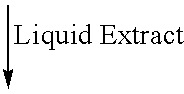Patents
Literature
108 results about "Aids patients" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lactobacillus reuteri to inhibit cryptosporidiosis in mammals
InactiveUS6103227AAvoid infectionImprove the immunityBiocideBacteriaChronic diarrheaImmune compromised
Cryptosporidium parvum (the cause of cryptosporidiosis) has become one of the most common enteropathogens causing diarrhea worldwide. Symptoms associated with cryptosporidiosis are very debilitating especially in the immunocompromised subject (e.g., AIDS patient). Clinical features include severe, chronic diarrhea, abdominal cramps, fatigue, weight loss, etc. which lead to increased health care costs and increased mortality. There is disclosed herein a method of inhibiting the severity of Cryptosporidium parvum infection by enterally administering a therapeutically effective amount of Lactobacillus reuteri.
Owner:WOLF BRYAN WARREN +1
Reduction in bacterial colonization by administering bacteriophage compositions
InactiveUS7459272B2Reduce and eliminate colonizationReduce riskAntibacterial agentsBiocideBacteroidesAcquired immunodeficiency
The present invention provides a method for reducing the risk of bacterial infection or sepsis in a susceptible patient by treating the susceptible patient with a pharmaceutical composition containing bacteriophage of one or more strains which produce lytic infections in pathogenic bacteria. Preferably, treatment of the patient reduces the level of colonization with pathogenic bacteria susceptible to the bacteriophage by at least one log. In a typical embodiment, the susceptible patient is an immunocompromised patient selected from the group consisting of leukemia patients, lymphoma patients, carcinoma patients, sarcoma patients, allogeneic transplant patients, congenital or acquired immunodeficiency patients, cystic fibrosis patients, and AIDS patients. In a preferred mode, the patients treated by this method are colonized with the pathogenic bacteria subject to infection by said bacteriophage.
Owner:INTRALYTIX
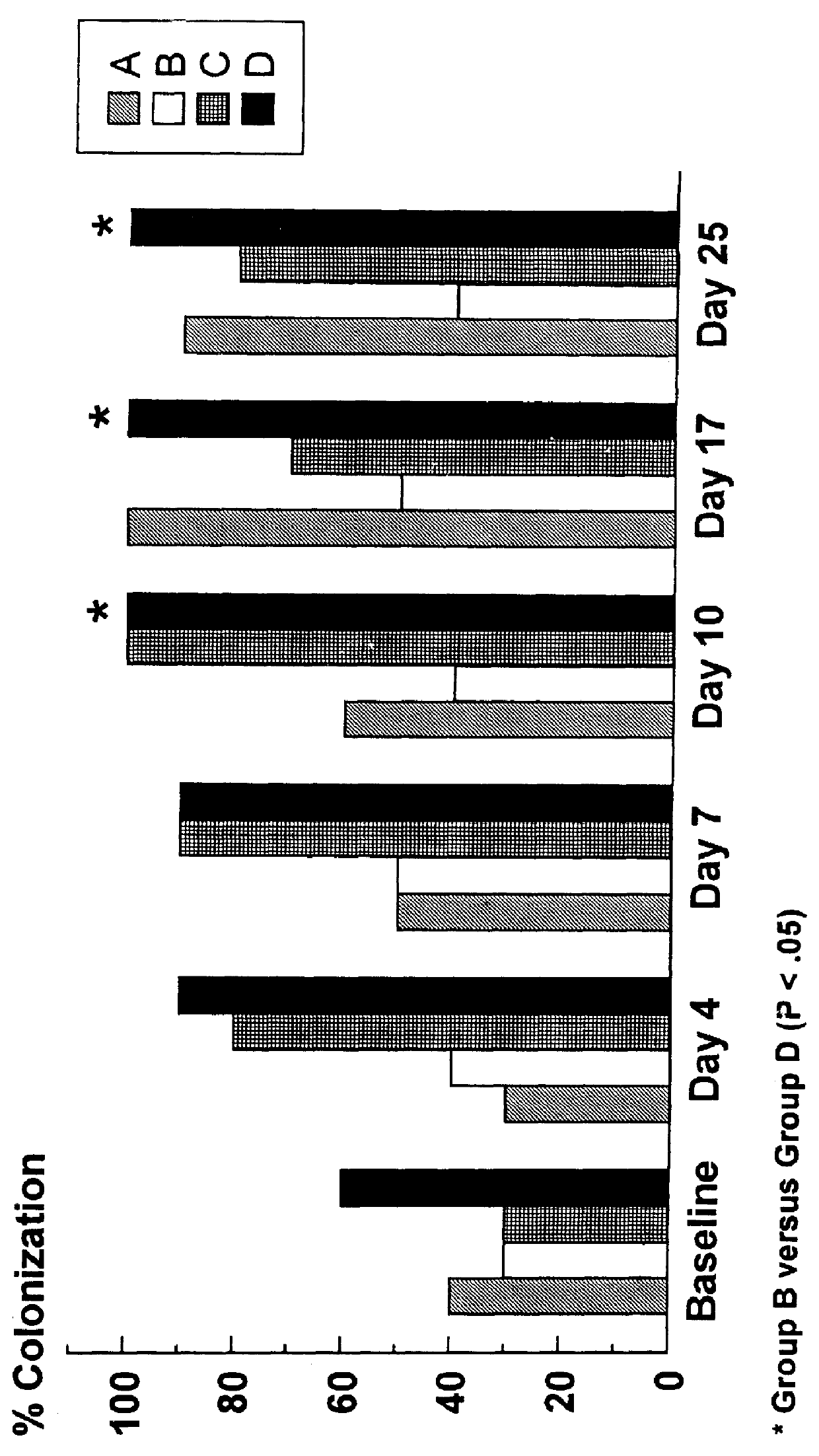
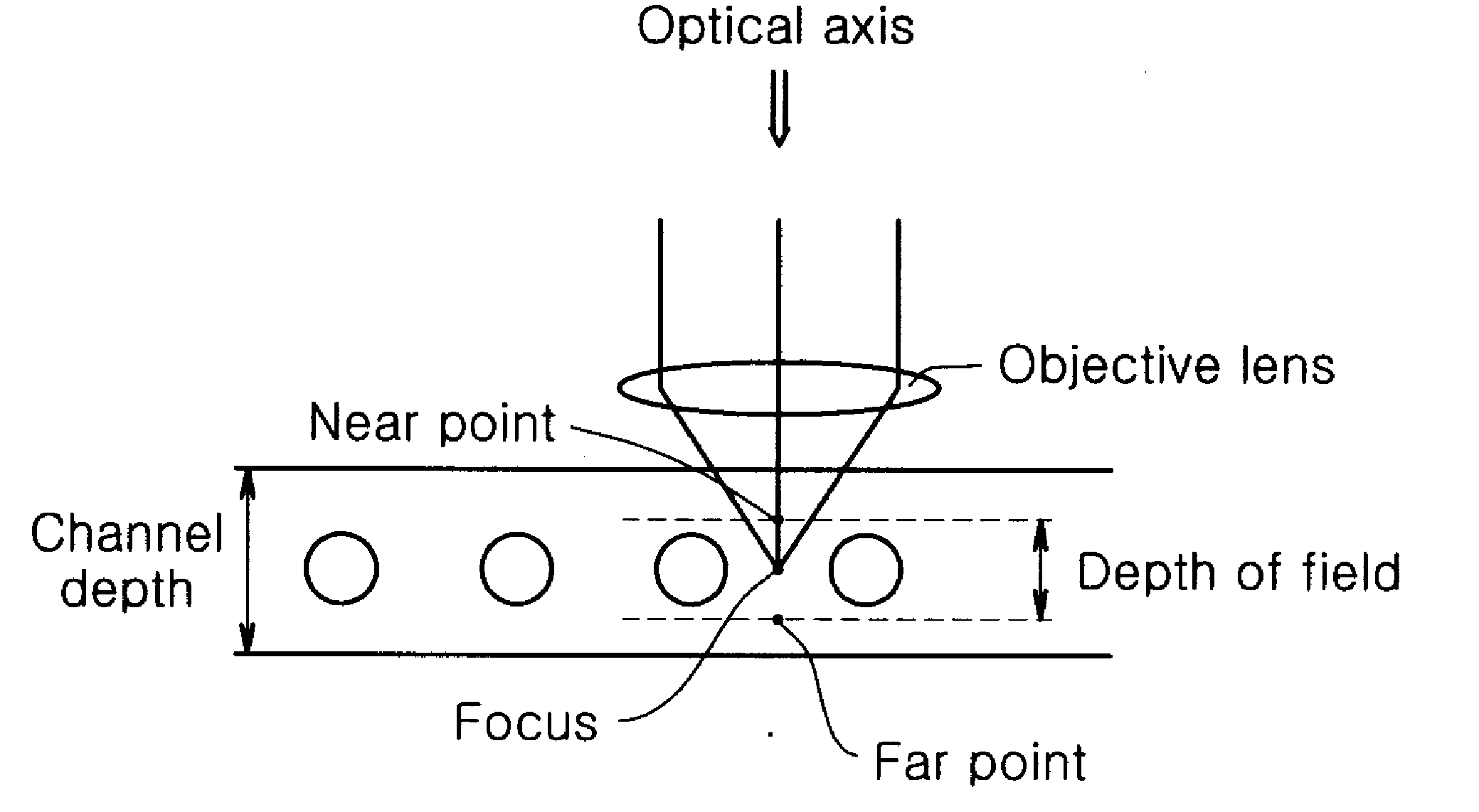
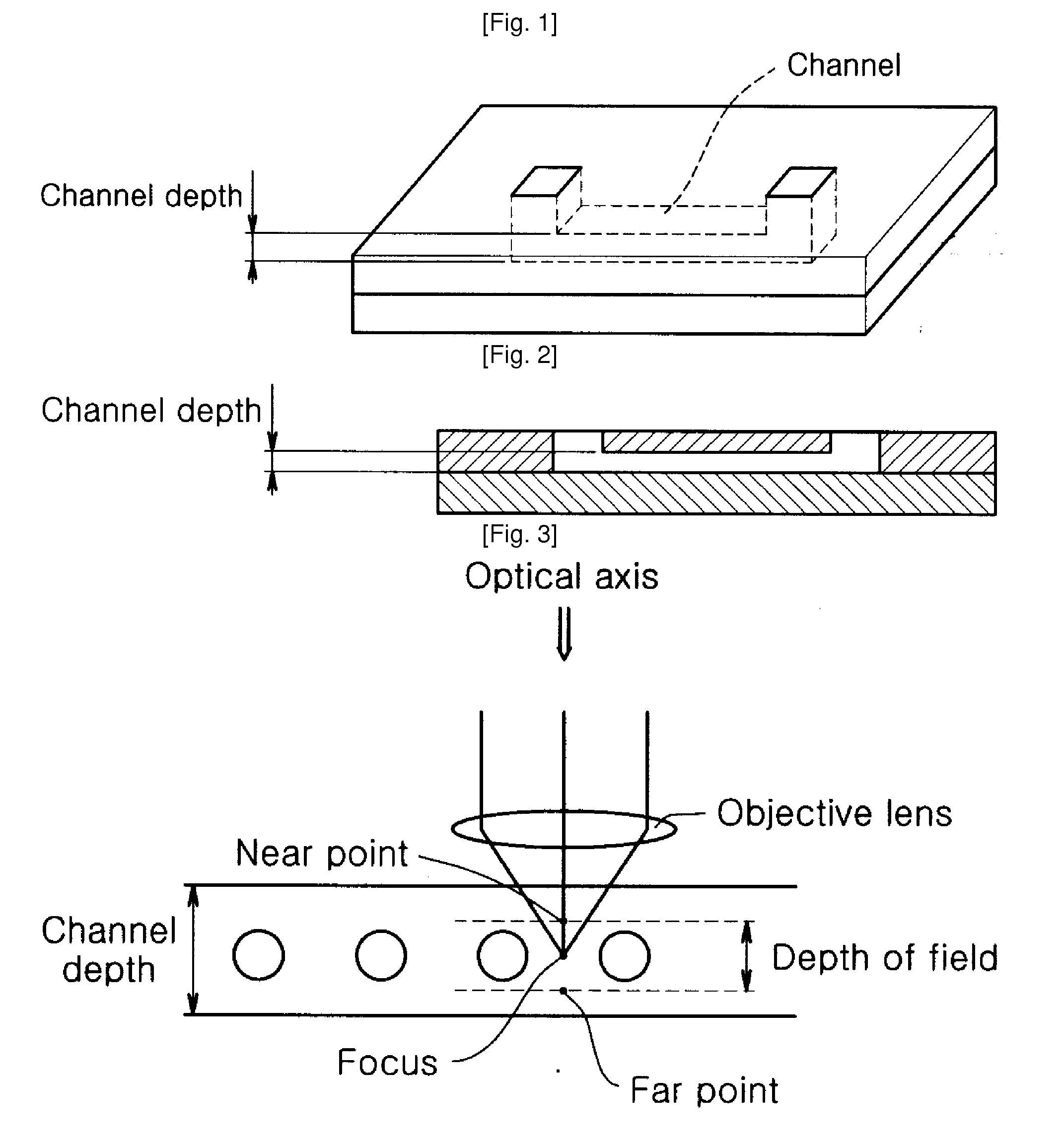
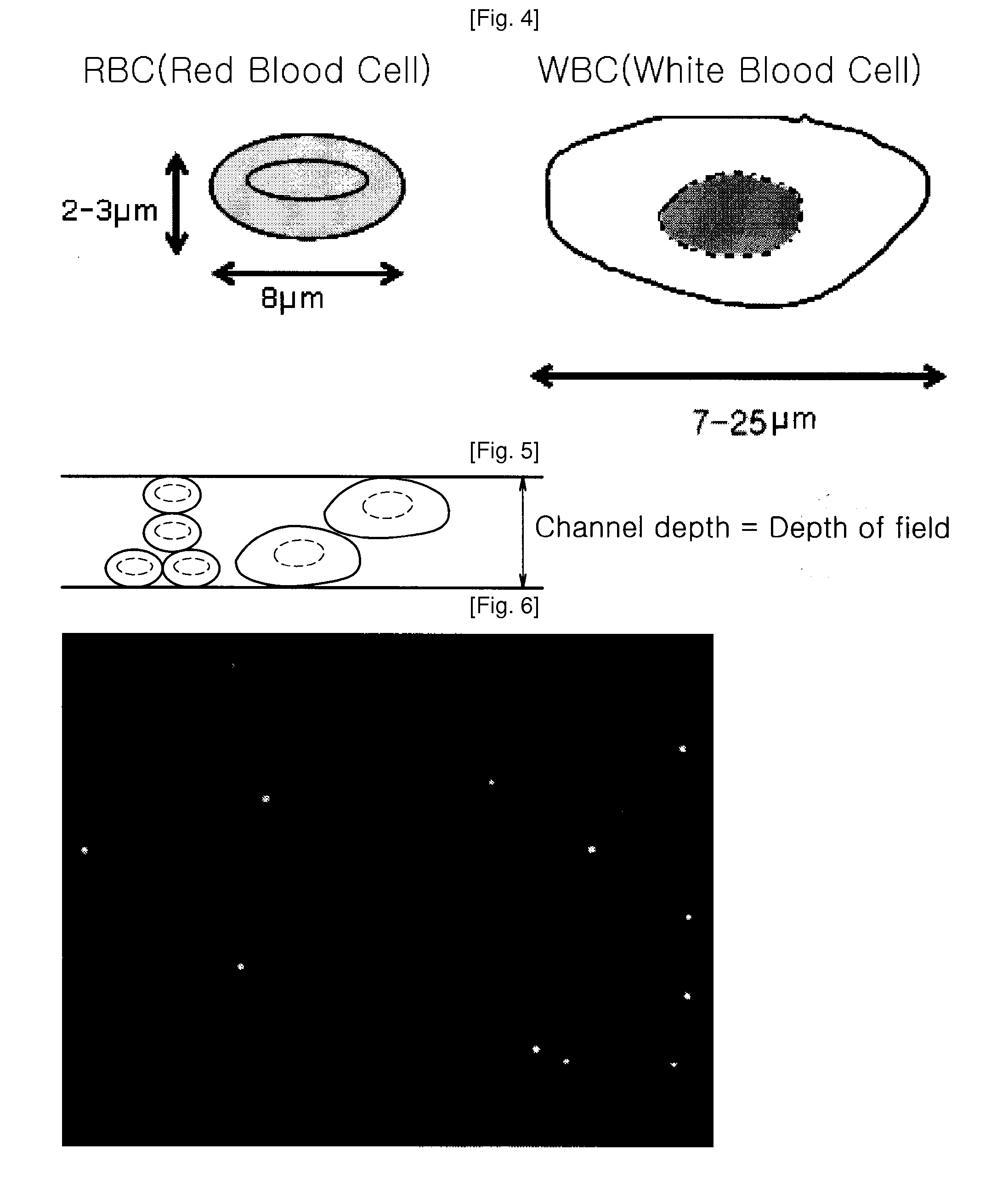
Chip Having Microchannel For Counting Specific Micro Particles Among Floating Micro Particle Mixture By Optical Means And A Method For Counting Micro Particles Using The Same
InactiveUS20100112631A1Clear and sharp optical imageEasily countDiagnostics using lightMicrobiological testing/measurementRed blood cellWhite blood cell
A microchannel chip for counting floating microparticles with an optical method is provided. The depth of the microchannel chip is made to be a depth of field such that a clear and sharp optical image of microparticles can be obtained, without necessarily diluting the microparticle mixture inside the microchannel. Consequently, a microparticle counting instrument can easily count microparticles shown on an optical image of the microchannel. Particularly, it enables to count more easily the number of CD3 cells CD4 cells or CD8 cells in white blood cells being stained, without lysing or diluting red blood cells. Therefore, the microchannel chip can advantageously used for counting the number of CD3 cells, CD4 cells or CD8 cells in blood of an AIDS patient to monitor the progression of AIDS.
Owner:NANOENTEK
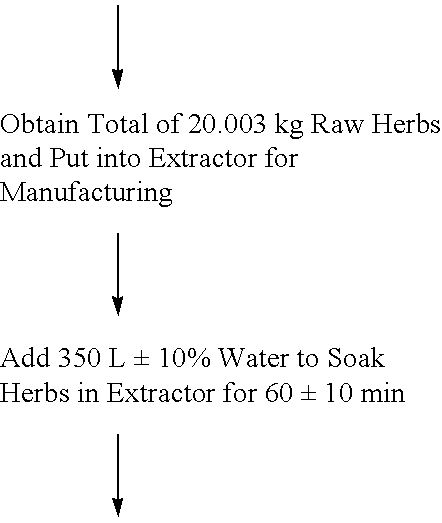
Herbal pharmaceutical composition for treatment of HIV/AIDS patients
Owner:WU TZU SHENG
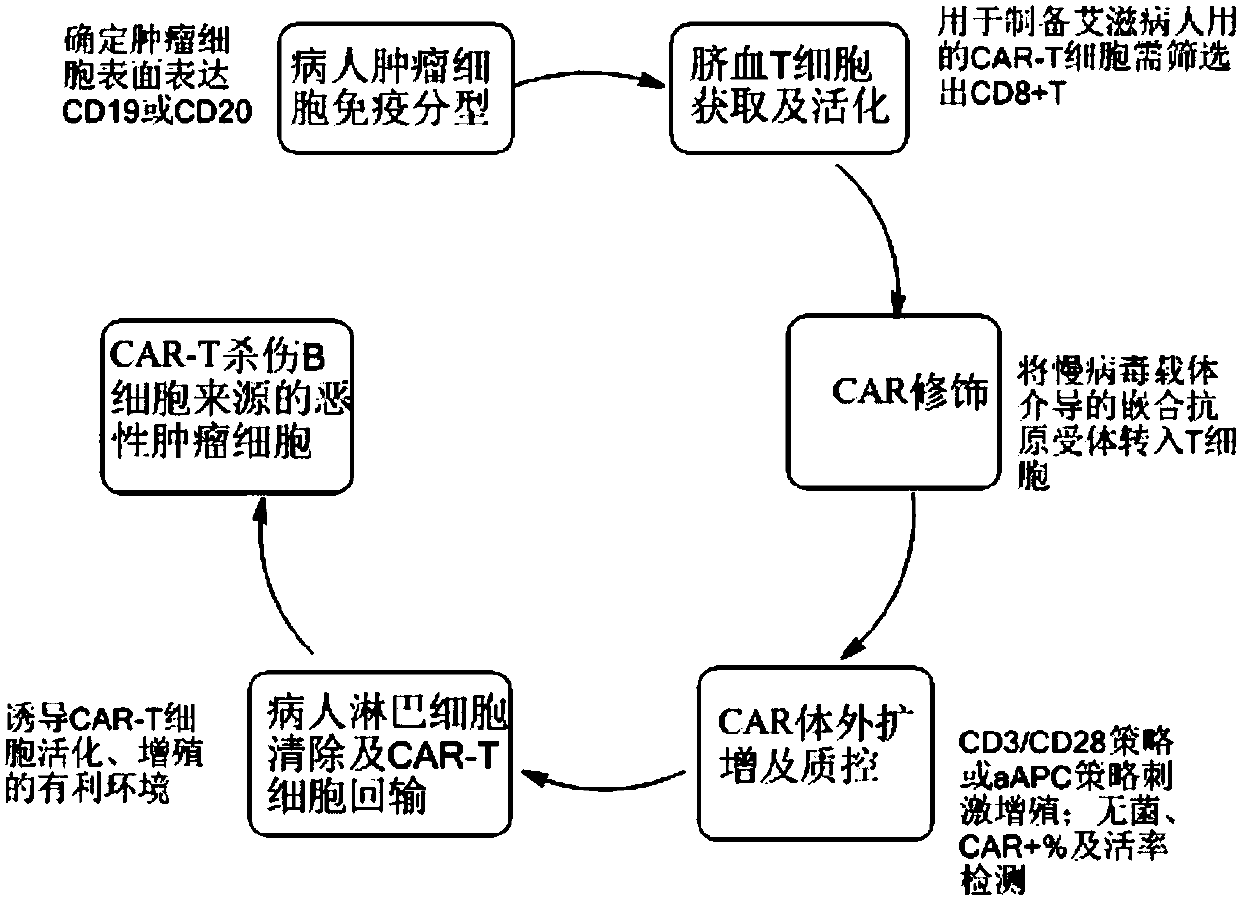
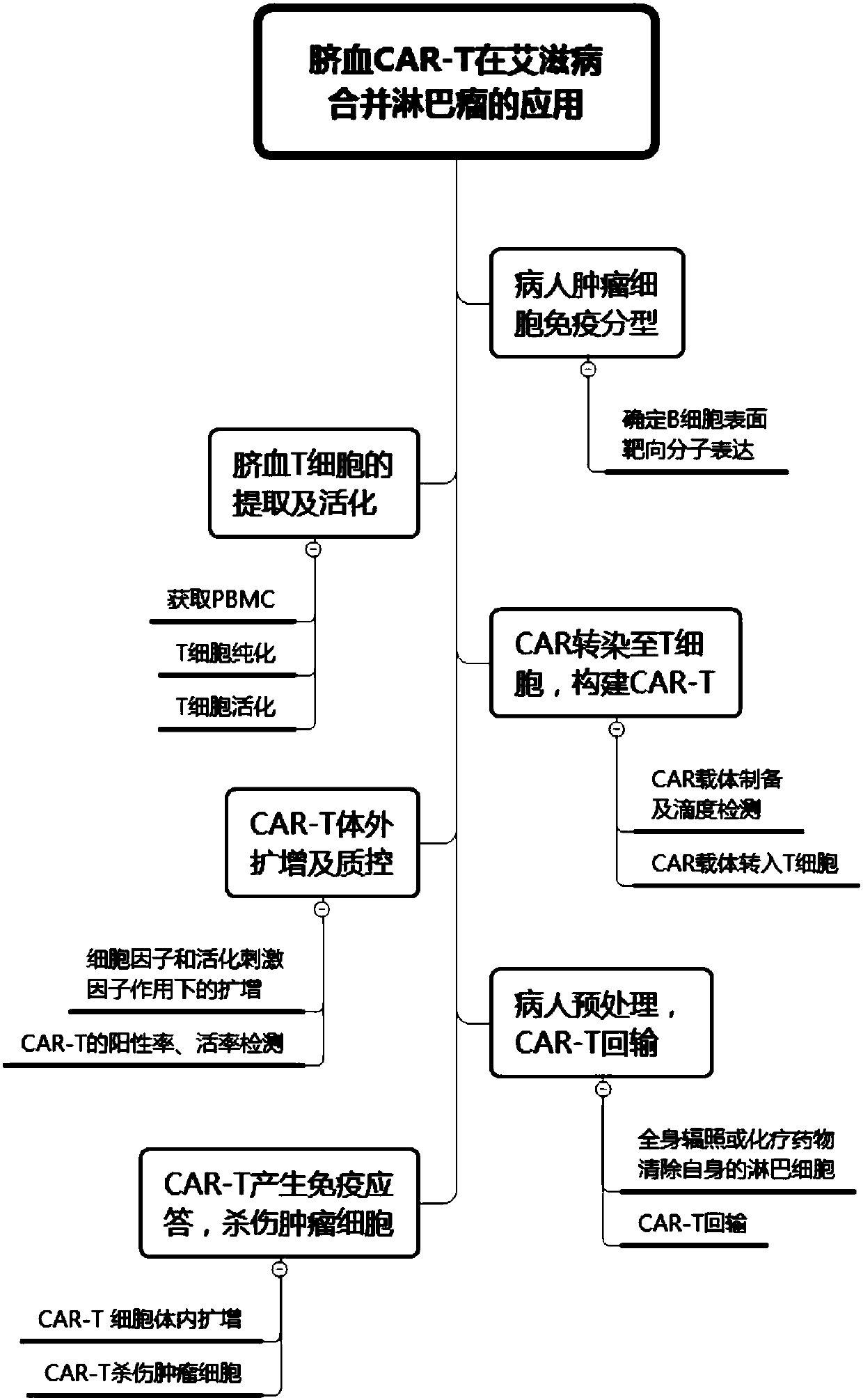
CAR-T cell for treating AIDS-associated lymphoma, and preparation method and application thereof
PendingCN107904259ALow storm riskSmall storm riskGenetically modified cellsAntiviralsAIDS-related lymphomaCord blood stem cell
The invention discloses a preparation method of a CAR-T cell for treating AIDS-related lymphoma. A CD8+ T cell is used to produce the CAR-T cell, and the CD8-T cell derives from a cord blood T cell. The method includes the following steps: preparing cord blood mononuclear cells from cord blood, removing tumors and other cells by using a human T cell purification kit to obtain T cells, and carryingout separation by using magnetic beads to obtain the CD8+ T cell; activating the CD8+ T cell by using an appropriate medium and appropriate stimulation conditions; transferring CAR to the CD8+ T cellby using lentivirus to prepare the CAR-T cell; and amplifying the CAR-T cell in vitro by using cytokines and activating stimulators to achieve the desired effective dose. The invention also relates to the CAR-T cell prepared by the method, and an application thereof. The CAR-T cell prepared in the invention has a good cell activity and a high proliferation speed, and reduces the attack risk of GVHD; and only the CD8+T cell of the umbilical blood T cells of AIDS patients is used to prepare the CAR-T cell for, so the risk of input CAR-T infected with in-vivo HIV is reduced.
Owner:SHANGHAI LONGYAO BIOTECH CO LTD
Highly sensitive method for detection of viral HIV DNA remaining after antiretroviral therapy of aids patients
InactiveUS20110027774A1High sensitivityBioreactor/fermenter combinationsBiological substance pretreatmentsNucleotideImmunodeficiency virus
Methods for detecting polynucleotides, especially the DNA replicated from samples obtained from subjects infected with pathogenic viruses such as human immunodefiency virus, by detecting electromagnetic signals (“EMS”) emitted by such polynucleotides, and methods for improving the sensitivity of the polymerase chain reaction (“PCR”).
Owner:MONTAGNIER LUC
Primer pair and probe used for detecting AIDS treatment medicine 3TC and FTC drug-resistance mutation sites and application thereof
ActiveCN104911252AStrong specificityEnrichmentMicrobiological testing/measurementDNA/RNA fragmentationPatient survivalPharmaceutical drug
The invention discloses a primer pair and a probe used for detecting AIDS treatment medicine 3TC and FTC drug-resistance mutation sites, which comprise an ARMS primer and a Taqman probe of mutation sites M184V, M184I, Q151M and K65R at 184th site, 151st site and 65th site of pol gene for detecting HIV-1 virus RNA. The invention also provides the application of the primer pair and the probe on detection of 3TC and FTC main drug-resistance mutation sites M184V, M184I, Q151M and K65R. The kit has the advantages of high detection sensitivity, good specificity, and low detection cost, and provides medicine usage guidance for treating clinic AIDS patient, individuation treatment for AIDS patient can be realized, medicine effectiveness can be increased, AIDS patient living time can be prolonged, and the primer pair and the probe have wide application prospect and social benefit.
Owner:江苏亦文生物科技有限公司
Herbal pharmaceutical composition for treatment of HIV/AIDS patients
InactiveUS20030091658A1Inhibit pathological changesPowder deliveryCosmetic preparationsAdditive ingredientBaical Skullcap Root
The present invention provides a pharmaceutical composition for treating patients with HIV infection. The pharmaceutical composition is in the form of an intravenous injection solution and optionally capsules. The pharmaceutical composition contains fourteen (14) ingredients, i.e., diffuse hedyotis, bistort rhizome, giant knotweed rhizome, Asiatic moonseed rhizome, baical skullcap root, bovine biliary powder, milkvetch root, barbary wolfberry fruit, sanqi, figwort root, Chinese magnoliavine fruit, turmeric root-tuber, hawthorn fruit, and Chinese angelica.
Owner:WU TZU SHENG
Acquired immune deficiency syndrome personnel behavior analysis method based on deep learning
The invention discloses an AIDS (acquired immune deficiency syndrome) personnel behavior analysis method based on deep learning. The method comprises the following steps: acquiring user behavior data;and based on the user behavior data, analyzing the multi-dimensional space-time information of the user, and constructing a user behavior portrait. Behavior portraying is performed on the AIDS patient based on network, geographic position and social communication behavior analysis, and descriptive label attributes for the user are constructed in multiple dimensions such as network, geographic position and social communication. The label attributes are utilized to describe and outline the real personal characteristics of the AIDS patient in multiple aspects, and are used for describing relatedcharacteristics, behaviors and preferences. Potential social communication rules of AIDS crowds are discovered, high-risk AIDS crowds, potential AIDS propagators and AIDS propagation paths are discovered, and the AIDS intervention link work is assisted to be intervened in advance.
Owner:UNIV OF ELECTRONIC SCI & TECH OF CHINA
Medicine composition for preventing and treating AIDS and its prepn process and use
The present invention discloses one kind of medicine composition for preventing and treating AIDS and its preparation process and use. The medicine composition consists of active ingredient and auxiliary ingredient, and may be prepared into granule, tablet, capsule, oral liquid or injection. It has the functions of inhibiting the copying of HIV, promoting immunologic reconstruction of the AIDS patient, delaying disease and clinical complication, and synergizing and attenuating in high efficiency retrovirus resisting therapy. The present invention has wide application foreground.
Owner:广州中医药大学热带医学研究所
Highly sensitive method for detection of viral HIV DNA remaining after antiretroviral therapy of aids patients
InactiveUS20130143205A1High sensitivityBioreactor/fermenter combinationsBiological substance pretreatmentsNucleotideImmunodeficiency virus
Owner:MONTAGNIER LUC
GST fusion expression of conotoxin MVII A gene and its use
InactiveCN1473935ASimple processQuality improvementNervous disorderPeptide/protein ingredientsEscherichia coliHalf-life
The present invention provides the GST fusion expression and application of a kind of conotoxin MVII A gene. GST-CTX MVII A recombinant fusion protein is cloned and expressed in colibacillus, and through GST affinity chromatography purified GST-CTX MVII A fusion protein is obtained. The fusion protein the present invention obtains may be applied in preparing medicine for treating intractable painof cancer and AIDS patient in the late stage, preparing analgesic for treating post-operation acute pain, pain of burn, biliary colic, intractable neuralgia, etc., and preparing medicine with nerve protecting function for treating cerebral ischemia and cerebral damage. The obtained fusion protein has relatively great molecular weight and relatively long half life in body and may be enzyme incisedto obtain active omiga-conotoxin MVII A small peptide for direct medicine development.
Owner:ZHEJIANG UNIV
Traditional Chinese medicine composition and preparation method and use thereof
InactiveCN101450173AEnhance immune functionHas anti-HIV effectAntinoxious agentsAntiviralsLycium barbarum fruitOphiopogon japonicus
The invention discloses a traditional Chinese medicine composition for preventing and treating AIDS and preparation method thereof. The medicament composition is mainly made from the following raw material medicaments of weight proportions of: astragalus 15-20 parts, Ginseng 7-12 parts, Chinese angelica 8-13 parts, wolfberry 9-14 parts, Chinese magnoliavine 7-12 parts, Tuber of Dwarf Lilyturf 7-12 parts, trichosanthes root 8-13 parts, Poria cocos 7-12 parts, licorice 7-12 parts, radix bupleuri 3-8 parts and cimicifuga foetida 3-8 parts. The traditional Chinese medicine composition of the invention has the functions of supplementing center, boosting qi, nourishing blood and nourishing yin; it can not only resist AIDS virus, but also increase the immunization function of patient substantially, and can eliminate the common symptoms of AIDS patients.
Owner:HUNAN YANDI BIOLOGICAL ENG
Compsns. contg. itraconazole with improved bioavailability and narrow intra-and inter-individual variation of its absorption
InactiveCN1398184ALarge specific surface areaNot hydrophobicPowder deliveryOrganic active ingredientsNormal peopleItraconazole
The present invention relates to agent containing itraconazole with both improved bioavailability, due to higher water-solubility and impressively reduced difference of pH-dependent solubility, and narrow intra- and inter-individual variation of its absorption- and a manufacturing method thereof. Agent containing itraconazole of the present invention consists of itraconazole, water-soluble macromolecule of 10-100 % w / w to itracotazole, solubilizing agent of 0.1-100 % w / w to itraconazole and pharmaceutically allowed additives. Agent containing itraconazole of the present invention minimizes absorption variation by dozing time after food intake as well as is available for adults with hypoacidity, AIDS patients and normal people. In addition, the manufacturing method introduces the elementary process, the spray drying, thereby control of physical properties of particles containing drug is easier and economy / production is improved.
Owner:DONG A PHARMA
J2EE development framework and development method based on J2EE development framework
InactiveCN108536433AImprove development efficiencyQuality improvementProgramming languages/paradigmsSoftware designAnti virusSoftware development process
The invention belongs to the technical field of software development and specifically discloses a J2EE development framework for a software product for AIDS antiviral therapy and a development methodbased on the J2EE development framework. The J2EE development framework is mainly composed of a view module, a permission control module, a service support module, a data persistence layer module, a server monitoring module, a report module, and a log module. Based on the J2EE development framework and development method, the program developer invokes through a convenient API, achieves the development of anti-viral treatment services and management software products for AIDS patients with high efficiency and high quality, and develops anti-virus treatment services and management software products for AIDS patients with strong compatibility and scalability. Through the application of Internet medical technology for anti-viral treatment services and management of AIDS patients, AIDS antiviral treatment and management systems are optimized and innovated.
Owner:云南软捷科技有限公司
Protocol for AIDS prevention and treatment by nutritional methods
InactiveUS20090104287A1Easy to manageOrganic active ingredientsBiocideTryptophanGlutathione peroxidase
HIV-1 is a slow virus that has a genome that requires the removal of glutathione, glutathione peroxidase and of selenium, cysteine, glutamine and tryptophan from its host as it is replicated. Although various clinical trials have improved the health of AIDS patients by correcting one or more of these nutritional deficiencies, they have never been addressed together. The current invention combines selenium, cysteine, tryptophan, and glutamine in powder, capsule, or pill form for the creation of glutathione and glutathione peroxidasae by the patient and the correction of deficiencies of other biochemical substances that require selenium, cysteine, tryptophan, and glutamine as precursors. Such a treatment protocol will prevent HIV-1 seropositive individuals from developing AIDS and reverse most of the symptoms of AIDS in those with this disorder.
Owner:FOSTER HAROLD DOUGLAS
Chinese medicine for treating AIDS
InactiveCN1666762AEnhance physical fitnessPhysically strongAntiviralsUnknown materialsPatient constitutionPatients symptoms
The invention discloses a Chinese medicine for treating AIDS, which has the functions of fast increasing immunocyte of viral infection AIDS patients, recovering immunological system performance, reducing virus replication, killing AIDS viruses, reinforcing patient constitution and improving patient symptoms.
Owner:王太兴
Anti-AIDS traditional Chinese medicine compound composite and preparation method thereof
ActiveCN101417049AImprove immunityEliminate symptomsAntiviralsUnknown materialsSide effectRaw material
The invention relates to a compound composition of a traditional Chinese medicine, in particular to an anti-AIDS compound composition and a preparation method thereof. Pilos antler, wolfberry fruits, herba epimedii, rhizoma curculiginis, herba cistanches and other traditional Chinese raw materials are extracted, concentrated, decocted, filtered, concentrated, dried in vacuum, smashed and mixed to prepare the anti-AIDS compound composition. The compound composition is mainly used for treating AIDS patients and HIV-infected patients, and can restrain and kill AIDS virus improve the immunity of human bodies and increase the number of CD4 cells in human body, thereby eliminating all symptoms of patients and restoring the strength and weight of patients so that the patients can work and live healthily. The anti-AIDS compound composition is efficient and safe, causes no drug resistance and has no toxic side effects, no rebound after drug withdraw and wide application range. In addition, the anti-AIDS compound composition can be applied to children and pregnant women as well as all varieties and stages of HIV-infected patients.
Owner:北京同馨堂中医药科技发展有限公司
Chronic kidney disease follow-up visit assistant
InactiveCN106971064ARealize the whole process of informatizationImprove work efficiencyTelemedicineComputer-assisted diets prescription/deliveryDecision takingSelf supervision
The invention provides a platform convenient for doctor management and patient self-supervision, and aims to assist a patient to know a health condition and remind the patient to timely go back to a hospital for follow-up visit. The platform has main functions of patient archiving, examination index input, follow-up visit plan and rehabilitation plan pushing and reminding, related follow-up visit data analysis and the like, and provides online expert consultation and wardmate communication, thereby facilitating self-management and self-supervision of the patient. While follow-up visit and diagnosis and treatment process management is covered, data accumulated in an operation process is subjected to statistical analysis, so that big data value is comprehensively reflected and powerful data support and decision support are provided for clinic scientific and research of nephrology departments. The follow-up visit is taken as a center; for the patient with the chronic kidney disease, the follow-up visit is performed in the way; the problem that a kidney disease assistant in China at present takes no count of the follow-up visit is solved; the burden of a doctor and the frequency of going to the hospital for the patient are reduced; and a few unnecessary expenses are saved.
Owner:SICHUAN UNIV
Medicinal composition for treating acquired immune deficiency syndrome (Aids) as well as preparation method, quality control method and application thereof
ActiveCN102641489AHas therapeutic effectInhibition efficacy indexAntiviralsCapsule deliveryDiseaseMonkshoods
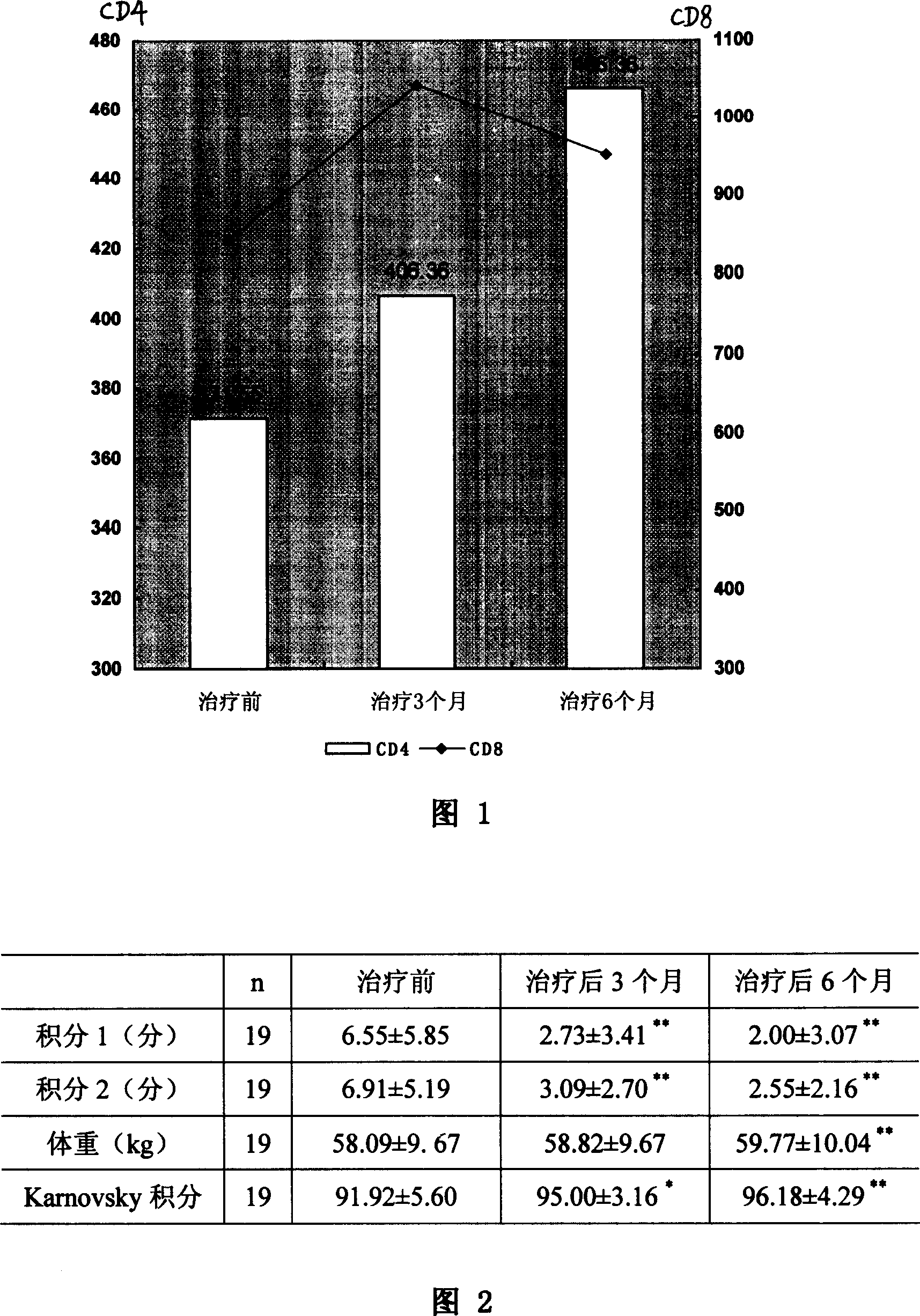
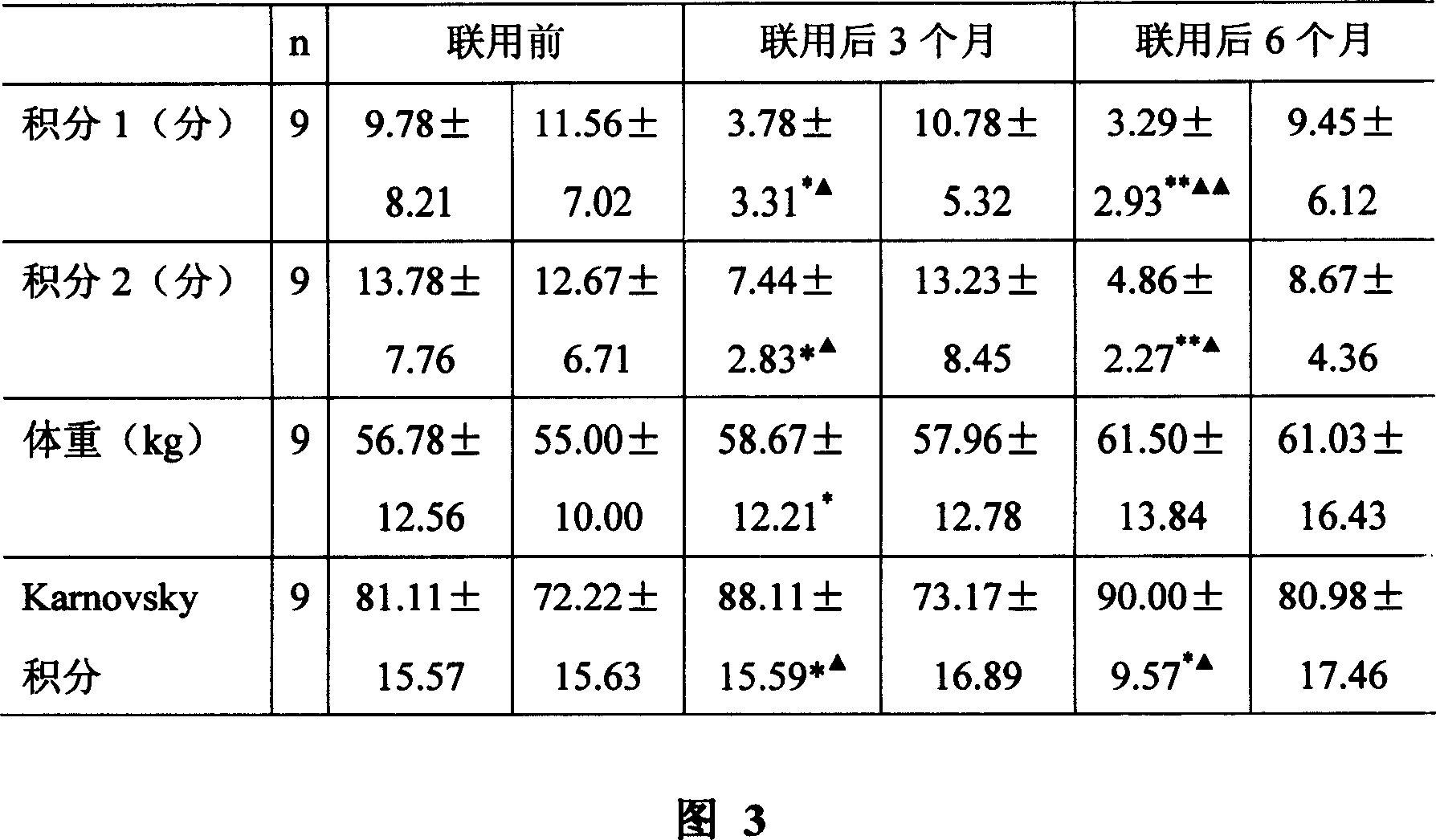
The invention discloses a medicinal composition for treating acquired immune deficiency syndrome (Aids) as well as a preparation method, quality control method and application of the medicinal composition. The raw materials of the medicinal composition comprise cooked monkshood, herba epimedii, dried ginger, liquorice, ginseng, the root of red-rooted salvia, giant knot weed, Poria cocos, golden cypress and Scutellaria baicalensis. Experimental data indicate that the medicinal composition preparation disclosed by the invention has Aids treating function on Aids model monkeys derived from rhesus monkeys infected by monkey Aids Virus, based on the curative effect on the Aids monkey models adaptive to Aids animal models, the medicinal composition preparation has the application of treating human Aids; after being given to HIV infected persons or Aids patients, the medicinal composition preparation can relieve disease, i stabilize or improvethe CD4+T lymphocyte count level of the HIV infected persons or the Aids patients, relieve clinical clinical symptoms and improve survival quality.
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE
Multi-objective transport path optimization method based on HIV epidemic dynamics
InactiveCN106126897AThe total number remains the sameImplement random searchEpidemiological alert systemsSpecial data processing applicationsCrowdsSexual intercourse
The invention discloses a multi-objective transport path optimization method based on HIV epidemic dynamics. It is assumed that an ecosystem consists of a number of crowds; an HIV virus spreads in the crowds, and people who are in effective contact with people with the HIV virus, are infected with the infectious disease; people who are not infected with the HIV virus are known as susceptible persons; after the susceptible persons are infected with the HIV virus, immediate onset does not occur, and the virus in the body enters an incubation period; people, whose HIV virus is in the incubation period, are known as HIV virus carriers, and such people are divided into two kinds, one kind is people who do not have the disease and have sexual intercourse, the kind of people become AIDS patients after a certain period of time, and ultimately die due to the AIDS; the other kind is people who have never had the disease and lose the sexual intercourse ability, and the kind of people eventually die a natural death; and under the action of the HIV virus, the growth state of each person is randomly changed, and by using the random variation and an HIV epidemic model, a global optimal solution of the multi-objective transport path combined optimization problem can be quickly obtained.
Owner:XI'AN UNIVERSITY OF ARCHITECTURE AND TECHNOLOGY
Primer pair and probe used for detecting AIDS treatment medicine DDI and TDF drug-resistance mutation sites and application thereof
ActiveCN104911255AStrong specificityHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationPharmaceutical drugResistance mutation
The invention discloses a primer pair and a probe used for detecting AIDS treatment medicine DDI and TDF drug-resistance mutation sites, which comprise an ARMS primer and a Taqman probe of mutation sites K65R, K70E and Q151M at 65th site, 70th and 151st site of pol gene for detecting HIV-1 virus RNA. The invention also provides the application of the primer pair and the probe. The kit has the advantages of high detection sensitivity, good specificity, and low detection cost, and provides a medicine usage guidance for treating clinic AIDS patient, individuation treatment for AIDS patient can be realized, medicine effectiveness can be timely reflected, AIDS patient living quality can be prolonged, and the primer pair and the probe have wide application prospect and long social benefit.
Owner:JIANGSU FAST BIOTECH
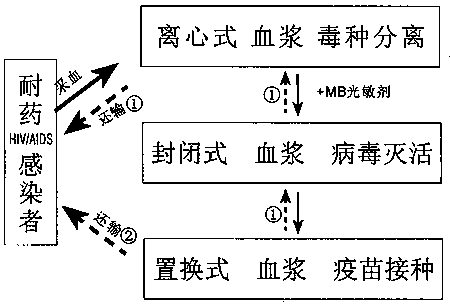
One-step method preparation process of treatment type drug resistant AIDS (acquired immunodeficiency syndrome) autologous plasma virus inactivated vaccine
PendingCN108498795AReduce intensityReduce the number of infectious agentsViral antigen ingredientsAntiviralsBlood collectionLarge dose
The invention relates to a one-step method preparation process of treatment type drug resistant AIDS (acquired immunodeficiency syndrome) autologous plasma virus inactivated vaccine. Through blood collection, plasma separation and plasma virus inactivation, the vaccine is directly prepared; then, intravenous vaccination is performed; the whole process is completed in a sealed disposable consumable; the pollution risk in the preparation process can be effectively avoided; the vaccine preparation safety is ensured. The preparation process is simple; the cost is low; the inoculation procedure canalso be simplified; the technical requirements of large-dose (600 to 800ml) plasma virus inactivated vaccine inoculation are met. More importantly, the virus strain of the inactivated vaccine is froma patient self; the plasma is also from the patient self; the obvious safety, effectiveness and feasibility are realized. The principle of medical ethics is conformed; the problem of animal model lack of AIDS vaccine development can also be solved. The vaccine is applicable to the continuous treatment of AIDS patients with drug resistance and treatment failure, initial and conventional treatmenton AIDS patients without drug resistance, and emergent blockade treatment on occupational exposure persons.
Owner:耿艳春 +1
Lyophilized hemsley rockvine root tuber used for treating AIDS
InactiveCN103127313AObvious side effectsReduce loadAntiviralsPlant ingredientsSide effectLife quality
The invention relates to an application of lyophilized hemsley rockvine root tuber in preparing a medicine used for treating AIDS. The lyophilized hemsley rockvine root tuber can be prepared into any medical preparation with traditional Chinese medicine preparation method. An effective dose for a patient a day comprises 1-300g of lyophilized hemsley rockvine root tuber. Compared with medicines prepared with commonly dried hemsley rockvine root tuber, the medicine prepared by using lyophilized hemsley rockvine root tuber has better functions in enhancing AIDS patient body immunity, inhibiting HIV virus, reducing patient clinical symptoms, and improving patient life qualities. Also, medicine resistance and toxic and side effects when HIV virus is treated by using antiretroviral can be effectively reduced.
Owner:胡馨尹
Application of a compound in preparing anti-virus medicament
InactiveCN101108187AInhibition of replicationSuitable for life-long medication needsOrganic active ingredientsAntiviralsChemical industryAnti virus
The invention relates to the technical field of chemical industry, in particular to a preparation method and application of micromolecule compound with HIV resisting activity. CyPA is able to combine with the Gag polymer protein in HIV-1. Silence or inhibit the activity of CypA with RNAi technology and disturb the replication of virus. The micromolecule compound in the invention refers to a CyPA inhibitor that has the effect of resisting HIV-1 virus. Besides, as the micromolecule compound is designed while aiming at cellular target, the invention is not easy to develop drug resistance, thus meeting the demands of lifetime medication for AIDS patients. Therefore, the micromolecule compound in the invention, which can be developed as a new type anti-AIDS drug, provides a new means for treating and curing AIDS.
Owner:FUDAN UNIV
Chinese herbal medicine gargle for preventing and treating AIDS stomatitis
The invention relates to a gargle of Chinese medicine for curing aids-induced stomatitis. The gargle is prepared in a way that Chinese medicinal materials including root of rehmannia, radix scrophulariae, radix ophiopogonis, root of straight ladybell, fiveleaf gynostemma herb, coptis chinensis and the like are mixed according to certain weight portion and decocted with water. The gargle has the advantages of convenience, efficiency, low price, no side effects, and acceptability for patients, is a new method for curing aids-induced stomatitis, and takes an active role in helping aids patients ease pains, improve life quality, and prolong life.
Owner:郑培秋
AIDS based preparation method of three-dimensional faulting specimens for researching and teaching
InactiveCN104077948AAchieve the perfect combinationCan be stored for a long timeEducational modelsMethyl violetMedicine
The invention relates to an AIDS based preparation method of three-dimensional faulting specimens for researching and teaching. The preparation method is characterized by comprising the following steps: preparing the operation environment and operation conditions; shaping a corpse specimen of an AIDS patient, and pouring a formalin solution through femoral artery intubation for anticorrosion; after pouring the formalin solution into the corpse specimen, placing for 10-12 hours, and fully soaking the corpse in a formalin solution with the weight ratio of 5% for fixing for 6 months; positioning and scribing on the corpse specimen surface in the methyl violet elastic line method; placing the corpse specimen at the environment of -30 DEG C for freezing for 7-10 days; sawing the corpse specimen through a wood serrulate band saw for traverse, coronary and sagittal faulting specimens; conducting pathologic sampling on the faulting specimens; processing and preserving the specimens. The AIDS three-dimensional faulting specimens prepared through the preparation method realize the general perfect combination of the faulting specimens, CT images and pathological corroboration.
Owner:BEIJING YOUAN HOSPITAL CAPITAL MEDICAL UNIV
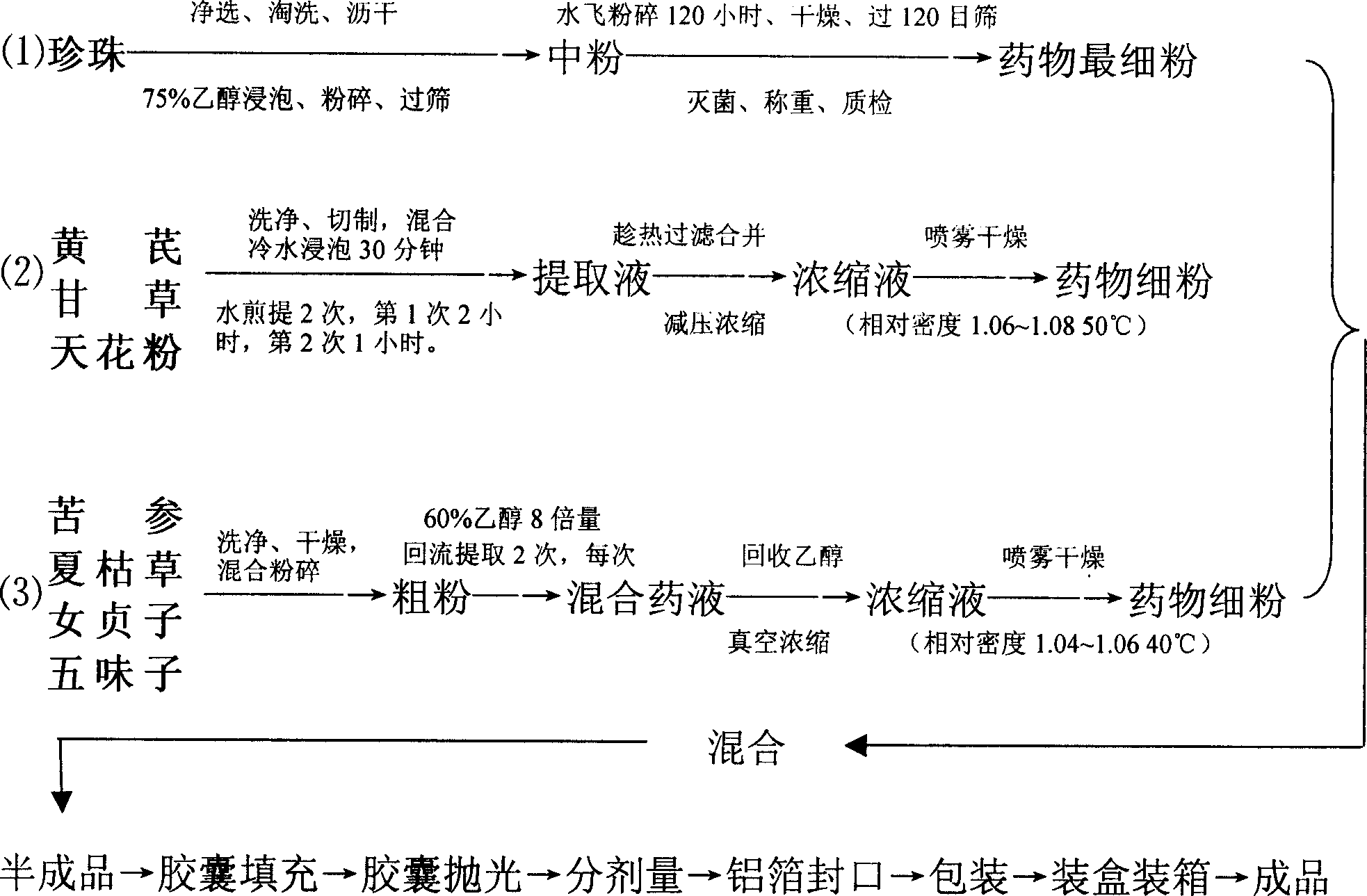
Chinese medicine capsule for treating AIDS and its preparing method
InactiveCN1857411AAbundant resourcesEasy to acceptAntiviralsCapsule deliveryUse medicationSophora Root
The present invention discloses AIDS treating Chinese medicine capsule and its preparation process, and relates to HIV treating medicine. The medicine is prepared with eight kinds of Chinese medicinal materials, including pearl, astragalus root, trichosantes root, licorice, flavescent sophora root, etc in certain weight proportion, and through grinding the Chinese medicinal materials, encapsulating and other steps. It has functions of benefiting Qi, nourishing Yin, cooling blood, detoxicating, clearing away heat, etc, and can inhibiting HIV and strengthen immunity, and has no toxic side effect.
Owner:汪贵昌
Chinese medicinal preparation for treating acquired immune deficiency syndrome (AIDS) and preparation method thereof
ActiveCN102805810AImprove immune functionGood treatment effectAntiviralsPlant ingredientsCD4 LymphocyteSide effect
The invention discloses a Chinese medicinal preparation for treating acquired immune deficiency syndrome (AIDS) and a preparation method thereof. The Chinese medicinal preparation is prepared from the following raw materials of baical skullcap root, sophora flower, astragalus, Chinese angelica, siberian solomonseal rhizome, prepared rehmannia root, liquoric root, weeping forsythia, Chinese caterpillar fungus and citric acid in a weight ratio. The Chinese medicinal preparation has the effects of clearing heat and removing toxicity, strengthening body resistance to eliminate pathogenic factors,activating blood circulation and tonifying qi, is used for human immunodeficiency virus (HIV) infected persons and AIDS patients, has an effect of improving cluster of differentiation 4 (CD4) lymphocyte count, can reduce the HIV viral load of peripheral blood gradually, improve the symptoms of the patients obviously and improve liver functions of the patients, and does not have toxic or side effects of antiviral medicines in western medicines.
Owner:张林
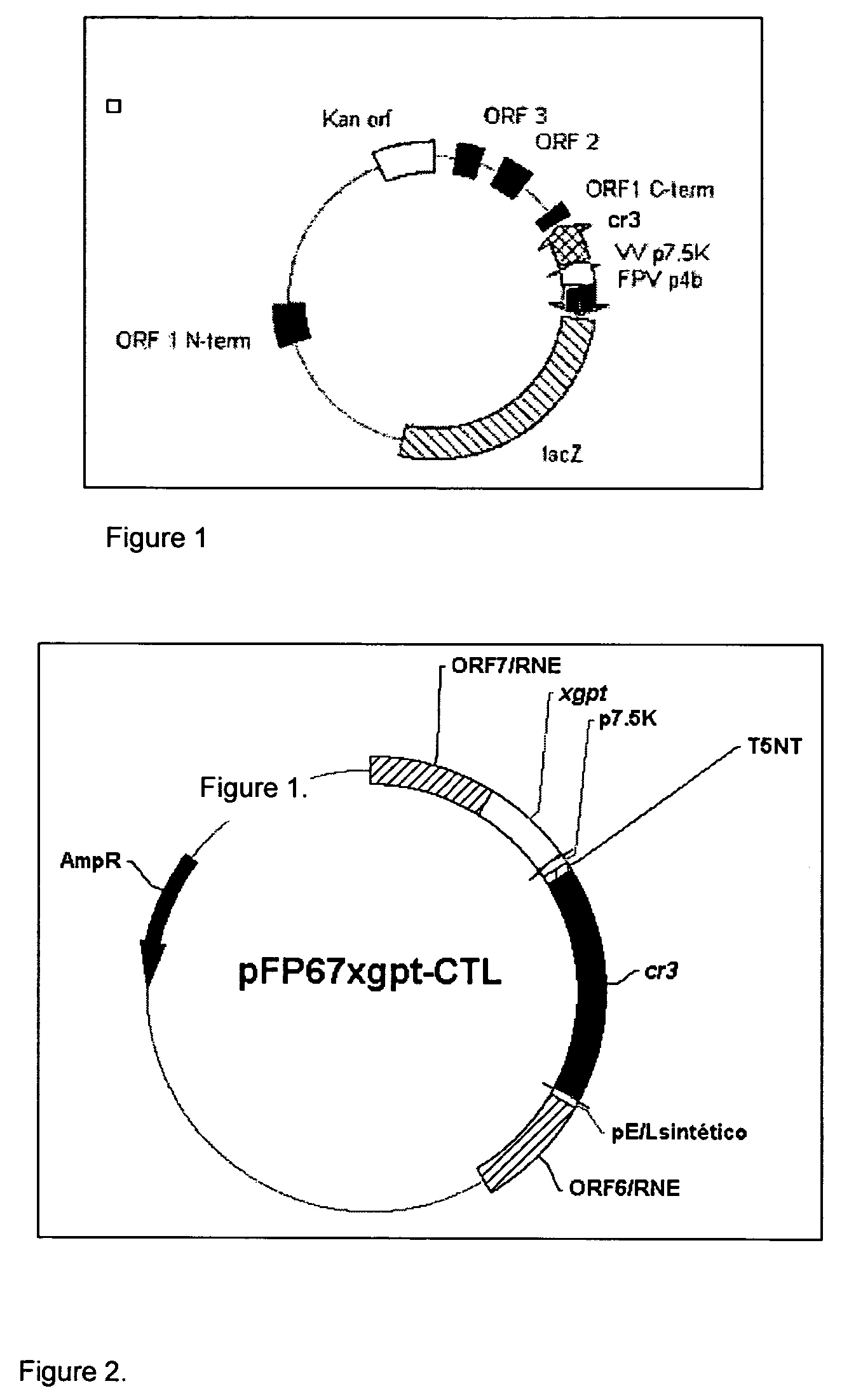
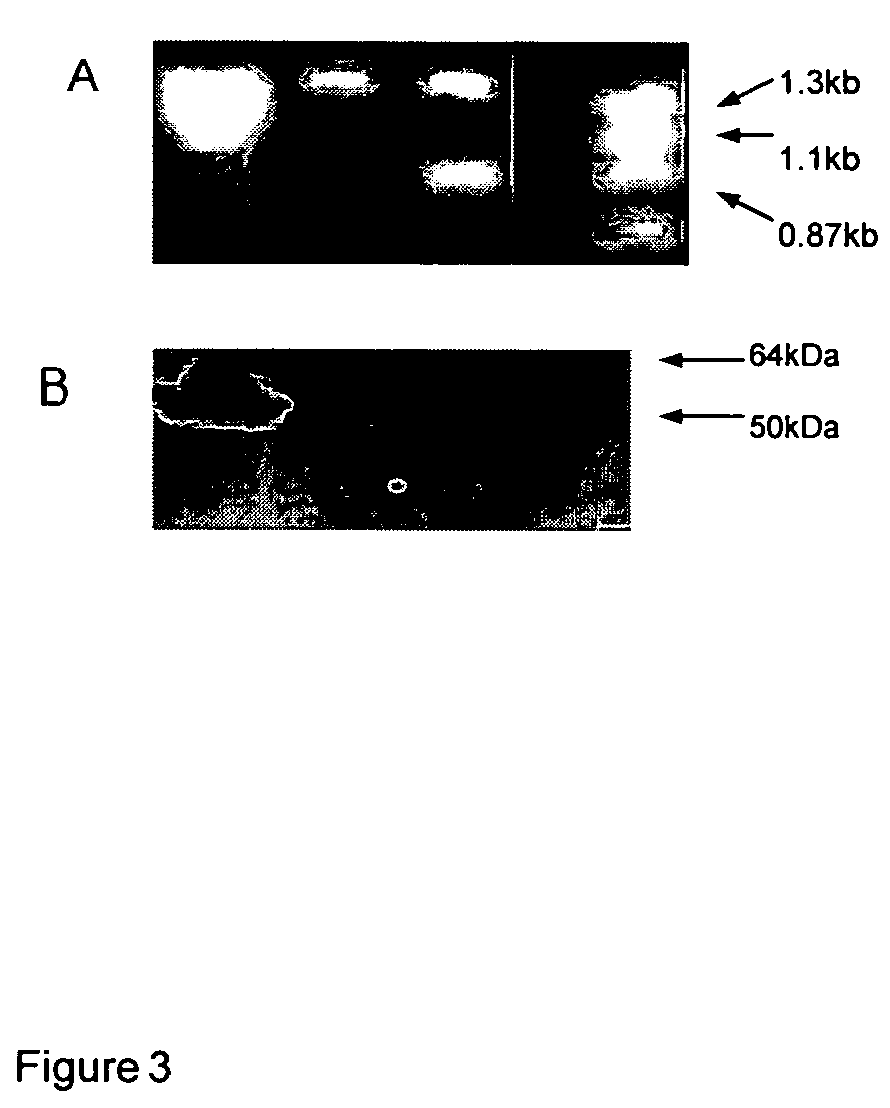
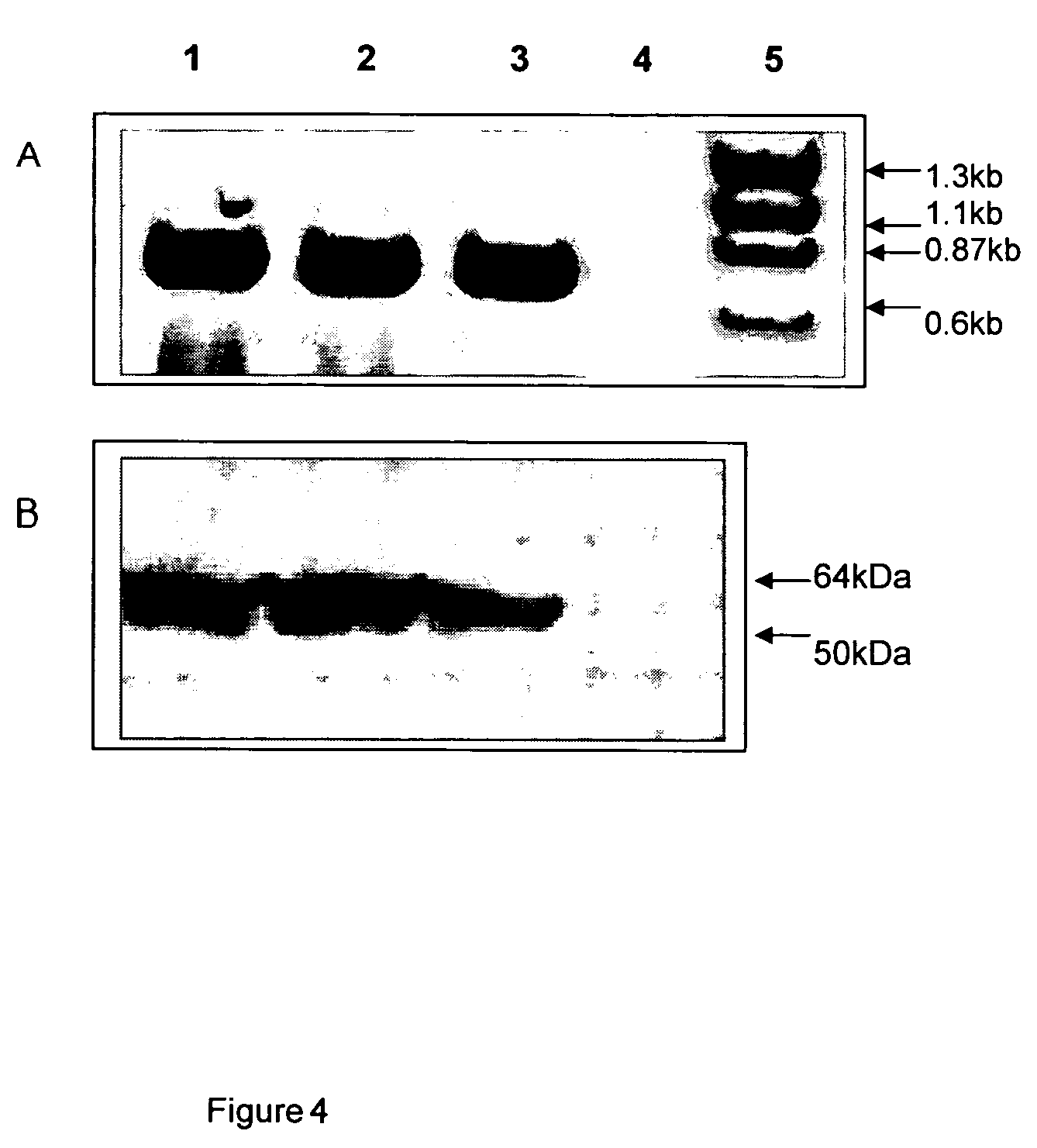
Recombinant poxvirus for chimeric proteins of the human immunodeficiency virus
InactiveUS7318927B2Promote generationEasy to combinePeptide/protein ingredientsGenetic material ingredientsVaccinationImmunodeficiency virus
The invention relates to HIV chimeric gene formed by the union of fragments of different genes of said virus, wherein said fragments contains epitopes for cytotoxic T cells (CTL) or HIV-1 auxiliary T cells, which are presented by a wide range of antigens of type Major Histocompatibility Complex (HLA-I). Recombinant poxviruses are obtained from said genes, which are useful for prophylactic and therapeutic vaccination against HIV / AIDS infections, are capable of generating a protective immune cell response in vaccinated laboratory animals and are recognized by the CTL lymphocytes of HIV / AIDS patients.
Owner:CENT DE ING GENETICA & BIOTECNOLOGIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com