Patents
Literature
43 results about "Treatment failure" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Treatment Failure. A measure of the quality of health care by assessment of unsuccessful results of management and procedures used in combating disease, in individual cases or series.
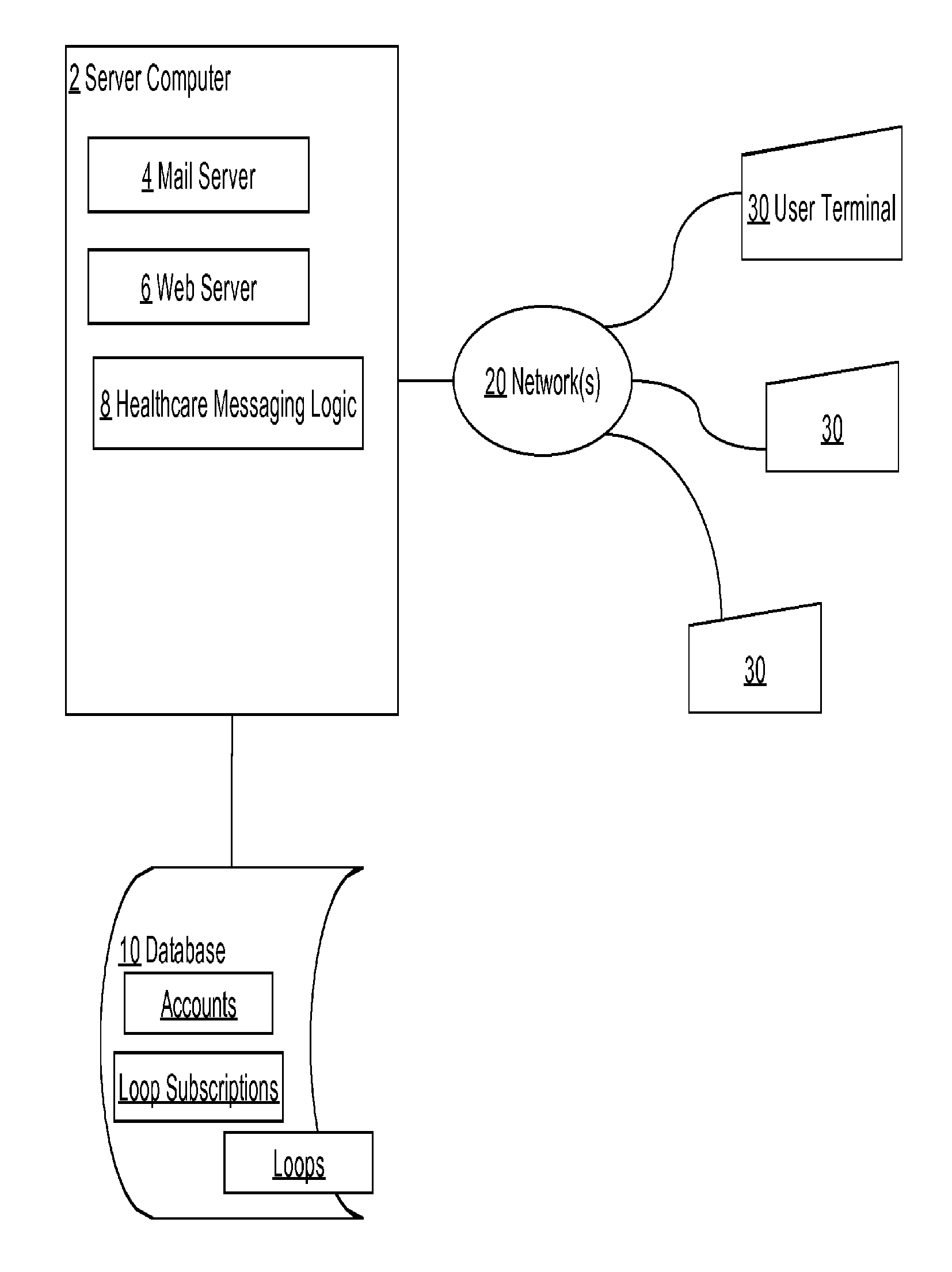
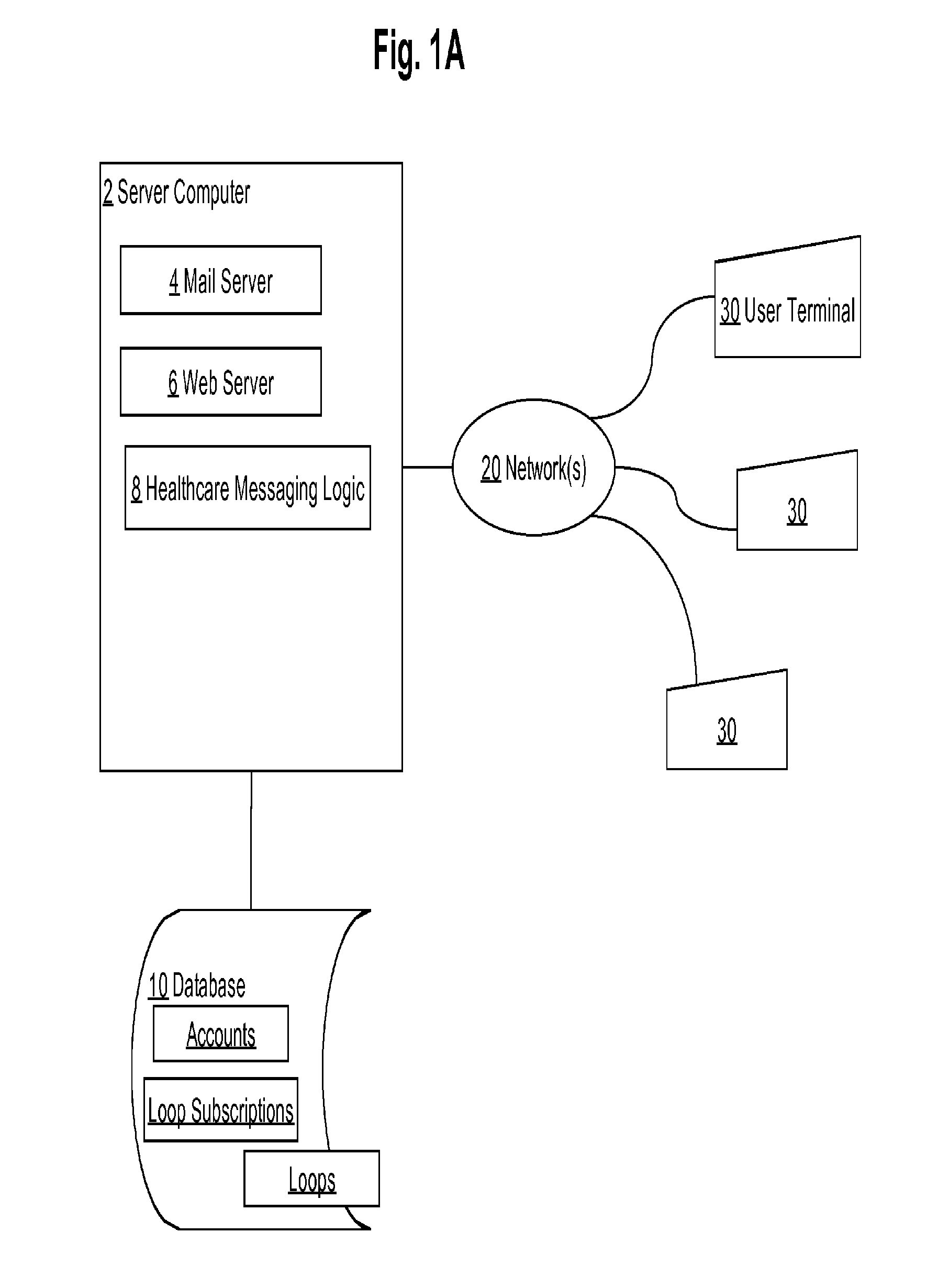
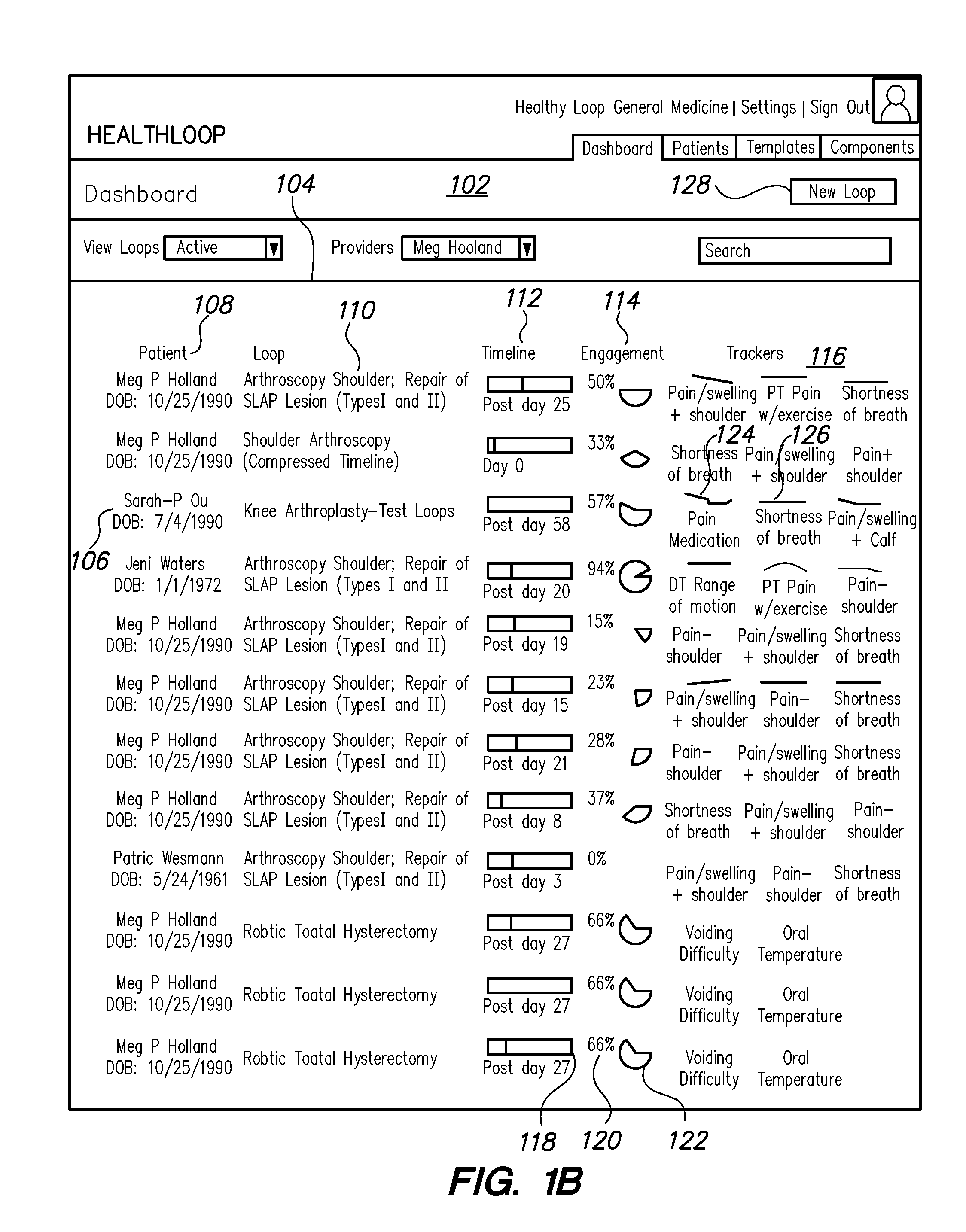
Healthcare pre-visit and follow-up system
Data processing methods facilitate an exchange, between healthcare providers and patients or other users, of structured data for objective health signs and subjective symptoms from the patient or caregiver during a pre-visit and / or follow-up period of care; standardized data-informed Loop protocols for following health conditions; data-informed ARC OF RECOVERY profiles that represent population-based recovery standards for signs, symptoms, or composites; automated alerts, based on analytics or machine learning, to warn health care providers regarding impending treatment failures or worsening of conditions. Patients in pre-visit or follow-up stages of healthcare respond to questions structured by a provider for objective signs and subjective symptoms, and may view questions and responses with associated alerts or status updates in a consolidated display that provides patient graphic images and comments about conditions. Aspects of pre-visit or follow-up care are Tracker historical graphical displays for evaluation by the physician against expected profiles, protocols or other standards.
Owner:GETWELLNETWORK INC
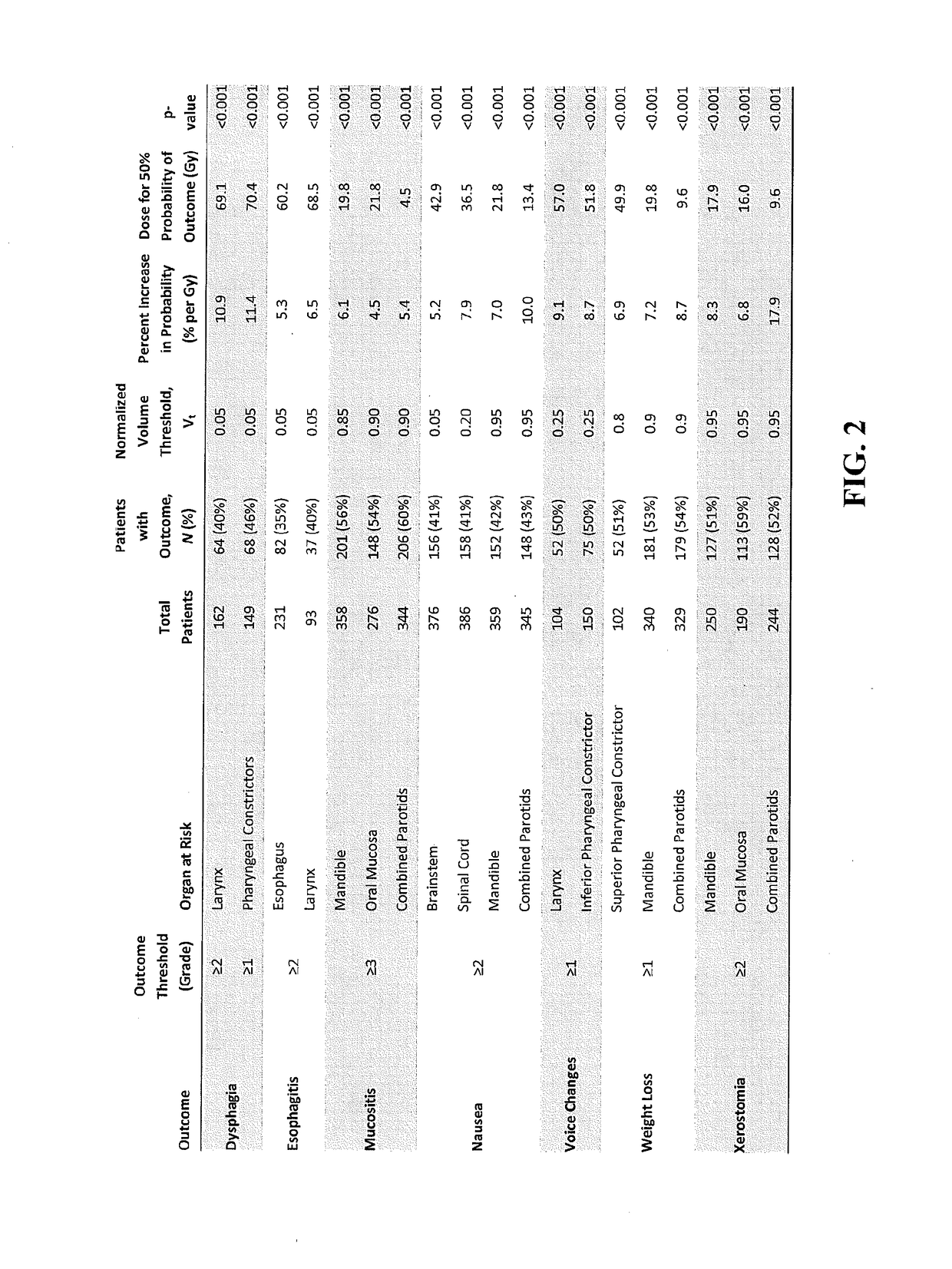
Method, system and computer-readable media for treatment plan risk analysis
ActiveUS20170083682A1Risk minimizationMedical simulationMedical data miningPatient dataOrgan at risk
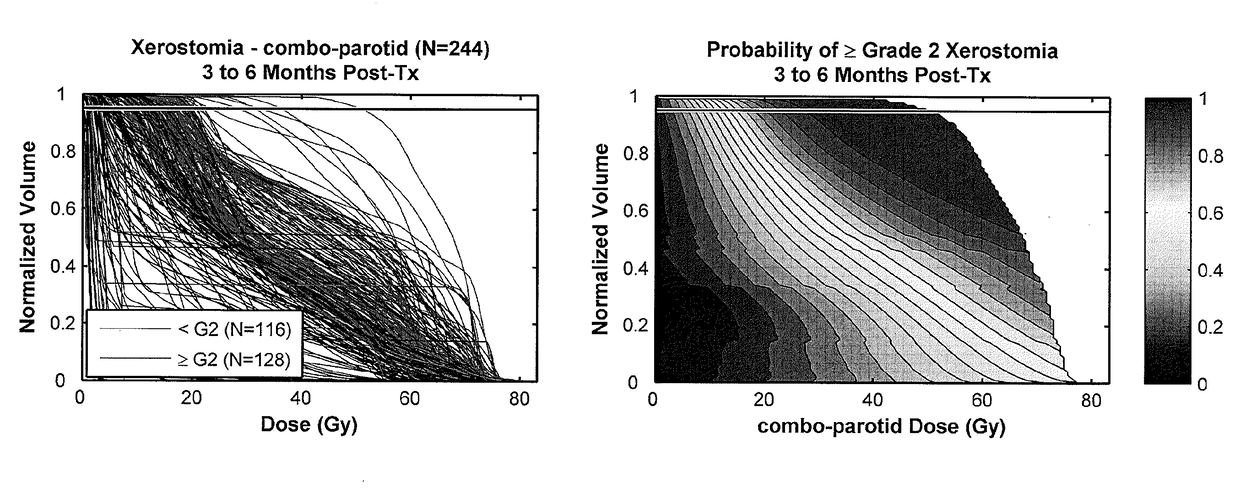
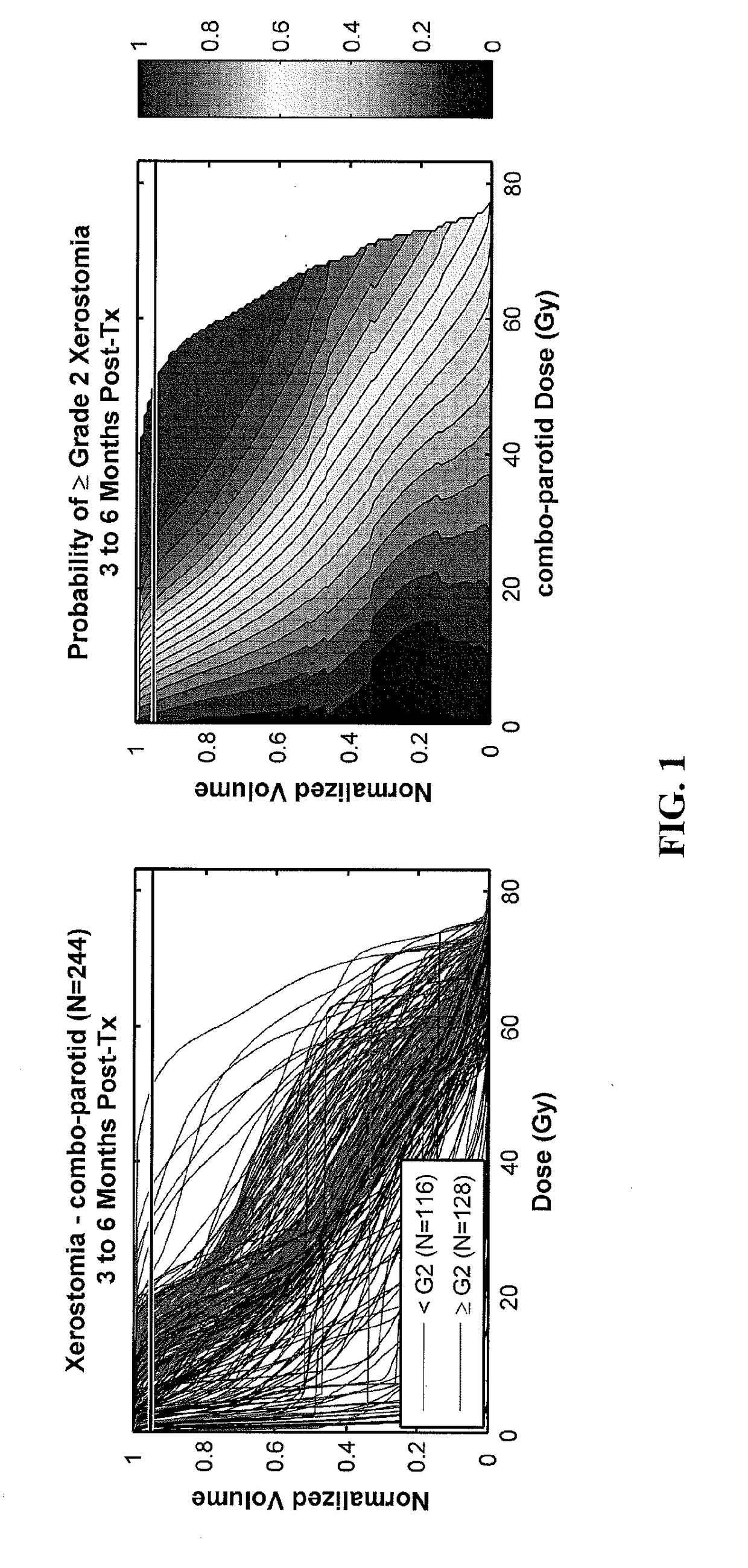
A method, system and computer readable medium of: providing feature data of at least one organ at risk or target volume of said patient from a database of non-transitory data stored on a data storage device of prior patients data; generating, using a data processor, a distribution of dose points of the at least one organ at risk or target volume of said patient based on said feature data; calculating, using the data processor, at least one of (i) a probability of toxicity for the at least one organ at risk or (ii) a probability of treatment failure for the at least one target volume, based on said distribution of dose points; assessing, using the data processor, a dosimetric-outcome relationship based on the calculated probability; and automatically formulating, using the data processor, a treatment plan using the dosimetric-outcome relationship to minimize the at least one treatment-related risk.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
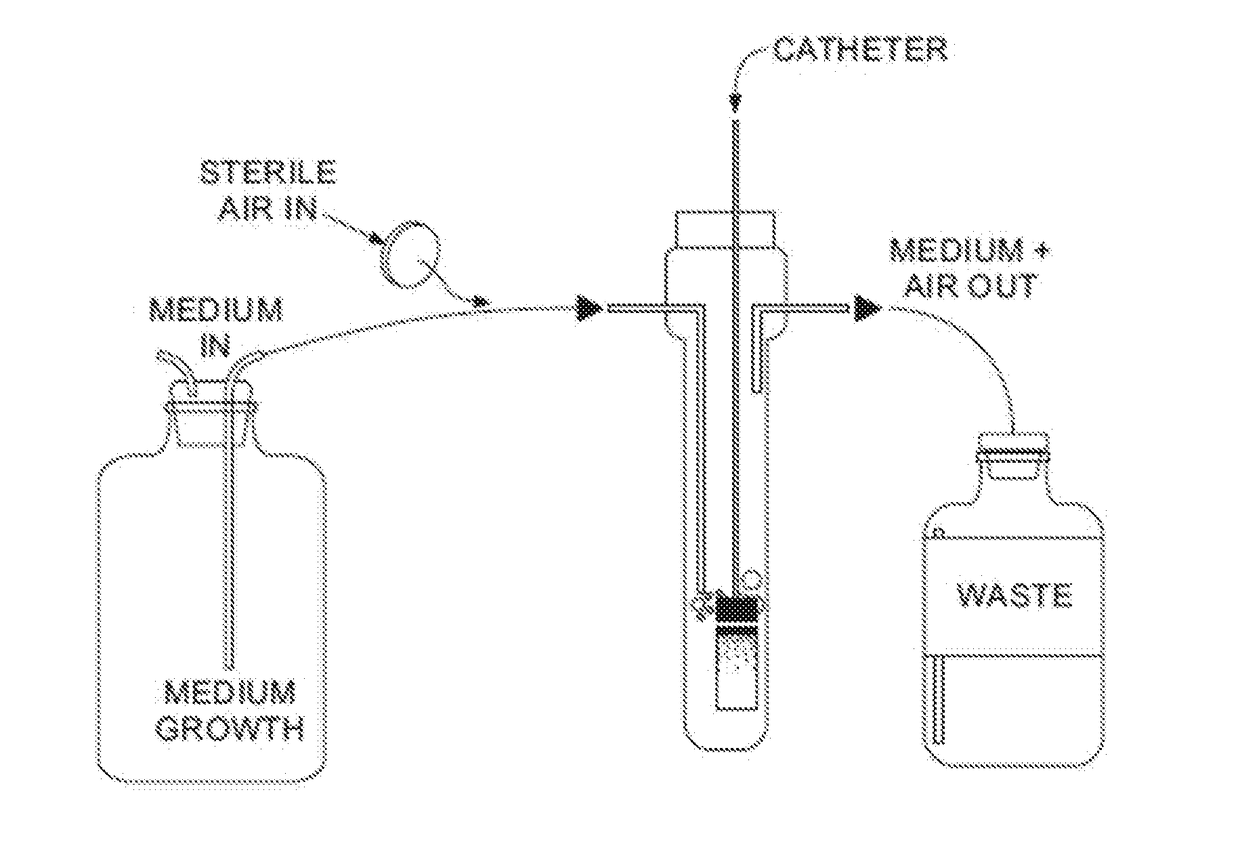
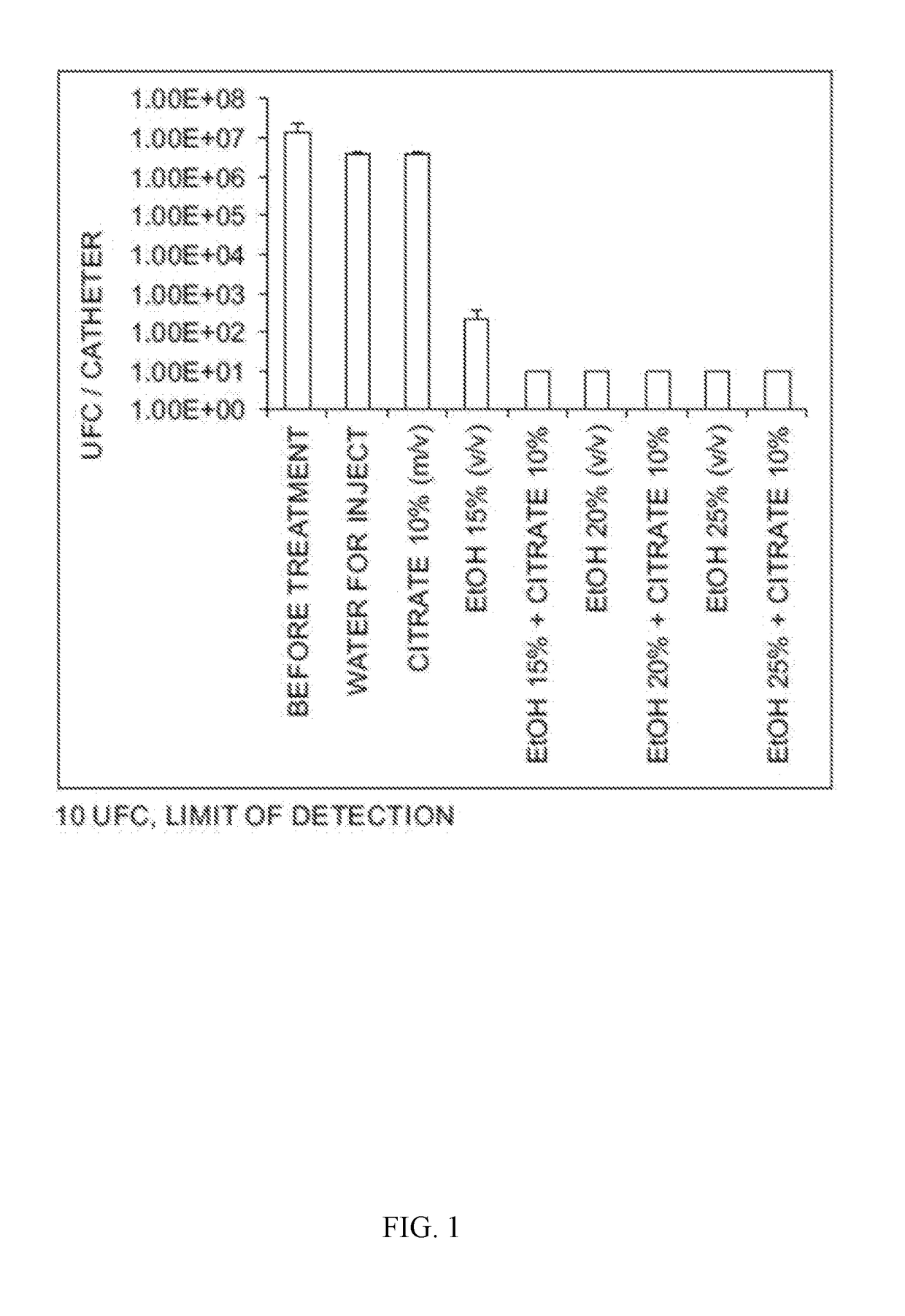
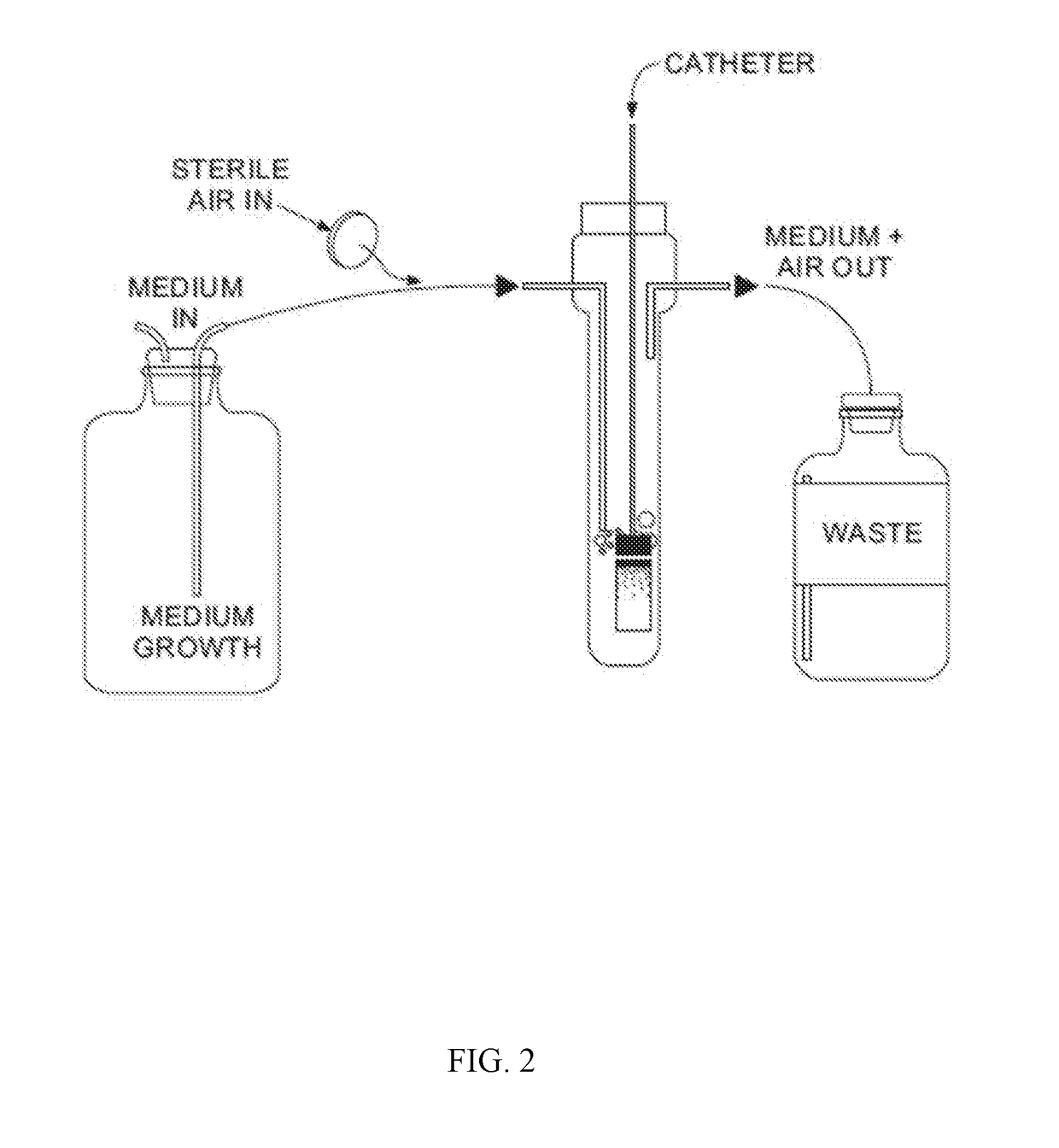
Catheter Locking Solution And Catheter Locking Therapy
ActiveUS20170232153A1Reduce failurePrevent coagulationCatheterLavatory sanitoryDiseaseTreatment failure
Various embodiments relate to catheter locking solutions and catheter locking therapies with use of trisodium citrate and ethyl alcohol, and in particular 4.0 to 15.0 weight / volume % trisodium citrate as an anticoagulant component and / or an antibacterial component and 15.0 to 25.0 volume / volume % ethyl alcohol as an antibacterial component. Use of the catheter locking solution and catheter locking therapy can reduce treatment failure during medical procedures that may employ catheters to supply treatment by at least significantly reducing the risks associated with bloodstream infections, catheter system malfunction, emboli formation, patient discomfort, and patient illness. These benefits can be partially due to the synergistic antibacterial effects of the trisodium citrate and ethyl alcohol in solution, generating an effective catheter locking solution with minimal concentrations of ethyl alcohol.
Owner:MEDICAL COMPONENTS INC
Method for increasing the replication of oncolytic HSVs in highly resistant tumor cells using mTOR pathway and PI3K inhibitors
InactiveUS20120100109A1Reduce healingImproved prognosisBiocideOrganic active ingredientsTherapy resistantWilms' tumor
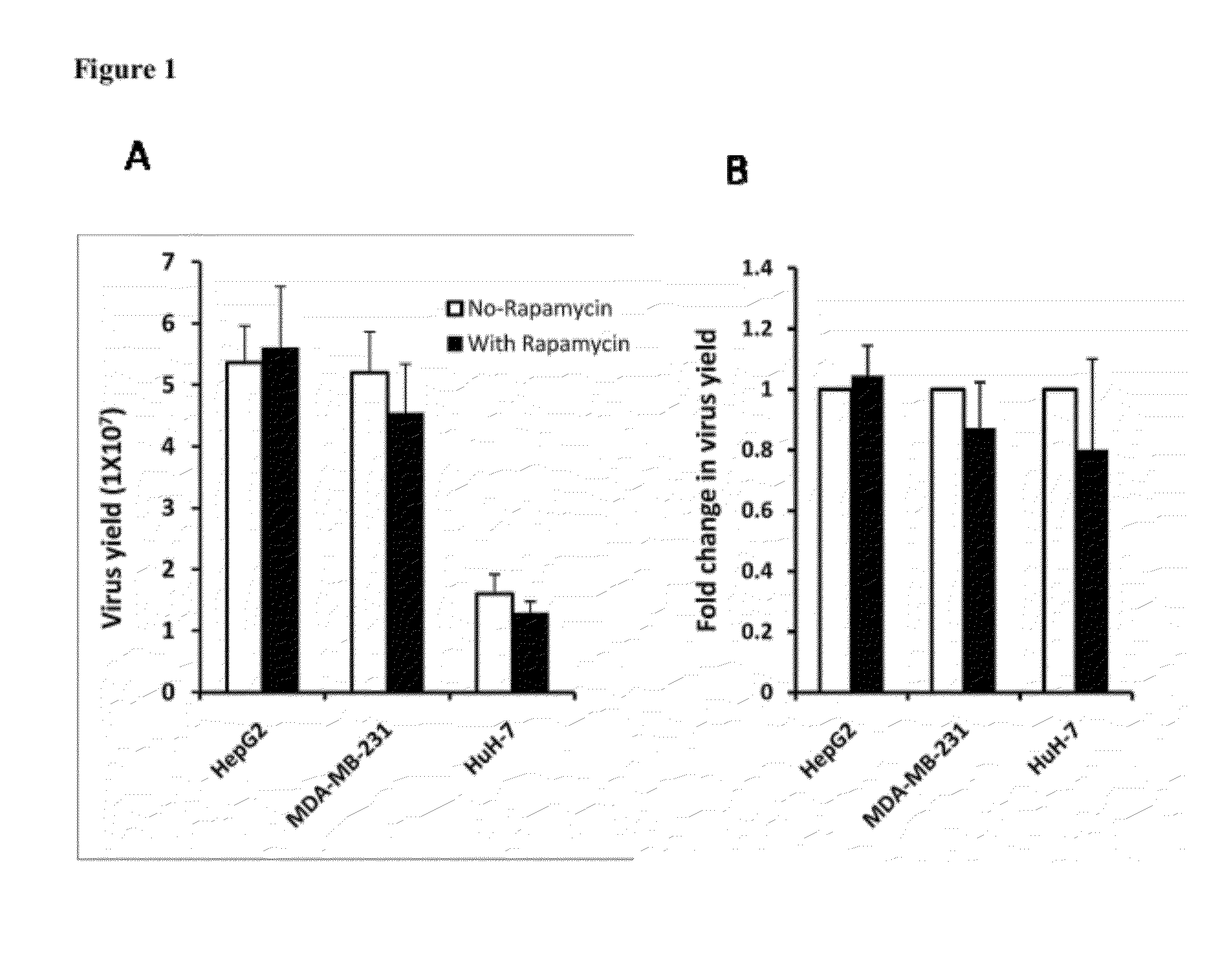
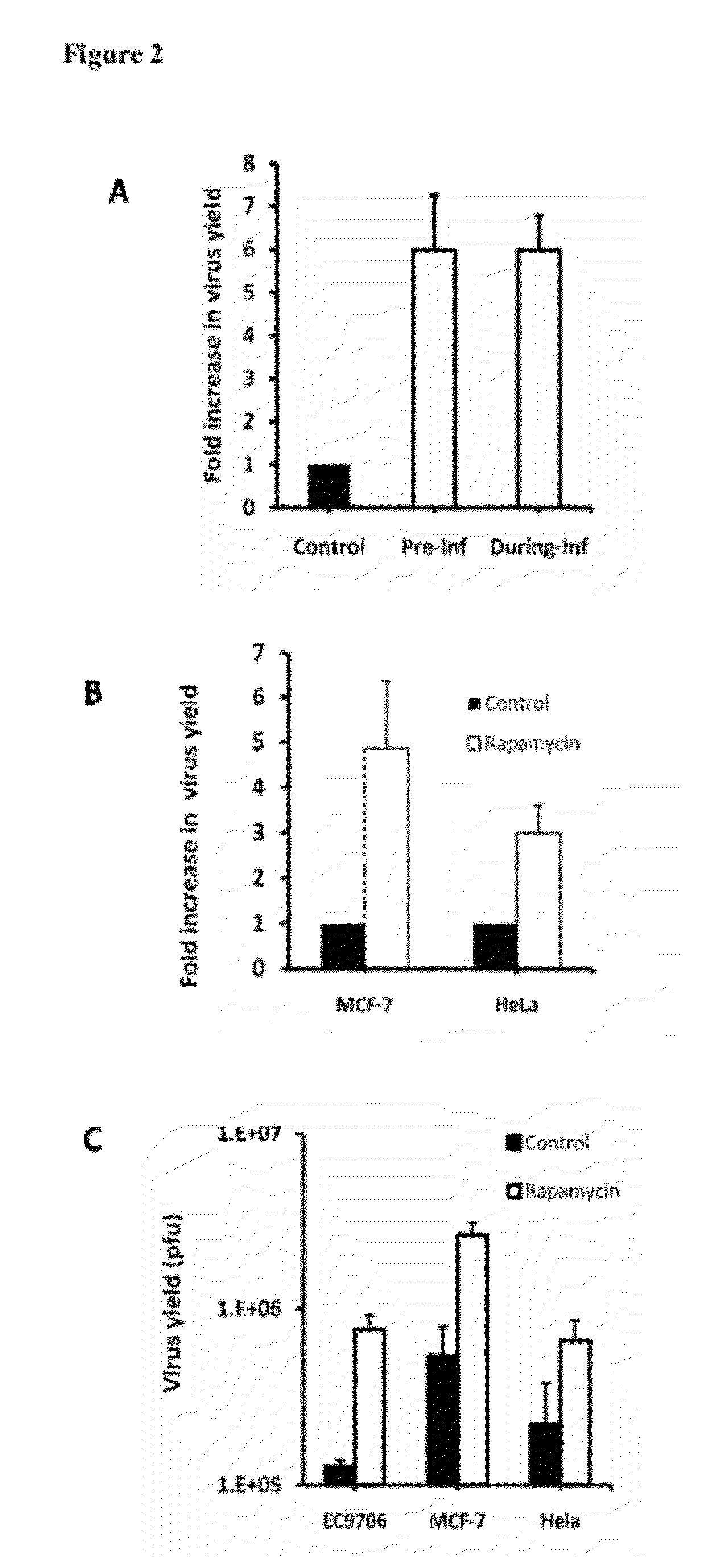
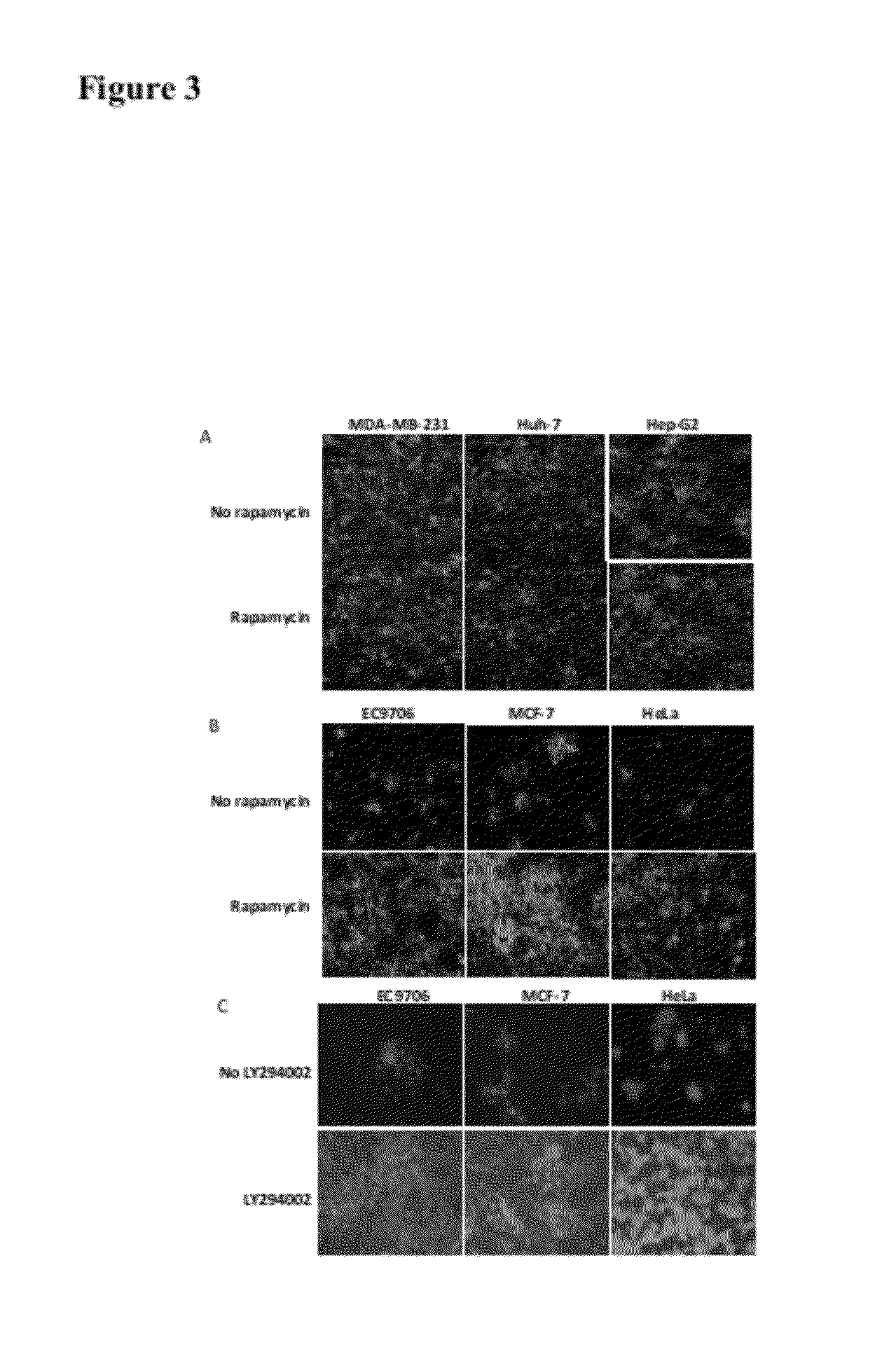
The present invention is directed to the administration of an HSV derived oncolytic virus and a PI3K / AKT / mTOR pathway inhibitor to treat various types of resistant tumors. Therapy-resistant tumor formation is one of the main causes for treatment failure in the clinic. The treatment methods and compositions disclosed herein sensitize resistant tumors to the treatment of herpes simplex virus (HSV)-based oncolytic virotherapy. Pre or co-treatment of resistant tumor cells with the mTOR inhibitor, rapamycin, or certain PI3K inhibitors, such as LY294002, can efficiently sensitize the tumors to HSV derived oncolytic viruses, whereby the replication and spread of the viruses are dramatically enhanced.
Owner:HOUSTON SYST UNIV OF
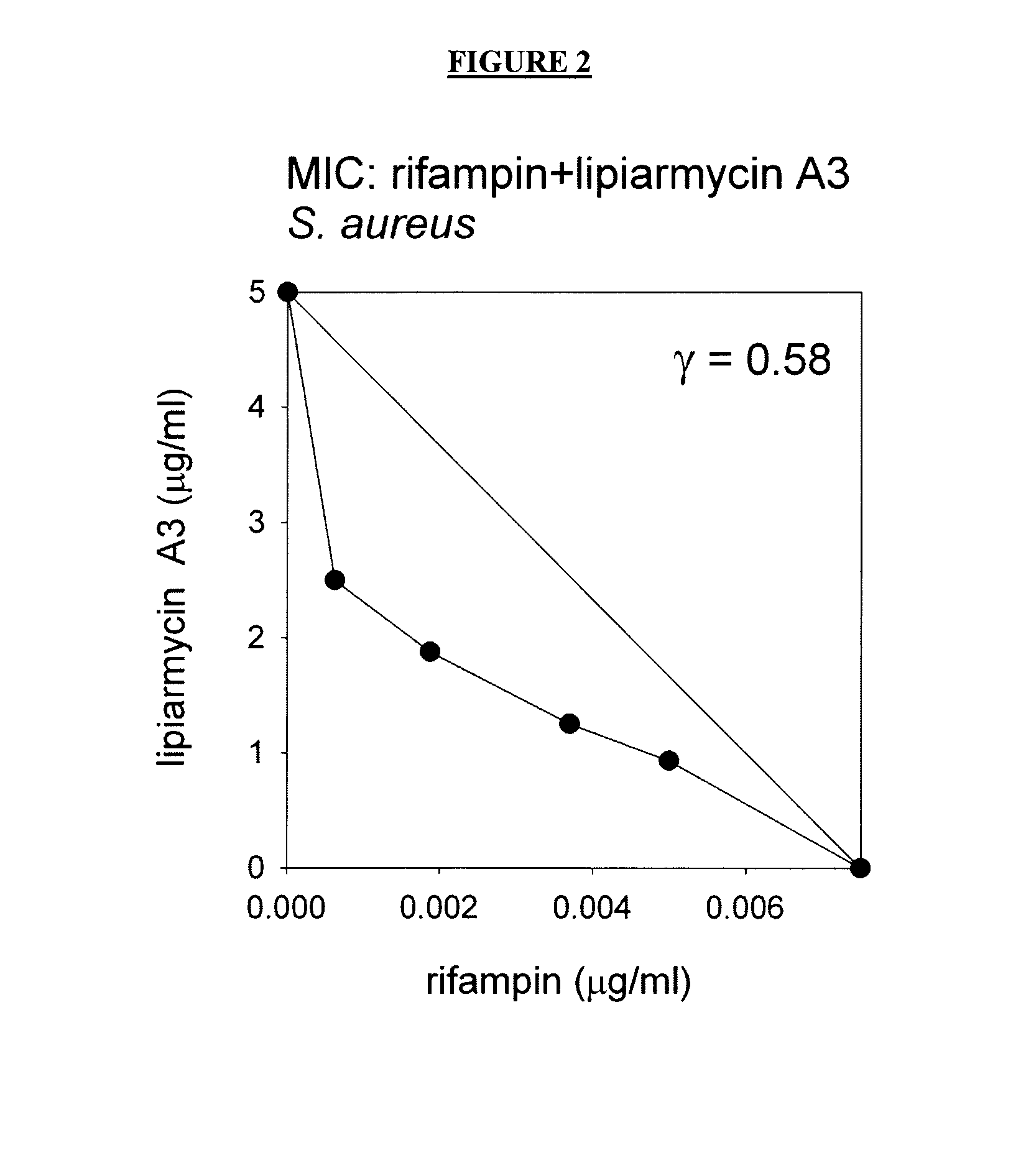
Antibacterial agents: combination of a rifamycin and a switch region inhibitor
ActiveUS20150031640A1Reduces minimum effective doseReduces spontaneous resistance frequencyBiocideCarbohydrate active ingredientsTreatment failureCo administration
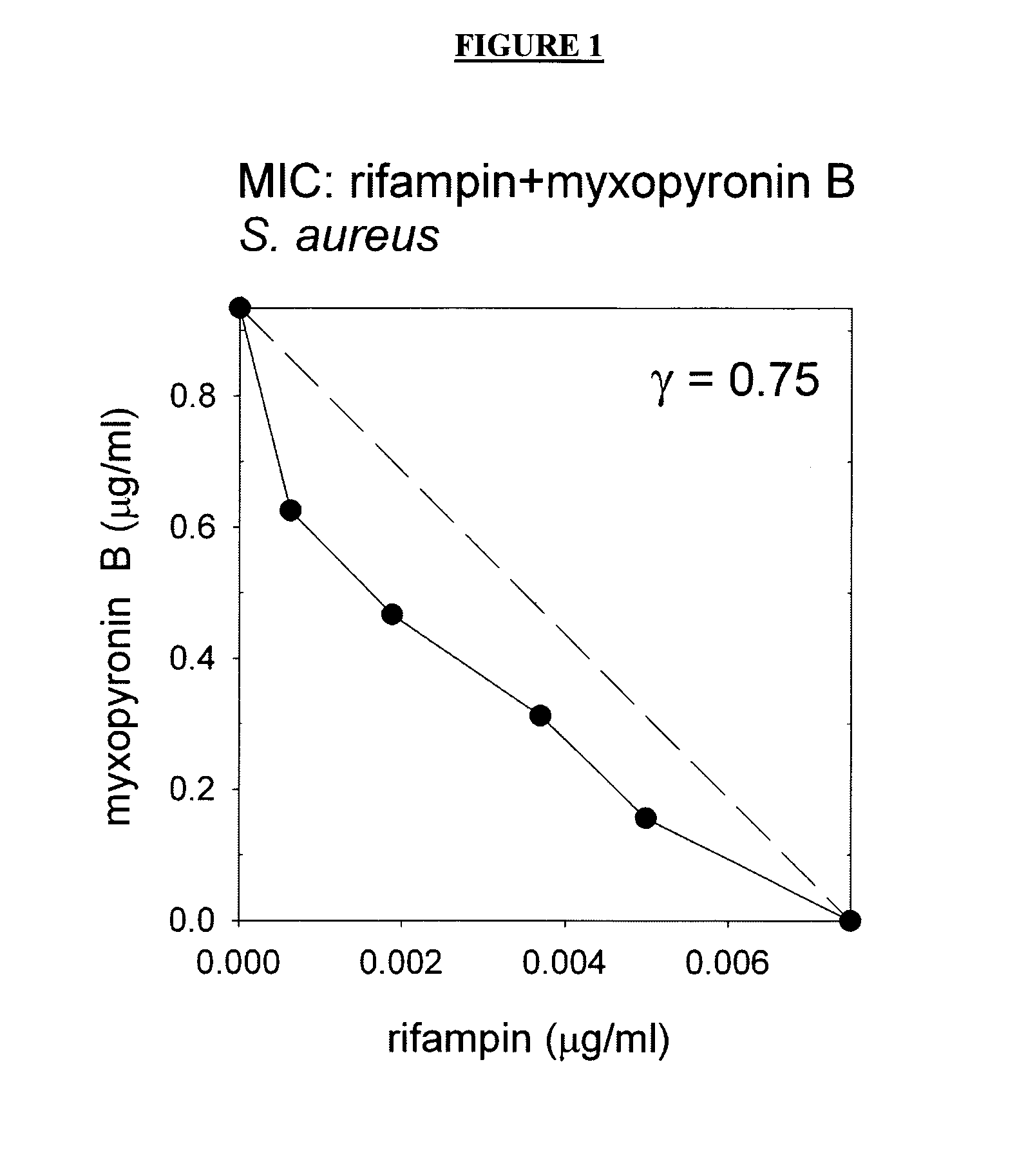
It has been determined that co-administration of a rifamycin and a switch-region inhibitor 1) results in synergistic antibacterial effects, enabling efficacy at low, subtoxic doses, and / or 2) results in a low spontaneous resistance frequency, enabling treatment of high-titer infections without treatment failure due to spontaneous resistance. Accordingly, certain embodiments provide composition comprising a rifamycin and a switch region inhibitor, as well as methods of use thereof.
Owner:RUTGERS THE STATE UNIV
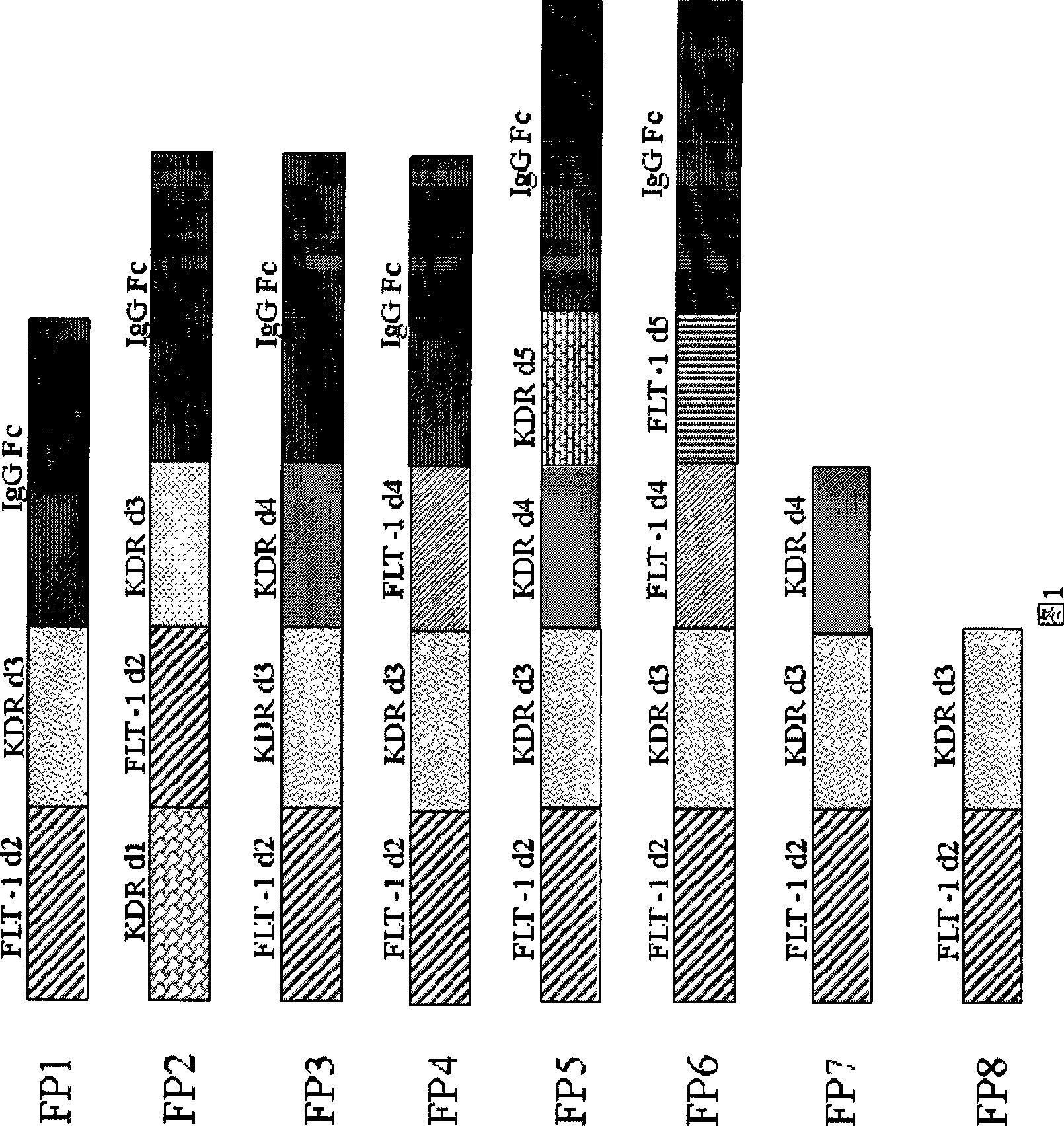
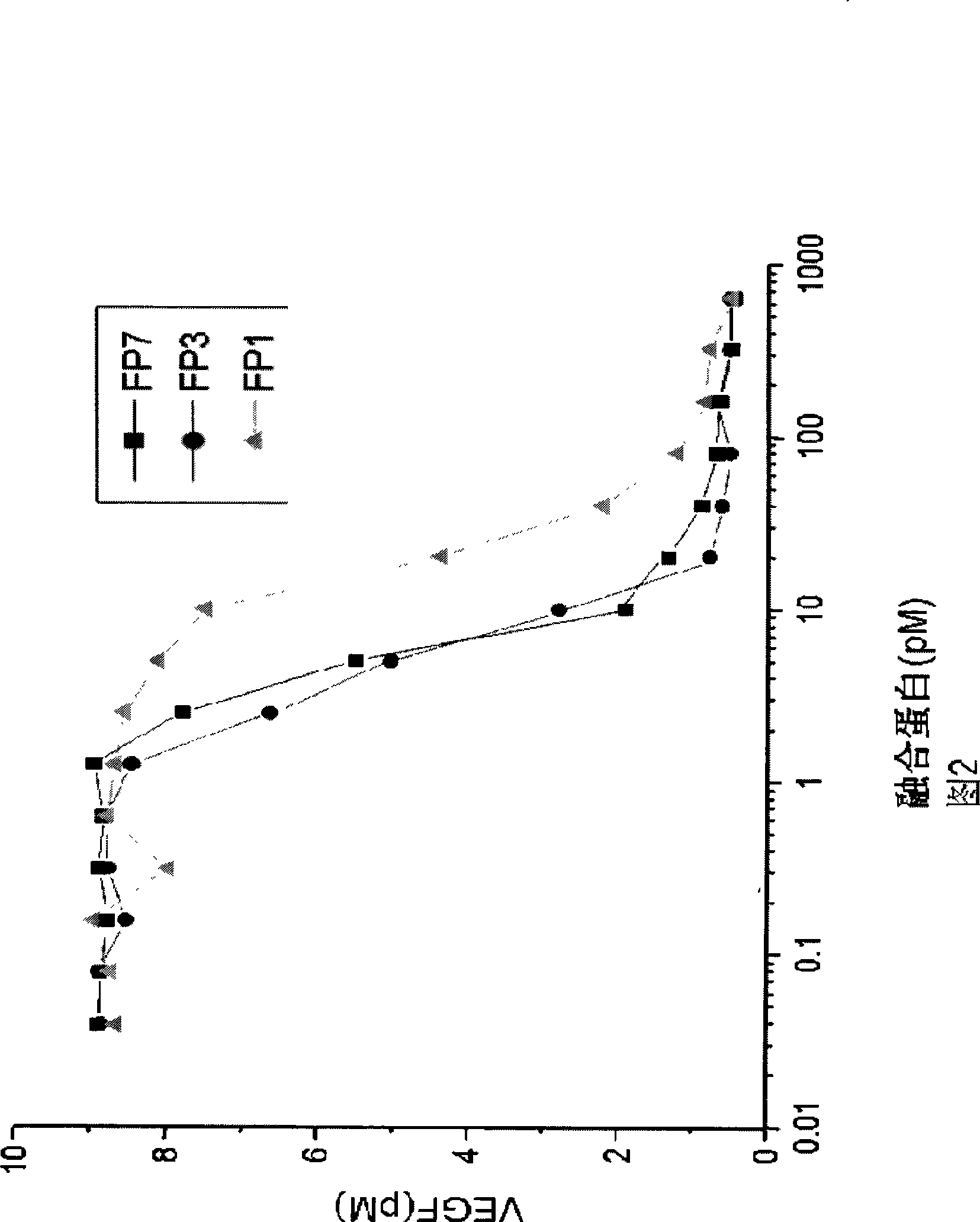
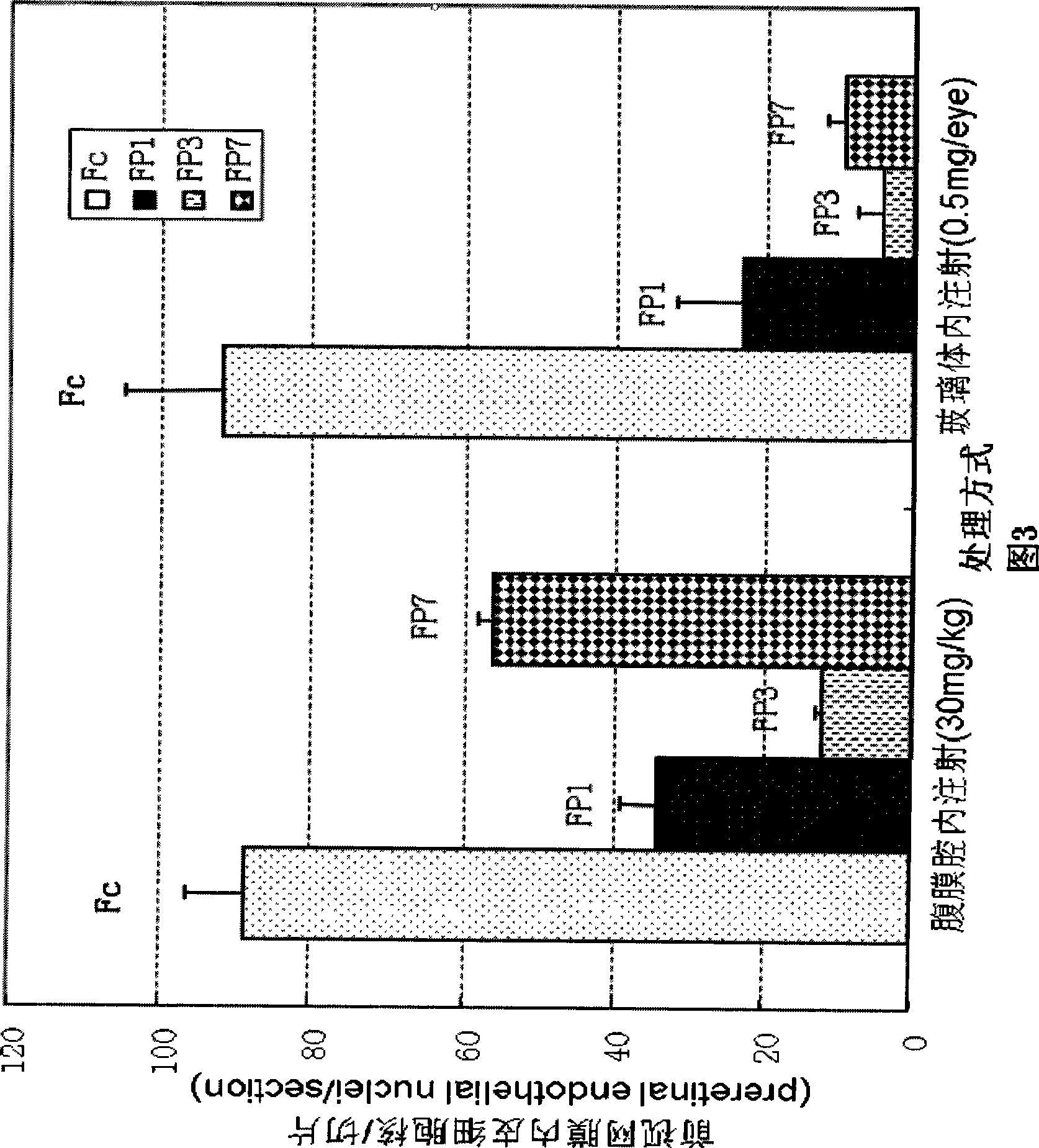
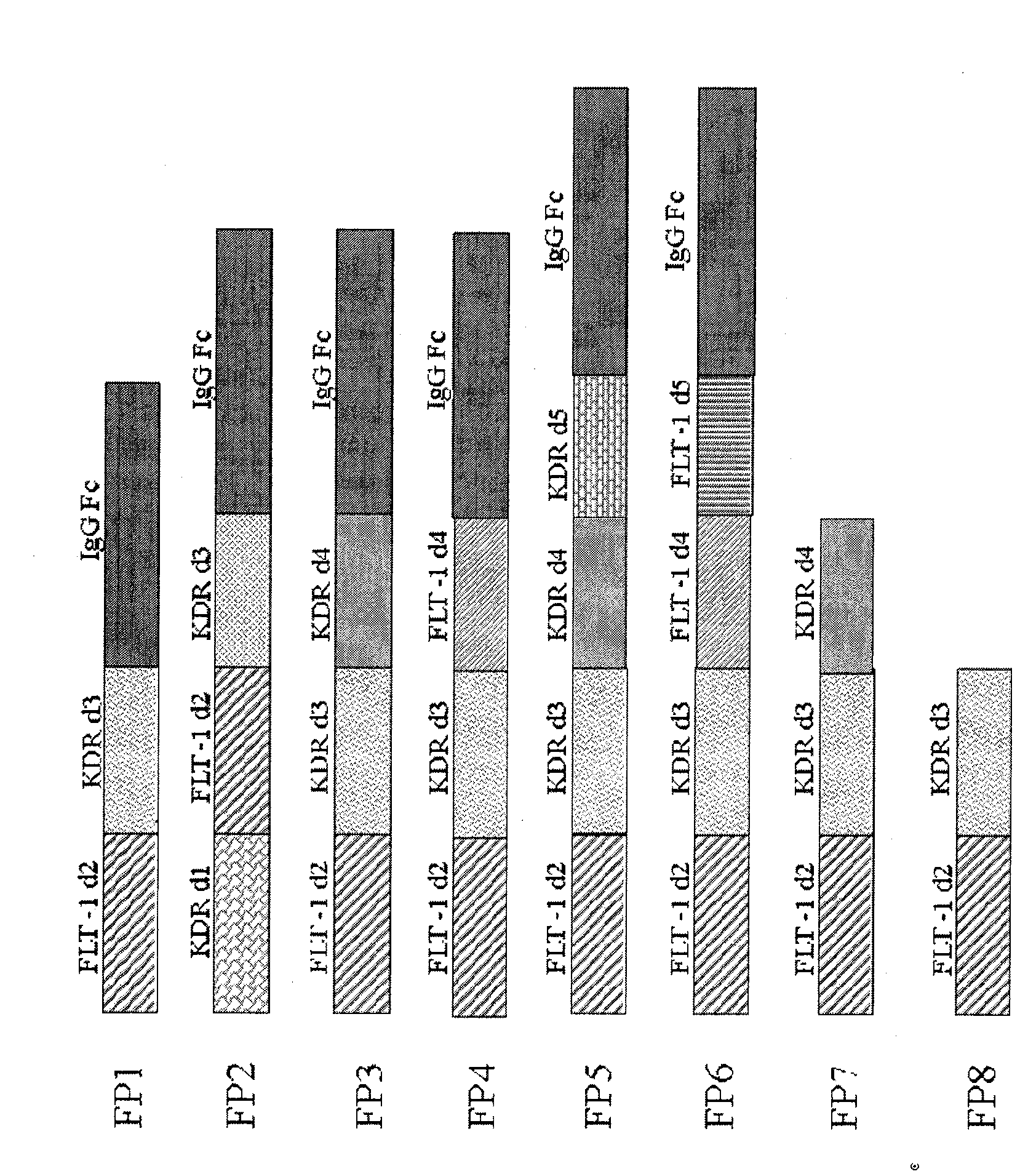
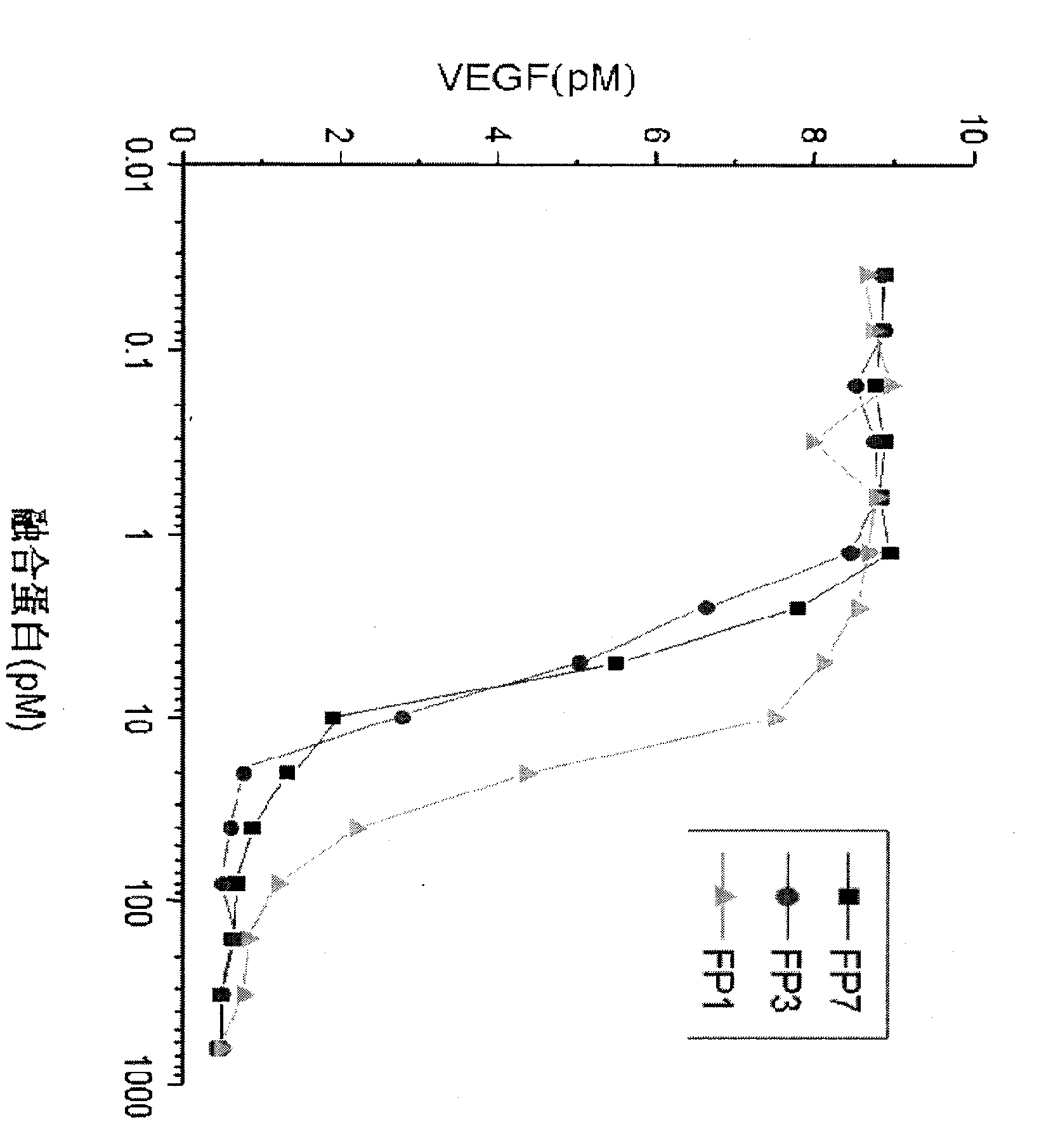
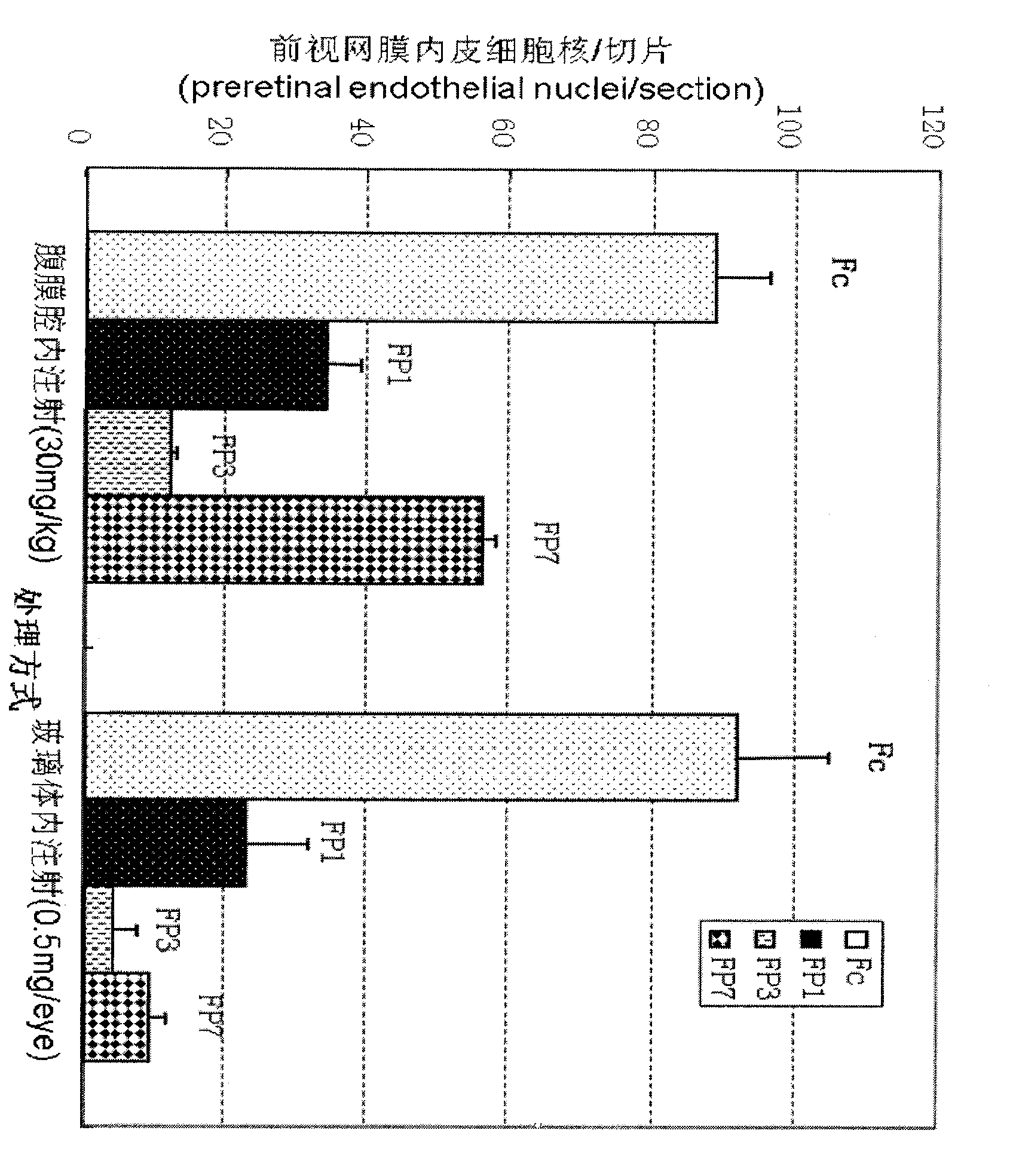
VEGF receptor fusion protein and its use in preparation of medicament for treating eye disease
ActiveCN101397343AExcellent eye disease treatment effectImprove stabilityPowder deliverySenses disorderDiseaseDiabetes retinopathy
The invention relates to two VEGF receptor fusion proteins, a preparation method thereof and the application in treating eye diseases, wherein, the eye diseases comprise age-retinal pigment epitheliitis, diabetic retinopathy, diabetic xanthelasma gland and treatment failure caused by neovascularization growth, such as laser photocoagulation body and surgery retina transplantation.
Owner:CHENGDU KANGHONG BIOTECH
VEGF receptor fusion protein and its use in preparation of medicament for treating eye disease
ActiveCN100567325CExcellent eye disease treatment effectImprove stabilityPowder deliverySenses disorderAbnormal tissue growthDiabetes retinopathy
The present invention relates to two VEGF receptor fusion proteins, their preparation methods and their use in the treatment of eye diseases, including age-related macular degeneration, diabetic retinopathy, diabetic xanthoma glands and tumors caused by neovascular growth Caused failure of treatments such as laser photocoagulation, surgical retinal transplantation.
Owner:CHENGDU KANGHONG BIOTECH
Method for treating hepatitis c virus infection in treatment failure patients
InactiveUS20050031586A1Prevent diseaseArresting its developmentOrganic active ingredientsPeptide/protein ingredientsDosing regimenTreatment failure
The present invention provides methods for treating individuals having an HCV infection, which individuals have failed to respond to therapy with an INF-α other than consensus interferon (CIFN), or who, following cessation of therapy with an INF-α other than CIFN, have suffered relapse. The methods generally involve a dosage regimen involving administering a dose of CIFN and a dose of ribavirin for a period of time, where the dosage regimen is effective to achieve a sustained viral response in the individual.
Owner:VALEANT PHARMACEUTICALS NORTH AMERICA LLC
DNA-based methods for clone-specific identification of staphylococcus aureus
InactiveUS20120208714A1Rapid and reliable identificationHigh variationNucleotide librariesMicrobiological testing/measurementBacteroidesTest performance
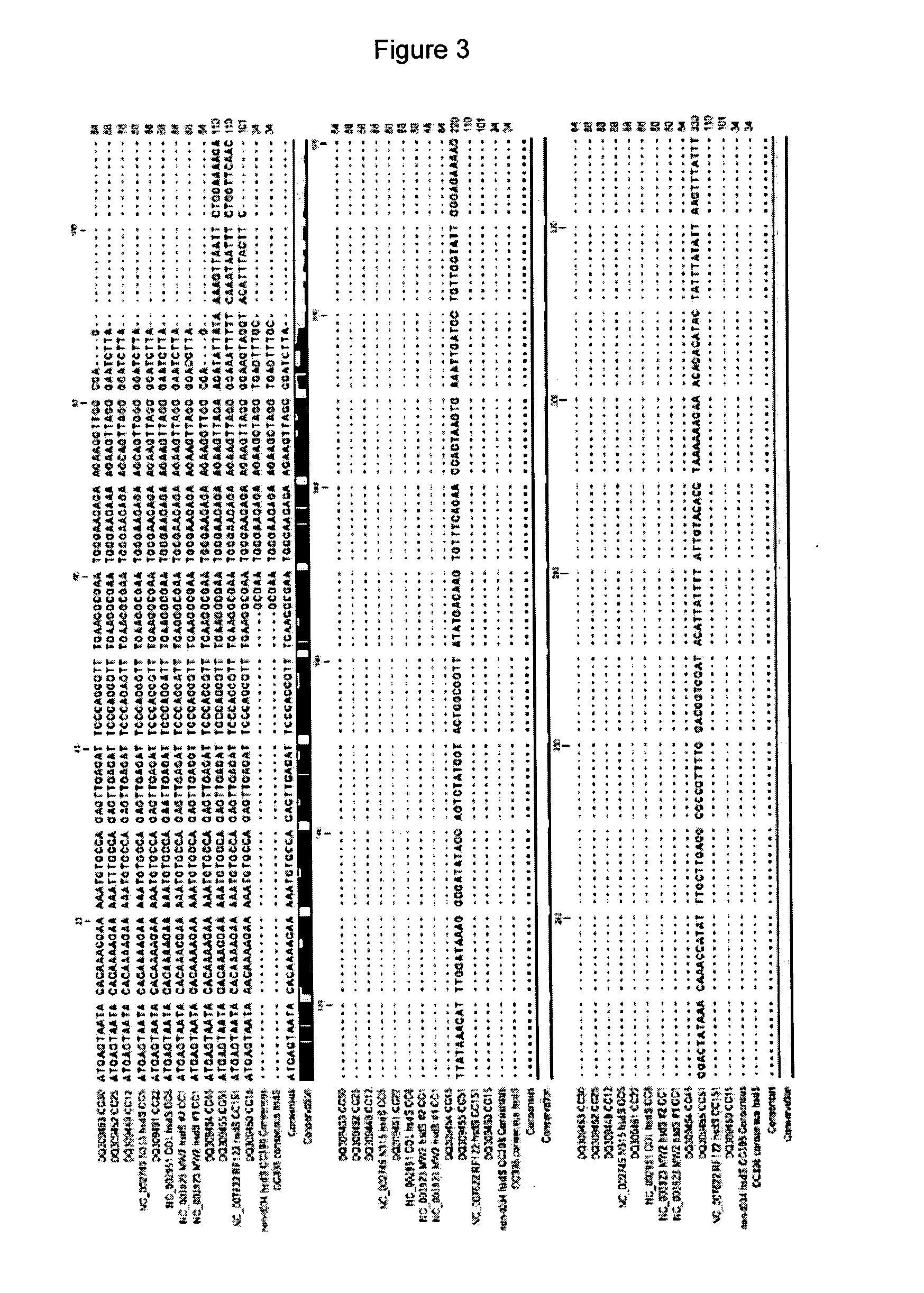
MRSA CC398 is a clone of S. aureus that has recently emerged in pigs and other domestic animals worldwide. As any other MRSA, the clone displays high levels of antibiotic resistance and poses a serious threat to human health because of the risk of antibiotic treatment failure in human patients. We developed a new diagnostic test for identification of MRSA CC398 using a single one-step PCR that is very easily performed within a few hours. The test is based on the principle that clonal differences within S. aureus are reflected in the sequence of a gene (sau1hsdS1) located on the chromosome of this bacterial species. Accordingly, such a gene represents an optimal target for S. aureus and MRSA identification at the clone level. The test includes detection of the gene conferring methicillin resistance (mecA), therefore allowing rapid discrimination between methicillin-susceptible and methicillin-resistant variants of the clone. A preliminary validation of the test was performed on a collection of CC398 and non-CC398 strains, resulting in 100% sensitivity and 100% specificity. The test can be combined to real-time PCR technology to further reduce simplify the test performance as well as to allow quantification of the target MRSA clone in biological specimens. The invention has important applications related to surveillance and control of MRSA CC398 in humans, animals and food products.
Owner:ST GEORGES HOSPITAL MEDICAL SCHOOL +2
One-step method preparation process of treatment type drug resistant AIDS (acquired immunodeficiency syndrome) autologous plasma virus inactivated vaccine
PendingCN108498795AReduce intensityReduce the number of infectious agentsViral antigen ingredientsAntiviralsBlood collectionLarge dose
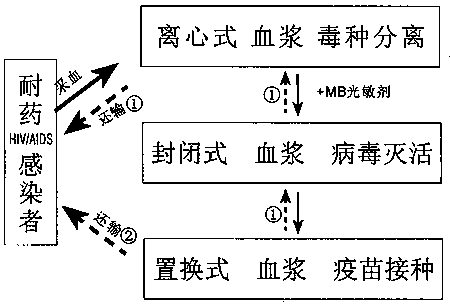
The invention relates to a one-step method preparation process of treatment type drug resistant AIDS (acquired immunodeficiency syndrome) autologous plasma virus inactivated vaccine. Through blood collection, plasma separation and plasma virus inactivation, the vaccine is directly prepared; then, intravenous vaccination is performed; the whole process is completed in a sealed disposable consumable; the pollution risk in the preparation process can be effectively avoided; the vaccine preparation safety is ensured. The preparation process is simple; the cost is low; the inoculation procedure canalso be simplified; the technical requirements of large-dose (600 to 800ml) plasma virus inactivated vaccine inoculation are met. More importantly, the virus strain of the inactivated vaccine is froma patient self; the plasma is also from the patient self; the obvious safety, effectiveness and feasibility are realized. The principle of medical ethics is conformed; the problem of animal model lack of AIDS vaccine development can also be solved. The vaccine is applicable to the continuous treatment of AIDS patients with drug resistance and treatment failure, initial and conventional treatmenton AIDS patients without drug resistance, and emergent blockade treatment on occupational exposure persons.
Owner:耿艳春 +1
Compound mequindox soluble powder for animals and preparation thereof
InactiveCN101301274ALess residueEasy to useSalicyclic acid active ingredientsPowder deliveryOral glucoseTreatment failure
The invention discloses a compound mequindox soluble powder for animals and a preparation method thereof, which aims to provide the compound mequindox soluble powder for the animals and the preparation method, wherein, the compound mequindox soluble powder for the animals can treat the complex infection of shigella shigae and colon bacillus of pigs, is quick to take effect, can solve the treatment failure phenomenon caused by feeding and drinking conditions of the pigs during the illness, and improve the rate of liveweight growth; and the preparation method has a simple process and is easy to realize. The powder comprises the compositions in percentage by weight: 2 to 10 percent of mequindox, 0.5 to 5 percent of colistin sulfate, 6 to 30 percent of sodium salicylate, and the balance being oral glucose. The soluble powder is a compound preparation formed by a double antimicrobial drug combined with a suspending agent, has quick effect on the complex infection of the shigella shigae and the colon bacillus of the pigs due to the fact that the mequindox and the colistin sulfate are jointly used, is effective and highly efficient, can solve the treatment failure phenomenon caused by feeding and drinking problems during the illness, accelerate the recovery of the pigs, and increase the weight of the pigs.
Owner:TIANJIN SHENGJI GRP CO LTD
Vaginal implant template for treatment
InactiveCN111659032AEasy to adjustKnow the exact locationX-ray/gamma-ray/particle-irradiation therapyTreatment failureEngineering
The invention discloses a vaginal implant template for treatment, including a bottom plate, a hollow long shaft is fixedly connected to the middle of the bottom plate; the inner wall of the long shaftis rotationally connected with a rotating rod; a disc is fixedly connected to the top end of the rotating rod; a containing plate is rotationally connected to the upper side of the rotating rod; thebottom end of the rotating rod is rotationally connected with the bottom plate; a transmission structure is arranged in the middle of the rotating rod; a first gear is arranged on the rear side of thetransmission structure; and the first gear is connected with a first hollow rack in an engaged mode. According to the invention, the problems that the traditional source applicator is not easy to fix, the source applicator cannot accurately reach the treatment position of the affected part of the patient, the fixing support is independent of the patient, and the position of the patient is changedin the transferring and treatment process, so that the source applicator deviates, falls off and the like relative to the implanting moment, dose distribution is affected, and treatment fails, are effectively solved.
Owner:HENAN CANCER HOSPITAL
Method for treating hepatitis c virus infection in treatment failure patients
InactiveUS20080213218A1Organic active ingredientsPeptide/protein ingredientsDosing regimenTreatment failure
The present invention provides methods for treating individuals having a hepatitis C virus (HCV) infection, which individuals have failed to respond to therapy with an IFN-α other than consensus interferon (CIFN), or who, following cessation of therapy with an IFN-α other than CIFN, have suffered relaspe. The methods generally involve a treatment regimen comprising administering a first dosing regimen of CIFN, followed by a second dosing regimen of CIFN. Ribavirin is administered with at least the second dosing regimen.
Owner:VALEANT PHARMA INT
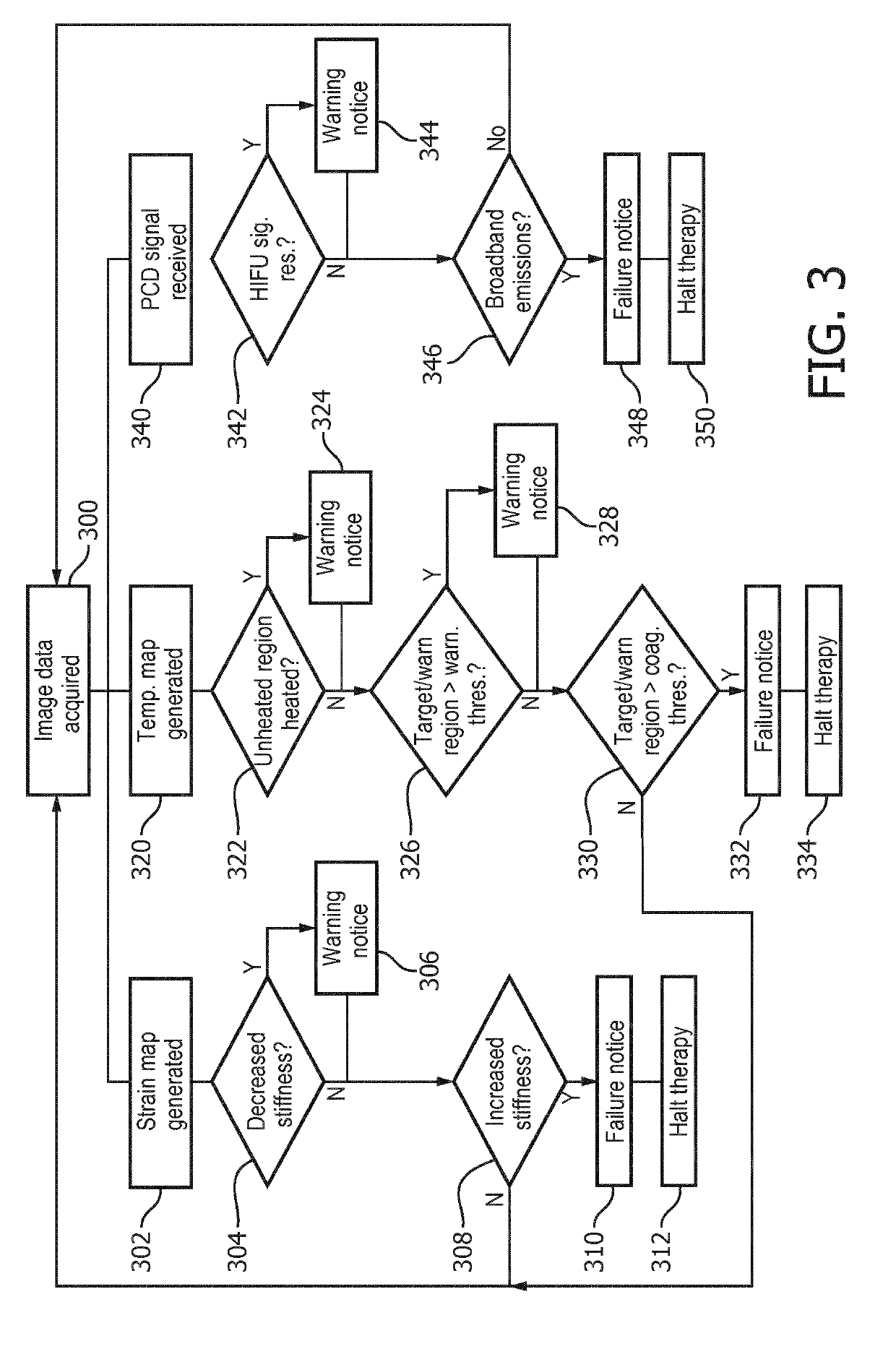
Detection of treatment failure for mild hyperthermia
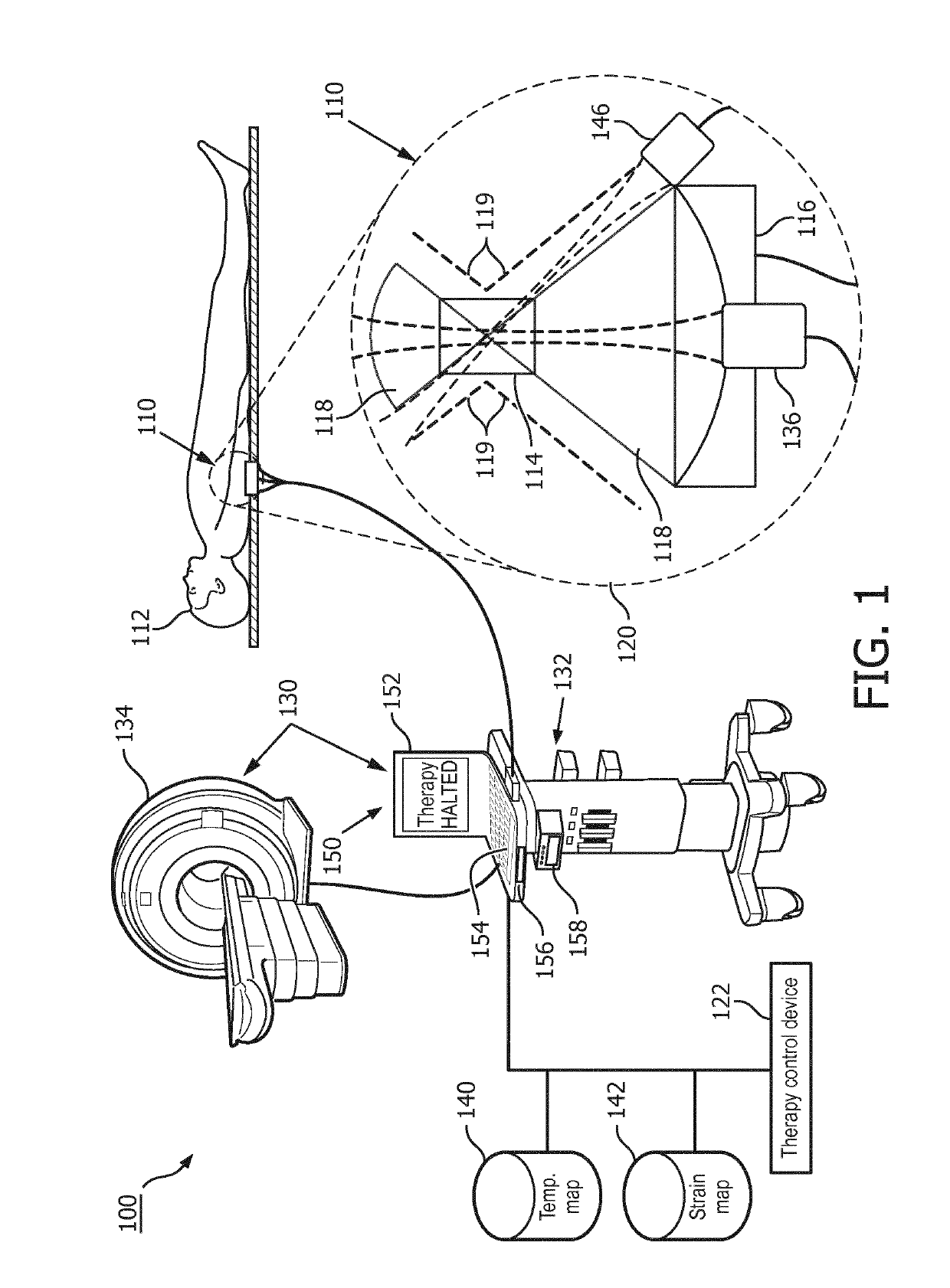
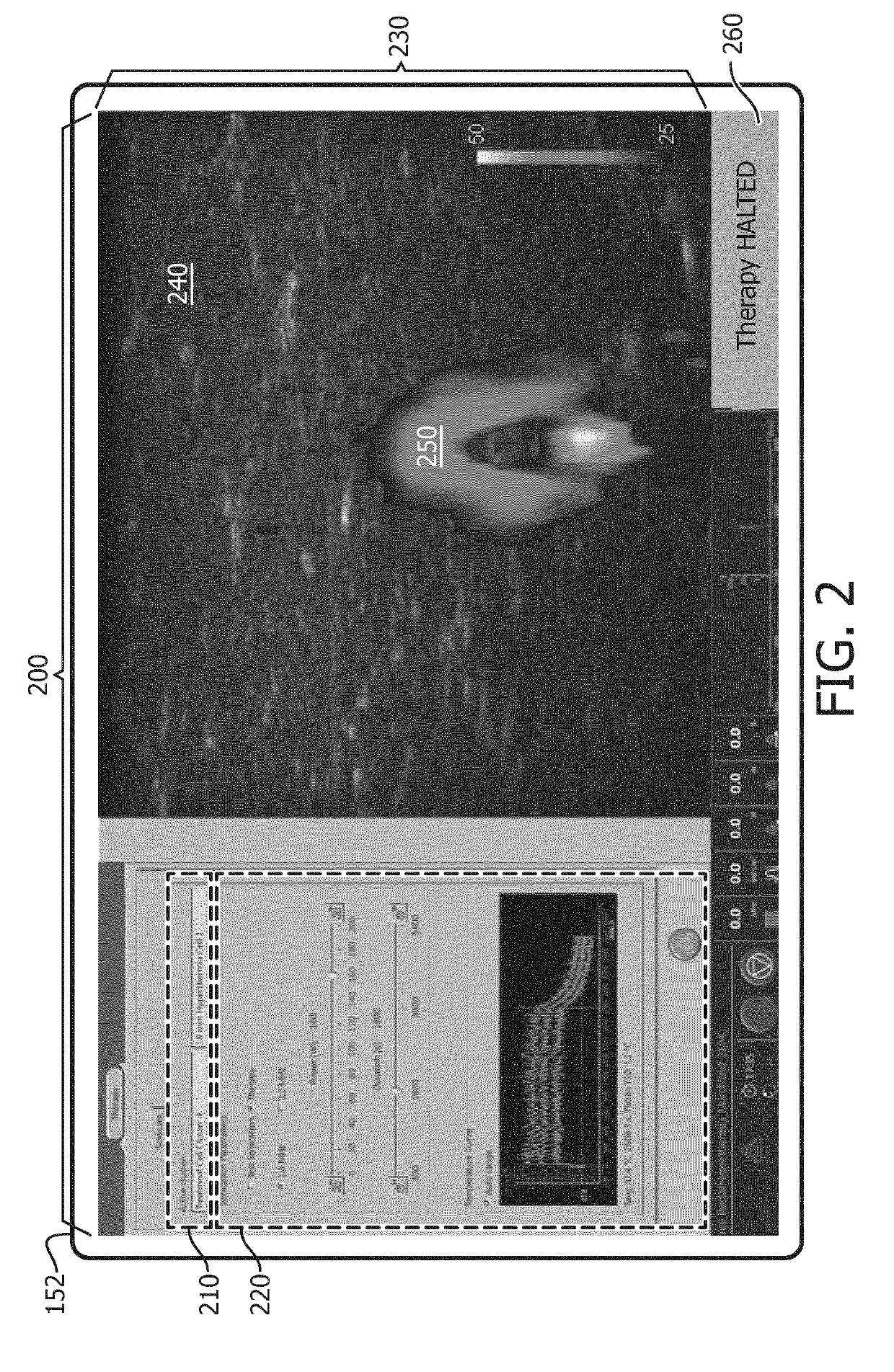
InactiveUS20190209872A1Mild hyperthermiaHighly focusedUltrasound therapyOrgan movement/changes detectionTreatment failureCavitation
A system (100) includes an imaging system (130), and a therapy control device (122). The imaging system (130) generates temperature maps (140) and strain maps (142) of localized tissues of a patient. The therapy control device (122) includes one or more computer processors configured to detect at least one failure mode (300, 302, 304, 400) of generated mild hyperthermia in the localized tissues of the patient according to at least one of the temperature maps, the strain maps, or a signal indicative of detected inertial cavitation. In some embodiments, the therapy control device either halts therapy or issues a warning.
Owner:KONINKLJIJKE PHILIPS NV
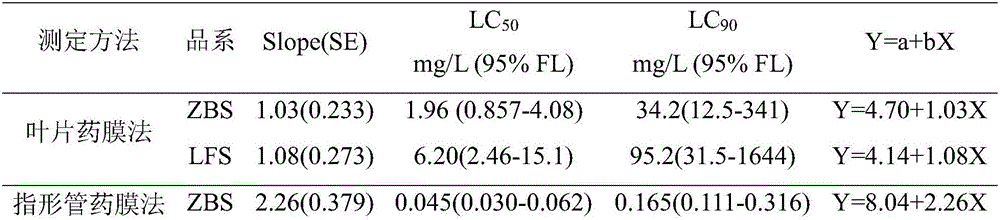
Diagnostic kit for fast determining aphis gossypii glover sensitivity to acetamiprid and use method thereof
InactiveCN106093368ALow costShorten diagnostic timeBiological material analysisBiological testingTreatment failureEngineering
The invention relates to a diagnostic kit for fast determining aphis gossypii glover sensitivity to acetamiprid. The diagnostic kit comprises a finger-type pipe, an agent film and a filler. The finger-type pipe is a glass pipe with one enclosed end. The agent film is an acetamiprid agent film uniformly adhering to the inner surface of the finger-type pipe. The opening end of the finger-type pipe is detachably filled with the filler. The diagnostic kit is used for fast diagnosis of field aphis gossypii glover sensitivity to acetamiprid, shortens diagnosis time, produces accurate and reliable results and prevents adverse results such as cost increasing, prevention and treatment failure and environment pollution caused by use of acetamiprid in a resistant population.
Owner:INST OF PLANT PROTECTION CHINESE ACAD OF AGRI SCI
Pharmaceutical tablet formulation for the veterinary medical sector, method of production and use thereof
The invention is directed to a pharmaceutical tablet formulation for the veterinary medical sector containing an instable ACE inhibitor or a pharmaceutically acceptable salt thereof as a first pharmaceutically active substance, and pimobendan or a pharmaceutically acceptable salt thereof as a second pharmaceutically active substance, comprising granules which contain carrier core particles coated with at least one layer wherein the first pharmaceutically active substance is present, the granules being embedded in a tablet matrix wherein the second pharmaceutically active substance is present. It is provided a “fixed-dose-combination” which allows to ease the treatment and administration of the medication, improves the medication compliance by reducing the pill burden to the animal holder and enables the better observation of and adherence to the therapy by decreasing the number of tablets to be administered. The lower number of tablets leads to a lower treatment failure rate, minimizes dosage mistakes and avoids confusions by false dose intake and slower development of resistance.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
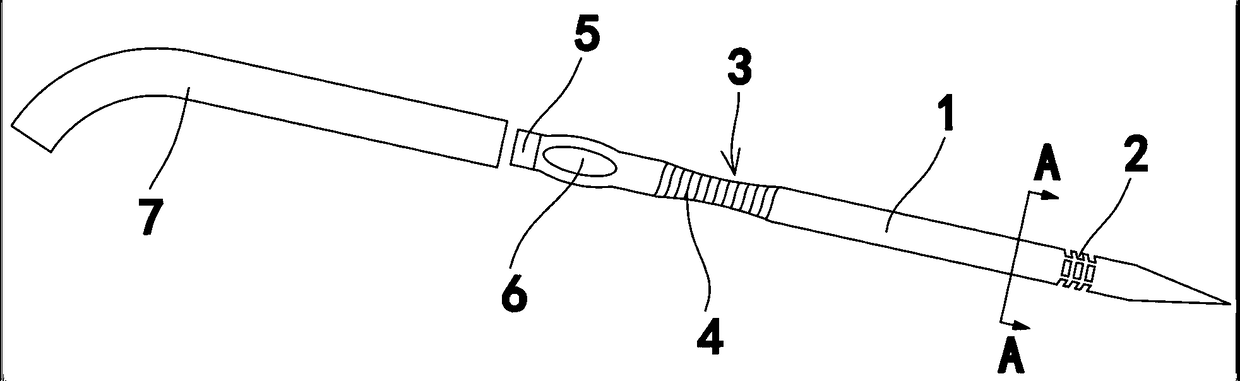
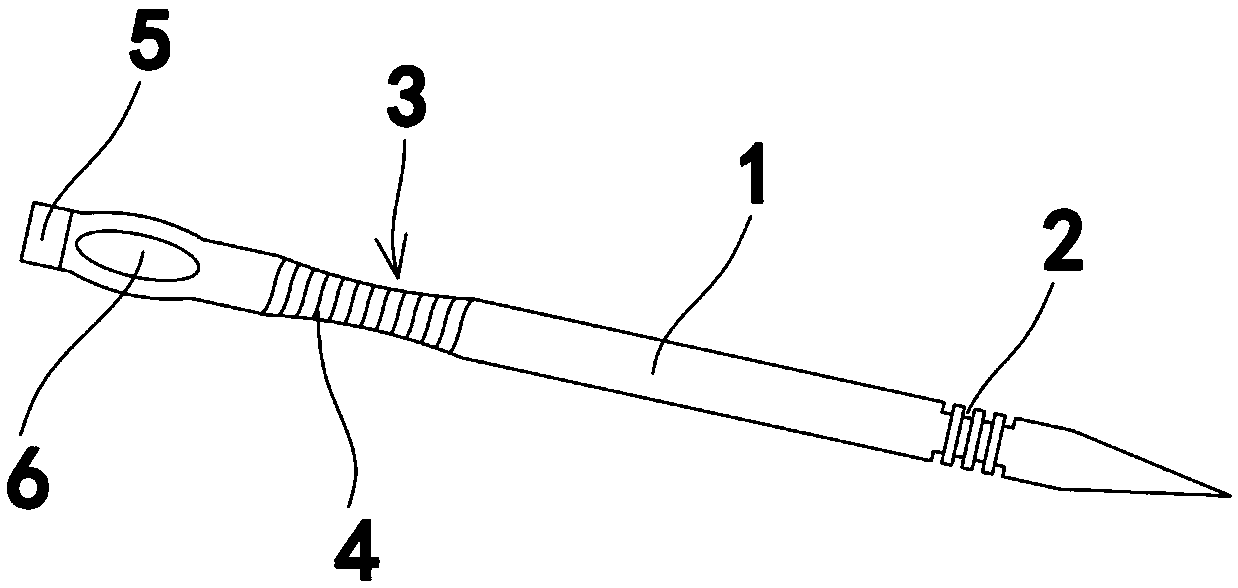
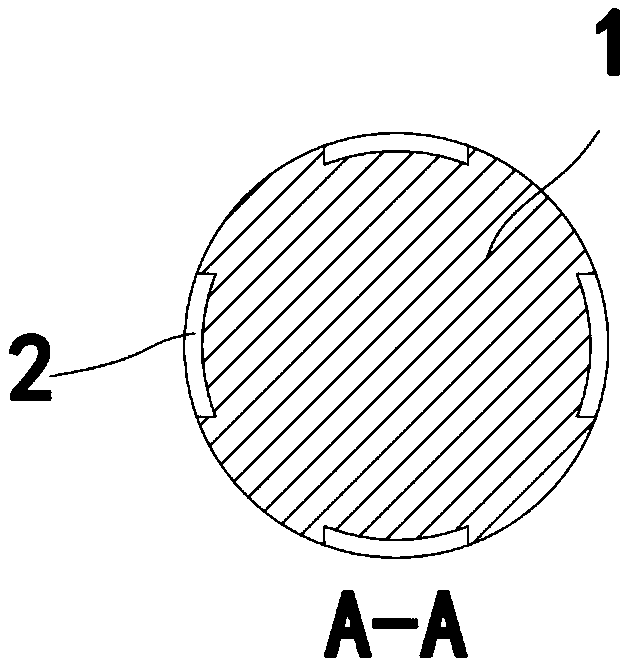
Instrument for needle-picking therapy for lumbar, neck and shoulder diseases and use method thereof
The invention relates to the technical field of medical appliances, in particular to an instrument for the needle-picking therapy for lumbar, neck and shoulder diseases and a use method thereof. The instrument includes a needle body, the part, near the needle tip, of the needle body is provided with a slip stitch groove, and the part, near the needle end, of the needle body is like a flat notch. The use method of the instrument includes the following steps that 1, an adjustment needle rod does not need to be used, and people hold the flat notch of the needle body to make the needle tip pass through the ill skin of the waist, the neck and the shoulder until a disease source enters the slip stitch groove; 2, the needle tip vertically pick up the ill skin through force from the wrist, and blades cut off the ill skin from the root; 3, if the adjustment needle rod needs to be used, the adjustment needle rod is fixedly connected with a connection part, and the step 1 and the step 2 are repeated. In the pick-up process, the treatment failure rate caused by slippage of the disease source can be largely reduced, and the needle body can be used independently and can be connected with the adjustment needle rod for use in cooperation according to different locations of the ill skin of a patient.
Owner:高关会
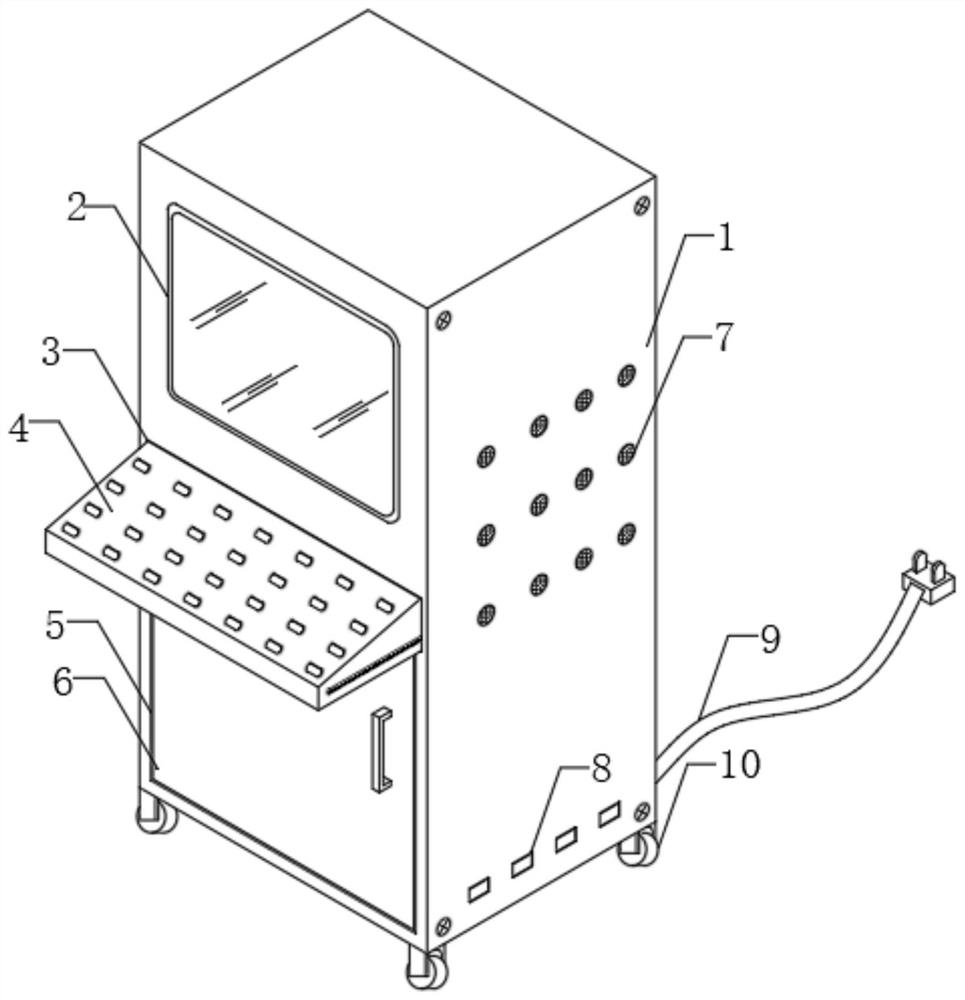
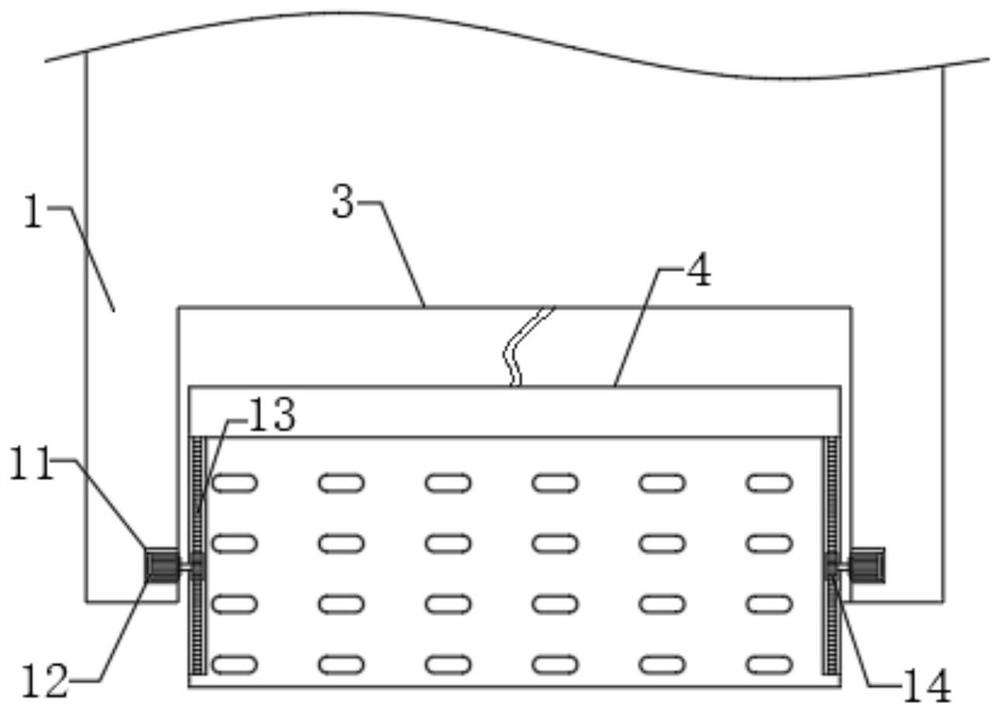
Electrical impedance detection and online treatment equipment and method for flowing thrombus
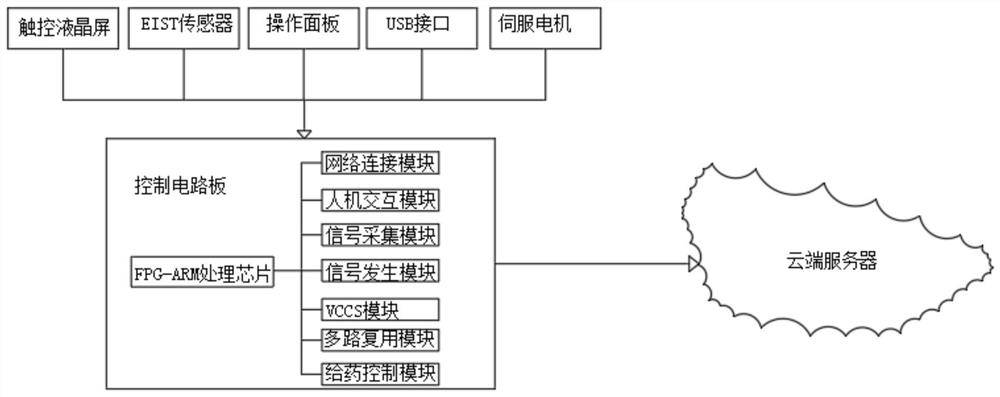
PendingCN112790755AAccurate quantityThe location information is accurateOther blood circulation devicesDiagnostic recording/measuringExtracorporeal circulationTreatment failure
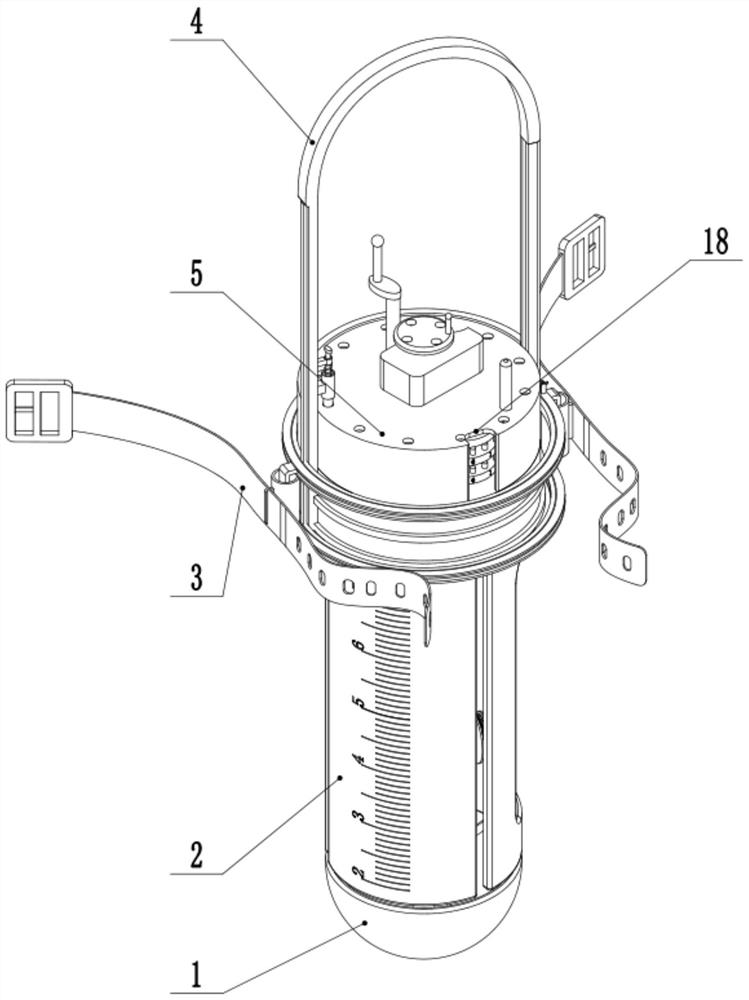
The invention discloses an electrical impedance detection and online treatment device and method for flowing thrombus, and the device comprises a device housing, the top of the front surface of the device housing is fixedly provided with a touch liquid crystal screen, the middle part of the front surface of the device housing is provided with a packaging groove, and the interior of the packaging groove is provided with an operation panel in a packaging manner. A moving mechanism is installed between the operation panel and the equipment shell, an equipment cavity is formed in the bottom of the front face of the equipment shell, and a control circuit board and an EIST sensor are fixedly installed in the equipment cavity. According to the invention, the blood state in the extracorporeal circulation system can be accurately detected; the size, the number and the position information of flowing thrombus in the extracorporeal circulation system on the cross section of the circulation pipeline can be accurately detected. The blood environment in the circulation pipeline can be controlled, the detected information is used for controlling the administration dosage of the anticoagulant, the administration dosage can be automatically regulated and controlled, and damage to a patient caused by excessive administration or treatment failure caused by insufficient administration can be relieved.
Owner:NANJING UNIV OF AERONAUTICS & ASTRONAUTICS
Method for treating hepatitis c virus infection in treatment failure patients
InactiveUS20050031585A1Arresting its developmentDisease reliefBiocidePeptide/protein ingredientsTreatment failureDosing regimen
The present invention provides methods for treating individuals having a hepatitis C virus (HCV) infection, which individuals have failed to respond to therapy with an IFN-α other than consensus interferon (CIFN), or who, following cessation of therapy with an IFN-α other than CIFN, have suffered relapse. The methods generally involve a treatment regimen comprising administering a first dosing regimen of CIFN, followed by a second dosing regimen of CIFN. Ribavirin is administered with at least the second dosing regimen.
Owner:THREE RIVERS PHARMA LLC
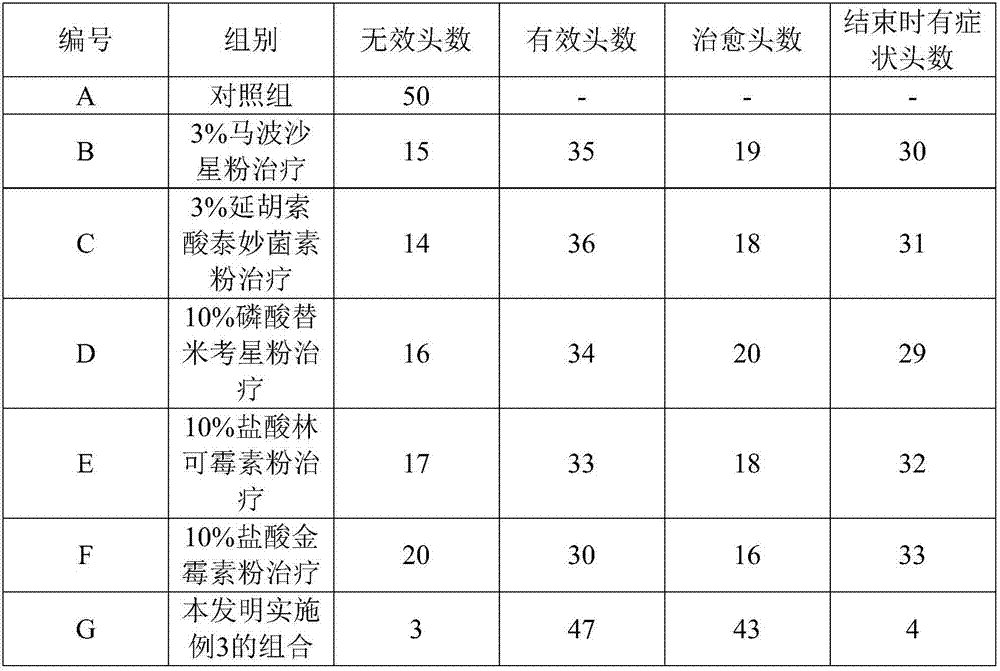
Composition for treating porcine respiratory tract infection and preparation method of composition
InactiveCN107441111AReduce chance of drug resistanceImprove antibacterial propertiesTetracycline active ingredientsAntiinfectivesChlortetracycline HydrochlorideFumaric acid
The invention relates to a composition for treating porcine respiratory tract infection and a preparation method of the composition. The composition is prepared from the following raw materials in percentage by weight: 1-5 % of marbofloxacin, 1-5 % of tiamulin fumarate, 5-15 % of tilmicosin phosphate, 5-15 % of lincomycin hydrochloride, 5-15 % of chlortetracycline hydrochloride, the balance of anhydrous glucose. All the components are grinded, the obtained powder is screened by adopting a No.5 sieve, the screened powder is sufficiently and uniformly mixed, and thus the composition for treating porcine respiratory tract infection is obtained. Compared with the prior art, the composition has the advantages that the plurality of medicines which can be mixed for use are comprehensively selected, the situation that some pathogenic bacteria have drug resistance to some medicines, consequently, treatment failure is caused, is avoided, the drug resistance probability of pathogens is reduced, so that the pathogenic microorganism resisting range is wide, the antibacterial effect is strong, and disease recovery is facilitated.
Owner:JIANGXI AOXIN BIOTECH CO LTD +1
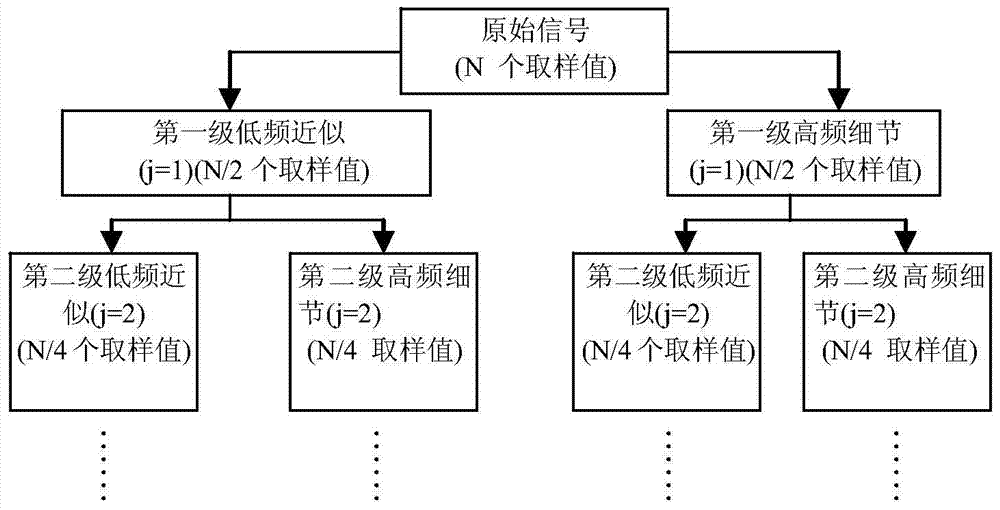
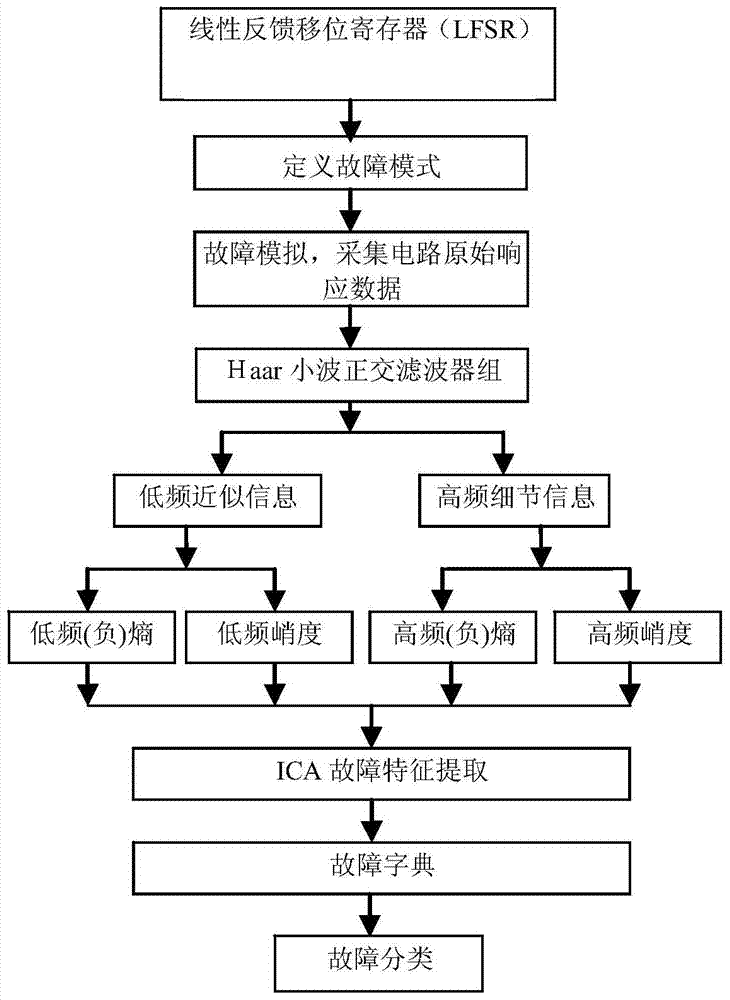
Fault Diagnosis Method of Switching Circuit Based on Wavelet Transform and ICA Feature Extraction
InactiveCN104793124BThe classification result is accurateAccurate diagnosisElectronic circuit testingTreatment failureFeature extraction
The invention discloses a switching circuit fault diagnosis method based on wavelet transform and ICA feature extraction, step 1: classifier training and constructing a fault dictionary, based on circuit simulation, adopting a method based on wavelet transform and ICA feature extraction to obtain characteristic parameters, based on The characteristic parameters construct the fault dictionary and train the classifier; Step 2: Fault diagnosis: refer to the fault dictionary, use the method based on wavelet transform and ICA feature extraction to obtain the characteristic parameters for the switching current circuit to be diagnosed, and input the characteristic parameters into the training Fault diagnosis is carried out in the classifier of the switch current circuit to be tested, and the output signal of the classifier is the fault diagnosis result. The invention has an ingenious concept and is easy to implement. The simulation proves that compared with the existing method, various fault types can be more accurately distinguished .
Owner:CHANGSHA UNIVERSITY
Suppression of cancer metastasis
ActiveUS20160051634A1Inhibit transferEfficient dosingSsRNA viruses positive-sensePeptide/protein ingredientsBreast cancer metastasisTreatment failure
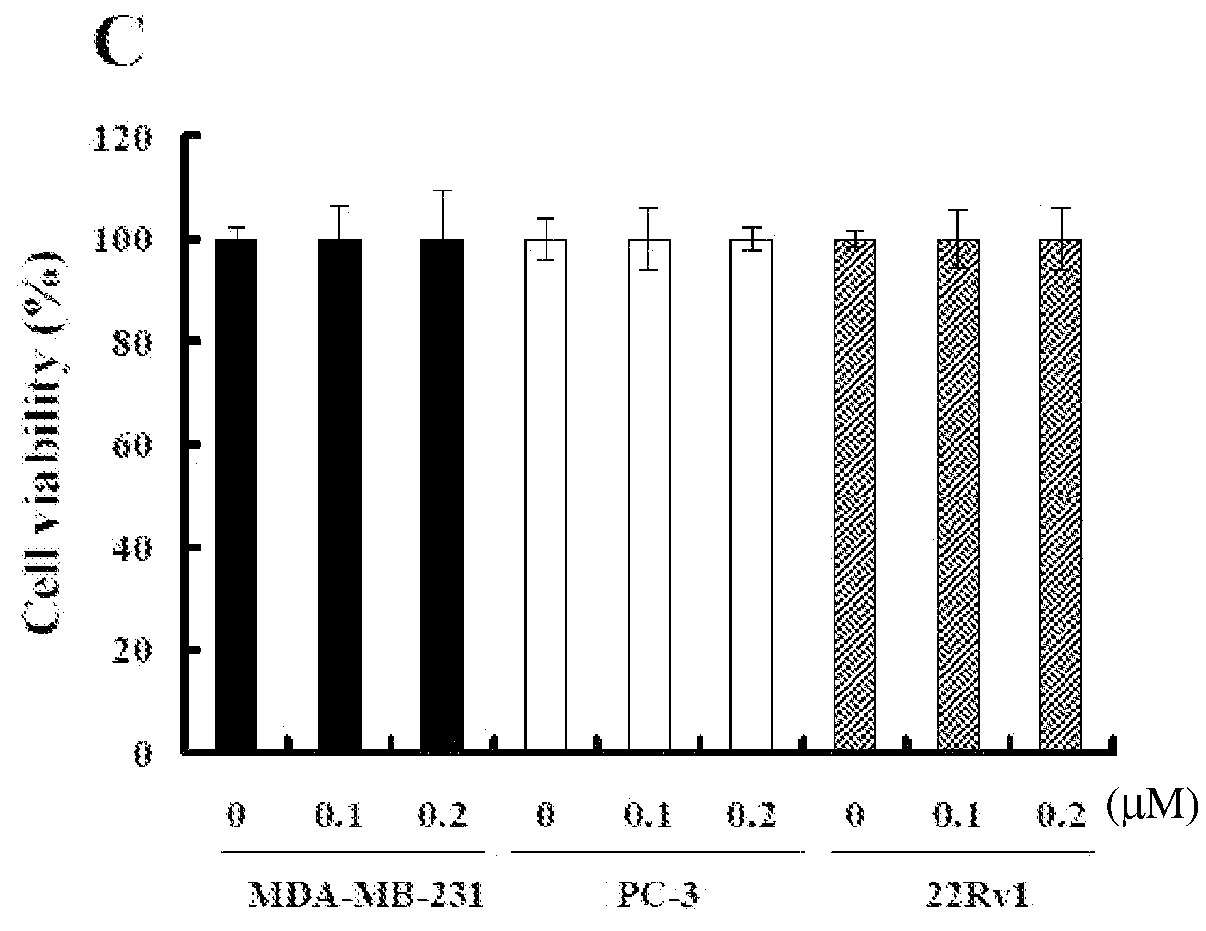
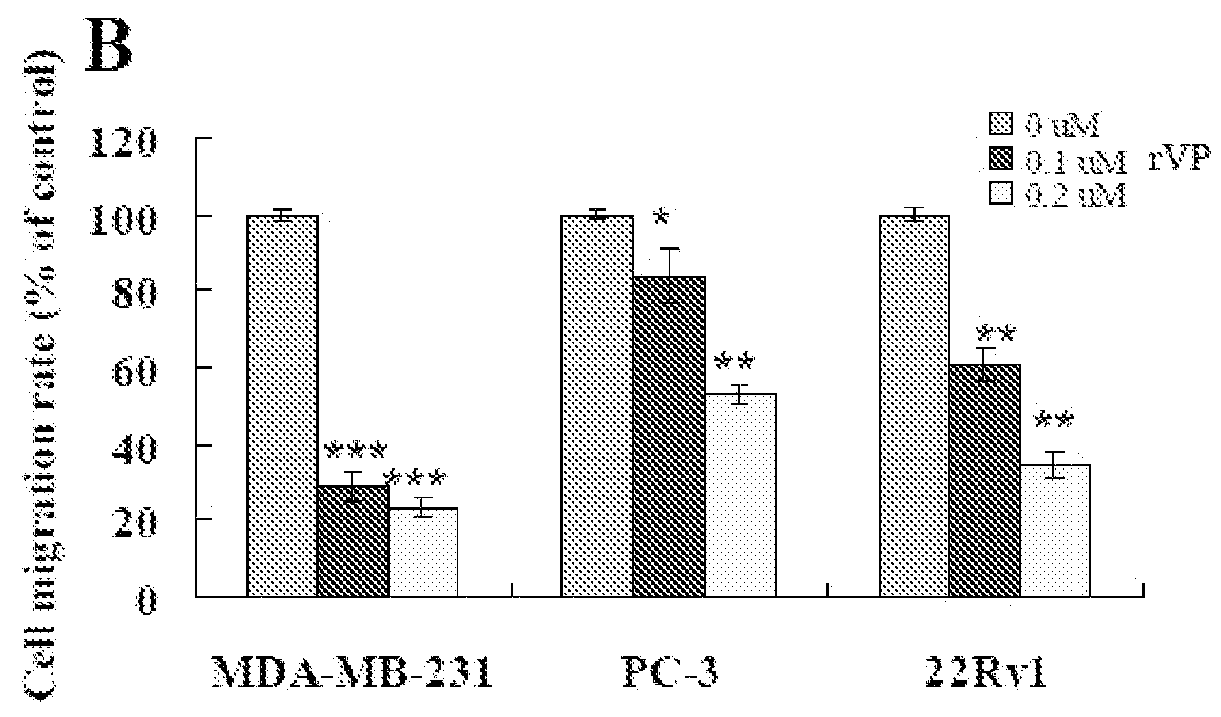
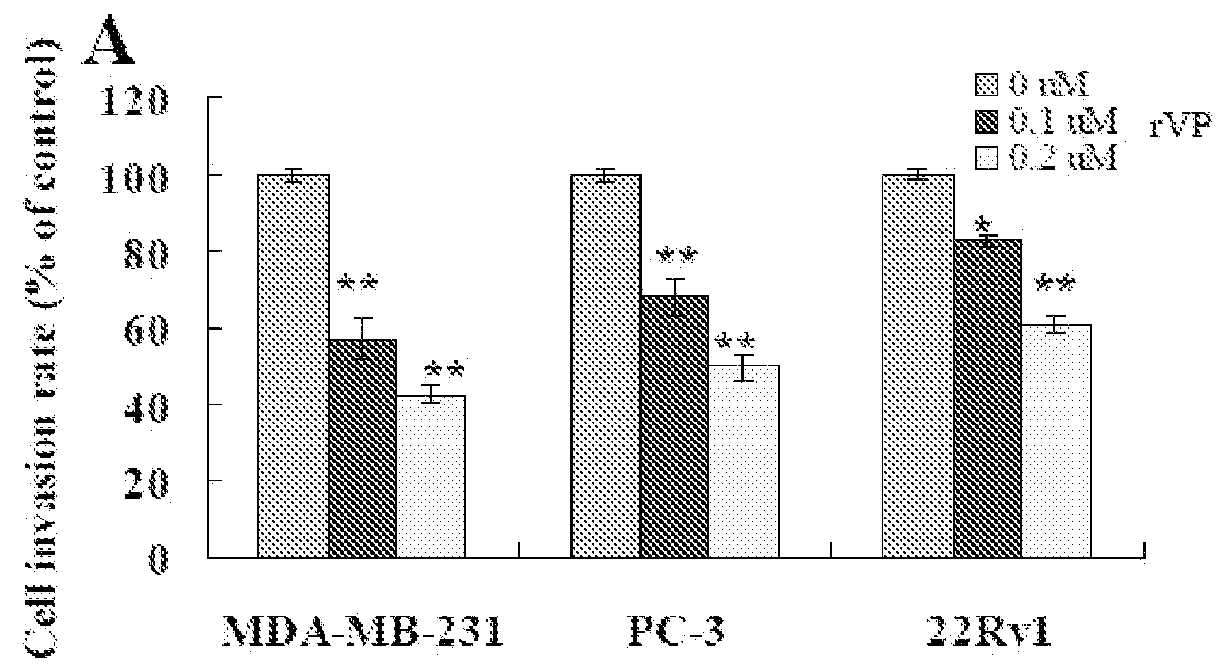
Methods of using fibrillar proteins are provided for suppressing cancer metastasis. Cancer metastasis is the most common cause of treatment failure and death in cancer patients. Tumor cell invasion and / or migration can be significantly inhibited after fibrillar proteins (rVP1, F-HSA, and F-BSA) treatment in vitro. In addition, rVP1 can significantly suppress murine and human breast cancer metastasis and human prostate and ovarian cancer metastasis in vivo while F-HSA can significantly suppress murine breast cancer metastasis.
Owner:ACAD SINIC
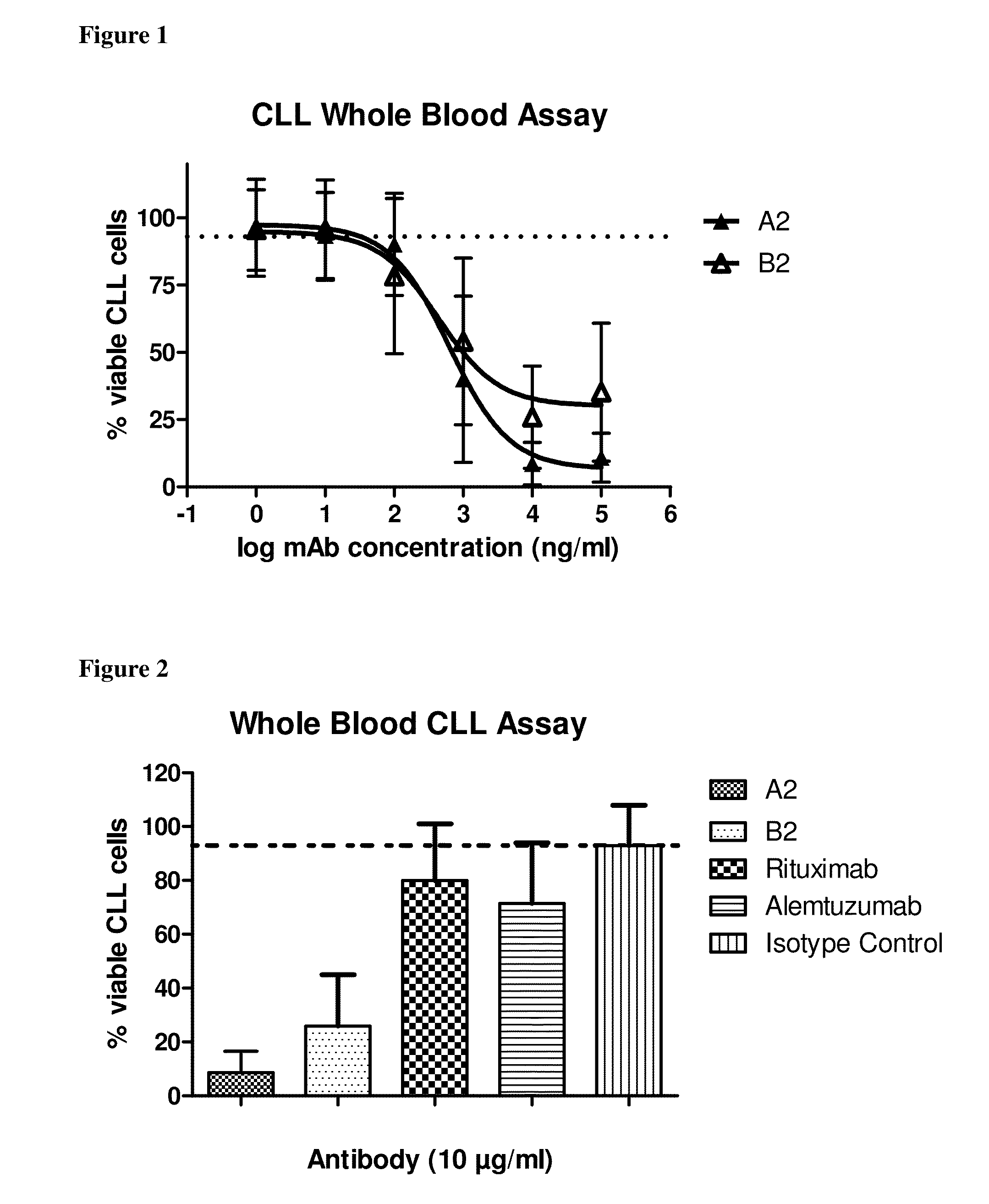
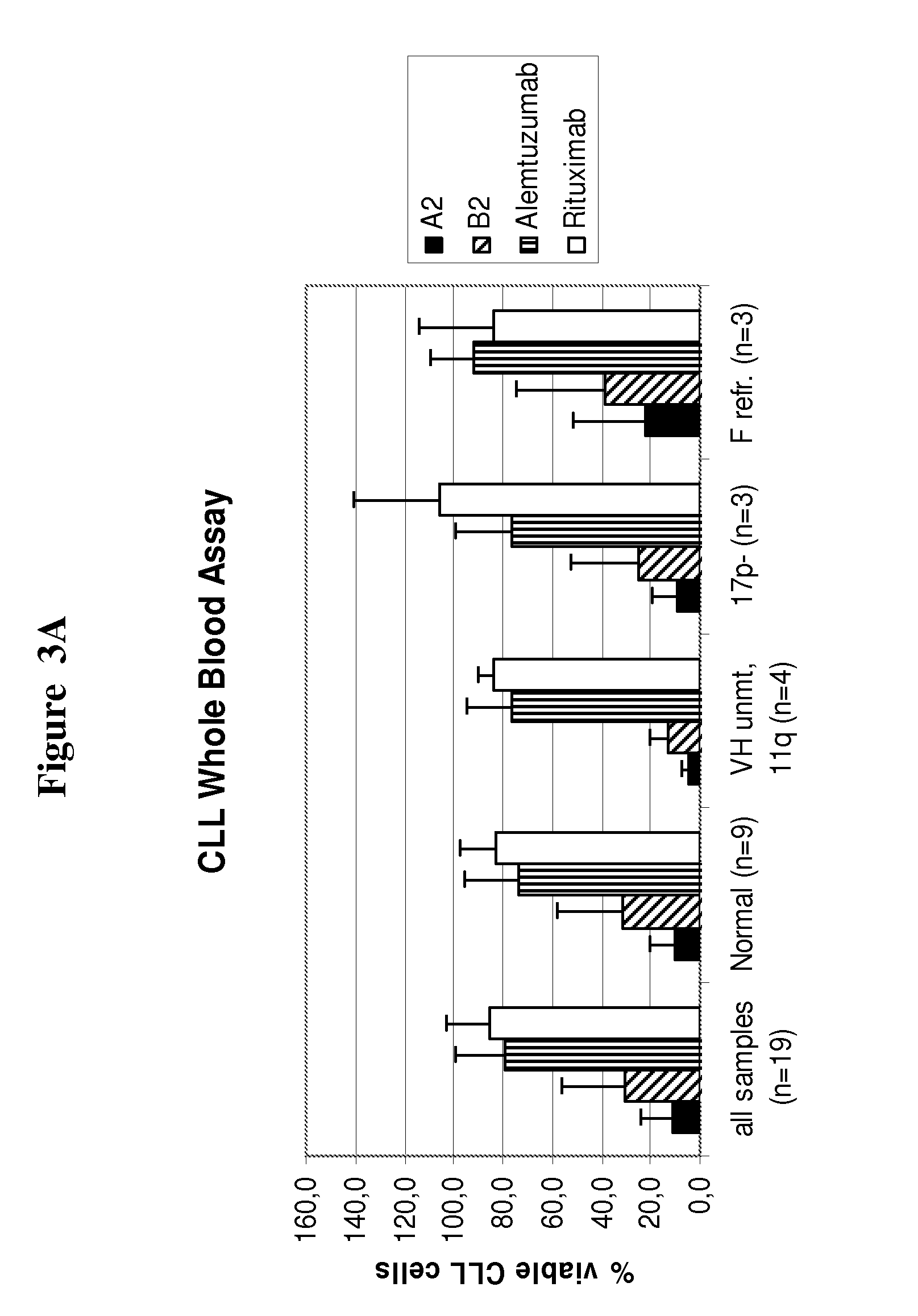
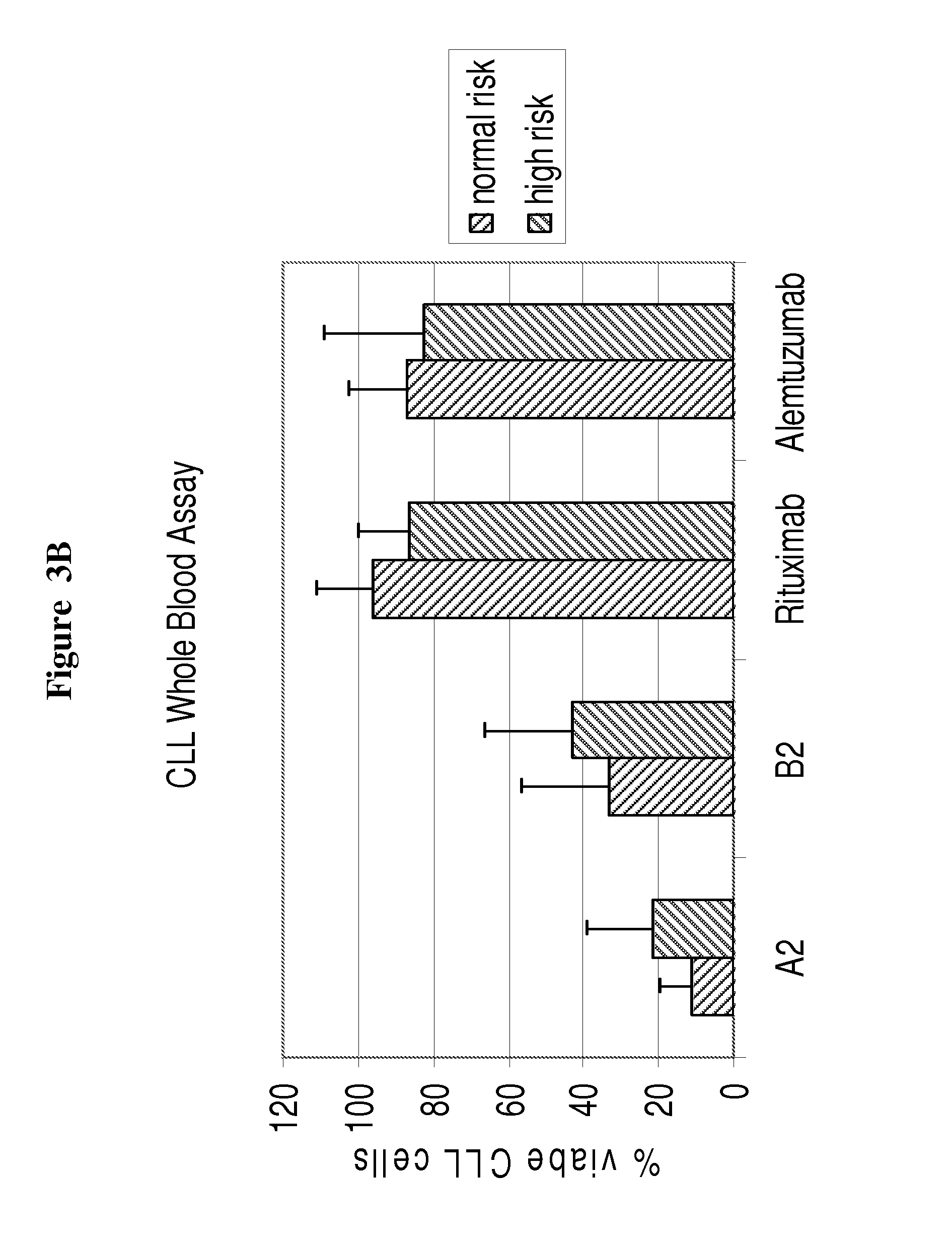
Superior efficacy of cd37 antibodies in cll blood samples
InactiveUS20150266967A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCvd riskAlemtuzumab
The present invention describes CD37 antibodies, especially A2 and B2, for the treatment of patients with CLL, especially of patients belonging to a “high risk” or “ultra-high risk” group of patients. Those patients are either patients who are refractory to fludarabine treatment or patients who carry a genetic marker which is indicative for poor prognosis or increased risk of treatment failure, e.g. patients with TP53 dysfunction or deletion of chromosome 17p13, or patients after failure to previous anti-CD20 treatment. The ability of A2 and B2 to deplete CLL cells is high both in patient samples derived from patients with normal risk and with increased risk (“high risk” patients) and clearly superior to that of rituximab and alemtuzumab.
Owner:BOEHRINGER INGELHEIM INT GMBH
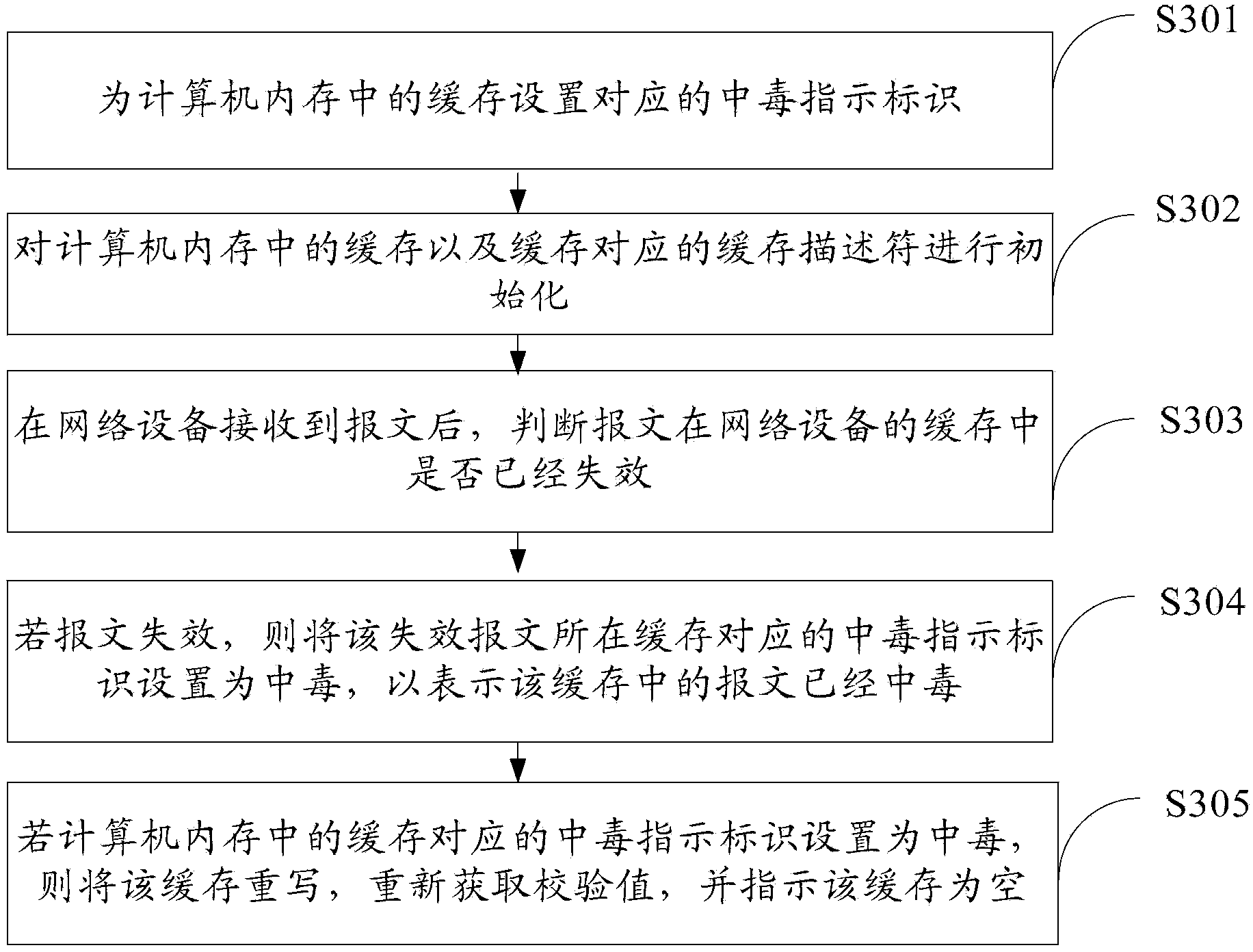
Method of treatment failure packets, network device and processor
InactiveCN103534704AImprove reliabilityError preventionError detection/correctionTreatment failureComputer memory
The present invention provides a method of treatment failure packets, network device and processor, relates to a field of a computer, the packet in a cache has failed, indicating that the message has failed to the invention, and discard invalid packets to avoid the influence of the system reset or will fail to process the data as normal data brought to improve the reliability of the system. The methods are: by computer memory cache settings packets corresponding signs of poisoning, and the network device receives the packet, the packet will fail the corresponding cache poisoning where the identity is set to indicate intoxication, and failure messages where the cache rewrite, then indicates that the cache is empty. The invention is used for processing a packet failure.
Owner:HUAWEI TECH CO LTD
Use of 3-[5-amino-4-(3-cyanobenzoyl)-pyrazol-1-yl]-n-cyclopropyl-4-methylbenzamide in the prevention or reduction of acute exacerbations of chronic obstructive pulmonary disease
Owner:MEREO BIOPHARMA 1
Use of 3-[5-amino-4-(3-cyanobenzoyl)-pyrazol-1-yl]-n-cyclopropyl-4-methylbenzamide in the prevention or reduction of acute exacerbations of chronic obstructive pulmonary disease
The invention relates to dosage regimens for administering pharmaceutical compositions comprising 3-[5-amino-4-(3-cyanobenzoyl)-pyrazol-1-yl]-N-cyclopropyl-4-methylbenzamide for the treatment of a human patient suffering from COPD, or suffering from an acute exacerbation of COPD, or at risk of developing an acute exacerbation of COPD, or to prevent a reoccurrence of an acute exacerbation of COPD, or to prevent a treatment failure of an acute exacerbation of COPD, and to methods of prevention or reduction in the rate of acute exacerbations of COPD in a human patient.
Owner:MEREO BIOPHARMA 1
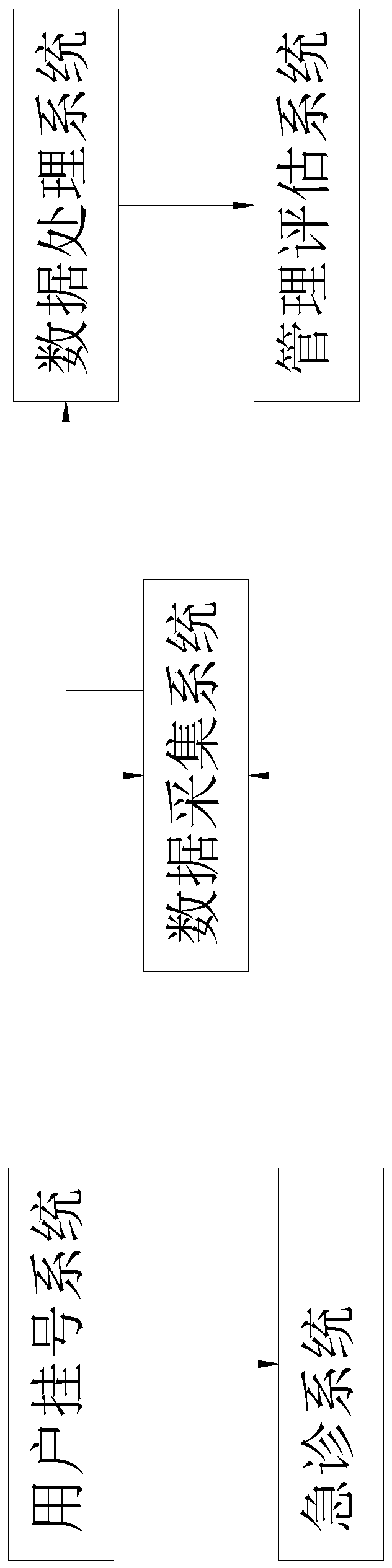
Emergency treatment quality evaluation management system
PendingCN111048213AImproved treatment methodsImprove the success rate of treatmentMedical data miningPatient-specific dataTreatment successData acquisition
An emergency treatment quality evaluation management system comprises a patient registration system, an emergency treatment system, a data acquisition system, a data processing system and a managementevaluation system. The patient registration system is used for collecting basic identity information of a patient; the emergency treatment system is used for recording treatment first-aid informationof the patient, wherein the treatment first-aid information comprises disease information, treatment duration information and treatment result information of the patient; the data processing system comprises a data classification module, a data proofreading module, a data analysis module and a data evaluation summary sheet; and the management evaluation system comprises a data query module, a statistics module, a case classification module and a key case storage module. According to the system, not only can the treatment process of the disease be analyzed and evaluated, but also treatment failure cases can be analyzed, and thus the overall treatment success rate is improved.
Owner:张彩东
Kosteletzkya virginica traditional Chinese medicine composite composition for preventing and treating coxey and application of kosteletzkya virginica traditional Chinese medicine composite composition
ActiveCN110141590AEffective controlAvoid problems such as drug resistanceAntiparasitic agentsPlant ingredientsCoccidiosisTreatment failure
The invention provides an application of kosteletzkya virginica to preparation of a traditional Chinese medicine preparation for preventing and treating coxey, and further provides a kosteletzkya virginica traditional Chinese medicine composite composition for preventing and treating coxey and an application of the kosteletzkya virginica traditional Chinese medicine composite composition. The composition is prepared from the following raw materials in parts by mass of 40-50 parts of kosteletzkya virginica, 25-30 parts of spartina alterniflora, 8-12 parts of sweet wormwood herbs, 8-12 parts ofbrucea amarissima, and 4-6 parts of radix scutellariae. The traditional Chinese medicine composite composition can effectively prevent and treat coxey. The composite plant extract preparation can prevent problems of reverse tolerance and the like, so that the probability of prevention and treatment failure can be greatly reduced. The composition is a purely plant extract compound recipe, and is low in remaining in bodies of chickens, and favorable in biological safety.
Owner:JIANGSU INST OF POULTRY SCI
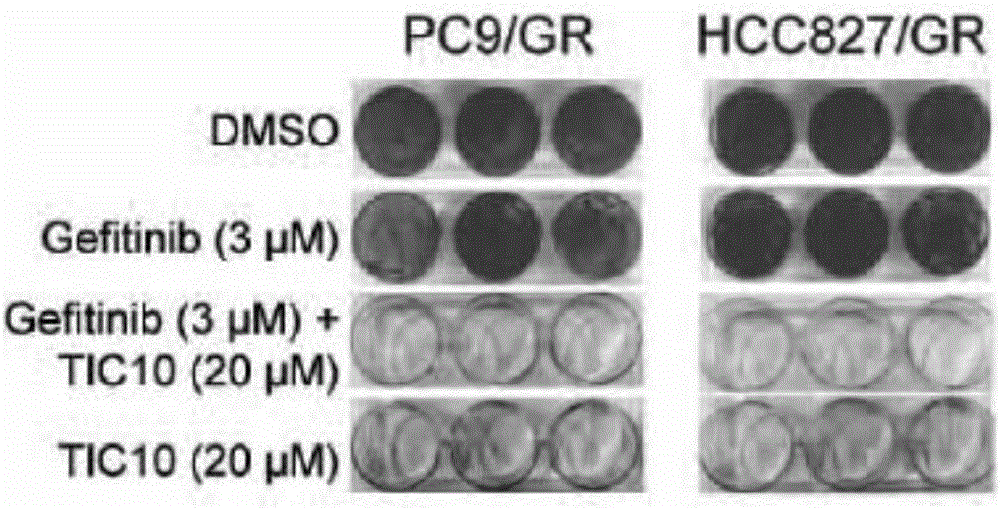
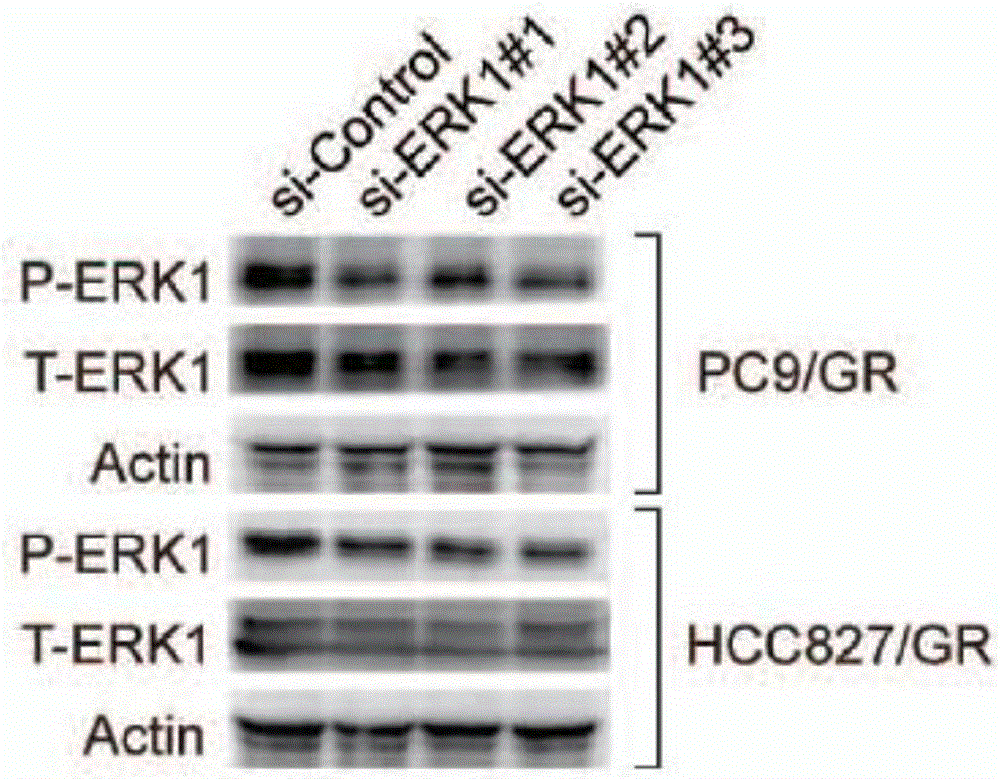
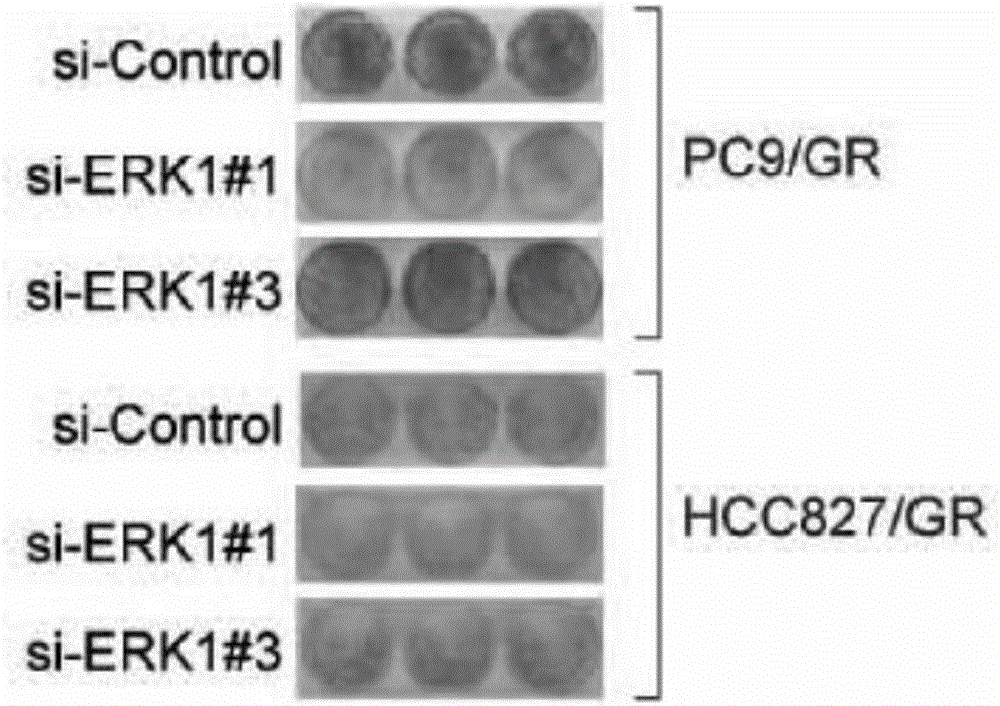
Application of ERK micro-molecular inhibitor in inhibition of drug-resistant lung cancer cells
InactiveCN106729715AInhibitionReceive treatment wellOrganic active ingredientsAntineoplastic agentsTreatment effectPhosphorylation
The invention relates to an application of an ERK micro-molecular inhibitor in the preparation of drugs for inhibiting drug-resistant lung cancer cells. The ERK micro-molecular inhibitor inhibits the phosphorylation level of ERK kinase, prevents cell autophagy, and substantially inhibits generation of gefitinib-resistant lung cancer cells in vivo and in vitro, and the ERK micro-molecular inhibitor is TIC10. The lung cancer drug-resistant cell proliferation inhibition molecular mechanism of the ERK micro-molecular inhibitor TIC10 is explicated in the invention, the in-vitro and in-vitro drug-resistant lung cancer cell treatment effect of the ERK micro-molecular inhibitor TIC10 is researched on the basis of the molecular mechanism, and the ERK micro-molecular inhibitor TIC10 can be used for preparing drugs for inhibiting drug-resistant lung cancer cells, solves the problem of treatment failure caused by resistance of lung cancer cells to targeting drugs, reduces the generation of the drug-resistant lung cancer cells, and prolongs the life of lung cancer patients.
Owner:SHANGHAI PULMONARY HOSPITAL
Novel chain tightener
InactiveCN107010363ASafe operationReduce complex work proceduresConveyorsTreatment failureSurface mounting
The invention discloses a novel chain tensioner, and the invention relates to a chain stop device for a scraper conveyor. It includes a cover plate and a pressure plate. The pressure plate is installed in the middle of the cover plate. At least three pin shafts are installed on the surface of the cover plate. The bottom of the chute of the scraper conveyor is provided with a pin hole matching the pin shaft. The two ends of the pressure plate are installed on the Inside the chute. The present invention has the following advantages. 1. When using, combine the three parts of the cover plate, the pressure plate and the pin shaft into a whole, insert them into the limit hole in the chute, use the motor to reverse the direction, and pinch off the remaining chain, so as to achieve the purpose of tightening the chain ; This device has been used on site for more than half a year. The maintenance workers generally report that it is safe and convenient to operate, which reduces the complicated work procedures of the operators, reduces the labor intensity of the workers, and protects the personal safety of the workers. 2. The device is easy to operate, which can help maintenance personnel reduce the time for troubleshooting and improve work efficiency.
Owner:姜涛
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
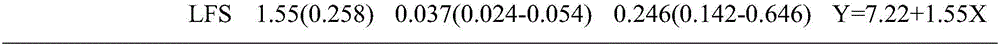


![Use of 3-[5-amino-4-(3-cyanobenzoyl)-pyrazol-1-yl]-n-cyclopropyl-4-methylbenzamide in the prevention or reduction of acute exacerbations of chronic obstructive pulmonary disease Use of 3-[5-amino-4-(3-cyanobenzoyl)-pyrazol-1-yl]-n-cyclopropyl-4-methylbenzamide in the prevention or reduction of acute exacerbations of chronic obstructive pulmonary disease](https://images-eureka.patsnap.com/patent_img/fa48557e-d4ac-4c48-8792-f36bed708473/US11234967-D00001.png)
![Use of 3-[5-amino-4-(3-cyanobenzoyl)-pyrazol-1-yl]-n-cyclopropyl-4-methylbenzamide in the prevention or reduction of acute exacerbations of chronic obstructive pulmonary disease Use of 3-[5-amino-4-(3-cyanobenzoyl)-pyrazol-1-yl]-n-cyclopropyl-4-methylbenzamide in the prevention or reduction of acute exacerbations of chronic obstructive pulmonary disease](https://images-eureka.patsnap.com/patent_img/fa48557e-d4ac-4c48-8792-f36bed708473/US11234967-D00002.png)
![Use of 3-[5-amino-4-(3-cyanobenzoyl)-pyrazol-1-yl]-n-cyclopropyl-4-methylbenzamide in the prevention or reduction of acute exacerbations of chronic obstructive pulmonary disease Use of 3-[5-amino-4-(3-cyanobenzoyl)-pyrazol-1-yl]-n-cyclopropyl-4-methylbenzamide in the prevention or reduction of acute exacerbations of chronic obstructive pulmonary disease](https://images-eureka.patsnap.com/patent_img/fa48557e-d4ac-4c48-8792-f36bed708473/US11234967-D00003.png)
![Use of 3-[5-amino-4-(3-cyanobenzoyl)-pyrazol-1-yl]-n-cyclopropyl-4-methylbenzamide in the prevention or reduction of acute exacerbations of chronic obstructive pulmonary disease Use of 3-[5-amino-4-(3-cyanobenzoyl)-pyrazol-1-yl]-n-cyclopropyl-4-methylbenzamide in the prevention or reduction of acute exacerbations of chronic obstructive pulmonary disease](https://images-eureka.patsnap.com/patent_img/0cbddc36-7a65-4879-a8e4-b3be37076625/US20200383950A1-D00001.png)
![Use of 3-[5-amino-4-(3-cyanobenzoyl)-pyrazol-1-yl]-n-cyclopropyl-4-methylbenzamide in the prevention or reduction of acute exacerbations of chronic obstructive pulmonary disease Use of 3-[5-amino-4-(3-cyanobenzoyl)-pyrazol-1-yl]-n-cyclopropyl-4-methylbenzamide in the prevention or reduction of acute exacerbations of chronic obstructive pulmonary disease](https://images-eureka.patsnap.com/patent_img/0cbddc36-7a65-4879-a8e4-b3be37076625/US20200383950A1-D00002.png)
![Use of 3-[5-amino-4-(3-cyanobenzoyl)-pyrazol-1-yl]-n-cyclopropyl-4-methylbenzamide in the prevention or reduction of acute exacerbations of chronic obstructive pulmonary disease Use of 3-[5-amino-4-(3-cyanobenzoyl)-pyrazol-1-yl]-n-cyclopropyl-4-methylbenzamide in the prevention or reduction of acute exacerbations of chronic obstructive pulmonary disease](https://images-eureka.patsnap.com/patent_img/0cbddc36-7a65-4879-a8e4-b3be37076625/US20200383950A1-D00003.png)