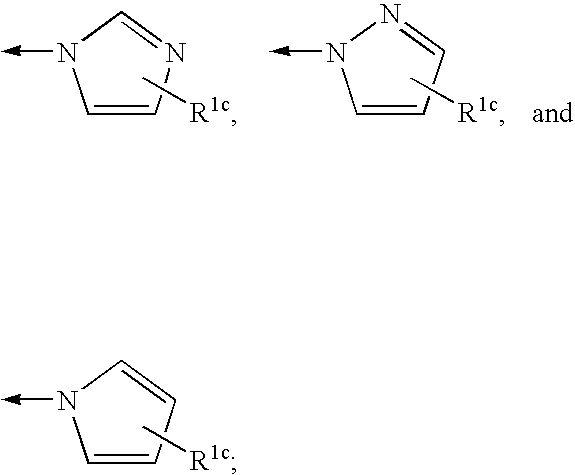
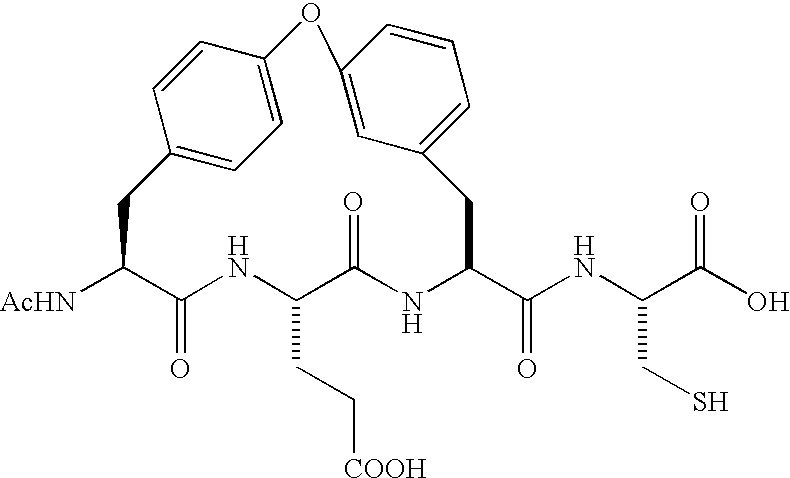
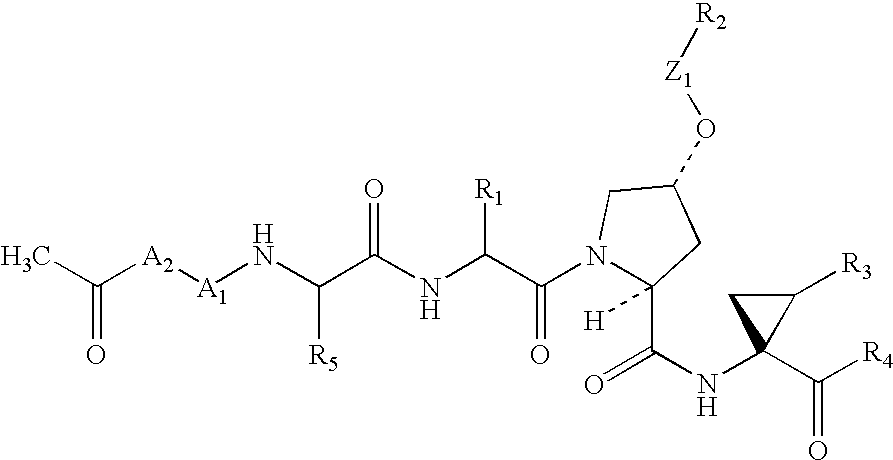
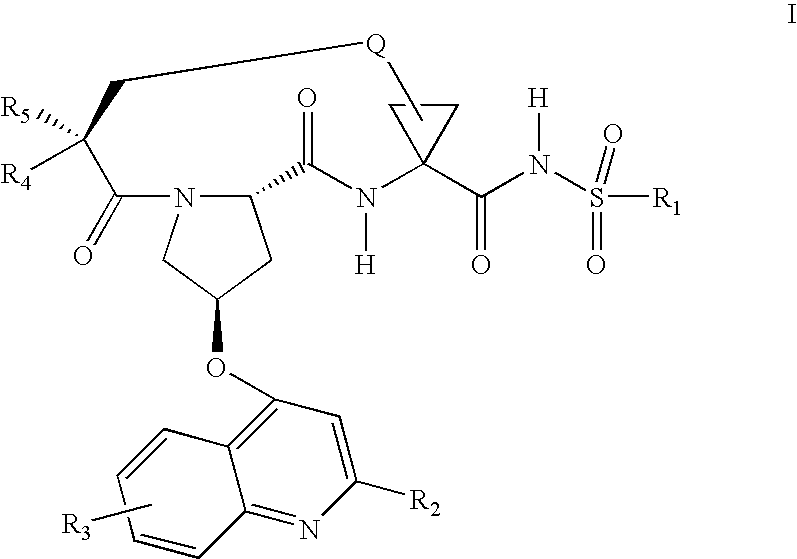
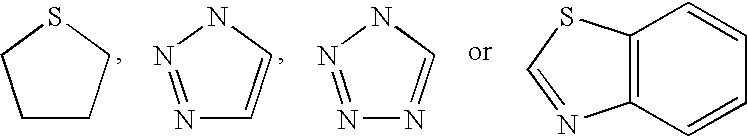
Patents
Literature
4980results about "Cyclic peptide ingredients" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pharmaceutical dosage forms for highly hydrophilic materials
Pharmaceutical dosage forms having a highly hydrophilic fill material and a shell encapsulating the fill material are disclosed and described. Generally, the shell has at least one plasticizing agent therein in order to provide the shell with an effective plasticity. In one aspect, the shell may have included therein an amount of plasticizing agent that is sufficient to provide the shell with an effective plasticity upon migration of a portion of the plasticizing agent into the fill material. In another aspect, the plasticizing agent may have a solubility in the fill material of less than about 10% w / w. In yet another aspect, a combination of a plasticizing agent, and a plasticizing agent having a solubility in the fill material of less than about 10% w / w, may be presented in a total amount sufficient to provide the shell with an effective plasticity upon migration of plasticizing agent into the fill material.
Owner:LIPOCINE
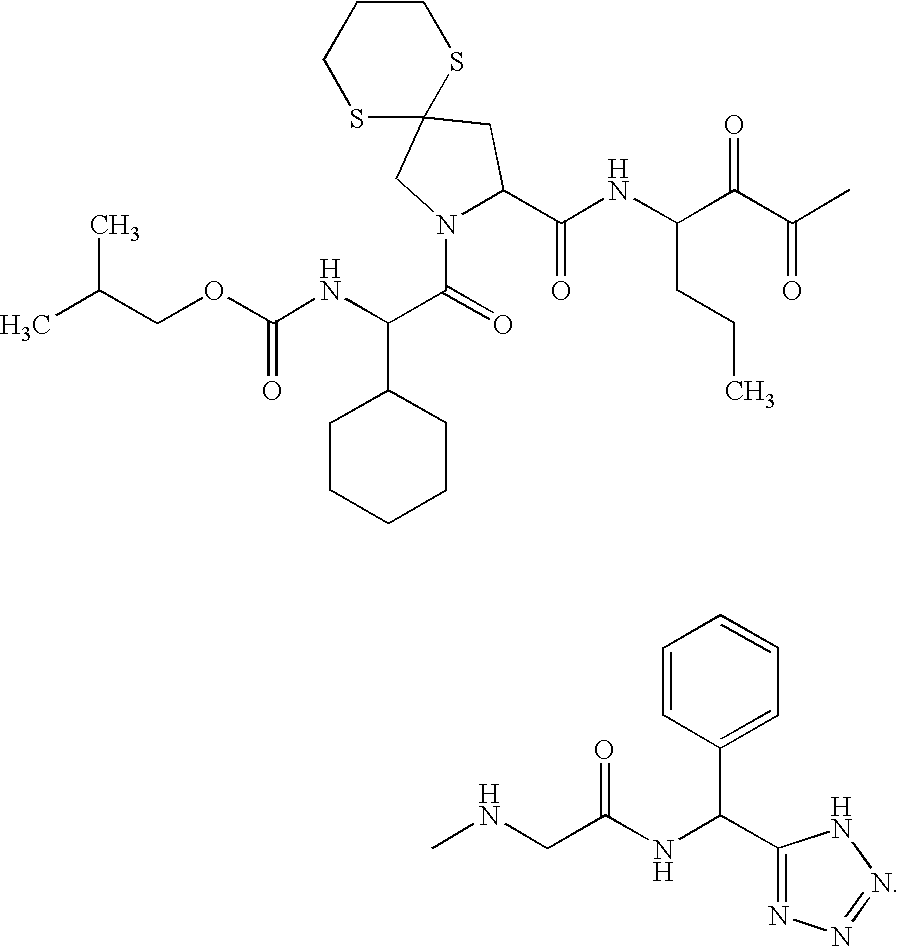
Cyclosporin analogues and their pharmaceutical uses
Owner:ALBANY MOLECULAR RESEARCH INC
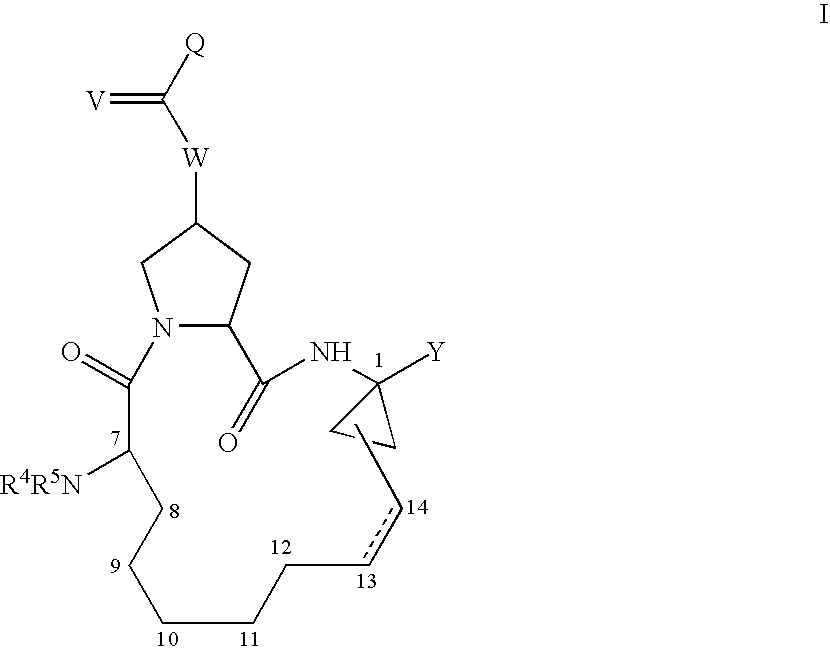
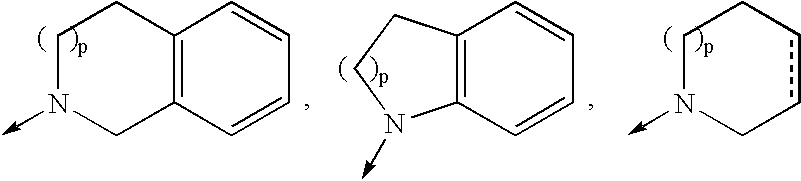
Macrocyclic hepatitis C serine protease inhibitors
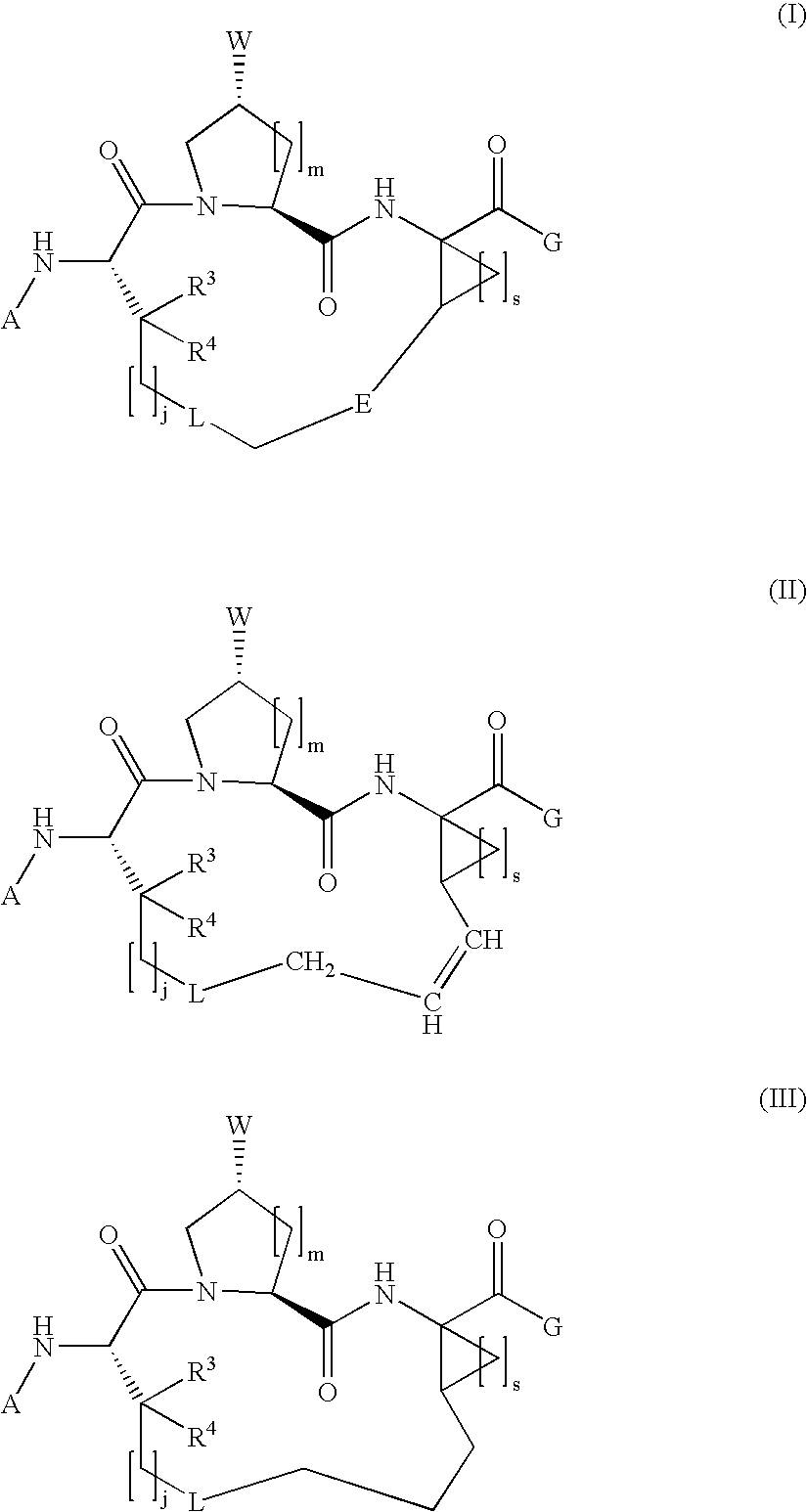
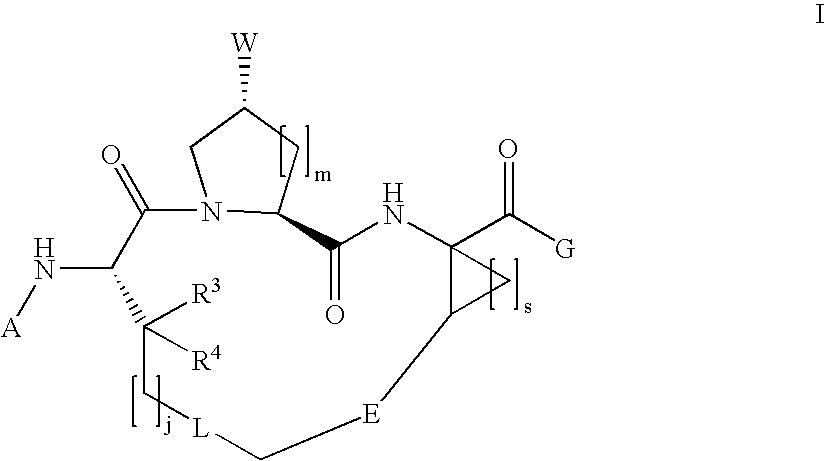
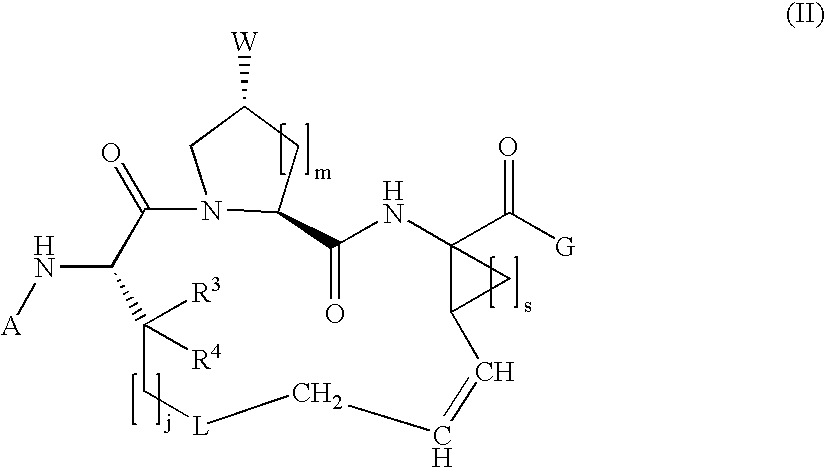
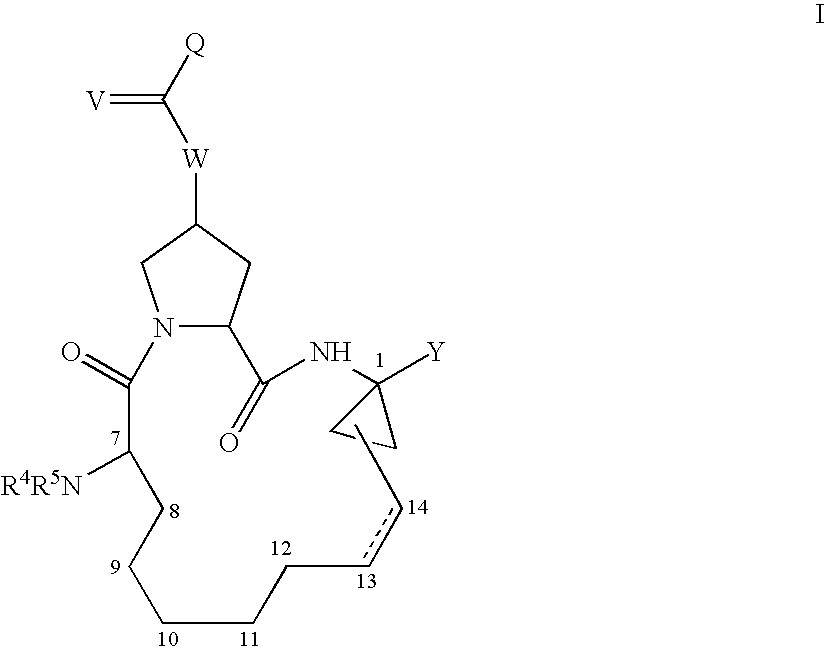
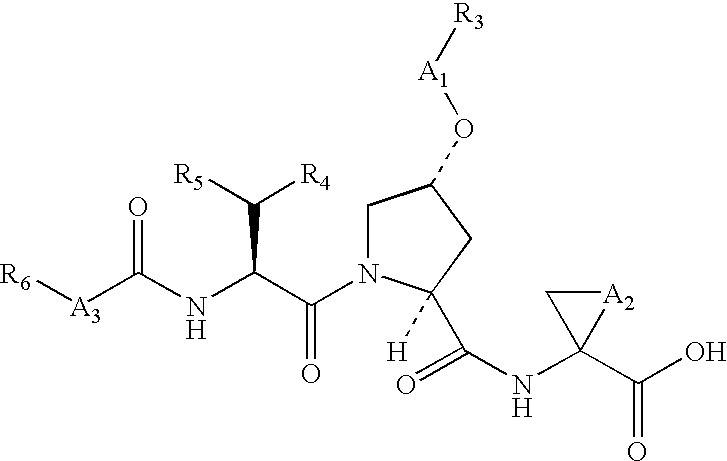
The present invention relates to compounds of Formula I, II or Ill, or a pharmaceutically acceptable salt, ester, or prodrug, thereof: wherein W is a substituted or unsubstituted heterocyclic ring system. The compounds inhibit serine protease activity, particularly the activity of hepatitis c virus (HCV) NS3-NS4A protease. Consequently, the compounds of the present invention interfere with the life cycle of the hepatitis c virus and are also useful as antiviral agents. The present invention further relates to pharmaceutical compositions comprising the aforementioned compounds for administration to a subject suffering from HCV infection. The invention also relates to methods of treating an HCV infection in a subject by administering a pharmaceutical composition comprising the compounds of the present invention.
Owner:ENANTA PHARM INC
Pharmaceutical formulations and systems for improved absorption and multistage release of active agents
InactiveUS7374779B2Improve bioavailabilityLow variabilityPowder deliveryOrganic active ingredientsActive agentFast release
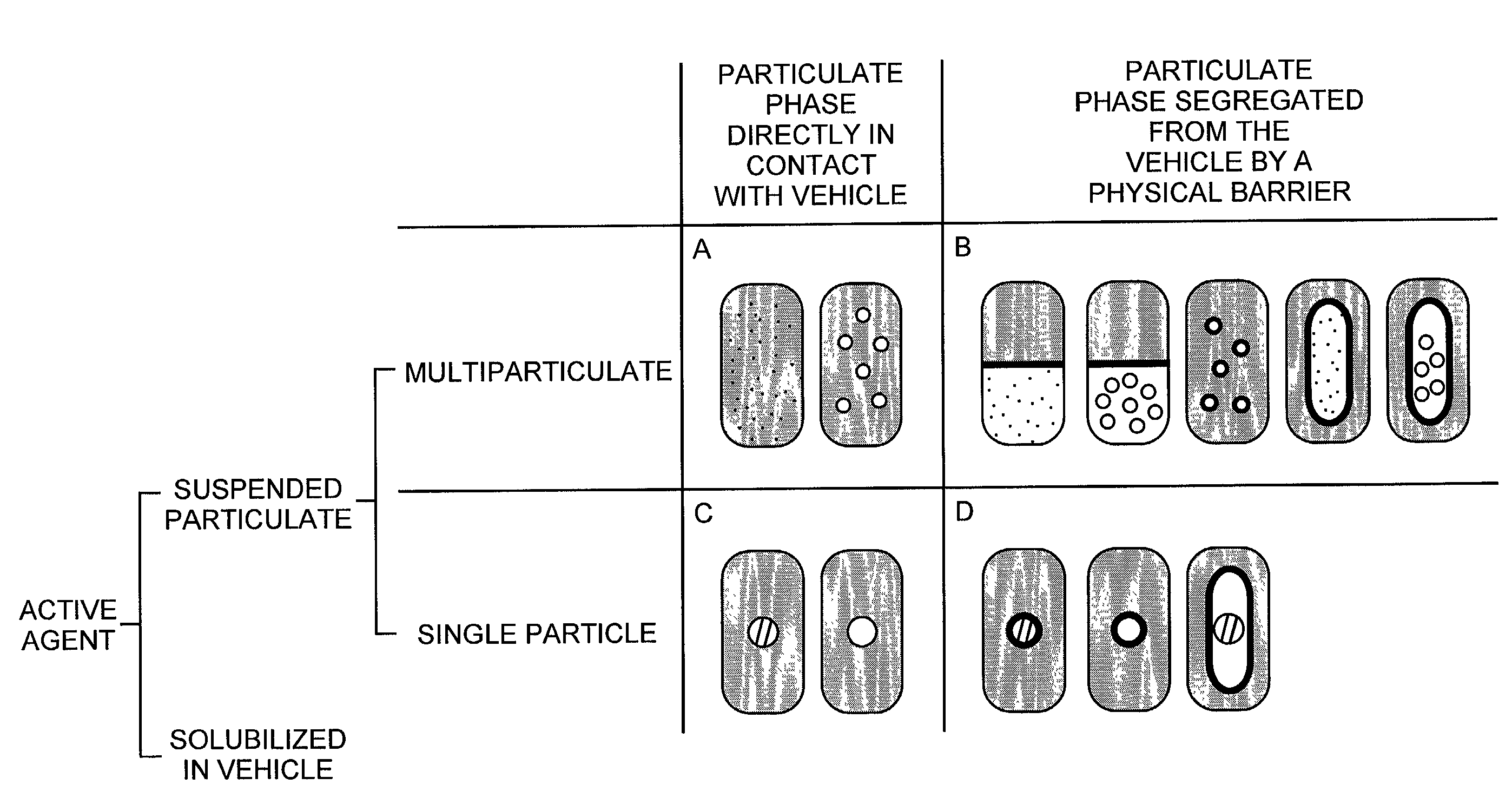
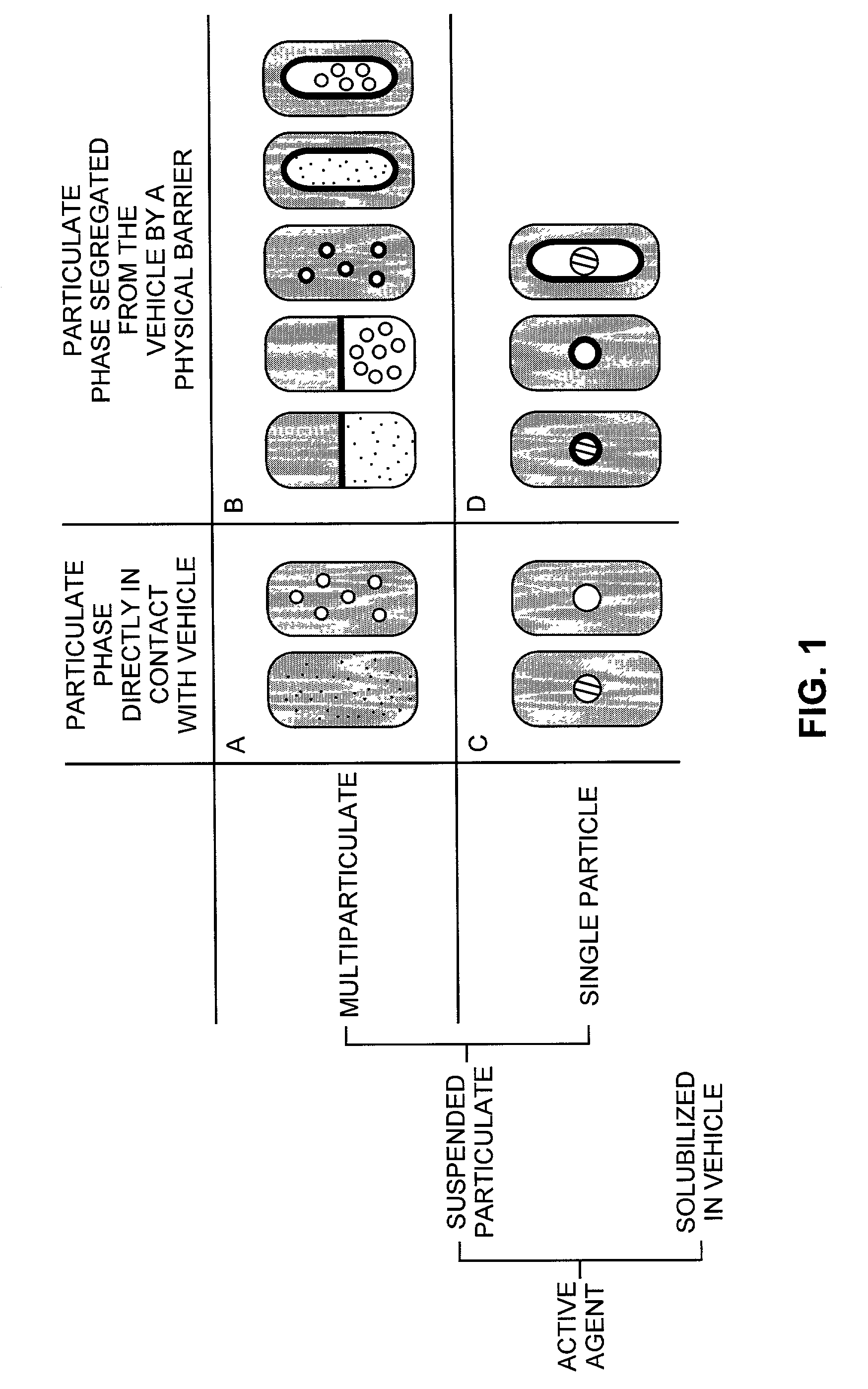
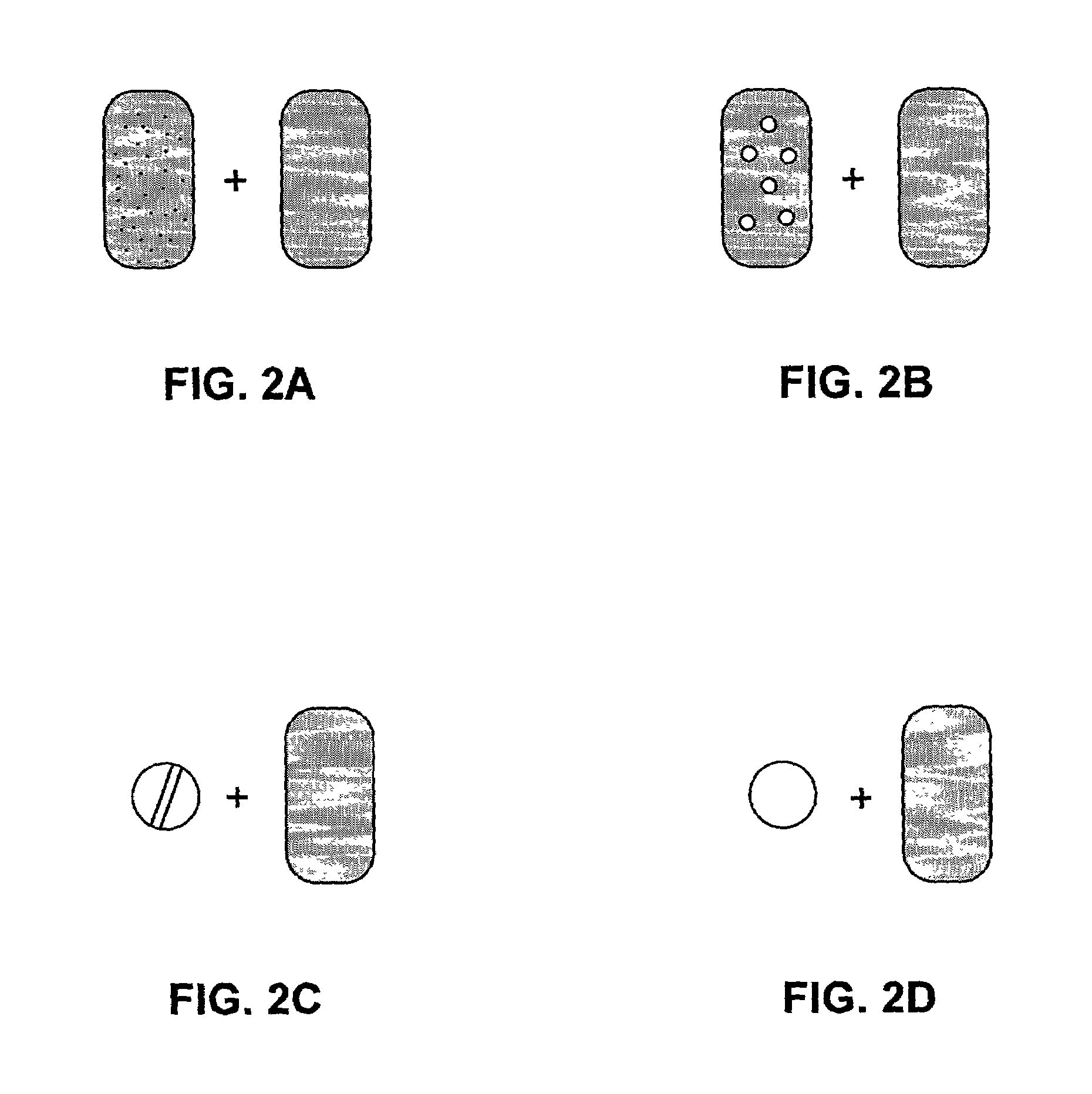
The present invention pertains to pharmaceutical formulations and systems for delivery of active agents, wherein a first fraction of an active agent is suspended in a vehicle and a second fraction of active agent is solubilized in the vehicle, with the suspended fraction representing about 5 wt. % to about 80 wt. % of the active agent and the second fraction representing about 20 wt. % to about 95 wt. % of the active agent. One or more additional active agents, which may be fully solubilized, partially solubilized, or suspended, may also be present. The first and second fractions of the active agent may or may not have different release profiles. Generally, a significant fraction of the solubilized drug will release rapidly, providing for rapid onset, while the suspended drug may be formulated for delayed and / or sustained release.
Owner:LIPOCINE
Pharmaceutical compositions for lipophilic drugs
InactiveUS7070802B1Good self-emulsifying performanceShelf-stableCyclic peptide ingredientsCapsule deliveryMonoglycerideCyclosporins
Stable solutions of lipophilic drugs, such as cyclosporin, forming a polar lipid self-emulsifying drug delivery system. The solutions can include lipophilic drugs, such as cyclosporin, dissolved in a polar lipid, such as having a C6-C12 fatty acid monoglyceride content of at least about 50%, surfactants and triglycerides. The composition forms a fine emulsion on exposure to water. The encapsulated dosage form of this composition needs neither a hydrophilic component nor air-tight blister packaging, and is particularly suitable for oral administration.
Owner:WATSON LAB INC
Macrocyclic compounds as inhibitors of viral replication
The embodiments provide compounds of the general formulas I-XIX, as well as compositions, including pharmaceutical compositions, comprising a subject compound. The embodiments further provide treatment methods, including methods of treating flaviviral infection, including hepatitis C virus infection and methods of treating liver fibrosis, the methods generally involving administering to an individual in need thereof an effective amount of a subject compound or composition.
Owner:F HOFFMANN LA ROCHE & CO AG
Oleaginous pharmaceutical and cosmetic foam
ActiveUS20050031547A1Pleasant and easy to spreadPatient compliance is goodAntibacterial agentsCosmetic preparationsActive agentNon ionic
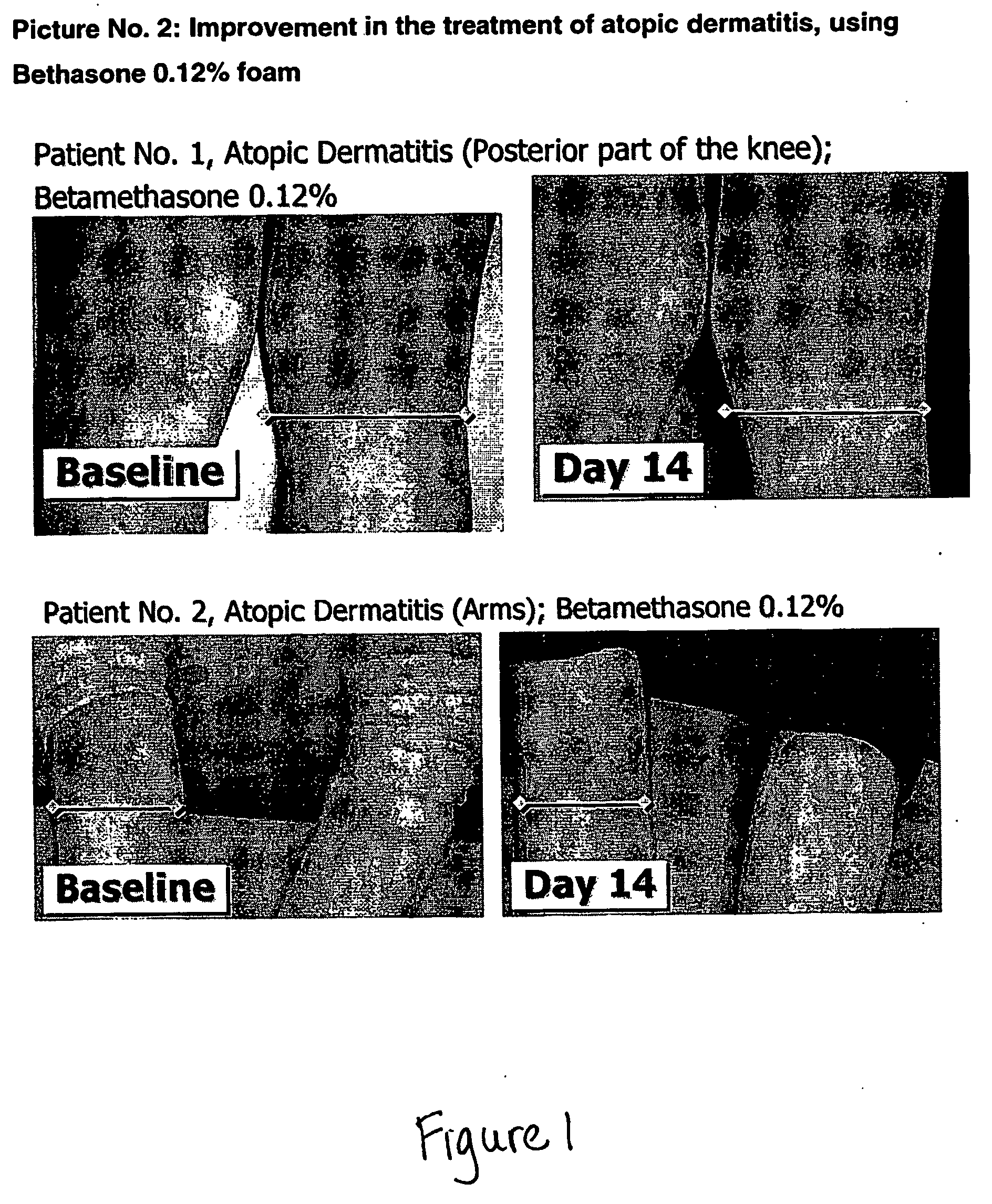
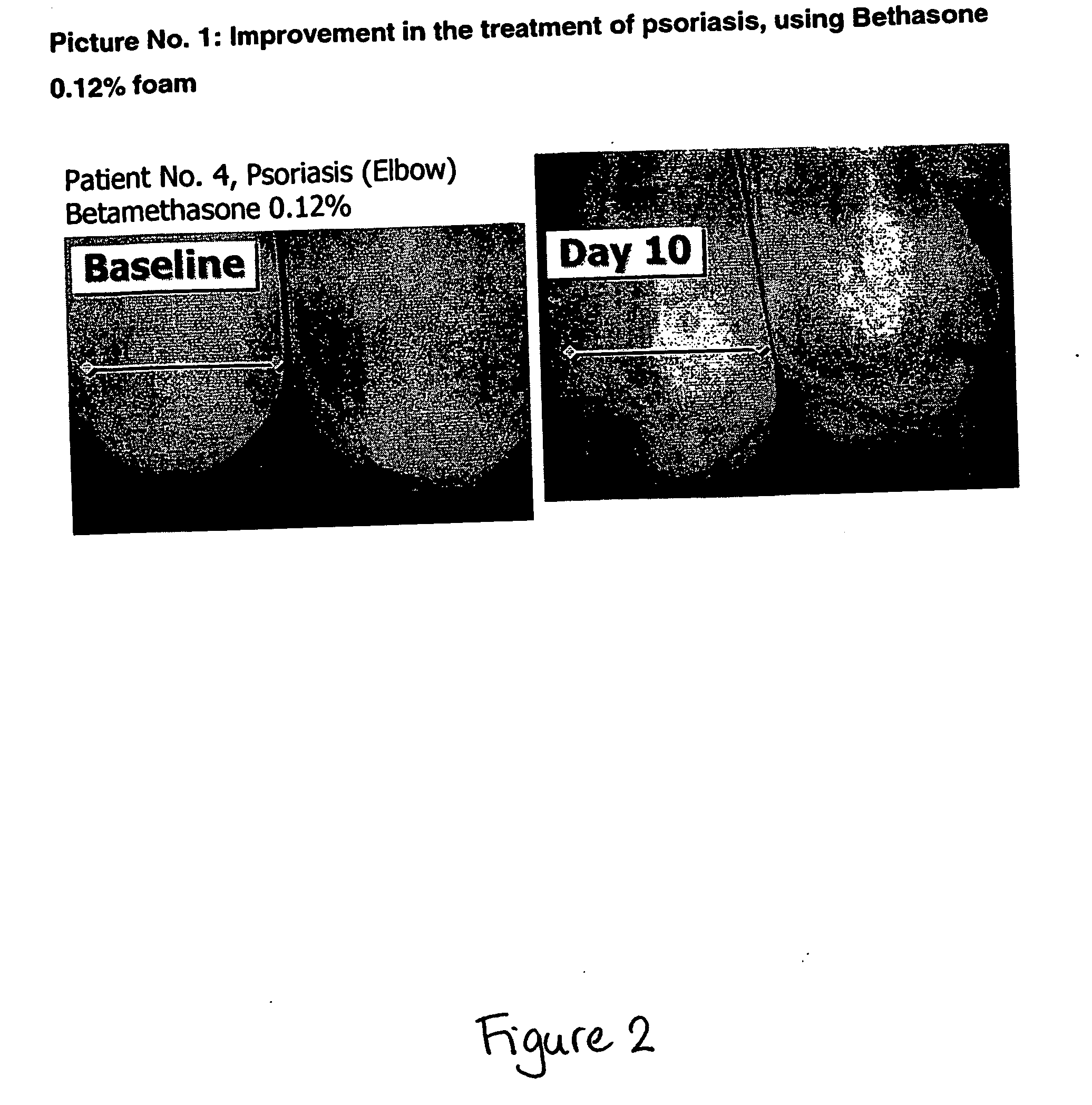
The invention relates to stable oleaginous cosmetic or therapeutic foam compositions containing certain active agents, having unique therapeutic properties and methods of treatment using such compositions. The foamable composition includes at least one solvent selected from a hydrophobic solvent, a silicone oil, an emollient, a co-solvent, and mixtures thereof, wherein the solvent is present at a concentration of about 70% to about 96.5% by weight of the total composition, at least a non-ionic surface-active agent at a concentration of about 0.1% to less than about 10% by weight of the total composition; at least one gelling agent at a concentration of about 0.1% to about 5% by weight of the total composition; a therapeutically effective amount of at least one active agent; and at least one liquefied or compressed gas propellant, at a concentration of about 3% to about 25% by weight of the total composition.
Owner:VYNE THERAPEUTICS INC
Methods and topical formulations comprising colloidal metal for treating or preventing skin conditions
In preferred embodiments, the present invention relates to compositions comprising colloidal metals and / or metals for the treatment and prevention of skin conditions and / or diseases. More specifically, the disclosed metal containing compositions are useful as antioxidants, anti-aging agents, anti-wrinkle agents, anti-peroxidation agents, antimicrobial agents, anti-inflammatory agents, pain-relieving agents, wound recovery agents, sun-screens, sunblocks, and integument and skin-supporting agents when applied to the skin / integument, or administered generally to an animal or human body.
Owner:MARGULIES JOEL +1
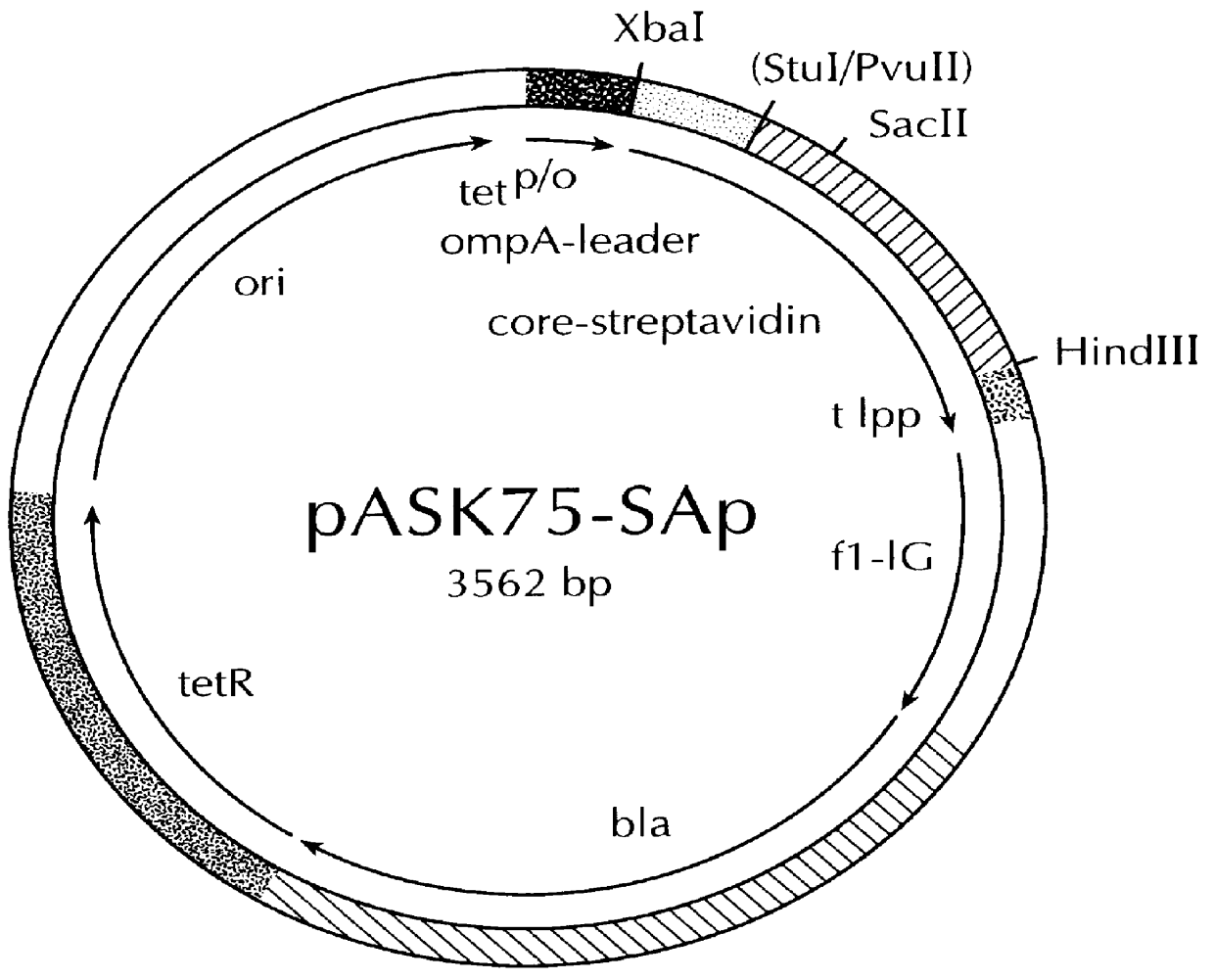
Streptavidin muteins
InactiveUS6103493AHigh affinityElution can be checked visuallyBacteriaAntibody mimetics/scaffoldsPeptide ligandSubject matter
The invention concerns a polypeptide selected from muteins of streptavidin which is characterized in that it (a) contains at least one mutation in the region of the amino acid positions 44 to 53 with reference to wild type-(wt)-streptavidin and (b) has a higher binding affinity than wt-streptavidin for peptide ligands comprising the amino acid sequence Trp-X-His-Pro-Gln-Phe-Y-Z in which X represents an arbitrary amino acid and Y and Z either both denote Gly or Y denotes Glu and Z denotes Arg or Lys. In addition nucleic acids coding for the polypeptide, a vector containing this nucleic acid, a cell transfected with the vector as well as the use of a polypeptide in a method for the isolation, purification or determination of proteins are disclosed. Yet a further subject matter is a reagent kit containing the polypeptide.
Owner:INST FUR BIOANALYTIC
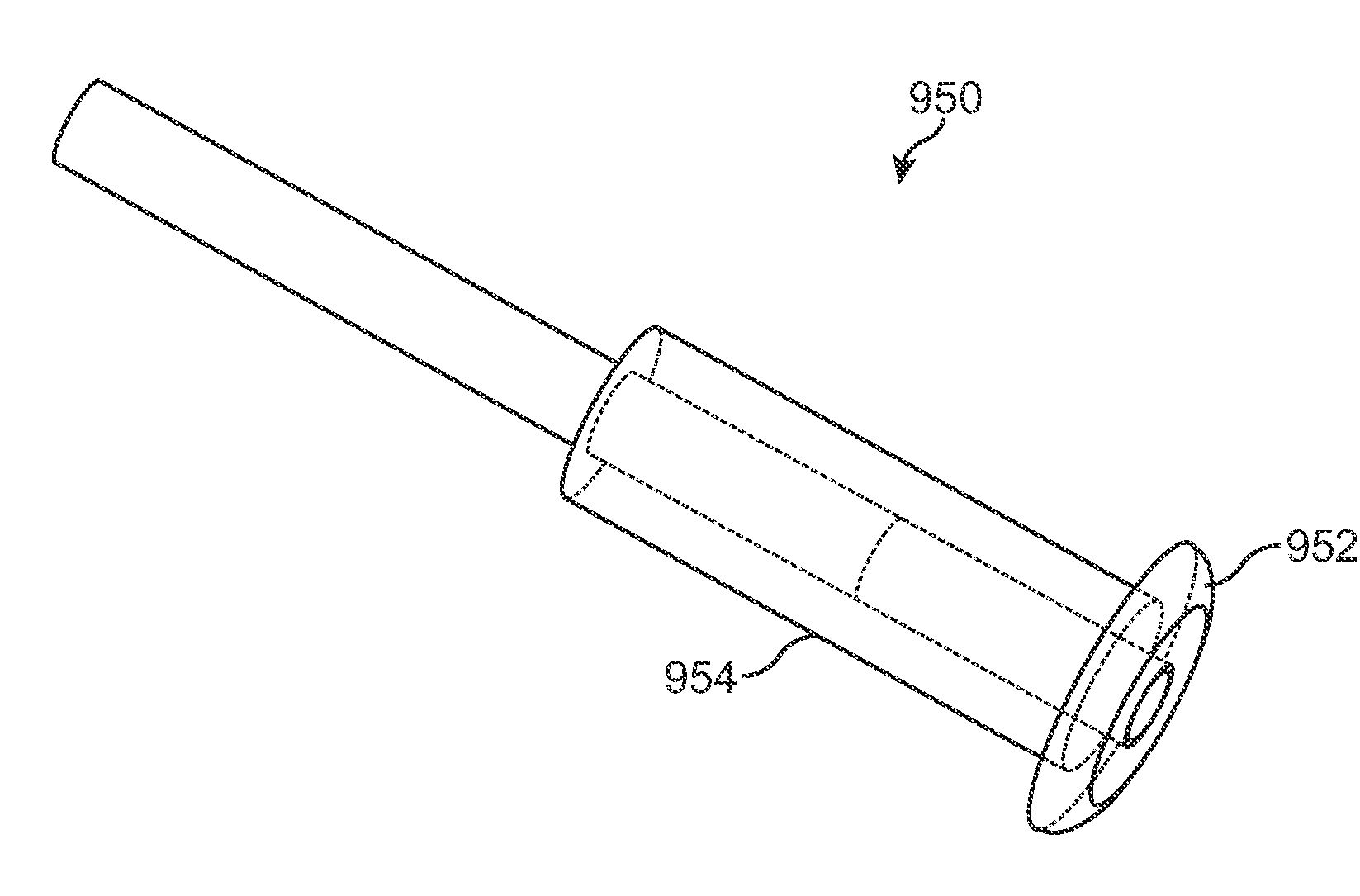
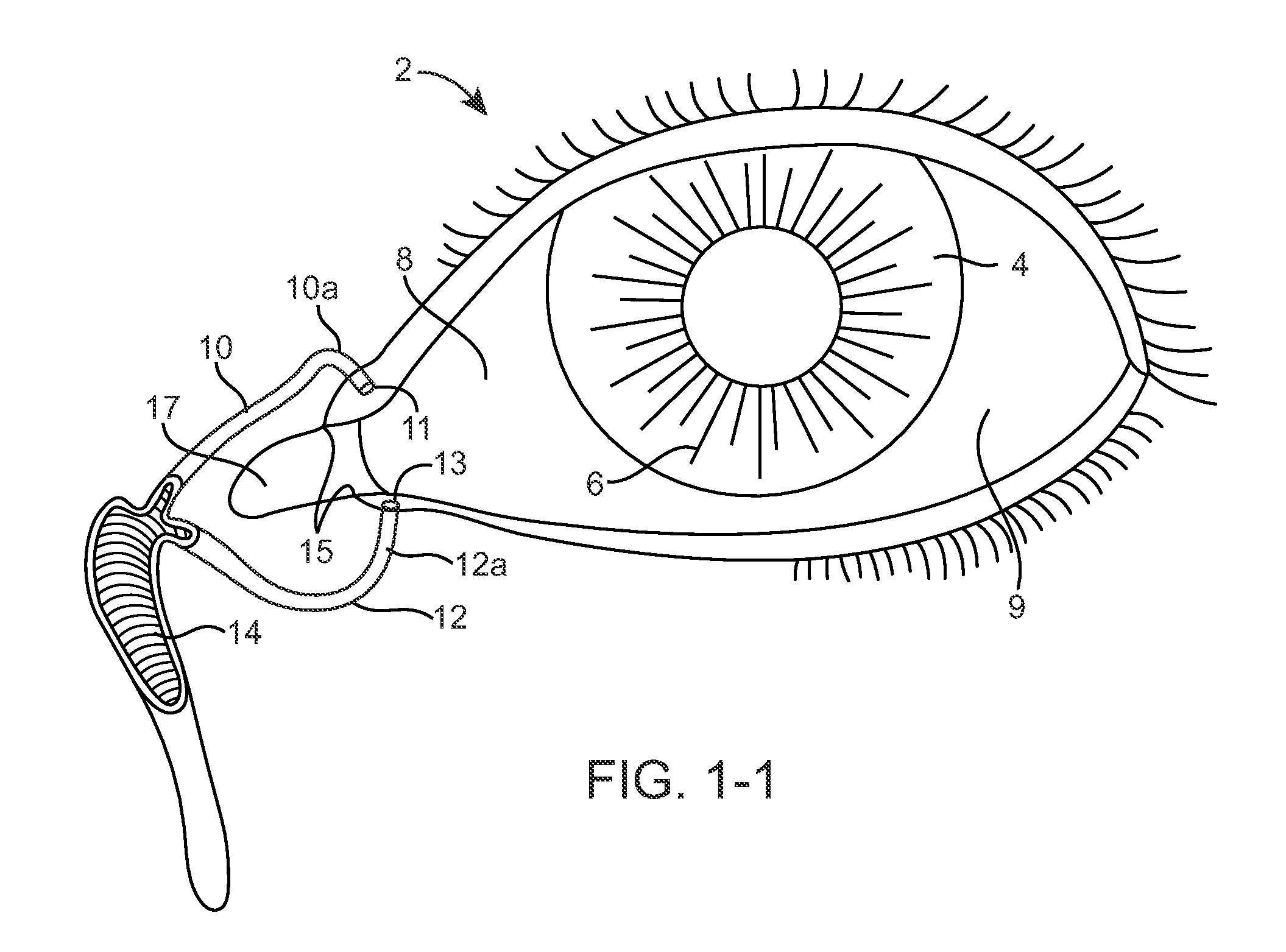
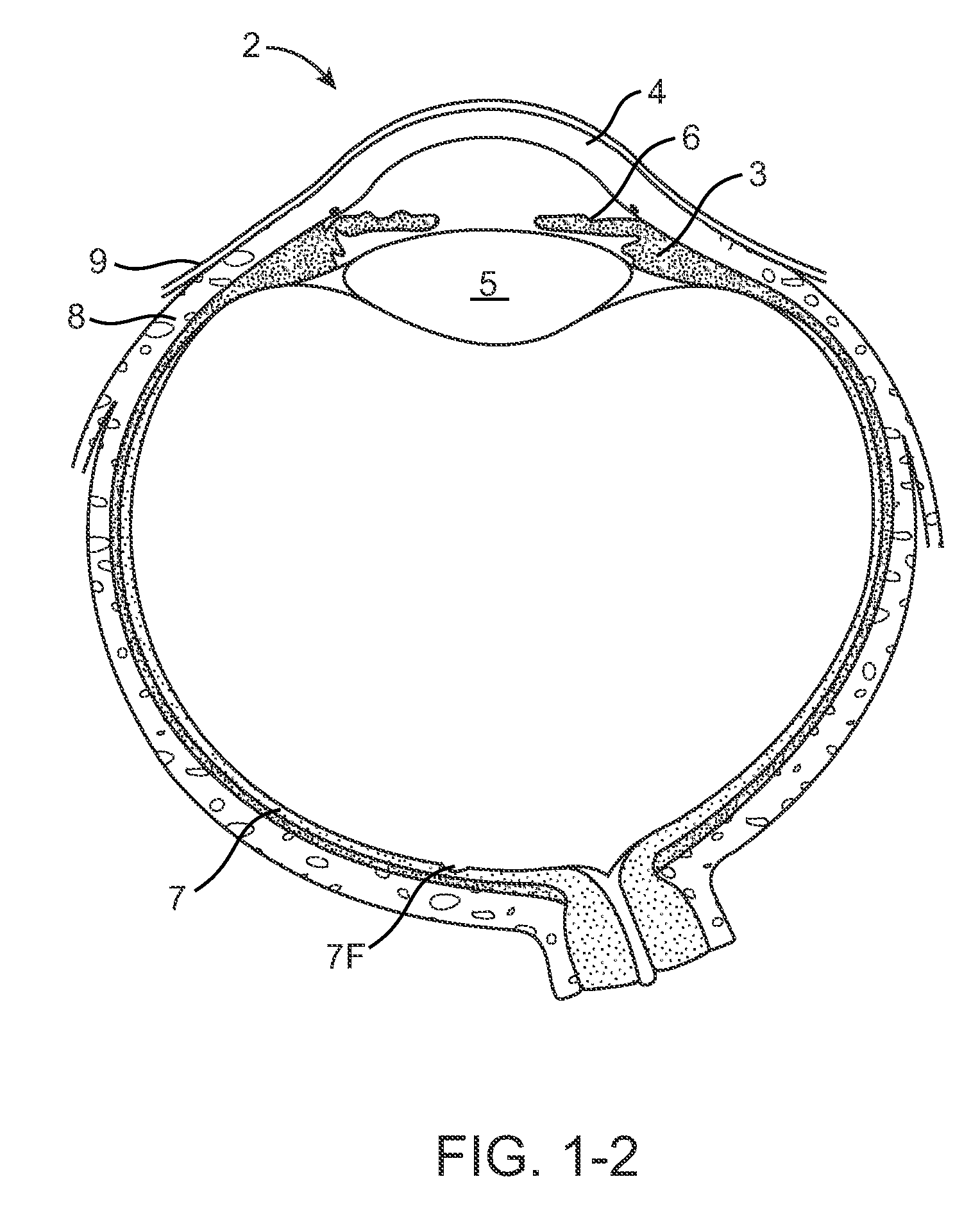
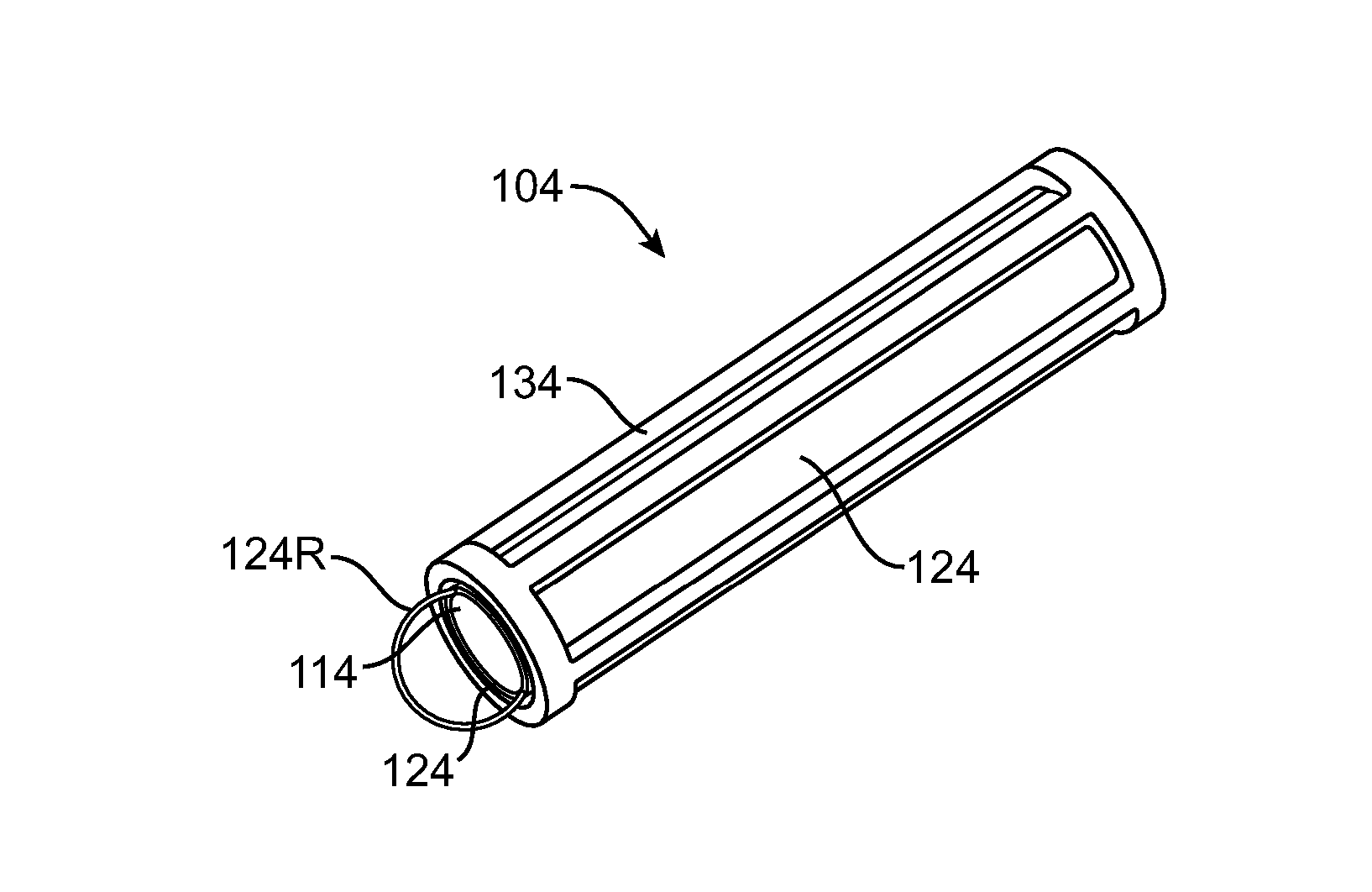
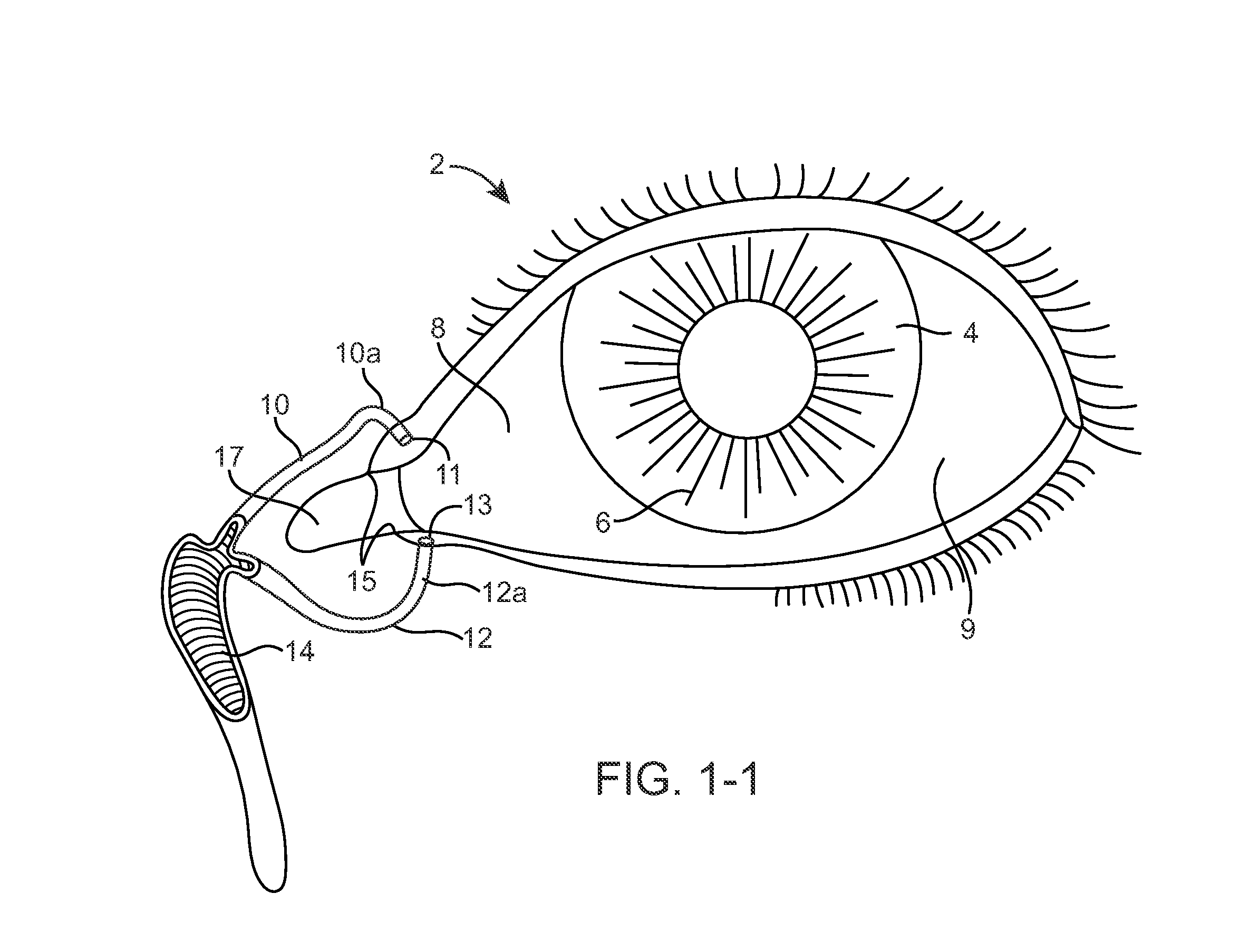
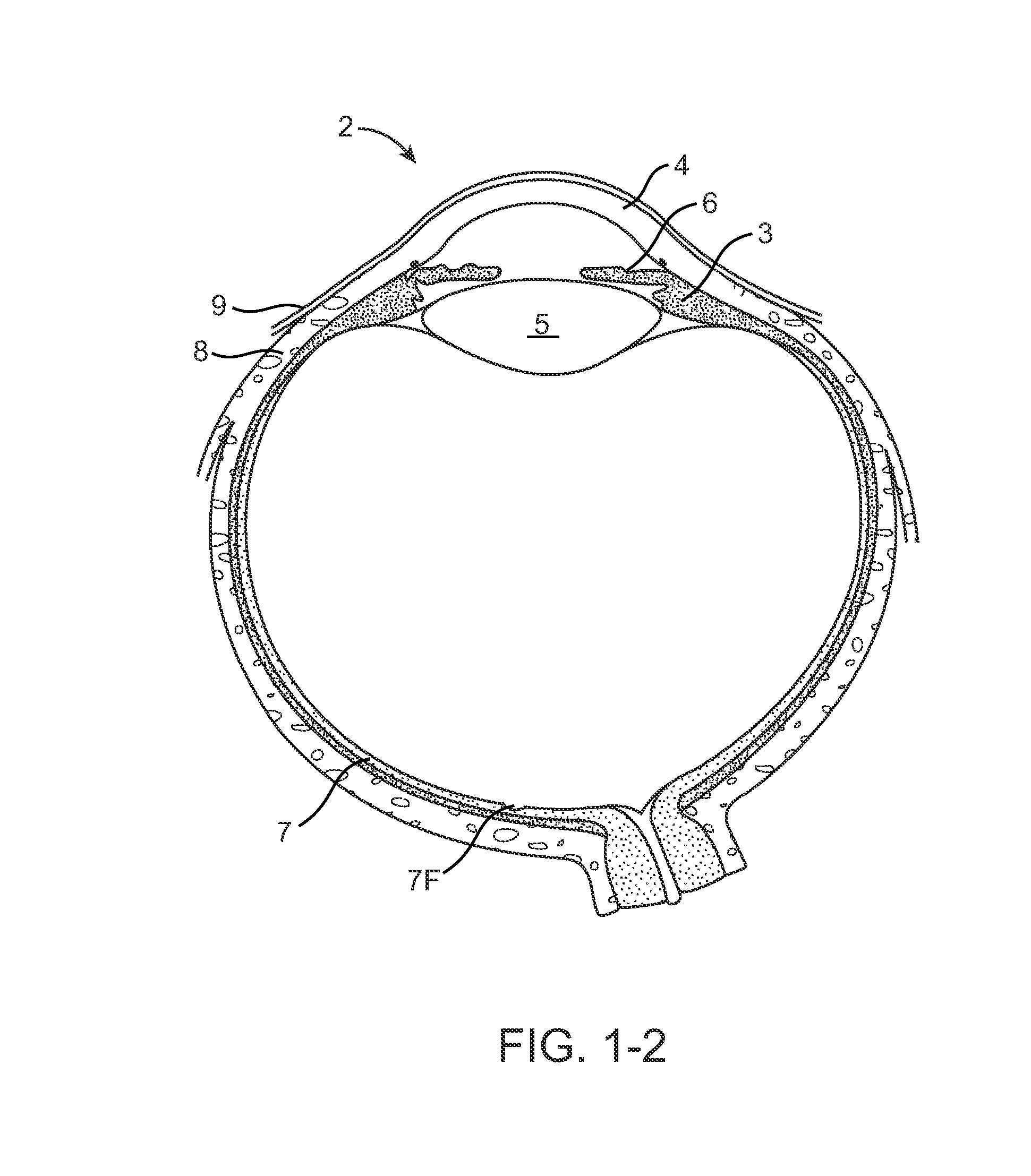
Nasolacrimal Drainage System Implants for Drug Therapy
ActiveUS20070243230A1Reduce deliveryAvoid flowAntibacterial agentsSenses disorderShape-memory alloyImplanted device
Implant devices, systems and methods for insertion into a punctum of a patient optionally comprises a drug core and a sheath body disposed over the drug core. The drug core includes a therapeutic agent deliverable into the eye, and the sheath defines at least one exposed surface of the drug core. The exposed surface(s) of the drug core may contact a tear or tear film fluid and release the therapeutic agent at therapeutic levels over a sustained period when the implant is implanted for use. The implant may include a retention element to retain the drug core and sheath body near the punctum, optionally comprising a shape memory alloy that can resiliently expand. An occlusive element may be attached to the retention element to at least partially occlude tear flow through the canalicular lumen.
Owner:MATI THERAPEUTICS
Spontaneous emulsions containing cyclosporine
A pharmaceutical composition contains cyclosporine as the active ingredient. More specifically, the composition is an orally administered pharmaceutical formulation in the form of a spontaneous emulsion comprising cyclosporine, ethanol ethyl oleate and polyoxyethylene glycerol trioleate. A method for preparing an orally administered pharmaceutical composition involves first dissolving cyclosporine in ethanol. Polyoxyethylene glycerol trioleate and an oil component are then added, mixed and diluted in an aqueous media to form a spontaneous emulsion.
Owner:WOCKHARDT EU OPERATIONS SWISS
Conveniently implantable sustained release drug compositions
InactiveUS20080038316A1Economical and practical and efficientEasy to produceBiocideOrganic active ingredientsDiseaseSustained release drug
This invention provides for biocompatible and biodegradable syringeable liquid, implantable solid, and injectable gel pharmaceutical formulations useful for the treatment of systemic and local disease states.
Owner:RAMSCOR
Drug Delivery Methods, Structures, and Compositions for Nasolacrimal System
ActiveUS20070269487A1Avoid expulsionInhibition releaseAntibacterial agentsPowder deliveryEffective treatmentBiomedical engineering
Owner:MATI THERAPEUTICS
Methods of using and compositions comprising immunomodulatory compounds for the treatment and management of asbestos-related diseases and disorders
Methods of treating, preventing and managing an asbestos-related disease or disorder are disclosed. Specific embodiments encompass the administration of an immunomodulatory compound, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof, alone or in combination with a second active agent and / or chemotherapy, surgery, or radiation therapy. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in the methods of the invention are also disclosed.
Owner:CELGENE CORP
Macrocyclic NS3-serine protease inhibitors of hepatitis C virus comprising N-cyclic P2 moieties
The present invention discloses novel macrocyclic compounds which have HCV protease inhibitory activity as well as methods for preparing such compounds. In another embodiment, the invention discloses pharmaceutical compositions comprising such macrocycles as well as methods of using them to treat disorders associated with the HCV protease.
Owner:SCHERING CORP
Composition and methods for treatment of neurological disorders and neurodegenerative diseases
InactiveUS6187756B1Increased formationPromote activationBiocideElcosanoid active ingredientsDiseaseGlial fibrillary acidic protein
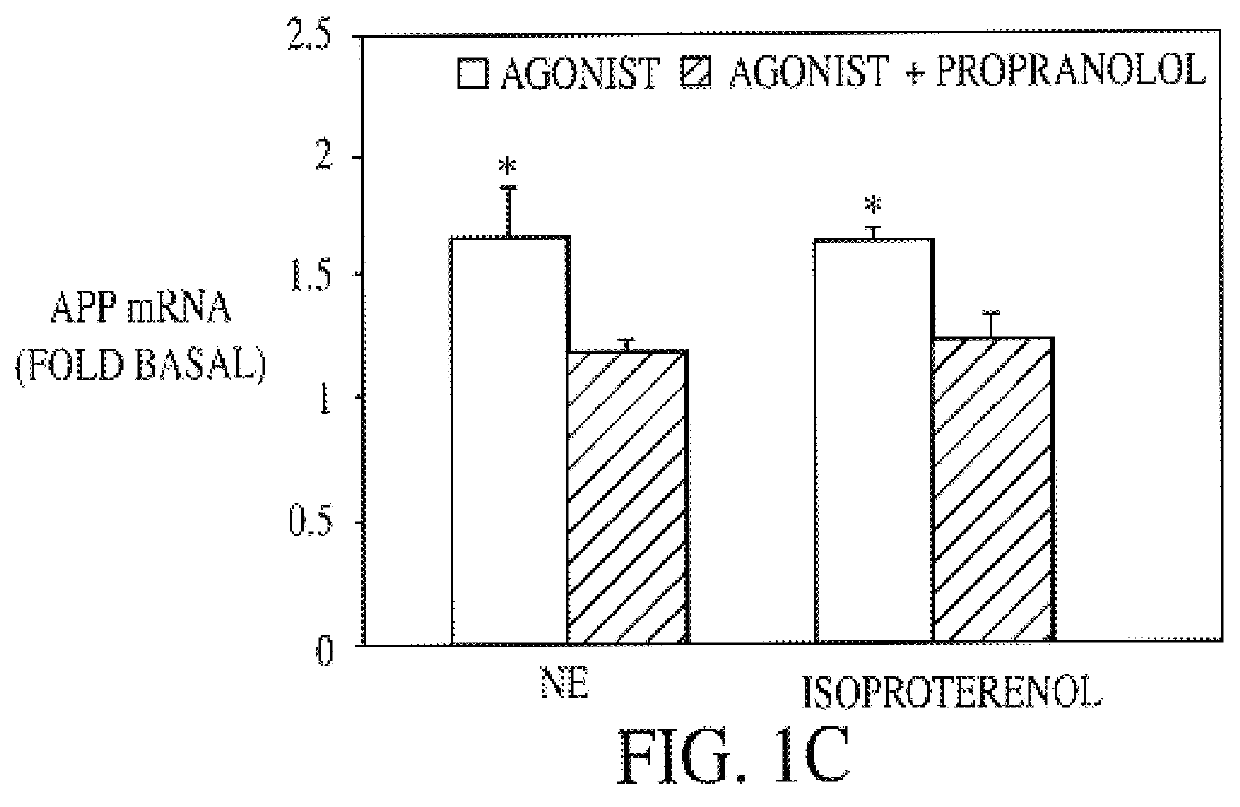
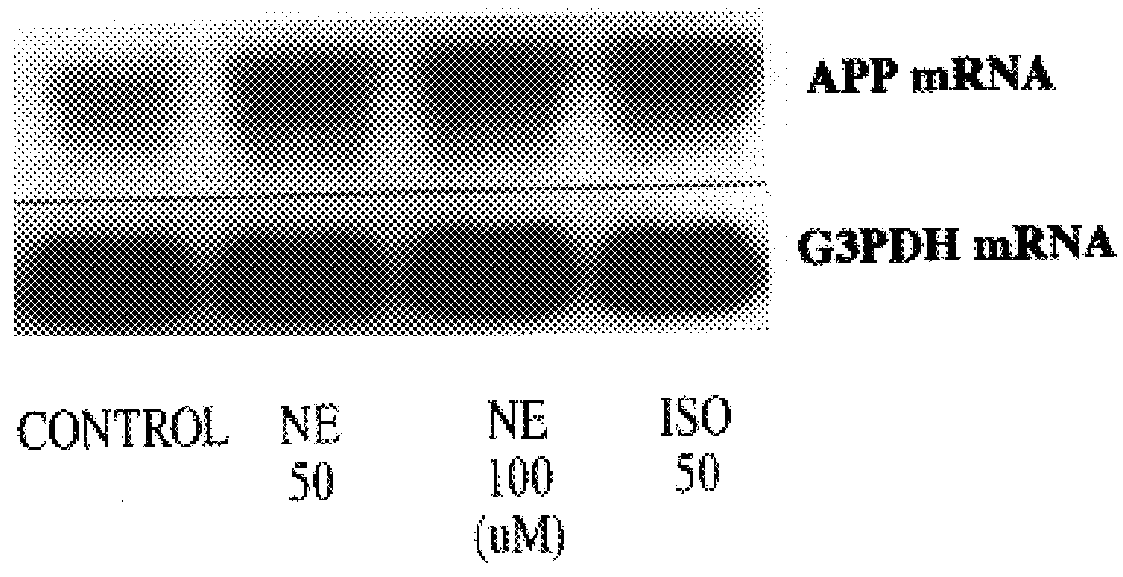
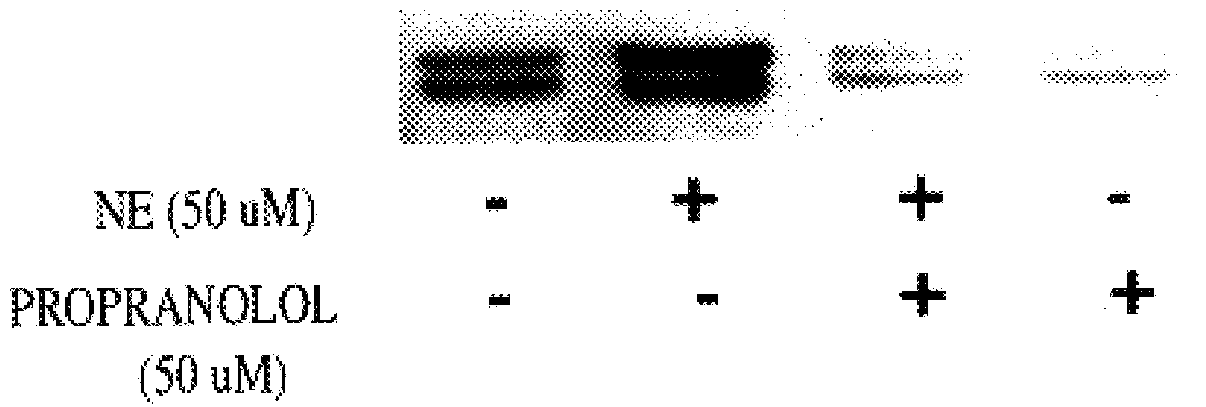
It has been discovered that the stimulation of beta-adrenergic receptors, which activate cAMP formation, give rise to increased APP and GFAP synthesis in astrocytes. Hence, the in vitro or in vivo exposure of neuronal cells to certain compositions comprising beta-adrenergic receptor ligands or agonists, including, e.g., norepinephrine, isoproterenol and the like, increases APP mRNA transcription and consequent APP overproduction. These increases are blocked by beta-adrenergic receptor antagonists, such as propranolol. The in vitro or in vivo treatment of these cells with 8Br-cAMP, prostaglandin E2 (PG E2), forskolin, and nicotine ditartrate also increased APP synthesis, including an increase in mRNA and holoprotein levels, as well as an increase in the expression of glial fibrillary acidic protein (GFAP). Compositions and methods are disclosed of regulating APP overexpression and mediating reactive astrogliosis through cAMP signaling or the activation of beta-adrenergic receptors. It has further been found that the increase in APP synthesis caused by 8Br-cAMP, PG E2, forskolin, or nicotine ditartrate is inhibited by immunosuppressants or anti-inflammatory agents, such as cyclosporin A, and FK-506 (tacrolimus), as well as ion-channel modulators, including ion chelating agents such as EGTA, or calcium / calmodulin kinase inhibitors, such as KN93. The present invention has broad implications in the alleviation, treatment, or prevention of neurological disorders and neurodegenerative diseases, including Alzheimer's Disease.
Owner:MASSACHUSETTS INST OF TECH
Cosmetic and pharmaceutical foam
InactiveUS20060140984A1Lower yield strengthRubbing easy and efficientCosmetic preparationsBiocideAlcohol freeAdjuvant
The invention relates to an alcohol-free cosmetic or pharmaceutical foam carrier comprising water, a hydrophobic solvent, a foam adjuvant agent, a surface-active agent and a water gelling agent. The cosmetic or pharmaceutical foam carrier does not contain aliphatic alcohols, making it non-irritating and non-drying. The alcohol-free foam carrier is suitable for inclusion of both water-soluble and oil soluble pharmaceutical and cosmetic agents.
Owner:FOAMIX PHARMACEUTICALS LIMITED
Immunostimulatory nucleic acids for inducing a Th2 immune response
Owner:OTTAVA HEALTH RES INST (CA)
Methods, compositions and systems for local delivery of drugs
ActiveUS20110195123A1Reduce degradationImprove permeabilityBiocidePowder deliveryCell-Extracellular MatrixBrachytherapy
Implantable medical device eluting drug locally and in prolonged period is provided, including several types of such a device, the treatment modes of implementation and methods of implantation. The device comprising of polymeric substrate, such as a matrix for example, that is used as the device body, and drugs, and in some cases additional scaffolding materials, such as metals or additional polymers, and materials to enhance visibility and imaging. The selection of drug is based on the advantageous of releasing drug locally and in prolonged period, where drug is released directly to the extracellular matrix (ECM) of the diseased area such as tumor, inflammation, degeneration or for symptomatic objectives, or to injured smooth muscle cells, or for prevention. One kind of drug is the gene silencing drugs based on RNA interference (RNAi), including but not limited to si RNA, sh RNA, or antisense RNA / DNA, ribozyme and nucleoside analogs. The modes of implantation in some embodiments are existing implantation procedures that are developed and used today for other treatments, including brachytherapy and needle biopsy. In such cases the dimensions of the new implant described in this invention are similar to the original implant. Typically a few devices are implanted during the same treatment procedure.
Owner:SILENSEED LTD
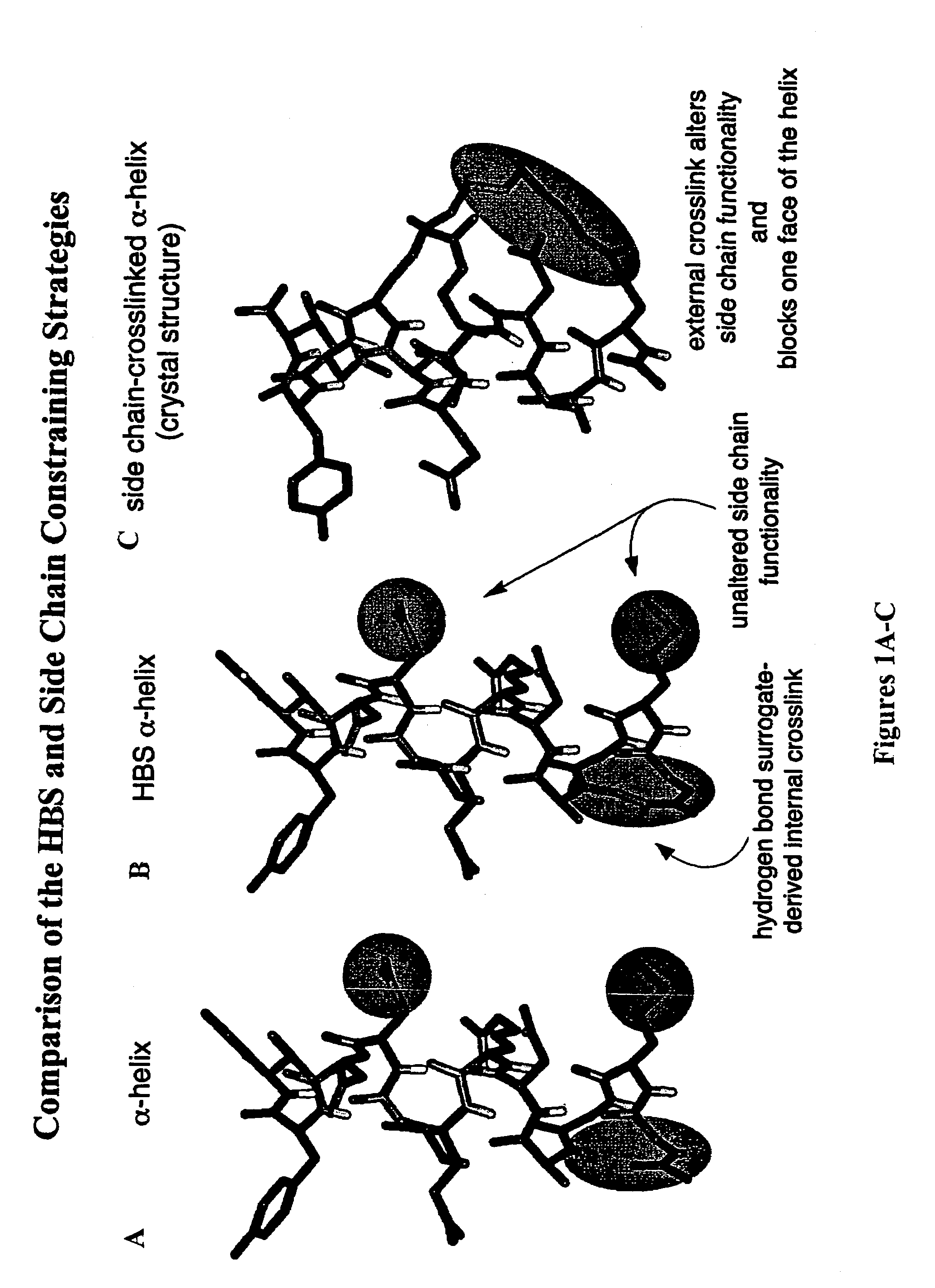
Methods for preparing internally constrained peptides and peptidomimetics
ActiveUS7202332B2Stable and irreversible bondPeptide preparation methodsCyclic peptide ingredientsBeta sheetHelix
The present invention relates to a method for preparing a peptide having a stable, internally constrained alpha-helical, beta-sheet / beta-turn, 310-helical, or pi-helical region and a method of stabilizing an alpha-helical, beta-sheet / beta-turn, 310-helical, or pi-helical region within a peptide structure. The resulting peptides and methods of using them are also disclosed.
Owner:NEW YORK UNIV
Methods of generating novel peptides
The present invention describes peptides capable of specifically binding to preselected micromolecules or to their natural receptor. The preselected molecules include but are not limited to drugs, vitamins, neuromediators and steroid hormones. Methods of using the phage display libraries to identify peptide compositions in preselected binding interactions are also disclosed. The retrieved peptides mimicking a natural receptor binding site to preselected molecules are used as is or as ligands to re-screen the same or different libraries to find and / or derive new receptor ligands, or are used to elicit the production of antibodies capable of binding to the natural receptor. The two categories of effector molecules (peptides or antibodies) may find diagnostic, therapeutic or prophylactic uses. The peptides directly derived from the phage display libraries may be used as drug detectors or antidotes. The others may be used to identify, target, activate or neutralize the receptor for the preselected micromolecules, the receptor being known or unknown.
Owner:BIOPHAGE
Ionic silicone hydrogels
Owner:JOHNSON & JOHNSON VISION CARE INC
Novel macrocyclic inhibitors of hepatitis C virus replication
InactiveUS20070054842A1Improve liver functionBiocideOrganic active ingredientsHepatitis c viralLiver fibrosis
The embodiments provide compounds of the general Formulae I through general Formula VIII, as well as compositions, including pharmaceutical compositions, comprising a subject compound. The embodiments further provide treatment methods, including methods of treating a hepatitis C virus infection and methods of treating liver fibrosis, the methods generally involving administering to an individual in need thereof an effective amount of a subject compound or composition.
Owner:ARRAY BIOPHARMA +1
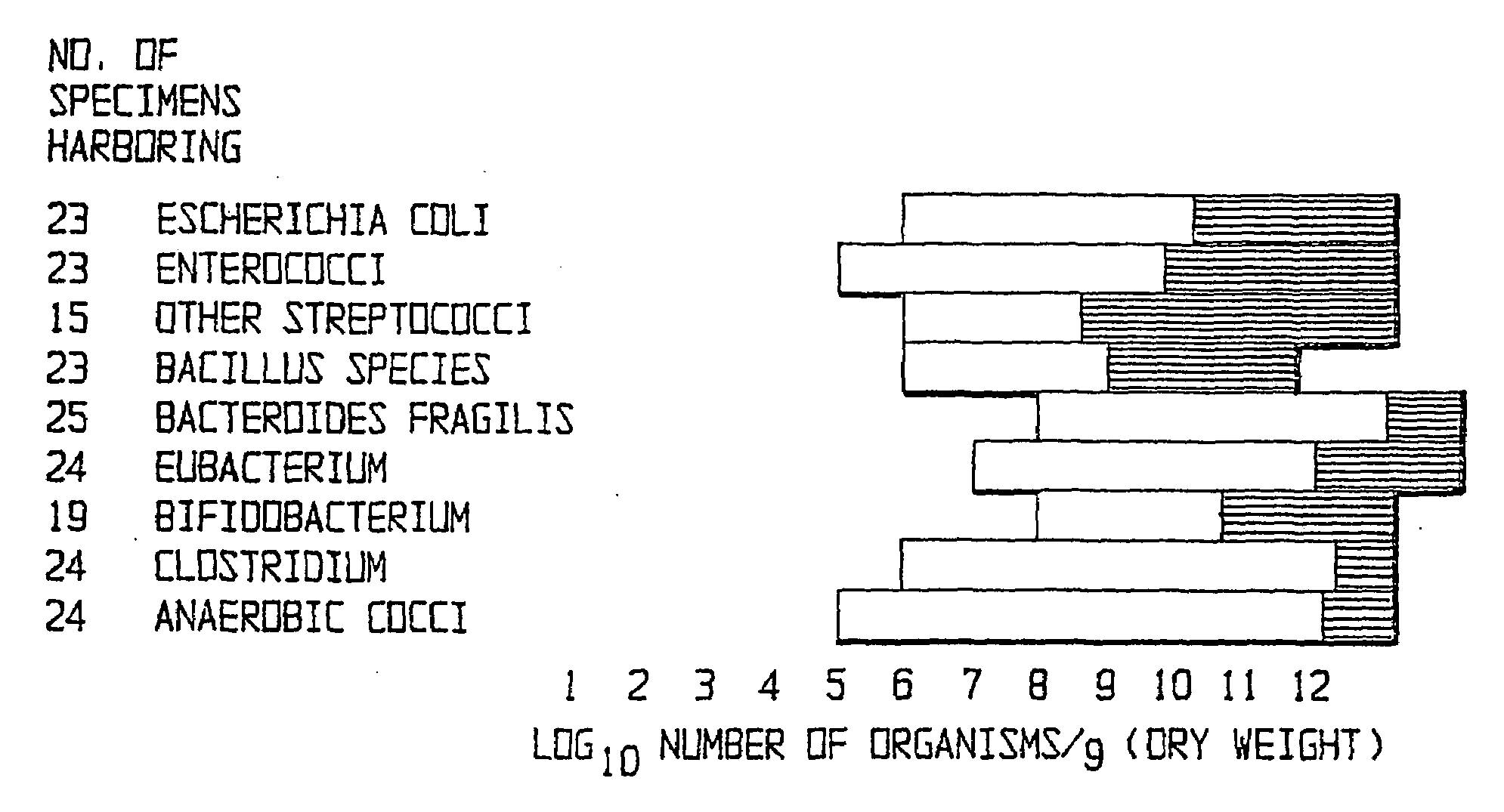
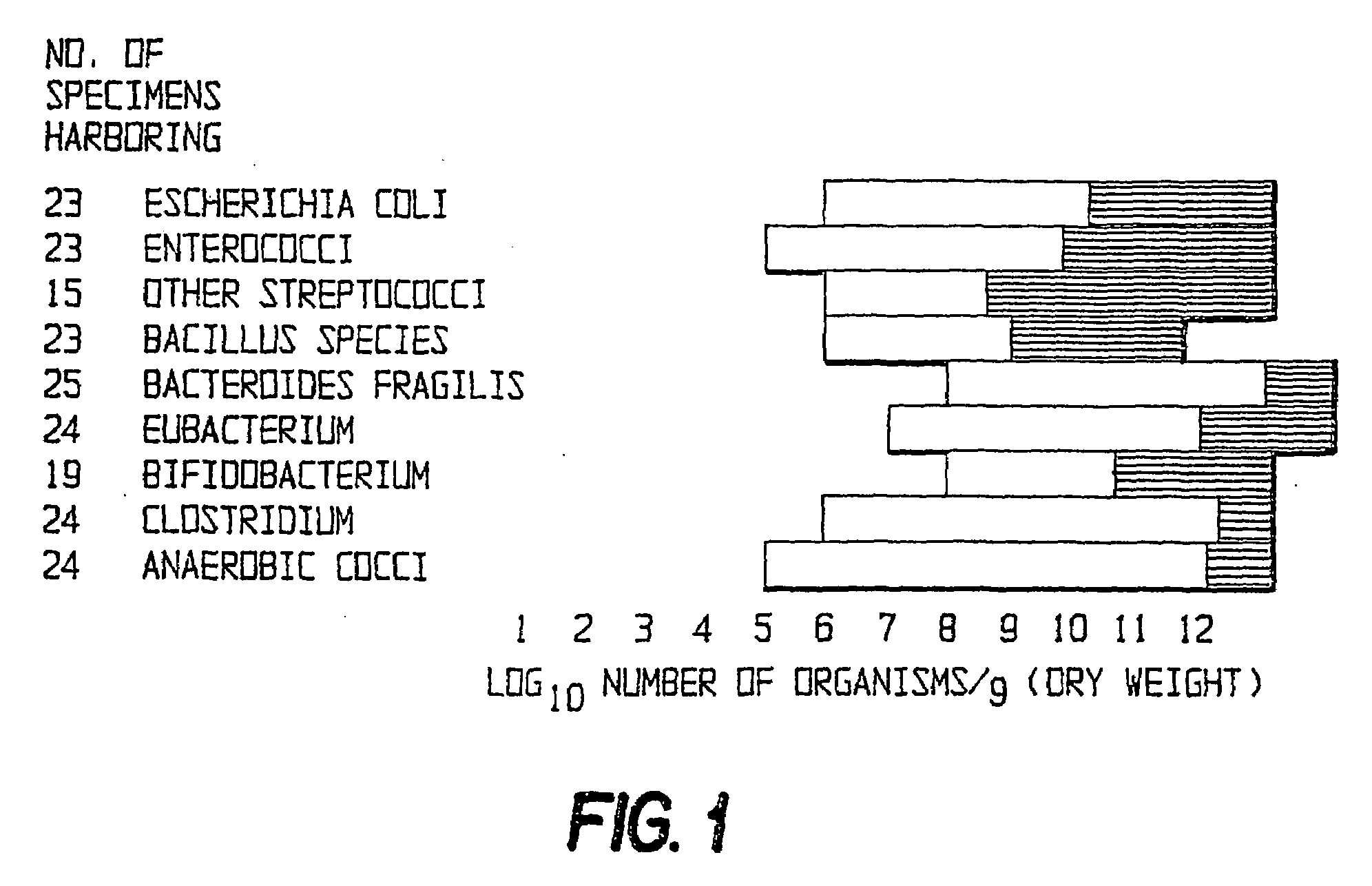
Method of treating gastrointestinal diseases associated with species of genus clostridium
The invention includes a method of treating gastrointestinal diseases associated with species of genus Clostridium such as clostridium deficit in human patients with gastrointestinal disorders having an etiological component such as a microbial agent producing a toxin where treated with an antimicrobial composition an amount effective to inhibit or eliminate the microbial agent. The antimicrobial composition in a form of probiotic mixture can be administrated alone or in combination with an antimicrobial agent, such as a bacteriophage which is specific for a bacterium producing toxin or antibiotics which are then used to eliminate or inhibit the clostridial species overgrown in a patient's gastrointestinal tract. Disorders that can be treated by the method of the invention include diarrhea or inflammatory bowel diseases such as colitis or Crohn's disease.
Owner:UNITED STATES OF AMERICA
Macrocyclic compounds as inhibitors of viral replication
The embodiments provide compounds of the general formulas I-XIX, as well as compositions, including pharmaceutical compositions, comprising a subject compound. The embodiments further provide treatment methods, including methods of treating flaviviral infection, including hepatitis C virus infection and methods of treating liver fibrosis, the methods generally involving administering to an individual in need thereof an effective amount of a subject compound or composition.
Owner:F HOFFMANN LA ROCHE & CO AG
Body cavity foams
The invention relates to an alcohol-free cosmetic or therapeutic foam carrier comprising water, a hydrophobic organic carrier, a foam adjuvant agent, a surface-active agent and a gelling agent. The cosmetic or therapeutic foam carrier does not contain aliphatic alcohols, making it non-irritating and non-drying. The alcohol-free foam carrier is suitable for inclusion of both water-soluble and oil soluble therapeutic and cosmetic agents.
Owner:VYNE THERAPEUTICS INC
Method of Drug Formulation Based on Increasing the affinity of Crystalline Microparticle Surfaces for Active Agents
Methods are provided for coating crystalline microparticles with an active agent by altering the surface properties of the microparticles in order to facilitate favorable association on the microparticle by the active agent. Type of surface properties that are altered by the disclosed methods include by electrostatic properties, hydrophobic properties and hydrogen bonding properties.
Owner:MANNKIND CORP
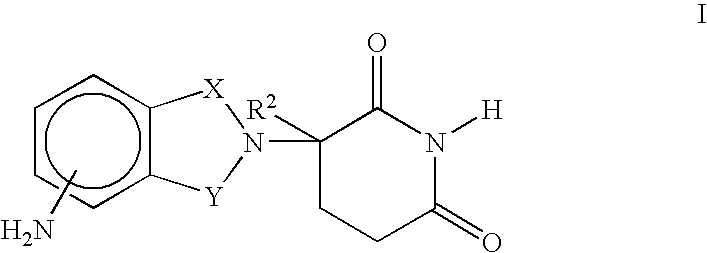
Macrocyclic peptides active against the hepatitis C virus
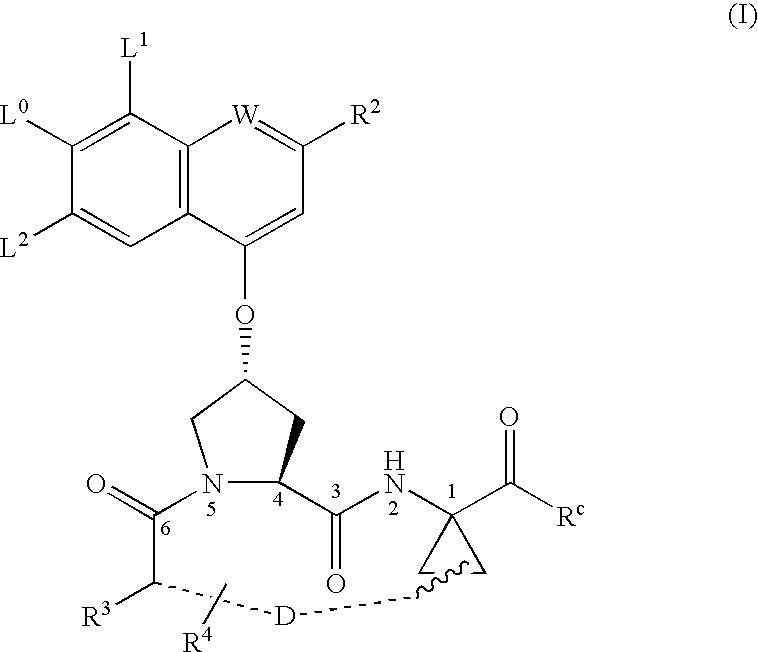
ActiveUS20050080005A1Avoid virus infectionBiocideTripeptide ingredientsMacrocyclic peptideHcv ns3 protease
Compounds of formula I: wherein D, R4, R3, L0, L1, L2, R2 and Rc are defined herein; or a pharmaceutically acceptable salt thereof, useful as inhibitors of the HCV NS3 protease.
Owner:BOEHRINGER INGELHEIM INT GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com