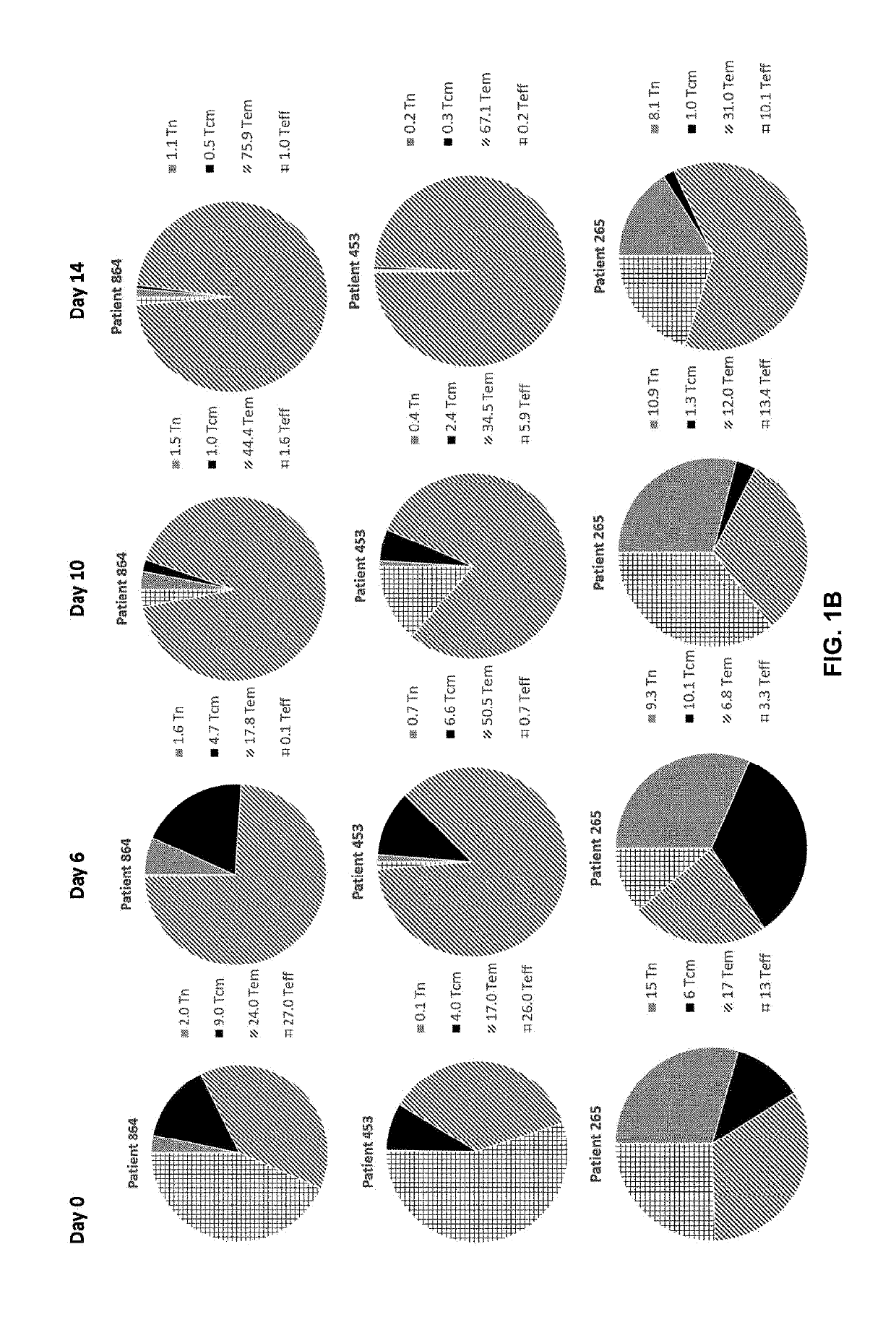
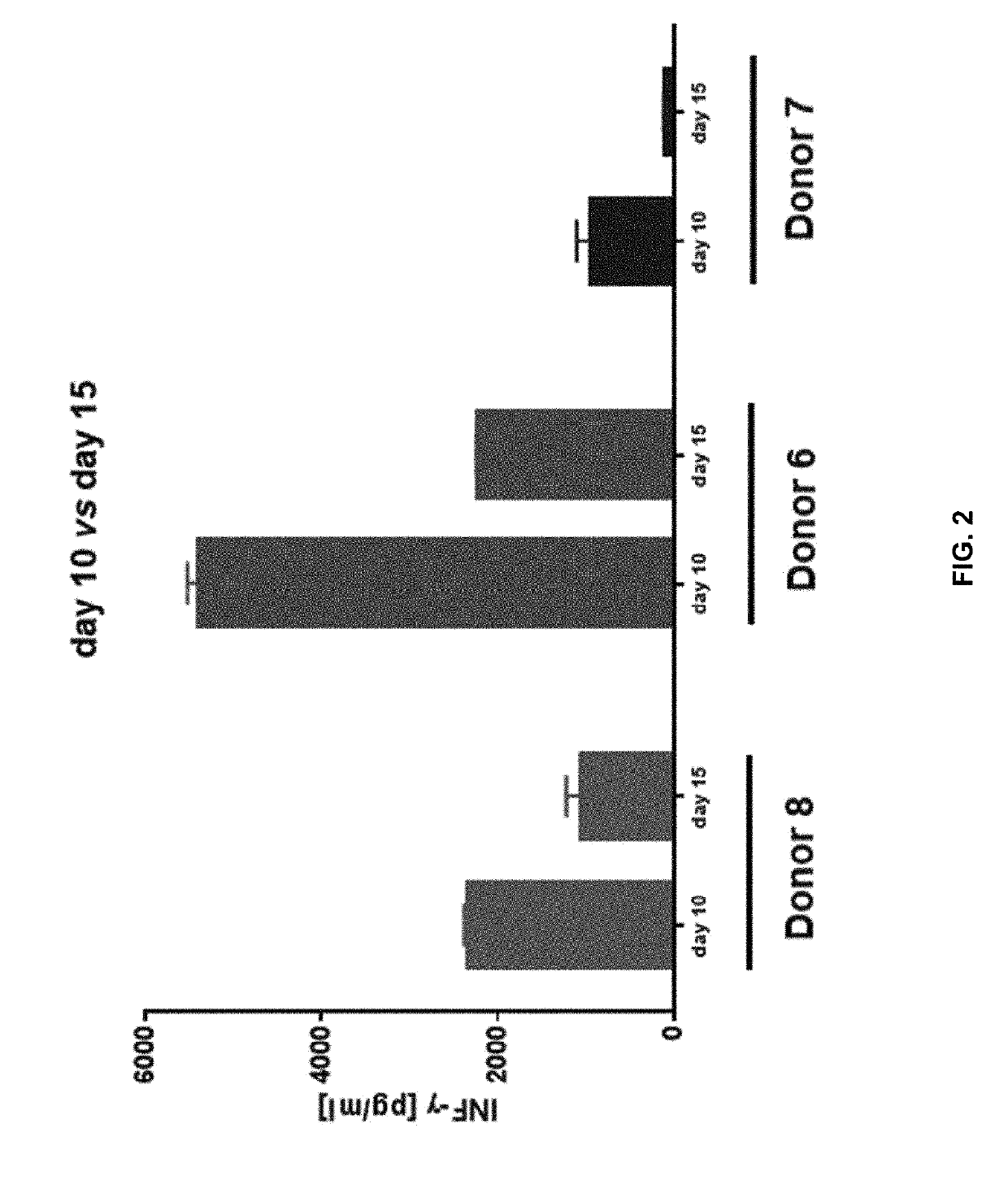
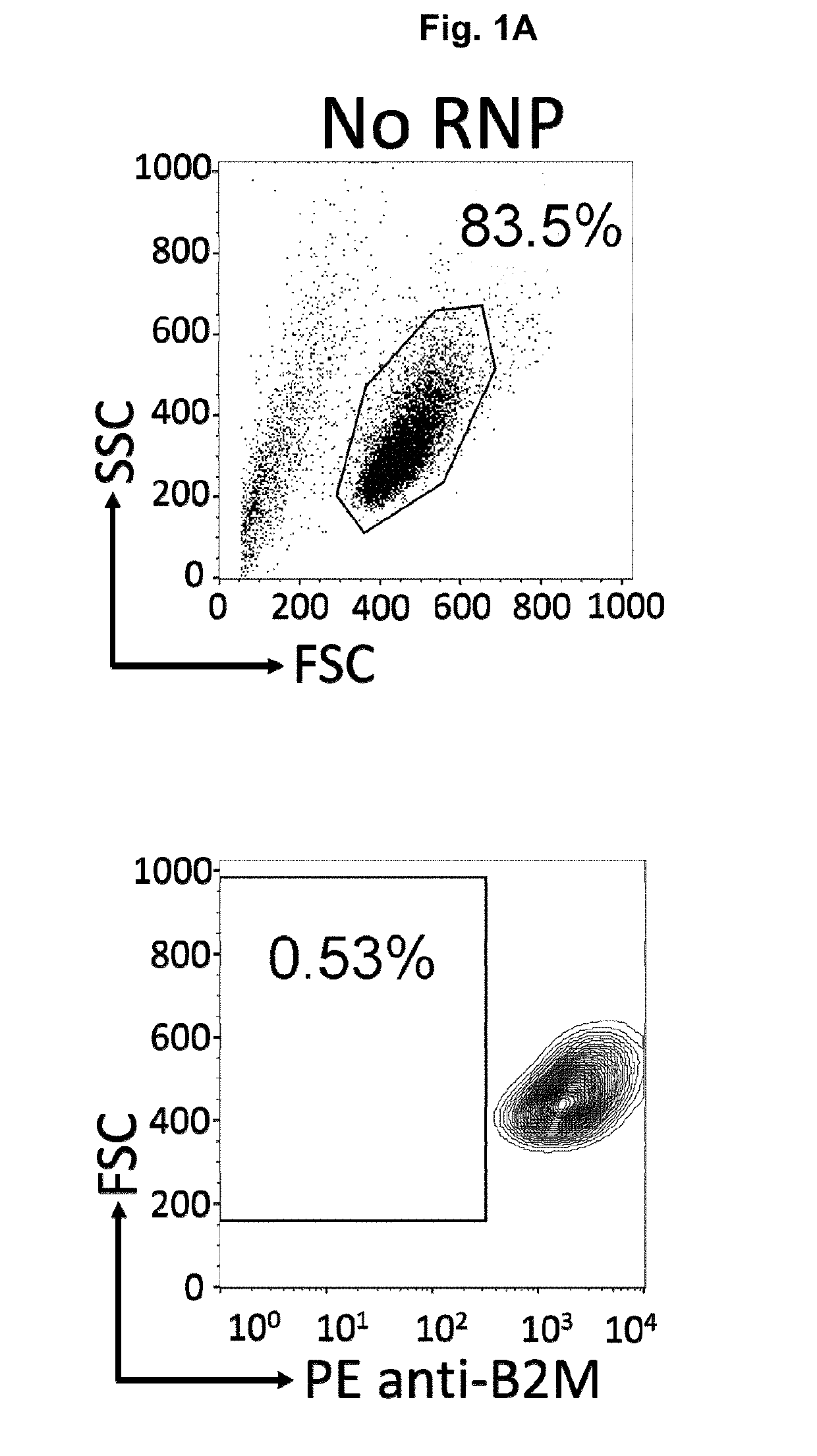
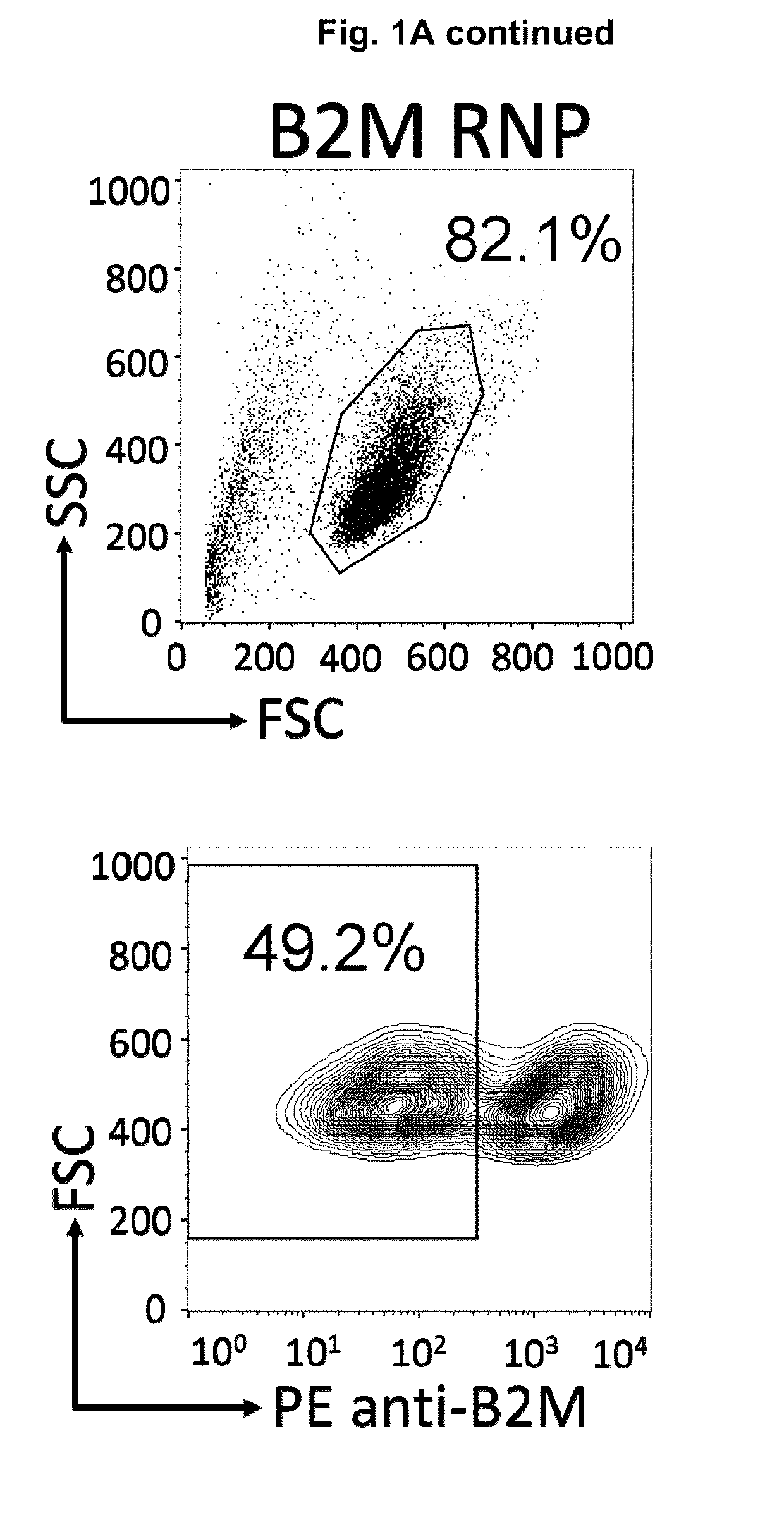
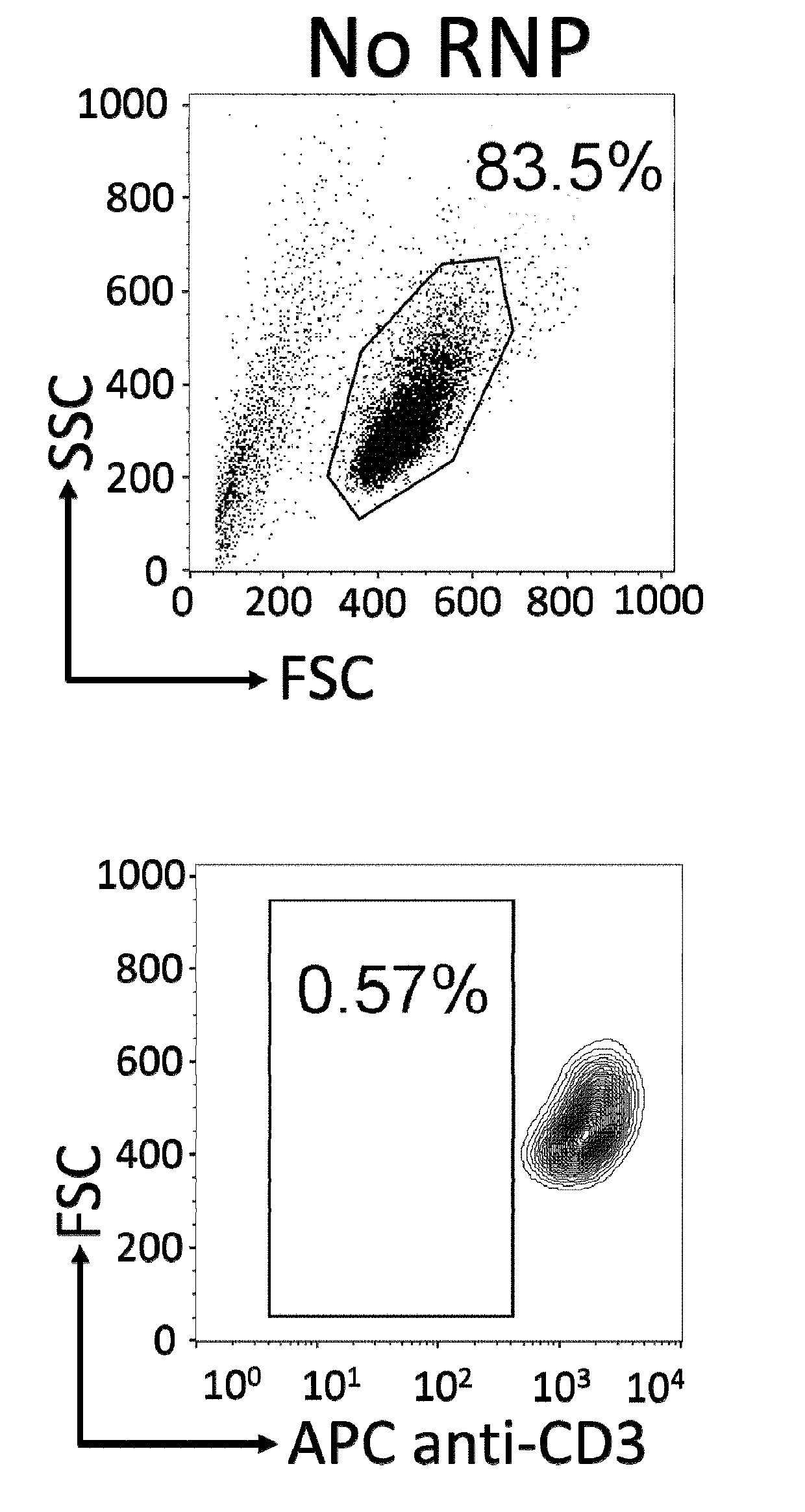
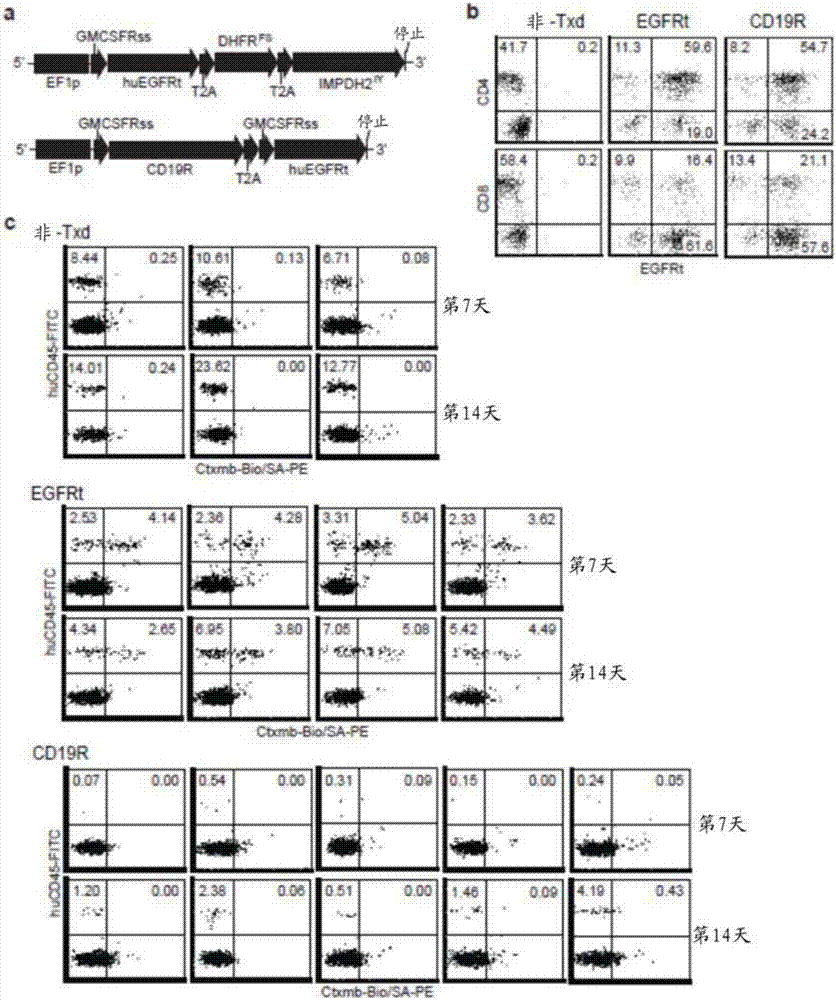
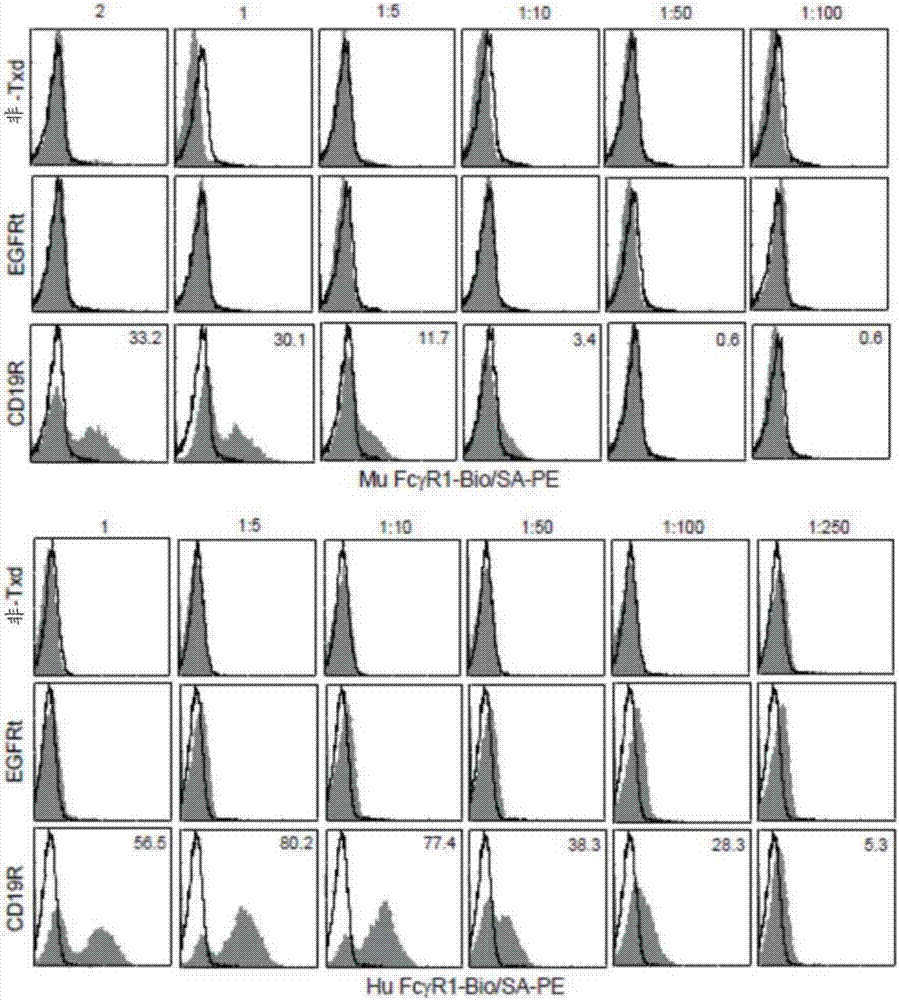
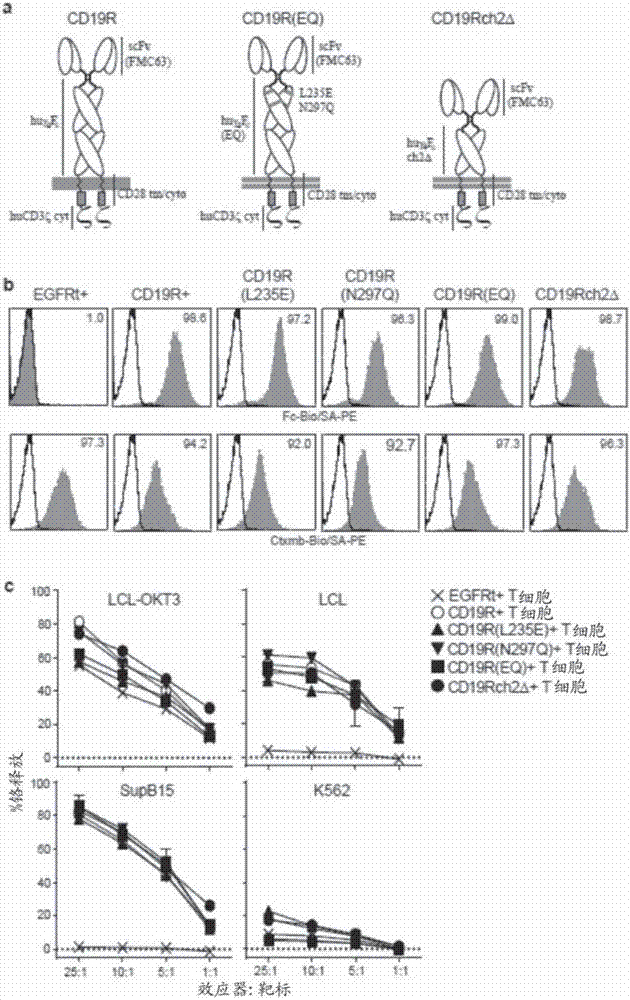
Patents
Literature
148 results about "Adoptive immunity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
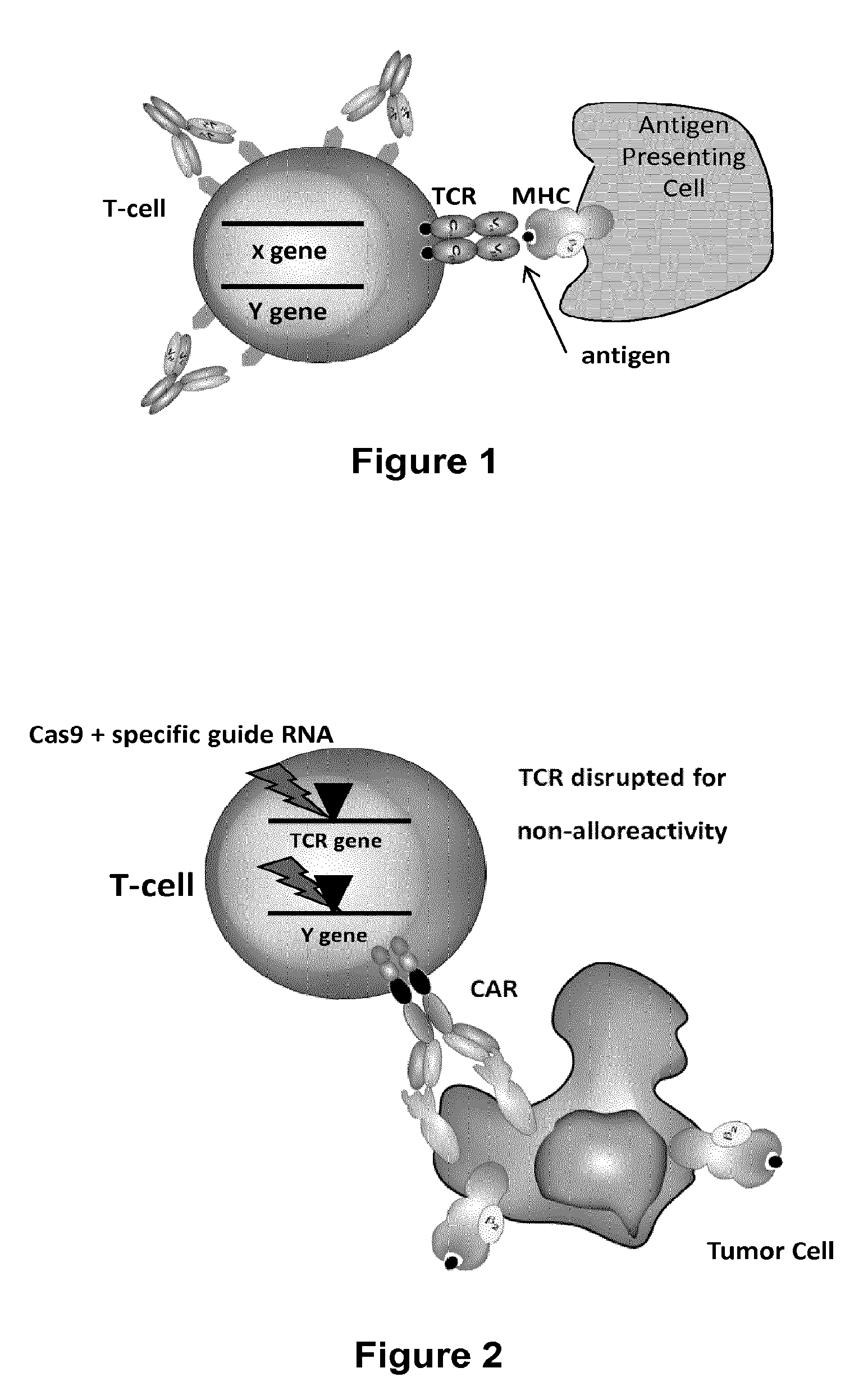
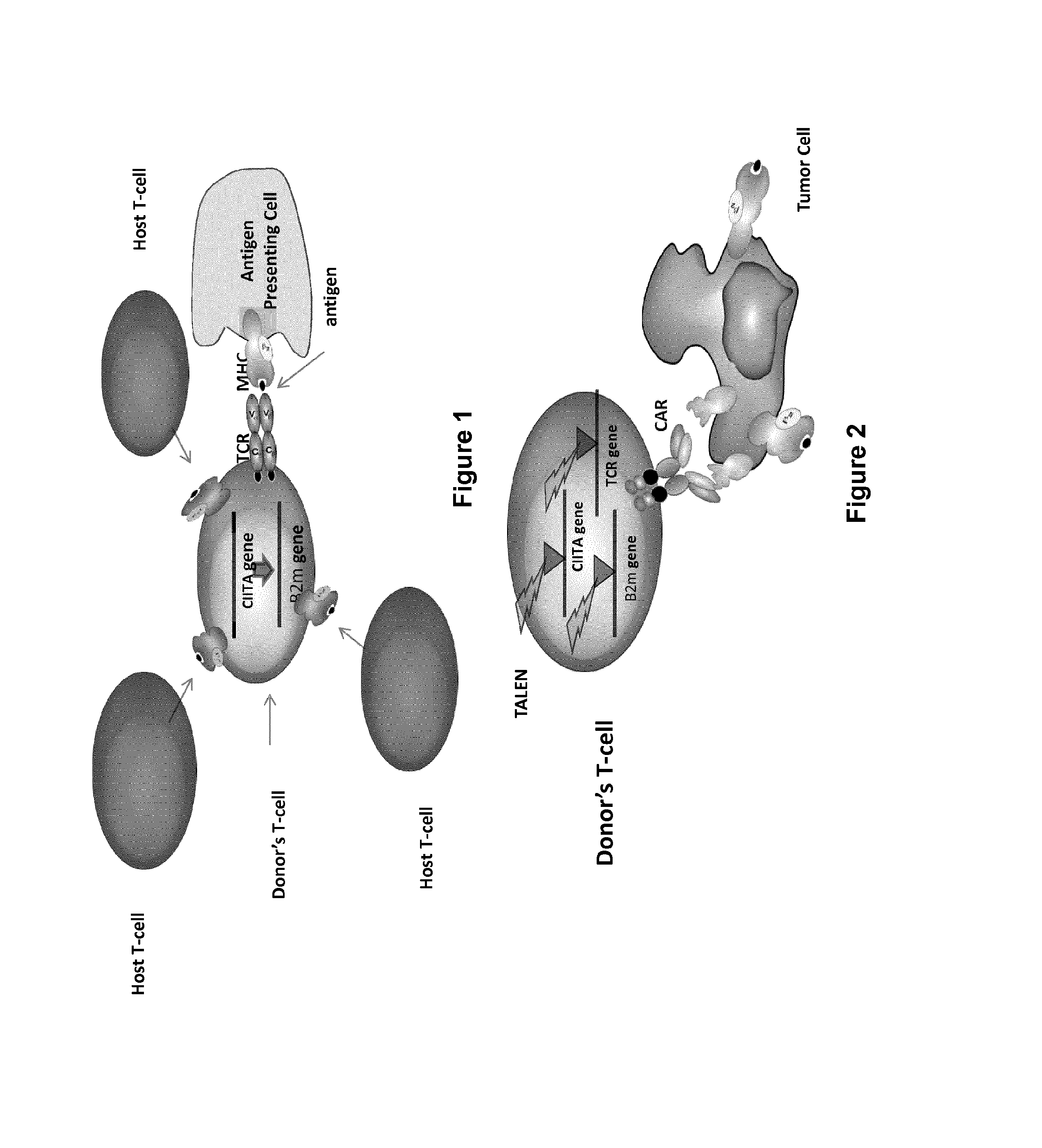
Adoptive immunity acts in a host after their immunological components are withdrawn, their immunological activity is modified extracorporeally, and then reinfused into the same host. This process in its former part is analogous to adoption: a child is once adopted out from their home, grown up, and then returned to their home of birth. Transferred immunological components include immune cells such as T lymphocytes or tumour-infiltrating lymphocytes, NK cells, macrophages, or B cells.
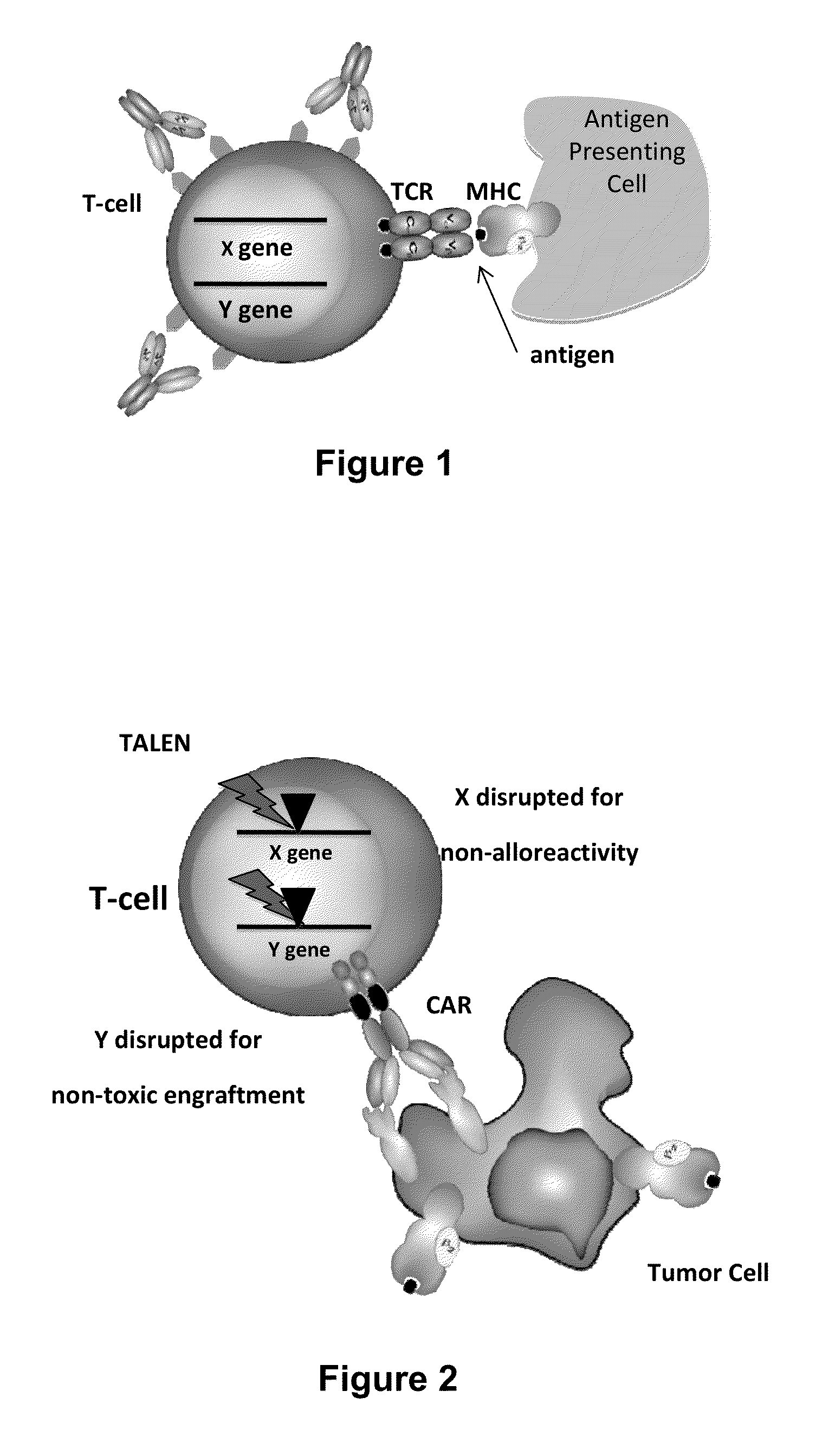
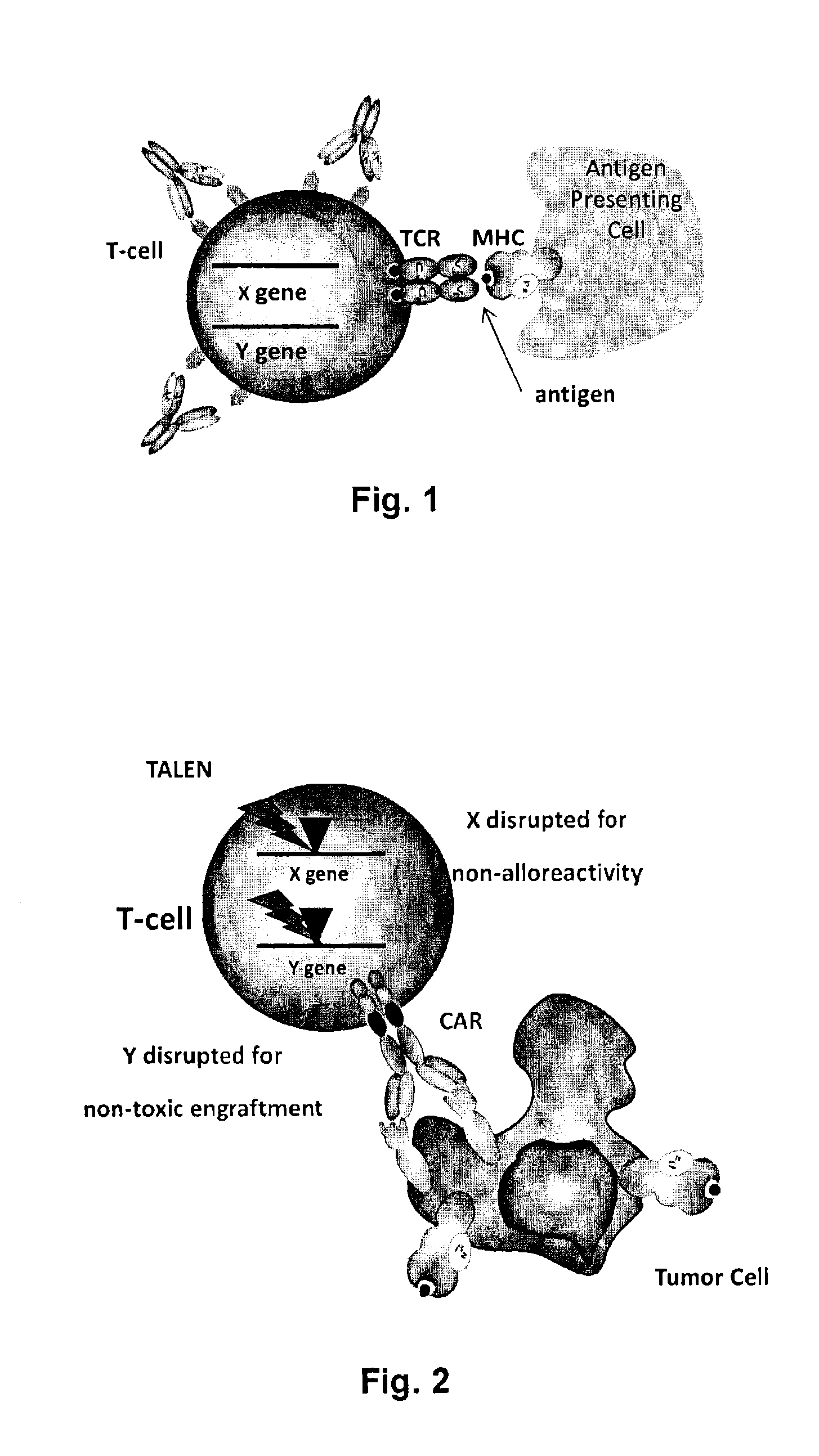
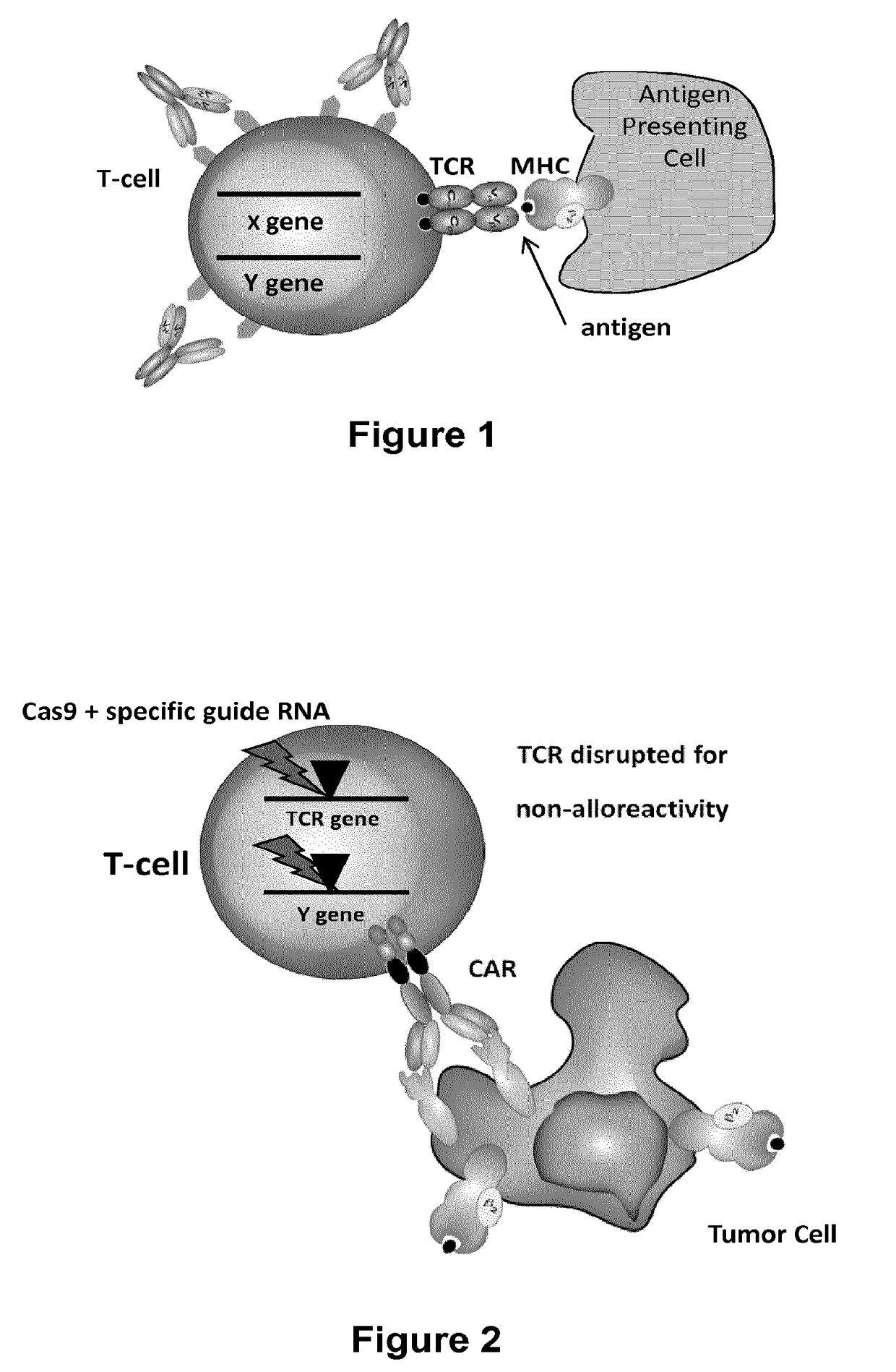
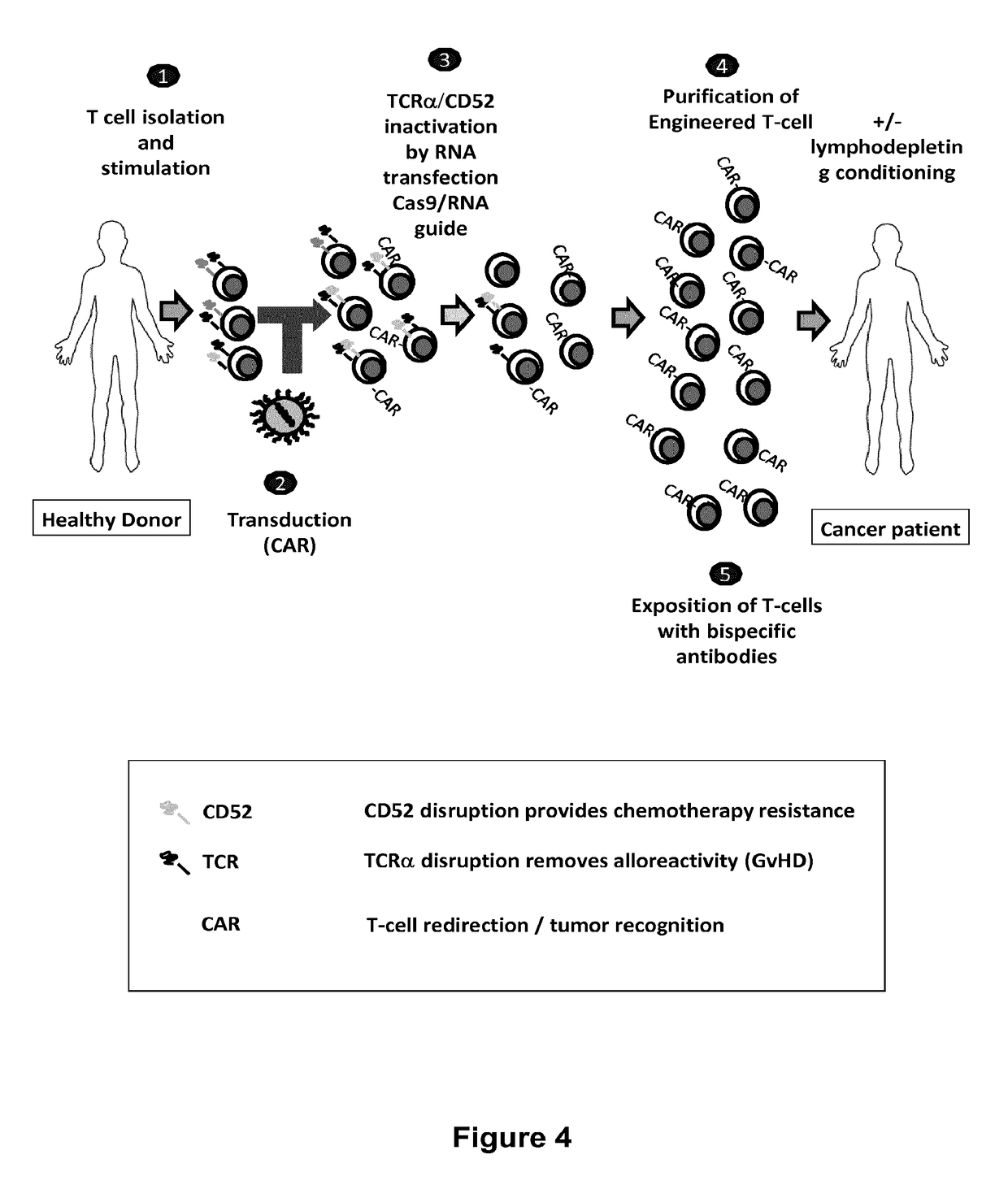
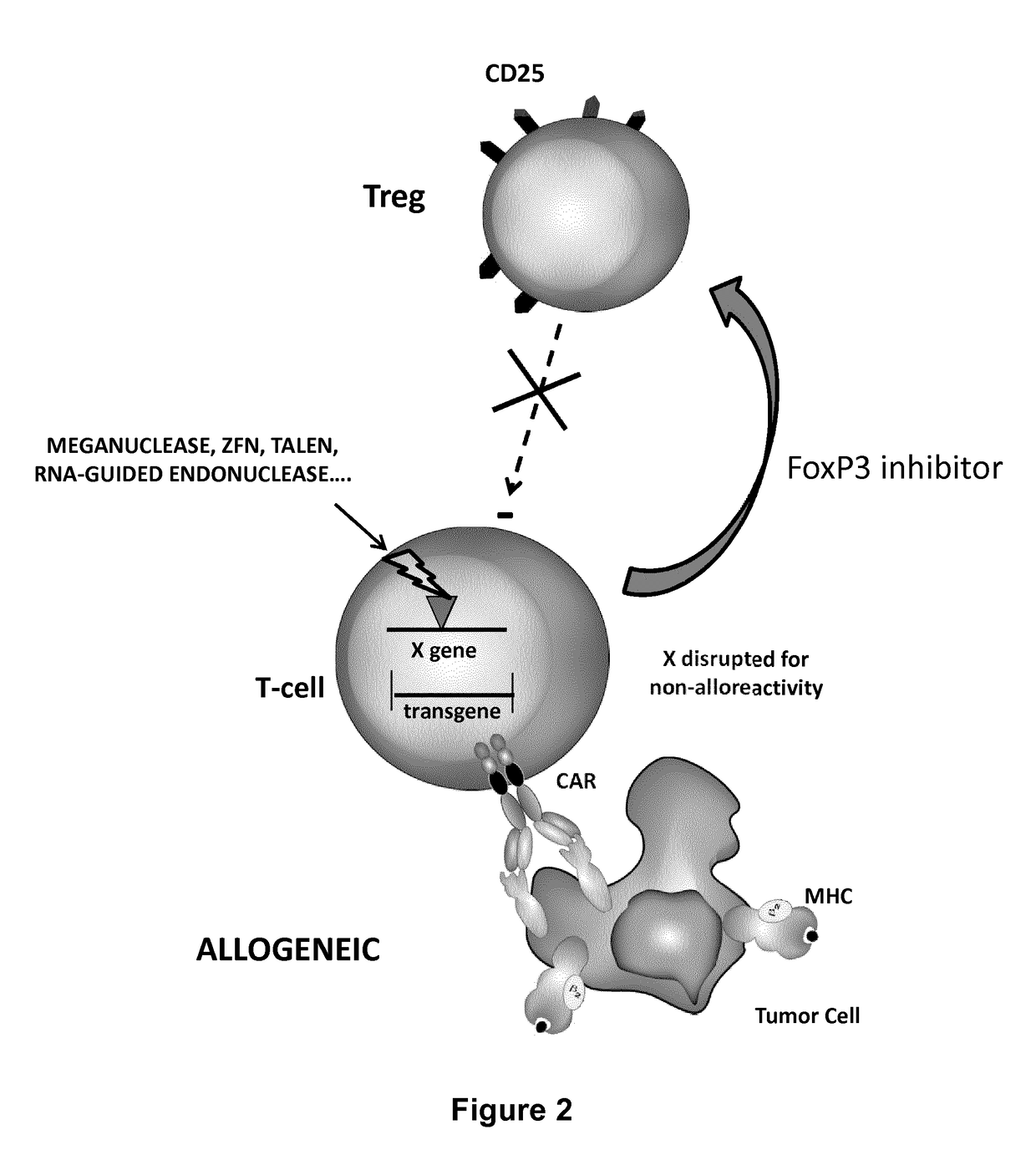
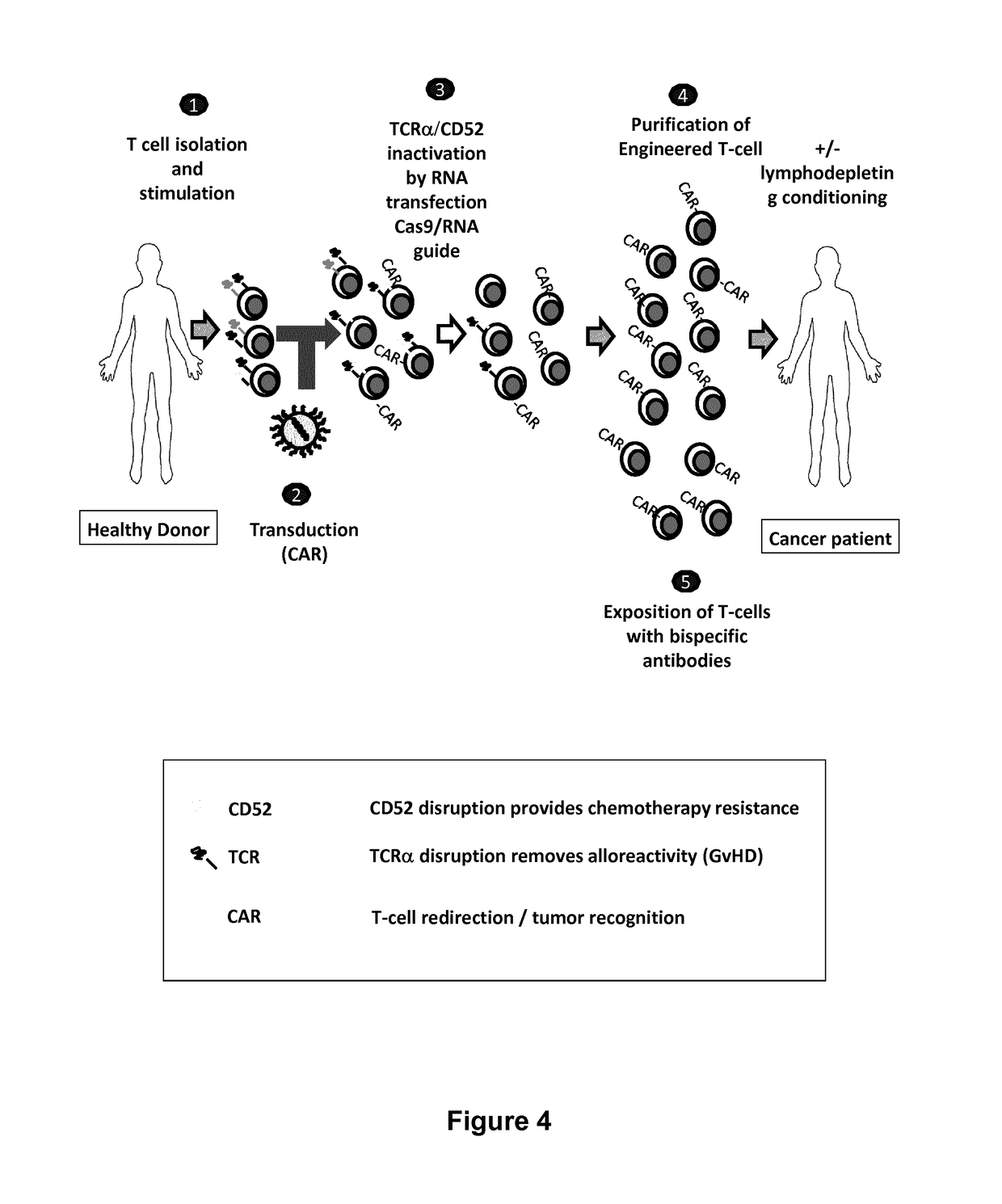
Methods for engineering allogeneic and immunosuppressive resistant t cell for immunotherapy
ActiveUS20130315884A1Precise positioningPeptide/protein ingredientsAntibody mimetics/scaffoldsImmunosuppressive drugPrimary cell
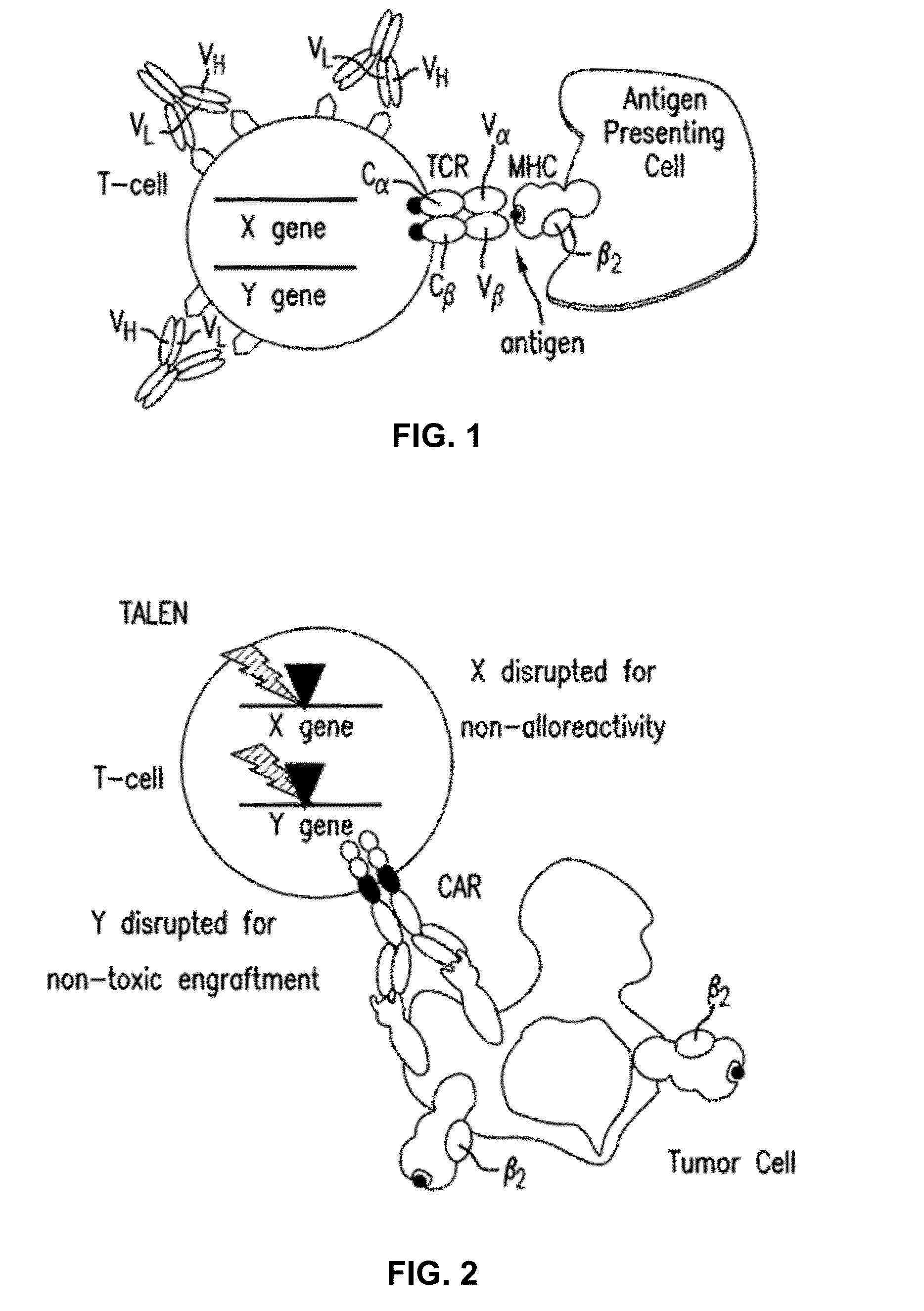
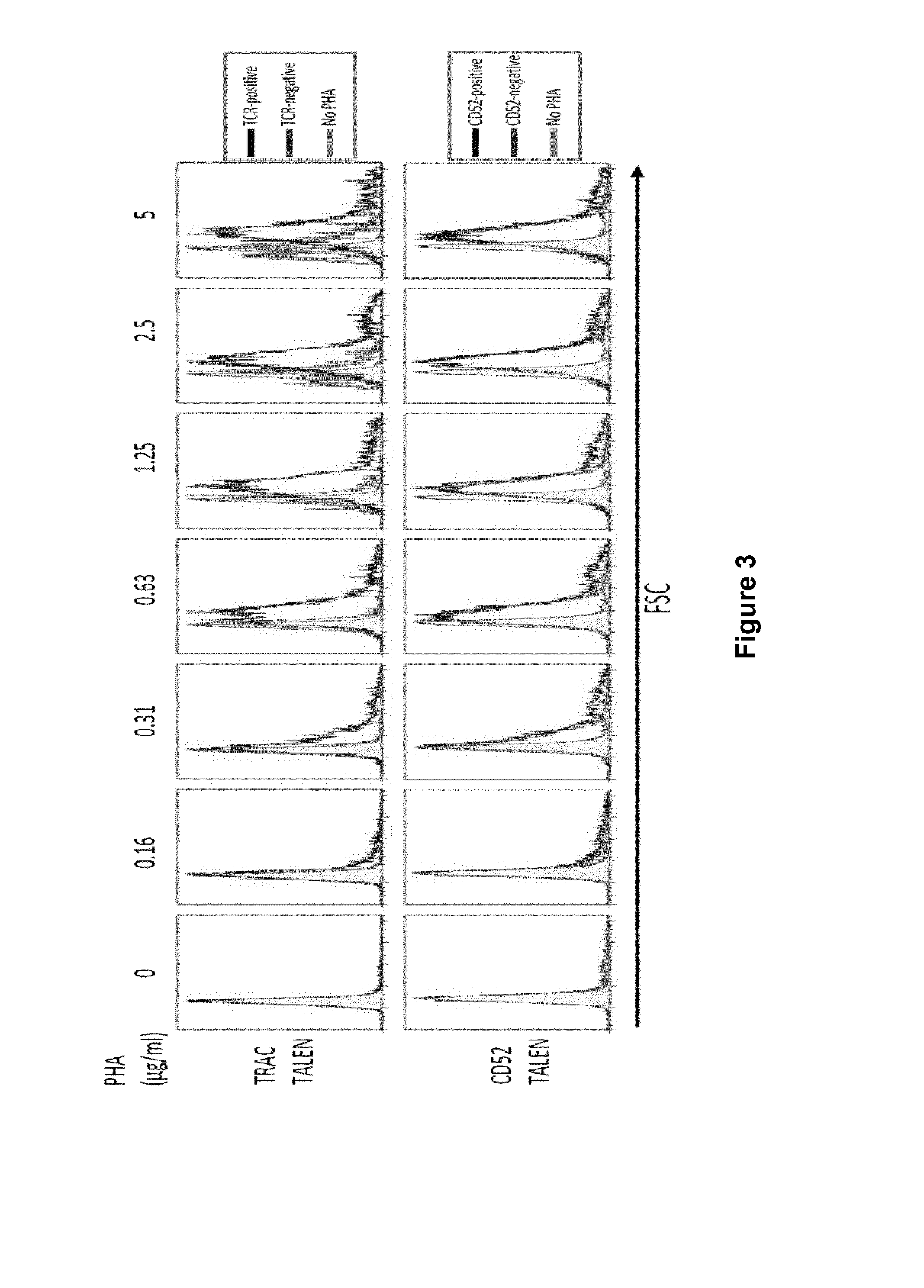
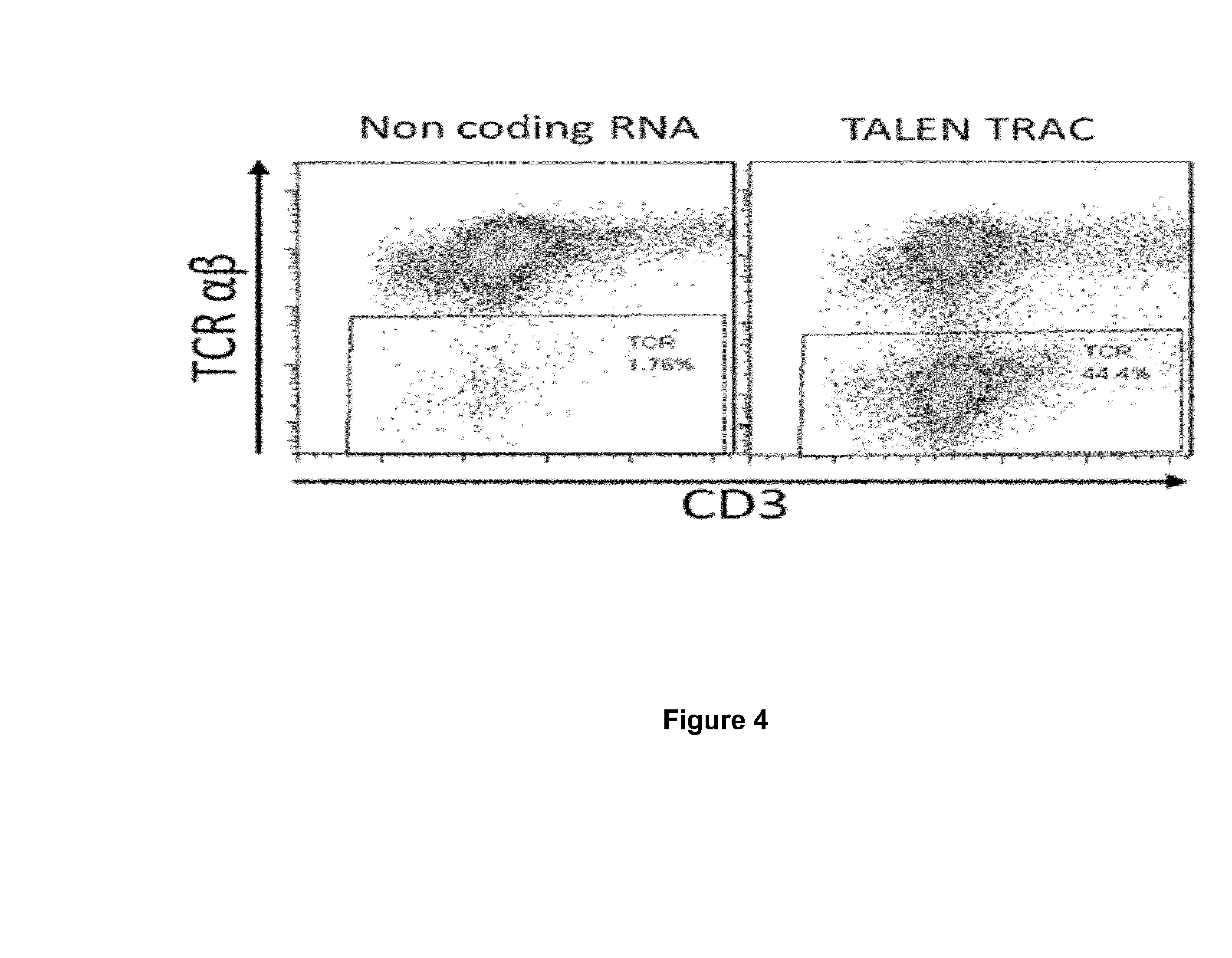
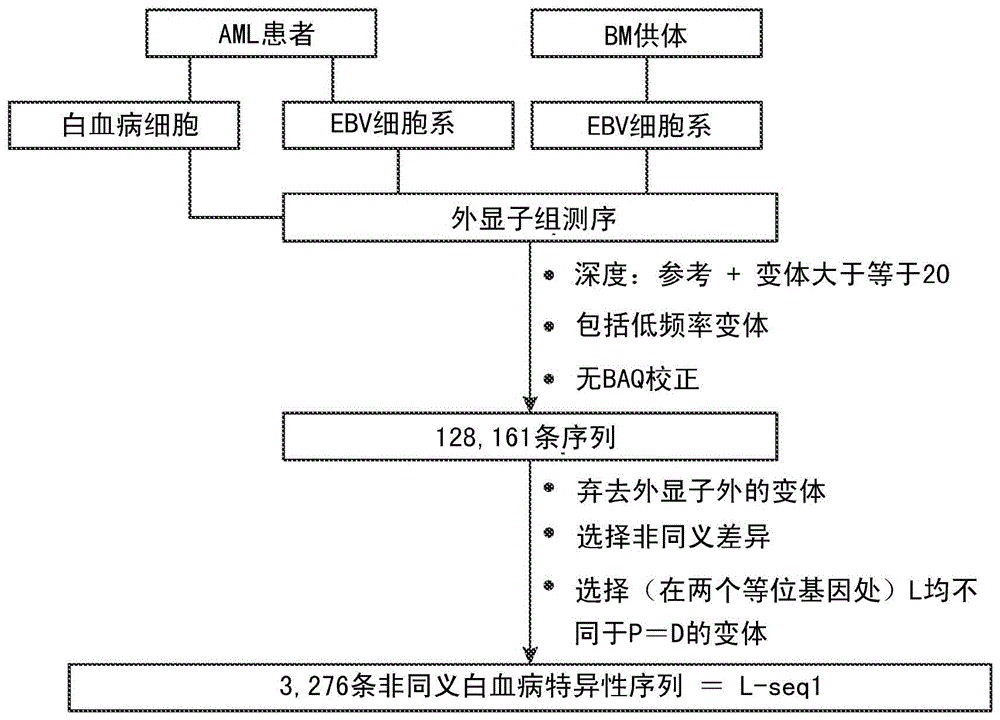
Methods for developing engineered T-cells for immunotherapy that are both non-alloreactive and resistant to immunosuppressive drugs. The present invention relates to methods for modifying T-cells by inactivating both genes encoding target for an immunosuppressive agent and T-cell receptor, in particular genes encoding CD52 and TCR. This method involves the use of specific rare cutting endonucleases, in particular TALE-nucleases (TAL effector endonuclease) and polynucleotides encoding such polypeptides, to precisely target a selection of key genes in T-cells, which are available from donors or from culture of primary cells. The invention opens the way to standard and affordable adoptive immunotherapy strategies for treating cancer and viral infections.
Owner:CELLECTIS SA
Compositions and methods for treatment of neoplastic disease
InactiveUS20050112141A1High productFacilitates their targetingFusions for specific cell targetingReceptors for cytokines/lymphoines/interferonsAbnormal tissue growthDisease
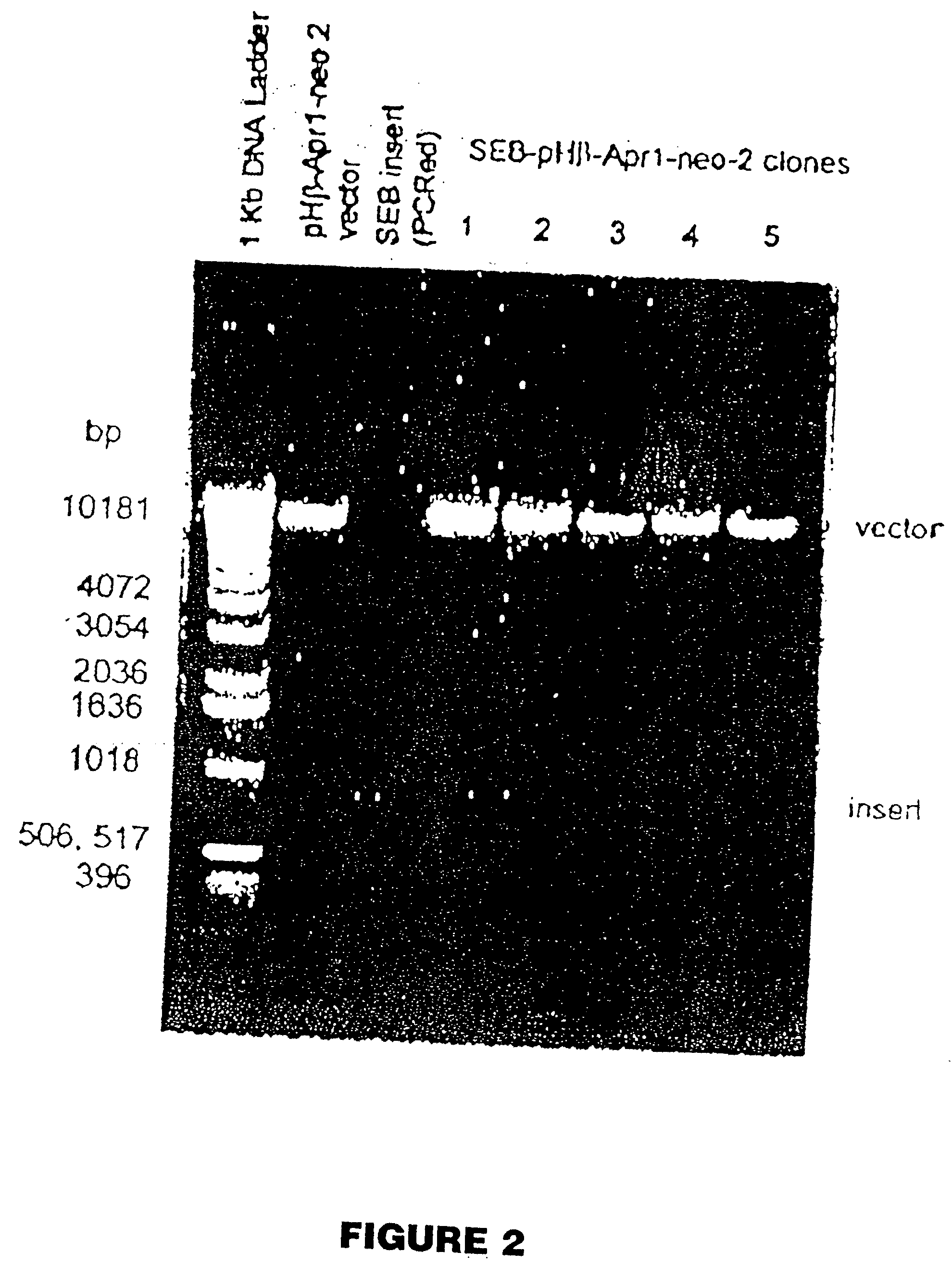
The present invention comprises compositions and methods for treating a tumor or neoplastic disease in a host, The methods employ conjugates comprising superantigen polypeptides or nucleic acids with other structures that preferentially bind to tumor cells and are capable of inducing apoptosis. Also provided are superantigen-glycolipid conjugates and vesicles that are loaded onto antigen presenting cells to activate both T cells and NKT cells. Cell-based vaccines comprise tumor cells engineered to express a superantigen along with glycolipids products which, when expressed, render the cells capable of eliciting an effective anti-tumor immune response in a mammal into which these cells are introduced. Included among these compositions are tumor cells, hybrid cells of tumor cells and accessory cells, preferably dendritic cells. Also provided are T cells and NKT cells activated by the above compositions that can be administered for adoptive immunotherapy.
Owner:TERMAN DAVID
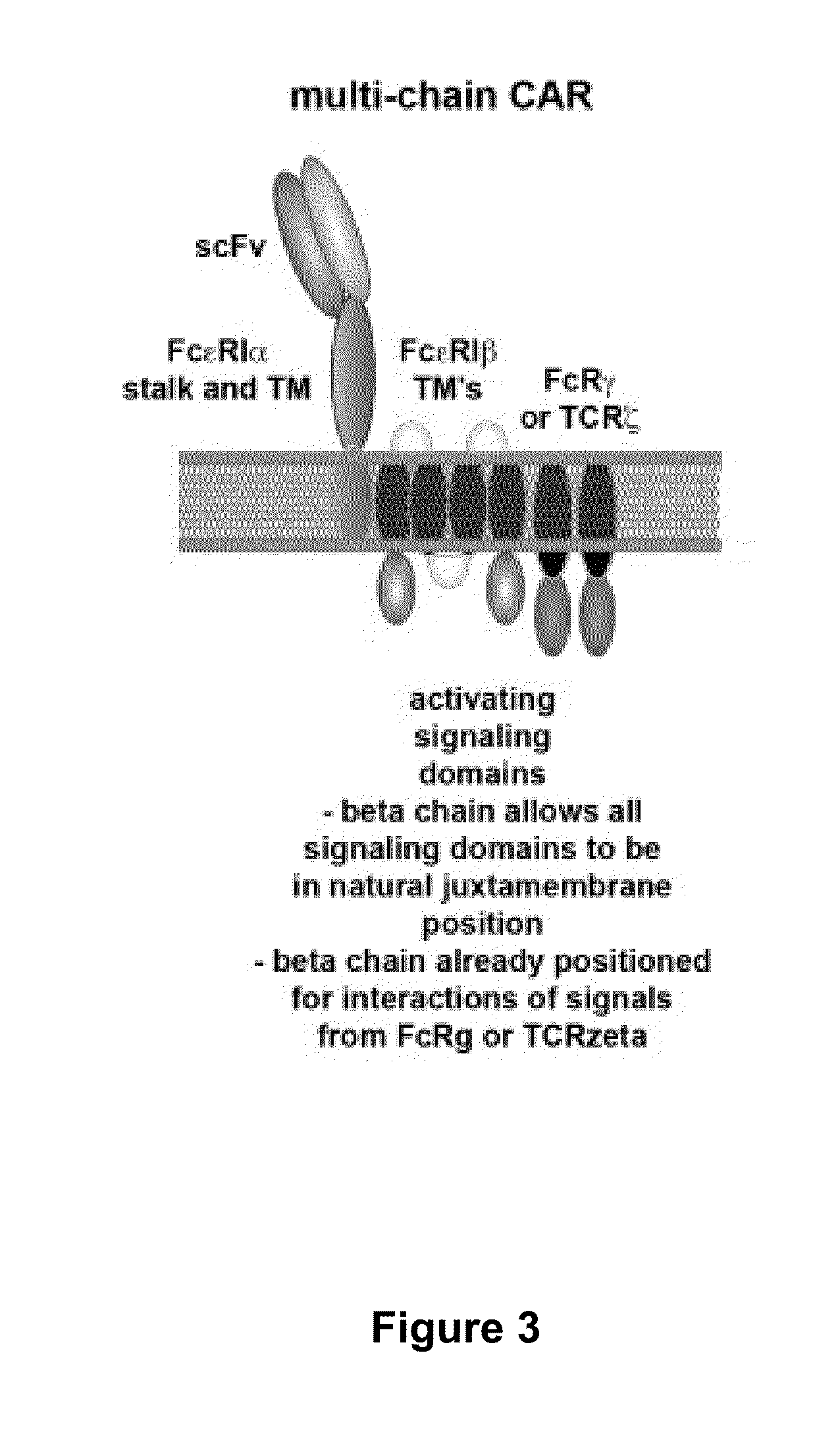
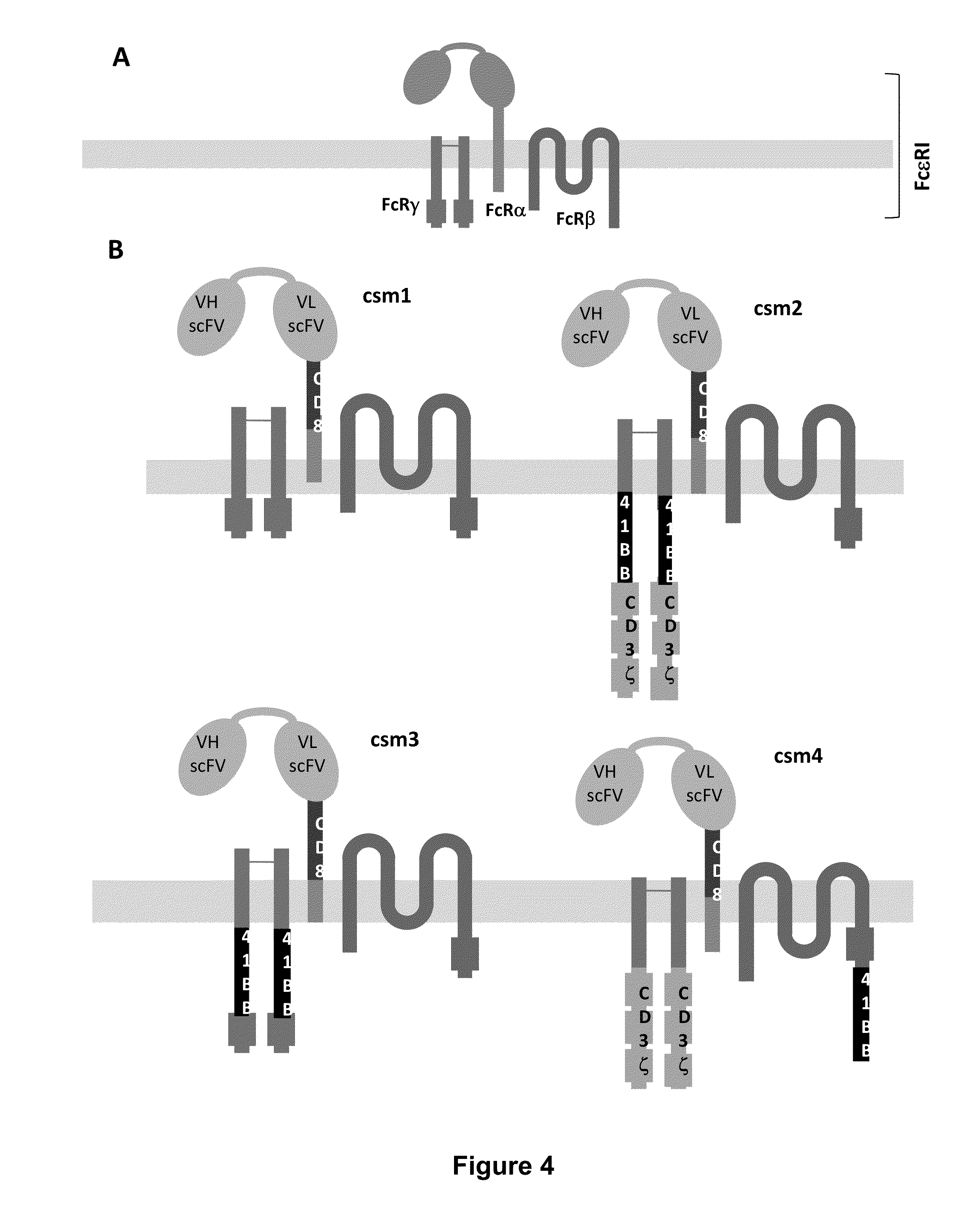
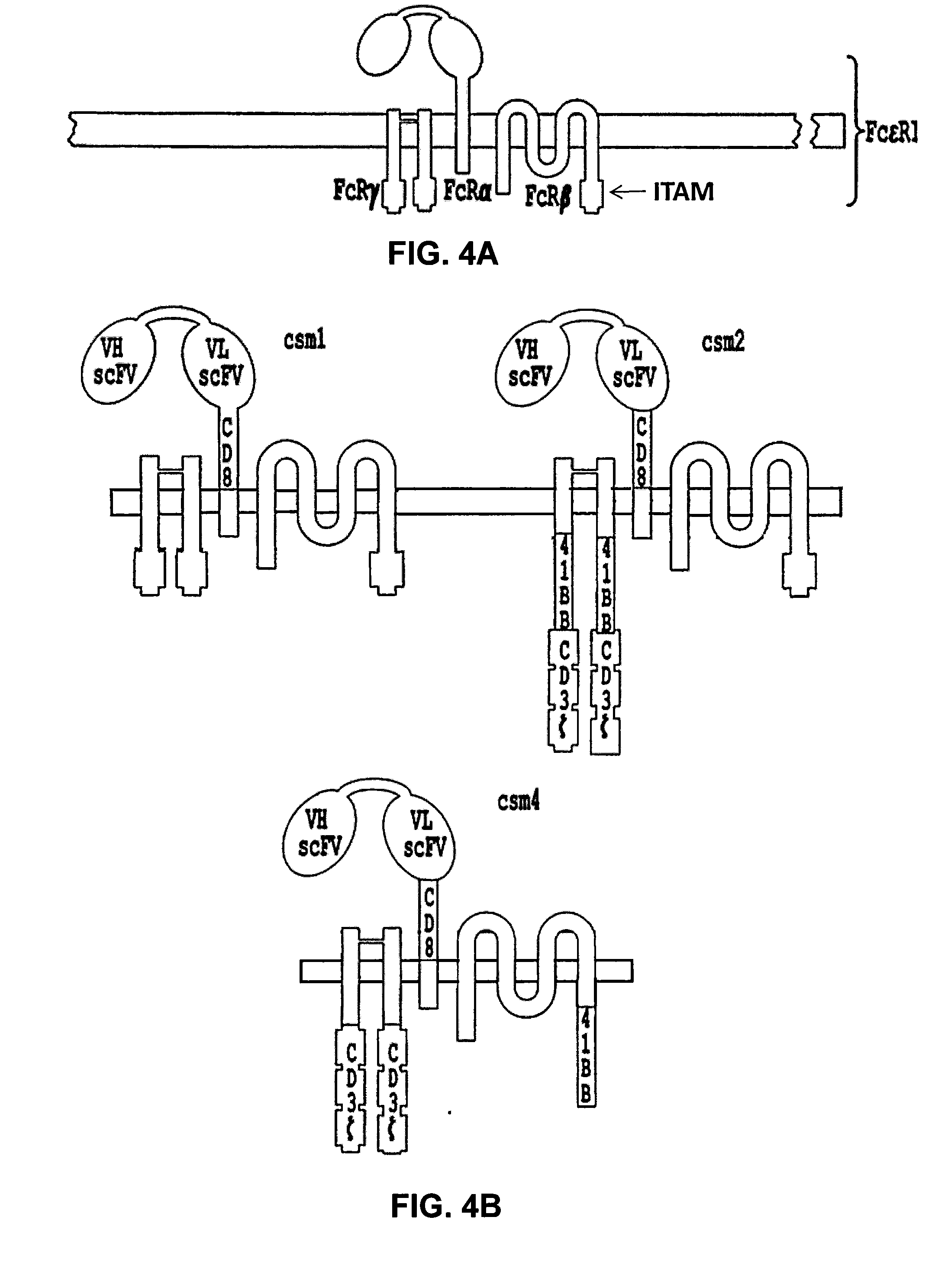
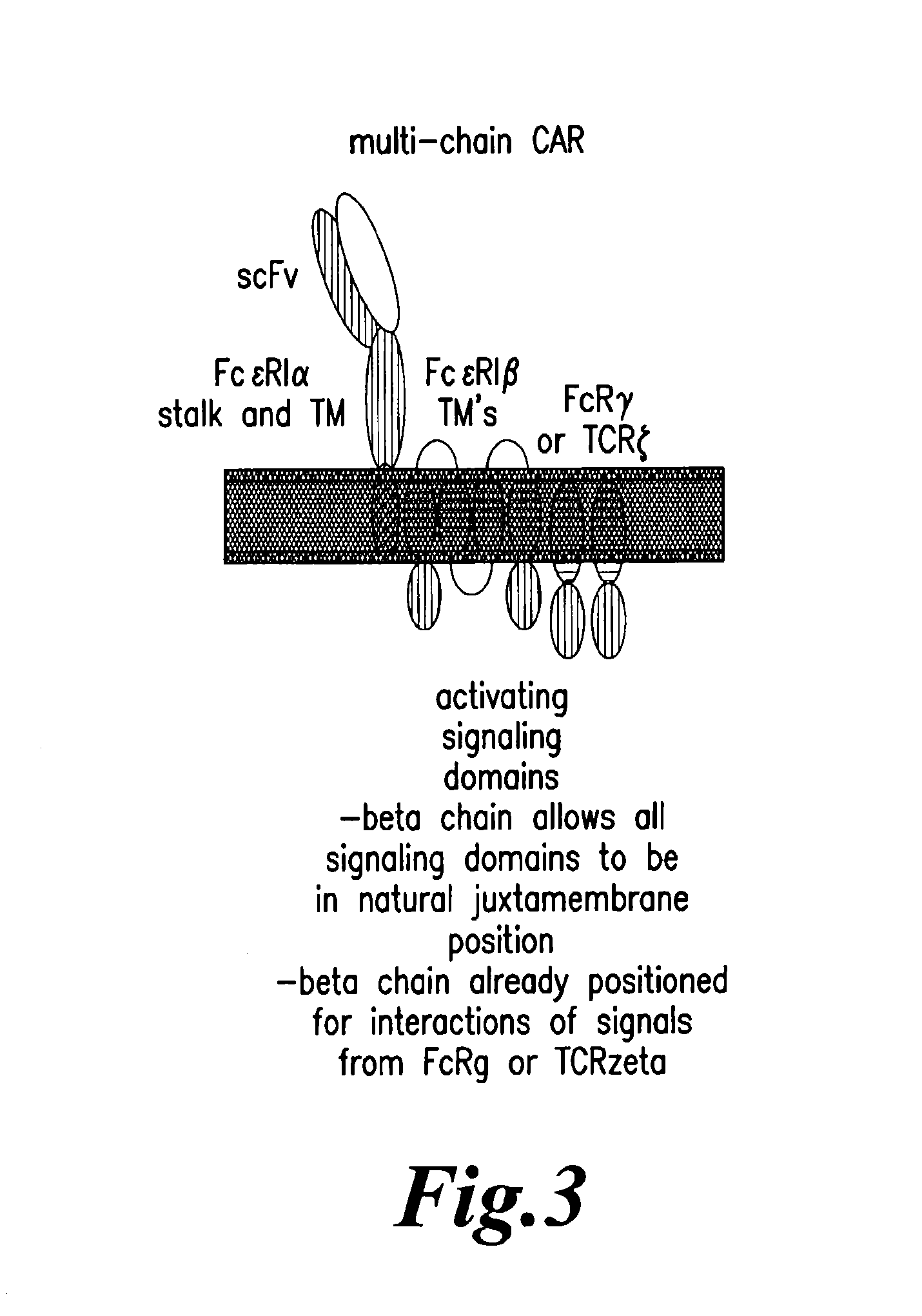
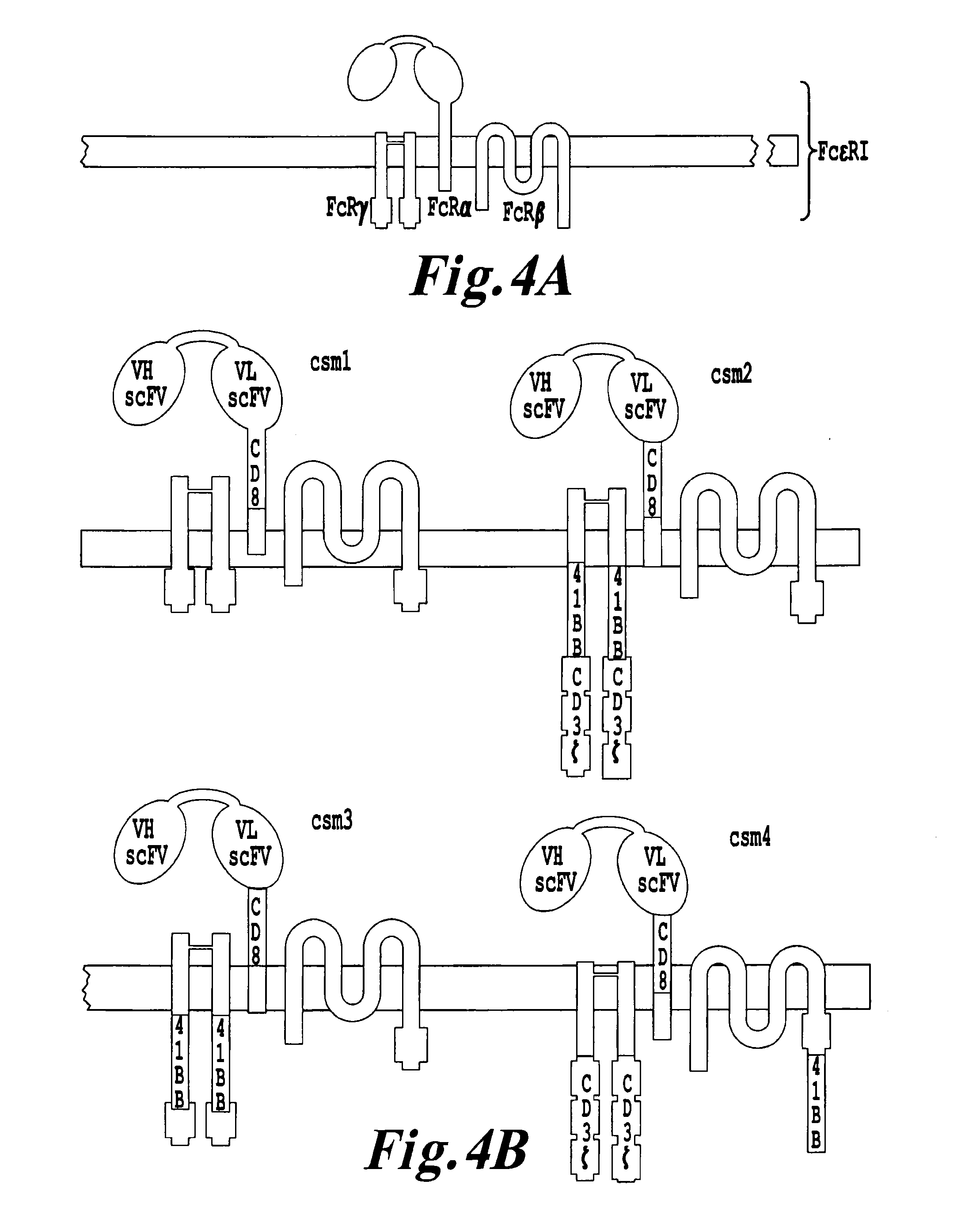
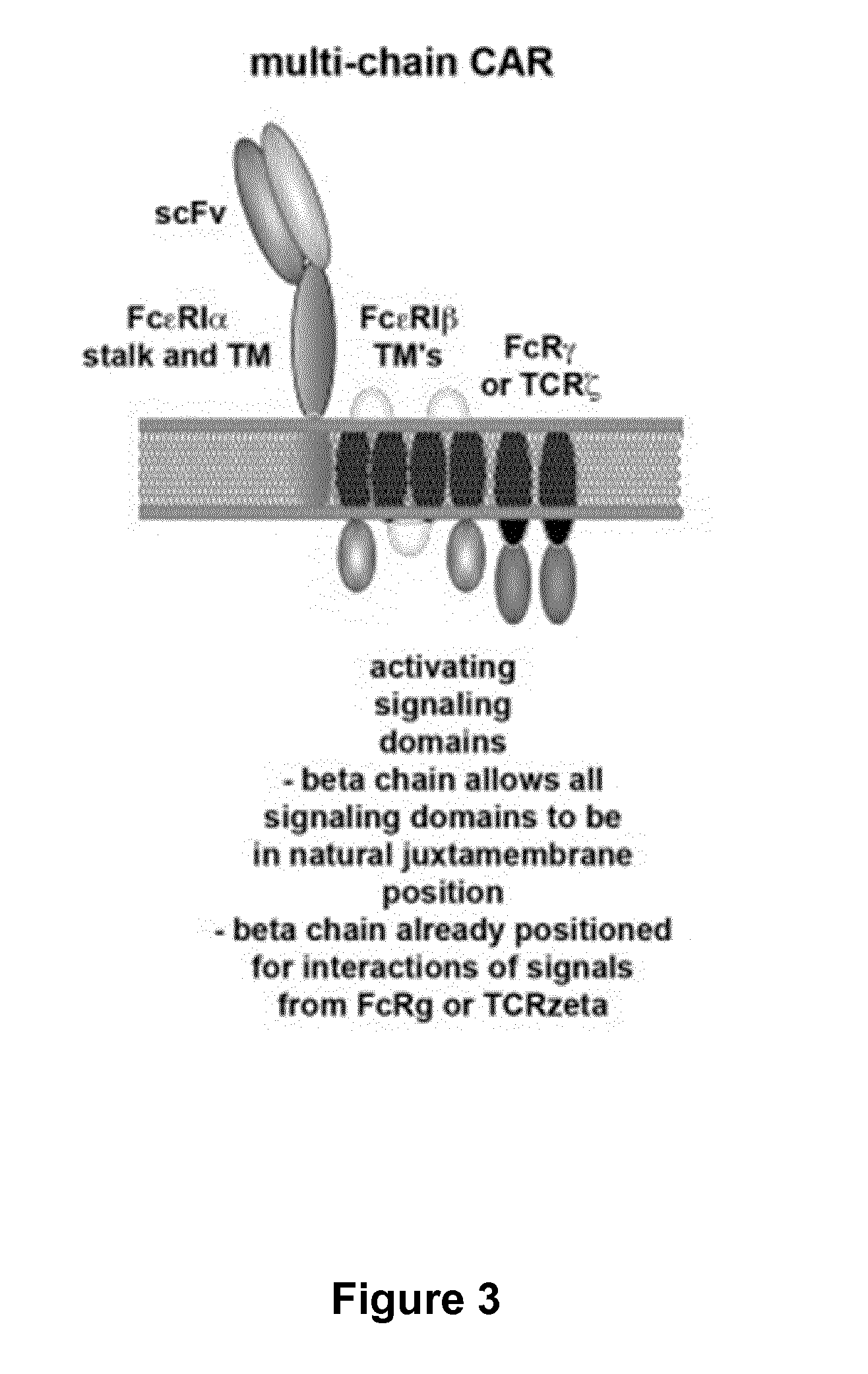
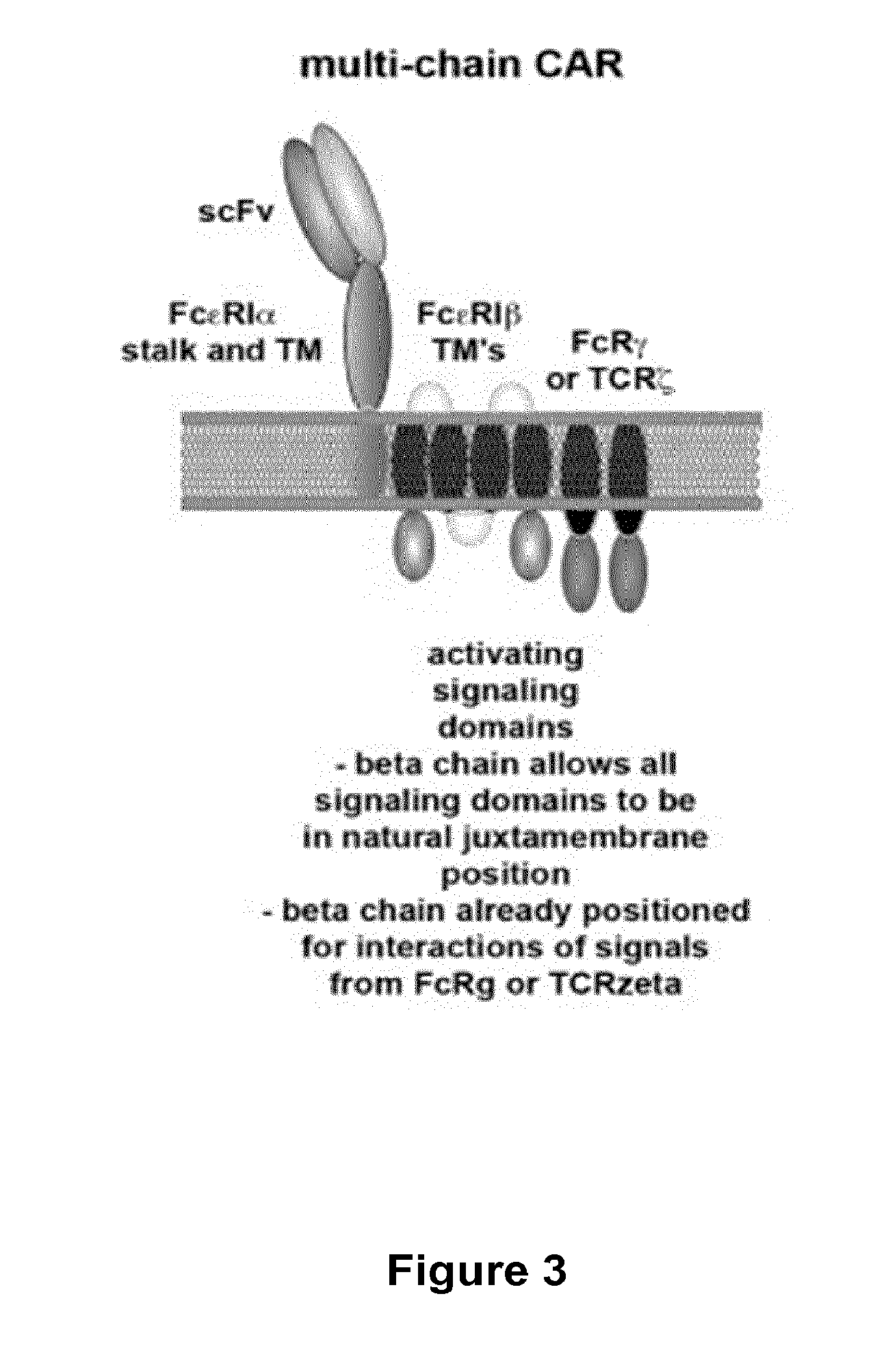
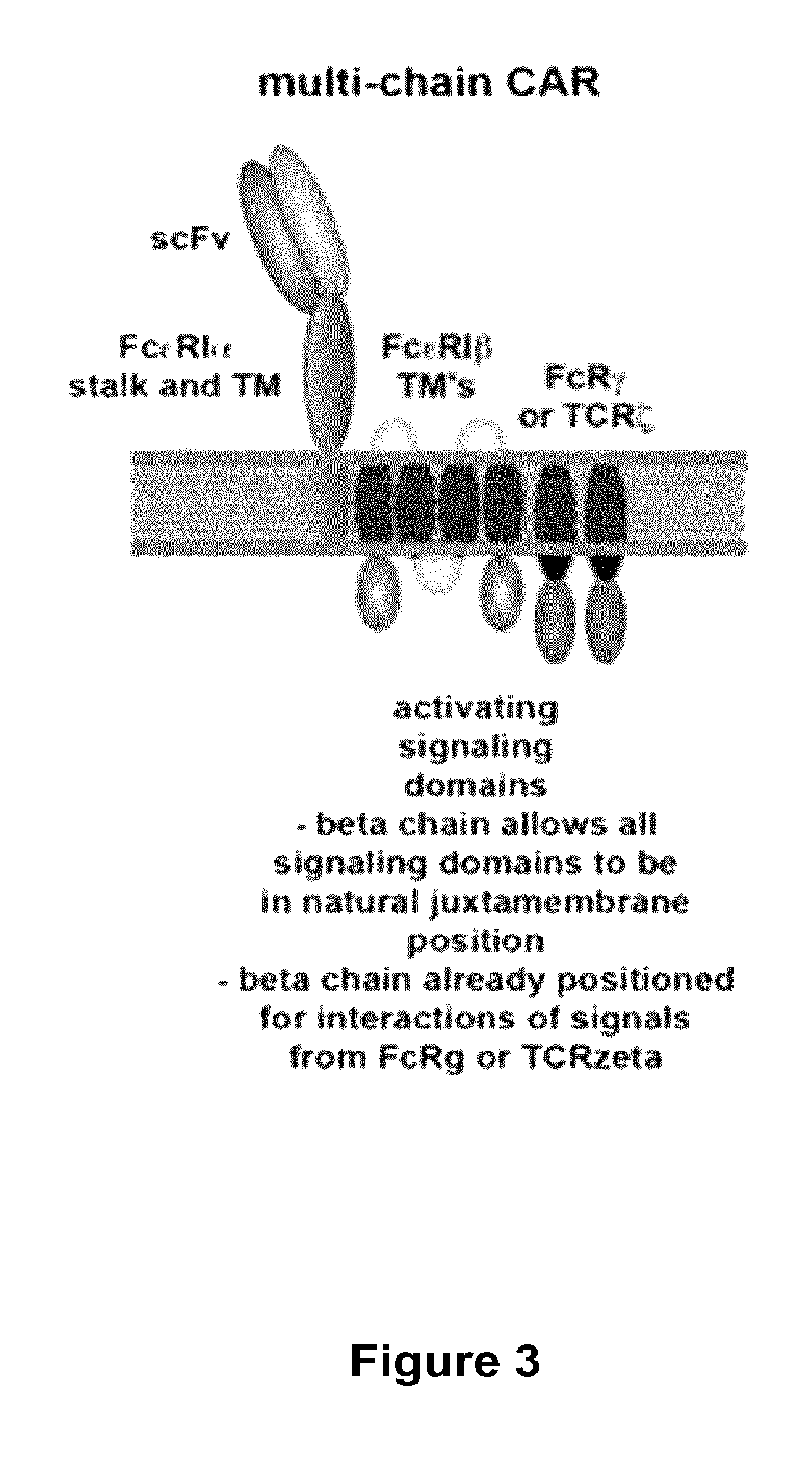
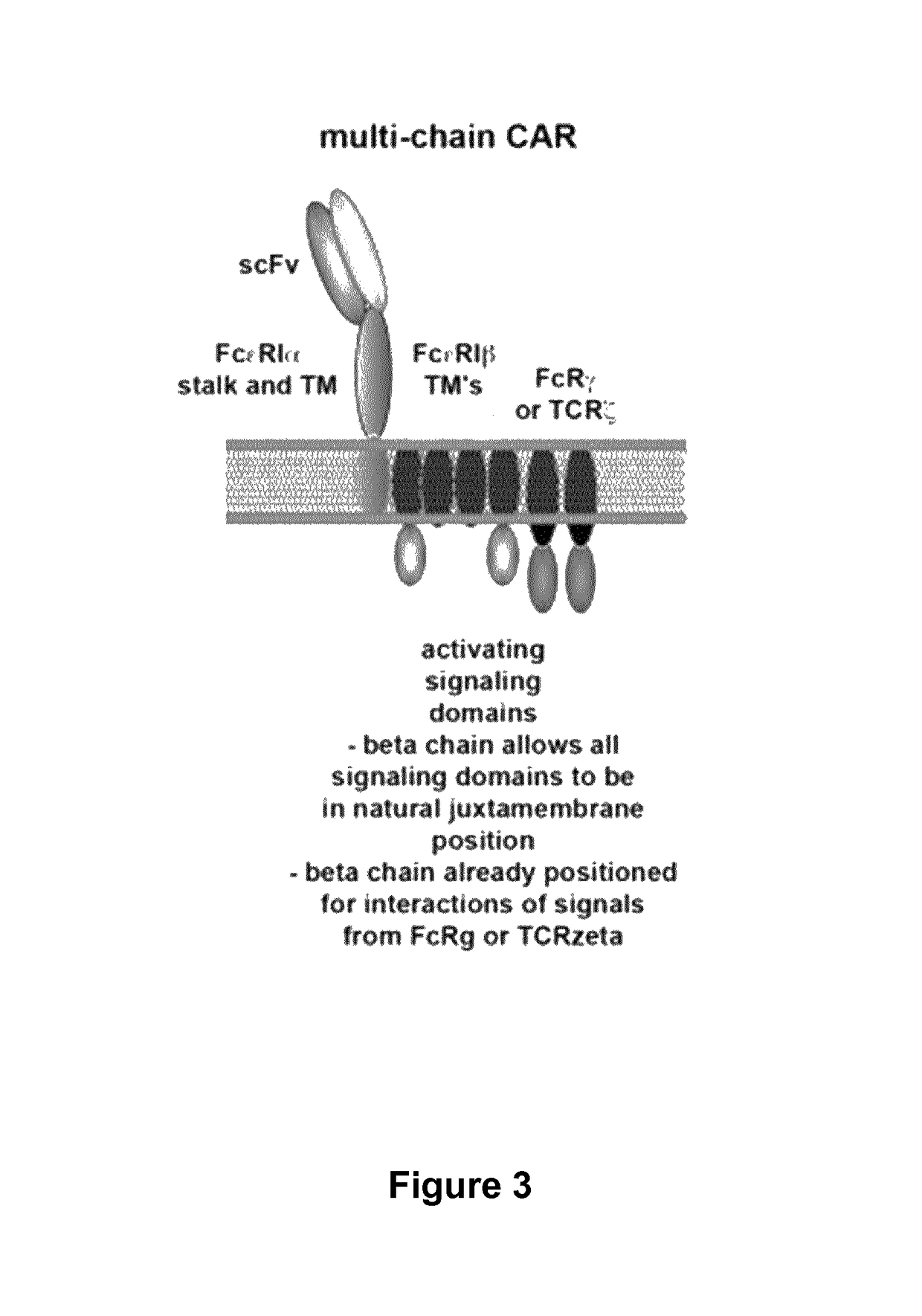
Multi-Chain Chimeric Antigen Receptor and Uses Thereof
ActiveUS20140134142A1Modulate activityStrong specificityPeptide/protein ingredientsAntibody mimetics/scaffoldsAntigen receptorReceptor
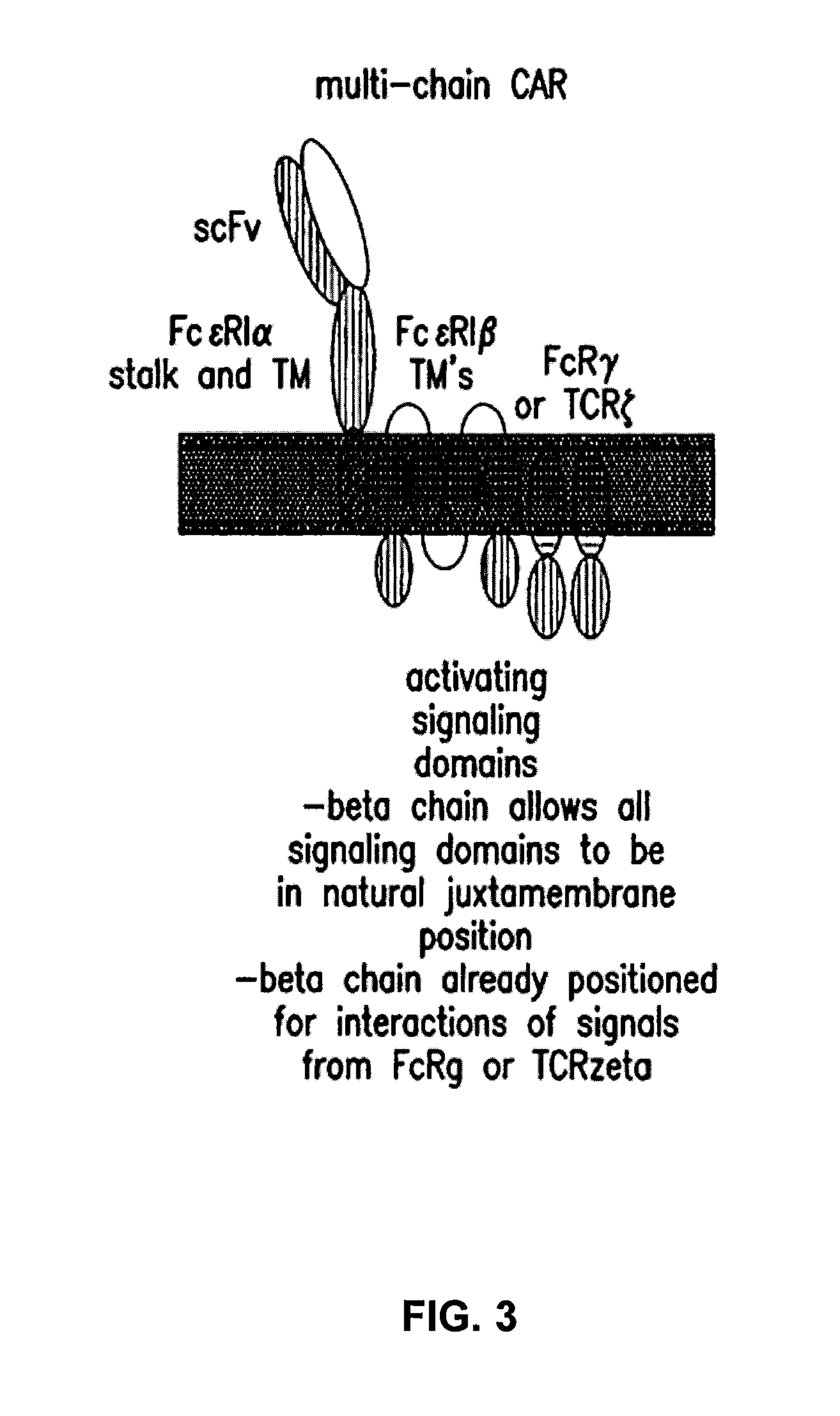
The present invention relates to a new generation of chimeric antigen receptors (CAR) referred to as multi-chain CARs. Such CARs, which aim to redirect immune cell specificity and reactivity toward a selected target exploiting the ligand-binding domain properties, comprise separate extracellular ligand binding and signaling domains in different transmembrane polypeptides. The signaling domains are designed to assemble in juxtamembrane position, which forms flexible architecture closer to natural receptors, that confers optimal signal transduction. The invention encompasses the polynucleotides, vectors encoding said multi-chain CAR and the isolated cells expressing them at their surface, in particularly for their use in immunotherapy. The invention opens the way to efficient adoptive immunotherapy strategies for treating cancer and viral infections.
Owner:CELLECTIS SA
Methods for engineering highly active t cell for immunotheraphy
The present invention relates to methods for developing engineered T-cells for immunotherapy and more specifically to methods for modifying T-cells by inactivating at immune checkpoint genes, preferably at least two selected from different pathways, to increase T-cell immune activity. This method involves the use of specific rare cutting endonucleases, in particular TALE-nucleases (TAL effector endonuclease) and polynucleotides encoding such polypeptides, to precisely target a selection of key genes in T-cells, which are available from donors or from culture of primary cells. The invention opens the way to highly efficient adoptive immunotherapy strategies for treating cancer and viral infections.
Owner:CELLECTIS SA
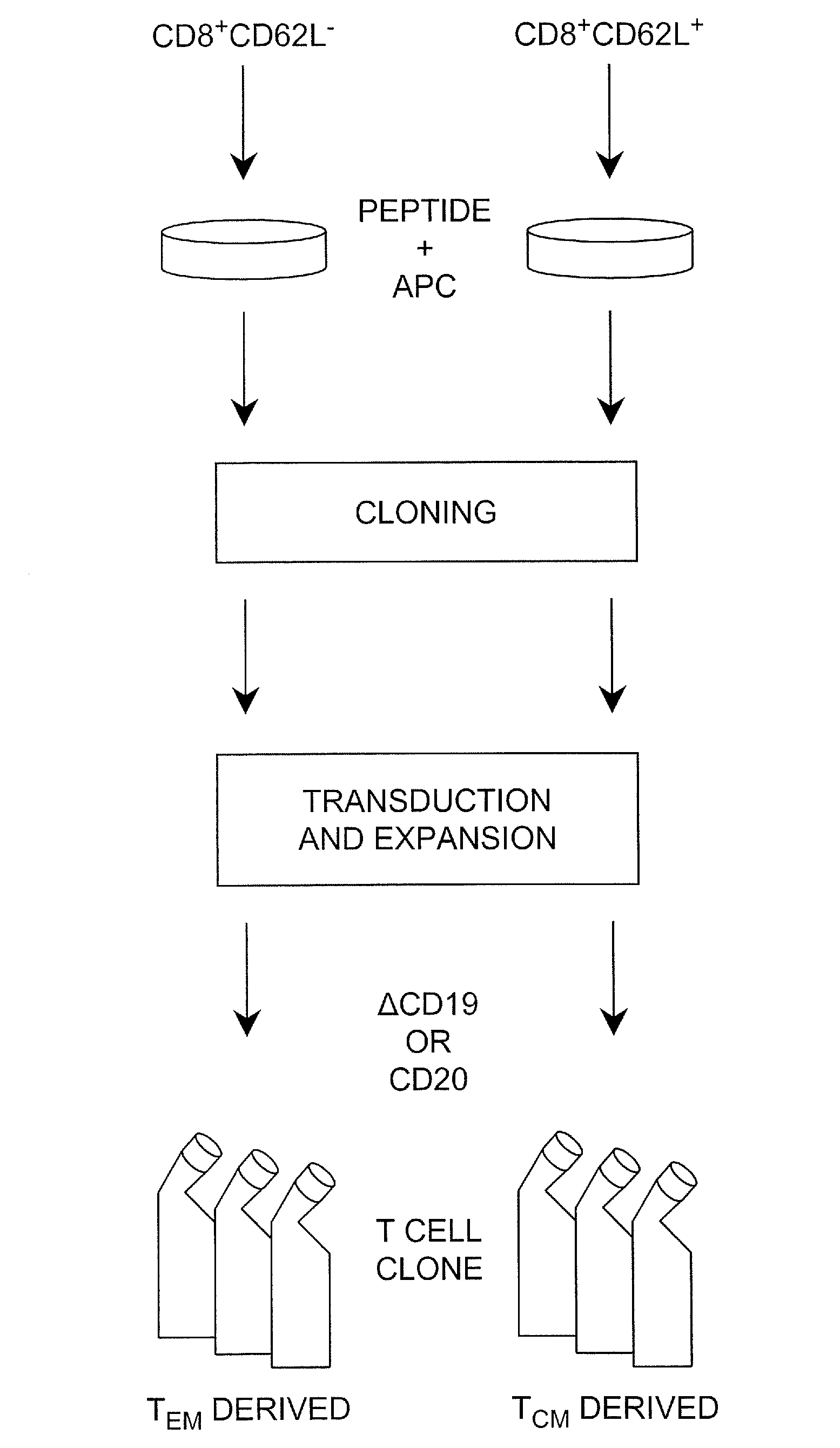
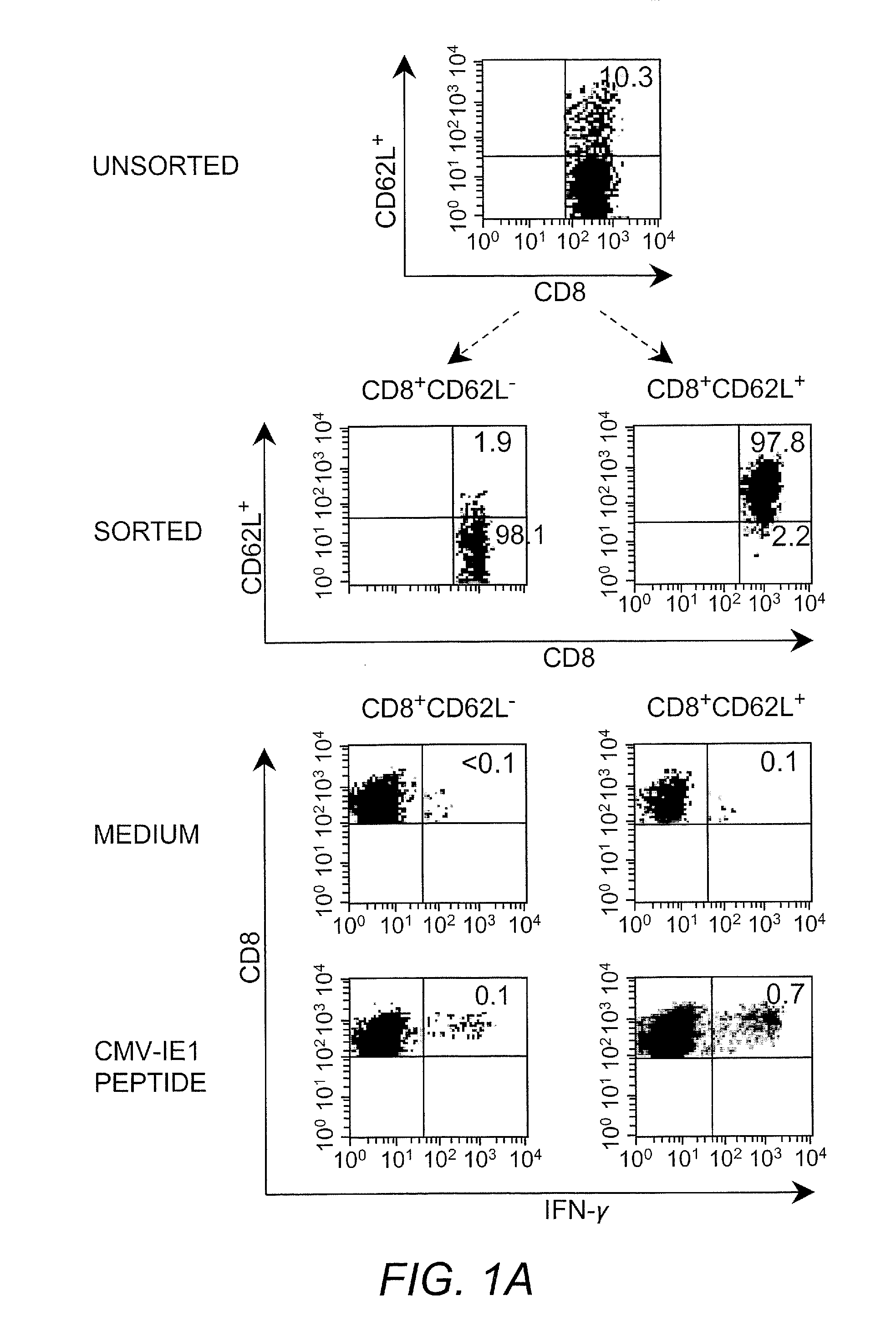
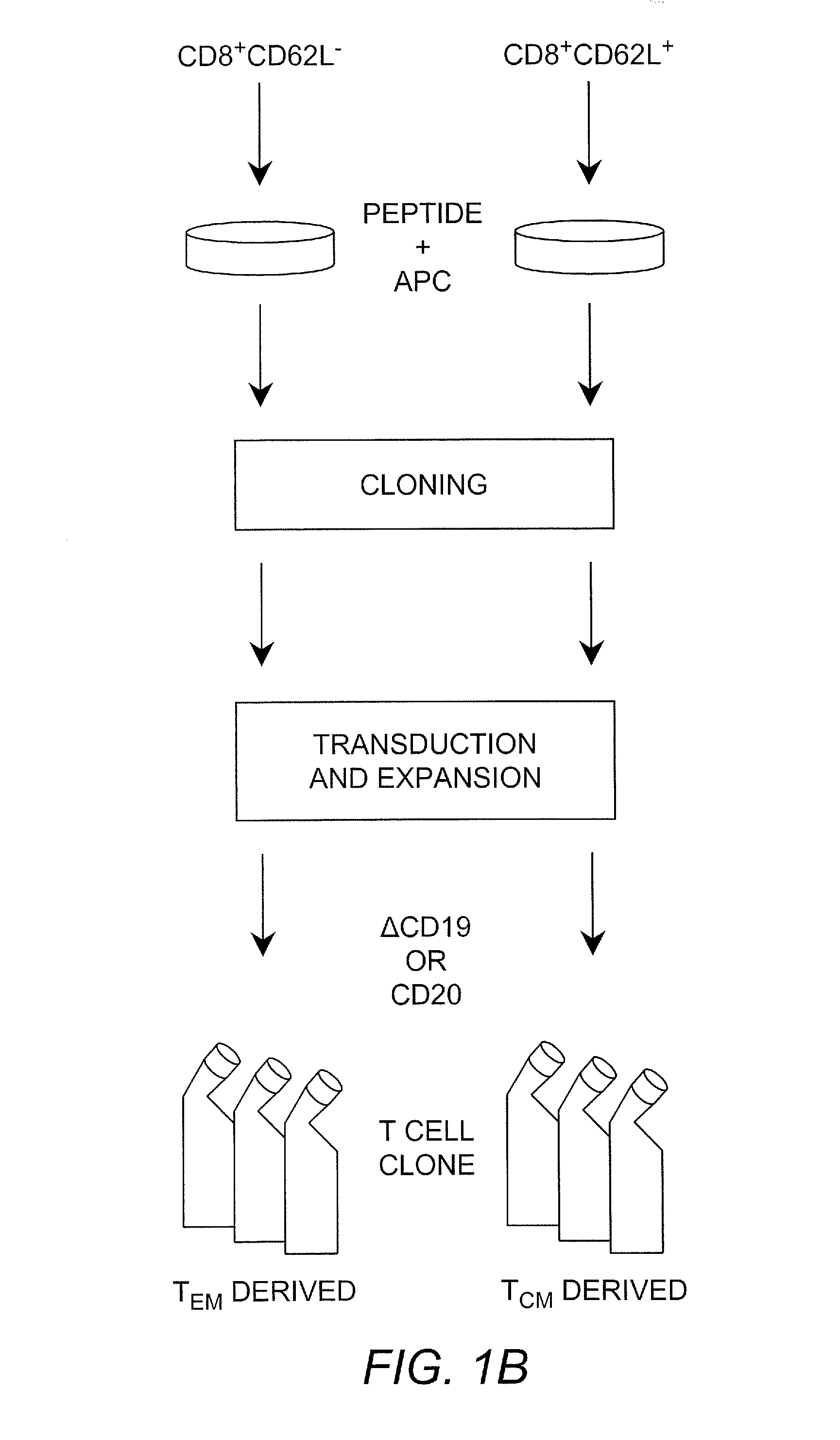
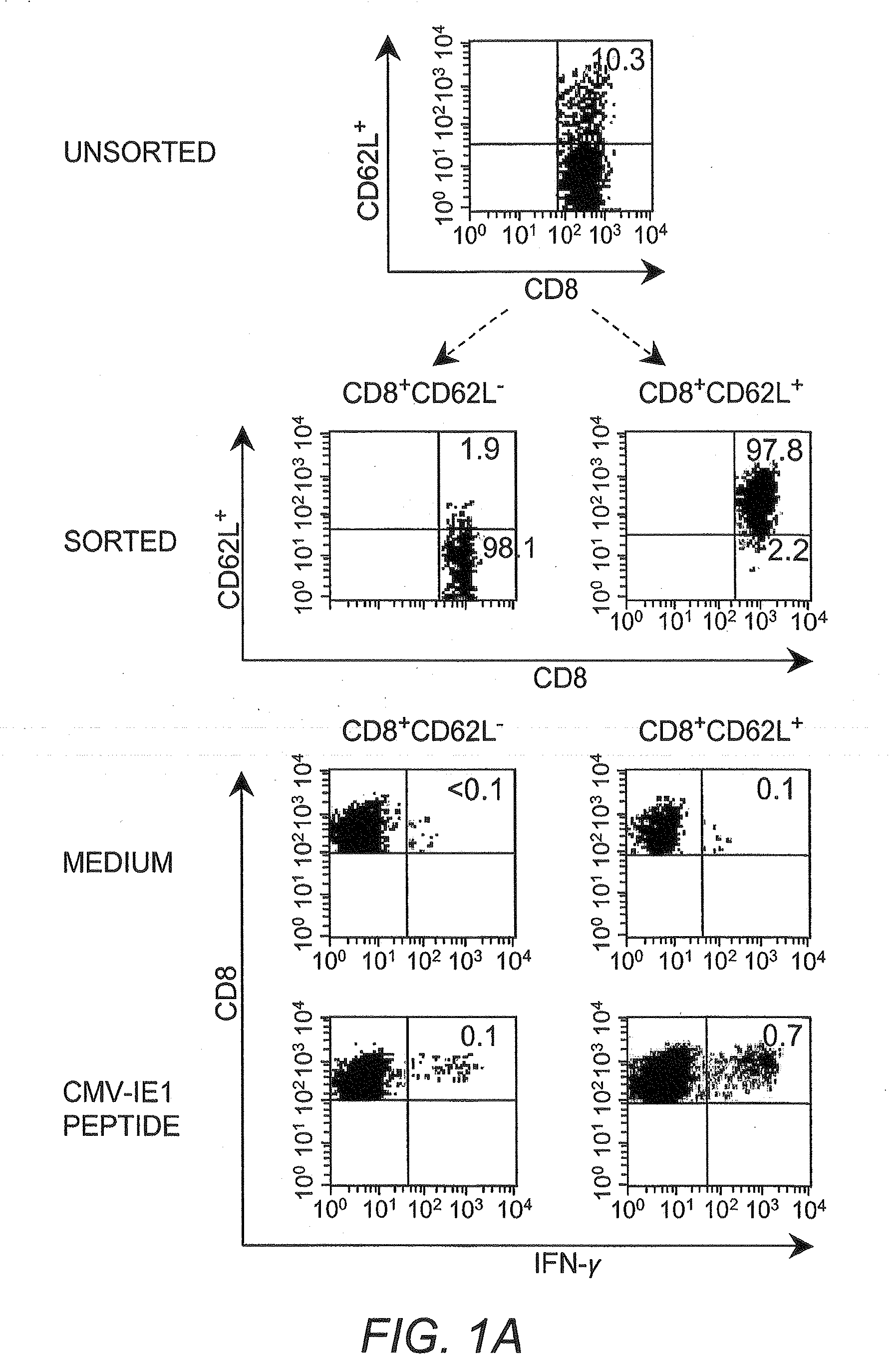
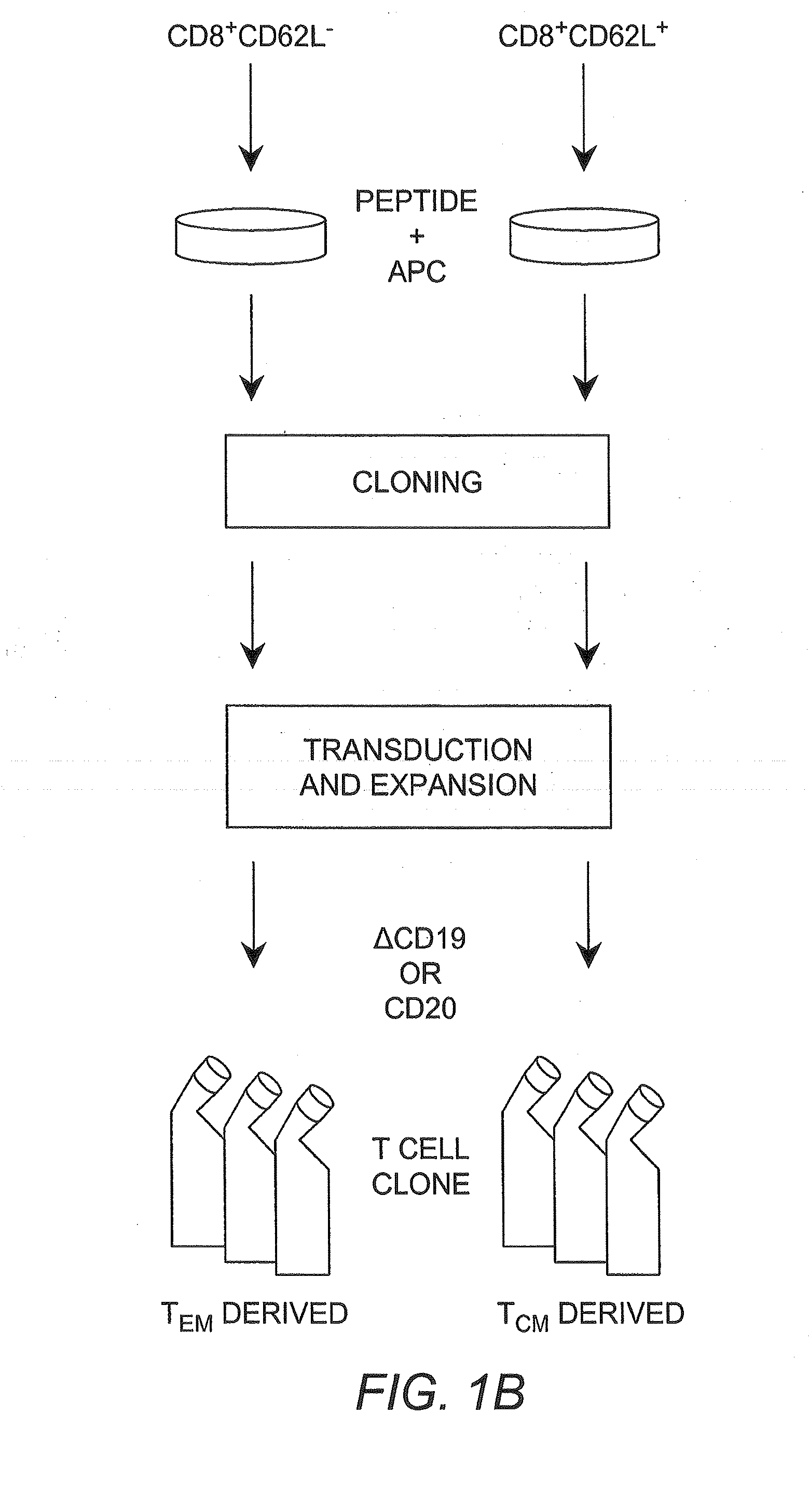
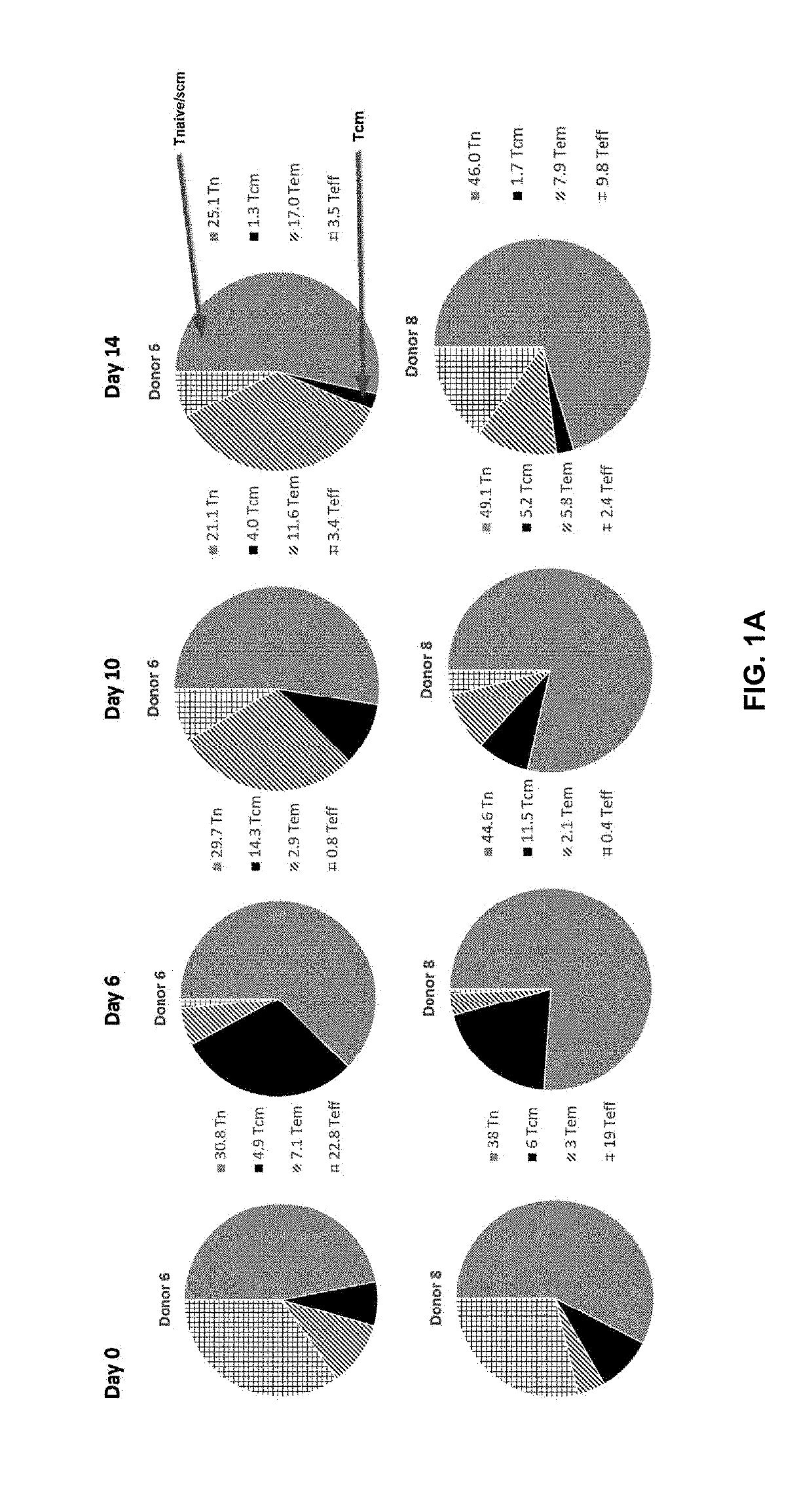
Adoptive transfer of cd8 + t cell clones derived from central memory cells
InactiveUS20080131415A1Increased proliferationBiocideArtificial cell constructsWhite blood cellPharmaceutical formulation
The present invention provides a method of carrying out adoptive immunotherapy in a primate subject in need thereof by administering the subject a cytotoxic T lymphocytes (CTL) preparation in a treatment-effective amount. The method comprises administering as the CTL preparation a preparation consisting essentially of an in vitro expanded primate CTL population, the CTL population enriched prior to expansion for central memory T lymphocytes, and depleted prior to expansion of effector memory T lymphocytes. In some embodiments, the method may further comprise concurrently administering Interleukin-15 to the subject in an amount effective to increase the proliferation of the central memory T cells in the subject. Pharmaceutical formulations produced by the method, and methods of using the same, are also described.
Owner:CITY OF HOPE +1
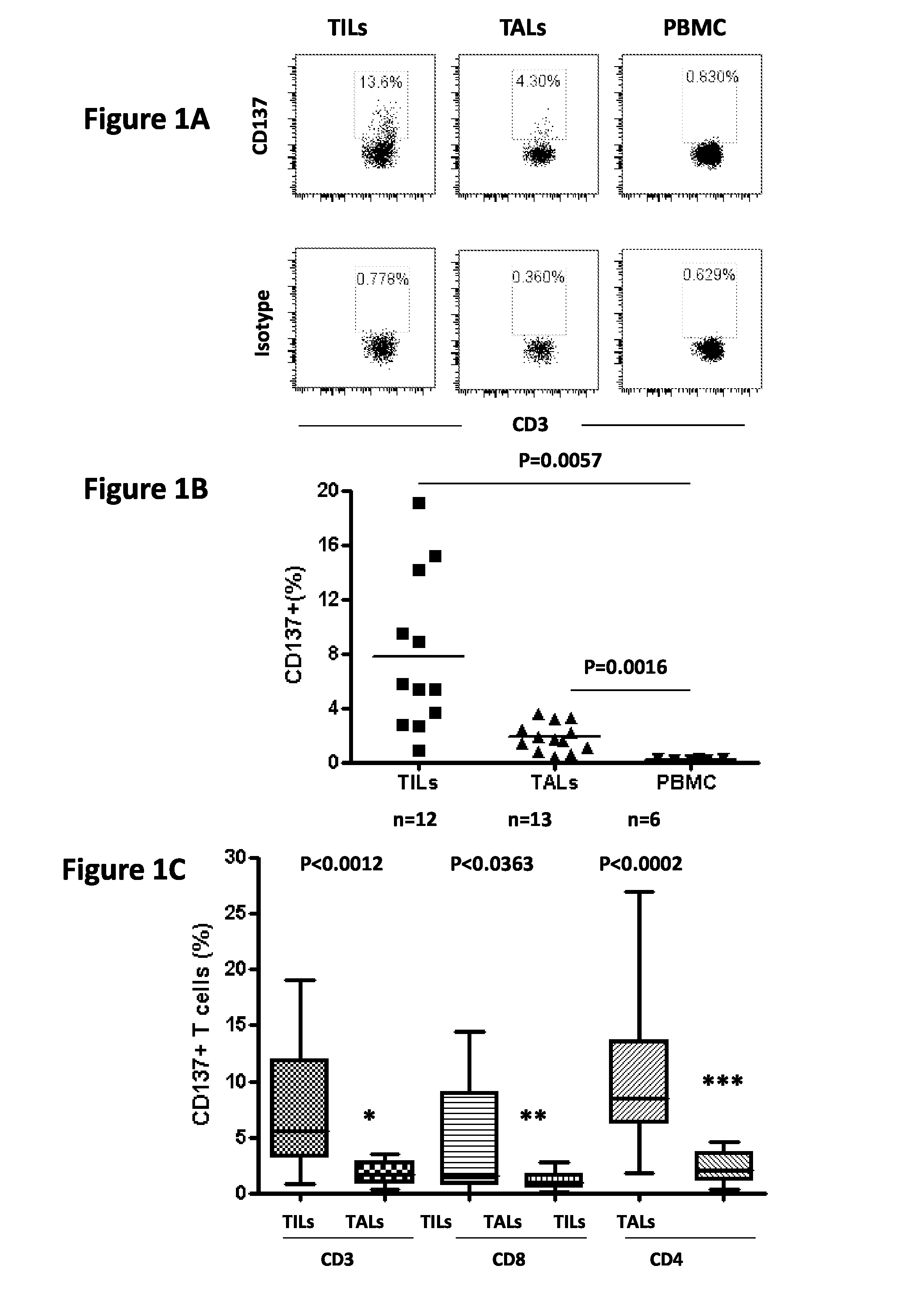
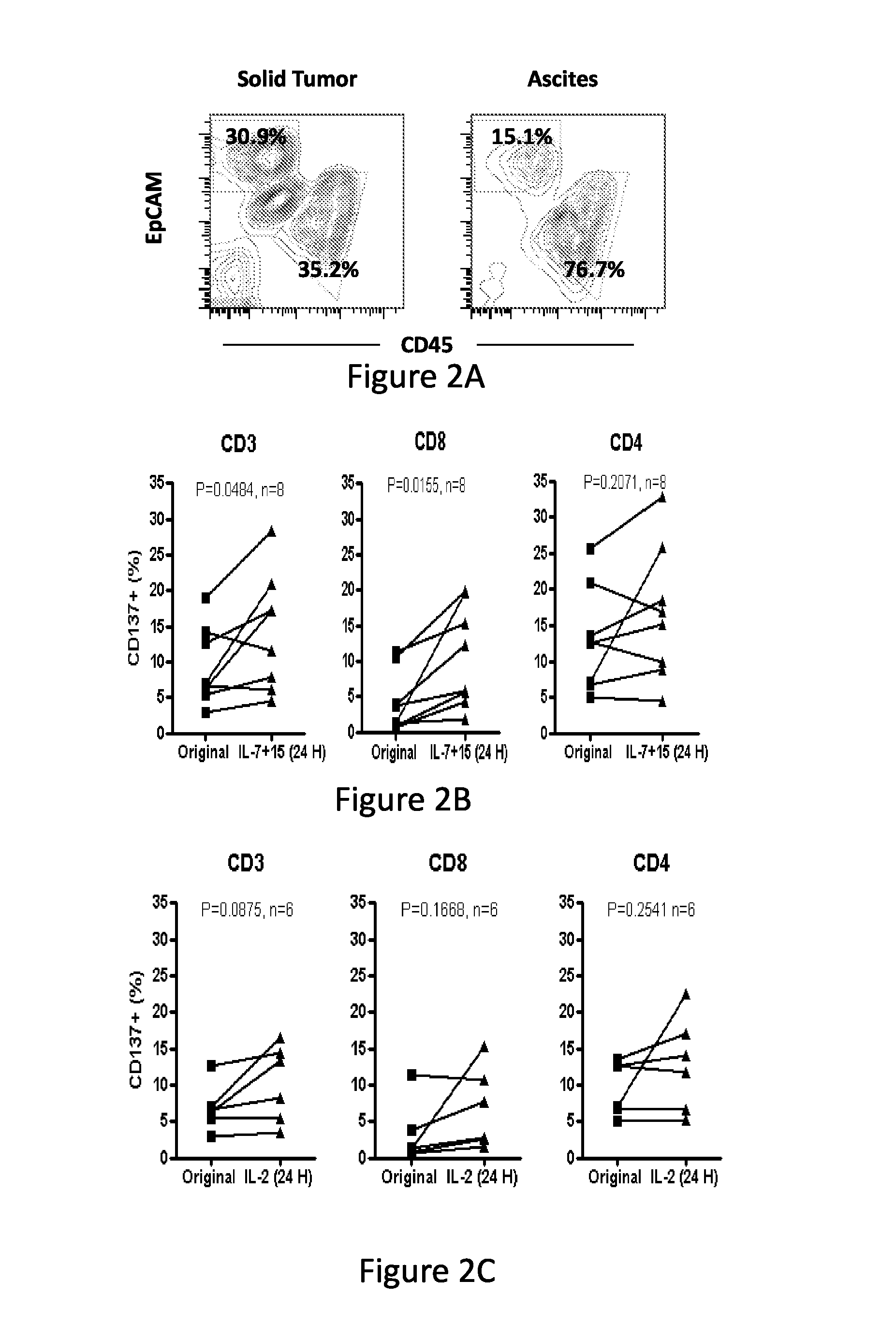
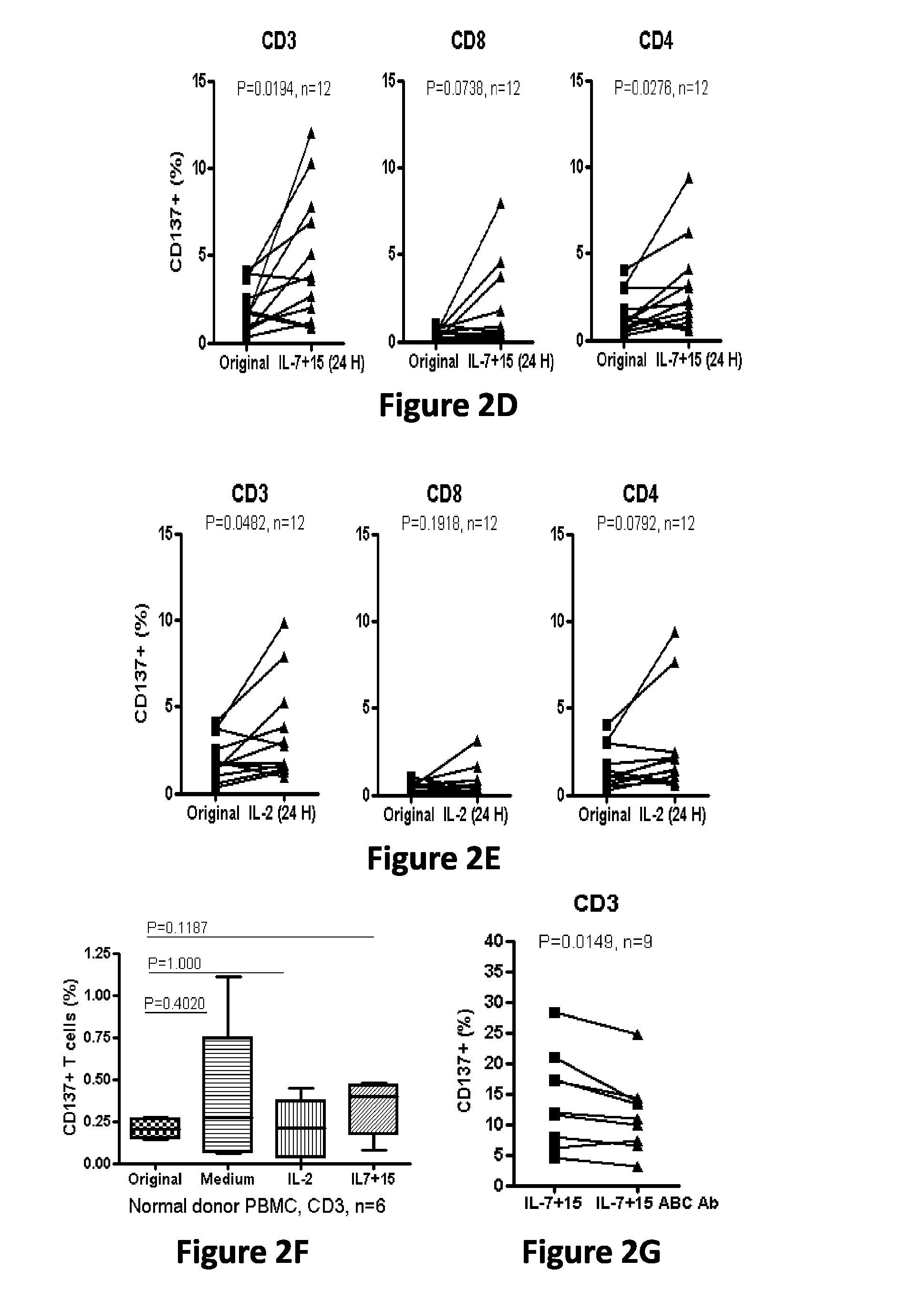
Cd137 enrichment for efficient tumor infiltrating lymphocyte selection
ActiveUS20160215262A1Effective anti-cancer T cell productMammal material medical ingredientsBlood/immune system cellsAbnormal tissue growthCD137
The invention includes compositions and methods to rapidly isolate and culture cells that are potent for use in adoptive immunotherapy. In one embodiment, the isolated cells of the invention are tumor infiltrating lymphocytes (TIL) that express CD137 (also known as 4-1BB and TNFSFR9).
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
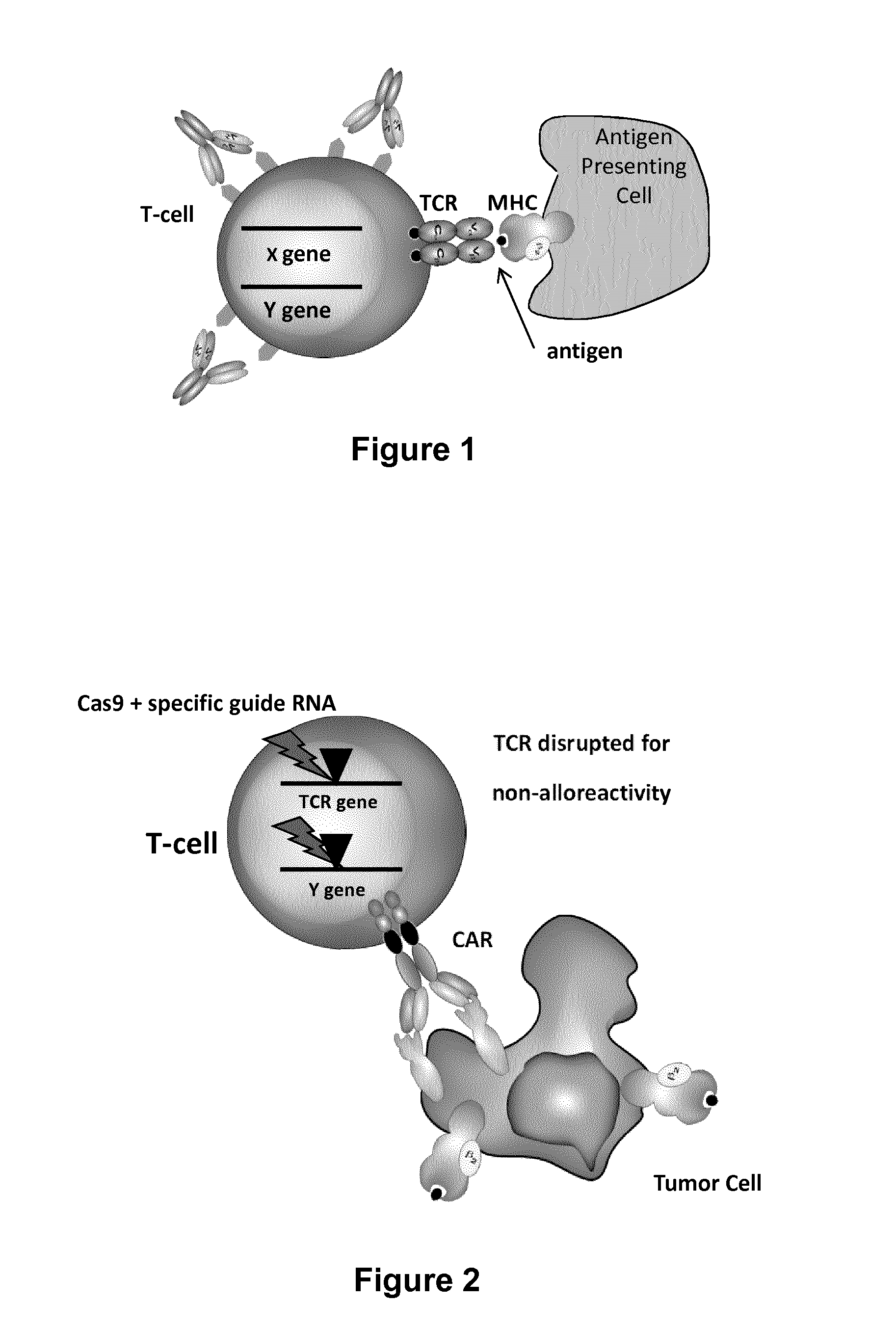
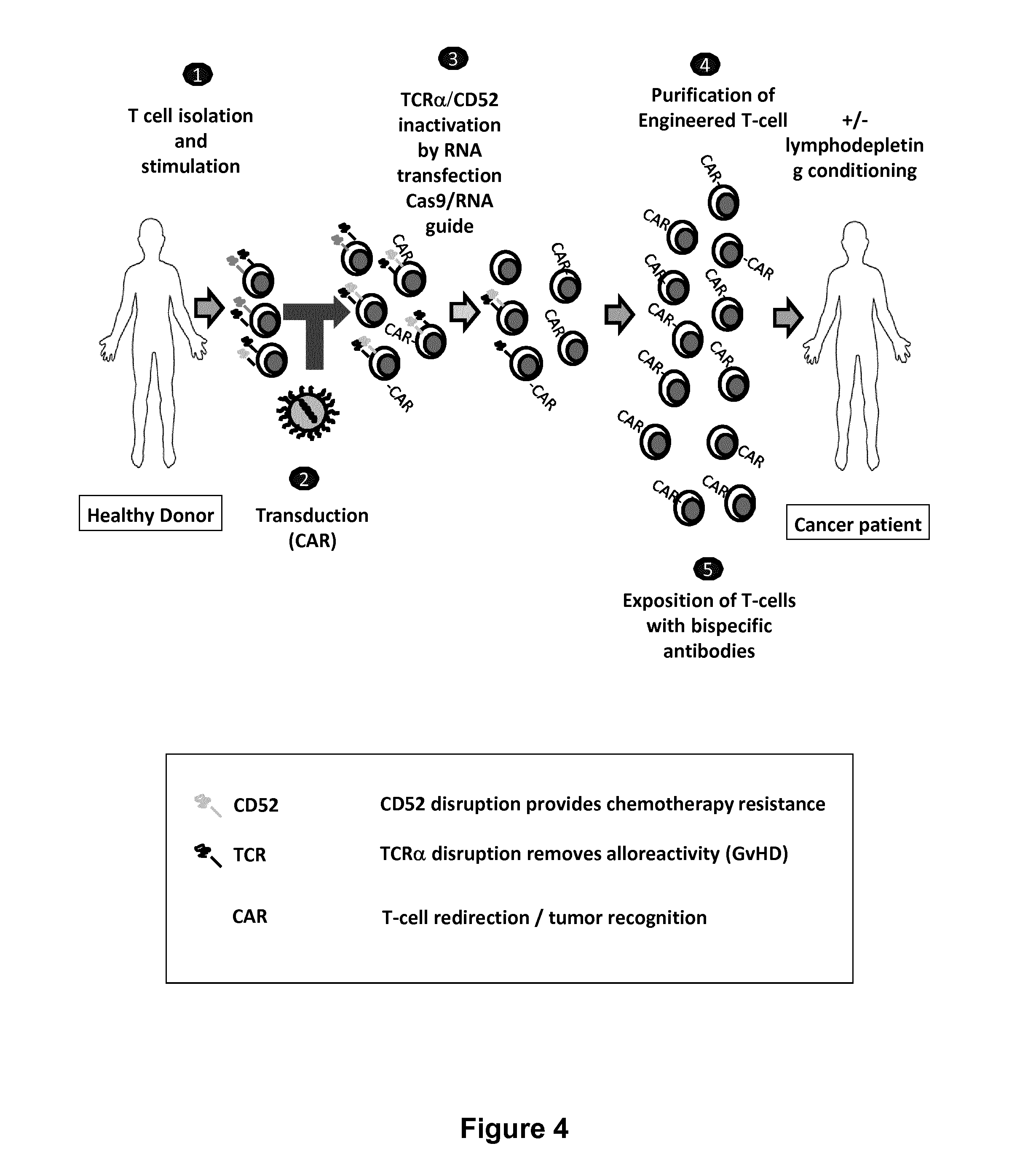
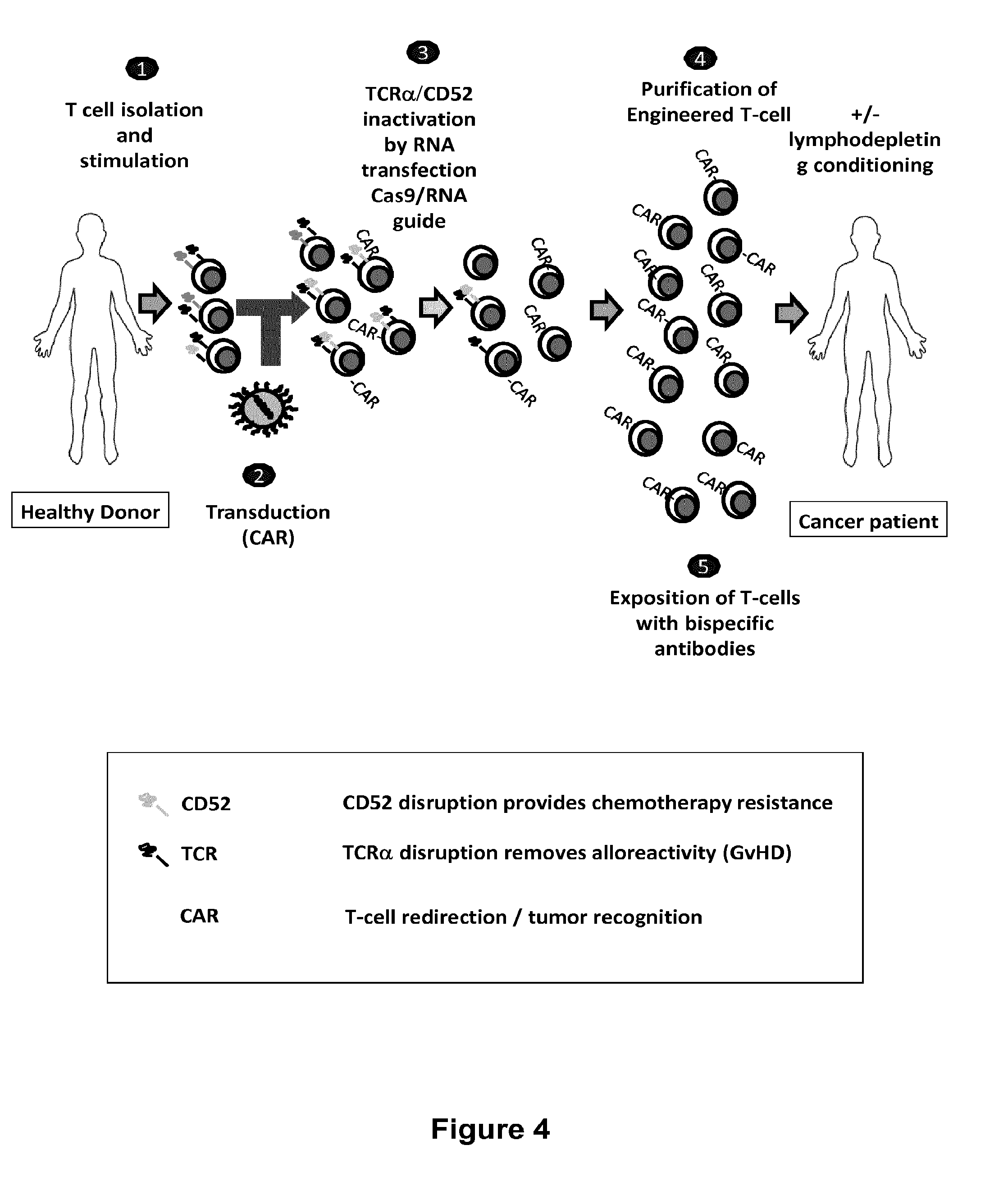
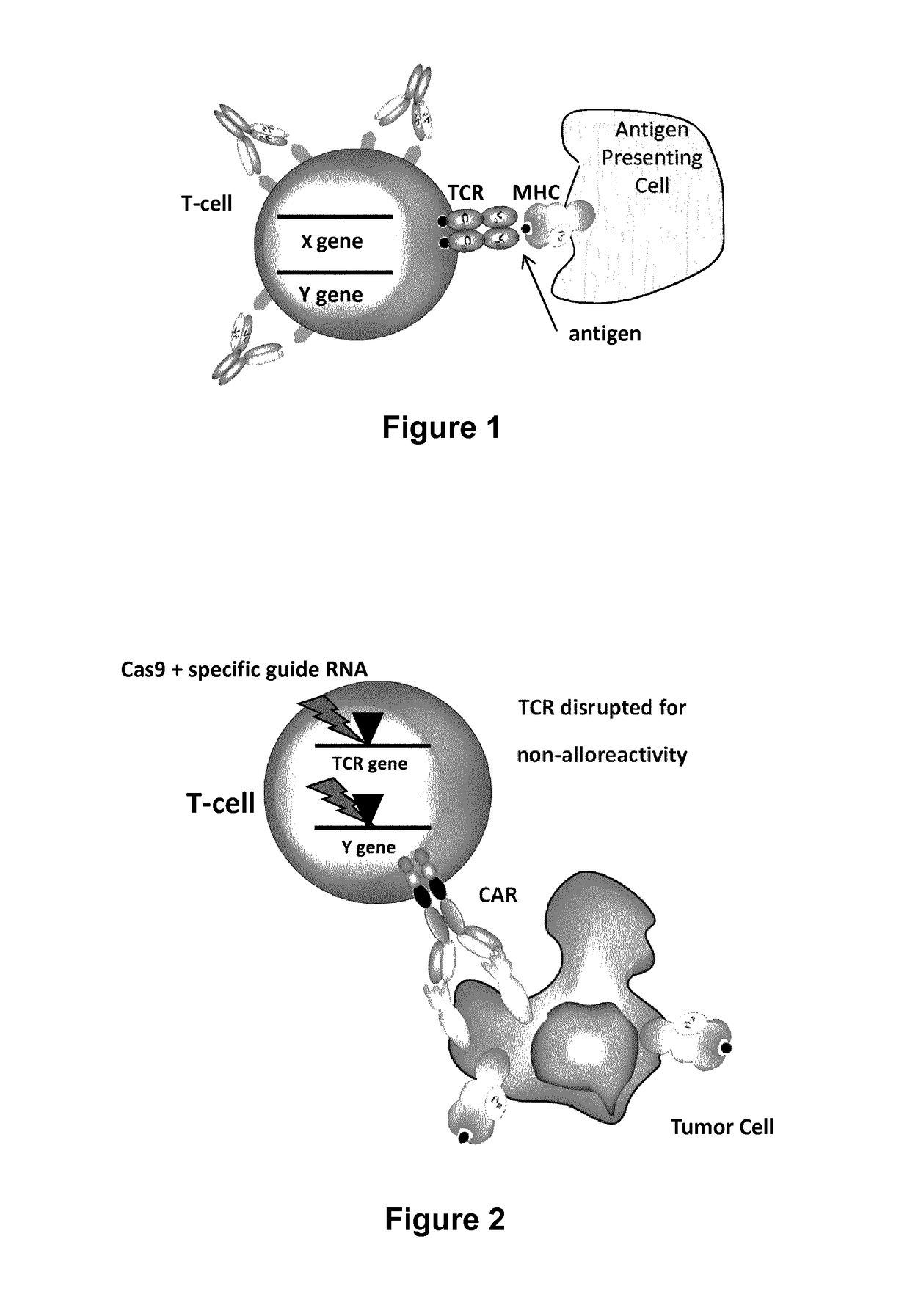
Methods for engineering t cells for immunotherapy by using rna-guided cas nuclease system
ActiveUS20160272999A1Accurate modificationHydrolasesGenetically modified cellsInfected cellAntigen receptors
The present invention relates to methods of developing genetically engineered, preferably non-alloreactive T-cells for immunotherapy. This method involves the use of RNA-guided endonucleases, in particular Cas9 / CRISPR system, to specifically target a selection of key genes in T-cells. The engineered T-cells are also intended to express chimeric antigen receptors (CAR) to redirect their immune activity towards malignant or infected cells. The invention opens the way to standard and affordable adoptive immunotherapy strategies using T-Cells for treating cancer and viral infections.
Owner:CELLECTIS SA
Methods for engineering t cells for immunotherapy by using rna-guided cas nuclease system
ActiveUS20160184362A1Accurate modificationHydrolasesGenetically modified cellsInfected cellAntigen receptors
The present invention relates to methods of developing genetically engineered, preferably non-alloreactive T-cells for immunotherapy. This method involves the use of RNA-guided endonucleases, in particular Cas9 / CRISPR system, to specifically target a selection of key genes in T-cells. The engineered T-cells are also intended to express chimeric antigen receptors (CAR) to redirect their immune activity towards malignant or infected cells. The invention opens the way to standard and affordable adoptive immunotherapy strategies using T-Cells for treating cancer and viral infections.
Owner:CELLECTIS SA
Adoptive immunotherapy using macrophages sensitized with heat shock protein-epitope complexes
InactiveUS6156302AEnhancing host 's immunocompetenceHigh activityBiocideOrganic active ingredientsDiseaseInterleukin 6
The present invention relates to methods and compositions for enhancing immunological responses and for the prevention and treatment of infectious diseases or primary and metastatic neoplastic diseases based on the administration of macrophages and / or other antigen presenting cells (APC) sensitized with heat shock proteins non-covalently bound to peptide complexes and / or antigenic components. APC are incubated in the presence of hsp-peptide complexes and / or antigenic components in vitro. The sensitized cells are reinfused into the patient with or without treatment with cytokines including but not limited to interferon- alpha , interferon- alpha , interleukin-2, interleukin-4, interleukin-6 and tumor neurosis factor.
Owner:FORDHAM UNIVERSITY
Method for generating t-cells compatible for allogenic transplantation
InactiveUS20170016025A1Polypeptide with localisation/targeting motifImmunoglobulin superfamilyCIITAAuto immune disease
The present invention pertains to engineered T-cells, method for their preparation and their use as medicament, particularly for immunotherapy. The engineered T-cells of the invention are characterized in that the expression of beta 2-microglobulin (B2M) and / or class II major histocompatibility complex transactivator (CIITA) is inhibited, e.g., by using rare-cutting endonucleases able to selectively inactivating by DNA cleavage the gene encoding B2M and / or CIITA, or by using nucleic acid molecules which inhibit the expression of B2M and / or CIITA. In order to further render the T-cell non-alloreactive, at least one gene encoding a component of the T-cell receptor is inactivated, e.g., by using a rare-cutting endonucleases able to selectively inactivating by DNA cleavage the gene encoding said TCR component. In addition, expression of immunosuppressive polypeptide can be performed on those modified T-cells in order to prolong the survival of these modified T cells in host organism. Such modified T-cell is particularly suitable for allogeneic transplantations, especially because it reduces both the risk of rejection by the host's immune system and the risk of developing graft versus host disease. The invention opens the way to standard and affordable adoptive immunotherapy strategies using T-Cells for treating cancer, infections and auto-immune diseases.
Owner:CELLECTIS SA
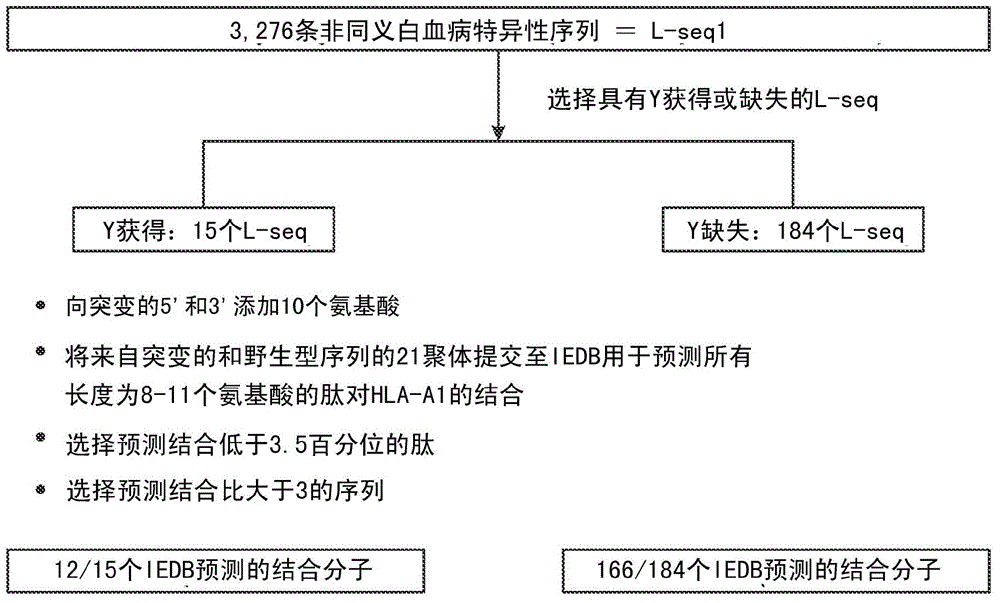
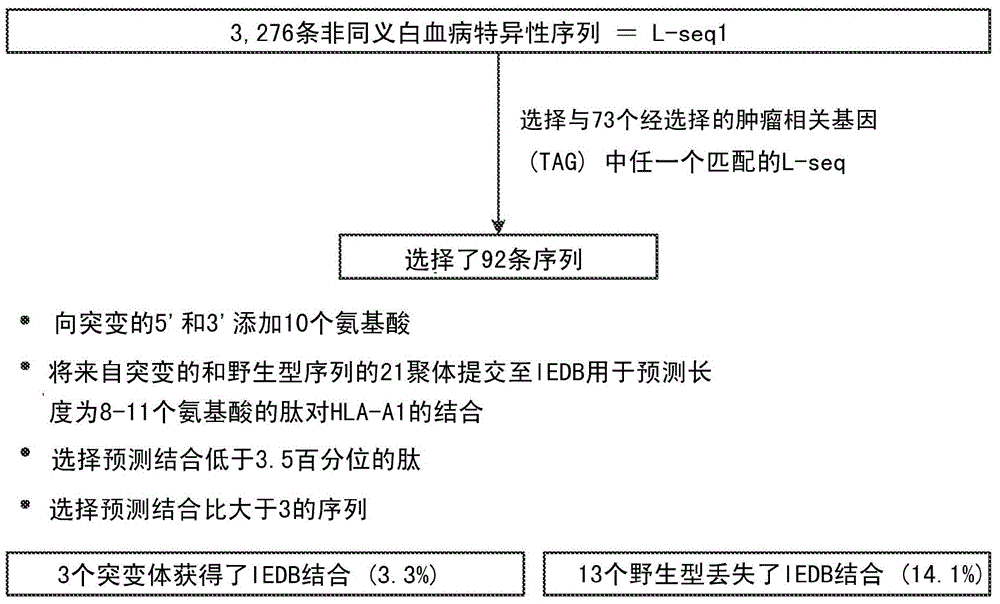
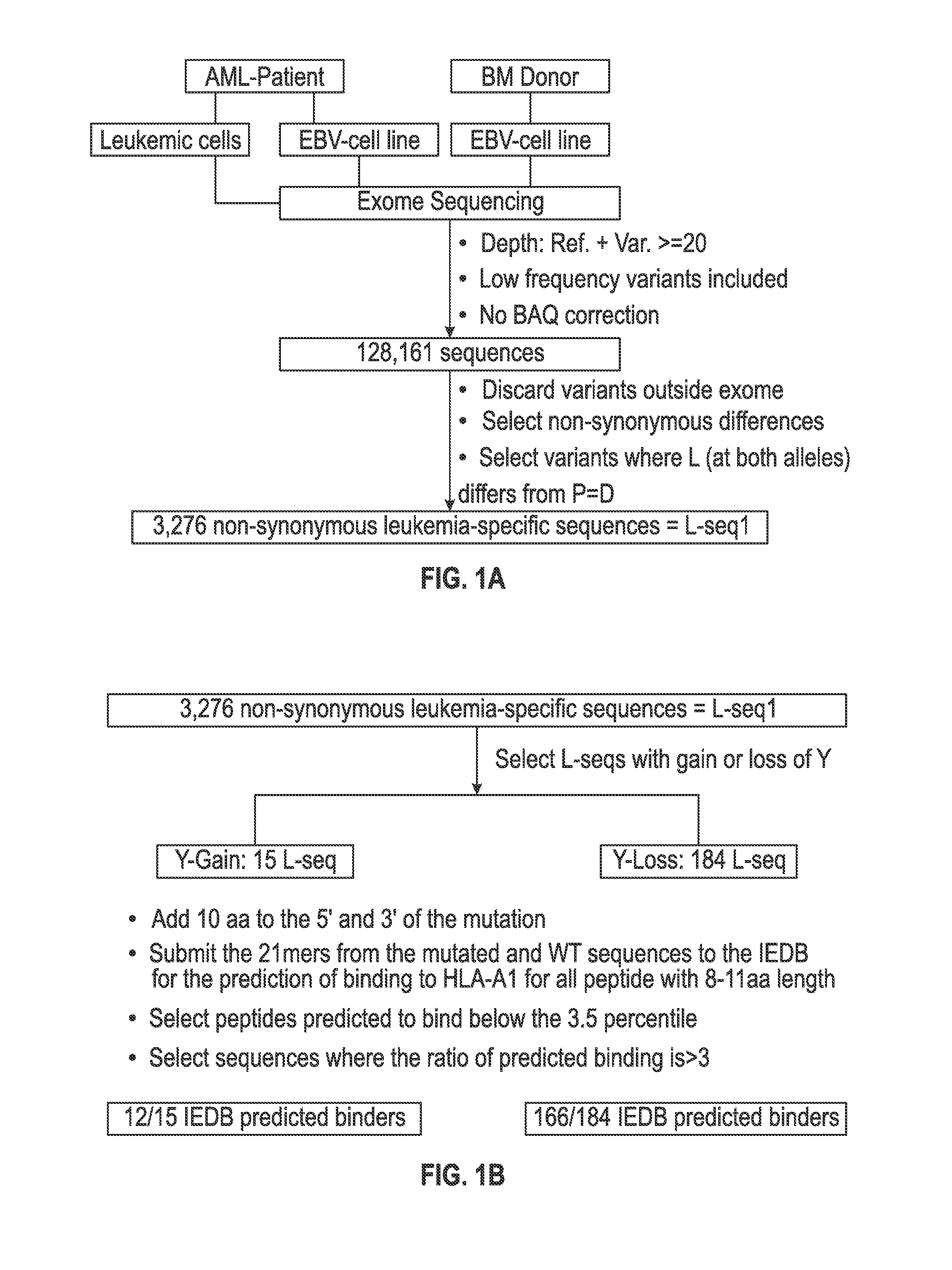
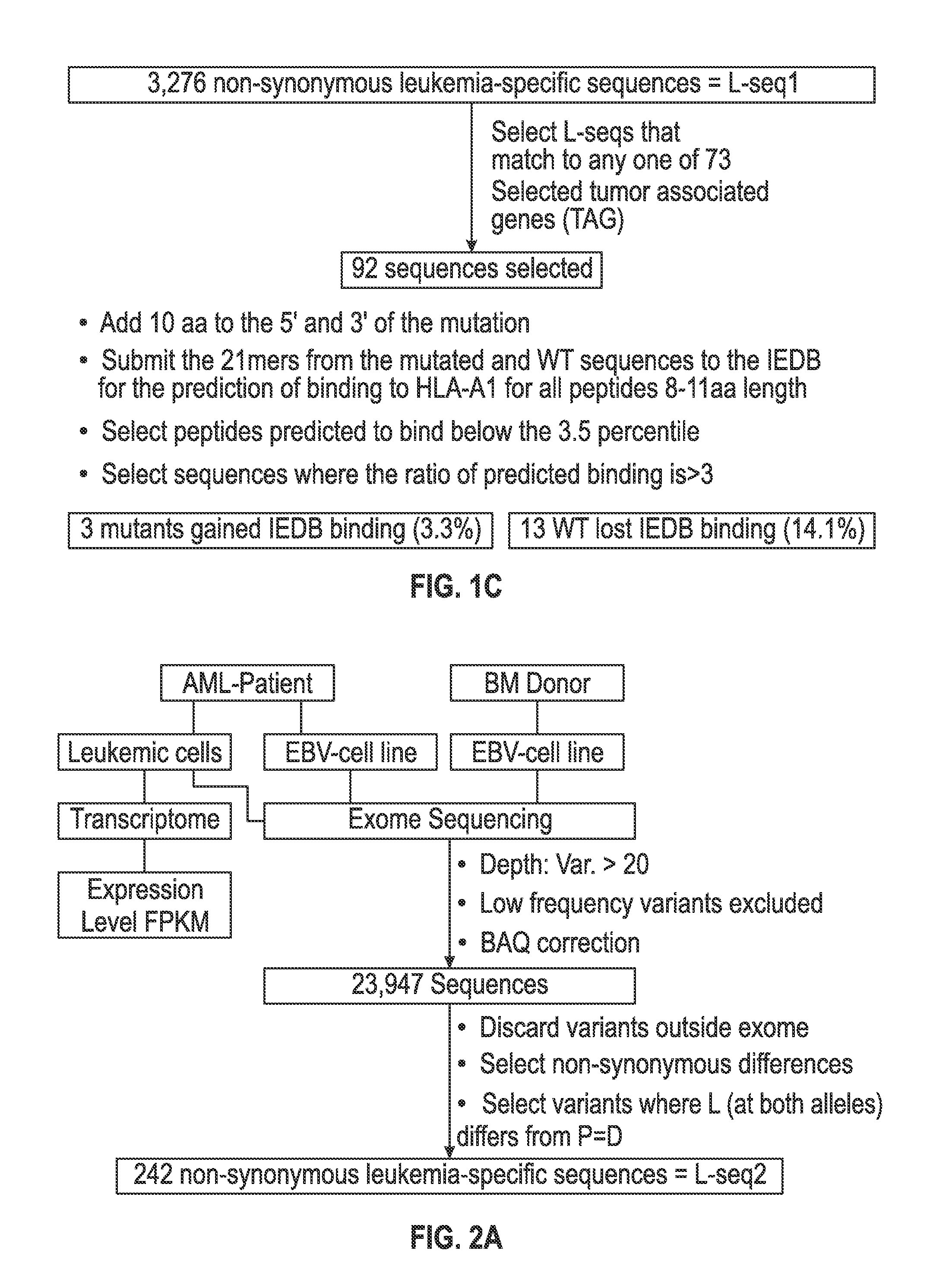
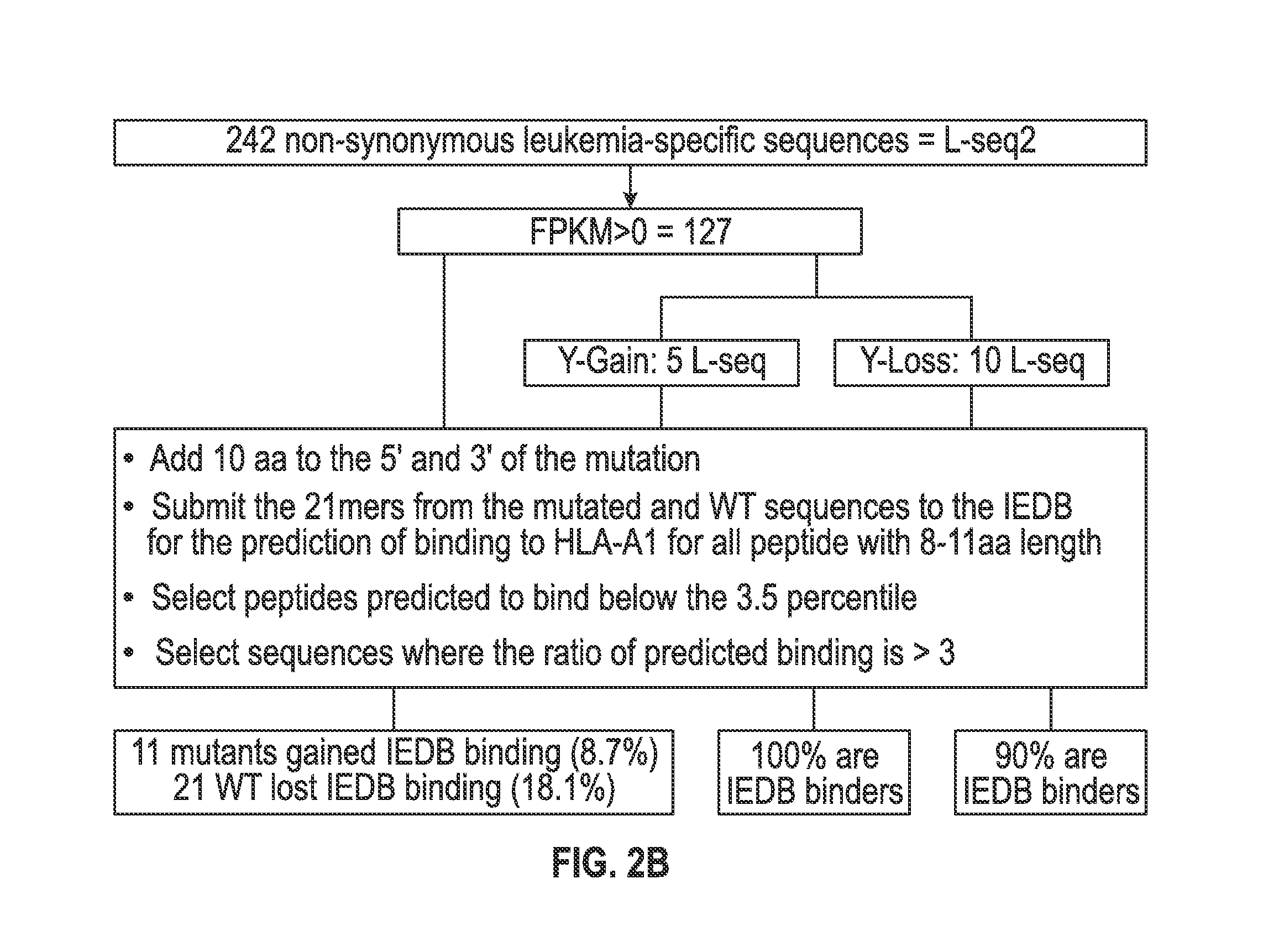
Personalized cancer vaccines and adoptive immune cell therapies
InactiveCN104662171AMicrobiological testing/measurementBiological material analysisCancer cellWild type
Cancer antigens containing mutations in an expressed gene of cancer cells from a cancer patient are identified. Sequences from cancer cells obtained using a parallel sequencing platform are selected by comparing to the patient's normal genes or to normal genes from an HLA-matched individual. Sequences are further selected by identifying an HLA supertype of the cancer patient and selecting for that HLA supertype, sequences that have a particular amino acid at the mutant position and / or corresponding wild-type position in the effected gene. Peptides containing cancer antigens (i.e., mutations—once a mutation is defined, what makes it an immunogen is its ability to induce an immune response) are optionally tested for binding to HLA antigens of the cancer patient. Peptides containing the cancer antigens are evaluated for activating T cells (e.g., helper T lymphocytes and cytotoxic T lymphocytes (CTL)) cell lines from the cancer patient or from an HLA-matched donor. The cancer antigen(s) identified for a cancer patient are used to prepare a cancer vaccine and to treat the cancer patient.
Owner:PERSIMMUNE
Genes differentially expressed in cancer cells to design cancer vaccines
Owner:GENZYME CORP
Method of preparing adenosine-resistant anti-tumor T lymphocytes for adoptive immunotherapy
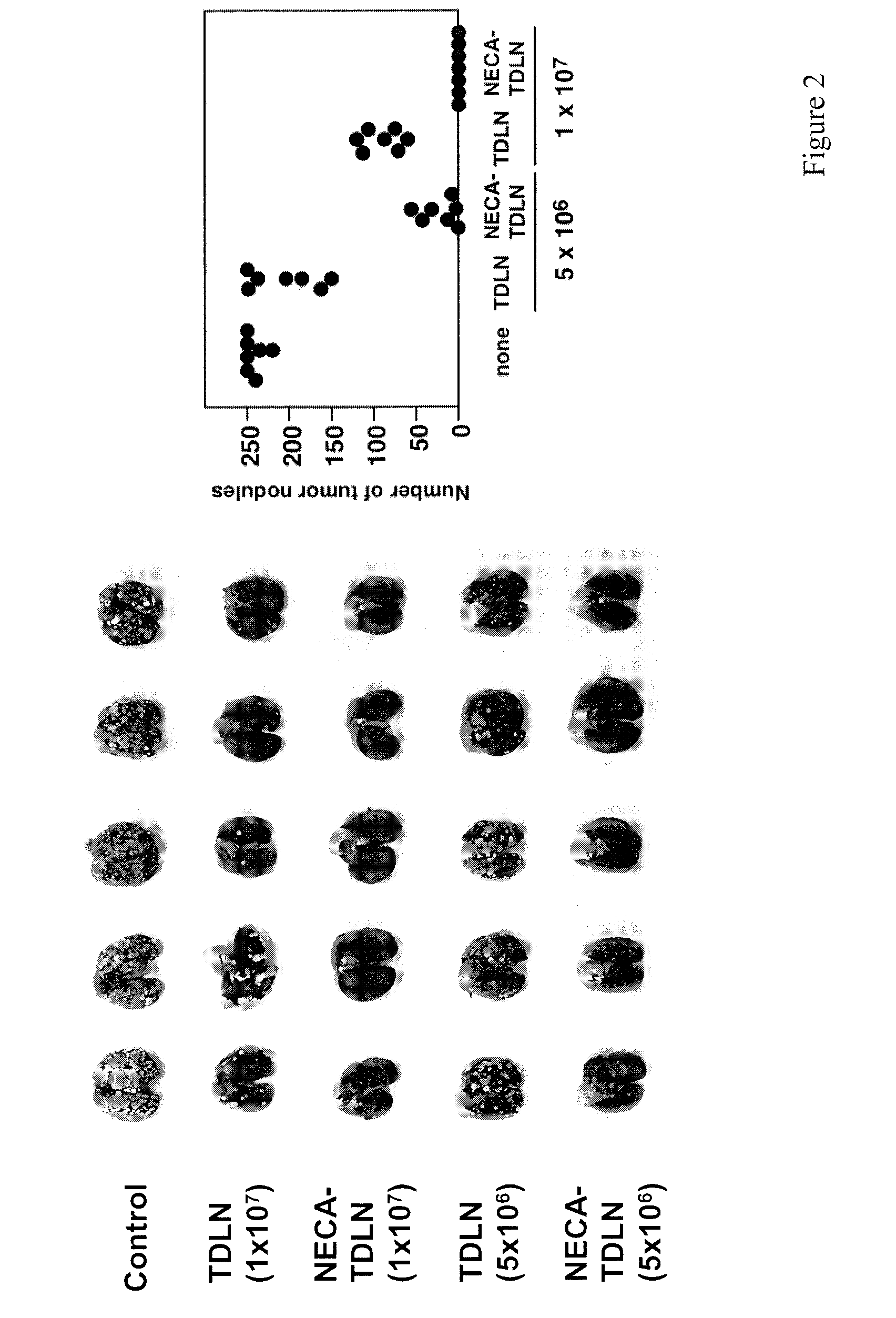
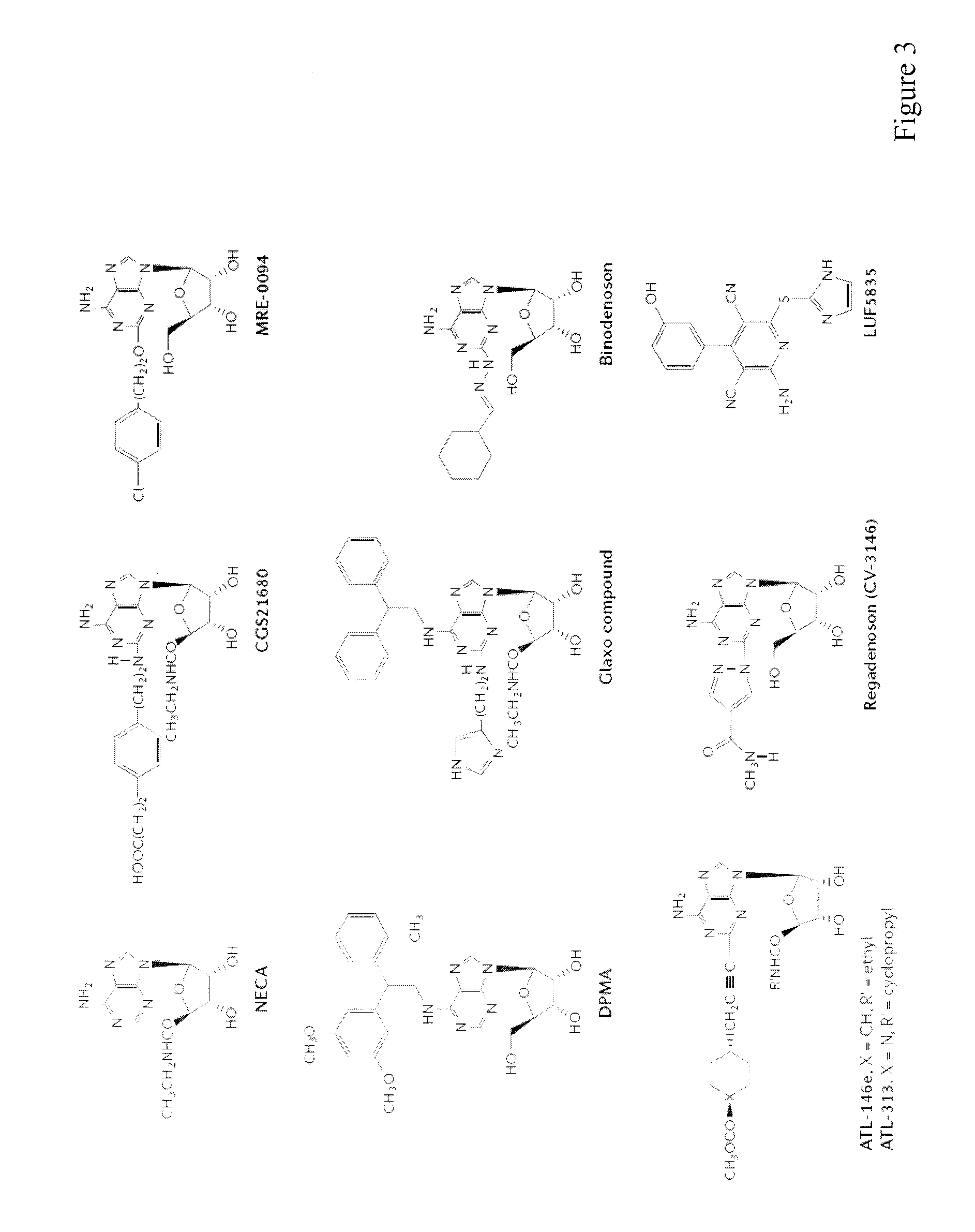
In the past, adoptive immunotherapy often failed because the transferred immune cells were inactive in vivo. This disclosure provides a method of producing immune cells that are highly active in vivo. The immune cells may be expanded in vitro in the presence of an adenosine receptor agonist or an antisense nucleic acid that downregulates expression of an adenosine receptor, for example. The immune cells may be tumor-infiltrating lymphocytes (TIL), cytotoxic T lymphocytes (CTL), natural killer (NK) cells, or lymphokine-activated killer (LAK) cells, for example. The methods described herein may be used to treat a number of diseases including cancer, infectious diseases, and immunodeficiencies.
Owner:NORTHEASTERN UNIV
Methods for engineering T cells for immunotherapy by using RNA-guided CAS nuclease system
ActiveUS9855297B2Accurate modificationHydrolasesGenetically modified cellsInfected cellAntigen receptors
The present invention relates to methods of developing genetically engineered, preferably non-alloreactive T-cells for immunotherapy. This method involves the use of RNA-guided endonucleases, in particular Cas9 / CRISPR system, to specifically target a selection of key genes in T-cells. The engineered T-cells are also intended to express chimeric antigen receptors (CAR) to redirect their immune activity towards malignant or infected cells. The invention opens the way to standard and affordable adoptive immunotherapy strategies using T-Cells for treating cancer and viral infections.
Owner:CELLECTIS SA
Personalized cancer vaccines and adoptive immune cell therapies
Cancer antigens containing mutations in an expressed gene of cancer cells from a cancer patient are identified. Sequences from cancer cells obtained using a parallel sequencing platform are selected by comparing to the patient's normal genes or to normal genes from an HLA-matched individual. Sequences are further selected by identifying an HLA supertype of the cancer patient and selecting for that HLA supertype, sequences that have a particular amino acid at the mutant position and / or corresponding wild-type position in the effected gene. Peptides containing cancer antigens (i.e., mutations—once a mutation is defined, what makes it an immunogen is its ability to induce an immune response) are optionally tested for binding to HLA antigens of the cancer patient. Peptides containing the cancer antigens are evaluated for activating T cells (e.g., helper T lymphocytes and cytotoxic T lymphocytes (CTL)) cell lines from the cancer patient or from an HLA-matched donor. The cancer antigen(s) identified for a cancer patient are used to prepare a cancer vaccine and to treat the cancer patient.
Owner:PERSIMMUNE
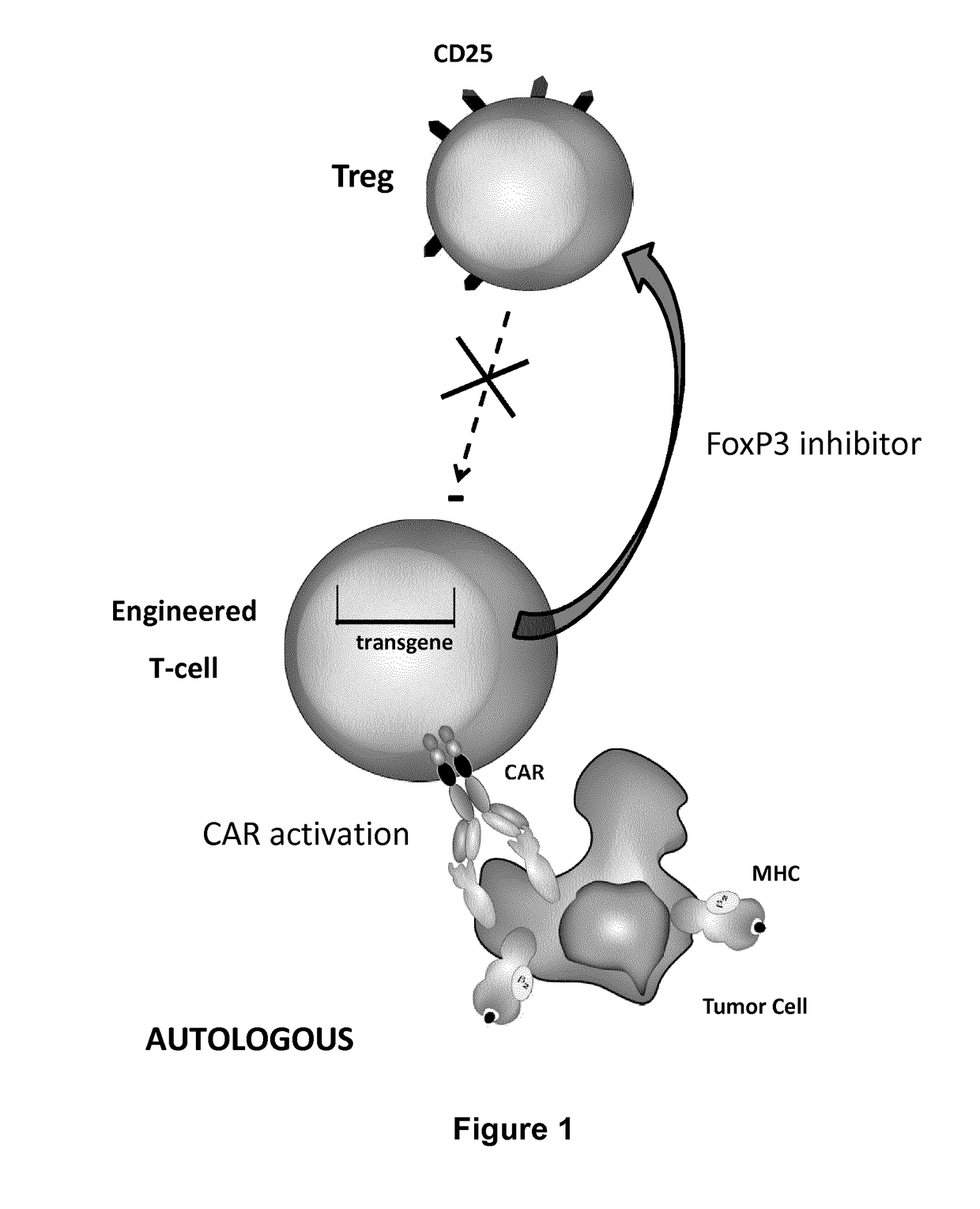
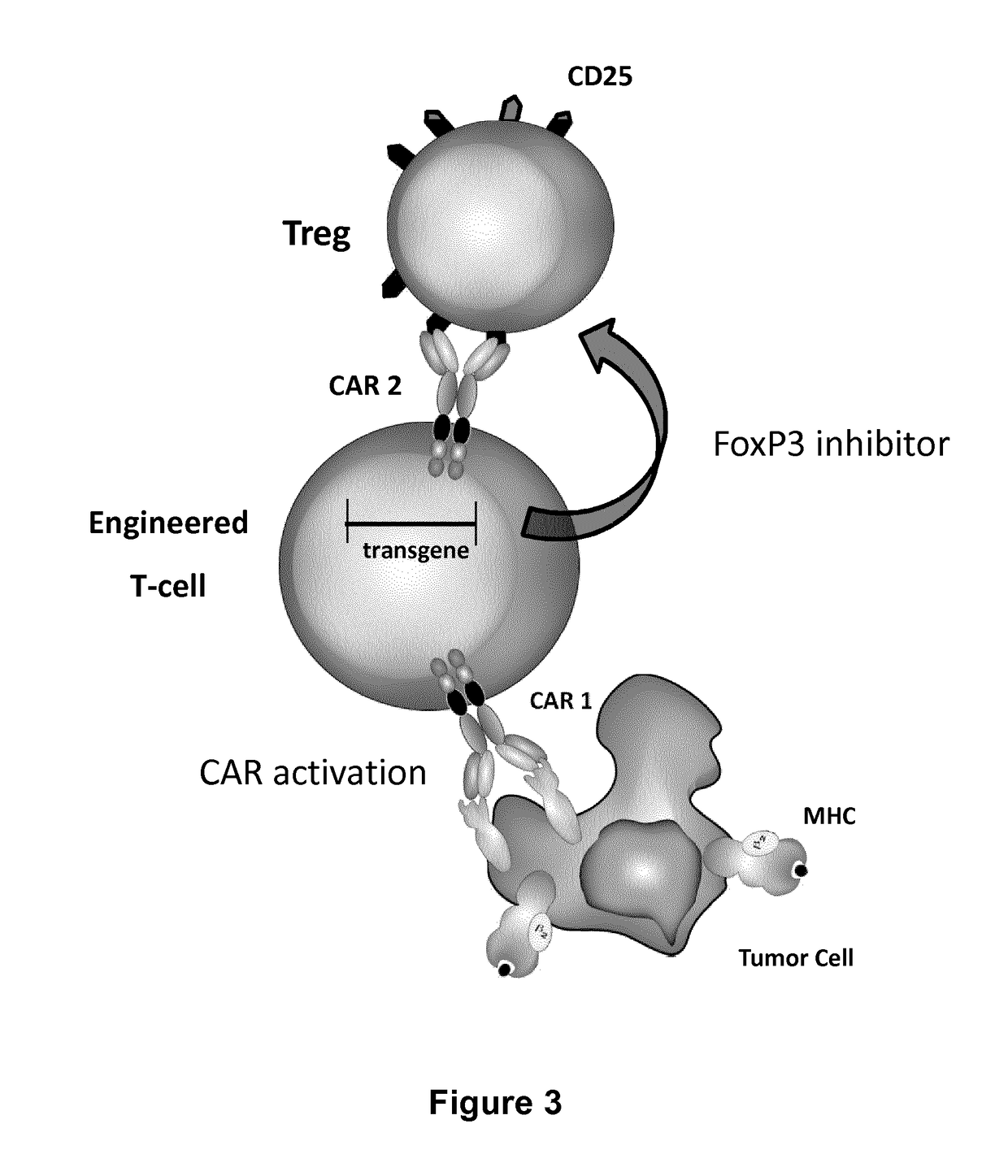
Method for in situ inhibition of regulatory t cells
ActiveUS20170067022A1Reducing FoxP transcriptional activityHydrolasesAntibody mimetics/scaffoldsAntigenInfected cell
The present invention pertains to engineered T-cells, method for their preparation and their use as medicament, particularly for immunotherapy. The engineered T-cells of the invention are designed to express both a Chimeric Antigen Receptor (CAR) directed against at least one antigen expressed at the surface of a malignant or infected cell, and a secreted inhibitor of regulatory T-cells (Treg). Preferably, such secreted inhibitor is a peptide inhibitor of forkhead / winged helix transcription factor 3 (FoxP3), a specific factor involved into the differentiation of T-cells into regulatory T-cells. The engineered T-cells of the invention direct their immune activity towards specific malignant or infected cells, while at the same time will prevent neighbouring regulatory T-cells from modulating the immune response. The invention opens the way to standard and affordable adoptive immunotherapy strategies, especially for treating or preventing cancer, and bacterial or viral infections.
Owner:CELLECTIS SA
Methods for engineering T cells for immunotherapy by using RNA-guided CAS nuclease system
ActiveUS9890393B2Accurate modificationHydrolasesGenetically modified cellsInfected cellAntigen receptors
The present invention relates to methods of developing genetically engineered, preferably non-alloreactive T-cells for immunotherapy. This method involves the use of RNA-guided endonucleases, in particular Cas9 / CRISPR system, to specifically target a selection of key genes in T-cells. The engineered T-cells are also intended to express chimeric antigen receptors (CAR) to redirect their immune activity towards malignant or infected cells. The invention opens the way to standard and affordable adoptive immunotherapy strategies using T-Cells for treating cancer and viral infections.
Owner:CELLECTIS SA
Adoptive transfer of cd8+ t cell clones derived from central memory cells
ActiveUS20140356398A1Mammal material medical ingredientsArtificial cell constructsWhite blood cellPharmaceutical formulation
The present invention provides a method of carrying out adoptive immunotherapy in a primate subject in need thereof by administering the subject a cytotoxic T lymphocytes (CTL) preparation in a treatment-effective amount. The method comprises administering as the CTL preparation a preparation consisting essentially of an in vitro expanded primate CTL population, the CTL population enriched prior to expansion for central memory T lymphocytes, and depleted prior to expansion of effector memory T lymphocytes. In some embodiments, the method may further comprise concurrently administering Interleukin-15 to the subject in an amount effective to increase the proliferation of the central memory T cells in the subject. Pharmaceutical formulations produced by the method, and methods of using the same, are also described.
Owner:FRED HUTCHINSON CANCER CENT
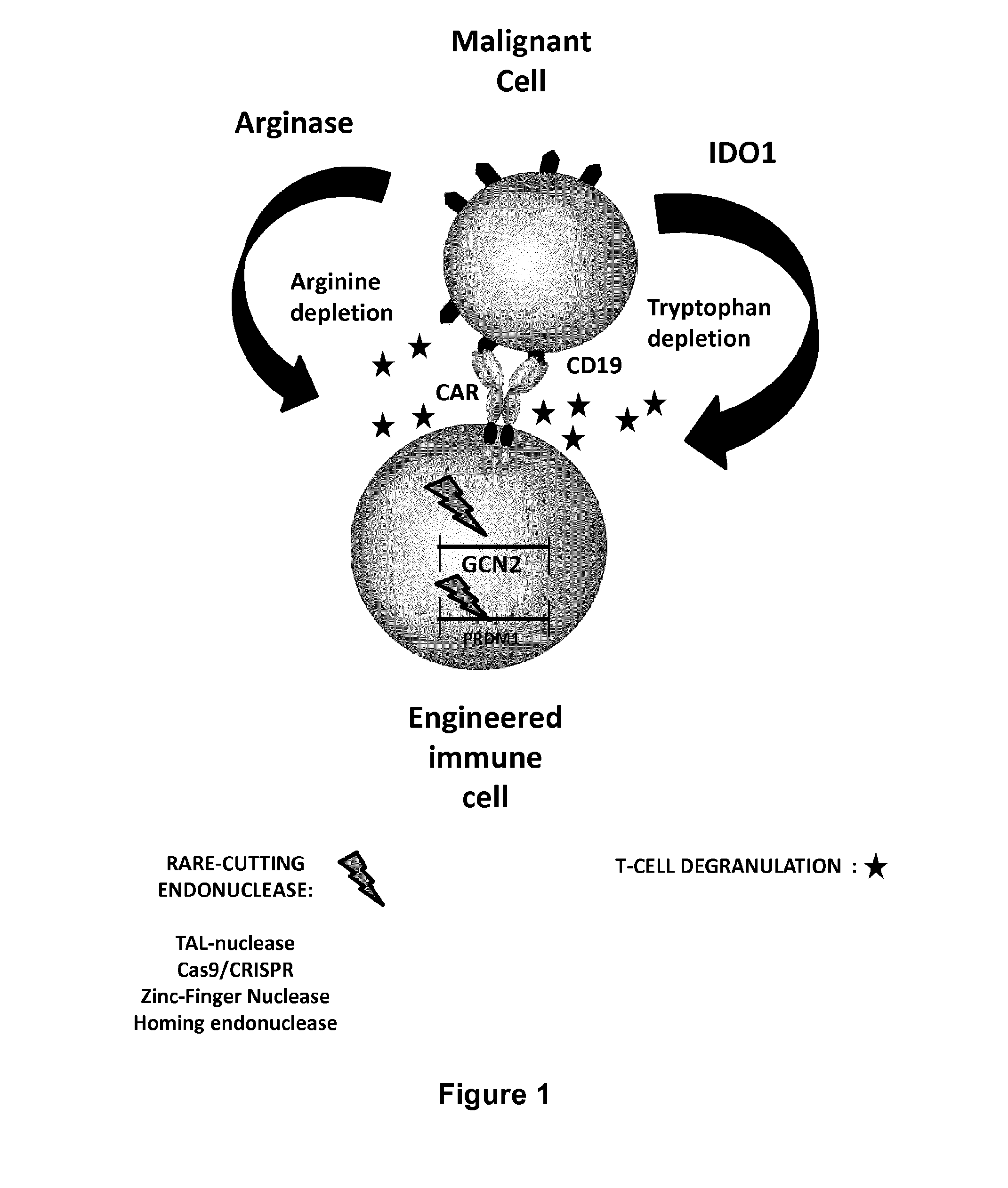
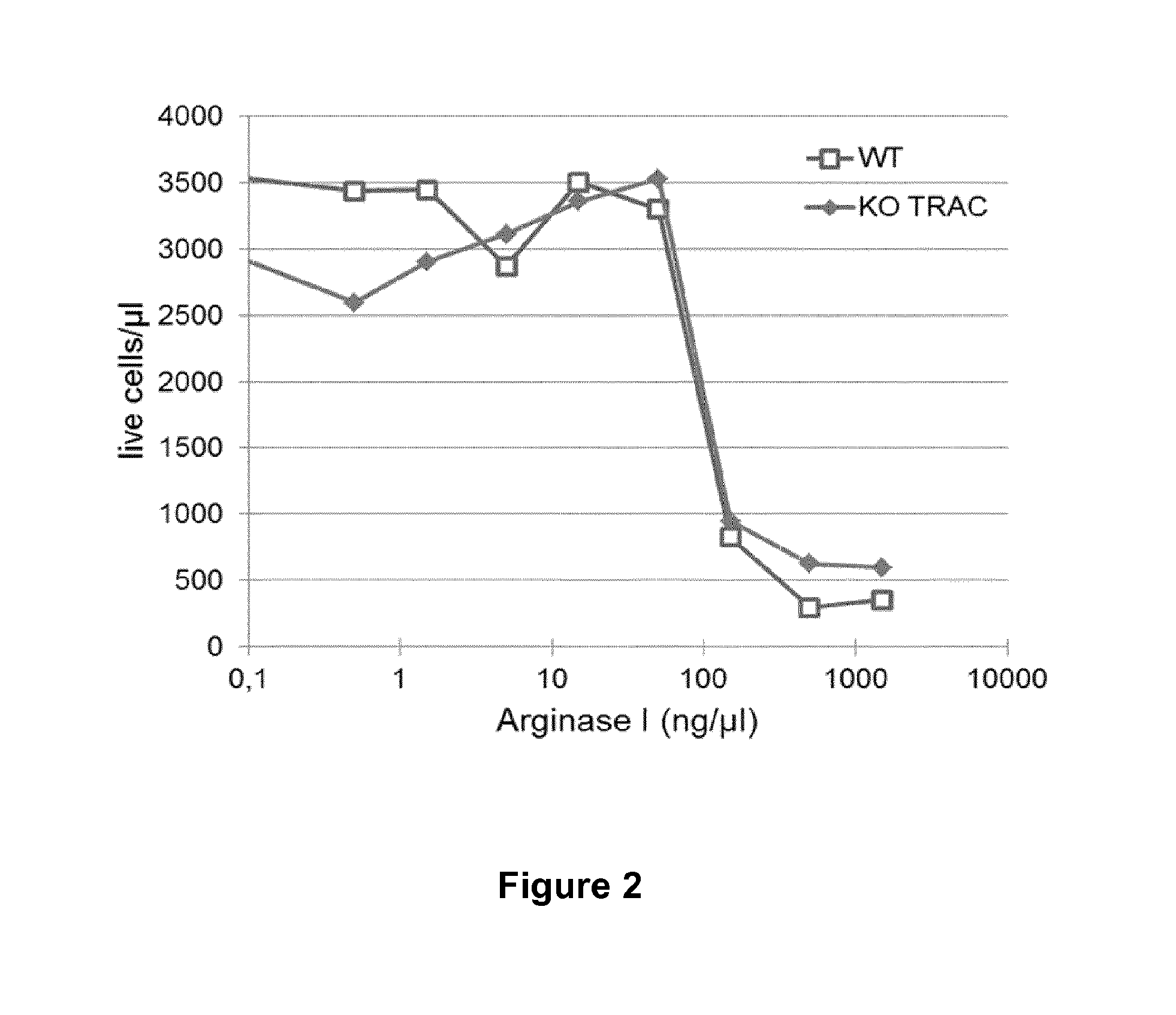
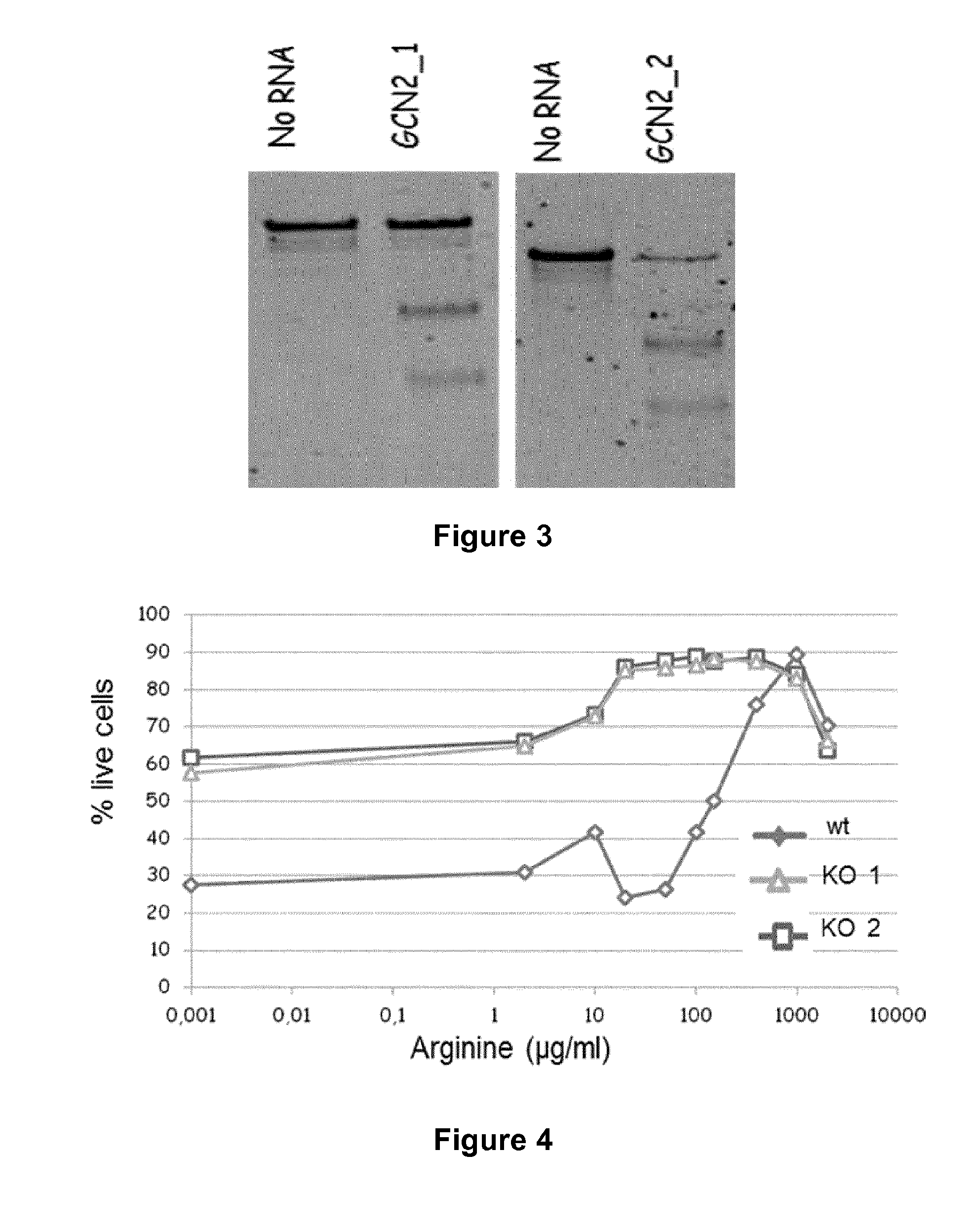
Method for generating immune cells resistant to arginine and/or tryptophan depleted microenvironment
ActiveUS20170035866A1Enhance immune responseImmunoglobulin superfamilyTransferasesArginineTryptophan
The present invention pertains to engineered immune cells, method for their preparation and their use as medicament, particularly for immunotherapy. The engineered immune cells of the present invention are characterized in that at least one gene selected from a gene encoding GCN2 and a gene encoding PRDM1 is inactivated or repressed. Such modified Immune cells are resistant to an arginine and / or tryptophan depleted microenvironment caused by, e.g., tumor cells, which makes the immune cells of the invention particularly suitable for immunotherapy. The invention opens the way to standard and affordable adoptive immunotherapy strategies using immune cells for treating different types of malignancies.
Owner:CELLECTIS SA
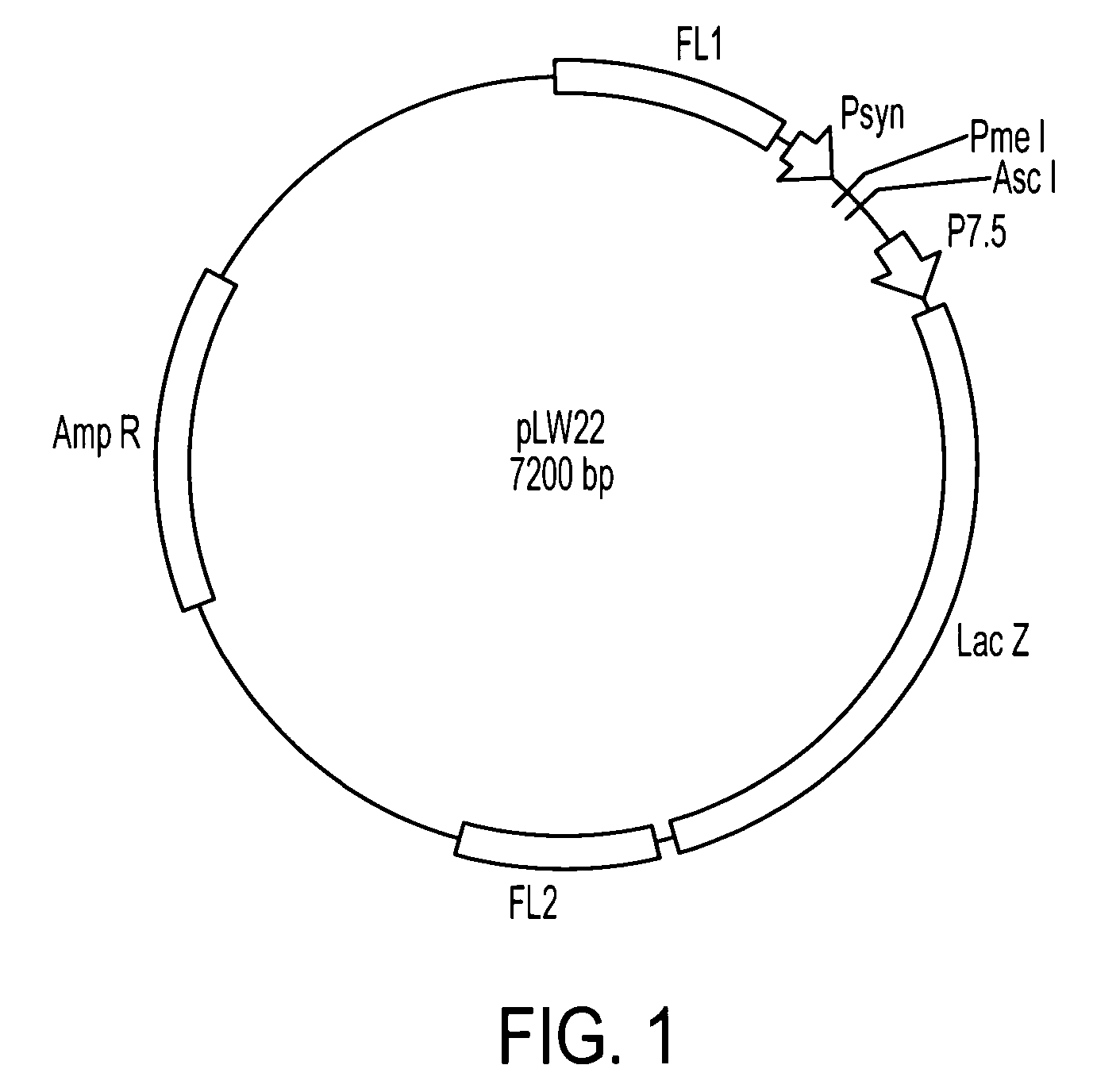
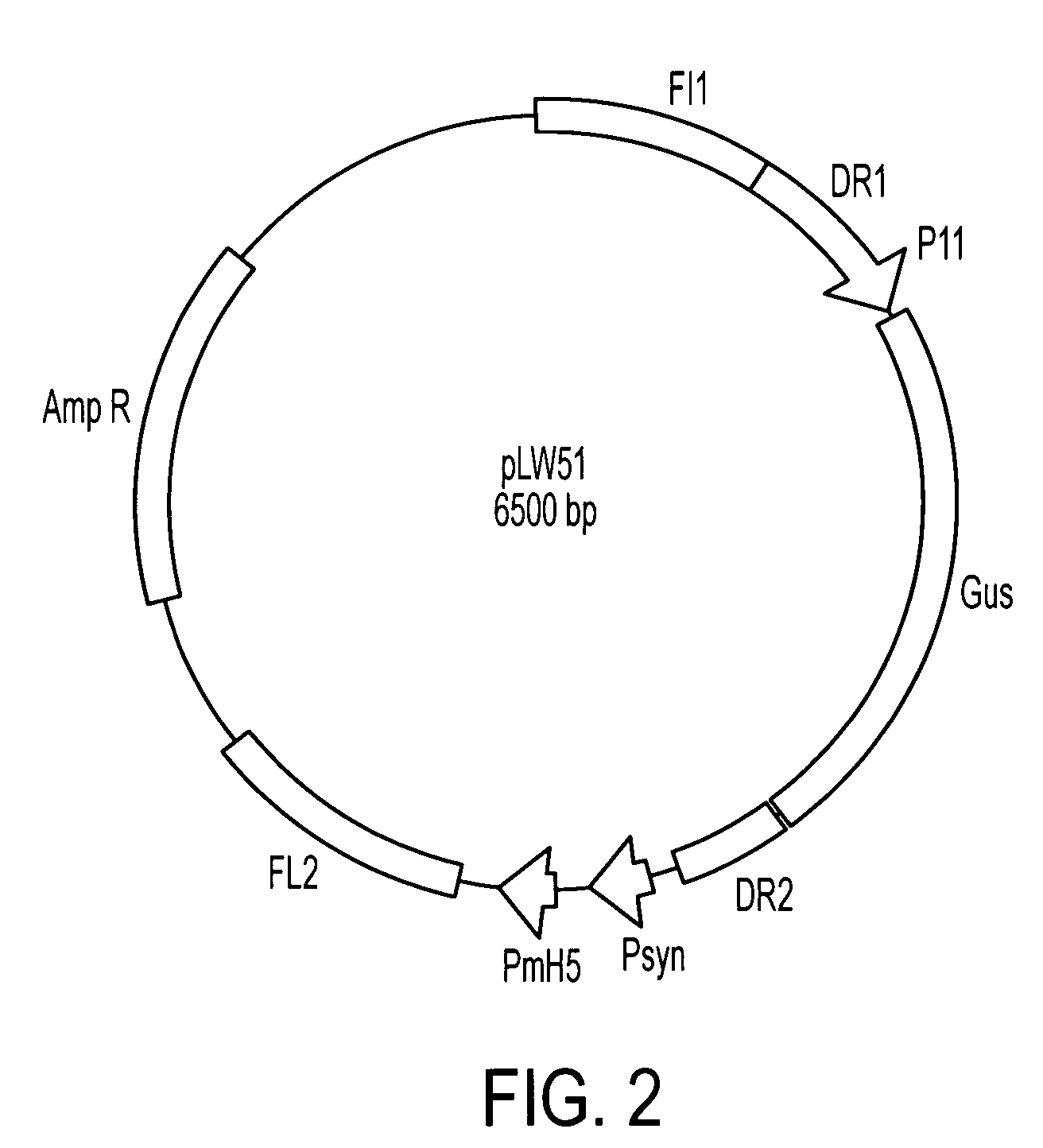
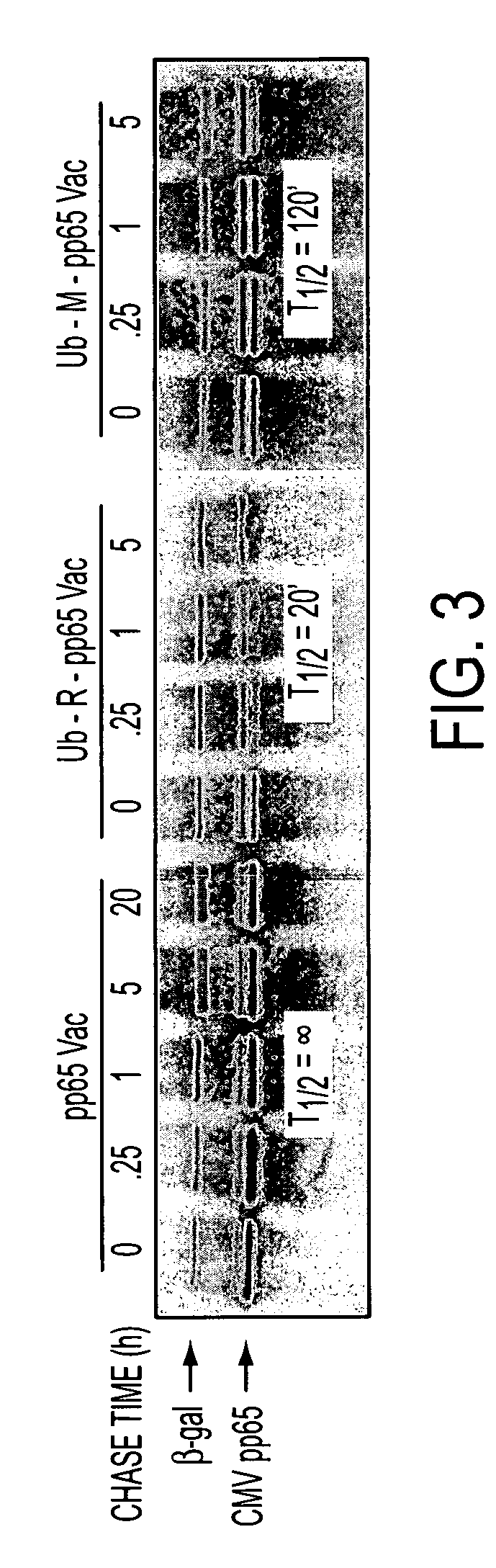
Human cytomegalovirus antigens expressed in MVA and methods of use
ActiveUS7163685B2Improve immunitySugar derivativesViral antigen ingredientsModified vaccinia AnkaraVaccine virus
DNA and protein constructs useful in producing vaccines against human cytomegalovirus contain optionally N-end modified and N-terminal ubiquitinated human cytomegalovirus antigenic proteins, including pp65, pp150, IE1, gB and antigenic fragments thereof. Vaccine viruses, in particular poxviruses such as vaccinia and Modified Vaccinia Ankara, that express the constructs may be used as vaccines to augment the immune response to human cytomegalovirus, both prophylatically and in patients already carrying human cytomegalovirus, as well as to create and expand cytomegalovirus-reactive T cells for transfer of adoptive immunity.
Owner:CITY OF HOPE
Methods for manufacturing t cells
ActiveUS20190247433A1Shorten the resting timeGenetically modified cellsGenetic material ingredientsVirus typeCD28
The disclosure relates to methods of manufacturing T cells for adoptive immunotherapy. The disclosure further provides for methods of genetically transducing T cells, methods of using T cells, and T cell populations thereof. In an aspect, the disclosure provides for methods of thawing frozen peripheral blood mononuclear cells (PBMC), resting the thawed PBMC, activating the T cell in the cultured PBMC with an anti-CD3 antibody and an anti-CD28 antibody immobilized on a solid phase, transducing the activated T cell with a viral vector, expanding the transduced T cell, and obtaining expanded T cells.
Owner:IMMATICS US INC
Immortalized car-t cells genetically modified to elminate t-cell receptor and beta 2-microglobulin expression
InactiveUS20190175651A1None is suitable for purposeTumor rejection antigen precursorsGenetic material ingredientsT cellAuto immune disease
The present invention pertains to engineered immortalized T-cell lines, method for their preparation and their use as medicament, particularly for immunotherapy. The engineered immortalized T-cell lines of the invention are characterized in that the expression of endogenous T-cell receptors (TCRs) and beta 2-microglobulin (B2M) is inhibited, e.g., by using an endonuclease able to selectively inactivate the TCR and B2M genes in order to render the immortalized T-cells non-alloreactive. In addition, expression of immunosuppressive polypeptide can be performed on those engineered immortalized T-cells in order to prolong the survival of these T-cells in host organisms. Such engineered immortalized T-cells are particularly suitable for allogeneic transplantations, especially because it reduces both the risk of rejection by the host's immune system and the risk of developing graft versus host disease. The invention opens the way to standard and affordable adoptive immunotherapy strategies using immortalized T-cells for treating cancer, infections and auto-immune diseases.
Owner:JANSSEN BIOTECH INC
Chimeric antigen receptors (CARs) having mutations in the Fc spacer region and methods for their use
ActiveCN107074957APrevent identification and damageAvoid clearingPolypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigen receptorsChimeric antigen receptor
Adoptive immunotherapy using T cells genetically redirected via expression of chimeric antigen receptors (CARs) is a promising approach for cancer treatment. However, this immunotherapy is dependent in part on the optimal molecular design of the CAR, which involves an extracellular ligand-binding domain connected to an intracellular signaling domain by spacer and / or transmembrane sequences.
Owner:CITY OF HOPE
Adoptive immunotherapy with enhanced T lymphocyte survival
Owner:UNITED STATES OF AMERICA
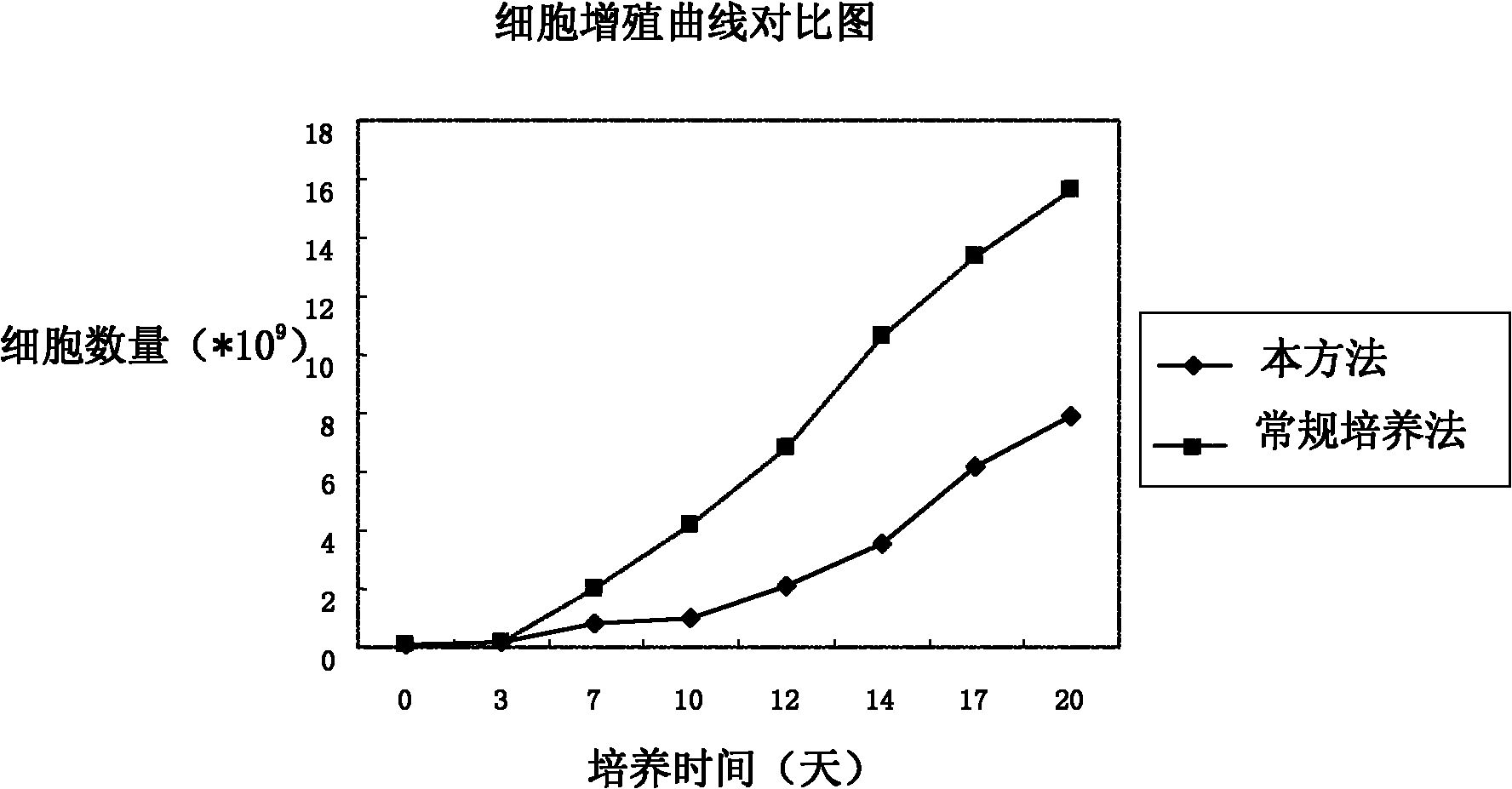
Preparation method of antitumor adoptive immune cells and prepared immune cells
InactiveCN102321577AIncrease multiplierImprove tumor killing abilityBlood/immune system cellsImmunity activeCD28
The invention discloses a preparation method of antitumor adoptive immune cells. The method comprises the following steps of: sampling and separating a single peripheral blood karyocyte; and adding a cell factor for inducting and culturing to obtain a cytokine induced kill cell (CIK), wherein the cell factor comprises CD28. In a CIK induced system, the cell factor CD28 is induced, so that the inducing effect is enhanced, and the cell proliferation multiple is increased simultaneously.
Owner:深圳市中美康士生物科技有限公司
Recombinant immunoreceptors
InactiveUS20050118185A1Restored cell surface expressionImpaired stabilitySugar derivativesAntibody mimetics/scaffoldsIntracellular signallingCell activation
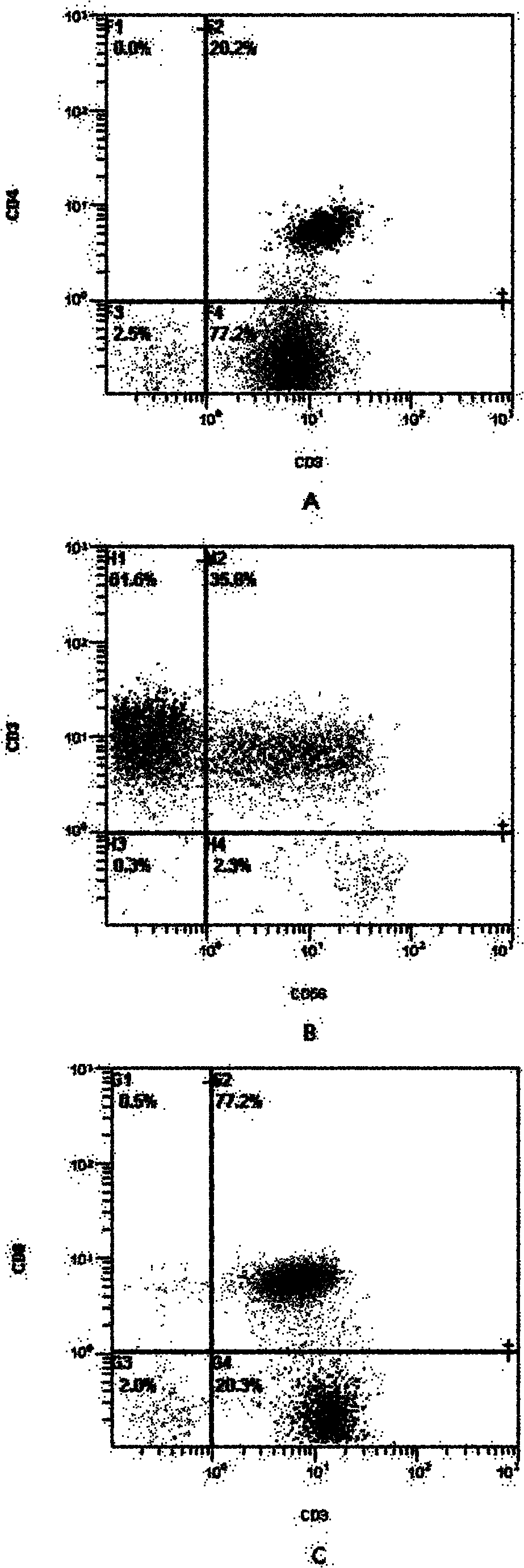
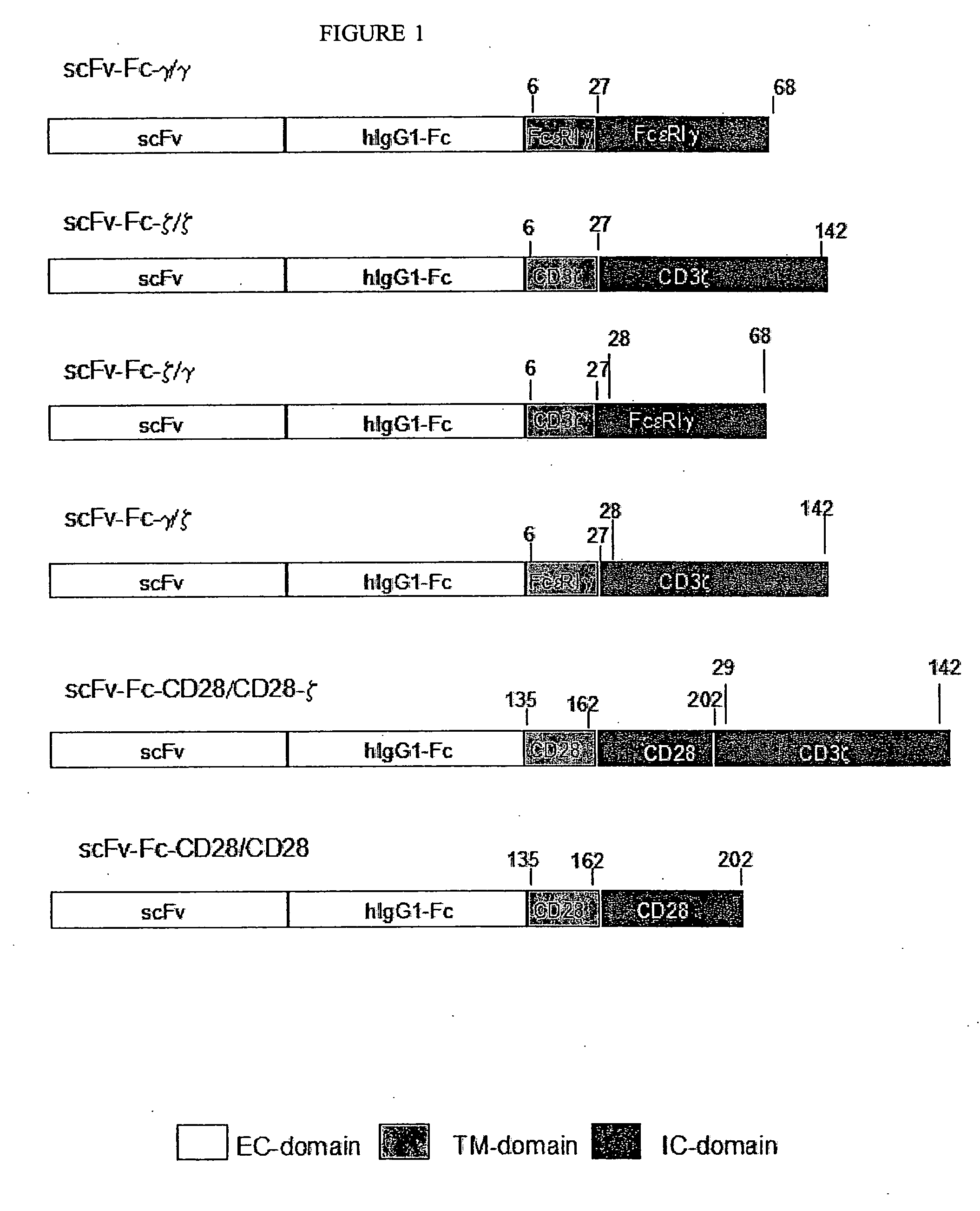
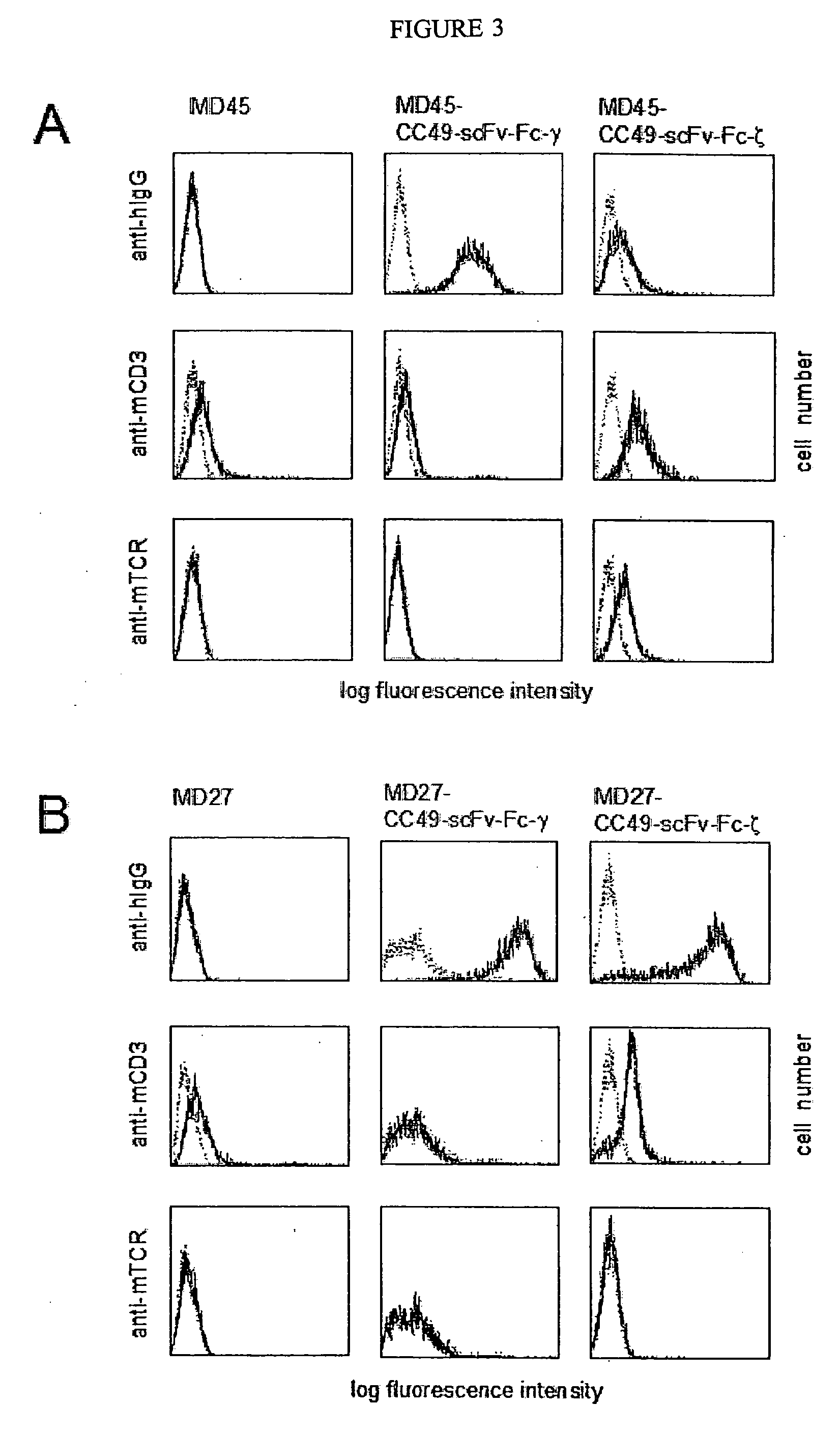
Described are recombinant immunoreceptors composed of an extracellular antigen binding domain (scFv) and a transmembrane region derived from the CD3ζ-chain and / or the FcεRIγ-chain linked to an intracellular signaling domain with cell activation properties. Moreover, nucleic acid sequences encoding said immunoreceptor, immune cells expressing said immunoreceptor as well as therapeutic uses of said immunoreceptor, e.g. adoptive immunotherapy, are described.
Owner:CELL CENT COLOGNE
Selection of antigen-specific t cells
InactiveUS20090004742A1Altering cellular behaviorPeptide/protein ingredientsVertebrate cellsDiagnostic Radiology ModalityDendritic cell
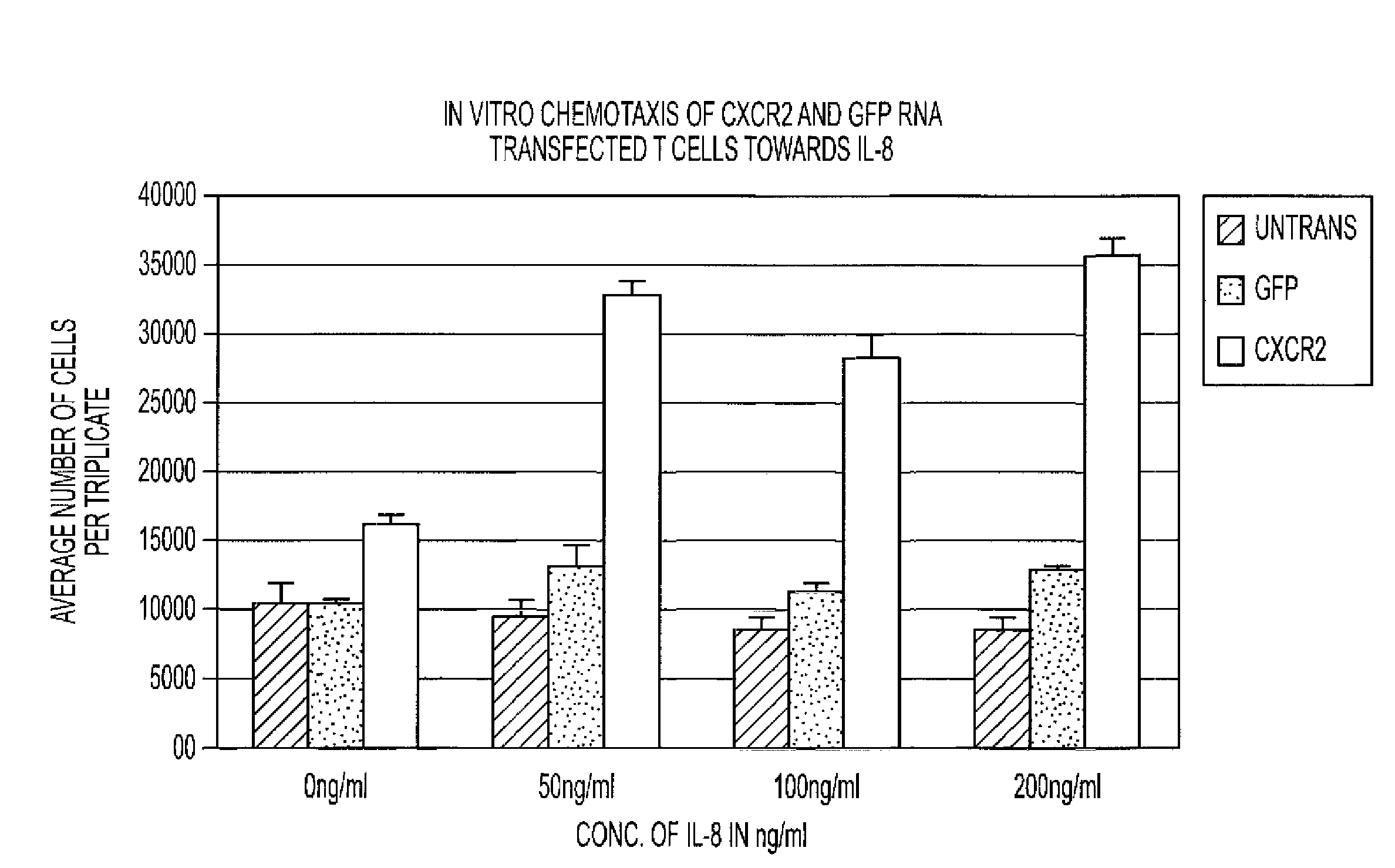
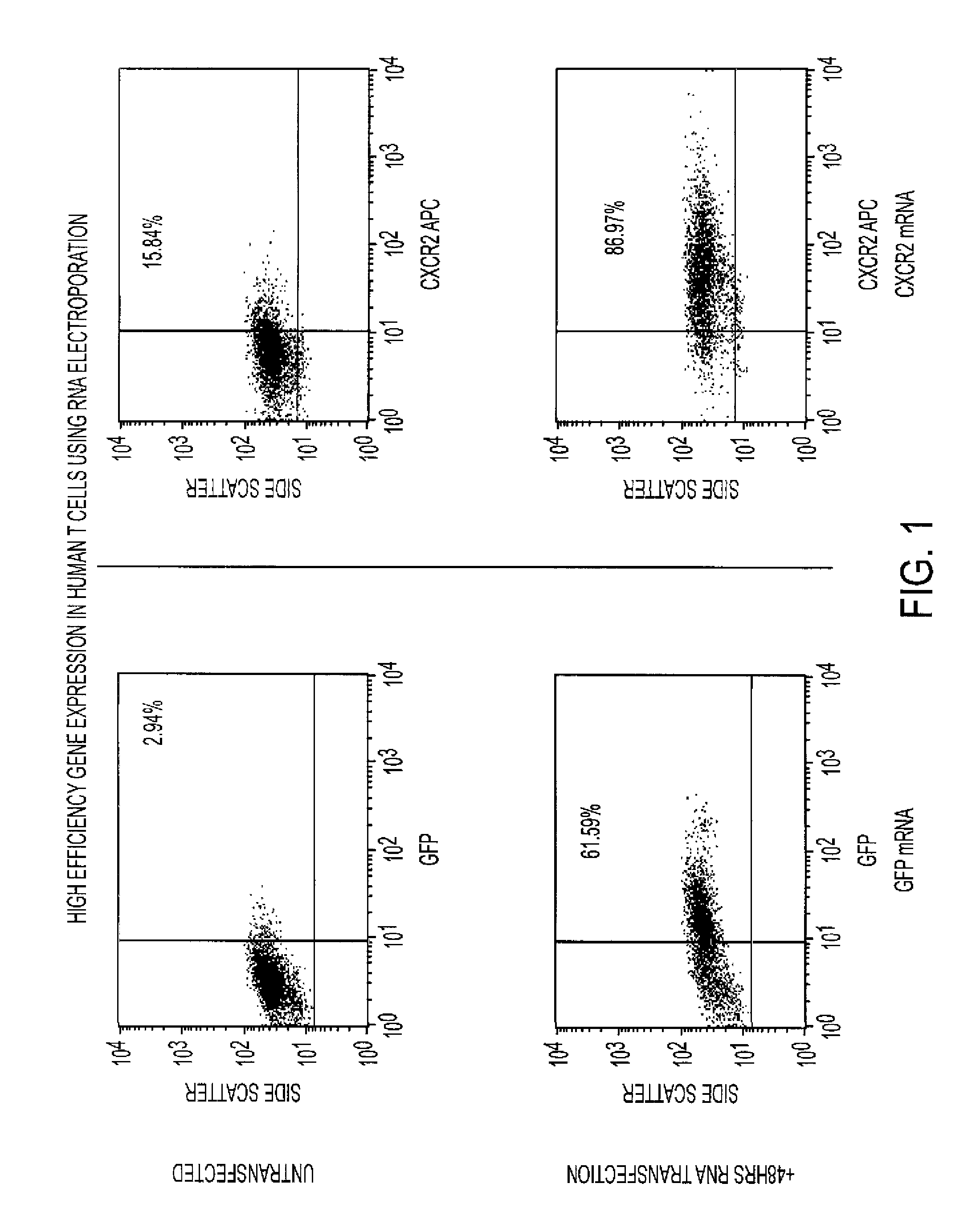
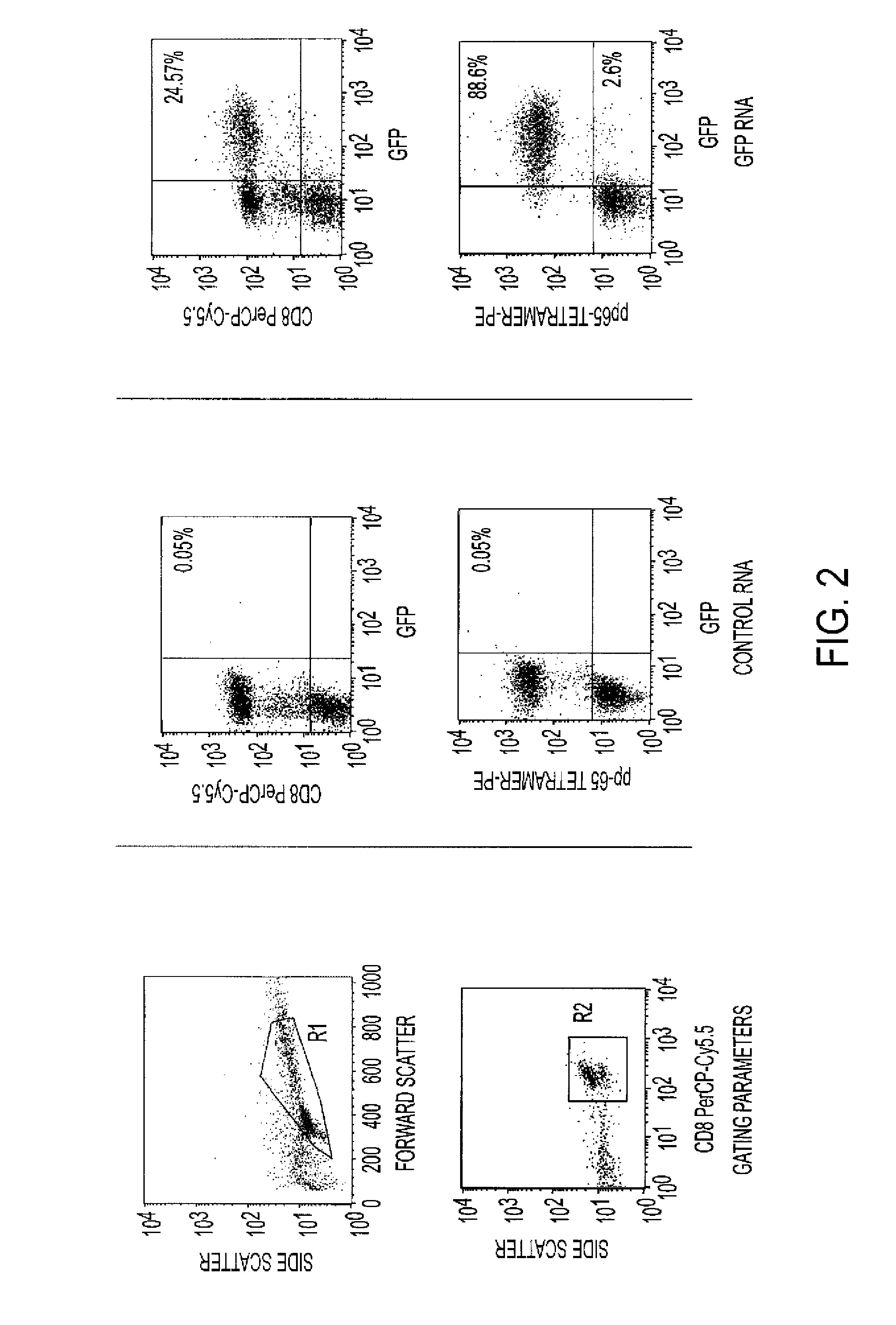
The requirement of T cell activation for efficient expression of genes after messenger ribonucleic acid (mRNA) transfection is leveraged to identify and enrich antigen-specific T cells responding to antigen-pulsed dendritic cells (DCs). RNA transfection of marker genes is used for the selection and enrichment of antigen-specific T cells for use in adoptive immunotherapy. RNA-modified T cells are also used for the generation of enhanced effector populations for use in adoptive immunotherapy. Genes whose transient expression may significantly enhance the in vivo function of T cells (i.e., migratory receptors, anti-apoptotic genes or cytokines enhancing T cell proliferation / differentiation) are used in this modality.
Owner:DUKE UNIV
Application of vitamin C in in-vitro expansion of number of effector memory T cells
InactiveCN102925411AEfficient in vitro expansionImprove securityBlood/immune system cellsVitamin CVitamin
The invention discloses application of vitamin C in in-vitro expansion of number of effector memory T cells, and the optimal concentration for promoting in-vitro expansion is found. The method has the advantages of high safety, low cost and the like, and provides a good technical support for the wide development of adoptive immunotherapy.
Owner:SUN YAT SEN UNIV
Methods and compositions for treating cancer and infectious diseases
ActiveUS20170007698A1Prevention and reduction of severityShorten the progressOrganic active ingredientsAntibody ingredientsInfectious DisorderImmune modulator
The invention relates to compositions comprising a CD4 lymphocyte depleting agent; and methods of using the compositions to treat, prevent, reduce the severity of and / or slow the progression of a condition in a subject. The invention also relates to use of combinations of a CD4 lymphocyte depleting agent and at least one additional agent to treat, prevent, reduce the severity of and / or slow the progression of a condition in a subject. The additional agent may be an immune check point inhibitor, an adoptive immune therapeutic, an immune adjuvant, or an immune modulating agent, or their combinations.
Owner:CEDARS SINAI MEDICAL CENT
Method of extensive culture of antigen-specific cytotoxic t cells
InactiveUS20040115809A1High antigen-specific cytotoxic activityHigh cytotoxic activitySsRNA viruses negative-senseOrganic active ingredientsAdditive ingredientOligosaccharide
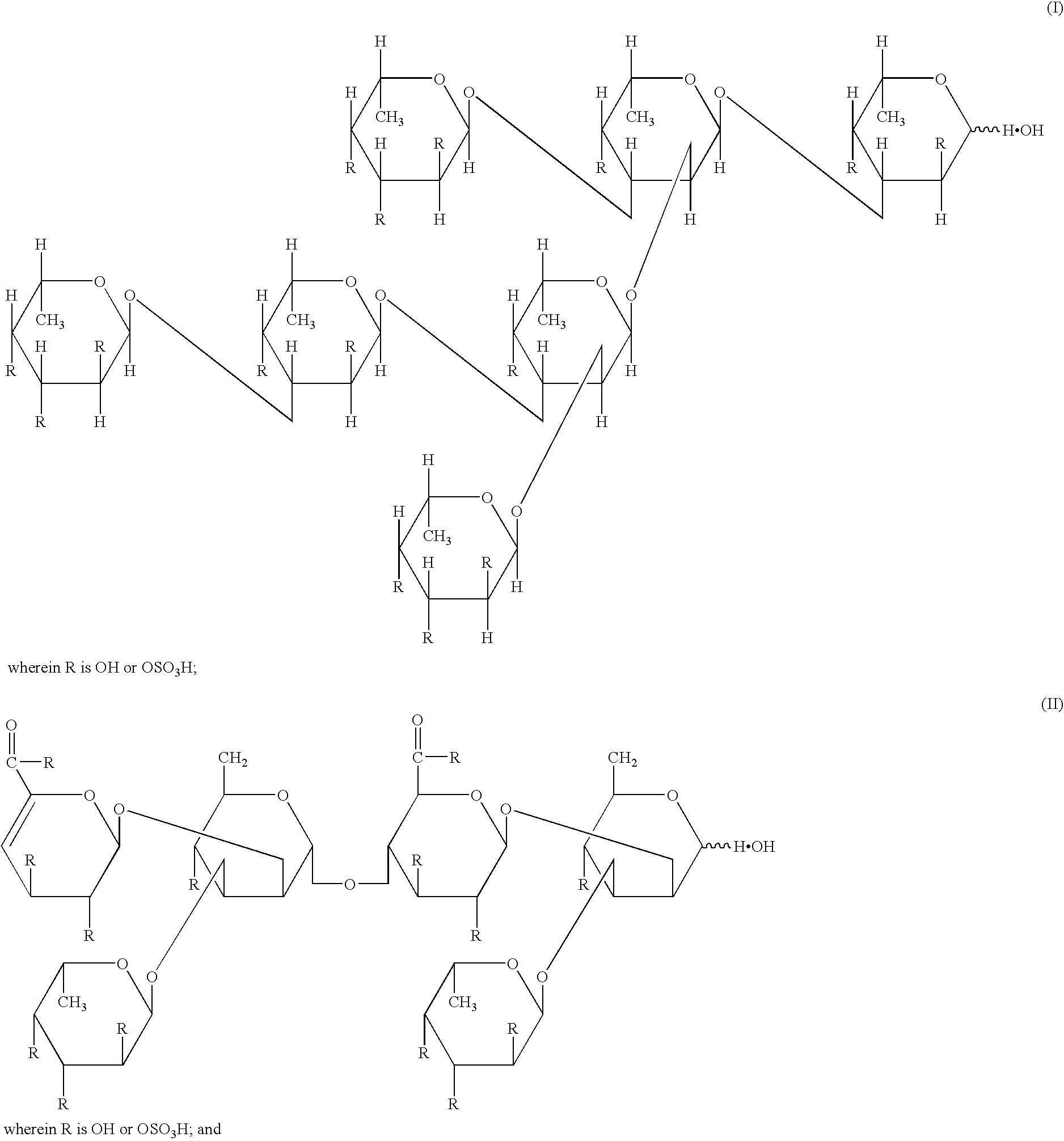
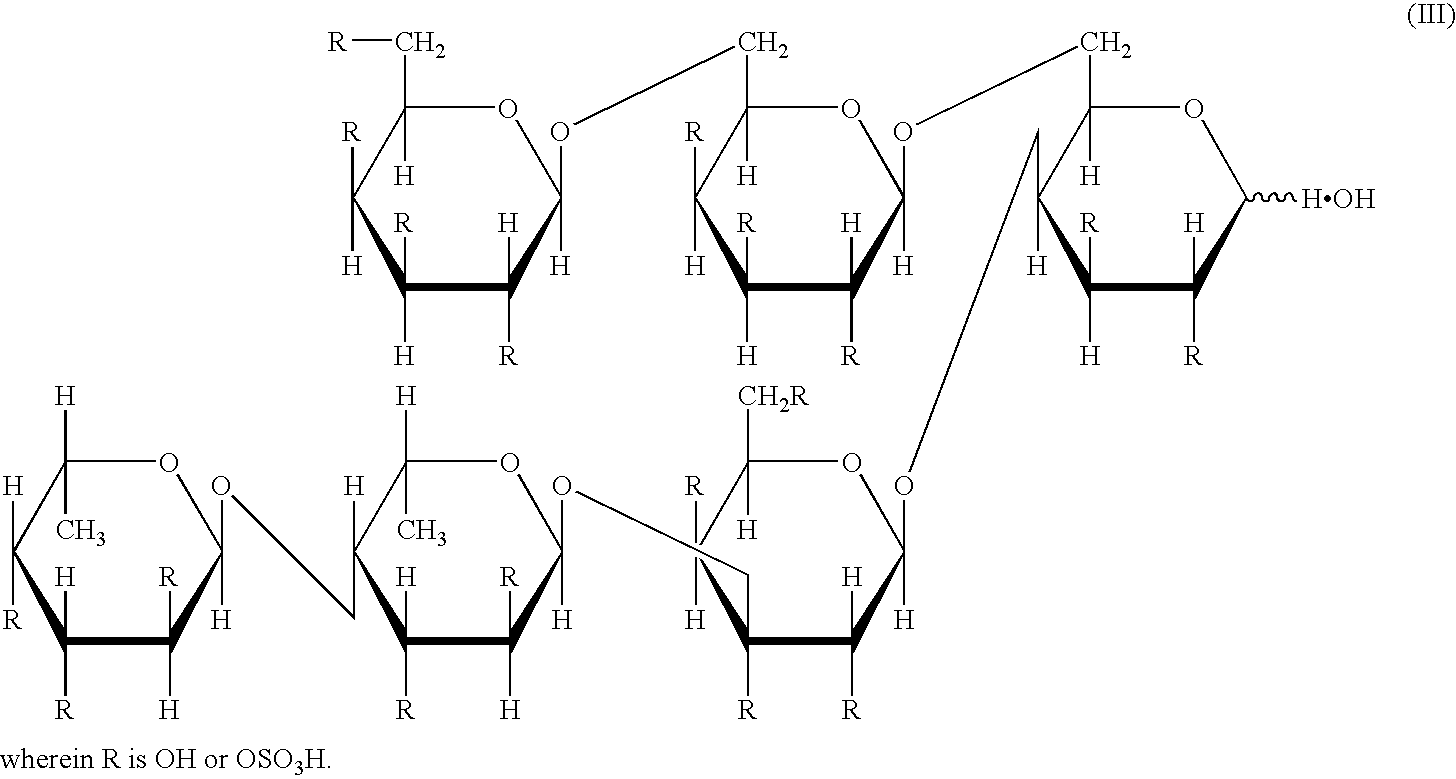
The present invention provides methods for inducing, maintaining and expanding CTL (cytotoxic T cell) having an antigen-specific cytotoxic activity at a high level, which is useful in the adoptive immunotherapy, by using as an effective ingredient at least one compound selected from the group consisting of acidic polysaccharides, acidic oligosaccharides, acidic monosaccharides, and salts thereof. The above-mentioned compounds include fucoidans, heparins, alginic acid, chondroitin sulfate A, chondroitin sulfate B, pectic acid, hyaluronic acid, degradation products of fucoidans, sulfated glucose, sulfated fucose and salts thereof.
Owner:TAKARA HOLDINGS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com