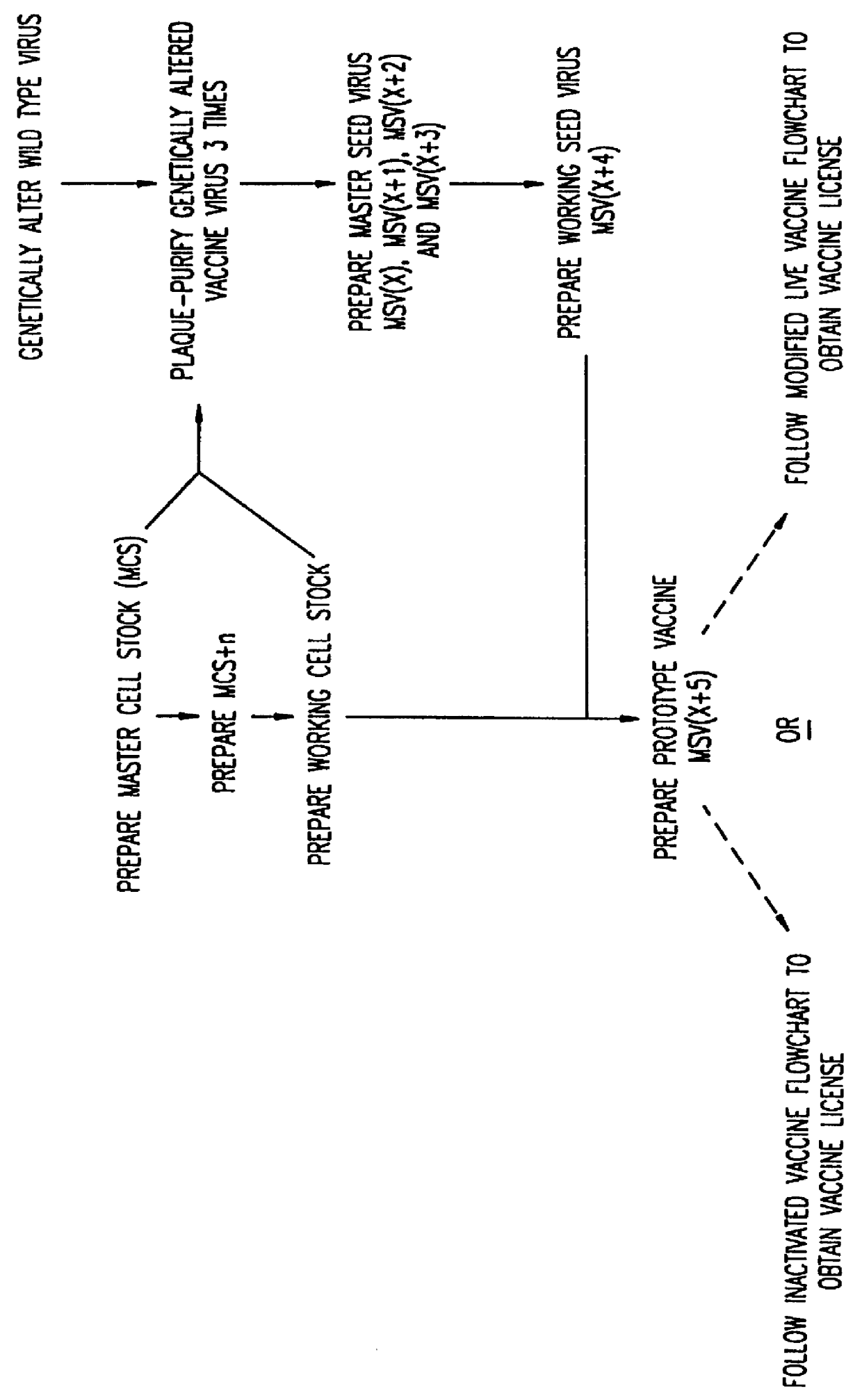
Modified Vaccinia Ankara virus variant and cultivation method
InactiveUS7445924B2Reduce riskGenetic material ingredientsVirus peptidesSerum free mediaModified vaccinia Ankara
The present invention provides an attenuated virus, which is derived from Modified Vaccinia Ankara virus and characterized by the loss of its capability to reproductively replicate in human cell lines. It further describes recombinant viruses derived from this virus and the use of the virus, or its recombinants, as a medicament or vaccine. A method is provided for inducing an immune response in individuals who may be immune-compromised, receiving antiviral therapy, or have a pre-existing immunity to the vaccine virus. In addition, a method is provided for the administration of a therapeutically effective amount of the virus, or its recombinants, in a vaccinia virus prime / vaccinia virus boost innoculation regimen. The present invention relates to a method of virus amplification in primary cells which are cultivated in a serum free medium. Viruses produced by this method are advantageously free of any infectious agents comprised in animal sera.
Owner:BAVARIAN NORDIC AS
Methods and compositions for analyte detection
InactiveUS20100323343A1Bioreactor/fermenter combinationsAnalysis using chemical indicatorsAnalyteInfectious agent
The present invention is directed to methods and apparatus for detection of one or more analytes. Analytes include agents or components of infectious agents such as pathogenic virus, as well as enzymes, proteins and biomarkers.
Owner:NEXUS DX
Targeted multi-epitope dosage forms for induction of an immune response to antigens
InactiveUS20120070493A1Induce and enhance and cytokine productionAntibacterial agentsPowder deliveryAntigenBinding peptide
Provided herein are compositions and methods related to MHC II binding peptides. In some embodiments, the peptides are obtained or derived from a common source. In other embodiment, the peptides are obtained or derived from an infectious agent to which a subject has been repeatedly exposed.
Owner:SELECTA BIOSCI
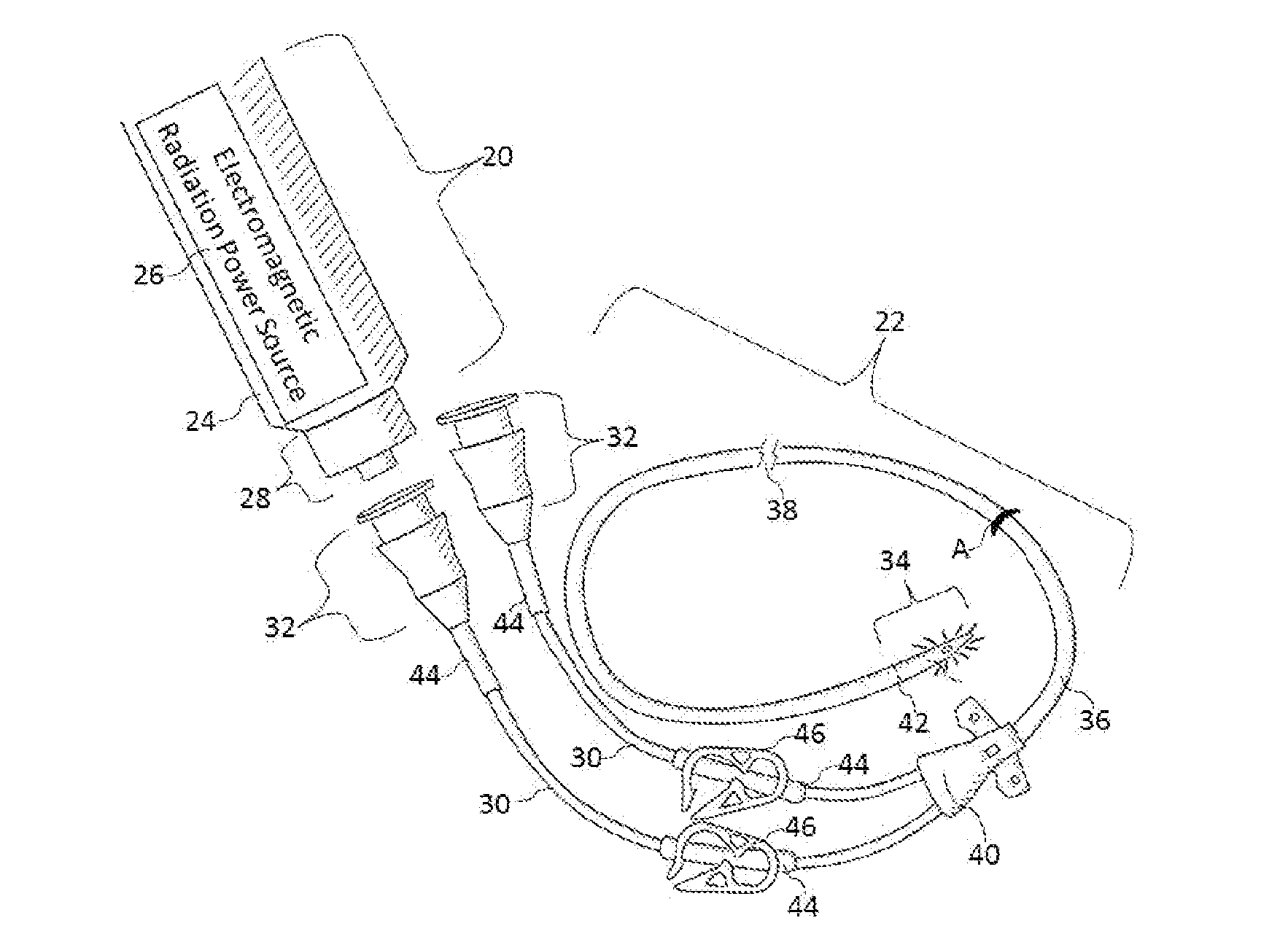
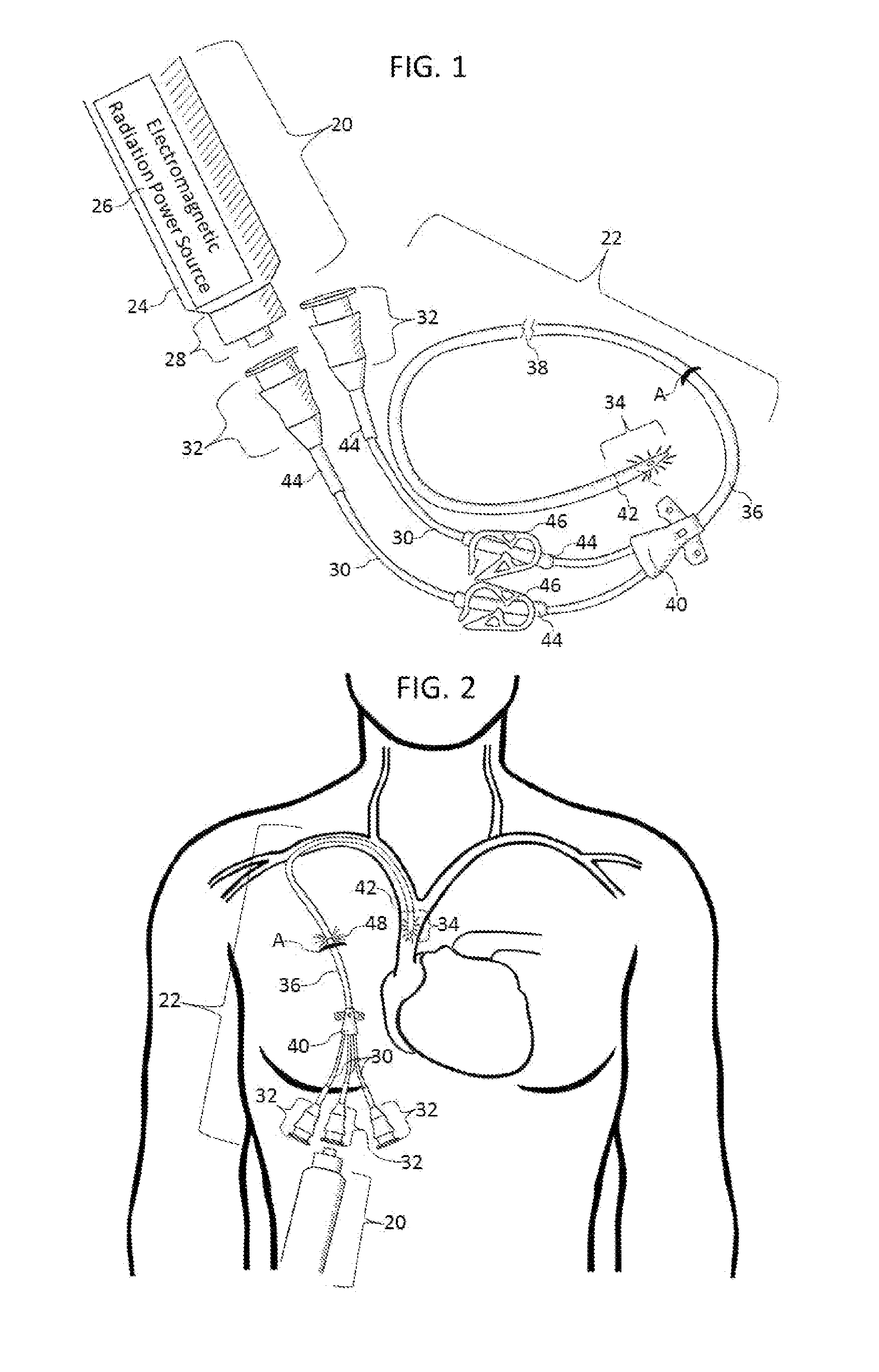
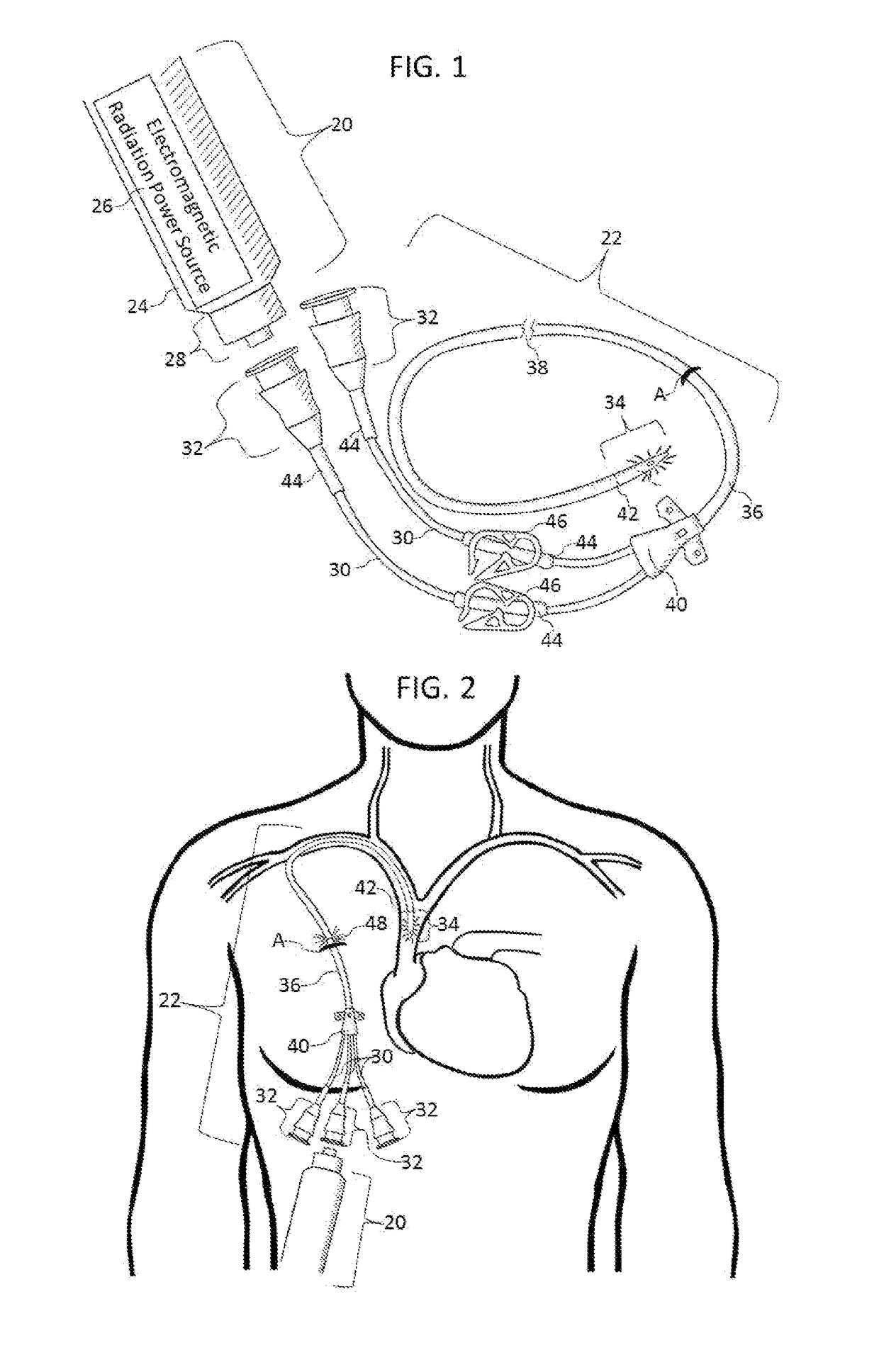
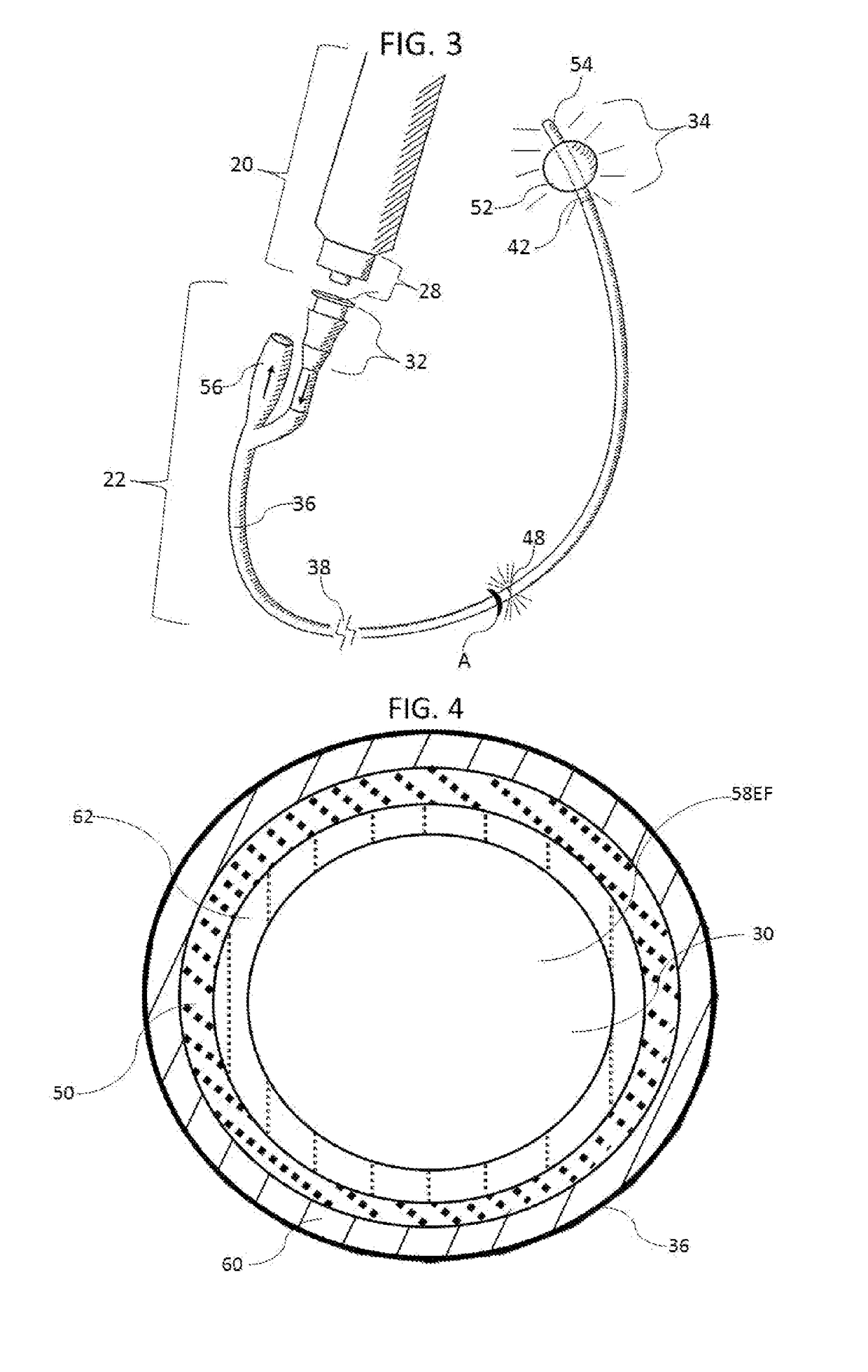
Methods and apparatus to inactivate infectious agents on a catheter residing in a body cavity
ActiveUS20130267888A1Reduce and eliminate countEffective sterilizationElectrotherapyMulti-lumen catheterMedicineUltraviolet
Methods and apparatus for the inactivation of infectious agents in, on or around a catheter residing in a patient's body cavity. The method comprises transmitting non-ultraviolet sterilizing electromagnetic radiation (EMR) substantially axially along an optical element in the catheter body. Through delivery of the sterilizing EMR to particular areas of highest infection, the present disclosure is able to inactivate the major sources of infection in catheters.
Owner:LIGHT LINE MEDICAL INC
Methods and compositions for analyte detection
ActiveUS7932099B2Avoid readingBioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteInfectious agent
The present invention is directed to methods and apparatus for detection of one or more analytes. Analytes include agents or components of infectious agents such as pathogenic virus, as well as enzymes, proteins and biomarkers.
Owner:NEXUS DX
Anti tubercular drug: compositions and methods
Methods and compositions for treating disease caused by infectious agents, particularly tuberculosis. In particular, methods and compositions comprising substituted ethylene diamines for the treatment of infectious diseases are provided. In one embodiment, these methods and compositions are used for the treatment of mycobacterial infections, including, but not limited to, tuberculosis.
Owner:SEQUELLA +1
Human immune therapies using a CD27 agonist alone or in combination with other immune modulators
ActiveUS8481029B2Promotes strong expression of 4-1BBImprove responseAntibacterial agentsNervous disorderAutoimmune responsesImmune modulator
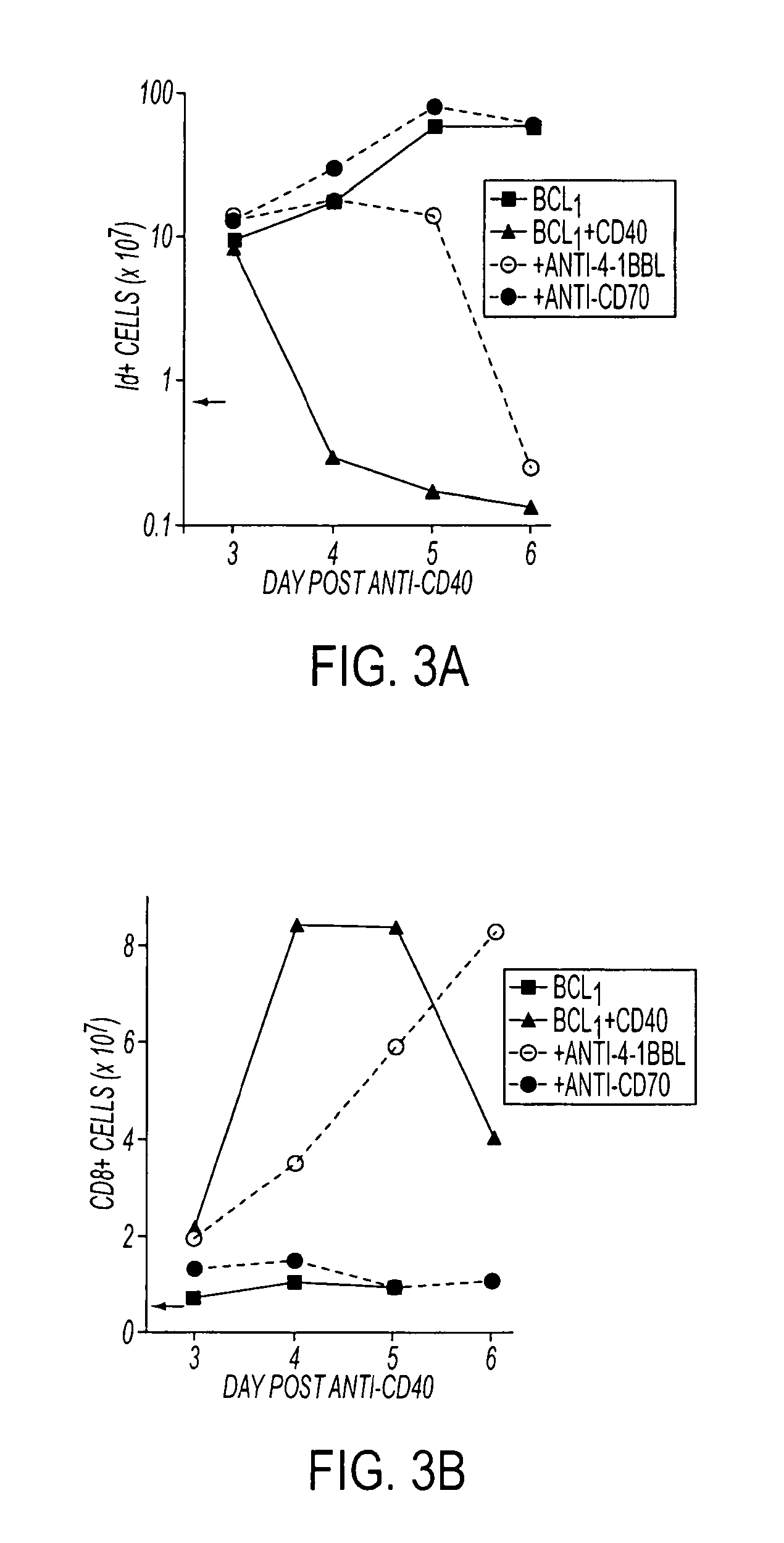
Methods of inducing T cell proliferation and expansion in vivo for treating conditions wherein antigen-specific T cell immune response are therapeutically desirable such as cancer, infection, inflammation, allergy and autoimmunity and for enhancing the efficacy of vaccines are provided. These methods comprise the administration of at least one CD27 agonist, preferably an agonistic CD27 antibody, alone or in association with another moiety such as immune stimulant or immune modulator such as an anti-CD40, OX-40, 4-IBB, or CTLA-4 antibody or an agent that depletes regulatory cells, or a cytokine. These mono and combination therapies may also optionally include the administration of a desired antigen such as a tumor antigen, an allergen, an autoantigen, or an antigen specific to an infectious agent or pathogen against which a T cell response (often CD8+) is desirably elicited.
Owner:UNIV OF SOUTHAMPTON
Cytomegalovirus gene function and methods for developing antivirals, anti-CMV vaccines, and CMV-based vectors
ActiveUS20050064394A1Enhance their long-term survivabilitySugar derivativesMicrobiological testing/measurementSurvivabilityORFS
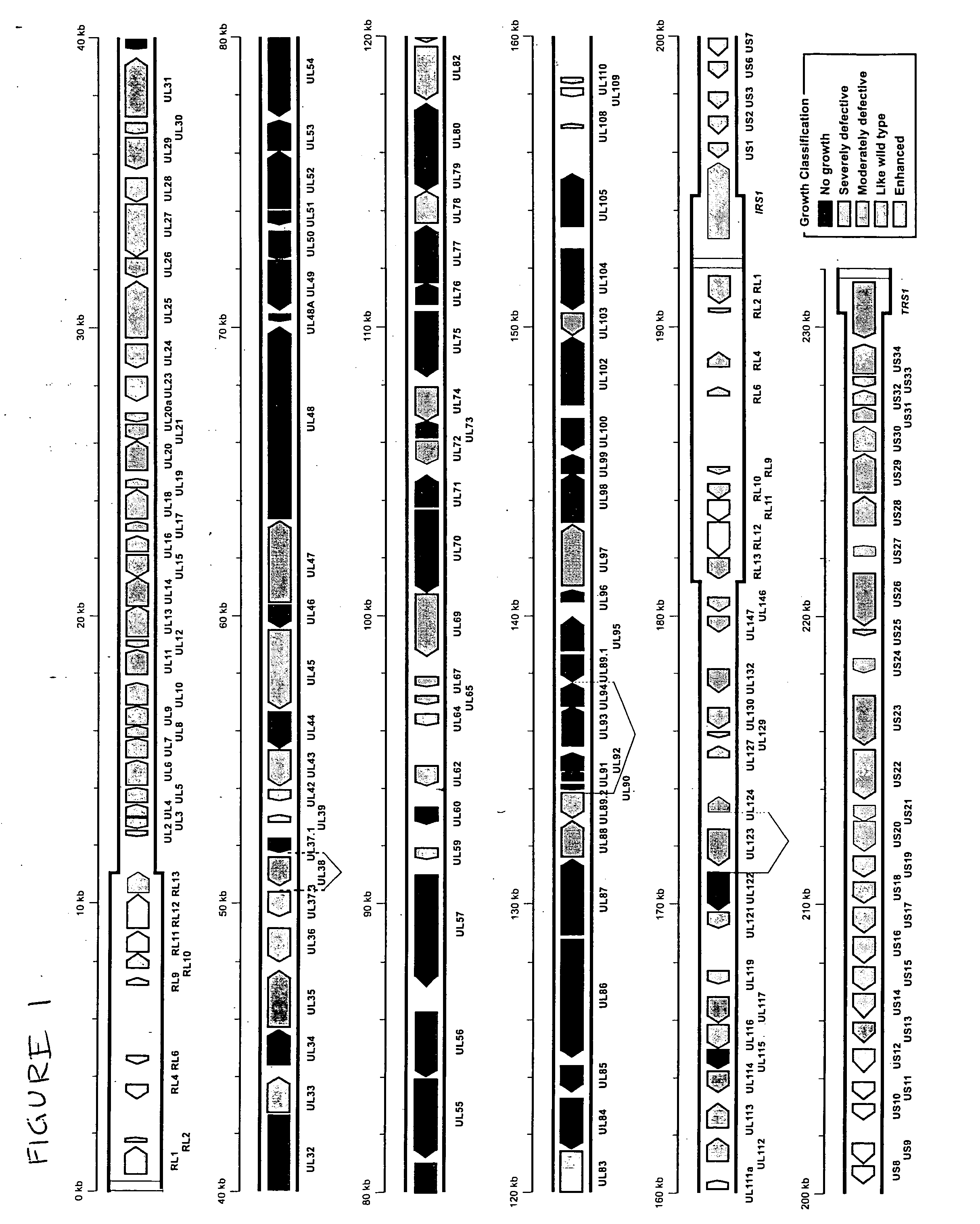
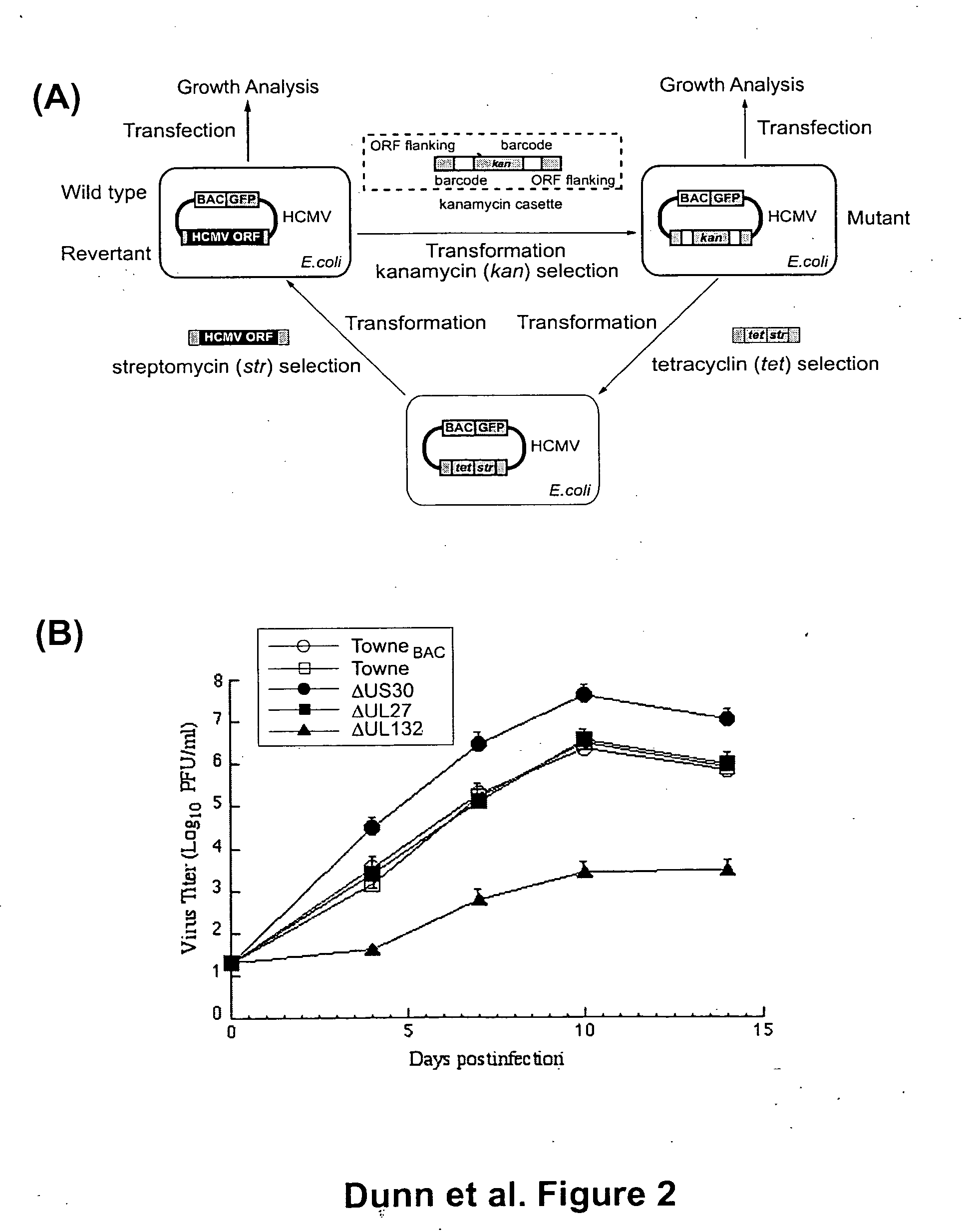
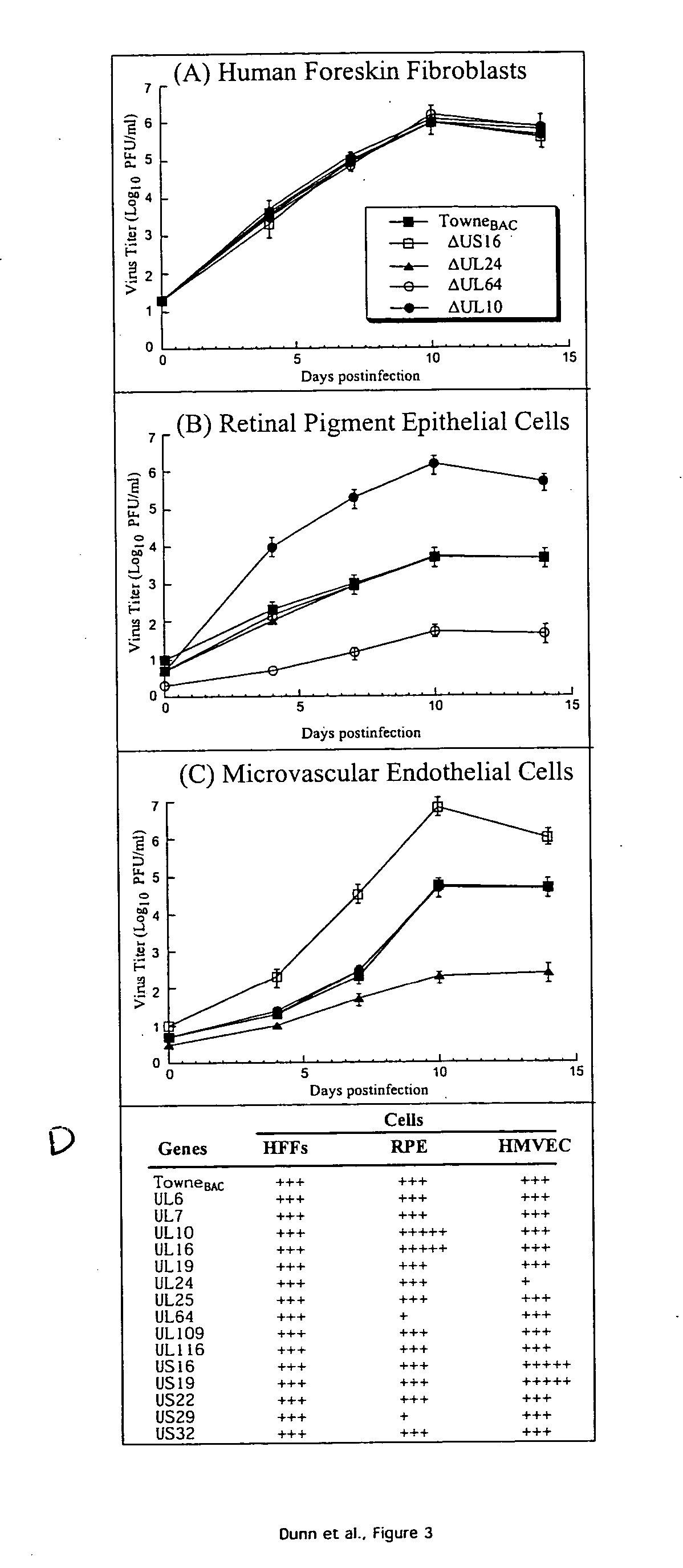
A global functional analysis of HCMV genes is performed by constructing virus gene-deletion mutants and examining their growth phenotypes in different natural HCMV host cells. This systematic analysis of the HCMV genome identified 45 viral ORFs essential for viral replication and characterizes of 115 growth-dispensable viral genes. Of particular interest is the finding that HCMV encodes genes (temperance factors) that repress its own replication on a cell type-specific basis. In addition to HCMV, pathogen temperance may be a strategy employed by other infectious agents to enhance their long-term survivability within their respective host population.
Owner:RGT UNIV OF CALIFORNIA
Systems and methods that kill infectious agents (bacteria) without the use of a systemic anti-biotic
InactiveUS20110200655A1Inhibition of colonizationReduce the prevalenceBiocidePowder deliveryWound dressingMedical product
A medical product is provided that is selected from at least one of, nasal cannulas, oxygen masks, wound dressings, bandages, band aids, catheters, endotrachial tubes, condoms, surgical and other gloves, sheaths for endoscopy probes, and medical products that physically touch the body. A coating is included with at least one of, a non-antibiotic, antimicrobial and / or antiviral substance that prevents further local, non-systemic, colonization of infections.
Owner:BLACK MICHAEL DARRYL +1
Zinc salt compositions for the prevention of dermal and mucosal irritation
InactiveUS7563461B2Minimize and prevent irritationReduce transmissionCosmetic preparationsBiocideIrritationMedicine
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Methods and apparatus to inactivate infectious agents on a catheter residing in a body cavity
ActiveUS9808647B2Effective sterilizationReduce and eliminate countElectrotherapyMulti-lumen catheterMedicineUltraviolet
Methods and apparatus for the inactivation of infectious agents in, on or around a catheter residing in a patient's body cavity. The method comprises transmitting non-ultraviolet sterilizing electromagnetic radiation (EMR) substantially axially along an optical element in the catheter body. Through delivery of the sterilizing EMR to particular areas of highest infection, the present disclosure is able to inactivate the major sources of infection in catheters.
Owner:LIGHT LINE MEDICAL INC
Non-hormonal vaginal contraceptive
InactiveUS7521064B2Easy to useGood health careAntibacterial agentsPowder deliveryInfectious agentSexually transmitted disease
The present invention relates to a non-hormonal, biocompatible, and biodegradable intravaginal device for the delivery of spermiostatic, spermicidal and anti-infectious agents. The present invention also relates to methods of contraception using such a device, as well as the prevention and treatment of sexually transmitted diseases and vaginal infections through the application of the device.
Owner:SAXENA BRIJ +2
Modified vaccinia ankara virus variant and cultivation method
InactiveUS20050214323A1Reduce riskViral antigen ingredientsGenetic material ingredientsSerum free mediaModified vaccinia Ankara
The present invention provides an attenuated virus, which is derived from Modified Vaccinia Ankara virus and characterized by the loss of its capability to reproductively replicate in human cell lines. It further describes recombinant viruses derived from this virus and the use of the virus, or its recombinants, as a medicament or vaccine. A method is provided for inducing an immune response in individuals who may be immune-compromised, receiving antiviral therapy, or have a pre-existing immunity to the vaccine virus. In addition, a method is provided for the administration of a therapeutically effective amount of the virus, or its recombinants, in a vaccinia virus prime / vaccinia virus boost innoculation regimen. The present invention relates to a method of virus amplification in primary cells which are cultivated in a serum free medium. Viruses produced by this method are advantageously free of any infectious agents comprised in animal sera.
Owner:BAVARIAN NORDIC AS
Methods and compositions for a multipurpose, lab-on-chip device
InactiveUS20140030800A1Sturdy in constructionReduce usageBioreactor/fermenter combinationsBiological substance pretreatmentsDisaster areaTerrain
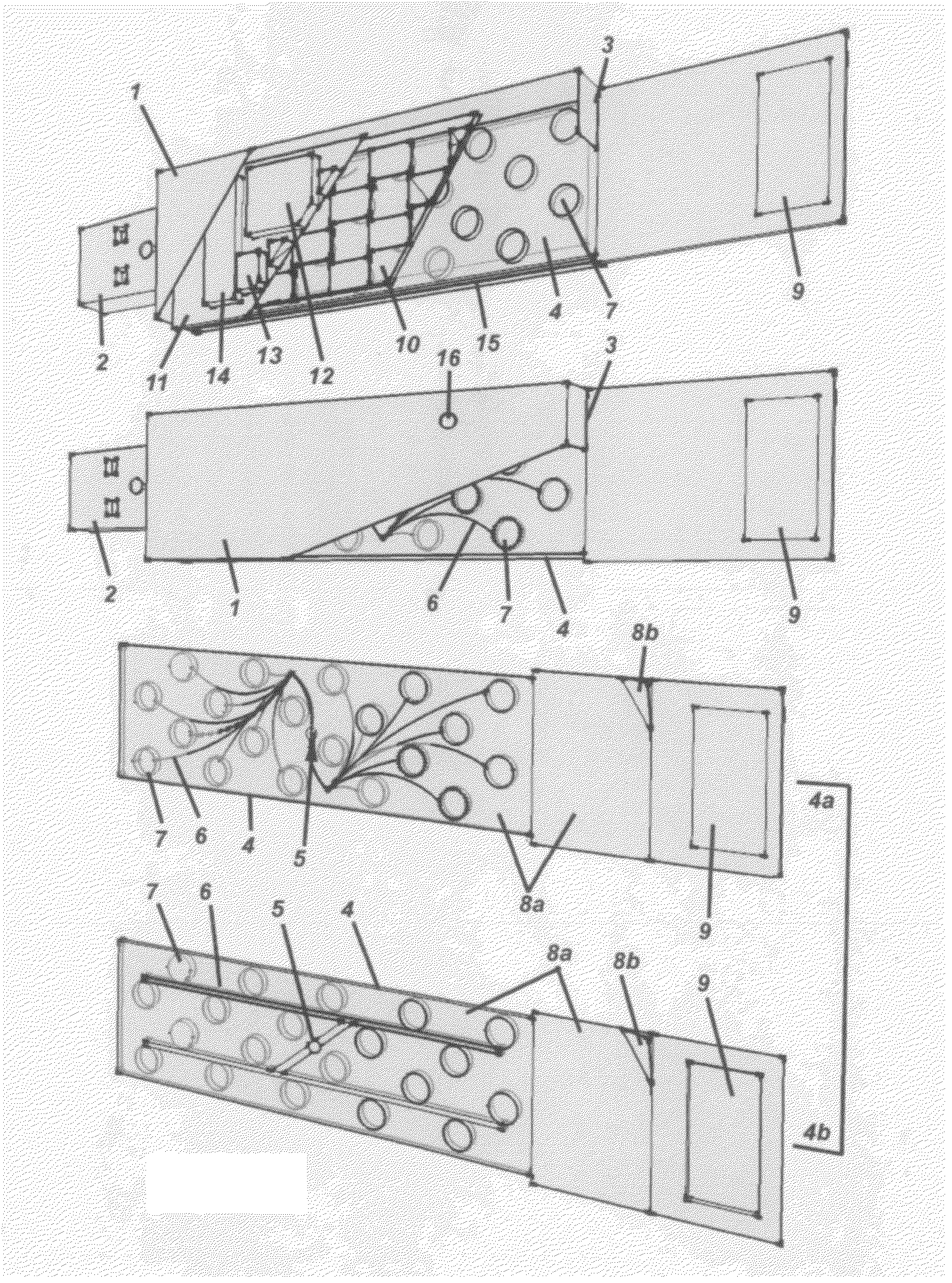
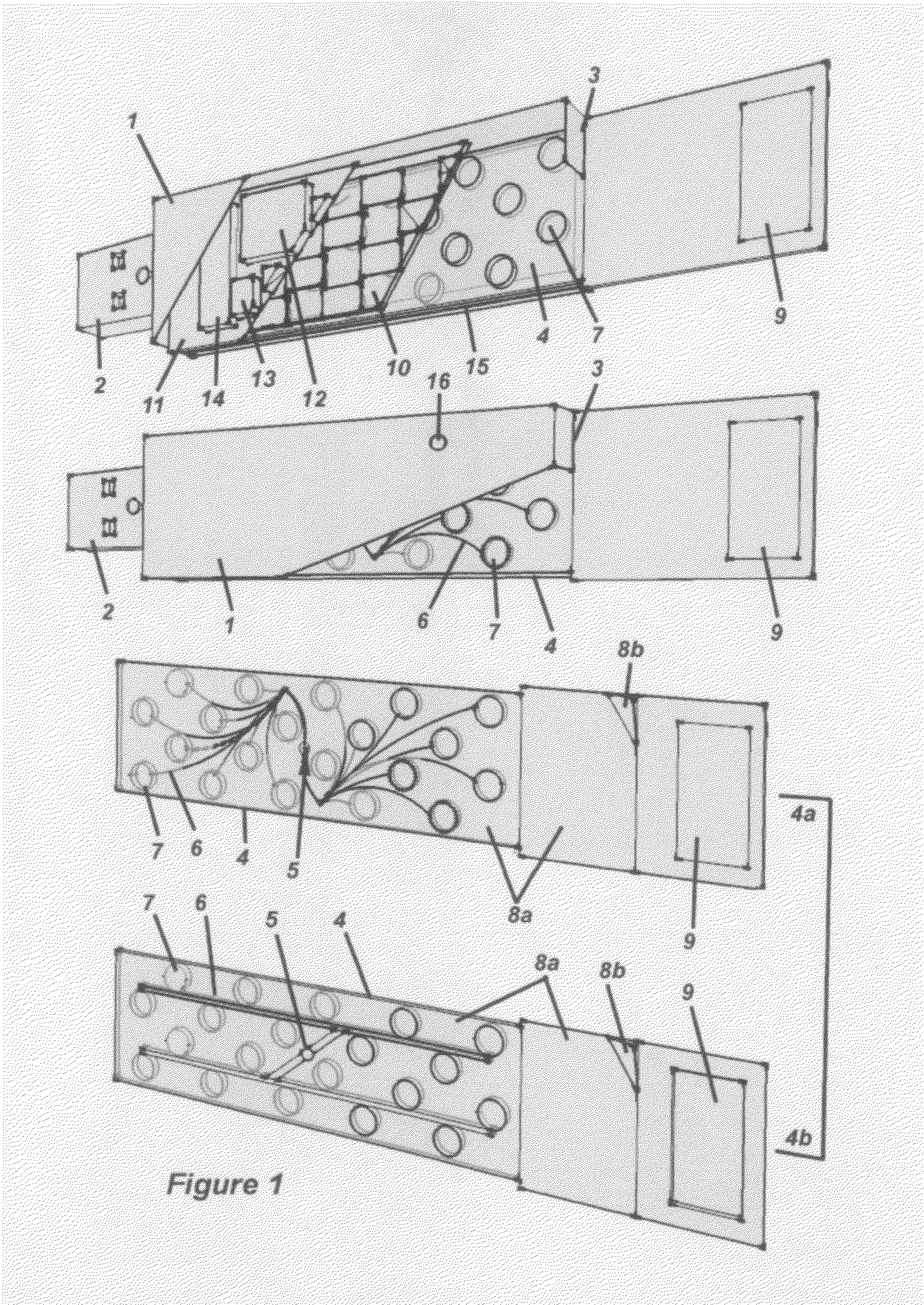
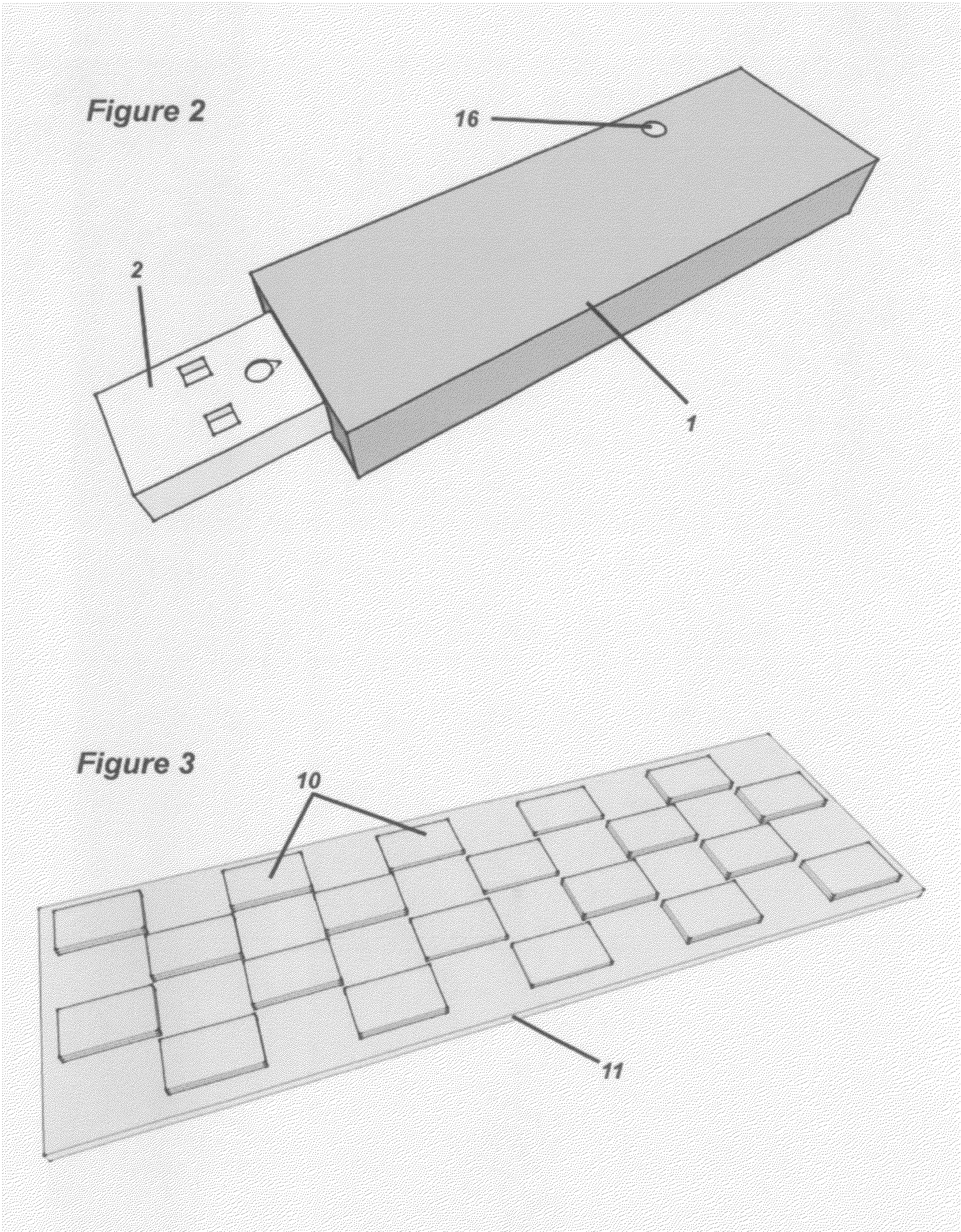
Methods and compositions for developing a series of microfluidic, USB-enabled, wireless-enabled, lab-on-chip devices, designed to reduce the chain-of-custody handling of samples between sample acquisition and final reporting of data, to a single individual. These devices provide on-the-spot testing for micro- and nanoscale (molecular) analysis of blood, urine, infectious agents, toxins, measurement of therapeutic drug levels, purity-of-sample testing and presence of contaminants (toxic and non-toxic, volatile and non-volatile); and for the identification of individual components and formal compounds—elemental, biological, organic and inorganic—inclusive of foodstuffs, air, water, soil, oil and gas samples. These devices may be relatively inexpensive, ruggedly designed, lightweight and capable of being employed—depending upon the specific application—by individuals with limited training, in remote and extreme environments and settings: including combat zones, disaster areas, rural communities, tropical / arctic / desert and other inhospitable climates and challenging terrains. The device may be comprised of materials that are reclaimed, are re-usable and are recyclable.
Owner:MOSES JONAS +1
Methods for generating immunity to antigen
InactiveUS20050226888A1Tumor rejection antigen precursorsTumor specific antigensInfectious agentTumor antigen
Provided are methods of generating an immune response to an antigen. The method comprises priming an individual by administering an expression vector encoding the antigen. The vectors comprises a transcription unit encoding a secretable fusion protein, the fusion protein containing an antigen and CD40 ligand. Administration of a fusion protein containing the antigen and CD40 ligand is used to enhance the immune response above that obtained by vector administration alone. The invention methods may be used to generate an immune response against cancer expressing a tumor antigen such as a mucin or human papilloma viral tumor antigen and to generate an immune response against an infectious agent. Also provided is a method for simultaneously producing the expression vector and the fusion protein.
Owner:MICROVAX +1
High Throughput Proteomics
InactiveUS20080260763A1Successful recoveryPeptide/protein ingredientsVirus peptidesNucleotideInfectious agent
Methods to obtain expression systems and proteins in a high-throughput protocol by utilizing mixtures of cells cultured from those transformed with a desired nucleotide sequence permit rapid production of protein for use in arrays to assess activity. In one embodiment, the proteins (or peptides) in the array are assessed for their immunological activity with regard to an infectious agent.
Owner:RGT UNIV OF CALIFORNIA
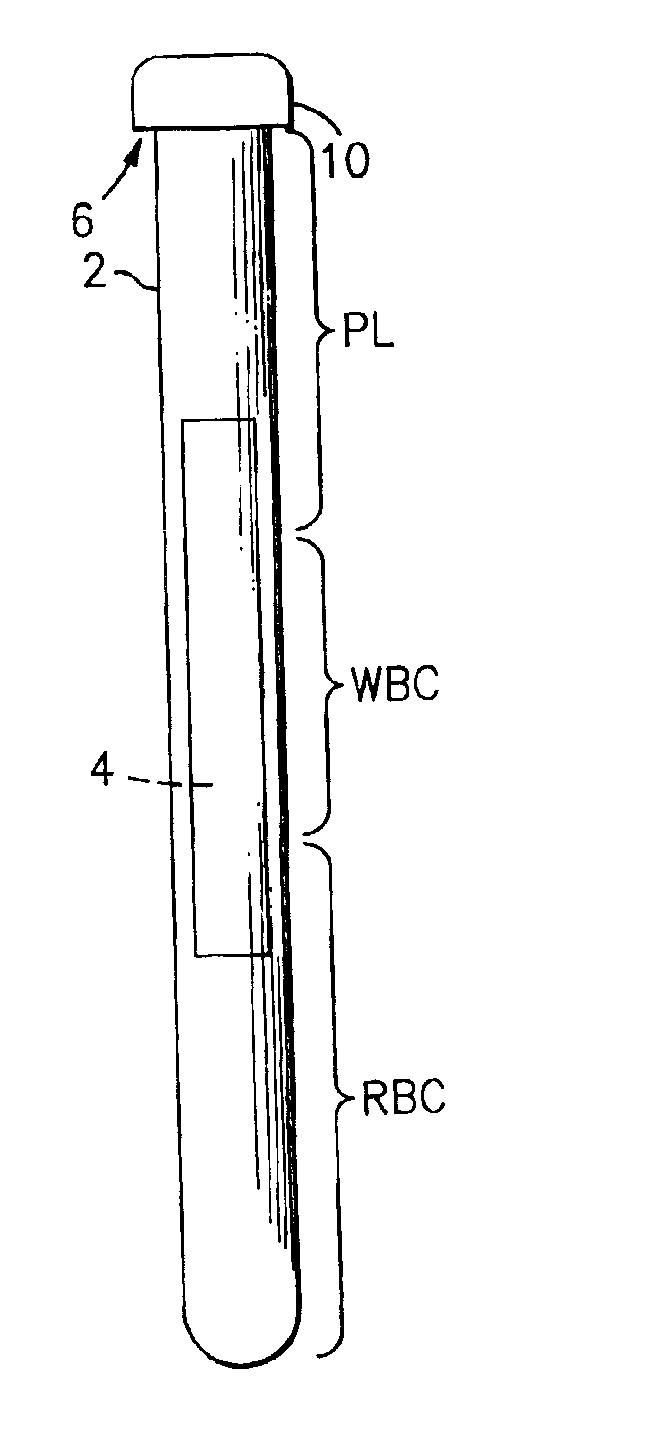
Method for the detection, identification, enumeration and confirmation of virally infected cells and other epitopically defined cells in whole blood
InactiveUS6911315B2Reduce the possibilityAvoid pollutionMicrobiological testing/measurementImmunoglobulins against virusesInfected cellFluorophore
A method for analyzing blood enables one to isolate, detect, enumerate and confirm under magnification the presence of target cells which have expressed surface epitopes that indicate intracellular infection by various viruses or other infectious agents, and also cells which have expressed surface epitopes that indicate the presence of non-infectious medical conditions. The analysis involves the examination of cells in the blood sample for the presence or absence of particular surface epitopes while the blood sample is disposed in a centrifuged blood sampling container. The epitopic analysis for the presence or absence of infected cells, or cells which indicate the presence of non-infectious medical conditions relies on the detection of known target expressed epitopes. The target epitopes on the target cell types are epitopes which are also known to be absent on normal circulating cells in the blood. Fluorophores or other labels with distinct wavelength emissions are coupled with specific binding agents such as lectins, antibodies, aptamers, or the like, which are directed against the target expressed epitopes. The epitopic analyses may be performed in or near the expanded buffy coat layer in the centrifuged blood sample. The epitopic analysis may be performed under magnification either visually and / or photometrically. The blood sampling container is sized to hold between about 1 and about 20 ml, preferably about 10 ml of blood, and contains an insert that occupies about 90-98% of the volume of the container bore in the area of the container where the target cells will, if present, be detected. The insert forces the target cells in question to reside in an annular space in the container which is adjacent to the circumference of the container bore. The entire analysis can be performed in a relatively short period of time which is typically a matter of minutes to single digit hours.
Owner:LEVINE ROBERT A +1
Methods for measuring the rates of replication and death of microbial infectious agents in an infected
InactiveUS20060105339A1Inhibit growthAvoid maintenanceMicrobiological testing/measurementDisease diagnosisMicroorganismBiological body
The present invention provides methods and kits useful for determining rates of replication and destruction of an infectious agent within an infected host organism. In the methods of the invention, an isotopically-labeled precursor molecule is administered to an infected host, and is given sufficient time to pass into the host's metabolic pools into a biochemical component of the infectious agent. The isotopic content and / or pattern or the rate of change of the isotopic content and / or pattern of the biochemical component is then measured to determine the rate of replication (growth) of the infectious organism while in the host. Alternatively, isotopic decay of labeled molecular components of the infectious agent is measured over time after discontinuing administration of the isotopically labeled precursor molecule to determine the rate of destruction (death) of the infectious agent while in the host. Thus, using methods of the invention, in vivo sensitivity of infectious agents to drug agents may be determined, in order to optimize therapy of the infected host.
Owner:RGT UNIV OF CALIFORNIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com