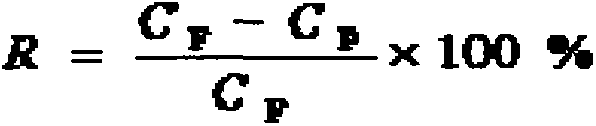Patents
Literature
650 results about "Supporting electrolyte" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A supporting electrolyte, in electrochemistry, according to an IUPAC definition, is an electrolyte containing chemical species that are not electroactive (within the range of potentials used) and which has an ionic strength and conductivity much larger than those due to the electroactive species added to the electrolyte. Supporting electrolyte is also sometimes referred to as inert electrolyte or inactive electrolyte.
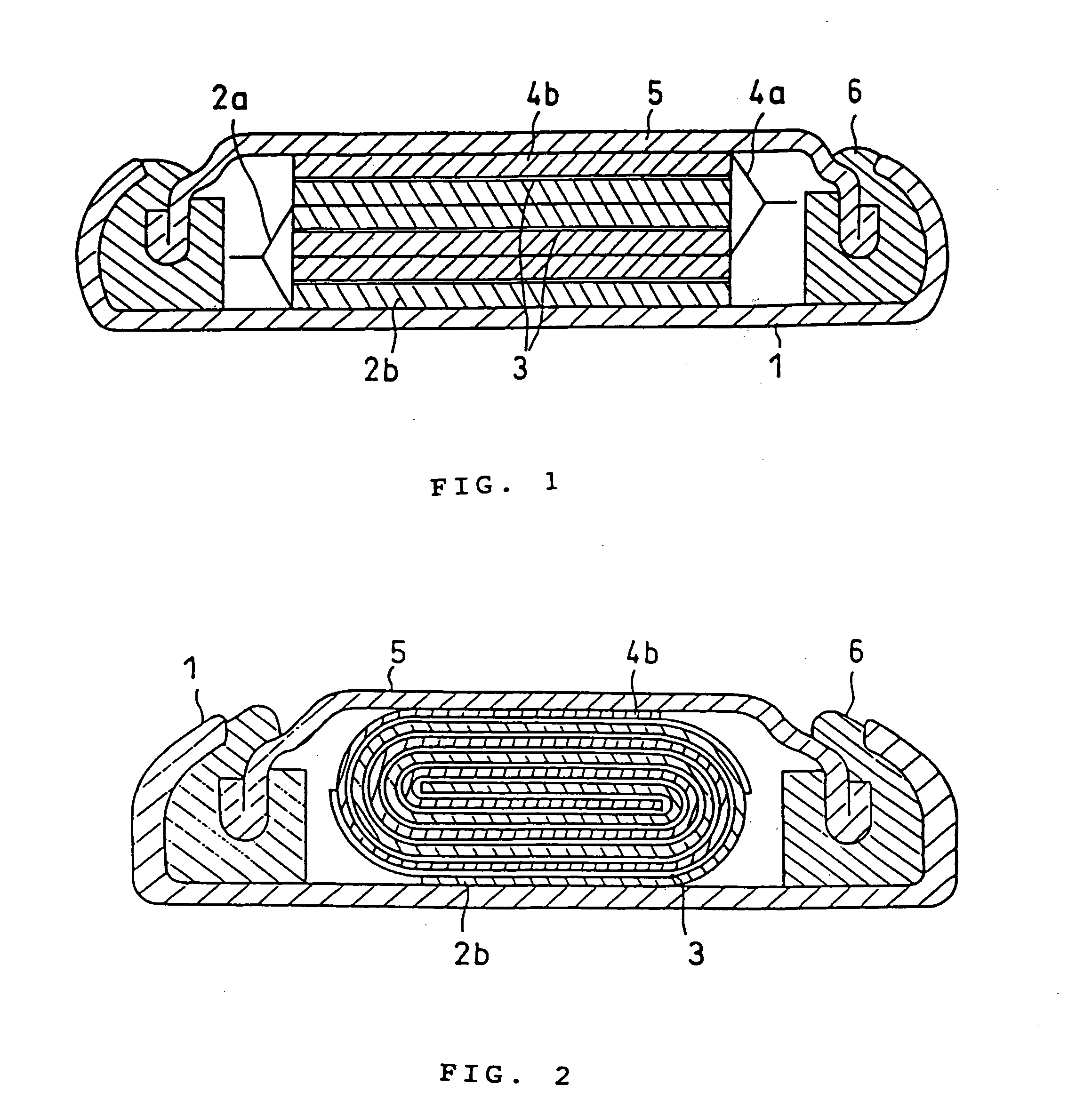
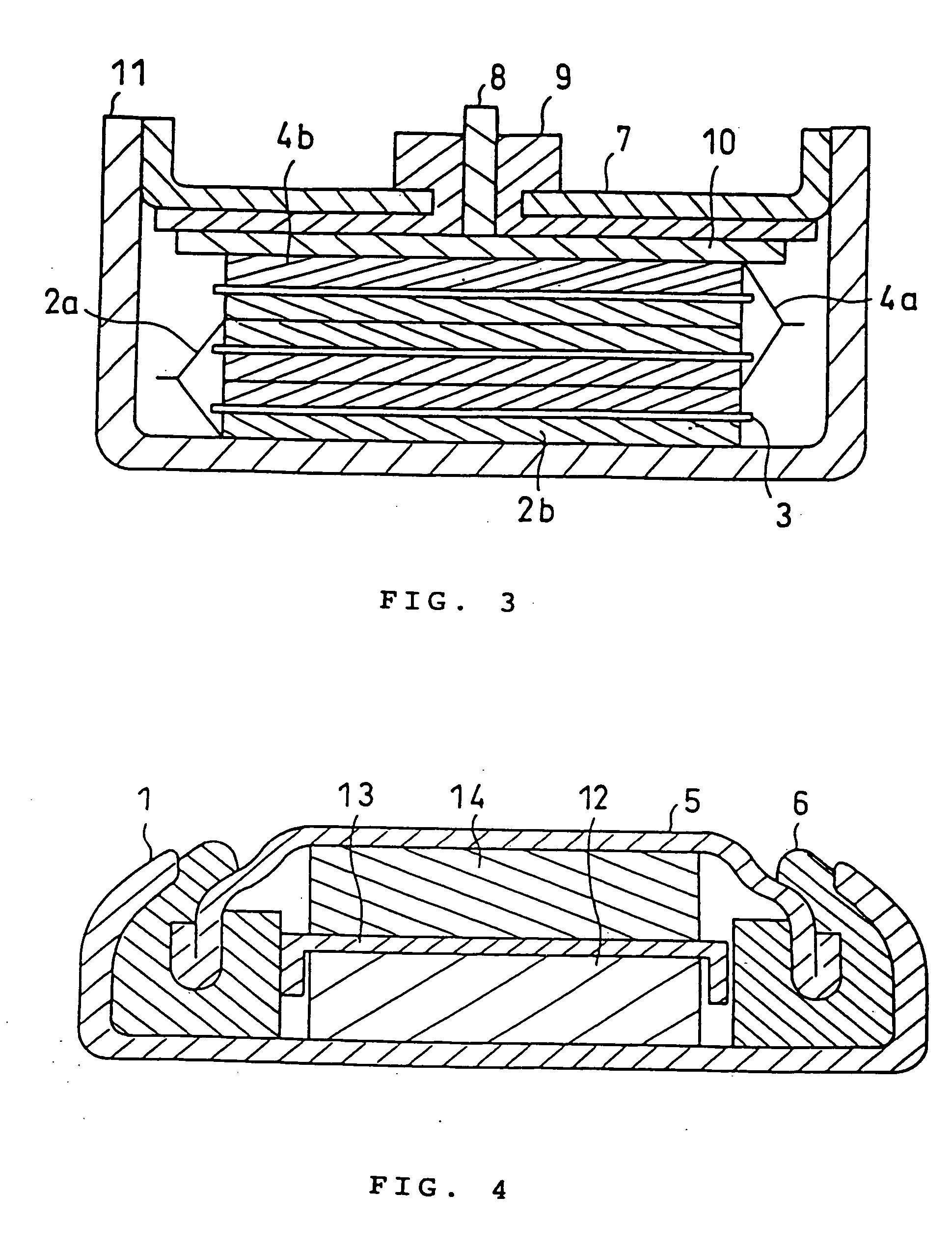
Battery electrolytic solution with fluoride-ion-dissociating salt and battery using the same
InactiveUS6306540B1Improve stabilityAvoid conductivitySolid electrolyte cellsLi-accumulatorsSupporting electrolyteDecomposition
The present invention relates to an electrolytic solution excellent in stability, and also relates to a battery excellent in battery performance and having an outer structure having light weight. The electrolytic solution contains a supporting electrolyte and a gas formation inhibitor in the solvent. The gas formation inhibitor contains a decomposition product of the supporting electrolyte with formation of a gas in the solvent. It functions as controlling to solution equilibrium in the electrolytic solution participating in decomposition reaction of the supporting electrolyte. A battery is obtained by filling the electrolytic solution between a positive electrode and a negative electrode.
Owner:MITSUBISHI ELECTRIC CORP
Perfluorinated Membranes and Improved Electrolytes for Redox Cells and Batteries
ActiveUS20080292964A1Improve performanceReduce resistanceFinal product manufactureSecondary cellsSupporting electrolyteVanadyl ion
A vanadium redox cell having a positive half cell containing a positive half cell solution comprising a supporting electrolyte selected from H2SO4, HBr / HCl mixtures and one or more ions selected from the group vanadium (EI), Vanadium (IV), Vanadium (V), Br3 and Br2Cl; a negative half cell containing a negative half cell solution comprising a supporting electrolyte selected from H2SO4, HBr and HBr / HCl mixtures and one or more vanadium ions selected from the group Vanadium (II), Vanadium (III) and Vanadium (FV) and a perfluorinated cast cation exchange membrane or separator disposed between the positive and negative half cells and in contact with the positive and negative half cell solutions.
Owner:NEWSOUTH INNOVATIONS PTY LTD
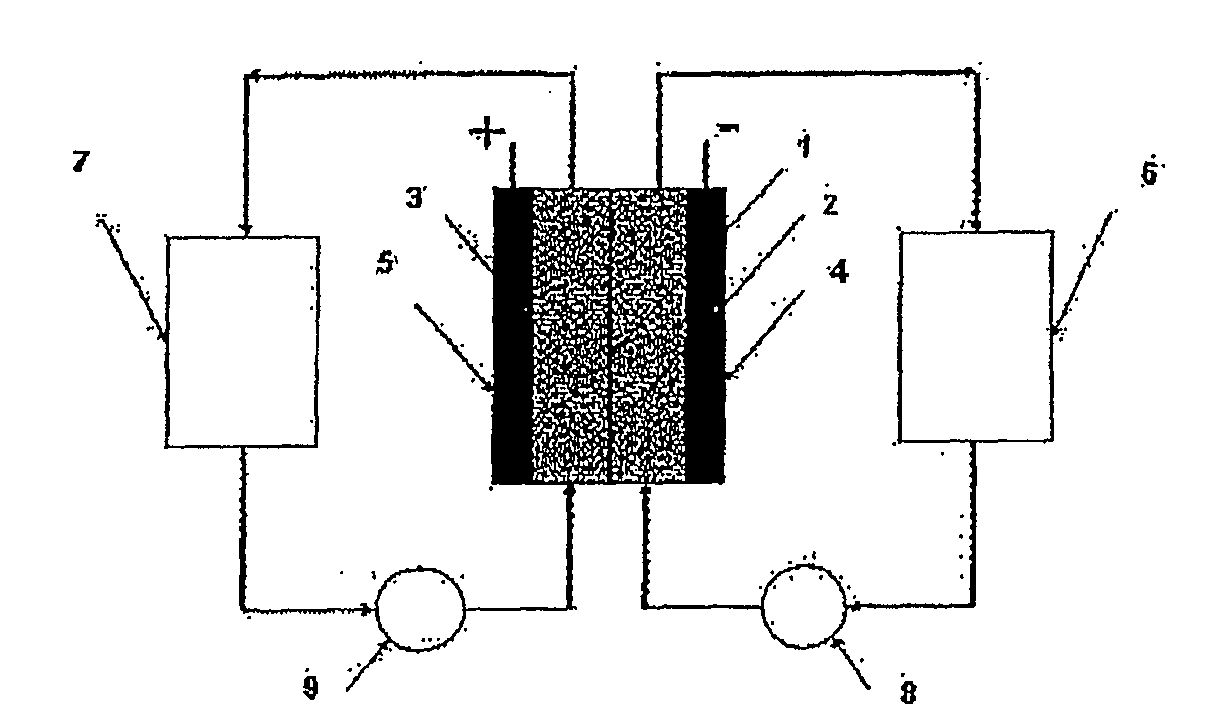
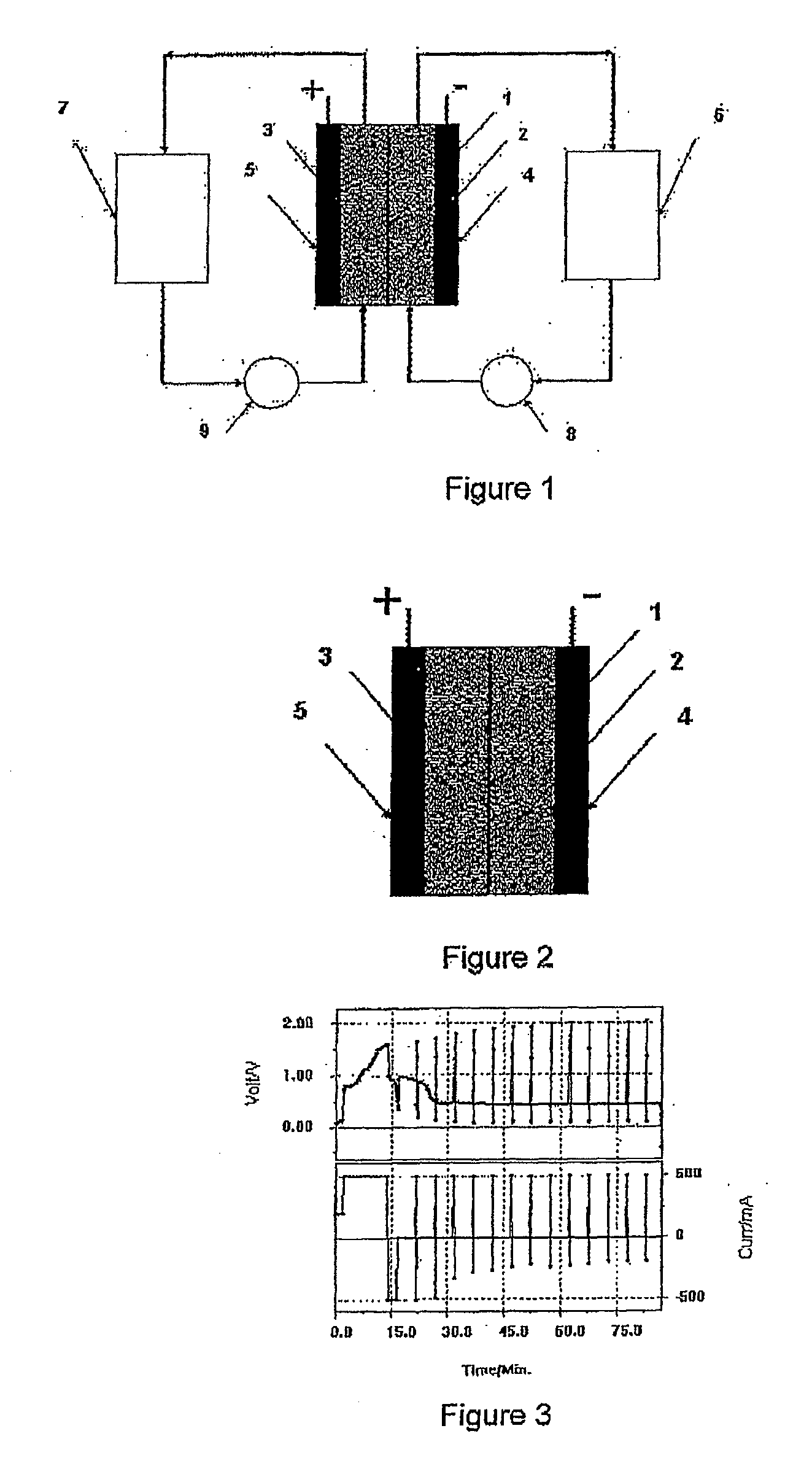
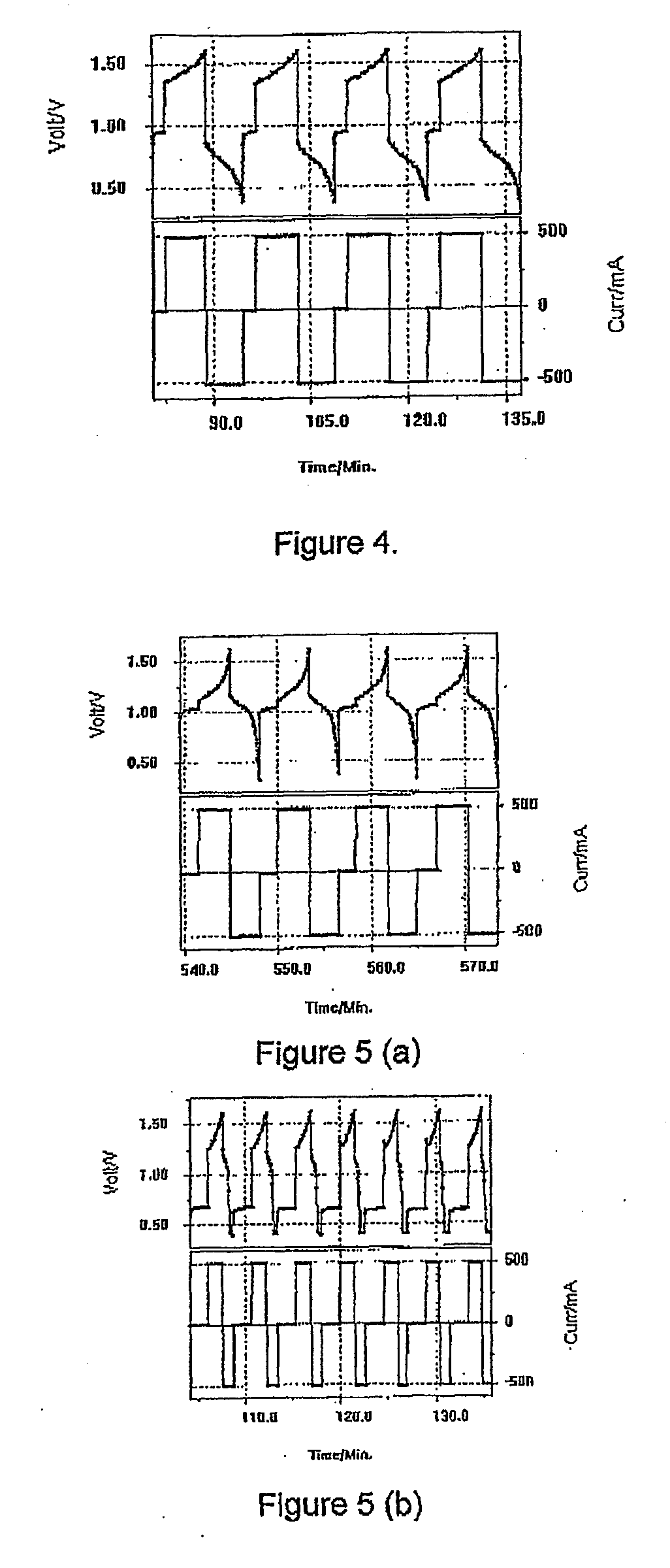
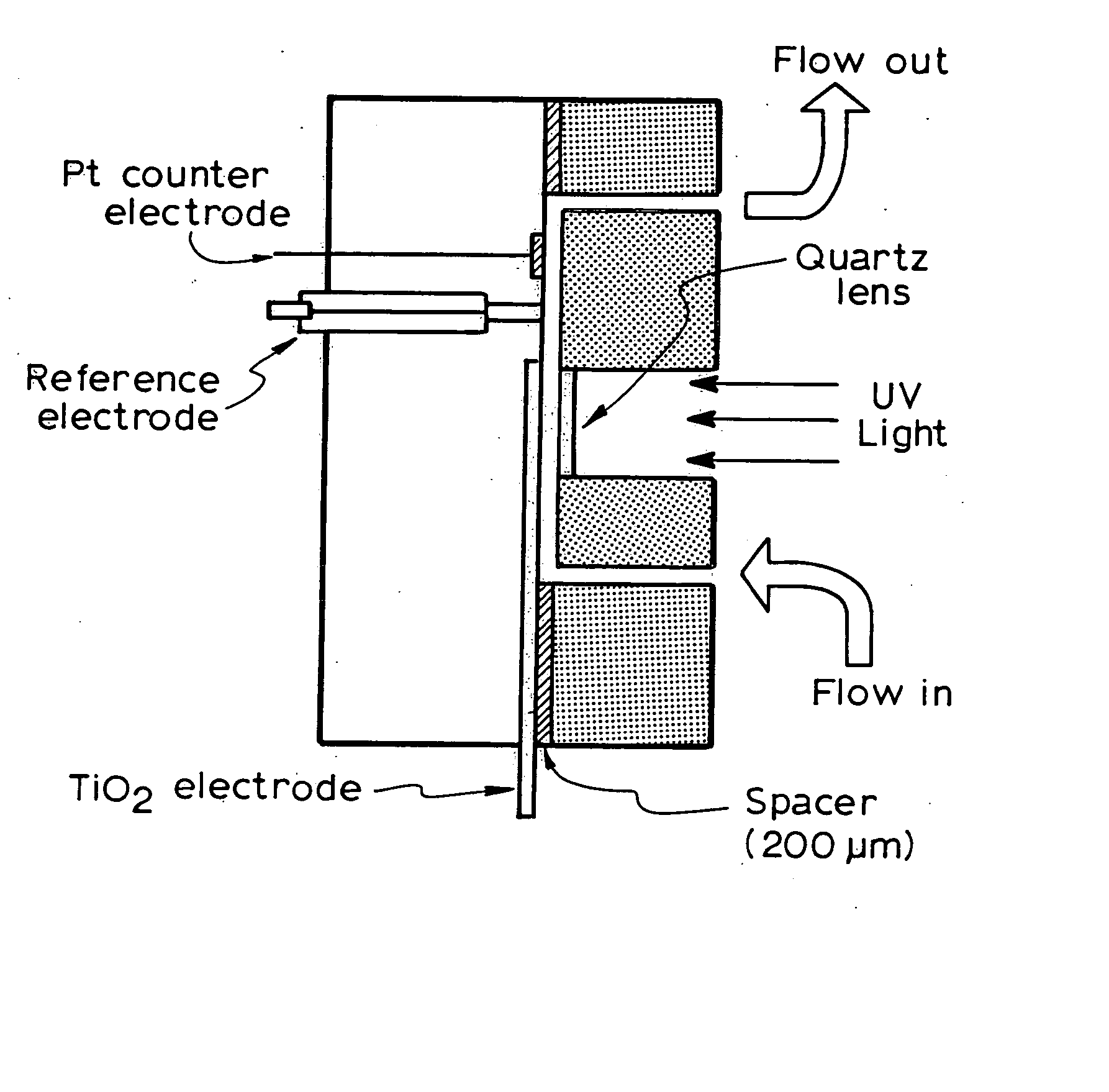
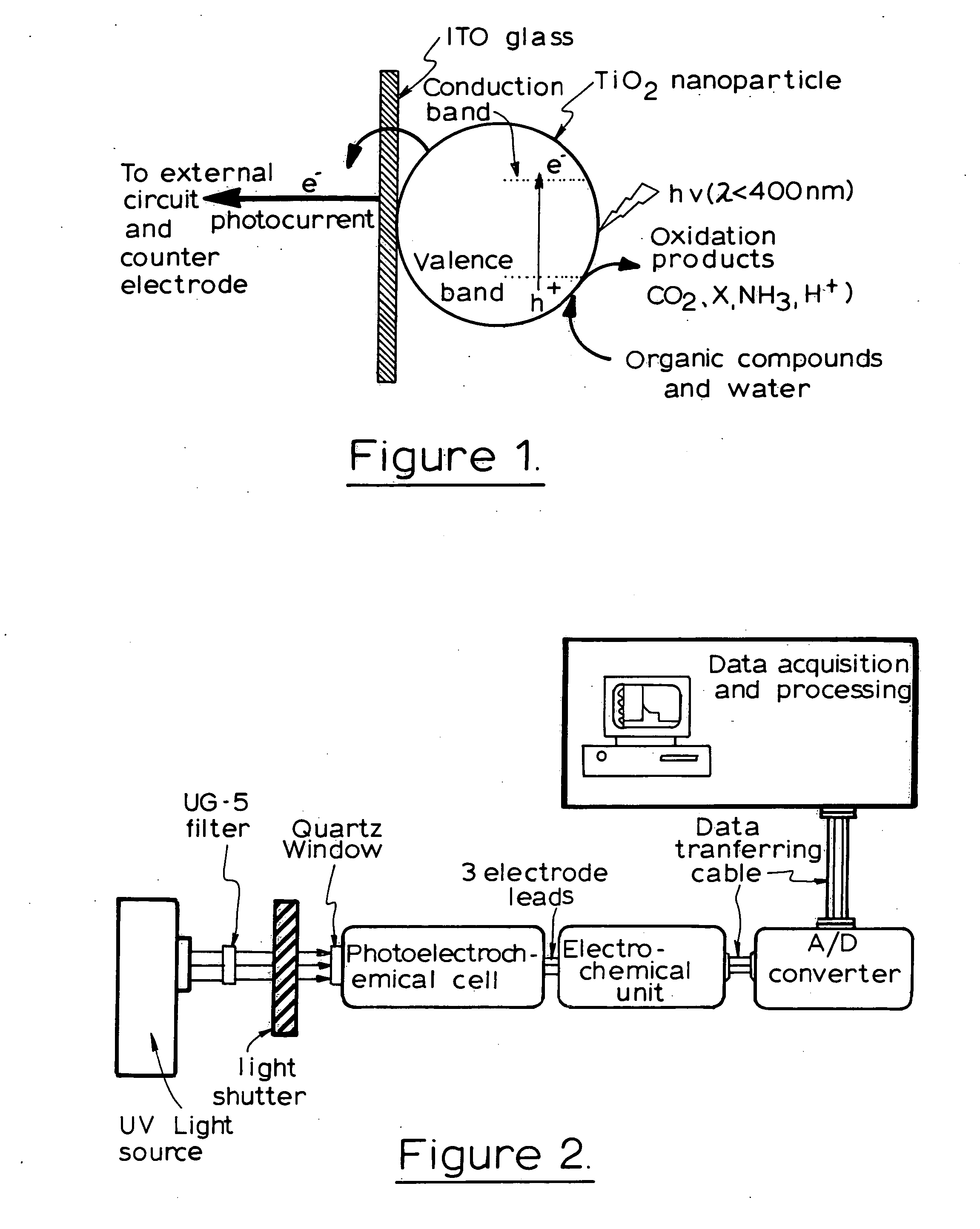
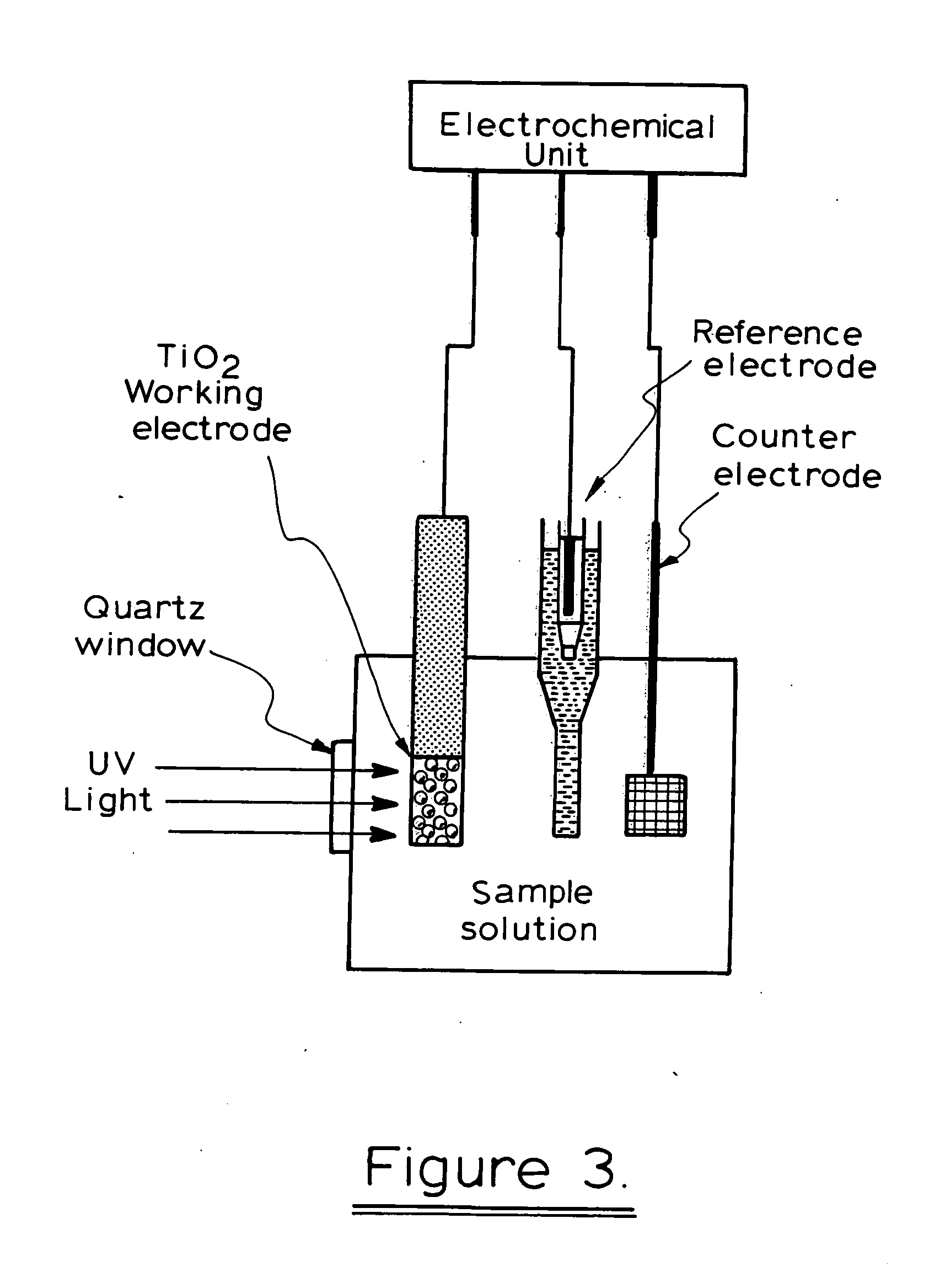
Photoelectrochemical determination of chemical oxygen demand
ActiveUS20060240558A1High sensitivityWide linear rangeChemical analysis using catalysisChemical analysis using combustionSupporting electrolytePotential measurement
A photoelectrochemical assay apparatus for determining chemical oxygen demand (COD) of a water sample which consists of a) a measuring cell for holding a sample to be analysed b) a titanium dioxide nanoparticle photoelectric working electrode and a counter electrode disposed in said cell, c) a UV light source adapted to illuminate the photoelectric working electrode d) control means to control the illumination of the working electrode e) potential measuring means to measure the electrical potential at the working and counter electrodes f) analysis means to derive a measure of oxygen demand from the measurements made by the potential measuring means. The method of determining chemical oxygen demand of a water sample, comprises the steps of a) applying a constant potential bias to a photoelectrochemical cell, containing a supporting electrolyte solution; b) illuminating the working electrode with a UV light source and recording the background photocurrent produced at the working electrode from the supporting electrolyte solution; c) adding a water sample, to be analysed, to the photoelectrochemical cell; d) illuminating the working electrode with a UV light source and recording the total photocurrent produced; e) determining the chemical oxygen demand of the water sample according to the type of degradation conditions employed. The determination may be under exhaustive degradation conditions, in which all organics present in the water sample are oxidised or under non-exhaustive degradation conditions, in which the organics present in the water sample are partially oxidised.
Owner:579453 ONTARIO INC
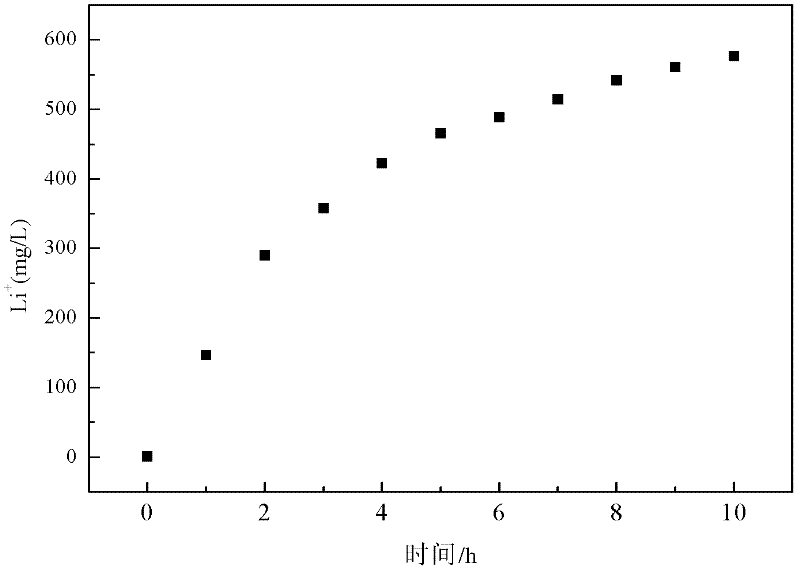
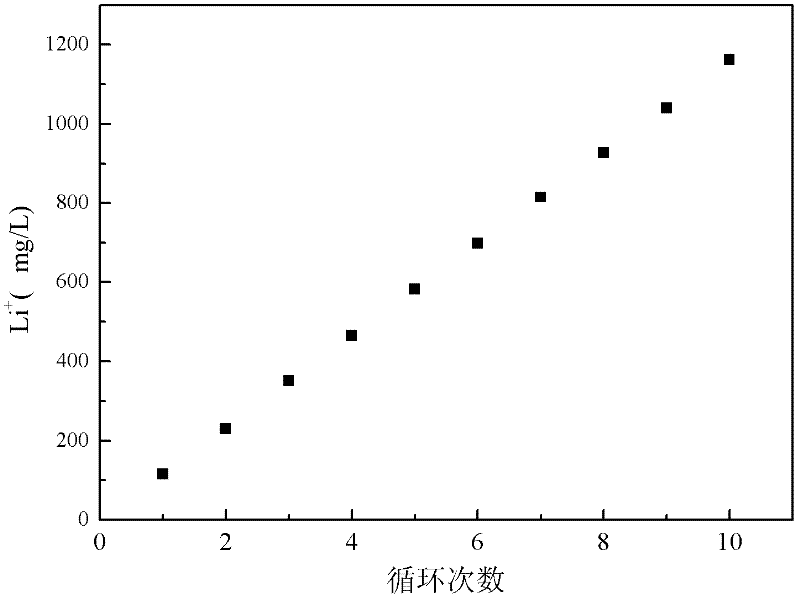
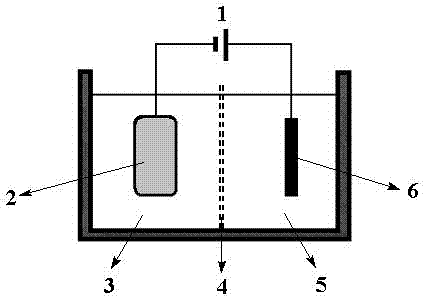
Method and device for separating magnesium and lithium and enriching lithium from salt lake brine
ActiveCN102382984AGood choiceImprove stabilityProcess efficiency improvementSupporting electrolyteIon-exchange membranes
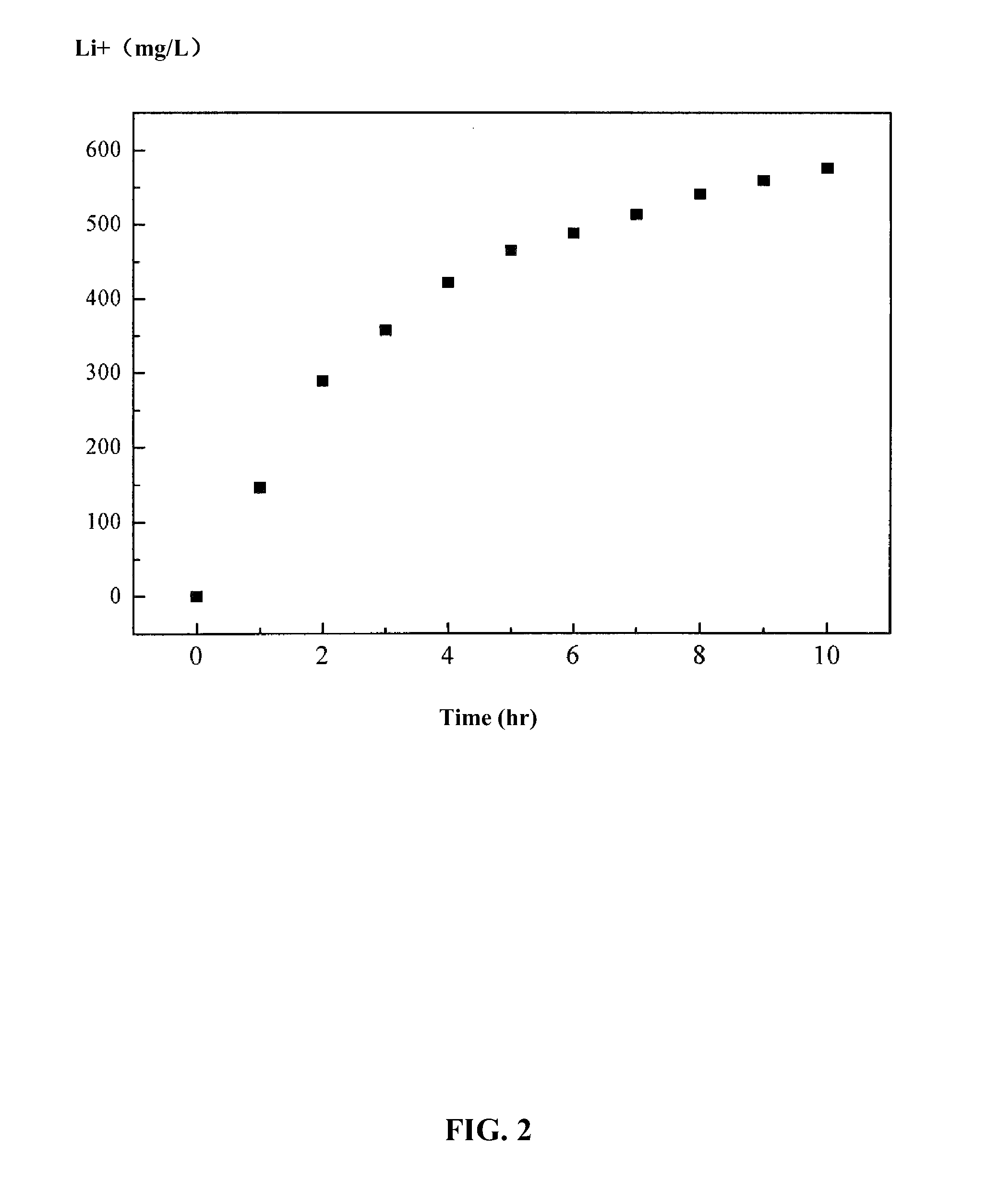
The invention relates to a method and a device for separating magnesium and lithium and enriching the lithium from salt lake brine. The method comprises the following steps of: separating an electrodialyzing device into two areas by using an anion exchange membrane, namely a lithium salt chamber and a brine chamber, filling the salt lake brine in the brine chamber, and filling a supporting electrolyte solution which does not contain Mg<2+> in the lithium salt chamber; placing a conducting matrix coated by an ionic sieve in the brine chamber as a cathode; placing the conducting matrix coated by a lithium-embedded ionic sieve in the lithium salt chamber as an anode; under the driving of an external electric potential, embedding Li <1+> in the brine in the brine chamber into the ionic sieve to form the lithium-embedded ionic sieve, and recovering the lithium-embedded ionic sieve into the ionic sieve after the lithium-embedded ionic sieve in the lithium salt chamber releases the Li <1+> into a conducting solution; and discharging a liquid in the brine chamber after the lithium is embedded, adding the salt lake brine again, alternatively placing electrodes in the two chambers, and repeating and circulating operations. Through the method and the device for separating magnesium and lithium and enriching lithium in the salt lake brine, the separation of the lithium and other ions is effectively realized, and a lithium-enriched solution is synchronously obtained. The method has a short flow and low production cost, is simple to operate, can be operated continuously, and is easy to industrially apply.
Owner:CENT SOUTH UNIV
Method for preparing formic acid through electrochemical catalytic reduction of carbon dioxide
InactiveCN102190573AGood solubility and absorption propertiesImprove conductivityOrganic compound preparationCarboxylic compound preparationSupporting electrolyteOrganic solvent
The invention relates to a method for preparing formic acid through electrochemical catalytic reduction of carbon dioxide, and belongs to the technical field of carbon dioxide recycling. In the method, a proton exchange membrane separates an electrolytic tank into a cathode chamber and an anode chamber, organic solvent / ionic liquid / water mixed solution in which a large amount of carbon dioxide is dissolved is injected into the cathode chamber, and aqueous solution containing supporting electrolyte is injected into the anode chamber; and after an electrolysis power supply is connected, the carbon dioxide undergoes electroreduction reaction on the cathode to form the formic acid. By the method, the organic solvent / ionic liquid / water mixed solution with the advantages of good conductivity, low viscosity, high capacity of dissolving the carbon dioxide, wide electrochemical window, and low use cost can be obtained, and when the carbon dioxide is electrically reduced in the mixed solution, the current density in the electroreduction reaction of the carbon dioxide can be improved and the electrocatalytic activity and long-time stability of a cathode material are improved.
Owner:KUNMING UNIV OF SCI & TECH
High-performance composite nanofiltration membrane with resistance to oxidation of organic solvent and chlorine, as well as preparation method and application of membrane
InactiveCN104258743ALoose structureImprove throughputSemi-permeable membranesSupporting electrolyteOrganic solvent
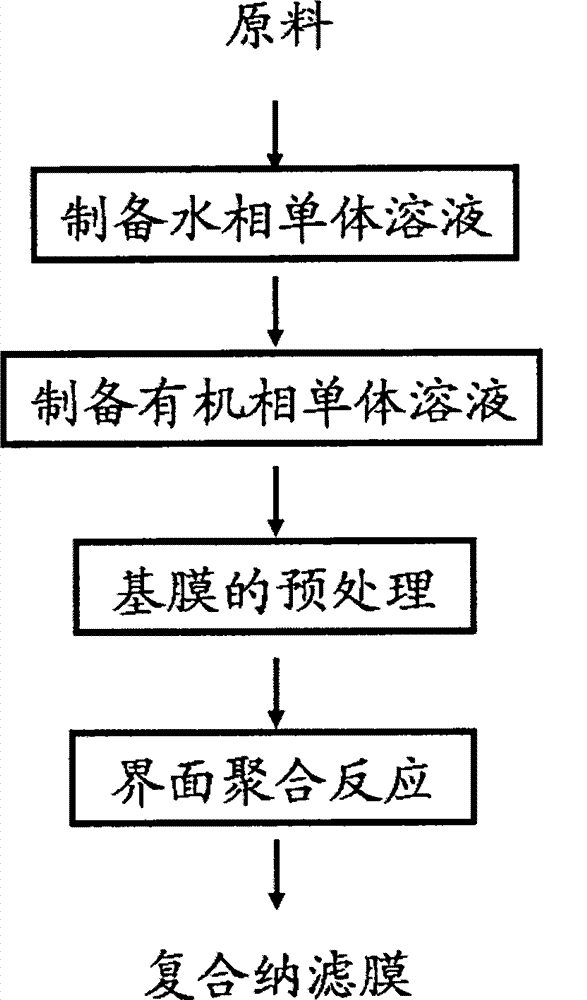
The invention relates to a high-performance composite nanofiltration membrane with resistance to oxidation of an organic solvent and chlorine, as well as a preparation method and application of the membrane. According to the preparation method, an interfacial polymerization process is interfered by supporting electrolyte, so that the density of aqueous phase monomers adsorbed on the surface of the membrane is increased, a base membrane is closely connected with an active layer, and the active layer is compact while the thickness of the active layer is reduced; therefore, high permeation flux can be obtained while low-concentration monomers are used, and high selectivity is maintained. The composite nanofiltration membrane has the properties of resisting to oxidation of the organic solvent and chlorine, under the pressure of 1.0 MPa and at the temperature of 25 DEG C, the flux of 2g / L sodium sulfate aqueous solution is 25-65 L / (m<2>.h), and retention rate is 85-99%; the flux of 2g / L sodium chloride aqueous solution is 30-60 L / (m<2>.h), and the retention rate is 50-75%.
Owner:OCEAN UNIV OF CHINA
Lithium ion battery gel polymer electrolyte and preparation method thereof
InactiveCN103840198AAddress effectivenessSolving the problem of delithiation cycleSecondary cellsSupporting electrolyteCharge discharge
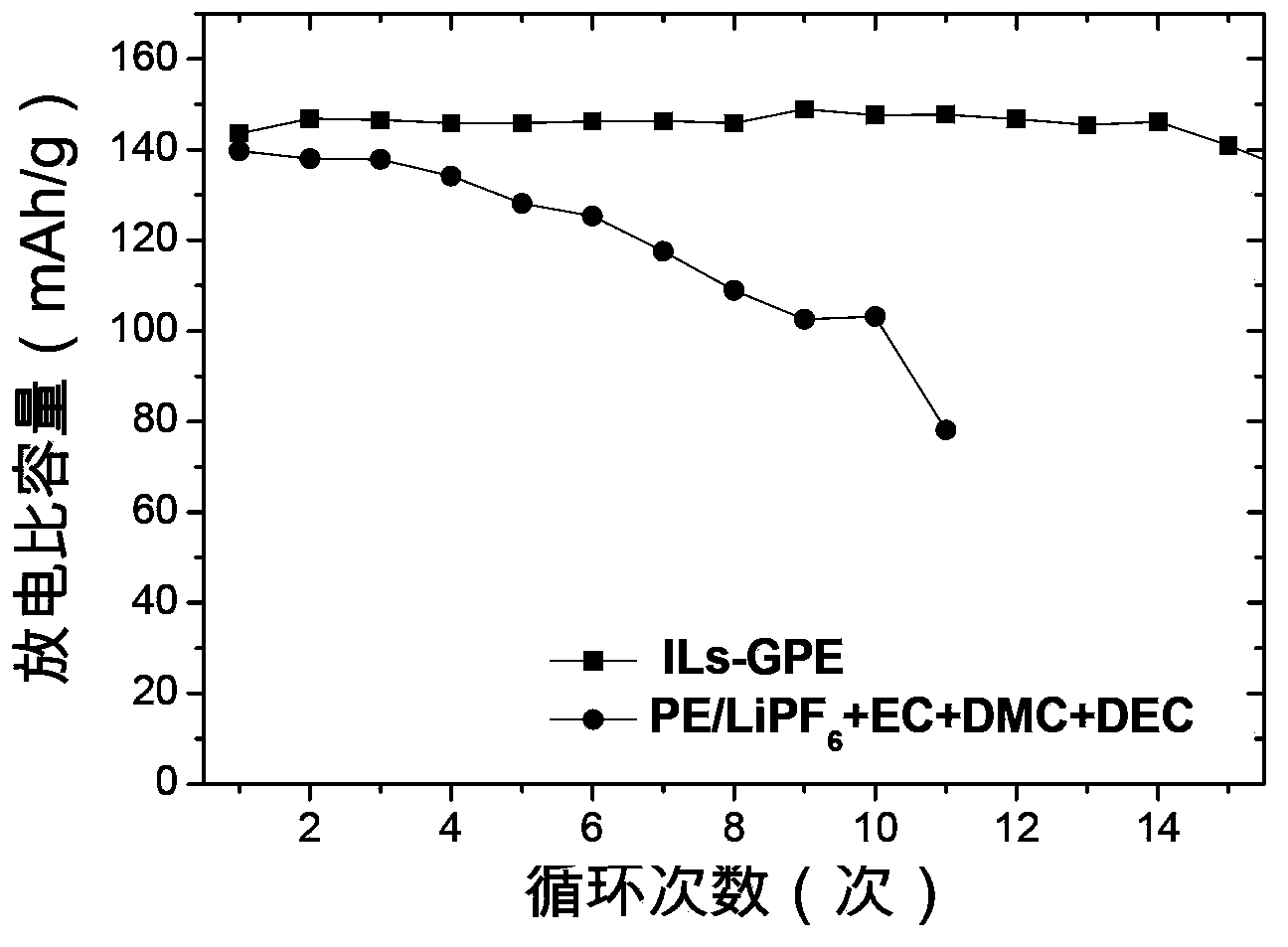
The invention provides a lithium ion battery gel polymer electrolyte and a preparation method thereof. The lithium ion battery electrolyte is composed of a macromolecular polymer, an ionic liquid, an organic solvent, a lithium salt and a film-forming additive. Through preparation of the gel polymer electrolyte, the disadvantages of leakage, easy corrosion of electrode materials and the like can be eliminated. The high temperature performance of the electrolyte can be improved by using an ionic liquid. By adding the organic solvent, the viscosity of the ionic liquid is reduced and the conductivity is enhanced. And by adding the film-forming additive, the problem of poor compatibility between the ionic liquid and graphite or a lithium electrode material can be solved. Experiments prove that the lithium ion battery gel polymer electrolyte provided by the invention is an elastic self-supporting electrolyte membrane, which has ionic conductivity up to the 10<-3>S / cm magnitude order, good high temperature stability and safety, and has good compatibility with a lithium cathode or a graphite cathode material. Lithium ions can undergo effective lithium insertion and removal circulation, and can achieve high capacity when applied to lithium ion battery charge-discharge cycle.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI
Cyanide-free silver plating solution containing auxiliary complexing agent
InactiveCN102277601AGood binding and brightPlating solution is stableSupporting electrolyteContinuous use
The invention discloses cyanogen-free silver-plating electroplating liquid containing an auxiliary complexing agent. The cyanogen-free silver-plating electroplating liquid containing the auxiliary complexing agent is composed of a silver ion source material, a complexing agent, the auxiliary complexing agent, a supporting electrolyte, an electroplating additive, a pH adjusting agent and the like;the components of the electroplating liquid and the contents are as follows: 30-60 g / L of silver ion source material, 140-200 g / L of complexing agent, 10-50 g / L of auxiliary complexing agent, 10-30 g / L of supporting electrolyte, 100-800 mg / L of electroplating additive and 10-30 g / L of pH adjusting agent. The cyanogen-free silver-plating electroplating liquid containing the auxiliary complexing agent provided by the invention has the advantages that: the electroplating liquid is stable and has low toxicity, the anodic passivation in the electroplating process can be better inhibited, the anodeis normally dissolved, the electroplating liquid can be continuously utilized for long time, the electroplating layer has a good bonding force and is bright, and the electroplating liquid can be applied to multiple fields including decorative electroplating, functional electroplating and the like.
Owner:NANJING UNIV
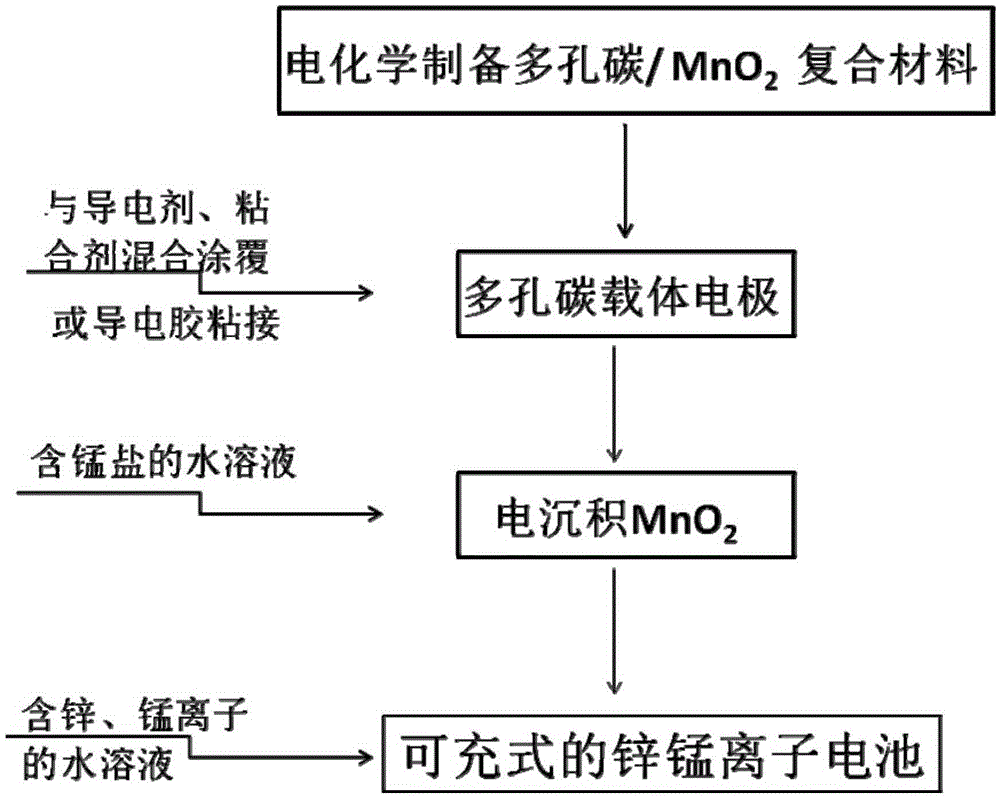
Porous carbon/manganese dioxide composite electrode, preparation method of porous carbon/manganese dioxide composite electrode and rechargeable zinc-manganese ion battery
ActiveCN105390697AControl particle sizeControl distribution densityCell electrodesFinal product manufactureSupporting electrolyteElectrolytic agent
The invention relates to a porous carbon / manganese dioxide composite electrode, a preparation method of the porous carbon / manganese dioxide composite electrode and a rechargeable zinc-manganese ion battery, and belongs to the technical field of electrochemistry. Porous carbon with high specific surface area and favorable electric conductivity is taken as a carrier electrode; the carrier electrode is electrolyzed in flowing water solution which contains a manganous salt precursor and a supporting electrolyte; and manganese dioxide is deposited on the surface of the porous carbon. Through selecting the concentration of the manganous salt as well as the concentration, pH value, current, temperature and time of the supporting electrolyte in the electric deposition process, a porous carbon / manganese dioxide composite is prepared, so that the regulation and control of the particle size and distribution density of the manganese dioxide are realized, and the active substance utilization rate of the manganese dioxide is improved. By taking the obtained porous carbon / manganese dioxide composite as the electrode, and by using the water solution containing zinc and manganese ions as electrolyte to assemble secondary batteries, the specific capacity of the electrode is above 200mAh / g and the electrode has the characteristics of being high in capacity and long in service life. The preparation method is easy to operate, green and environmental.
Owner:中国人民解放军军事科学院防化研究院
Non-aqueous electrolyte secondary battery
ActiveUS20060286458A1Large capacityImprove featuresNegative electrodesElectrolytesSupporting electrolyteDissolution reaction
A non-aqueous electrolyte secondary battery including: a positive electrode; a negative electrode, and a non-aqueous electrolyte. The negative electrode includes a negative electrode active material that includes at least Si. The non-aqueous electrolyte includes lithium hexafluorophosphate as a main supporting electrolyte and has an acid content of not less than 50 ppm and not more than 200 ppm. The negative electrode has a potential of not less than 0.6 V and not more than 1.5 V relative to a Li electrode at an end-of-discharge voltage of the battery. The battery is prevented from suffering degradation of storage characteristics caused by the dissolution reaction of Si from the negative electrode during charging and the precipitation reaction of the dissolved Si.
Owner:PANASONIC CORP
Electrochemical oxidation processing method for wastewater containing anthraquinone dye
InactiveCN101508477AGood oxidative degradation abilityHigh drop rateWater contaminantsWater/sewage treatmentElectrochemical responseSupporting electrolyte
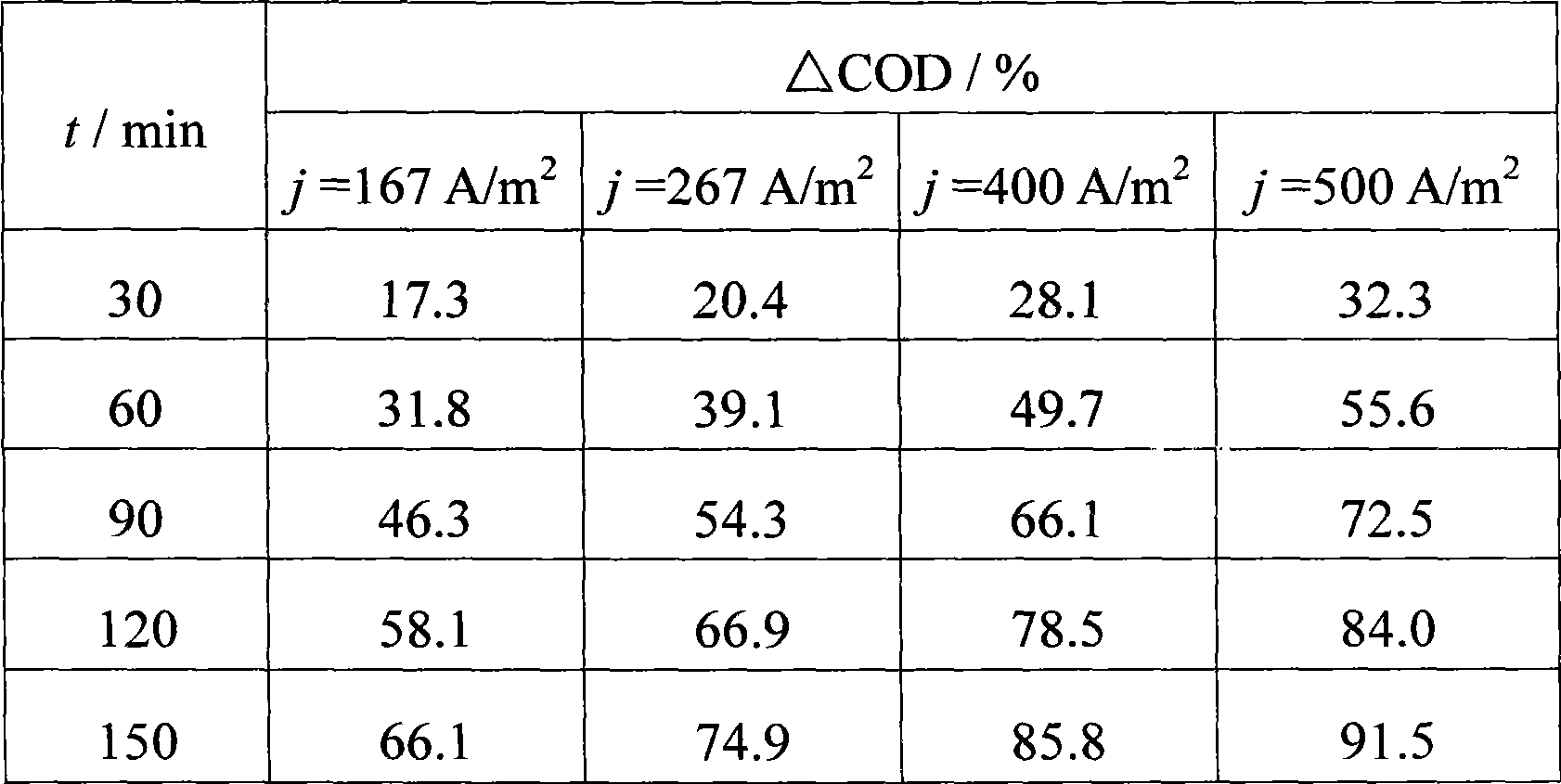
The invention provides a method for electrochemical oxidation treatment of waste water containing anthraquinone dye, and relates to the technical field of water treatment. In the method, a titanium-based metal oxide electrode is taken as an anode, copper or stainless steel is taken as a cathode, a flat plate or three-dimensional fixed bed is taken as an electrochemical reactor, under conditions of the current density of between 10 and 1,500A / m, the content of supporting electrolyte, namely Na2SO4 of between 1 and 20g / L and the temperature of the waste water of between 5 and 95 DEG C, the electrochemical oxidation treatment is performed. The method has the advantages of the degradation of the anthraquinone dye in a short time, quick reaction rate, good decolorization effect, high decrease rate of COD, simple technological flow, little equipment investment, easy operational control and easy industrialized application.
Owner:YANGZHOU UNIV
Efficient treatment of wastewater using electrochemical cell
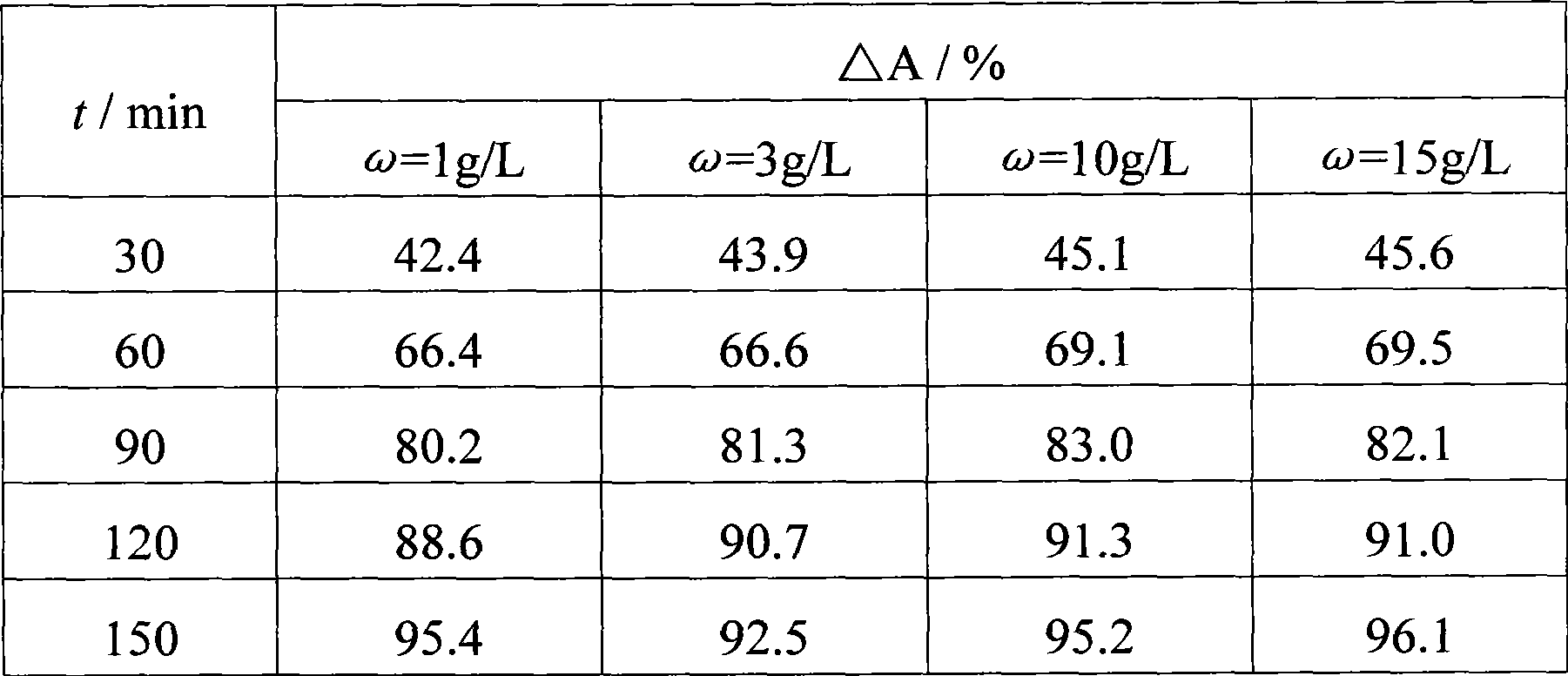
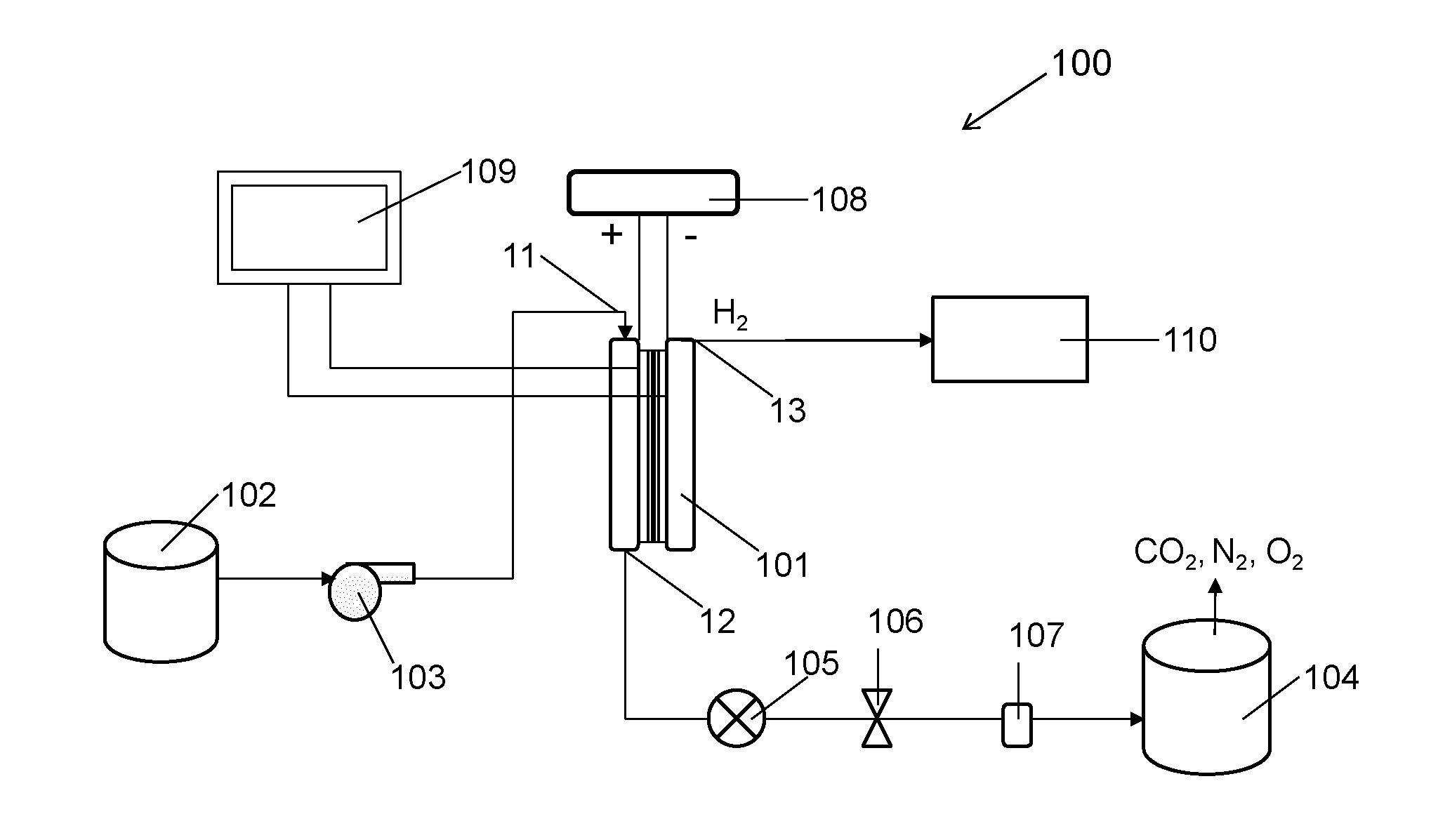
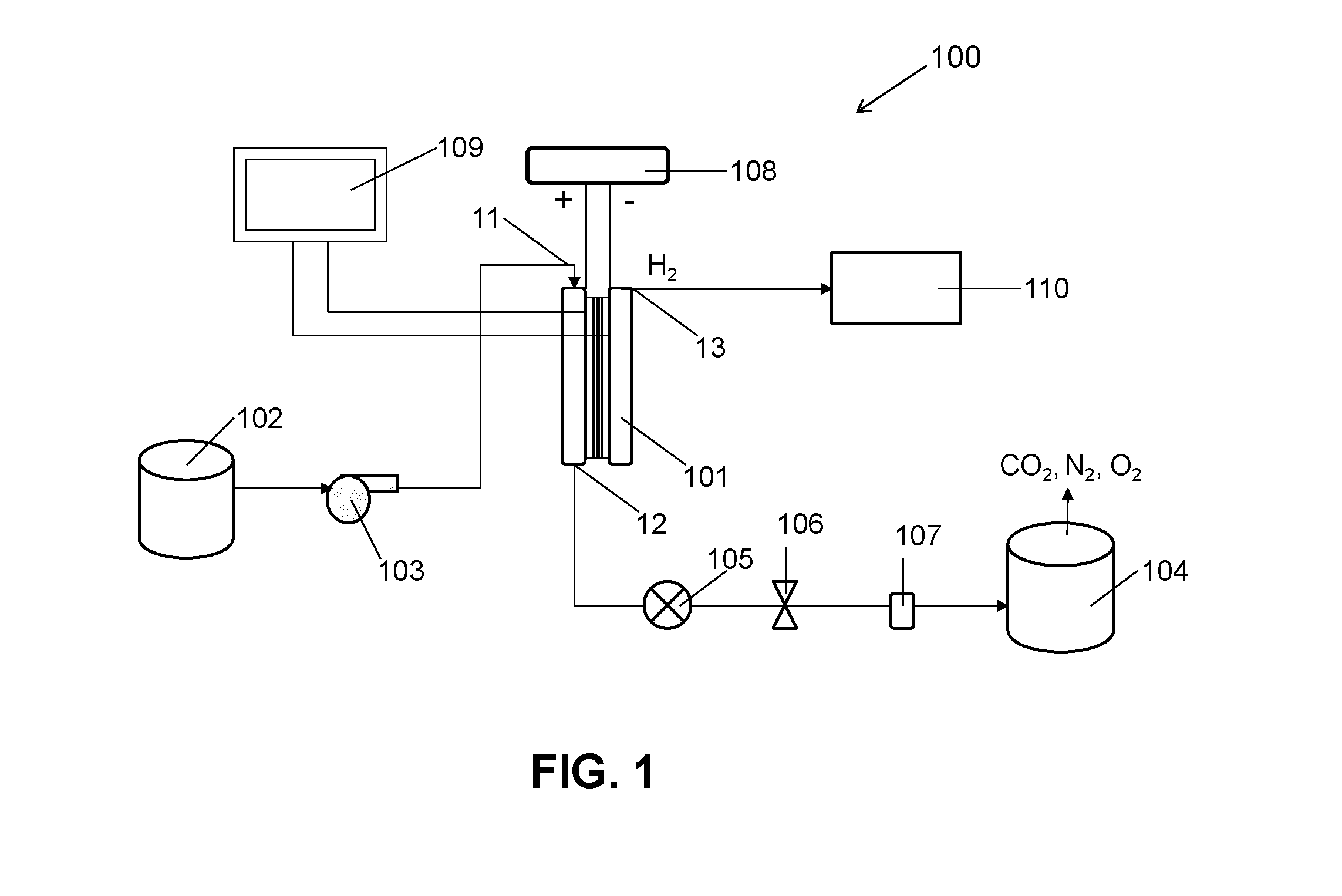
ActiveUS20140183054A1Energy efficiencyLow densityCellsWater treatment parameter controlSupporting electrolyteWastewater
An efficient method and system for the electrochemical treatment of waste water comprising organic and / or inorganic pollutants is disclosed. The system comprises an electrolytic cell comprising a solid polymer, proton exchange membrane electrolyte operating without catholyte or other supporting electrolyte. The cell design and operating conditions chosen provide for significantly greater operating efficiency.
Owner:AXINE WATER TECH
Improved perfluorinated membranes and improved electrolytes for redox cells and batteries
InactiveCN101257121AElectrode manufacturing processesRegenerative fuel cellsSupporting electrolytePolyolefin
The invention relates to an electrode used for vanadium redox flow battery, a preparation of electrolyte and a rebalance method. The preparation comprises thermally sticking a carbon felt or agraphite felt to at least one side of a carbon-filled polyolefine substrate. A flat, low-resistance electrode having good mechanical property can be obtained by the preparation. The preparation includes dissolving at least one vanadium oxide powder to a supporting electrolyte and optional electrolysis steps. The preparation needs no toxic SO2 gas, by separating the power dissolving stage and the electrolysis stage, problems related to suspension powder electrolysis are eliminated. The rebalance method comprises partially reducing a half-cell electrolyte in the cathode chamber of an electrolytic cell.
Owner:NEWSOUTH INNOVATIONS PTY LTD
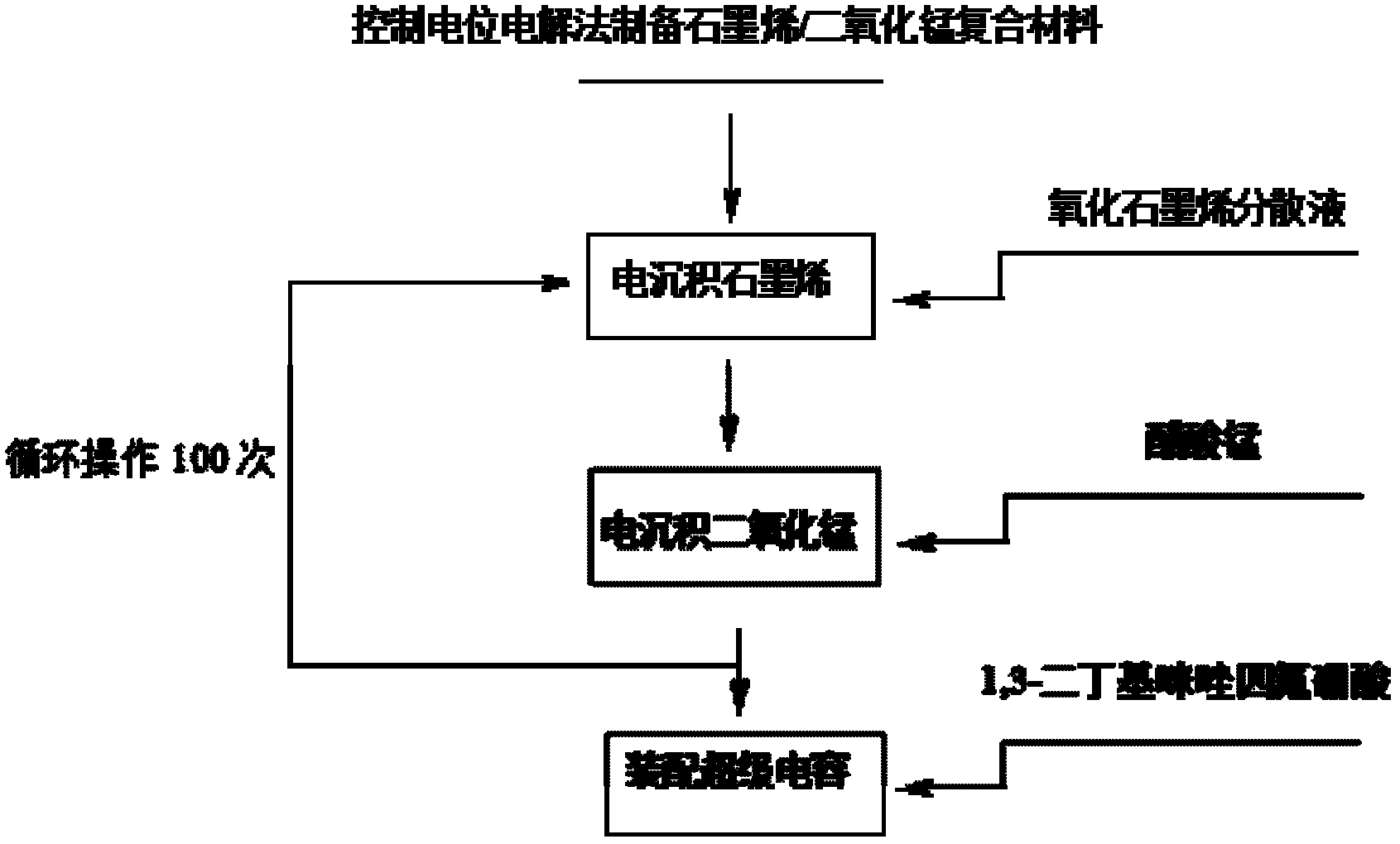
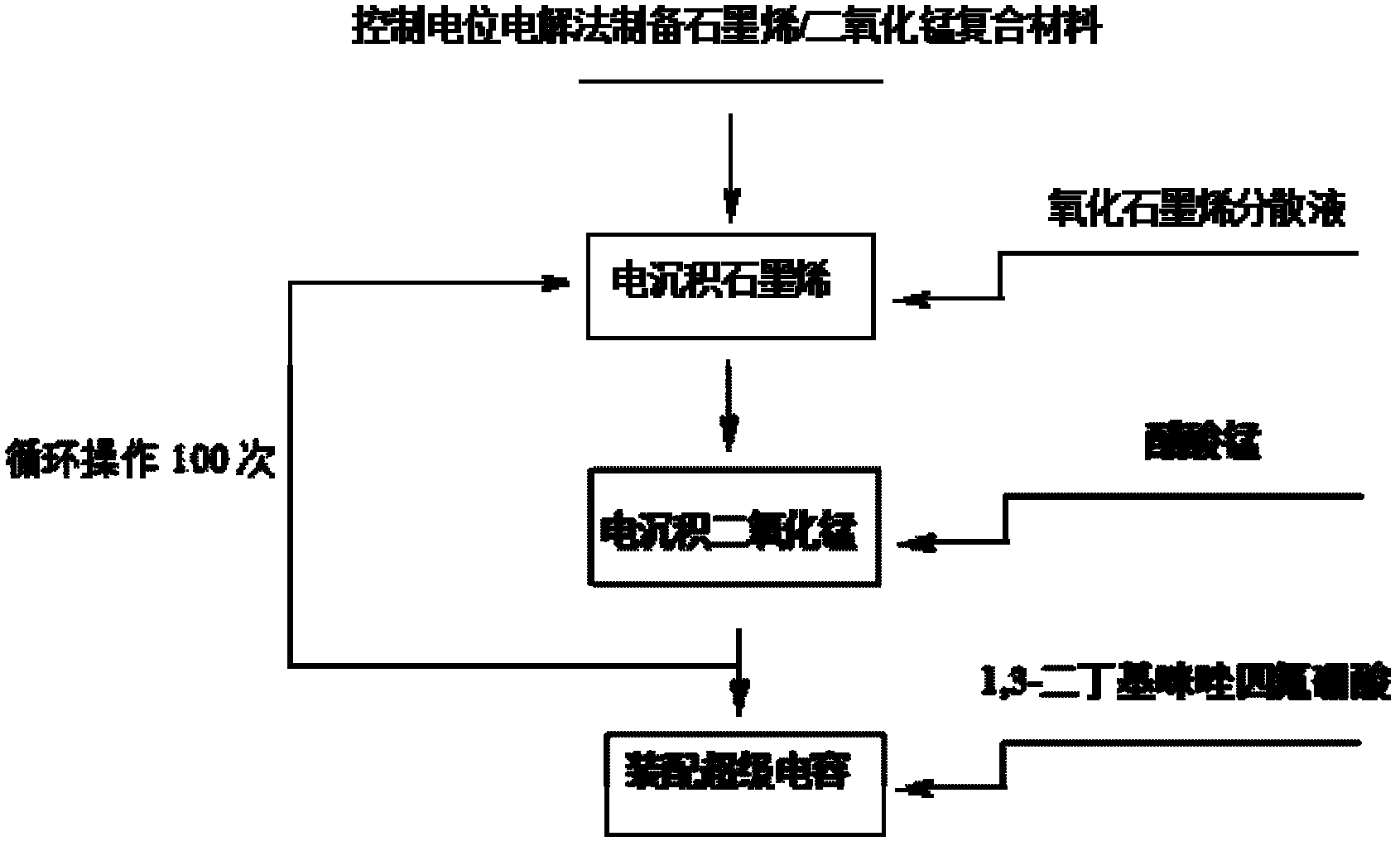
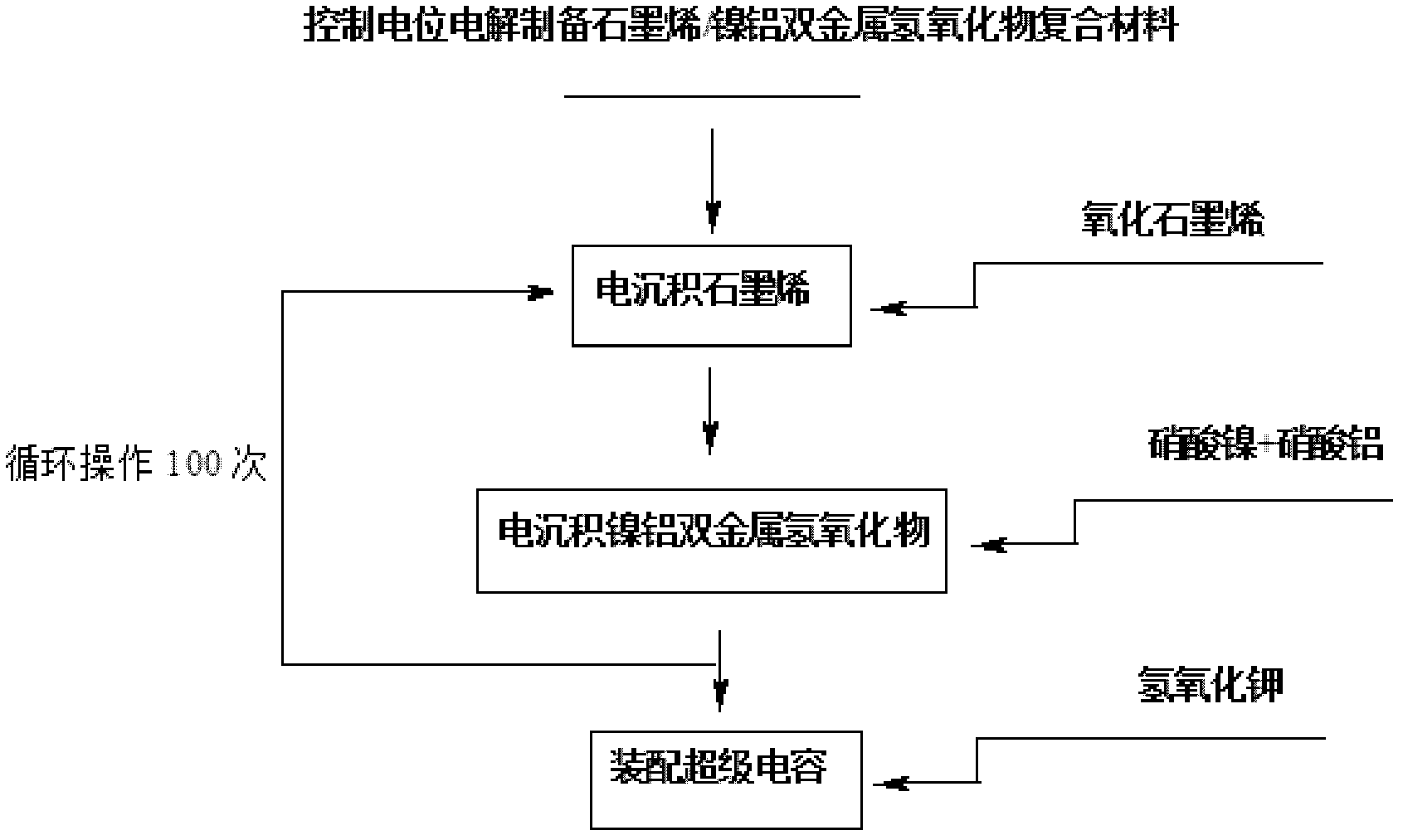
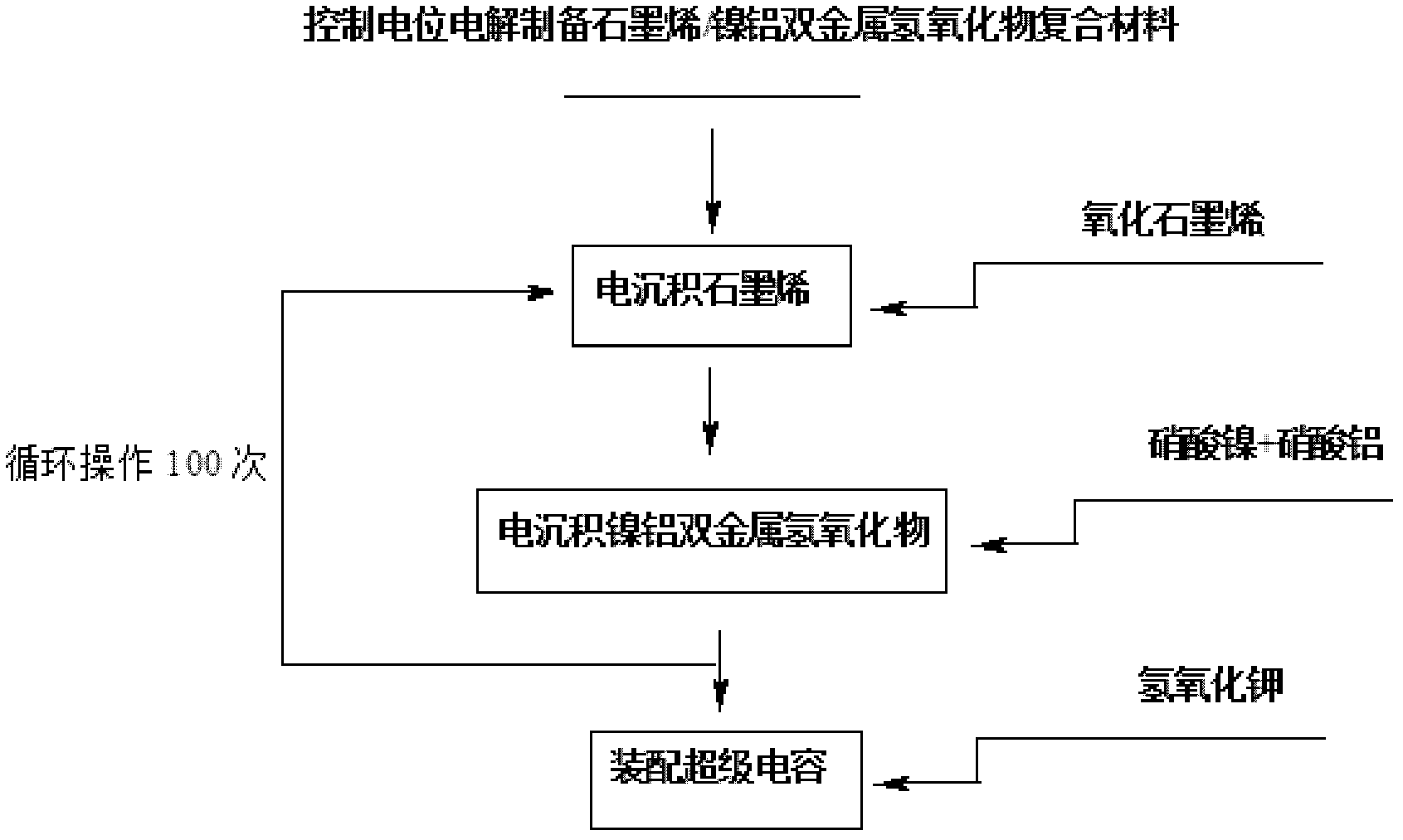
Method for electrochemically preparing graphene/manganese dioxide composite material, and application of graphene/manganese dioxide composite material
ActiveCN102568847ASmall particle sizeControl distribution densityElectrolytic capacitorsMANGANESE ACETATESupporting electrolyte
The invention relates to a method for electrochemically preparing a graphene / manganese dioxide composite material, and application of the graphene / manganese dioxide composite material, and belongs to the technical field of electrochemistry. The method comprises the following steps of: taking sodium carbonate as a supporting electrolyte; reducing a graphene oxide into graphene by a controlled potential electrolysis method, and uniformly fixing the graphene on a surface of an electrode; and precisely controlling the thickness of a graphene coating layer according to the concentration of the graphene oxide, potential, temperature and time during electro-deposition; respectively taking manganese acetate and sodium sulfate as a manganese precursor and a supporting electrolyte; adjusting the acidity of the electrolyte by sulfuric acid, performing controlled potential electrolysis, electrically depositing manganese dioxide on the surface of the graphene, and precisely controlling the particle size and the distribution density of the manganese dioxide; and repeating the operation for 100 times, and preparing the graphene / manganese dioxide composite material. Test shows that the obtained graphene / manganese dioxide composite material is an electrode or a super assembly capacitor, in which ionic liquid is used as the electrolyte; the capacity is more than 500 F / g; and after the super assembly capacitor is cyclically charged and discharged for 1,000 times, the capacity can still be kept over 99 percent.
Owner:JIANGSU SUNPOWER
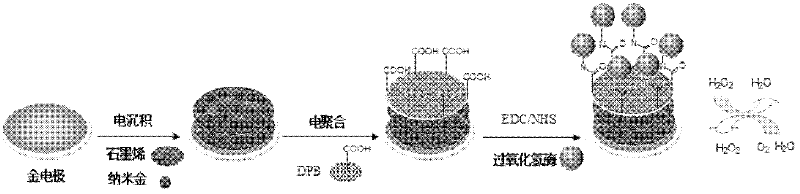
Method for preparing graphene biosensor
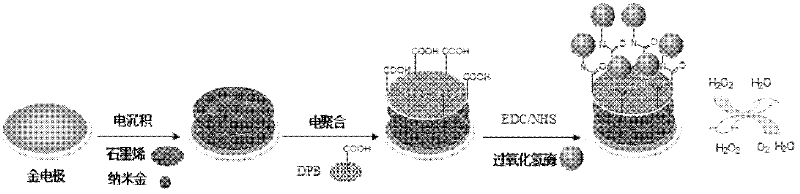
ActiveCN102520038ASensitive electrochemical responsePrecise size controlMaterial analysis by electric/magnetic meansSupporting electrolyteConductive polymer
The invention relates to a method for preparing a graphene biosensor, belonging to the technical field of electrochemistry. The method comprises the following steps of: immersing a processed gold electrode into graphene oxide and a sodium sulphate solution, electrically depositing the electrode through a control electric potential, taking out the electrode, washing the electrode by using water, after drying the electrode at room temperature, putting the electrode in a chloroauric acid solution, electrically depositing the electrode through the control electric potential, taking out the electrode, washing the electrode by using water, and drying the electrode at room temperature; putting a modified electrode in a conductive polymer monomer and a supporting electrolyte solution, polymerizing the electrode through the control electric potential by adopting a cyclic voltammetry, taking out the electrode, washing the electrode by using water, and drying the electrode at room temperature; and activating the modified electrode in an EDC / NHS (Dichloroethane / N-Hydroxysuccinimide) solution, and immersing the modified electrode in a vomiting toxin antibody. The method disclosed by the invention is used for fixing graphene, gold nanoparticles and conducting polymers through electro-deposition; therefore, the method is very green and environment-friendly; furthermore, the thickness of a coating and sizes and distribution densities of the gold nanoparticles can be precisely controlled, thus, the batch production repeatability of the modified electrode is good.
Owner:盐城福万家保温板有限公司
Preparation method of conductive polypyrrole
InactiveCN101979438ARegulate polymerization speedHigh polymerization efficiencyElectrolysis componentsElectrolytic organic productionSupporting electrolytePolypyrrole
The invention discloses a preparation method of conductive polypyrrole. The method comprises the following steps of: dissolving a pyrrole monomer in an organic solvent and dissolving a supporting electrolyte in water so as to prepare solutions respectively; transferring the solutions into a reactor in two steps so as to form a liquid-liquid interface; directly inserting a working electrode into the liquid-liquid interface; putting an auxiliary electrode and a reference electrode into solution of supporting electrolyte; and performing electrochemical polymerization by an electrochemical measure. A prepared conductive polypyrrole film is a polymer of pyrrole, which has the thickness of between 1 and 500mu m, the tensile strength of more than or equal to 0.5MPa and high electrical conductivity, and can be used in the aspects of super capacitors, anode and cathode materials of batteries, electromagnetic shielding and the like.
Owner:WUHAN UNIV
Method of depositing metal organic framework material by oxygen auxiliary cathode
InactiveCN108130574AAvoid depositionHigh selectivityElectrolytic organic material coatingSupporting electrolyteOrganic solvent
The invention provides a method of depositing a metal organic framework material by an oxygen auxiliary cathode. The method comprises the following steps: (1) preparing a reaction precursor solution which is composed of a 5-50mM metal source, a 10-250mM organic ligand and an organic solvent; and (2) carrying out electrochemical deposition on the surface of a conducting substrate of the cathode toobtain the material. The method provided by the invention does not corrode the deposited electrode, the selecting range of the conducting substrate is wide, the prepared MOF material is regulatable inshape, dimension and thickness in nanoscale, pure, free of adding a supporting electrolyte and low in cost, and can be synthesized in one step. The metal organic framework is simple in method and operation, and industrial production is conveniently achieved.
Owner:SUZHOU UNIV
Process for preparing shape controllable cuprous oxide micro/nano crystal by electrochemical deposition
InactiveCN1807688AThe experimental equipment is simpleGood symmetryPolycrystalline material growthElectrolysis componentsSupporting electrolyteChange density
The invention discloses a shape-controlled cuprous oxide micron / nanometer crystal preparing method with electrochemical deposition, which comprises the following steps: pre-processing conductive basal body; formulating electrolyte solution; carrying on constant current electrochemical deposition; gaining Cu2O micron / nanometer crystal. The method is characterized by the following: the cuprous oxide micron / nanometer crystal is gained by changing density of electrolyte solution and electrochemical parameter by constant current electrodeposition method at room temperature, which needn't any supporting electrolytes and surface activators; the form is composed of octahedron, top rake octahedron, top rake cube and cube.
Owner:XIAMEN UNIV
Air cathode, metal-air battery and method for producing air cathode for metal-air battery
InactiveUS20130202974A1Increased durabilityIncrease capacityFuel and primary cellsFuel and secondary cellsSupporting electrolyteEngineering
An object of the present invention is to provide a metal-air battery having excellent durability and capacity by facilitating a reaction of oxygen radicals and metal ions at an air cathode.Disclosed are an air cathode used for a metal-air battery comprising an air cathode, an anode and an electrolyte layer which is present between the air cathode and the anode and which conducts metal ions between the air cathode and the anode, wherein the air cathode comprises an air cathode layer comprising at least an electroconductive material and a supporting electrolyte salt, a metal-air battery comprising the air cathode, and a method for producing the air cathode for the metal-air battery.
Owner:TOYOTA JIDOSHA KK
Electrochemical preparation method of graphene/nickel-aluminum bimetal hydroxide composite material for super capacitor
ActiveCN102509640ANo pollutionGood dispersionElectrolytic capacitorsCapacitanceSupporting electrolyte
The invention relates to an electrochemical preparation method of a graphene / nickel-aluminum bimetal hydroxide composite material for a super capacitor, belonging to the technical field of capacitor preparation. The method comprises the following steps of: putting graphite oxide in a beaker; adding deionized water and supporting electrolyte; performing ultrasonic oscillation and constant-potential electrolysis; taking out the electrode; washing the electrode with the deionized water and drying; putting the obtained graphene-modified electrode into a solution containing the precursor of nickel salt and aluminum salt; adding the supporting electrolyte; performing constant-potential electrolysis for 10 seconds; taking out the electrode; and washing the electrode with the deionized water and drying. In the invention, the electrolysis is performed under controlled potential to electrically and alternately deposit the graphene and bimetal hydroxide on the surface of the electrode, therefore, the reduction and deposition of the graphene oxide are finished at the same time, and more importantly, the accurate control on the layer thickness of graphene as well as the size and distribution density of bimetal hydroxide particles is realized; and moreover, the preparation of the material does not produce the 'three wastes' (waste gas, waste water and waste residues).
Owner:盐城市纺织染整产业园实业开发有限公司
Method for treating organic wastewater through synergistic activation of persulfate using electrochemistry and Ni-Fe-LDH/rGO catalyst
InactiveCN105731606AHigh catalytic activityImprove magnetic propertiesWater treatment compoundsWater/sewage treatmentSupporting electrolyteElectrolysis
The invention provides a method for treating organic wastewater through synergistic activation of persulfate using electrochemistry and a Ni-Fe-LDH / rGO catalyst. The method comprises the specific steps: adding persulfate and the Ni-Fe-LDH / rGO catalyst in an electrolysis device containing organic wastewater, degrading the organic wastewater under the effect of external electric field, providing a constant current for the electrolysis process by using a constant potential rectifier, using 0.1M of Na2SO4 as a support electrolyte in the electrolysis process, and continuously performing magnetic stirring in the test process so as to guarantee the sufficient contact of the catalyst and solution. The method not only can activate the persulfate through the electrochemical manner, but also can enhance the capability of the original catalyst to activate the persulfate, and multiple paths are used for synergistically degrading the organic pollutants. The method is simple in operation, flexible in reaction, high in catalysis efficiency, and good in degradation effect; the catalyst has good magnetism and stability, and has wide application prospect in the environment pollution governance field.
Owner:DALIAN UNIV OF TECH
A bright cyanide-free silver plating solution and preparation method thereof
The invention discloses non-cyanide bright silver electroplating bath, which consists of the following components in mass concentration: 50 to 800mg / L brightener, 25 to 60g / L silver ion source substance, 130 to 190g / L coordinating agent, 10 to 40g / L supporting electrolyte and 10 to 50g / L electroplating bath pH regulator, wherein the brightener is one or more kinds of amino acid compounds, imidazole, polyethylene glycol, quinoline derivatives and saccharin. Compared with the prior art, the non-cyanide bright silver electroplating bath has the outstanding advantages that: the electroplating bath is stable and low in toxicity; and an extremely small amount of brightener can obviously improve the performance of the electroplating bath and the quality of a coating. The coating has fine crystals, good bonding force, smooth and bright surface and high anti-tarnish property, can meet the requirement of application in multiple fields such as decorative electroplating, functional electroplatingand the like, has high practicability and can generate good economic and social benefits.
Owner:NANJING UNIV
Preparation method of composite anode of microbial fuel cell with carbon-base material modified by conductive complex
InactiveCN102780010AReduce usageReduce production processCell electrodesSupporting electrolyteInternal resistance
The invention relates to a preparation method of a composite anode of a microbial fuel cell with a carbon-base material modified by a conductive complex. The preparation method comprises purifying a multi-wall carbon nanotube; placing the purified multi-wall carbon nanotube into a 0.1 to 0.5mol / L supporting electrolyte solution, and dispersing the carbon nanotube in the solution, with 5% to 20% of carbon nanotube; adding 3,4-ethylenedioxythiophene monometers into the solution to obtain a well-dispersed 3,4-ethylenedioxythiophene / multi-wall carbon nanotube suspending solution; electrically depositing a complex on the anode surface by cyclic voltammetry; and carrying out vacuum drying, washing with deionized water, and room-temperature airing in sequence to obtain the modified anode. The method provided by the invention can reduce use amount of toxic reactants and shorten the preparation process so as to save the preparation cost. The modified anode has the advantages of peculiar surface effect, good conductivity and electrochemical activity, is remarkably improved in the maximum power density and open-circuit voltage in comparison with the unmodified anode when used in a battery, and can greatly reduce the battery internal resistance.
Owner:QINGDAO UNIV OF SCI & TECH
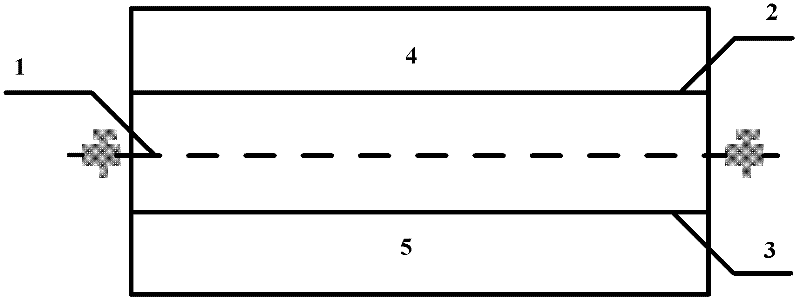
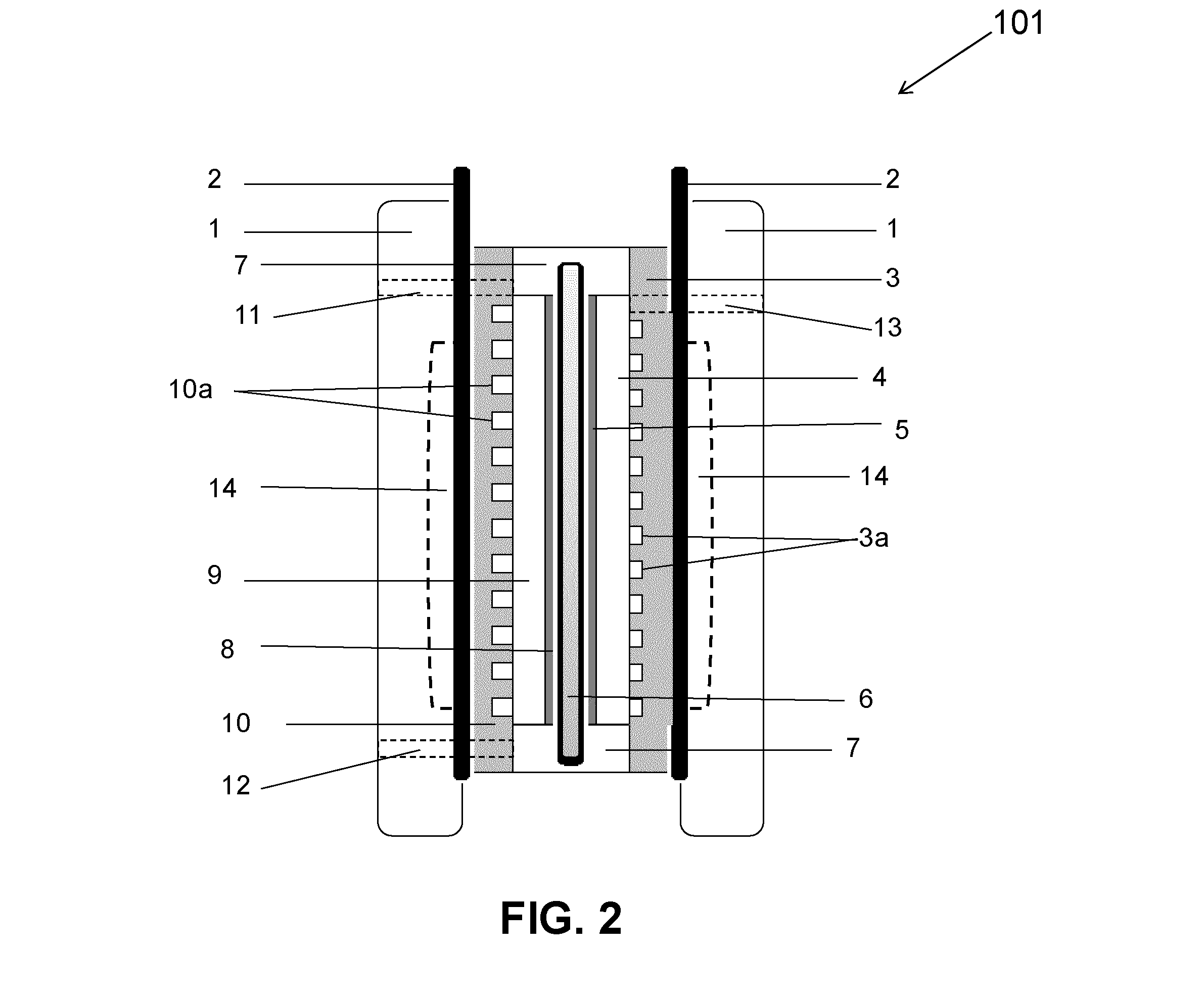
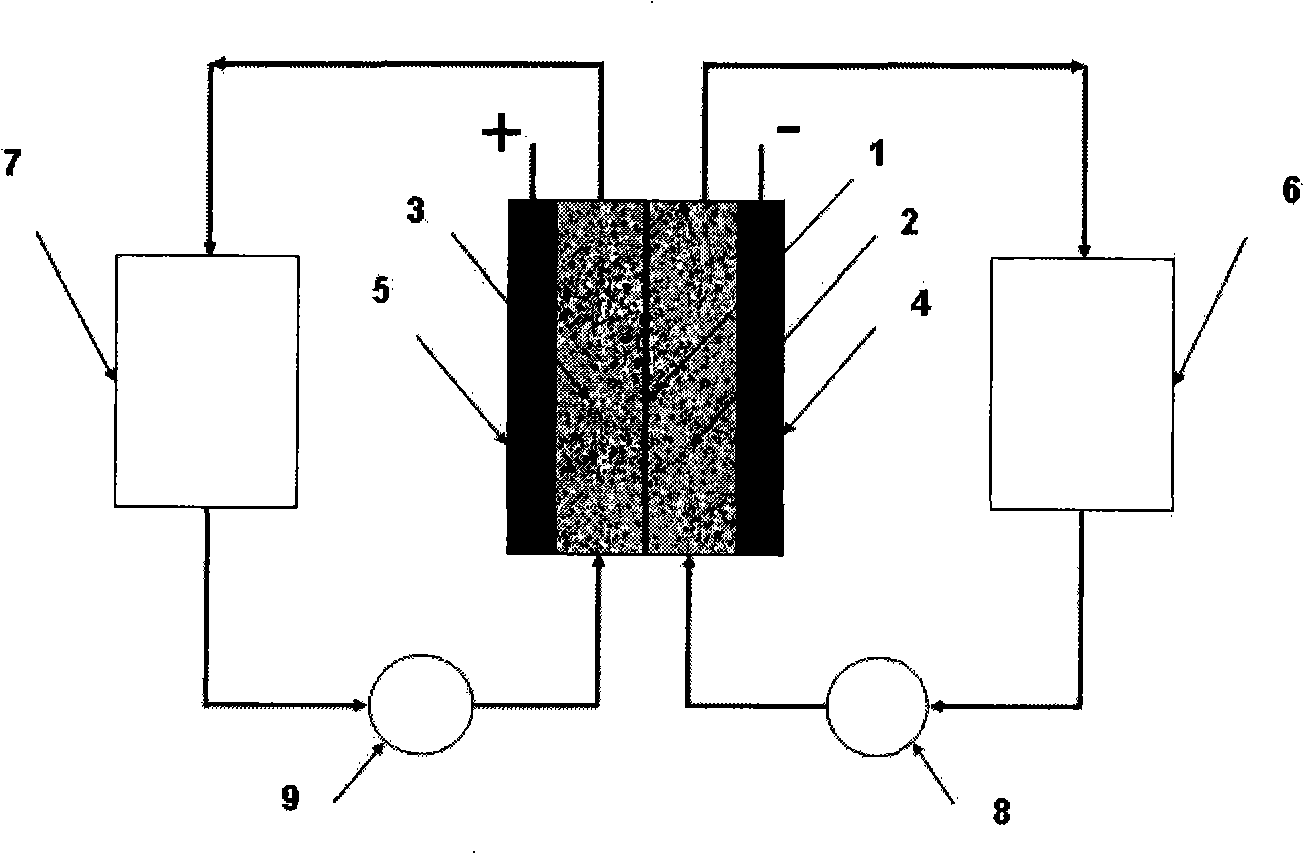
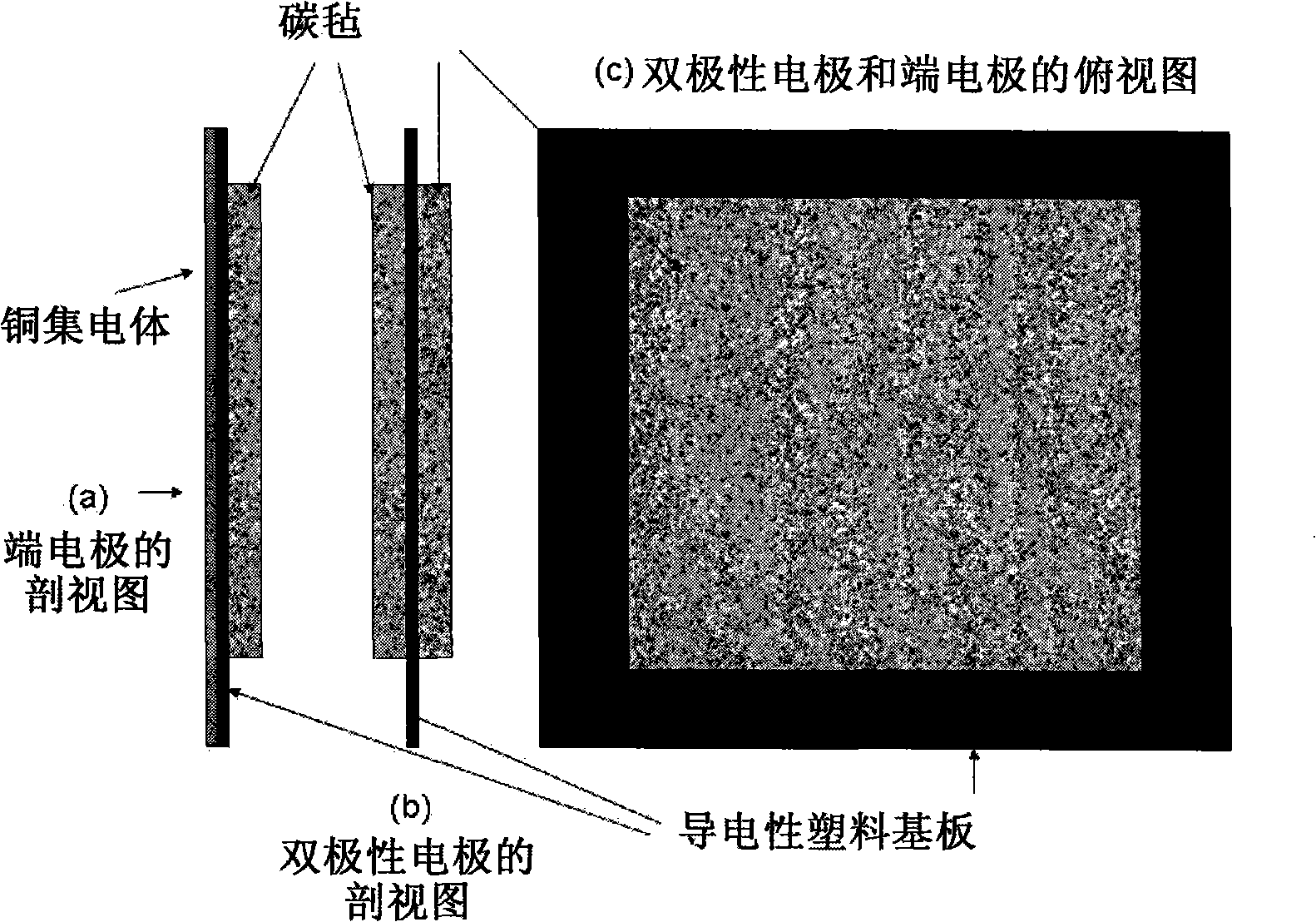
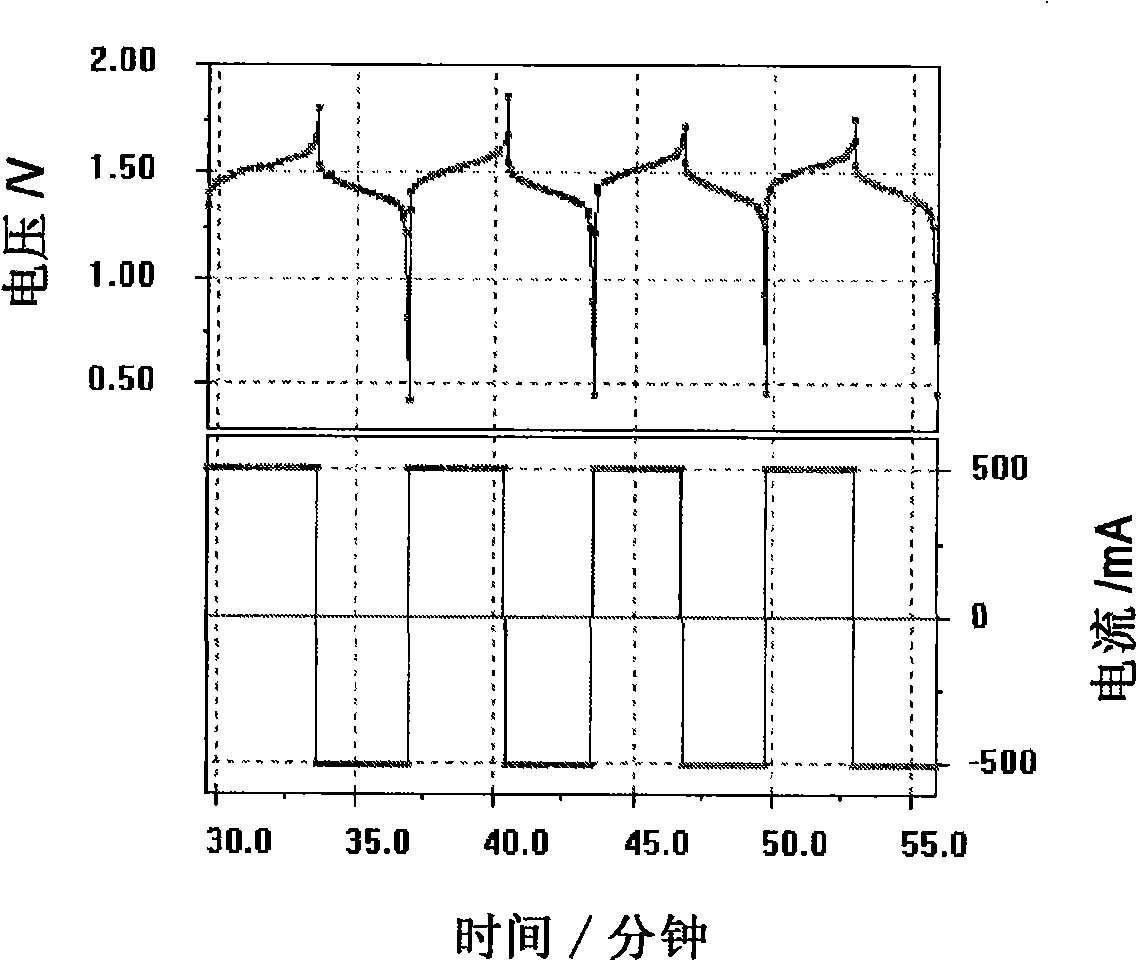
Neutral zinc iron double fluid flow battery
The invention discloses a neutral zinc iron double fluid flow battery, a single cell comprises positive and negative electrode end plates, a collector, a positive electrode, a film, a negative electrode, positive and negative electrode liquid storage tanks, a pipeline and a pump, wherein the positive electrode is a porous electrode, the negative electrode is a deposition type electrode, a positive electrode electrolyte solution is a neutral K4Fe (CN) 6 solution, and a negative electrolyte solution is a neutral zinc-ion-containing; when in charging, the electrolyte solutions respectively pass through the positive and negative electrode liquid storage tanks to be respectively sent to the positive electrode and the negative electrode, K3Fe (CN) 6 is produced by oxidation reaction of active material K4Fe (CN) 6 in the positive electrode storage tank, and zinc ions are directly deposited in the form of elemental zinc in the negative electrode; when in discharge, K4Fe (CN) 6 is produced by reduction reaction of active material K3Fe (CN) 6 in the positive electrode electrolyte solution, and the elemental zinc is oxidized into zinc ions to return through the pump back to the negative electrode liquid storage tank. The positive and negative electrode electrolyte solutions are neutral solutions, overcome the corrosion problems caused by a strong acid and a strong alkali as support electrolytes in the traditional liquid flow battery.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
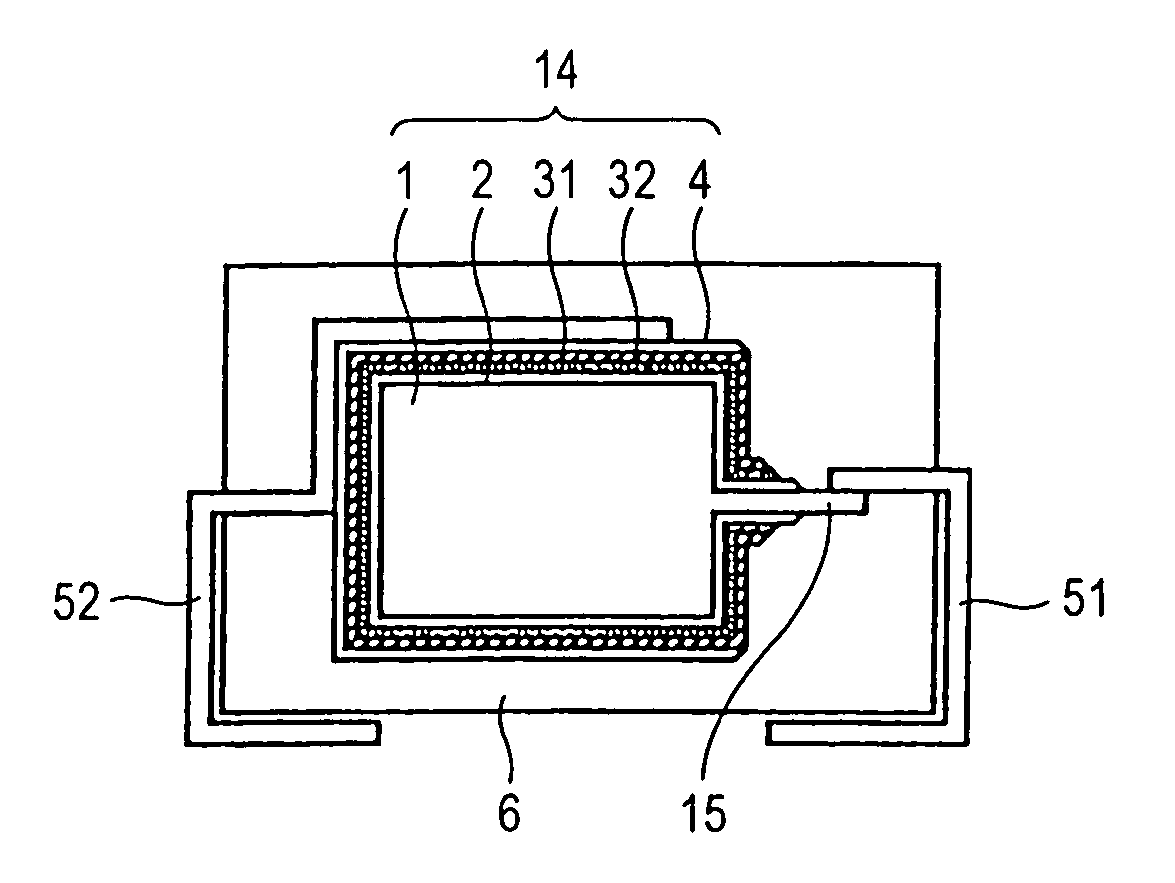
Flat non-aqueous electrolyte secondary cell
InactiveUS20050271938A1Improve discharge characteristicsImprovement of the heavy-loading discharge characteristicsFinal product manufactureElectrode carriers/collectorsSupporting electrolyteElectricity
In a flat non-aqueous electrolyte secondary cell comprising an electricity-generating element including at least a cathode, a separator and an anode and a non-aqueous electrolyte in the inside of a cathode case, a plurality of electrode units each consisting of the cathode and the anode opposite to each another via the separator are laminated to form an electrode group, or an electrode unit in a sheet form consisting of the cathode and the anode opposite to each another via the separator is wound to form an electrode group, or a sheet-shape cathode is wrapped with the separator except for a part contacting at inner face of cathode case and a sheet-shaped anode is set on the sheet-shaped cathode in a right angled position each other and then these cathode and anode are bent alternately to form an electrode group, and the total sum of the areas of the opposing cathode and anode in this electrode group is larger than the area of the opening of an insulating gasket in a sealed portion in the cathode case or than the area of an opening in a sealed plate in a sealed portion in the cathode case, whereby the discharge capacity upon heavy-loading discharge is significantly increased as compared with the conventional cells. Accordingly, while the size of the cell is small, the discharge capacity is increased as described above, and thus it is possible to provide a highly utilizable flat non-aqueous electrolyte secondary cell. Further, in said flat non-aqueous electrolyte secondary cell, problems which may be caused by the increased discharge capacity in the cell can be solved by improving the solvent and supporting electrolyte for the electrolyte or by various improvements in the cathode and anode cases.
Owner:MAXELL HLDG LTD
Method and device for extracting and enriching lithium
ActiveUS9062385B2Efficient separationShort processing flowSludge treatmentVolume/mass flow measurementSupporting electrolyteIon-exchange membranes
A method for extracting and enriching lithium, including: (a) providing an electrodialysis device including an electrodialysis cell; (b) dividing the electrodialysis cell into a lithium salt chamber and a brine chamber using an anion exchange membrane; (c) filling the brine chamber with salt lake brine; (d) filling the lithium salt chamber with a Mg2+ free supporting electrolyte solution; (e) placing a conductive substrate coated with an ion sieve in the brine chamber to operate as a cathode; (f) placing a conductive substrate coated with a lithium-intercalated ion sieve in the lithium salt chamber to operate as an anode; and (g) carrying out an electrodialysis.
Owner:CENT SOUTH UNIV
Treatment technology for lithium ion battery current collector with conductive polymer film on surface
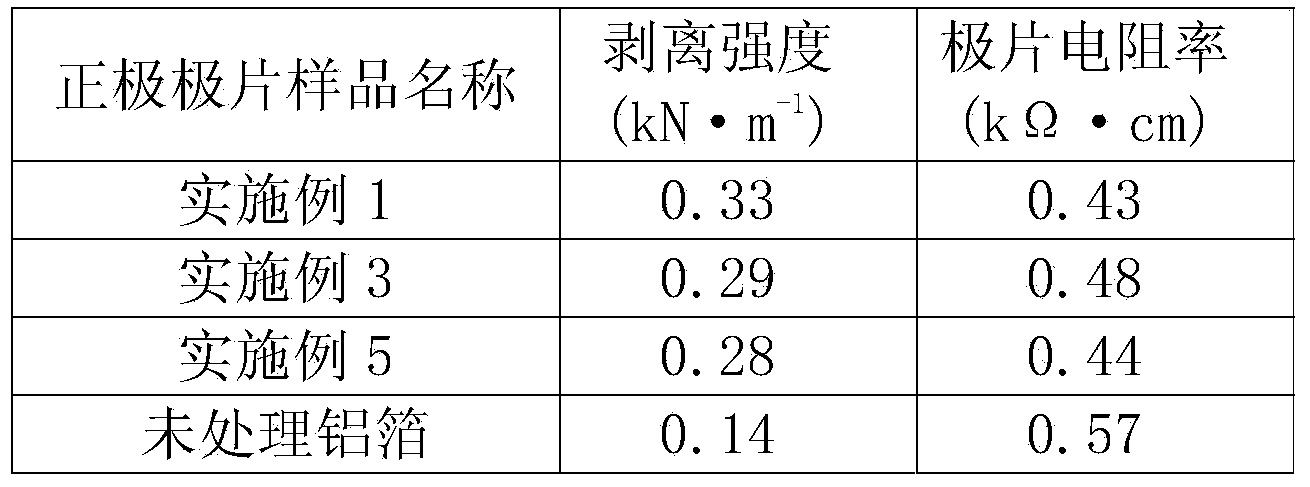
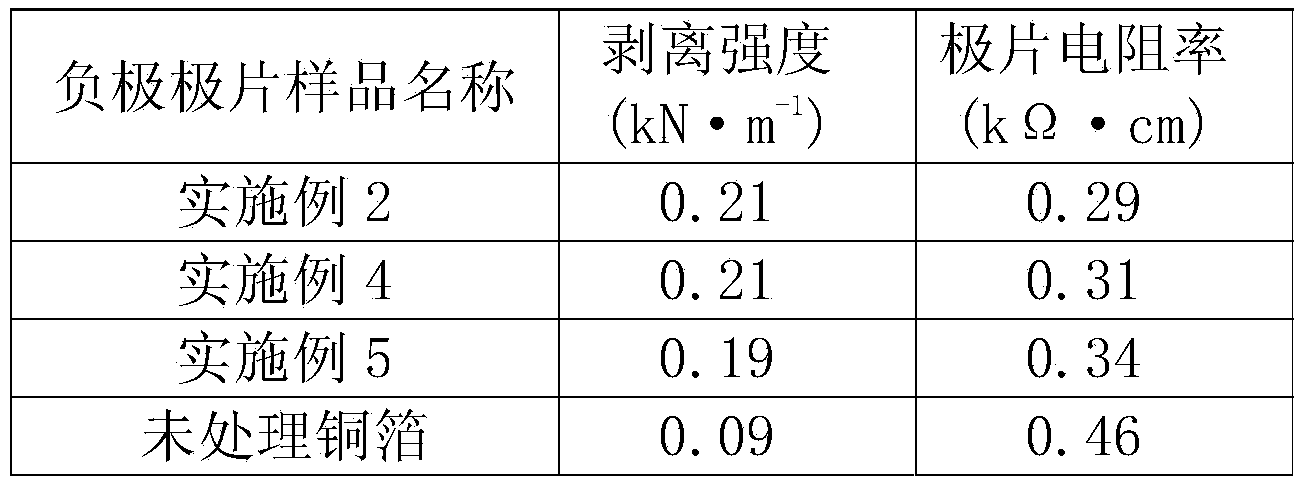
ActiveCN103985877AImprove corrosion resistanceStop erosionElectrode manufacturing processesElectrode carriers/collectorsSupporting electrolyteInternal resistance
The invention discloses a treatment technology for a lithium ion battery current collector with a conductive polymer film on a surface, relating to the technical field of lithium ion battery manufacturing. The treatment technology comprises the steps of dissolving a conductive polymer monomer into acidic liquid to obtain supporting electrolyte, enabling the supporting electrolyte to permeate into the lithium ion battery current collector subjected to surface cleaning so as to be used as a working electrode, and depositing a conductive polymer film layer which is 0.5-2 microns thick on the surface of the lithium ion battery current collector by regulating corresponding electrochemical parameters by an electrochemical method. The deposited conductive polymer film layer of the lithium ion battery current collector is uniform and compact, is firmly adhered to substrate metal, and is high in bonding force with an active substance; the peeling strength of the active substance can be enhanced, the using amount of an adhesive is reduced, and the contact internal resistance is reduced. The conductive polymer film layer is high in corrosion resistance so as to effectively prevent corrosion of the electrolyte to the current collector; meanwhile, due to the excellent conductivity, side effects on the charging and discharging performance of the battery are avoided. The equipment is simple and is convenient to control, treatment can be realized at normal temperature, and large-scale production can be realized.
Owner:HEFEI GUOXUAN HIGH TECH POWER ENERGY
Method of producing solid electrolytic capacitor
InactiveUS7125764B2Increase capacitanceReduce leakage currentEmergency protective circuit arrangementsCapacitor electrolytes/absorbentsDielectricSupporting electrolyte
Owner:SAN DENSHI INDS
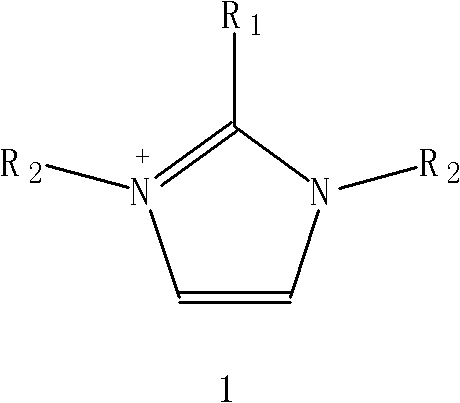
Electrochromic Display Device
InactiveUS20100134865A1Static indicating devicesNon-linear opticsSupporting electrolytePotential difference
Disclosed is an electrochromic display device comprising: a first substrate; a first electrode; a second substrate; a second electrode; and an electrochromic composition layer, wherein the device is of a passive matrix drive where the device performs a display by an energization between the electrodes, and performs a erasion of the display, wherein the first electrode comprises electrodes, the second electrode comprises a plurality of transparent display electrodes, a pixel is formed where the electrodes are in a grade separated crossing, at least a surface of the electrodes is respectively oxidized, the electrochromic composition layer comprising (i) insulative partition walls and (ii) an electrochromic composition including a supporting electrolyte, a polar solvent, and a leuco dye, and wherein the device displays a selected pixel by applying a voltage of a first potential difference, and applies the voltage of a second potential difference so as not to cause any energization.
Owner:FUNAI ELECTRIC CO LTD
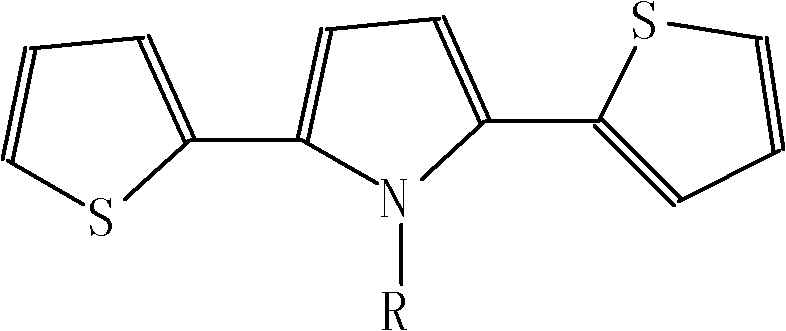
Poly (3,4-alkylenedioxythiophene)-based capacitors using ionic liquids as supporting electrolytes
ActiveUS6965509B2Liquid electrolytic capacitorsFinal product manufactureLithiumSupporting electrolyte
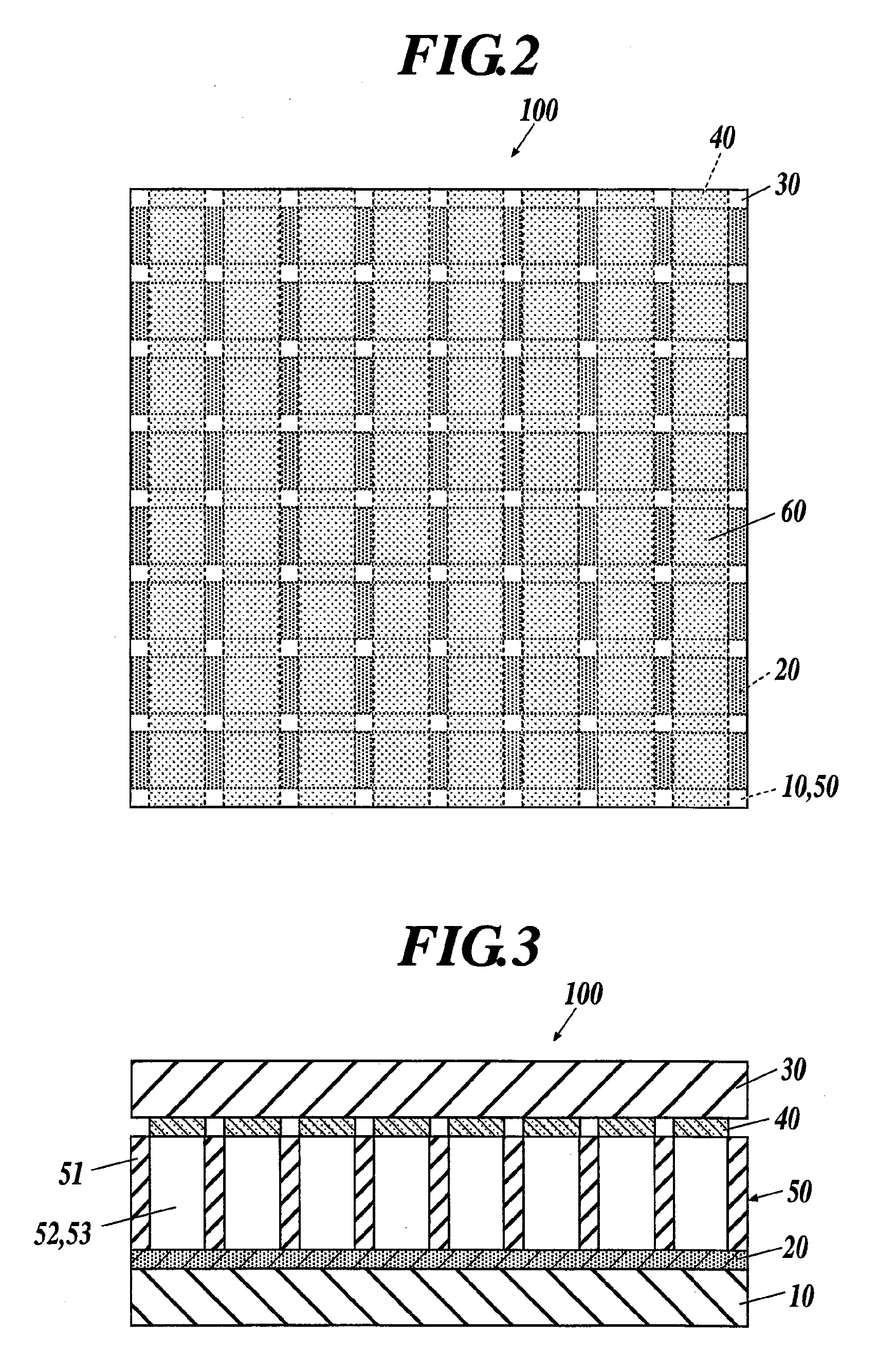
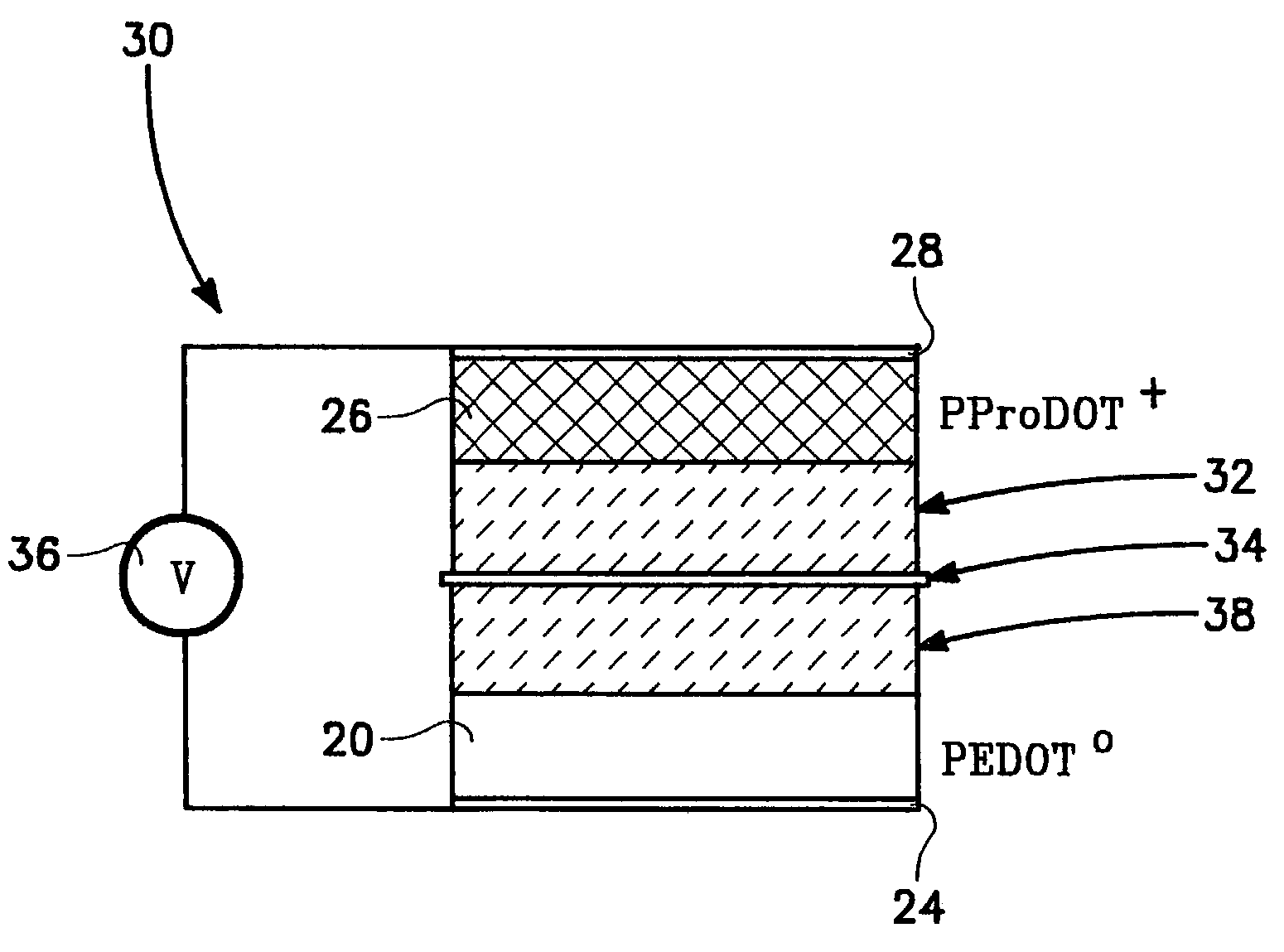
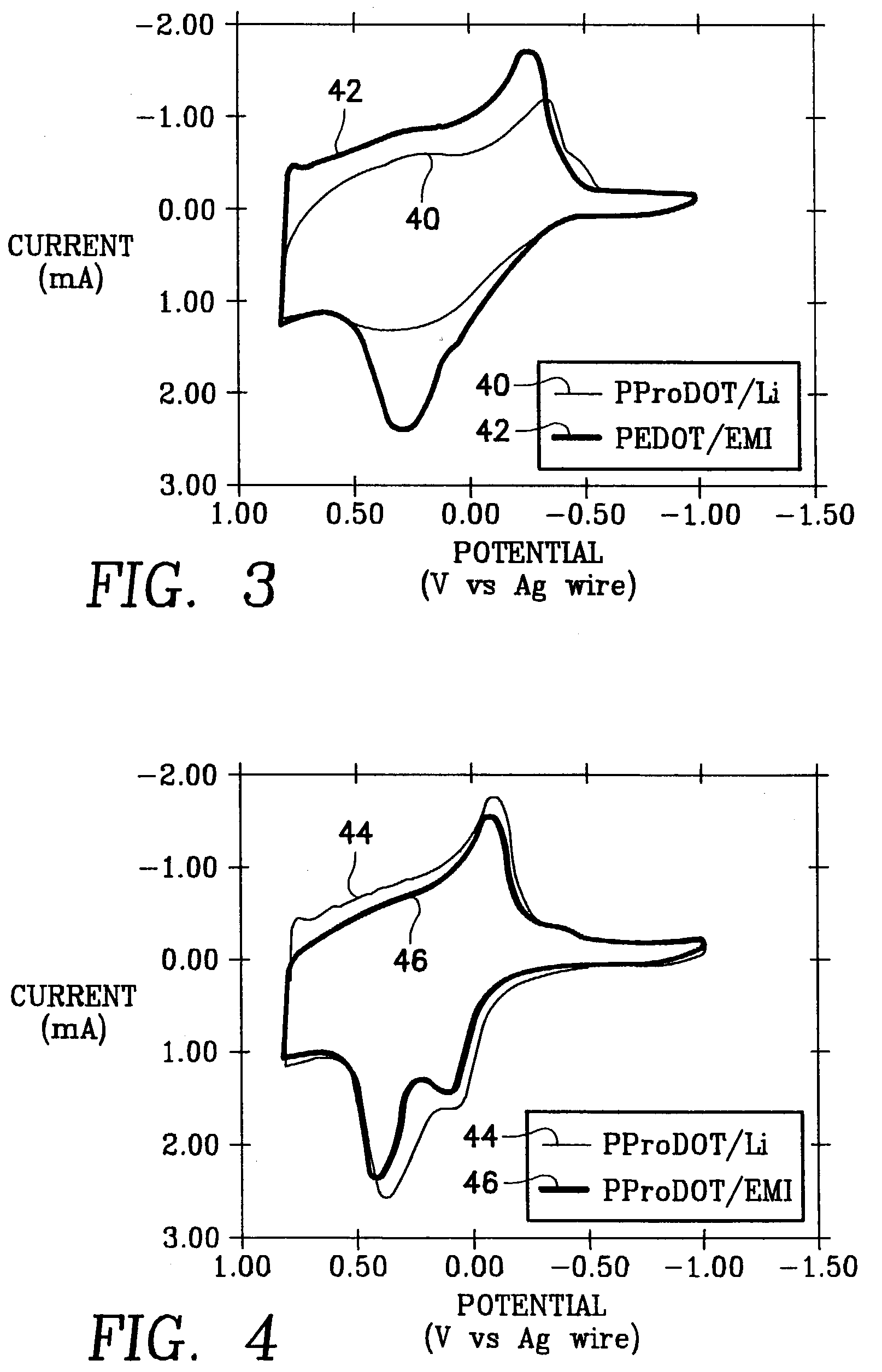
A supercapacitor comprising a poly(3,4-ethylendioxythiophene) (PEDOT) and poly(3,4-propylenedioxythiophene) (PProDOT) as electrode couples for the capacitor and a pair of gel electrolyte layers disposed between the electrodes. The gel electrolytes are separated by a battery paper and are selected from a group consisting of a lithium salt and an organic electrolyte.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com