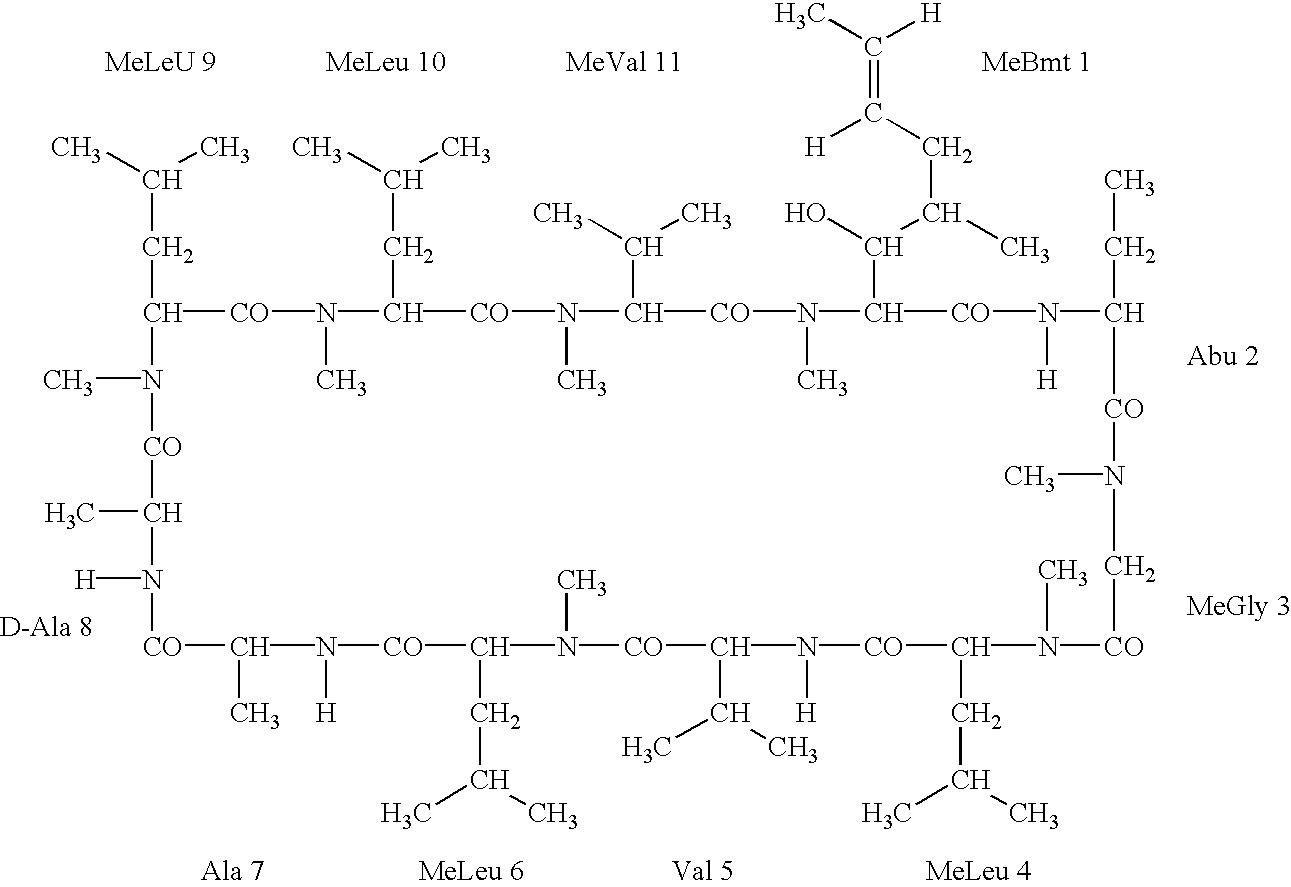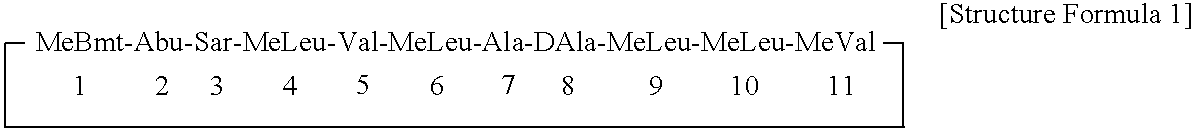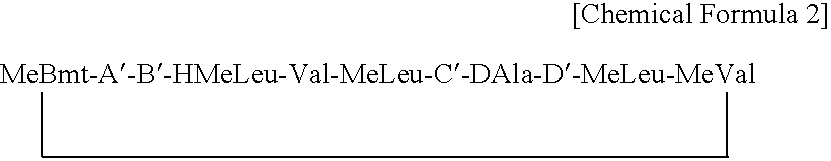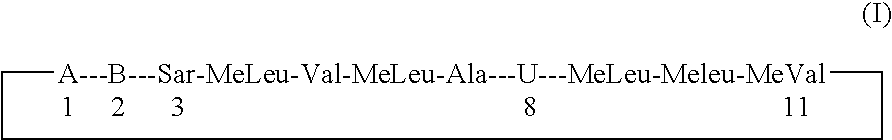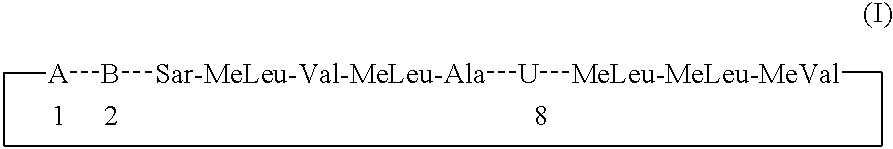Patents
Literature
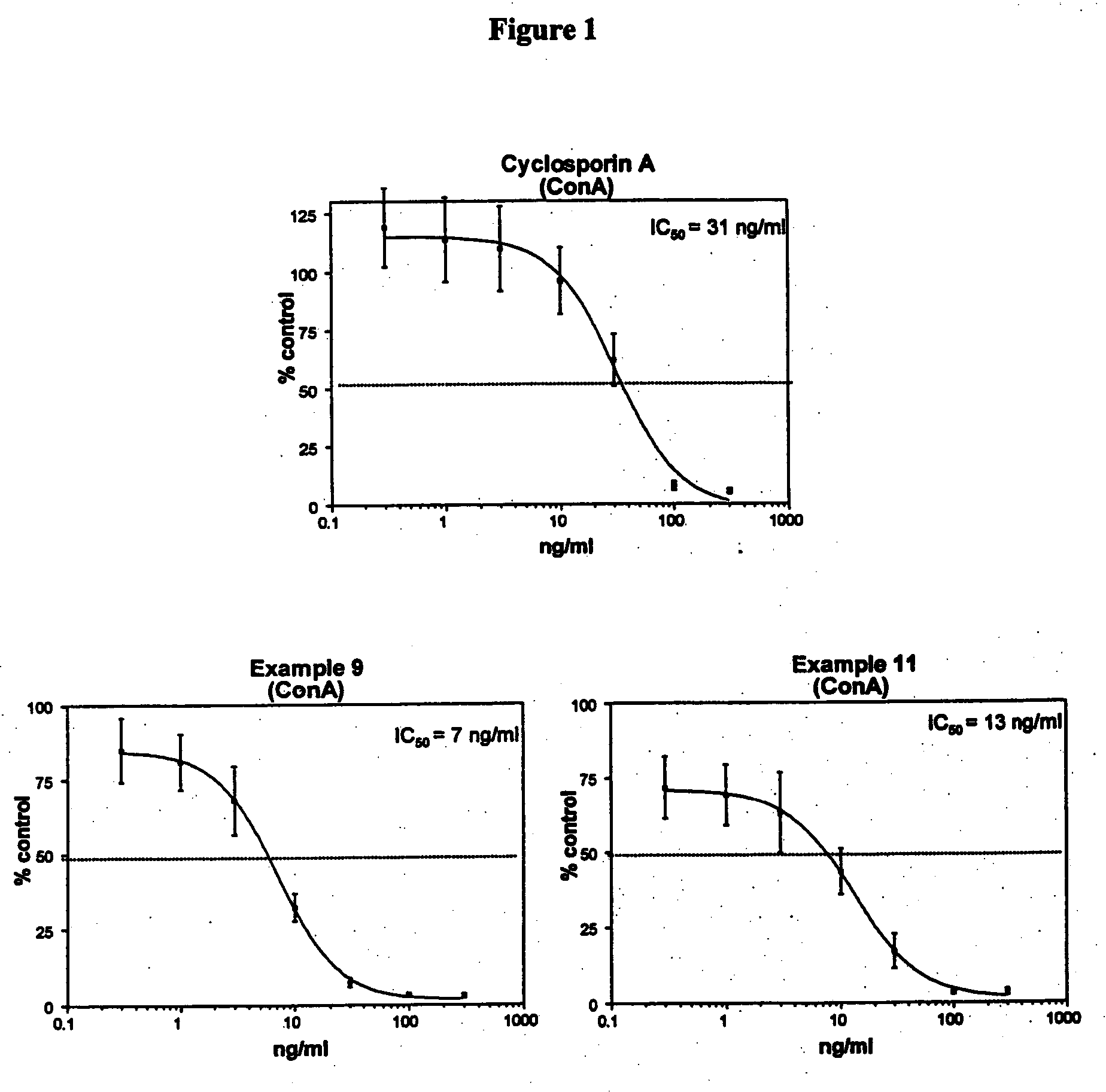
277 results about "Cyclosporins" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
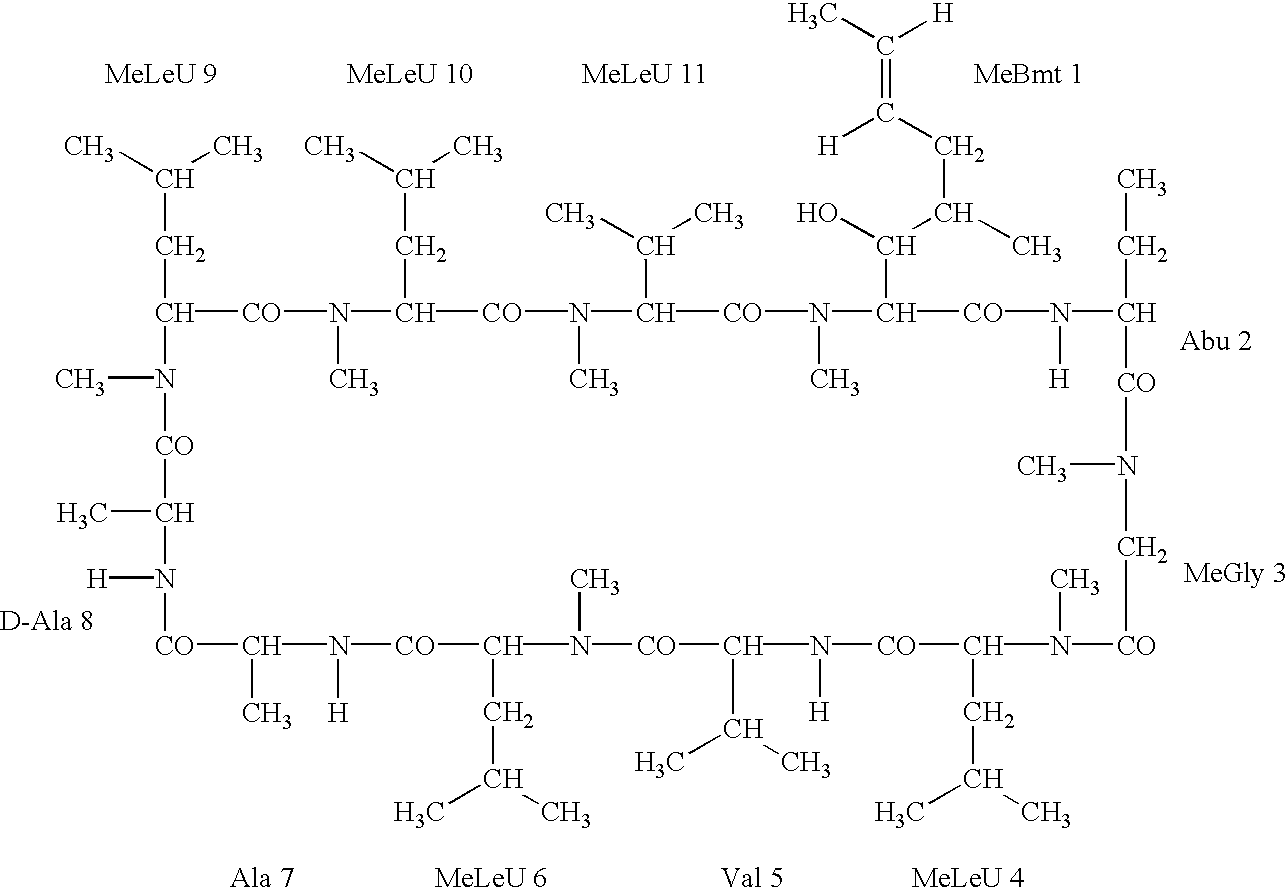
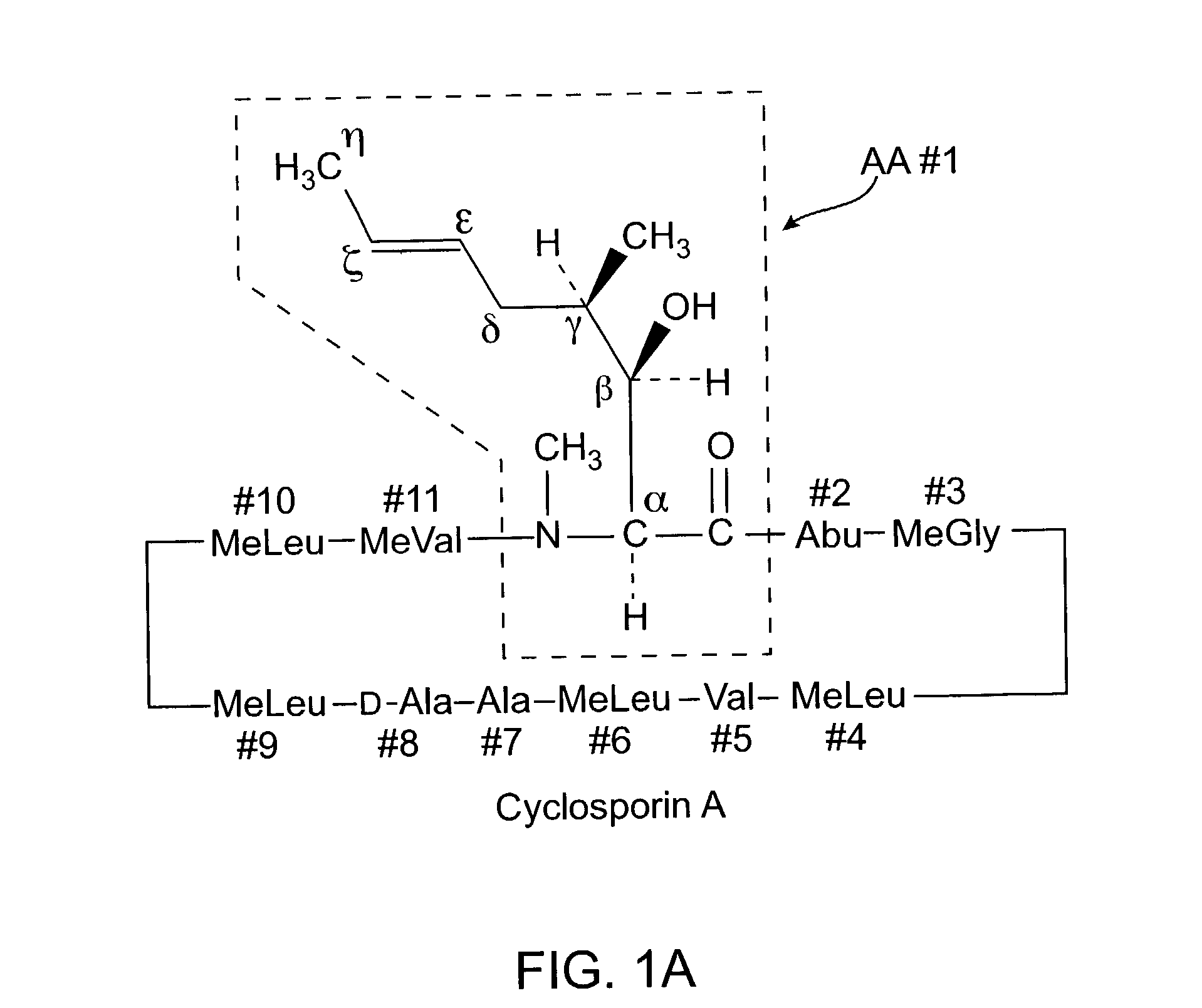
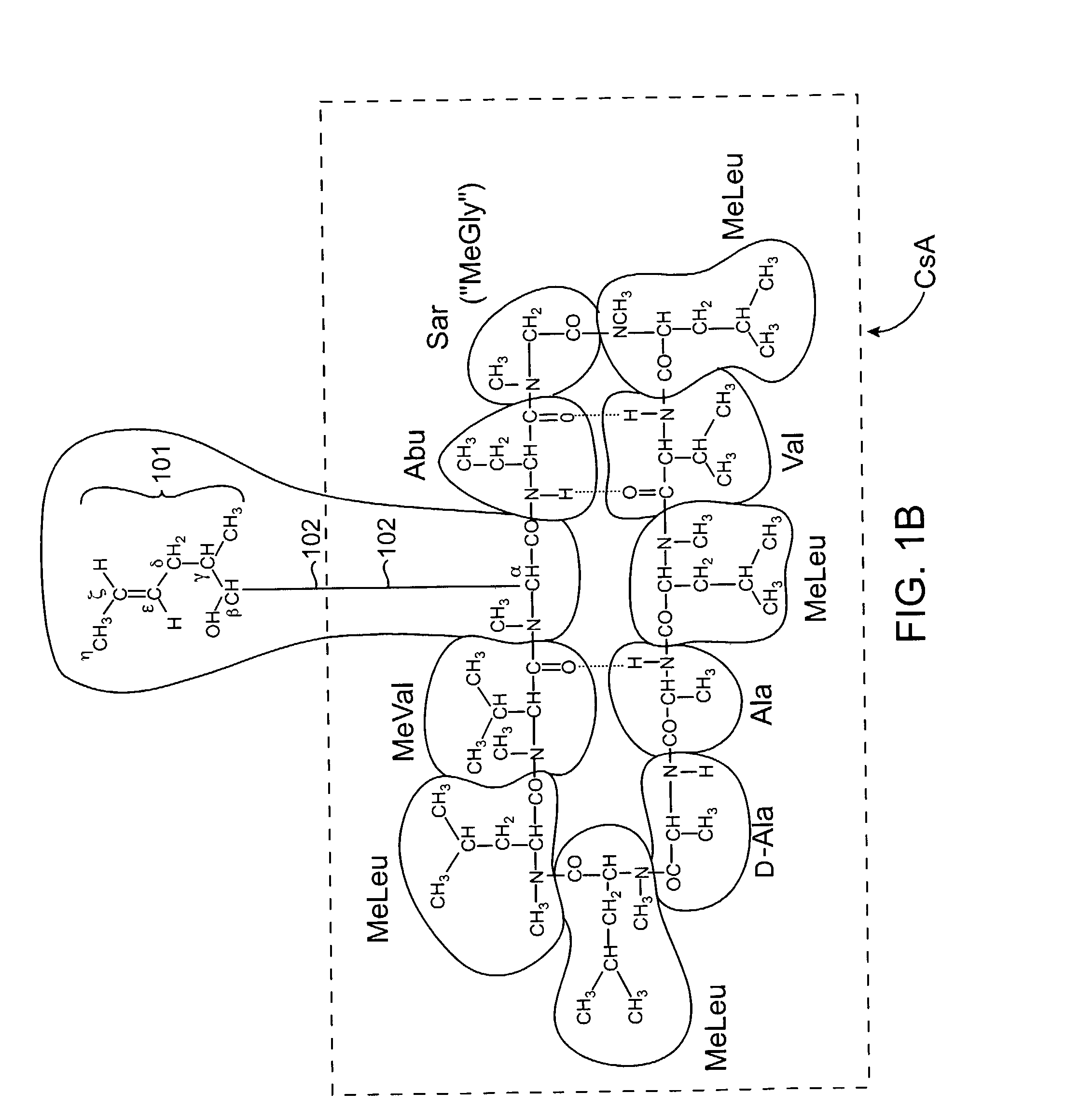
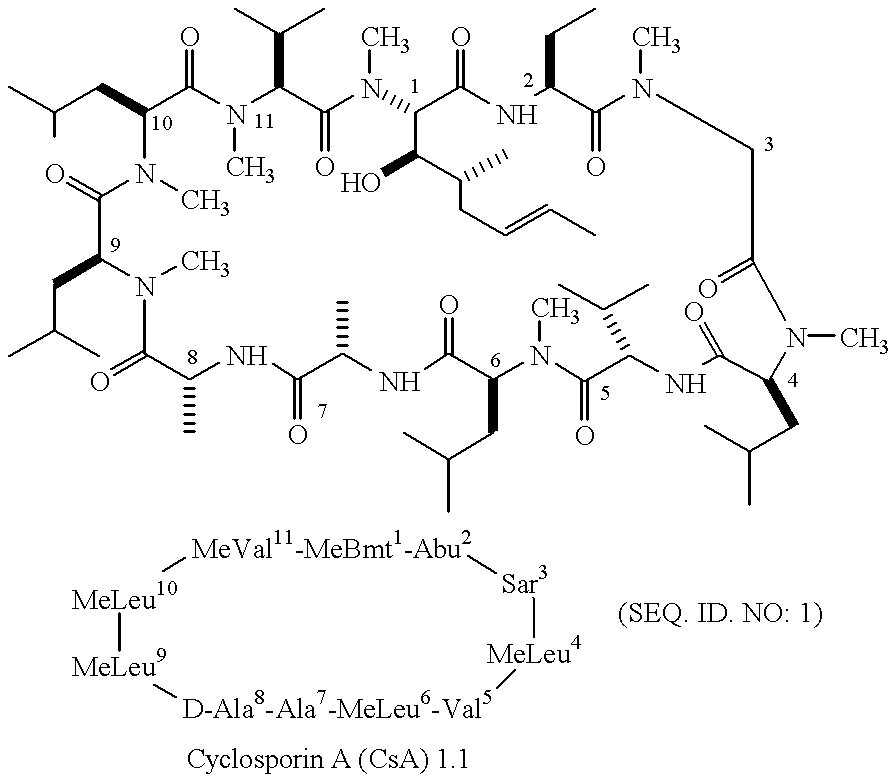
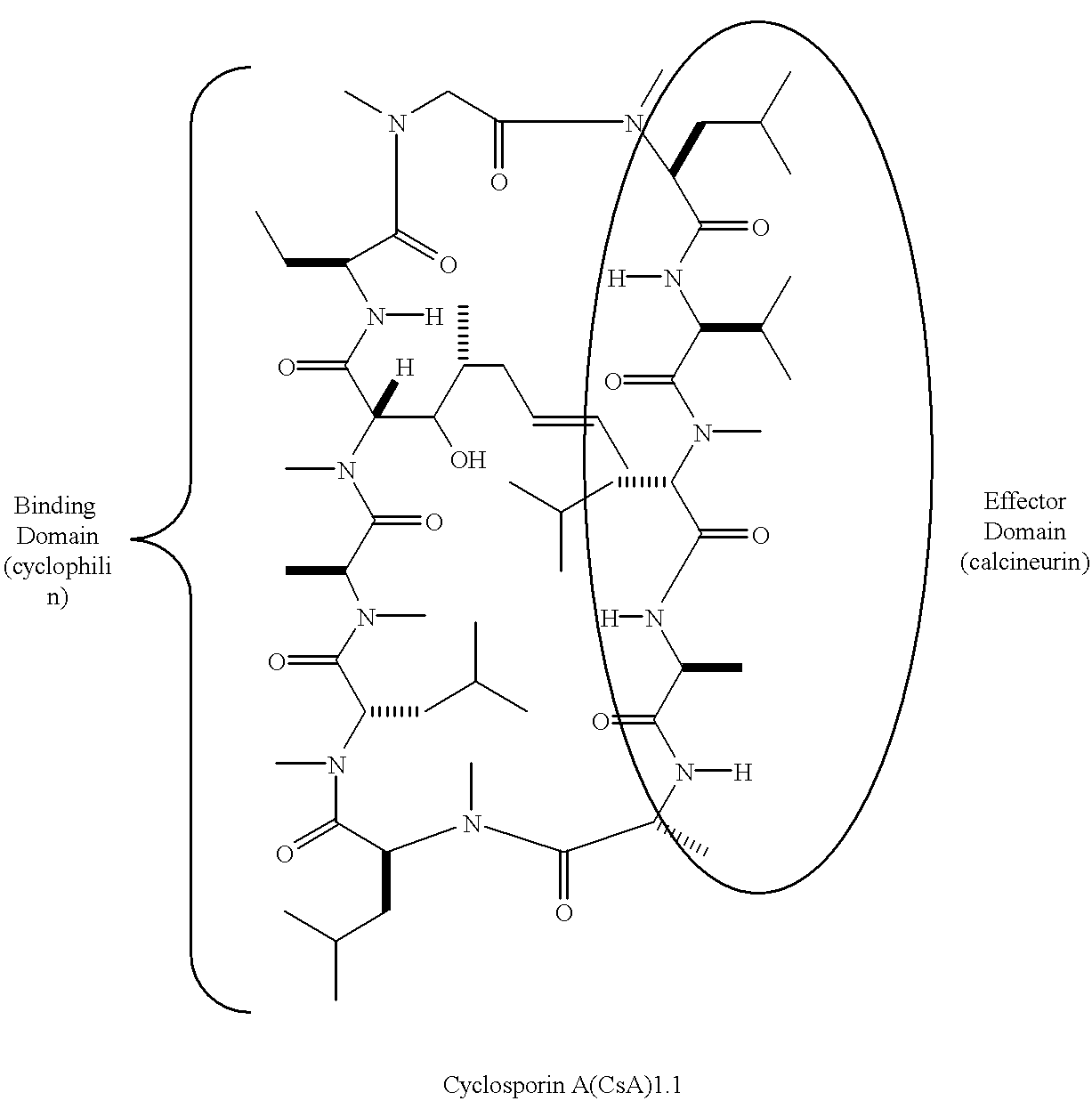
The cyclosporins are a group of macrolides isolated from fungi and used as immunosuppresant drugs, for example after transplant surgery. They are nonribosomal peptide synthesized by cyclosporin synthetase.
Cyclosporin analogues and their pharmaceutical uses
Owner:ALBANY MOLECULAR RESEARCH INC
Pharmaceutical compositions for lipophilic drugs
InactiveUS7070802B1Good self-emulsifying performanceShelf-stableCyclic peptide ingredientsCapsule deliveryMonoglycerideCyclosporins
Stable solutions of lipophilic drugs, such as cyclosporin, forming a polar lipid self-emulsifying drug delivery system. The solutions can include lipophilic drugs, such as cyclosporin, dissolved in a polar lipid, such as having a C6-C12 fatty acid monoglyceride content of at least about 50%, surfactants and triglycerides. The composition forms a fine emulsion on exposure to water. The encapsulated dosage form of this composition needs neither a hydrophilic component nor air-tight blister packaging, and is particularly suitable for oral administration.
Owner:WATSON LAB INC
Spontaneous emulsions containing cyclosporine
A pharmaceutical composition contains cyclosporine as the active ingredient. More specifically, the composition is an orally administered pharmaceutical formulation in the form of a spontaneous emulsion comprising cyclosporine, ethanol ethyl oleate and polyoxyethylene glycerol trioleate. A method for preparing an orally administered pharmaceutical composition involves first dissolving cyclosporine in ethanol. Polyoxyethylene glycerol trioleate and an oil component are then added, mixed and diluted in an aqueous media to form a spontaneous emulsion.
Owner:WOCKHARDT EU OPERATIONS SWISS
Drug eluting coatings for medical implants
ActiveUS20040037886A1Minimizing restenosisMinimizing thrombosisSuture equipmentsBiocideEverolimusCyclosporins
Drug eluting coating compositions are composed of at least one therapeutic agent dispersed in modified, biologically active binders. The therapeutic agents included in the coating composition are paclitaxel, sirolimus, tacrolimus, everolimus, actinomycin-D, dexamethasone, mycophenolic acid, cyclosporins, estradiol, and derivatives and analogs thereof. These therapeutic agents are applied to the surface of the medical device by a modified, biologically active binders. By using these biologically active binders, the therapeutic agents can be applied to at least one surface of a medical implant without using inert polymer carriers.
Owner:BIOVENTION INC
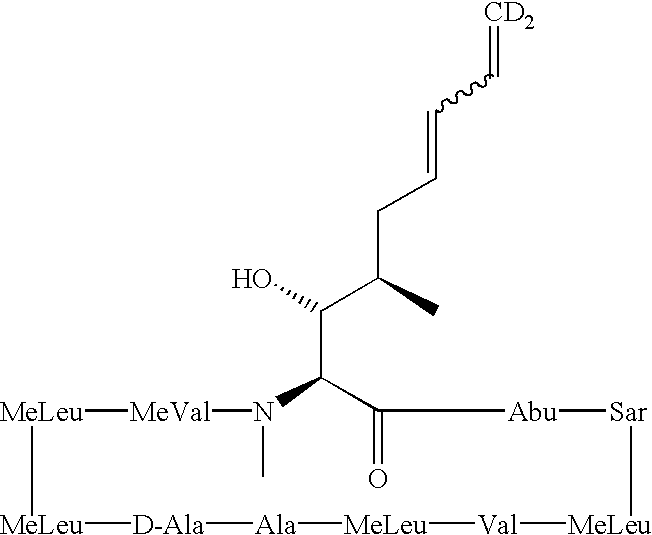
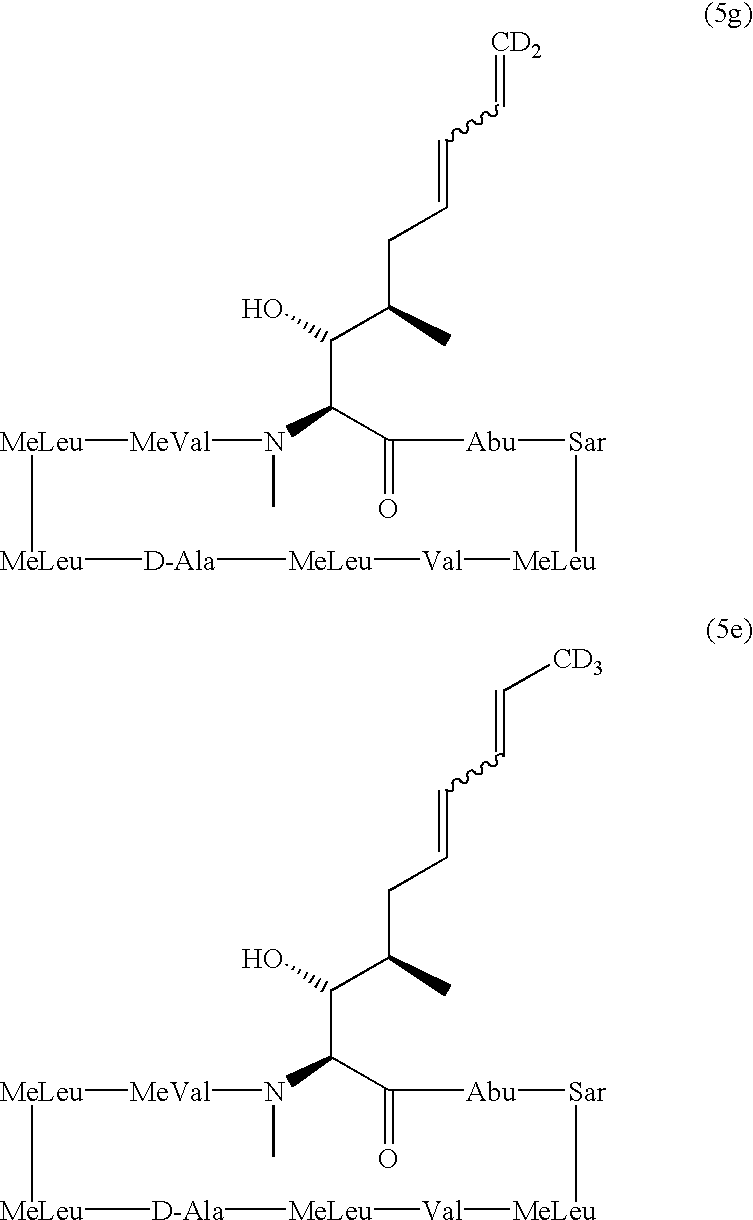
Deuterated cyclosporine analogs and their use as immunomodulating agents
InactiveUS6605593B1Improve practicalityAltered physicochemical and pharmacokinetic propertyNervous disorderAntipyreticCyclosporinsImmunomodulating Agent
Cyclosporine derivatives are disclosed which possess enhanced efficacy and reduced toxicity over naturally occurring and other presently known cyclosporins and cyclosporine derivatives. The cyclosporine derivatives of the present invention are produced by chemical and isotopic substitution of the cyclosporine A (CsA) molecule by: (1) Chemical substitution and optionally deuterium substitution of amino acid 1; and (2) deuterium substitution at key sites of metabolism of the cyclosporine A molecule such as amino acids 1, 4, 9. Also disclosed are methods of producing the cyclosporine derivatives and method of producing immunosuppression with reduced toxicity with the disclosed cyclosporine derivatives.
Owner:AURINIA PHARMA
Pharmaceutical composition comprising cyclosporin solid-state microemulsion
InactiveUS6306434B1Easy to controlMaintaining blood concentrationPowder deliveryCyclic peptide ingredientsIntestinal fluidBlood concentration
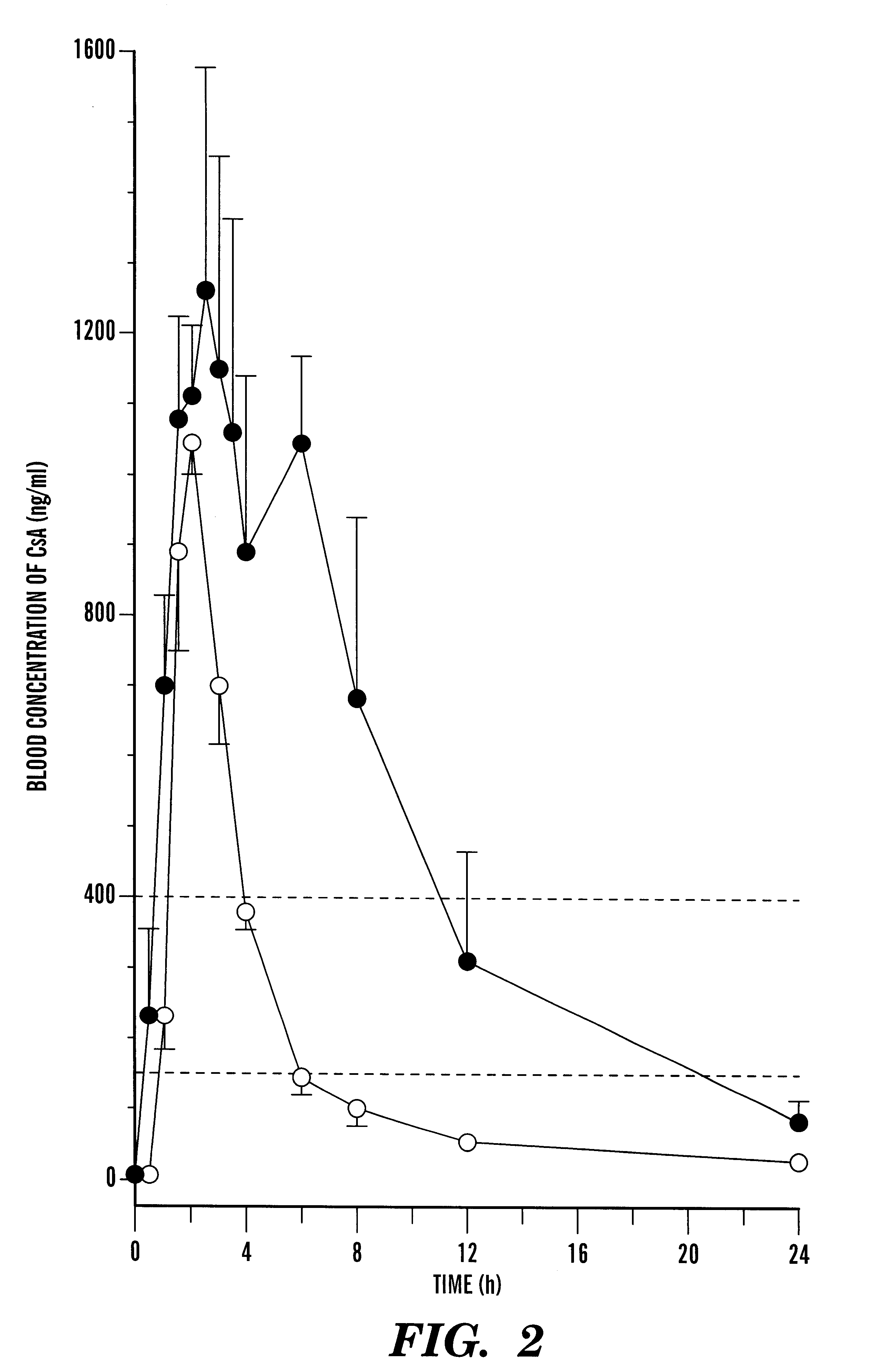
A pharmaceutical composition comprising a cyclosporin solid-state microemulsion is disclosed. In a preferred embodiment, the composition comprises a cyclosporin microemulsion dispersed in an enteric carrier. The composition does not dissolve in external phases such as artificial gastric fluid, but dissolves rapidly in artificial intestinal fluid, whereby it releases the cyclosporin microemulsion, providing rapid delivery of cyclosporin. The composition effectively maintains a therapeutic blood concentration of cyclosporin with once a day dosing, providing for convenience of administration and avoiding adverse effects induced by increasing peak blood cyclosporin concentrations associated with conventional cyclosporin formulations.
Owner:CHONG KUN DANG PHARMA CORP
Ophthalmic formulations
InactiveUS6953776B2Improve bioavailabilityImprove toleranceOrganic active ingredientsBiocideCyclosporinsAqueous solution
A topical ophthalmic formulation in the form of an aqueous solution comprising a cyclosporin, hyaluronic acid or one of its salts, and polysorbate 80 is described.
Owner:LABE MEDIDOM
Cyclosporin compositions
A composition is disclosed herein comprising from about 0.001% to about 0.4% cyclosporin A, castor oil, and a surfactant selected from the group consisting of alcohol ethoxylates, alcohols, alkyl glycosides, alkyl polyglycosides, alkylphenol ethoxylates, amine oxides, block polymers, carboxylated alcohol or alkylphenol ethoxylates, carboxylic acids / fatty acids, cellulose derivatives, ethoxylated alcohols, ethoxylated alkylphenols, ethoxylated aryl phenols, ethoxylated fatty acids, ethoxylated fatty acids, ethoxylated fatty esters and oils, fatty alcohols, fatty esters, glycol esters, lanolin-based derivatives, lecithin and lecithin derivatives, lignin and lignin derivatives, methyl esters, monoglycerides and derivatives , phosphalipids, polyacrylic acids, polyethylene glycols, polyethylene oxide-polypropylene oxide copolymers, polyethylene oxides, polymeric surfactants, polypropylene oxides, propoxylated alcohols, propoxylated alkyl phenols, propoxylated fatty acids, protein-based surfactants, sarcosine derivatives, silicone-based surfactants, sorbitan derivatives, stearates, sucrose and glucose esters and derivatives, and combinations thereof.
Owner:SAINT REGIS MOHAWK TRIBE
Pharmaceutical compositions comprising cyclosporins
A liquid comprising a therapeutically effective concentration of a cyclosporin and a vitamin E tocopherol polyethylene glycol succinate, wherein said liquid is an aqueous solution, and wherein no hydrophilic organic solvent is present at a concentration greater than half of that of the cyclosporin is also disclosed herein. A composition comprising a therapeutically effective concentration of cyclosporin A and an effective amount of a vitamin E tocopherol polyethylene glycol succinate, wherein said composition is an aqueous liquid solution which is intended for ophthalmic use, and wherein no hydrophilic organic solvent is present at a mass concentration greater than or equal to that of the cyclosporin, is disclosed herein. A composition comprising a therapeutically effective concentration of cyclosporin A and an effective amount of a vitamin E tocopherol polyethylene glycol succinate, wherein said composition is an aqueous liquid solution which is intended for parenteral use, and wherein no hydrophilic organic solvent is present at a mass concentration greater than or equal to that of the cyclosporin, is disclosed herein. Methods of treating diseases or conditions using said compositions, and medicaments related thereto, are also disclosed herein.
Owner:ALLERGAN INC
Methods for prevention and treatment of septic shock
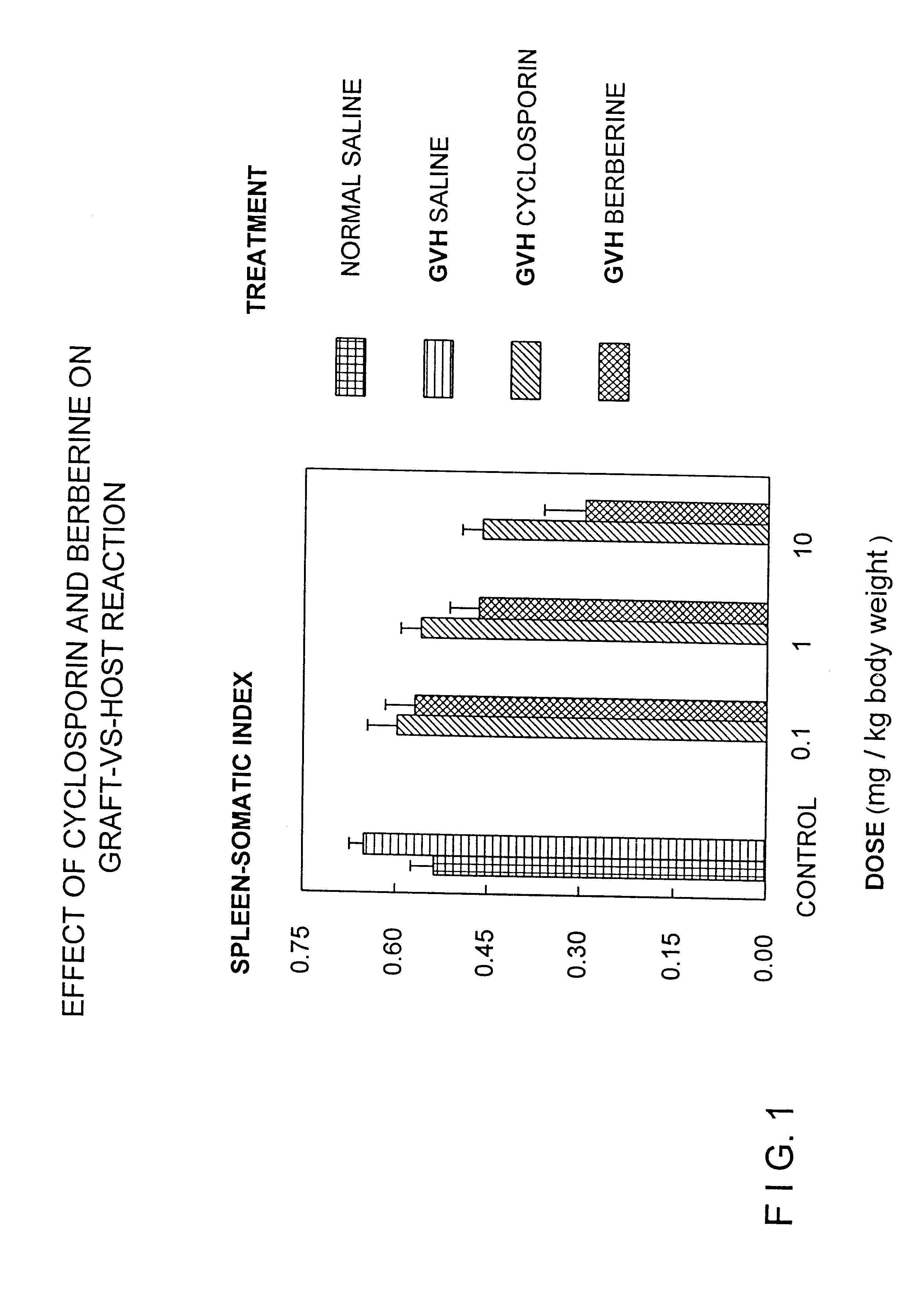
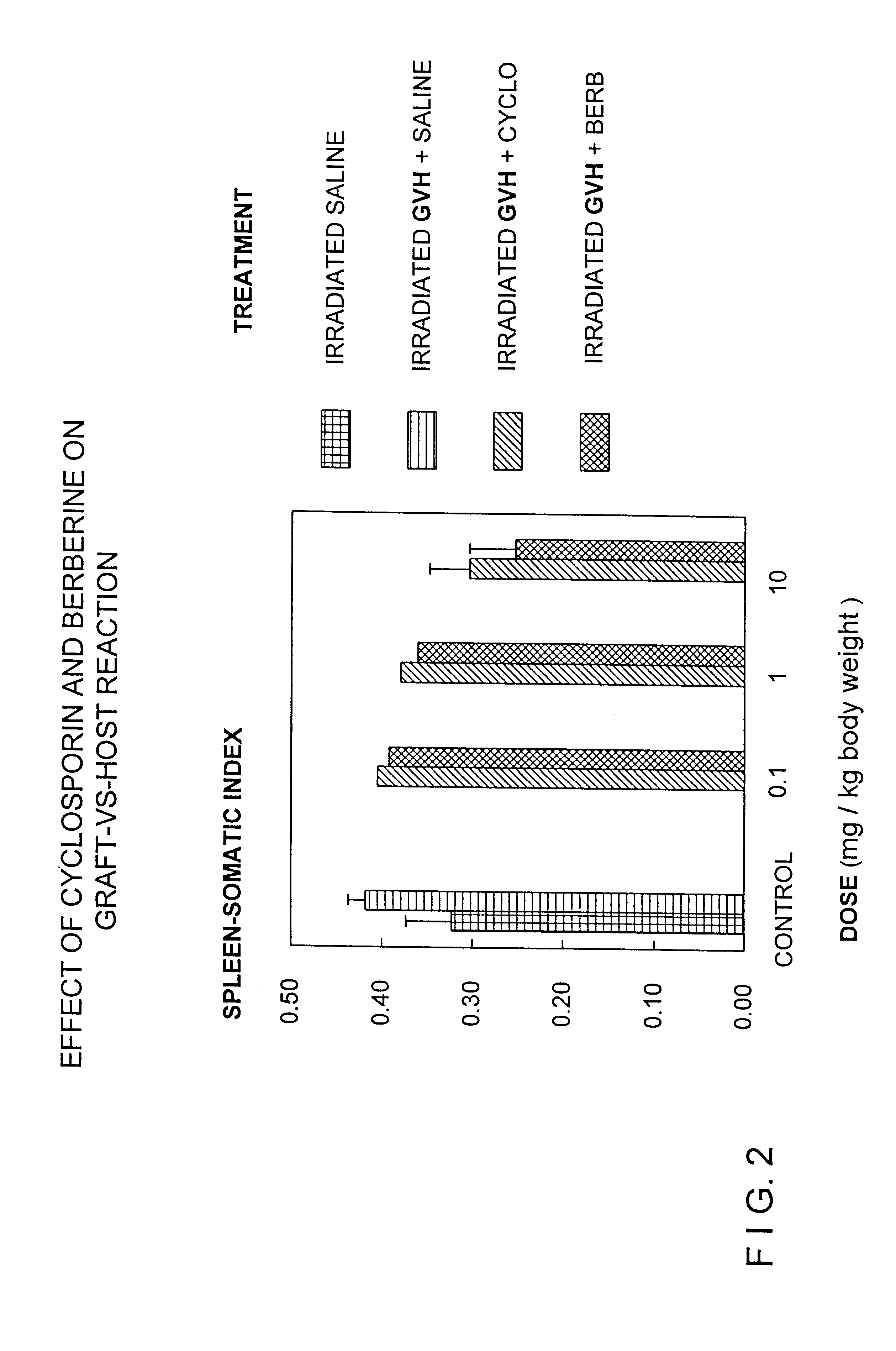
Berberine or pharmaceutically acceptable salts thereof either alone or in combination with cyclosporin can be used to treat or prevent graft vs. host disease. Berberine or pharmaceutically acceptable salts thereof can also be used to treat or prevent septic shock syndrome.
Owner:NATIONAL INSTUTUTE OF IMMUNOLOGY
Deuterated cyclosporine analogs and their use as immunomodulating agents
InactiveUS20020132763A1Improve practicalityAltered physicochemical and pharmacokinetic propertyNervous disorderAntipyreticCyclosporinsImmunomodulating Agent
Cyclosporine derivatives are disclosed which possess enhanced efficacy and reduced toxicity over naturally occurring and other presently known cyclosporins and cyclosporine derivatives. The cyclosporine derivatives of the present invention are produced by chemical and isotopic substitution of the cyclosporine A (CsA) molecule by: (1) Chemical substitution and optionally deuterium substitution of amino acid 1; and (2) deuterium substitution at key sites of metabolism of the cyclosporine A molecule such as amino acids 1, 4, 9. Also disclosed are methods of producing the cyclosporine derivatives and method of producing immunosuppression with reduced toxicity with the disclosed cyclosporine derivatives.
Owner:NAICKER SALVARAJ +2
Novel cyclosporin analogues and their pharmaceutical uses
Owner:ALBANY MOLECULAR RESEARCH INC
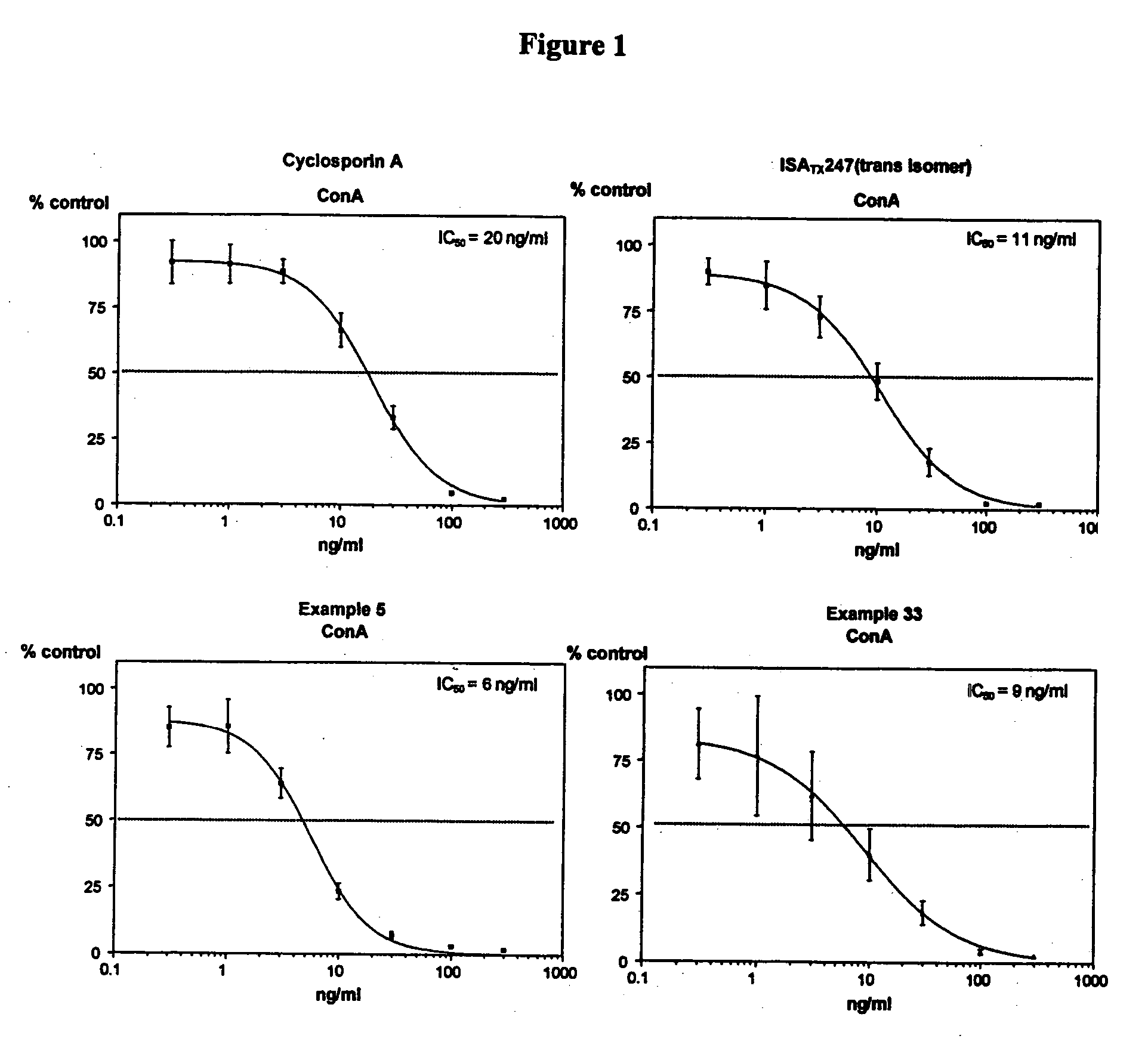
Cyclosporine analogue mixtures and their use as immunomodulating agents
ActiveUS6998385B2Potent immunosuppressantImprove effectivenessSenses disorderNervous disorderCyclosporinsImmunomodulating Agent
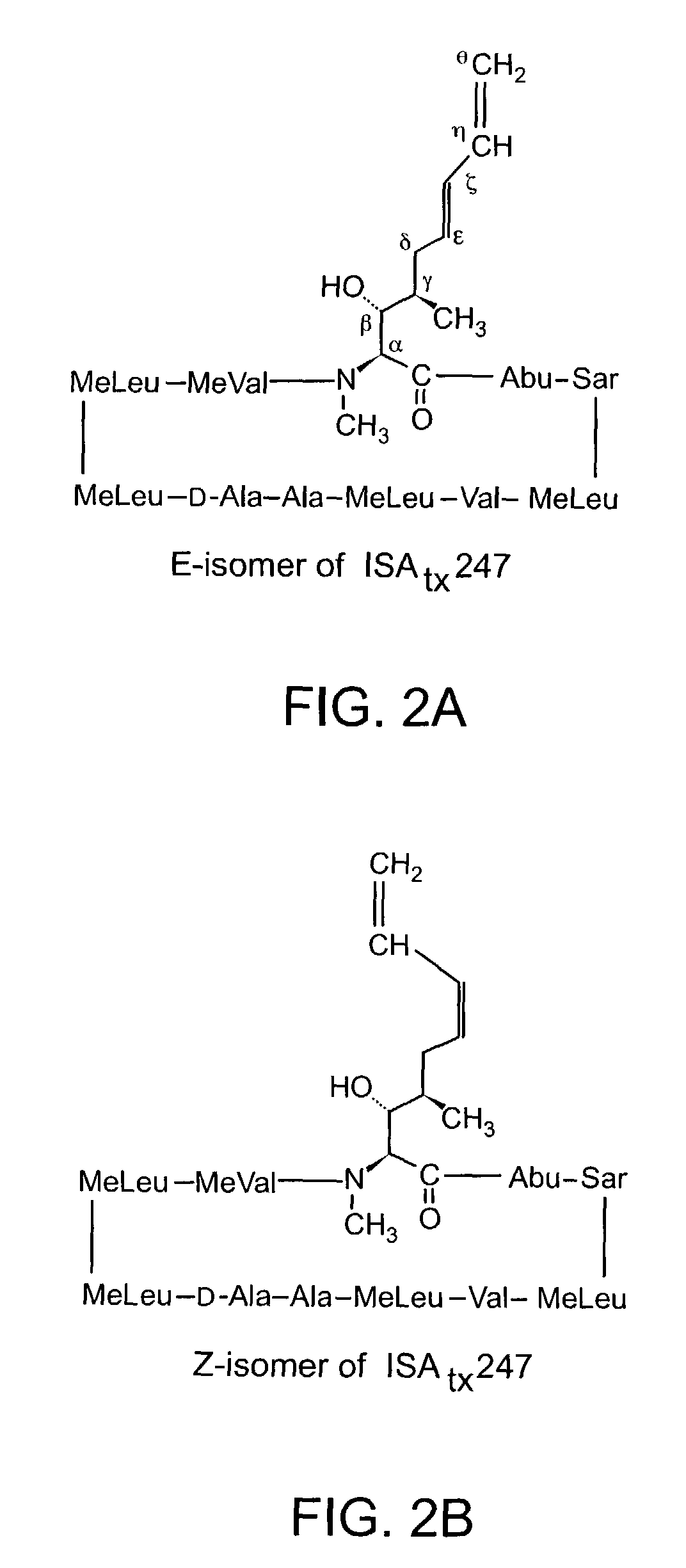
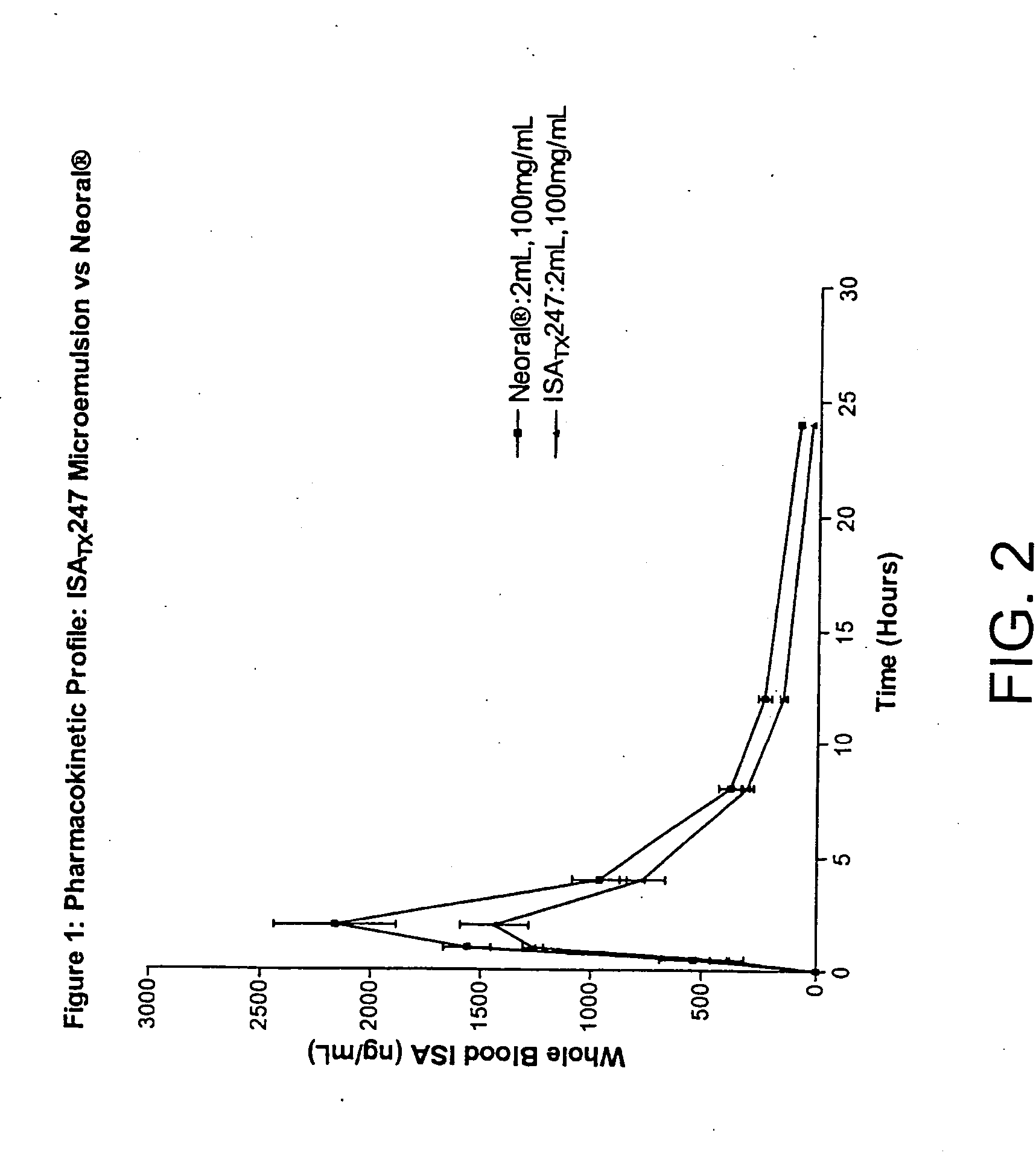
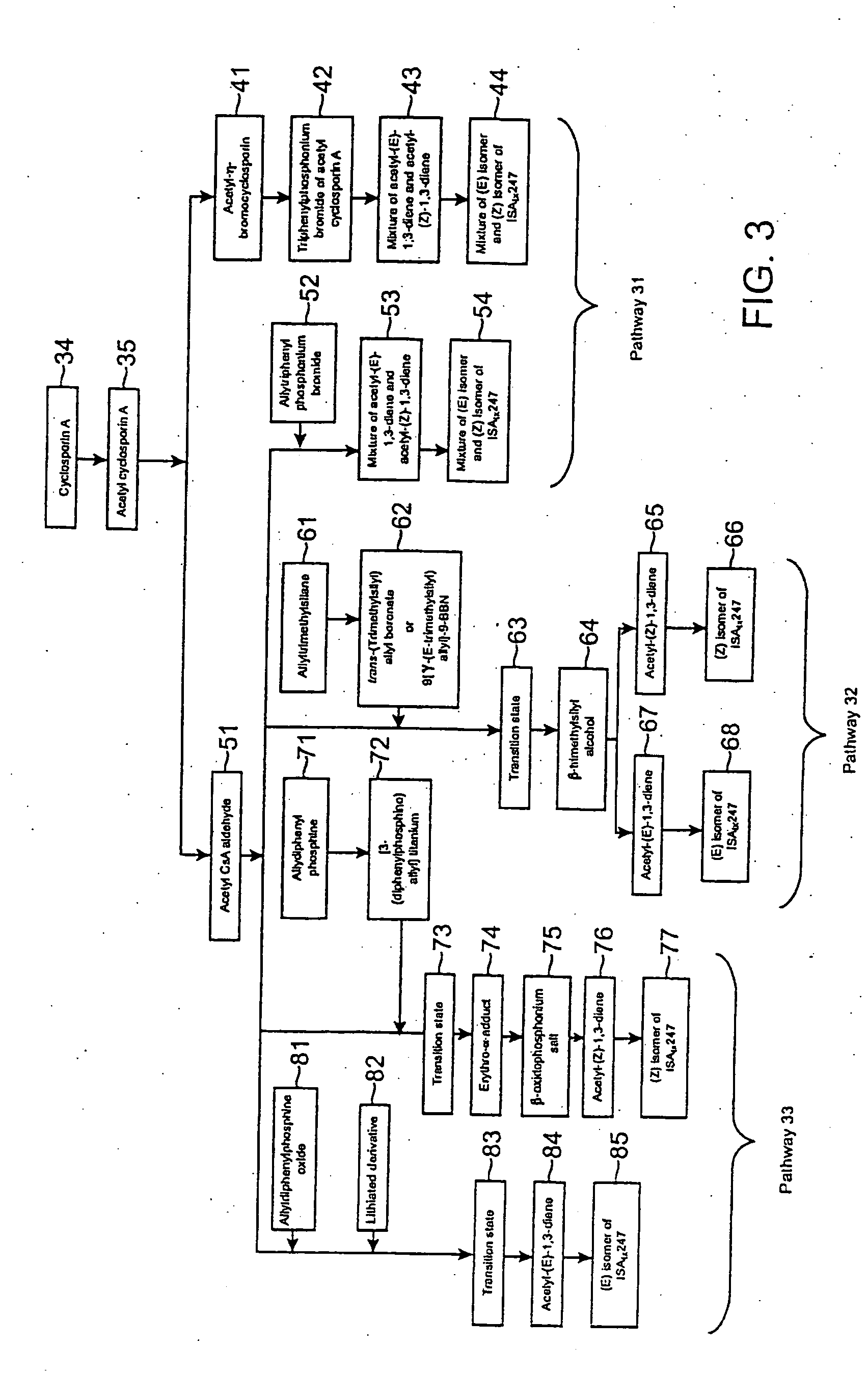
The invention is directed to isomeric mixtures of cyclosporine analogues that are structurally similar to cyclosporine A. The mixtures possess enhanced efficacy and reduced toxicity over the individual isomers and over naturally occurring and other presently known cyclosporines and cyclosporine derivatives. Embodiments of the present invention are directed toward cis and trans-isomers of cyclosporin A analogs referred to as ISATX247, and derivatives thereof. Mixtures of ISATX247 isomers exhibit a combination of enhanced potency and reduced toxicity over the naturally occurring and presently known cyclosporins. ISATX247 isomers and alkylated, arylated, and deuterated derivatives are synthesized by stereoselective pathways where the particular conditions of a reaction determine the degree of stereoselectivity. The ratio of isomers in a mixture may range from about 10 to 90 percent by weight of the (E)-isomer to about 90 to 10 percent by weight of the (Z)-isomer, based on the total weight of the mixture.
Owner:AURINIA PHARMA
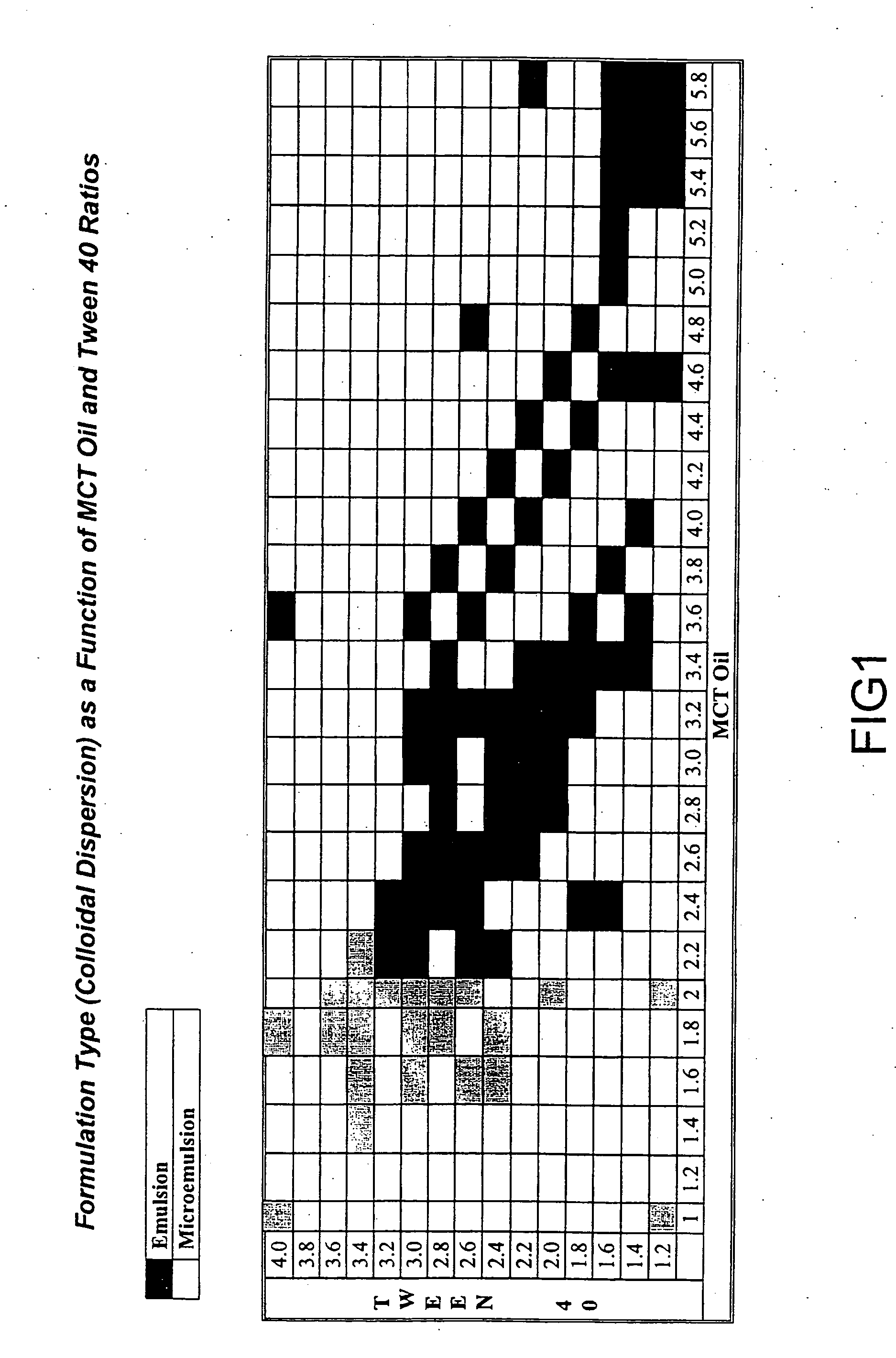
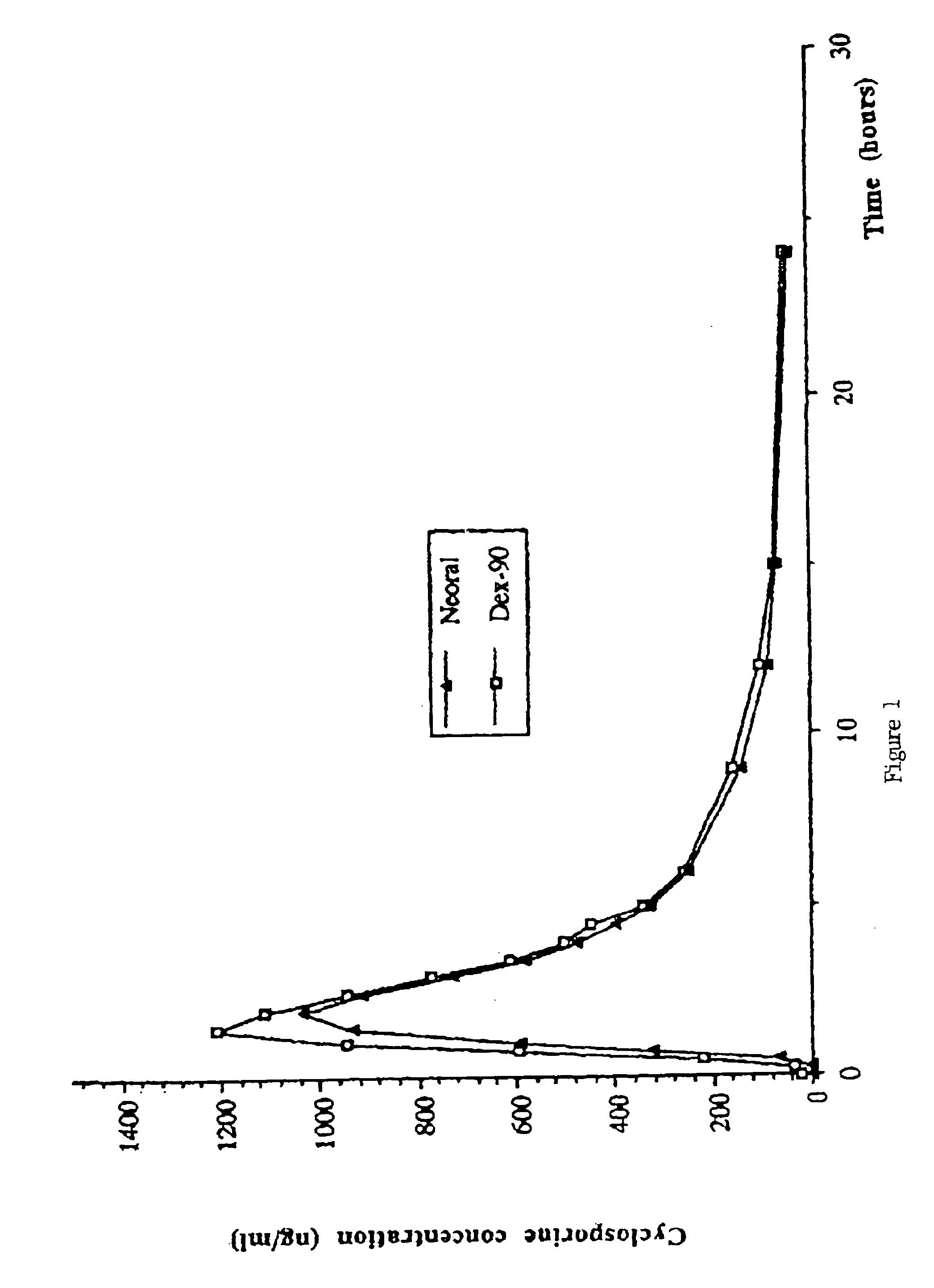
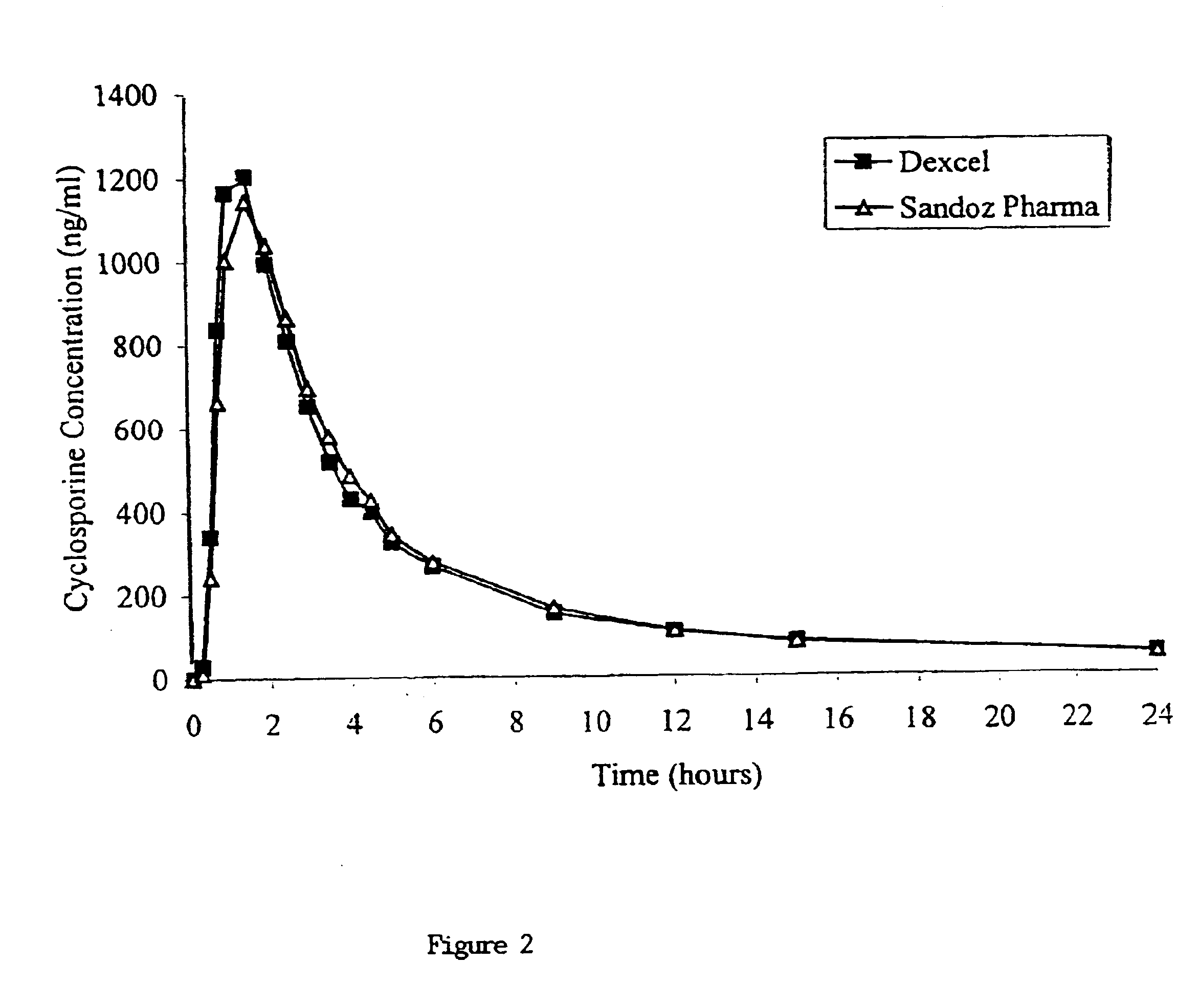
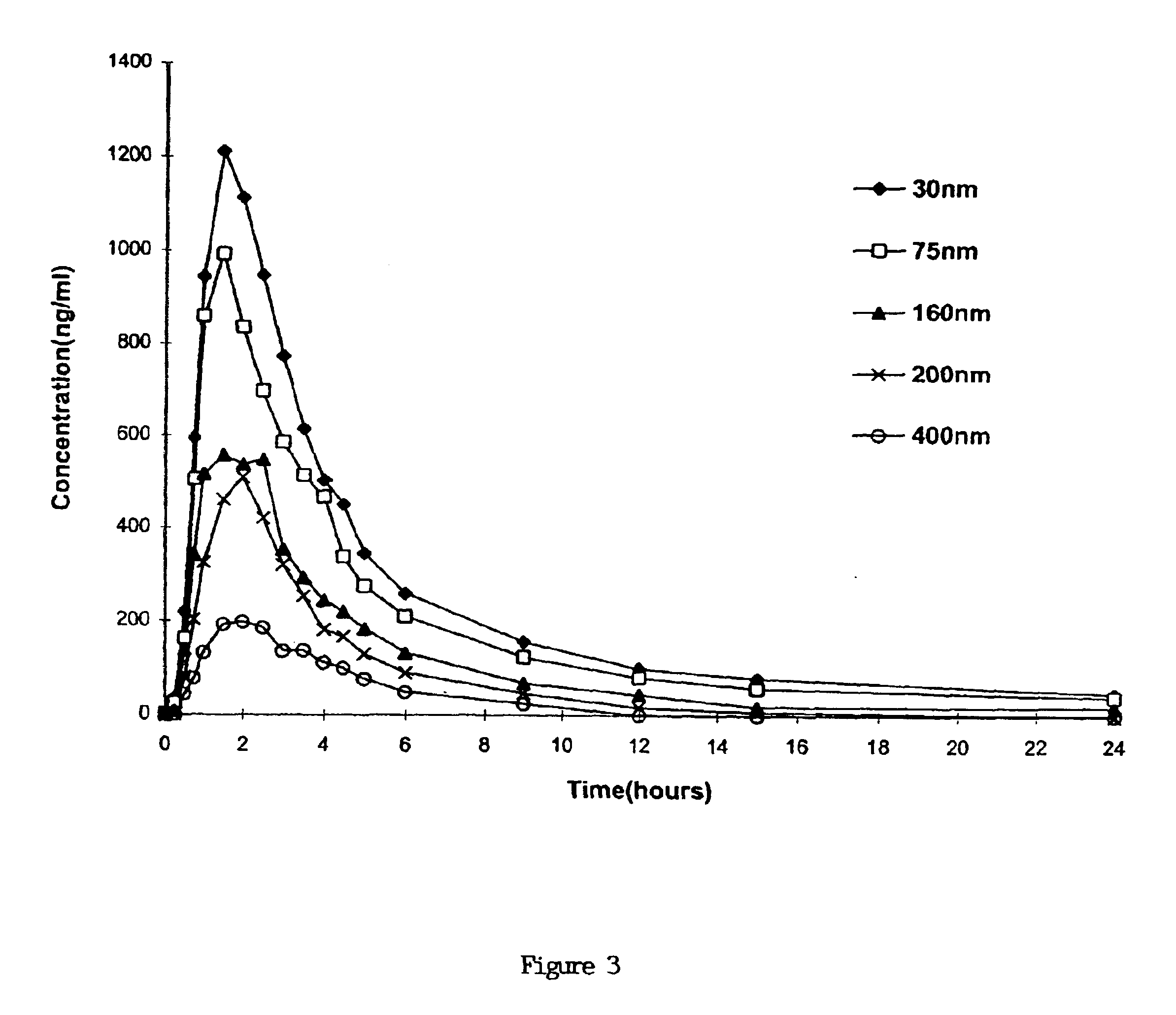
Pro-nanodispersion for the delivery of cyclosporin
InactiveUS20060205639A1Small particle sizeIncrease volumeSenses disorderNervous disorderMedicineCyclosporins
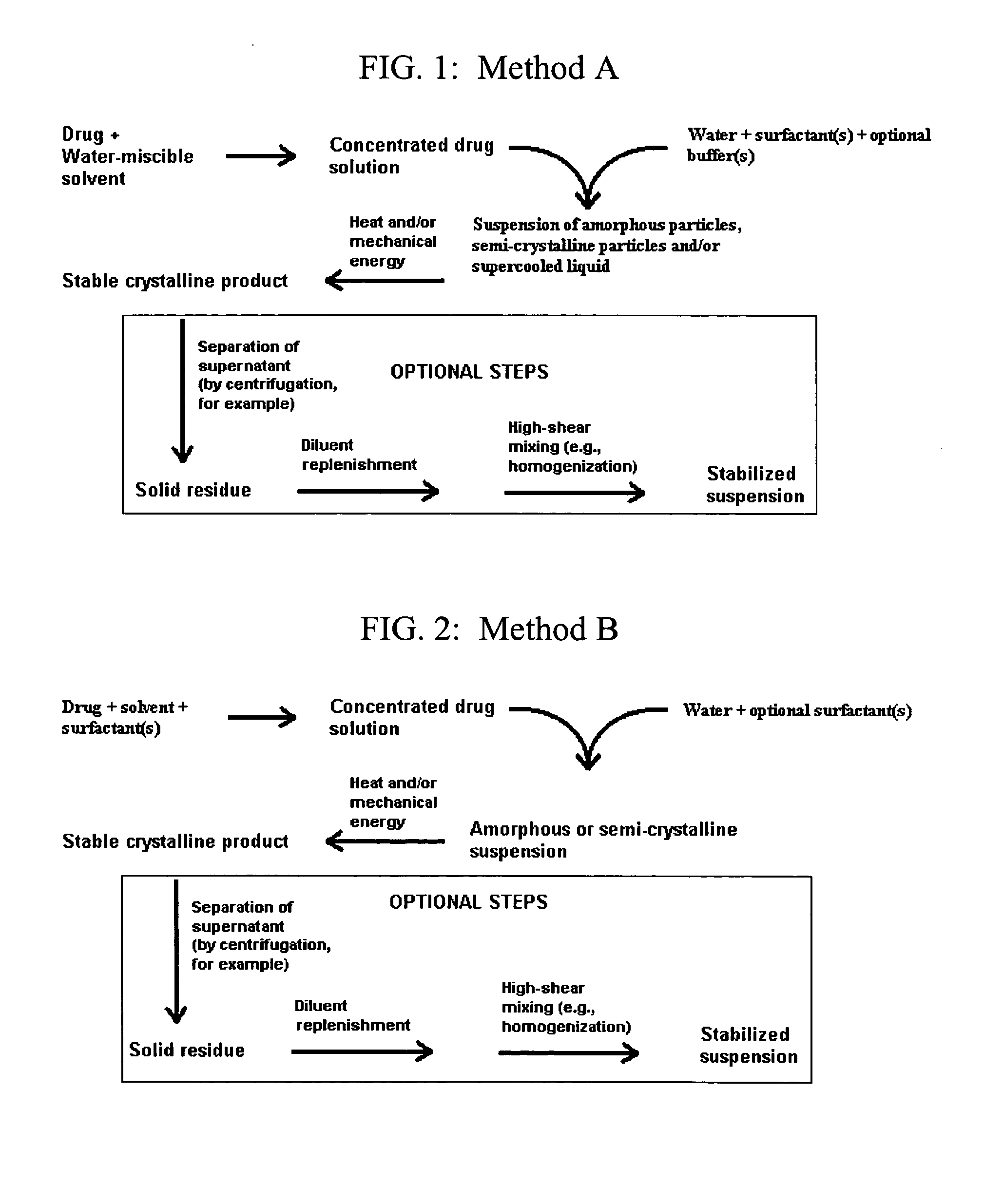
A novel cyclosporine formulation, which is a pro-nanodispersion at room temperature, featuring solid particles of a relatively large particle size (at least about 150 nm) and yet which is a nanodispersion at body temperature.
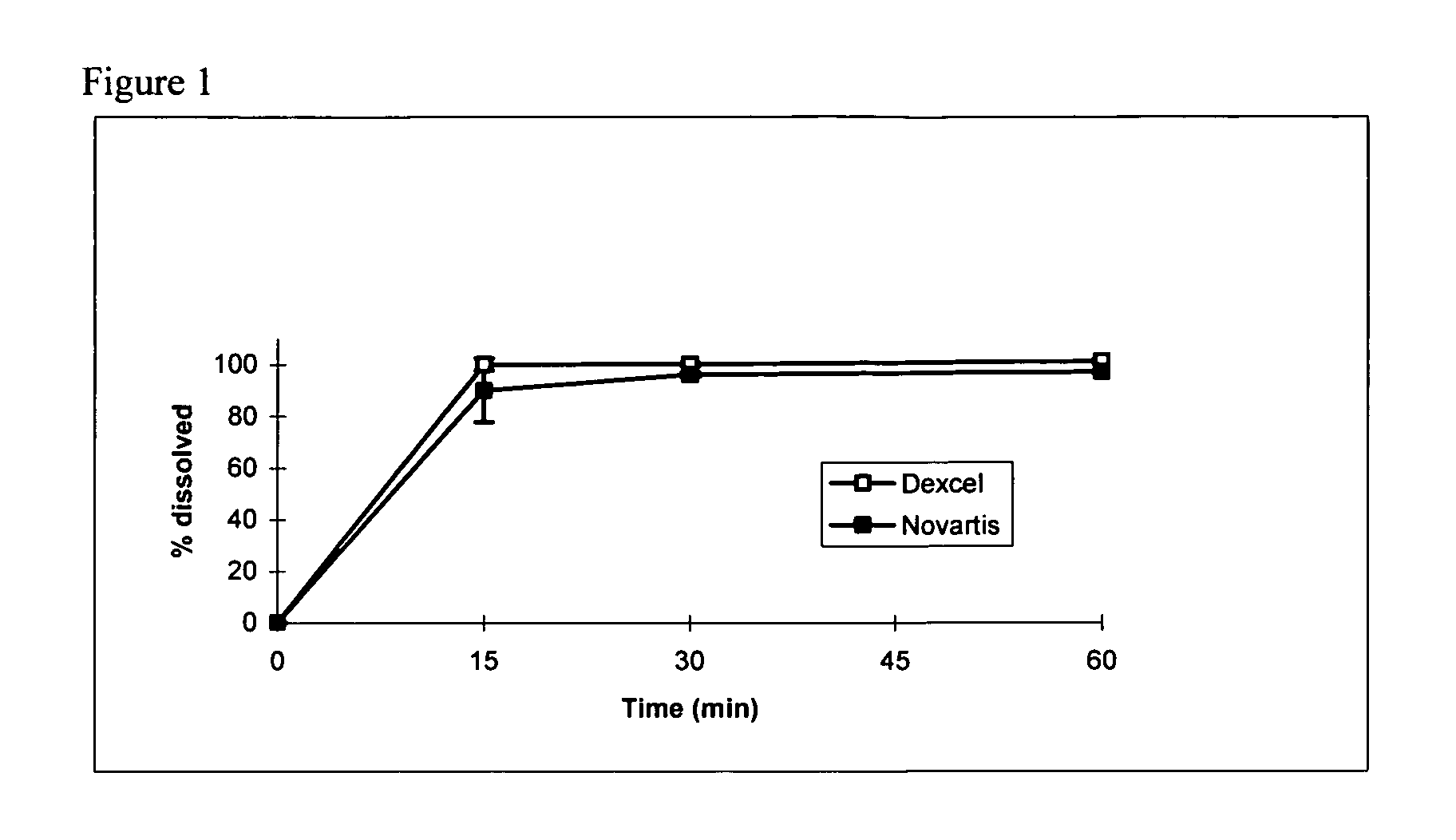
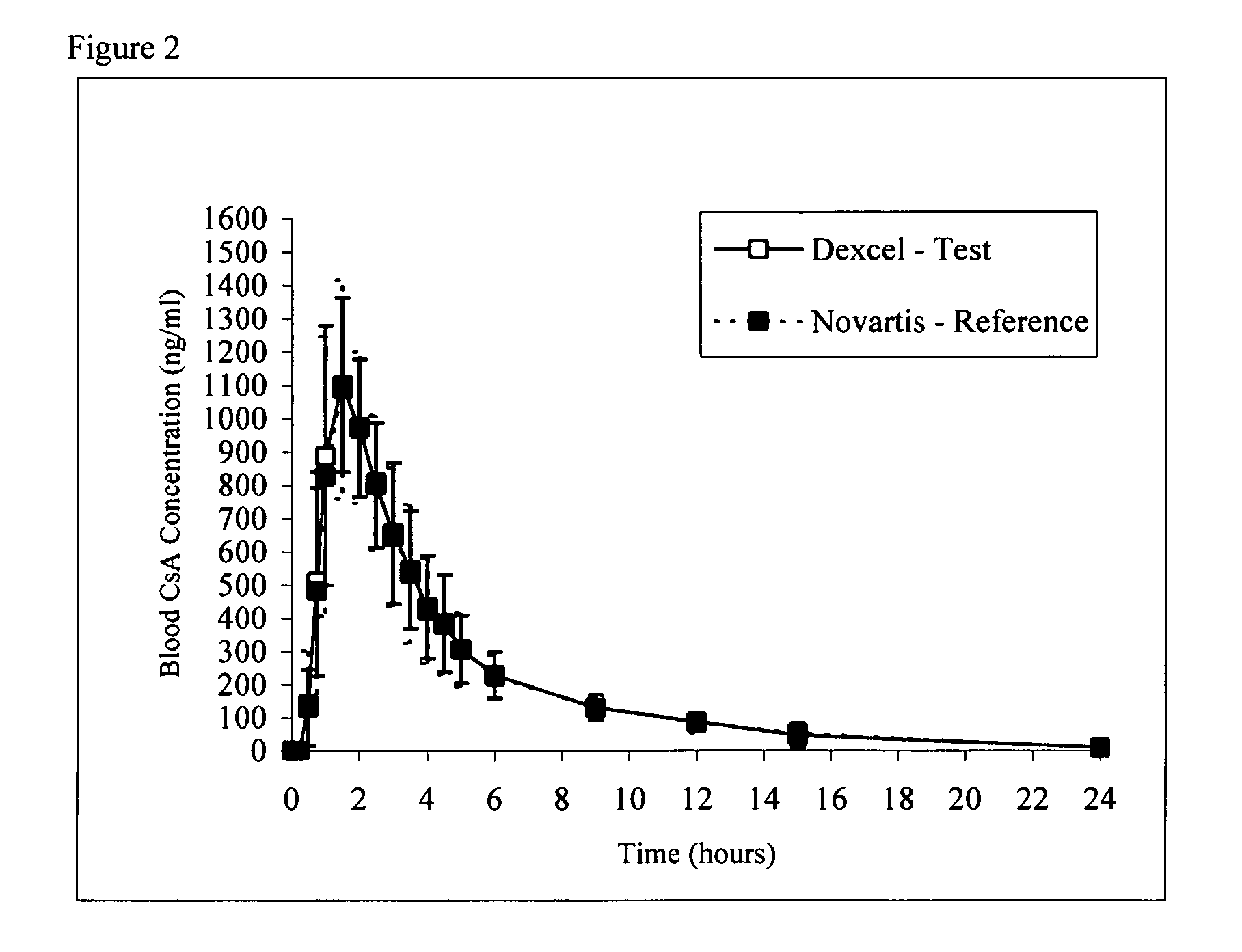
Owner:DEXCEL
Small-particle pharmaceutical formulations of antiseizure and antidementia agents and immunosuppressive agents
InactiveUS20050244503A1High drug loadingMinimize side effectsPowder deliveryCyclic peptide ingredientsMedicineCyclosporins
This invention pertains to the formulation of small-particle suspensions of anticonvulsants and antidementia, particularly carbamazepine, for pharmaceutical use. This invention also pertains to the formulation of small-particle suspensions of immunosuppressive agents, particularly cyclosporin, for pharmaceutical use.
Owner:BAXTER INT INC +1
Cyclosporin compositions
A composition is disclosed herein comprising from about 0.001% to about 0.4% cyclosporin A, castor oil, and a surfactant selected from the group consisting of alcohol ethoxylates, alcohols, alkyl glycosides, alkyl polyglycosides, alkylphenol ethoxylates, amine oxides, block polymers, carboxylated alcohol or alkylphenol ethoxylates, carboxylic acids / fatty acids, cellulose derivatives, ethoxylated alcohols, ethoxylated alkylphenols, ethoxylated aryl phenols, ethoxylated fatty acids, ethoxylated fatty acids, ethoxylated fatty esters and oils, fatty alcohols, fatty esters, glycol esters, lanolin-based derivatives, lecithin and lecithin derivatives, lignin and lignin derivatives, methyl esters, monoglycerides and derivatives, phosphalipids, polyacrylic acids, polyethylene glycols, polyethylene oxide-polypropylene oxide copolymers, polyethylene oxides, polymeric surfactants, polypropylene oxides, propoxylated alcohols, propoxylated alkyl phenols, propoxylated fatty acids, protein-based surfactants, sarcosine derivatives, silicone-based surfactants, sorbitan derivatives, stearates, sucrose and glucose esters and derivatives, and combinations thereof.
Owner:SAINT REGIS MOHAWK TRIBE
Pharmaceutical compositions
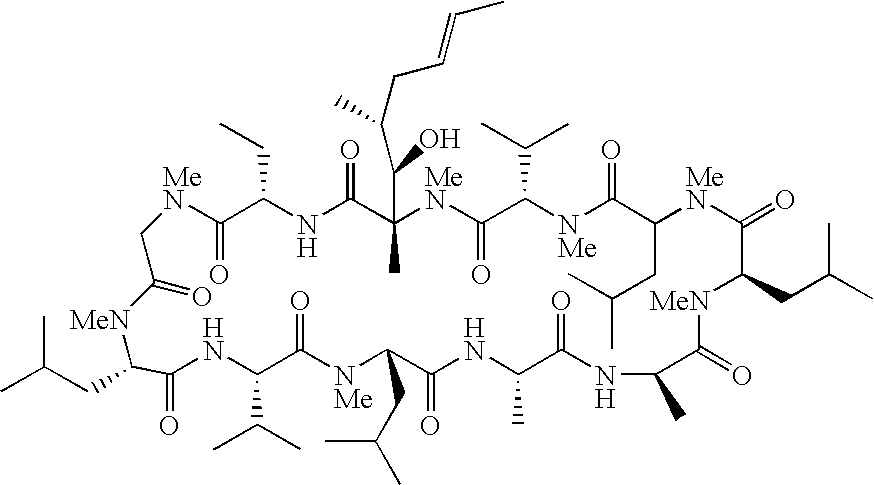
This invention relates to combinations comprising 3-[(R)-2-(N,N-dimethylamino)ethylthio-Sar]-4-(gammahydroxymethylleucine)cyclosporine, or a pharmaceutically acceptable salt, solvate or hydrate thereof; and certain nucleoside analogues, and their use in the treatment of hepatitis C virus.
Owner:SCYNEXIS INC
Topical compositions containing nonimmunosuppressive cyclosporin derivatives for treating hair loss
InactiveUS20050074468A1Good hair growthGood effectCosmetic preparationsHair cosmeticsCyclosporinsTert-leucine
The present invention discloses a topical scalp and transdermal preparation with excellent penetration to the skin and follicle, containing a [γ-hydroxy-N-methyl-L-leucine4] cylosporin derivative which is a non-immunosuppressive component with hair growth stimulating ability. The topical scalp and transdermal preparation is prepared by incorporating the cyclosporin derivative into a liposome, microcapsule, micro-sphere, composite particle or emulsion, capable of being employed as a hair growth stimulating agent and applied for the prevention of hair loss.
Owner:LG HOUSEHOLD & HEALTH CARE LTD
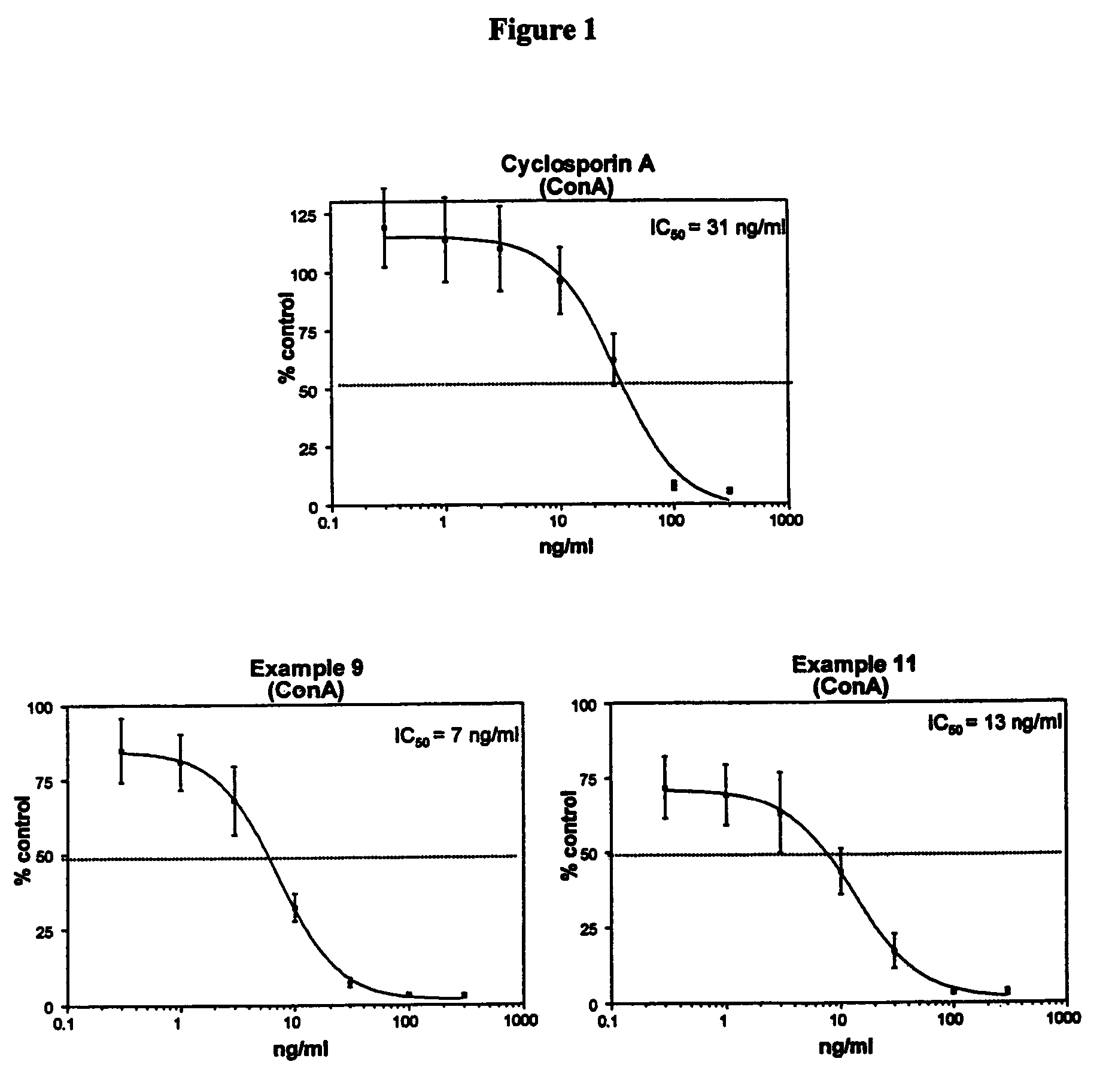
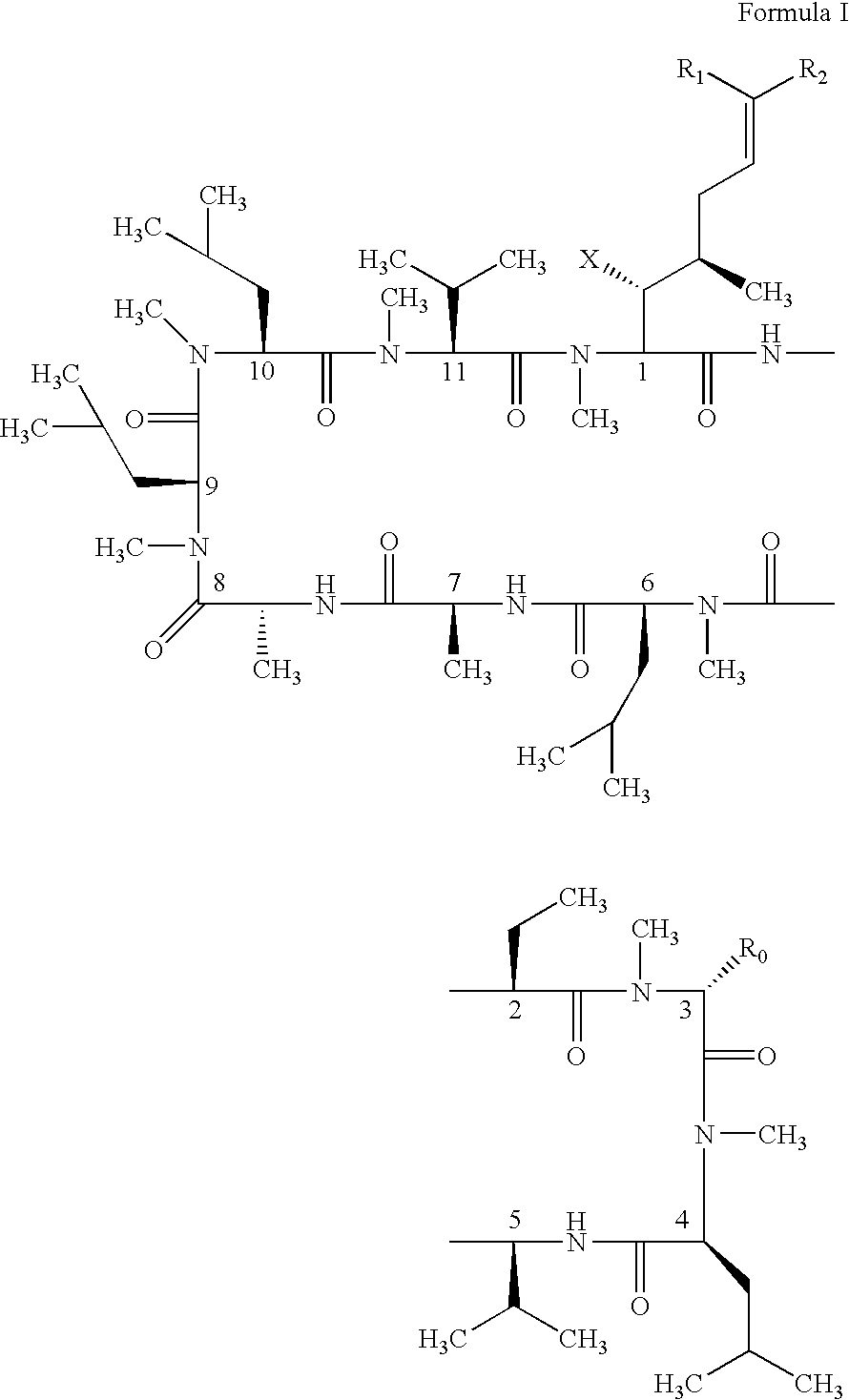
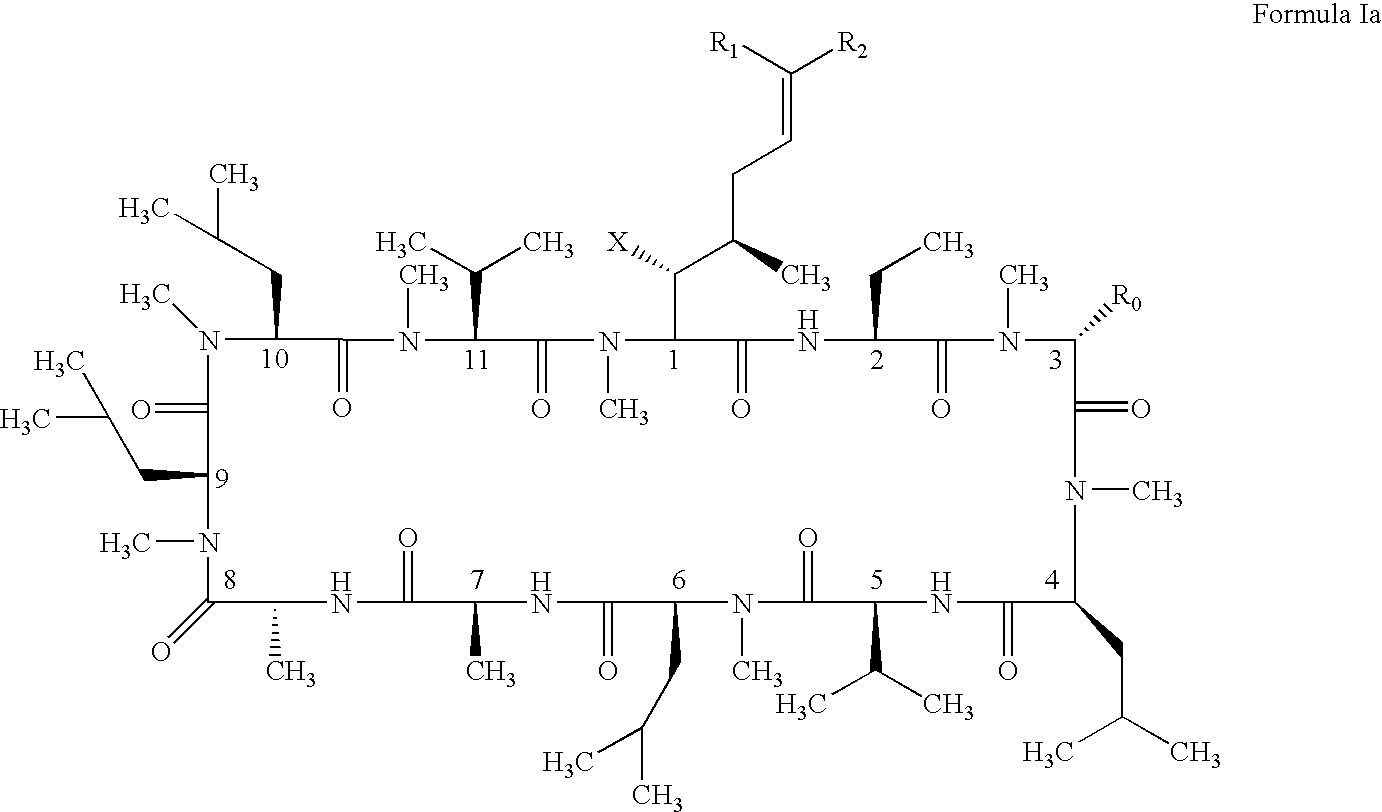
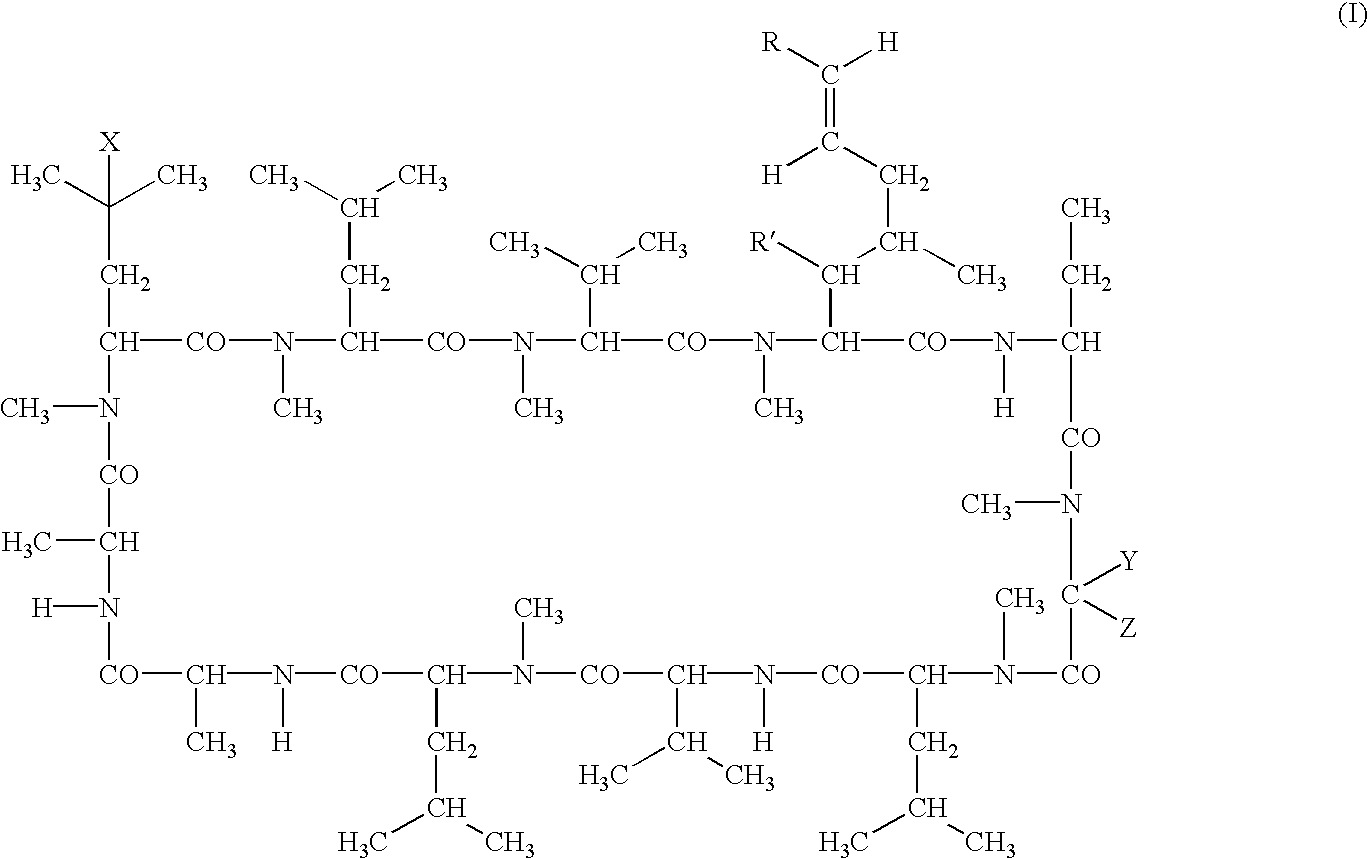
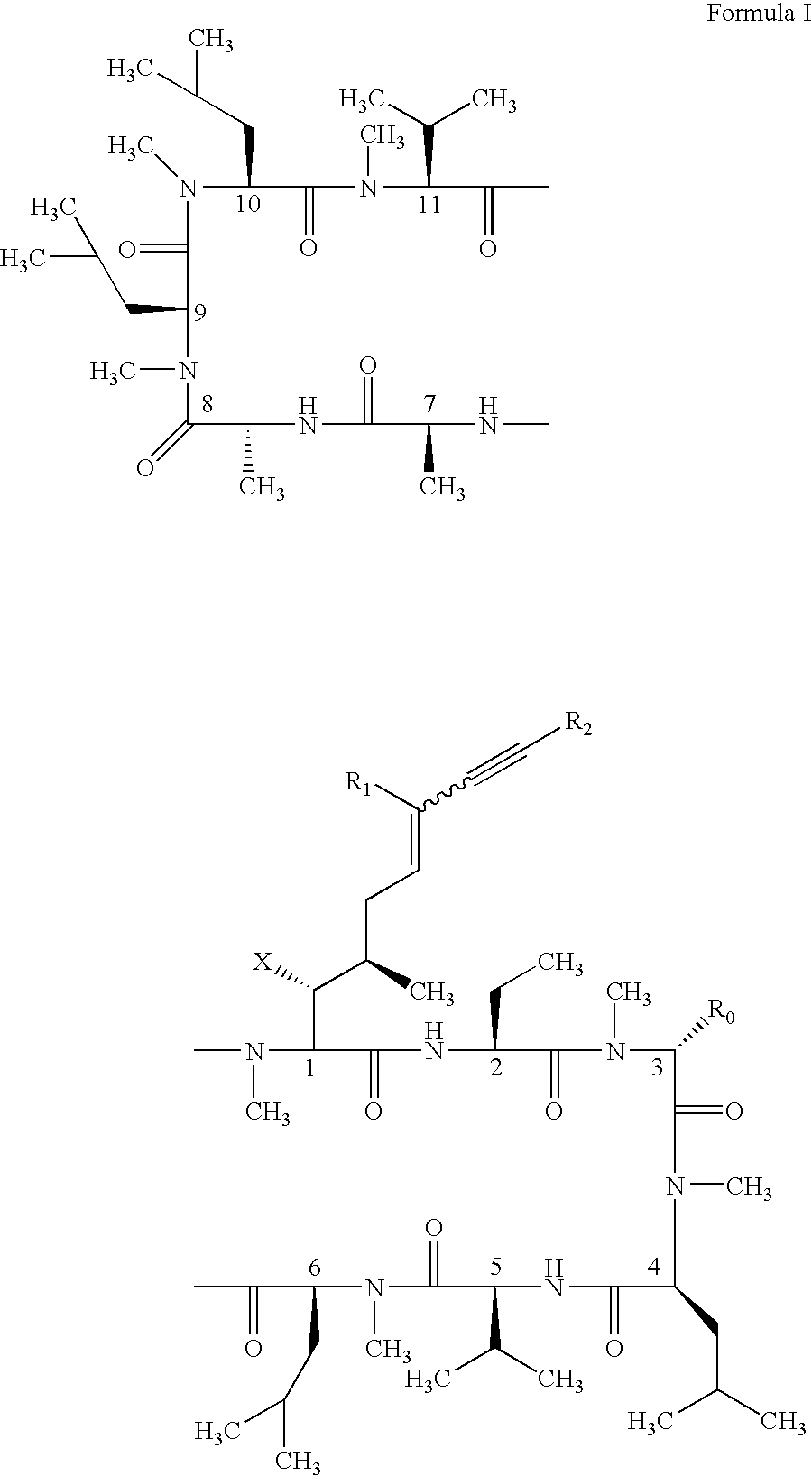
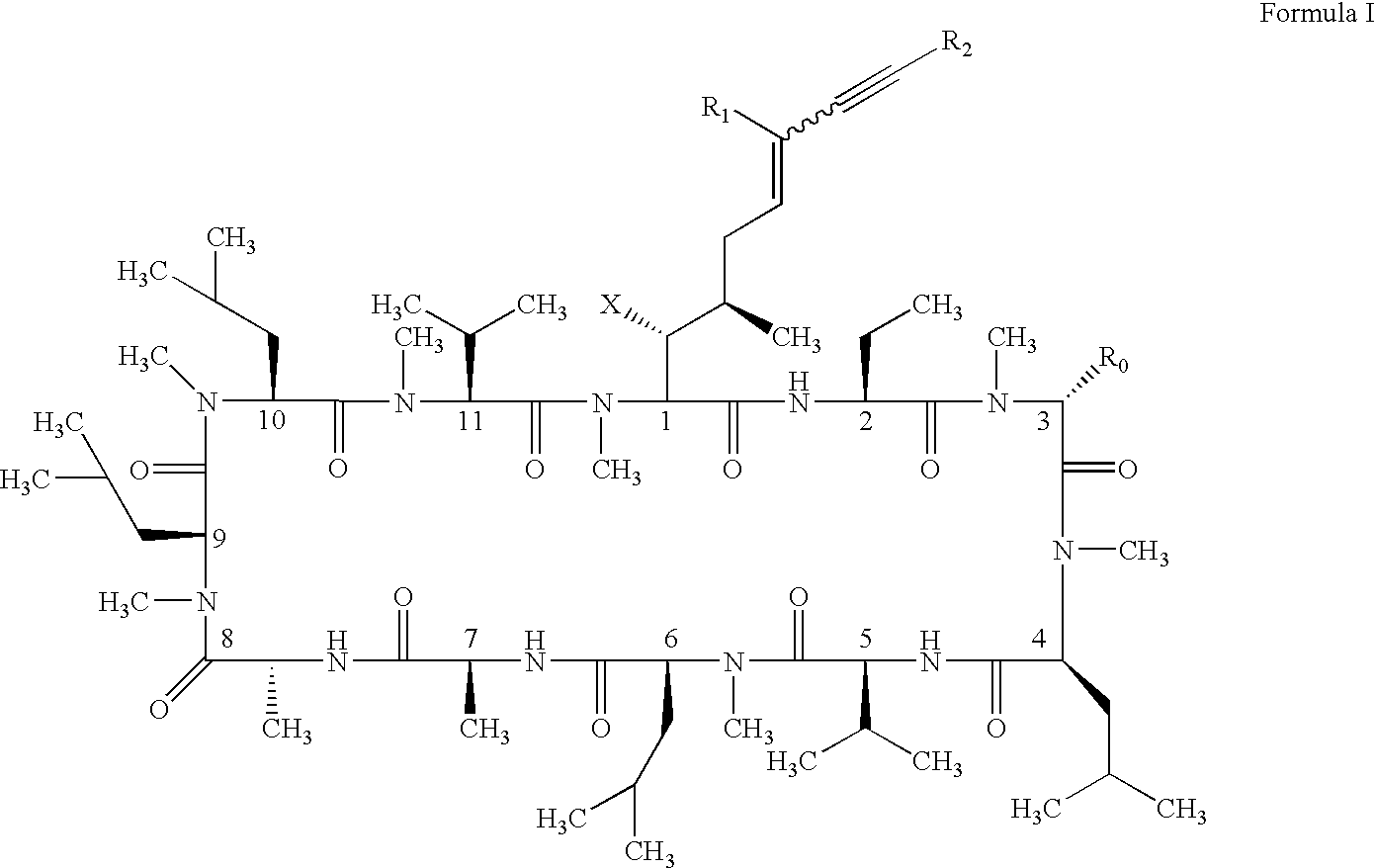
Cyclosporins for the treatment of immune disorders
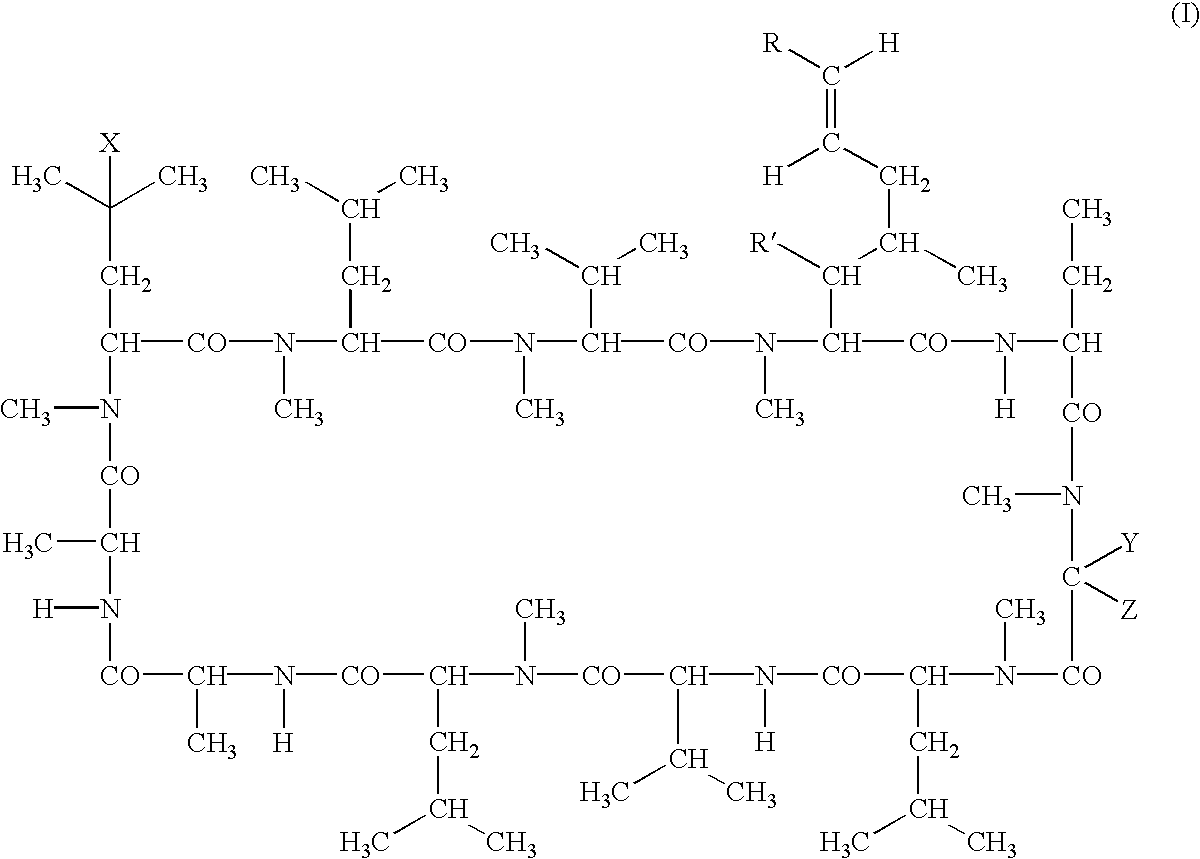
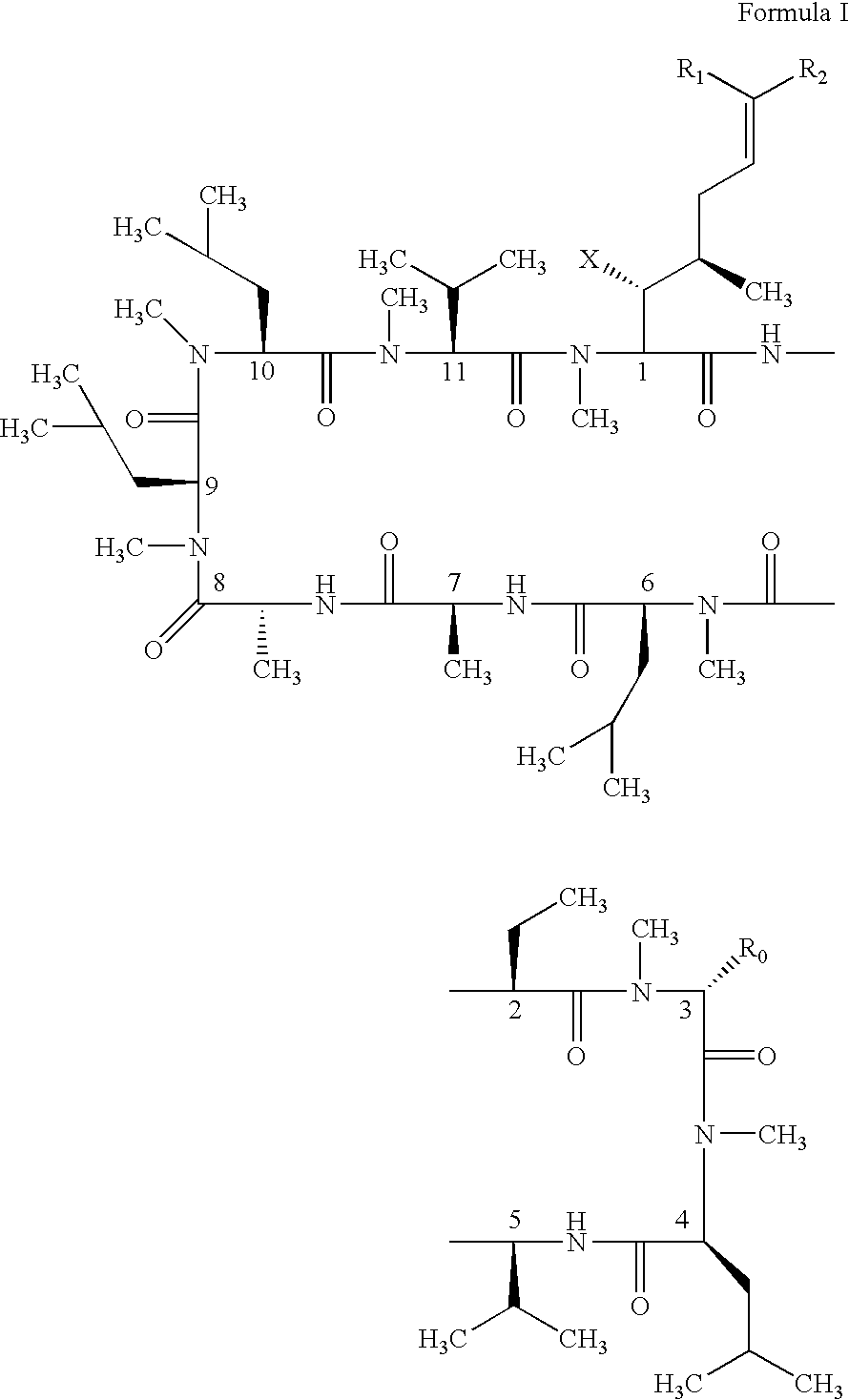
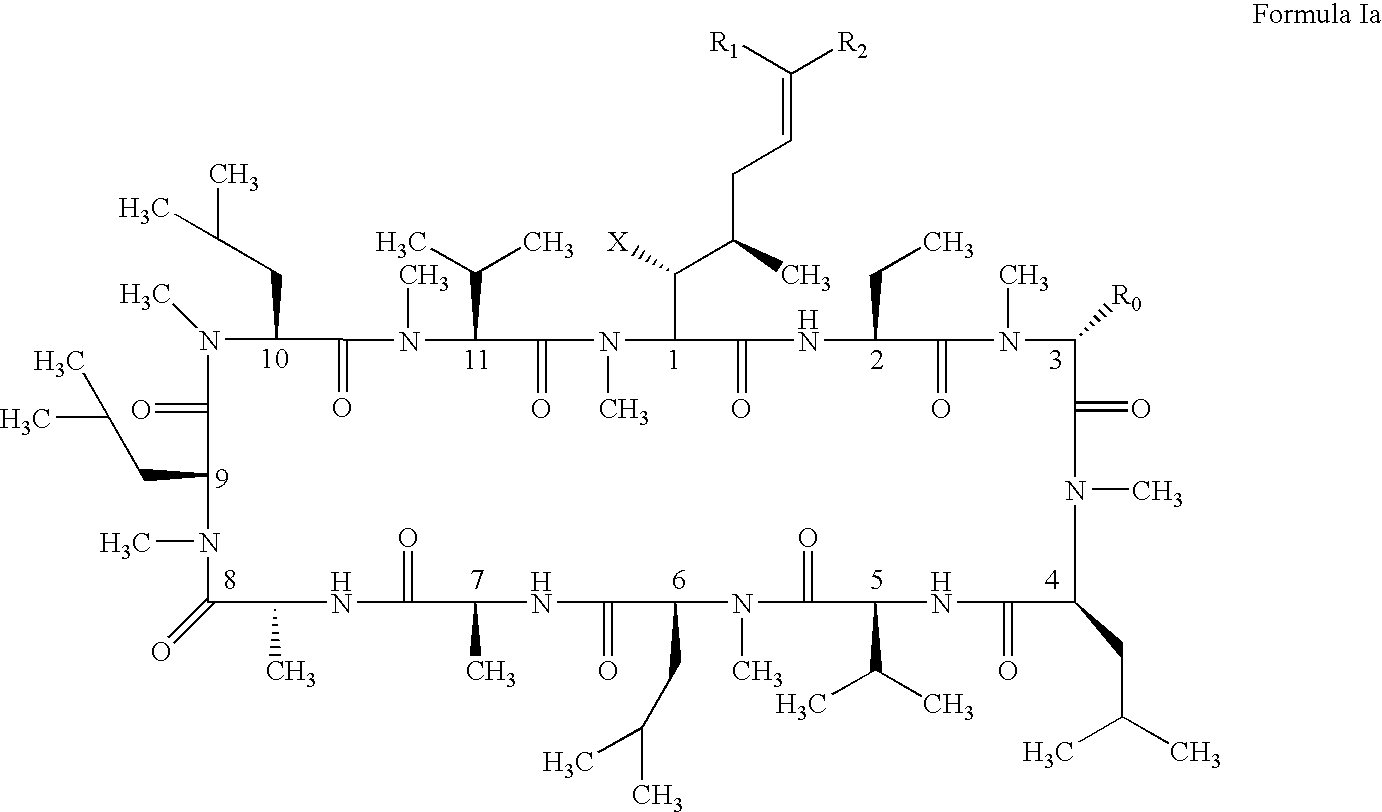
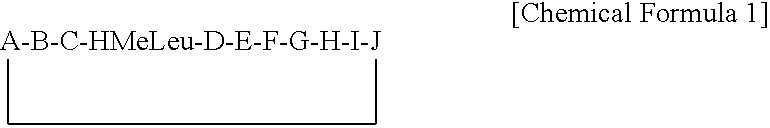
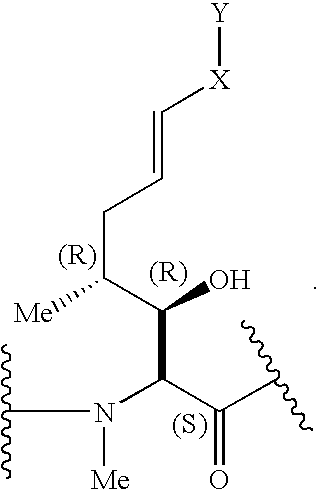
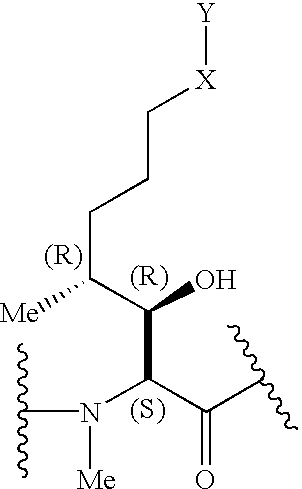
The present invention relates to methods of treating or preventing an inflammatory or immune disorder in a subject while eliminating or reducing the toxicity associated with the administration of cyclosporin A, comprising systemically administering to said subject a pharmaceutical composition comprising a therapeutically effective amount of at least one compound of Formula (I) or a pharmaceutically acceptable salt, ester or prodrug thereof, in combination with a pharmaceutically acceptable carrier or excipient: in Formula (I), the formula for residue A is:
Owner:ENANTA PHARM INC
Cyclosporins for the treatment of immune disorders
The present invention relates to a cyclosporin analog of the following formula (I) or its pro-drug or pharmaceutically acceptable salt: In formula I, the formula for residue A is: where X and Y are defined according to the claimed invention the present invention also relates to pharmaceutical compositions comprising pro-drugs or pharmaceutically acceptable salts of the compounds of the present invention and the use thereof for treating an inflammatory or immune disorder in a subject need of such treatment.
Owner:ENANTA PHARM INC
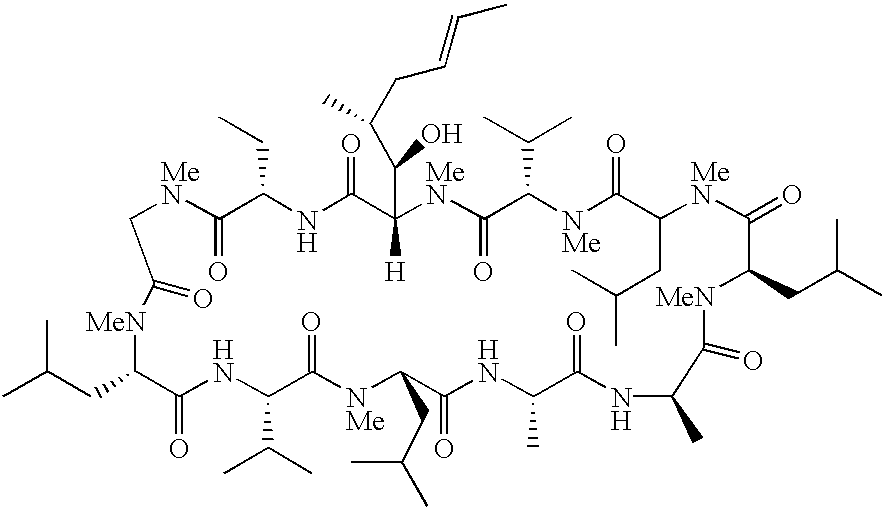
Cyclosporin alkyne analogues and their pharmaceutical uses
InactiveUS20060069016A1High activityPossess utility in the treatmentSenses disorderNervous disorderChemical structureCyclosporins
The compounds of the present invention are represented by the chemical structure found in Formula I: or a pharmaceutically acceptable salt thereof, with X, R0, R1, and R2 defined herein.
Owner:ALBANY MOLECULAR RESEARCH INC
Pharmaceutical cyclosporin compositions
ActiveUS20100203120A1Maximize absorptionMaximize activityAntibacterial agentsOrganic active ingredientsDiseaseCyclosporins
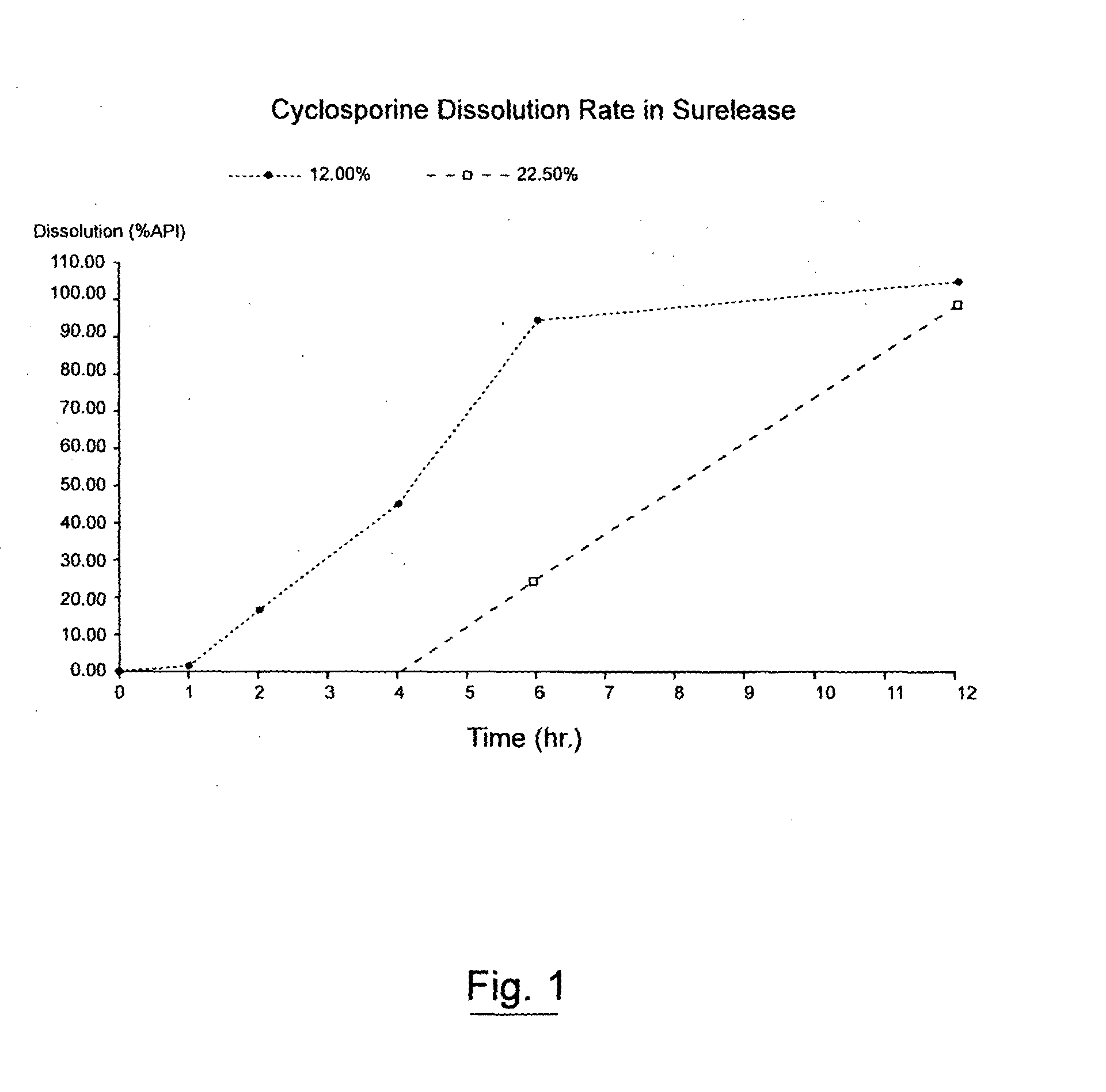
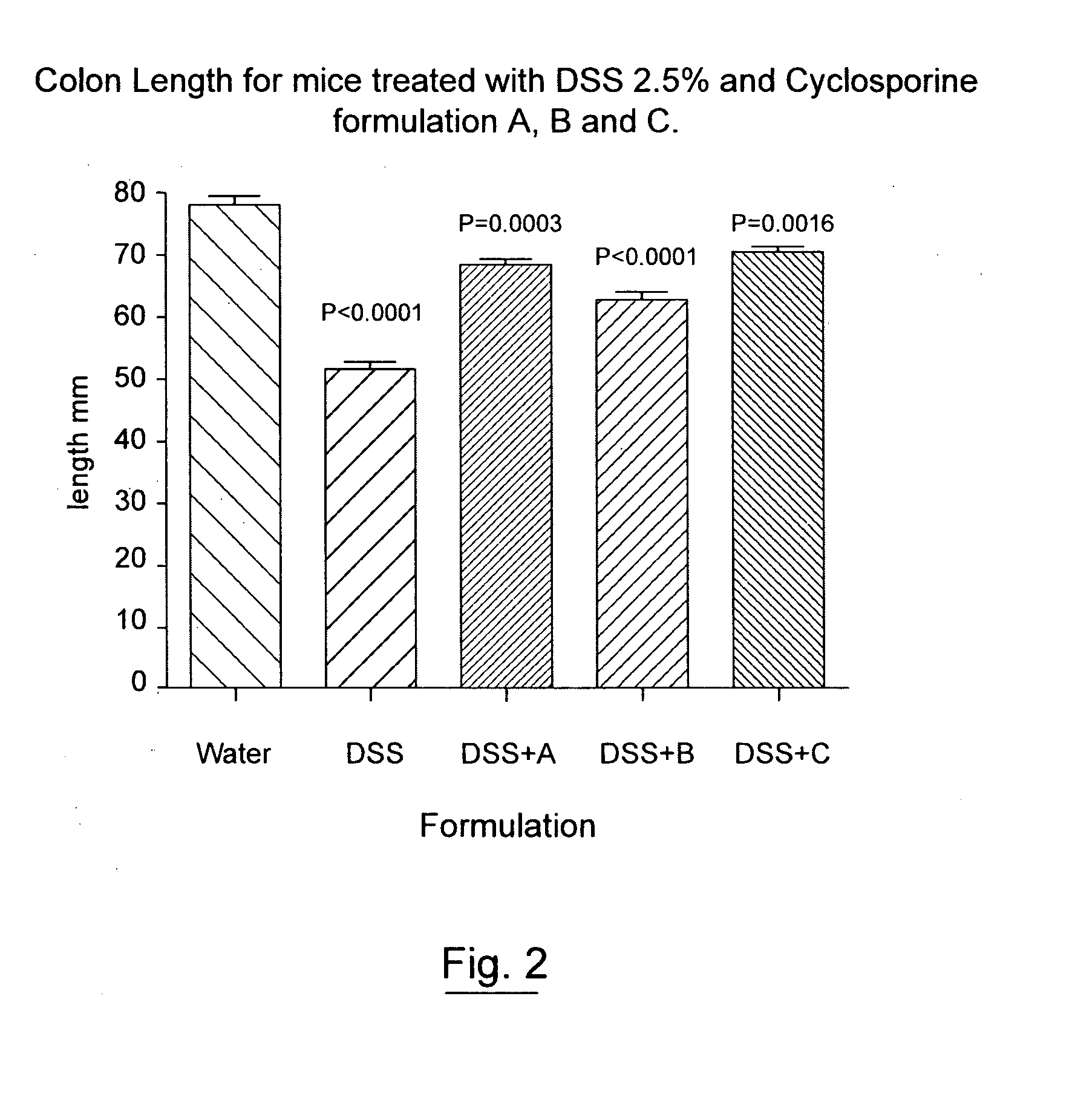
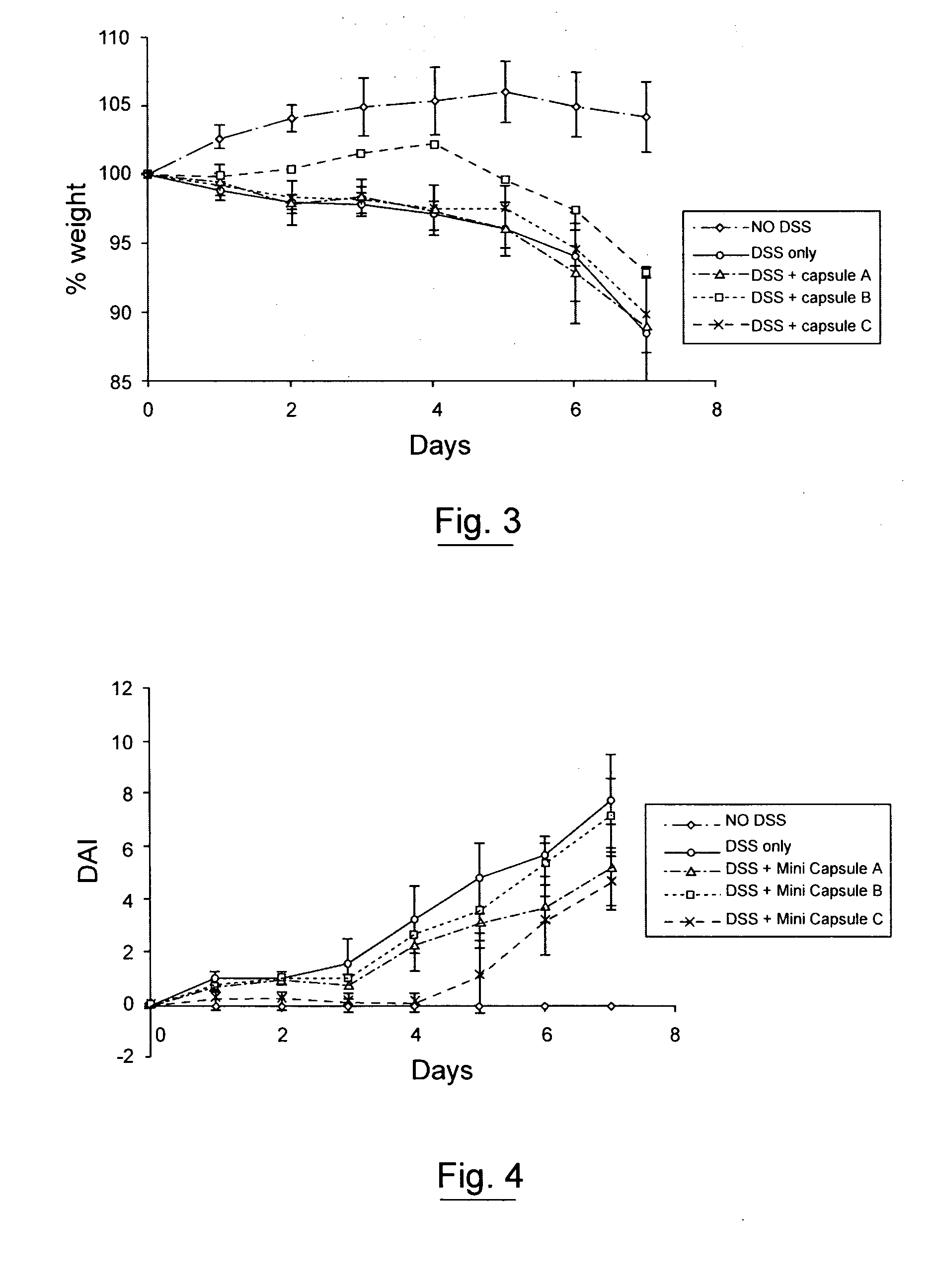
An oral cyclosporin composition comprises minicapsules having a core containing a cyclosporin, especially cyclosporin A in a solubilised liquid form. The minicapsules have a release profile to release the pre-solubilised cyclosporin, at least in the colon. The composition may be used for treating a range of intestinal diseases [FIG. 10].
Owner:SUBLIMITY THERAPEUTICS LTD
Novel cyclosporine analog formulations
The present invention relates to formulations containing cyclosporin analogs that are structurally similar to cyclosporin A, in particular isomeric mixtures of cyclosporin analogs that are structurally similar to cyclosporin A. The formulations form stable microemulsion preconcentrates and may provide superior drug bioavailability and / or may reduce one or more adverse effects associated with the administration of cyclosporin. Also disclosed are methods for using and preparing the formulations.
Owner:AURINIA PHARMA
Pharmaceutical compositions comprising a cyclosporin, a hydrophilic surfactant and a lipophilic surfactant
InactiveUS20050129718A1Cyclic peptide ingredientsPharmaceutical non-active ingredientsHigh absorptionCyclosporins
Pharmaceutical compositions, which enable high absorption when administered orally, and which comprise a cyclosporin or cyclosporin derivative dissolved in a solvent-surfactant system further comprising a hydrophilic surfactant and a lipophilic surfactant, with minimal quantities of solvents.
Owner:SHERMAN
Cyclosporin compositions
A composition comprising a therapeutically effective amount of cyclosporin A, a blend of oils having a specific gravity of from 0.90 to 1.07, and a surfactant is disclosed herein.
Owner:SAINT REGIS MOHAWK TRIBE
Cyclosporin a conjugates and uses therefor
InactiveUS6316405B1Highly effective to treat or prevent neurological disordersAct synergisticallyNervous disorderMetabolism disorderAmyotrophic lateral sclerosisAmyloid
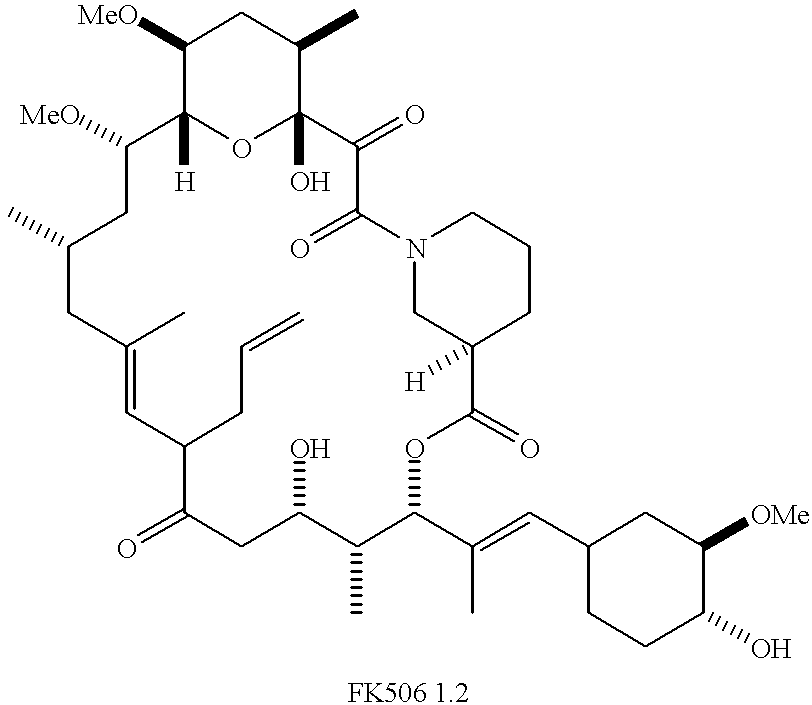
Disclosed are conjugates of Abeta-binding peptides and CsA analogs and conjugates of Abeta-binding peptides and FK506 Binding Peptide inhibitors. These conjugates chemically induce dimerization of either cyclophilin or FK506 Binding Peptide with Abeta peptide, a major component of amyloid plaques found in neurological disorders such as Alzheimer's disease, multiple sclerosis, and amyotrophic lateral sclerosis. The conjugates are useful in the treatment of neurological diseases involving the formation of amyloid plaques because they inhibit and / or prevent the aggregation and deposition of Abeta peptide into plaques.
Owner:WISCONSIN ALUMNI RES FOUND
Dispersible concentrate for the delivery of cyclosprin
InactiveUS7026290B1Suitable for useImprove bioavailabilityPowder deliveryAntipyreticCyclosporinsAdditive ingredient
A formulation for the administration of a cyclosporin. This formulation features a hydrophilic solvent which is characterized by being a lower alkyl ester of hydroxyalkanoic acid; and a surfactant, preferably a combination of a surfactant with a high HLB (hydrophilic / lipophilic balance) of at least about 8 and a surfactant with a low HLB of less than about 5. Other ingredients are optional, such as a fatty acid ester such as tricaprin, a phospholipid, and an ethoxylated fat such as Cremophor or another similar substance. Optionally, the ethoxylated fat is substituted for the surfactant. The preferred particle size of the resultant formulation is less than about 100 nm, more preferably less than about 60 nm, and most preferably from about 5 nm to about 50 nm. The formulation of the present invention is characterized by having high bioavailability.
Owner:DEXCEL
Cyclosporin compositions
A composition is disclosed herein comprising from about 0.001% to about 0.4% cyclosporin A, castor oil, and a surfactant selected from the group consisting of alcohol ethoxylates, alcohols, alkyl glycosides, alkyl polyglycosides, alkylphenol ethoxylates, amine oxides, block polymers, carboxylated alcohol or alkylphenol ethoxylates, carboxylic adds / fatty acids, cellulose derivatives, ethoxylated alcohols, ethoxylated alkylphenols, ethoxylated aryl phenols, ethoxylated fatty acids, ethoxylated fatty acids, ethoxylated fatty esters and oils, fatty alcohols, fatty esters, glycol esters, lanolin-based derivatives, lecithin and lecithin derivatives, lignin and lignin derivatives, methyl esters, monoglycerides and derivatives, phosphalipids, polyacrylic acids, polyethylene glycols, polyethylene oxide-polypropylene oxide copolymers, polyethylene oxides, polymeric surfactants, polypropylene oxides, propoxylated alcohols, propoxylated alkyl phenols, propoxylated fatty acids, protein-based surfactants, sarcosine derivatives, silicone-based surfactants, sorbitan derivatives, stearates, sucrose and glucose esters and derivatives, and combinations thereof.
Owner:SAINT REGIS MOHAWK TRIBE
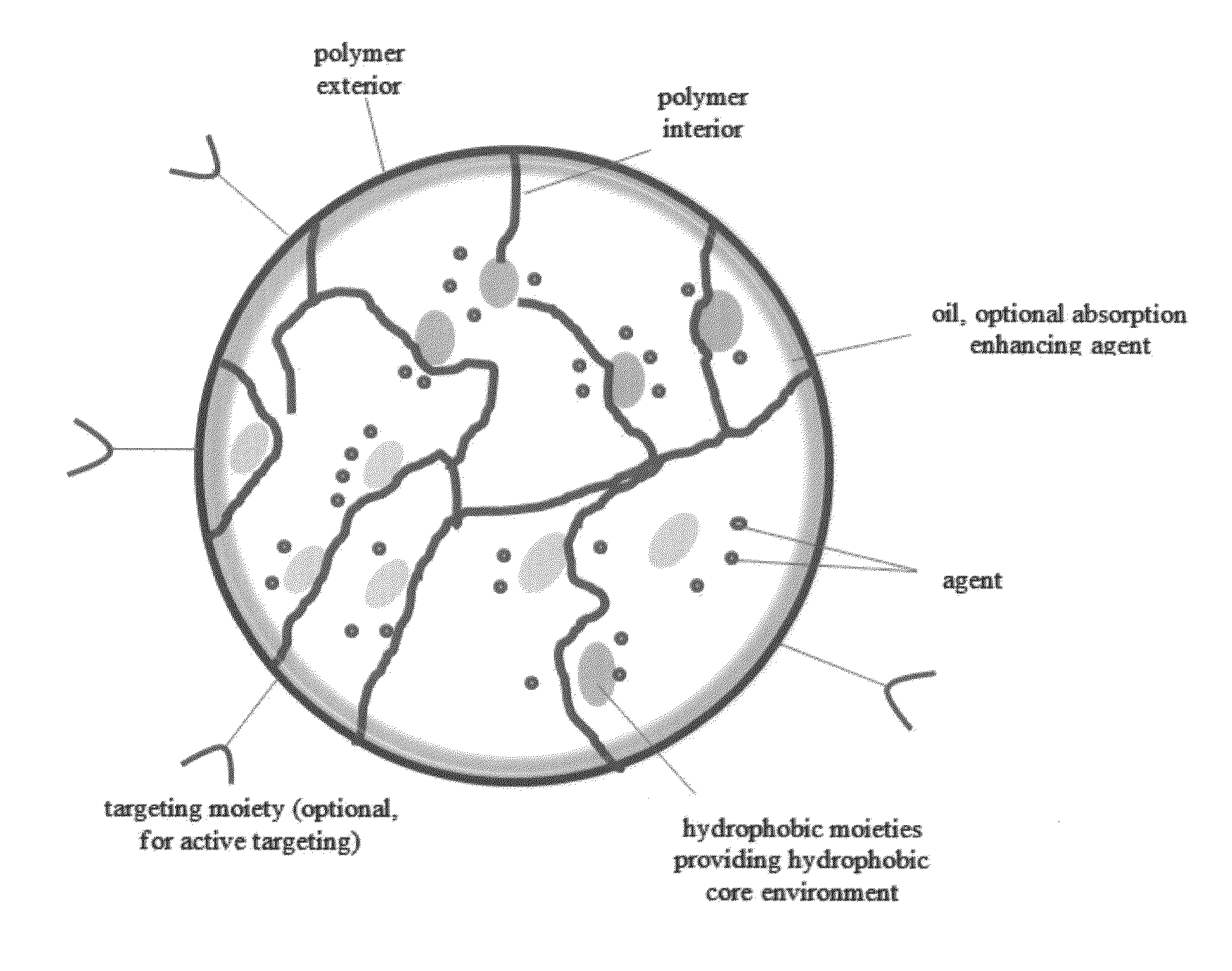
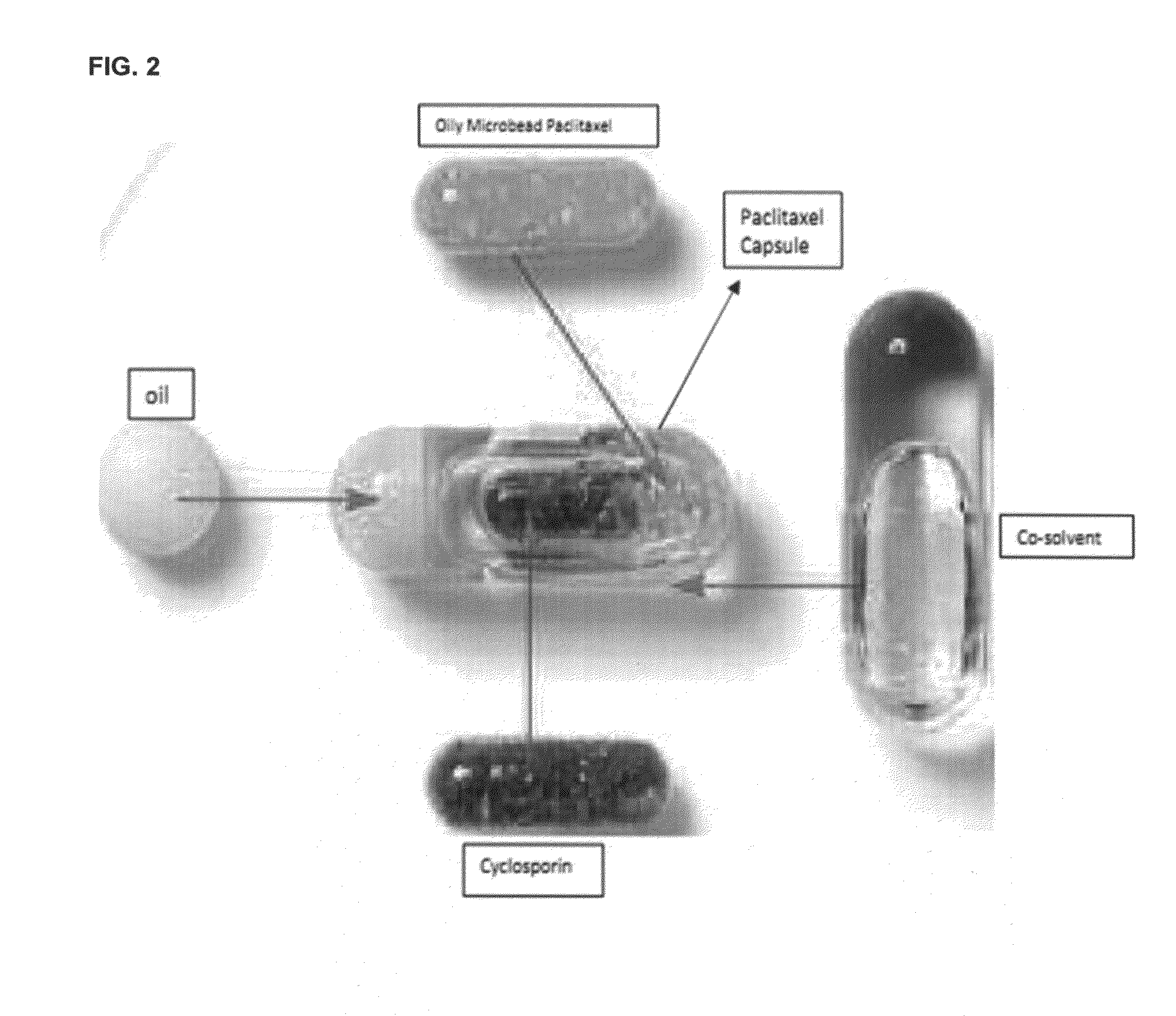
Oral formulations of chemotherapeutic agents
InactiveUS20150231069A1Increase probabilityEffectively infiltrate across the inflamed leakyPowder deliveryOrganic active ingredientsCyclosporinsEnhanced absorption
A composition and method of using the composition for treating a patient in need thereof, the composition comprising an oral formulation for enhanced bioavailability of therapeutic agents such as the taxane chemotherapeutic agents. The composition comprises the therapeutic agent and an absorption enhancing agent either co-administered with the agent or administered separately, the therapeutic agent in a polymer matrix resulting in a microbead and including an edible oil resulting in an emulsion. The absorption enhancing agent is a cyclosporin in one embodiment. The absorption enhancing agent is a P glycoprotein inhibitor in another embodiment.
Owner:MODI PANKAJ
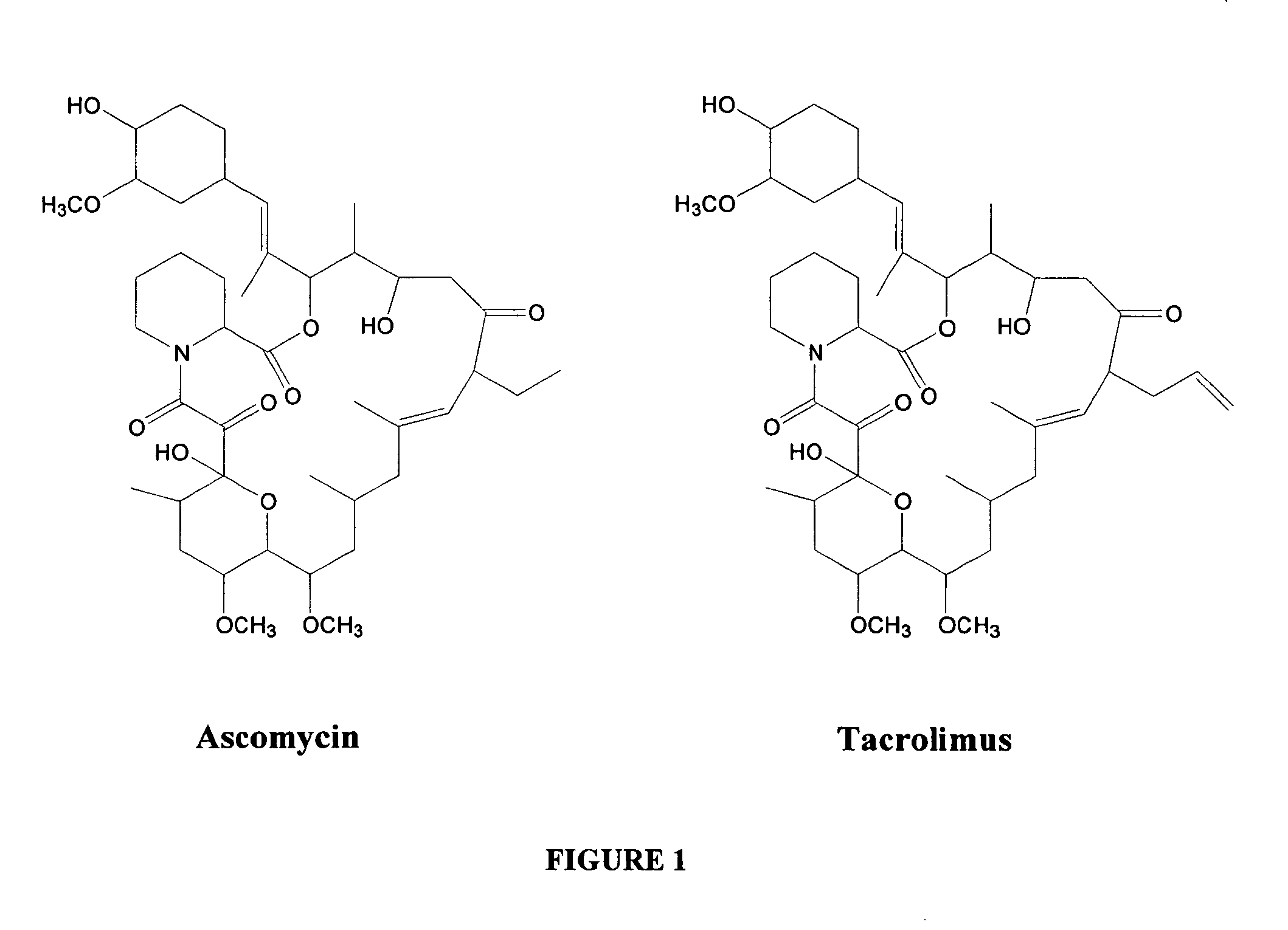
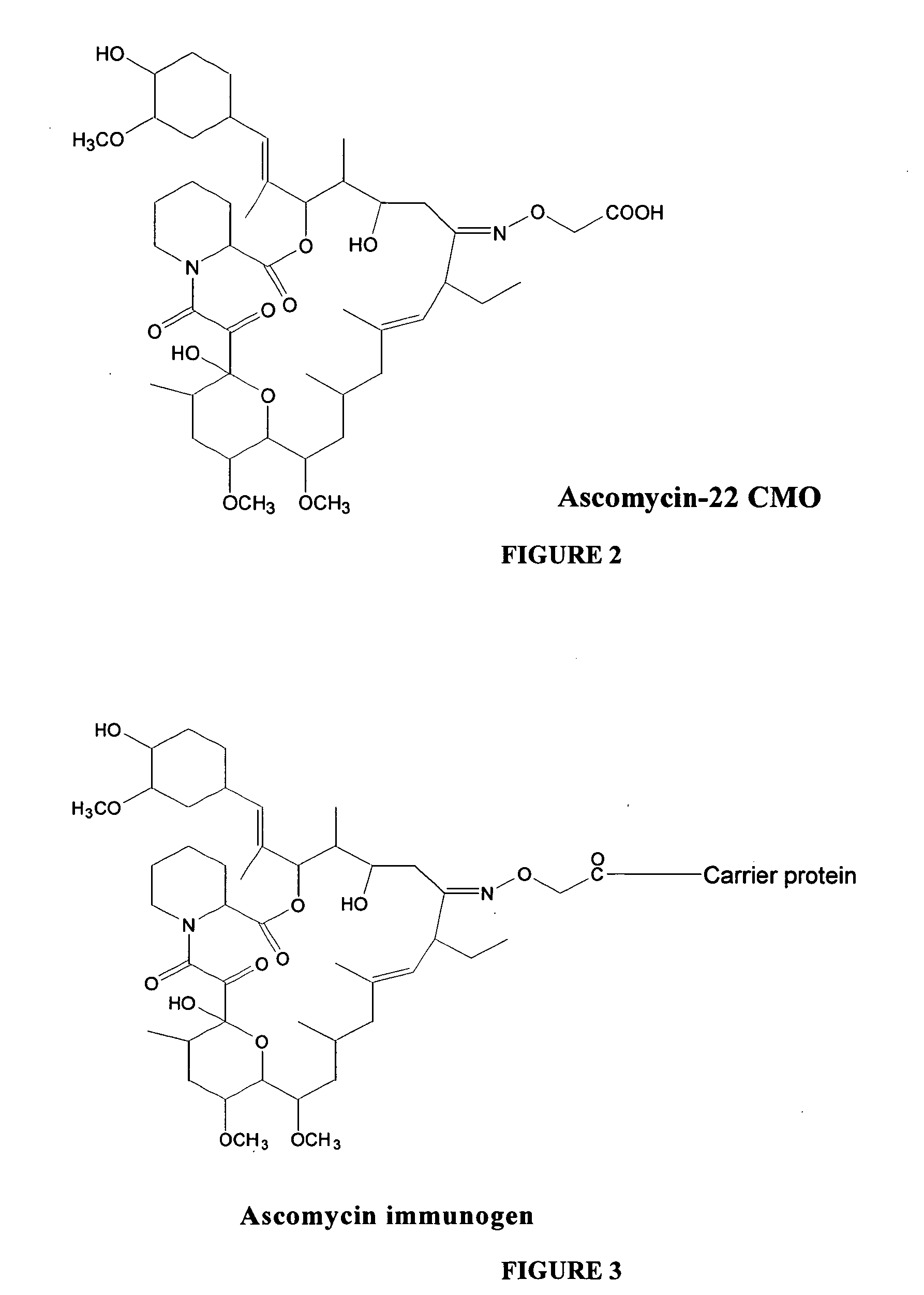
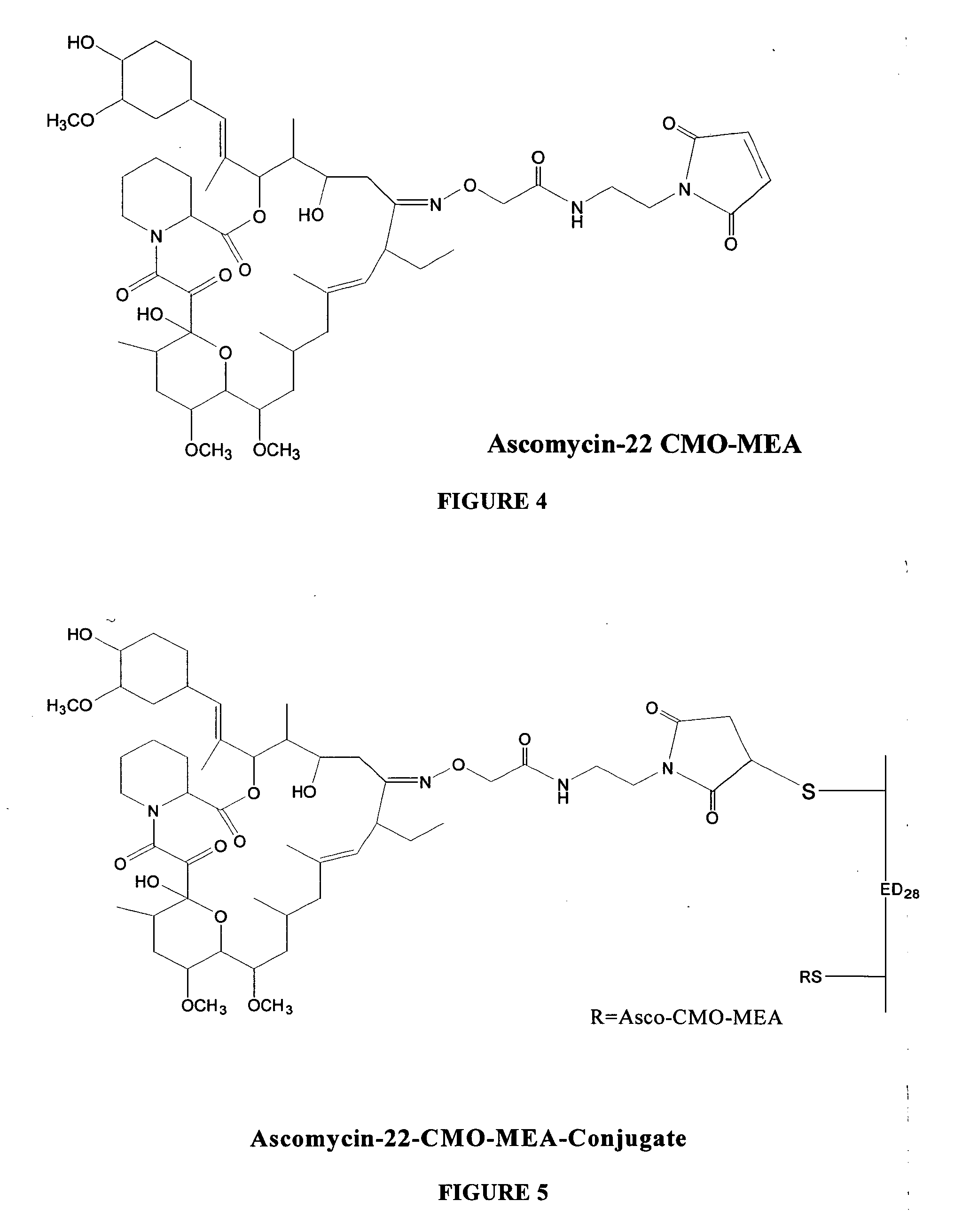
Hapten, immunogens and derivatives of ascomycin useful for preparation of antibodies and immunoassays
The invention teaches derivatives of ascomycin and methods of preparing immunogens and other conjugates useful in immunoassays for quantitatively measuring concentrations of tacrolimus in patient specimens. Antibodies produced from the disclosed immunogens capable of binding to tacrolimus with cross-reactivity of no more than 5% with each of 15-O-demethyl tacrolimus, 31-O-demethyl tacrolimus, and 13,31-O-didemethyl tacrolimus, less than 40% with 13-O-demethyl tacrolimus, and less than 1% with cyclosporin, rapamycin, mycophenolic acid, prednisone, hydrocortisol, and prednisolone are described. Further, immunoassays for measuring the concentration of tacrolimus using such antibodies are taught.
Owner:MICROGENICS CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com