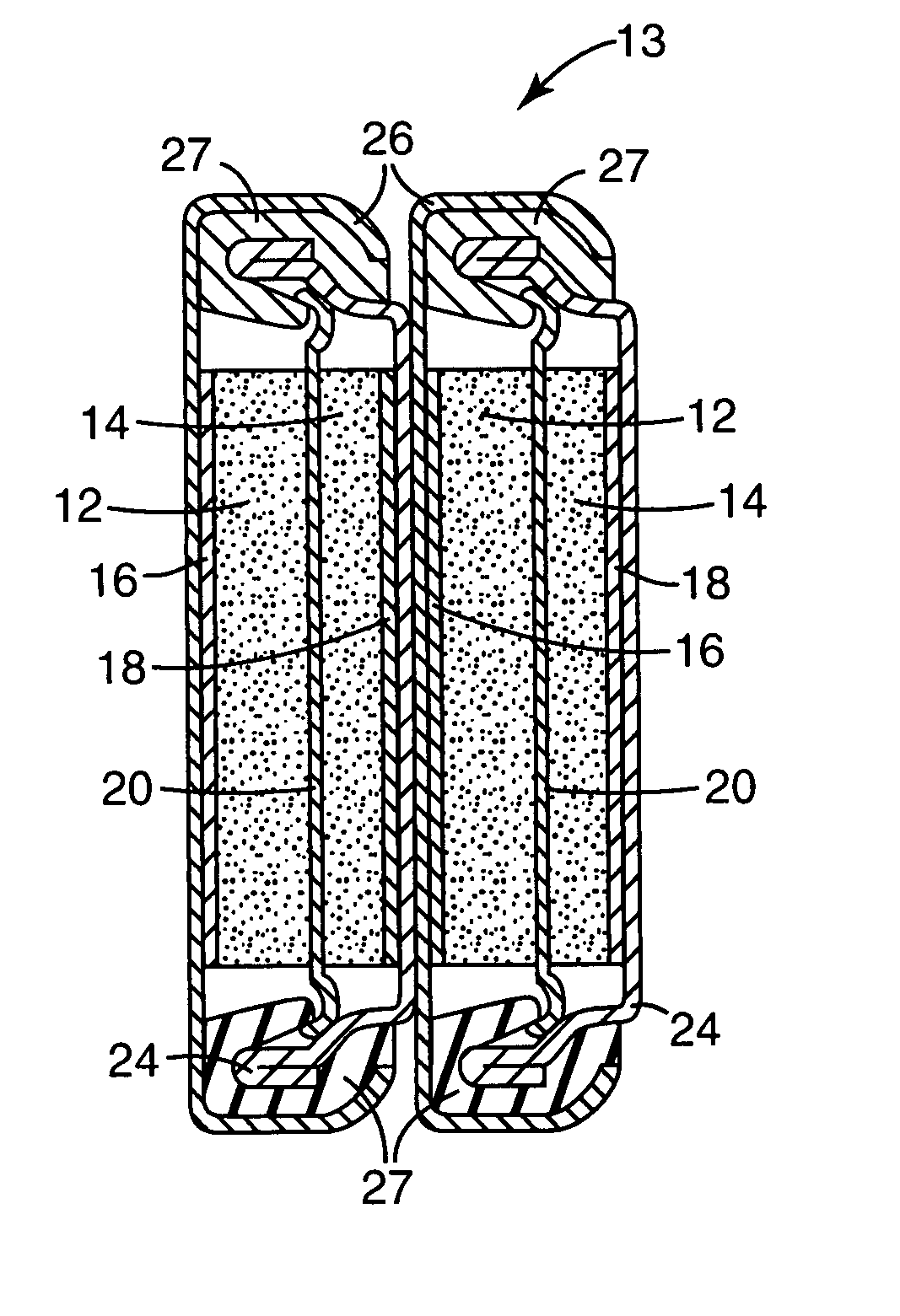
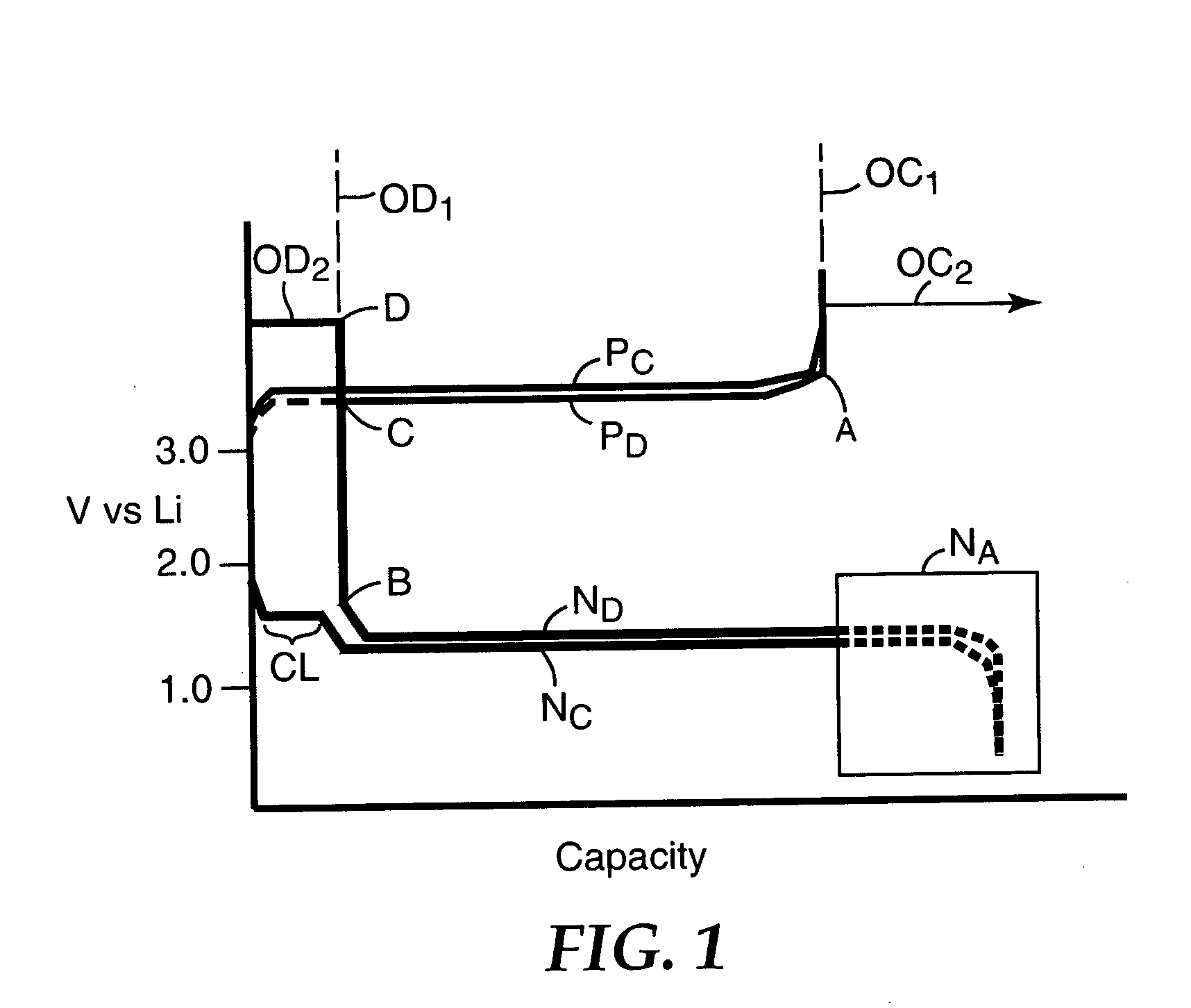
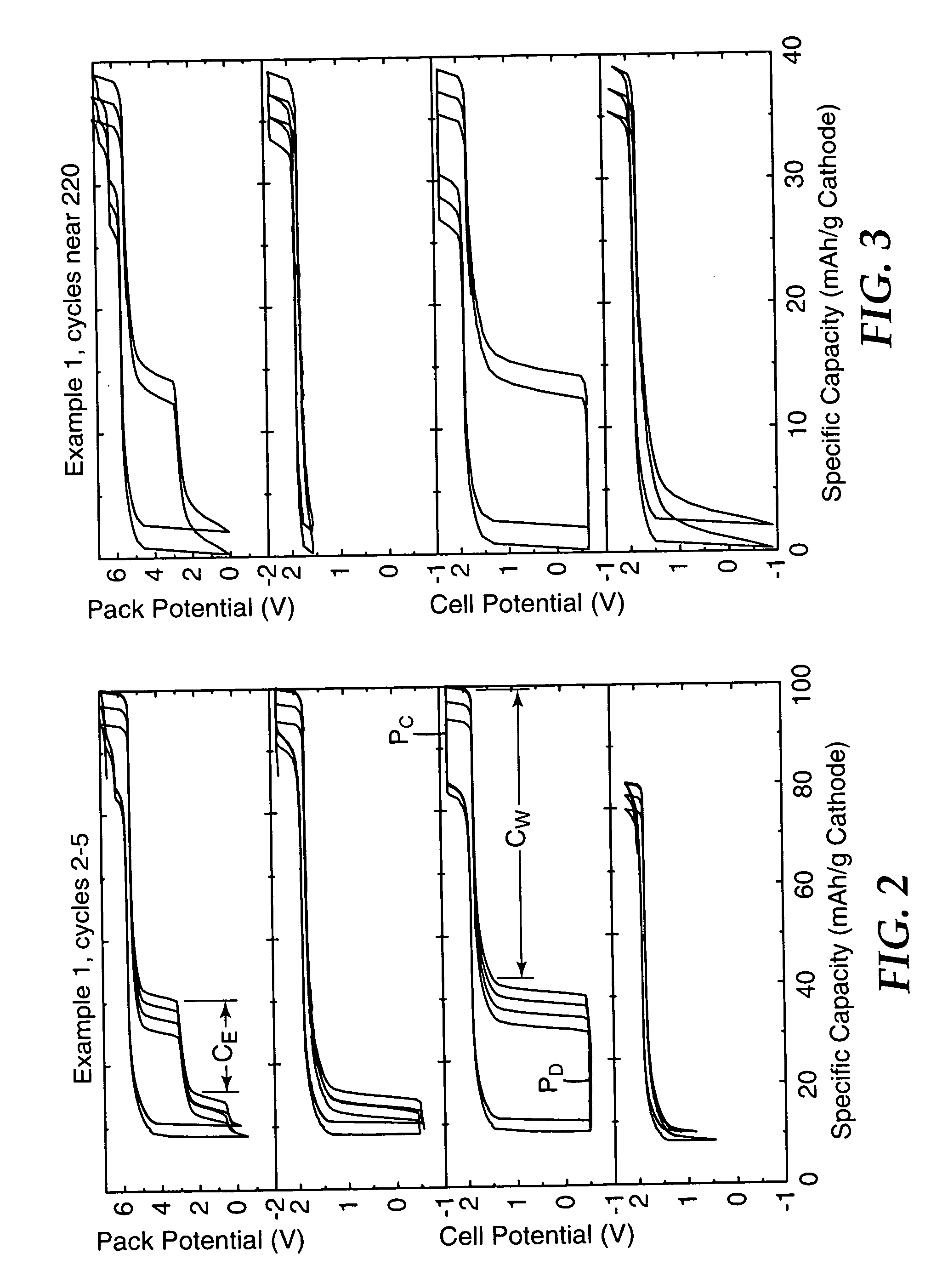
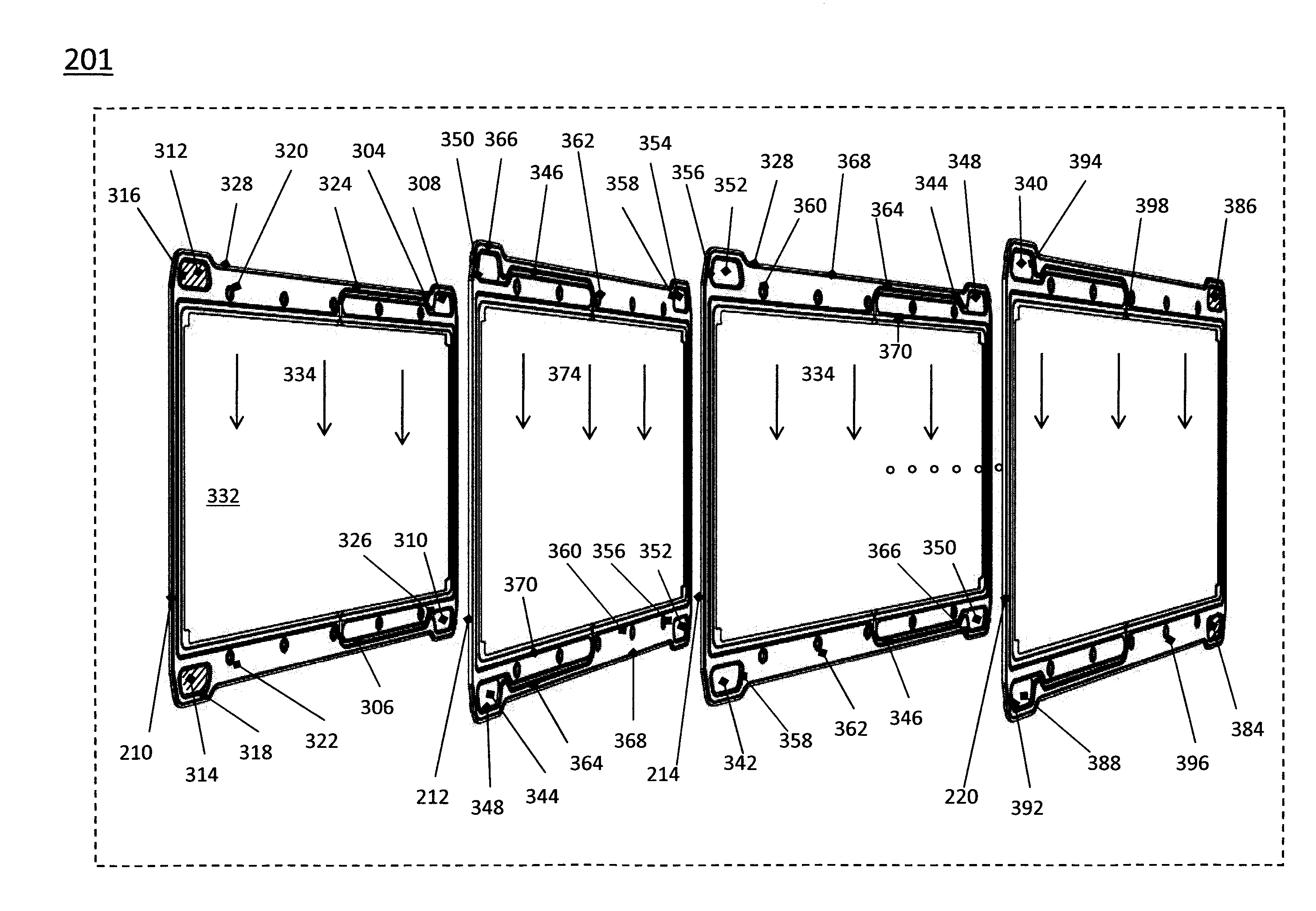
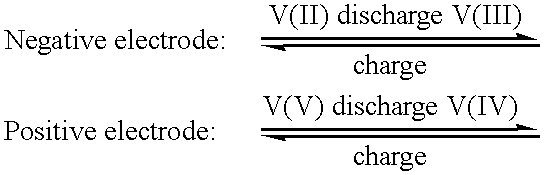Patents
Literature
641results about "Indirect fuel cells" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
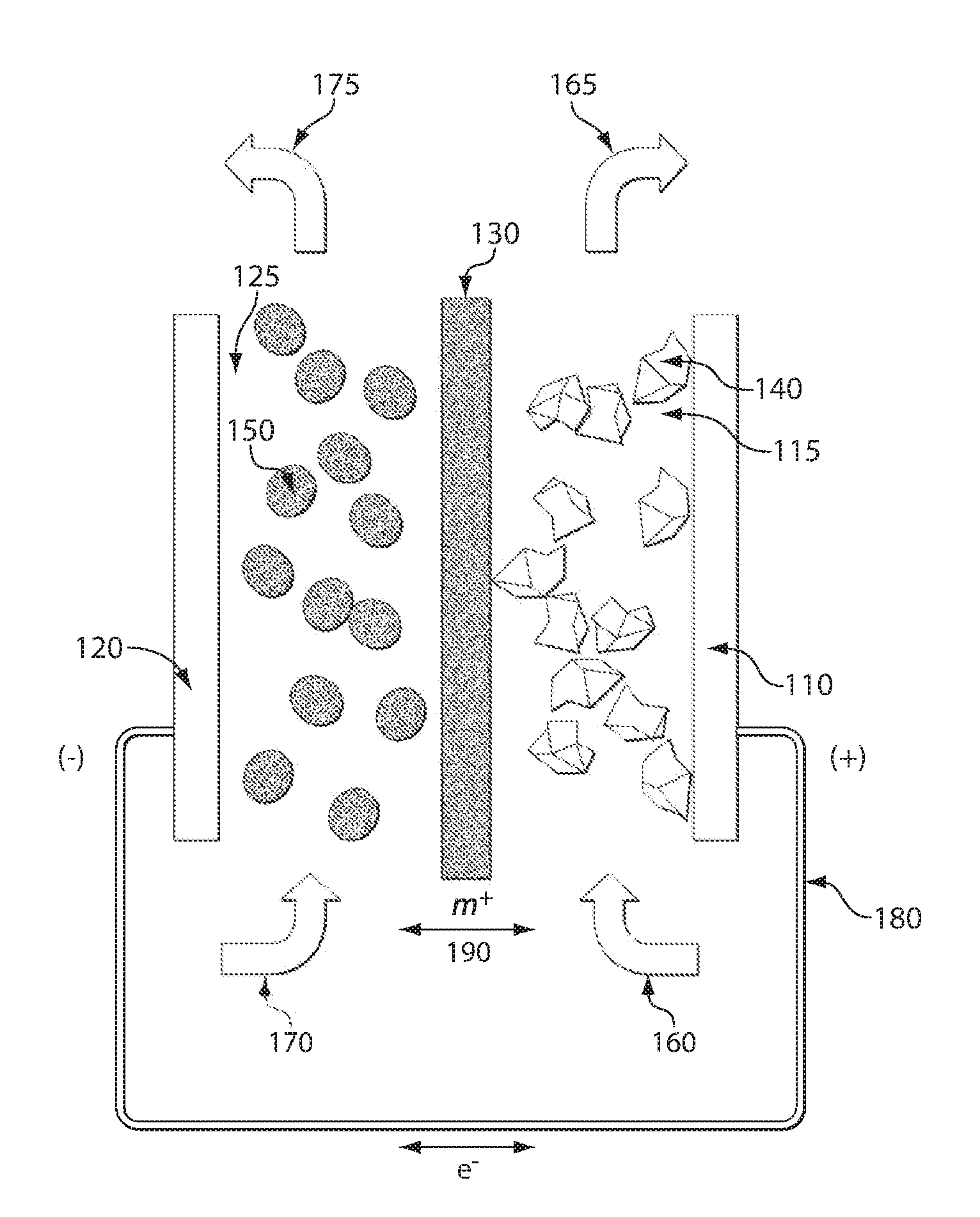
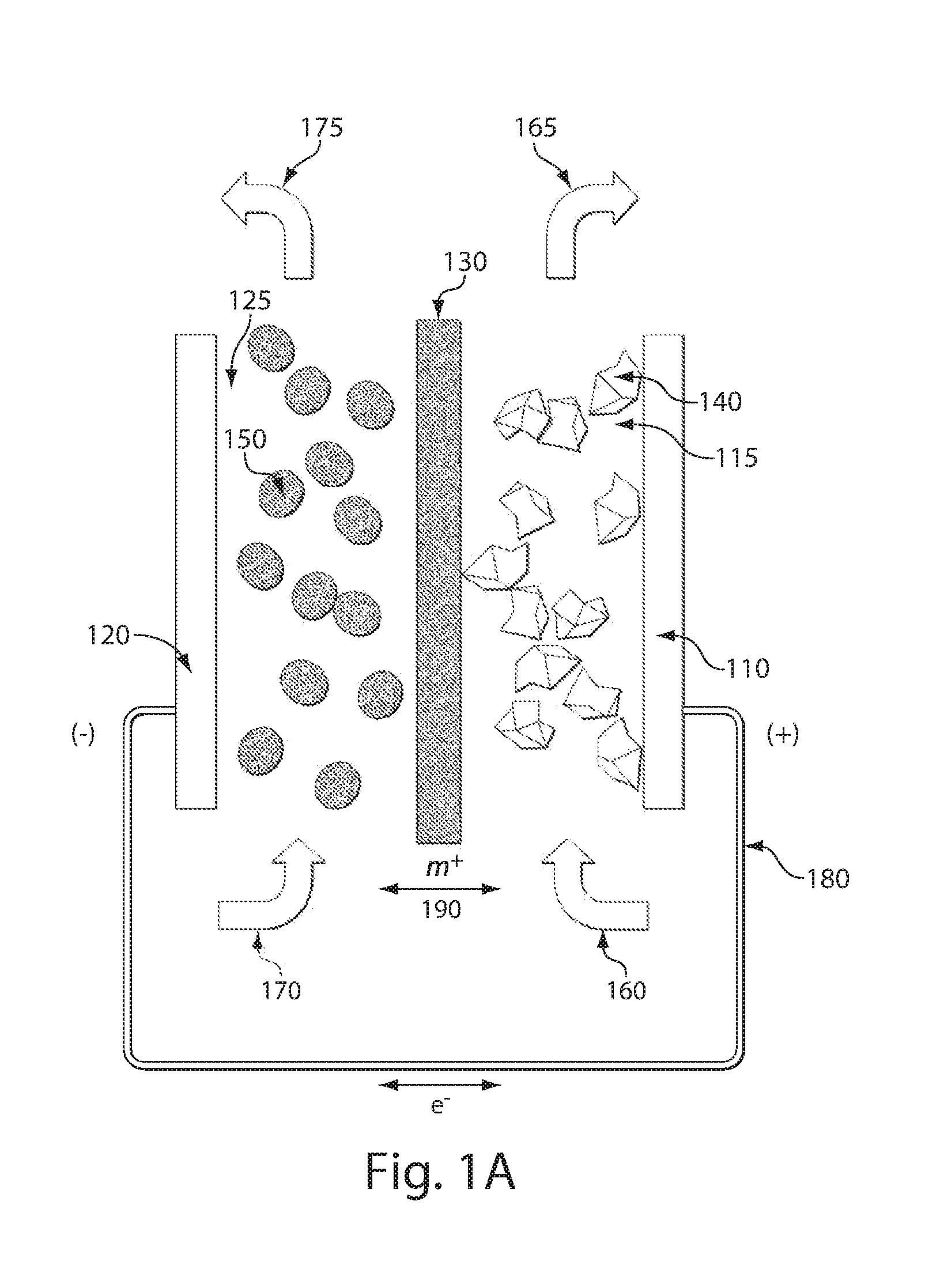
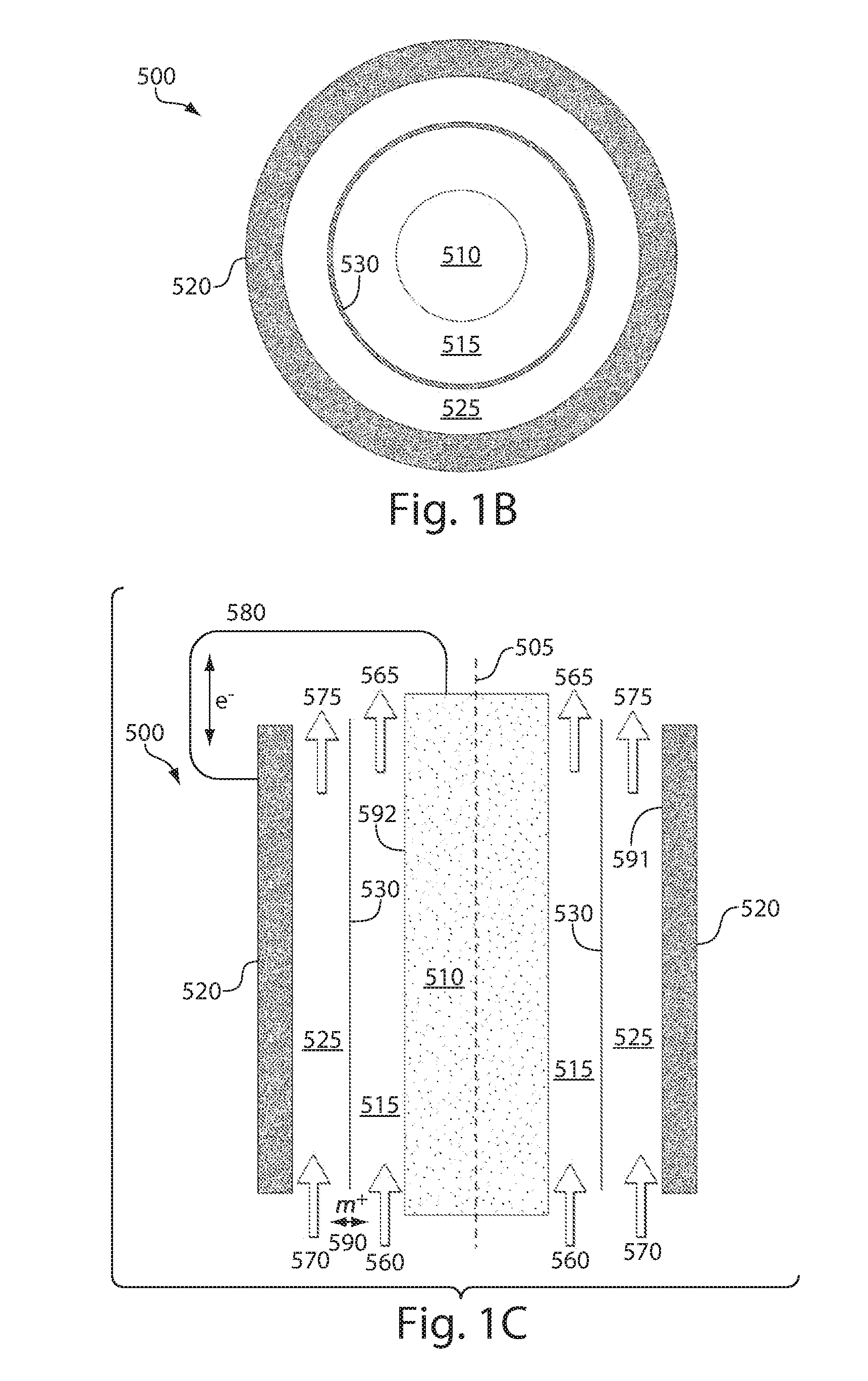
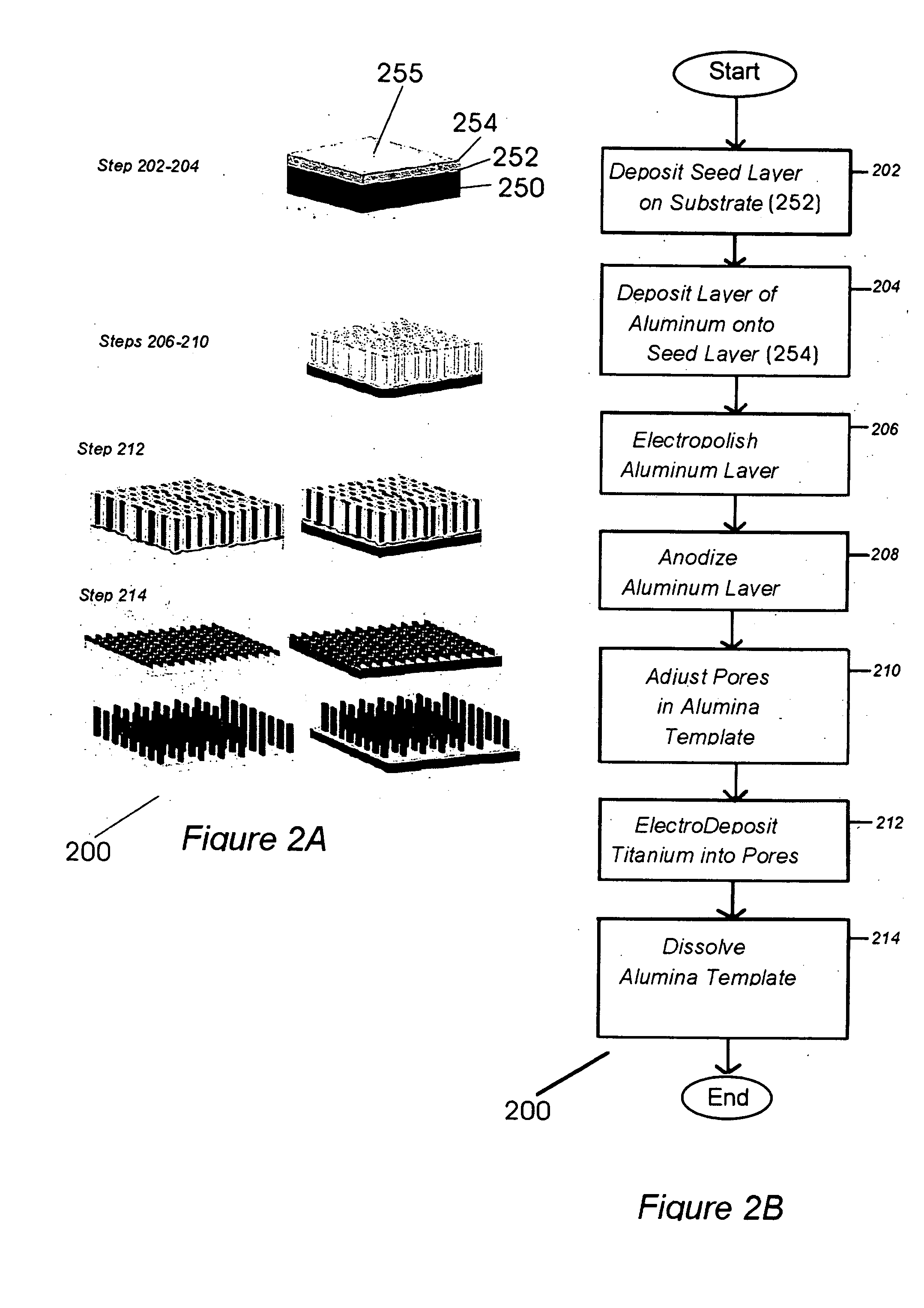
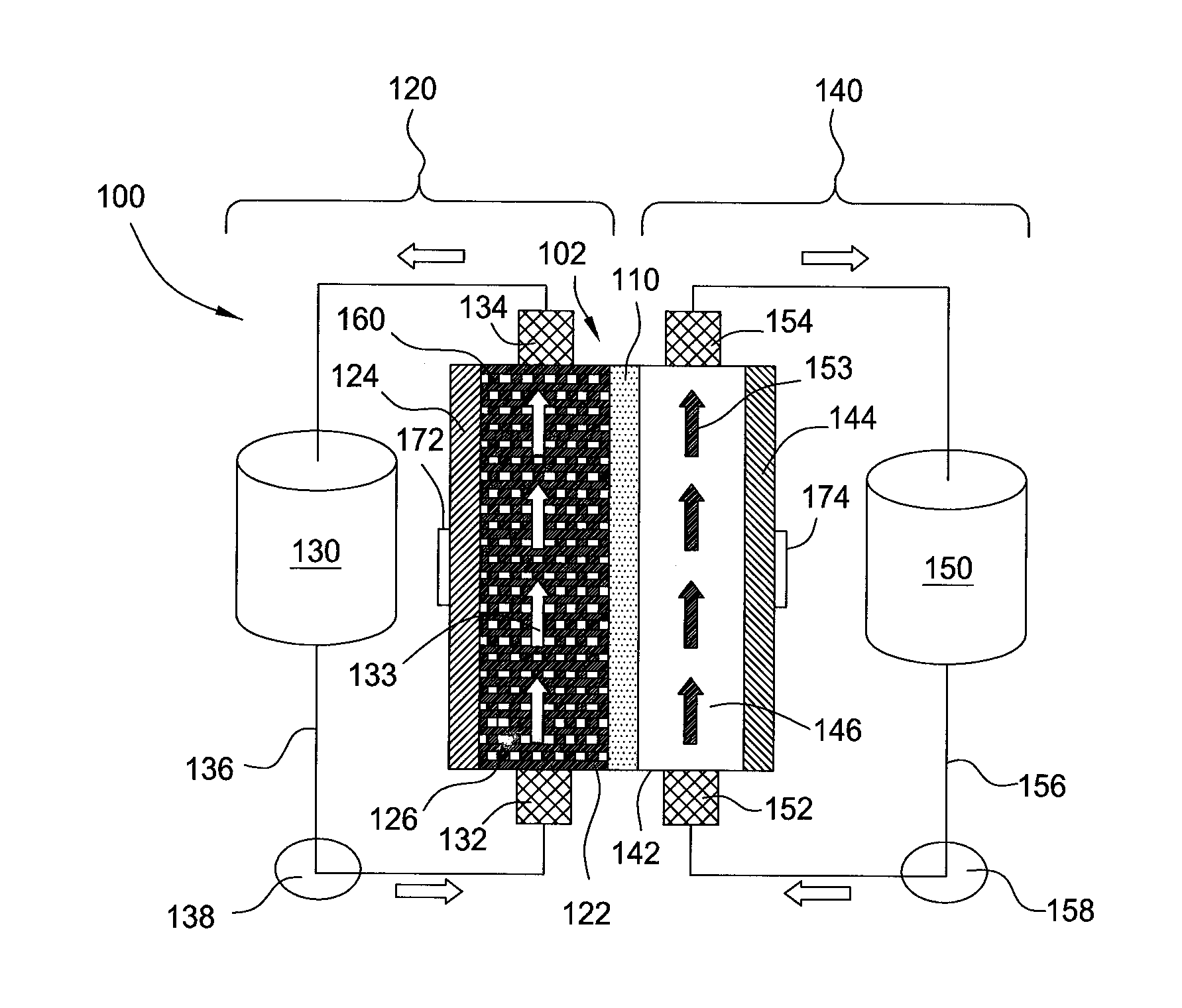
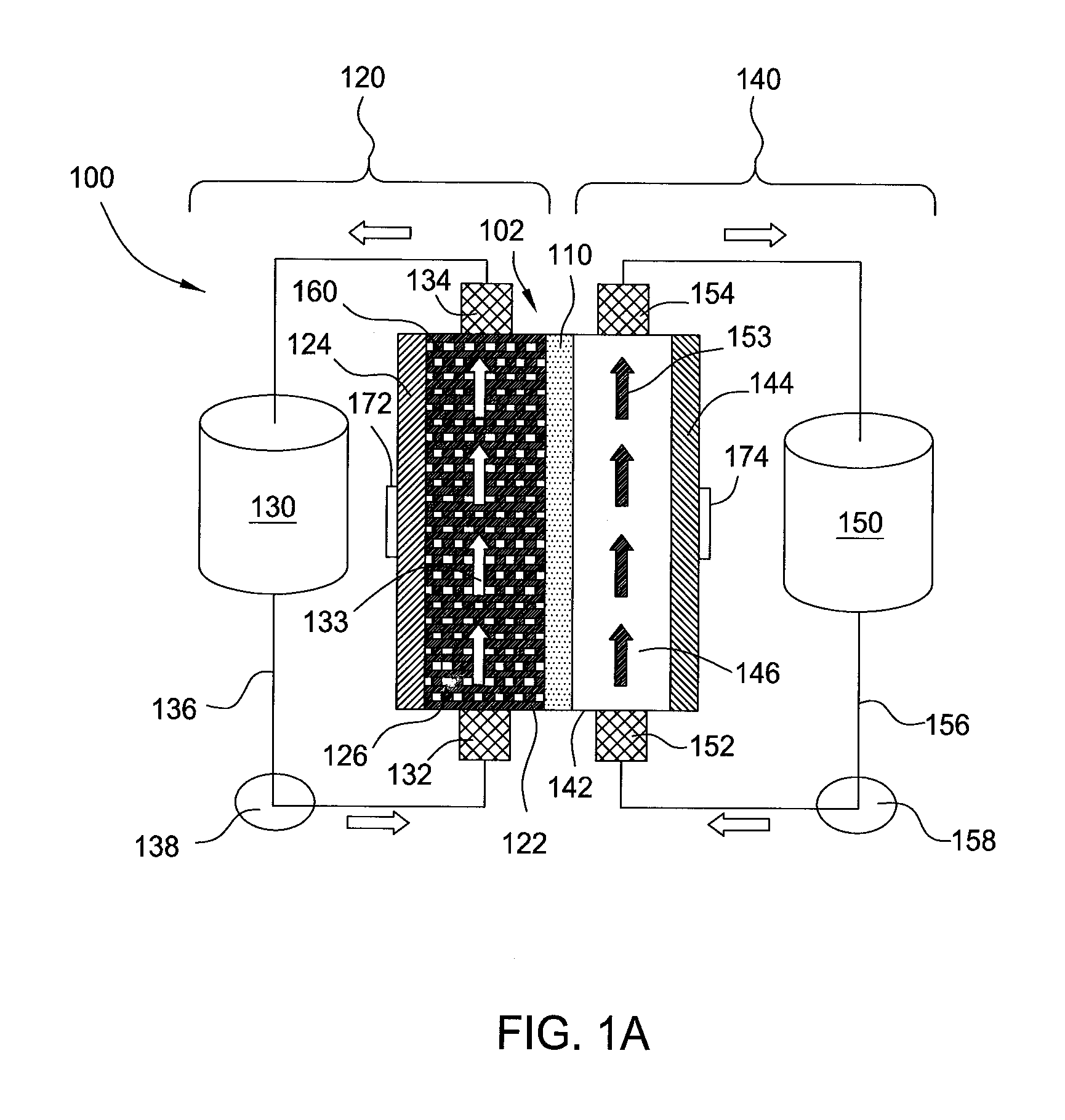
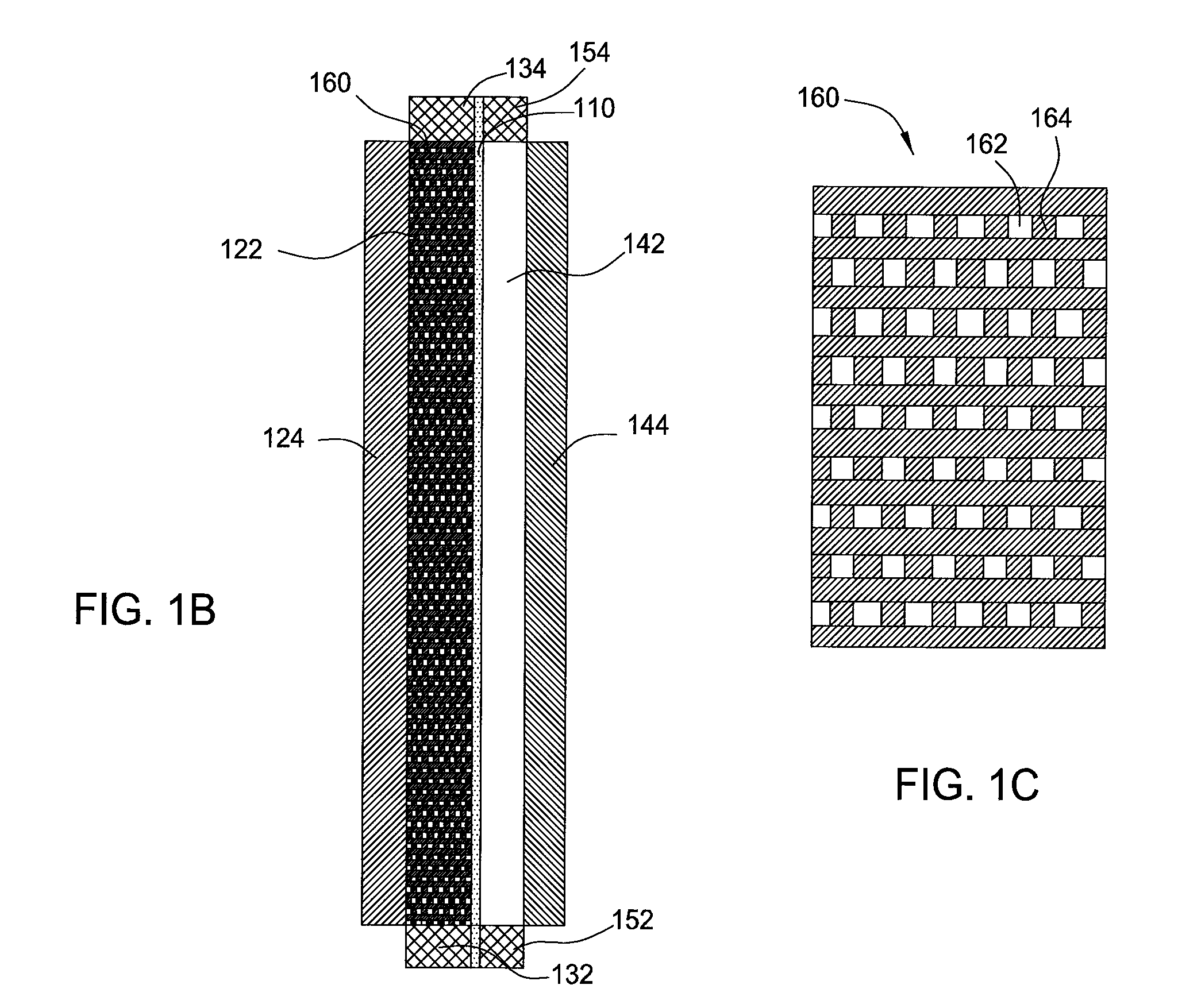
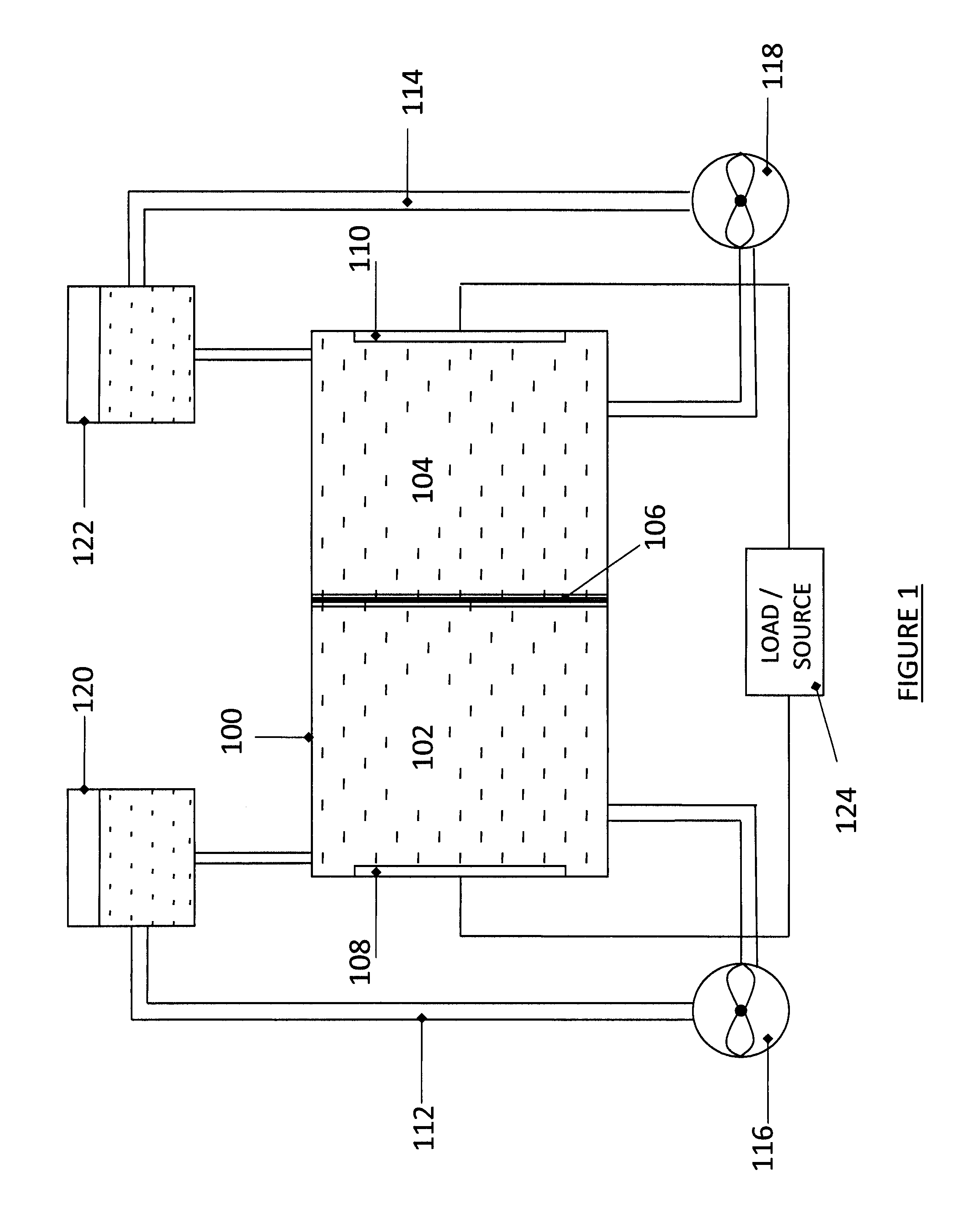
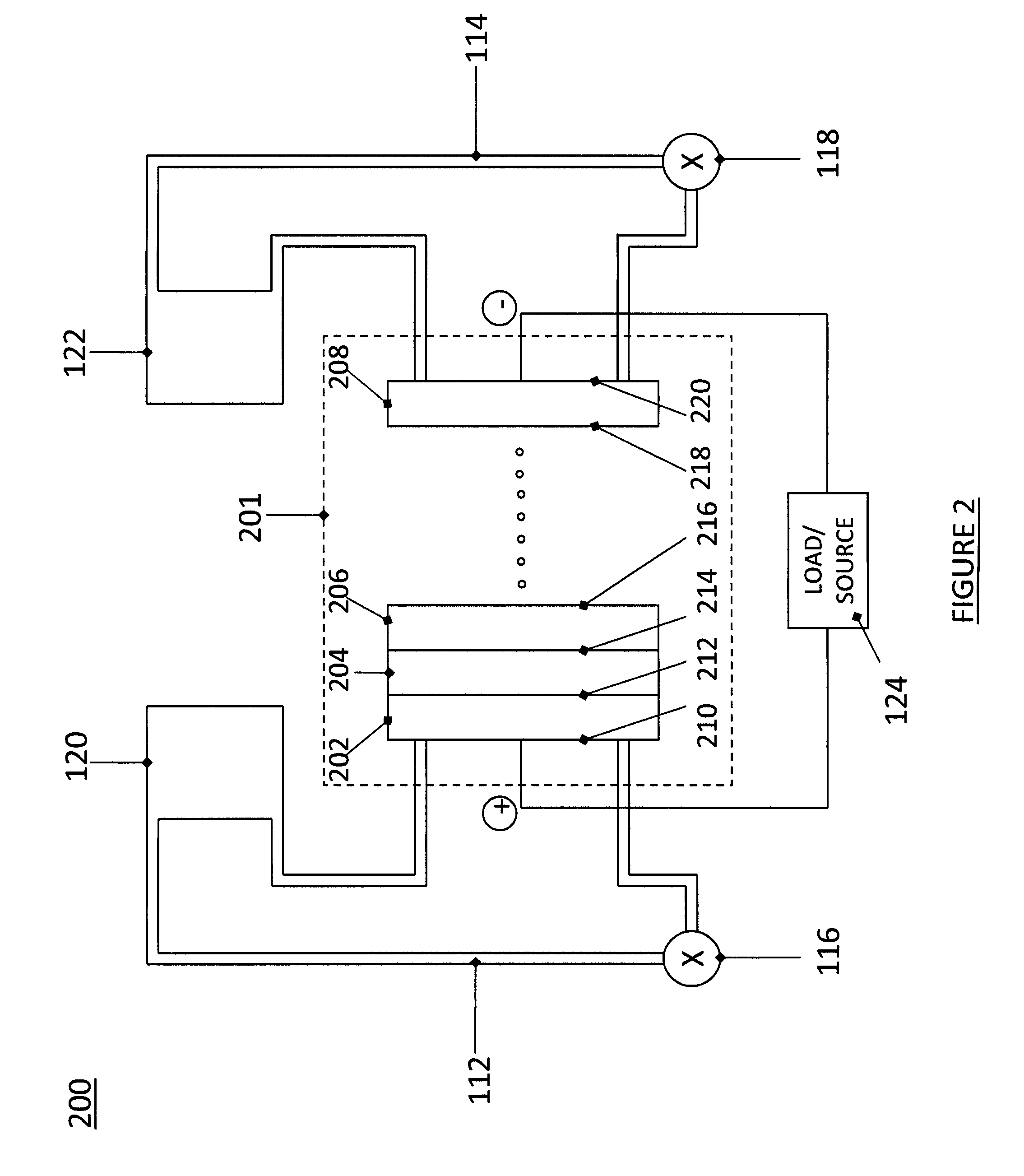
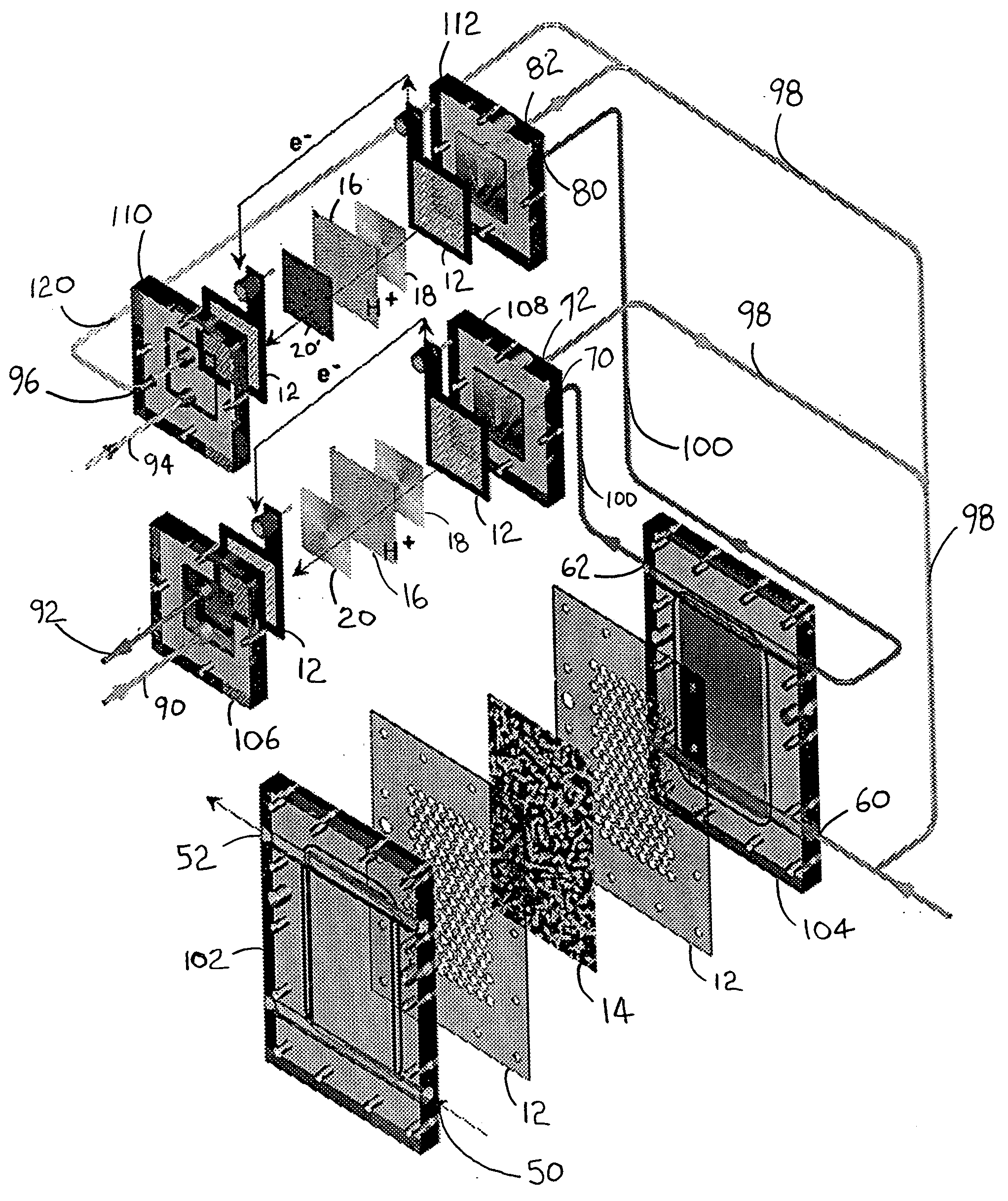
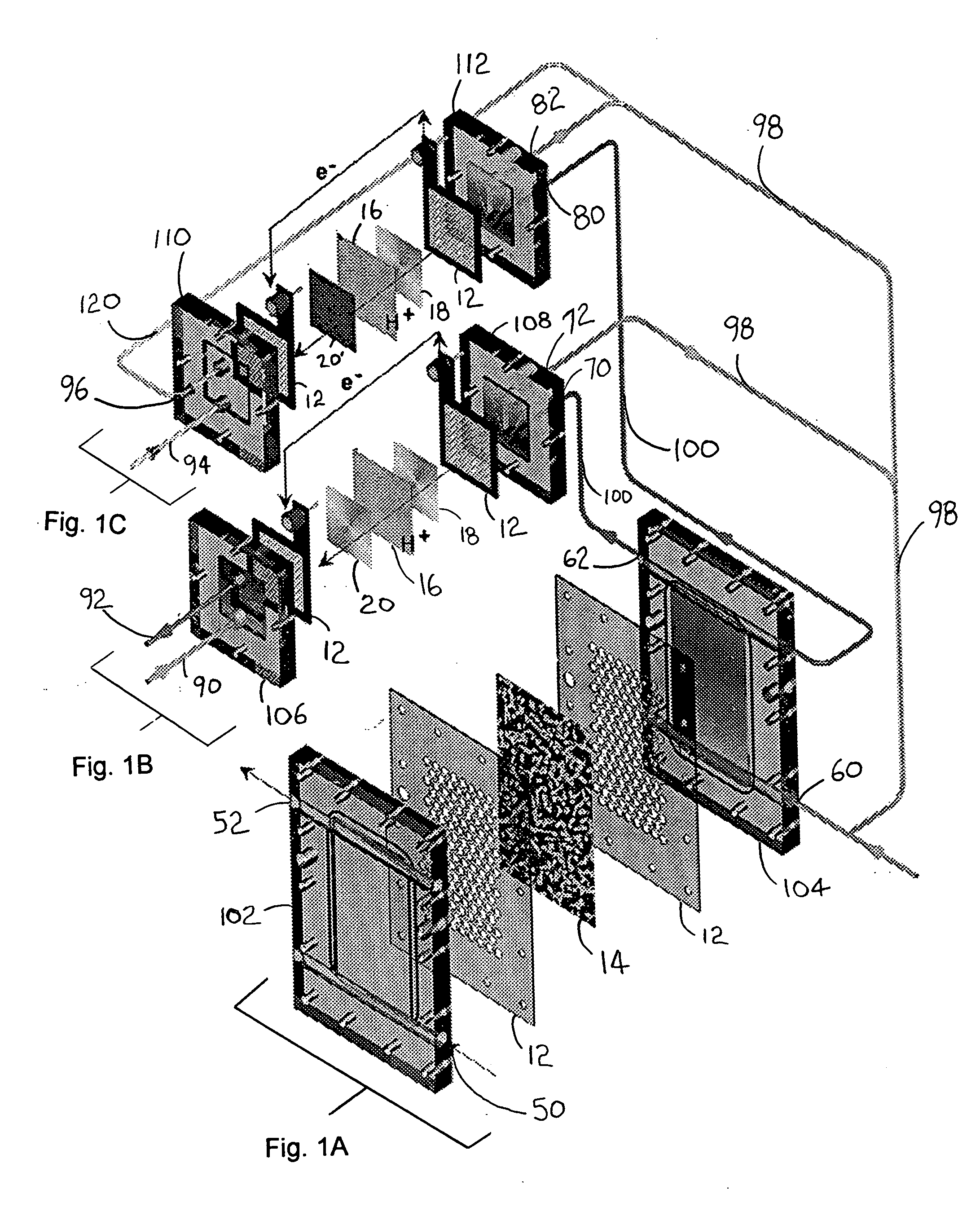
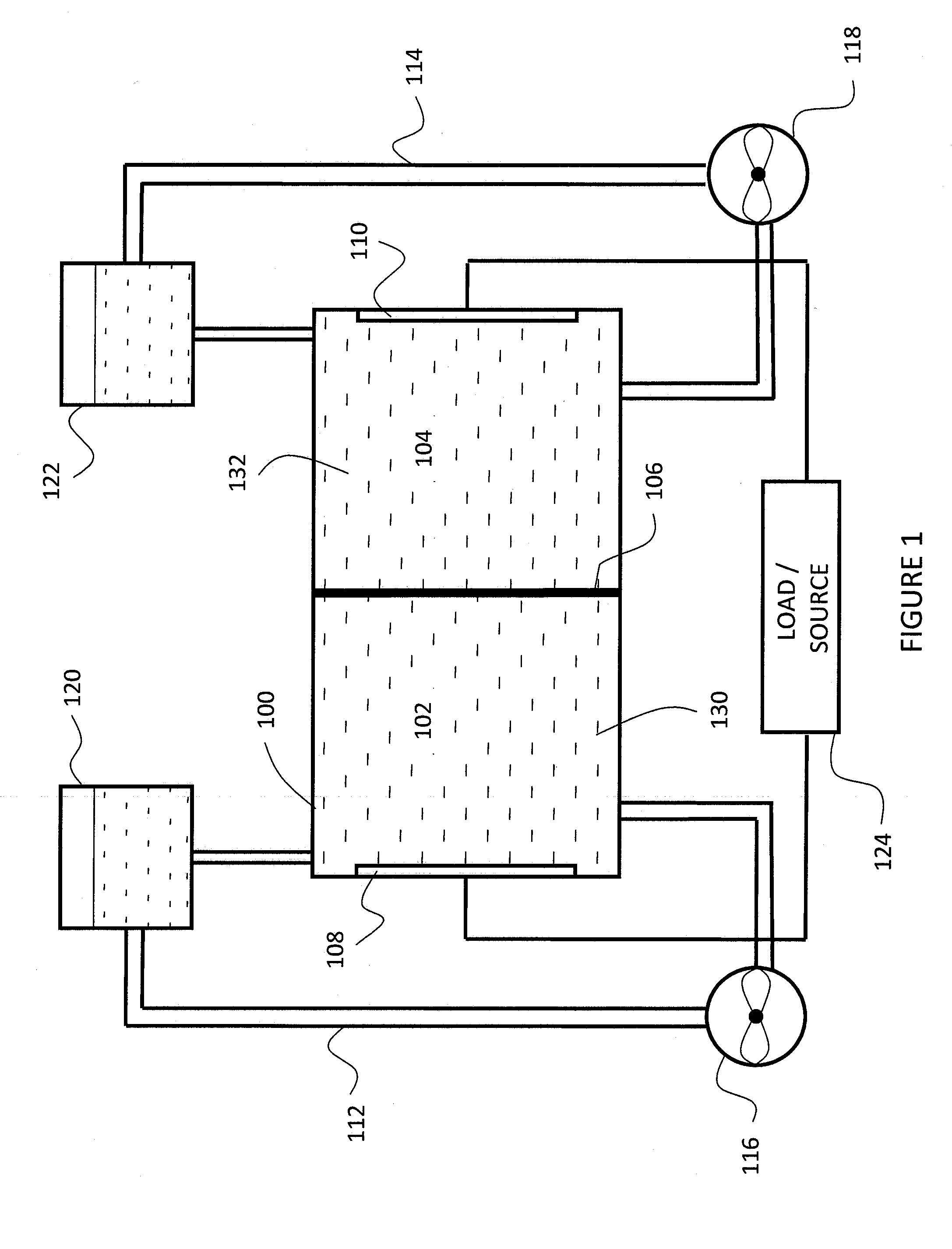
High energy density redox flow device
ActiveUS20110200848A1Avoid accumulationHigh enough specific energyOrganic chemistryFlow propertiesElectrochemical responseHigh energy
Redox flow devices are described in which at least one of the positive electrode or negative electrode-active materials is a semi-solid or is a condensed ion-storing electroactive material, and in which at least one of the electrode-active materials is transported to and from an assembly at which the electrochemical reaction occurs, producing electrical energy. The electronic conductivity of the semi-solid is increased by the addition of conductive particles to suspensions and / or via the surface modification of the solid in semi-solids (e.g., by coating the solid with a more electron conductive coating material to increase the power of the device). High energy density and high power redox flow devices are disclosed. The redox flow devices described herein can also include one or more inventive design features. In addition, inventive chemistries for use in redox flow devices are also described.
Owner:MASSACHUSETTS INST OF TECH +2
High energy density vanadium electrolyte solutions, methods of preparation thereof and all-vanadium redox cells and batteries containing high energy vanadium electrolyte solutions
InactiveUS20010028977A1Effective amountEasy to modifyCharging stationsCell electrodesElectricityVanadium redox battery
Disclosed is a method for preparing a high energy density (HED) electrolyte solution for use in an all-vanadium redox cells, a high energy density electrolyte solution, in particular an all-vanadium high energy density electrolyte solution, a redox cell, in particular an all-vanadium redox cell, comprising the high energy density electrolyte solution, a redox battery, in particular an all-vanadium redox battery, comprising the HED electrolyte solution, a process for recharging a discharged or partially discharged redox battery, in particular an all-vanadium redox battery, comprising the HED electrolyte solution, a process for the production of electricity from a charged redox battery, and in particular a charged all-vanadium redox battery, comprising the HED electrolyte, a redox battery / fuel cell and a process for the production of electricity from a redox battery / fuel cell. A method for stabilising an electrolyte solution for use in a redox cell, in particular for stabilising an electrolyte solution for use in an all-vanadium redox cell, a stabilised electrolyte solution, in particular an all-vanadium stabilised electrolyte solution, a redox cell, in particular an all-vanadium redox cell, comprising the stabilised electrolyte solution, a redox battery, in particular an all-vanadium redox battery comprising the stabilised electrolyte solution, a process for recharging a discharged or partially discharged redox battery, in particular an all-vanadium redox battery, comprising the stabilised electrolyte solution, and a process for the production of electricity from a charged redox battery, and in particular a charged all-vanadium redox battery, comprising the stabilised electrolyte solution are disclosed. Also disclosed are a redox battery / fuel cell and a process for the production of electricity from a redox battery / fuel cell.
Owner:JD HLDG INC
Stabilized vanadium electrolyte solutions for all-vanadium redox cells and batteries
InactiveUS6562514B1Effective amountEasy to modifyFinal product manufactureRegenerative fuel cellsRedoxPhysical chemistry
Owner:JD HLDG INC
Perfluorinated Membranes and Improved Electrolytes for Redox Cells and Batteries
ActiveUS20080292964A1Improve performanceReduce resistanceFinal product manufactureSecondary cellsSupporting electrolyteVanadyl ion
A vanadium redox cell having a positive half cell containing a positive half cell solution comprising a supporting electrolyte selected from H2SO4, HBr / HCl mixtures and one or more ions selected from the group vanadium (EI), Vanadium (IV), Vanadium (V), Br3 and Br2Cl; a negative half cell containing a negative half cell solution comprising a supporting electrolyte selected from H2SO4, HBr and HBr / HCl mixtures and one or more vanadium ions selected from the group Vanadium (II), Vanadium (III) and Vanadium (FV) and a perfluorinated cast cation exchange membrane or separator disposed between the positive and negative half cells and in contact with the positive and negative half cell solutions.
Owner:NEWSOUTH INNOVATIONS PTY LTD
High Voltage and High Specific Capacity Dual Intercalating Electrode Li-Ion Batteries
ActiveUS20060269834A1High voltageHigh energyElectrode carriers/collectorsOrganic electrolyte cellsPresent methodElectrode pair
The present invention provides high capacity and high voltage Li-ion batteries that have a carbonaceous cathode and a nonaqueous electrolyte solution comprising LiF salt and an anion receptor that binds the fluoride ion. The batteries can comprise dual intercalating electrode Li ion batteries. Methods of the present invention use a cathode and electrode pair, wherein each of the electrodes reversibly intercalate ions provided by a LiF salt to make a high voltage and high specific capacity dual intercalating electrode Li-ion battery. The present methods and systems provide high-capacity batteries particularly useful in powering devices where minimizing battery mass is important.
Owner:CALIFORNIA INST OF TECH
Redox flow battery
InactiveUS20120135278A1High charge-discharge efficiencyIncrease energy densityCell electrodesFinal product manufactureRedoxSlurry
A redox flow battery comprising an electrode cell including a negative electrode cell, a positive electrode cell and a separator for separating them, in which at least one of the negative electrode cell and the positive electrode cell includes a slurry type electrode solution, a porous current collector and a casing; a tank for storing the slurry type electrode solution; and a pipe for circulating the slurry type electrode solution between the tank and the electrode cell.
Owner:SHARP KK
Part solid, part fluid and flow electrochemical cells including metal-air and li-air battery systems
PendingUS20130189592A1Provide integrityAvoid shortingPrimary cell to battery groupingFuel and primary cellsLithium–air batteryEngineering
Owner:CALIFORNIA INST OF TECH
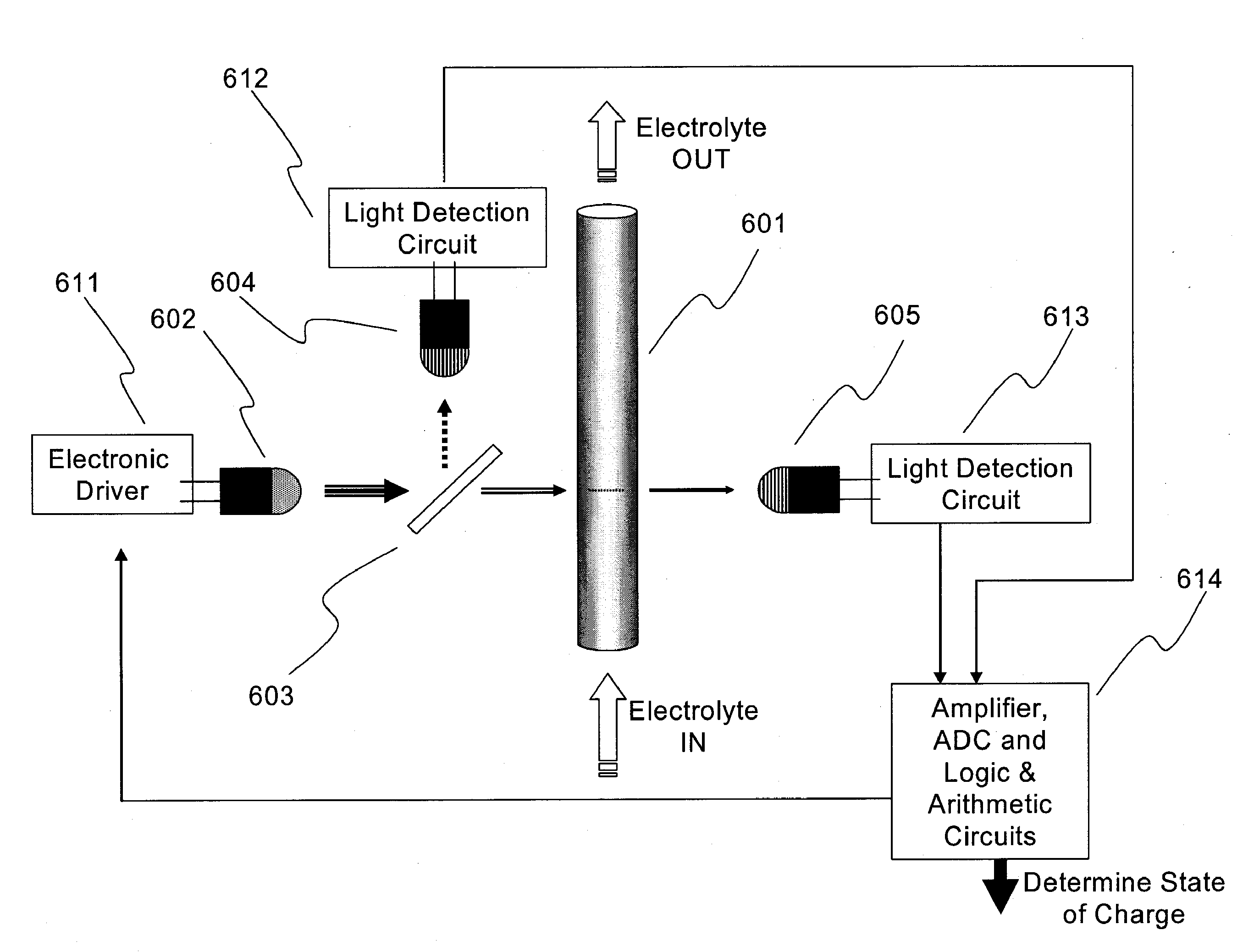
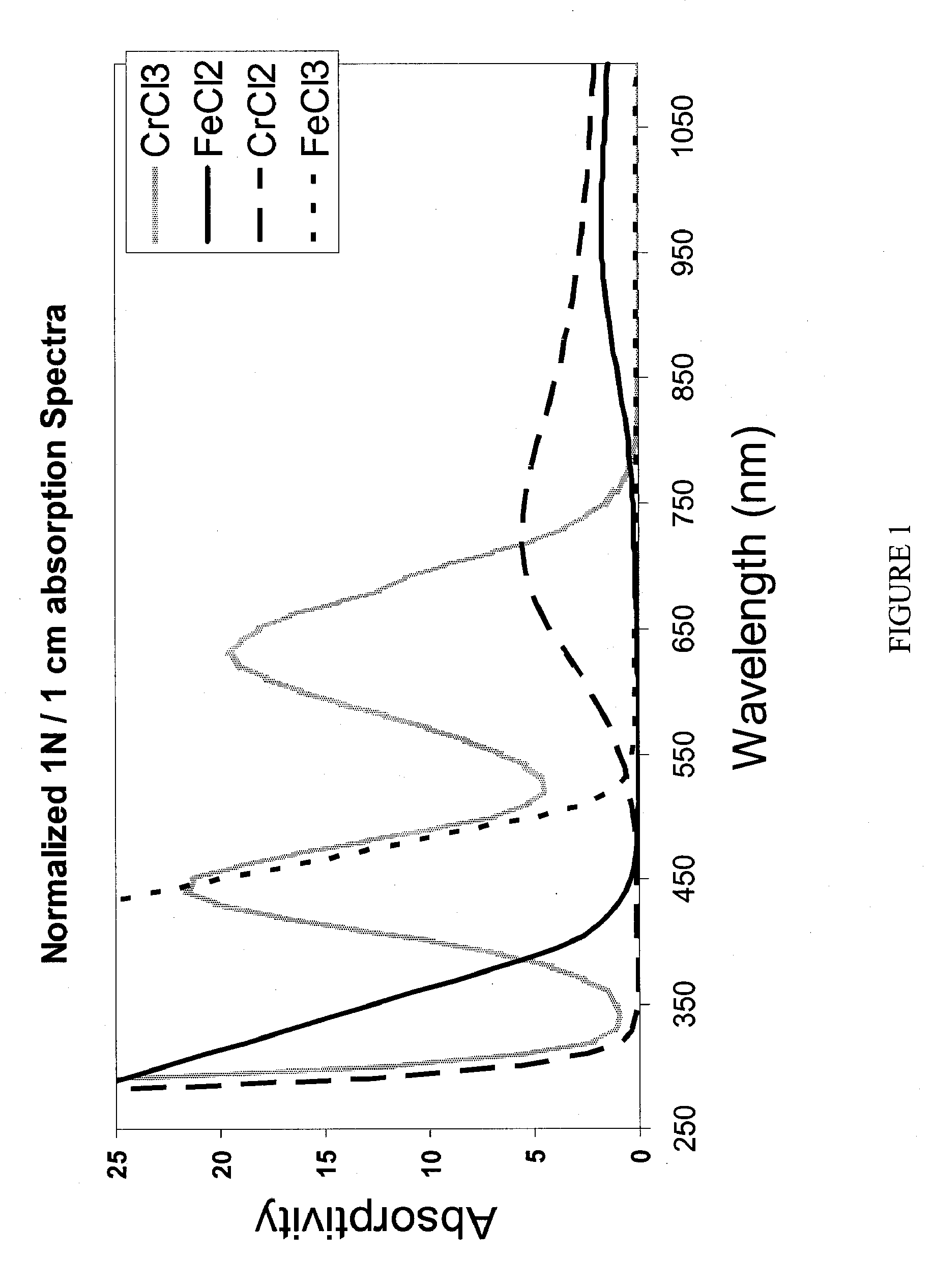
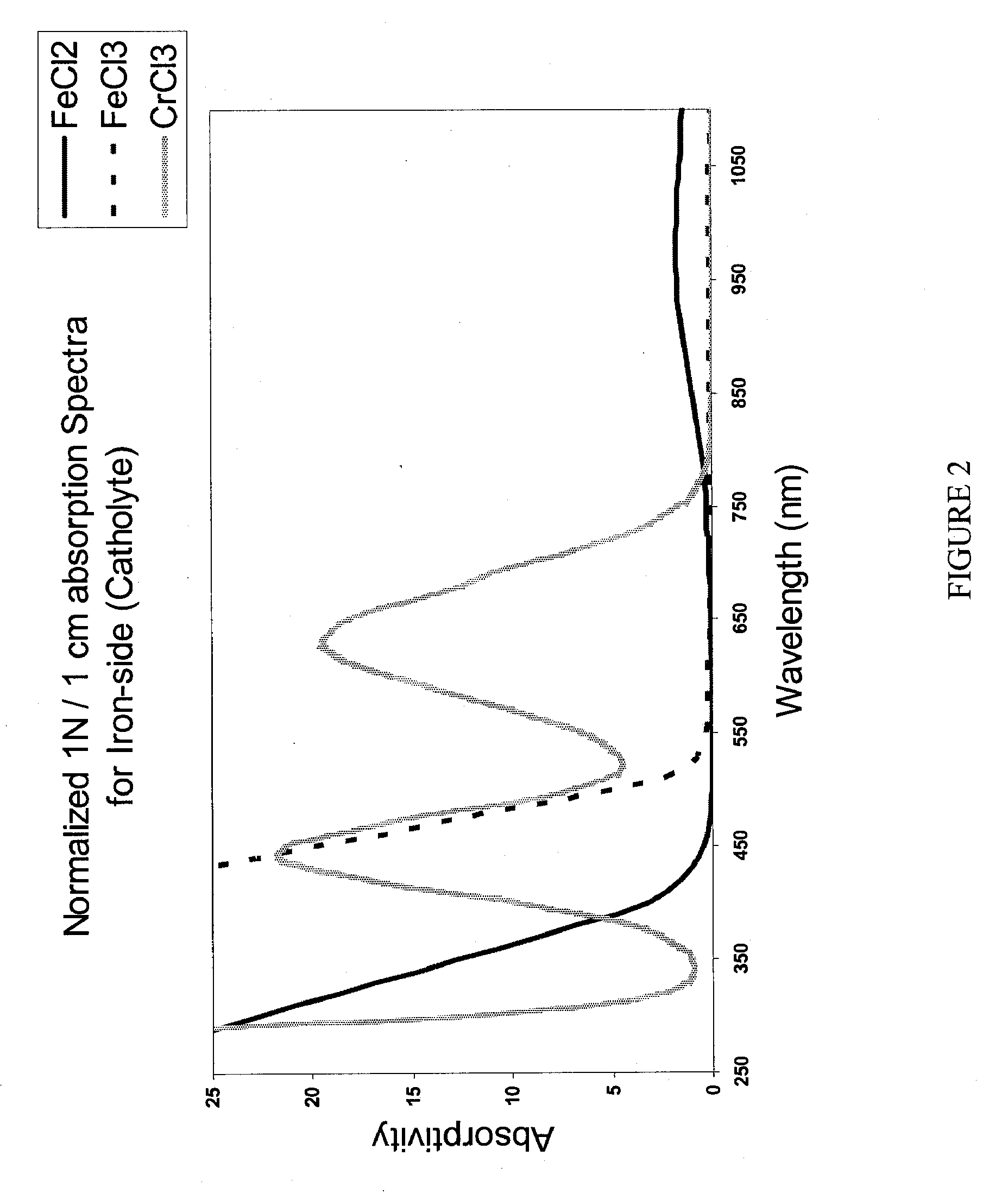
Apparatus and Methods of Determination of State of Charge in a Redox Flow Battery
InactiveUS20080193828A1Batteries circuit arrangementsMaterial analysis by observing effect on chemical indicatorRedoxState of charge
Apparatus and methods for determining the individual states of charge of electrolytes in a redox battery with mixed or unmixed reactants by optical absorption spectrophotometry are disclosed. The state of charge thus obtained may serve as a gauge for the amount of electro-chemical energy left in the system. Further, the information on anolyte and catholyte charge states may be used for any rebalancing mechanism if the states are different.
Owner:IMERGY POWER SYST
High energy density vanadium electrolyte solutions, methods of preparation thereof and all-vanadium redox cells and batteries containing high energy vanadium electrolyte solutions
InactiveUS6468688B2Effective amountEasy to modifyCharging stationsCell electrodesElectricityVanadium redox battery
Disclosed is a method for preparing a high energy density (HED) electrolyte solution for use in an all-vanadium redox cells, a high energy density electrolyte solution, in particular an all-vanadium high energy density electrolyte solution, a redox cell, in particular an all-vanadium redox cell, comprising the high energy density electrolyte solution, a redox battery, in particular an all-vanadium redox battery, comprising the HED electrolyte solution, a process for recharging a discharged or partially discharged redox battery, in particular an all-vanadium redox battery, comprising the HED electrolyte solution, a process for the production of electricity from a charged redox battery, and in particular a charged all-vanadium redox battery, comprising the HED electrolyte, a redox battery / fuel cell and a process for the production of electricity from a redox battery / fuel cell. A method for stabilising an electrolyte solution for use in a redox cell, in particular for stabilising an electrolyte solution for use in an all-vanadium redox cell, a stabilised electrolyte solution, in particular an all-vanadium stabilised electrolyte solution, a redox cell, in particular an all-vanadium redox cell, comprising the stabilised electrolyte solution, a redox battery, in particular an all-vanadium redox battery comprising the stabilised electrolyte solution, a process for recharging a discharged or partially discharged redox battery, in particular an all-vanadium redox battery, comprising the stabilised electrolyte solution, and a process for the production of electricity from a charged redox battery, and in particular a charged all-vanadium redox battery, comprising the stabilised electrolyte solution are disclosed. Also disclosed are a redox battery / fuel cell and a process for the production of electricity from a redox battery / fuel cell.
Owner:JD HLDG INC
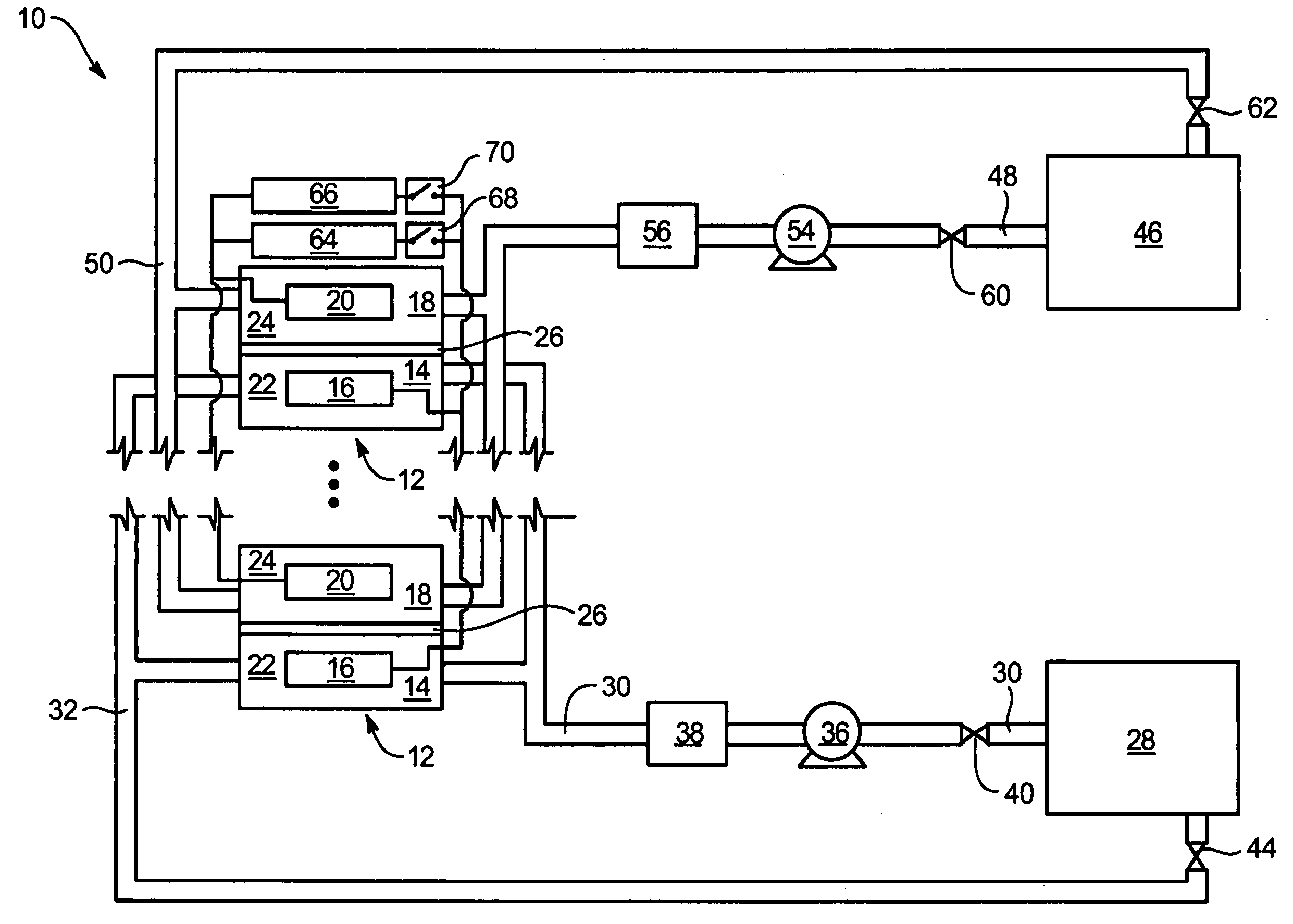
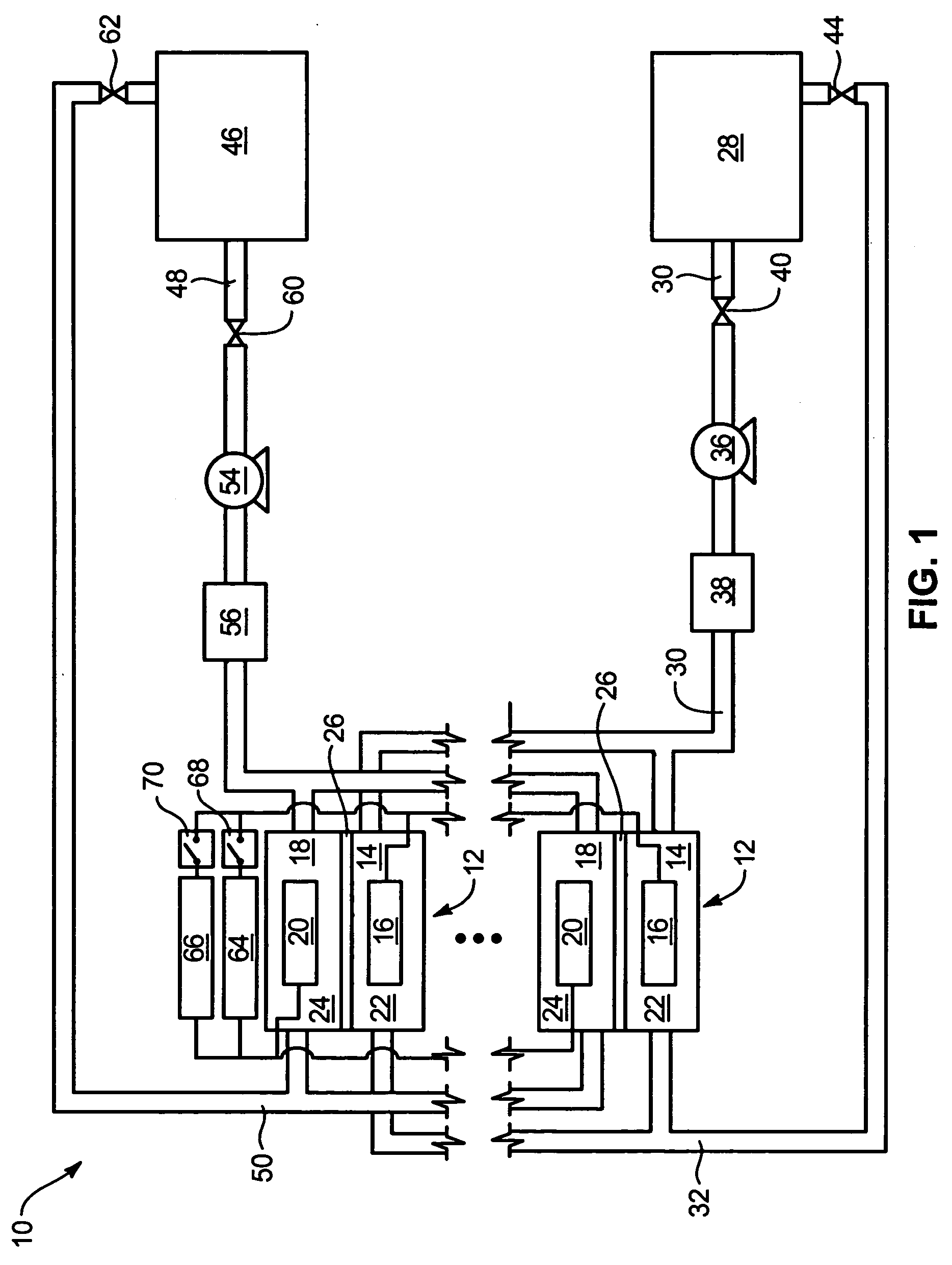
System and method for optimizing efficiency and power output from a vanadium redox battery energy storage system
An energy storage system includes a vanadium redox battery that interfaces with a control system to optimize performance and efficiency. The control system calculates optimal pump speeds, electrolyte temperature ranges, and charge and discharge rates. The control system instructs the vanadium redox battery to operate in accordance with the prescribed parameters. The control system further calculates optimal temperature ranges and charge and discharge rates for the vanadium redox battery.
Owner:VRB ENERGY INC
Implantable biofuel cell system based on nanostructures
InactiveUS20050118494A1Increase powerImprove power densityMaterial nanotechnologyFuel cell auxillariesCarbon nanotubeMolecular level
A bio-implantable electrochemical cell system for active implantable medical devices. In one embodiment, the fuel cell includes an electrode structure consisting of immobilized anode and cathode enzymes deposited on nanostructured high-surface-area metal nanowires or carbon nanotube electrodes. The anode enzyme comprises immobilized glucose oxidase and the cathode enzyme comprises immobilized laccase. Glucose is oxidized at the surface of the anode and oxygen is reduced at the surface of the cathode. The coupled glucose oxidation-oxygen reduction reactions provide a self-generating current source. In another embodiment, the nanowires or carbon nanotubes, along with the adjacent surface anode and cathode electrodes, are coated with immobilized glucose oxidase and immobilized laccase containing biocolloidal substrates, respectively. This results in the precise construction of an enzyme architecture with control at the molecular level, while increasing the reactive surface area and corresponding output power by at least two orders of magnitude.
Owner:NANOSOLUTIONS
Flow battery systems
ActiveUS20120052347A1Uniform metal platingHigh cell current densityFuel and secondary cellsCell electrodesEngineeringMetal
Embodiments of the invention generally provide for flow battery cells and systems containing a plurality of flow battery cells, and methods for improving metal plating within the flow battery cell, such as by flowing and exposing the catholyte to various types of cathodes. In one embodiment, a flow battery cell is provided which includes a cathodic half cell and an anodic half cell separated by an electrolyte membrane, wherein the cathodic half cell contains a plurality of cathodic wires extending perpendicular or substantially perpendicular to and within the catholyte pathway and in contact with the catholyte, and each of the cathodic wires extends parallel or substantially parallel to each other. In some examples, the plurality of cathodic wires may have at least two arrays of cathodic wires, each array contains at least one row of cathodic wires, and each row extends along the catholyte pathway.
Owner:APPLIED MATERIALS INC
Redox shuttle for overdischarge protection in rechargeable lithium-ion batteries
InactiveUS20050221168A1Wide windowLarge capacityNon-aqueous electrolyte accumulatorsElectrolytic capacitorsElectrode potentialCapacity loss
A battery of series-connected rechargeable lithium ion cells each of which contains a negative electrode; a negative electrode current collector; a positive electrode; a positive electrode current collector; and an electrolyte comprising charge carrying medium, lithium salt and cyclable redox chemical shuttle. The negative electrode has a larger irreversible first cycle capacity loss than that of the positive electrode, and is driven to a potential above that of the positive electrode if the cell is discharged to a state of cell reversal. The shuttle has an electrochemical potential above the positive electrode maximum normal operating potential, and prevents the negative electrode potential from reaching even higher and more destructive positive values during overdischarge. The current collector has a lithium alloying potential below the negative electrode minimum normal operating potential. The battery chemically limits or eliminates cell damage due to repeated overdischarge, and may operate without electronic overdischarge protection circuitry.
Owner:3M INNOVATIVE PROPERTIES CO
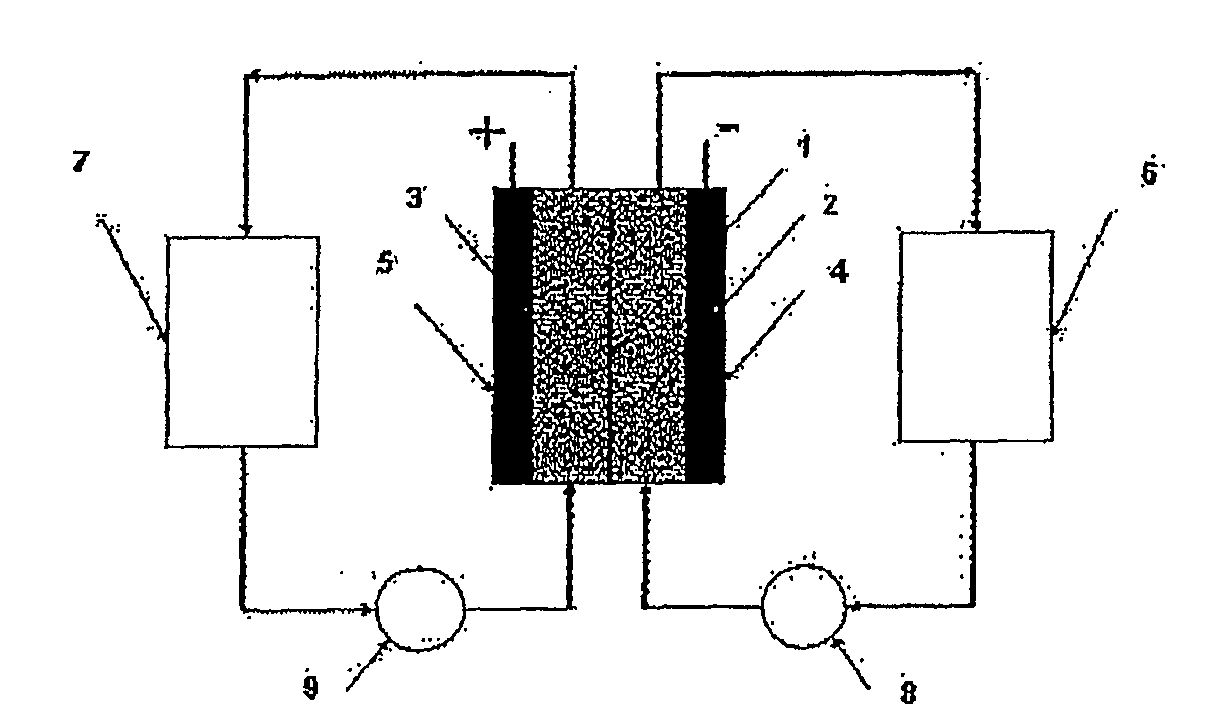
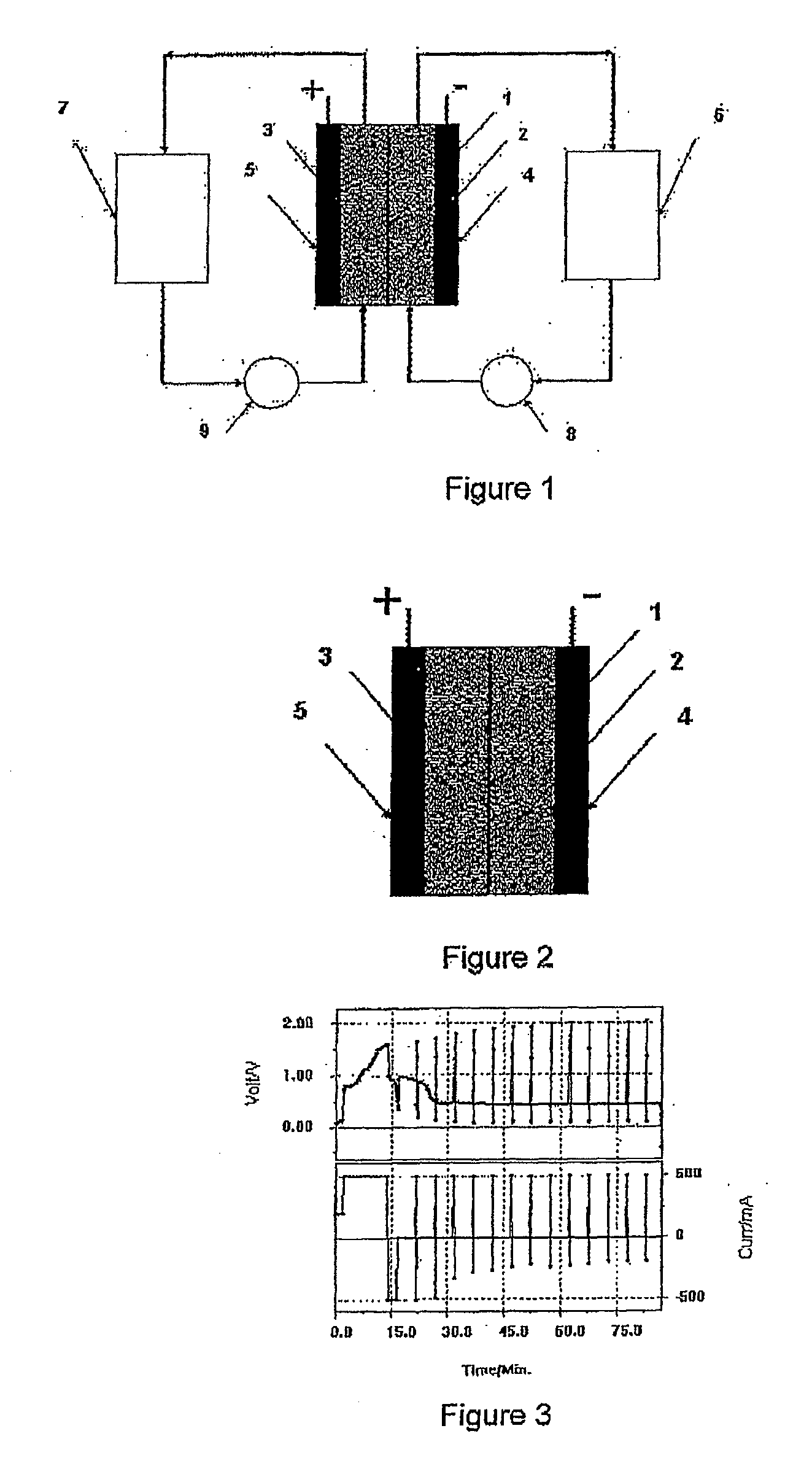
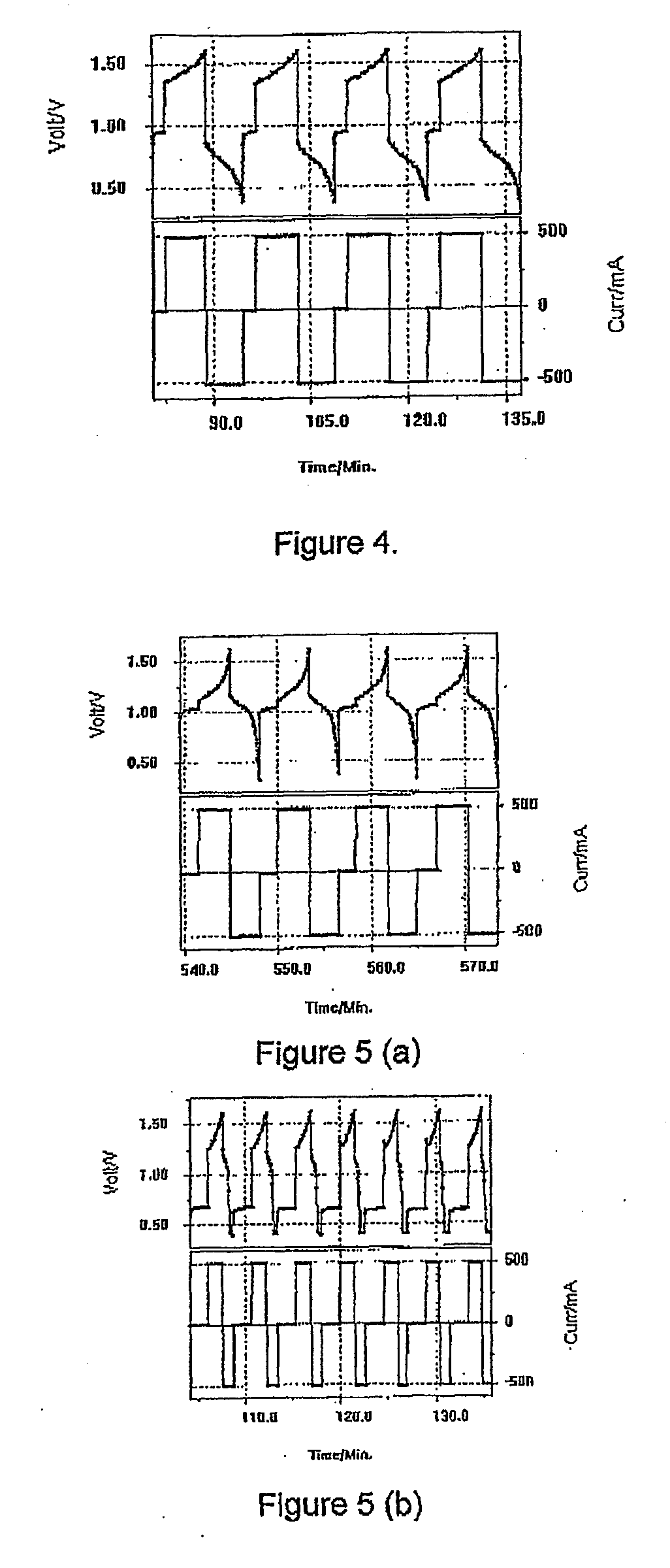
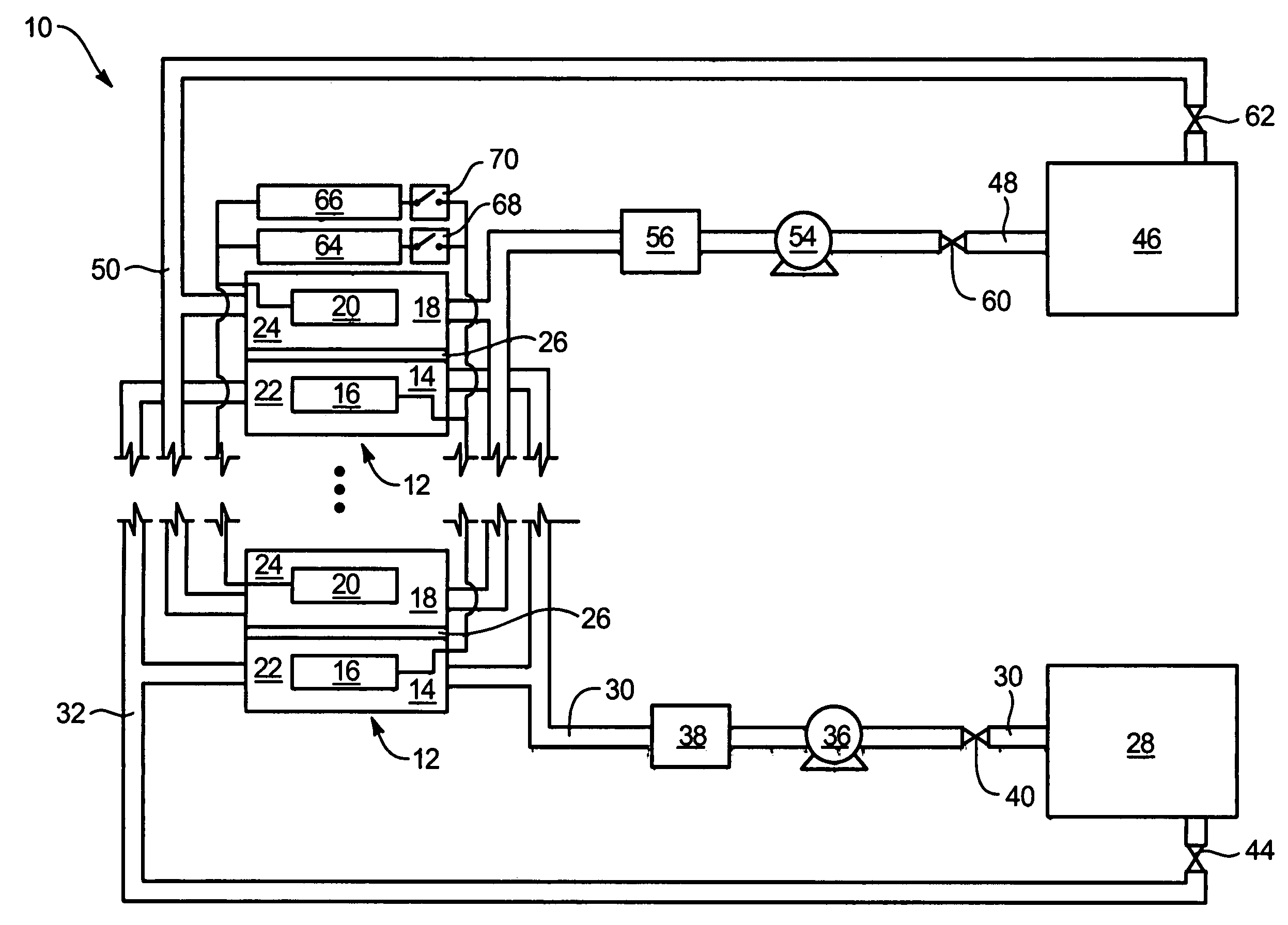
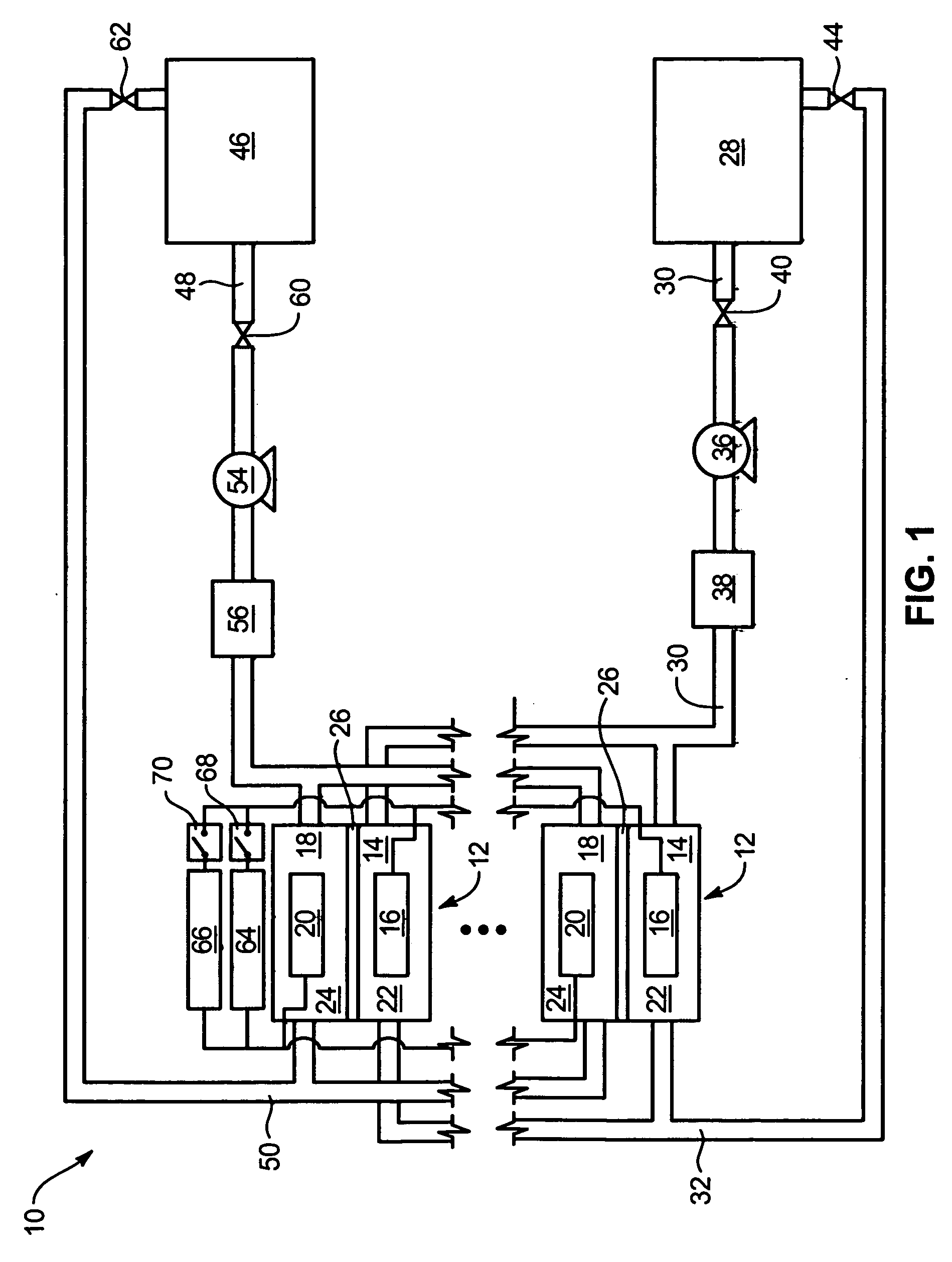
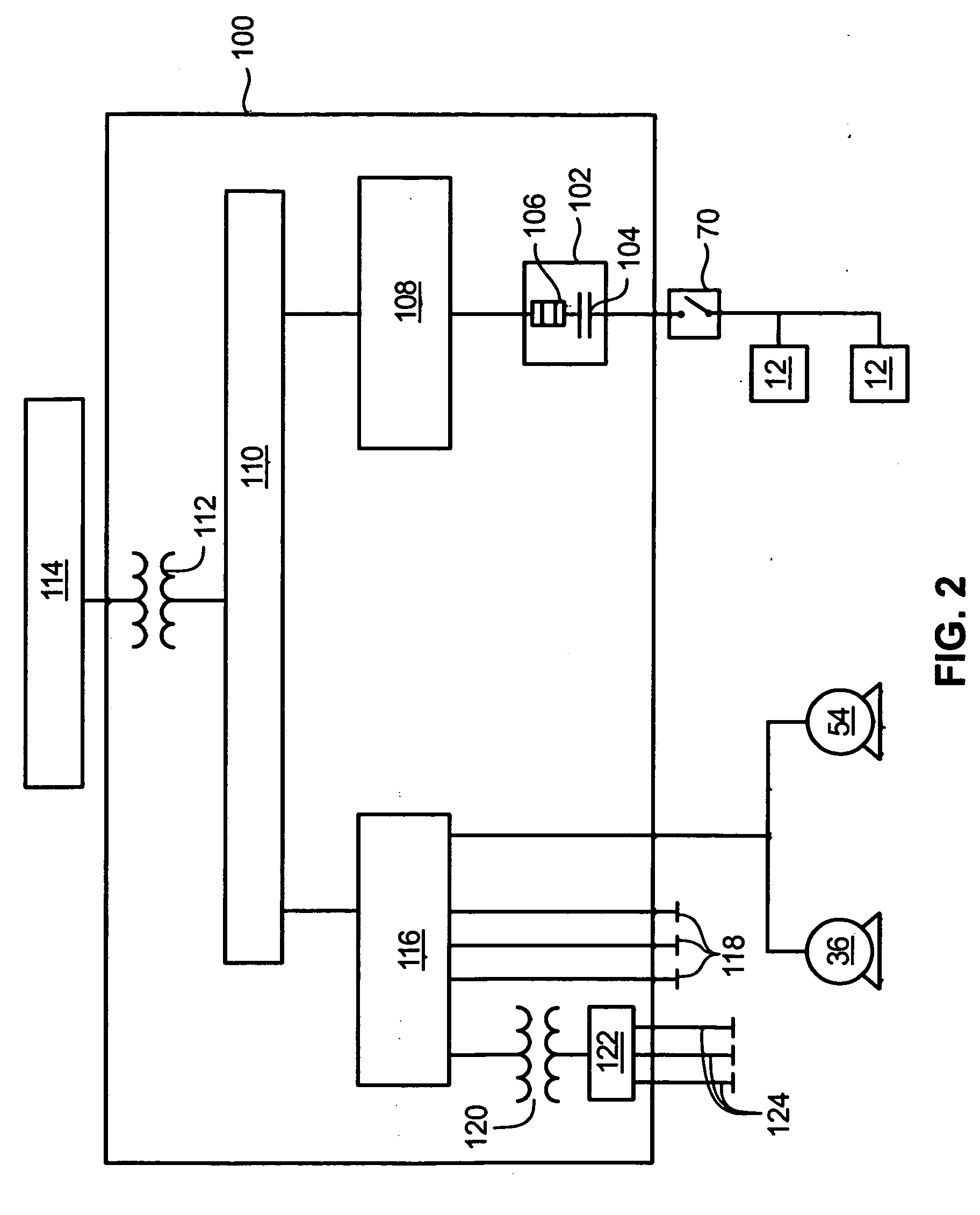
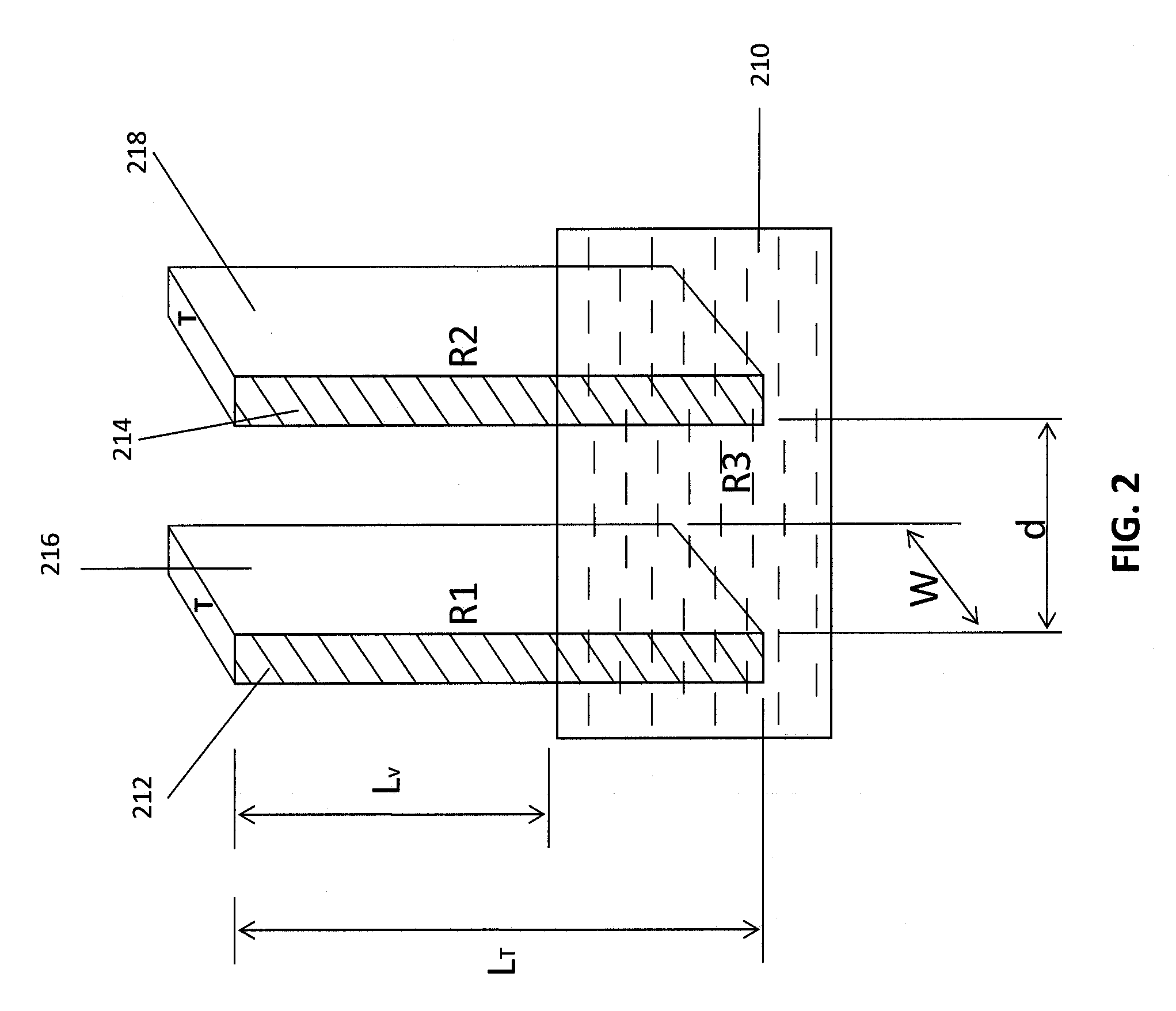
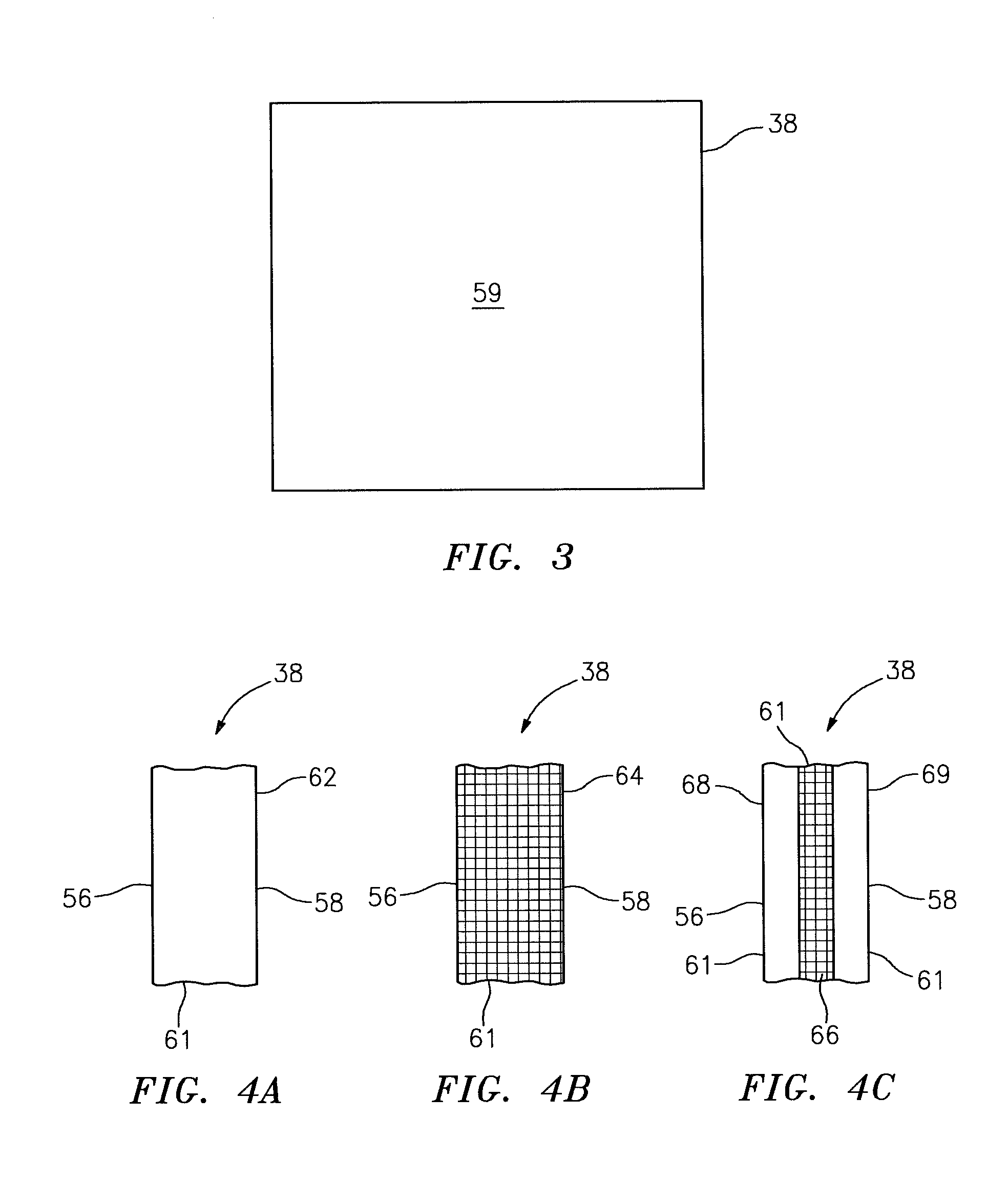
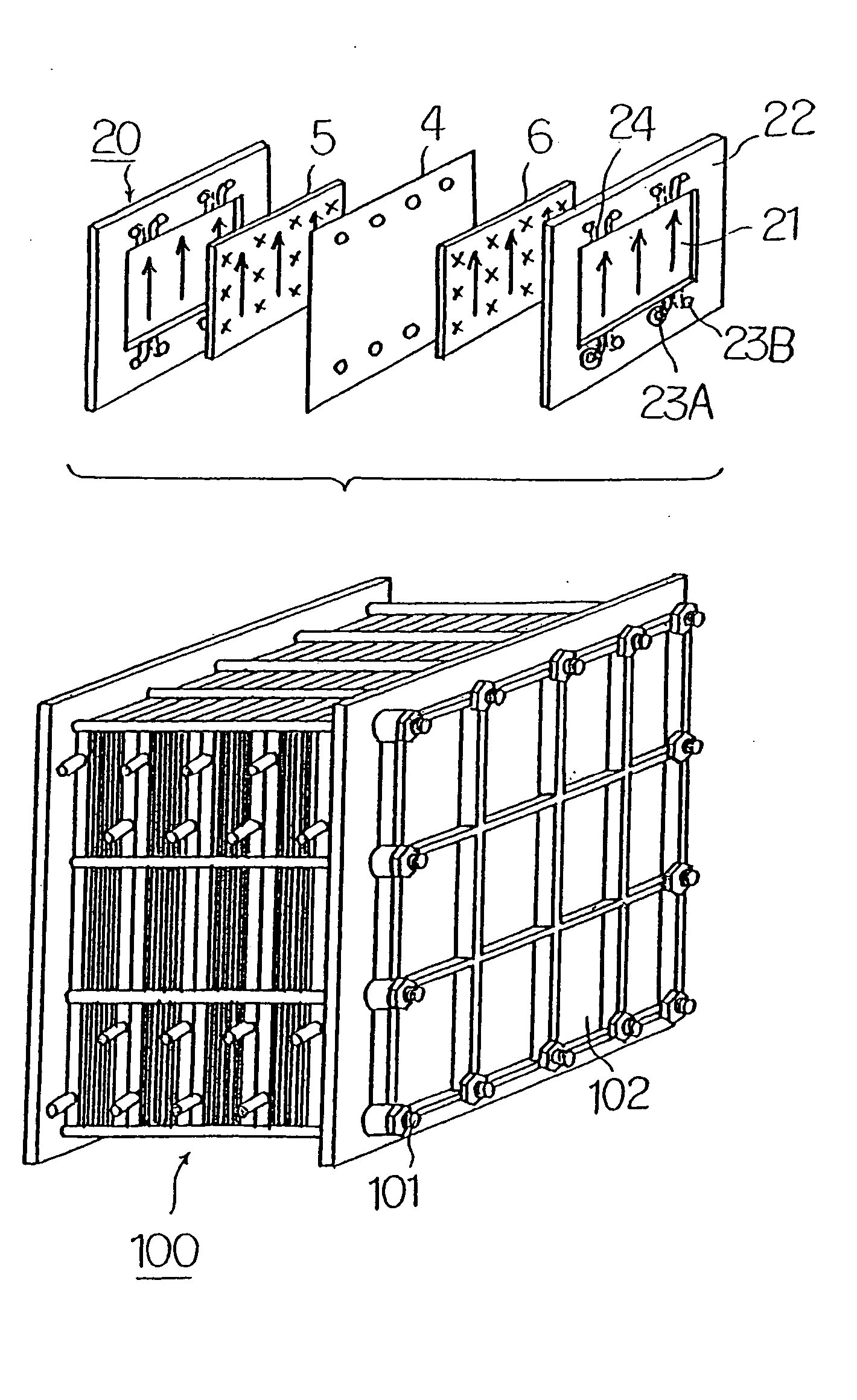
Common Module Stack Component Design
A stack for use in a flow battery, the stack comprising an odd number of interior elements positioned between two end elements, the two end elements each including an electrode, and the odd number of interior elements including membrane elements alternating with electrode elements, wherein the membrane elements include an interior frame and a membrane, the electrode elements include the interior frame and an electrode, wherein the interior frame is rotated by 180° from the frame of the membrane elements.
Owner:IMERGY POWER SYST
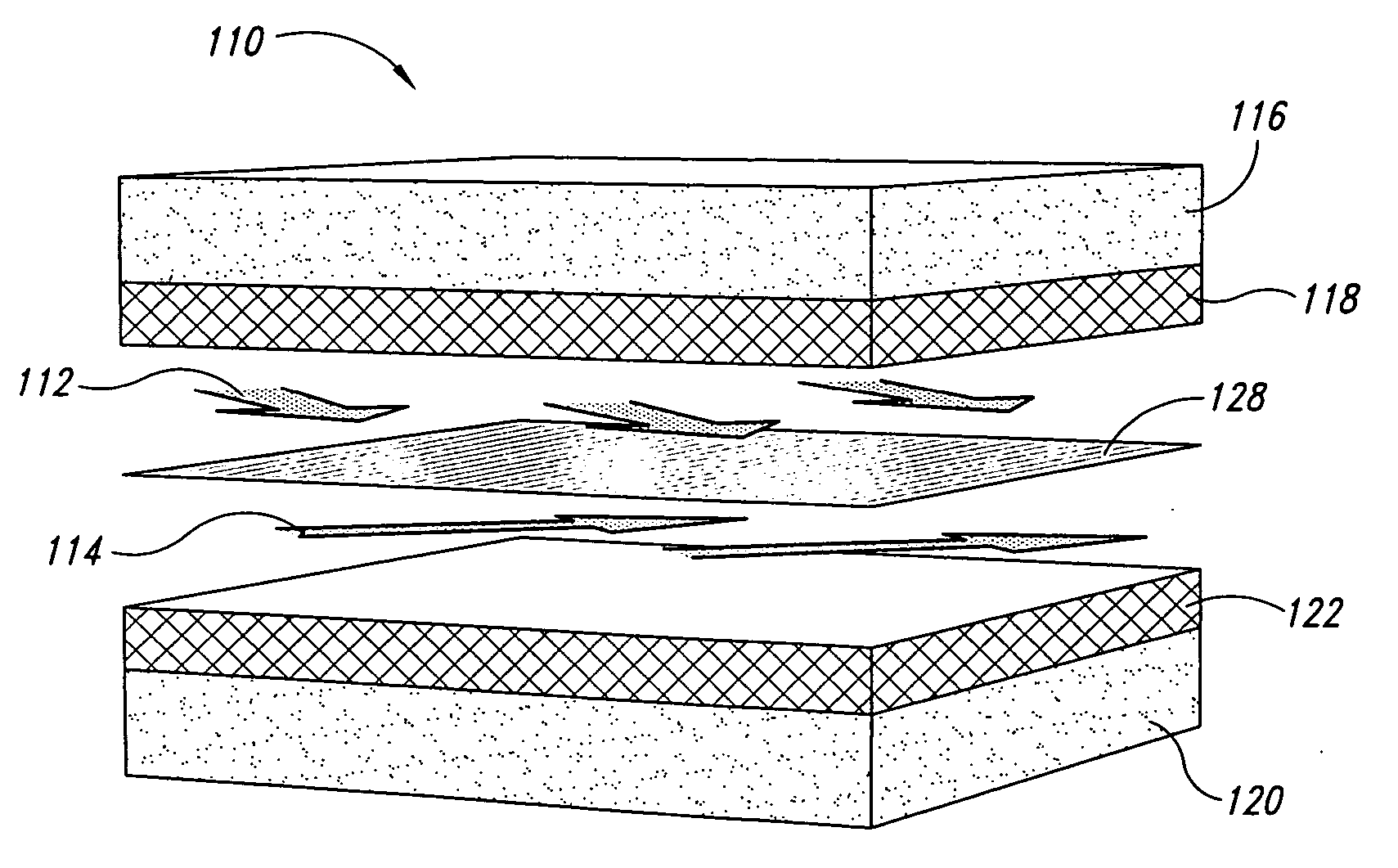
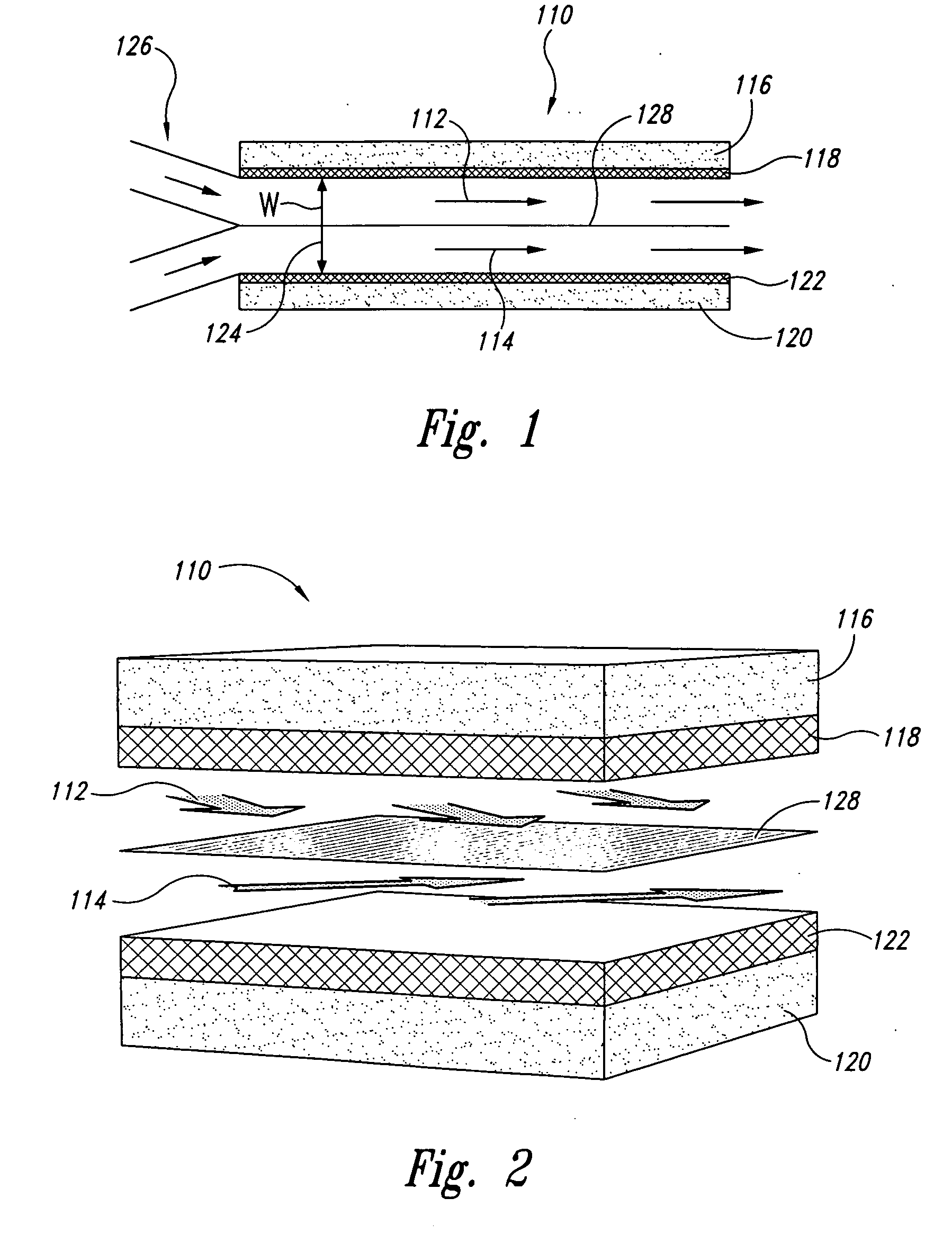
Fuel cells having cross directional laminar flowstreams
The invention disclosed herein relates to fuel cell and electrochemical cells having internal multistream laminar flow and, more specifically, to microfluidic fuel cell and electrochemical cells having two or more adjacent and cross-flowing (i.e., non-parallel) laminar flowstreams positioned within an electrode pair assembly. In one embodiment, an electrochemical cell is disclosed that comprises: a first electrode; a second electrode that opposes the first electrode; and a channel or plenum interposed between and contiguous with at least a portion of the first and second electrodes. The electrochemical cell of this embodiment is configured such that a first fluid enters the channel or plenum and laminarly flows adjacent to the first electrode in a first flow direction, and a second fluid enters the channel or plenum and laminarly flows adjacent to the second electrode in a second flow direction, wherein the first and second flow directions are different from each other.
Owner:EPD INVESTMENT CO
Venturi pumping system in a hydrogen gas circulation of a flow battery
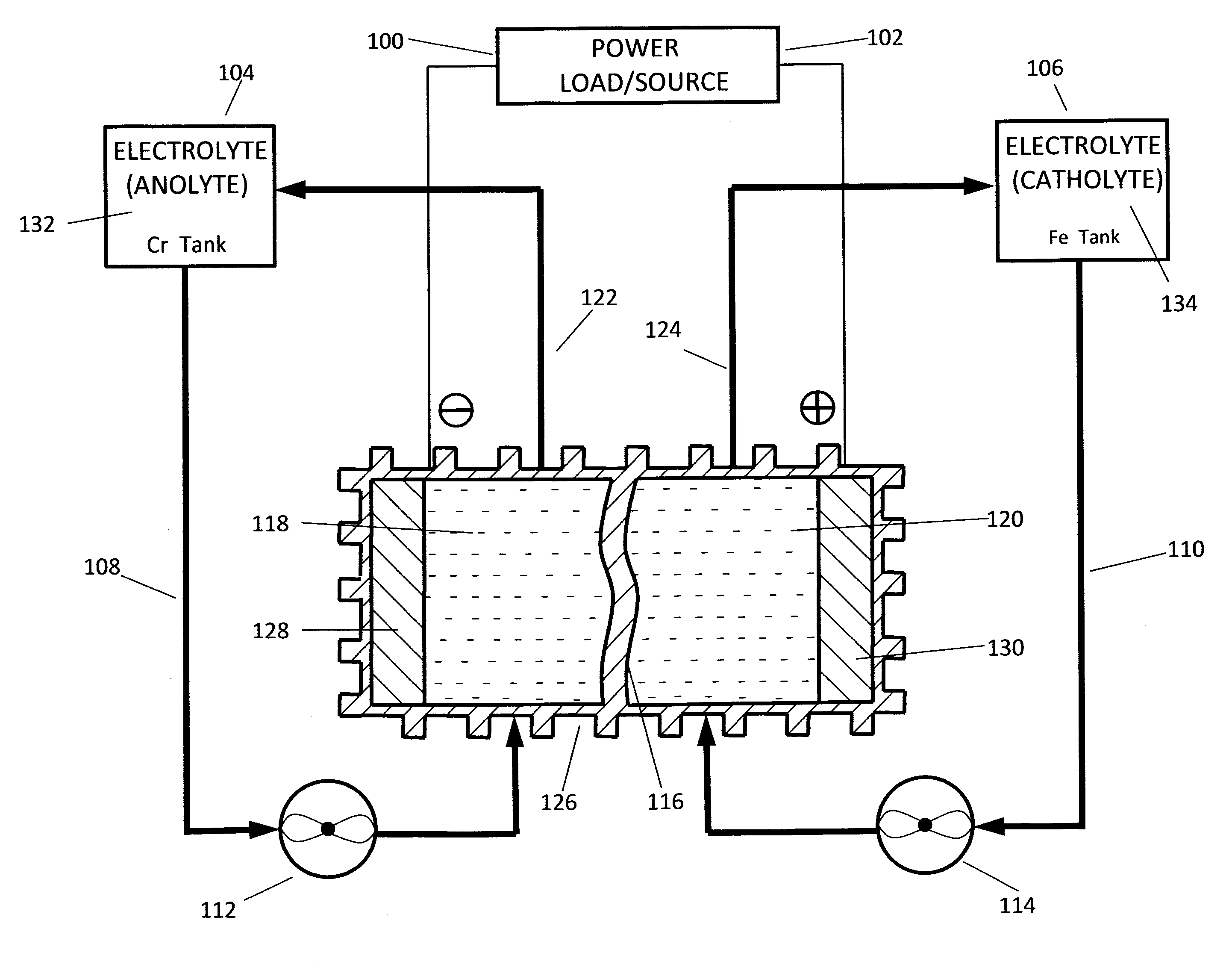
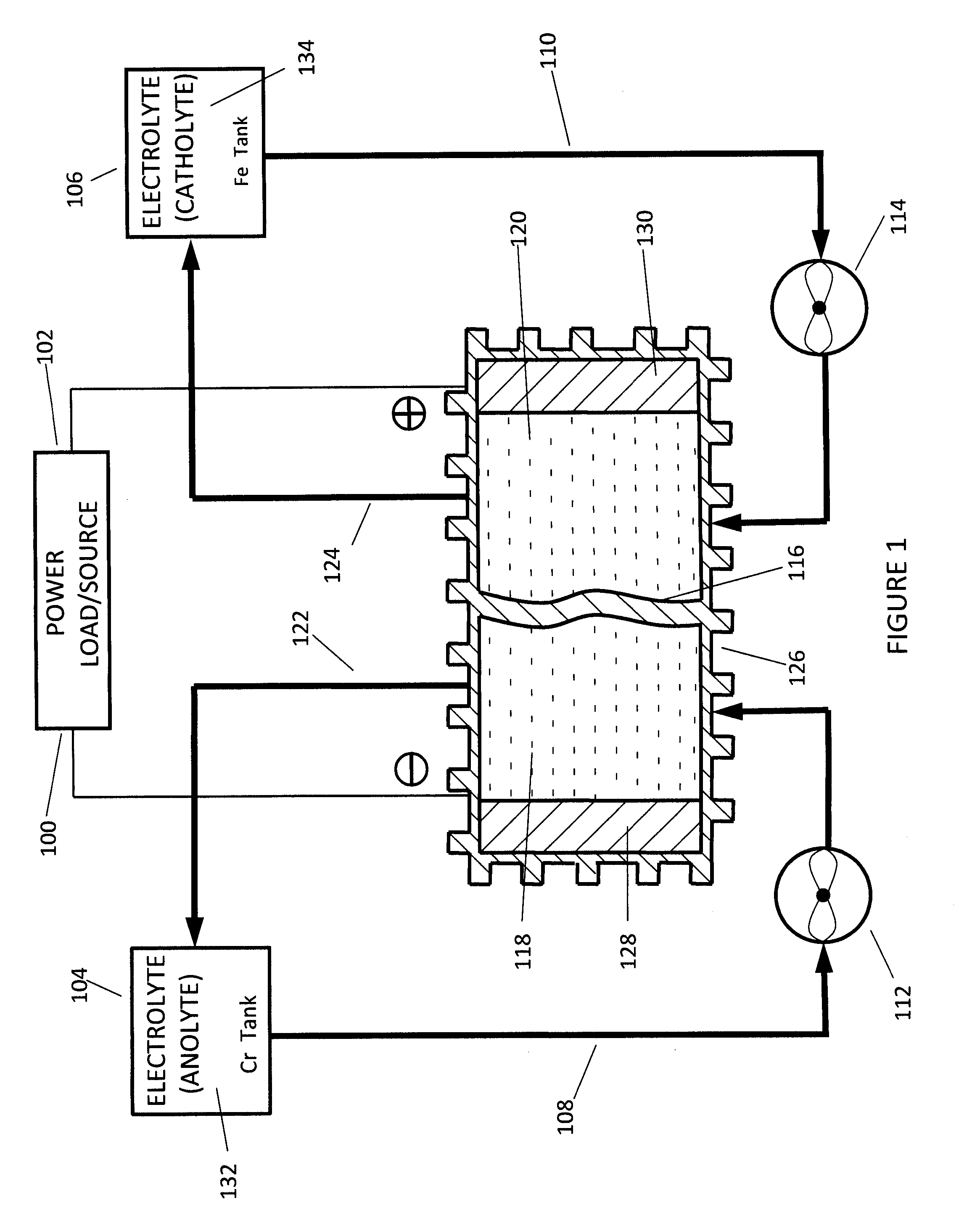
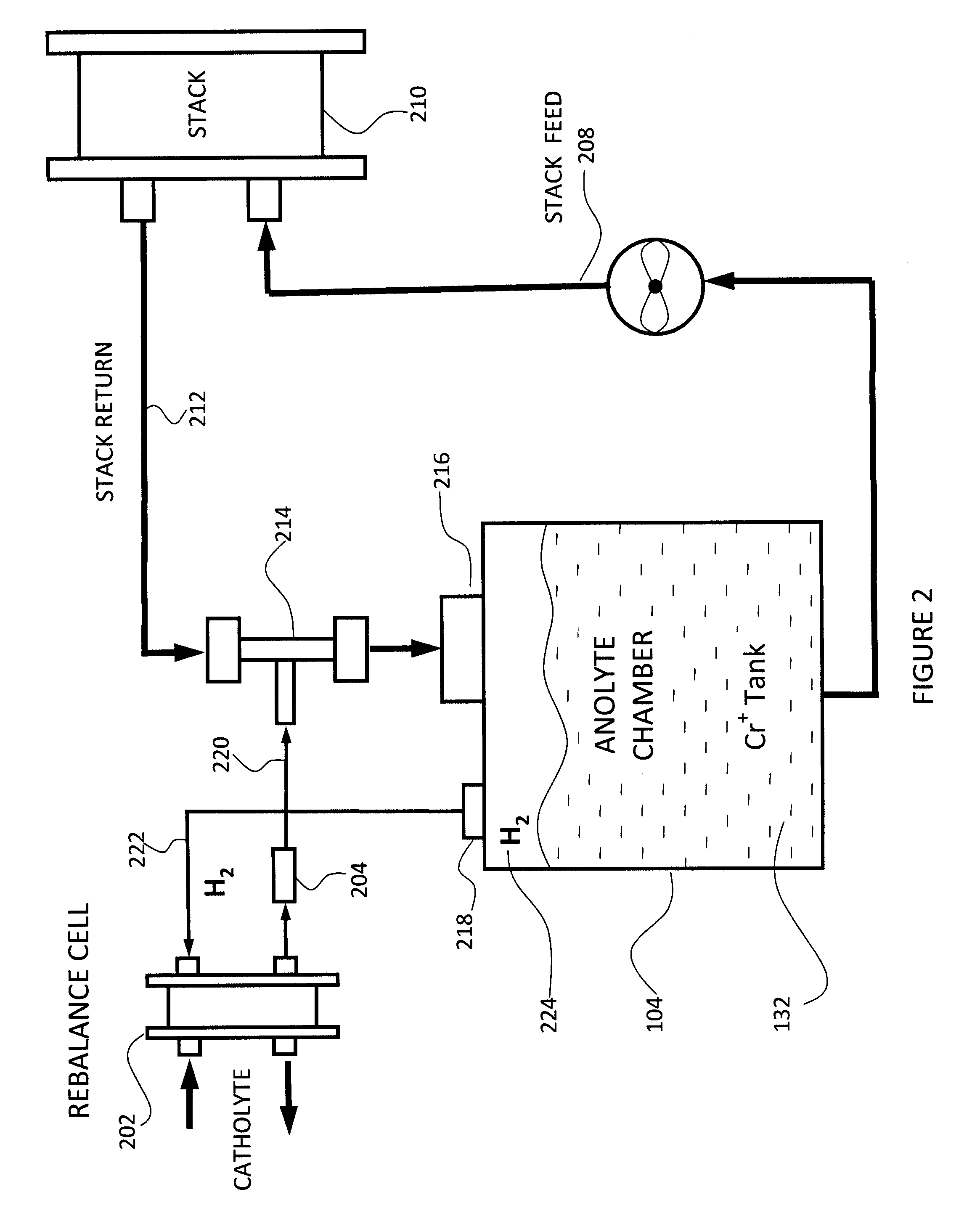
InactiveUS20100092843A1Increase capacity and performanceElectrolyte stream managementIndirect fuel cellsHydrogenRedox
A redox flow battery system is presented that utilizes a rebalancing cell. A pump based on the Venturi principle is coupled to the rebalancing cell in order to actively circulate hydrogen gas through the rebalancing cell. The venturi pump requires no moving parts which eliminates problems of reliability and cost. Utilizing the venturi pump to actively circulate gas can significantly enhanced the function of the rebalance cell thereby providing enhanced capacity and performance of the flow battery system.
Owner:DEEYA ENERGY TECH INC
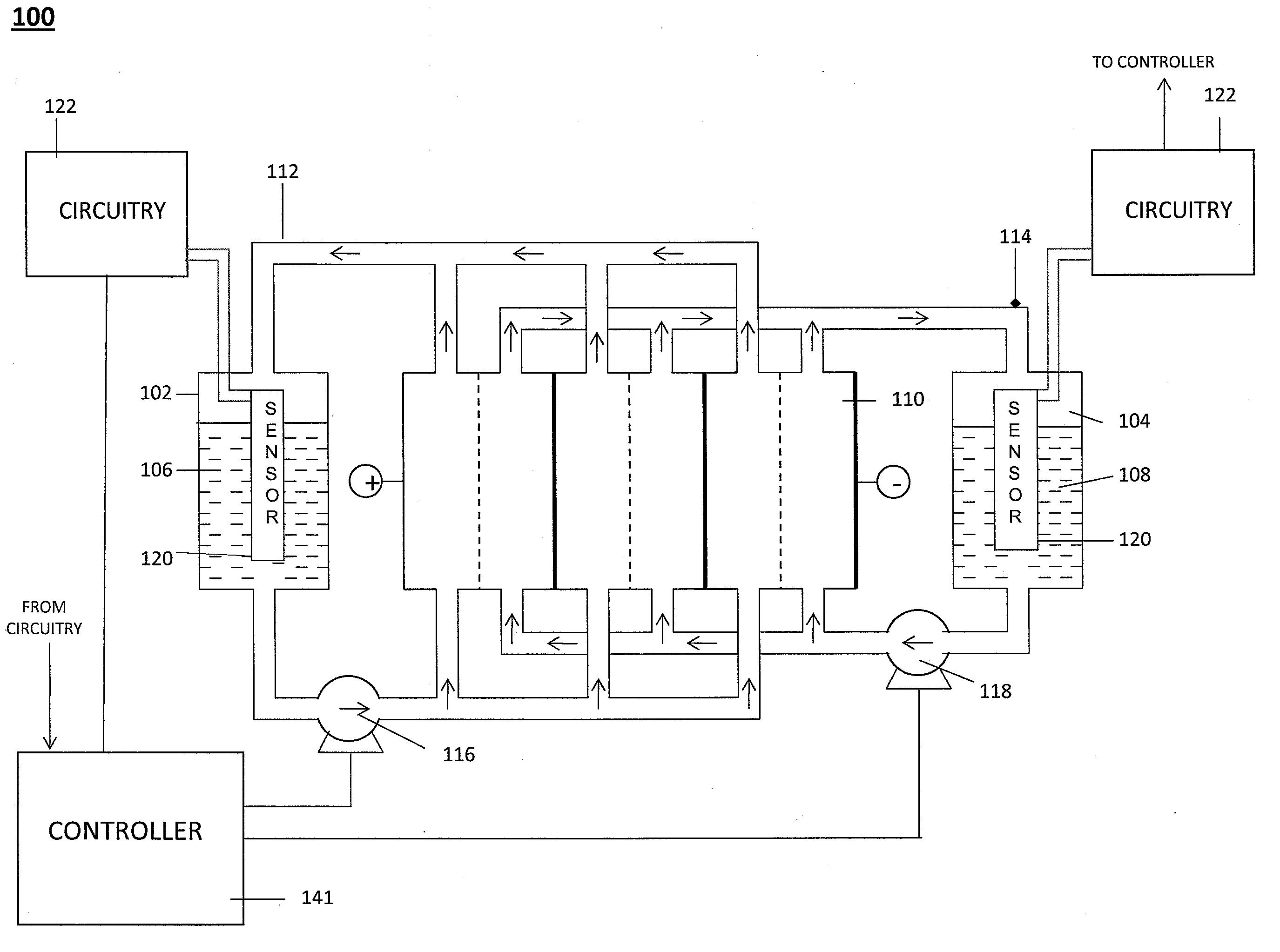
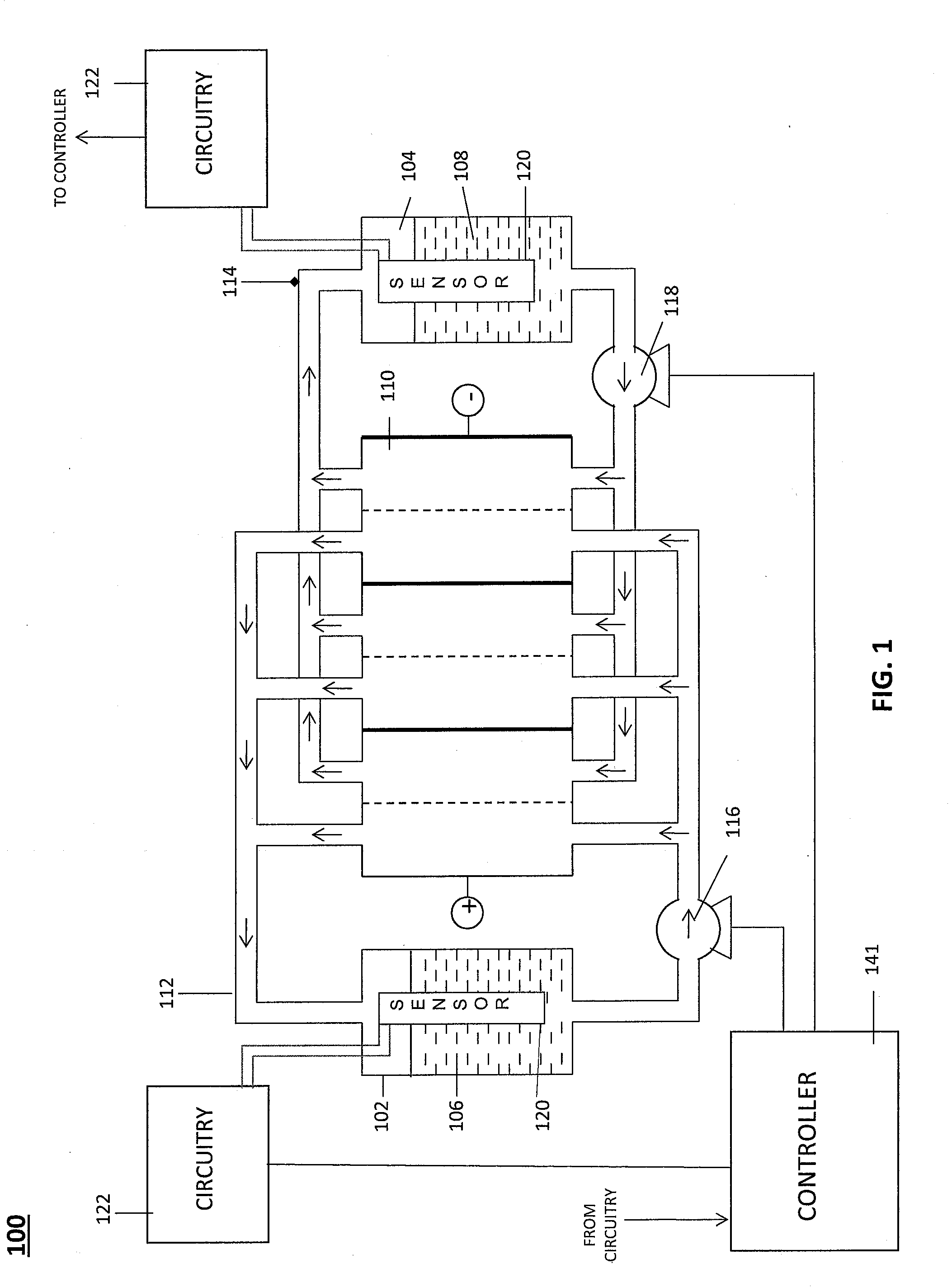
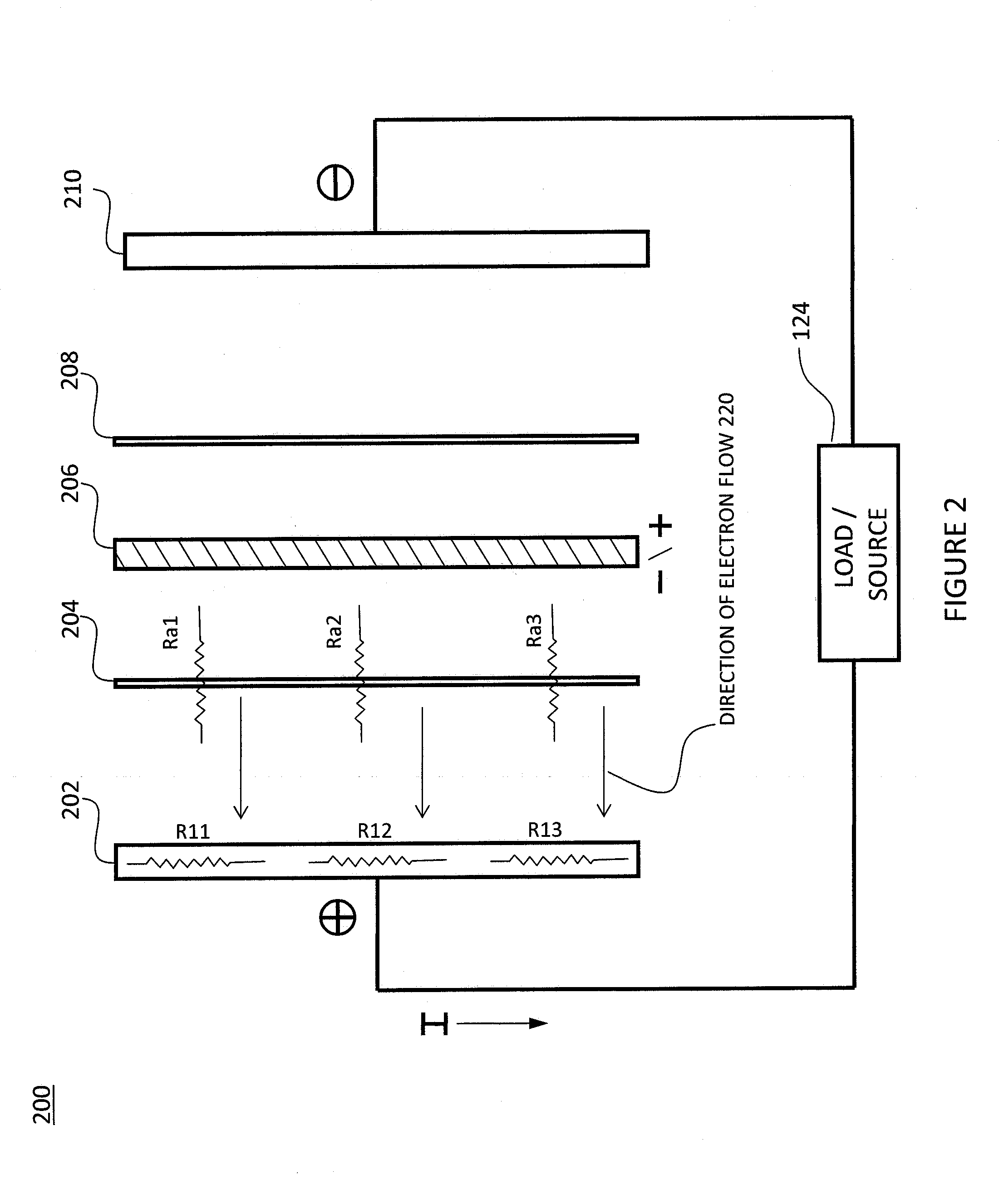
Level Sensor for Conductive Liquids
A sensor for measuring a level of a conductive liquid, is provided. The sensor includes at least two electrodes that can be positioned in a holding tank so as to be partially submerged in the conductive liquid, sensor leads coupled to the at least two electrodes, and circuitry and a controller for determining the properties of the electrolyte, the circuitry being coupled to the at least two electrodes via the sensor leads, and the controller being coupled to the circuitry. The sensor may be used as an electrolyte level sensor in a flow battery system.
Owner:IMERGY POWER SYST
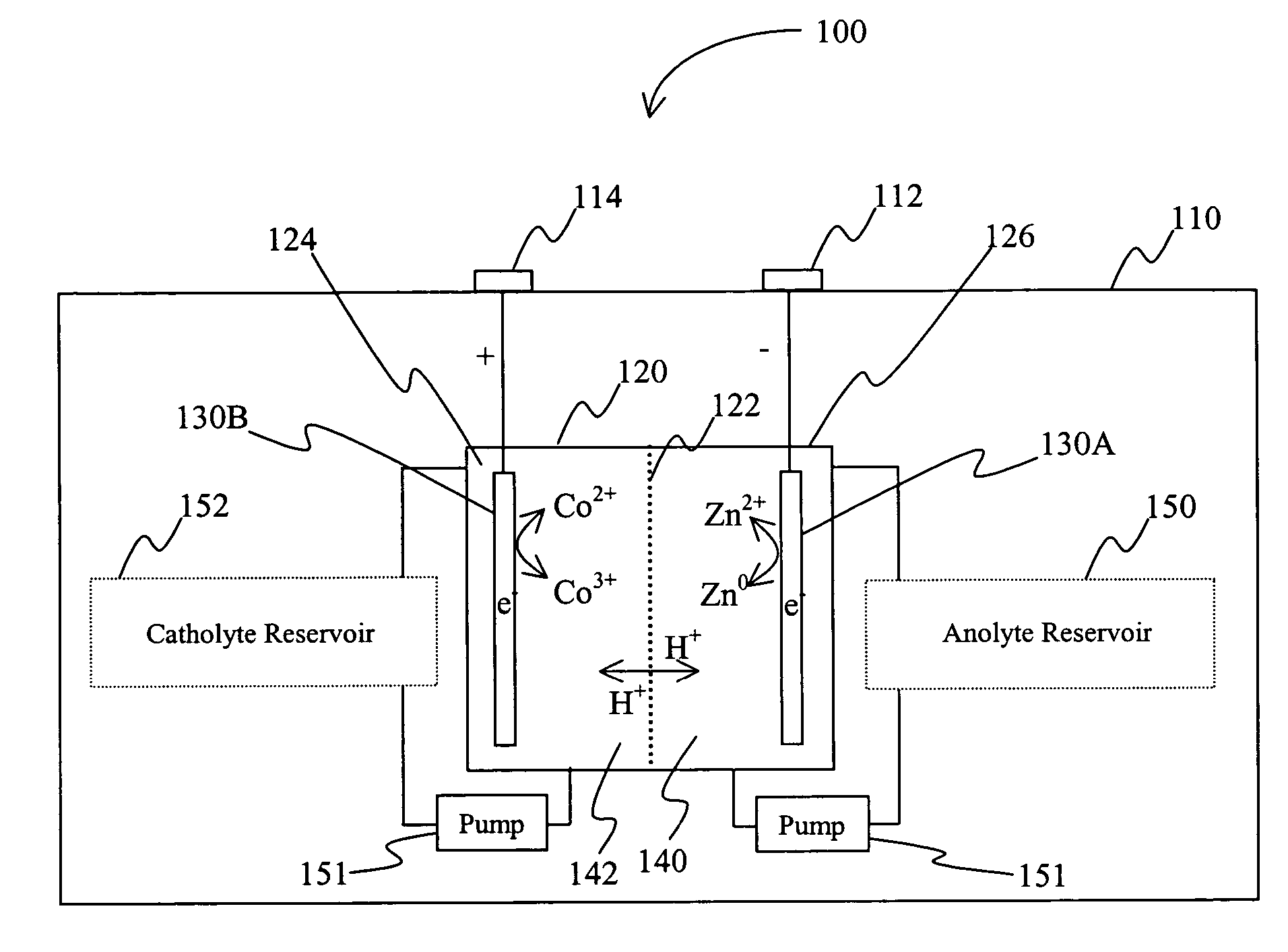
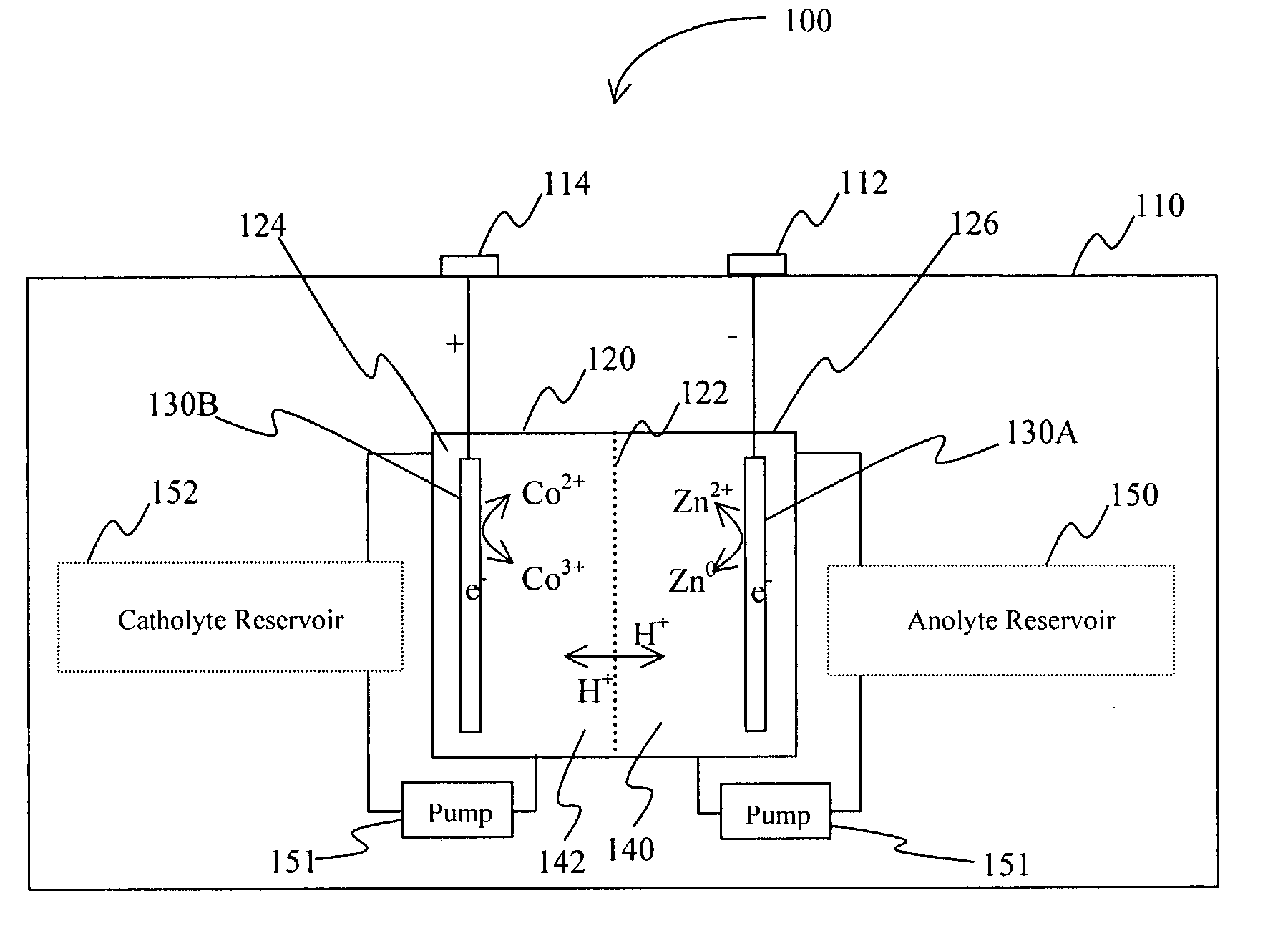
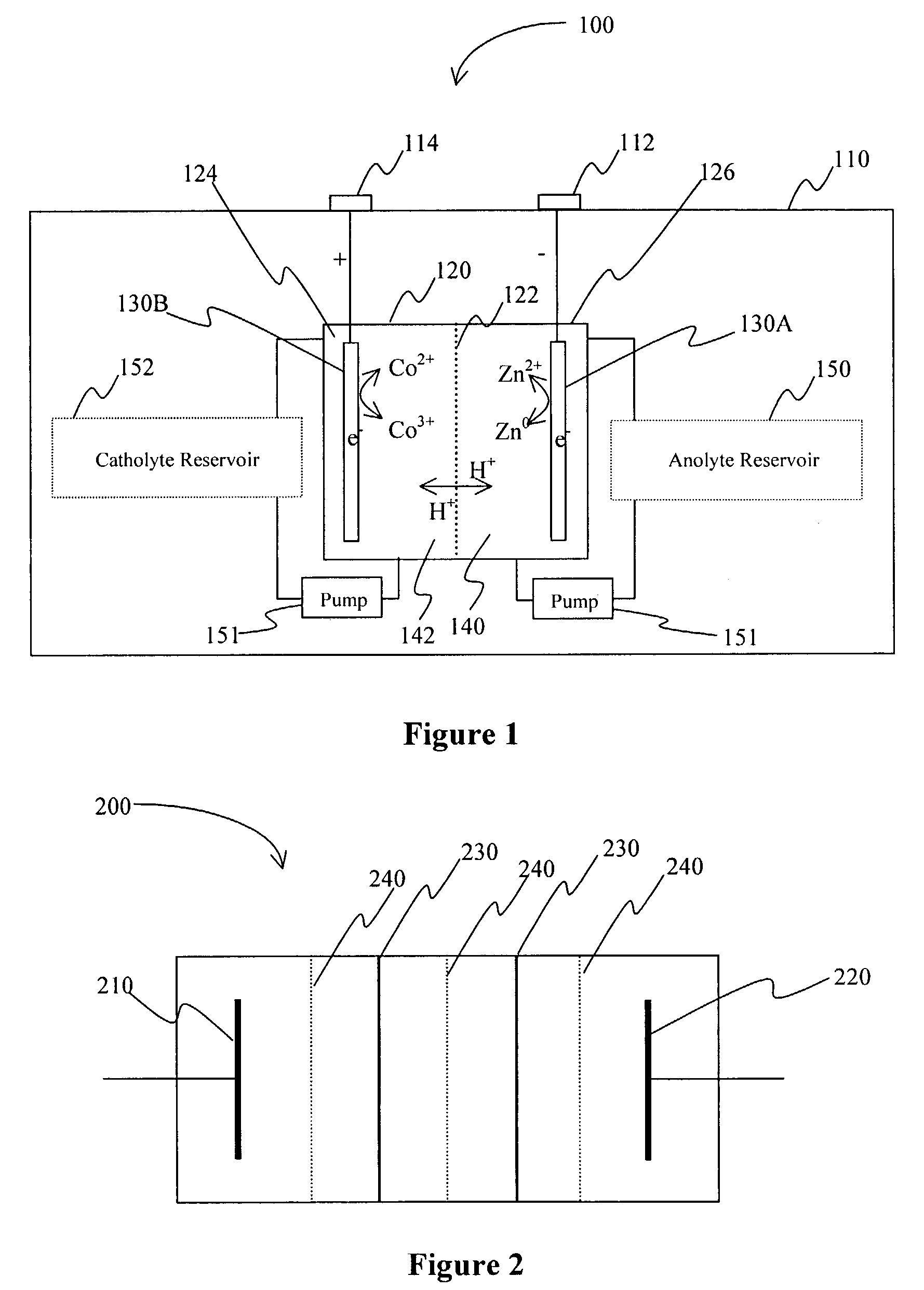
Battery with bifunctional electrolyte
InactiveUS20060063065A1Improve solubilityCell electrodesRegenerative fuel cellsSolubilityOxidation-Reduction Agent
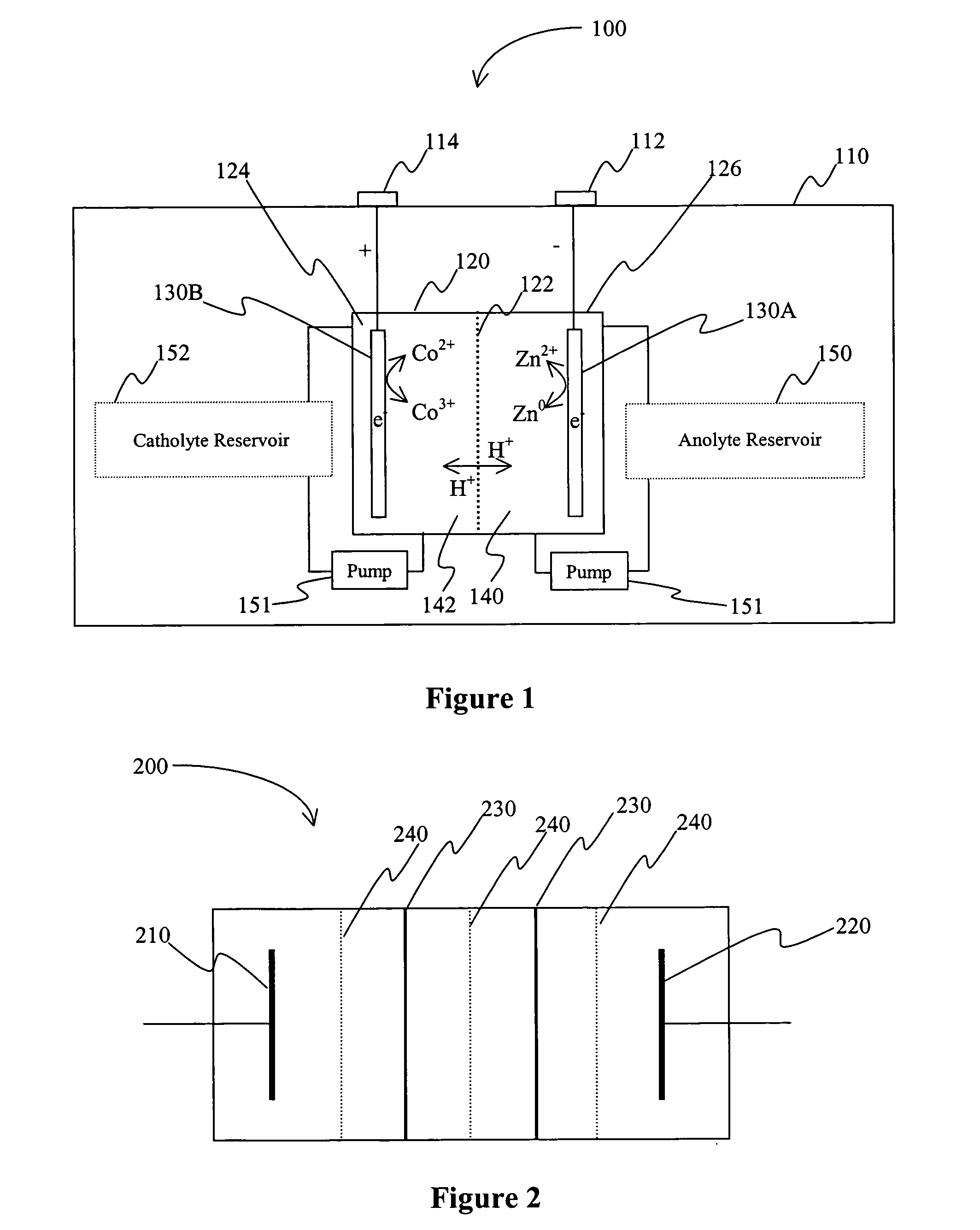
A battery comprises an acid electrolyte in which a compound provides acidity to the electrolyte and further increases solubility of at least one metal in the redox pair. Especially preferred compounds include alkyl sulfonic acids, amine sulfonic acids, and alkyl phosphonic acids, and particularly preferred redox coupled include Co3+ / Zn0, Mn3+ / Zn0, Ce4+ / V2+, Ce4+ / Ti3+, Ce4+ / Zn0, and Pb4+ / Pb0.
Owner:PLURION LTD
Preparation method for commercial vanadium battery electrolyte
ActiveCN103606694AHigh purityLimited service lifeIndirect fuel cellsAcid electrolytesSocial benefitsHydrogen
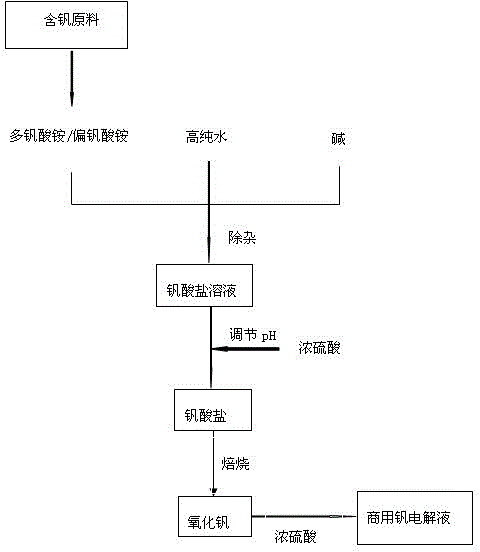
The invention discloses a preparation method for commercial vanadium battery electrolyte. According to the preparation method, vanadium electrolyte is prepared by one step through a chemical method; the preparation method comprises the following steps: dissolving raw materials containing vanadium by using an alkali; adjusting a pH (Potential of Hydrogen) value by acid-alkali regulation; repeatedly precipitating the vanadium to remove impurity elements; then roasting to obtain an oxide of the vanadium; finally, dissolving the oxide of the vanadium by using concentrated sulfuric acid to obtain the vanadium electrolyte. With the adoption of the method, impurities including iron, chrome, silicon, manganese and the like can be removed effectively and a process flow is shortened; the vanadium yield is improved, the impurity content is reduced and the process flow is shortened; the product purity can also be improved. The preparation method for the commercial vanadium battery electrolyte has important meanings on production technology levels and market competitiveness of the vanadium electrolyte; the method has good economic benefits and social benefits.
Owner:HEBEI IRON AND STEEL
Novel vanadium halide redox flow battery
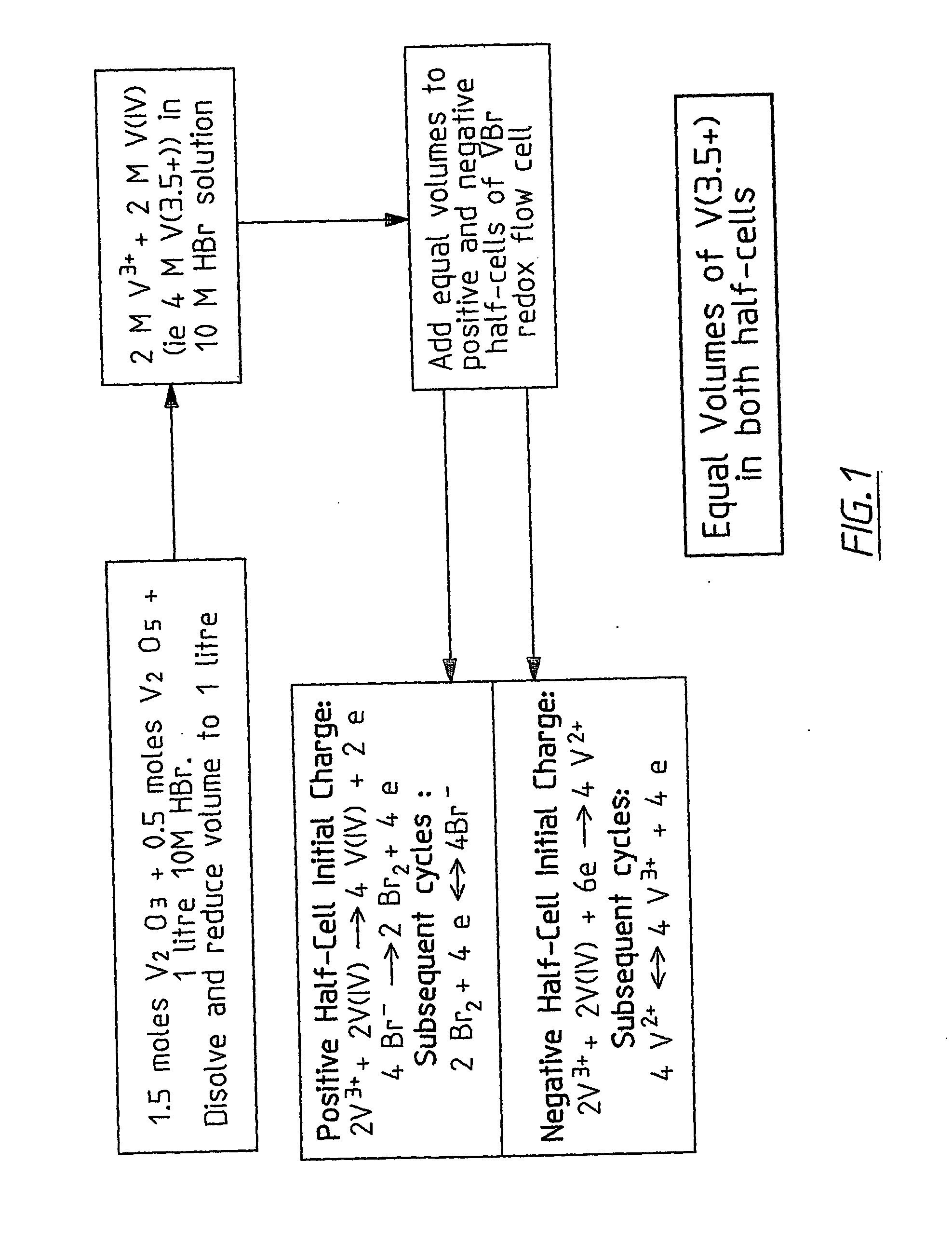
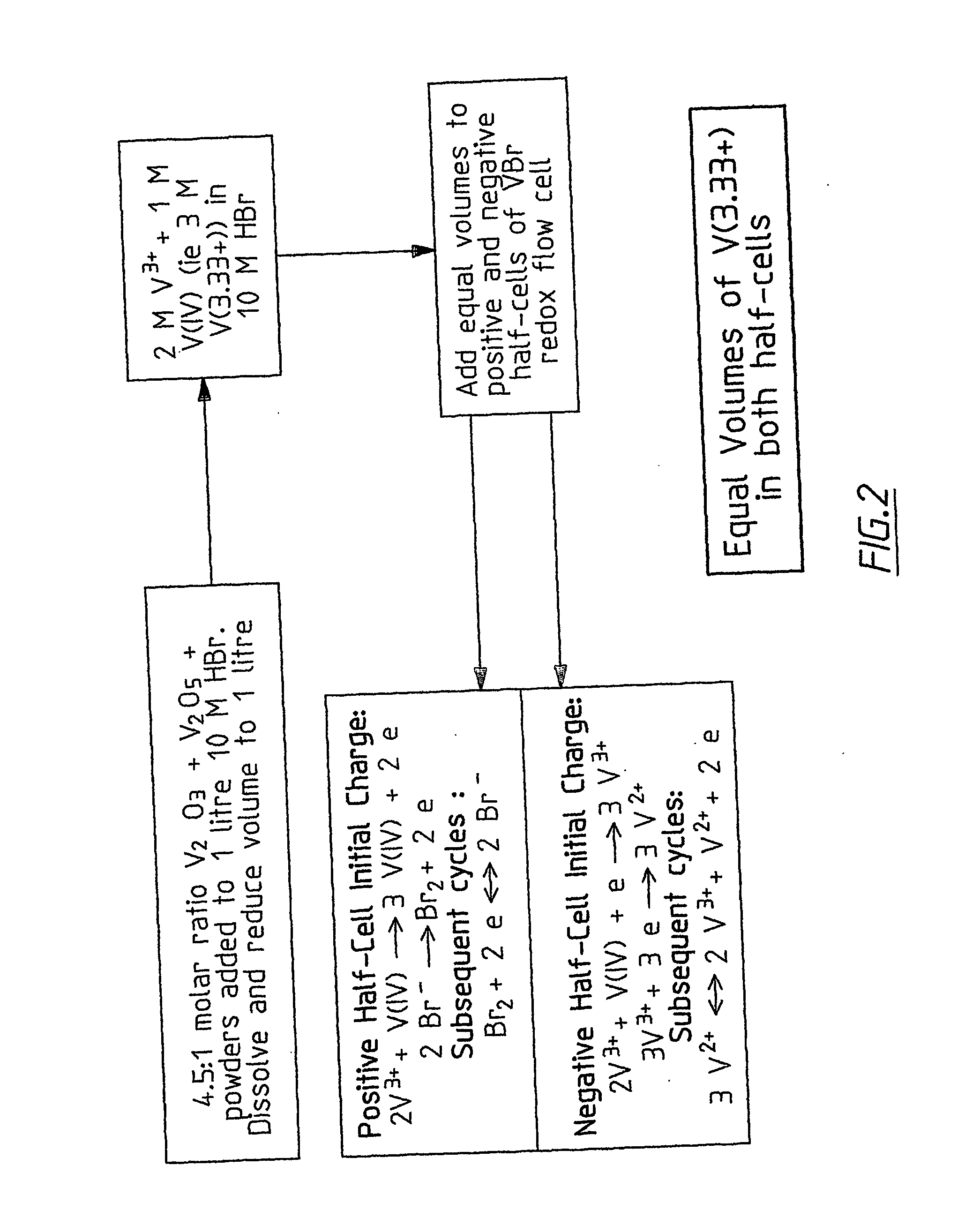
InactiveUS20060183016A1Avoid excessive bromine generationStabilise the bromine producedCharging stationsCell electrodesRedoxPhysical chemistry
A prior to charge vanadium halide redox cell, a vanadium halide redox cell which is at a state of charge selected from the group consisting of a zero state of charge and a near zero state of charge and vanadium halide redox cell which are fully charged and partially charged are described. The prior to charge vanadium halide redox cell comprises a positive half cell containing a positive half cell solution comprising a halide electrolyte, vanadium (III) halide and vanadium (IV) halide, a negative half cell containing a negative half cell solution comprising a halide electrolyte, vanadium (III) halide and vanadium (N) halide wherein the amounts of vanadium (III) halide, vanadium (IV) halide and halide ions in the positive and negative half cell solutions are such that in a first charging step comprising charging the prior to charge vanadium halide redox cell, a vanadium halide redox cell having a state of charge selected from the group consisting of a zero state of charge and a near zero state of charge comprising predominantly vanadium (N) halide in the positive half cell solution and predominantly V(III) halide in the negative half cell solution can be prepared. The vanadium halide redox cell which is at a state of charge selected from the group consisting of a zero state of charge and a near zero state of charge comprises a positive half cell containing a positive half cell solution comprising a halide electrolyte and a vanadium halide which is predominantly vanadium (N) halide, a negative half cell containing a negative half cell solution comprising a halide electrolyte and a vanadium halide which is predominantly vanadium (III) halide wherein the amount of vanadium (N) halide in the positive half cell solution and the amount of vanadium (III) halide in the negative half cell solution are such that the vanadium halide redox cell is at a state of charge selected from the group consisting of a zero state of charge and a near zero state of charge. The vanadium halide redox cell which is fully charged comprises a positive half cell containing a positive half cell solution comprising a halide electrolyte, a polyhalide complex, vanadium (IV) halide and vanadium (V) halide, a negative half cell containing a negative half cell solution comprising a halide electrolyte and vanadium (II) halide wherein the molar concentration of vanadium (V) and polyhalide complex:molar concentration of vanadium (II) halide is about stoichiometrically balanced. The vanadium halide redox cell which is partially charged comprises a positive half cell containing a positive half cell solution comprising a halide electrolyte, a polyhalide complex, vanadium (IV) halide and vanadium (V) halide, a negative half cell containing a negative half cell solution comprising a halide electrolyte, vanadium (II) halide and vanadium (III) halide wherein the number of moles of moles of polyhalide complex and vanadium (V): number of moles of vanadium (II) halide is about stoichiometrically balanced.
Owner:NEWSOUTH INNOVATIONS PTY LTD
Battery with bifunctional electrolyte
A battery comprises an acid electrolyte in which a compound provides acidity to the electrolyte and further increases solubility of at least one metal in the redox pair. Especially preferred compounds include alkyl sulfonic acids and alkyl phosphonic acids, and particularly preferred redox coupled include Co3+ / Zn0, Mn3+ / Zn0, Ce4+ / V2+, Ce4+ / Ti3+, Ce4+ / Zn0, and Pb4+ / Pb0.
Owner:ITI SCOTLAND +1
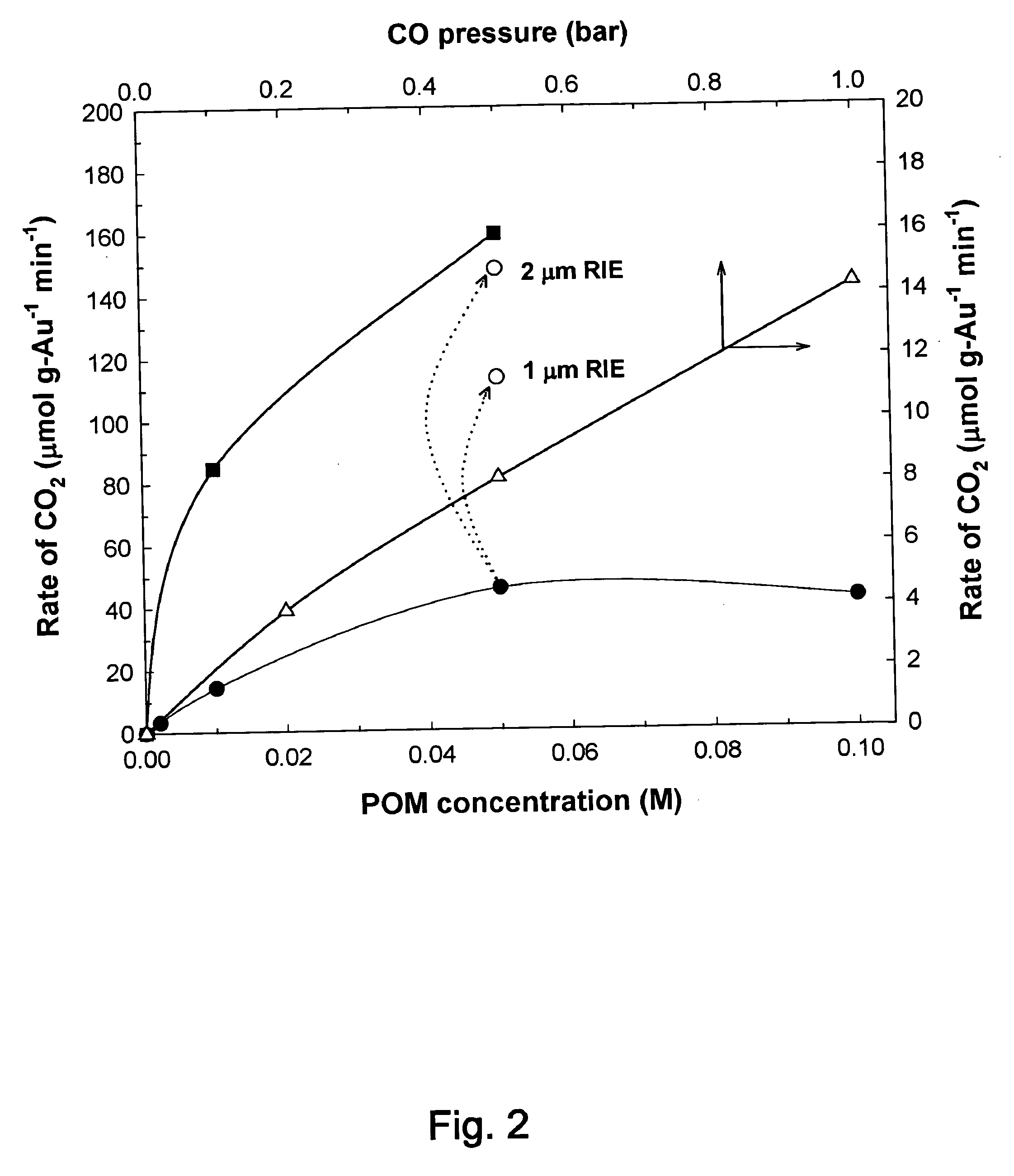
Catalytic method to remove CO and utilize its energy content in CO-containing streams
InactiveUS20060024539A1Low costUse minimizedMaterial nanotechnologyGas treatmentFuel cellsCatalytic method
Disclosed are a reactor and a corresponding method for producing electrical energy using a fuel cell by selectively oxidizing CO at room temperature using polyoxometalate compounds and transition metal compounds over metal-containing catalysts, thereby eliminating the water-gas shift reaction and the need to transport and vaporize liquid water in the production of H2 for fuel cells. The reactor also functions to deplete CO from an incoming gas stream.
Owner:WISCONSIN ALUMNI RES FOUND
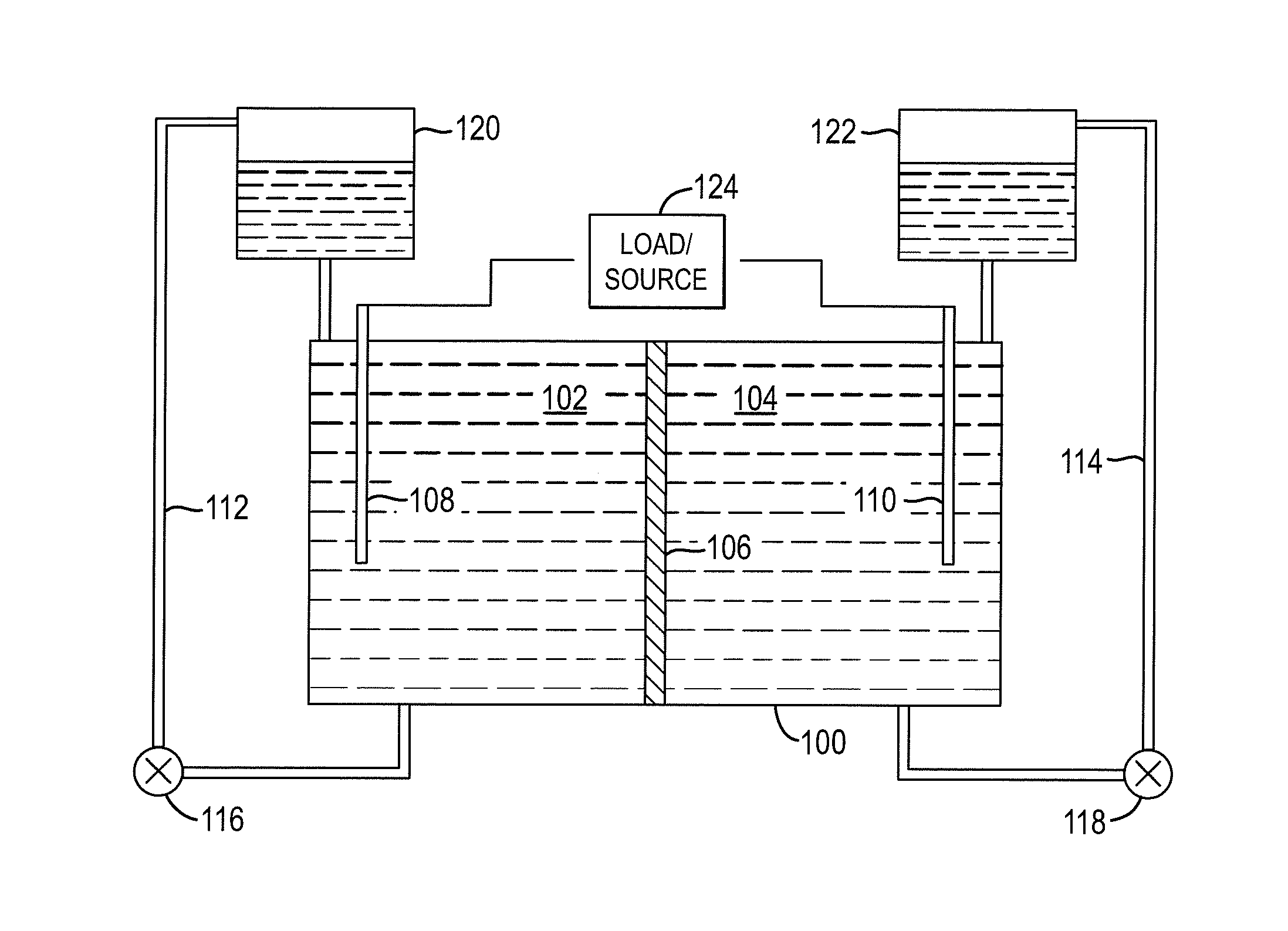
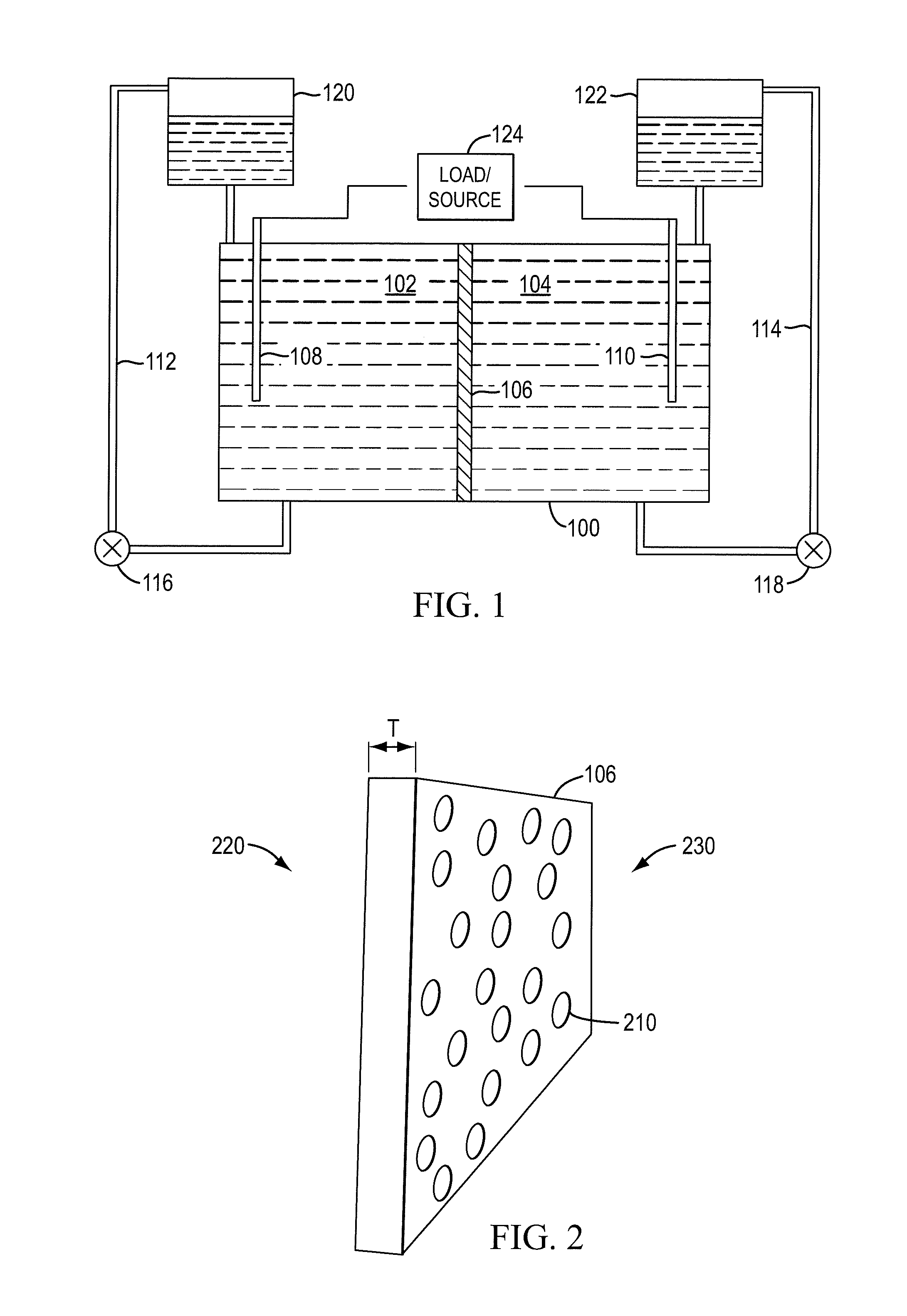
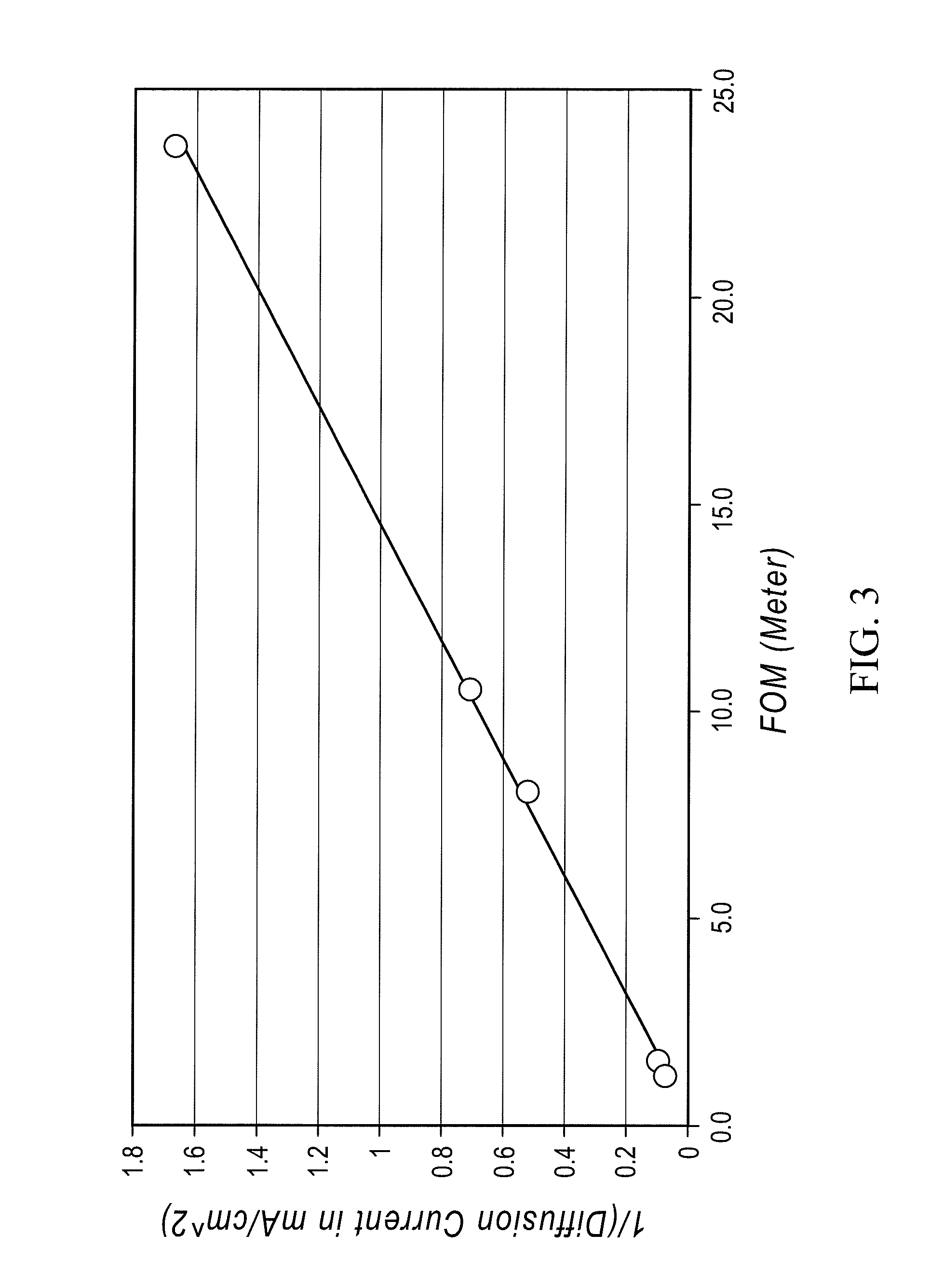
Redox flow cell
A redox flow cell is presented that utilizes a porous membrane separating a first half cell and a second half cell. The porous membrane is chosen to have a figure of merit (FOM) is at least a minimum FOM. A method of providing a porous membrane for a flow cell can include determining a figure of merit; determining a first parameter from a pore size or a thickness for the porous membrane; determining a second parameter from the pore size or the thickness that is not the first parameter for the porous membrane, based on the figure of merit; and constructing a porous membrane having the pore size and the thickness.
Owner:IMERGY POWER SYST
Flow battery having a low resistance membrane
A flow battery includes a membrane having a thickness of less than approximately one hundred twenty five micrometers; and a solution having a reversible redox couple reactant, wherein the solution wets the membrane.
Owner:UNITED TECH CORP
Additives for non-aqueous electrolyte and lithium secondary battery using the same
ActiveUS20070166609A1Improved overcharge stabilityCell electrodesOrganic electrolyte cellsOrganic solventLithium-ion battery
Disclosed is an electrolyte for batteries, comprising: (a) an electrolyte salt; (b) an organic solvent; (c) a first compound having an oxidation initiation voltage (vs. Li / Li+) higher than the operating voltage of a cathode; and (d) a second reversible compound having an oxidation initiation voltage higher than the operating voltage of the cathode, but lower than the oxidation initiation voltage of the first compound. Also disclosed is a lithium secondary battery comprising said electrolyte. In the lithium secondary battery, two compounds having different safety improvement actions at a voltage higher than the operating voltage of the cathode are used in combination as electrolyte components. Thus, the safety of the secondary battery in an overcharged state can be ensured, and at the same time, the deterioration of the battery can be prevented from occurring when it is repeatedly cycled, continuously charged and stored at high temperature for a long time.
Owner:LG ENERGY SOLUTION LTD
Vanadium redox battery energy storage and power generation system incorporating and optimizing diesel engine generators
ActiveUS20050156431A1Batteries circuit arrangementsLevel controlVanadium redox batteryElectrical battery
A power generation system includes a vanadium redox battery that interfaces with a control system to optimize performance and efficiency. The power generation system may include one or more wind turbine generators and one or more diesel fuel generators. The control system manages the vanadium redox battery's absorption and power generation to control system stability and system frequency. The control system further manages the operation of the wind turbine generators and diesel fuel generators to control system stability and voltage.
Owner:VRB ENERGY INC
Method for designing redox flow battery system
InactiveUS20050181273A1Improve system efficiencySure easyCell electrodesFuel cell auxillariesOxidation-Reduction AgentRedox
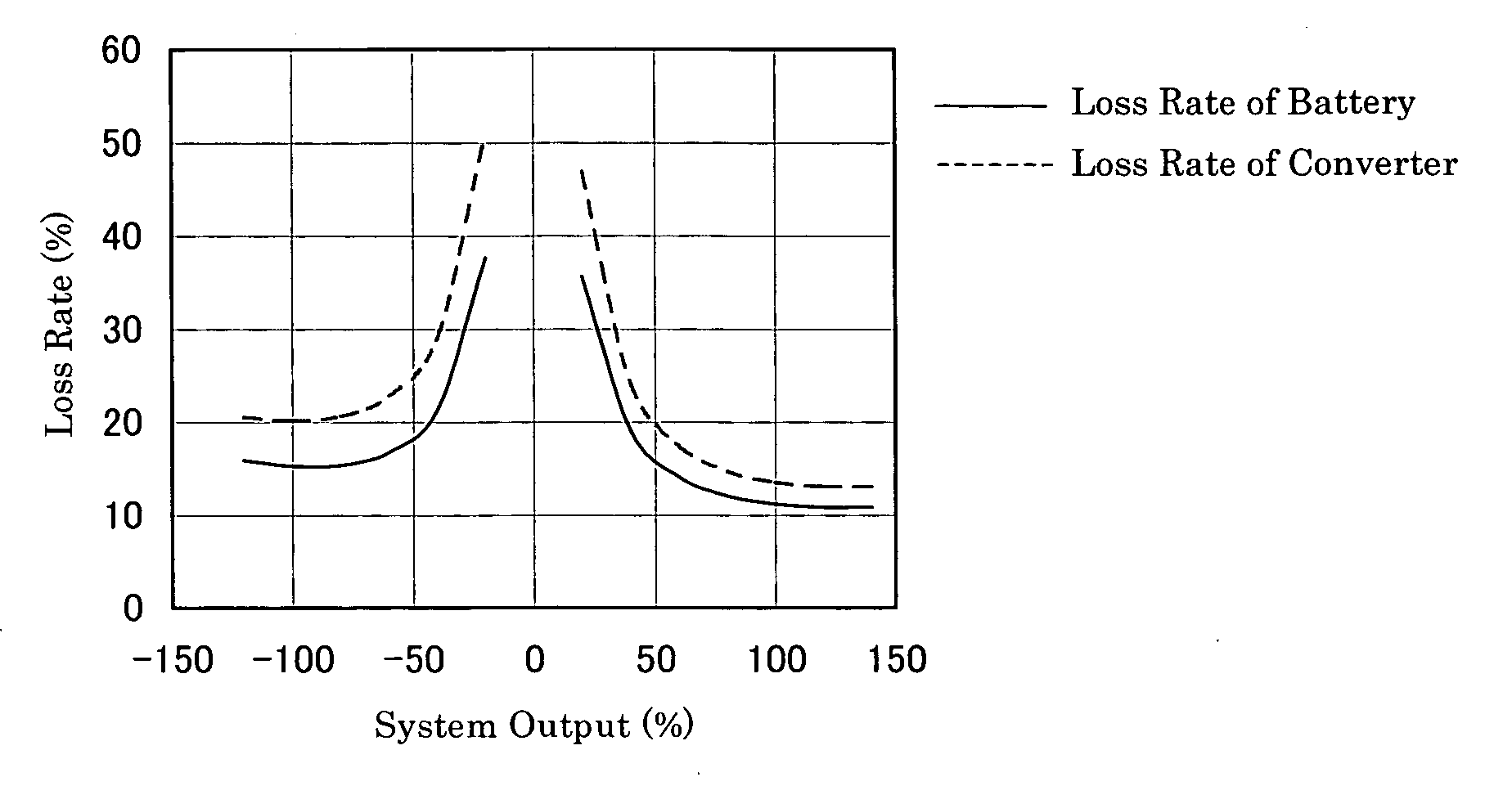
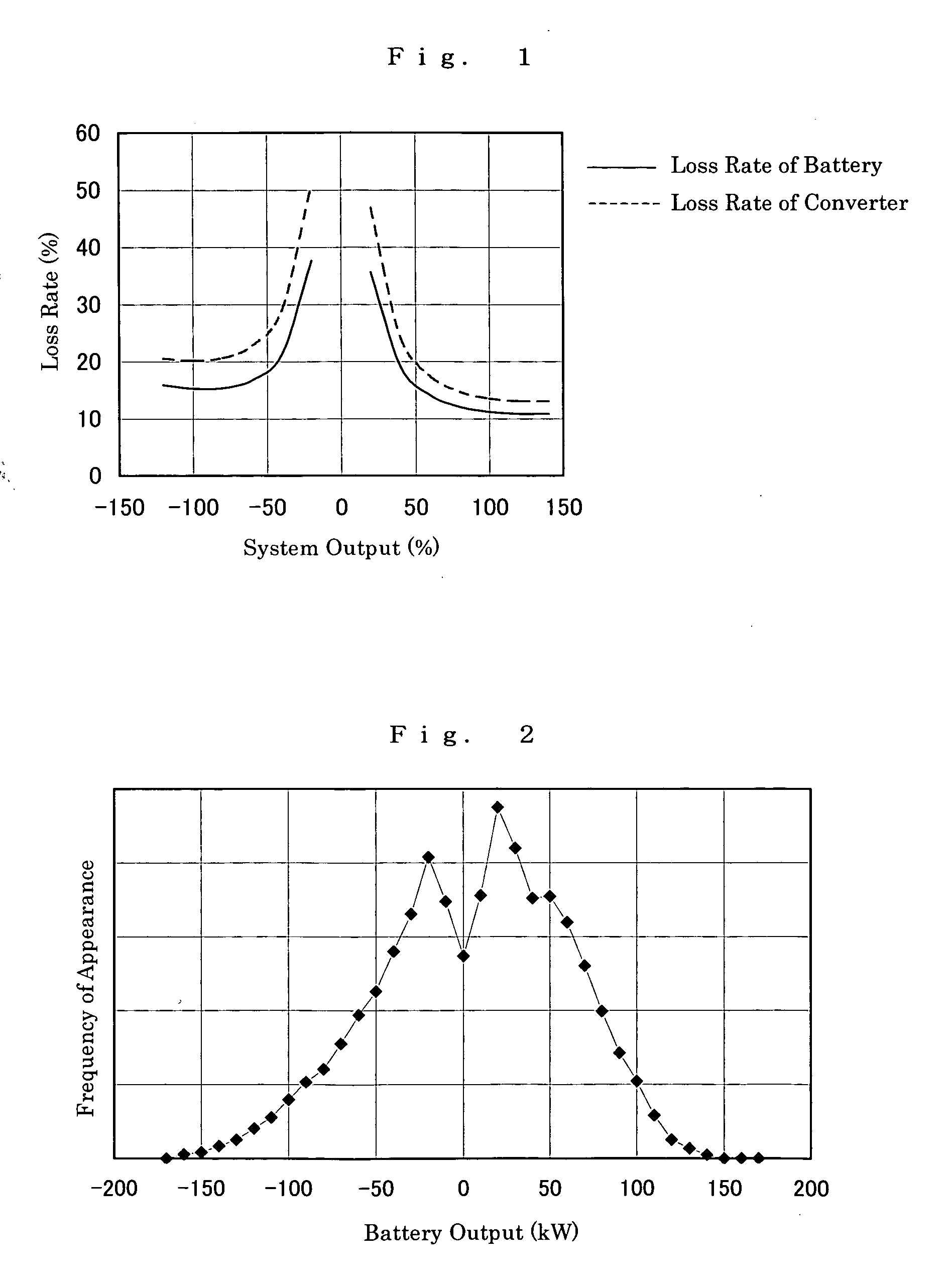
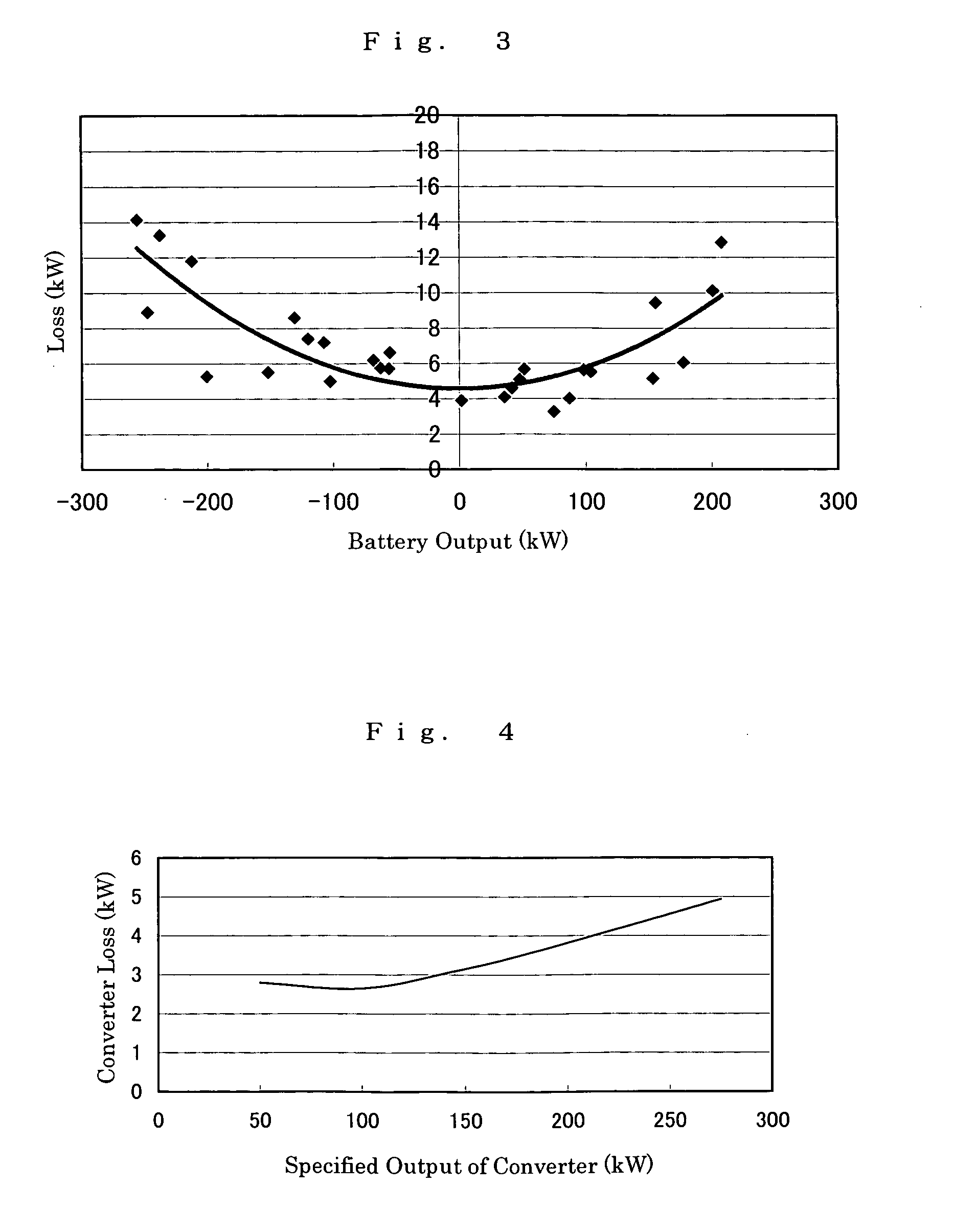
The present invention provides a method of designing a redox flow battery system that can prevent system efficiency loss caused by weak generation power or load power at the time of electric charge or discharge, without using any lead storage battery, and can also provide further improved system efficiency. In the present invention, generating equipment that varies irregularly in output of power generation is provided with the redox flow battery to smooth the output of power generation. An average value of output distribution of the battery with respect to the smoothed output of power generation and standard deviation are determined. Then, at least either of a specified output of the battery and a specified output of the converter for converting the battery output is determined based on the standard deviation.
Owner:SUMITOMO ELECTRIC IND LTD +1
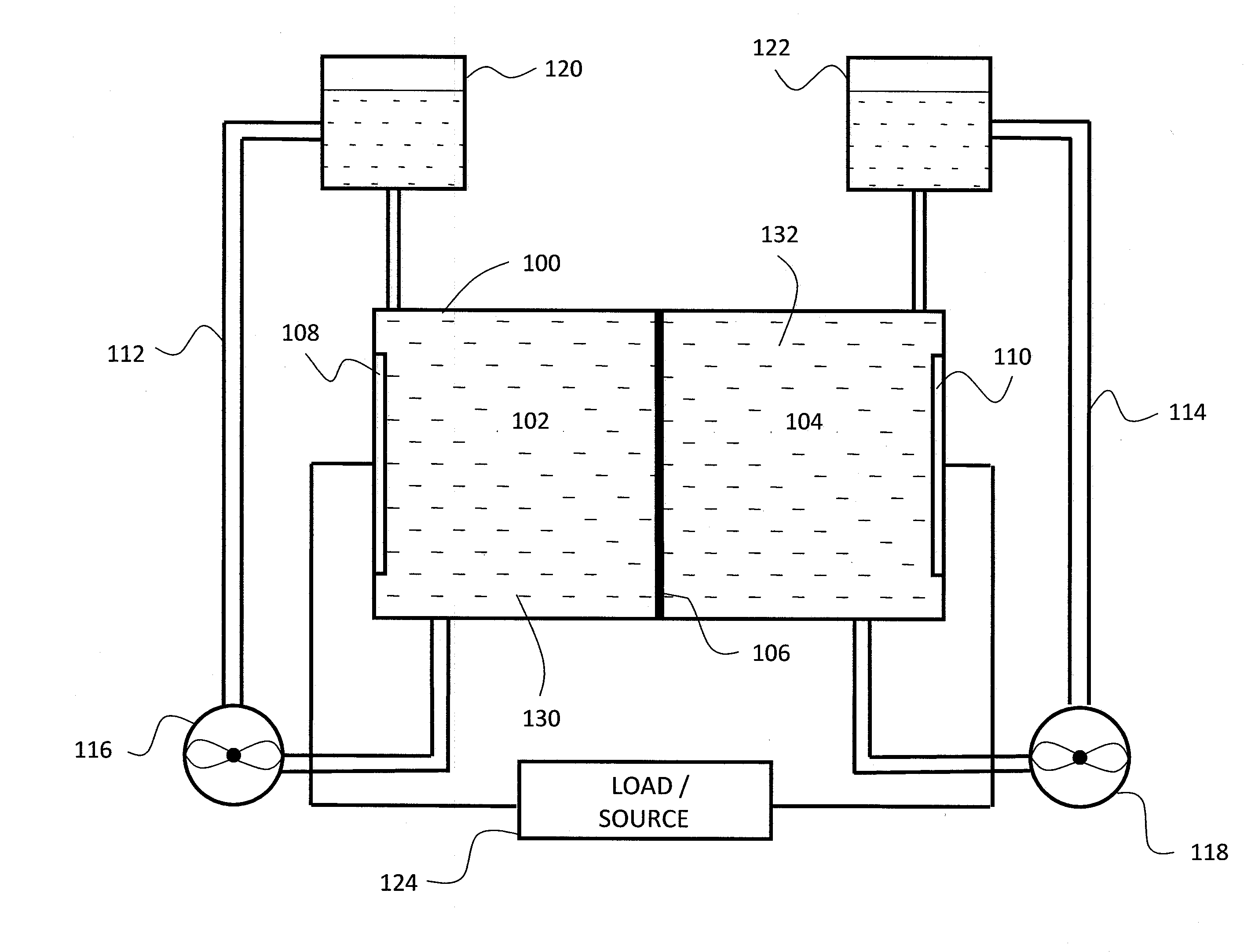
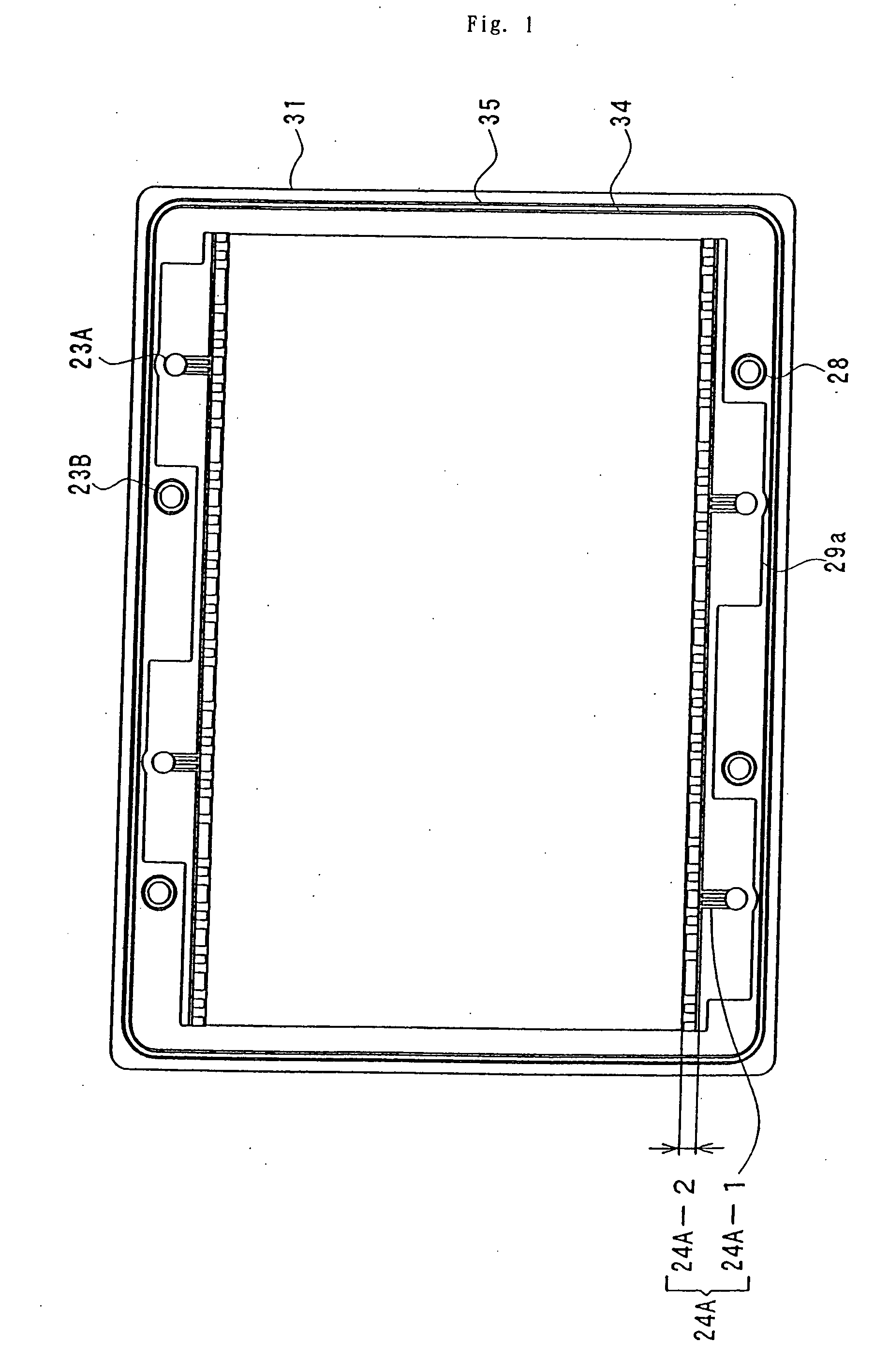
Cell frame for redox flow battery, and redox flow battery
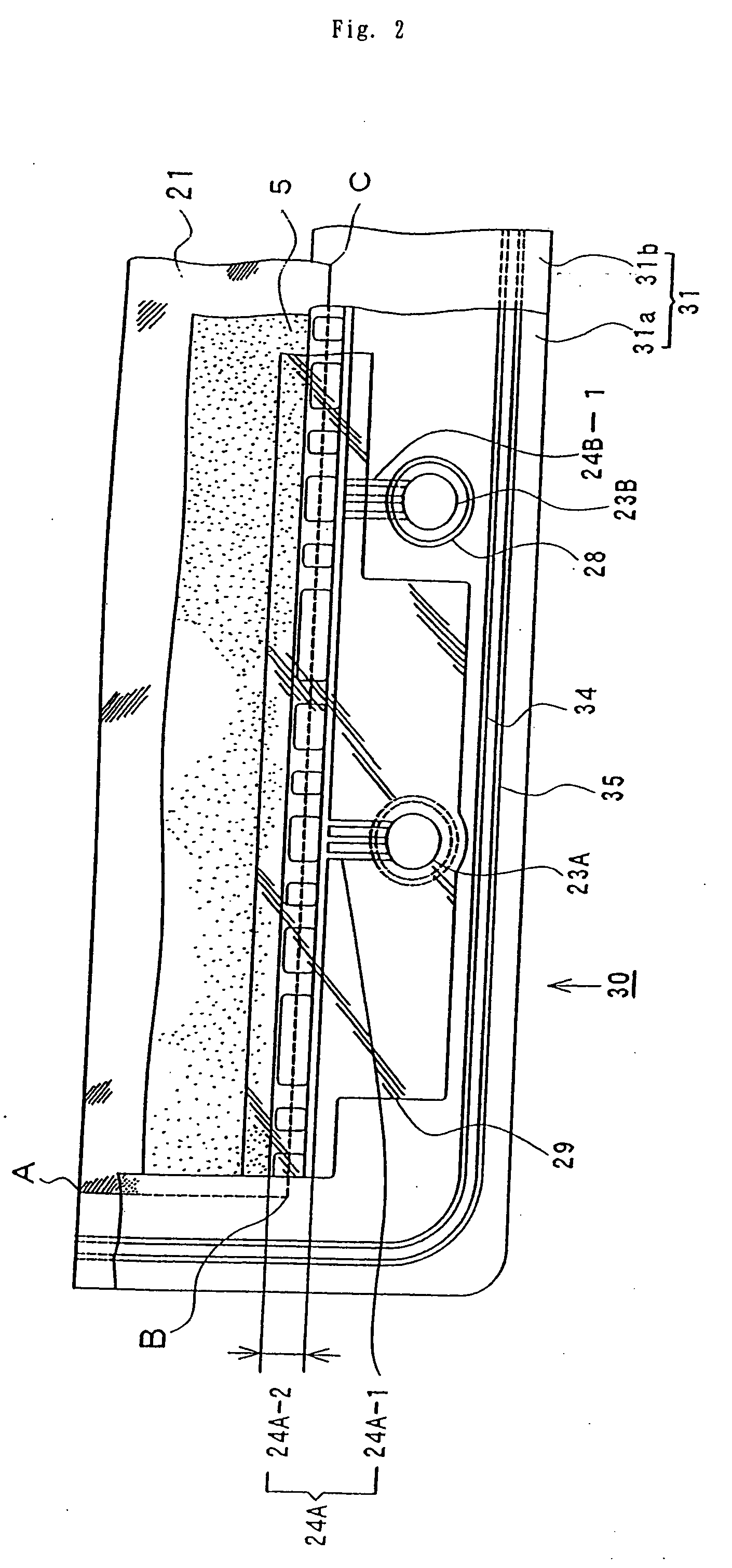
InactiveUS20080081247A1Avoid breakingPrevent a break in the membraneElectrolyte holding meansEngine sealsElectrolyte leakageOxidation-Reduction Agent
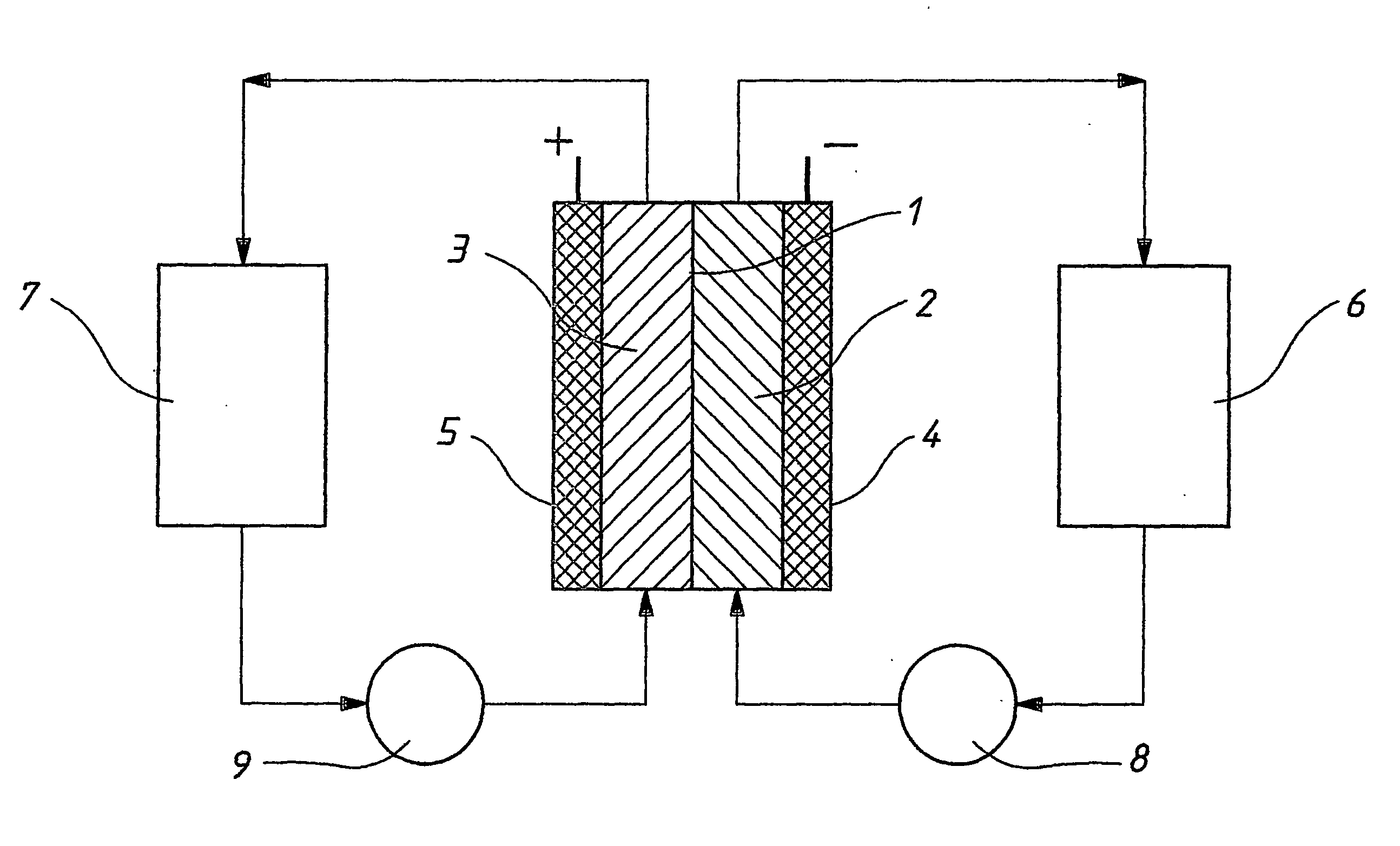
This invention provides a cell frame for a redox flow battery that prevents leakage of electrolyte out of the cell frame and also provides a good workability in assembling the redox flow battery. Also, this invention provides a redox flow battery using the cell frame. In the cell frame 30 for the redox flow battery 30 comprising a bipolar plate 21 and a frame 31 fitted around a periphery of the bipolar plate 21, the frame 31 has, on each side thereof, an inner seal and an outer seal to press-contact with a membrane and also seal electrolyte. The frame 31 has, on each side thereof, an inner seal groove 34 and an outer seal groove 35 for placing therein the inner seal and the outer seal, respectively, to prevent the electrolyte from leaking out, and O-rings are placed in the respective seal grooves.
Owner:SUMITOMO ELECTRIC IND LTD +1
Vanadium/polyhalide redox flow battery
InactiveUS7320844B2Minimise potential cross-contaminationCell electrodesRegenerative fuel cellsElectricityRedox
The invention relates to a redox flow cell containing a polyhalide / halide redox couple in the positive half-cell electrolyte and a V(III) / V(II) redox couple in the negative half-cell electrolyte. The invention also relates to a method of producing electricity by discharging the fully charged or partially charged redox flow cell, and a method of charging the discharged or partially discharged redox flow cell.
Owner:WATTJOULE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com