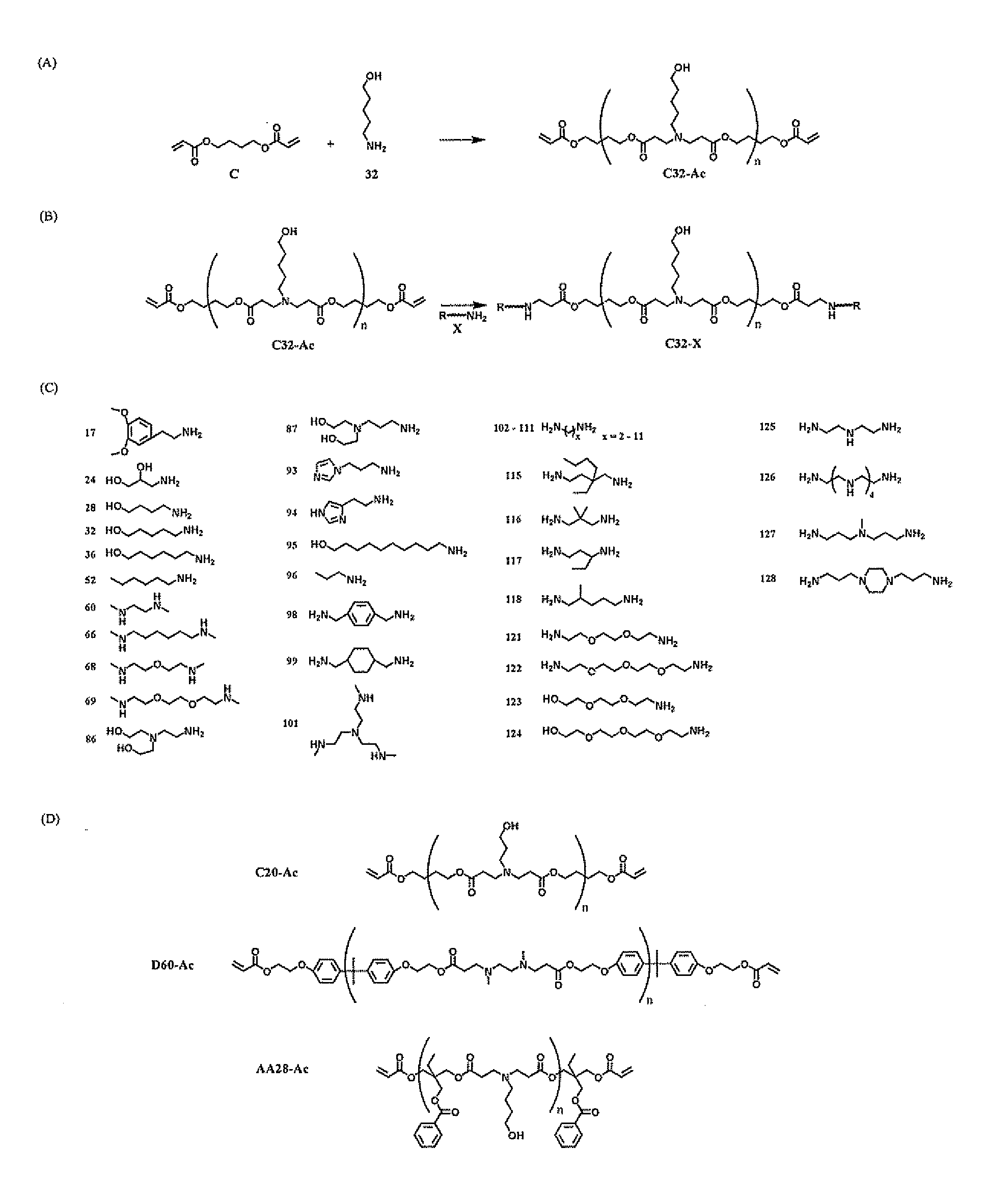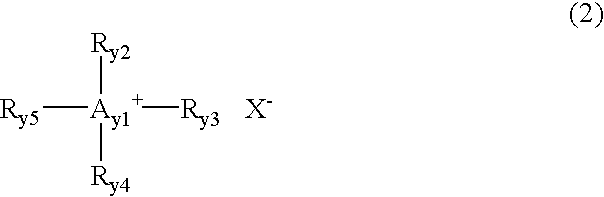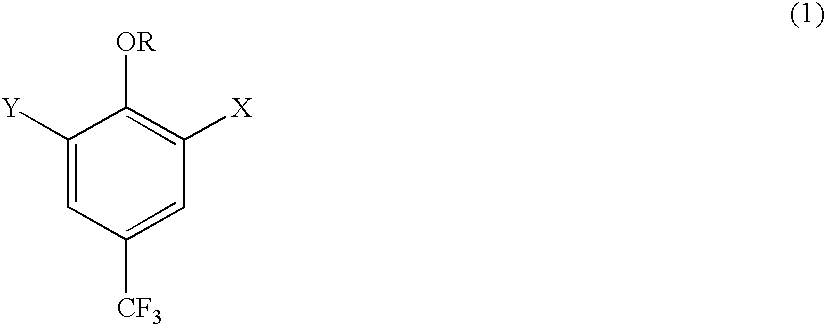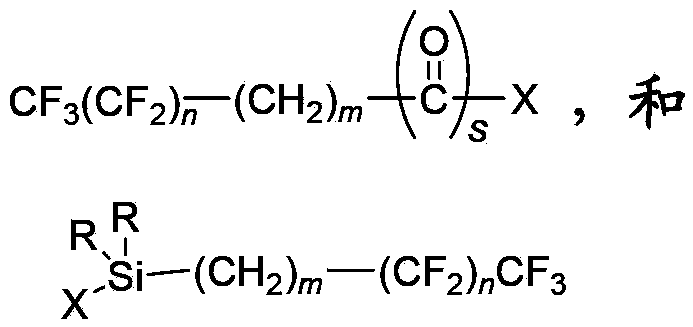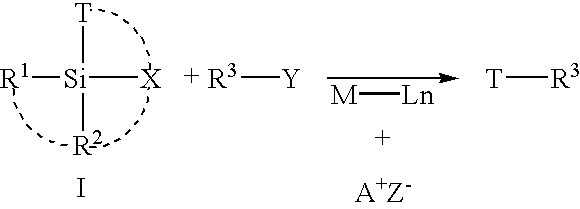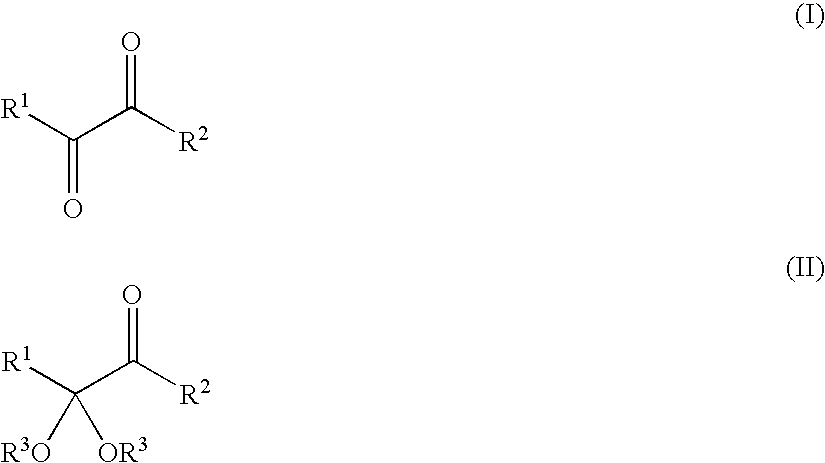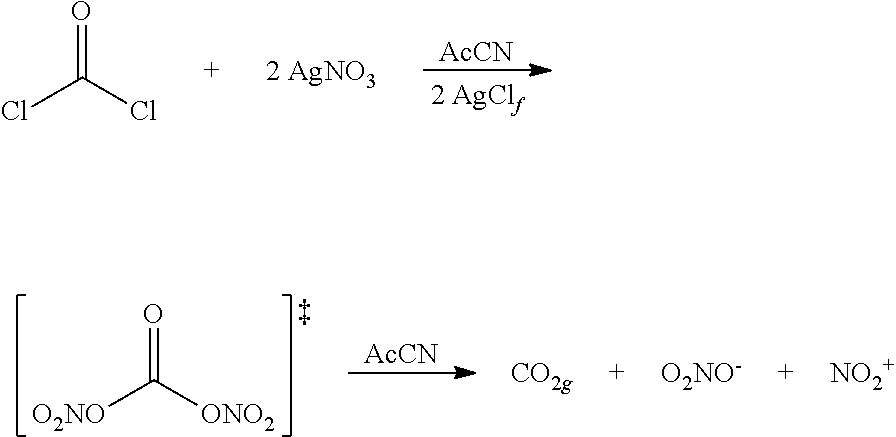Patents
Literature
100 results about "Electrophile" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In organic chemistry, an electrophile is an electron pair acceptor. Electrophiles are positively charged or neutral species having vacant orbitals that are attracted to an electron rich centre. It participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile. Because electrophiles accept electrons, they are Lewis acids (see acid-base reaction theories). Most electrophiles are positively charged, have an atom that carries a partial positive charge, or have an atom that does not have an octet of electrons. They appear to attract electrons as well and seem to behave as though they are partially empty. These partially empty substances thus require an electron rich center, and thus they are filled. Electrophiles can be observed as electron-sensitive or photo-sensitive.
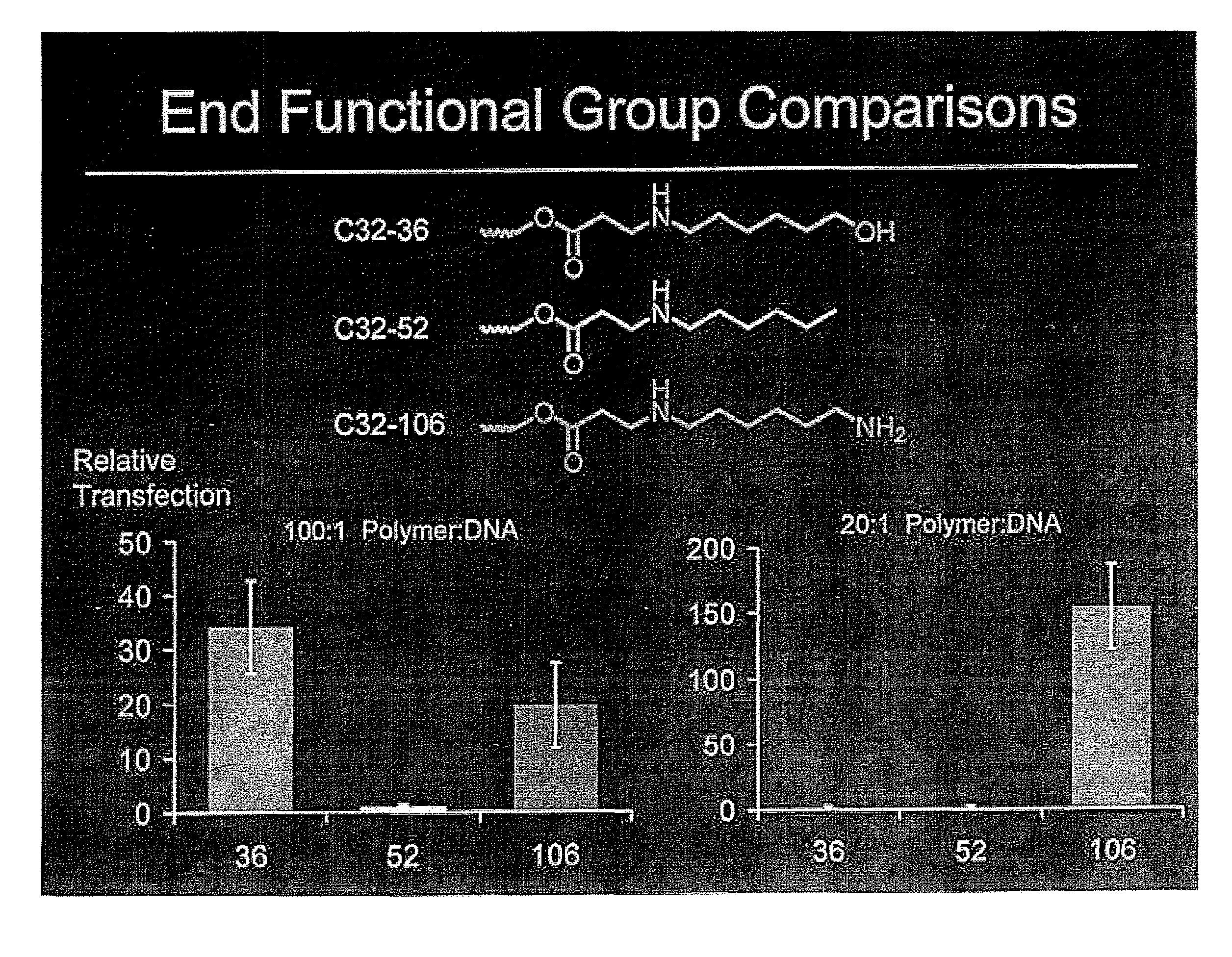
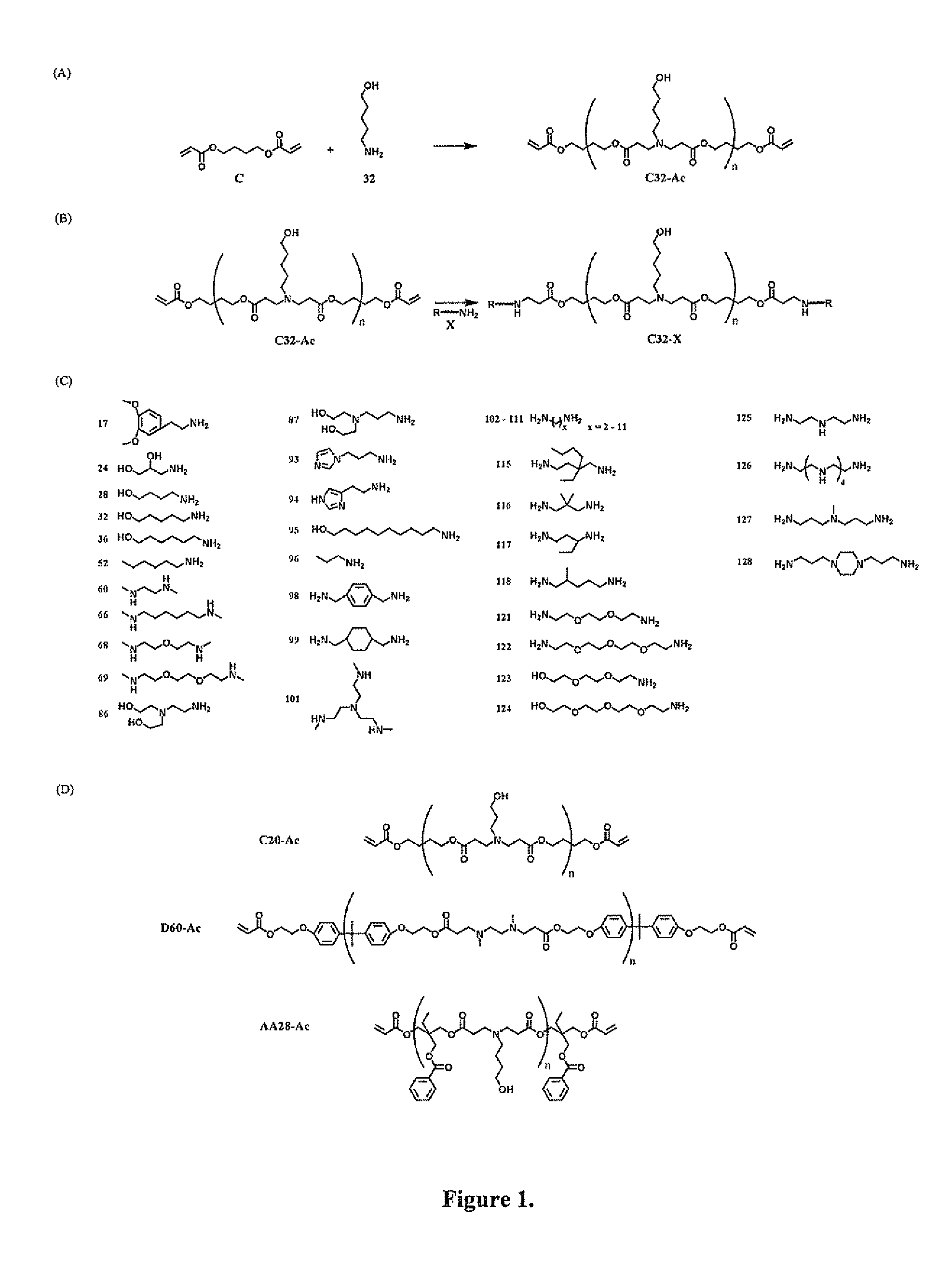
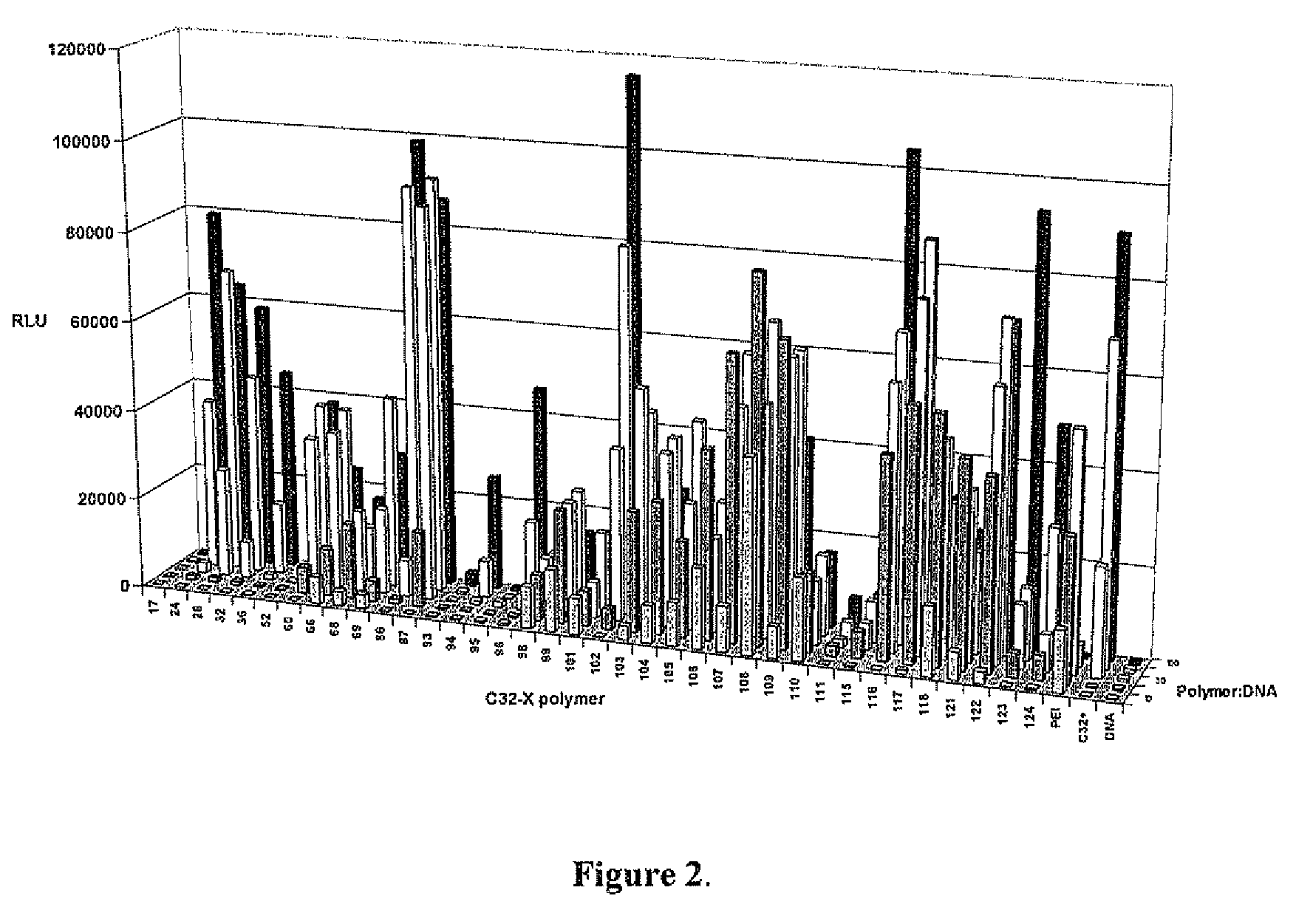
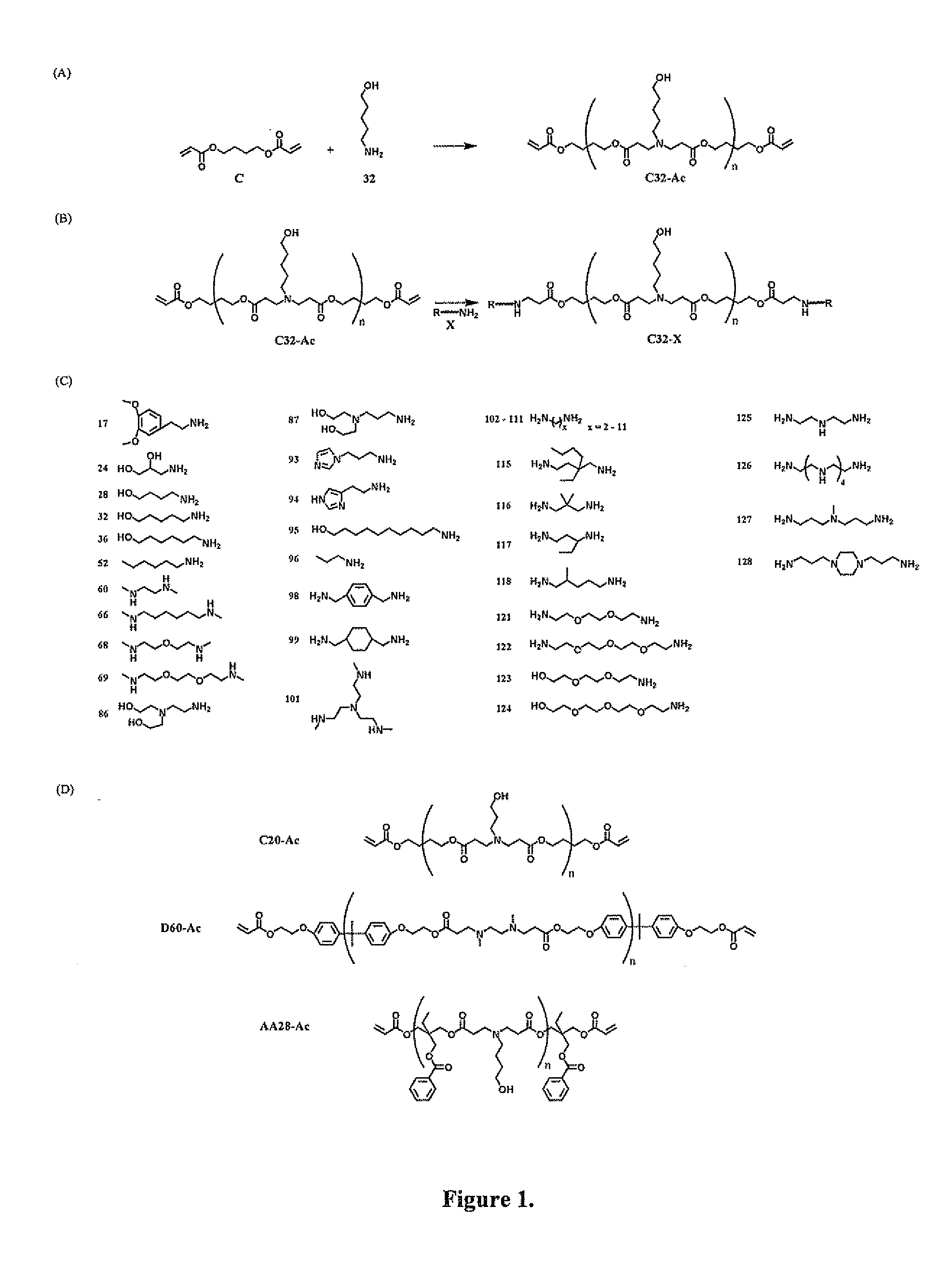
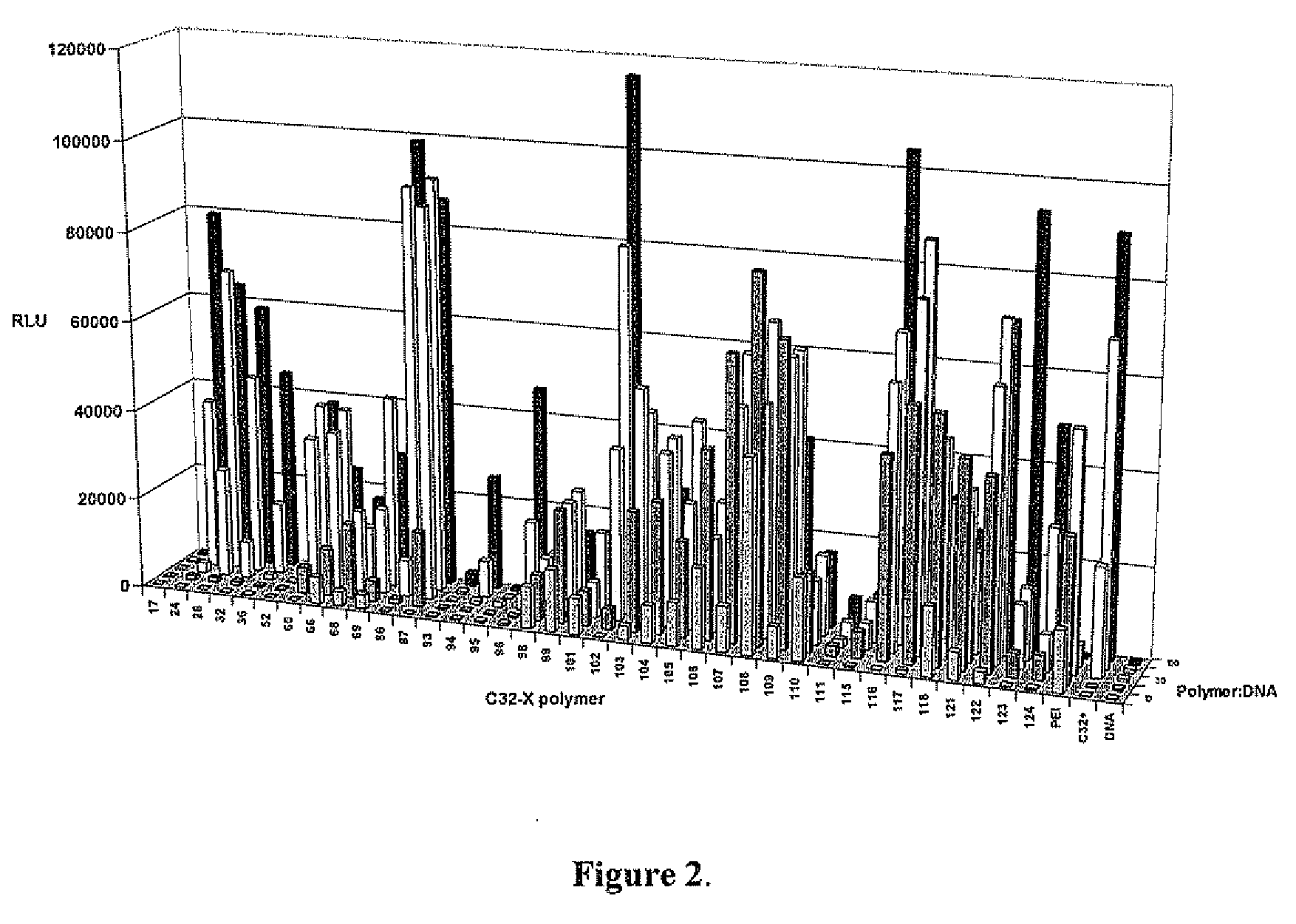
End-modified poly(beta-amino esters) and uses thereof
Poly(beta-amino esters) are end-modified to form materials useful in the medical as well as non-medical field. An amine-terminated poly(beta-amino ester) is reacted with an electrophile, or an acrylate-terminated poly(beta-amino ester) is reacted with a nucleophile. The inventive end-modified polymers may be used in any field where polymers have been found useful including the drug delivery arts. The end-modified polymers are particularly useful in delivery nucleic acids such as DNA or RNA. The invention also provides compositions including the inventive end-modified polymers, methods of preparing the inventive polymers, and method of using the inventive polymers.
Owner:MASSACHUSETTS INST OF TECH
End-Modified Poly(beta-amino esters) and Uses Thereof
ActiveUS20080242626A1Organic active ingredientsOther foreign material introduction processesAmino estersElectrophile
Poly(beta-amino esters) are end-modified to form materials useful in the medical as well as non-medical field. An amine-terminated poly(beta-amino ester) is reacted with an electrophile, or an acrylate-terminated poly(beta-amino ester) is reacted with a nucleophile. The inventive end-modified polymers may be used in any field where polymers have been found useful including the drug delivery arts. The end-modified polymers are particularly useful in delivery nucleic acids such as DNA or RNA. The invention also provides compositions including the inventive end-modified polymers, methods of preparing the inventive polymers, and method of using the inventive polymers.
Owner:MASSACHUSETTS INST OF TECH
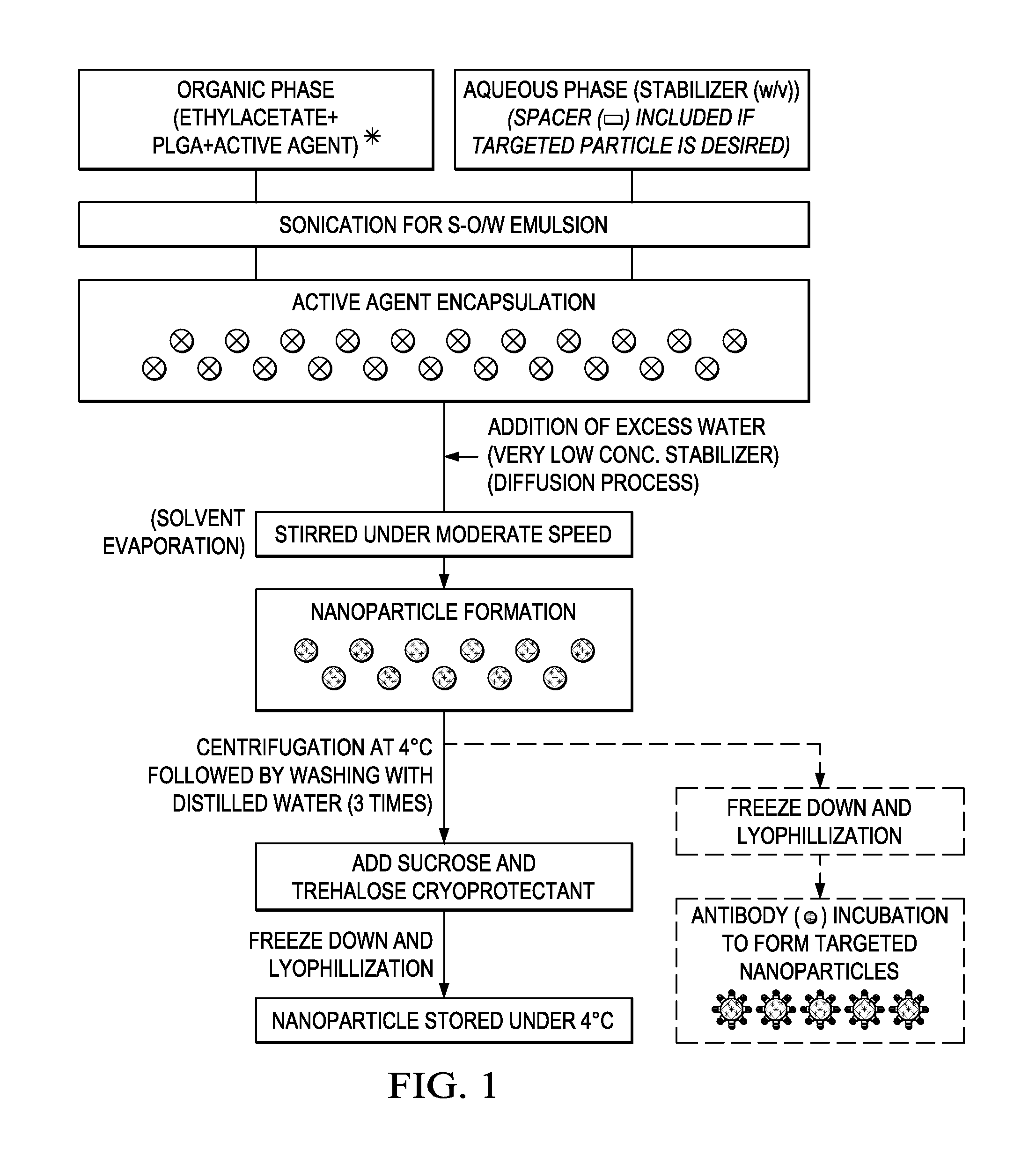
Formulation of Active Agent Loaded Activated PLGA Nanoparticles for Targeted Cancer Nano-Therapeutics
The present invention includes compositions and methods of making an activated polymeric nanoparticle for targeted drug delivery that includes a biocompatible polymer and an amphiphilic stabilizing agent non-covalently associated with a spacer compound that includes at least one electrophile that selectively reacts with any nucleophilic on a targeting agent and places the targeting agent on the exterior surface of a biodegradable nanoshell, wherein an active agent is loaded with the nanoshell.
Owner:UNIV OF NORTH TEXAS HEALTH SCI CENT
Functionalized block copolymers, method for making same, and various uses for such block copolymers
The present invention is a, solid block copolymer comprising at least two polymer end blocks A and at least one polymer interior block B wherein each A block is a polymer block resistant to lithiation and each B block is a polymer block susceptible to lithiation, and wherein said A and B blocks do not contain any significant levels of olefinic unsaturation. Preferably, each A block comprising one or more segments selected from polymerized (i) para-substituted styrene monomers not having hydrogen on a para benzylic carbon center, (ii) ethylene, (iii) alpha olefins of 3 to 18 carbon atoms; (iv) hydrogenated 1,3-cyclodiene monomers, (v) hydrogenated monomers of conjugated dienes having a vinyl content less than 35 mol percent prior to hydrogenation, (vi) acrylic esters, (vii) methacrylic esters, and (viii) mixtures thereof; and each B block comprising segments of one or more polymerized vinyl aromatic monomers selected from (i) unsubstituted styrene monomers, (ii) ortho-substituted styrene monomers, (iii) meta-substituted styrene monomers, (iv) alpha-methylstyrene, (v) para-substituted styrene having hydrogen on a para benzylic carbon center (vi) 1,1-diphenylethylene, (vii) 1,2-diphenylethylene and (viii) mixtures thereof. After lithiation, the lithiated polymer is reacted with at least one graftable functional molecule selected from the group consisting of an electrophilic graftable molecule containing a functional group and an electrophile. Preferred are carbon monoxide and ethylene oxide. Also claimed are processes for making such block copolymers, and the various end uses and applications for such block copolymers.
Owner:KRATON POLYMERS US LLC
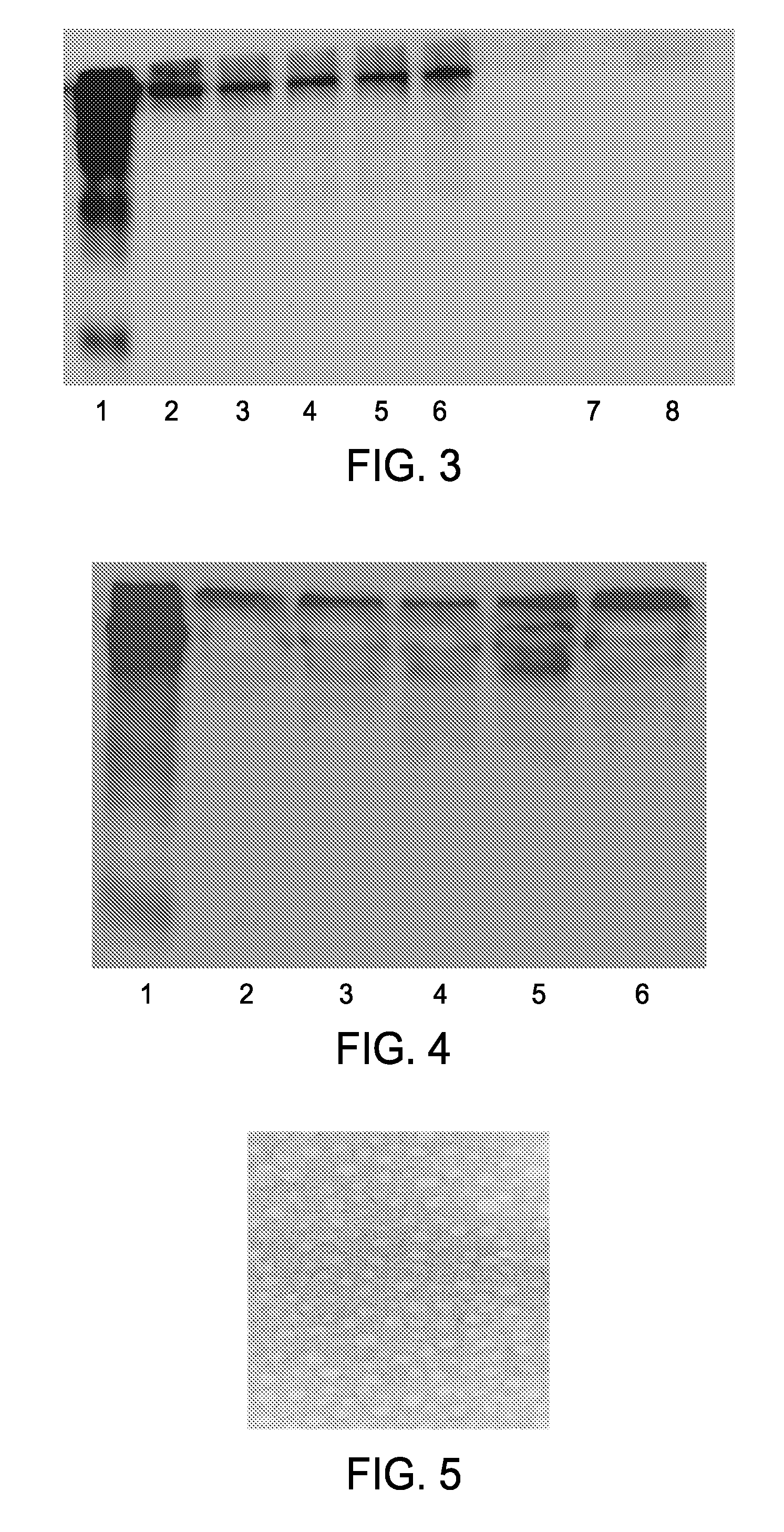
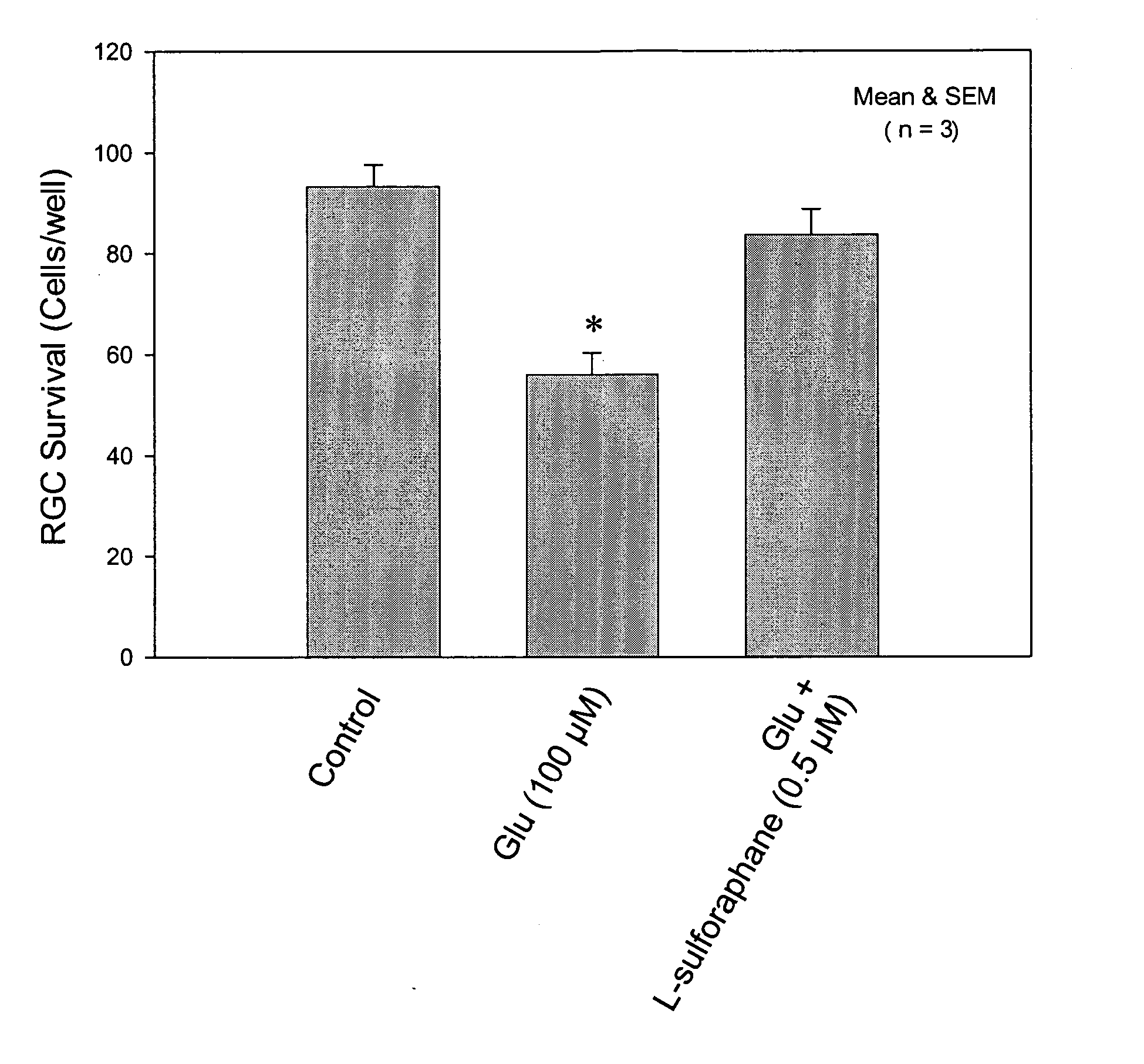
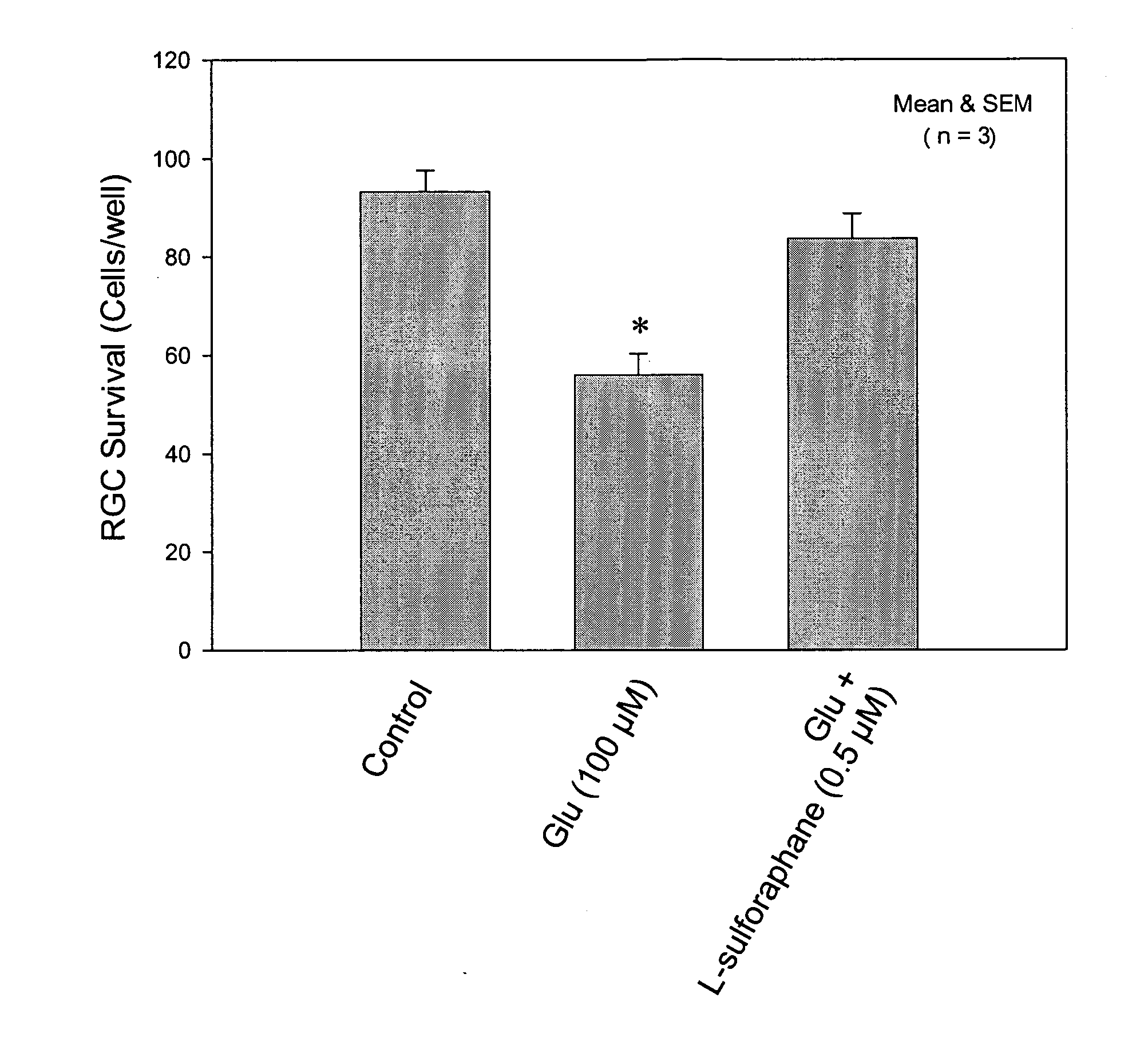
Agents for treatment of glaucomatous retinopathy and optic neuropathy
InactiveUS20050137146A1Delaying and preventing lossImprove availabilityBiocideSenses disorderQuinoneThiocarbamate
Agents that stimulate nuclear translocation of Nrf2 protein and the subsequent increases in gene products that detoxify and eliminate cytotoxic metabolites are provided in a method for treating glaucomatous retinopathy or optic neuropathy. The structurally diverse agents that act on the Nrf2 / ARE pathway induce the expression of enzymes and proteins that possess chemically versatile cytoprotective properties and are a defense against toxic metabolites and xenobiotics. Agents include certain electrophiles and oxidants such as a Michael Addition acceptor, diphenol, thiocarbamate, quinone, 1,2-dithiole-3-thione, butylated hydroxyanisole, flavonoid, an isothiocyanate, 3,5-di-tert-butyl-4-hydroxytoluene, ethoxyquin, a coumarin, combinations thereof, or a pharmacologically active derivative or analog thereof.
Owner:ALCON INC
Electrolyte composition and non-aqueous electrolyte secondary cell
InactiveUS20030013021A1Maintain good propertiesPreventing leakage and depletionSolid electrolytesNon-aqueous electrolyte accumulatorsMolten saltElectrolyte composition
An electrolyte composition excellent in charge-transporting property that can be prepared with ease, and a non-aqueous electrolyte secondary cell that comprises the electrolyte composition to exhibit excellent cell characteristics while preventing leakage or depletion of the electrolyte composition. The electrolyte composition comprises: a particular molten salt; a polymer prepared by a reaction between an electrophile having at least two unsaturated bonds polarized by an electron-withdrawing group and a nucleophile having a plurality of nucleophilic groups; and a metal salt containing a Group IA metal ion or a Group IIA metal ion.
Owner:FUJIFILM CORP +1
Universal linker compositions for the release or transfer of chemical agents from a polynucleotide
InactiveUS20060199192A1Simple and inexpensive to prepareMonoazo dyesNanotechLeaving groupReaction rate
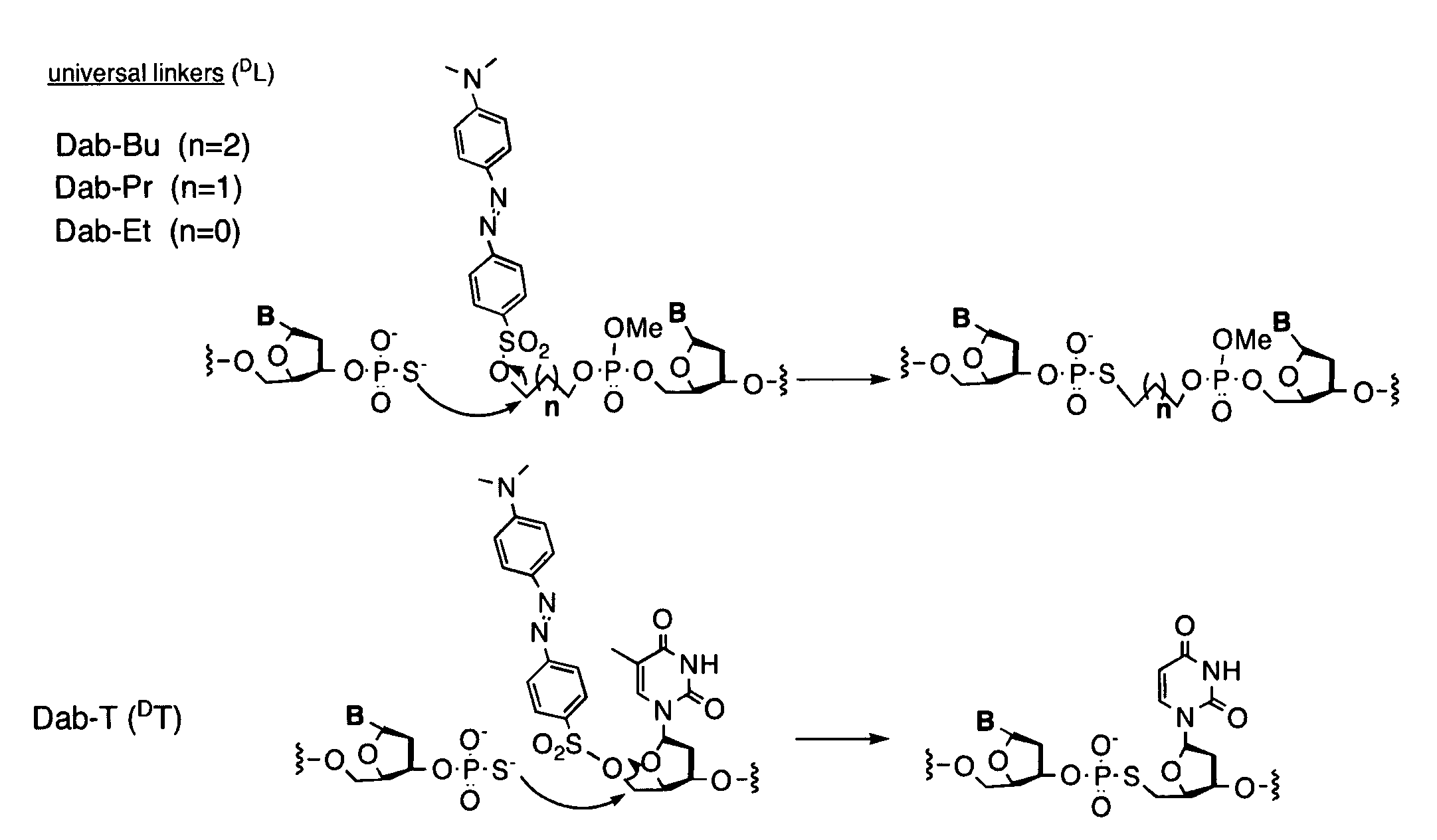
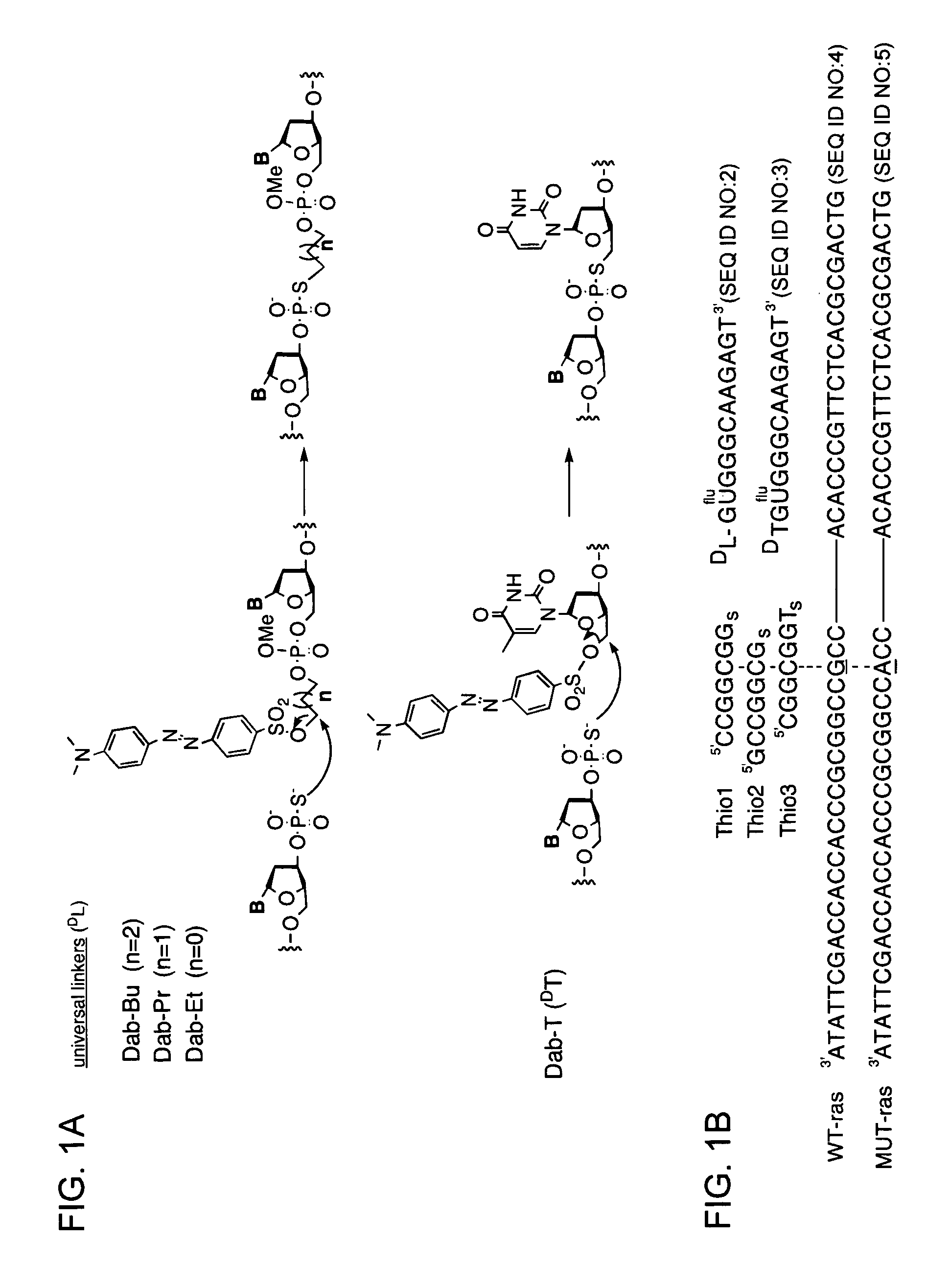
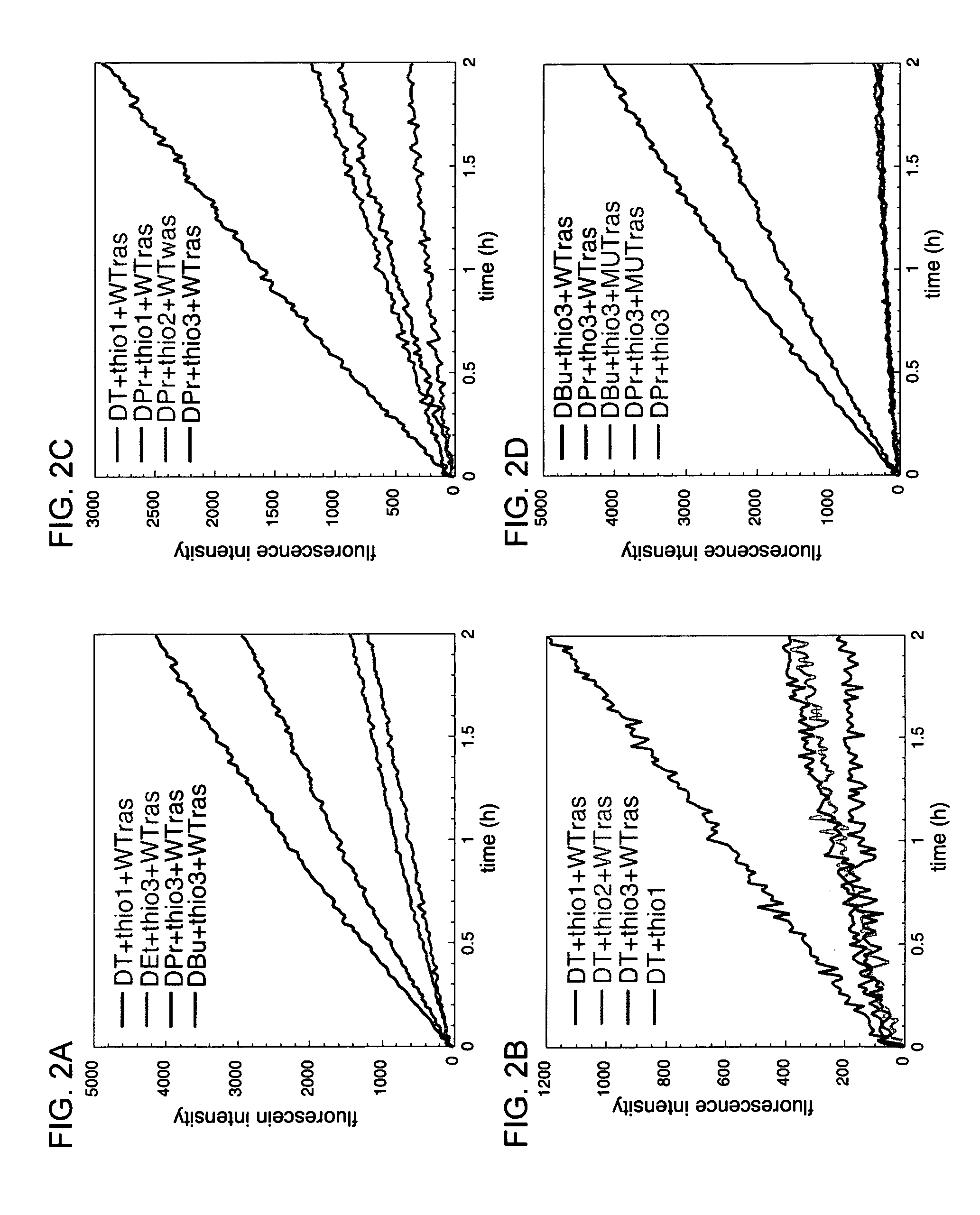
A universal linker structure is provided, in which a functional group and activating leaving group are placed on a tether, allowing the placement of an electrophile at the end of any nucleic acid sequence. The electrophile on the tether can react with a second nucleic acid carrying a nucleophile when the two nucleic acids are hybridized near one another, resulting in release of the leaving group, and creation of a functional change. The linker can be designed to destabilize the ligation product without slowing the rate of reaction. This lowers product inhibition, and the target DNA or RNA can become a catalyst for isothermally generating multiple signals for detection. This enhanced signal is demonstrated in solution experiments and in solid supported assays. The universal linkers of the present invention are simple and inexpensive to prepare, and can be appended to any polynucleotide in automated steps on a standard DNA synthesizer.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Polymers and other groups attached to pigment and subsequent reactions
A method of making a modified pigment by reacting a first chemical group and a second chemical group to form a pigment having attached a third chemical group. The first chemical group includes at least one nucleophile and the second chemical group includes at least one electrophile, or vice versa. Resulting modified pigments, and ink compositions containing such pigments, are also described.
Owner:CABOT CORP
Agents for treatment of diabetic retinopathy and drusen formation in macular degeneration
InactiveUS20050137147A1Enhances availability and transportAvoid damageBiocideSenses disorderThiocarbamateQuinone
Agents that stimulate nuclear translocation of Nrf2 protein and the subsequent increases in gene products that detoxify and eliminate cytotoxic metabolites are provided in a method for treating diabetic retinopathy or drusen formation in age-related macular degeneration. The structurally diverse agents that act on the Nrf2 / ARE pathway induce the expression of enzymes and proteins that possess chemically versatile cytoprotective properties and are a defense against toxic metabolites and xenobiotics. Agents include certain electrophiles and oxidants such as a Michael Addition acceptor, diphenol, thiocarbamate, quinone, 1,2-dithiole-3-thione, butylated hydroxyanisole, flavonoid other than genistein, an isothiocyanate, 3,5-di-tert-butyl-4-hydroxytoluene, ethoxyquin, a coumarin, combinations thereof, or a pharmacologically active derivative or analog thereof.
Owner:ALCON INC
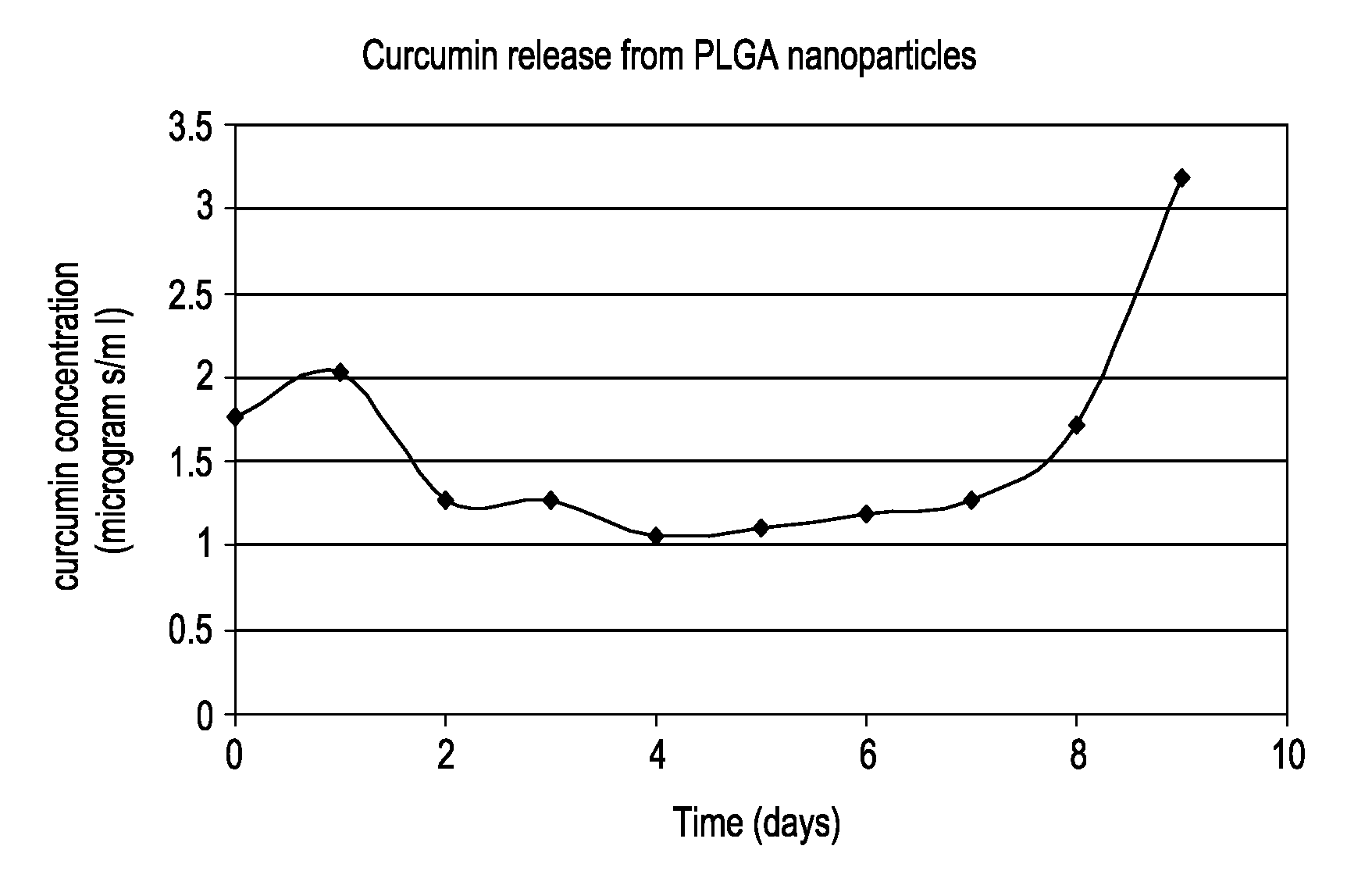
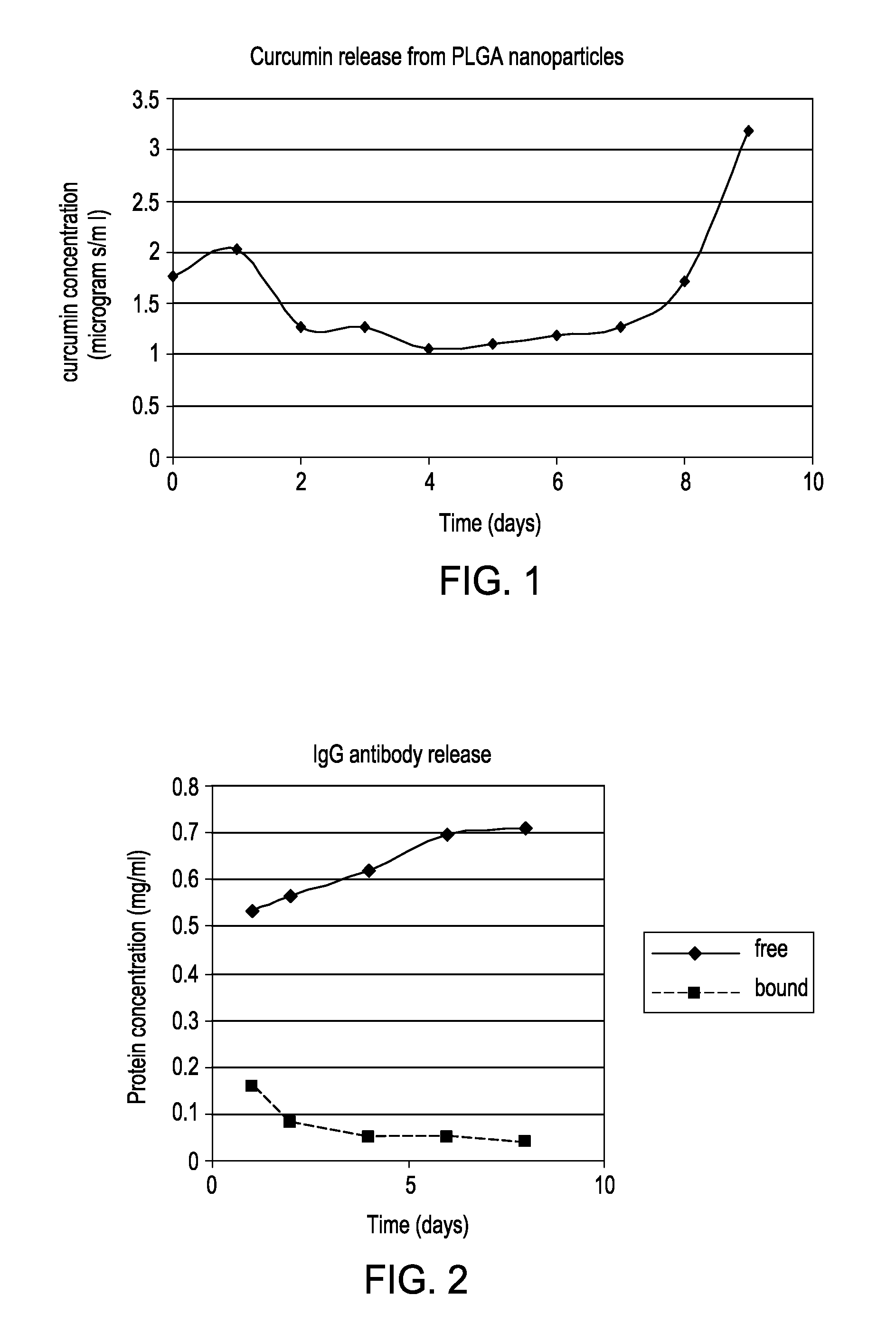
Solid in oil/water emulsion-diffusion-evaporation formulation for preparing curcumin-loaded plga nanoparticles
The present invention includes compositions and methods of making an activated polymeric nanoparticle for targeted drug delivery that includes a biocompatible polymer and an amphiphilic stabilizing agent non-covalently associated with a spacer compound that includes at least one electrophile that selectively reacts with any nucleophilic on a targeting agent and places the targeting agent on the exterior surface of a biodegradable nanoparticle, wherein an active agent is encapsulated in or about the nanoparticle.
Owner:UNIV OF NORTH TEXAS HEALTH SCI CENT
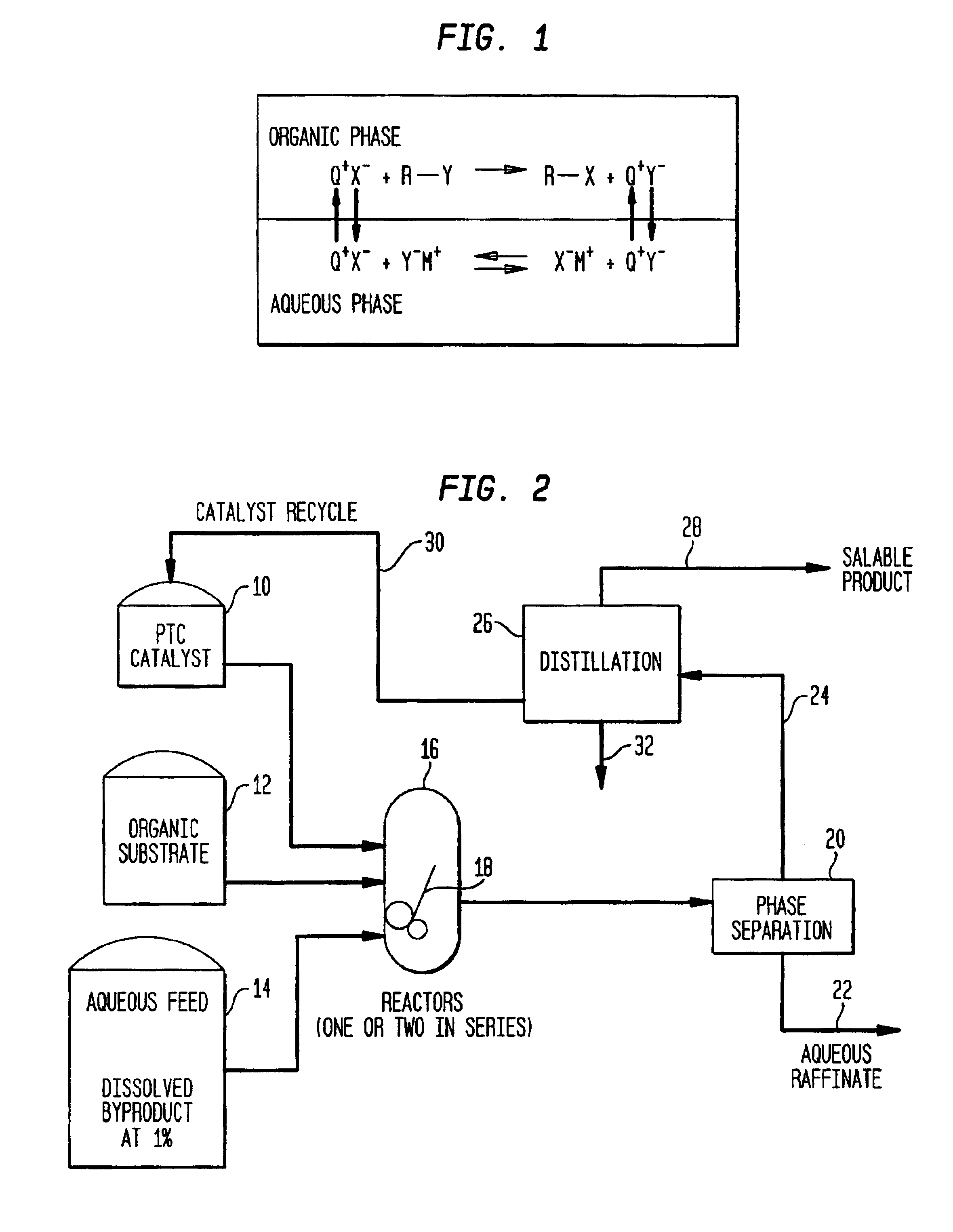
Process for making organic products and improving the quality of non-product streams using phase transfer catalysis
InactiveUS6846946B2Low costLow cost of treatmentCarbamic acid derivatives preparationOrganic compound preparationChemical speciesOrganic solvent
A method for preparing organic products from aqueous solutions, such as waste or byproduct liquid streams and waste or byproduct gas or vapor streams, uses phase transfer catalysis to transfer a chemical species in low concentration from the aqueous solution to the organic phase or the aqueous-organic interface. The system has little or no organic solvent, and the organic phase contains an electrophile which participates in the reaction. In one embodiment, the aqueous solution is contacted with the electrophile and a phase transfer catalyst and, optionally, a pH adjusting agent in the event that the chemical species in the aqueous solution is not sufficiently ionized to react with the electrophile, and optionally an organic solvent. A method for continuously converting a chemical species involves this contacting step, separating the phases, then dividing the organic phase into the product, the phase transfer catalyst, and the optional organic solvent.
Owner:VALUE RECOVERY
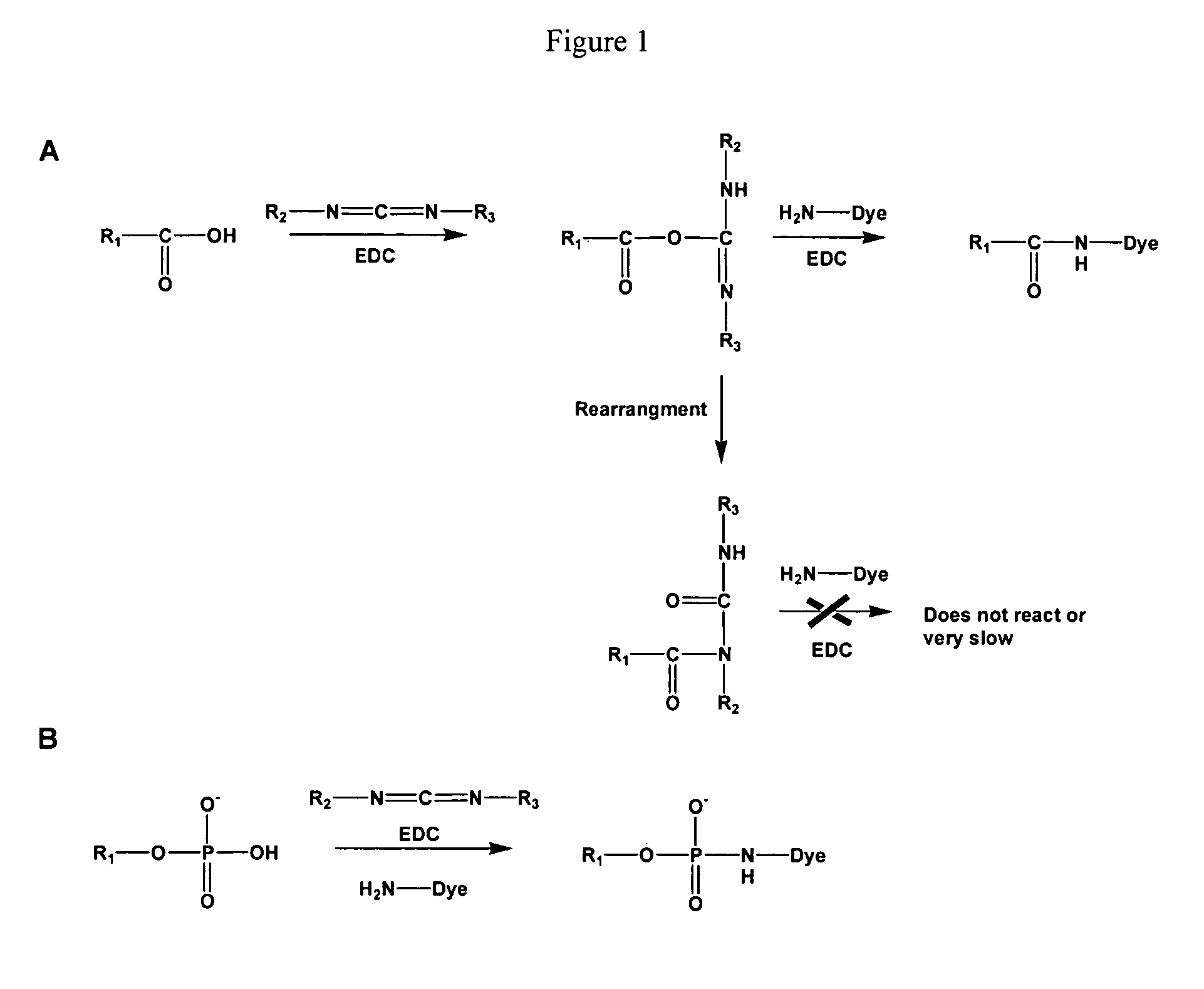
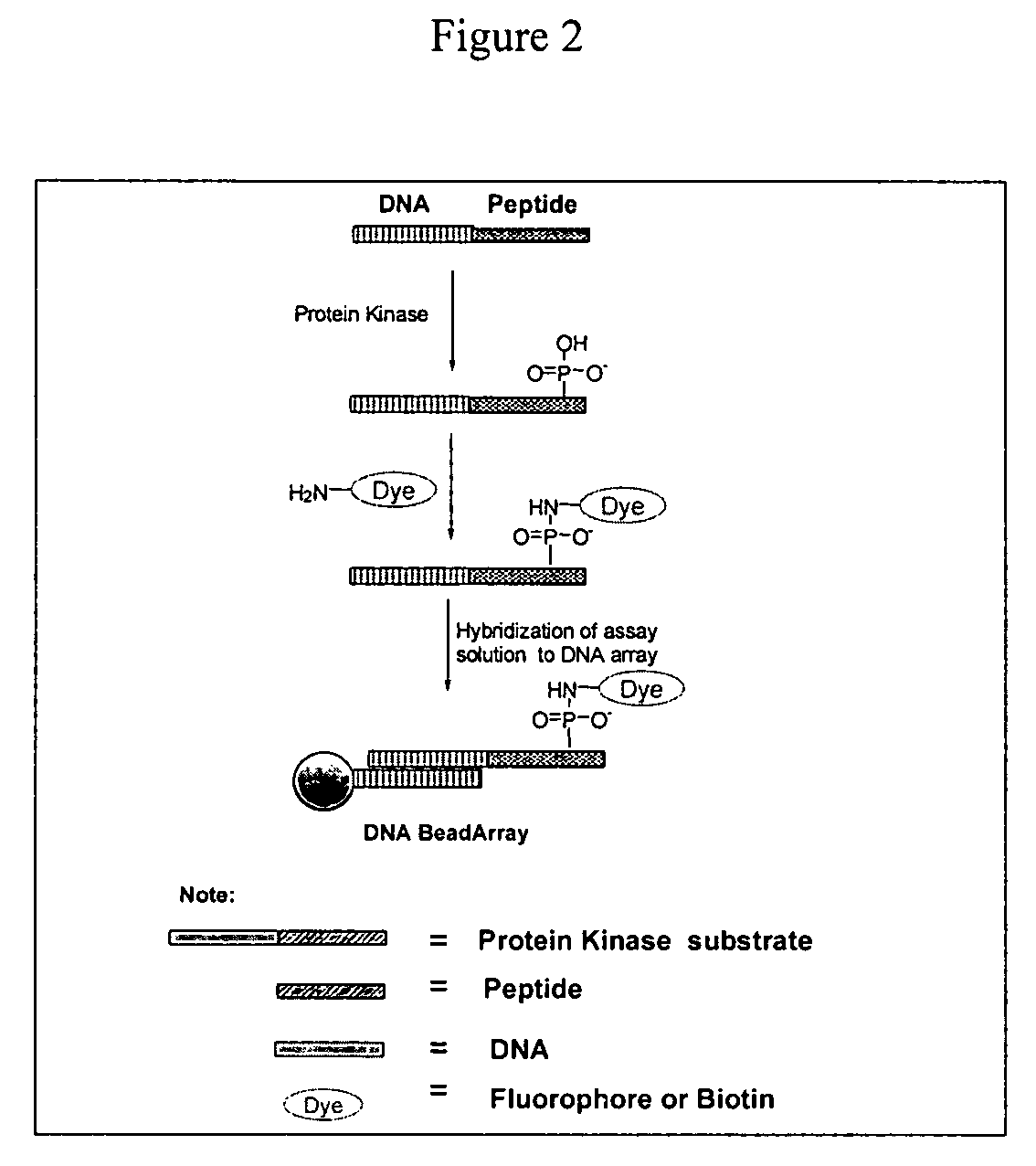
Compositions and methods for detecting phosphomonoester
The invention provides a method of modifying a phosphomonoester moiety of a target compound. The method can include the steps of (a) providing a target compound having an electrophilic moiety and a phosphomonoester moiety; (b) contacting the target compound with a first carbodiimide compound under conditions for preferential addition of the first carbodiimide compound to the electrophilic moiety over the phosphomonoester moiety, thereby forming an electrophile-protected target compound; and (c) contacting the electrophile-protected target compound with a second carbodiimide compound and a nucleophilic compound under conditions for addition of the nucleophilic compound to the phosphomonoester.
Owner:ILLUMINA INC
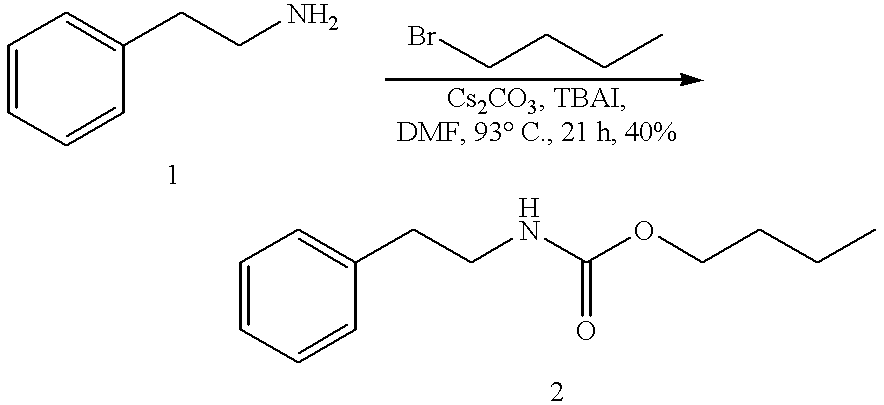
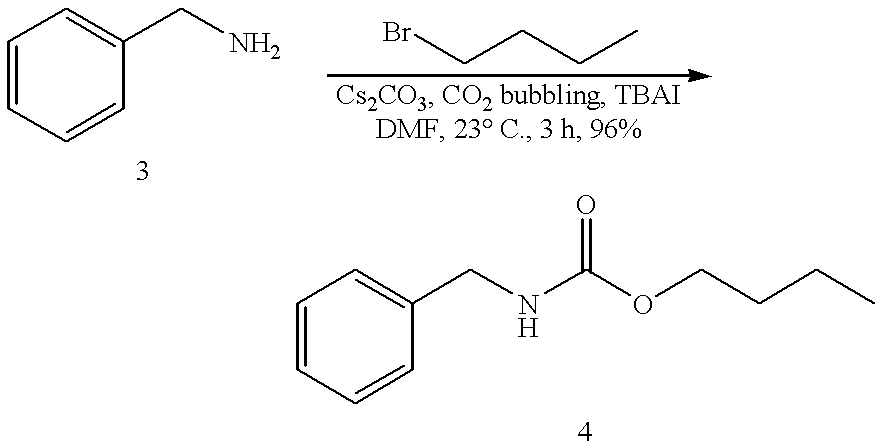
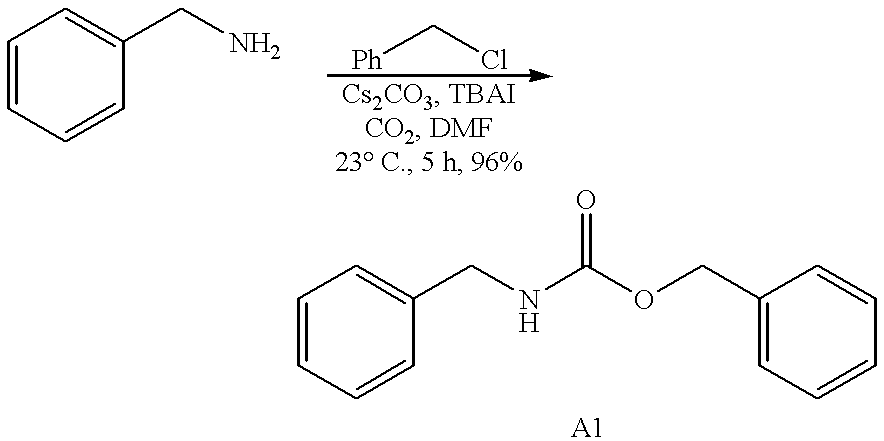
Efficient carbamate synthesis
InactiveUS6399808B1Simple and efficientPreserve the enantiomeric purity of the starting materialsGroup 4/14 element organic compoundsCarbamic acid derivatives preparationHydrogenSilylene
A method of preparing carbamates of the general formula RR'-N-CO2-R'' by reacting an amine with carbon dioxide and an organic electrophile in an anhydrous solvent in the presence of a cesium base, whereby carbamate synthesis is accomplished in good yield at mild temperatures, either in solution or on a solid support, and wherein R, R', and R'' are hydrogen, alkyl of 1-18 carbon atoms, silyl, phenyl, benzyl, cyclopentyl, cyclohexyl, cycloheptyl, cyclooctyl, allyl, or heterocycle, which are widely used as industrial products, and as intermediates in organic synthesis.
Owner:SOUTH FLORIDA UNIVESITY OF
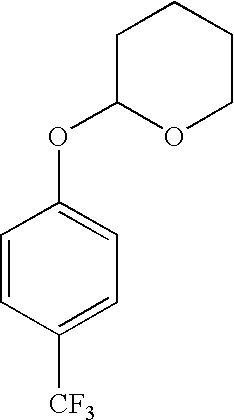
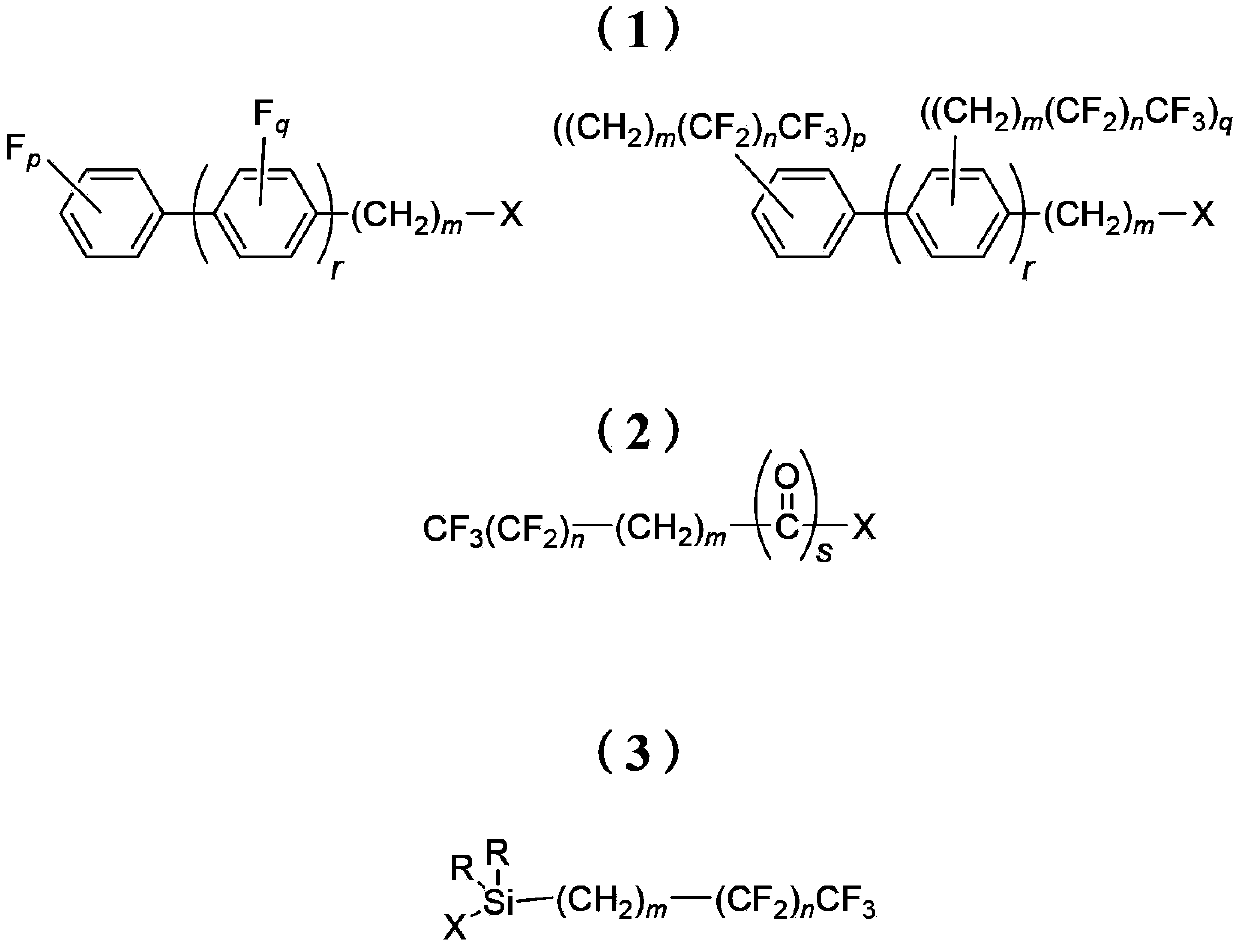
Derivatives of 4-(trifluoromethyl)-phenol and 4-(trifluoromethylphenyl)-2-(tetrahydropyranyl) ether and method for producing the same
InactiveUS20040092754A1High yieldLower requirementSilicon organic compoundsOrganic compound preparationEtherElectrophile
The invention relates to a method for producing 2- and 2,5-substituted derivatives of 4-(trifluoromethyl)-phenol and 4-(2-trifluoromethyl)-phenyl)-2-tetrahydropyranyl) ether and to novel derivatives. Said method is characterised in that a compound of formula (2) 4-(trifluoromethylphenyl)-2-(tetrahydropyranyl) ether is reacted with an electrophile E-X or a combination of electrophiles E-X and E-Y in the presence of a base, X and Y having the meanings given in the description.
Owner:BASF AG
Method for preparing reinforced fluoropolymer composites comprising surface functionalized nanocrystalline cellulose
A method of preparing a reinforced fluoropolymer composite is presented, which includes reacting a surface of a nanocrystalline cellulose with a fluorinated electrophile to form a fluoro-functionalized nanocrystalline in which the outer circumference of the nanocrystalline cellulose has been functionalized with fluorinated substrates, and contacting the fluoro-functionalized nanocrystalline cellulose with a fluoropolymer to form a fluoropolymer composite. Also presented is a method of preparing fluoro-functionalized nanocrystalline cellulose, including reacting a surface of a nanocrystalline cellulose with a fluorinated electrophile forming fluoro-functionalized nanocrystalline cellulose in which the outer circumference of the nanocrystalline cellulose has been functionalized with fluorinated substrates, precipitating the fluoro-functionalized nanocrystalline cellulose and isolating and purifying the fluoro-functionalized nanocrystalline cellulose.
Owner:XEROX CORP
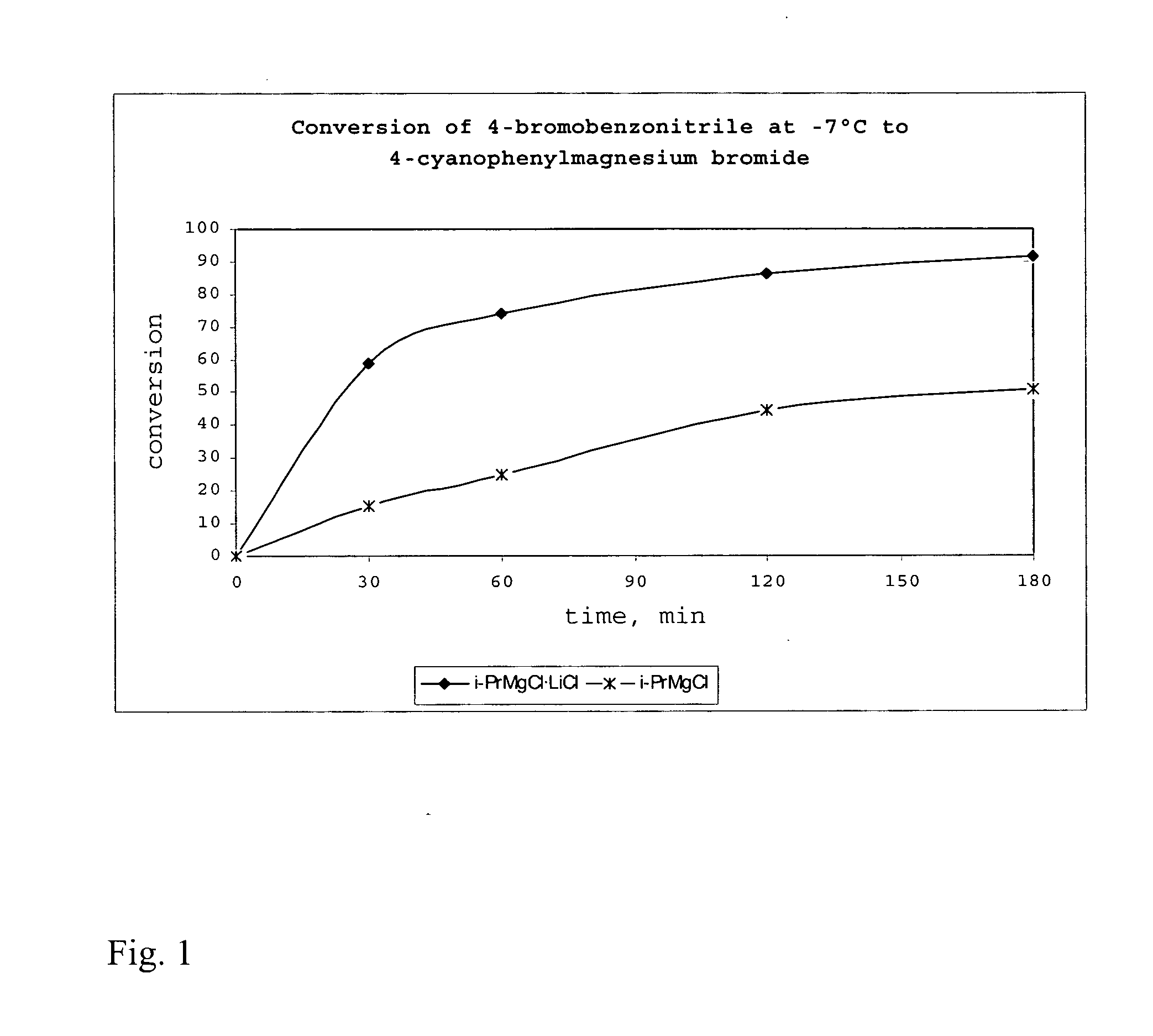
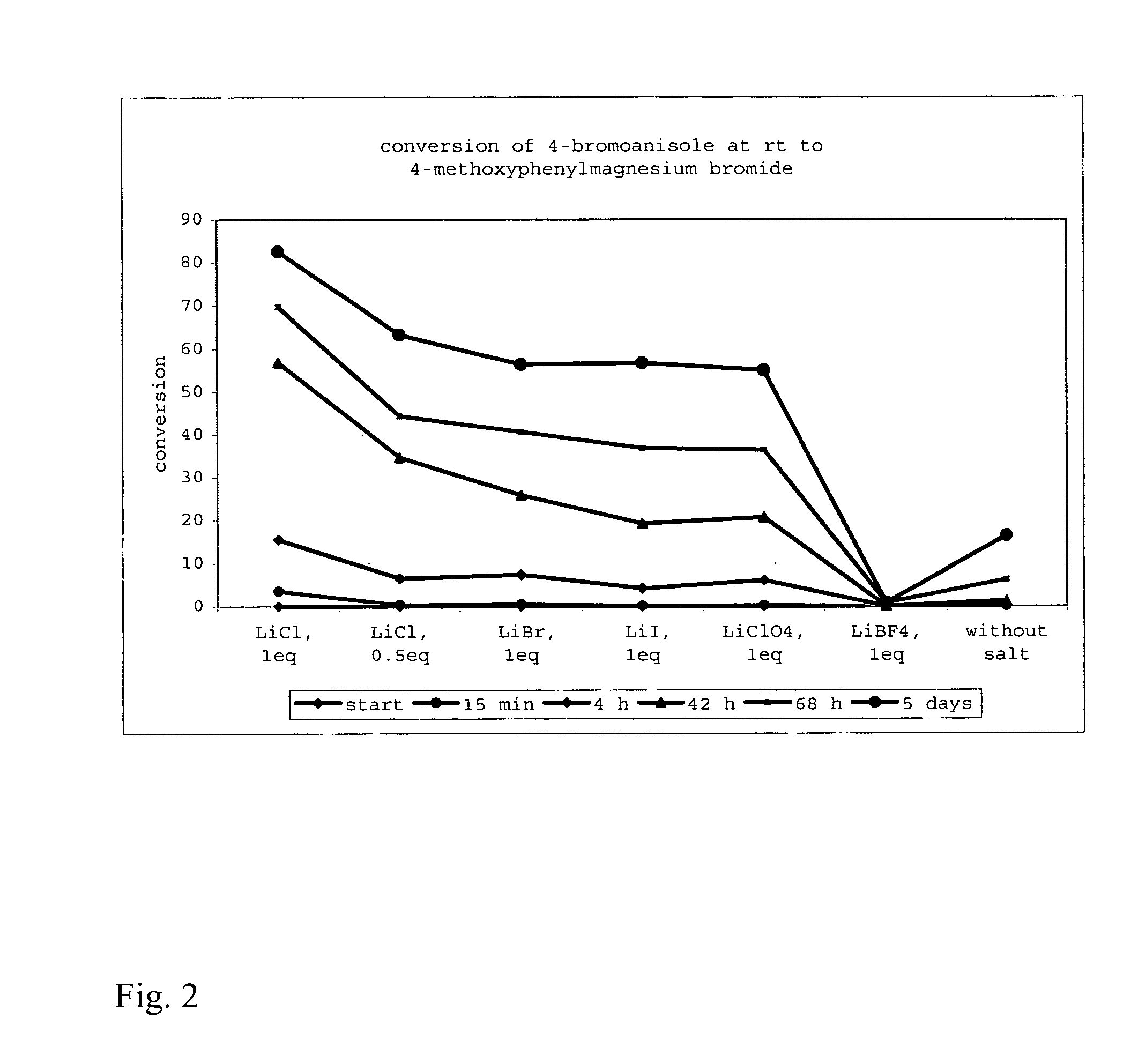
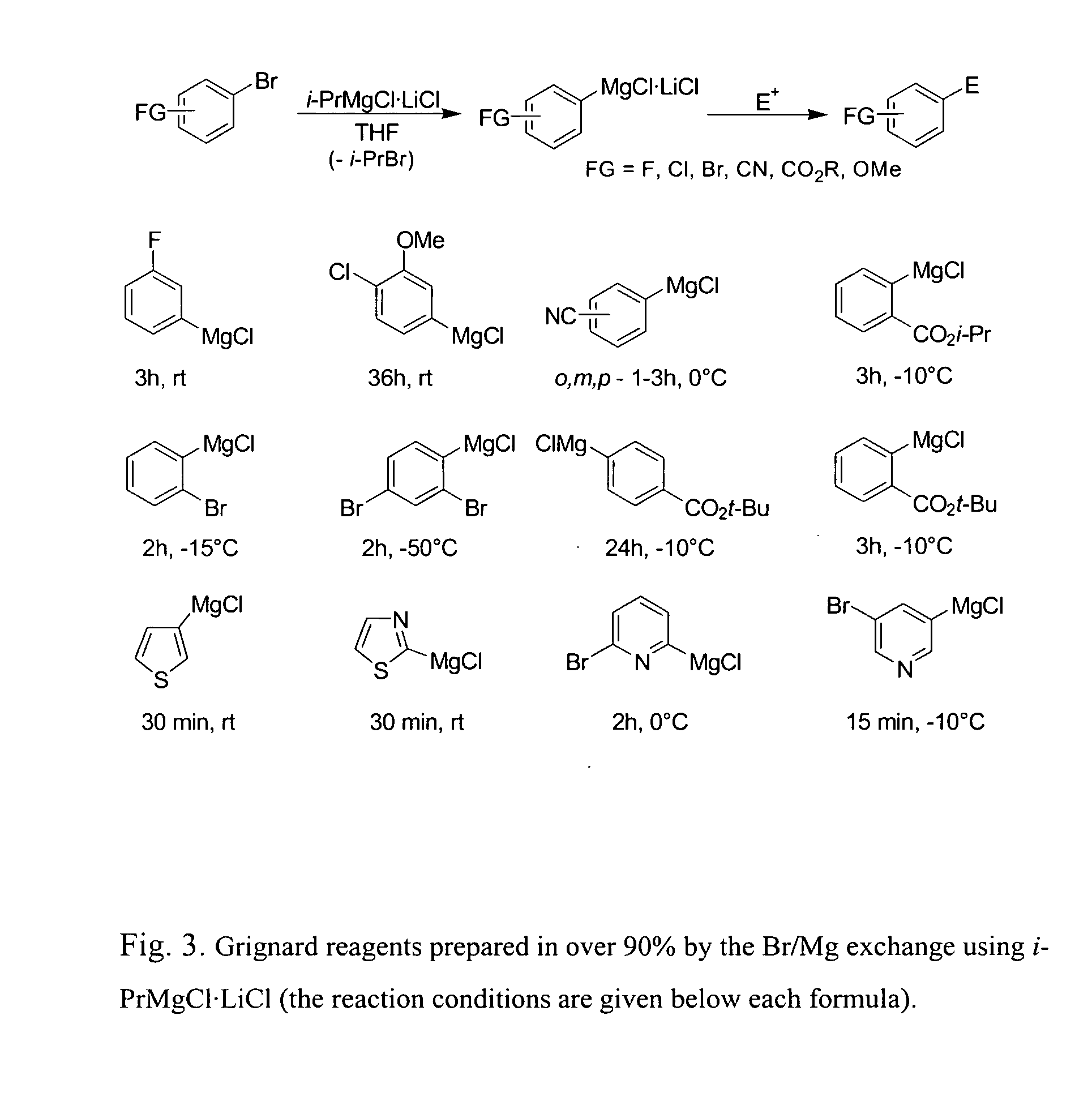
Method of preparing organomagnesium compounds
ActiveUS20050218532A1High yieldAllows preparationCarboxylic acid nitrile preparationGroup 5/15 element organic compoundsSimple Organic CompoundsLithium
The present invention is directed to a reagent for use in the preparation of organomagnesium compounds as well as to a method of preparing such organomagnesium compounds. The present invention furthermore provides a method of preparing functionalized or unfunctionalized organic compounds as well as the use of the reagents of the present invention in the preparation of organometallic compounds and their reaction with electrophiles. Finally, the present invention is directed to the use of lithium salts—LiY in the preparation of organometallic compounds and their reactions with electrophiles and to an organometallic compound which is obtainable by the disclosed method.
Owner:LUDWIG MAXIMILIANS UNIV MUNCHEN
Electrolyte composition with a molten salt and crosslinked polymer
InactiveUS6841299B2Maintain good propertiesPreventing leakage and depletionSolid electrolytesNon-aqueous electrolyte accumulatorsPhysical chemistryElectrolyte composition
An electrolyte composition excellent in charge-transporting property that can be prepared with ease, and a non-aqueous electrolyte secondary cell that comprises the electrolyte composition to exhibit excellent cell characteristics while preventing leakage or depletion of the electrolyte composition. The electrolyte composition comprises: a particular molten salt; a polymer prepared by a reaction between an electrophile having at least two unsaturated bonds polarized by an electron-withdrawing group and a nucleophile having a plurality of nucleophilic groups; and a metal salt containing a Group IA metal ion or a Group IIA metal ion.
Owner:FUJIFILM CORP +1
Topical use of plant extract for skin detoxification
InactiveUS20090306219A1Maintain cellular redox homeostasisReduce hydrogen peroxideBiocideSulfur/selenium/tellurium active ingredientsPeroxideCollard Green
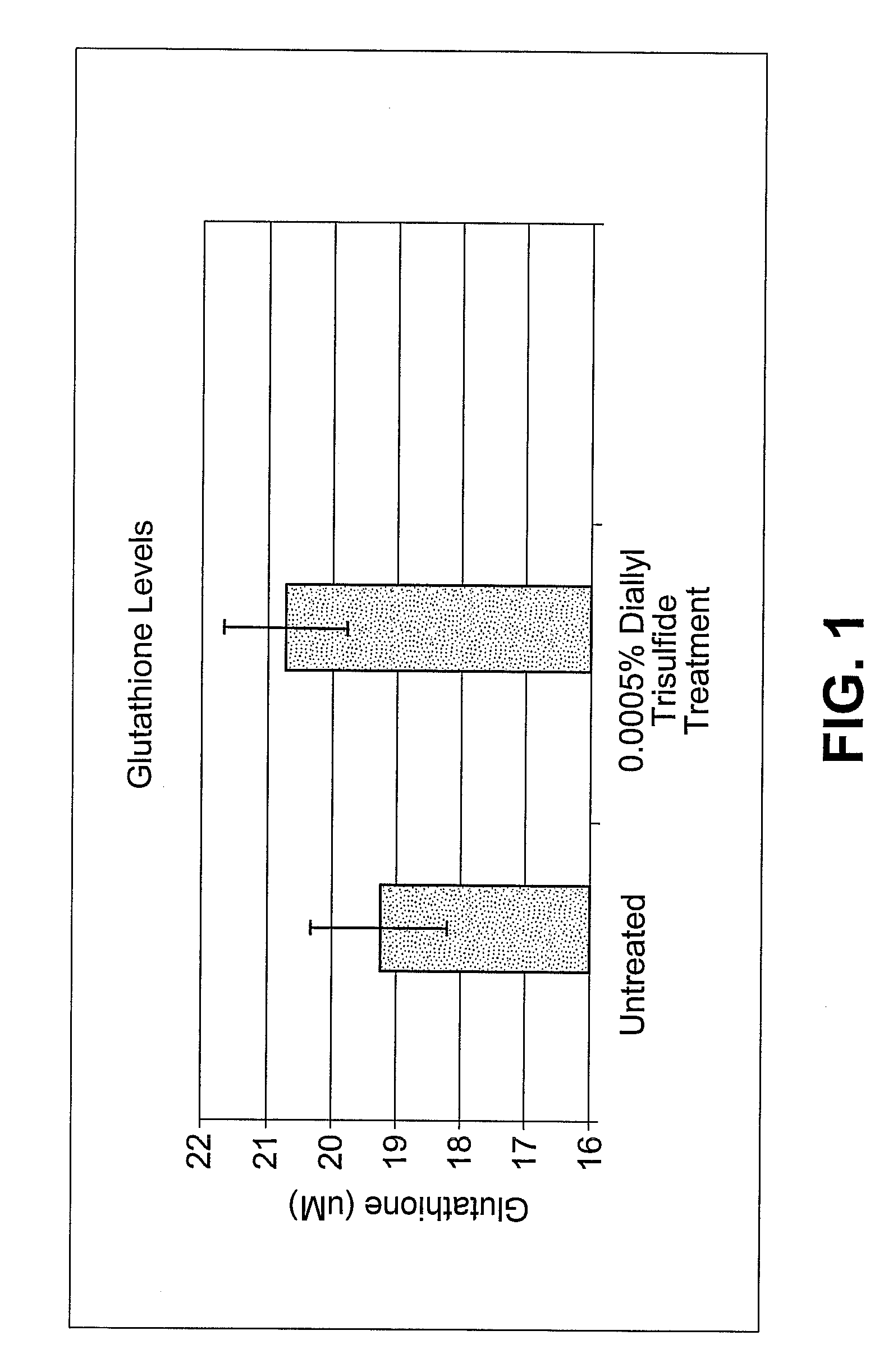
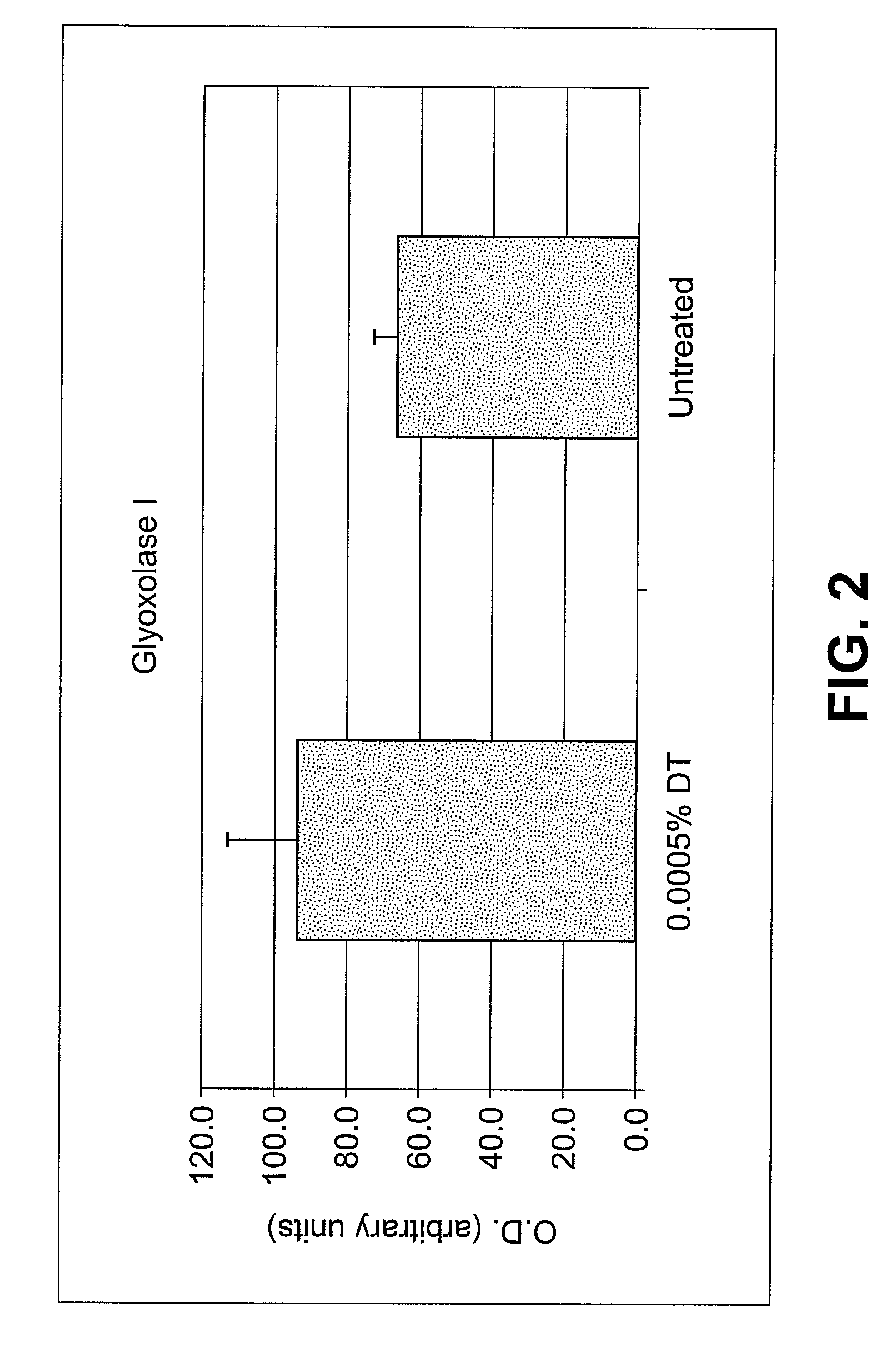
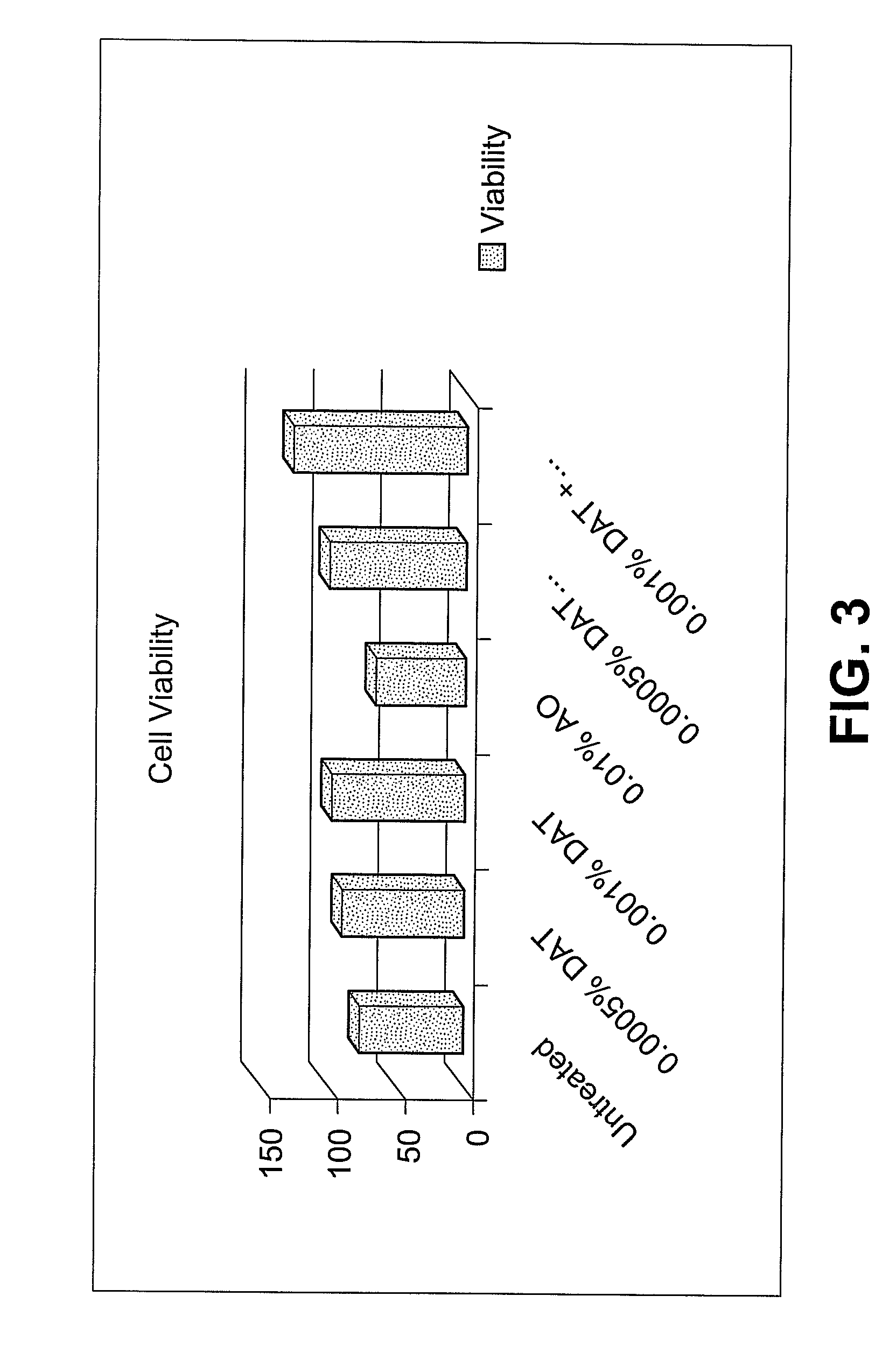
Specific plant extracts are applied topically to upregulate genes which code for proteins to prevent the build-up of and mitigate the activity of various harmful materials within the skin. These upregulated proteins include glutathione transferases, peroxiredoxins, oxidoreductases, glutaredoxins and glutathione syntheses. These proteins produce additional glutathione, glyoxalase I, maintain cellular redox homeostasis, reduce hydrogen peroxide and alkylhydroperoxides, and conjugate glutathione to a wide number of exogenous and endogenous hydrophobic electrophiles. Keratinocytes have been induced to significantly upregulate glutathione and glyoxalase I protein expression. Additionally, keratinocytes pre-treated with diallyl trisulfide have increased viability when exposed to ozone, UV radiation or cigarette smoke extract. The active material can be extracted from plants such as garlic, onions, shallots, leeks, chives, scallions, brussel sprouts, broccoli, cabbage, cauliflower, bok choy, kale, mustard, turnip or radish, may include any compound of the allyl sulfide family, and may be of synthetic origin.
Owner:BEILIS DEV
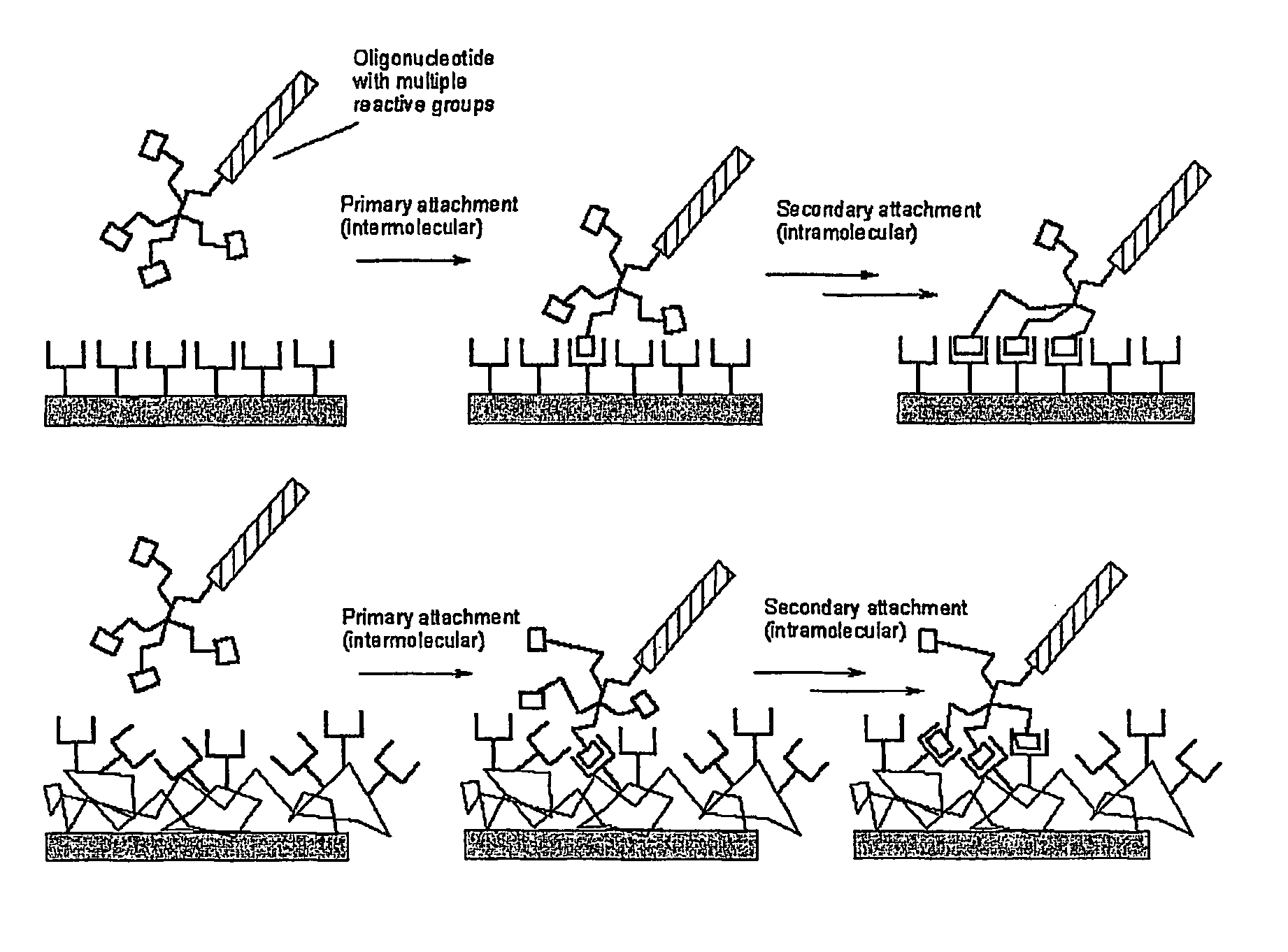
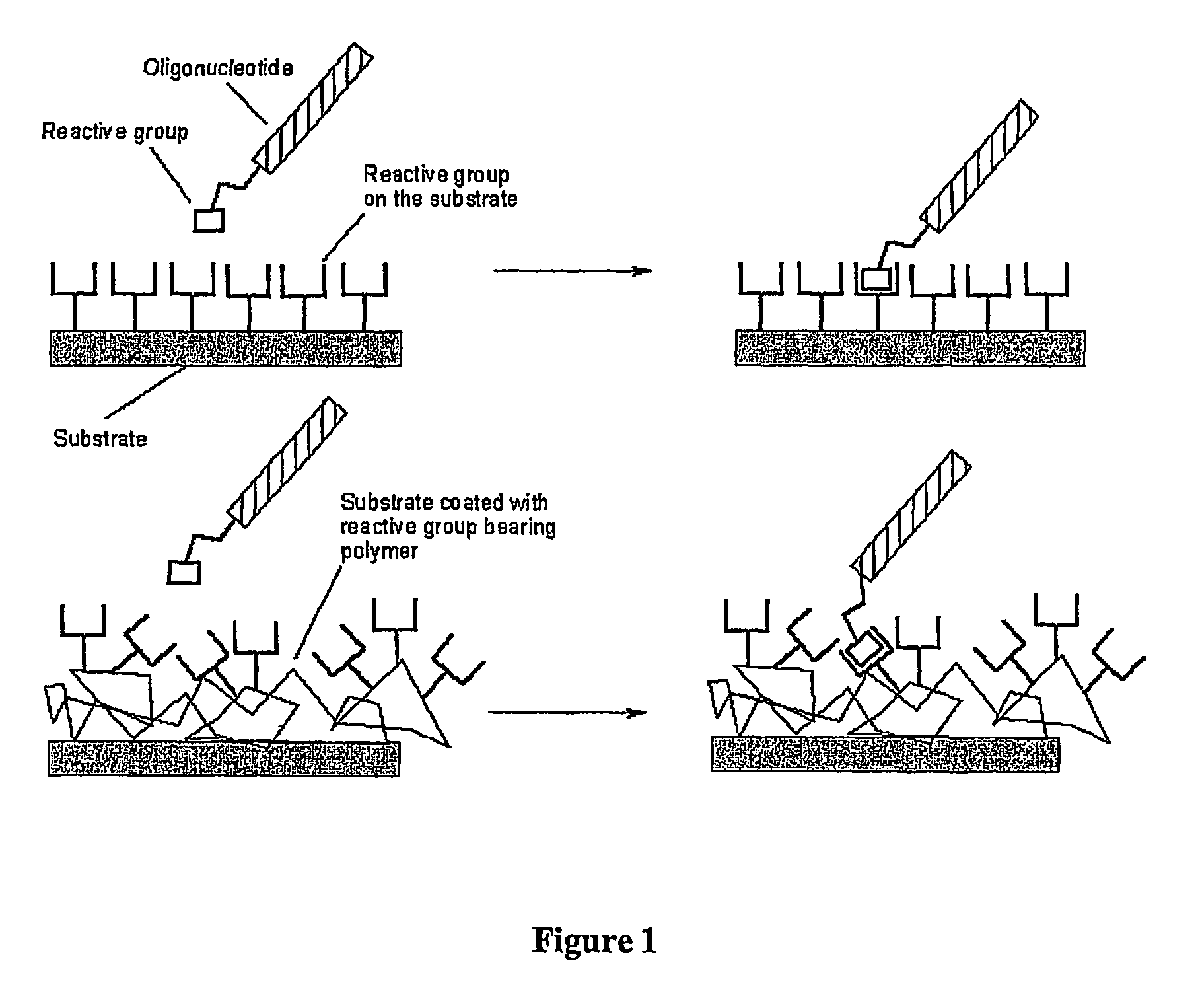
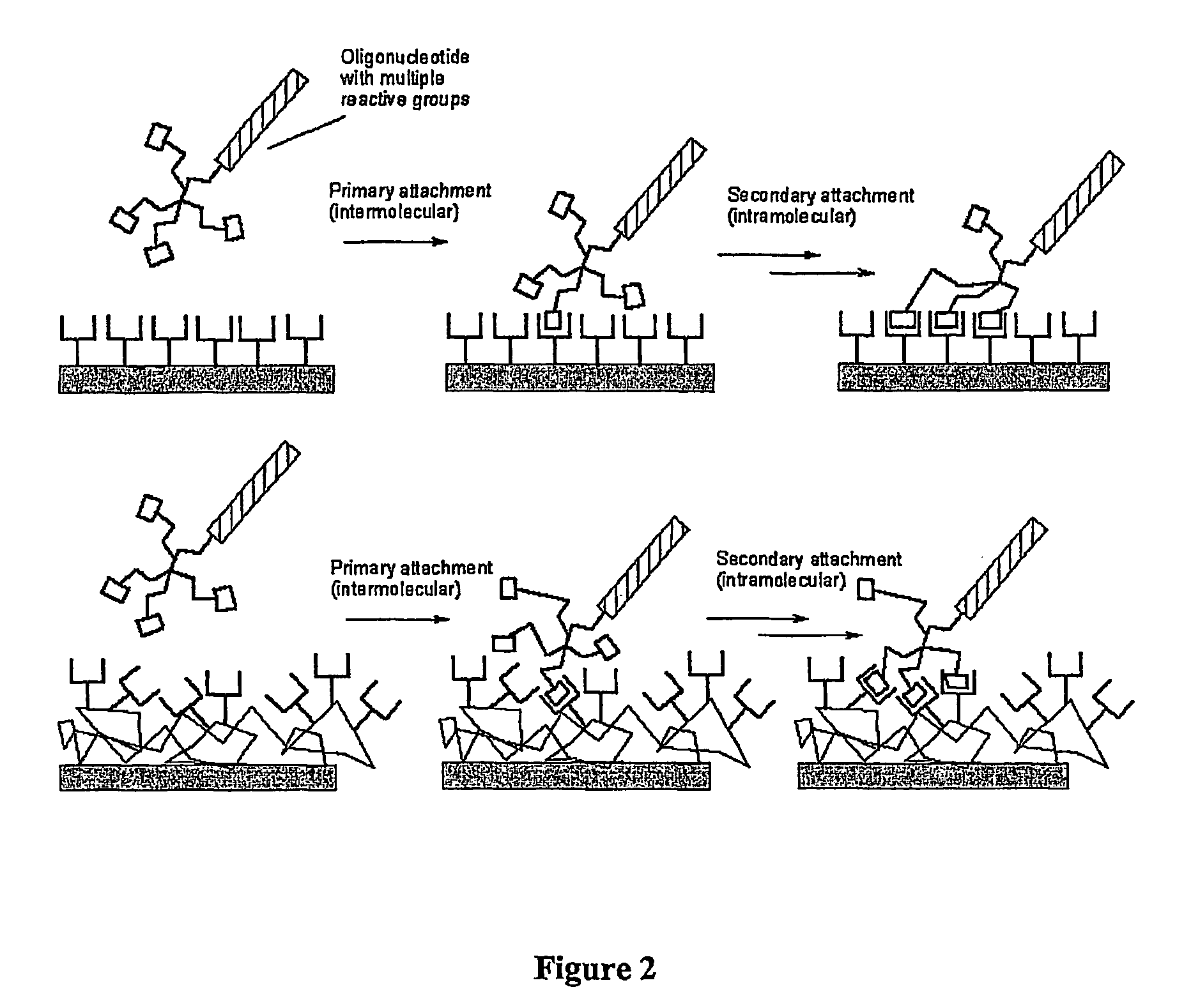
Biomolecules having multiple attachment moieties for binding to a substrate surface
InactiveUS7833715B1Increase speedHigh immobilization rateSugar derivativesMicrobiological testing/measurementElectrophileSubstrate surface
Methods of binding biomolecules to a substrate are provided that include contacting the biomolecule with a branched linking moiety to form a branched linking structure. The branched linking structure is then contacted with a binding moiety on the substrate to form a coupled substrate binding structure, thereby binding the biomolecule to the substrate. The biomolecule may contain a Lewis base or a nucleophile to react with a Lewis acid or electrophile in the branched linking moiety. Alternatively, the biomolecule may contain a Lewis acid or electrophile that can react with a Lewis base or nucleophile in the branched linking moiety. Additionally, the biomolecule can be bound to the substrate through a covalent or non-covalent bond.
Owner:SANOFI AVENTIS SA
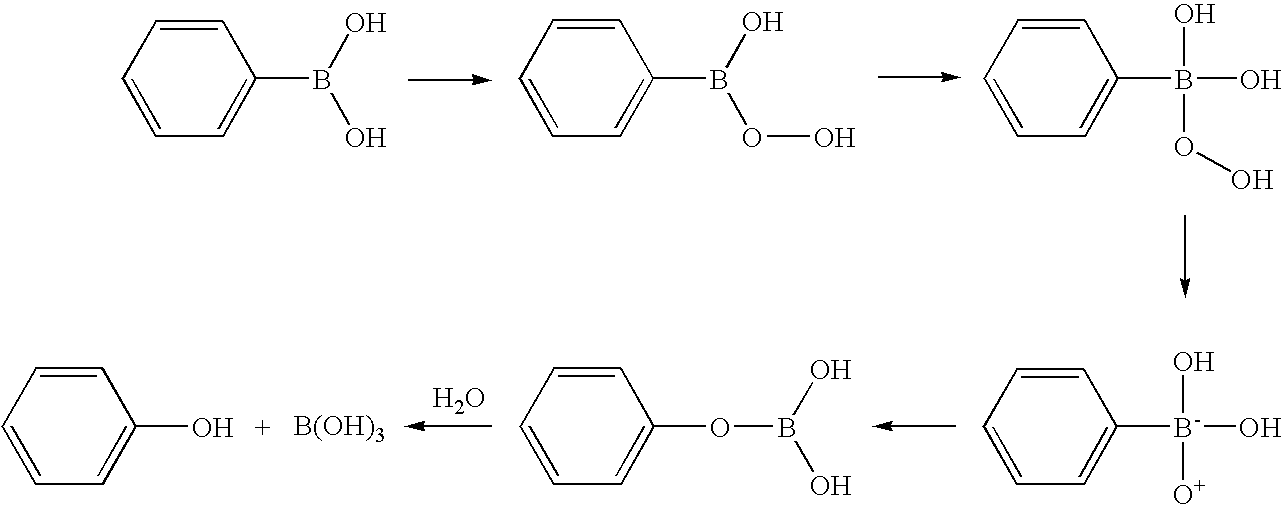
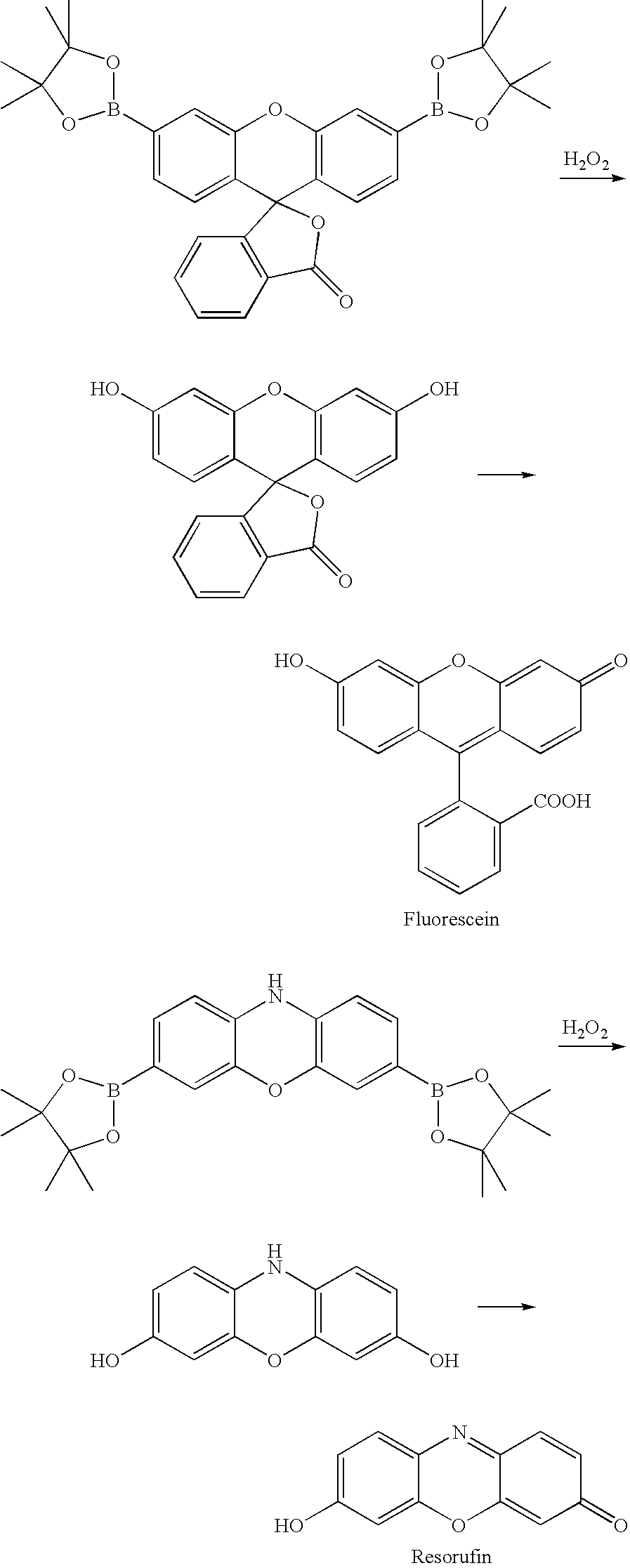
Compositions for oxidatively dyeing keratin fibers and methods for using such compositions
Compositions for dyeing keratin fibers comprise (a) at least one keratin dyeing compound selected from aromatic systems which comprise at least one boronic acid or boronic ester moiety and which are capable of forming upon oxidation a nucleophile or an electrophile, (b) at least one additional keratin dyeing compound selected from the group consisting of auxiliary developers and auxiliary couplers, and (c) a cosmetically suitable medium. Methods for oxidatively dyeing keratin fibers comprise the steps of applying such compositions in the presence of an oxidizing agent and rinsing the hair. A hair coloring product in kit form comprises a first separately packaged container comprising a composition as described above and a second separately packaged container comprising an oxidizing agent.
Owner:PROCTER & GAMBLE CO
Method for identifying electrophiles and nucleophiles in a sample
InactiveUS20090258430A1Analysis using chemical indicatorsMaterial thermal conductivityChemical reactionElectrophile
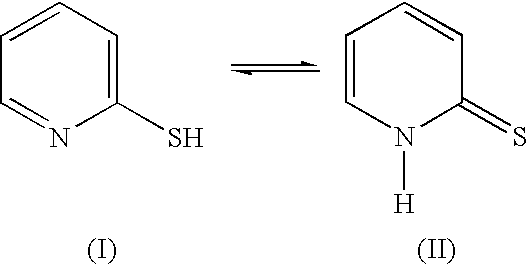
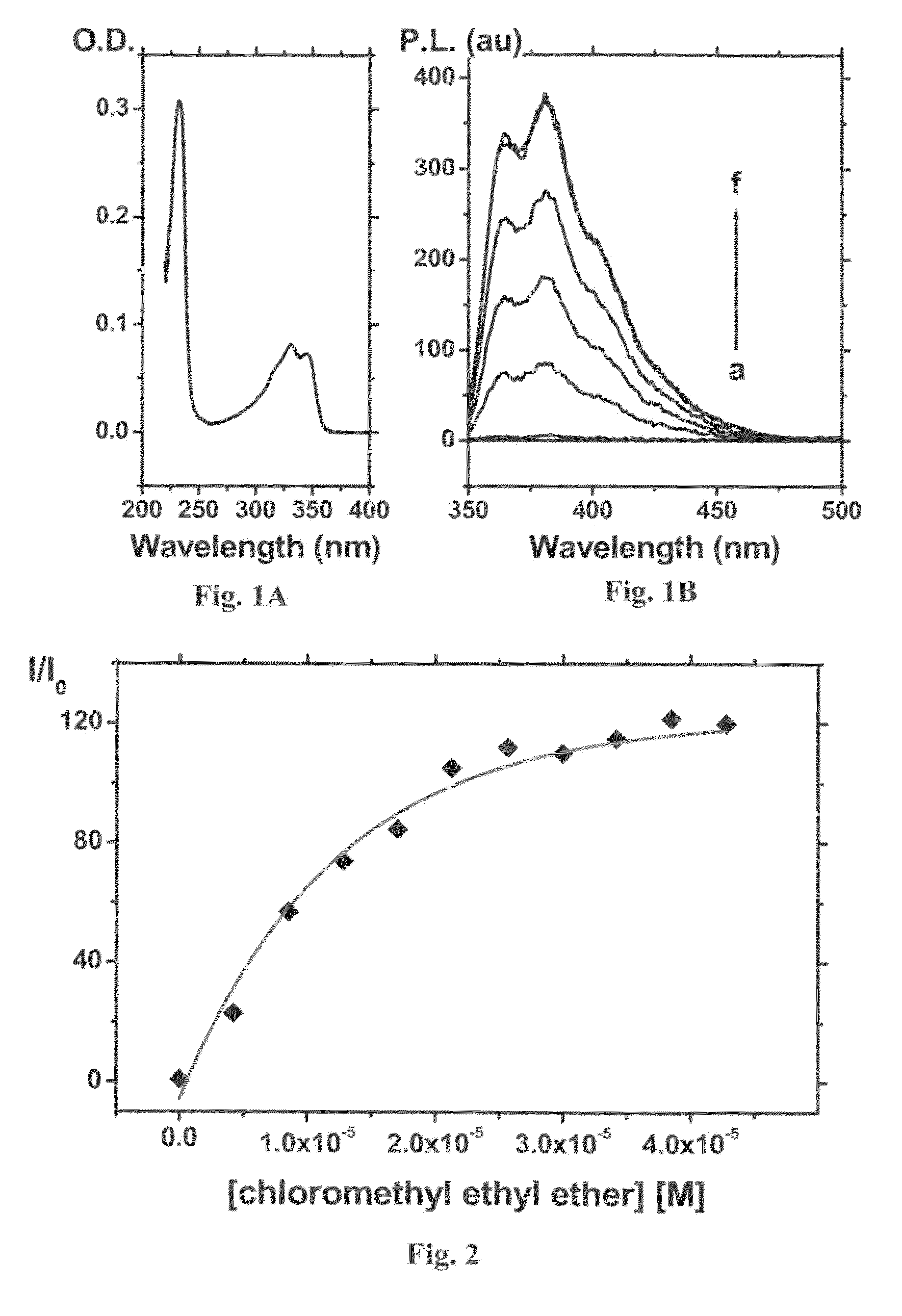
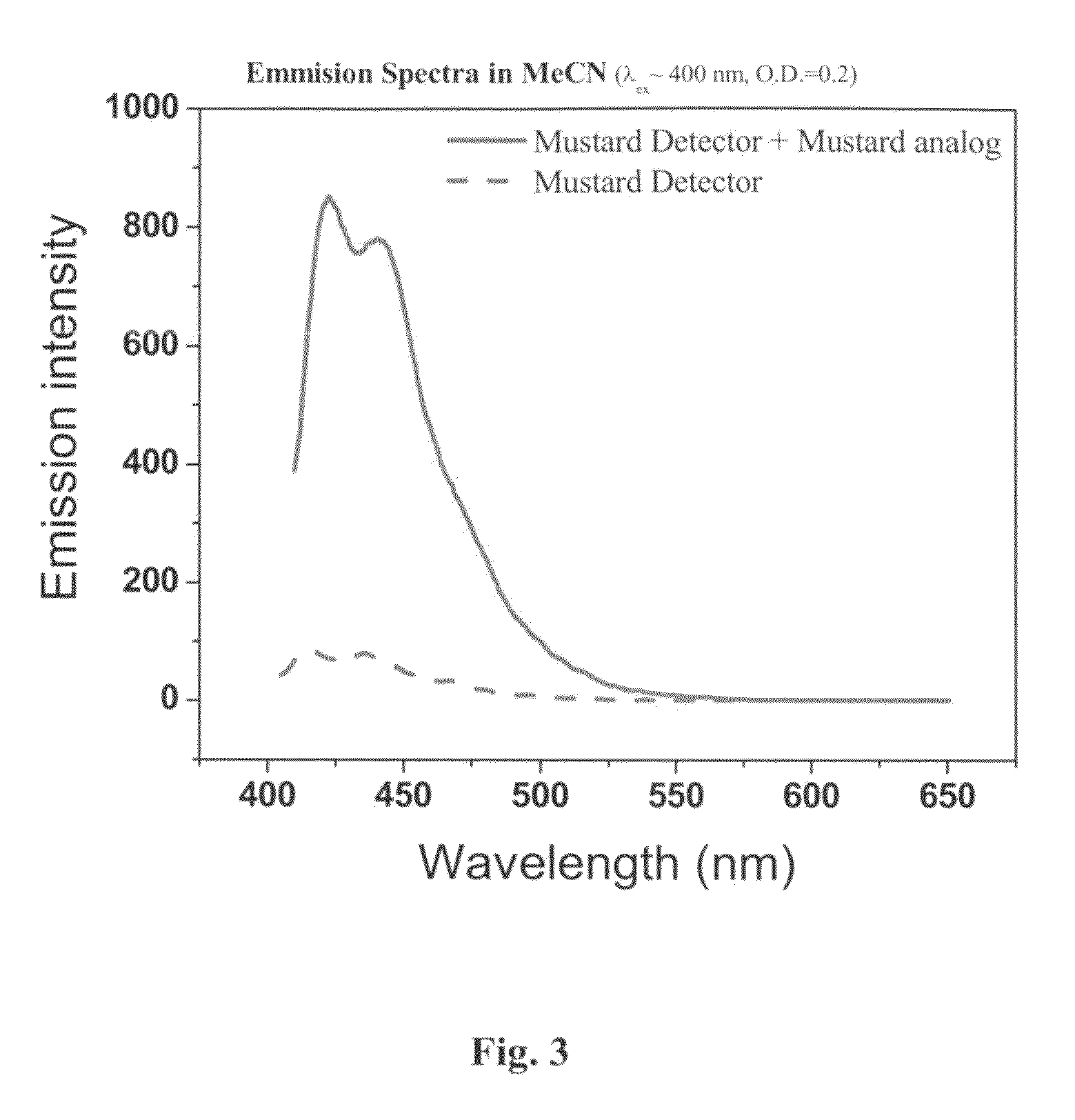
A method and device for identifying a molecule in a sample, the molecule comprising an electrophilic or nucleophilic moiety. The method comprises contacting the sample with a plurality of chemosensors, each of the chemosensors comprising a π-conjugated system and a moiety having a nucleophilic property or an electrophilic property; and measuring an electromagnetic property of each of the chemosensors in the sample; whereby the pattern of changes in the electromagnetic properties of the plurality of chemosensors after chemically reacting with the electrophile or nucleophile of the molecule identifies the molecule in said sample. The device comprises a substrate carrying a plurality of chemosensor molecules having at least one predetermined electromagnetic property, the at least one electromagnetic property being changeable by subjecting the chemosensor molecules to a sample containing at least one electrophile or nuclephile, wherein the pattern of change of the electromagnetic property of the plurality of chemosensor molecules allows the device to identify the electrophile or nuclephile in the sample.
Owner:TECHNION RES & DEV FOUND LTD
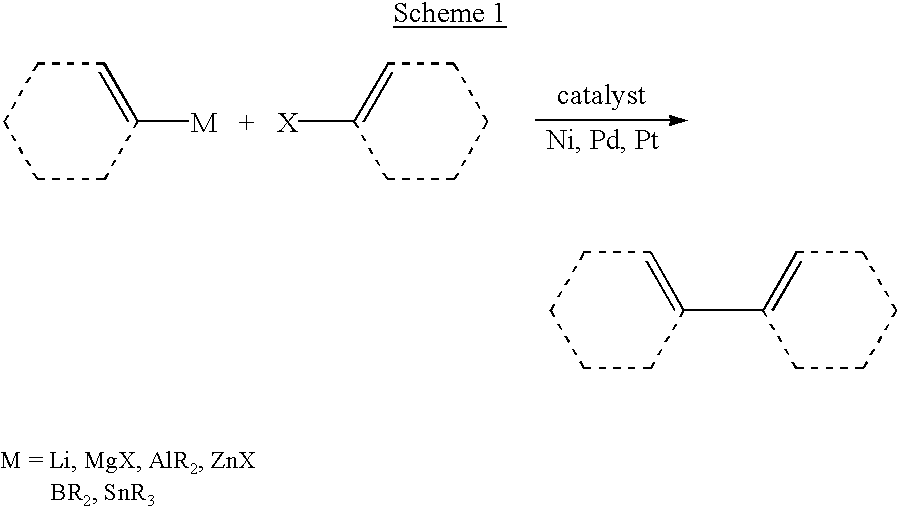
Cross-coupling reaction of organosilicon nucleophiles
InactiveUS6867323B2Easy to prepareImprove production yieldSilicon organic compoundsOxygen-containing compound preparationRing-closing metathesisHydrosilylation
Improved methods for generating a —C—C— bond by cross-coupling of a transferable group with an acceptor group. The transferable group is a substituent of an organosilicon nucleophile and the acceptor group is provided as an organic electrophile. The reaction is catalyzed by a Group 10 transition metal complex (e.g., Ni, Pt or Pd), particularly by a palladium complex. Certain methods of this invention use improved organosilicon nucleophiles which are readily prepared, can give high product yields and exhibit high stereoselectivity. Methods of this invention employ activating ions such as halides, hydroxide, hydride and silyloxides. In specific embodiments, organosilicon nucleophilic reagents of this invention include siloxanes, particularly cyclic siloxanes. The combination of the cross-coupling reactions of this invention with ring-closing metathesis, hydrosilylation and intramolecular hydrosilylation reactions provide useful synthetic strategies that have wide application.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Universal linker compositions for the release or transfer of chemical agents from a polynucleotide
A universal linker structure is provided, in which a functional group and activating leaving group are placed on a tether, allowing the placement of an electrophile at the end of any nucleic acid sequence. The electrophile on the tether can react with a second nucleic acid carrying a nucleophile when the two nucleic acids are hybridized near one another, resulting in release of the leaving group, and creation of a functional change. The linker can be designed to destabilize the ligation product without slowing the rate of reaction. This lowers product inhibition, and the target DNA or RNA can become a catalyst for isothermally generating multiple signals for detection. This enhanced signal is demonstrated in solution experiments and in solid supported assays. The universal linkers of the present invention are simple and inexpensive to prepare, and can be appended to any polynucleotide in automated steps on a standard DNA synthesizer.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Composition containing n-(n-butyl) thiophosphoric triamide adducts and reaction products
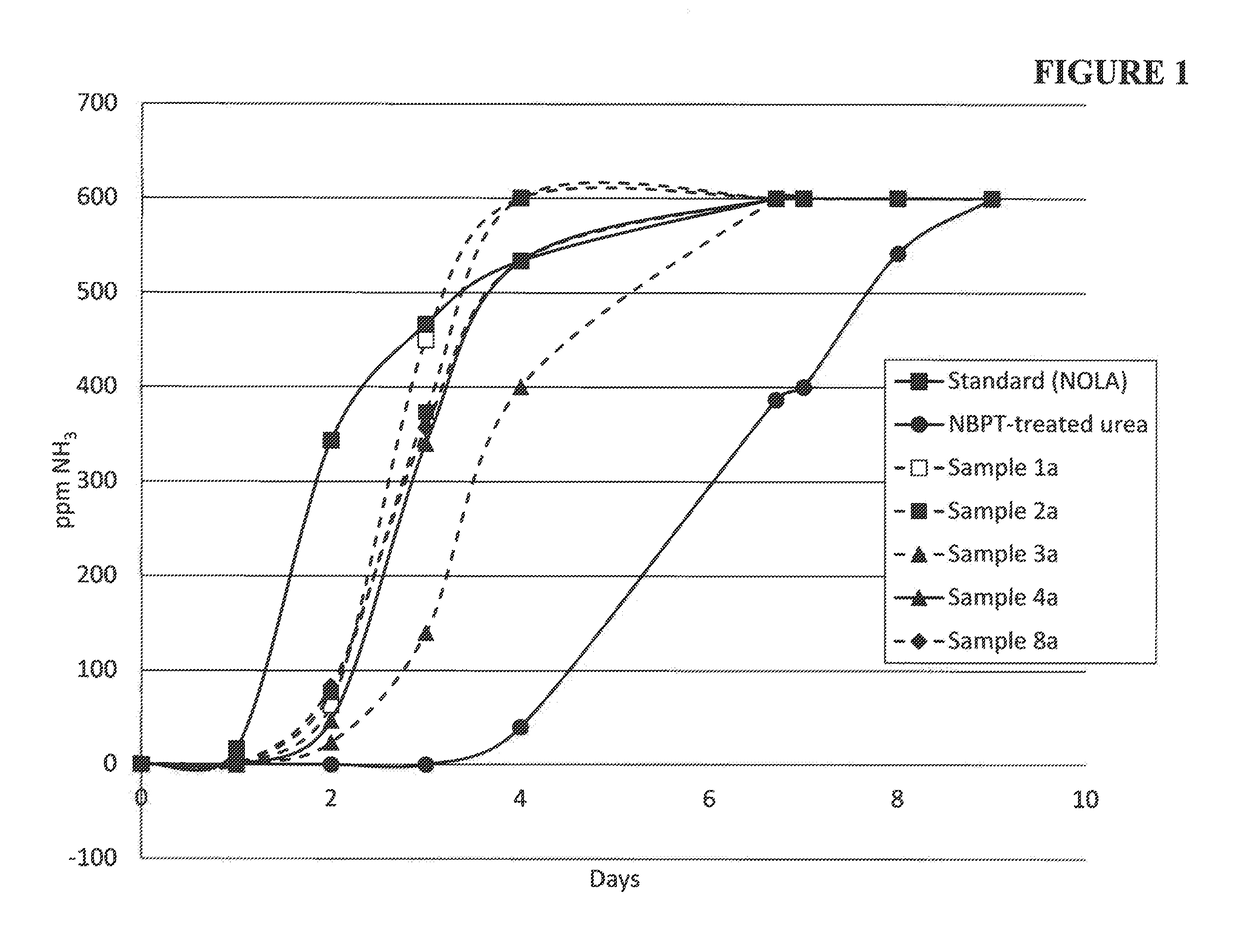
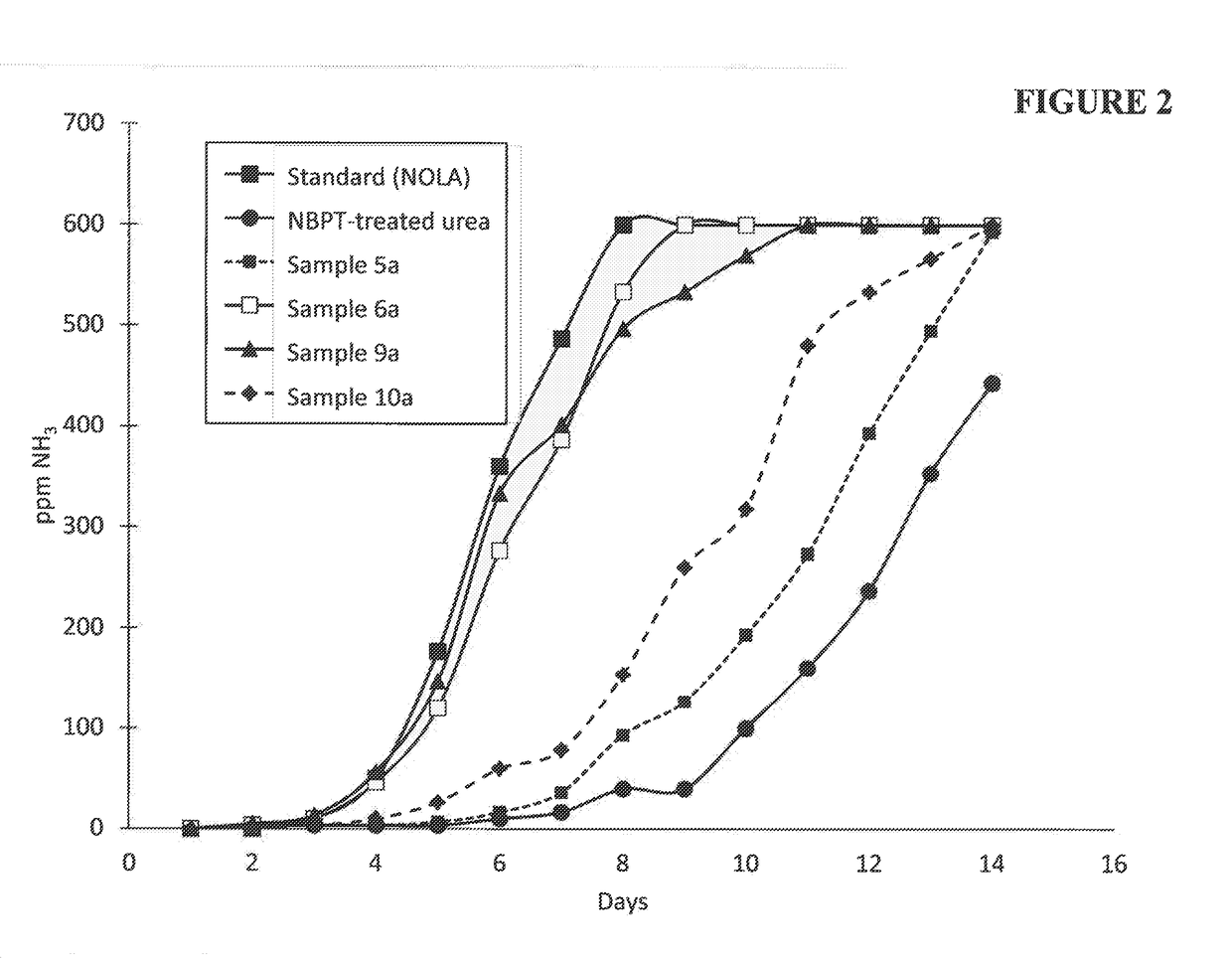
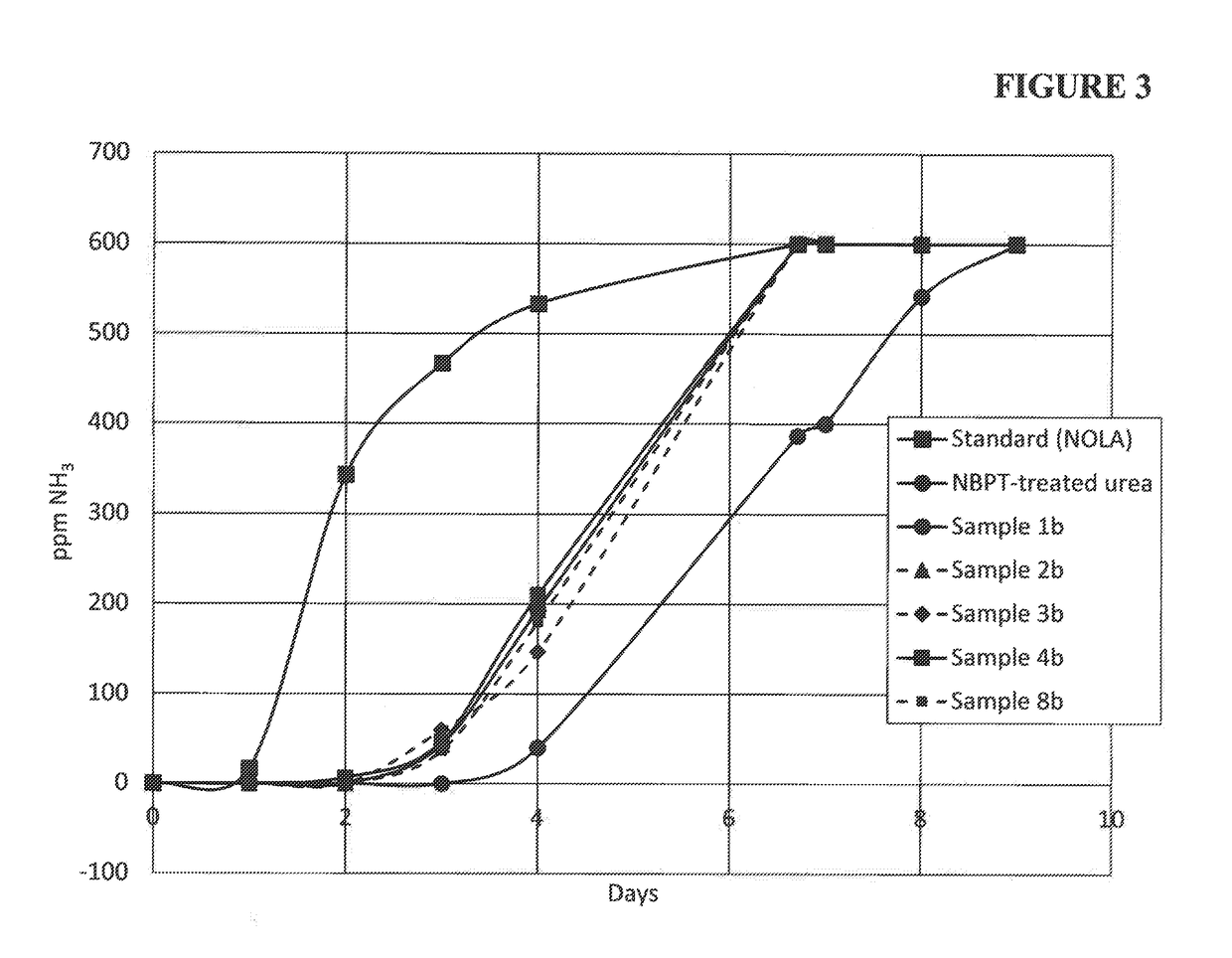
ActiveUS20180208520A1Substantial urease inhibitory effectLow volatilization lossGroup 5/15 element organic compoundsAgriculture gas emission reductionNitrogenUrease Inhibitors
Reaction products and methods for making and using such reaction products are provided. For example, a reaction product comprising an adduct formed from two or more of a first nucleophile (e.g., including, but not limited to, a urease inhibitor), a second nucleophile, and an electrophile is described, which can be provided in various forms. Such a reaction product can be in the form of a solid or solution. Such a reaction product can also be combined with one or more additional components, including but not limited to, free urease inhibitor and / or a nitrogen-based fertilizer composition.
Owner:KOCH AGRONOMIC SERVICES LLC
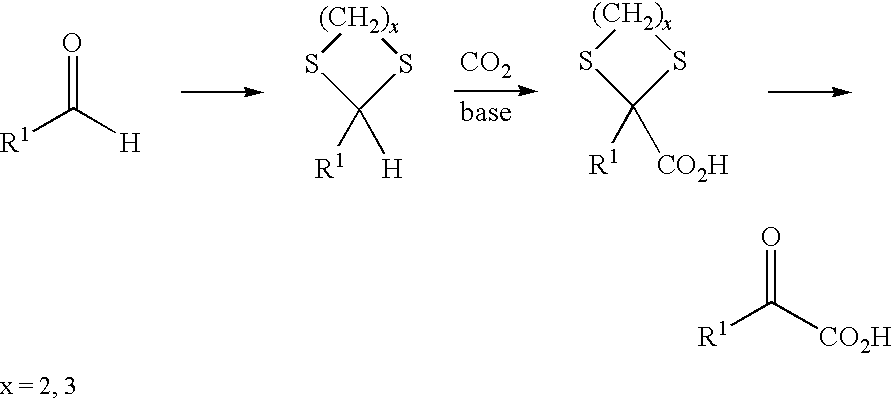
Process for preparing α-keto acids and derivatives thereof
A method for preparing α-keto acids, especially α-ketomethionine, and / or derivatives thereof, whereby an aldehyde is reacted with thiols to give a corresponding dithioacetal, the dithioacetal formed, is reacted with an electrophile in the presence of a strong base, and the resulting α,α-(dithio)carboxylic acid is solvolyzed with acid-catalysis to release thiol and give the α-keto acid or a derivative thereof. Umpolung of aliphatic or aromatic aldehydes is effected by reaction with thiols.
Owner:EVONIK DEGUSSA GMBH
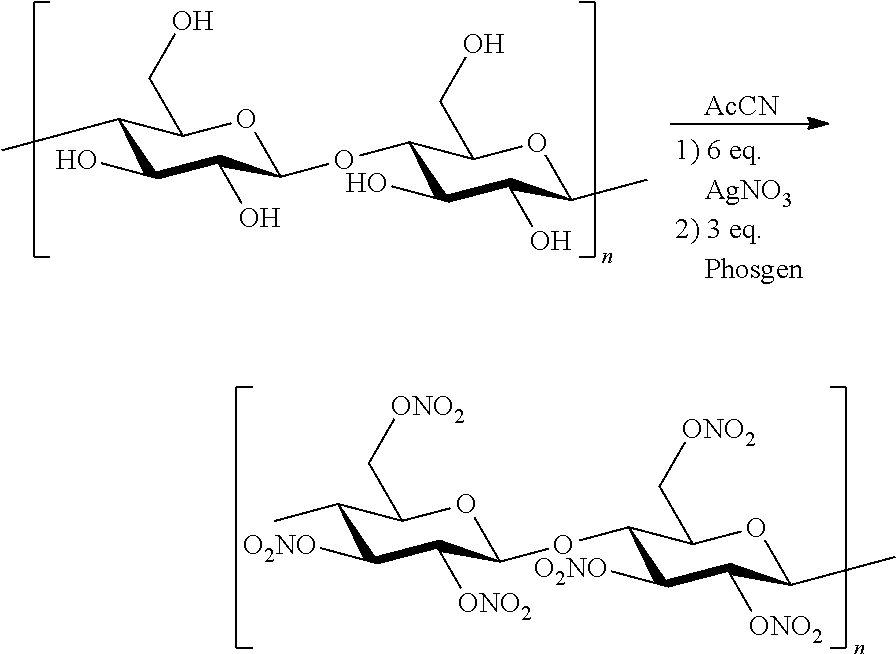
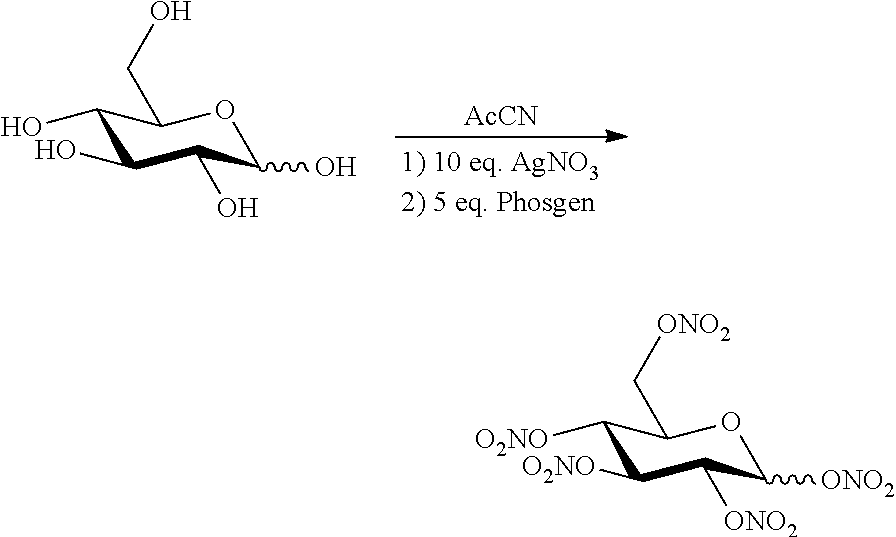
Novel Method for Directly Nitration of OH-, SH-and NHR-Functions in Organic Molecules by Means of in Situ Generated Carbonic Acid Dinitrate
The invention relates to a nitration method having the following principles: a phosgene species is converted with two equivalent silver nitrates into a double-mixed anhydride of carbonic acid and nitric acid, known here as carbonic acid dinitrate (I). Said operation is carried out in situ, and the formed dinitrate decomposes spontaneously. In addition to carbon dioxide, nitrate ions and nitronium ions are formed, said ions comprising electrophiles which are necessary for nitration. The solution which is used is acetonitrile, and is insignificant if the alcohol species is dissolved or suspended. The necessary equivalent silver nitrates are introduced into the system and optionally heated or cooled to the desired temperature. Subsequently, the acid chloride is introduced slowly, drop by drop or slowly little by little. Phosgene, diphosgene, triphosgene and chloroformic acid ester can be used as carbonic acid dichloride and monochloride, and their thiocarbonic acid analogues. A brown colouration and precipitated silver chloride display the formation of the carbonic acid reactants, said brown colouration rapidly discolouring due to an immediate reaction of the nitronium ions with the substrate with is to be nitrated. Towards the end of the addition of phosgene, the brown colouration remains for longer and longer until it no longer disappears. Then, it is stirred for another hour at room temperature. In the event of high acid-sensitive educts, non-nucleophilic nitrogen bases such as DBU can be added to the system in order to intercept the formation of nitric acid.
Owner:SYNOVO
Method for producing polyalkylene glycol derivative having amino group at end
ActiveUS20160159831A1High yield rateAdverse effectSilicon organic compoundsOrganic compound preparationPtru catalystCombinatorial chemistry
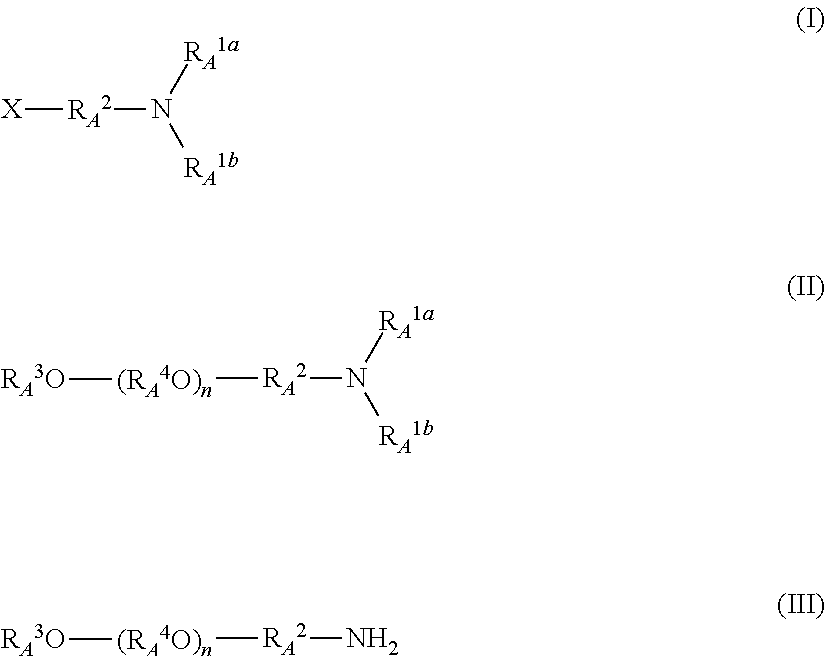
A method simply produces a narrowly distributed and high-purity polyalkylene glycol derivative having an amino group at an end without using a heavy metal catalyst. A method for producing a polyalkylene glycol derivative having an amino group at the end by reacting a compound represented by the general formula (V) with an alkylene oxide, then reacting a reaction product with an electrophile represented by the general formula (I), and deprotecting the obtained product without using a heavy metal:RA3O(RA4O)k-1RA4O−M+ (V)wherein RA3 represents a linear; branched, or cyclic hydrocarbon group having 1 to 20 carbon atoms; RA4 represents an alkylene group having 2 to 8 carbon atoms; k represents an integer of 2 to 5; and M represents an alkali metal;wherein RA1a and RA1b each independently represent a protective group of the amino group, or one of RA1a and RA1b represents H and the other represents a protective group of the amino group, or RA1a and RA1b bind to each other to form a cyclic protective group, and the protective group is deprotectable without using a heavy metal; RA2 represents a linear, branched, or cyclic hydrocarbon group having 1 to 6 carbon atoms; and X represents a leaving group.
Owner:SHIN ETSU CHEM IND CO LTD
Chiral Phosphorus Compounds
InactiveUS20080255391A1Influence on reaction selectivityHigh yieldPhosphorus organic compoundsCompound aAlcohol
A process for the stereoselective preparation of a P-chiral four-co-ordinated phosphorus compound, the process comprising reacting a first reactant selected from the group consisting of a chiral alcohol, chiral amine or chiral thiol, with a second reactant comprising a P-chiral three-co-ordinated phosphorus compound, in the presence of an electrophile.
Owner:UNIV COLLEGE DUBLIN NAT UNIV OF IRELAND DUBLIN
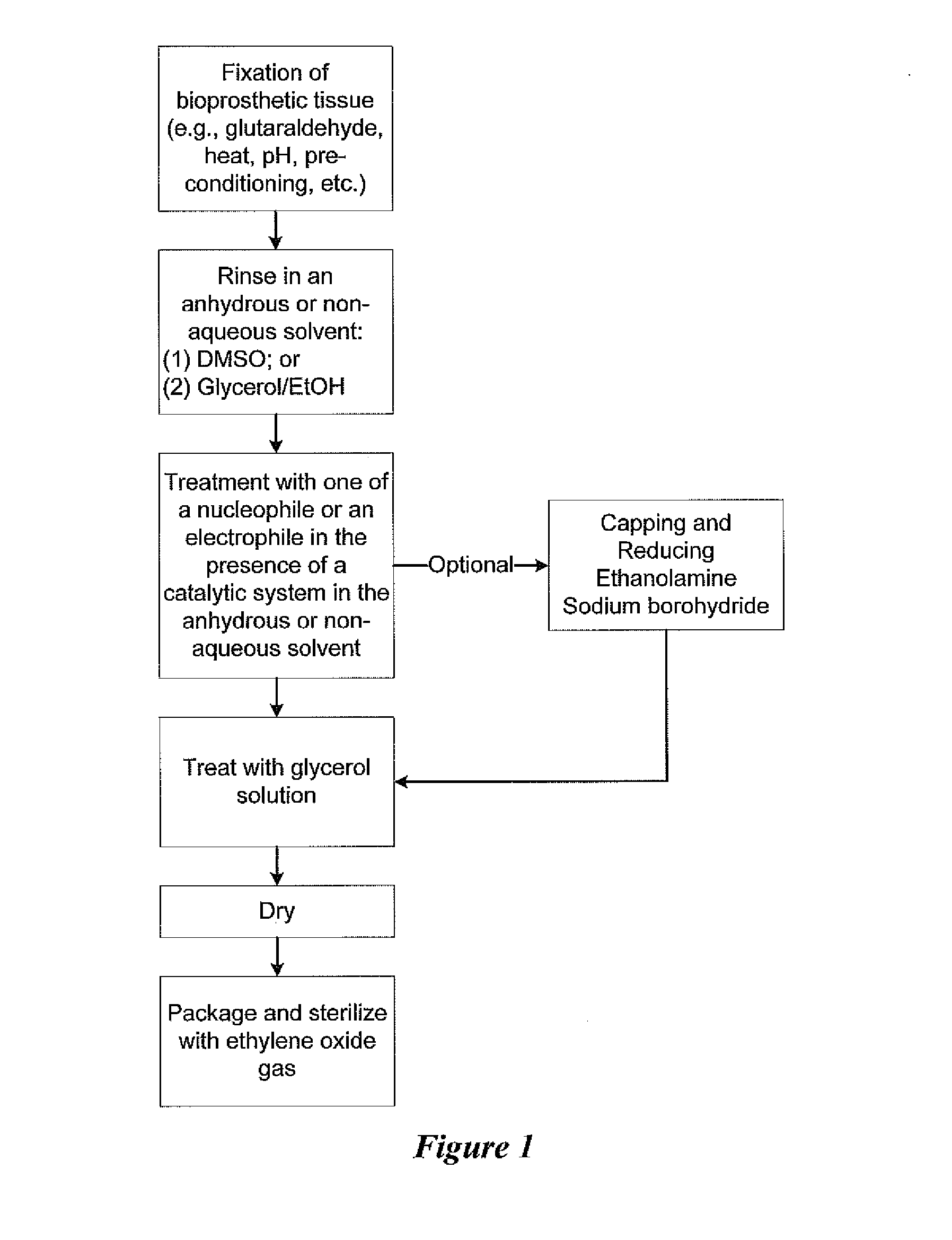
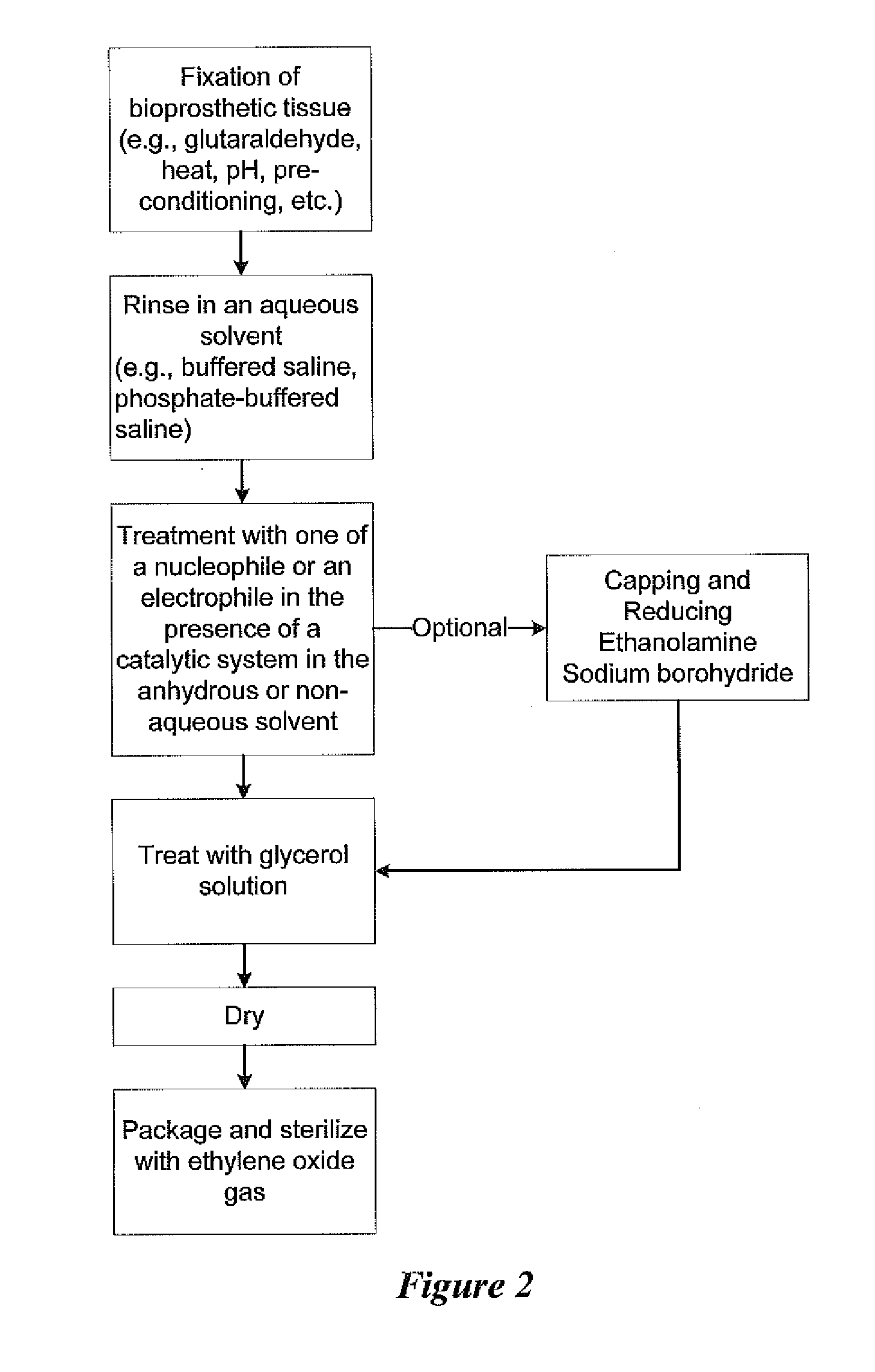
Methods for treating bioprosthetic tissue using a nucleophile/electrophile in a catalytic system
ActiveUS20140127804A1Pharmaceutical delivery mechanismSkeletal/connective tissue cellsCross-linkRubidium
Methods for treating a bioprosthetic tissue are described herein. The methods comprise contacting the bioprosthetic tissue with at least one nucleophile and / or at least one electrophile in the presence of a catalytic system comprising at least one or a combination of a fluoride-based salt, a cesium-based salt, a potassium-based salt, a rubidium-based salt, or a carbonate-based salt. The methods may be used to alter functional groups on biological tissue which represent actual and potential calcium binding sites and also processes for cross-linking bioprosthetic tissue. Both processes may be used in conjunction with known fixative techniques, such as glutaraldehyde fixation, or may be used to replace known fixative techniques.
Owner:EDWARDS LIFESCIENCES CORP
Aromatic sulfonated ketals
The present invention advantageously provides ketal functional compounds that can be strong electrophiles under conditions compatible with ketal groups, are stable, crystalline solids at room temperature, and are much safer to handle than ketal iodides. The present invention accomplishes by incorporating aromatic sulfonyl moieties into ketal functional materials. The compounds are useful starting materials or intermediates in the synthesis of more complex organic molecules.
Owner:ROCHE COLORADO CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com