CAR-T cell capable of efficiently and stably expressing inhibiting antibody and application thereof
An inhibitory and cellular technology, applied in the field of CAR-T cells, can solve the problems of lack of CAR-T therapeutic targets, difficulties in packaging and preparation of retroviruses or lentiviruses, and functional insufficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
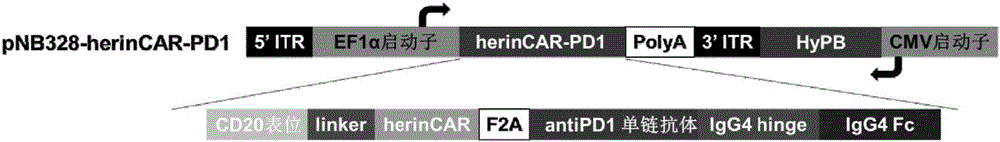
[0106] Example 1: Construction of recombinant plasmid pNB328-herinCAR-PD1
[0107] According to the herinCAR coding sequence shown in SEQ ID NO: 1 containing the CD20-Rituximab molecular brake (targeting the CAR of the EGFR family, see CN 201510812654.9) and the coding sequence of herinCAR-PD1 shown in SEQ ID NO: 2 (targeting the EGFR family and containing CD20-Rituximab Molecular Brake CAR and PD1 single-chain-Fc antibody, connected with 2A in the middle), entrusted Shanghai Jereh Biological Company to synthesize, and introduced EcoRI and SalI restriction sites in the upstream and downstream respectively, loaded into pNB328 vector, respectively Named pNB328-herinCAR, pNB328-herinCAR-PD1.
[0108] HerinCAR coding sequence containing CD20-rituximab molecular brake:
[0109] GCCACCATGGAGTTTTGGCTGAGCTGGGTTTTCCTTGTTGCTATTTTAAAAGGTGTCCAGTGT GGTGGAGGTGGAGGTGGAGGTGGAGGTGGTACCCACTCACTGCCCCCGAGGCCAGCTGCAGTTCCTGTCCCTCTGCGCATGCAGCCTGGCCCAGCCCACCCTGTCCTATCCTTCCTCAGACCCTCTTGGGACCT...
Embodiment 2
[0113] Example 2: Construction of herinCAR-PD1 cells
[0114] Prepare 1×10 7 Freshly isolated peripheral blood mononuclear cells (PBMC) were transfected with 6 μg of the pNB328-herinCAR-PD1 plasmid into the nucleus through a Lonza 2b-Nucleofector instrument, and placed at 37°C, 5% CO 2 Culture in an incubator; transfer to a 6-well plate containing 30ng / mL anti-CD3 antibody and 3000IU / mL IL-2 (purchased from Novoprotein Company) after 6 hours, and place at 37°C, 5% CO 2 Incubator culture. After the cells were confluent, they were subcultured at a ratio of 1:5. That is, genetically modified T cells that simultaneously express the targeting EGFR family and contain CD20-rituximab molecular brake CAR and PD1 single-chain-Fc antibody, referred to as herinCAR-PD1 cells. At the same time, PBMCs from the same source were transfected with the pNB328-herinCAR plasmid to obtain herinCAR-T cells.
Embodiment 3
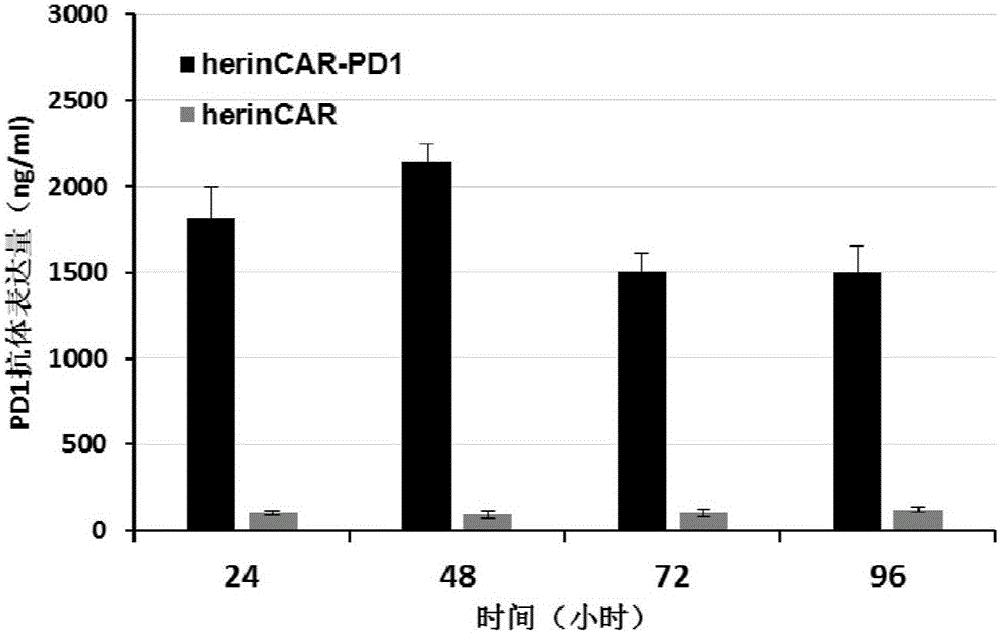
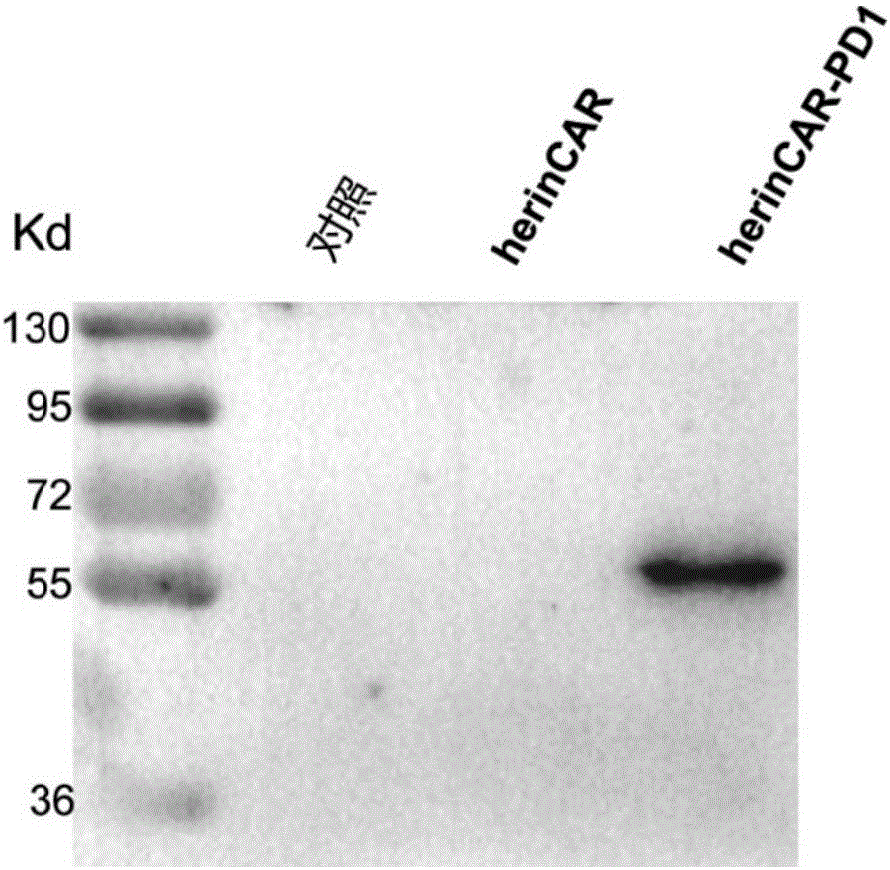
[0115] Example 3: Quantitative detection of PD1 antibody expression in herinCAR-PD1 cells
[0116] Subculture the herinCAR-PD1 and herinCAR cells prepared in Example 2 at a dilution ratio of 1:3. 6 Cells / well were spread in a 6-well plate with 4ml of AIM-V medium (purchased from Gibco), placed at 37°C, 5% CO 2 Culture in an incubator, and collect 800 μl of cell supernatant after 24 hours, 48 hours, 72 hours, and 96 hours of culture, and store at -20°C for future use. Human PD1 recombinant protein was used to coat the microtiter plate (purchased from SinoBiological Company), and HRP-labeled mouse anti-human IgG mAb (purchased from Abcam Company) was used for detection, and commercialized anti-PD1 antibody (purchased from Merck Company) was used as For standard products, the expression level of PD1 antibody was detected by double-sandwich ELISA method.
[0117] turn out, herin CAR-PD1 The cells can express PD1 antibody stably at a high level, as shown in figure 2 shown...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com