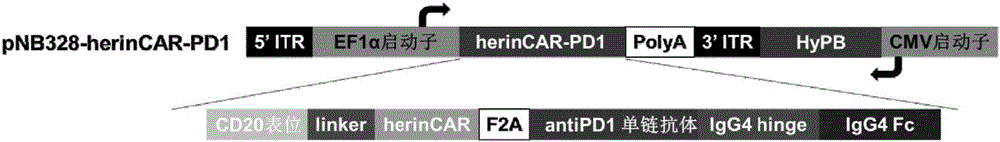
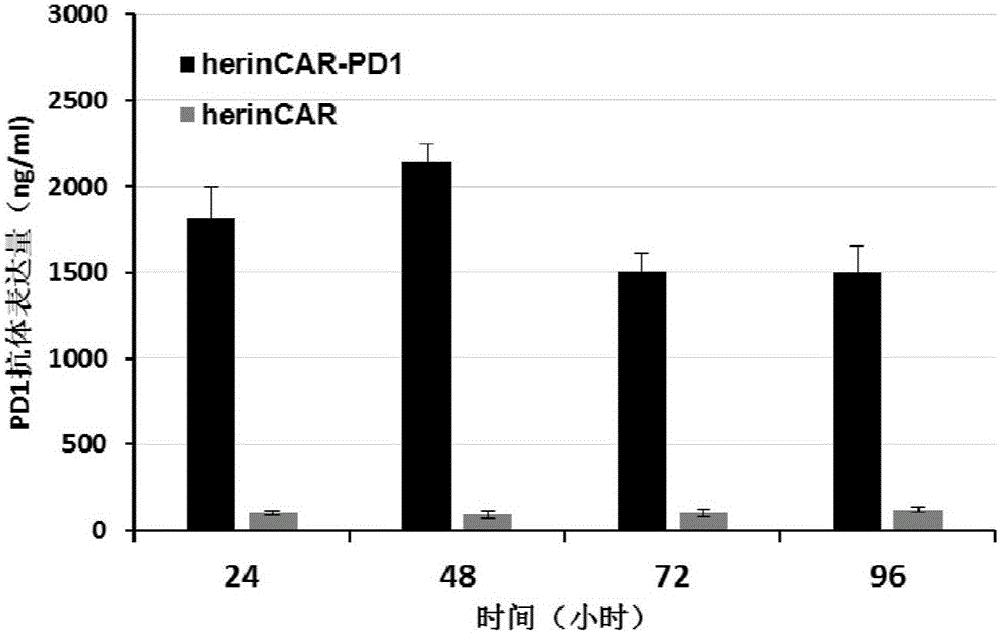
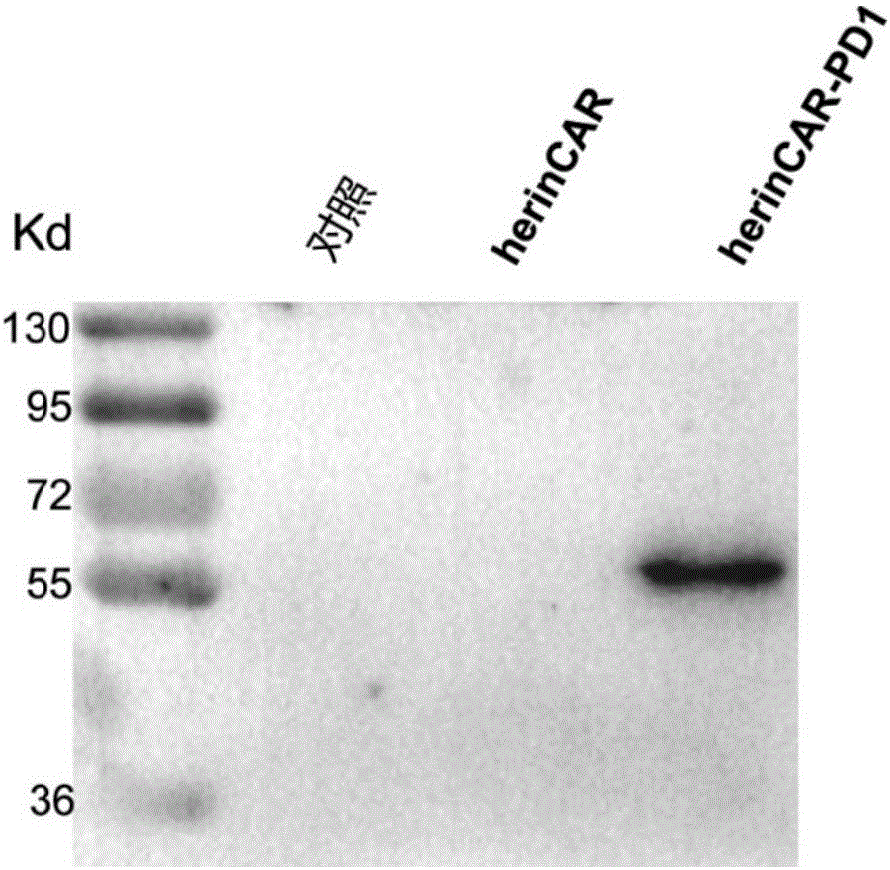
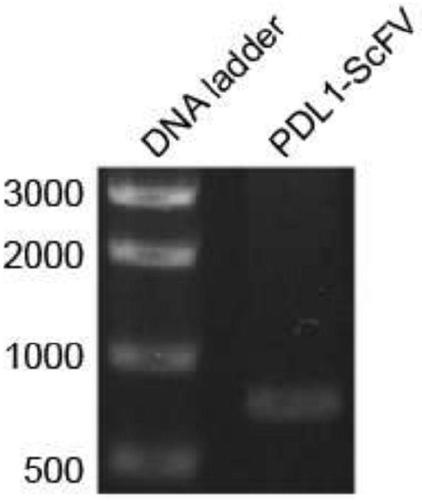
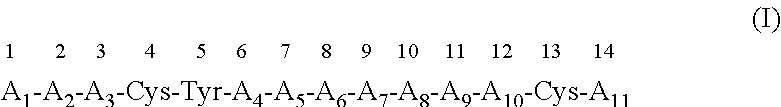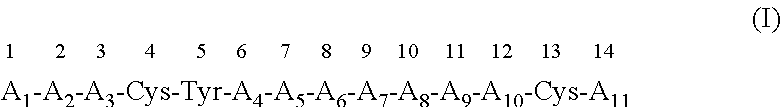Patents
Literature
384 results about "Immune checkpoint" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Immune checkpoints are regulators of the immune system. These pathways are crucial for self-tolerance, which prevents the immune system from attacking cells indiscriminately. However, some cancers can protect themselves from attack by stimulating immune checkpoint targets.
Targeted tgfß inhibition
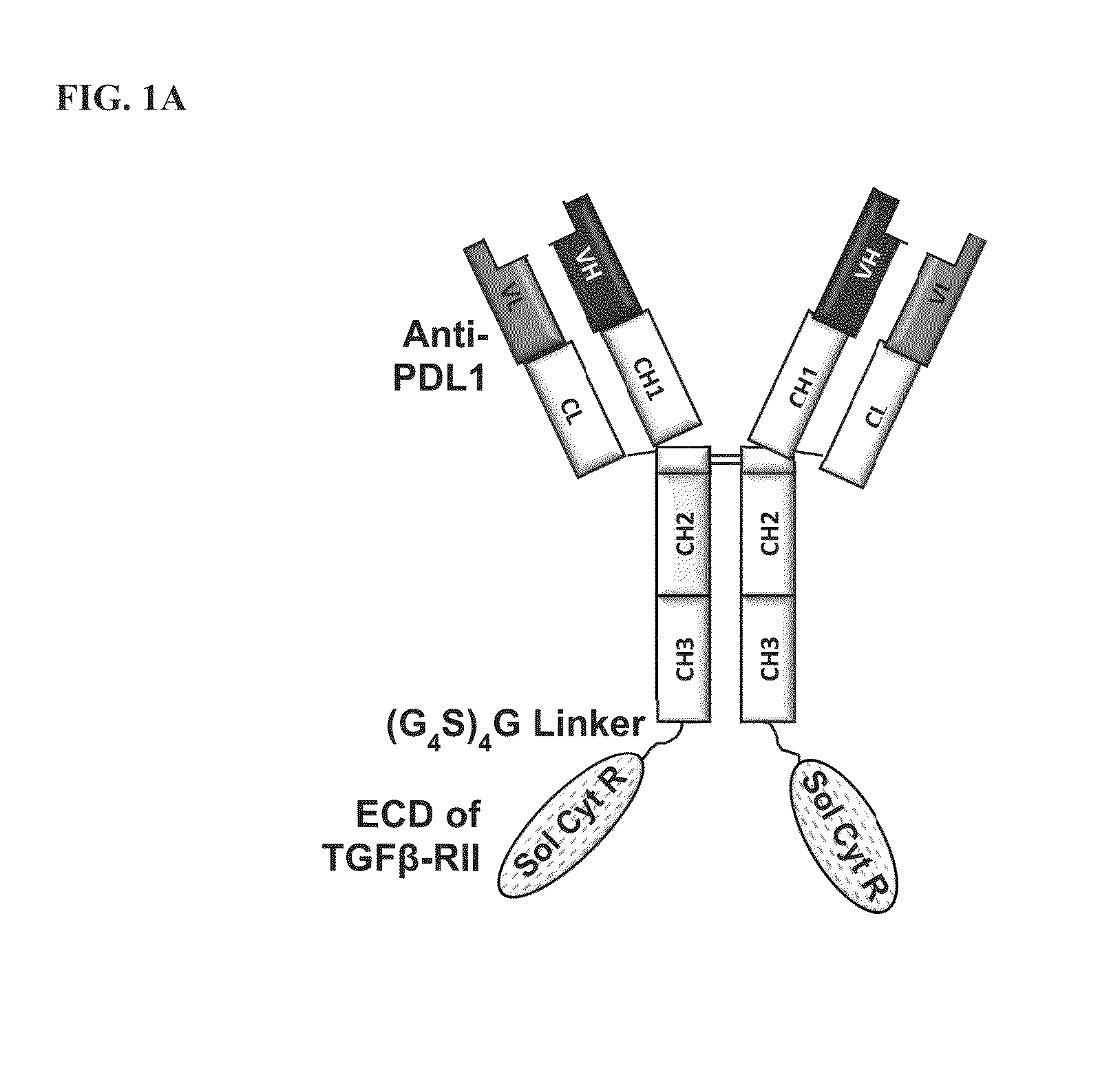
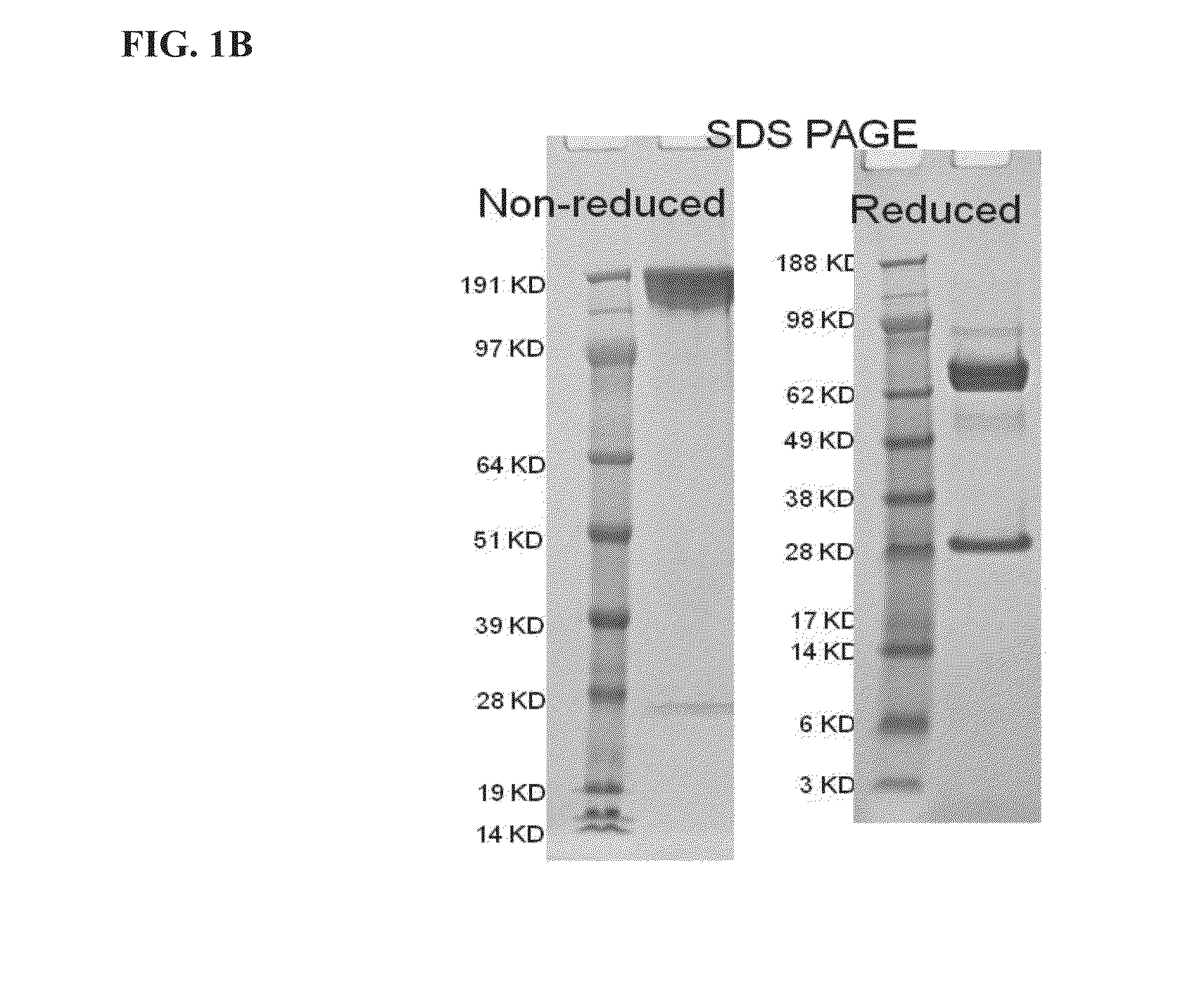
ActiveUS20150225483A1Effective therapyPeptide/protein ingredientsAntibody mimetics/scaffoldsAntigenAntigen Binding Fragment
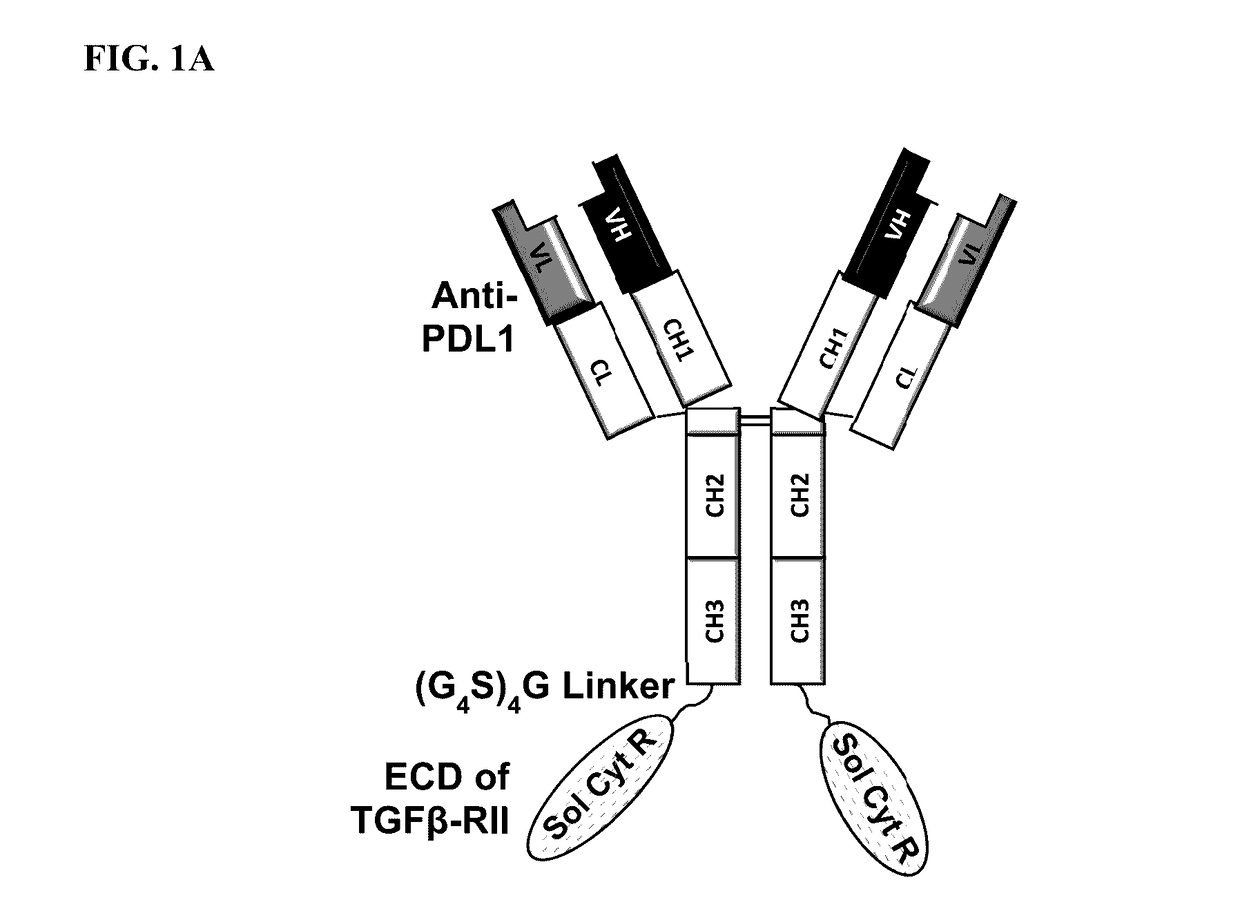
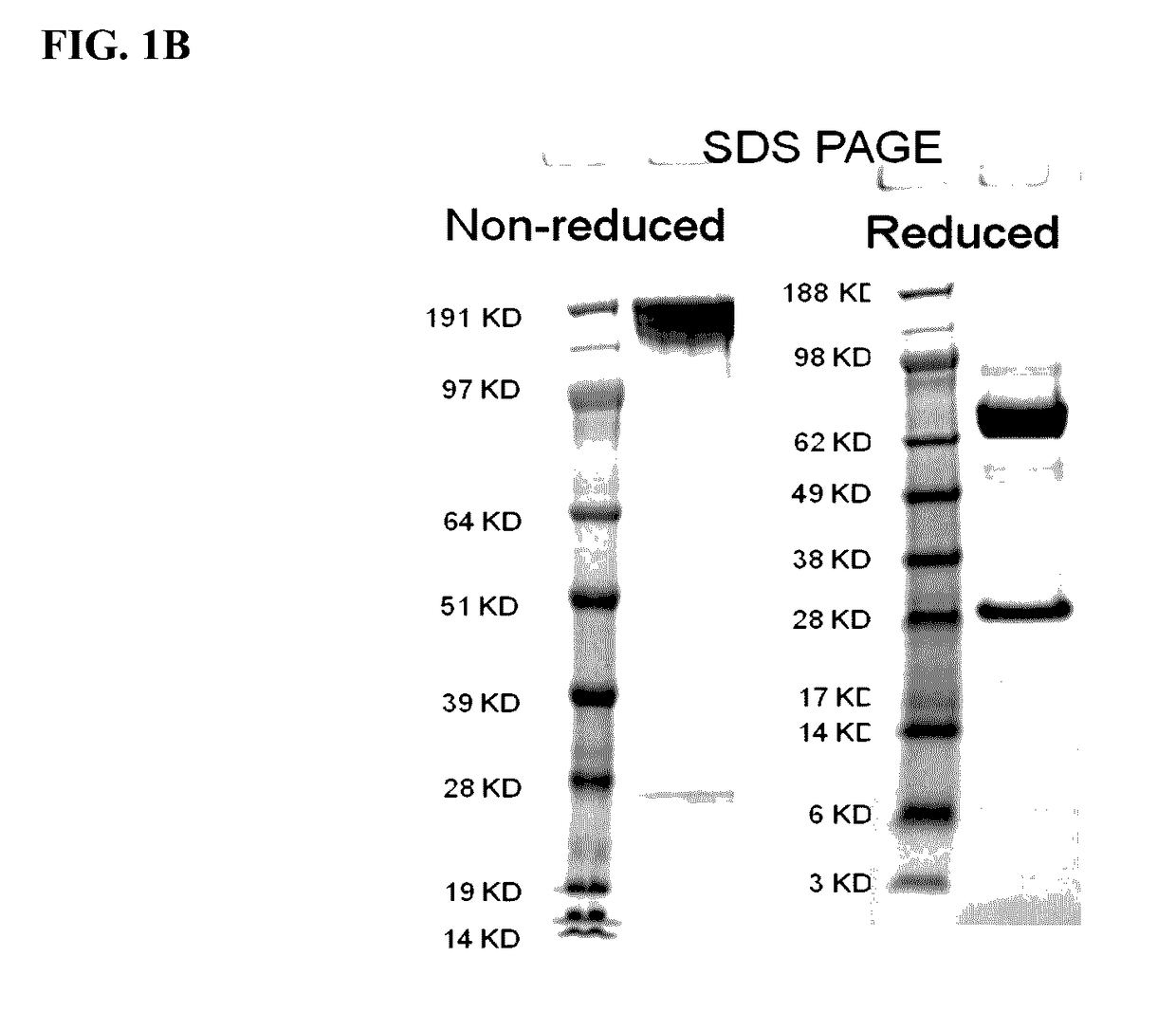
This invention relates generally to bifunctional molecules including (a) a TGFβRII or fragment thereof capable of binding TGFβ and (b) an antibody, or antigen binding fragment thereof, that binds to an immune checkpoint protein, such as Programmed Death Ligand 1 (PD-L1), uses of such molecules (e.g., for treating cancer), and methods of making such molecules.
Owner:MERCK PATENT GMBH
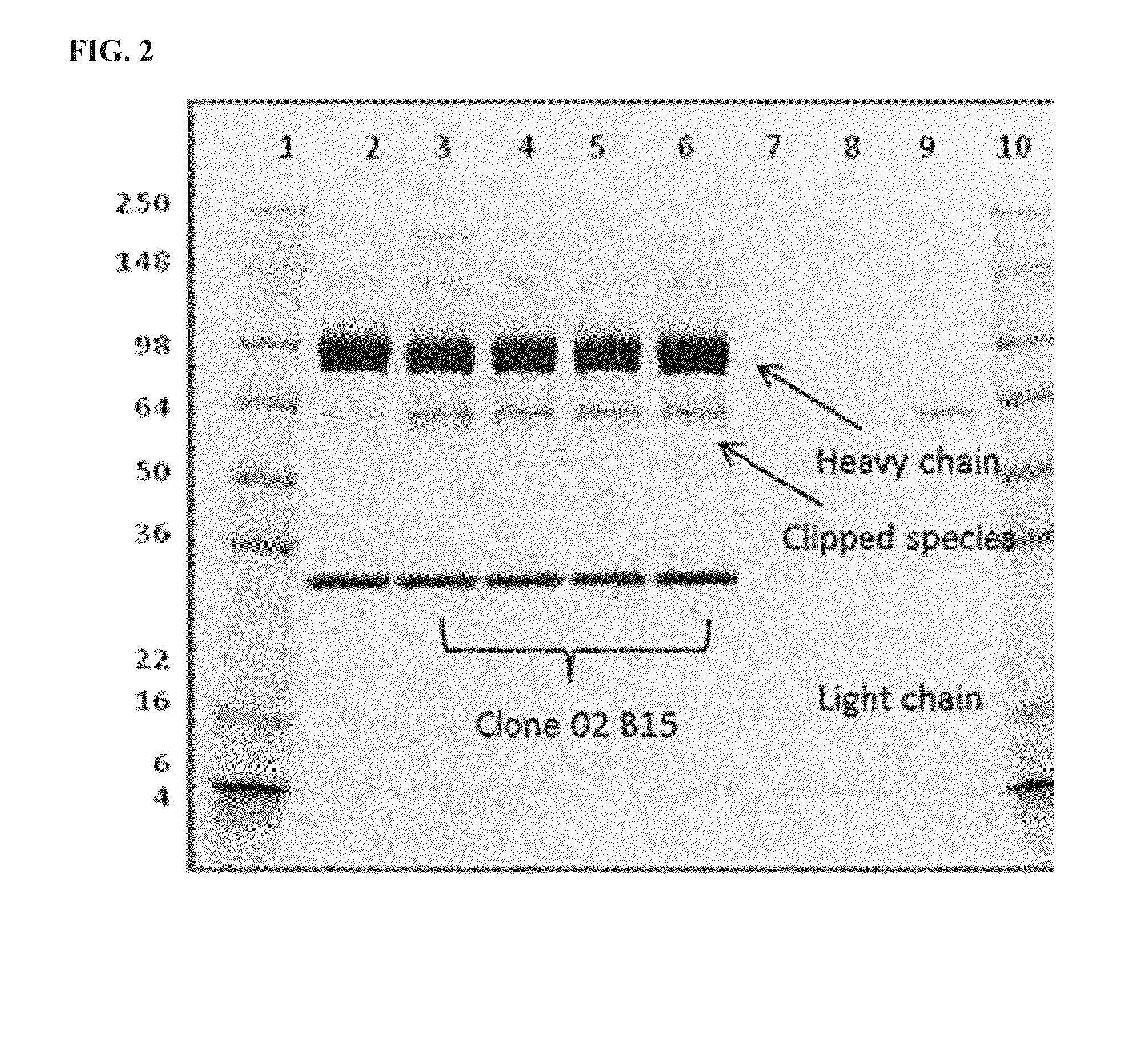
Methods for engineering highly active t cell for immunotheraphy
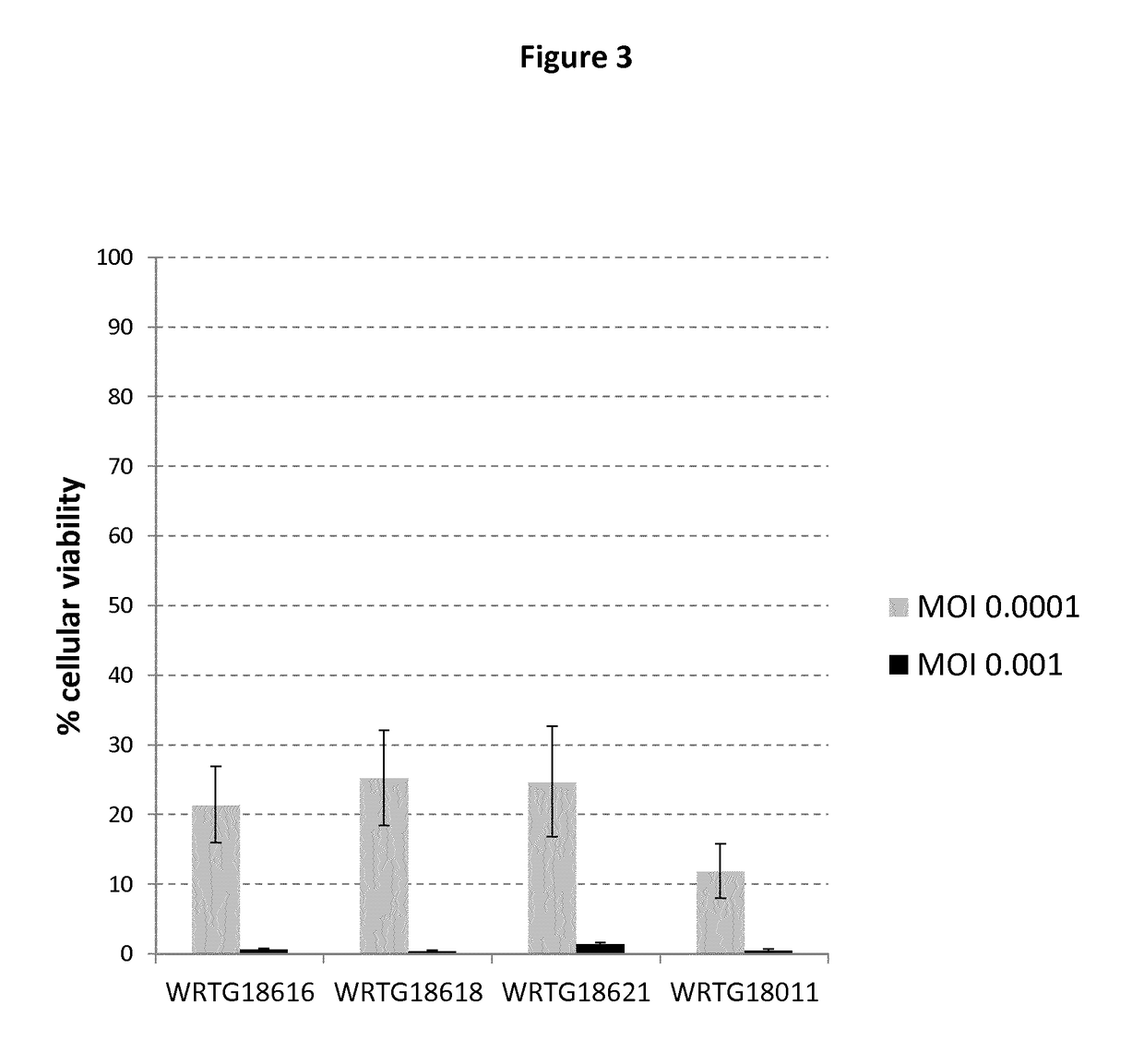
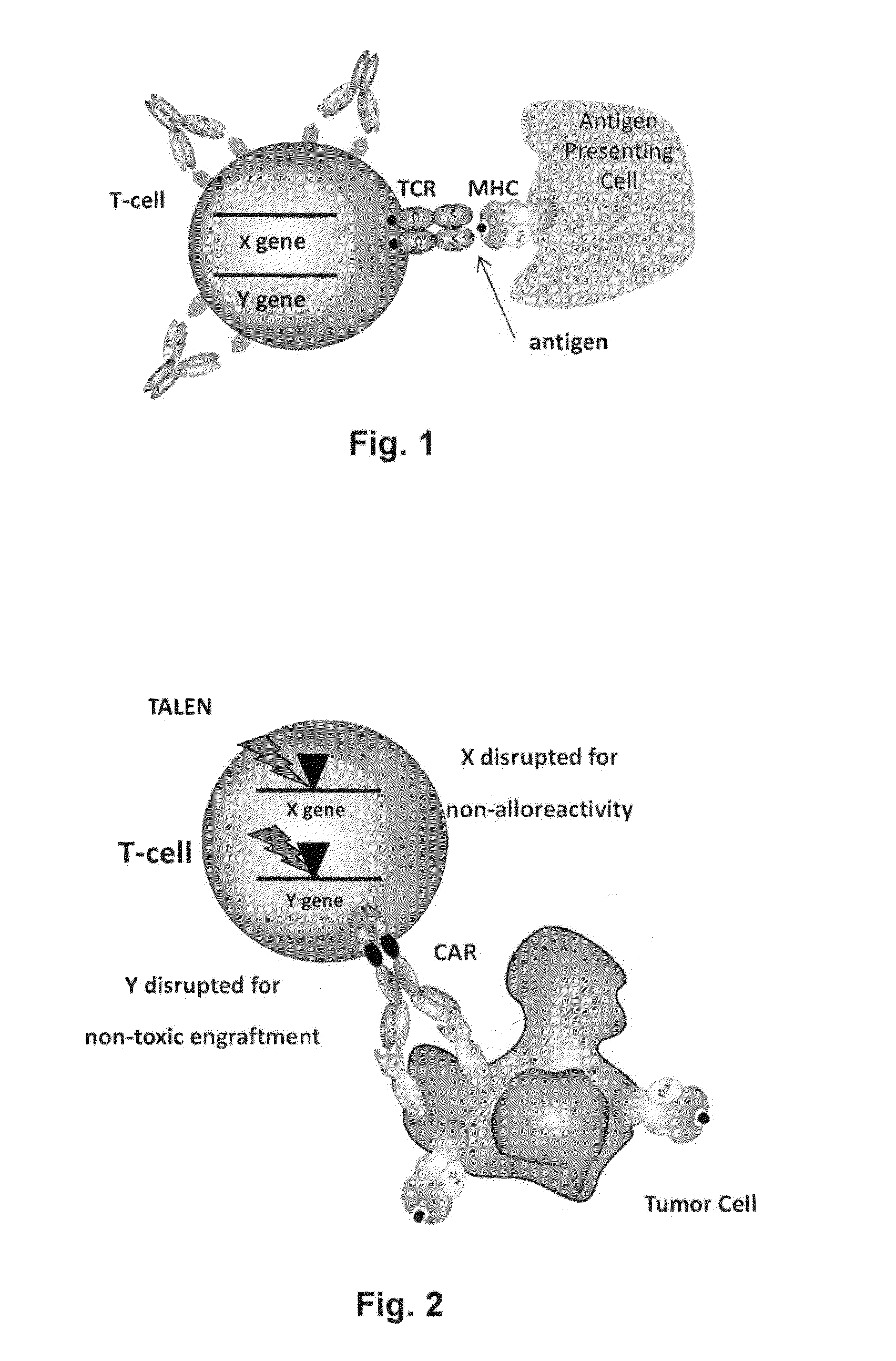
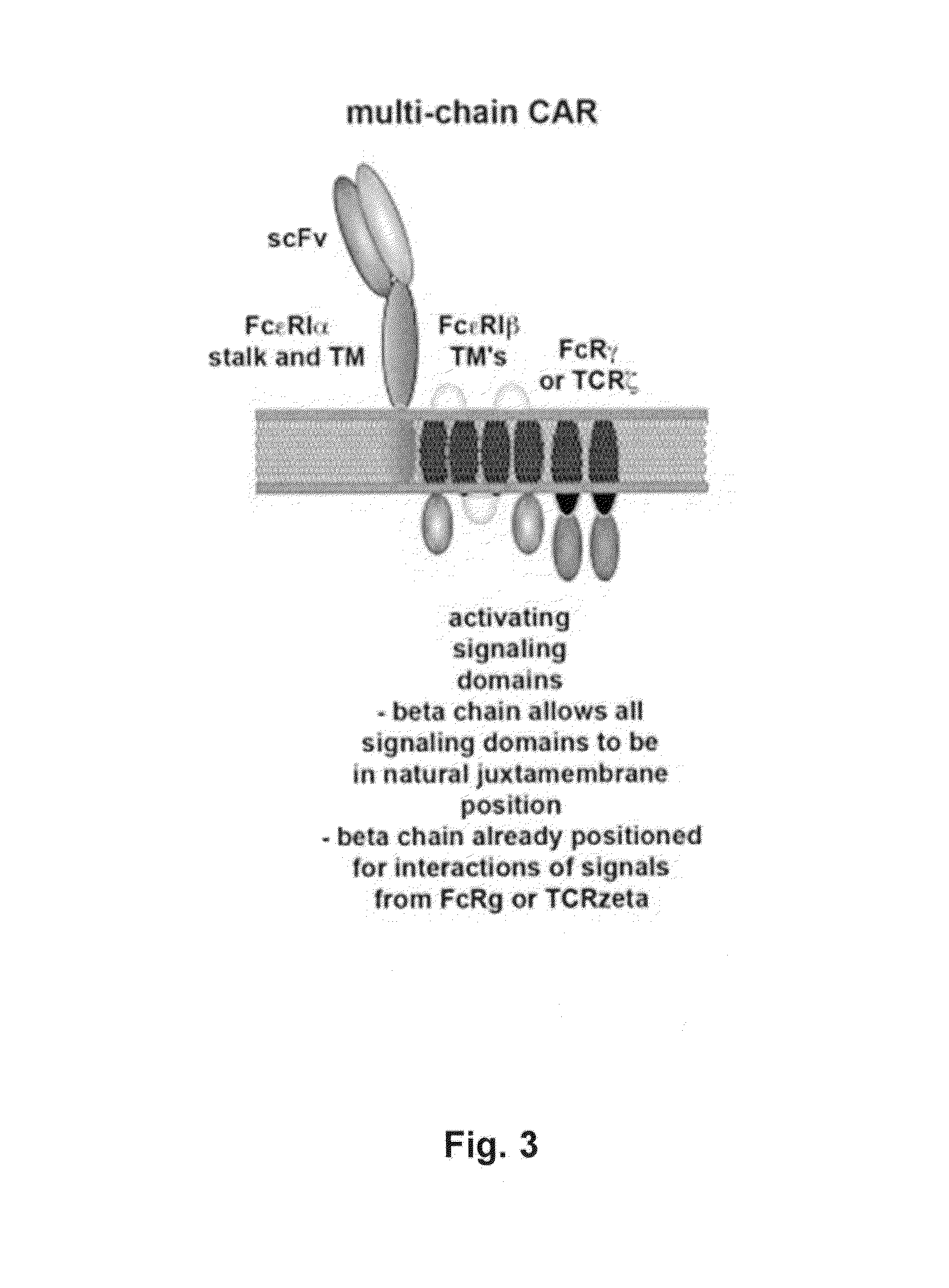
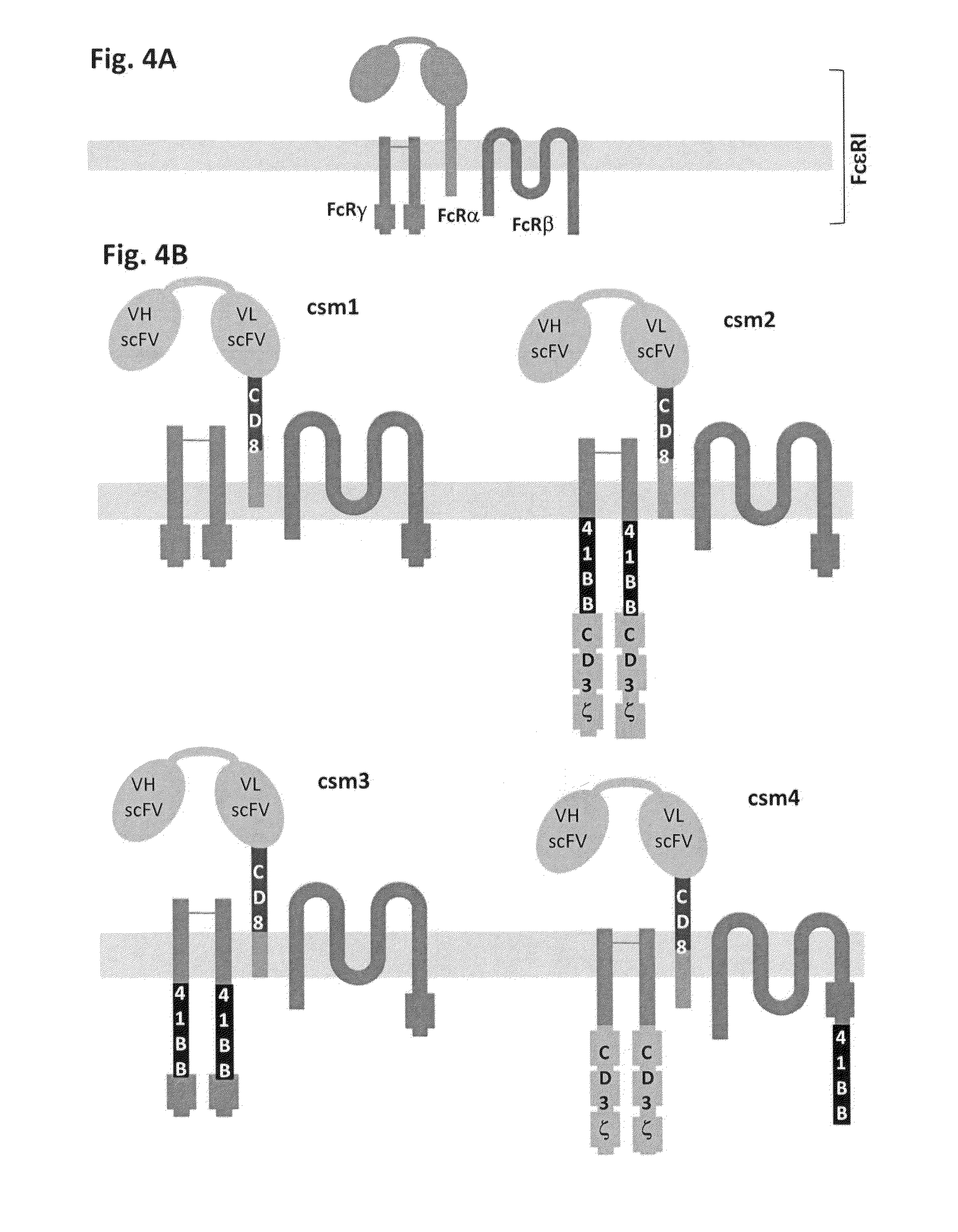
The present invention relates to methods for developing engineered T-cells for immunotherapy and more specifically to methods for modifying T-cells by inactivating at immune checkpoint genes, preferably at least two selected from different pathways, to increase T-cell immune activity. This method involves the use of specific rare cutting endonucleases, in particular TALE-nucleases (TAL effector endonuclease) and polynucleotides encoding such polypeptides, to precisely target a selection of key genes in T-cells, which are available from donors or from culture of primary cells. The invention opens the way to highly efficient adoptive immunotherapy strategies for treating cancer and viral infections.
Owner:CELLECTIS SA
Targeted TGFβ inhibitors
ActiveUS9676863B2Effective therapyNervous disorderPeptide/protein ingredientsAntigenAntigen Binding Fragment
This invention relates generally to bifunctional molecules including (a) a TGFβRII or fragment thereof capable of binding TGFβ and (b) an antibody, or antigen binding fragment thereof, that binds to an immune checkpoint protein, such as Programmed Death Ligand 1 (PD-L1), uses of such molecules (e.g., for treating cancer), and methods of making such molecules.
Owner:MERCK PATENT GMBH
Compositions And Methods For Modulating And Redirecting Immune Responses
InactiveUS20170015758A1Immunoglobulins against cell receptors/antigens/surface-determinantsImmunological disordersAntigenT cell
Provided herein are methods of modulating and redirecting an immune response. Compositions and methods for killing targeted cells in a cell population are also provided wherein, a cell population containing target cells expressing a target associated antigen and T cells are contacted with 1, 2, or more immune checkpoint antagonists and a multispecific T cell-redirecting agent that specifically binds the target associated antigen expressed on the target cells and specifically binds a T cell surface antigen.
Owner:DUKE UNIV +1
PD-1-Binding Molecules and Methods of Use Thereof
ActiveUS20190127467A1Low affinityIncreased serum half-lifeAntibacterial agentsNervous disorderEpitopeCheck point
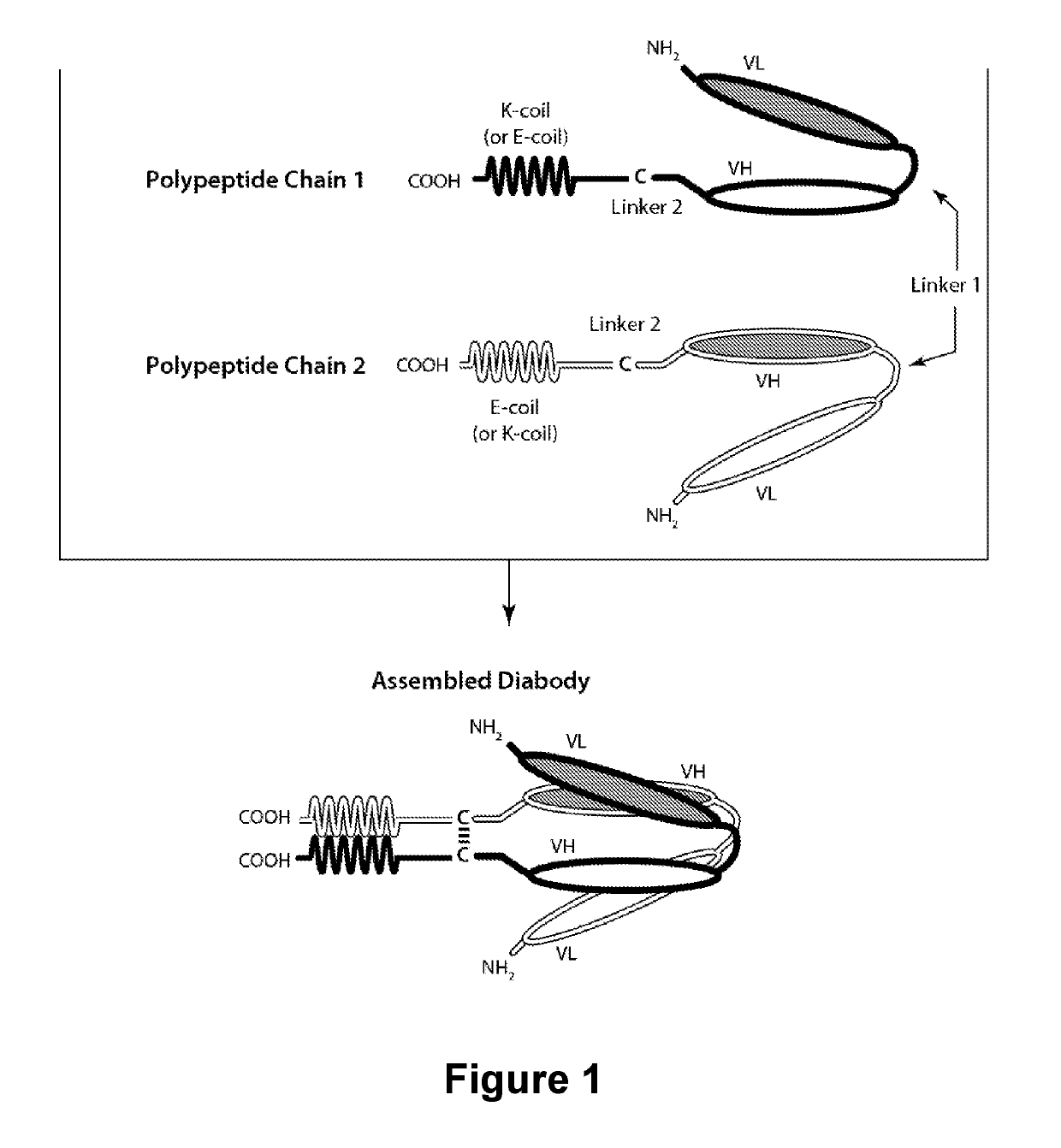
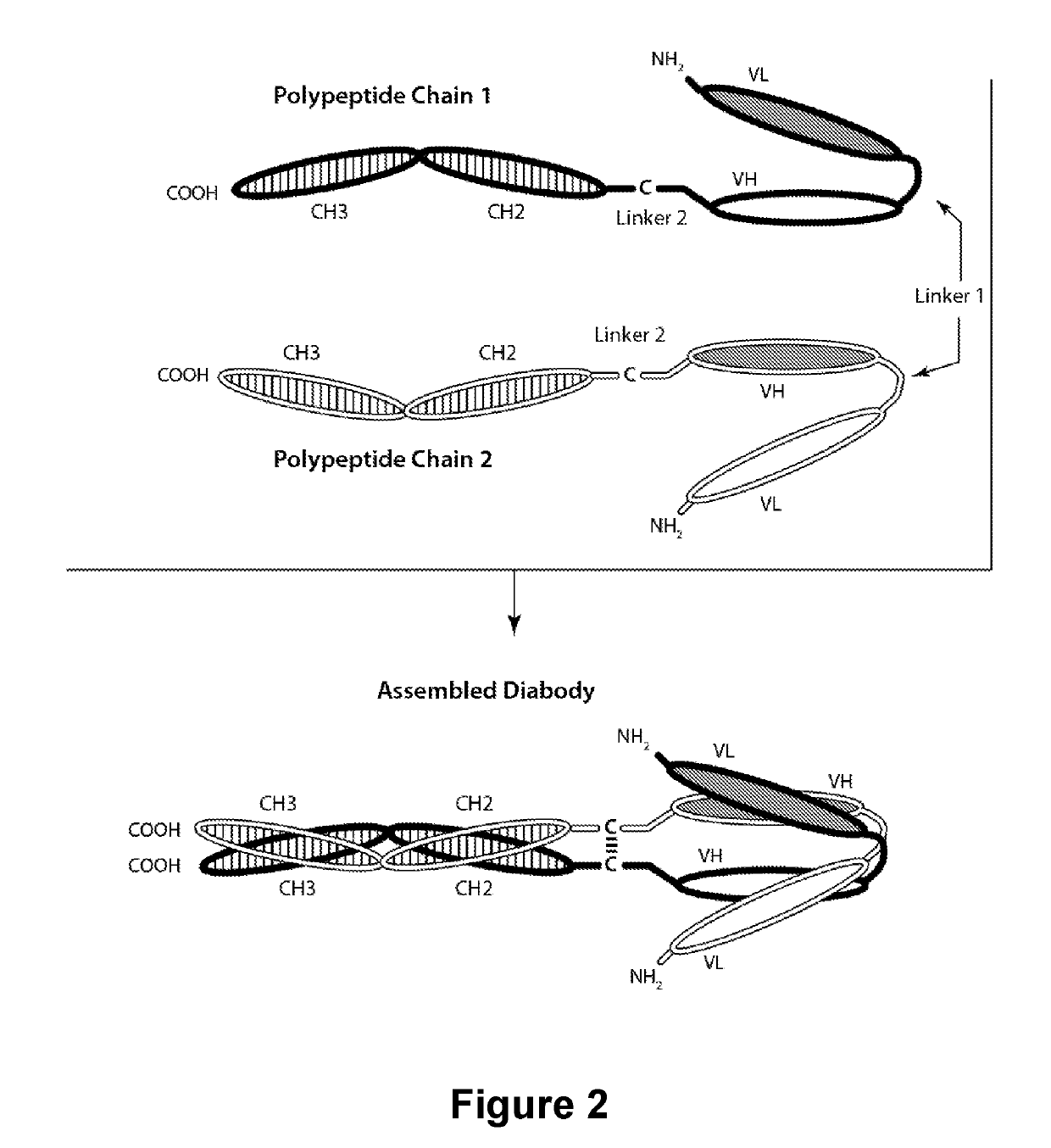
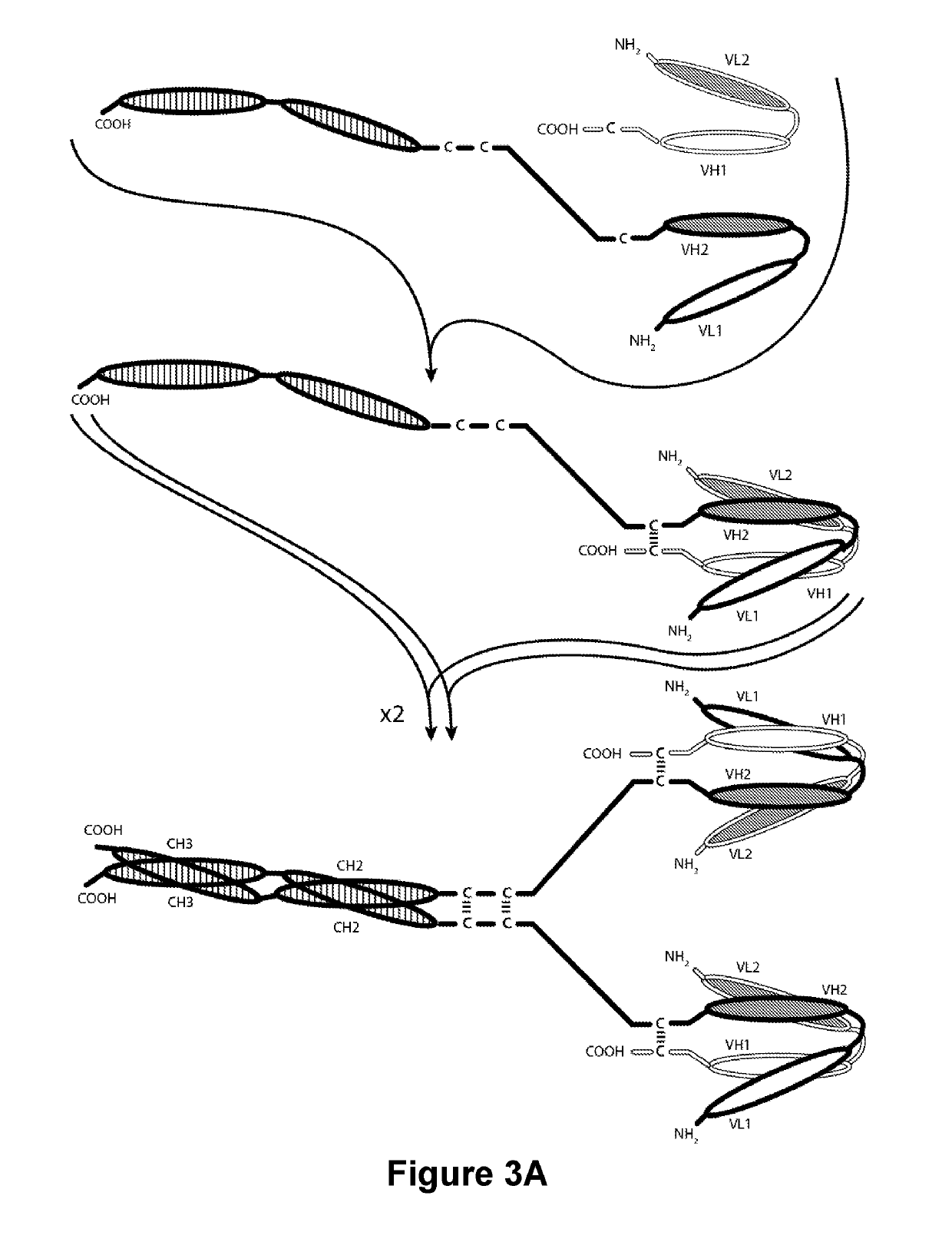
The present invention is directed to selected anti-PD-1 antibodies capable of binding to both cynomolgus monkey PD-1 and to human PD-1: PD-1 mAb 1, PD-1 mAb 2, PD-1 mAb 3, PD-1 mAb 4, PD-1 mAb 5, PD-1 mAb 6, PD-1 mAb 7, PD-1 mAb 8, PD-1 mAb 9, PD-1 mAb 10, PD-1 mAb 11, PD-1 mAb 12, PD-1 mAb 13, PD-1 mAb 14, or PD-1 mAb 15, and to humanized and chimeric versions of such antibodies. The invention additionally pertains to PD-1-binding molecules that comprise PD-1 binding fragments of such anti-PD-1 antibodies, immunocongugates, and to bispecific molecules, including diabodies, BiTEs, bispecific antibodies, etc., that comprise (i) such PD-1-binding fragments, and (ii) a domain capable of binding an epitope of a molecule involved in regulating an immune check point present on the surface of an immune cells. The present invention also pertains to methods of using molecules that bind PD-1 for stimulating immune responses, as well as methods of detecting PD-1.
Owner:MACROGENICS INC
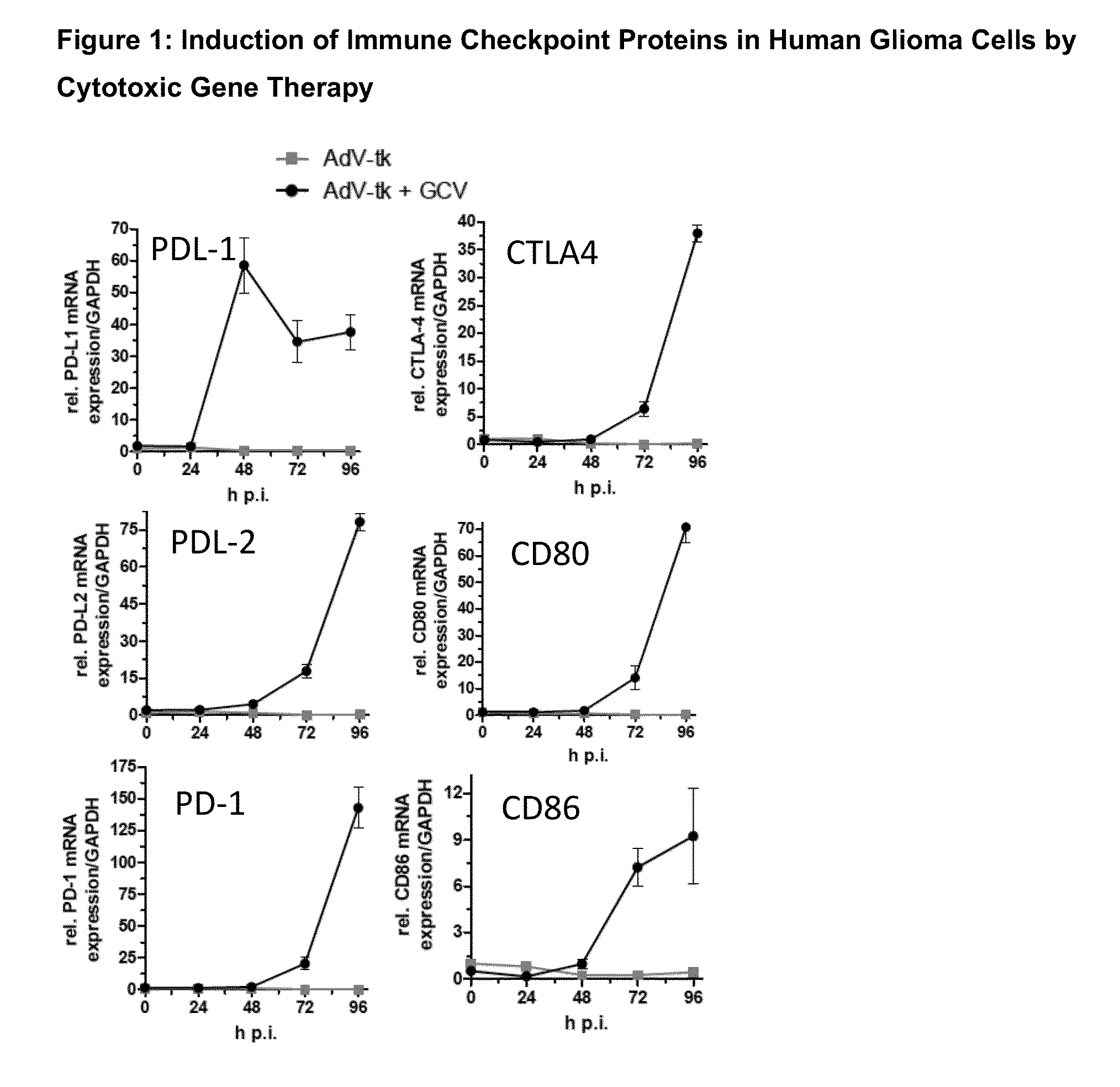
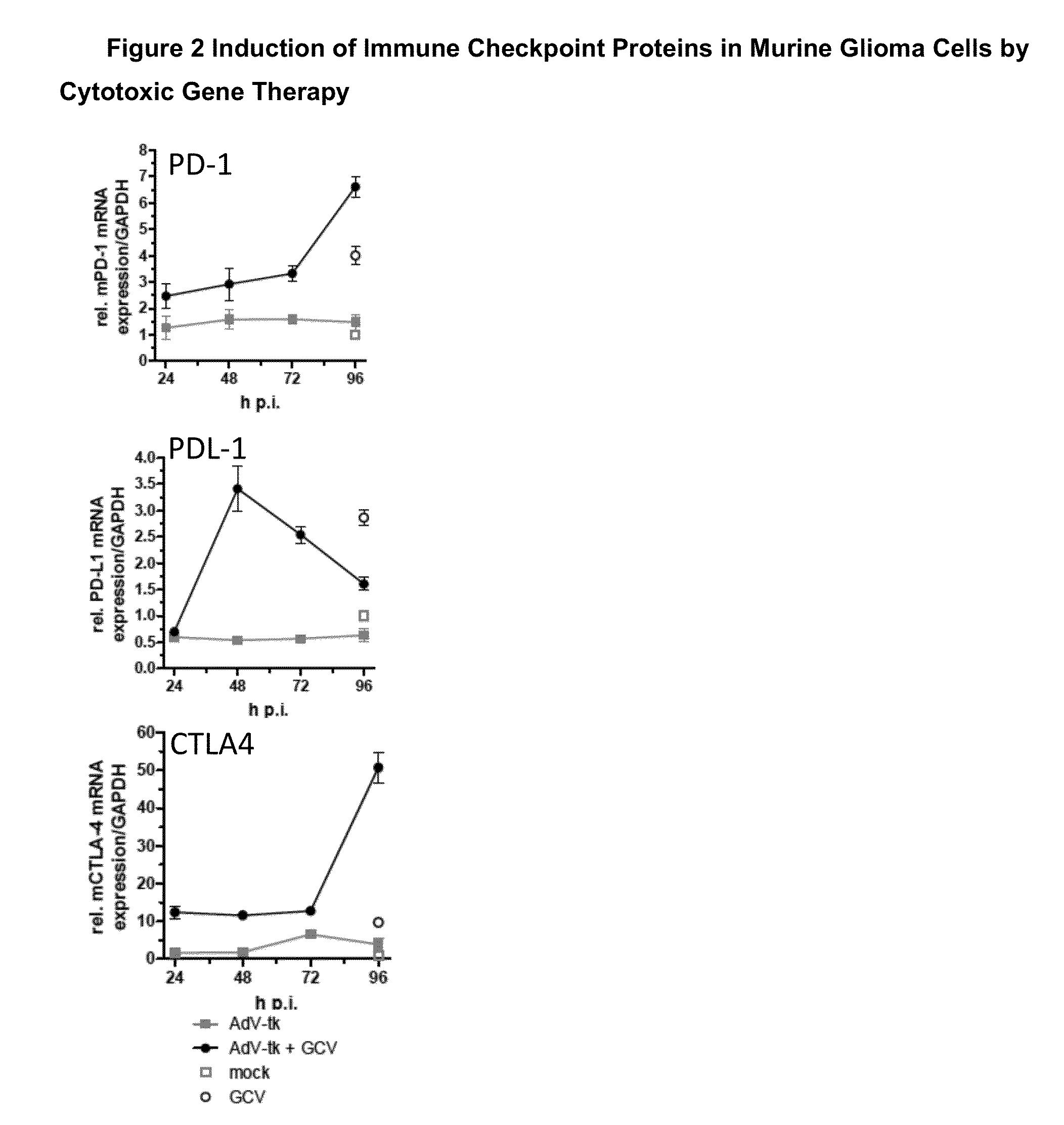
Methods of Cytotoxic Gene Therapy To Treat Tumors
A method is disclosed for decreasing or retarding an increase in the size of a localized or metastatic tumor by using a combination of an immune stimulating cytotoxic gene therapy and immune-checkpoint modulating agent, in conjunction with other therapies, including radiation therapy, chemotherapy, surgery, and immunotherapies.
Owner:CANDEL THERAPEUTICS INC
Oncolytic virus for expression of immune checkpoint modulators
ActiveUS20170157188A1High activityEasy to identifyHydrolasesPharmaceutical delivery mechanismNucleotideOncolytic adenovirus
The present invention provides an oncolytic virus comprising nucleotide sequence(s) encoding one or more immune checkpoint modulator(s). It also concerns a pharmaceutical composition comprising effective amount of said oncolytic virus and, eventually, a pharmaceutically acceptable vehicle and its use for treating proliferative diseases such as cancers.
Owner:TRANSGENE SA
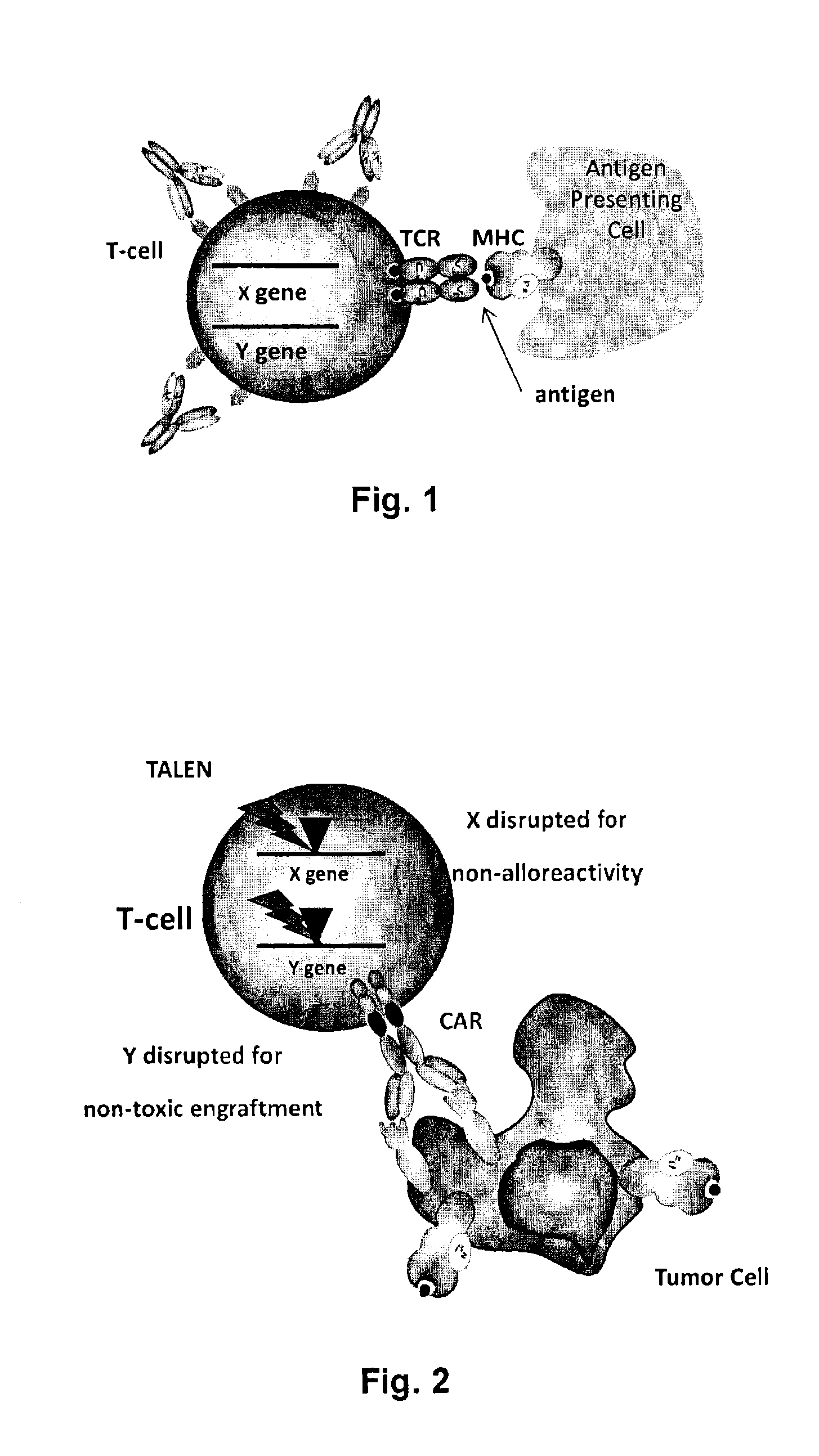
Methods for engineering allogeneic and highly active t cell for immunotherapy
InactiveUS20150017136A1Precise positioningPeptide/protein ingredientsAntibody mimetics/scaffoldsPrimary cellNuclease
The present invention relates to methods for developing engineered T-cells for immunotherapy that are non-alloreactive. The present invention relates to methods for modifying T-cells by inactivating both genes encoding T-cell receptor and an immune checkpoint gene to unleash the potential of the immune response. This method involves the use of specific rare cutting endonucleases, in particular TALE-nucleases (TAL effector endonuclease) and polynucleotides encoding such polypeptides, to precisely target a selection of key genes in T-cells, which are available from donors or from culture of primary cells. The invention opens the way to standard and affordable adoptive immunotherapy strategies for treating cancer and viral infections.
Owner:CELLECTIS SA
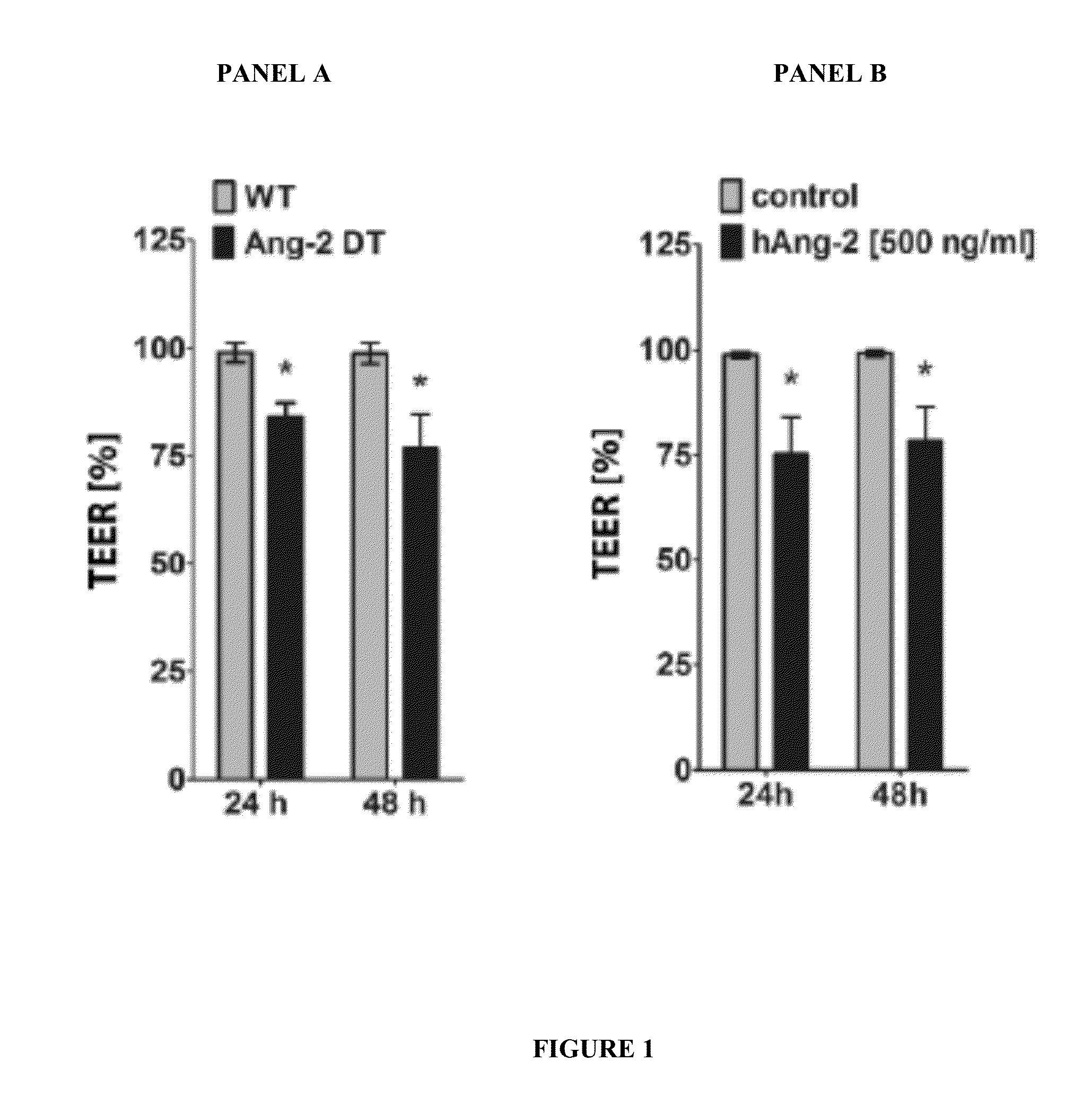
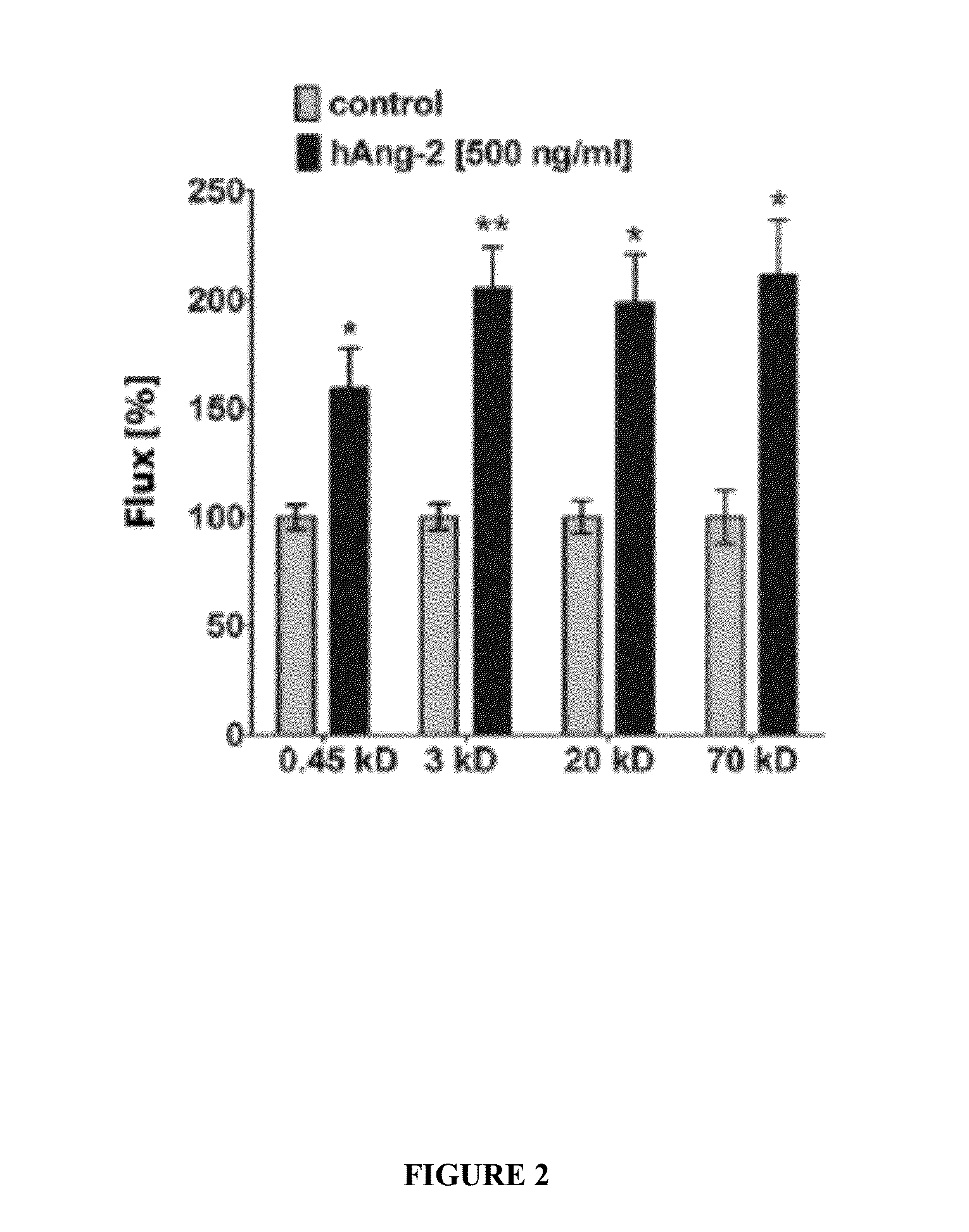
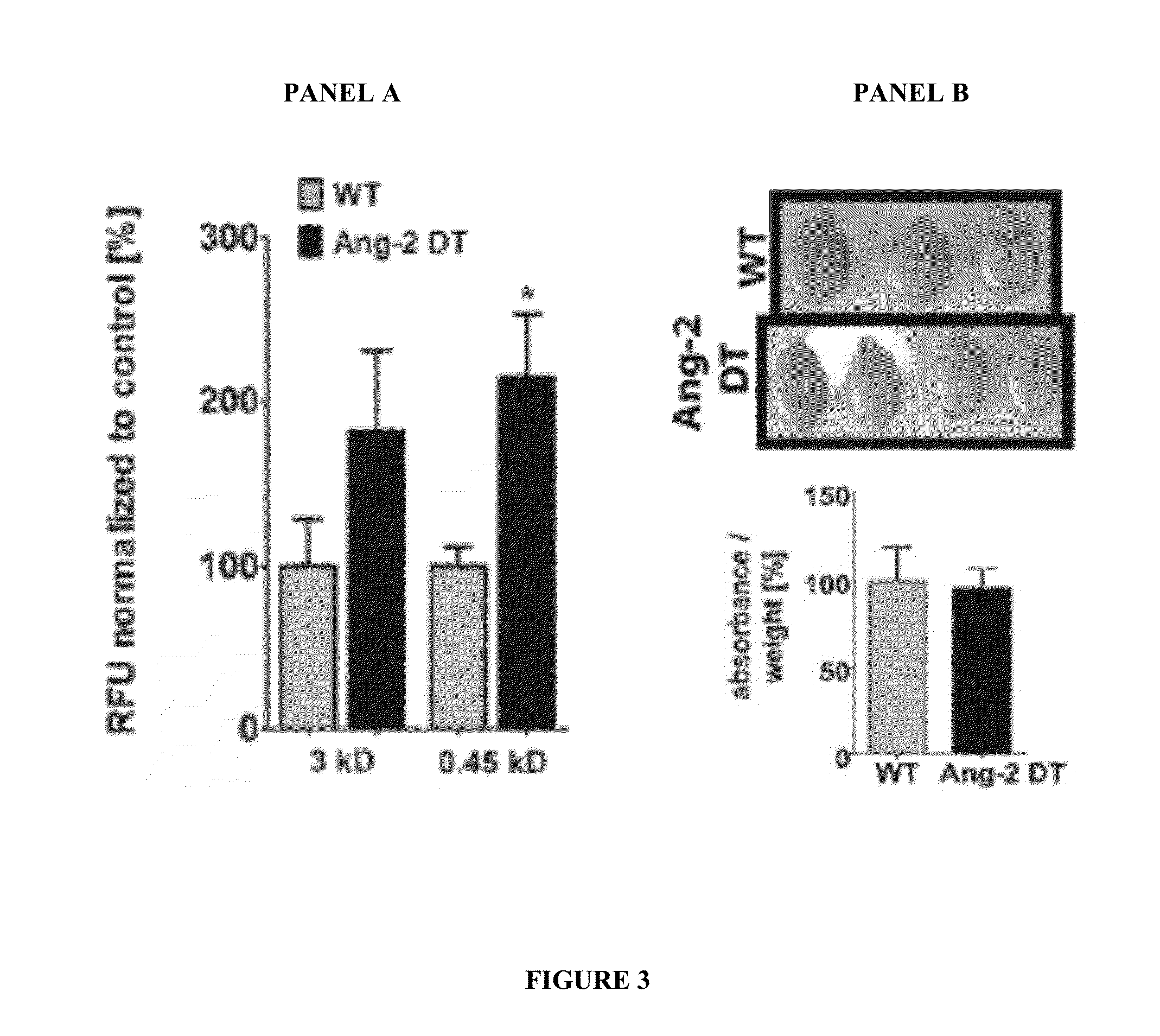
Combination of immunotherapies with activators of Tie-2
The present invention relates to combination therapies comprising at least one activator of Tie-2 and immunotherapies that target immune checkpoints. Combination therapies of the disclosure can provide therapeutic effects not obtained with singly-administered immunotherapies. Combination therapies can be used to increase the therapeutic efficacy of an immunotherapy, to provide lower dosages of an immunotherapy being administered, to lower a toxicity of an immunotherapy, or to manage a side effect of an immunotherapy.
Owner:EYEPOINT PHARMA INC
Combination of oncolytic virus with immune checkpoint modulators
ActiveUS20170143780A1Antagonize activityEasy to getPeptide/protein ingredientsPharmaceutical delivery mechanismOncolytic adenovirusVirology
The present invention provides a combination comprising at least an oncolytic virus and one or more immune checkpoint modulator(s) for use for the treatment of a proliferative disease such as cancer. It also relates to a kit comprising said oncolytic virus and said one or more immune checkpoint modulator(s) in separate containers. It also concerns a pharmaceutical composition comprising effective amount of said oncolytic virus and said one or more immune checkpoint modulator(s).
Owner:INSTITUT GUSTAVE ROUSSY +1
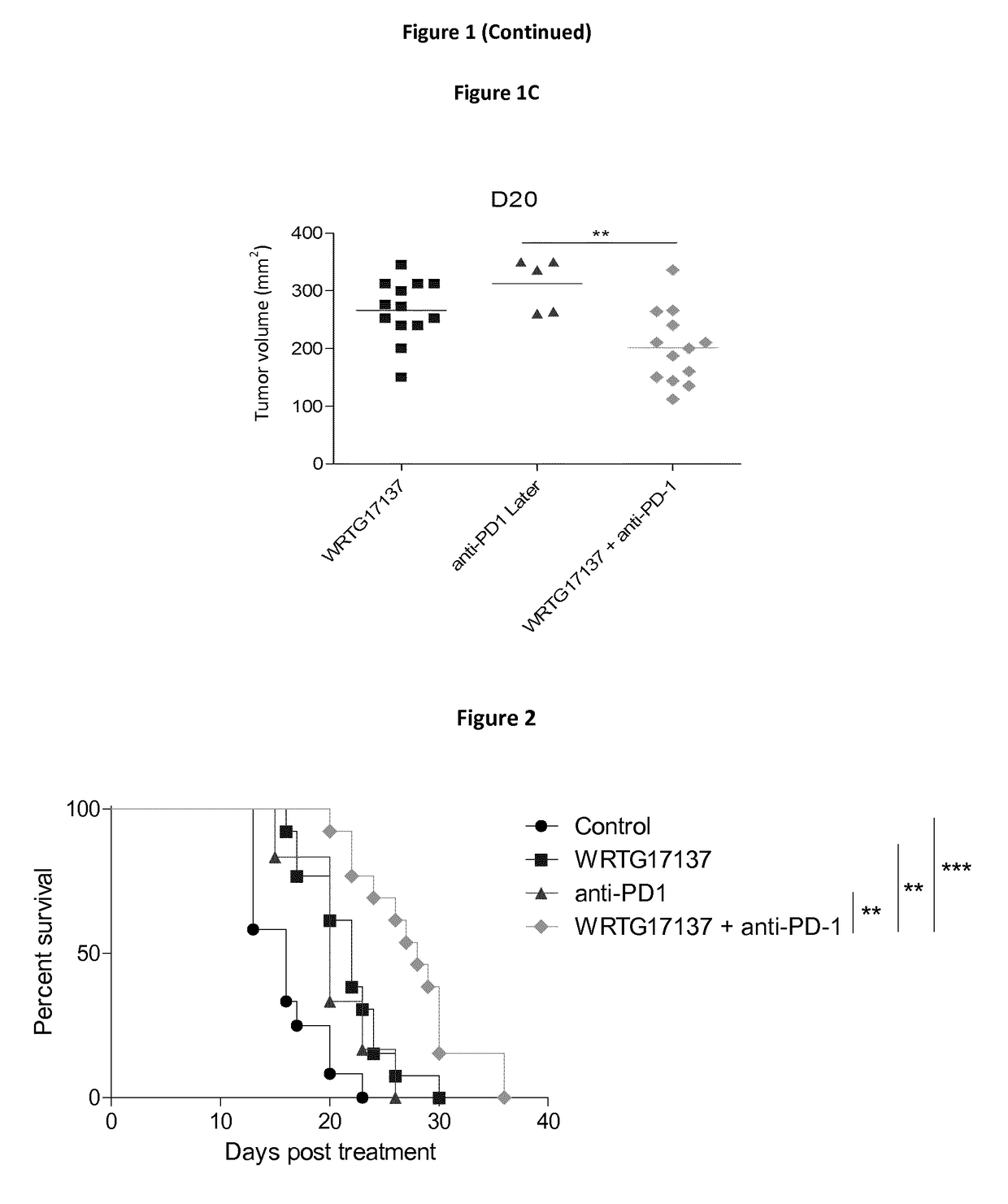
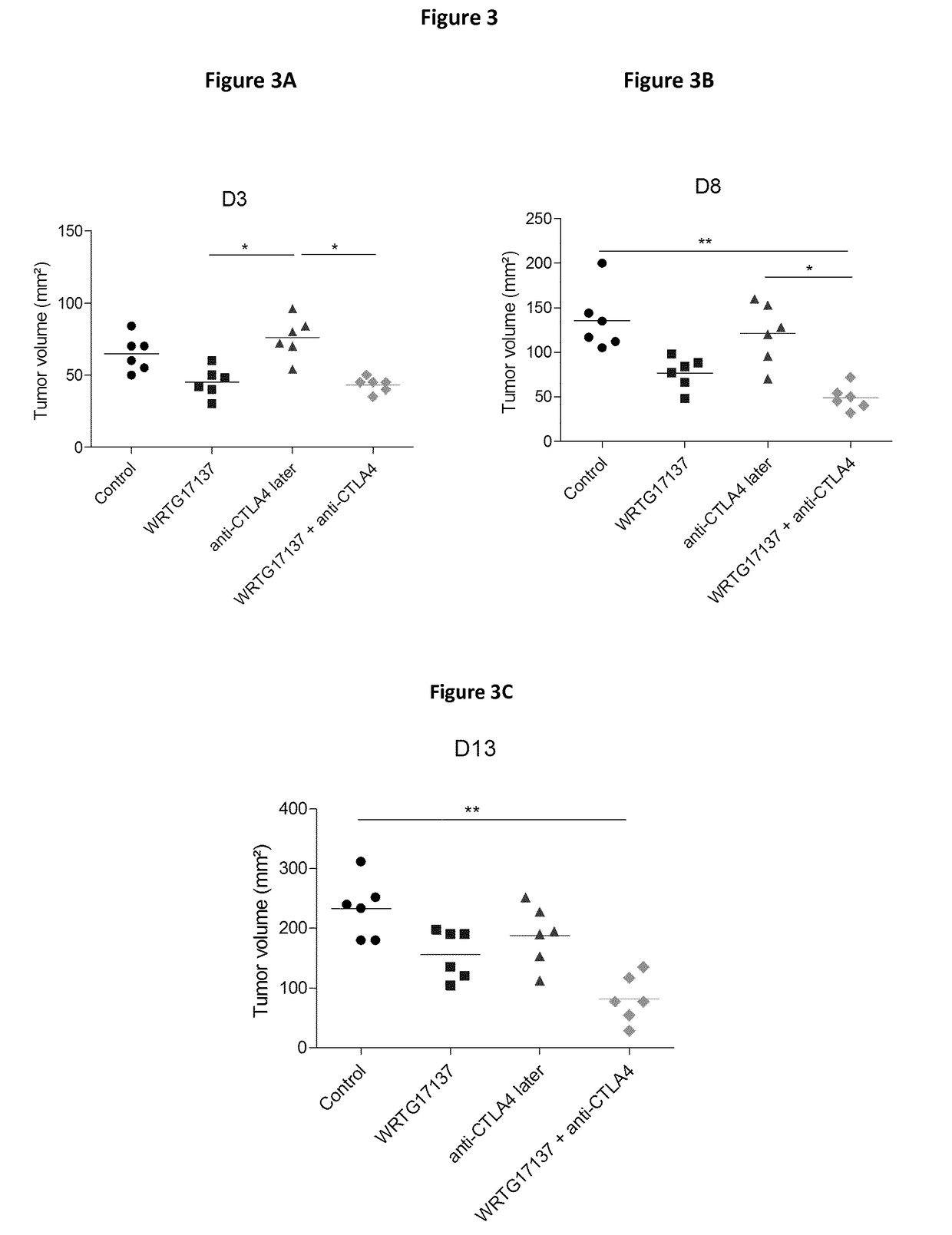
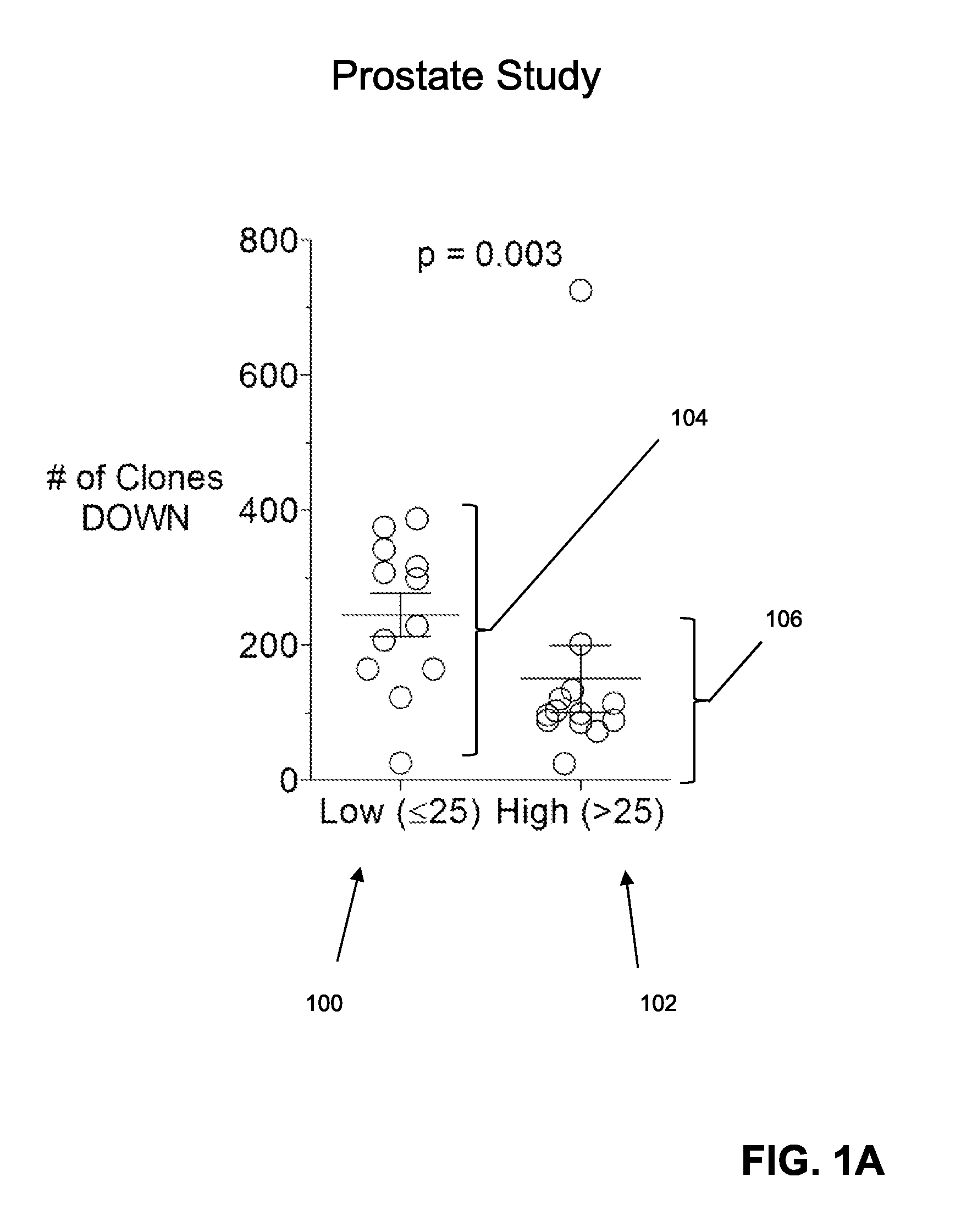
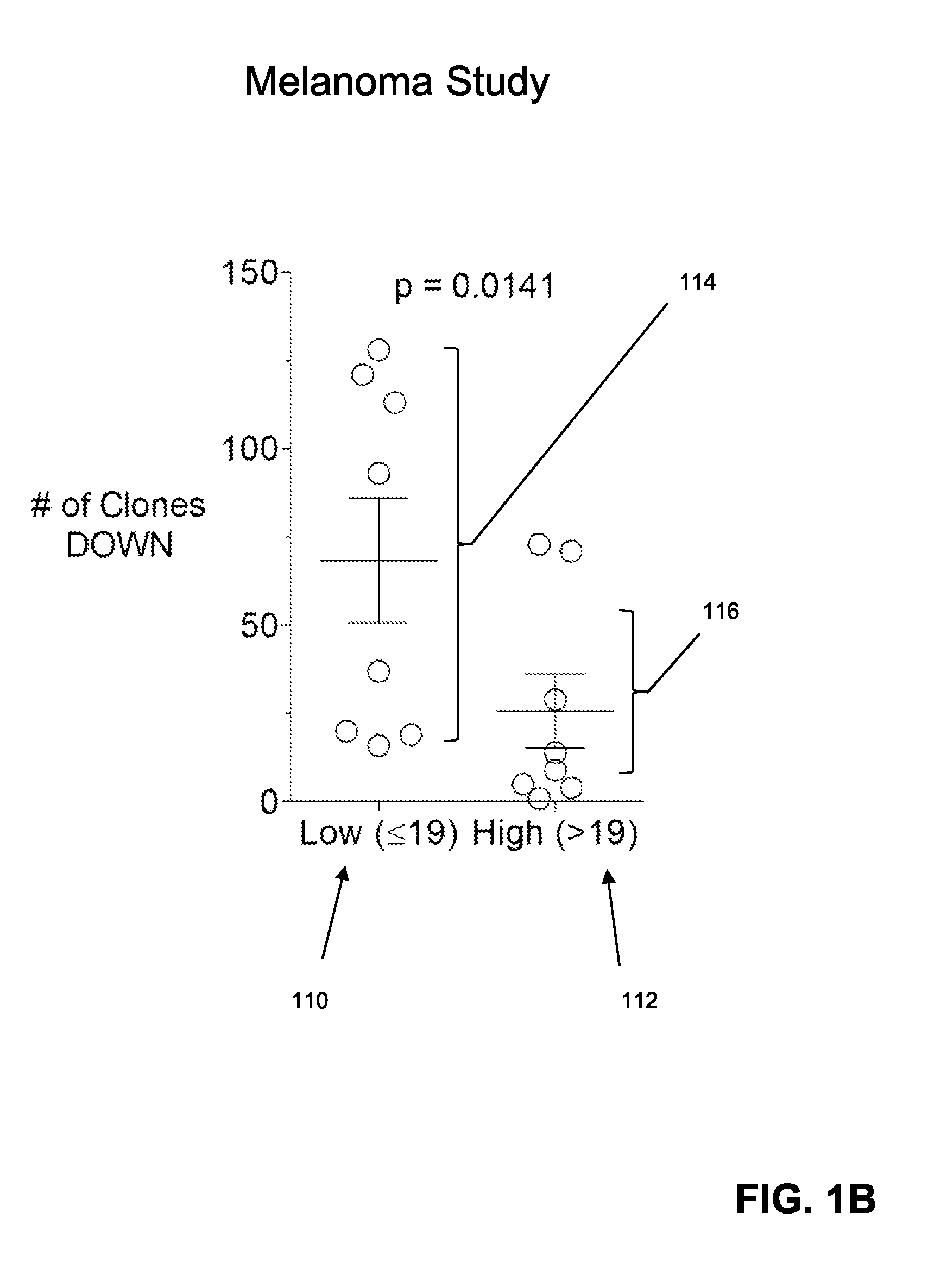
Predicting patient responsiveness to immune checkpoint inhibitors
The invention is directed to a method of predicting clinical response of a patient to treatment of a cancer by an immune checkpoint pathway inhibitor, such as an anti-CTLA-4 or anti-PD-1 antibody binding compound. In one aspect the method comprises generating pre- and post-treatment clonotype profiles, determining a number of clonotypes that decrease in frequency between the first and second clonotype profiles, and predicting a lack of responsiveness in the patient to the treatment whenever the number of clonotypes that decrease in frequency is greater than a predetermined value.
Owner:ADAPTIVE BIOTECH +1
Method for assessing a prognosis and predicting the response of patients with malignant diseases to immunotherapy
The invention relates to, among others, a method for assessing a prognosis of a patient with a malignant disease and / or for predicting the response of a patient with a malignant disease to immunotherapy. For this purpose, a DNA methylation analysis is carried out on at least one immunoregulatory gene of cells of the malignant disease and / or T lymphocytes which interact with the cells of the malignant disease, said gene coding for an immune checkpoint selected from B7 proteins and the receptors thereof, MHC-peptide complex-binding co-receptors, the members of the tumor necrosis factor receptorsuperfamily TNFRSF9, CD40, TNFRSF4, TNFRSF18, and CD27, the members of the immunoglobulin superfamily TIGIT, BTLA, HAVCR2, BTNL2, and CD48, and the andenosine-binding adenosine 2A receptor.
Owner:迪莫迪特里希
Combination of Immunotherapies with Activators of Tie-2
ActiveUS20160038467A1Organic active ingredientsPeptide/protein ingredientsTreatment effectSide effect
The present invention relates to combination therapies comprising at least one activator of Tie-2 and immunotherapies that target immune checkpoints. Combination therapies of the disclosure can provide therapeutic effects not obtained with singly-administered immunotherapies. Combination therapies can be used to increase the therapeutic efficacy of an immunotherapy, to provide lower dosages of an immunotherapy being administered, to lower a toxicity of an immunotherapy, or to manage a side effect of an immunotherapy.
Owner:EYEPOINT PHARMA INC
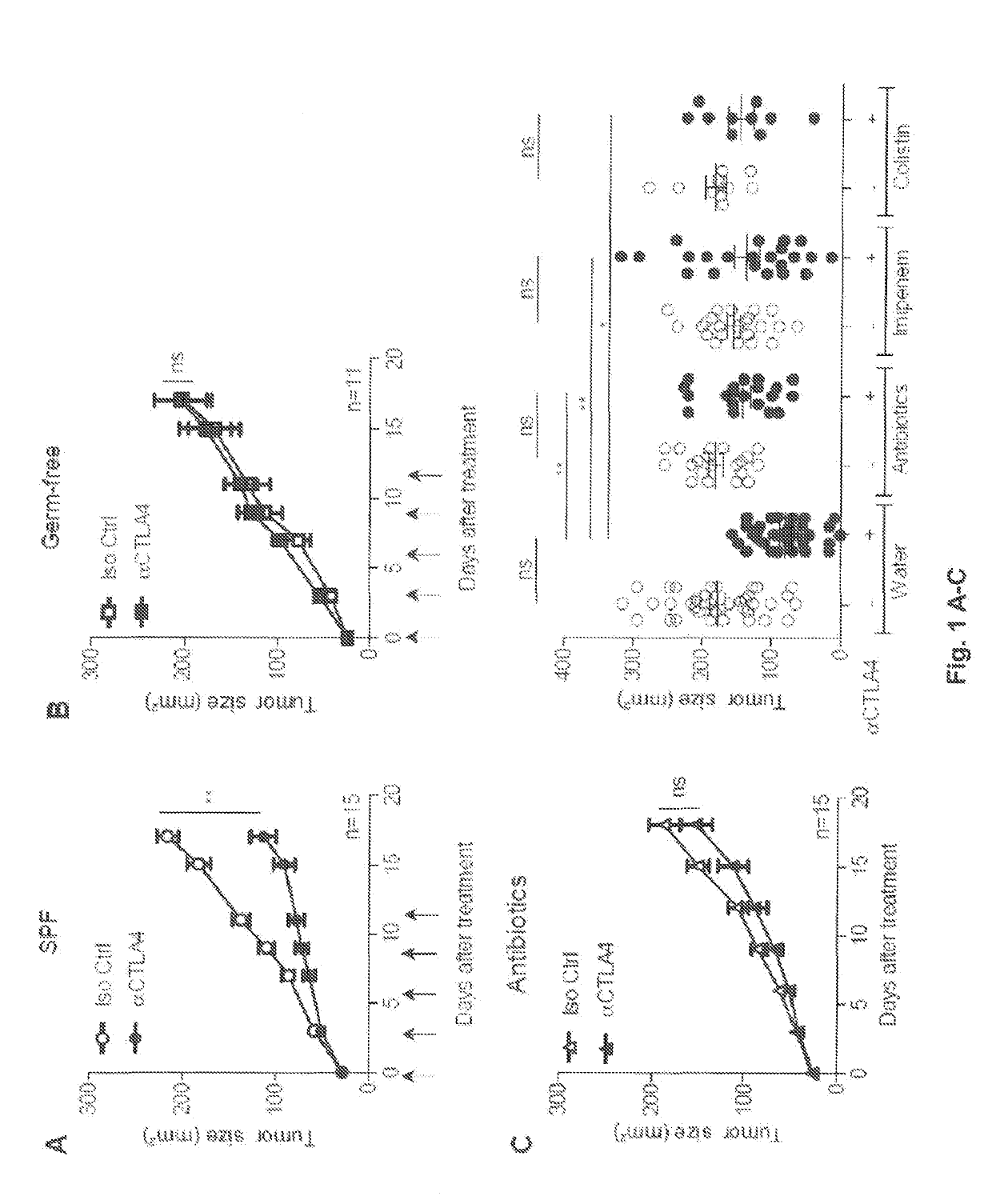
Methods and products for modulating microbiota composition for improving the efficacy of a cancer treatment with an immune checkpoint blocker
PendingUS20190282632A1Pharmaceutical delivery mechanismImmunoglobulins against cell receptors/antigens/surface-determinantsFecesIntestinal microorganisms
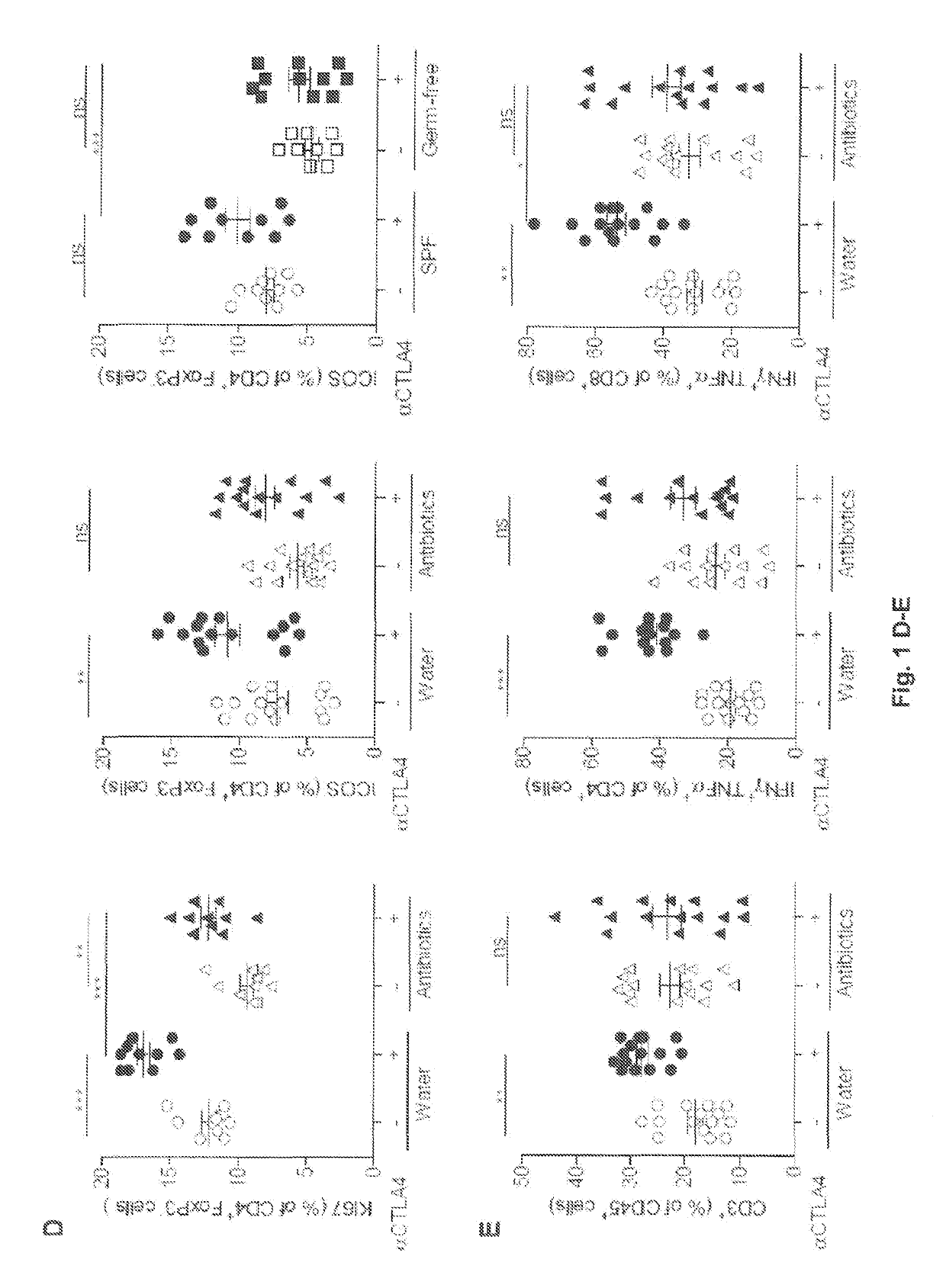
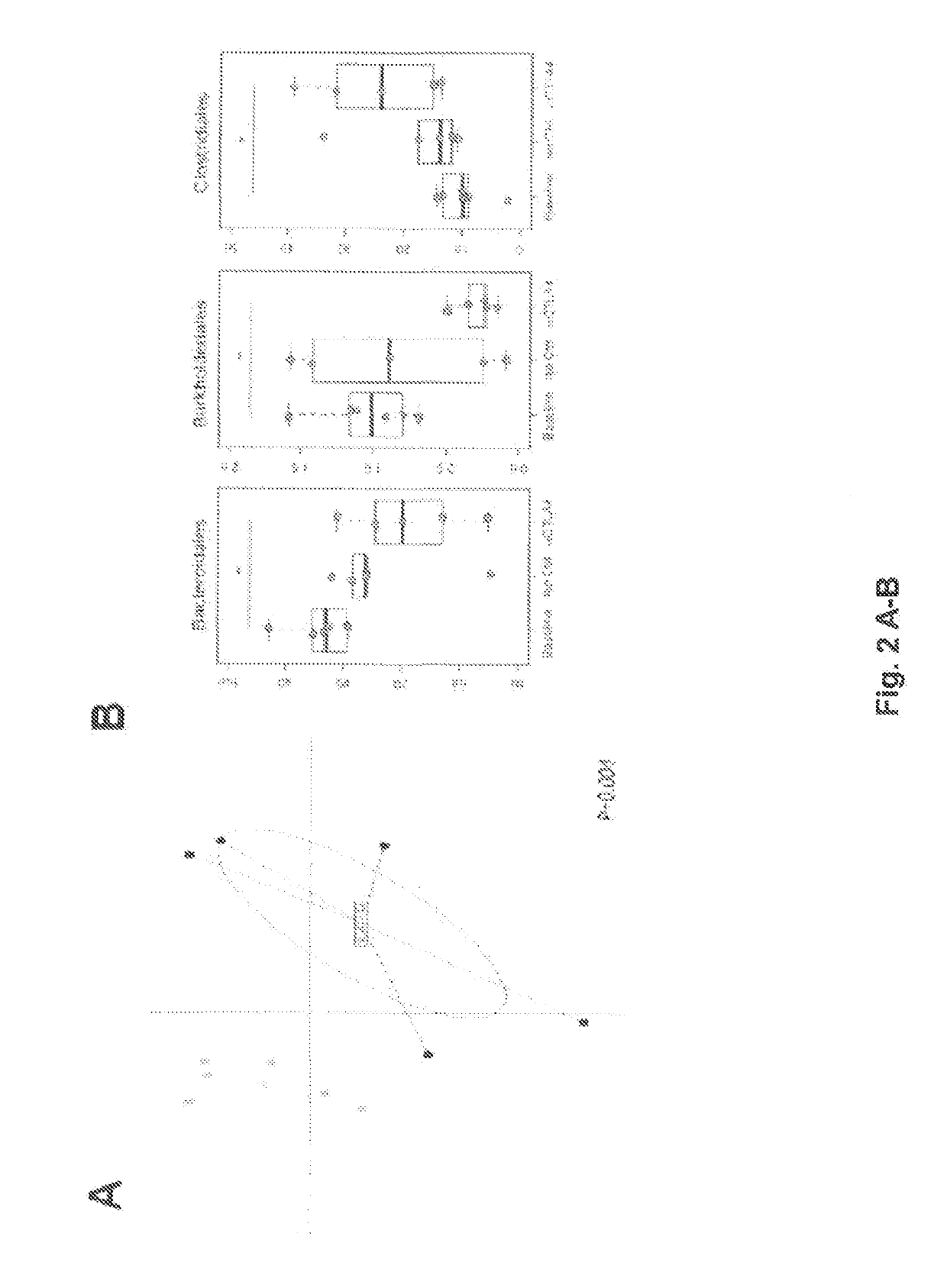
The present invention relates to the role of the microbiota in the efficacy of cancer treatments with a drug blocking an. immune checkpoint and provides methods and probiotics to improve the efficacy of such a treatment in patients in need thereof. More particularly, the invention pertains to the use of vancomycin or penicillin to modulate the gut microbiota to potentiate the anticancer effects of anti-CTLA4 molecules. B. fragilis or fecal microbial transplantation of a defined composition enriched in immunogenic Bacteroides spp. can also be used as a probiotic to that aim.
Owner:INSTITUT GUSTAVE ROUSSY +3
Chemical modulators of immune checkpoints and therapeutic use
Compounds and pharmaceutical compositions that down-regulate immune checkpoints such as PD-1, PD-L1 and CTLA-4 are provided. Also provided are methods of treating a disease by down-regulating immune checkpoints such as PD-1, PD-L1 and CTLA-4. The methods are useful for treating cancer and viral infection in a subject.
Owner:DUKE UNIV
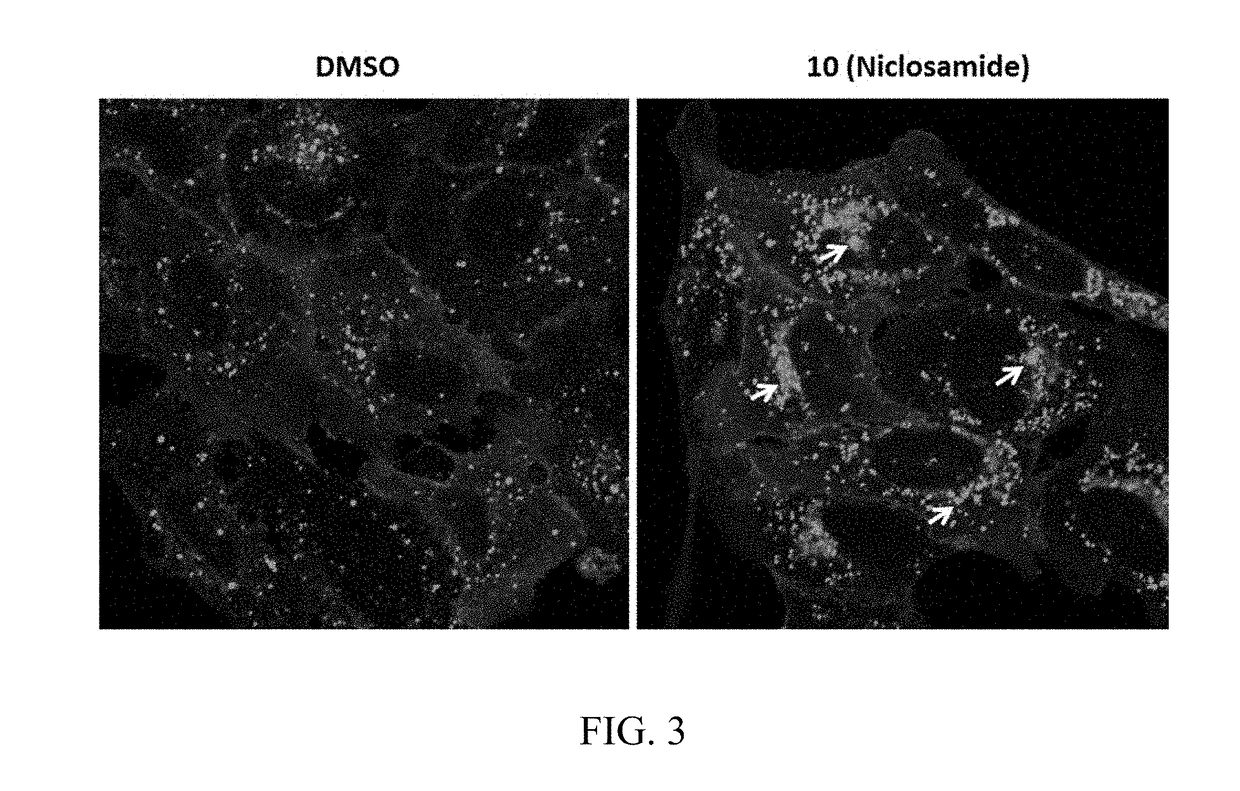
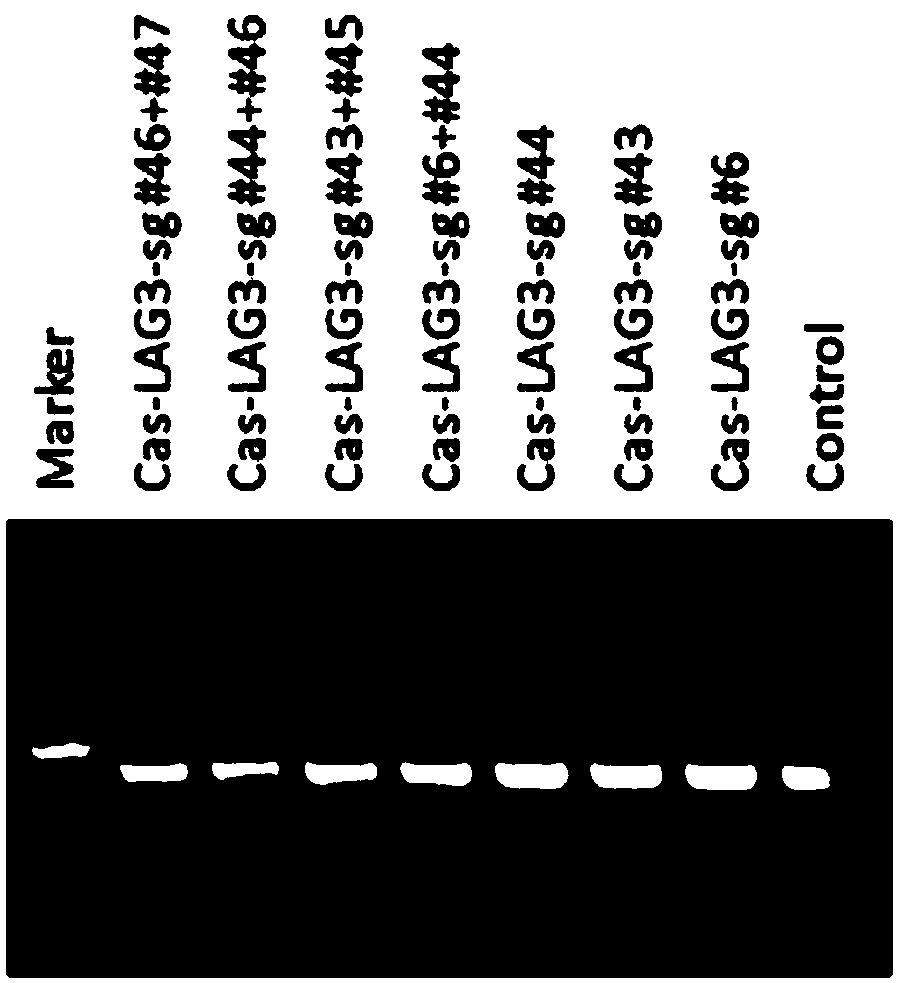
SgRNAs specifically targeting to LAG-3 gene and method for specifically knocking out LAG-3 gene
ActiveCN107746845ARapid knockoutSuppress lung cancerImmunoglobulin superfamilyVectorsHuman cellT cell
Based on a CRISPR system, the invention provides sgRNAs specifically targeting to a human LAG-3 gene and a method for specifically knocking out the LAG-3 gene in human cells. The sgRNAs provided by the invention can accurately and effectively target to the human LAG-3 gene and precisely knock out the gene, and has excellent characteristics of high efficiency, short period and low cost. The methodcan be used for preparing human T cells having LAG-3 immune checkpoints knocked out, and then the human T cells are used in for tumor immunotherapy.
Owner:BEIJING MICRO HELIX GENE TECH
Oncolytic virus construction body, oncolytic virus and application thereof
InactiveCN108728488APrevent proliferationSignificant effectAntibody mimetics/scaffoldsUnknown materialsOncolytic adenovirusTumor specific
The invention provides an oncolytic virus construction body, an oncolytic virus and application thereof. The oncolytic virus construction body comprises a first nucleic acid molecule and a second nucleic acid molecule, wherein the first nucleic acid molecule encodes a secreted fusion protein molecule; the secreted fusion protein molecule is used for inhibiting immune checkpoints; and the second nucleic acid molecule encodes an immunostimulant molecule. According to the embodiment of the invention, the construction body encodes fusion protein and immunostimulant molecules for inhibiting the immune checkpoints and can inhibit immunosuppression mechanism mediated by the immune checkpoints and immunoreactions which causes individual tumor specificity so as to realize generalized and effectivetumor cell killing.
Owner:LIFESEQ LTDRP
Compositions and methods for treating cancer
Use of a CXCR4 antagonistic peptide and an immune-check point regulator in the treatment of cancer is provided. Accordingly there is provided a method of treating cancer in a subject in need thereof, the method comprising administering to the subject a therapeutically effective amount of a peptide having an amino acid sequence as set forth in SEQ ID NO: 1 or an analog or derivative thereof; and a therapeutically effective amount of a PD1 antagonist, a PDL-1 antagonist, a CTLA-4 antagonist, a LAG-3 antagonist, a TIM-3 antagonist, a KIR antagonist, an IDO antagonist, an OX40 agonist, a CD137 agonist, a CD27 agonist, a CD40 agonist, a GITR agonist, a CD28 agonist or an ICOS agonist, thereby treating the cancer in the subject. Also provided are pharmaceutical compositions and articles of manufacture.
Owner:BIOKINE THERAPEUTICS LTD +1
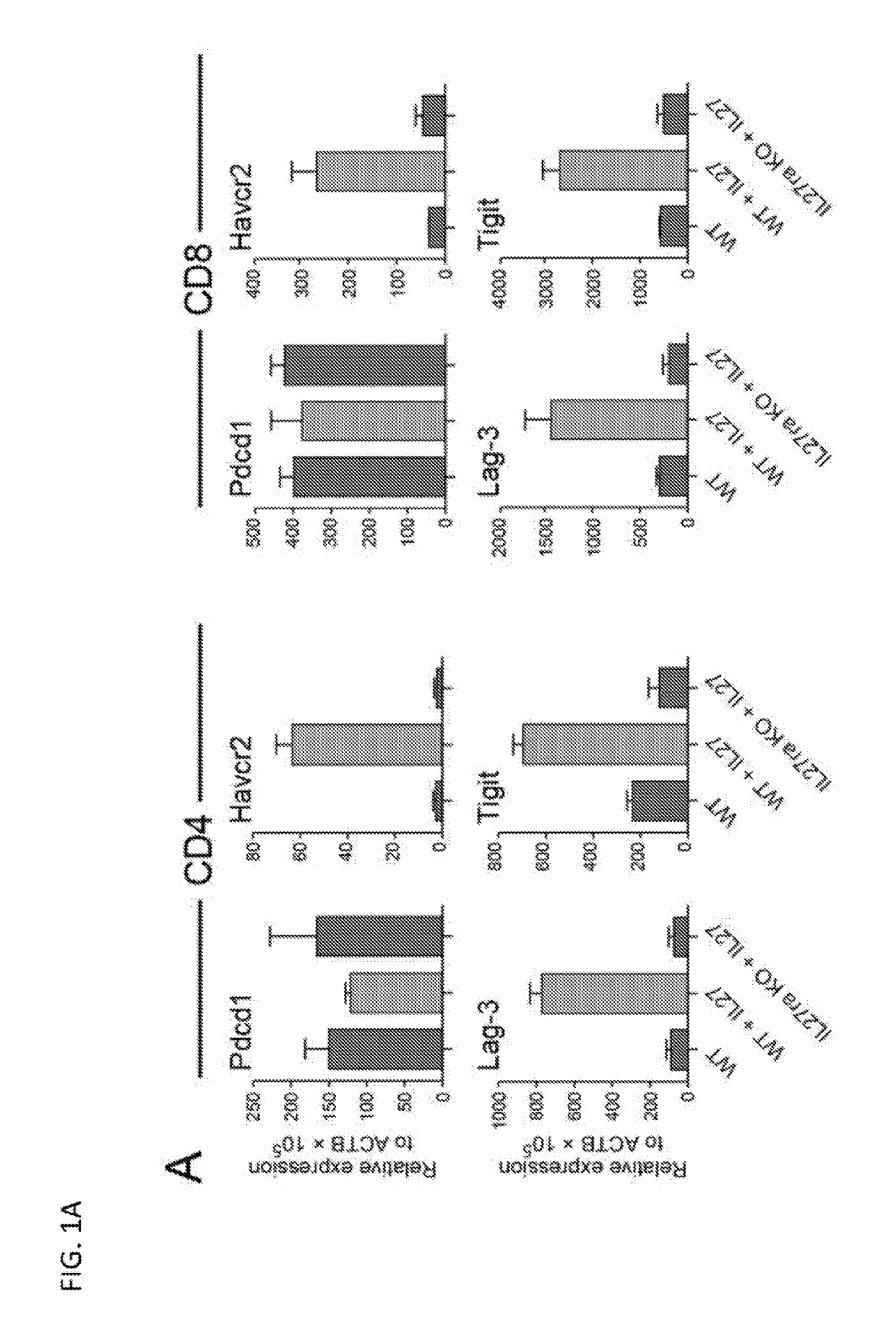
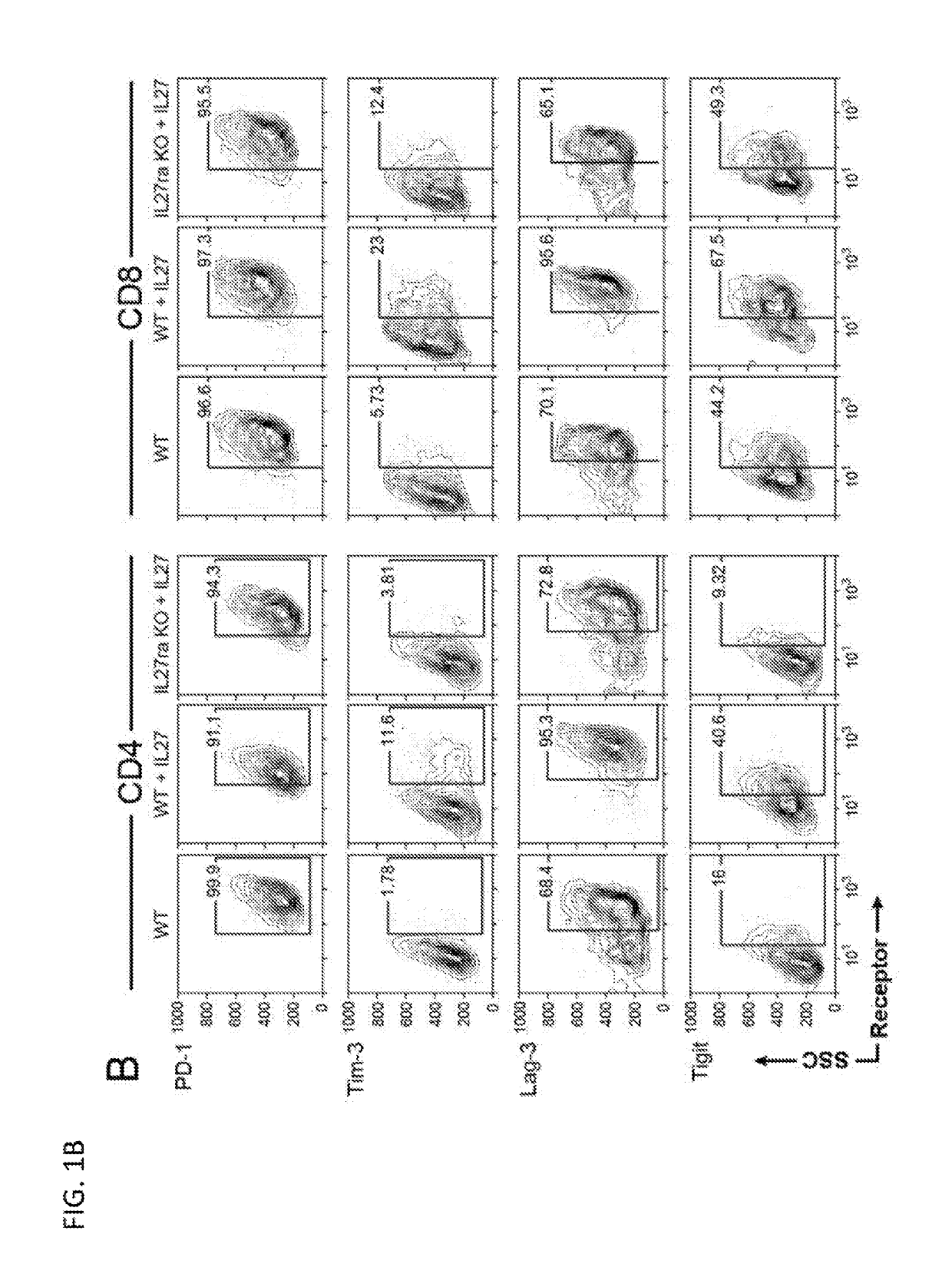
Modulation of novel immune checkpoint targets
PendingUS20190255107A1Decrease in exhausted T cell phenotypeIncrease T cell activationAntibacterial agentsCell receptors/surface-antigens/surface-determinantsDiseaseClinical efficacy
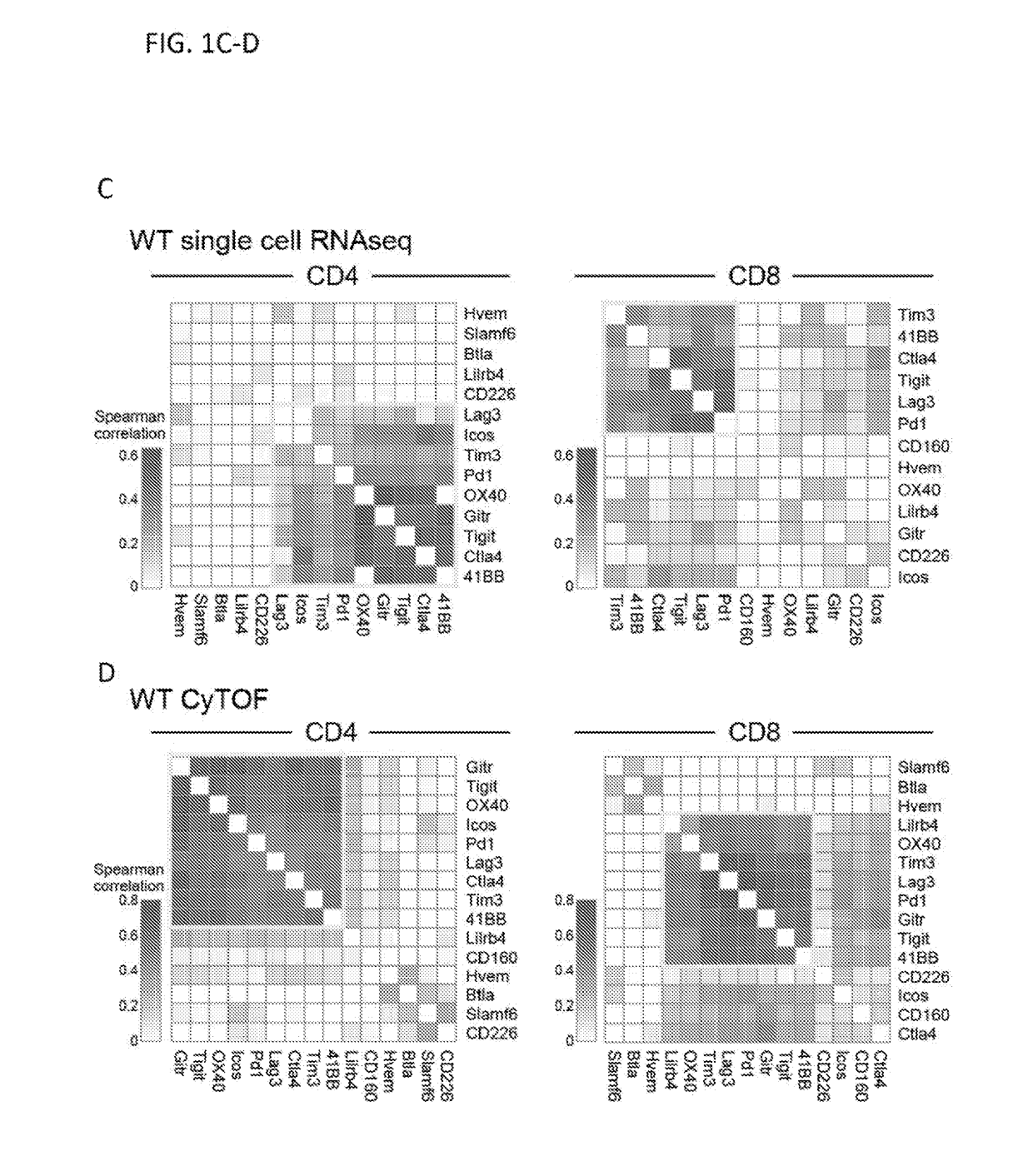
Dysfunctional or exhausted T cells arise in chronic diseases including chronic viral infections and cancer, and express high levels of co-inhibitory receptors. Therapeutic blockade of these receptors has clinical efficacy in the treatment of cancer. While co-inhibitory receptors are co-expressed, the triggers that induce them and the transcriptional regulators that drive their co-expression have not been identified. The immunoregulatory cytokine IL-27 induces a gene module in T cells that includes several known co-inhibitory receptors (Tim-3, Lag-3, and TIGIT). The present invention provides a novel immunoregulatory network as well as novel cell surface molecules that have an inhibitory function in the tumor microenvironment. The present invention further provides the novel discovery that the transcription factors Prdm1 and c-Maf cooperatively regulate the expression of the co-inhibitory receptor module. This critical molecular circuit underlies the co-expression of co-inhibitory receptors in dysfunctional T cells and identifies novel regulators of T cell dysfunction.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC +2
Novel replicating oncolytic adenovirus capable of simultaneously inhibiting immune checkpoints of PD-L1 (programmed cell death ligand 1) and TIGIT (T cell immunoreceptor with Ig (immune globulin) and ITIM (immunoreceptor tyrosine-based inhibition motif) domain) and application thereof
ActiveCN110128550ASignificant activation of anti-tumor immunityGrowth inhibitionPeptide/protein ingredientsAntibody mimetics/scaffoldsImmune checkpointAnti-Tumor Drugs
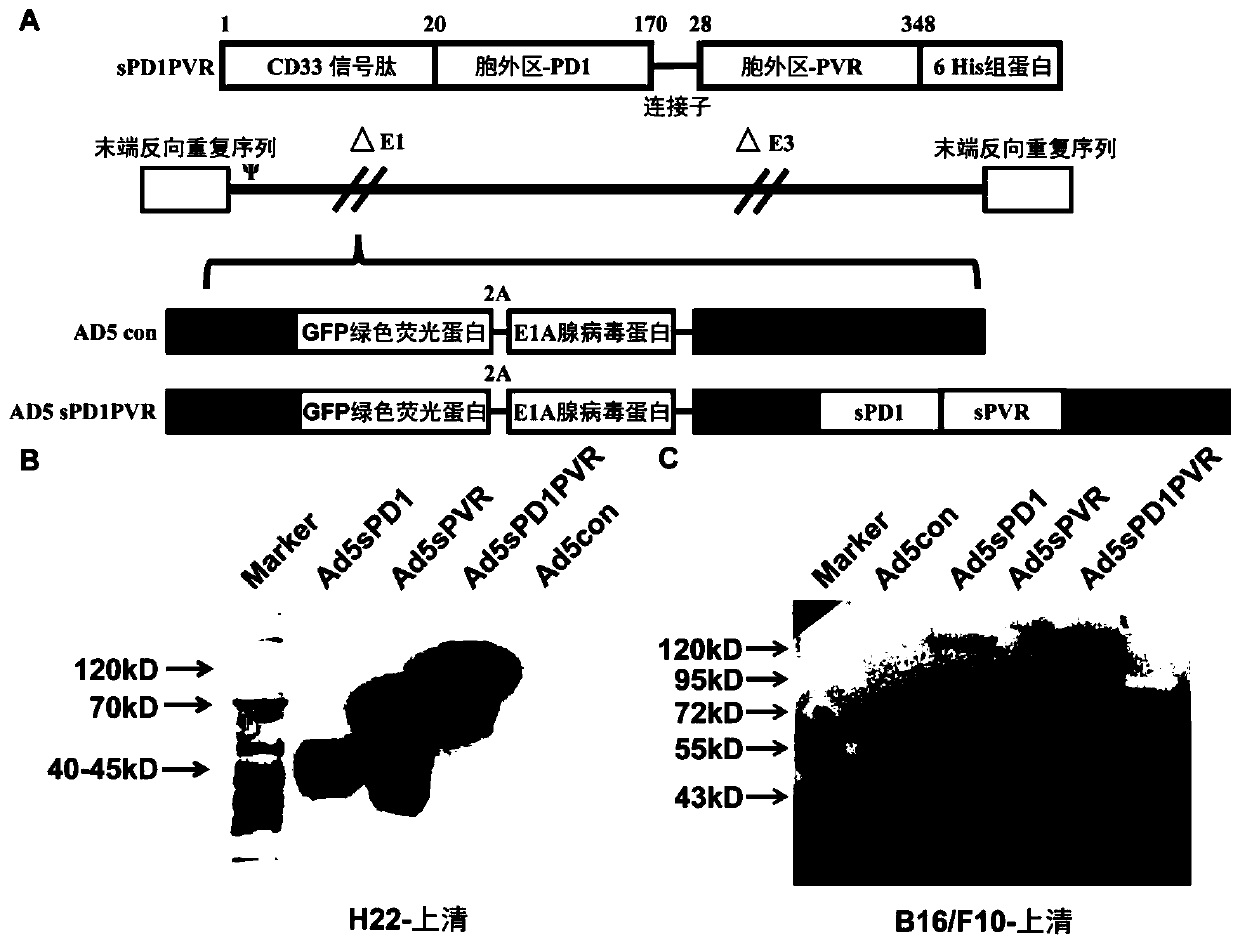
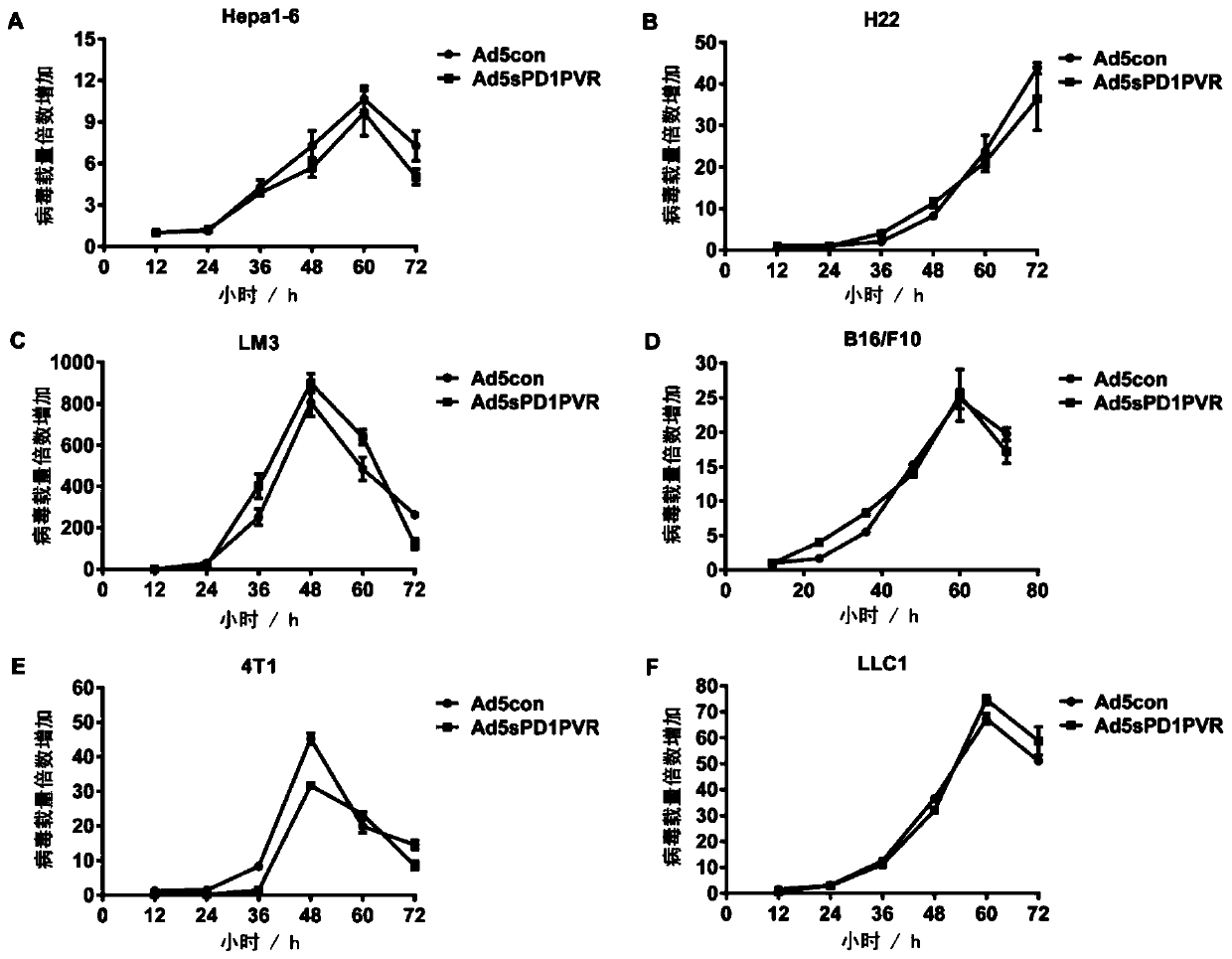
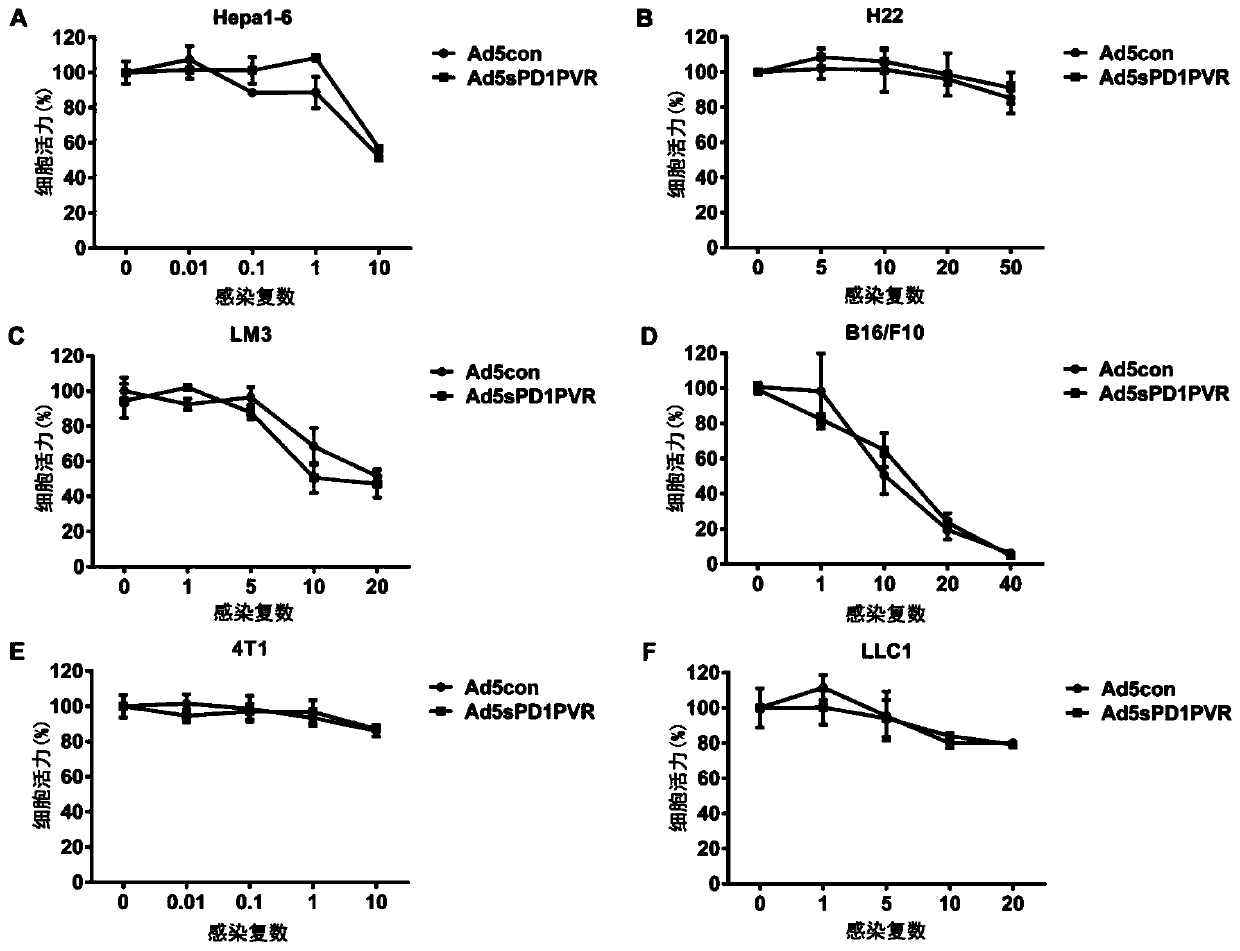
The invention discloses a novel replicating oncolytic adenovirus capable of simultaneously inhibiting immune checkpoints of PD-L1 and TIGIT and application thereof. According to the novel replicatingoncolytic adenovirus capable of simultaneously inhibiting immune checkpoints of PD-L1 and TIGIT, a soluble fusion protein capable of simultaneously inhibiting immune checkpoints of PD-L1 and TIGIT andactivating immune costimulatory pathways is characterized in that both ends of the soluble fusion protein are a PD1 (programmed cell death protein 1) extracellular region for binding PD-L1 and a PVR(poliovirus receptor) extracellular region for binding TIGIT respectively, PD1 fragments and PVR fragments are connected through a linker sequence, and both ends of the soluble fusion protein can simultaneously inhibiting the immune checkpoints of PD-L1 and TIGIT after binding PD-L1 and TIGIT respectively. Experiments show that the novel replicating oncolytic adenovirus (ad5sPD1PVR) can significantly inhibit the immune checkpoints and activate antitumor immunity effects, achieve significant antitumor activity and accordingly have a good prospect and high values for developing antitumor drugs.
Owner:NANJING VIROTHER BIOPHARMACEUTICAL CO LTD
CAR-T cell capable of efficiently and stably expressing inhibiting antibody and application thereof
PendingCN107523547AAntibacterial agentsGenetically modified cellsCAR T-cell therapyAntigen receptors
The invention provides a CAR-T cell capable of efficiently and stably expressing an inhibiting antibody and application thereof. Specifically, the invention provides a transgenic T cell, and the genome of the transgenic T cell is stably integrated with expression cassettes containing nucleic acid sequences coding chimeric antigen receptors and immune checkpoint inhibiting antibodies, wherein two ends of the expression cassettes contain inverted terminal repeats of transposons. In the genome of the pluripotent CAR-T cell, the expression cassettes of the inhibiting antibodies are stably integrated via a transposon system, so the CAR-T cell has the activity of stably and efficiently expressing the inhibiting antibodies on the premise that original killing activity of the T cell is maintained, transfer-back CAR-T cells are prevented from inhibition by a tumor microenvironment, and residual tumor-specific T cells can be activated in situ. Meanwhile, a molecular brake system is introduced to ensure the security of CAR-T cell therapy, and transfer-back pluripotent CAR-T cells can be timely removed if necessary.
Owner:SHANGHAI CELL THERAPY RES INST +1
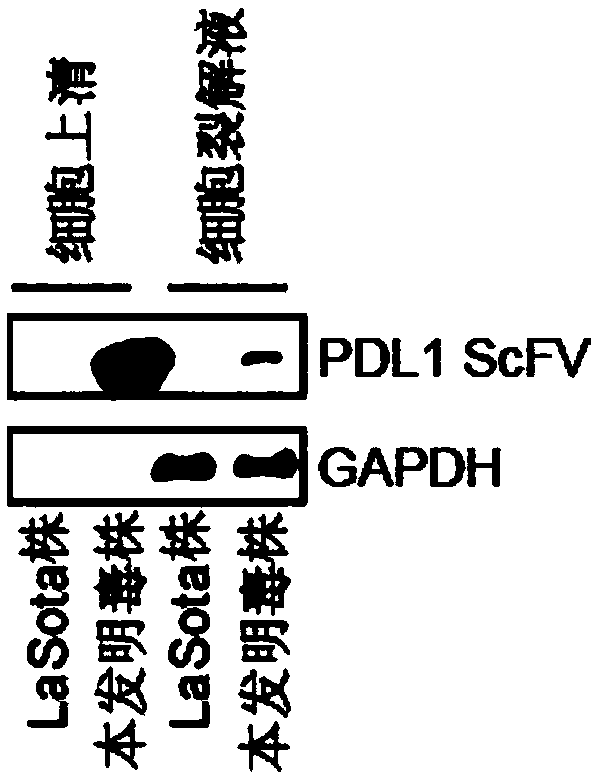
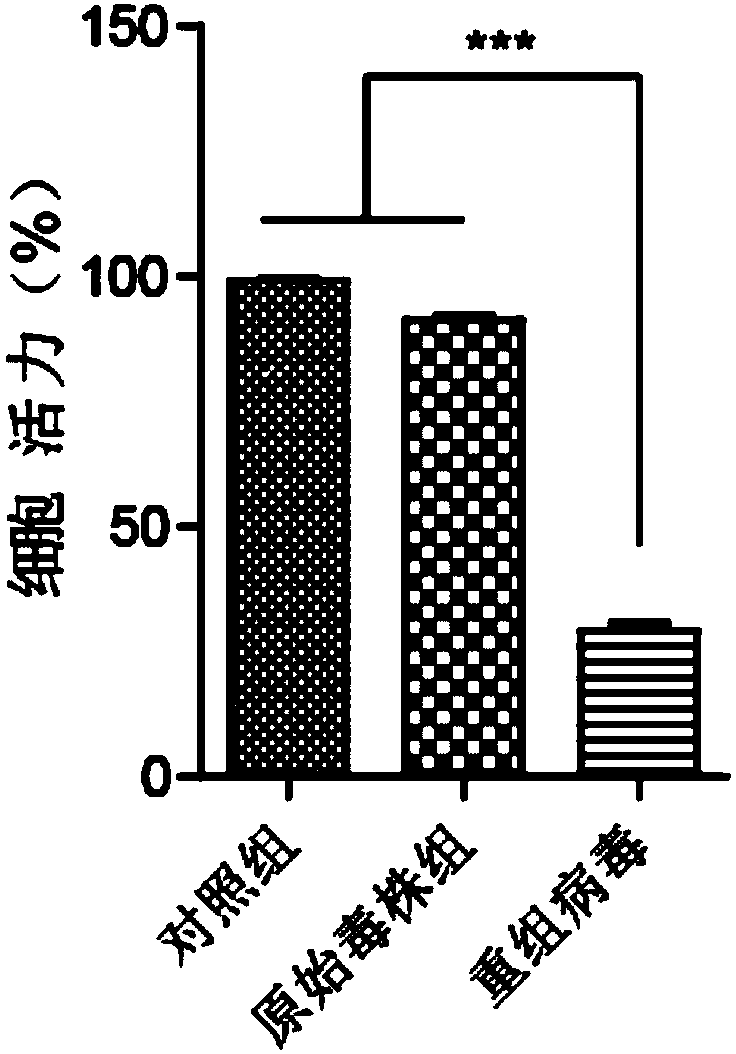
Preparation method and application of newcastle disease oncolytic virus expressing PD-L1 single chain antibody
InactiveCN109627336AOncolytic abilityFunctionalSsRNA viruses negative-senseStable introduction of DNASingle-Chain AntibodiesF protein
The invention discloses a preparation method and application of a newcastle disease oncolytic virus expressing PD-L1 single chain antibody and an application thereof. The amino acid residues at sits of 112-117 of F protein of mutant recombinant LaSota strain newcastle disease virus is mutated into 112-R-R-Q-R-F-117, so that the newcastle disease oncolytic virus has independent infection capability; a PD-L1 single chain antibody gene is inserted between a P gene and M gene of a full-length clone of the newcastle disease virus LaSota strain through homologous recombination to obtain a finally modified full-length clone pBRN-FL (112-RRQRRF-117)-PDL1(ScFV); the recombinant virus is purified by chick embryo proliferation and ultracentrifugation. The rNDV-LaSota (112-RRQRRF-117)-PDL1(ScFV) recombinant virus has safety comparable to that of a LaSota original strain, and also has tumor killing capability and immune checkpoint inhibition function of a virulent strain.
Owner:南京昂科利医药科技创新研究院有限公司
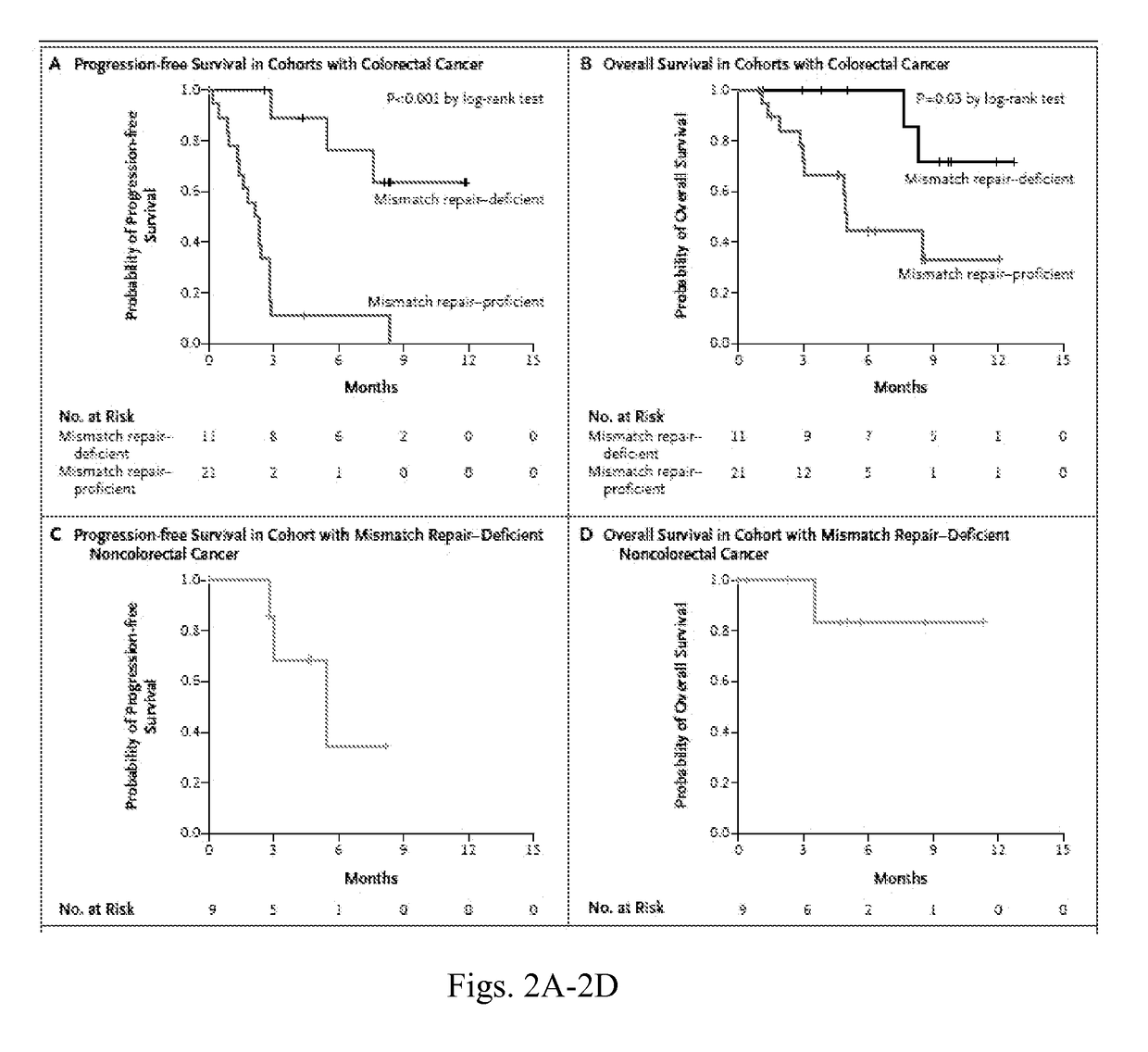
Checkpoint Blockade and Microsatellite Instability
InactiveUS20170267760A1High mutational burdenMicrobiological testing/measurementDigestive systemProgrammed deathImmune tolerance
Blockade of immune checkpoints such as cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed death-1 (PD-1) shows promise in patients with cancer. Inhibitory antibodies directed at these receptors have been shown to break immune tolerance and promote anti-tumor immunity. These agents work particularly well in patients with a certain category of tumor. Such tumors may be particularly susceptible to treatment because of the multitude of neoantigens which they produce.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
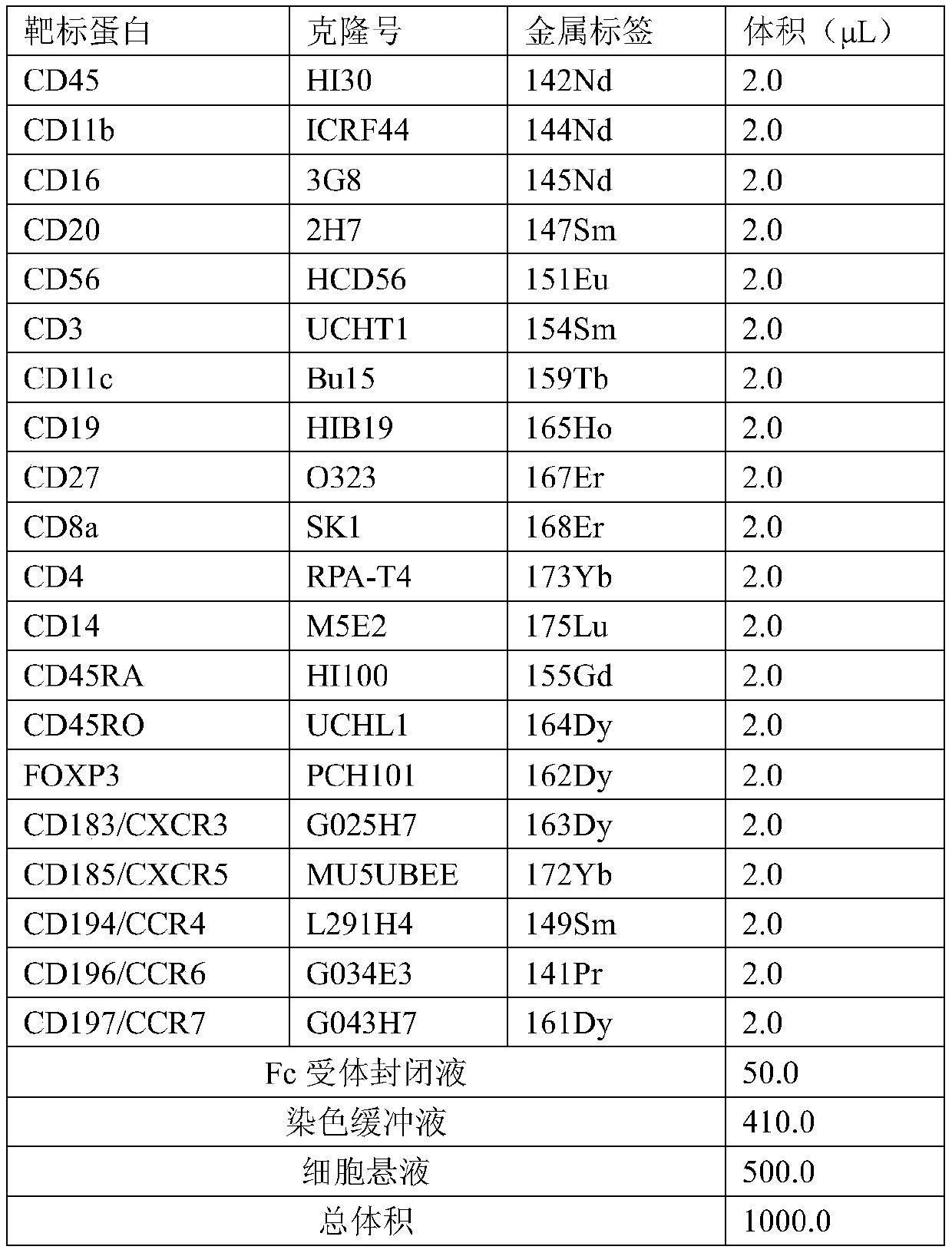
Unicell detection method for tumor sample by means of mass spectrum flow system
PendingCN110412286AHigh precisionEarly prognostic effectBiological testingTissue biopsySingle cell suspension
The invention provides a unicell detection method for a tumor sample by means of a mass spectrum flow system. The method comprises the following steps that (1) a cancer tissue biopsy sample or blood sample of a tumor patient is collected and processed to prepare a unicell suspension; (2) an antibody labeled with metal elements is used to mark the suspended unicell suspension with multiple targetsat the same time; (3) the marked unicell sample is detected by the mass spectrum flow system; and (4) on the basis of obtained mass spectrum flow type detection data, different immunocyte subgroups are clustered and analyzed, and expression levels of protein in tumor immunization detection points in different subgroup cells are quantitatively analyzed. Thus, the immucyte subgroups that can reflectthe clinical response degree of tumor immunotherapy can be accurately found in the immunocyte cells related to tumor of high heteroplasmy, the protein expression level threshold of the tumour immunization detection pointd can be determined appropriately, and a detection result is high in clinical correlation. Thus, the method has good clinical application prospects and higher social benefit.
Owner:SHANGHAI POLARIS BIOLOGY CO LTD
Fusion protein for restoring functions of exhaustion immune cells and application thereof
ActiveCN107082812AMeet the needs of immune recoveryGrowth inhibitionPeptide/protein ingredientsAntibody mimetics/scaffoldsImmune checkpointMutant
The invention relates to a fusion protein for restoring functions of debilitating immune cells. The fusion protein comprises a functional zone for identifying exhaustion immune cells and a functional zone for activating and amplifying the exhaustion immune cells. The two functional zones are connected through a non-functional amino acid fragment. According to the identification functional zone, an immune checkpoint specific antibody is utilized for identifying a phenotypic receptor of the exhaustion immune cells. According to the activation and amplification functional zone, cytokine or functional analogy mutant, or a ligand of surface receptor or functional analogy mutant, or an activated antibody is adopted for activation of the exhaustion immune cells. The invention also relates to application of the fusion protein. Tests show that the prepared fusion protein can identify exhaustion immune cells and also can activate and amplify the identified immune cells, restore the immune cells' function of killing antigen positive cells and enhance the functions of inhibiting tumor growth and controlling virus infection, and has a good clinical prospect and a wide application range.
Owner:科弈(浙江)药业科技有限公司
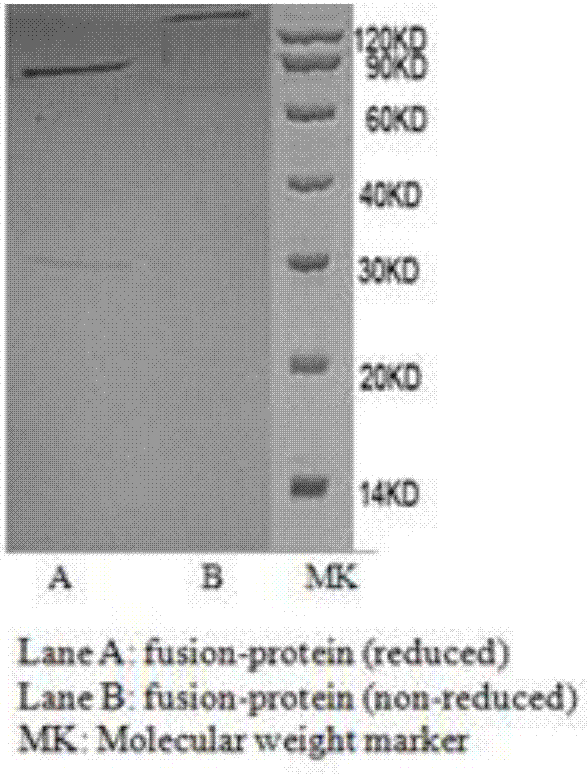
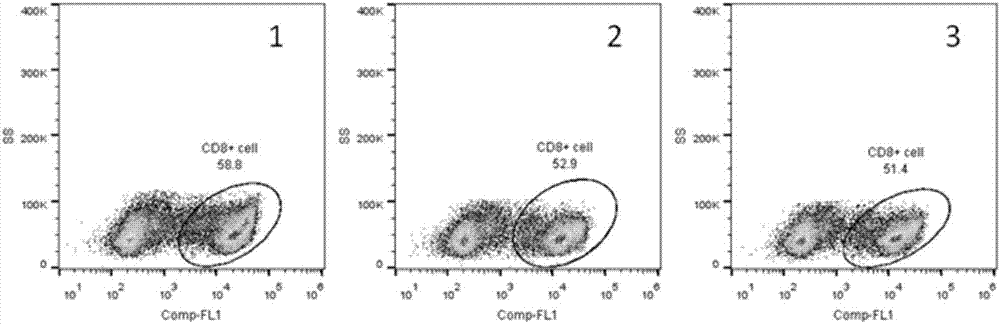
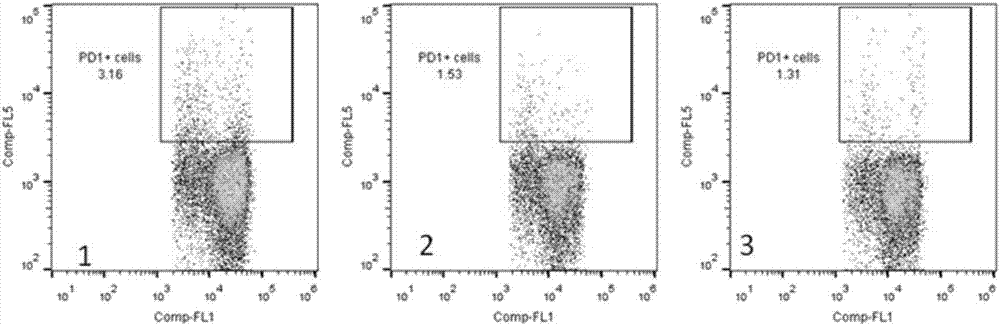
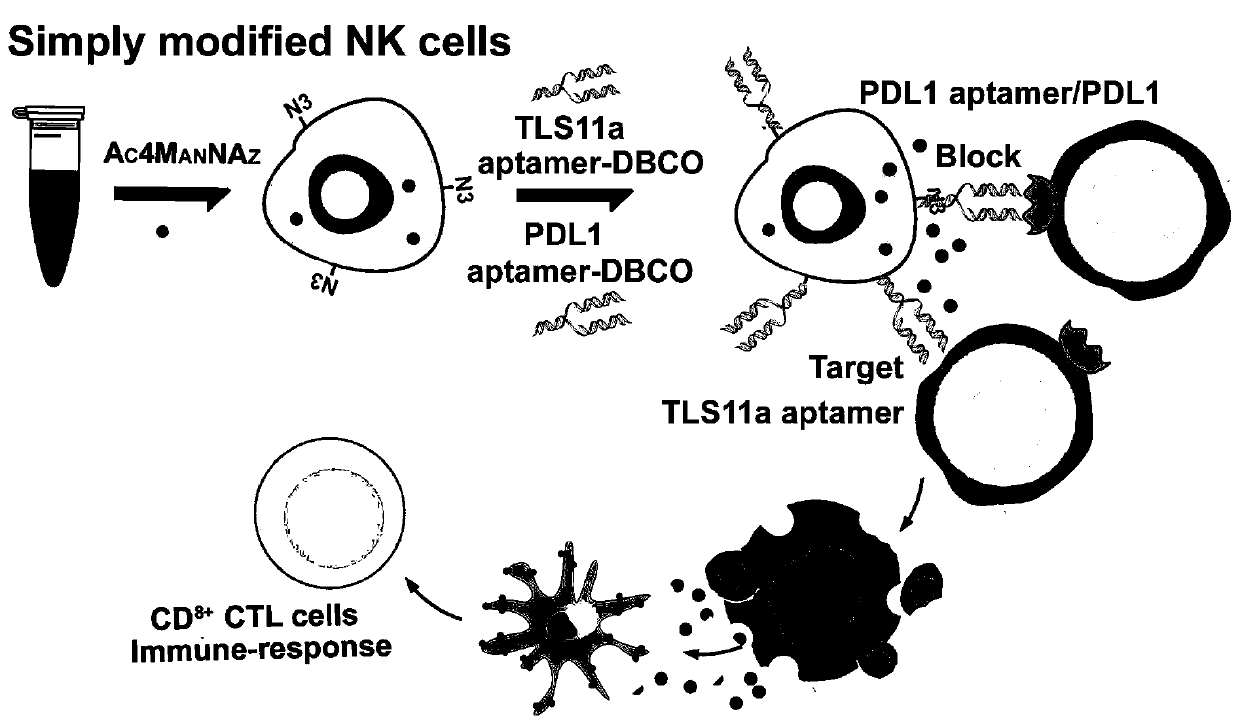
Artificial modified natural killer cell and preparation and application thereof
ActiveCN110055218AHigh stability in vivoGood biocompatibilityLiver cancer vaccineDigestive systemAbnormal tissue growthTreatment effect
Owner:上海瑞可新生物科技有限公司 +1
Compositions and methods for treating cancer
Use of a CXCR4 antagonistic peptide and an immune-check point regulator in the treatment of cancer is provided. Accordingly there is provided a method of treating cancer in a subject in need thereof, the method comprising administering to the subject a therapeutically effective amount of a peptide having an amino acid sequence as set forth in SEQ ID NO: 1 or an analog or derivative thereof; and a therapeutically effective amount of a PD1 antagonist, a PDL-1 antagonist, a CTLA-4 antagonist, a LAG-3 antagonist, a TIM-3 antagonist, a KIR antagonist, an IDO antagonist, an OX40 agonist, a CD137 agonist, a CD27 agonist, a CD40 agonist, a GITR agonist, a CD28 agonist or an ICOS agonist, thereby treating the cancer in the subject. Also provided are pharmaceutical compositions and articles of manufacture.
Owner:BIOKINE THERAPEUTICS LTD +1
Chemical modulators of immune checkpoints and therapeutic use
Compounds and pharmaceutical compositions that down-regulate immune checkpoints such as PD-1, PD-L1 and CTLA-4 are provided. Also provided are methods of treating a disease by down-regulating immune checkpoints such as PD-1, PD-L1 and CTLA-4. The methods are useful for treating cancer and viral infection in a subject.
Owner:DUKE UNIV
Gene combination and application thereof in preparation of reagent for predicting prognosis of patient treated by immune checkpoint inhibitor
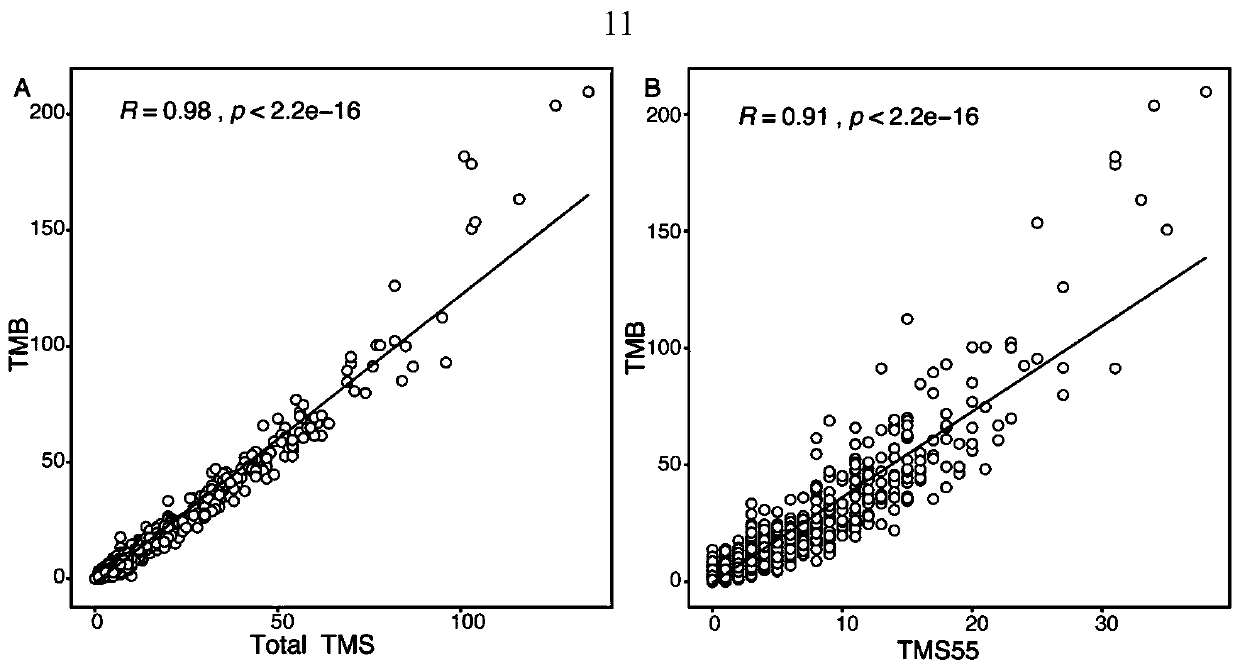
ActiveCN110229894AImproved prognosisReduce financial burdenMicrobiological testing/measurementDNA/RNA fragmentationWilms' tumorBiology
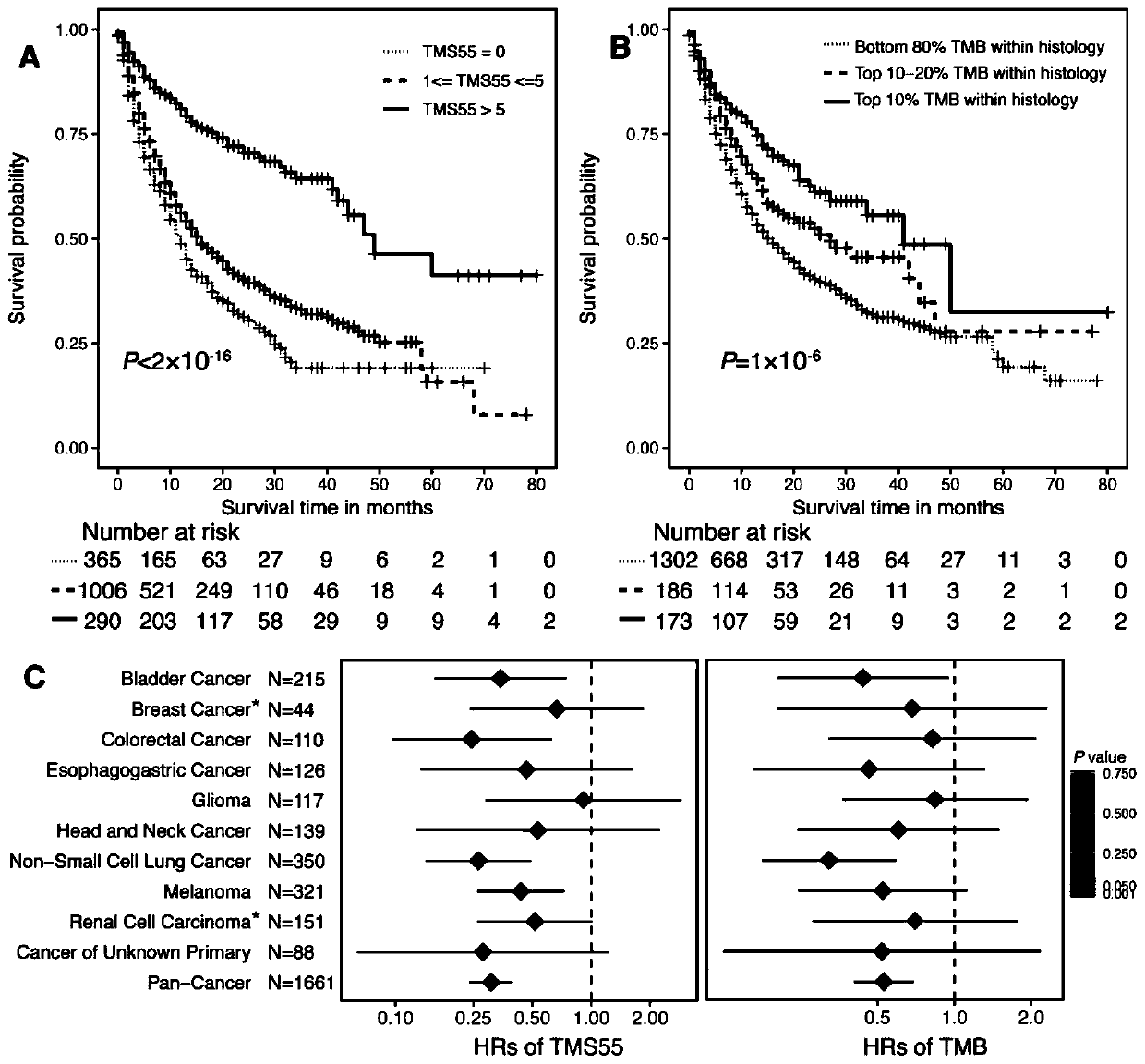
The invention provides a gene combination and application thereof in preparation of a reagent for predicting prognosis of a patient treated with an immune checkpoint inhibitor. Based on the combination of 55 genes, the invention develops a tumor mutation score TMS32055 method through the gene combination, and TMS is defined as the number of genes containing non-synonymous mutations in a specific gene combination. TMS 55 is divided into 3 groups: TMS 55 is equal to 0, TMS 55 is greater than or equal to 1 and is less than or equal to 5, and TMS 55 is greater than 5. Compared with a group that TMS 55 is equal to 0, the patients of groups that TMS 55 is greater than or equal to 1 and is less than or equal to 5 and TMS 55 is greater than 5 have better prognosis and longer overall survival time.The TMS55 based on the 55-gene combination has higher prediction efficiency than TMB in predicting the overall survival after immunotherapy, and has uniform cutoff value. Therefore, the TMS55 can beused for predicting the prognosis of patients receiving immune checkpoint inhibition treatment, and is easier to popularize and apply in clinical practice.
Owner:WUHAN UNIV
Multi-immunohistochemical analysis kit of lung cancer, and using method and application thereof
ActiveCN109596831AGood treatment effectStrong detection signalBiological testingMonoclonal antibodyImmunohistochemistry technique
The invention provides a multi-immunohistochemical analysis kit of lung cancer, and a using method and application thereof, and relates to the field of the multi-immunohistochemical technology. The multi-immunohistochemical analysis kit defines that the monoclonal antibody group comprises more than two types of a CD163 monoclonal antibody, a CD68 monoclonal antibody, a PDL1 monoclonal antibody, aCD8 monoclonal antibody, a PD1 monoclonal antibody and a CD57 monoclonal antibody. The multi-immunohistochemical analysis kit uses the human or animal in vitro tissue, which is given the immune checkpoint inhibitor, as the sample to test, so as to judge the lung cancer effect validity of the multi-immunohistochemical analysis kit. The invention further provides a using method of the multi-immunohistochemical analysis kit; the method can effectively mark not less than 6 types of immune checkpoints on the same one tissue; and the cross reaction does not exist among multiple marks.
Owner:ZHENYUE BIOTECHNOLOGY JIANGSU CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com