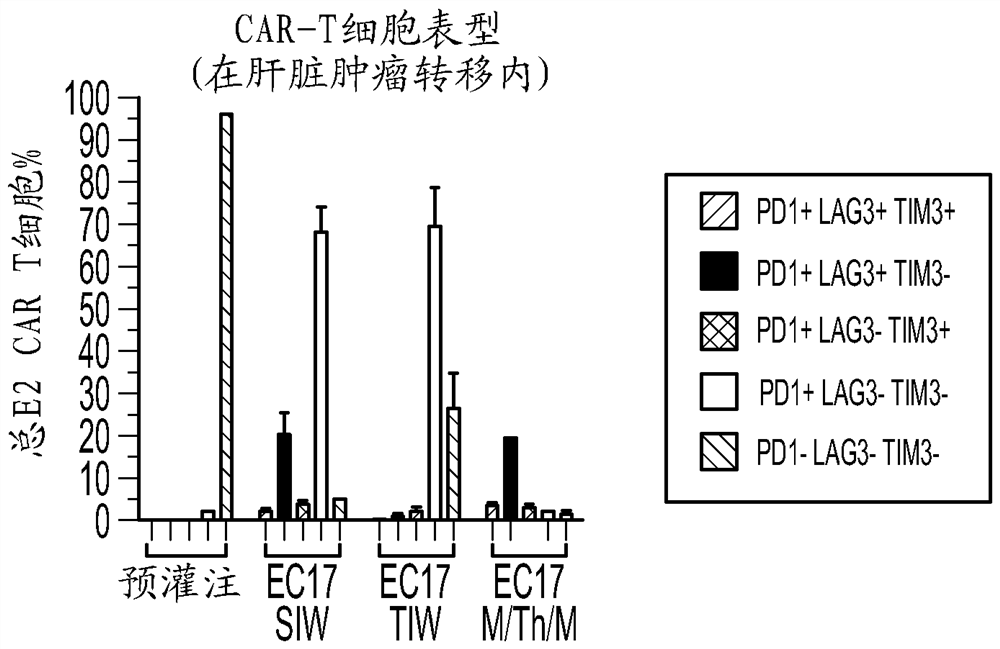
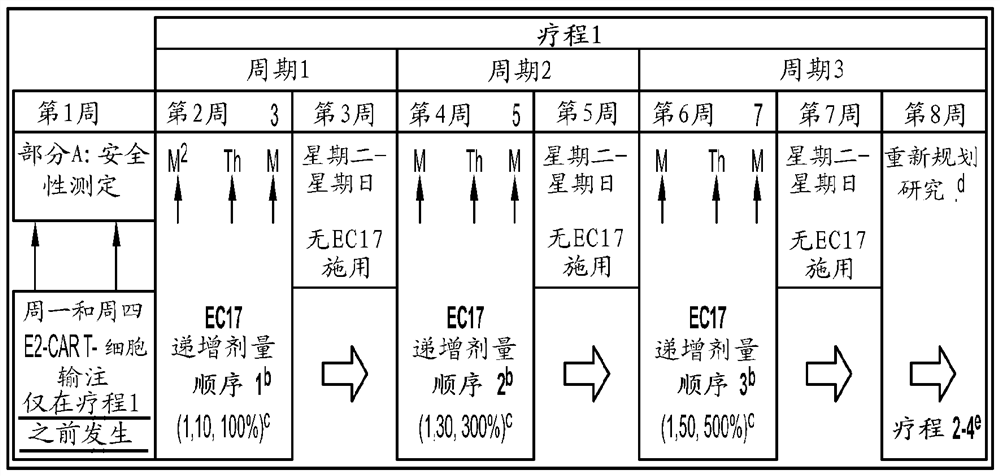
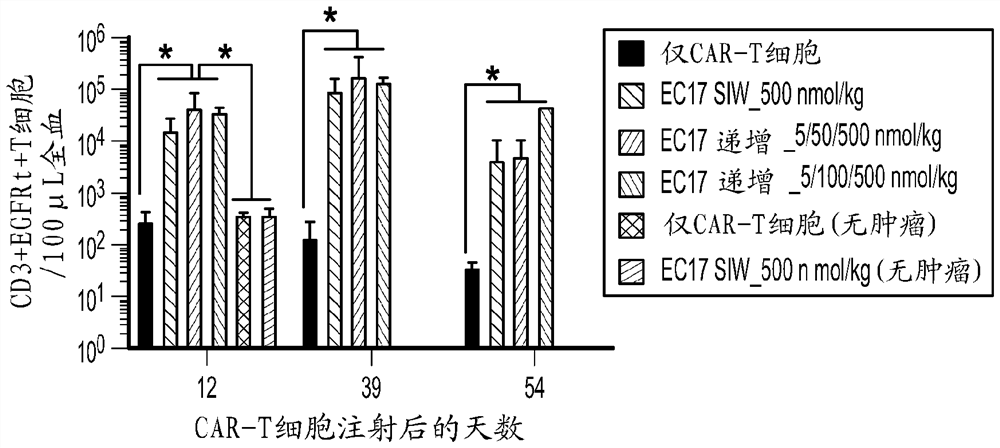
Sequencing method for car t cell therapy
A sequential and cell-based technology, applied in the direction of targeting specific cell fusion, chemical instruments and methods, biochemical equipment and methods, etc., can solve problems such as inability to prolong activation and expand CART cell populations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0478] Synthesis of FITC-Folic Acid
[0479]
[0480] Folic acid-γ-ethylenediamine and fluorescein isothiocyanate (FITC) isomer I ( Sigma-Aldrich) coupling. The crude product was loaded onto an Xterra RP18 preparative HPLC column (Waters) and started with 99% 5 mM sodium phosphate (mobile phase A, pH 7.4) and 1% acetonitrile (mobile phase B) at 20 mL / min The flow rate reached 90% A and 10% B gradient condition elution within 10 min. Under these conditions, the main FITC-folate peak usually elutes at 27-50 min. The quality of the FITC-folate fraction was monitored by analytical reverse-phase HPLC with UV detector. Fractions with a purity greater than 98.0% (LCMS) were lyophilized to obtain the final FITC-folate product. Compounds with this structure are also known as EC17, as known in the art.
Embodiment 2
[0482] Synthesis of FITC-PEG12-folate
[0483]
[0484] Apply Universal Polyethylene Glycol (PEG) Nova Tag TM The resin (0.2 g) was loaded into a peptide synthesis vessel and washed with isopropanol ( i-PrOH) (3 × 10 mL) and dimethylformamide (DMF, 3 × 10 mL). 9-Fluorenylmethoxycarbonyl (Fmoc) deprotection was performed using 20% piperidine in DMF (3 x 10 mL). A Kaiser test was performed to assess response progression. A DMF solution of Fmoc-L-glutamic acid 5-tert-butyl ester (Fmoc-Glu-(O-t-Bu)-OH) (23.5 mg), N,N-diisopropylethylamine ( i -Pr 2 NEt) (4 equiv) and benzotriazol-1-yl-oxytripyrrolidinylphosphonium hexafluorophosphate (PyBOP) (2 equiv). Fmoc deprotection was performed using 20% piperidine in DMF (3 x 10 mL). Then introduce N into the container 10 -TFA-Pte-OH (22.5 mg), DMF, i -Pr 2 A solution of NEt (4 equiv) and PyBOP (2 equiv). Argon bubbled for 2 h, and with DMF (3 × 3 mL) and i -PrOH (3 x 3 mL) to wash the resin. After the resin was swollen i...
Embodiment 3
[0486] Synthesis of FITC-PEG20-folate
[0487]
[0488] Ethylenediamine, polymer bound (200-400 mesh) resin (50 mg) was charged into a peptide synthesis vessel and swelled with DCM (3 mL) followed by DMF (3 mL). Subsequent introduction of Fmoc-PEG in DMF to the container 20 -COOH solution (131 mg, 1.0 equiv), i -Pr 2 NEt (6.0 equiv) and PyBOP (4.0 equiv). Argon was bubbled for 6 h, the coupling solution was discharged, and the solution was washed with DMF (3 × 10 mL) and i -PrOH (3 x 10 mL) to wash the resin. A Kaiser test was performed to assess response progression. Fmoc deprotection was performed using 20% piperidine in DMF (3 x 10 mL) prior to coupling of each amino acid. The above sequence was repeated to complete the reaction with Fmoc-Glu-OtBu (72 mg, 2.0 equiv) and the coupling step of Tfa.pteroic acid (41 mg, 1.2 equiv). The resin was washed with DMF 3 × 10 mL (5 min) containing 2% hydrazine to cleave the trifluoro-acetyl protecting group on pteroic acid, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com