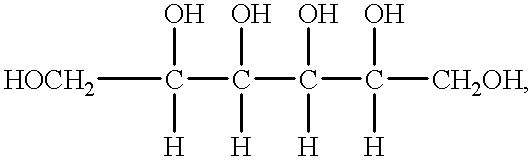
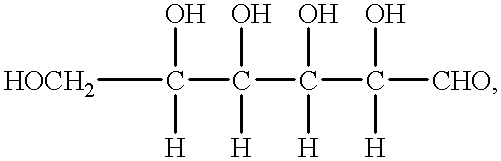
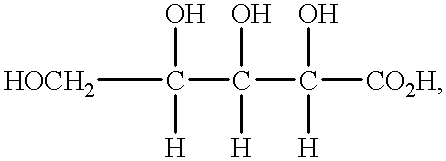
Patents
Literature
154results about "Two electrolyte cells" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methods and reagents for enhancing the cycling efficiency of lithium polymer batteries
InactiveUS6017651AImprove efficiencyElectrode rolling/calenderingElectrochemical processing of electrodesLithium metalSulfur electrode
Batteries including a lithium electrode and a sulfur counter electrode that demonstrate improved cycling efficiencies are described. In one embodiment, an electrochemical cell having a lithium electrode and a sulfur electrode including at least one of elemental sulfur, lithium sulfide, and a lithium polysulfide is provided. The lithium electrode includes a surface coating that is effective to increase the cycling efficiency of said electrochemical cell. In a more particular embodiment, the lithium electrode is in an electrolyte solution, and, more particularly, an electrolyte solution including either elemental sulfur, a sulfide, or a polysulfide. In another embodiment, the coating is formed after the lithium electrode is contacted with the electrolyte. In a more particular embodiment, the coating is formed by a reaction between the lithium metal of the lithium electrode and a chemical species present in the electrolyte.
Owner:POLYPLUS BATTERY CO INC
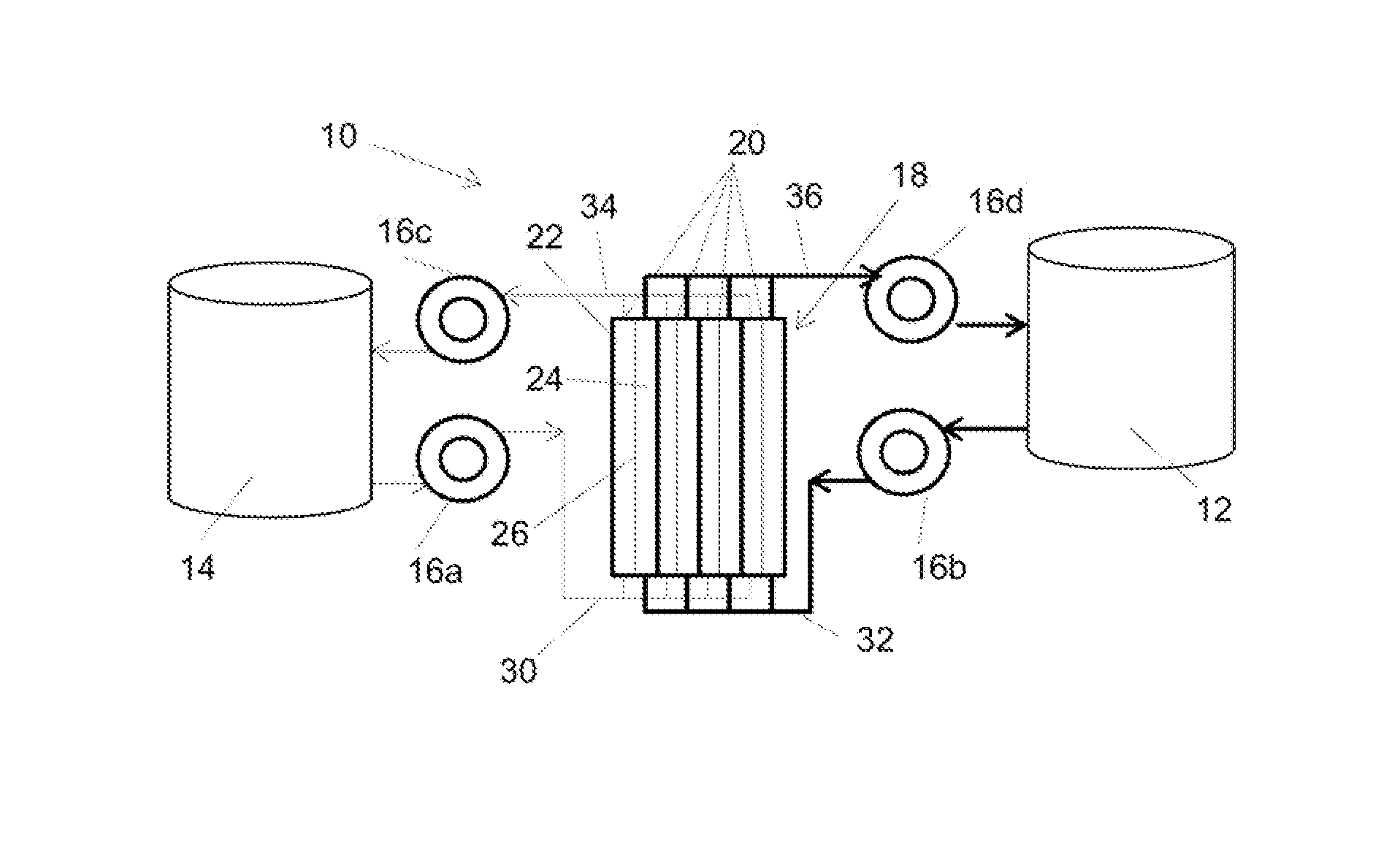
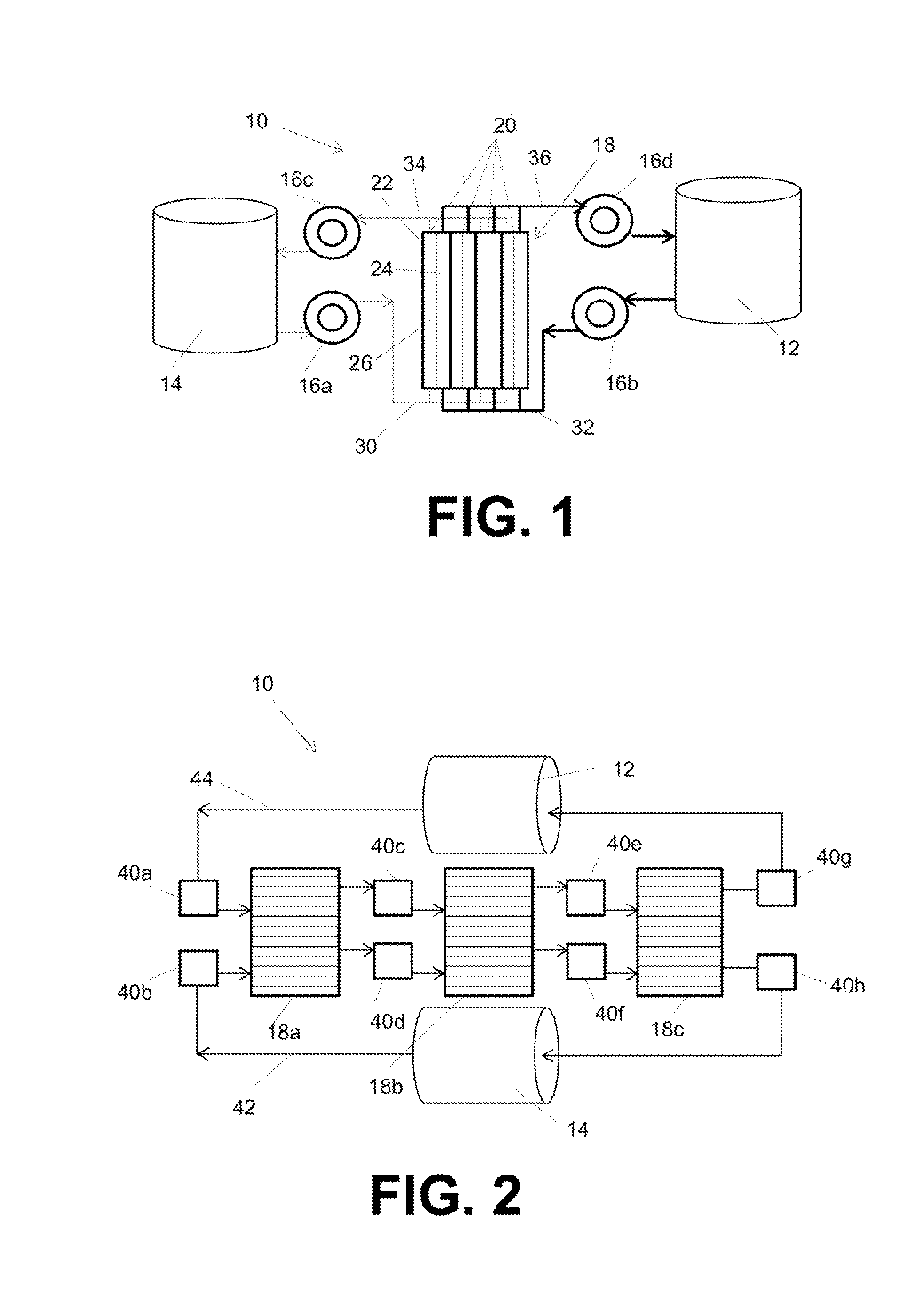
High energy density redox flow device
ActiveUS20110200848A1Avoid accumulationHigh enough specific energyOrganic chemistryFlow propertiesElectrochemical responseHigh energy
Redox flow devices are described in which at least one of the positive electrode or negative electrode-active materials is a semi-solid or is a condensed ion-storing electroactive material, and in which at least one of the electrode-active materials is transported to and from an assembly at which the electrochemical reaction occurs, producing electrical energy. The electronic conductivity of the semi-solid is increased by the addition of conductive particles to suspensions and / or via the surface modification of the solid in semi-solids (e.g., by coating the solid with a more electron conductive coating material to increase the power of the device). High energy density and high power redox flow devices are disclosed. The redox flow devices described herein can also include one or more inventive design features. In addition, inventive chemistries for use in redox flow devices are also described.
Owner:MASSACHUSETTS INST OF TECH +2
Separation of electrolytes
Methods and articles relating to separation of electrolyte compositions within lithium batteries are provided. The lithium batteries described herein may include an anode having lithium as the active anode species and a cathode having sulfur as the active cathode species. Suitable electrolytes for the lithium batteries can comprise a heterogeneous electrolyte including a first electrolyte solvent (e.g., dioxolane (DOL)) that partitions towards the anode and is favorable towards the anode (referred to herein as an “anode-side electrolyte solvent”) and a second electrolyte solvent (e.g., 1,2-dimethoxyethane (DME)) that partitions towards the cathode and is favorable towards the cathode (and referred to herein as an “cathode-side electrolyte solvent”). By separating the electrolyte solvents during operation of the battery such that the anode-side electrolyte solvent is present disproportionately at the anode and the cathode-side electrolyte solvent is present disproportionately at the cathode, the battery can benefit from desirable characteristics of both electrolyte solvents (e.g., relatively low lithium reactivity of the anode-side electrolyte solvent and relatively high polysulfide solubility of the cathode-side electrolyte solvent).
Owner:SION POWER CORP
Stabilized vanadium electrolyte solutions for all-vanadium redox cells and batteries
InactiveUS6562514B1Effective amountEasy to modifyFinal product manufactureRegenerative fuel cellsRedoxPhysical chemistry
Owner:JD HLDG INC
Advanced Metal-Air Battery Having a Ceramic Membrane Electrolyte Background of the Invention
ActiveUS20080268327A1Reduce oxygenReduce layeringFuel and primary cellsSolid electrolytesOxygenCeramic membrane
A metal-air battery is disclosed in one embodiment of the invention as including a cathode to reduce oxygen molecules and an alkali-metal-containing anode to oxidize the alkali metal (e.g., Li, Na, and K) contained therein to produce alkali-metal ions. An aqueous catholyte is placed in ionic communication with the cathode to store reaction products generated by reacting the alkali-metal ions with the oxygen containing anions. These reaction products are stored as solutes dissolved in the aqueous catholyte. An ion-selective membrane is interposed between the alkali-metal containing anode and the aqueous catholyte. The ion-selective membrane is designed to be conductive to the alkali-metal ions while being impermeable to the aqueous catholyte.
Owner:FIELD UPGRADING USA INC
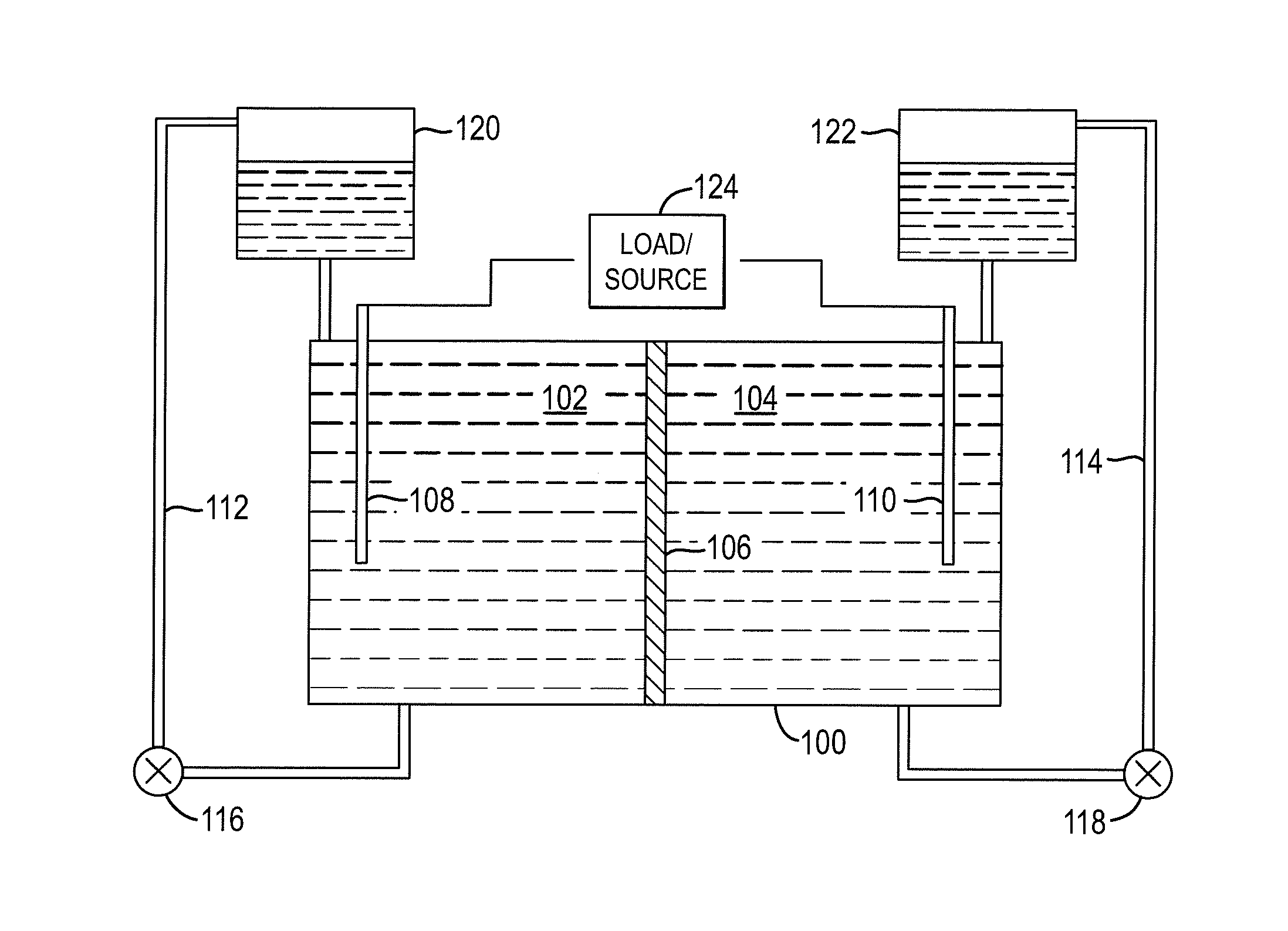
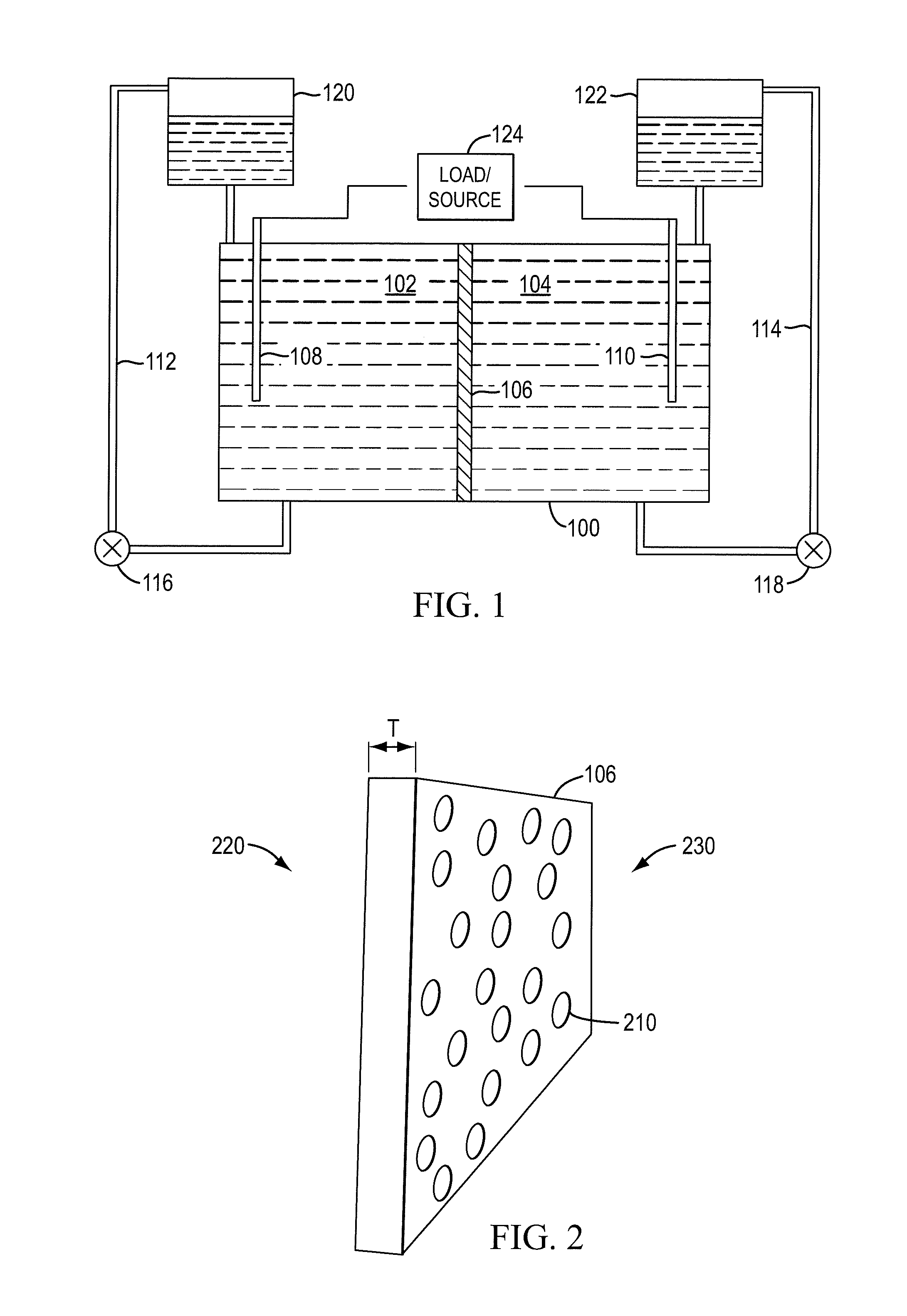
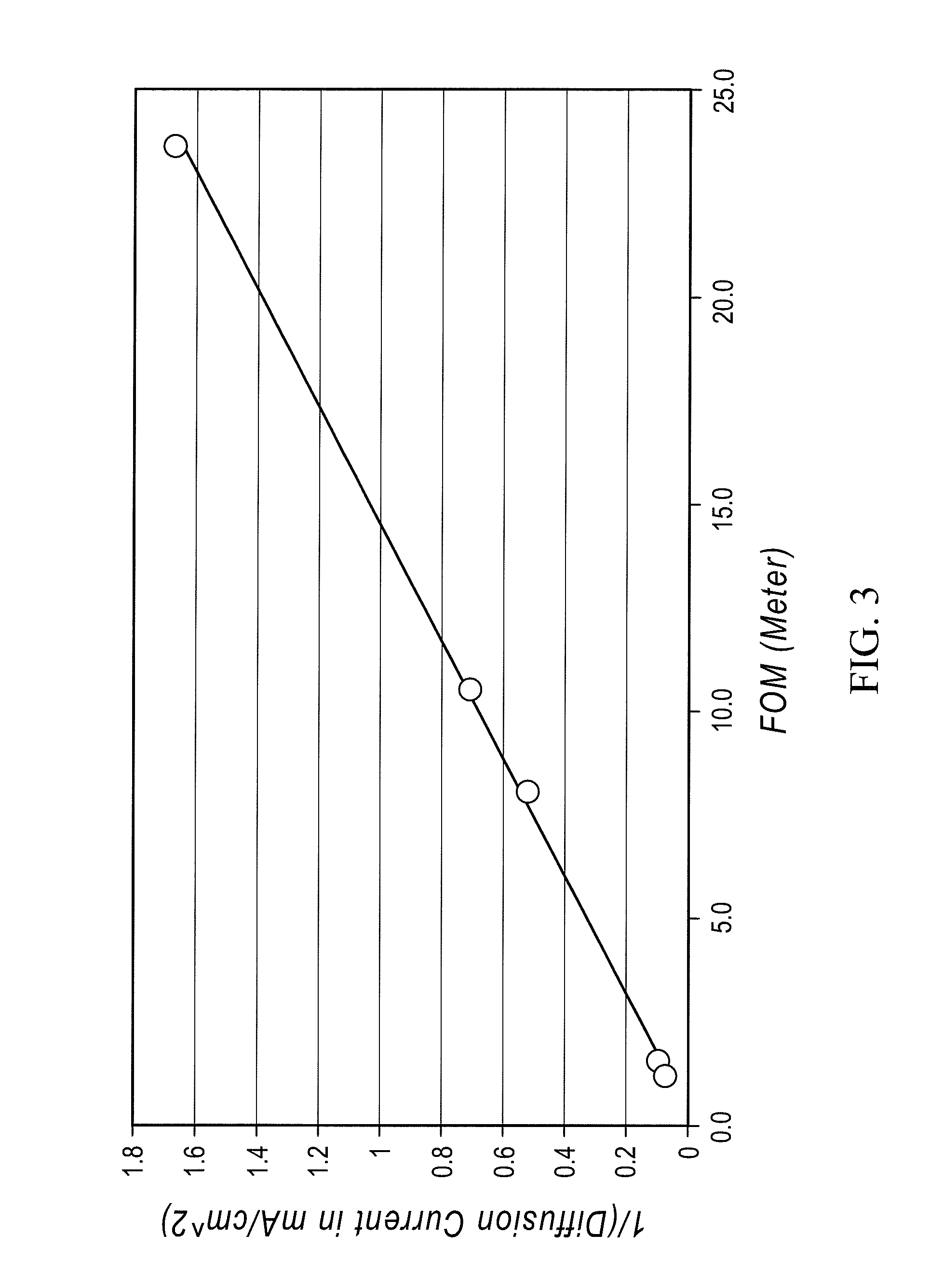
Redox flow cell
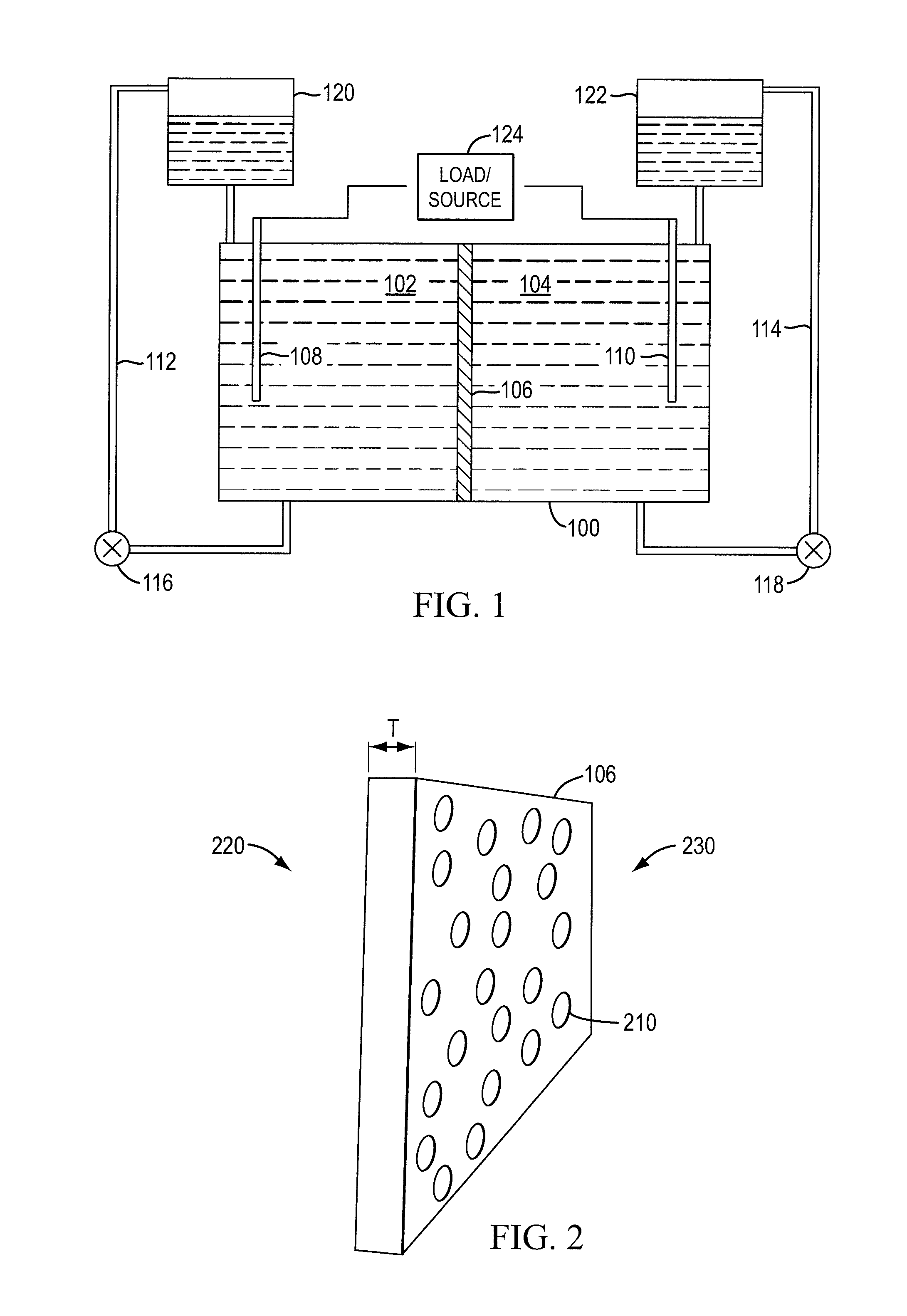
InactiveUS20100003586A1Facilitate ion exchangeCell electrodesFuel cell auxillariesFlow cellPorous membrane
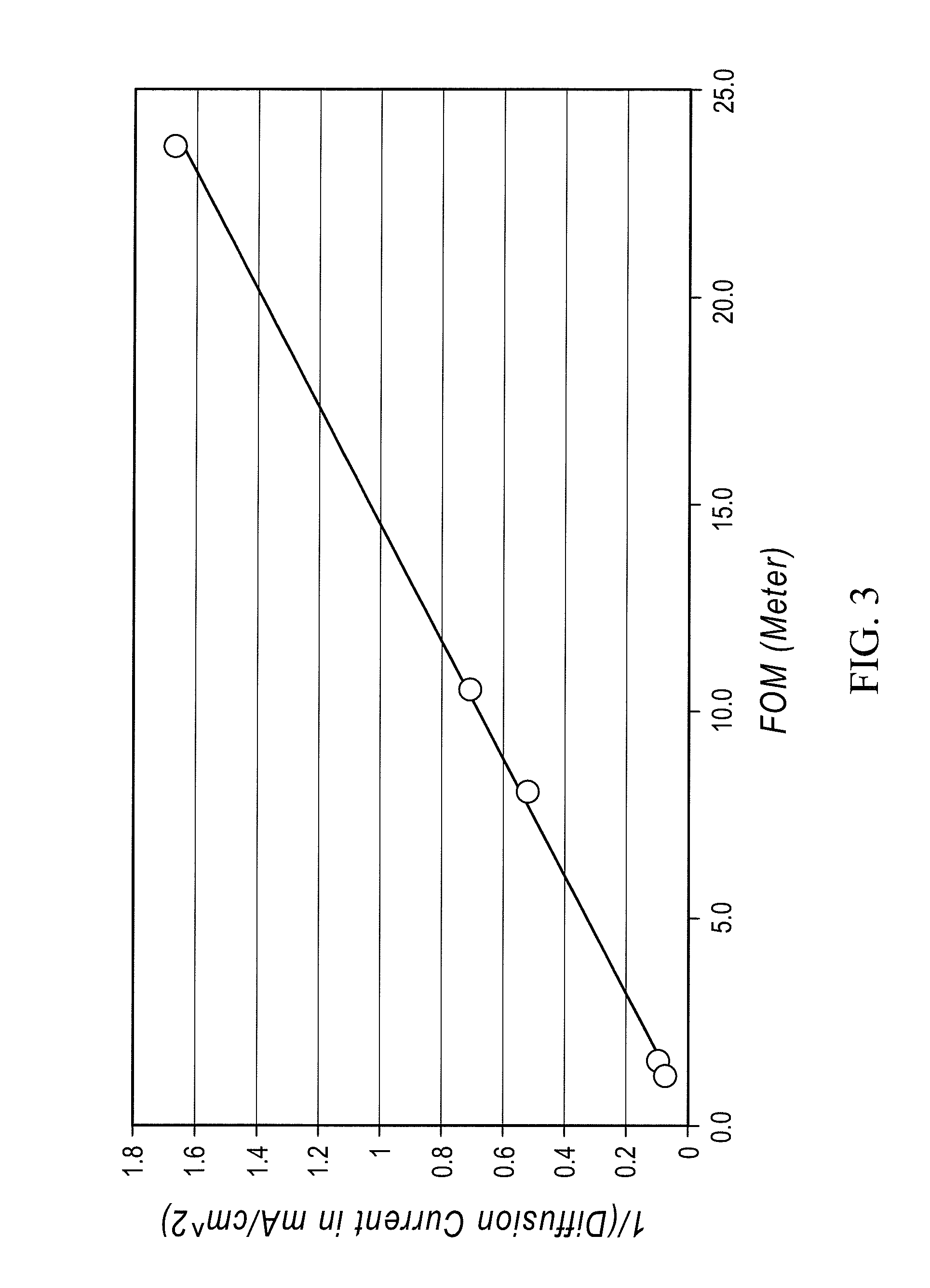
A redox flow cell is presented that utilizes a porous membrane separating a first half cell and a second half cell. The porous membrane is chosen to have a figure of merit (FOM) is at least a minimum FOM. A method of providing a porous membrane for a flow cell can include determining a figure of merit; determining a first parameter from a pore size or a thickness for the porous membrane; determining a second parameter from the pore size or the thickness that is not the first parameter for the porous membrane, based on the figure of merit; and constructing a porous membrane having the pore size and the thickness.
Owner:IMERGY POWER SYST
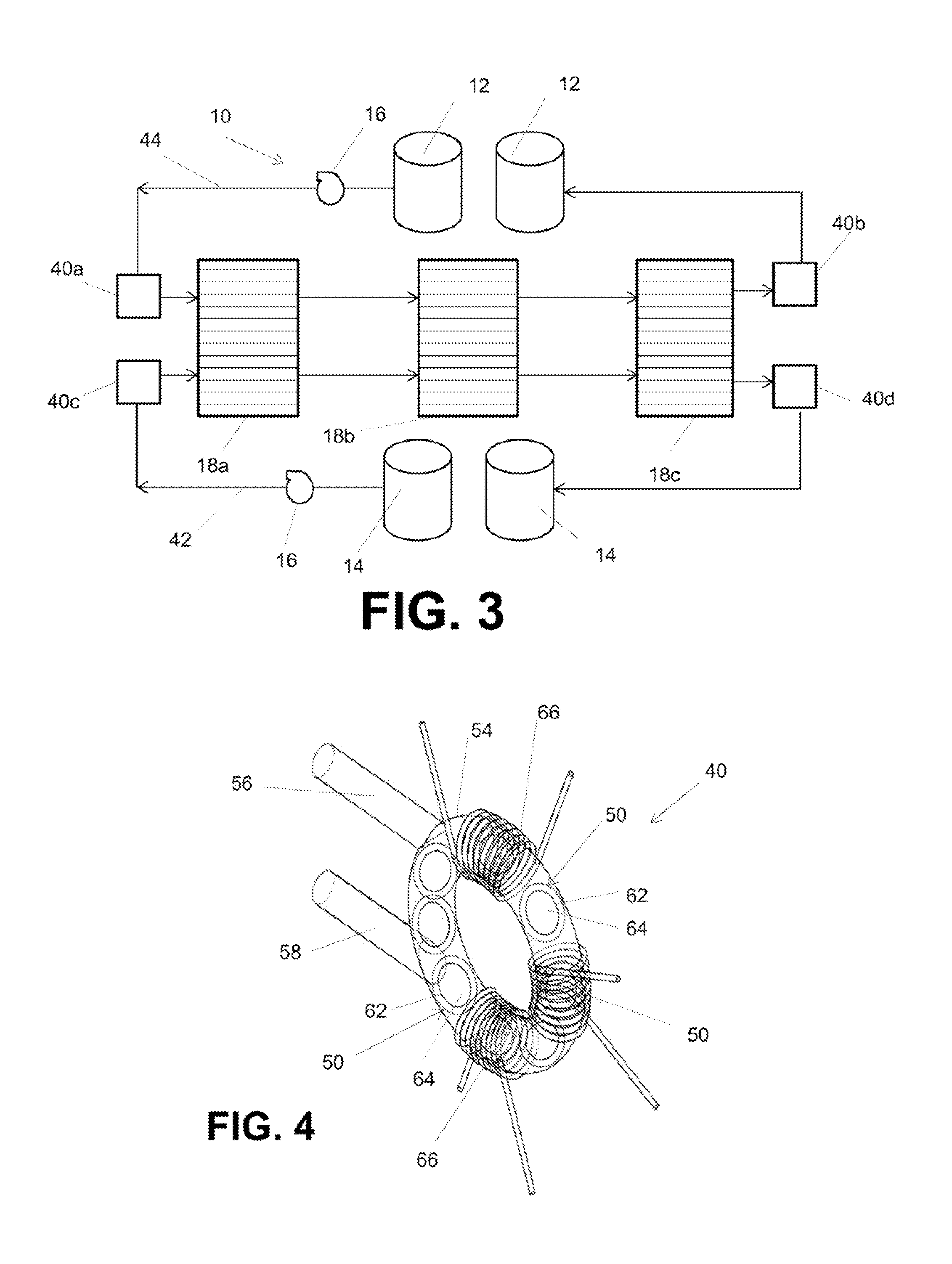
Electrode array for use in electrochemical cells
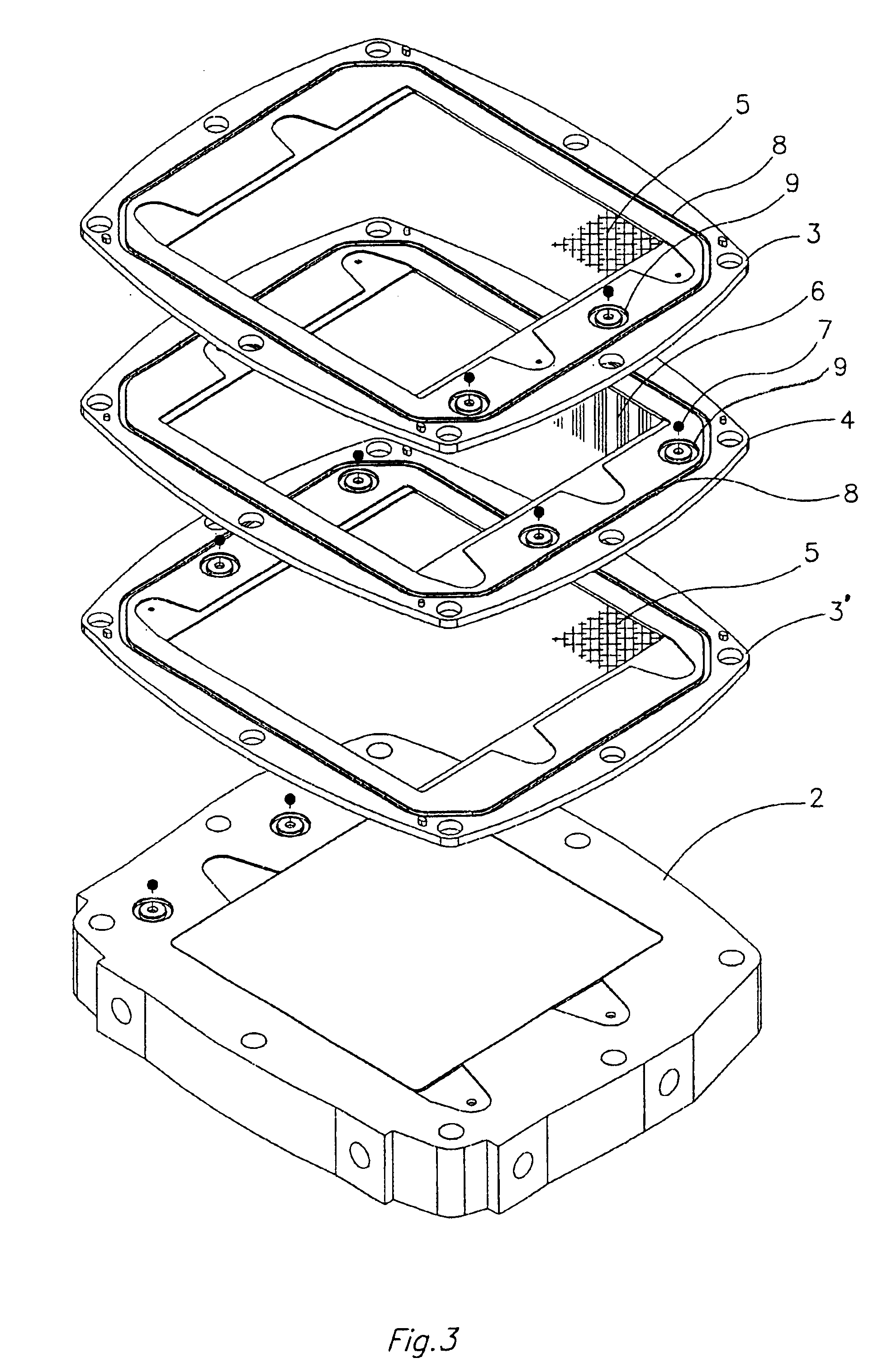
InactiveUS7368191B2Reduce leakageReduced series resistanceFinal product manufactureFuel cell auxillariesFuel cellsEngineering
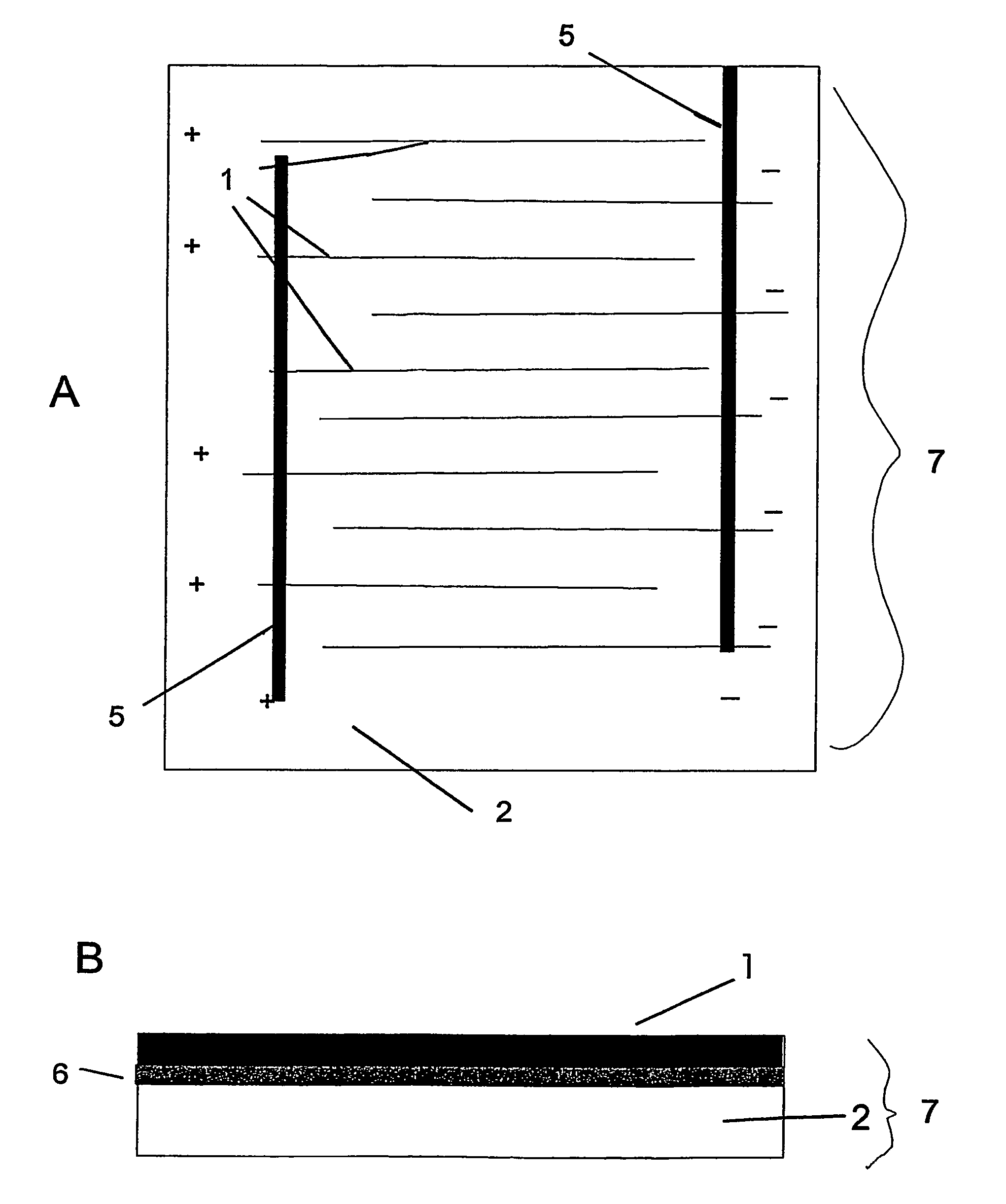
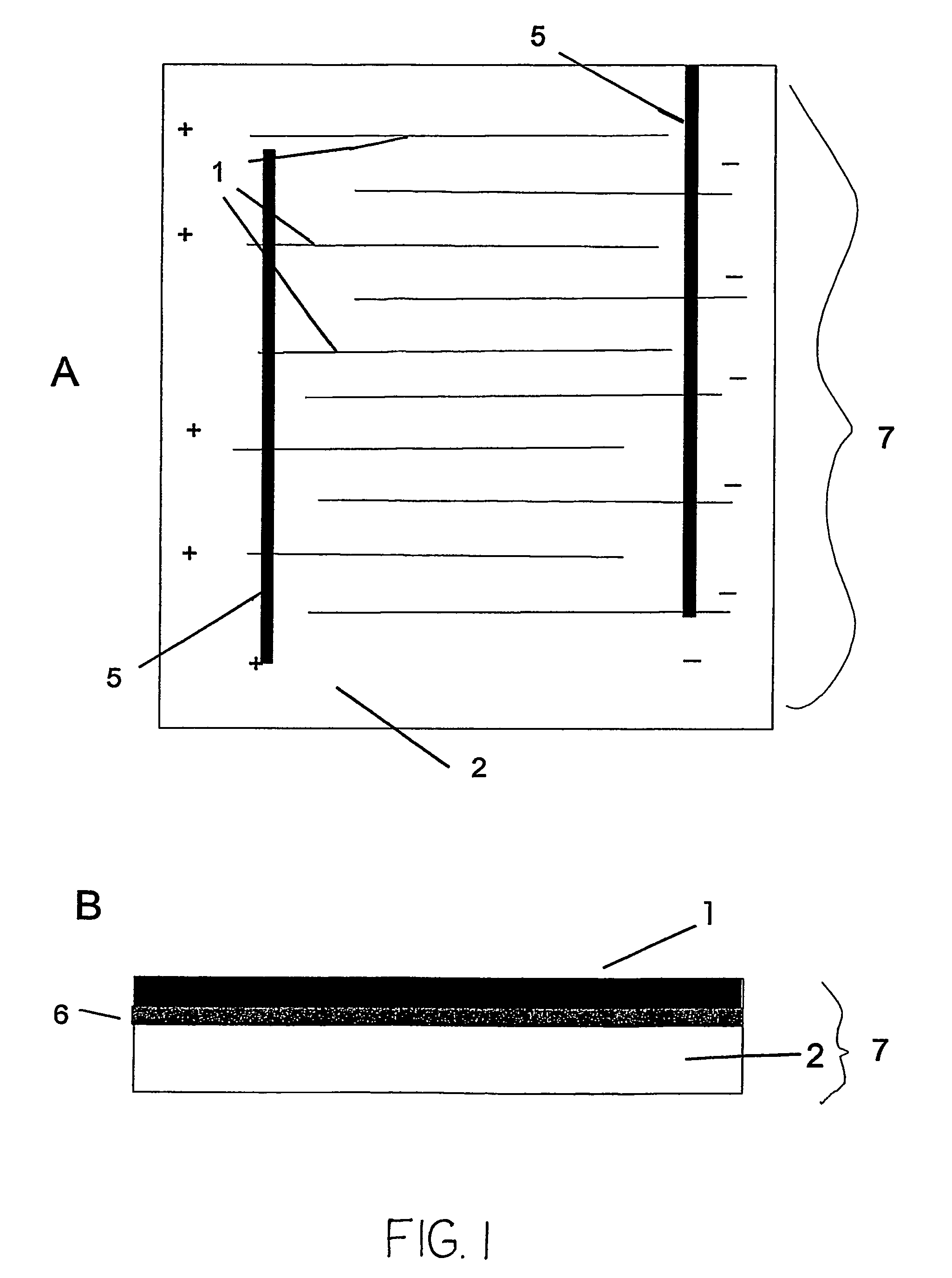
The invention features an electrode array (7) in which pairs of electrodes (1) are geometrically arranged so that the broadest faces of the exposed electrodes are not directly opposing to each other. Rather, the broadest facing surfaces of the electrodes in the array are parallel, adjacent, or offset at an angle. The electrode geometry of an electrode array of the invention permits electrodes to be in close proximity, thereby lowering series resistance, while minimizing the possibility for short circuits that can cause electrical leakage. An electrode array of the invention can be used in an electrochemical cell, such as a battery, e.g., a lithium battery, a capacitor, a flow-through capacitor, or a fuel cell.
Owner:BIOSOURCE INC
Redox flow battery and method of operating it
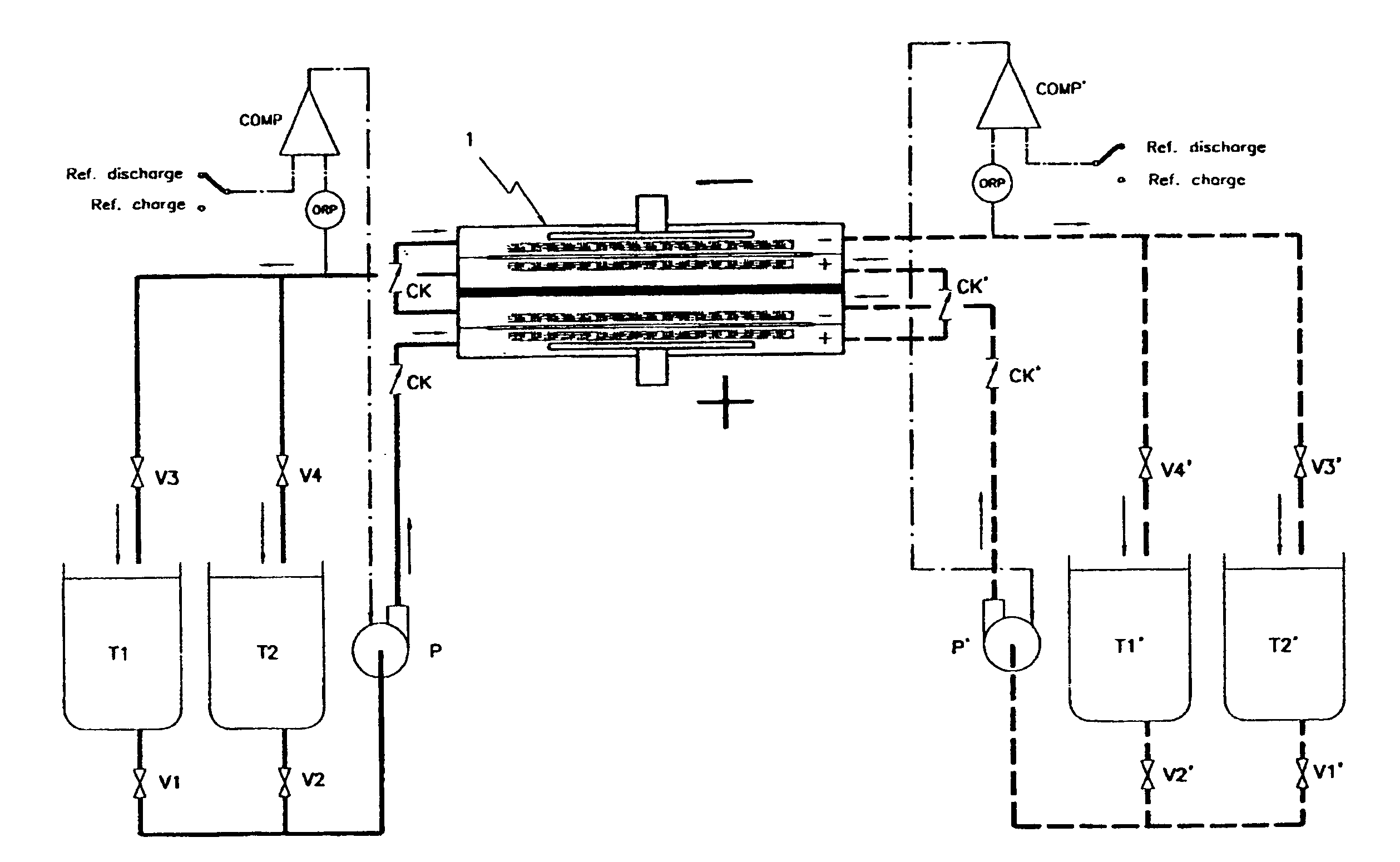
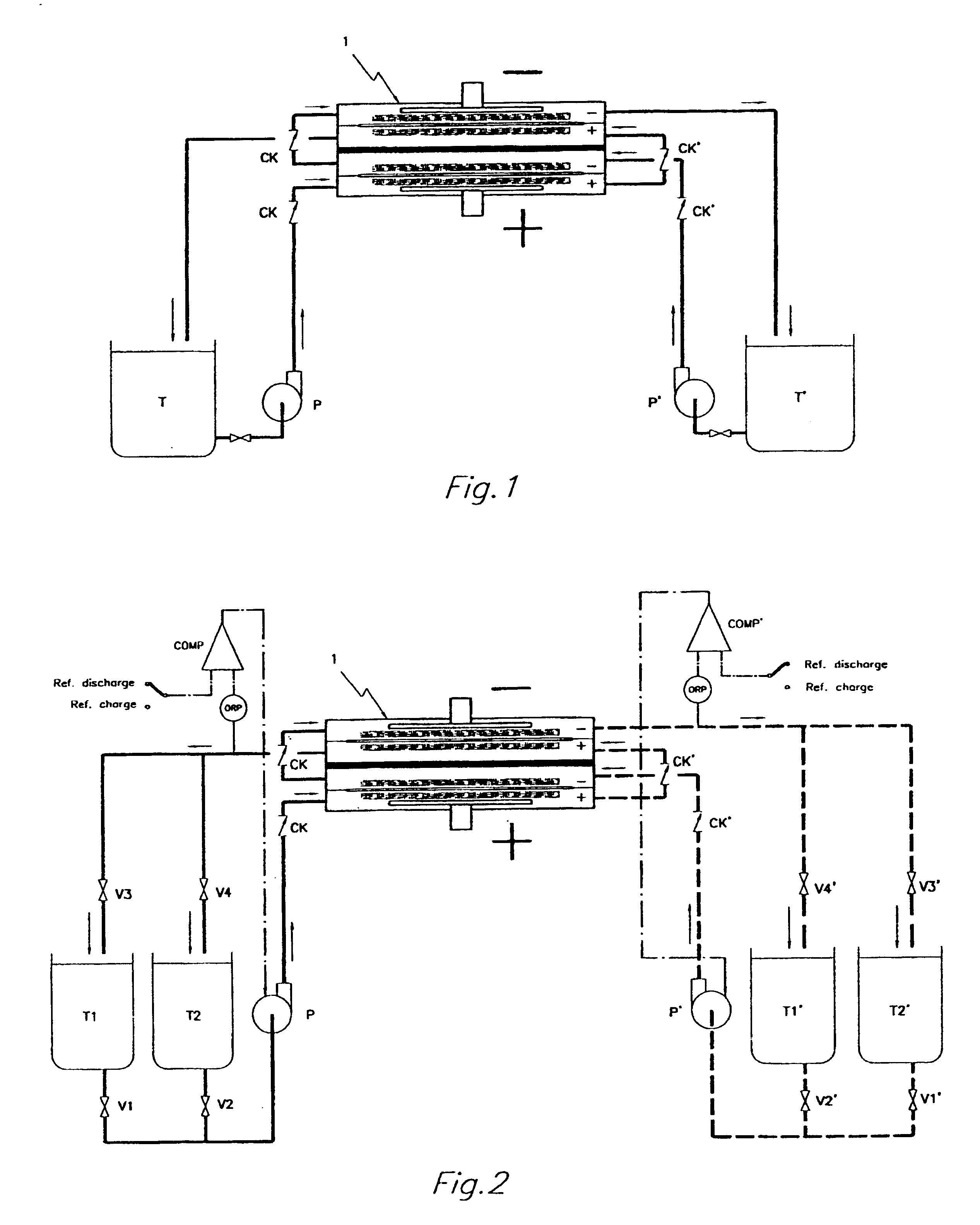
InactiveUS6692862B1Easy dischargeImprove efficiencyElectrolyte moving arrangementsFuel cells groupingWork cycleRedox
By realizing or installing check valve liquid vein interrupters in each compartment of the battery the phenomenon of slow discharge of the retained volumes of electrolytes during long periods of inactivity of a redox flow battery, with the electrolyte pumps stopped altogether, can be practically eliminated with the effect that the battery is perfectly ready to deliver electric power immediately upon request even after prolonged periods of inactivity. Moreover, the presence of liquid vein interrupters on each compartment in either an outlet or an inlet port substantially preventing by-pass current during a not pumping phase, permits to increase the by pumping the electrolytes through the compartments of a battery stack intermittently, in other words in a pulsed manner, with a certain duty-cycle. Relatively brief pumping phases at relatively high flow rate alternated to phases of not pumping provide for a volumetrically adequate refreshing of the electrolytes present in the battery compartments and contrast the formation of gradients in the bodies of electrolyte.
Owner:SQUIRREL HLDG
High energy density vanadium electrolyte solutions, methods of preparation thereof and all-vanadium redox cells and batteries containing high energy vanadium electrolyte solutions
InactiveUS6468688B2Effective amountEasy to modifyCharging stationsCell electrodesElectricityVanadium redox battery
Disclosed is a method for preparing a high energy density (HED) electrolyte solution for use in an all-vanadium redox cells, a high energy density electrolyte solution, in particular an all-vanadium high energy density electrolyte solution, a redox cell, in particular an all-vanadium redox cell, comprising the high energy density electrolyte solution, a redox battery, in particular an all-vanadium redox battery, comprising the HED electrolyte solution, a process for recharging a discharged or partially discharged redox battery, in particular an all-vanadium redox battery, comprising the HED electrolyte solution, a process for the production of electricity from a charged redox battery, and in particular a charged all-vanadium redox battery, comprising the HED electrolyte, a redox battery / fuel cell and a process for the production of electricity from a redox battery / fuel cell. A method for stabilising an electrolyte solution for use in a redox cell, in particular for stabilising an electrolyte solution for use in an all-vanadium redox cell, a stabilised electrolyte solution, in particular an all-vanadium stabilised electrolyte solution, a redox cell, in particular an all-vanadium redox cell, comprising the stabilised electrolyte solution, a redox battery, in particular an all-vanadium redox battery comprising the stabilised electrolyte solution, a process for recharging a discharged or partially discharged redox battery, in particular an all-vanadium redox battery, comprising the stabilised electrolyte solution, and a process for the production of electricity from a charged redox battery, and in particular a charged all-vanadium redox battery, comprising the stabilised electrolyte solution are disclosed. Also disclosed are a redox battery / fuel cell and a process for the production of electricity from a redox battery / fuel cell.
Owner:JD HLDG INC
Separation of electrolytes
Methods and articles relating to separation of electrolyte compositions within lithium batteries are provided. The lithium batteries described herein may include an anode having lithium as the active anode species and a cathode having sulfur as the active cathode species. Suitable electrolytes for the lithium batteries can comprise a heterogeneous electrolyte including a first electrolyte solvent (e.g., dioxolane (DOL)) that partitions towards the anode and is favorable towards the anode (referred to herein as an “anode-side electrolyte solvent”) and a second electrolyte solvent (e.g., 1,2-dimethoxyethane (DME)) that partitions towards the cathode and is favorable towards the cathode (and referred to herein as an “cathode-side electrolyte solvent”). By separating the electrolyte solvents during operation of the battery such that the anode-side electrolyte solvent is present disproportionately at the anode and the cathode-side electrolyte solvent is present disproportionately at the cathode, the battery can benefit from desirable characteristics of both electrolyte solvents (e.g., relatively low lithium reactivity of the anode-side electrolyte solvent and relatively high polysulfide solubility of the cathode-side electrolyte solvent).
Owner:SION POWER CORP
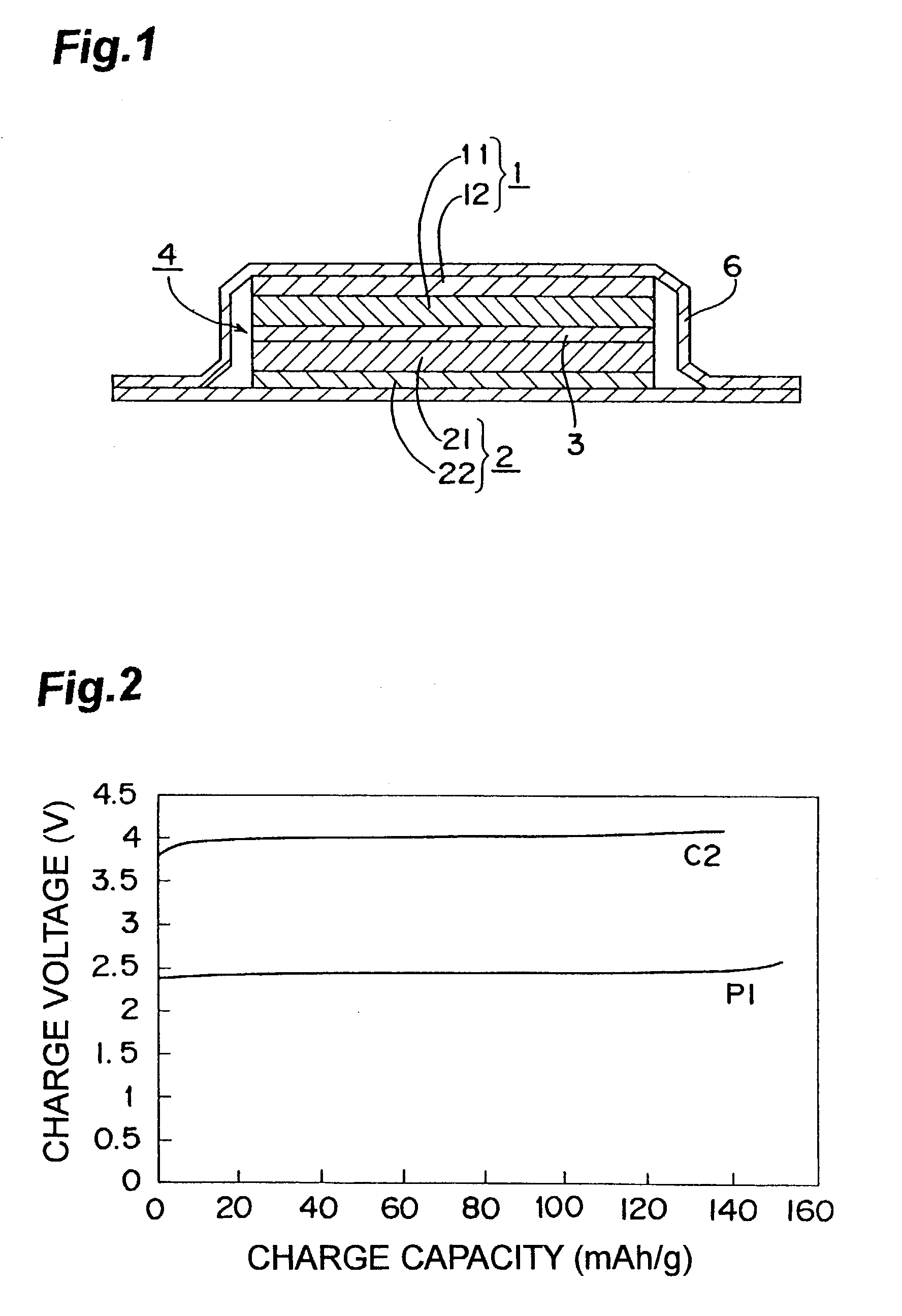
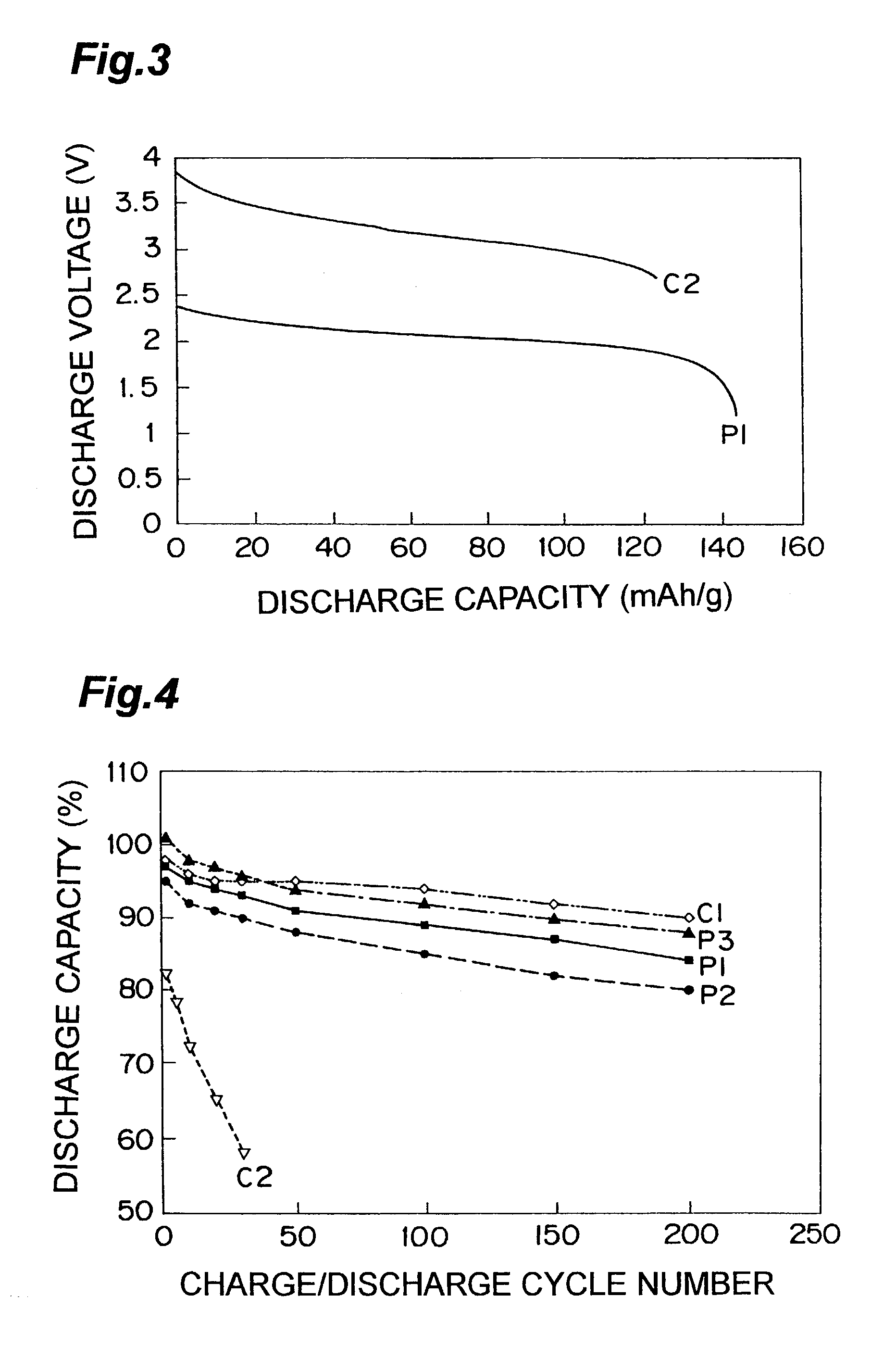
Nonaqueous electrolyte lithium secondary cell
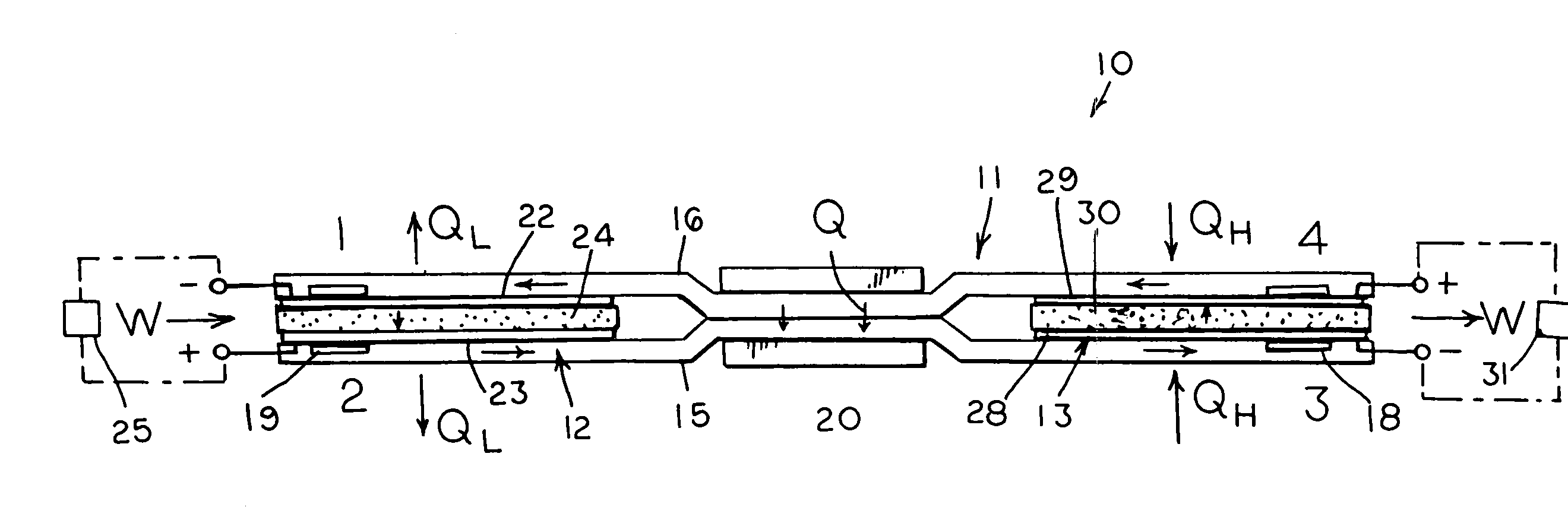
InactiveUS7029793B2Improve securityImprove battery performanceSolid electrolyte cellsTwo electrolyte cellsMetallic lithiumMolten salt
A nonaqueous electrolyte lithium secondary cell comprising a positive electrode (1), a negative electrode (2) and a nonaqueous electrolyte containing a lithium salt is characterized by that the nonaqueous electrolyte contains a room temperature molten salt as a main component, a material wherein a working potential of the negative electrode (2) is nobler by above 1V than a potential of a metallic lithium is used for a negative active material of the negative electrode. This nonaqueous electrolyte lithium secondary cell has excellent safety and cell performance.
Owner:GS YUASA INT LTD +1
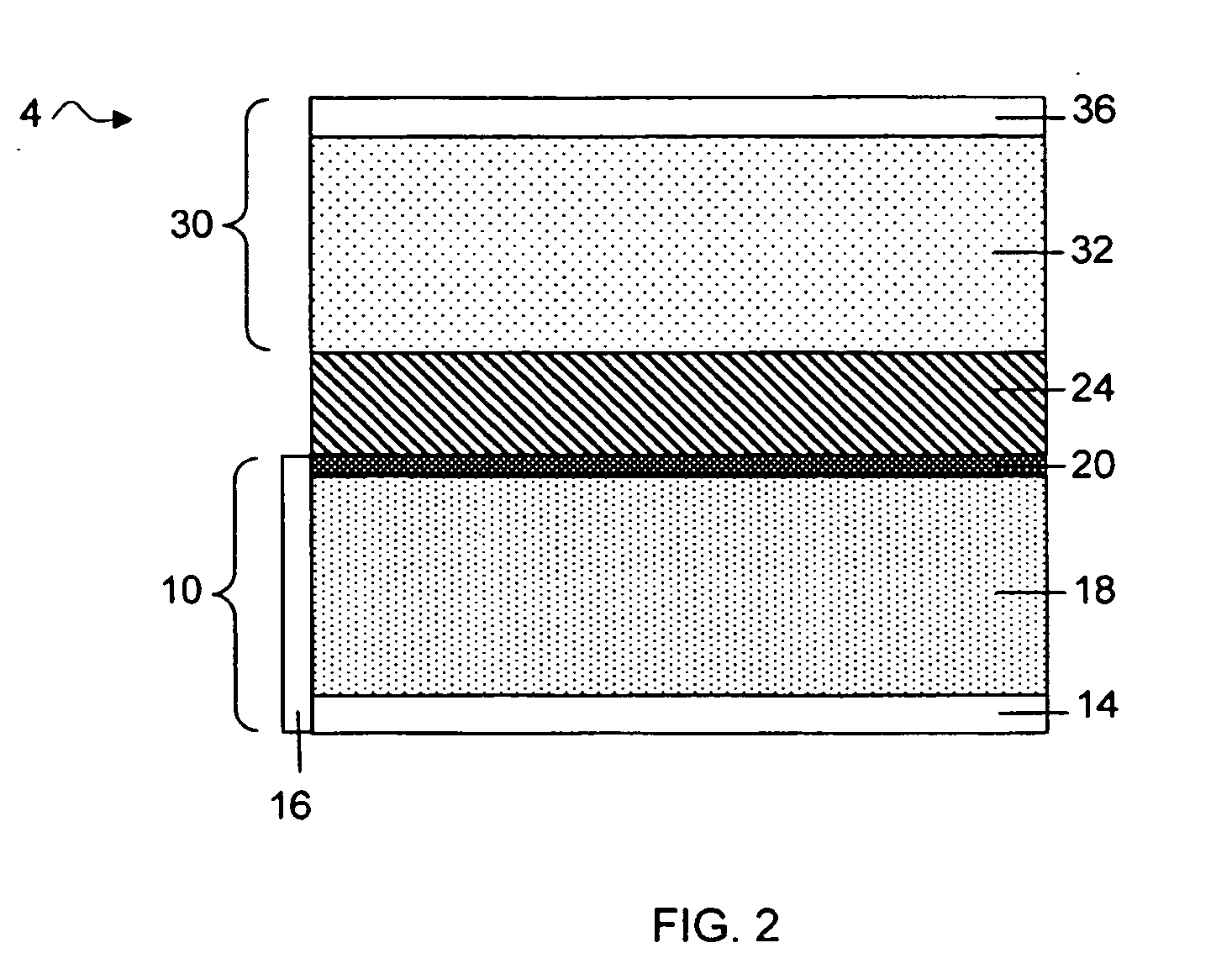
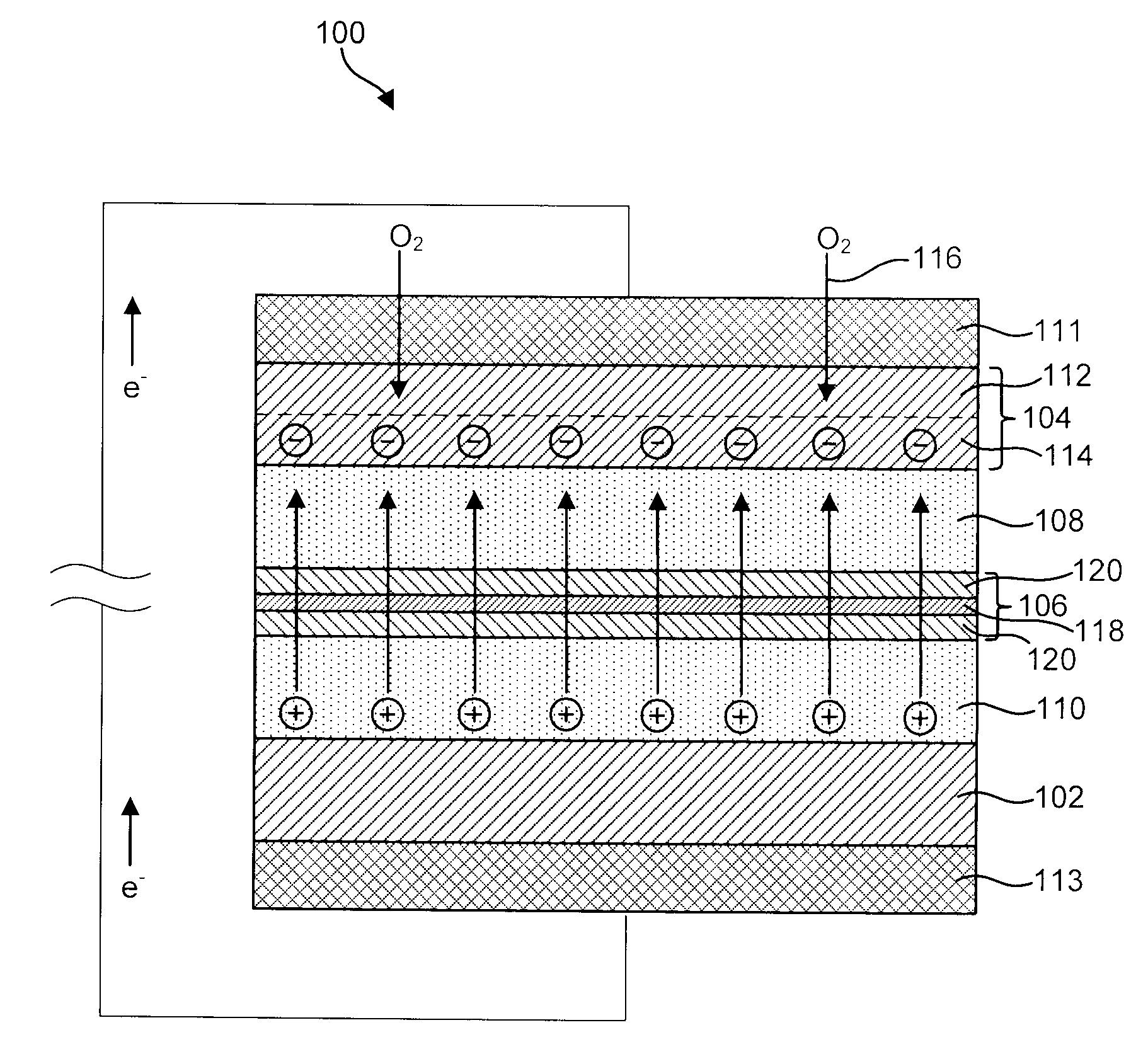
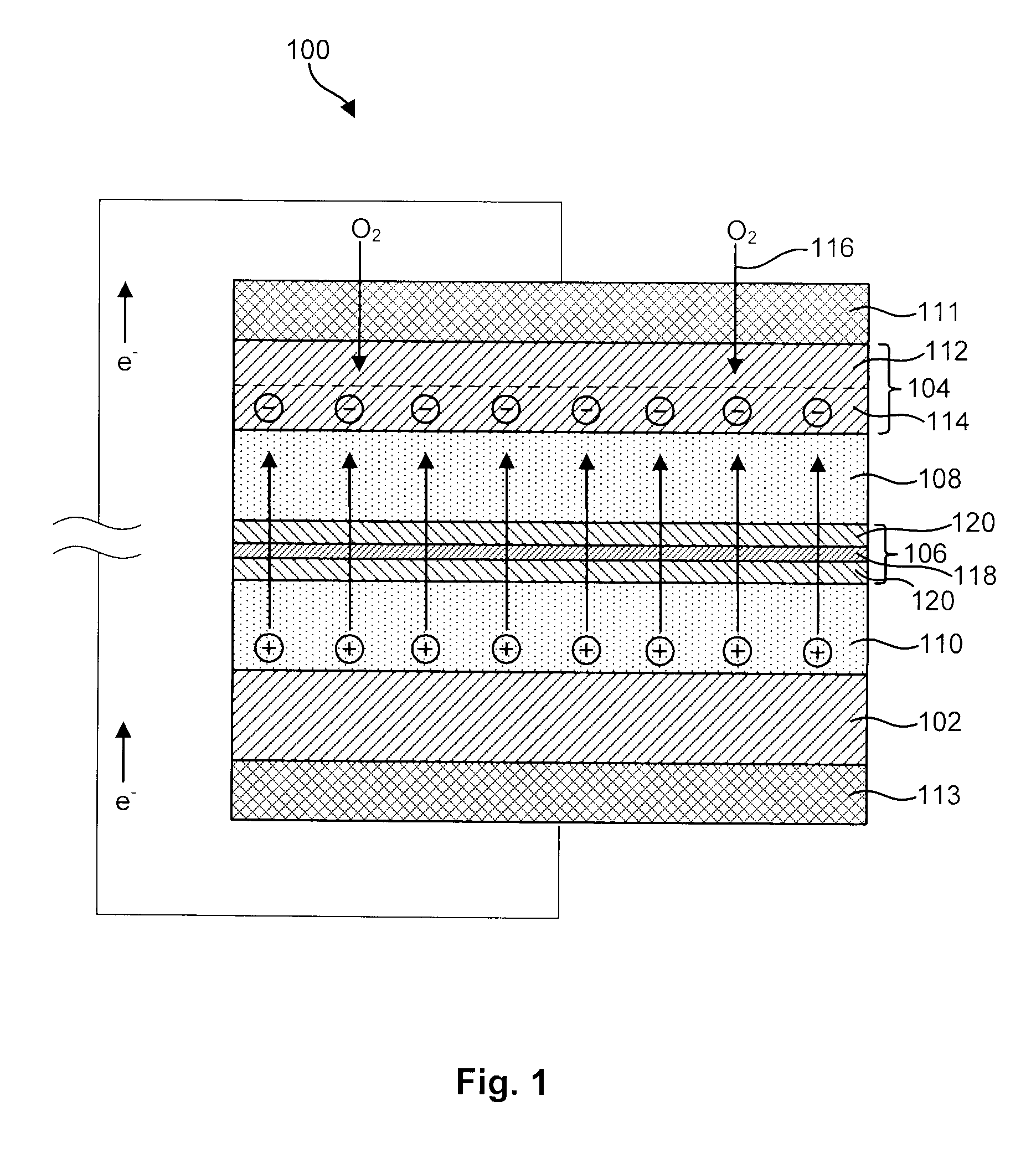
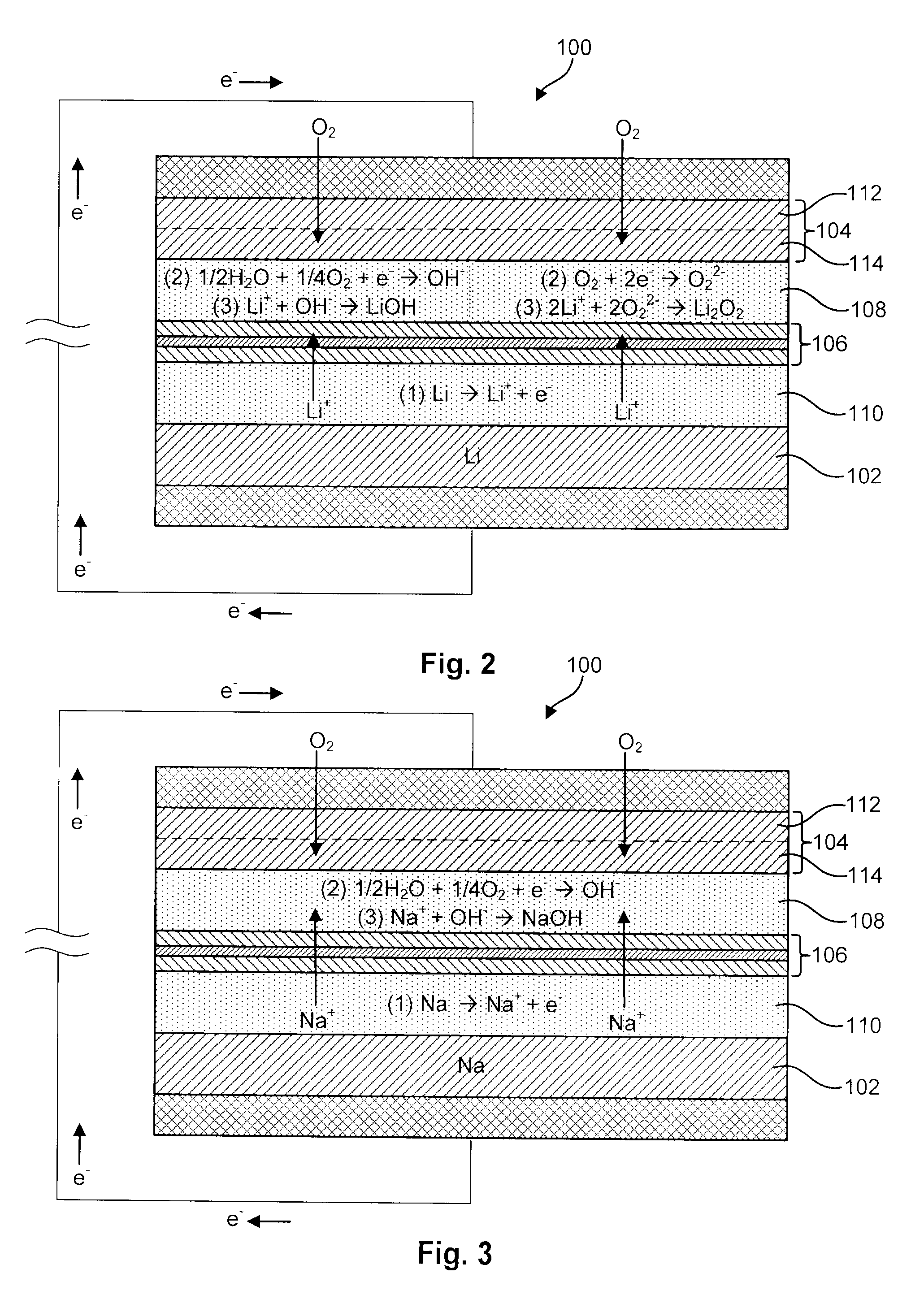
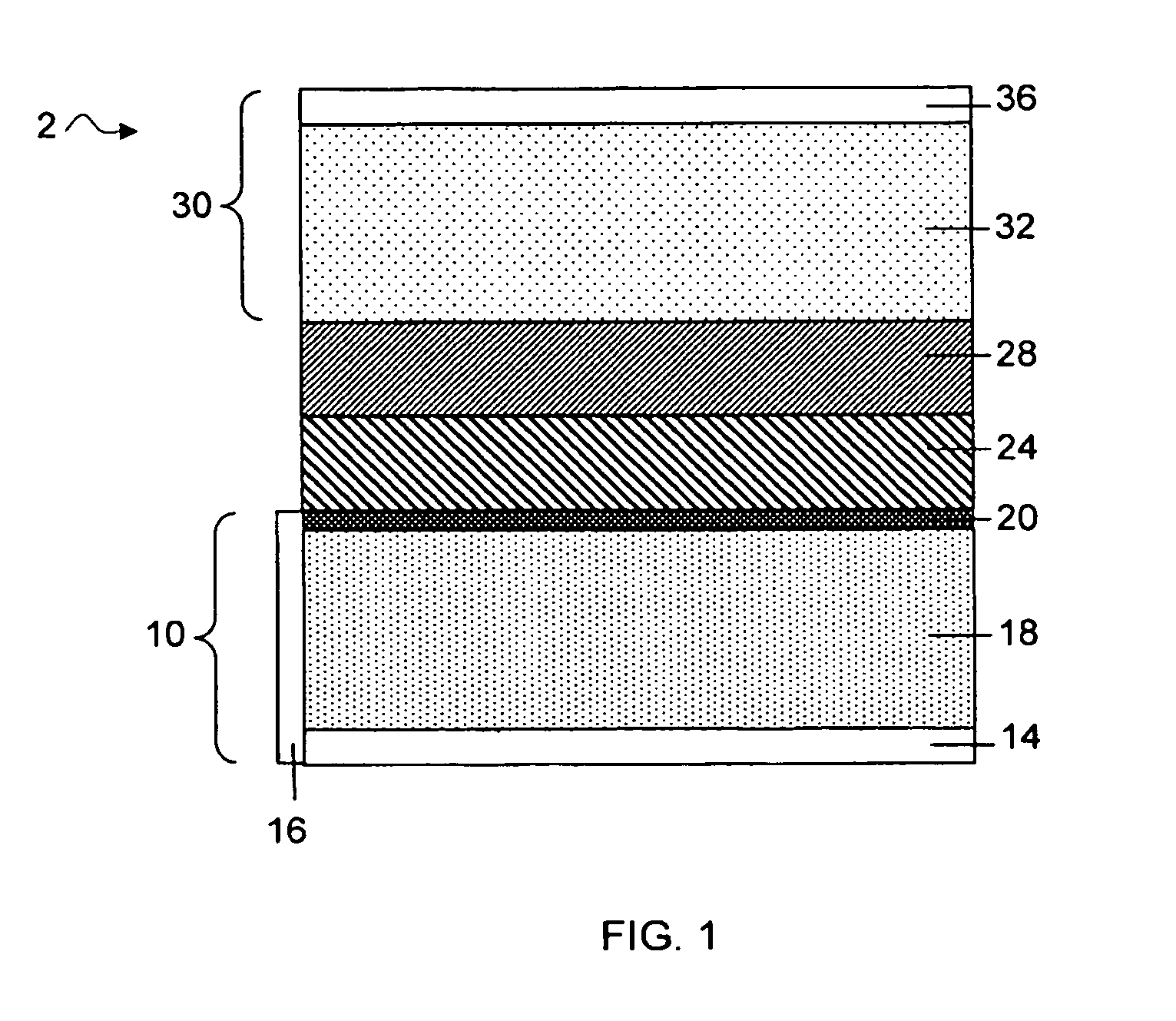
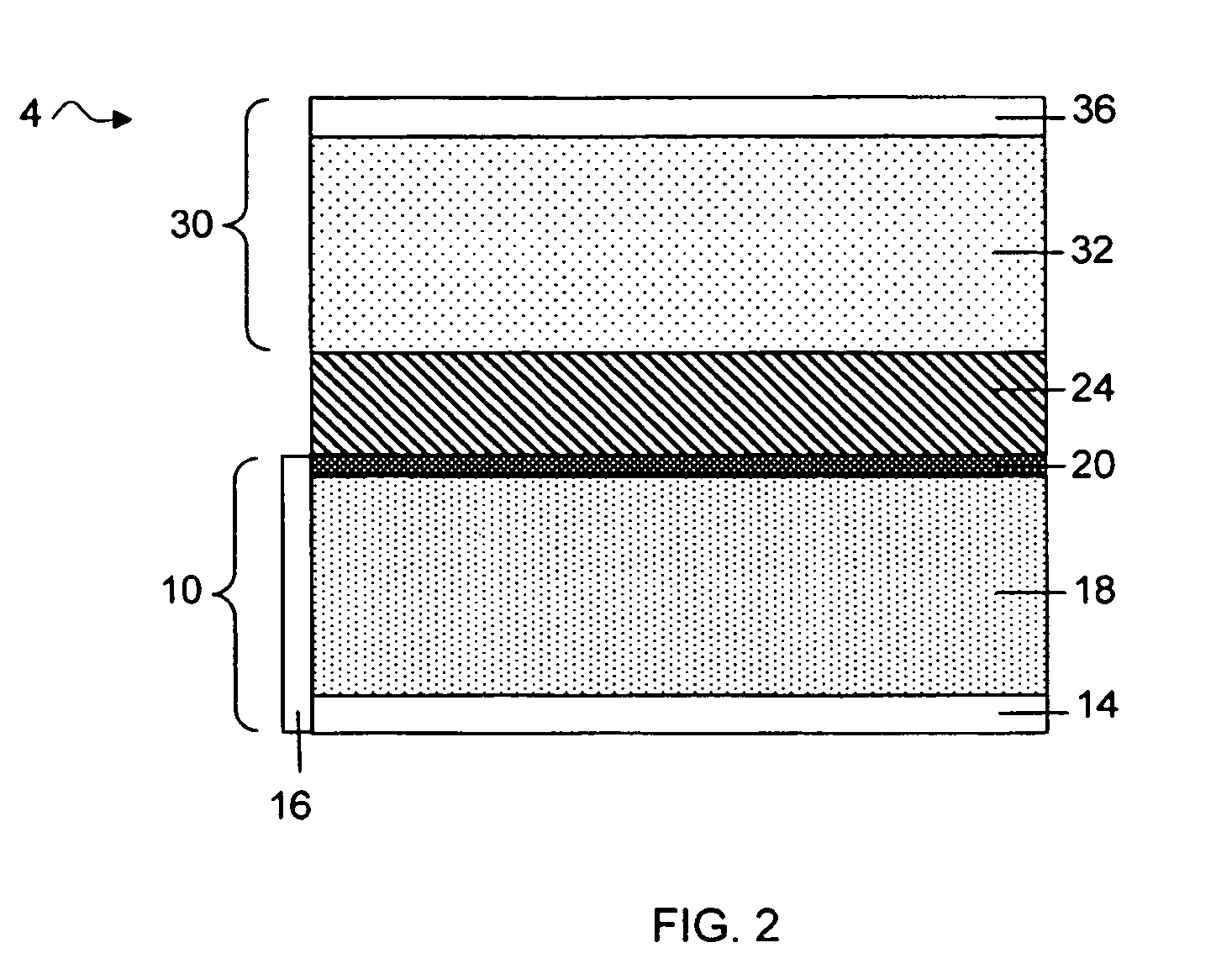
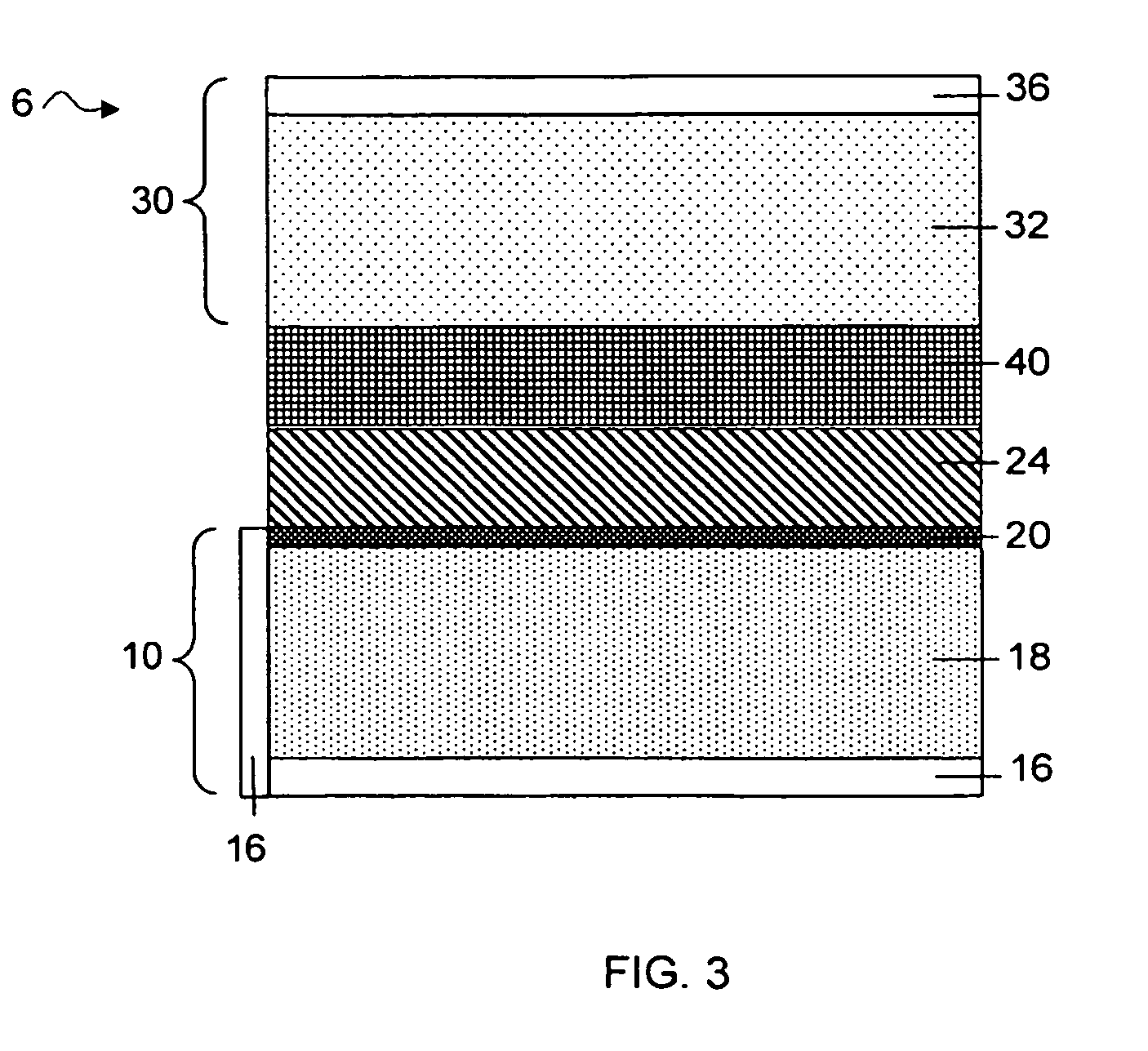
MultiLayer Solid Electrolyte for Lithium Thin Film Batteries
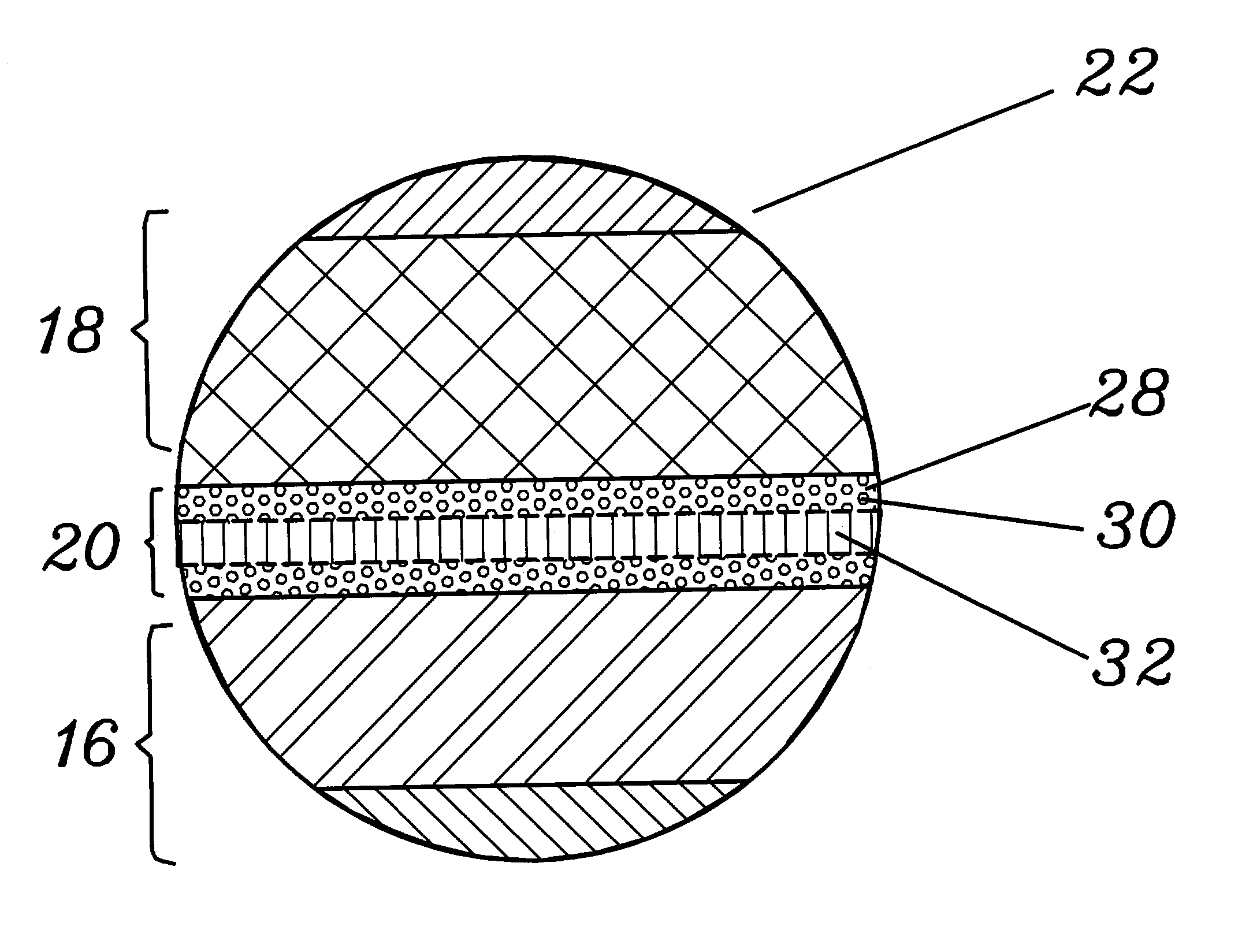
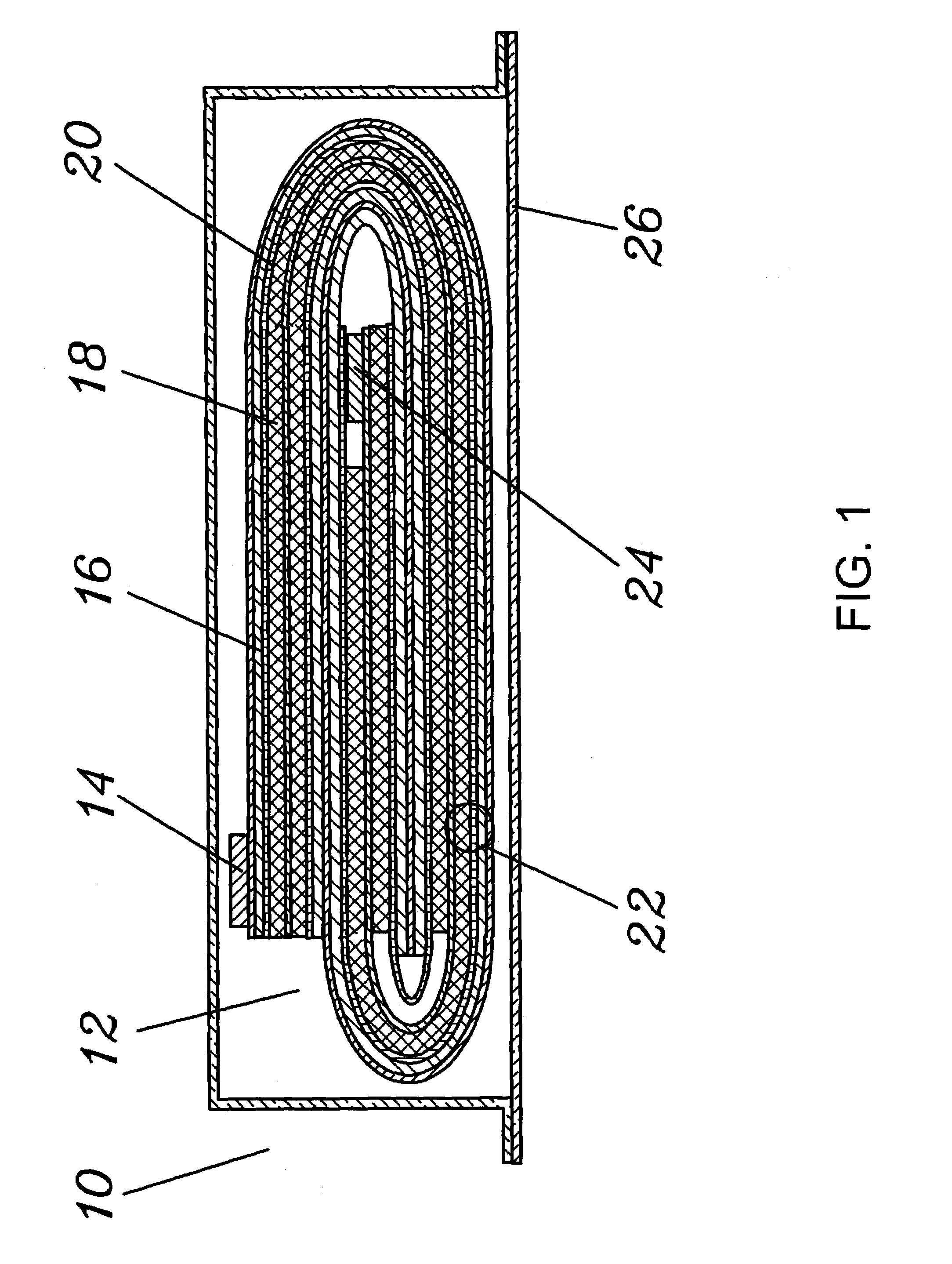
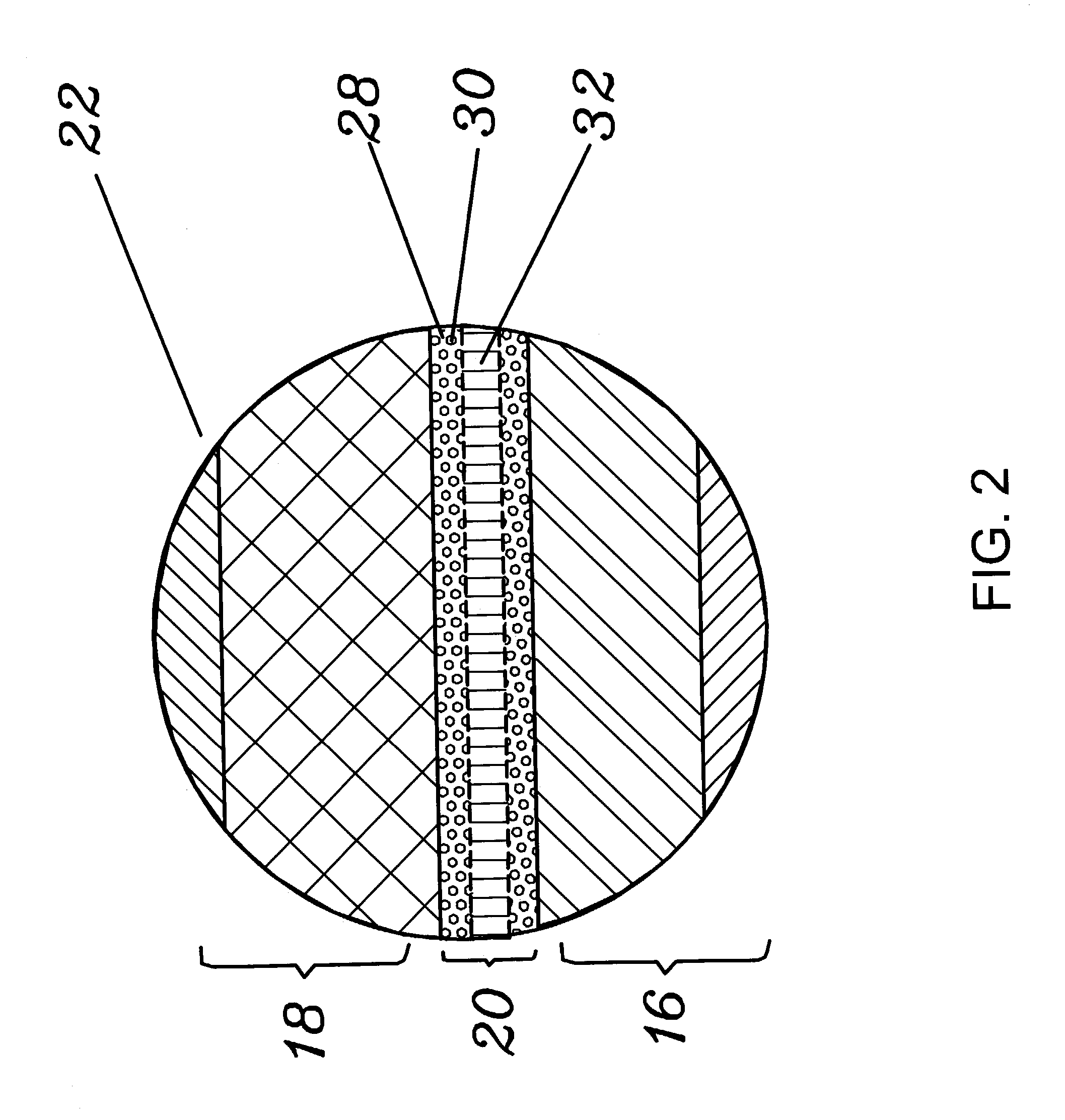
ActiveUS20100285372A1Rapidly and efficiently producedImprove the immunityBatteries circuit arrangementsFinal product manufactureLithium metalRechargeable cell
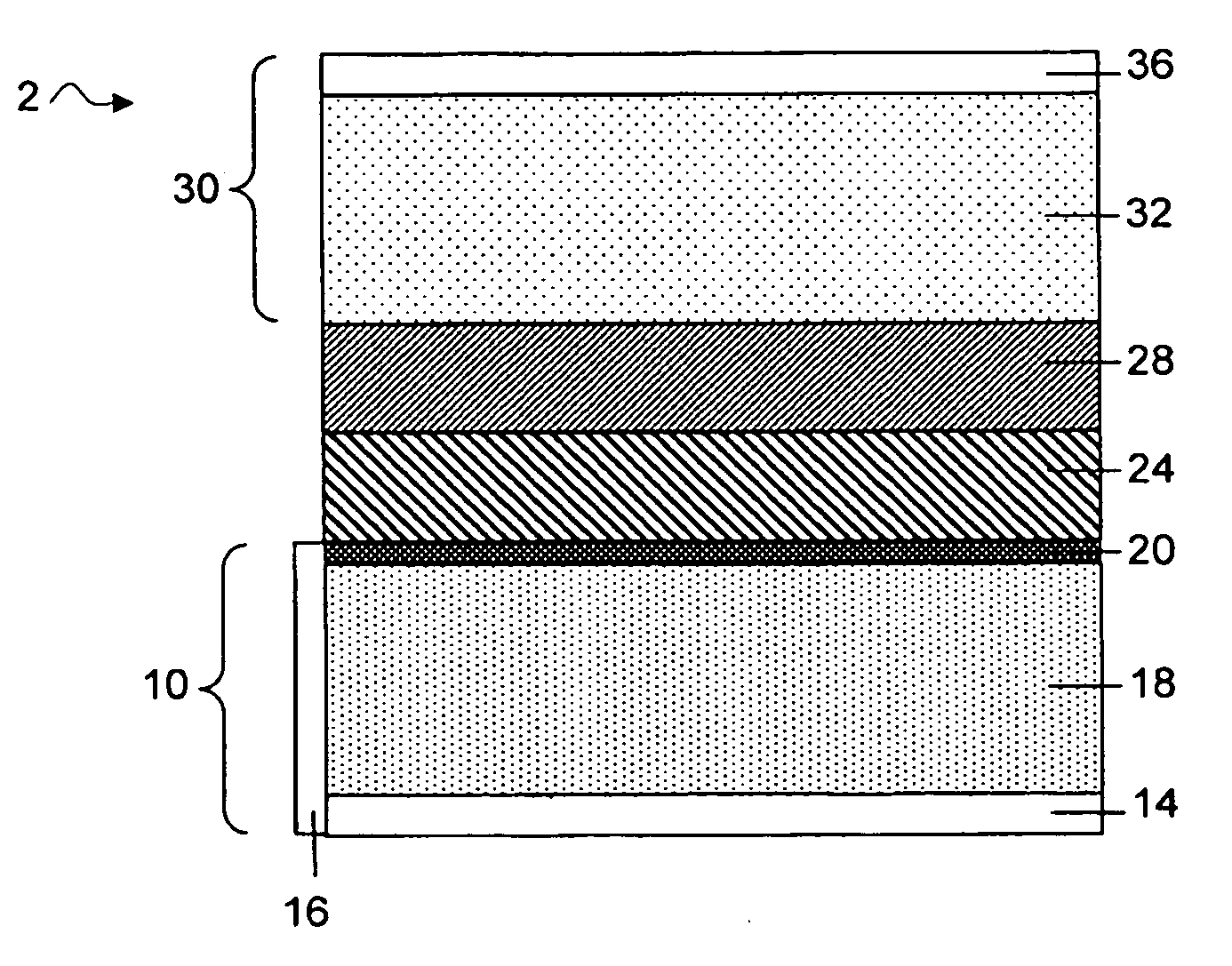
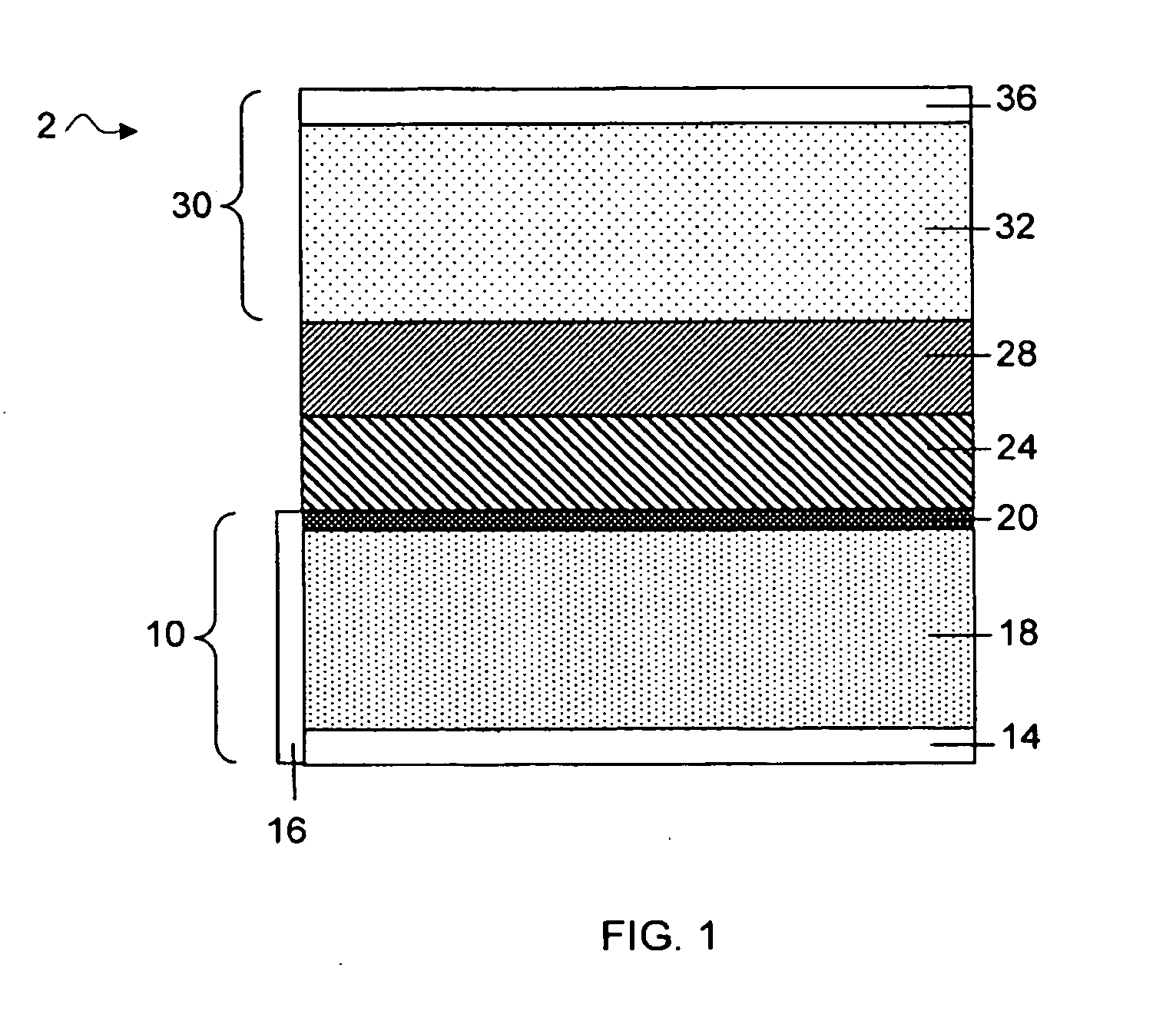
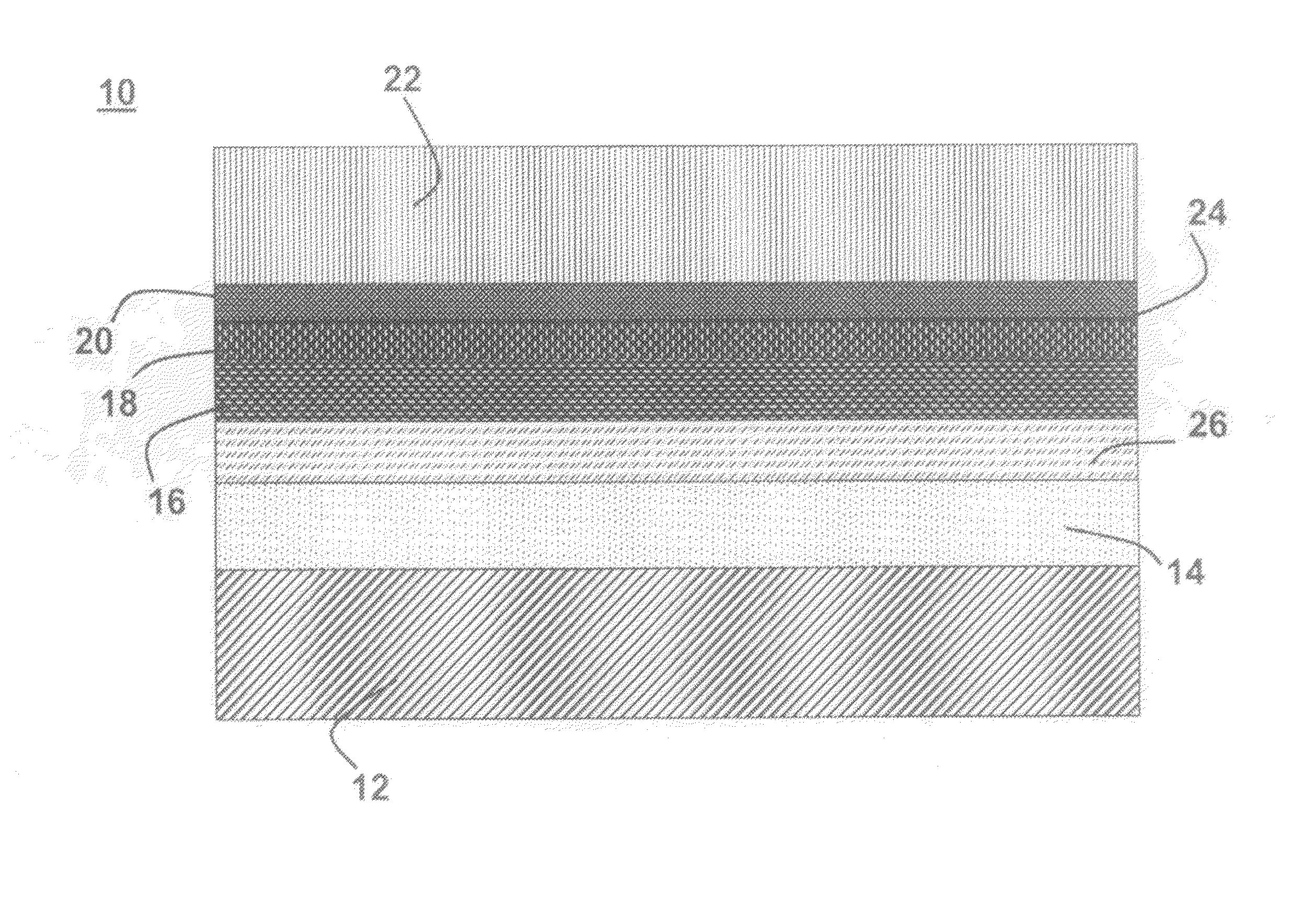
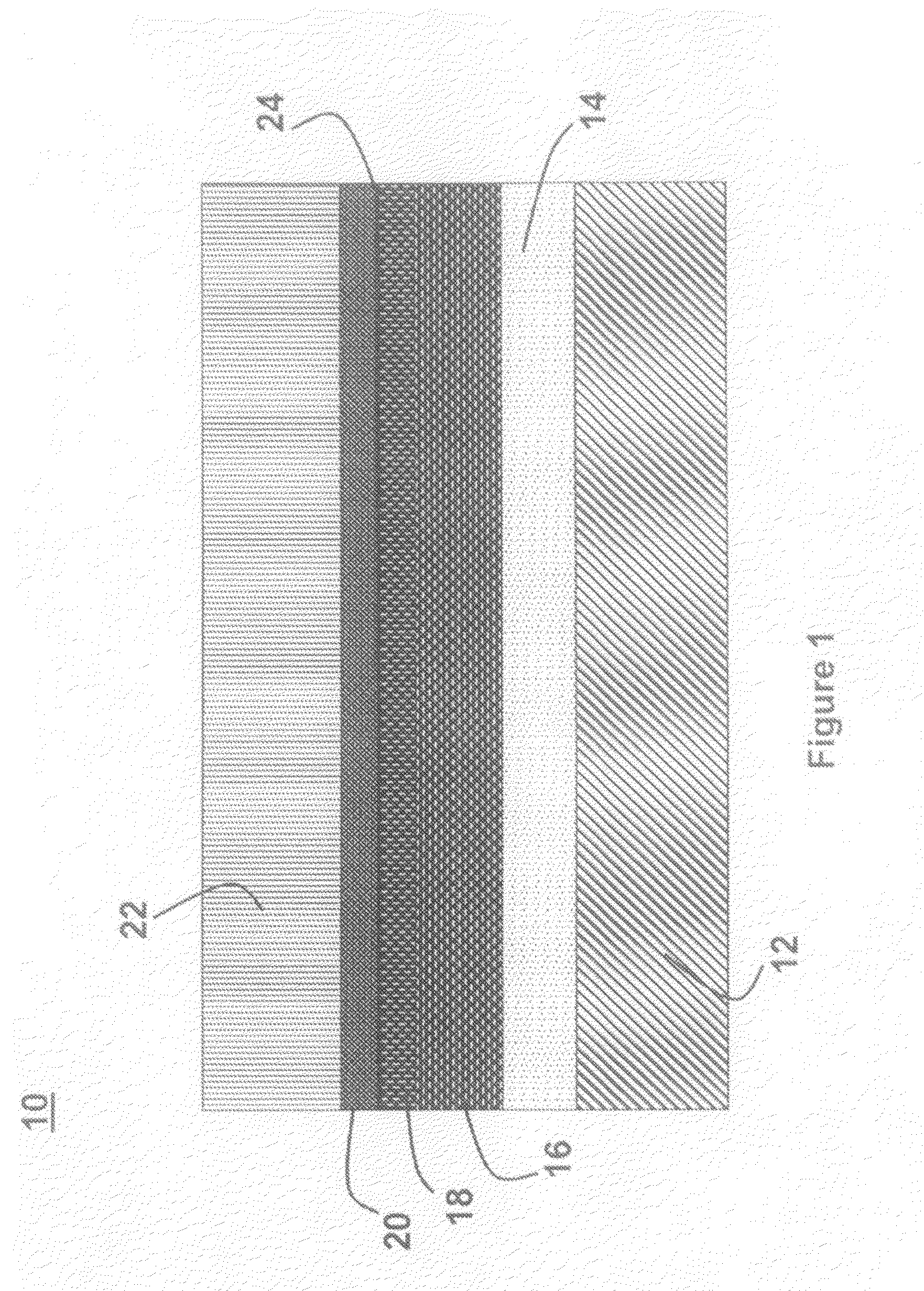
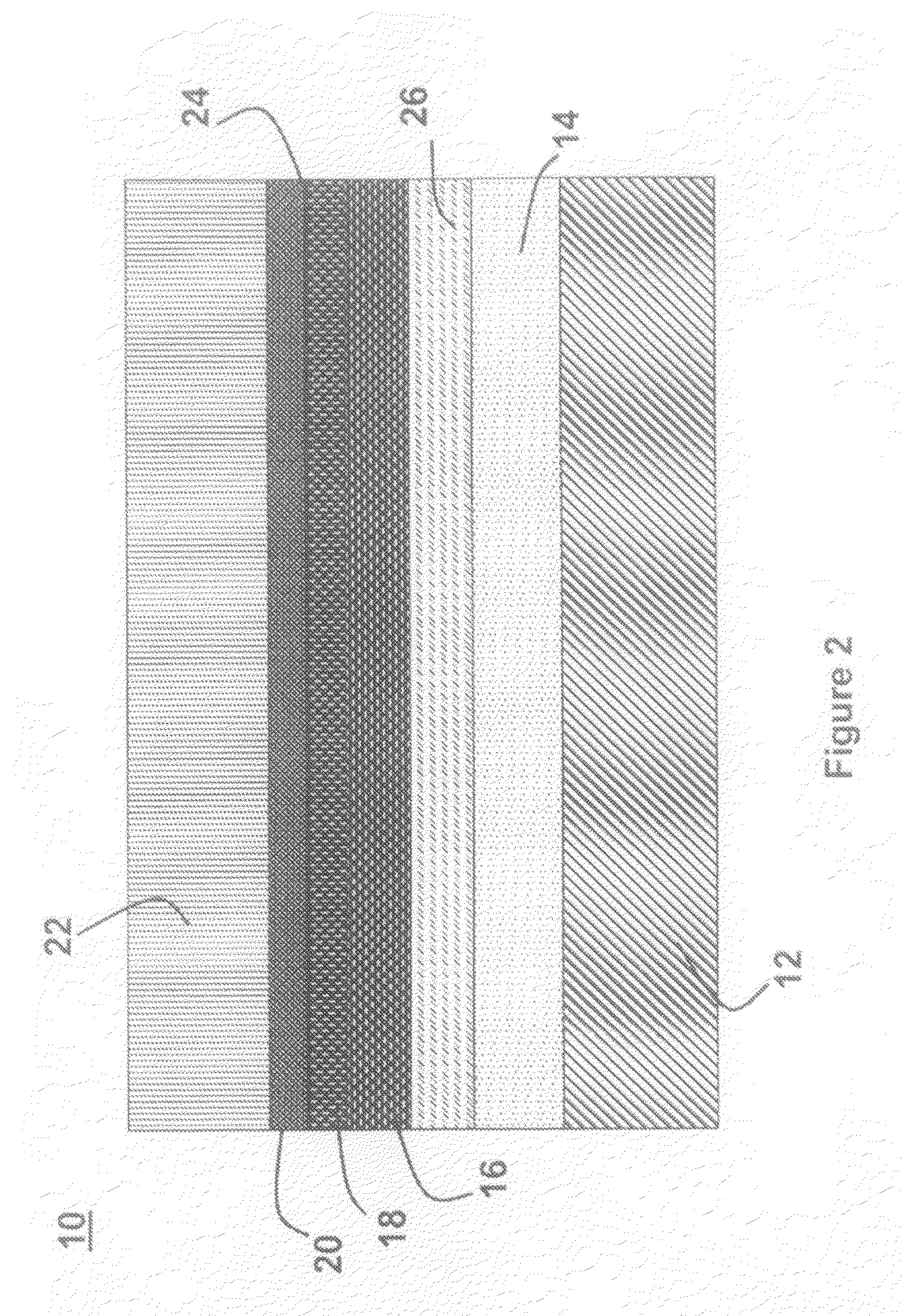
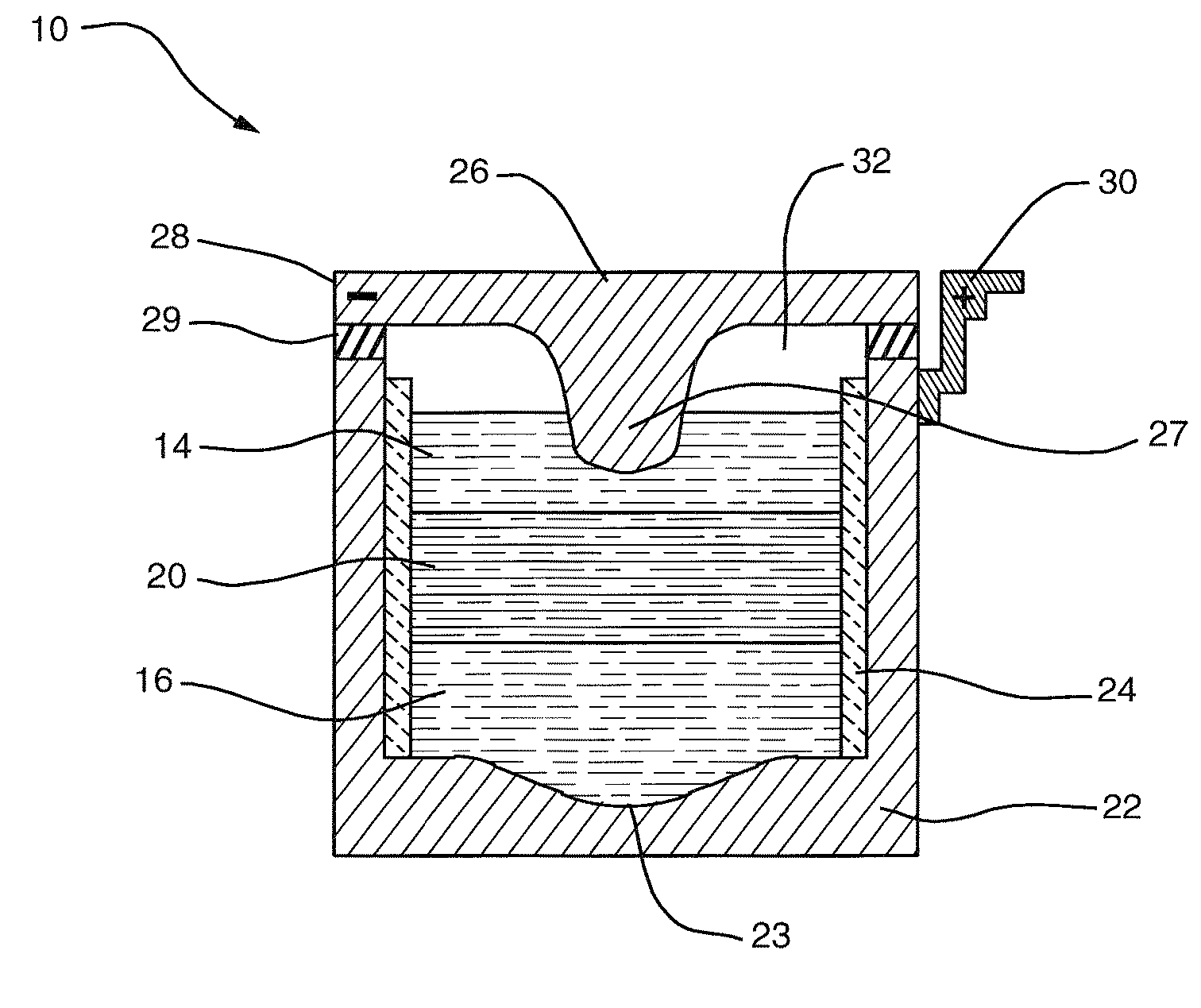
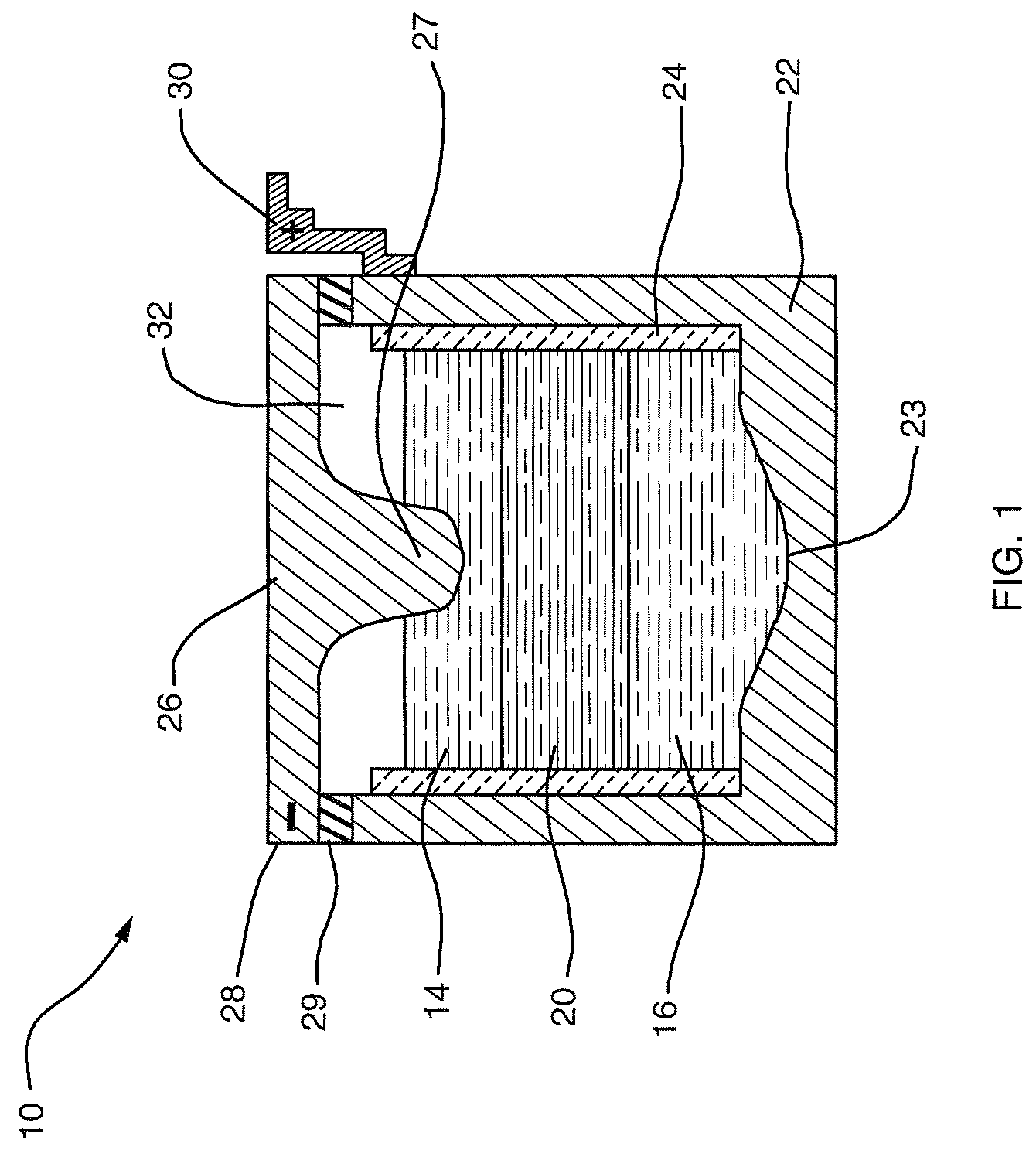
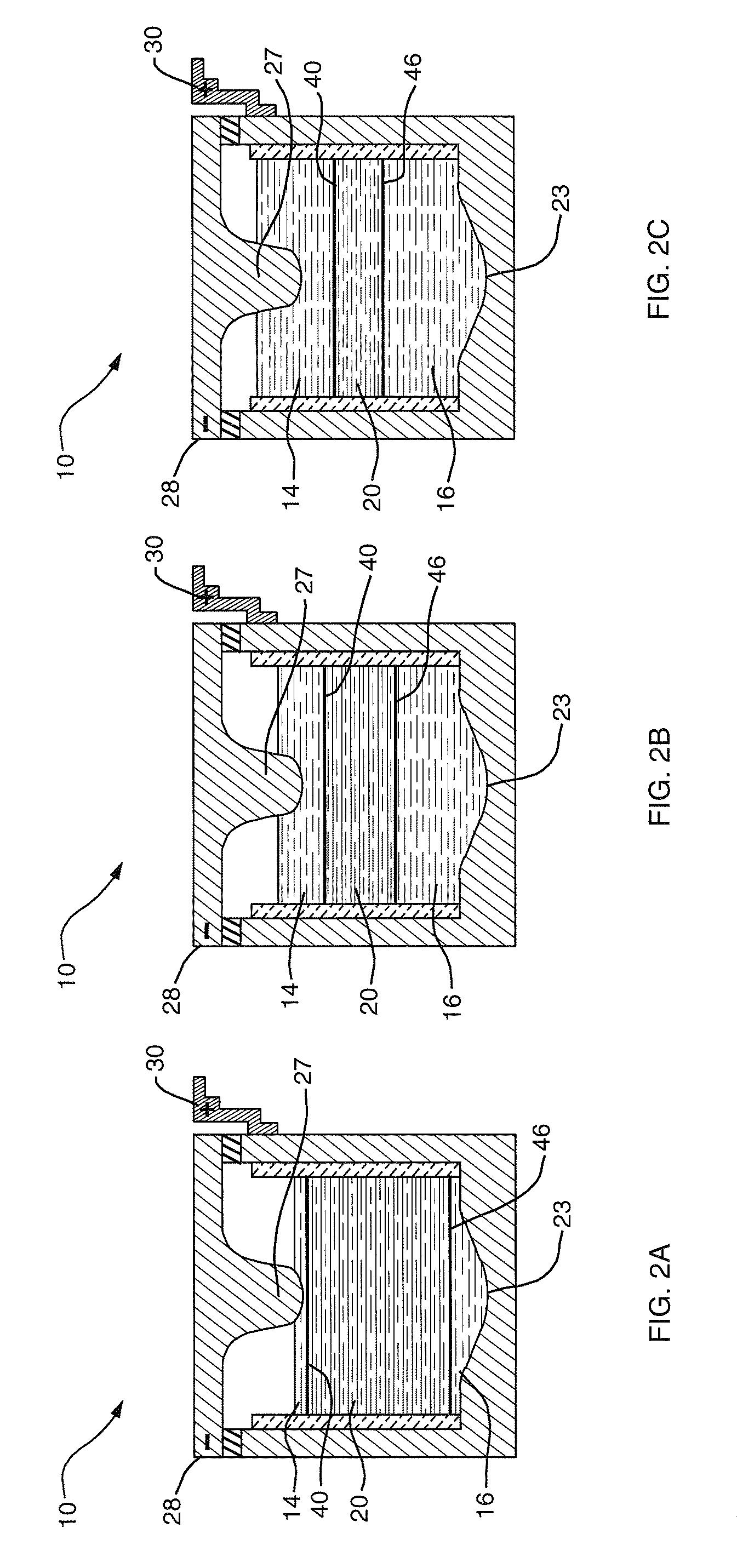
A lithium metal thin-film battery composite structure is provided that includes a combination of a thin, stable, solid electrolyte layer [18] such as Lipon, designed in use to be in contact with a lithium metal anode layer; and a rapid-deposit solid electrolyte layer [16] such as LiAlF4 in contact with the thin, stable, solid electrolyte layer [18]. Batteries made up of or containing these structures are more efficient to produce than other lithium metal batteries that use only a single solid electrolyte. They are also more resistant to stress and strain than batteries made using layers of only the stable, solid electrolyte materials. Furthermore, lithium anode batteries as disclosed herein are useful as rechargeable batteries.
Owner:ALLIANCE FOR SUSTAINABLE ENERGY
Redox flow cell
A redox flow cell is presented that utilizes a porous membrane separating a first half cell and a second half cell. The porous membrane is chosen to have a figure of merit (FOM) is at least a minimum FOM. A method of providing a porous membrane for a flow cell can include determining a figure of merit; determining a first parameter from a pore size or a thickness for the porous membrane; determining a second parameter from the pore size or the thickness that is not the first parameter for the porous membrane, based on the figure of merit; and constructing a porous membrane having the pore size and the thickness.
Owner:IMERGY POWER SYST
Additive for non-aqueous liquid electrolyte, non-aqueous liquid electrolyte secondary cell and non-aqueous liquid electrolyte electric double layer capacitor
InactiveUS20030170548A1Improve the immunityImprove securityLight-sensitive devicesCell electrodesElectrolytic agentPhysical chemistry
The present invention provides an additive for a non-aqueous electrolyte comprising a phosphazene derivative represented by the following formula (1): (PNR2)n formula (1) wherein R represents a fluorine-containing substituent or fluorine, at least one of all R's is a fluorine-containing substituent, and n represents 3 to 14. More particularly, the present invention provides a non-aqueous electrolyte secondary cell and a non-aqueous electrolyte electric double layer capacitor comprising the additive for a non-aqueous electrolyte which exhibit good low temperature characteristics, good resistance to deterioration, and good incombustibility, and accordingly are significantly high in safety.
Owner:BRIDGESTONE CORP
Method for designing redox flow battery system
InactiveUS20050181273A1Improve system efficiencySure easyCell electrodesFuel cell auxillariesOxidation-Reduction AgentRedox
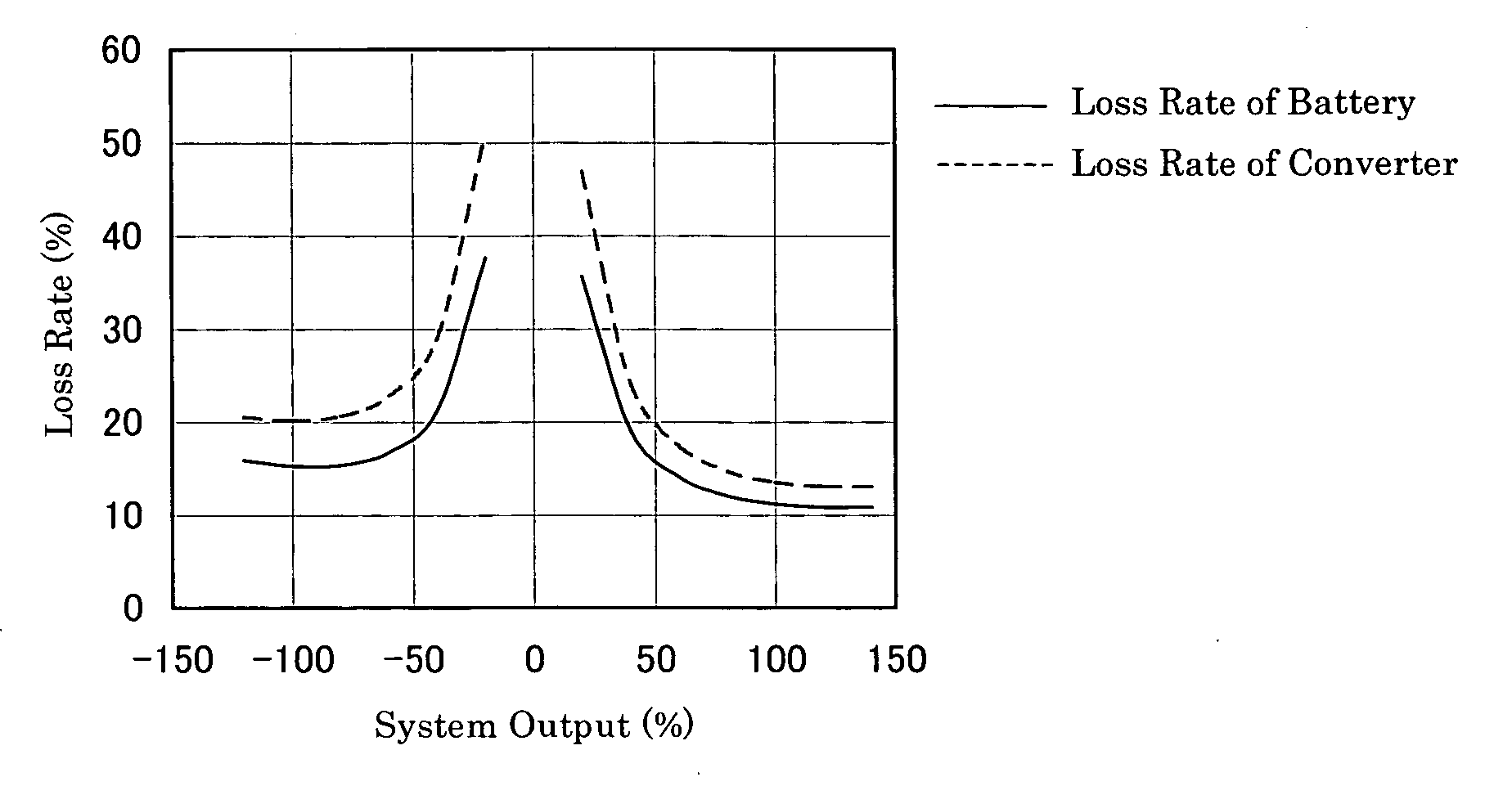
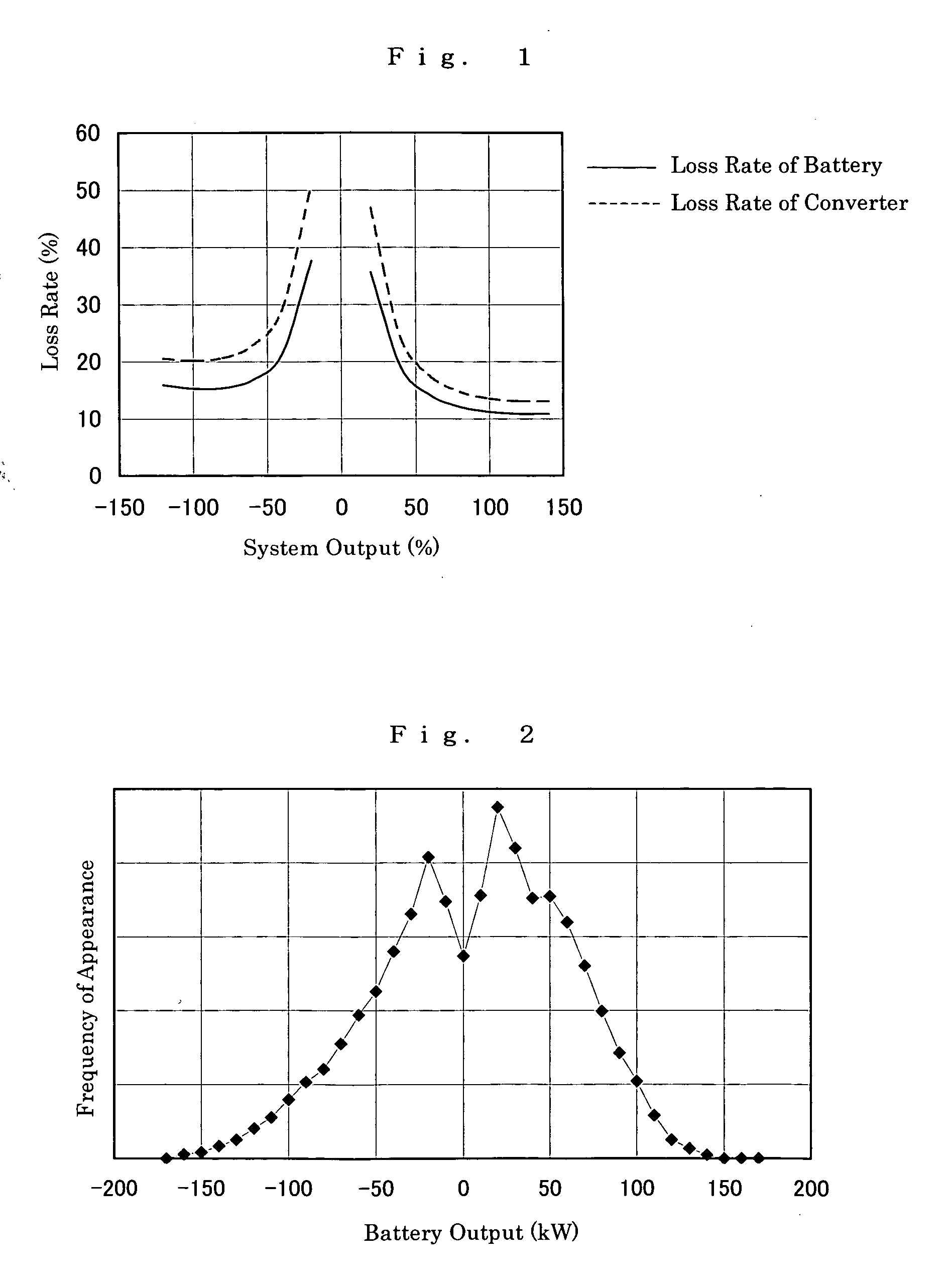
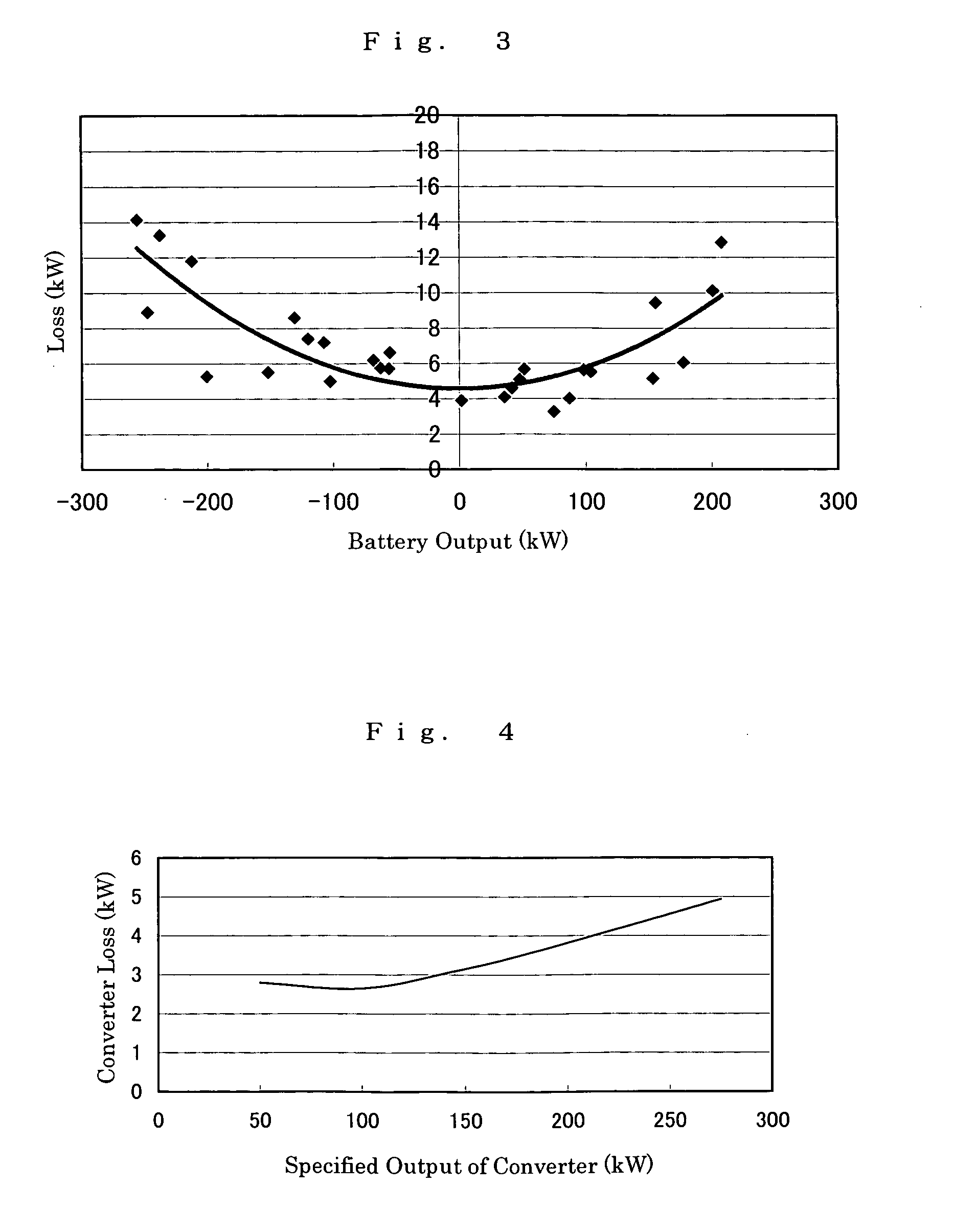
The present invention provides a method of designing a redox flow battery system that can prevent system efficiency loss caused by weak generation power or load power at the time of electric charge or discharge, without using any lead storage battery, and can also provide further improved system efficiency. In the present invention, generating equipment that varies irregularly in output of power generation is provided with the redox flow battery to smooth the output of power generation. An average value of output distribution of the battery with respect to the smoothed output of power generation and standard deviation are determined. Then, at least either of a specified output of the battery and a specified output of the converter for converting the battery output is determined based on the standard deviation.
Owner:SUMITOMO ELECTRIC IND LTD +1
Johnson reversible engine
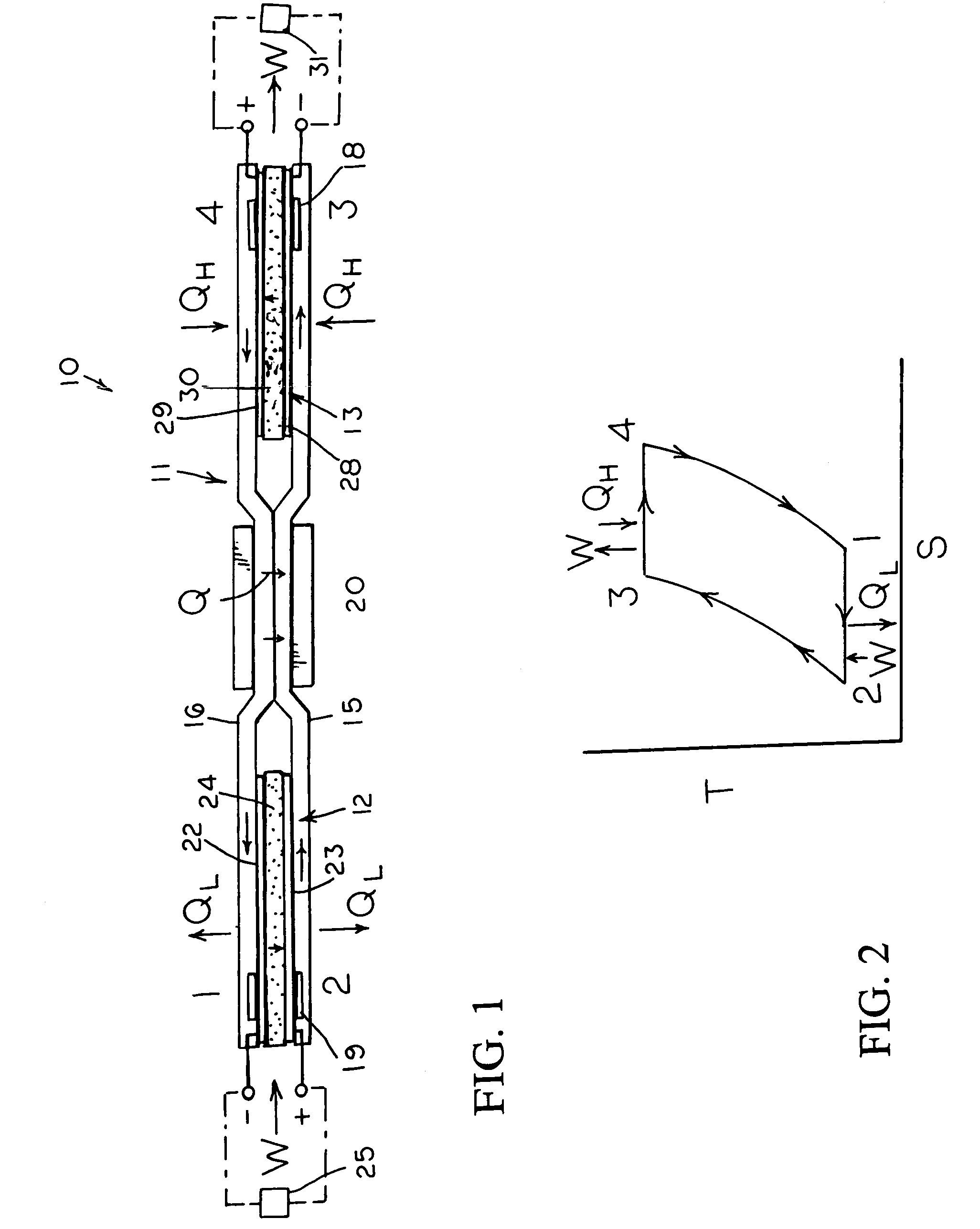
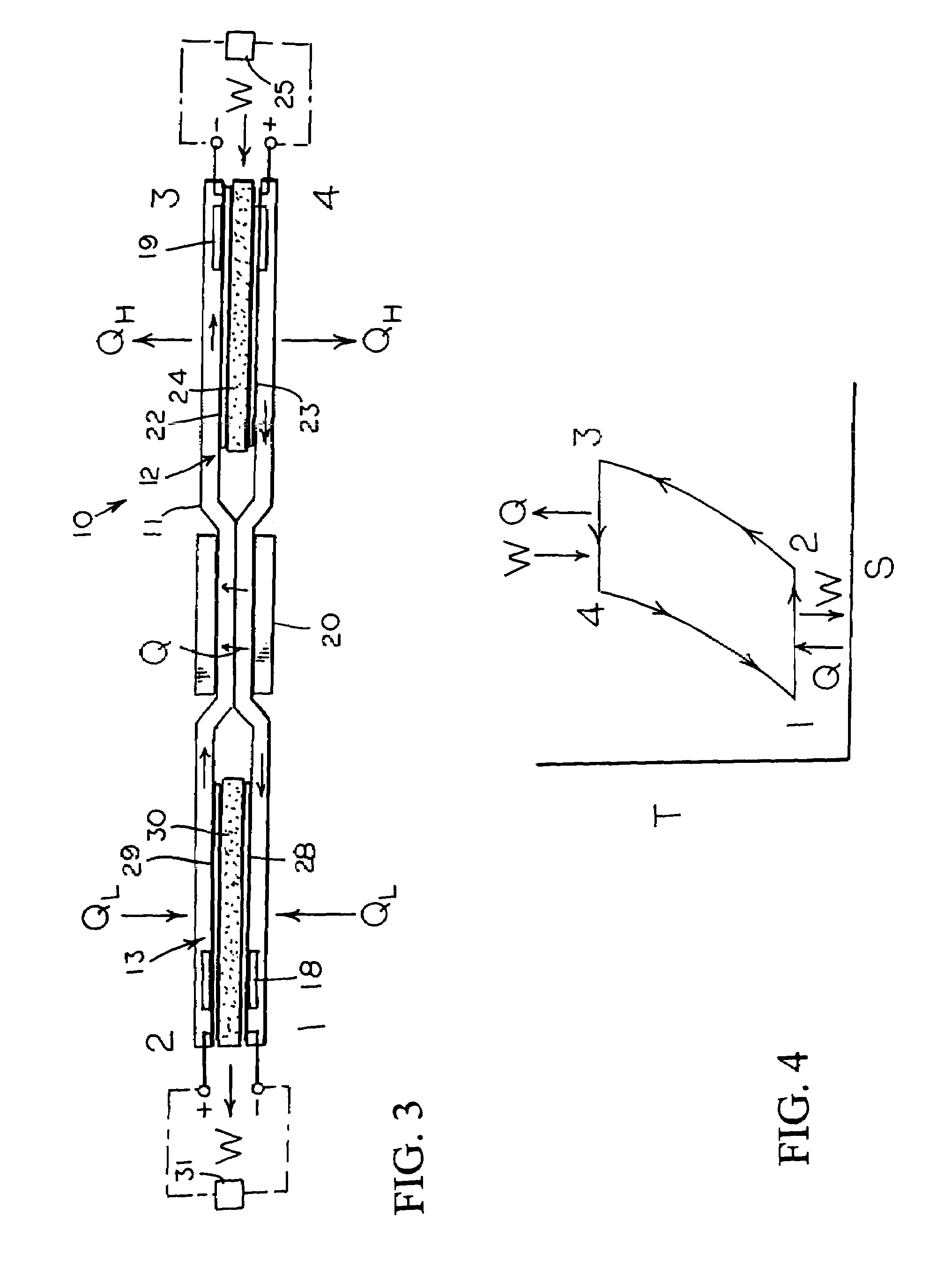
InactiveUS7160639B2Increase pressureReduce pressureHeat pumpsFuel cell heat exchangeEngineeringElectrochemical cell
An reversible engine (10) is disclosed having a conduit system (11), a first electrochemical cells (12), and a second electrochemical cell (13). The conduit system (11) includes a first conduit (15) extending from the first electrochemical cell (12) to the second electrochemical cell (13), and a second conduit (16) extending from the second electrochemical cell (13) to the first electrochemical cell (12). The heat engine (10) also includes a heater (18) mounted in thermal communication with the conduit system (11) adjacent the second electrochemical cell (13), a cooler (19) mounted in thermal communication with the conduit system (11) adjacent the first electrochemical cell (12), and a regenerative heat exchanger (20) thermally coupled to the first and second conduits (15) and (16) for the transfer of heat therebetween.
Owner:JTEC ENERGY INC
Current collector of positive electrode and sodium-sulfur battery using the same
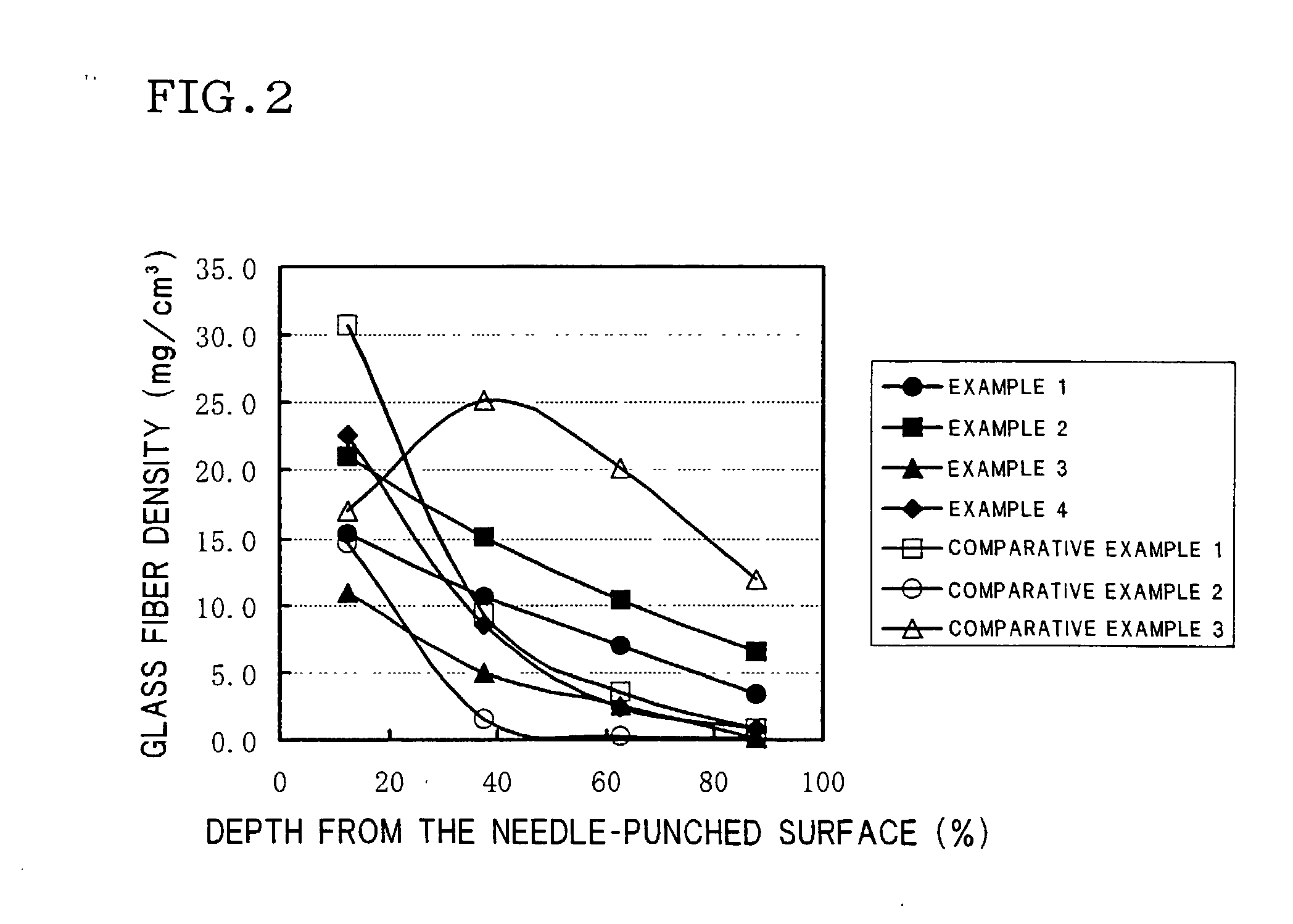
InactiveUS20030054255A1Electrode carriers/collectorsSolid electrolyte cellsHigh resistanceGlass fiber
A current collector of positive electrode enabling a NaS battery to be excellent in the charge recovery characteristic and low in internal resistance is provided, which collector has a high resistance layer formed by needle-punching glass fibers with 5 to 15 mum fiber diameters into a felt substrate made of carbon fibers or graphite fibers by needle-punching from the one surface of the substrate. The density of the glass fibers needle-punched into the substrate is gradually decreased in the direction from the above mentioned surface to the other surface of the substrate, and the deepest portions of the needle-punched glass fibers reach the depths of 85 to 100% of the substrate thickness.
Owner:NGK INSULATORS LTD
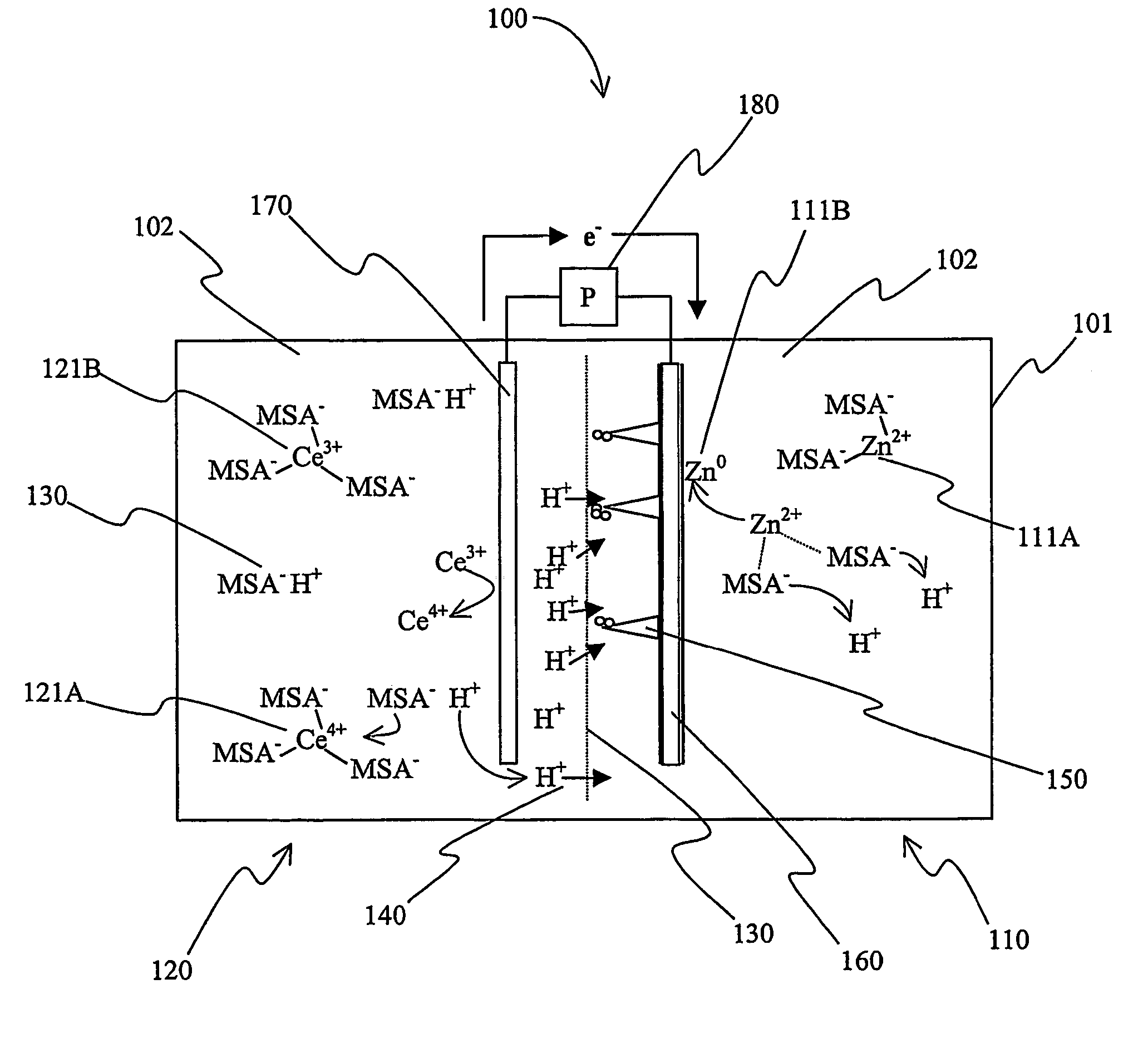
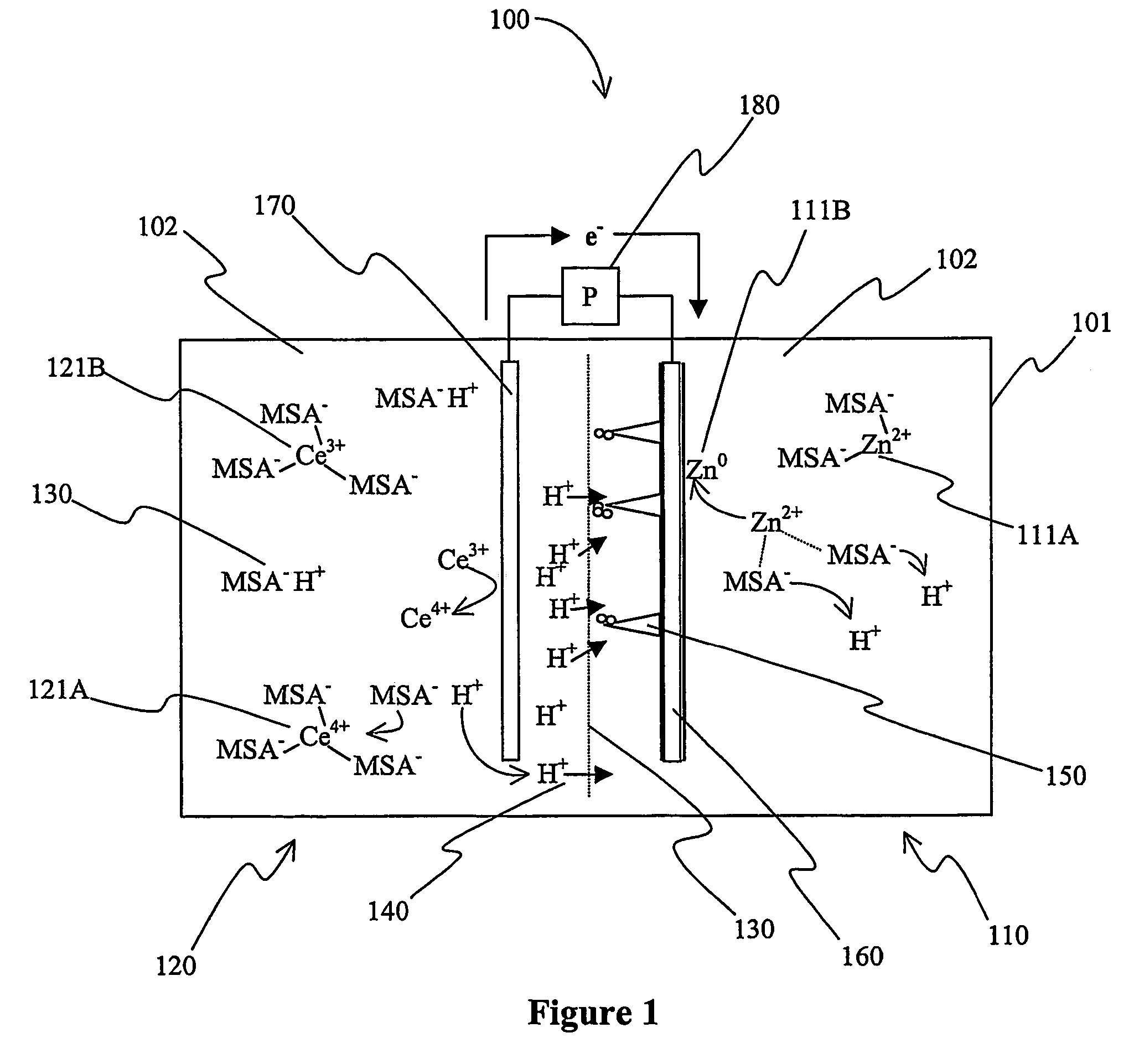
Secondary battery with autolytic dendrites
A battery (100) comprises a cell having a cathode compartment (120) that includes an element that is oxidized during charging of the battery (100), wherein the oxidized element forms a salt with an acid and thereby increases the H+ concentration in the cathode compartment (120) sufficient to promote an H+ flux into the anode compartment (110) across the separator (130), wherein the H+ flux across the separator (130) is sufficient to disintegrate a zinc dendrite proximal to the separator (130).
Owner:PLURION LTD
Lithium-sulfur secondary battery containing gradient electrolyte
ActiveUS20140342209A1Reduce electrical conductivityLow ionic conductivityElectrode carriers/collectorsTwo electrolyte cellsLithium sulfurBattery cell
A rechargeable lithium-sulfur cell comprising a cathode, an anode, a separator electronically separating the two electrodes, a first electrolyte in contact with the cathode, and a second electrolyte in contact with the anode, wherein the first electrolyte contains a first concentration, C1, of a first lithium salt dissolved in a first solvent when the first electrolyte is brought in contact with the cathode, and the second electrolyte contains a second concentration, C2, of a second lithium salt dissolved in a second solvent when the second electrolyte is brought in contact with the anode, wherein C1 is less than C2. The cell exhibits an exceptionally high specific energy and a long cycle life.
Owner:GLOBAL GRAPHENE GRP INC
Electrode array for use in electrochemical cells
InactiveUS20050079409A1Reduce leakageLower ESRFinal product manufactureDouble layer capacitorsElectrical resistance and conductanceFuel cells
The invention features an electrode array (7) in which pairs of electrodes (1) are geometrically arranged so that the broadest faces of the exposed electrodes are not directly opposing to each other. Rather, the broadest facing surfaces of the electrodes in the array are parallel, adjacent, or offset at an angle. The electrode geometry of an electrode array of the invention permits electrodes to be in close proximity, thereby lowering series resistance, while minimizing the possibility for short circuits that can cause electrical leakage. An electrode array of the invention can be used in an electrochemical cell, such as a battery, e.g., a lithium battery, a capacitor, a flow-through capacitor, or a fuel cell.
Owner:BIOSOURCE INC
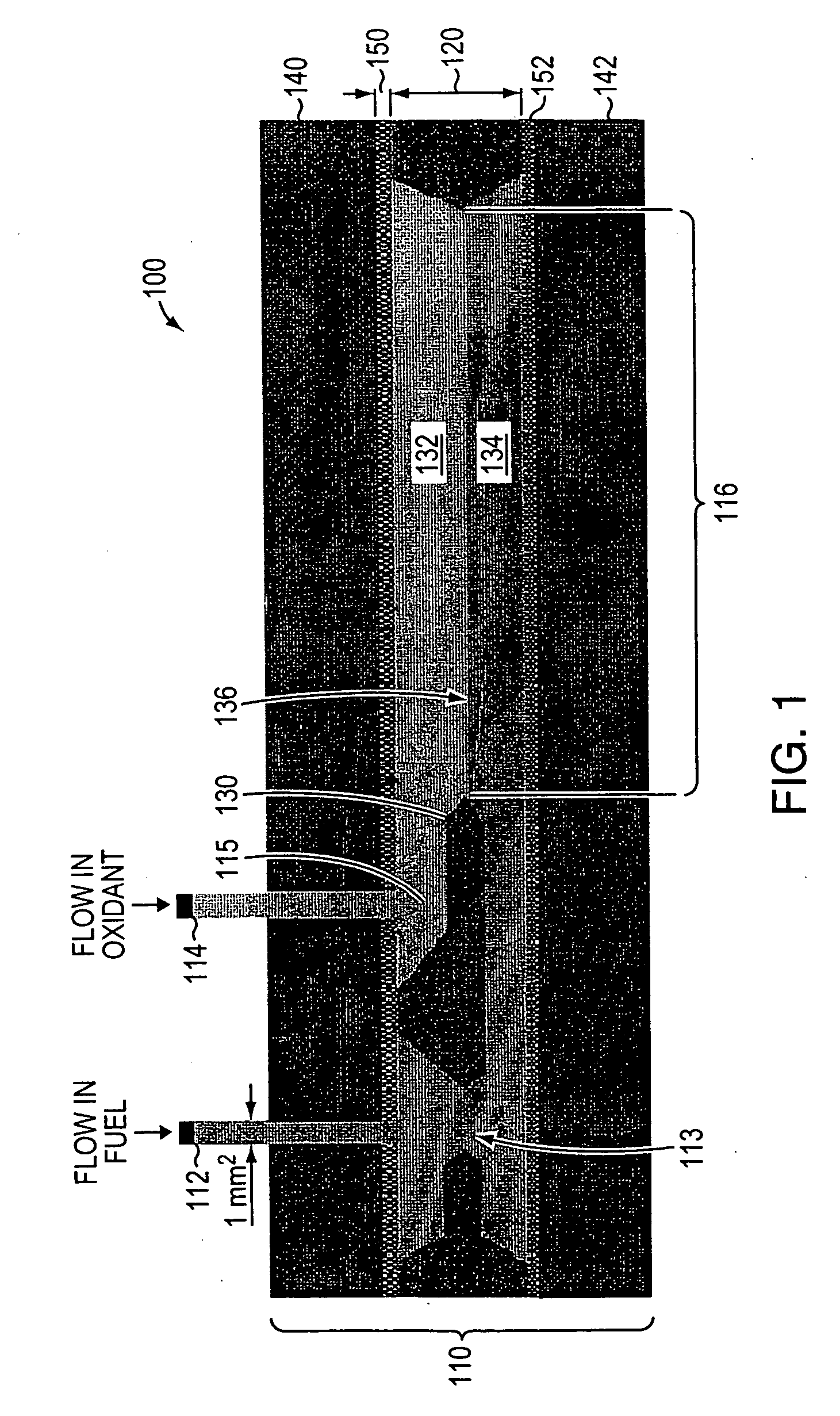
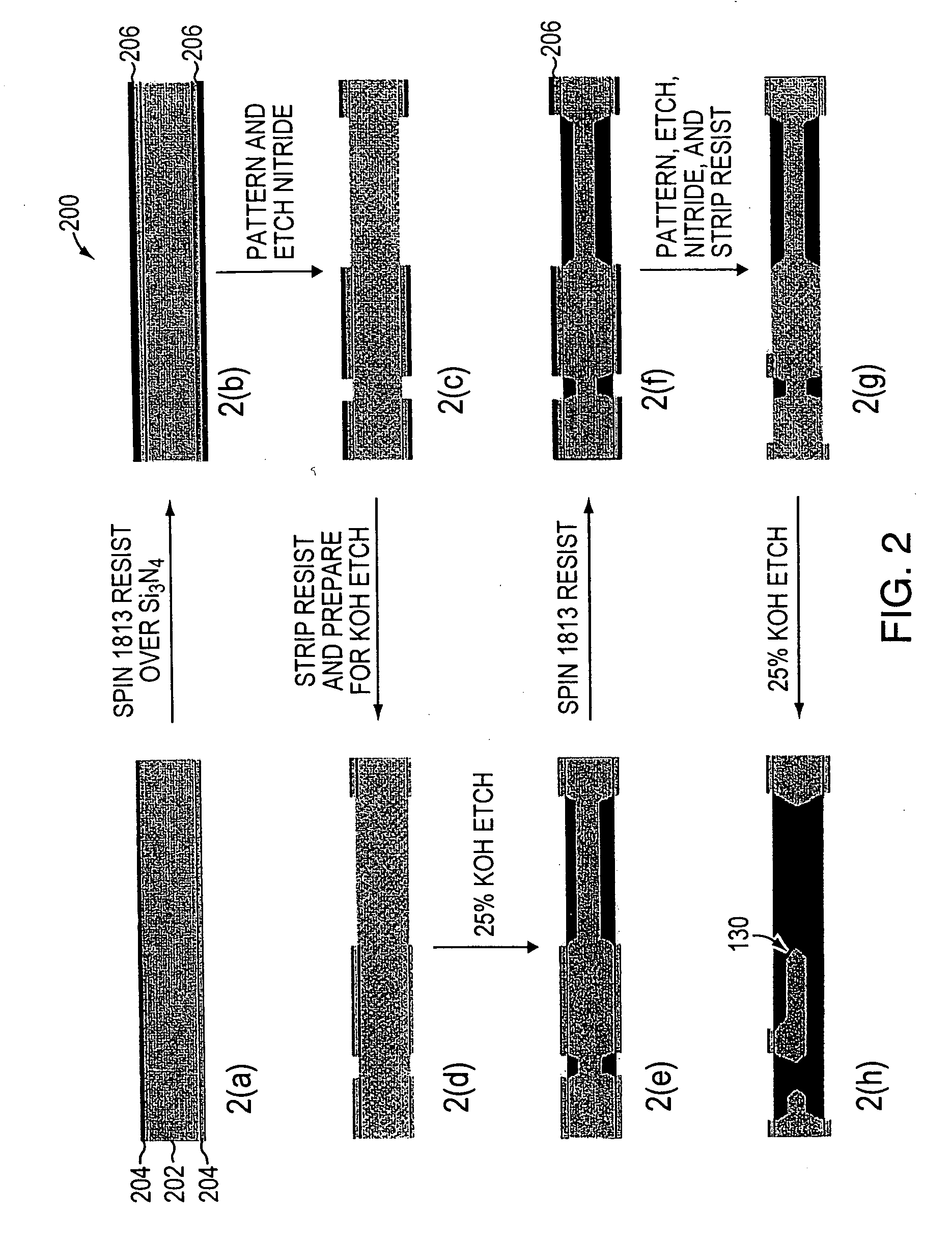
Dual electrolyte membraneless microchannel fuel cells
InactiveUS20060228622A1Maintain structural integrityHigh power deviceElectrolyte holding meansCell electrodesFlow cellEngineering
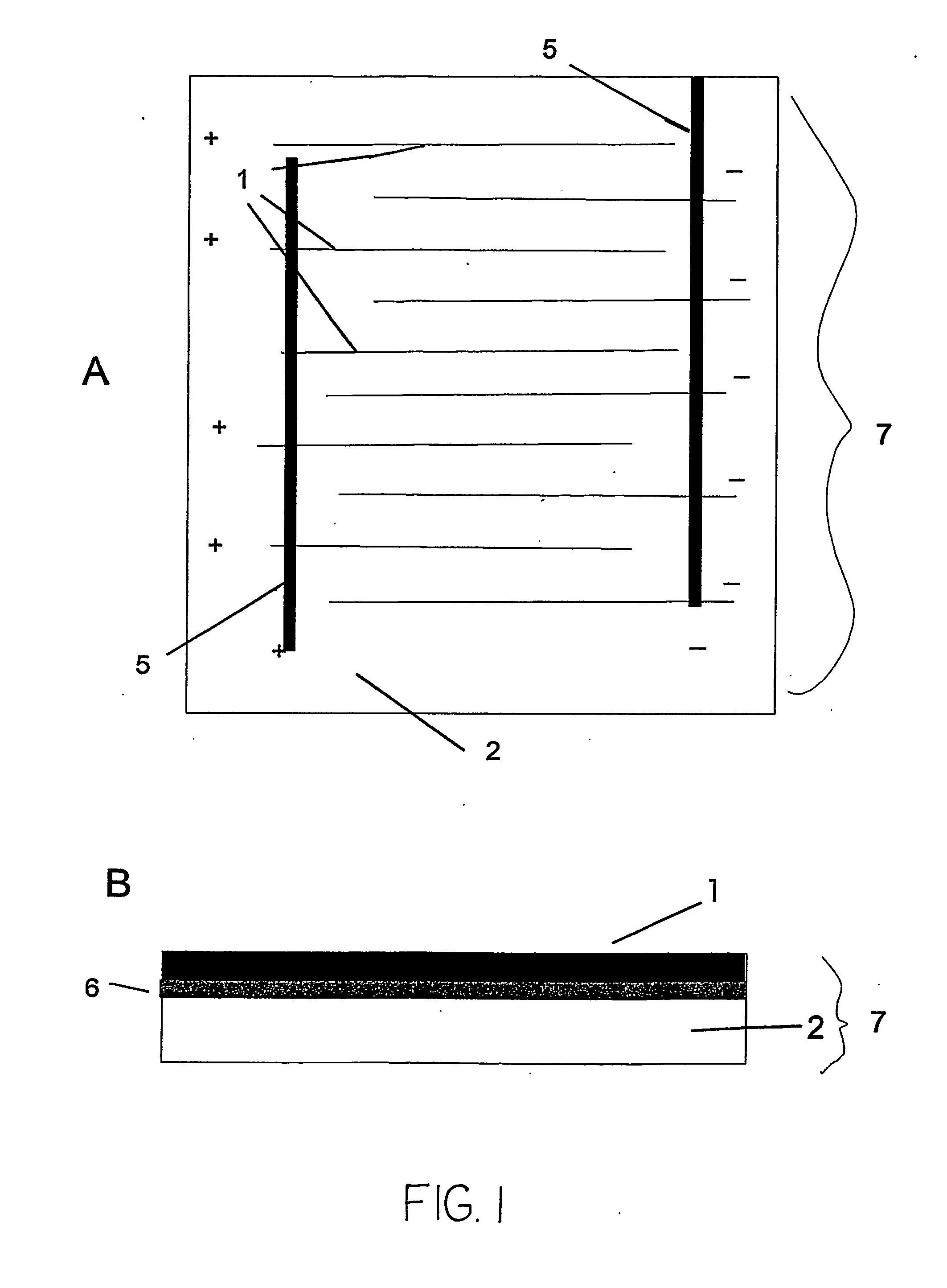
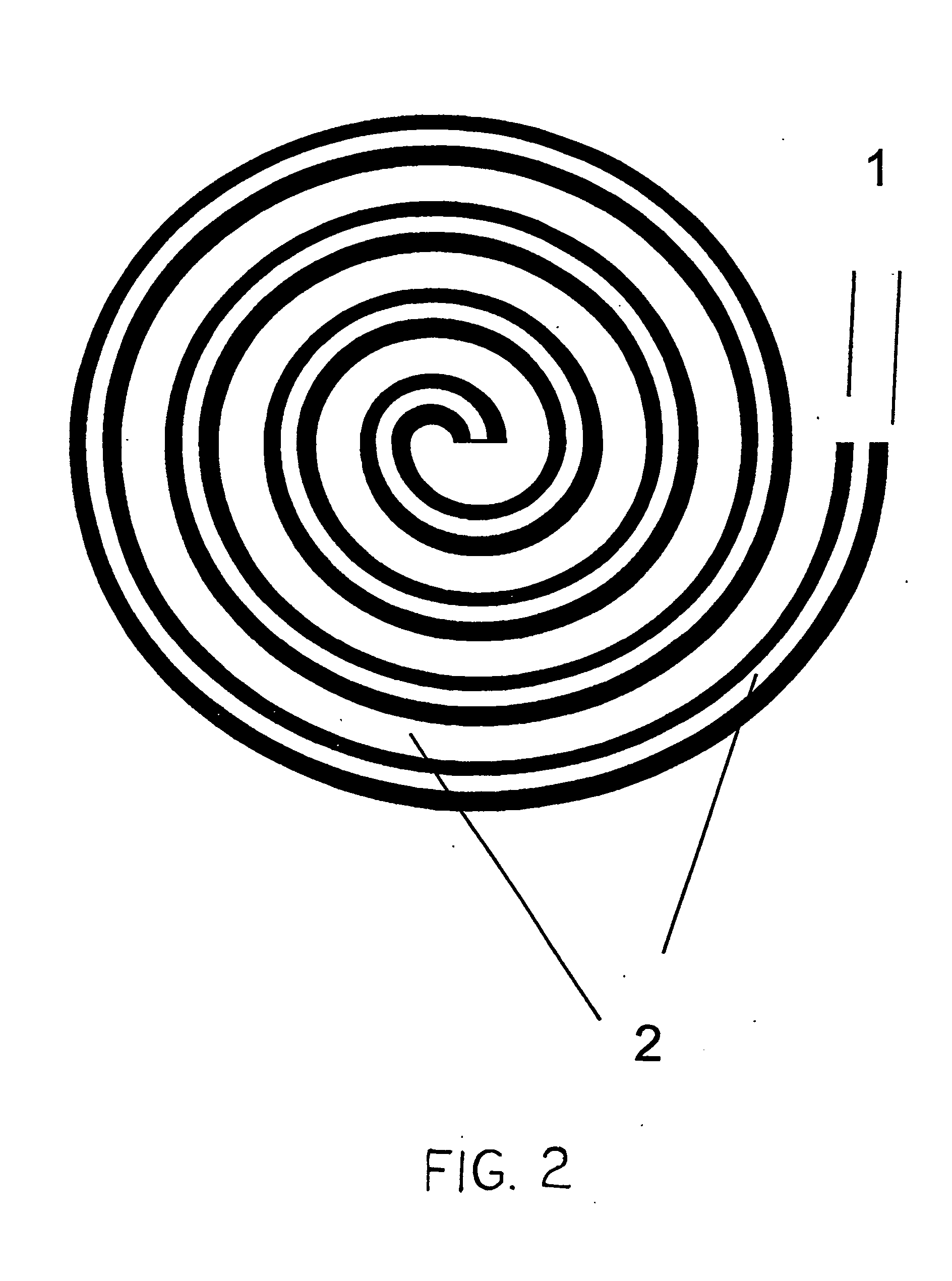
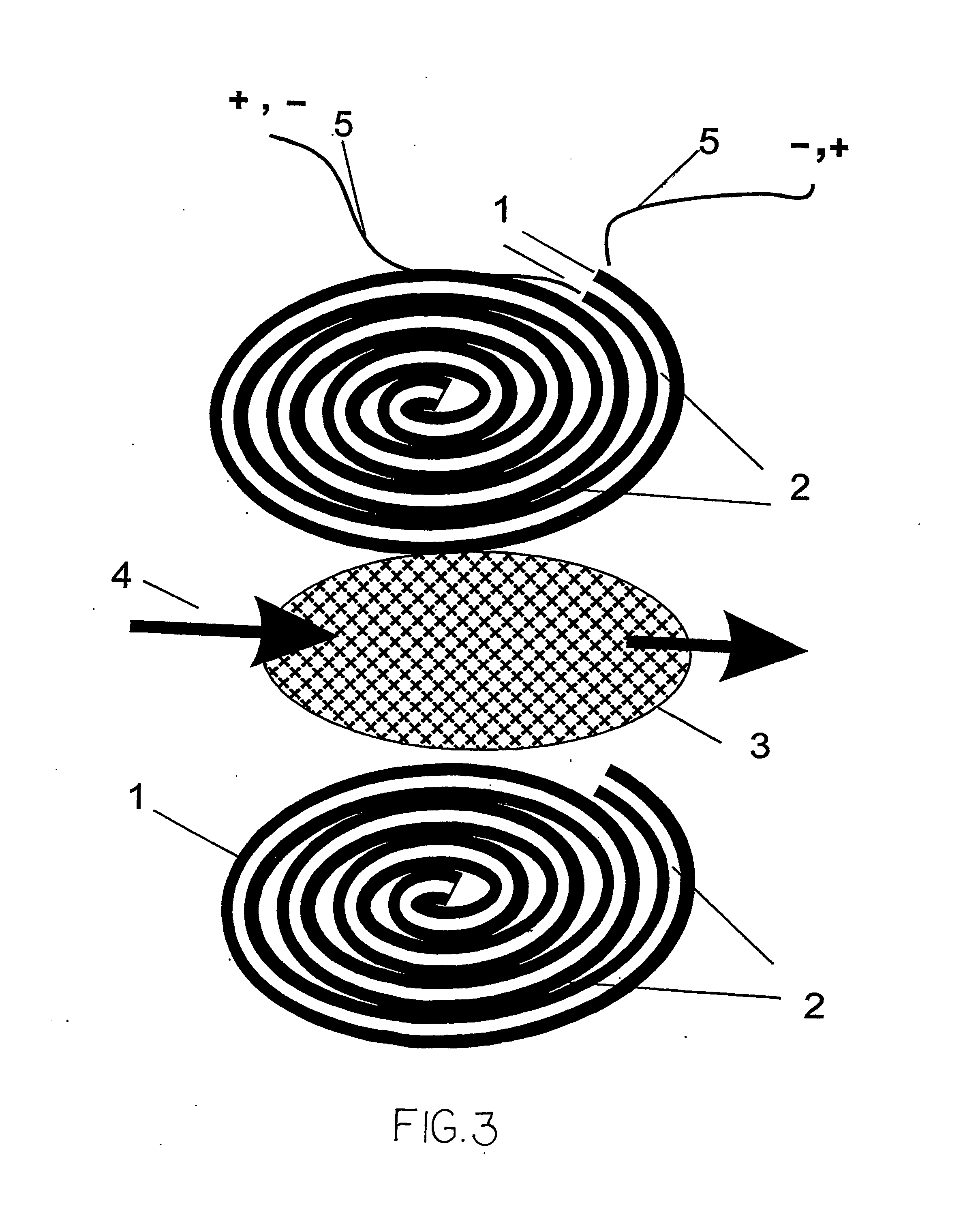
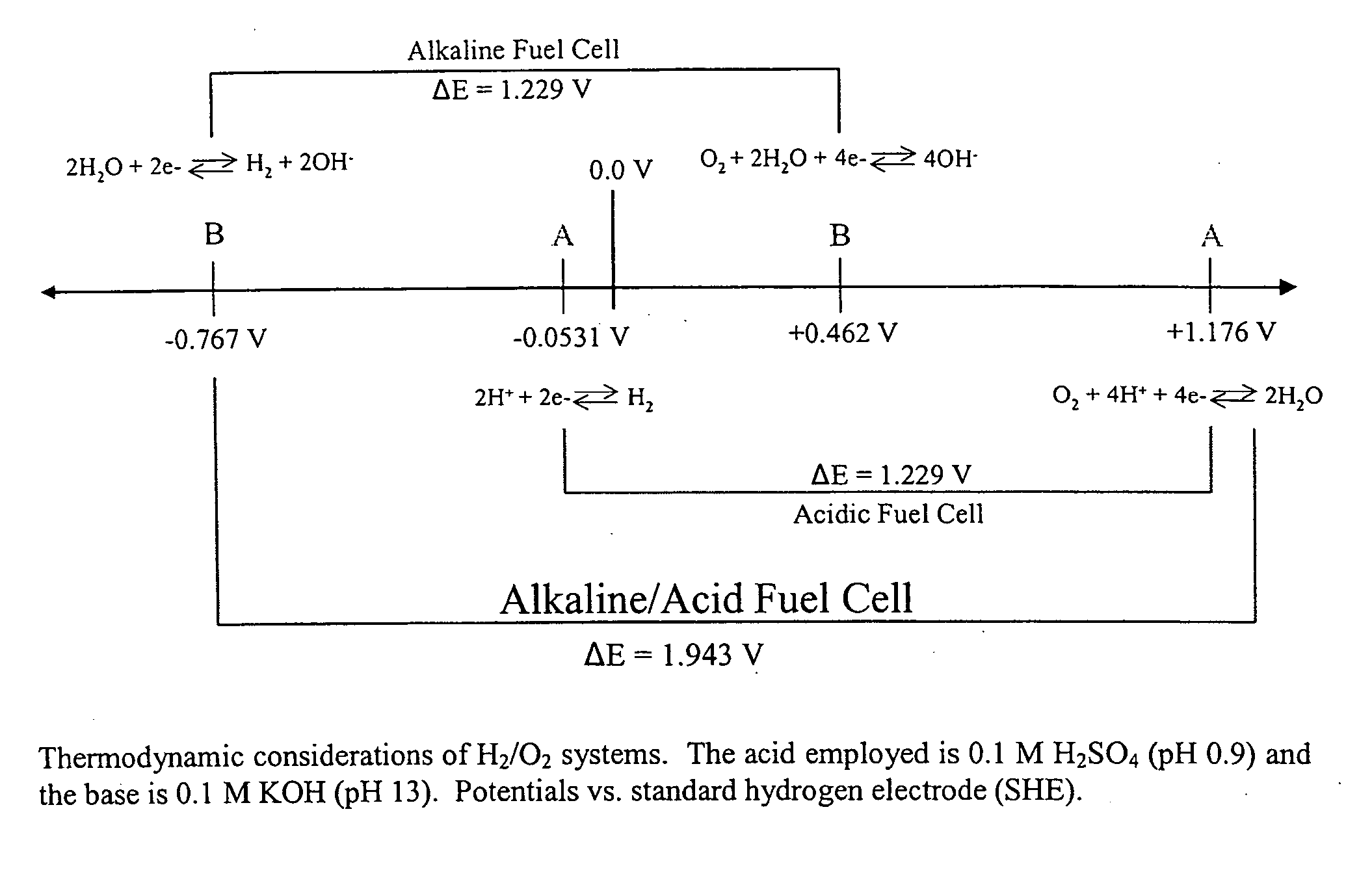
A microfluidic membraneless flow cell formed with multiple acidic / alkaline electrolyte solutions. The flow cell can be adapted to provide a dual electrolyte H2 / O2 fuel cell that generates thermodynamic potentials of up to 1.943 V or possibly greater. The selected fuel can be hydrogen dissolved in 0.1 M KOH, and the selected oxidant can be oxygen dissolved in 0.1 M H2SO4. Individual fuel cells can be combined to form fuel cell stacks to generate increased power output. Furthermore, microchannels of varying dimensions may be selected, including thickness variations, and different flow rates of acid / base electrolyte solutions can be applied to satisfy predetermined power generation needs. Some (micro-) fuel cell embodiments can be formed with silicon microchannels of fixed length and variable width and height, and can be used with hydrogen or formic acid as a fuel and oxygen as an oxidant, each dissolved in different acid / base electrolyte solutions. Micro-fuel cells are also provided which can be designed to generate different power levels for various applications including portable electronic devices such as wireless communication handsets and cellular telephones.
Owner:CORNELL RES FOUNDATION INC
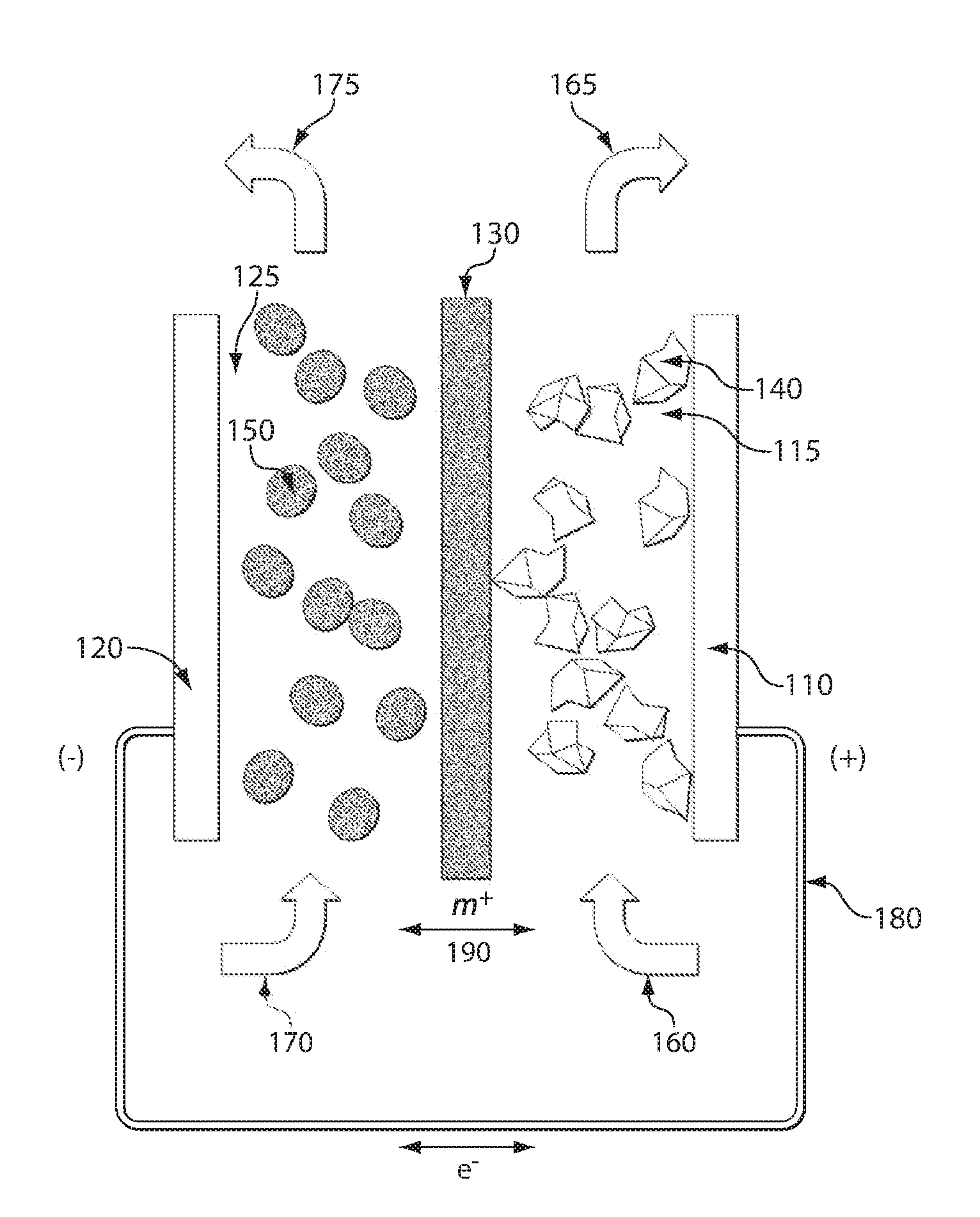
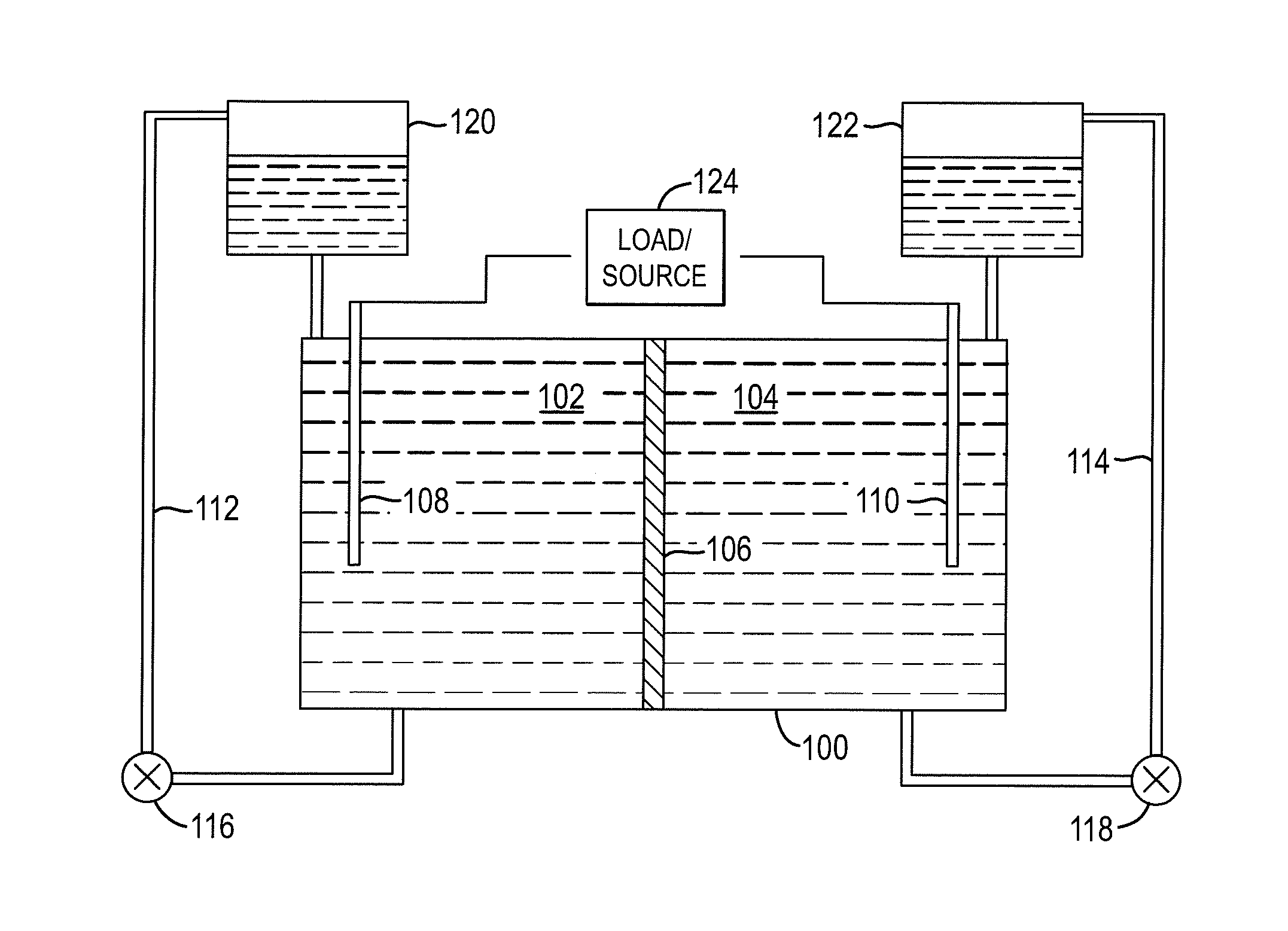
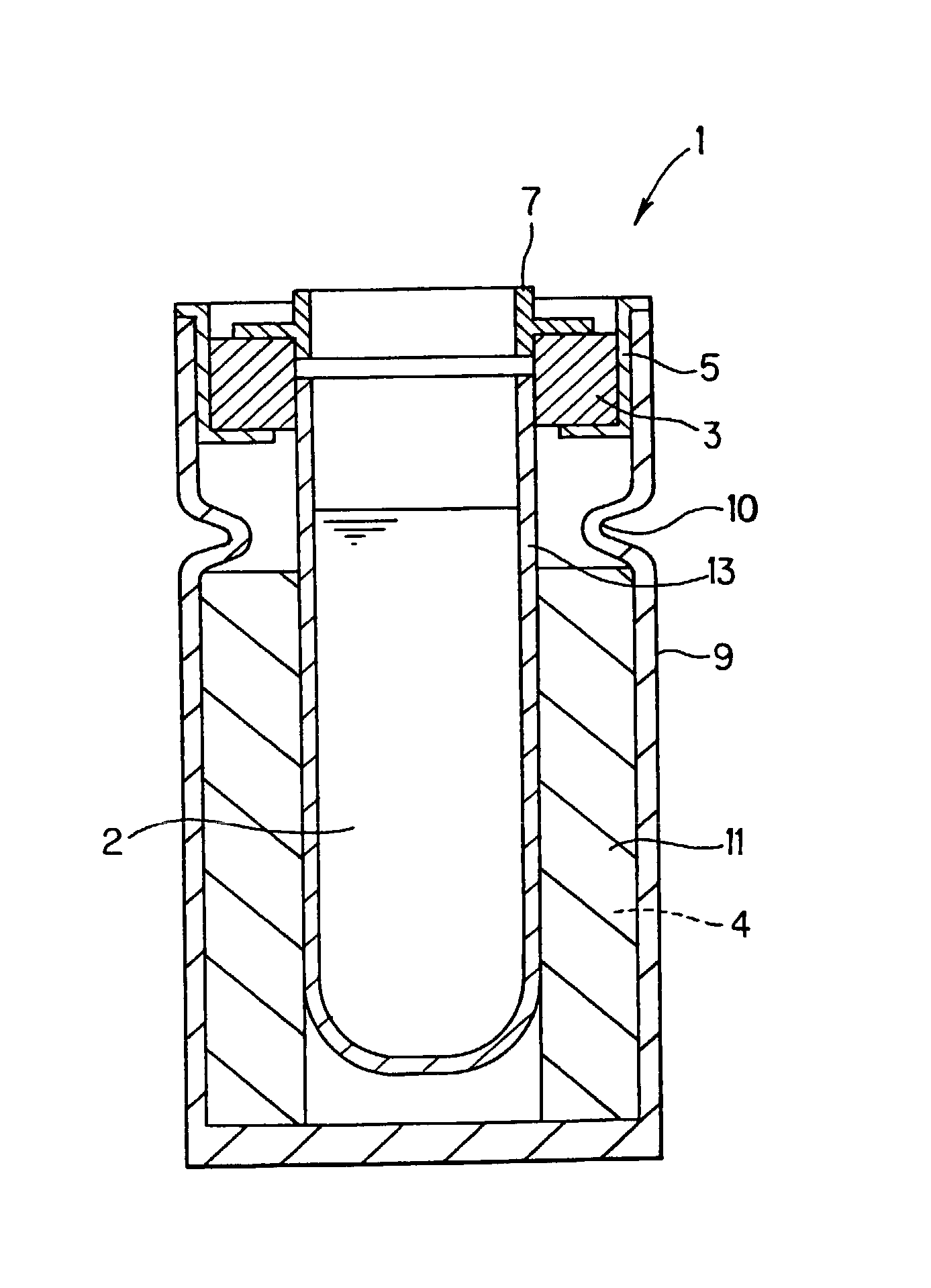
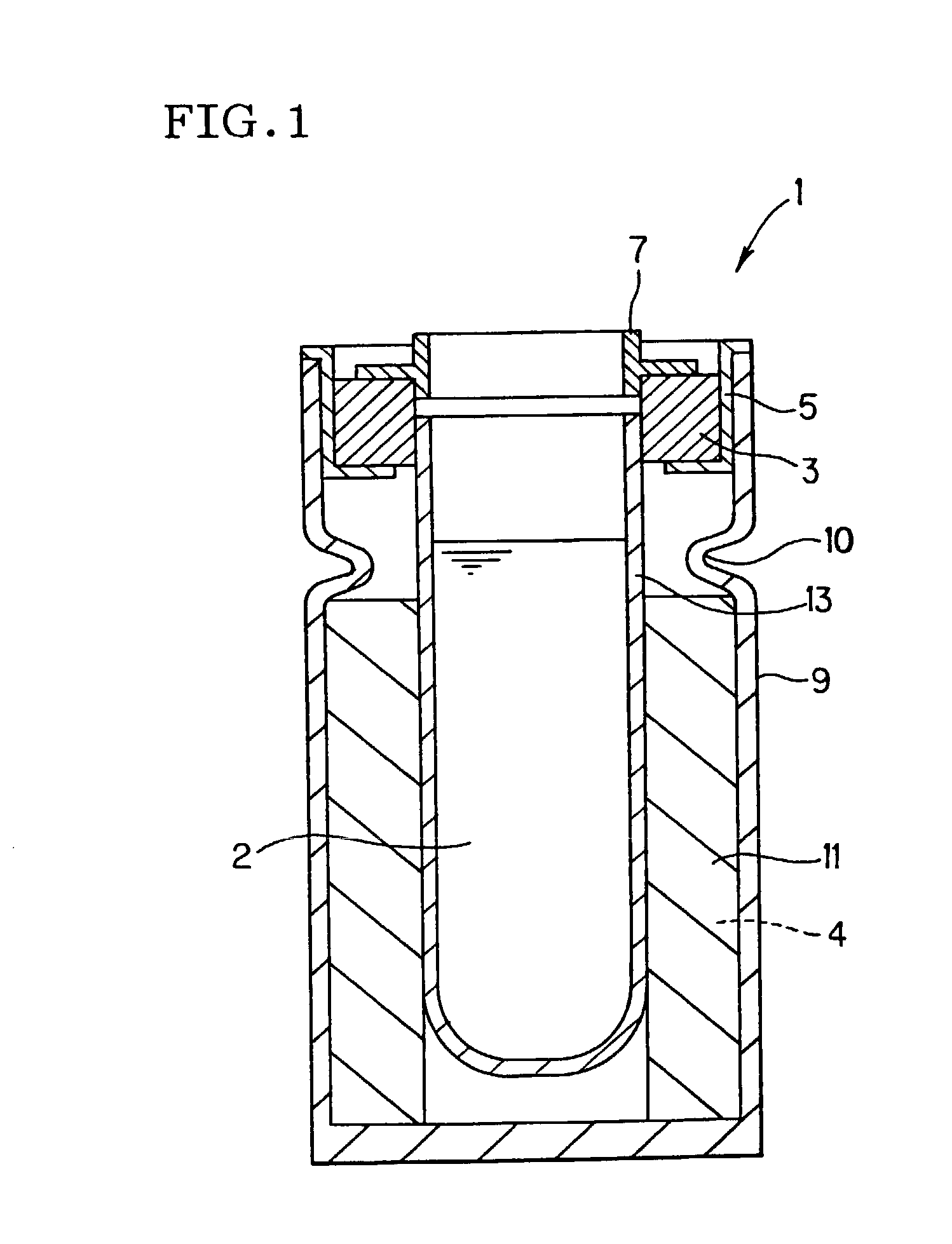
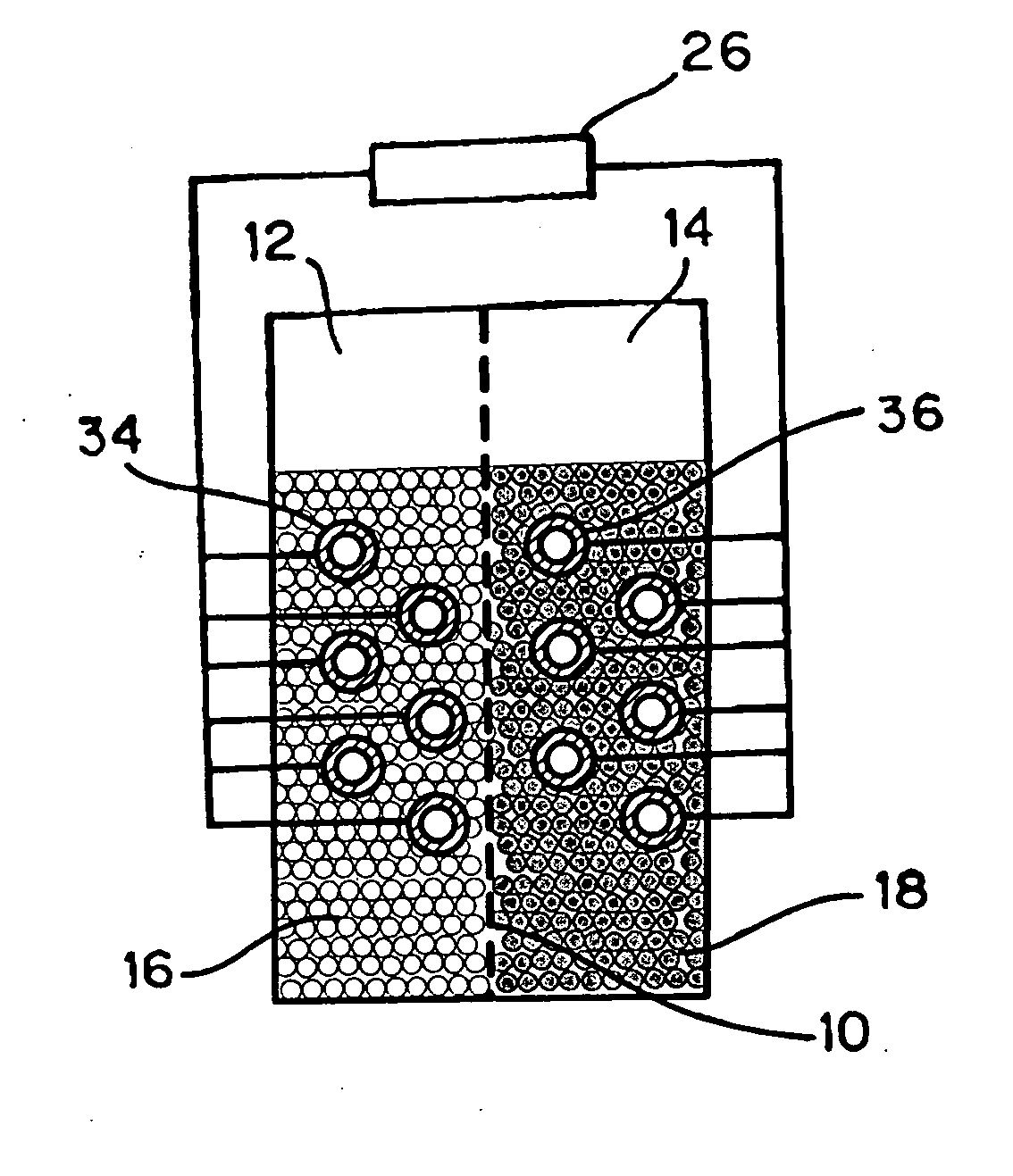
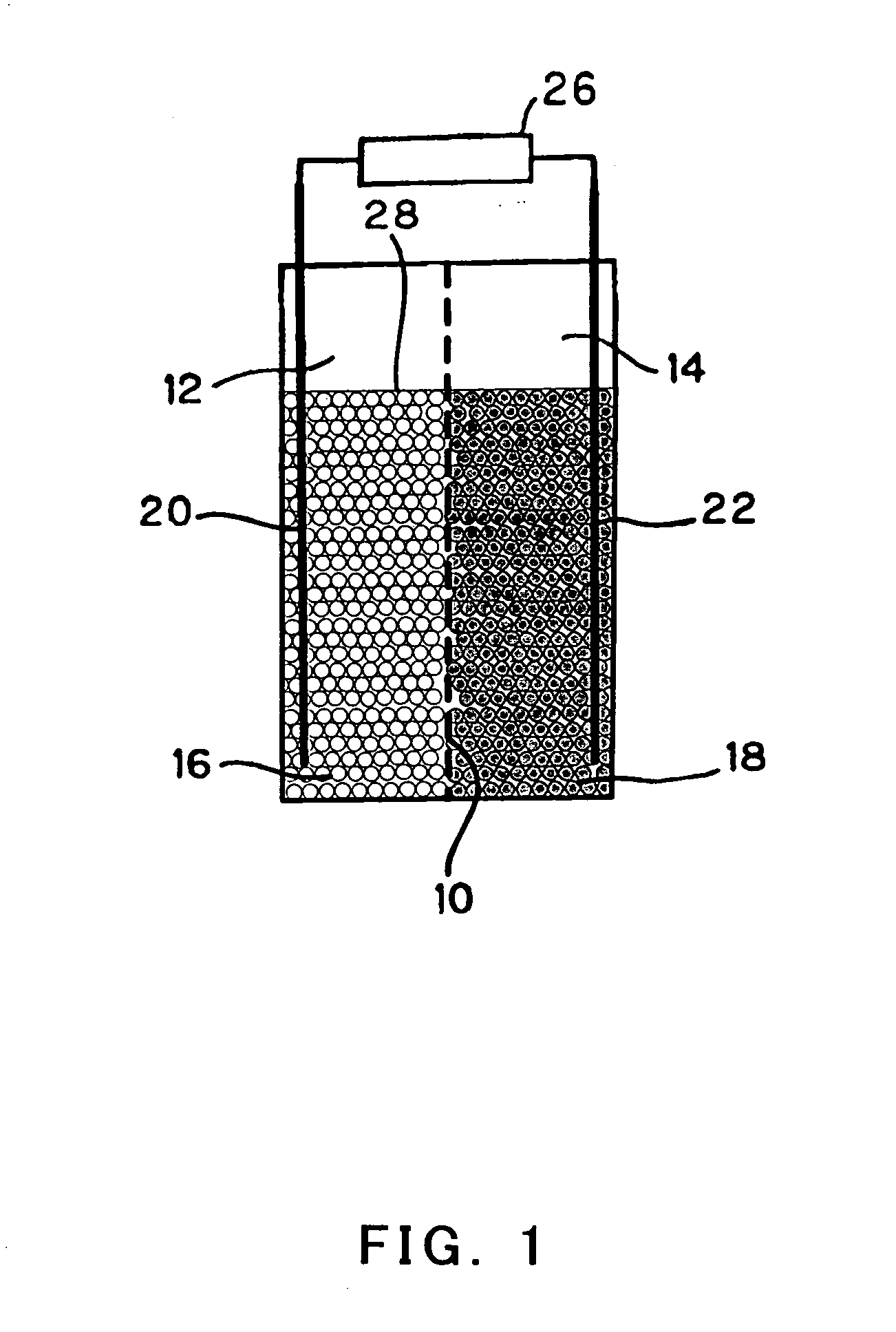
Battery
InactiveUS20050175890A1Production cost advantageIncrease capacityPrimary cell to battery groupingMoving electrode arrangementsEngineeringIon
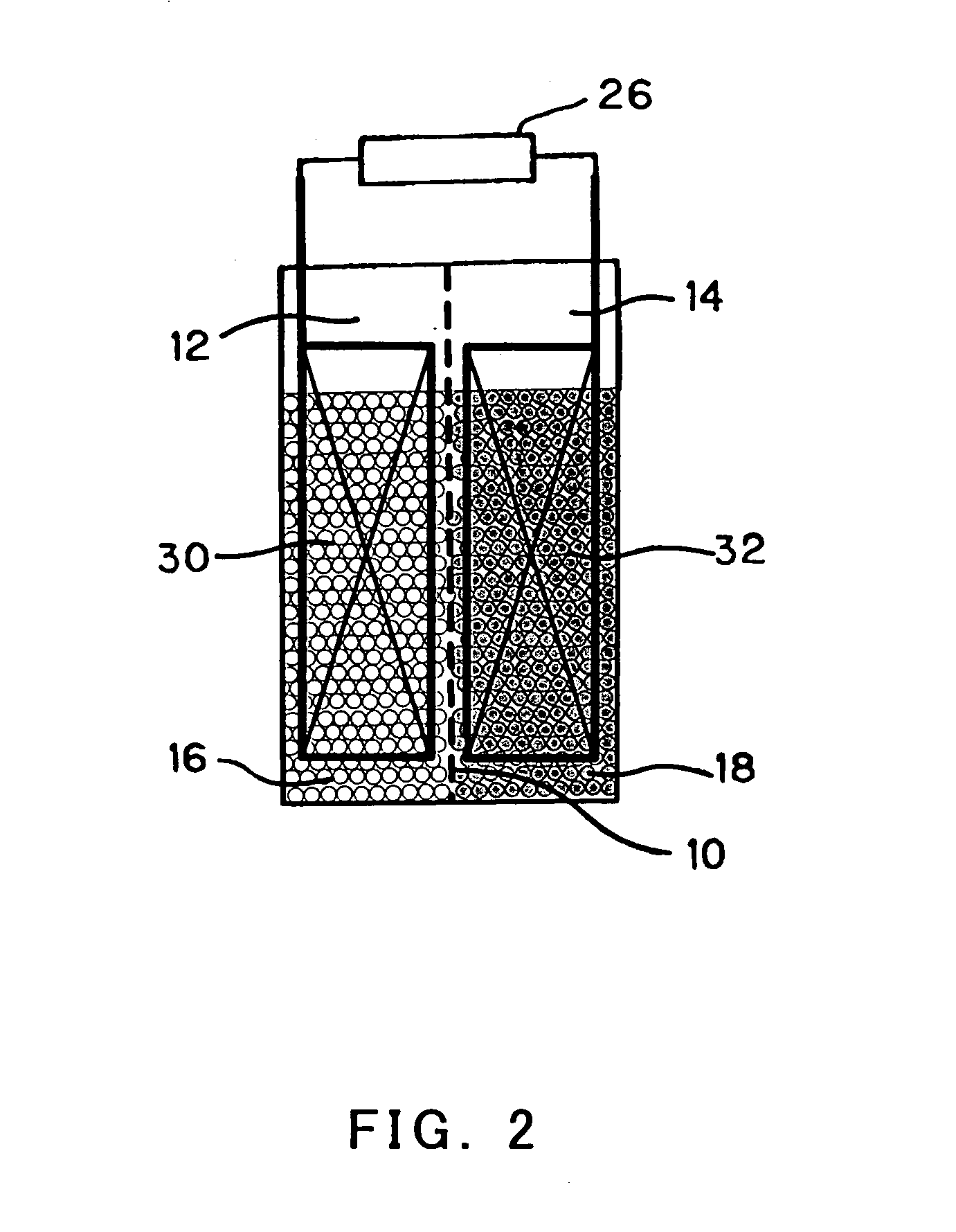
Anode active material particles and an electrolytic solution 16 are filled in an anode cell 12 as one of two vessels connected to each other with an ion-permeable separator 10 interposed therebetween, and cathode active material particles and an electrolytic solution 18 are filled in a cathode cell 14 as the other vessel. Electrically conductive current collectors 20 and 22 are provided in contact with the active material particles within the two vessels. The active material particles form fixed layers.
Owner:KAWASAKI HEAVY IND LTD
High-amperage energy storage device with liquid metal negative electrode and methods
ActiveUS8268471B2Improve ionic conductivityIncrease response ratePrimary cell to battery groupingCell electrodesElectrolysisEngineering
Owner:MASSACHUSETTS INST OF TECH
Nonaqueous electrolyte solution and secondary battery employing the same
InactiveUS6942948B2Organic electrolyte cellsActive material electrodesHigh temperature storageLithium
A nonaqueous electrolyte solution for secondary batteries which is an electrolyte solution for secondary batteries obtained by dissolving a lithium salt in a nonaqueous solvent, wherein the nonaqueous solvent is a solvent mainly comprising a lactone compound and the content of hydroxy carboxylic acids in the electrolyte solution is 1 mmol / kg or lower and a secondary battery employing the same are excellent in high-temperature storage characteristics, cycle characteristics, and capacity retention characteristics and in various cell characteristics in a wide temperature range and safety such as firing properties.
Owner:MU IONIC SOLUTIONS CORP +1
Polymer-gel lithium ion battery
InactiveUS7008722B2Quality improvementBe consistentPrimary cell to battery groupingFinal product manufacturePolymer sciencePolyolefin
An embodiment of the invention is a new method of making a polymer lithium ion battery with low cost, high efficiency and excellent quality. The new polymer lithium ion battery comprises four major components, each of which is a composite: an anode, a cathode, a polymer-gel-electrolyte-separator system and a soft packaging laminate. Adherent particles are introduced into the electrolyte and deposited on the surfaces of both separators and electrodes by Chemical Liquid Deposition (CLD) in-situ the battery cell during the battery assembly process. Those adherent particles not only serve as glue to strongly hold both the anode and cathode together with polyolefin separators, but also form a polymer-gelling electrolyte through the Polymer Gel Formation (PGF) process. The fabrication method creates a self-supporting and self-strengthening battery cell and allows a soft coffee bag laminate to be used as packing shell.
Owner:HUANG SUI YANG
Methods of forming biocompatible rechargable energization elements for biomedical devices
Methods and apparatus to form biocompatible energization elements are described. In some embodiments, the methods and apparatus to form the biocompatible energization elements involve forming cavities comprising active cathode chemistry. The active elements of the cathode and anode are sealed with a laminate stack of biocompatible material. In some embodiments, a field of use for the methods and apparatus may include any biocompatible device or product that requires energization elements.
Owner:JOHNSON & JOHNSON VISION CARE INC
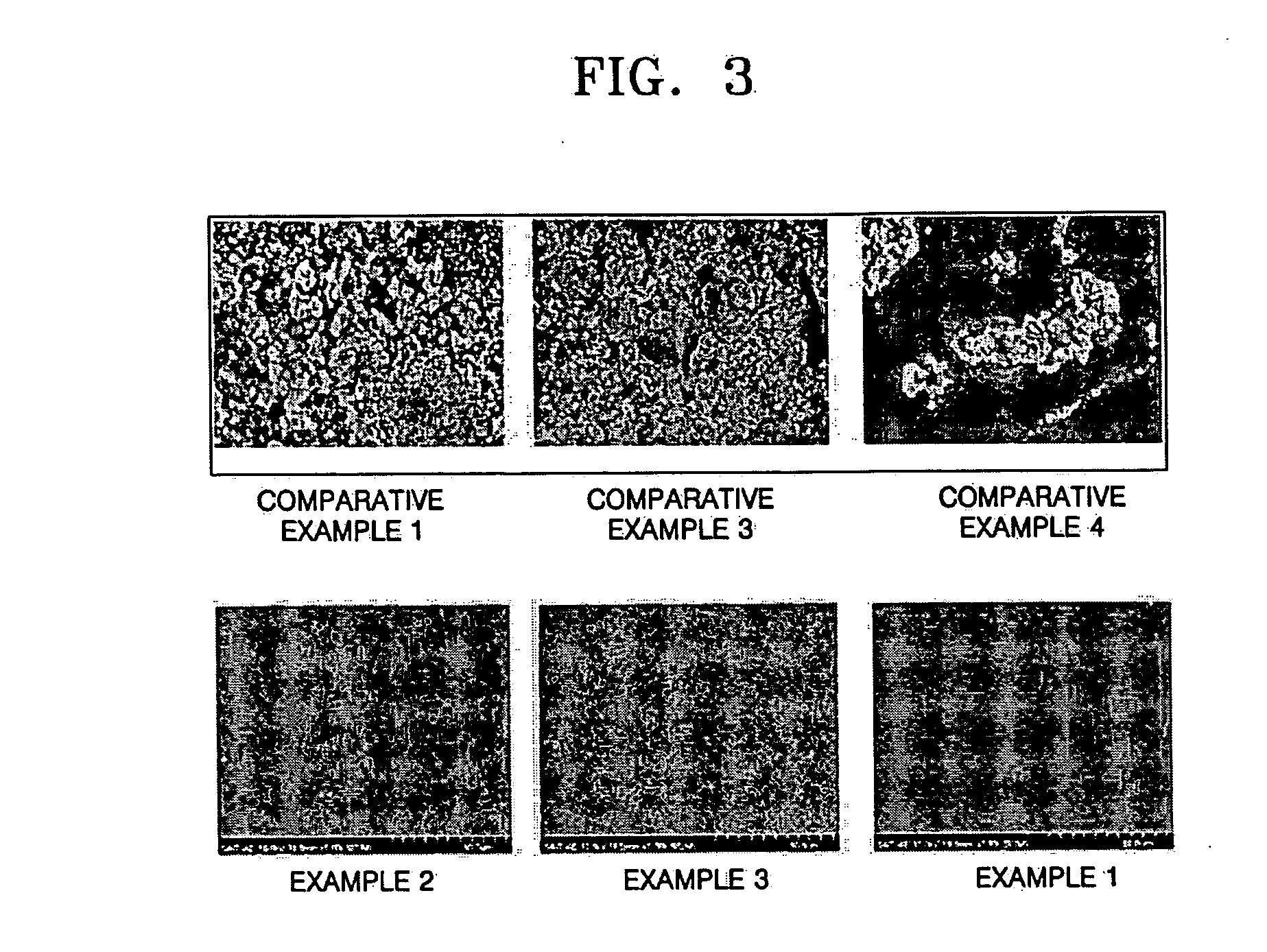
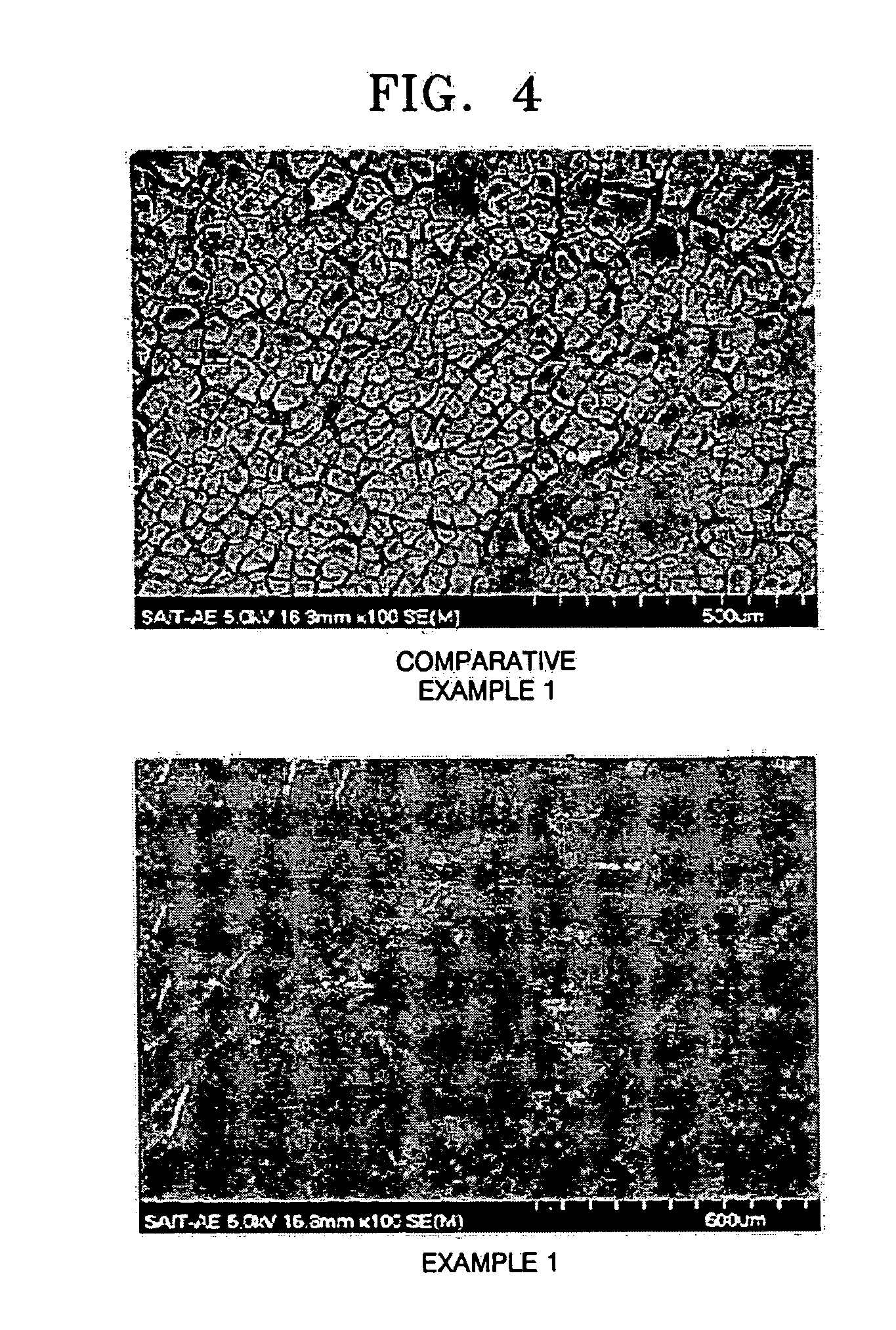
Lithium-sulfur battery
InactiveUS20050042503A1Improve charge and discharge efficiencyTwo electrolyte cellsNon-aqueous electrolyte accumulator electrodesDischarge efficiencyDecomposition
A lithium-sulfur battery includes a cathode, a lithium metal anode, and a separator interposed between the cathode and the anode. The separator contains less than two fluorine atoms per carbon atom to enable a protective layer to form on a surface of the lithium metal anode. The lithium-sulfur battery forms a uniform and dense LiF protective layer on the surface of the lithium metal and stabilizes the lithium metal during its operation. The lithium-sulfur battery prevents the formation of lithium dendrites and inhibits the decomposition of an electrolytic solution to provide improved cycle characteristics and excellent charging / discharging efficiency. In addition, the lithium-sulfur battery blocks the reaction of polysulfide with the surface of lithium metal to prevent a reduction of the lifetime of the battery.
Owner:SAMSUNG SDI CO LTD
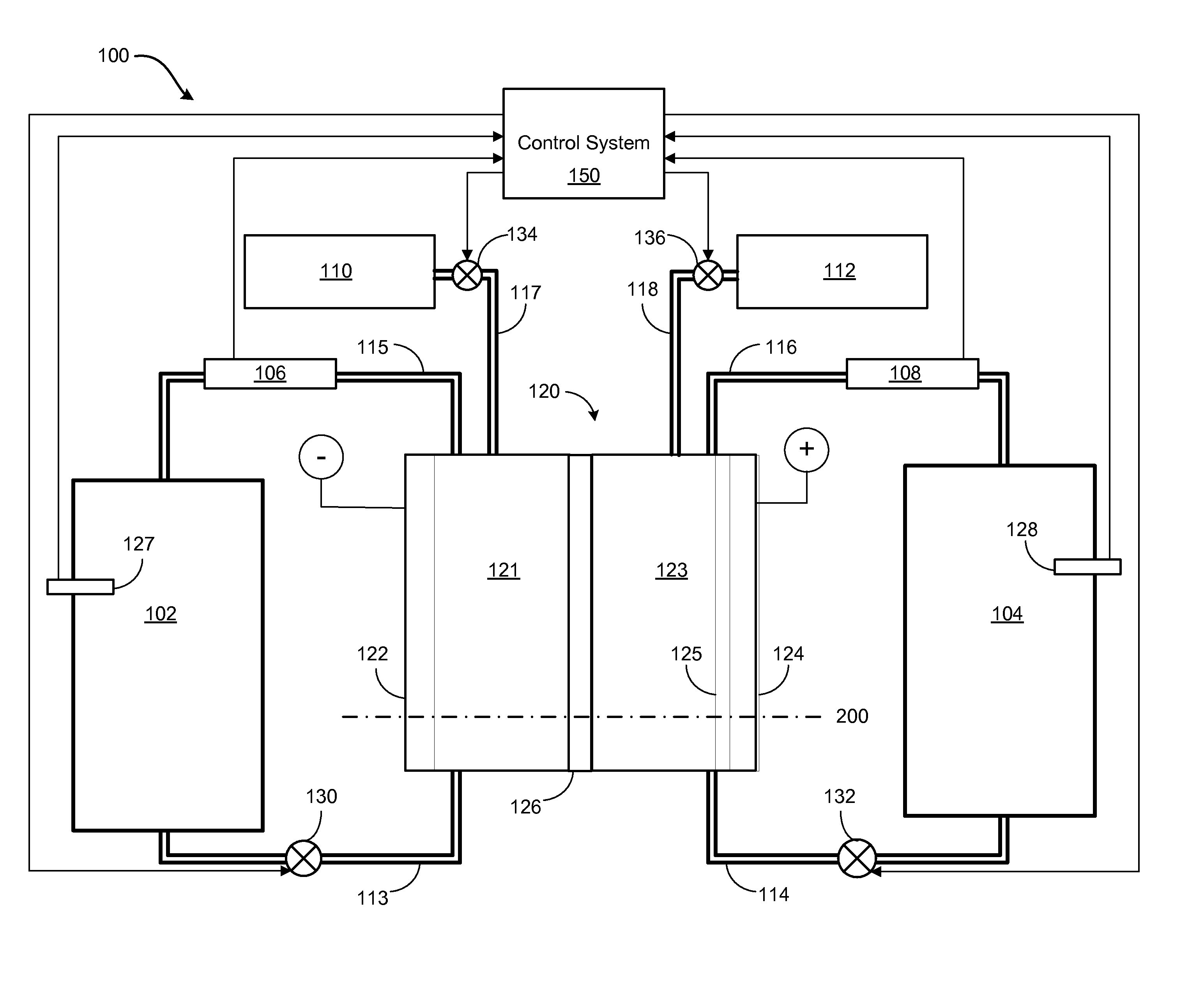
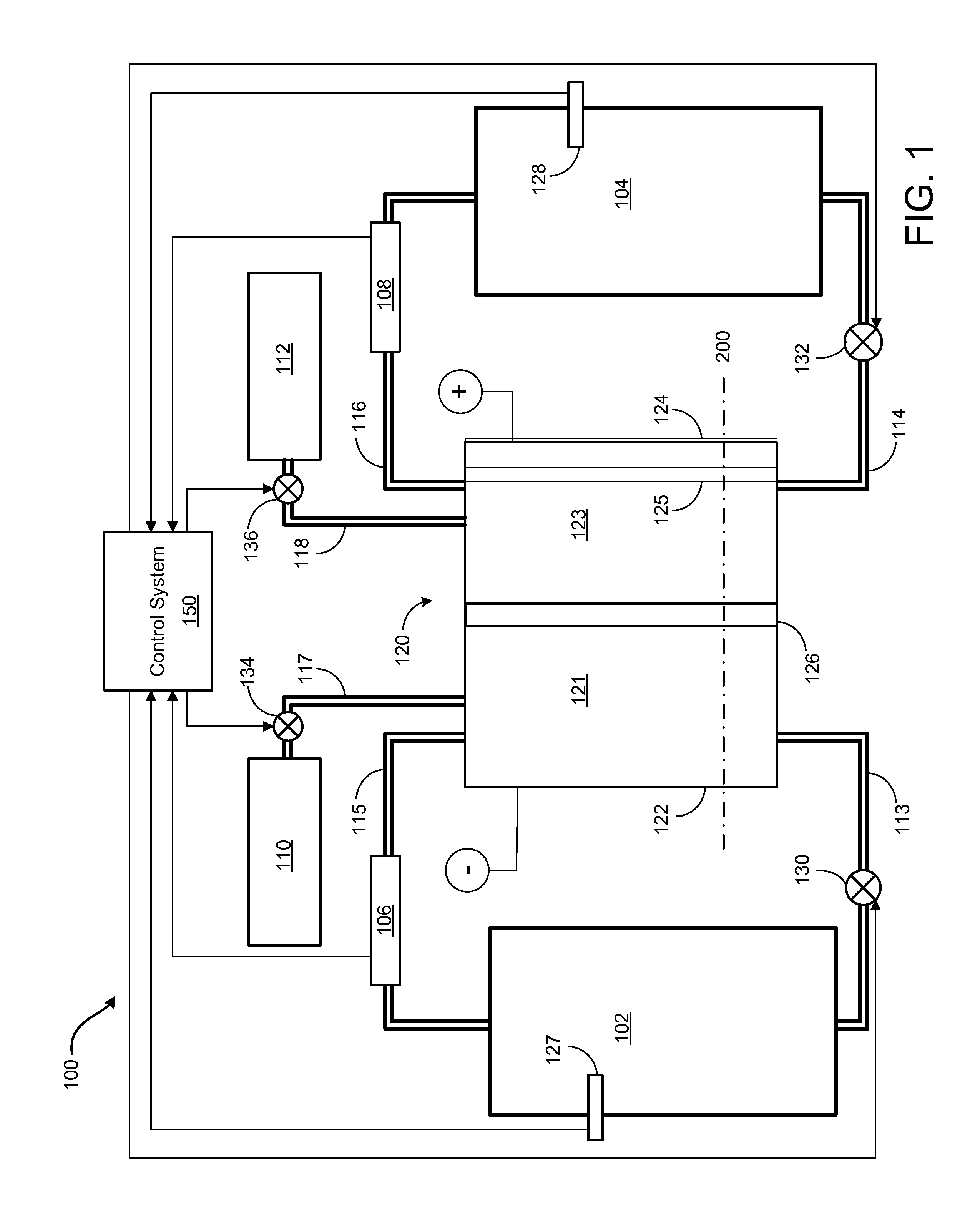
Pressure balancing of electrolytes in redox flow batteries
Methods and apparatuses are disclosed for mitigating electrolyte migration in a redox flow battery system. A first parameter of a first electrolyte in a first flow path of a redox flow battery cell block may be measured. The first flow path may have an inlet to and an outlet from the redox flow battery cell block. A second parameter of a second electrolyte in a second flow path of the redox flow battery cell block may be measured. The second flow path may have an inlet to and an outlet from the redox flow battery cell block. The first parameter may be detected to be greater than the second parameter. A first device coupled to the redox flow battery cell block in the second flow path may be operated to increase the second parameter in the second flow path.
Owner:ENERVAULT CORP
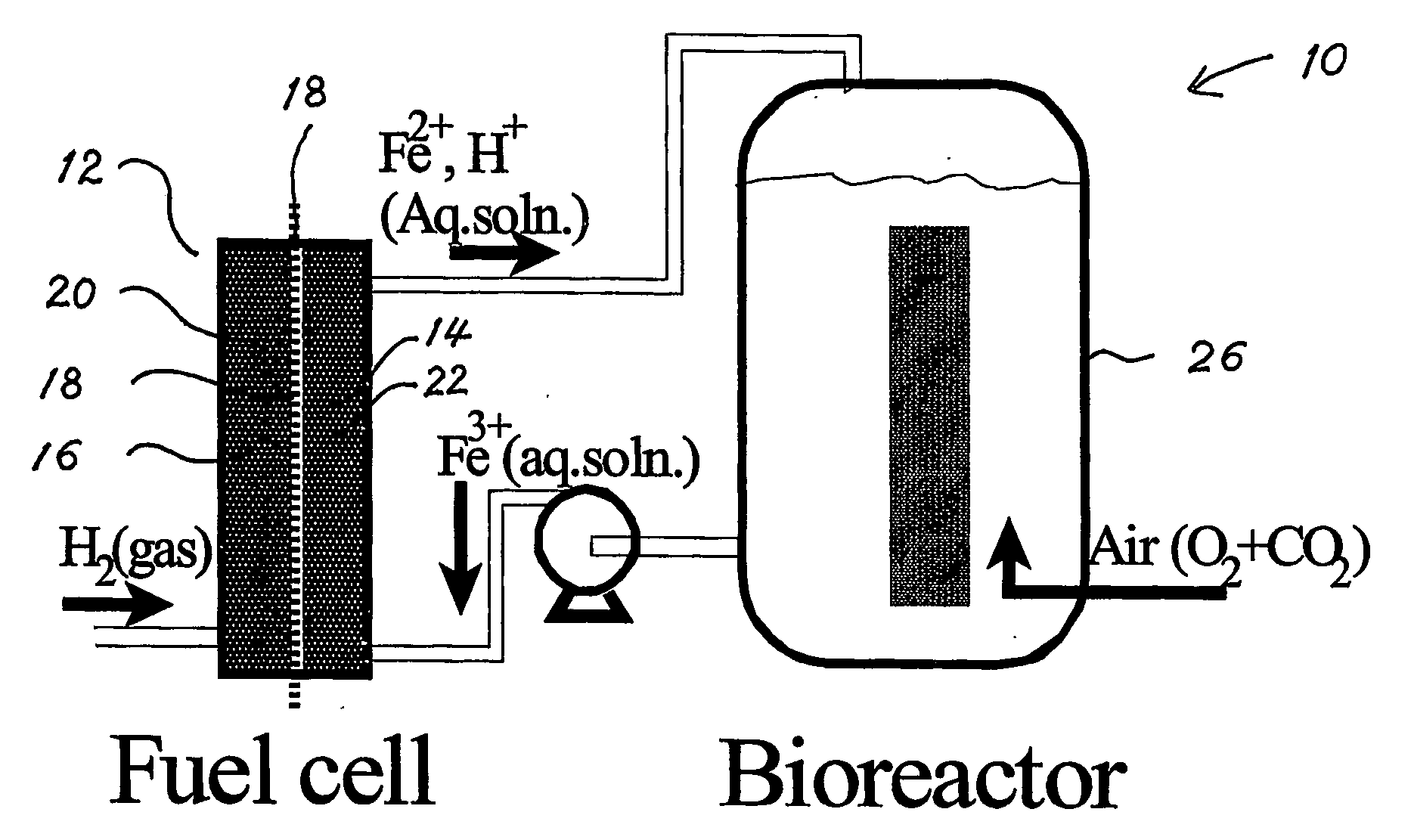
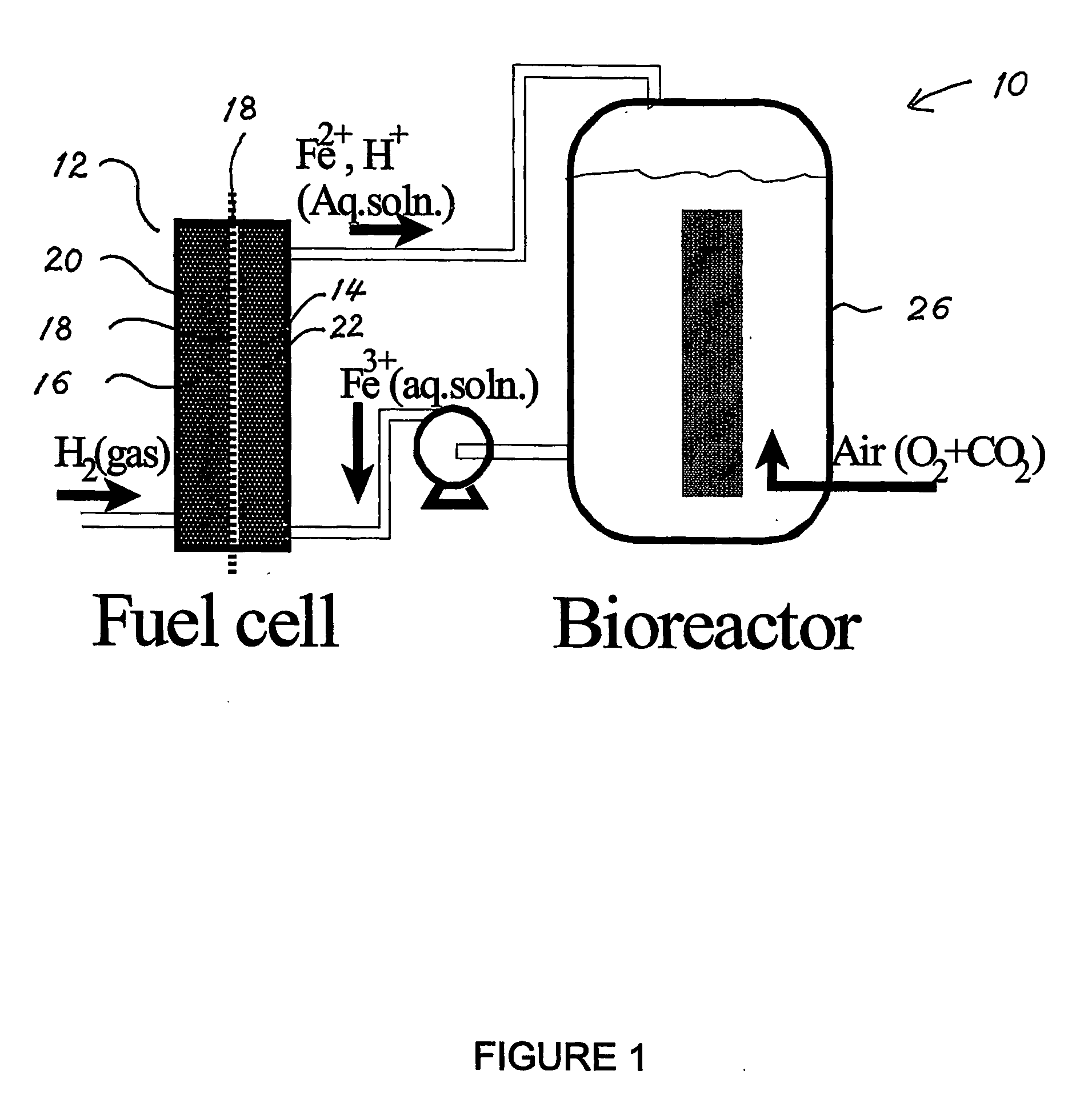
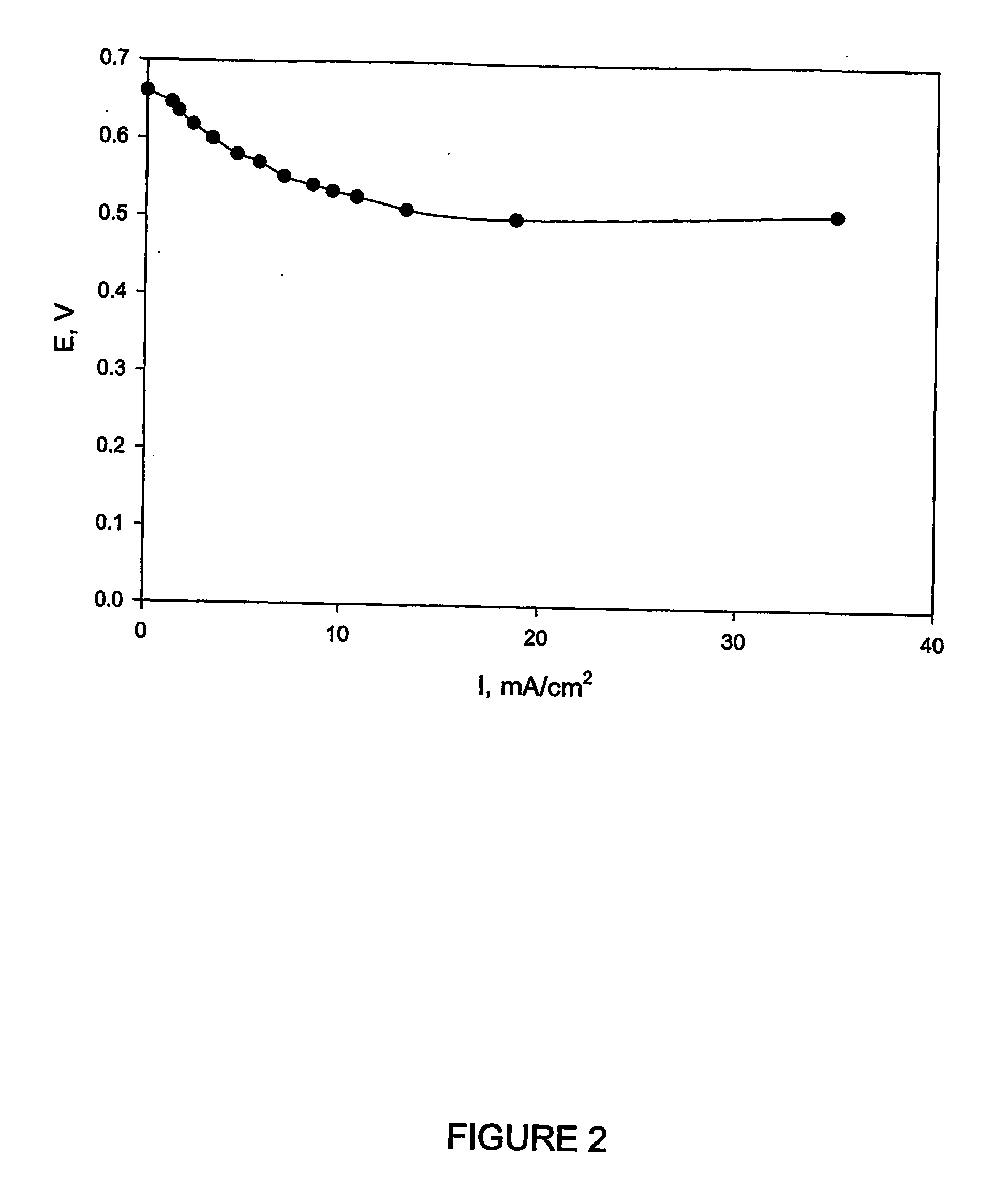
Biofuel cell
InactiveUS20060251959A1Promote growthElectrolyte holding meansRegenerative fuel cellsMicroorganismHydrogen
The present invention discloses a new type of biofuel cell, based on the microbial regeneration of the oxidant, ferric ions. The bio-fuel cell is based on the cathodic reduction of ferric to ferrous ions, coupled with the microbial regeneration of ferric ions by the oxidation of ferrous ions, with fuel (such as hydrogen) oxidation on the anode. The microbial regeneration of ferric ions is achieved by chemolithotrophic microorganisms such as Acidithiobacillus ferroxidans. Electrical generation is coupled with the consumption of carbon dioxide from atmosphere and its transformation into microbial cells, which can be used as a single-cell protein.
Owner:UNIV OF WESTERN ONTARIO
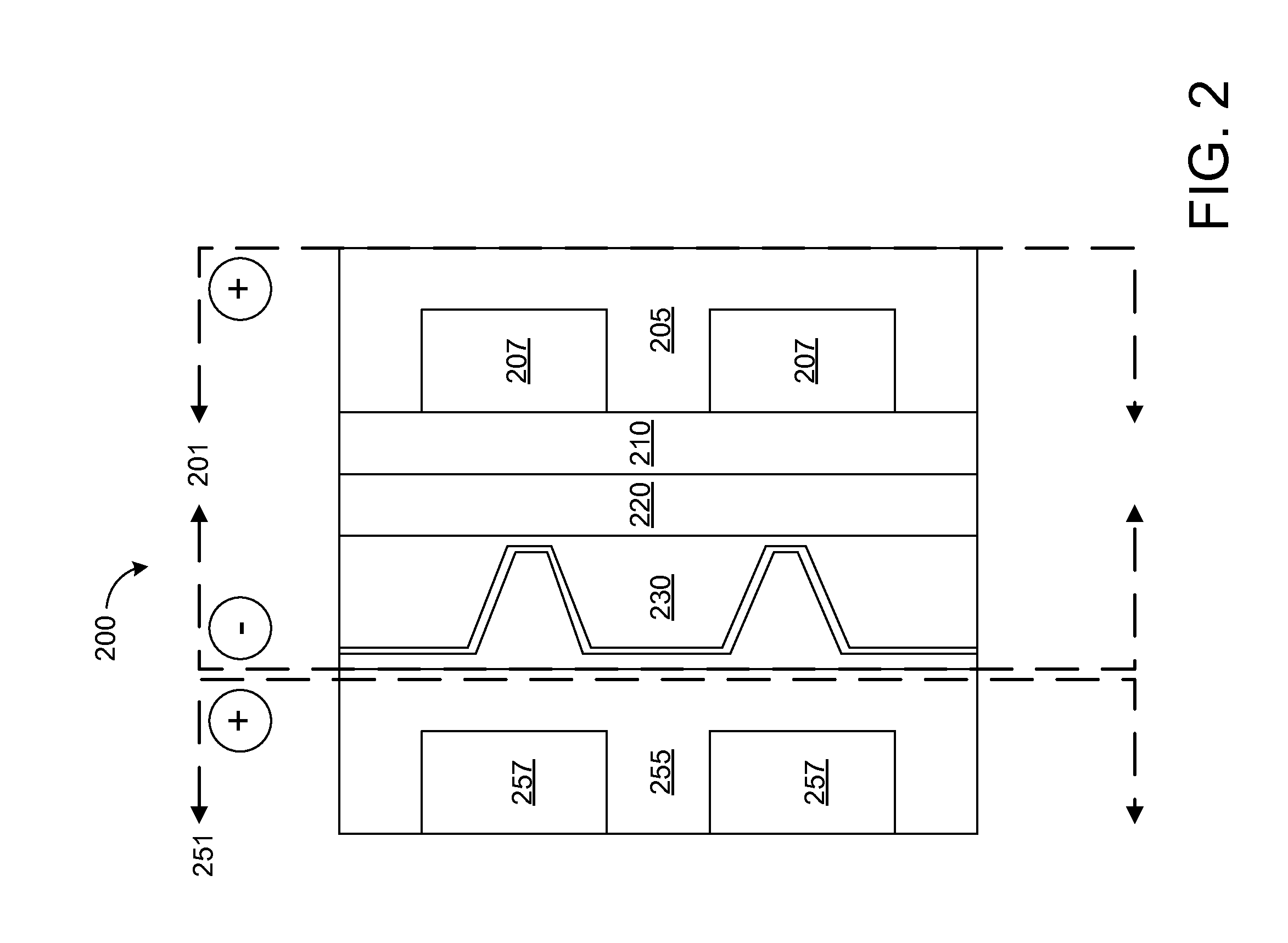
Redox and plating electrode systems for an all-iron hybrid flow battery
A system for a flow cell for a hybrid flow battery, comprising: a redox plate comprising a plurality of electrolyte flow channels; conductive inserts attached to the redox plate between adjacent electrolyte flow channels; a redox electrode attached to a surface of the redox plate; a plating electrode, comprising: a plurality of folded fins with an oscillating cross-section, the plurality of folded fins comprising: a first planar surface; a second planar surface, parallel to the first planar surface; a plurality of ridges intersecting the first and second planar surfaces such that the plurality of ridges divide the first planar surface into a first plurality of strips, and divide the second planar surface into a second plurality of strips; and a membrane barrier. In this way, the capacity and performance of hybrid flow batteries may be maximized, through decreasing the reaction kinetics, mass transport and ohmic resistance losses at both electrodes.
Owner:ESS TECHNOLOGY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com