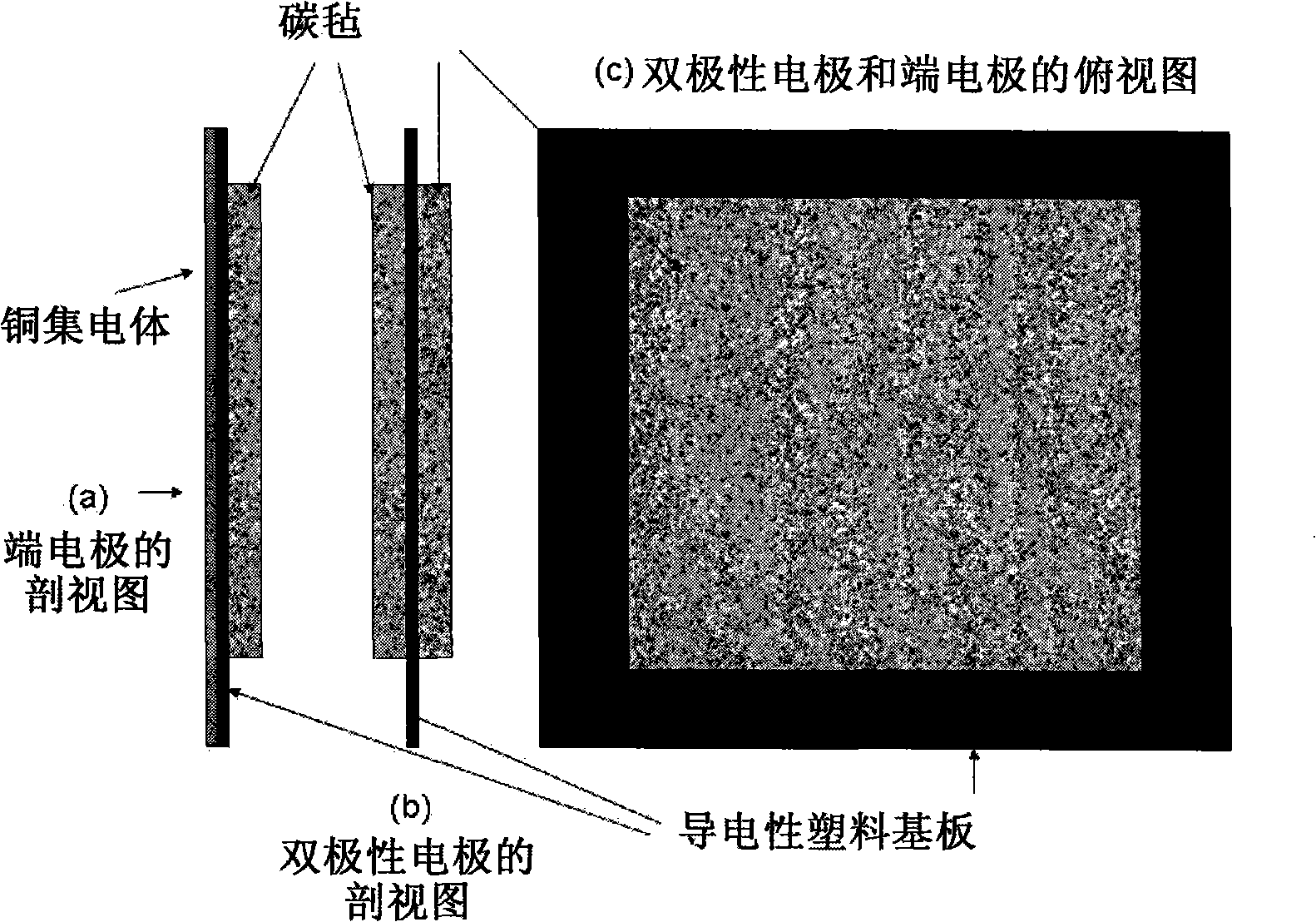
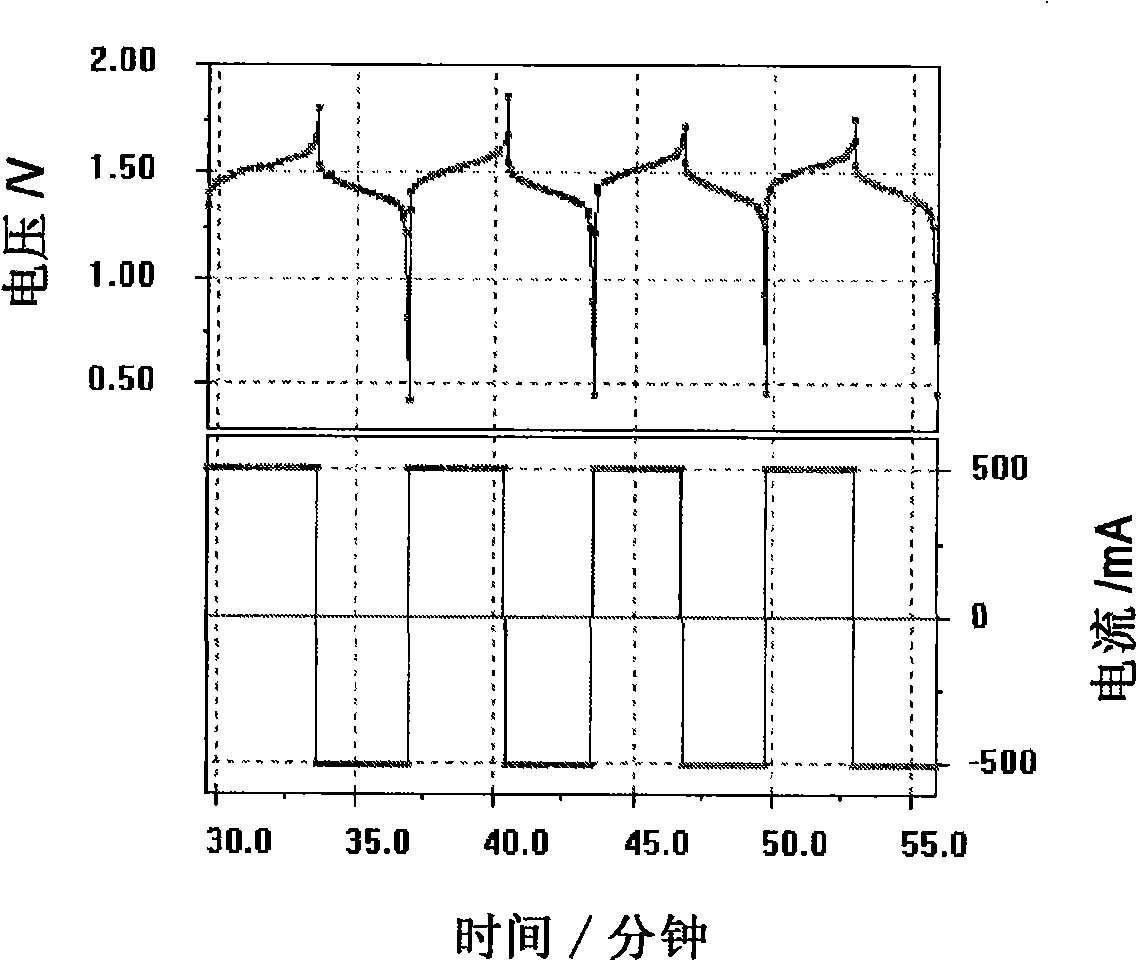
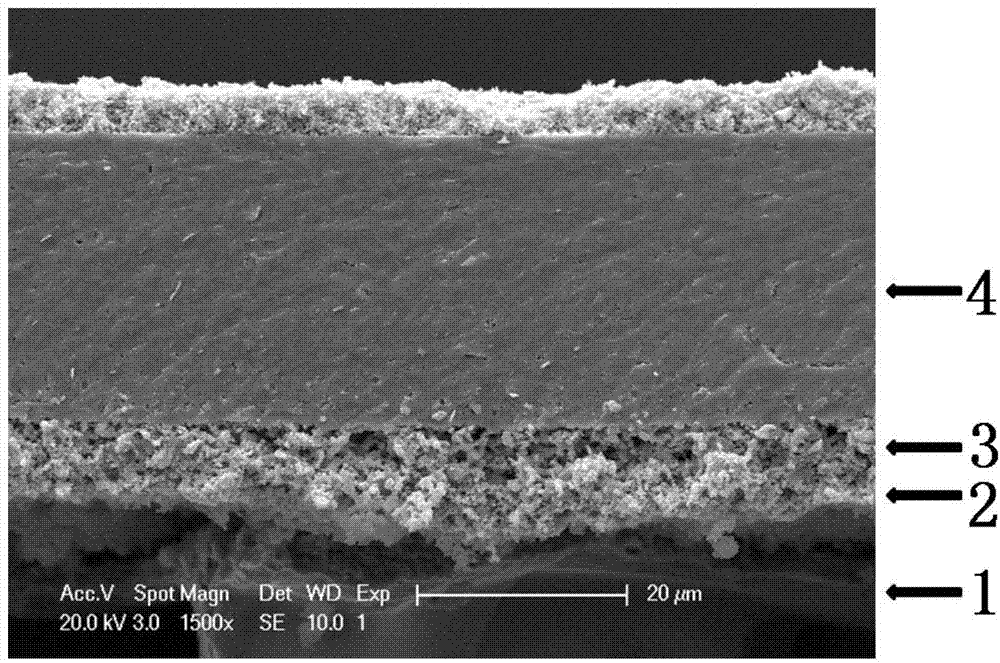
Patents
Literature
470 results about "Half-cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
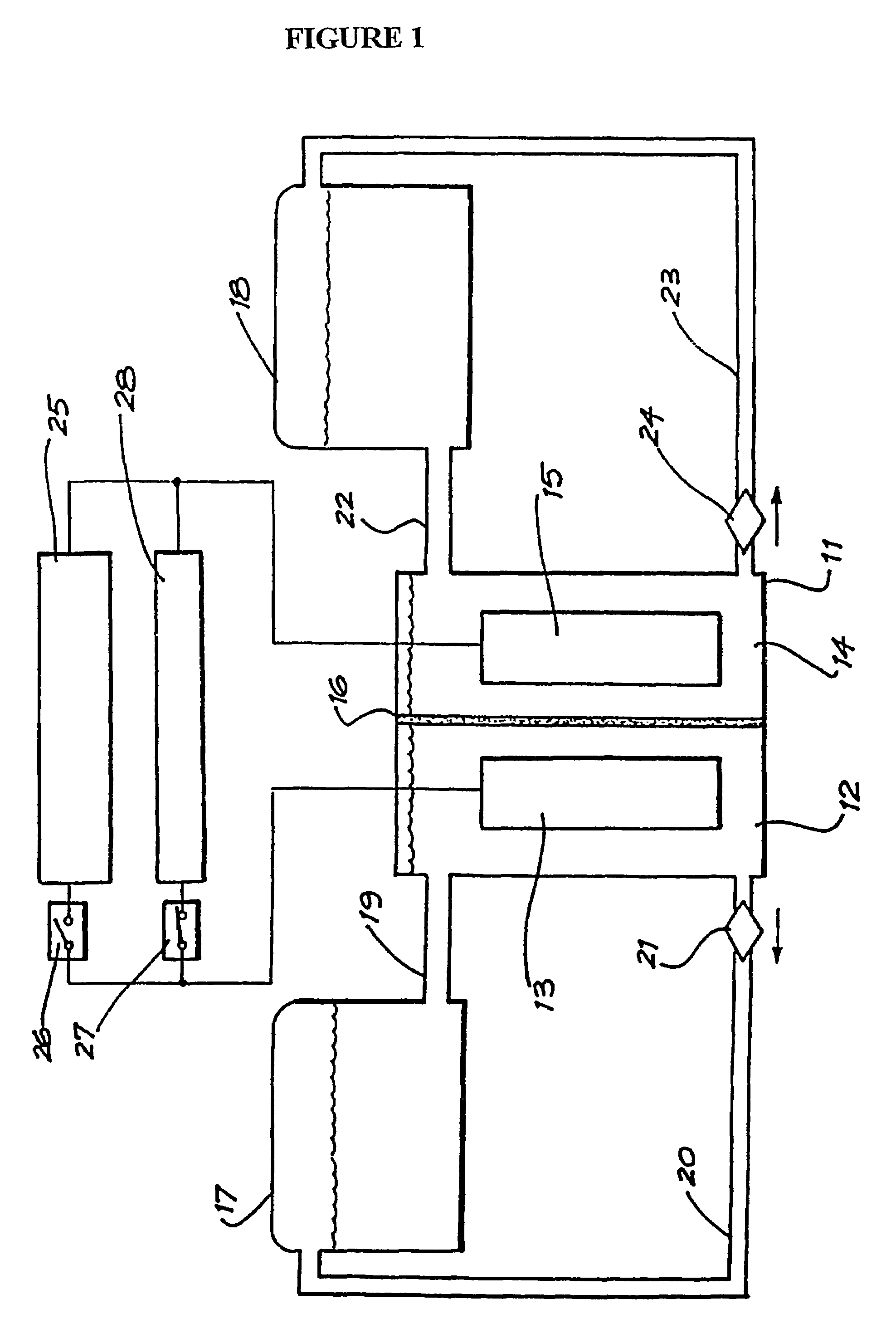
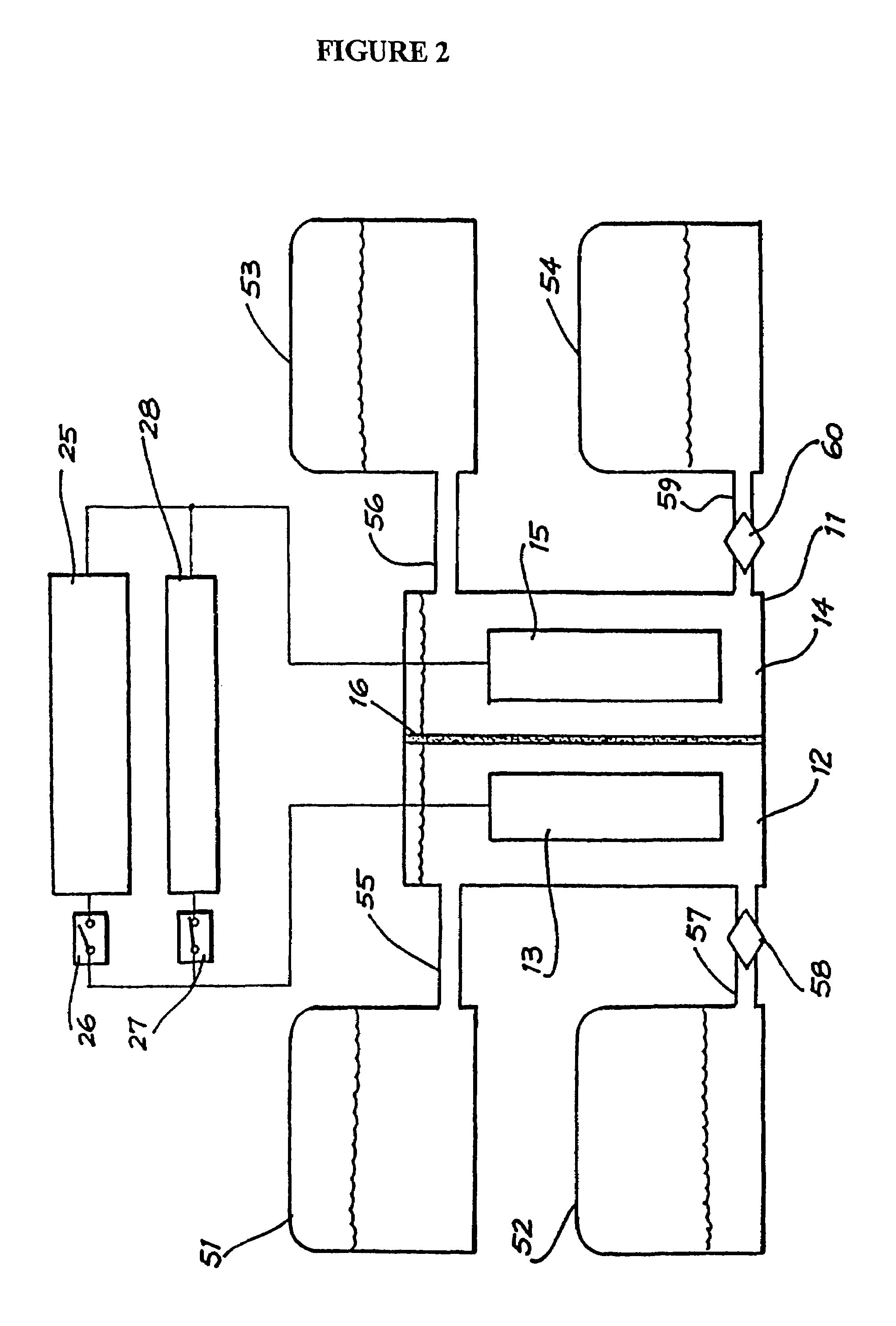
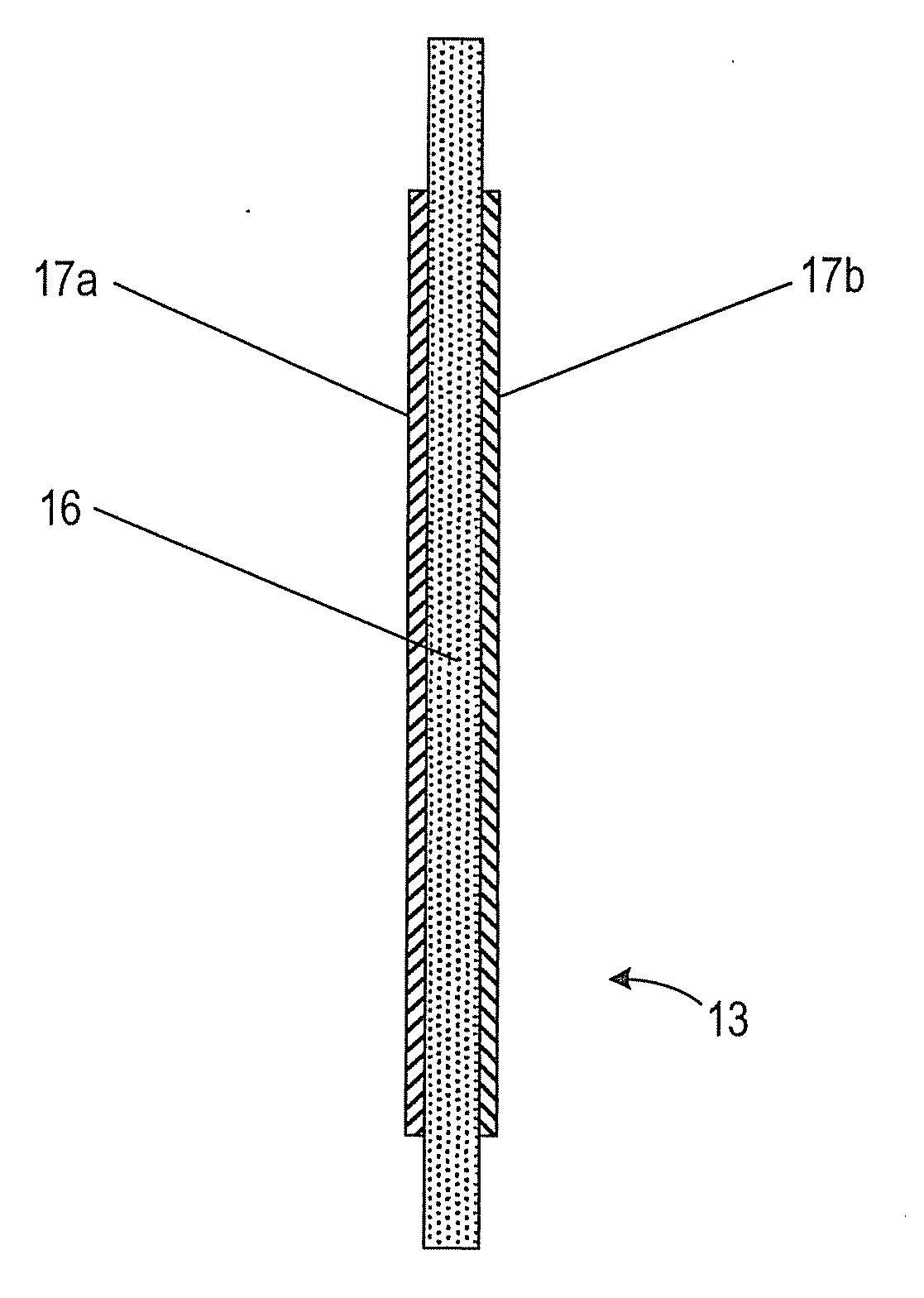
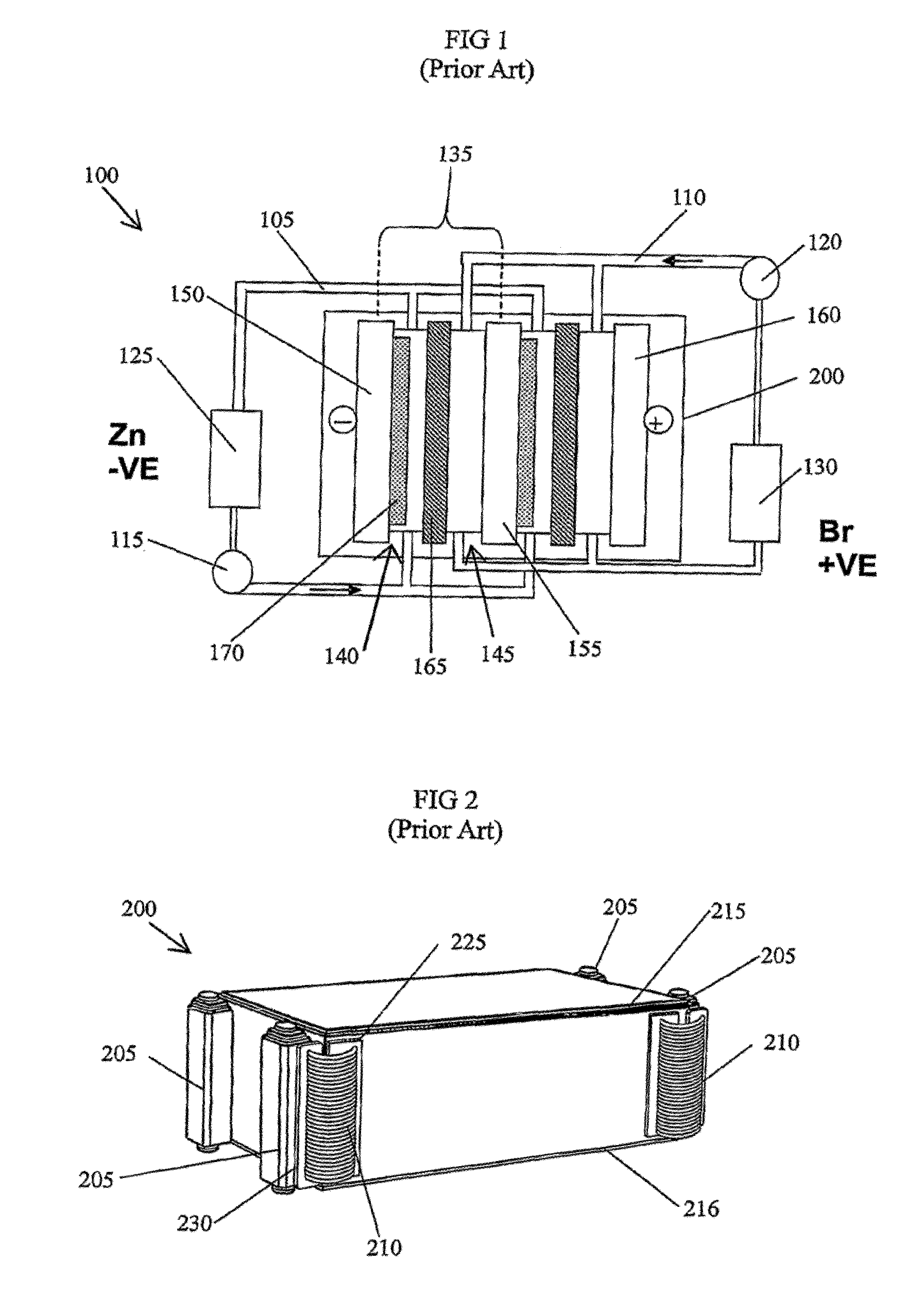
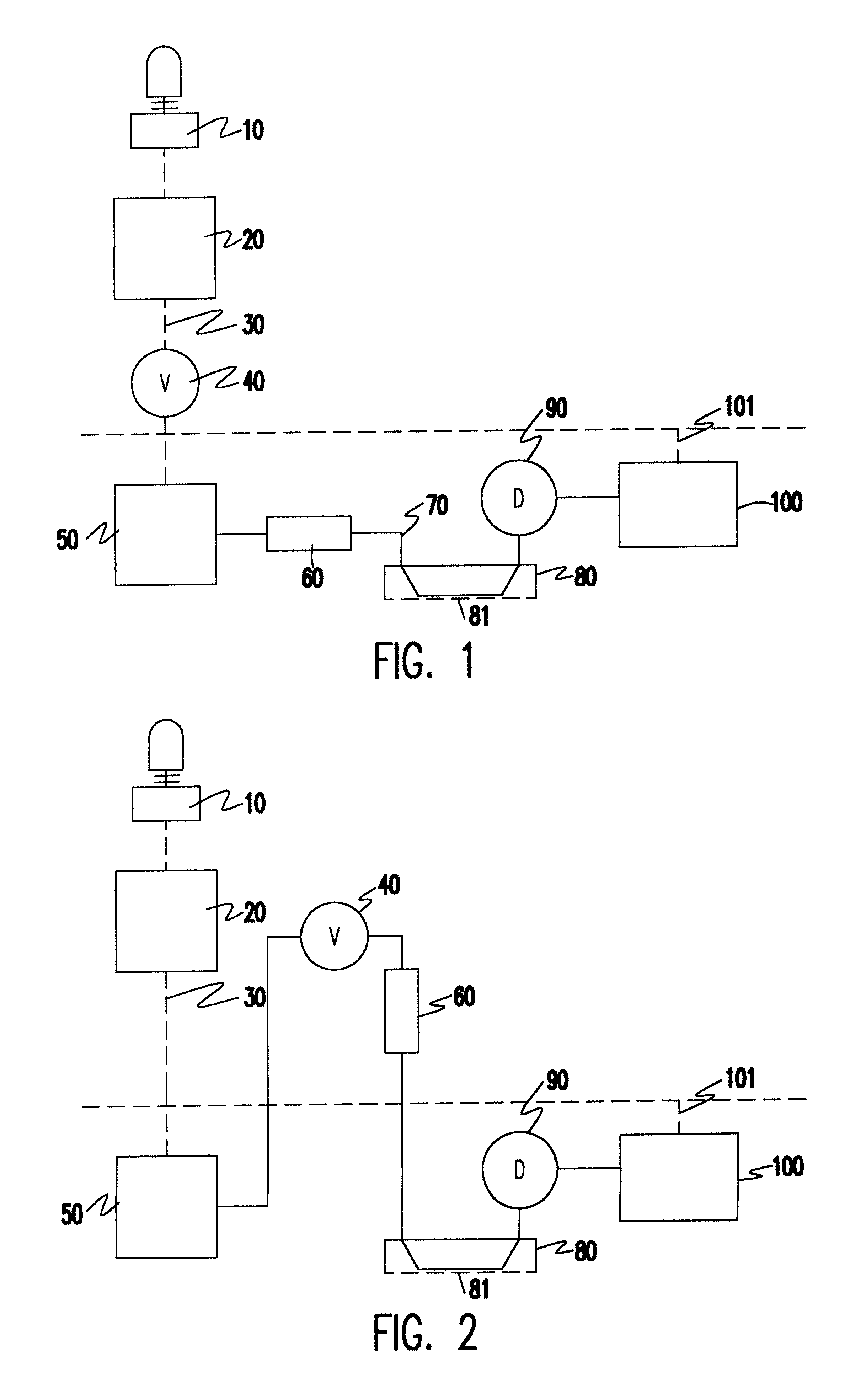
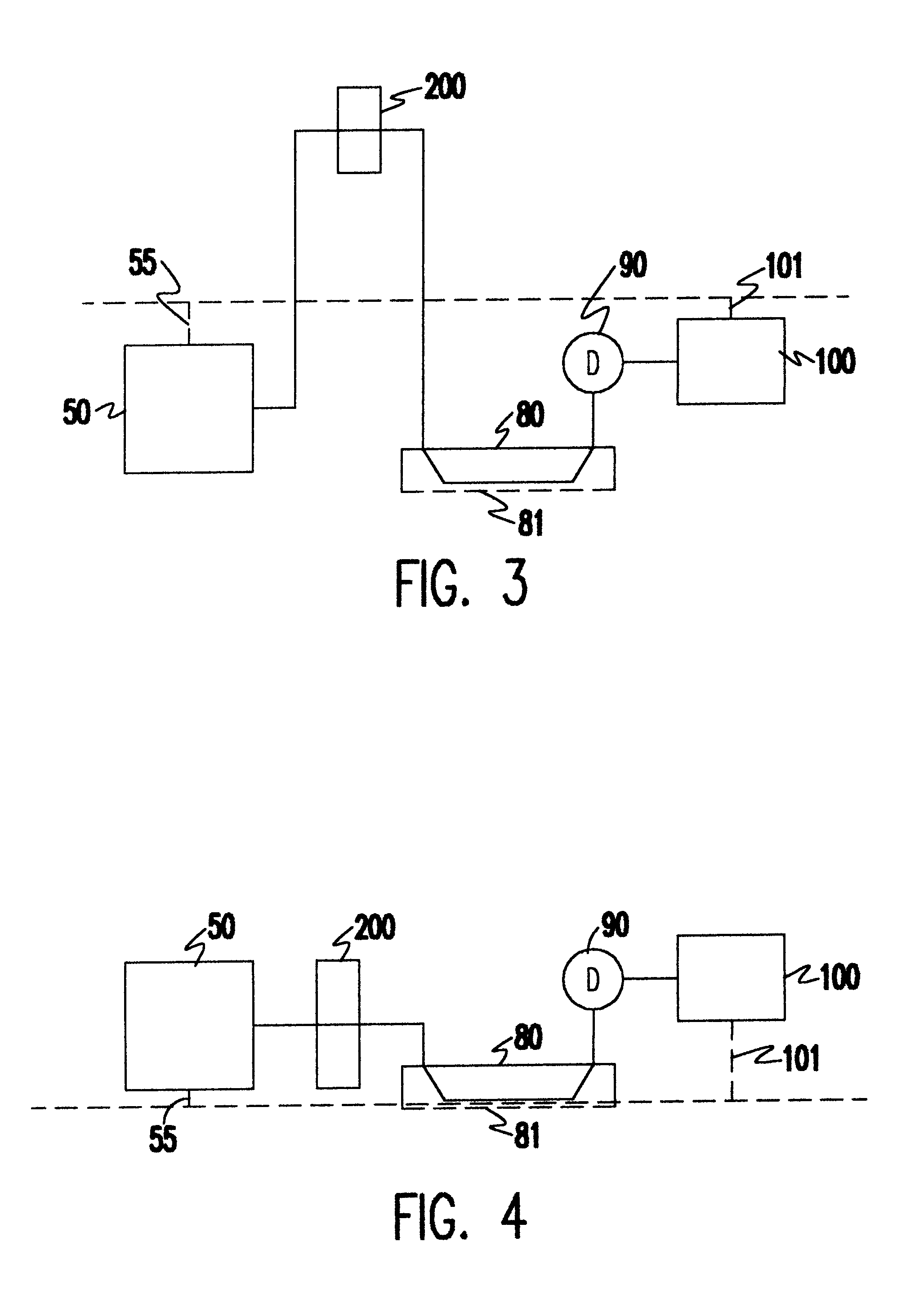
In electrochemistry, a half-cell is a structure that contains a conductive electrode and a surrounding conductive electrolyte separated by a naturally occurring Helmholtz double layer. Chemical reactions within this layer momentarily pump electric charges between the electrode and the electrolyte, resulting in a potential difference between the electrode and the electrolyte. The typical anode reaction involves a metal atom in the electrode dissolved and transported as a positive ion across the double layer, causing the electrolyte to acquire a net positive charge while the electrode acquires a net negative charge. The growing potential difference creates an intense electric field within the double layer, and the potential rises in value until the field halts the net charge-pumping reactions. This self-limiting action occurs almost instantly in an isolated half-cell; in applications two dissimilar half-cells are appropriately connected to constitute a Galvanic cell.
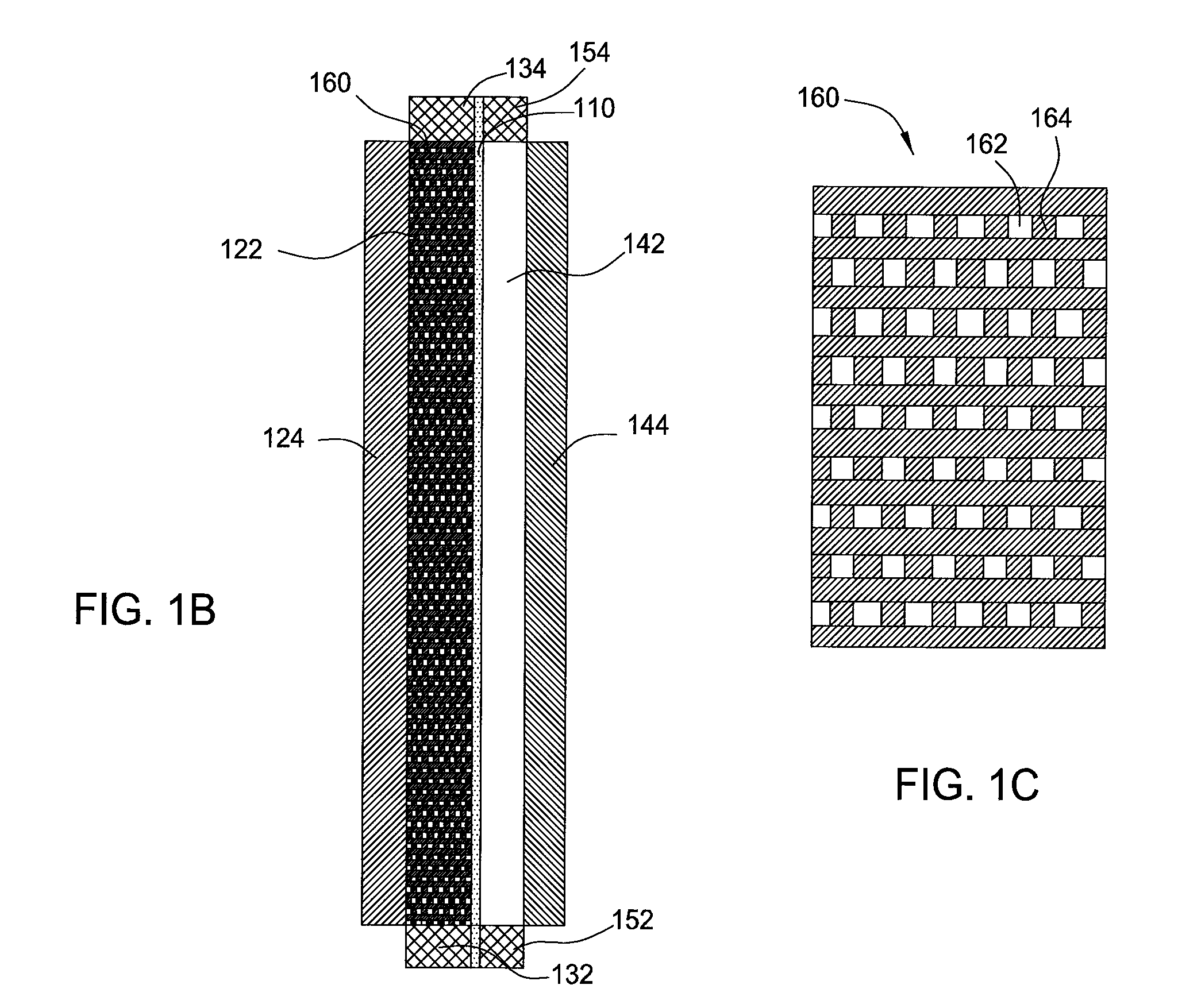
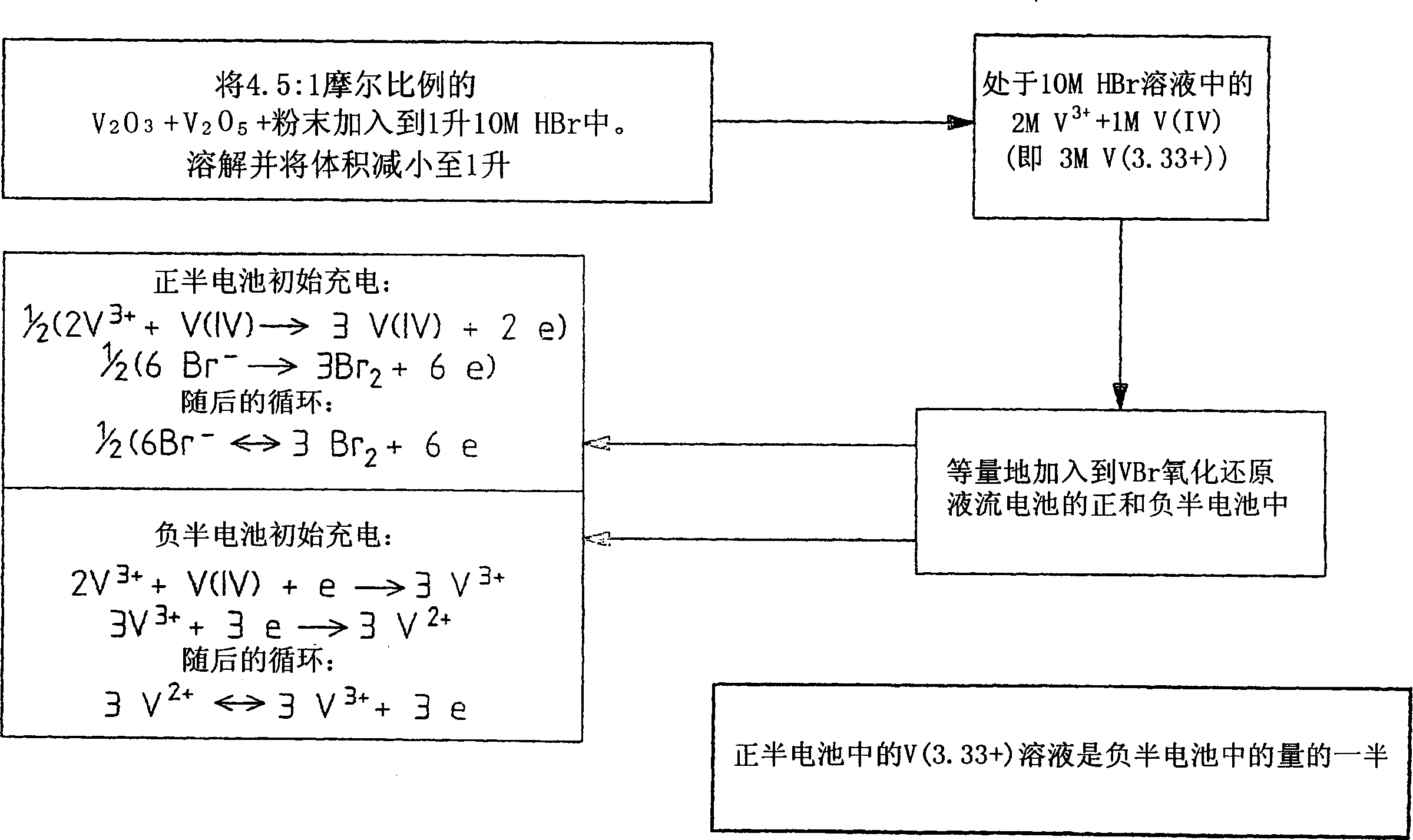
Perfluorinated Membranes and Improved Electrolytes for Redox Cells and Batteries
ActiveUS20080292964A1Improve performanceReduce resistanceFinal product manufactureSecondary cellsSupporting electrolyteVanadyl ion
A vanadium redox cell having a positive half cell containing a positive half cell solution comprising a supporting electrolyte selected from H2SO4, HBr / HCl mixtures and one or more ions selected from the group vanadium (EI), Vanadium (IV), Vanadium (V), Br3 and Br2Cl; a negative half cell containing a negative half cell solution comprising a supporting electrolyte selected from H2SO4, HBr and HBr / HCl mixtures and one or more vanadium ions selected from the group Vanadium (II), Vanadium (III) and Vanadium (FV) and a perfluorinated cast cation exchange membrane or separator disposed between the positive and negative half cells and in contact with the positive and negative half cell solutions.
Owner:NEWSOUTH INNOVATIONS PTY LTD
Redox flow cell
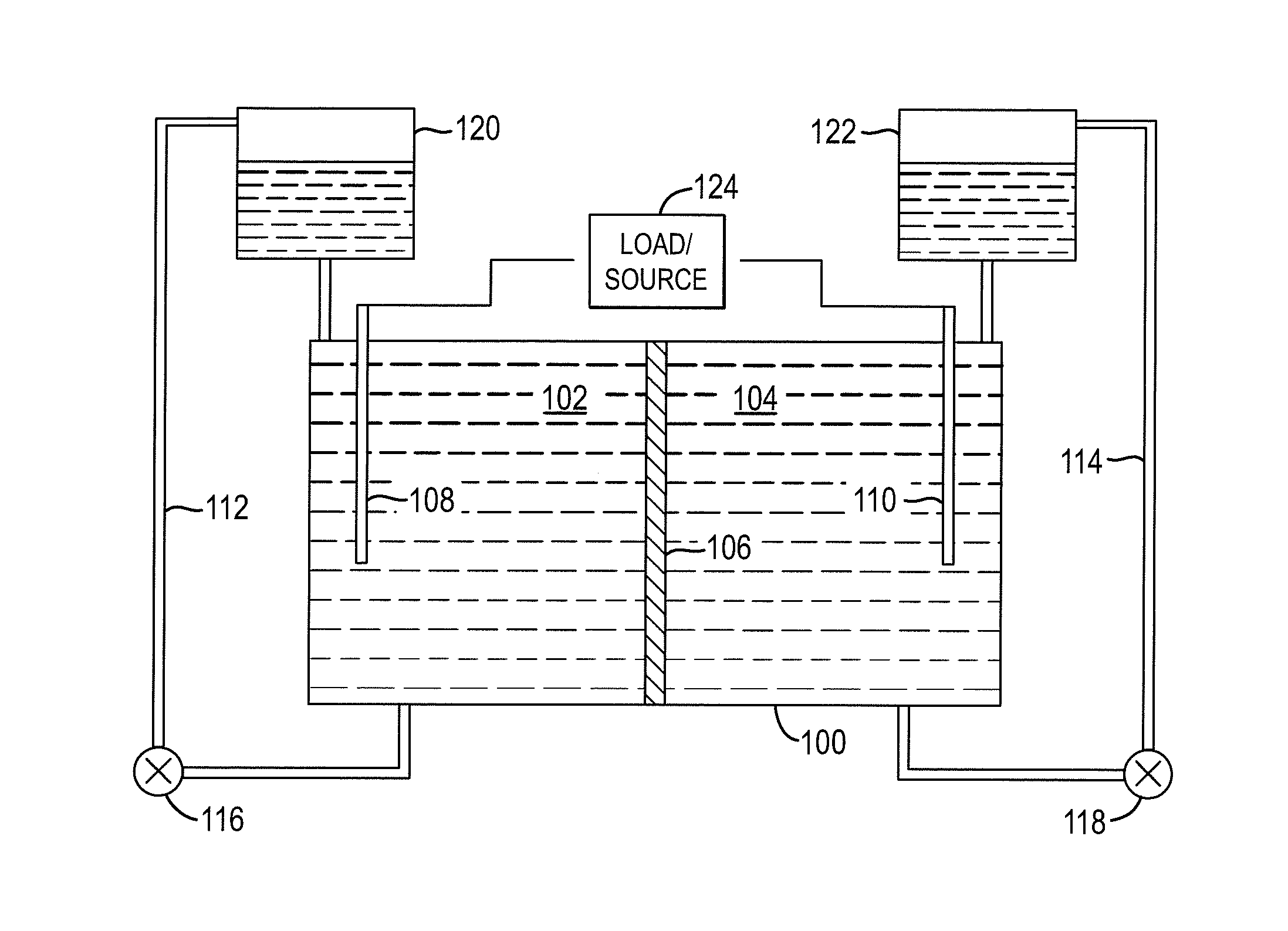
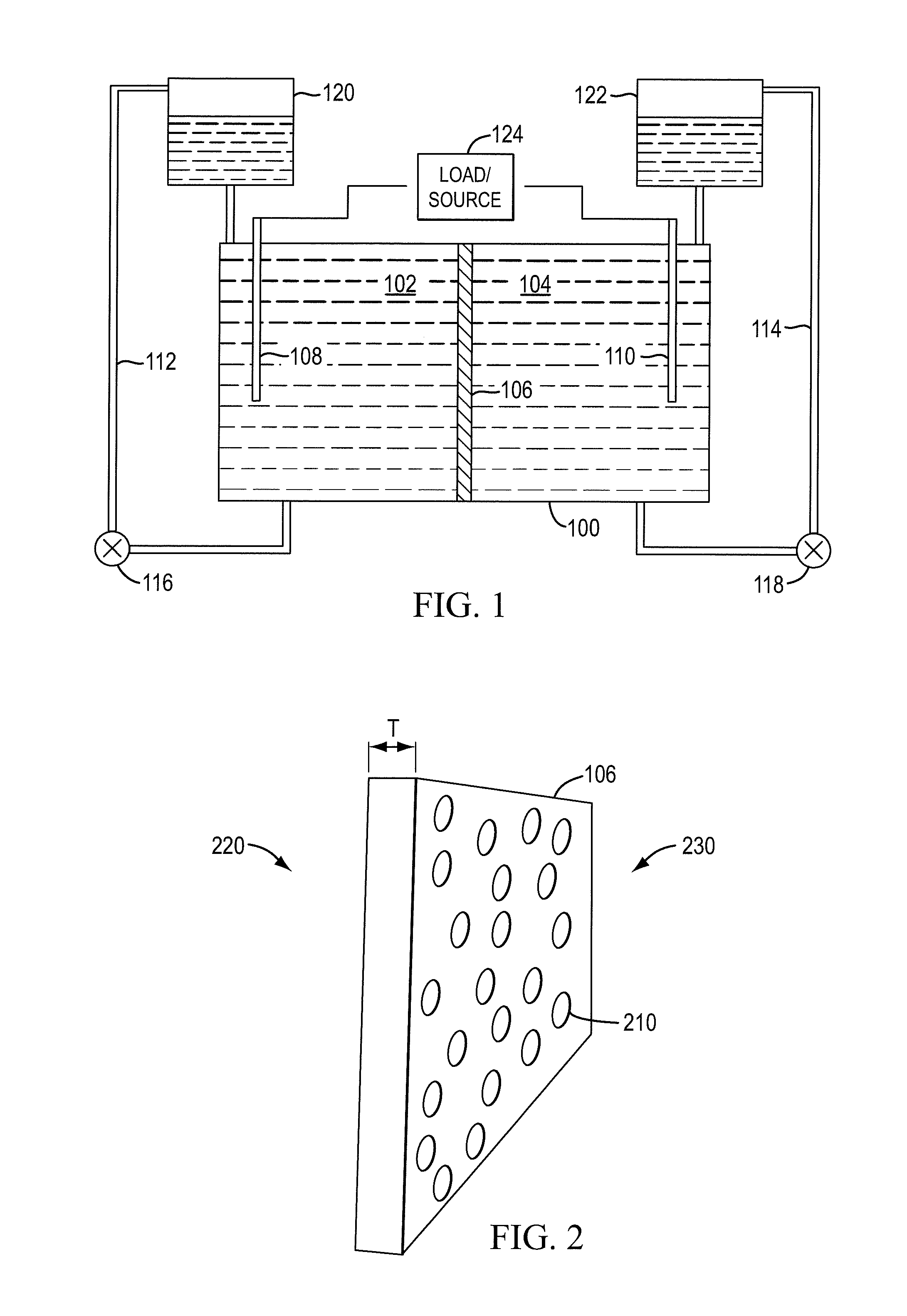
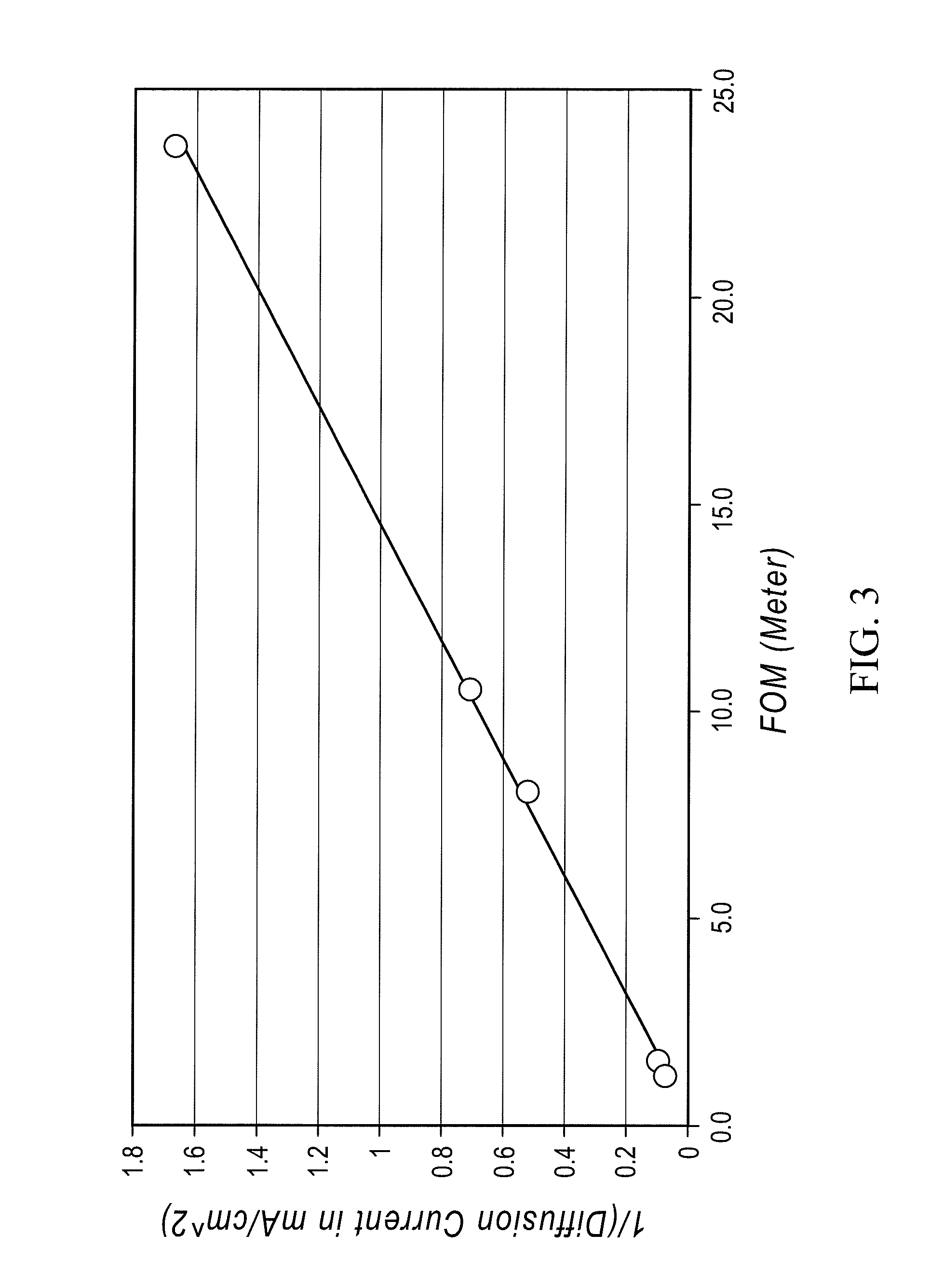
InactiveUS20100003586A1Facilitate ion exchangeCell electrodesFuel cell auxillariesFlow cellPorous membrane
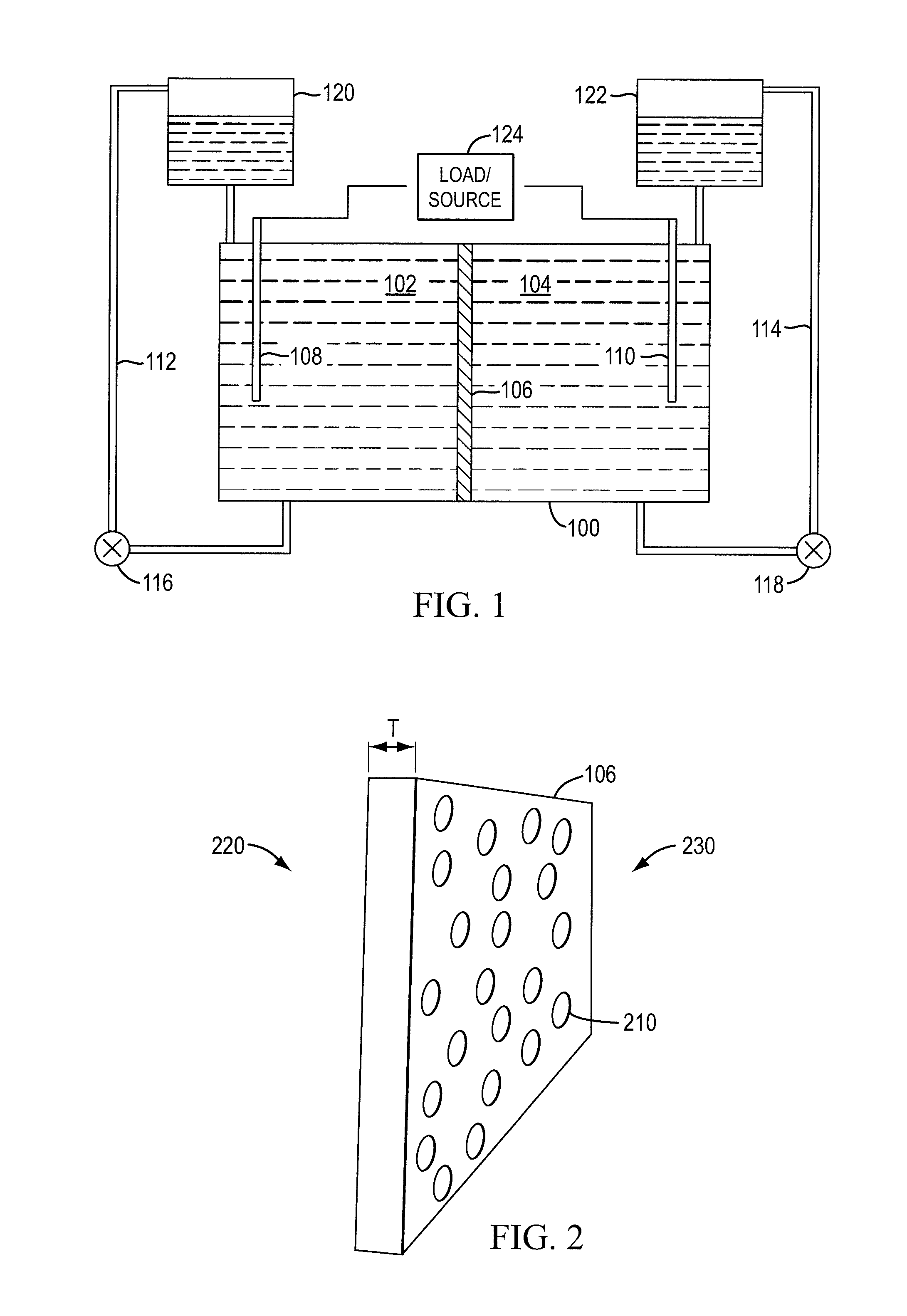
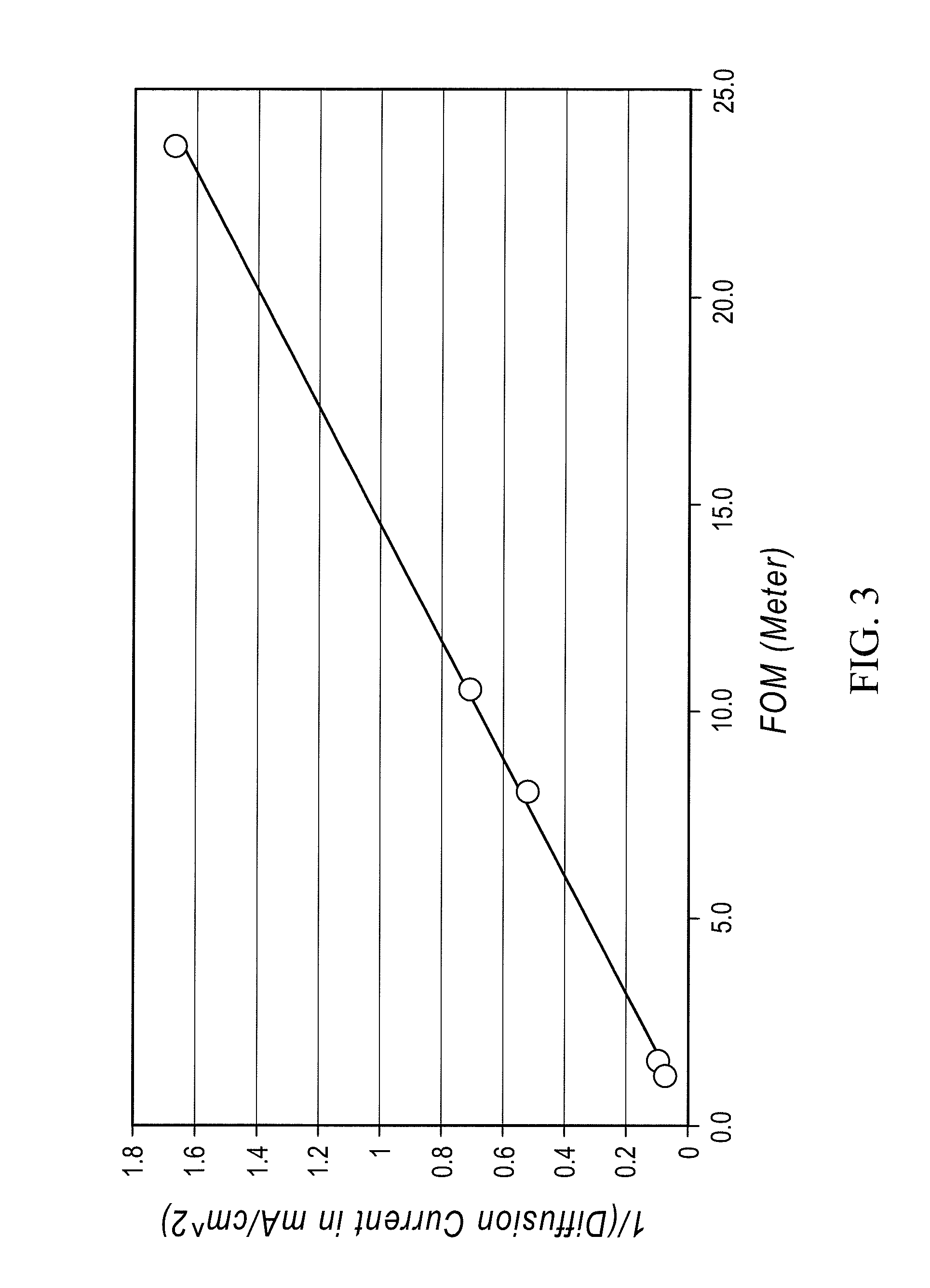
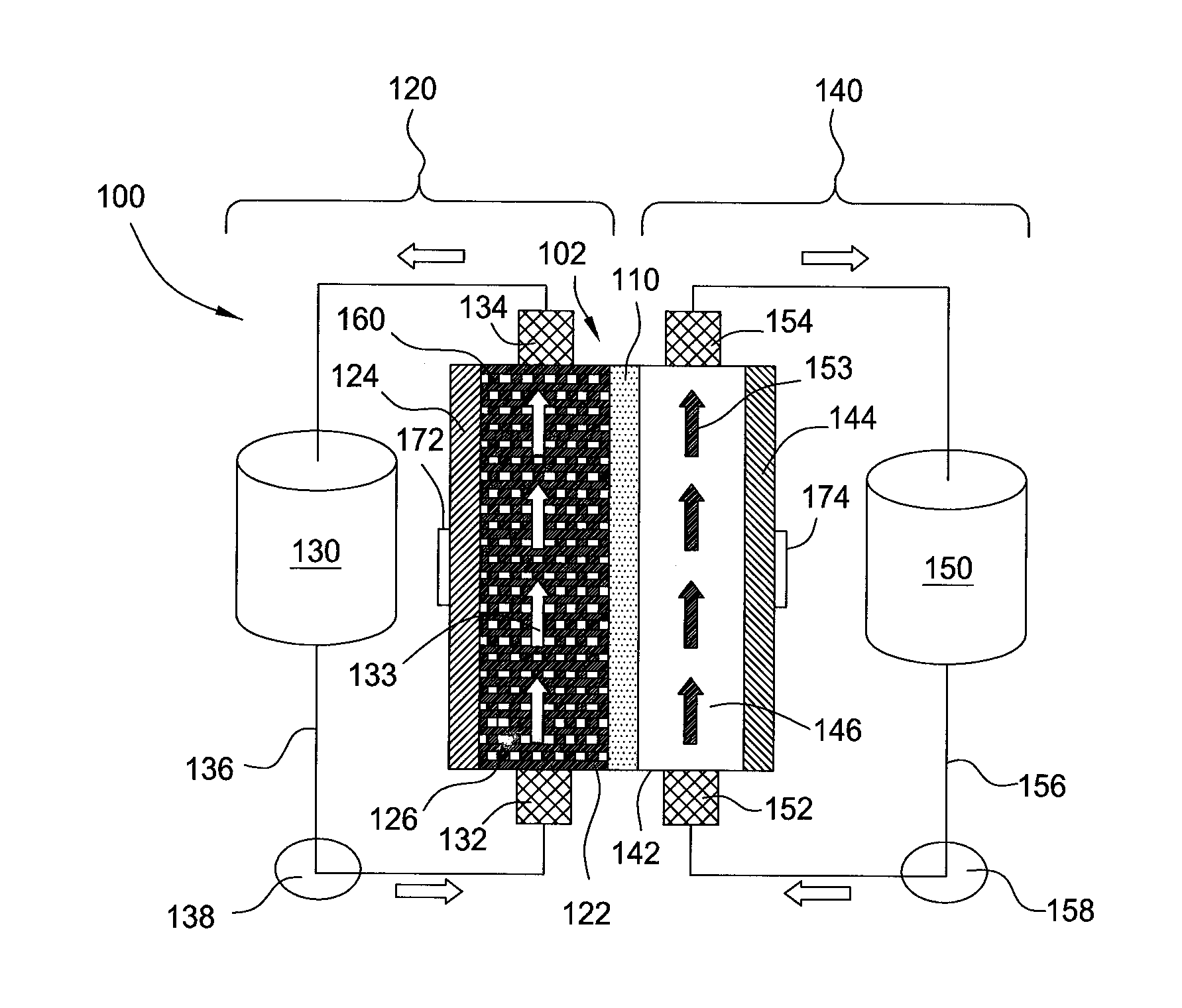
A redox flow cell is presented that utilizes a porous membrane separating a first half cell and a second half cell. The porous membrane is chosen to have a figure of merit (FOM) is at least a minimum FOM. A method of providing a porous membrane for a flow cell can include determining a figure of merit; determining a first parameter from a pore size or a thickness for the porous membrane; determining a second parameter from the pore size or the thickness that is not the first parameter for the porous membrane, based on the figure of merit; and constructing a porous membrane having the pore size and the thickness.
Owner:IMERGY POWER SYST
Flow battery systems
ActiveUS20120052347A1Uniform metal platingHigh cell current densityFuel and secondary cellsCell electrodesEngineeringMetal
Embodiments of the invention generally provide for flow battery cells and systems containing a plurality of flow battery cells, and methods for improving metal plating within the flow battery cell, such as by flowing and exposing the catholyte to various types of cathodes. In one embodiment, a flow battery cell is provided which includes a cathodic half cell and an anodic half cell separated by an electrolyte membrane, wherein the cathodic half cell contains a plurality of cathodic wires extending perpendicular or substantially perpendicular to and within the catholyte pathway and in contact with the catholyte, and each of the cathodic wires extends parallel or substantially parallel to each other. In some examples, the plurality of cathodic wires may have at least two arrays of cathodic wires, each array contains at least one row of cathodic wires, and each row extends along the catholyte pathway.
Owner:APPLIED MATERIALS INC
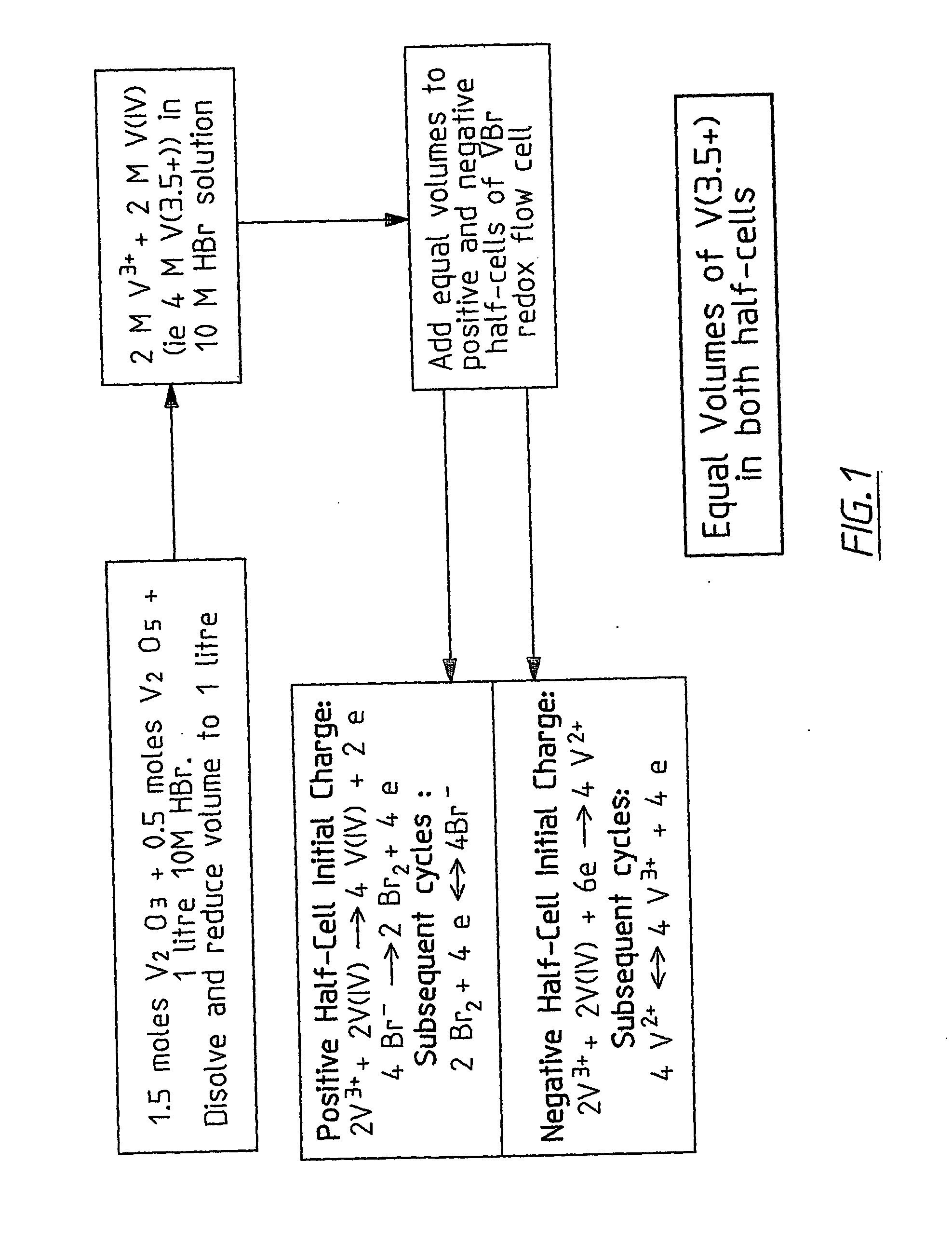
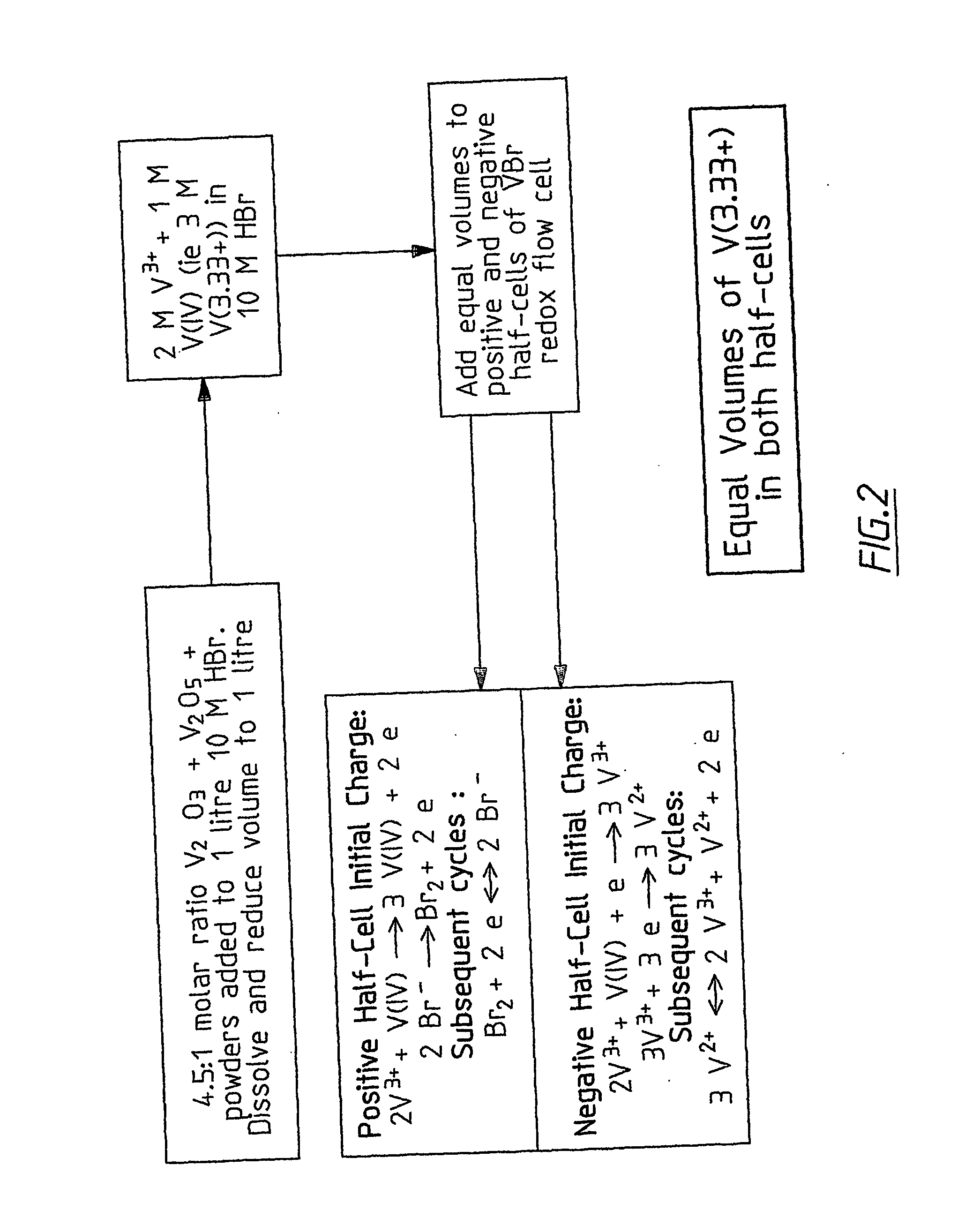
Novel vanadium halide redox flow battery
InactiveUS20060183016A1Avoid excessive bromine generationStabilise the bromine producedCharging stationsCell electrodesRedoxPhysical chemistry
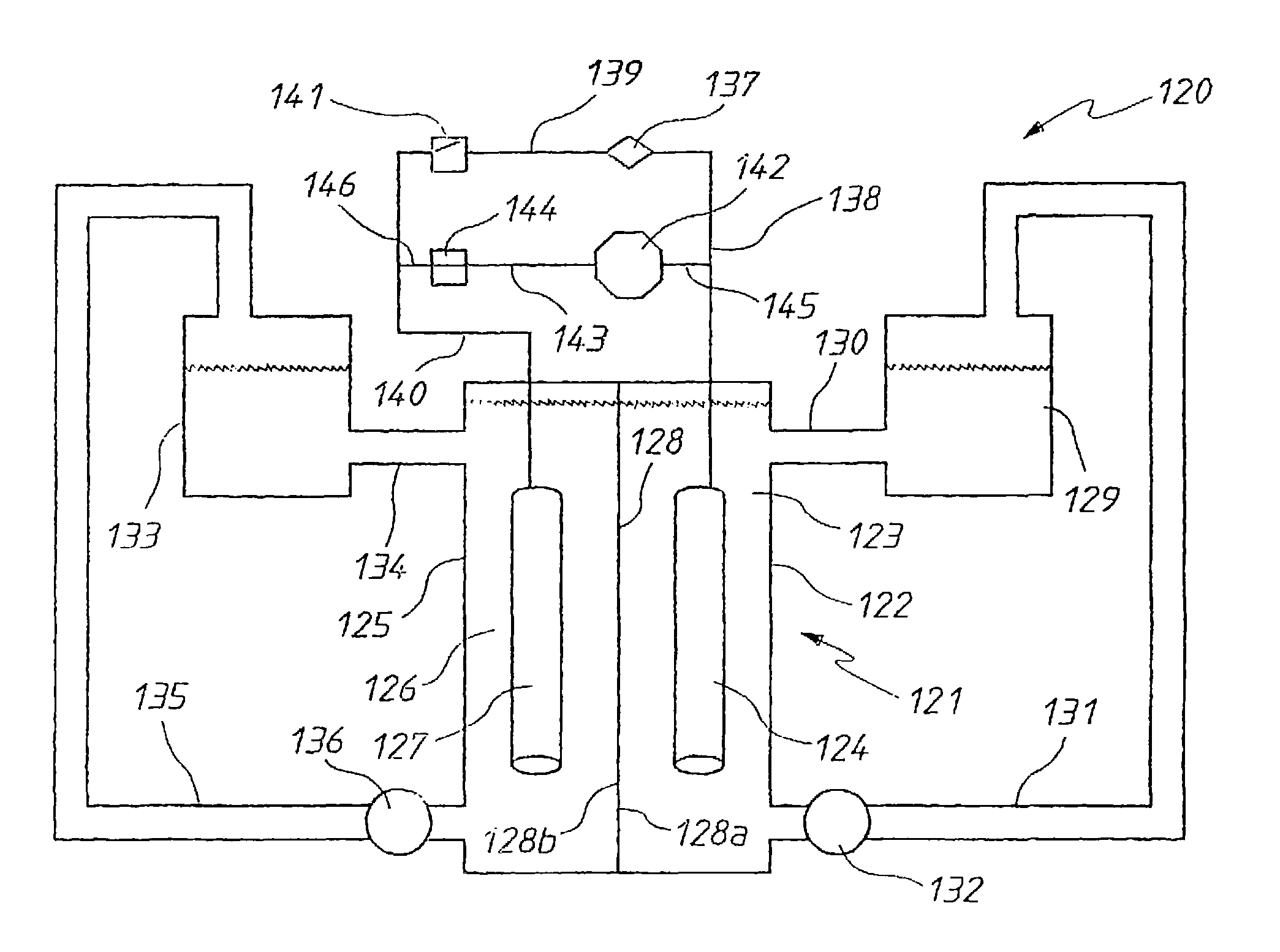
A prior to charge vanadium halide redox cell, a vanadium halide redox cell which is at a state of charge selected from the group consisting of a zero state of charge and a near zero state of charge and vanadium halide redox cell which are fully charged and partially charged are described. The prior to charge vanadium halide redox cell comprises a positive half cell containing a positive half cell solution comprising a halide electrolyte, vanadium (III) halide and vanadium (IV) halide, a negative half cell containing a negative half cell solution comprising a halide electrolyte, vanadium (III) halide and vanadium (N) halide wherein the amounts of vanadium (III) halide, vanadium (IV) halide and halide ions in the positive and negative half cell solutions are such that in a first charging step comprising charging the prior to charge vanadium halide redox cell, a vanadium halide redox cell having a state of charge selected from the group consisting of a zero state of charge and a near zero state of charge comprising predominantly vanadium (N) halide in the positive half cell solution and predominantly V(III) halide in the negative half cell solution can be prepared. The vanadium halide redox cell which is at a state of charge selected from the group consisting of a zero state of charge and a near zero state of charge comprises a positive half cell containing a positive half cell solution comprising a halide electrolyte and a vanadium halide which is predominantly vanadium (N) halide, a negative half cell containing a negative half cell solution comprising a halide electrolyte and a vanadium halide which is predominantly vanadium (III) halide wherein the amount of vanadium (N) halide in the positive half cell solution and the amount of vanadium (III) halide in the negative half cell solution are such that the vanadium halide redox cell is at a state of charge selected from the group consisting of a zero state of charge and a near zero state of charge. The vanadium halide redox cell which is fully charged comprises a positive half cell containing a positive half cell solution comprising a halide electrolyte, a polyhalide complex, vanadium (IV) halide and vanadium (V) halide, a negative half cell containing a negative half cell solution comprising a halide electrolyte and vanadium (II) halide wherein the molar concentration of vanadium (V) and polyhalide complex:molar concentration of vanadium (II) halide is about stoichiometrically balanced. The vanadium halide redox cell which is partially charged comprises a positive half cell containing a positive half cell solution comprising a halide electrolyte, a polyhalide complex, vanadium (IV) halide and vanadium (V) halide, a negative half cell containing a negative half cell solution comprising a halide electrolyte, vanadium (II) halide and vanadium (III) halide wherein the number of moles of moles of polyhalide complex and vanadium (V): number of moles of vanadium (II) halide is about stoichiometrically balanced.
Owner:NEWSOUTH INNOVATIONS PTY LTD
Redox flow cell
A redox flow cell is presented that utilizes a porous membrane separating a first half cell and a second half cell. The porous membrane is chosen to have a figure of merit (FOM) is at least a minimum FOM. A method of providing a porous membrane for a flow cell can include determining a figure of merit; determining a first parameter from a pore size or a thickness for the porous membrane; determining a second parameter from the pore size or the thickness that is not the first parameter for the porous membrane, based on the figure of merit; and constructing a porous membrane having the pore size and the thickness.
Owner:IMERGY POWER SYST
Vanadium/polyhalide redox flow battery
InactiveUS7320844B2Minimise potential cross-contaminationCell electrodesRegenerative fuel cellsElectricityRedox
The invention relates to a redox flow cell containing a polyhalide / halide redox couple in the positive half-cell electrolyte and a V(III) / V(II) redox couple in the negative half-cell electrolyte. The invention also relates to a method of producing electricity by discharging the fully charged or partially charged redox flow cell, and a method of charging the discharged or partially discharged redox flow cell.
Owner:WATTJOULE
PEM water electrolyser module
ActiveUS20110042228A1Maintain inherent scalabilityRelieve pressureCellsDiaphragmsHydrogenEngineering
Owner:NEXT HYDROGEN CORP
Electrolyser module
ActiveUS20100012503A1Avoid difficultyInterior pressure is increasedCellsPhotography auxillary processesMechanical engineeringHalf-cell
An electrolyser module comprising a plurality of structural plates each having a sidewall extending between opposite end faces with a half cell chamber opening and at least two degassing chamber openings extending through the structural plate between the opposite end faces.
Owner:NEXT HYDROGEN CORP
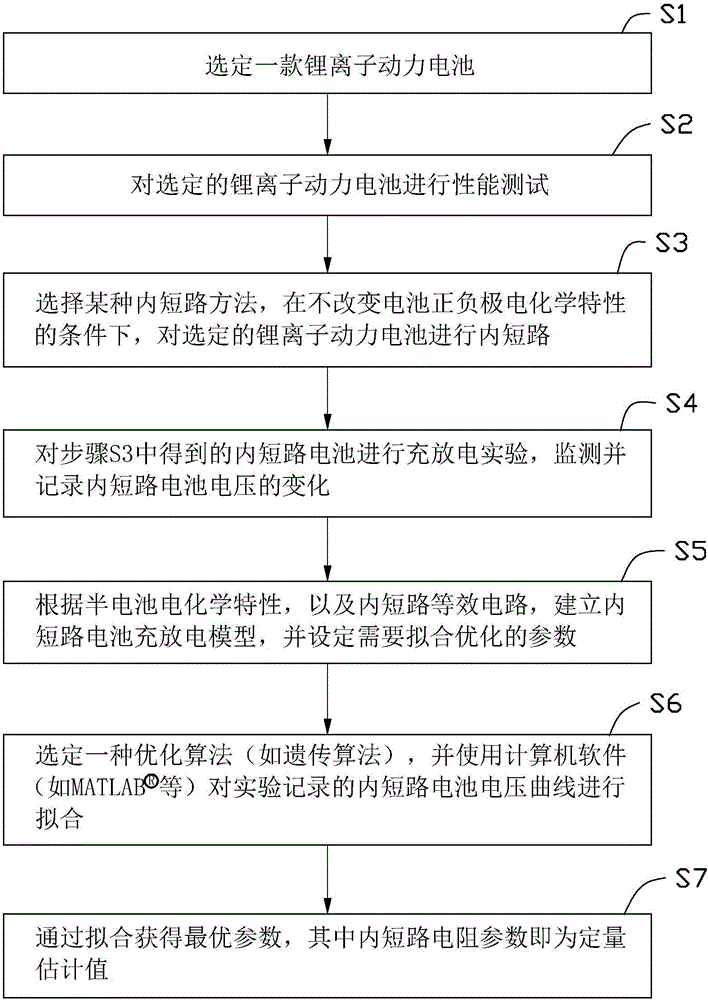
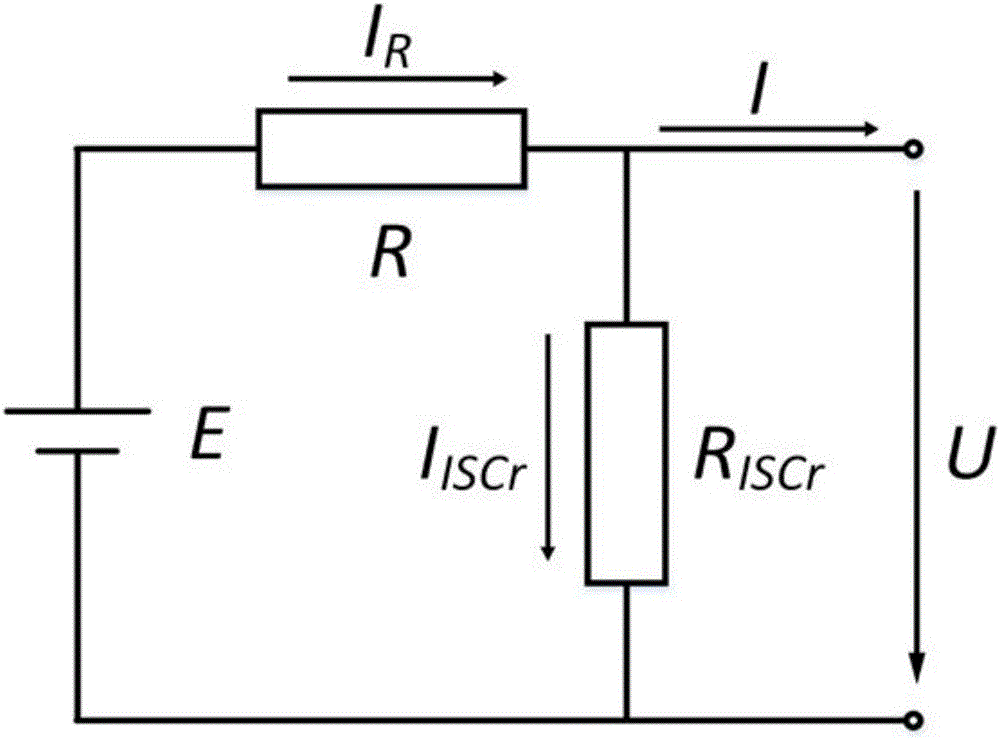
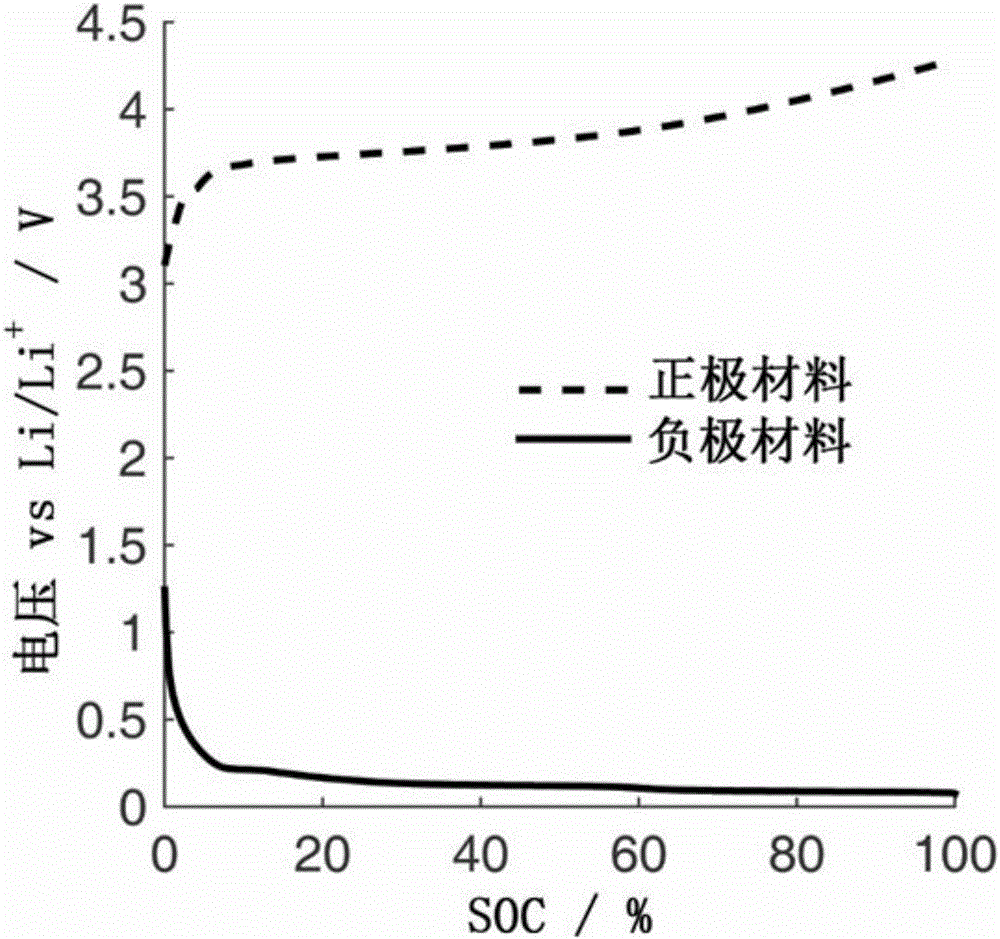
Quantitative estimation method of lithium ion power battery internal short-circuit degree
ActiveCN106154172AImprove reliabilityReduce safety incidentsElectrical testingModel parametersEngineering
The invention provides a quantitative estimation method of a lithium ion power battery internal short-circuit degree, and the quantitative estimation method belongs to the technical field of batteries. The invention provides the estimation method based on a battery electrochemical model, which identifies model parameters by adopting an optimization method, and through establishing an internal short-circuit equivalent circuit model and utilizing variations of half-cell voltage along with the state of charge (SOC) and a discharge voltage curve of an internal short-circuit battery, thereby acquiring an estimated value of internal short-circuit resistance. The quantitative estimation method has good repeatability, high adaptability, can be used for evaluating the internal short-circuit degree of the battery with triggered internal short-circuit, and can complete the estimation of magnitude of the internal short-circuit resistance in different battery operating states. The quantitative estimation method provides valid internal short-circuit evaluation data for an internal short-circuit early detection algorithm, and has important practical significance.
Owner:TSINGHUA UNIV
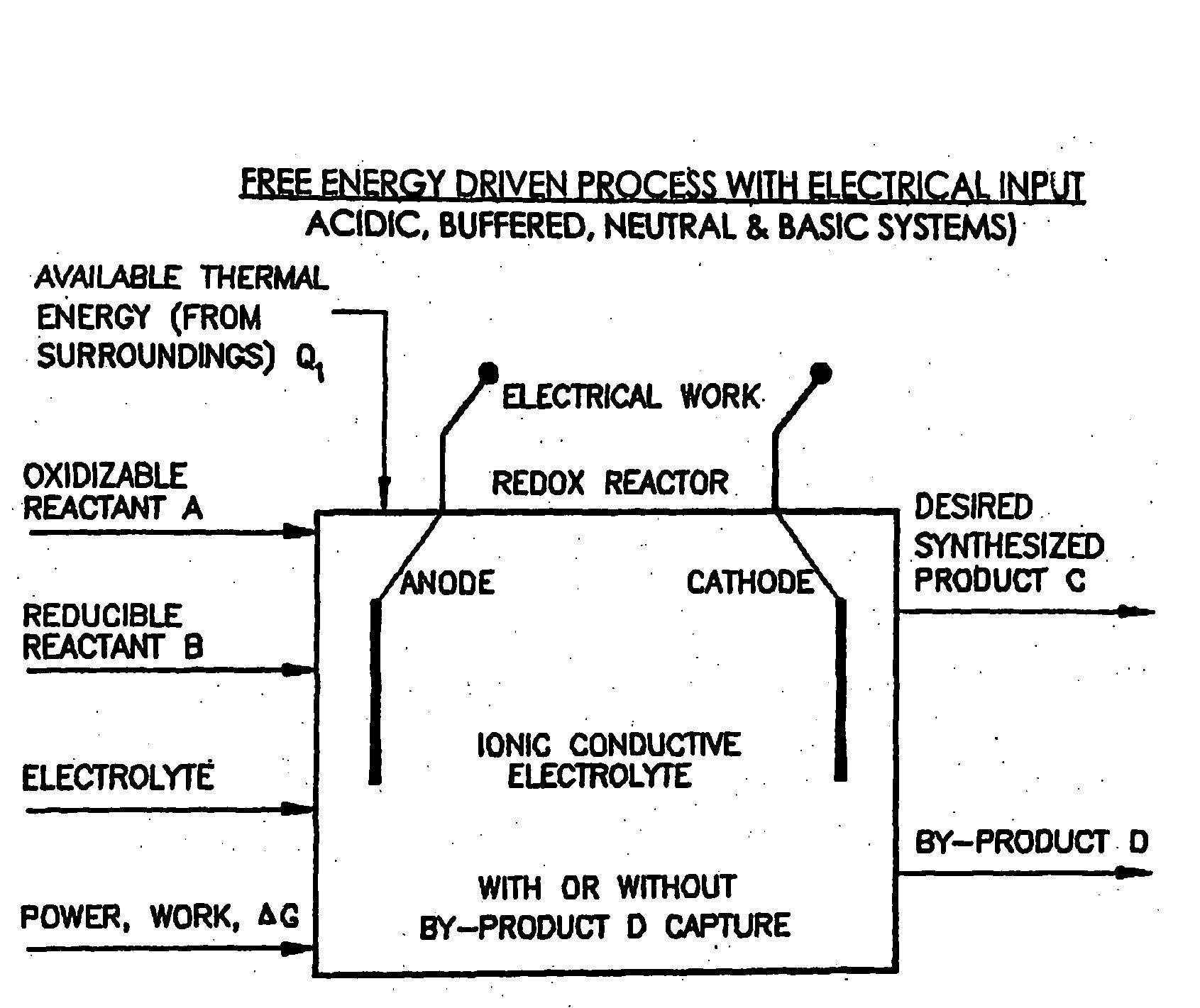
Efficient Production of Fuels
ActiveUS20090277799A1Efficient productionHydrogenBicarbonate preparationFluid phaseElectrical battery
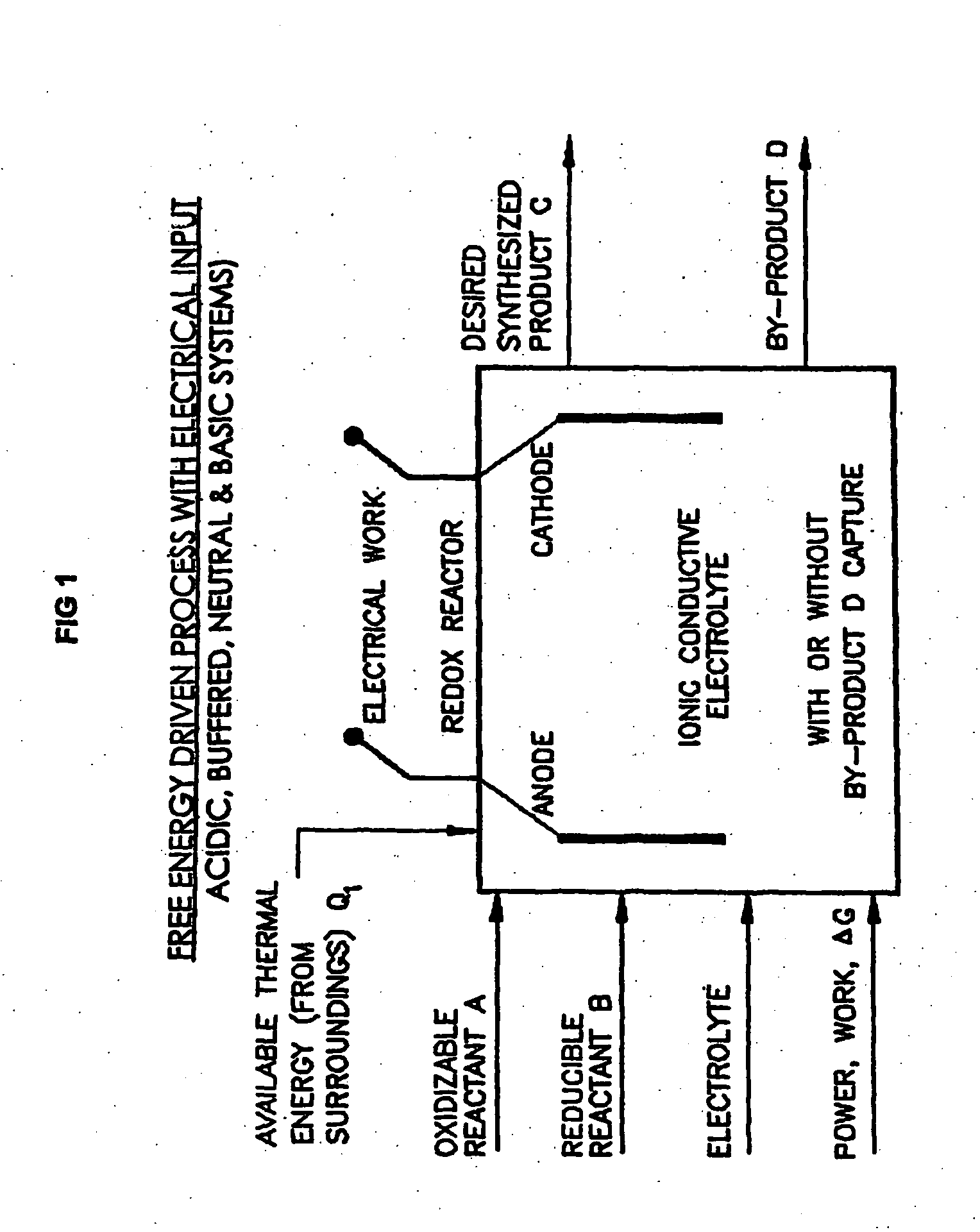
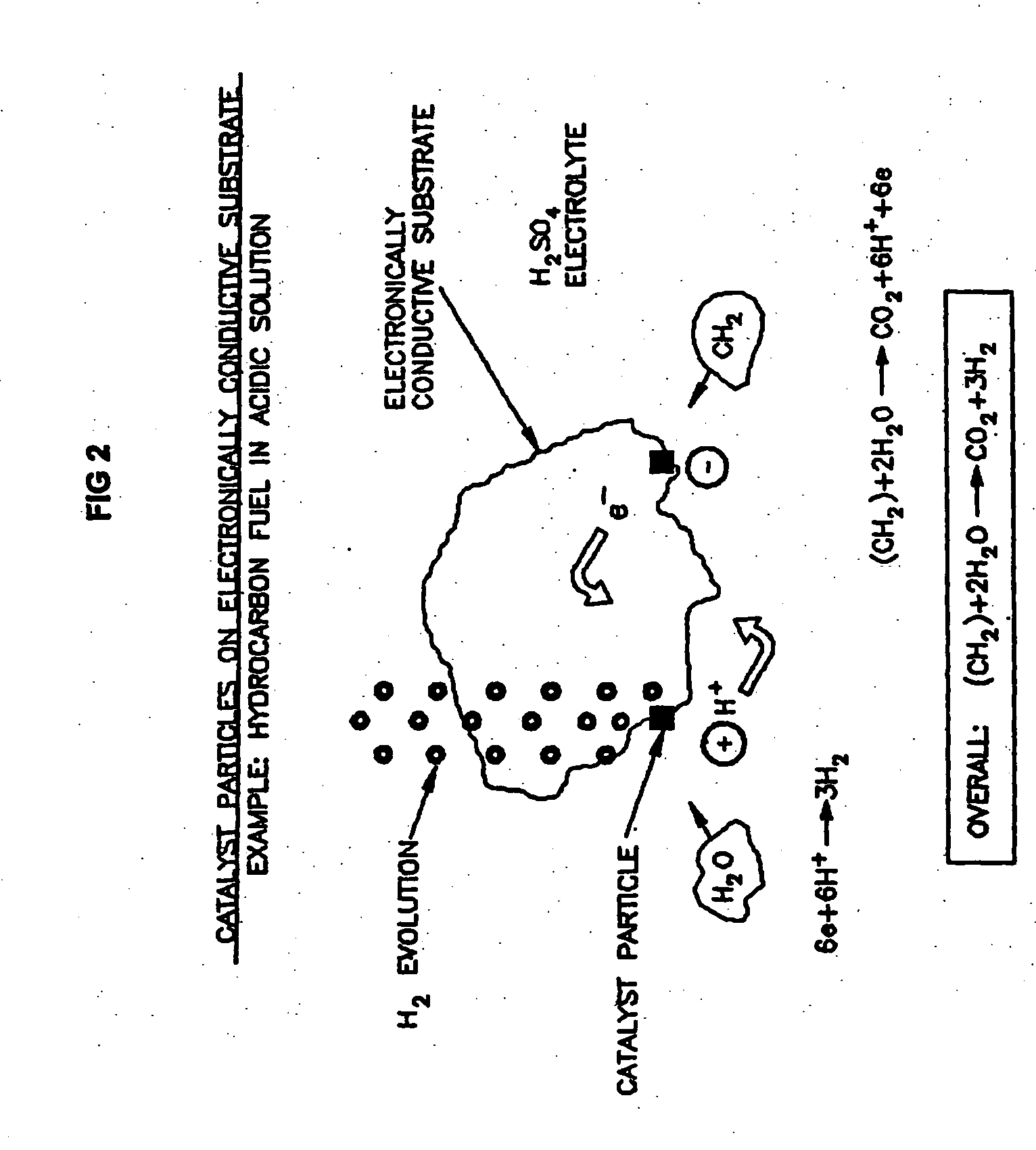
Liquid phase processes for producing fuel in a reactor comprising the step of combining at least one oxidizable reactant with liquid water and at least one electrolyte to form a mixture and conducting a fuel-producing reaction in the presence of an electron transfer material, wherein the mixture permits the movement or transport of ions and electrons to facilitate the efficient production of the fuel. An alternative embodiment produces fuel in an electrochemical cell, the reaction characterized by an overall thermodynamic energy balance according to the half-cell reactions occurring at the anode and cathode. Energy generated and / or required by the system components is directed according to the thermodynamic requirements of the half-cell reactions, thereby realizing improved fuel production efficiency.
Owner:GAS TECH INST
Robust potentiometric sensor
InactiveUS20110048971A1Weather/light/corrosion resistanceVolume/mass flow measurementElectricityElectrical conductor
A modular potentiometric sensor includes a housing having measuring and reference half-cells, a temperature sensor, and solution ground combination assembly. An electrical conductor of the combination assembly extends through the housing, while remaining electrically isolated from the housing and half-cells, terminating at an electrically and thermally conductive end cap. Seals at opposite ends of the housing permit portions of the half-cells and the combination assembly to extend therethrough. The seals, measuring half-cell, and the combination assembly define an electrolyte compartment for the reference half-cell. The end cap provides close thermal coupling to the test fluid while also serving as a test fluid ground that is electrically isolated from the electrolyte compartment. The housing, measuring half-cell, reference half-cell, and combination assembly are modular, with the measurement sensor configurable in a plurality of lengths by altering the length of the housing independently of the half-cells and combination assembly.
Owner:INVENSYS SYST INC
Device and method of skin care and treatment via microneedles having inherent anode and cathode properties, with or without cosmetic or pharmacological compositions
InactiveUS20170028184A1Good lookingImproves user 's experienceMicroneedlesMedical devicesMedicineElectrochemistry
A device with in situ anode and cathode microneedles is used, either with or without any cosmetic, cosmeceutical, nutraceutical, and / or pharmaceutical composition or formulation. The in situ anode and cathode microneedles form battery cells or half-cells when placed in contact with an electrolyte found in bodily interface tissue, to produce an electromotive force without any additional chemical battery or power source. The microneedles may be composed of, or may carry (e.g., be coated with) electrical potential material(s) that has or have an electrical potential relative to an electrolyte in the bodily interface material. A first number of microneedles may, for instance, include a first electrical potential material having a first electrical potential. A second number of microneedles may, for instance, include a second electrical potential material having a second electrical potential. The first number of microneedles may thus serve as an anode, while the second number of microneedles may thus serve as a cathode. Alternatively, a number of microneedles may, for instance, include both a first electrical potential material and a second electrical potential material, having a first electrical potential and a second electrical potential, respectively. Respective portions of each of the microneedles may thus serve as an anode and a cathode. Again, such may be accomplished without any other or additional electrochemical battery.
Owner:CATURA CORP
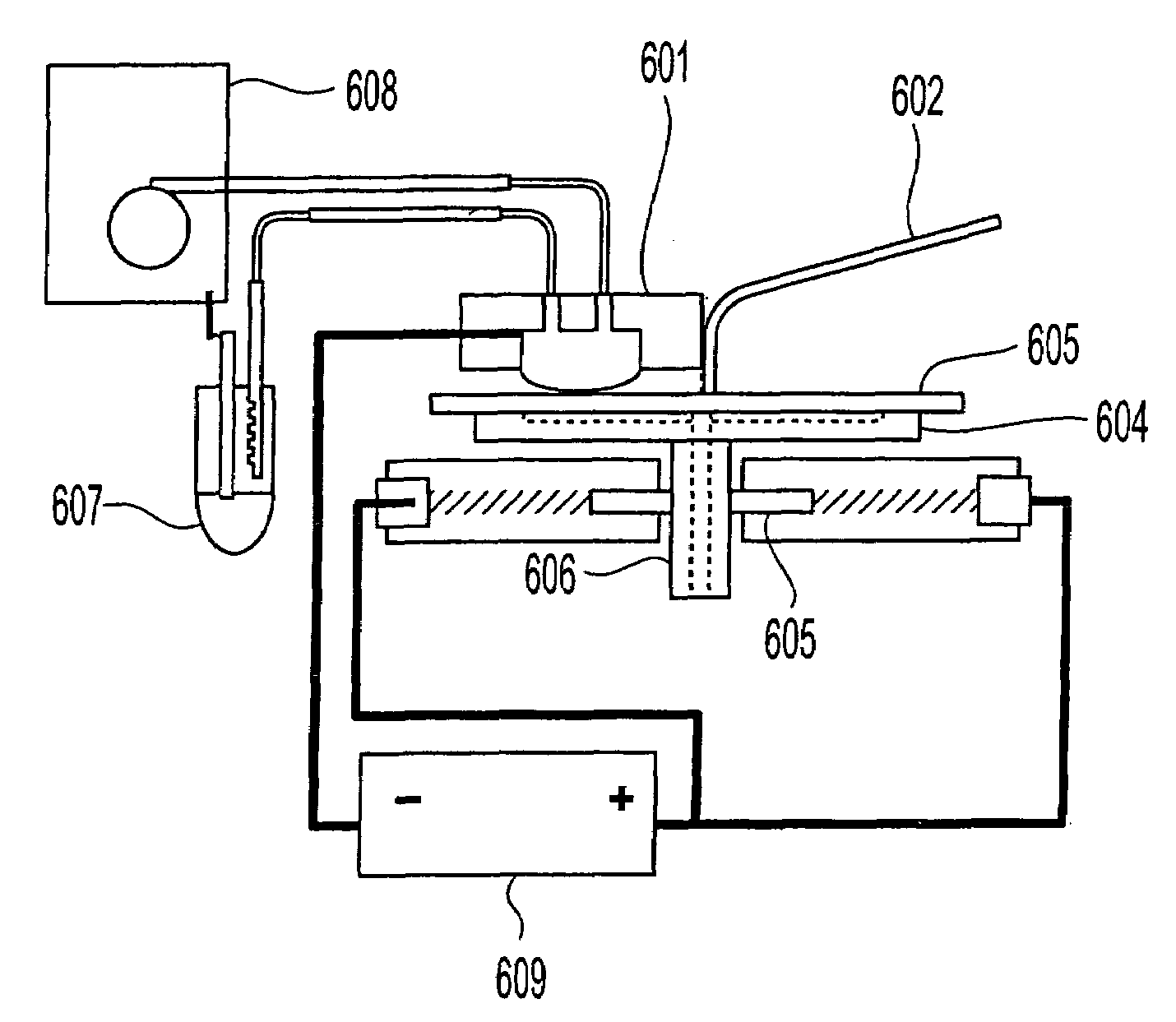
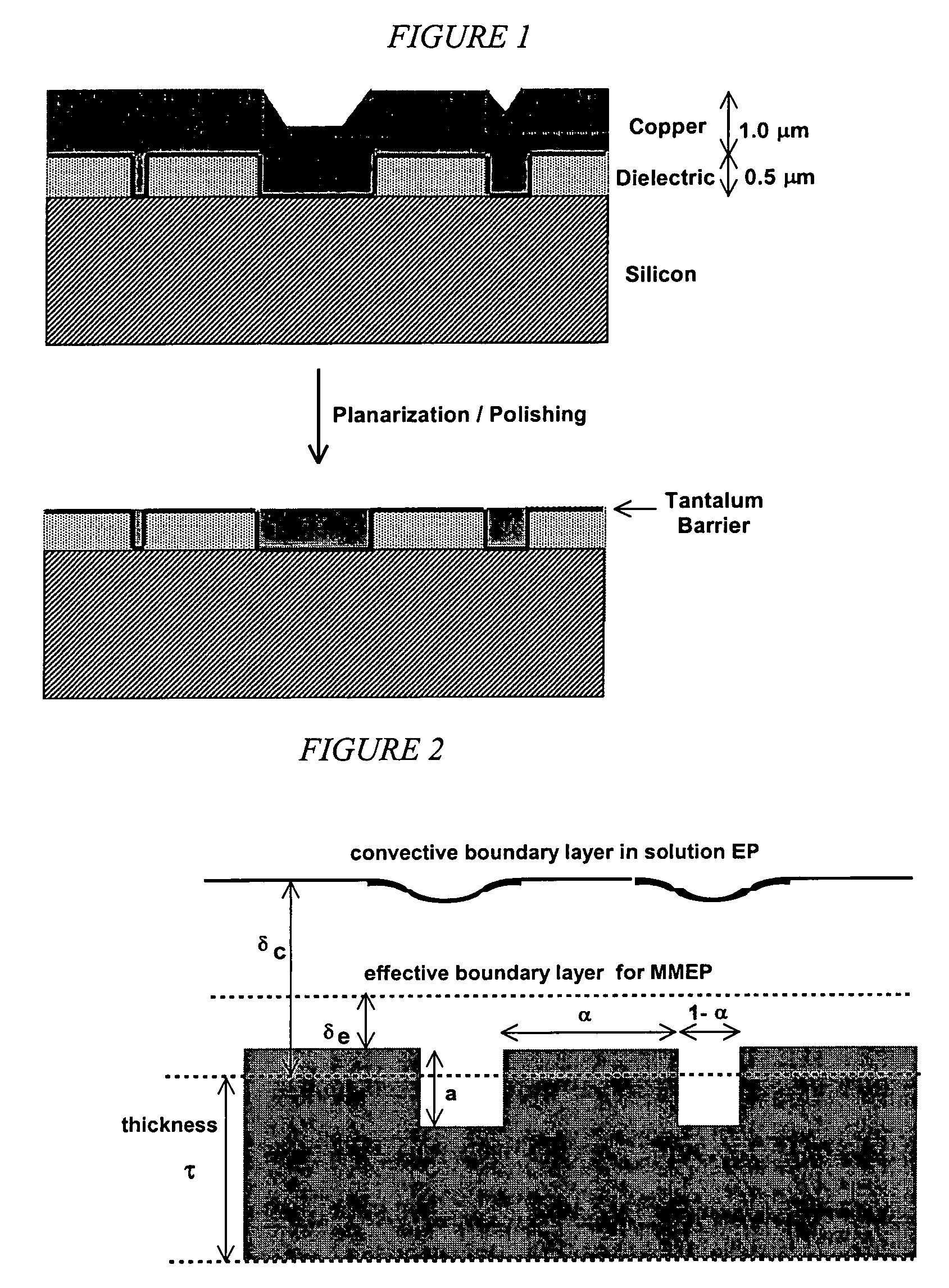
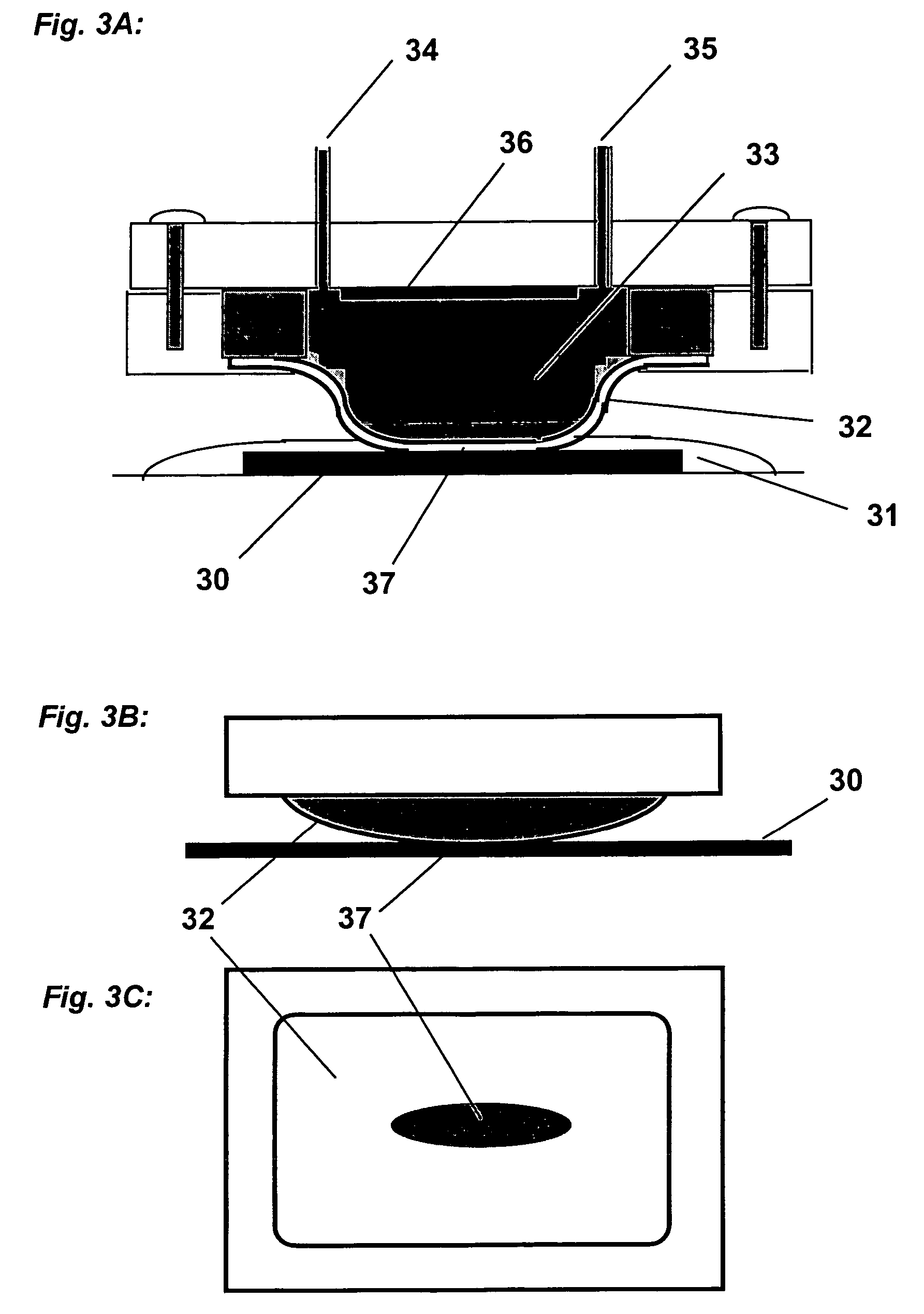
Apparatus adapted for membrane-mediated electropolishing
InactiveUS20070051619A1Reduce roughnessImprove abilitiesCellsSemiconductor/solid-state device manufacturingElectricityElectrolysis
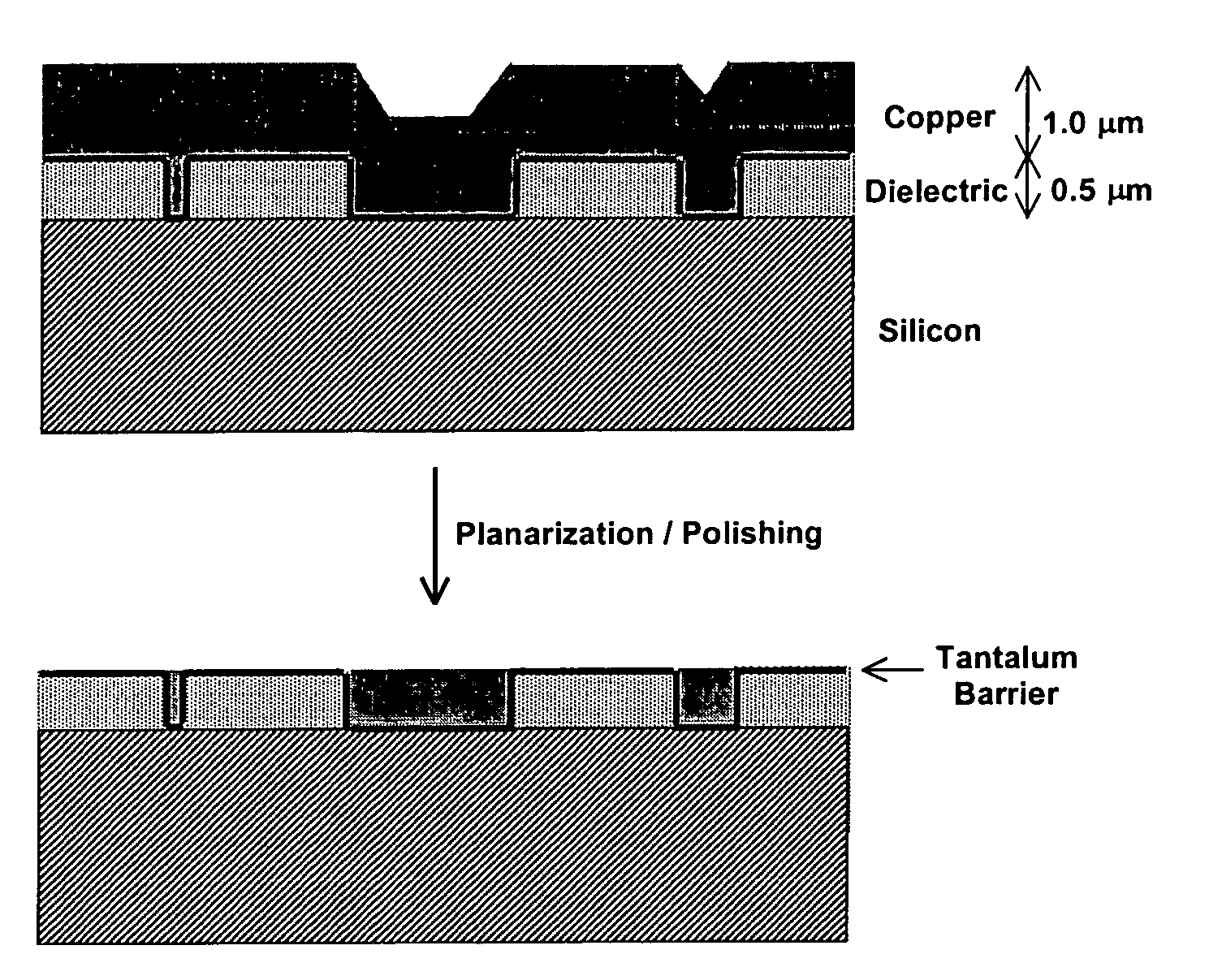
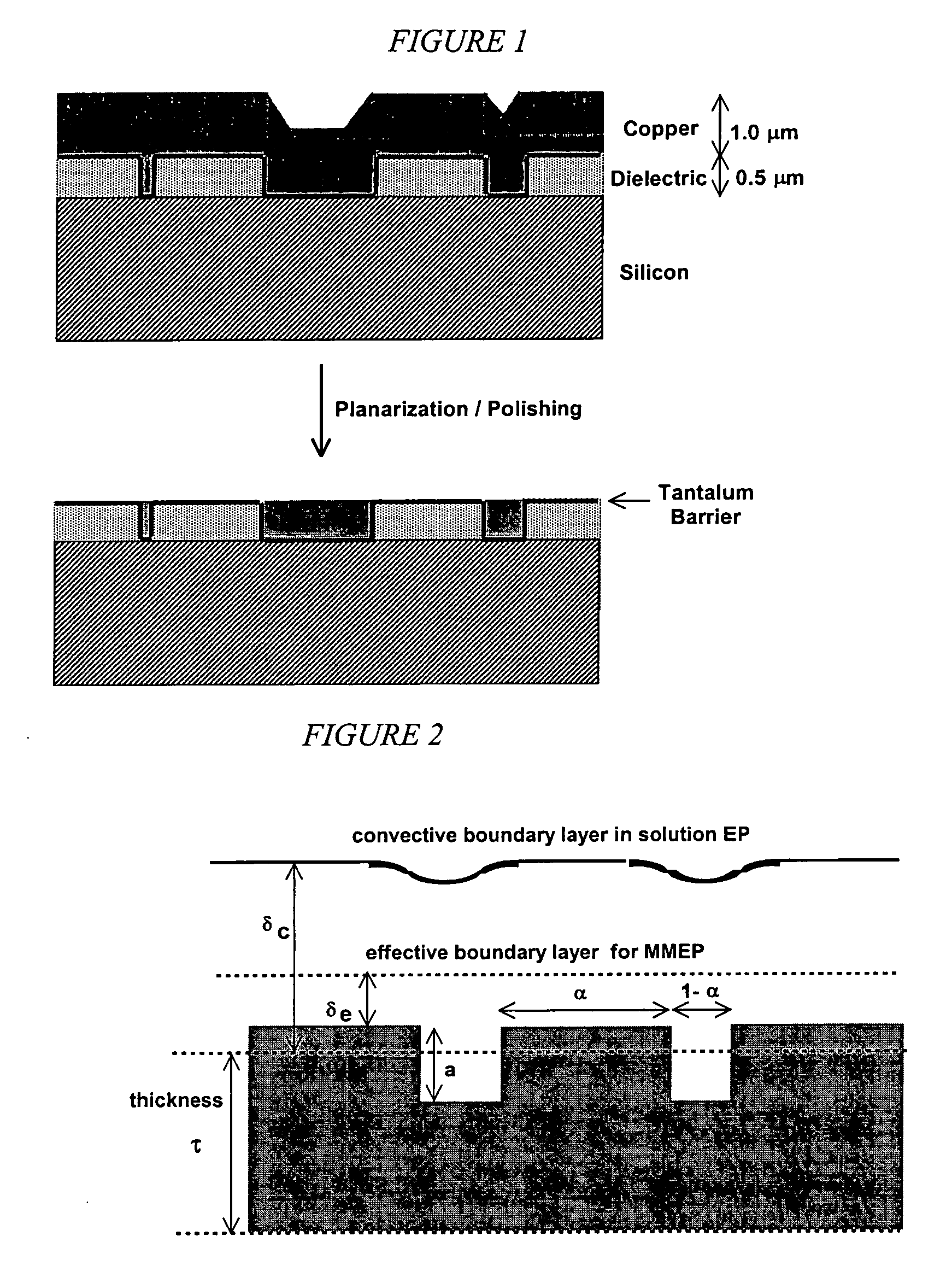
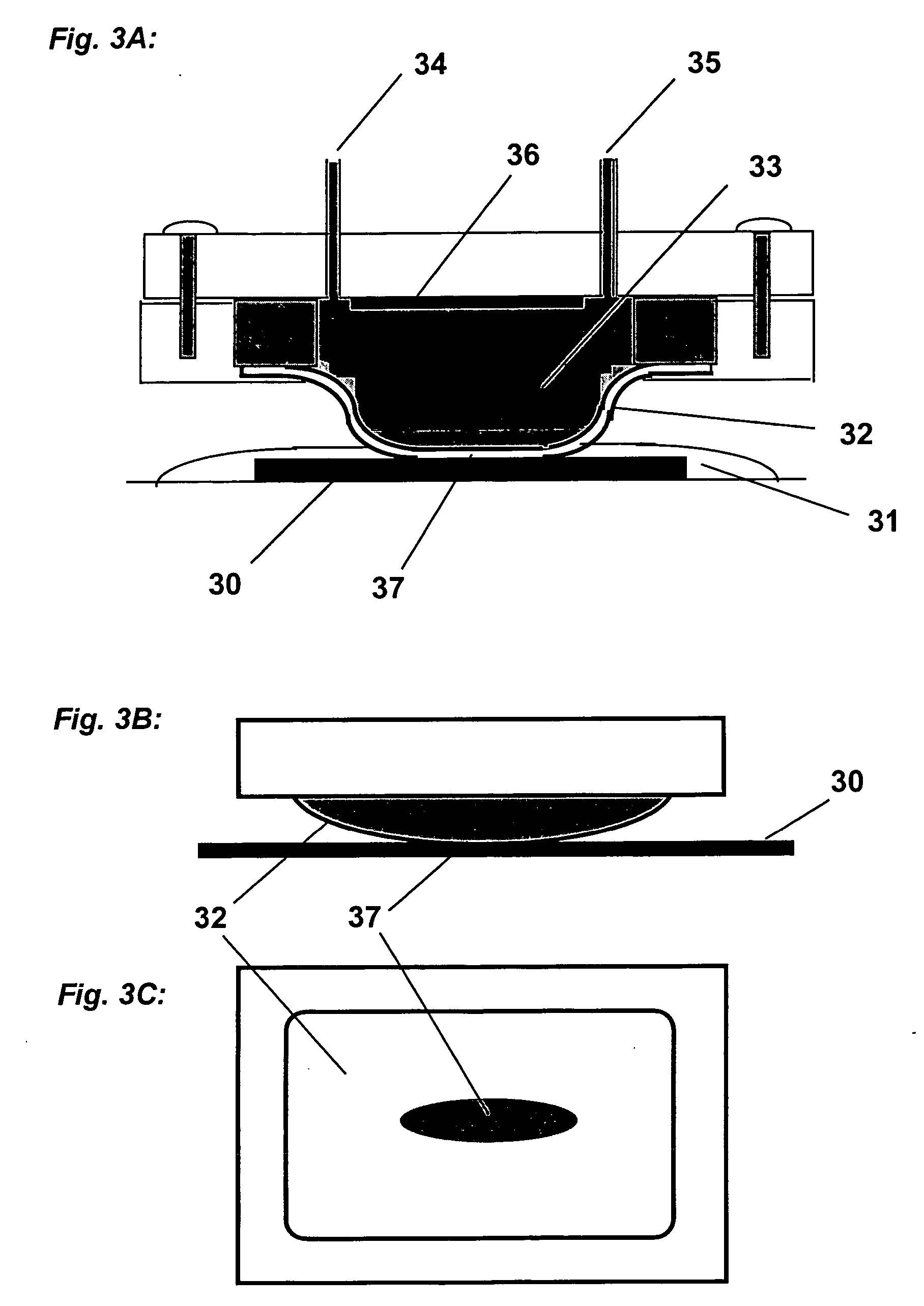
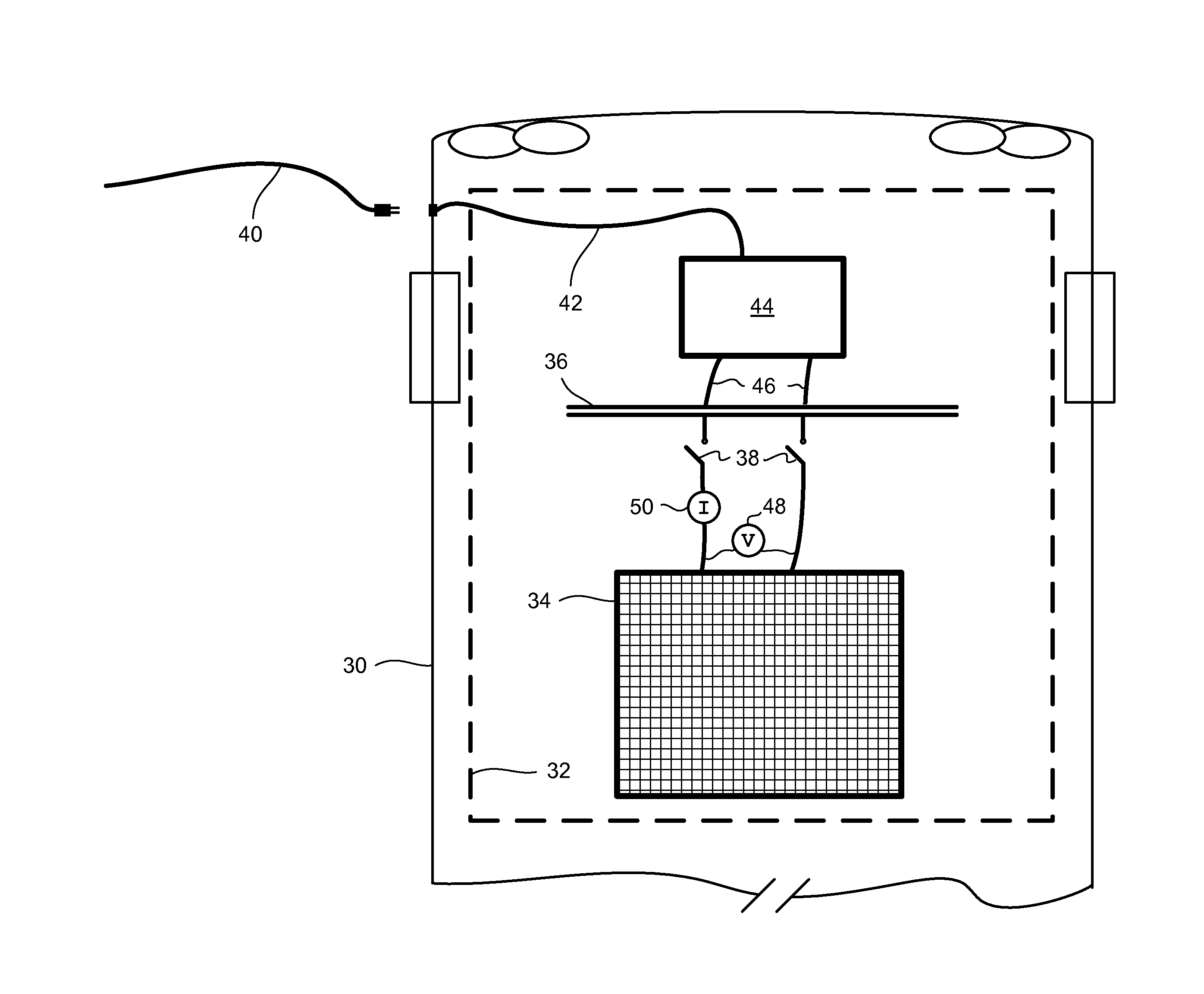
This invention provides a membrane-mediated electropolishing apparatus for polishing and / or planarizing metal work-pieces. The work-piece is wetted with a low-conductivity fluid. The wetted work-piece is contacted with a first side of a charge-selective ion-conducting membrane, wherein the second side contacts a conductive electrolyte solution in electrical contact with a electrode. Current flow between the electrode and the work-piece electropolishes metal from the work-piece. This invention also provides a half-cell adapted for use in membrane-mediated electropolishing having a fully or partially enclosed volume, a conductive electrolyte which partially or essentially fills the enclosed volume, an electrode which is in contact with the electrolyte, and a charge-selective ion-conducting membrane which seals one surface of the enclosed volume, cavity or vessel in such a way that the internal surface of said membrane contacts the electrolyte solution or gel and the external surface is accessible to contact the work-piece.
Owner:EI DU PONT DE NEMOURS & CO
Method to detect open-circuit voltage shift through optimization fitting of the anode electrode half-cell voltage curve
InactiveUS20140214347A1Electrical testingSpecial data processing applicationsState of chargeEngineering
Methods are disclosed for modeling changes in capacity and the state of charge vs. open circuit voltage (SOC-OCV) curve for a battery cell as it ages. During battery pack charging, voltage and current data are gathered for a battery cell. In one method, using multiple data points taken during the plug-in charge event, data optimization is used to determine values for two parameters which define a scaling and a shifting of the SOC-OCV curve from its original shape at the cell's beginning of life to its shape in the cell's current condition. In a second method, only initial and final voltages and current throughput data are needed to determine the values of the two parameters. With the scaling and shifting parameters calculated, the cell's updated capacity and updated SOC-OCV curve can be determined. The methods can also be applied to data taken during a discharge event.
Owner:GM GLOBAL TECH OPERATIONS LLC
Method for preparing low temperature solid oxide fuel cell supported by porous metal
InactiveCN1960047AAffect performanceAffect lifeSolid electrolyte fuel cellsElectrochemical responseAir atmosphere
Method for preparing the disclosed fuel cell includes steps: (1) using porous stainless steel as supportor; depositing porous anodal thin film, compact solid electrolyte film, and reaction barrier layer in sequence on the supportor to be as half cell; carrying out sintering under reducing atmosphere or inert atmosphere; (2) cooling after sintering, depositing active layer of cathode and contact layer of cathode on solid electrolyte film right along; carrying out sintering under air atmosphere so as to obtain cell; (3) dipping reforming catalyst for supportor of porous stainless steel so as to obtain fuel cell. Through the supportor, various fuel gases reformed to anodic gas rich in hydrogen enters into anode to carry out electrochemical reaction.
Owner:SHANGHAI JIAO TONG UNIV
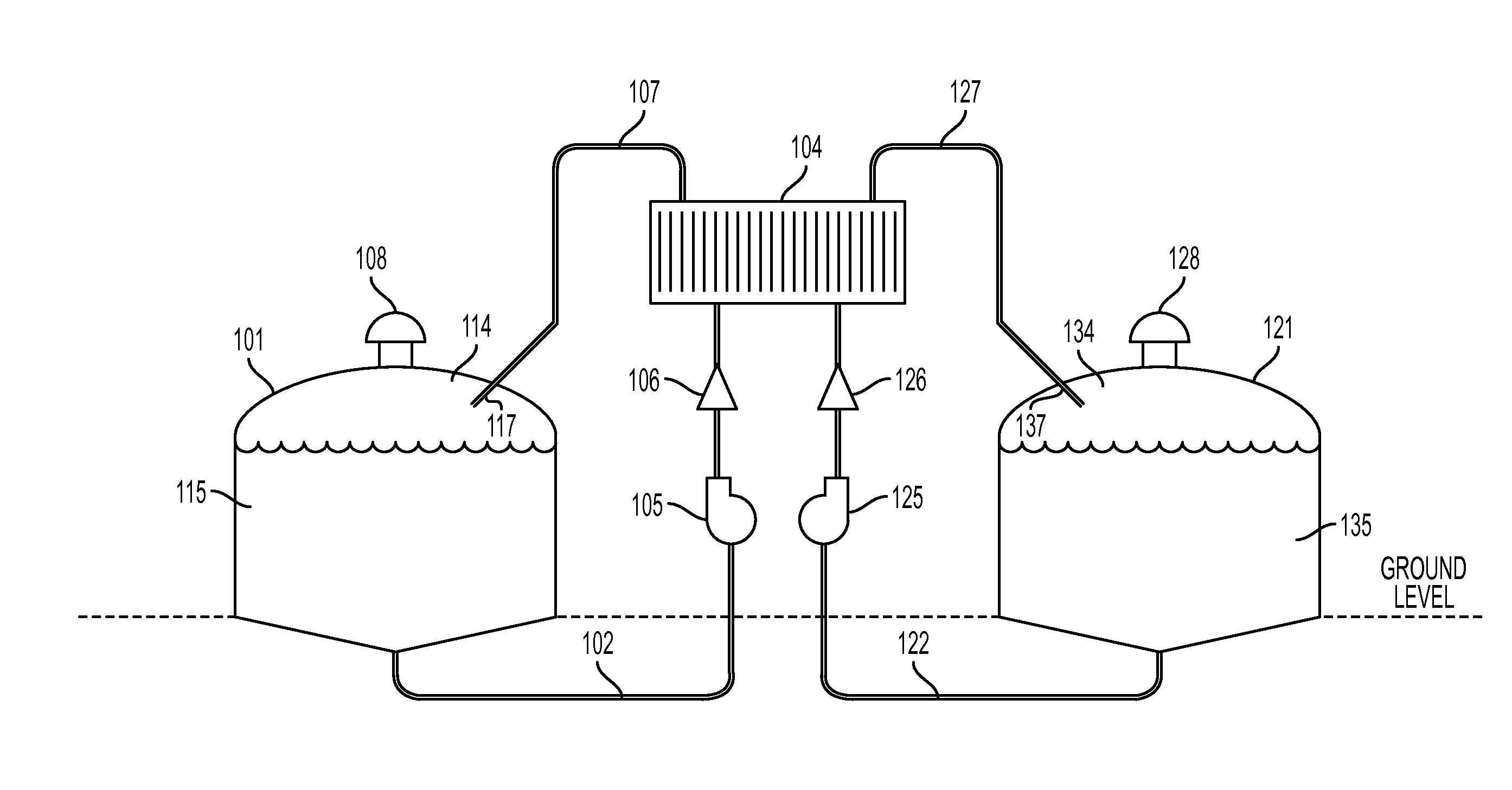
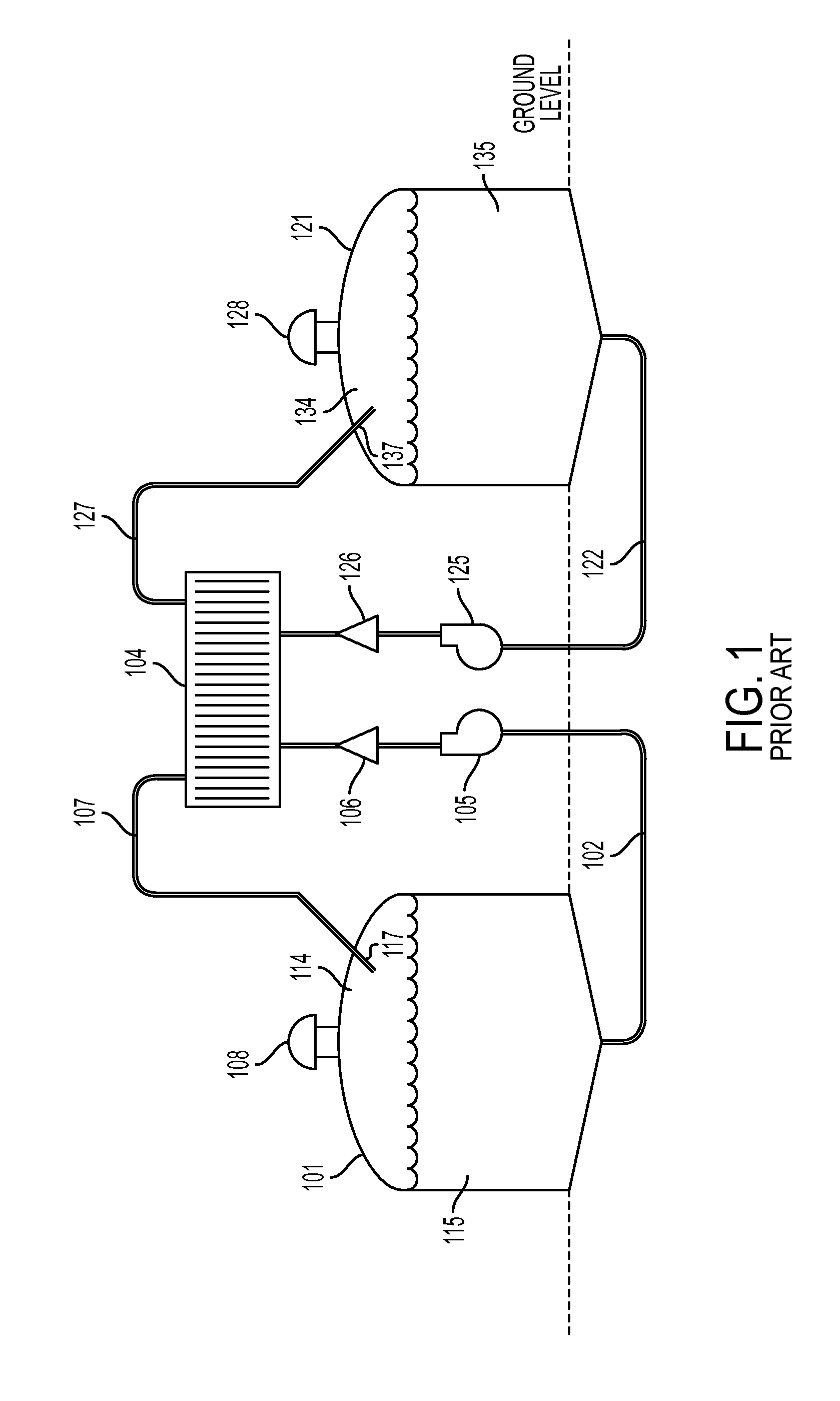
Cell stack for a flowing electrolyte battery
ActiveUS20100119937A1Increase in sizeReduce manufacturing costElectrolyte moving arrangementsAlkaline accumulatorsReduced sizeEngineering
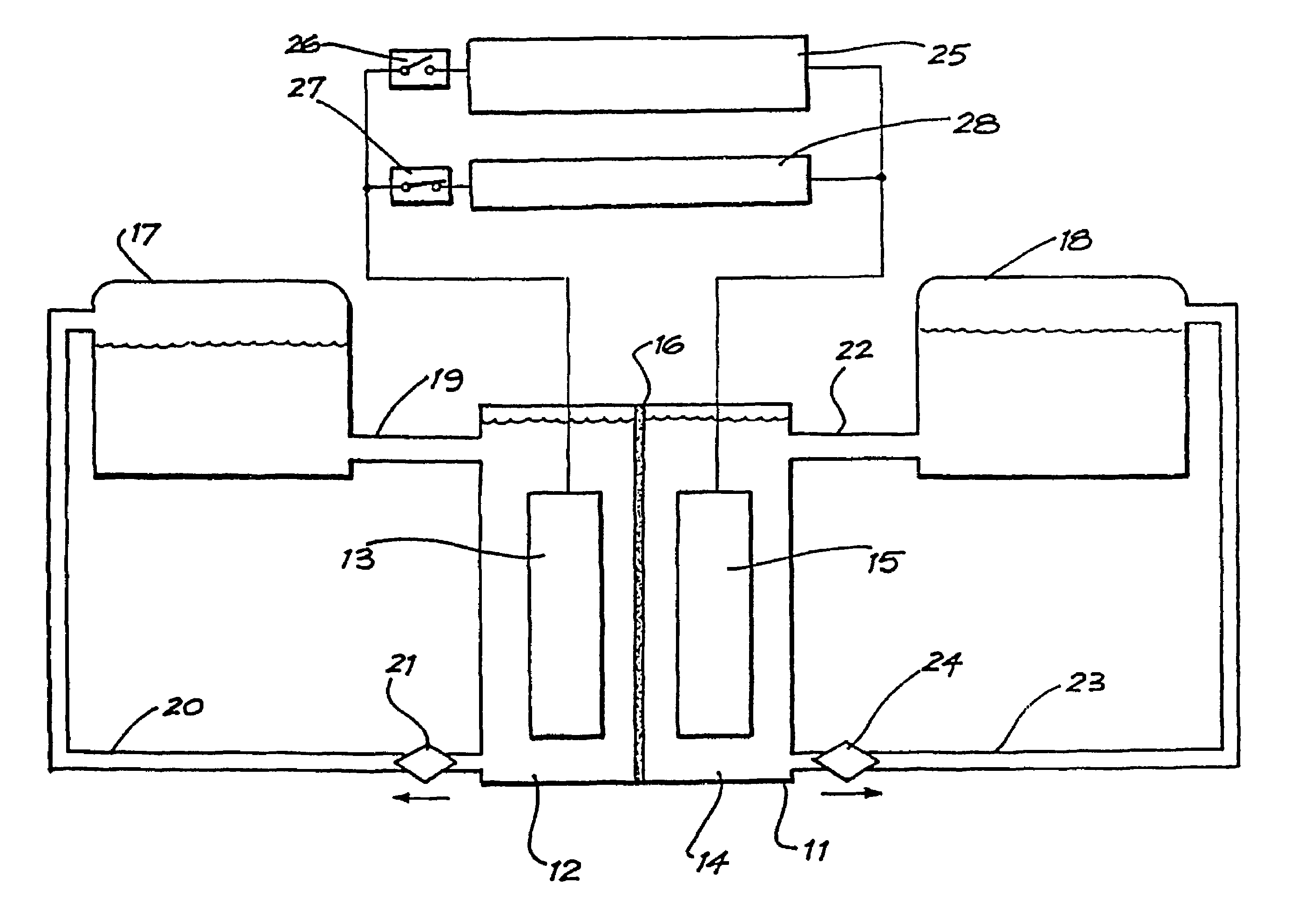
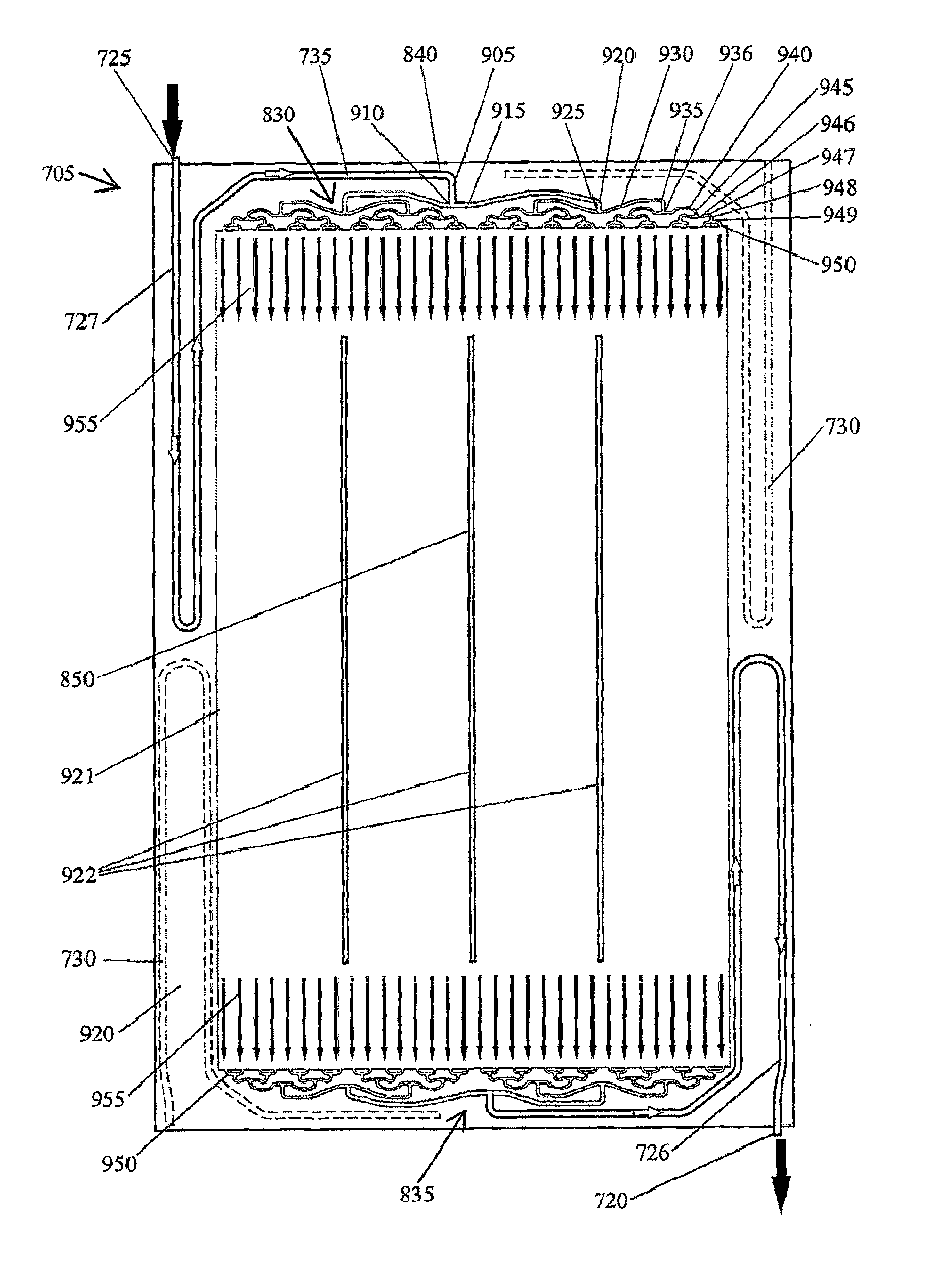
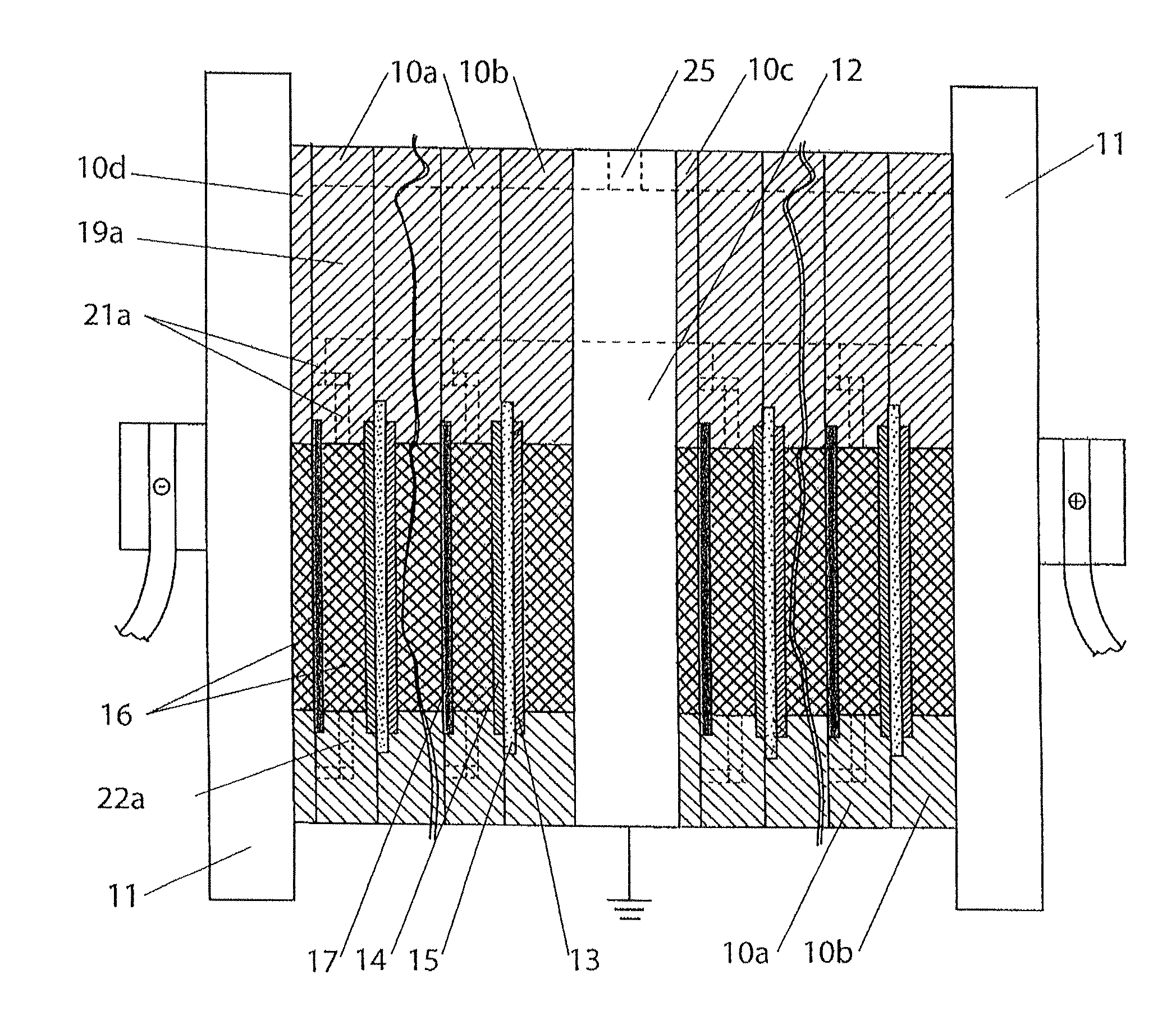
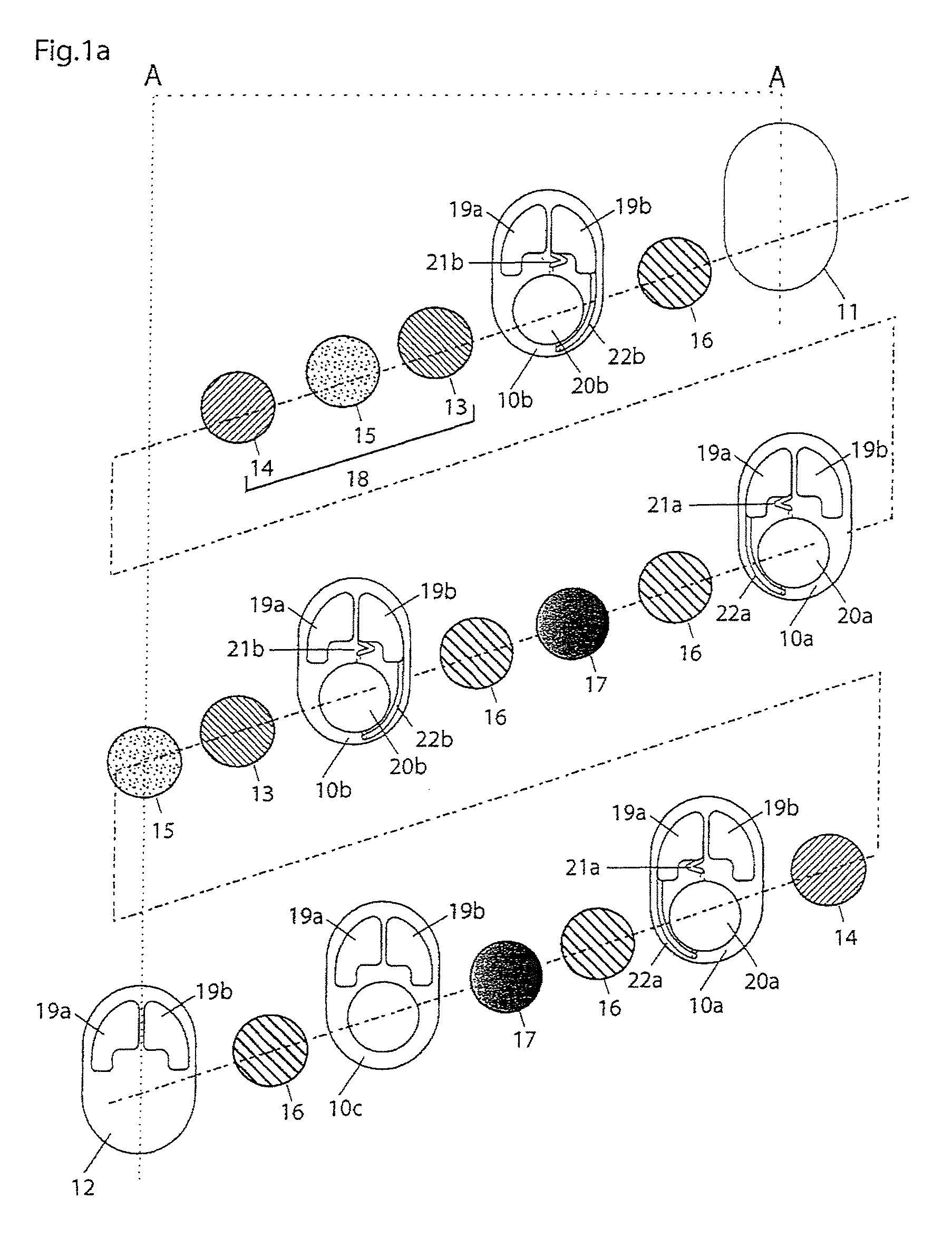
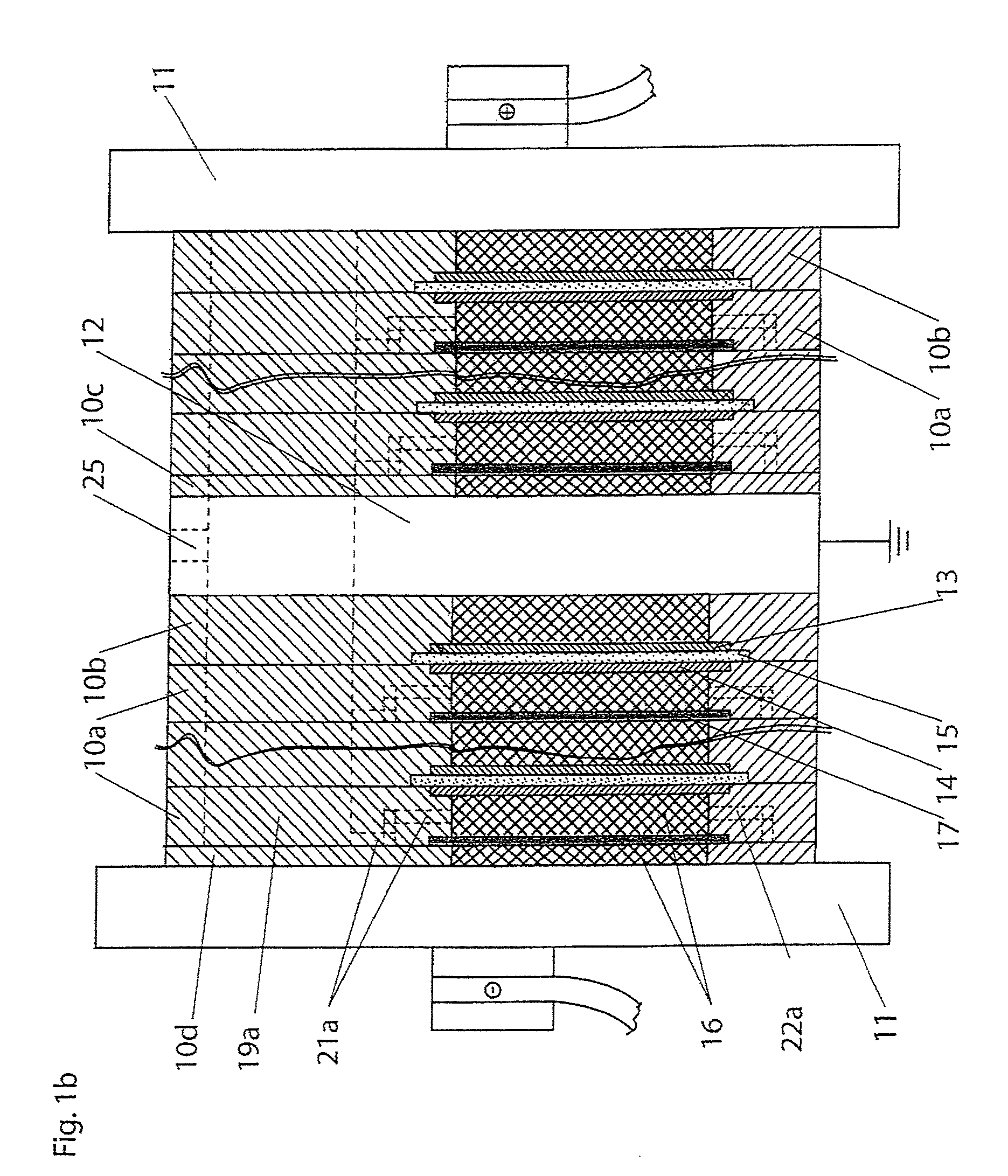
A cell stack (700) as provided enables a flowing electrolyte battery to have a reduced size and weight. The cell stack (700) includes a casing having a positive polarity end and a negative polarity end. A plurality of half cells (805) are inside the casing, and each half cell (805) includes an electrode plate (705), an adjacent separator plate (715), and at least one capillary tube (727) positioned between the electrode plate (705) and the adjacent separator plate (715). The capillary tube (727) has a first end extending outside of the half cell (805) and a second end located inside the half cell (805). At least one manifold (530) is in hydraulic communication with a plurality of capillary tube ends including the first end of the capillary tube (727) in each half cell (805). The capillary tube (727) in each half cell (805) enables electrolyte to circulate through the plurality of half cells (805) via the at least one manifold (530).
Owner:REDFLOW PTY LTD
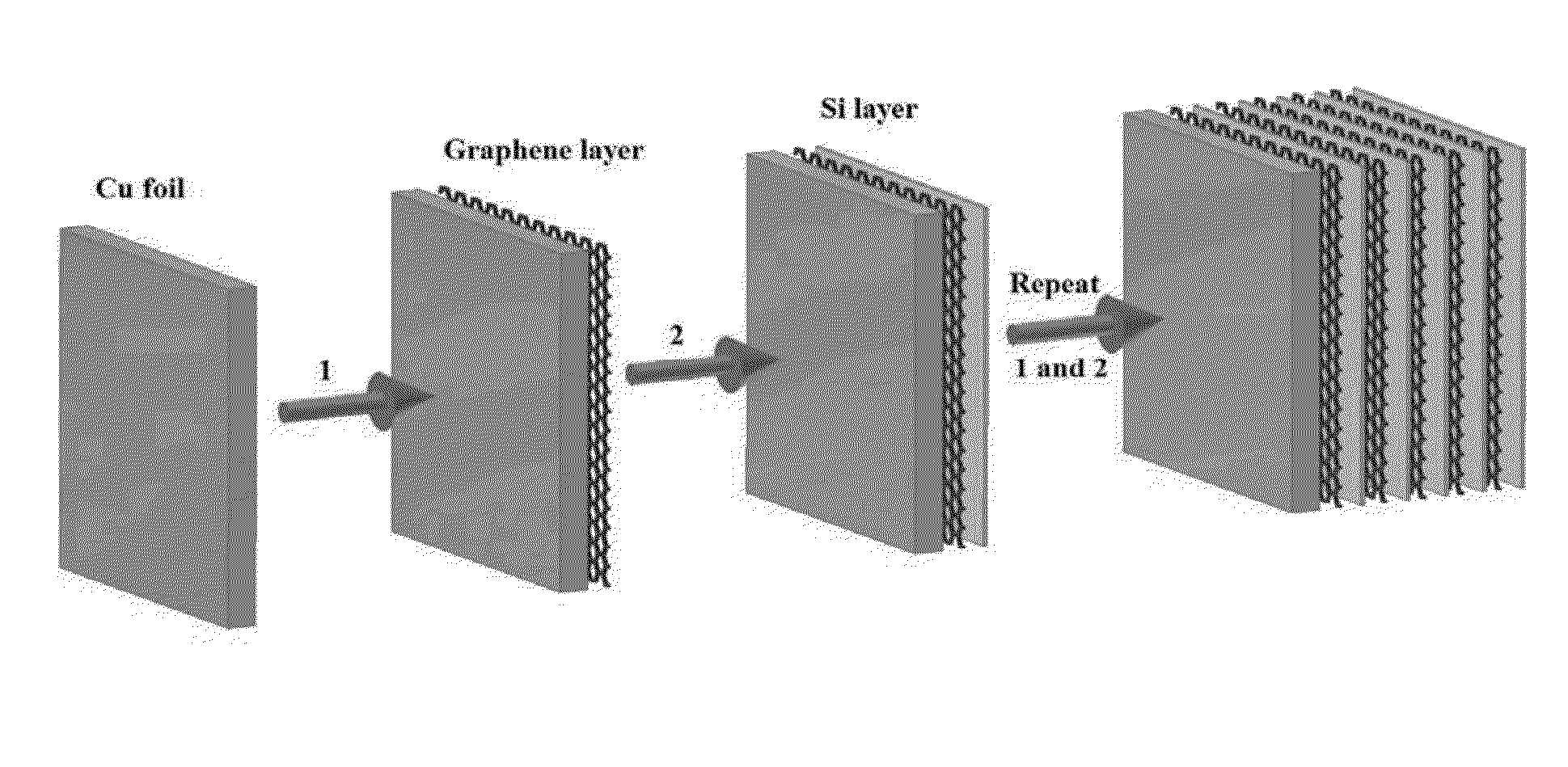
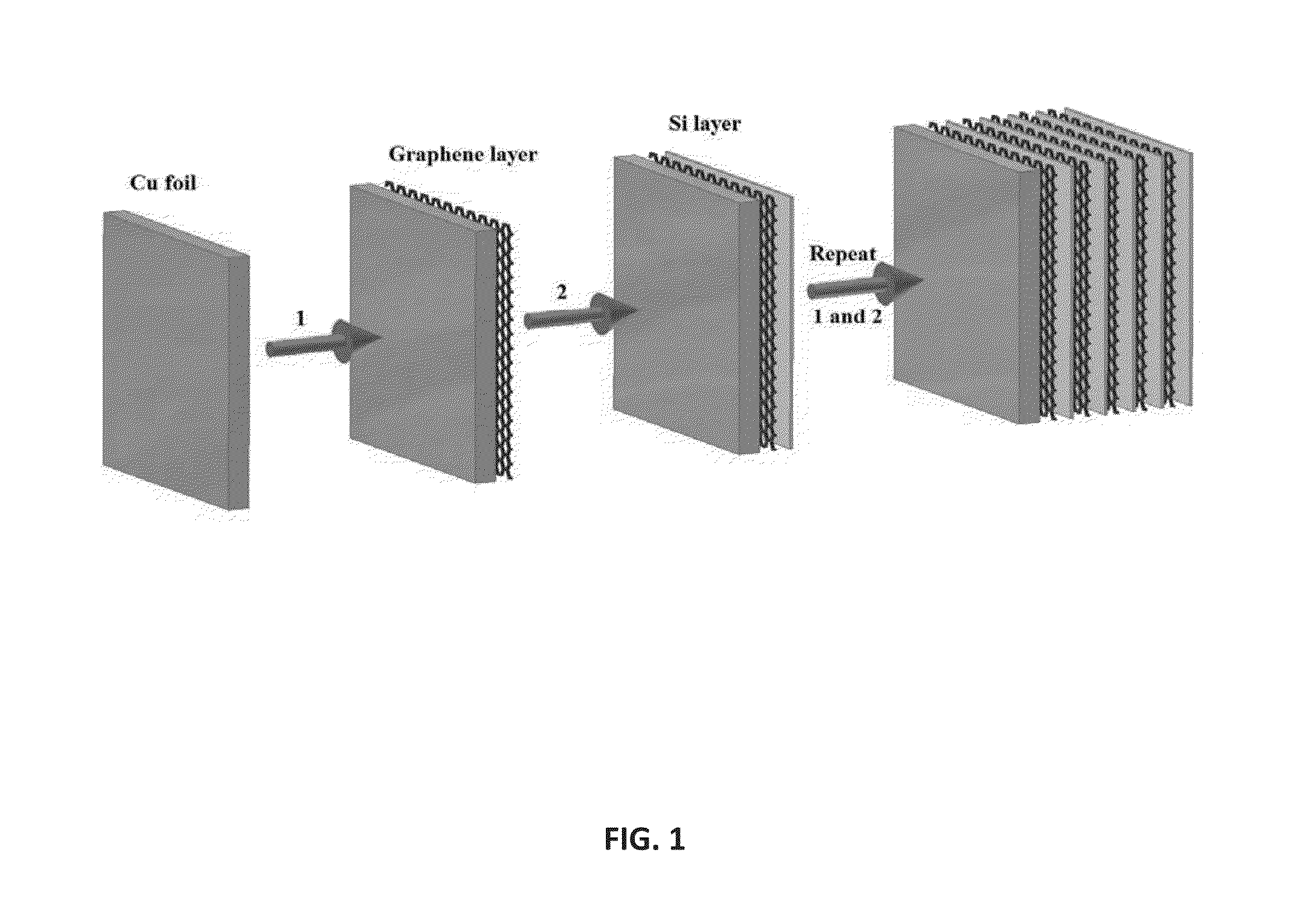
Method for the preparation of graphene/silicon multilayer structured anodes for lithium ion batteries
InactiveUS20140170483A1Effectively improves adhesionPrevent crushingActive material electrodesNon-aqueous electrolyte accumulator electrodesAluminium-ion batteryComposite film
Multilayer structures with alternating graphene and Si thin films were constructed by a repeated process of filtering liquid-phase exfoliated grapheme film and subsequent coating of amorphous Si film using plasma-enhanced chemical vapor deposition (PECVD) method. The multilayer-structure composite films, fabricated on copper current collectors, can be directly used as anodes for rechargeable lithium-ion batteries (LIBs) without the addition of polymer binders or conductive additives. Fabricated coin-type half cells based on the new anode materials easily achieved a capacity almost four times higher than the theoretical value of graphite even after 30 cycles. These cells also demonstrated improved capacity retention and enhanced rate capability during charge / discharge processes compared to those of pure Si film-based anodes.
Owner:RGT UNIV OF CALIFORNIA
Improved perfluorinated membranes and improved electrolytes for redox cells and batteries
InactiveCN101257121AElectrode manufacturing processesRegenerative fuel cellsSupporting electrolytePolyolefin
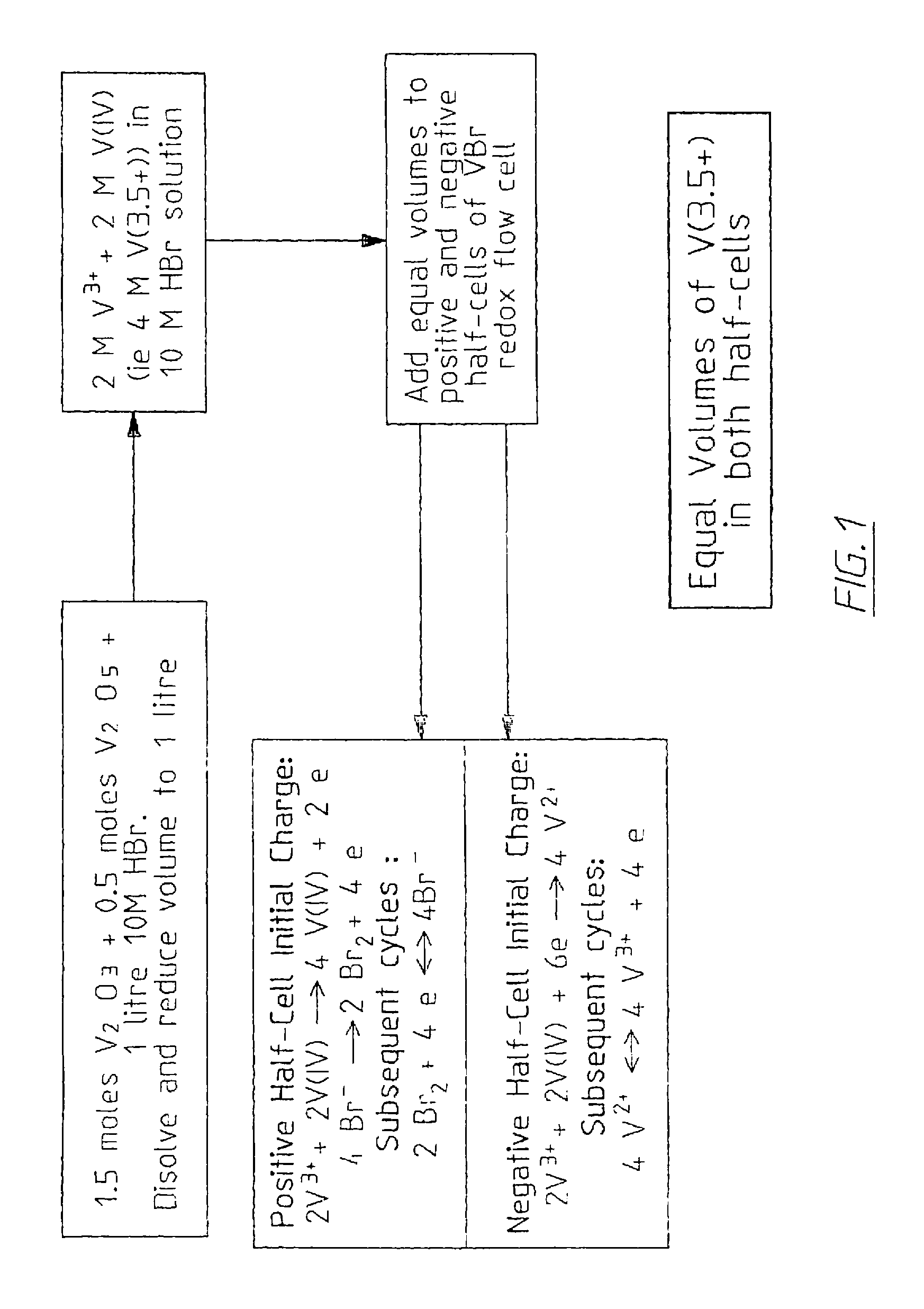
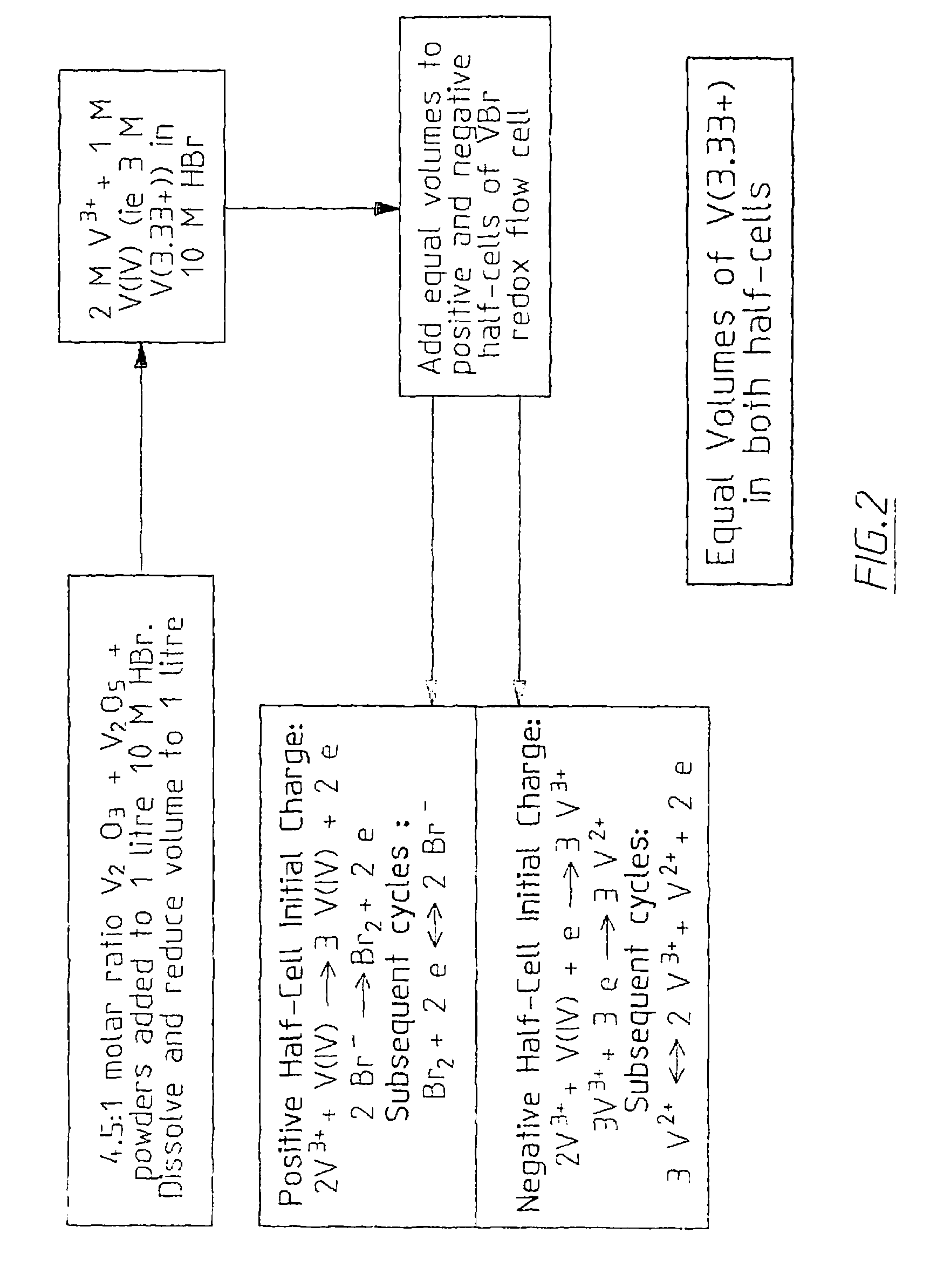
The invention relates to an electrode used for vanadium redox flow battery, a preparation of electrolyte and a rebalance method. The preparation comprises thermally sticking a carbon felt or agraphite felt to at least one side of a carbon-filled polyolefine substrate. A flat, low-resistance electrode having good mechanical property can be obtained by the preparation. The preparation includes dissolving at least one vanadium oxide powder to a supporting electrolyte and optional electrolysis steps. The preparation needs no toxic SO2 gas, by separating the power dissolving stage and the electrolysis stage, problems related to suspension powder electrolysis are eliminated. The rebalance method comprises partially reducing a half-cell electrolyte in the cathode chamber of an electrolytic cell.
Owner:NEWSOUTH INNOVATIONS PTY LTD
Metal support half-cell of solid oxide fuel cell and preparation method thereof
ActiveCN103928693AAvoid direct contactReduce interdiffusionCell electrodesSolid electrolyte fuel cellsFuel cellsMixed oxide
The invention discloses a metal support half-cell of a solid oxide fuel cell and a preparation method thereof. The half-cell comprises a porous metal supporting layer thick membrane, a porous cermet gradient transition layer film, a porous anode layer and a compact electrolyte layer film from down to up. The porous gradient transition layer composed of a mixed oxide and a oxide with a fluorite structuring can avoid the direct contact of the porous metal supporting layer and the porous anode layer, and the mutual diffusion of Fe / Cr elements in the metal supporting layer and Ni element in the porous anode layer can be reduced under high temperature sintering condition. The mixed oxide is reduced to an alloy under the work condition of the cell; a high anode active material is formed at a side interface of the anode, a high conductivity composite material which takes the alloy as a main phase is formed on the side interface of a metal support body, so that higher conductivity is presented, ohmic resistance is reduced, electrocatalytic activity is not reduced, long-term stability for operation of the cell can be ensured, and good combination of the porous metal supporting layer and the porous anode layer can be simultaneously realized.
Owner:中弗(无锡)新能源有限公司
Membrane-mediated electropolishing with topographically patterned membranes
This invention provides membrane-mediated electropolishing (MMEP) processes for polishing and / or planarizing metal work pieces using topographically patterned membranes. The processes can be used for both pure metals and alloys, and provide advantages over conventional electropolishing processes and known MMEP processes using smooth membranes. This invention also provides a cathode half-cell and an apparatus useful in membrane-mediated electropolishing processes. The invention also provides processes for electroengraving and electromachining topographic patterns, holes and / or grooves into the surface of a metal work piece.
Owner:EI DU PONT DE NEMOURS & CO
Electrolyser module
Owner:NEXT HYDROGEN CORP
Process and measuring system for detection of substances emitted or perspired through the skin
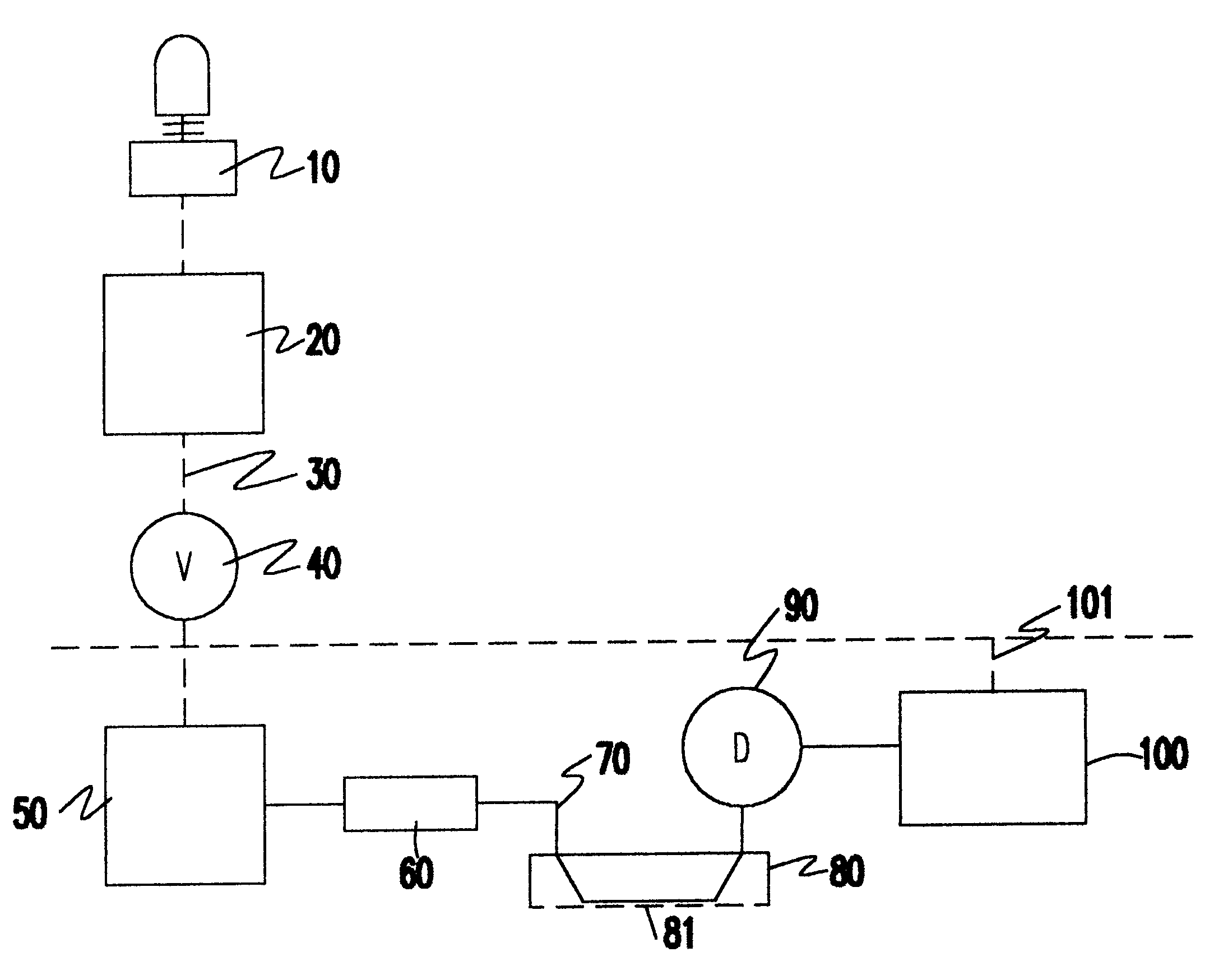
InactiveUS6183418B1Minimize impactInexpensive productionTesting beveragesPreparing sample for investigationDiffusionQuantitative determination
The process for detection and for quantitative determination of substances emitted or perspired through the skin is derived from flow diffusion analysis. The measuring system conceived for this purpose uses a diffusion half cell through which an acceptor medium flows and which is closed by a membrane. For the duration of the measurement, the membrane is brought into contact with the skin or a closed gas volume formed over the skin. With the process and the related measuring system, the blood alcohol level can be determined with a good degree of precision indirectly via the quantity of (gaseous) ethanol emitted through the skin.
Owner:MOLLER MEDICAL +2
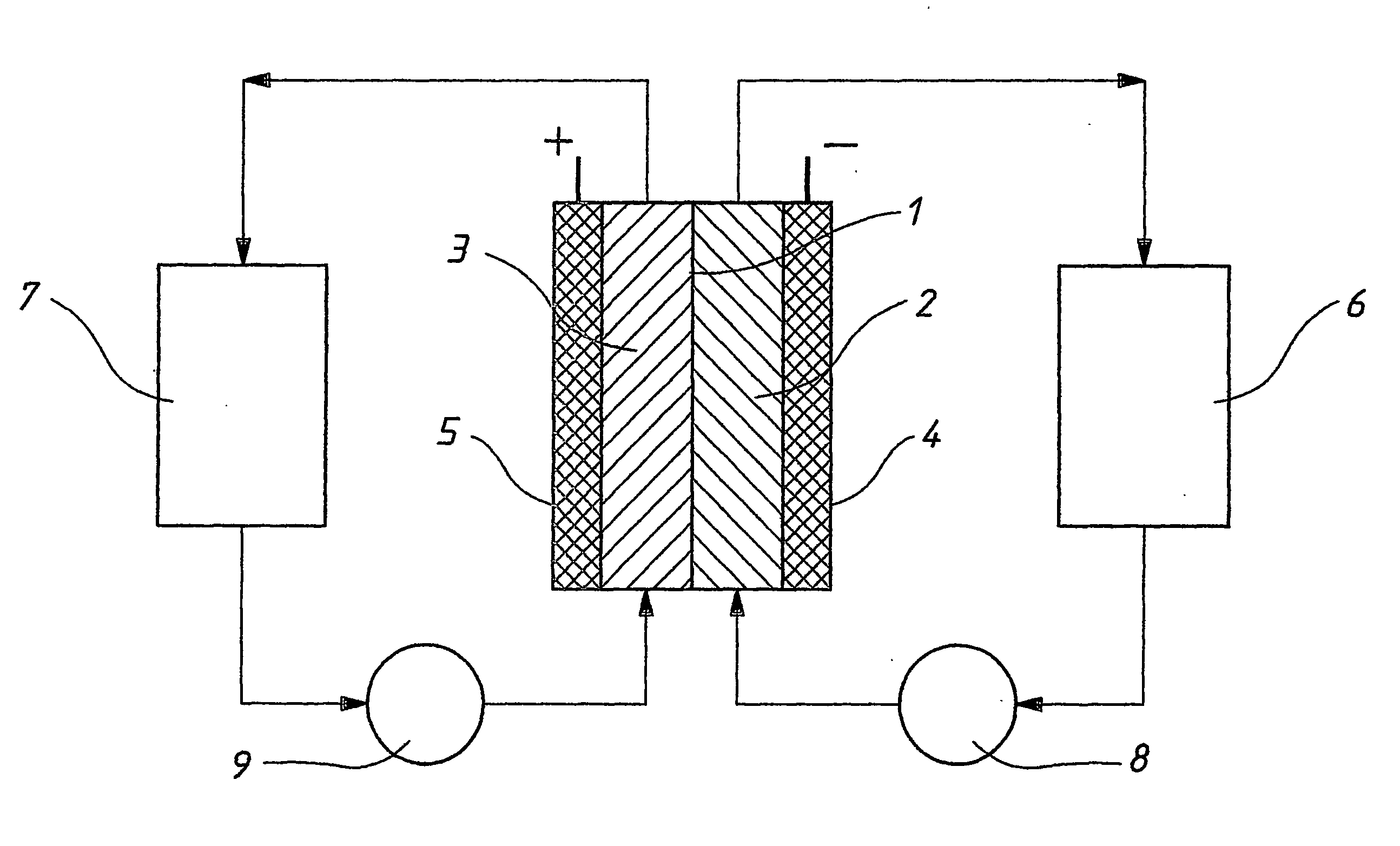
Pressure feed flow battery system and method
InactiveUS20140220463A1Electrolyte stream managementRegenerative fuel cellsEngineeringFluid electrolytes
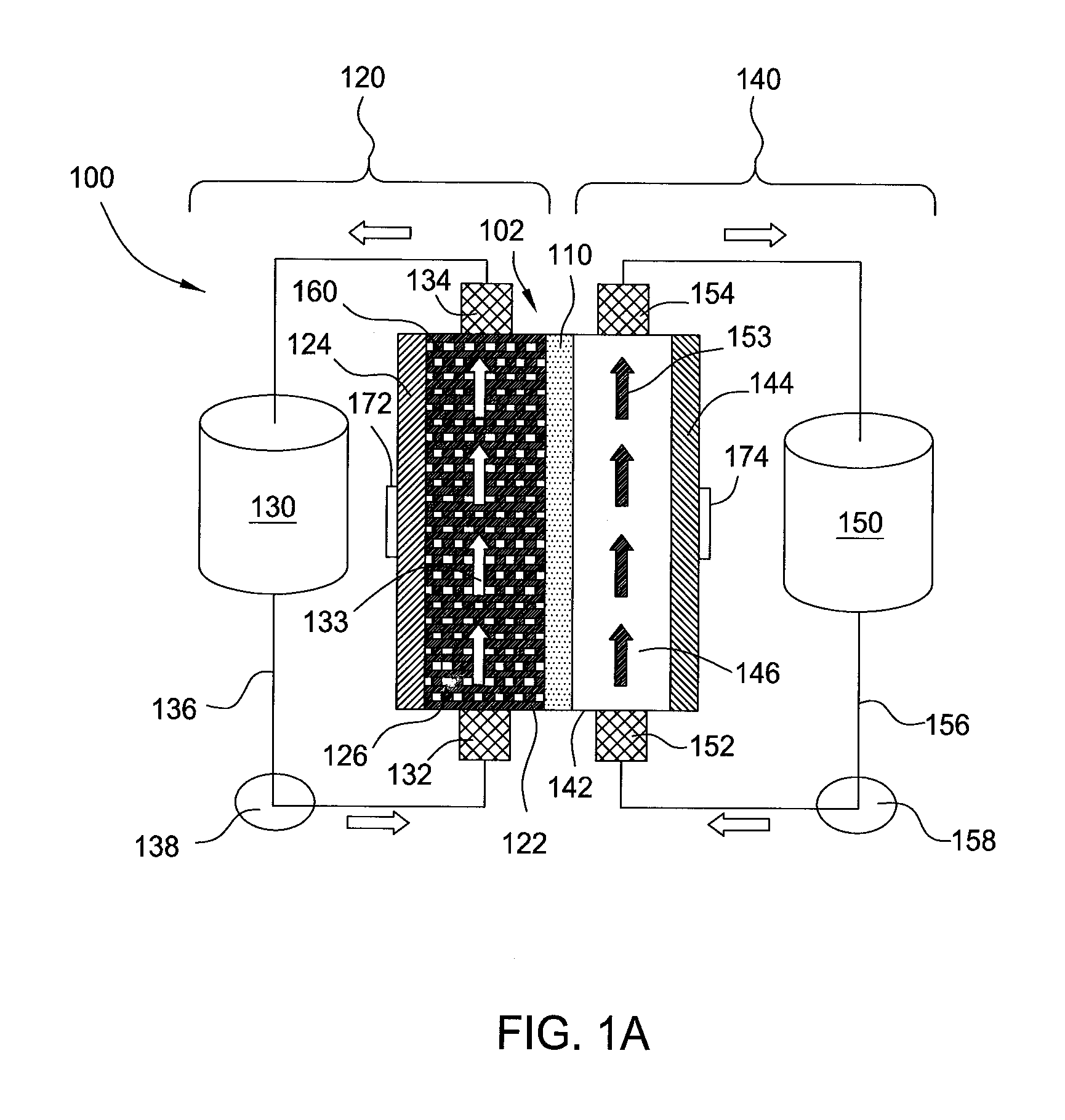
A flow battery system and method are provided. The flow battery system includes a first battery stack including a first half-cell, a first pressure feed system, including at least a first storage tank and a first booster tank to store a liquid electrolyte, designed to generate a first booster pressure in the first booster tank sufficient to force the liquid electrolyte to be fed from the first pressure feed system through the first half-cell, and a return system to return the liquid electrolyte from the first half-cell to the first pressure feed system. The return system may include a gravity feed system returning liquid electrolyte from the first half-cell to a collection tank, and a pump to return the collected liquid electrolyte from the collection tank to the first storage tank. The pressure feed flow battery system may have a two-tank, divided 2-tank, or four-tank flow battery configurations.
Owner:ASHLAWN ENERGY
Cross-linked polymer-based all-solid-state electrolyte material and application of cross-linked polyoxyethylene ether
ActiveCN103500845AImprove conductivityImprove conduction abilitySecondary cellsCross-linkAll solid state
The invention discloses a cross-linked polymer-based all-solid-state electrolyte material and an application of cross-linked polyoxyethylene ether. The cross-linked polymer-based all-solid-state electrolyte material comprises cross-linked polyoxyethylene ether, lithium salt and a modifying agent. An electrolyte membrane prepared from the electrolyte material containing the cross-linked polyoxyethylene ether has relatively high conductivity and a wide electrochemical window; and an assembled half-cell has good high-temperature cycle performance, and the retention rate of the charge-discharge specific capacity of the half-cell is high.
Owner:王海斌
Vanadium halide redox flow battery
InactiveUS7976974B2Avoid excessive bromine generationStabilise the bromine producedCharging stationsCell electrodesOxidation-Reduction AgentRedox
A vanadium halide redox cell including: a positive half cell containing a positive half cell solution including a halide electrolyte, a polyhalide complex, vanadium (IV) halide and vanadium (V) halide; a negative half cell containing a negative half cell solution including a halide electrolyte, vanadium (II) halide and vanadium (III) halide; wherein the ratio of the number of moles of polyhalide complex and vanadium (V):number of moles of vanadium (II) halide is about stoichiometrically balanced and wherein the ratio of the number of moles of polyhalide complex:the number of moles of vanadium (II) halide is in the range of from about 0.7:2 to about 1.3:2.
Owner:WATTJOULE
Reversible polarity operation and switching method for ZnBr flow battery when connected to common DC bus
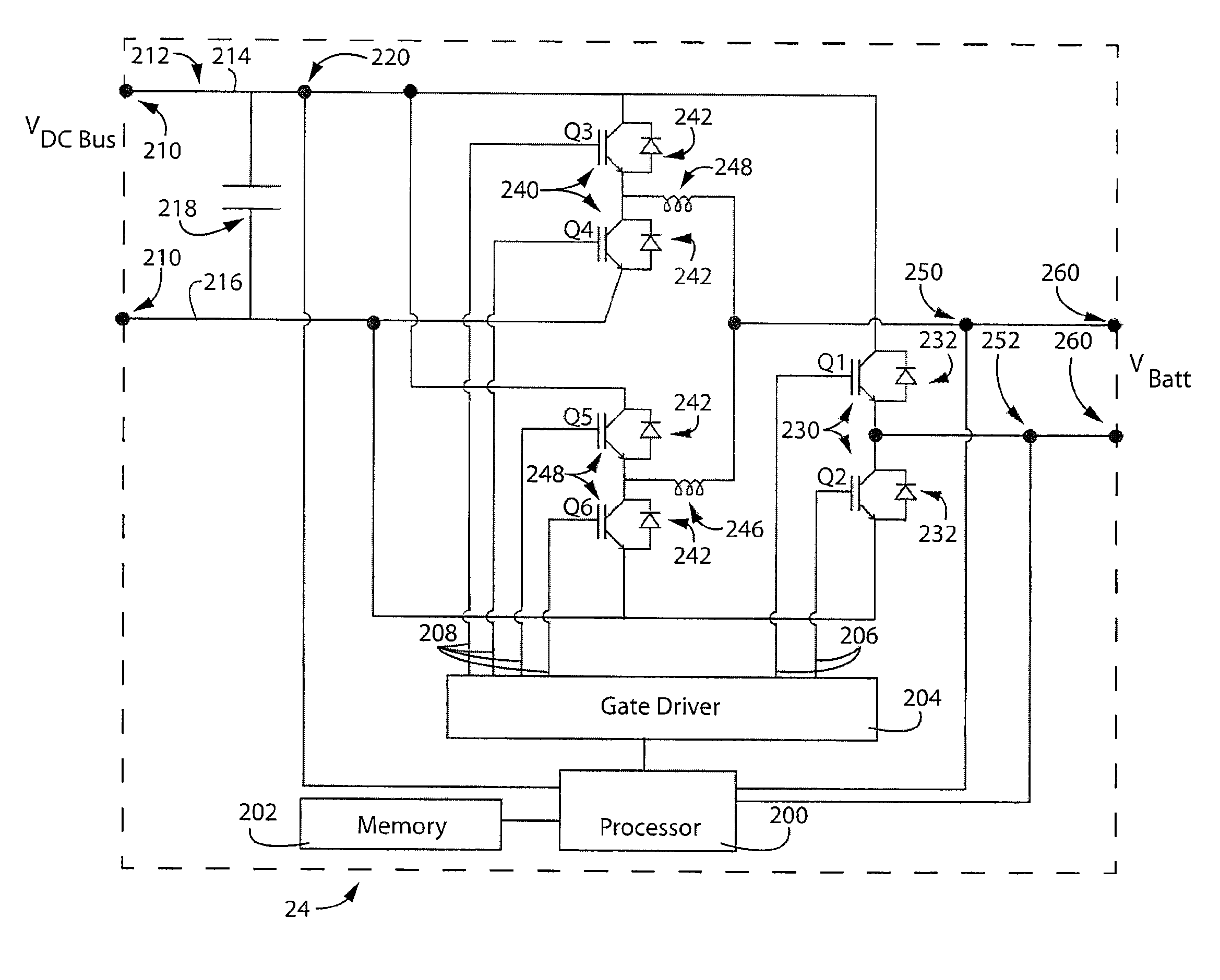
ActiveUS20120326672A1Reduce manufacturing costSimple structureBatteries circuit arrangementsElectrode thermal treatmentElectrical polarityEngineering
An improved electrolyte battery is provided that includes a tank assembly adapted to hold an amount of an anolyte and a catholyte, a number of cell stacks operably connected to the tank assembly, each stack formed of a number of flow frames disposed between end caps and a number of power converters operatively connected to the cell stacks. The cell stacks are formed with a number of flow frames each including individual inlets and outlets for anolyte and catholyte fluids and a separator disposed between flow frames defining anodic and cathodic half cells between each pair of flow frames. The power converter is configured to connect the battery with either forward or reverse polarity to a DC power source, such as a DC bus. The anodic and cathodic half cells switch as a function of the polarity by which the battery is connected to the Dc power source.
Owner:LOTTE CHEM CORP +1
Novel vanadium halide redox flow battery
The present invention describes a vanadium halide redox cell prior to charging, a vanadium halide redox cell in a state of charge selected from the group below, and fully charged or partially charged vanadium halide redox cells, wherein the group Consists of zero state of charge and near zero state of charge. A vanadium halide redox cell prior to charging includes a positive half-cell having a positive half-cell solution including a halide electrolyte, a vanadium(III) halide, and a vanadium(IV) halide, and a negative half-cell having a A negative half-cell solution comprising a halide electrolyte, a vanadium(III) halide and a vanadium(IV) halide, wherein the amounts of the vanadium(III) halide, vanadium(IV) halide and halide ions in the positive and negative half-cell solutions are set to such that in the first charging step comprising charging the vanadium halide redox cell prior to charging, it is possible to prepare a vanadium halide redox cell having a state of charge selected from the group consisting of zero state of charge and With a near-zero state-of-charge composition, the vanadium halide redox cell mainly includes vanadium(IV) halide in the positive half-cell solution and V(III) halide in the negative half-cell solution. A vanadium halide redox cell at a state of charge selected from the group consisting of a positive half-cell and a negative half-cell consisting of zero and near-zero states of charge, the positive half-cell having a halide electrolyte comprising: and a positive half-cell solution of a vanadium halide mainly vanadium(IV) halide, a negative half-cell having a negative half-cell solution comprising a halide electrolyte and a vanadium halide mainly of a vanadium(III) halide, wherein the positive half-cell solution The amount of vanadium(IV) halide and the amount of vanadium(III) halide in the negative half-cell solution are set such that the vanadium halide redox cell is at a state of charge selected from the group consisting of zero state of charge and close to zero state of charge composition. A fully charged vanadium halide redox cell consists of a positive half cell with a positive half cell comprising a halide electrolyte, a polyhalide complex, a vanadium(IV) halide, and a vanadium(V) halide solution, the negative half-cell has a negative half-cell solution comprising a halide electrolyte and a vanadium(II) halide, wherein the molar concentration of vanadium(V) and polyhalide complexes: the molar concentration of vanadium(II) halide is approximately stoichiometrically balanced. A partially charged vanadium halide redox cell includes a positive half cell with a positive half cell including a halide electrolyte, a polyhalide complex, a vanadium(IV) halide, and a vanadium(V) halide solution, the negative half-cell has a negative half-cell solution comprising a halide electrolyte, a vanadium (II) halide and a vanadium (III) halide, wherein the number of moles of the polyhalide complex and the vanadium (V) halide: vanadium halide ( The moles of II) are approximately stoichiometrically balanced.
Owner:NEWSOUTH INNOVATIONS PTY LTD
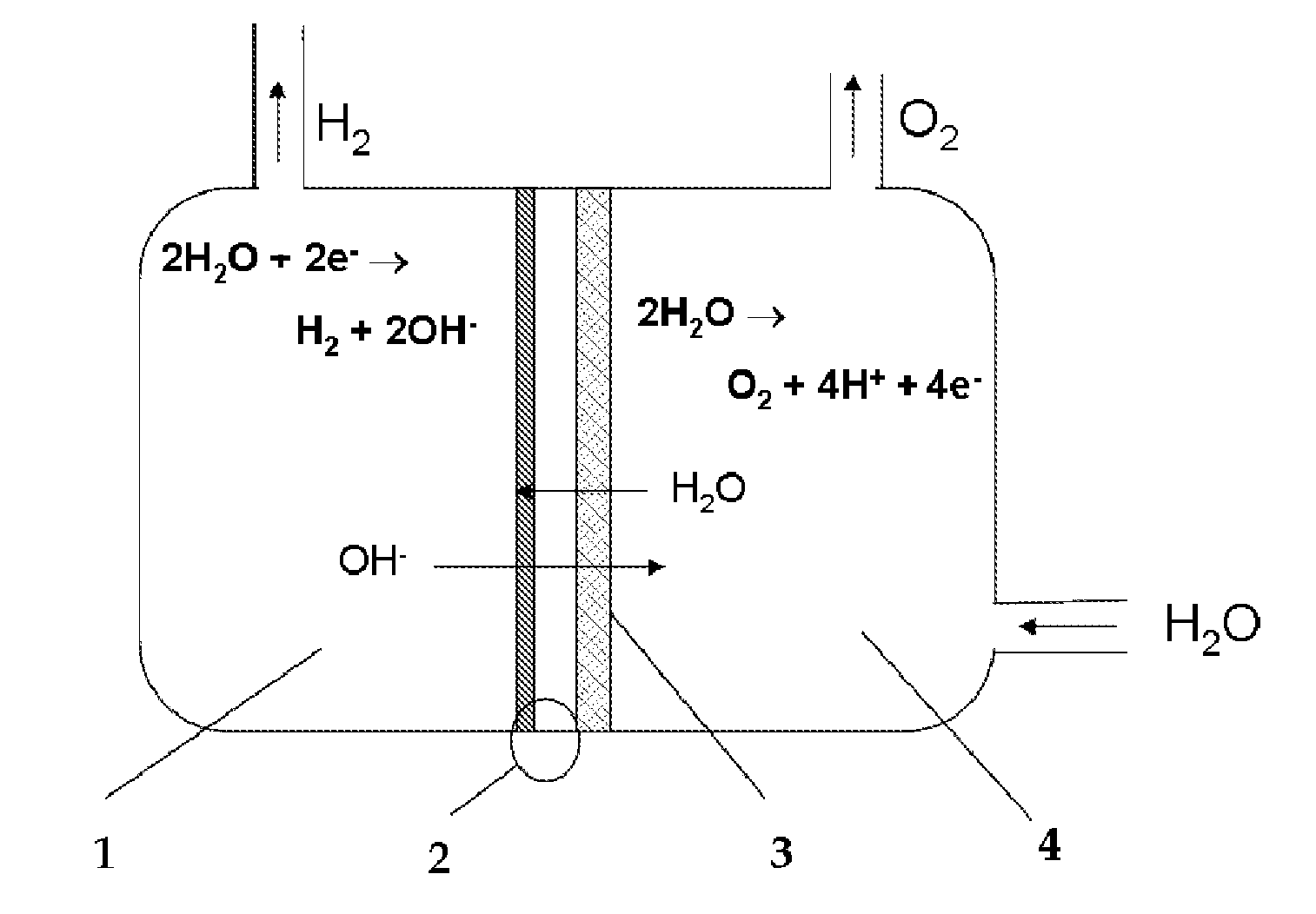
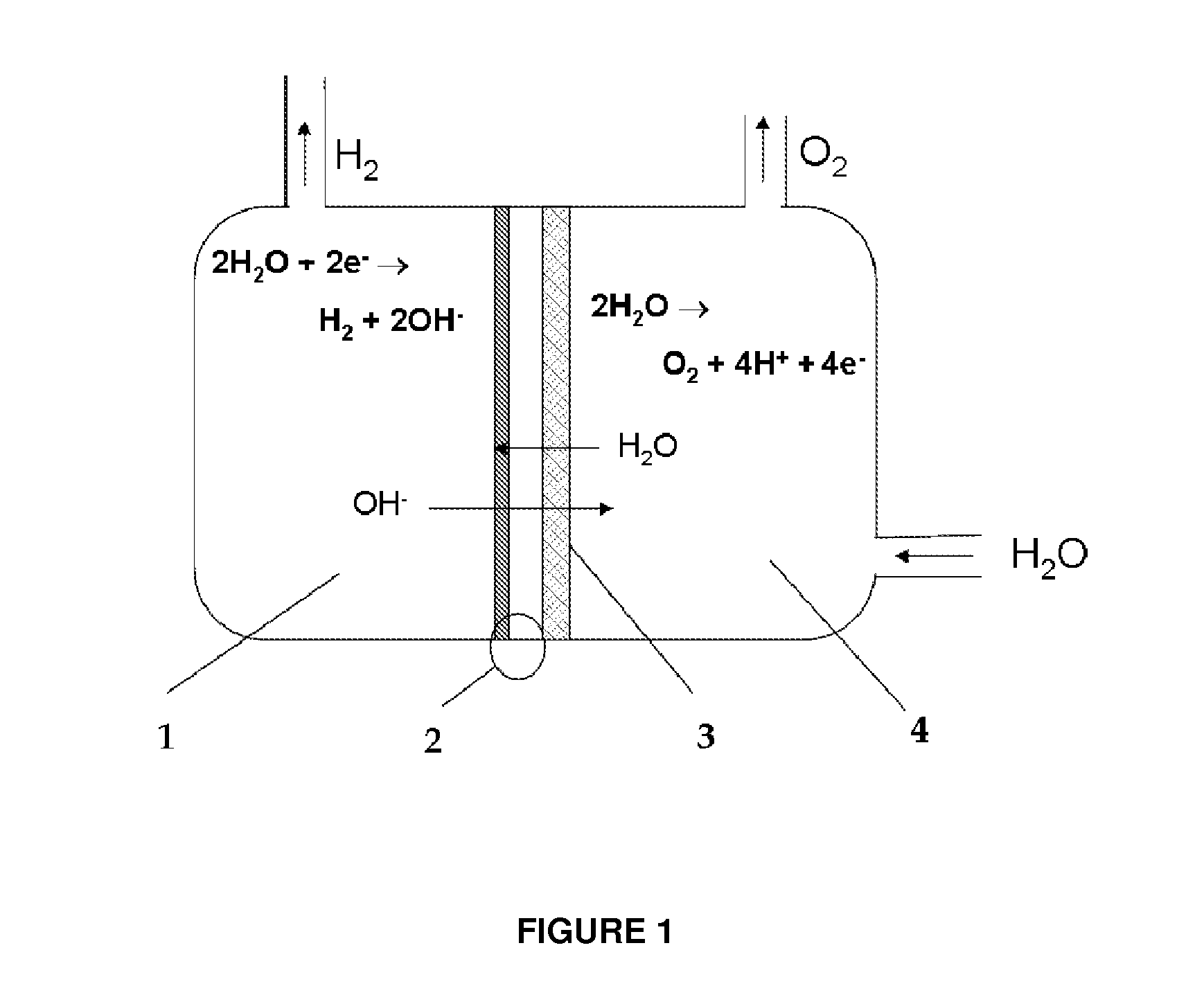
Device for the production on-demand of hydrogen by electrolysis of aqueous solutions from dry cathode
This invention relates to a device for the electrolytic production of hydrogen which can operate discontinuously or associated to strong power fluctuations and provide dry pressurized directly hydrogen, with high purity. The device for the electrolytic production of hydrogen from an alkaline aqueous solution, starting from dry cathode, comprises two half-cell, anodic and cathodic, separated by an anionic exchange membrane whose surface in contact with the cathodic half-cell is a membrane-electrode assembly (MEA), and the alkaline solution is present only in the anodic half-cell
Owner:ENAPTER SRL
Producing method for a high strength ultra-thin anode supporting type solid oxide fuel cell
ActiveCN101399352AHigh mechanical strengthImprove bindingFinal product manufactureCell electrodesFuel cellsSlurry
The invention discloses a method for preparing a high-strength ultra-thin anode supported solid oxide fuel cell, the method comprises: the slurry of an anode support is firstly used for preparing an anode support green compact with a certain thickness by a tape casting method, then an active anode slurry and an electrolyte slurry are sprayed onto the anode support green compact by using an air direct spraying method, the green compact of the composite material is further increased to a certain temperature by a certain heating rate for the calcination, then a half cell is obtained, then the cathode slurry is finally sprayed on the half cell by the air spraying method, and a single cell is obtained by drying, calcinating and other steps. The obtained flat-plate single cell has the advantages of high mechanical strength of the anode support, even thin active anode, conditional method, easy control, simple process, easy industrial enlargement, etc.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI
Apparatus adapted for membrane-mediated electropolishing
InactiveUS7566385B2Generates virtually no waste productEfficient processingCellsSemiconductor/solid-state device manufacturingElectrolysisElectrical battery
This invention provides a membrane-mediated electropolishing apparatus for polishing and / or planarizing metal work-pieces. The work-piece is wetted with a low-conductivity fluid. The wetted work-piece is contacted with a first side of a charge-selective ion-conducting membrane, wherein the second side contacts a conductive electrolyte solution in electrical contact with a electrode. Current flow between the electrode and the work-piece electropolishes metal from the work-piece. This invention also provides a half-cell adapted for use in membrane-mediated electropolishing having a fully or partially enclosed volume, a conductive electrolyte which partially or essentially fills the enclosed volume, an electrode which is in contact with the electrolyte, and a charge-selective ion-conducting membrane which seals one surface of the enclosed volume, cavity or vessel in such a way that the internal surface of said membrane contacts the electrolyte solution or gel and the external surface is accessible to contact the work-piece.
Owner:EI DU PONT DE NEMOURS & CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com