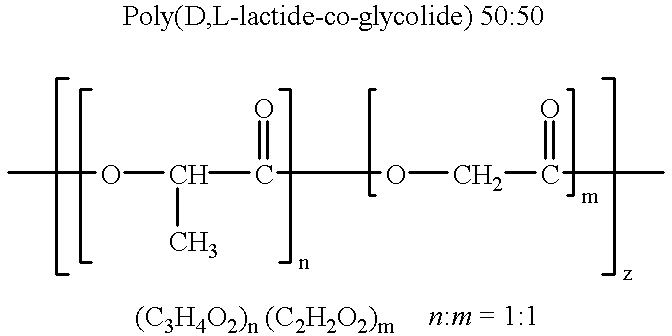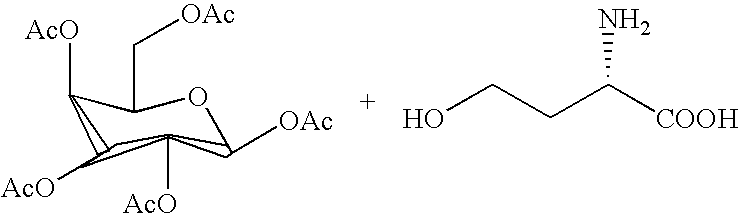Patents
Literature
8415 results about "Adjuvant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An adjuvant is a pharmacological or immunological agent that modifies the effect of other agents. Adjuvants may be added to a vaccine to boost the immune response to produce more antibodies and longer-lasting immunity, thus minimizing the dose of antigen needed. Adjuvants may also be used to enhance the efficacy of a vaccine by helping to modify the immune response to particular types of immune system cells: for example, by activating T cells instead of antibody-secreting B cells depending on the purpose of the vaccine. Adjuvants are also used in the production of antibodies from immunized animals. There are different classes of adjuvants that can push immune response in different directions, but the most commonly used adjuvants include aluminum hydroxide and paraffin oil.
Therapeutic treatment and prevention of infections with a bioactive materials encapsulated within a biodegradable-biocompatible polymeric matrix
InactiveUS6309669B1Sustained release of active agent over timeEfficient and effective usePowder deliveryPeptide/protein ingredientsAdjuvantEnd-group
Novel burst-free, sustained release biocompatible and biodegrable microcapsules which can be programmed to release their active core for variable durations ranging from 1-100 days in an aqueous physiological environment. The microcapsules are comprised of a core of polypeptide or other biologically active agent encapsulated in a matrix of poly(lactide / glycolide) copolymer, which may contain a pharmaceutically-acceptable adjuvant, as a blend of upcapped free carboxyl end group and end-capped forms ranging in ratios from 100 / 0 to 1 / 99.
Owner:ARMY GOVERNMENT OF THE UNITED STATES AS REPRESENTED BY THE SEC OF THE
Potentiation of immune responses with liposomal adjuvants
InactiveUS6090406AGood water solubilityPractical and convenientBacterial antigen ingredientsViral antigen ingredientsLipid formationOrganic acid
A high integrity liposome comprising at least one stabile lipid and at least one peptide-like therapeutic agent associated with said liposome, adapted for parenteral administration to an animal, including a human, and method according to manufacture and use. Immunizing dosage forms comprising a liposome and an immunogen, wherein said liposome and immunogen are present in an immunization dose. Additionally, a dosage form, including such form particularly adapted to producing an immune response, comprising a salt according to an organic acid derivative of a sterol and an immunogen wherein said organic acid derivative of a sterol and immunogen are present in an immunization dose, and method according to use is disclosed. Further, a dosage form, including such form particularly adapted to producing an immune response, comprising dimyristoylphosphatidylcholine (DMPC) / cholesterol liposomes, optionally in an aluminum hydroxide gel, and an immunogen wherein said DMPC / cholesterol and immunogen are present in an immunization dose, and method according to use.
Owner:TRANSAVE
Plant extracts for the preparation of pharmaceutical compositions for the treatment of hepatitis
The use of extracts from the plant Hypericum perforatum in the preparation of pharmaceutical compositions for the treatment of hepatitis C, chronic hepatitis C and related viruses, said pharmaceutical compositions comprising at least one extract of the plant Hypericum perforatum and optionally in addition, one or more pharmaceutically acceptable inactive components, selected from, carriers, coatings, diluents and adjuvants.
Owner:LAVIE DAVID +1
New adjuvant composition
InactiveUS20060009360A1Improve efficiencyImprove area coverageBiocideDead animal preservationGlycineAdjuvant
The present invention is directed to pesticidal formulations utilizing novel alkylpolyglycoside amines as adjuvants. The pesticidal formulation need not, but may, include an additional eye irritation-reducing complex. The adjuvant is particularly useful with glyphosate (N-(phosphonomethyl)glycine) compositions. Methods for using these pesticidal formulations are also disclosed.
Owner:COGNIS IP MANAGEMENT GMBH
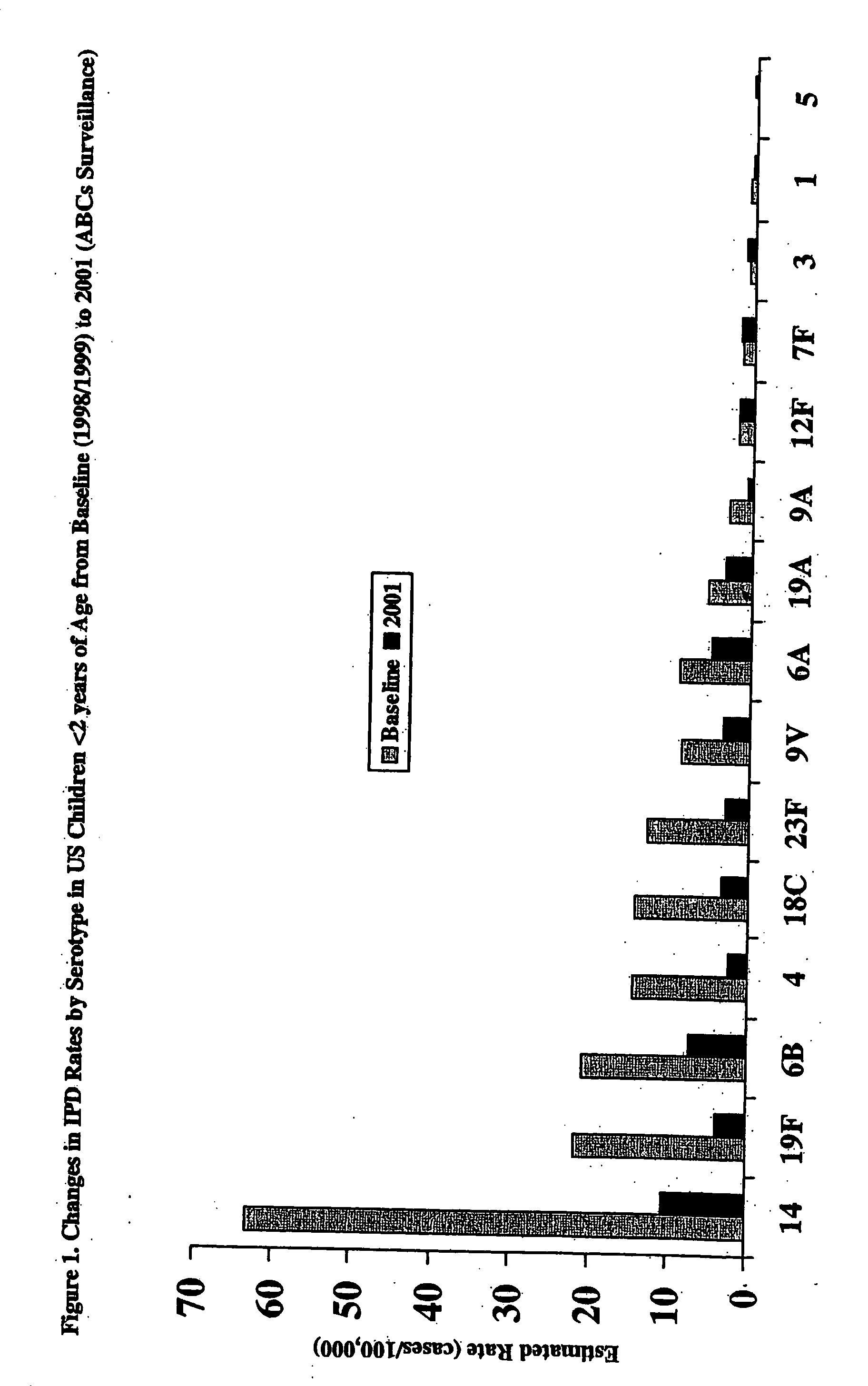
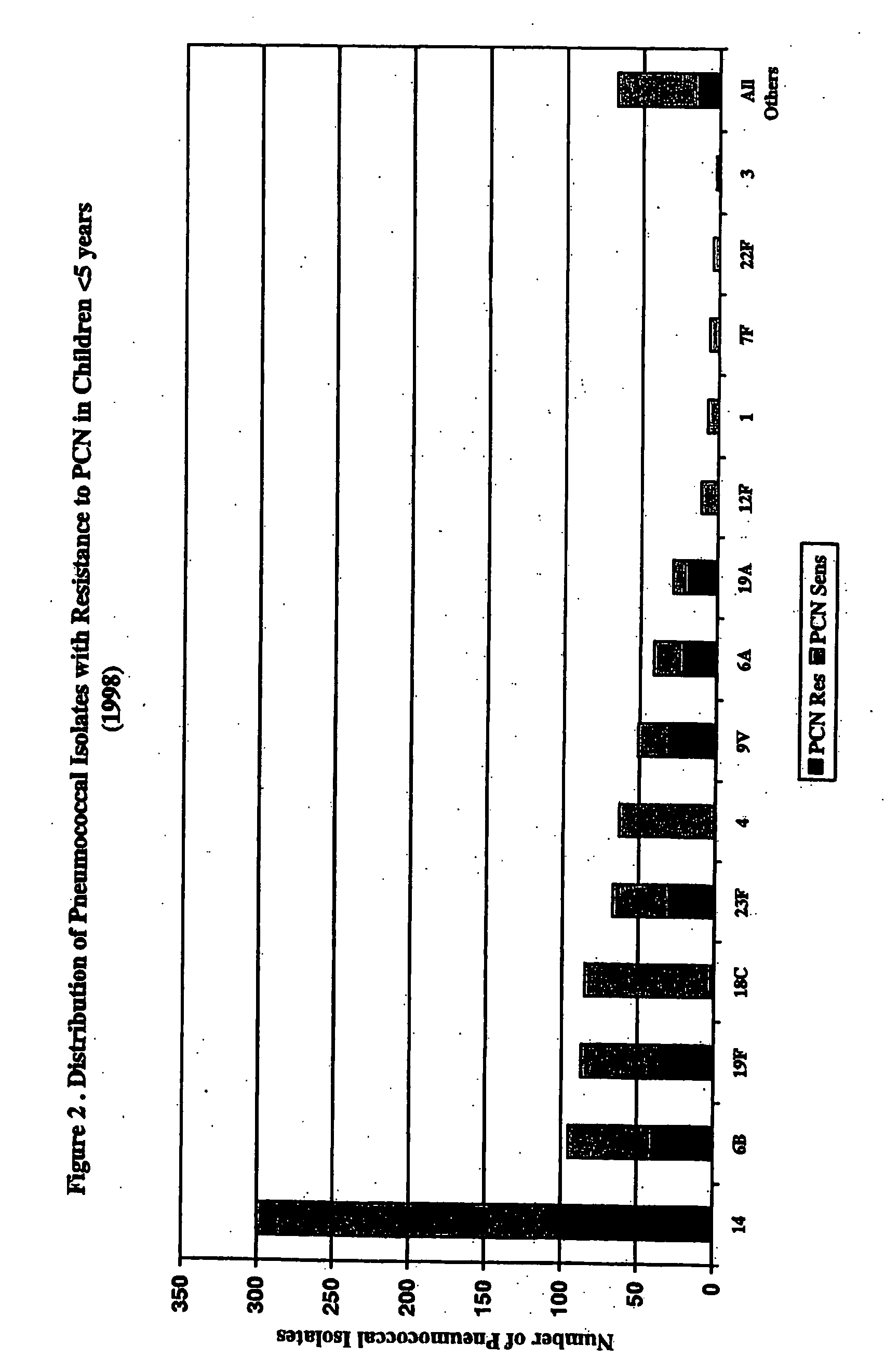
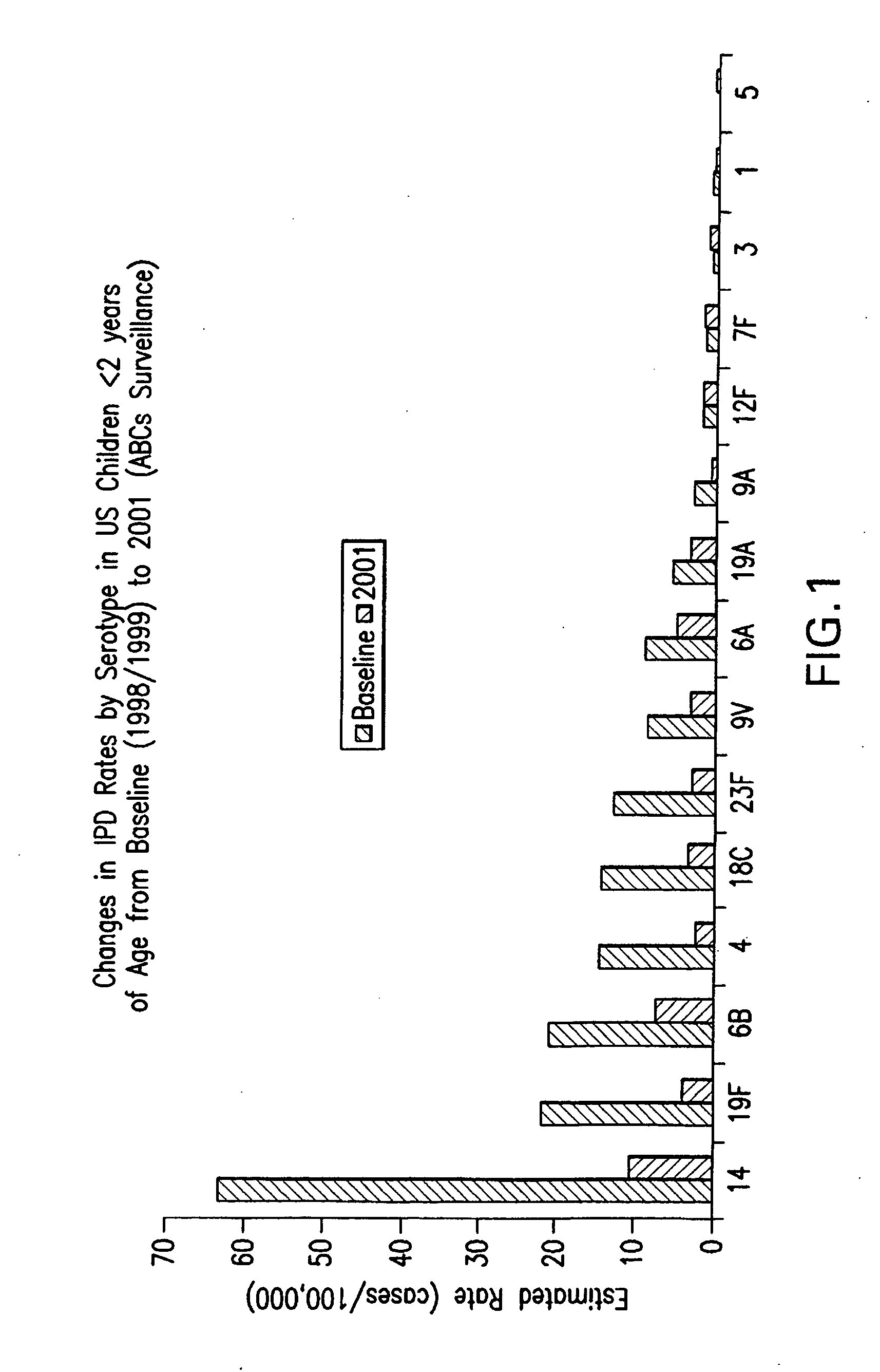
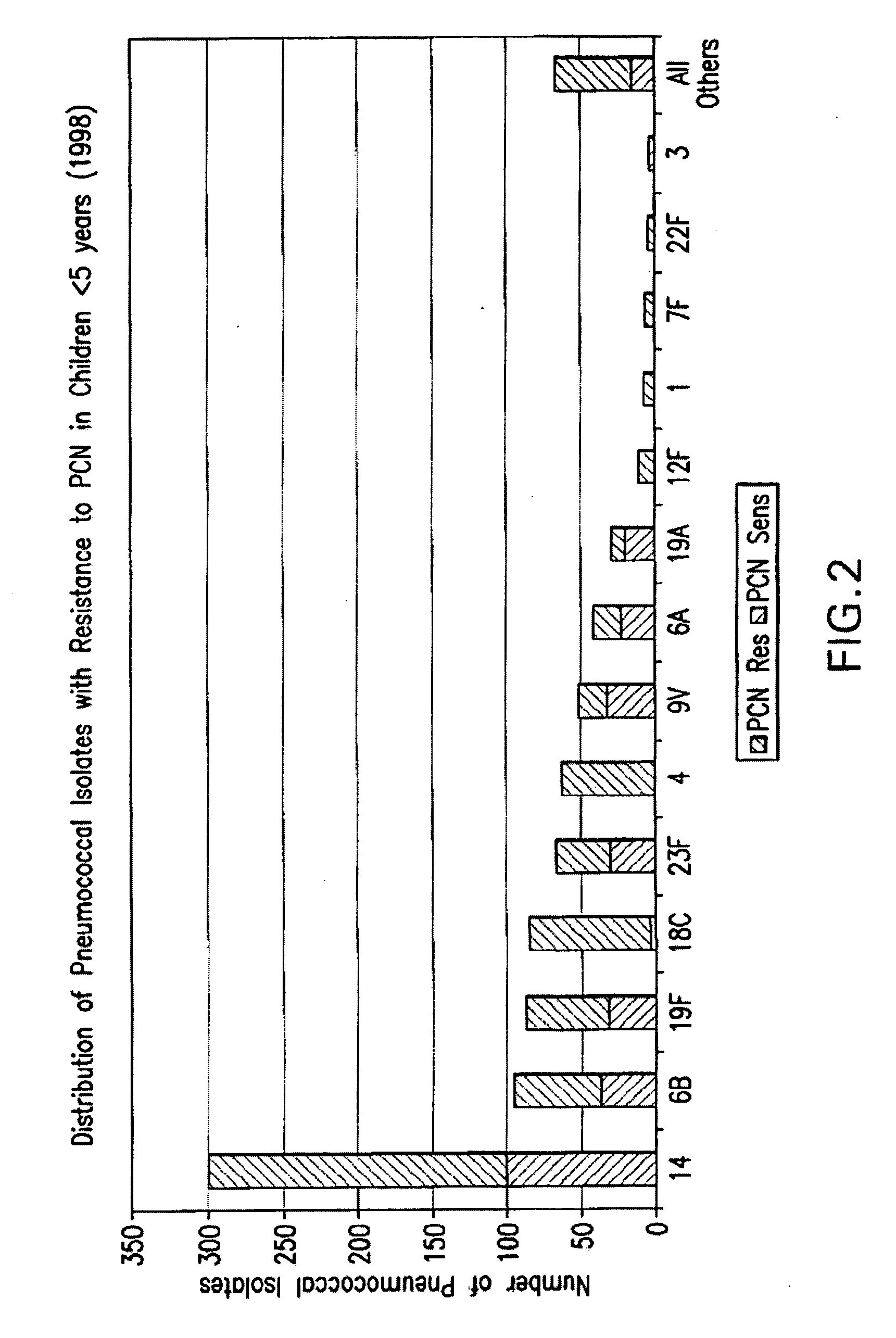
Multivalent pneumococcal polysaccharide-protein conjugate composition
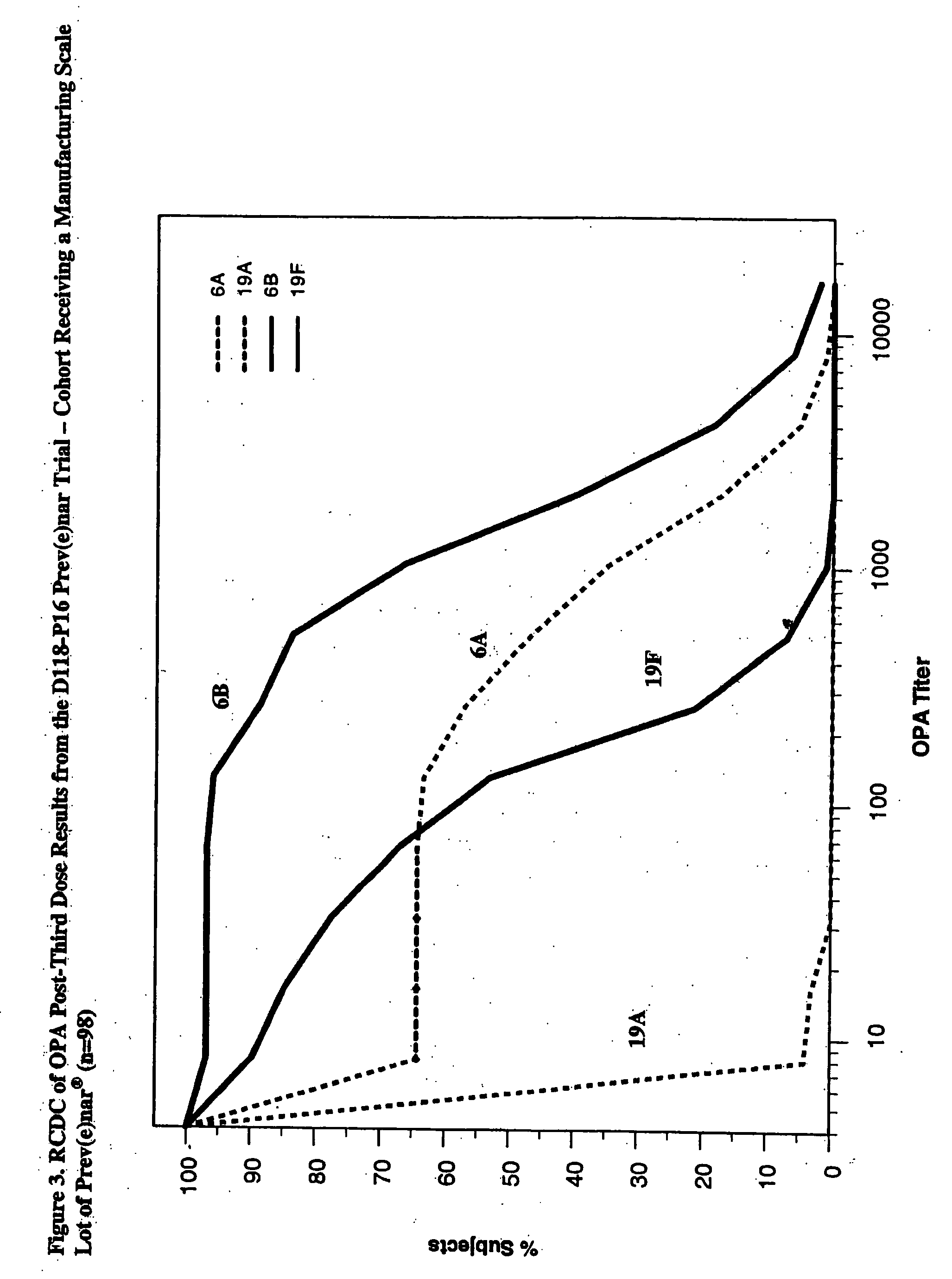
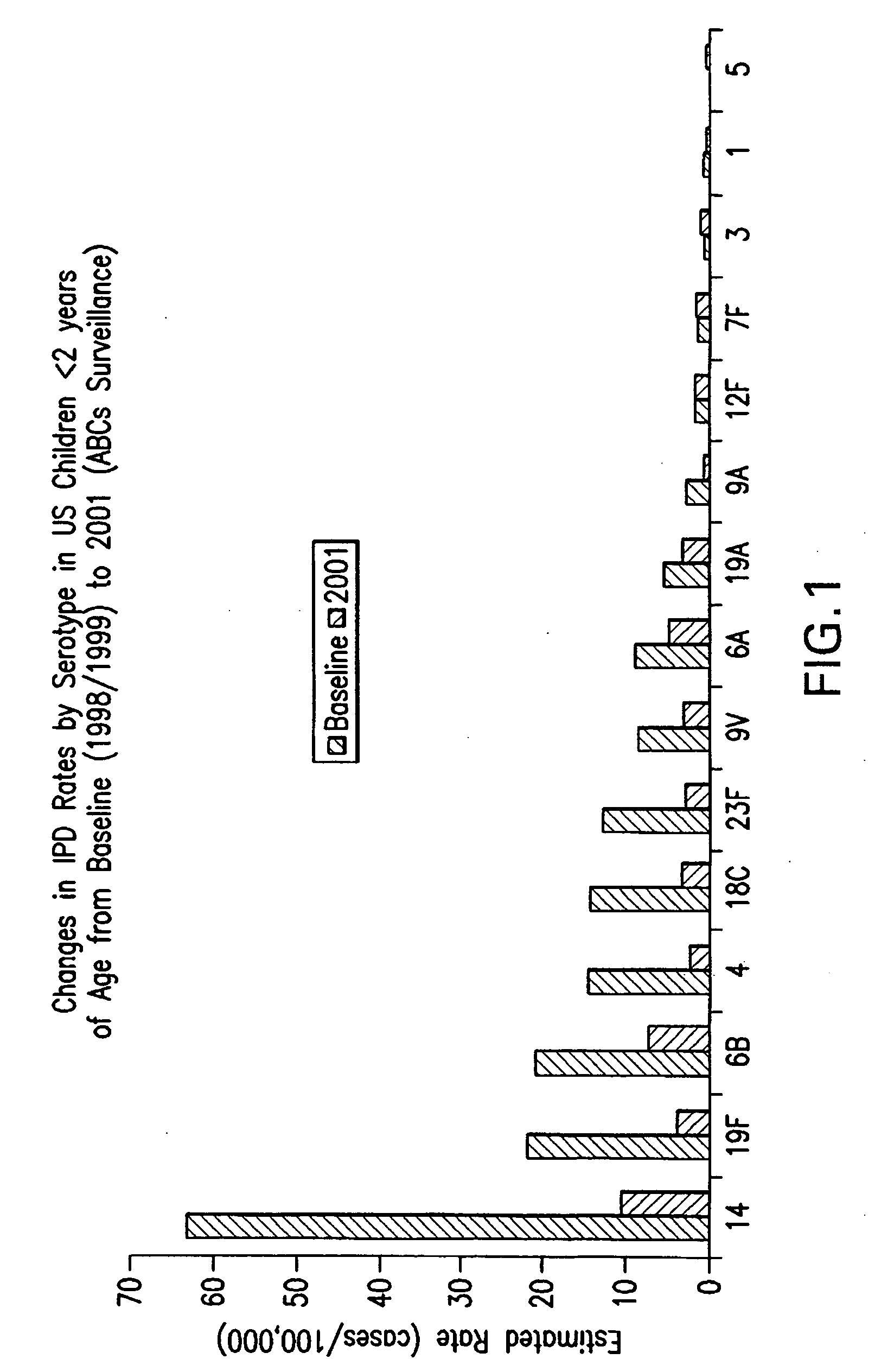
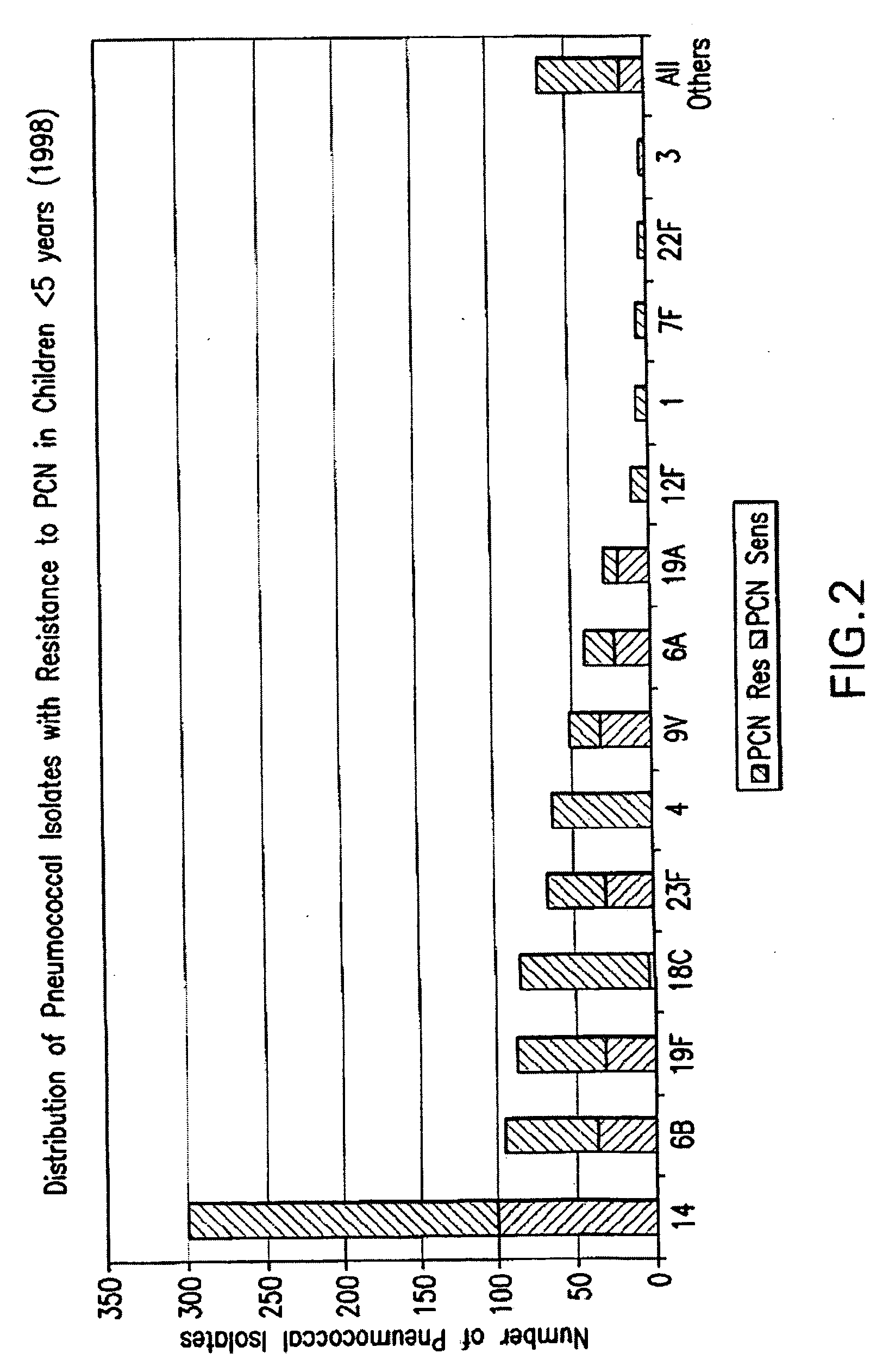
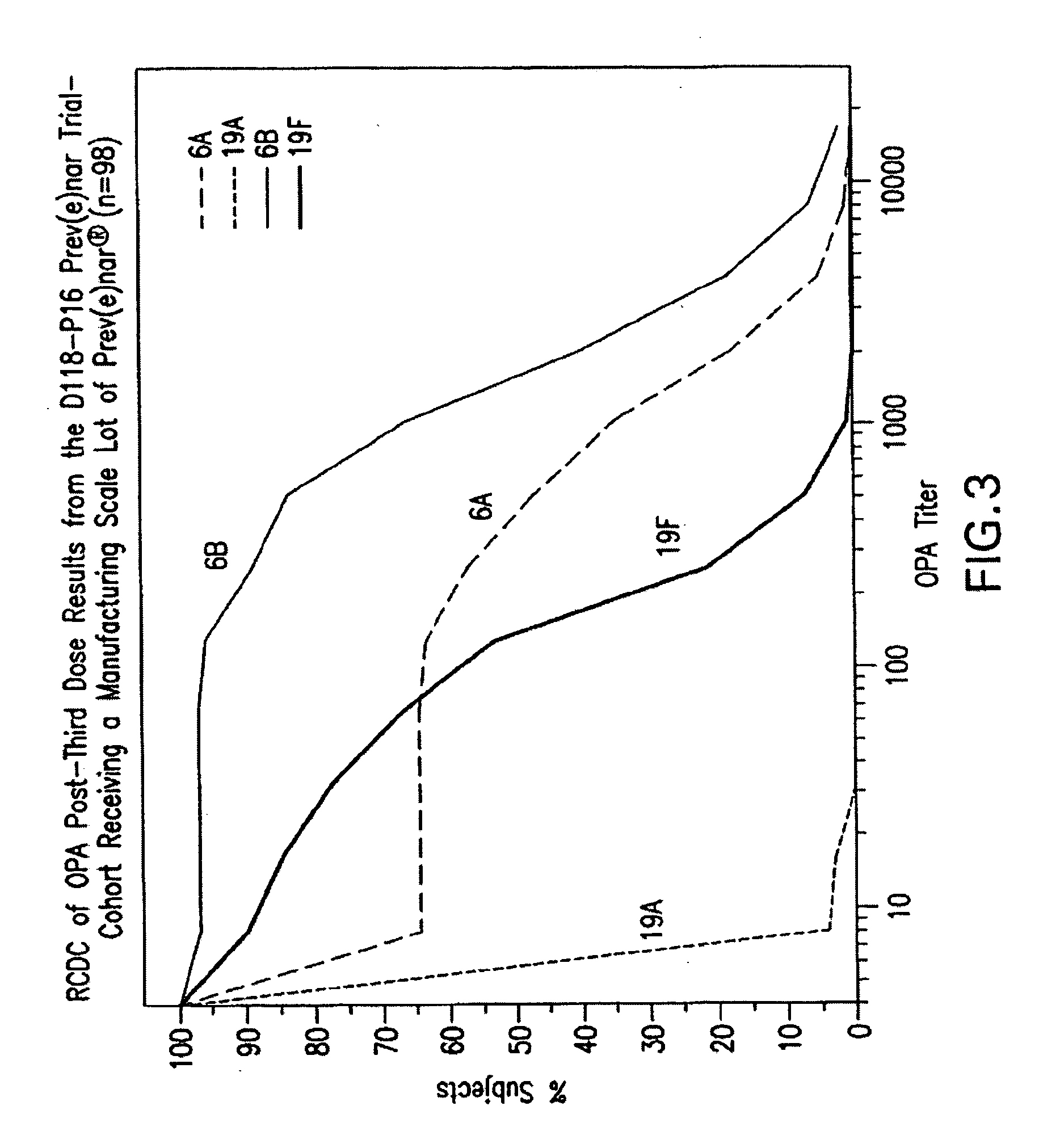
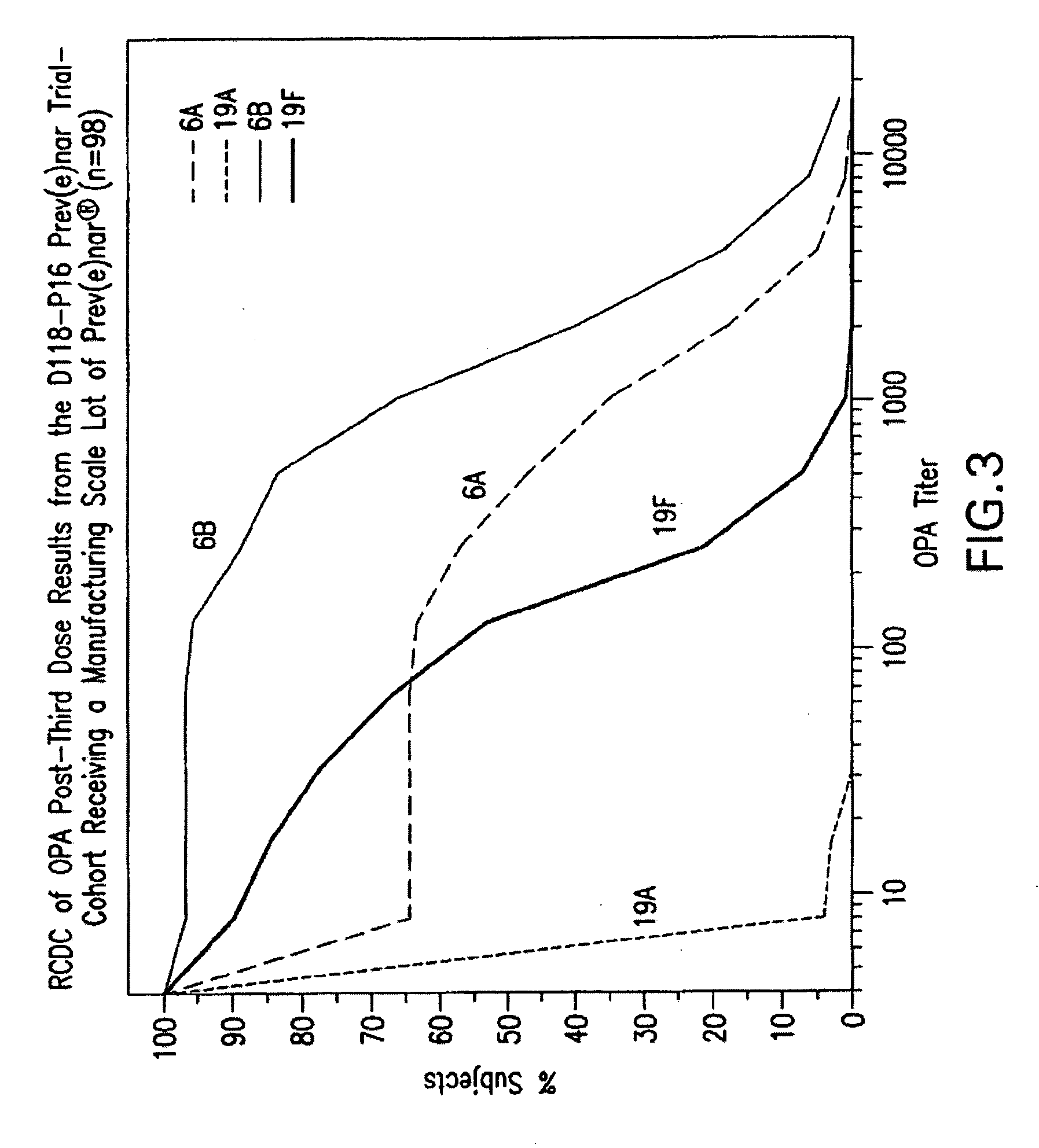
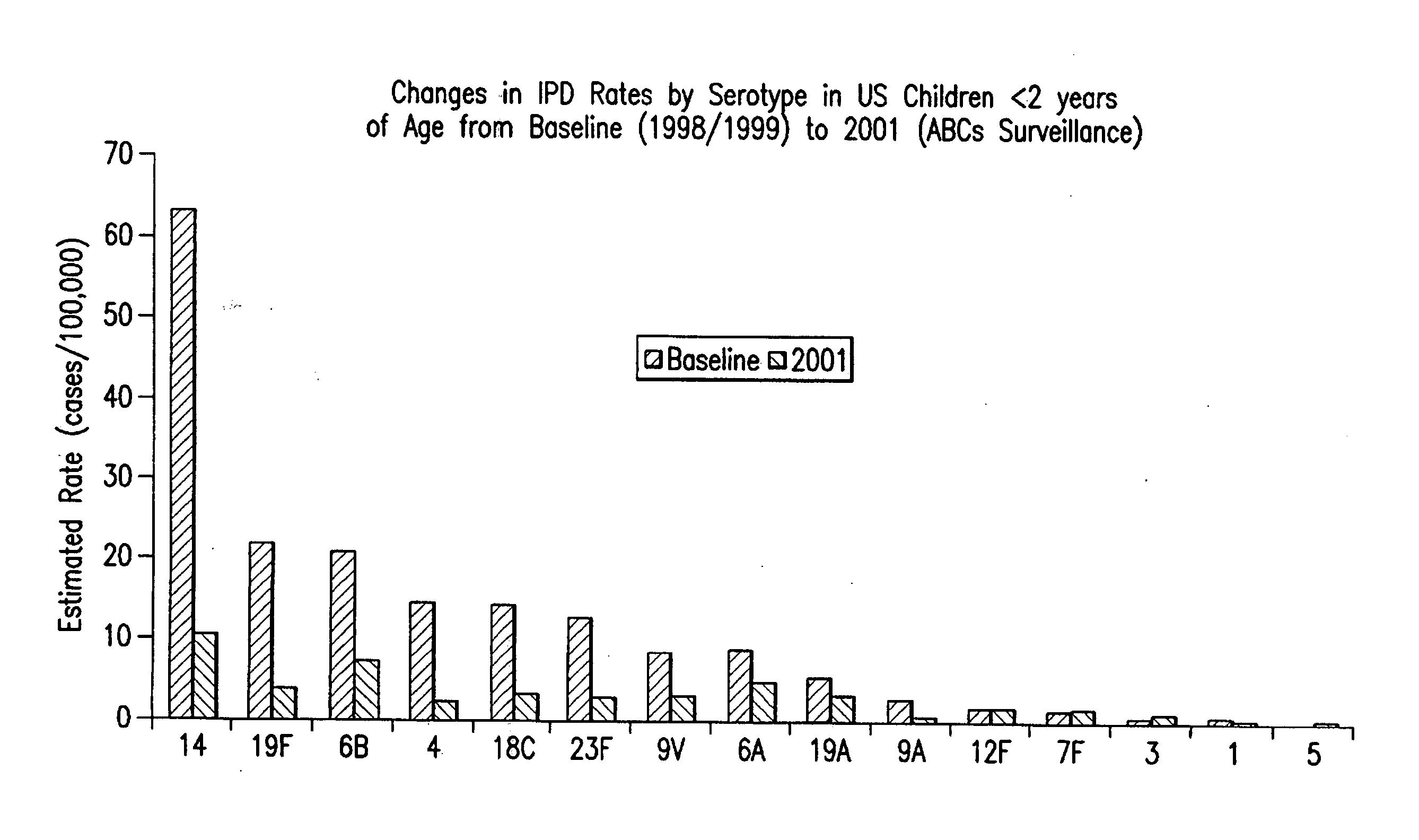
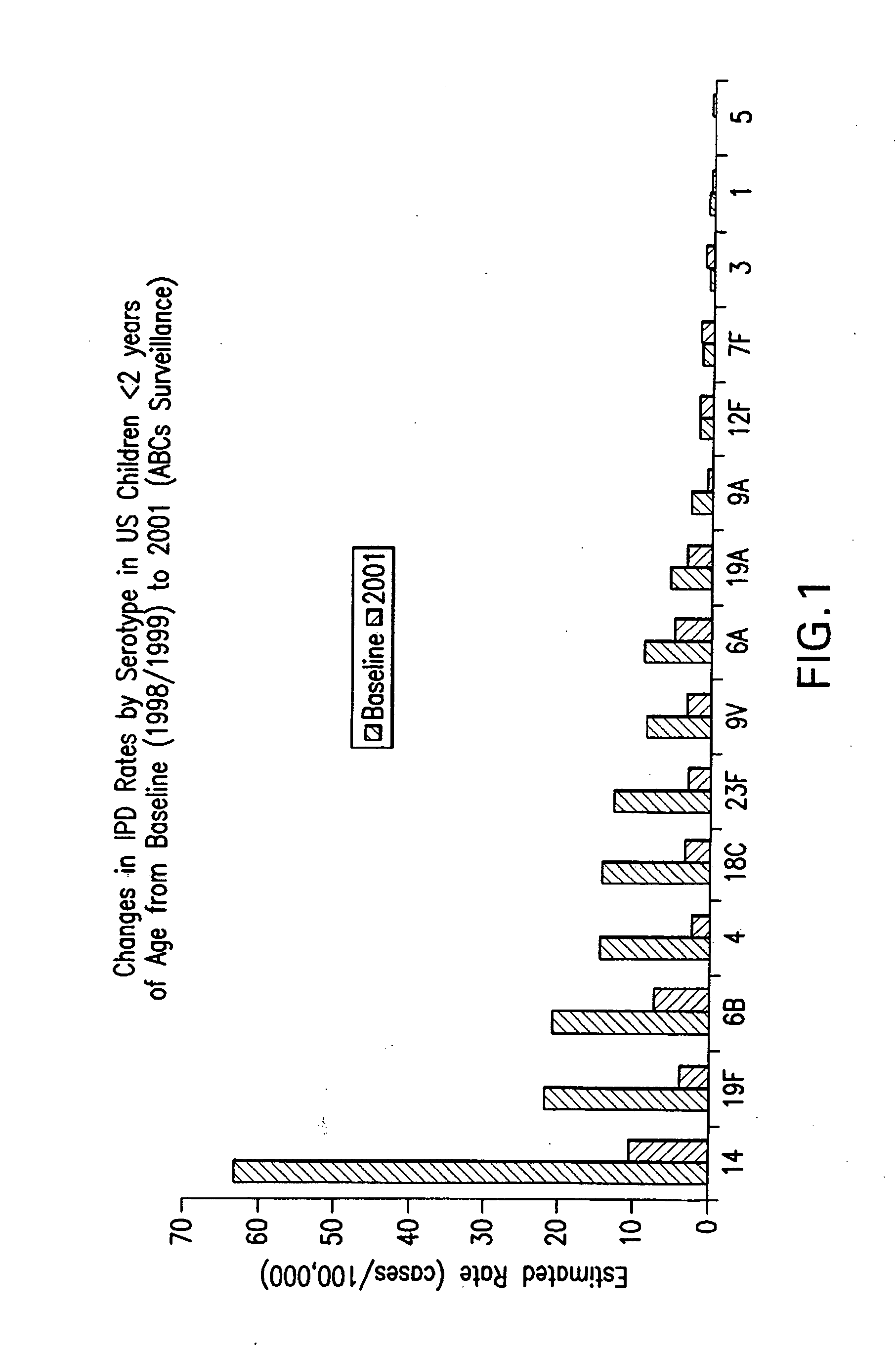
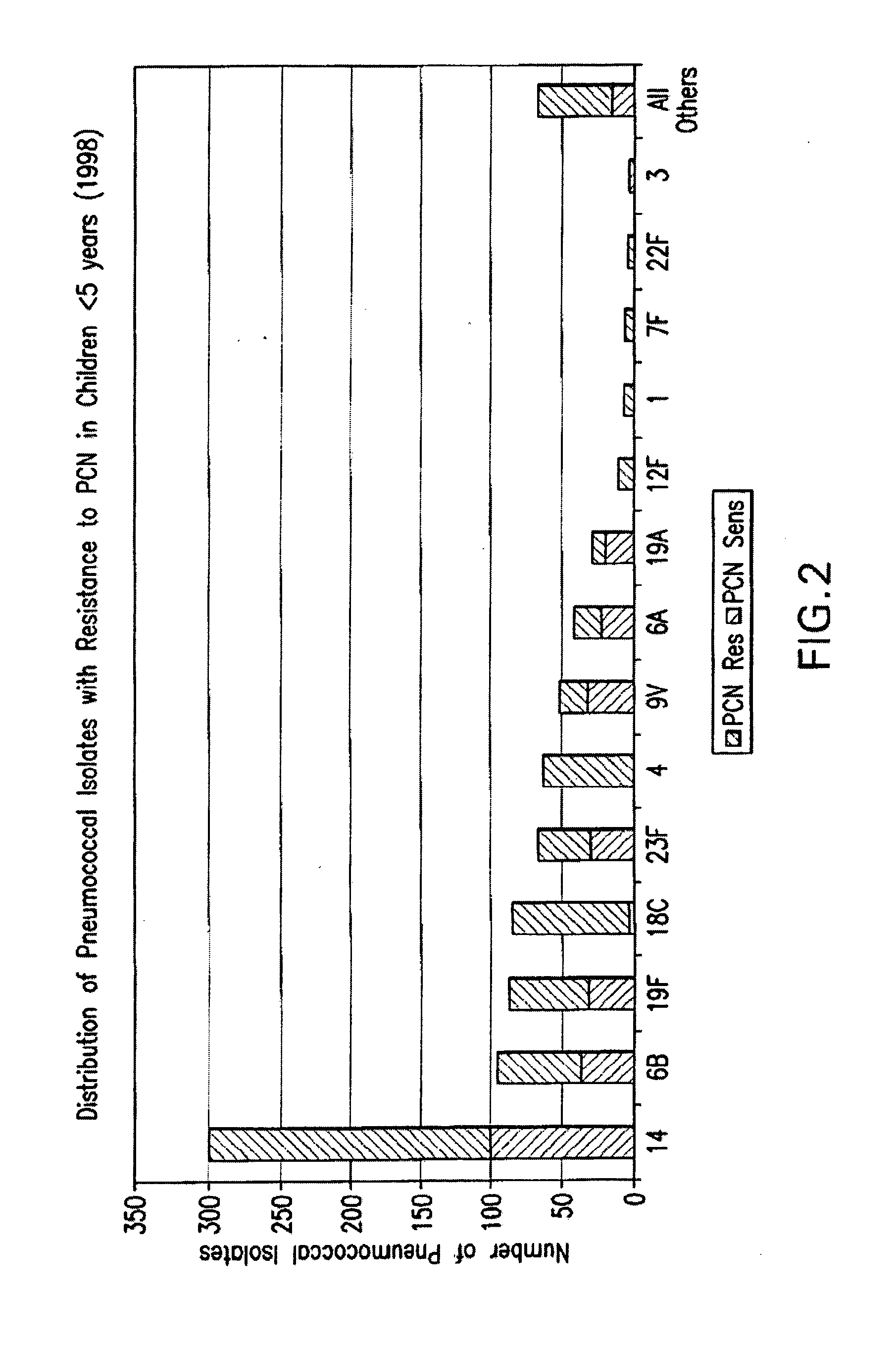
An immunogenic composition having 13 distinct polysaccharide-protein conjugates and optionally, an aluminum-based adjuvant, is described. Each conjugate contains a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F) conjugated to a carrier protein. The immunogenic composition, formulated as a vaccine, increases coverage against pneumococcal disease in infants and young children globally, and provides coverage for serotypes 6A and 19A that is not dependent on the limitations of serogroup cross-protection.
Owner:WYETH LLC
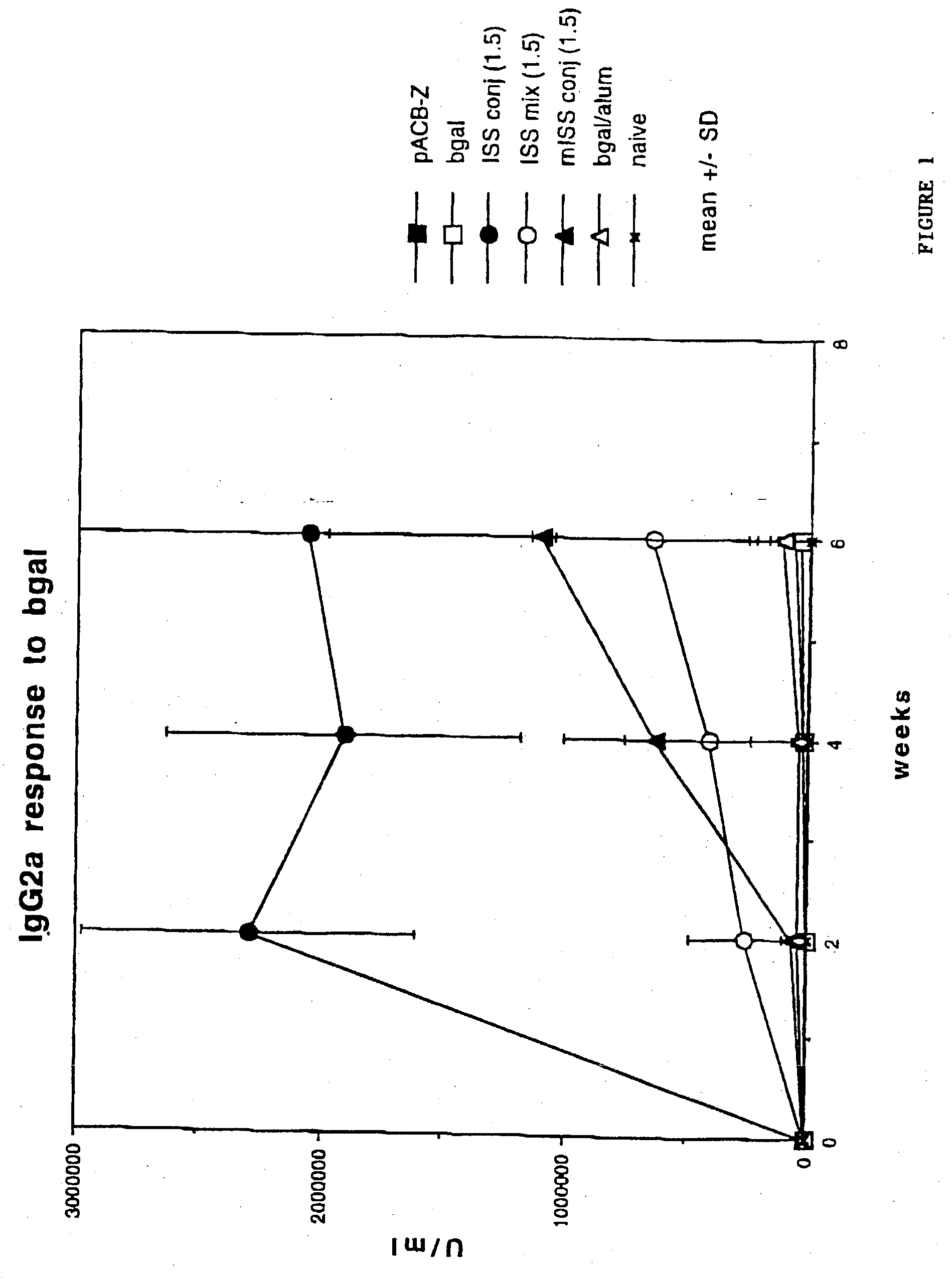
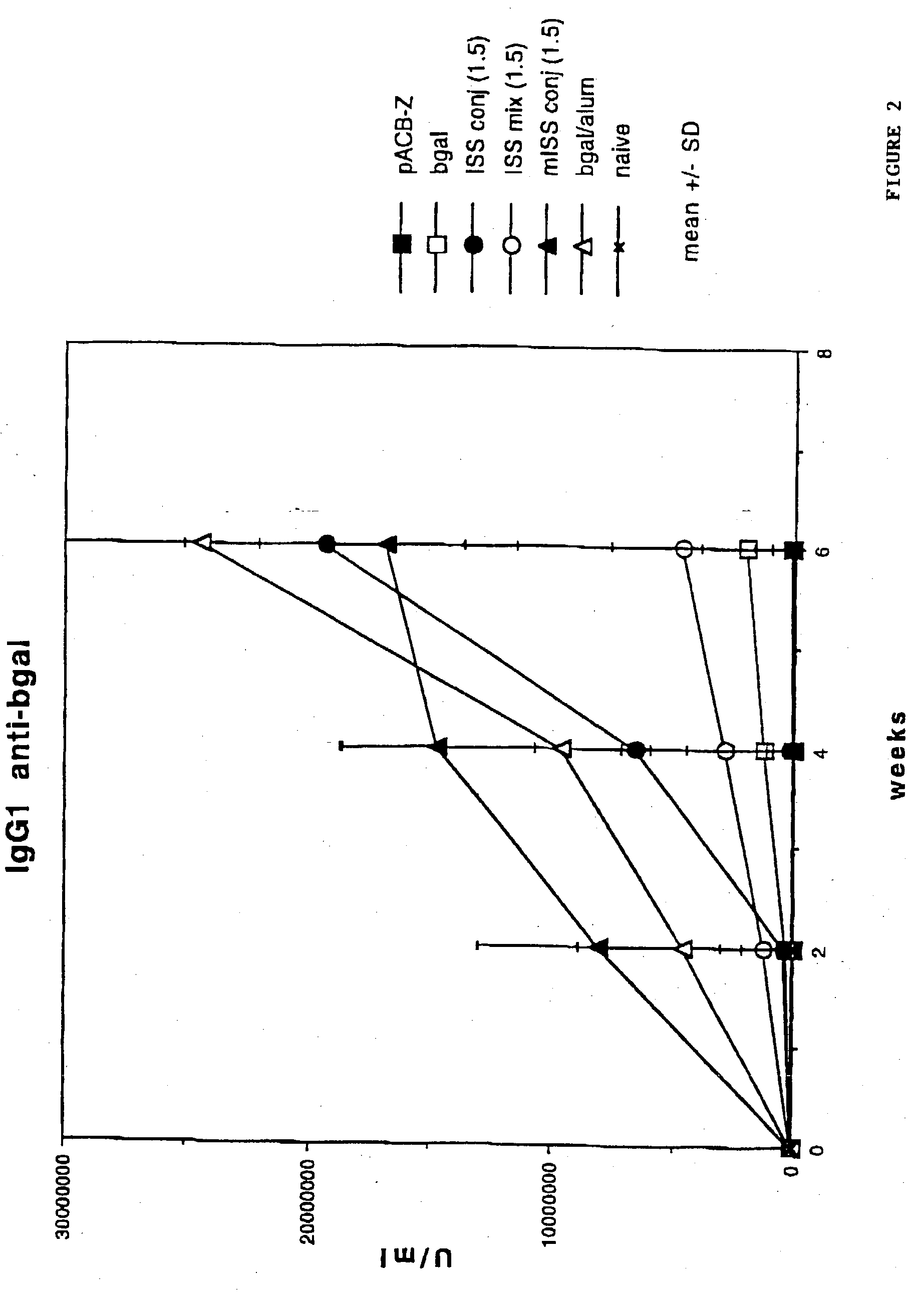
Immunostimulatory polynucleotide/immunomodulatory molecule conjugates
InactiveUS6610661B1Boost magnitudeBoost both humoral (antibody)Peptide/protein ingredientsGenetic material ingredientsAntigenAdjuvant
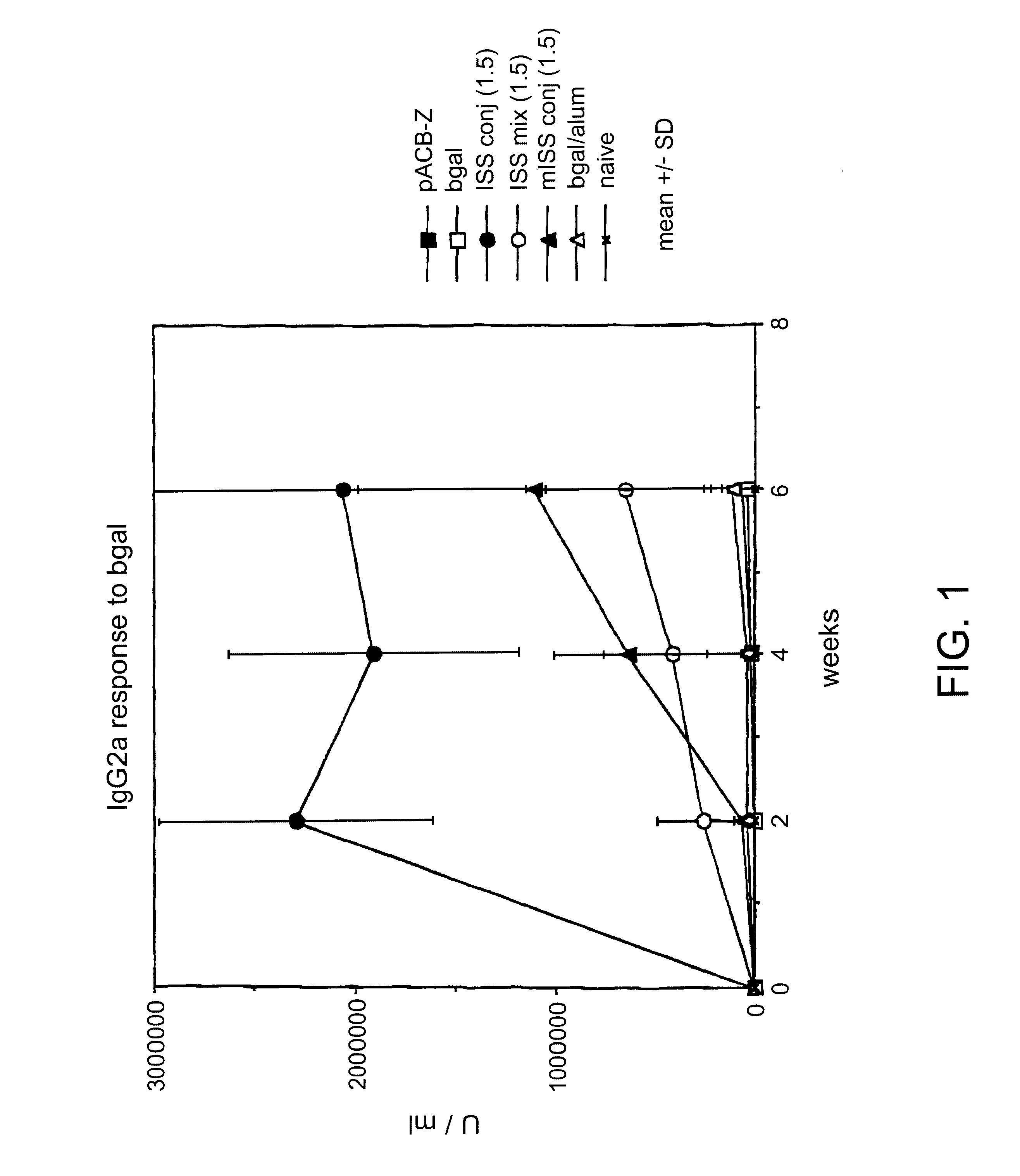
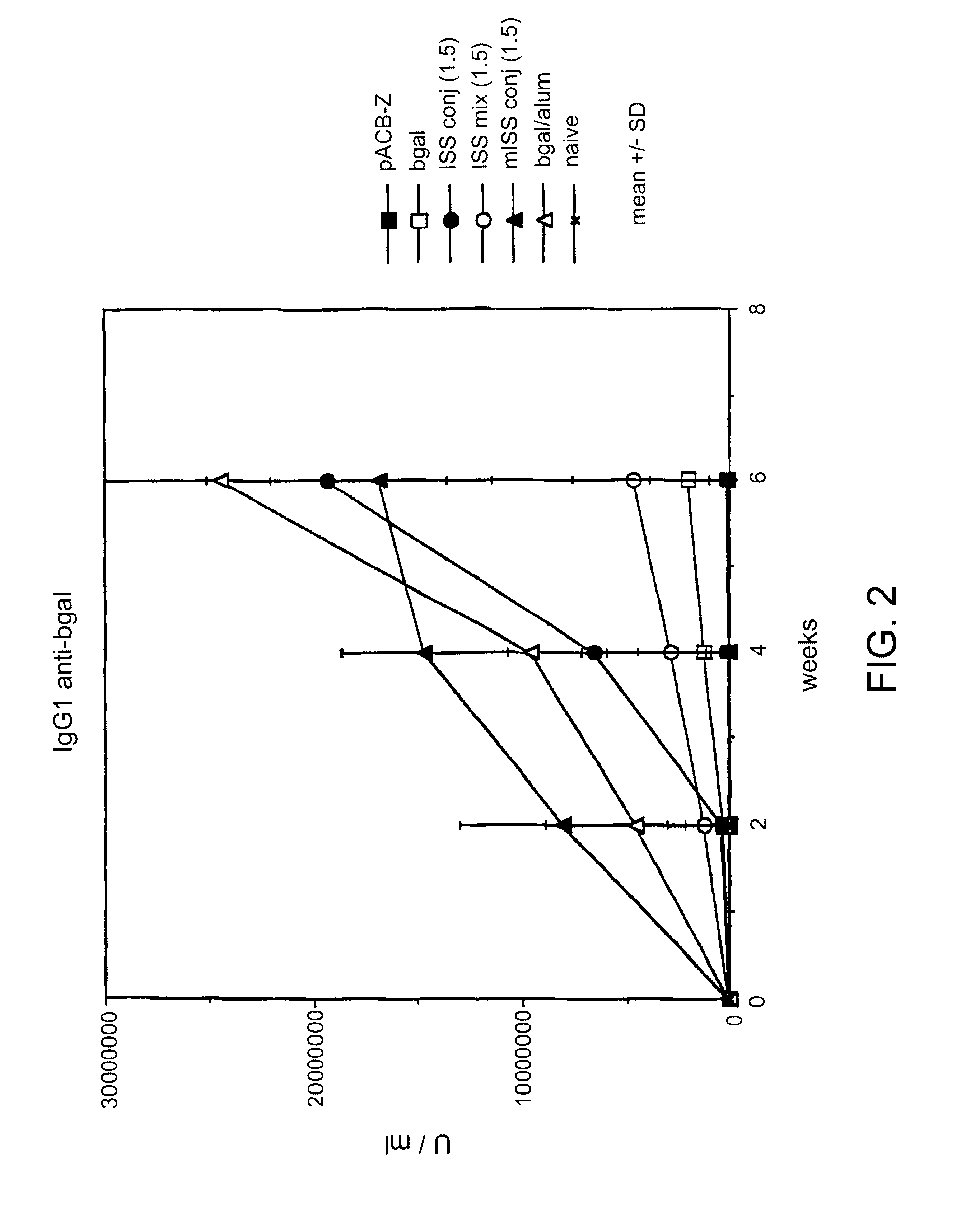
Immunostimulatory polynucleotide-immunomodulatory molecule conjugate compositions are disclosed. These compositions include a polynucleotide that is linked to an immunomodulatory molecule, which molecule comprises an antigen and may further comprise immunomodulators such as cytokines and adjuvants. The polynucleotide portion of the conjugate includes at least one immunostimulatory oligonucleotide nucleotide sequence (ISS). Methods of modulating an immune response upon administration of the polynucleotide-immunomodulatory conjugate preparation to a vertebrate host are also disclosed.
Owner:RGT UNIV OF CALIFORNIA
Multivalent pneumococcal polysaccharide-protein conjugate composition
An immunogenic composition having 13 distinct polysaccharide-protein conjugates and optionally, an aluminum-based adjuvant, is described. Each conjugate contains a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F) conjugated to a carrier protein. The immunogenic composition, formulated as a vaccine, increases coverage against pneumococcal disease in infants and young children globally, and provides coverage for serotypes 6A and 19A that is not dependent on the limitations of serogroup cross-protection. Methods for making an immunogenic conjugate comprising Streptococcus pneumoniae serotype 19A polysaccharide are also provided in which the serotype 19A polysaccharide is co-lyophilized with a carrier protein and conjugation is carried out in dimethyl sulfoxide (DMSO) via a reductive amination mechanism.
Owner:WYETH LLC
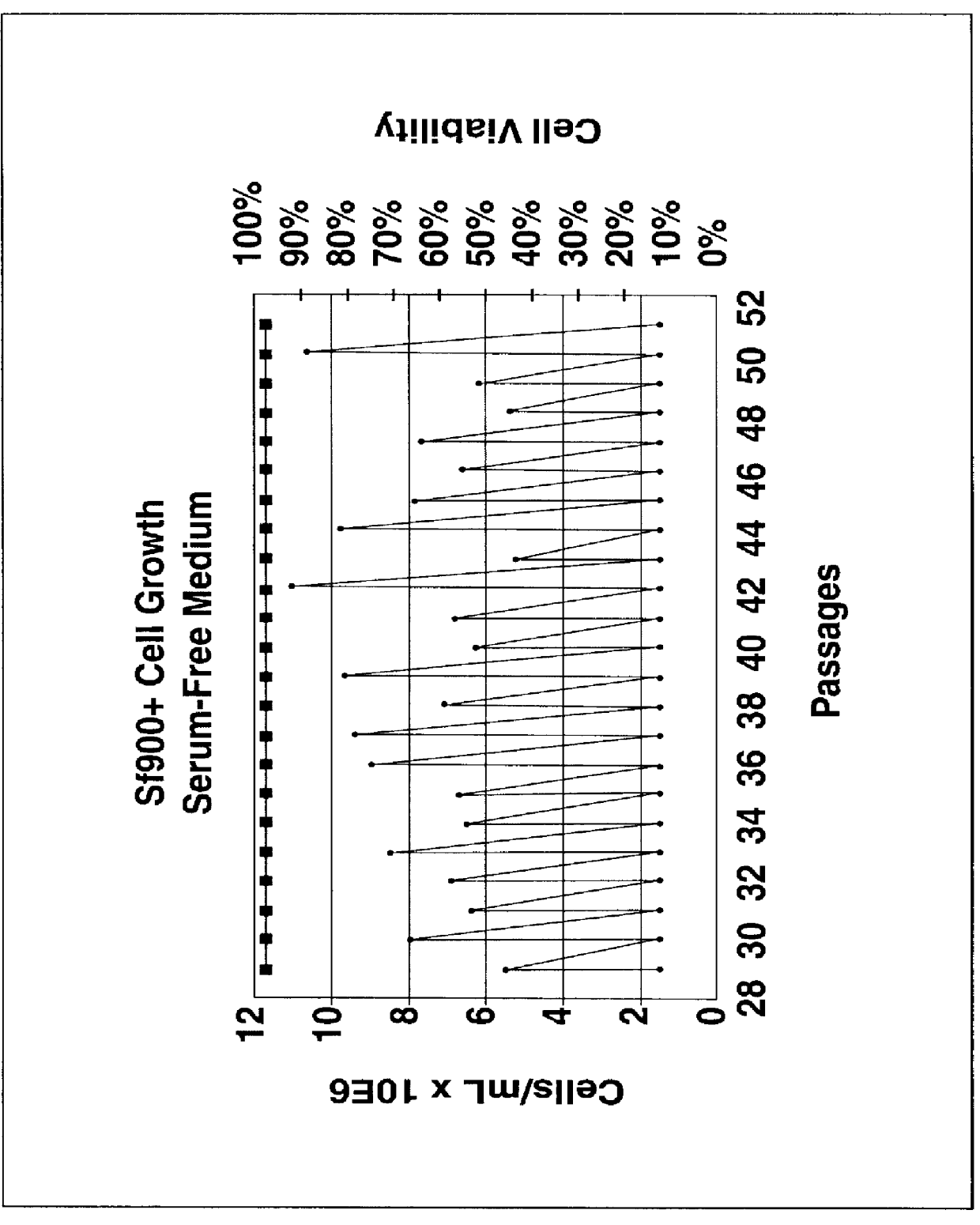
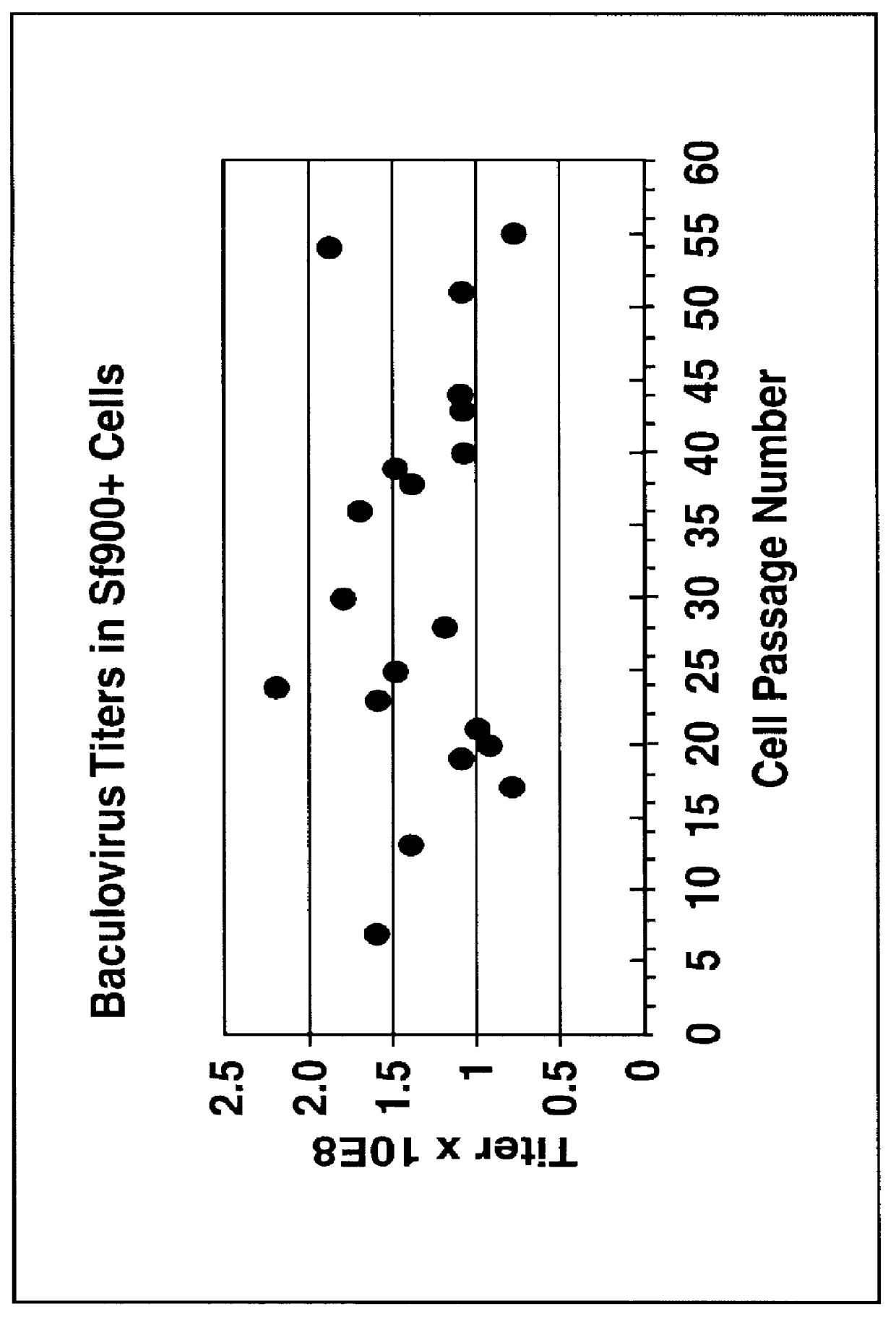
Spodoptera frugiperda single cell suspension cell line in serum-free media, methods of producing and using
InactiveUS6103526AAvoid infectionHigh densityConnective tissue peptidesInvertebrate cellsSerum free mediaAdjuvant
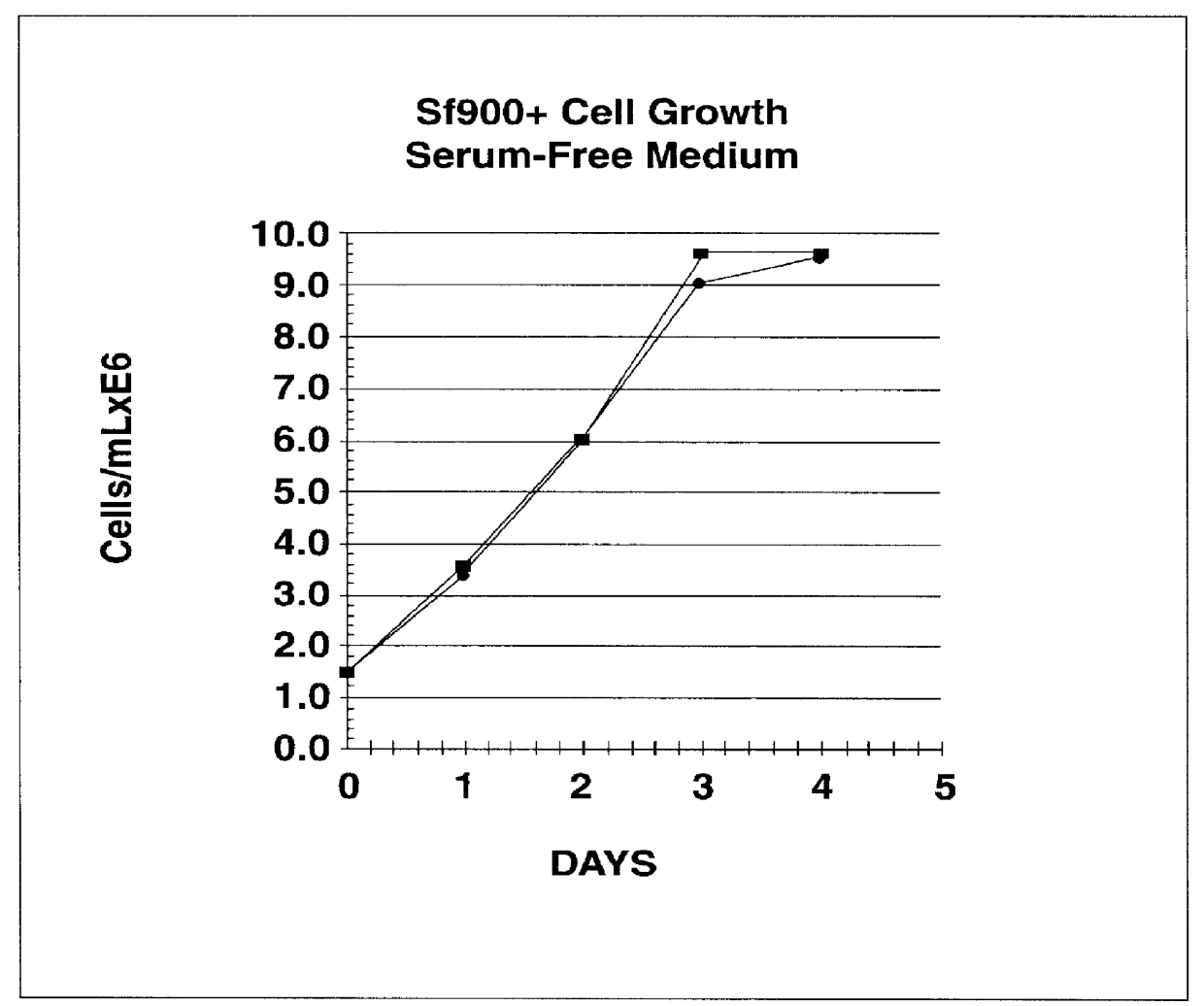
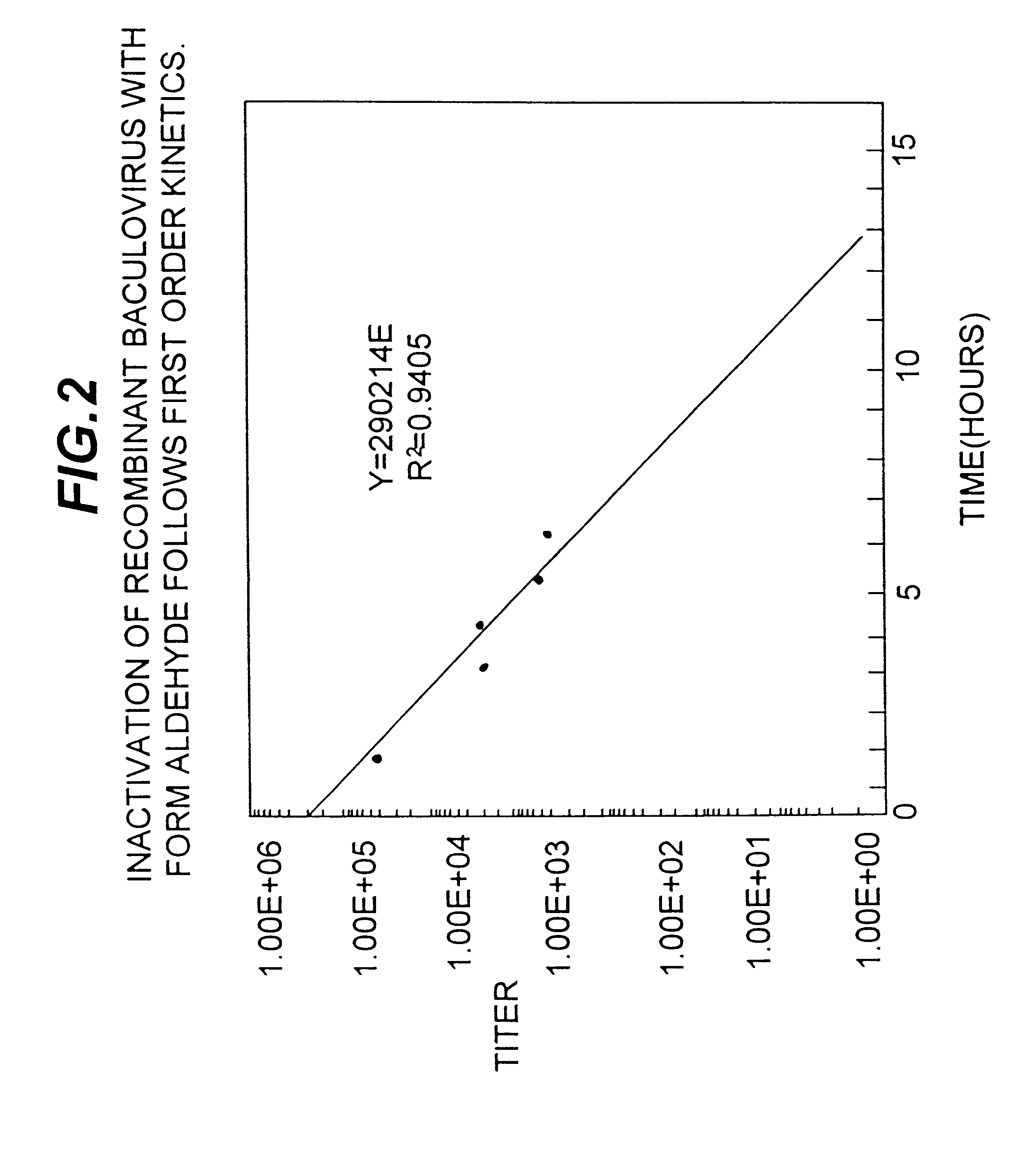
Disclosed and claimed is a new insect cell line, Sf900+, ATCC CRL-12579. The insect cell line was established from Lepidoptera, Noctuidae, Spodoptera frugiperda Sf-9 (ATCC CRL-1711) through multiple rounds of limiting dilution and selection in a serum-free insect medium supplemented with added human insulin. The insect cell line is useful in BEVS or as an adjuvant and has many characteristics and advantages. Also disclosed and claimed are recombinant proteins from recombinant baculovirus expression in insect cells such as Sf900+ cells, for instance, HA, NA, EPO, CD4, CEA, and thrombospondin.
Owner:PROTEIN SCI
Multivalent pneumococcal polysaccharide-protein conjugate composition
An immunogenic composition having 13 distinct polysaccharide-protein conjugates and optionally, an aluminum-based adjuvant, is described. Each conjugate contains a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F) conjugated to a carrier protein. The immunogenic composition, formulated as a vaccine, increases coverage against pneumococcal disease in infants and young children globally, and provides coverage for serotypes 6A and 19A that is not dependent on the limitations of serogroup cross-protection. Also described is a method for making an immunogenic conjugate comprising Streptococcus pneumoniae serotype 1 polysaccharide covalently linked to a carrier protein, the method including partial de-O-acetylation of the polysaccharide by mild hydrolysis in an alkaline pH buffer.
Owner:WYETH LLC
Multivalent pneumococcal polysaccharide-protein conjugate composition
An immunogenic composition having 13 distinct polysaccharide-protein conjugates and optionally, an aluminum-based adjuvant, is described. Each conjugate contains a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F) conjugated to a carrier protein. The immunogenic composition, formulated as a vaccine, increases coverage against pneumococcal disease in infants and young children globally, and provides coverage for serotypes 6A and 19A that is not dependent on the limitations of serogroup cross-protection. Also described is a method for making an immunogenic conjugate comprising Streptococcus pneumoniae serotype 3 polysaccharide covalently linked to a carrier protein, the method including periodic acid oxidation of the polysaccharide in the presence of bivalent cations.
Owner:WYETH LLC
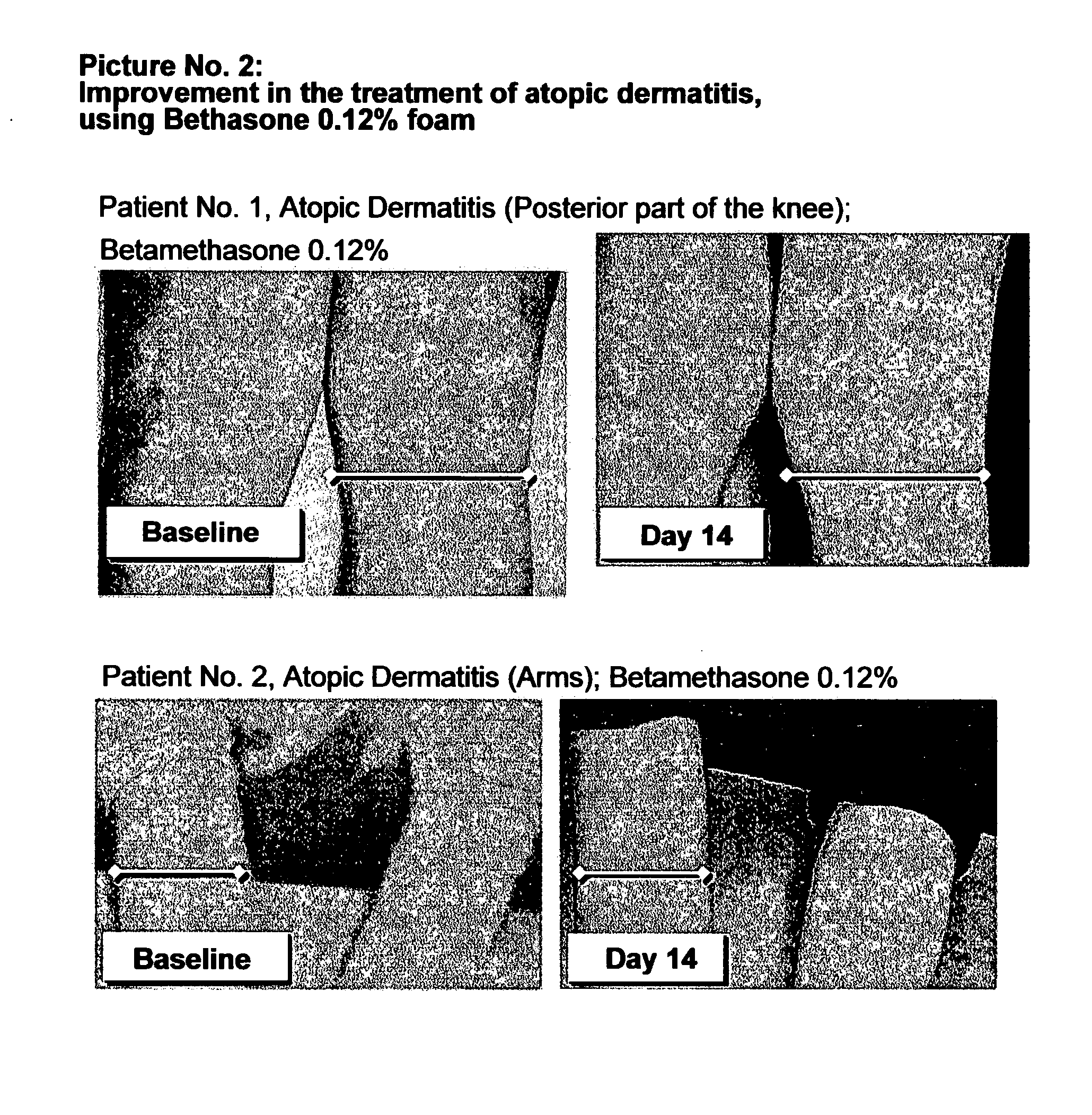
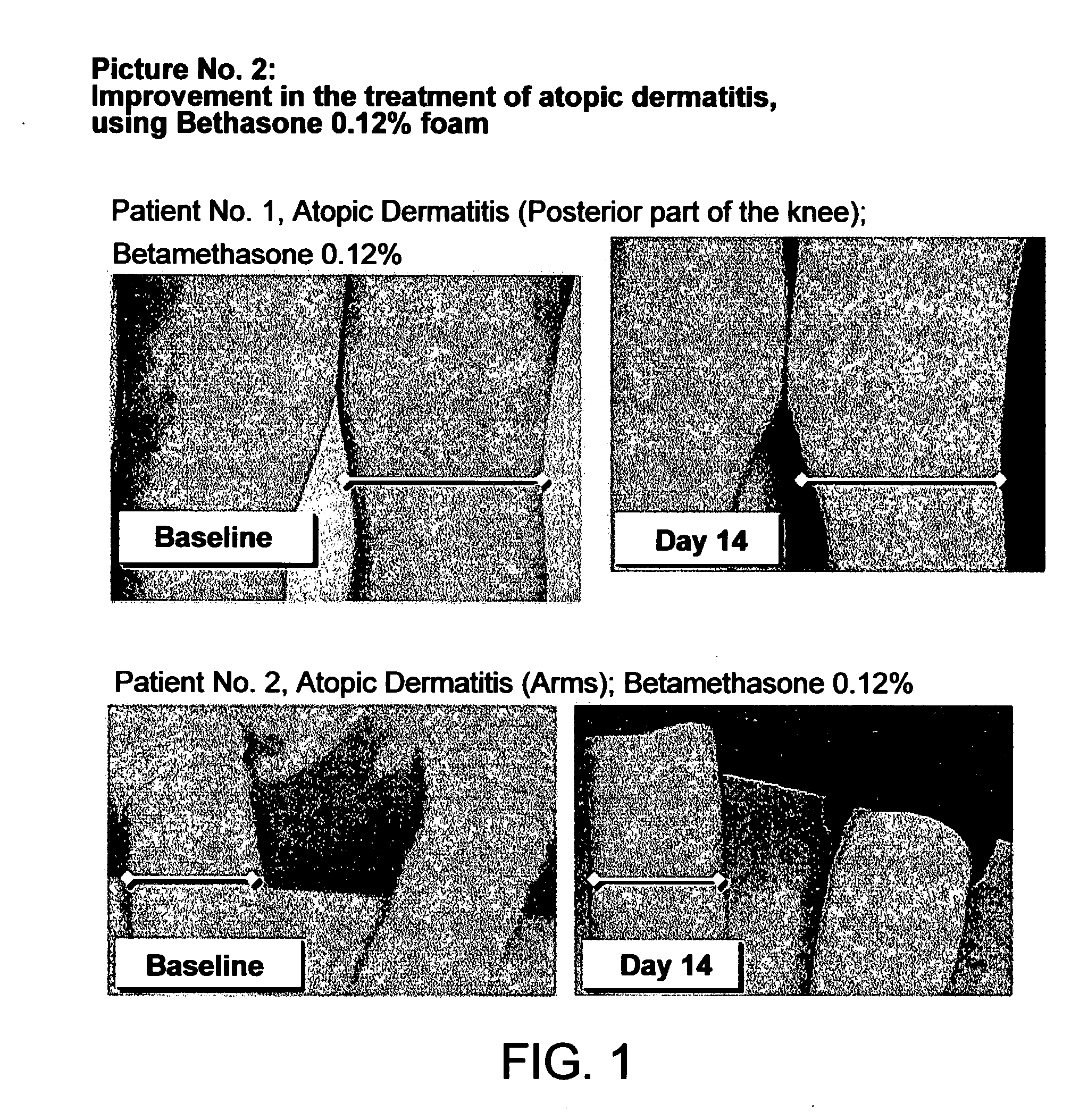
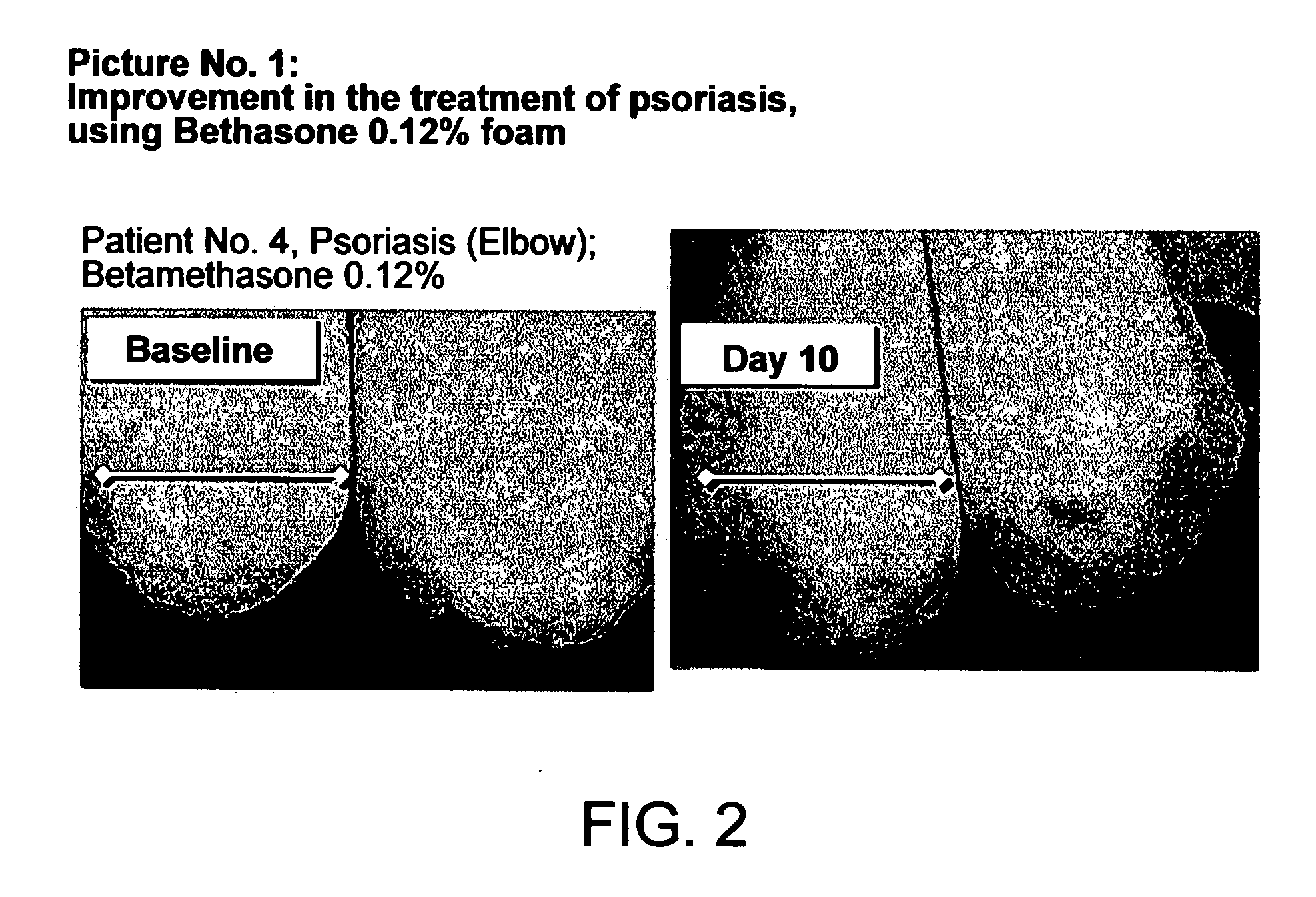
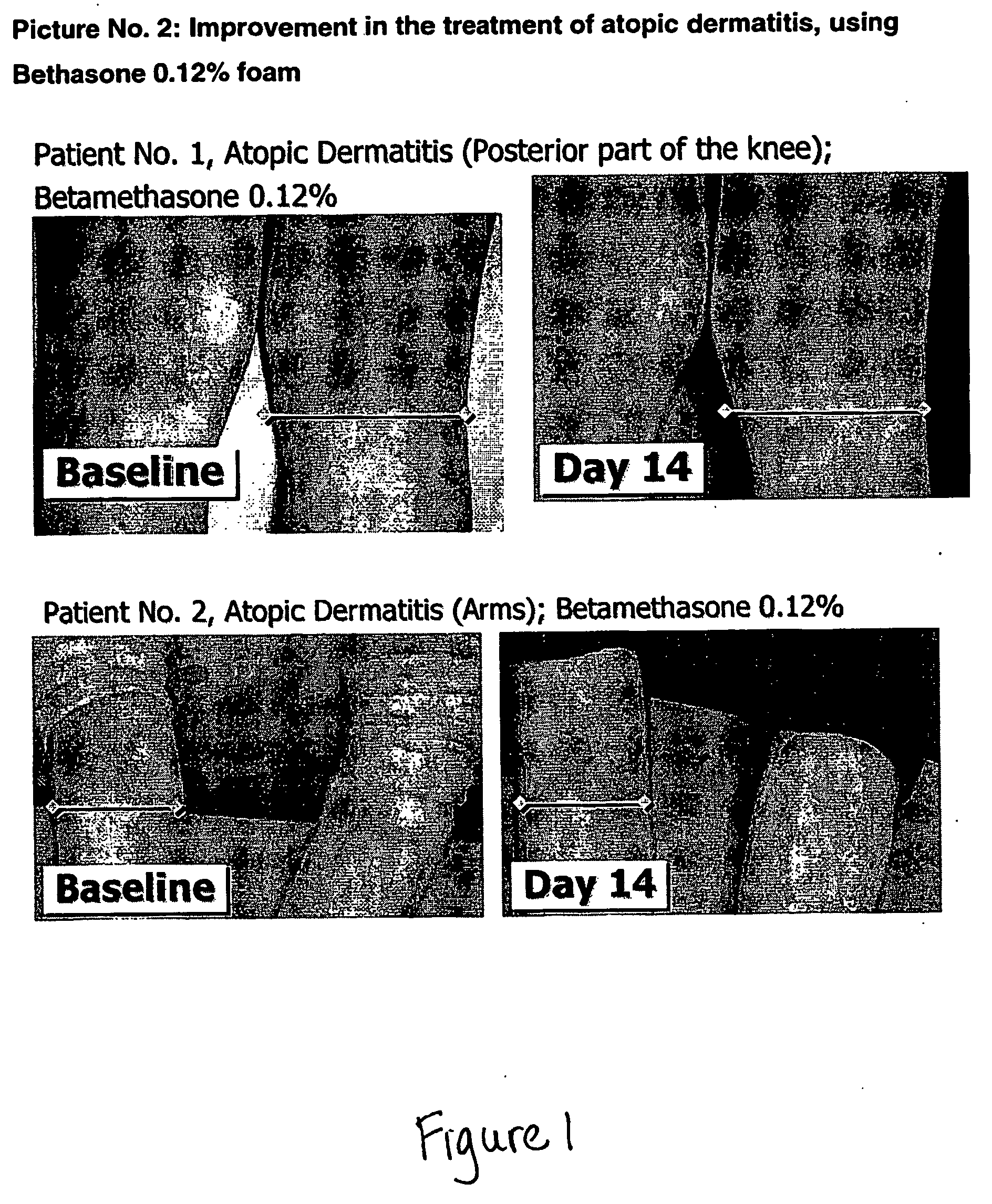
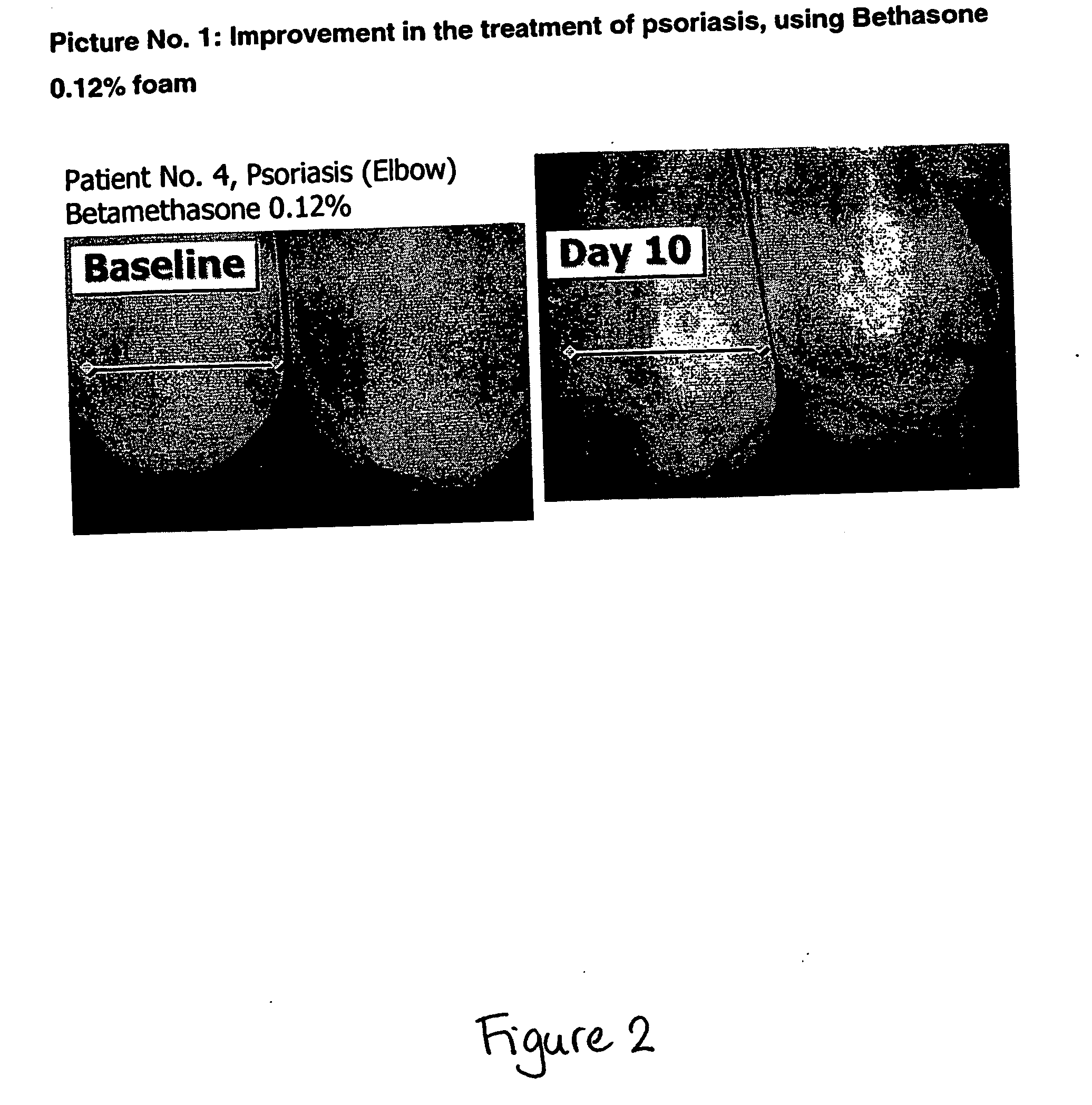
Cosmetic and pharmaceutical foam
InactiveUS20080031907A1Efficient ConcentrationReduce sensitivityAntibacterial agentsBiocideAlcohol freeVegetable oil
The invention relates to uses of an alcohol-free cosmetic or pharmaceutical foam carrier comprising water, a hydrophobic solvent, a foam adjuvant agent, a surface-active agent and a water gelling agent as a flame retardant or flame resistant foam. The hydrophobic solvent is preferably mineral oil; medium chain triglycerides; isopropyl myristearate or octyl dodecanol, silicone oil or vegetable oil or mixtures thereof. The cosmetic or pharmaceutical foam carrier does not contain aliphatic alcohols, also making it non-irritating and non-drying. The alcohol-free foam carrier is suitable for inclusion of both water-soluble and oil-soluble pharmaceutical and cosmetic agents.
Owner:FOAMIX PHARMACEUTICALS LIMITED
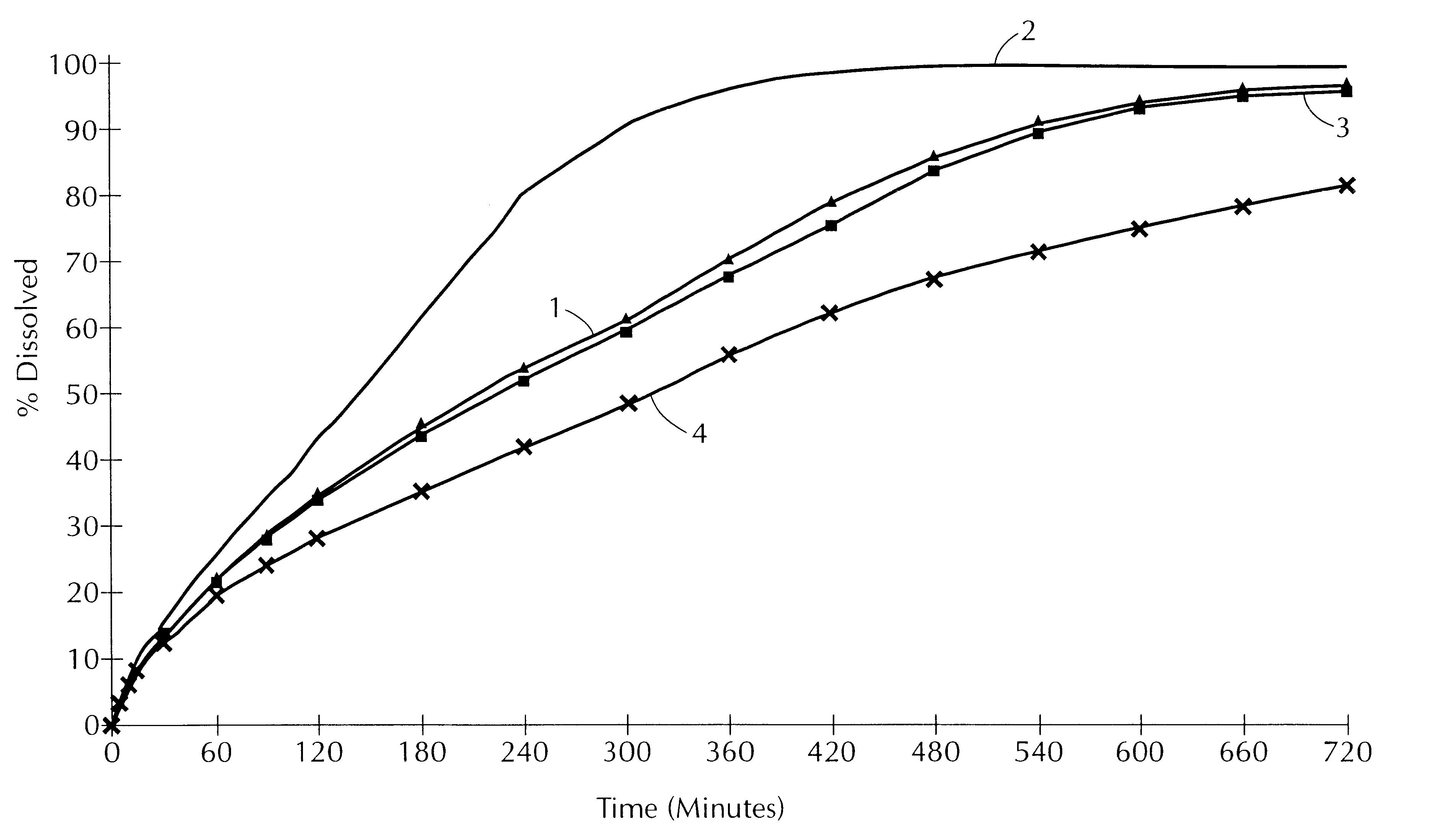
Process for the manufacture of pharmaceutical composition with modified release of active principle comprising the matrix
The invention relates to a process for the manufacture of a pharmaceutical composition with modified release of active principle comprising at least one active principle, a lipid matrix agent composed of ester of alcohol with at least one fatty acid and at least one adjuvant.This process is characterized in that:a powder composed of at least one component selected in the group comprising the active principle and the adjuvant, is mixed, while heating and fluidizing, in order to obtain individual grains;the said lipid matrix agent is liquefied separately under warm conditions;the said powder is then coated under warm conditions by spraying the said lipid matrix agent over the individual grains;finally, the temperature of the combined product is lowered in order to allow the lipid matrix agent to solidify.
Owner:GATTEFOSSE HLDG
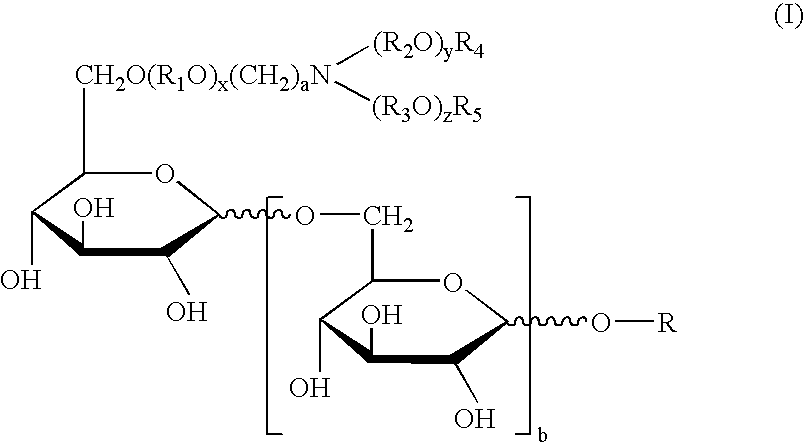
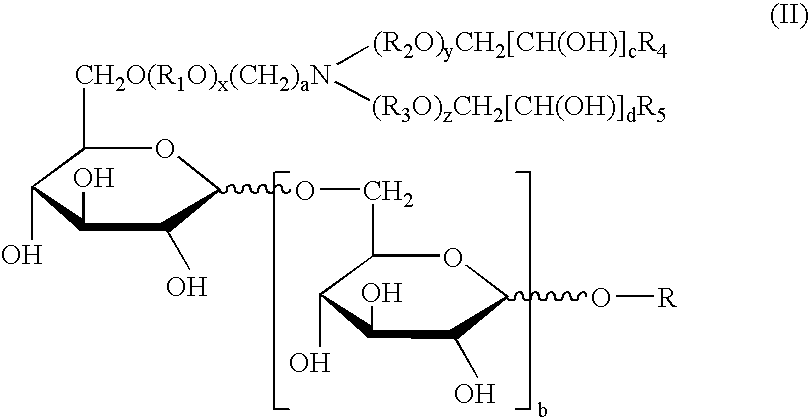
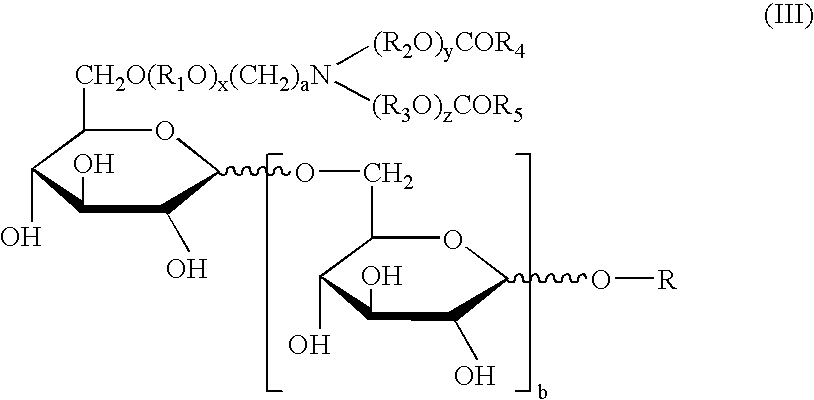
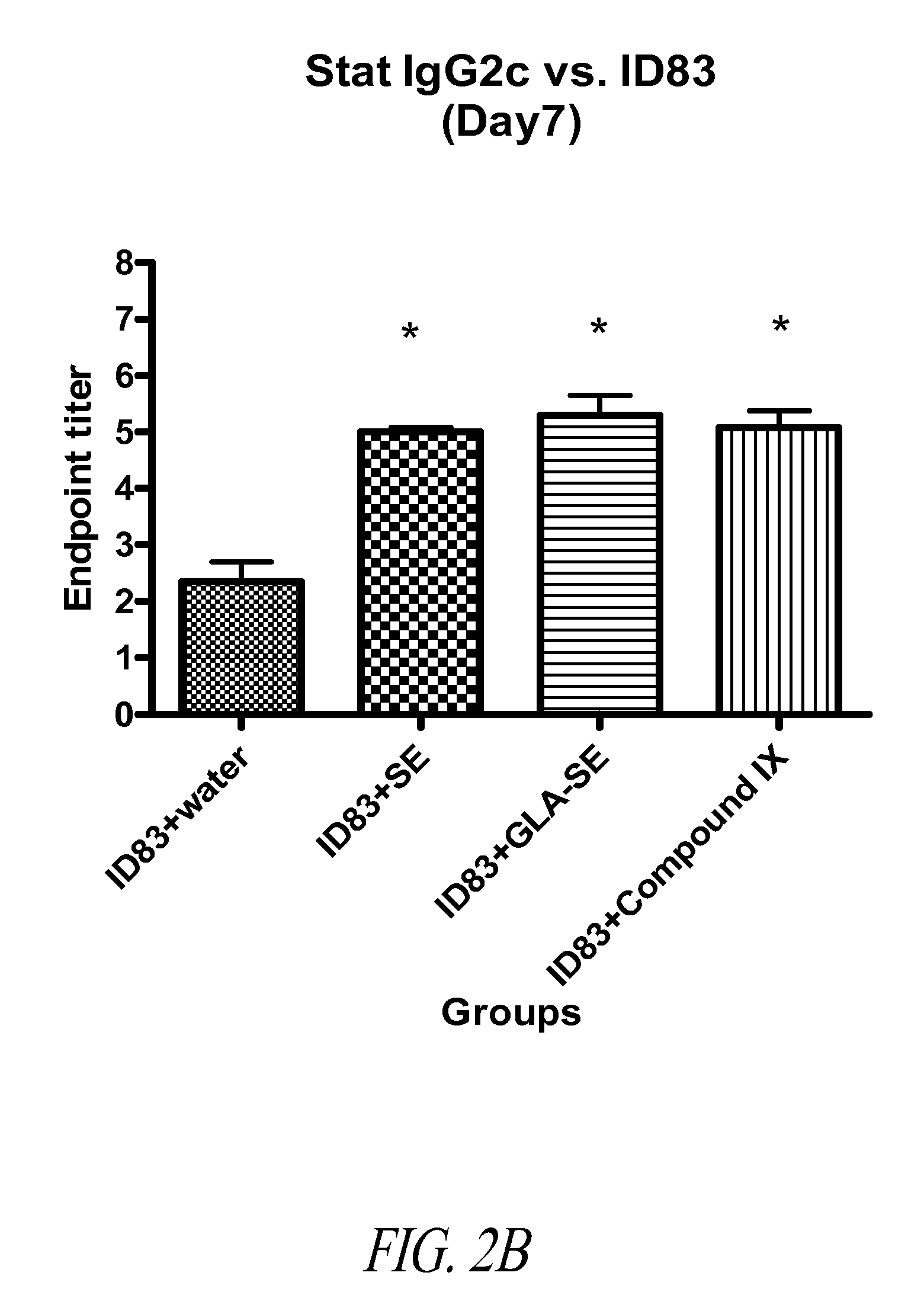
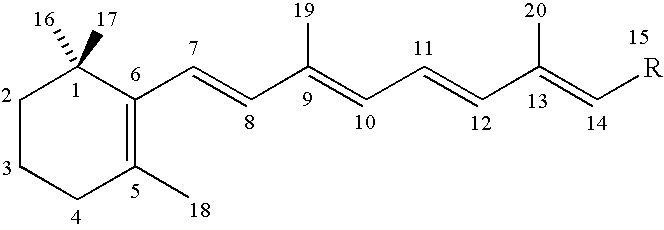
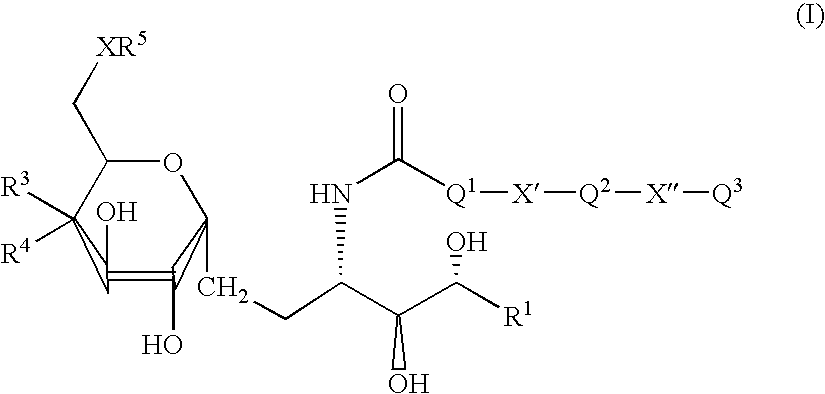
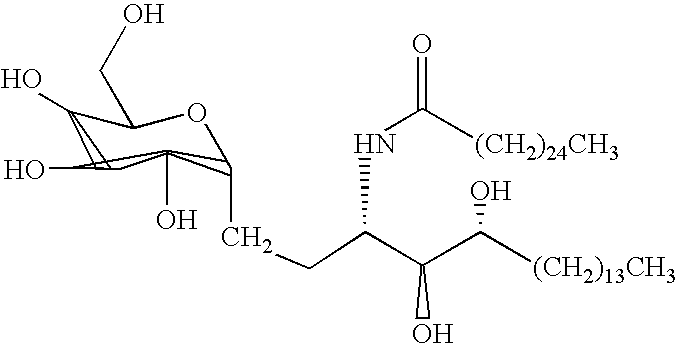
Synthetic glucopyranosyl lipid adjuvants
Compounds, particularly, glucopyranosyl lipid adjuvant (GLA) compounds, having the following structure (I) are provided:or a pharmaceutically acceptable salt thereof, wherein L1, L2, L3, L4, L5, L6, L7, L8, L9, L10, Y1, Y2, Y3, Y4, R1, R2, R3, R4, R5, R6, are as defined herein. Pharmaceutical compositions, vaccine compositions, and related methods for inducing or enhancing immune responses, are also provided.
Owner:ACCESS TO ADVANCED HEALTH INST
Cosmetic and pharmaceutical foam
InactiveUS20060140984A1Lower yield strengthRubbing easy and efficientCosmetic preparationsBiocideAlcohol freeAdjuvant
The invention relates to an alcohol-free cosmetic or pharmaceutical foam carrier comprising water, a hydrophobic solvent, a foam adjuvant agent, a surface-active agent and a water gelling agent. The cosmetic or pharmaceutical foam carrier does not contain aliphatic alcohols, making it non-irritating and non-drying. The alcohol-free foam carrier is suitable for inclusion of both water-soluble and oil soluble pharmaceutical and cosmetic agents.
Owner:FOAMIX PHARMACEUTICALS LIMITED
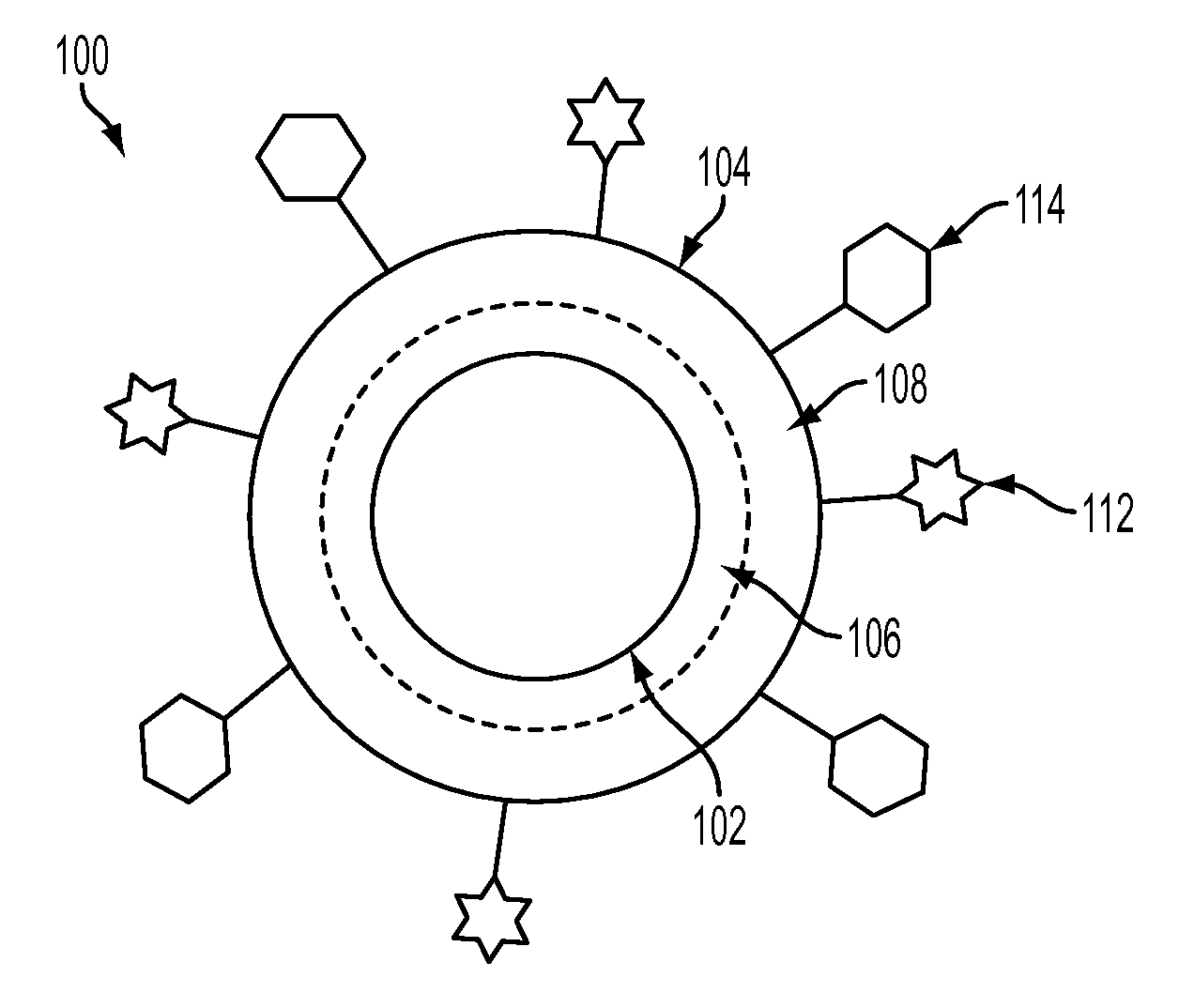
Nanoparticle Based Immunological Stimulation
A nanoparticle-based delivery system and methods for its use are disclosed. In one aspect, a nanoparticle-based delivery system comprising at least one molecule such as proteins, DNA / RNA or fragments thereof, carbohydrates, enzymes, chemicals, virus cells, bacteria, parts of a virus, parts of a bacteria, parts of a cell, part of a tissue, or a combination of one or more of these, which shall be referred to as immunogens, are chemically or physically combined with water soluble nanoparticles which, when administered to a living system, is capable of eliciting a desired immunological response. More particularly, the invention relates to nanoparticle-based delivery systems that are specifically engineered to enhance humoral or cellular immune response without the use of adjuvants.
Owner:UNIV OF HAWAII +1
Body cavity foams
The invention relates to an alcohol-free cosmetic or therapeutic foam carrier comprising water, a hydrophobic organic carrier, a foam adjuvant agent, a surface-active agent and a gelling agent. The cosmetic or therapeutic foam carrier does not contain aliphatic alcohols, making it non-irritating and non-drying. The alcohol-free foam carrier is suitable for inclusion of both water-soluble and oil soluble therapeutic and cosmetic agents.
Owner:VYNE THERAPEUTICS INC
Retinoid immunomodulating kit and composition and uses thereof
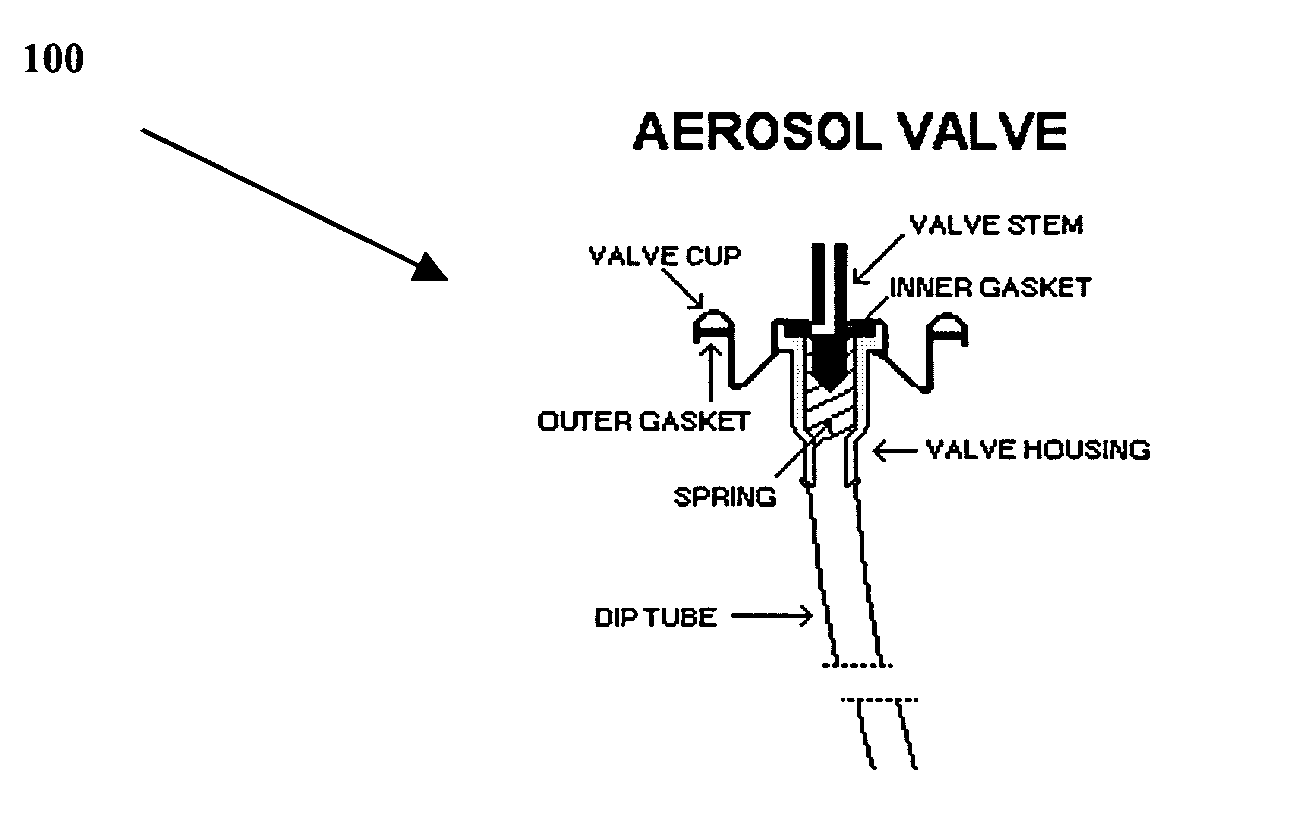
A composition and therapeutic kit including an aerosol packaging assembly including a container accommodating a pressurized product and an outlet capable of releasing a foamable composition, including a retinoid as a foam. The pressurized product includes a foamable composition including: a container accommodating a pressurized product; and an outlet capable of releasing the pressurized product as a foam; wherein the pressurized product comprises a foamable composition including: i. a retinoid; ii. at least one organic carrier selected from the group consisting of a hydrophobic organic carrier, a polar solvent, an emollient and mixtures thereof, at a concentration of about 2% to about 50% by weight; iii. a surface-active agent; iv. about 0.01% to about 5% by weight of at least one polymeric additive selected from the group consisting of a bioadhesive agent, a gelling agent, a film forming agent and a phase change agent; v. water; and vi. liquefied or compressed gas propellant at a concentration of about 3% to about 25% by weight of the total composition. The composition further may include a therapeutically active foam adjuvant, selected from the group consisting of a fatty alcohol, a fatty acid, a hydroxyl fatty acid; and mixtures thereof.
Owner:FOAMIX PHARMACEUTICALS LIMITED
Adjuvant in the form of a lipid-modified nucleic acid
The present invention relates to an immune-stimulating adjuvant in the form of a lipid-modified nucleic acid, optionally in combination with further adjuvants. The invention relates further to a pharmaceutical composition and to a vaccine, each containing an immune-stimulating adjuvant according to the invention, at least one active ingredient and optionally a pharmaceutically acceptable carrier and / or further auxiliary substances and additives and / or further adjuvants. The present invention relates likewise to the use of the pharmaceutical composition according to the invention and of the vaccine according to the invention for the treatment of infectious diseases or cancer diseases. Likewise, the present invention includes the use of the immune-stimulating adjuvant according to the invention in the preparation of a pharmaceutical composition for the treatment of cancer diseases or infectious diseases.
Owner:CUREVAC GMBH
Methods for treating and preventing infectious disease
InactiveUS20050101554A1High sensitivityAntibacterial agentsGenetic material ingredientsAdjuvantNucleic acid sequencing
Nucleic acid sequences containing unmethylated CpG dinucleotides that modulate an immune response including stimulating a Th1 pattern of immune activation, cytokine production, NK lytic activity, and B cell proliferation are disclosed. The sequences are also useful as a synthetic adjuvant.
Owner:UNIV OF IOWA RES FOUND +2
Materials and process for enhancing selective separations
Use of a Maillard reaction product as an adjuvant in a variety of applications including solid-liquid separations, corrosion inhibition, emulsification, dust suppression, slow release fertilization, viscosity modification and others and especially as a depressant or collector in separation processes, including the selective separation of solids and / or ionic species from aqueous media, such as in the process of froth flotation.
Owner:GEORGIA PACIFIC CHEM LLC
Method for stimulating the immune, inflammatory or neuroprotective response
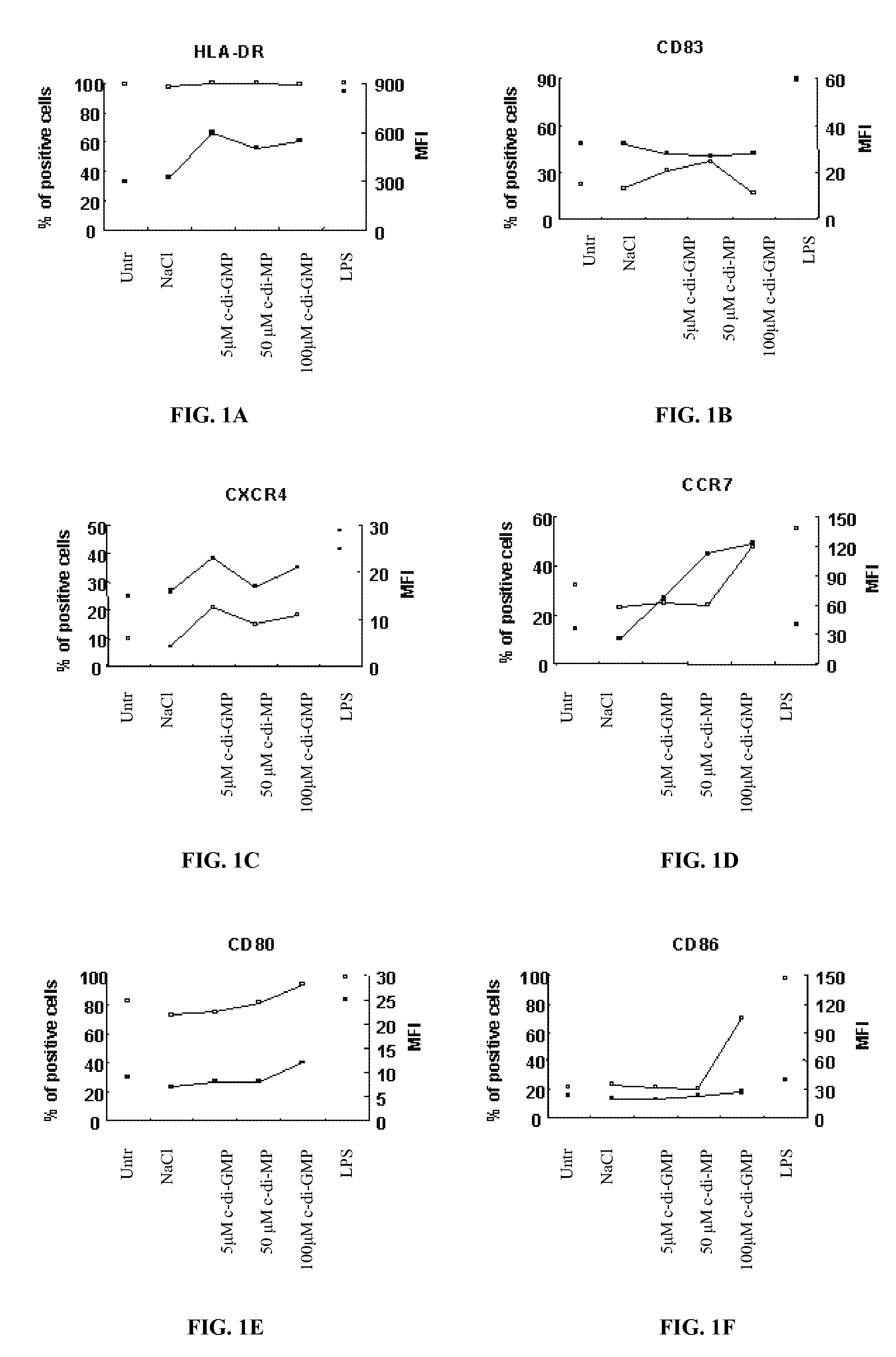
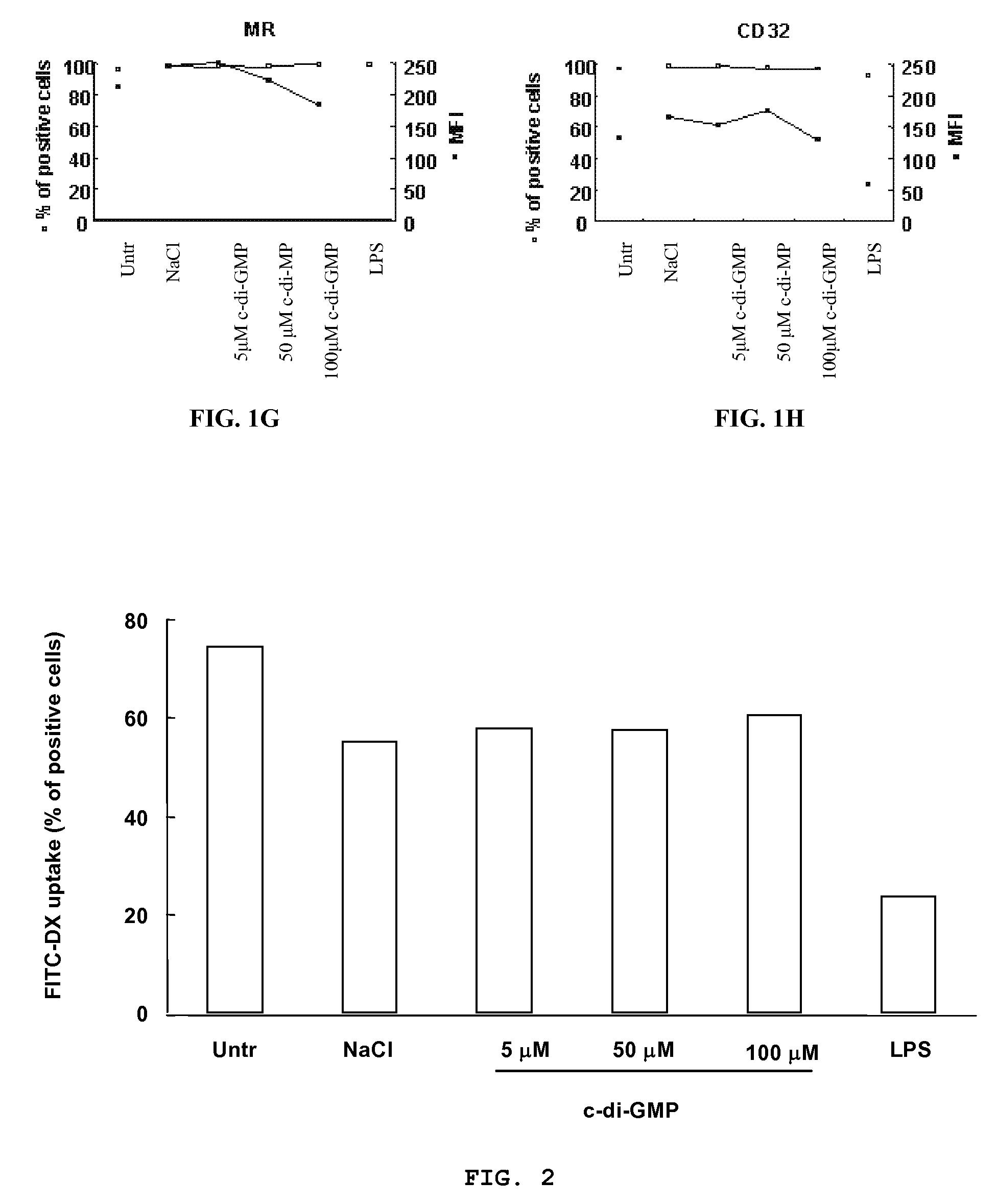
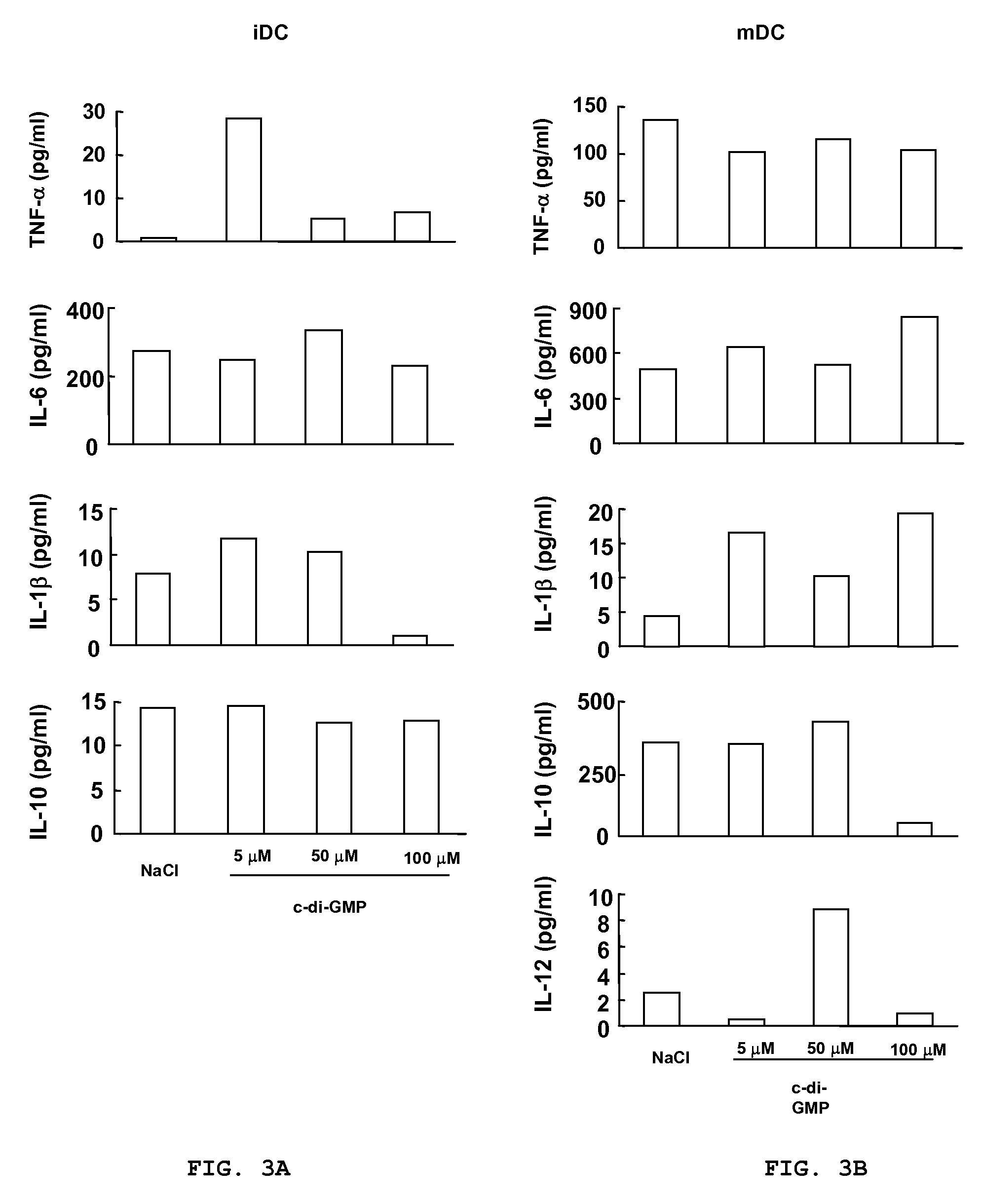
Cycic di-GMP, or a cyclic dinucleotide analogue thereof that has the same effect as cyclic di-GMP, stimulates or enhances immune or inflammatory response in a patient or enhances the immune response to a vaccine by serving as an adjuvant. Cyclic di-GMP, or a cyclic dinucleotide analogue thereof, also has neuroprotective properties for use as a neuroprotective agent to inhibit, treat, or ameliorate the effects of injuries, diseases,
Owner:KARAGEN PHARMA INC +1
Use of synthetic glycolipids as universal adjuvants for vaccines against cancer and infectious diseases
InactiveUS20050192248A1Enhancement and extension of durationBiocideOrganic active ingredientsDiseaseAdjuvant
The present invention relates to methods and compositions for augmenting an immunogenicity of an antigen in a mammal, comprising administering said antigen together with an adjuvant composition that includes a synthetic glycolipid compound of Formula I, as described herein. According to the present invention, the use of a compound of Formula I as an adjuvant is attributed at least in part to the enhancement and / or extension of antigen-specific Th1-type responses, in particular, CD8+ T cell responses. The methods and compositions of the present invention can be useful for prophylaxis and treatment of various infectious and neoplastic diseases.
Owner:NEW YORK UNIV +1
Natural Juncao liver-nourishing and sobering-up agent
The invention describes a health product additive and preparation for sots or hepatitis B pathogen carriers By adding medicinal fungus fermentation liquor as additive and using effective components and fine powder extracted from plants and herbal medicines by solvent as adjuvant, the health product can be made into forms of effervescent tablet, buccal tablet, chewable tablet, oral taken tablet, candy, chocolate, chewing gum, oral liquid, particle, soluble granules, capsule, aerosol, liquid beverage and solid beverage. The product can relieve alcohol effect, nourish stomach, protect liver, and help to remit hepatitis B virus and hepatitis liver cancer patient condition, achieves a quite important effect for protecting the health of the sots or hepatitis B pathogen carriers in daily or social occasions. The invention provides a good idea to the utilization of the large amount of active fermentation liquid generated with the thallus pharmacy in the medicinal fungus fermentation industries, and also provides a effective approach for increasing the utilization value of the large amount of wild plant resources such as wild jujube and haw widely distributed in the north areas.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Immunostimulatory polynucleotide/immunomodulatory molecule conjugates
InactiveUS20040006010A1Boost magnitudeBoost both humoral (antibody)BiocideOrganic active ingredientsAntigenAdjuvant
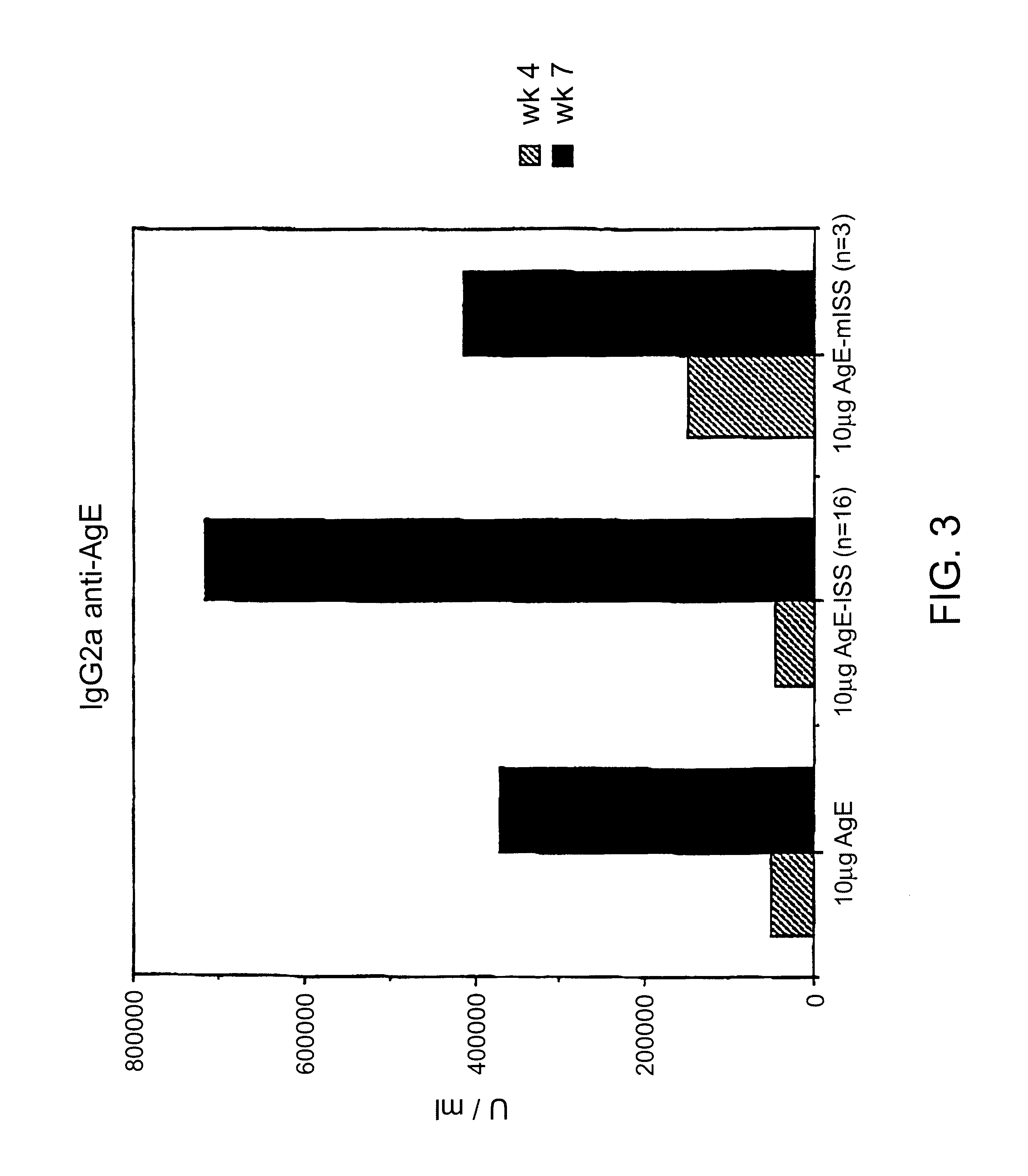
Immunostimulatory polynucleotide-immunomodulatory molecule conjugate compositions are disclosed. These compositions include a polynucleotide that is linked to an immunomodulatory molecule, which molecule comprises an antigen and may further comprise immunomodulators such as cytokines and adjuvants. The polynucleotide portion of the conjugate includes at least one immunostimulatory oligonucleotide nucleotide sequence (ISS). Methods of modulating an immune response upon administration of the polynucleotide-immunomodulatory conjugate preparation to a vertebrate host are also disclosed.
Owner:RGT UNIV OF CALIFORNIA
Method of inducing an antigen-specific immune response by administering a synergistic combination of adjuvants comprising unmethylated CpG-containing nucleic acids and a non-nucleic acid adjuvant
The present invention relates generally to adjuvants, and in particular to methods and products utilizing a synergistic combination of immunostimulatory oligonucleotides having at least one unmethylated CpG dinucleotide (CpG ODN) and a non-nucleic acid adjuvant. Such combinations of adjuvants may be used with an antigen or alone. The present invention also relates to methods and products utilizing immunostimulatory oligonucleotides having at least one unmethylated CpG dinucleotide (CpG ODN) for induction of cellular immunity in infants.
Owner:UNIV OF IOWA RES FOUND +2
HERCEPTIN adjuvant therapy
ActiveUS20060275305A1Decrease subject ' likelihood of cancer recurrenceIncrease subject ' likelihood of survivalBiocideOrganic active ingredientsAdjuvantAdjuvant therapy
Owner:GENENTECH INC
Insect cells or fractions as adjuvant for antigens
Disclosed and claimed is an adjuvant for immunogenic, immunological, antigenic or vaccine compositions. The adjuvant is composed of insect cells or fractions thereof. Disclosed and claimed are also methods for preparing and using the adjuvant and compositions containing the adjuvant. Advantageously, a recombinant baculovirus containing DNA encoding and expressing an epitope of interest or antigen can be infected into insect cells such as insect cells derived from a Lepidopteran species such as S. frugiperda for expression, and the infected insect cells or a fraction thereof can be used with the expressed epitope of interest or antigen as an inventive antigen or in an inventive immunological, antigen or vaccine composition.
Owner:MERIAL LTD
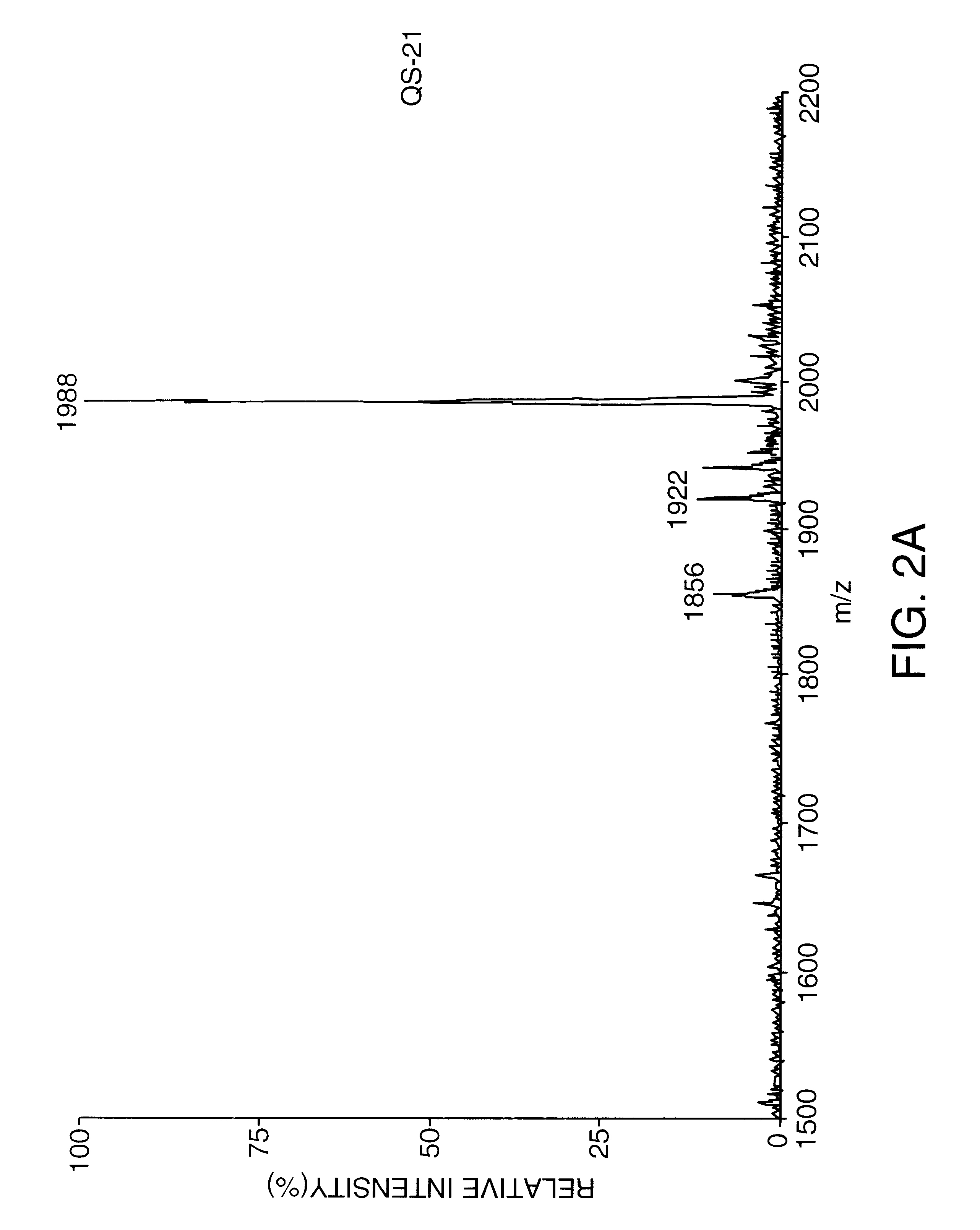
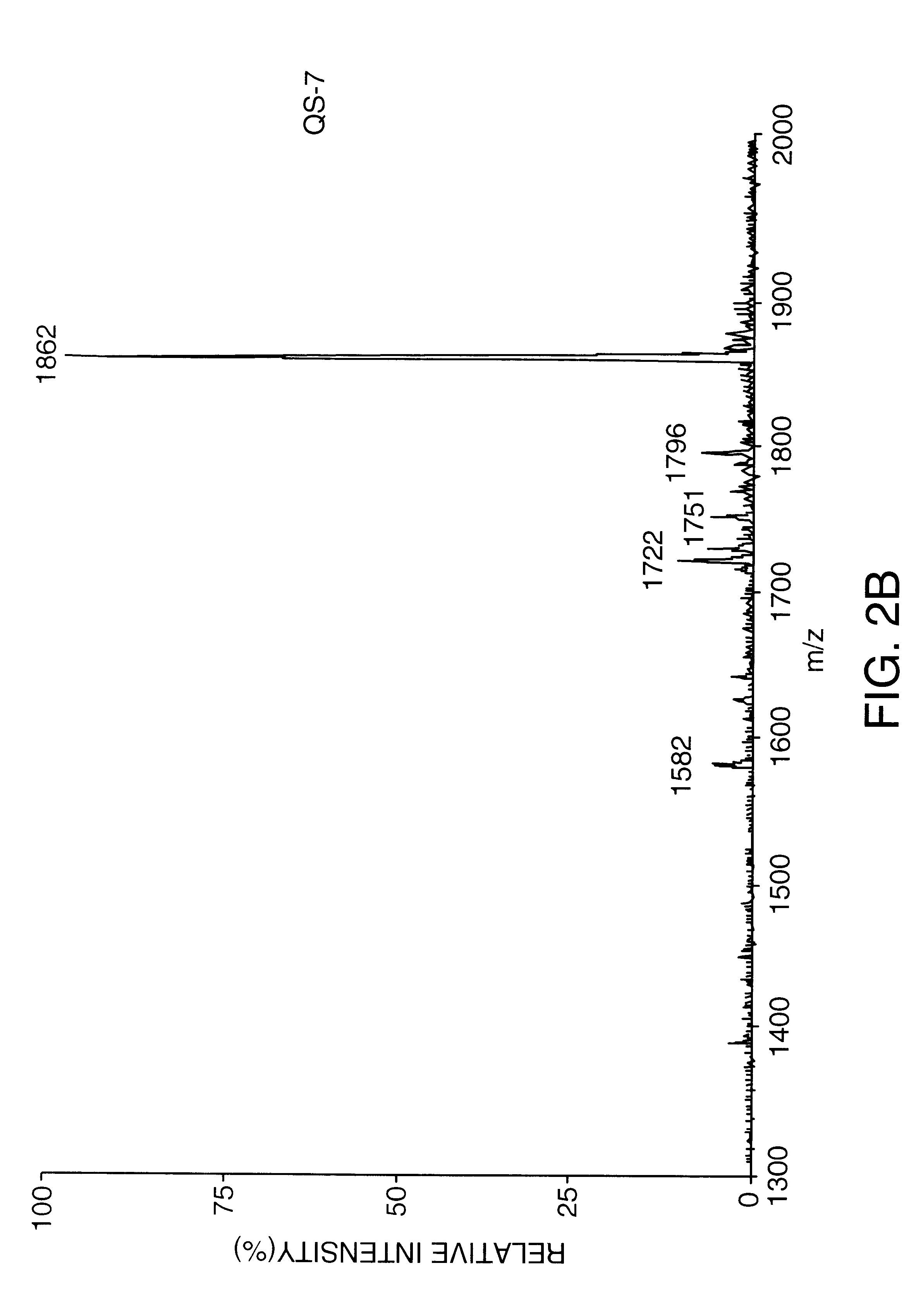
Saponin compositions and uses thereof
Owner:ANTIGENICS
High-frequency electroporation for cancer therapy
ActiveUS20120109122A1Enhanced couplingMore predictable and uniform treatment outcomesElectrotherapySurgical instruments for heatingDiseaseAdjuvant
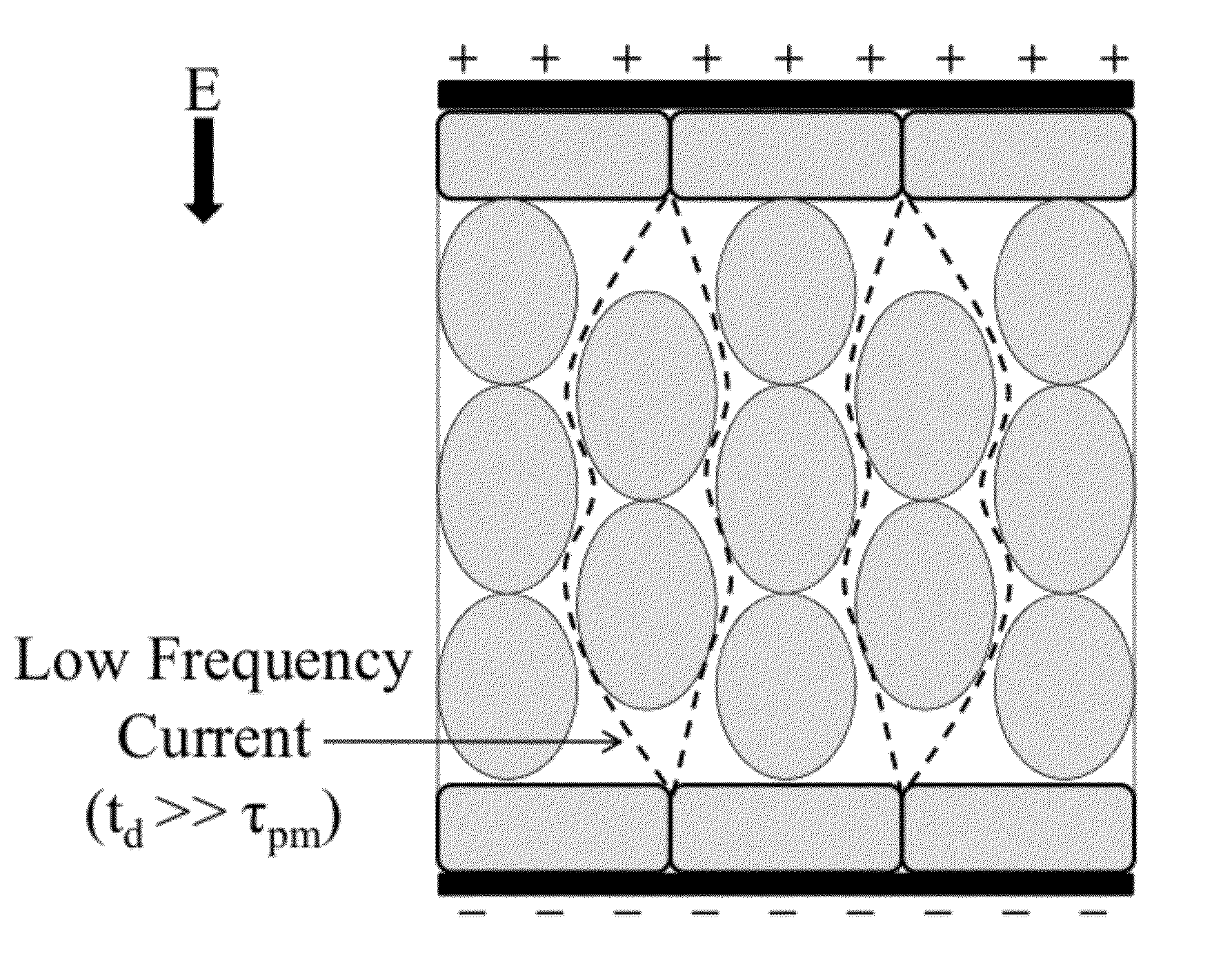
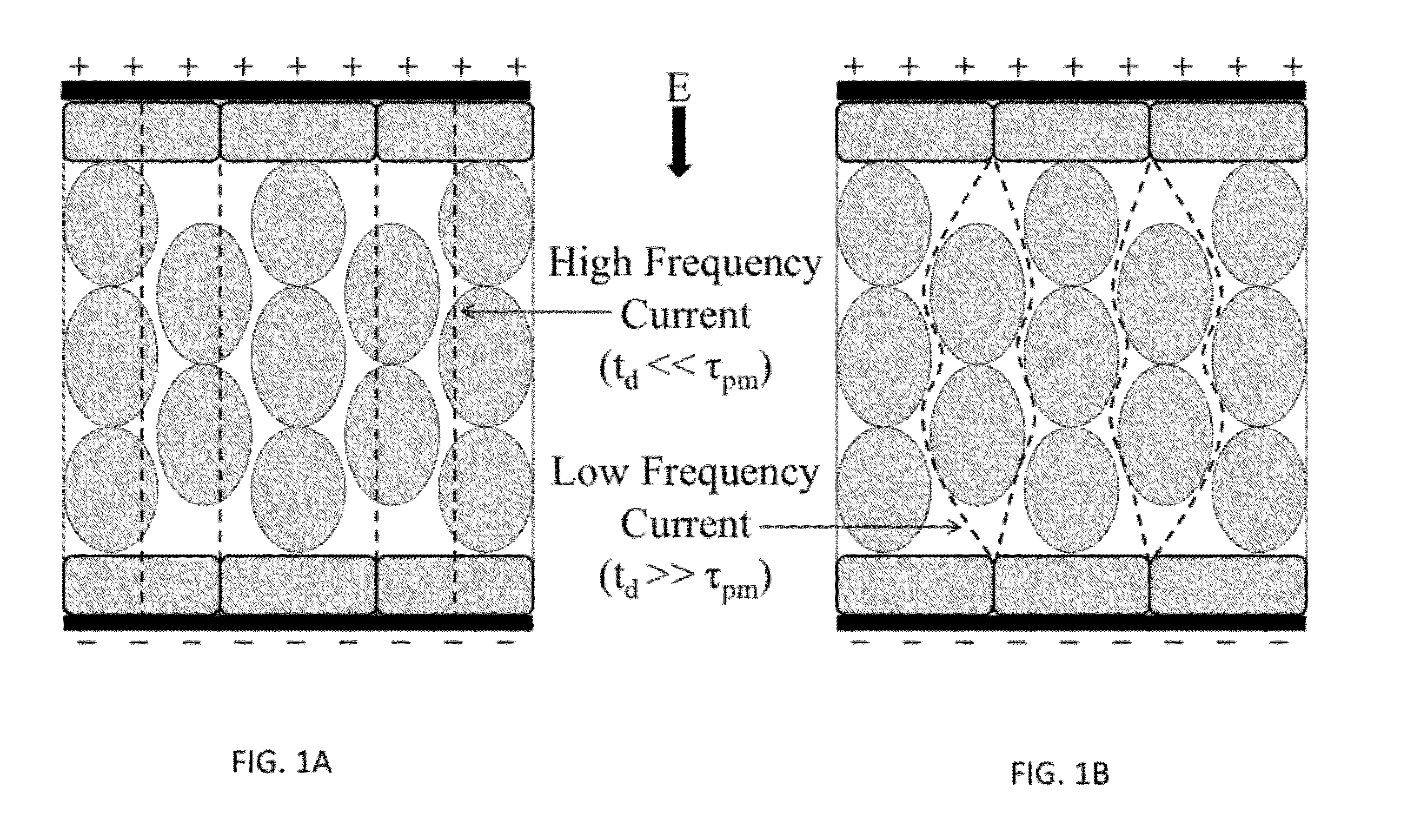
The present invention relates to the field of biomedical engineering and medical treatment of diseases and disorders. Methods, devices, and systems for in vivo treatment of cell proliferative disorders are provided. In embodiments, the methods comprise the delivery of high-frequency bursts of bipolar pulses to achieve the desired modality of cell death. More specifically, embodiments of the invention relate to a device and method for destroying aberrant cells, including tumor tissues, using high-frequency, bipolar electrical pulses having a burst width on the order of microseconds and duration of single polarity on the microsecond to nanosecond scale. In embodiments, the methods rely on conventional electroporation with adjuvant drugs or irreversible electroporation to cause cell death in treated tumors. The invention can be used to treat solid tumors, such as brain tumors.
Owner:VIRGINIA TECH INTPROP INC
Mutant cholera holotoxin as an adjuvant
InactiveUS7384640B1Low toxicityEnhance immune responseSsRNA viruses negative-senseBacteriaAntigenAdjuvant
A mutant cholera holotoxin featuring a point mutation at amino acid 29 of the A subunit, wherein the glutamic acid residue is replaced by an amino acid other than aspartic acid, is useful as an adjuvant in an antigenic composition to enhance the immune response in a vertebrate host to a selected antigen from a pathogenic bacterium, virus, fungus or parasite. In a particular embodiment, the amino acid 29 is histidine. The mutant cholera holotoxin may contain at least one additional mutation in the A subunit at a position other than amino acid 29. The antigenic composition may include a second adjuvant in addition to the mutant cholera holotoxin.
Owner:UNIFORMED SERVICES UNIV OF HEALTH SCI UNITED STATES OF AMERICA AS REPRESENTED BY THE +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com