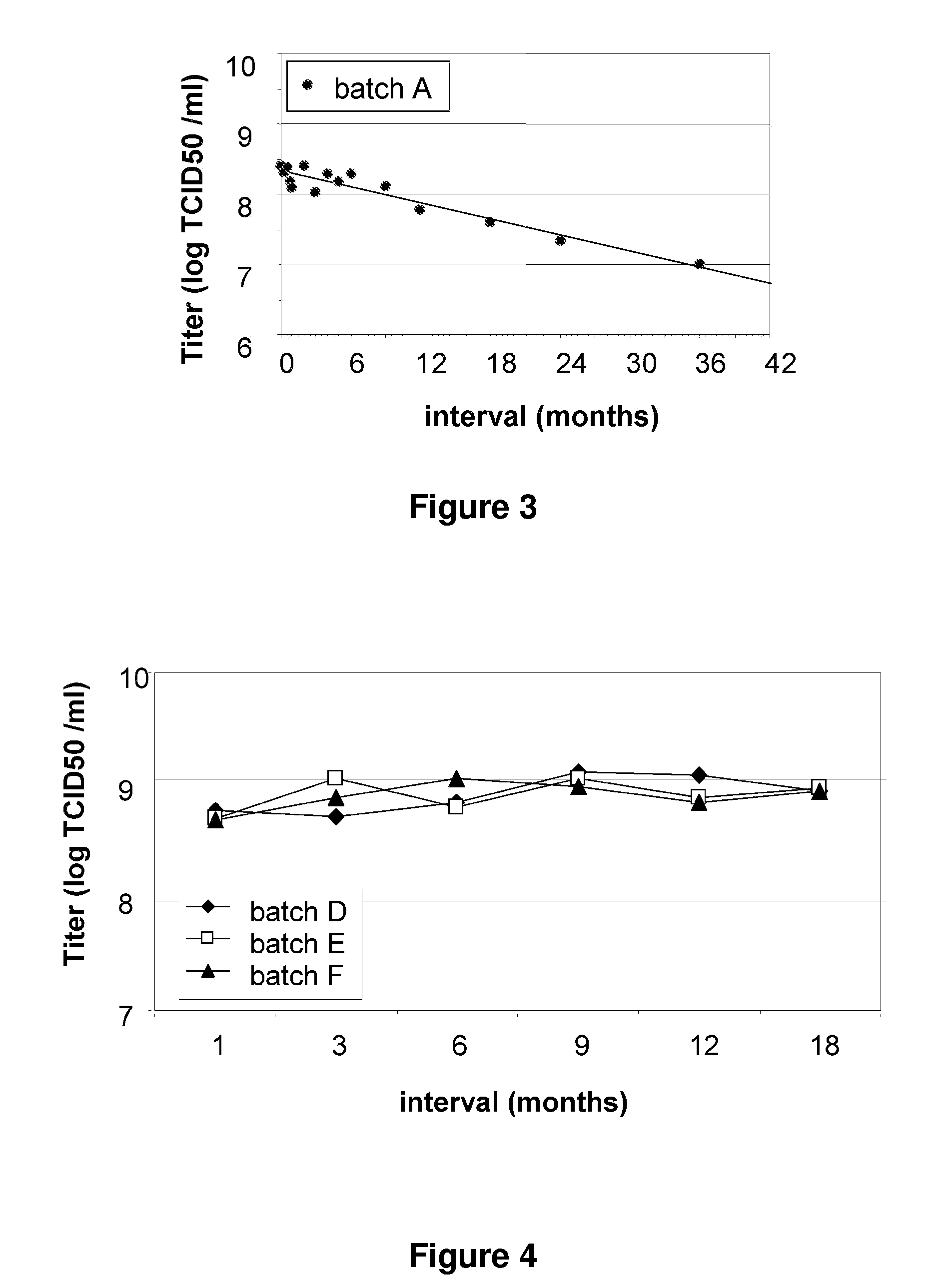
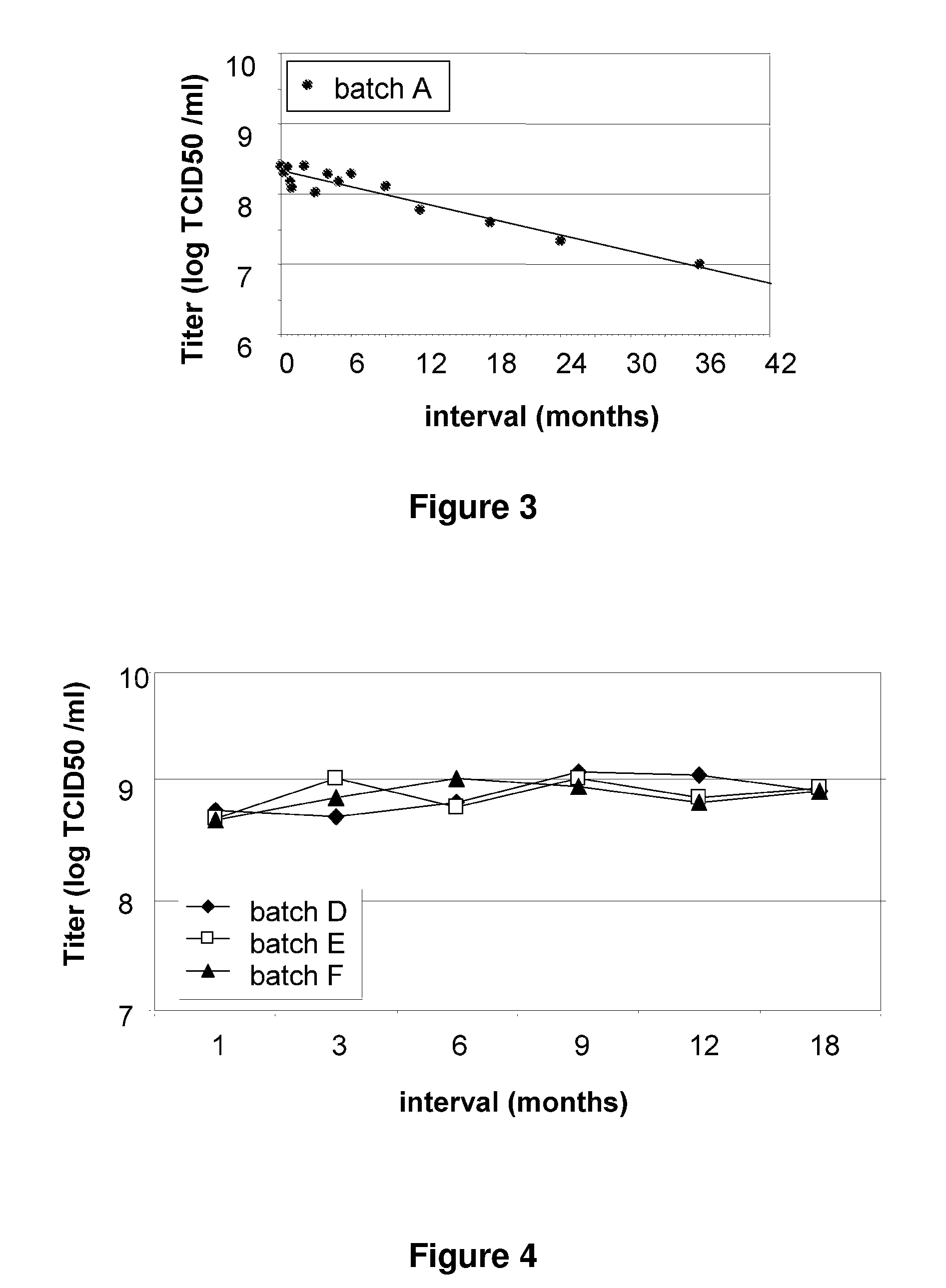
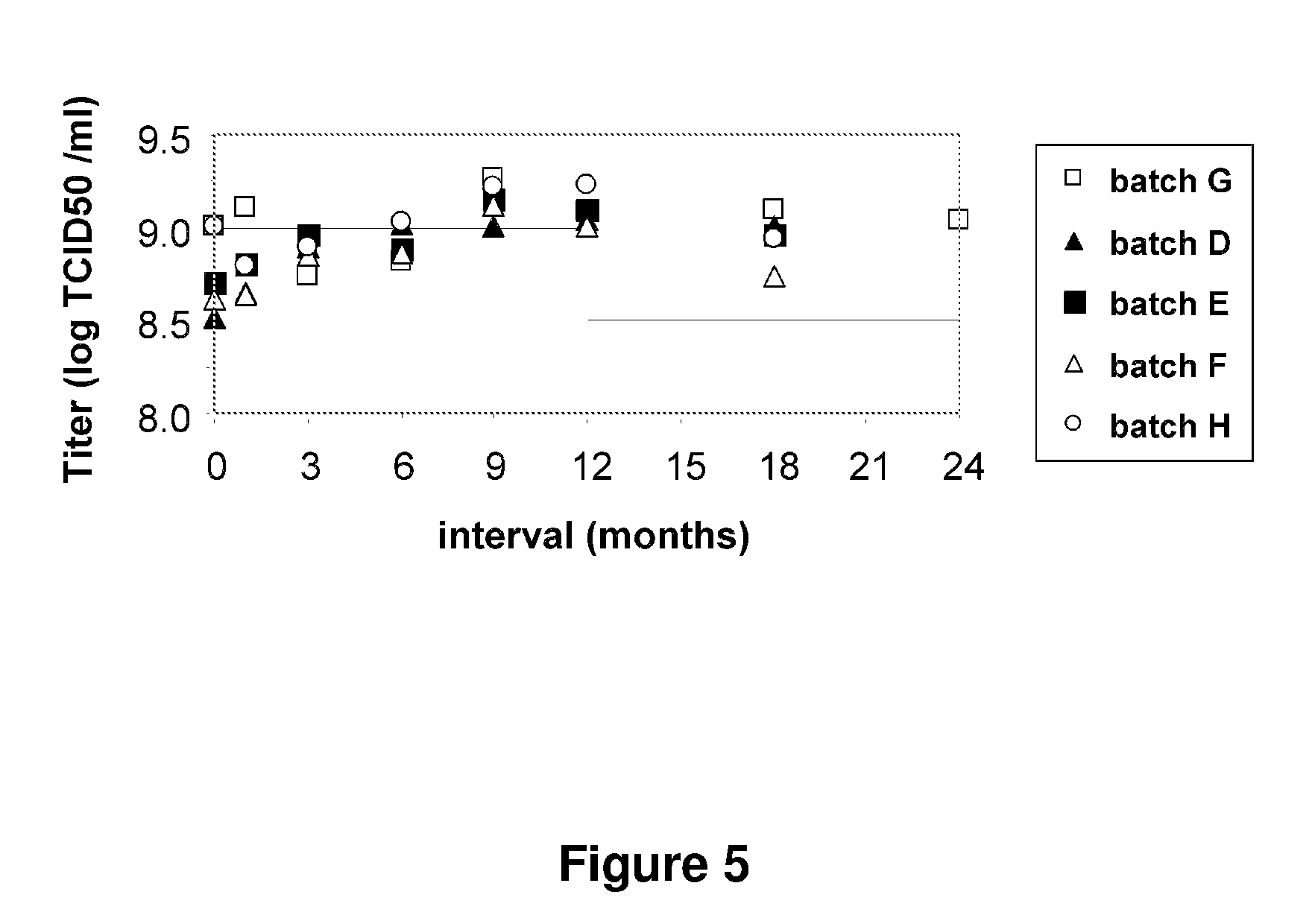
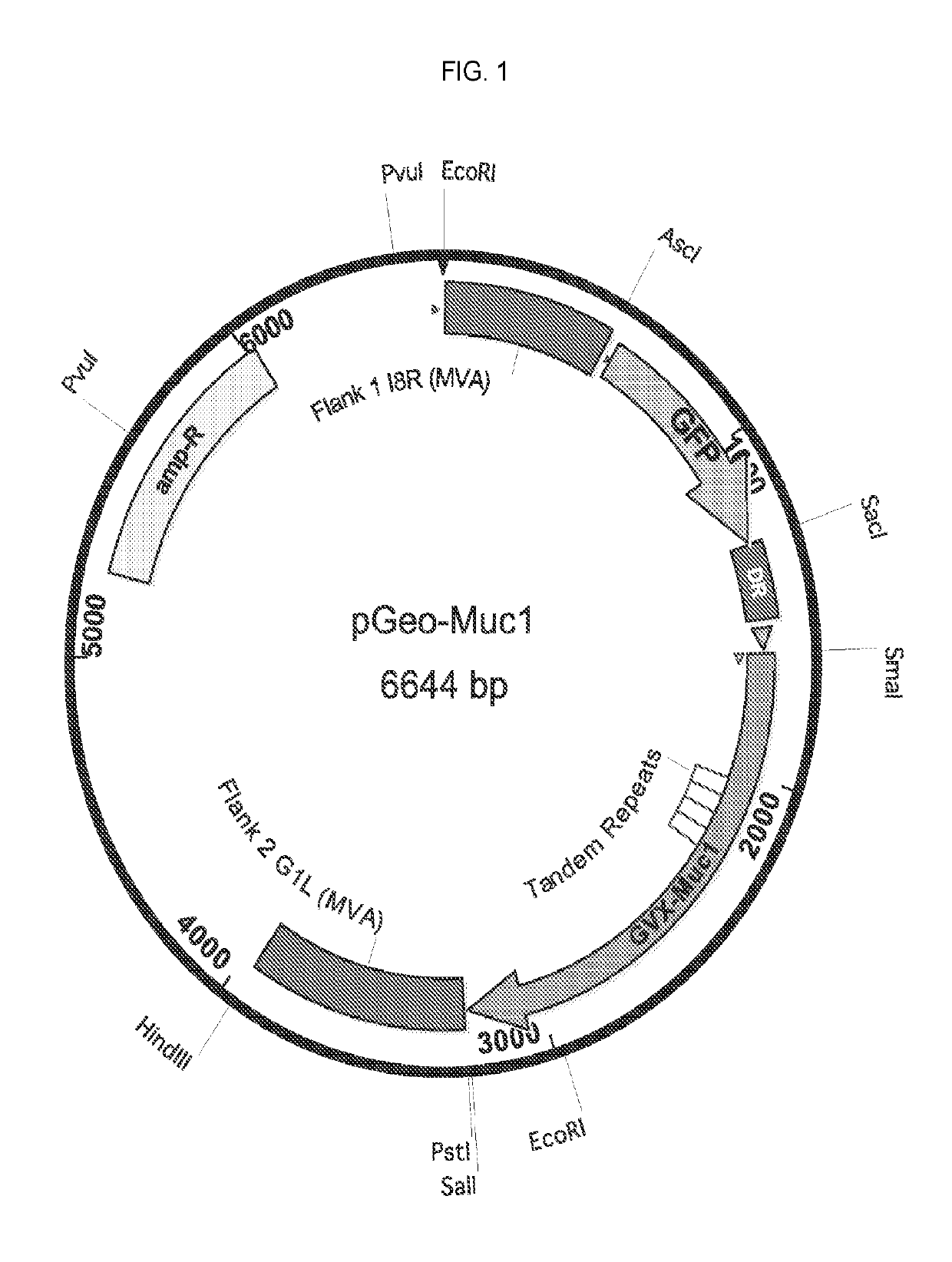
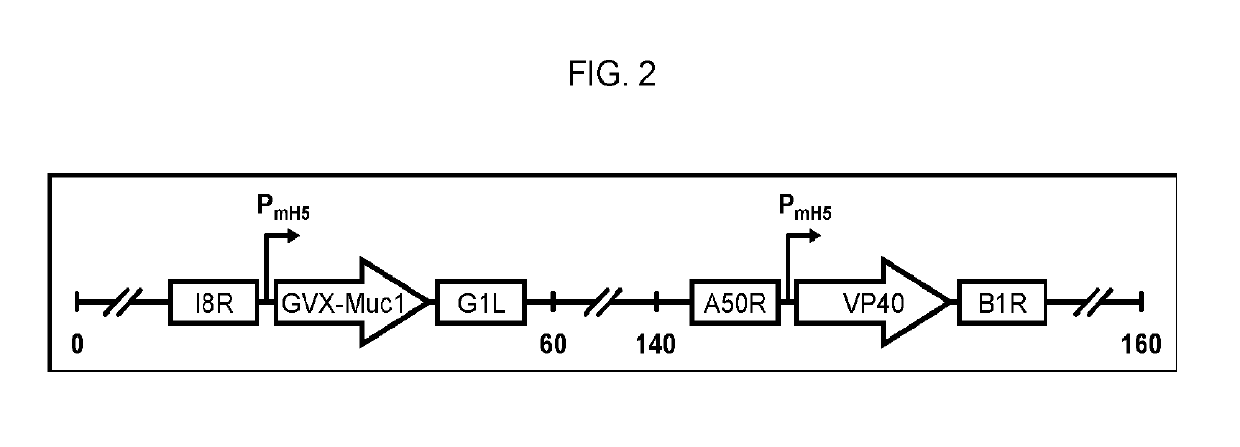
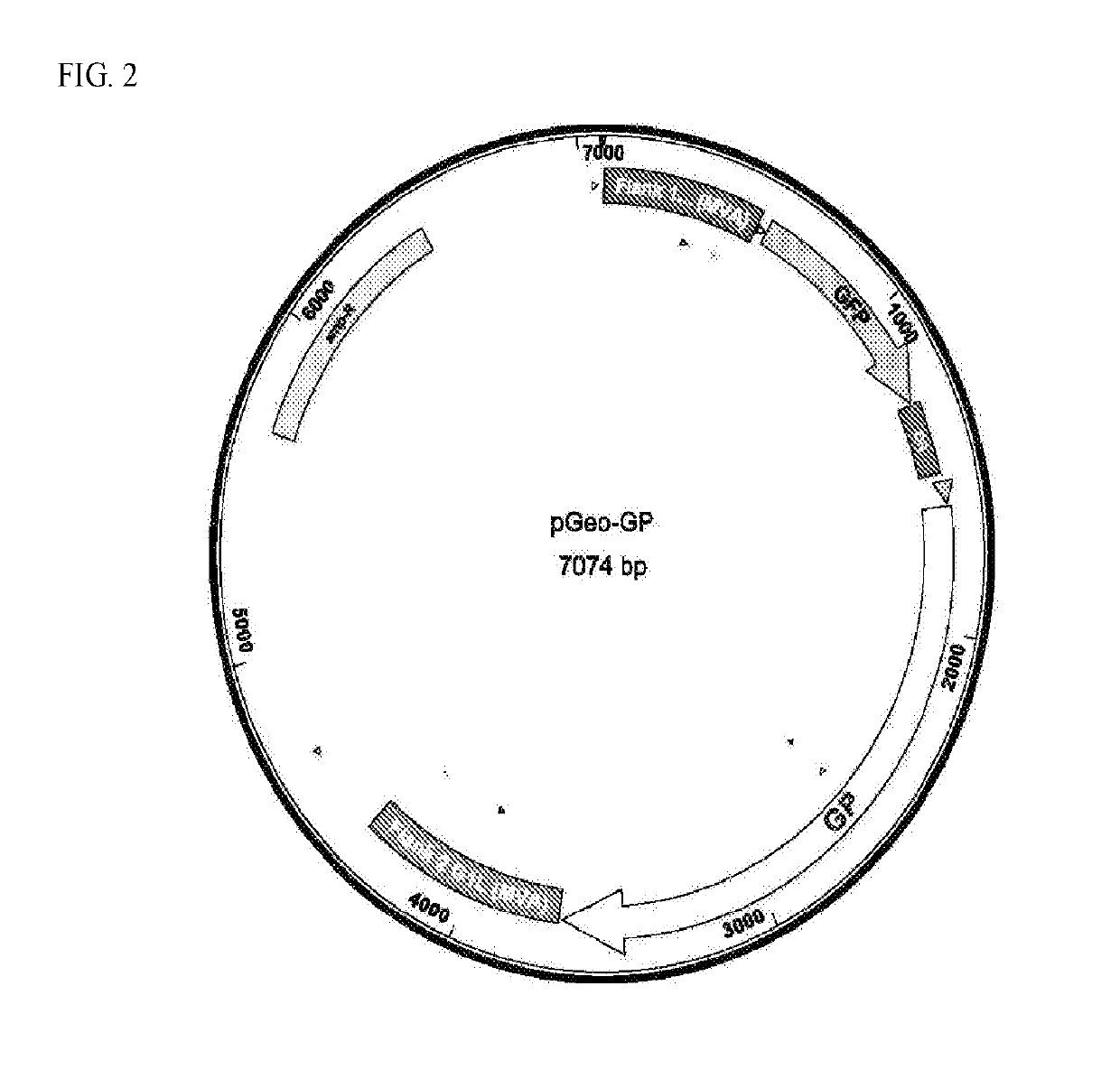
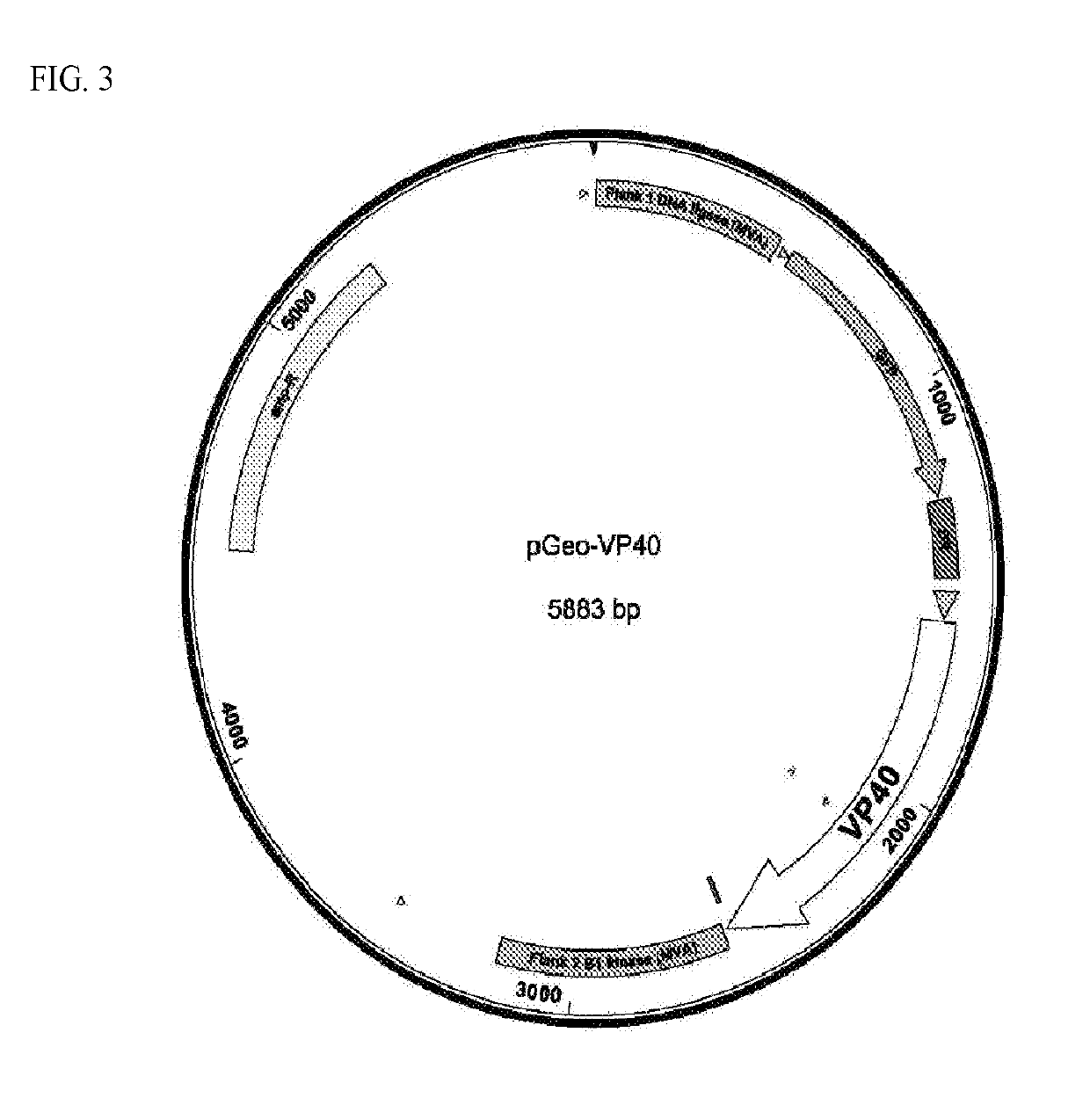
Patents
Literature
66 results about "Modified vaccinia Ankara" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The Modified Vaccinia Ankara (MVA) is an attenuated vaccine of a poxvirus. It was licensed and used as a poxvirus vaccine in Bavaria and is a vector for vaccination against non-poxvirus diseases.
Modified Vaccinia Ankara virus variant and cultivation method
InactiveUS7445924B2Reduce riskGenetic material ingredientsVirus peptidesSerum free mediaModified vaccinia Ankara
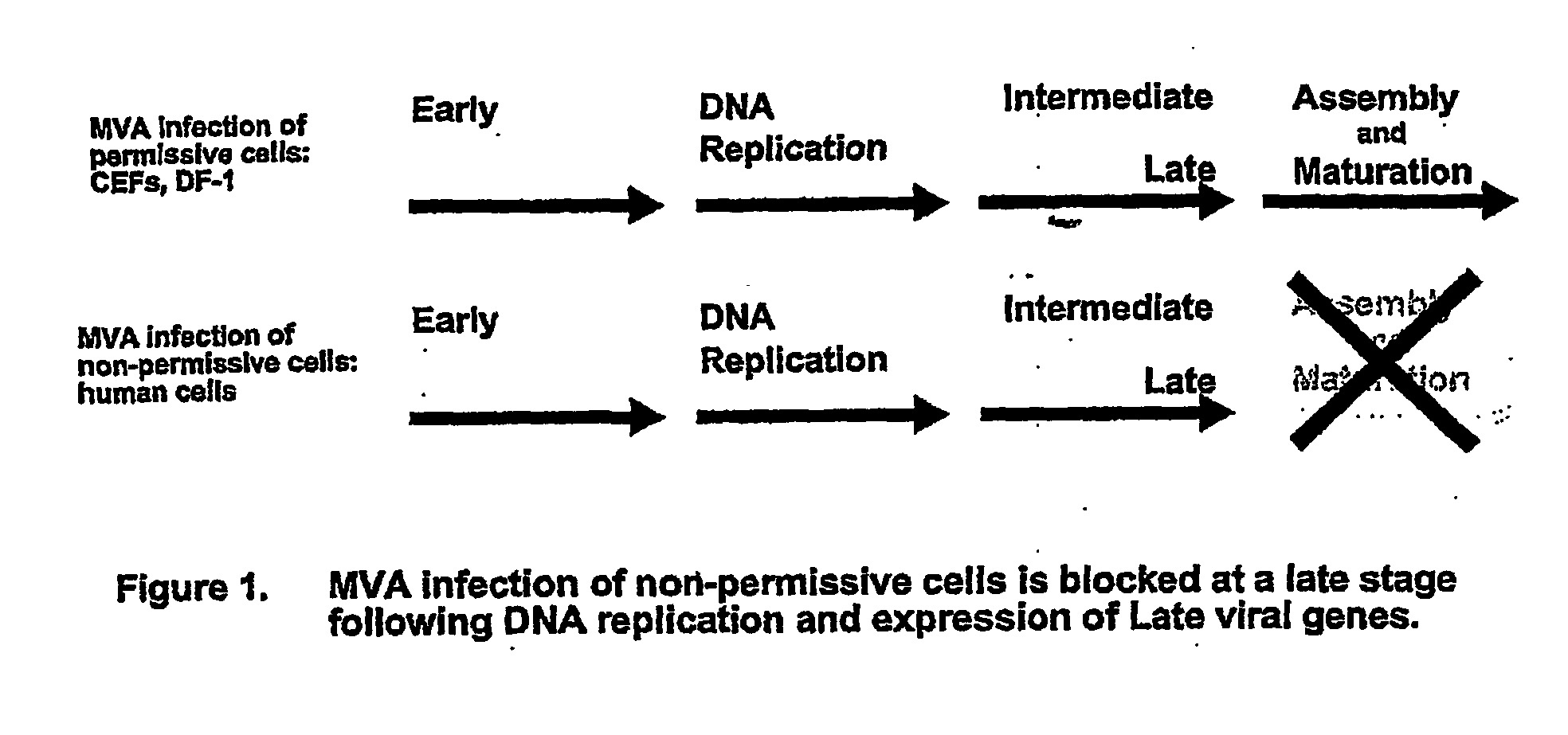
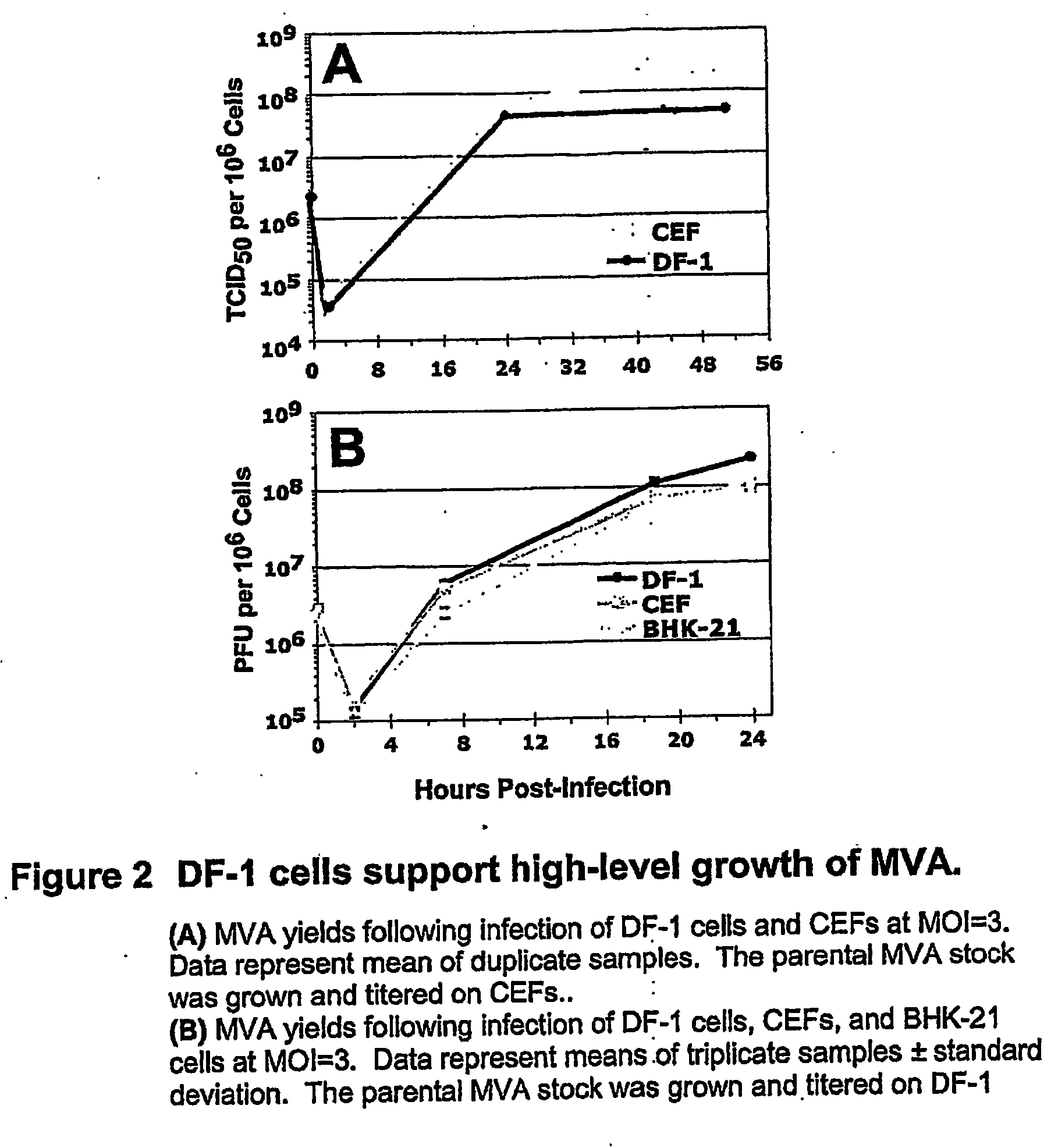
The present invention provides an attenuated virus, which is derived from Modified Vaccinia Ankara virus and characterized by the loss of its capability to reproductively replicate in human cell lines. It further describes recombinant viruses derived from this virus and the use of the virus, or its recombinants, as a medicament or vaccine. A method is provided for inducing an immune response in individuals who may be immune-compromised, receiving antiviral therapy, or have a pre-existing immunity to the vaccine virus. In addition, a method is provided for the administration of a therapeutically effective amount of the virus, or its recombinants, in a vaccinia virus prime / vaccinia virus boost innoculation regimen. The present invention relates to a method of virus amplification in primary cells which are cultivated in a serum free medium. Viruses produced by this method are advantageously free of any infectious agents comprised in animal sera.
Owner:BAVARIAN NORDIC AS
Modified vaccinia ankara virus variant and cultivation method
InactiveUS20050214323A1Reduce riskViral antigen ingredientsGenetic material ingredientsSerum free mediaModified vaccinia Ankara
The present invention provides an attenuated virus, which is derived from Modified Vaccinia Ankara virus and characterized by the loss of its capability to reproductively replicate in human cell lines. It further describes recombinant viruses derived from this virus and the use of the virus, or its recombinants, as a medicament or vaccine. A method is provided for inducing an immune response in individuals who may be immune-compromised, receiving antiviral therapy, or have a pre-existing immunity to the vaccine virus. In addition, a method is provided for the administration of a therapeutically effective amount of the virus, or its recombinants, in a vaccinia virus prime / vaccinia virus boost innoculation regimen. The present invention relates to a method of virus amplification in primary cells which are cultivated in a serum free medium. Viruses produced by this method are advantageously free of any infectious agents comprised in animal sera.
Owner:BAVARIAN NORDIC AS
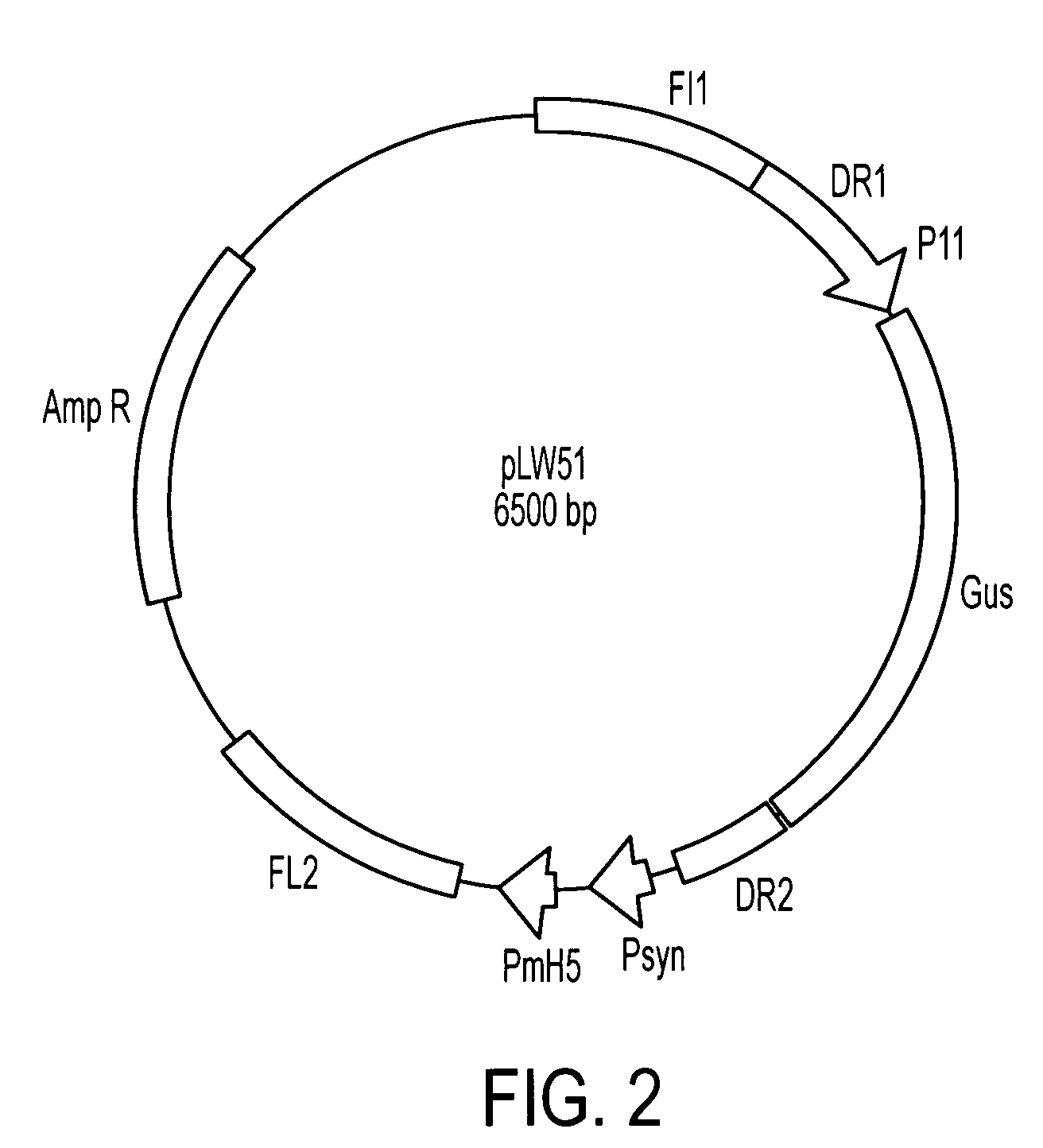
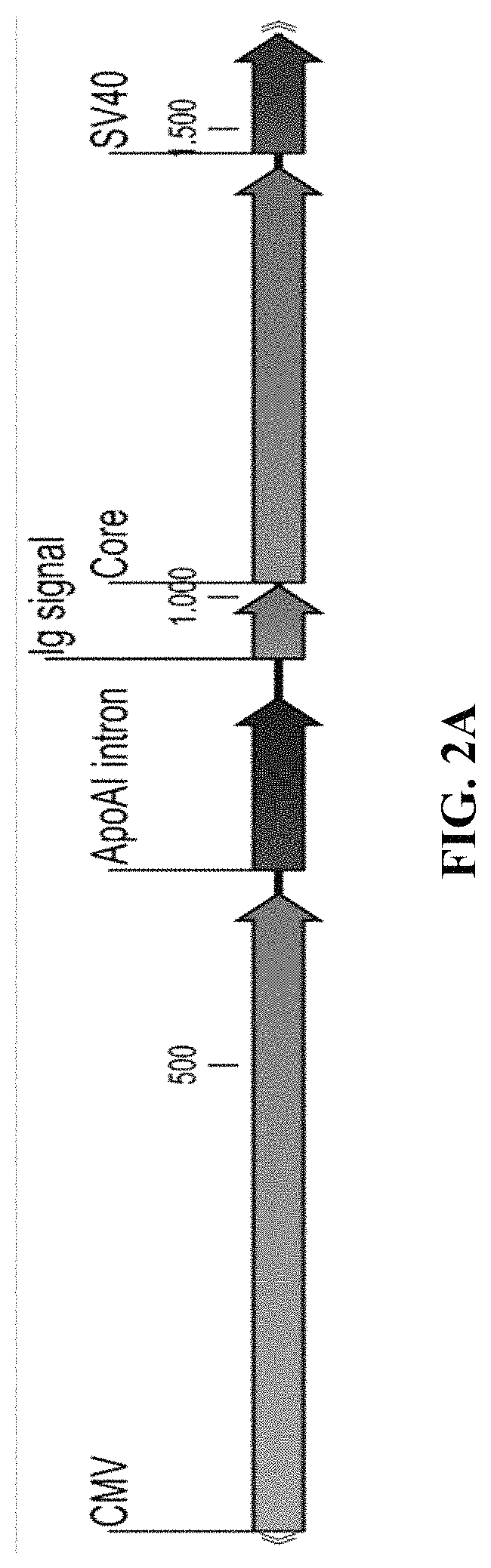
Genetically stable recombinant modified vaccinia ankara (RMVA) vaccines and methods of preparation thereof
ActiveUS20100316667A1Elicit immune responseVectorsSugar derivativesHeterologousModified vaccinia Ankara
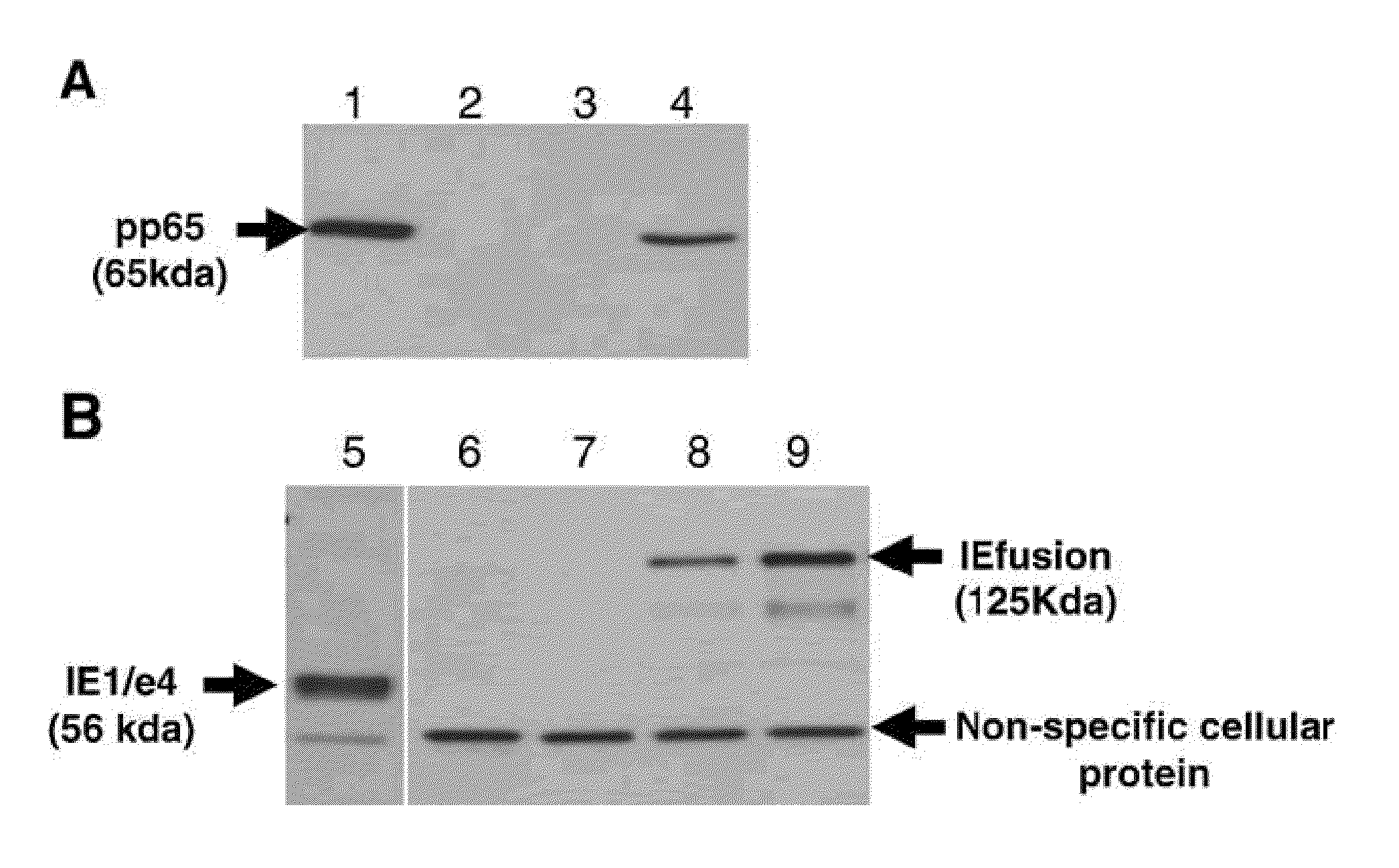
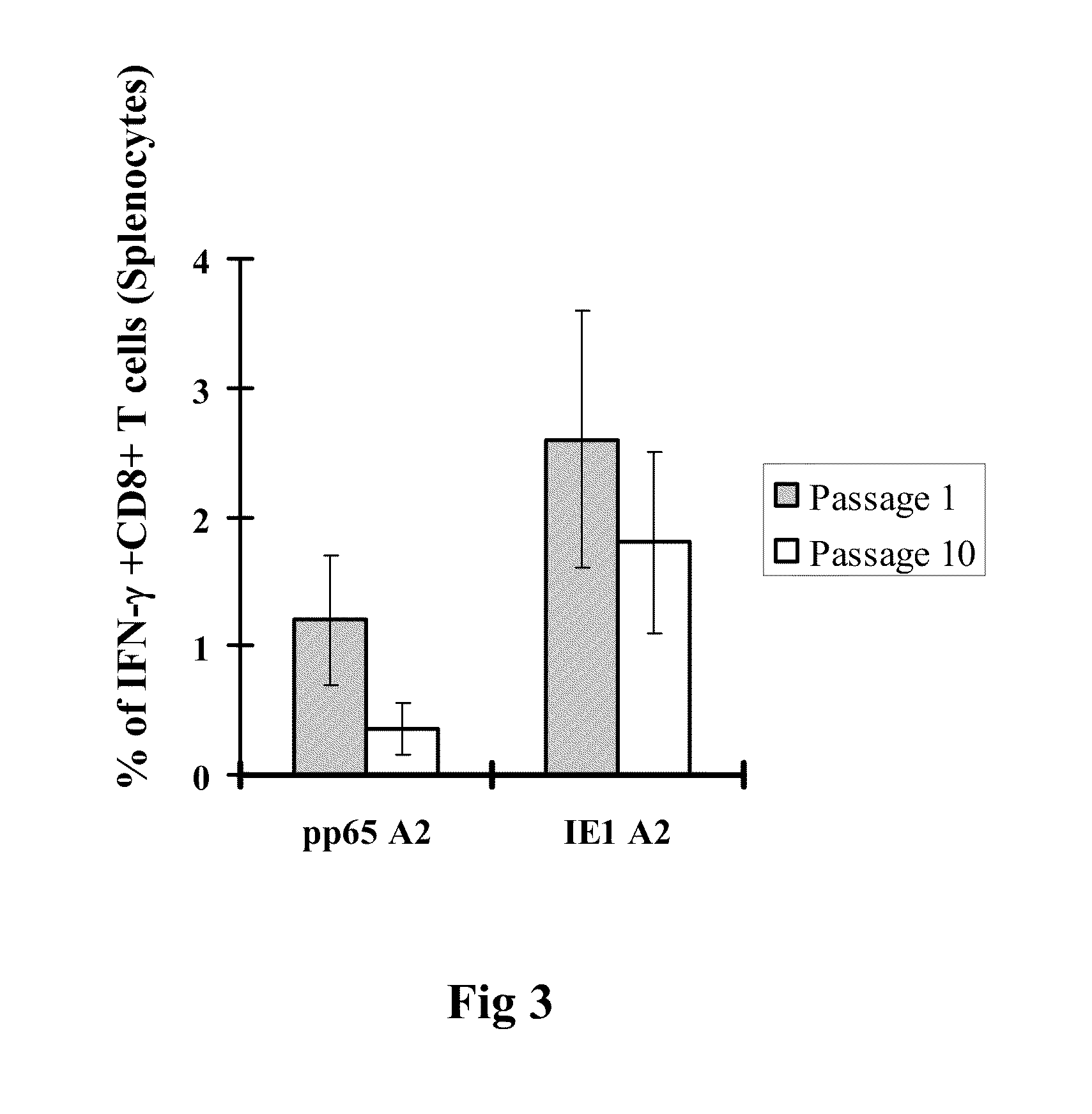
A vaccine comprising an immunologically effective amount of recombinant modified vaccinia Ankara (rMVA) virus which is genetically stable after serial passage and produced by a) constructing a transfer plasmid vector comprising a modified H5 (mH5) promoter operably linked to a DNA sequence encoding a heterologous foreign protein antigen, wherein the expression of said DNA sequence is under the control of the mH5 promoter; b) generating rMVA virus by transfecting one or more plasmid vectors obtained from step a) into wild type MVA virus; c) identifying rMVA virus expressing one or more heterologous foreign protein antigens using one or more selection methods for serial passage; d) conducting serial passage; e) expanding an rMVA virus strain identified by step d); and f) purifying the rMVA viruses from step e) to form the vaccine. One embodiment is directed to a fusion cytomegalovirus (CMV) protein antigen comprising a nucleotide sequence encoding two or more antigenic portions of Immediate-Early Gene-1 or Immediate-Early Gene-2 (IEfusion), wherein the antigenic portions elicit an immune response when expressed by a vaccine.
Owner:CITY OF HOPE
Modified vaccinia Ankara expressing p53 in cancer immunotherapy
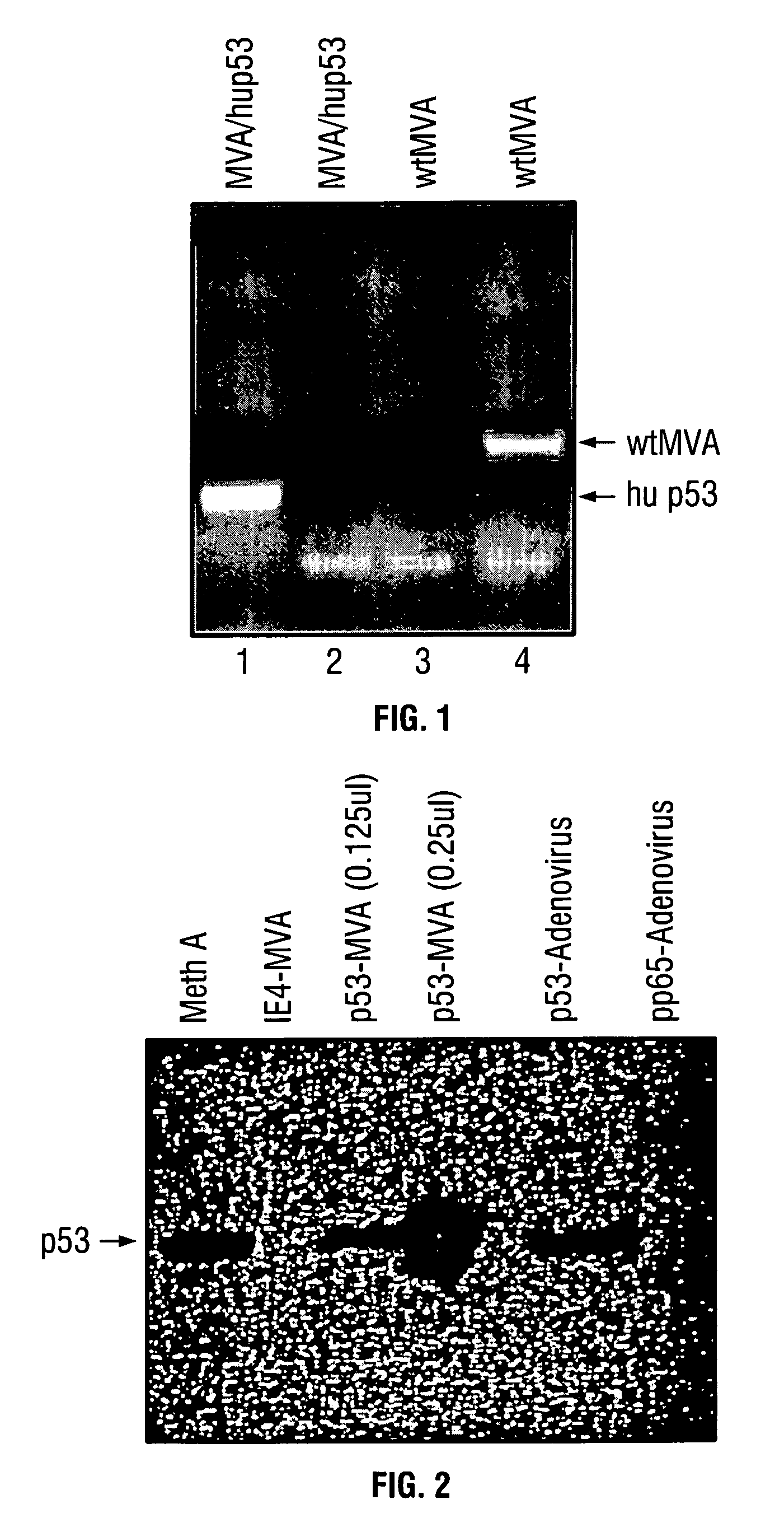
ActiveUS7256037B2Limit therapeutic efficacyReduce developmentBiocideGenetic material ingredientsModified vaccinia AnkaraADAMTS Proteins
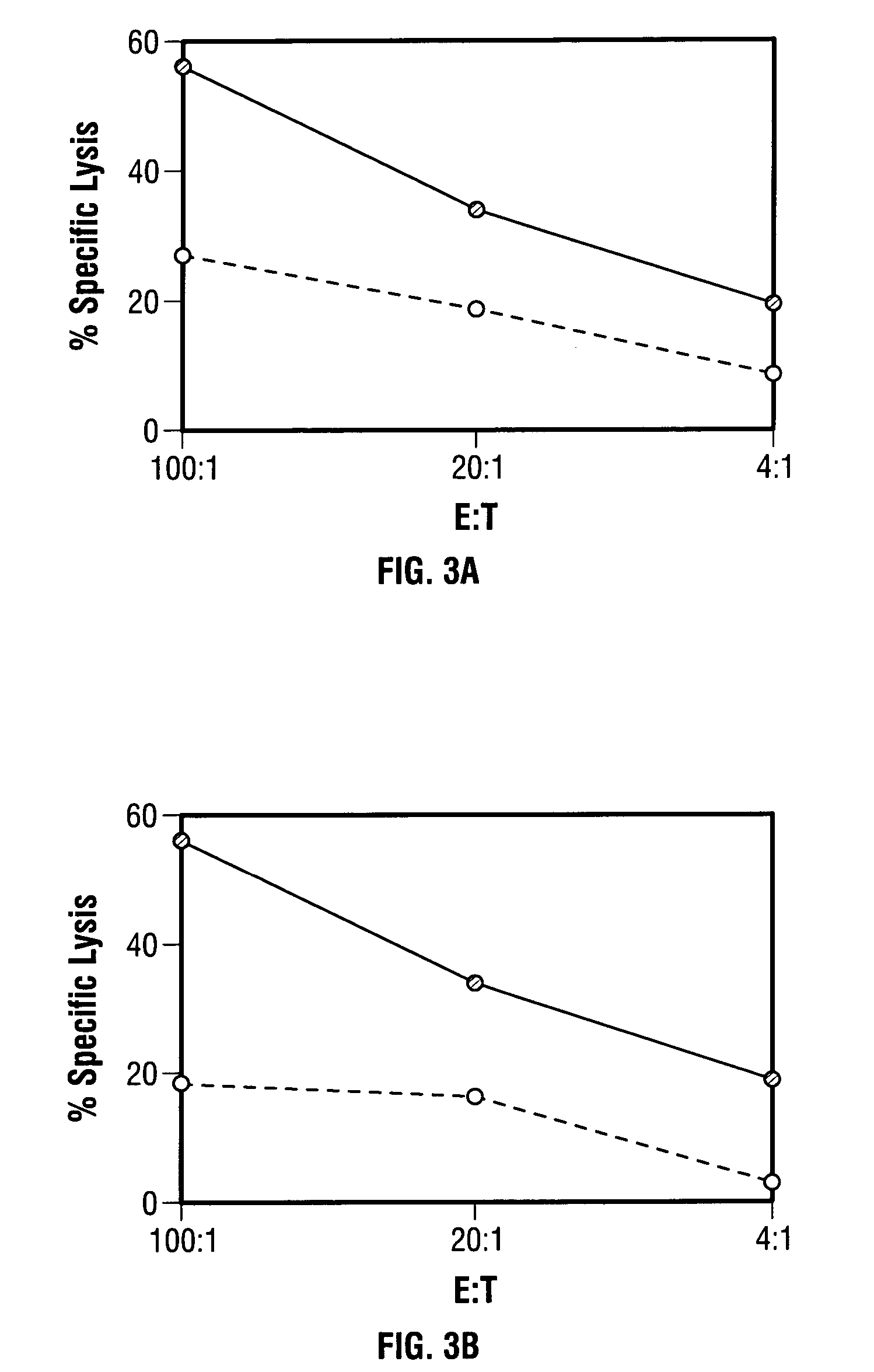
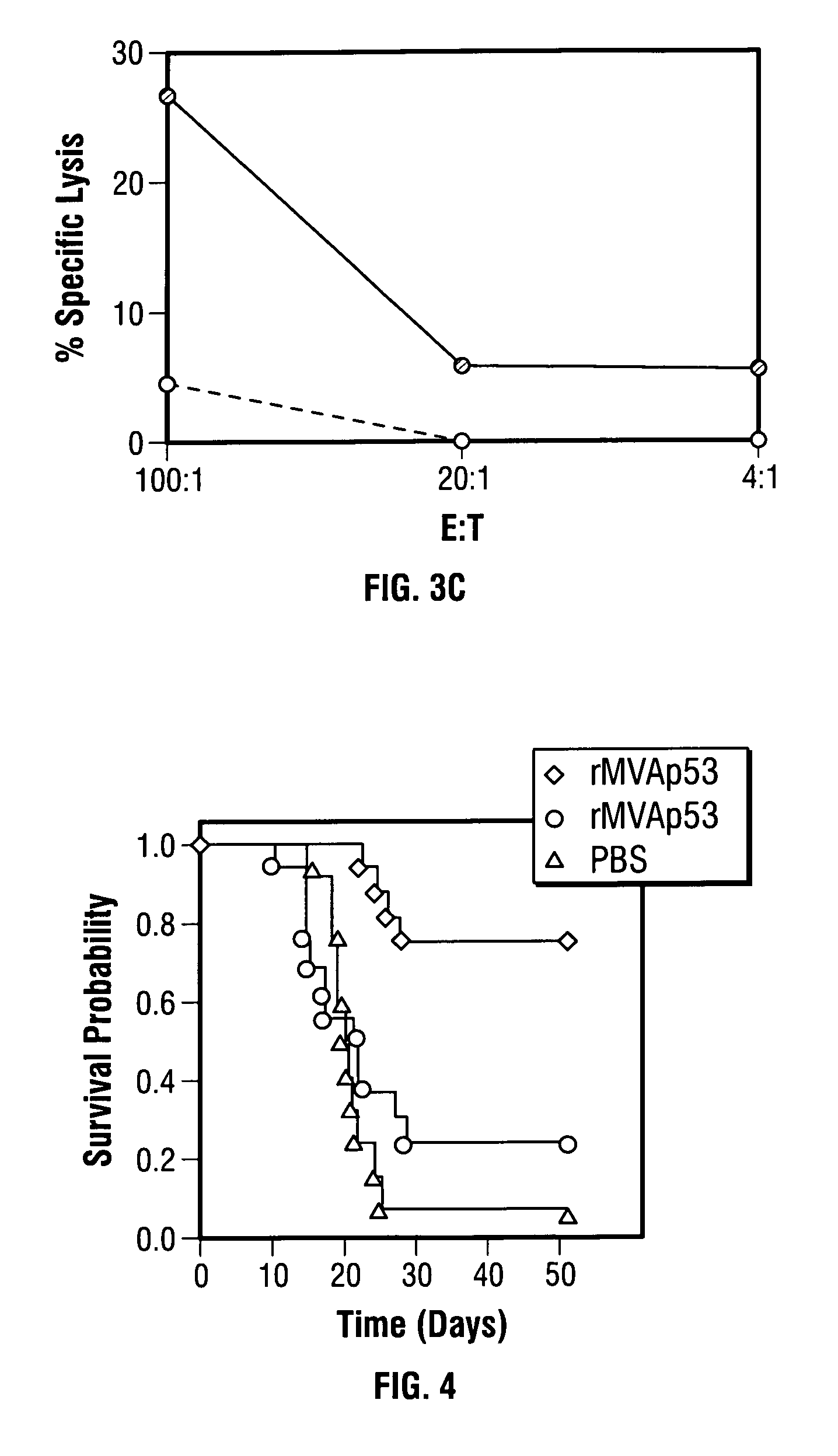
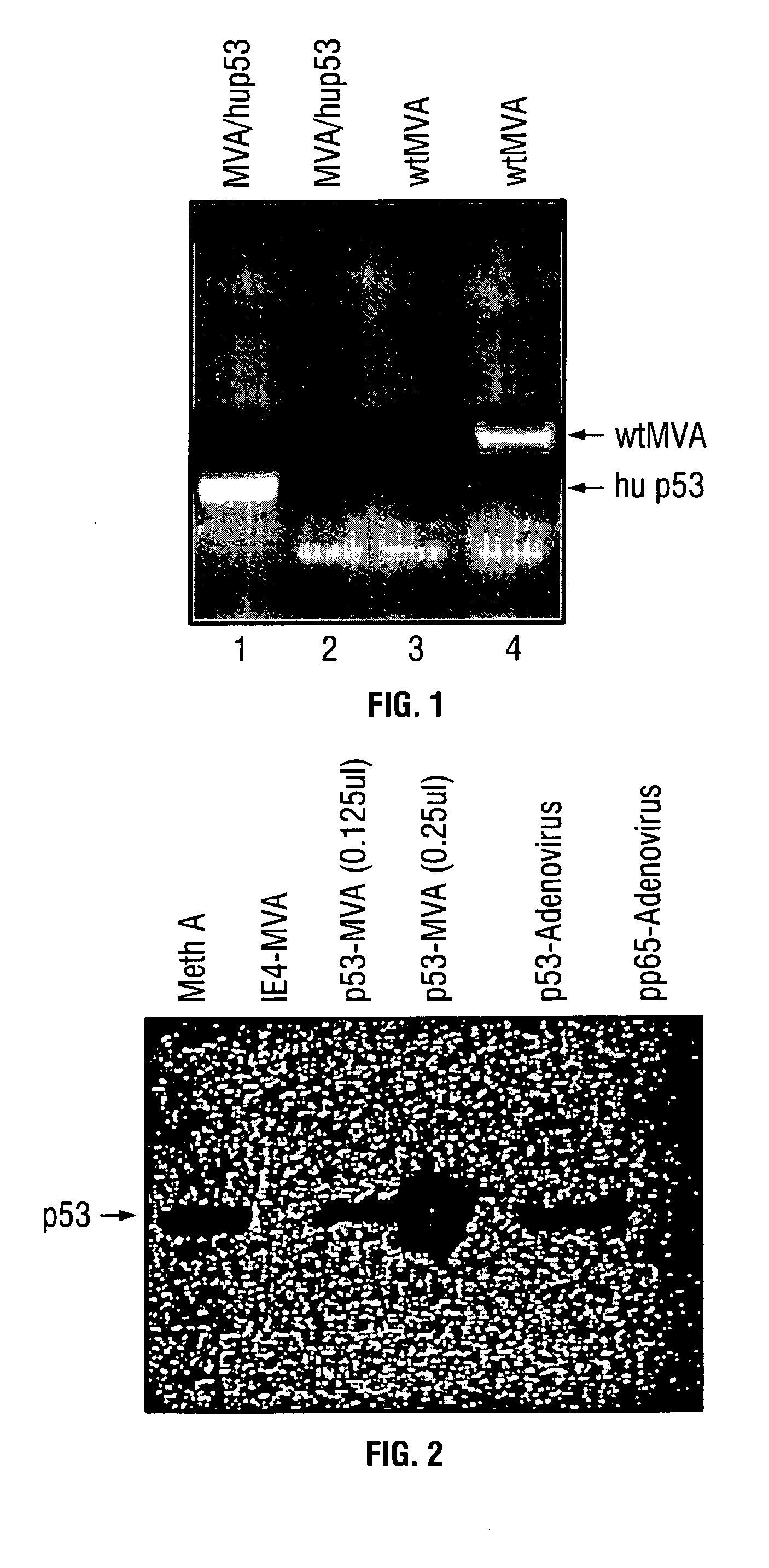
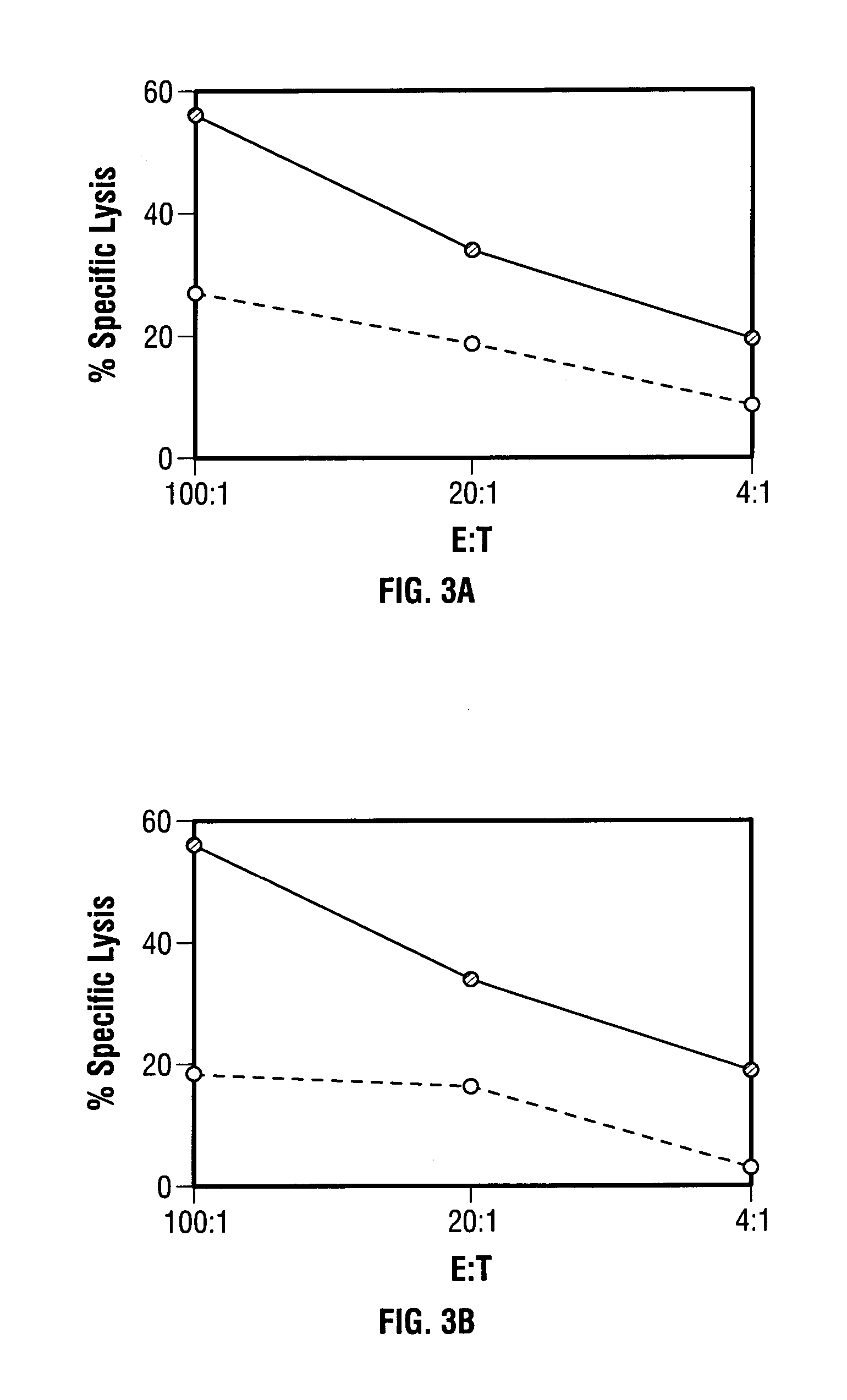
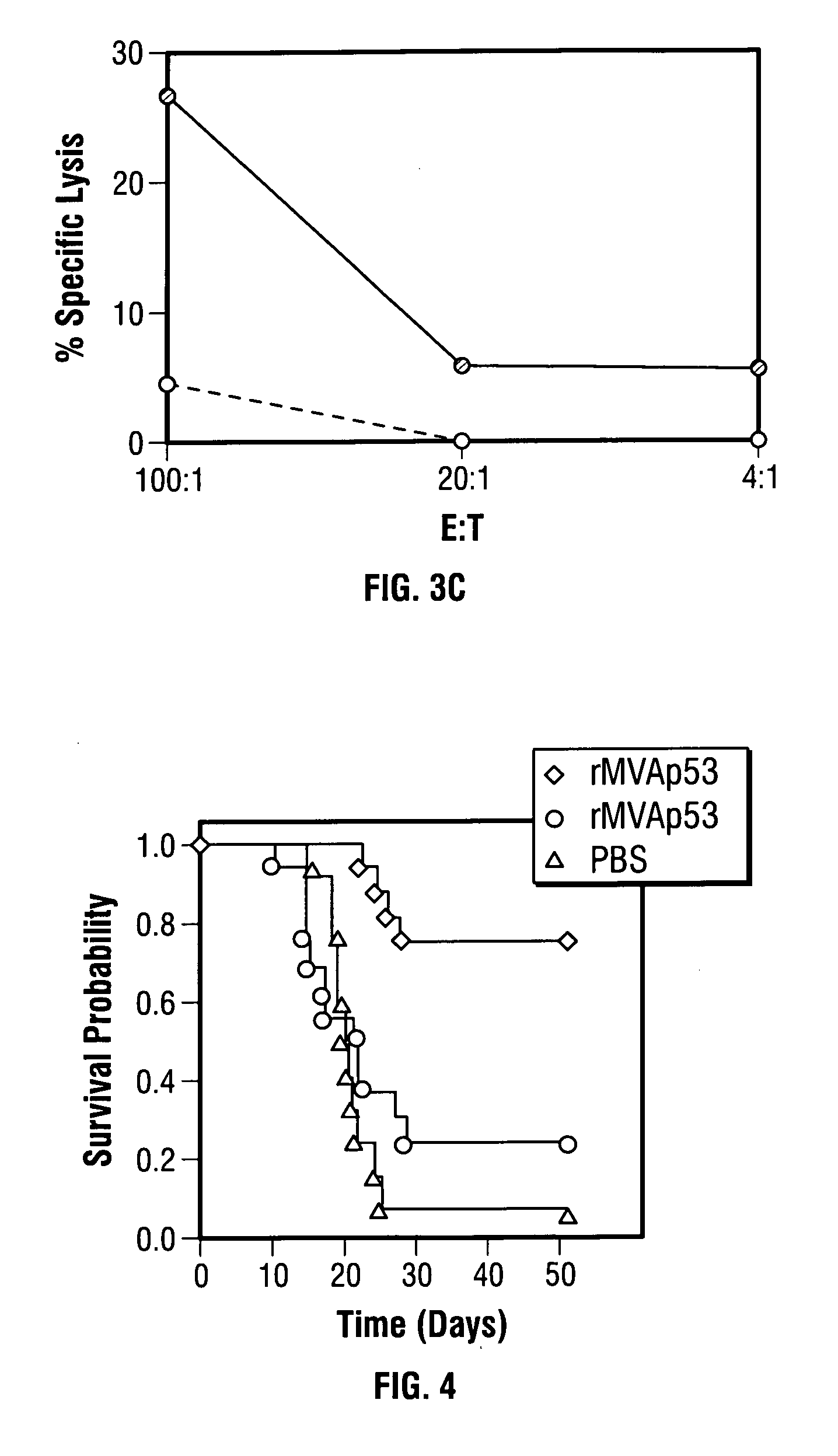
Mutations to the tumor suppressor protein p53 have been observed in 40-60% of all human cancers. These mutations are often associated with high nuclear and cytoplasmic concentrations of p53. Since many tumors exhibit highly elevated p53 levels, the protein is an attractive target for cancer immunotherapy. Unfortunately, p53 is an autoantigen that is likely to be tolerated as a self-protein by the immune system. The present invention is based on the discovery that this self-tolerance can be overcome by administration of recombinant modified vaccinia Ankara (MVA) containing a nucleic acid that encodes p53 (rMVAp53). The invention discloses a method of generating a p53-specific CTL-response to tumor cells expressing mutated p53 by administering a composition comprising rMVAp53. Administration of rMVAp53 decreases tumor development, tumor growth, and mortality in a variety of malignant cell types. These effects are enhanced by administration of CTLA-4 blocker and / or CpG oligodeoxynucleotide immunomodulators.
Owner:CITY OF HOPE
Modified vaccinia ankara expressing p53 in cancer immunotherapy
Mutations to the tumor suppressor protein p53 have been observed in 40-60% of all human cancers. These mutations are often associated with high nuclear and cytoplasmic concentrations of p53. Since many tumors exhibit highly elevated p53 levels, the protein is an attractive target for cancer immunotherapy. Unfortunately, p53 is an autoantigen that is likely to be tolerated as a self-protein by the immune system. The present invention is based on the discovery that this self-tolerance can be overcome by administration of recombinant modified vaccinia Ankara (MVA) containing a nucleic acid that encodes p53 (rMVAp53). The invention discloses a method of generating a p53-specific CTL-response to tumor cells expressing mutated p53 by administering a composition comprising rMVAp53. Administration of rMVAp53 decreases tumor development, tumor growth, and mortality in a variety of malignant cell types. These effects are enhanced by administration of CTLA-4 blocker and / or CpG oligodeoxynucleotide immunomodulators.
Owner:CITY OF HOPE
Genetically stable recombinant modified vaccinia ankara (rMVA) vaccines and methods of preparation thereof
A vaccine comprising an immunologically effective amount of recombinant modified vaccinia Ankara (rMVA) virus which is genetically stable after serial passage and produced by a) constructing a transfer plasmid vector comprising a modified H5 (mH5) promoter operably linked to a DNA sequence encoding a heterologous foreign protein antigen, wherein the expression of said DNA sequence is under the control of the mH5 promoter; b) generating rMVA virus by transfecting one or more plasmid vectors obtained from step a) into wild type MVA virus; c) identifying rMVA virus expressing one or more heterologous foreign protein antigens using one or more selection methods for serial passage; d) conducting serial passage; e) expanding an rMVA virus strain identified by step d); and f) purifying the rMVA viruses from step e) to form the vaccine. One embodiment is directed to a fusion cytomegalovirus (CMV) protein antigen comprising a nucleotide sequence encoding two or more antigenic portions of Immediate-Early Gene-1 or Immediate-Early Gene-2 (IEfusion), wherein the antigenic portions elicit an immune response when expressed by a vaccine.
Owner:CITY OF HOPE
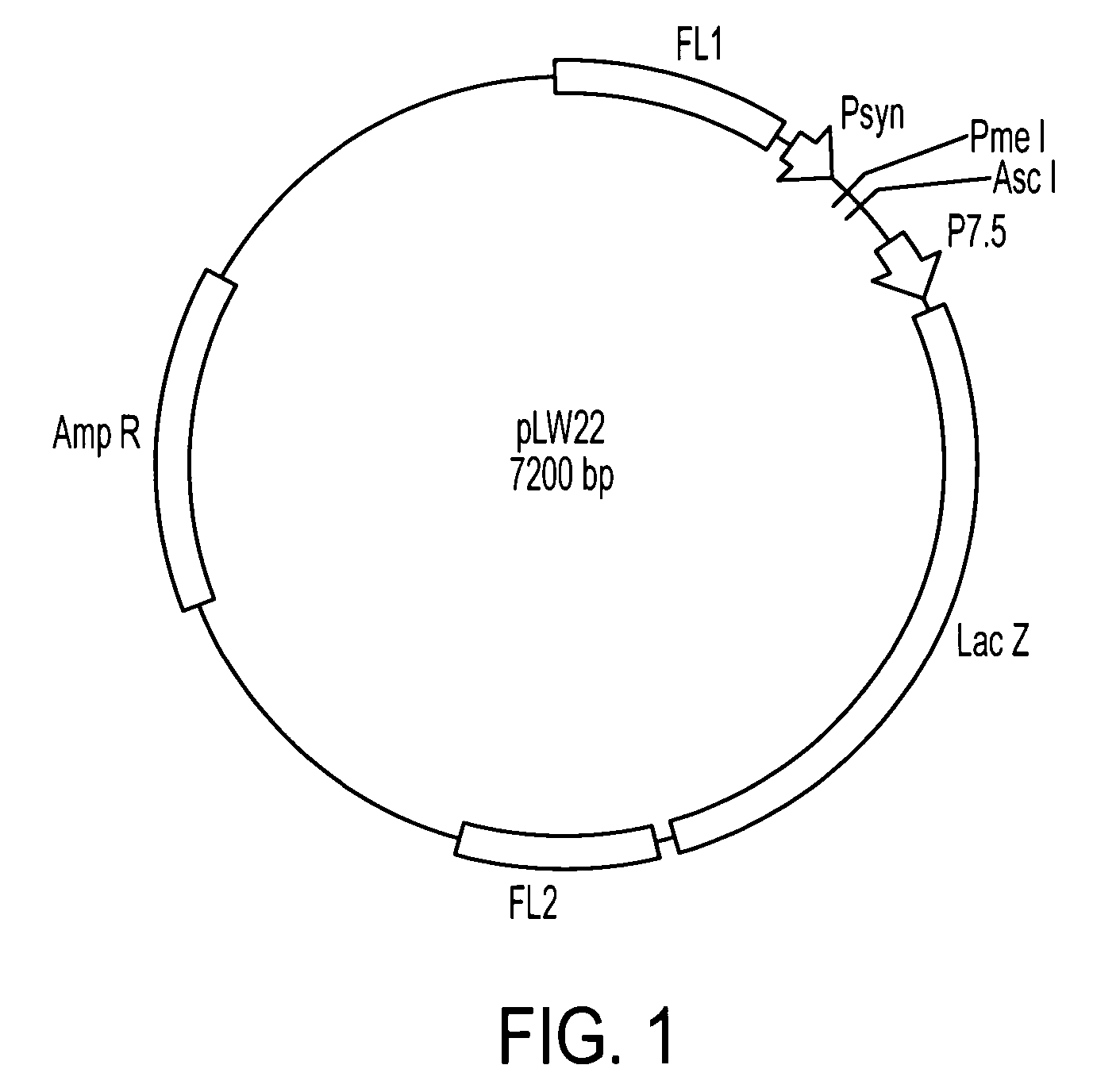
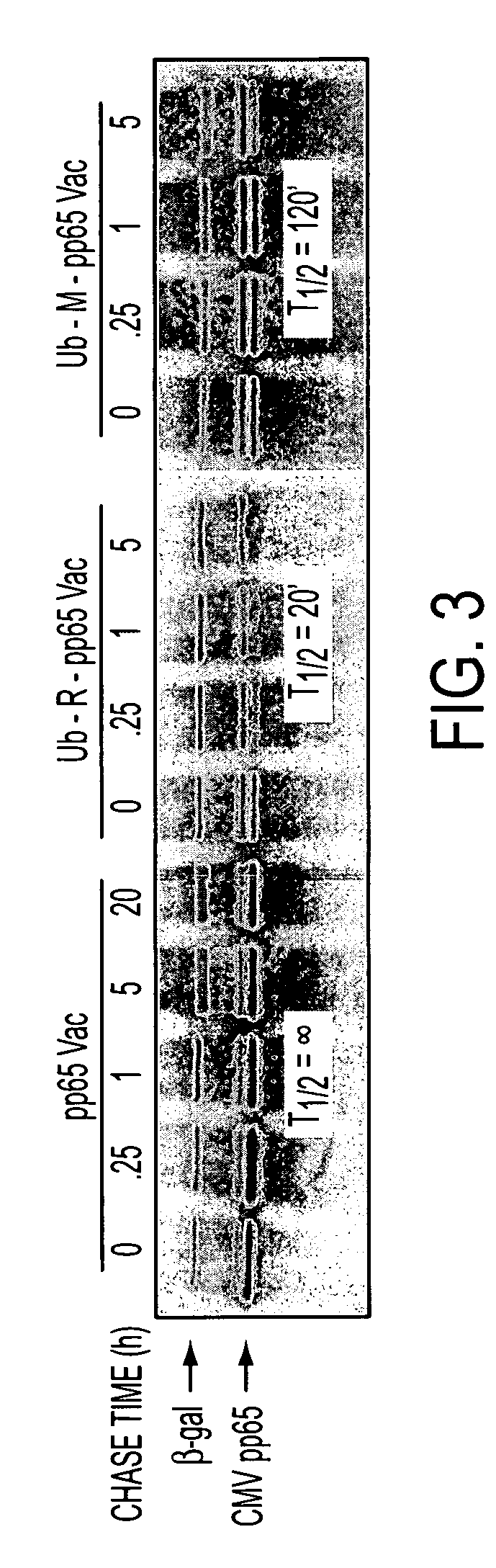
Human cytomegalovirus antigens expressed in MVA and methods of use
ActiveUS7163685B2Improve immunitySugar derivativesViral antigen ingredientsModified vaccinia AnkaraVaccine virus
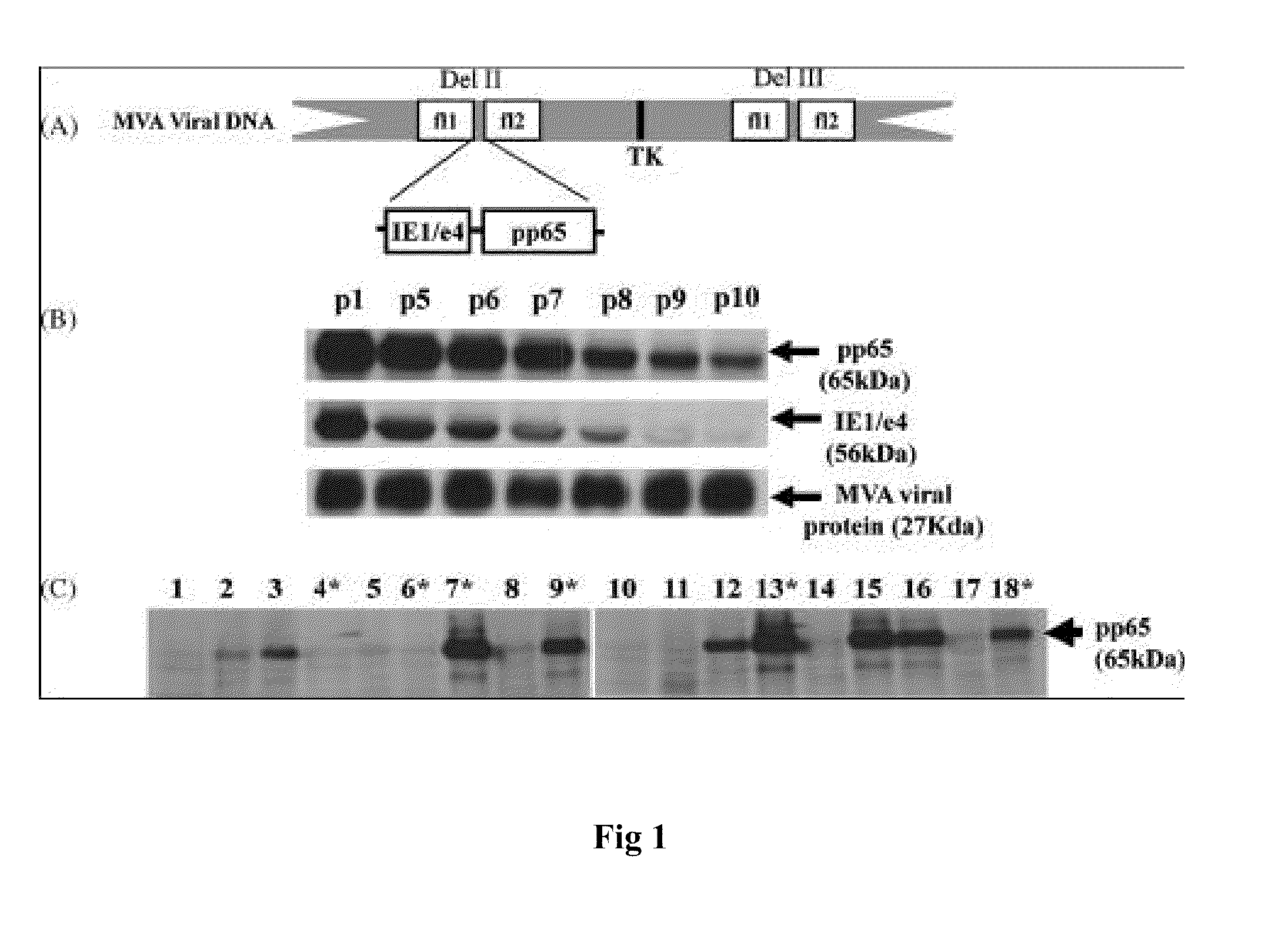
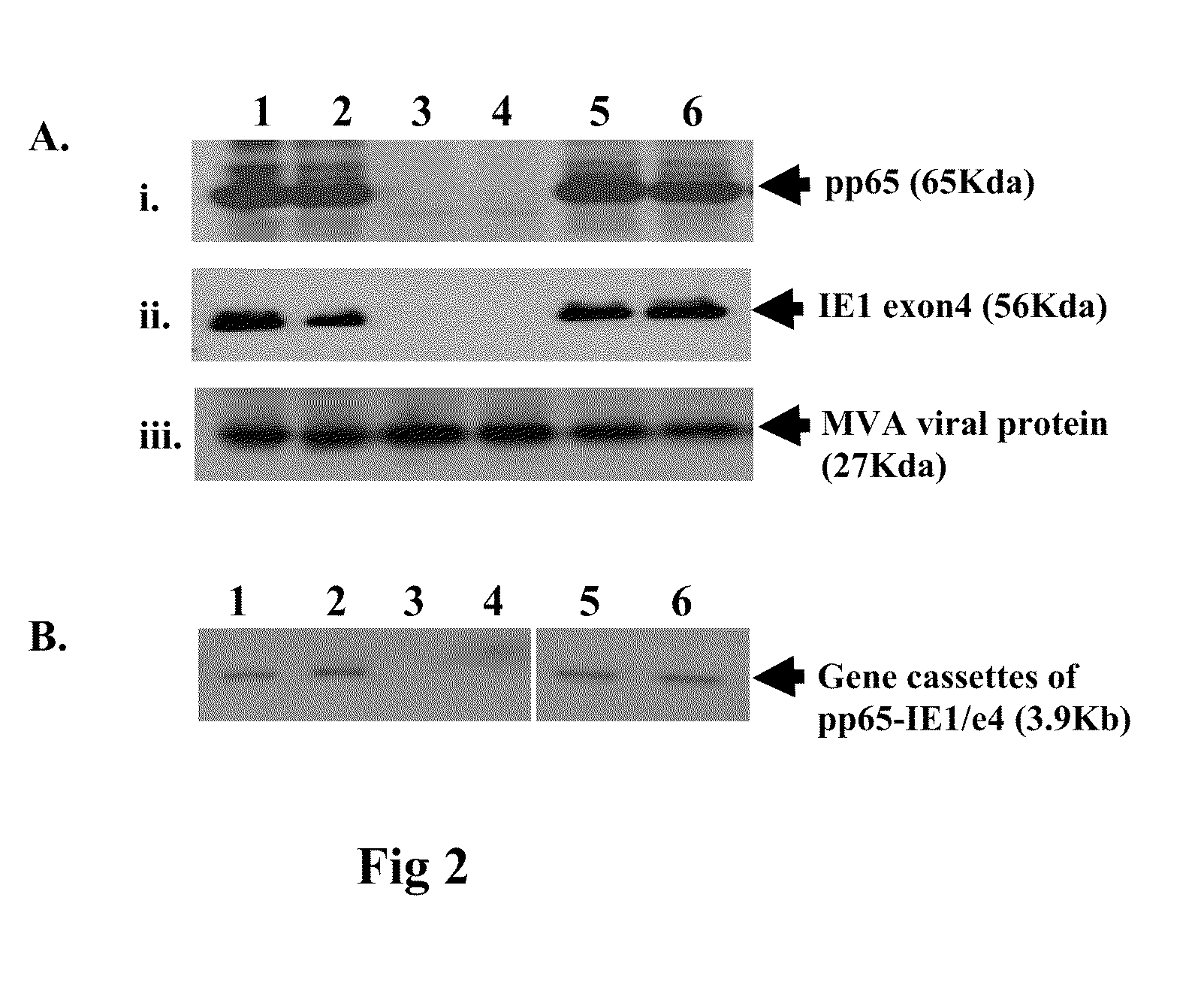
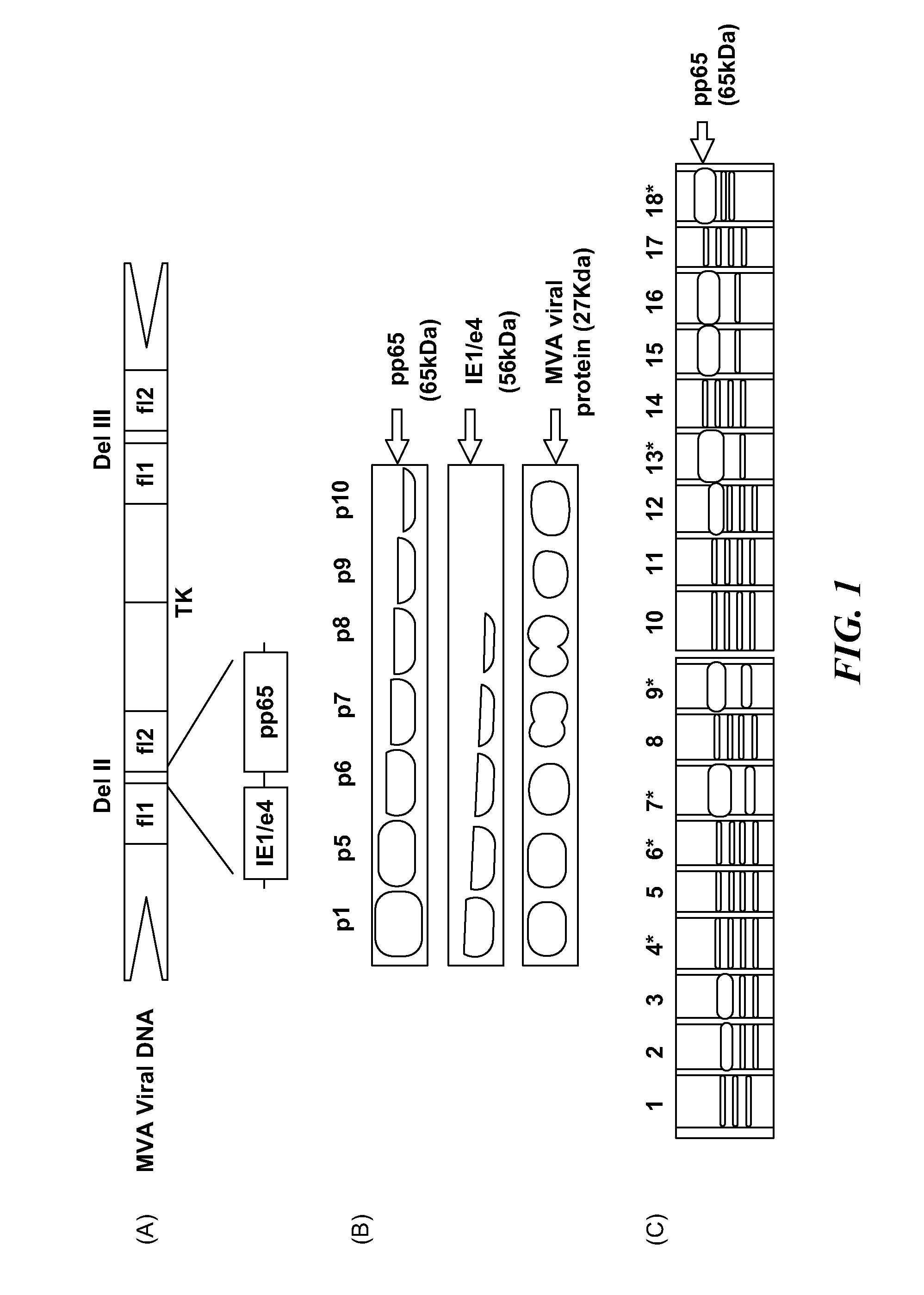
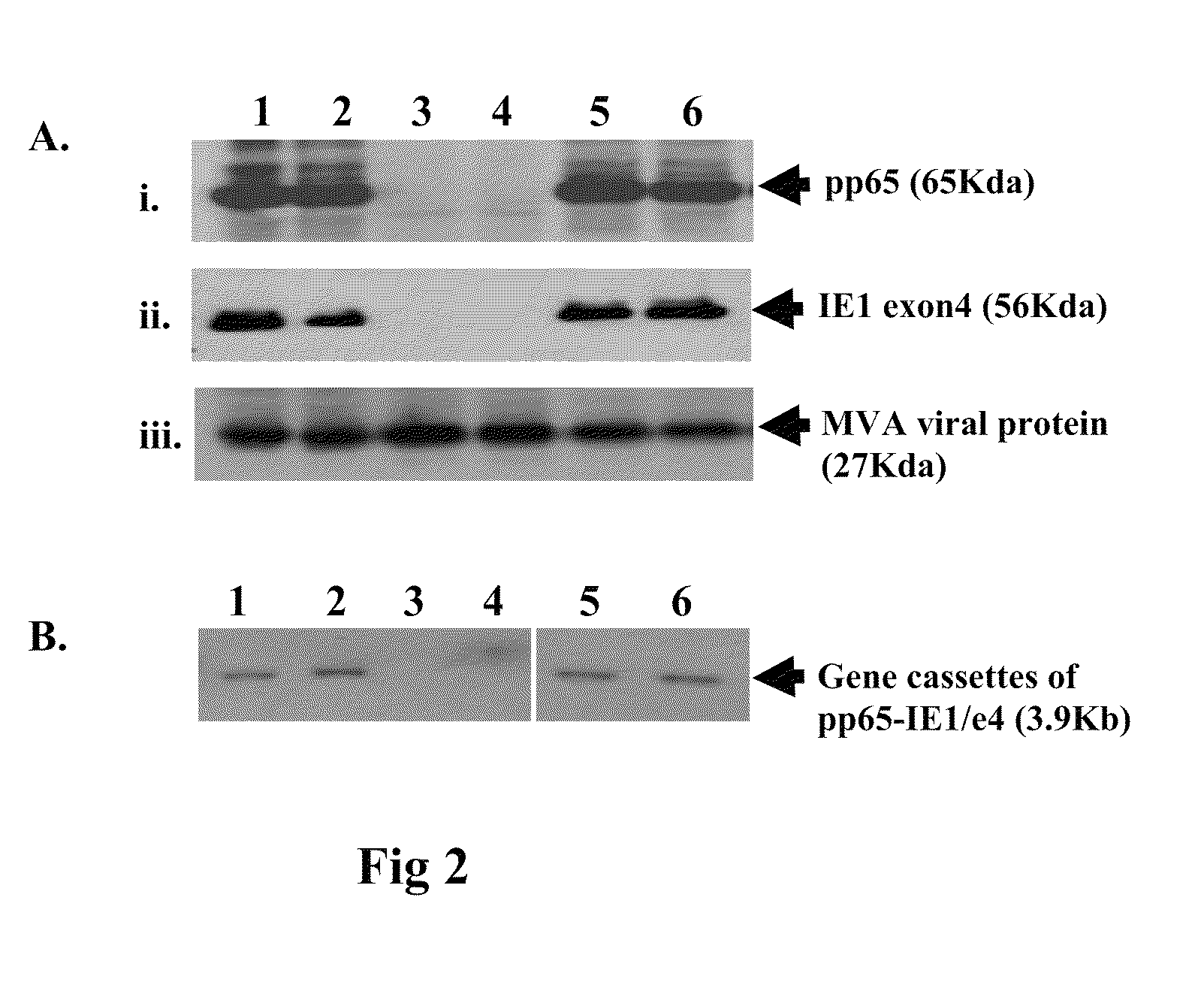
DNA and protein constructs useful in producing vaccines against human cytomegalovirus contain optionally N-end modified and N-terminal ubiquitinated human cytomegalovirus antigenic proteins, including pp65, pp150, IE1, gB and antigenic fragments thereof. Vaccine viruses, in particular poxviruses such as vaccinia and Modified Vaccinia Ankara, that express the constructs may be used as vaccines to augment the immune response to human cytomegalovirus, both prophylatically and in patients already carrying human cytomegalovirus, as well as to create and expand cytomegalovirus-reactive T cells for transfer of adoptive immunity.
Owner:CITY OF HOPE
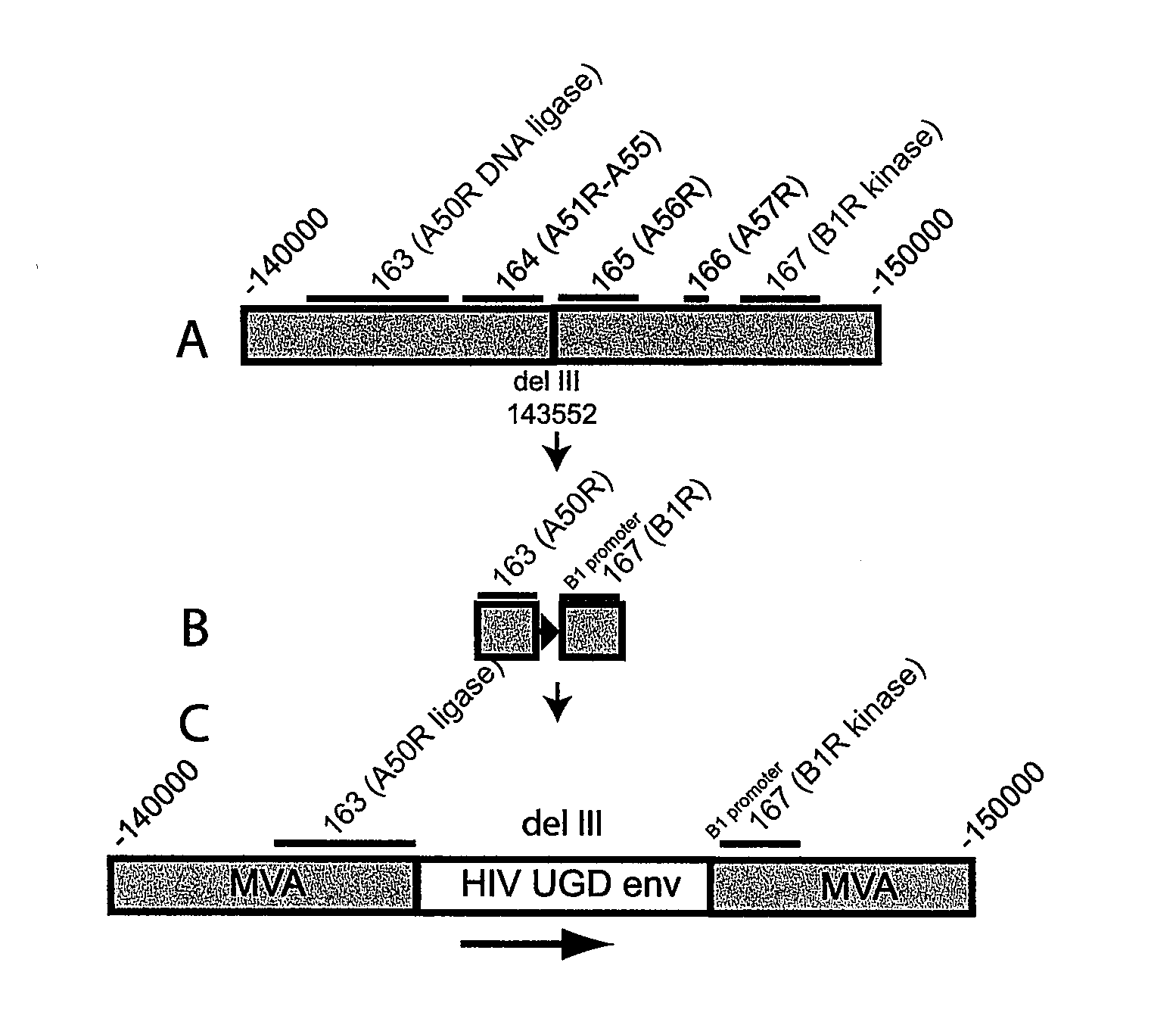
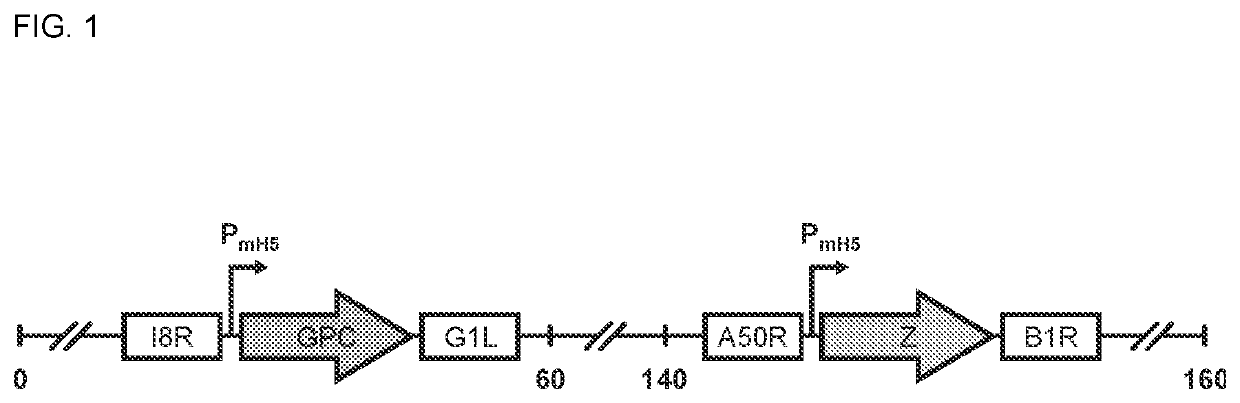
Recombinant modified vaccinia ankara (MVA) vaccinia virus containing restructured insertion sites
The present invention relates to recombinant modified vaccinia Ankara (MVA) virus containing restructured sites useful for the integration of heterologous nucleic acid sequences into an intergenic region (IGR) of the virus genome, where the IGR is located between two adjacent, essential open reading frames (ORFs) of the vaccinia virus genome, wherein the adjacent essential ORFs are non-adjacent in a parental MVA virus used to construct the recombinant MVA virus, and to related nucleic acid constructs useful for inserting heterologous DNA into the genome of a vaccinia virus, and further to the use of the disclosed viruses as a medicine or vaccine.
Owner:UNITED STATES OF AMERICA
Vaccine formulations and uses thereof
ActiveUS20100124557A1Improve stabilityHigh mechanical strengthViral antigen ingredientsViruses/bacteriophagesModified vaccinia AnkaraMANNITOL/SORBITOL
A liquid or liquid-frozen composition comprising: a modified vaccinia Ankara (MVA) virus or variant or derivative thereof and mannitol, wherein mannitol is the sole stabilization agent of the composition. The mannitol may provide a stabilizing effect at 0 to +10° C. or in a liquid-frozen composition, for example between −10° C. and −30° C. or between −20° C. and −23.5° C. The MVA may be used as a vaccine or for use in gene therapy, virotherapy, immunotherapy, or cancer therapy in a mammal, preferably a human.
Owner:RESILIENCE GOVERNMENT SERVICES INC
Intergenic regions as insertion sites in the genome of modified vaccinia virus ankara (mva)
ActiveUS20050244428A1Insert firmlyFungiSsRNA viruses positive-senseModified vaccinia AnkaraPlasmid Vector
The present invention relates to novel insertion sites useful for the integration of exogenous sequences into the Modified Vaccinia Ankara (MVA) virus genome. The present invention further provides plasmid vectors to insert exogenous DNA into the genome of MVA. Furthermore, the present invention provides recombinant MVA comprising an exogenous DNA sequence inserted into said new insertion site as medicine or vaccine.
Owner:BAVARIAN NORDIC AS
Mva Vaccines
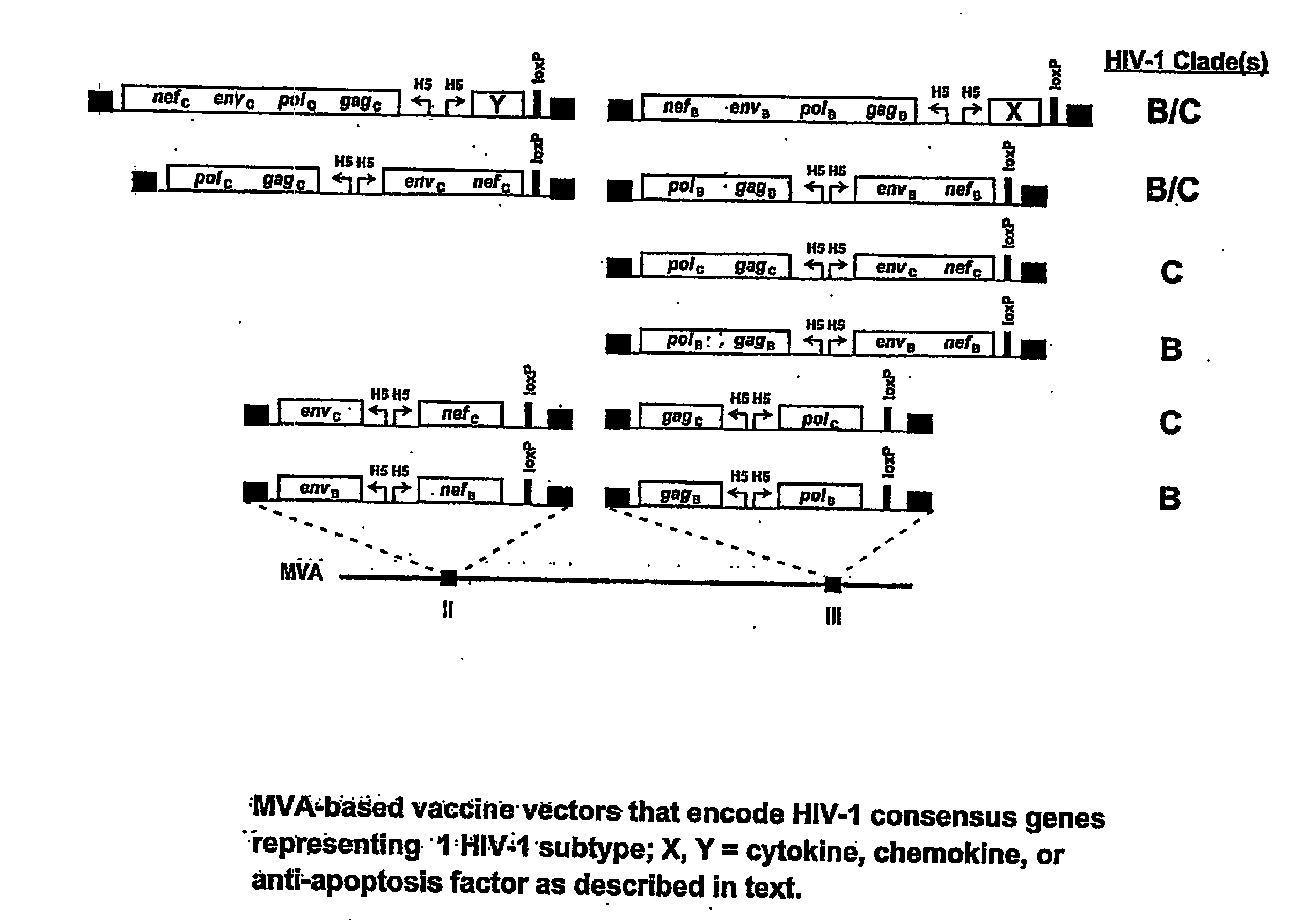
Recombinant modified vaccinia Ankara vectors are provided having a null mutation in a gene necessary for replication of the recombinant modified vaccinia Ankara virus and at least one heterologous antigen. The disclosed vectors optionally encode at least one pro-apoptotic factor, at least one anti-apoptotic factor, at least one immunomodulator, and combinations thereof. Cells complementing the null mutation the disclosed vectors are also provided.
Owner:FEINBERG MARK +1
Modified vaccinia ankara virus variant and cultivation method
InactiveUS20080317778A1Reduce riskBiocideGenetic material ingredientsSerum free mediaModified vaccinia Ankara
The present invention provides an attenuated virus, which is derived from Modified Vaccinia Ankara virus and characterized by the loss of its capability to reproductively replicate in human cell lines. It further describes recombinant viruses derived from this virus and the use of the virus, or its recombinants, as a medicament or vaccine. A method is provided for inducing an immune response in individuals who may be immune-compromised, receiving antiviral therapy, or have a pre-existing immunity to the vaccine virus. In addition, a method is provided for the administration of a therapeutically effective amount of the virus, or its recombinants, in a vaccinia virus prime / vaccinia virus boost inoculation regimen. The present invention relates to a method of virus amplification in primary cells which are cultivated in a serum free medium. Viruses produced by this method are advantageously free of any infectious agents comprised in animal sera.
Owner:BAVARIAN NORDIC AS
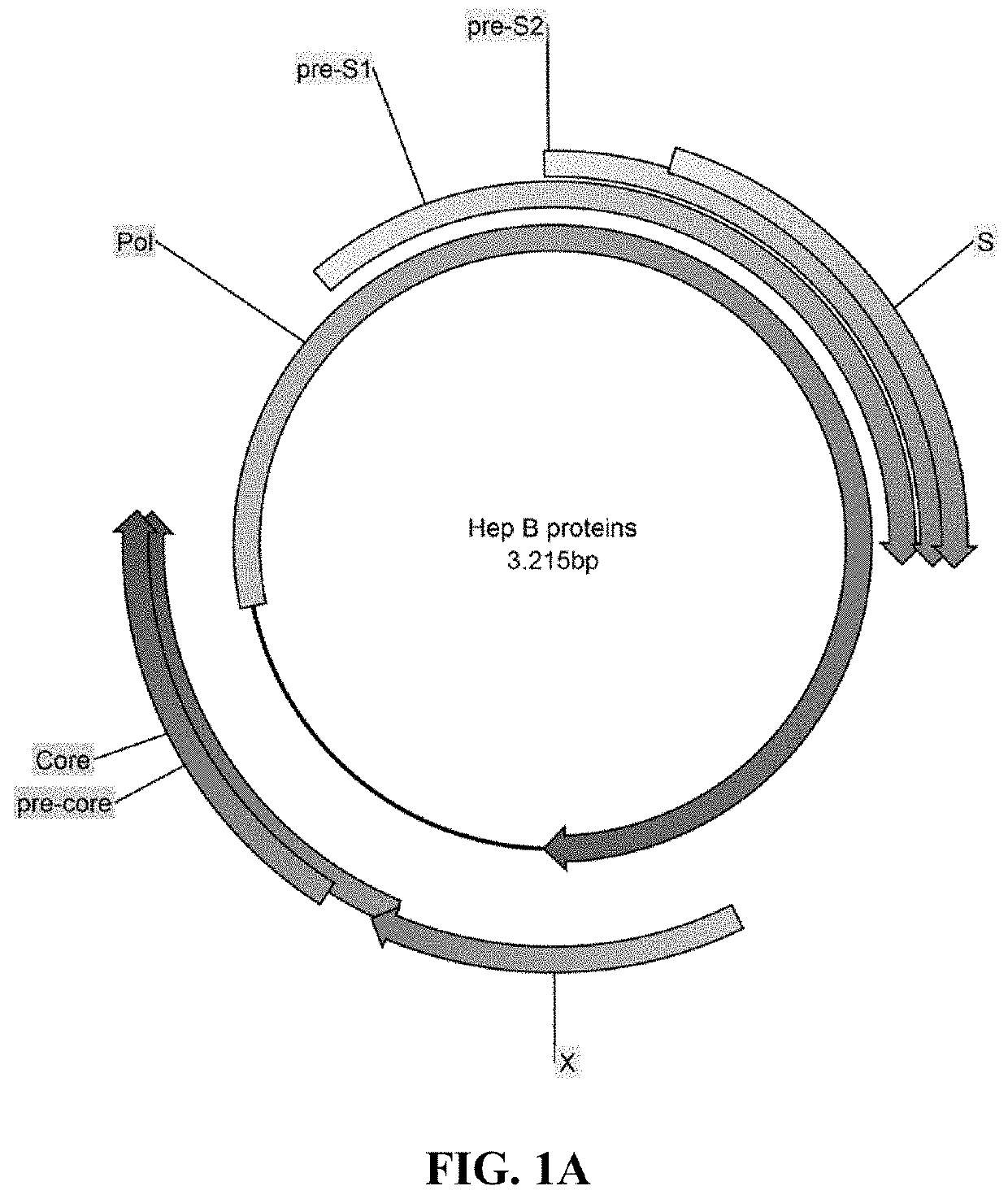
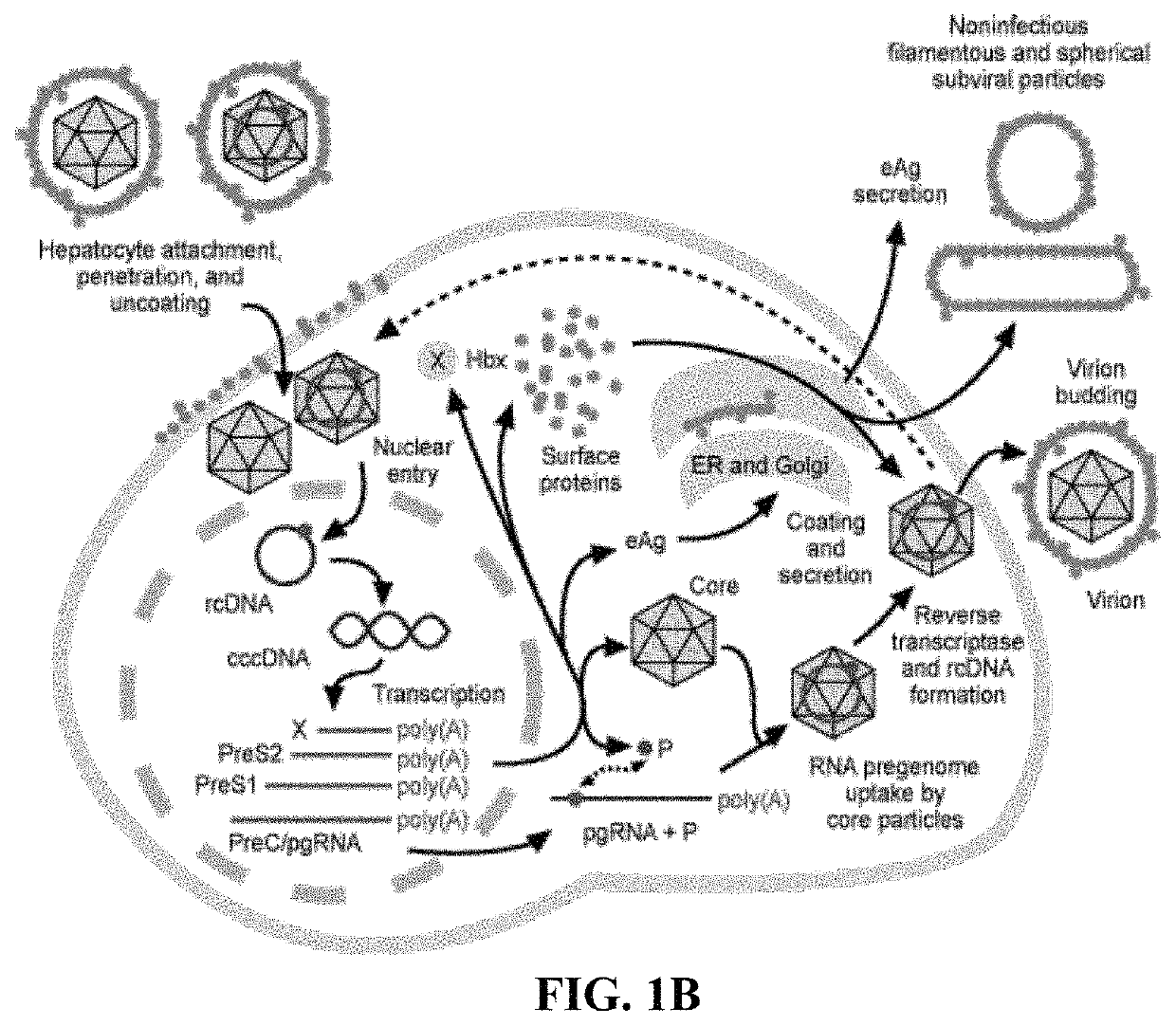
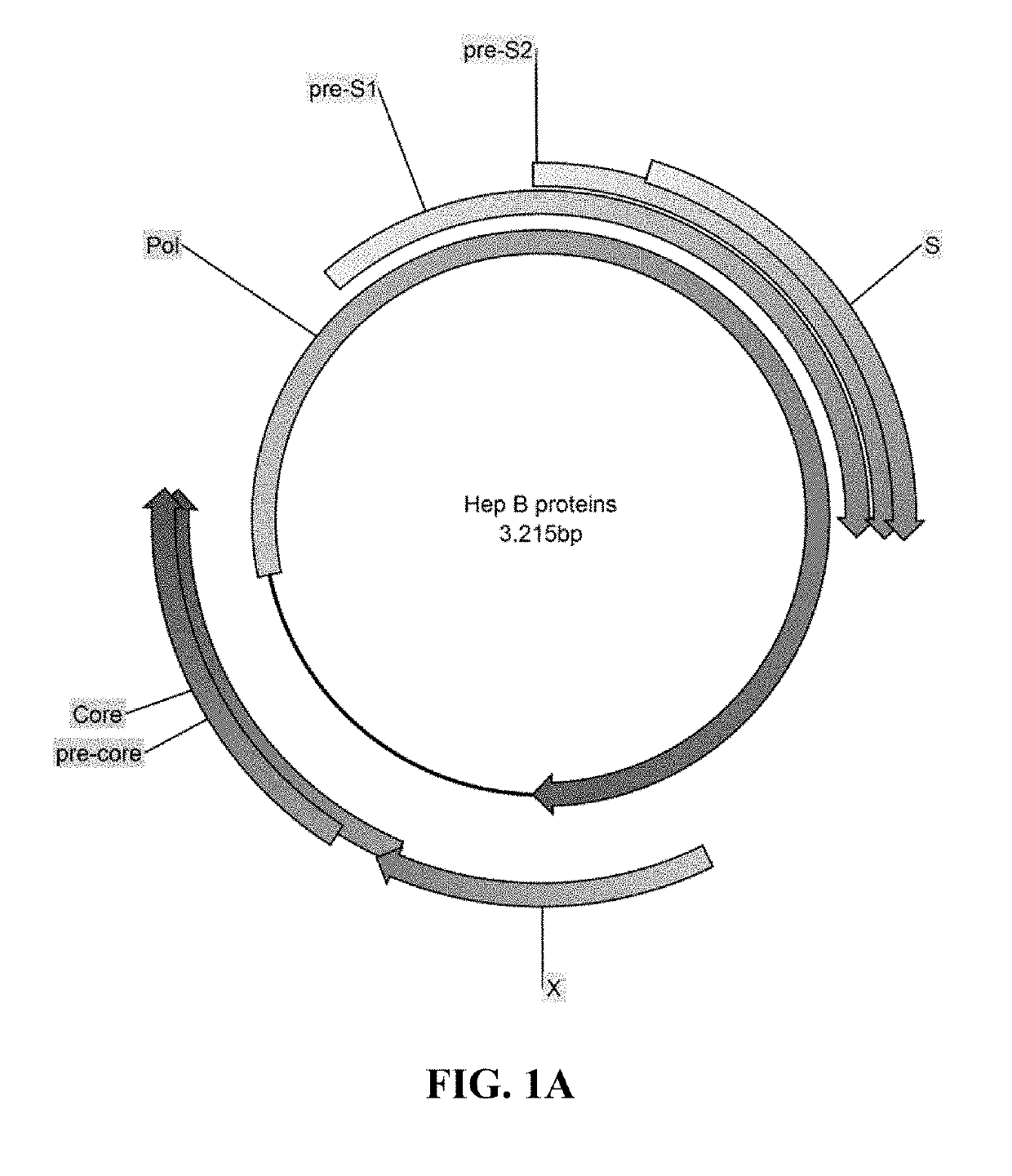
Methods and compositions for inducing an immune response against Hepatitis B Virus (HBV)
Provided herein are Modified Vaccinia Ankara (MVA) vectors and adenovirus vectors encoding HBV antigens. Also provided are methods of enhancing an immune response in a human subject by utilizing the MVA and adenovirus vectors encoding HBV antigens in a prime / boost regimen to the enhance the immune response in the human subject.
Owner:JANSSEN SCI IRELAND UC +1
Recombinant modified vaccinia ankara (MVA) vaccinia virus containing restructured insertion sites
ActiveUS20120263750A1Viral antigen ingredientsVirus peptidesModified vaccinia AnkaraOpen reading frame
The present invention relates to recombinant modified vaccinia Ankara (MVA) virus containing restructured sites useful for the integration of heterologous nucleic acid sequences into an intergenic region (IGR) of the virus genome, where the IGR is located between two adjacent, essential open reading frames (ORFs) of the vaccinia virus genome, wherein the adjacent essential ORFs are non-adjacent in a parental MVA virus used to construct the recombinant MVA virus, and to related nucleic acid constructs useful for inserting heterologous DNA into the genome of a vaccinia virus, and further to the use of the disclosed viruses as a medicine or vaccine.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Modified vaccinia ankara virus variant
InactiveCN1476483AAntibacterial agentsGenetic material ingredientsModified vaccinia AnkaraHuman cell line
The present invention provides an attenuated virus derived from a modified vaccinia Ankara virus characterized by loss of the ability to replicate repeatedly in human cell lines. The present invention further describes recombinant viruses derived from the virus and the use of the virus or recombinants thereof as drugs or vaccines. Furthermore, the present invention provides a method of inducing an immune response even in immunocompromised patients, patients who are already immune to vaccine viruses, or patients who are receiving antiviral therapy.
Owner:BAVARIAN NORDIC AS
Recombinant Modified Vaccinia Ankara (MVA) Vaccinia Virus Containing Restructured Insertion Sites
The present invention relates to recombinant modified vaccinia Ankara (MVA) virus containing restructured sites useful for the integration of heterologous nucleic acid sequences into an intergenic region (IGR) of the virus genome, where the IGR is located between two adjacent, essential open reading frames (ORFs) of the vaccinia virus genome, wherein the adjacent essential ORFs are non-adjacent in a parental MVA virus used to construct the recombinant MVA virus, and to related nucleic acid constructs useful for inserting heterologous DNA into the genome of a vaccinia virus, and further to the use of the disclosed viruses as a medicine or vaccine.
Owner:UNITED STATES OF AMERICA
Vaccine formulations and uses thereof
ActiveUS7998488B2Improve stabilityHigh mechanical strengthAntibacterial agentsViral antigen ingredientsModified vaccinia AnkaraMANNITOL/SORBITOL
A liquid or liquid-frozen composition comprising: a modified vaccinia Ankara (MVA) virus or variant or derivative thereof and mannitol, wherein mannitol is the sole stabilization agent of the composition. The mannitol may provide a stabilizing effect at 0 to +10° C. or in a liquid-frozen composition, for example between −10° C. and −30° C. or between −20° C. and −23.5° C. The MVA may be used as a vaccine or for use in gene therapy, virotherapy, immunotherapy, or cancer therapy in a mammal, preferably a human.
Owner:RESILIENCE GOVERNMENT SERVICES INC
Compositions and Methods for Generating an Immune Response to a Tumor Associated Antigen
ActiveUS20190290745A1Reduce the overall heightSsRNA viruses positive-senseInactivation/attenuationModified vaccinia AnkaraNeoplasm
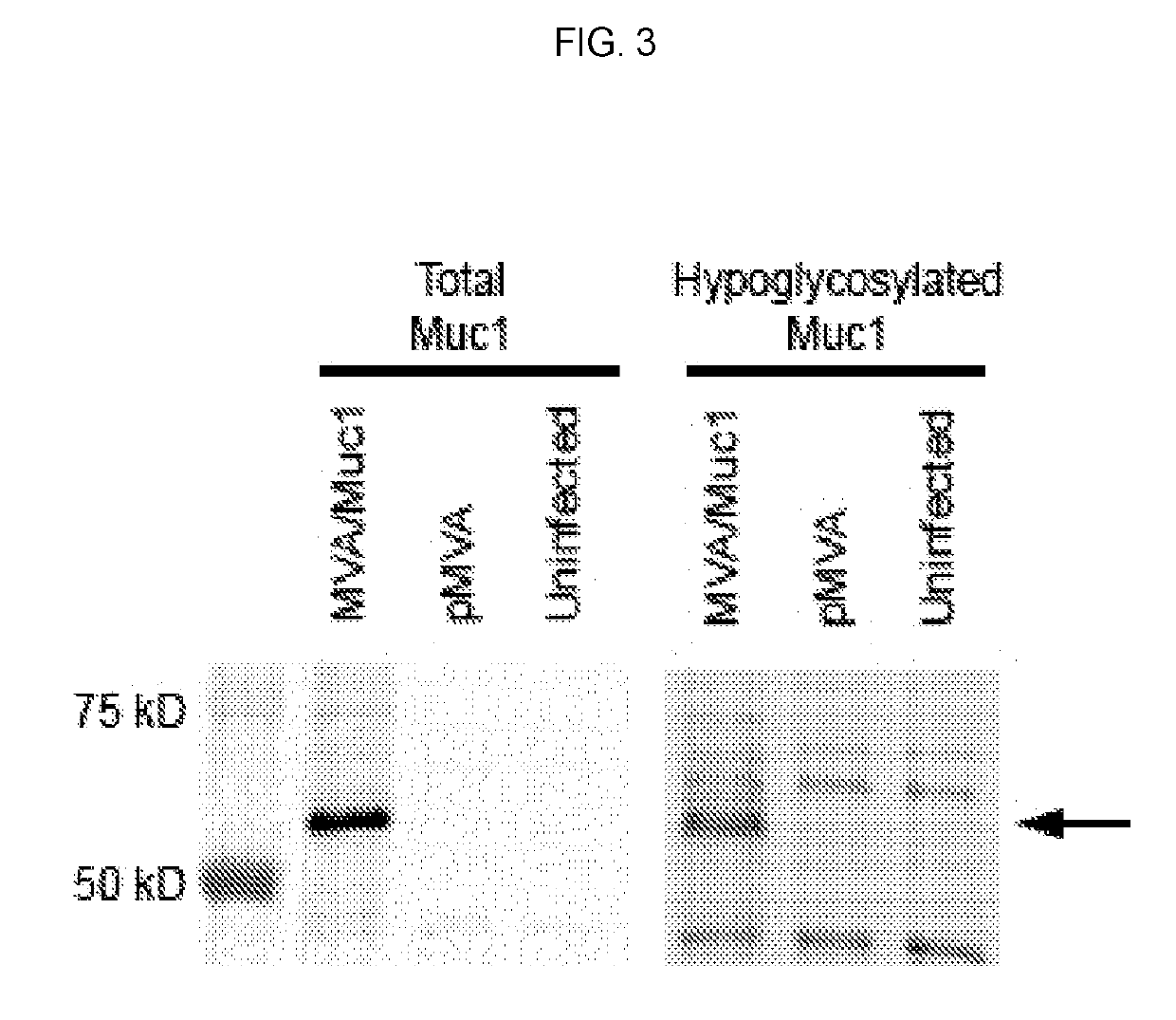
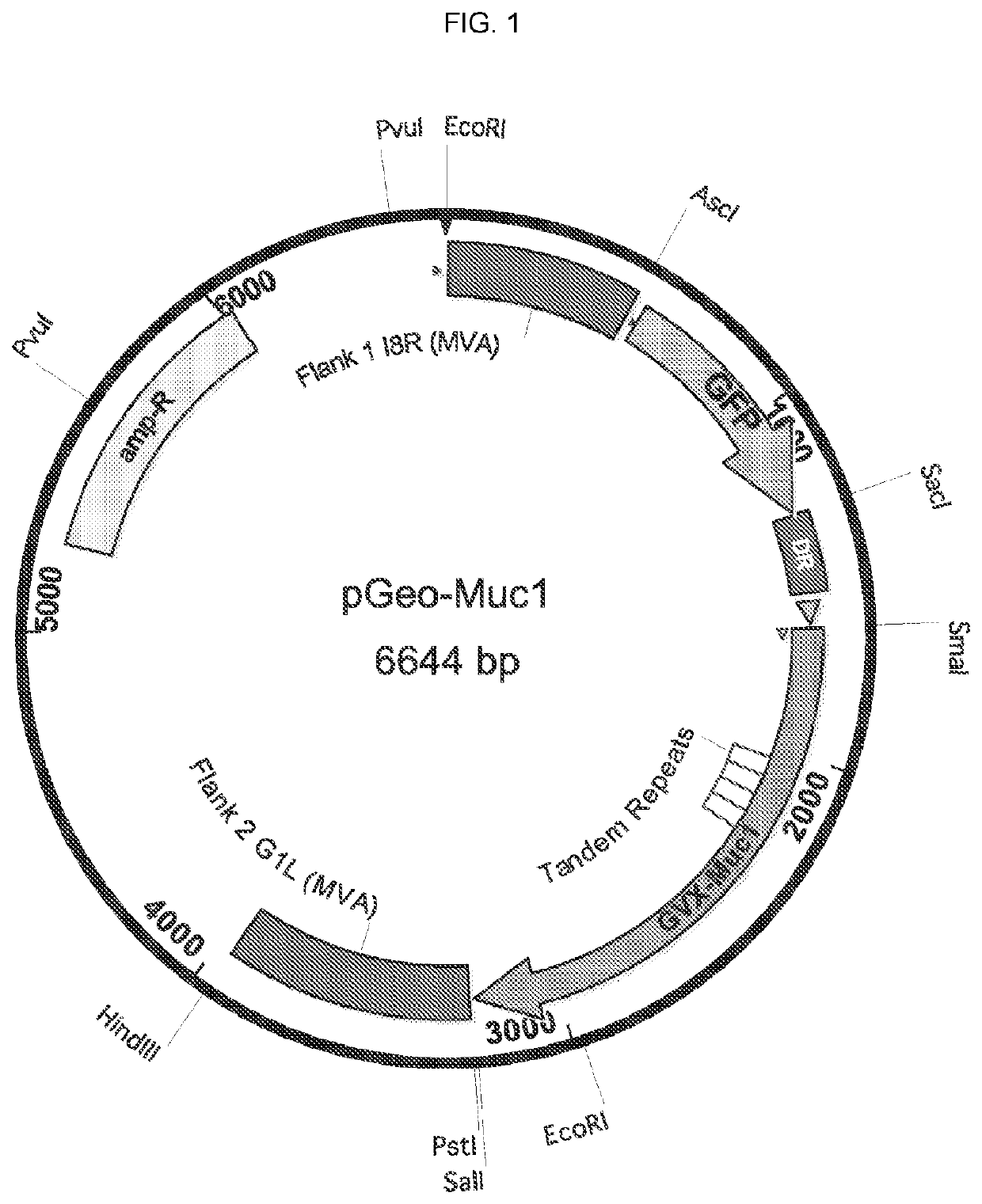
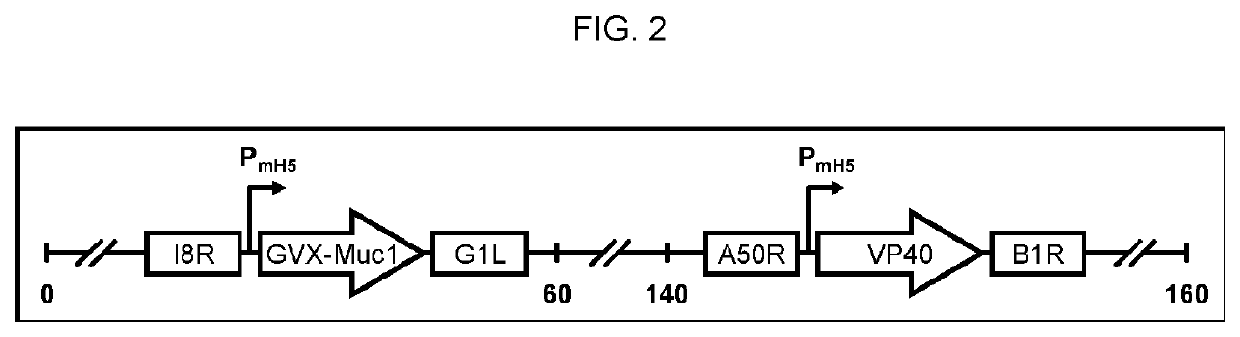
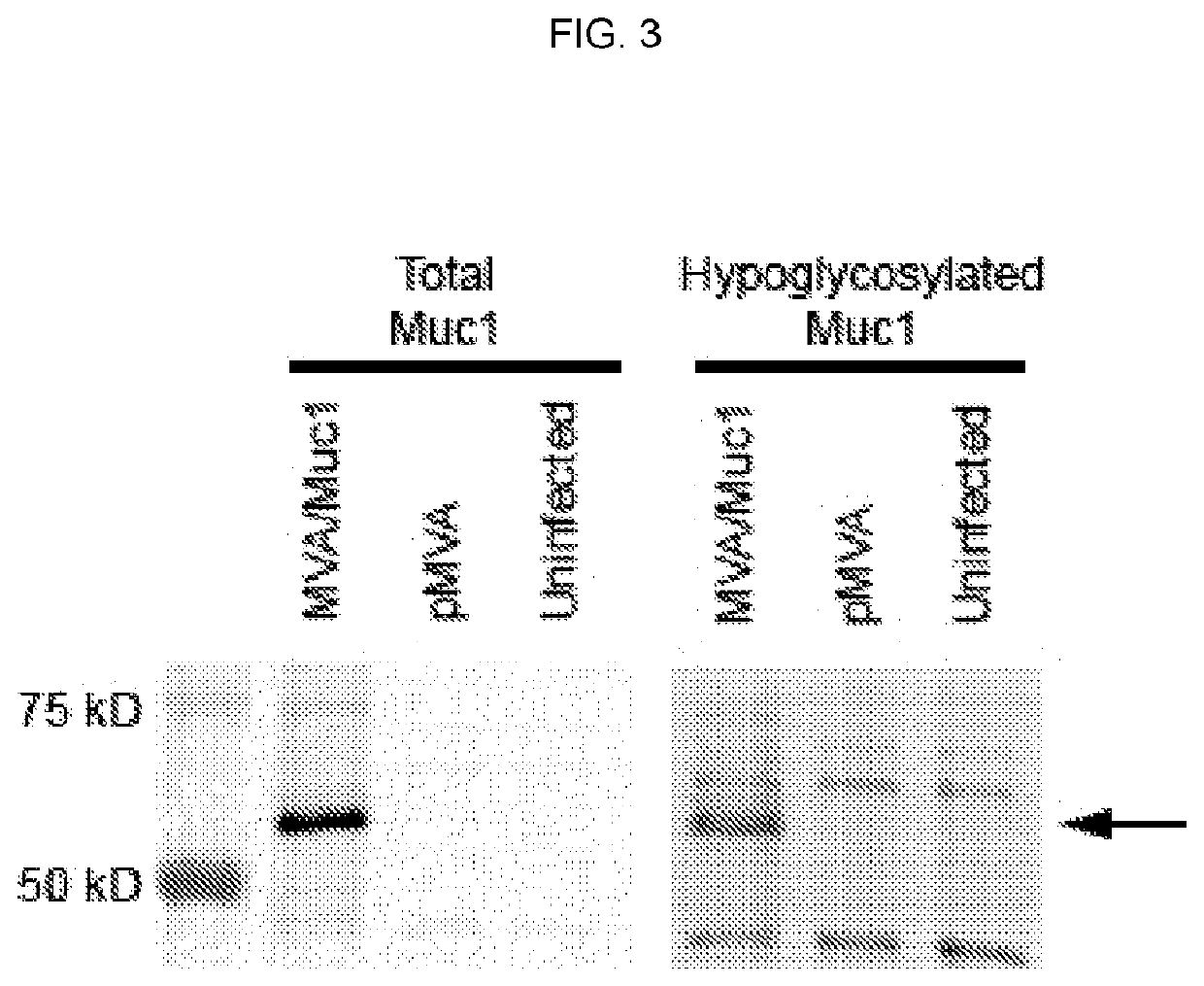
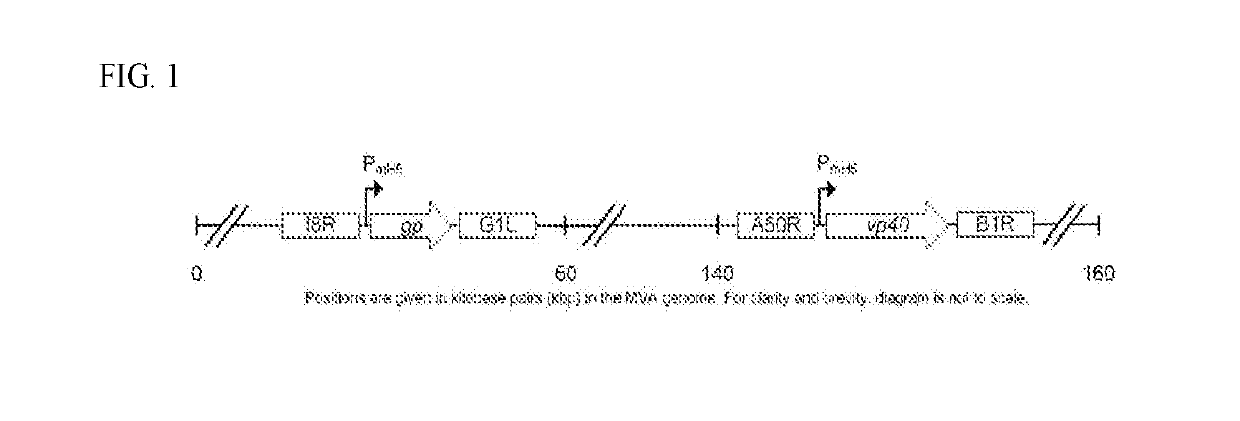
The compositions and methods are described for generating an immune response to a tumor associated antigen such as MUC1. The compositions and methods described herein relate to a modified vaccinia Ankara (MVA) vector encoding one or more viral antigens for generating a protective immune response to a neoplasm expressing the tumor associated antigen in the subject to which the vector is administered. The compositions and methods of the present invention are useful both prophylactically and therapeutically and may be used to prevent and / or treat neoplasms and associated diseases.
Owner:GEOVAX INC
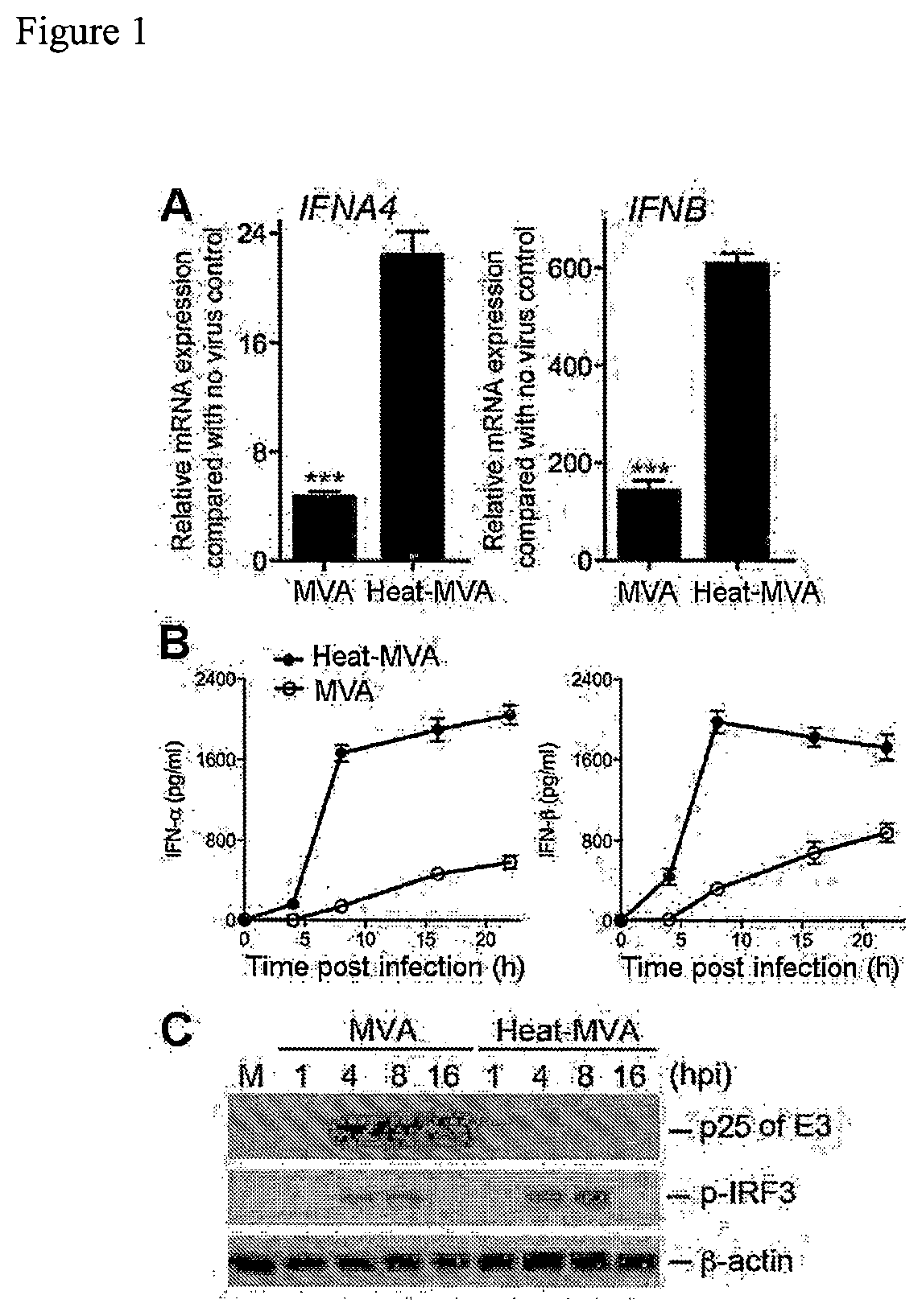
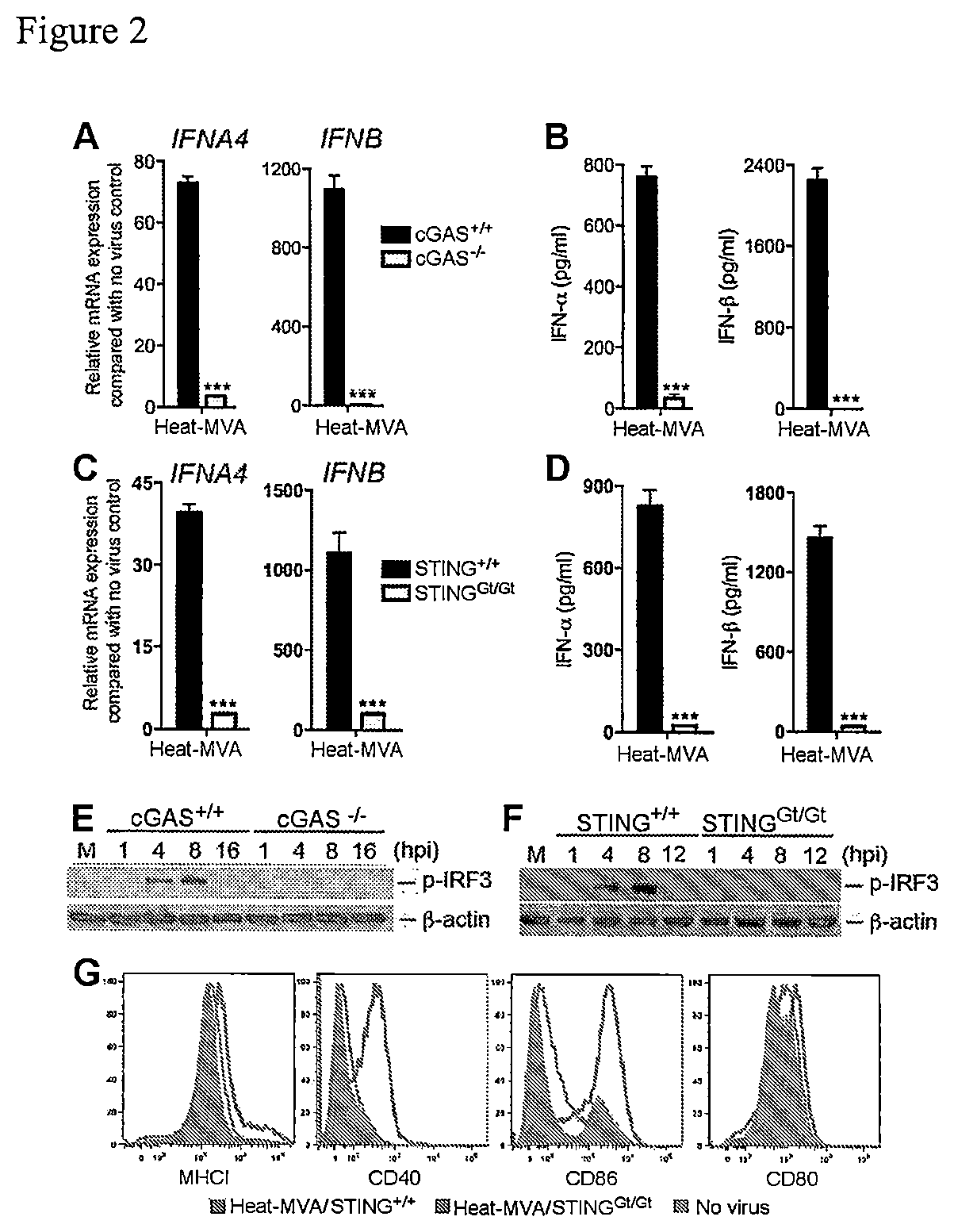
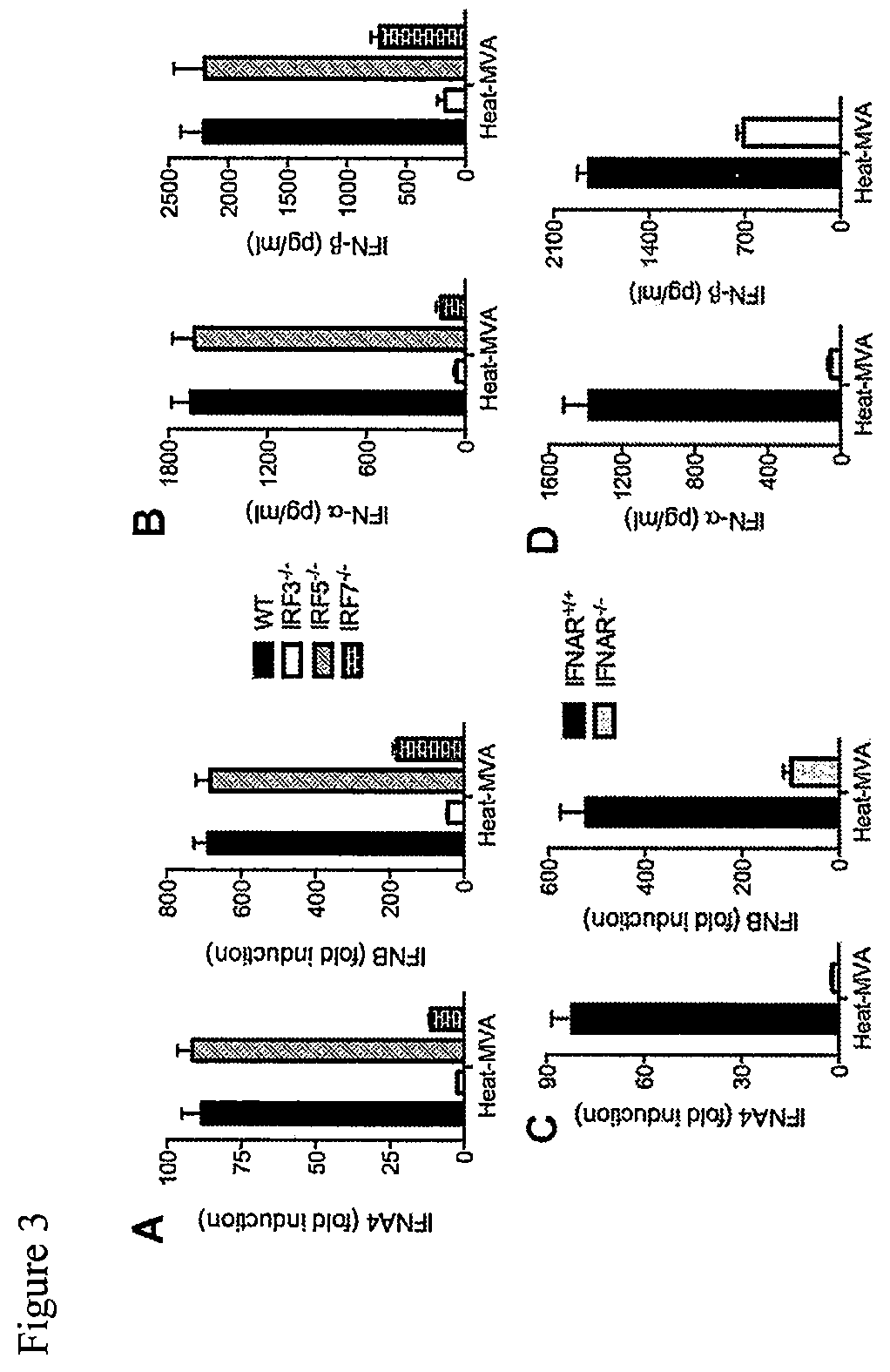
Use of inactivated nonreplicating modified vaccinia virus Ankara (MVA) as monoimmunotherapy or in combination with immune checkpoint blocking agents for solid tumors
ActiveUS10639366B2Reduce doseSynergistic effectViral antigen ingredientsDigestive systemModified vaccinia AnkaraLow Grade Malignant Neoplasm
The present disclosure relates to infection-competent, but nonreplicative inactivated modified vaccinia Ankara (MVA) and its use as immunotherapy, alone, or in combination with immune checkpoint blocking agents for the treatment of malignant solid tumors. Particular embodiments relate to inducing an immune response in a subject diagnosed with a solid malignant tumor.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
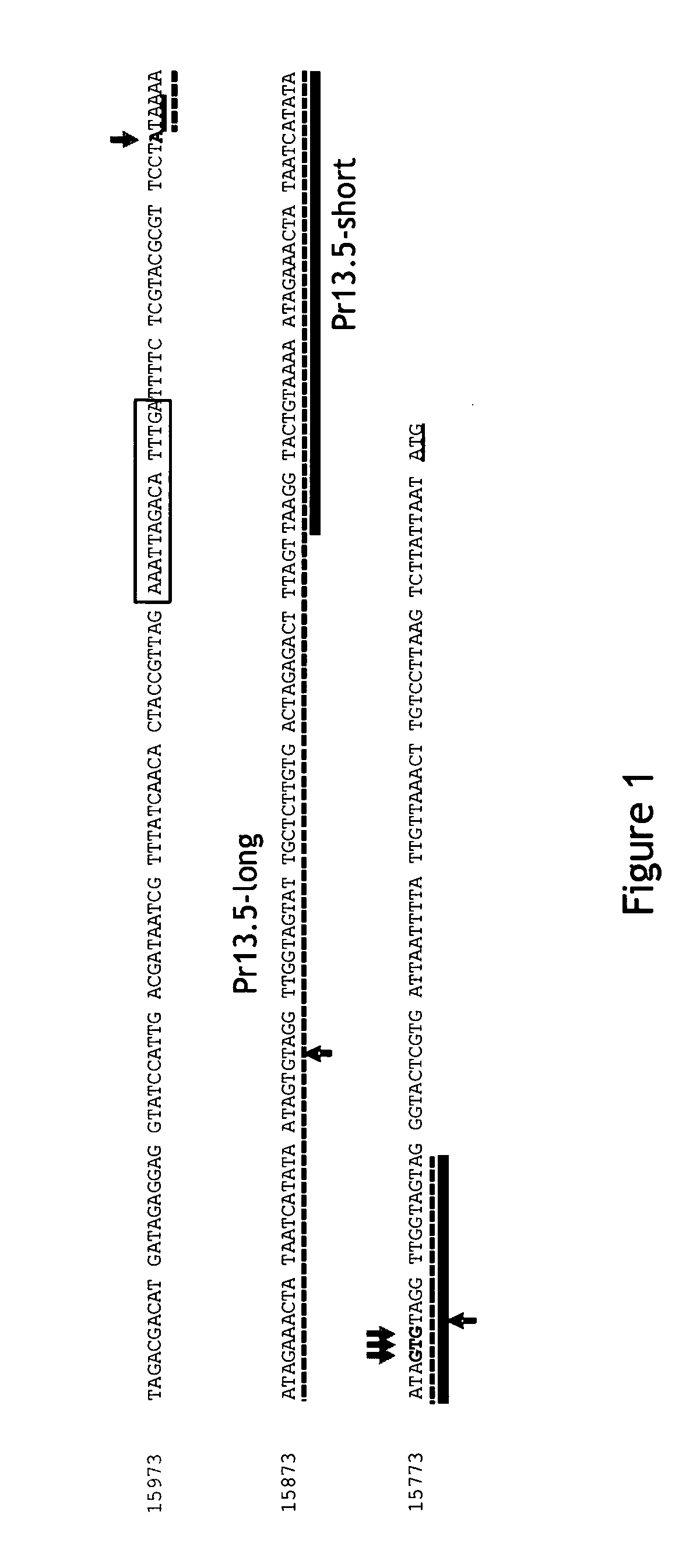
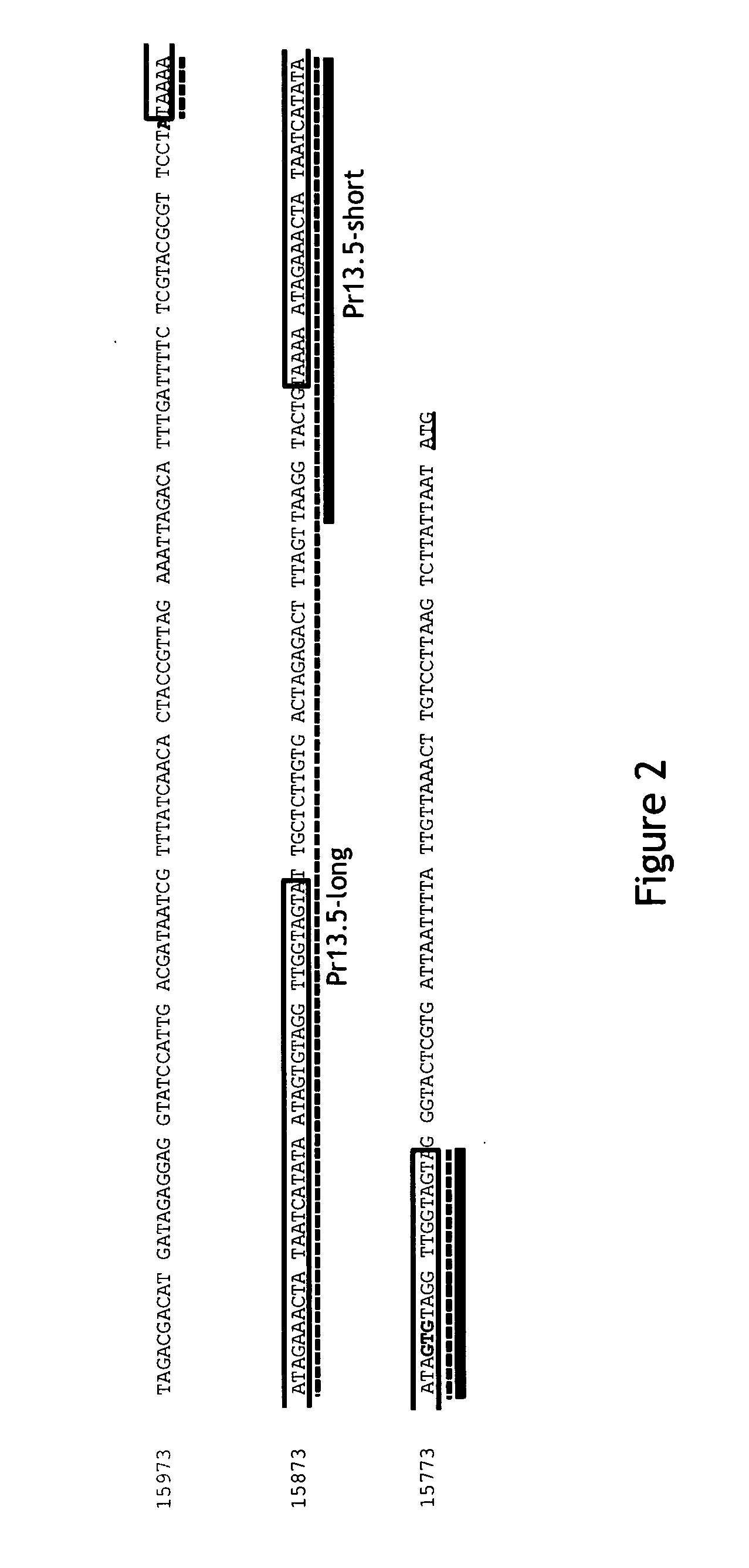
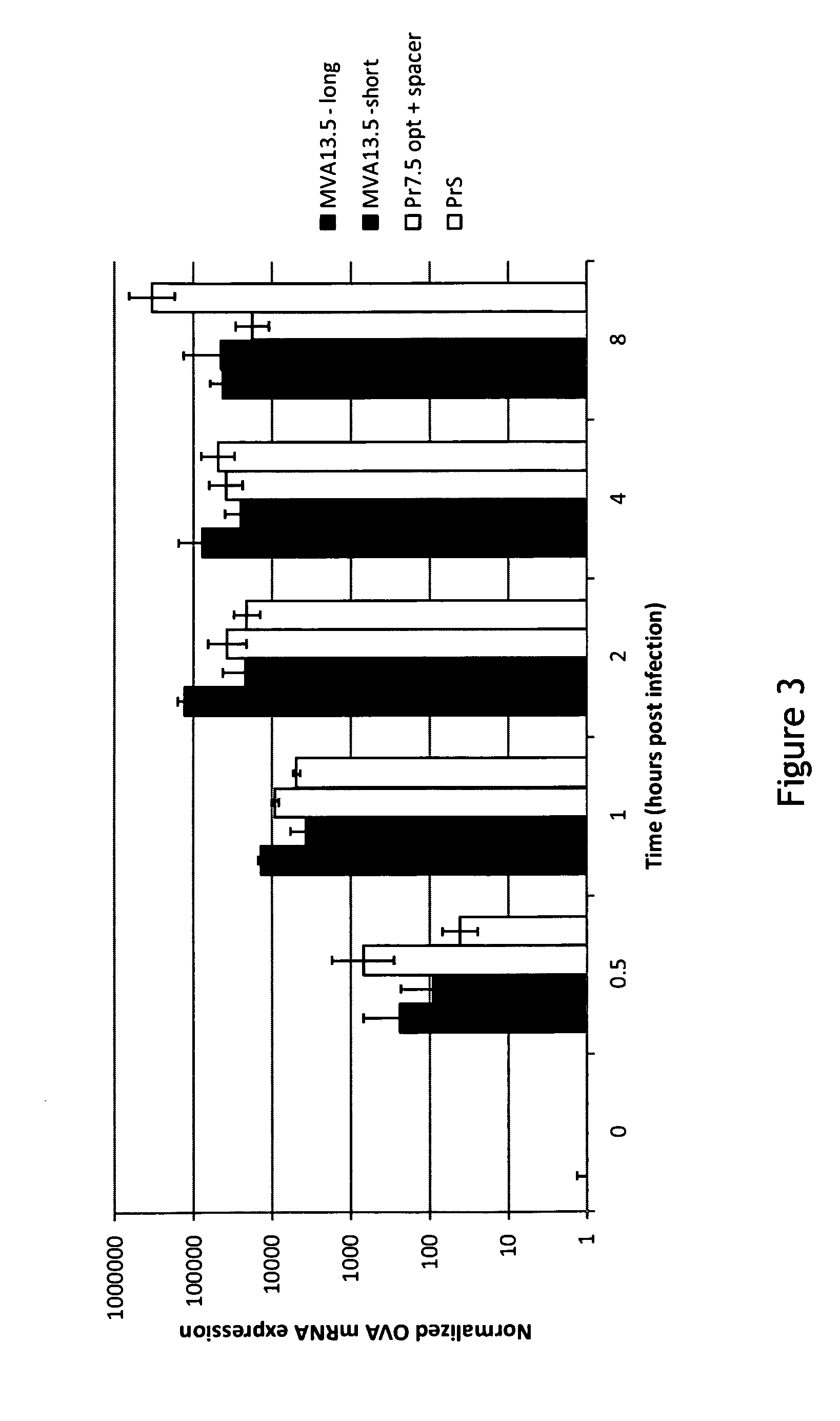
Pr13.5 promoter for robust t-cell and antibody responses
ActiveUS20150299267A1Improving immunogenicityAccurate replacementVectorsViral antigen ingredientsModified vaccinia AnkaraNucleotide
The invention encompasses recombinant poxviruses, preferably modified Vaccinia Ankara (MVA) viruses, comprising a Pr13.5 promoter operably linked to a nucleotide sequence encoding an antigen and uses thereof. The invention is drawn to compositions and methods for the induction of strong CD8 T cell and antibody responses to a specific antigen(s) by administering one or more immunizations of the recombinant MVA to a mammal, preferably a human.
Owner:BAVARIAN NORDIC AS
Compositions and Methods for Generating an Immune Response to LASV
InactiveUS20200171141A1Reduction and elimination of abilityAvoid virus infectionSsRNA viruses negative-senseViral antigen ingredientsModified vaccinia AnkaraImmunity response
Compositions and methods are described for generating an immune response to an arenavirus. The compositions and methods described herein relate to a modified vaccinia Ankara (MVA) vector encoding one or more viral antigens for generating a protective immune response to a member of genus Arenavirus (such as a member of species Lassa virus) in the subject to which the vector is administered. The compositions and methods of the present invention may be used to prevent and / or treat an infection caused by arenavirus.
Owner:GEOVAX INC
Modified vaccinia ankara virus vaccine
The present invention relates, in general, to vaccines and, in particular, to a modified vaccinia Ankara (MVA) virus, to a composition comprising such a virus and to methods of using same to induce an immune response.
Owner:DUKE UNIV
Methods and compositions for inducing an immune response against hepatitis b virus (HBV)
Provided herein are Modified Vaccinia Ankara (MVA) vectors and adenovirus vectors encoding HBV antigens. Also provided are methods of enhancing an immune response in a human subject by utilizing the MVA and adenovirus vectors encoding HBV antigens in a prime / boost regimen to the enhance the immune response in the human subject.
Owner:JANSSEN SCI IRELAND UC +1
Vaccinia viral vectors encoding chimeric virus like particles
ActiveUS20200289633A1SsRNA viruses negative-senseSsRNA viruses positive-senseModified vaccinia AnkaraDisease
Owner:GEOVAX INC
Compositions and Methods for Generating an Immune Response to a Hemorrhagic Fever Virus
ActiveUS20190117758A1Avoid infectionPrevents and ameliorates BundibugyoSsRNA viruses negative-senseViral antigen ingredientsModified vaccinia AnkaraGenus Ebolavirus
The compositions and methods are described for generating an immune response to a hemorrhagic fever virus such as ebolavirus, Marburgvirus, or arenavirus. The compositions and methods described herein relate to a modified vaccinia Ankara (MVA) vector encoding one or more viral antigens for generating a protective immune response to a member of genus Ebolavirus (such as a member of species Zaire ebolavirus), a member of genus Marburgvirus (such as a member of species Marburg marburgvirus), or a member of genus Arenavirus (such as a member of species Lassa virus) in the subject to which the vector is administered. The compositions and methods of the present invention are useful both prophylactically and therapeutically and may be used to prevent and / or treat an infection caused by ebolavirus, Marburgvirus, or arenavirus.
Owner:GEOVAX INC
Universal influenza vaccine based on recombinant modified vaccine ankara virus (MVA)
InactiveUS20120141525A1SsRNA viruses negative-senseViral antigen ingredientsModified vaccinia AnkaraAvian influenza virus
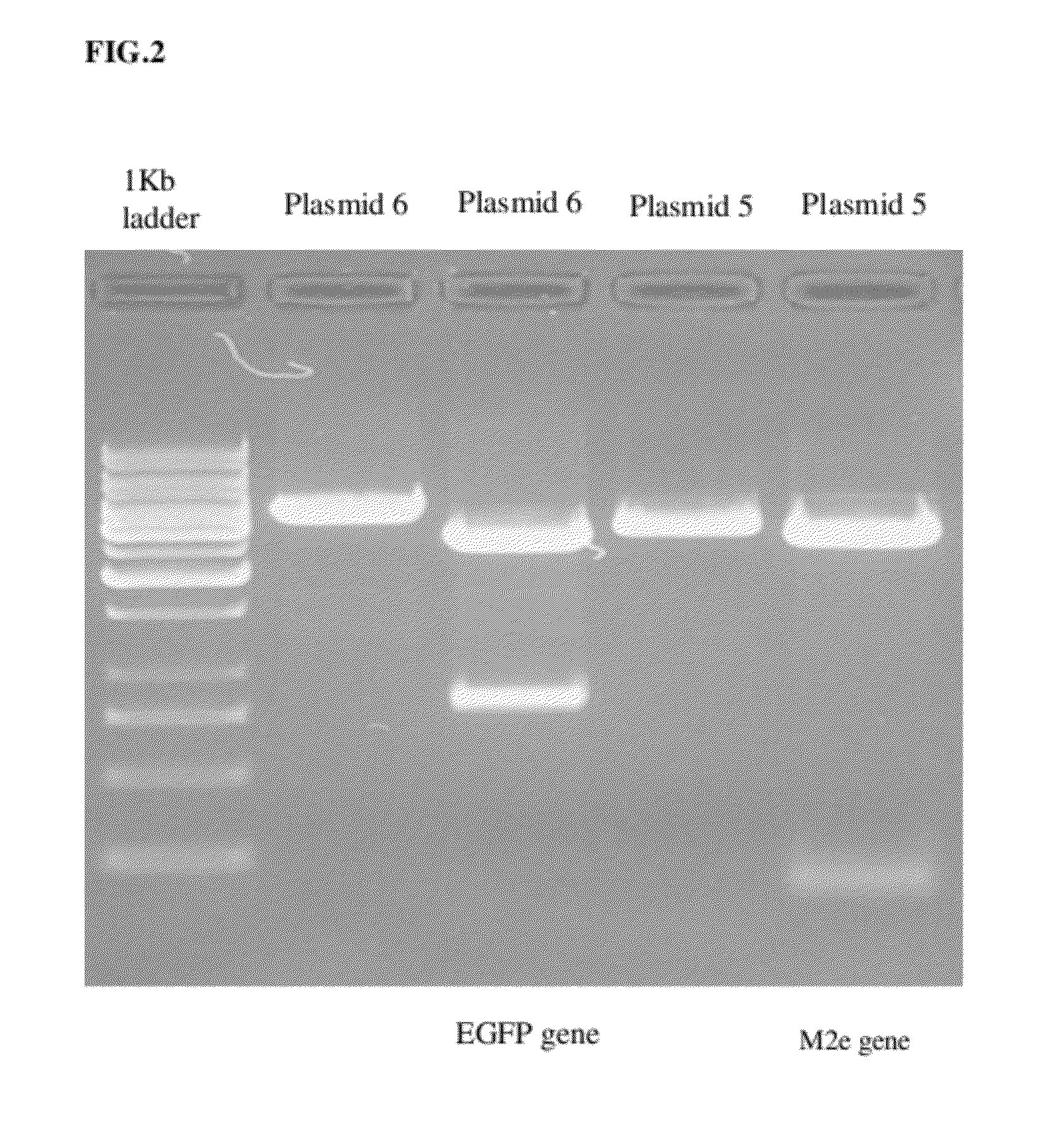
The present invention relates to a novel influenza vaccine, a novel plasmid for preparing the same and a novel dosage form comprising the same. The present invention in particular relates to a recombinant modified vaccinia Ankara (MVA) virus comprising and capable of simultaneously expressing a cassette of at least four foreign genes from influenza virus, specifically an avian influenza virus, wherein the said genes are inserted at a non-essential site, within the MVA genome. The invention further relates to a recombinant modified vaccinia Ankara (MVA) virus comprising and capable of simultaneously expressing a cassette of not less than two foreign genes from influenza virus, wherein the said genes are inserted at a non-essential site, within the MVA genome, with the provision that at least one foreign gene is either PB2 or M2e. The invention also provides composition and methods of making the universal influenza vaccine.
Owner:PANACEA BIOTEC
Vaccinia virus mutants useful for cancer immunotherapy
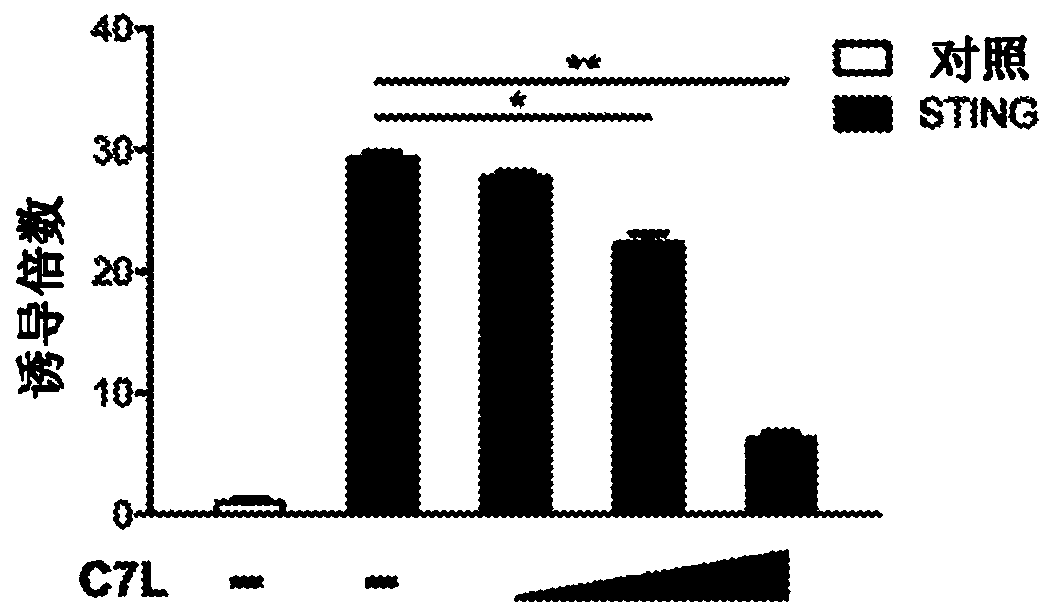
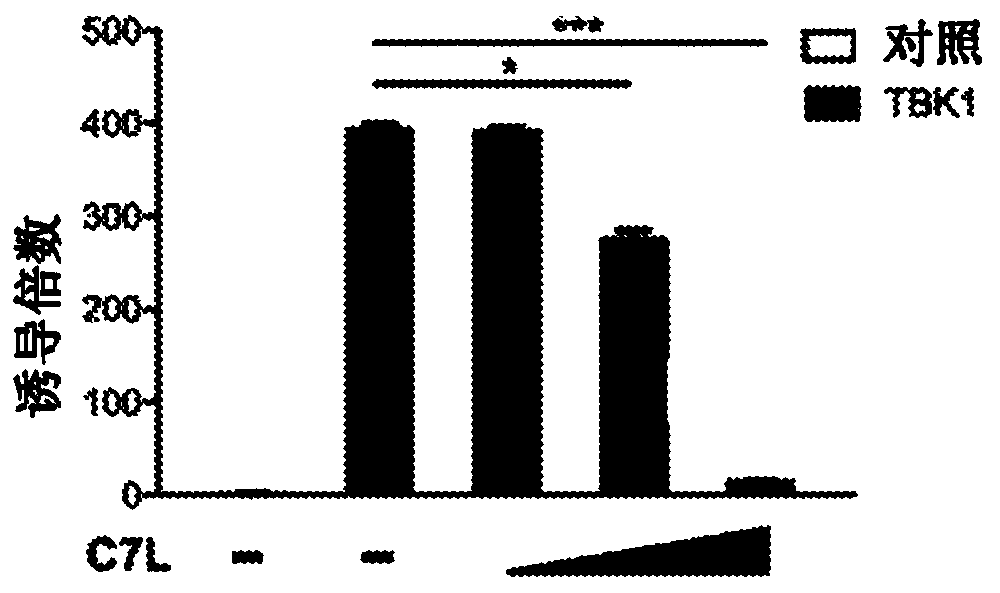
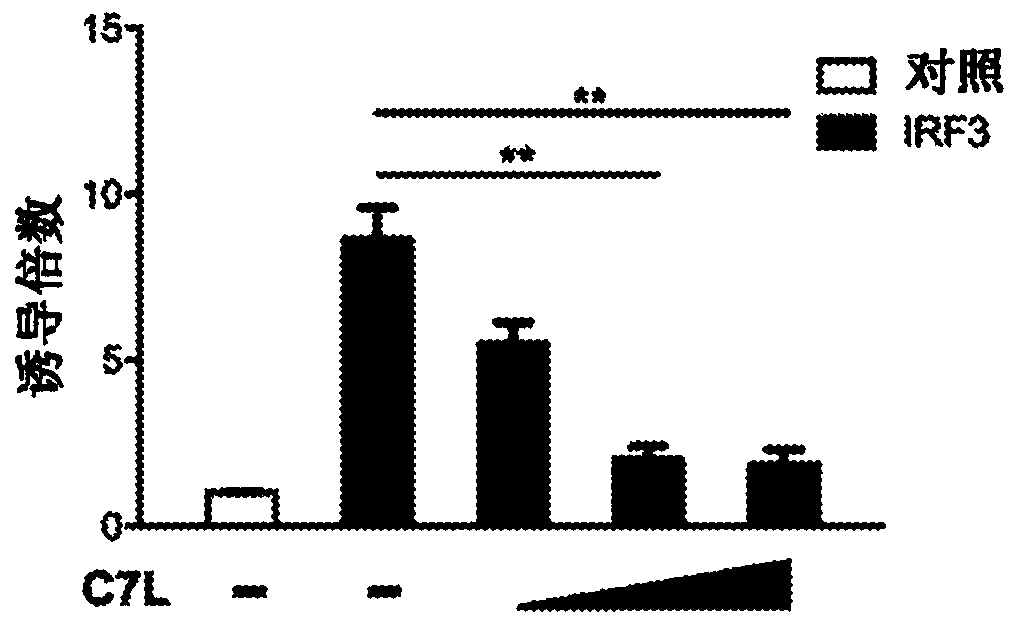
Disclosed herein are methods and compositions related to the treatment, prevention, and / or amelioration of cancer in a subject in need thereof. In particular aspects, the present technology relates tothe use of poxviruses, including a recombinant modified vaccinia Ankara (MVA) virus or vaccinia virus with deletion of vaccinia host-range factor C7 (MVA[delta]C7L and VACV[delta]C7L, respectively),alone or in combination with immune checkpoint blocking agents, as an oncolytic and immunotherapeutic composition. In some embodiments, the technology of the present disclosure relates to a MVA[delta]C7L or VACV[delta]C7L virus further modified to express human Fms-like tyrosine kinase 3 ligand (Flt3L).
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Compositions and Methods for Generating an Immune Response to Hepatitis B Virus
ActiveUS20190184009A1Reduce eliminateAvoid virus infectionViral antigen ingredientsPharmaceutical delivery mechanismModified vaccinia AnkaraHepatitis B virus
The compositions and methods are described for generating an immune response to a hepatitis B virus. The compositions and methods described herein relate to a modified vaccinia Ankara (MVA) vector encoding one or more viral antigens for generating a protective immune response to a hepatitis B virus, in the subject to which the vector is administered. The compositions and methods of the present invention are useful both prophylactically and therapeutically and may be used to prevent and / or treat an infection caused by hepatitis B virus.
Owner:GEOVAX INC +1
MVA virus and uses thereof
ActiveUS9732325B2Improve industrial productionHigh yieldVirus peptidesImmunological disordersModified vaccinia AnkaraDiluent
The present invention relates to a novel Modified Vaccinia Ankara (MVA) virus. The present invention also relates to a method for culturing said MVA virus and to a method for producing said MVA virus. Further, the present invention relates to a pharmaceutical composition comprising said MVA virus and one or more pharmaceutical acceptable excipient(s), diluent(s), and / or carrier(s). Furthermore, the present invention relates to a vaccine comprising said MVA virus. In addition, the present invention relates to said MVA virus for use in medicine.
Owner:PROBIOGEN AG
Compositions and methods for generating an immune response to treat or prevent malaria
ActiveUS11311612B2Pharmaceutical delivery mechanismPharmaceutical non-active ingredientsModified vaccinia AnkaraImmunity response
The compositions and methods are described for generating an immune response to a Plasmodium antigen. The compositions and methods described herein relate to a modified vaccinia Ankara (MVA) vector encoding one or more viral antigens for generating a protective immune response to malaria by expressing the Plasmodium antigen in the subject to which the MVA vector is administered. The compositions and methods of the present invention are useful both prophylactically and therapeutically and may be used to prevent and / or treat malaria.
Owner:GEOVAX INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com