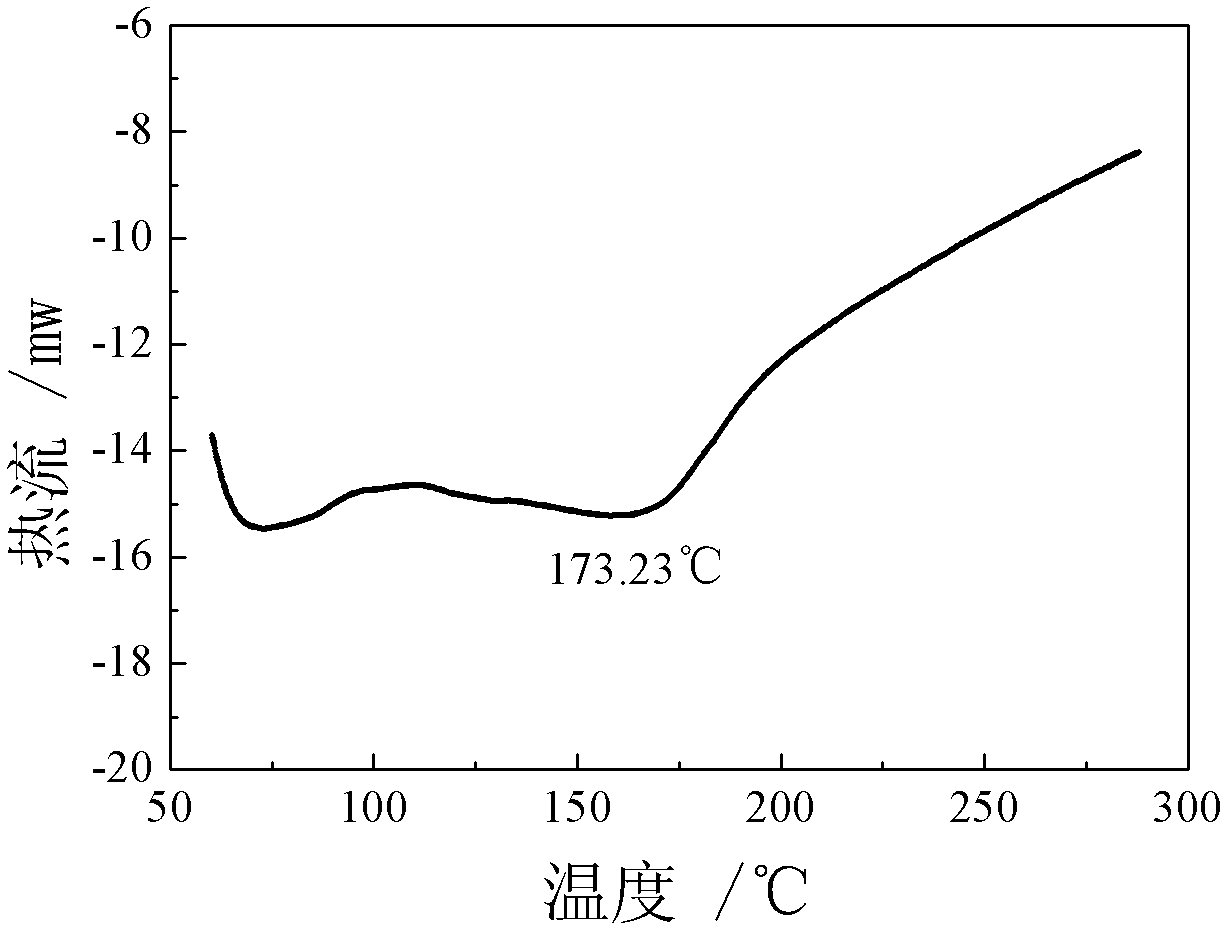
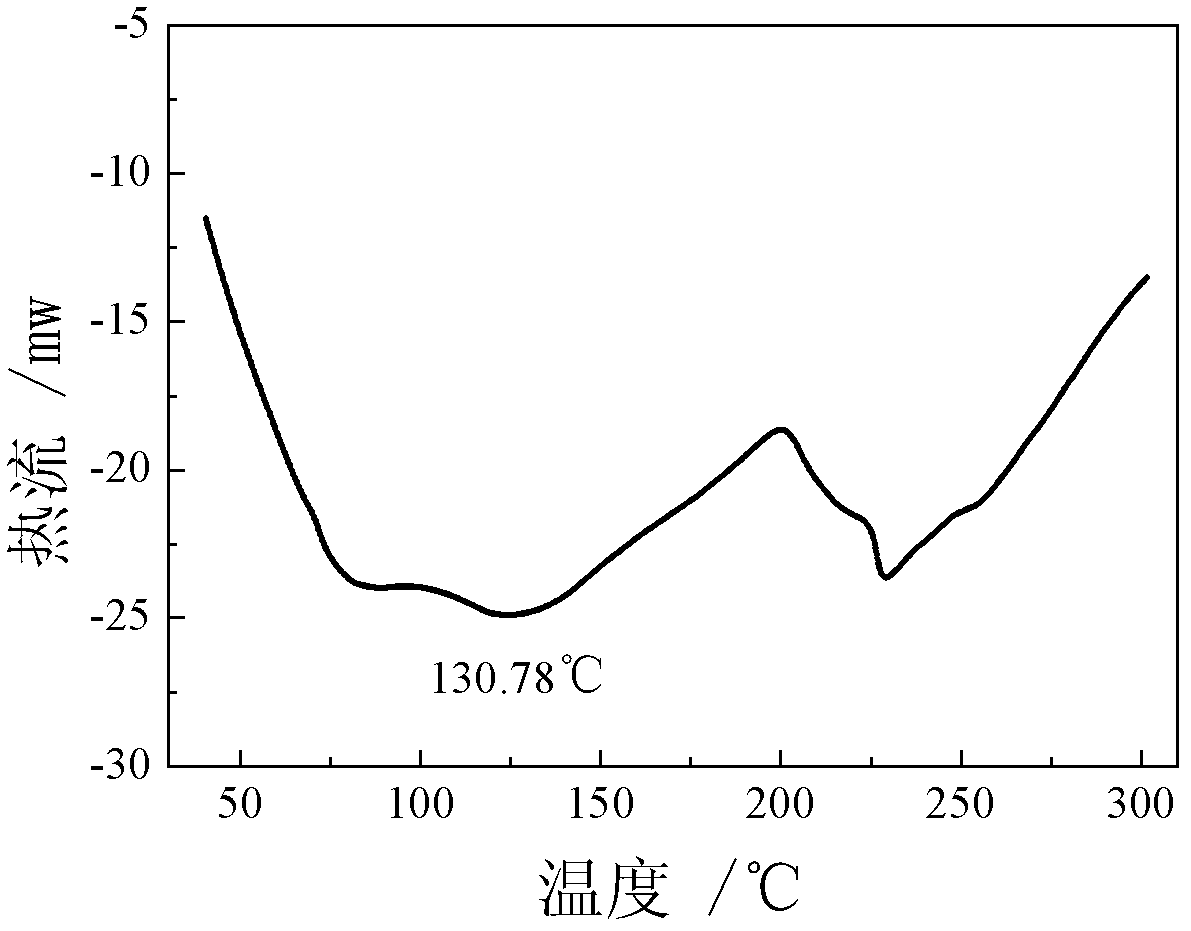
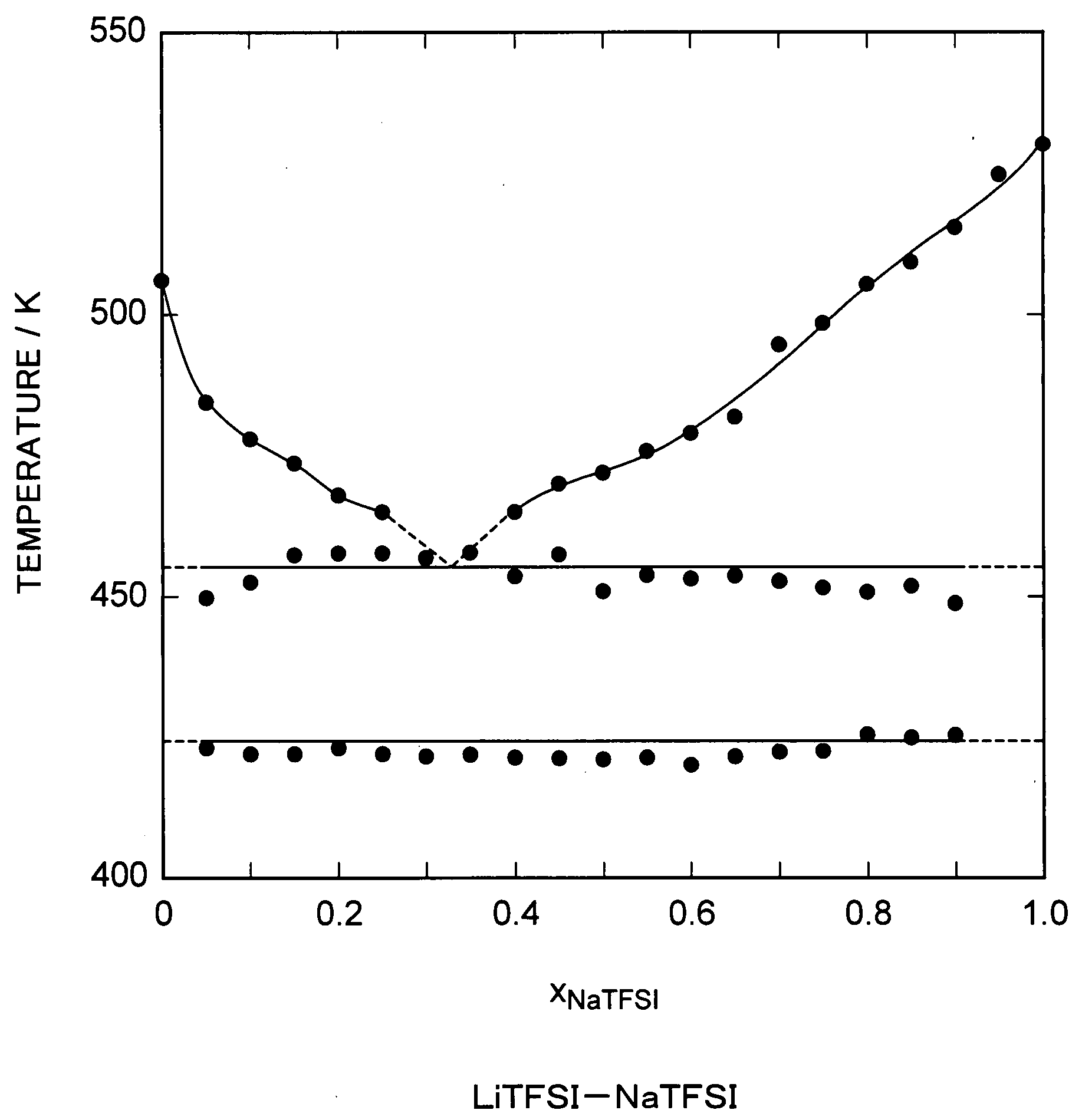
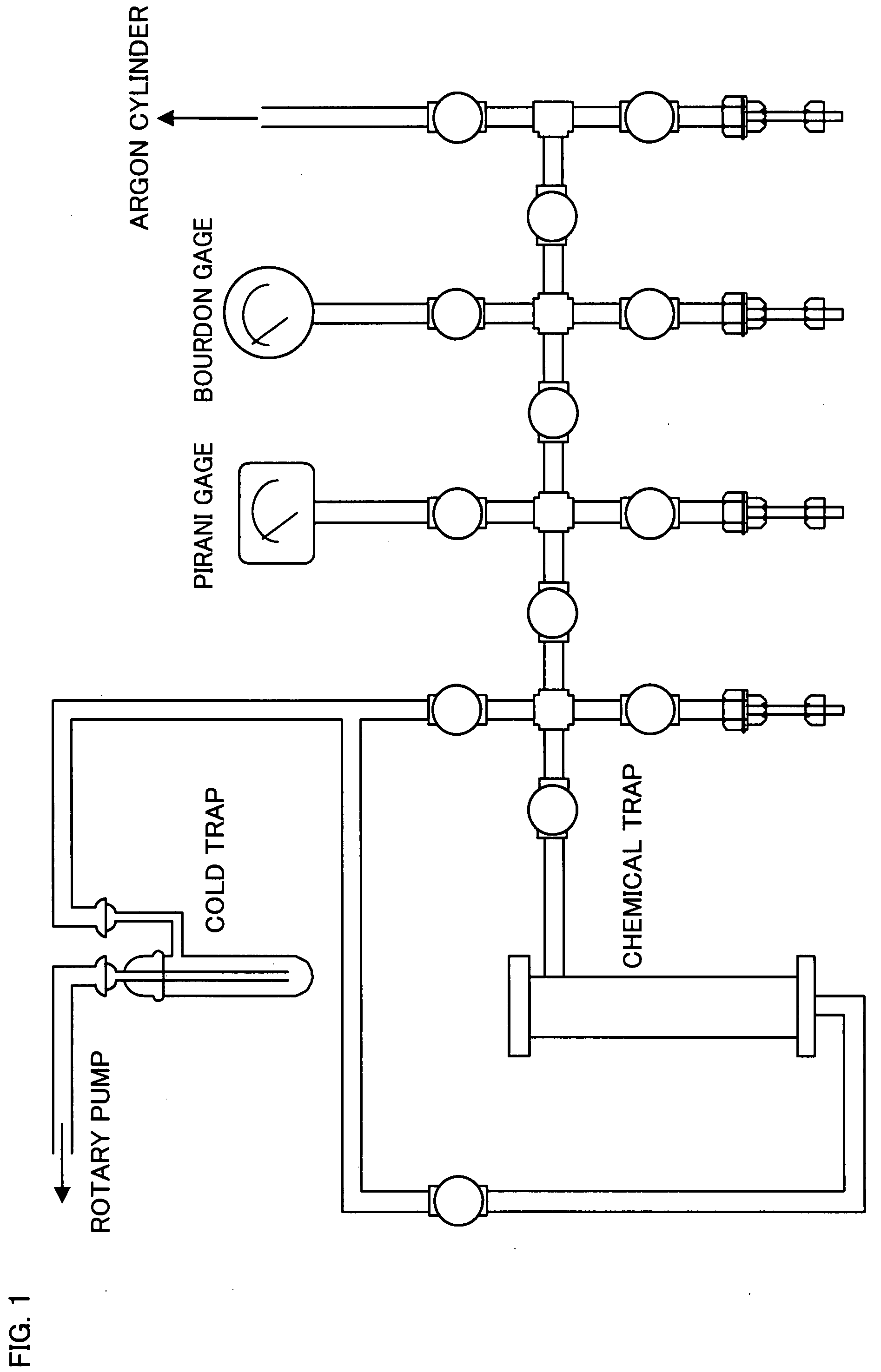
Patents
Literature
6798 results about "Molten salt" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Molten salt is salt which is solid at standard temperature and pressure (STP) but enters the liquid phase due to elevated temperature. A salt that is normally liquid even at STP is usually called a room temperature ionic liquid, although technically molten salts are a class of ionic liquids.
Low emission polymer compositions
InactiveUS6331264B1Improve stabilityEmission reductionCeramic shaping apparatusMelt spinning methodsMolten stateMolten salt
The invention comprises polymer compositions containing 3-hydroxypropanoxy terminated polymer that exhibit reduced levels of degradation product emissions during processing, by contacting the polymer in the molten state with a melt stable, organic nitrogen-containing stabilizing compound, such as polyamide.
Owner:DUPONT IND BIOSCIENCES USA LLC
Method for producing chemically tempered glass
To provide a method for producing chemically tempered glass, whereby frequency of replacement of the molten salt can be reduced. A method for producing chemically tempered glass, which comprises repeating ion exchange treatment of immersing glass in a molten salt, wherein the glass comprises, as represented by mole percentage, from 61 to 77% of SiO2, from 1 to 18% of Al2O3, from 3 to 15% of MgO, from 0 to 5% of CaO, from 0 to 4% of ZrO2, from 8 to 18% of Na2O and from 0 to 6% of K2O; SiO2+Al2O3 is from 65 to 85%; MgO+CaO is from 3 to 15%; and R calculated by the following formula by using contents of the respective components, is at least 0.66:R=0.029×SiO2+0.021×Al2O3+0.016×MgO−0.004×CaO+0.016×ZrO2+0.029×Na2O+0×K2O−2.002
Owner:ASAHI GLASS CO LTD
Non-aqueous electrolyte battery
InactiveUS20060068282A1Improve output performanceImprove cycle performanceNon-aqueous electrolyte accumulatorsCell seperators/membranes/diaphragms/spacersMolten saltNon aqueous electrolytes
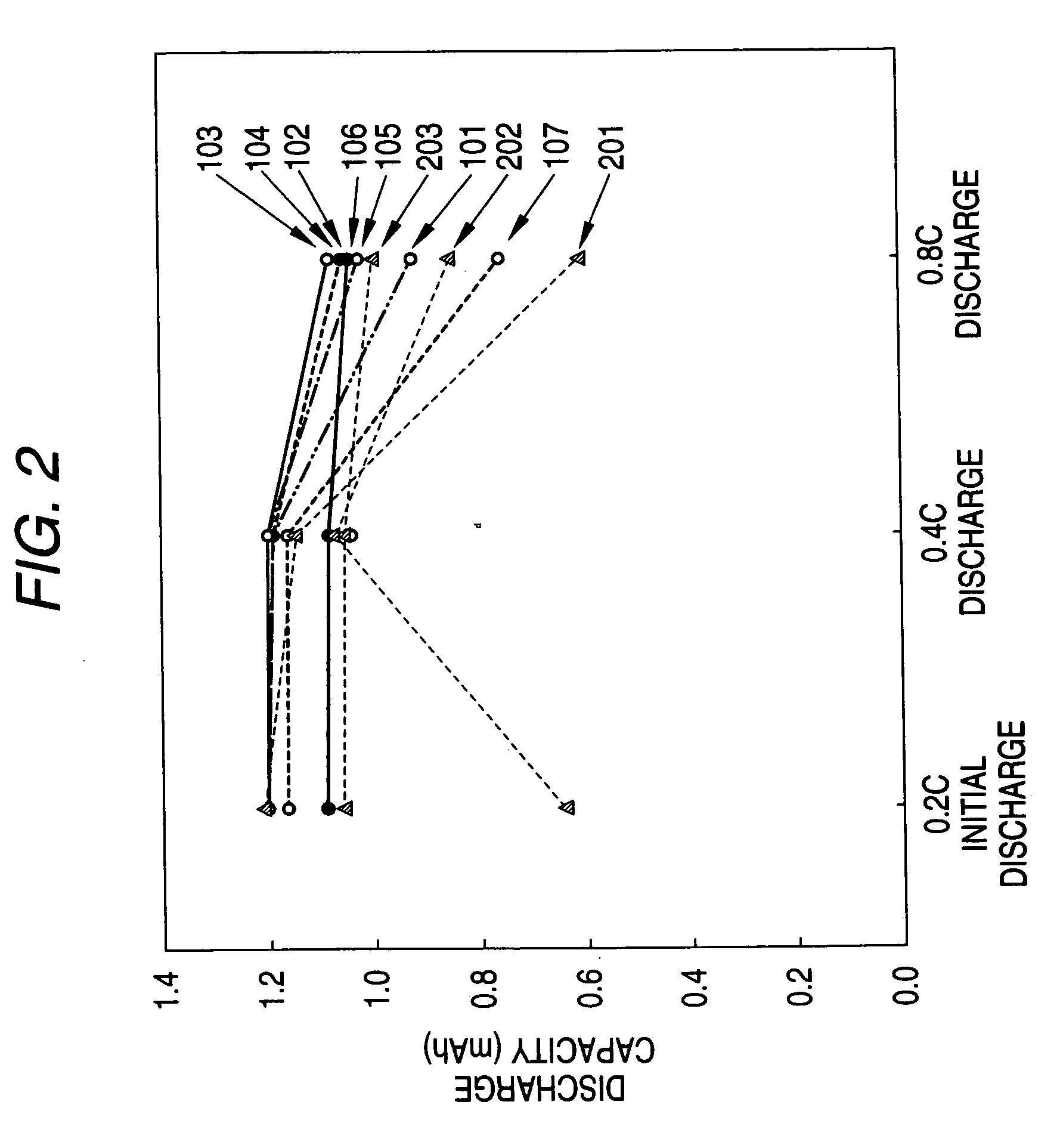
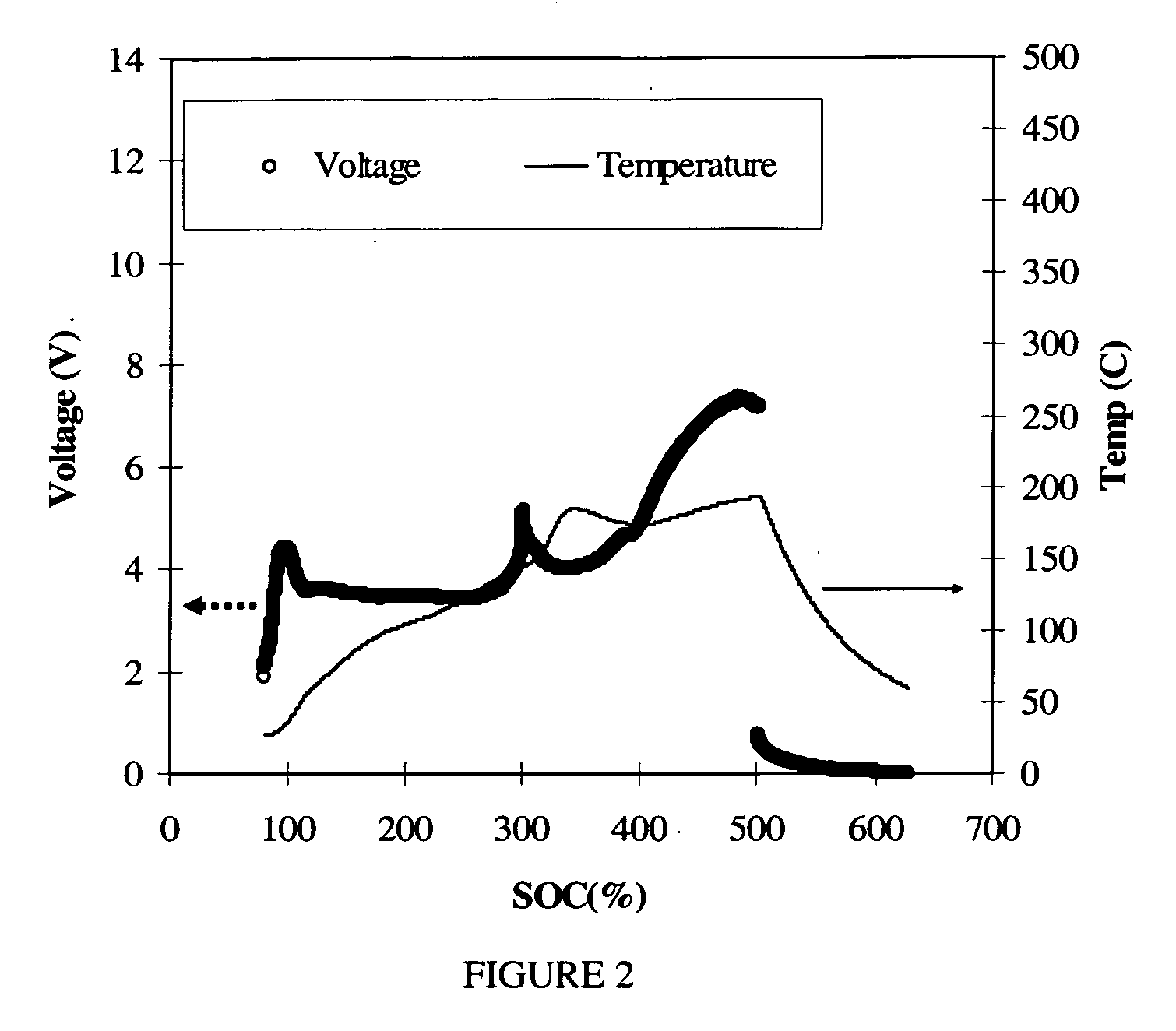
A non-aqueous electrolyte battery that contains a molten salt electrolyte and has the enhanced output performances and cycle performances can be provided. The electrolyte has a molar ratio of lithium salt to molten salt of from 0.3 to 0.5, and the non-aqueous electrolyte battery has a positive electrode having a discharge capacity of 1.05 or more times that of a negative electrode thereof.
Owner:KK TOSHIBA
Display device and electric apparatus using the same
InactiveUS20070040982A1Improve the display effectReduce power consumptionNon-linear opticsOptical elementsLow voltageMolten salt
In a display device that includes a display space provided on a display surface side and a liquid sealed inside the display space so as to be operable and is constituted so as to be able to change a display color on the display surface side according to an application of an electric field to the liquid, the liquid is an ionic liquid containing an ambient temperature molten salt combining a cation and an anion. Further, an amount of water blended in the liquid is 0 to 10 parts by weight with respect to 100 parts by weight of the ionic liquid. This makes it possible both to reduce a voltage to be applied to the liquid so as to allow driving at a low voltage and to improve an operation performance.
Owner:SHARP KK
Nonaqueous electrolyte battery
InactiveUS20050164082A1Good rate characteristicsExcellent cycle characteristicsFinal product manufactureCylindrical casing cells/batteryAlkaline earth metalPotential difference
A nonaqueous electrolyte battery includes a positive electrode, a negative electrode containing an active material providing a negative electrode working potential which is nobler than a lithium electrode potential, and whose potential difference from the lithium electrode potential is 0.5V or more, and an electrolyte containing molten salt, ester phosphate and metal salt including at least one of alkaline metal salt and alkaline earth metal salt, the electrolyte satisfying the following formula (1): 0.5≦(M2 / M1)≦1 (1) where M1 is a molar number of the metal salt and M2 is a molar number of the ester phosphate.
Owner:KK TOSHIBA
Corrosion protection using protected electron collector
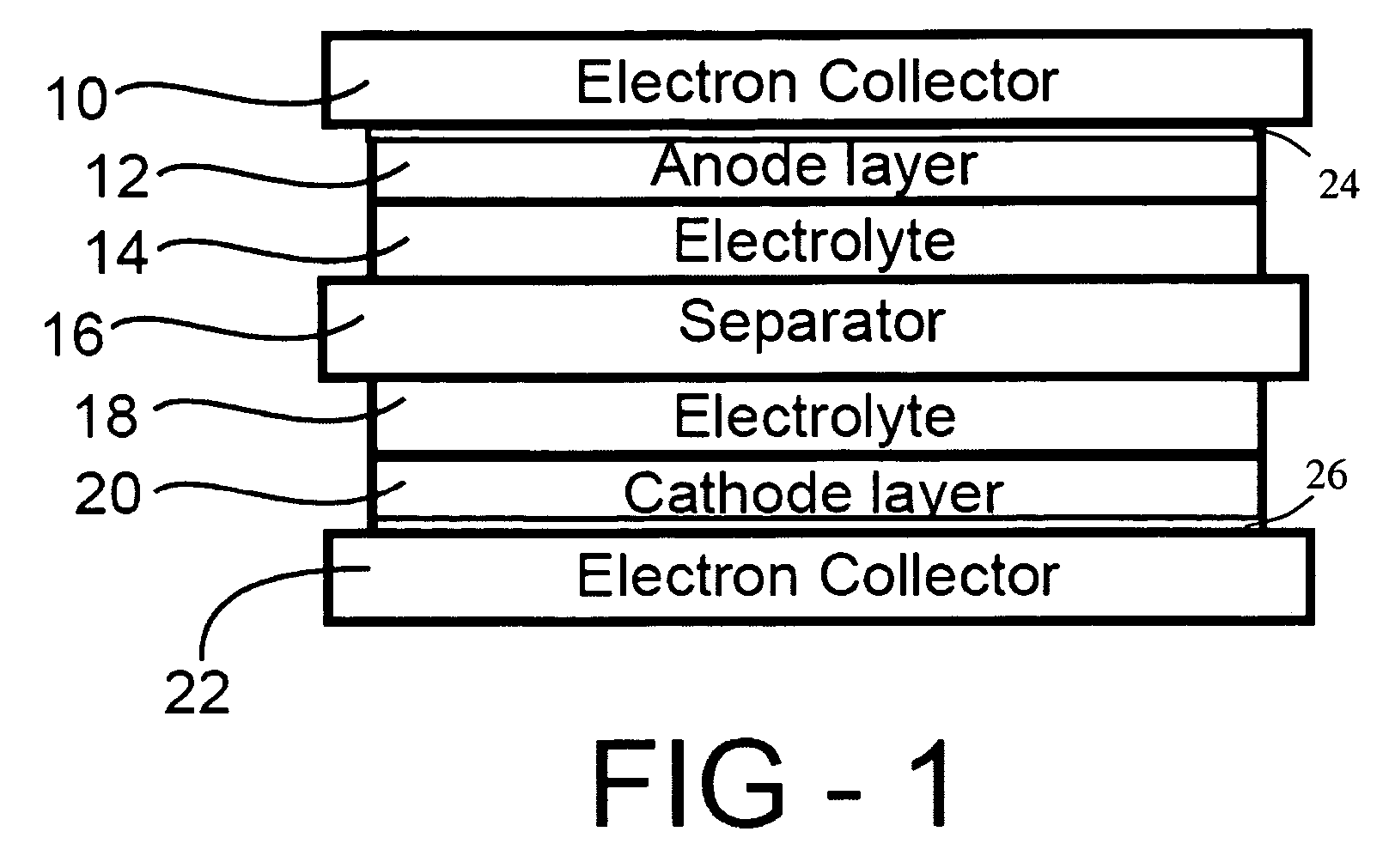
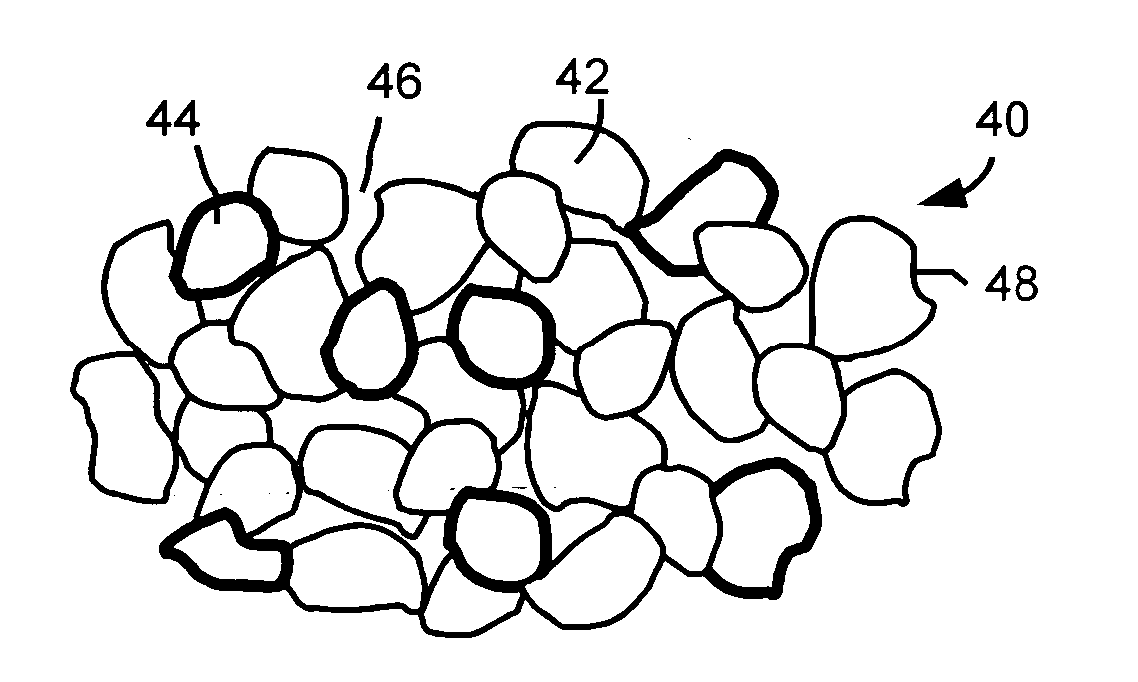
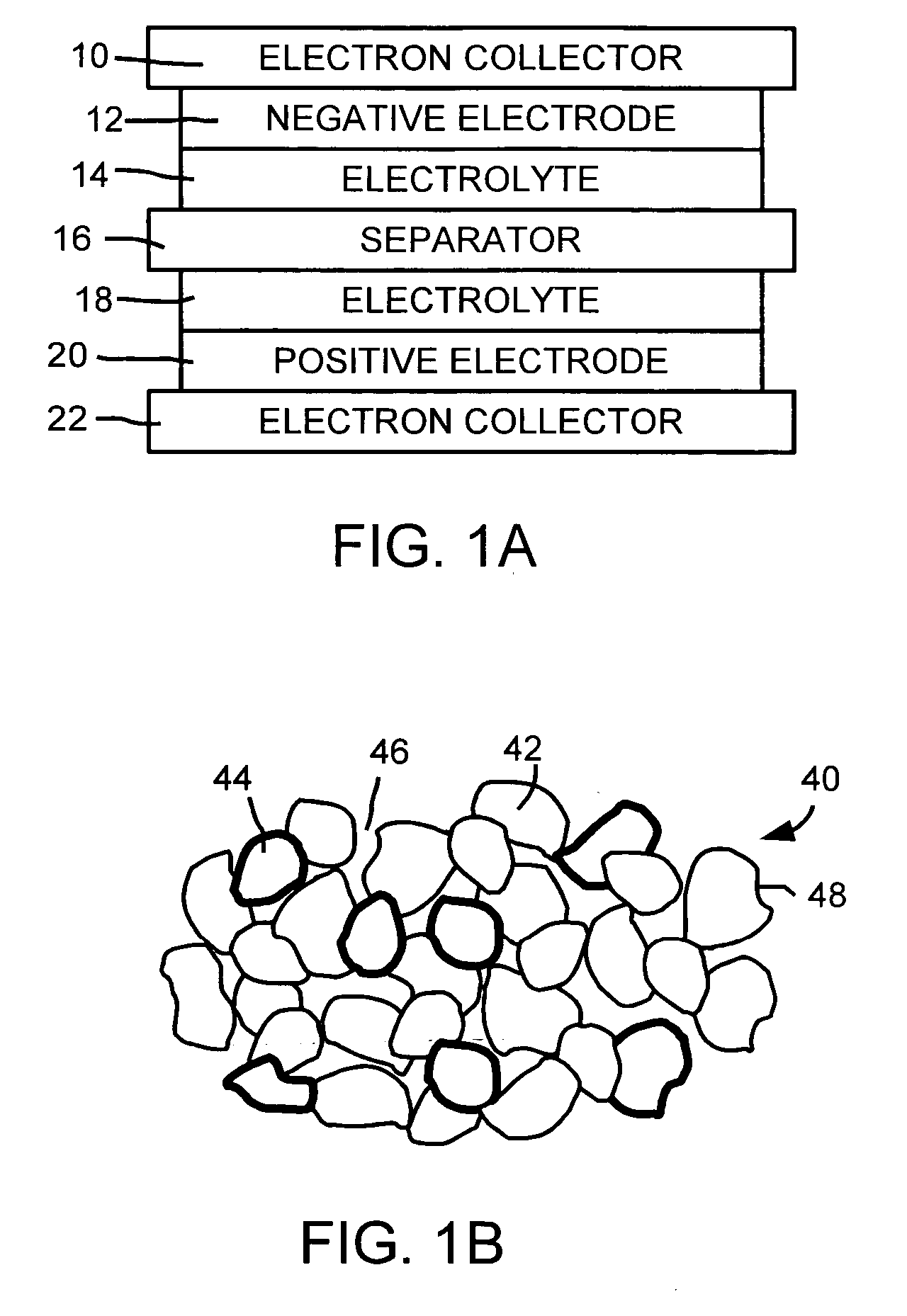
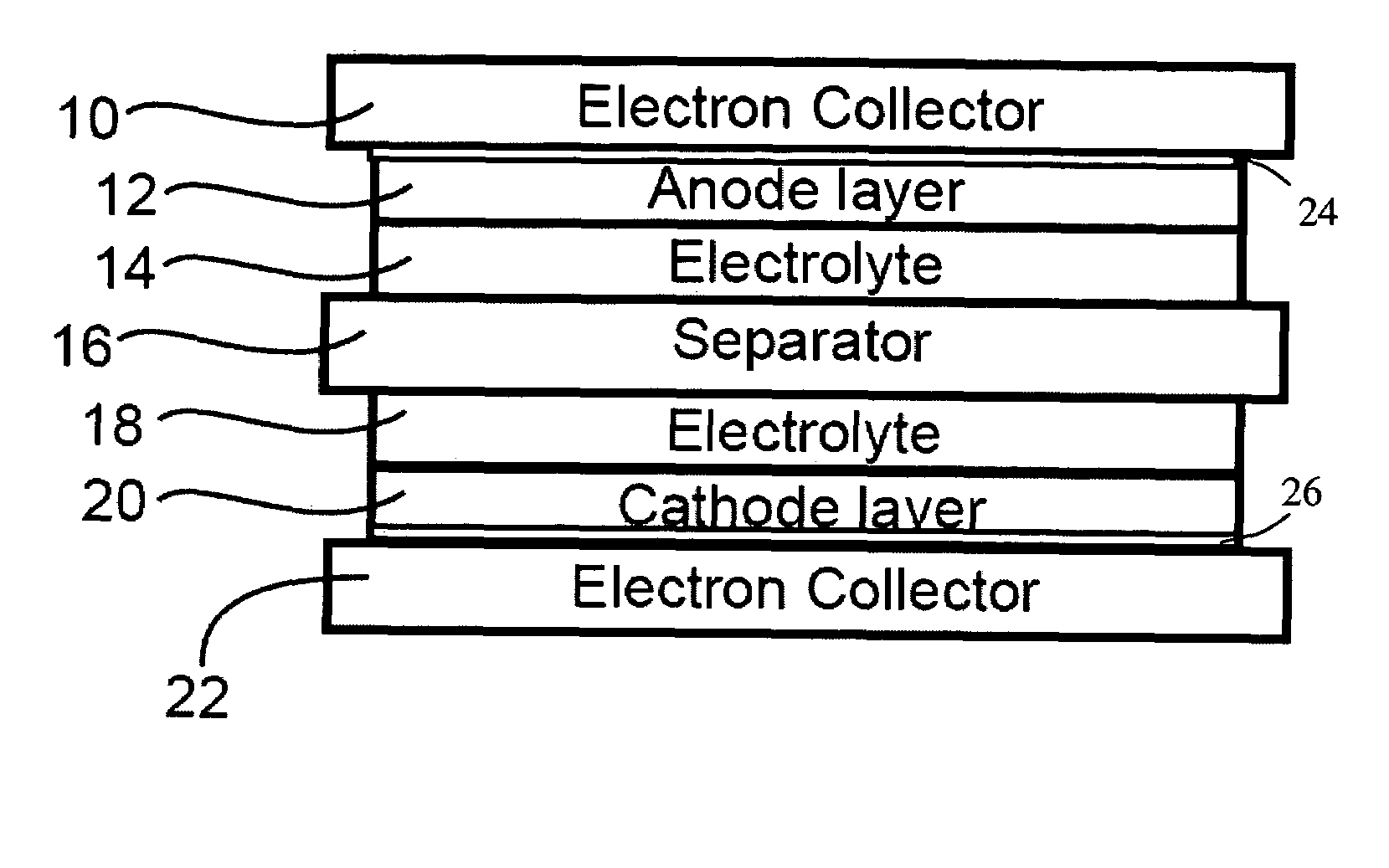
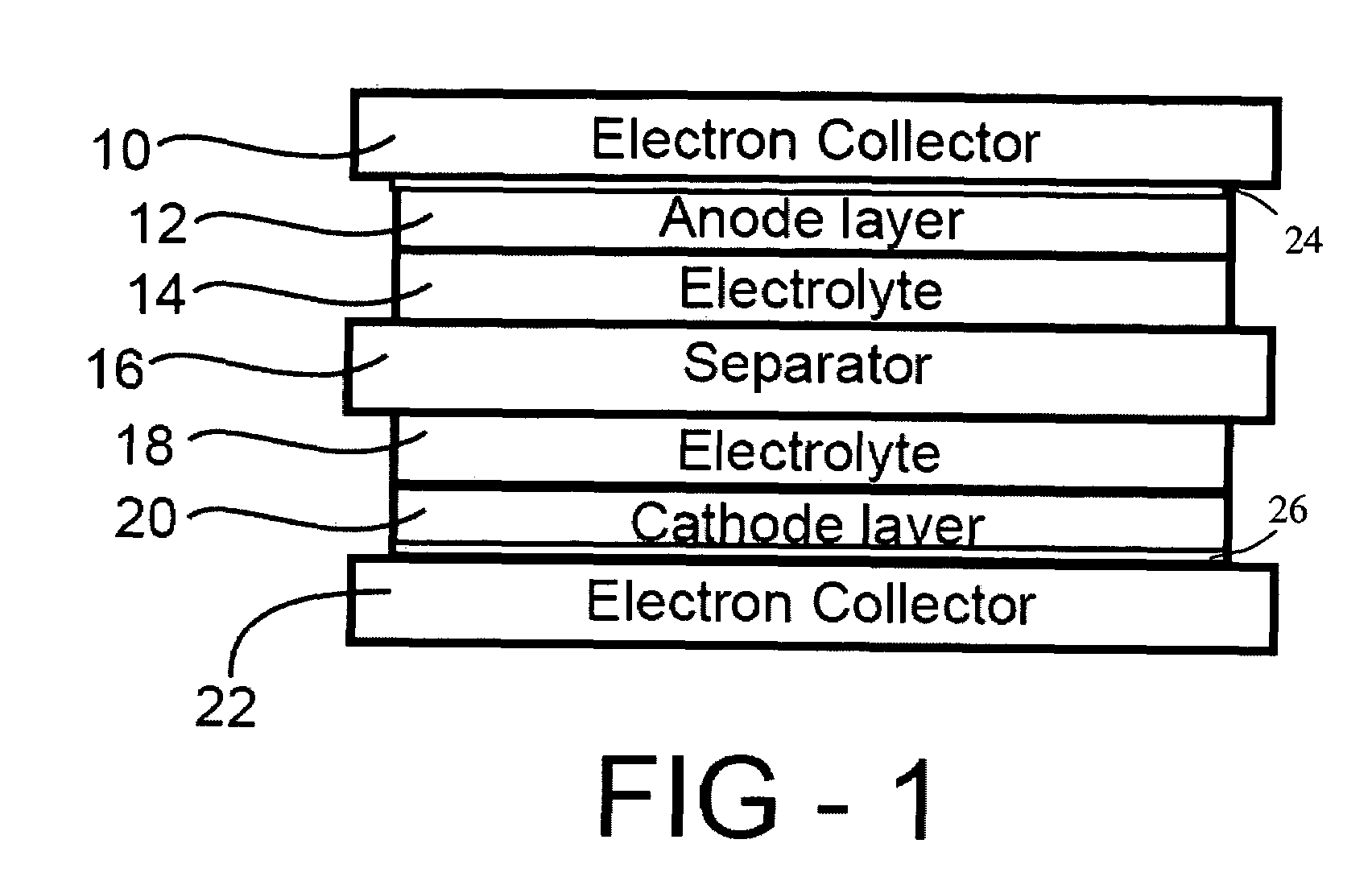
ActiveUS20060019168A1Improve electrochemical stabilityImproved batteryElectrode carriers/collectorsLi-accumulatorsMolten saltProtection layer
A battery comprises a first electrode, a second electrode, an electrolyte, and an electron collector associated with the first electrode, the electron collector having a surface treatment, such as a protection layer, that reduces corrosion of the electron collector by the molten salt electrolyte.
Owner:TOYOTA MOTOR CO LTD
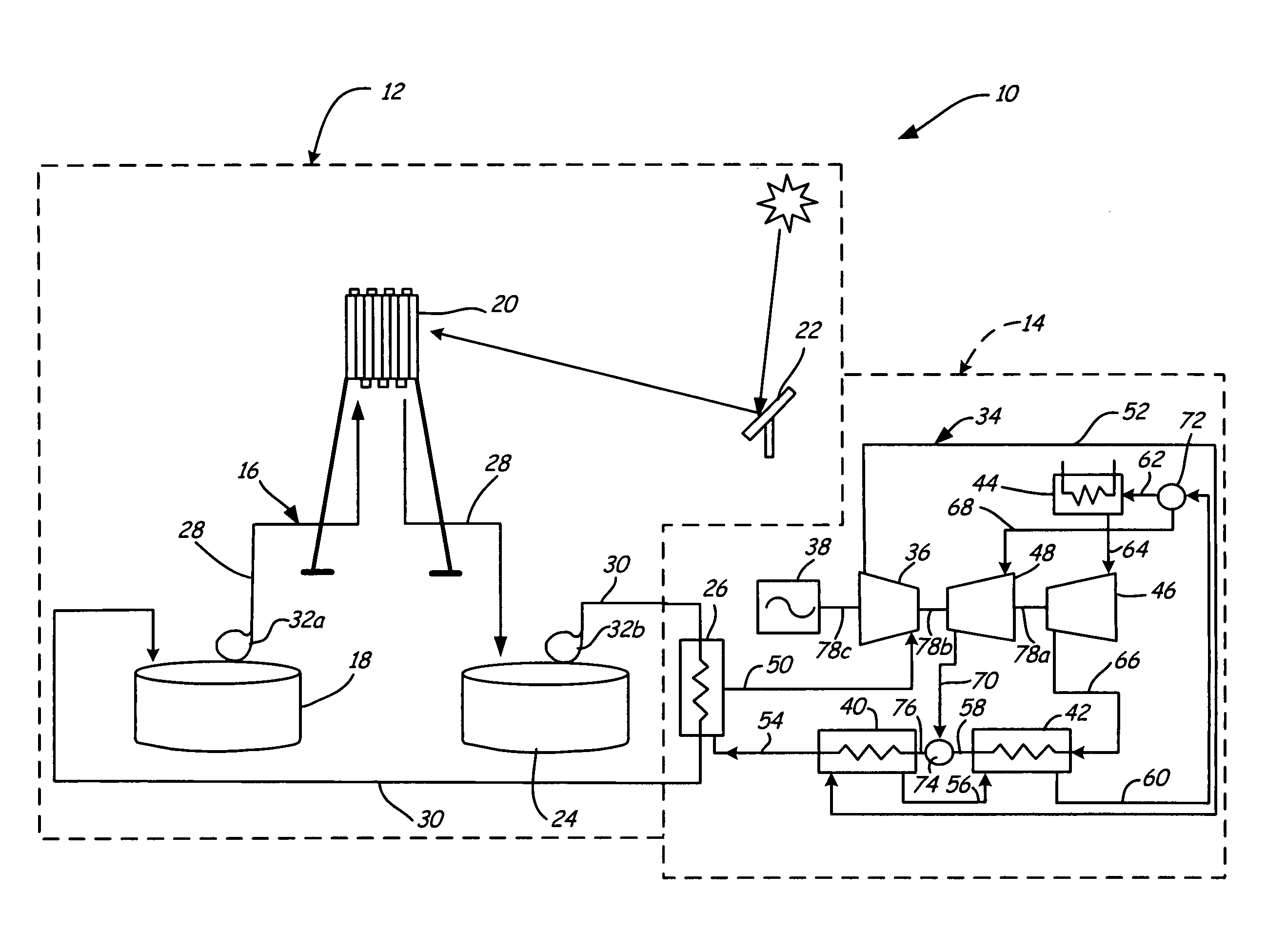
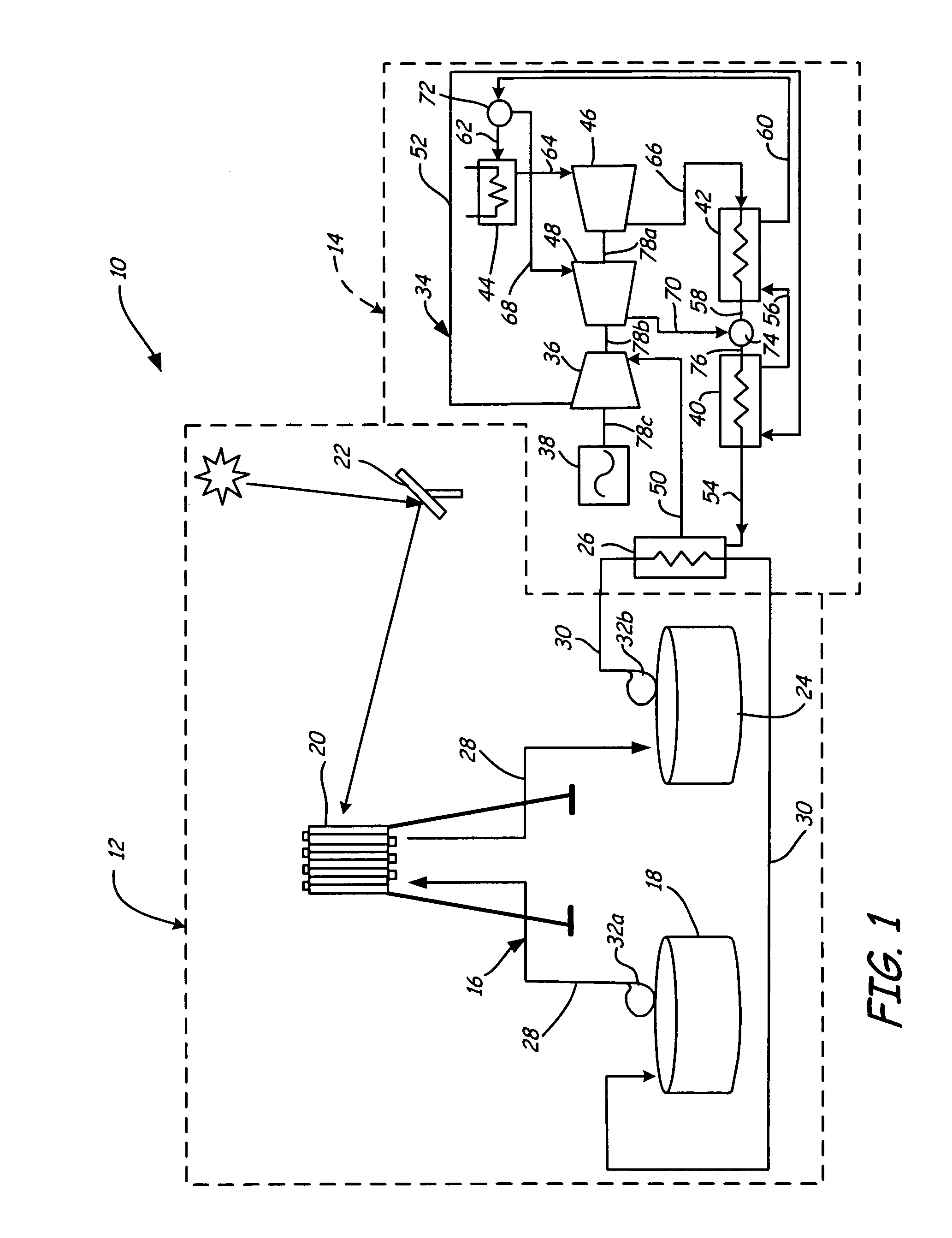
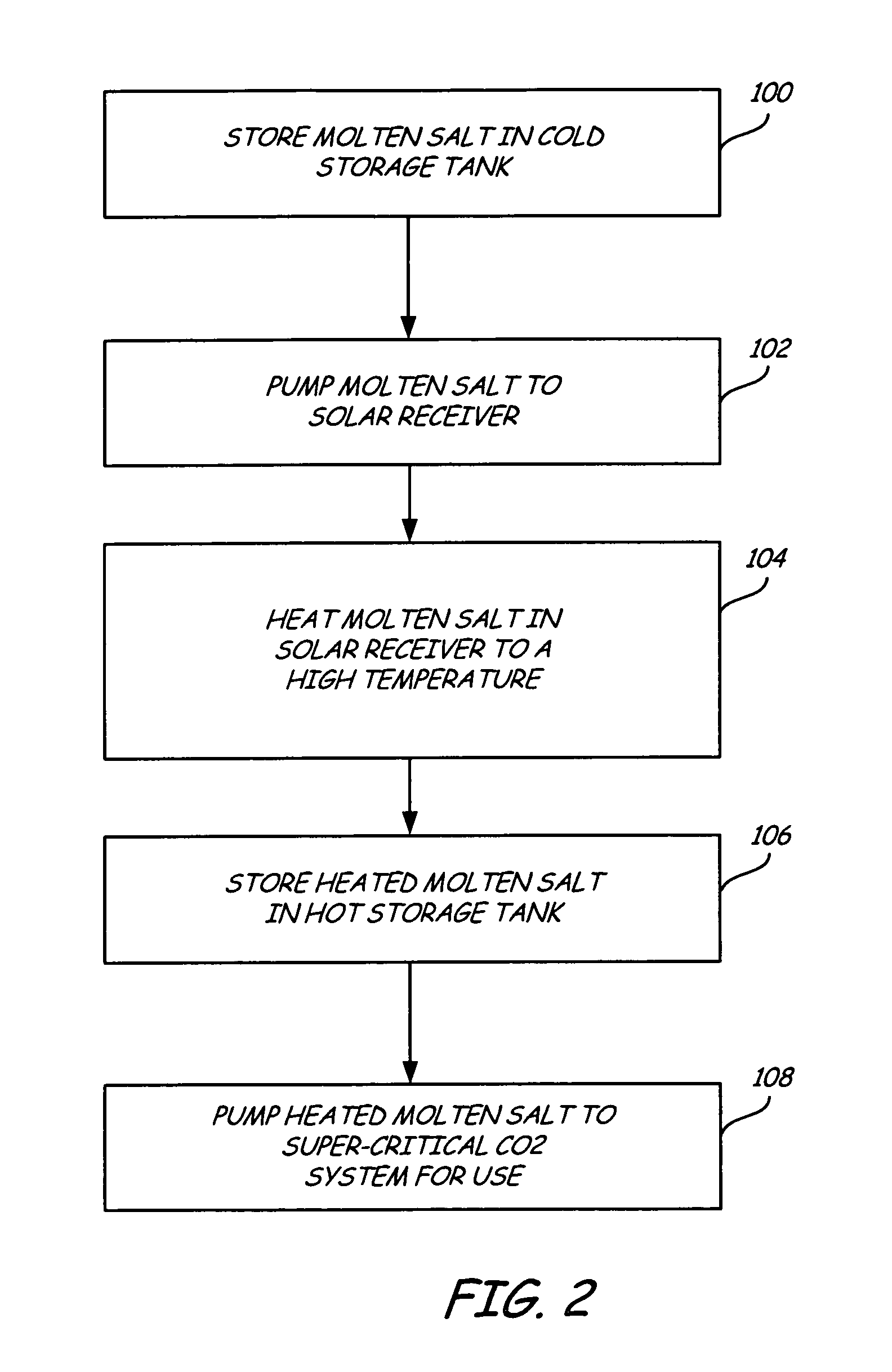
Supercritical CO2 turbine for use in solar power plants
Owner:SOLARRESERVE TECH
Battery with molten salt electrolyte and high voltage positive active material
InactiveUS20060088767A1High voltageActive material electrodesSecondary cellsMolten saltRechargeable cell
A lithium-based rechargeable battery comprises a positive electrode, a negative electrode, and a molten salt electrolyte that is electrically conductive lithium ions. The positive electrode includes a positive active material that has an electrochemical potential of at least approximately 4.0 volts relative to lithium, and more preferably at least approximately 4.5 V relative to lithium. The electrolyte may further include a source of lithium ions, such as a lithium compound. Other rechargeable batteries using other ionic species can be fabricated to an analogous design.
Owner:TOYOTA MOTOR ENGINEERING & MANUFACTURING NORTH AMERICA +1
Electrolytes for electrooptic devices comprising ionic liquids
Electrolyte solutions of soluble bifunctional redox dyes in molten salt solvent may be used to prepare electrooptic devices with enhanced stability toward ultraviolet radiation. The solvents include lithium or quaternary ammonium cations, and perfluorinated sulfonylimide anions selected from trifluoromethylsulfonate (CF3SO3−), bis(trifluoromethylsulfonyl)imide ((CF3SO2)2N−), bis(perfluoroethylsulfonyl)imide ((CF3CF2SO2)2N−) and tris(trifluoromethylsulfonyl)methide ((CF3SO2)3C−).
Owner:TRIAD NAT SECURITY LLC +1
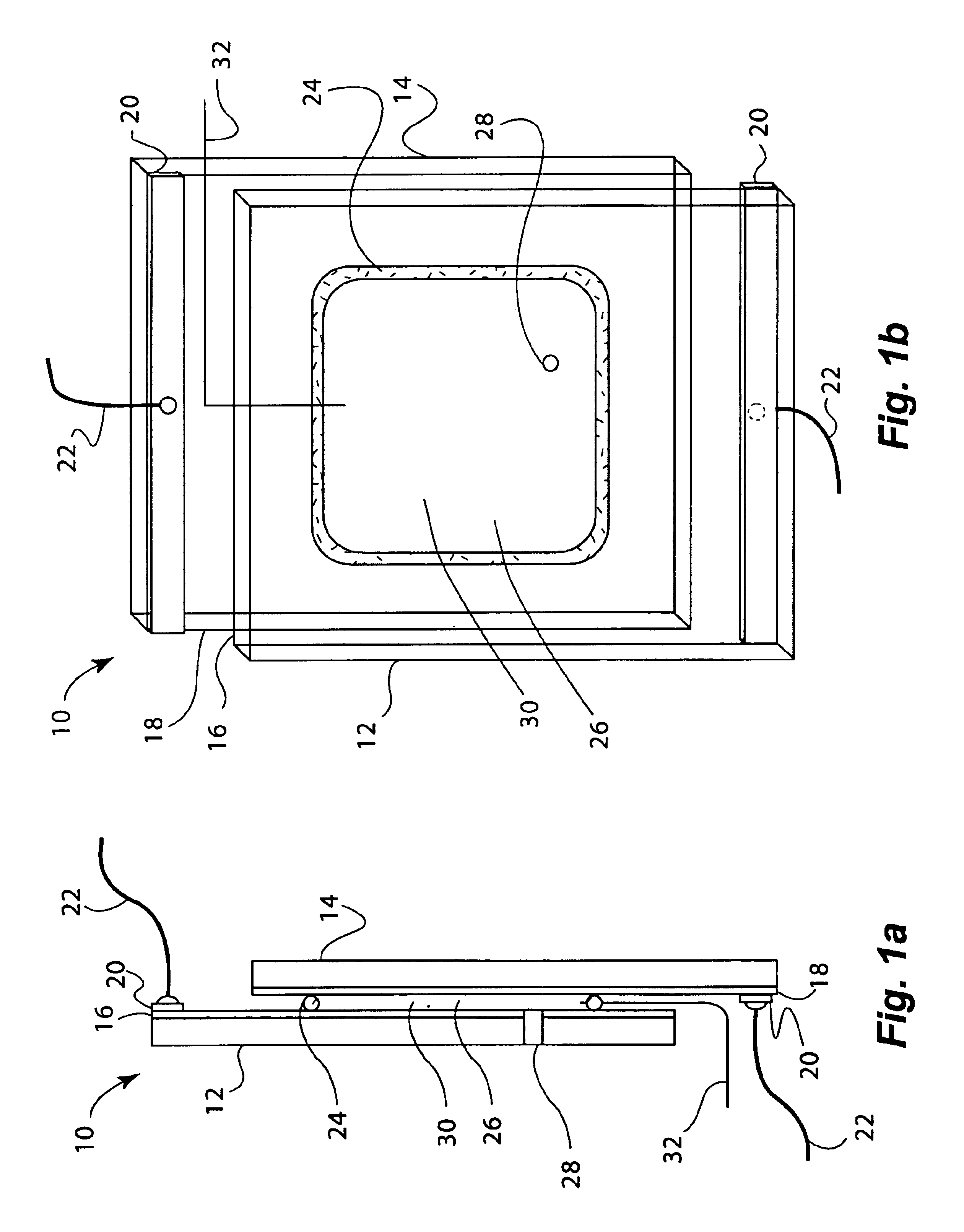
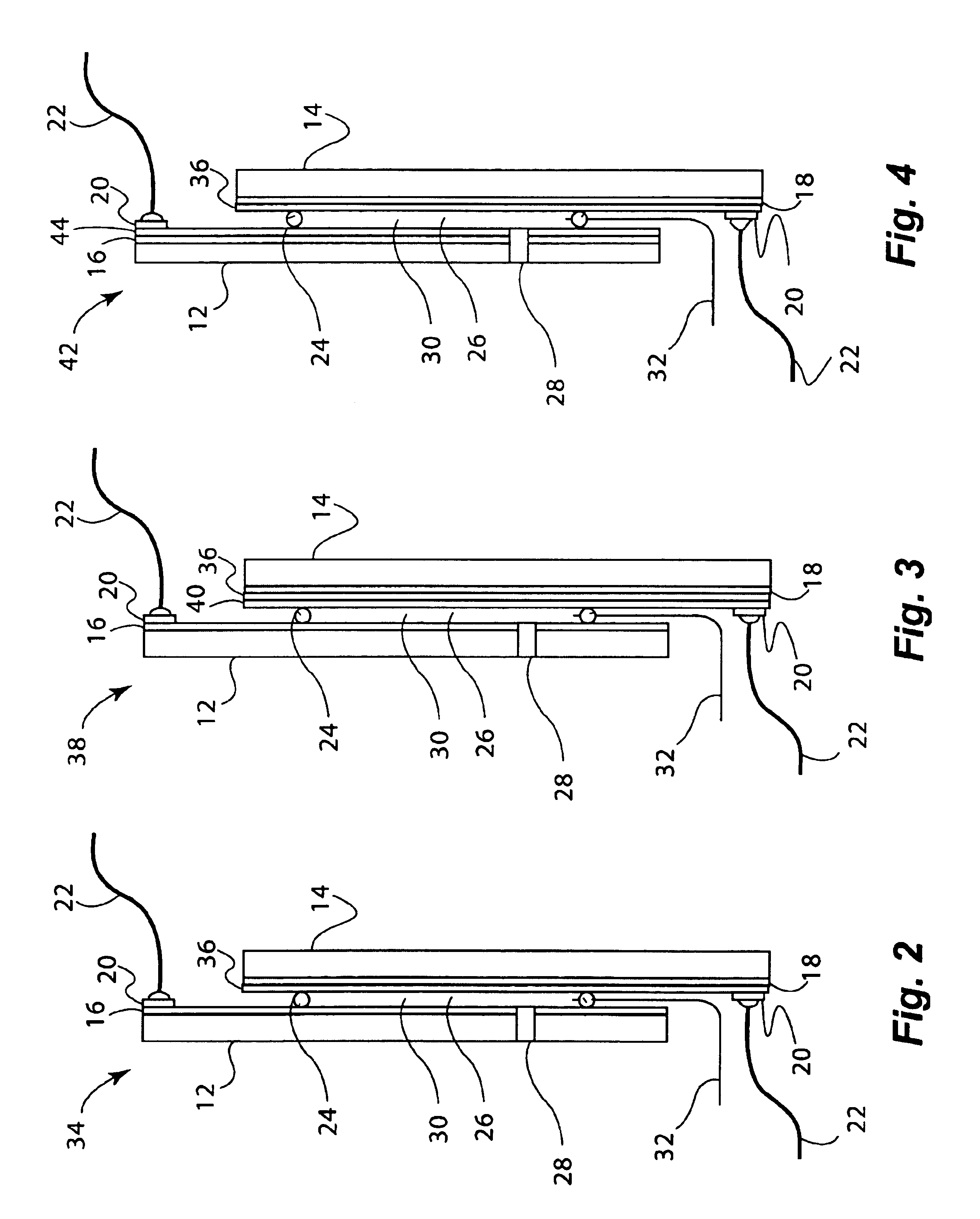
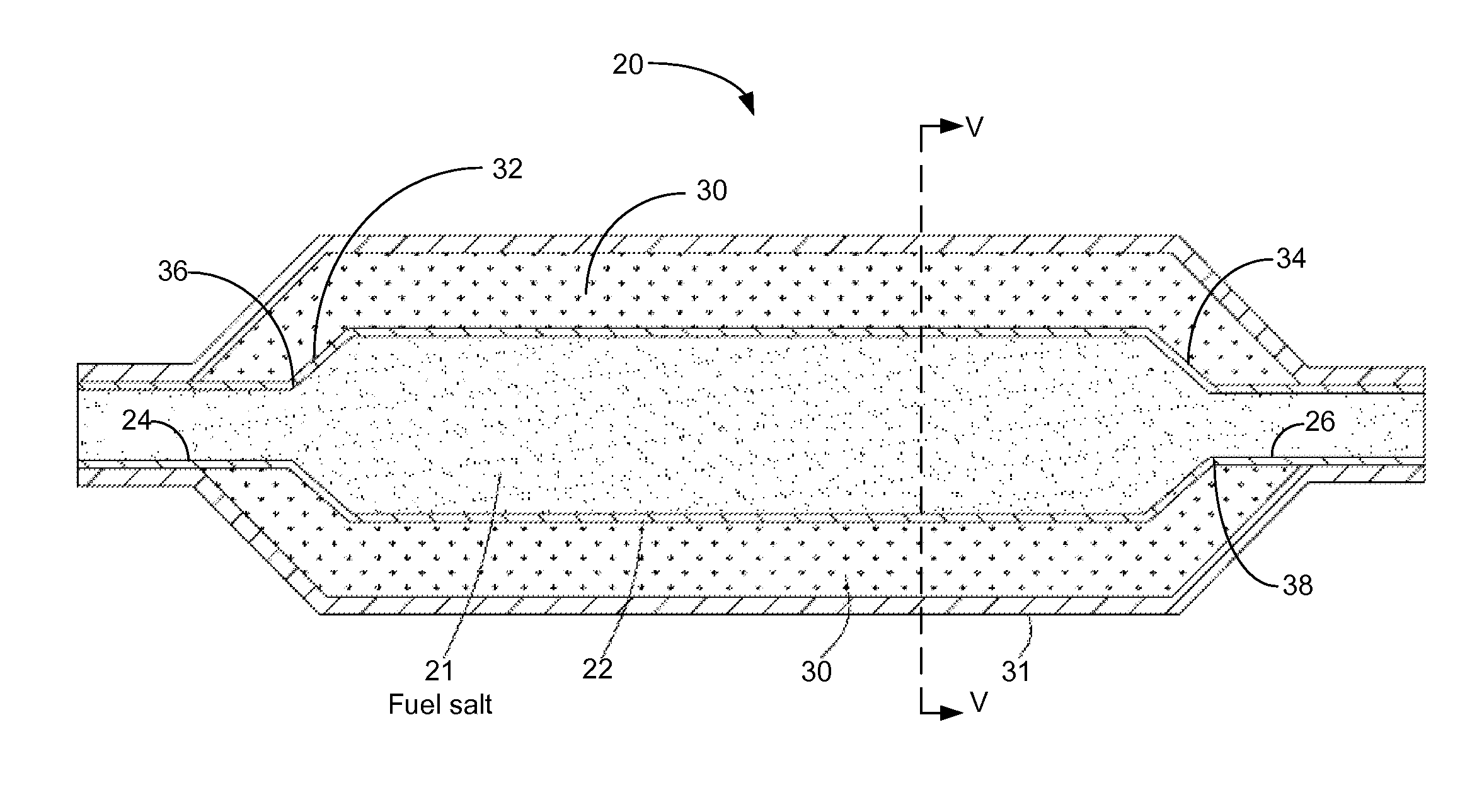
Molten salt nuclear reactor
InactiveUS20090279658A1Maximize probabilityFuel elementsNuclear energy generationBreeder reactorNuclear reactor
A molten salt breeder reactor that has fuel conduit surrounded by a fertile blanket. The fuel salt conduit has an elongated core section that allows for the generation of electrical power on a scale comparable to commercially available nuclear reactors. The geometry of the fuel conduit is such that sub-critical conditions exist near the input and output of the fuel salt conduit and the fertile blanket surrounds the input and output of the fuel salt conduit, thereby minimizing neutron losses.
Owner:OTTAWA VALLEY RES ASSOCS
NANO silicon-carbon composite material and preparation method thereof
ActiveUS20140302396A1Improve cycle stabilityShort production processMaterial nanotechnologyElectrolysis componentsCarbon compositesSilicon dioxide
The invention relates to a nano silicon-carbon composite negative material for lithium ion batteries and a preparation method thereof. A porous electrode composed of silica and carbon is taken as a raw material, and a nano silicon-carbon composite material of carbon-loaded nano silicon is formed by a molten salt electrolysis method in a manner of silica in-situ electrochemical reduction. Silicon and carbon of the material are connected by nano silicon carbide, and are metallurgical-grade combination, so that the electrochemical cycle stability of the nano silicon-carbon composite material is improved. The preparation method of the nano silicon-carbon composite material provided by the invention comprises the following steps: compounding a porous block composed of carbon and silica powder with a conductive cathode collector as a cathode; using graphite or an inert anode as an anode, and putting the cathode and anode into CaCl2 electrolyte or mixed salt melt electrolyte containing CaCl2 to form an electrolytic cell; applying voltage between the cathode and the anode; controlling the electrolytic voltage, the electrolytic current density and the electrolytic quantity, so that silica in the porous block is deoxidized into nano silicon by electrolytic reduction, and the nano silicon-carbon composite material for lithium ion batteries is prepared at the cathode.
Owner:CHINA AUTOMOTIVE BATTERY RES INST CO LTD
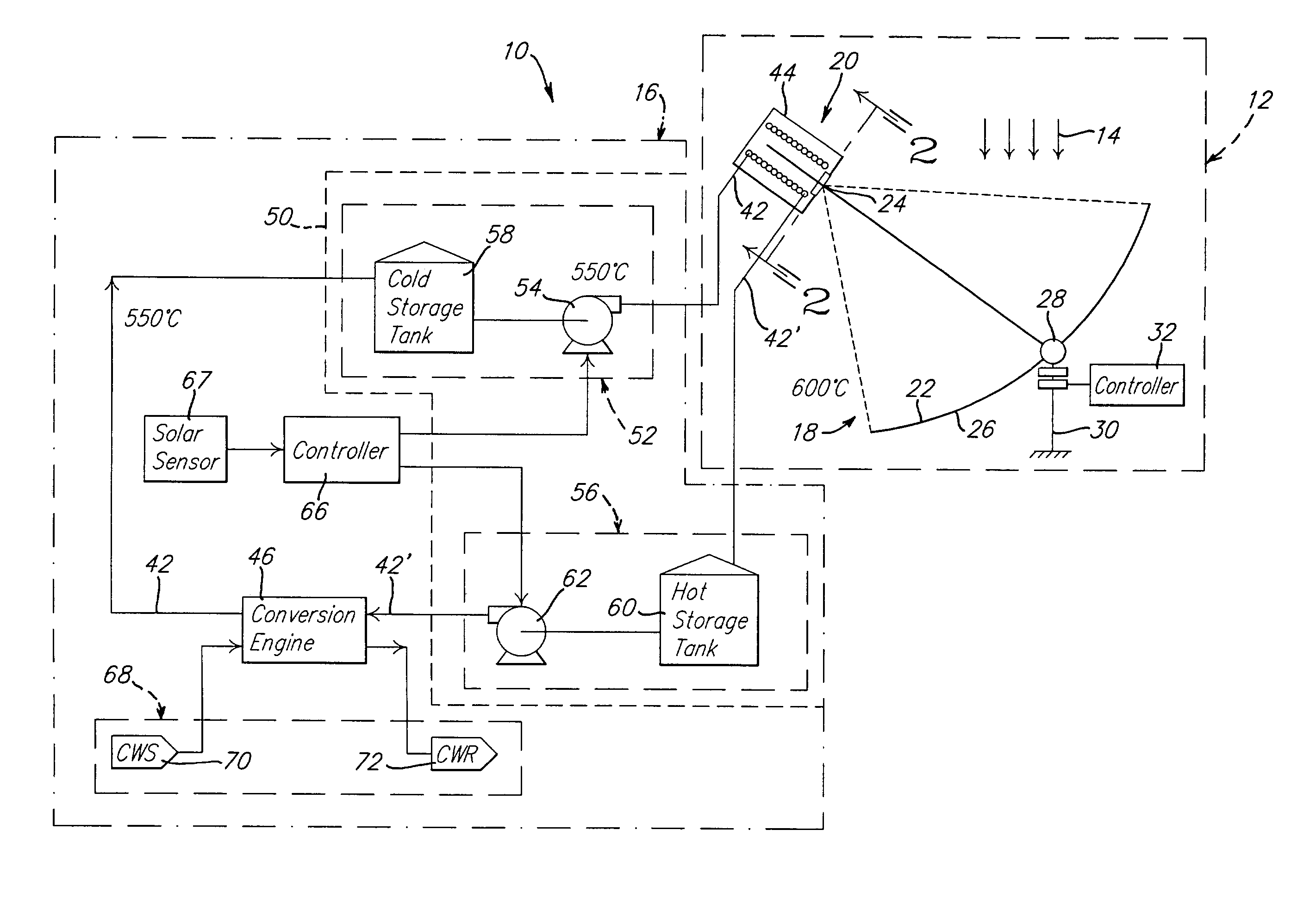
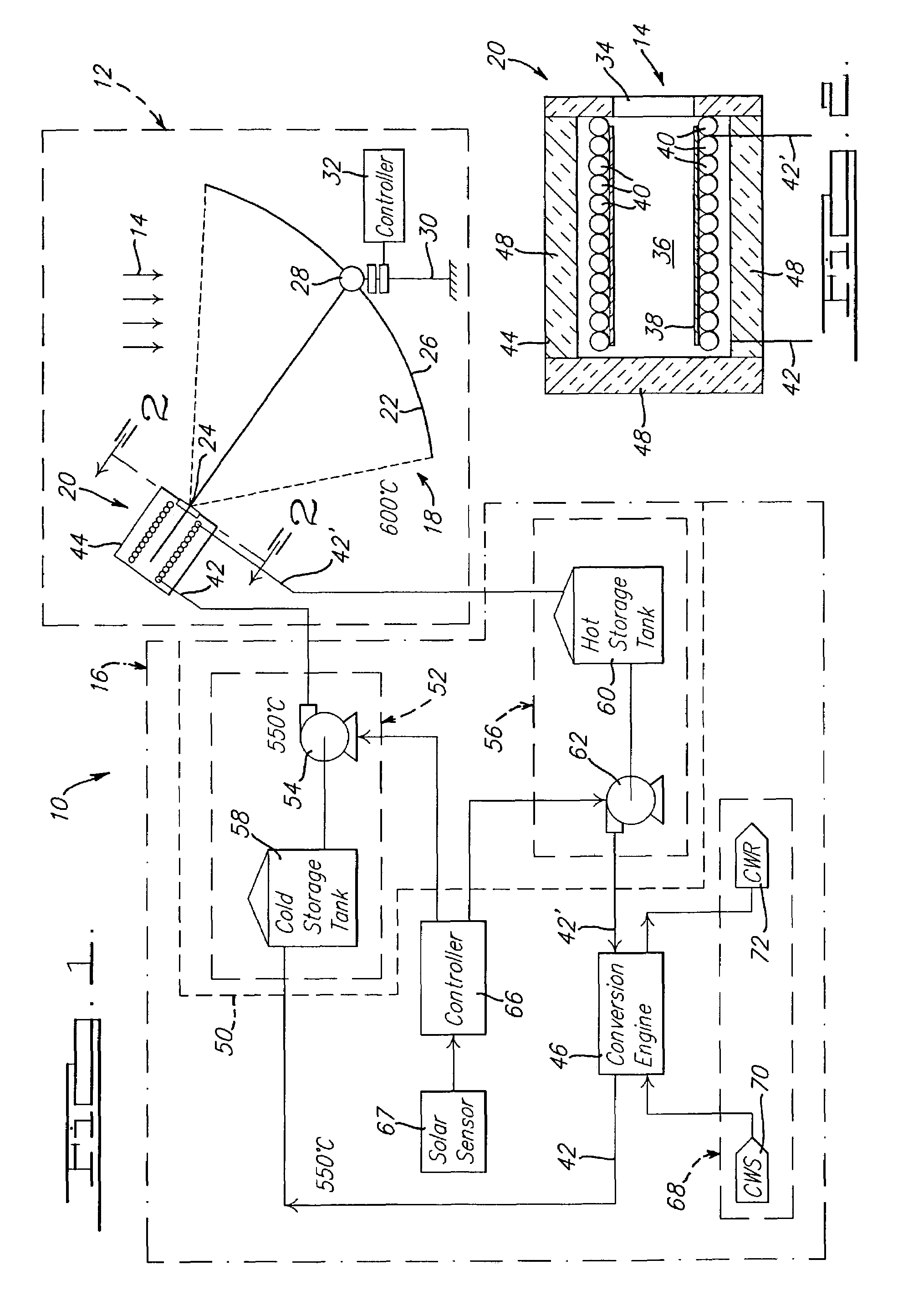
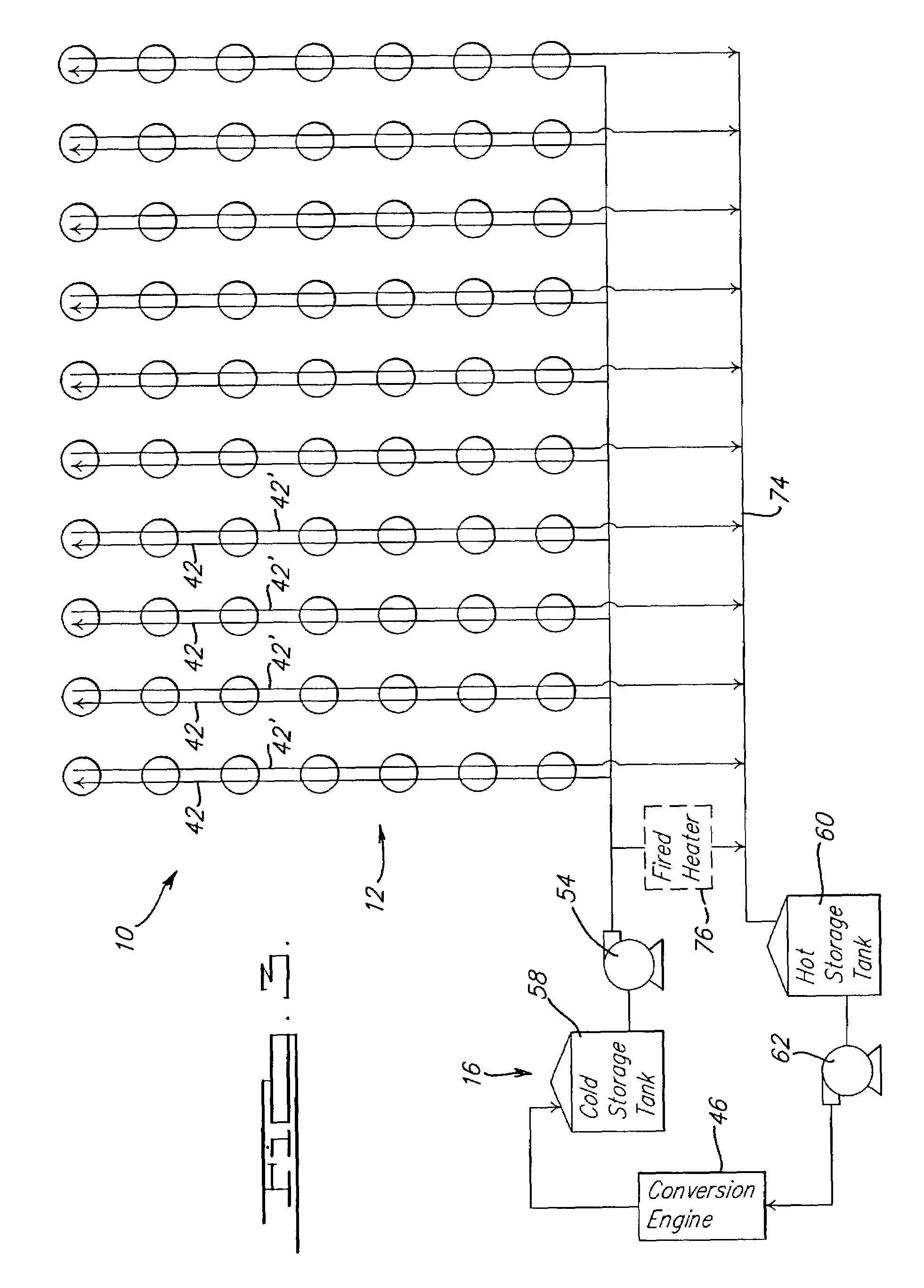
Solar dish concentrator with a molten salt receiver incorporating thermal energy storage
A solar power system capable of storing heat energy and converting sun light to electrical power. The solar power system includes a solar collection system which gathers and transmits concentrated solar energy to an absorber / cavity. The thermal energy is extracted from the absorber / cavity via a fluid and transported to a heat conversion system. The heat conversion system uses the thermal energy to create electricity.
Owner:SOLARRESERVE TECH +1
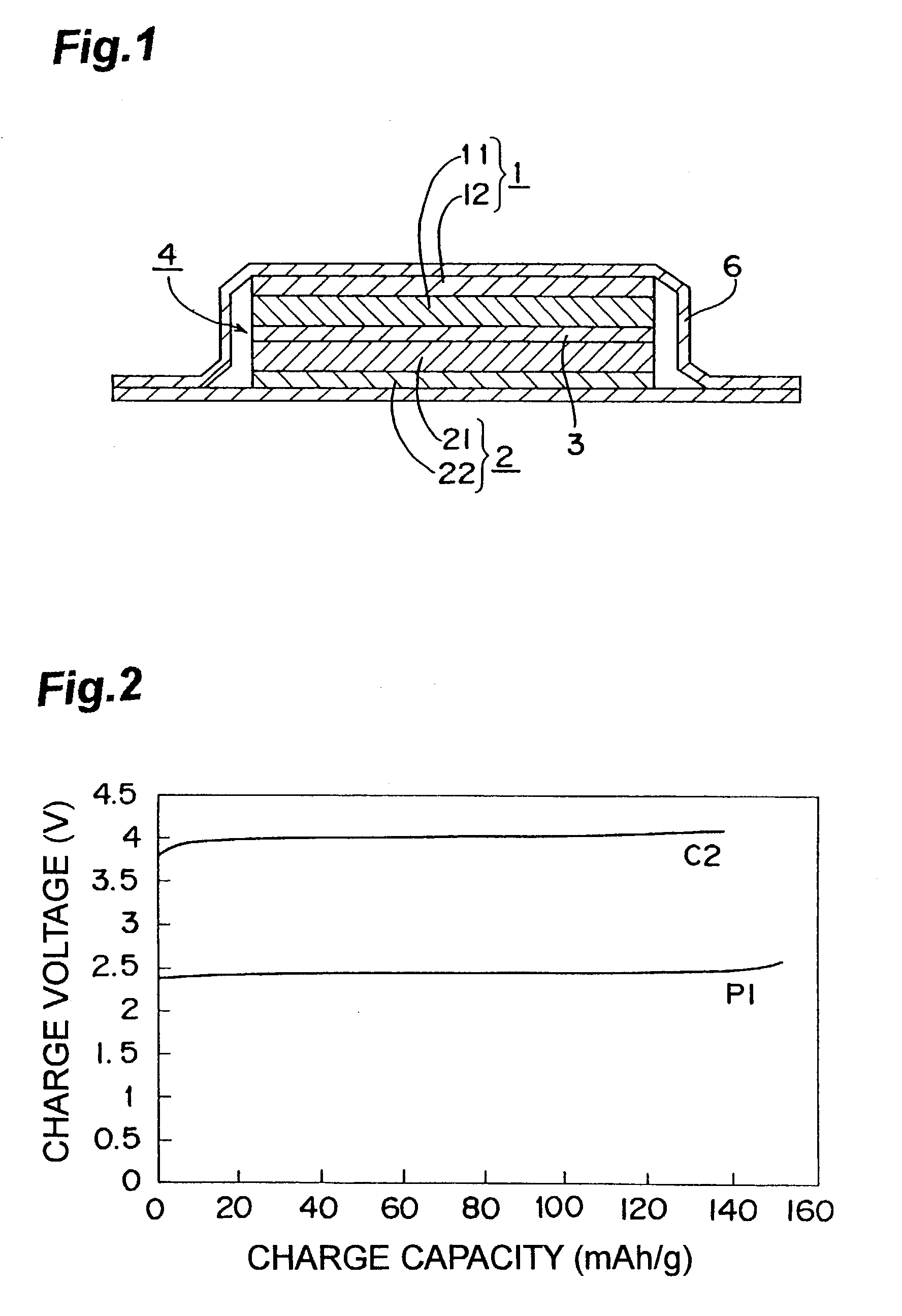
Nonaqueous electrolyte lithium secondary cell
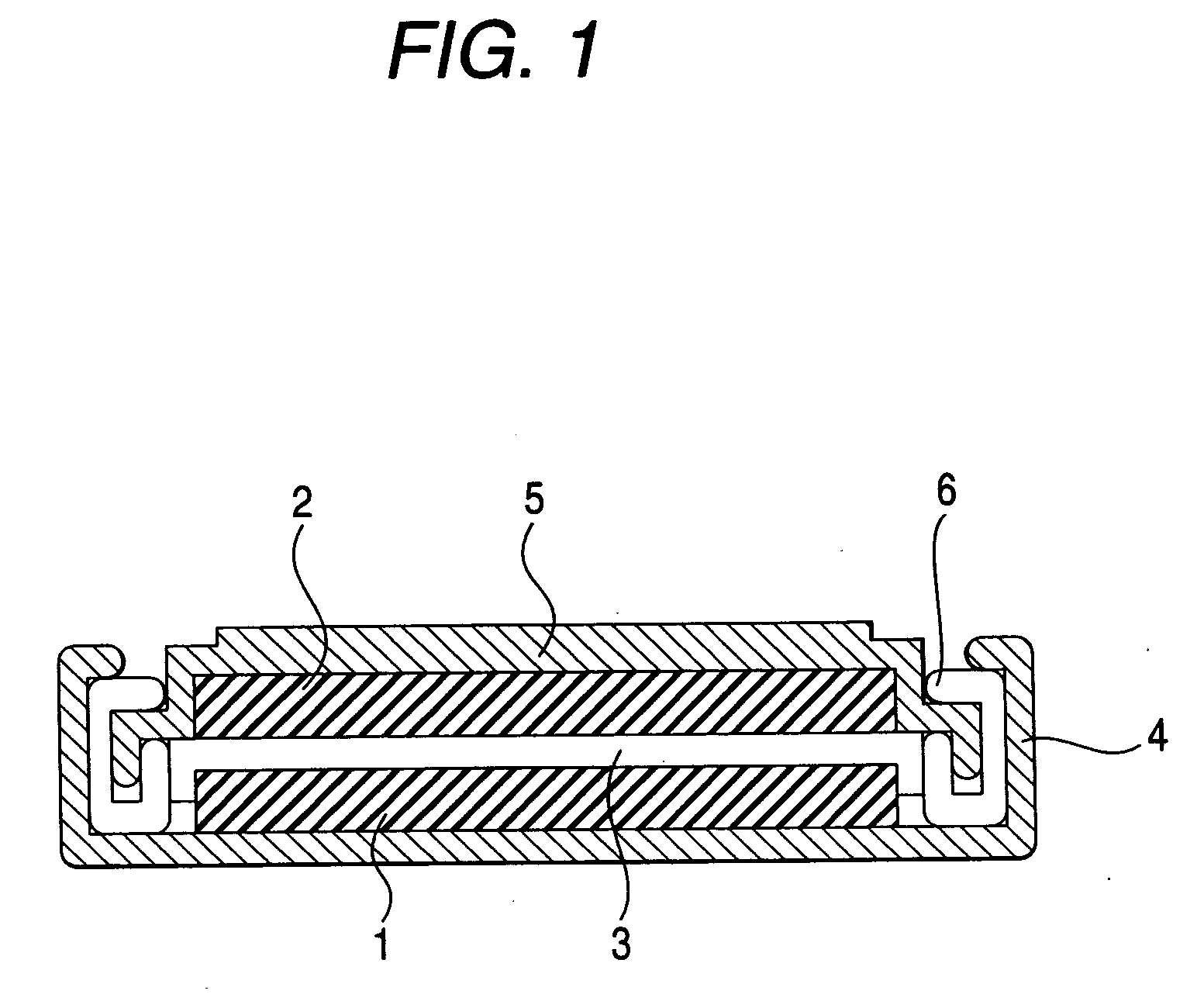
InactiveUS7029793B2Improve securityImprove battery performanceSolid electrolyte cellsTwo electrolyte cellsMetallic lithiumMolten salt
A nonaqueous electrolyte lithium secondary cell comprising a positive electrode (1), a negative electrode (2) and a nonaqueous electrolyte containing a lithium salt is characterized by that the nonaqueous electrolyte contains a room temperature molten salt as a main component, a material wherein a working potential of the negative electrode (2) is nobler by above 1V than a potential of a metallic lithium is used for a negative active material of the negative electrode. This nonaqueous electrolyte lithium secondary cell has excellent safety and cell performance.
Owner:GS YUASA INT LTD +1
Method and system for combing solar energy thermal power generation with biomass power generation
ActiveCN101876299AReduce dosageReduce procurement costsSteam generation heating methodsFrom solar energyThermal energyCounter flow
The invention relates to a method and a system for combining solar energy thermal power generation with biomass power generation. When the method is used for generating electricity in the daytime (a fine day), one path of heat transfer oil heated in a heat collection field passes through a heat exchanger to ensure that counter-flow water is heated up into superheated steam at the temperature of between 360 and 380 DEG C; and the other path of the heat transfer oil heated in the heat collection field passes through the heat exchanger to heat up a fused salt so as to perform energy storage. The water heated in a water cooled wall of a biomass boiler is changed into steam which enters a steam header and a steam-water separator, then is heated to the temperature of between 535 and 545 DDEG C after being sent into a super-heater of the boiler together with the steam at the temperature of between 360 and 380 DEG C, and then is supplied to a steam turbine to drive a generator to finish the power generation process. During receiving electric valley adjustment, the biomass boiler maintains the minimum stable combustion state. The self power generation of the method and the system can be used for supplying electricity to an electric heater in a fused salt heat storage system to heat the fused salt and perform secondary energy storage for a fused salt heat tank. During the night, or when a solar energy condition is not good, the heat energy stored in the fused salt is released by the heat exchanger to generate electricity.
Owner:北京京仪集团有限公司
Electrochemical synthesis of ammonia
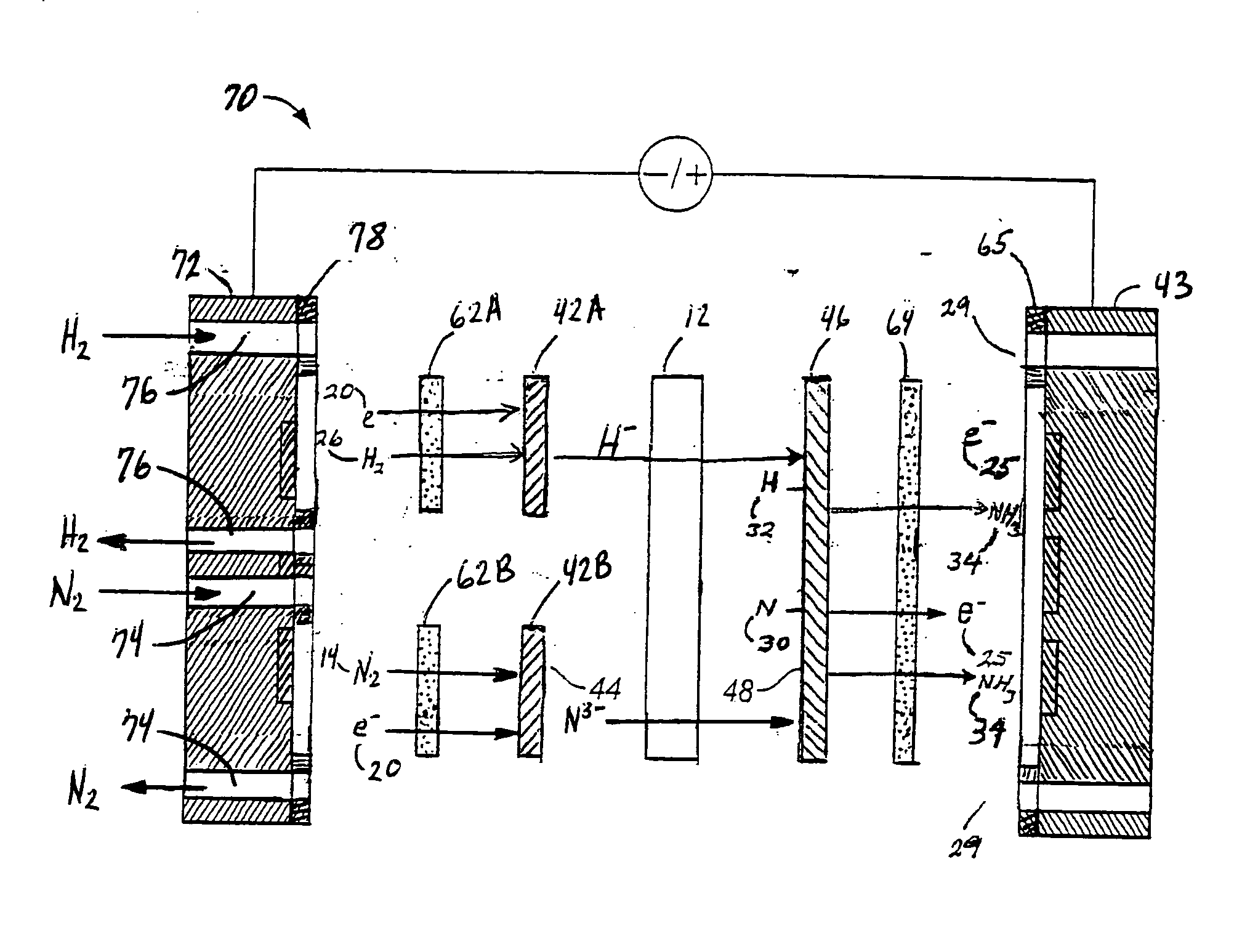
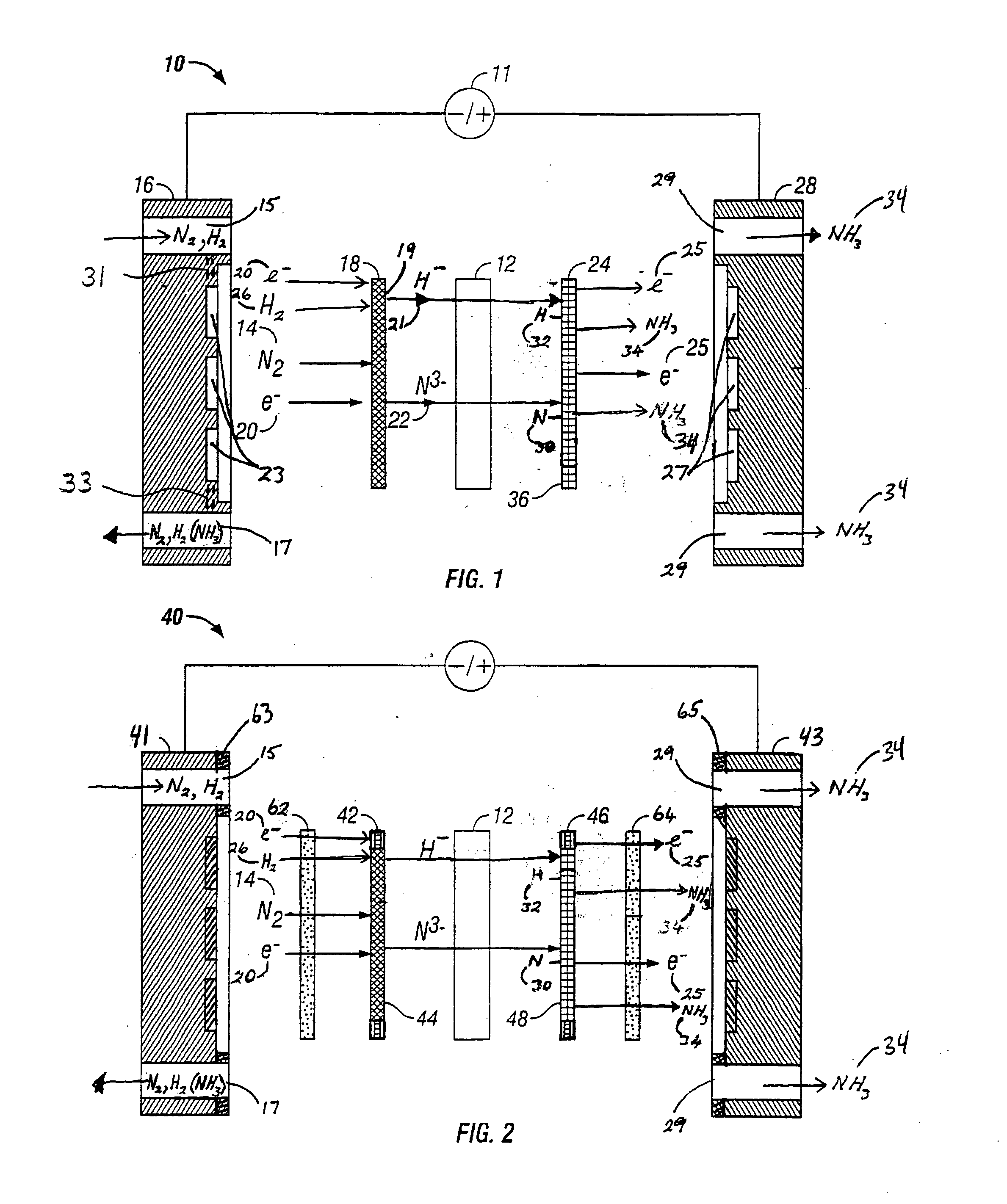
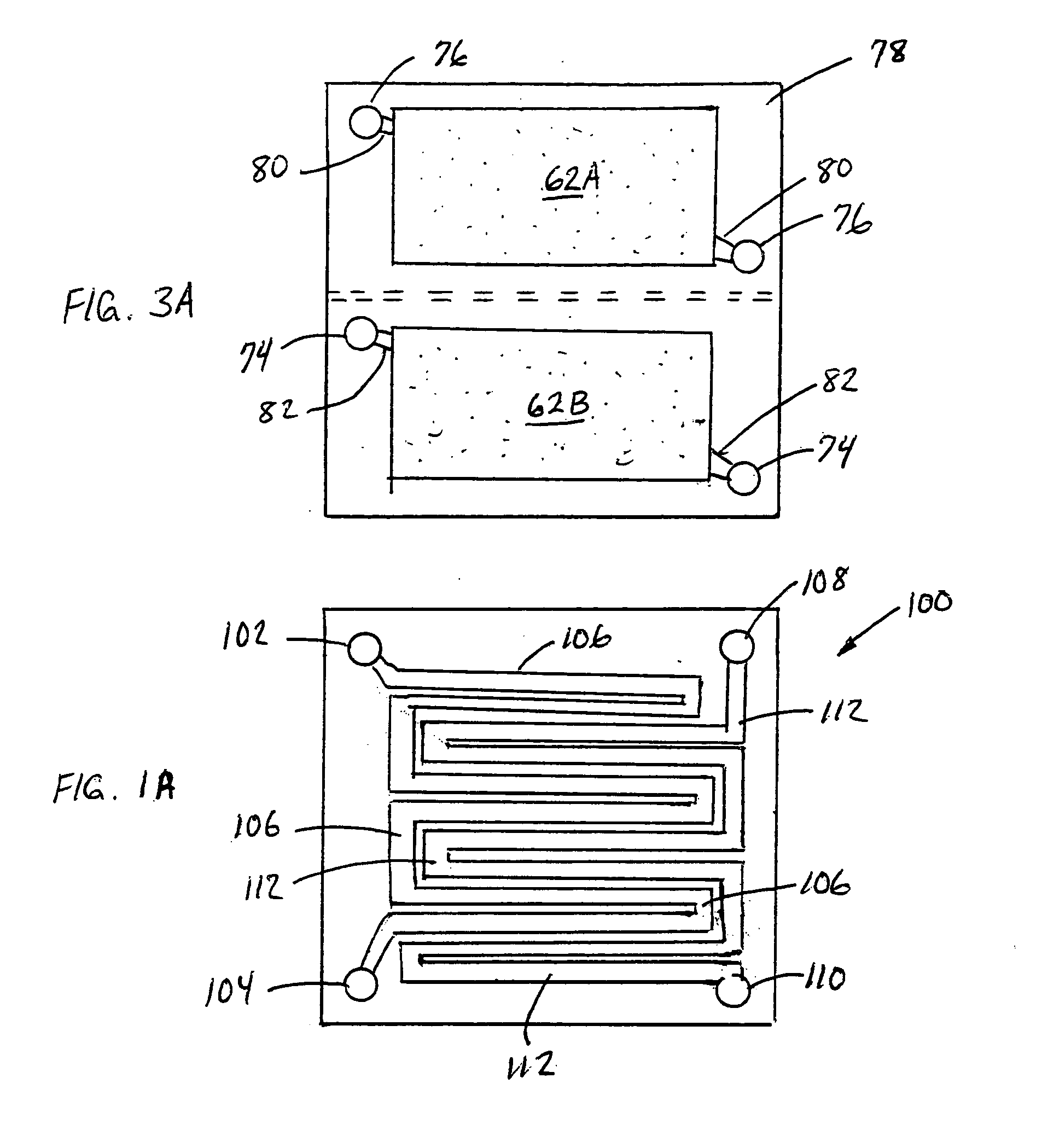
A method for the anodic electrochemical synthesis of ammonia gas. The method comprises providing an electrolyte between an anode and a cathode, providing nitrogen and hydrogen gases to the cathode, oxidizing negatively charged nitrogen-containing species and negatively charged hydrogen-containing species present in the electrolyte at the anode to form adsorbed nitrogen species and adsorbed hydrogen species, respectively, and reacting the adsorbed nitrogen species with the adsorbed hydrogen species to form ammonia. Nitrogen and hydrogen gases may be provided through a porous cathode substrate. The negatively charged nitrogen-containing species in the electrolyte may be produced by reducing nitrogen gas at the cathode and / or by supplying a nitrogen-containing salt, such as lithium nitride, into the molten salt electrolyte. Similarly, the negatively charged hydrogen-containing species in the electrolyte may be produced by reducing hydrogen gas at the cathode and / or by supplying a hydrogen-containing salt, such as lithium hydride, into the molten salt electrolyte.
Owner:LYNTECH INC
Alumina carrier and preparation method thereof
The invention discloses an alumina carrier and a preparation method thereof. In the alumina carrier, aluminum hydroxide gel prepared by a molten salt supersolubilizing micelle method is taken as a raw material. After a surfactant and a hydrocarbon component contained in the gel are molded and baked, nano-alumina particles formed by removing water from the polymerized aluminum hydroxide still have a basic rod-shaped structure and are piled up in an unordered way so as to form a frame structure. The alumina carrier has the characteristics of large pore volume, large pore diameter, high porosity, large orifice on the outer surface and high pore canal penetrability. In particular for macromolecules, the alumina carrier prevents catalyst inactivation caused by the blocking of an ink bottle-shaped orifice, so that impurity deposition is increased, and the running period of a catalyst is prolonged. The alumina carrier can be applied to a catalytic reaction which contains a macromolecule reactant or a macromolecule product.
Owner:CHINA PETROLEUM & CHEM CORP +1
Ambient temperature, rechargeable cells with metal salt-based electrodes and a system of cell component materials for use therein
InactiveUS6187479B1Improve battery performanceImprove performanceLead-acid accumulatorsNon-aqueous electrolyte cellsHalogenRechargeable cell
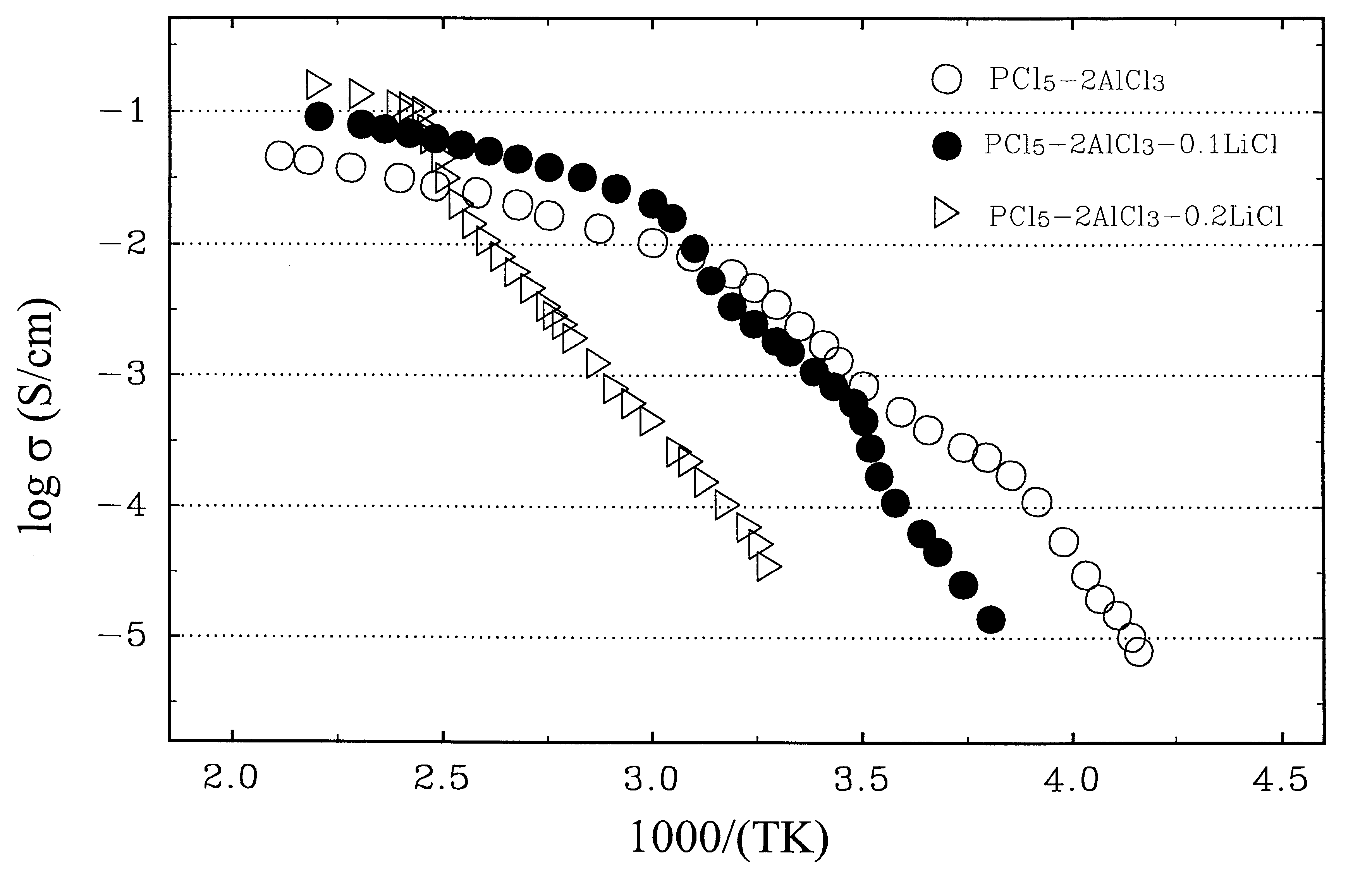
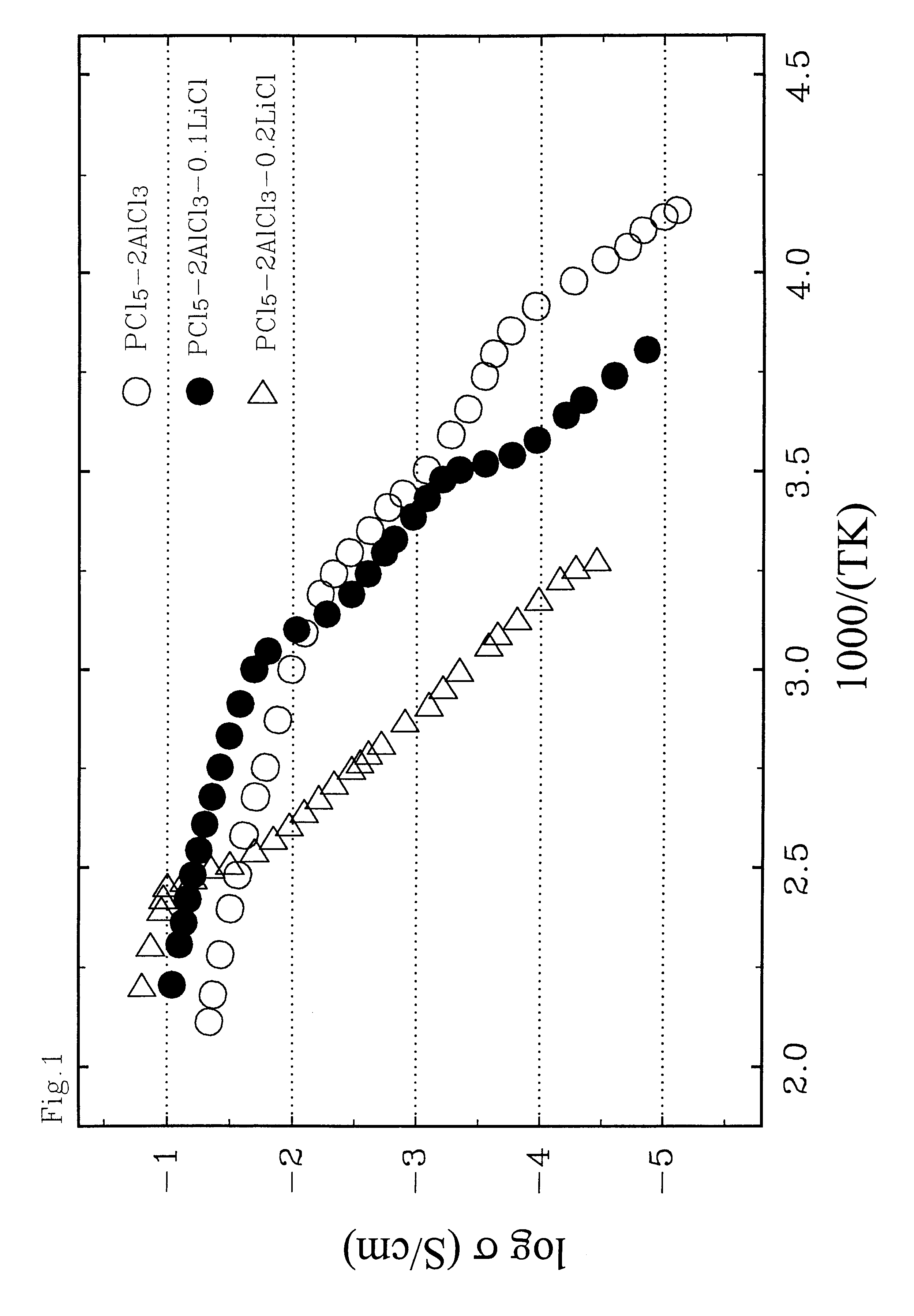
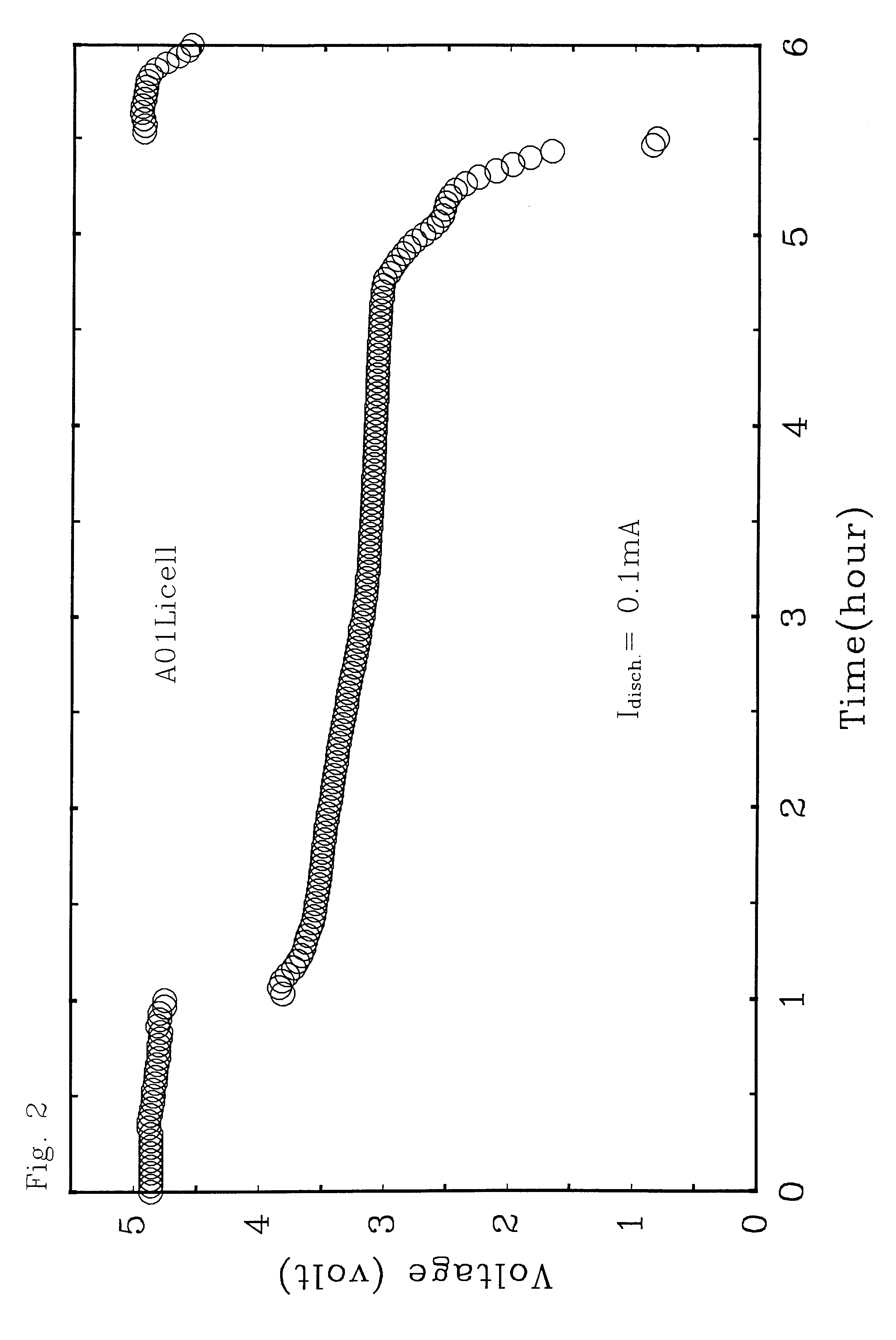
A rechargeable battery or cell is disclosed in which the electrode active material consists of at least one nonmetallic compound or salt of the electropositive species on which the cell is based, and the electrolyte or electrolyte solvent consists predominantly of a halogen-bearing or chalcogen-bearing covalent compound such as SOCl2 or SO2Cl2. Also disclosed are cell component materials which include electrodes that consist primarily of salts of the cell electropositive species and chemically compatible electrolytes. These latter electrolytes include several newly discovered ambient temperature molten salt systems based on the AlCl3-PCl5 binary and the AlCl3-PCl5-PCl3 ternaries.
Owner:LIU CHANGLE
Durable electrooptic devices comprising ionic liquids
InactiveUS20080266642A1NanoopticsTenebresent compositionsElectrical conductorQuaternary ammonium cation
Electrolyte solutions for electrochromic devices such as rear view mirrors and displays with low leakage currents are prepared using inexpensive, low conductivity conductors. Preferred electrolytes include bifunctional redox dyes and molten salt solvents with enhanced stability toward ultraviolet radiation. The solvents include lithium or quaternary ammonium cations, and perfluorinated sulfonylimide anions selected from trifluoromethylsulfonate (CF3SO3−), bis(trifluoromethylsulfonyl)imide ((CF3SO2)2N−), bis(perfluoroethylsulfonyl)imide ((CF3CF2SO2)2N−) and tris(trifluoromethylsulfonyl)methide ((CF3SO2)3C−). Electroluminescent, electrochromic and photoelectrochromic devices with nanostructured electrodes include ionic liquids with bifunctional redox dyes. Some of the electrolyte solutions color to red when devices employing the solutions are powered, leading to red or neutral electrooptic devices.
Owner:TRIAD NAT SECURITY LLC +2
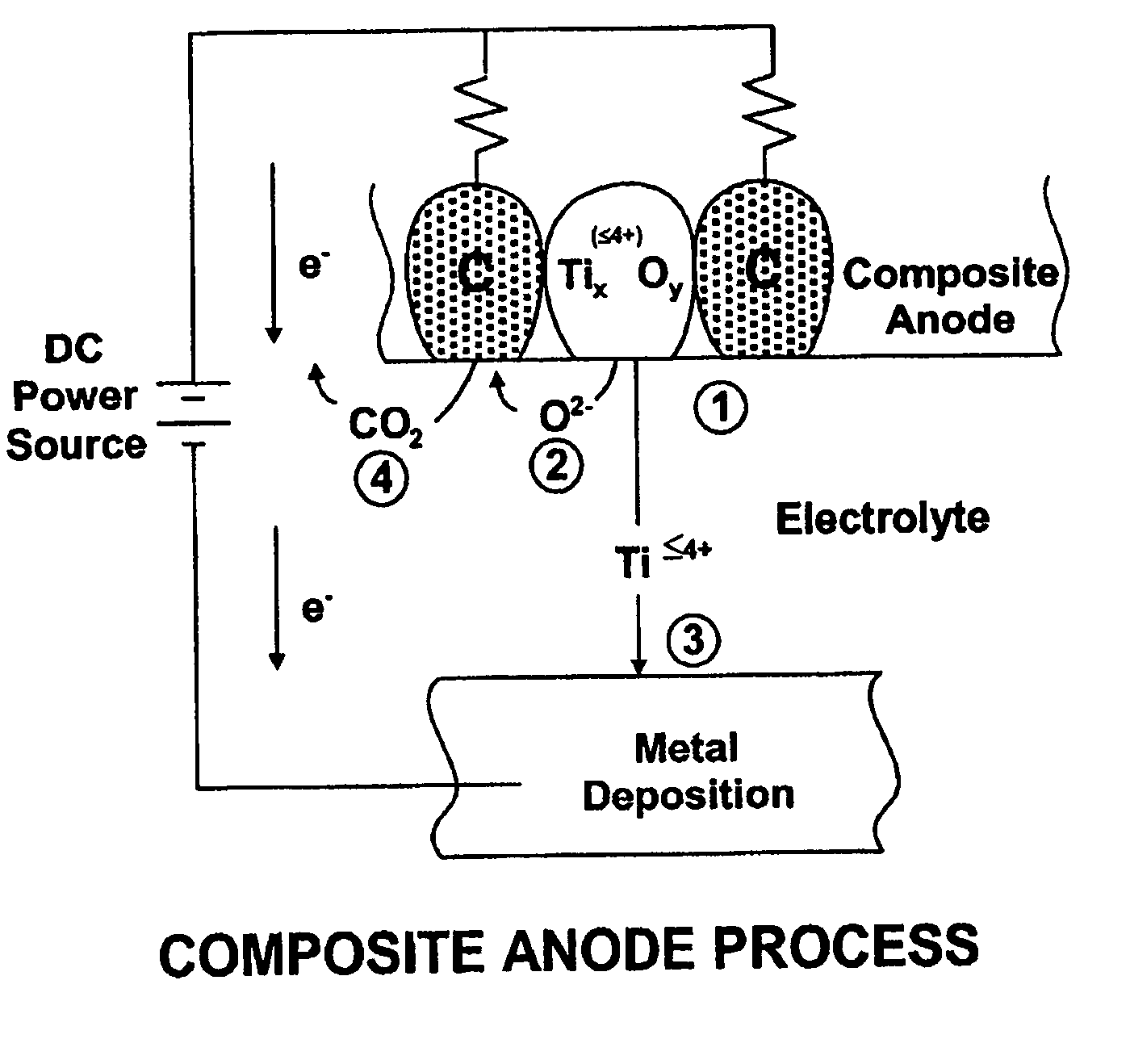
Thermal and electrochemical process for metal production
A system for purification of high value metals comprises an electrolytic cell in which an anode formed of a composite of a metal oxide of the metal of interest with carbon is electrochemically reduced in a molten salt electrolyte.
Owner:ATS MER LLC
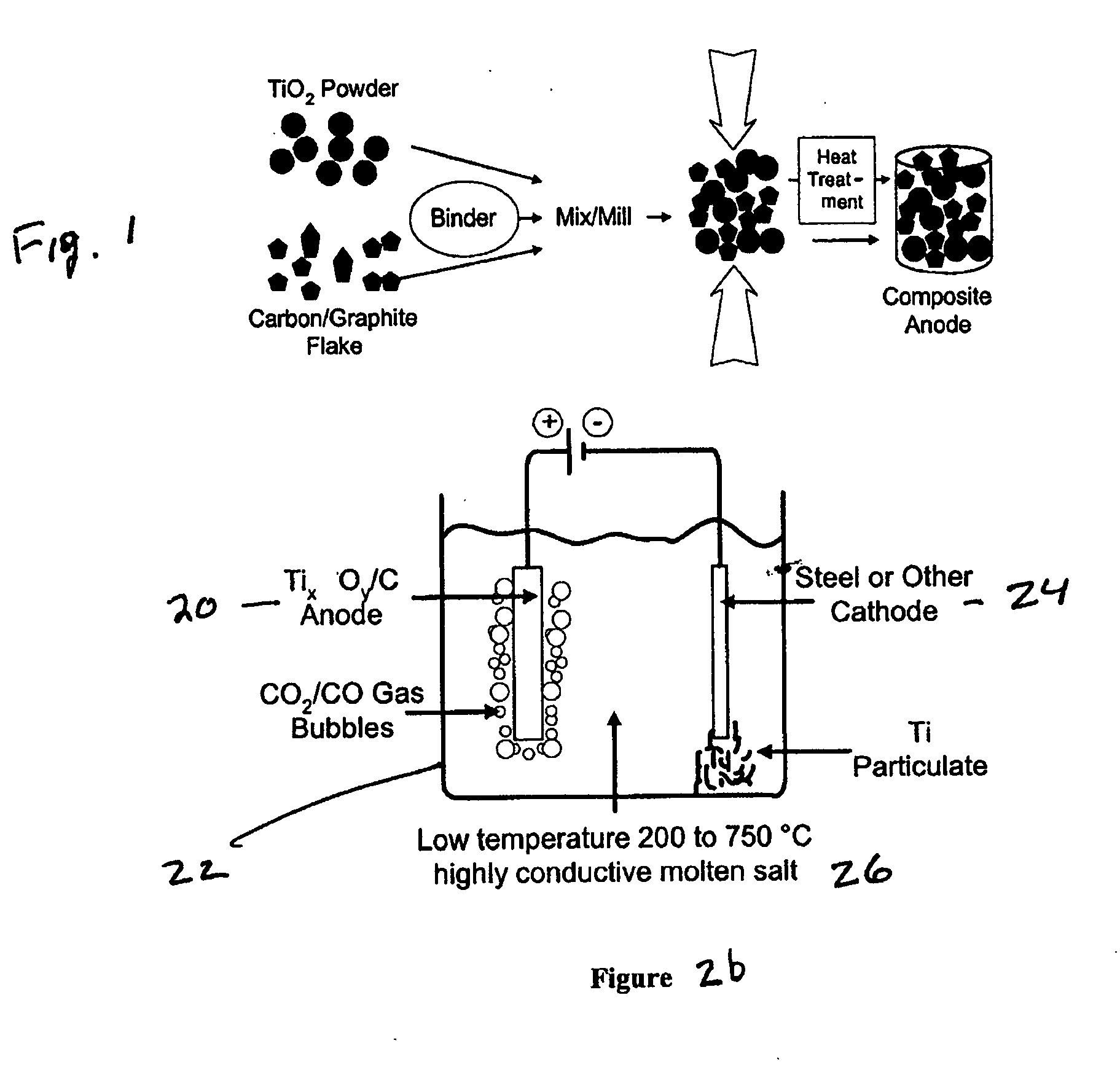
Pure titanium production from titanium monoxide/titanium carbide soluble solid anode electrolysis
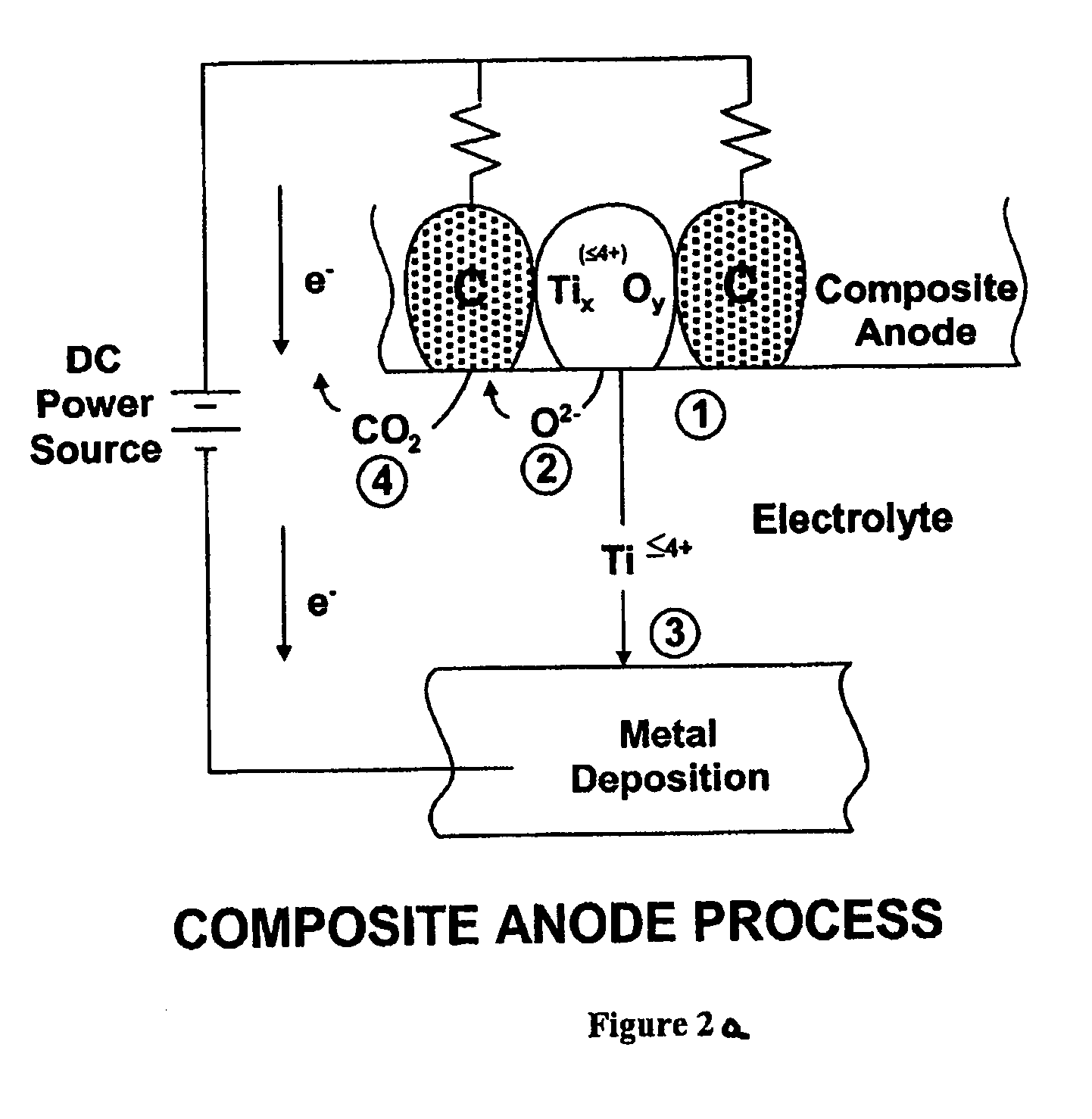
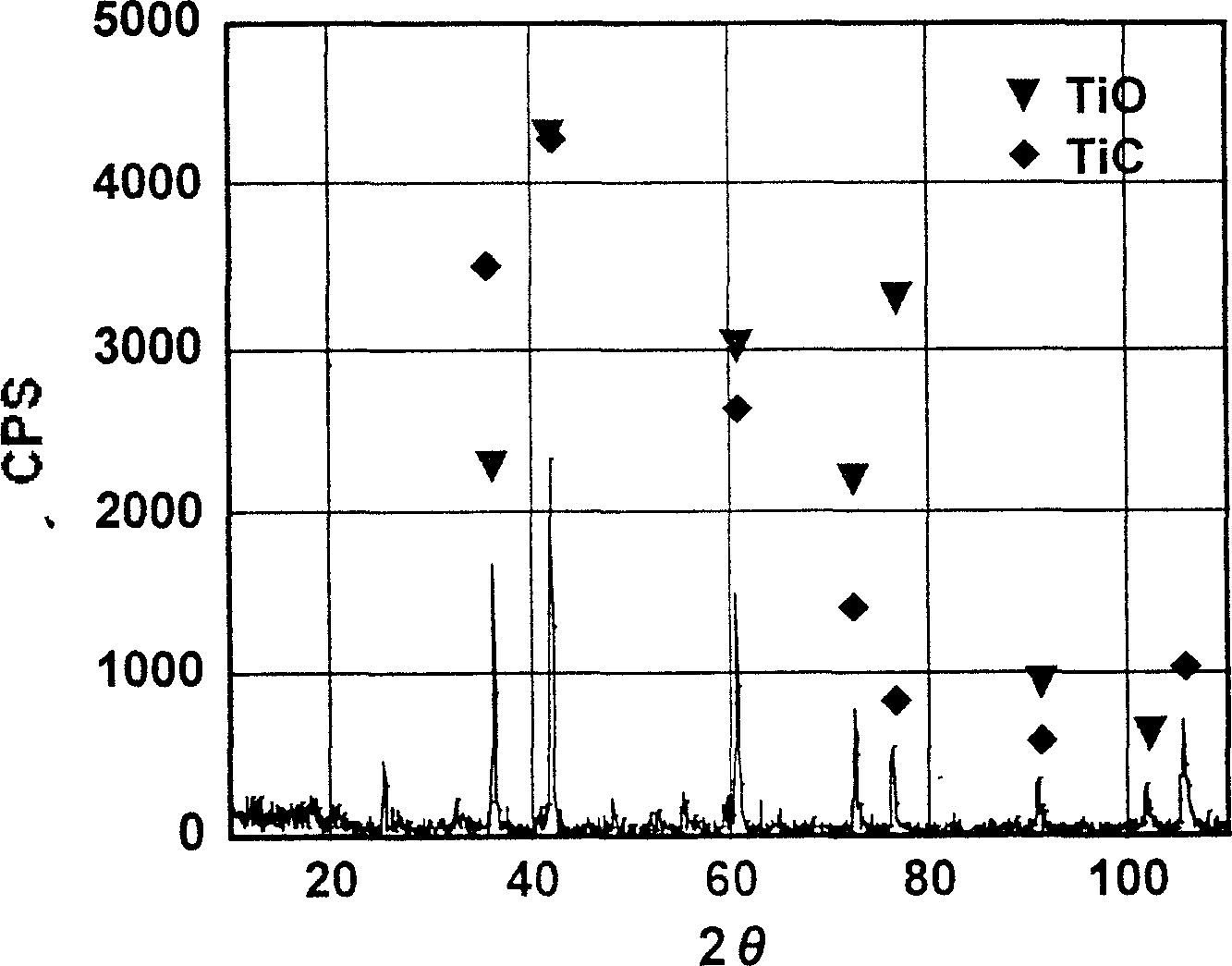
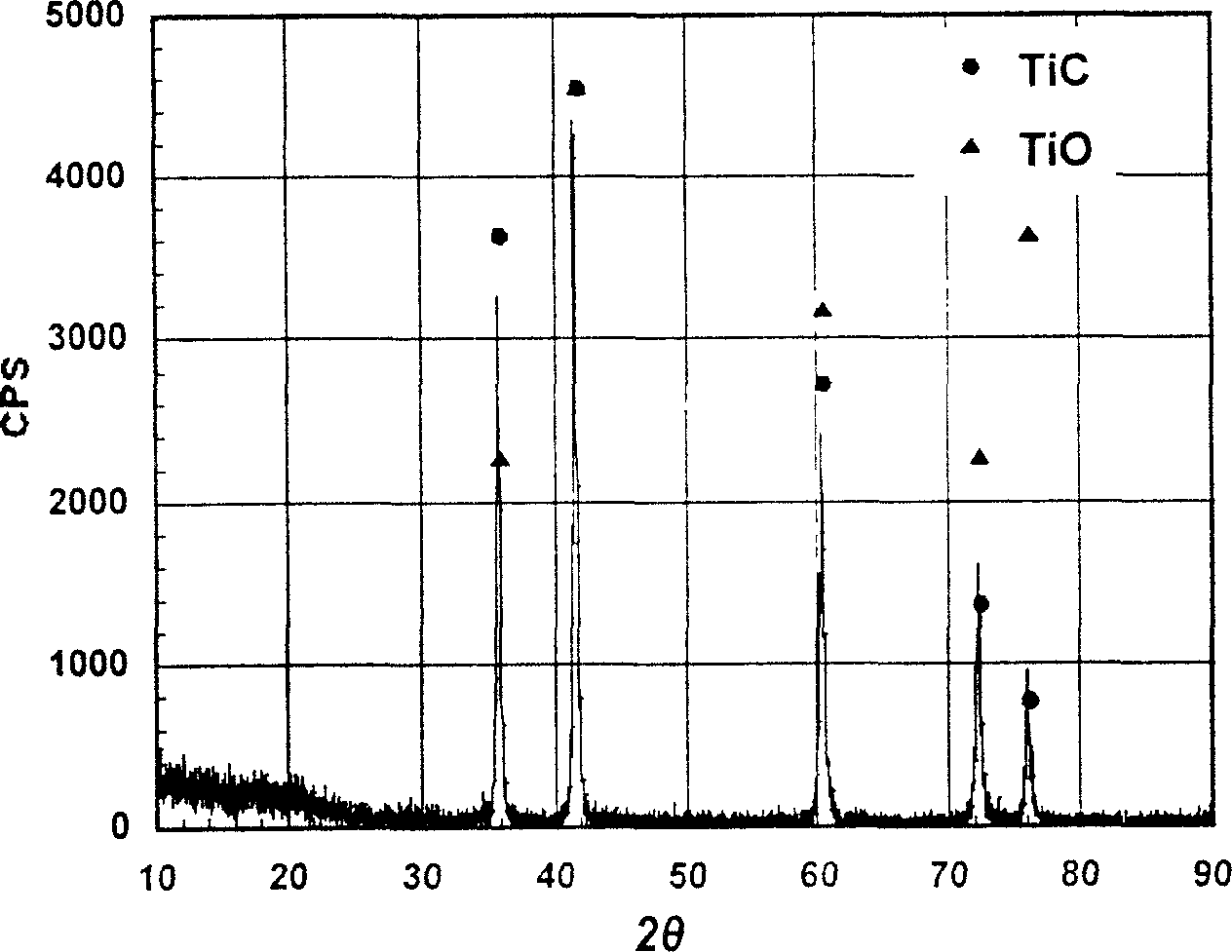
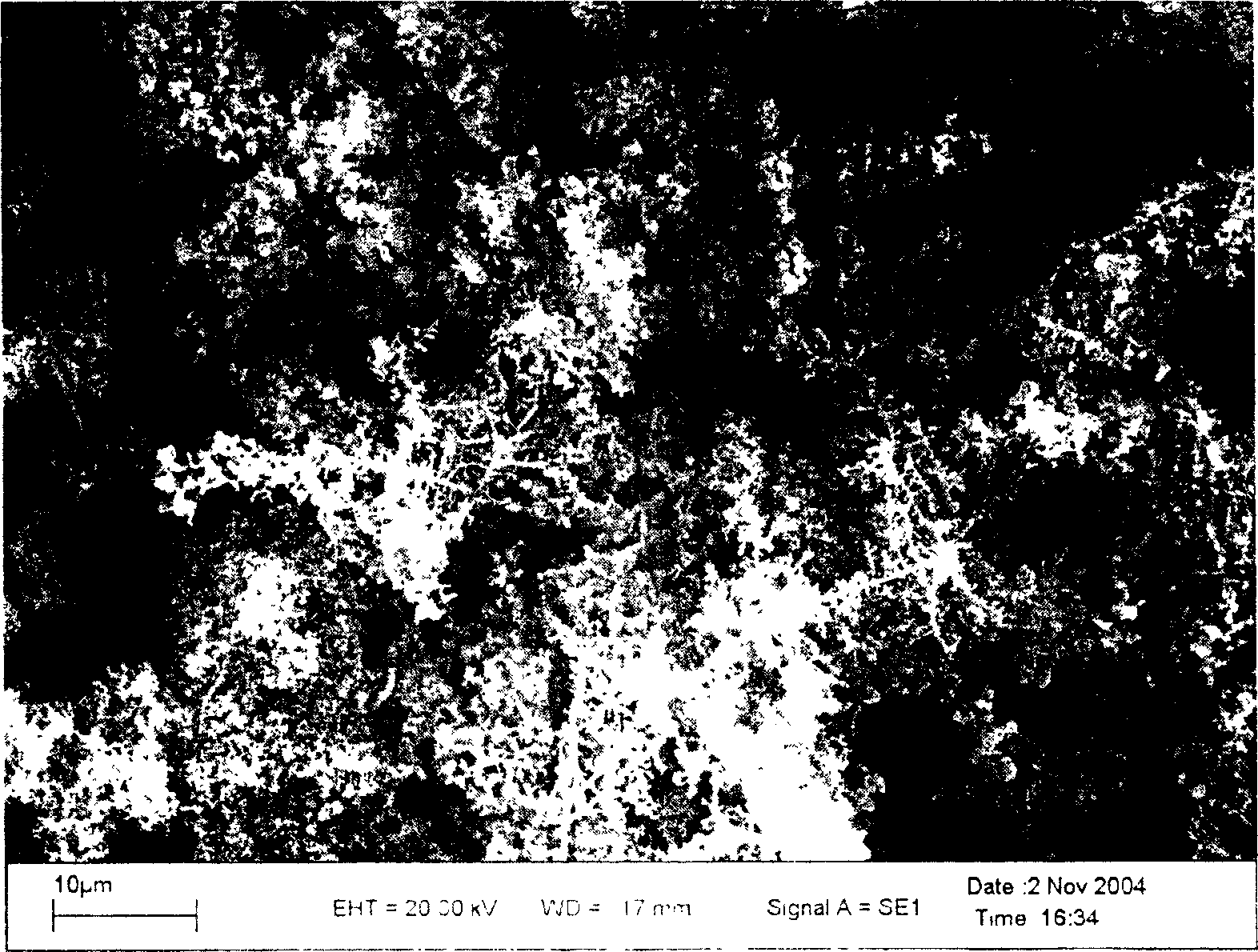
A process of preparing purified Ti by the way of electrolysis directly from sosoloid positive pole, TiO?mTiC. Mix the powder of C and TiO2 or TiC as measurement of chemical reaction. Then press to be certain size to make positive pole, TiO?mTiC in airvoid at 600-1600íµ. Electrolyte is halide fused salt of alkali. Electrolysis at 400-1000íµ. C and O in the positive pole form CO, CO2 and O2. Purified Ti is obtained from negative pole.
Owner:鸿钛(北京)科技有限公司
Strengthened glass articles and methods of making
InactiveCN102149649APerformance fragilityPerformance non-fragileGlass tempering apparatusThin material handlingMolten saltIon exchange
A strengthened glass article having a central tension that is below a threshold value above which the glass exhibits frangible behavior. The central tension varies non-linearly with the thickness of the glass. The glass may be used as a cover plate or window for portable or mobile electronic devices such as cellular phones and the like. The article is strengthened by alkal ion-exchange in a molten salt bath.
Owner:CORNING INC
Nitrate molten salt heat transferring and reserving medium and preparation method and application thereof
InactiveCN102533226AWide operating temperature rangeImprove thermal stabilityHeat-exchange elementsDecompositionInstability
The invention discloses a nitrate molten salt heat transferring and reserving medium and a preparation method and application thereof. The nitrate molten salt heat transferring and reserving medium is prepared with 5 to 40 percent of potassium nitrate, 5 to 25 percent of sodium nitrate, and 10 to 70 percent of calcium nitrate. The melting point of the nitrate molten salt heat transferring and reserving medium can be as low as 120 DEG C, and the upper limit of temperature for use can reach 550 DEG C, the nitrate molten salt heat transferring and reserving medium has a wide temperature scope of application, can work normally at the temperature scope of 180 DEG C to 550 DEG C, and has good thermal stability; the shortcoming of high melting point of binary nitrate molten salt system can be overcome, and the problem of instability caused by easy oxidative decomposition at high temperature of NaNO2 in the Chinese patent 200110027954.1 and ternary nitrate salt system can be solved, and the problems of corrosion and cost increase caused by the existing LiNO3 in the Chinese patent 00111406.9 and the American patent US007588694B1 can also be solved.
Owner:SUN YAT SEN UNIV +1
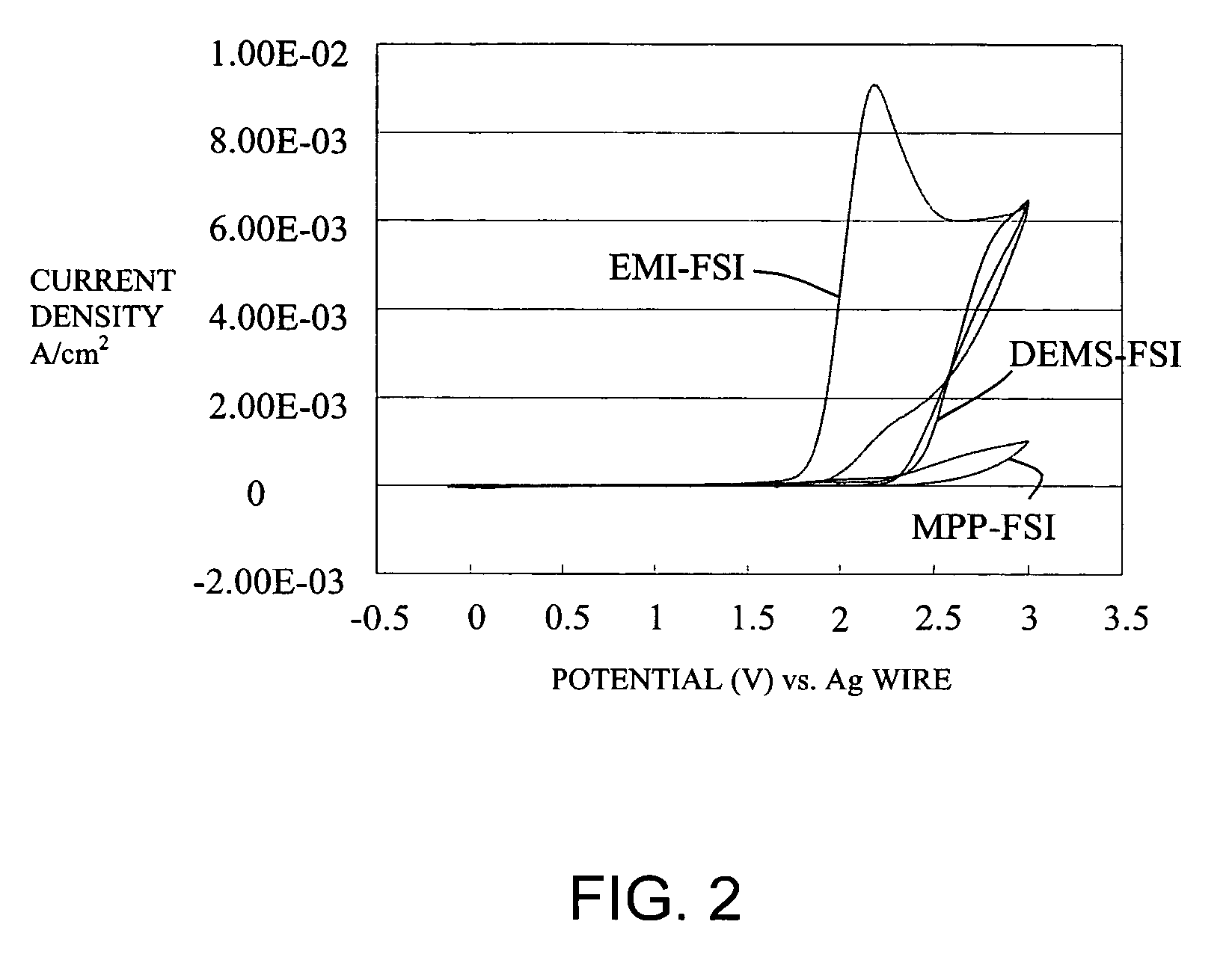
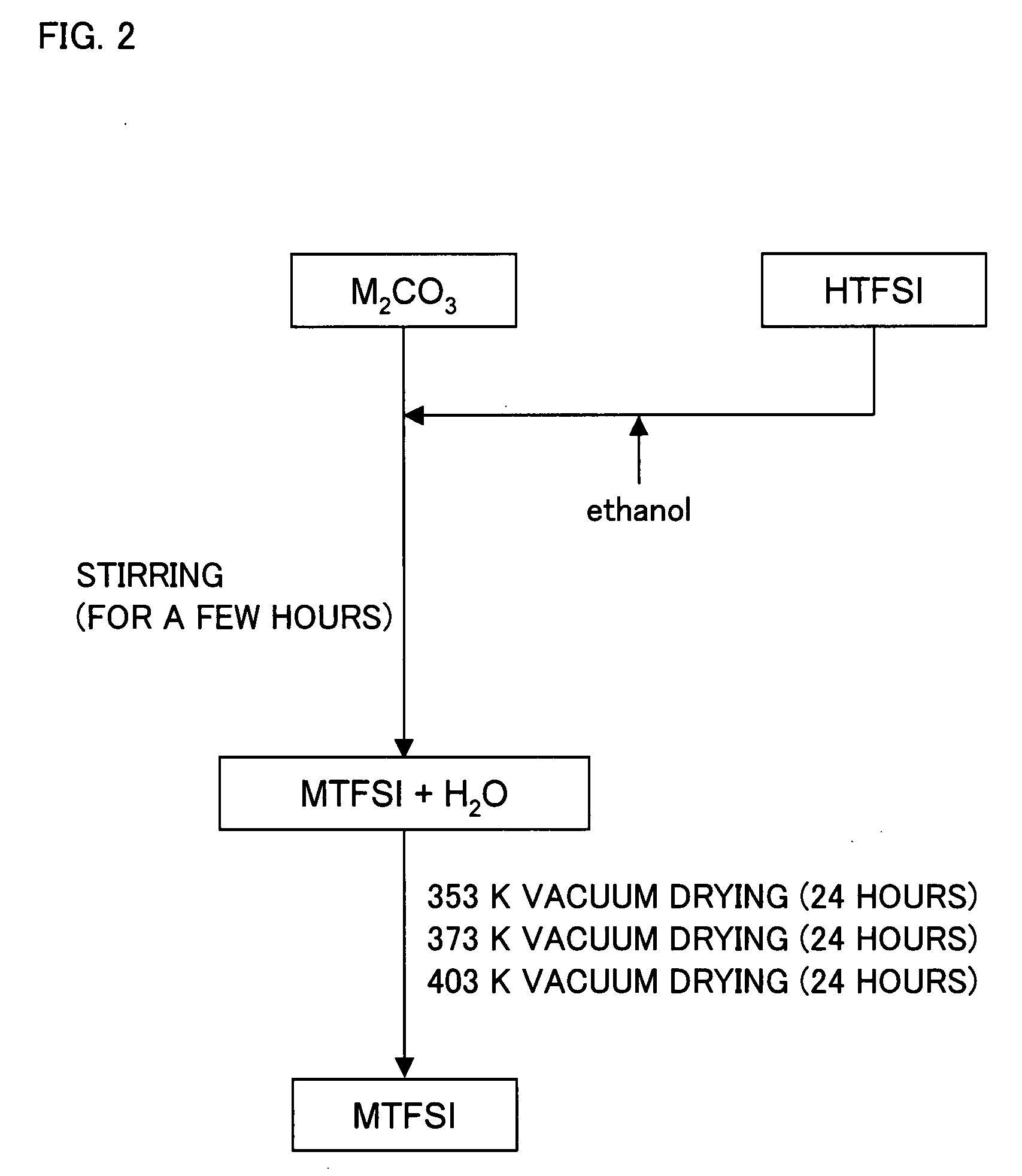
Molten Salt Composition and Use Thereof
ActiveUS20090212743A1High surface finishUniform platingNon-metal conductorsBatteries circuit arrangementsImideMolten salt
A molten salt composition is disclosed containing two or more types of molten salt MTFSI whose anion is an imide anion TFSI and whose cation is an alkali metal M exhibits a lower electrolyte melting point and a wider operating temperature range than a simple salt does. This brings about various advantages such as a wider range of materials that are chosen for use in batteries and the like.
Owner:KYOTO UNIV
Aluminum electrolysis inert anode
InactiveCN103757661AAvoid consumptionWith energy saving and emission reductionElectrolysisHigh entropy alloys
The invention provides an aluminum electrolysis inert anode of which the material is a high-entropy alloy. The high-entropy alloy contains 5-10 alloy elements of which the mole ratio can be identical or different; and the atomic percent of each main element is 5-35%. The high-entropy alloy inert anode has the characteristics of favorable high-temperature oxidation resistance, high aluminum electrolysis salt corrosion resistance, high electric conductivity and the like. The high-entropy alloy prepared by the smelting method has the advantages of simple technique and low cost, and is easy for connection. When being used for aluminum electrolysis, the high-entropy alloy can avoid the consumption of the carbon anode and the emission of the CO2 gas, and has the advantages of energy saving and emission reduction.
Owner:FUJIAN UNIV OF TECH
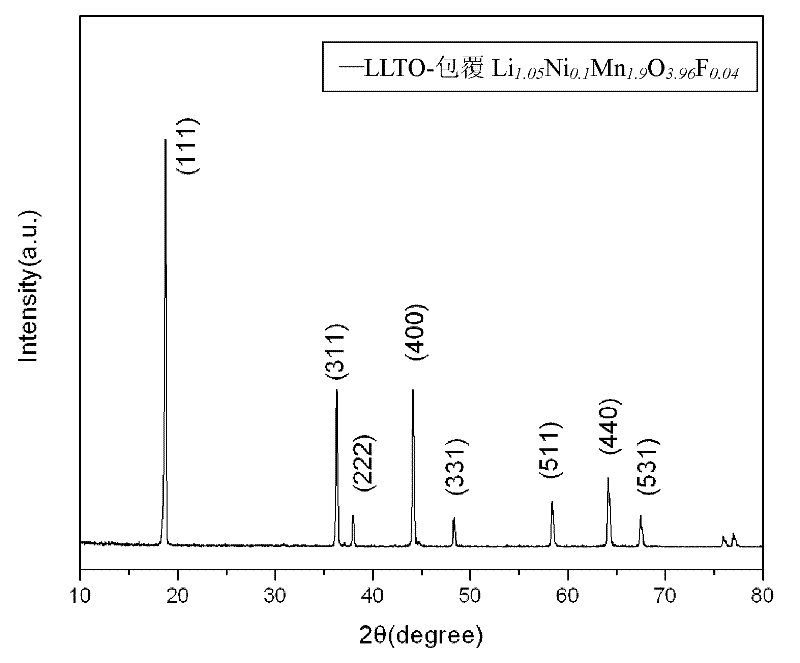
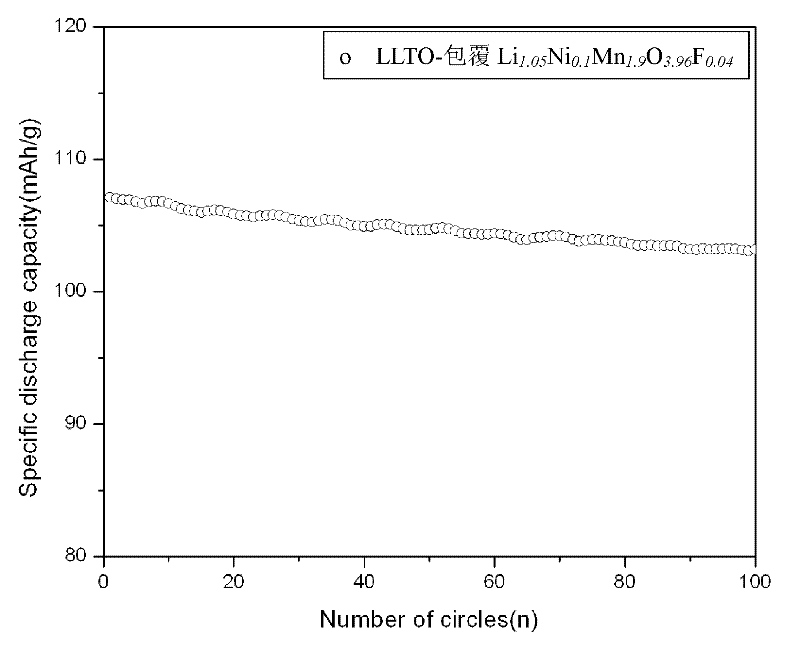
High-temperature manganic acid lithium cathode material and preparation method thereof
ActiveCN102244257AUniform and perfect spinel structureSimple processCell electrodesSolid state electrolytePower battery
The invention discloses a high-temperature manganic acid lithium cathode material and a preparation method thereof. Single crystal particles of the cathode material consists of a matrix and solid electrolyte which clads the surface of the matrix; the matrix is spinel manganic acid lithium which is doped with anions and cations; and the chemical formula of the material is Li1.05MxMn2-xO4-yQy, wherein M represents the doped cations; and Q represents the doped anions. The preparation method comprises the following steps of: firstly, preparing the spinel manganic acid lithium which is doped with the anions and the cations through solid phase reaction; secondly, synthesizing by using a molten-salt growth method to control a crystal form, and preparing the spinel manganic acid lithium having the particle size of 3 to 5 microns and the characteristic of an octahedron single crystal; and finally, cladding the spinel manganic acid lithium by using the solid electrolyte after the crystal form is controlled. The cathode material has high high-temperature performance and high rate cycle performance, and can be applied to electric automobiles or other kinds of lithium ion power batteries.
Owner:淮安新能源材料技术研究院
Method of manufacturing an ion-exchanged glass article
ActiveUS20130061636A1Maintain stable propertiesLittle change in intensityBase layer manufactureMolten saltIon exchange
An ion-exchanged glass article manufacturing method includes an ion-exchange step of bringing a glass article with a composition containing Li into contact with a molten salt dissolved solution containing an alkali metal element having an ionic radius larger than an ionic radius of the Li contained in the glass article, thereby ion-exchanging the Li in the glass article with the alkali metal element in the molten salt dissolved solution. At least one kind of additive selected from the group consisting of NaF, KF, K3AlF6, Na2CO3, NaHCO3, K2CO3, KHCO3, Na2SO4, K2SO4, KAl(SO4)2, Na3PO4, and K3PO4 is added to the molten salt dissolved solution so that the ion-exchange step is carried out while the additive is in a solid state.
Owner:HOYA CORP
Method and Apparatus for a Porous Metal Electrospray Emitter
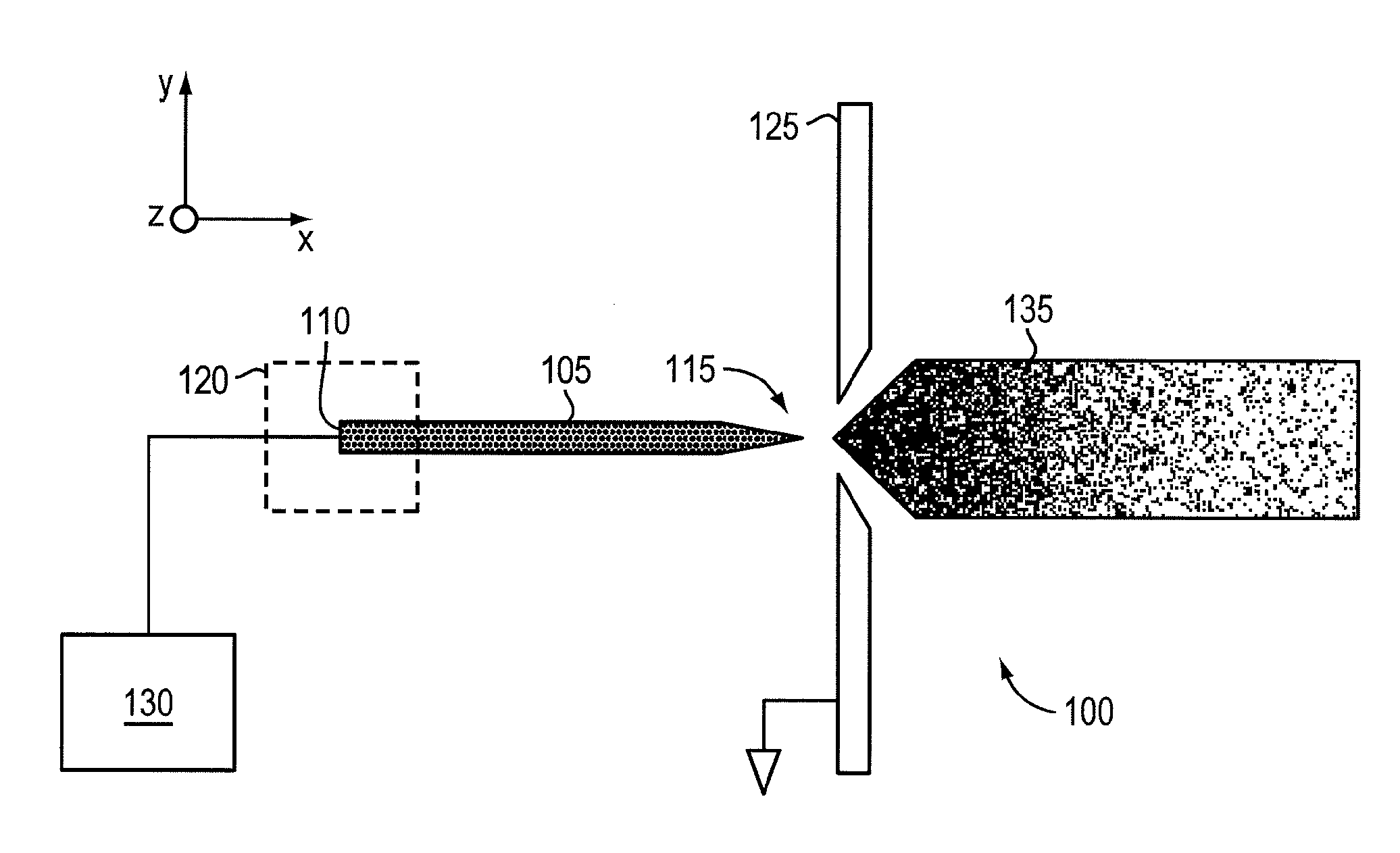
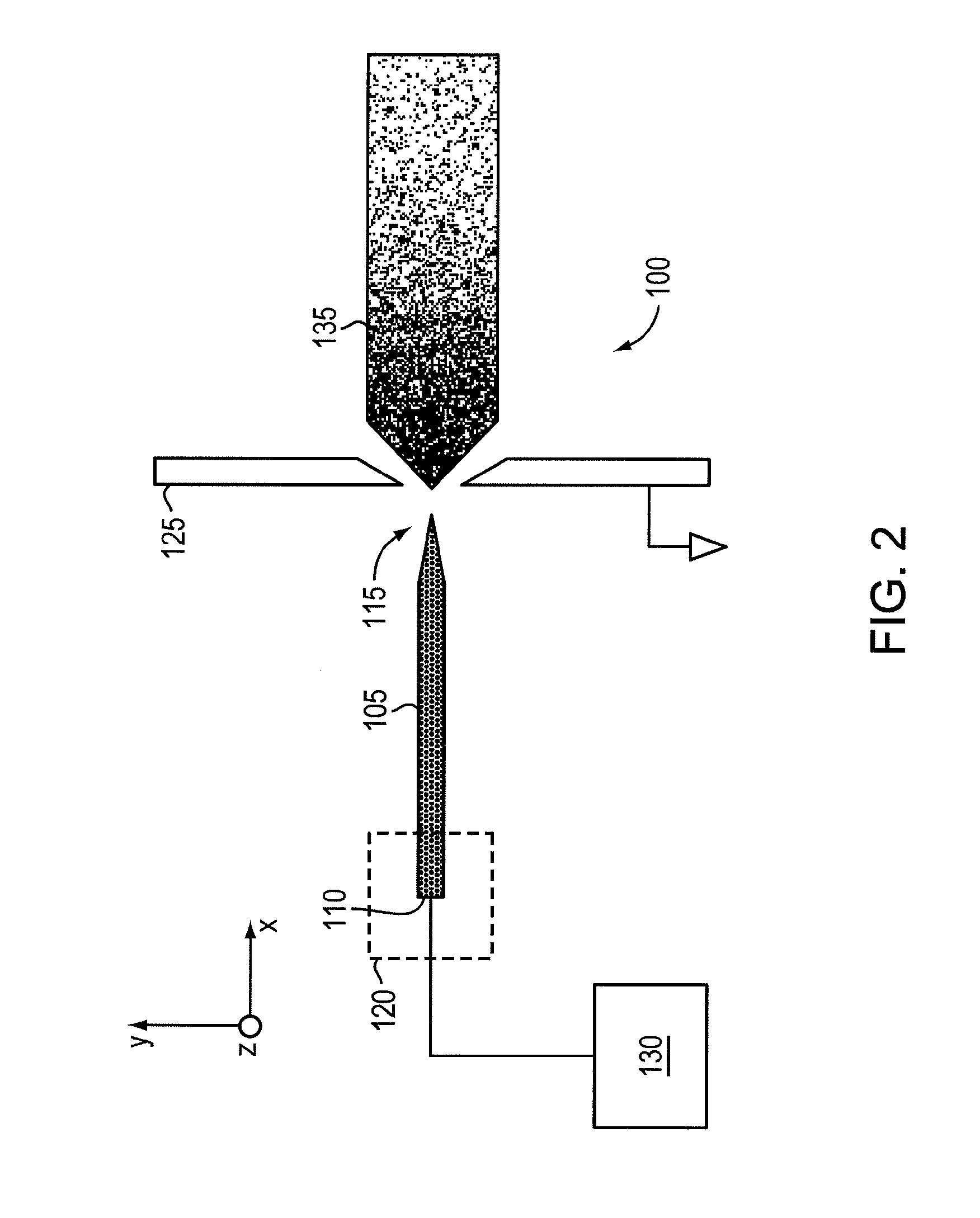
ActiveUS20110210265A1Increased capillary flow capacityReduce complexityElectrolysis componentsParticle separator tubesRoom temperatureMolten salt
An ionic liquid ion source can include a microfabricated body including a base and a tip. The microfabricated body can be formed of a porous metal compatible (e.g., does not react or result in electrochemical decaying or corrosion) with an ionic liquid or a room-temperature molten salt. The microfabricated body can have a pore size gradient that decreases from the base of the body to the tip of the body, so that the ionic liquid can be transported through capillarity from the base to the tip.
Owner:MASSACHUSETTS INST OF TECH
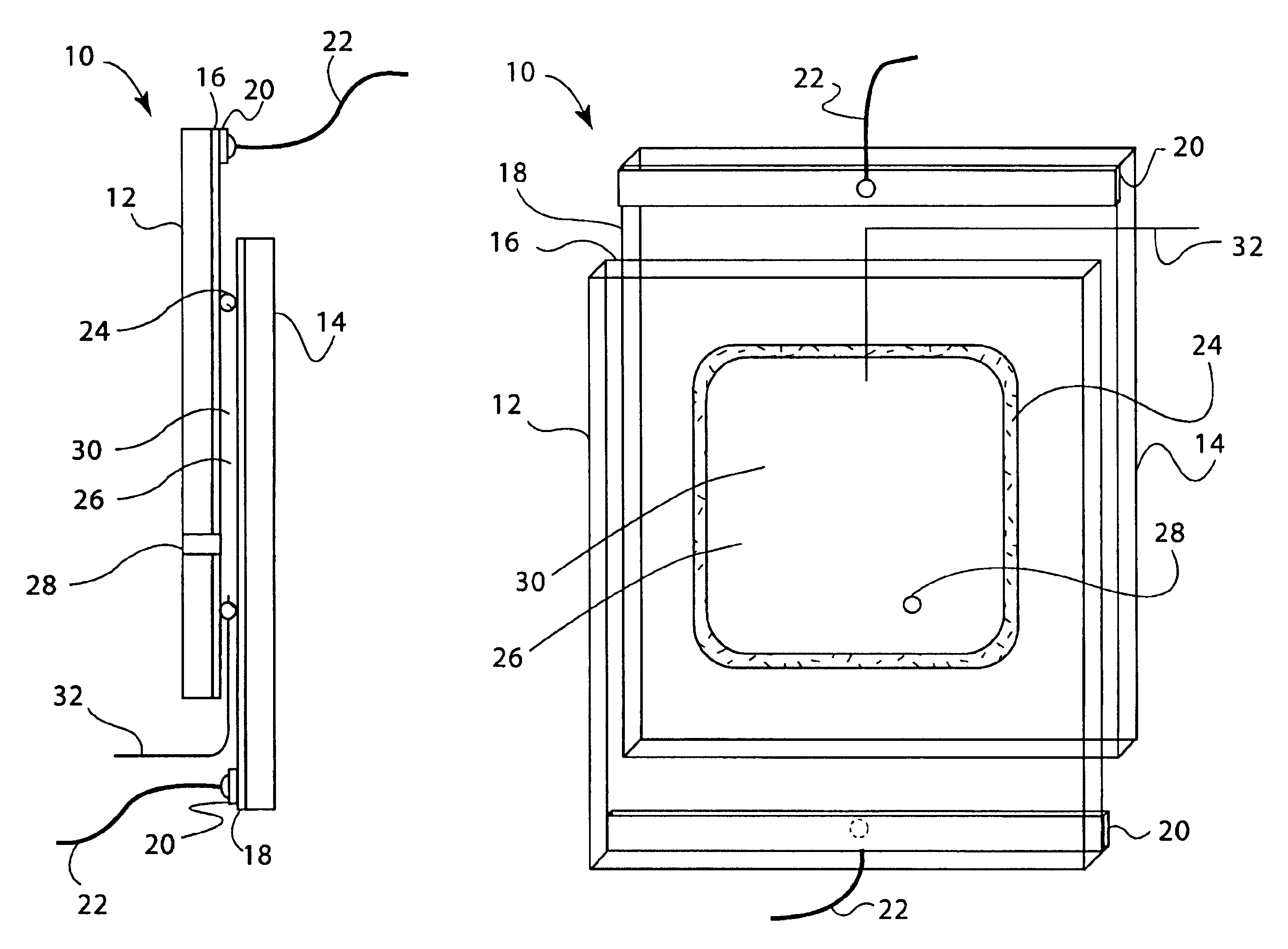
Battery with molten salt electrolyte and phosphorus-containing cathode
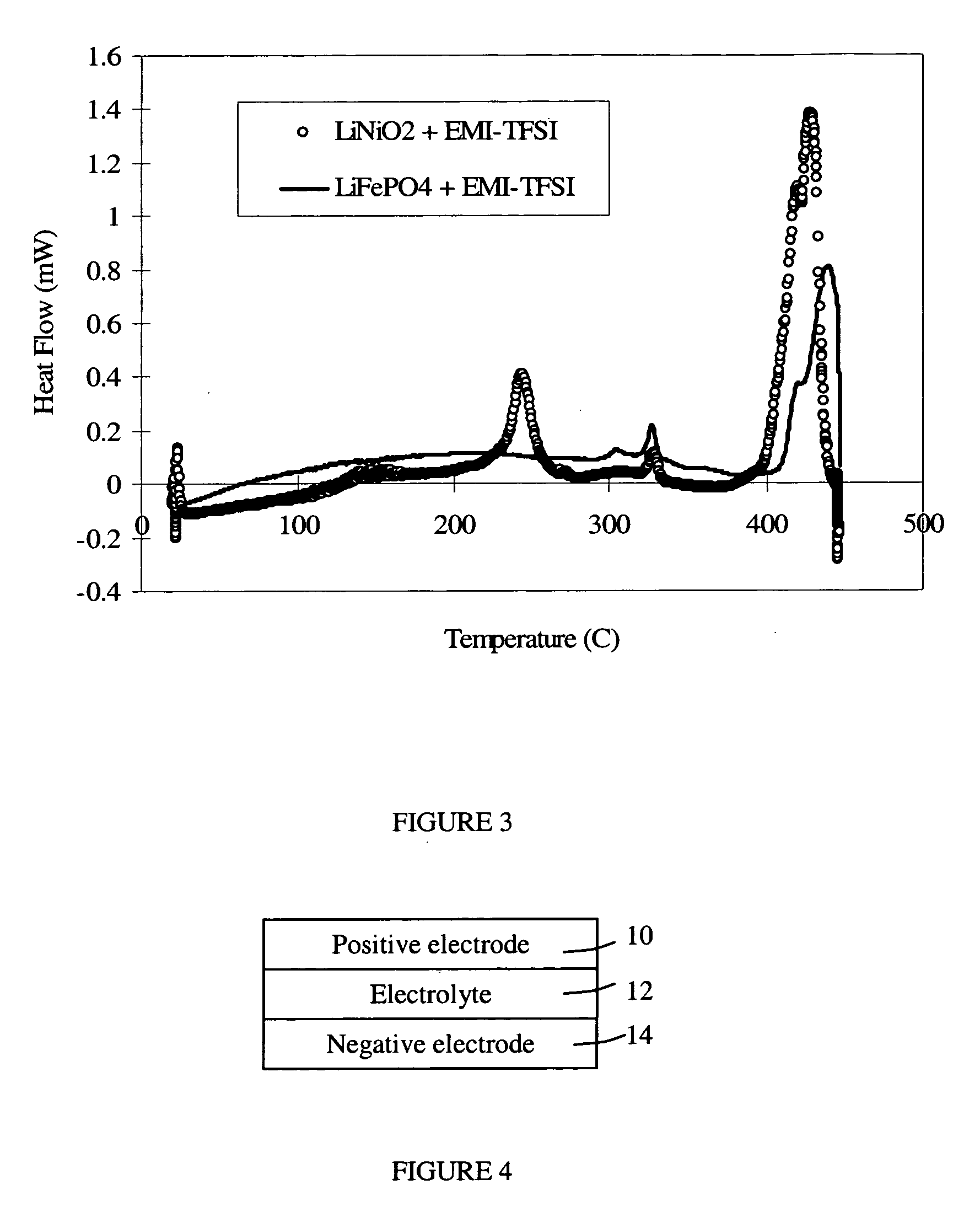
A lithium-ion battery comprises a negative electrode, a positive electrode, and an electrolyte including a molten salt. The positive electrode comprises an electroactive compound including phosphorus, oxygen, lithium, and at least one other metal or semi-metal. The combination of such electrode compositions and a molten salt electrolyte provides a battery with very high thermal stability. Other ions, such as alkal metal ions, may be used in place of lithium ions for applications in other battery technologies.
Owner:TOYOTA MOTOR CO LTD +2
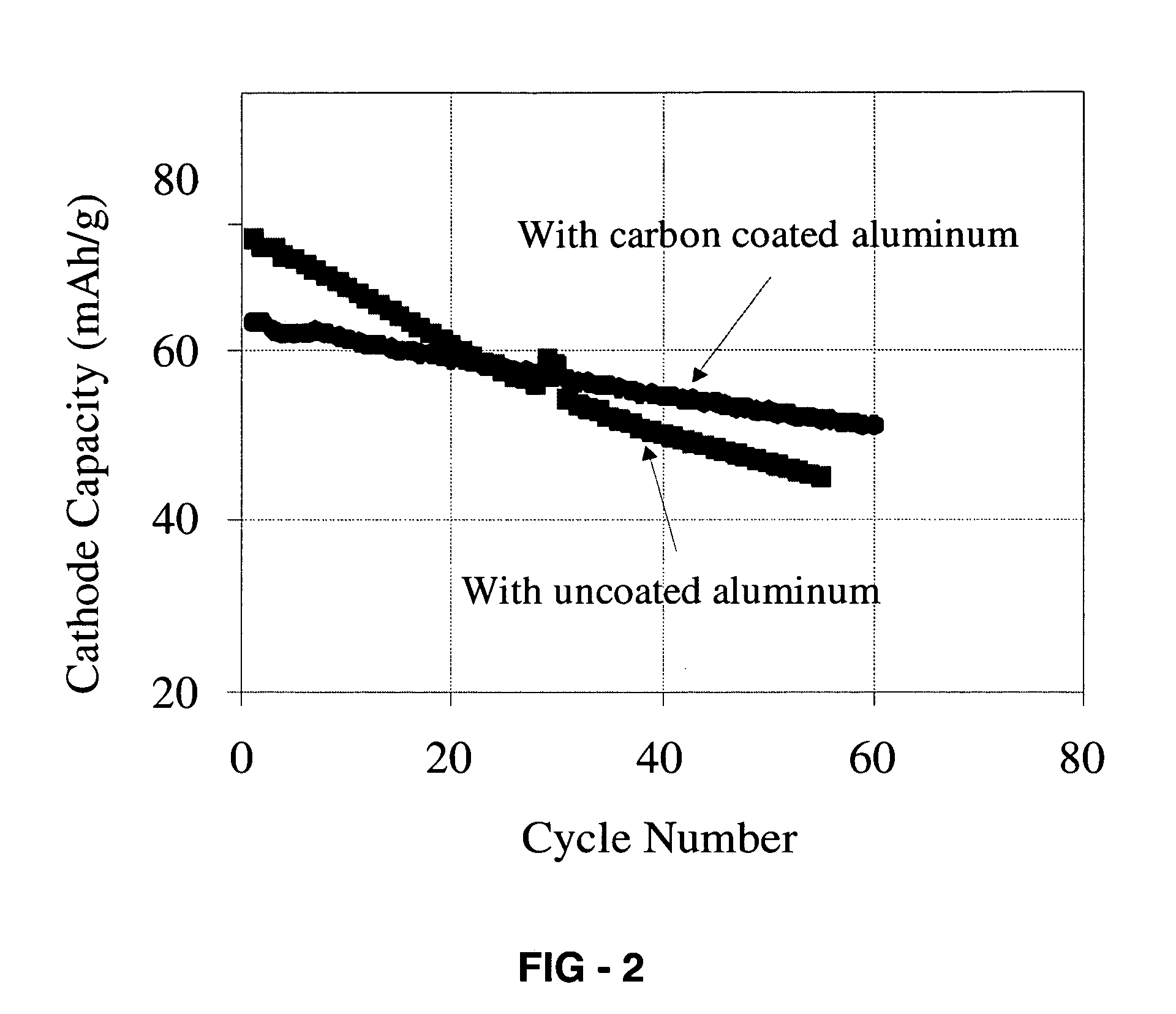
Corrosion protection using carbon coated electron collector for lithium-ion battery with molten salt electrolyte
InactiveUS7348102B2Material nanotechnologyElectrode carriers/collectorsConductive polymerCarbon nanotube
A battery, such as a lithium-ion battery, comprises a first electrode, a second electrode, a molten salt electrolyte, and an electron collector, associated with the first electrode, the electron collector comprising an electrically conducting film. The battery further includes a protection layer separating the electron collector and the first electrode, the protection layer comprising a carbon-containing material. The electron collector may be an electrically conducting material such as aluminum, aluminum alloy, copper, nickel, other metal (such as alloys), conducting polymer, and the like. In one example, the protection layer is a graphite layer. In other examples, the protection layer may be a fullerene film, carbon nanotube film, or other carbon-containing material.
Owner:TOYOTA MOTOR CO LTD +2
Method for preparing titanium solution by wet-processing on vanadium-titanium magnetite concentrates
ActiveCN103276207ASolve unexploitable puzzlesImprove resource utilizationProcess efficiency improvementSlagResource utilization
The invention belongs to the wet-process metallurgical filed and in particular relates to a method for preparing titanium solution by wet-processing on vanadium-titanium magnetite concentrates. The method for preparing titanium solution by wet-processing on vanadium-titanium magnetite concentrates comprises the following steps of: (1), mixing the vanadium-titanium magnetite concentrate with hydrochloric acid, and leaching to obtain an intermediate sizing agent; (2), filtering the intermediate sizing agent to obtain leaching liquor and leaching residues; (3), carrying out water-washing on the leaching slag to obtain washing water and washing slag; (4), carrying out fused-salt reaction on the washing slag to obtain fused-salt reaction materials; (5), carrying out water-washing and filtering on the fused-salt reaction materials to obtain water-washing materials; (6), carrying out acid pickling on the water-washing materials to obtain sizing agent, and filtering to obtain the acid-pickled sizing agent; (7), carrying out acid dissolving on the acid-pickled materials by using a sulfuric acid solution to obtain acid-dissolved materials; and (8), adding the acid-dissolved materials to the sulfuric acid solution for extracting, and filtering to obtain extracting solution which is the titanium solution. According to the method for preparing titanium solution by wet-processing on vanadium-titanium magnetite concentrates disclosed by the invention, the titanium in the iron concentrate is sufficiently utilized, so that the titanium resource utilization rate is high and the recovery rate of the titanium in the titanium concrete is higher than 90%.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com