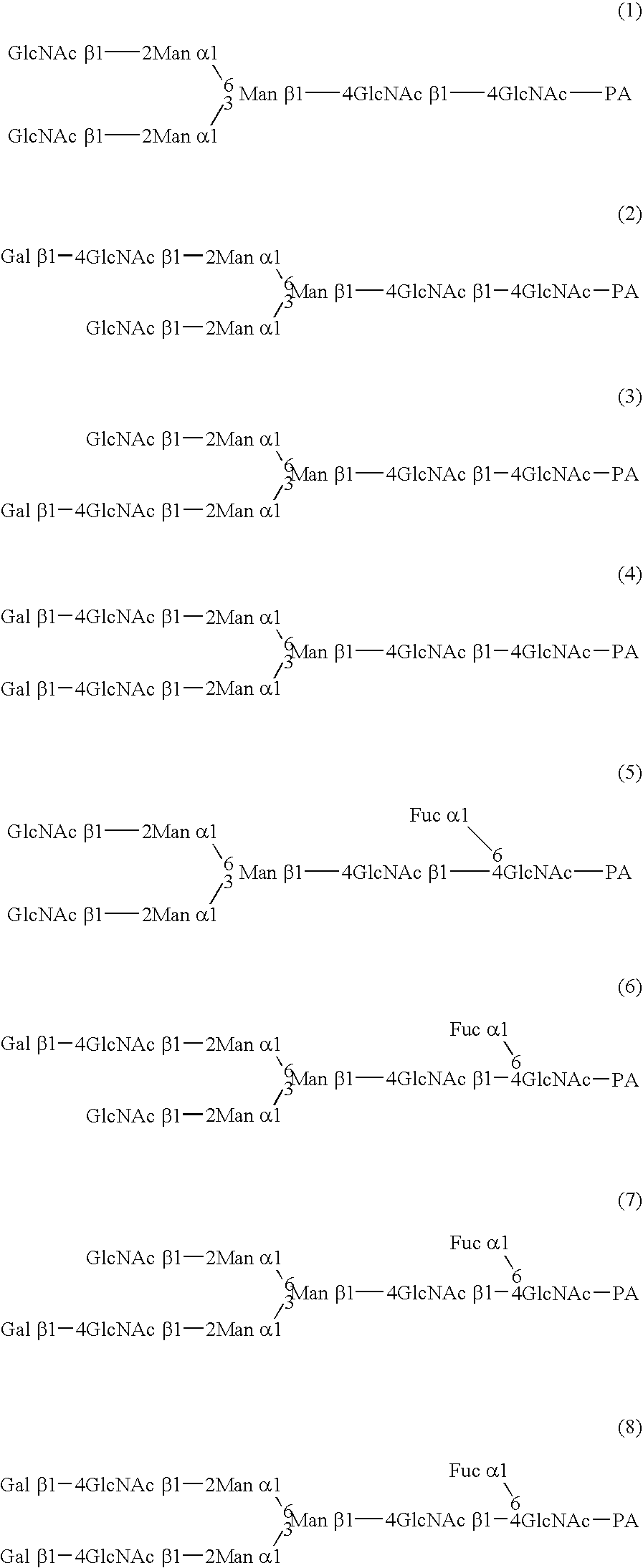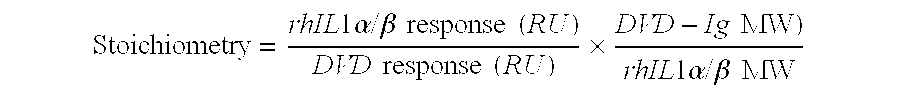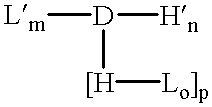Patents
Literature
60165results about "Antivirals" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
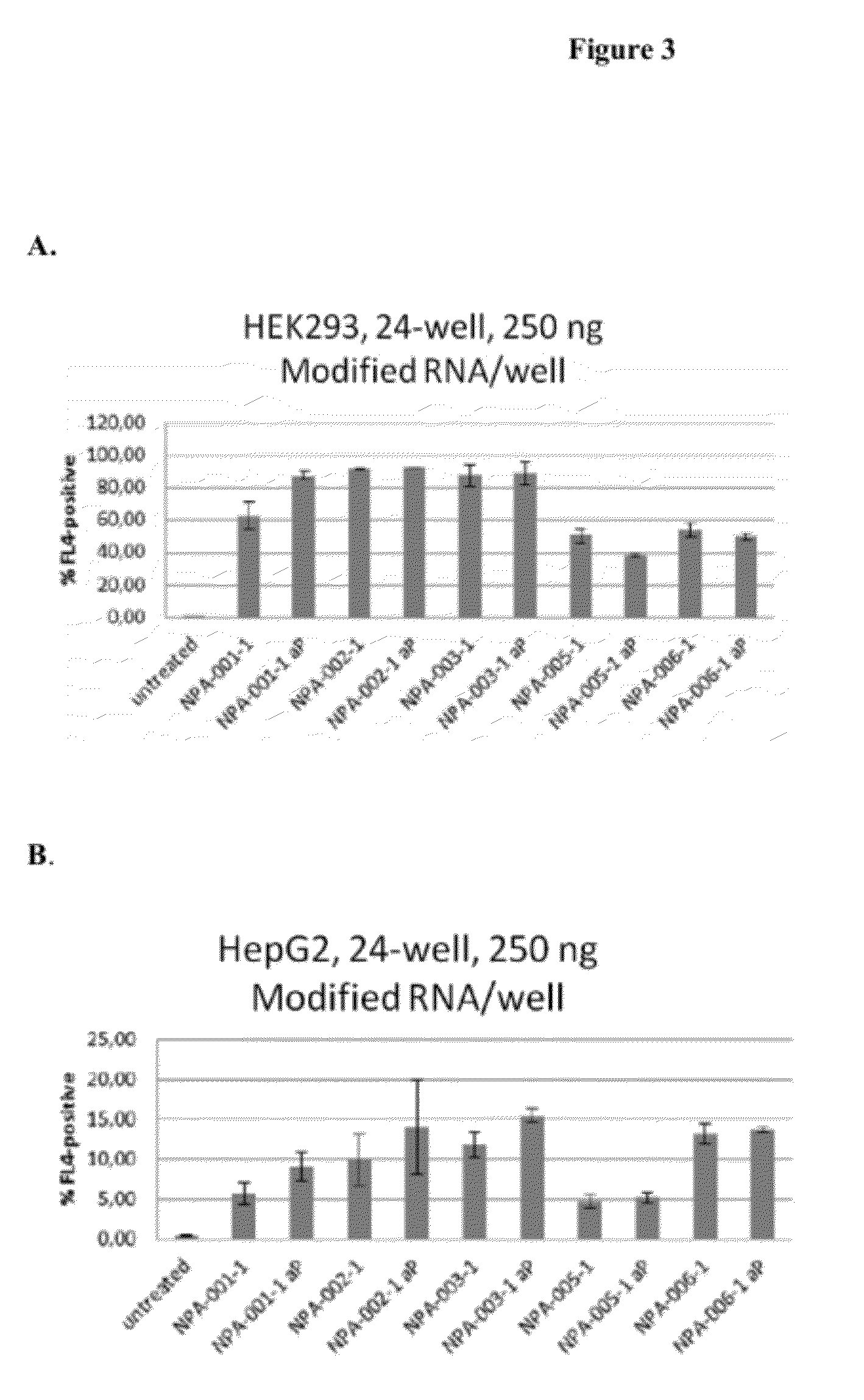
Cells of which genome is modified
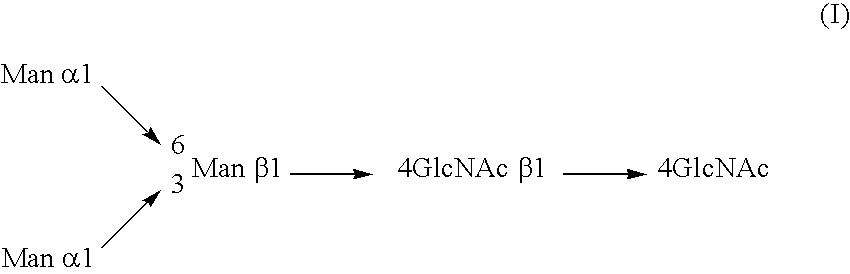
InactiveUS20040110704A1Raise the ratioDecreased and deleted activityAntibacterial agentsAntipyreticGlycosideN-Acetylglucosamine
A cell in which genome is modified so as to have a more decreased or deleted activity of an enzyme relating to modification of a sugar chain in which 1-position of fucose is bound to 6-position of N-acetylglucosamine in the reducing end through alpha-bond in a complex N-glycoside-linked sugar chain than its parent cell, and a process for producing an antibody composition using the cell.
Owner:KYOWA HAKKO KOGYO CO LTD
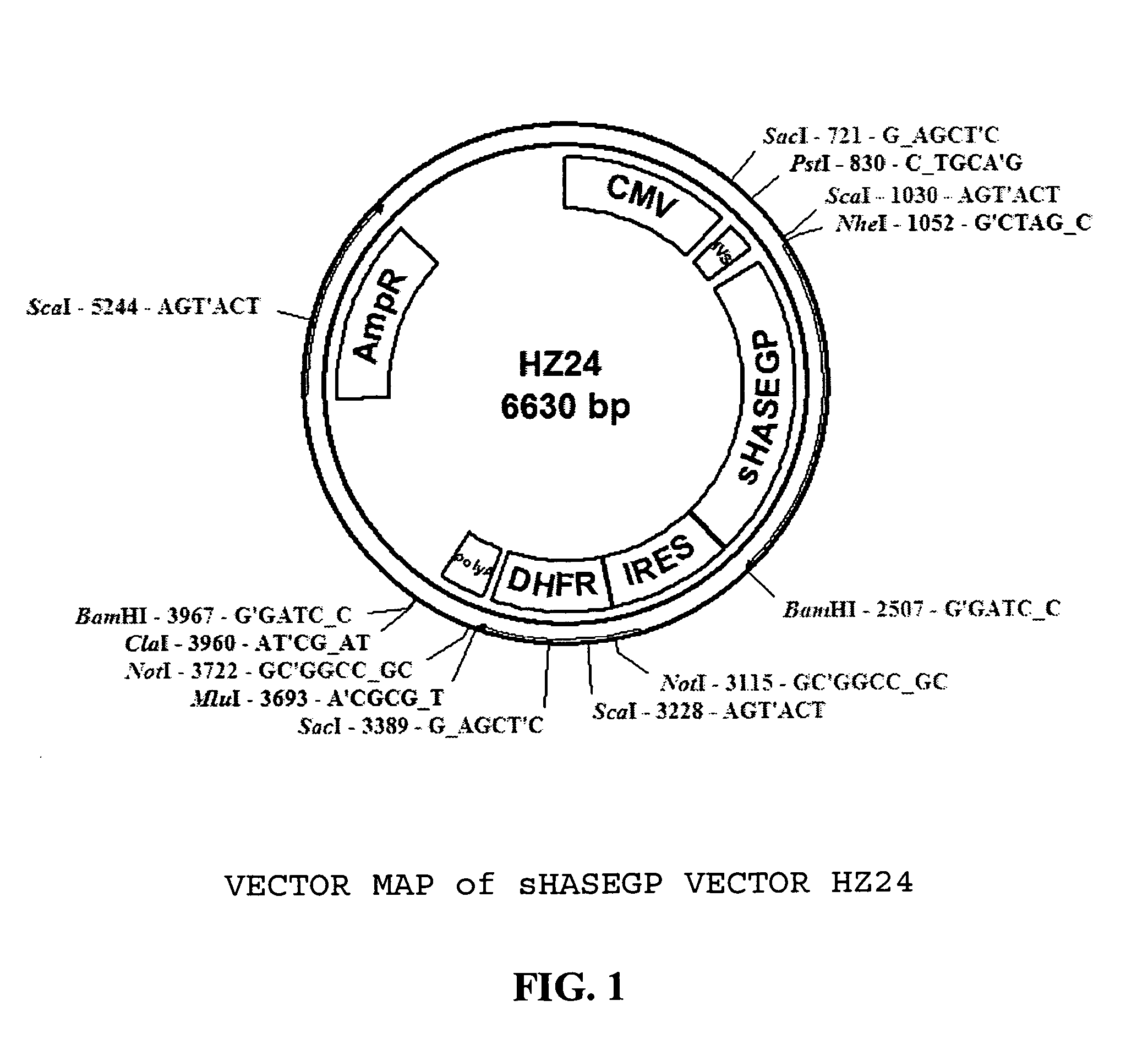
Soluble glycosaminoglycanases and methods of preparing and using soluble glycosaminoglycanases
ActiveUS20050260186A1Improve extentIncrease ratingsAntibacterial agentsSenses disorderHyaluronidasePathology diagnosis
The invention relates to the discovery of novel soluble neutral active Hyaluronidase Glycoproteins (sHASEGPs), methods of manufacture, and their use to facilitate administration of other molecules or to alleviate glycosaminoglycan associated pathologies. Minimally active polypeptide domains of the soluble, neutral active sHASEGP domains are described that include asparagine-linked sugar moieties required for a functional neutral active hyaluronidase domain. Included are modified amino-terminal leader peptides that enhance secretion of sHASEGP. The invention further comprises sialated and pegylated forms of a recombinant sHASEGP to enhance stability and serum pharmacokinetics over naturally occurring slaughterhouse enzymes. Further described are suitable formulations of a substantially purified recombinant sHASEGP glycoprotein derived from a eukaryotic cell that generate the proper glycosylation required for its optimal activity.
Owner:HALOZYME
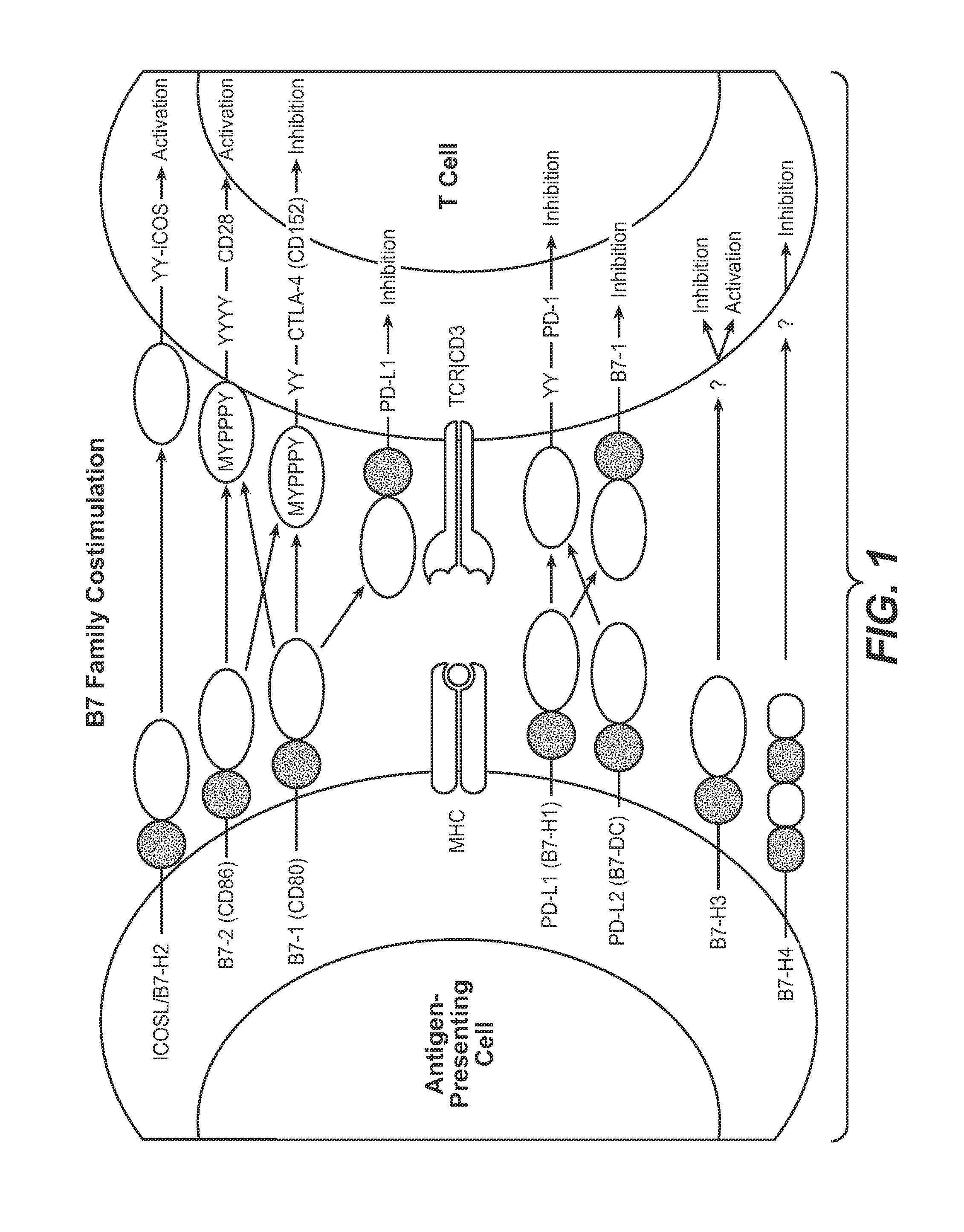
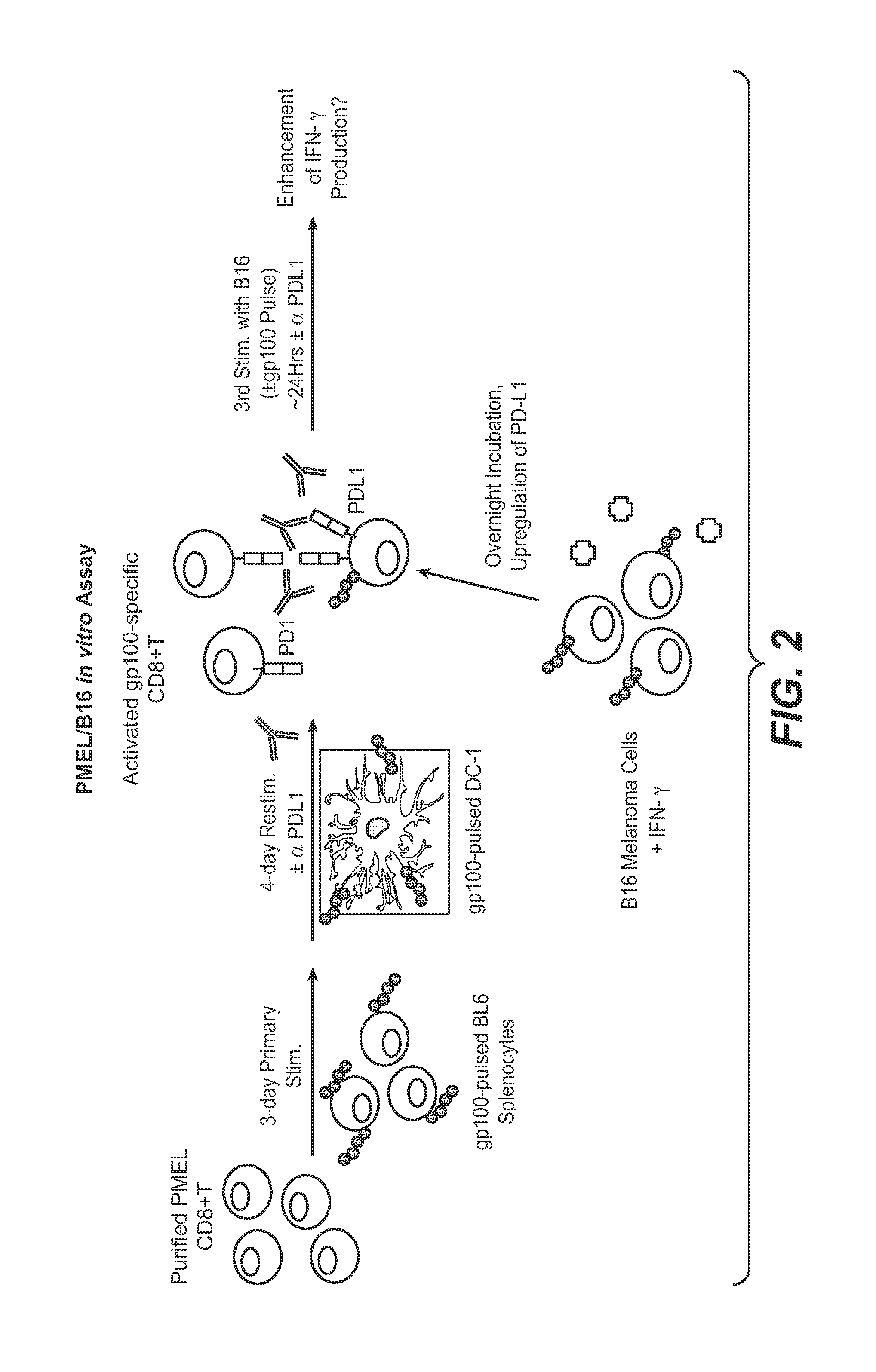
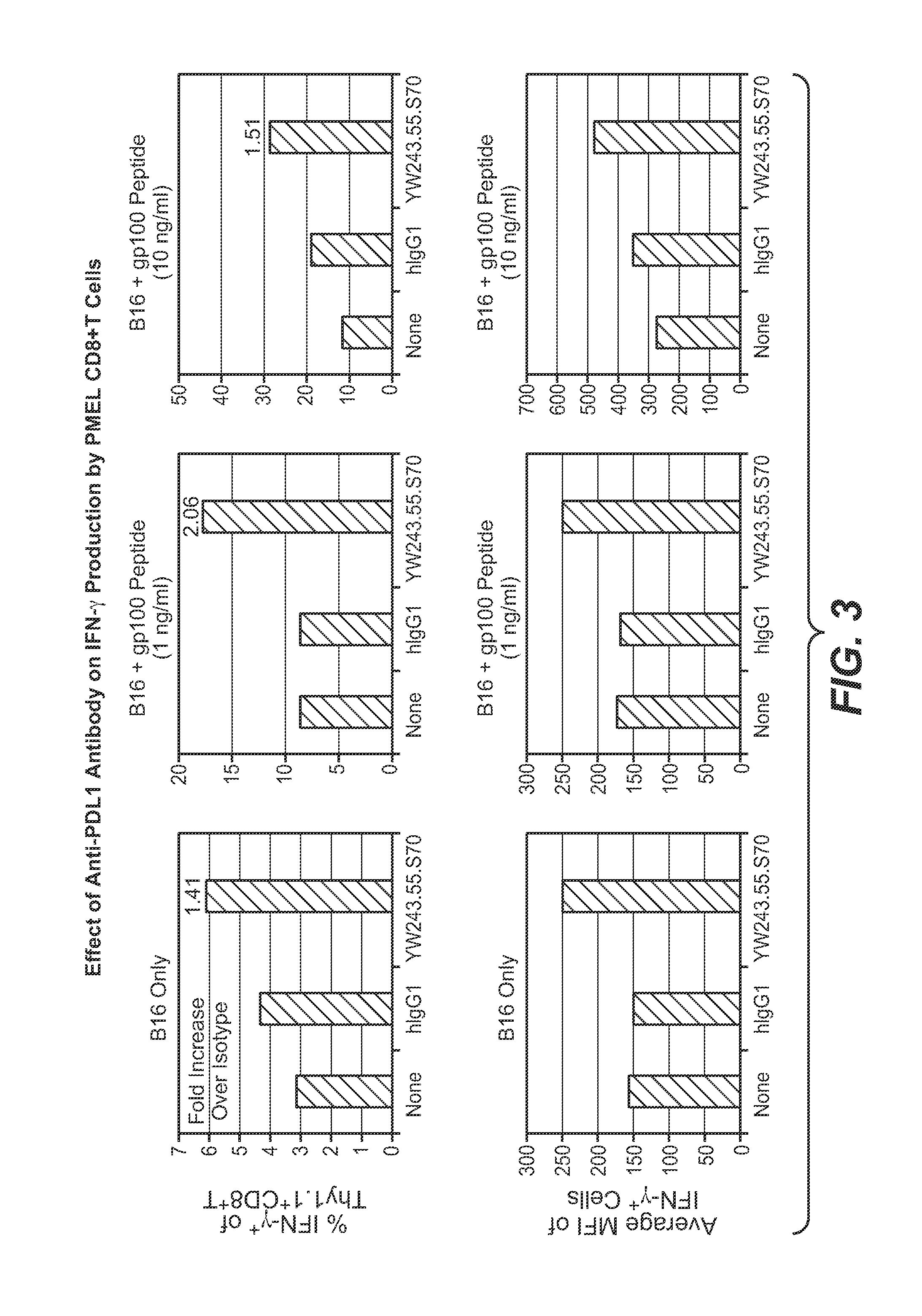
Anti-PD-L1 antibodies, compositions and articles of manufacture
The present application relates to anti-PD-L1 antibodies, nucleic acid encoding the same, therapeutic compositions thereof, and their use enhance T-cell function to upregulate cell-mediated immune responses and for the treatment of T cell dysfunctional disorders, including infection (e.g., acute and chronic) and tumor immunity.
Owner:F HOFFMANN LA ROCHE & CO AG
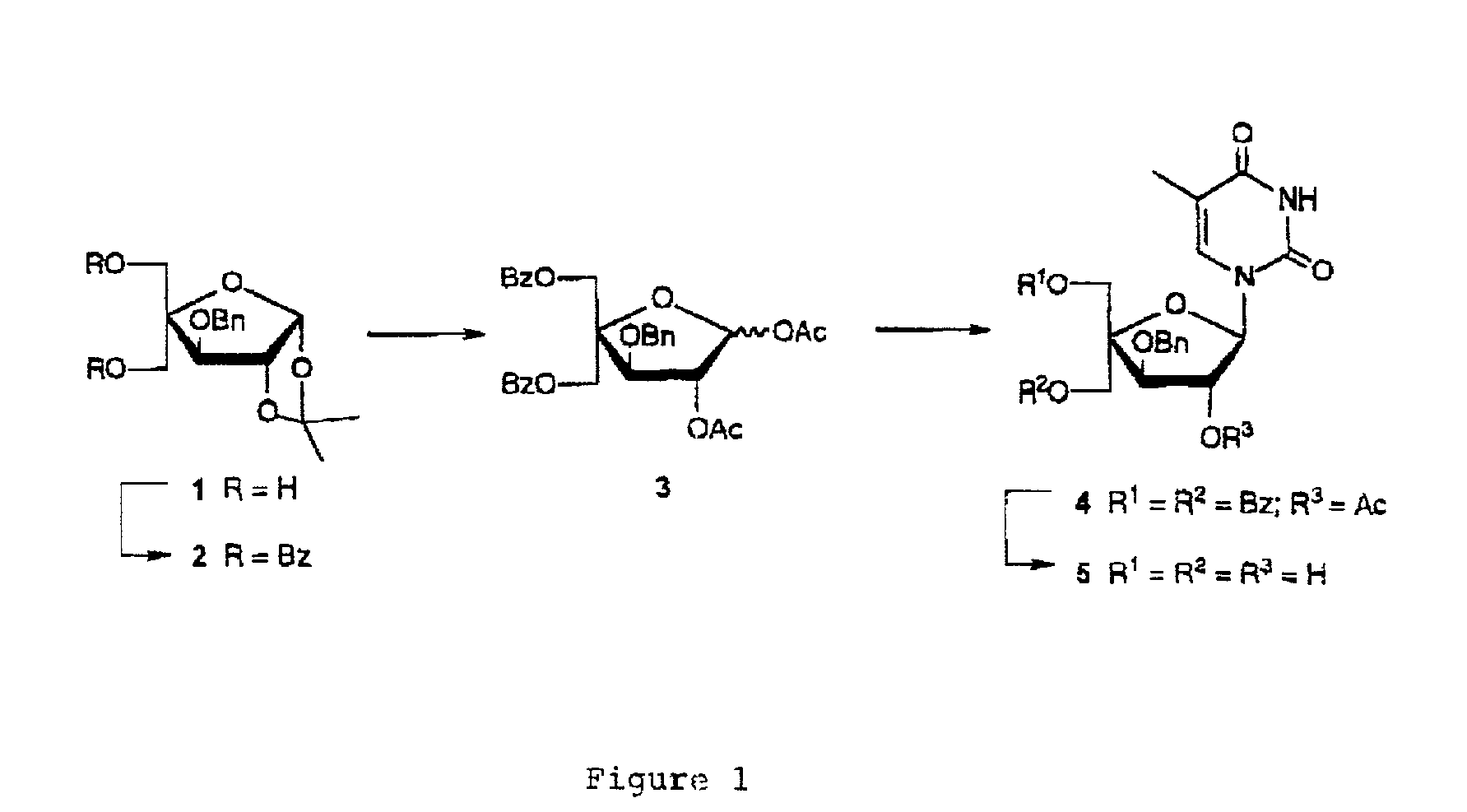
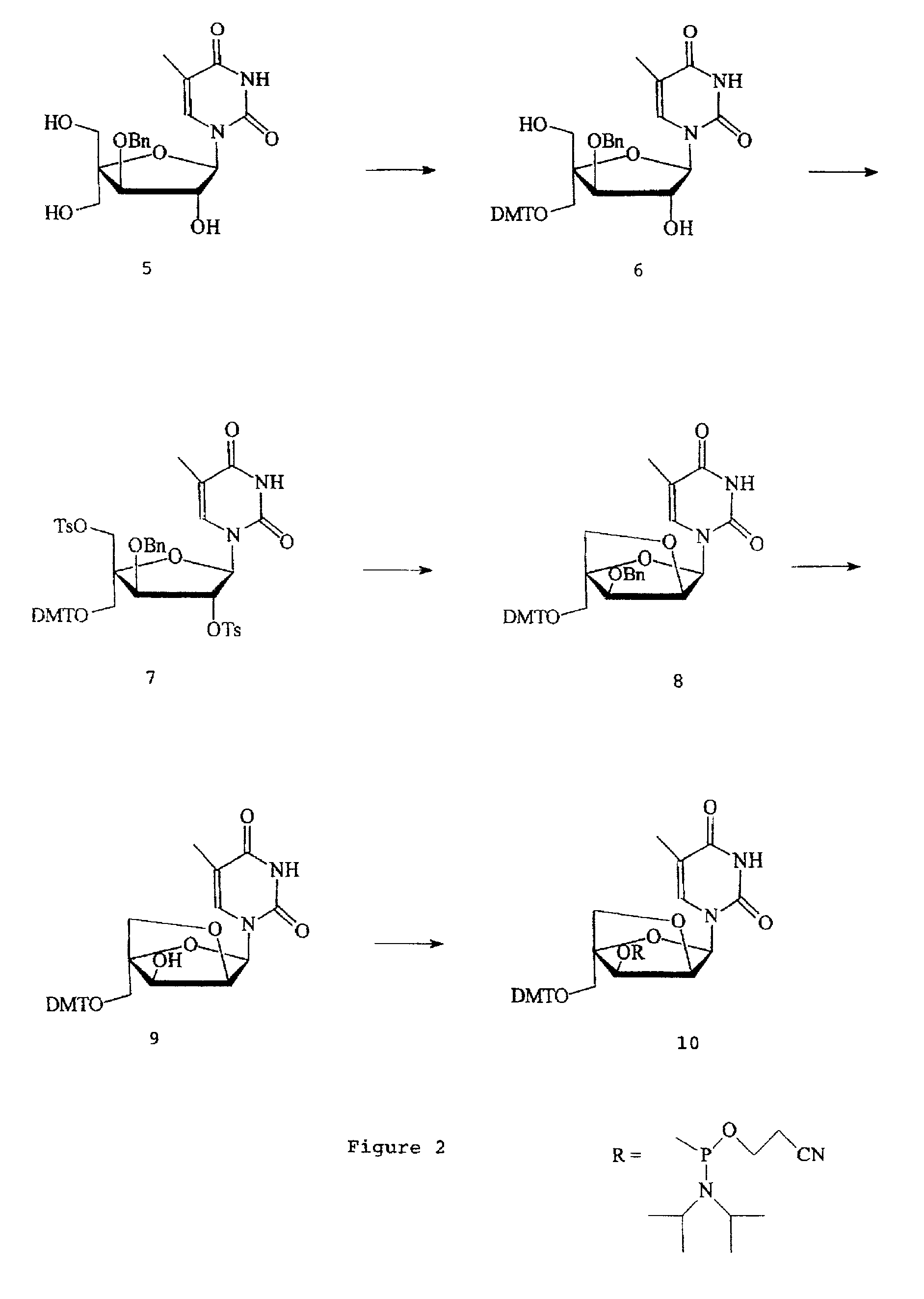
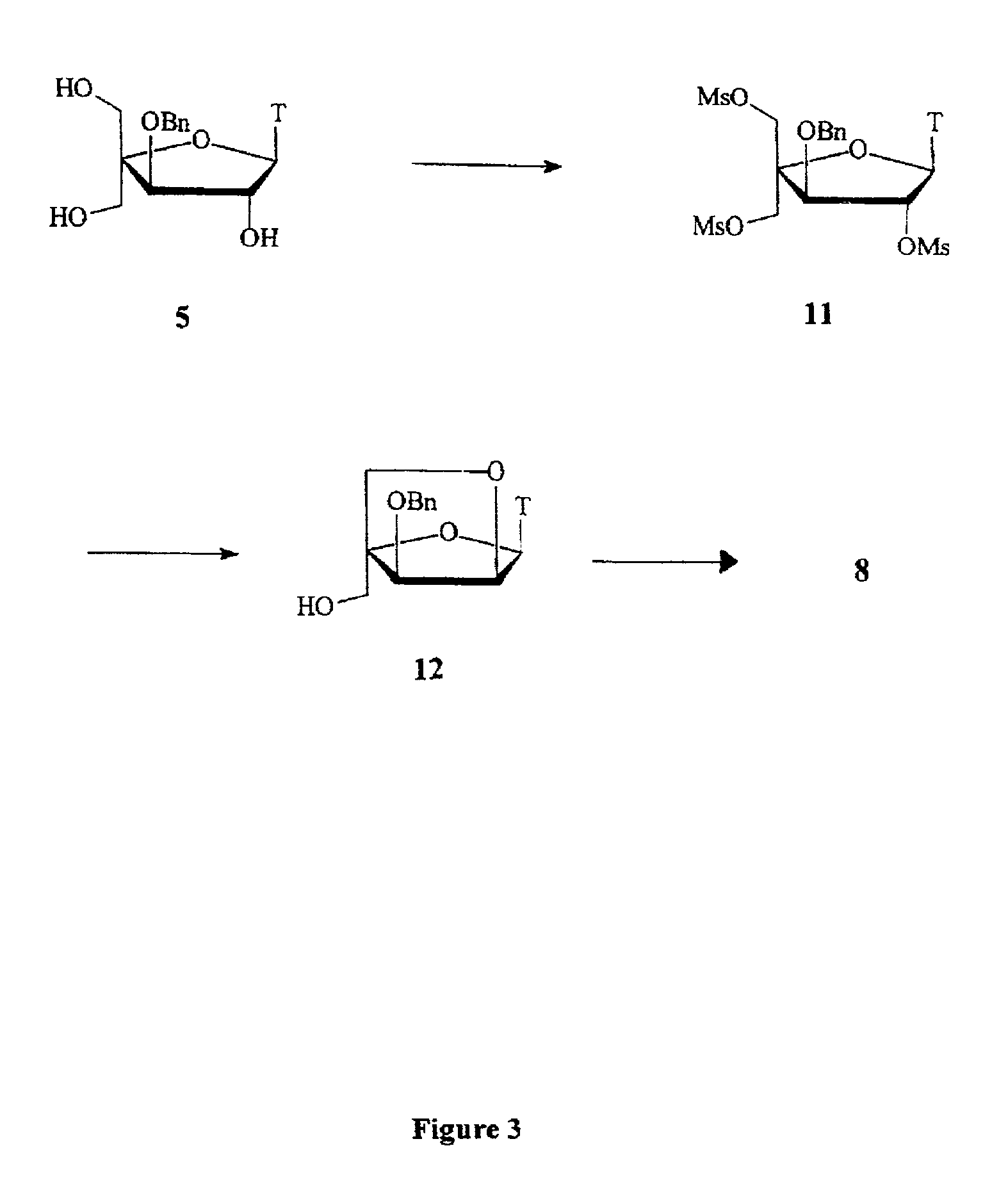
L-ribo-LNA analogues
Provided are L-ribo bicyclic nucleotide compounds as well as syntheses of such compounds. The nucleoside compounds of the invention are useful in forming oligonucleotides that can produce nucleobase specific duplexes with complementary single stranded and double stranded nucleic acids.
Owner:SANTARIS PHARMA AS
Monomethylvaline compounds capable of conjugation to ligands
ActiveUS20050238649A1Improve bioavailabilityImprove compoundAntibacterial agentsBiocideD-norephedrineBiochemistry
Owner:SEAGEN INC
Human CTLA-4 antibodies
The present invention provides human sequence antibodies against CTLA-4 and methods of treating human diseases, infections and other conditions using these antibodies.
Owner:ER SQUIBB & SONS INC
Molecules with extended half-lives, compositions and uses thereof
The present invention provides molecules, including IgGs, non-IgG immunoglobulin, proteins and non-protein agents, that have increased in vivo half-lives due to the presence of an IgG constant domain, or a portion thereof that binds the FcRn, having one or more amino acid modifications that increase the affinity of the constant domain or fragment for FcRn. Such proteins and molecules with increased half-lives have the advantage that smaller amounts and or less frequent dosing is required in the therapeutic, prophylactic or diagnostic use of such molecules.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
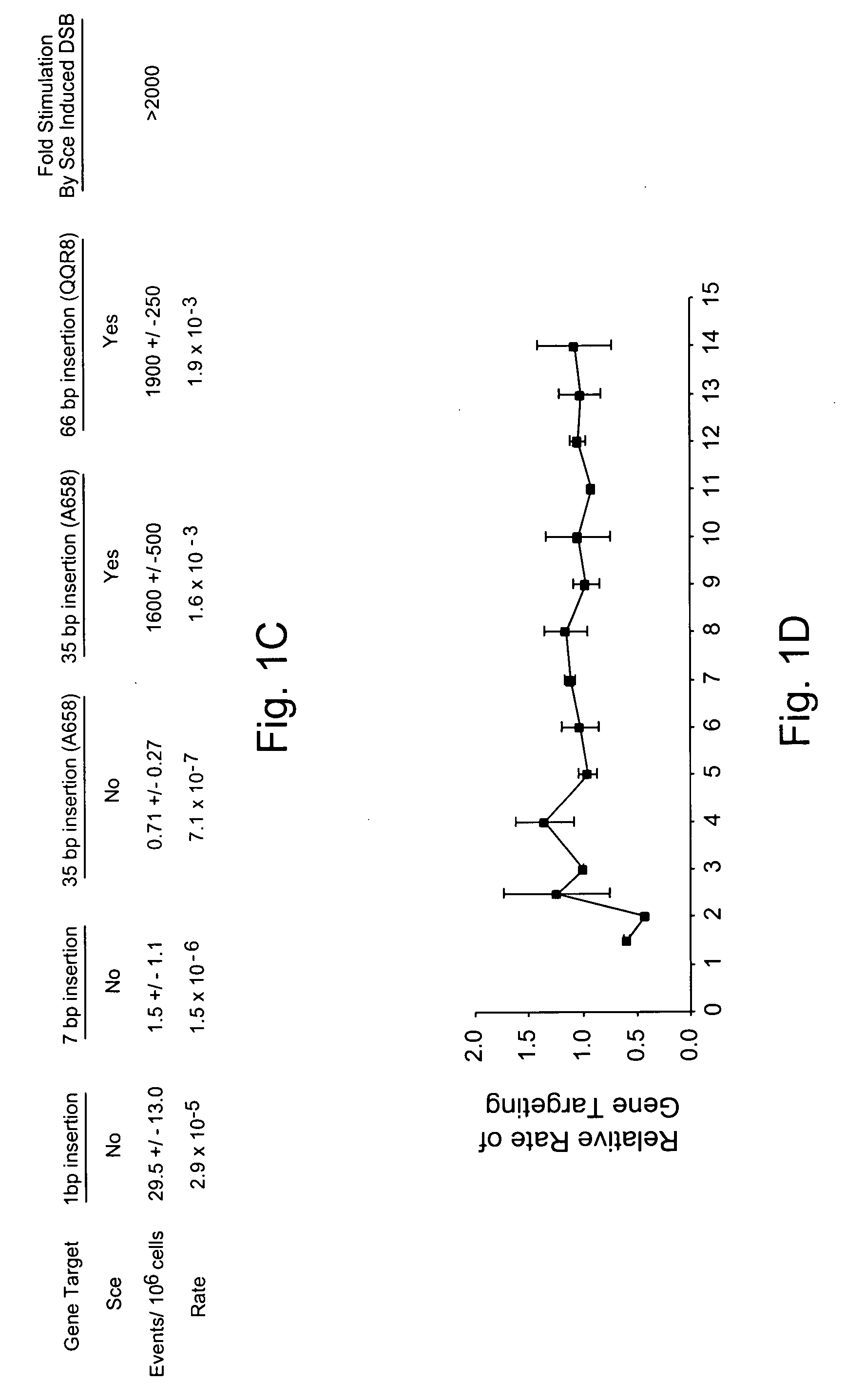
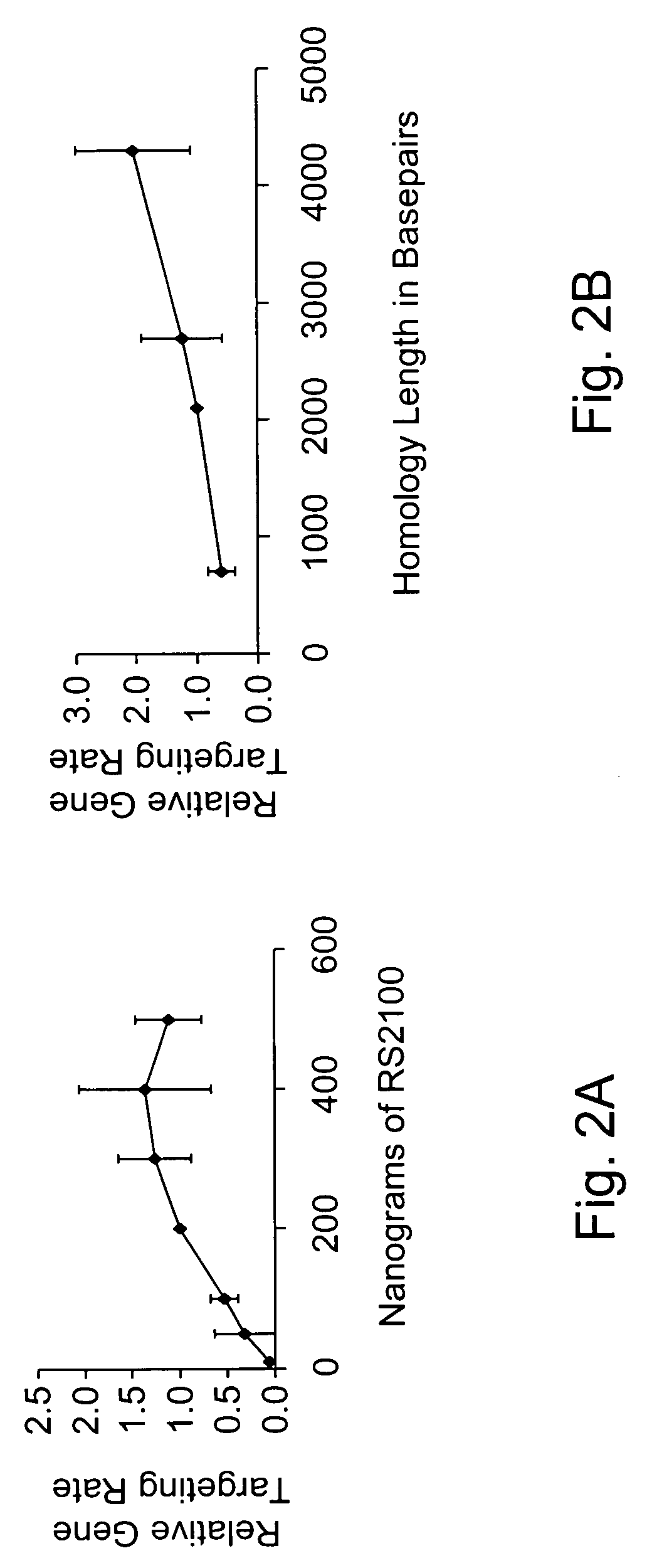
Use of chimeric nucleases to stimulate gene targeting
ActiveUS20050026157A1Ameliorate genetic disorderIncrease productionAntibacterial agentsFusion with DNA-binding domainGene targetsGenetic Change
Gene targeting is a technique to introduce genetic change into one or more specific locations in the genome of a cell. For example, gene targeting can introduce genetic change by modifying, repairing, attenuating or inactivating a target gene or other chromosomal DNA. In one aspect, this disclosure relates to methods and compositions for gene targeting with high efficiency in a cell. This disclosure also relates to methods of treating or preventing a genetic disease in an individual in need thereof. Further disclosed are chimeric nucleases and vectors encoding chimeric nucleases.
Owner:CALIFORNIA INST OF TECH
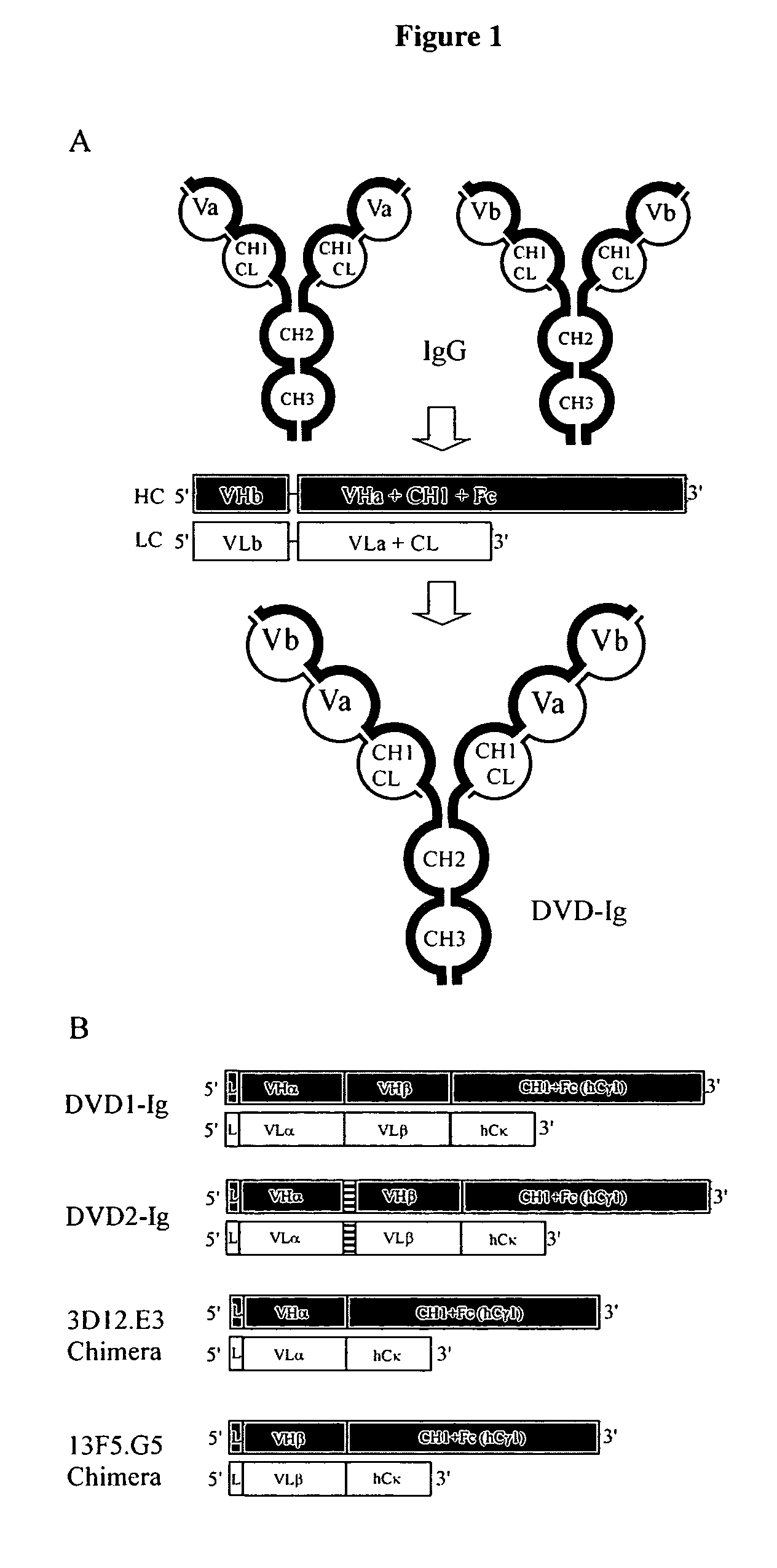
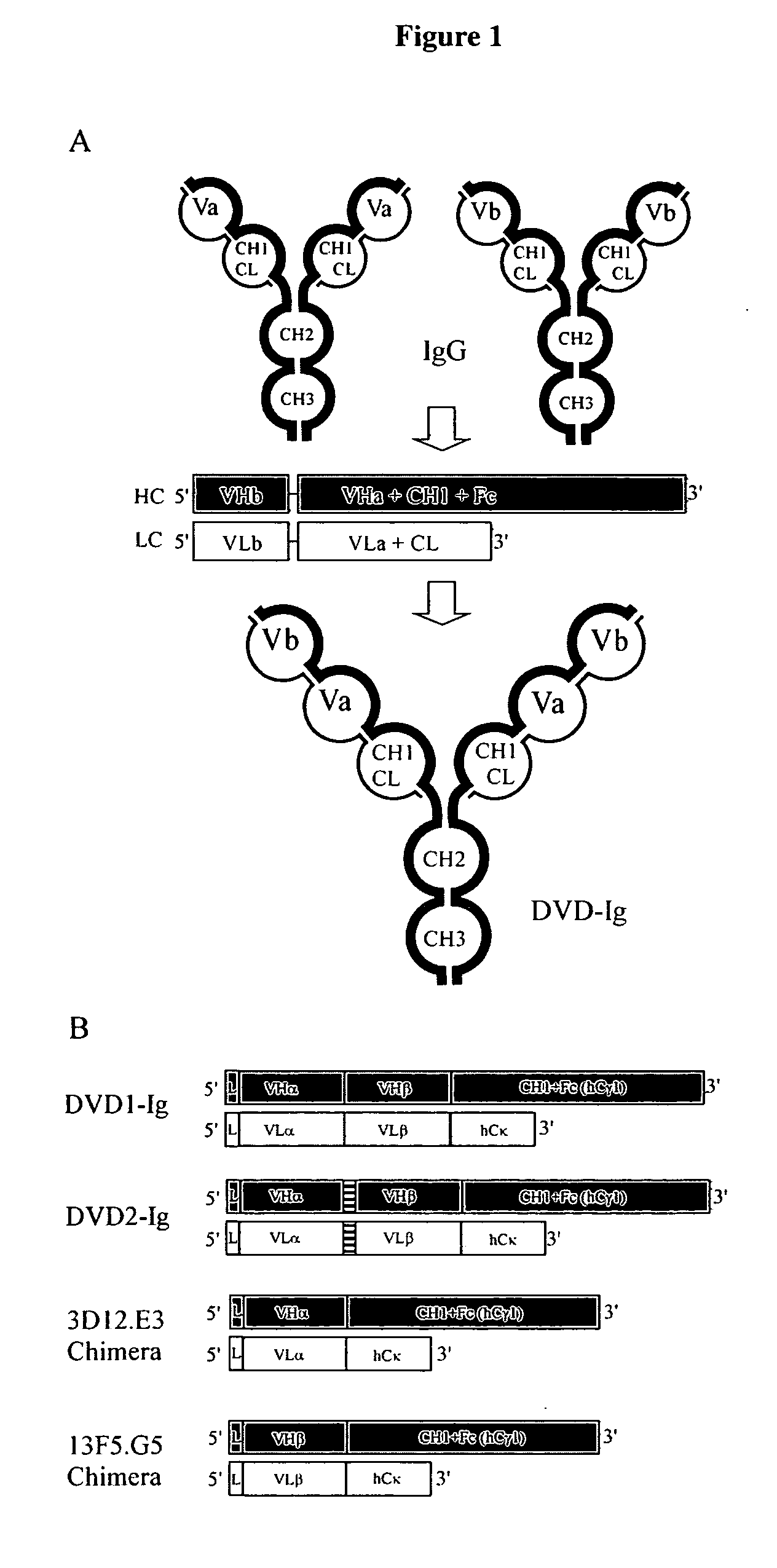
Dual variable domain immunoglobulin and uses thereof
Owner:ABBVIE INC
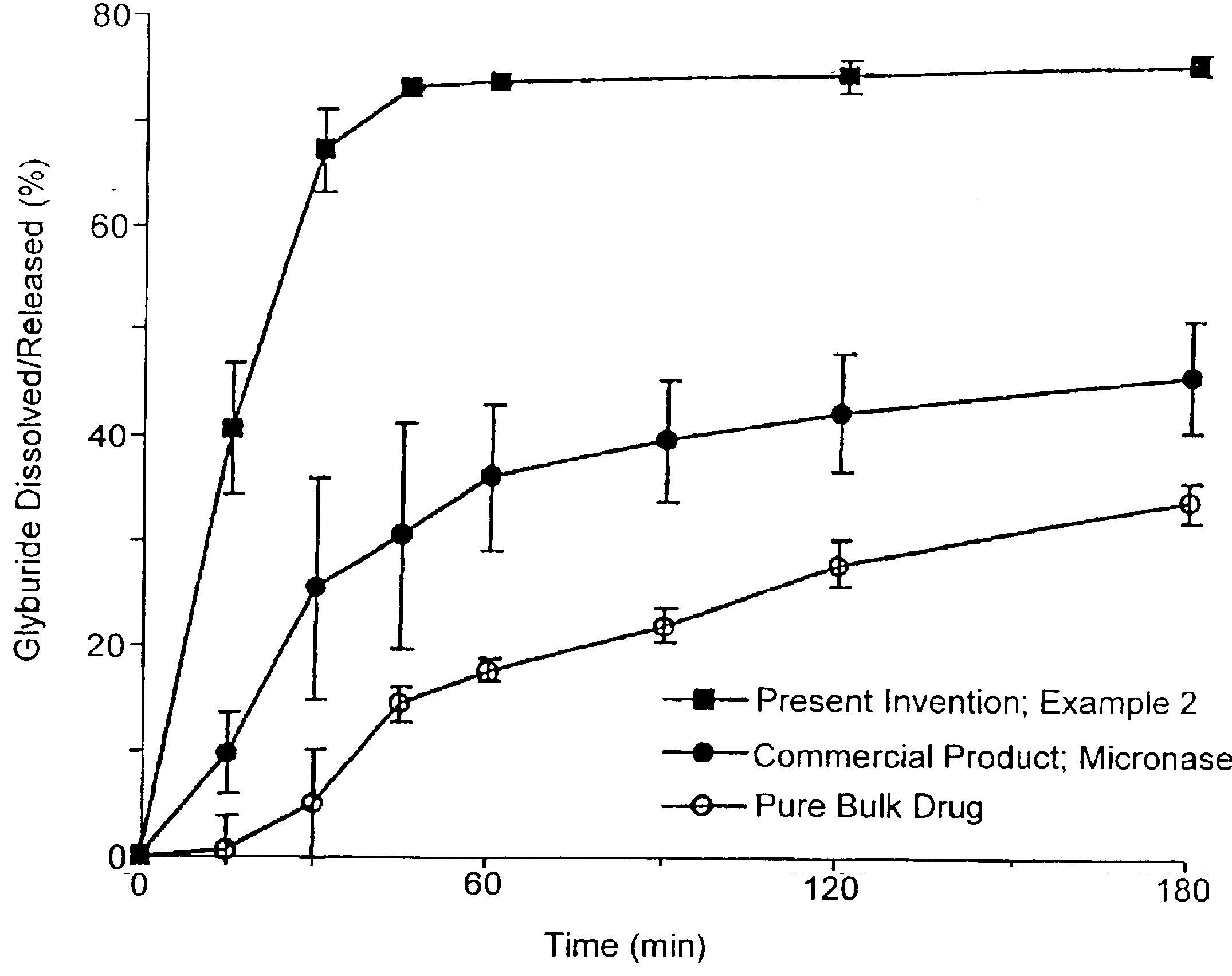
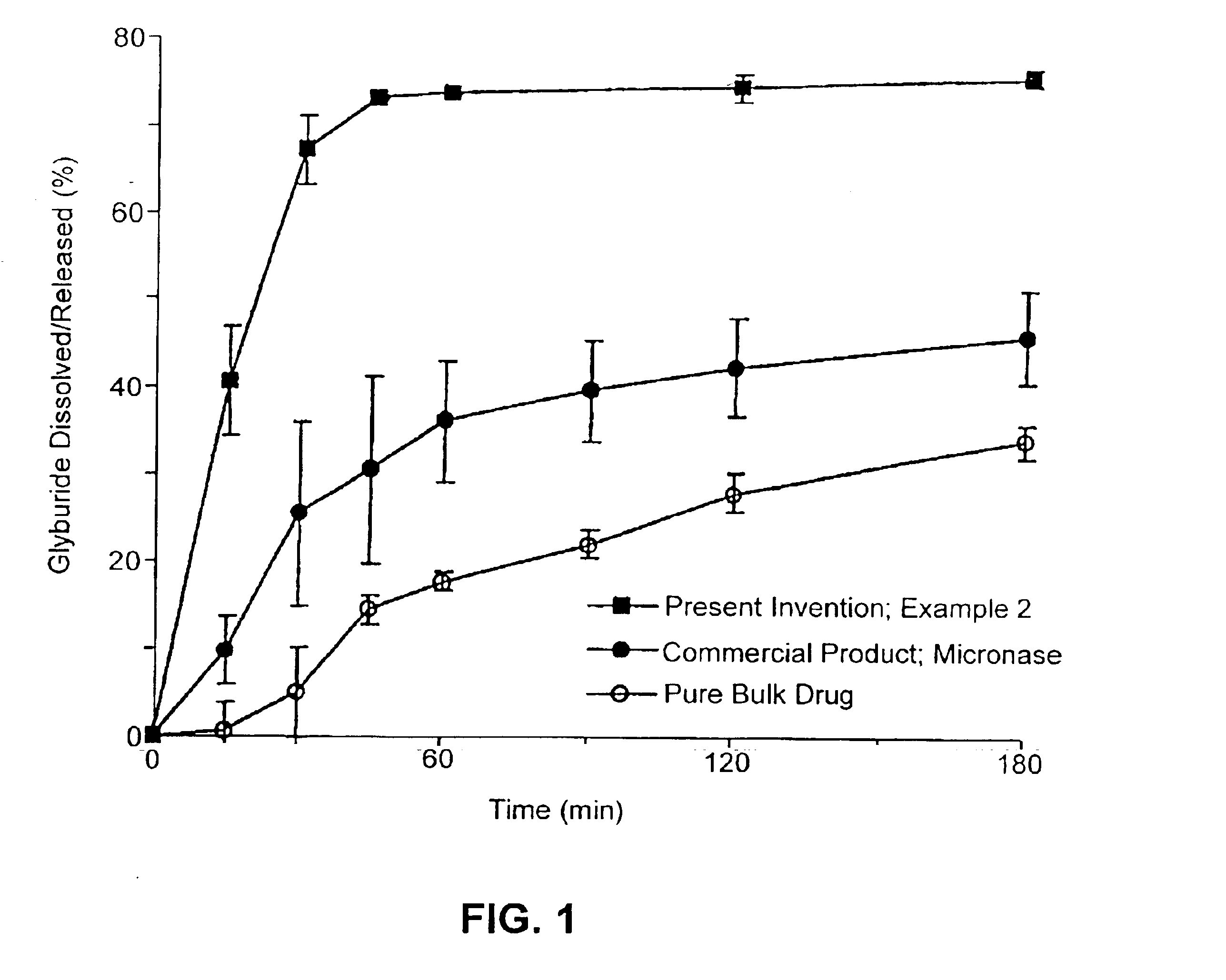
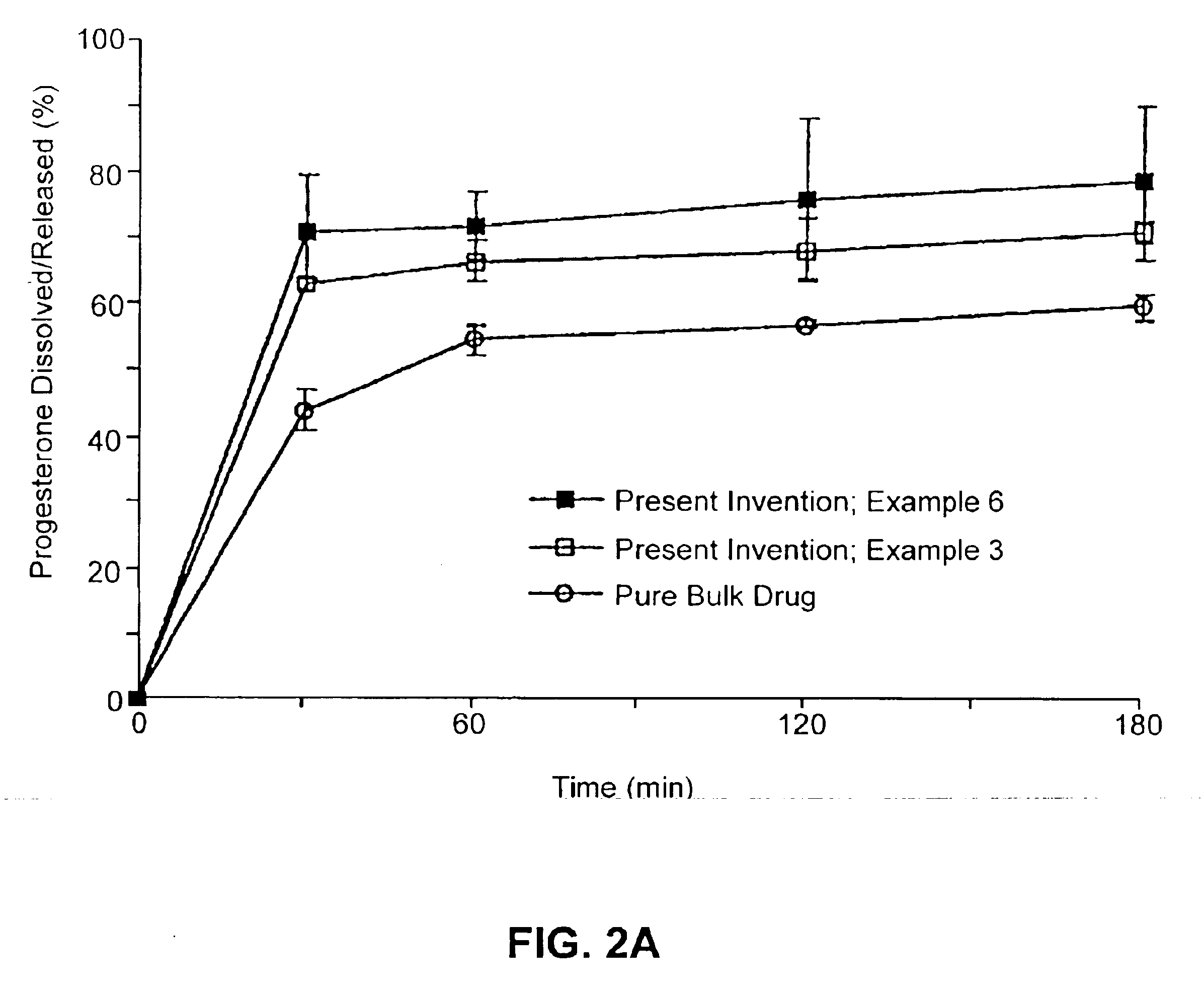
Solid carriers for improved delivery of active ingredients in pharmaceutical compositions
InactiveUS6923988B2Rapid dissolvableMore solubilizedAntibacterial agentsOrganic active ingredientsDiagnostic agentTG - Triglyceride
The present invention provides solid pharmaceutical compositions for improved delivery of a wide variety of pharmaceutical active ingredients contained therein or separately administered. In one embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier including a substrate and an encapsulation coat on the substrate. The encapsulation coat can include different combinations of pharmaceutical active ingredients, hydrophilic surfactant, lipophilic surfactants and triglycerides. In another embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier being formed of different combinations of pharmaceutical active ingredients, hydrophilic surfactants, lipophilic surfactants and triglycerides. The compositions of the present invention can be used for improved delivery of hydrophilic or hydrophobic pharmaceutical active ingredients, such as drugs, nutritional agents, cosmeceuticals and diagnostic agents.
Owner:LIPOCINE
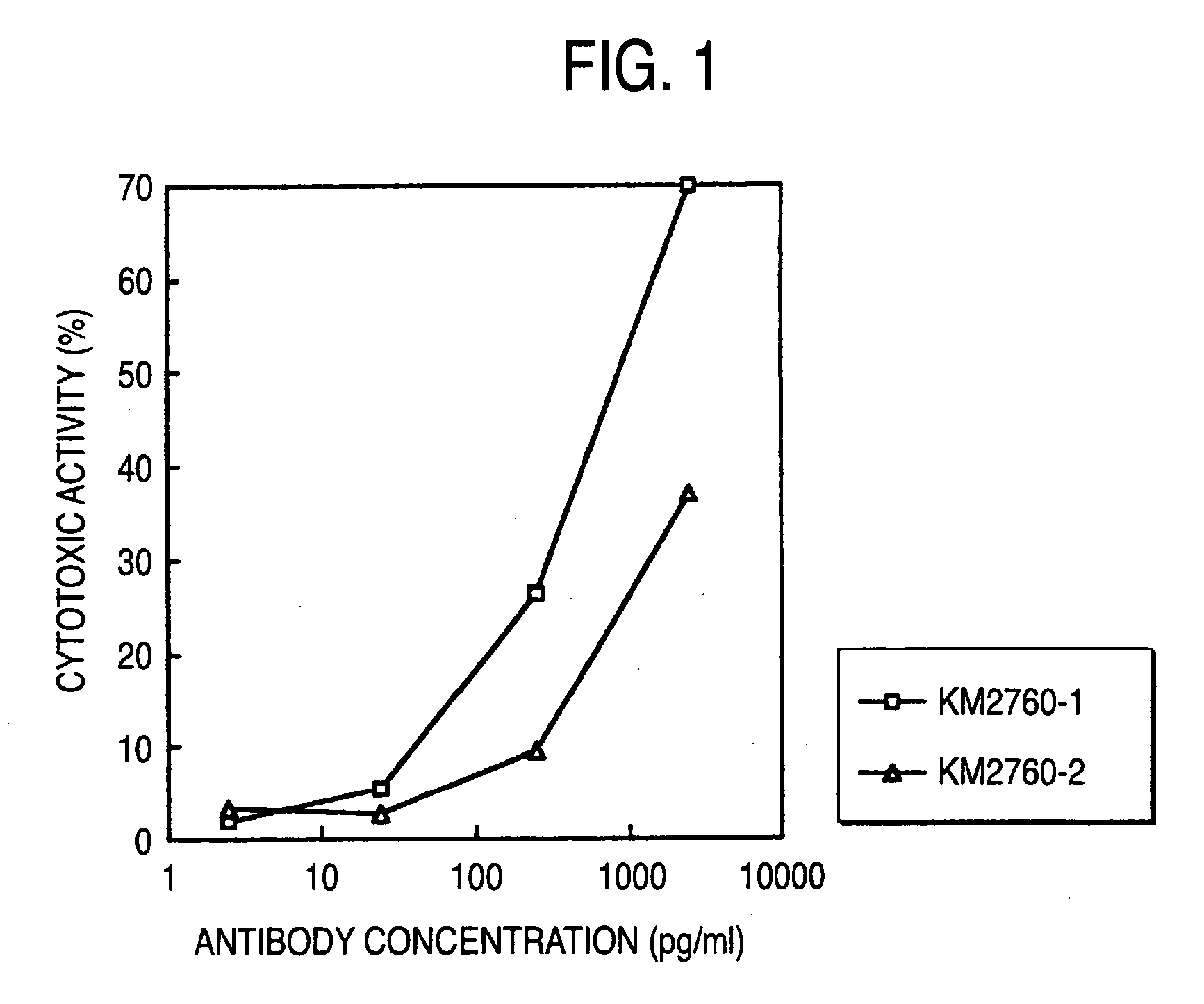
Cells producing antibody compositions with increased antibody dependent cytotoxic activity
The present invention relates to a cell for the production of an antibody molecule such as an antibody useful for various diseases having high antibody-dependent cell-mediated cytotoxic activity, a fragment of the antibody and a fusion protein having the Fc region of the antibody or the like, a method for producing an antibody composition using the cell, the antibody composition and use thereof.
Owner:KYOWA HAKKO KIRIN CO LTD
Method of producing sustained-release preparation
InactiveUS6267981B1Maintain good propertiesEnhancement of entrapmentPowder deliveryPeptide/protein ingredientsEntrapmentBiodegradable polymer
This invention provides a sustained-release preparation comprising a biodegradable polymer metal salt and broactive polypeptide, with enhanced entrapment of the bioactive polypeptides, a suppression of initial burst, and a constant long-term release of the bioactive polypeptides.
Owner:TAKEDA PHARMA CO LTD
Dual variable domain immunoglobulin and uses thereof
Owner:ABBVIE INC
Polymer-based, sustained release drug delivery system
InactiveUS20120016467A1Reduce interactionSuture equipmentsAntibacterial agentsRate limitingSolubility
Disclosed is a sustained release system that includes a polymer and a prodrug having a solubility less than about 1 mg / ml dispersed in the polymer. Advantageously, the polymer is permeable to the prodrug and may be non-release rate limiting with respect to the rate of release of the prodrug from the polymer. This permits improved drug delivery within a body in the vicinity of a surgery via sustained release rate kinetics over a prolonged period of time, while not requiring complicated manufacturing processes.
Owner:PSIVIDA INC
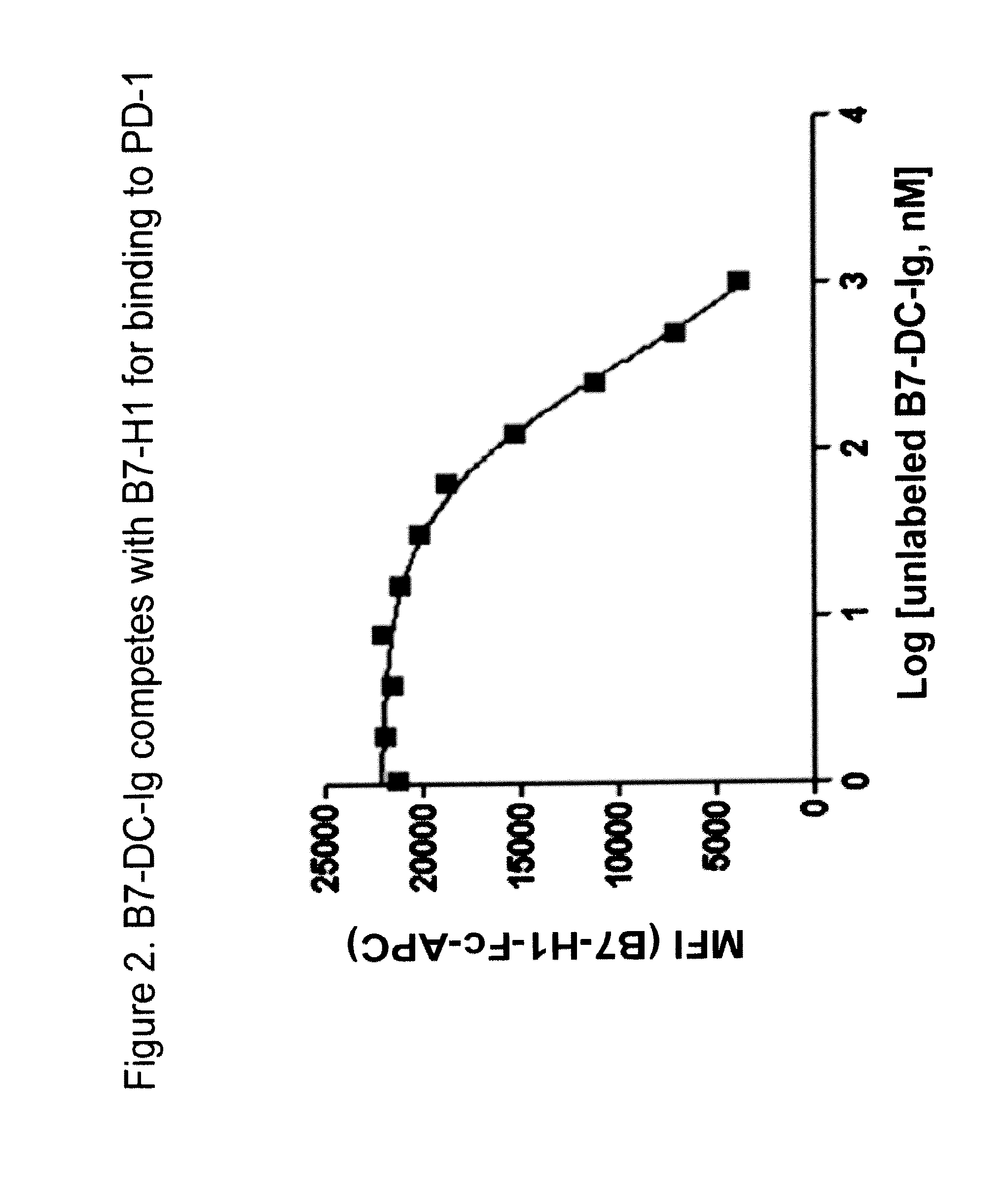
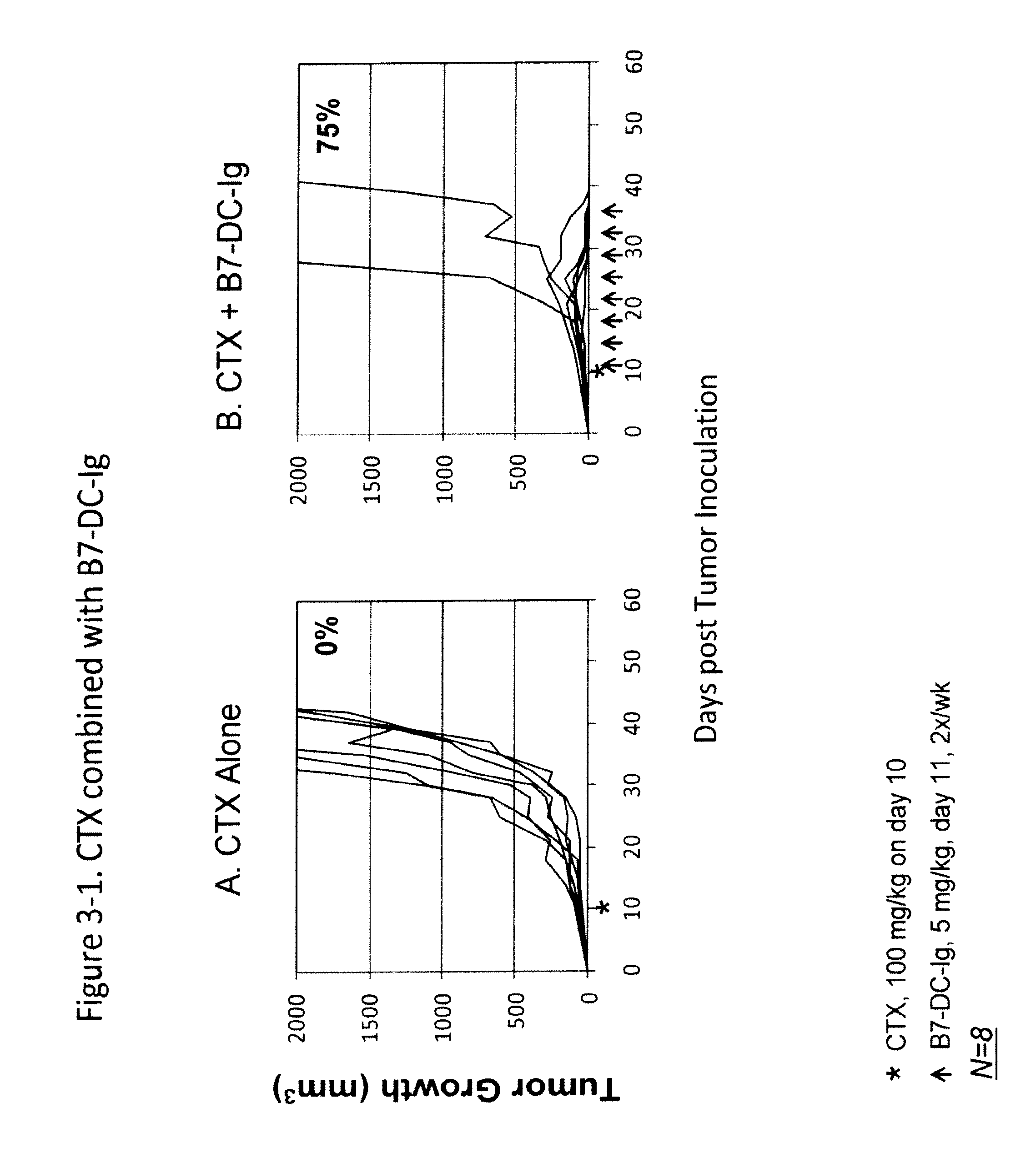
Compositions of pd-1 antagonists and methods of use
InactiveUS20120114649A1Improve responseInhibitory signal transductionAntibacterial agentsOrganic active ingredientsT cellInfective disorder
Methods of treating cancer and infectious diseases utilizing a treatment regimen comprising administering a compound that reduces inhibitory signal transduction in T cells, in combination with a potentiating agent, such as cyclophosphamide, to produce potent T cell mediated responses, are described. Compositions comprising the PD-1 antagonists and potentiating agents useful in the methods of the invention are also disclosed.
Owner:MEDIMMUNE LLC
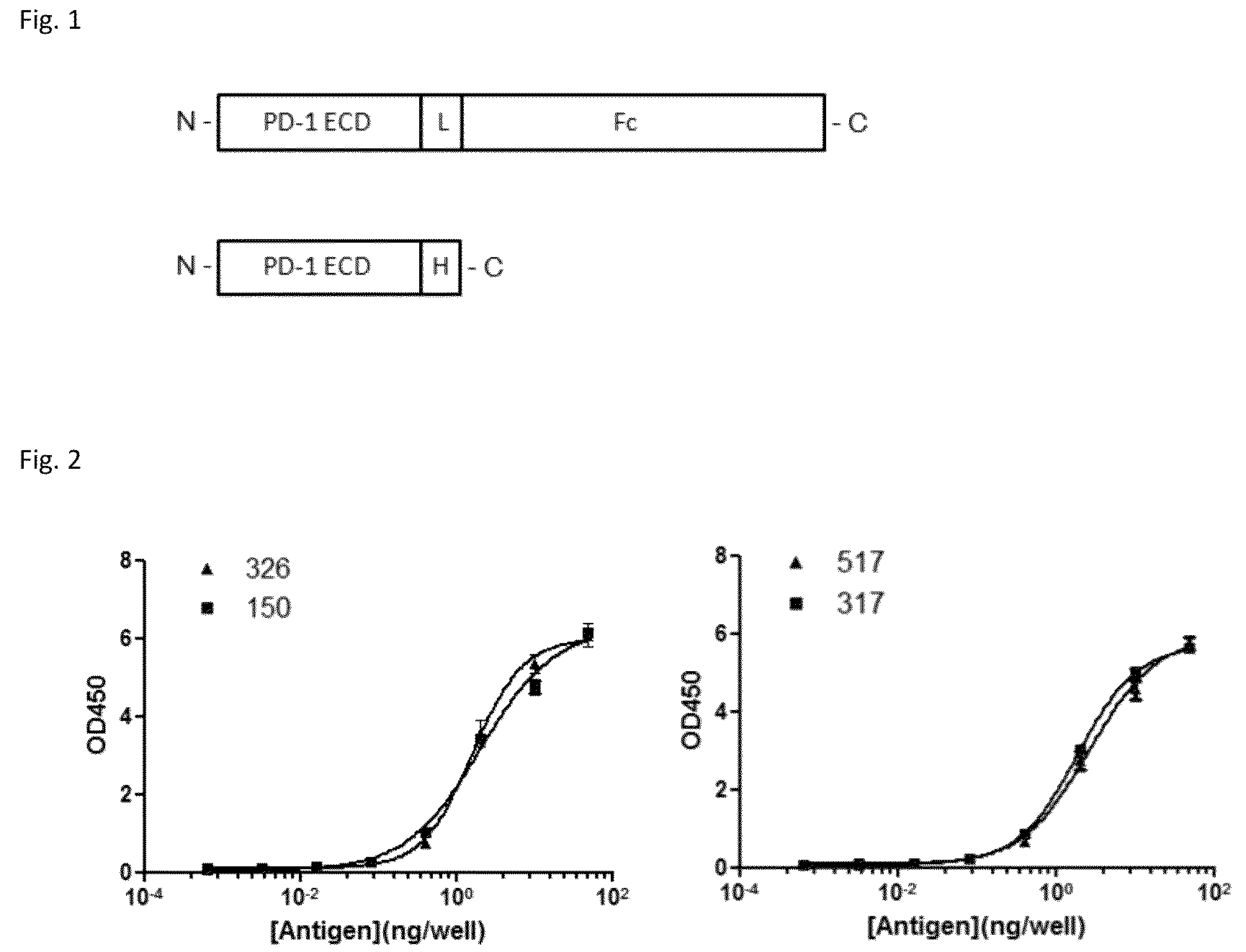
Anti-PD1 antibodies and their use as therapeutics and diagnostics
ActiveUS8735553B1Inhibition of secretionNervous disorderImmunoglobulins against cell receptors/antigens/surface-determinantsProgrammed deathAnti pd1
Owner:BEIGENE SWITZERLAND GMBH
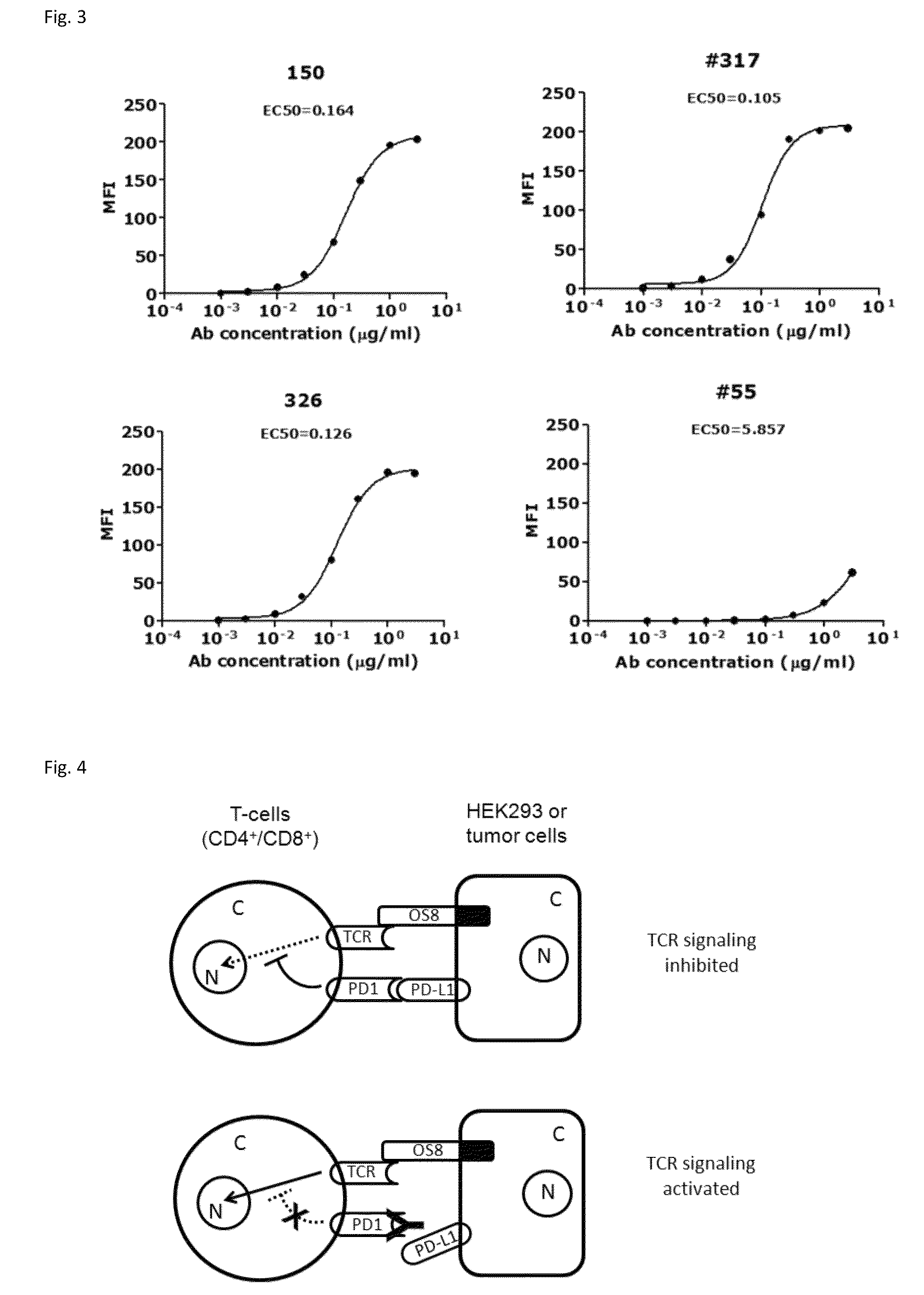
Anti-PD-L1 antibodies and uses therefor
The present invention is based, in part, on the identification of novel human anti-PD-1, PD-L1, and PD-L2 antibodies. Accordingly, the invention relates to compositions and methods for diagnosing, prognosing, and treating conditions that would benefit from modulating PD-1, PD-L1, and / or PD-L2 activity (e.g., persistent infectious diseases, autoimmune diseases, asthma, transplant rejection, inflammatory disorders and tumors) using the novel human anti-PD-1, PD-L1, and PD-L2 antibodies described herein.
Owner:DANA FARBER CANCER INST INC +2
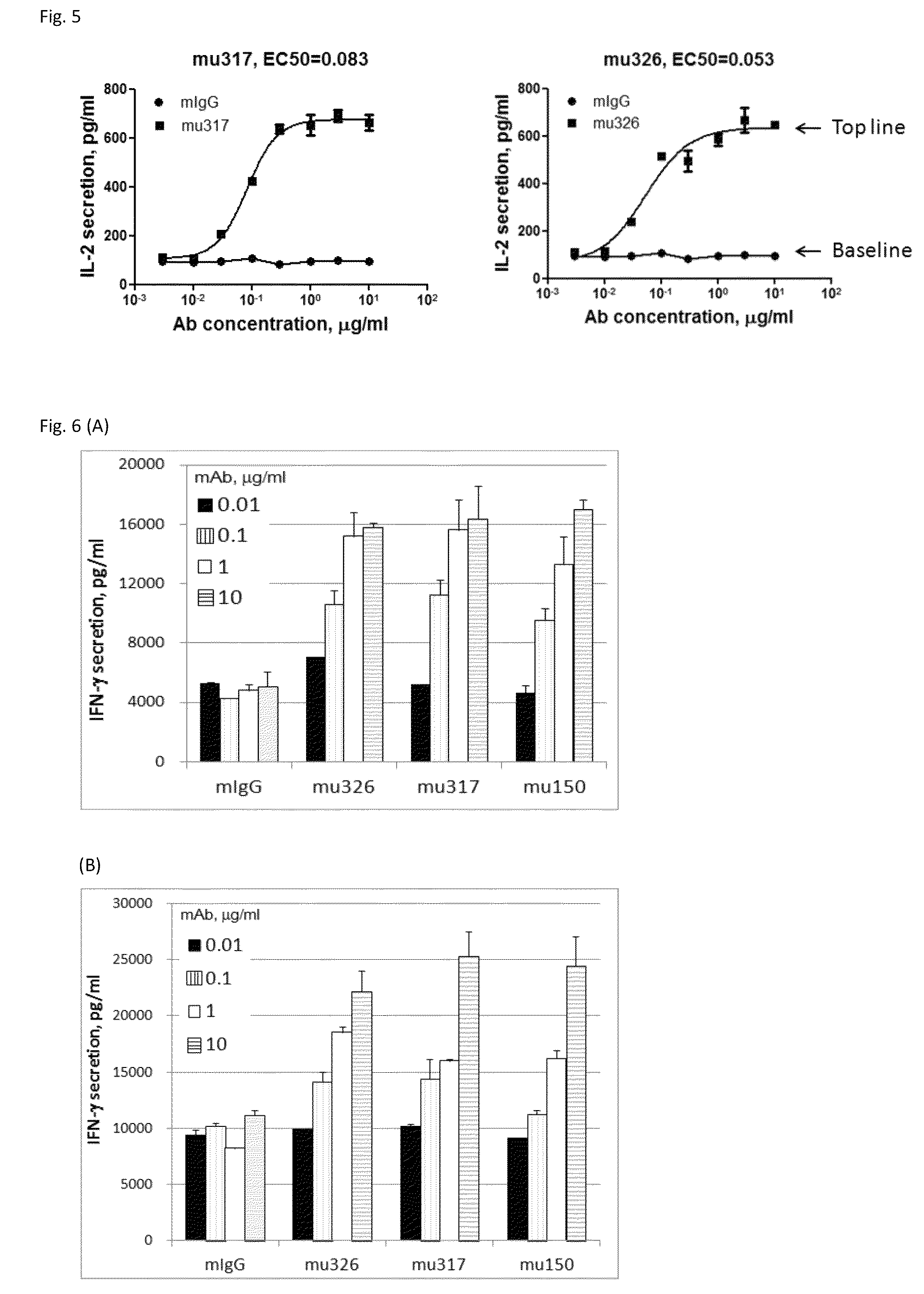
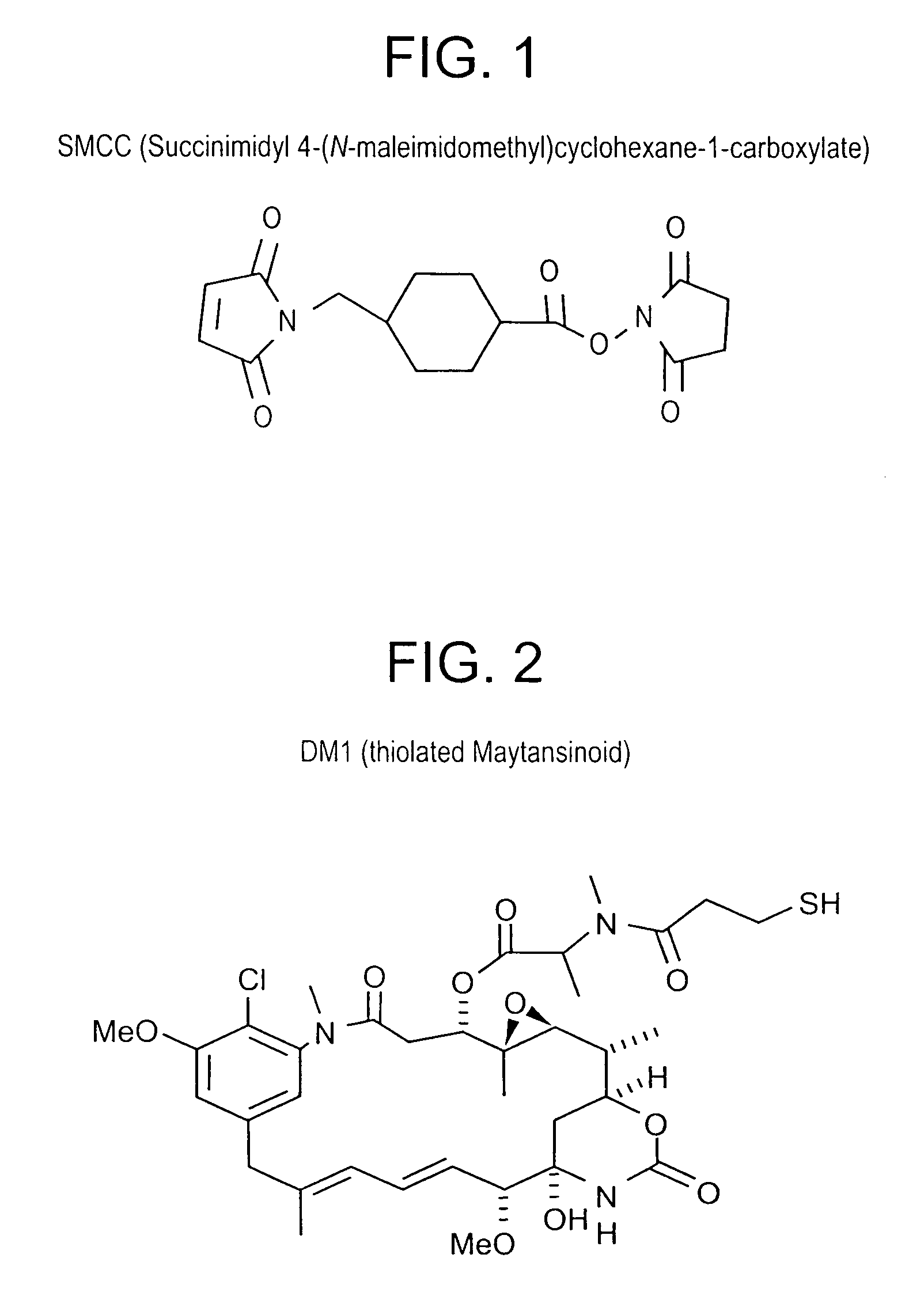
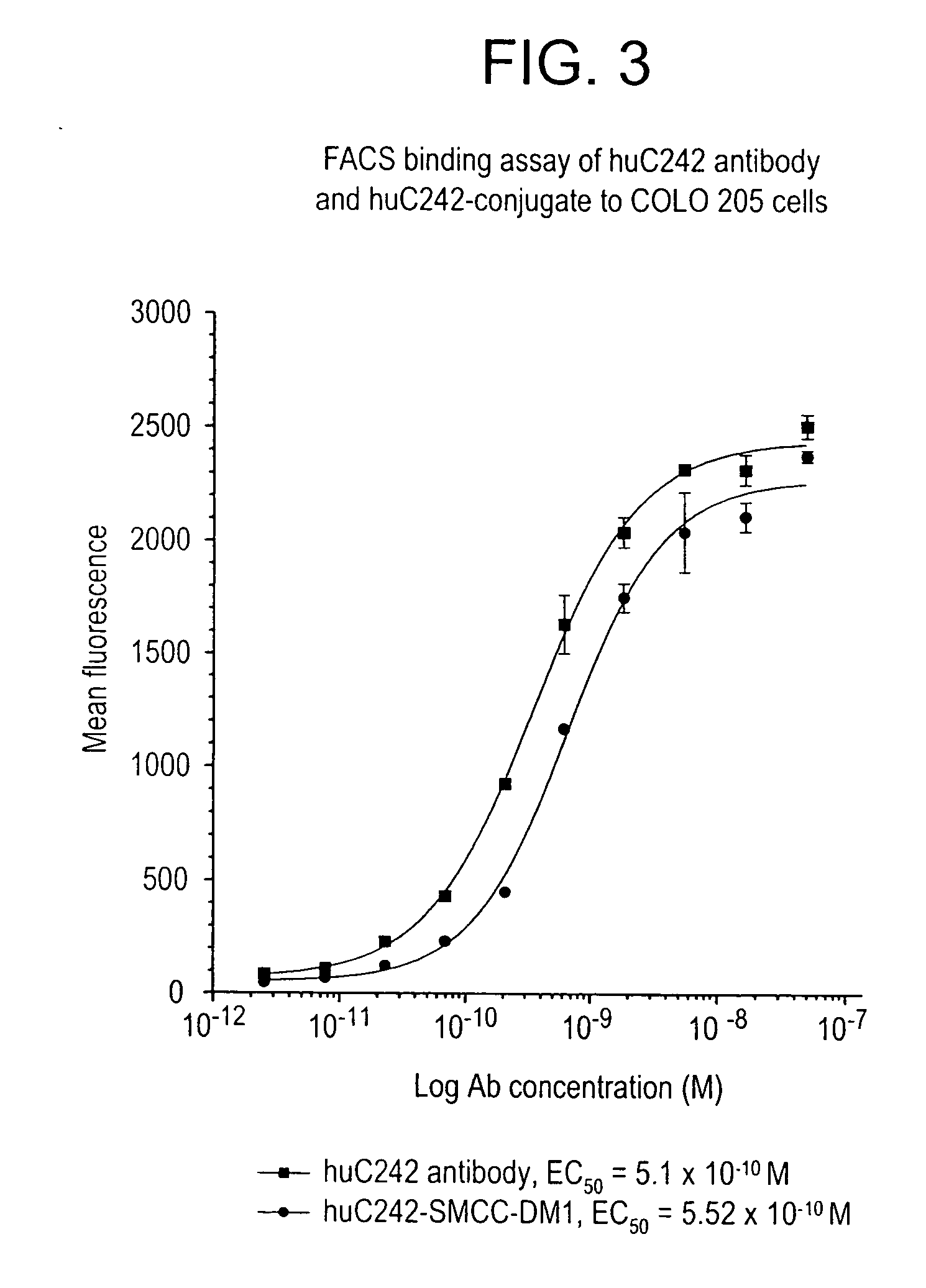
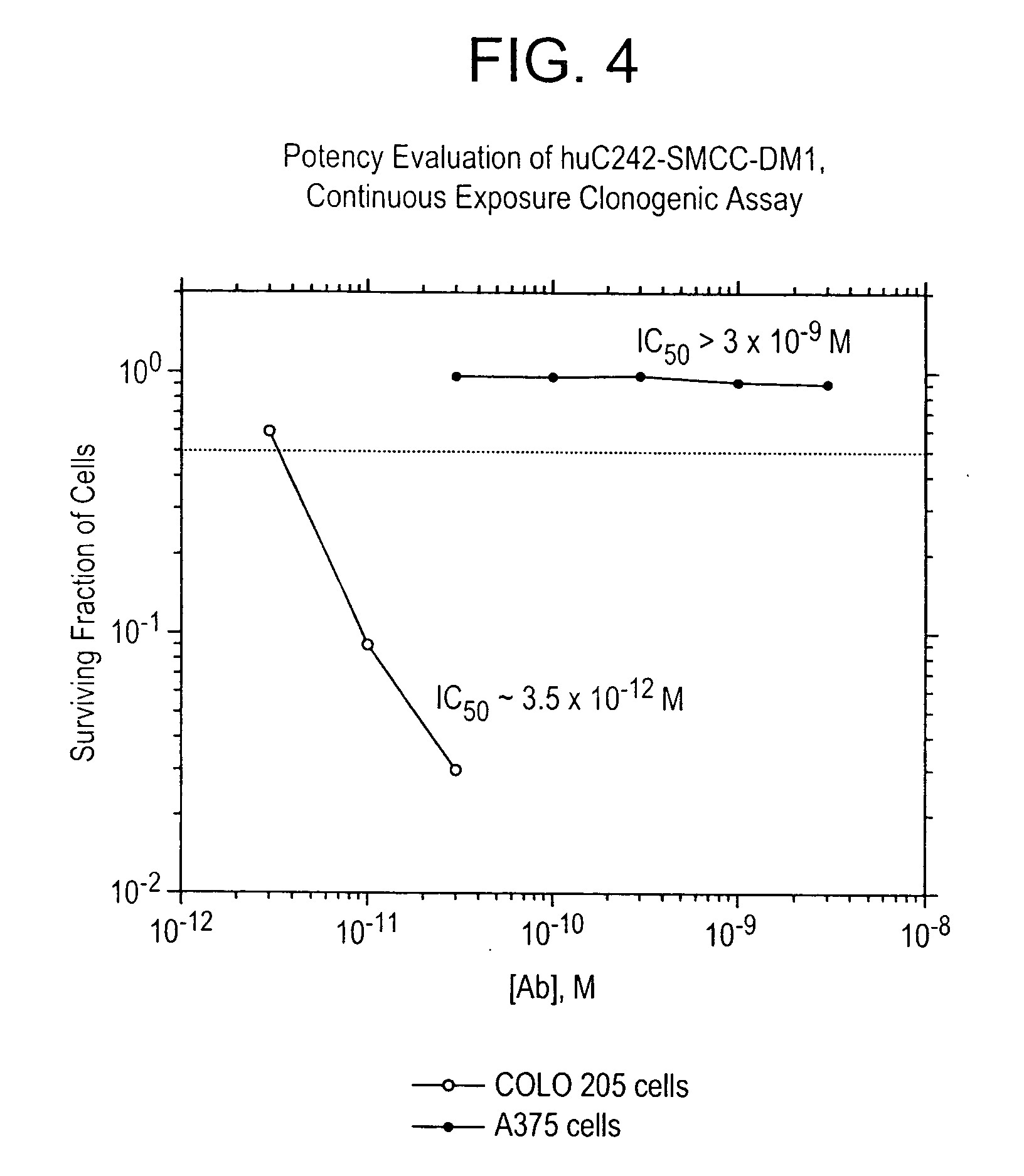
Method of targeting specific cell populations using cell-binding agent maytansinoid conjugates linked via a non-cleavable linker, said conjugates, and methods of making said conjugates
The present invention discloses a method for targeting maytansinoids to a selected cell population, the method comprising contacting a cell population or tissue suspected of containing the selected cell population with a cell-binding agent maytansinoid conjugate, wherein one or more maytansinoids is covalently linked to the cell-binding agent via a non-cleavable linker and the cell-binding agent binds to cells of the selected cell population.
Owner:IMMUNOGEN INC
Oxazolo, thiazolo and selenazolo [4,5-c]-quinolin-4-amines and analogs thereof
Thiazolo-, oxazolo- and selenazolo[4,5-c]quinolin-4-amines and analogs thereof are described including methods of manufacture and the use of novel intermediates. The compounds are immunomodulators and induce cytokine biosynthesis, including interferon and / or tumor biosynthesis, necrosis factor, and inhibit the T-helper-type 2 immune response. The compounds are further useful in the treatment of viral and neoplastic diseases.
Owner:3M INNOVATIVE PROPERTIES CO
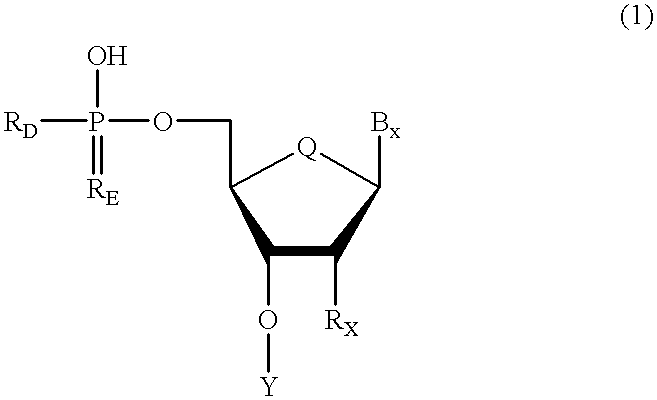
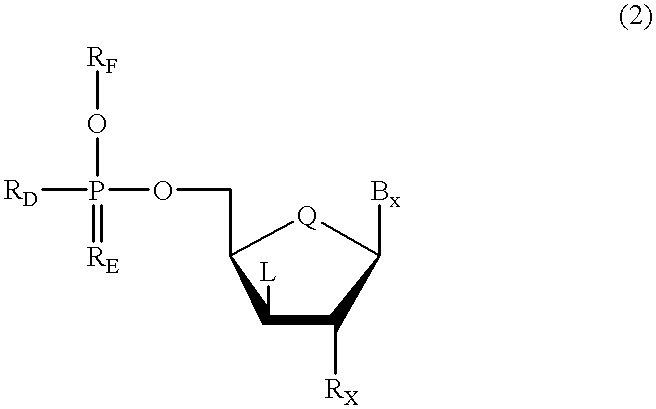
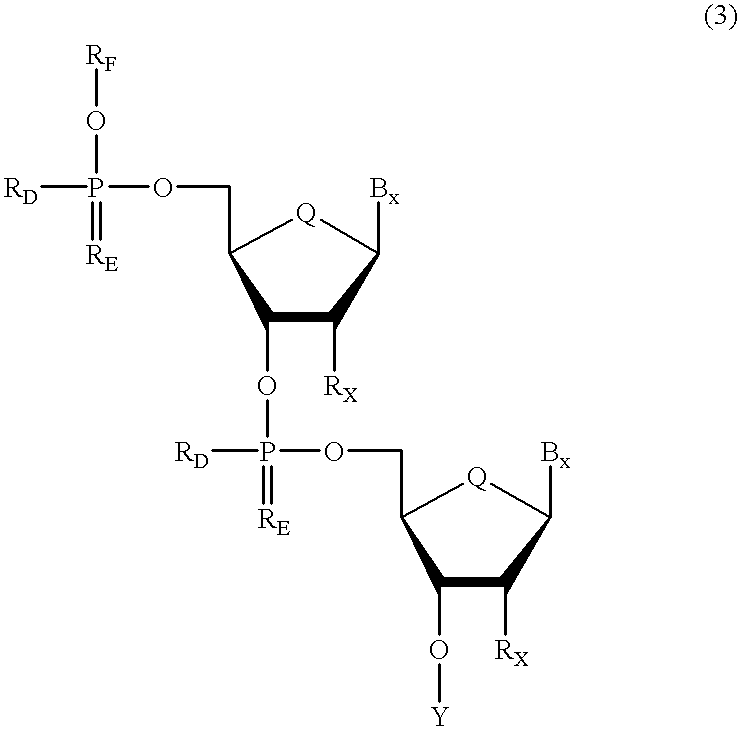
Oligonucleotides having chiral phosphorus linkages
InactiveUS6239265B1Improved pharmacokinetic propertiesImprove propertiesPeptide/protein ingredientsGenetic material ingredientsSugar moietyPhosphoramidate
Sequence-specific oligonucleotides are provided having substantially pure chiral Sp phosphorothioate, chiral Rp phosphorothioate, chiral Sp alkylphosphonate, chiral Rp alkylphosphonate, chiral Sp phosphoamidate, chiral Rp phosphoamidate, chiral Sp phosphotriester, and chiral Rp phosphotriester linkages. The novel oligonucleotides are prepared via a stereospecific SN2 nucleophilic attack of a phosphodiester, phosphorothioate, phosphoramidate, phosphotriester or alkylphosphonate anion on the 3' position of a xylonucleotide. The reaction proceeds via inversion at the 3' position of the xylo reactant species, resulting in the incorporation of phosphodiester, phosphorothioate, phosphoramidate, phosphotriester or alkylphosphonate linked ribofuranosyl sugar moieties into the oligonucleotide.
Owner:IONIS PHARMA INC
PD-1 Antibodies and PD-L1 Antibodies and Uses Thereof
ActiveUS20120039906A1Reduced activityStrong cytotoxicityAntibacterial agentsAnimal cellsPD-L1Antibody
Owner:INST JEAN PAOLI & IRENE CALMETTES +2
Chimeric receptor genes and cells transformed therewith
ActiveUS7741465B1Limit acquisitionMicroorganismsGenetic material ingredientsAntibody typesLymphocyte
Chimeric receptor genes suitable for endowing lymphocytes with antibody-type specificity include a first gene segment encoding a single-chain Fv domain of a specific antibody and a second gene segment encoding all or part of the transmembrane and cytoplasmic domains, and optionally the extracellular domain, of an immune cell-triggering molecule. The chimeric receptor gene, when transfected to immune cells, expresses the antibody-recognition site and the immune cell-triggering moiety into one continuous chain. The transformed lymphocytes are useful in therapeutic treatment methods.
Owner:HEALTH & HUMAN SERVICES GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF +1
Identification and engineering of antibodies with variant Fc regions and methods of using same
ActiveUS20050037000A1High affinityAltered affinityAntibacterial agentsSenses disorderTherapeutic antibodyWild type
The present invention relates to molecules, particularly polypeptides, more particularly immunoglobulins (e.g., antibodies), comprising a variant Fc region, wherein said variant Fc region comprises at least one amino acid modification relative to a wild-type Fc region, which variant Fc region binds FcgammaRIIA and / or FcgammaRIIA with a greater affinity, relative to a comparable molecule comprising the wild-type Fc region. The molecules of the invention are particularly useful in preventing, treating, or ameliorating one or more symptoms associated with a disease, disorder, or infection. The molecules of the invention are particularly useful for the treatment or prevention of a disease or disorder where an enhanced efficacy of effector cell function (e.g., ADCC) mediated by FcgammaR is desired, e.g., cancer, infectious disease, and in enhancing the therapeutic efficacy of therapeutic antibodies the effect of which is mediated by ADCC.
Owner:MARCOGENICS INC +1
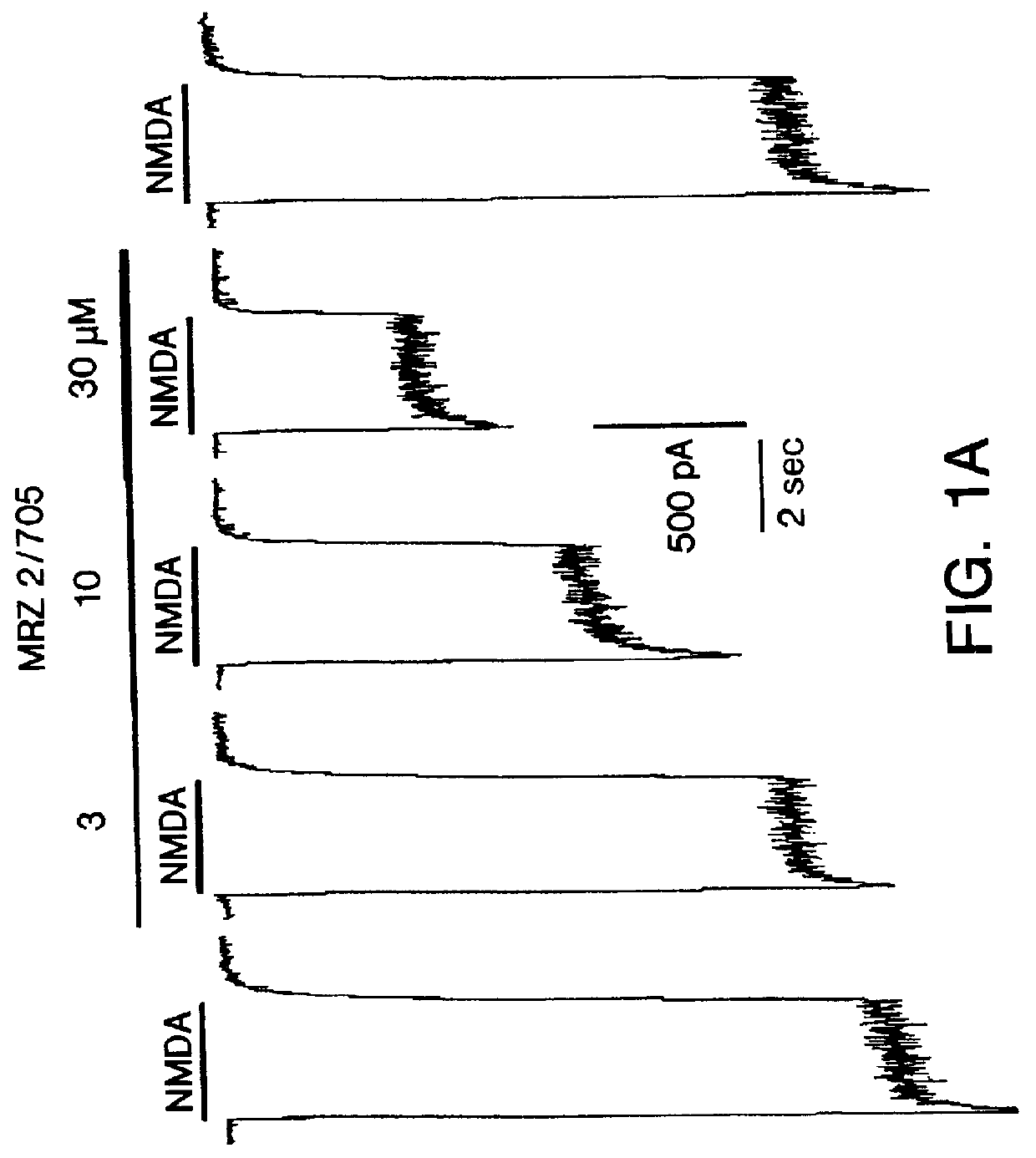
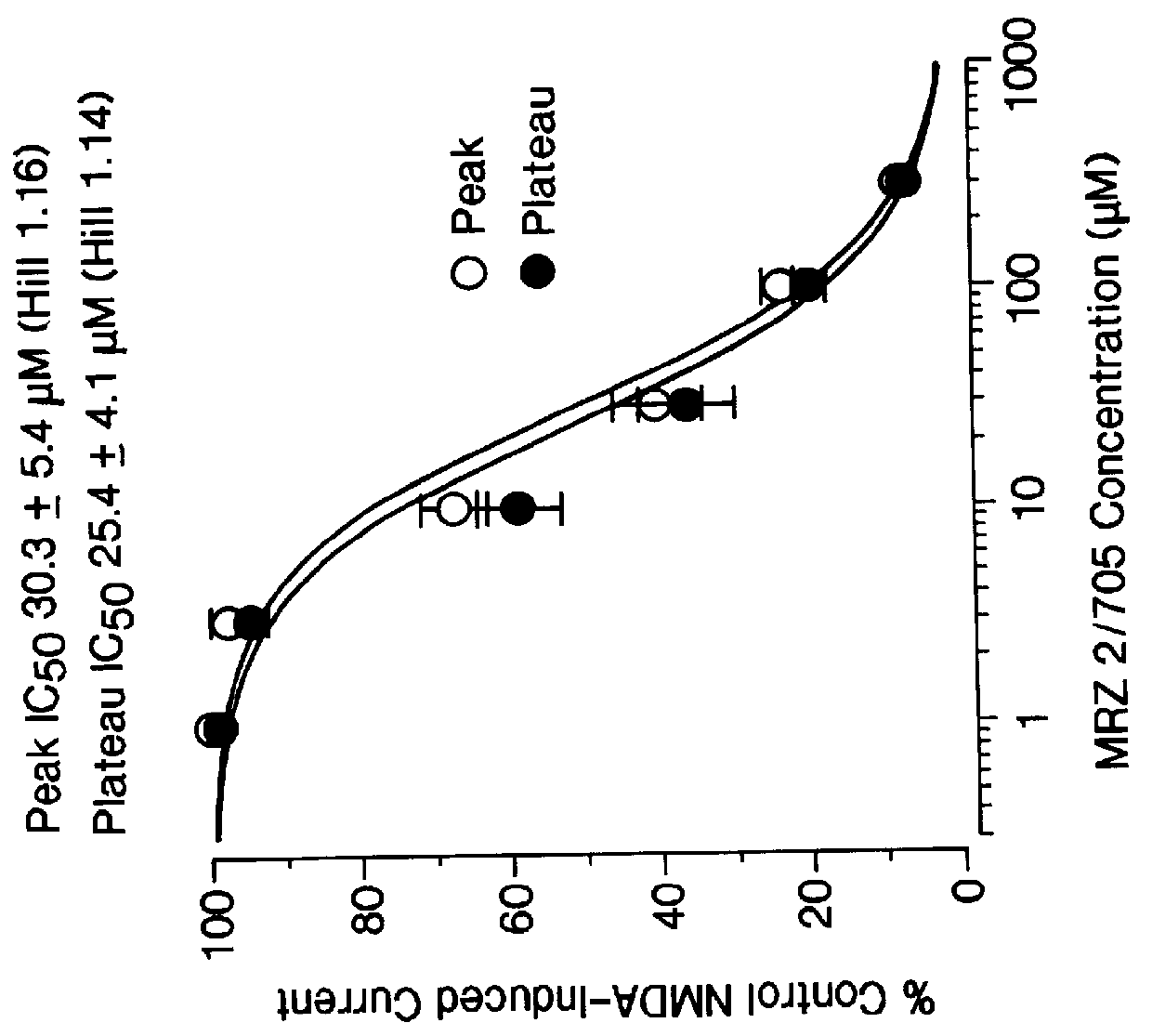
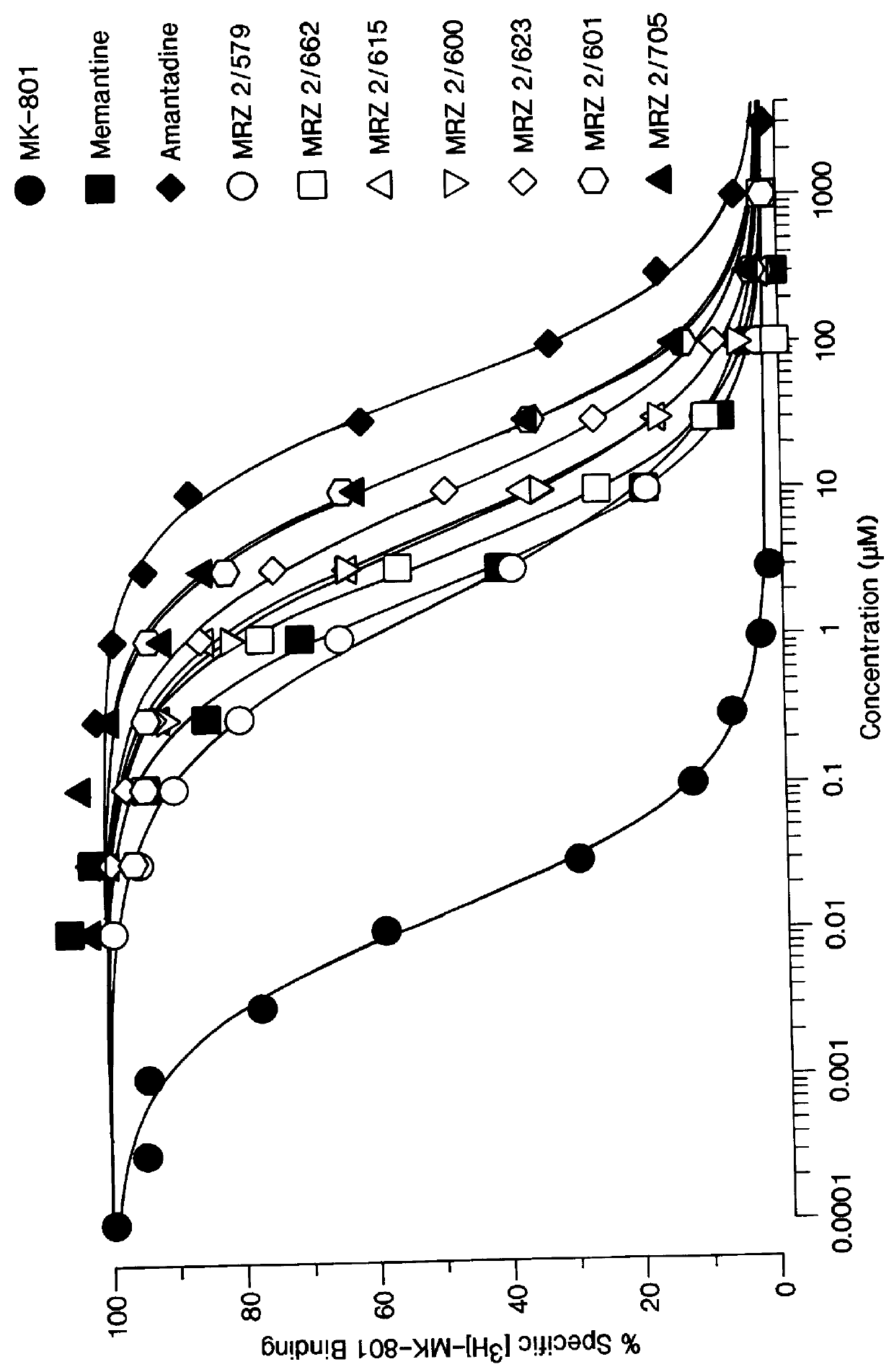
1-Amino-alkylcyclohexane NMDA receptor antagonists
Certain 1-aminoalkylcyclohexanes are systemically-active uncompetitive NMDA receptor antagonists having rapid blocking / unblocking kinetics and strong voltage-dependency and are therefore useful in the alleviation of conditions resulting from disturbances of glutamatergic transmission giving them a wide range of utility in the treatment of CNS disorders involving the same, as well as in non-NMDA indications, due to their immunomodulatory, antimalarial, anti-Borna virus, and anti-Hepatitis C activities and utilities. Pharmaceutical compositions thereof and a method-of-treating conditions which are alleviated by the employment of an NMDA receptor antagonist, as well as the aforementioned non-NMDA indications, and a method for the preparation of the active 1-aminoalkylcyclohexane compounds involved.
Owner:MERZ PHARMA GMBH & CO KGAA
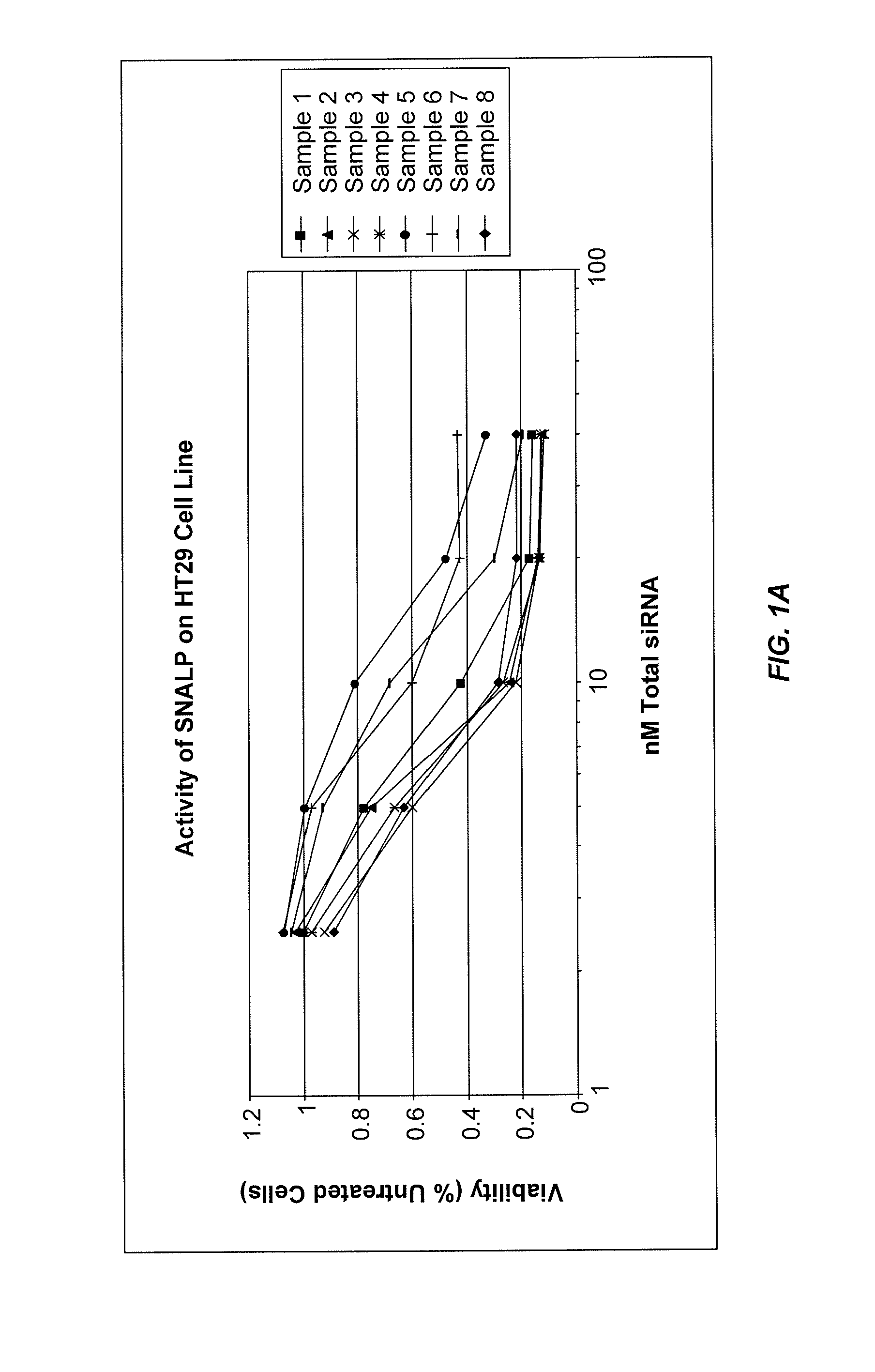
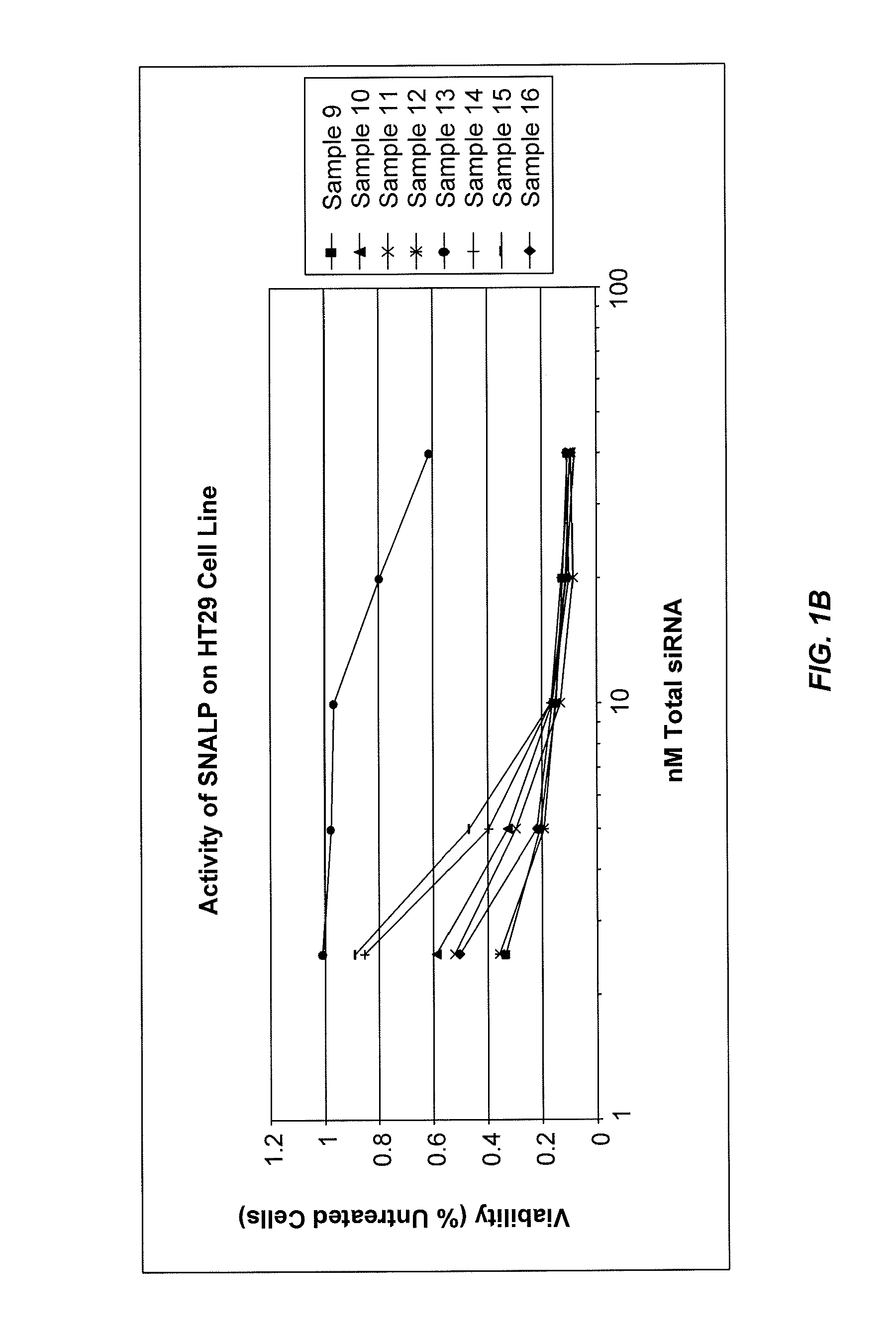
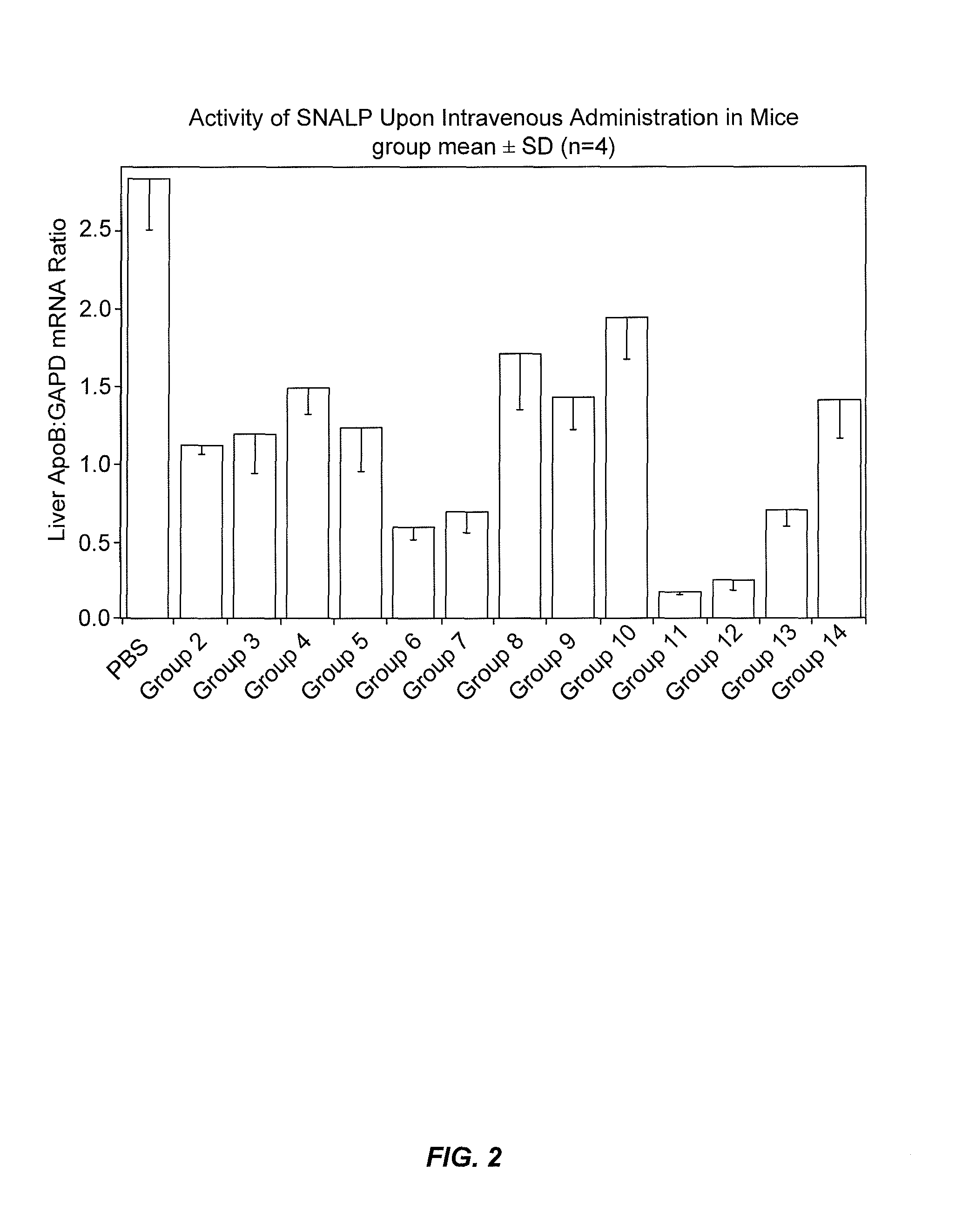
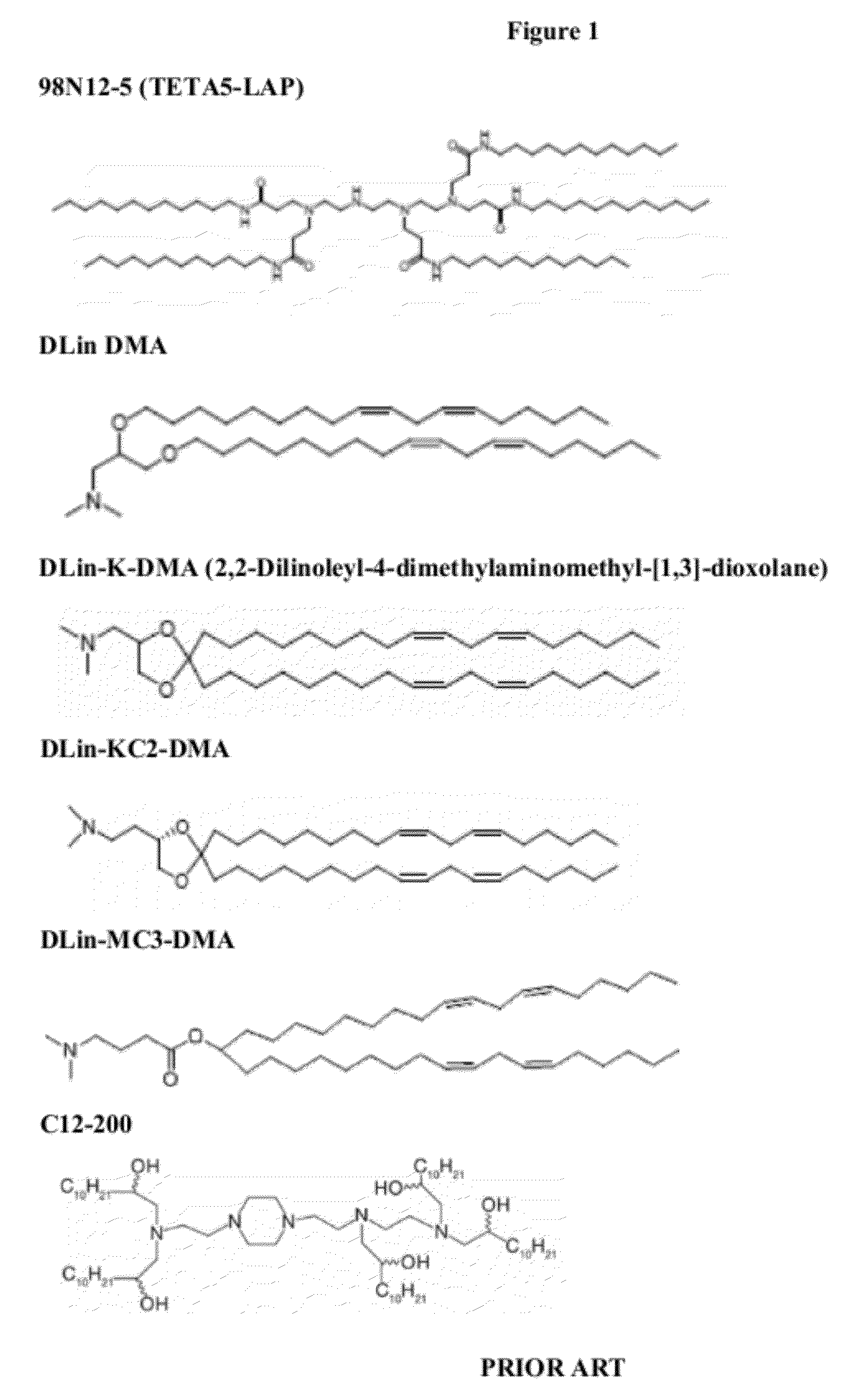
Lipid formulations for nucleic acid delivery
The present invention provides novel, stable lipid particles comprising one or more active agents or therapeutic agents, methods of making the lipid particles, and methods of delivering and / or administering the lipid particles. More particularly, the present invention provides stable nucleic acid-lipid particles (SNALP) comprising a nucleic acid (such as one or more interfering RNA), methods of making the SNALP, and methods of delivering and / or administering the SNALP.
Owner:ARBUTUS BIOPHARMA CORPORAT ION
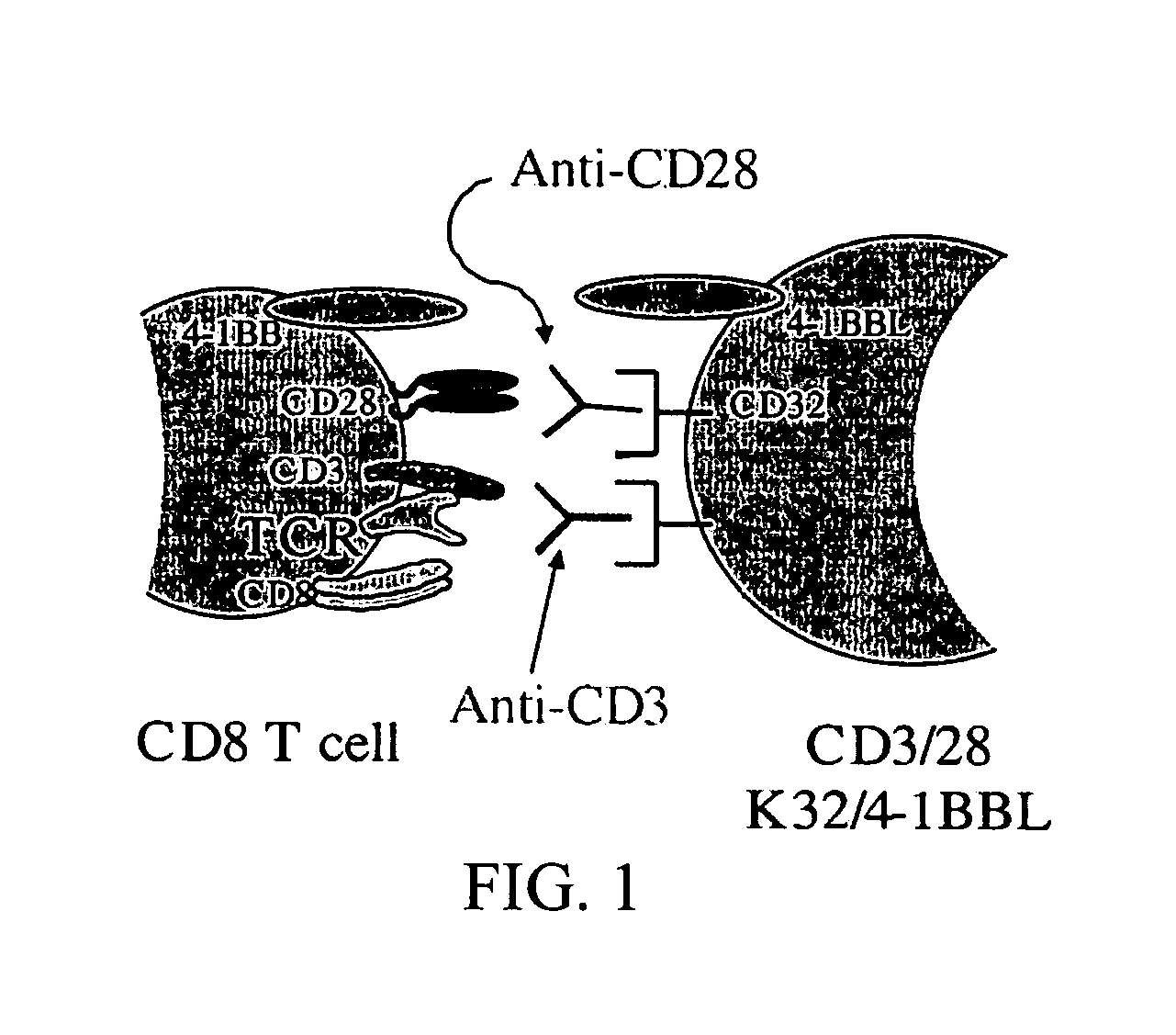
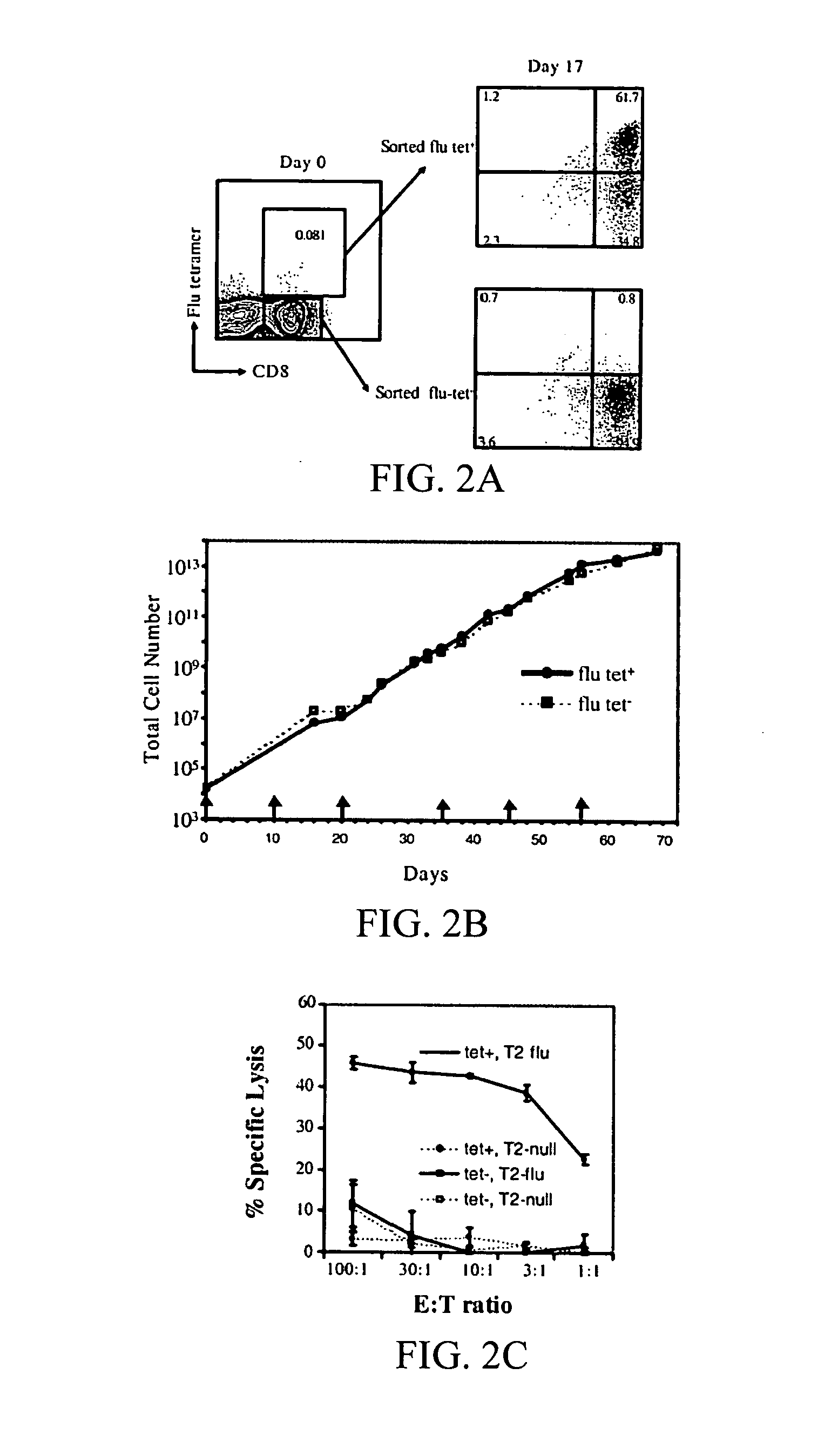
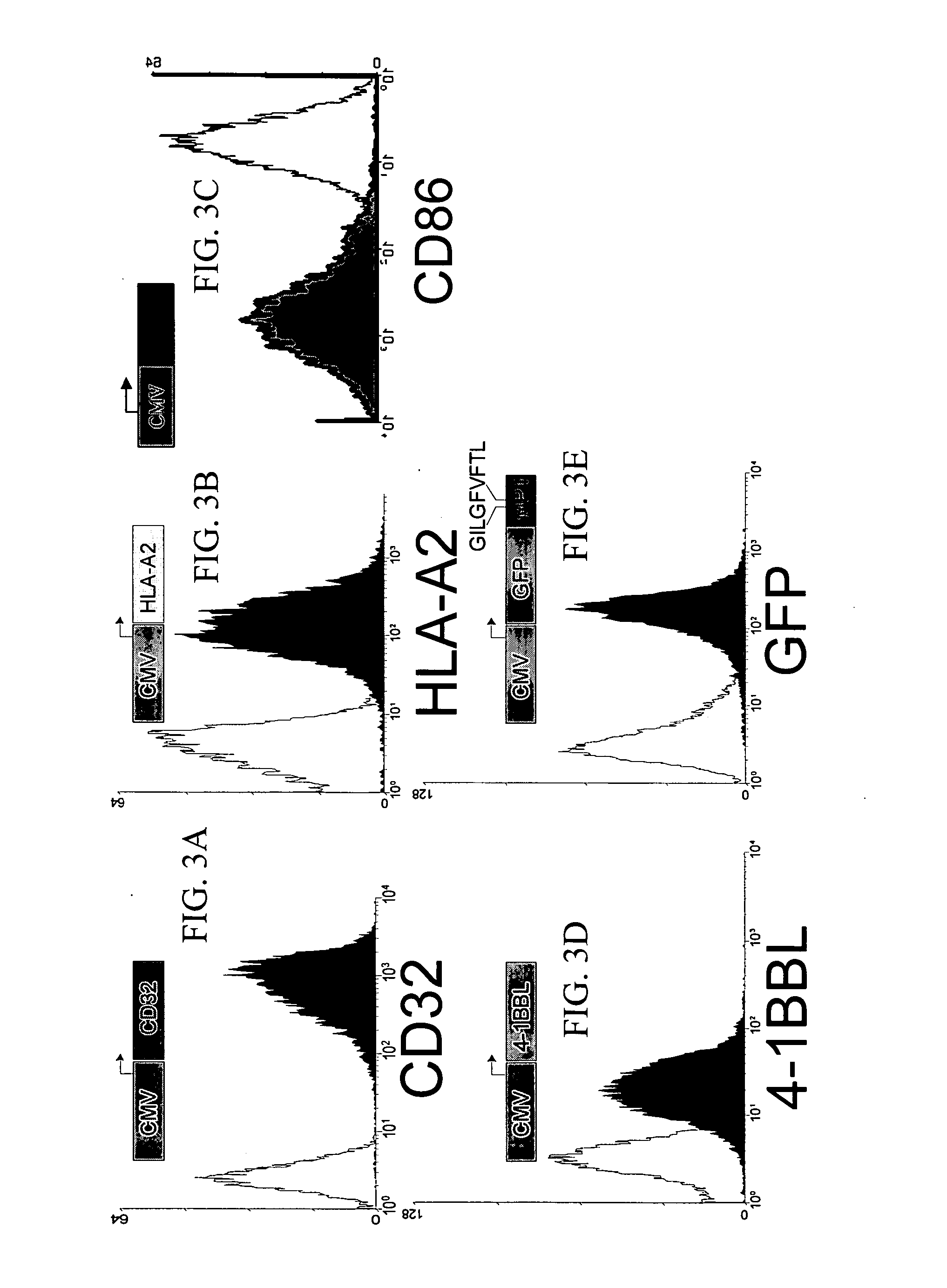
Novel artificial antigen presenting cells and uses therefor
The invention relates to novel artificial antigen presenting cells (aAPCs). The aAPC comprises at least one stimulatory ligand and at least one co-stimulatory ligand where the ligands each specifically bind with a cognate molecule on a T cell of interest, thereby mediating expansion of the T cell. The aAPC of the invention can further comprise additional molecules useful for expanding a T cell of interest. The aAPC of the invention can be used as an “off the shelf” APC that can be readily designed to expand a T cell of interest. Also, the aAPC of the invention can be used identify the stimulatory, co-stimulatory, and any other factors that mediate growth and expansion of a T cell of interest. Thus, the present invention provides powerful tools for development of novel therapeutics where activation and expansion of a T cell can provide a benefit.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Identification and engineering of antibodies with variant Fc regions and methods of using same
ActiveUS7355008B2Function increaseGood curative effectAntibacterial agentsSenses disorderTherapeutic antibodyEffector cell
Owner:MARCOGENICS INC +1
Delivery and formulation of engineered nucleic acids
ActiveUS20120251618A1Improve the level ofIncrease in level of polypeptideNervous disorderAntipyreticNucleic acidProtein expression
Provided are formulations, compositions and methods for delivering biological moieties such as modified nucleic acids into cells to modulate protein expression. Such compositions and methods include the delivery of biological moieties, and are useful for production of proteins.
Owner:MODERNATX INC
Isoindole-imide compounds, compositions, and uses thereof
The invention relates to isoindole-imide compounds and pharmaceutically acceptable salts, hydrates, solvates, clathrates, enantiomers, diastereomers, racemates, or mixtures of stereoisomers thereof, pharmaceutical compositions comprising these isoindole-imide compounds, and methods for reducing the level of cytokines and their precursors in mammals. In particular, the invention pertains to isoindole-imide compounds that are potent inhibitors of the production of TNF-alpha in mammals. The isoindole-imides described herein are useful for treating or preventing diseases or disorders in mammals, for example, cancers, such as solid tumors and blood-born tumors; heart disease, such as congestive heart failure; osteoporosis; and genetic, inflammatory; allergic; and autoimmune diseases.
Owner:CELGENE CORP
Amphiphilic drug-oligomer conjugates with hydroyzable lipophile components and methods for making and using the same
InactiveUS6309633B1Reduce deliveryExtended durationAntibacterial agentsOrganic active ingredientsTherapeutic proteinCholesterol
The invention provides a drug-oligomer conjugate having the following general formula:wherein D is a therapeutic drug moiety; H and H' are each a hydrophilic moiety, independently selected from the group consisting of straight or branched PEG polymers having from 2 to 130 PEG subunits, and sugars; L is a lipophilic moiety selected from the group consisting of alkyl groups having 2-26 carbon atoms, cholesterol, adamantane and fatty acids; o is a number from 1 to the maximum number of covalent bonding sites on H; m+n+p together have a value of at least one and not exceeding the total number of covalent bonding sites on D for the -H', -L and -H-L substituents; the H-L bond(s) are hydrolyzable and the D-L' bond(s), when present, are hydrolyzable; the conjugate being further characterized by one of the following: (i) m is 0 and p is at least 1; (ii) n is 0 and p is at least 1; (iii) m and n are each 0 and p is at least 1; (iv) p is 0 and m and n are each at least 1. The therapeutic drug moiety is preferably a therapeutic protein or peptide, preferably insulin or a functional equivalent thereof.
Owner:BIOCON LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com