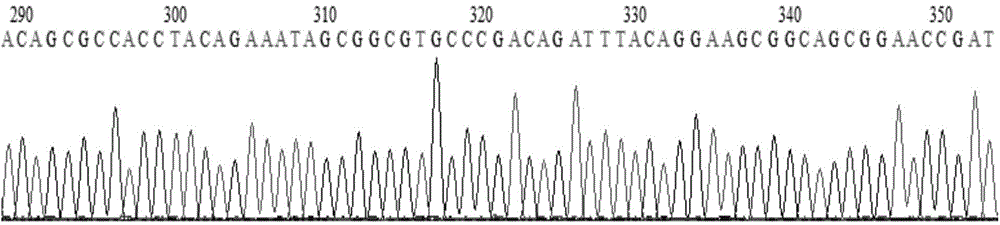
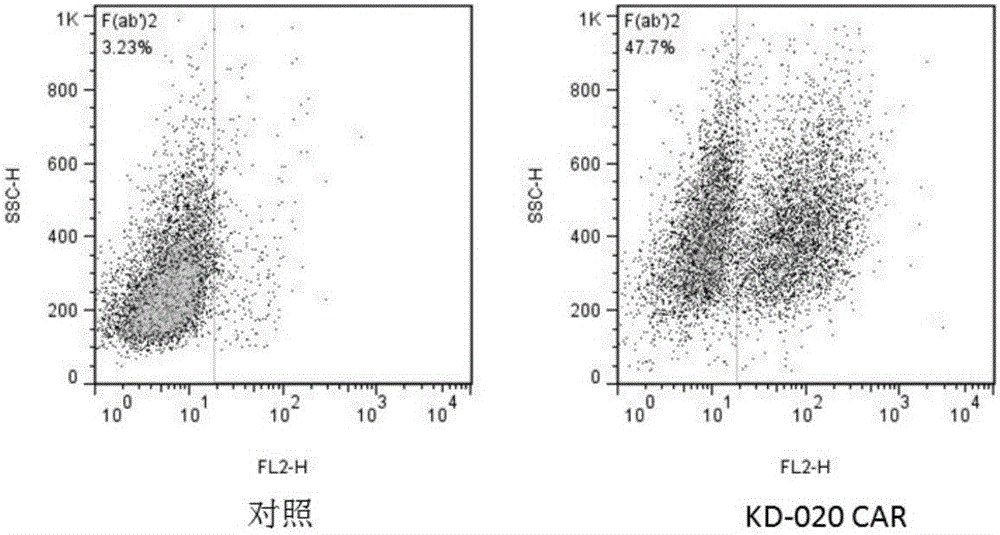
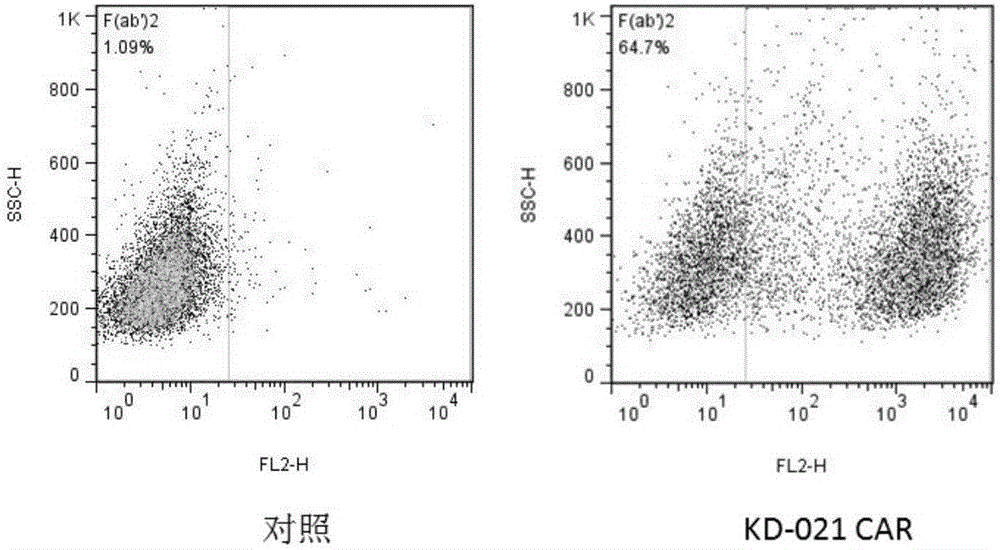
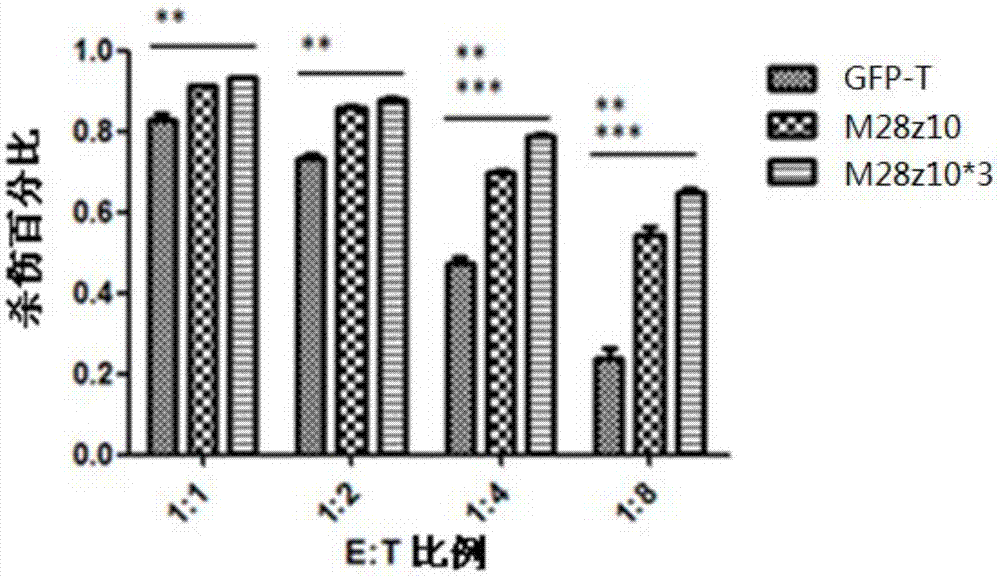
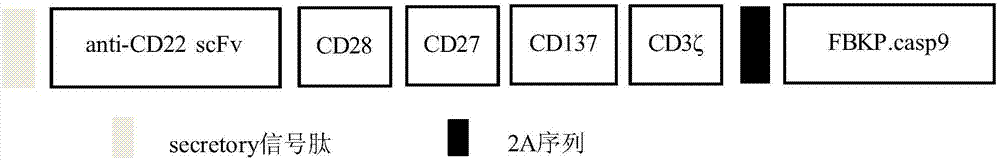
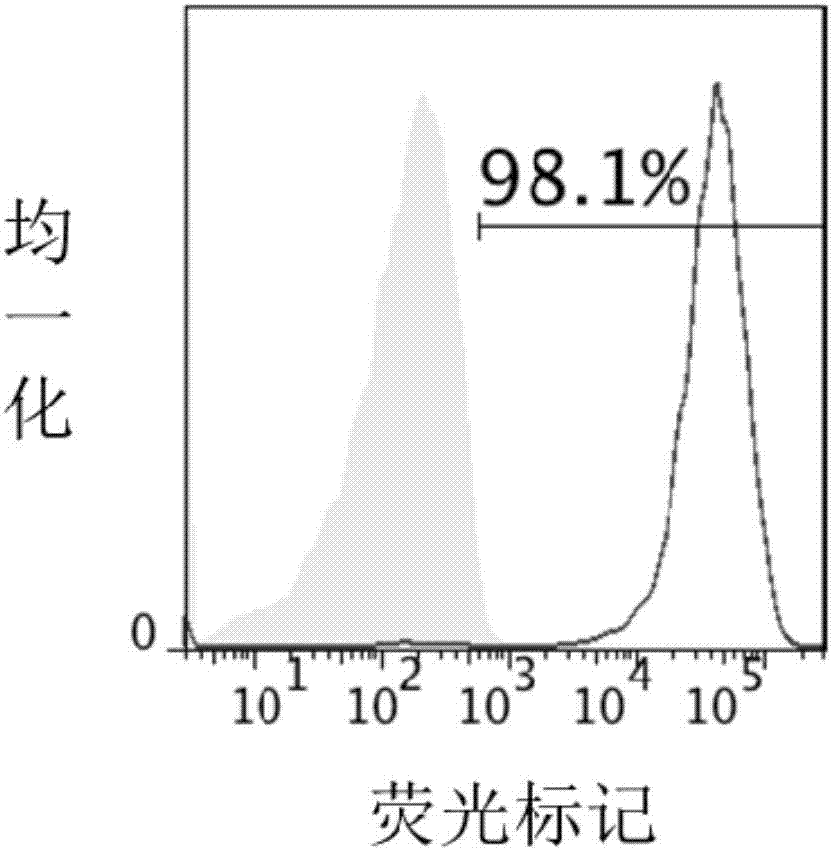
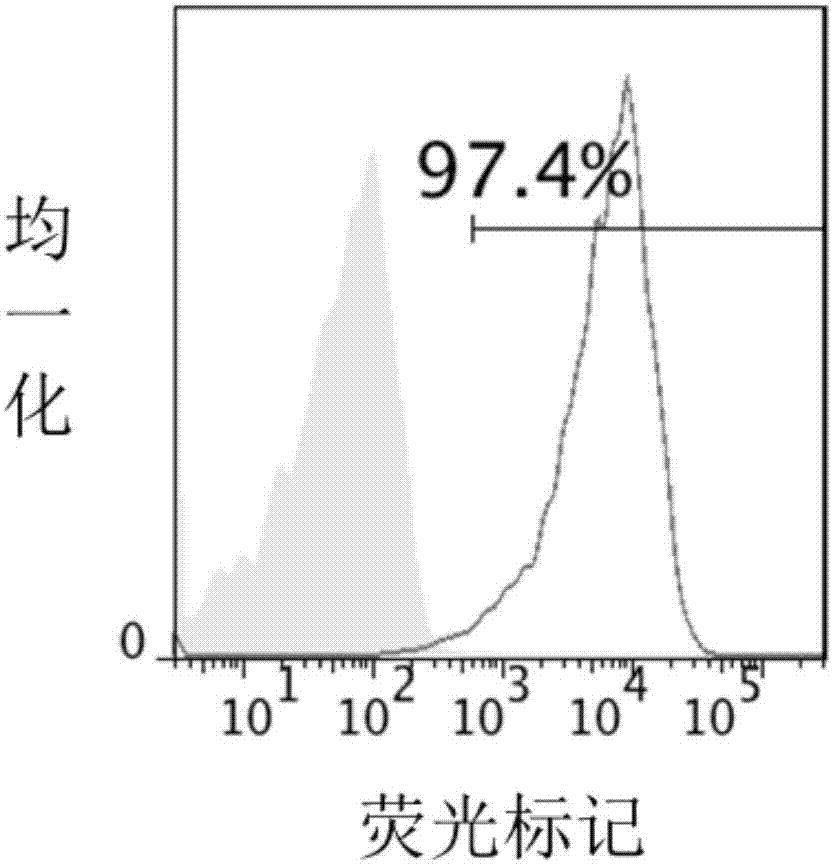
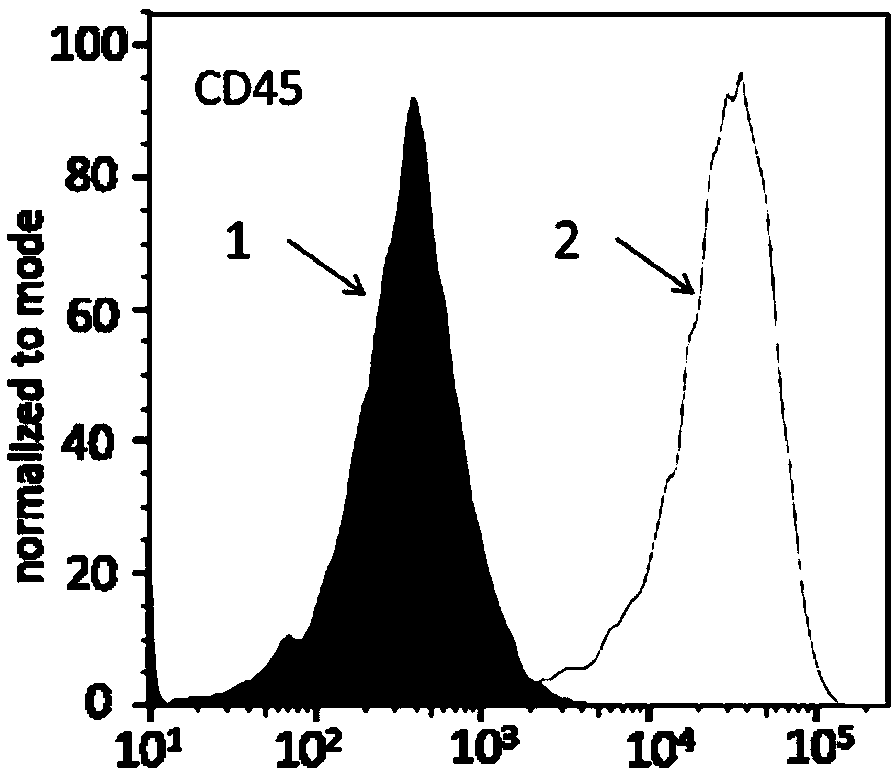
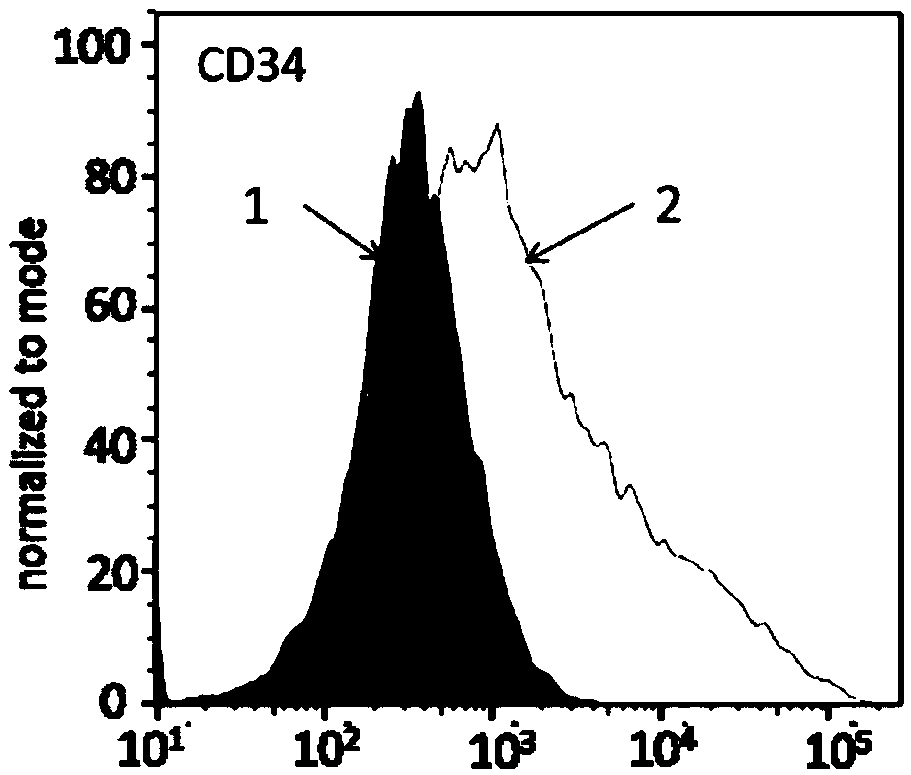
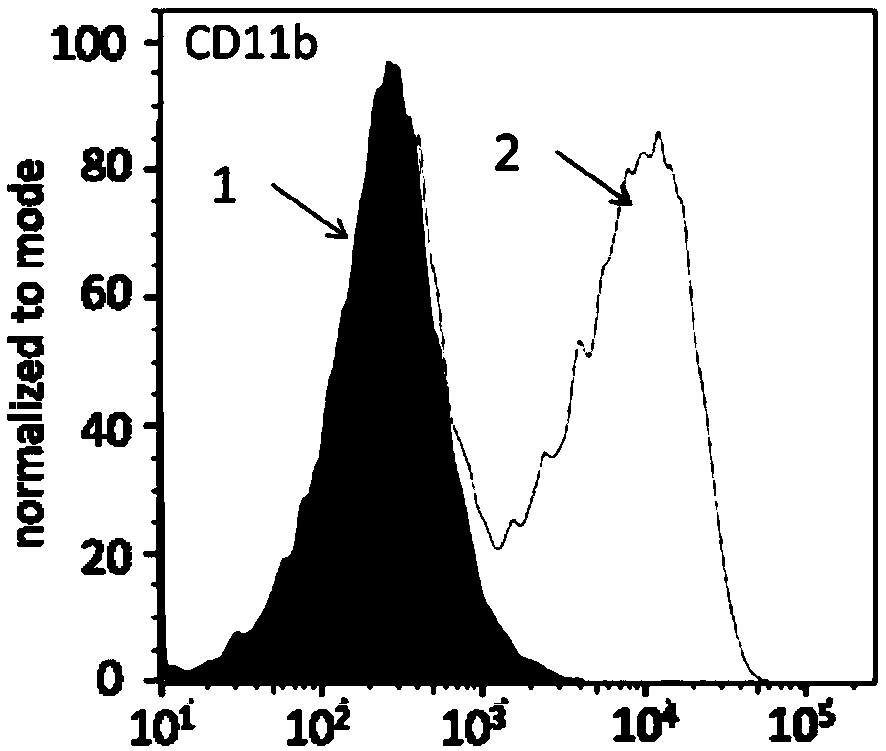
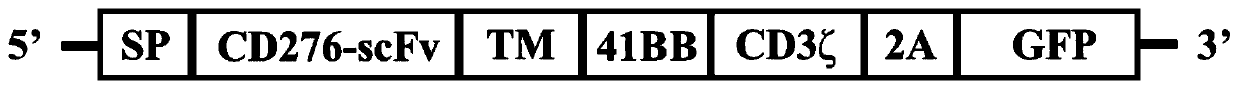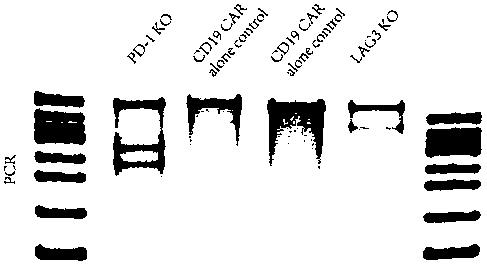Patents
Literature
358 results about "Car t cells" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
CAR T is a personalized therapy using the patient’s own immune cells, or T cells, to fight cancer. For this treatment, a patient’s T cells are removed from their blood and sent to a lab where the cells are genetically modified to better enable them to identify and attack cancer cells.
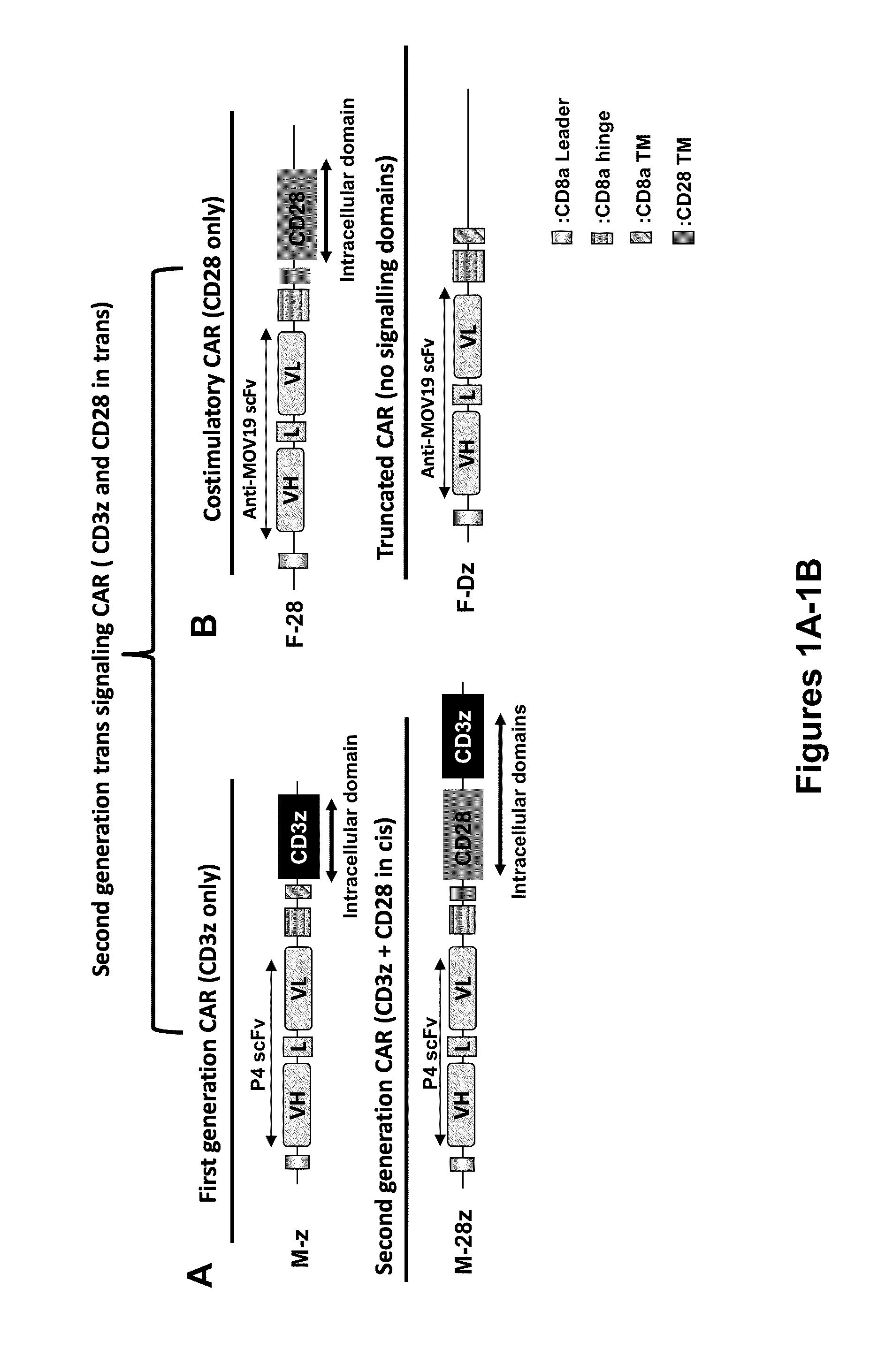
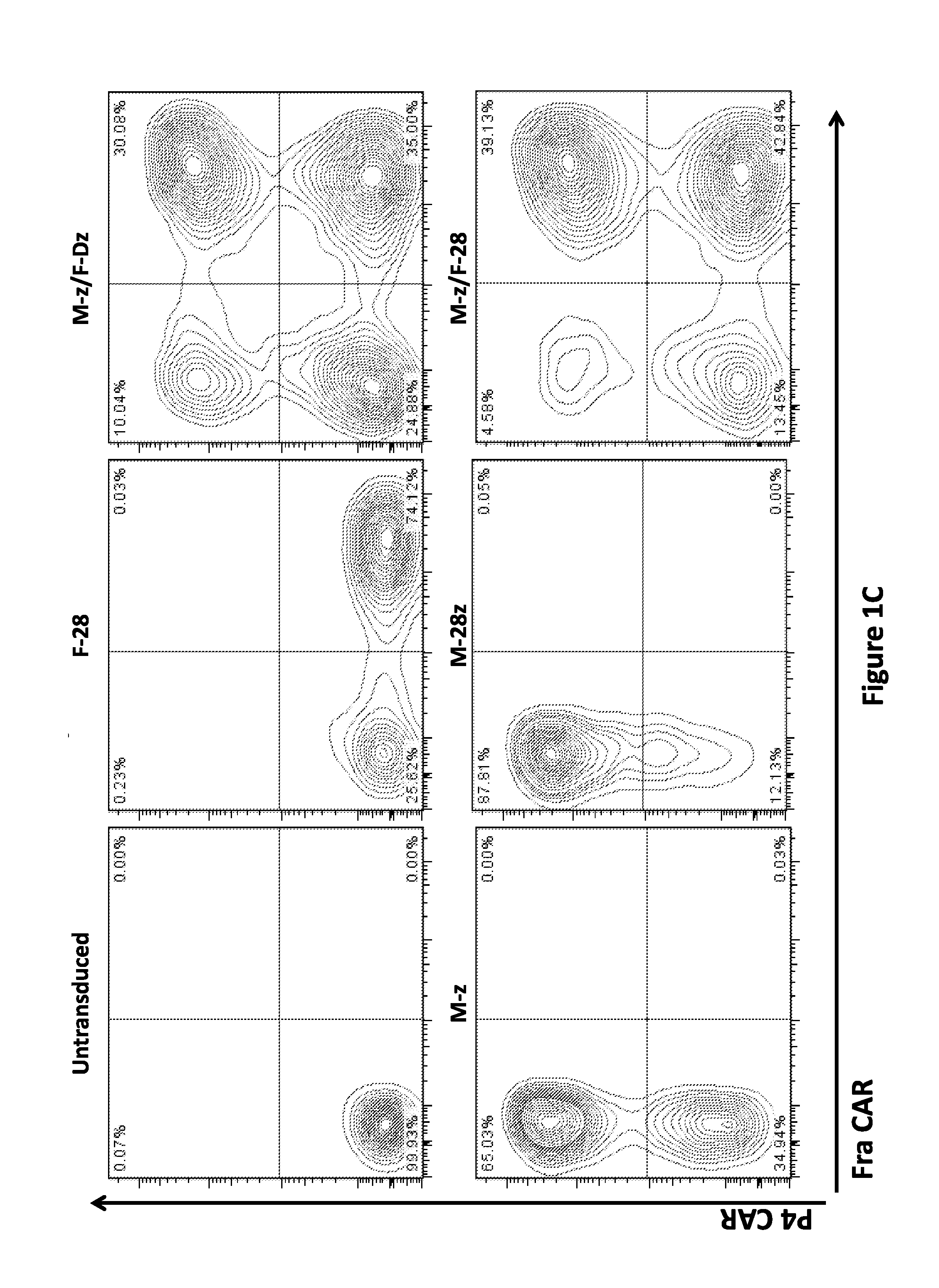
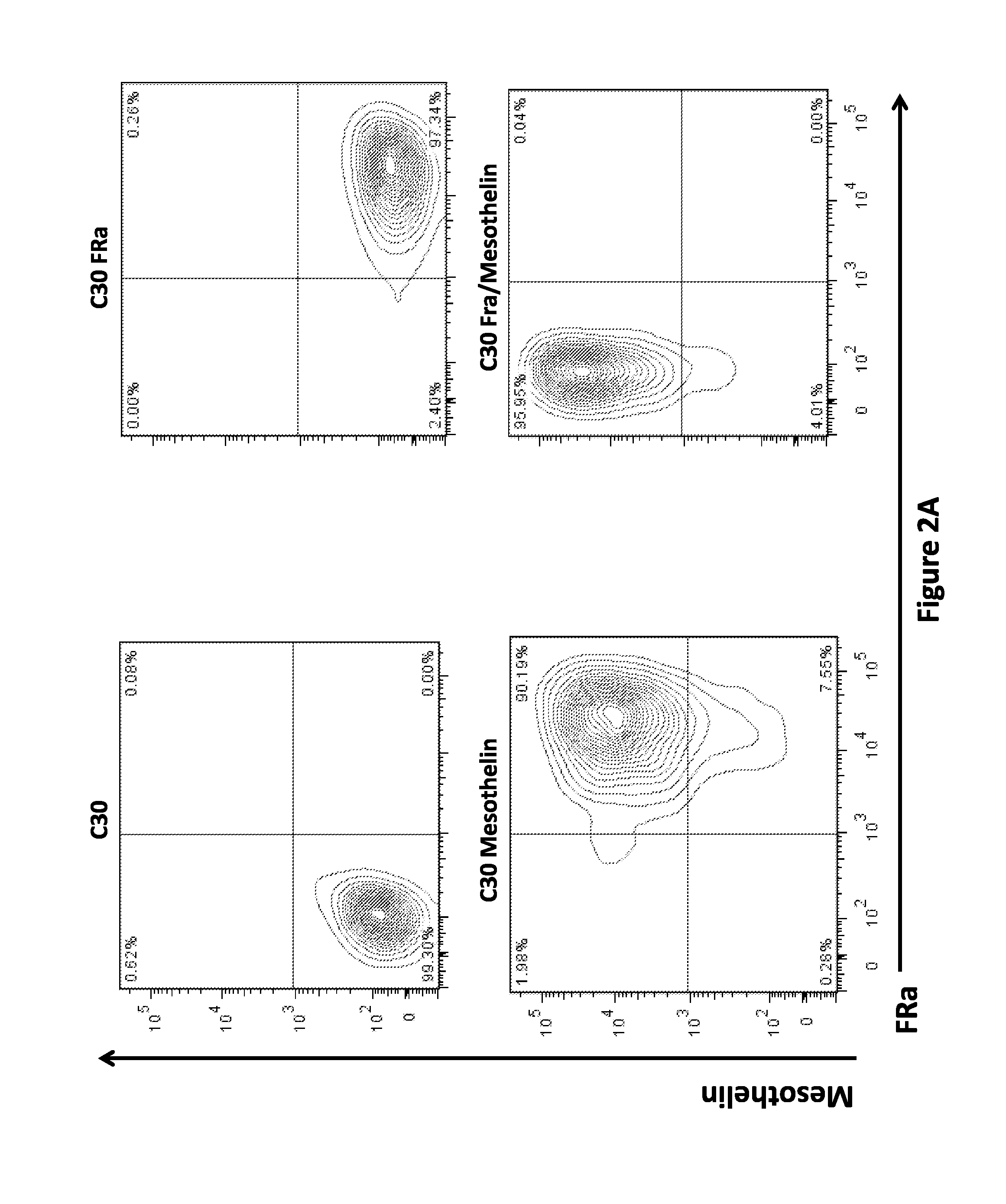
Use of a Trans-Signaling Approach in Chimeric Antigen Receptors
ActiveUS20140099309A1Heightened tumor specificitySugar derivativesAntibody mimetics/scaffoldsTrans signalingActivation cells
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
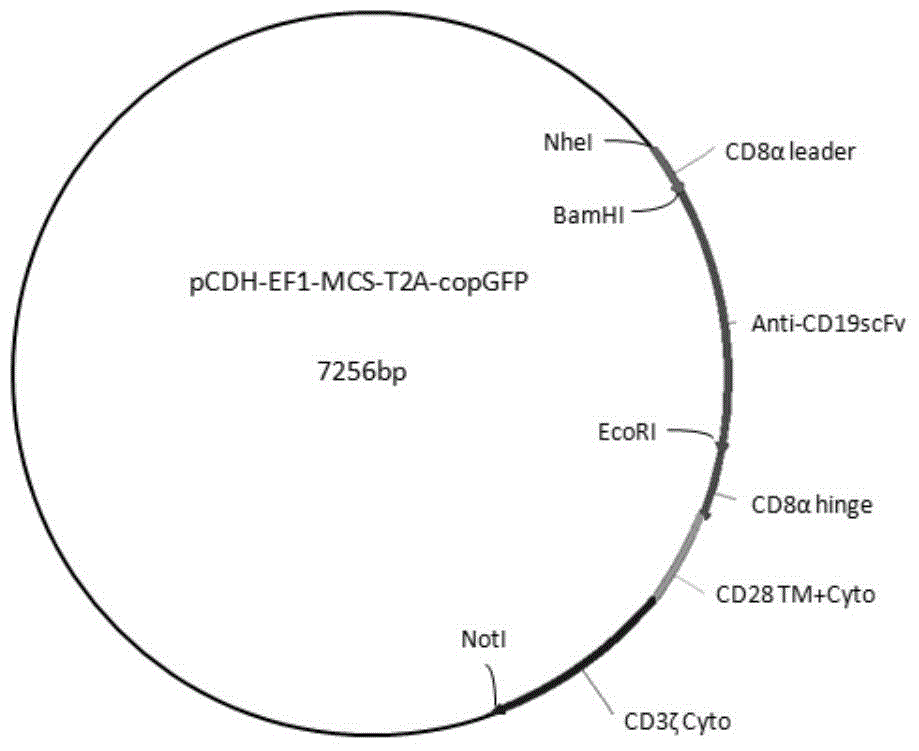
Chimeric antigen receptor hCD19scFv-CD8a-CD-28-CD3zata and application thereof
ActiveCN104788573AConfirmed specific killing effectMammal material medical ingredientsHybrid peptidesAntigen receptorHeavy chain
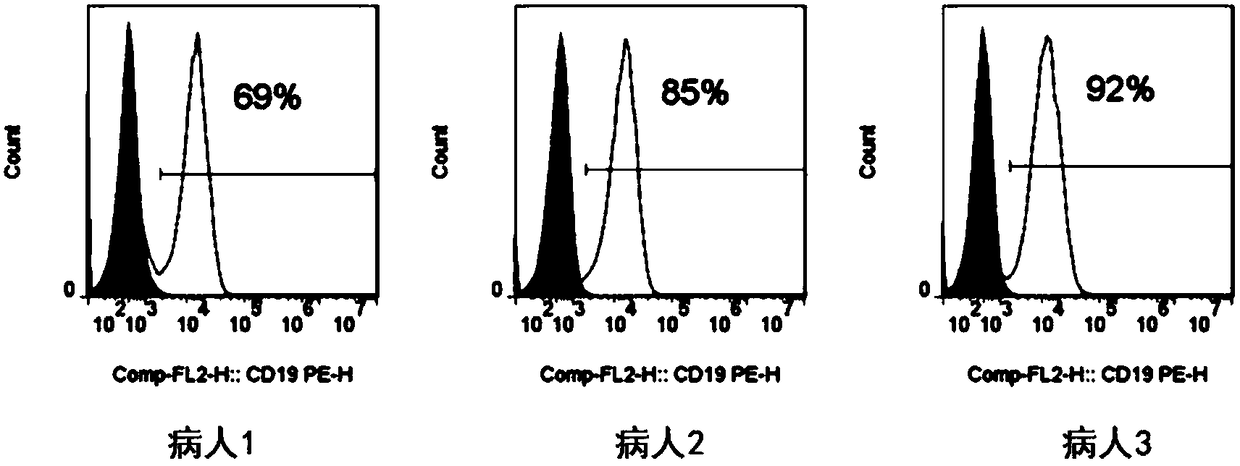
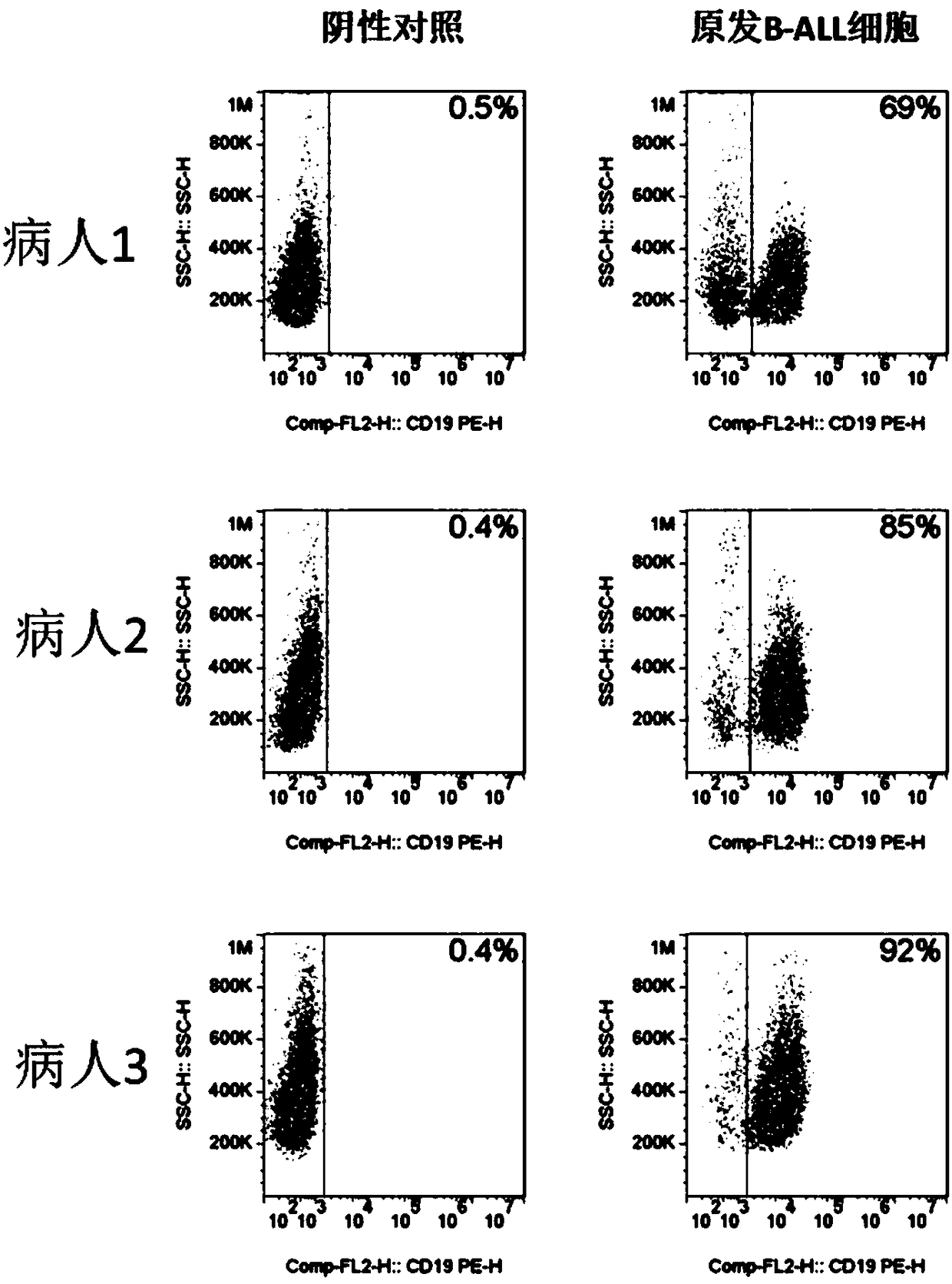
The invention discloses a chimeric antigen receptor hCD19scFv-CD8a-CD-28-CD3zata and application thereof. The chimeric antigen receptor is serially connected by an anti-human CD19 monoclonal antibody H119a light chain and heavy chain variable region (hCD19scFv), human CD8a hinge region, a human CD28 transmembrane region, an intracellular region and a human CD3zata intracellular region. The chimeric antigen receptor is used for modifying T lymphocyte, and the modified T lymphocyte (CAR-T cell) can be used for treating the tumor with positive surface CD19.
Owner:JUVENTAS CELL THERAPY LTD
Method for knocking out PD-1 gene by utilizing CRISPR/Cas9 technology to construct MSLN-targeted novel CAR-T cell and application of method
PendingCN106480097ABlock escapeBlocking inhibitoryGenetically modified cellsMammal material medical ingredientsT cellSolid tumor
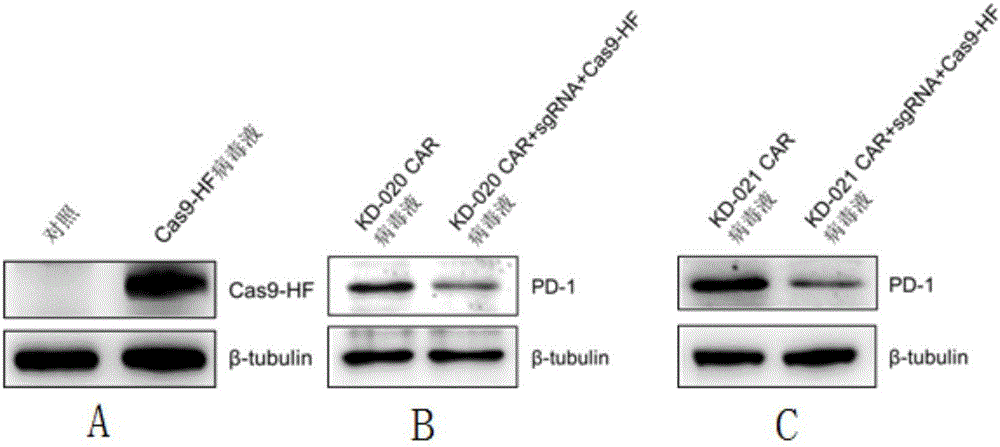
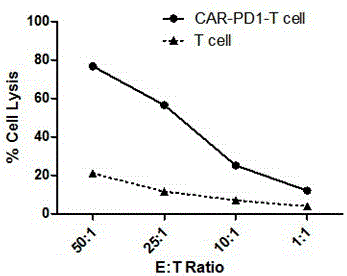
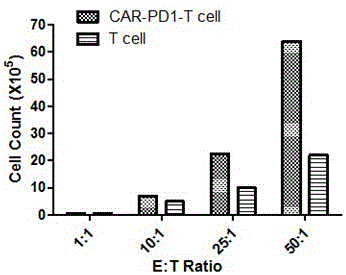
The invention discloses a method for knocking out a PD-1 gene by utilizing a CRISPR / Cas9 technology to construct an MSLN-targeted novel CAR-T cell. According to the method, a CAR-T cell is synchronously infected with a virus solution carrying sgRNA and Cas9-HF nuclease of a human PD-1 gene by utilizing the CRISPR / Cas9 technology to knock out the human PD-1 gene, so that the MSLN-targeted novel CAR-T cell is obtained. The novel CAR-T cell can be used for preparing a preparation used for treating solid tumors. A preparation method is simple in step, and the obtained CAR-T cell has high killing rate for solid tumor cells.
Owner:NANJING KAEDI BIOTECH INC
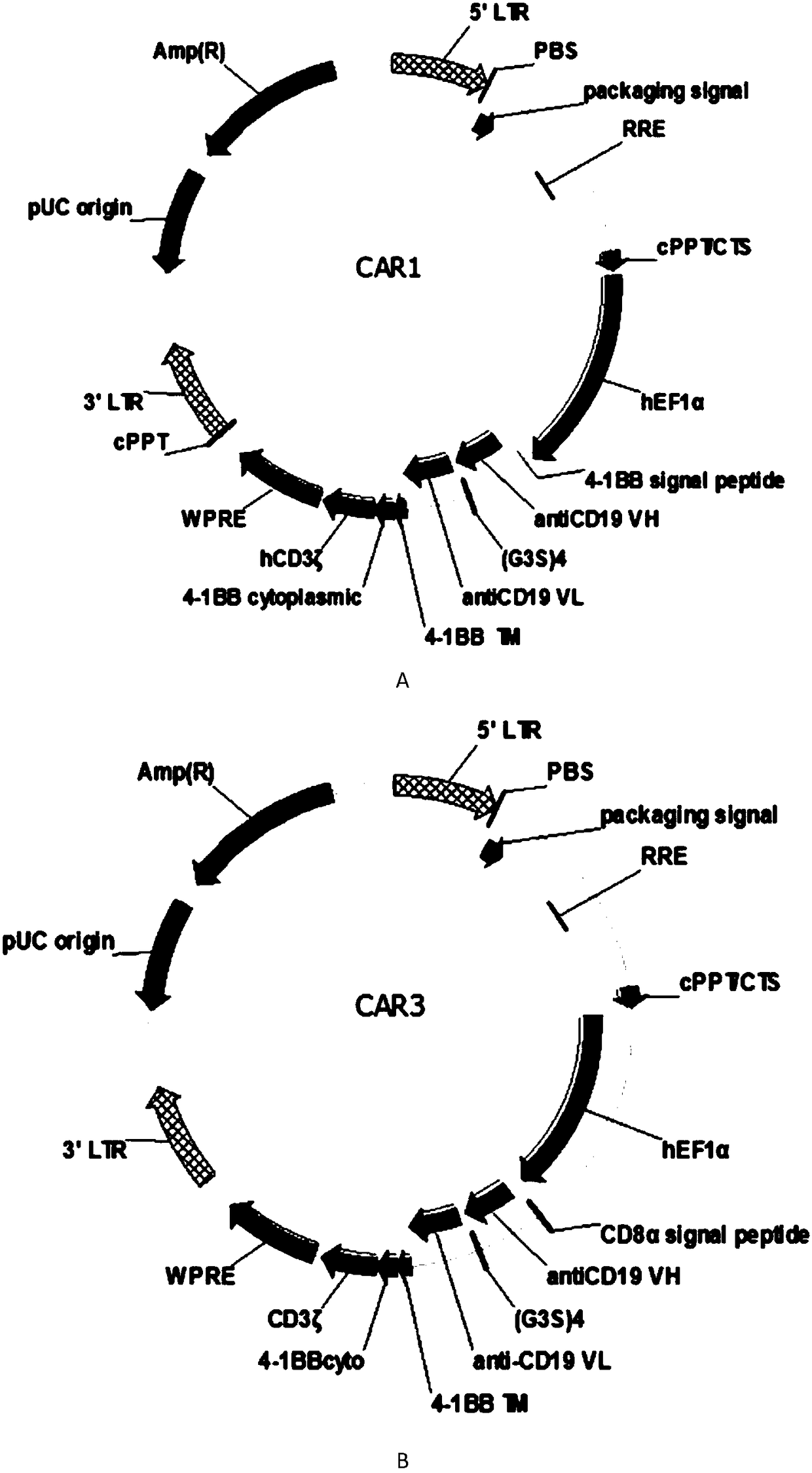
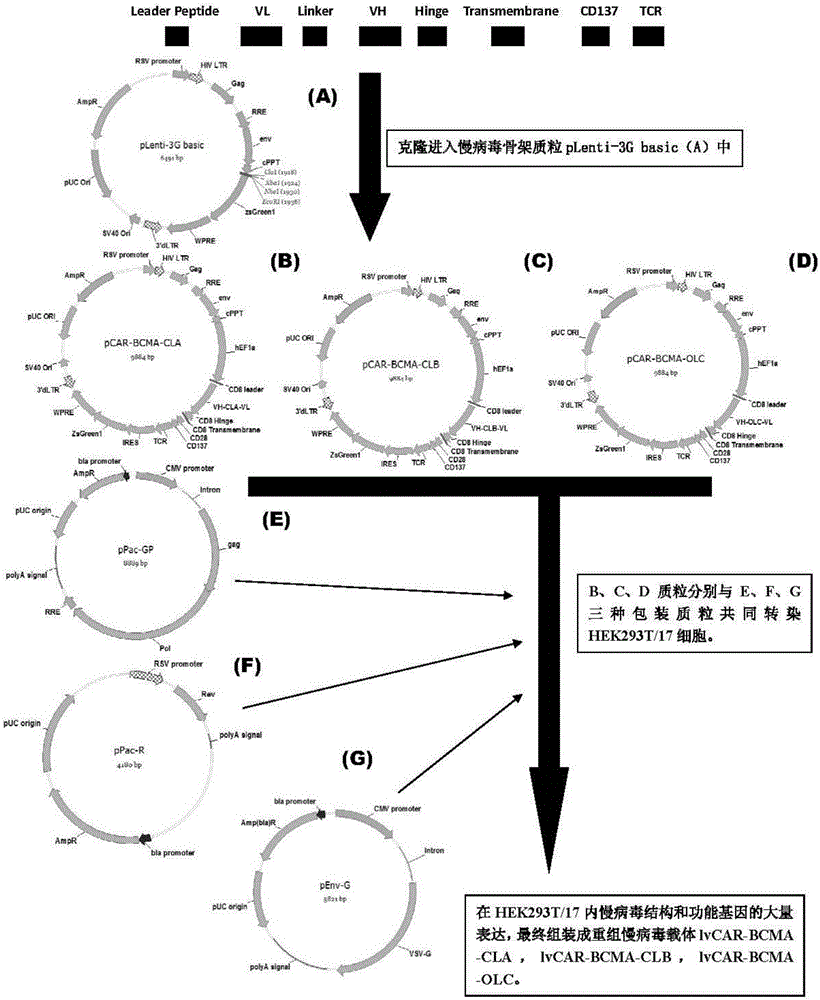
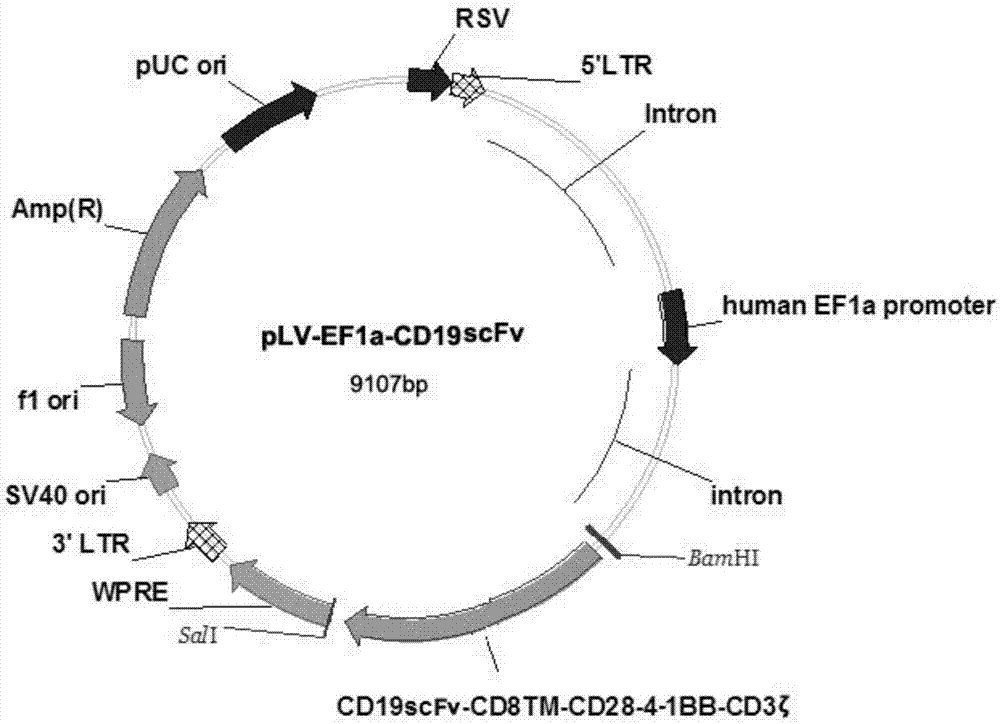
CAR-T transgene vector based on replication defective recombinant lentivirus and construction method and application of CAR-T transgene vector
ActiveCN105602992ASignificant effectPromote secretionGenetic material ingredientsFermentationEucaryotic cellAmpicillin
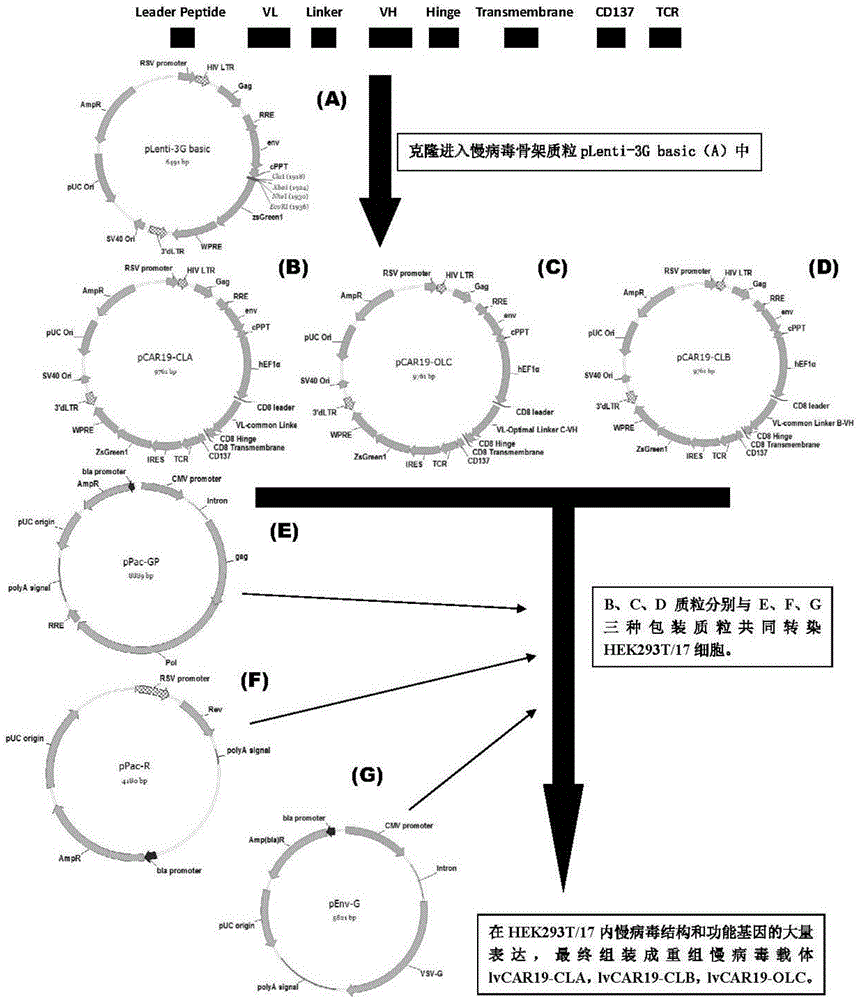
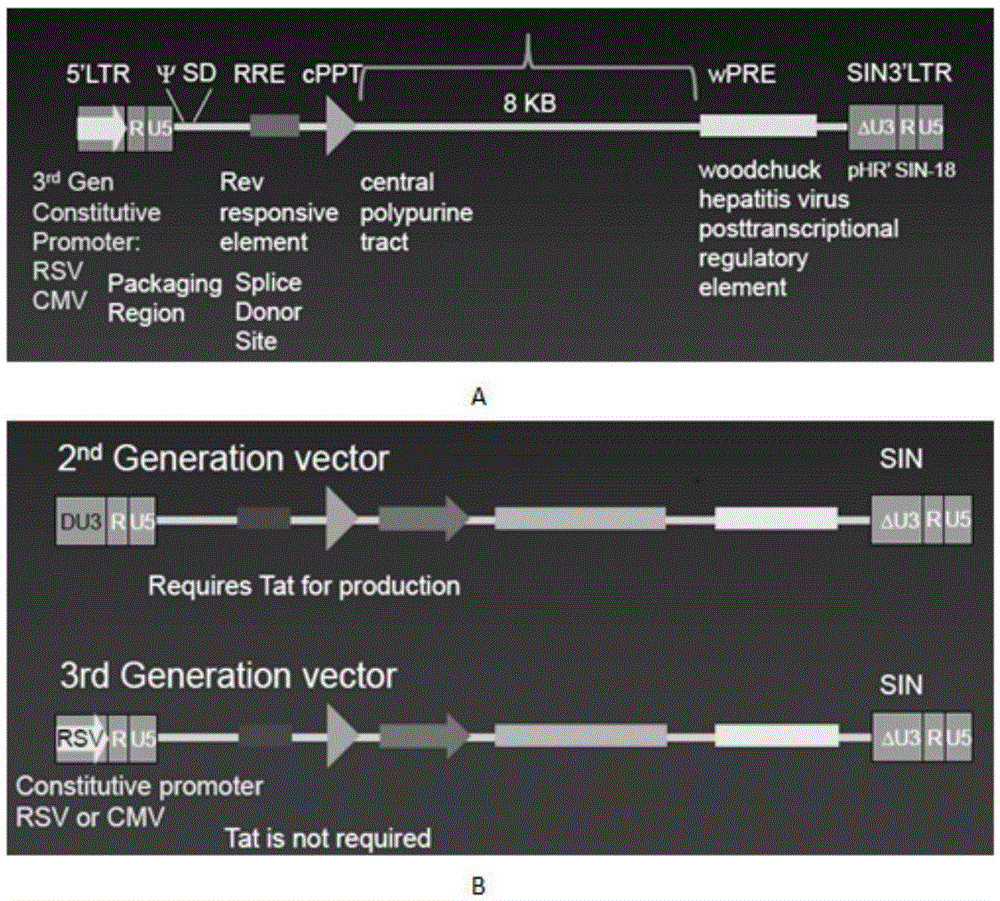
The invention discloses a CAR-T transgene vector based on replication defective recombinant lentivirus. The CAR-T transgene vector comprises an original nuclear replicon pUCOri sequence, a resistance gene AmpR sequence containing ampicillin, a virus replicon SV40 Ori sequence, a lentivirus packaging cis element, ZsGreen1 green fluorescent protein, an IRES ribosome binding sequence, a human EF1 alpha promoter , a chimeric antigen receptor of second-generation CAR or third-generation CAR and a regulating element, wherein the original nuclear replicon pUCOri sequence is used for plasmid replication; the resistance gene AmpR sequence is used for massively proliferating target strains; the virus replicon SV40 Ori sequence is used for enhancing replication in eukaryocyte; the lentivirus packaging cis element is used for lentivirus packaging; the ZsGreen1 green fluorescent protein is used for expressing green fluorescent for eukaryocyte; the IRES ribosome binding sequence is used for jointly transcribing and expressing protein; the human EF1 alpha promoter is used for conducting eukaryotic transcription on antigen receptor genes; the chimeric antigen receptor is used for forming the second-generation CAR or the third-generation CAR integrating recognition, transfer and start; the regulating element is used for enhancing expression efficiency of transgenes and used after eWPRE-enhanced type woodchuck hepatitis b virus is transcribed. Besides, the invention further discloses a construction method and application of the vector. By means of the CAR-T transgene vector and the construction method and application of the vector, secretion of cell factors and an in vitro killing effect of CAR-T cells can be remarkably improved, and the clinical treatment effect is remarkable.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
Novel chimeric antigen receptor and applications thereof
PendingCN108276493AMild release responseHigh ability to target and recognize tumor antigensPolypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigen receptorsAntigen binding
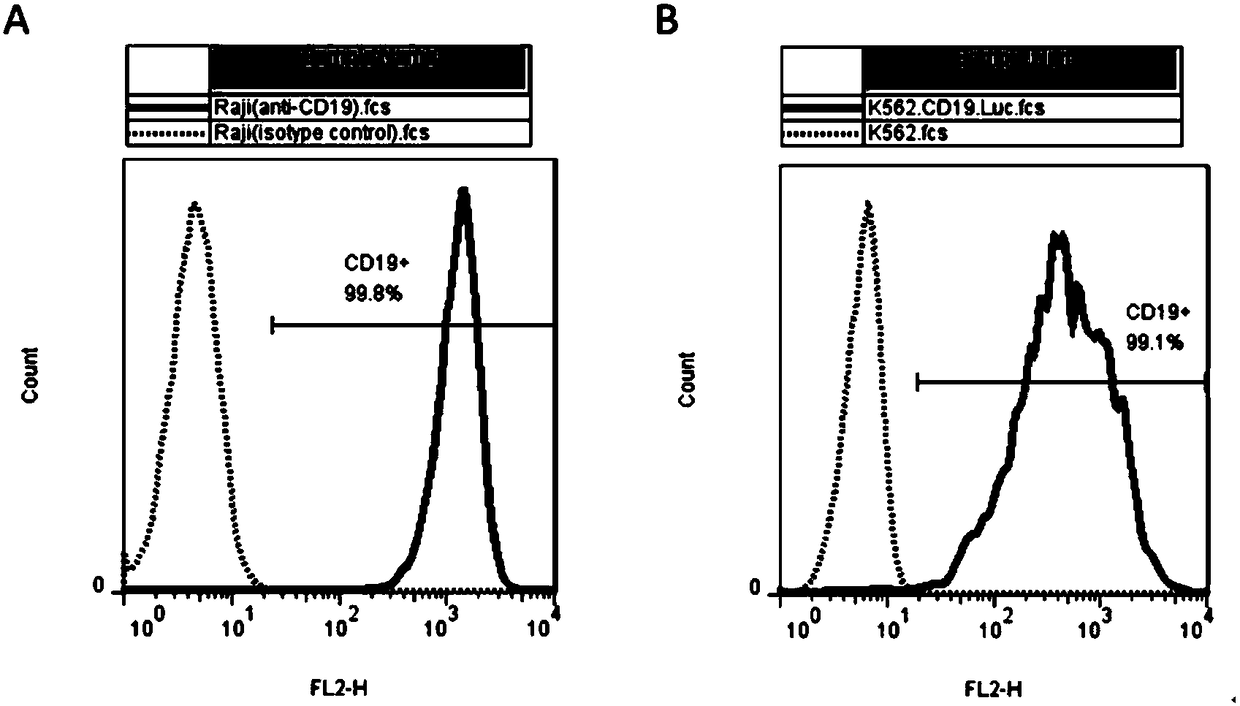
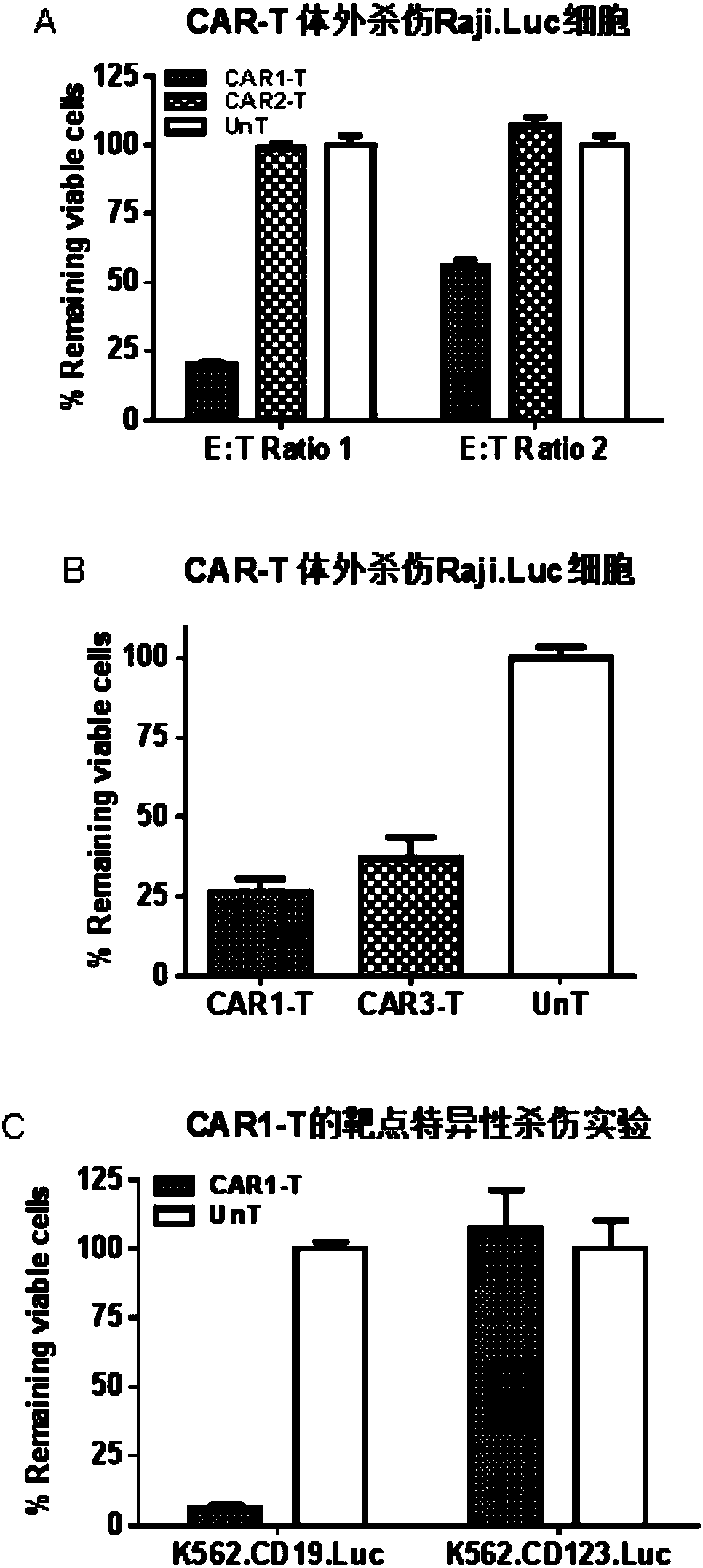
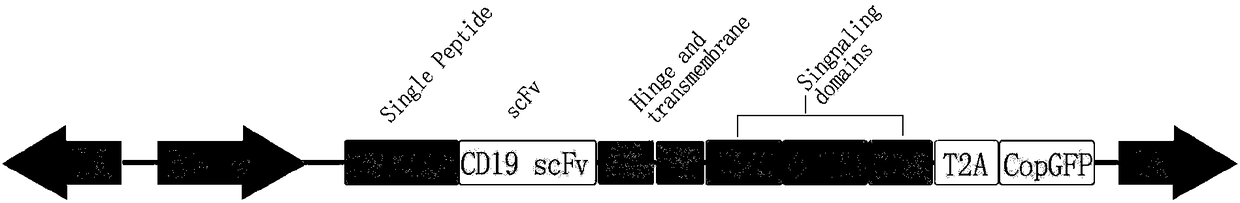
The present invention discloses a novel chimeric antigen receptor and applications thereof, wherein the novel chimeric antigen receptor comprises a signal peptide, an antigen binding domain, a transmembrane domain and an intracellular signal domain, and comprises a 4-1BB signal peptide and / or a 4-1BB molecular transmembrane domain. According to the present invention, a variety of chimeric antigenreceptor nucleic acid sequences are separated and purified, the chimeric antigen receptor specifically for CD19 malignant tumor antigens and the CAR-T cells are provided, and the blood cell line malignant tumor killing test results show that the tumor-cell-targeting ability of immune cells is significantly enhanced, and the tumor cell killing activity is enhanced.
Owner:NANJING LEGEND BIOTECH CO LTD
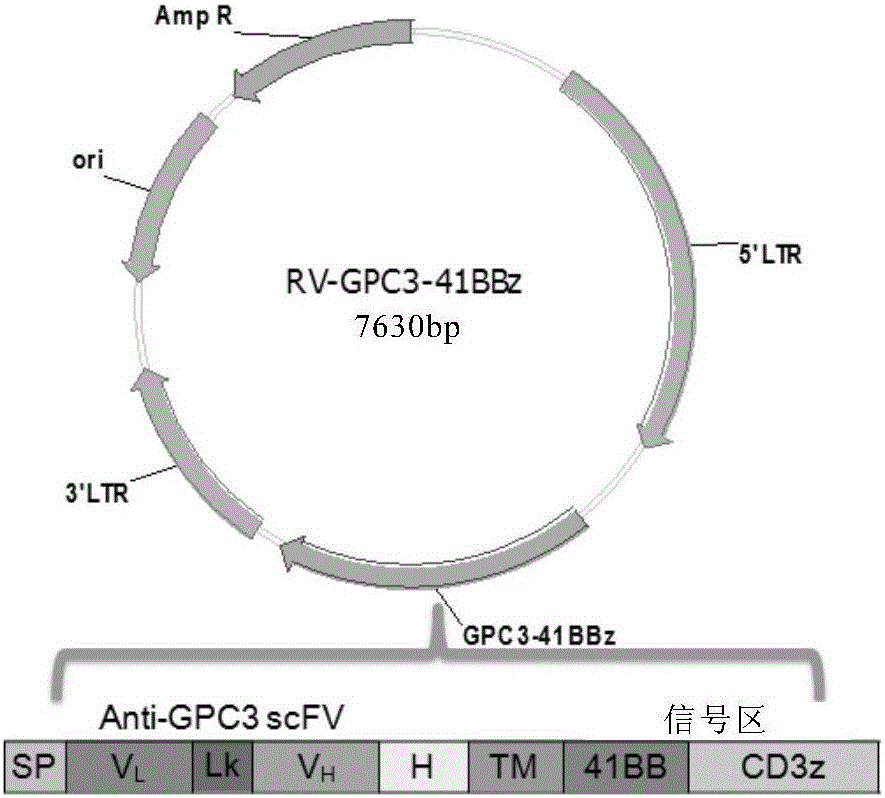
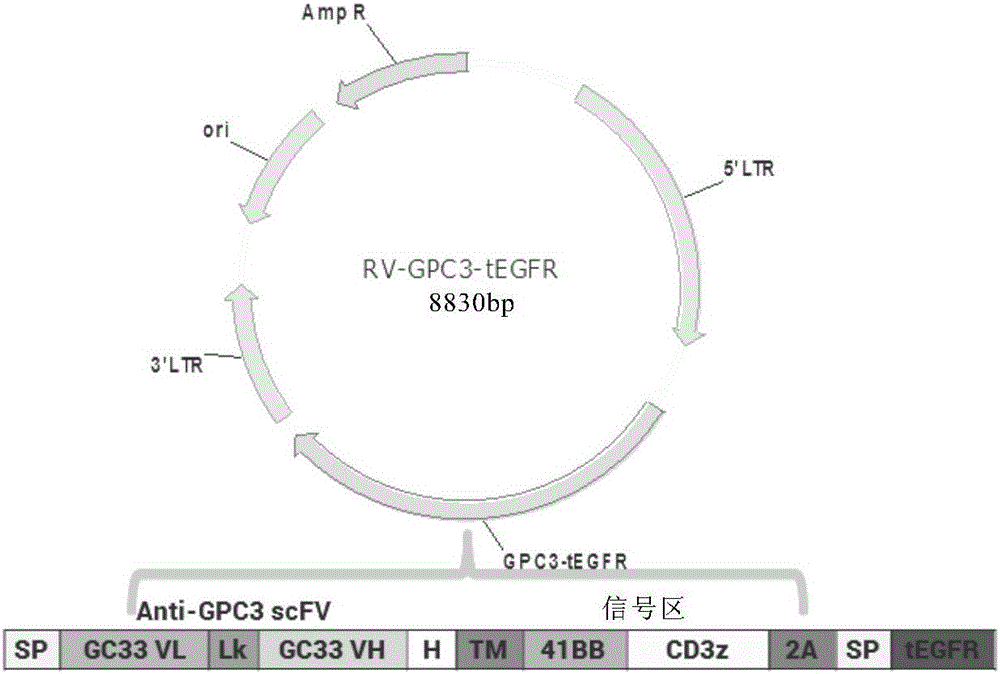
Chimeric antigen receptor of targeted GPC3 (Glypican 3) and application thereof
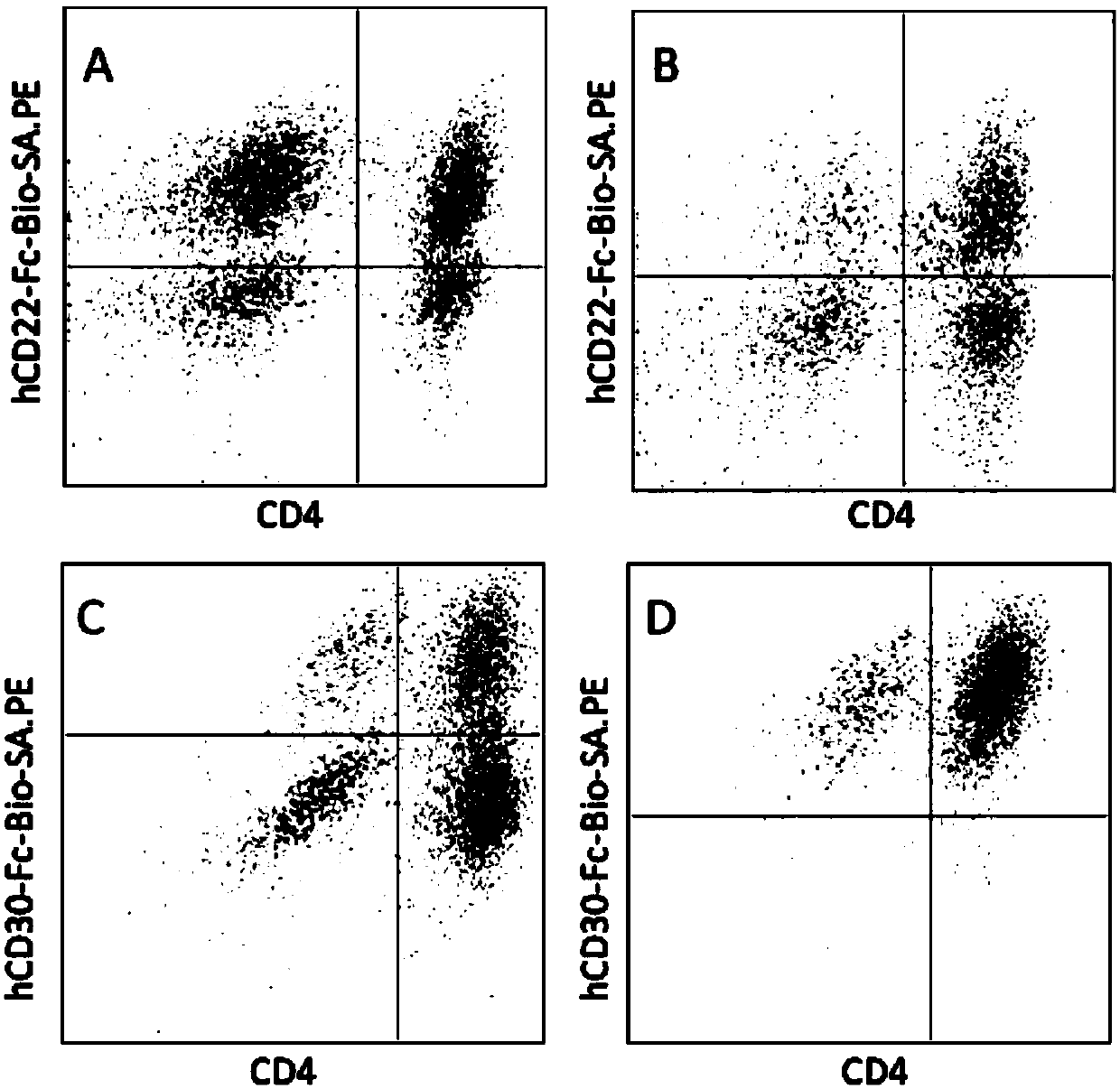
The invention relates to a chimeric antigen receptor of a targeted GPC3 (Glypican 3) and an application thereof. Specifically, the invention provides a polynucleotide sequence, wherein the polynucleotide sequence is selected from: polynucleotide sequences (1) containing a coding sequence of a CD8 antigen leading peptide, a coding sequence of an anti-GPC3 single-chain antibody, a coding sequence of a human CD8 alpha hinge region, a coding sequence of a human CD8 transmembrane region, a coding sequence of a human 41BB intracellular region, and a coding sequence of a human CD3 zeta intracellular region, and a coding sequence of an optional fragment of an EGFR (Epidermal Growth Factor Receptor) sequence containing an extracellular domain III and an extracellular domain IV, wherein the coding sequences are sequentially connected; and complementary sequences (2) of the polynucleotide sequences (1). The invention provides a CAR (chimeric antigen receptor) coded by the polynucleotide sequences and a T cell expressing the CAR. The prepared CAR-T cell has a function of strongly killing a specific tumor cell, and the kill effectiveness is more than 90% under the condition that the effector-target ratio is 20:1.
Owner:HRAIN BIOTECHNOLOGY CO LTD
Universal CAR-T cell and preparation method and application thereof
ActiveCN107723275AImprove expression rateImprove effectiveness and safetyMammal material medical ingredientsNucleic acid vectorAbnormal tissue growthDisease
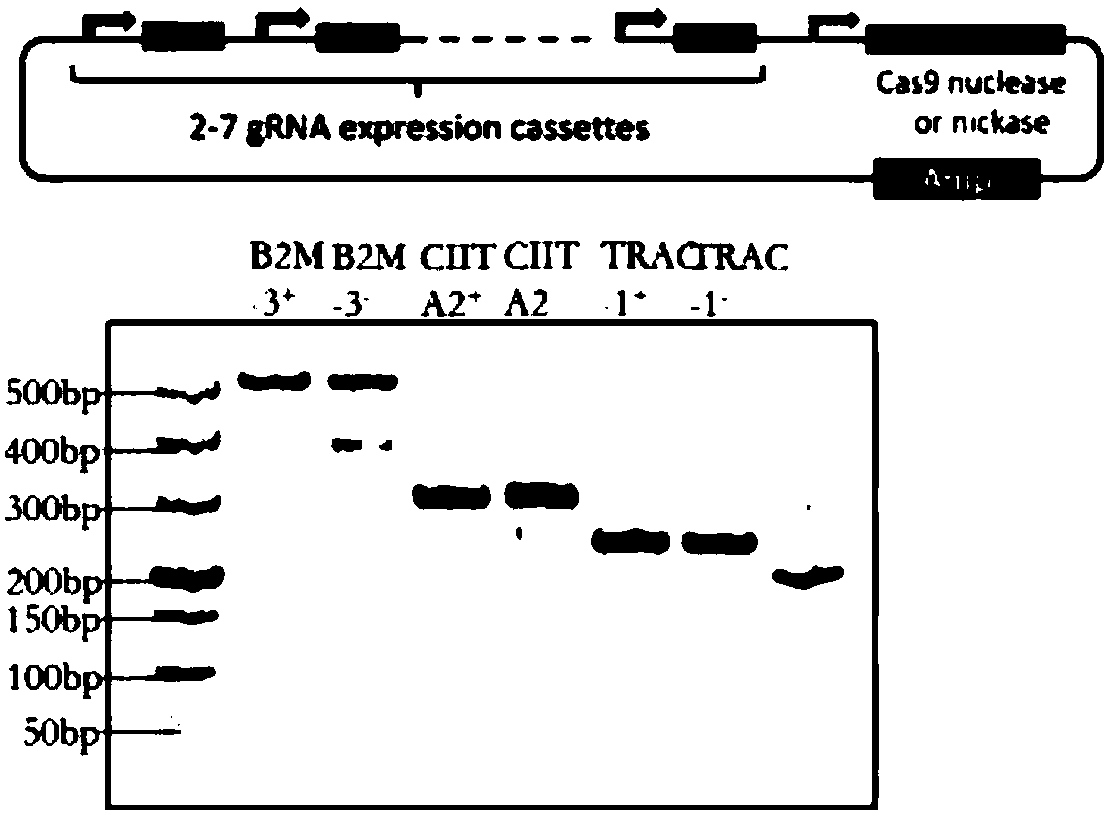
The invention belongs to the field of immunotherapy and relates to a universal CAR-T cell and a preparation method and application thereof. In the universal CAR-T cell, the functions of a T cell antigen receptor (TCR) and major histocompatibility complexes (MHC I and MHC II) in the T cell are inhibited while multi-gene knockout is performed; a gene encoding the TCR includes TRAC and / or TRBC; genesencoding the major histocompatibility complexes include HLA-A, B2MH and CIITA. The universal CAR-T cell can target relevant markers of specific tumors and inactivate the functions of the TCR and theMHC on the cell surface, can reduce immunological rejection caused by allogeneic cell therapy and safely and effectively remove tumor cells in the diseased human body, is not affected by the disease or a treatment mode of a patient in use and can be prepared at any time, treatment can be provided at the optimum time, and treatment effectiveness is ensured.
Owner:CHONGQING PRECISION BIOTECH CO LTD
Preparation method and application of autologous CAR (chimeric antigen receptor)-T cell
InactiveCN106755088ALittle side effectsNo MHC restrictionGenetically modified cellsMammal material medical ingredientsSide effectAntigen binding
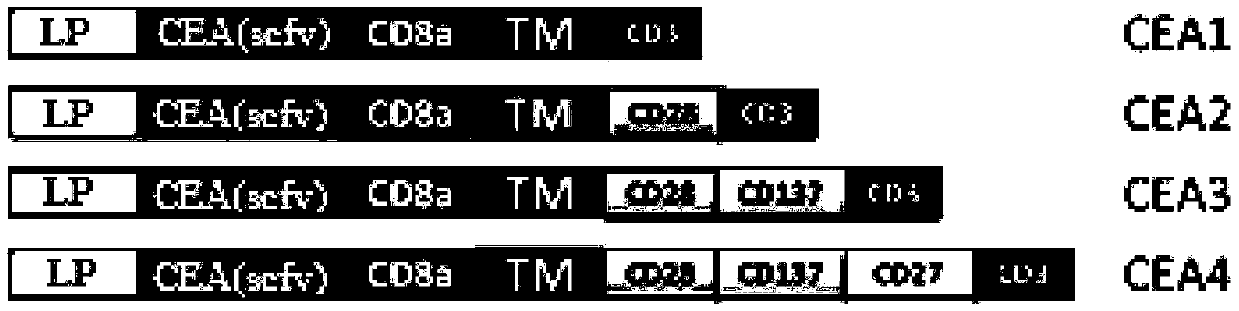
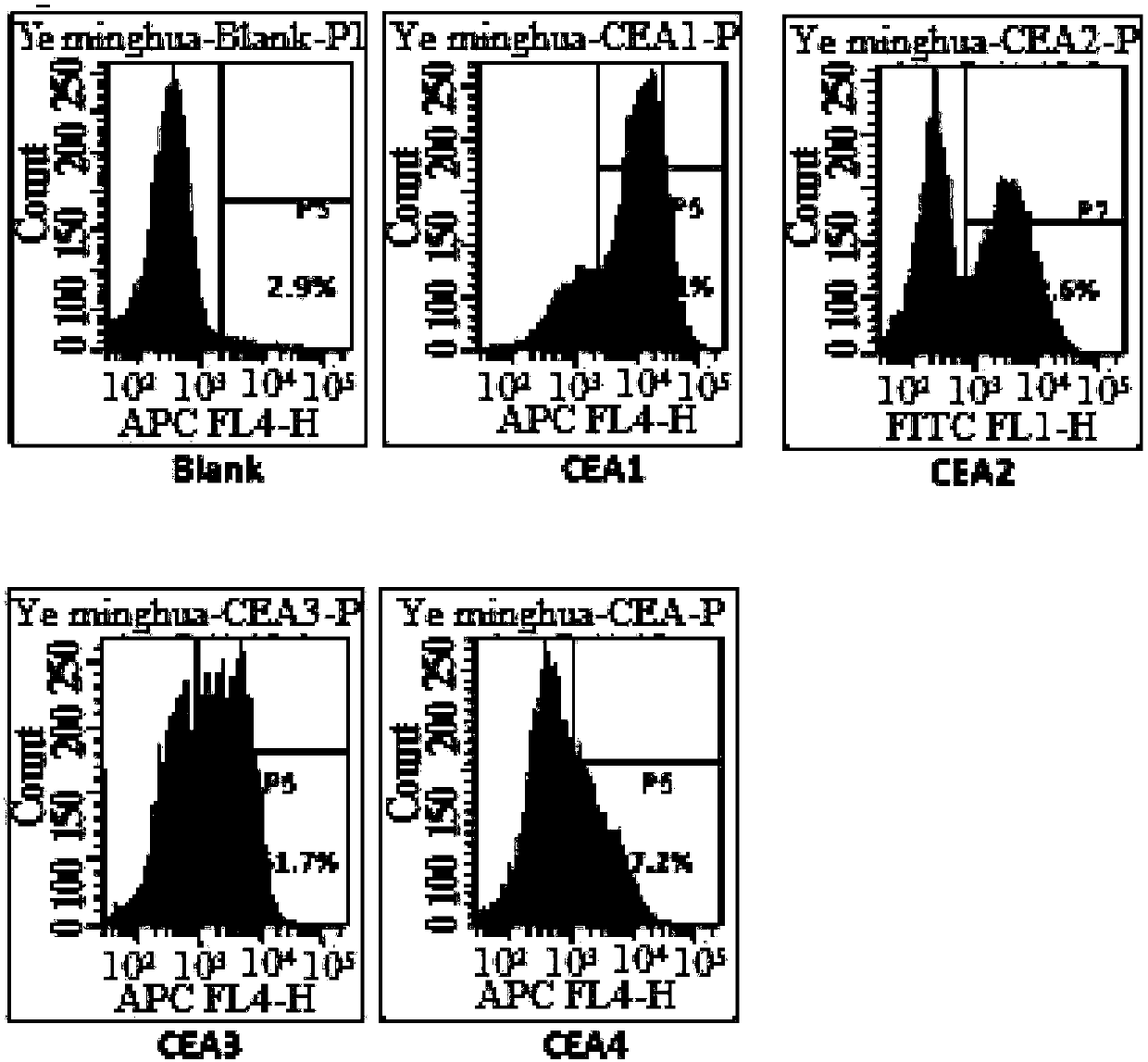
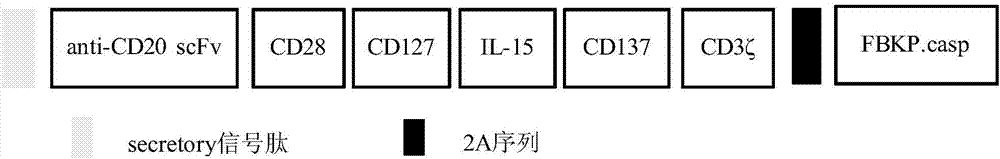
The invention relates to a preparation method and application of an autologous CAR (chimeric antigen receptor)-T cell. An established CD28-CD137-CD19-CD3 full-length gene is guided into a T-cell of a patient by a CRISPR / Cas9 technology to prepare the CAR-T cell, and the CAR-T cell is subjected to expansion in vitro and then returns in the body of the patient to perform anti-tumor treatment. Compared with the traditional tumor treatment method, the method has the advantages that the method is cell targeted therapy and small in side effect; the gene modified T cell can stably express an antigen binding domain on the surface and identify a target antigen, and does not have MHC limit; and the tumor treatment effect is improved.
Owner:GUANGDONG PANGUARD CELL BIOLOGICAL TECH CO LTD
Toxicity Management for Anti-Tumor Activity of CARs
InactiveUS20150202286A1Reducing and avoiding adverse effectReducing and avoiding adverseOrganic active ingredientsBiocideAbnormal tissue growthAntigen binding
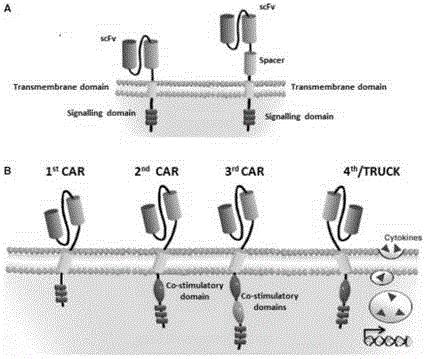
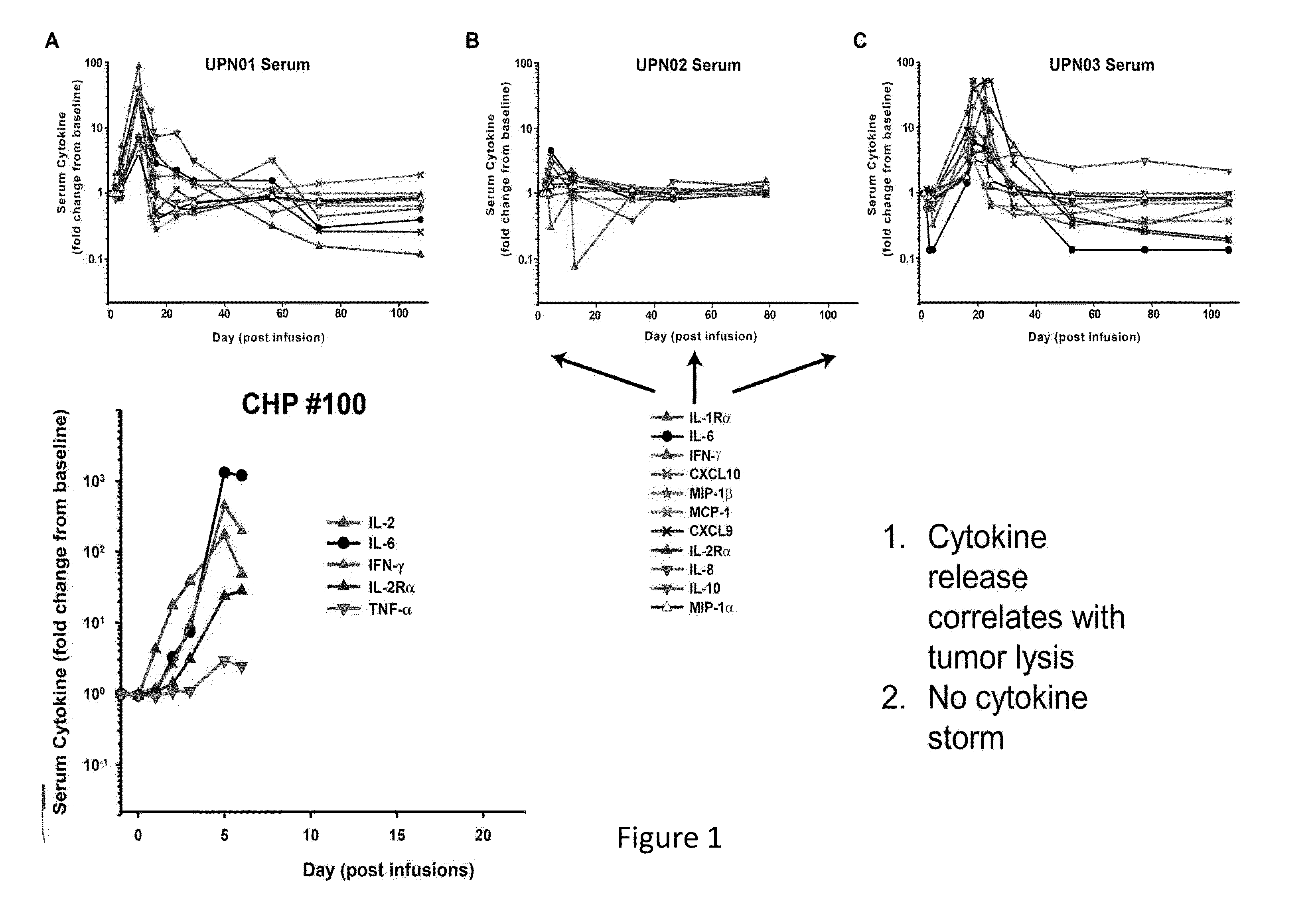
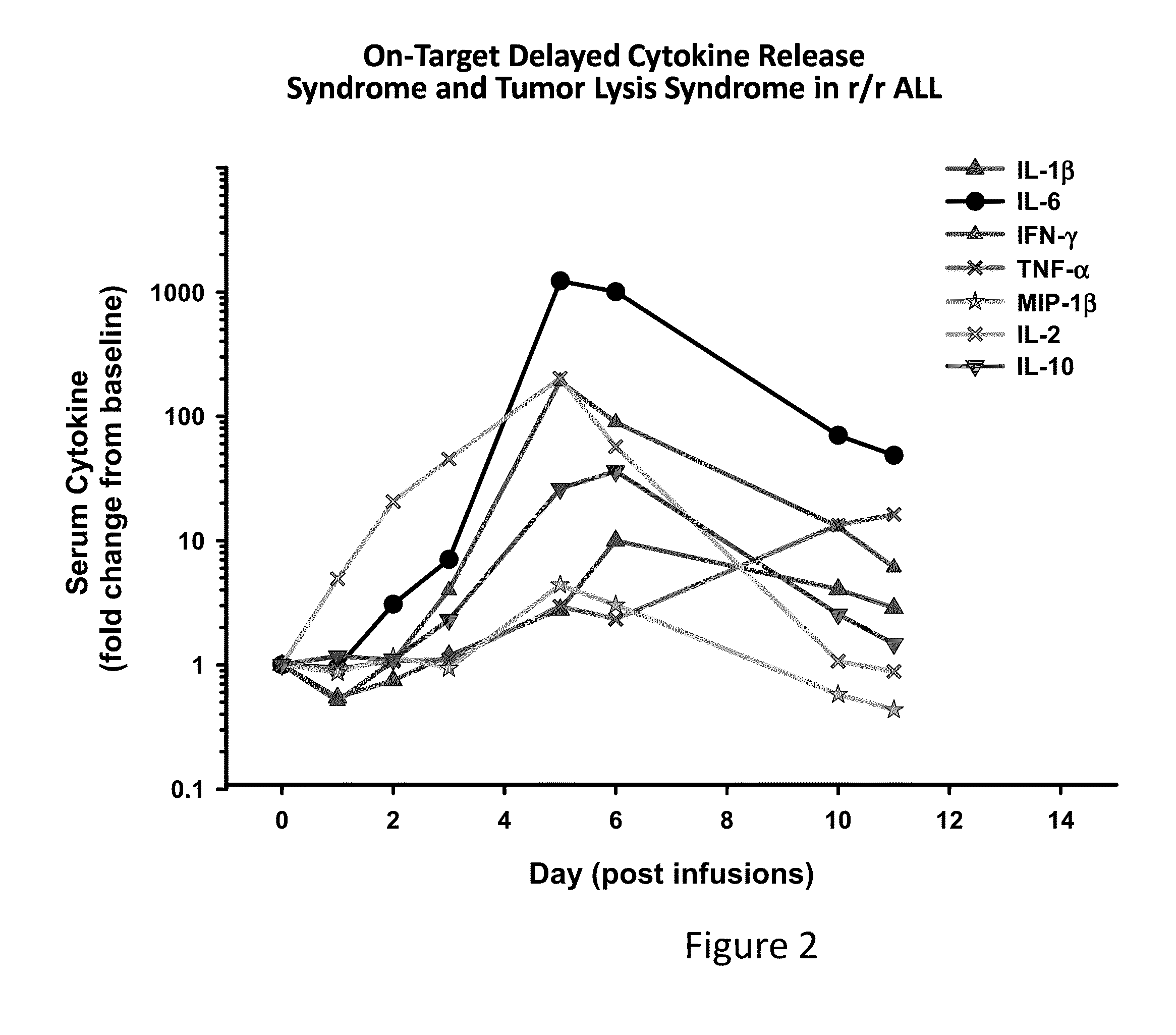
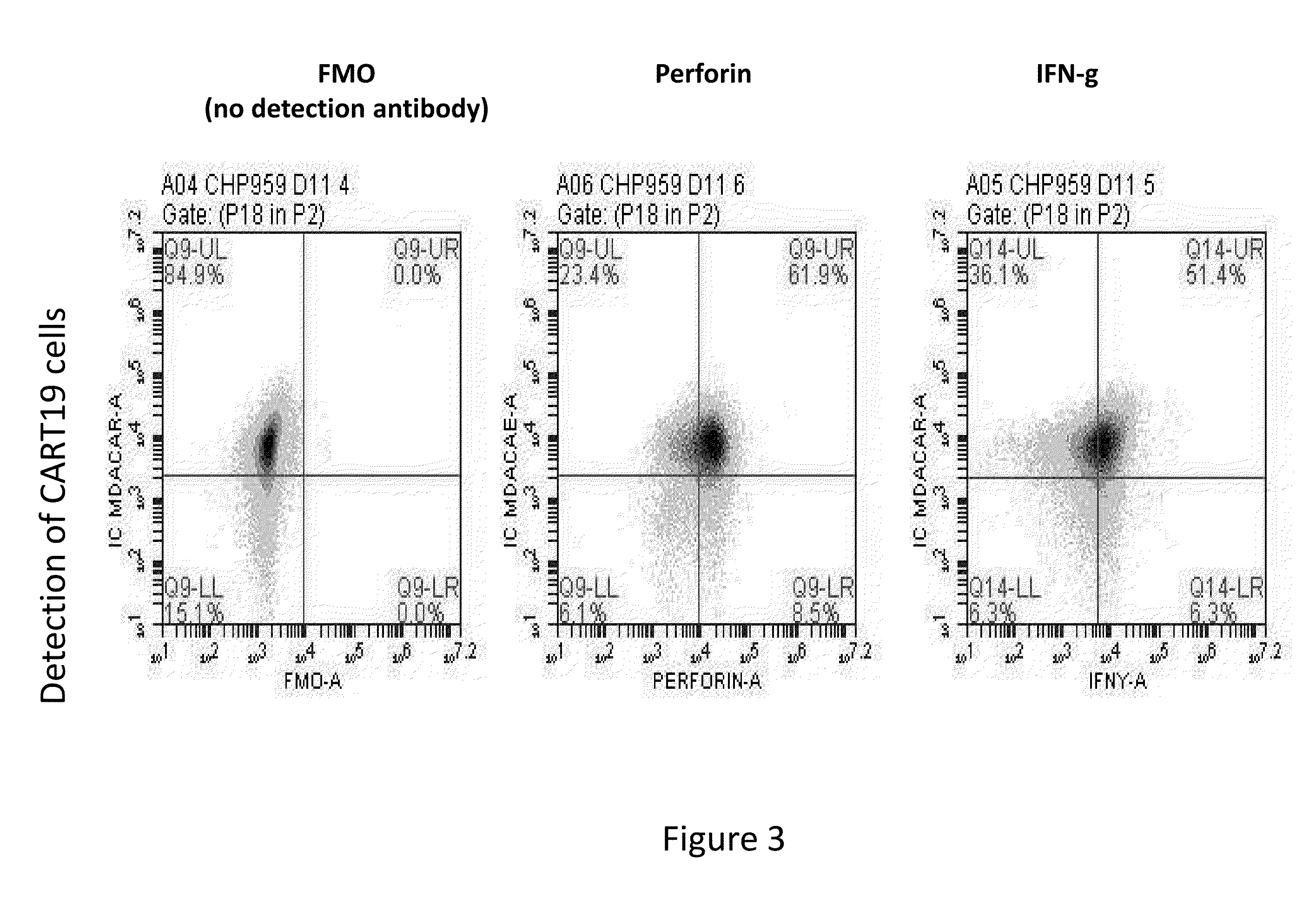
The present invention provides compositions and methods for treating cancer in a patient. In one embodiment, the method comprises a first-line therapy comprising administering to a patient in need thereof a genetically modified T cell expressing a CAR wherein the CAR comprises an antigen binding domain, a transmembrane domain, a costimulatory signaling region, and a CD3 zeta signaling domain and monitoring the levels of cytokines in the patient post T cell infusion to determine the type of second-line of therapy appropriate for treating the patient as a consequence of the presence of the CAR T cell in the patient.
Owner:THE CHILDRENS HOSPITAL OF PHILADELPHIA +1
Chimeric antigen receptors (CAR) and methods for making and using the same
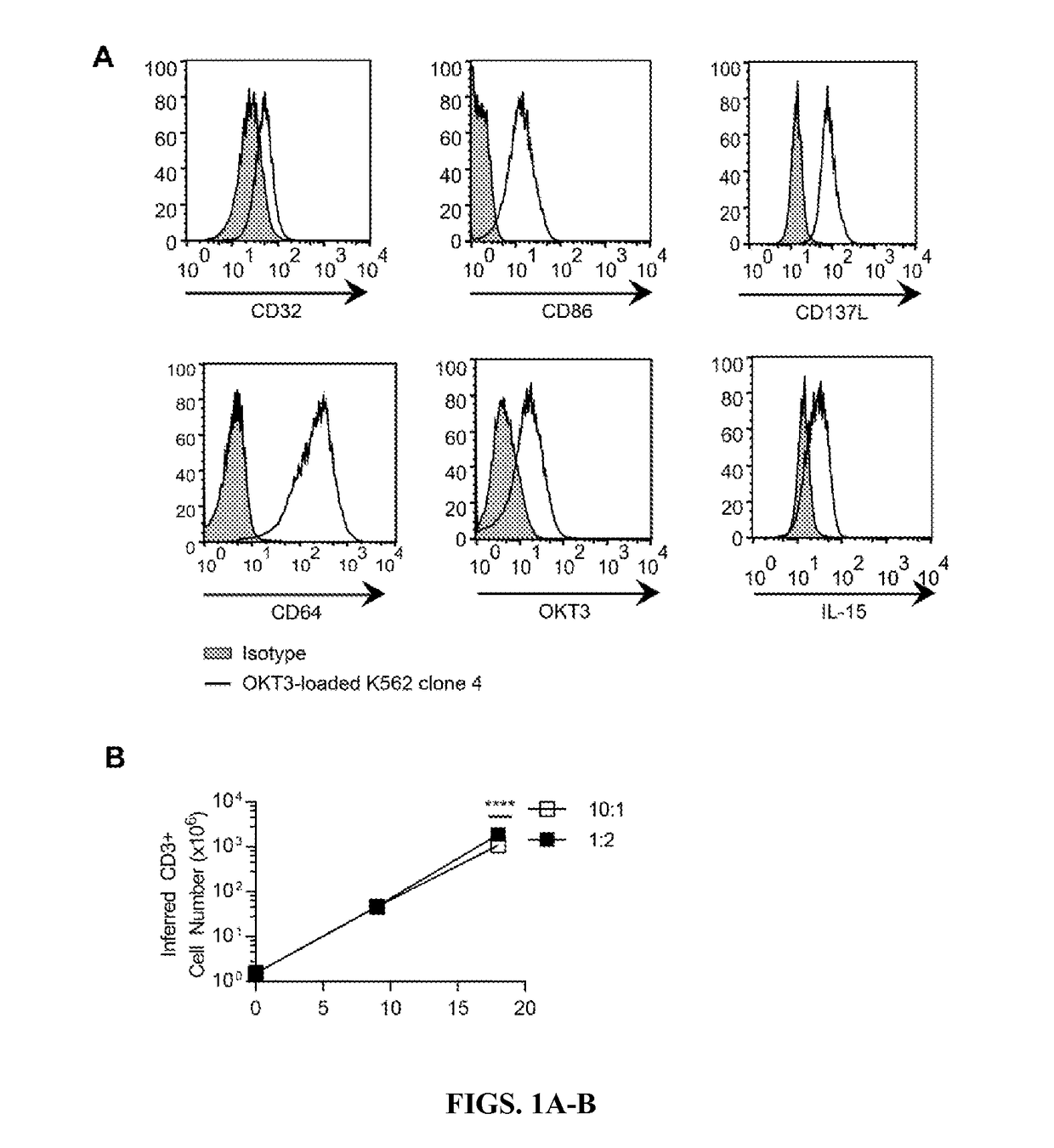
InactiveUS20170158749A1Facilitate cell targetingReduce off-target cytotoxicity of cellAntibody mimetics/scaffoldsMammal material medical ingredientsAntigenCAR T-cell therapy
Chimeric antigen receptors (CARs) and CAR-expressing T cells are provided that can specifically target cells that express an elevated level of a target antigen. Likewise, methods for specifically targeting cells that express elevated levels of antigen (e.g., cancer cells) with CAR T-cell therapies are provided.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Anti-BCMA chimeric antigen receptor, encoding gene, recombinant expression vector and establishing method and application of anti-BCMA chimeric antigen receptor, encoding gene and recombinant expression vector
ActiveCN105777911AImprove in vitro killing effectGood clinical effectPeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsSequence signalSingle-Chain Antibodies
The invention discloses an anti-BCMA chimeric antigen receptor, an encoding gene, a recombinant expression vector and an establishing method and application of the anti-BCMA chimeric antigen receptor, the encoding gene and the recombinant expression vector. The receptor comprises a CD8 leader chimeric receptor signal peptide, a BCMA single-chain antibody heavy chain VH, an Optimal Linker C, a BCMA single-chain antibody light chain VL, a CD8 Hinge chimeric receptor hinge, a CD8 Transmembrane chimeric receptor transmembrane domain, a CD137 chimeric receptor co-stimulatory factor and a TCR chimeric receptor T cell activating domain which are sequentially connected in series. In addition, the invention further discloses the encoding gene and the recombinant expression vector of the anti-BCMA chimeric antigen receptor and the establishing method and application of the encoding gene and the recombinant expression vector. The secretion of cell factors and the cytotoxicity in vitro of CAR-T cells can be remarkably improved, and the clinical treatment effect is outstanding.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
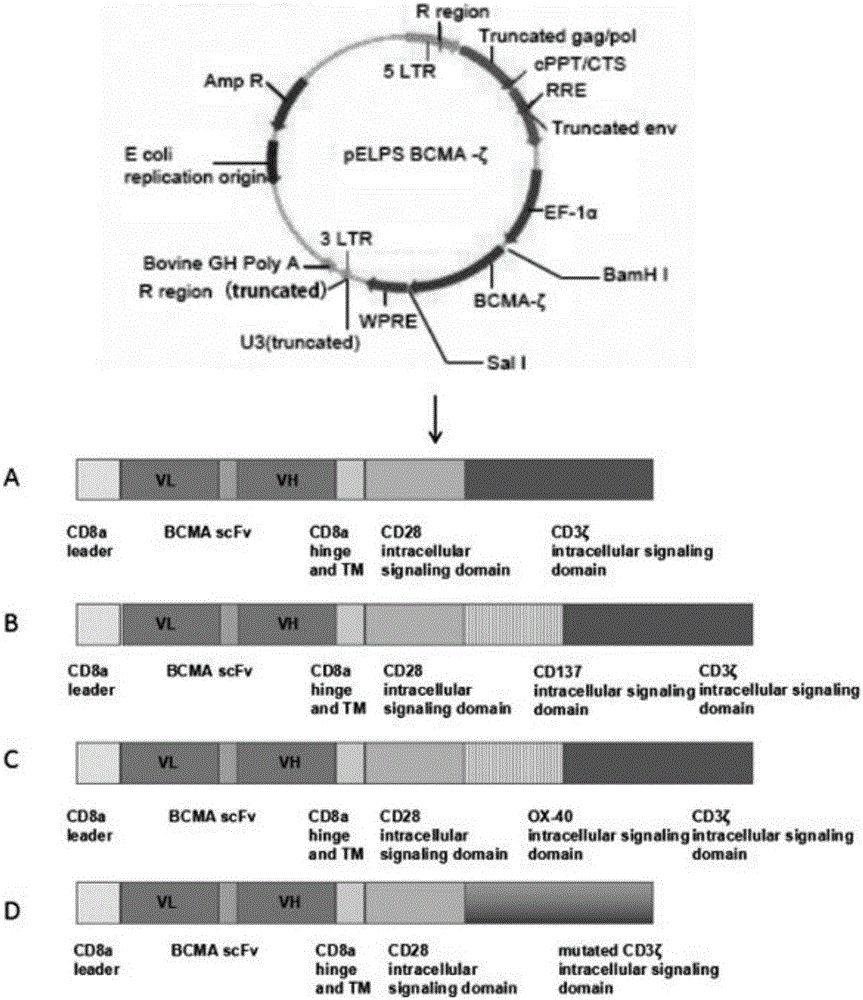
BCMA-based (B cell maturation antigen-based) chimeric antigen receptor and preparation method and application thereof
InactiveCN105837693AImprove anti-apoptotic abilityImprove bindingAntibody mimetics/scaffoldsMammal material medical ingredientsTumor targetAntigen
The invention provides a BCMA-based (B cell maturation antigen-based) chimeric antigen receptor, comprising following cis-form cascade domains: CD8a leader region, scFv fragment BCMA scFv of anti-BCMA antibody, CD8a hinge region and transmembrane region, CD28 intracellular signal domain and CD3 Zeta intracellular signal domain; the CD3 Zeta intracellular signal domain is wild CD3 Zetaintracellular signal domain or mutant CD3 Zeta mut intracellular signal domain; the BCMA-based chimeric antigen receptor can enhance anti-apoptotic capability of T-cells and enhance CAR T-cell and antigen bonding and signal conduction; T-cells with the BCMA-based chimeric antigen receptor show good tumor targeting performance in in-vivo experiments, tumors diminish significantly after two weeks of dosage, and the T-cells have excellent therapeutic effect in vivo.
Owner:李斯文 +1
CD19 targeted CAR (chimeric antigen receptor)-T cell, preparation method and application
InactiveCN107287164AIncrease lethalityHigh transduction efficiencyGenetically modified cellsMammal material medical ingredientsCAR T-cell therapySingle-Chain Antibodies
The invention provides a CD19 targeted CAR (chimeric antigen receptor)-T cell, a preparation method and an application and relates to the field of immune cells. The activation capacity of T cells in CAR-T cell therapy can be improved, the problem of insufficient transfection efficiency can be solved, and the CAR-T cell has a high killing capacity for CD19. The CD19 targeted CAR-T cell expresses a CD19 targeted CAR gene on surface, and the CD19 targeted CAR gene is formed by connecting a single-chain antibody CD19ScFv of CD19, a hinge region and a transmembrane region of CD8, an intracellular signal structure of CD28, an intracellular signal structure of 4-1BB and an intracellular signal structural domain of CD3zeta in series, and the base sequence of the CD19 targeted CAR gene is shown as SEQ ID NO:2.
Owner:青岛见康华美医学检验有限公司
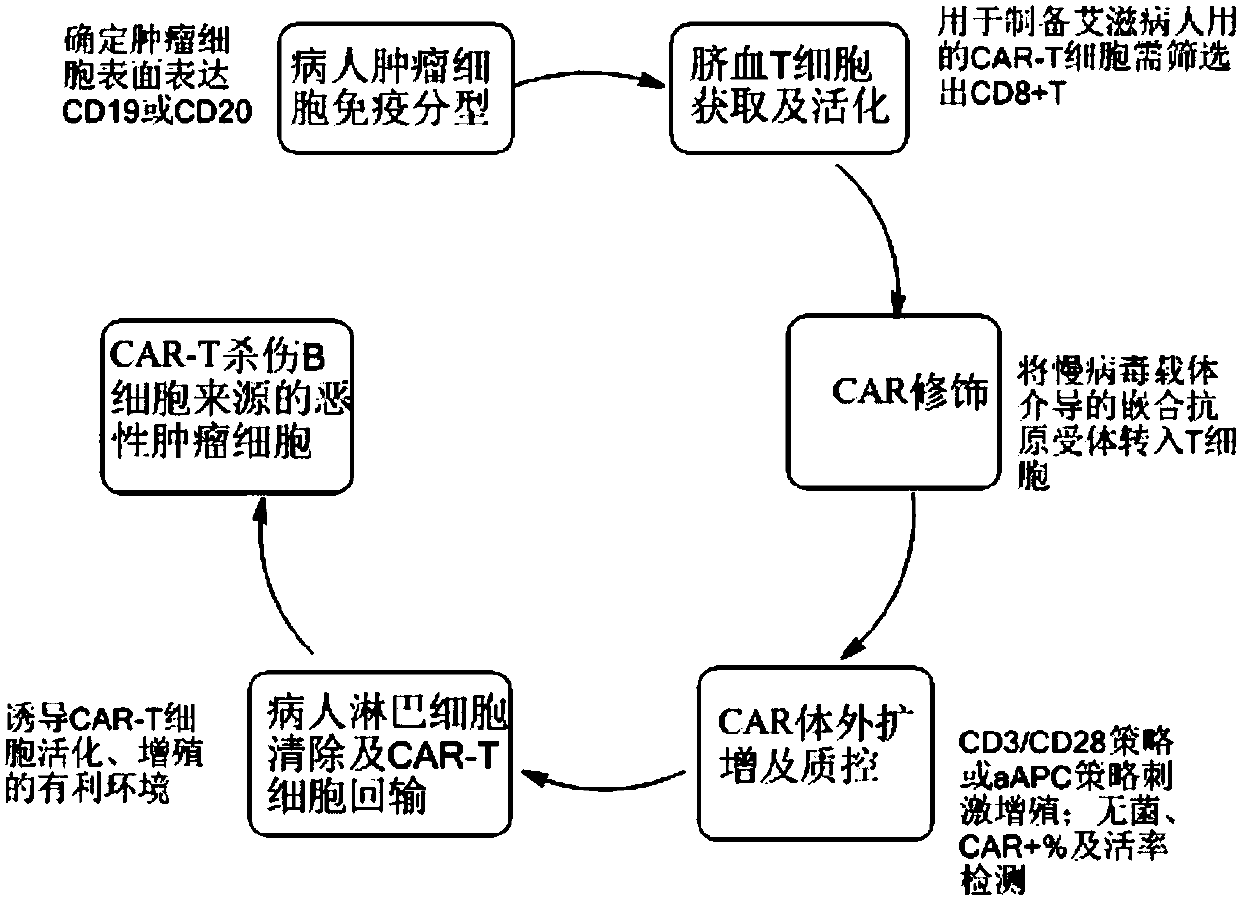
CAR-T cell for treating AIDS-associated lymphoma, and preparation method and application thereof
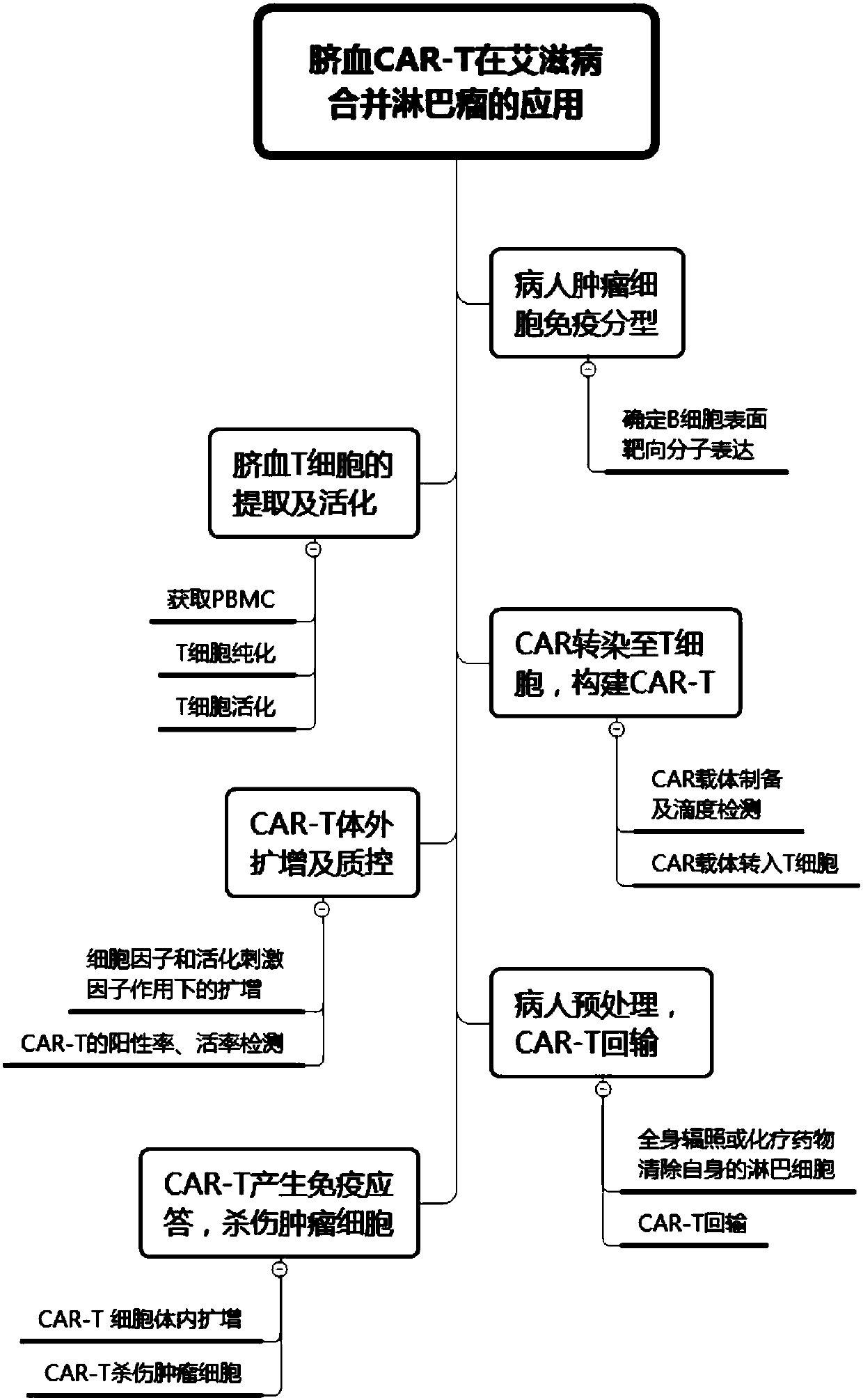
PendingCN107904259ALow storm riskSmall storm riskGenetically modified cellsAntiviralsAIDS-related lymphomaCord blood stem cell
The invention discloses a preparation method of a CAR-T cell for treating AIDS-related lymphoma. A CD8+ T cell is used to produce the CAR-T cell, and the CD8-T cell derives from a cord blood T cell. The method includes the following steps: preparing cord blood mononuclear cells from cord blood, removing tumors and other cells by using a human T cell purification kit to obtain T cells, and carryingout separation by using magnetic beads to obtain the CD8+ T cell; activating the CD8+ T cell by using an appropriate medium and appropriate stimulation conditions; transferring CAR to the CD8+ T cellby using lentivirus to prepare the CAR-T cell; and amplifying the CAR-T cell in vitro by using cytokines and activating stimulators to achieve the desired effective dose. The invention also relates to the CAR-T cell prepared by the method, and an application thereof. The CAR-T cell prepared in the invention has a good cell activity and a high proliferation speed, and reduces the attack risk of GVHD; and only the CD8+T cell of the umbilical blood T cells of AIDS patients is used to prepare the CAR-T cell for, so the risk of input CAR-T infected with in-vivo HIV is reduced.
Owner:SHANGHAI LONGYAO BIOTECH CO LTD
Polypeptide, nucleic acid for coding polypeptide, polypeptide-modified T lymphocyte and application of T lymphocyte
ActiveCN107827990AEffective treatmentIncrease the number ofMammal material medical ingredientsImmunoglobulinsFunctional activitySignalling pathways
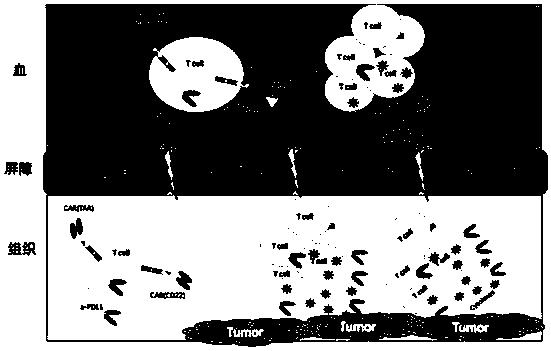
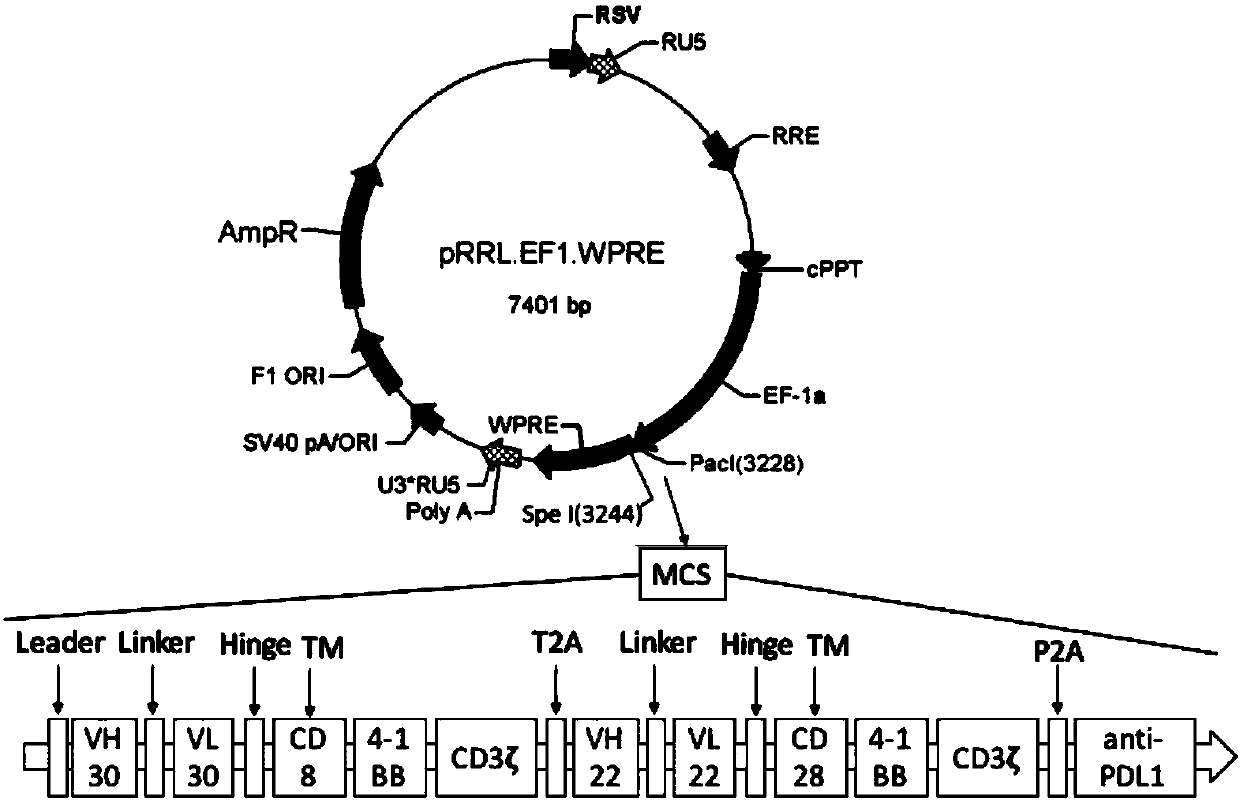
The invention belongs to the technical field of biology, and discloses a polypeptide containing two chimeric antigen receptor structures, nucleic acid for coding the polypeptide, a modified T lymphocyte, and a preparation method and application of the T lymphocyte. The polypeptide provided by the invention contains the two chimeric antigen receptor (CAR) structures, wherein one chimeric antigen receptor structure specifically targets a tumor cell, and the other one specifically targets a normal B lymphocyte, so that a CAR-T cell is effectively amplified through recognizing the B lymphocyte ofa blood circulation system, and more CAR-T cells arrive at a solid tumor part so as to specifically kill the tumor cell for improving a treatment effect on the solid tumor. The polypeptide further comprises an ScFv structure of an anti-PD-L1 monoclonal antibody, and the CAR-T cells can simultaneously secrete a PD-L1 monoclonal antibody which interdicts an immunosuppressive action medicated by a PD1 / PDL1 signal pathway and T cell failure, so that the treatment effect on the solid tumor is improved, the functional activity of immune cells in vivo is prolonged at the same time, and finally the tumor recurrence is retarded and even avoided.
Owner:HEBEI SENLANG BIOTECH CO LTD
Use of the cd2 signaling domain in second-generation chimeric antigen receptors
InactiveCN104136458AAvoid deathPolypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigen receptorsAntigen binding
The present invention provides compositions and methods for treating cancer in a human. The invention includes relates to administering a genetically modified T cell expressing a CAR having an antigen binding domain, a transmembrane domain, a CD2 signaling domain, and a CD3 zeta signaling domain. The invention also includes incorporating CD2 into the CAR to alter the cytokine production of CAR-T cells in both negative and positive directions.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
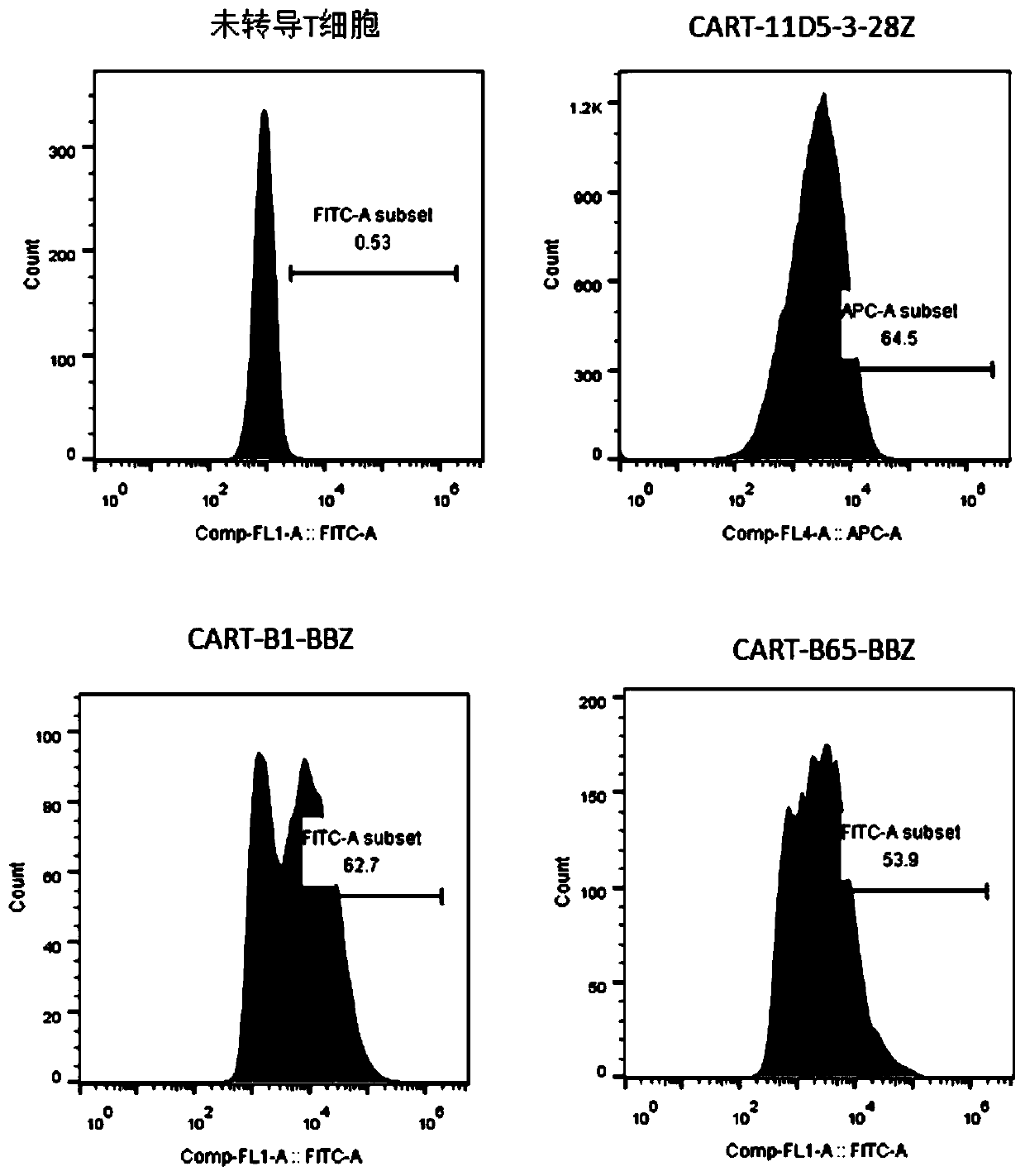
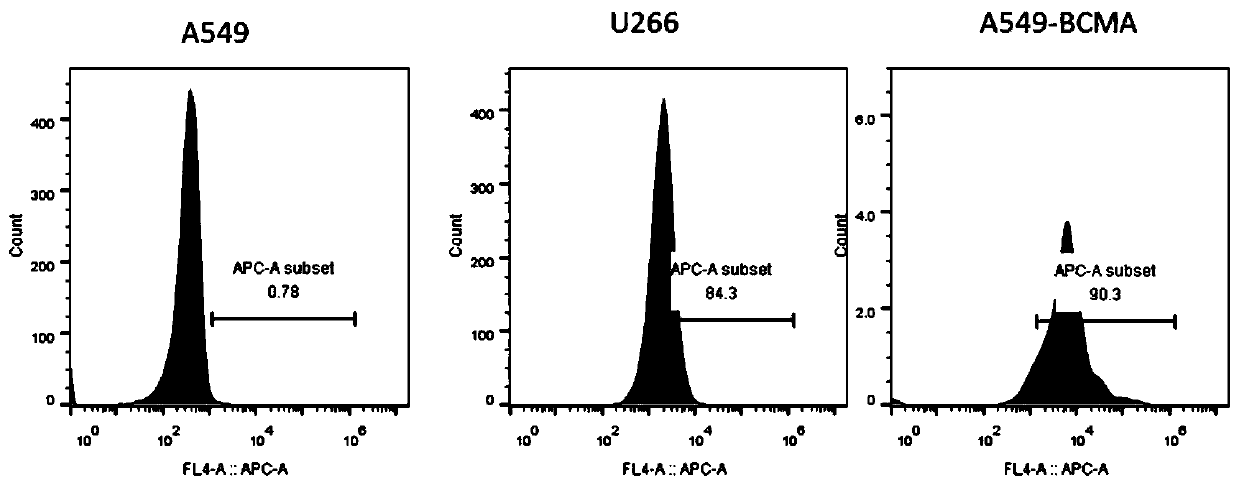
Chimeric antigen acceptor of target BCMA (B cell maturation antigen) and application of chimeric antigen acceptor
ActiveCN110041433ASmall molecular weightHighly effective specific therapyMammal material medical ingredientsImmunoglobulinsBCMA ProteinB-Cell Maturation Antigen
The invention relates to a chimeric antigen acceptor of a target BCMA (B cell maturation antigen). The chimeric antigen acceptor comprises an extracellular identification region, a hinge region, a transmembrane region and an intracellular signal region, wherein the extracellular identification region has a BCMA resisting nanometer antibody sequence, and the BCMA resisting nanometer antibody sequence is a heavy-chain variable region sequence combining with BCMA and from alpacas. More specifically, the BCMA resisting nanometer antibody sequence is BCMA monoclonal antibody B1 or B65, the amino acid sequence of the B1 is as shown in SEQID NO.1, and the amino acid sequence of the B65 is as shown in SEQID NO.2. According to the chimeric antigen acceptor disclosed by the invention, compared witha traditional mouse-derived SCFV or humanized SCFV, the nanometer antibody from the alpacas is used, and has small molecule quantity and immunogenicity, the possibility that CAR-T cells prepared on the base of the nanometer antibody produce HAMA effects in vivo is smaller, the remaining time of the CAR-T cells in vivo can be longer, the CAR-T cells pass through BCMA protein on the surfaces of target tumor cells and activate signal channels at the downstream part of T cells, the capacity for killing tumor cells having BCMA target points is given to the T cells, and BCMA positive blood tumor canbe efficiently and specifically treated.
Owner:上海科棋药业科技有限公司
CD19-based chimeric antigen receptor and application thereof
PendingCN108383914ALess prone to stormsGood killing effectPeptide/protein ingredientsAntibody mimetics/scaffoldsAbnormal tissue growthSide effect
The invention relates to a CD19-based chimeric antigen receptor and application thereof, in particular to a lentivirus vector material built by a chimeric antigen receptor T (CAR-T) cell technology using a tumor specific target point CD19 as the basis, a method, and application thereof to anti-tumor treatment. The chimeric antigen receptor is formed by serially connecting an antigen combination structure domain, a membrane spaning structure domain, a costimulatory signal conduction region, a CD3 zeta signal conduction structure domain and an inducible suicide fusion structure domain, wherein the antigen combination structure domain is combined with the tumor surface antigen; the tumor surface antigen is CD19. The chimeric antigen receptor is subjected to specific gene transformation on theT cell stimulation signals. Compared with other chimeric antigen receptors, the chimeric antigen receptor provided by the invention has a better reaction effect and higher safety, so that the CAR-T cells have higher immune effects and low side effects; the treatment effect and safety of the CAR-T cells are enhanced.
Owner:BEIJING MEIKANG JIMIAN BIOTECH CO LTD
Chimeric antigen receptor containing CD27 intracellular domain, lentiviral vector and application thereof
ActiveCN105949325AHave the ability to bind antigenEfficient killingMammal material medical ingredientsNucleic acid vectorAntigenSignalling molecules
The invention discloses a chimeric antigen receptor containing a CD27 intracellular domain, a lentiviral vector and application thereof. The immune receptor tyrosine activation sequence motif of the chimeric antigen receptor containing CD27 intracellular domain contains CD27molecule intracellular signal domain and T cell costimulatory signal molecules. The CD27molecule intracellular signal domain is connected with costimulatory signals related to T cell activation, so that the in-vitro proliferation and apoptosis effect of T cells can be obviously enhanced, and the ratio of young sample Tscm cells (CD45RA+CD62L+) with in vivo therapeutic action and central memory cells (CD45RO+CD62L+) in CAR-T cells is increased; expression of IL-10 factors capable of restraining immunization is reduced, an obvious effect of improving the CAR-T (T cell chimeric antigen receptor) cellular therapy effect is achieved, and a new idea and a new selection are provided for the field of CAR-T cellular therapy.
Owner:CHONGQING PRECISION BIOTECH CO LTD
Tumor immunization method combining with chimeric antigen T cells targeting at PD-1 (programmed cell death protein 1) and EGFR (epidermal growth factor receptor)
InactiveCN107034235AIncrease lethalityImprove homingGenetically modified cellsMammal material medical ingredientsTumor targetEGFR Antibody
The invention discloses a tumor immunization method combining with chimeric antigen T cells targeting at PD-1 (programmed cell death protein 1) and EGFR (epidermal growth factor receptor) and also discloses a plasmid vector for implementing the method. In combination with fourth-generation CAR-T (chimeric antigen receptor T) cells targeting at PD-1 and EGFR, a lentiviral vector is used as a CAR-T vector basic structure, and truncated EGFR antibody is selected as a CAR core to give tumor targeted enrichment to play, tumor-killing effect is given to play in conformity with overexpressed immune checkpoint inhibitor PD-1 monoclonal antibody; by constructing the fourth-generation CAR-T vector for co-expressing various regulatory factors such as IL21, CCR4 and Bcl2, the killing, homing and persistent proliferating abilities of CAR-T cells are improved. EP-CAR T (esophageal papilloma chimeric antigen receptor T) cells are treated by transducing patient's autologous T-lymphocytes in vitro, amplifying suitably and transducing back to the patient's body via autologous transfusion, and no reports on similar designs of CAR-T cells are provided at present.
Owner:尹荣
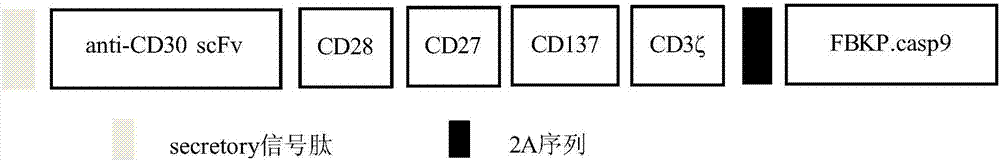
Embedded antigen receptor based on CD30 and application thereof
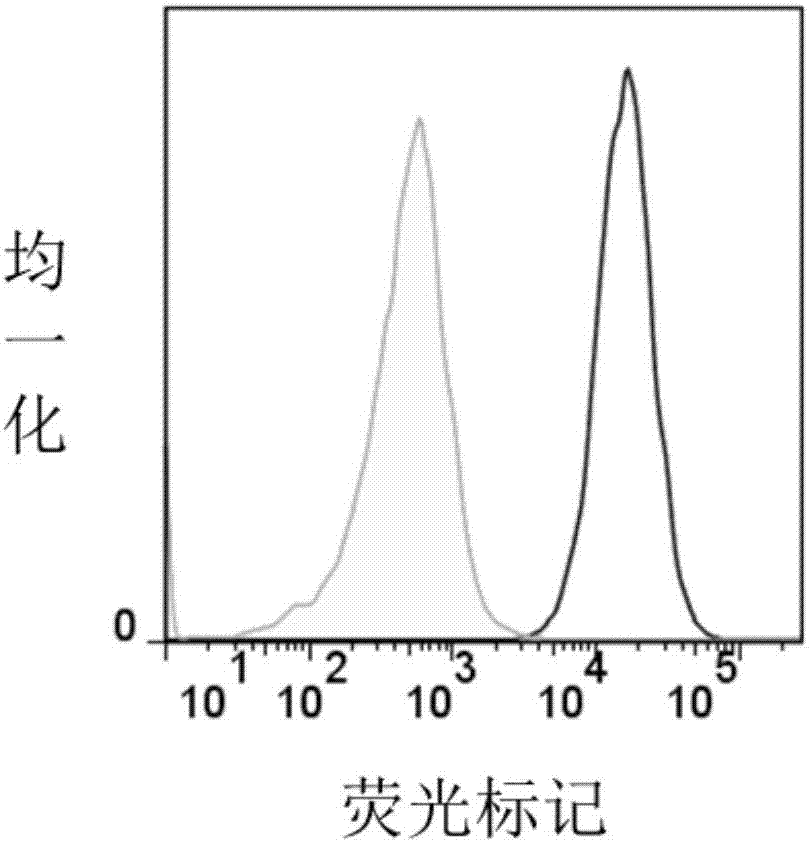
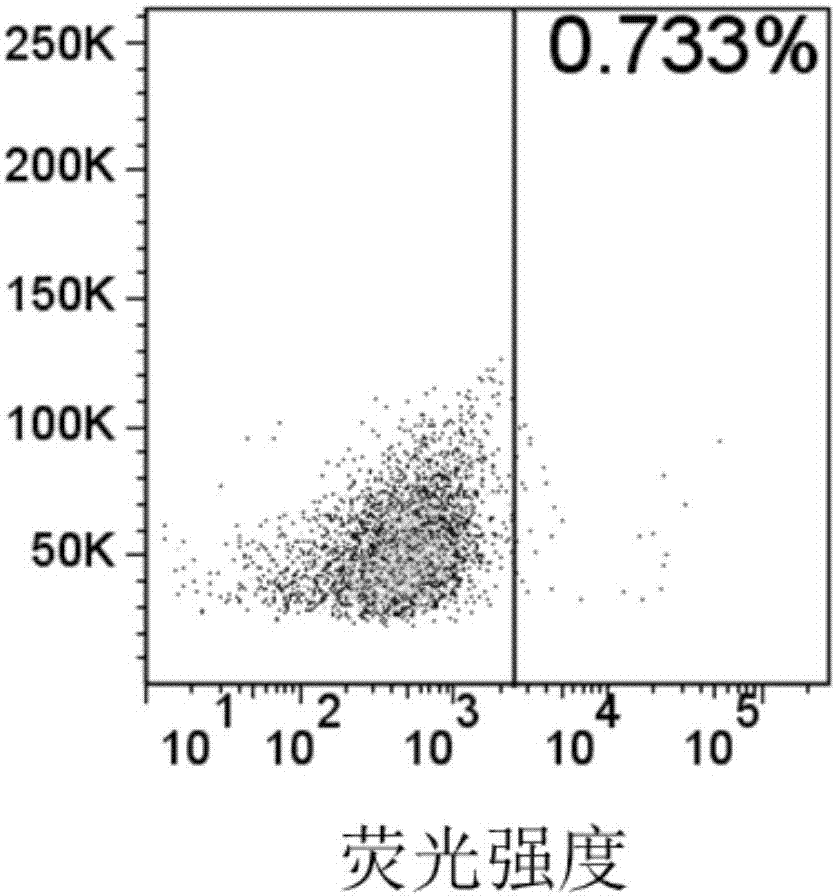
ActiveCN107312097AHigh expressionImprove immunityAntibody mimetics/scaffoldsMammal material medical ingredientsSingle-Chain AntibodiesAntigen receptors
The invention relates to an embedded antigen receptor based on CD30 and application thereof, and particularly relates to a building method of an embedded antigen receptor T (CAR-T) cell technology based on a tumor specific target site CD30 and application thereof in anti-tumor treatment. The embedded antigen receptor is prepared by connecting an antigen binding structural domain, a transmembrane structural domain, a costimulatory signal conducting region and a CD3 zeta signal conducting structural domain in series, wherein the antigen binding structural domain is combined with tumor-surface antigen, and the tumor-surface antigen is CD30. By adopting the embedded antigen receptor, specific gene transformation on a single chain antibody of the tumor-surface antigen CD30 is carried out, and because of the transformed antibody, the binding force of the antigen-antibody is stronger, and the mutation difficultly occurs; compared with other embedded antigen receptors and other tumor antigens, the embedded antigen receptor has a better effect, and the expression quantity of the target site is high, so that an immunization effect of CAR-T cells is enhanced, and a treatment effect on the CAR-T cells is enhanced.
Owner:SHENZHEN GENO IMMUNE MEDICAL INST
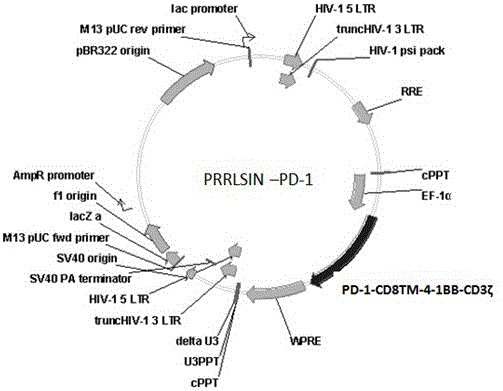
PD-1 CAR-T cell as well as preparation method and application thereof
ActiveCN106399255AEfficient tumor killing activityPeptide/protein ingredientsAntibody mimetics/scaffoldsAbnormal tissue growthDisease
The invention discloses a PD-1 CAR-T cell as well as a preparation method and an application thereof. Through a transformation method of modifying the T cell by virtue of a chimeric antigen receptor, PD1CD8TM41BBCD3[zeta] molecules are expressed in the T cell. The CAR-T cell prepared by the method can specifically recognize and bind tumor cells which achieve high-expression in PDL-1 protein; therefore, the CAR-T cell can be applied to the preparation of medicines for preventing and treating tumor diseases.
Owner:四川阿思科力生物科技有限公司
Nucleic acid molecule for enhancing antitumor activity of T cells
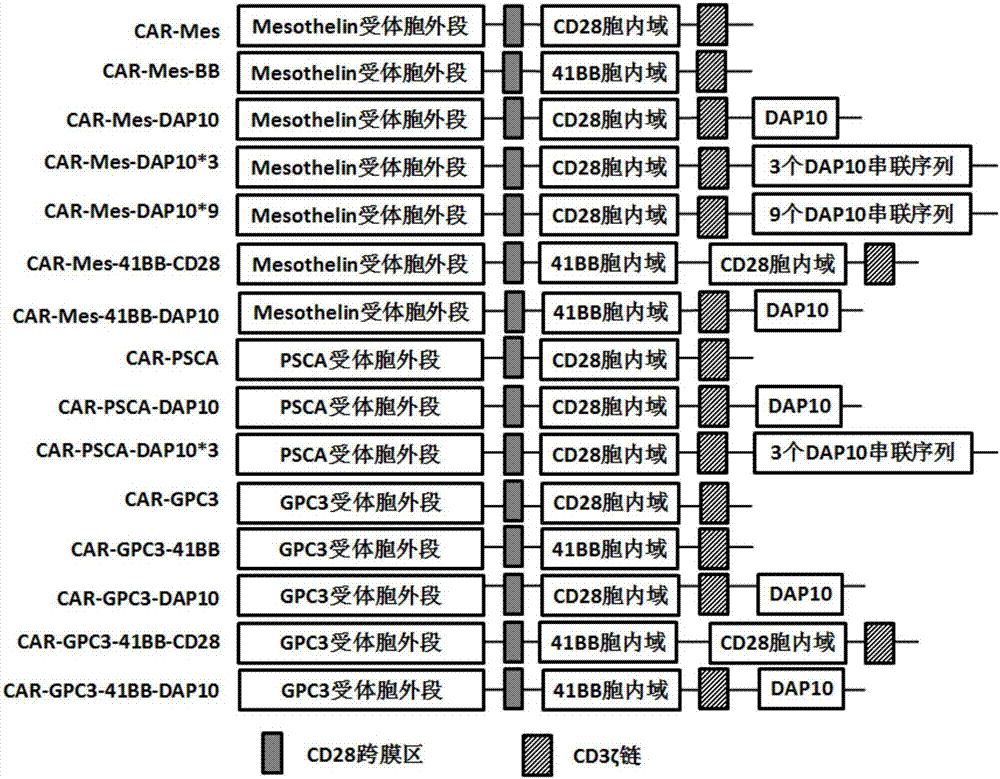
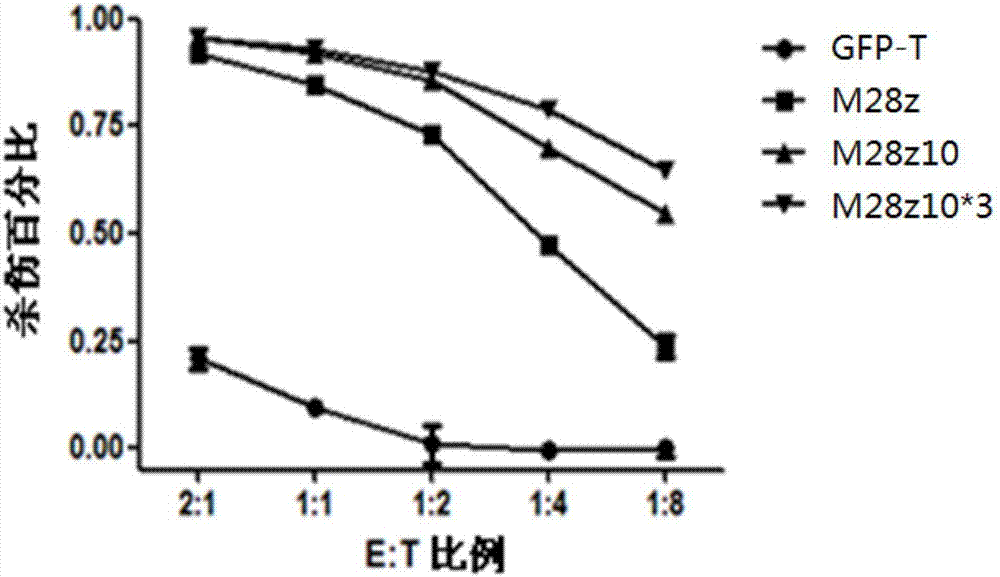
PendingCN107573419AGood immune effectImprove anti-tumor effectMammal material medical ingredientsFermentationIn vivoSolid tumor
The invention relates to a new chimeric antigen receptor (CAR) molecule containing an intracellular segment of DAP 10 protein, nucleic acid for encoding the CAR molecule, cells for expressing the CARmolecule, and use of the CAR molecule in preparation of medicines for treating tumors. The CAR molecule provided by the invention is realized by introducing the intracellular segment of the DAP 10 protein into the traditional second generation of CAR molecule; a downstream signaling pathway of the DAP10 of the T cells is activated after binding of the CAR molecule and a target antigen, so that thekilling effect of the corresponding CAR T cells on solid tumor in vivo and in vitro is improved, and the proliferation and survival abilities of the CAR T cells are enhanced.
Owner:SHENZHEN IN VIVO BIOMEDICINE TECH LTD
Embedded antigen receptor based on CD22 and application thereof
ActiveCN107312098AHigh expressionImprove immunityAntibody mimetics/scaffoldsMammal material medical ingredientsSingle-Chain AntibodiesTreatment effect
The invention relates to an embedded antigen receptor based on CD22 and application thereof, and particularly relates to a building method of an embedded antigen receptor T (CAR-T) cell technology based on a tumor specific target site CD22 and application thereof in anti-tumor treatment. The embedded antigen receptor is prepared by connecting an antigen binding structural domain, a transmembrane structural domain, a costimulatory signal conducting region and a CD3 zeta signal conducting structural domain in series, wherein the antigen binding structural domain is combined with tumor-surface antigen, and the tumor-surface antigen is CD22. By adopting the embedded antigen receptor, specific gene transformation on a single chain antibody of the tumor-surface antigen CD22 is carried out, and because of the transformed antibody, the binding force of the antigen-antibody is stronger, and the mutation difficultly occurs; compared with other embedded antigen receptors and other tumor antigens, the embedded antigen receptor has a better effect, and the expression quantity of the target site is high, so that an immunization effect of CAR-T cells is enhanced, and a treatment effect on the CAR-T cells is enhanced.
Owner:SHENZHEN GENO IMMUNE MEDICAL INST
Macrophages capable of targeting tumor cells and preparation method thereof
ActiveCN109266618AActivate phagocytosisEasy accessPolypeptide with localisation/targeting motifImmunoglobulin superfamilyCAR T-cell therapyAntigen receptors
Owner:赛元生物科技(杭州)有限公司
Chimeric antigen receptor based on CD276 antibody, lentivirus expression vector and application thereof
InactiveCN109929039AIncrease lethalityFunction increaseFermentationGenetic engineeringLentivirusAntigen receptors
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
Immortalized car-t cells genetically modified to elminate t-cell receptor and beta 2-microglobulin expression
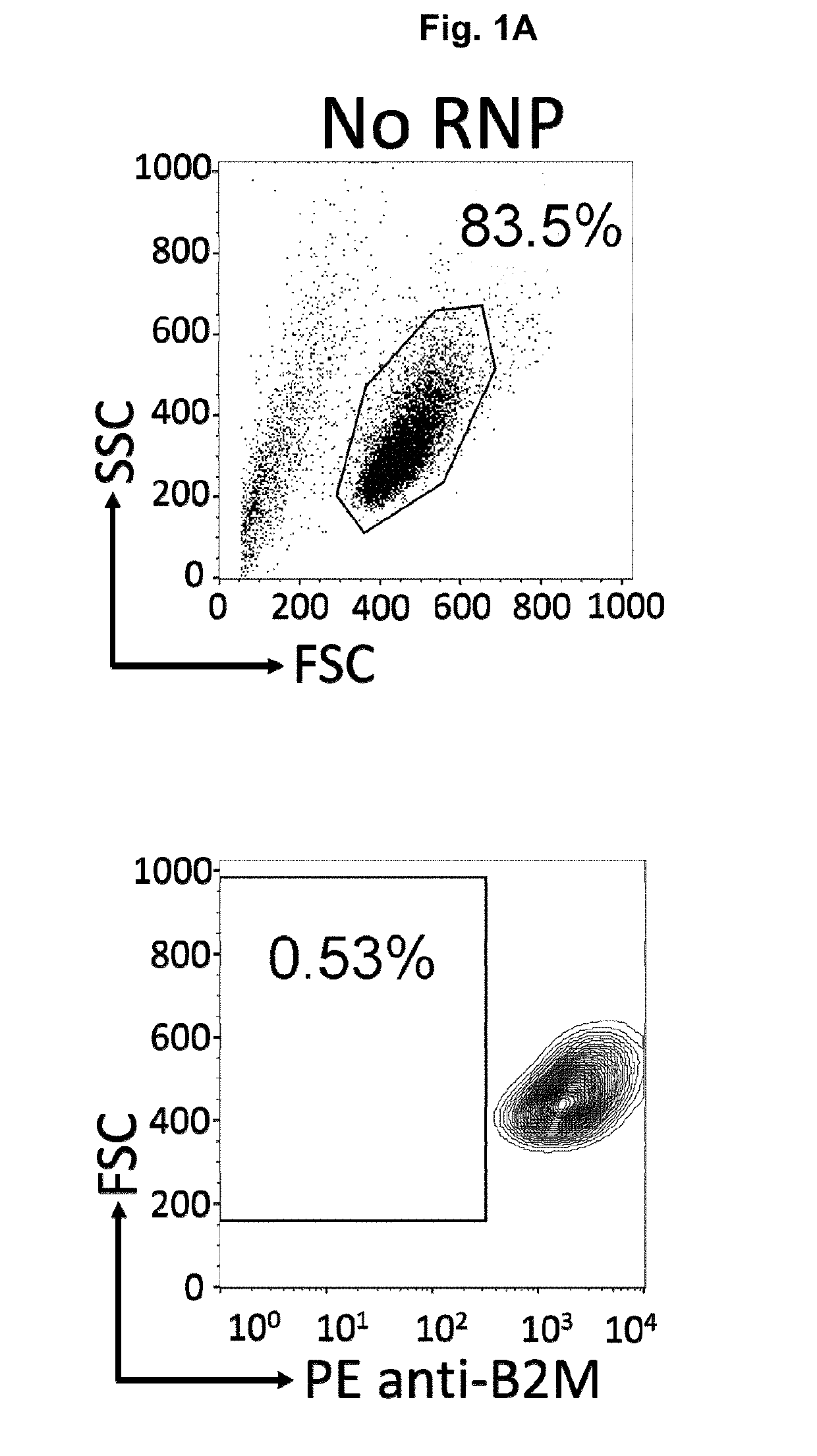
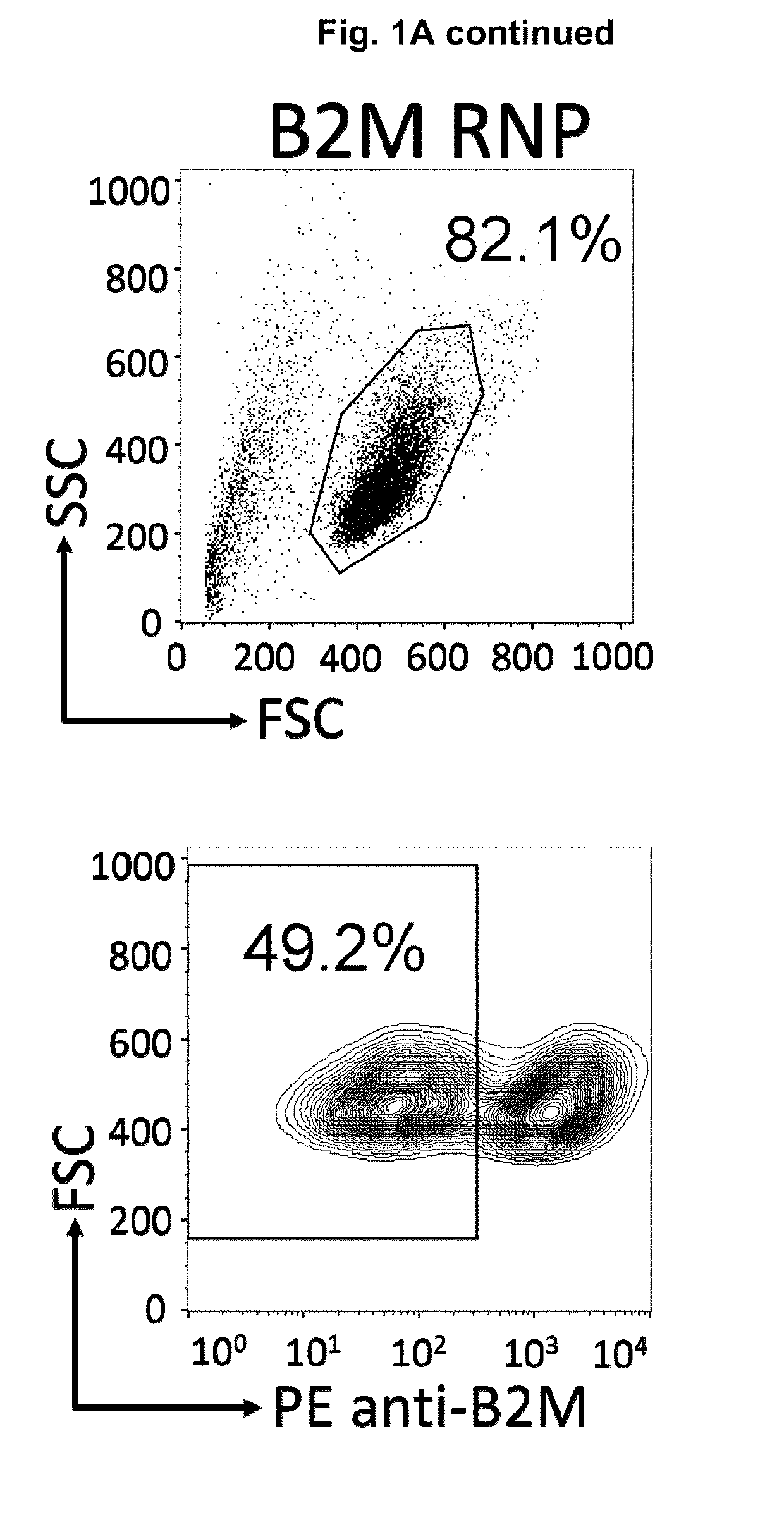
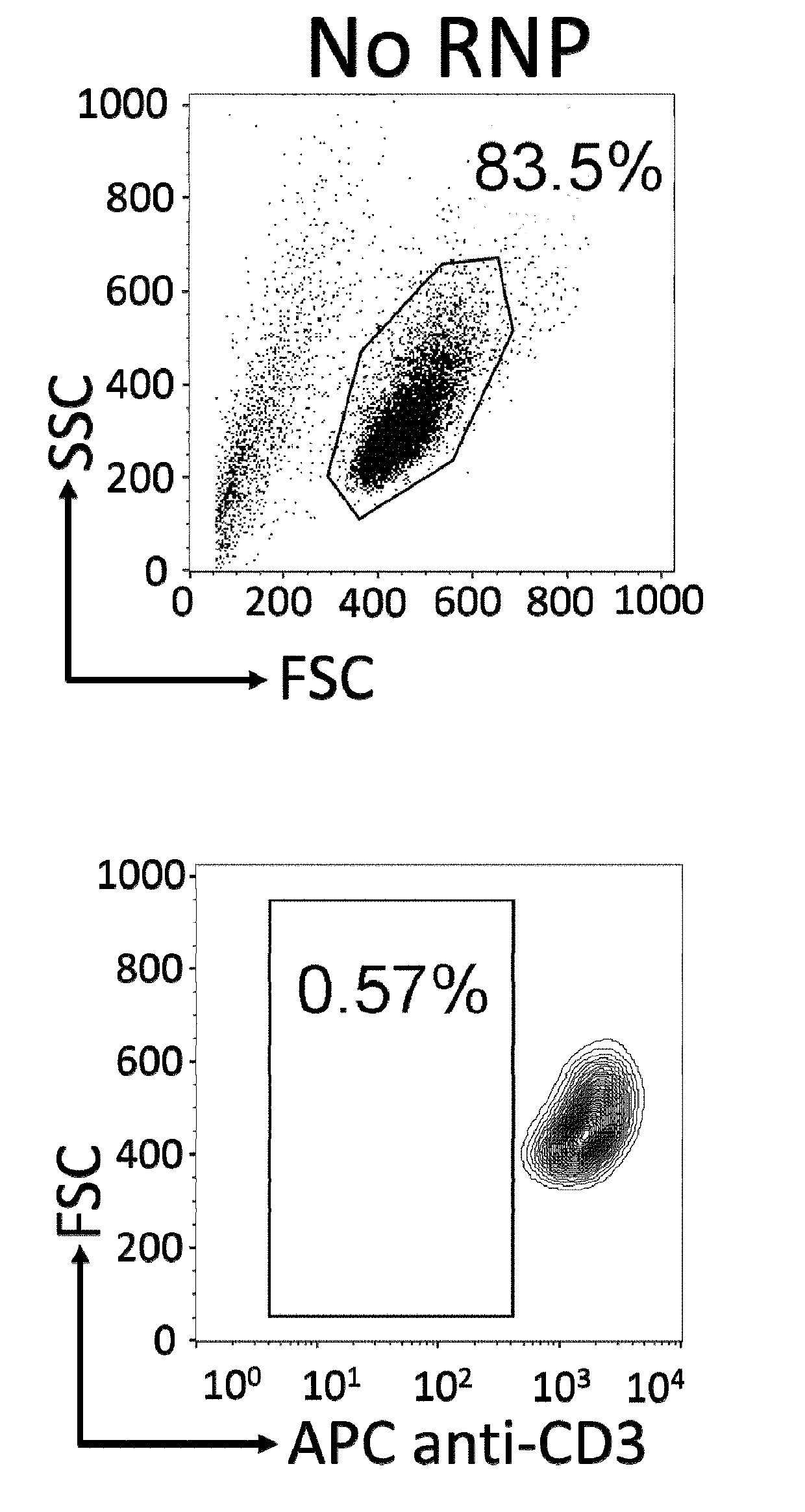
InactiveUS20190175651A1None is suitable for purposeTumor rejection antigen precursorsGenetic material ingredientsT cellAuto immune disease
The present invention pertains to engineered immortalized T-cell lines, method for their preparation and their use as medicament, particularly for immunotherapy. The engineered immortalized T-cell lines of the invention are characterized in that the expression of endogenous T-cell receptors (TCRs) and beta 2-microglobulin (B2M) is inhibited, e.g., by using an endonuclease able to selectively inactivate the TCR and B2M genes in order to render the immortalized T-cells non-alloreactive. In addition, expression of immunosuppressive polypeptide can be performed on those engineered immortalized T-cells in order to prolong the survival of these T-cells in host organisms. Such engineered immortalized T-cells are particularly suitable for allogeneic transplantations, especially because it reduces both the risk of rejection by the host's immune system and the risk of developing graft versus host disease. The invention opens the way to standard and affordable adoptive immunotherapy strategies using immortalized T-cells for treating cancer, infections and auto-immune diseases.
Owner:JANSSEN BIOTECH INC
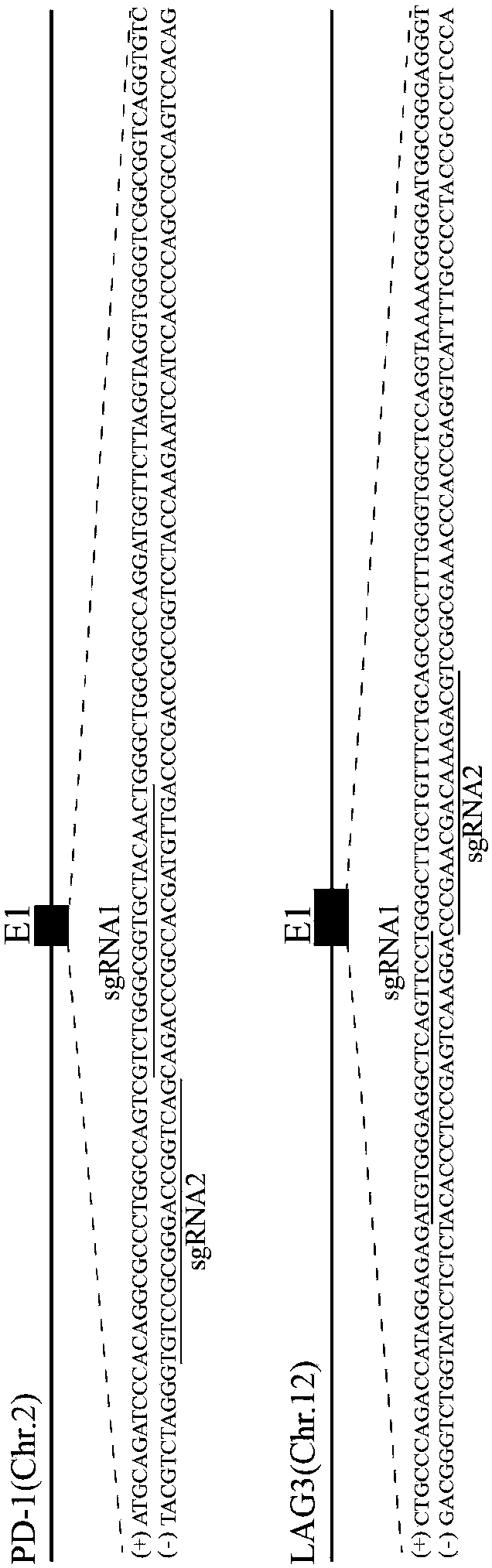
Cas9/RNP knockout T cell PD-1 and LAG3 genes and preparation method of CAR-T cells
InactiveCN108531457ADisinhibitionImprove securityImmunoglobulin superfamilyImmunoglobulinsLentivirusElectroporation
Owner:杭州荣泽生物科技集团有限公司
Chimeric antigen receptor based on CD20, and applications thereof
ActiveCN107245107AImprove bindingNot easy to mutateAntibody mimetics/scaffoldsMammal material medical ingredientsCD20Single-Chain Antibodies
The invention relates to a chimeric antigen receptor based on CD20, and applications thereof, and more specifically relates to a cell technology construction method of chimeric antigen receptor T (CAR-T) taking tumor specific target spot CD20 as a base, and applications of the chimeric antigen receptor T in treatment of tumor. The chimeric antigen receptor T is composed of an antigen binding domain, a transmembrane domain, a costimulatory signal transduction region, and a CD3 zeta signal transduction domine via series connection; wherein the antigen binding domain is used for binding tumor surface antigens, and the tumor surface antigen is CD20. According to applications, specific gene modification of single-chain antibody of tumor surface antigen CD20 is carried out, the modified antibody is capable of increasing antigen-antibody binding force, mutation is not easily caused. Compared with other chimeric antigen receptors and other tumor antigens, the chimeric antigen receptor possesses following advantages: the effect is better, target spot expression quantity is higher, immune effect on CAR-T cells is improved, and treatment effect of CAR-T cells is improved.
Owner:SHENZHEN GENO IMMUNE MEDICAL INST
Method for preparing chimeric antigen receptor-T (CAR-T) cells, and prepared CAR-T cells and application thereof
ActiveCN106222201AImprove effectivenessImprove securityGenetically modified cellsMammal material medical ingredientsIndividualized treatmentImmunologic function
The invention relates to the fields of immunology and molecular biology, in particular to a method for preparing chimeric antigen receptor-T (CAR-T) cells, and the prepared CAR-T cells and application thereof. According to the method for preparing the CAR-T cells, the CAR-T cells are obtained by enabling CD3 positive T cells to be infected by CAR-containing virus. The invention also discloses the prepared CAR-T cells and a composition containing the CAR-T cells. The method for preparing the CAR-T cells enables the CD3 positive T cells to be infected by the CAR-containing virus so as to obtain the CAR-T cells, thus being capable of realizing the industrial production and preparation of the CAR-T cells used for individualized treatment; the prepared CAR-T cells eliminate the repellency between individuals; the CAR-T cells prepared by the method are better in effectiveness and higher in safety. The invention also provides the application of the CAR-T cells in preparation of medicine for treating or preventing cancers.
Owner:BEIJING IMMUNOCHINA MEDICAL SCI & TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com