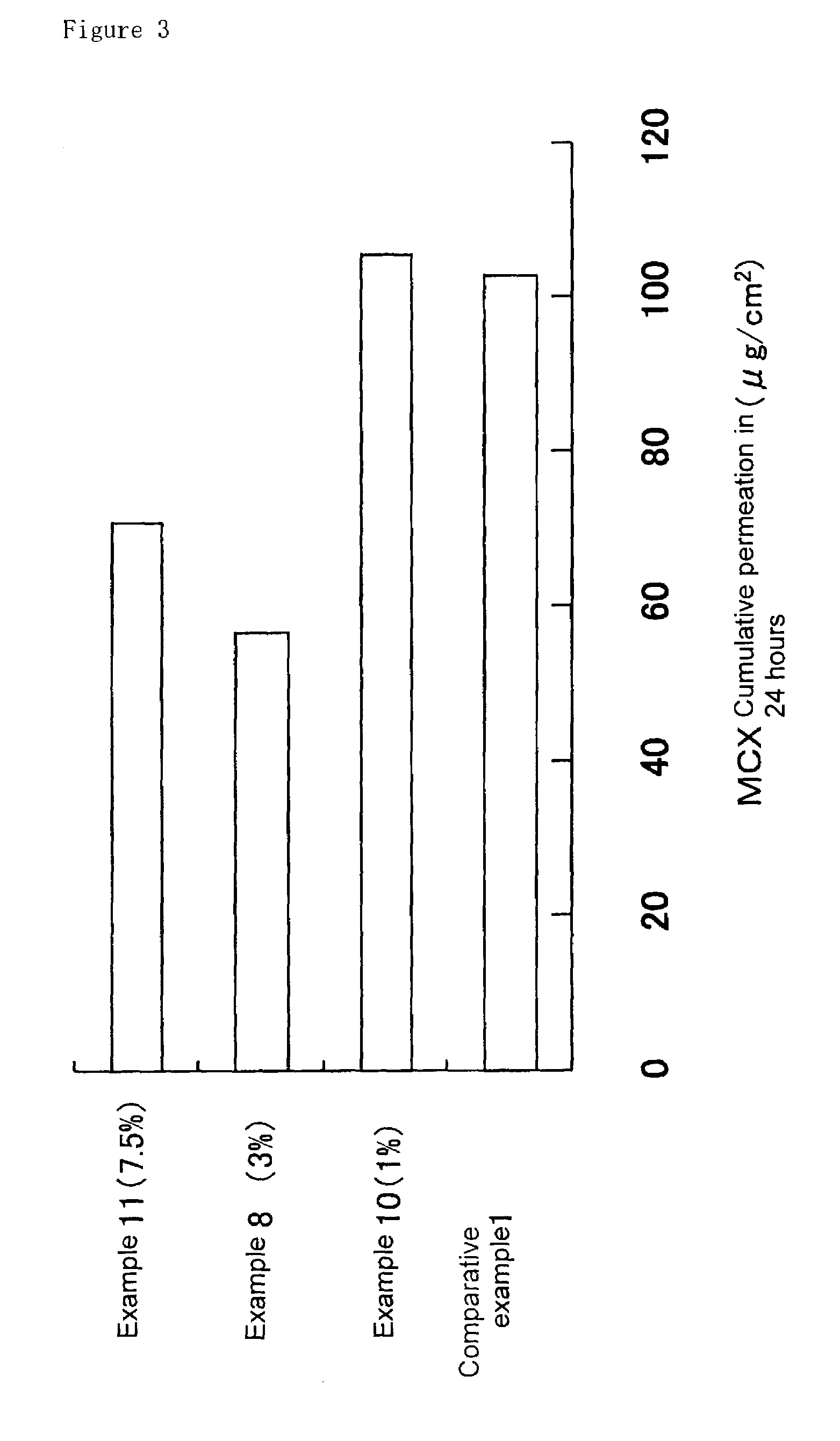
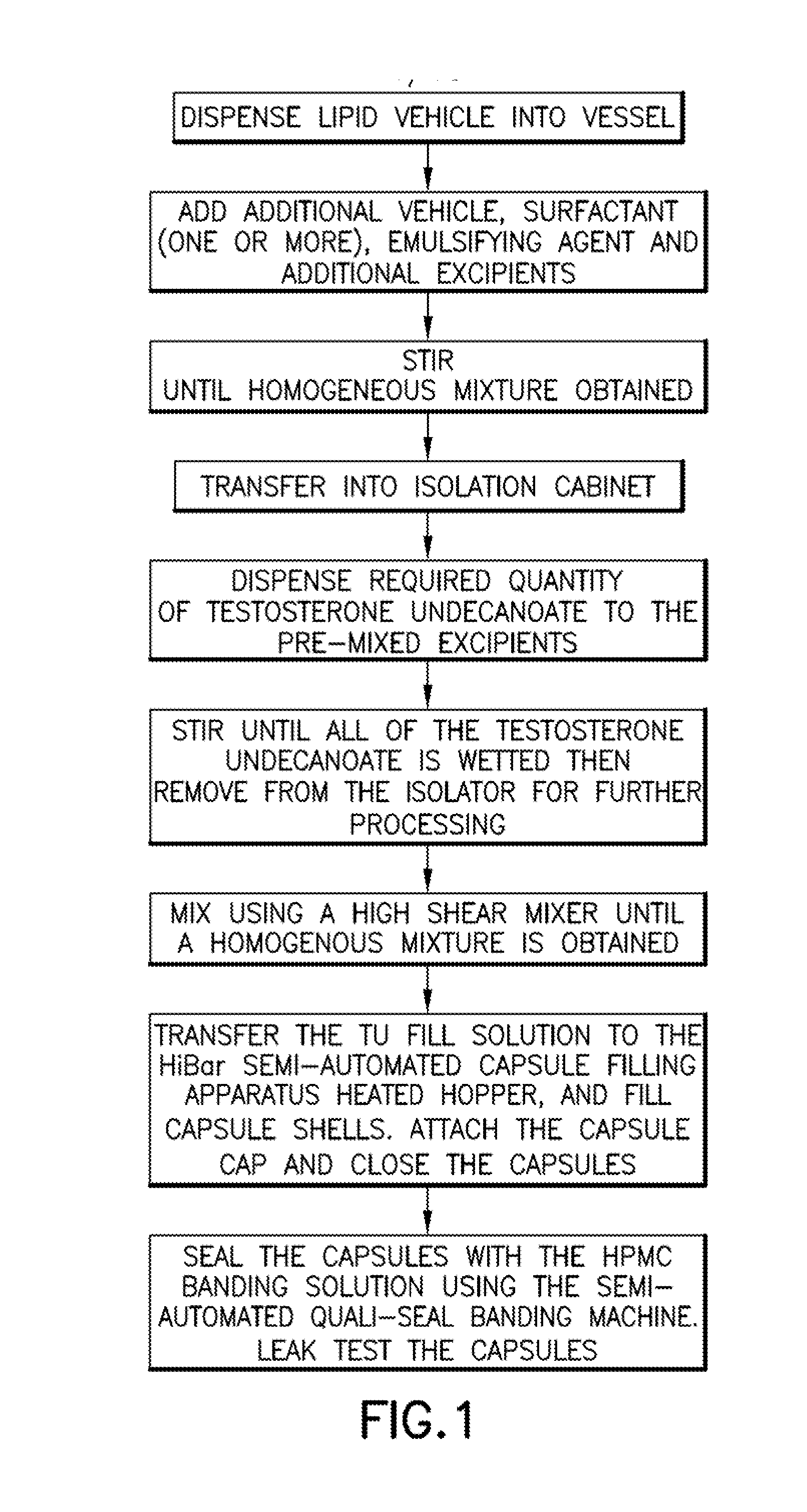
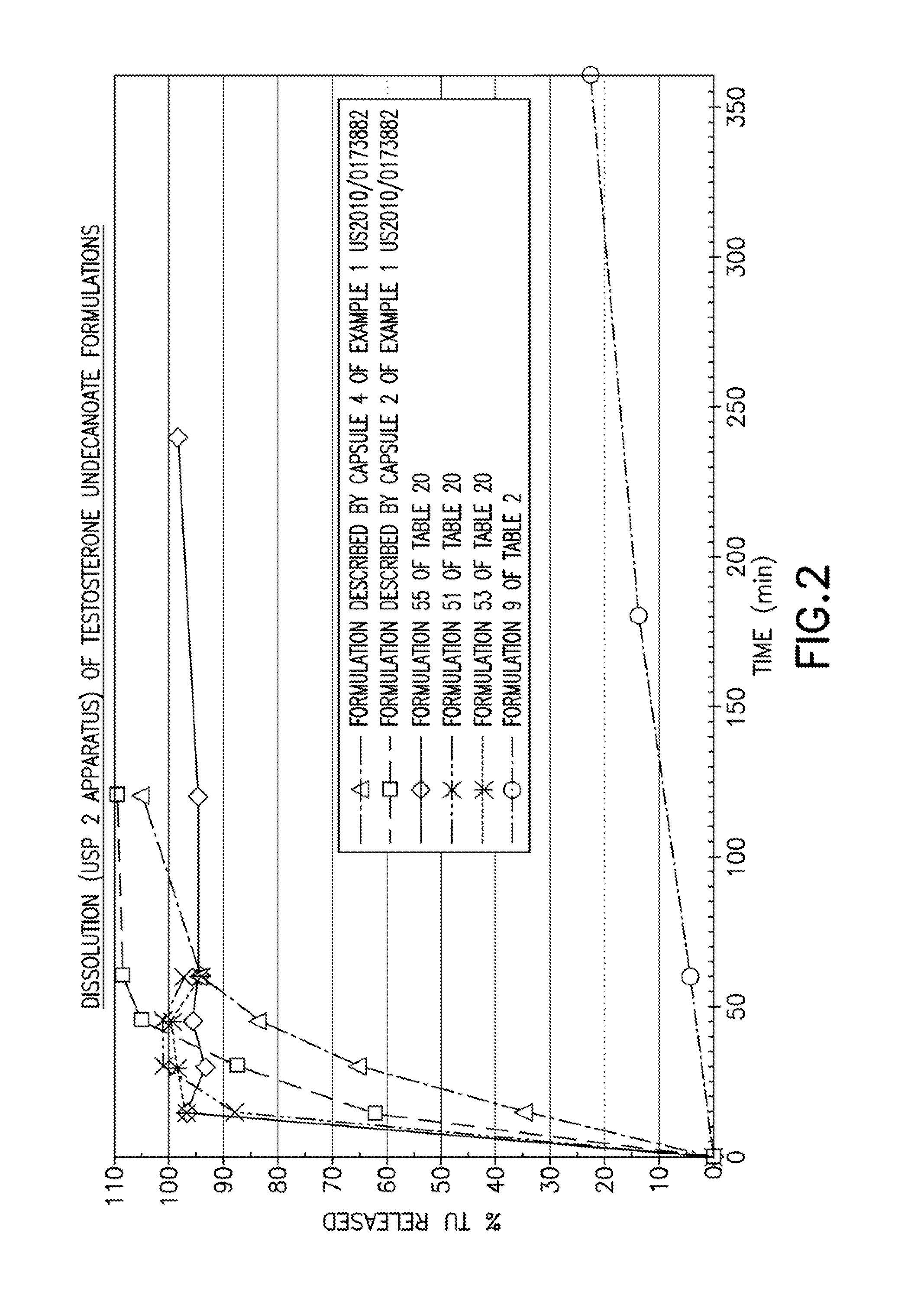
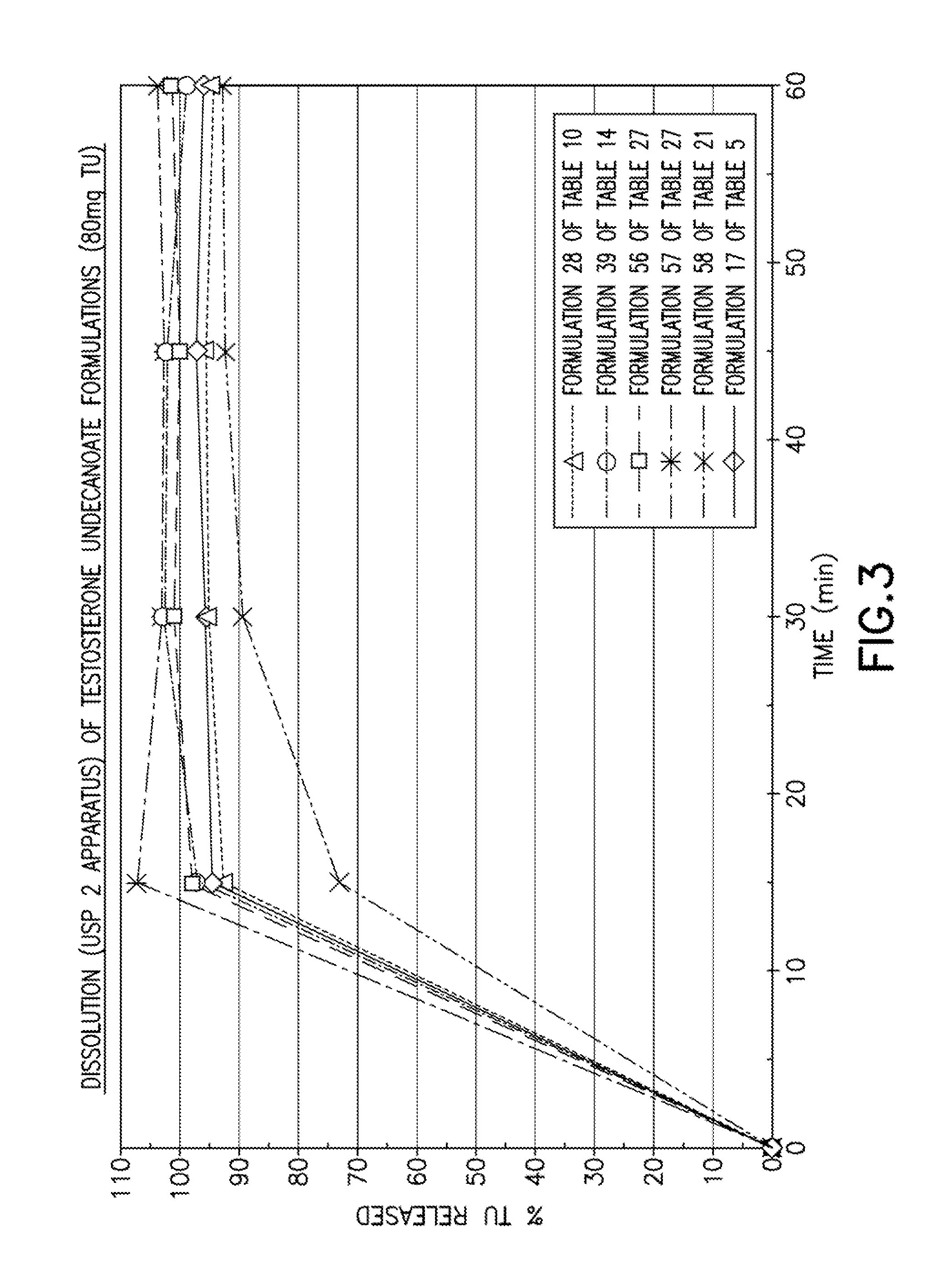
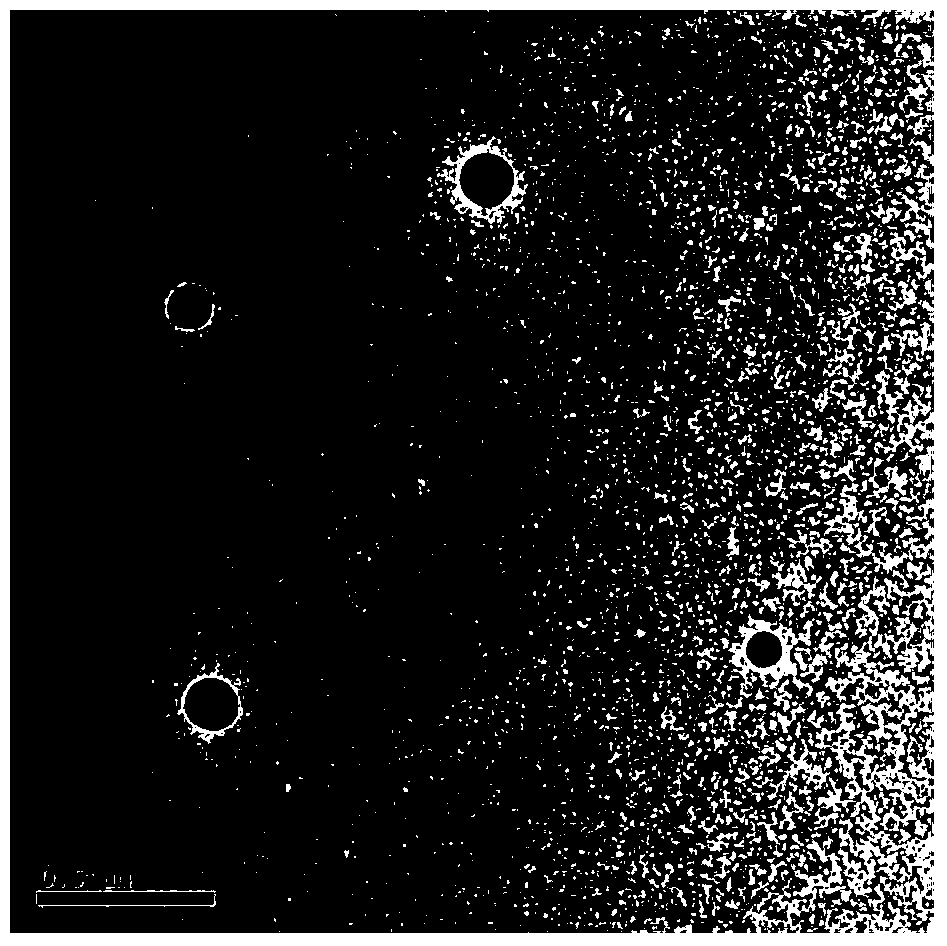
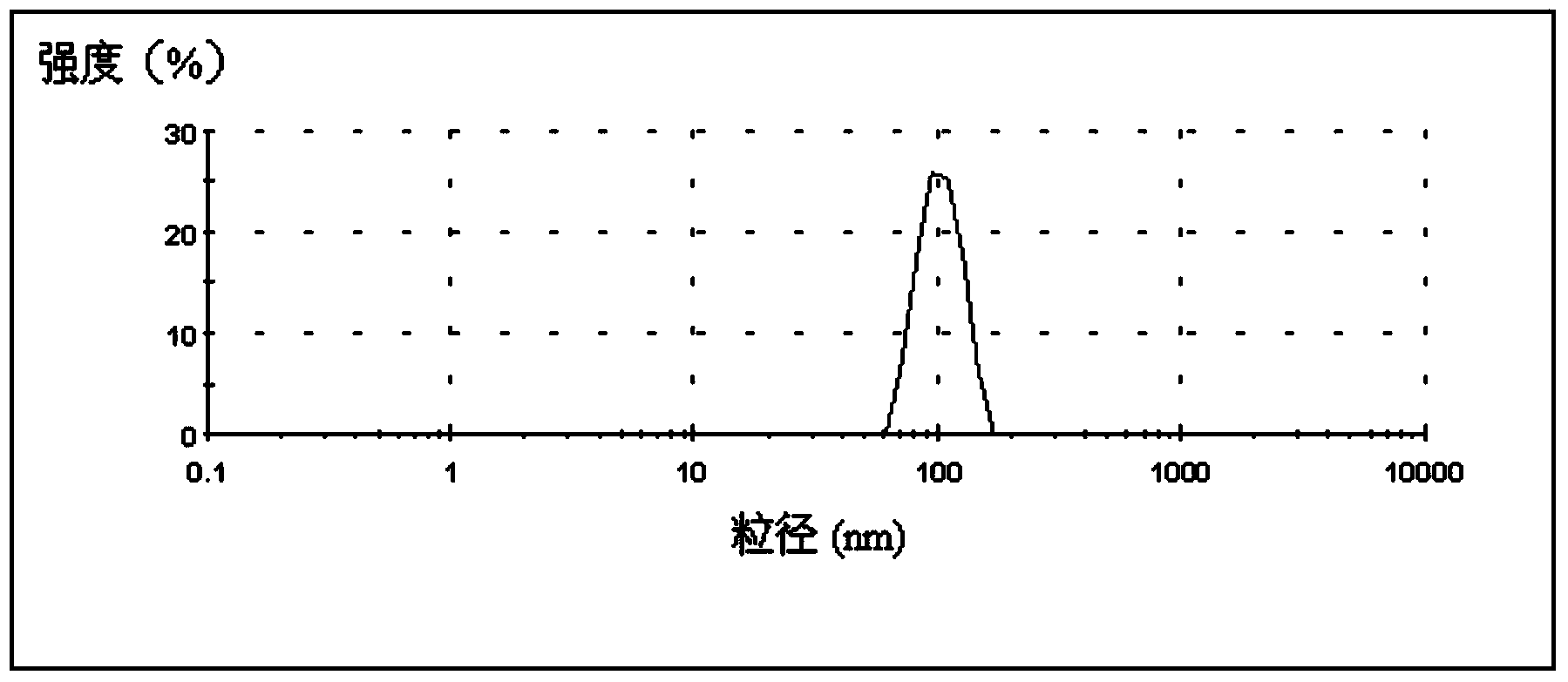
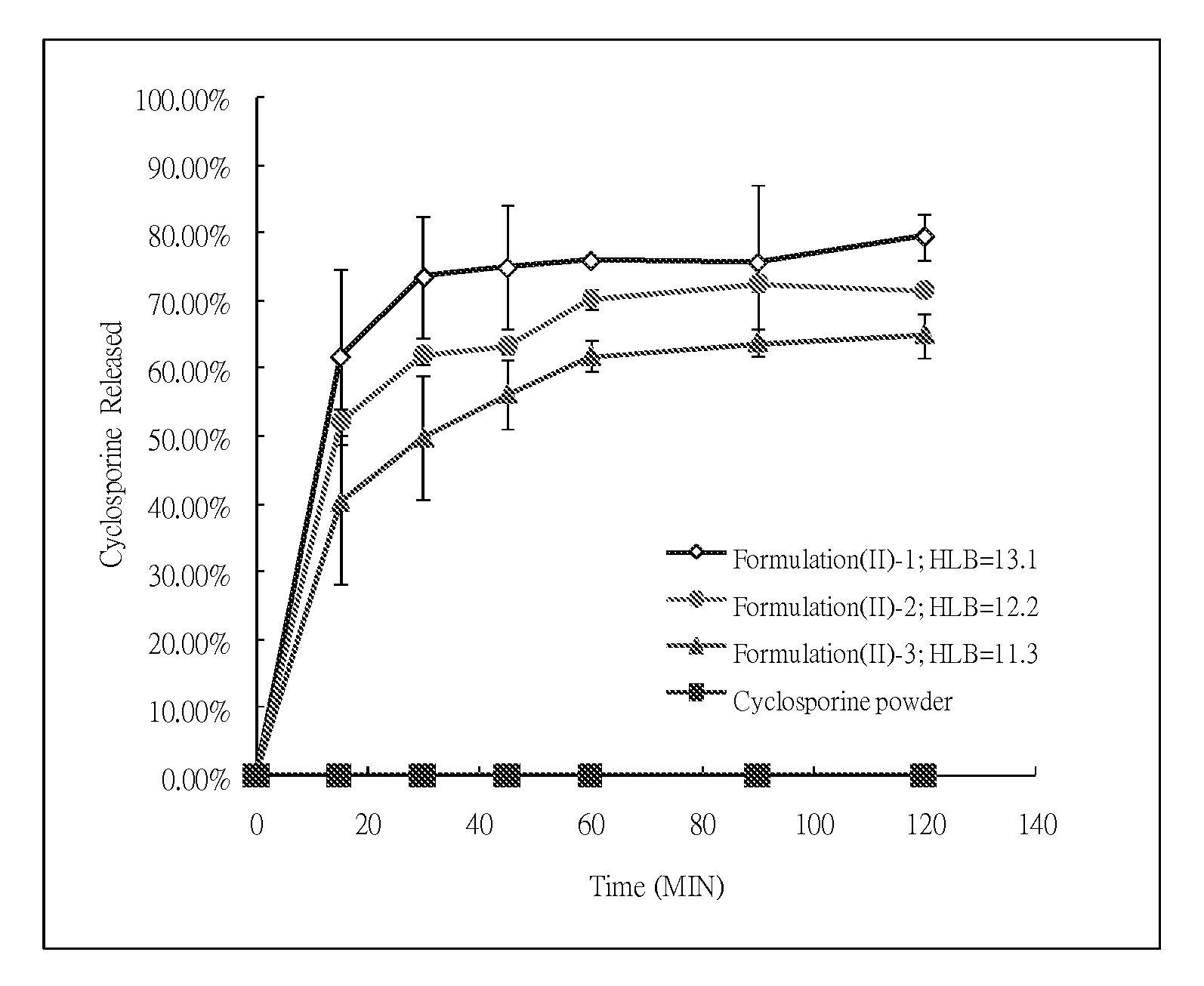
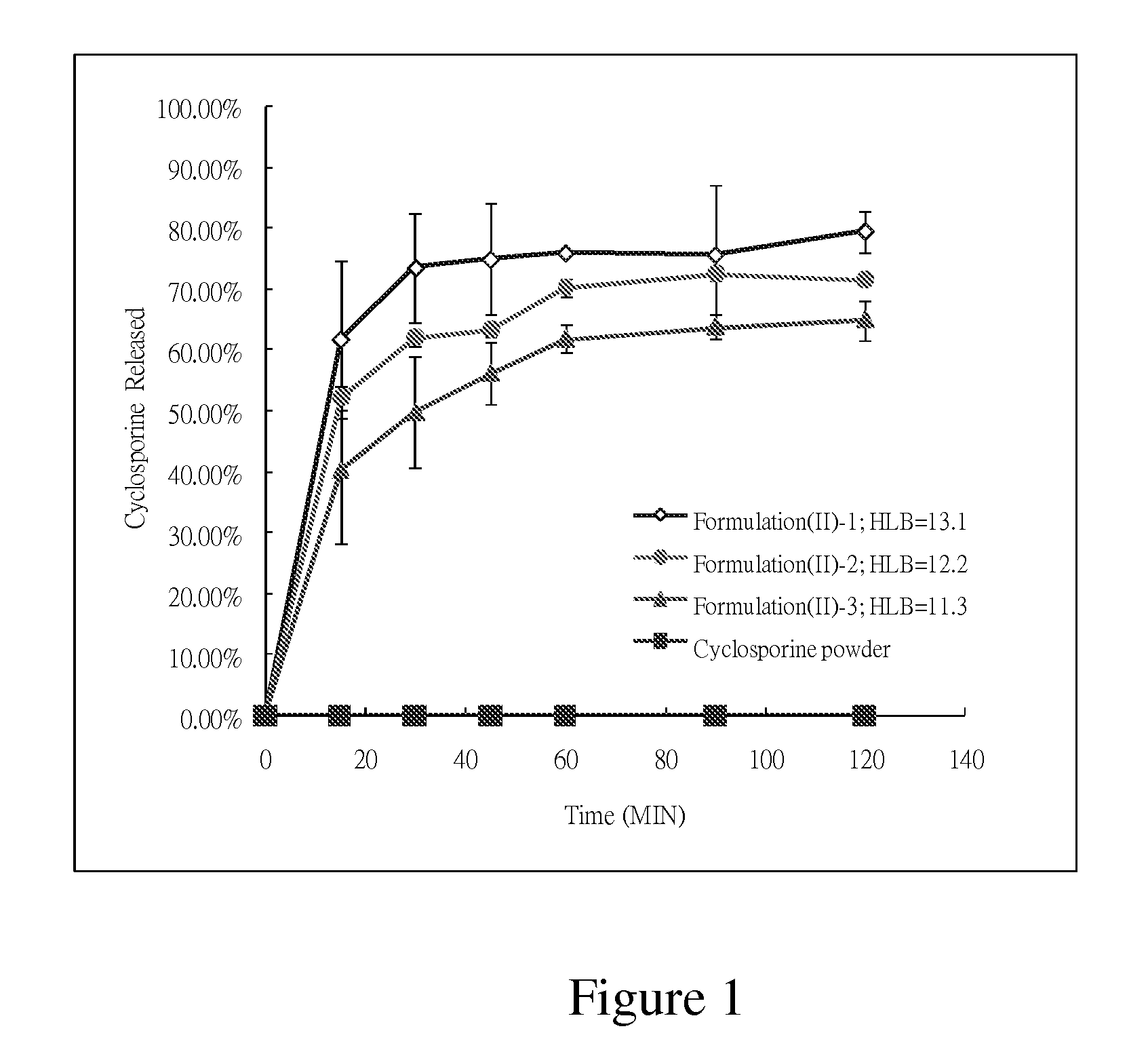
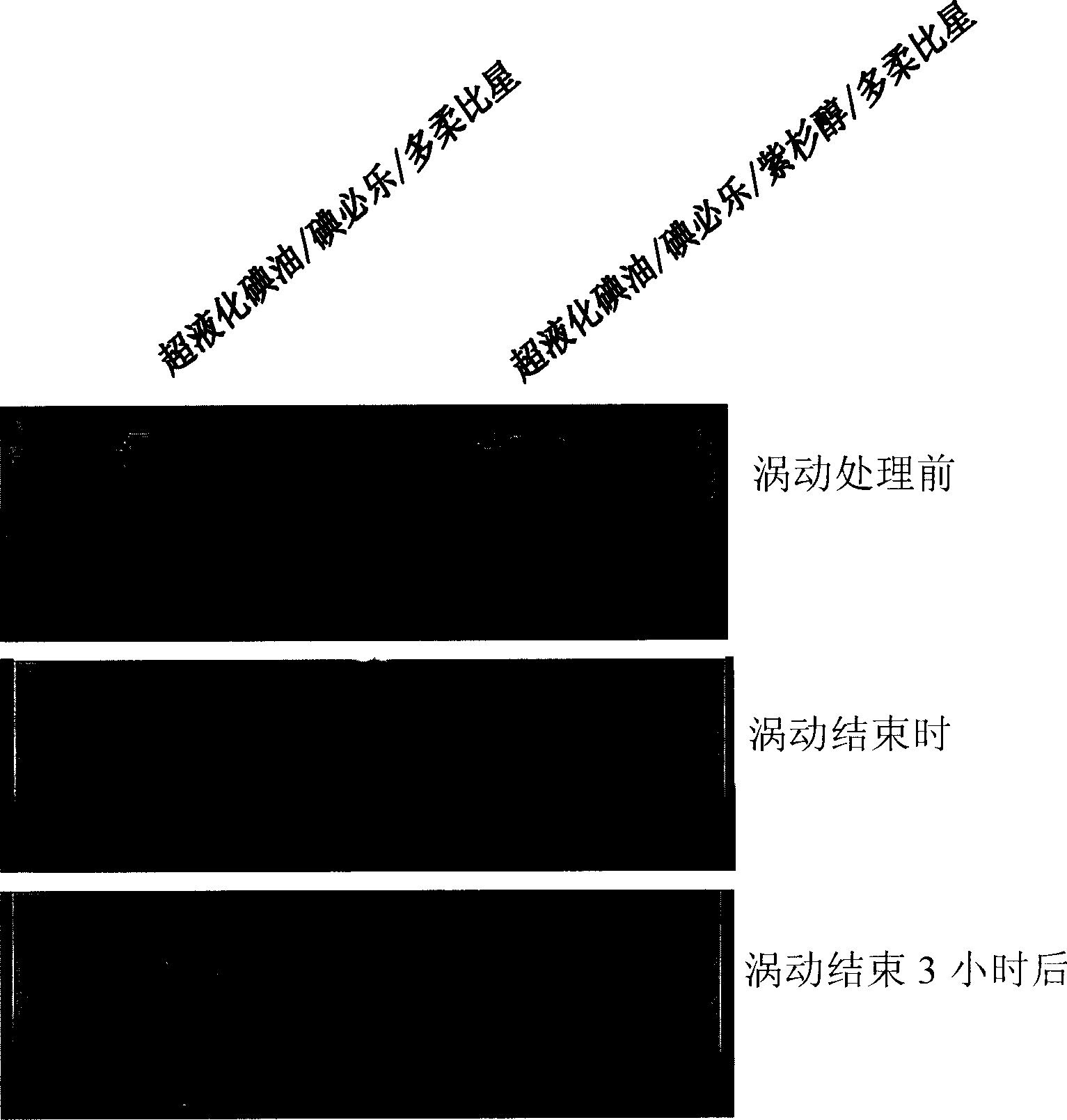
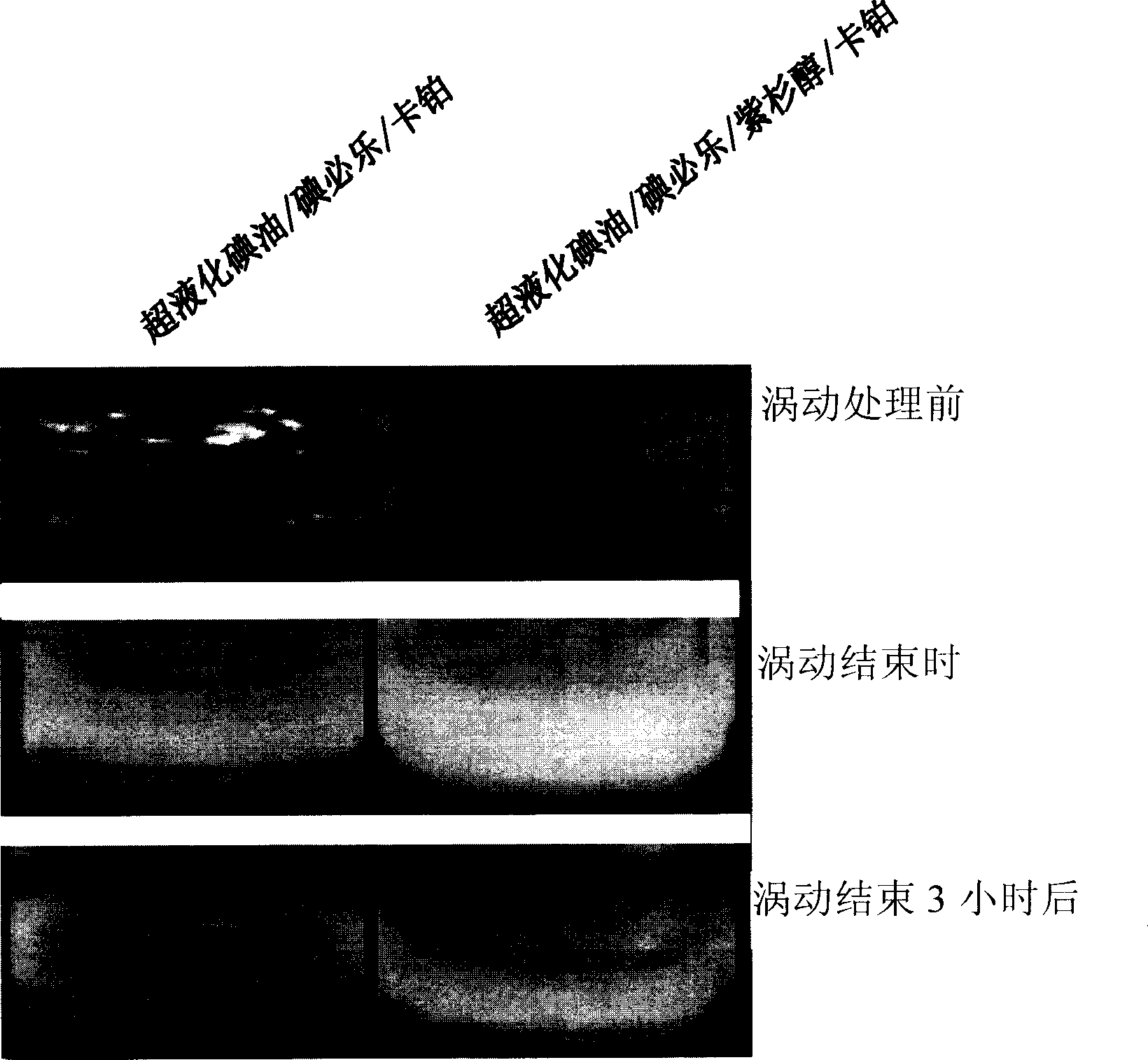
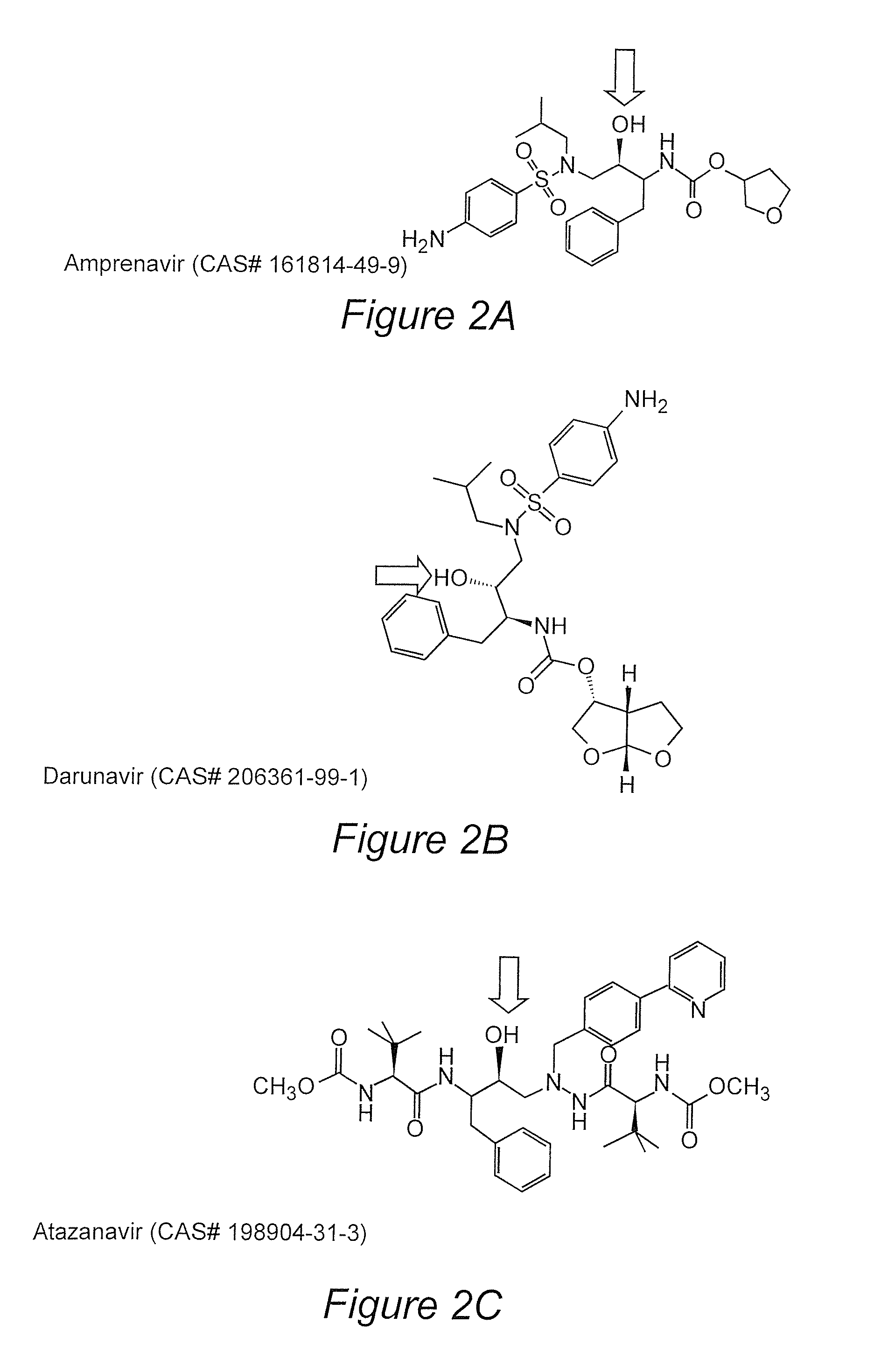
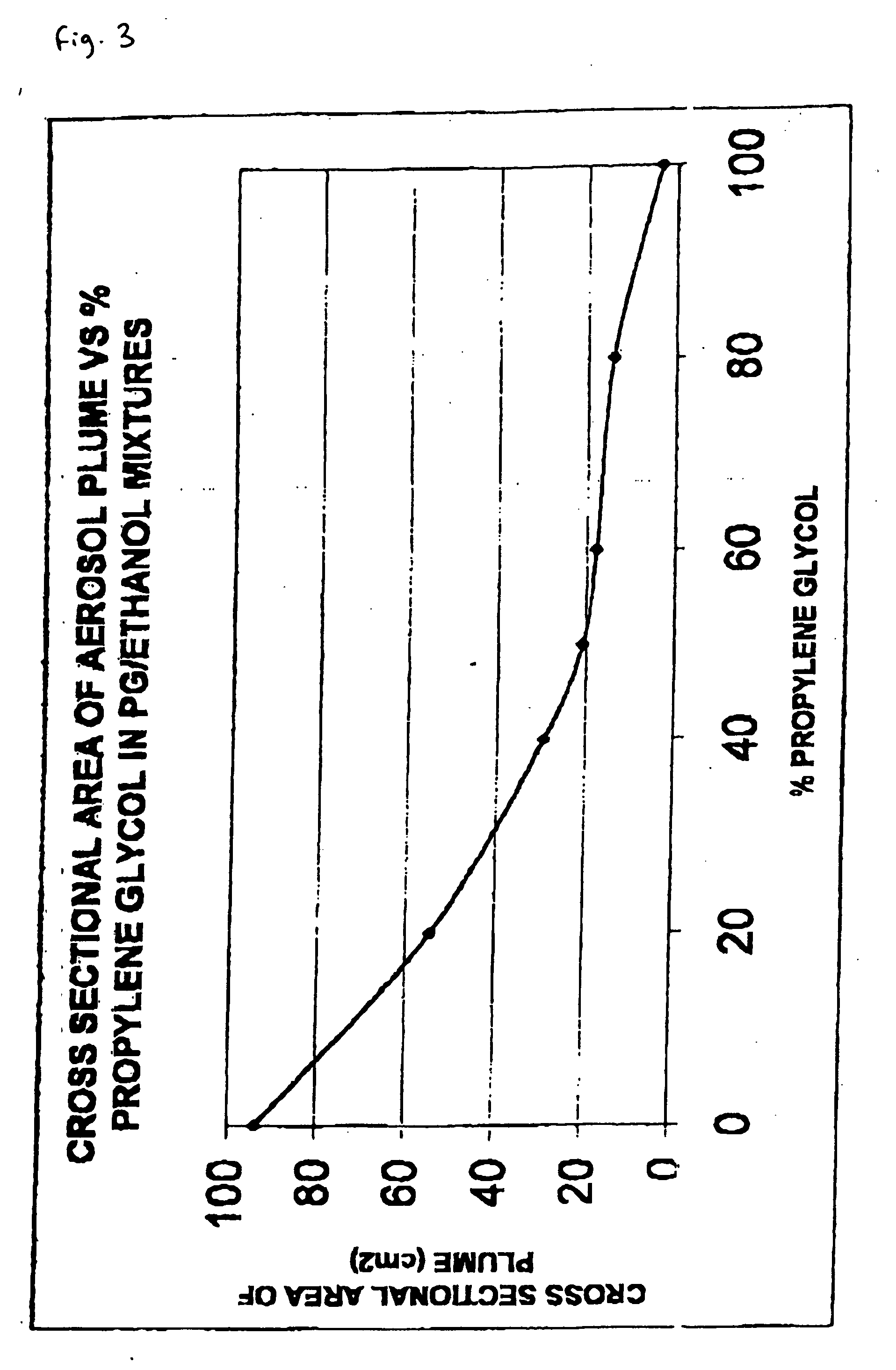
Patents
Literature
102 results about "Lipophilic drug" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
[2] Lipophilicity is defined as the affinity of a drug for a lipid environment. It has become a critical parameter in the Pharmaceutical industry, which indicates the relationship of a drug with their biological, pharmacokinetic, and metabolic properties.
Pharmaceutical compositions for lipophilic drugs
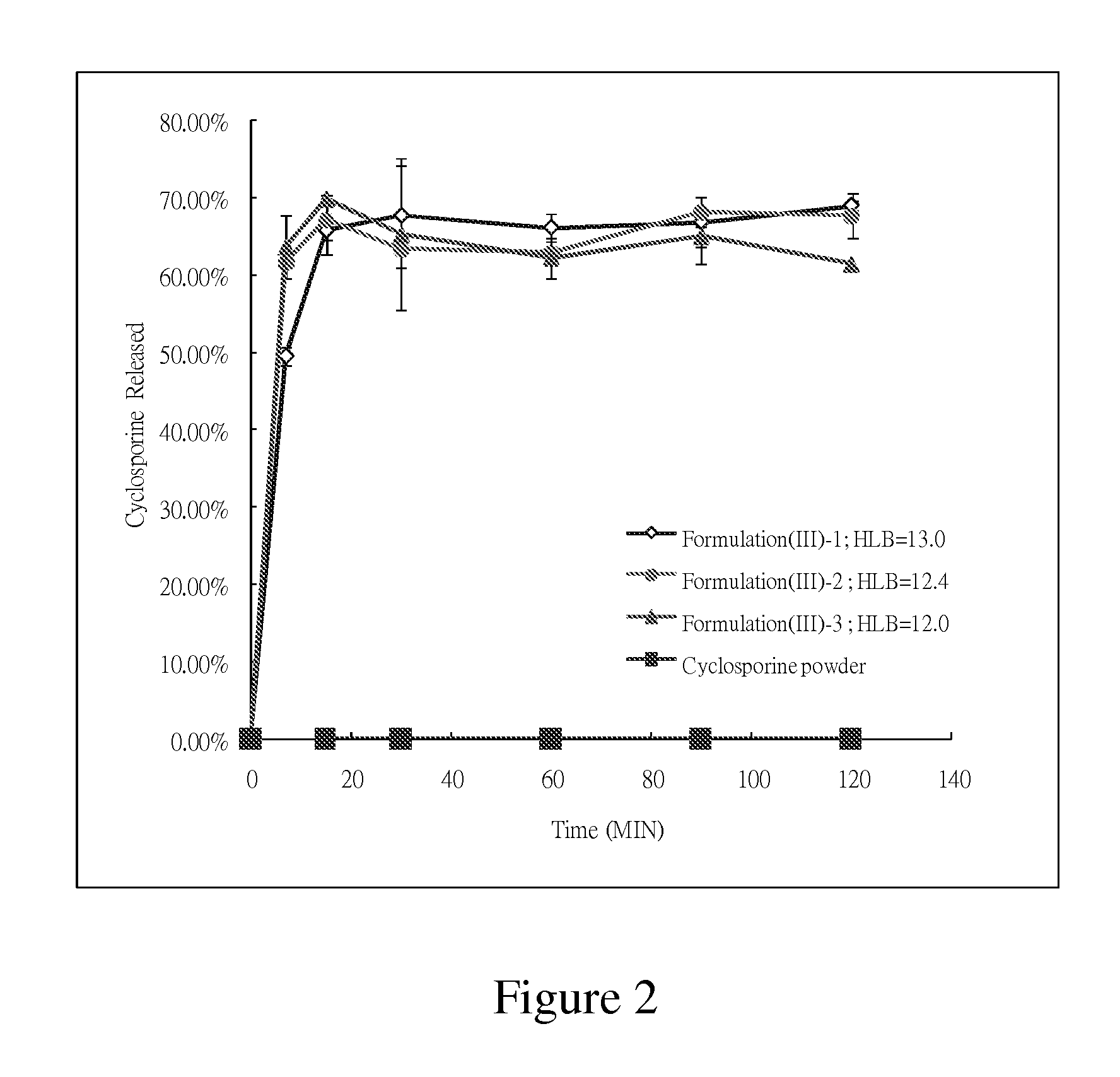
InactiveUS7070802B1Good self-emulsifying performanceShelf-stableCyclic peptide ingredientsCapsule deliveryMonoglycerideCyclosporins
Stable solutions of lipophilic drugs, such as cyclosporin, forming a polar lipid self-emulsifying drug delivery system. The solutions can include lipophilic drugs, such as cyclosporin, dissolved in a polar lipid, such as having a C6-C12 fatty acid monoglyceride content of at least about 50%, surfactants and triglycerides. The composition forms a fine emulsion on exposure to water. The encapsulated dosage form of this composition needs neither a hydrophilic component nor air-tight blister packaging, and is particularly suitable for oral administration.
Owner:WATSON LAB INC
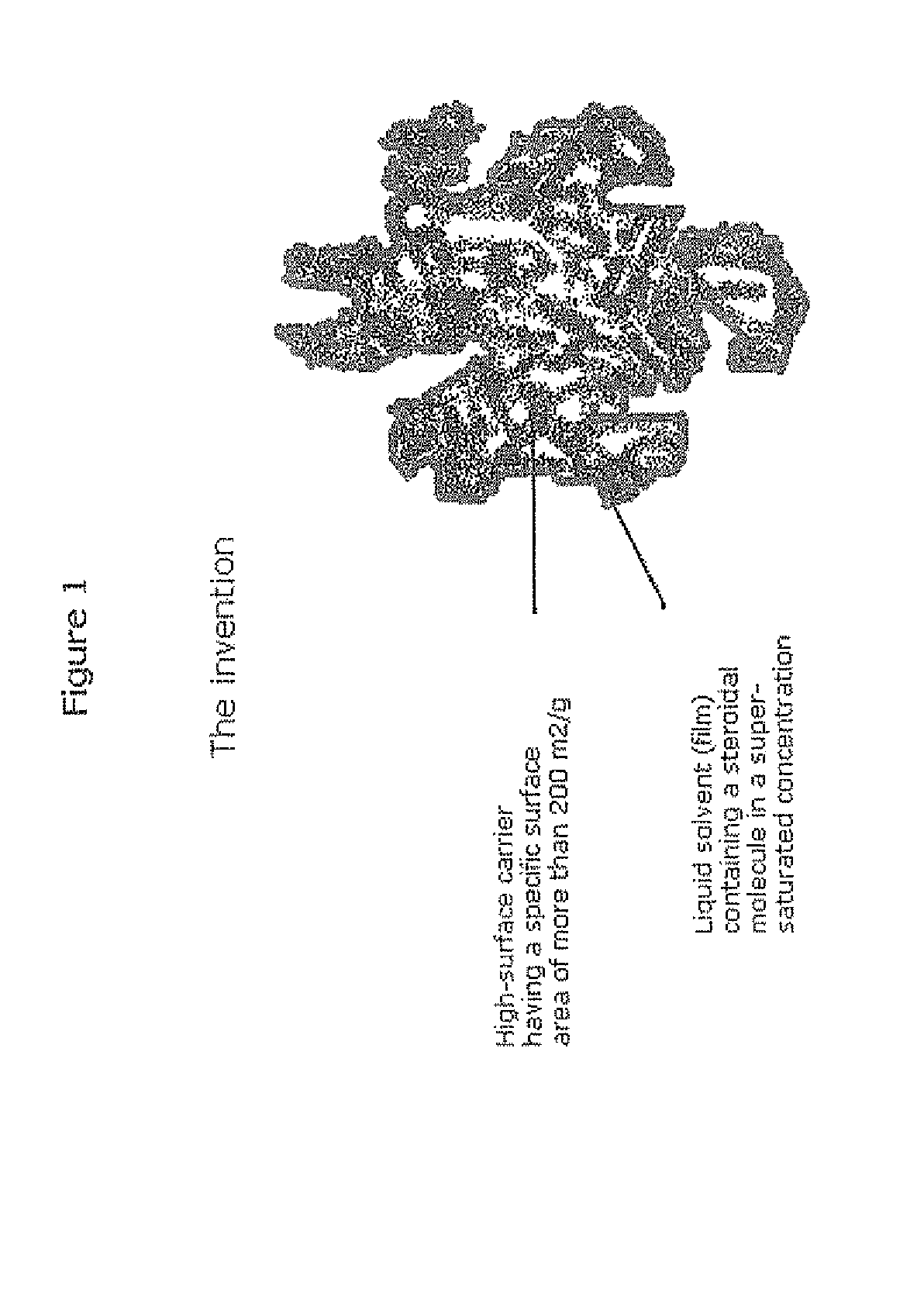
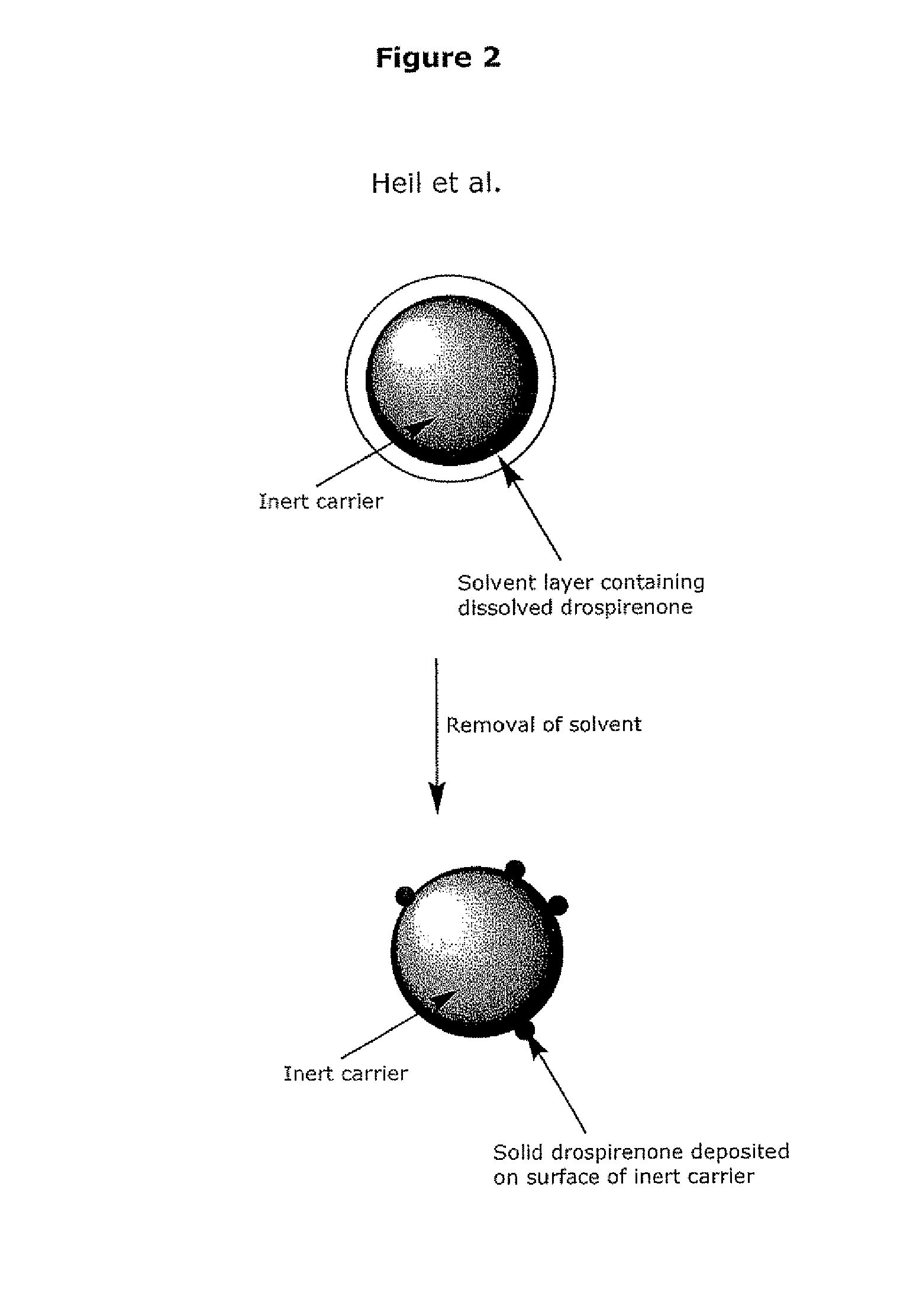
Stabilised supersaturated solids of lipophilic drugs
Methods for improving solubility and bioavailability of lipophilic compounds are described. Particularly, described are stabilized superstaturated solid solutions, particularly in power form, of lipophilic drugs, such as steroidal molecules.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
Novel Composition Of Matter For Delivering Lipid-Soluble Materials, And A Method For Producing It
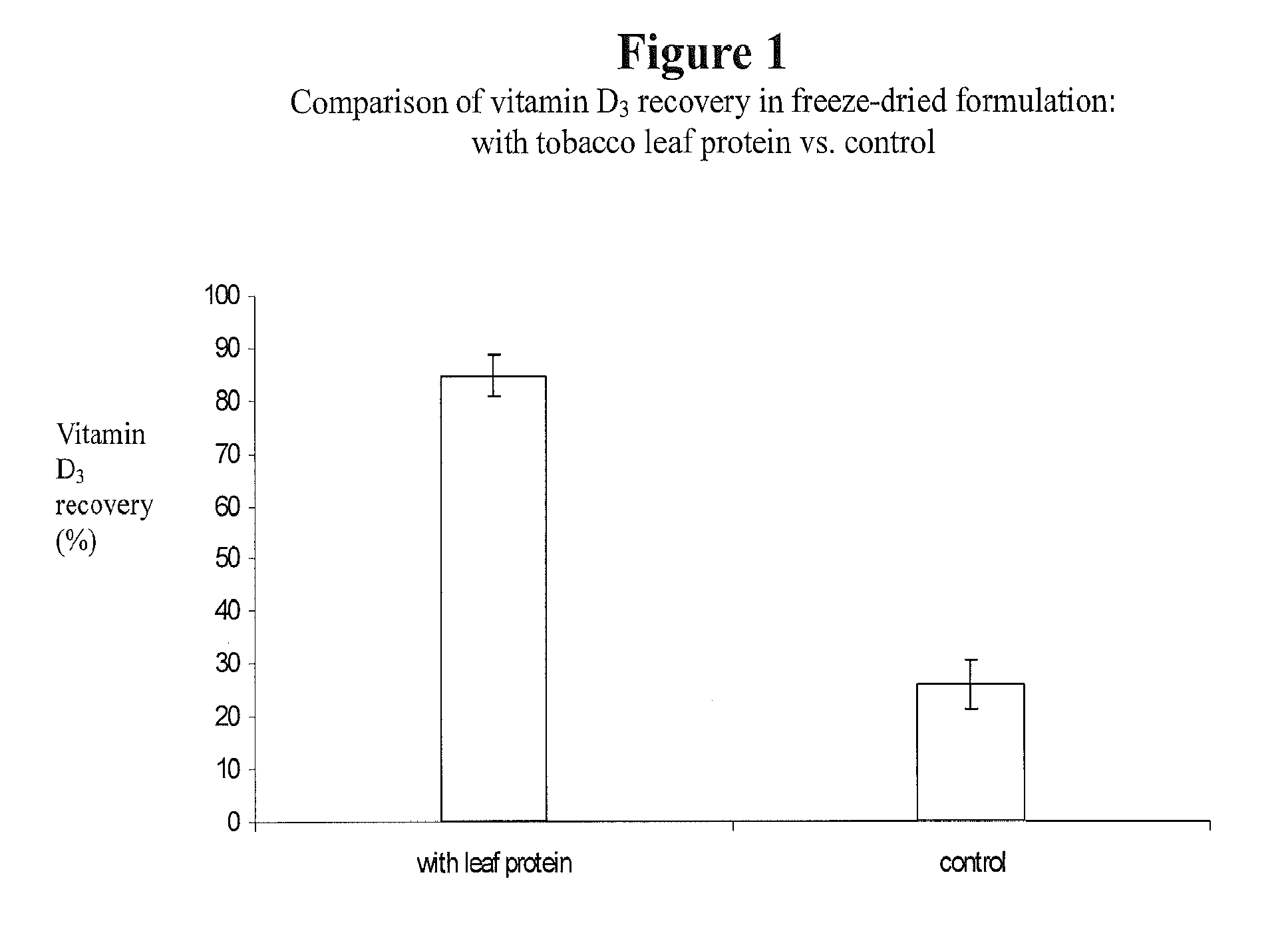
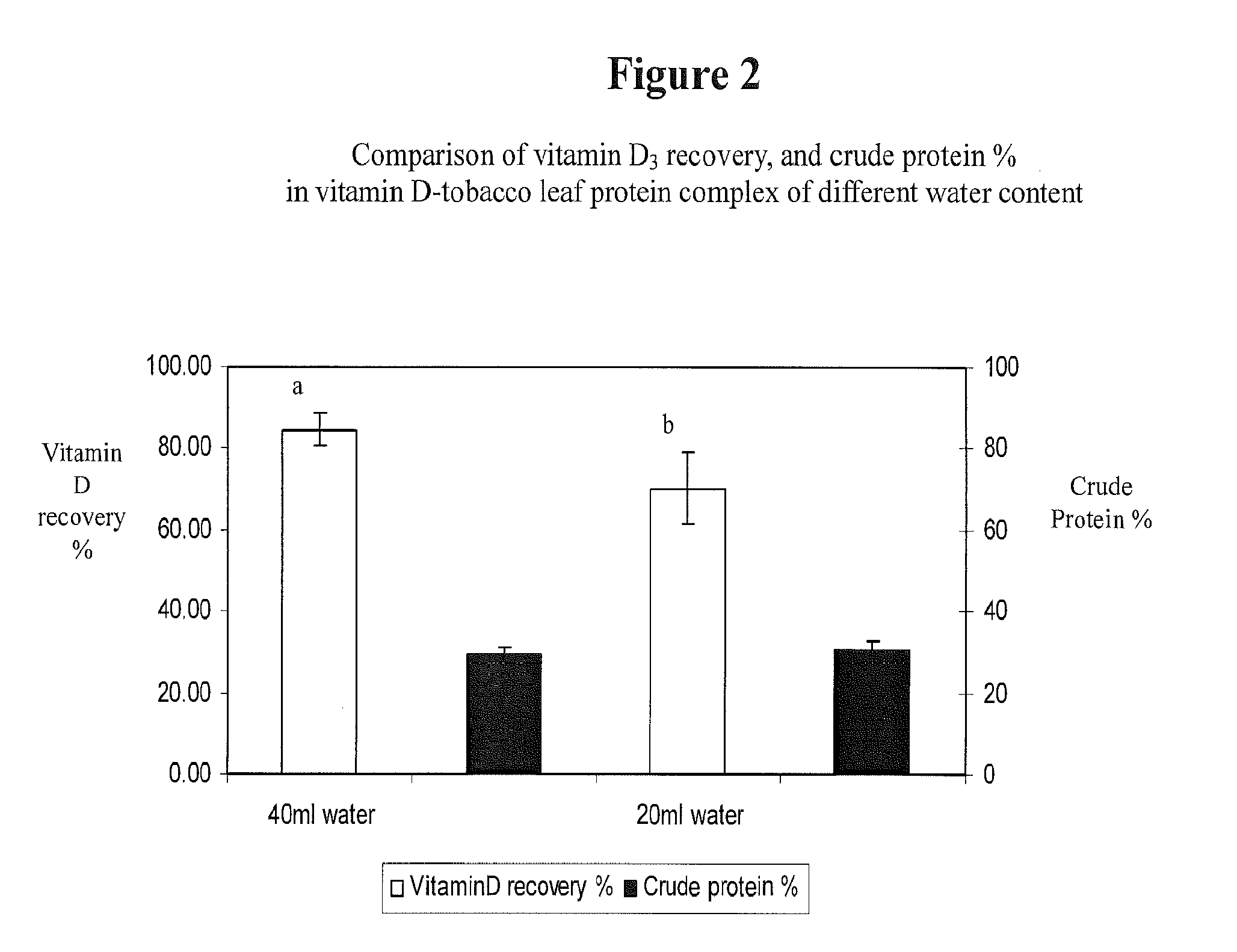
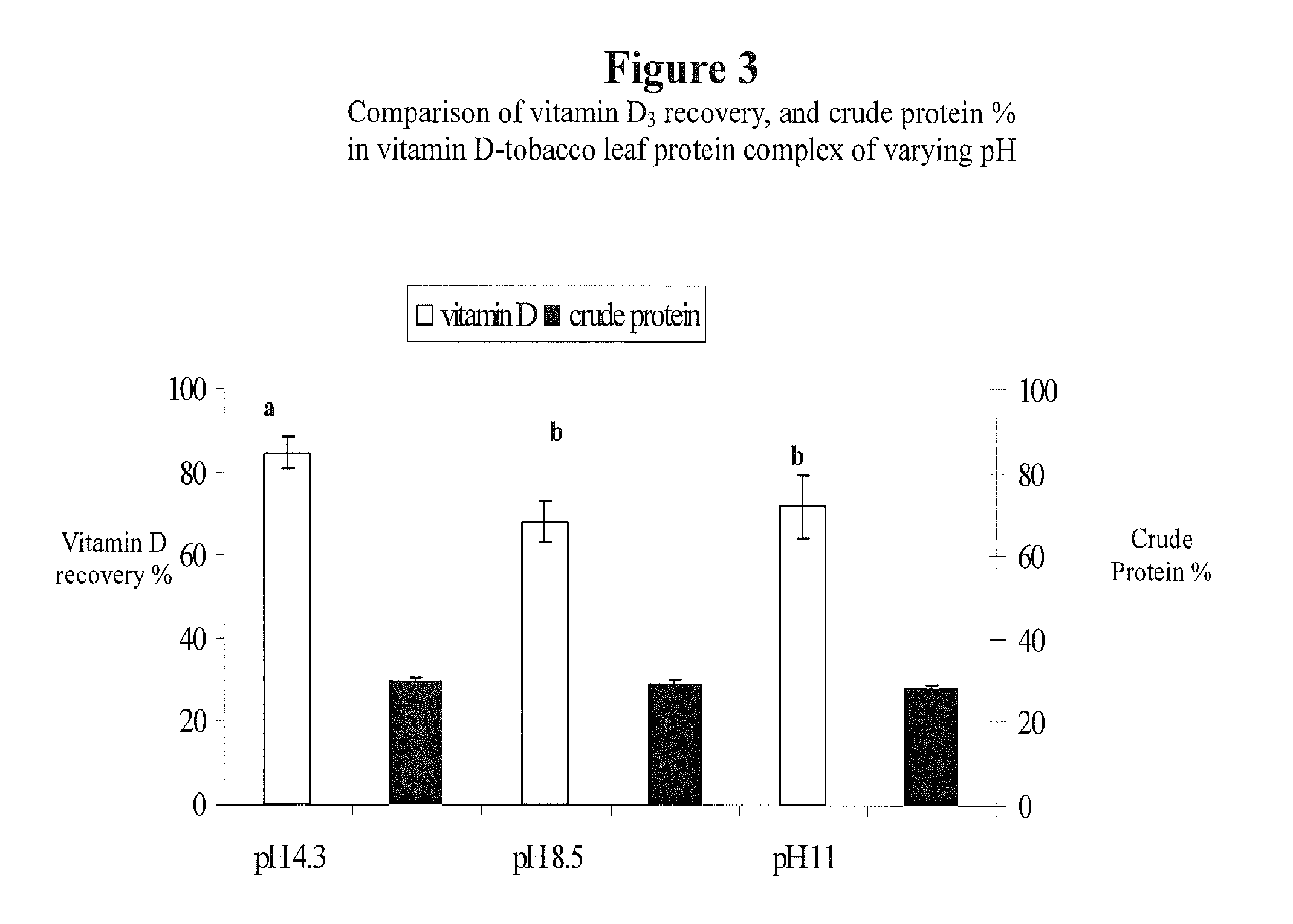
This invention describes a novel composition of matter describing a complex comprising leaf protein and a lipophilic substance(s), along with the method of producing it. Delivery of lipid-soluble materials into the body is challenging because they are generally highly insoluble in water and very subject to oxidative degradation. The inventors have found that leaf protein—the water-soluble proteins derived from plant leaves—can efficiently form a complex with lipophilic materials. This leaf protein—lipid-soluble material complex is an effective carrier of lipophilic substances. As such, the leaf protein—lipid-soluble material complex disclosed herein can be used for the delivery of lipophilic vitamins, fatty acids, caretenoids, lipophilic drugs, and other lipophilic materials. This complex can be used to deliver lipophiles in foods, nutritional and dietary supplements, topical compositions and in pharmaceutical products.
Owner:LEAFPRO LLC +1
Stabilised supersaturated solids of lipophilic drugs
Owner:BAYER INTELLECTUAL PROPERTY GMBH
Modulation of solubility, stability, absorption, metabolism, and pharmacokinetic profile of lipophilic drugs by sterols
InactiveUS20110160168A1Enhance biological absorptionGood metabolic stabilityBiocideMetabolism disorderSterol esterPharmaceutical drug
A formulation for drug delivery, providing enhanced modulation of solubility, stability, absorption, metabolism, and / or pharmacokinetic profile of a lipophilic therapeutic agent by formulation with sterols and / or sterol esters, resulting in higher bioavailability of a therapeutic agent administered to a subject in need of such therapeutic agent. The formulation contains a therapeutic agent and a sterol or sterol ester, and can, optionally, further contain a solubilizer and / or an enhancing agent. Also described are pharmaceutical compositions containing the formulations and methods of making and methods of using the formulations and pharmaceutical compositions. Formulations of the disclosure can be constituted to minimize the synthesis of dihydrotestosterone when the therapeutic agent includes testosterone or testosterone esters.
Owner:MARIUS PHARMA LLC
Methods and compositions for the treatment of ocular disorders
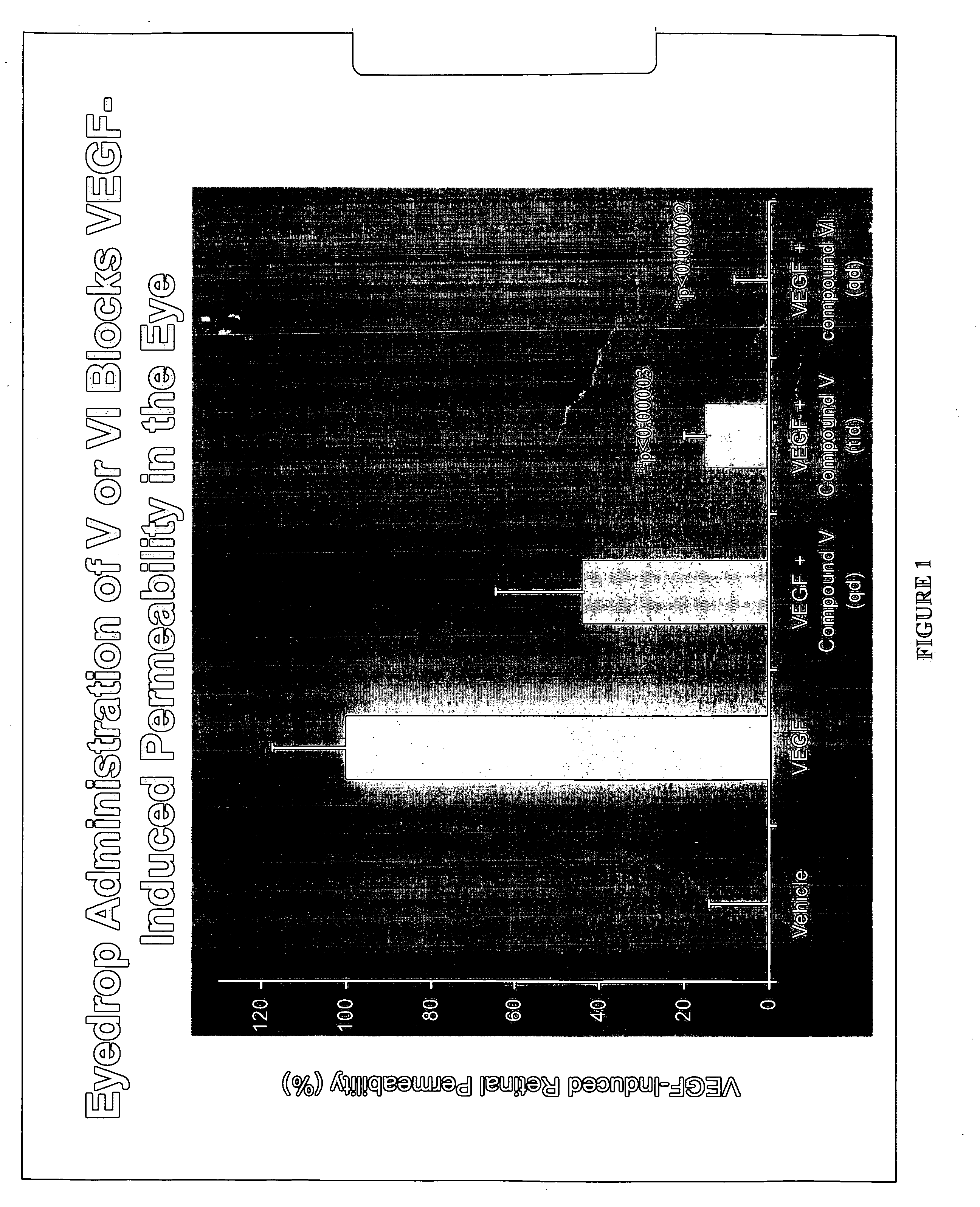
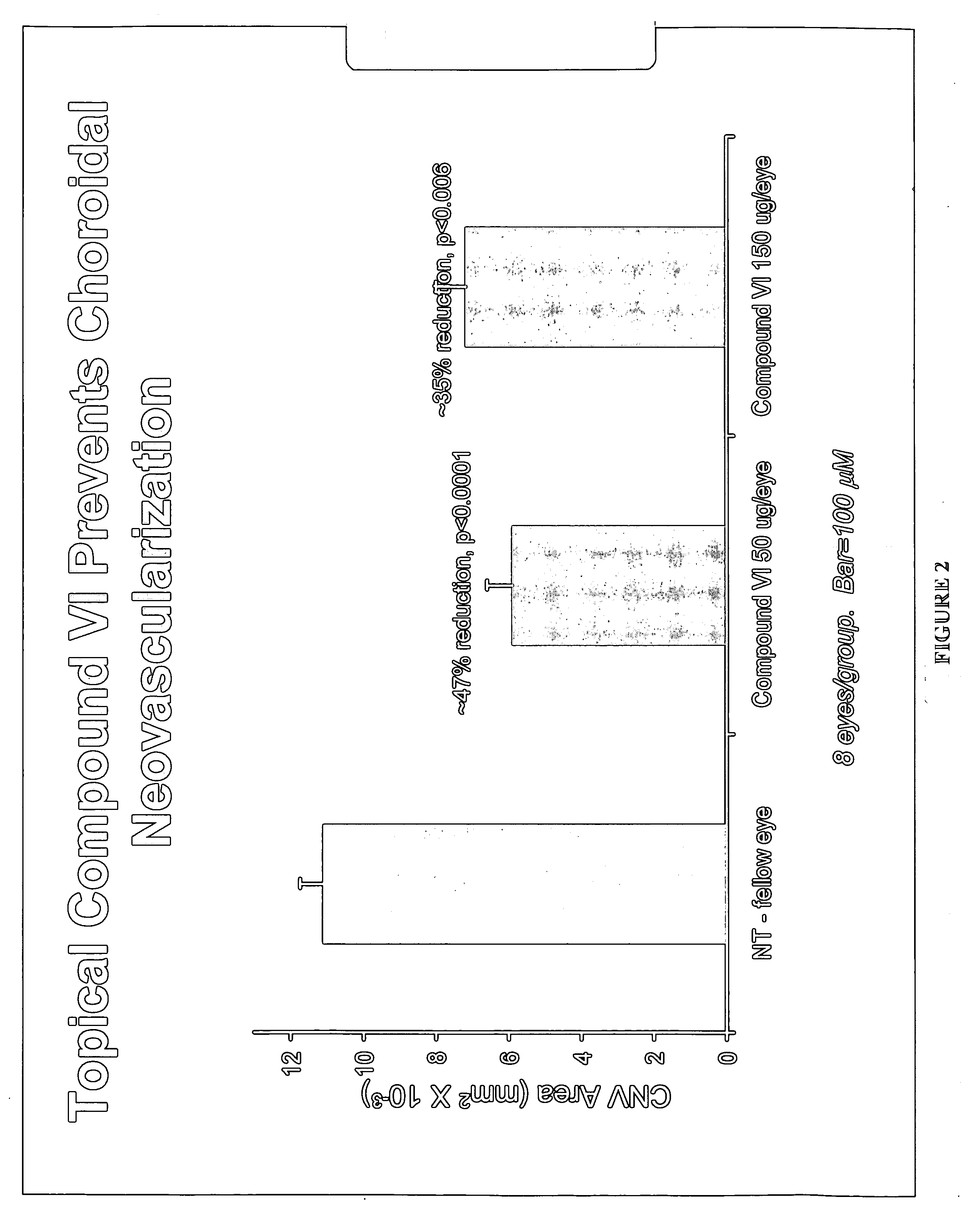
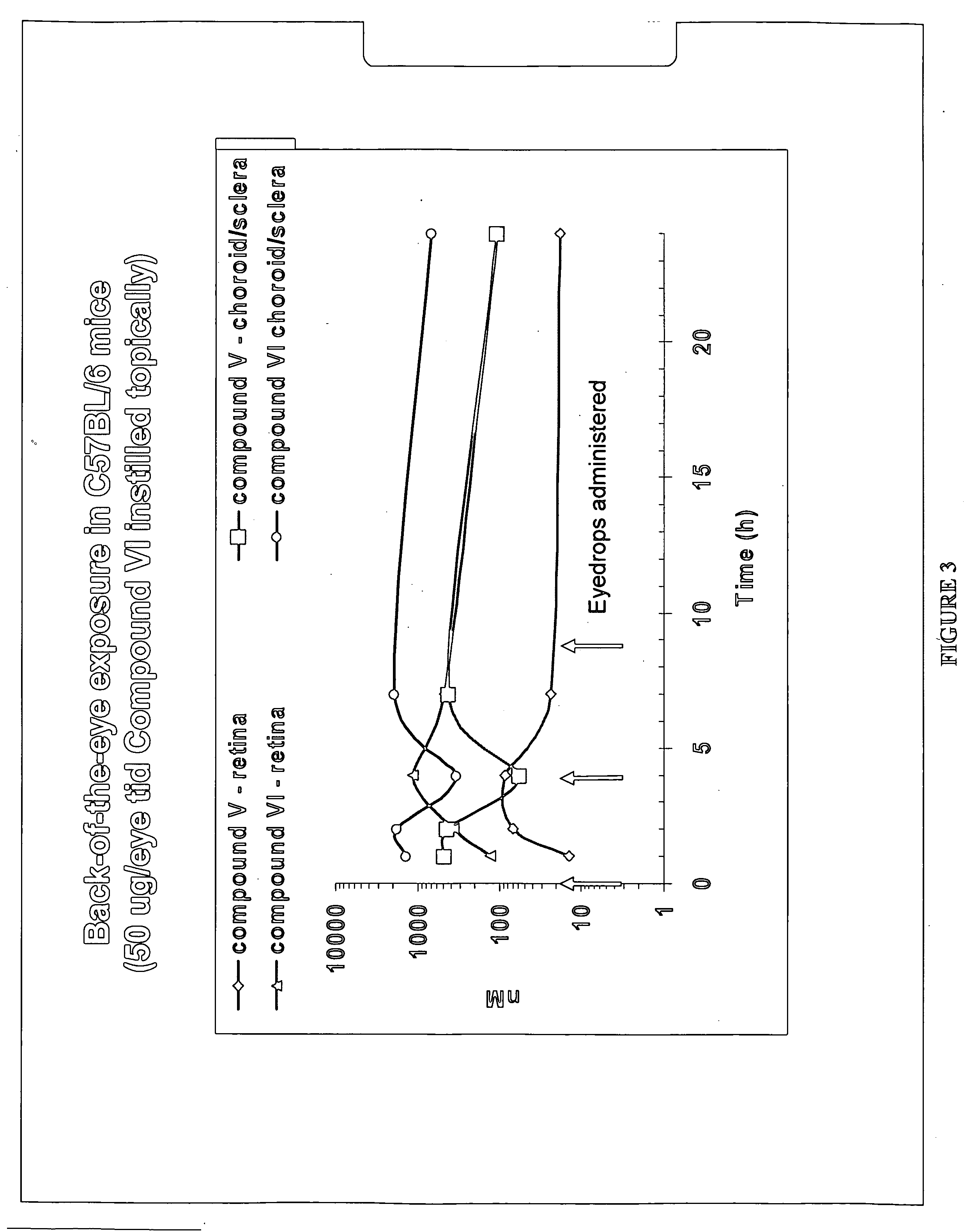
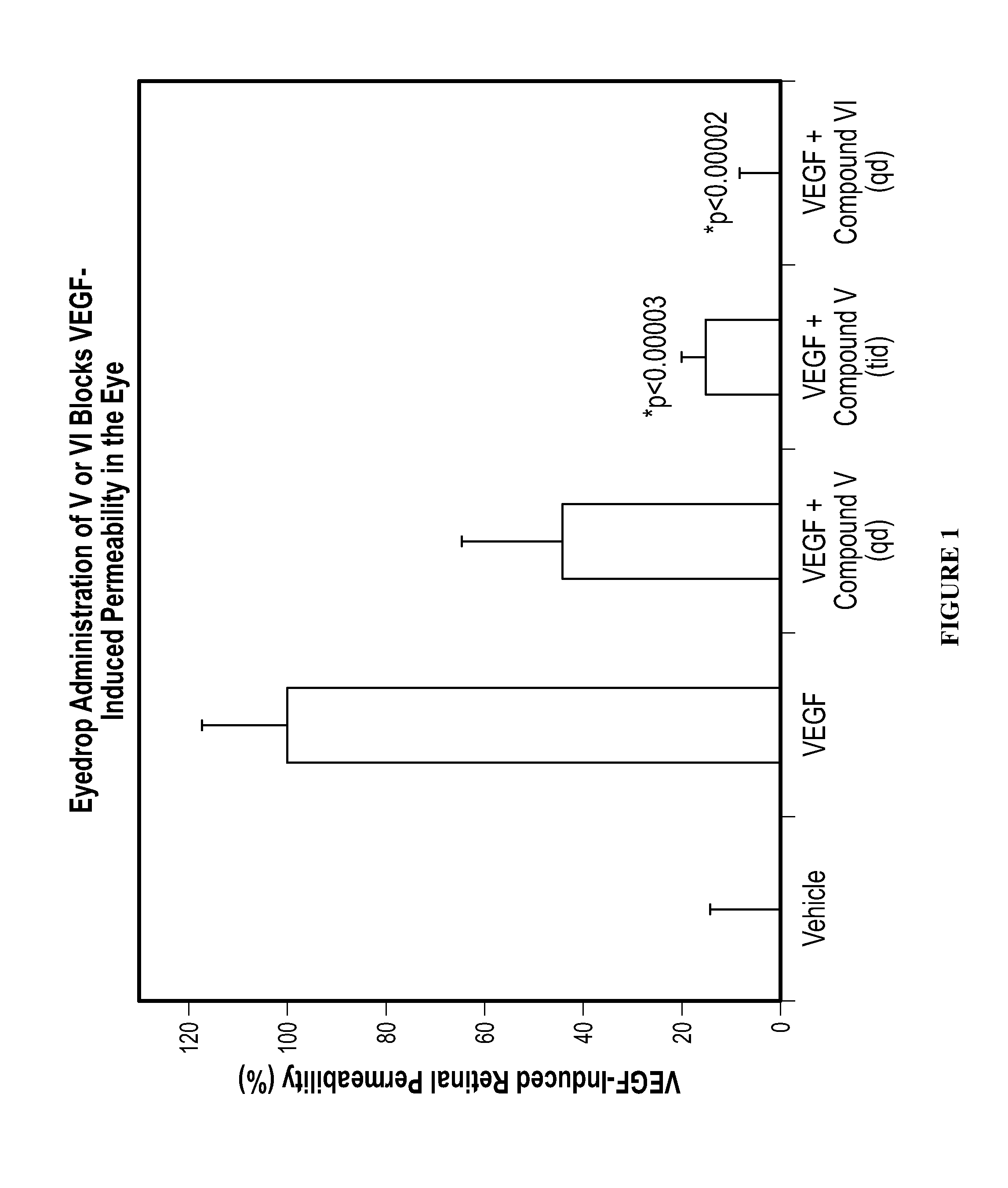
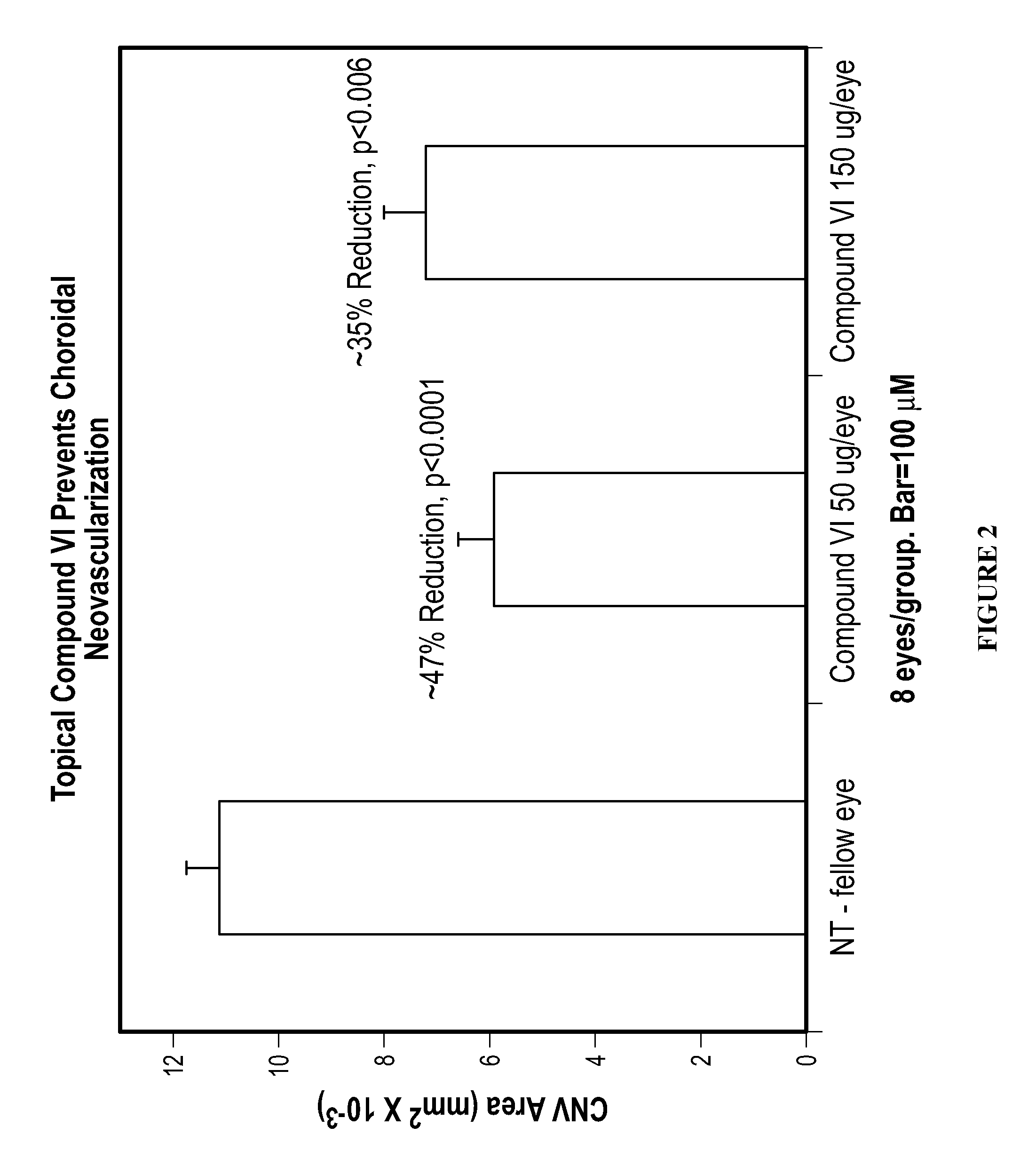
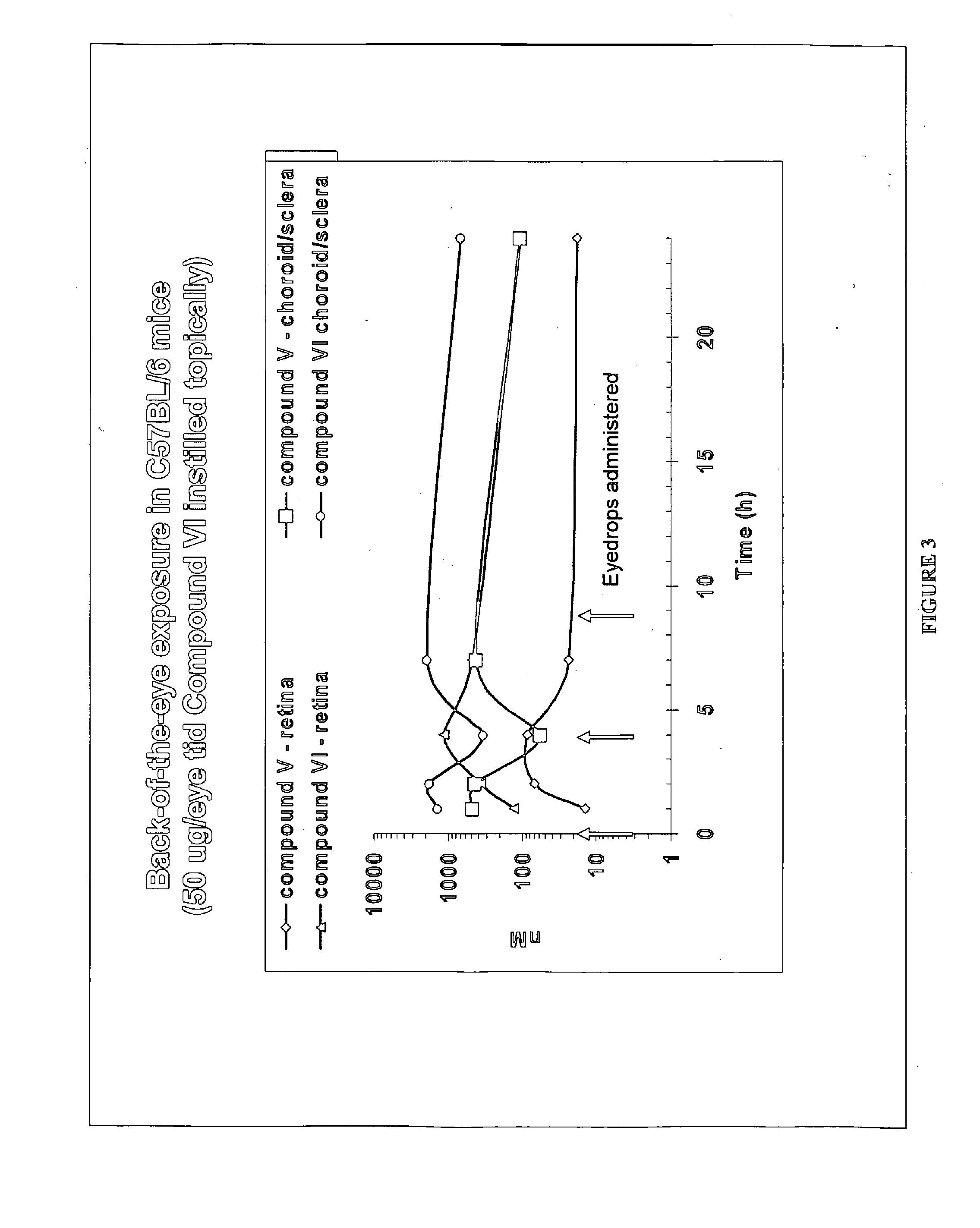
The invention provides methods and compositions for the delivery of lipophilic drugs that are useful for the treatment of various ophthalmological diseases, disorders, and pathologies, including the treatment of age-related macular degeneration, diabetic retinopathy, diabetic macular edema, cancer, and glaucoma.
Owner:TARGEGEN
Dispersible concentrate lipospheres for delivery of active agents
A formulation containing one or more lipophilic agents, methods of making and using the formulation are described herein. The formulation is formed by adding a pre-suspension concentrate composition to an aqueous medium. Upon contact with the aqueous medium, a solid nanoparticle suspension spontaneously forms. The resulting formulation is in the form of a microemulsion. The concentrate contains an amphiphilic solvent, a pharmaceutically acceptable solid carrier such as a solid fatty acid or ester, a surfactant, and an agent. Preferably the concentrate contains a combination of a surfactant with a high hydrophilic / lipophilic balance (HLB) of at least about 8 and a surfactant with a low HLB of less than about 5. The agent is preferably a lipophilic drug and other lipophilic ingredient, such as vitamins. The composition is suitable for use in medical and non-medical applications. The microemuslions described herein have increased stability was compared to the prior art.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Microspheres comprising nanocapsules containing a lipophilic drug
ActiveUS20090011009A1Improve bioavailabilityOrganic active ingredientsDispersion deliveryActive agentMicrosphere
The present invention provides microspheres comprising a plurality of nanocapsules accommodated in a gel forming polymer, the plurality of nanocapsules comprising an oil core carrying a non hydrophilic active agent and a shell of polymeric coating. The invention also provides a method for preparing the microspheres of the invention, pharmaceutical compositions comprising the same as well as methods of use of the microspheres, specifically, in therapeutic, cosmetic and diagnostic applications.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Gelled emulsion and microemulsion formulations for dermal drug delivery
The present invention is drawn to gelled emulsion and microemulsions formulations for dermal drug delivery, including transdermal drug delivery. In one embodiment, a drug-containing gelled emulsion can comprise a continuous gelled aqueous phase, and a discontinuous drug-containing oil phase dispersed within the continuous gelled aqueous phase, wherein the drug-containing gelled emulsion is present in a dermal delivery system. In another embodiment, a drug-containing microemulsion can comprise a continuous aqueous phase, a discontinuous oil phase including a lipophilic drug, and surfactant(s) substantially positioned interfacially between the continuous aqueous phase and the discontinuous oil phase. The discontinuous oil phase can be dispersed in the continuous aqueous phase, and the drug-containing microemulsion can be present in a dermal reservoir patch delivery system.
Owner:ZARS INC
Materials and methods for drug delivery and uptake
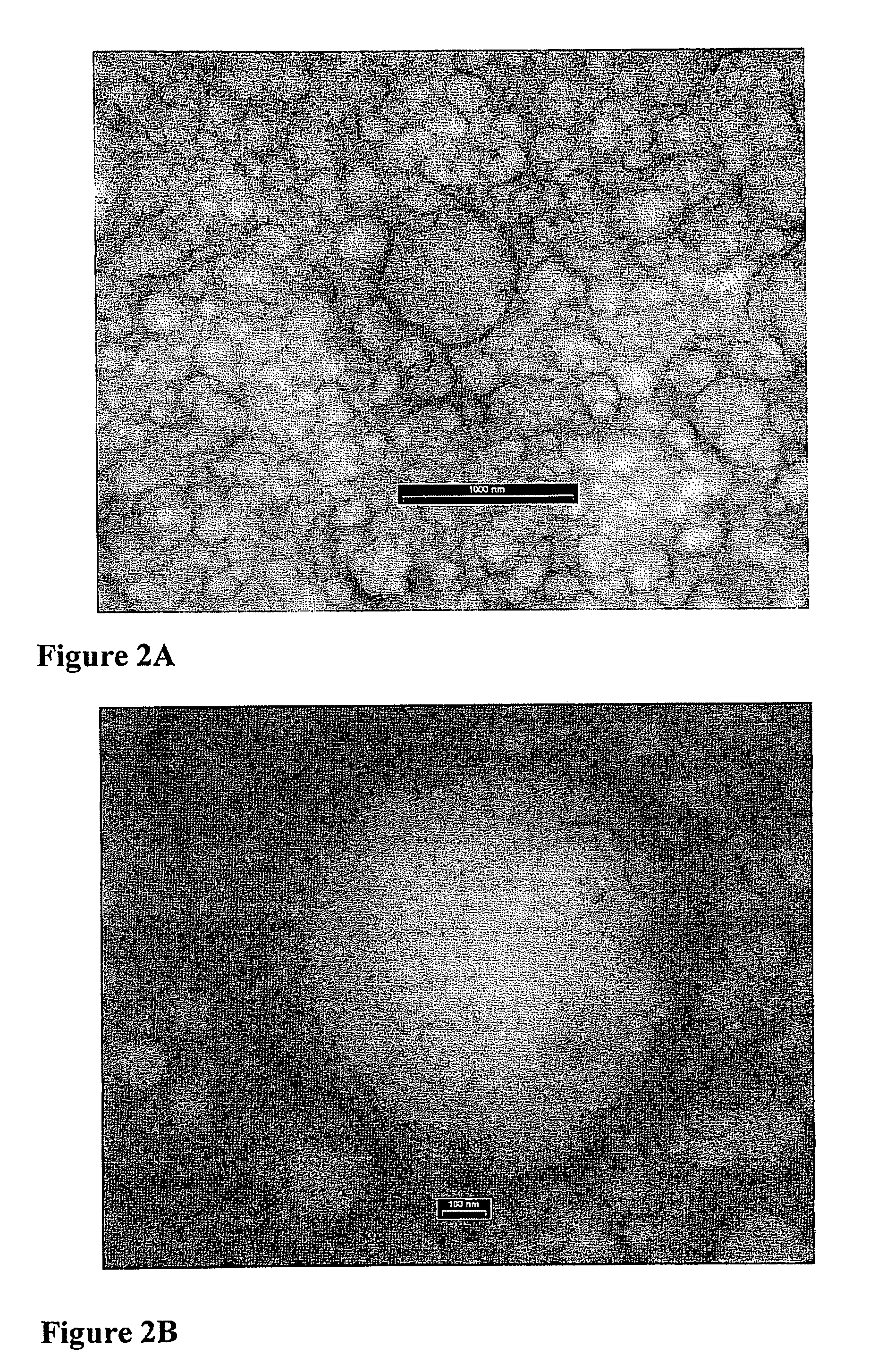
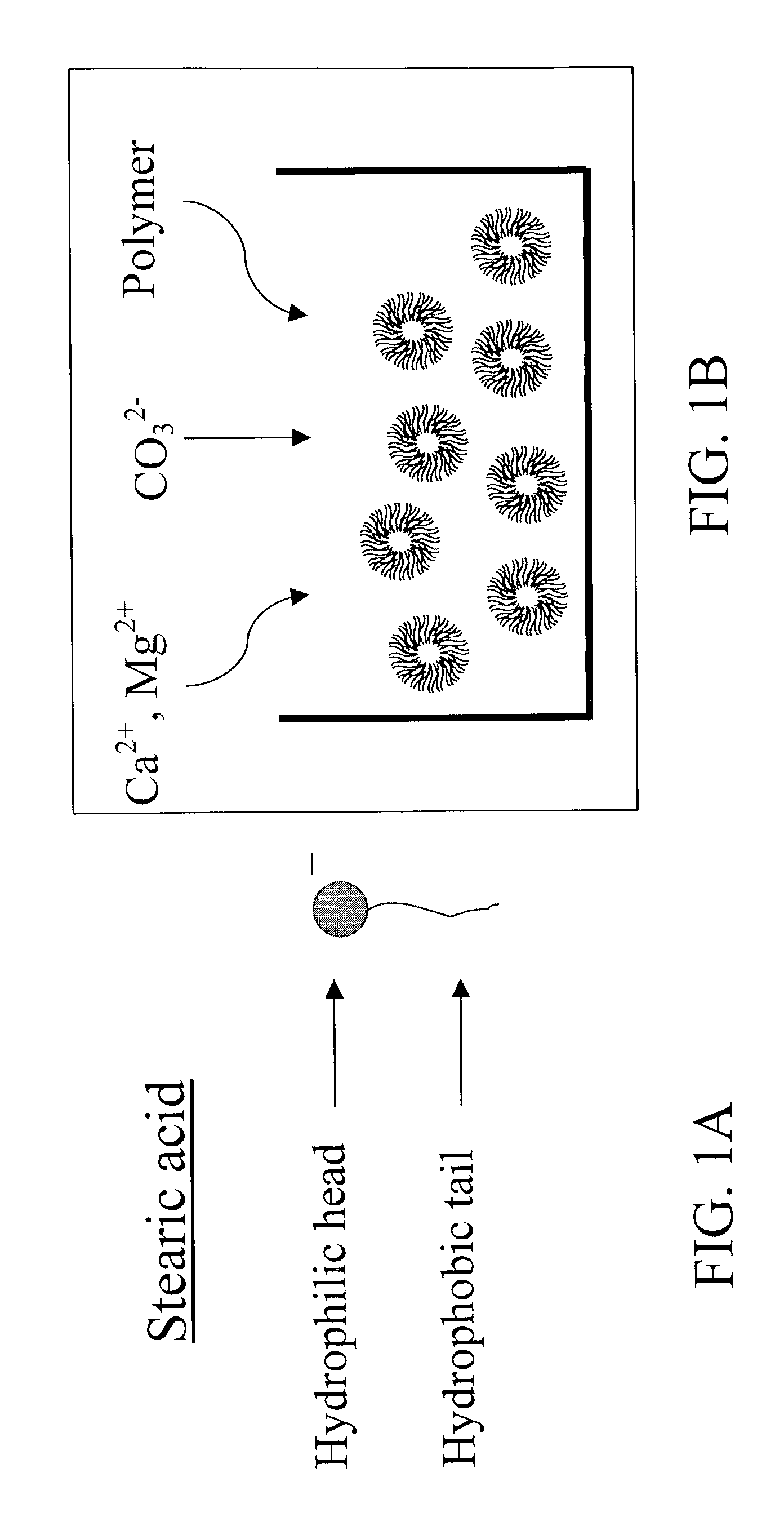
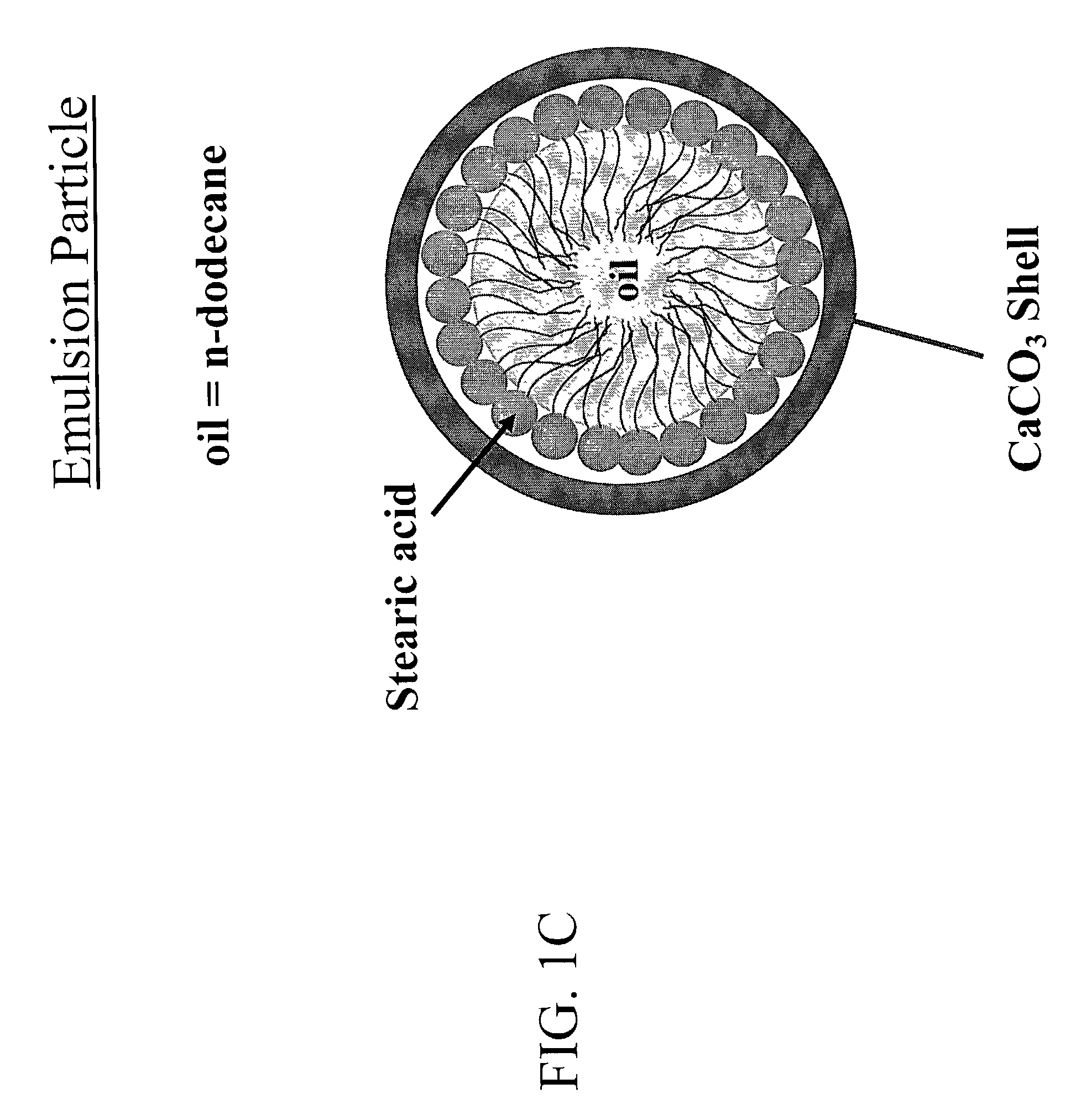
The subject invention pertains to novel materials and methods for use in delivering and sequestering substances, such as pharmacological agents, within a patient. One aspect of the invention is directed towards core-shell particles having a core encapsulated within a calcium carbonate shell, with an intermediate layer composed of an amphiphilic compound surrounding the core. When the particles of the subject invention are administered to a patient, they are capable of removing lipophilic drugs by absorption of the drug through their mineral shell and into their core. The particles of the subject invention can also be administered to a patient as controlled release, drug delivery vehicles. Thus, in another aspect, the subject invention concerns a method of delivering pharmacological agents by administering the core-shell particles of the subject invention to a patient in need of such administration.
Owner:UNIV OF FLORIDA RES FOUNDATION INC +1
Monoterpene compositions and uses thereof
The present invention relates to pharmaceutical compositions and methods for the mucosal and oral administration of monoterpenes and derivatives thereof. The compositions of this invention further comprise one or more surfactants and cosolvents and are in the form of self-emulsifying compositions. The compositions of the invention may further comprise water-insoluble therapeutic agents, vaccines and diagnostics. Such agents include but are not limited to taxanes, steroids, topoisomerase inhibitors such as etoposide and other water-insoluble or lipophilic drugs.
Owner:CONSTANTINIDES PANAYIOTISP +3
Dispersible concentrate lipospheres for delivery of active agents
A formulation containing one or more lipophilic agents, methods of making and using the formulation are described herein. The formulation is formed by adding a pre-suspension concentrate composition to an aqueous medium. Upon contact with the aqueous medium, a solid nanoparticle suspension spontaneously forms. The resulting formulation is in the form of a microemulsion. The concentrate contains an amphiphilic solvent, a pharmaceutically acceptable solid carrier such as a solid fatty acid or ester, a surfactant, and an agent. Preferably the concentrate contains a combination of a surfactant with a high hydrophilic / lipophilic balance (HLB) of at least about 8 and a surfactant with a low HLB of less than about 5. The agent is preferably a lipophilic drug and other lipophilic ingredient, such as vitamins. The composition is suitable for use in medical and non-medical applications. The microemuslions described herein have increased stability was compared to the prior art.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Prepn process of cyclodextrin inclusion for lipophilic medicine
InactiveCN1706501AImprove solubilityImprove stabilityMacromolecular non-active ingredientsSolubilityCyclodextrin
The present invention is the preparation process of new cyclodextrin inclusion of lipophilic medicine, and solves the problem in preparing inclusion of lipophilic medicine with poor water solubility and lower stability. The preparation process completed inside co-solvent system of tert-butyl alcohol and water includes the following steps: dissolving the lipophilic medicine and cyclodextrin as including material separately in tert-butyl alcohol and water, mixing the obtained two kinds of solution to obtain single phase solution, and drying the mixed solution to obtain powder of cyclodextrin inclusion of lipophilic medicine. The present invention may prepare powder for injection, injection, tablet, capsule or other form. The present invention has simple preparation process, easy operation, short preparation period, low power consumption and no organic solvent residue, and is suitable for industrial production.
Owner:SHENYANG PHARMA UNIVERSITY
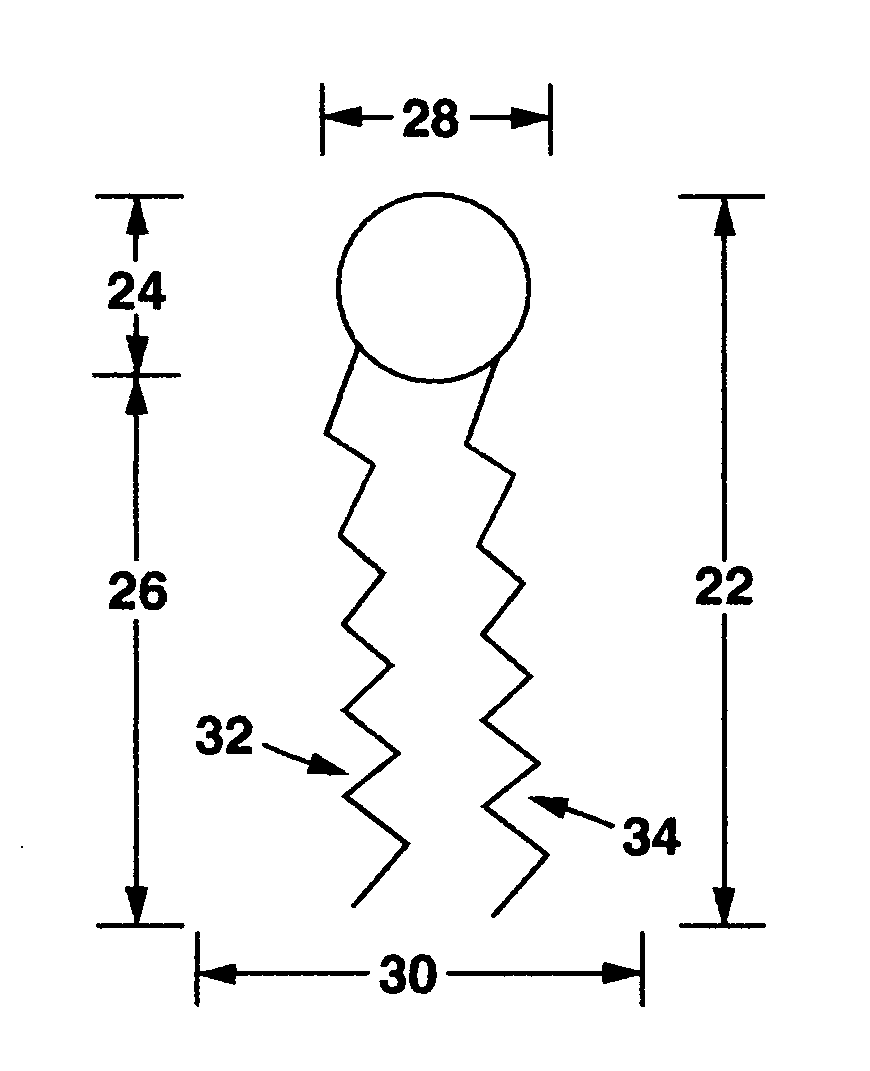
Lipophilic drug delivery vehicle and methods of use thereof
ActiveUS7824709B2Increase stability of particleAntibacterial agentsBiocideActive agentDelivery vehicle
The invention provides compositions and methods for delivery of a bioactive agent to an individual. Delivery vehicles are provided that include a bioactive agent in disc shaped particles that include one or more lipid binding polypeptides circumscribing the perimeter of a lipid bilayer in which the bioactive agent is localized. Chimeric lipid binding polypeptides are also provided and may be used to add additional functional properties to the delivery particles.
Owner:CHILDREN S HOSPITAL &RES CENT AT OAKLAN
Submicron liposome suspensions obtained from preliposome lyophilizates
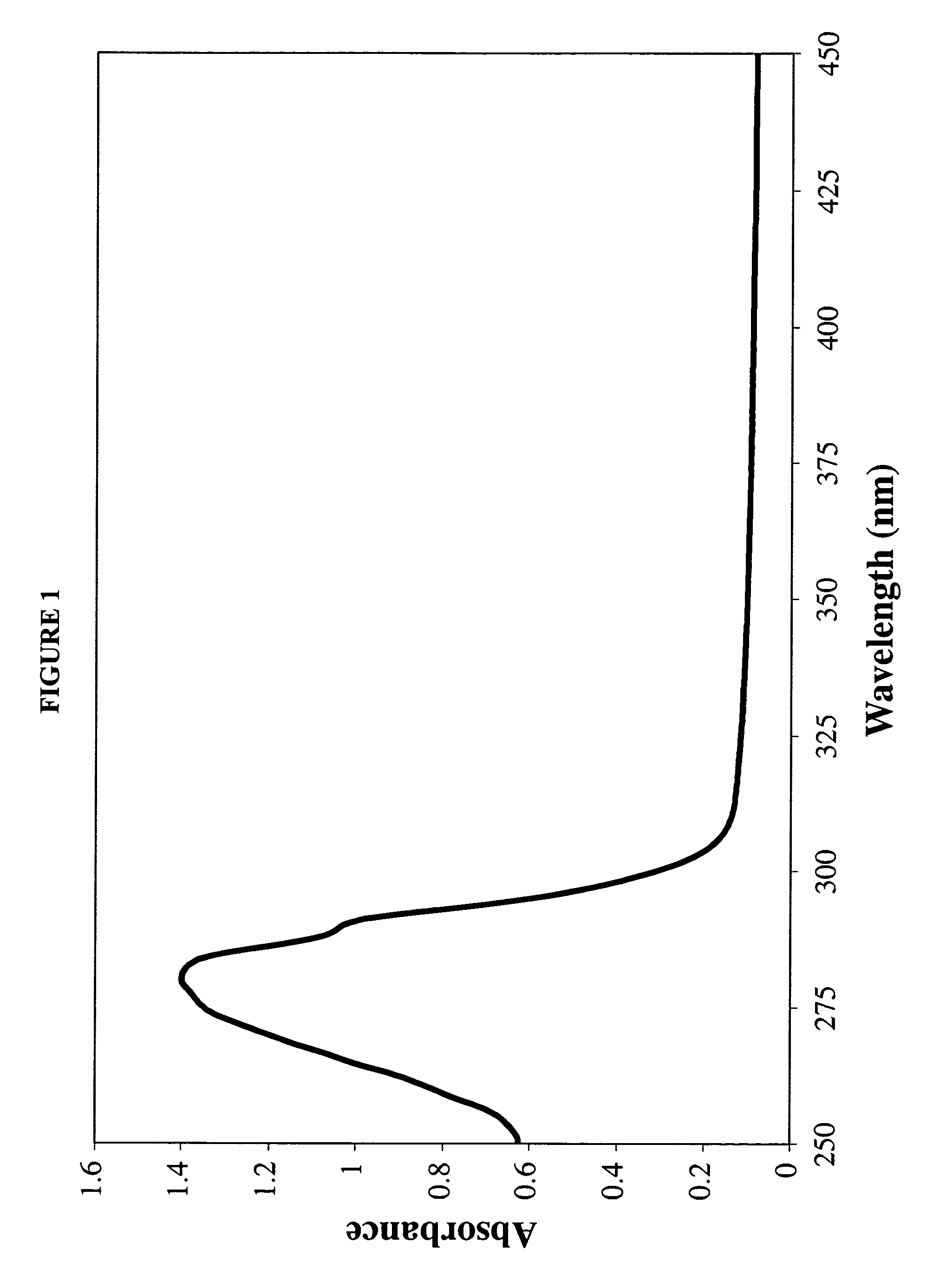
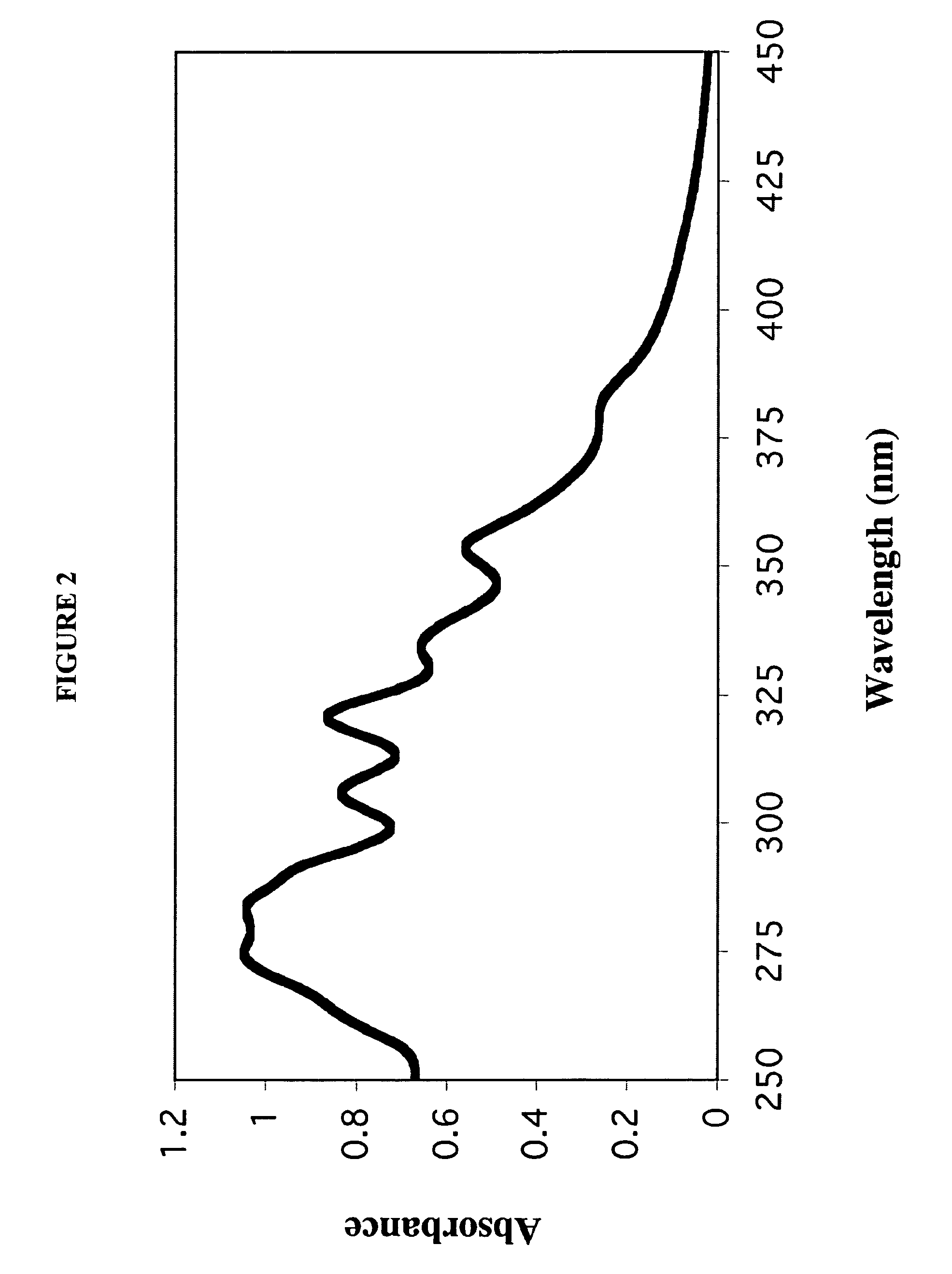
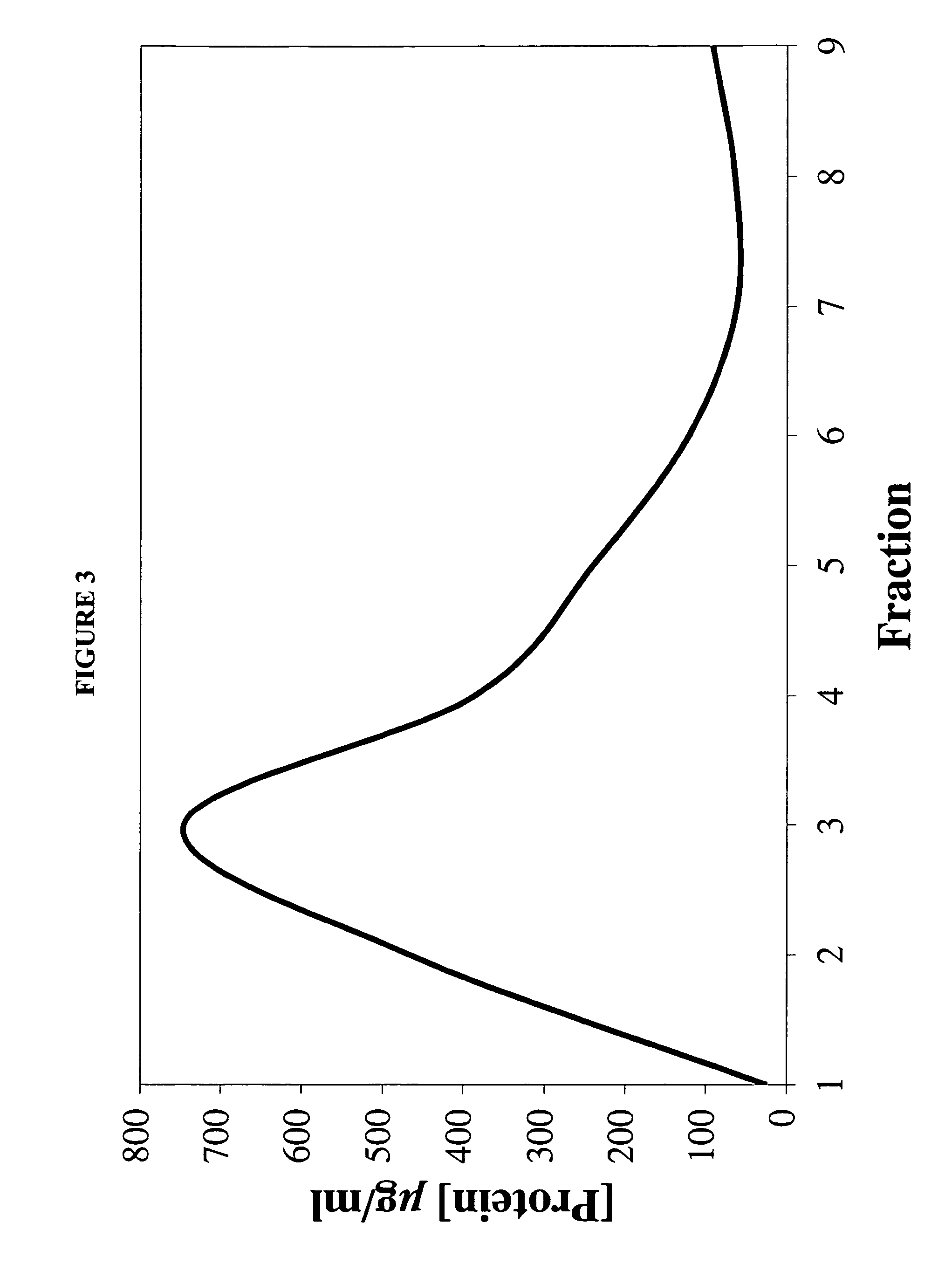
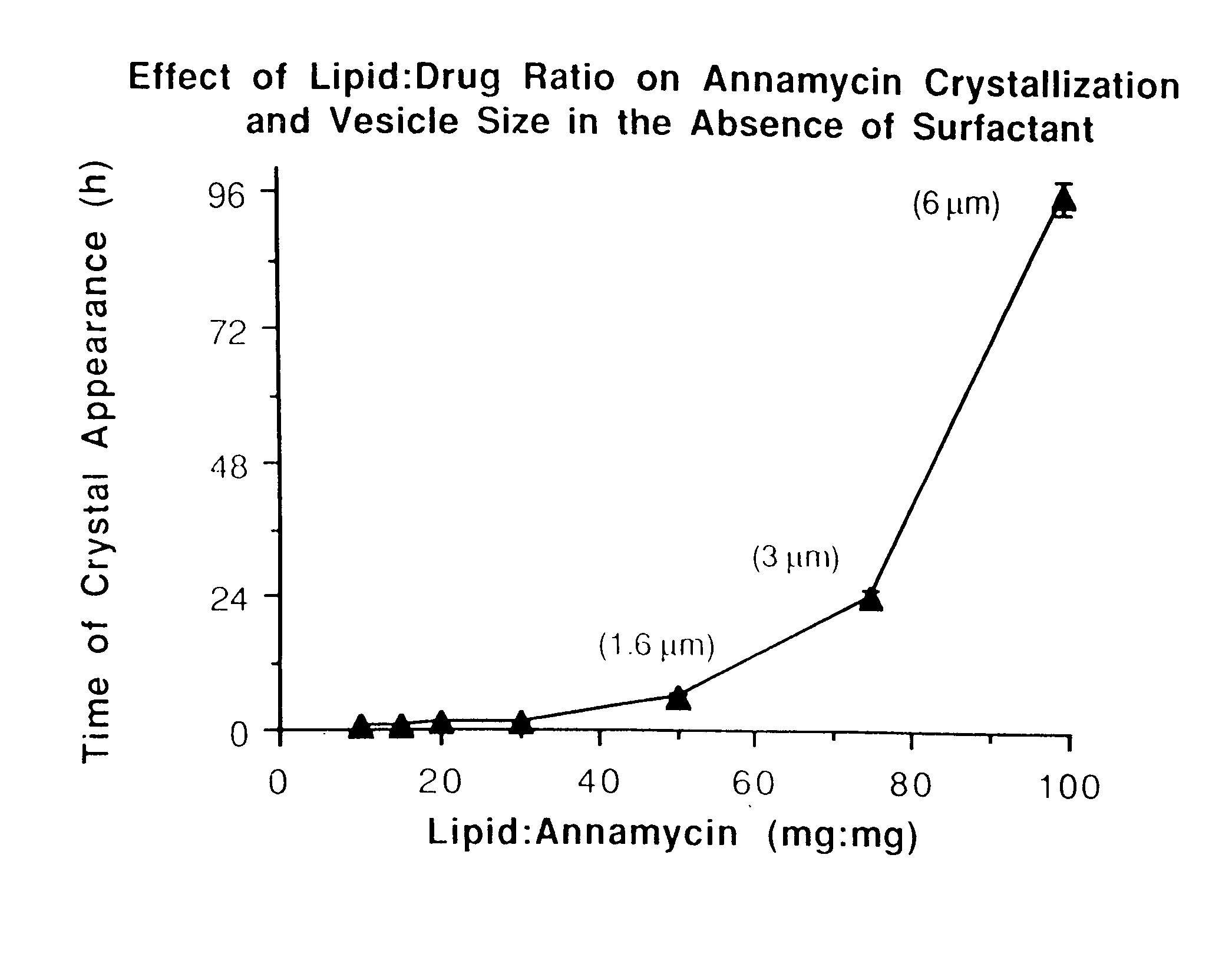
InactiveUS7238366B1Severe adverse effect on liposome integrityShorten hydration timePowder deliveryTetracycline active ingredientsLipid formationLiposome Vesicle
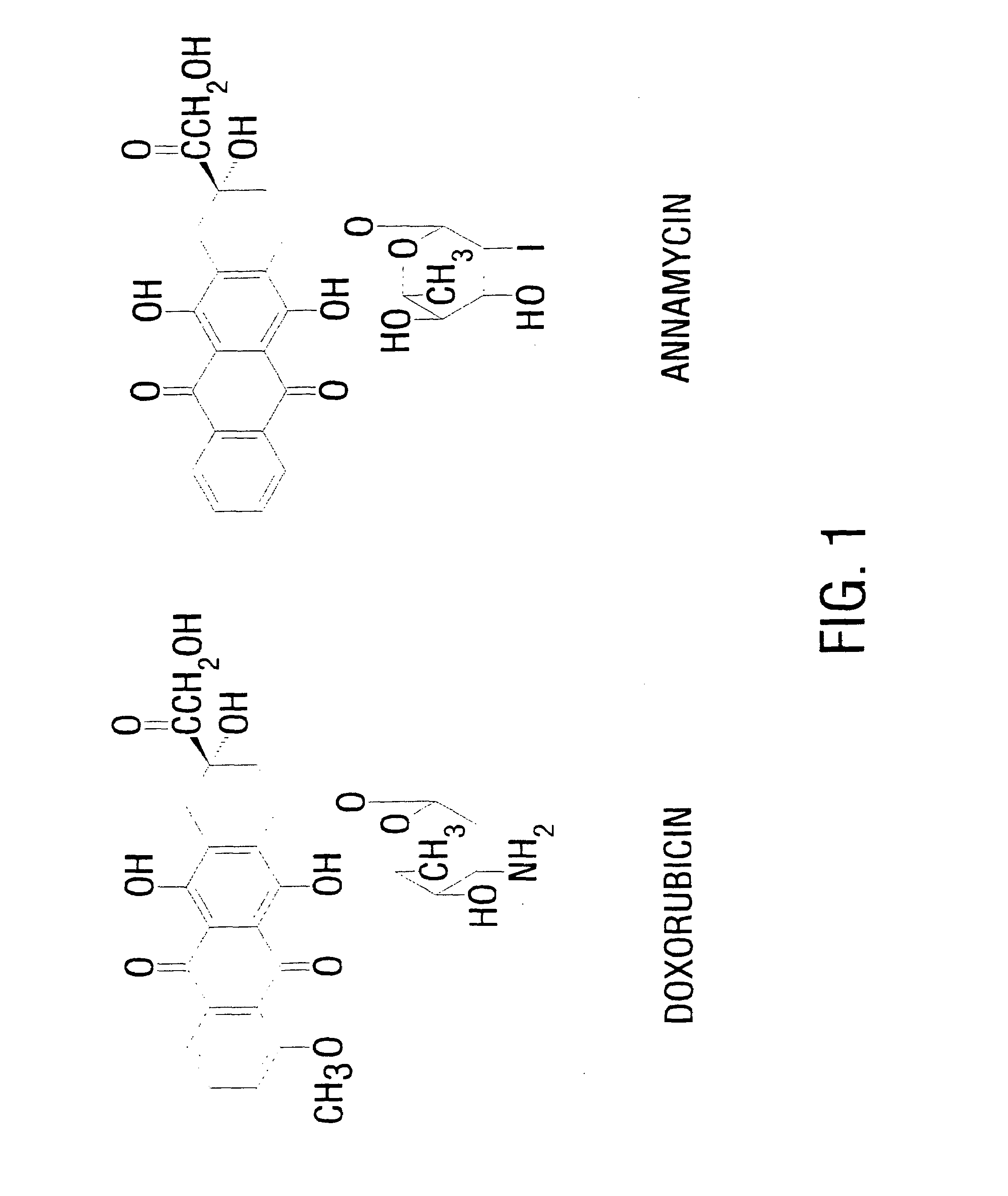
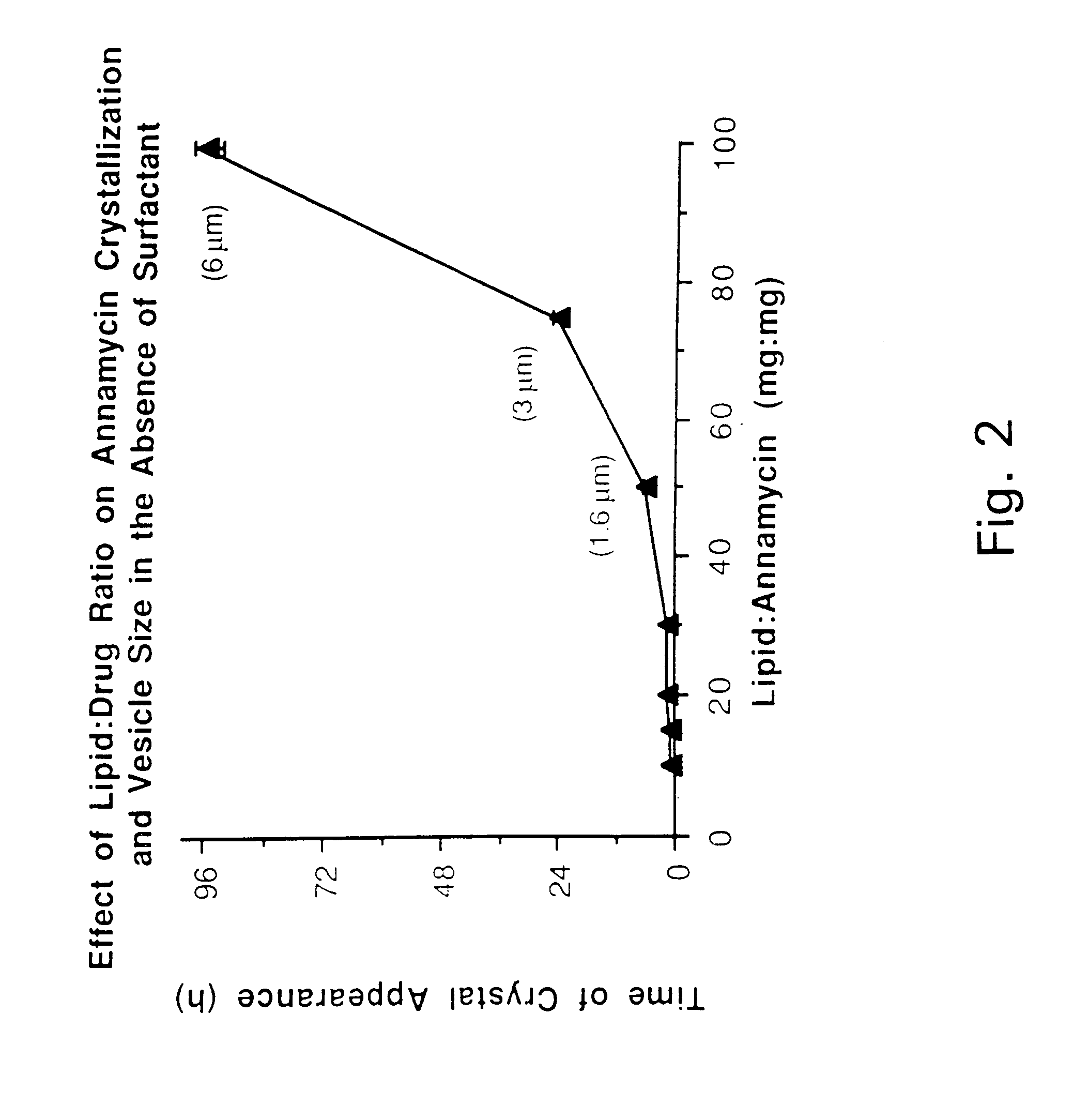
This invention provides an aqueous / t-butanol solvent-system, facile reconstitute, submicron-reconsitiute preliposome-lyophilaye and method of its preparation and use.In one embodiment this entails a modified method for the preparation of a submicron and stable liposome formulation of the non-cross-resistant anthracycline Annamycin is described. The optimal lipid composition was DMPC:DMPG at a 7:3 molar ratio and the optimal lipid:drug weight ratio 50:1. The selected formulation is a preliposome lyophilized powder that contains the phospholipids, Annamycin, and 1.7 mg Tween 20 per mg of Annamycin. The liposome suspension is obtained on the day of use by adding normal saline at 37° C. (1 ml per mg Annamycin) and hand-shaking for one minute. The presence of Tween 20 is essential in shortening the reconstitution step (from >2 hours to 1 minute), avoiding the early formation of free drug crystals, and reducing the median particle size (from 1.5 μm to 0.15-0.20 μm) without destruction of the liposome vesicles. The chemical stability of the preliposome powder at room temperature was >3 months and the chemical and physical stability of the liposome suspension at room temperature >24 hours. The in vitro cytotoxicity of the formulation was equivalent to that prepared by the standard evaporation method. The results of the study indicate that small amounts of surfactant may be used to enhance the reconstitution step and reduce the liposome size of lyophilized liposome formulations of lipophilic drugs.
Owner:BOARD OF REGENTS
Cosmetics
The cosmetic of the present invention is a cosmetic that alleviates skin irritation by blending in polypropylene glycol, a specific polar oil, or polybutylene glycol, as well as an ultraviolet absorbent. The present invention also relates to an agent and a method for alleviating irritation by lipophilic drugs. Since the present invention alleviates skin irritation due to ultraviolet absorbents and lipophilic drugs in cosmetics, any amount of ultraviolet absorbents and lipophilic drugs can be blended in cosmetics, and therefore cosmetics that can fully manifest their effects can be provided. Also, it is possible to prepare a safe sunblock cosmetic with superior ultraviolet protection effects because the ultraviolet absorbent is not absorbed through skin.
Owner:SHISEIDO CO LTD
Modulation of solubility, stability, absorption, metabolism, and pharmacokinetic profile of lipophilic drugs by sterols
ActiveUS20140011780A1Enhance biological absorption and metabolic stabilityPromote absorptionBiocideOrganic active ingredientsSolubilitySterol ester
A formulation for drug delivery, providing enhanced modulation of solubility, stability, absorption, metabolism, and / or pharmacokinetic profile of a lipophilic therapeutic agent by formulation with sterols and / or sterol esters, resulting in higher bioavailability of a therapeutic agent administered to a subject in need of such therapeutic agent. The formulation contains a therapeutic agent and a sterol or sterol ester, and can, optionally, further contain a solubilizer and / or an enhancing agent. Also described are pharmaceutical compositions containing the formulations and methods of making and methods of using the formulations and pharmaceutical compositions. Formulations of the disclosure can be constituted to minimize the synthesis of dihydrotestosterone when the therapeutic agent includes testosterone or testosterone esters.
Owner:MARIUS PHARMA LLC
Double-layer sustained and controlled release nanoparticle and preparation method thereof and application
ActiveCN103381146AImprove hydrophilic abilityImprove flexibilityPowder deliveryOrganic active ingredientsControlled releaseMedicine
The invention discloses a double-layer sustained and controlled release nanoparticle and a preparation method thereof and application in preparation of antitumor medicines. The double-layer sustained and controlled release nanoparticle is formed by a lactic acid-glycolic acid copolymer nanoparticle inner core and a polyethylene glycol questin grafting chitosan casing covering an outer layer of the lactic acid-glycolic acid copolymer nanoparticle inner core and can simultaneously load hydrophilic and lipophilic medicines. The drug entrapment rate is high, time-ordered release of inner layer medicines and outer layer medicines can be achieved, the double-layer sustained and controlled release nanoparticle has the effects of sustained release and controlled release, and in addition, the double-layer sustained and controlled release nanoparticle further has the advantages of being controllable in grain size, uniform in particle size distribution, smooth and round in forma and good in stability and dispersibility and the like.
Owner:XINXIANG MEDICAL UNIV
Pharmaceutical composition with enhanced bioavailability
InactiveUS20090130198A1Improve bioavailabilityAntibacterial agentsBiocideSelf emulsifyingEnhanced bioavailability
The invention pertains to a self-emulsifying pharmaceutical composition containing a lipophilic drug, a surfactant, and a hydrophilic carrier. The invention also provides a method for making the pharmaceutical composition for increasing the bioavailability of a drug by self-emulsification.
Owner:INNOPHARMAX
Iontophoretic transdermal drug delivery system
ActiveCN102430195AImprove compatibilityGood penetration enhancing effectElectrotherapyMedical devicesMacromolecular drugMiddle frequency
The invention discloses an iontophoretic transdermal drug delivery system which comprises a low and middle frequency pulse electronic generator, a conductor wire and a hydrosol pulse electrode; the low and middle frequency pulse electronic generator is connected with the hydrosol pulse electrode through the conductor wire; a drug carrier of the hydrosol pulse electrode of the system is prepared from a medical hydrosol and has the characteristics of good compatibility and permeability promoting property to drugs and good compatibility with human bodies; and the drug carrier can be used as a transdermal drug delivery carrier for macromolecular drugs, micromolecular drugs, hydrophilic drugs, lipophilic drugs and the like and can also be used as a transdermal drug delivery carrier of some biological drugs of polypeptide, protein and the like. Low and middle frequency pulse electrons generated by the system have a good permeability promoting action and a directional introduction function to the drugs.
Owner:太原市怀诚医疗器械有限公司
Paclitaxel mixed composition and water-in-oil type emulsion formulation for chemoembolization and preparation method thereof
InactiveCN1547469AHeavy metal active ingredientsOrganic active ingredientsAnticarcinogenWater soluble
The present invention relates to the paclitaxel mixed composition and water-in-oil type emulsion formulation for chemoembolization and preparation method thereof. The combinatory formulation of anticancer drugs can be easily prepared since the emulsion formulation of the present invention can include other hydrophilic or lipophilic drugs.
Owner:DAE HWA PHARMA
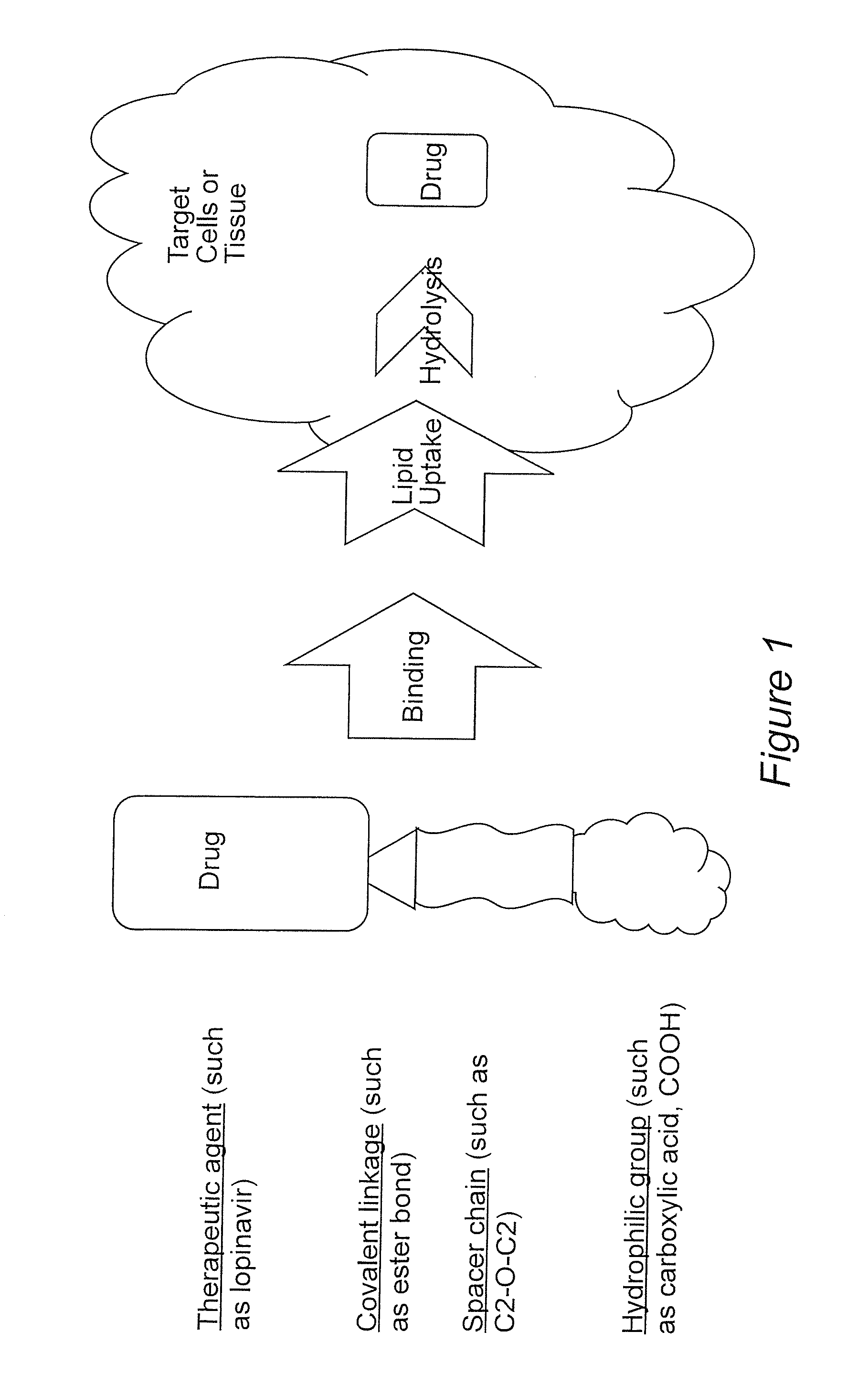
Prodrugs utilizing a transporter-directed uptake mechanism
Prodrugs comprising a lipophilic drug linked to a transport moiety that can be taken up by a fatty acid transporter are provided. The transport moiety comprises a lipid chain connected to a hydrophilic group (e.g. a carboxylic acid, a phosphate, or a sphingosine-like moiety). Due to the presence of the transport moiety, the prodrugs are substrates for endogenous fatty acid transporter systems. The transport moiety thus serves as a carrier or targeting moiety to facilitate uptake of the entire prodrug complex by endogenous fatty acid transporter systems, thereby moving the prodrug into cells and tissues where drug distribution and effects are desired. Hydrolysis of the chemical linkage between the lipid-like moiety and the lipophilic drug releases the drug in an active form within the cells or tissues.
Owner:VIRGINIA COMMONWEALTH UNIV
Pressure-sensitive adhesive and patch employing the same
InactiveUS20050053646A1Sufficient power and cohesive powerDesirable physical propertyNon-macromolecular adhesive additivesPharmaceutical non-active ingredientsPolymer scienceMethacrylic monomers
An adhesive which comprises aqueous and nonaqueous polymers suitable for holding an lipophilic drug, etc., and has tackiness and cohesiveness which are sufficient for the plaster of a patch; and a patch employing the adhesive. The adhesive contains a polymer which comprises one or more kinds of acrylic or methacrylic monomer units, at least one kind of the monomer units having a hydroxy group, and which has been crosslinked with a boron compound.
Owner:HISAMITSU PHARM CO INC
Nano lipid cubic crystal preparation and its preparation method and application
InactiveCN102274175AImprove stabilityAchieve autoclave sterilizationSenses disorderSolution deliveryLipid formationMedicine
The invention discloses a nanometer lipid cubic crystal preparation, which comprises fat-soluble medicine, amphiphilic lipid, stabilizer and water. The invention also discloses a preparation method of the nanometer lipid cubic crystal preparation and an application of the nanometer lipid cubic crystal in preparation of an ophthalmic drug preparation for treating ophthalmic diseases. The nano lipid cubic crystal preparation of the present invention can significantly improve the corneal penetration ability of drugs, has better stability than liposomes and nanoparticles, can realize high-pressure high-temperature sterilization, has good drug release characteristics, and has broad clinical applications and market prospects.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Peg-lipid conjugates for increasing the solubility of drug compounds
Diacyl lipid-polymer conjugates are used to enhance the solubility of lipophilic drugs in aqueous solution. The conjugates comprise a backbone, two lipophilic acyl groups and a hydrophilic polymer.
Owner:KELLER BRIAN CHARLES +1
Lipophilic drug delivery vehicle and methods of use thereof
The invention provides compositions and methods for delivery of a bioactive agent to an individual. Delivery vehicles are provided that include a bioactive agent in disc shaped particles that include one or more lipid binding polypeptides circumscribing the perimeter of a lipid bilayer in which the bioactive agent is localized. Chimeric lipid binding polypeptides are also provided and may be used to add additional functional properties to the delivery particles.
Owner:CHILDREN S HOSPITAL &RES CENT AT OAKLAN
Pharmaceutical formulation
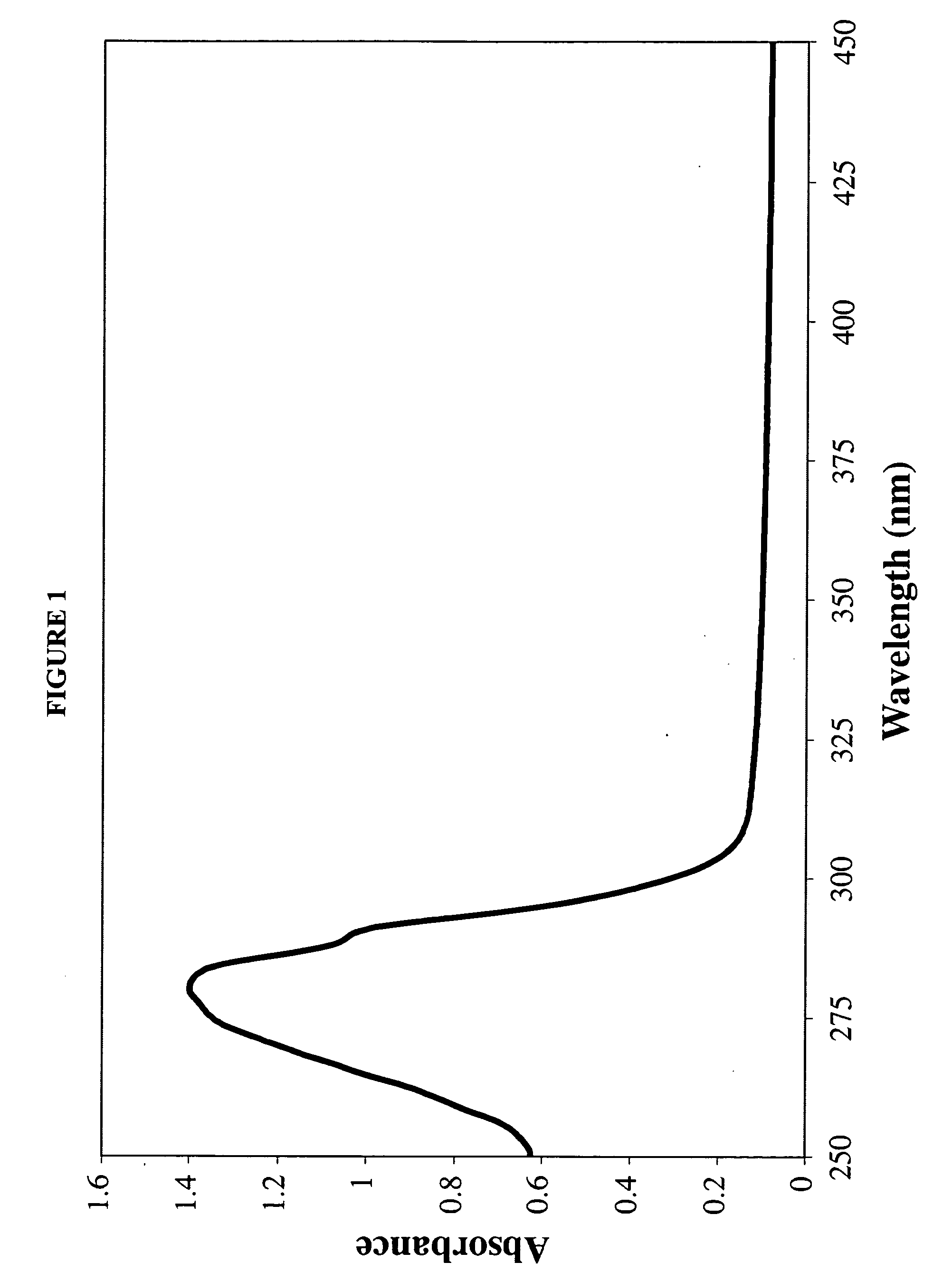
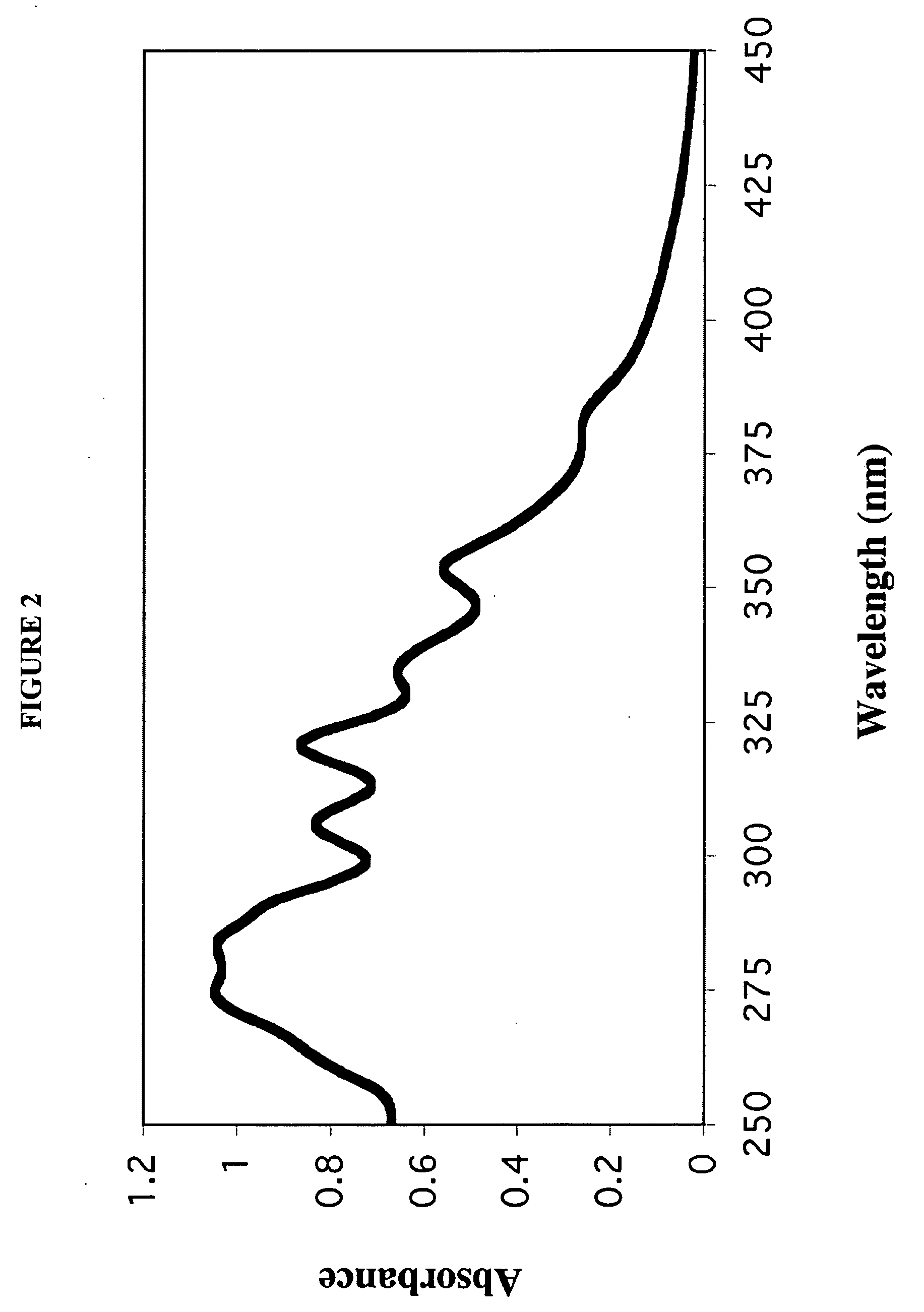
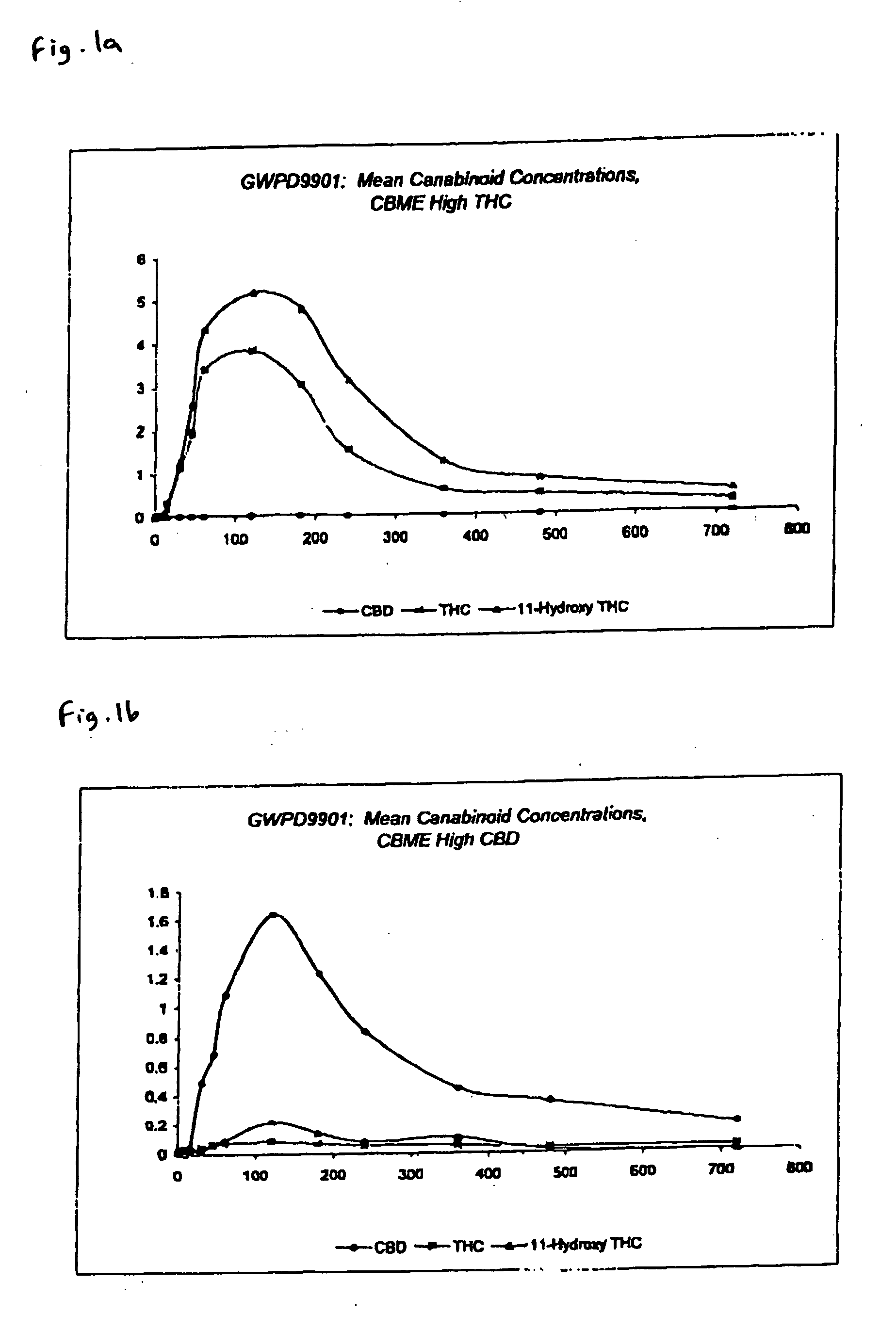
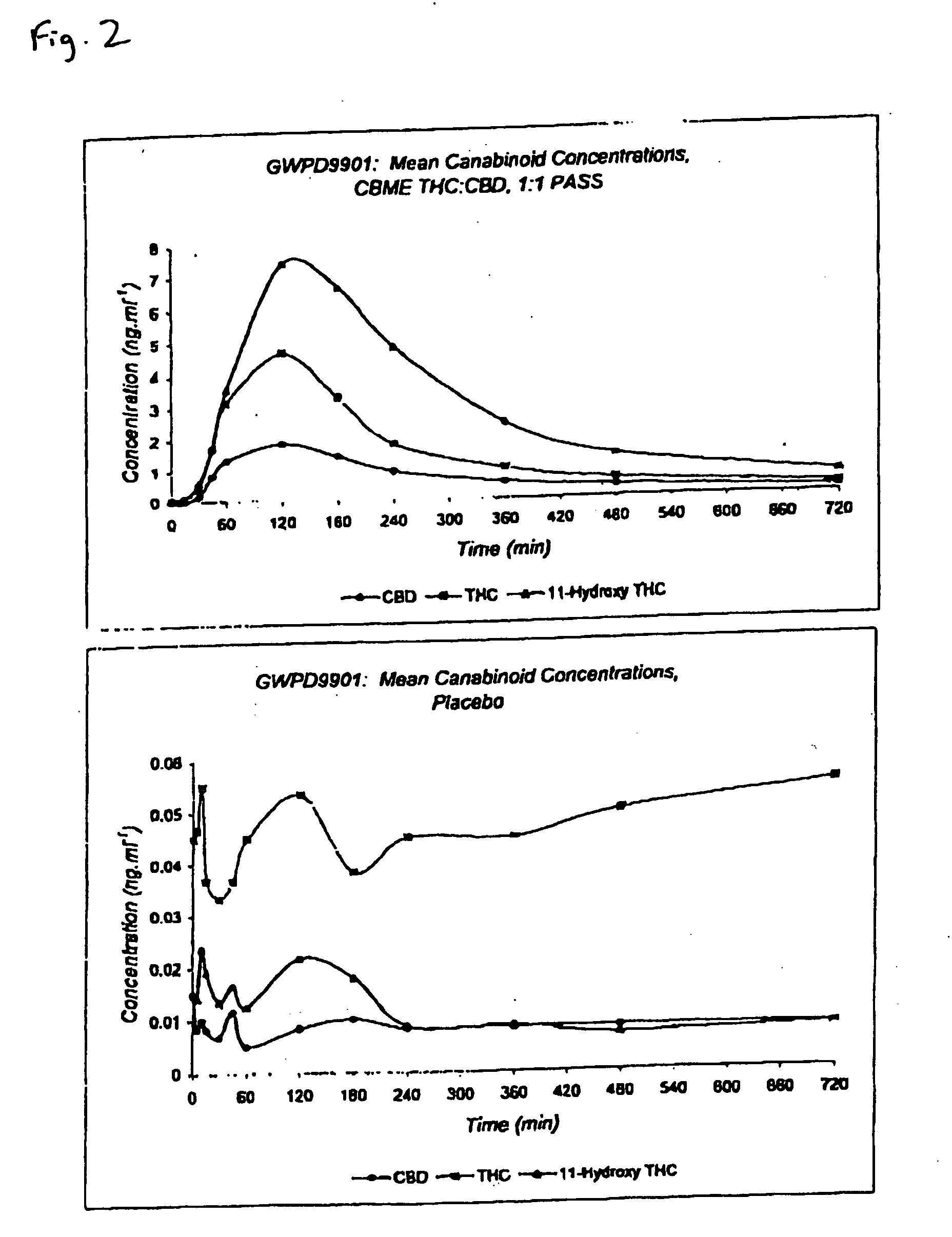
The invention relates to pharmaceutical formulations, and more particularly to formulations containing cannabinoids for administration via a pump action spray. In particular, the invention relates to pharmaceutical formulations, for use in administration of lipophilic medicaments via mucosal surfaces, comprising: at least one lipophilic medicament, a solvent and a co-solvent, wherein the total amount of solvent and co-solvent present in the formulation is greater than 55% wt / wt of the formulation and the formulation is absent of a self emulsifying agent and / or a fluorinated propellant.
Owner:GW RES LTD
Treatment methods requiring phyto-ingredients
ActiveUS20070196440A1Improve bioavailabilityAntibacterial agentsBiocideFood supplementPhytosterol esters
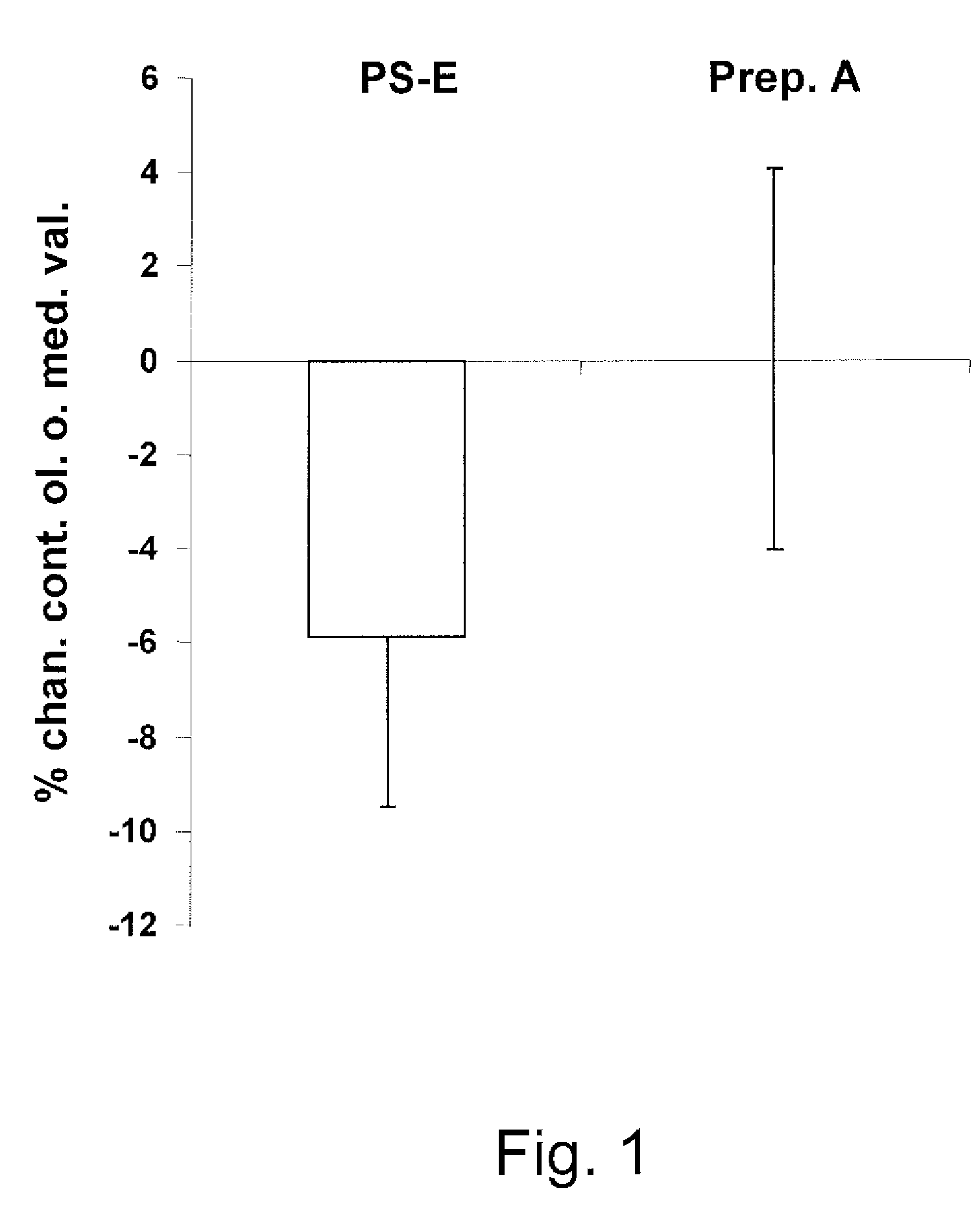
Methods of treatment of conditions which require phytosterol therapy without adversely affecting the bioavailability of lipophilic vitamins or lipophilic drugs, consisting of administering a mixture of phytosterol ester(s) (PS-E) and 1,3-diglyceride(s) (DAG) dissolved in an edible oil or fat are described herein, together with a method of improving weight management and a method of treating metabolic conditions that result in overweight. Dietary nutrients, food supplements and food articles containing said mixture are also described herein.
Owner:ENZYMOTEC
Preparation method and application of cyclodextrin-drug host-guest supramolecular modified hydrogel
InactiveCN109796607ADoes not affect function and effectAchieve sustained releaseProsthesisTissue repairAdditive ingredient
The invention discloses a preparation method and application of cyclodextrin-drug host-guest supramolecular modified hydrogel, which comprises the following steps: firstly, adopting cyclodextrin derivatives grafted with double bonds to include lipophilic drugs under the action of host-guest to form nano drug boxes which are uniformly dispersed under the condition of water phase; then introducing the nano drug boxes into a photocrosslinkable hydrogel solution to crosslink under ultraviolet light to form a corresponding liquid drug-carrying gel matrix. According to the invention, the crosslinking density, mechanical strength and drug-carrying density of the hydrogel can be adjusted by changing the grafting rate and mass fraction of the ingredients. The hydrogel has good biocompatibility andcan be used for three-dimensional culture of cells, can encapsulate stem cells with rapid proliferation and multiple differentiation potentials and achieves directional differentiation of the stem cells under the induction of drugs. The material is combined with stem cell therapy to achieve rapid tissue repair, which has obvious advantages.
Owner:SOUTH CHINA UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com