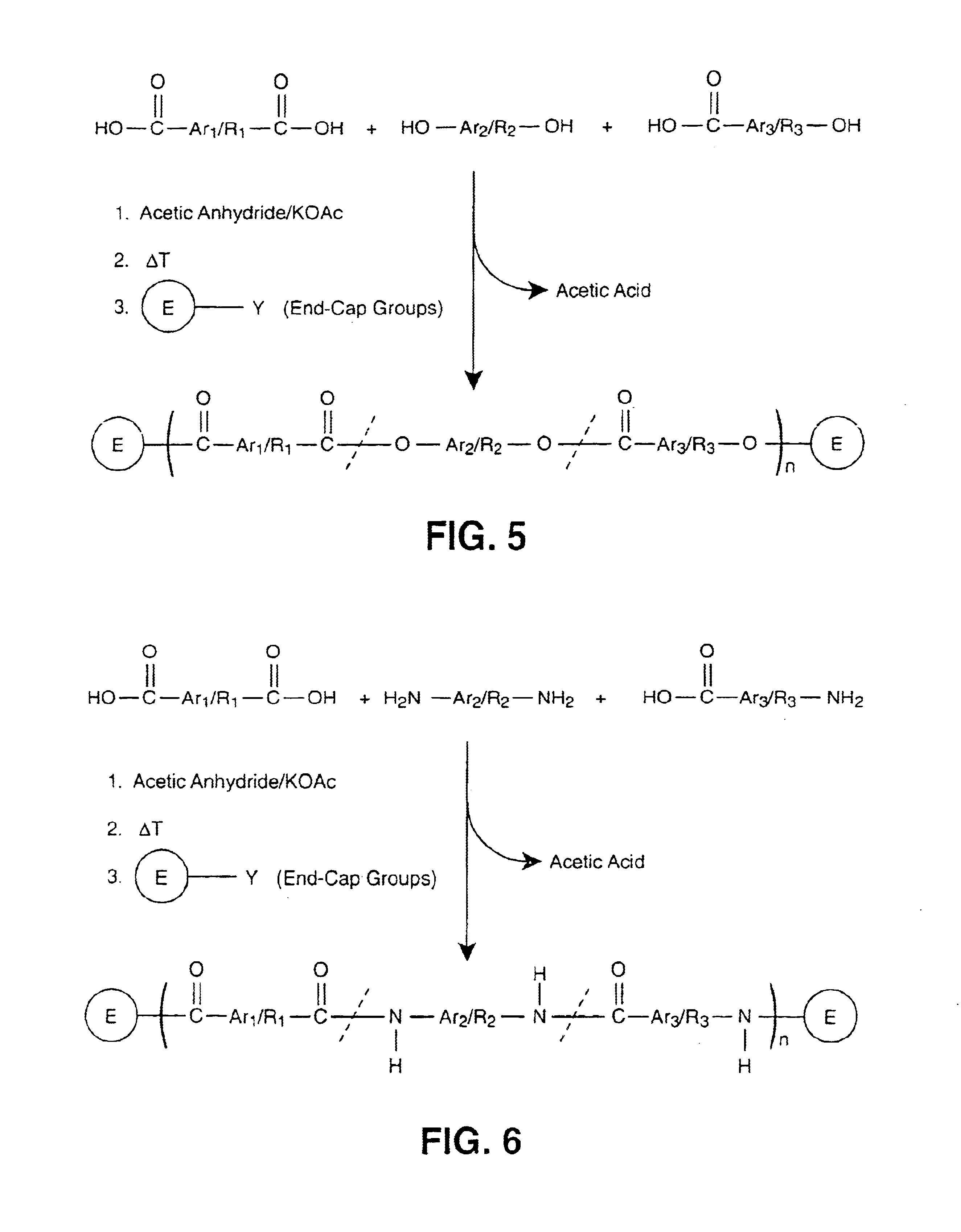
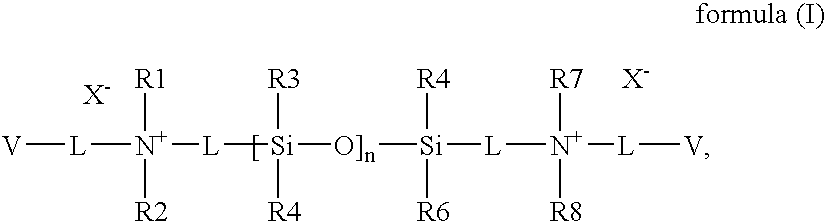
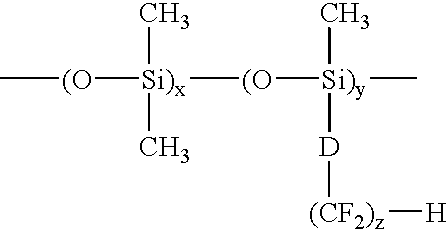
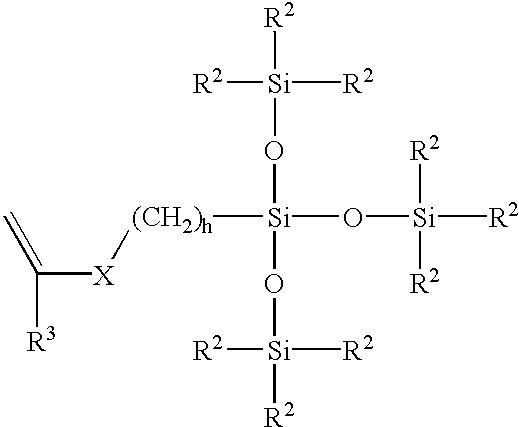
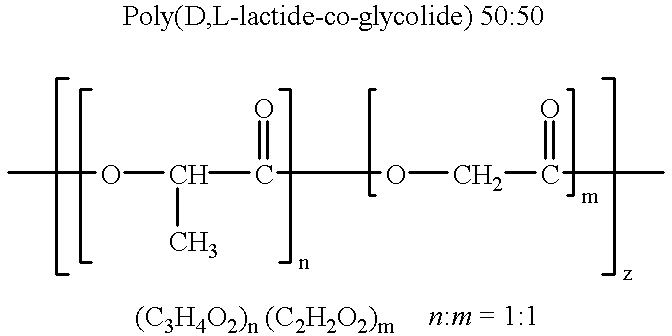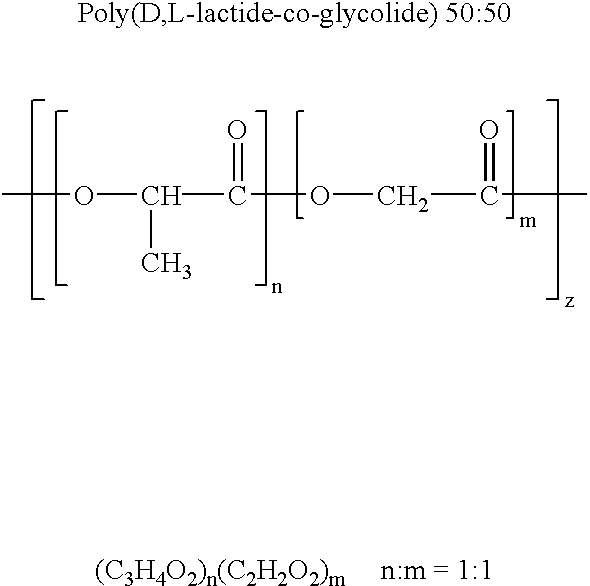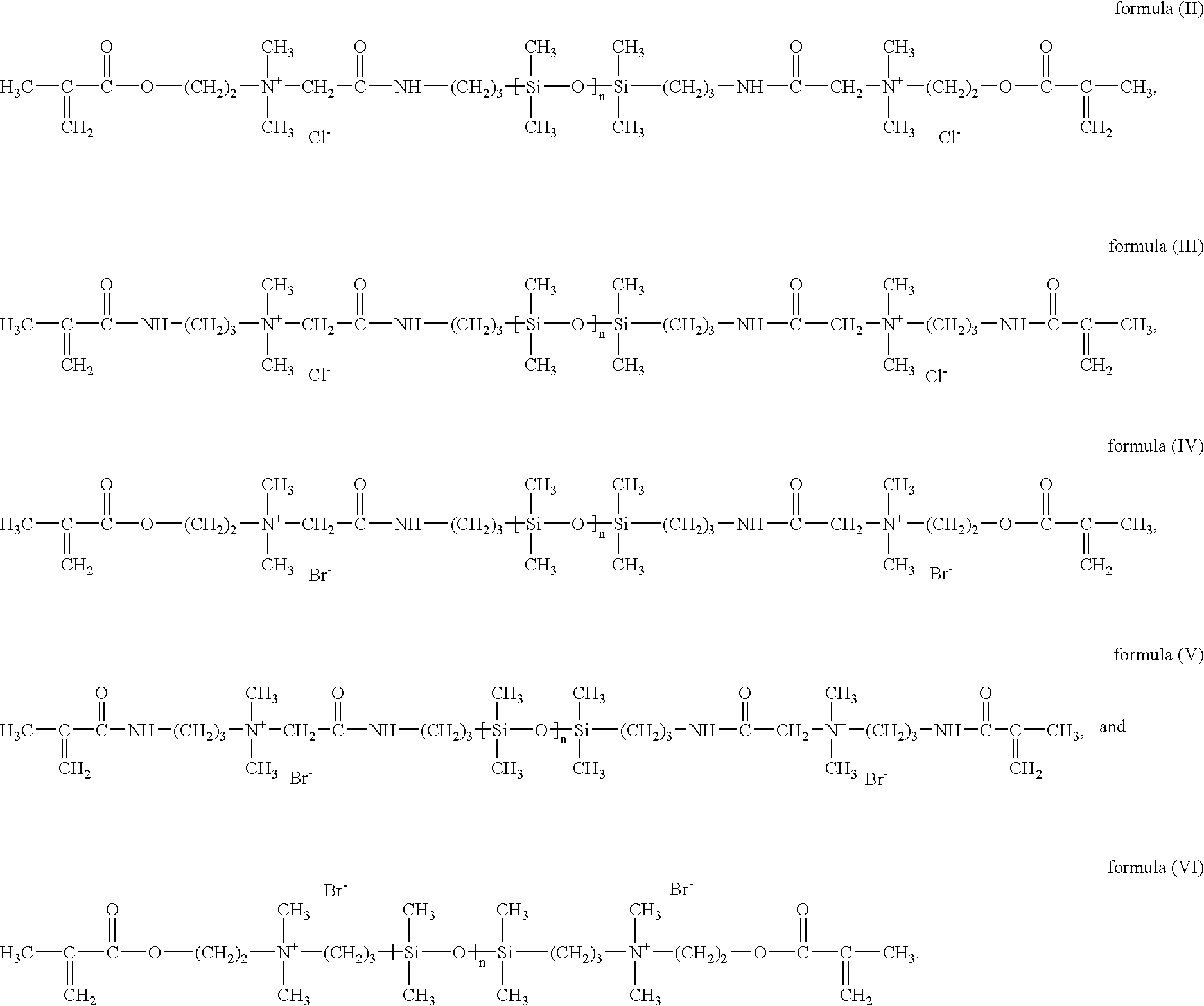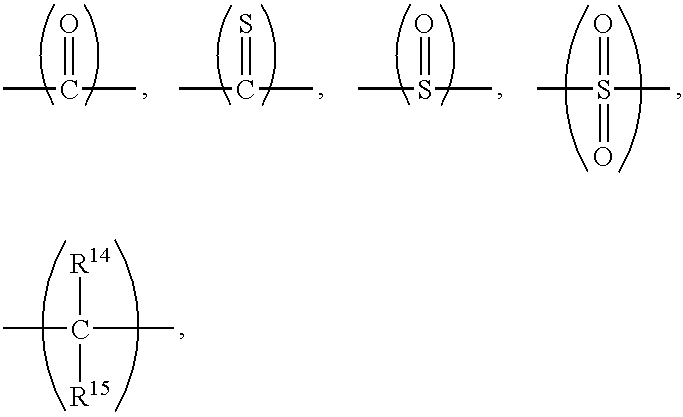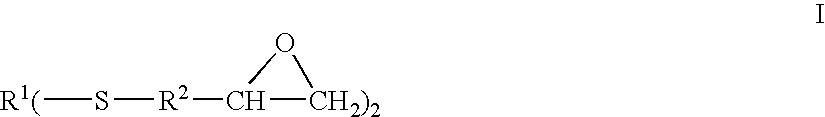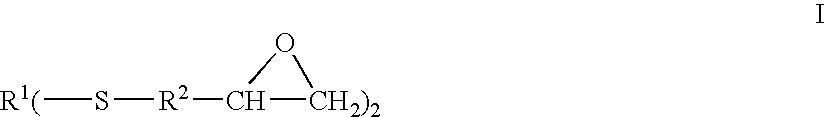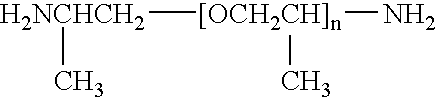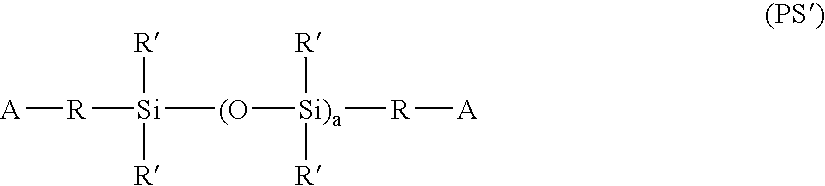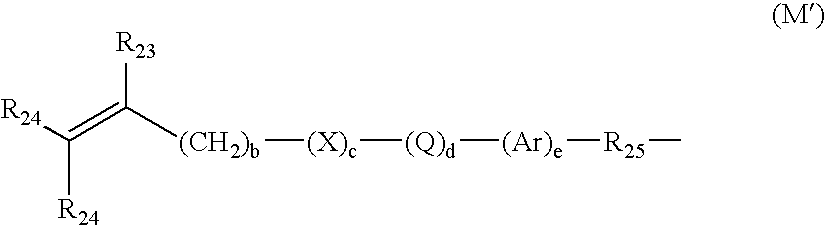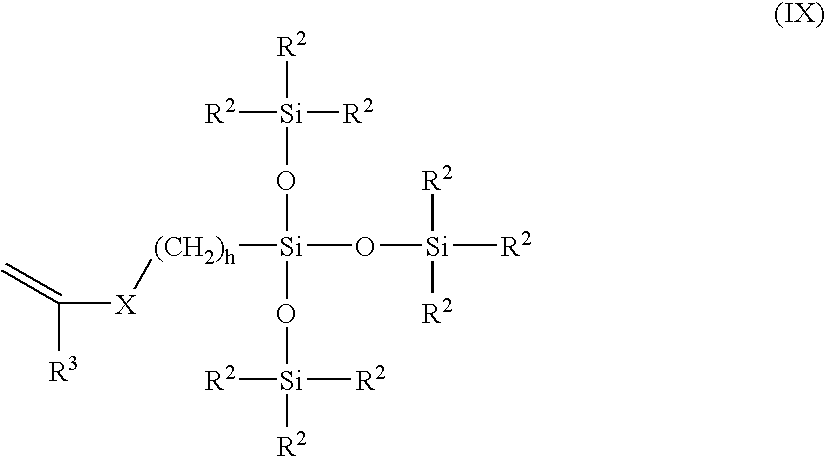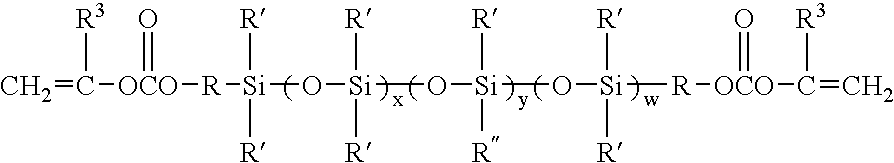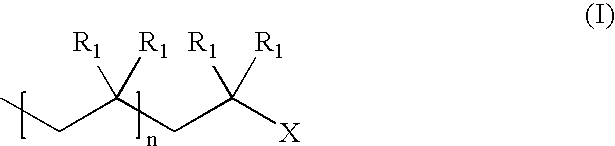Patents
Literature
2380 results about "Endcapping" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In chromatography, endcapping refers to the replacement of accessible silanol groups in a bonded stationary phase by trimethylsilyl groups. End-capped columns have much lower residual silanol group activity compared to non-endcapped columns.
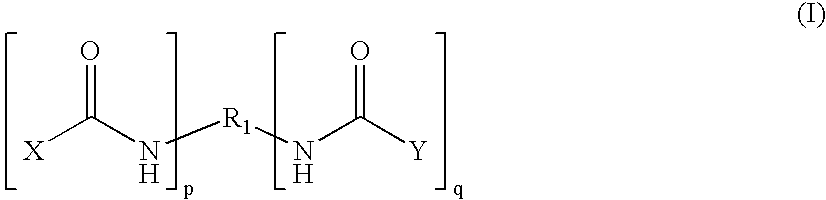
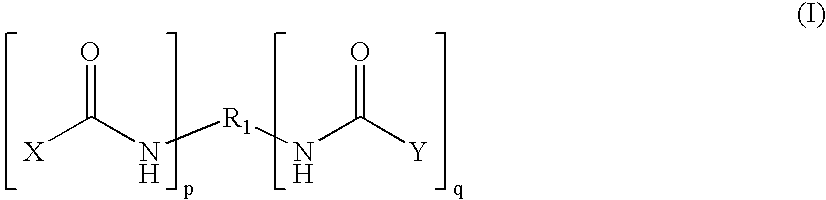
Therapeutic treatment and prevention of infections with a bioactive materials encapsulated within a biodegradable-biocompatible polymeric matrix
InactiveUS6309669B1Sustained release of active agent over timeEfficient and effective usePowder deliveryPeptide/protein ingredientsAdjuvantEnd-group
Novel burst-free, sustained release biocompatible and biodegrable microcapsules which can be programmed to release their active core for variable durations ranging from 1-100 days in an aqueous physiological environment. The microcapsules are comprised of a core of polypeptide or other biologically active agent encapsulated in a matrix of poly(lactide / glycolide) copolymer, which may contain a pharmaceutically-acceptable adjuvant, as a blend of upcapped free carboxyl end group and end-capped forms ranging in ratios from 100 / 0 to 1 / 99.
Owner:ARMY GOVERNMENT OF THE UNITED STATES AS REPRESENTED BY THE SEC OF THE
Process for applying a streamable epoxy adhesive
InactiveUS20050070634A1Low viscosityHigh strength bondAdhesive processes with adhesive heatingEpoxy resin adhesivesBENZYL ALCOHOL/WATERViscosity
The invention is a composition comprising applying to a substrate a stream of an adhesive comprising: one or more epoxy resins; one or more rubber modified epoxy resins; one or more toughening compositions comprising the reaction product of one or more isocyanate terminated prepolymers and one or more capping compounds having one or more phenolic, benzyl alcohol, aminophenyl, or, benzylamino groups wherein the reaction product is terminated with the capping compounds; one or more curing agents for epoxy resins and one or more catalysts which initiate cure at a temperature of about 100° C. or greater; and optionally; fillers adhesion promoters, wetting agents or rheological additives useful in epoxy adhesive compositions; wherein the adhesive composition has a viscosity at 45° C. of about 20 Pa.s to about 400 Pa.s. The composition can be used as an adhesive and applied as a stream using a high speed streaming process.
Owner:DOW GLOBAL TECH LLC
Sealants and potting formulations including mercapto-terminated polymers produced by the reaction of a polythiol and polyvinyl ether monomer
Sealant and potting formulations are provided which are prepared from components including ungelled mercapto-terminated polymer(s) prepared by reacting reactants comprising polyvinyl ether monomer(s) and polythiol material(s); curing agent(s) reactive with a mercapto group of the mercapto-terminated polymer; and additive(s) selected from the group consisting of fillers, adhesion promoters, plasticizers and catalysts.
Owner:PRC DE SOTO INT INC
Toughened epoxy adhesive composition
ActiveUS20060276601A1Improved lap shear and impact peel strengthGood storage stabilityPolyureas/polyurethane adhesivesSynthetic resin layered productsElastomerEnd-group
The invention is an epoxy resin based adhesive composition comprising an epoxy resin and a compound comprising an elastomeric prepolymer residue selected from the group of a polyurethane, a polyurea and a polyurea polyurethane having isocyanate end groups, the isocyanate end groups of said prepolymer residue being capped by a capping compound selected from the group consisting of a primary aliphatic, cycloaliphatic, heteroaromatic and araliphatic amine, a secondary aliphatic, cycloaliphatic, aromatic, heteroaromatic and araliphatic amine, a thiol and an alkyl amide, said capping compound being bound to the end of the polymer chain of the elastomeric prepolymer in a manner such that the end to which it is bonded no longer has a reactive group. In addition to the capping compound defined above above, a capping compound selected from the group consisting of a phenol and a polyphenol can be used for capping the isocyanate end groups of the prepolymer residue
Owner:DOW GLOBAL TECH LLC
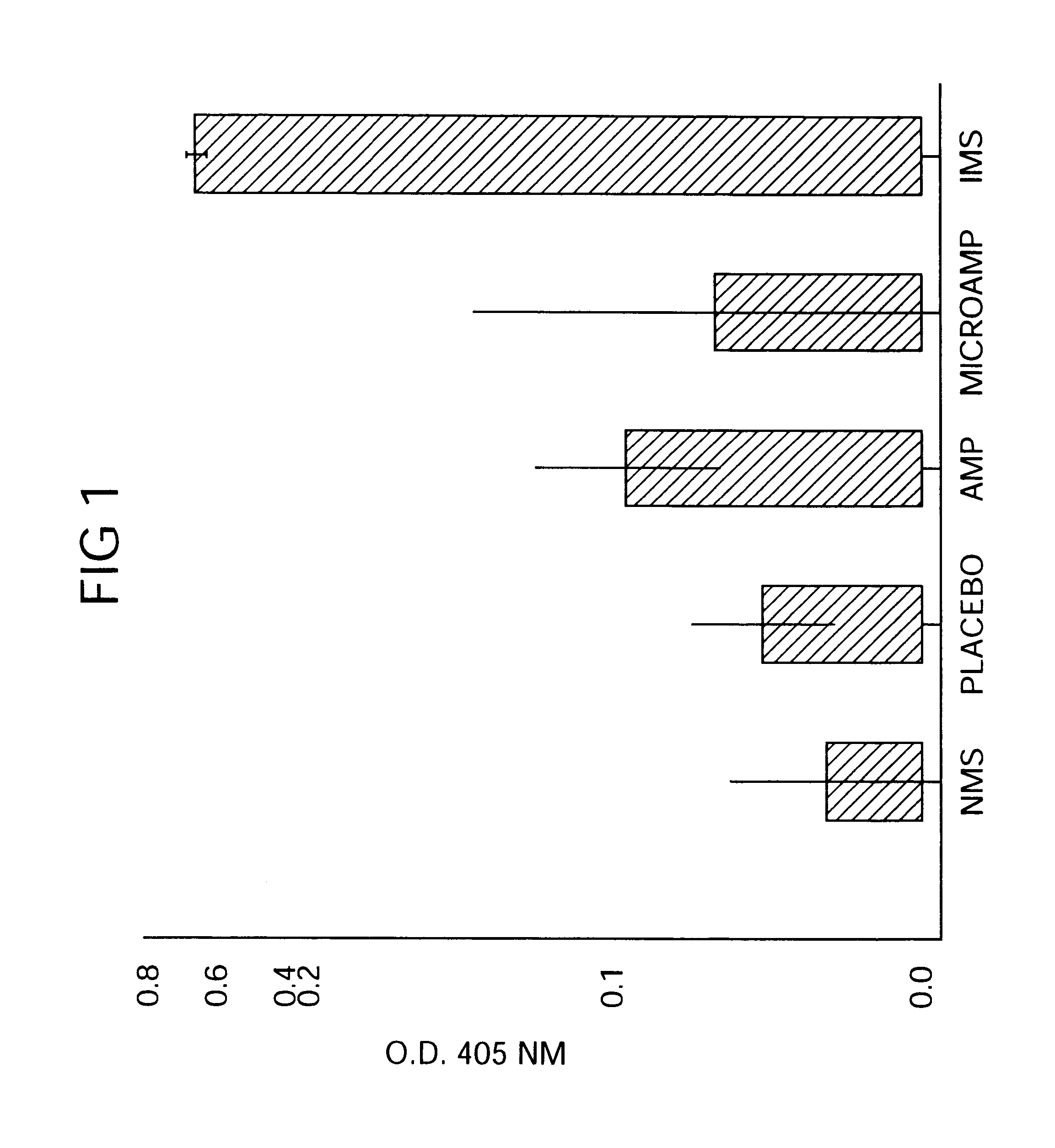
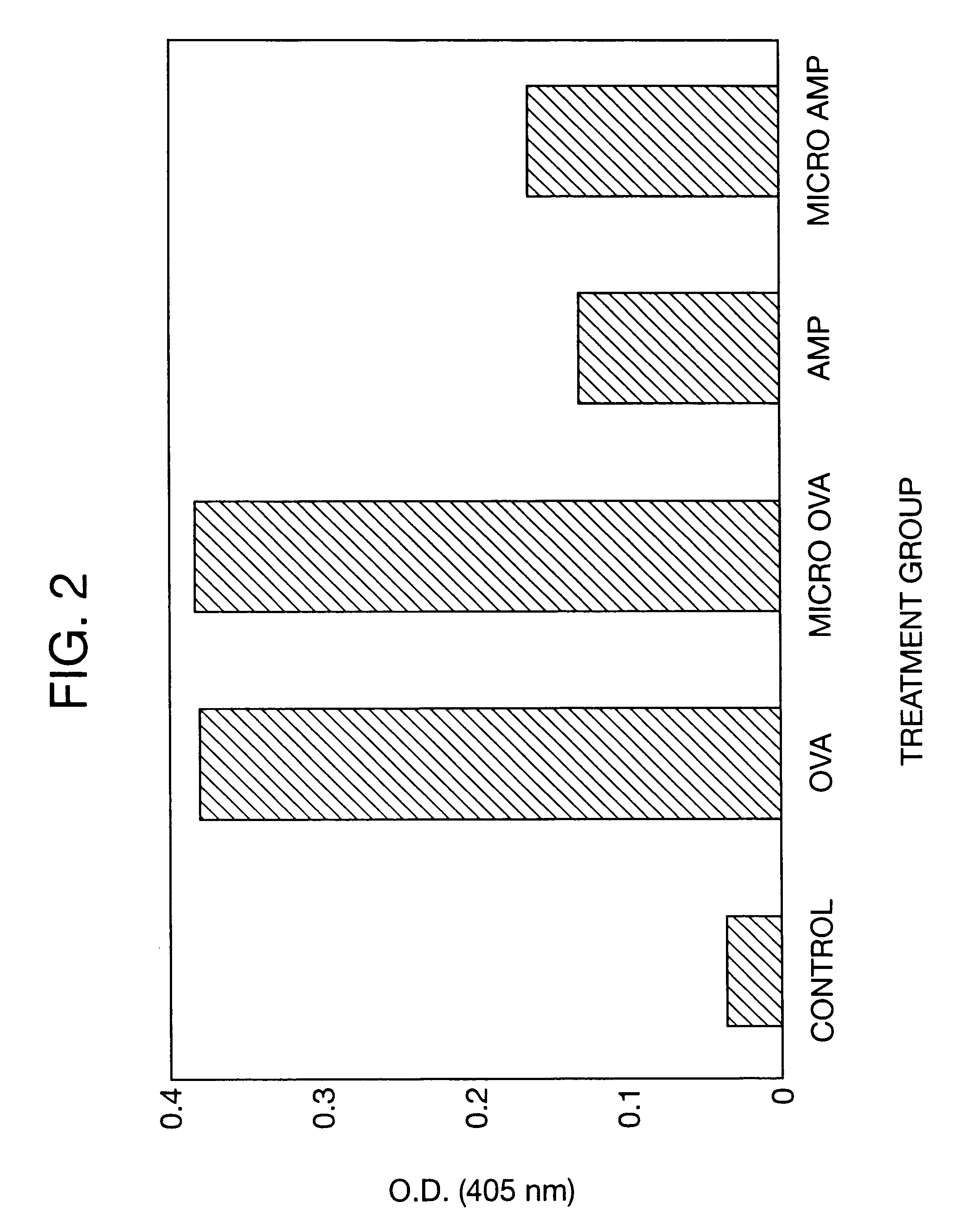
Therapeutic treatment and prevention of infections with a bioactive material(s) encapuslated within a biodegradable-bio-compatable polymeric matrix
InactiveUS6902743B1Induce productionSustained release of active agent over timePowder deliveryPeptide/protein ingredientsTherapeutic treatmentActive agent
Novel burst-free, sustained release biocompatible and biodegrable microcapsules which can be programmed to release their active core for variable durations ranging from 1-100 days in an aqueous physiological environment. The microcapsules are comprised of a core of polypeptide or other biologically active agent encapsulated in a matrix of poly(lactide / glycolide) copolymer having a molar composition of lactide / glycolide from 90 / 10 to 40 / 60, which may contain a pharmaceutically-acceptable adjuvant, as a blend of uncapped free carboxyl end group and end-capped forms ranging to ratios from 100 / 0 to 1 / 99.
Owner:ARMY UNITED STATES GOVERNMENT AS REPRESENTED BY THE SEC OF THE
Liquid crystalline thermosets from ester, ester-imide, and ester-amide oligomers
InactiveUS6939940B2Low viscosityLow dielectric constantLiquid crystal compositionsLiquid crystallineEnd-group
Main chain thermotropic liquid crystal esters, ester-imides, and ester-amides were prepared from AA, BB, and AB type monomeric materials and were end-capped with phenylacetylene, phenylmaleimide, or nadimide reactive end-groups. The resulting reactive end-capped liquid crystal oligomers exhibit a variety of improved and preferred physical properties. The end-capped liquid crystal oligomers are thermotropic and have, preferably, molecular weights in the range of approximately 1000-15,000 grams per mole. The end-capped liquid crystal oligomers have broad liquid crystalline melting ranges and exhibit high melt stability and very low melt viscosities at accessible temperatures. The end-capped liquid crystal oligomers are stable for up to an hour in the melt phase. These properties make the end-capped liquid crystal oligomers highly processable by a variety of melt process shape forming and blending techniques including film extrusion, fiber spinning, reactive injection molding (RIM), resin transfer molding (RTM), resin film injection (RFI), powder molding, pultrusion, injection molding, blow molding, plasma spraying and thermo-forming. Once processed and shaped, the end-capped liquid crystal oligomers were heated to further polymerize and form liquid crystalline thermosets (LCT). The fully cured products are rubbers above their glass transition temperatures. The resulting thermosets display many properties that are superior to their non-end-capped high molecular weight analogs.
Owner:NASA
Silicon-containing monomers end-capped with polymerizable cationic hydrophilic groups
InactiveUS20070142584A1High oxygen permeabilityDesirable biocompatibilitySilicon organic compoundsIntraocular lensEndcappingOrganic solvent
The present invention relates to polymeric compositions useful in the manufacture of biocompatible medical devices. More particularly, the present invention relates to certain cationic monomers capable of polymerization to form polymeric compositions having desirable physical characteristics useful in the manufacture of ophthalmic devices. Such properties include the ability to extract the polymerized medical devices with water. This avoids the use of organic solvents as is typical in the art. The polymer compositions comprise polymerized silicon-containing monomers end-capped with polymerizable cationic hydrophilic groups.
Owner:BAUSCH & LOMB INC
Poly(arylene ether)-containing thermoset composition, method for the preparation thereof, and articles derived therefrom
InactiveUS6812276B2Fast curingReduce sensitivitySynthetic resin layered productsSpecial tyresEndcappingHeat resistance
Owner:SABIC GLOBAL TECH BV
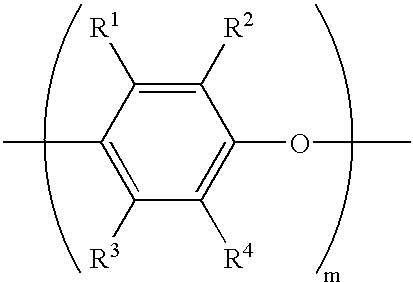
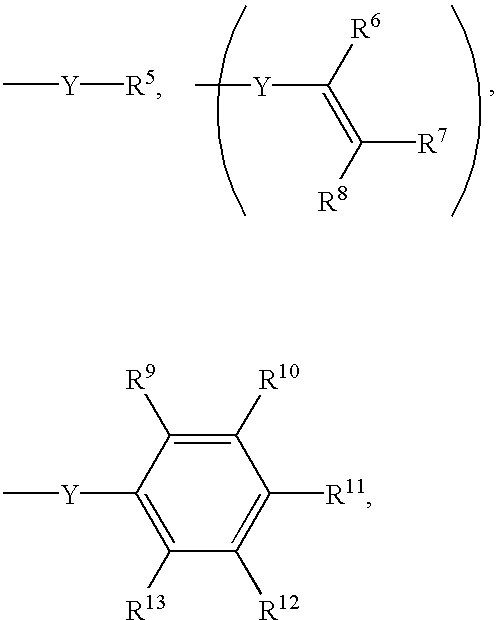
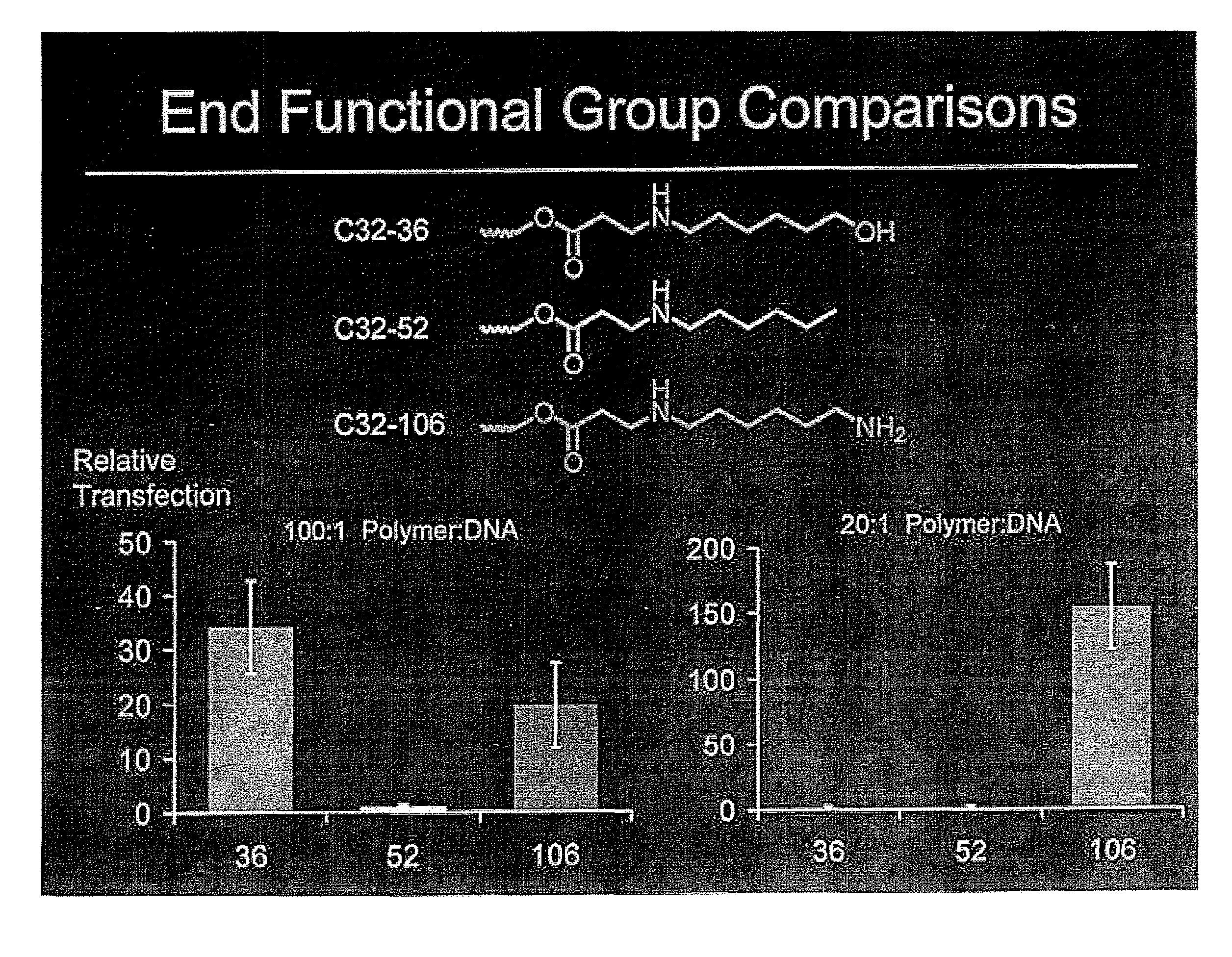
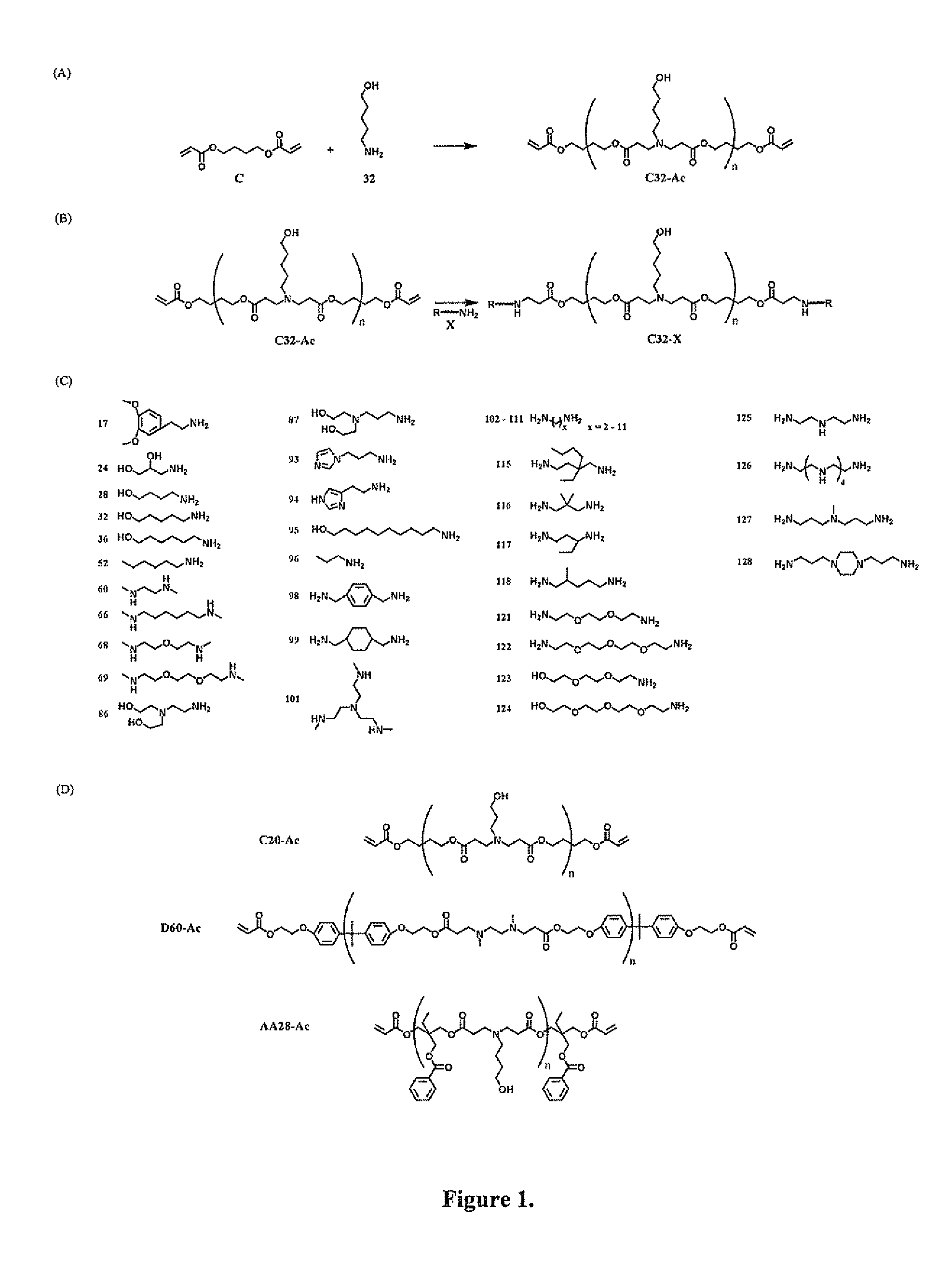
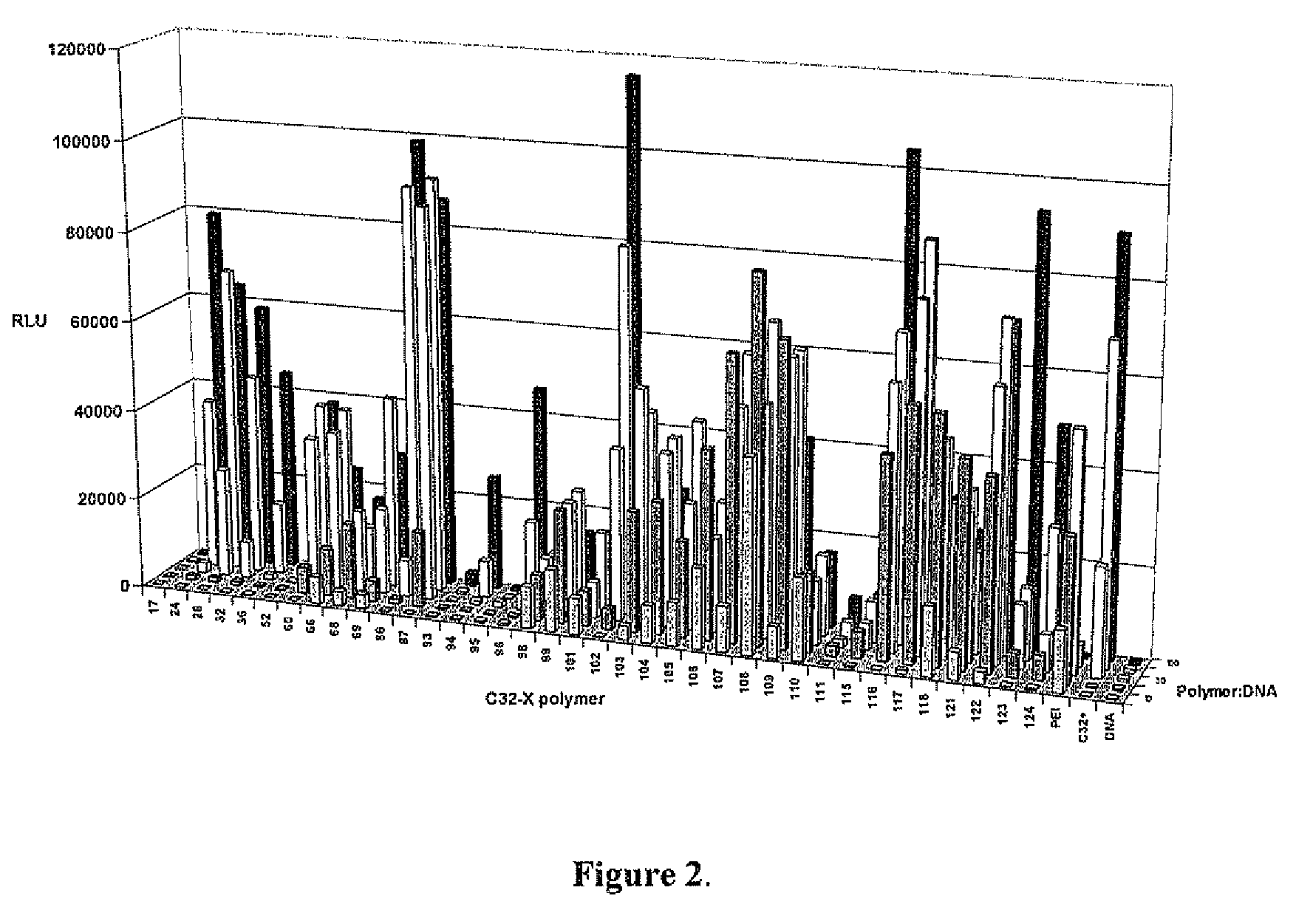
End-modified poly(beta-amino esters) and uses thereof
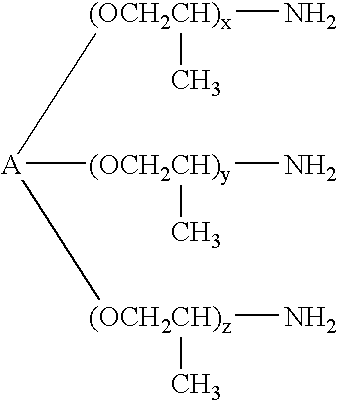
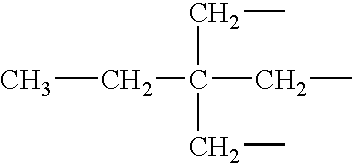
Poly(beta-amino esters) are end-modified to form materials useful in the medical as well as non-medical field. An amine-terminated poly(beta-amino ester) is reacted with an electrophile, or an acrylate-terminated poly(beta-amino ester) is reacted with a nucleophile. The inventive end-modified polymers may be used in any field where polymers have been found useful including the drug delivery arts. The end-modified polymers are particularly useful in delivery nucleic acids such as DNA or RNA. The invention also provides compositions including the inventive end-modified polymers, methods of preparing the inventive polymers, and method of using the inventive polymers.
Owner:MASSACHUSETTS INST OF TECH
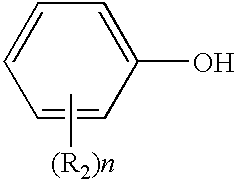
Peptide Nanostructures Containing End-Capping Modified Peptides And Methods Of Generating And Using The Same
InactiveUS20080009434A1Peptide/protein ingredientsSpace heating and ventilationEndcappingCrystallography
A nanostructure composed of a plurality of peptides, each peptide containing at least one aromatic amino acid, whereby one or more of these peptides is end-capping modified, is disclosed. The nanostructure can take a tubular, fibrillar, planar or spherical shape, and can encapsulate, entrap or be coated by other materials. Methods of preparing the nanostructure, and devices and methods utilizing same are also disclosed.
Owner:RAMOT AT TEL AVIV UNIV LTD
Epoxy-capped polythioethers
Owner:PRC DE SOTO INT INC
Novel reagents for directed biomarker signal amplification
ActiveUS20110256549A1Component separationOrganic compound preparationChain structureCombinatorial chemistry
Described herein are methods, compositions and articles of manufacture involving neutral conjugated polymers including methods for synthesis of neutral conjugated water-soluble polymers with linkers along the polymer main chain structure and terminal end capping units. Such polymers may serve in the fabrication of novel optoelectronic devices and in the development of highly efficient biosensors. The invention further relates to the application of these polymers in assay methods.
Owner:SIRIGEN II LTD
Crosslinked, degradable polymers and uses thereof
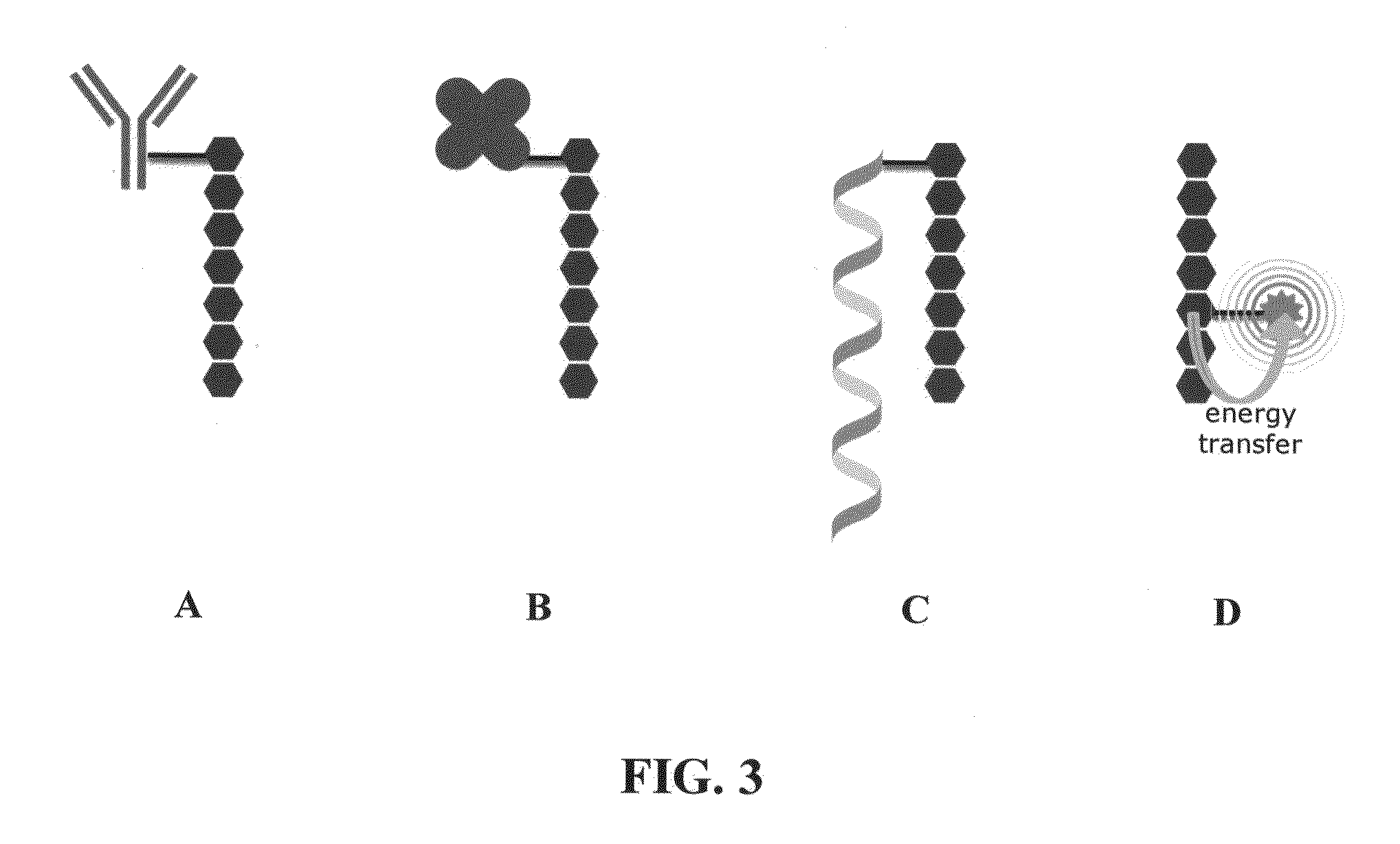
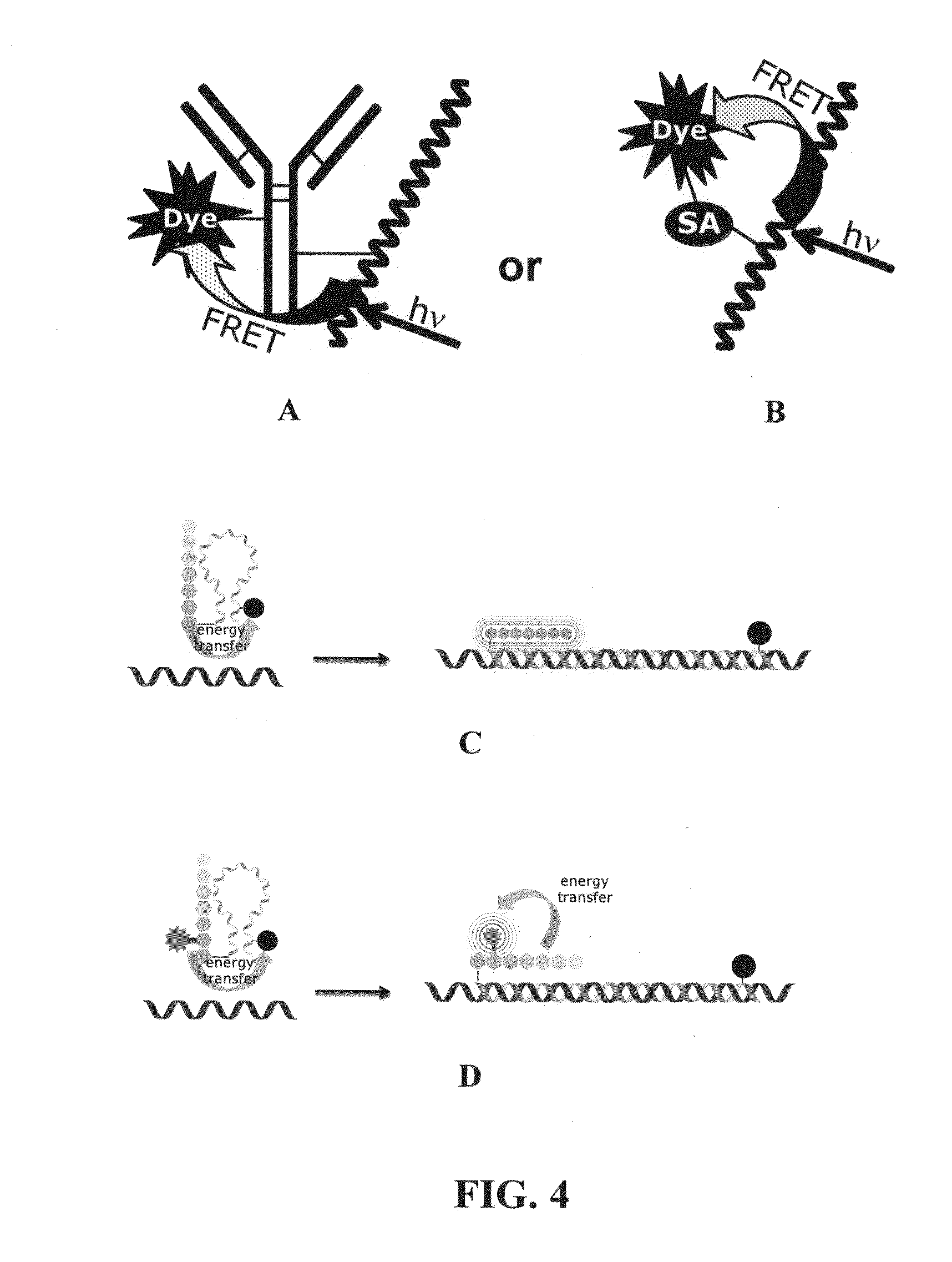
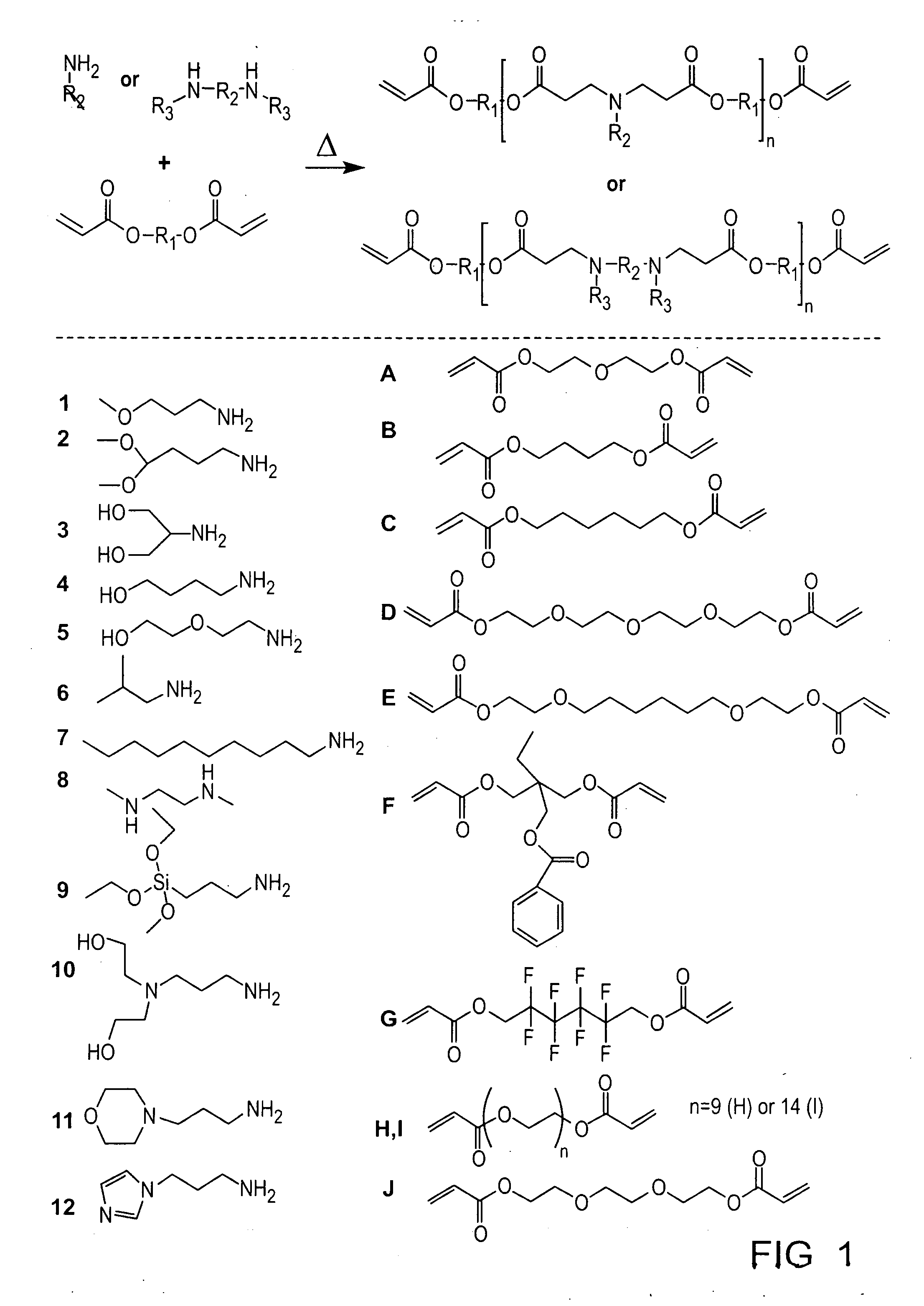
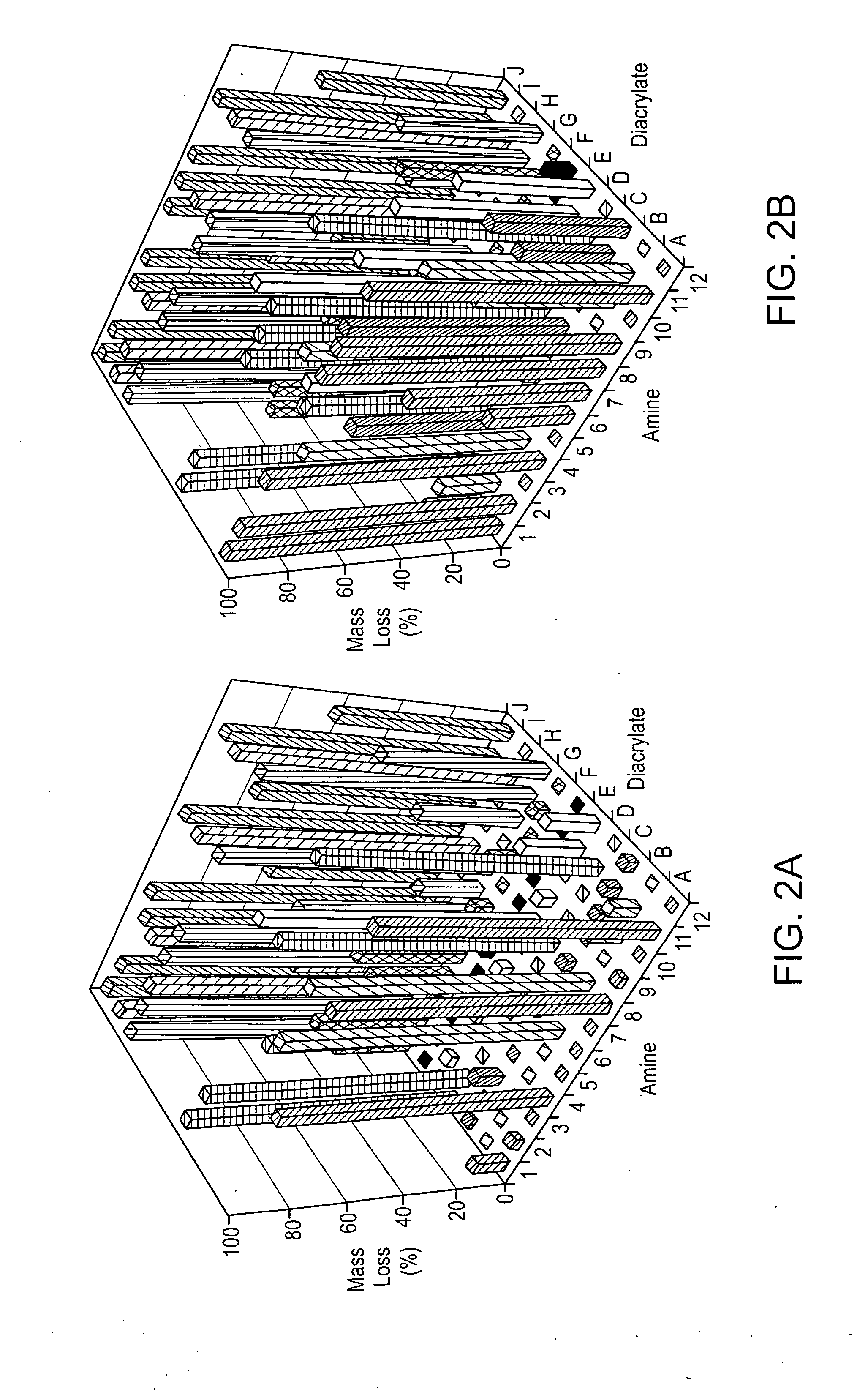
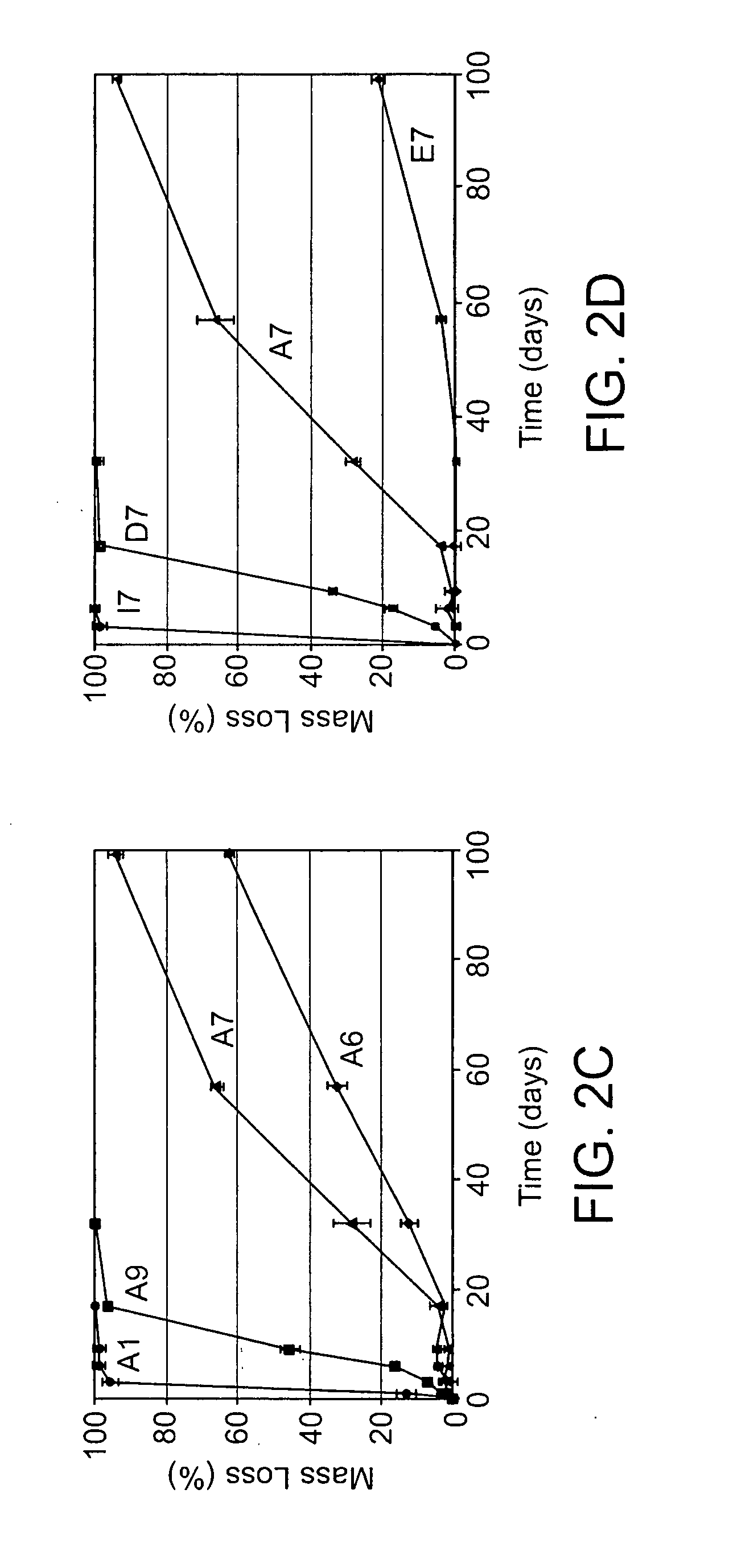
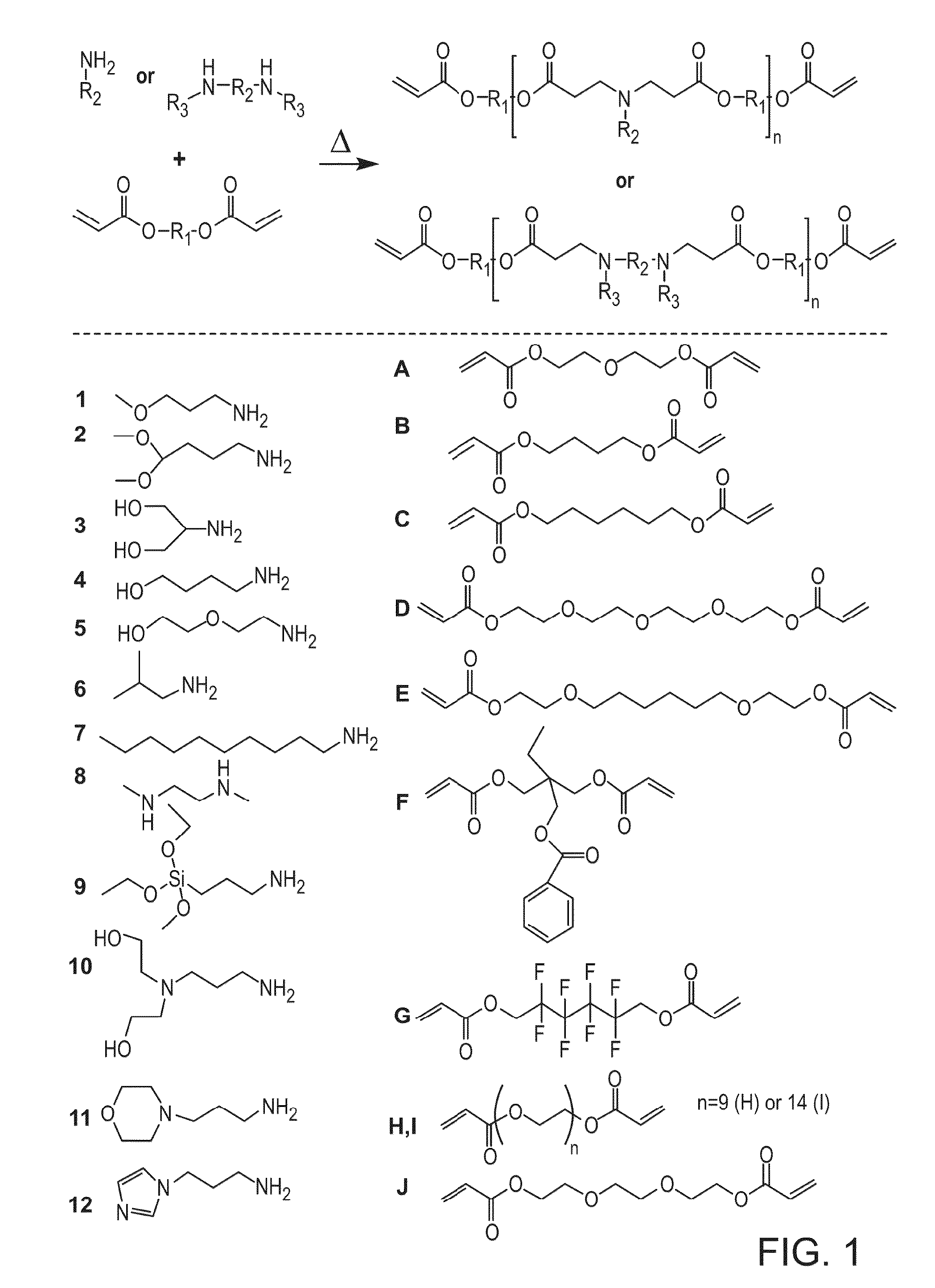
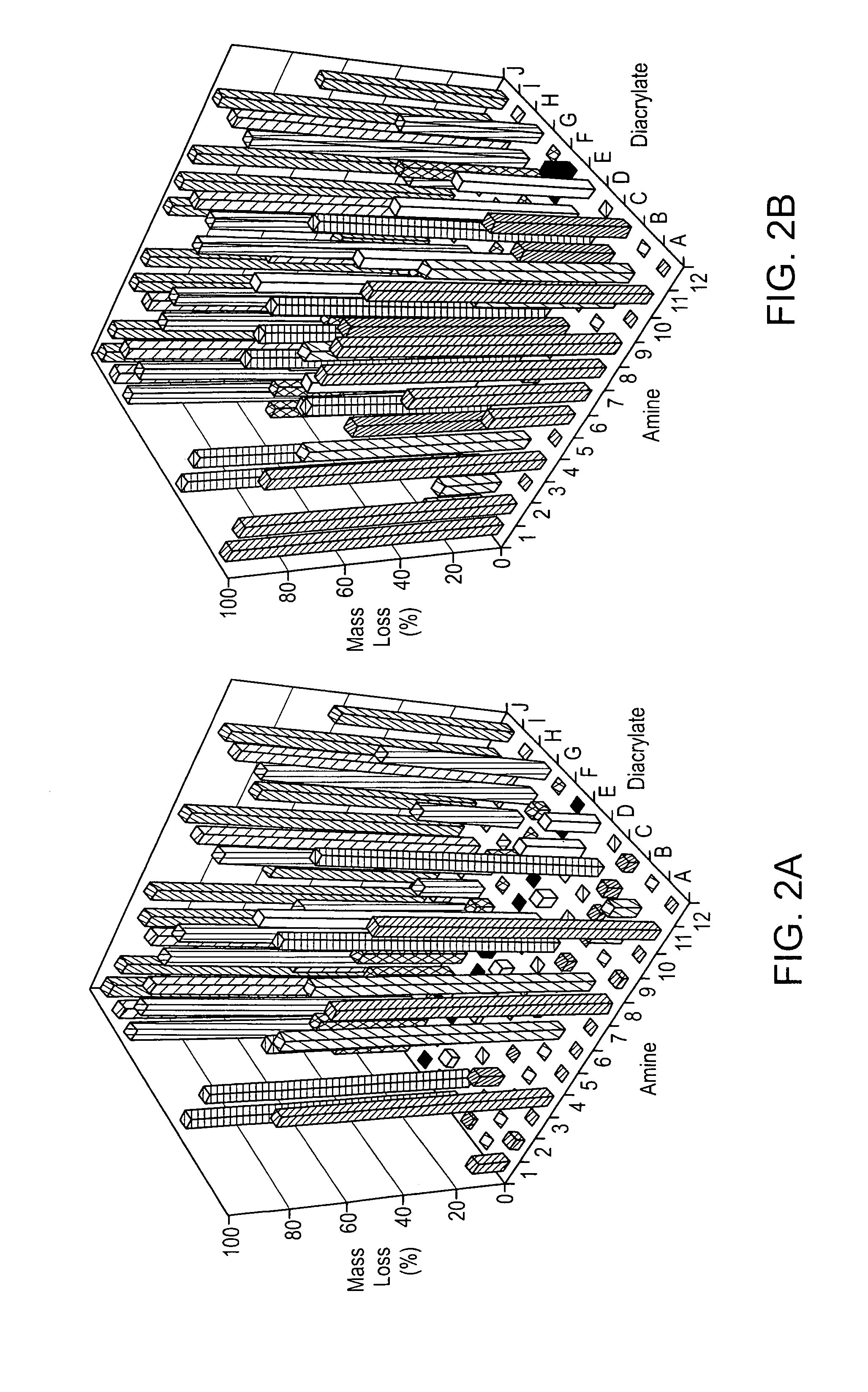
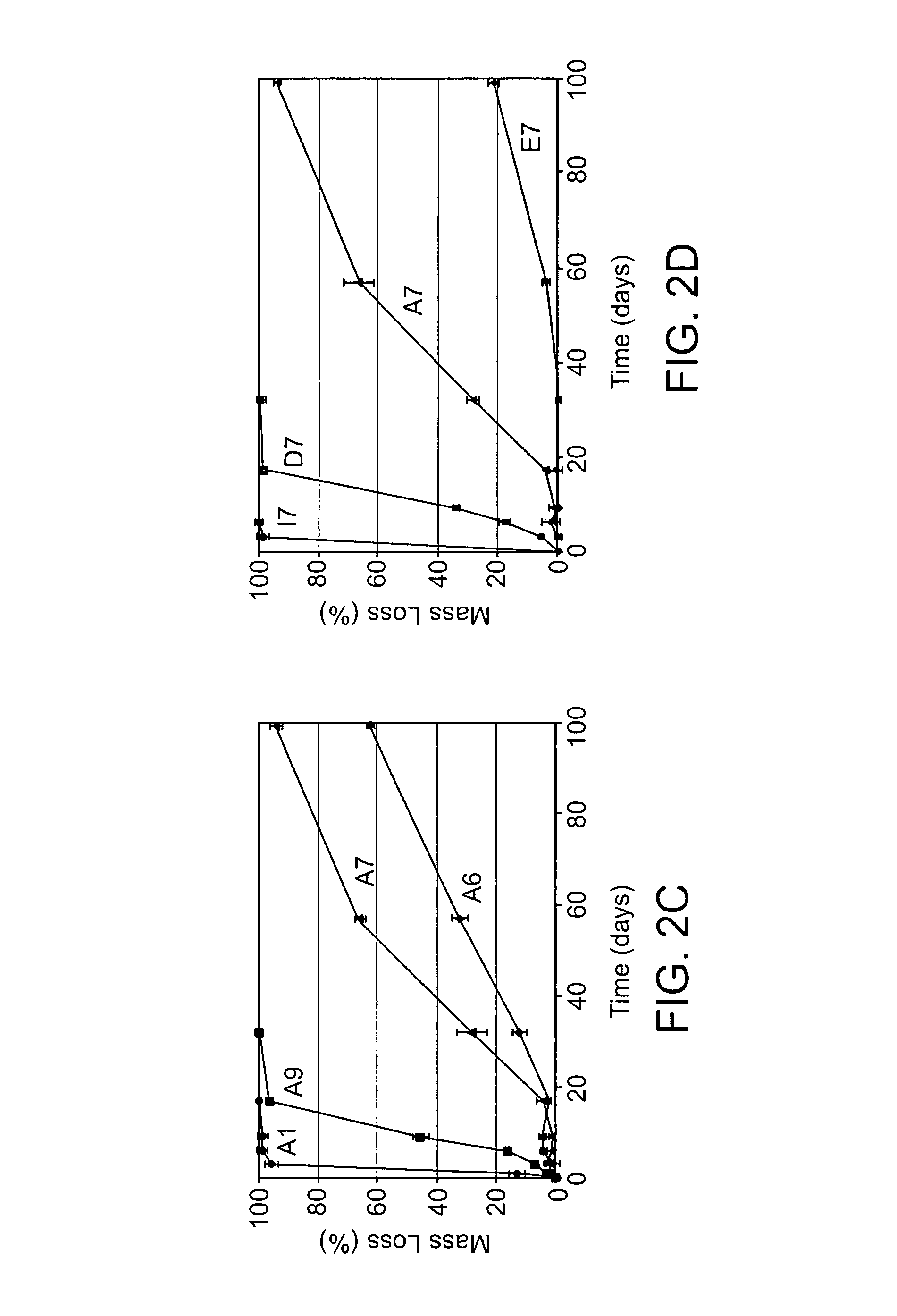
Acrylate-terminated poly(beta-amino esters) are cross-linked to form materials useful in the medical as well as non-medical field. The polymeric starting material is combined with a free radical initiator, either a thermal initiator or a photoinitiator, and the mixture for cross-linking is heated or exposed to light depending on the initiator used. The resulting materials due to the hydrolysable ester bond in the polymer backbone are biodegradable under physiological conditions. These cross-linked materials are particular useful as drug delivery vehicles, tissue engineering scaffolds, and in fabricating microdevices. The materials may also be used as plastics, coating, adhesives, inks, etc. The cross-linked materials prepared exhibit a wide range of degradation times, mass loss profiles, and mechanical properties. Therefore, the properties of the material may be tuned for the desired use. The high-throughput approach to preparing a library of cross-linked poly(beta-amino esters) allows for the rapid screening and design of degradable polymers for a variety of applications.
Owner:MASSACHUSETTS INST OF TECH
Epoxy adhesive having improved impact resistance
InactiveUS20030196753A1Polyureas/polyurethane adhesivesOrganic non-macromolecular adhesivePolyphenolExpandable microsphere
In the preparation of an improved adhesive composition, an epoxy-based prepolymer is obtained by the reaction of one or more epoxy resins with amino-terminated polyethers and / or carboxyl-terminated butadiene-nitrile rubbers. In one embodiment of the invention, both a solid epoxy resin and a liquid epoxy resin, each of which is a diglycidyl ether of a polyphenol such as bisphenol A, are used. The epoxy-based prepolymer is mixed with an acrylate-terminated urethane resin (preferably, one based on a polyol having a number average molecular weight of at least about 400) and a heat-activated latent curing agent to make an adhesive composition which can be pumpable at room temperature. Curable adhesives capable of expansion to about 100% with high impact resistance after curing may be obtained by inclusion of expanding agents such as expandable microspheres.
Owner:HENKEL KGAA
Polyurethane resins having multiple mechanisms of hardening for use in producing three-dimensional objects
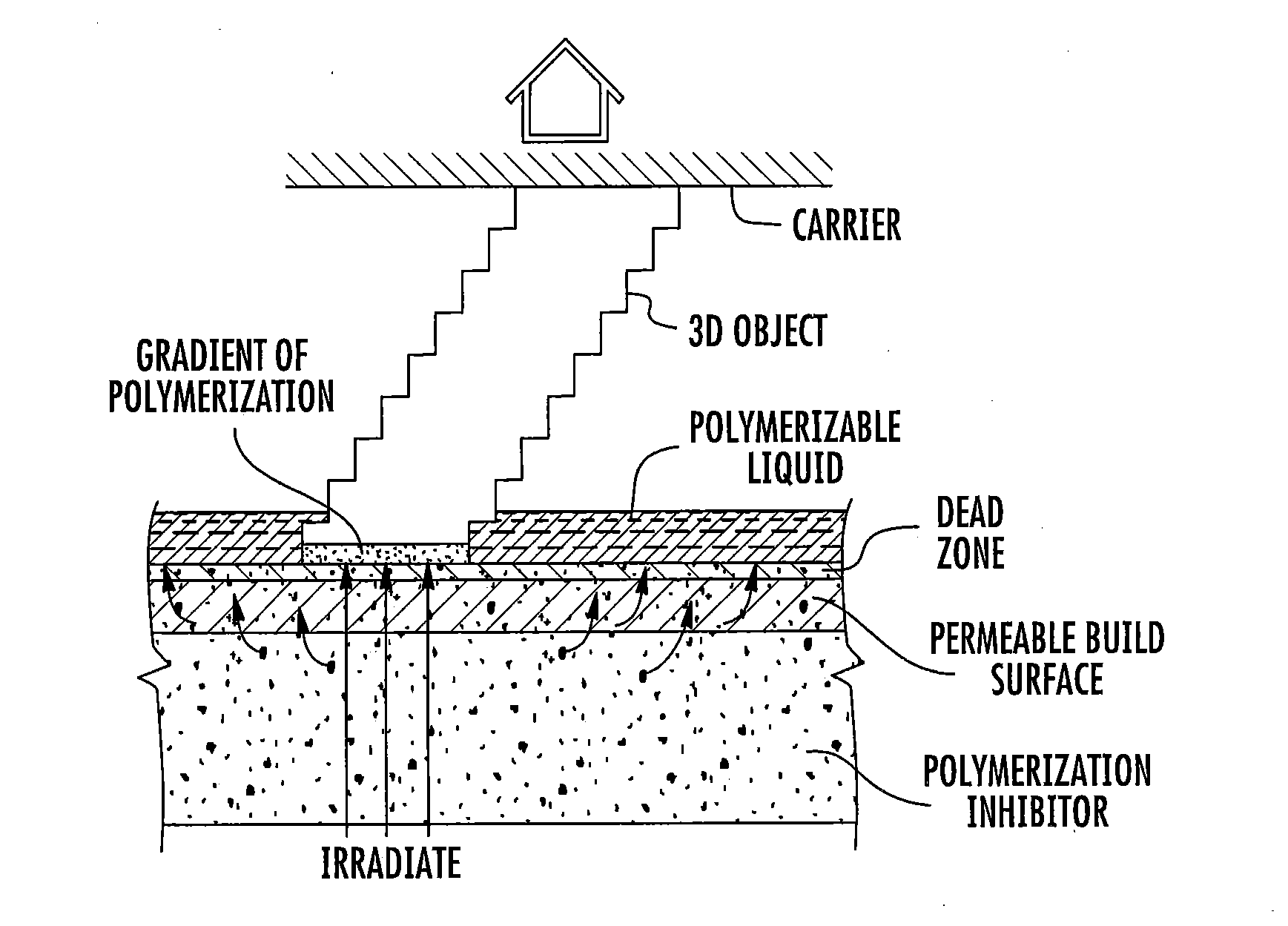
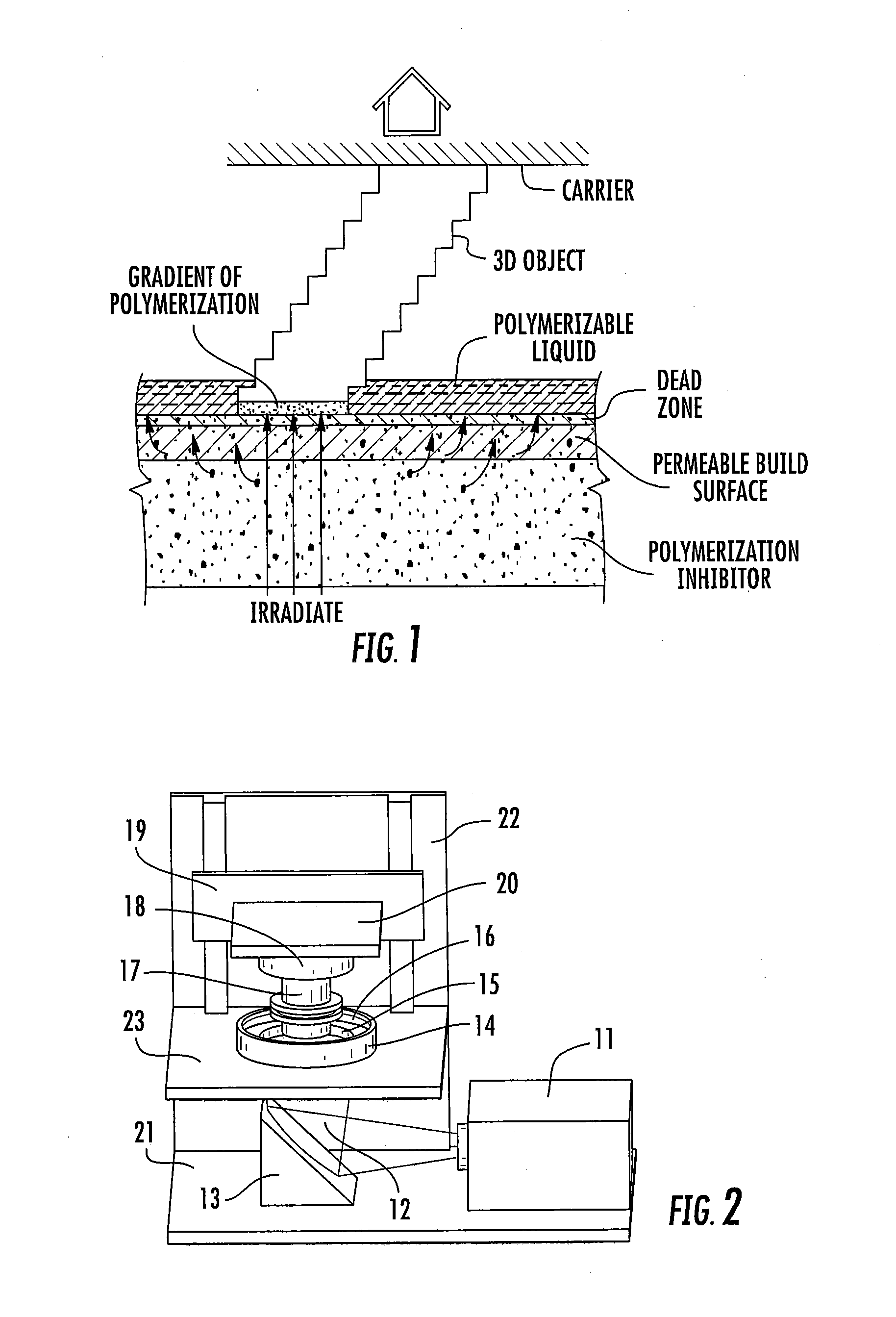
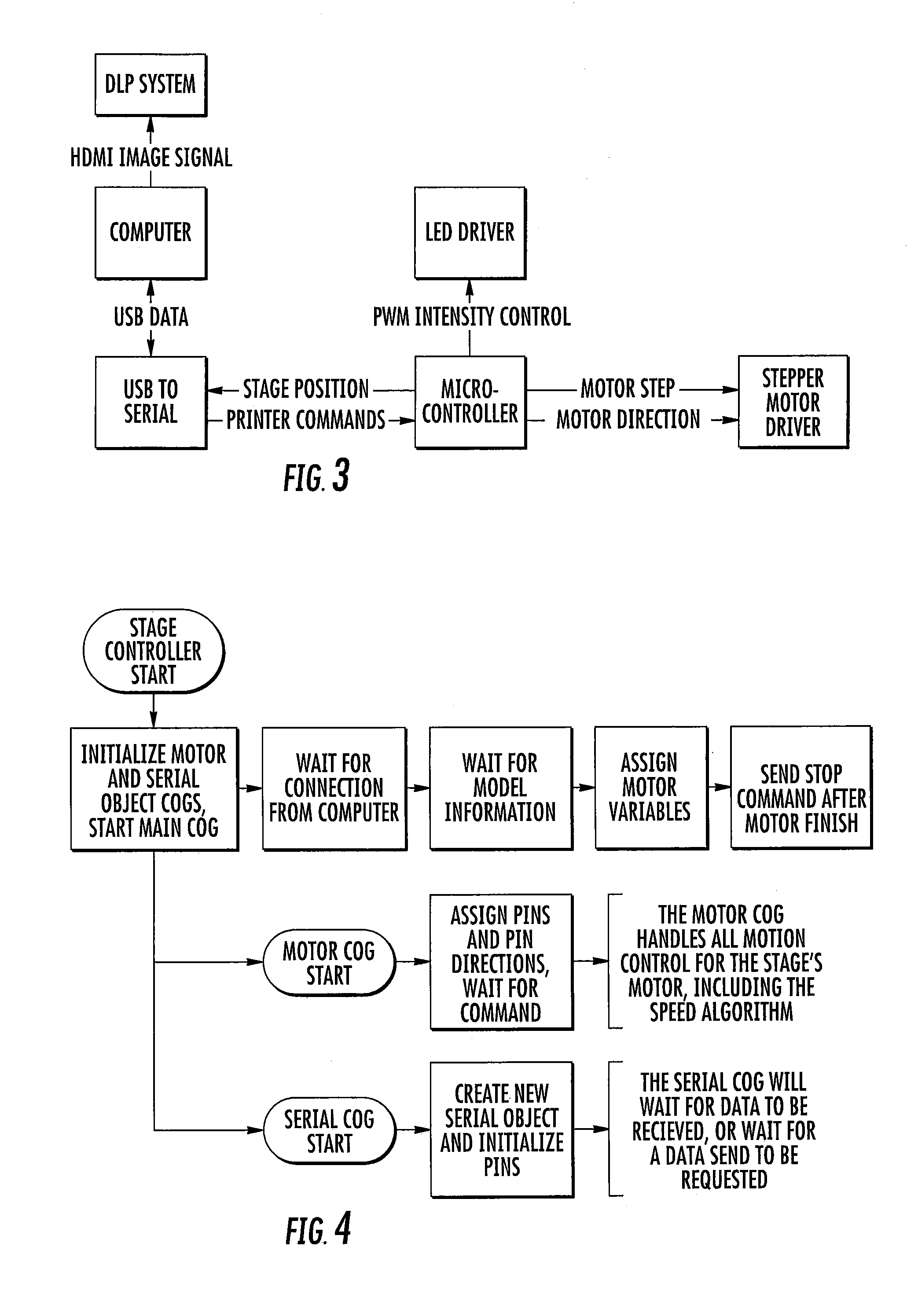
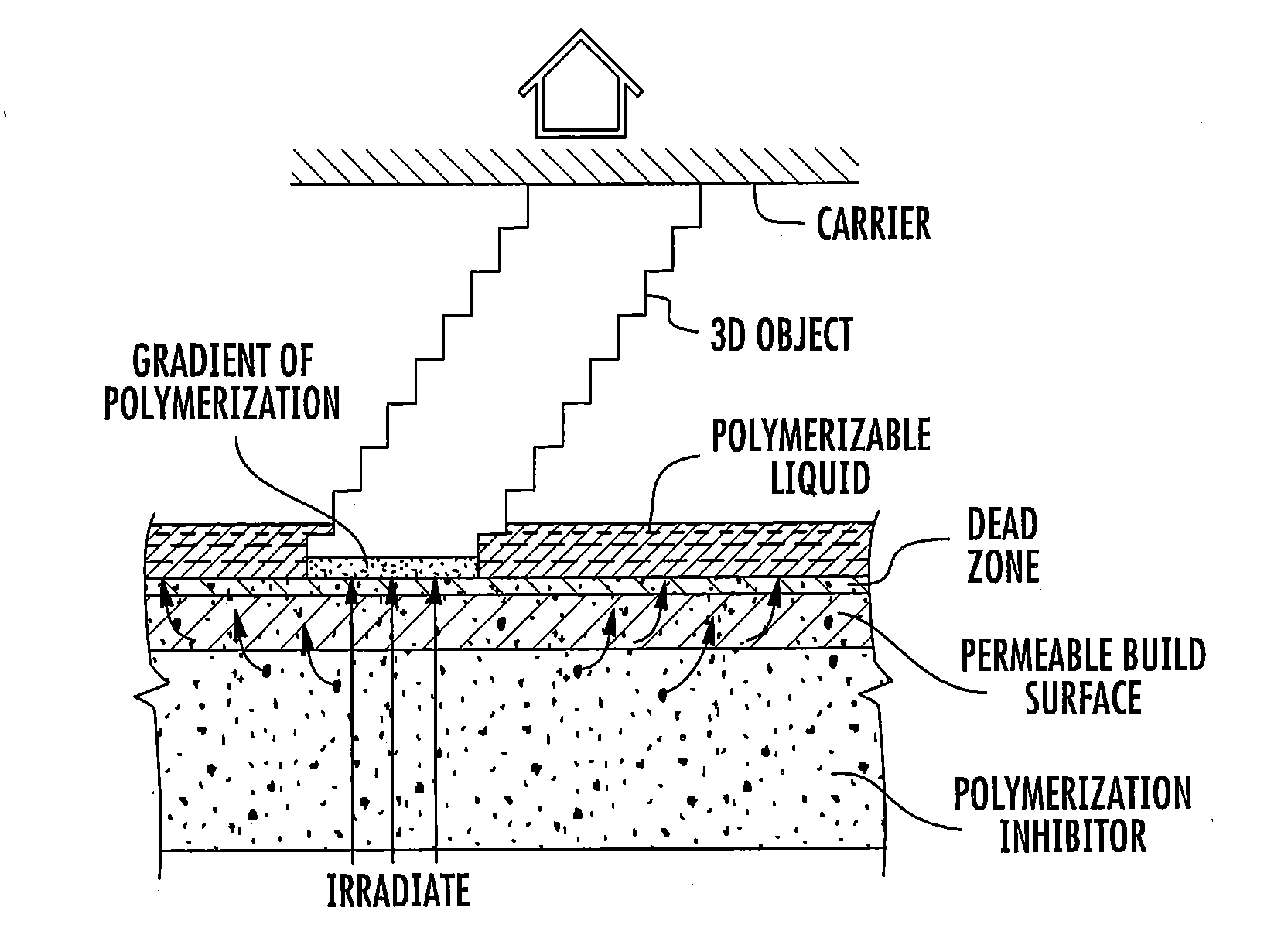
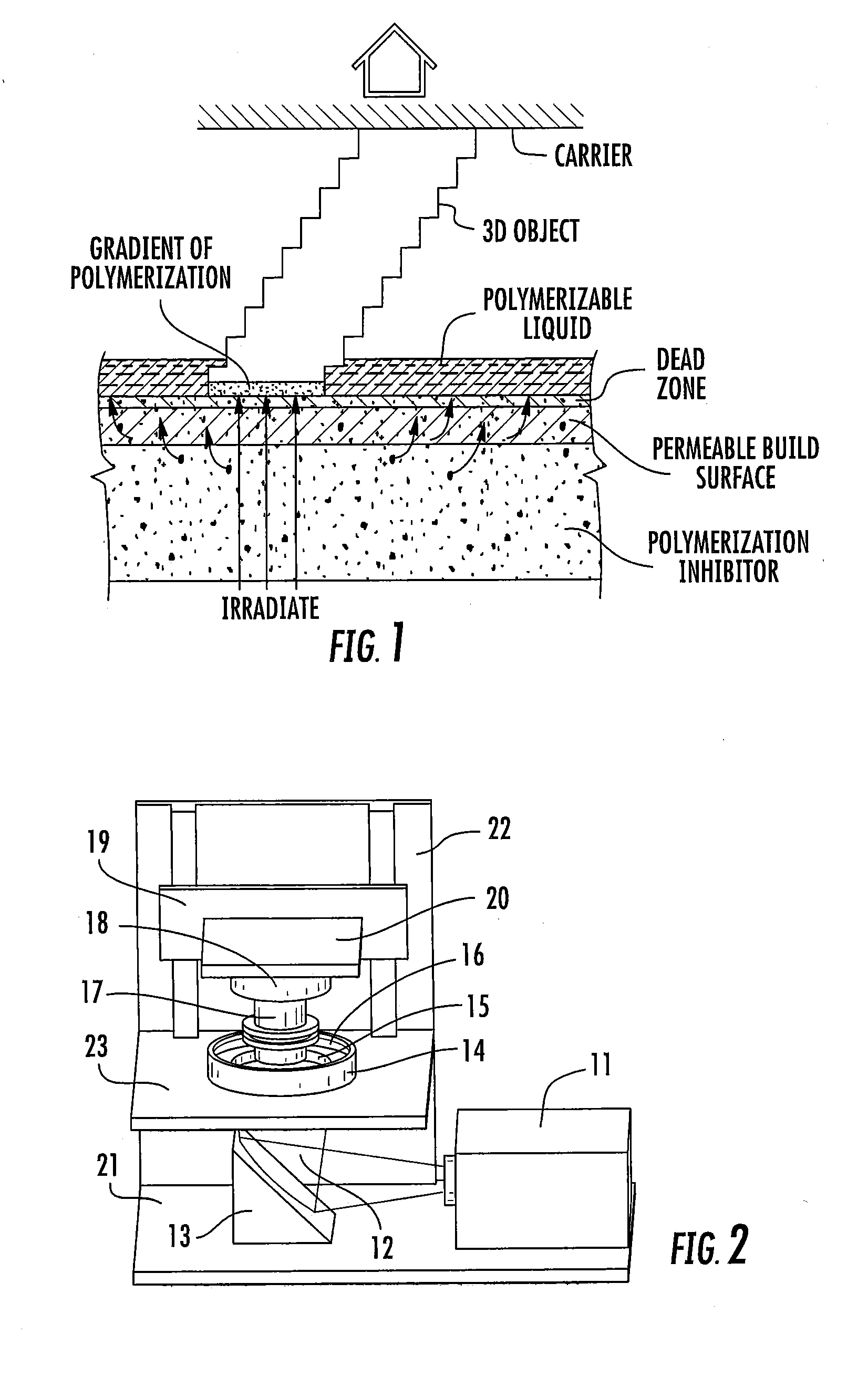
ActiveUS20160137839A1Increased formationEnhance or speed the refilling of the build regionImpression capsManufacturing enclosuresEndcappingPolymer science
A polymerizable liquid, or resin, useful for the production by additive manufacturing of a three-dimensional object of polyurethane, polyurea, or a copolymer thereof, is described. The resin includes at least one of (i) a blocked or reactive blocked prepolymer, (ii) a blocked or reactive blocked diisocyanate, or (iii) a blocked or reactive blocked diisocyanate chain extender.
Owner:CARBON INC
Rigid gas permeable lens material
A copolymer useful as a rigid gas permeable contact lens material is the polymerization product of a monomeric mixture comprising a polysiloxane-containing urethane or urea prepolymer endcapped with polymerizable ethylenically unsaturated radicals.
Owner:BAUSCH & LOMB INC
Methods of producing polyurethane three-dimensional objects from materials having multiple mechanisms of hardening
ActiveUS20160137838A1Increased formationEnhance or speed the refilling of the build regionManufacturing enclosuresOptical articlesEndcappingPolymer science
A method of forming a three-dimensional object of polyurethane, polyurea, or copolymer thereof is carried out by: (a) providing a carrier and an optically transparent member having a build surface, the carrier and the build surface defining a build region therebetween; (b) filling the build region with a polymerizable liquid, the polymerizable liquid including at least one of: (i) a blocked or reactive blocked prepolymer, (ii) a blocked or reactive blocked diisocyanate, or (iii) a blocked or reactive blocked diisocyanate chain extender; (c) irradiating the build region with light through the optically transparent member to form a solid blocked polymer scaffold and advancing the carrier away from the build surface to form a three-dimensional intermediate having the same shape as, or a shape to be imparted to, the three-dimensional object, with the intermediate containing the chain extender; and then (d) heating or microwave irradiating the three-dimensional intermediate sufficiently to form from the three-dimensional intermediate the three-dimensional object of polyurethane, polyurea, or copolymer thereof.
Owner:CARBON INC
Radiation-curable prepolymers
The present invention provides a radiation-curable prepolymer. The radiation-curable prepolymer of the invention is prepared by reacting an isocyanate-capped polyurethane with an ethylenically unsaturated amine or an ethylenically unsaturated monohydroxy compound or a mixture thereof, wherein the isocyanate-capped polyurethane is a copolymerization production of: (a) at least one polyalkylene glycol; (b) at least one branching agent having at least three hydroxy groups; and (c) at least one di- or polyisocyanate. The radiation-curable prepolymer of the invention can find use in economically producing contact lenses which have durable, highly elastic soft contact lenses with desired physical properties. In addition, the present invention provides method for making a radiation-curable prepolymer of the invention and for making a medical device, preferably an ophthalmic device, more preferably a contact lens.
Owner:ALCON INC
Low application temperature hot melt adhesive
InactiveUS20070088116A1High bond strength levelSame level of performanceAbsorbent padsAdhesivesElastomerCardboard
A hot melt adhesive composition, comprising a blend of components including about 10% to about 40% by weight of an elastomeric block copolymer, preferably styrene-isoprene-styrene (SIS) or styrene-butadiene-styrene (SBS), about 15% to about 70% by weight of a first midblock tackifying resin having a softening point of at least about 110° C. and having an aromatic content of at least about 1.5% by weight; about 0 to 55% of second midblock tackifying resin, about 5% to about 35% by weight of a plasticizer; and about 0% to about 20% by weight of an end block resin having a softening point lower than 125° C.; wherein the components total 100% by weight of the composition, the viscosity of the composition is equal to or less than about 20,000 mPa.s at 120° C., and is applied at a temperature lower that 150° C. and initial bond retention of the composition on elastic strands is at least about 60%. Also, the elastic modulus G′ of the composition is higher than about 5000 Pa, the vicous modules G″ is higher than about 50 Pa, and the tan delta value is between about 0.5 and about 60. Laminates, especially those used in disposable soft goods, and methods of making such laminates are also described. The adhesive composition and / or laminate may be used in making a variety of end products such as a disposable diaper, a sanitary napkin, a bed pad, a bandage, a surgical drape, a tape, a label, a plastic sheet, a nonwoven sheet, a paper sheet, a cardboard, a book, a filter, or a package.
Owner:BOSTIK INC
Silicone hydrogels based on vinyl carbonate endcapped fluorinated side chain polysiloxanes
InactiveUS6891010B2Maintain good propertiesLow modulusOptical partsProsthesisEndcappingSilicone Gels
Vinyl carbonate endcapped polysiloxanes containing a fluorinated side chain are useful as biomaterials, especially hydrogel biomaterials, including contact lens materials.
Owner:BAUSCH & LOMB INC
Capping reactions in cationic polymerization; kinetic and synthetic utility
A method of synthesizing an endcapped polymer, comprising reacting in a solvent a cationic living polymer with an optionally substituted conjugated diene as an endcapping reagent, whereby the solvent causes termination by halogenation to be faster than the addition of additional molecules of the conjugated diene, thereby producing an endcapped polymer having a halogenated endcap group.
Owner:MASSACHUSETTS LOWELL UNIV OF
Preparation of organyloxysilyl-terminated polymers
The present invention relates to a process for preparing organyloxysilyl-terminated polymers which have increased stability toward atmospheric moisture, by reacting hydroxy-terminated organic polymers with isocyanato-functional silanes in the presence of at least one catalyst selected from the group consisting of bismuth and zinc compounds, and to crosslinkable compositions comprising such polymers.
Owner:WACKER CHEM GMBH
Free flowing filler composition based on organofunctional silane
ActiveUS20070228322A1Improve performanceLong scorch timeSilicon organic compoundsOther chemical processesElastomerThiol
Owner:MOMENTIVE PERFORMANCE MATERIALS INC
Biocidal polyurethane compositions and methods of use
InactiveUS7459167B1Improve adhesionImprove hydrolytic resistanceOrganic active ingredientsBiocideMicroorganismAmmonium compounds
Polymeric compositions that include polyurethane polymers derived from a polyisocyanate compound and a polyactive hydrogen compound. The polyurethane compound is at least partially endcapped with a group including at least one antimicrobial quaternary ammonium compound. The polymeric composition of the present invention is capable of forming a self-supporting film. The polymeric compositions are suitable for coating substrates to effectively kill or prevent the growth of microorganisms such as bacteria, mold, mildew, algae fungi and the like. The polymeric compositions are particularly useful for protecting construction materials used in moist, outdoor environments to prevent discoloration or decay from microorganisms and for surfaces in health care facilities to mitigate the spread of pathogens.
Owner:3M INNOVATIVE PROPERTIES CO
Crosslinked, degradable polymers and uses thereof
Acrylate-terminated poly(beta-amino esters) are cross-linked to form materials useful in the medical as well as non-medical field. The polymeric starting material is combined with a free radical initiator, either a thermal initiator or a photoinitiator, and the mixture for cross-linking is heated or exposed to light depending on the initiator used. The resulting materials due to the hydrolysable ester bond in the polymer backbone are biodegradable under physiological conditions. These cross-linked materials are particular useful as drug delivery vehicles, tissue engineering scaffolds, and in fabricating microdevices. The materials may also be used as plastics, coating, adhesives, inks, etc. The cross-linked materials prepared exhibit a wide range of degradation times, mass loss profiles, and mechanical properties. Therefore, the properties of the material may be tuned for the desired use. The high-throughput approach to preparing a library of cross-linked poly(beta-amino esters) allows for the rapid screening and design of degradable polymers for a variety of applications.
Owner:MASSACHUSETTS INST OF TECH
Blocked mercaptosilane coupling agents for filled rubbers
Disclosed herein is a blocked mercaptosilane selected from the group consisting of[[(ROC(═O))p—(G)j]k—Y—S]r—G—(SiX3)s; and (1)[(X3Si)q—G]a—[Y—[S—G—SiX3]b]c (2)whereinY is a polyvalent species (Q)zA(═E) selected from the group consisting of —C(═NR)—; —SC(═NR)—; —SC(═O)—; —OC(═O)—; —S(═O)—; —S(═O)2—; —OS(═O)2—; (—NR)S(═O)2—; —SS(═O)—; —OS(═O)—; (—NR)S(═O)—; —SS(═O)2—; (—S)2P(═O)—; —(—S)P(═O)—; —P(═O)(—)2; (—S)2P(═S)—; —(—S)P(═S)—; —P(═S)(—)2; (—NR)2P(═O)—; (—NR)(—S)P(═O)—; (—O)(—NR)P(═O)—; (—O)(—S)P(═O)—; (—O)2P(═O)—; —(—O)P(═O)—; —(—NR)P(═O)—; (—NR)2P(═S)—; (—NR)(—S)P(═S)—; (—O)(—NR)P(═S)—; (—O)(—S)P(═S)—; (—O)2P(═S)—; —(—O)P(═S)—; and —(—NR)P(═S)—; wherein the atom A attached to unsaturated heteroatom E is attached to the sulfur which in turn is linked via a group G to the silicon atom;each R is chosen independently from hydrogen, straight, cyclic, or branched alkyl that may or may not contain unsaturation, alkenyl groups, aryl groups, and aralkyl groups, with each R containing from 1 to 18 carbon atoms;each G is independently a monovalent or polyvalent group derived by substitution of alkyl, alkenyl, aryl, or aralkyl, wherein G can contain from 1 to 18 carbon atoms, with the proviso that G is not such that the blocked mercaptosilane would contain an α,β-unsaturated carbonyl that can undergo polymerization reactions, and if G is univalent, G can be a hydrogen atom;X is independently selected from the group consisting of —Cl, —Br, RO—, RC(═O)O—, R2C═NO—, R2NO—, R2N—, —R, and —(OSiR2)t(OSiR3) wherein each R is as above and at least one X is not —R;p is 0 to 5; r is 1 to 3; z is 0 to 2; q is 0 to 6; a is 0 to 7; b is 1 to 3; j is 0 to 1, but it may be 0 only if p is 1; c is 1 to 6; t is 0 to 5; s is 1 to 3; k is 1 to 2; with the provisos that (I) if A is carbon, sulfur or sulfonyl, then (i) a+b is 2 and (ii) k is 1; (II) if A is phosphorus, then a+b is 3 unless both (i) c is greater than 1 and (ii) b is 1, in which case a is c+1; and (III) if A is phosphorus, then k is 2.
Owner:GENERAL ELECTRIC CO
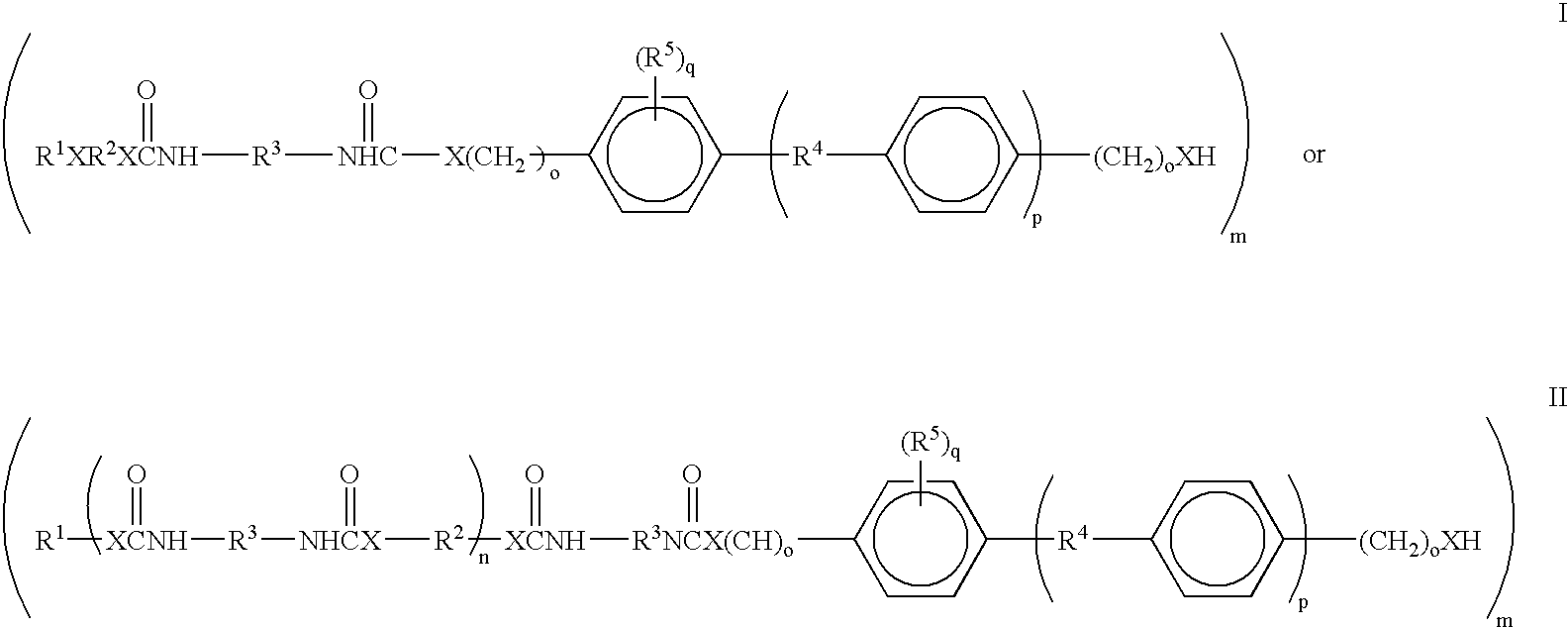
Adhesive compositions containing blocked polyurethane prepolymers
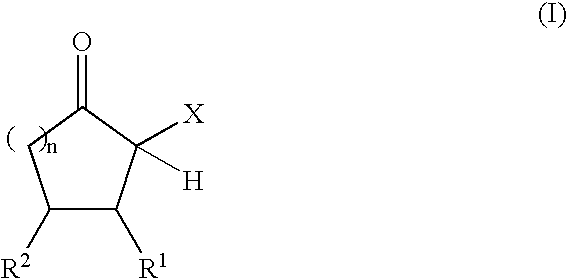
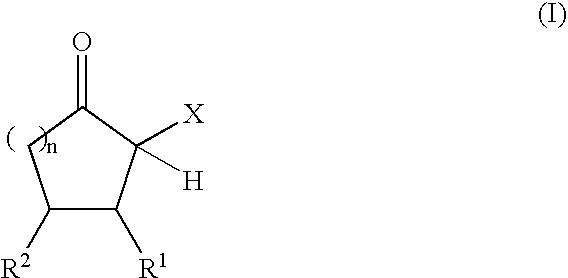
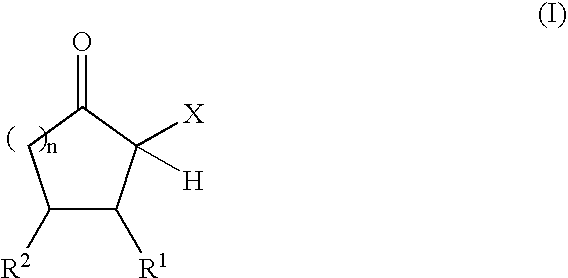
The present invention relates to reactive compositions containing A) one or more blocked polyurethane prepolymers which have a content of blocked isocyanate groups (calculated as NCO) of 0.1 to 20 wt. % and are prepared from i) at least one aromatic, aliphatic, araliphatic and / or cycloaliphatic diisocyanate having a content of free NCO groups of 5 to 60 wt. %, ii) a polyol component containing at least one polyester polyol, and / or at least one polyether polyol and / or at least one polycarbonate polyol, iii) CH-acidic cyclic ketones corresponding to formula (I) as blocking agents wherein X represents an electron-attracting group, R1 and R2 independently of one another represent the radicals H, C1-C20 (cyclo)alkyl, C6-C24 aryl, C1-C20 (cyclo)alkyl ester or amide, C6-C24 aryl ester or amide, mixed aliphatic / aromatic radicals with 1 to 24 carbon atoms that can also be part of a 4- to 8-membered ring, n is an integer from 0 to 5, and B) one or more OH-functional compounds in which the OH component undergoes activation by a deposition amine component. The present invention also relates to a composite system containing two adherends bonded together with the reactive composition according to the invention.
Owner:BAYER MATERIALSCIENCE AG
Method for end-capping polycarbonate resins and composition for use in same
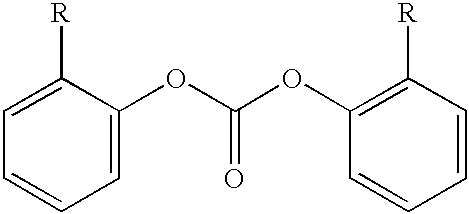
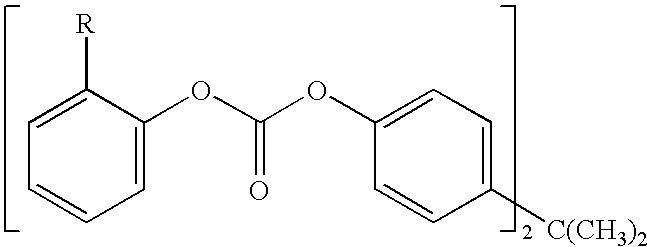
A method for end-capping polycarbonate resins, comprising the step of processing a mixture comprising a polycarbonate having free hydroxyl-end groups and an end-capping reagent in a melt transesterification reaction to produce a polycarbonate resin, wherein the end-capping reagent comprises a mixture of: (a) at least one species of a symmetrical activated aromatic carbonate, and (b) at least one species of an optionally-substituted phenol, whereby said end-capping reagent reacts with at least some of the free hydroxyl end-groups of the polycarbonate to produce an end-capped polycarbonate resin.
Owner:SABIC GLOBAL TECH BV
Silica-reinforced rubber compounded with blocked mercaptosilanes and alkyl alkoxysilanes
The invention provides a sulfur vulcanizable silica-reinforced elastomeric compound having improved tensile mechanical and dynamic viscoelastic properties. The compounds are formed by mixing an elastomer optionally having an alkoxysilane terminal group, with silica in the presence of an alkyl alkoxysilane and a blocked mercaptosilane. In particular, the mercaptosilane moiety of the blocked mercaptosilane and the alkyl alkoxysilane are present in a weight ratio of about 0.001:1 to about 0.20:1. Preferably, the blocked mercaptosilane and the alkyl alkoxysilane are compounded with the elastomer and the silica at high temperature to facilitate the silica-silane reaction. A deblocking agent is added at any desired mixing stage, optionally with the cure package, to allow binding of the mercaptosilane to the polymer.
Owner:BRIDGESTONE CORP
Peptide nanostructures containing end-capping modified peptides and methods of generating and using the same
A nanostructure composed of a plurality of peptides, each peptide containing at least one aromatic amino acid, whereby one or more of these peptides is end-capping modified, is disclosed. The nanostructure can take a tubular, fibrillar, planar or spherical shape, and can encapsulate, entrap or be coated by other materials. Methods of preparing the nanostructure, and devices and methods utilizing same are also disclosed.
Owner:RAMOT AT TEL AVIV UNIV LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com