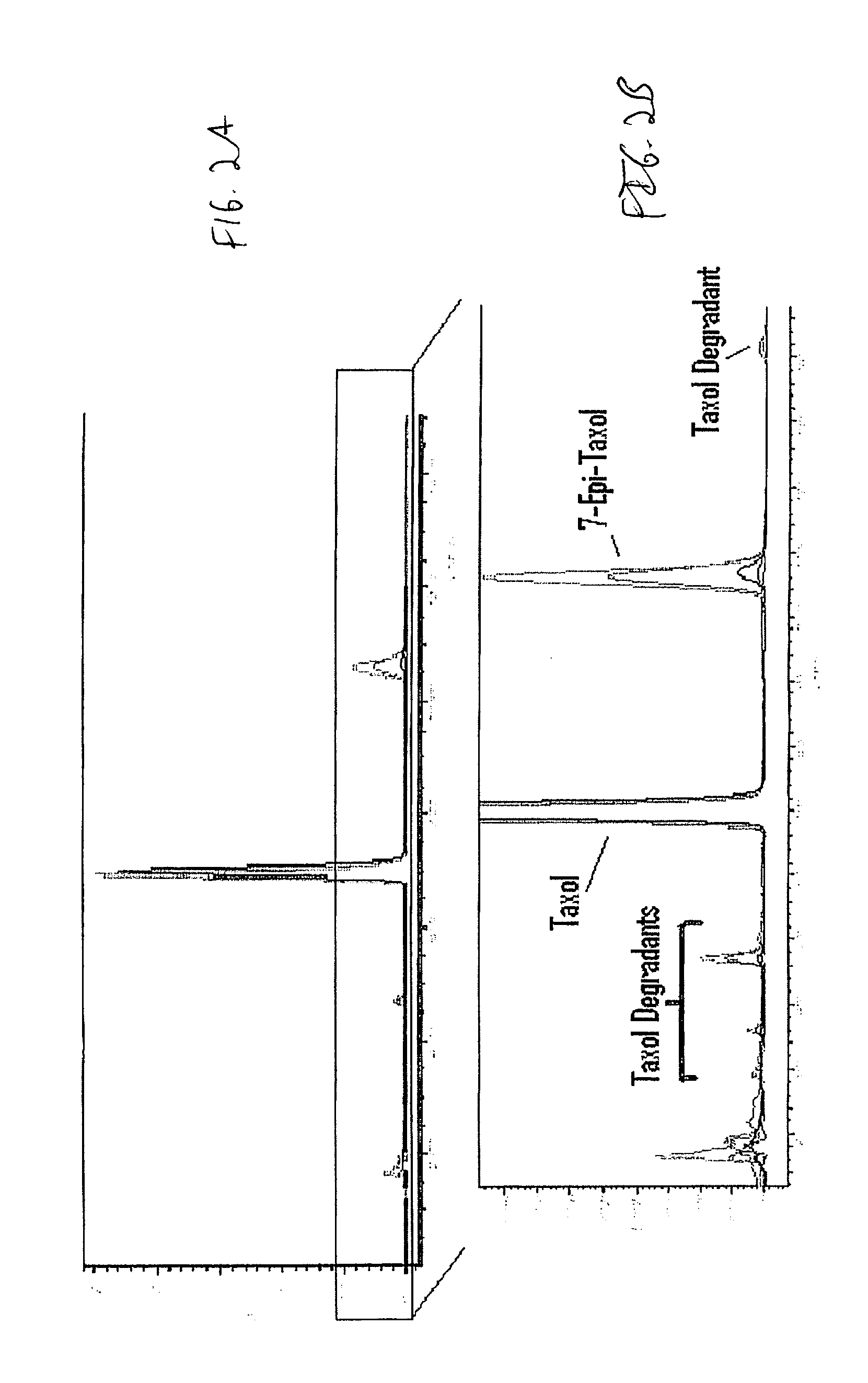
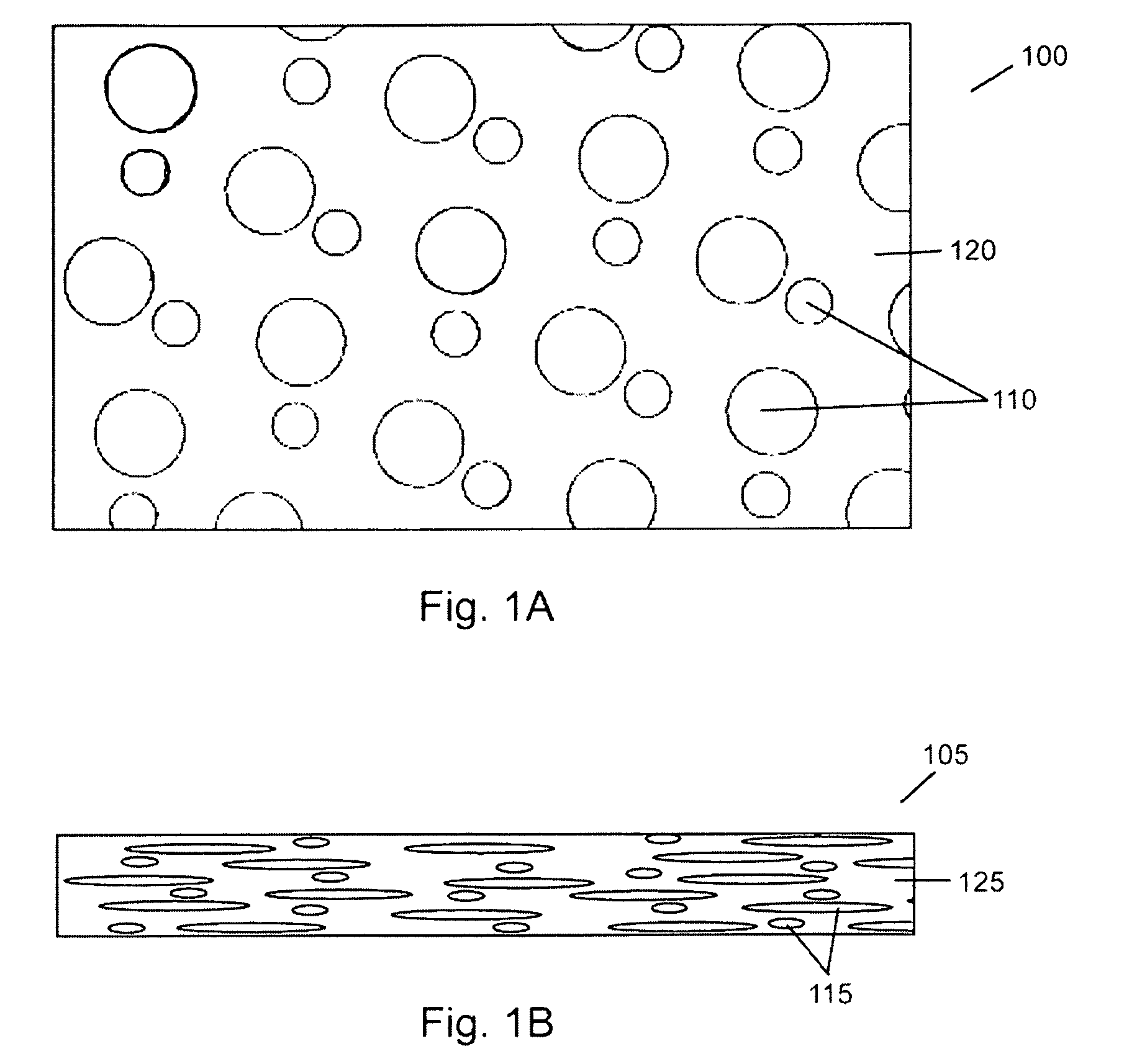
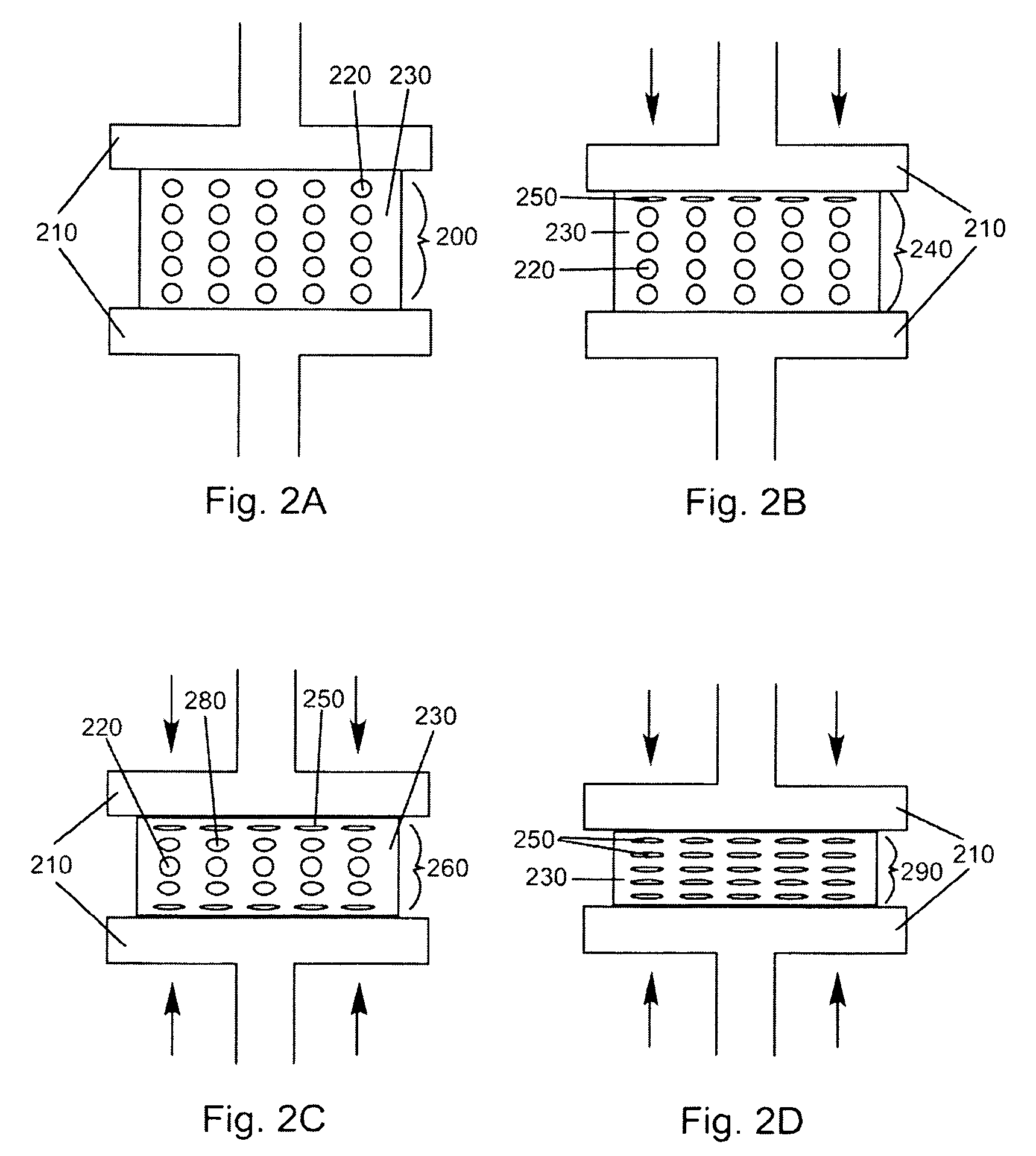
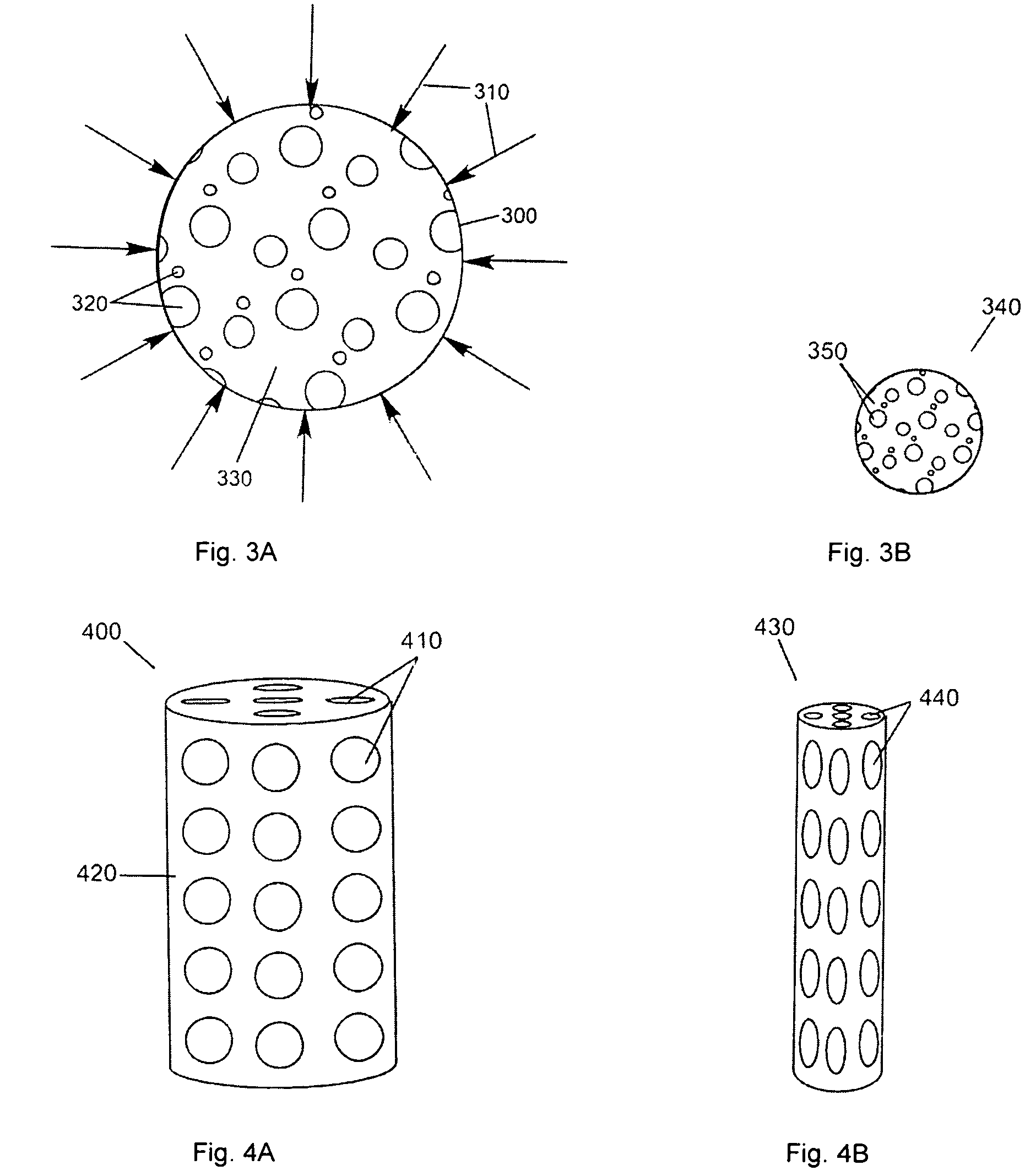
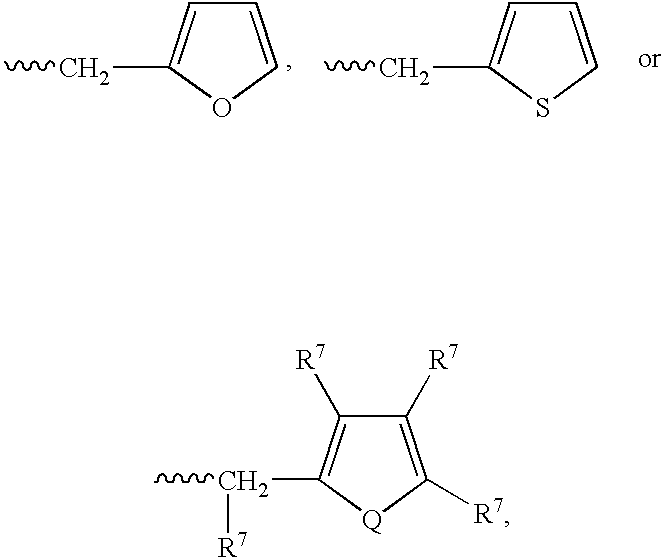
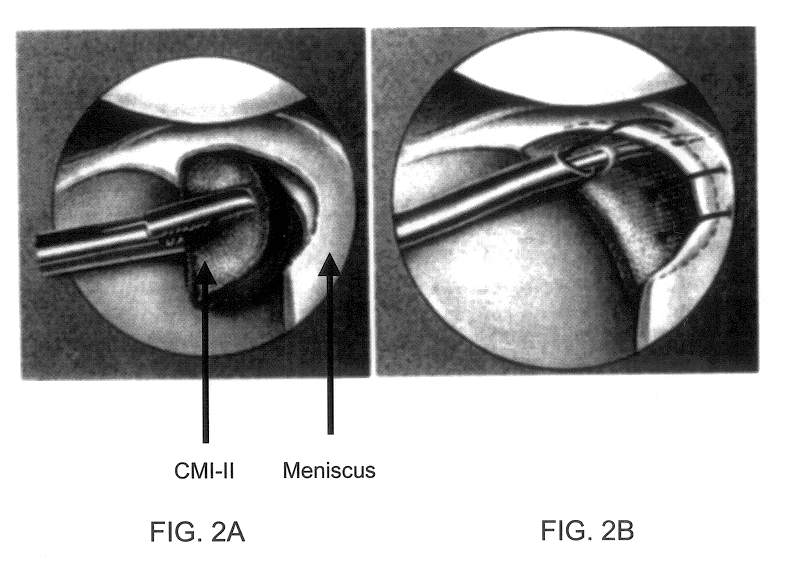
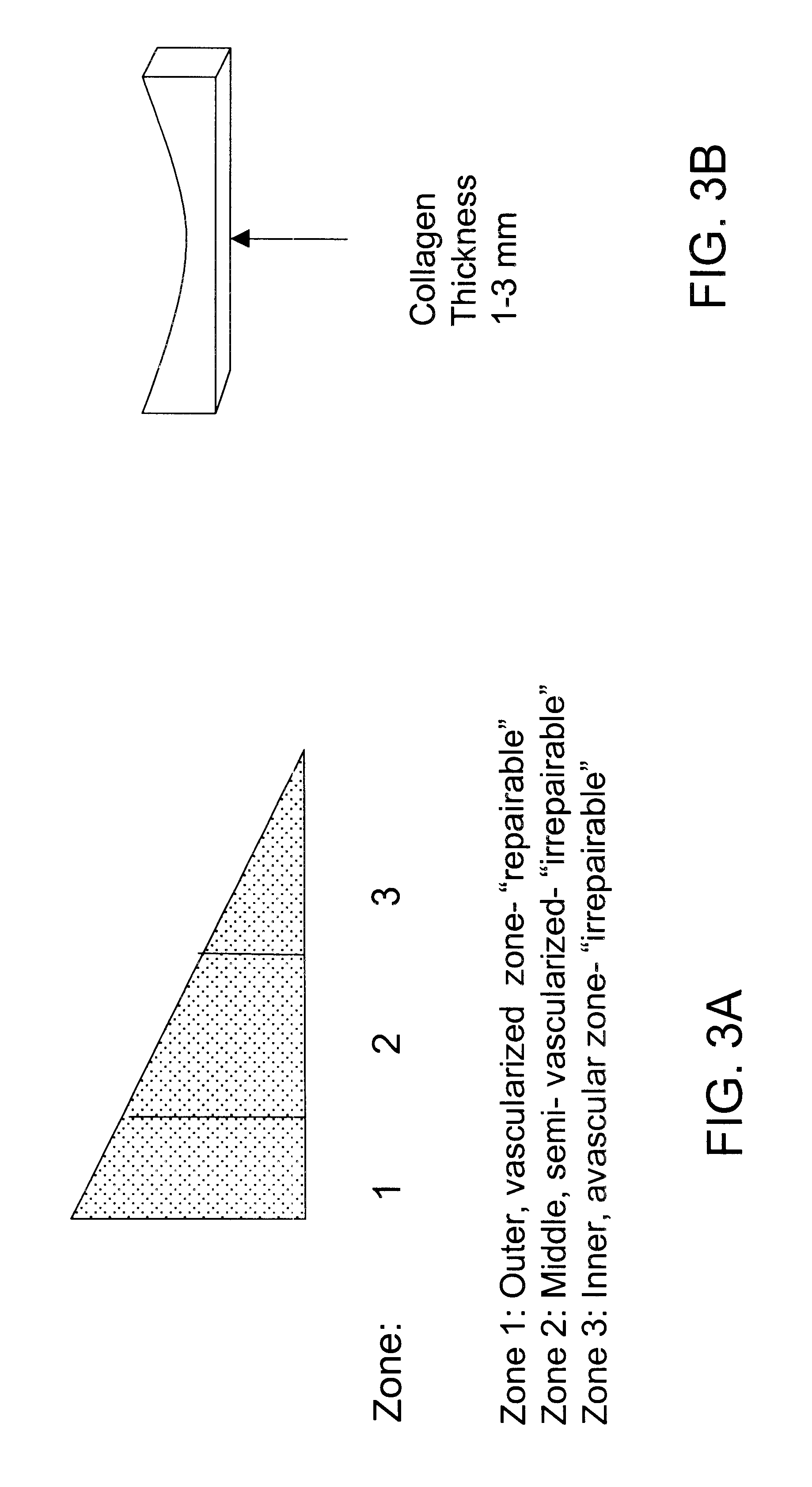
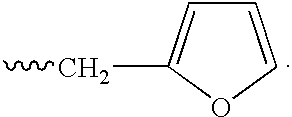Patents
Literature
55148results about "Skeletal disorder" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
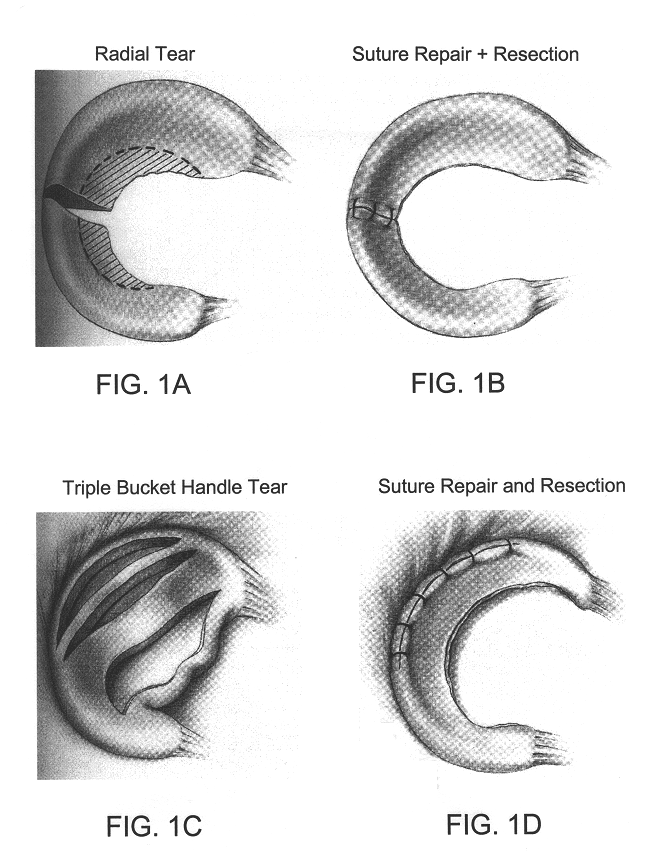
Devices and methods for treating defects in the tissue of a living being
InactiveUS7166133B2Restore mechanical and architectural and structural competenceSuture equipmentsPeptide/protein ingredientsHost tissueBiomedical engineering
Owner:DSM IP ASSETS BV
Plasma protein matrices and methods for their preparation
InactiveUS7009039B2Rapid cell growthRapid vascularizationBiocidePeptide/protein ingredientsBiological propertyFreeze-drying
A freeze dried biocompatible matrix comprising plasma proteins, useful as implants for tissue engineering as well as in biotechnology, and methods of producing the matrix are provided. Mechanical and physical parameters can be controlled by use of auxiliary components or additives which may be removed after the matrix is formed in order to improve the biological properties of the matrix. The matrices according to the present invention may be used clinically per se, or as a cell-bearing implant.
Owner:PROCHON BIOTECH
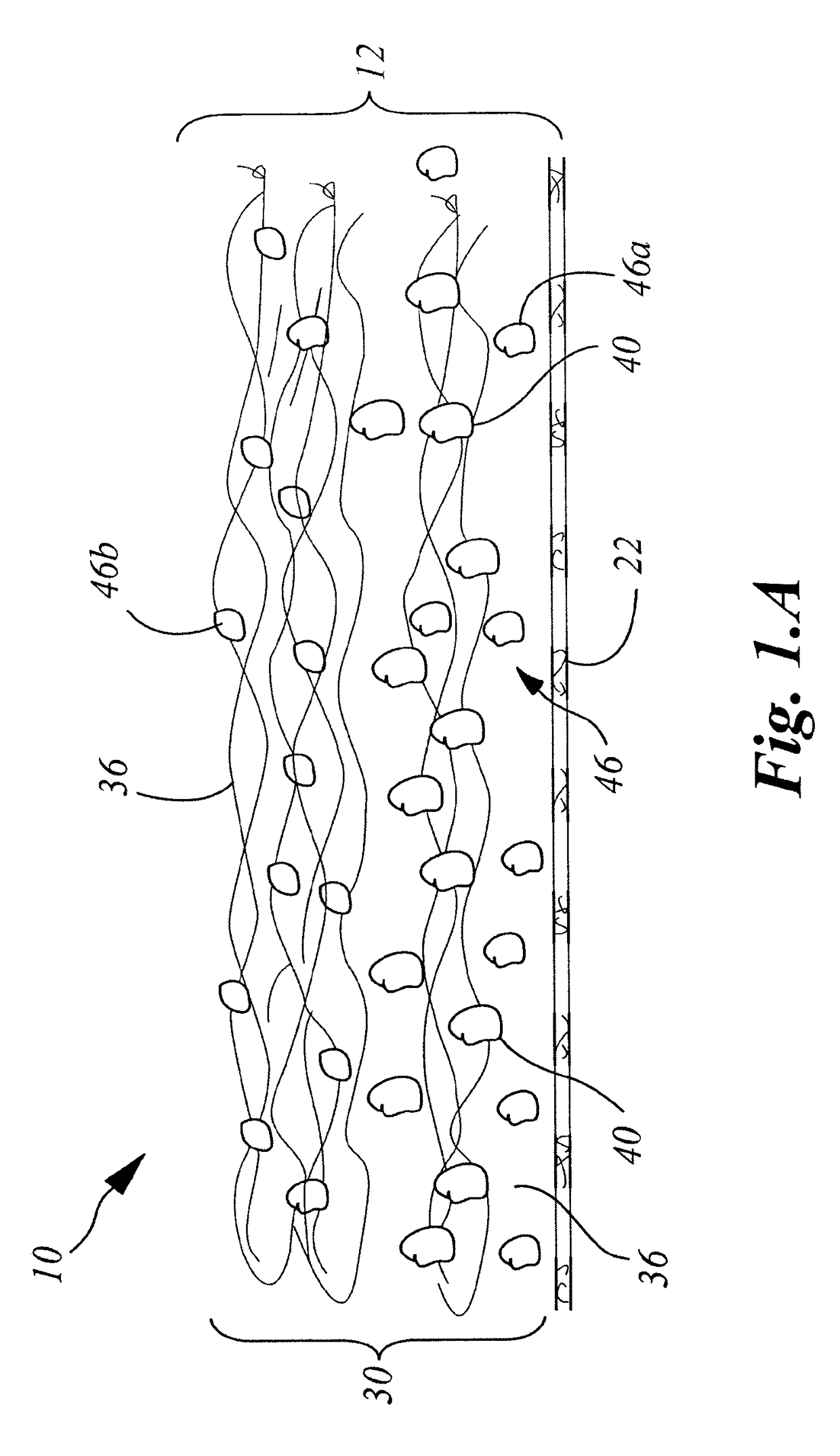
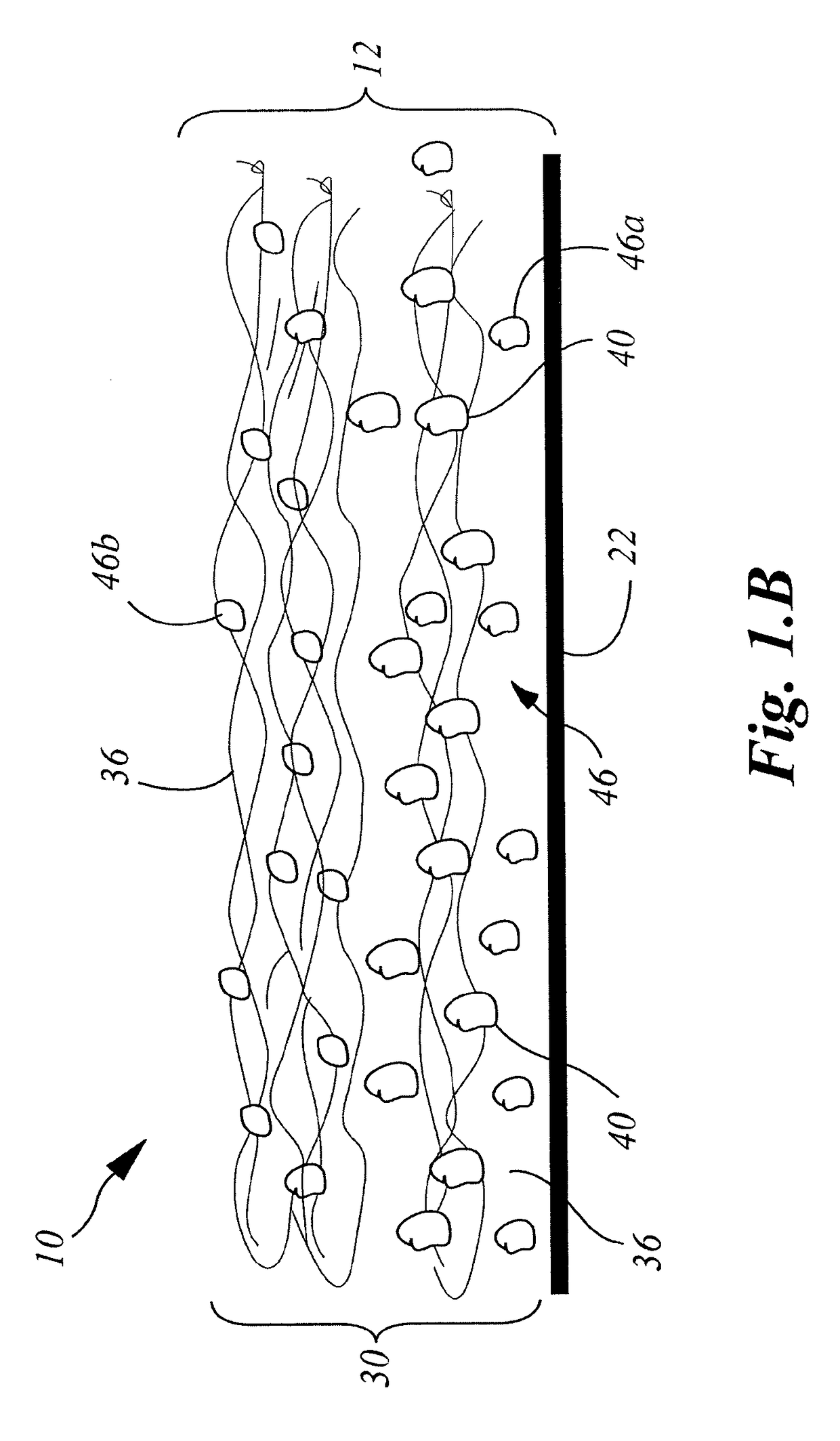
Wound closure material
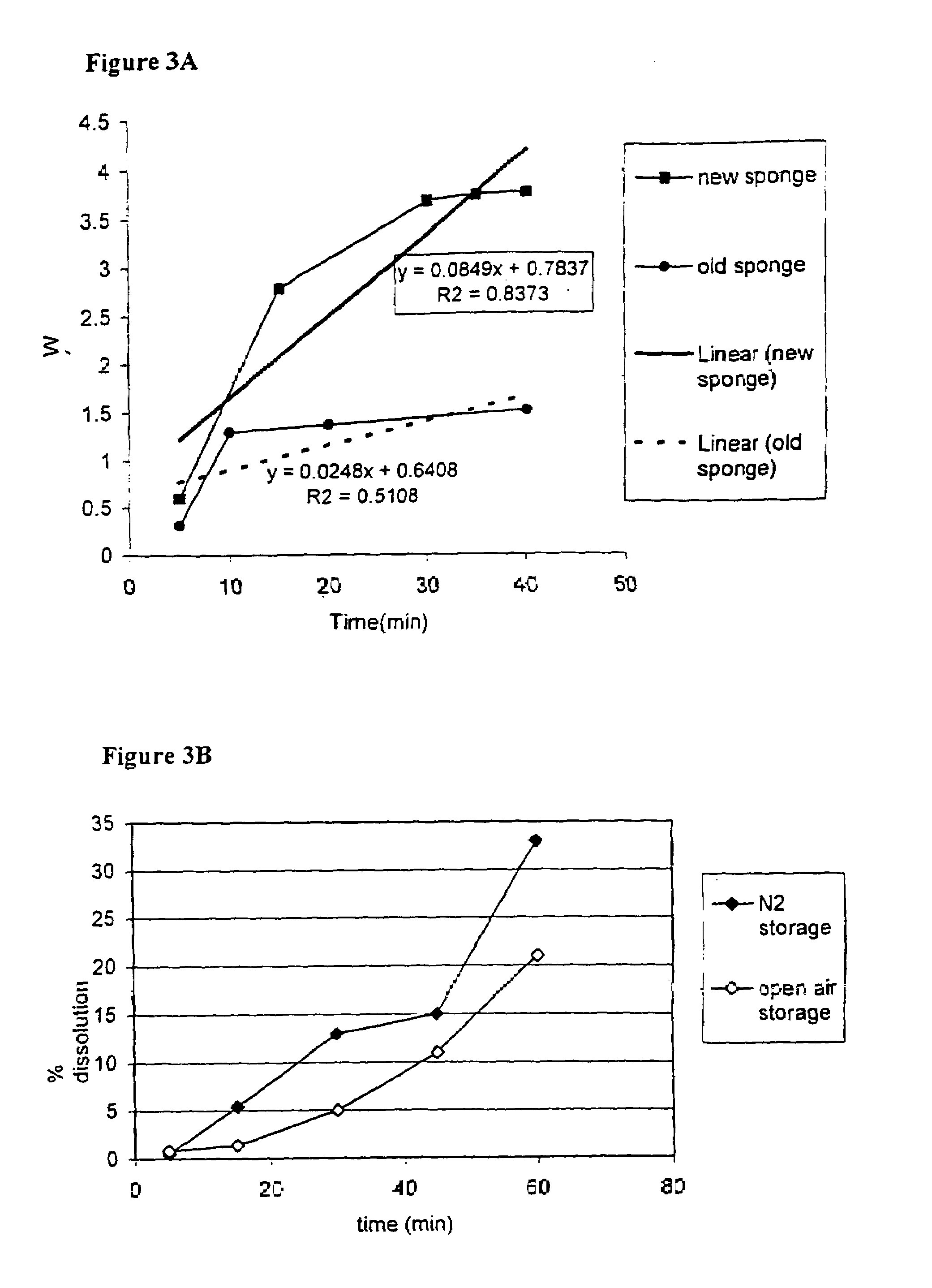
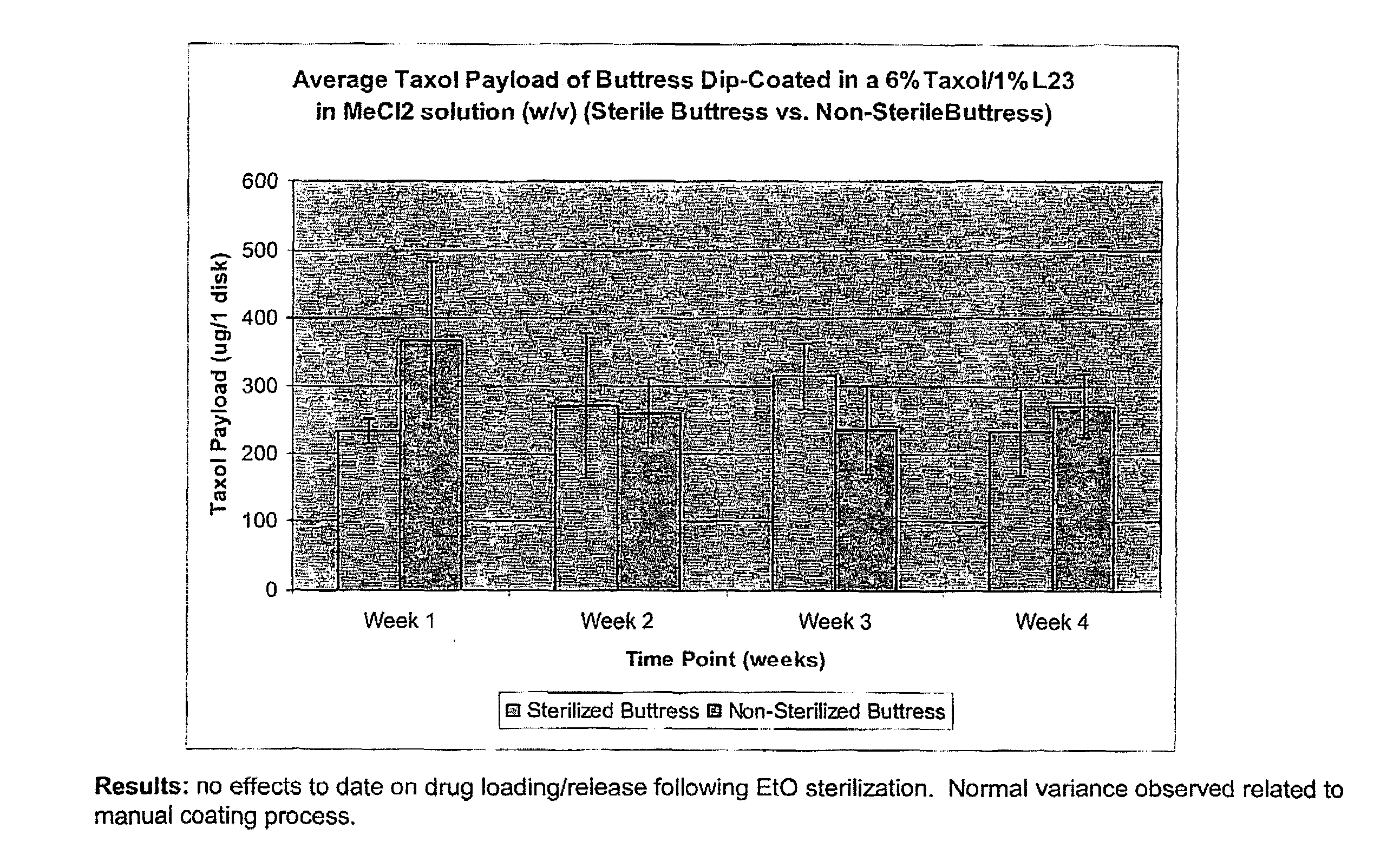
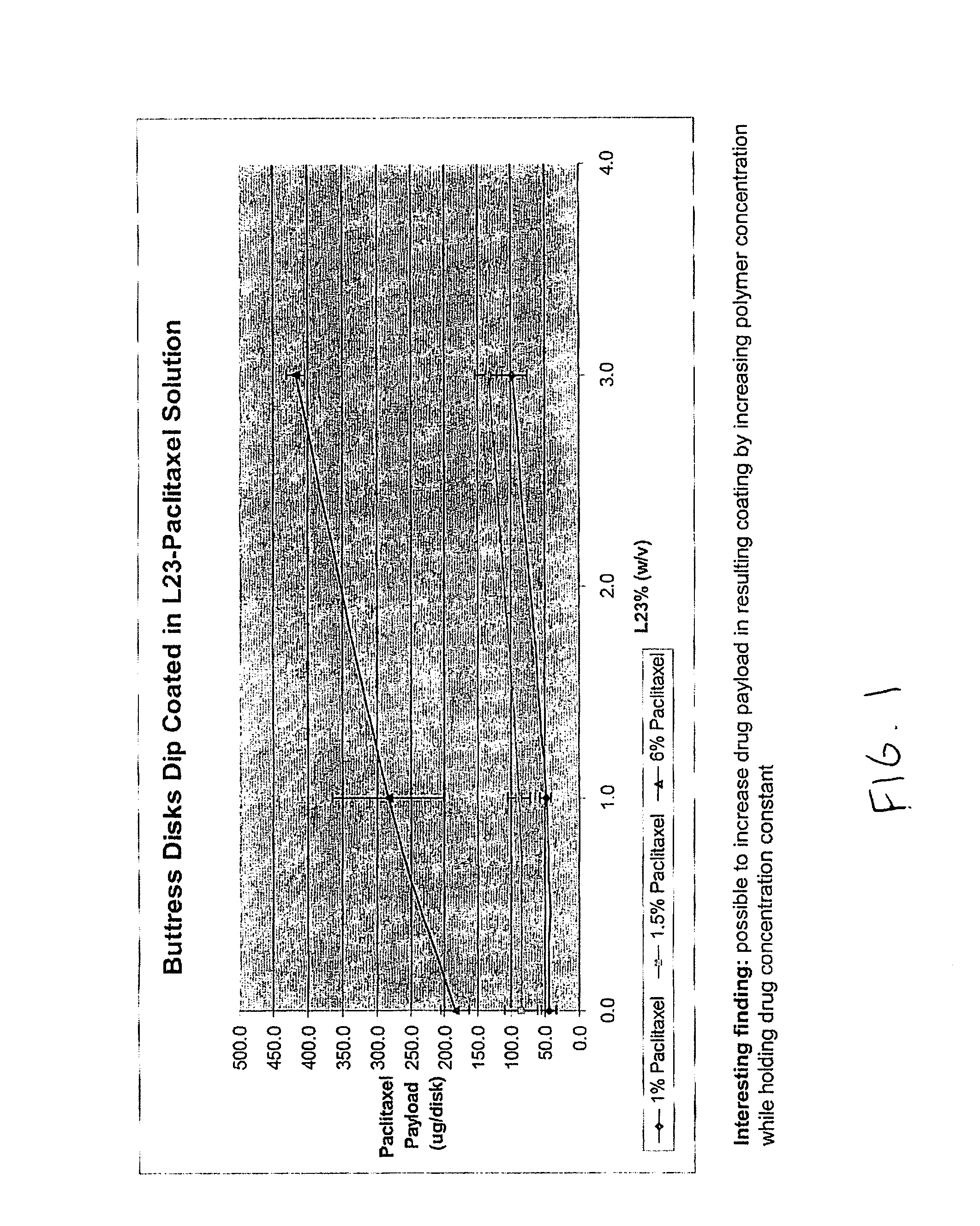
InactiveUS20100076489A1Prevent leakageSuture equipmentsHeavy metal active ingredientsButtressBiomedical engineering
Articles are provided having no orientation or a multi-directional orientation. Such articles may be in the form of films, ribbons, sheets, and / or tapes and may be utilized as buttresses with a surgical stapling apparatus or as reinforcing means for suture lines. The articles may be produced with a polymeric material having an agent, such as a chemotherapeutic agent or a radiotherapeutic agent, incorporated therein or applied as a coating thereon.
Owner:TYCO HEALTHCARE GRP LP
Bi-phasic compressed porous reinforcement materials suitable for implant
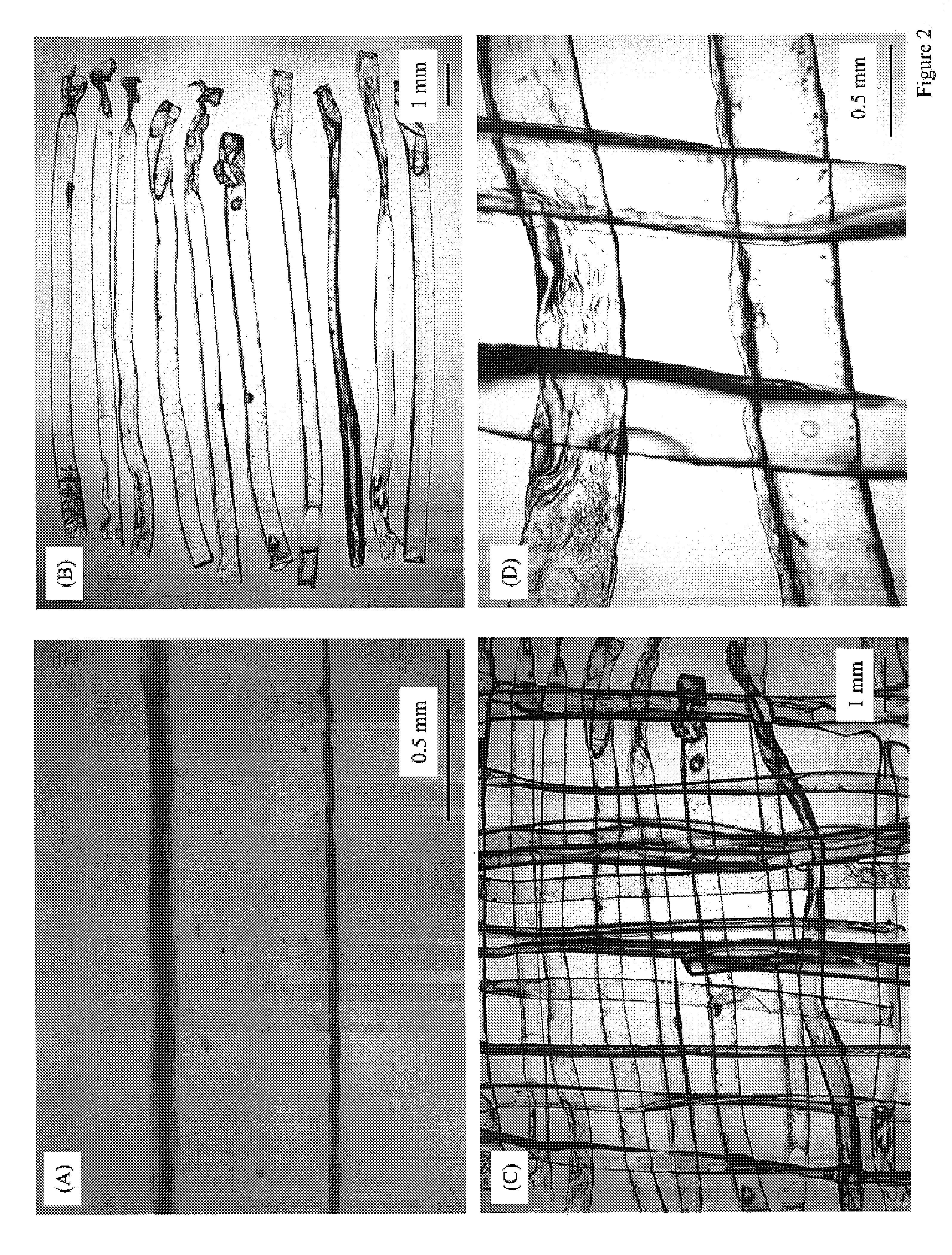
ActiveUS8389588B2Efficient use ofHigh and low porosity zoneBone implantSkeletal disorderFluid migrationUltimate tensile strength
Owner:DSM IP ASSETS BV
Human antibodies that bind human IL-12 and methods for producing
InactiveUS6914128B1Avoid interferencePreservationNervous disorderPeptide/protein ingredientsAntigen bindingIn vivo
Human antibodies, preferably recombinant human antibodies, that specifically bind to human interleukin-12 (hIL-12) are disclosed. Preferred antibodies have high affinity for hIL-12 and neutralize hIL-12 activity in vitro and in vivo. An antibody of the invention can be a full-length antibody or an antigen-binding portion thereof. The antibodies, or antibody portions, of the invention are useful for detecting hIL-12 and for inhibiting hIL-12 activity, e.g., in a human subject suffering from a disorder in which hIL-12 activity is detrimental. Nucleic acids, vectors and host cells for expressing the recombinant human antibodies of the invention, and methods of synthesizing the recombinant human antibodies, are also encompassed by the invention.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG
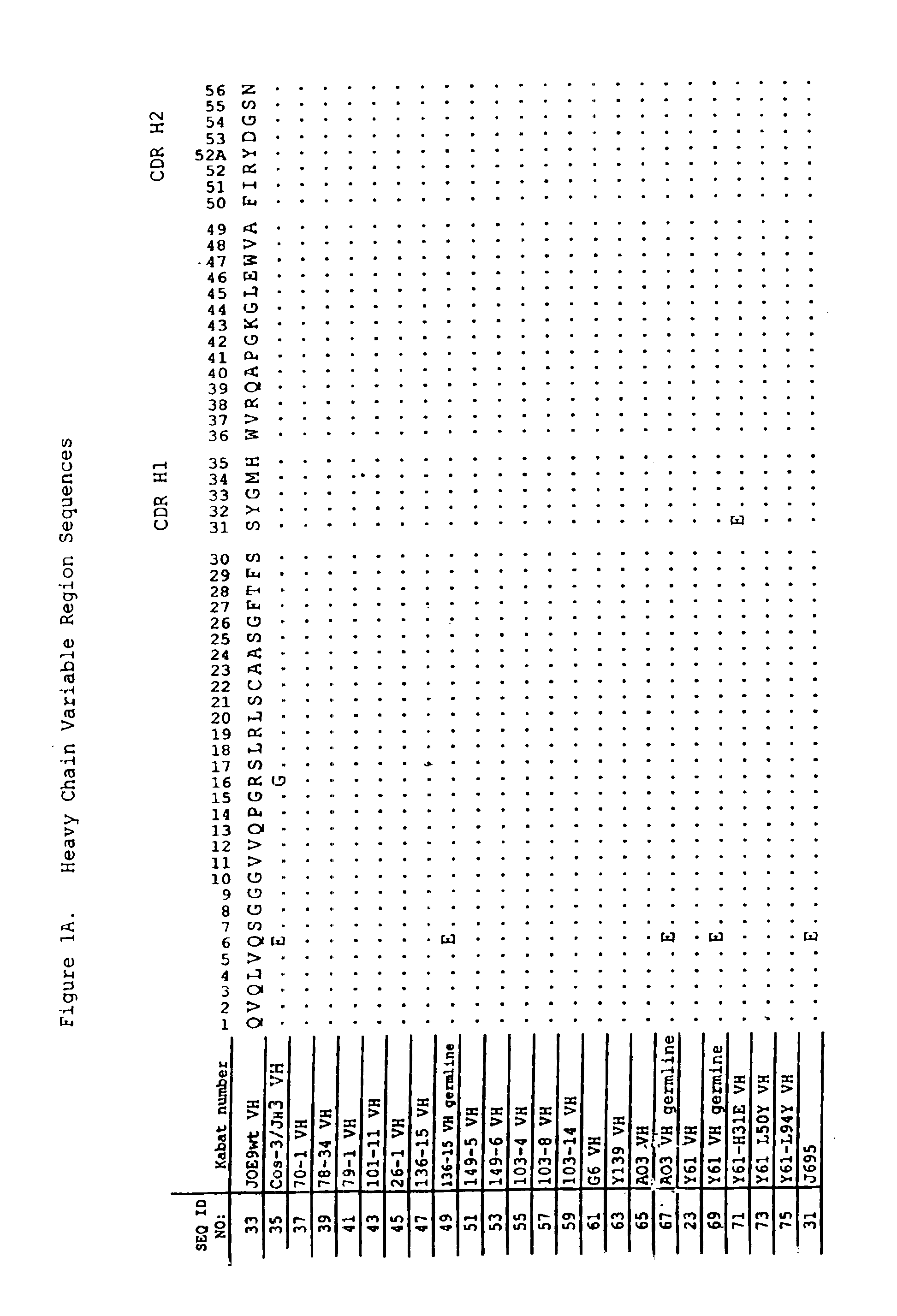
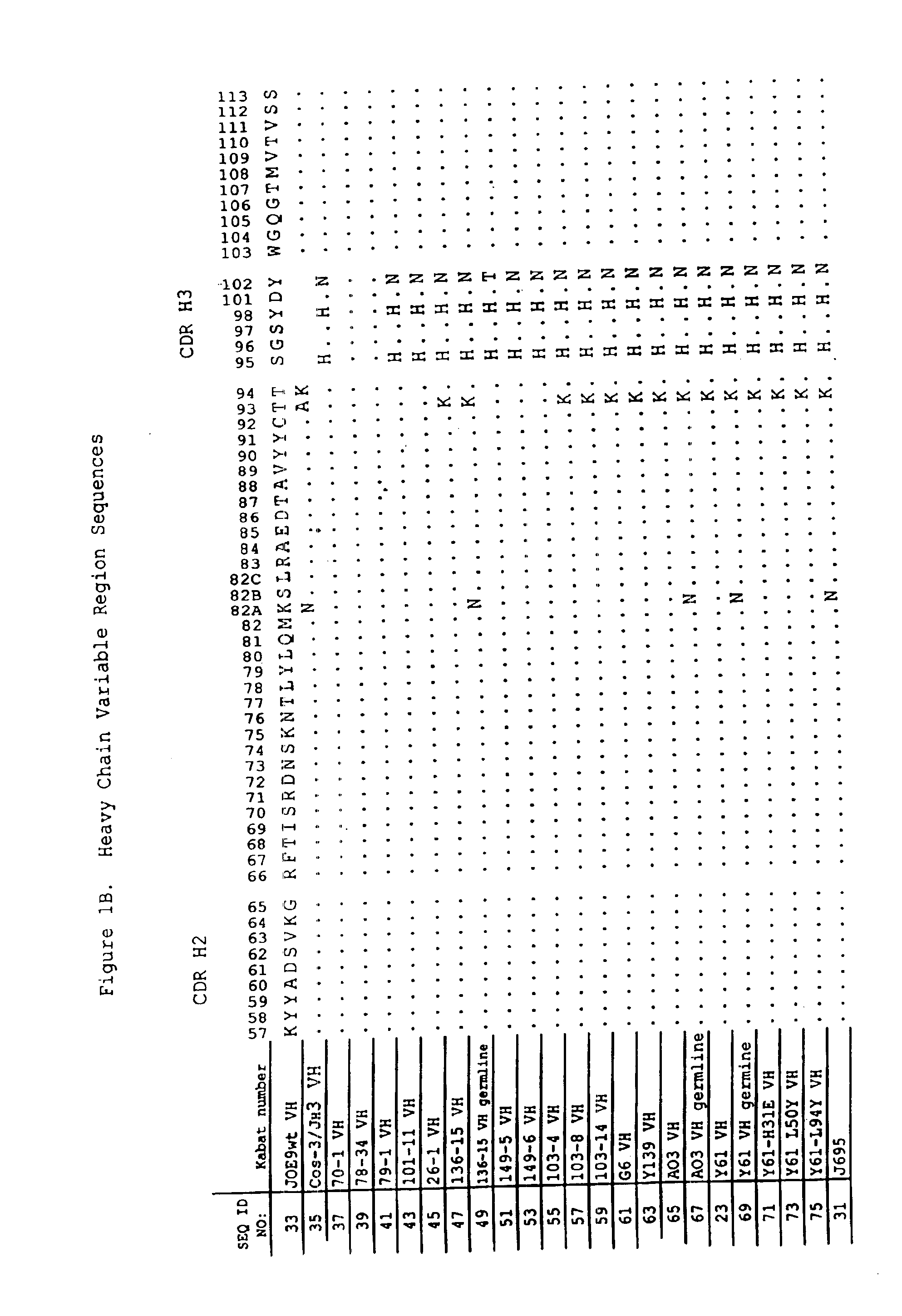
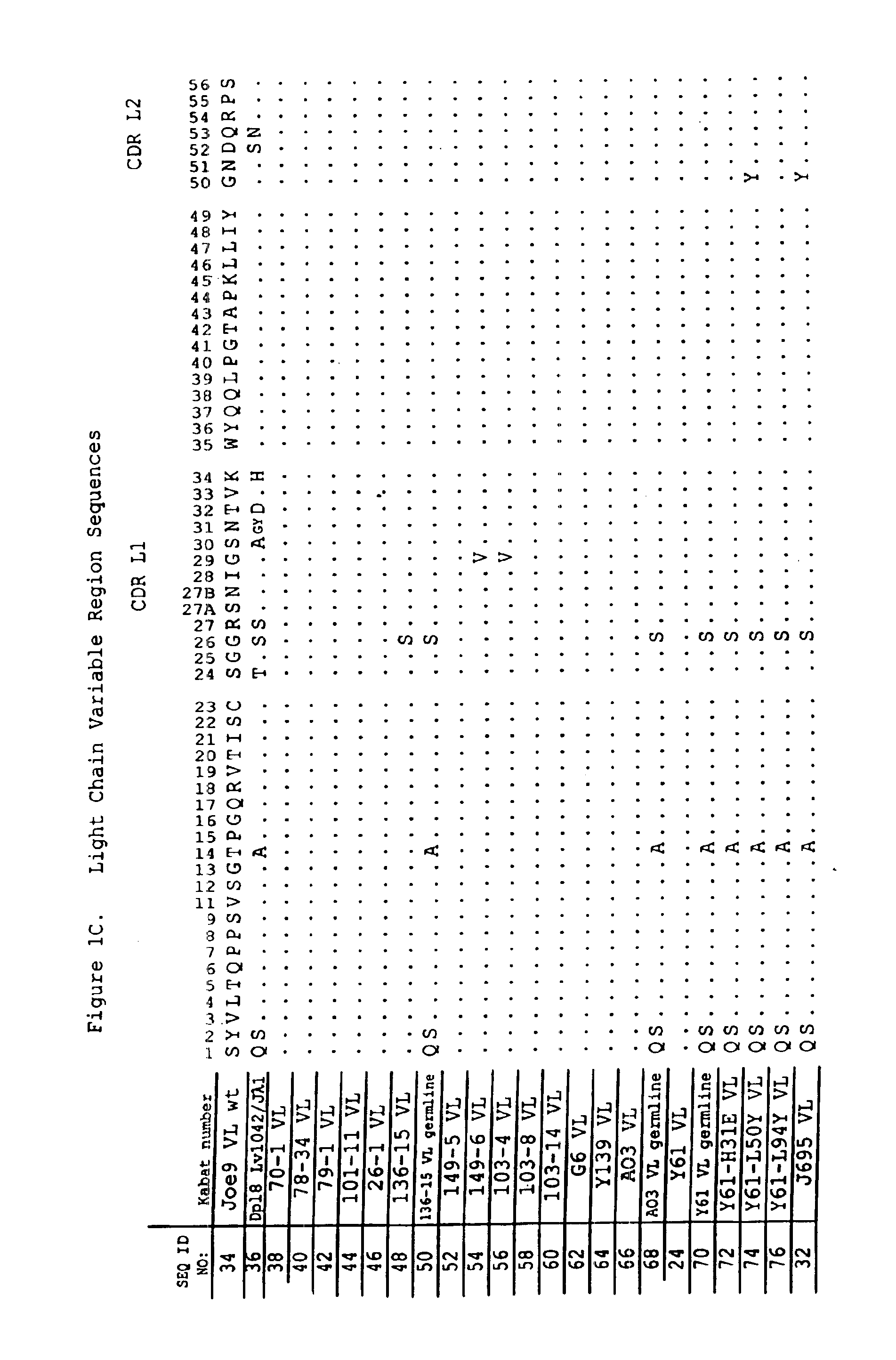
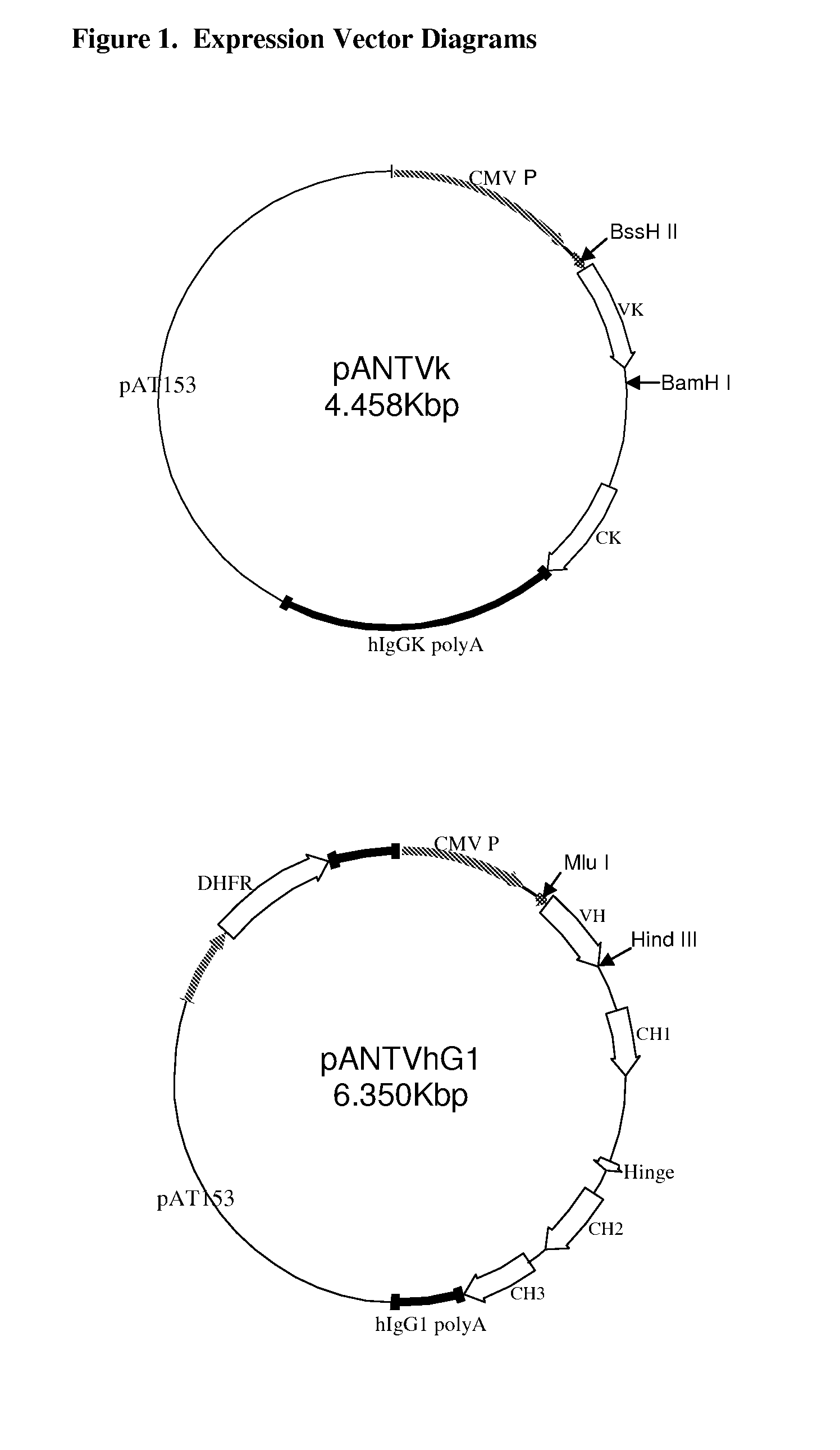
Anti-PD-L1 antibodies and uses therefor
The present invention is based, in part, on the identification of novel human anti-PD-1, PD-L1, and PD-L2 antibodies. Accordingly, the invention relates to compositions and methods for diagnosing, prognosing, and treating conditions that would benefit from modulating PD-1, PD-L1, and / or PD-L2 activity (e.g., persistent infectious diseases, autoimmune diseases, asthma, transplant rejection, inflammatory disorders and tumors) using the novel human anti-PD-1, PD-L1, and PD-L2 antibodies described herein.
Owner:DANA FARBER CANCER INST INC +2
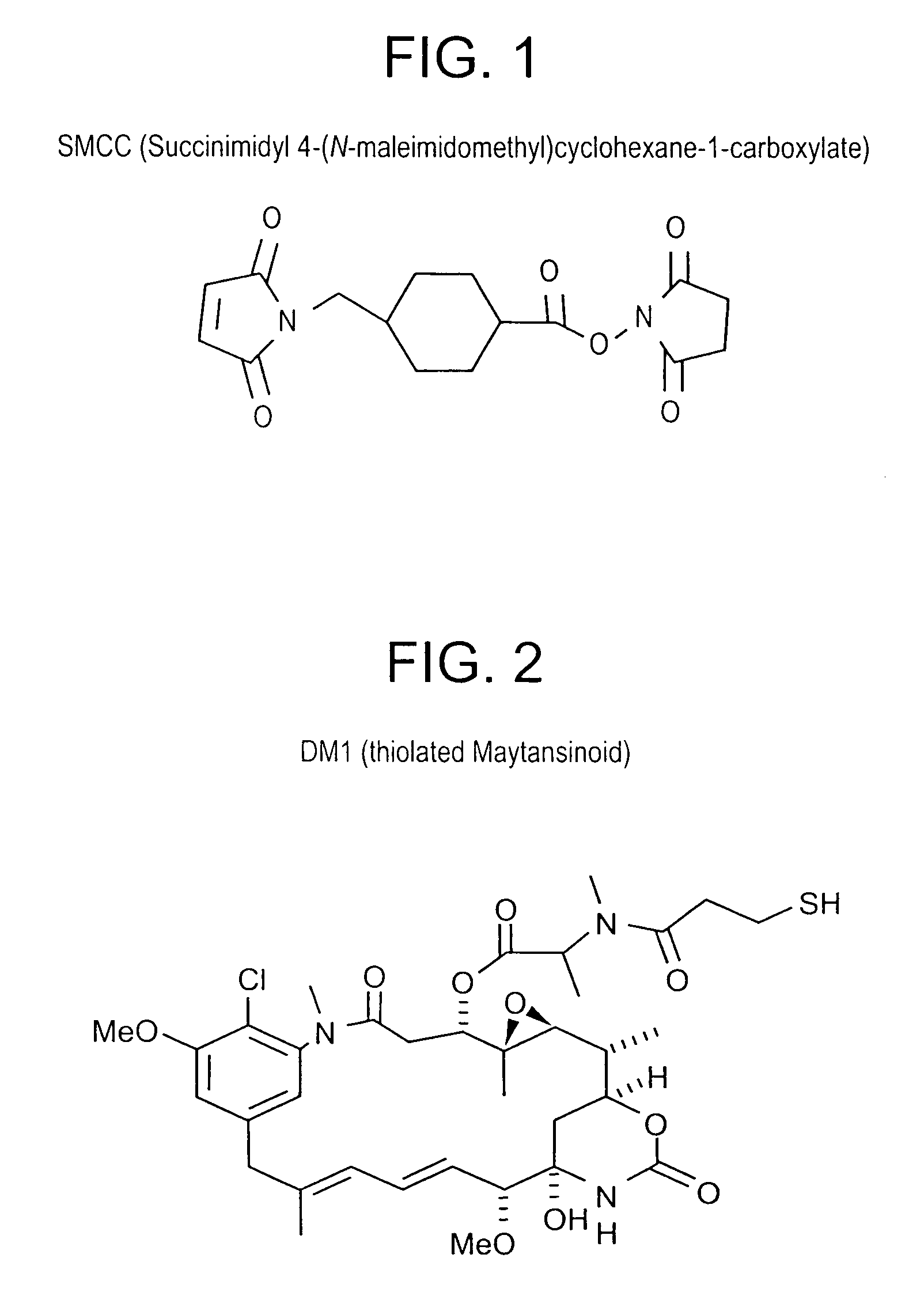
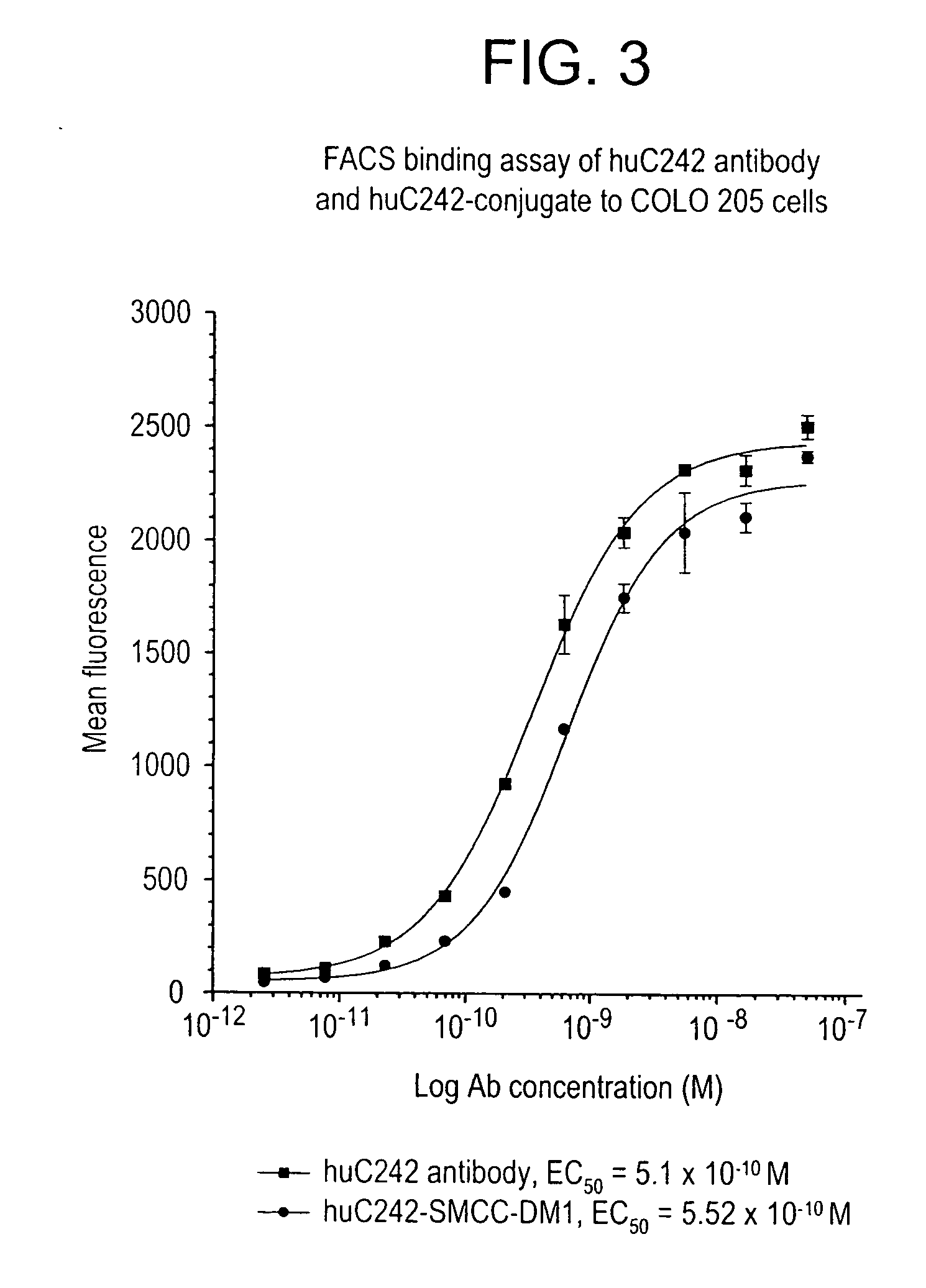
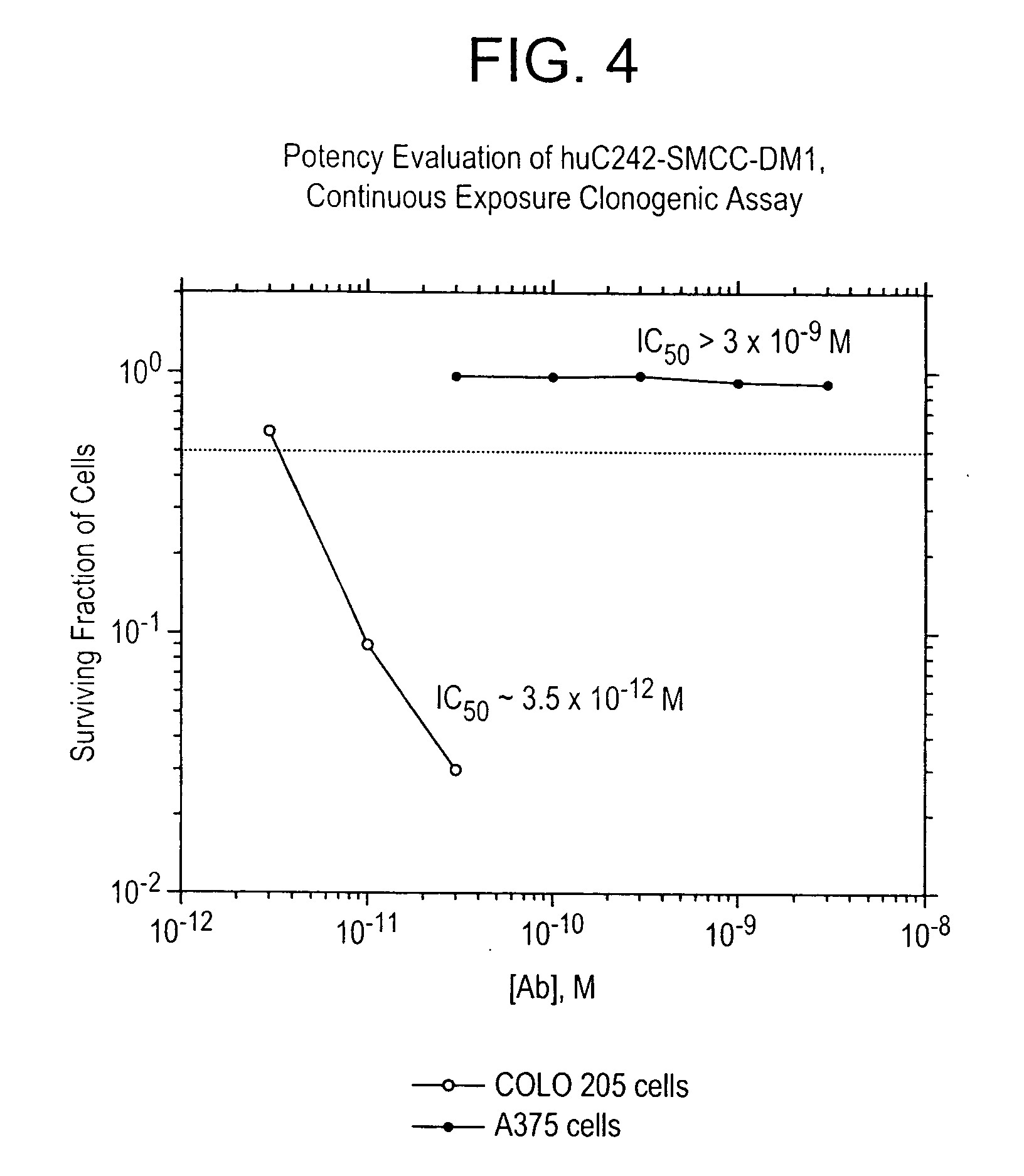
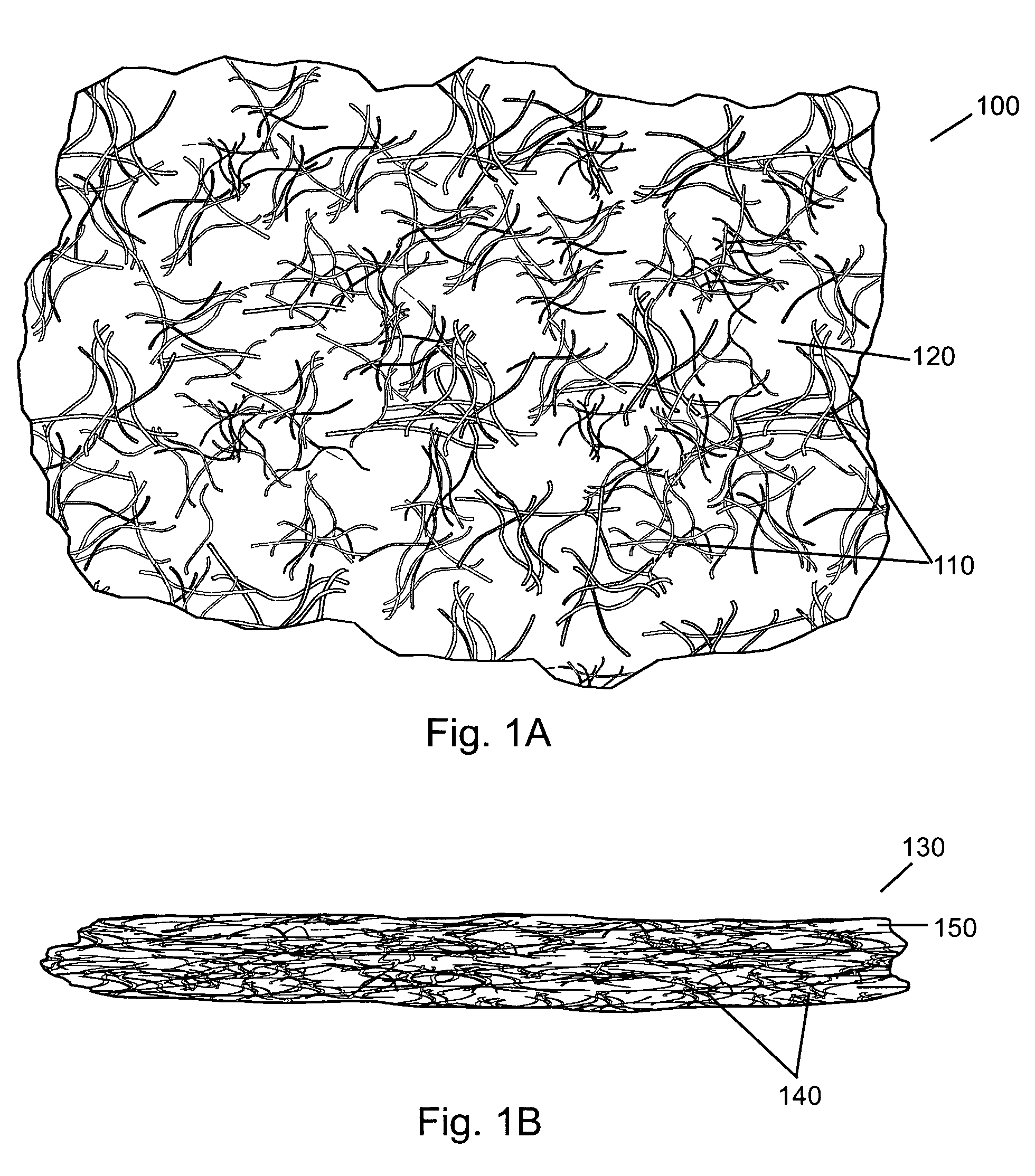
Method of targeting specific cell populations using cell-binding agent maytansinoid conjugates linked via a non-cleavable linker, said conjugates, and methods of making said conjugates
The present invention discloses a method for targeting maytansinoids to a selected cell population, the method comprising contacting a cell population or tissue suspected of containing the selected cell population with a cell-binding agent maytansinoid conjugate, wherein one or more maytansinoids is covalently linked to the cell-binding agent via a non-cleavable linker and the cell-binding agent binds to cells of the selected cell population.
Owner:IMMUNOGEN INC
Oxazolo, thiazolo and selenazolo [4,5-c]-quinolin-4-amines and analogs thereof
Thiazolo-, oxazolo- and selenazolo[4,5-c]quinolin-4-amines and analogs thereof are described including methods of manufacture and the use of novel intermediates. The compounds are immunomodulators and induce cytokine biosynthesis, including interferon and / or tumor biosynthesis, necrosis factor, and inhibit the T-helper-type 2 immune response. The compounds are further useful in the treatment of viral and neoplastic diseases.
Owner:3M INNOVATIVE PROPERTIES CO
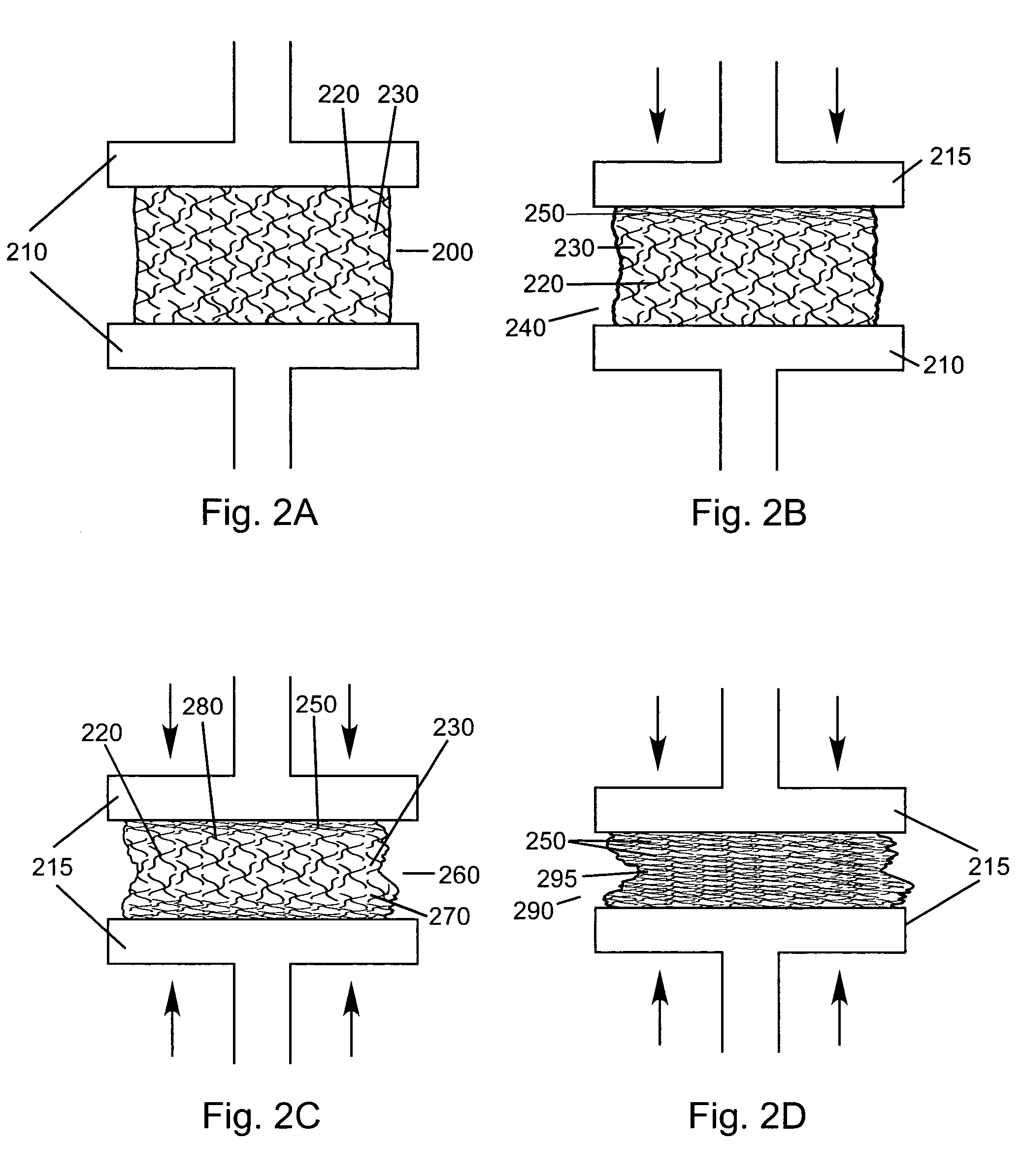
Compressed high density fibrous polymers suitable for implant
An embodiment of the present invention may be made by the following steps: providing a mixture comprising a plurality of fibers, a lubricant, and a suspension fluid, with the suspension fluid filling a void space between said fibers and subjecting said mixture to at least one compressive force. The compressive force causes the migration and alignment of said fibers; and may remove substantially all of the suspension fluid from said mixture. The mixture may further comprise a biologically active agent, or a reinforcing agent.
Owner:DSM IP ASSETS BV
Combination therapies employing GITR binding molecules
ActiveUS8591886B2Small sizeImprove securityOrganic active ingredientsNervous disorderInternal medicine
Owner:GITR
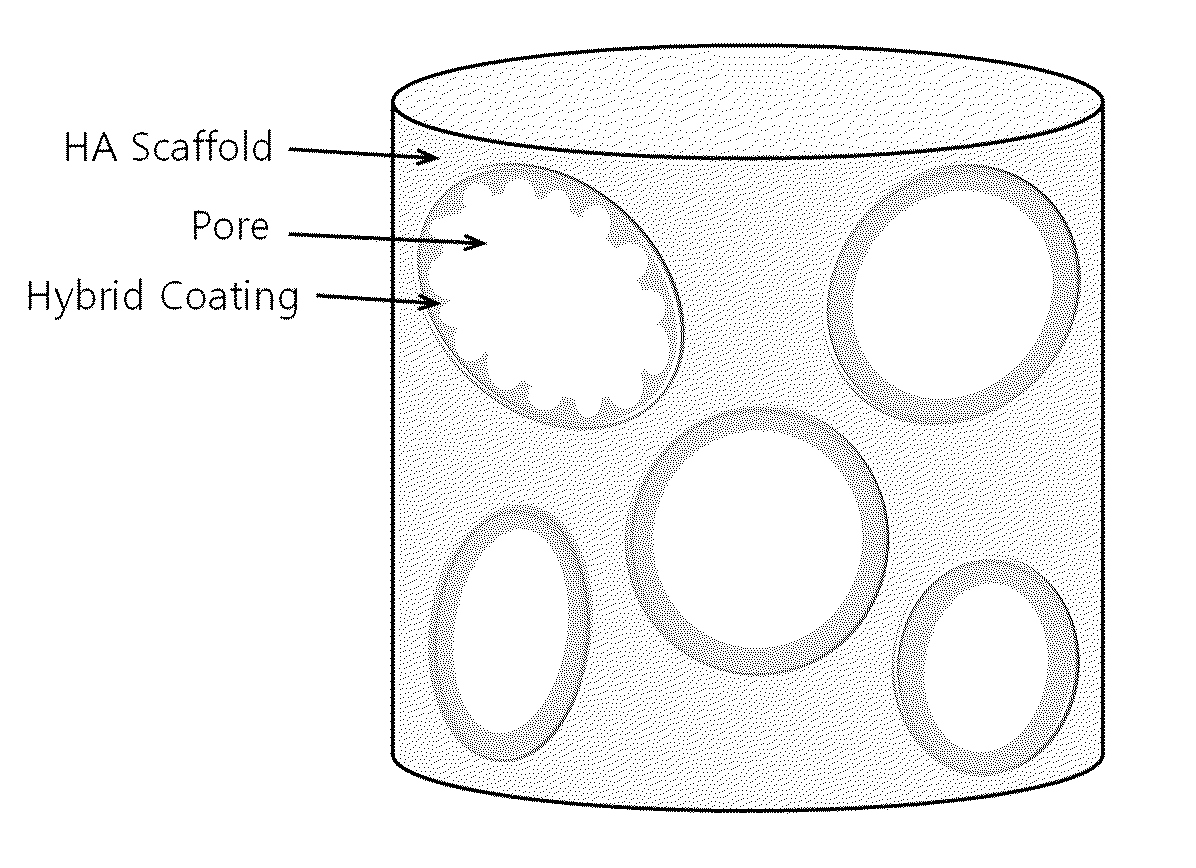
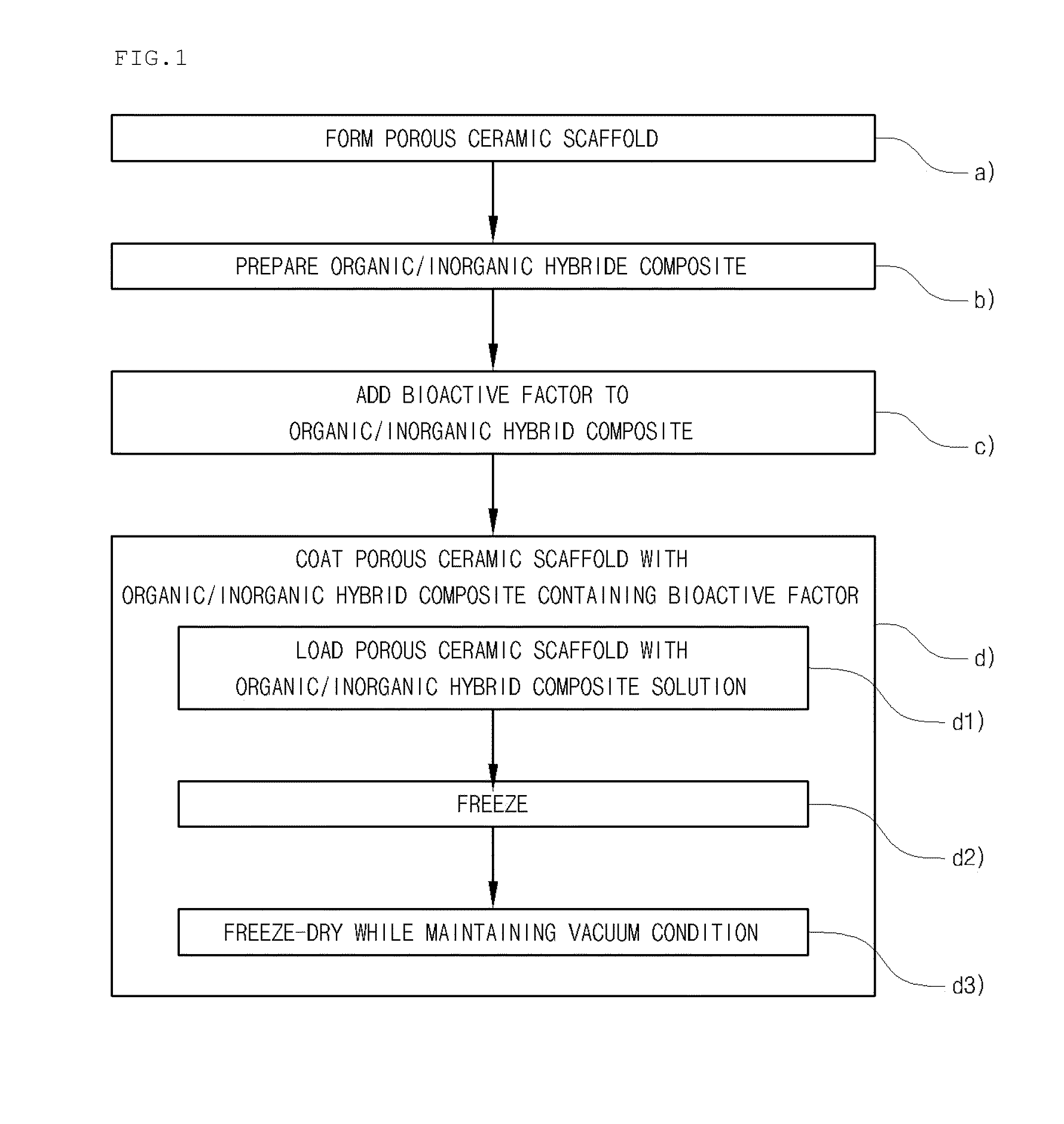
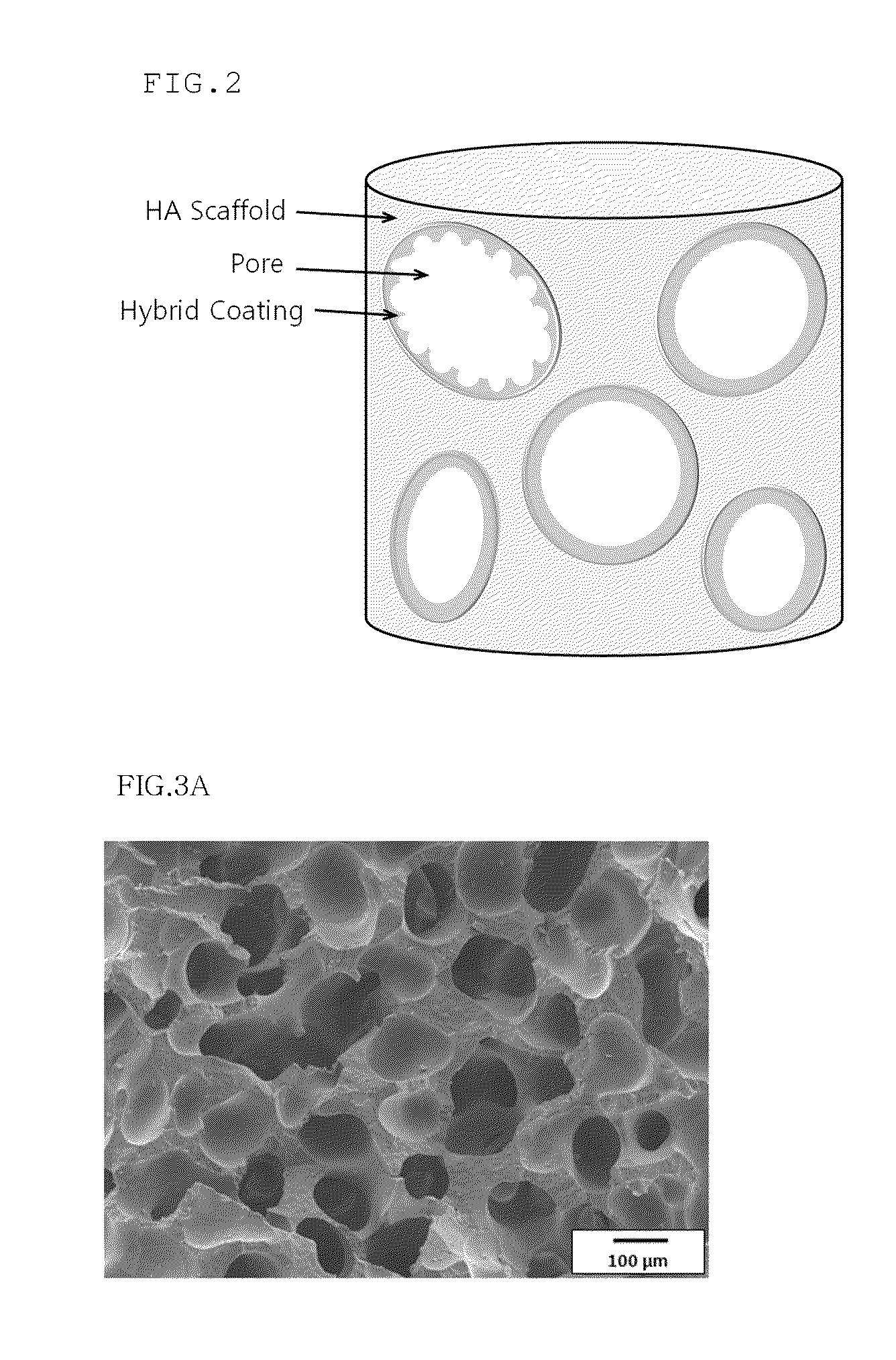
Method for manufacturing a porous ceramic scaffold having an organic/inorganic hybrid coating layer containing a bioactive factor
ActiveUS8734831B2Good biocompatibilityEasy to controlBiocideSurgical adhesivesOrganic matterPorous ceramics
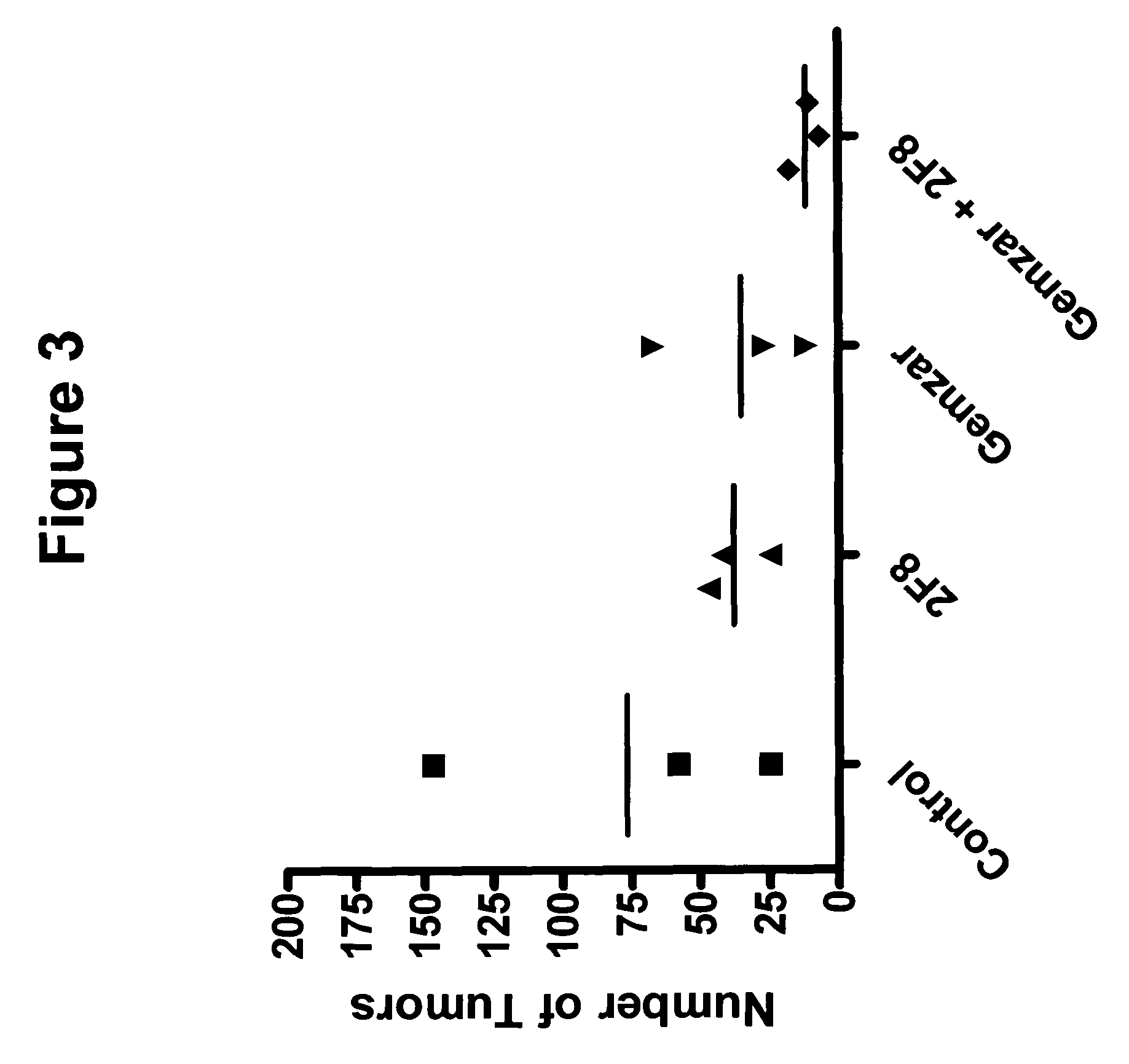
A method for manufacturing a porous ceramic scaffold having an organic / inorganic hybrid coating layer containing a bioactive factor includes (a) forming a porous ceramic scaffold; (b) mixing a silica xerogel and a physiologically active organic substance in a volumetric ratio ranging from 30:70 to 90:10 and treating by a sol gel method to prepare an organic / inorganic hybrid composite solution; (c) adding a bioactive factor to the organic / inorganic hybrid composite solution and agitating until gelation occurs; and (d) coating the porous ceramic scaffold with the organic / inorganic composite containing the bioactive factor added thereto. In accordance with the method, the porous ceramic scaffold may be uniformly coated with the organic / inorganic hybrid composite while maintaining an open pore structure, and stably discharge the bioactive factor over a long period of time.
Owner:SEOUL NAT UNIV R&DB FOUND
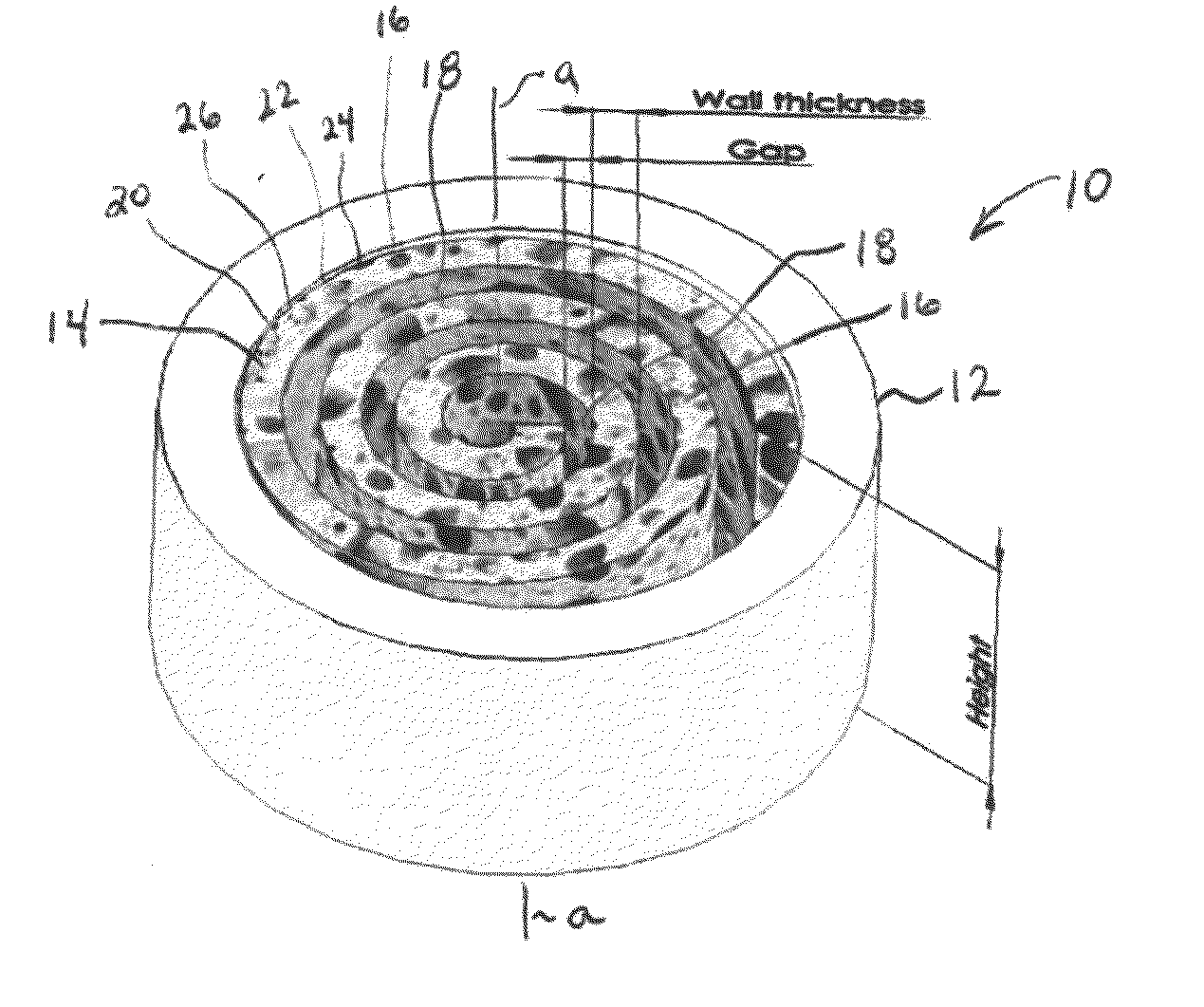
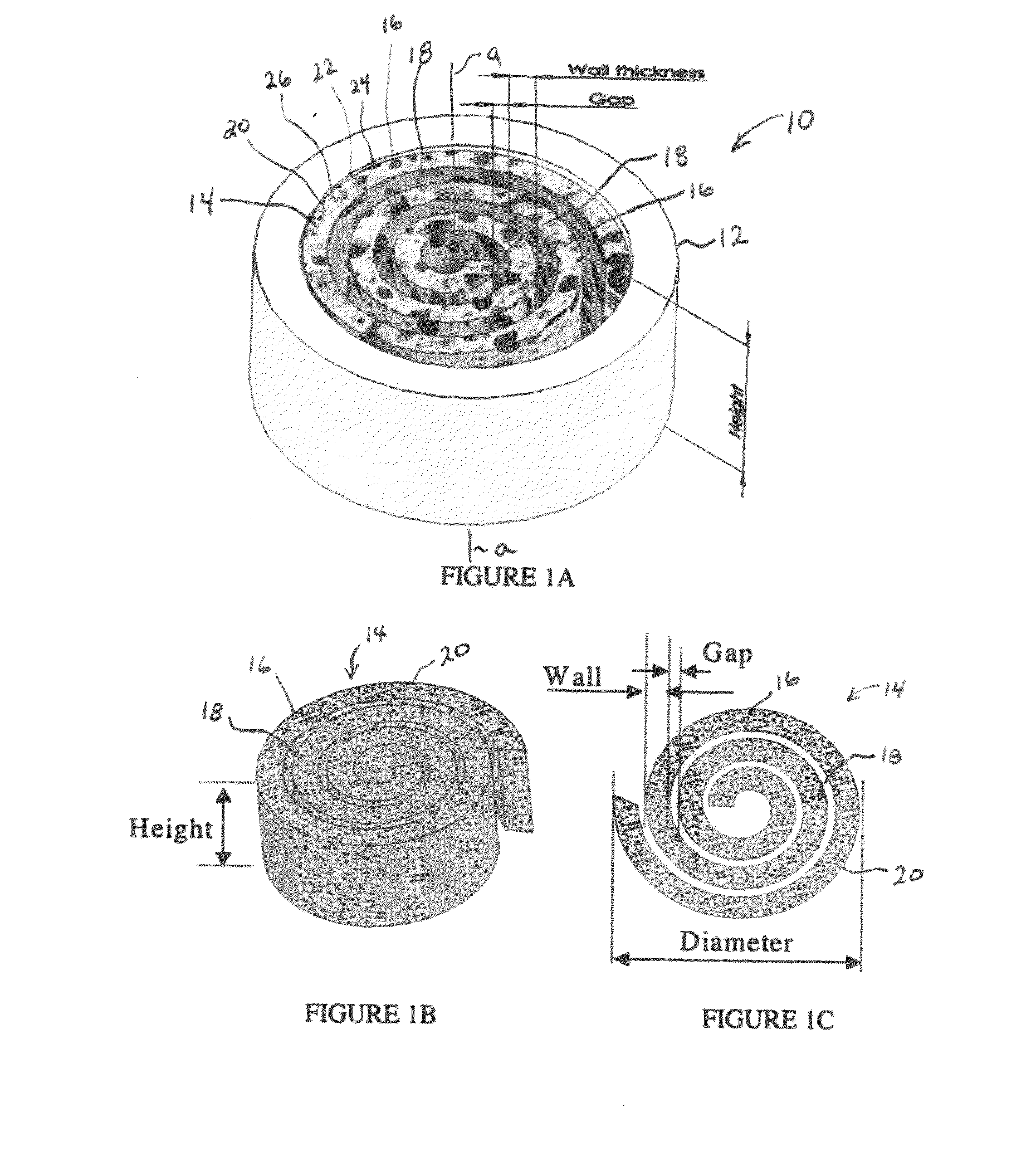
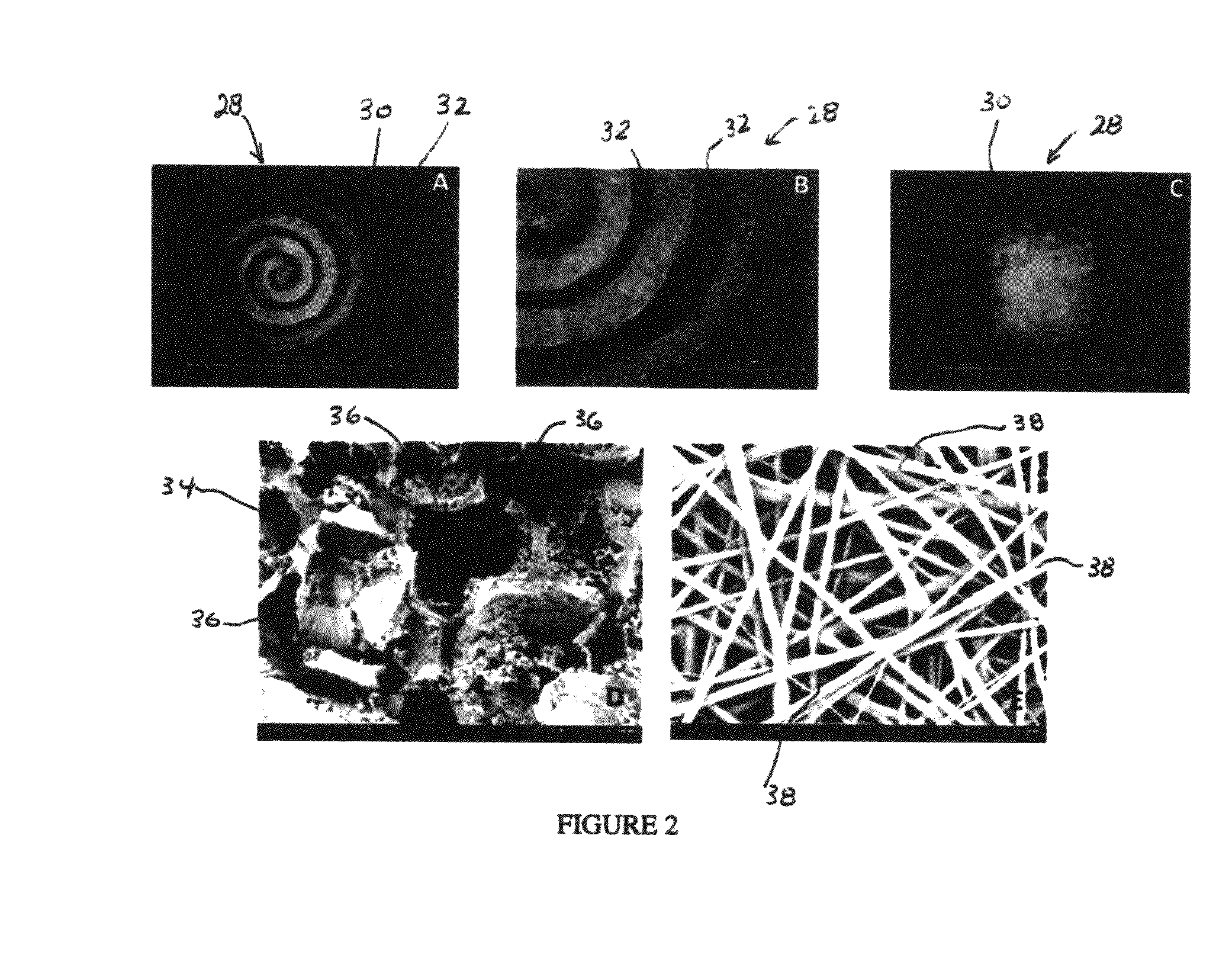
Synergetic functionalized spiral-in-tubular bone scaffolds
InactiveUS20100310623A1Increase the number ofIncrease alkaline phosphatase activityPeptide/protein ingredientsBone implantPorous sheetCell seeding
An integrated scaffold for bone tissue engineering has a tubular outer shell and a spiral scaffold made of a porous sheet. The spiral scaffold is formed such that the porous sheet defines a series of spiral coils with gaps of controlled width between the coils to provide an open geometry for enhanced cell growth. The spiral scaffold resides within the bore of the shell and is integrated with the shell to fix the geometry of the spiral scaffold. Nanofibers may be deposited on the porous sheet to enhance cell penetration into the spiral scaffold. The spiral scaffold may have alternating layers of polymer and ceramic on the porous sheet that have been built up using a layer-by-layer method. The spiral scaffold may be seeded with cells by growing a cell sheet and placing the cell sheet on the porous sheet before it is rolled.
Owner:UNIV OF CONNECTICUT
Three dimensional cell protector/pore architecture formation for bone and tissue constructs
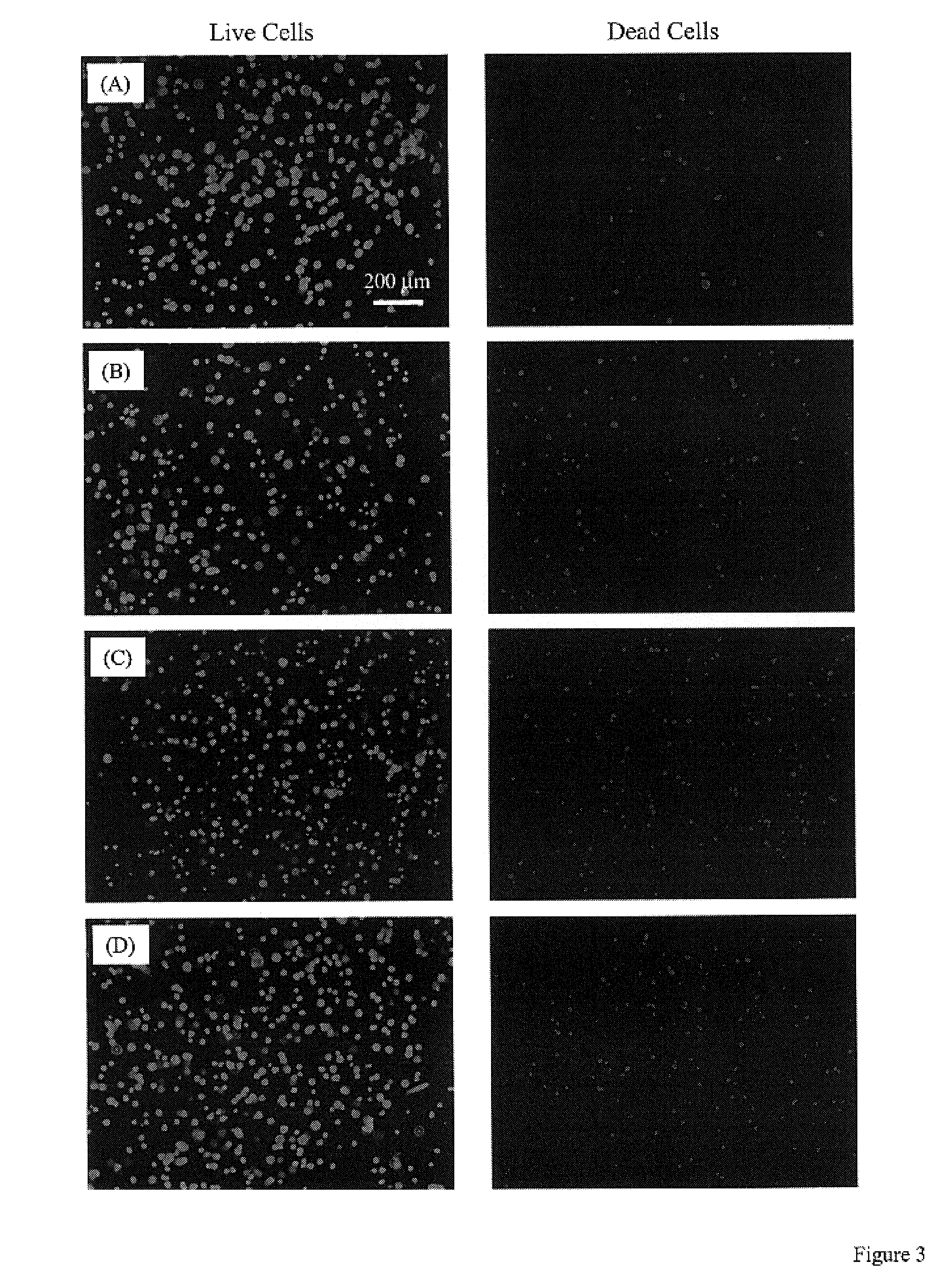
InactiveUS7713542B2Greatly multiplied in vitroProtection from damagePowder deliveryBiocideIn vivoLiving cell
Living cellular material is encapsulated or placed in a protective material (cell protector) which is biocompatible, biodegradable and has a three-dimensional form. The three dimensional form is incorporated into a matrix that maybe implanted in vivo, ultimately degrade and thereby by replaced by living cell generated material.
Owner:ADA FOUND
Identification and engineering of antibodies with variant Fc regions and methods of using same
ActiveUS20050037000A1High affinityAltered affinityAntibacterial agentsSenses disorderTherapeutic antibodyWild type
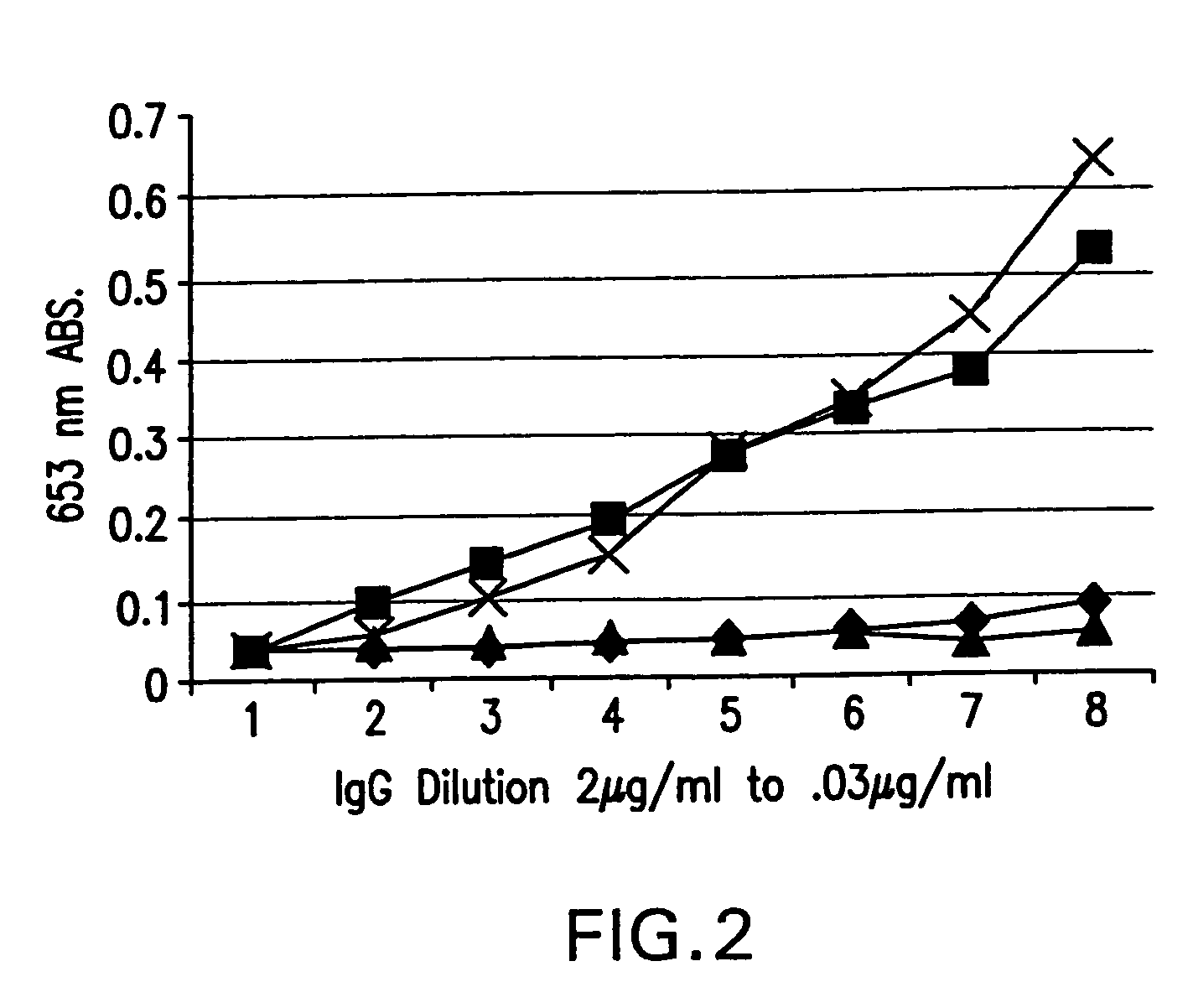
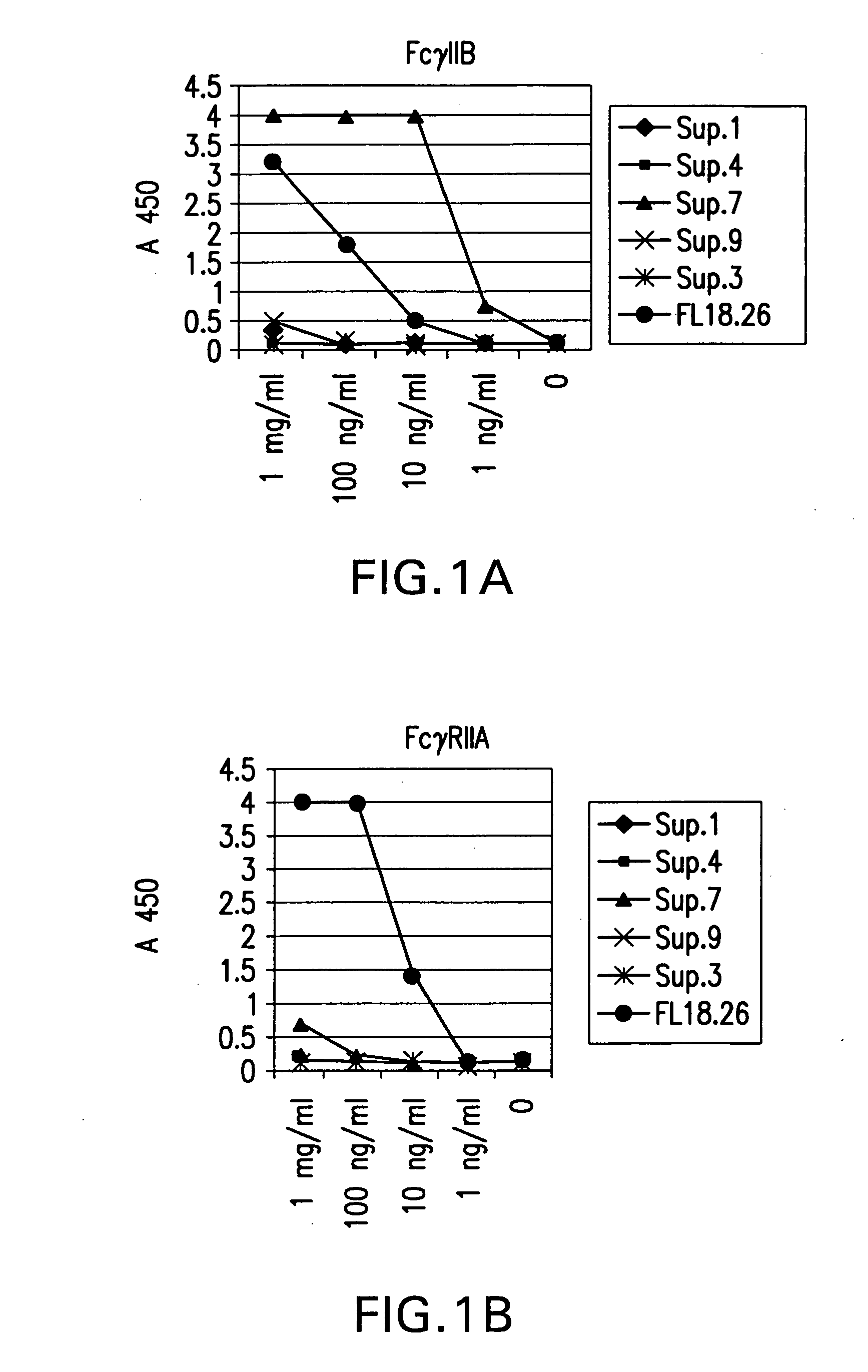
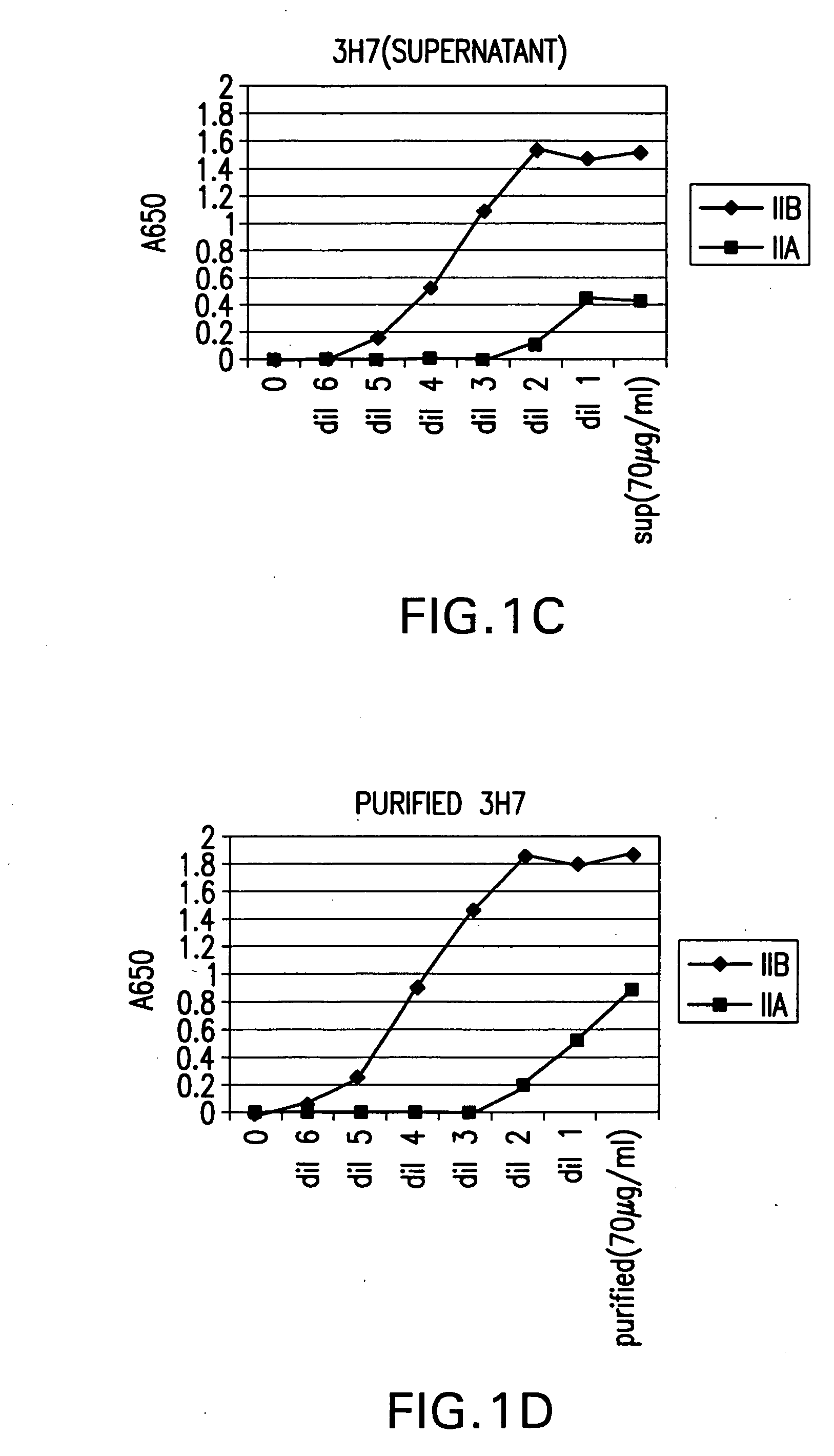
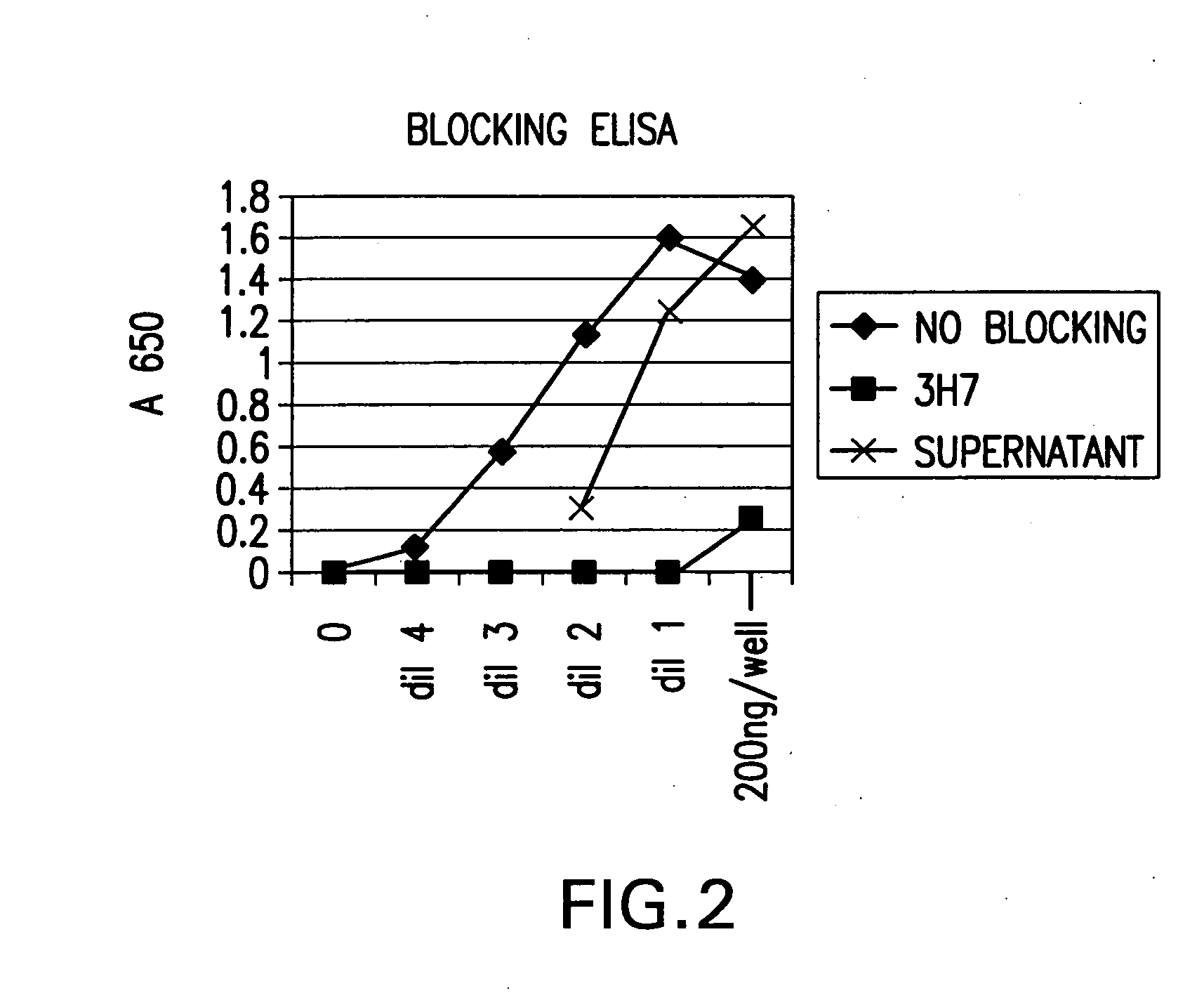
The present invention relates to molecules, particularly polypeptides, more particularly immunoglobulins (e.g., antibodies), comprising a variant Fc region, wherein said variant Fc region comprises at least one amino acid modification relative to a wild-type Fc region, which variant Fc region binds FcgammaRIIA and / or FcgammaRIIA with a greater affinity, relative to a comparable molecule comprising the wild-type Fc region. The molecules of the invention are particularly useful in preventing, treating, or ameliorating one or more symptoms associated with a disease, disorder, or infection. The molecules of the invention are particularly useful for the treatment or prevention of a disease or disorder where an enhanced efficacy of effector cell function (e.g., ADCC) mediated by FcgammaR is desired, e.g., cancer, infectious disease, and in enhancing the therapeutic efficacy of therapeutic antibodies the effect of which is mediated by ADCC.
Owner:MARCOGENICS INC +1
Identification and engineering of antibodies with variant Fc regions and methods of using same
ActiveUS7355008B2Function increaseGood curative effectAntibacterial agentsSenses disorderTherapeutic antibodyEffector cell
Owner:MARCOGENICS INC +1
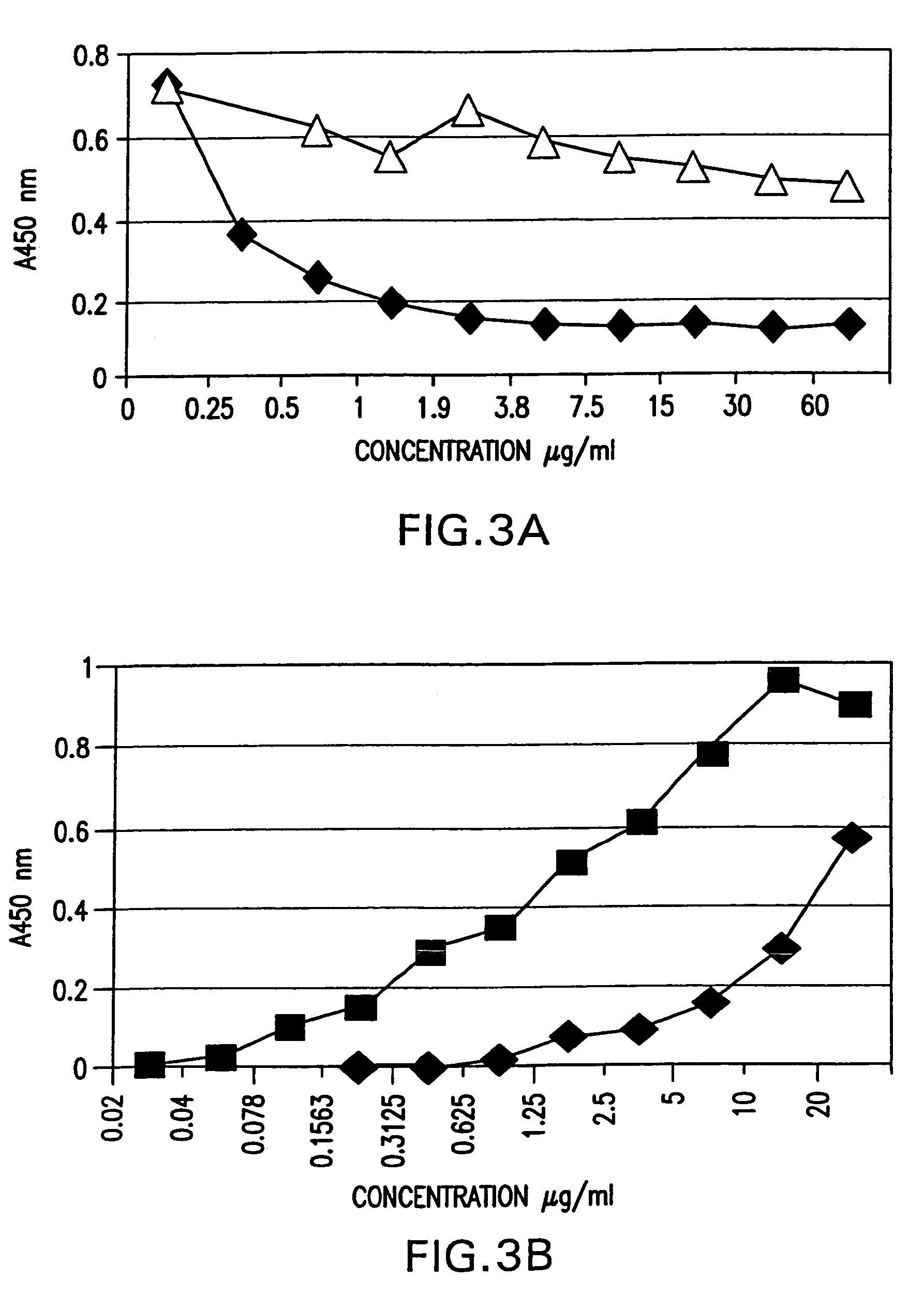
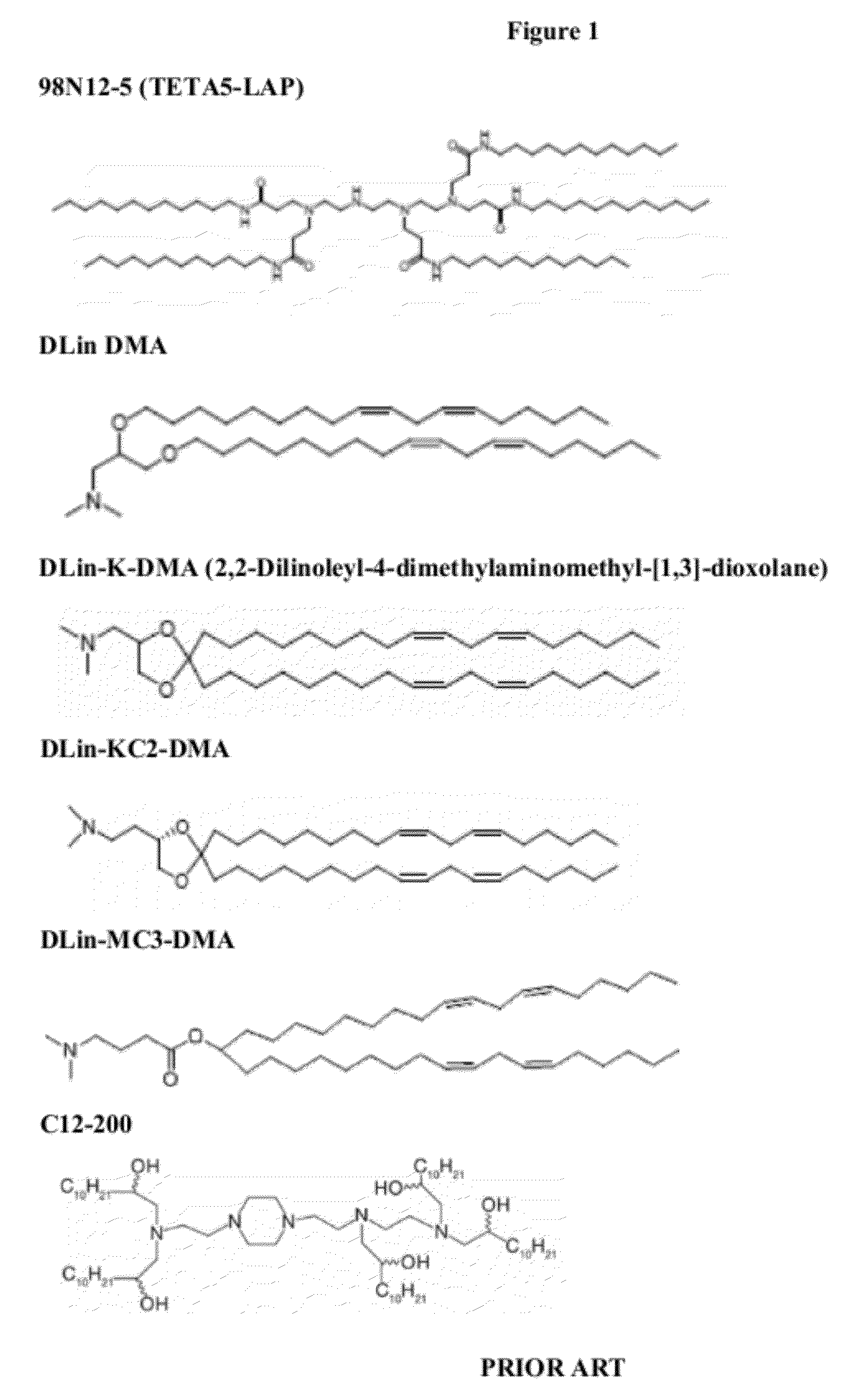
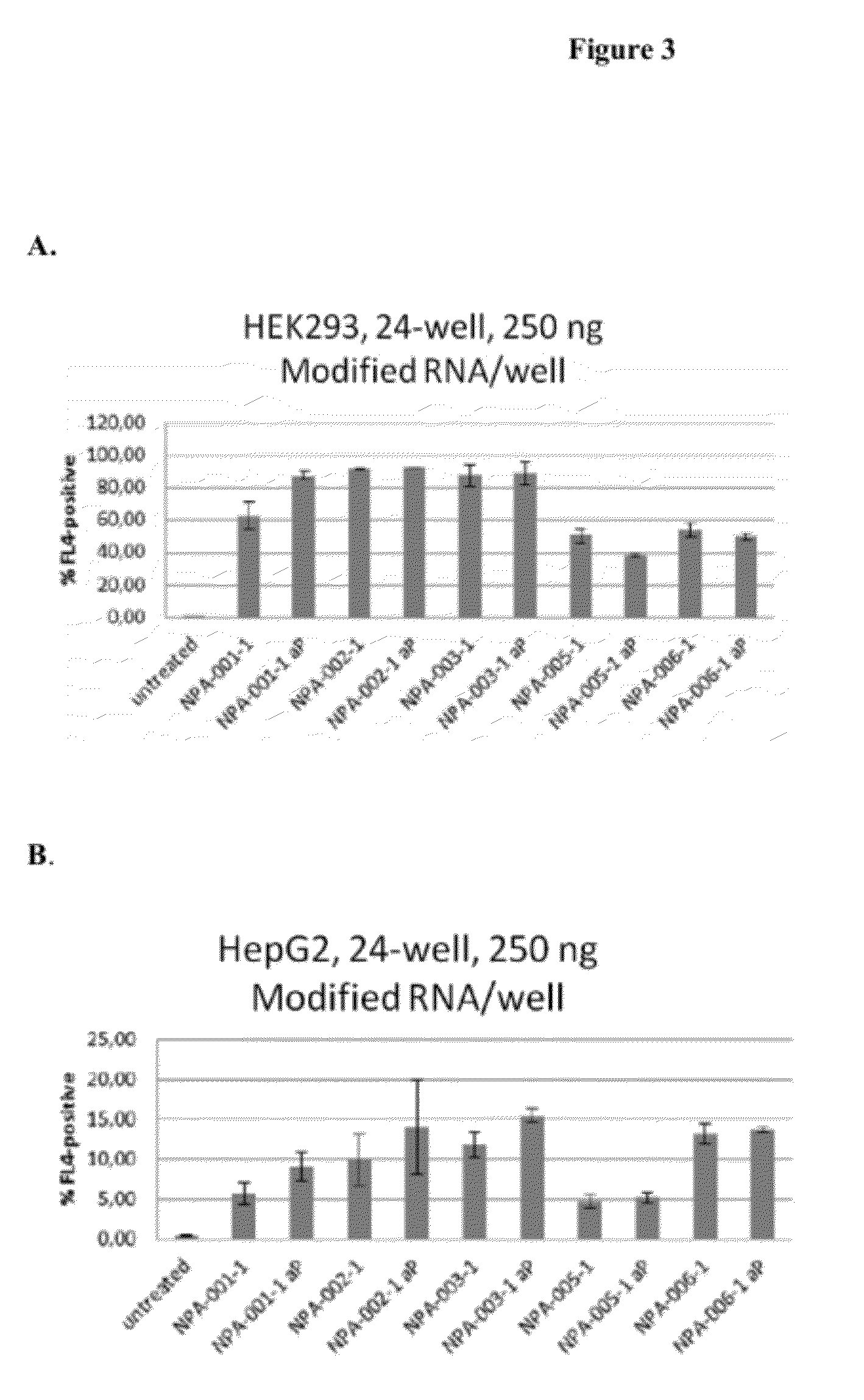
Delivery and formulation of engineered nucleic acids
ActiveUS20120251618A1Improve the level ofIncrease in level of polypeptideNervous disorderAntipyreticNucleic acidProtein expression
Provided are formulations, compositions and methods for delivering biological moieties such as modified nucleic acids into cells to modulate protein expression. Such compositions and methods include the delivery of biological moieties, and are useful for production of proteins.
Owner:MODERNATX INC
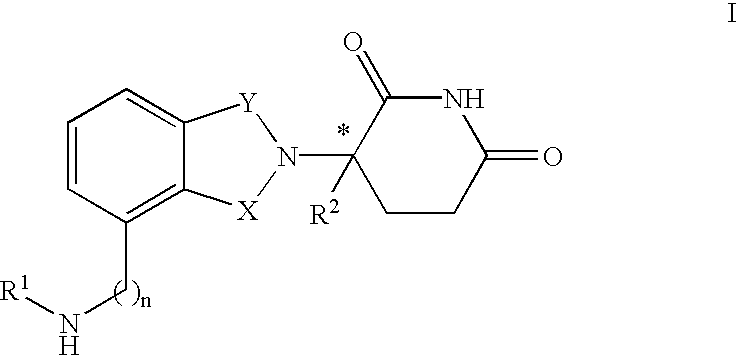
Isoindole-imide compounds, compositions, and uses thereof
The invention relates to isoindole-imide compounds and pharmaceutically acceptable salts, hydrates, solvates, clathrates, enantiomers, diastereomers, racemates, or mixtures of stereoisomers thereof, pharmaceutical compositions comprising these isoindole-imide compounds, and methods for reducing the level of cytokines and their precursors in mammals. In particular, the invention pertains to isoindole-imide compounds that are potent inhibitors of the production of TNF-alpha in mammals. The isoindole-imides described herein are useful for treating or preventing diseases or disorders in mammals, for example, cancers, such as solid tumors and blood-born tumors; heart disease, such as congestive heart failure; osteoporosis; and genetic, inflammatory; allergic; and autoimmune diseases.
Owner:CELGENE CORP
Isoindole-imide compounds, compositions, and uses thereof
The invention relates to isoindole-imide compounds and pharmaceutically acceptable salts, hydrates, solvates, clathrates, enantiomers, diastereomers, racemates, or mixtures of stereoisomers thereof, pharmaceutical compositions comprising these isoindole-imide compounds, and methods for reducing the level of cytokines and their precursors in mammals. In particular, the invention pertains to isoindole-imide compounds that are potent inhibitors of the production of TNF-alpha in mammals. The isoindole-imides described herein are useful for treating or preventing diseases or disorders in mammals, for example, cancers, such as solid tumors and blood-born tumors; heart disease, such as congestive heart failure; osteoporosis; and genetic, inflammatory; allergic; and autoimmune diseases.
Owner:CELGENE CORP
In situ formation of intervertebral disc implants
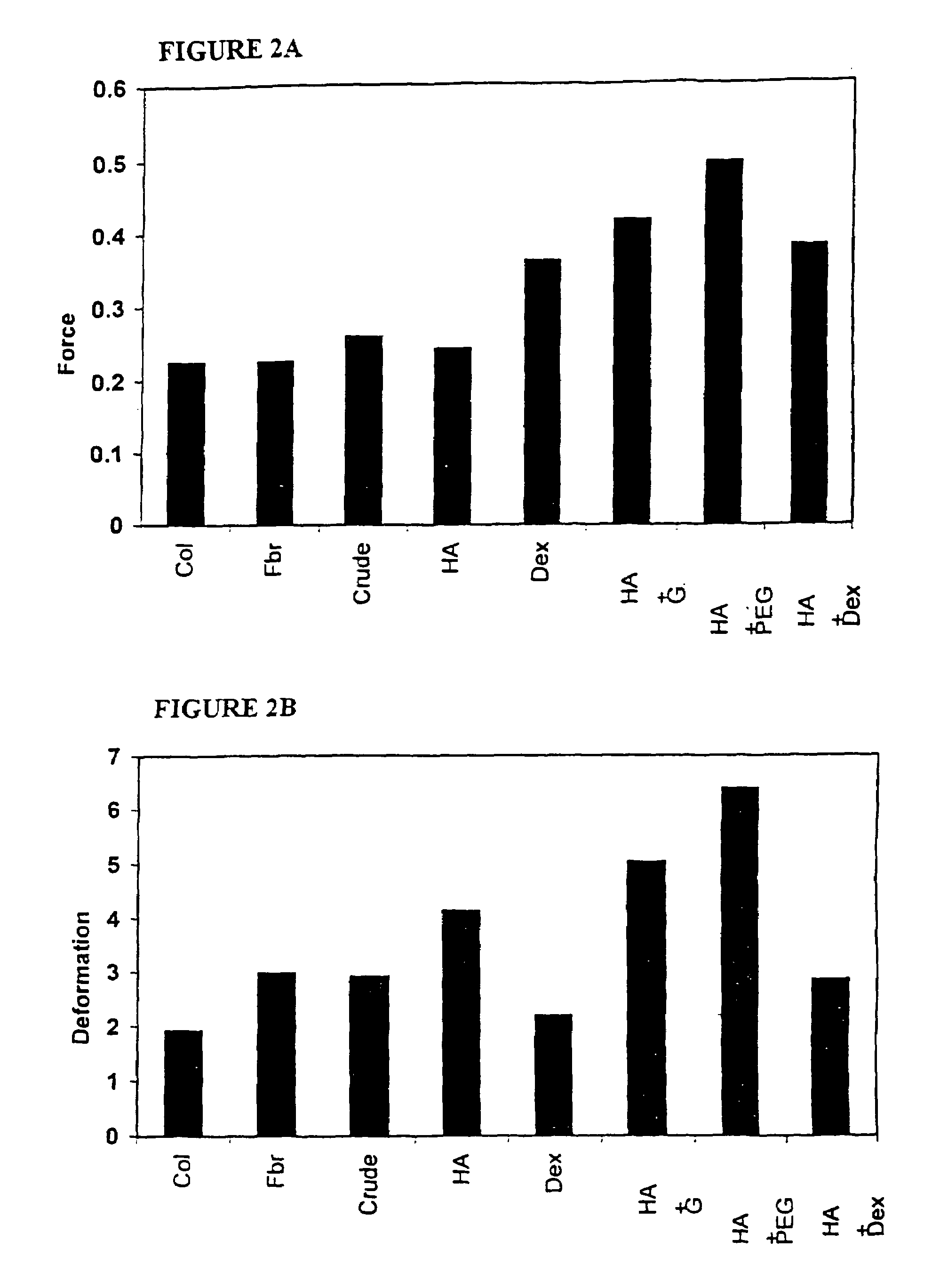
InactiveUS20060089719A1Improve bindingLarge deformationSkeletal disorderSpinal implantsSpinal Disk ImplantShort terms
Nucleus pulposus implants that are resistant to migration in and / or expulsion from an intervertebral disc space are provided. In one form of the invention, an implant includes a load bearing elastic body surrounded in the disc space by an anchoring, preferably resorbable, biocompatible material which may be in the form of an outer shell. In certain forms of the invention, the elastic body is surrounded by a supporting member, such as a band or jacket, and the supporting member is surrounded by the outer shell. Kits for forming such implants are also provided. In another form of the invention, an implant is provided that has locking features and optional shape memory characteristics. In yet another aspect of the invention, nucleus pulposus implants are provided that have shape memory characteristics and are configured to allow short-term manual, or other deformation without permanent deformation, cracks, tears, breakage or other damage. Methods of forming and implanting the implants are also described, as are delivery devices and components thereof for delivering the implants.
Owner:SDGI HLDG
FcgammaRIIB-specific antibodies and methods of use thereof
ActiveUS20040185045A1Strong therapeutic activityEnhancing antibody-mediated effector functionSenses disorderAntipyreticTherapeutic antibodyTreatment effect
The present invention relates to antibodies or fragments thereof that specifically bind FcgammaRIIB, particularly human FcgammaRIIB, with greater affinity than said antibodies or fragments thereof bind FcgammaRIIA, particularly human FcgammaRIIA. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention.
Owner:MACROGENICS INC
Fully human antibodies against human 4-1BB
Fully human antibodies and antigen-binding portions thereof that bind to human 4-1BB and that allow binding of human 4-1BB to a human 4-1BB ligand. In one aspect, the antibody is an IgG4 antibody. Also provided is a method for treating a disease in a subject comprising administering a therapeutically effective amount of the antibody to said subject.
Owner:BRISTOL MYERS SQUIBB CO
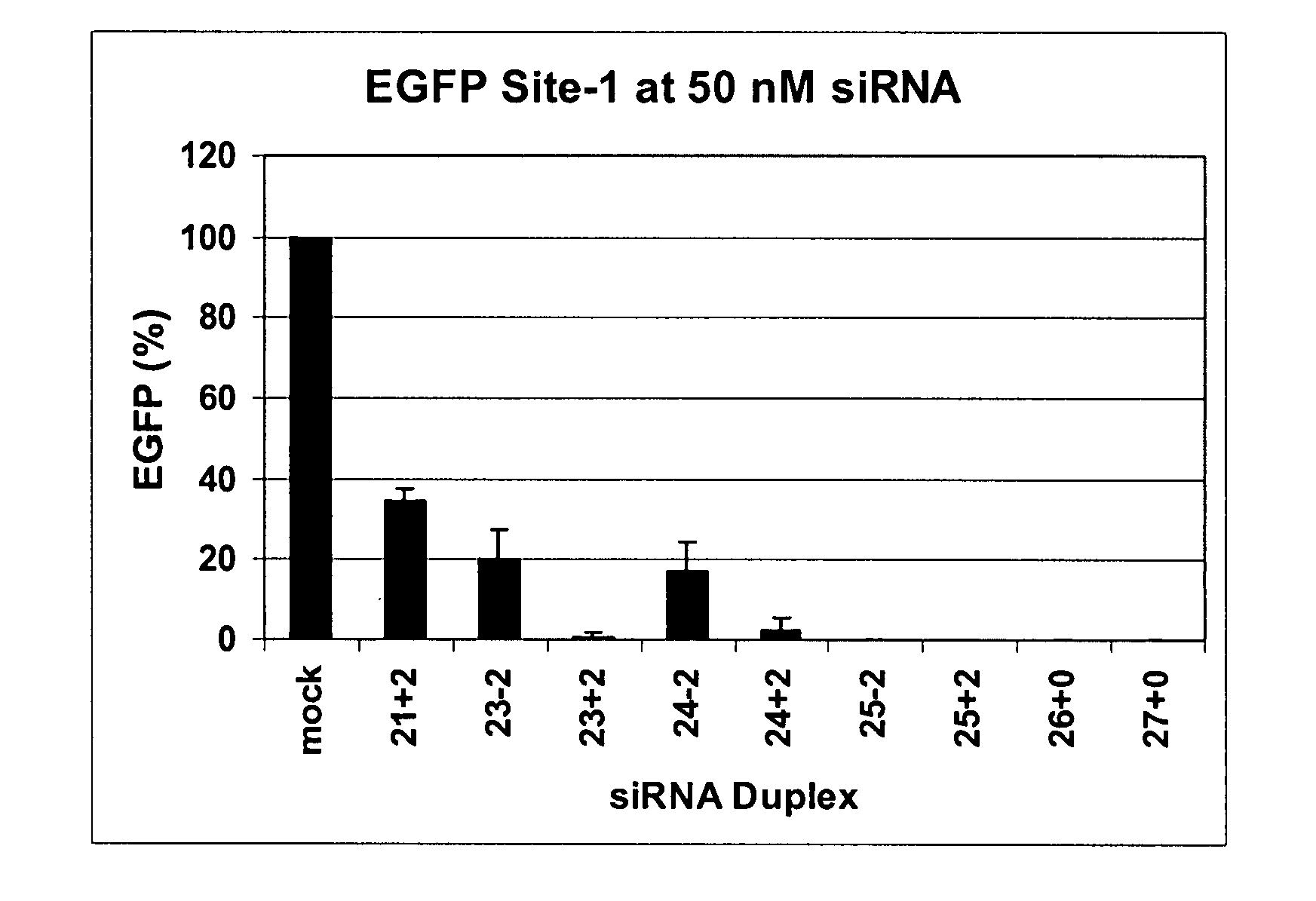
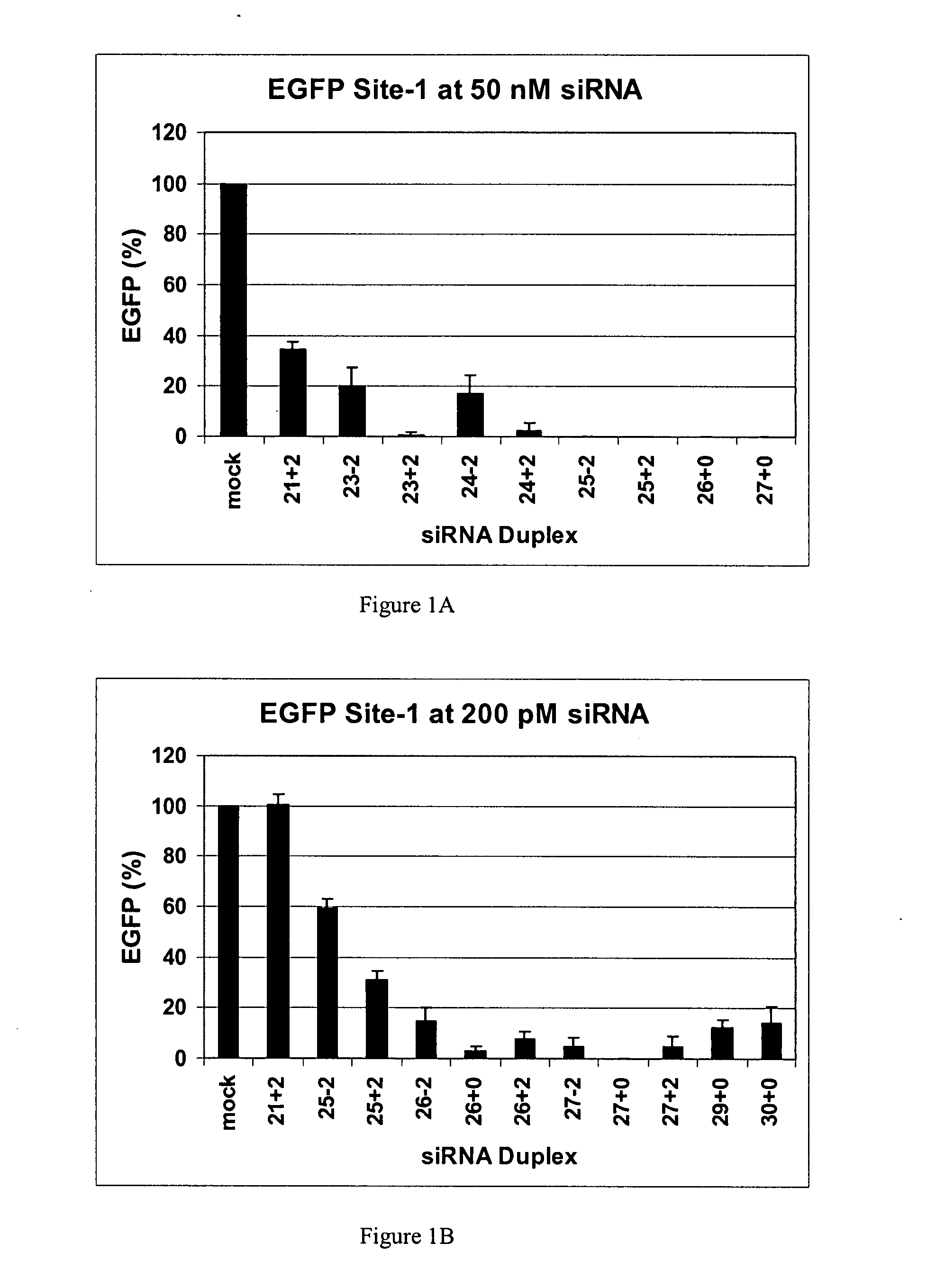
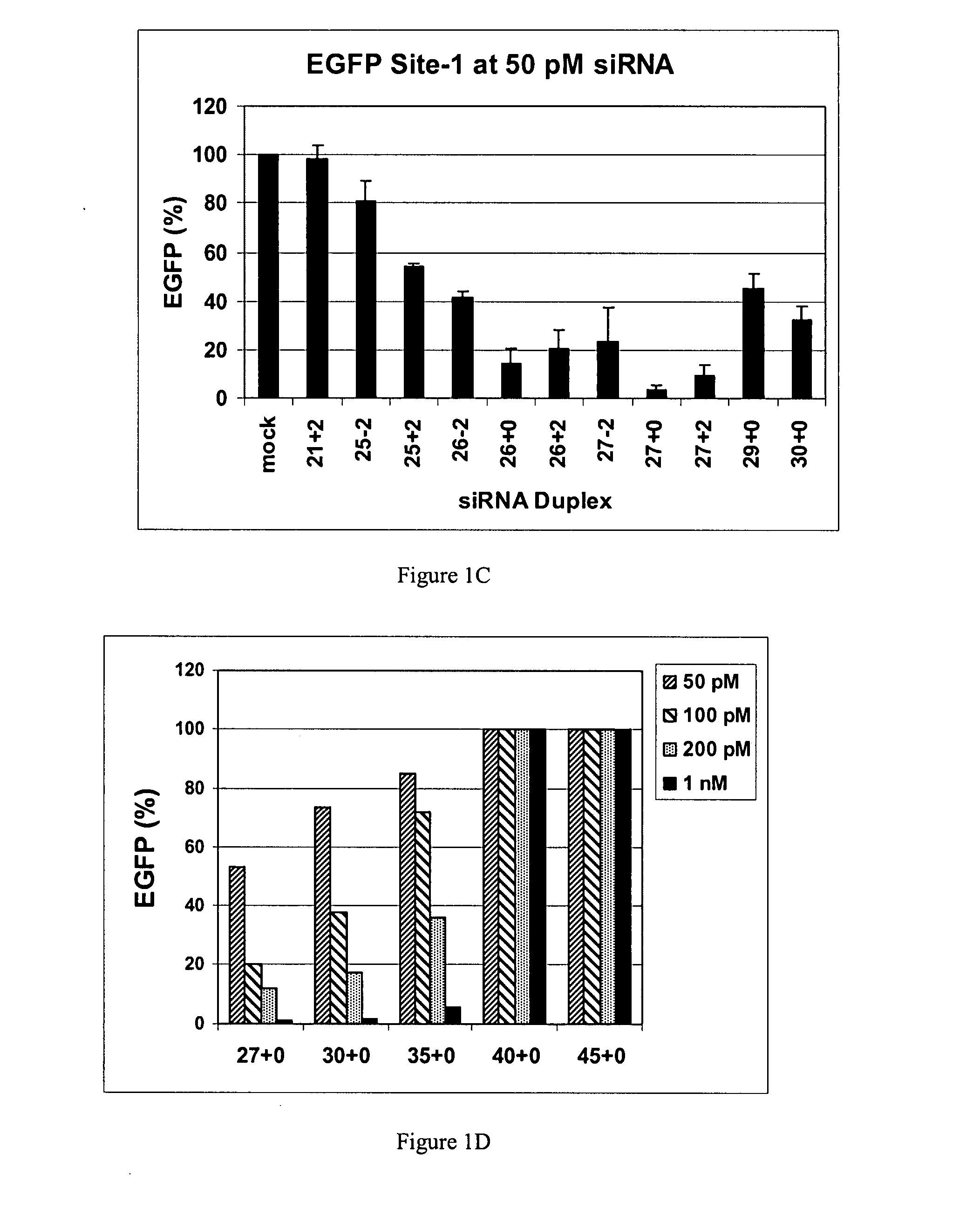
Methods and compositions for the specific inhibition of gene expression by double-stranded RNA
The invention is directed to compositions and methods for selectively reducing the expression of a gene product from a desired target gene in a cell, as well as for treating diseases caused by the expression of the gene. More particularly, the invention is directed to compositions that contain double stranded RNA (“dsRNA”), and methods for preparing them, that are capable of reducing the expression of target genes in eukaryotic cells. The dsRNA has a first oligonucleotide sequence that is between 25 and about 30 nucleotides in length and a second oligonucleotide sequence that anneals to the first sequence under biological conditions. In addition, a region of one of the sequences of the dsRNA having a sequence length of at least 19 nucleotides is sufficiently complementary to a nucleotide sequence of the RNA produced from the target gene to trigger the destruction of the target RNA by the RNAi machinery.
Owner:CITY OF HOPE +1
Methods and compositions using immunomodulatory compounds for treatment and management of cancers and other diseases
ActiveUS20040029832A1Prevent proliferationAntibacterial agentsBiocideSide effectBiologically-Based Therapy
Methods of treating, preventing and / or managing cancer as well as and diseases and disorders associated with, or characterized by, undesired angiogenesis are disclosed. Specific methods encompass the administration of an immunomodulatory compound alone or in combination with a second active ingredient. The invention further relates to methods of reducing or avoiding adverse side effects associated with chemotherapy, radiation therapy, hormonal therapy, biological therapy or immunotherapy which comprise the administration of an immunomodulatory compound. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in methods of the invention are also disclosed.
Owner:CELGENE CORP
Multispecific epitope binding proteins and uses thereof
InactiveUS20090155275A1Stimulate immune responseEnhanced interactionAntipyreticAnalgesicsEpitopeDisease
The present invention relates to multispecific epitope binding proteins, methods of making, and uses thereof in the prevention, management, treatment or diagnosis of acute or chronic diseases.
Owner:MEDIMMUNE LLC
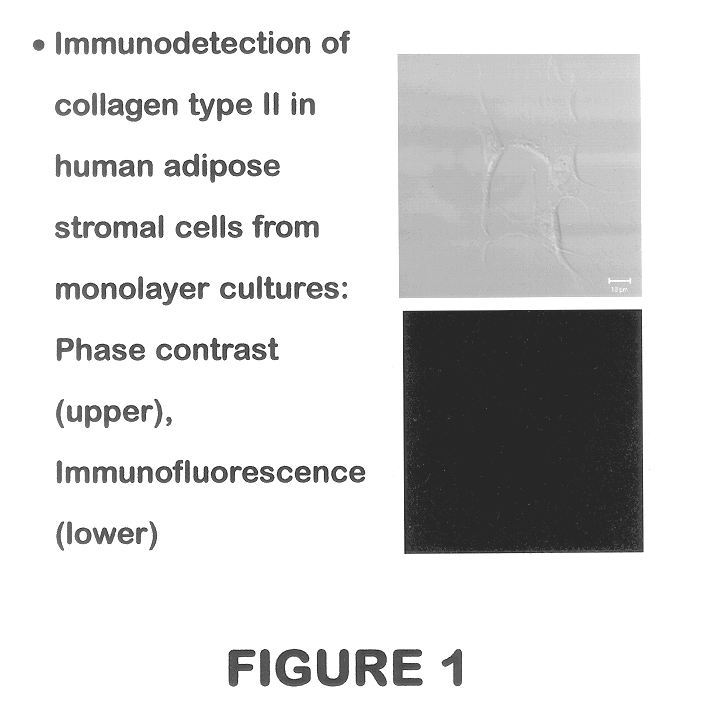
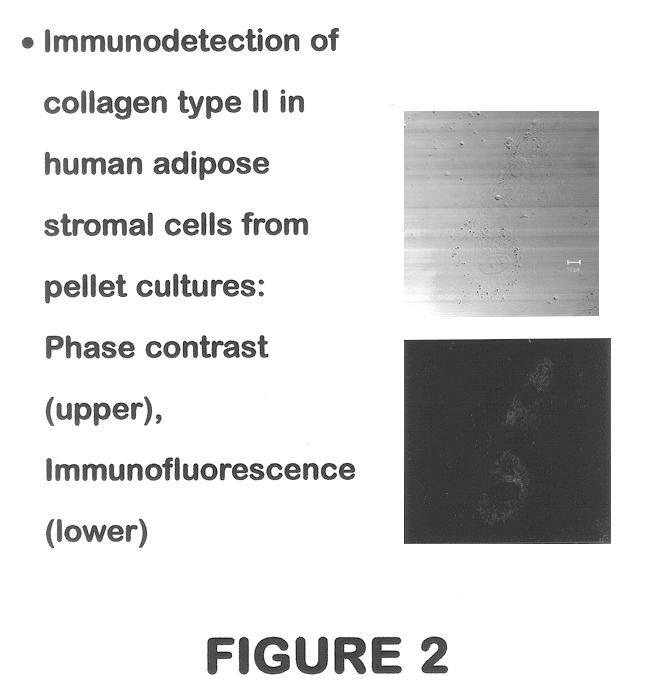
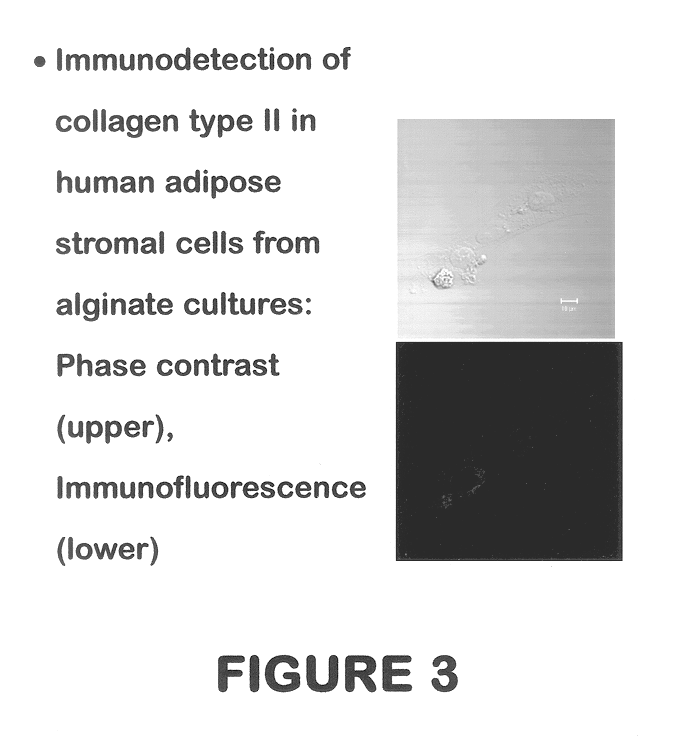
Use of adipose tissue-derived stromal cells for chondrocyte differentiation and cartilage repair
Methods and compositions for directing adipose-derived stromal cells cultivated in vitro to differentiate into cells of the chondrocyte lineage are disclosed. The invention further provides a variety of chondroinductive agents which can be used singly or in combination with other nutrient components to induce chondrogenesis in adipose-derived stromal cells either in cultivating monolayers or in a biocompatible lattice or matrix in a three-dimensional configuration. Use of the differentiated chondrocytes for the therapeutic treatment of a number of human conditions and diseases including repair of cartilage in vivo is disclosed.
Owner:COGNATE BIOSERVICES
Methods and compositions for the specific inhibition of gene expression by double-stranded RNA
InactiveUS20070265220A1Improve stabilityConvenience to mergeSenses disorderAntipyreticDouble strandOligonucleotide
The invention is directed to compositions and methods for selectively reducing the expression of a gene product from a desired target gene in a cell, as well as for treating diseases caused by the expression of the gene. More particularly, the invention is directed to compositions that contain double stranded RNA (“dsRNA”), and methods for preparing them, that are capable of reducing the expression of target genes in eukaryotic cells. The dsRNA has a first oligonucleotide sequence that is between 25 and about 30 nucleotides in length and a second oligonucleotide sequence that anneals to the first sequence under biological conditions. In addition, a region of one of the sequences of the dsRNA having a sequence length of at least 19 nucleotides is sufficiently complementary to a nucleotide sequence of the RNA produced from the target gene to trigger the destruction of the target RNA by the RNAi machinery.
Owner:CITY OF HOPE +1
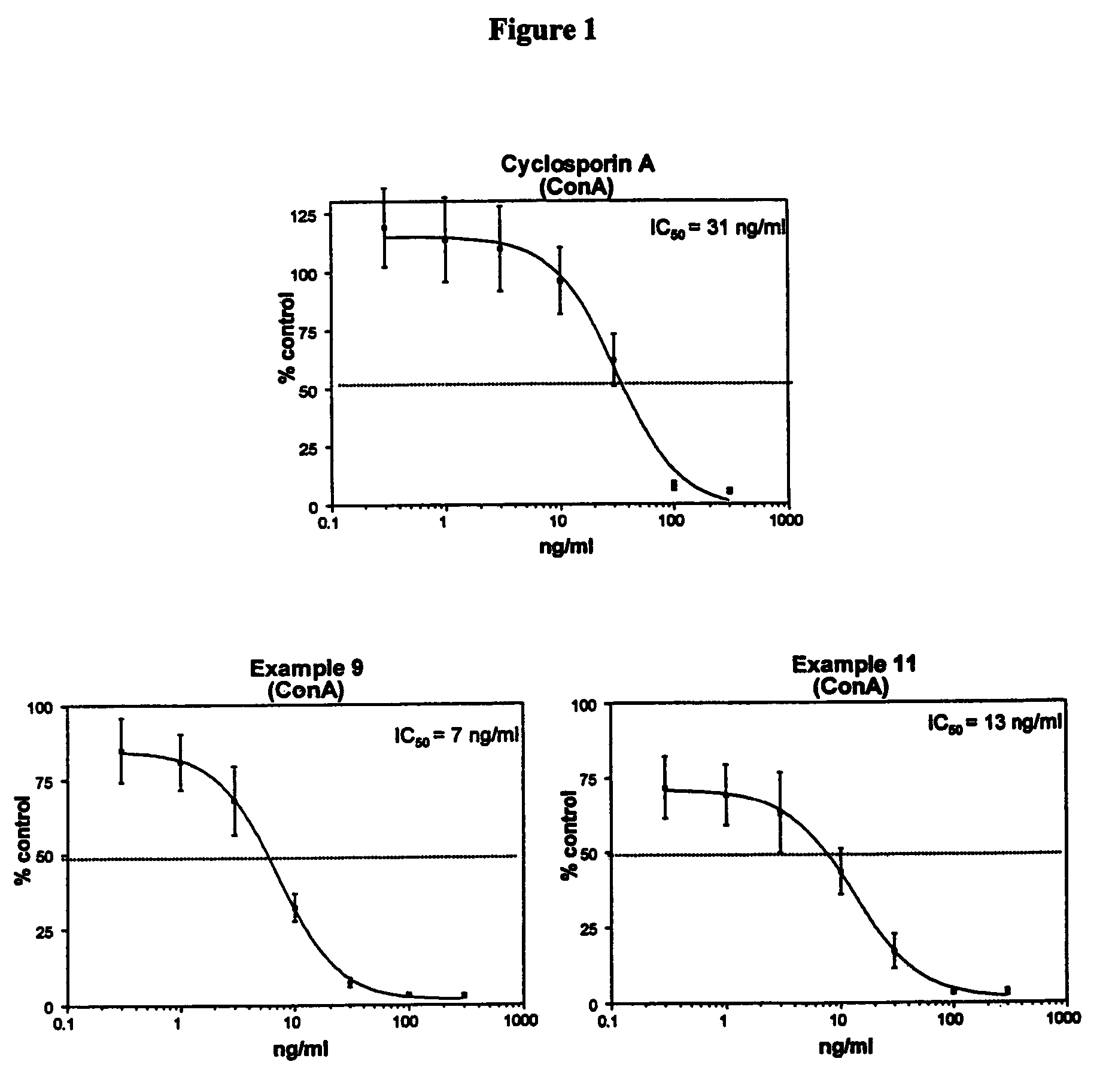
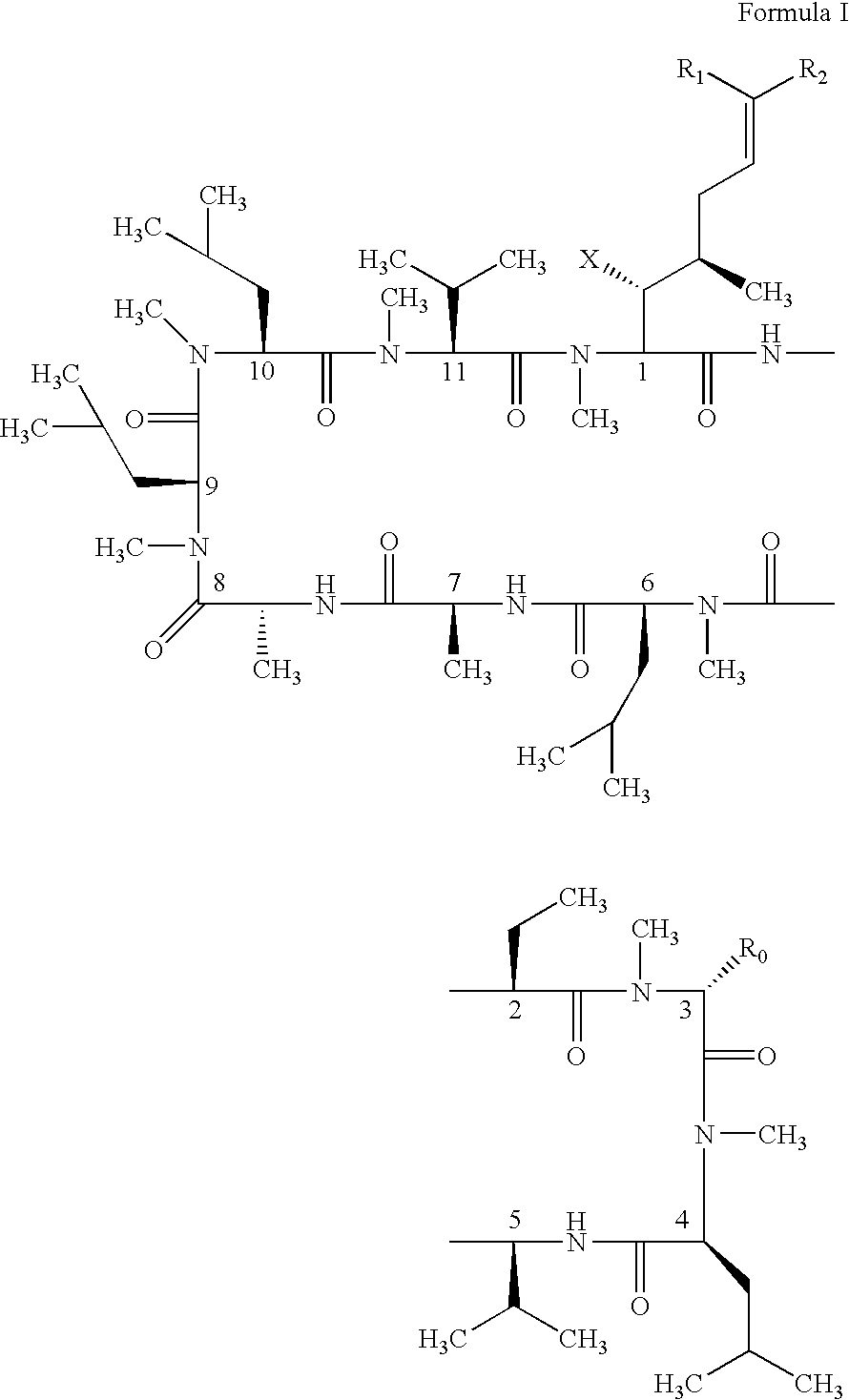
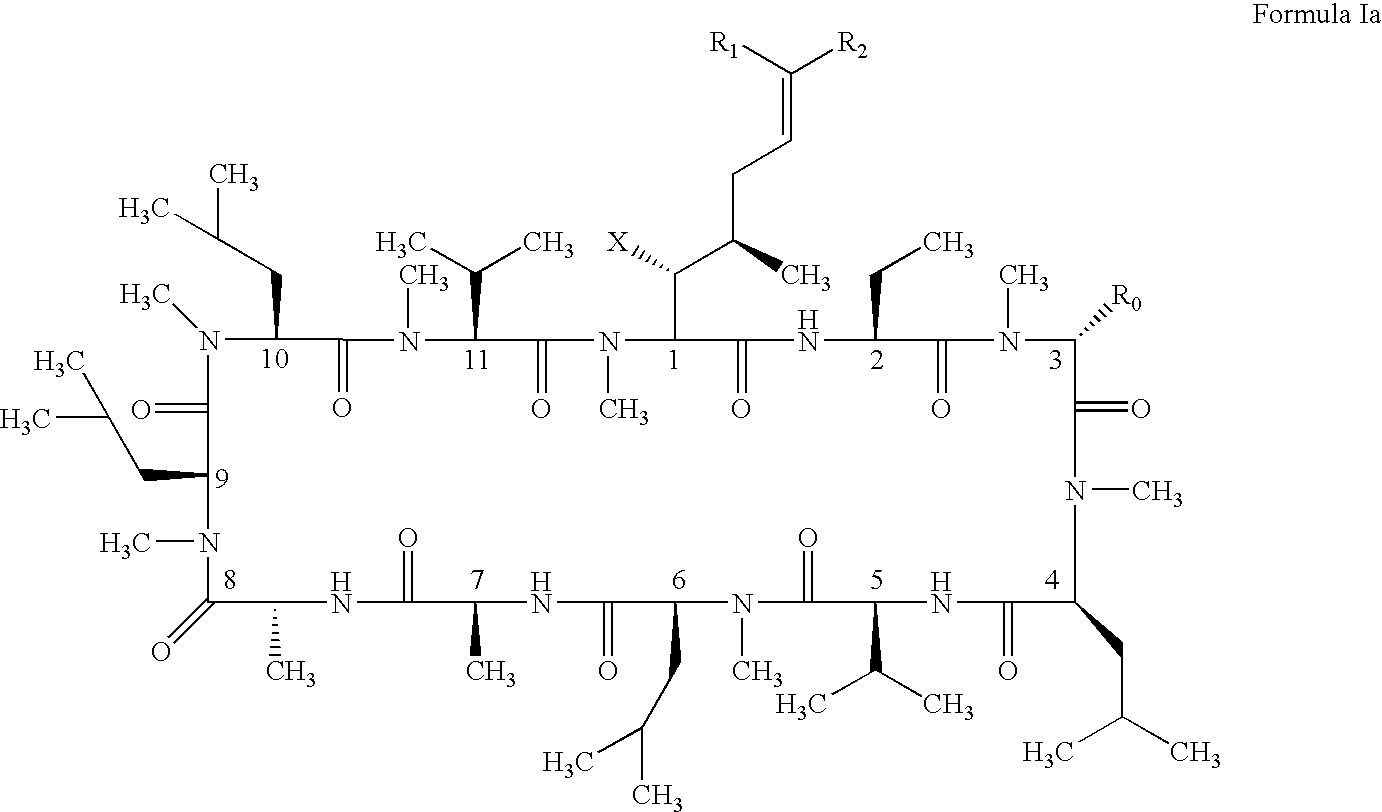
Cyclosporin analogues and their pharmaceutical uses
Owner:ALBANY MOLECULAR RESEARCH INC
Compositions for regeneration and repair of cartilage lesions
InactiveUS6511958B1Increase ratingsImprove repair qualitySuture equipmentsPowder deliveryMedicineCartilage lesion
Disclosed is a cartilage repair product that induces both cell ingrowth into a bioresorbable material and cell differentiation into cartilage tissue. Such a product is useful for regenerating and / or repairing both vascular and avascular cartilage lesions, particularly articular cartilage lesions, and even more particularly mensical tissue lesions, including tears as well as segmental defects. Also disclosed is a method of regenerating and repairing cartilage lesions using such a product.
Owner:ZIMMER ORTHOBIOLOGICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com