Patents
Literature
62 results about "Human sequence" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
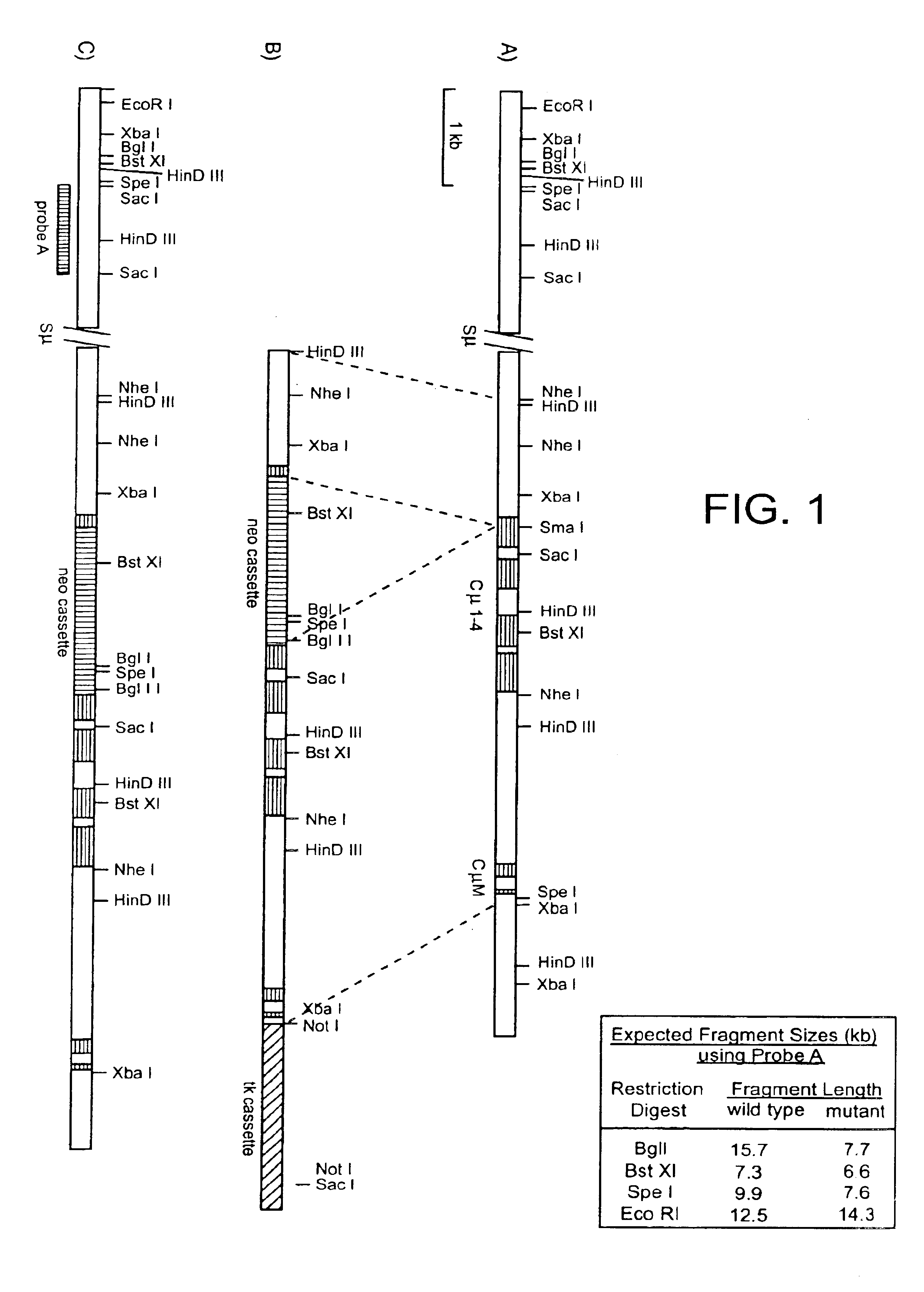
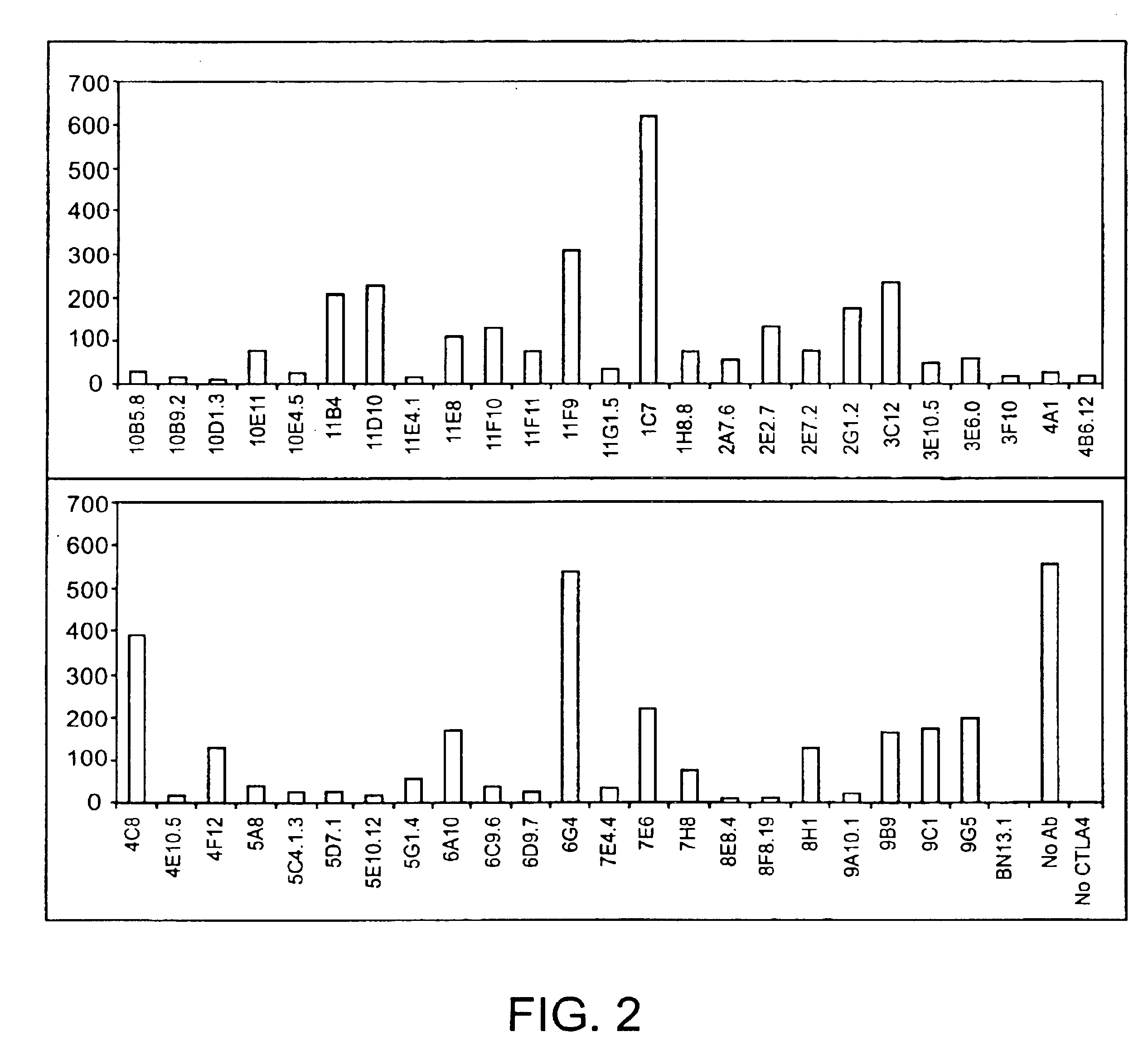
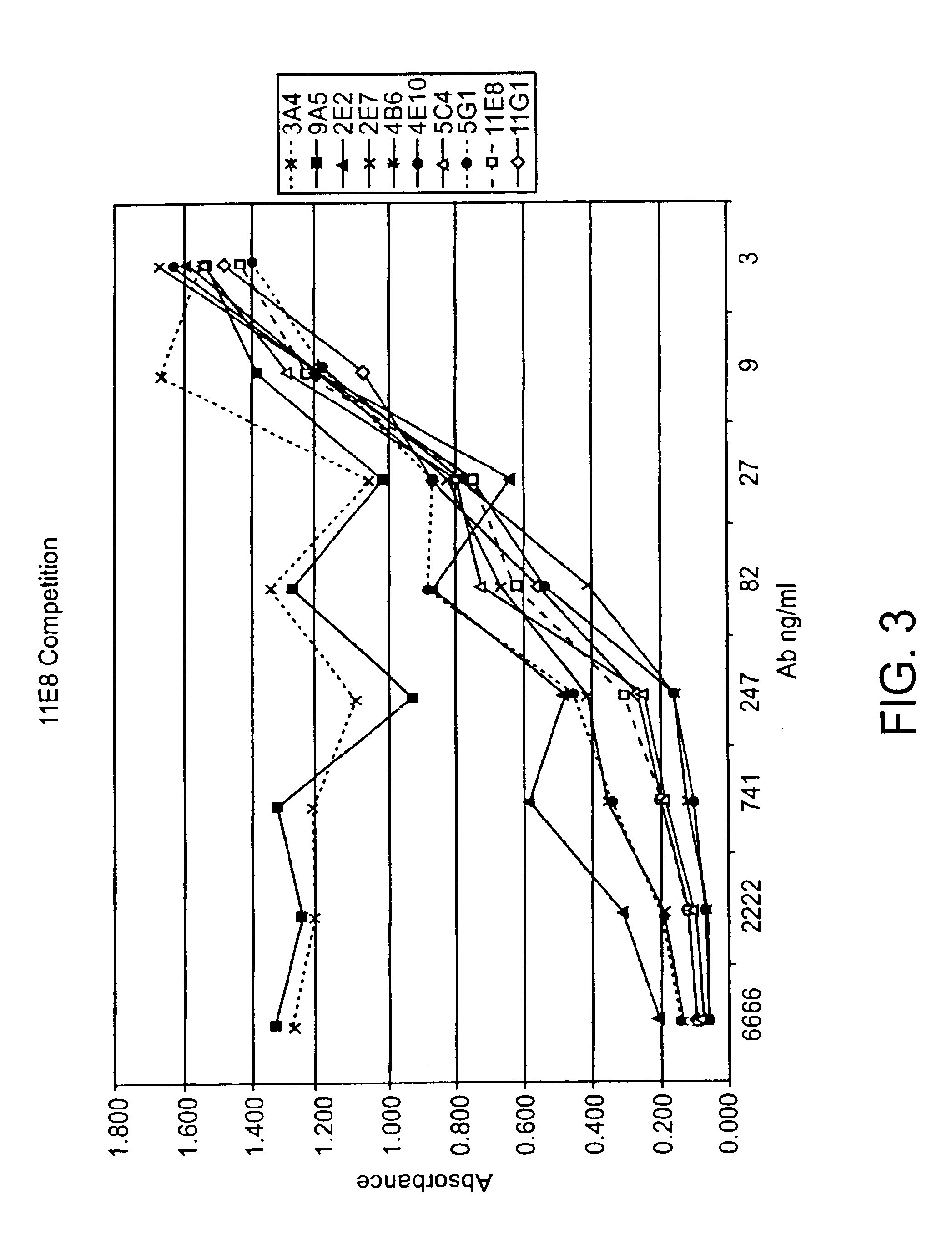
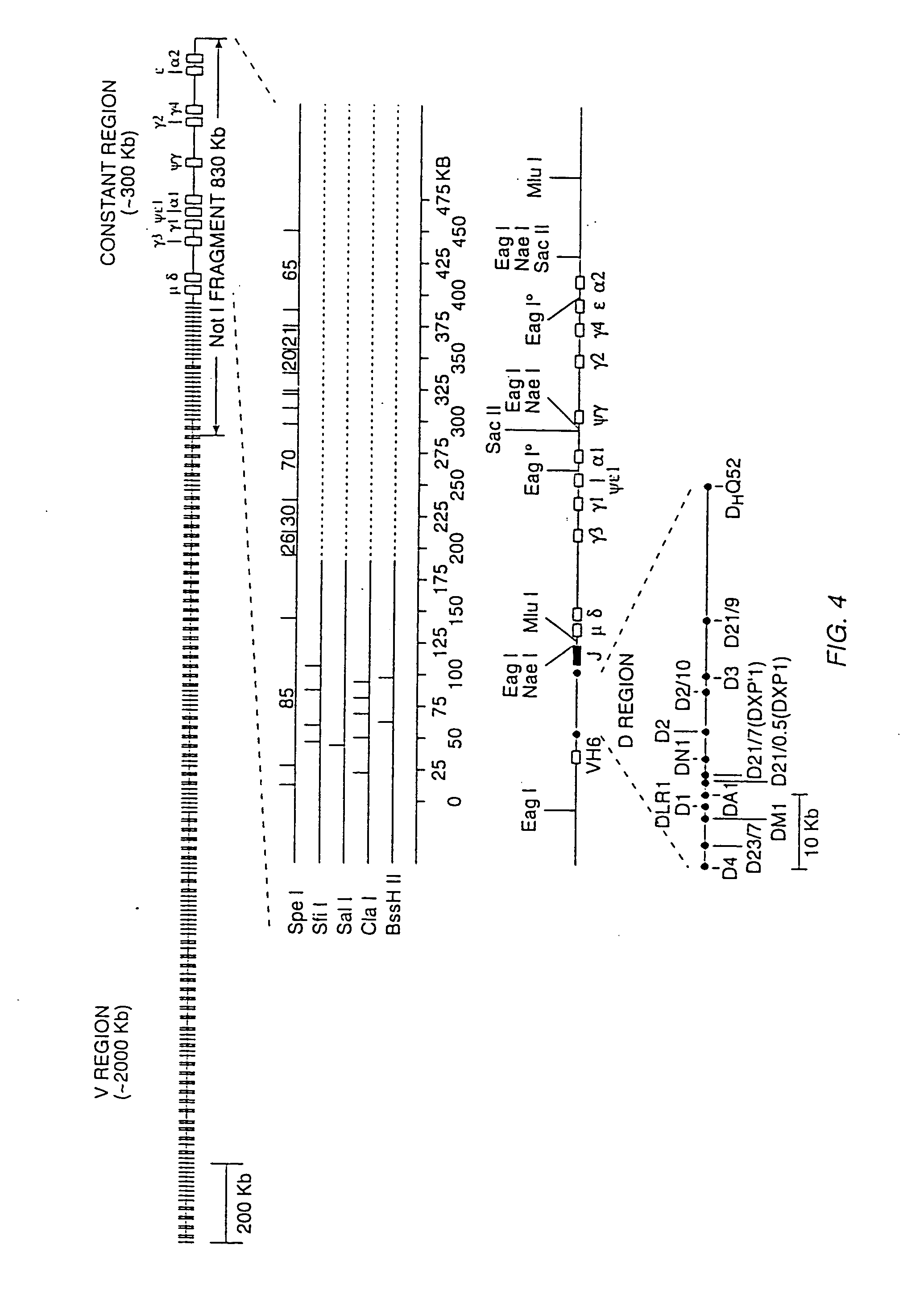
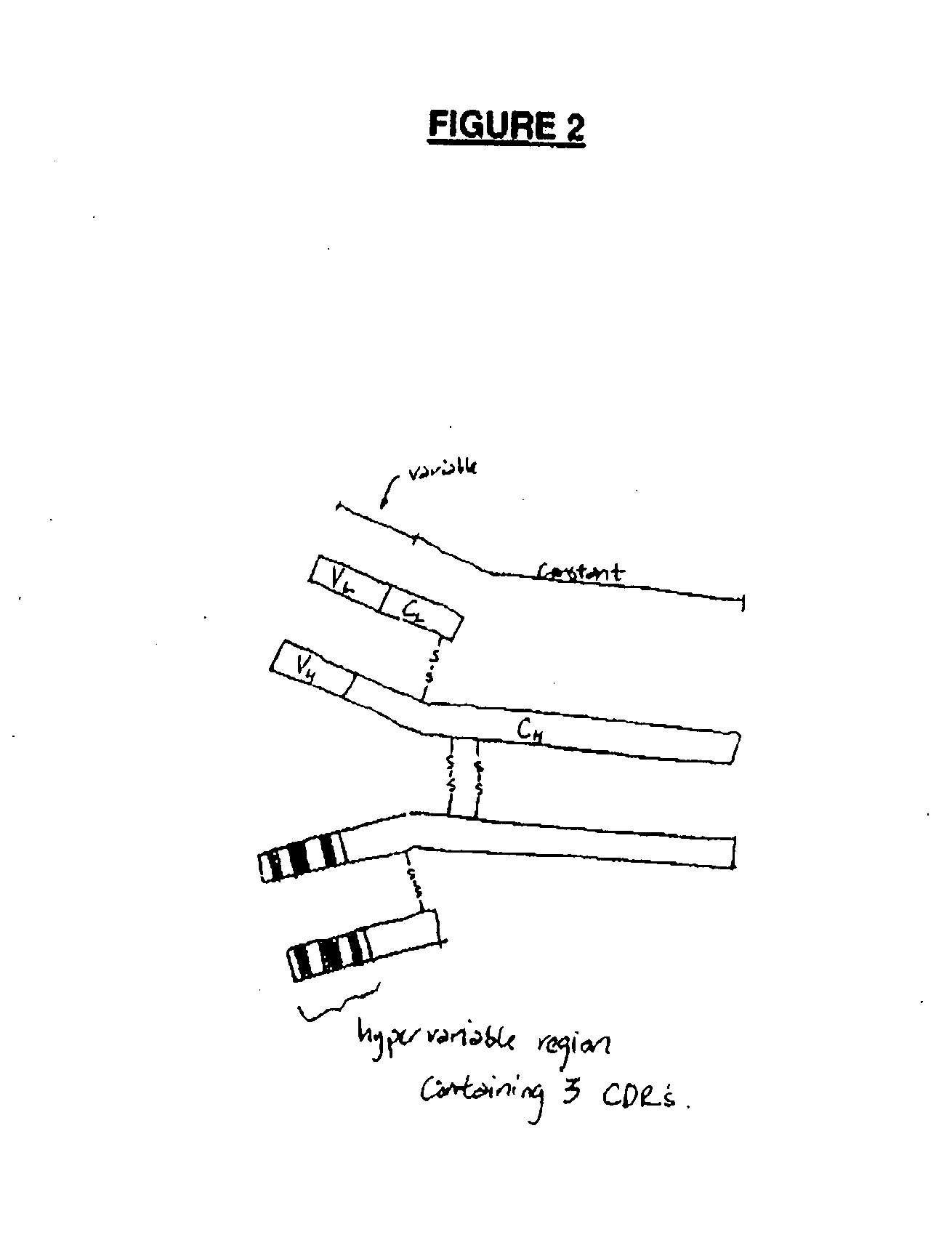
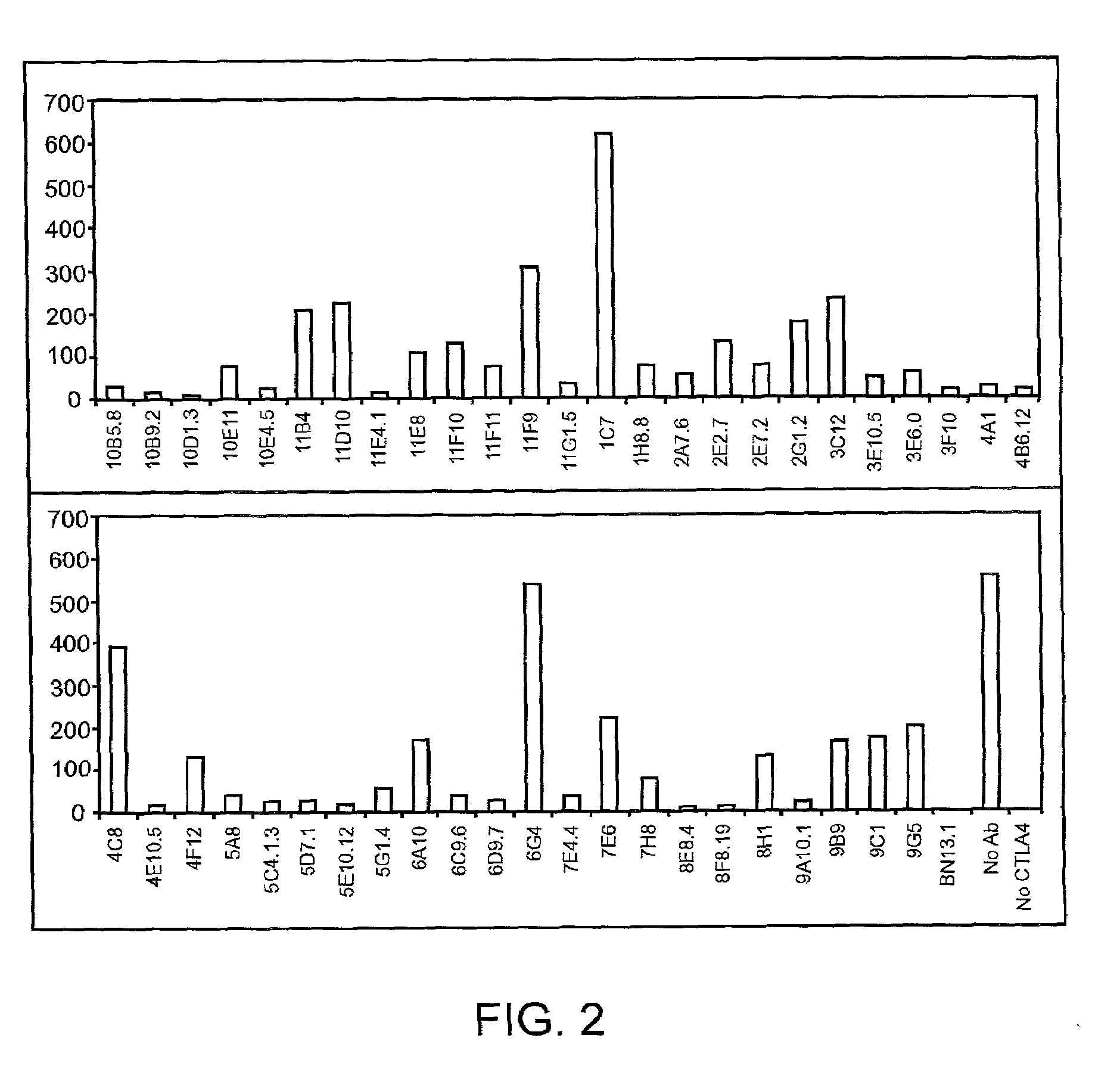
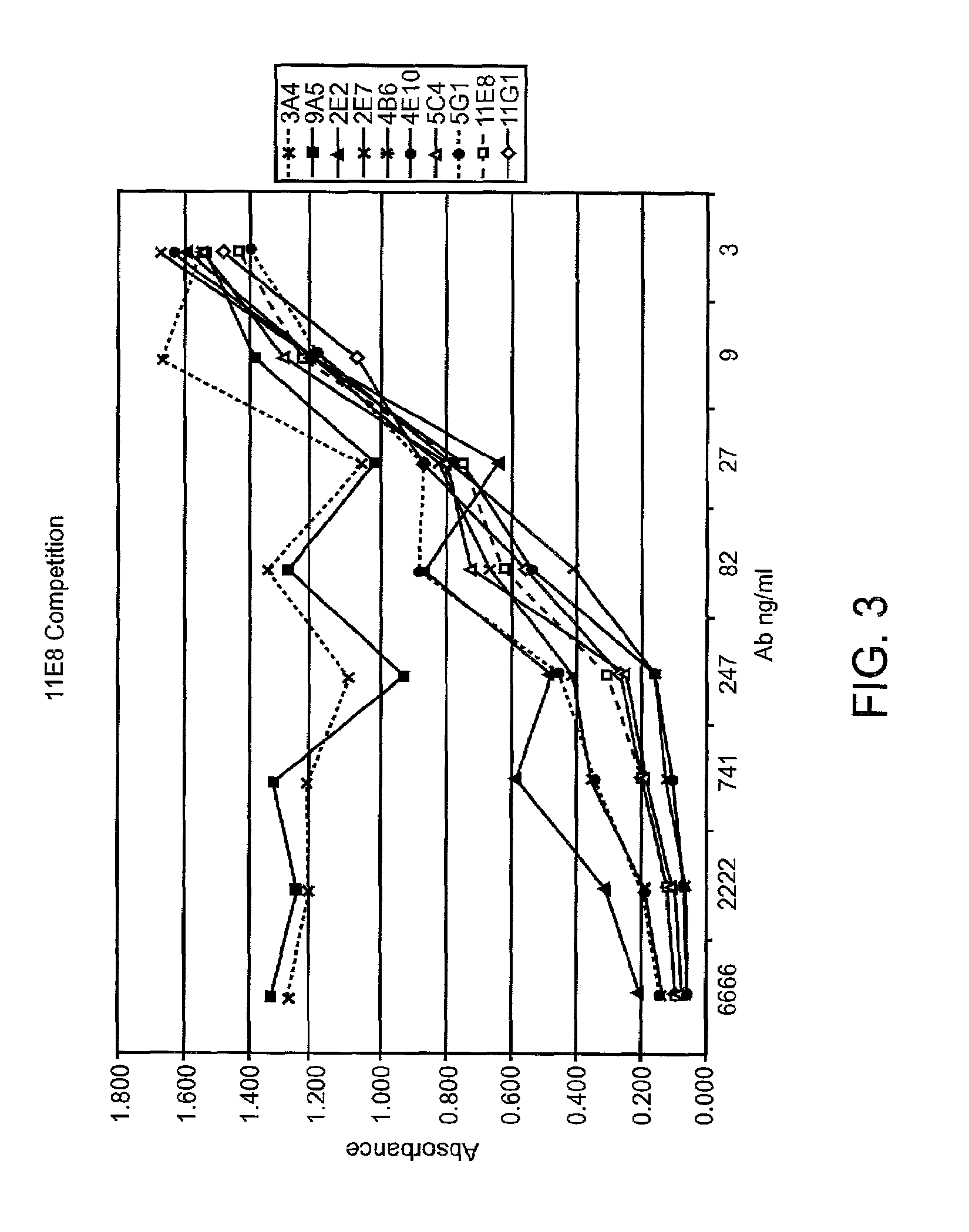
Human CTLA-4 antibodies
The present invention provides human sequence antibodies against CTLA-4 and methods of treating human diseases, infections and other conditions using these antibodies.
Owner:ER SQUIBB & SONS INC
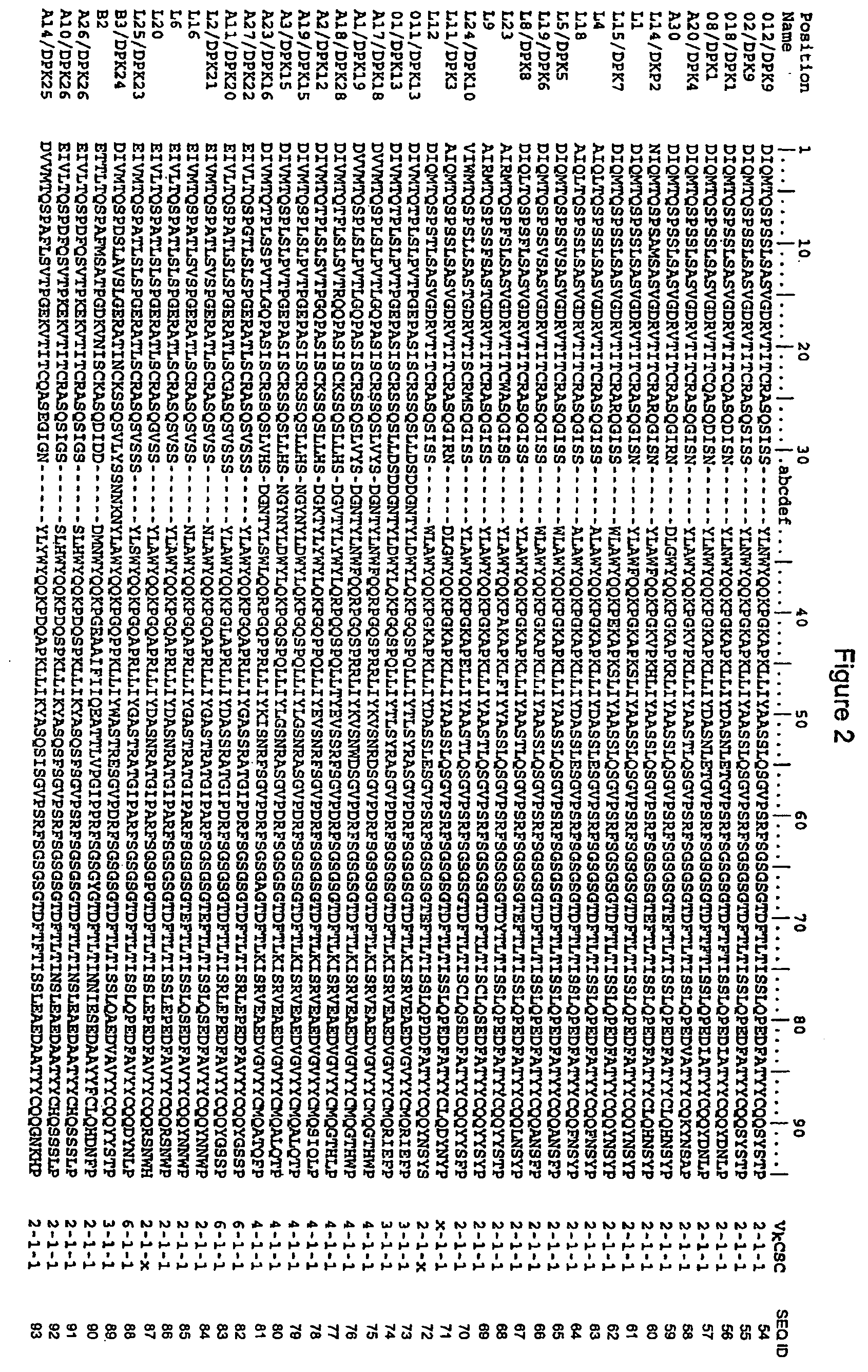
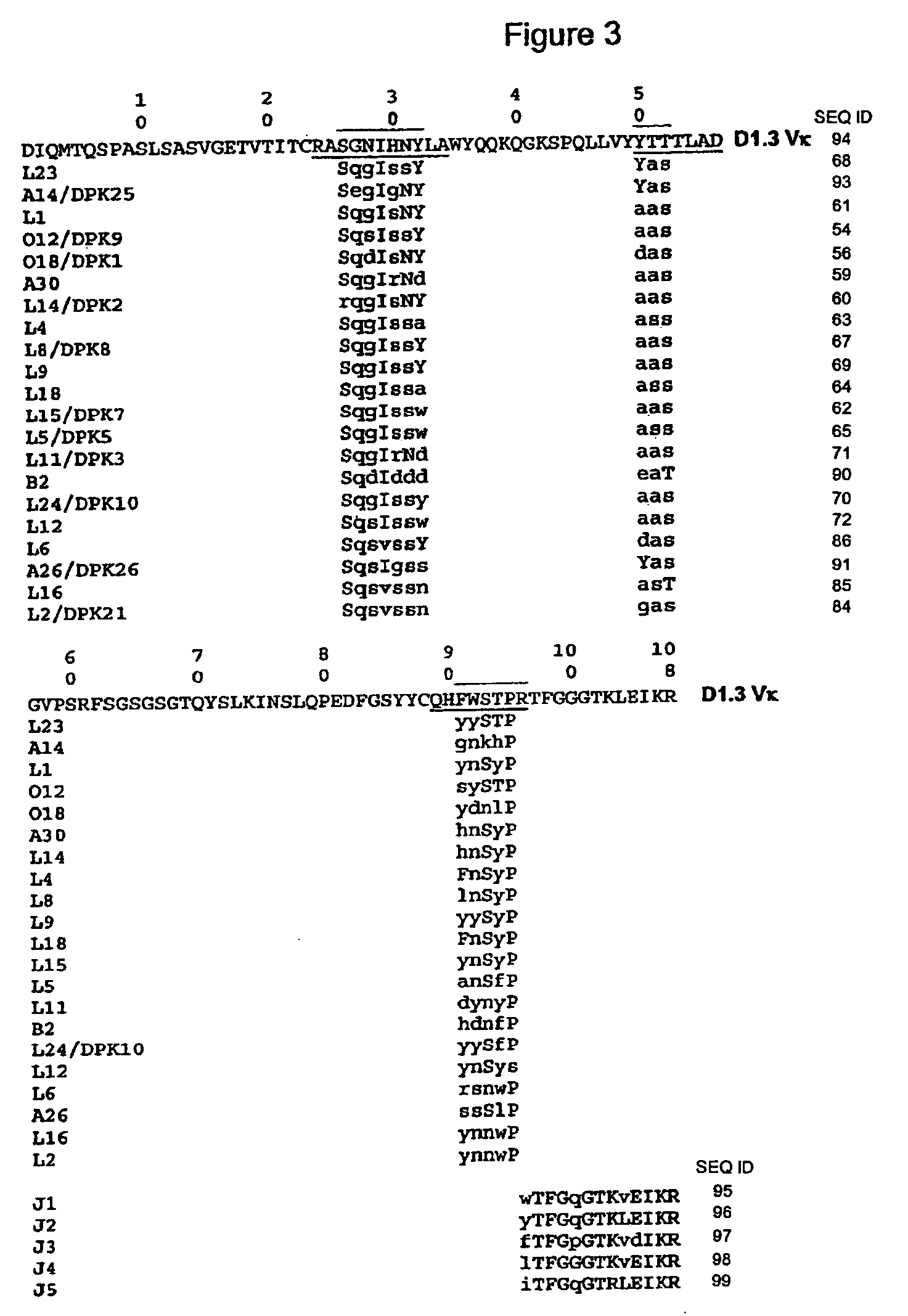
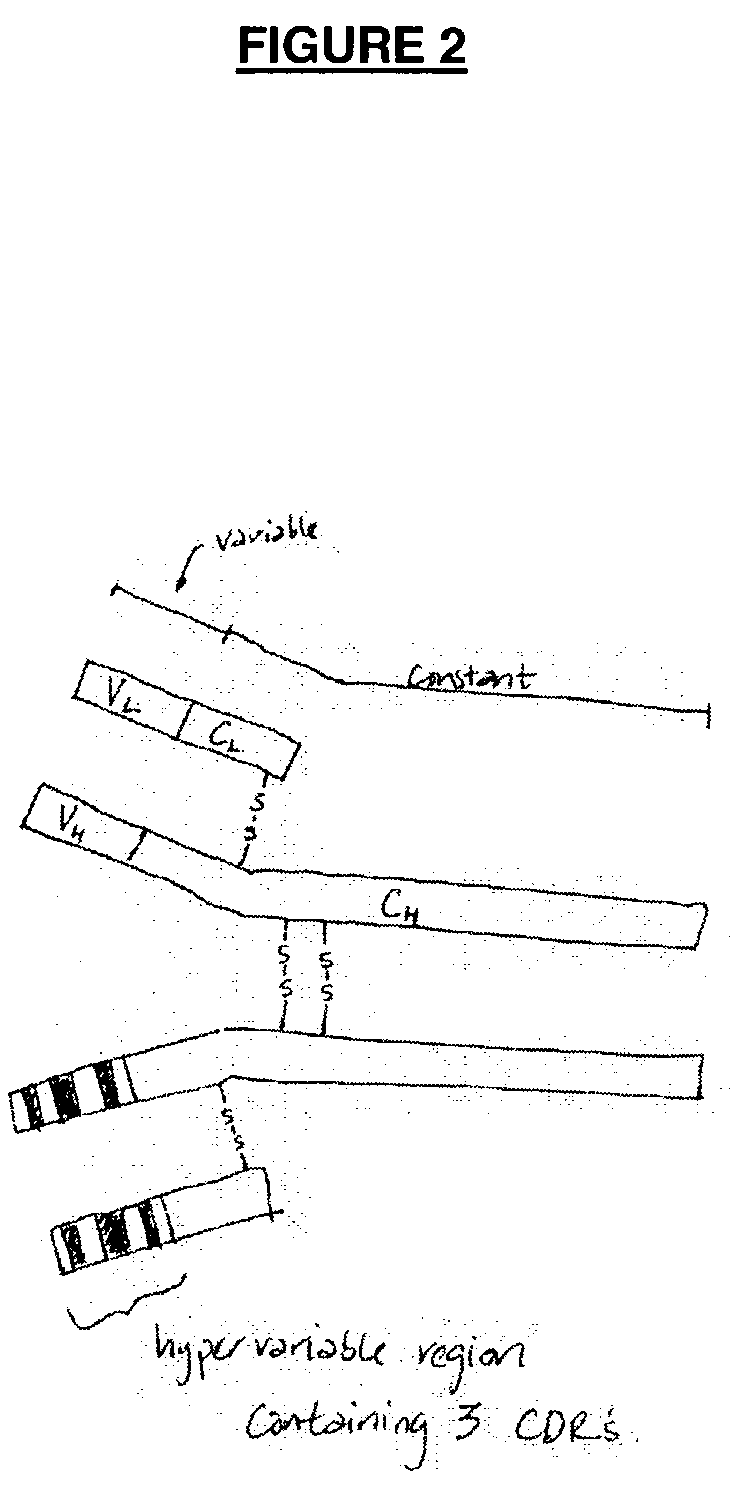
Super humanized antibodies
InactiveUS6881557B2Antibody mimetics/scaffoldsAnalogue computers for chemical processesHuman sequenceHumanized antibody
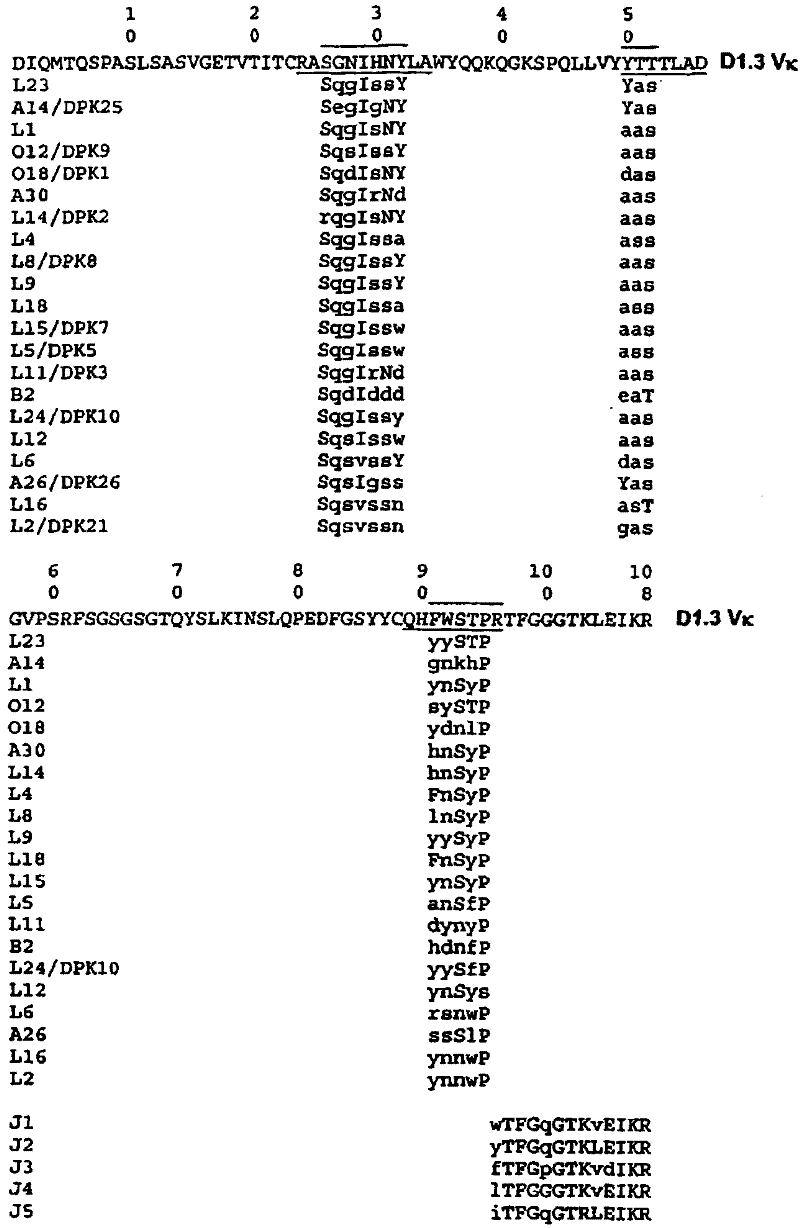
Disclosed herein are methods for humanizing antibodies based on selecting variable region framework sequences from human antibody genes by comparing canonical CDR structure types for CDR sequences of the variable region of a non-human antibody to canonical CDR structure types for corresponding CDRs from a library of human antibody sequences, preferably germline antibody gene segments. Human antibody variable regions having similar canonical CDR structure types to the non-human CDRs form a subset of member human antibody sequences from which to select human framework sequences. The subset members may be further ranked by amino acid similarity between the human and the non-human CDR sequences. Top ranking human sequences are selected to provide the framework sequences for constructing a chimeric antibody that functionally replaces human CDR sequences with the non-human CDR counterparts using the selected subset member human frameworks, thereby providing a humanized antibody of high affinity and low immunogenicity without need for comparing framework sequences between the non-human and human antibodies. Chimeric antibodies made according to the method are also disclosed.
Owner:ARROWSMITH TECH
Transgenic non-human animals for producing chimeric antibodies
InactiveUS20060015957A1Inhibit expressionEasy to switchImmunoglobulinsGenetic engineeringAntigenHuman animal
The invention relates to transgenic non-human animals capable of producing heterologous antibodies and methods for producing human sequence antibodies which bind to human antigens with substantial affinity.
Owner:GENPHARM INT INC
Human CTLA-4 antibodies and their uses
InactiveUS20050201994A1Augmenting and suppressing and prolonging immune responseBiocideAntibody mimetics/scaffoldsHuman sequenceCTLA4 Protein
The present invention provides novel human sequence antibodies against human CTLA-4 and methods of treating human diseases, infections and other conditions using these antibodies.
Owner:MEDAREX INC
Transgenic non-human animals capable of producing heterologous antibodies
The invention relates to transgenic non-human animals capable of producing heterologous antibodies and methods for producing human sequence antibodies which bind to human antigens with substantial affinity.
Owner:GENPHARM INT INC
Transgenic non-human animals for producing heterologous and chimeric antibodies
The invention relates to transgenic non-human animals capable of producing heterologous antibodies and methods for producing human sequence antibodies which bind to human antigens with substantial affinity.
Owner:GENPHARM INT INC
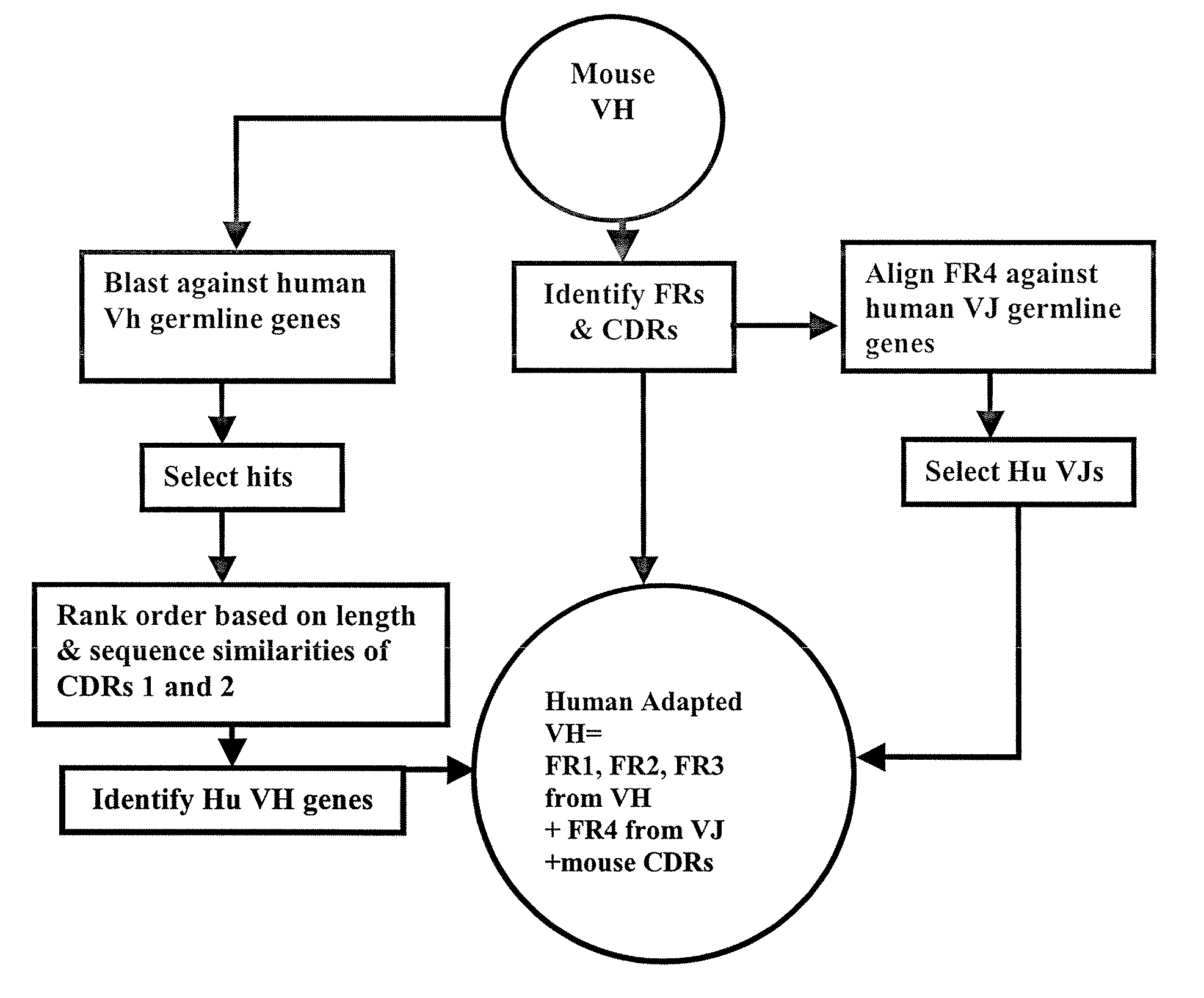
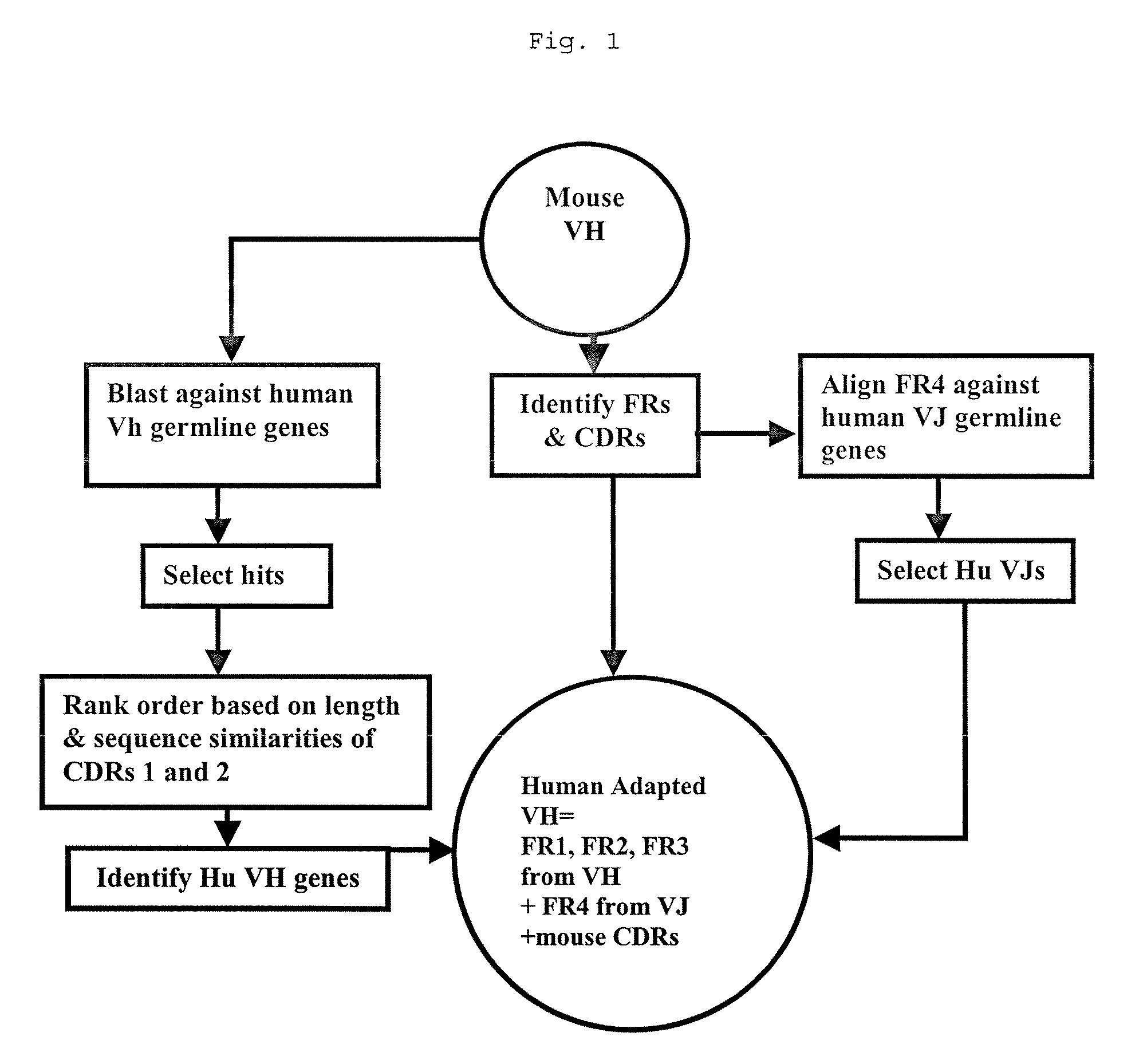
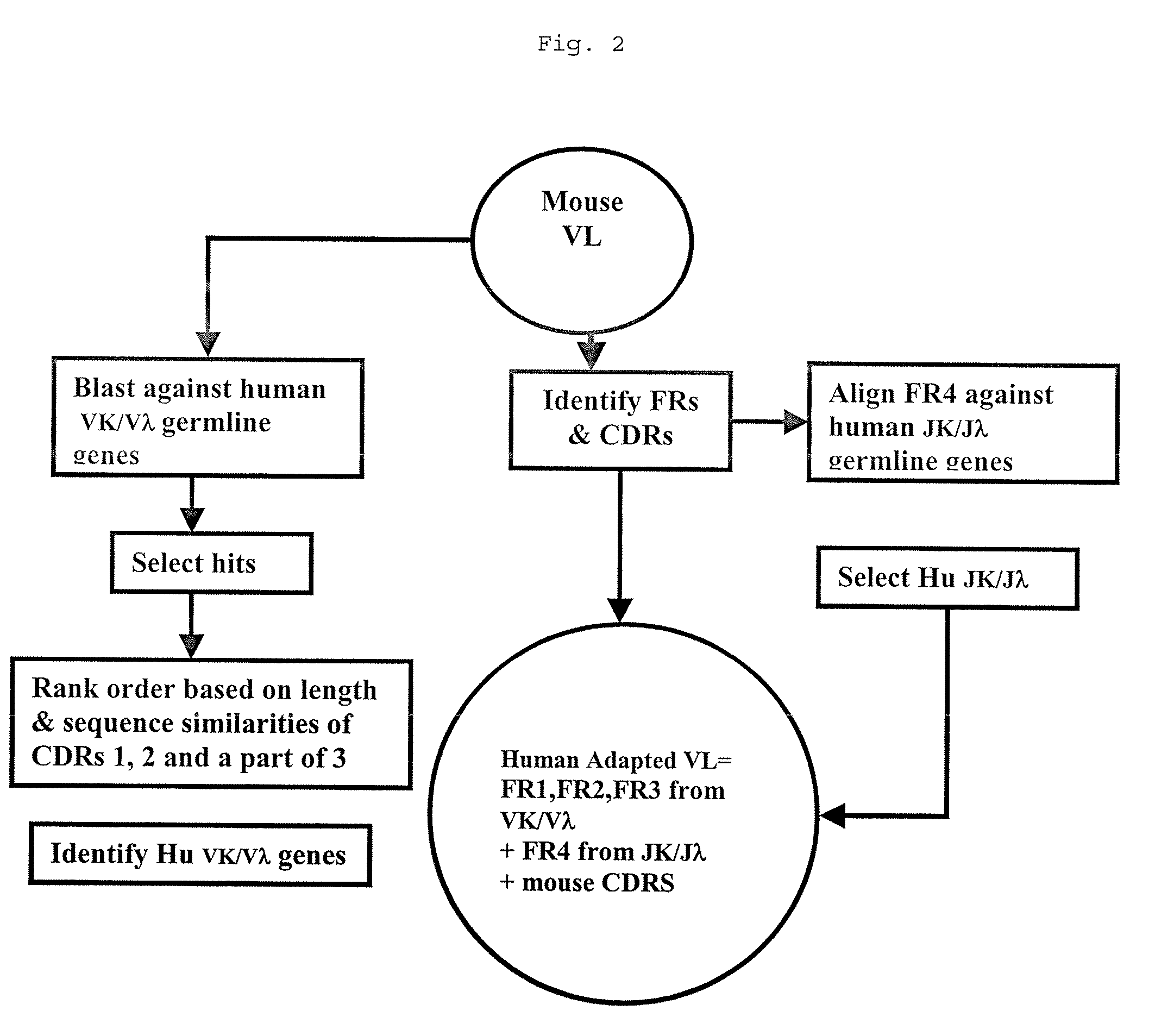
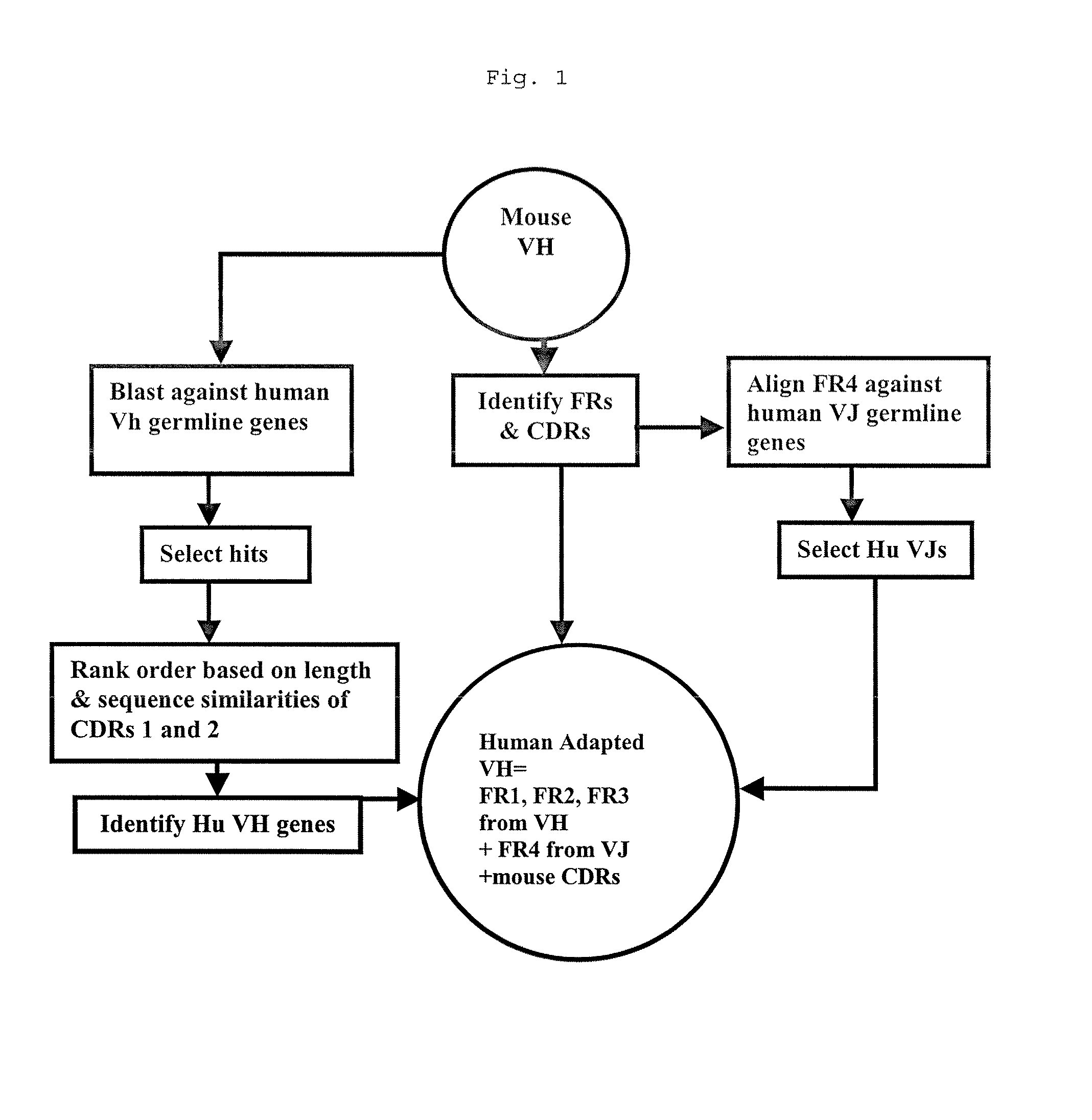
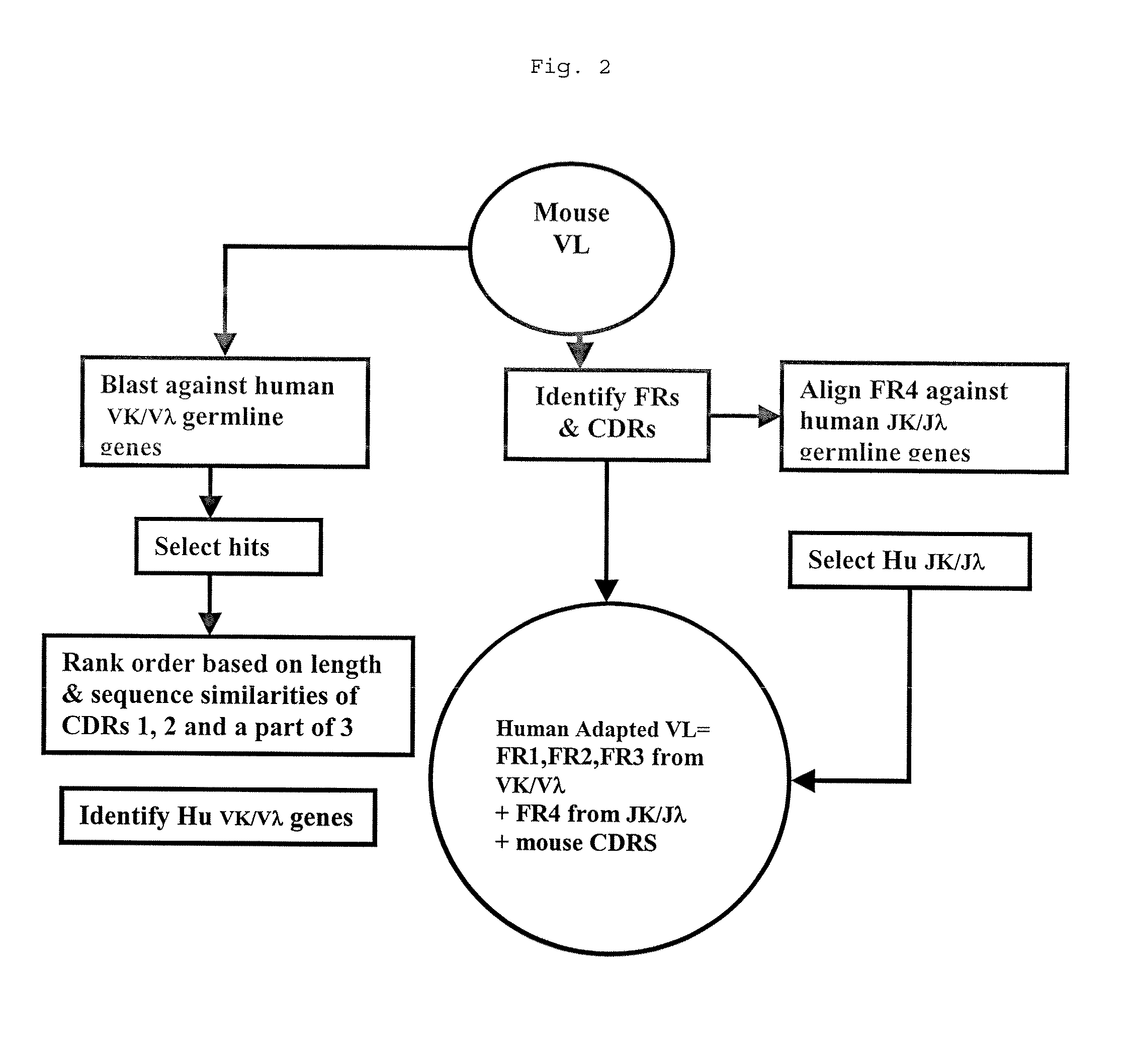
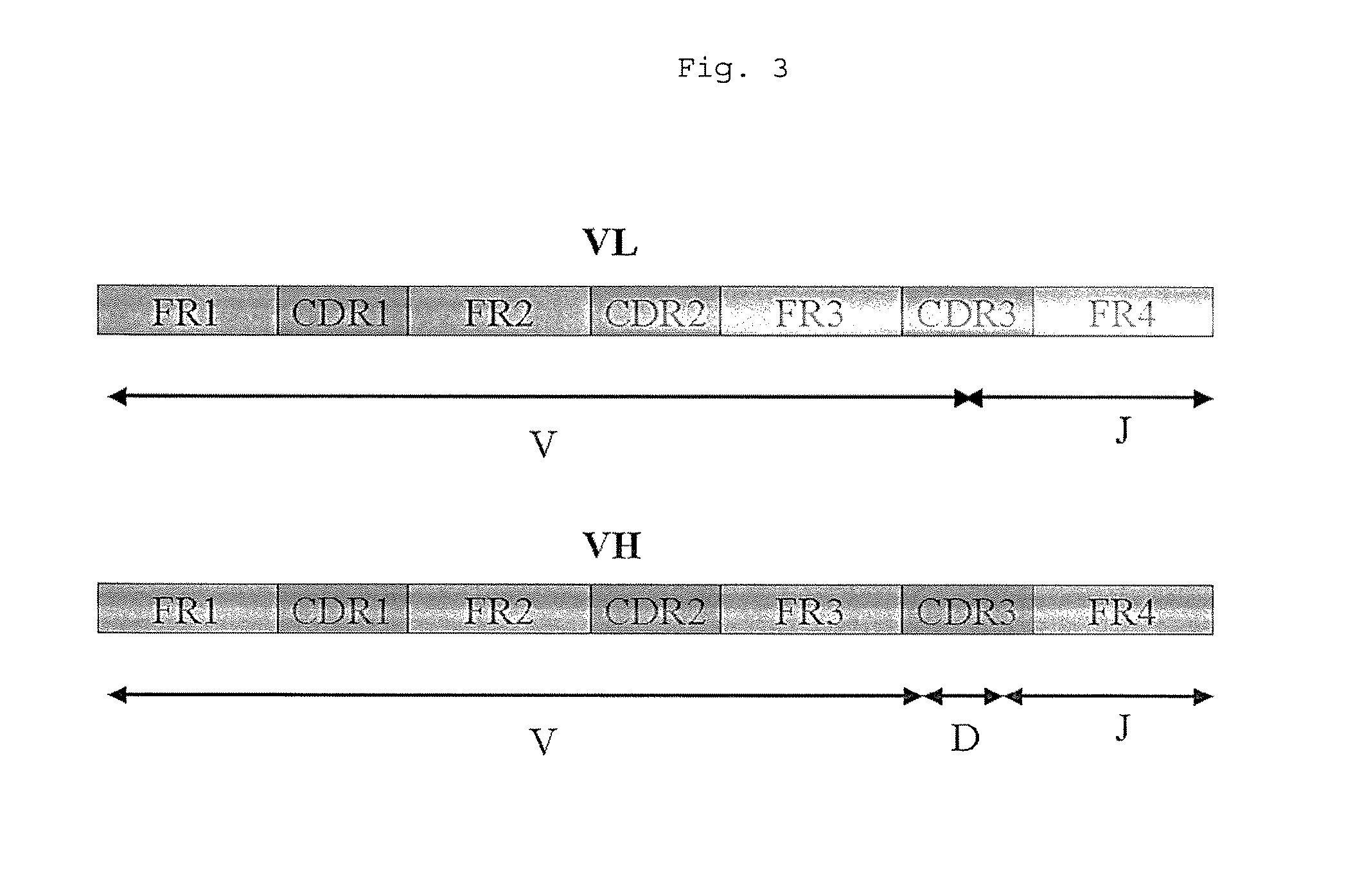
Methods for Use in Human-Adapting Monoclonal Antibodies
Methods useful for human adapting non-human monoclonal antibodies are disclosed. The methods select candidate human antibody framework sequences from a database of human germline genes or human sequences with somatic mutations.
Owner:JANSSEN BIOTECH INC
High affinity human antibodies and human antibodies against human antigens
The invention relates to transgenic non-human animals capable of producing high affinity human sequence antibodies. The invention is also directed to human sequence antibodies specific for human antigens, such as, human CD4. The invention also is directed to methods for producing human sequence antibodies.
Owner:GENPHARM INT INC
Methods for use in human-adapting monoclonal antibodies
Owner:JANSSEN BIOTECH INC
Super humanized antibodies
InactiveUS20050261480A1Antibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsHuman sequenceHumanized antibody
Disclosed herein are methods for humanizing antibodies based on selecting variable region framework sequences from human antibody genes by comparing canonical CDR structure types for CDR sequences of the variable region of a non-human antibody to canonical CDR structure types for corresponding CDRs from a library of human antibody sequences, preferably germline antibody gene segments. Human antibody variable regions having similar canonical CDR structure types to the non-human CDRs form a subset of member human antibody sequences from which to select human framework sequences. The subset members may be further ranked by amino acid similarity between the human and the non-human CDR sequences. Top ranking human sequences are selected to provide the framework sequences for constructing a chimeric antibody that functionally replaces human CDR sequences with the non-human CDR counterparts using the selected subset member human frameworks, thereby providing a humanized antibody of high affinity and low immunogenicity without need for comparing framework sequences between the non-human and human antibodies. Chimeric antibodies made according to the method are also disclosed.
Owner:ARROWSMITH TECH
Transgenic non-human animals for producing heterologous and chimeric antibodies
The invention relates to transgenic non-human animals capable of producing heterologous antibodies and methods for producing human sequence antibodies which bind to human antigens with substantial affinity.
Owner:GENPHARM INT INC
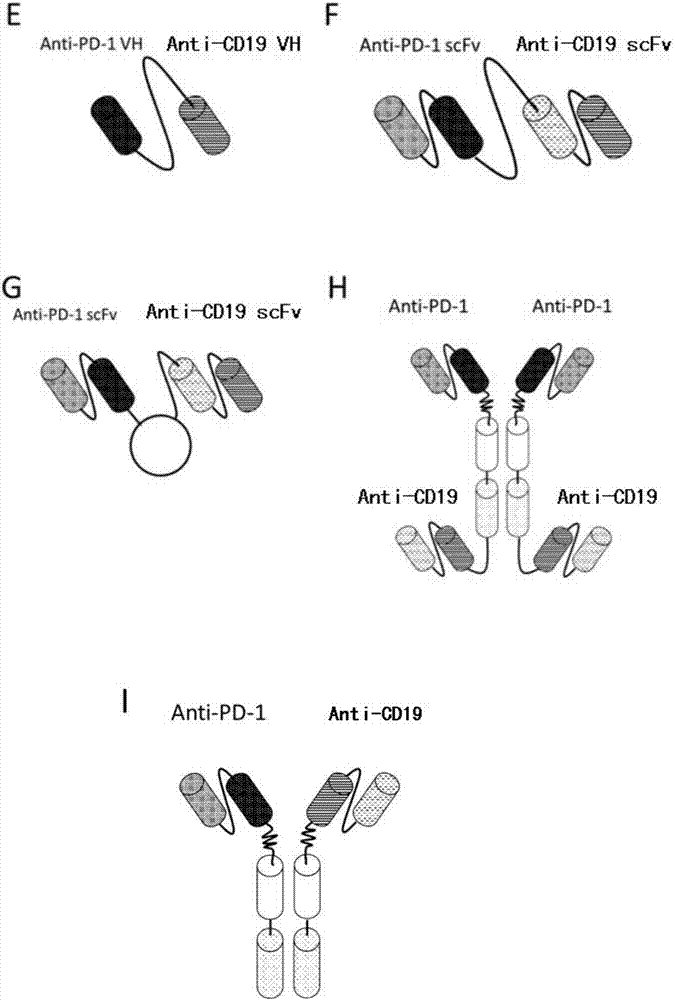
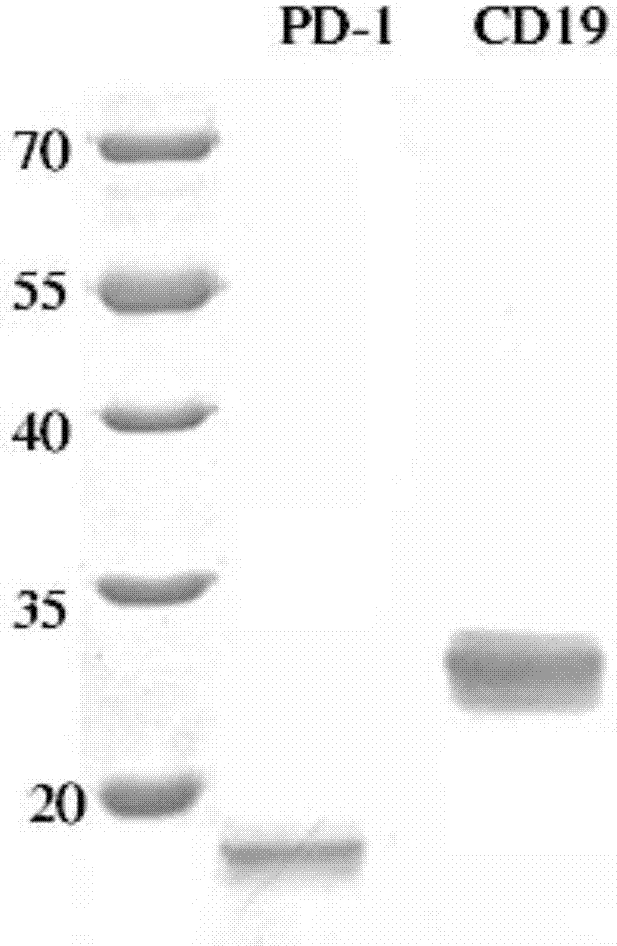
Anti-PD1 and anti-CD19 bispecific antibody and applications thereof
ActiveCN106939050AImprove biological activityGood biological stabilityHybrid immunoglobulinsAntibody ingredientsCell Surface AntigensTherapeutic effect
The present invention relates to anti-PD1 and anti-CD19 bispecific antibody and applications thereof, wherein the anti-PD1 and anti-CD19 bispecific antibody, or a variant, or a functional fragment thereof comprises: a domain capable of specifically recognizing and binding an immune cell surface antigen PD-1, wherein the domain comprises the heavy chain variable region (anti-PD-1 VH) of an anti-PD-1 specific antibody; and a domain capable of specifically recognizing and binding CD19, wherein the domain comprises the heavy chain variable region (anti-CD19 VH) of an anti-CD19 specific antibody. According to the present invention, the bispecific antibody has the whole human sequence, such that the occurrence of HAMA in clinical treatment application is effectively avoided, the occurrence of drug resistance can be effectively reduced, and the utilization efficiency and the treatment effect of the medicine can be improved; and the anti-PD-1 and anti-CD19 bispecific antibody can well mediate T cells to efficiently and specifically kill B-lymphocyte derived tumor cells compared to the existing anti-PD-1 specific antibody and the existing anti-CD19 specific antibody.
Owner:SHUNHAO CELL BIOTECHNOLOGY (TIANJIN) CO LTD
Transgenic non-human animals for producing heterologous antibodies
Owner:GENPHARM INT INC
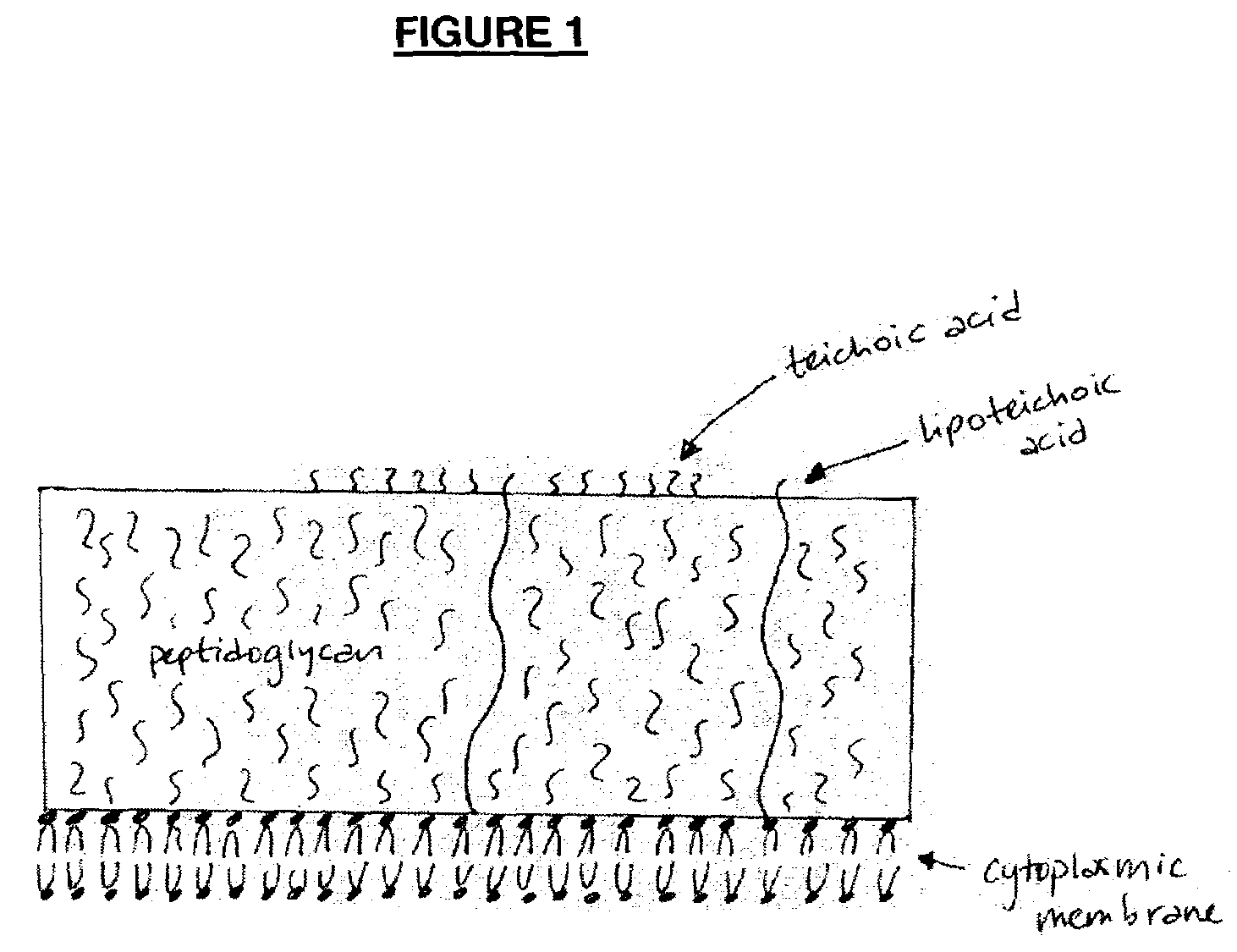
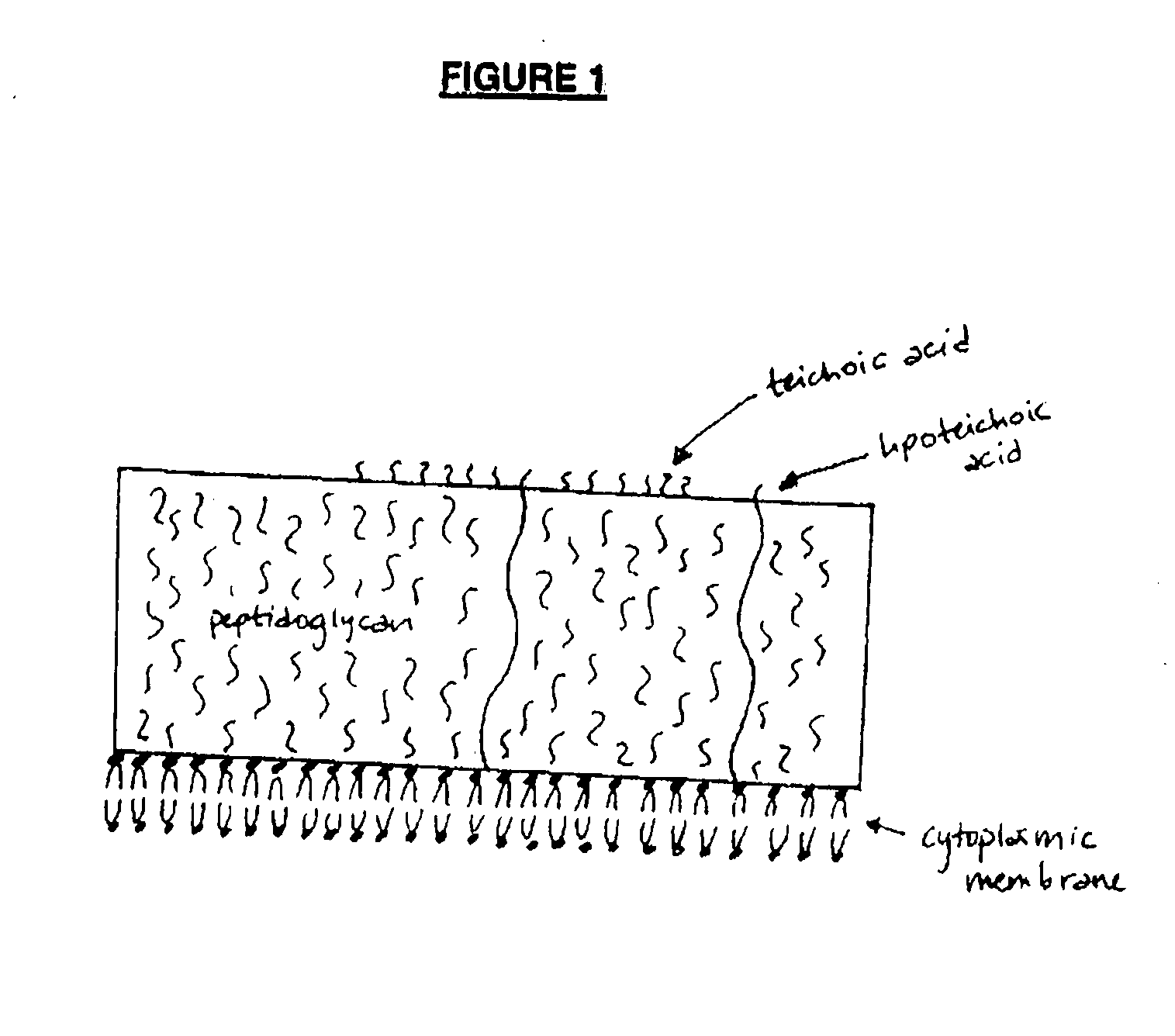
Opsonic monoclonal and chimeric antibodies specific for lipoteichoic acid of Gram positive bacteria
InactiveUS7250494B2High degree of sequence homologyIncrease infectionAntibacterial agentsAnimal cellsHuman sequenceGram-positive bacterium
The present invention encompasses monoclonal antibodies that bind to lipoteichoic acid (LTA) of Gram positive bacteria. The antibodies also bind to whole bacteria and enhance phagocytosis and killing of the bacteria in vitro. The invention also provides antibodies having human sequences (chimeric, humanized and human antibodies). The invention also sets forth the variable regions of three antibodies within the invention and presents the striking homology between them.
Owner:BIOSYNEXUS INC
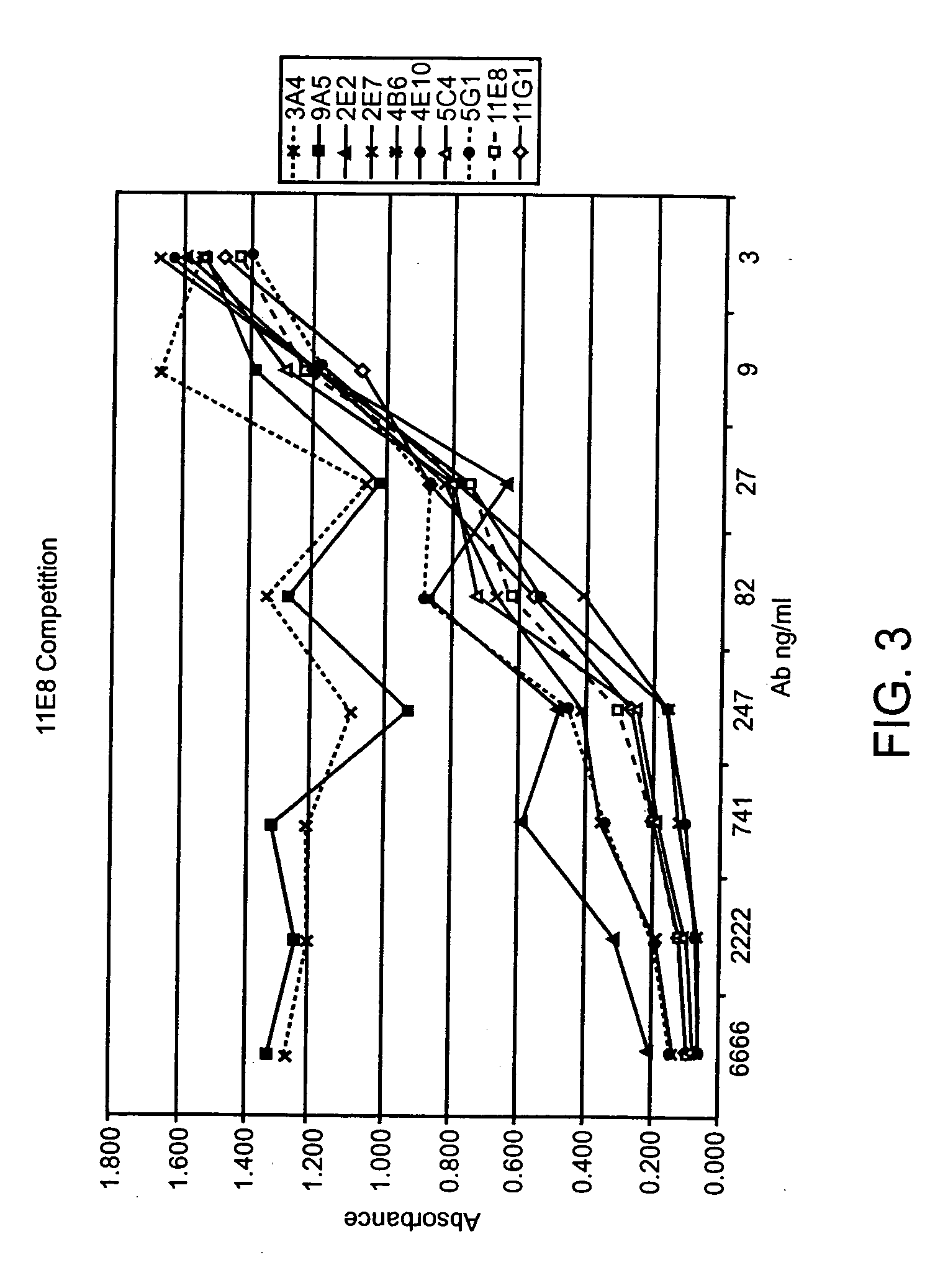
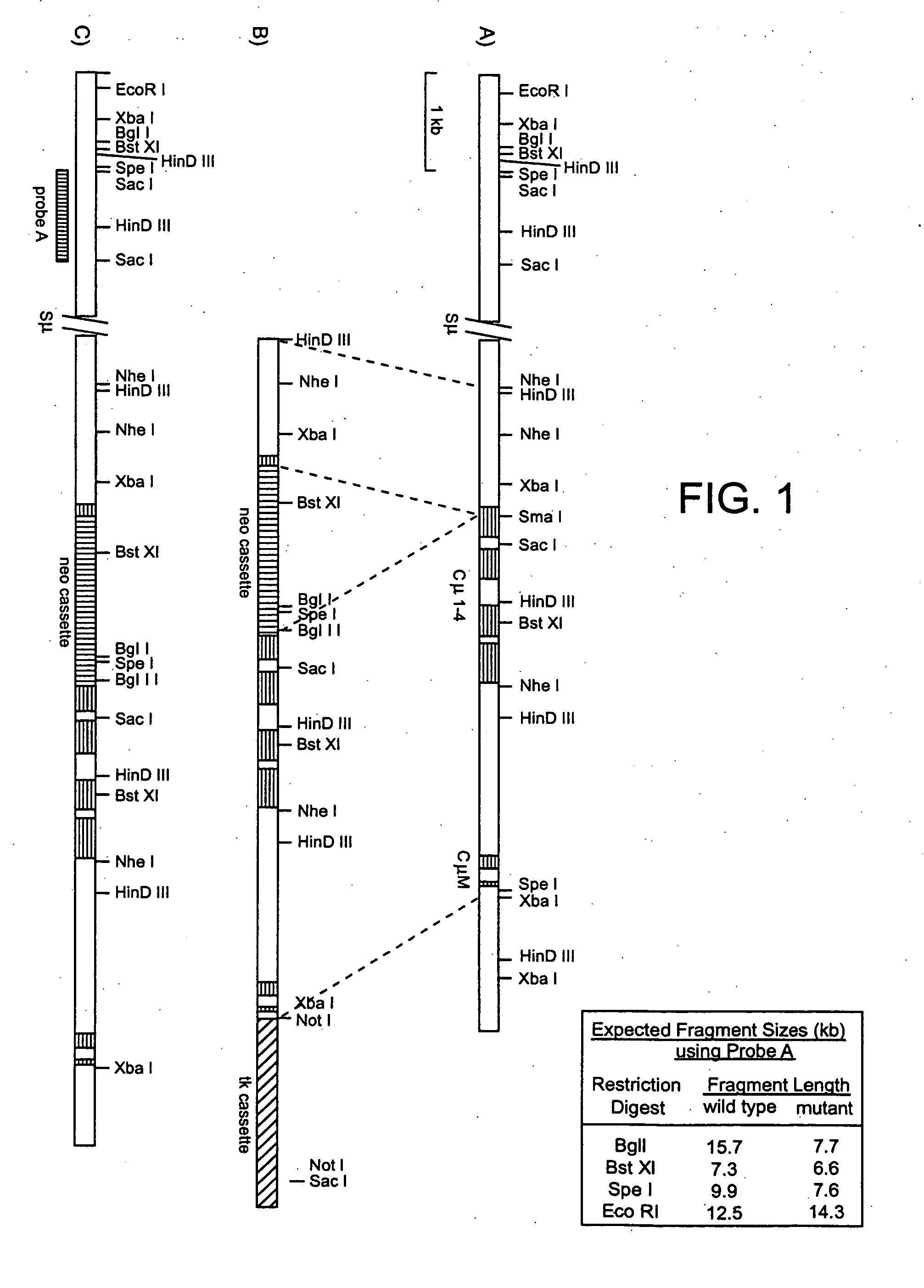
Transgenic transchromosomal rodents for making human antibodies
InactiveUS20060037093A1Microbiological testing/measurementImmunoglobulins against cytokines/lymphokines/interferonsMammalHuman sequence
The present invention provides novel transgenic nonhuman mammals capable of producing human sequence antibodies, as well as methods of producing and using these antibodies.
Owner:ER SQUIBB & SONS INC
Opsonic monoclonal and chimeric antibodies specific for lipoteichoic acid of gram positive bacteria
InactiveUS20080019976A1Enhance phagocytosis and killing of the bacteriaHigh degree of sequence homologyAntibacterial agentsPeptide/protein ingredientsHuman sequenceLipoteichoic acid binding
The present invention encompasses monoclonal antibodies that bind to lipoteichoic acid (LTA) of Gram positive bacteria. The antibodies also bind to whole bacteria and enhance phagocytosis and killing of the bacteria in vitro. The invention also provides antibodies having human sequences (chimeric, humanized and human antibodies). The invention also sets forth the variable regions of three antibodies within the invention and presents the striking homology between them.
Owner:BIOSYNEXUS INC
Super-humanized antibody
The invention discloses a method for humanizing an antibody. In the invention, through comparing a standard CDR (Complementarity-Determining Region) structure type of a CDR sequence in a variable region of an inhuman antibody with a standard CDR structure type of a corresponding CDR in a human antibody sequence library, a frame sequence of the variable region is selected from human antibody genes, preferably the gene segments of an embryonic system antibody. The variable region of the human antibody which has the standard CDR structure type similar to an inhuman CDR forms a subgroup of a member antibody sequence and a human frame sequence can be selected from the subgroup. The subgroup members can be further rated through the amino acid similarity between the human and inhuman CDR sequences. The ahead human sequence in the rating is selected to provide the frame sequence and construct a chimeric antibody by utilizing the selected the subgroup member human frame. The chimeric antibody replaces the human CDR sequence by the counterpart functionality of the inhuman CDR, therefore, the humanized antibody which has high affinity and low immunogenicity is provided without comparing the frame sequences between the inhuman antibody and the human antibody. The invention also discloses the chimeric antibody prepared according to the method of the invention.
Owner:杰斐逊 富特
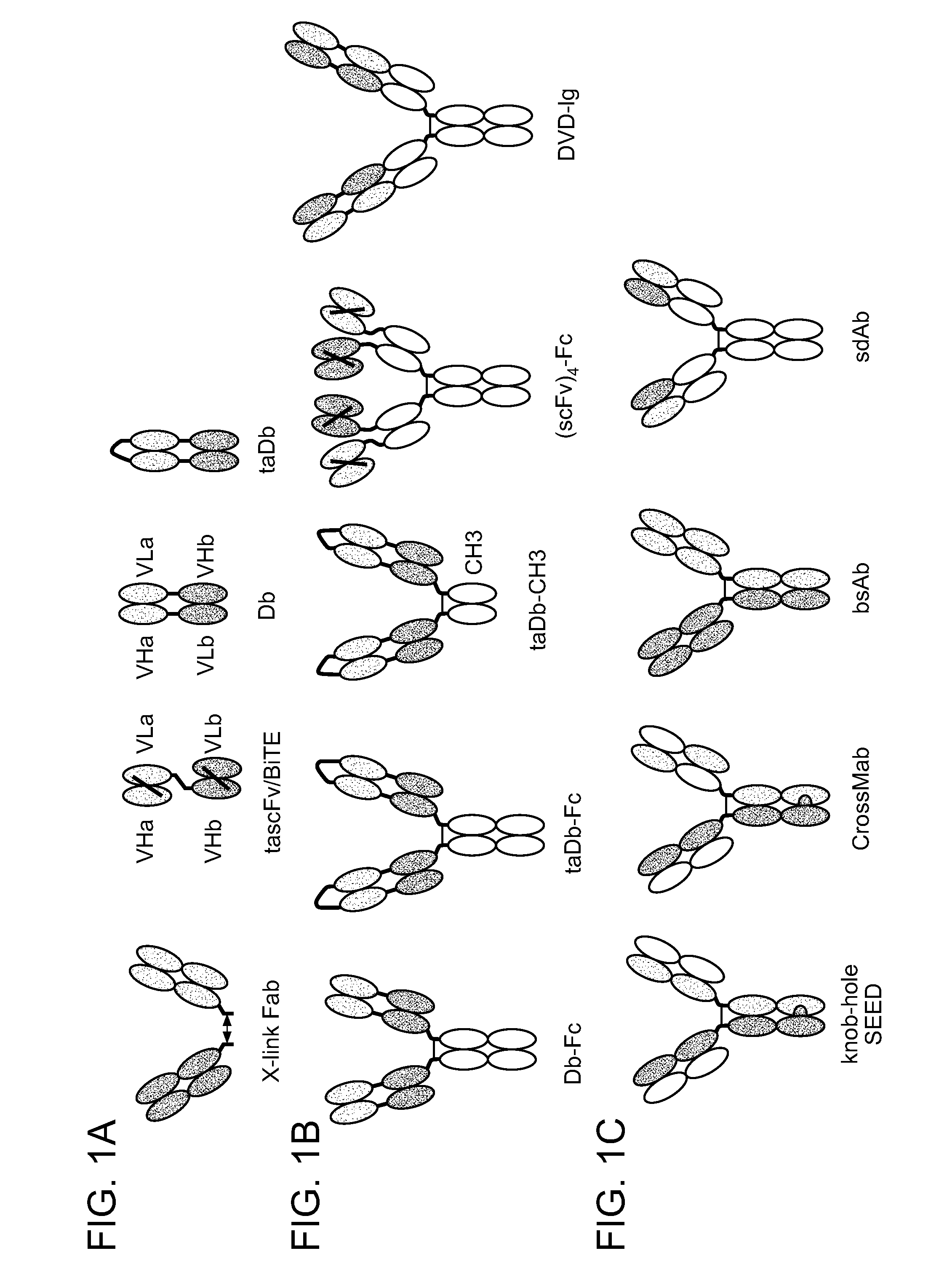
Methods For The Generation Of Multispecific And Multivalent Antibodies
ActiveUS20140179547A1Favorable manufacturing characteristicMaximize productionHybrid immunoglobulinsDNA preparationHeavy chainBispecific monoclonal antibody
Owner:NOVIMMUNE
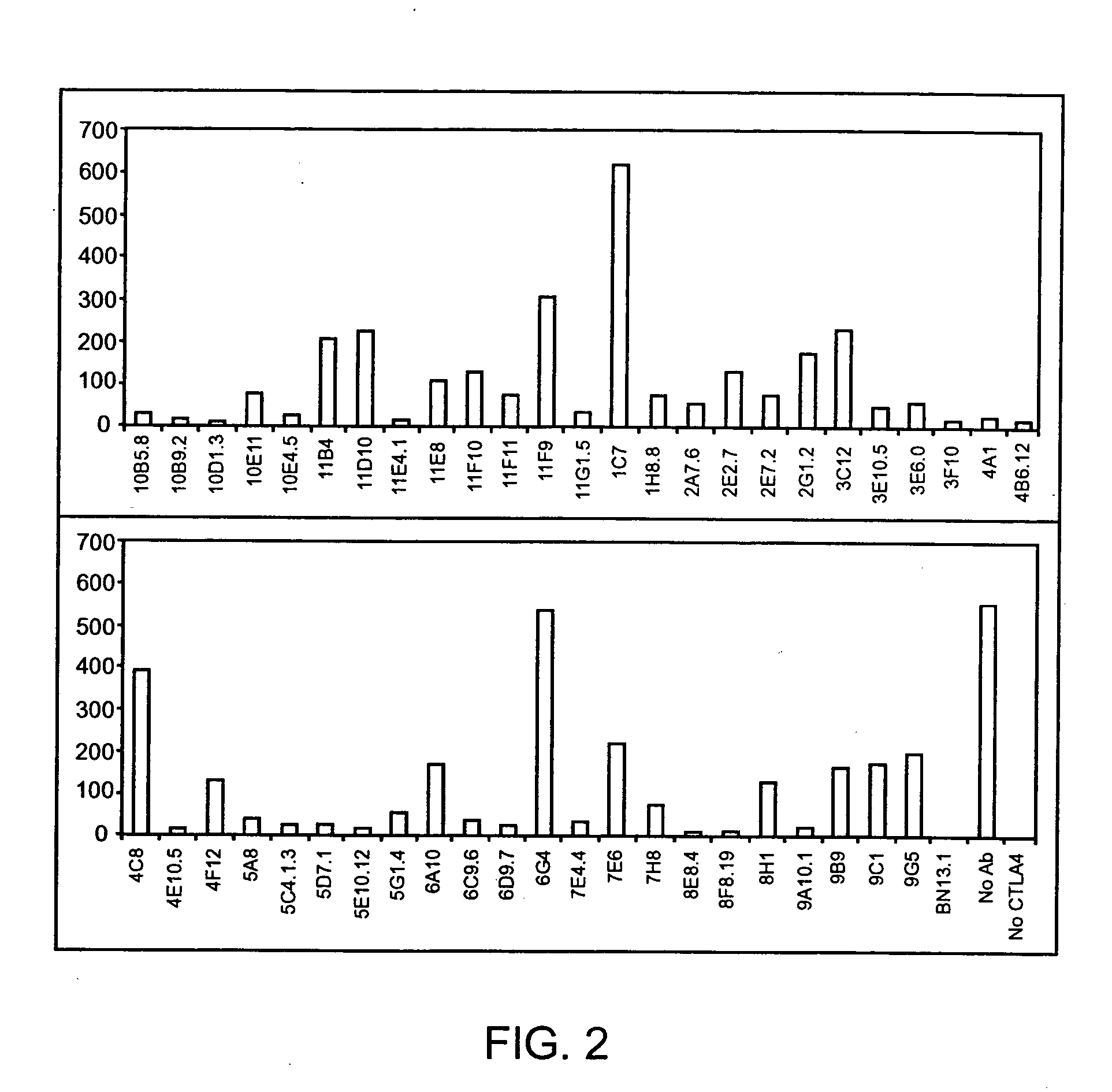
Methods Of Treatment Using CTLA-4 Antibodies
InactiveUS20090117037A1Induce and enhance immune responseAntibacterial agentsNervous disorderDiseaseHuman sequence
The present invention provides method of treatment using human sequence antibodies against human CTLA-4 linked to a cytotoxic agent. In particular, methods of treating cancer and autoimmune disease are provided.
Owner:ER SQUIBB & SONS INC
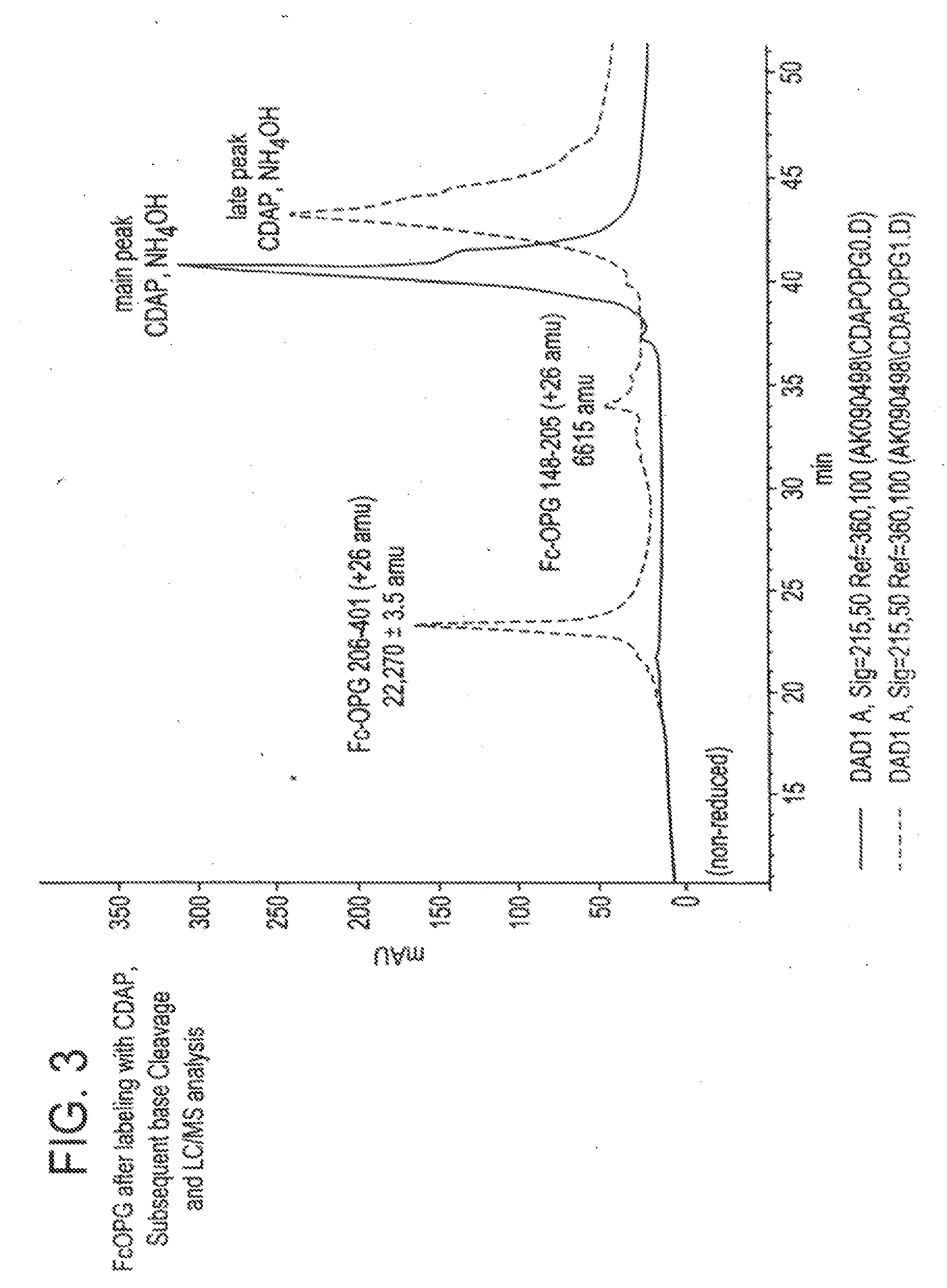
Process for correction of a disulfide misfold in Fc molecules
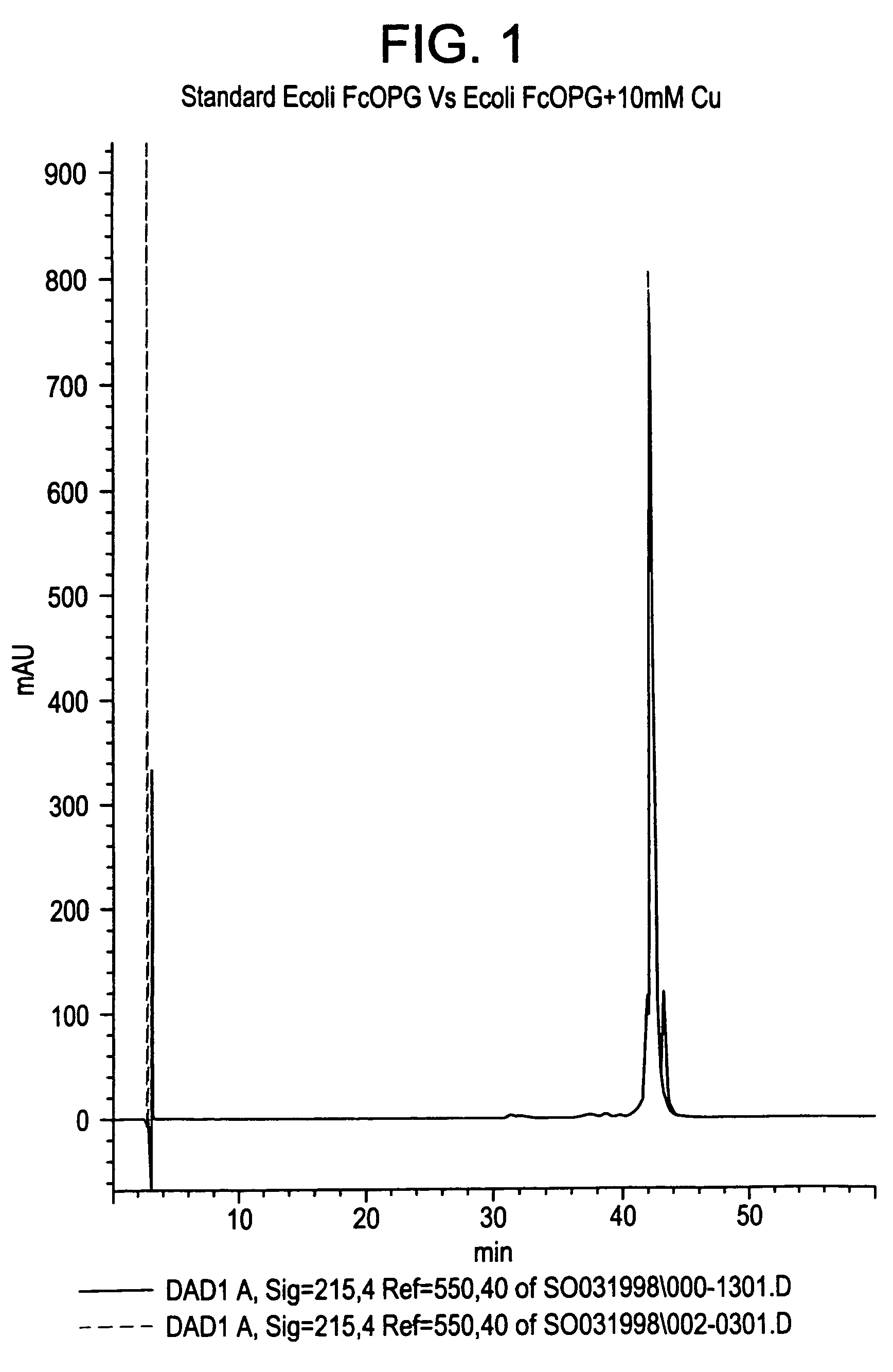
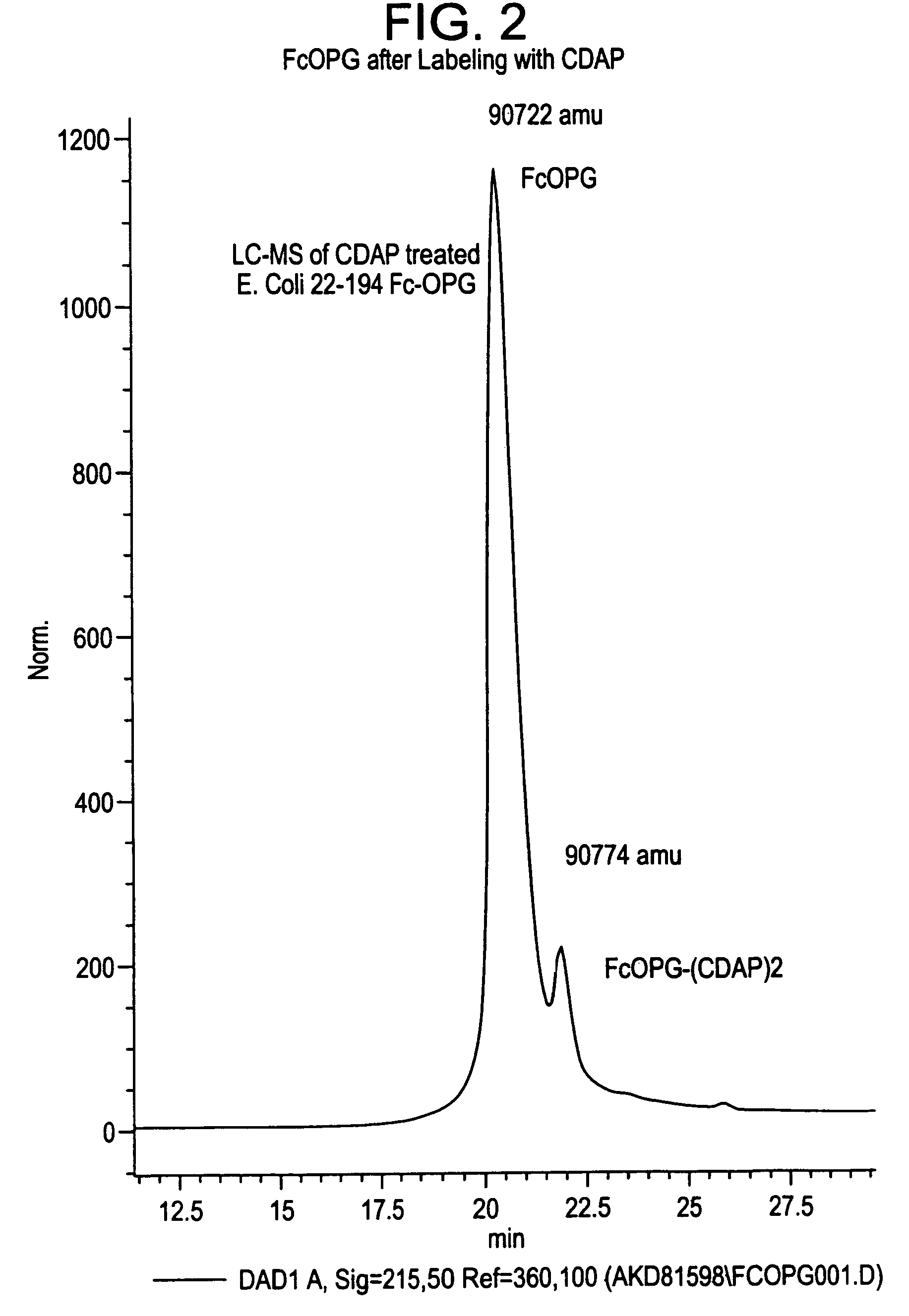
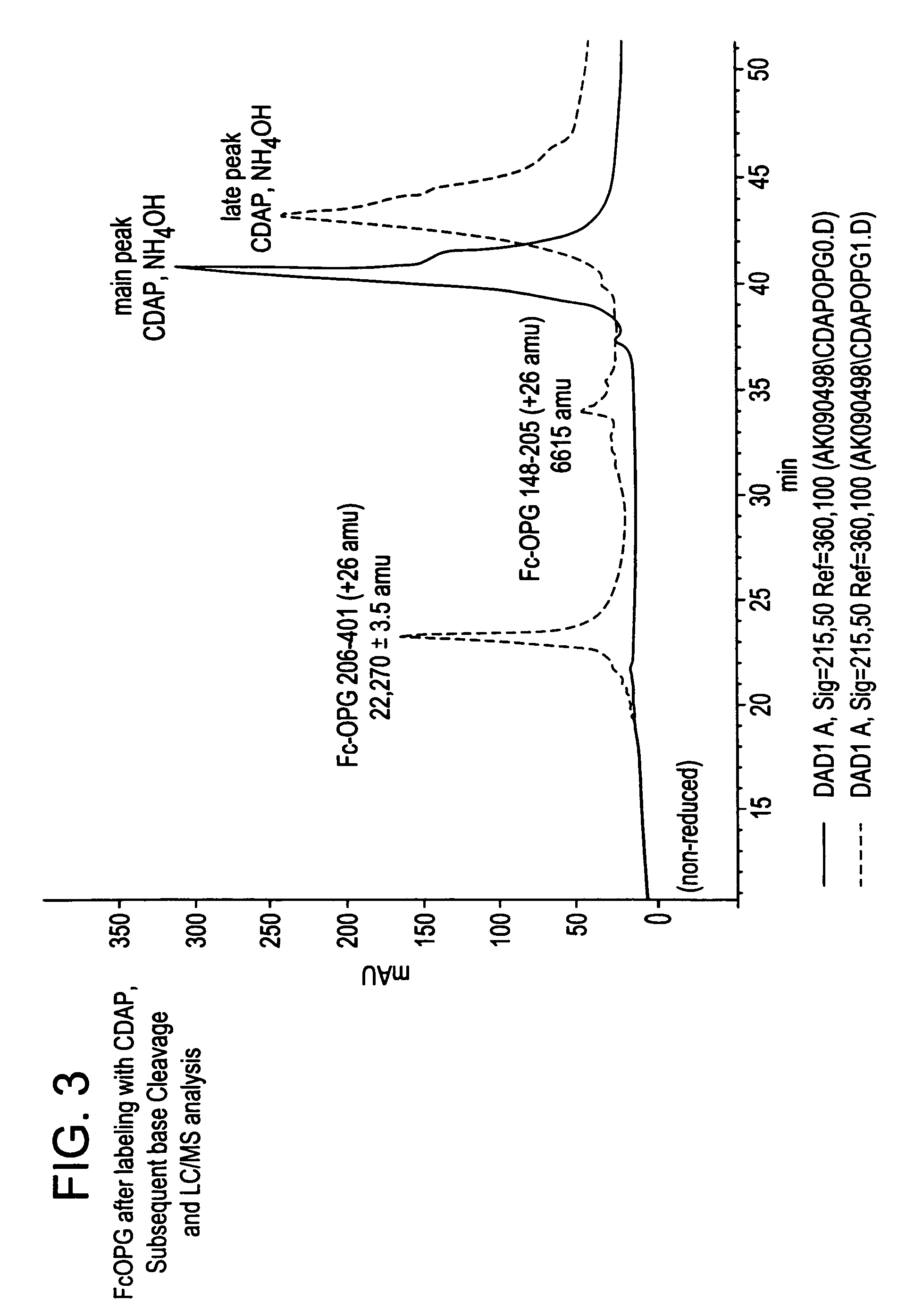
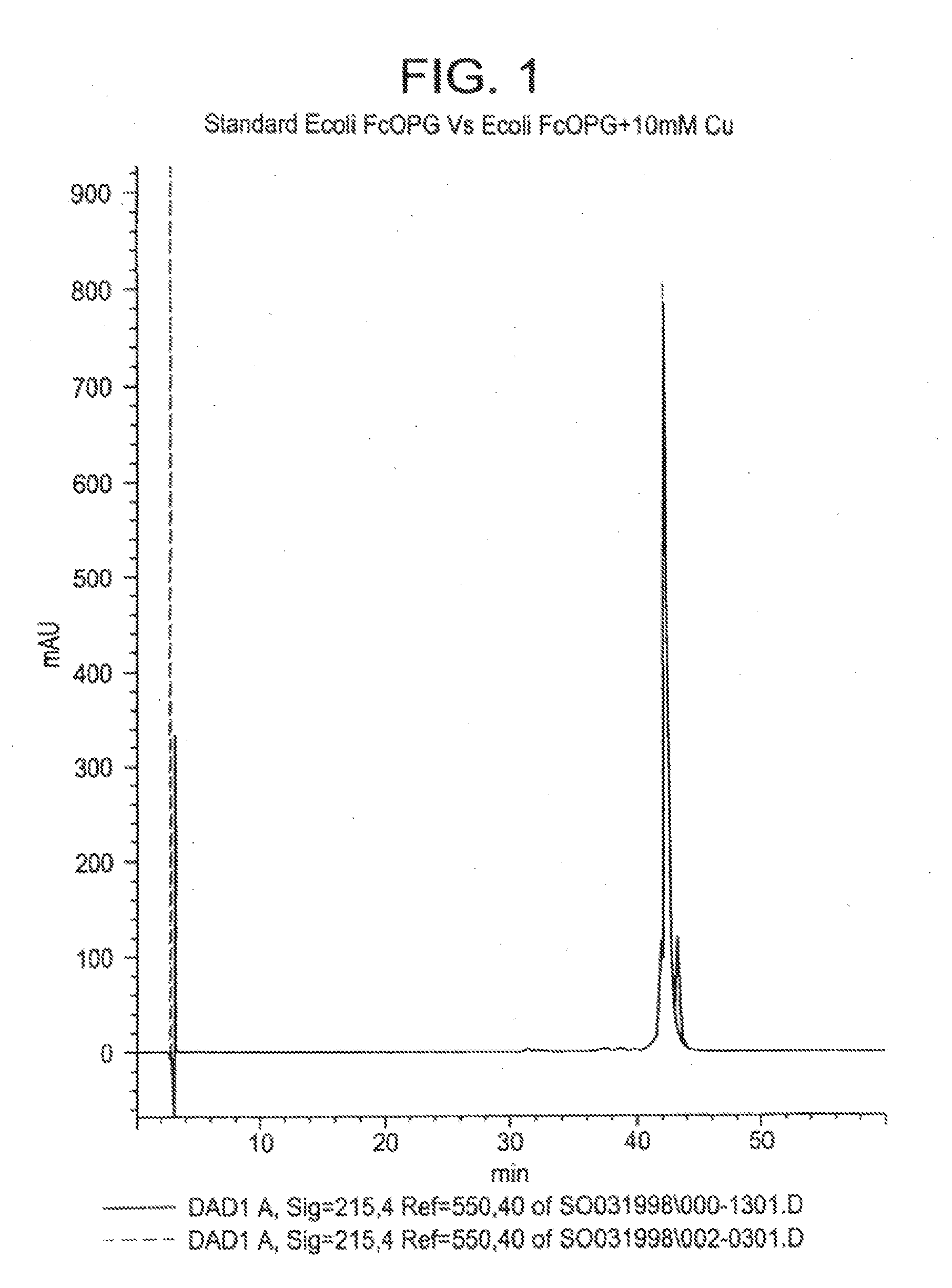
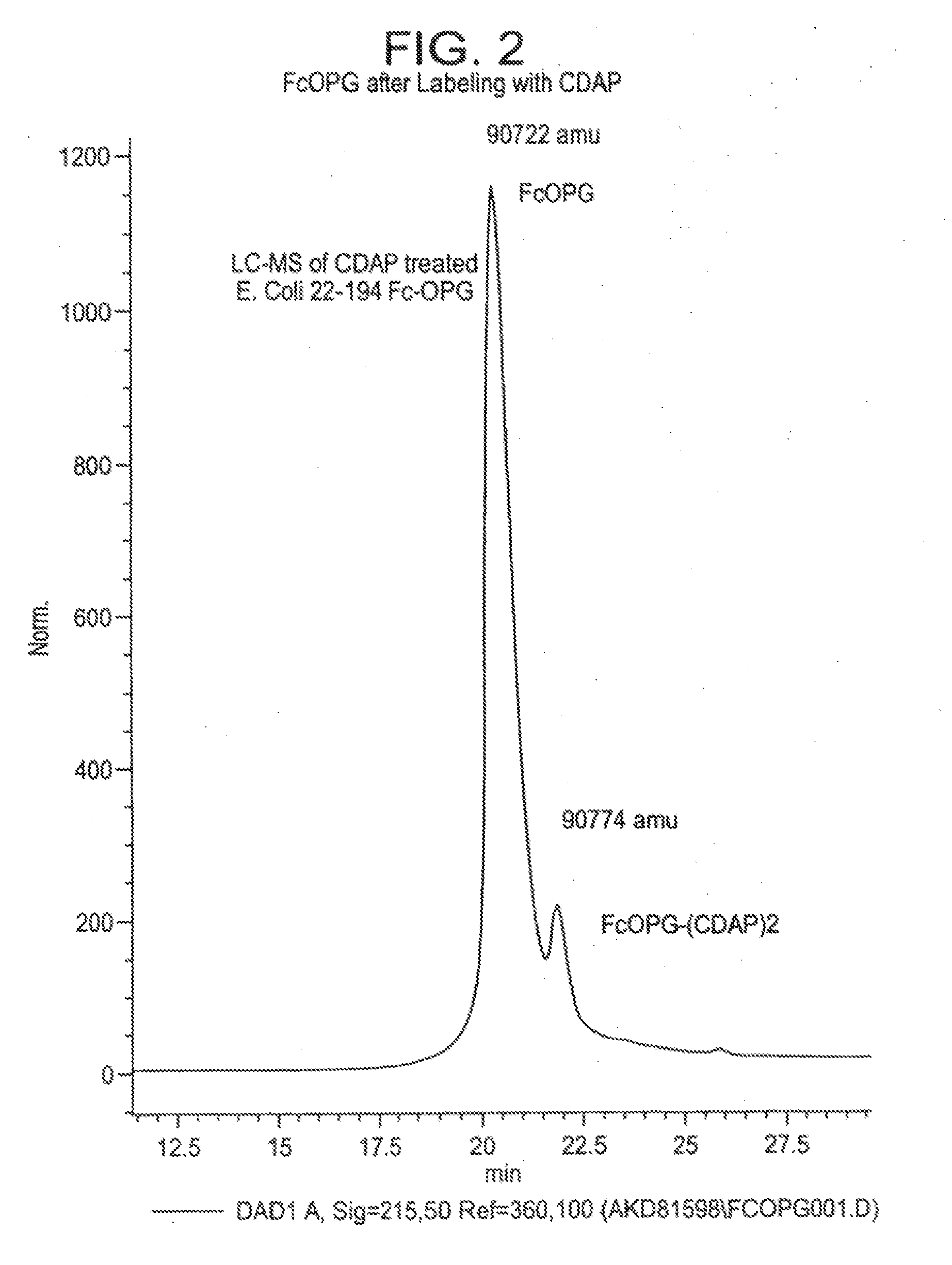
The present invention concerns a process by which a misfold in an Fc fusion molecule can be prevented or corrected. In one embodiment, the process comprises (a) preparing a pharmacologically active compound comprising an Fc domain; (b) treating the fusion molecule with a copper (II) halide; and (c) isolating the treated fusion molecule. The pharmacologically active compound can be an antibody or a fusion molecule comprising a pharmacologically active domain and an Fc domain. The preferred copper (II) halide is CuCl2. The preferred concentration thereof is at least about 10 mM for fusion molecules prepared in E. coli; at least about 30 mM for fusion molecules prepared in CHO cells. The process can be employed with any number of pharmacologically active domains. Preferred pharmacologically active domains include OPG proteins, leptin proteins, soluble portions of TNF receptors (e.g., wherein the fusion molecule is etanercept), IL-1ra proteins, and TPO-mimetic peptides. The Fc domain preferably has a human sequence, with an Fc sequence derived from IgG1 most preferred. An exemplary Fc sequence is shown in FIG. 5 hereinafter.
Owner:AMGEN INC
Human CTLA-4 Antibodies and Their Uses
InactiveUS20100047244A1Antibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsHuman sequenceCTLA4 Protein
The present invention provides novel human sequence antibodies against human CTLA-4 and methods of treating human diseases, infections and other conditions using these antibodies.
Owner:ER SQUIBB & SONS INC
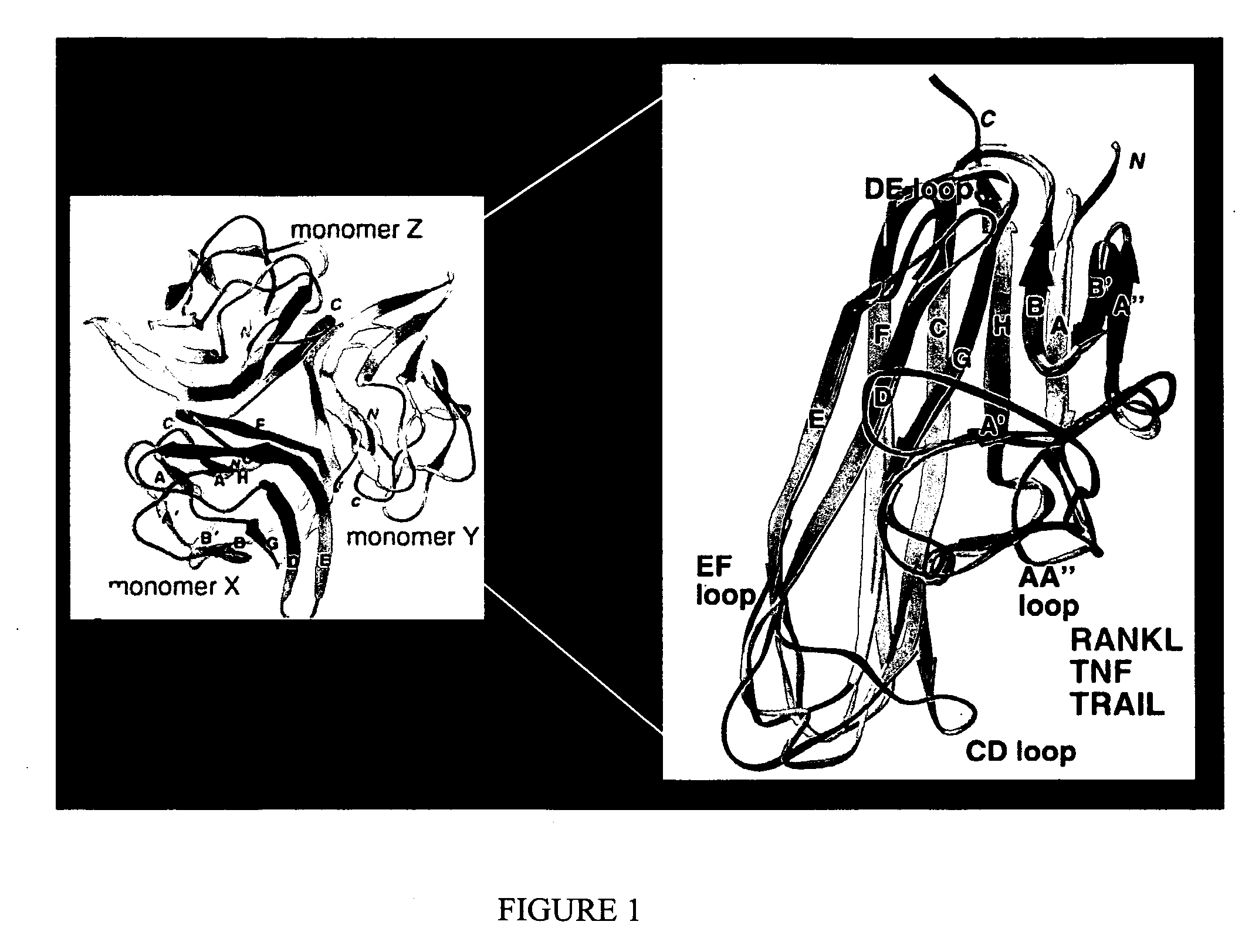
Isolation of antibodies that cross-react and neutralize rankl originating from multiple species
InactiveUS20080107597A1Promote reproductionLittle or no activityIn-vivo radioactive preparationsPeptide/protein ingredientsAnticarcinogenGlucocorticoid
The invention provides specific binding members (e.g., antibodies or antigen-binding fragments thereof) which bind to RANKL originating from multiple species. An epitope recognized by the specific binding members can be selected from surface exposed loop domains that bind to and activate its cognate receptor, RANK (Receptor Activator of NFkB), on the surface of osteoclast precursors and other cell types. The invention provides peptides for generating such anti-RANKL antibodies, including murine sequences, other non-human sequences and cross-reactive peptides. The specific binding members are useful in the diagnosis and treatment of lytic bone diseases, including osteoporosis, rheumatoid arthritis, bone metastasis and hypercalcemia of malignancy, glucocorticoid-induced bone loss, a periodontal disease or condition, a cancer and Juvenile Paget's Disease. The binding members can also be used in therapy in combination with chemotherapeutics or anti-cancer agents and / or with other antibodies or antigen-binding fragments thereof.
Owner:ANAPTYSBIO INC
Binding molecules
InactiveUS20090169548A1Improve in vivo stabilityReduce the possibilityOrganic active ingredientsGenetic material ingredientsNatural sourceHeterologous
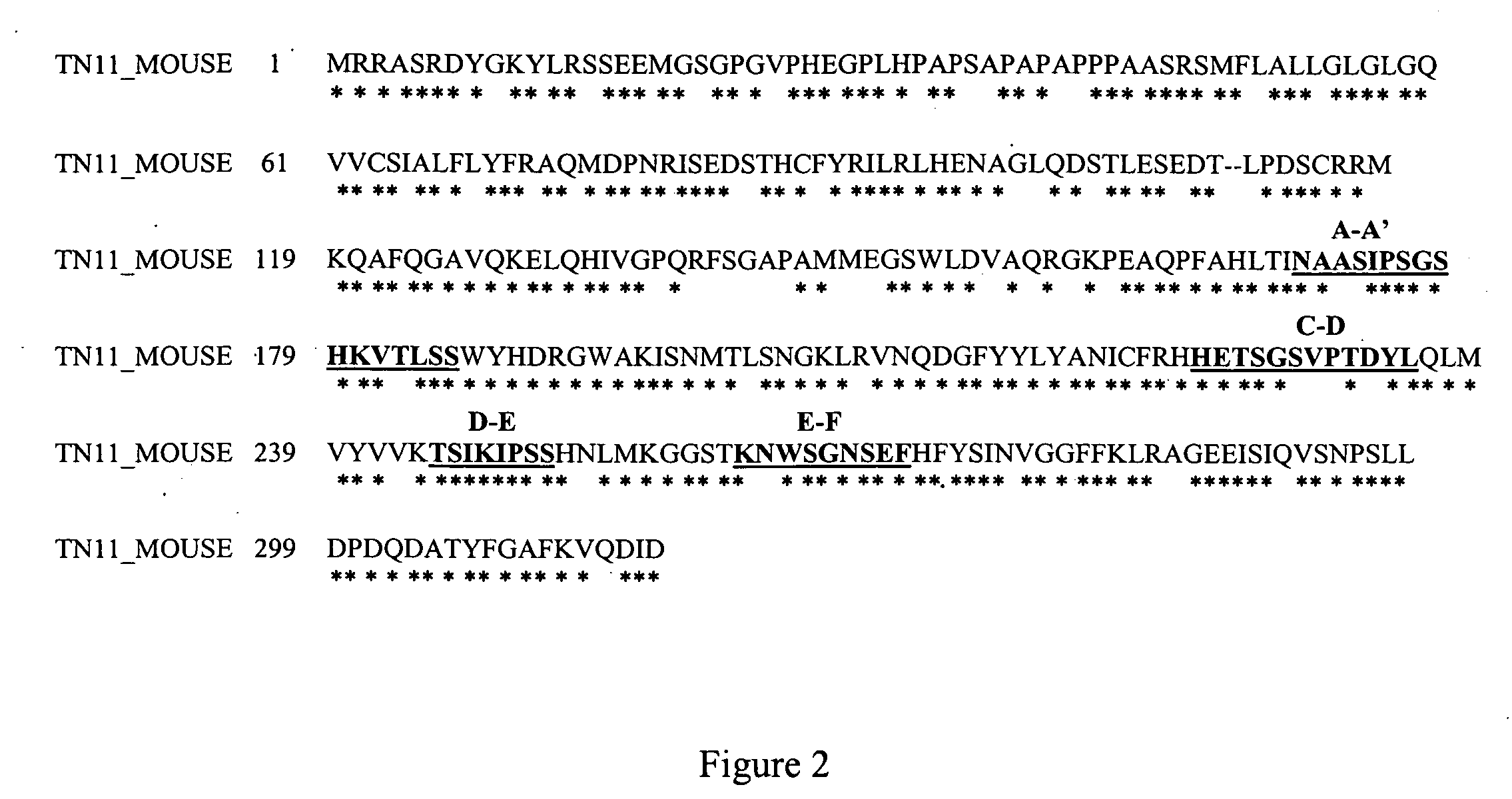
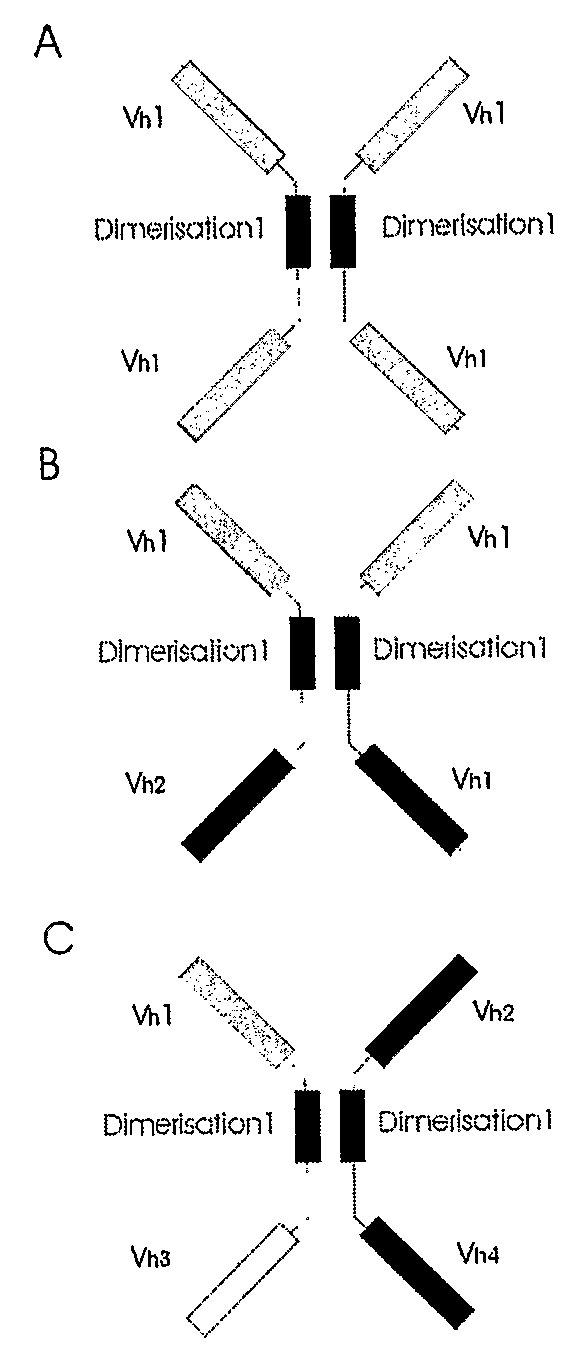
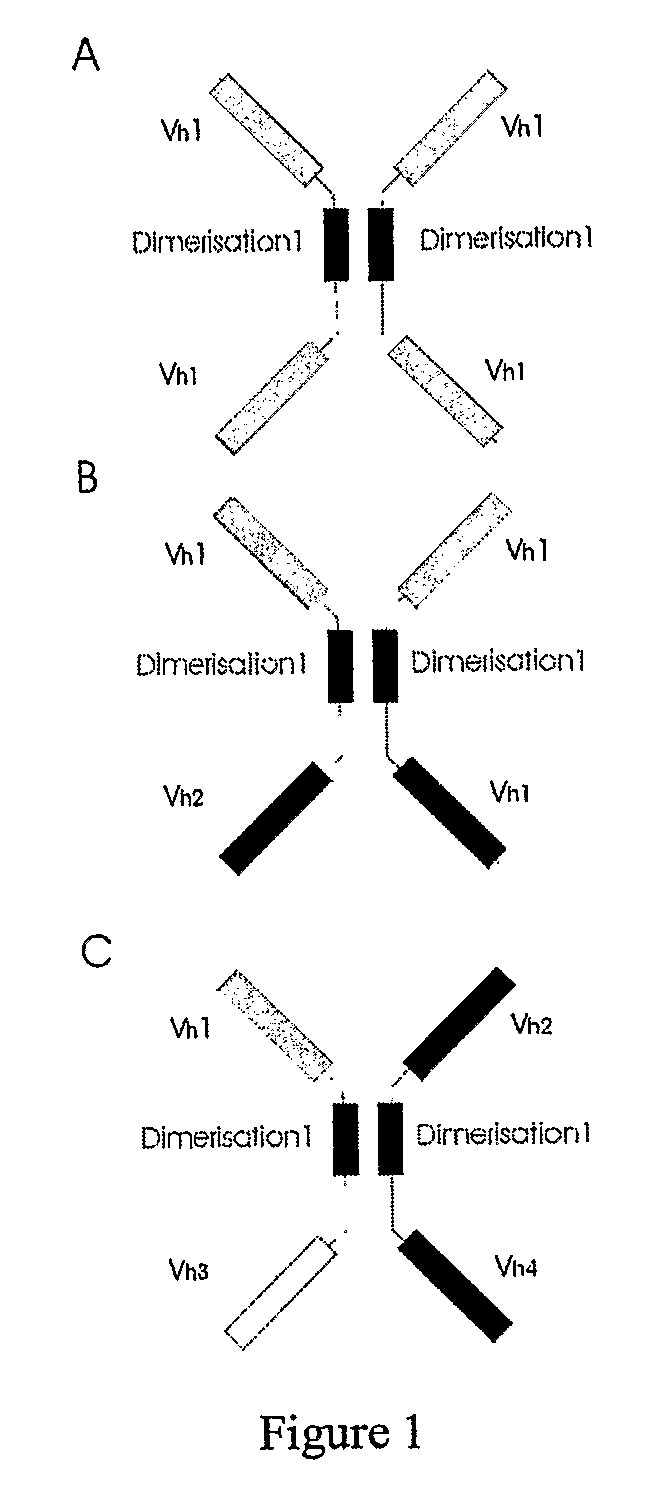
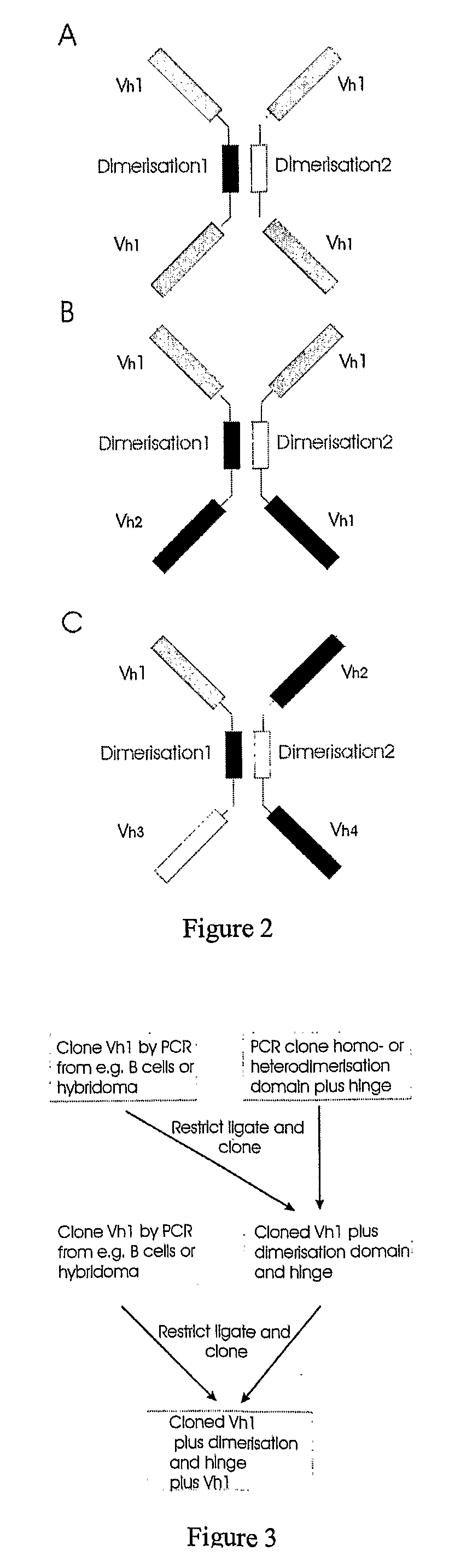
The present invention relates to the manufacture of mono, di and multivalent polypeptide binding complexes, also mono, di or multispecific polypeptide binding complexes and uses thereof. The invention also relates to the manufacture and use of a diverse repertoire of antigen specific VH binding domains derived from phage display libraries, transgenic animals or natural sources. Preferably the VH binding domains and the dimerisation domains comprise human sequences. The polypeptide binding complexes comprise homo or heterodimerisation domains with four antigen binding [VH] domains fused at the amino and carboxyl termini of the dimerisation domains preferably using natural hinge or linker peptides. Where the polypeptide binding complexes lack CH2-CH3 effector functions they are preferably less than 120 kDa in size. Routes of manufacture are described herein.
Owner:GROSVELD FRANKLIN GERARDUS +3
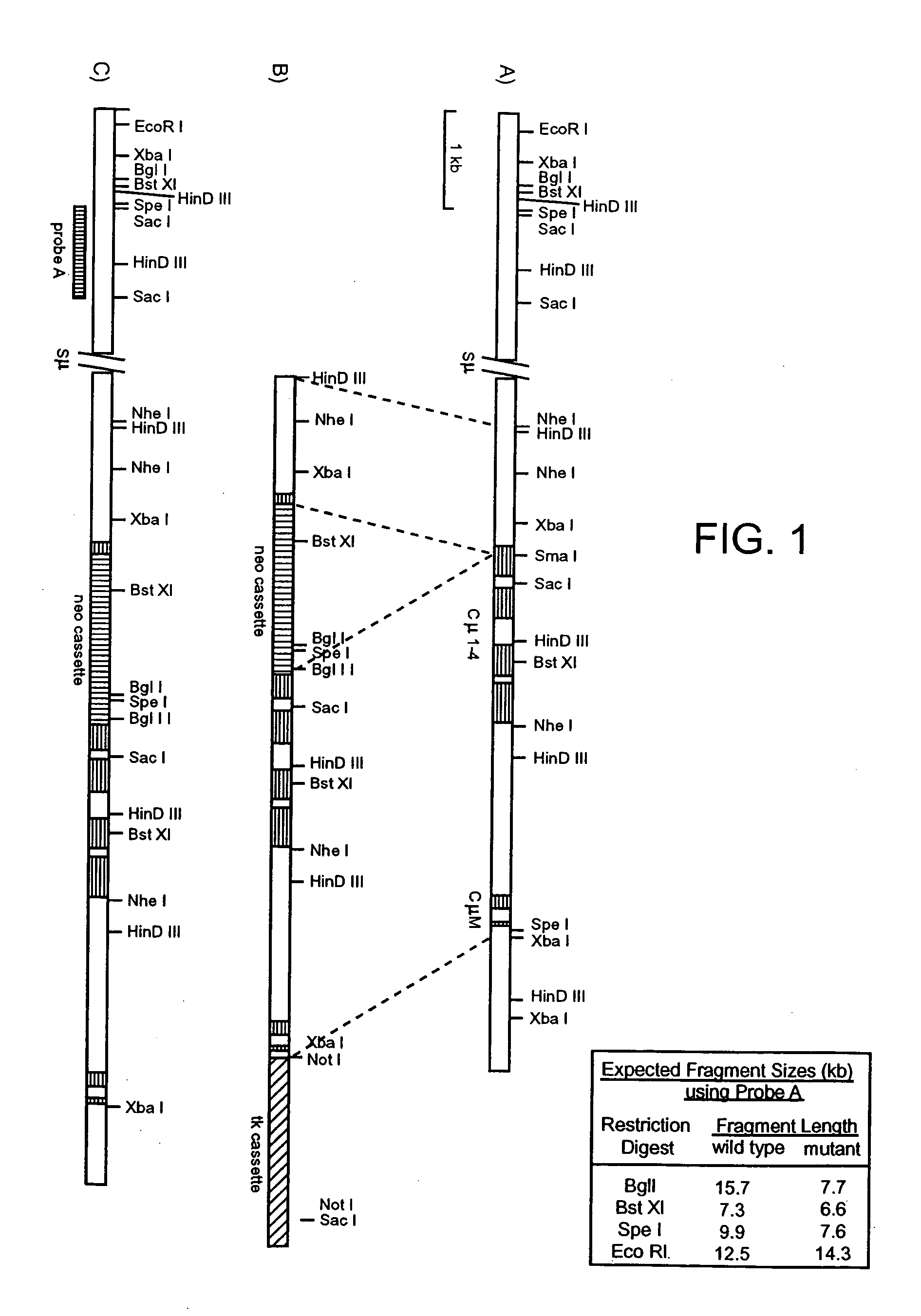
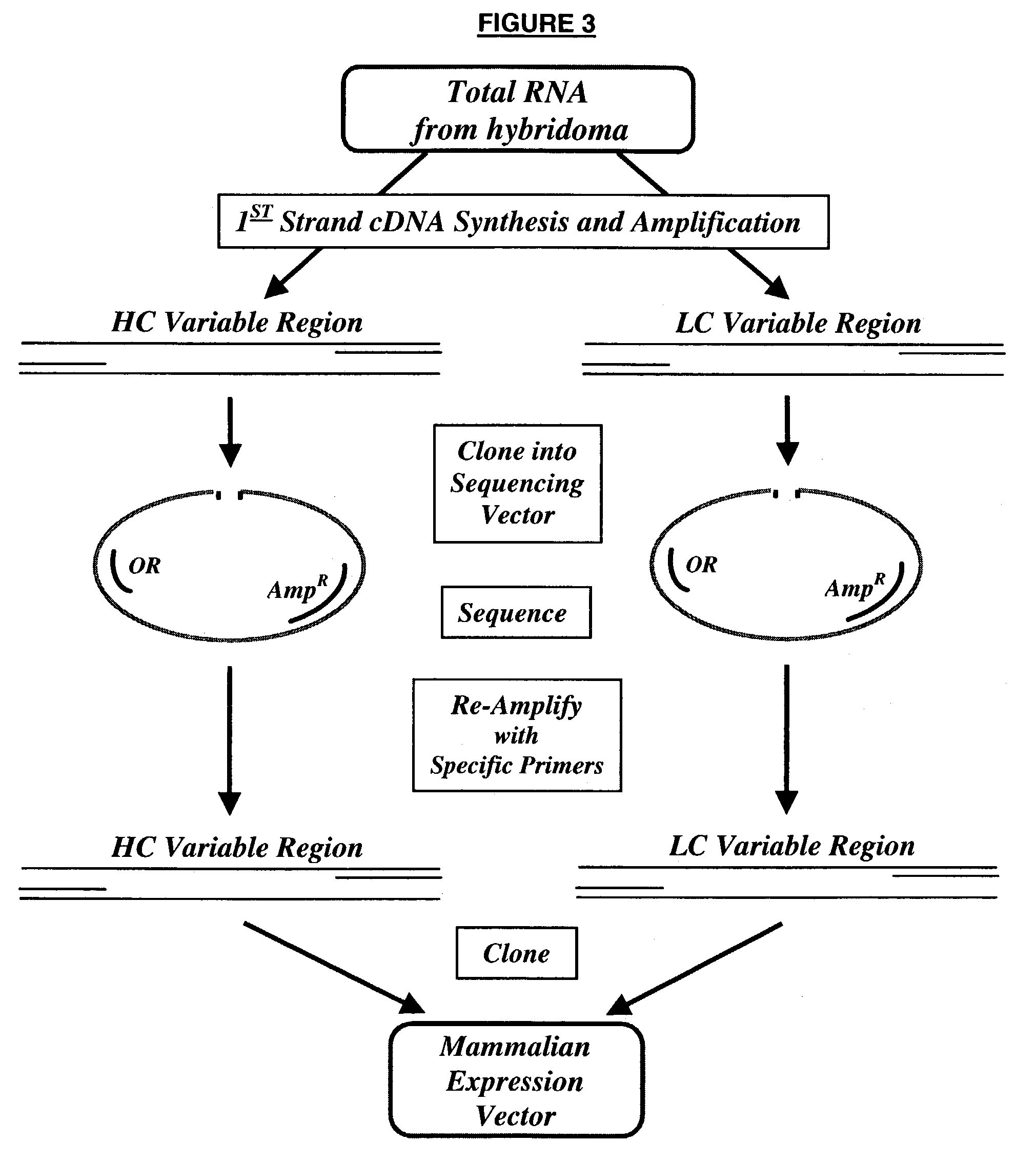
Hybridomas producing high levels human sequence antibody
A hybridoma cell line producing high levels of human sequence antibody. Also described is a hybridoma cell line producing human sequence antibodies that is adapted for growth in serum-free media or animal-derived-components-free media. A hybridoma cell line producing anti-CTLA4 antibodies is most preferred.
Owner:PFIZER INC
Three-dimensional human body movement data dividing method
InactiveCN1975779AReduce computational complexityImprove computing efficiencyCharacter and pattern recognitionImage data processing detailsCluster algorithmHuman body
The invention discloses a partition method on human motion 3D data. First, use manifold analysis method ISOMAP to map the original human sequence motion data to the low dimensional manifold space; secondly, utilize a heuristic method to detect rough partition points of different types of movement in the motion data sequence, then long sequence of human motion data are partitioned to several segments; last, for every two segments concatenated in time in the low dimensional manifold space use K-average clustering algorithm to compute the accurate partition point.
Owner:ZHEJIANG UNIV
New stapled-peptides and uses thereof
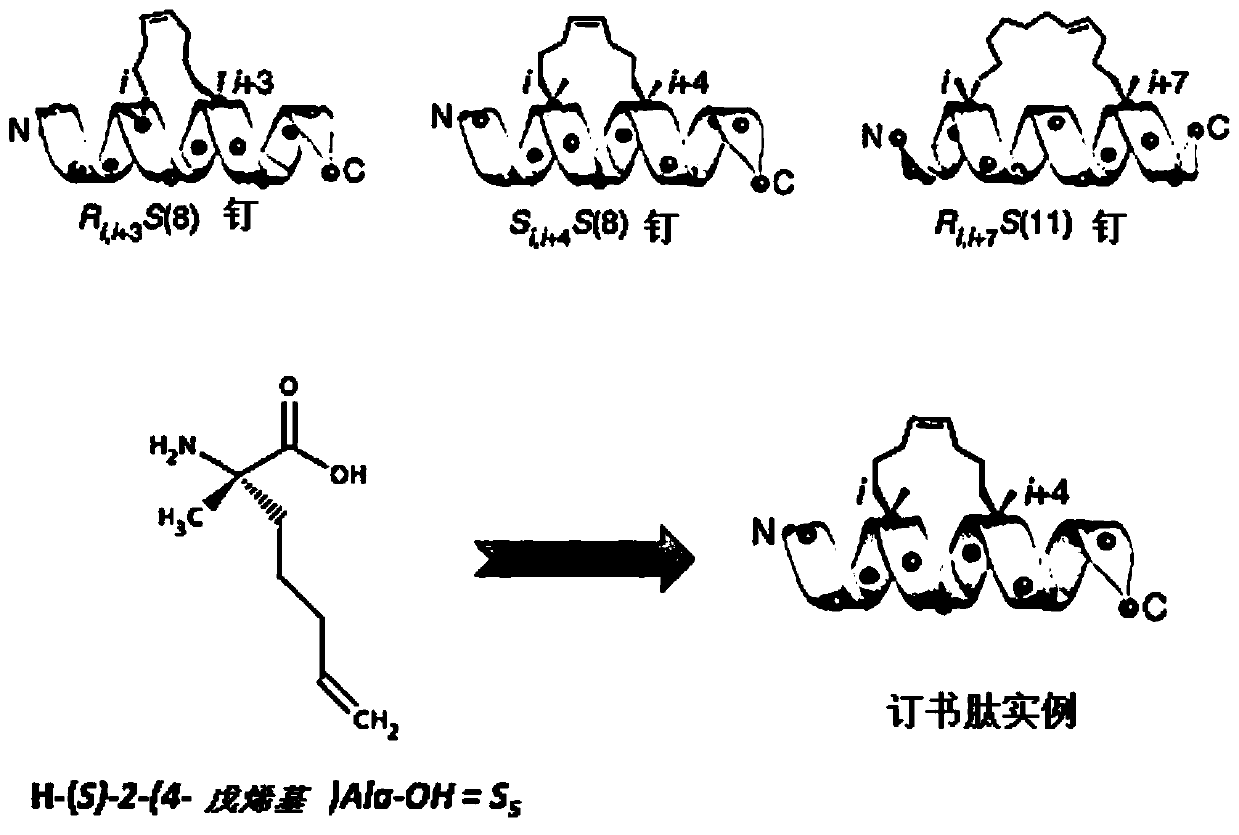
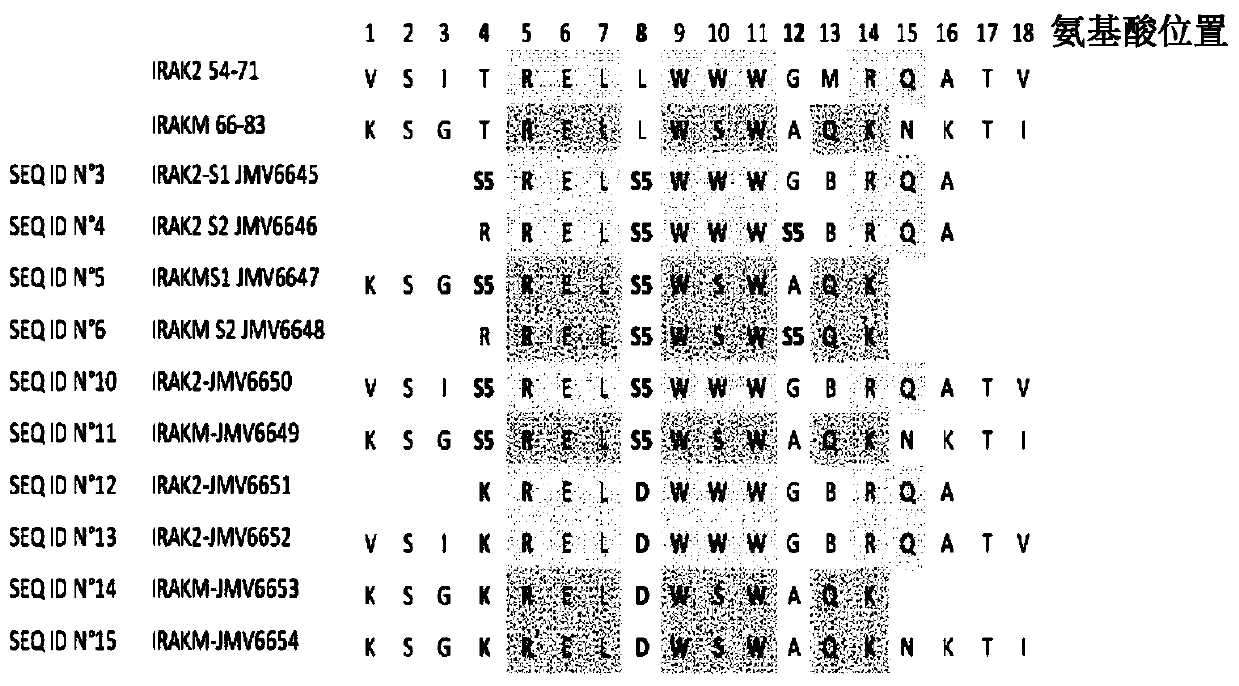
The present invention relates to peptidomimetic macrocycles comprising at least one macrocycle-forming linker and an amino acid sequence chosen from the group consisting of : i) an amino acid sequence with at least about 50%, 60%, 70%, 80%, 90%, or 95% sequence identity to a human sequence IRAK2 54-71 (SEQ ID No.1) and 100% identity with the amino acids in the positions 5-6, 9-11, 14-15 or ii) an amino acid sequence with at least about 50%, 60%, 70, 80%, 90%, or 95% sequence identity to a human sequence IRAKM 66-83 (SEQ ID No.2) and 100% identity with the amino acids in the positions 5-6, 9-11, 13-14, wherein the peptidomimetic macrocycle comprises an a-helix and at least two natural or two non-natural amino acids crosslinked by a macrocycle-forming linker. It also concerns method of preparation of said peptidomimetic macrocycles and uses thereof, pharmaceutical composition and uses thereof, in particular as inhibitors of inflammatory pathways.
Owner:UNIVERSITY OF MONTPELLIER +4
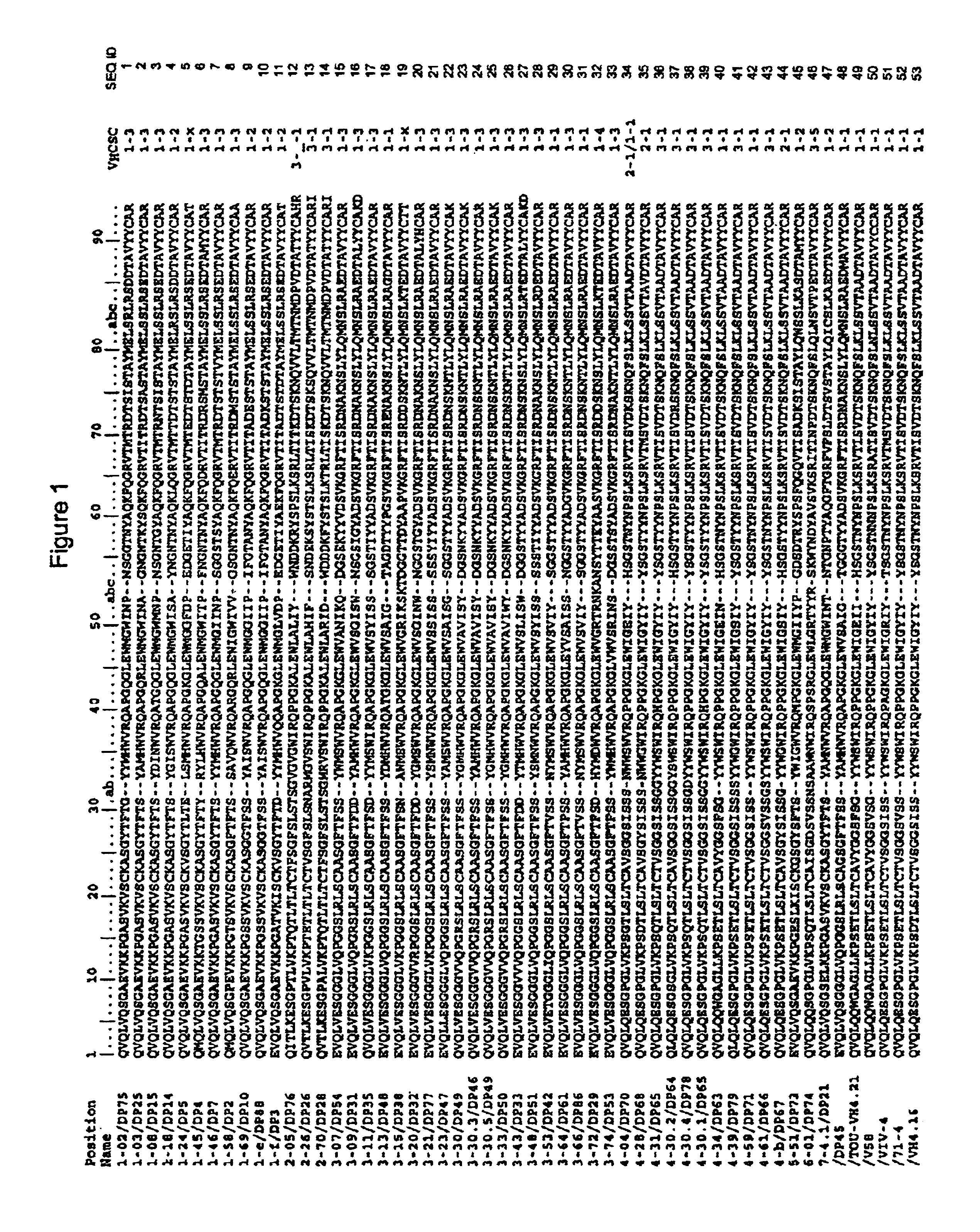
Functional humanization of complementarity determining regions (CDRS)
InactiveUS20100197896A1Maximizing numberAffecting specificityImmunoglobulinsFermentationComplementarity determining regionImmunglobulin e
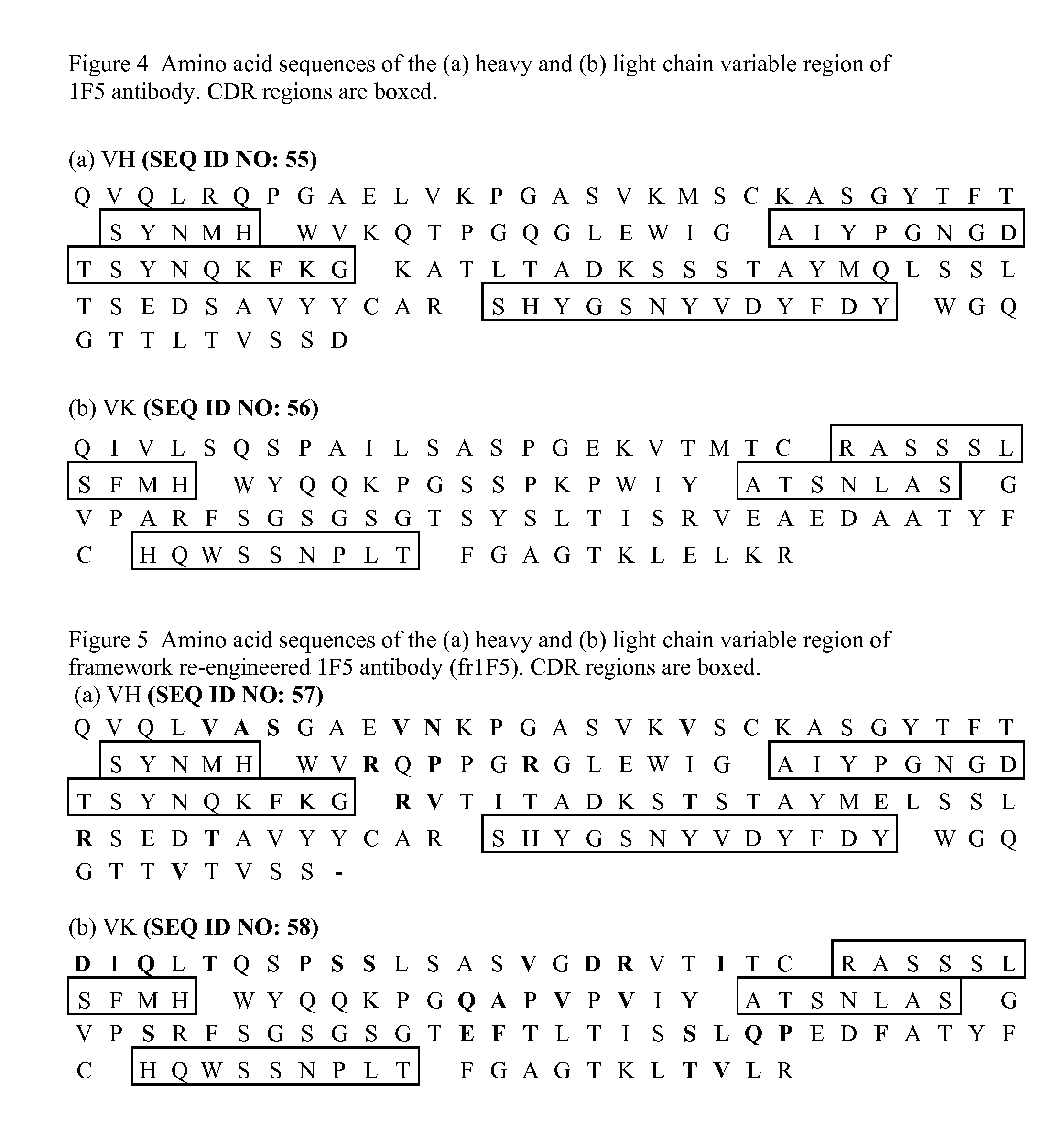
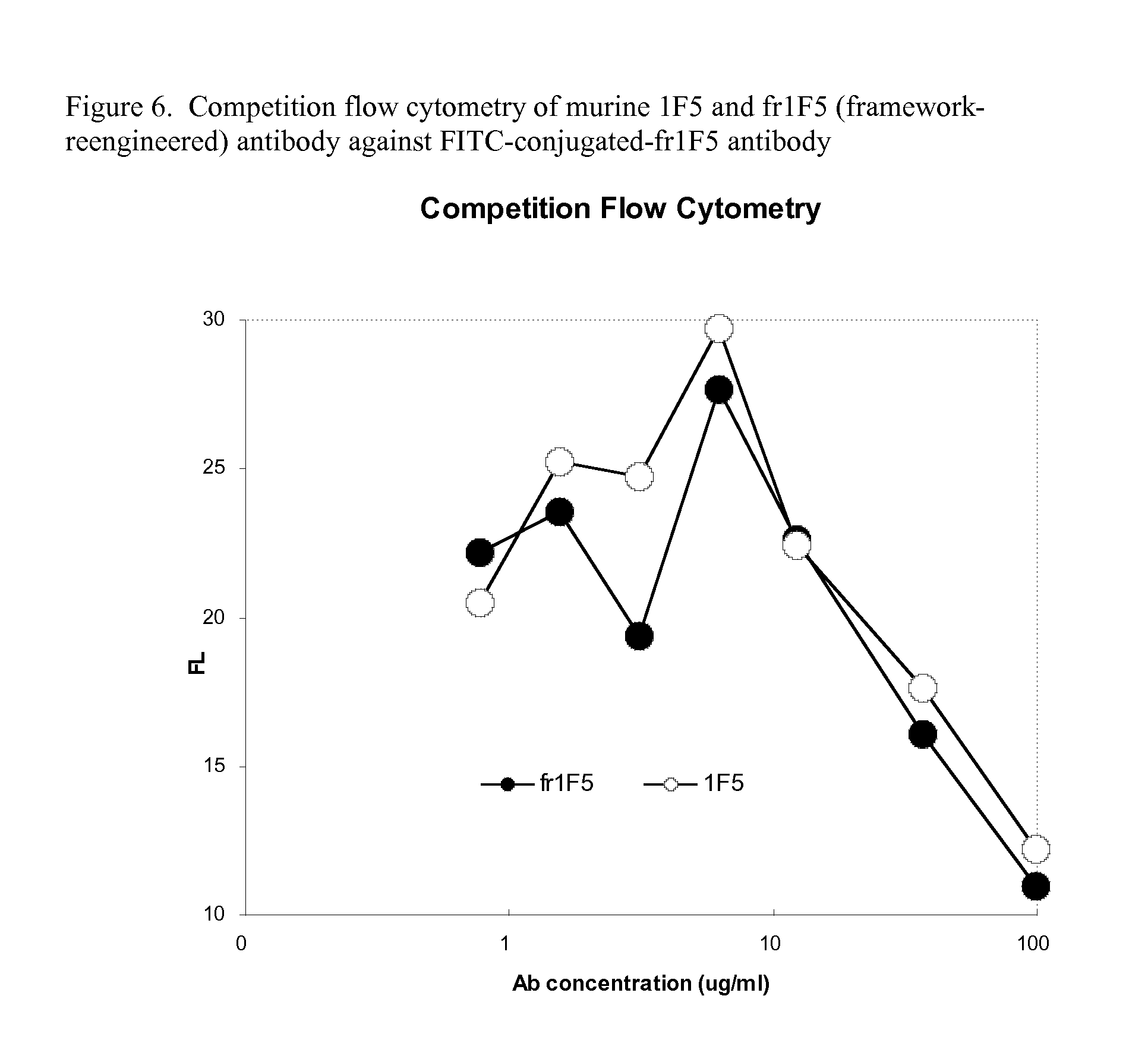
Current humanization approaches for immunoglobulins focus mostly on modifying the framework regions into human sequences. Herein is provided a method for humanizing antibody complementarity-determining regions (CDRs) through functional humanization to reduce the potential immunogenicity of non-human CDR-containing antibodies. CDRs with high sequence homology to the parent CDR are identified from a database of human CDR sequences. One or more human CDRs that are highly homologous to the parent CDR sequence can be used to replace the corresponding CDRs of murine immunoglobulins (or their humanized, or re-engineered versions). Human CDRs that improve or have minimal effects on the antigen binding affinity and specificity are adopted.
Owner:SINOMAB BIOSCI
Humanized model of kidney and liver disorders
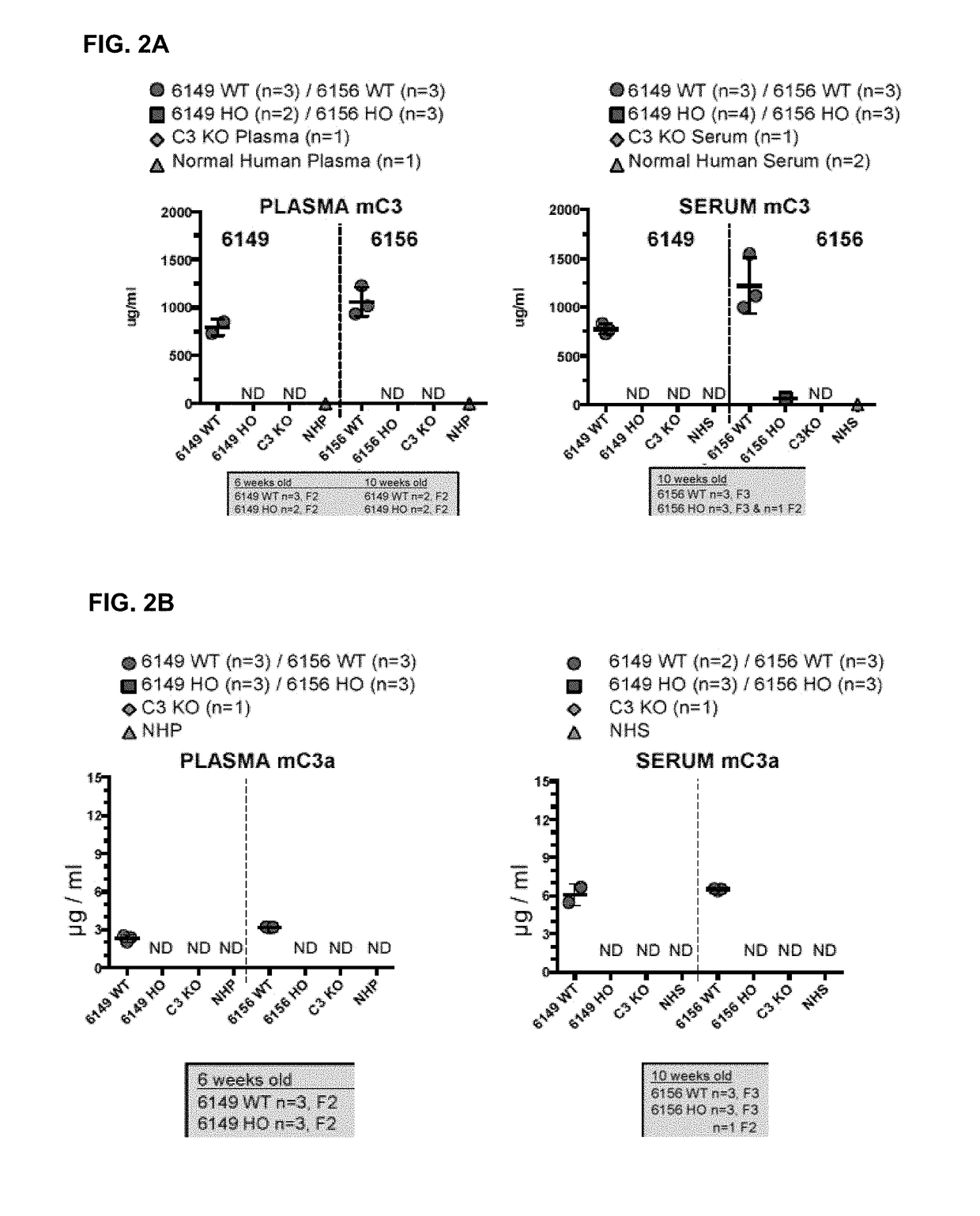
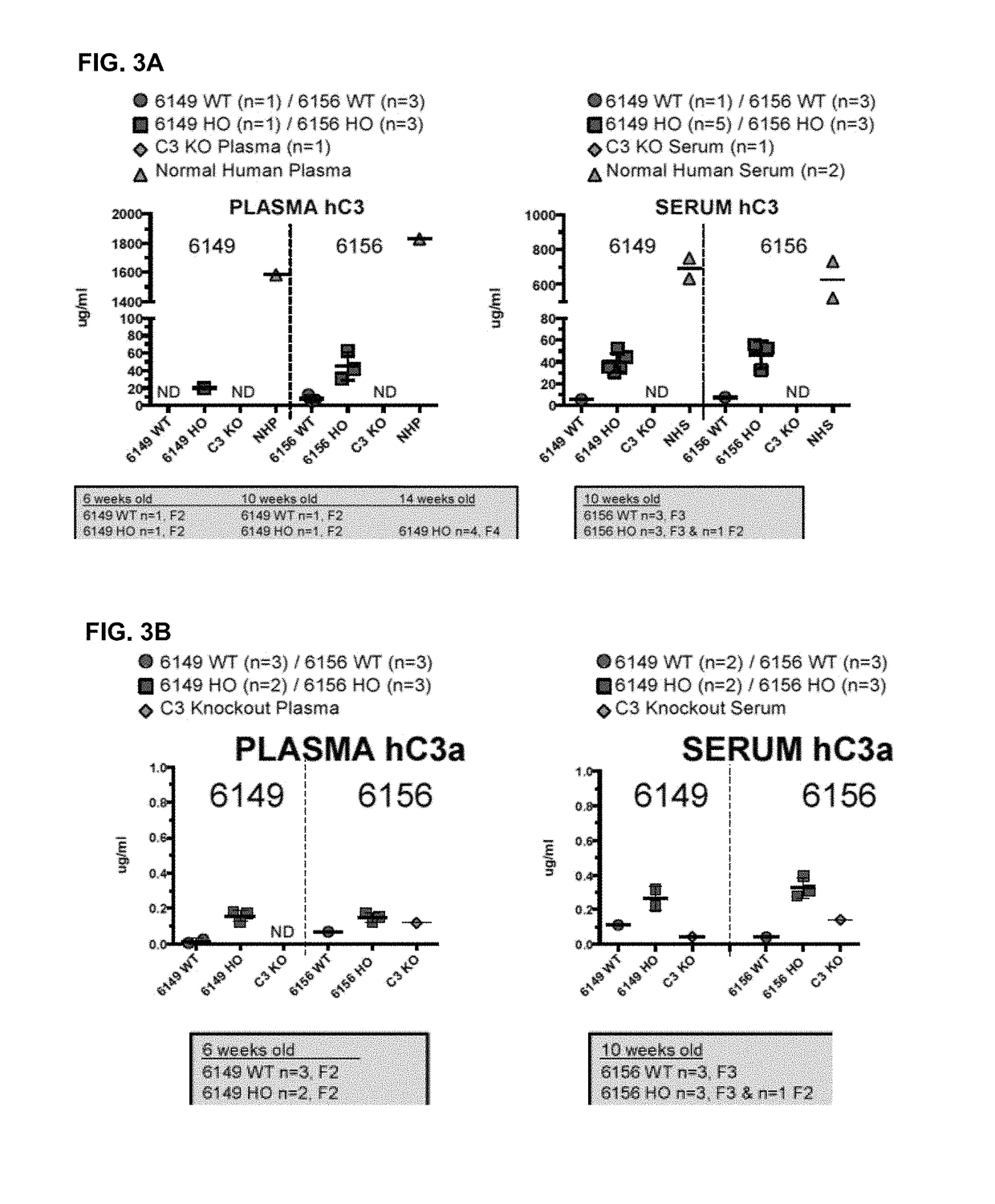
ActiveUS20180243450A1Improve kidney functionDecrease glomerular MAC formationCompounds screening/testingDigestive systemNephrosisHuman sequence
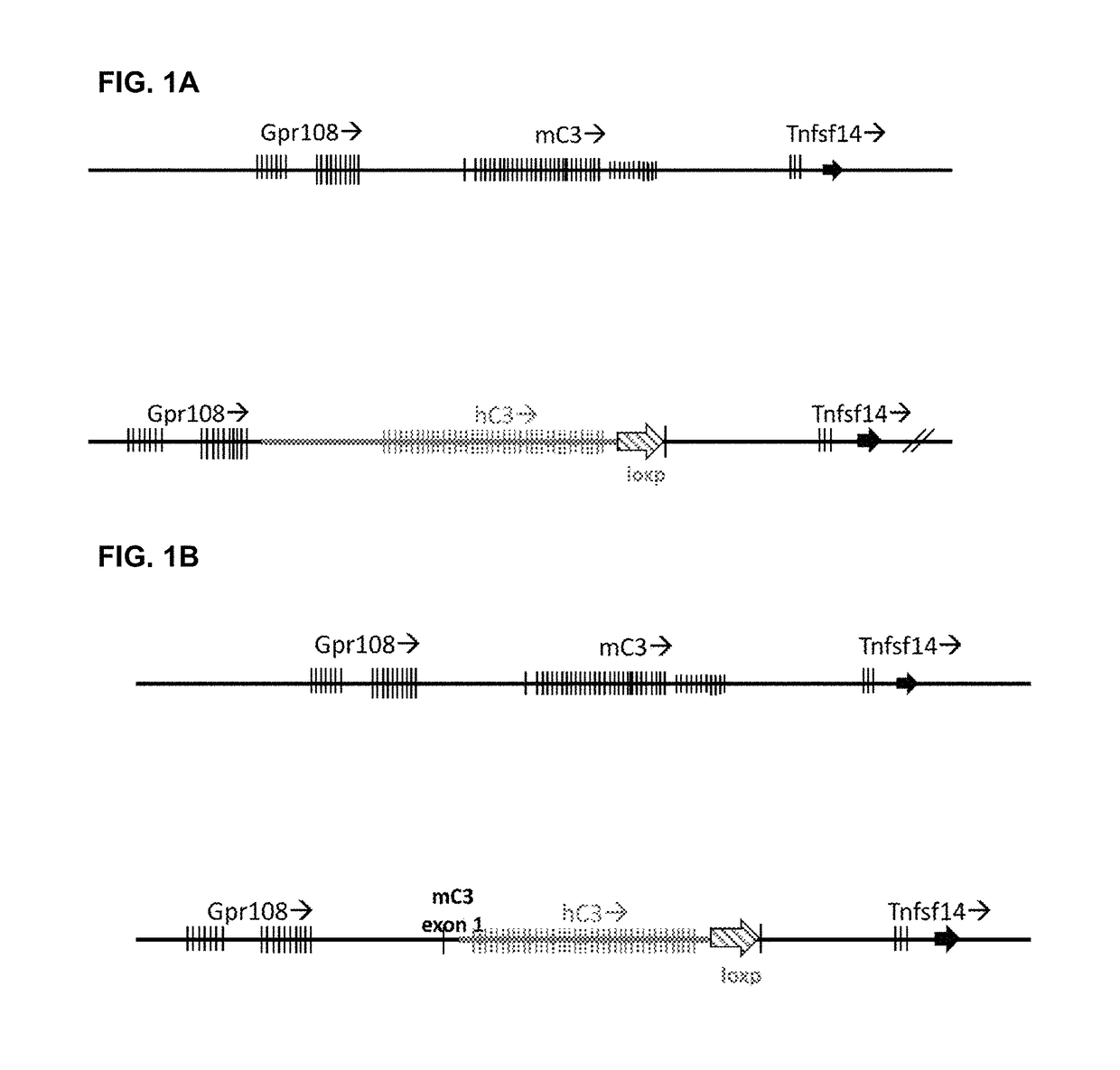
Provided herein, inter alia, are non-human animals comprising nucleic acid sequences encoding a C3 protein that comprises a human sequence as well as transgenic non-human animals comprising a C3 gene that is human in whole or in part as well as methods for using the same to screen for candidate therapeutic molecules to treat complement-related nephropathies. Also provided herein are methods for improving kidney function in an individual diagnosed with or thought to have a complement-related nephropathy.
Owner:REGENERON PHARM INC
PROCESS FOR CORRECTION OF A DISULFIDE MISFOLD IN Fc MOLECULES
InactiveUS20100267936A1Peptide/protein ingredientsAntibody mimetics/scaffoldsEscherichia coliImpurity
Owner:AMGEN INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com