PROCESS FOR CORRECTION OF A DISULFIDE MISFOLD IN Fc MOLECULES
a technology of fc molecules and disulfide misfolds, which is applied in the direction of drug compositions, peptide/protein ingredients, metabolic disorders, etc., can solve the problems of aggregation or stability problems in production, limited use of fc fusion molecules,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
working examples
[0149]The following disclosure(s) are illustrative rather than limiting.
example 1
Identification of Cysteines in a Fc Construct of Osteoprotegerin by CDAP Labeling and Alkaline-Induced Cleavage with LC-MS Analysis
Abstract
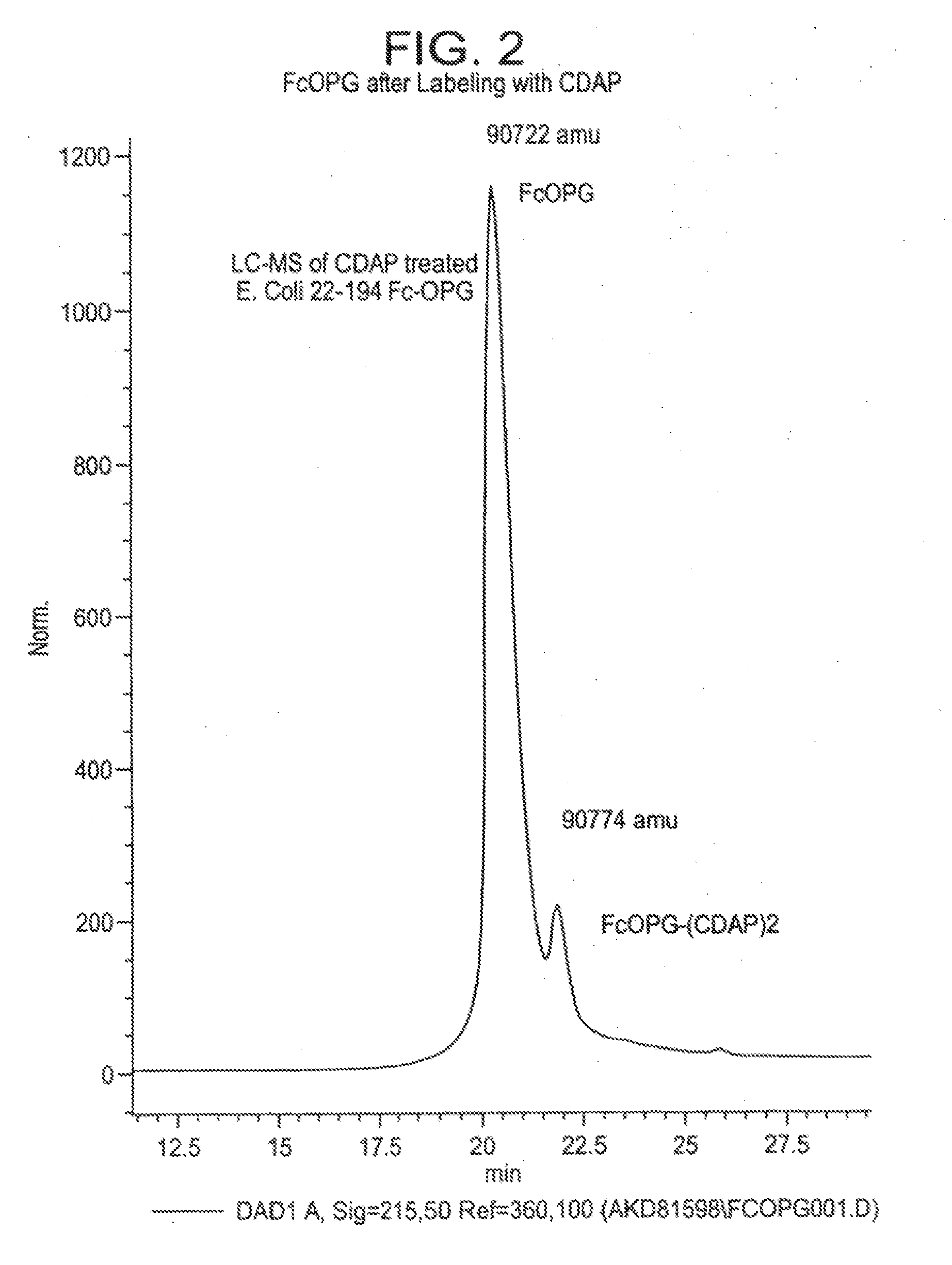
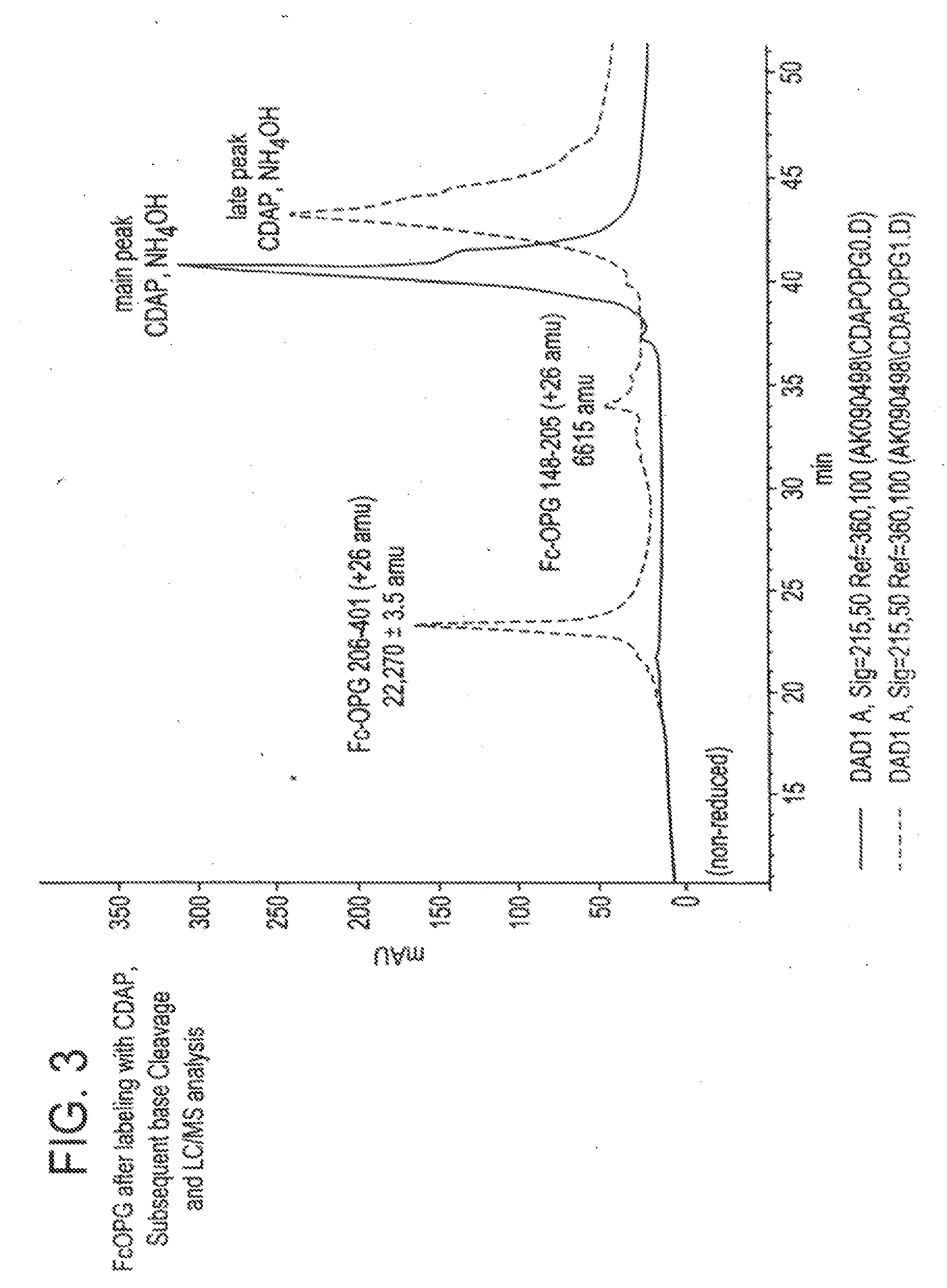
[0150]Purpose. To assay for cysteines in a Fc construct of Osteoprotegerin stored in acidic media and to ascertain their location.
[0151]Methods. The reagent 1-cyano-4-dimethylamino-pyridinium tetrafluoroborate (CDAP) was employed to selectively cyanylate stable free-sulfhydryl groups at pH 4. Cyanylation reactions were analyzed by HPLC coupled with mass-spectral analysis (LC-MS). Reversed-phase HPLC was used to separate component peaks from the native and CDAP-reacted samples. The reversed-phase samples were collected, introduced into a solution of aqueous ammonium hydroxide plus guanidine hydrochloride and subsequently analyzed by LC-MS. Results. Reversed-phase HPLC analysis of Fc-Osteoprotegerin gave two peaks. LC-MS of the primary CDAP reaction revealed selective cyanylation of two-sulfhydryl groups in a peak that represents approximately 10% ...
example 2
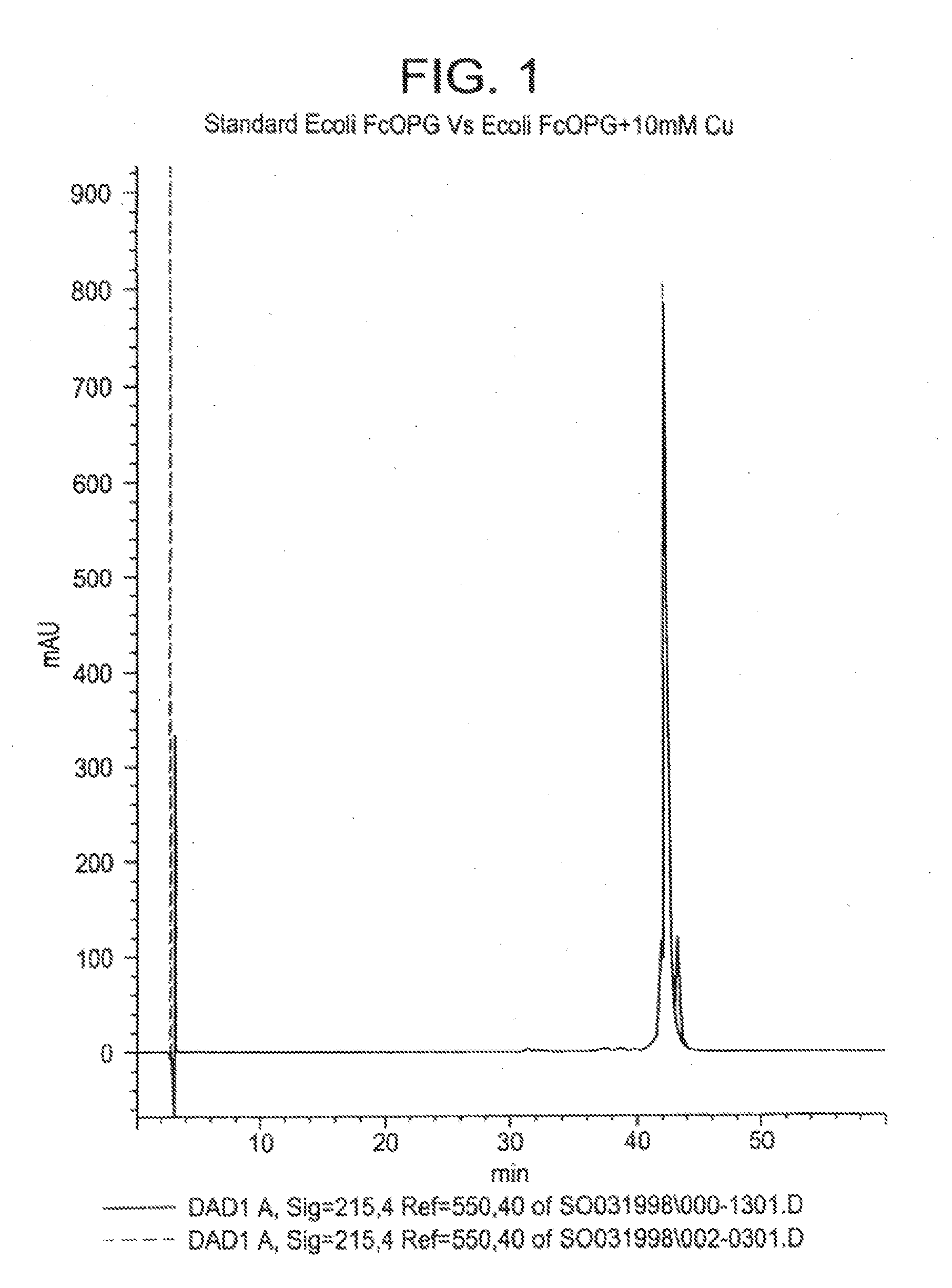
[0167]Purpose. Significant differences were observed in the stability of Fc-OPG as a function of copper treatment of the bulk protein, where Fc-OPG treated with copper was significantly more stable than Fc-OPG that was not. Specifically, the Fc-OPG that was not treated with copper was more prone to aggregation than the copper-treated Fc-OPG. The improved stability of Fc-OPG is thought to be due to the conversion of an unstable fraction of Fc-OPG with incomplete disulfide structure to a form with intact disulfide bonding upon treatment with copper ion.
[0168]Methods. Reversed-phase chromatography: Reversed-phase chromatography was performed on a Hewlett-Packard 1100 or 1090 HPLC system equipped with a diode-array detector and controlled by Chemstation Software. Fc-OPG samples were analyzed on a Zorbax (4.6 mm×25 cm) 300SB column. The column is initially equilibrated at 90% buffer A (HPLC grade water containing 0.1% TFA). The samples were eluted with a linear gradient of 4.2% buffer B / ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com