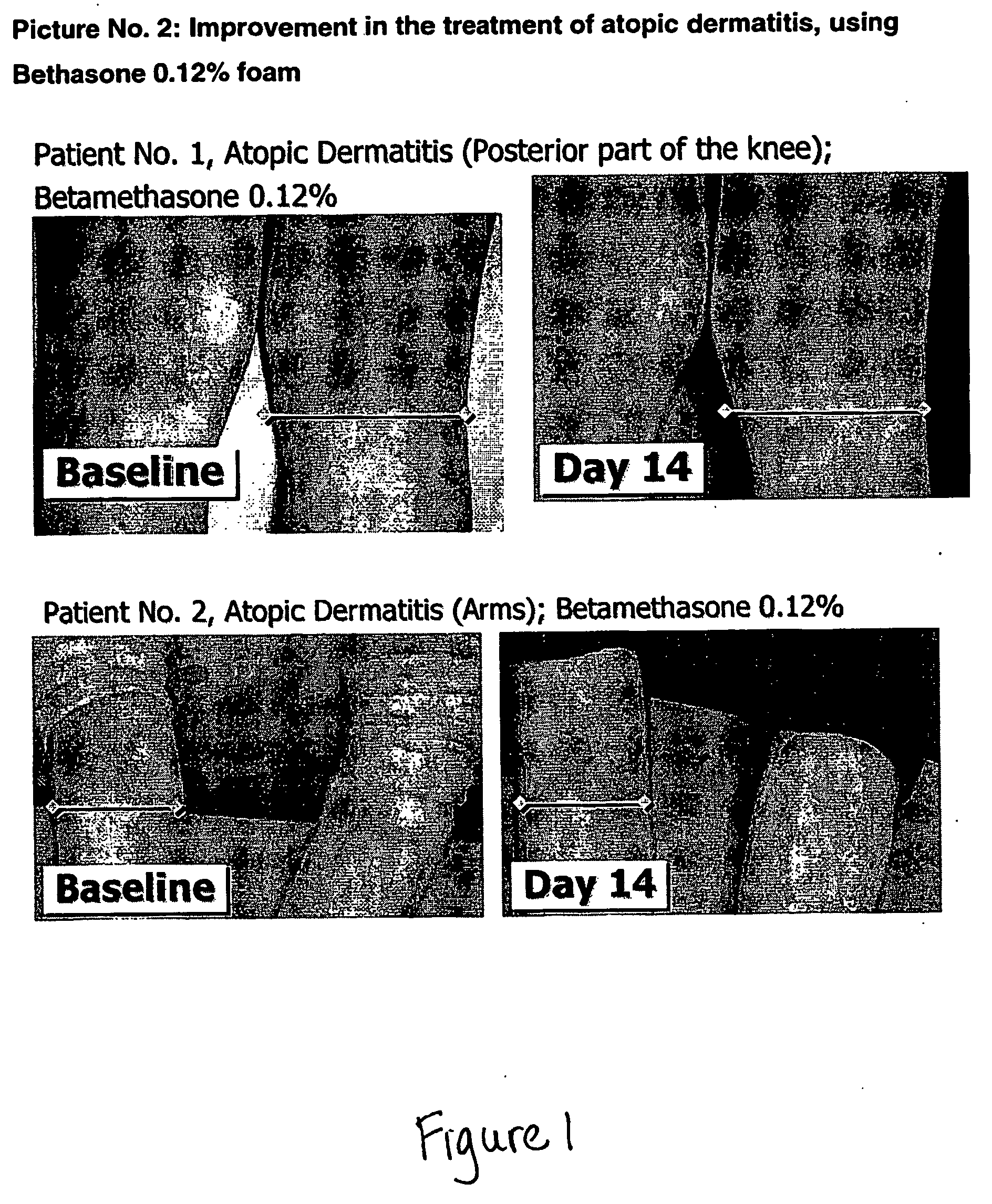
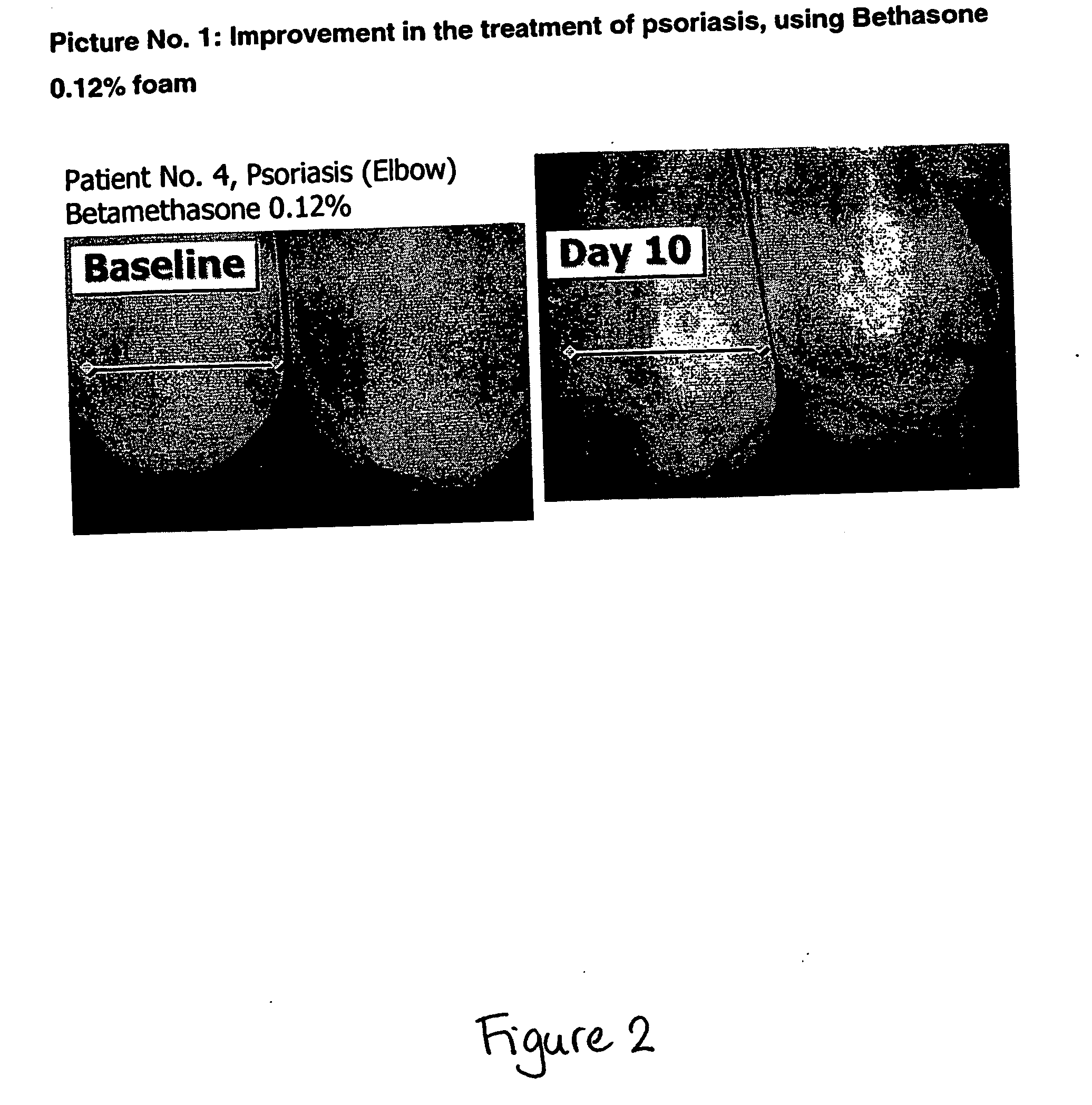
Cosmetic and pharmaceutical foam
a foam carrier and cosmetic technology, applied in the direction of aerosol delivery, drug compositions, immunological disorders, etc., can solve the problems of reducing the efficacy of drugs, affecting the appearance of cosmetics, so as to achieve the effect of improving the appearan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
General Procedure for Preparing Foamable Composition
[0338] Aqueous Phase: Water gelling agent and surface-active agent are dissolved in water, with agitation. The solution is warmed to 50-70° C. Water soluble cosmetic or pharmaceutical active ingredients and optional water soluble ingredients are added with agitation to the Aqueous Phase mixture.
[0339] Hydrophobic Phase: The hydrophobic solvent is heated to same temperature. Foam adjuvant agent is added to preheated hydrophobic solvent. Oil soluble cosmetic or pharmaceutical active ingredients* and optional oil soluble formulation ingredients are added with agitation to the Hydrophobic Phase mixture.
[0340] The warm Hydrophobic Phase is gradually poured into the warm Aqueous Phase, with agitation, followed by Ultraturax homogenization. The mixture is allowed to cool down to ambient temperature. In case of heat sensitive active ingredients, the active ingredient is added with agitation to the mixture after cooling to ambient temper...
example 2
Vegetable Oil-Based Foam Carrier Composition
[0341]
VersionVersionVersionNo. 1No. 2No. 3Ingredient% (W / W)Hydrophobic solventSoybean oil4030.520WaterWater48.532.561Foam adjuvant agentStearyl Alcohol0.81.050.73Surface-active agentSucrose ester SP700.640.450.8Water gelling agentXanthan Gum0.160.110.1Methocel ELV150.320.220.28Other IngredientsAntioxidant0.020.020.02Preservatives0.30.30.3Fragrance0.20.20.2Foam Specific gravity0.100.150.065(gr / mL)
[0342] The compositions use a non-ionic surfactant and contain a combined amount of surface-active agent, foam adjuvant and water gelling agent ranging from 1.83% to 1.92% (w / w). The foam of this example is useful as a carrier of active pharmaceutical and / or cosmetic active ingredients, as exemplified below. It also can be used as a protective product. Additionally, it is also useful as lubricating foam, for various purposes.
example 3
Silicone Oil-Based Foam Carrier Composition
[0343]
VersionVersionNo. 1No. 2Specific Ingredient% (W / W)Hydrophobic solventDimeticone 350*2510WaterWater7287Foam adjuvant agentStearyl Alcohol0.20.2Surface-active agentSucrose ester SP700.8—Myrj 49P—0.8Water gelling agentXanthan Gum0.20.2Methocel ELV150.40.4Other IngredientsAntioxidant0.020.02Preservatives11Fragrance0.20.2Foam Specific gravity (gr / mL)0.10ND
*Dimethylpolysiloxane of 350 cps viscosity.
[0344] The compositions use only non-ionic surfactant and contain a combined amount of surface-active agent, foam adjuvant and water gelling agent of 1.6% (w / w). The foam of this example is useful as a carrier of active pharmaceutical and / or cosmetic active ingredients, as exemplified below. It also can be used as a protective product. Additionally, it is also useful as lubricating foam, for various purposes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| average primary particle size | aaaaa | aaaaa |
| average primary particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com