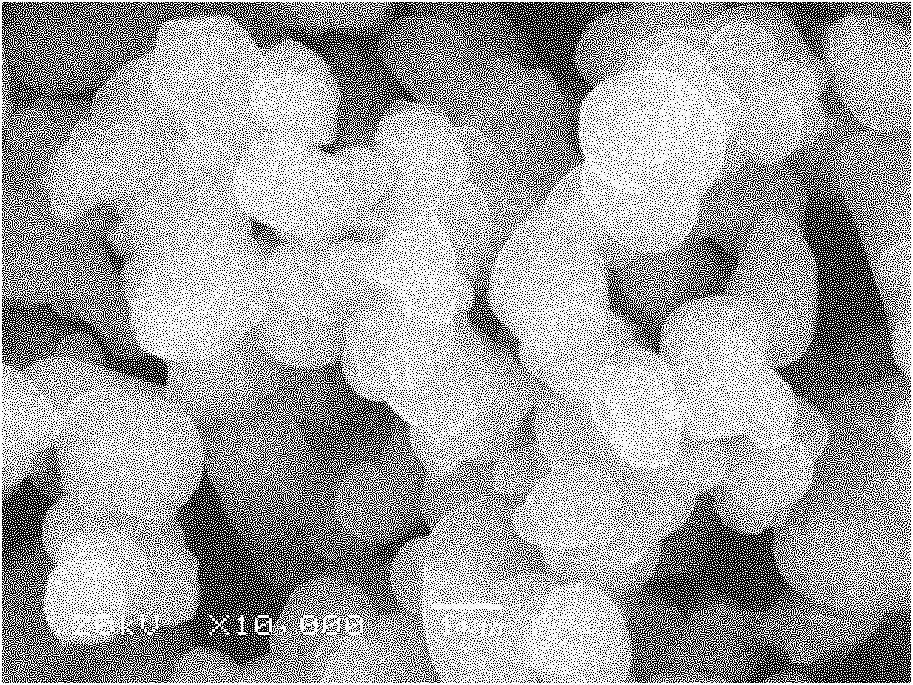
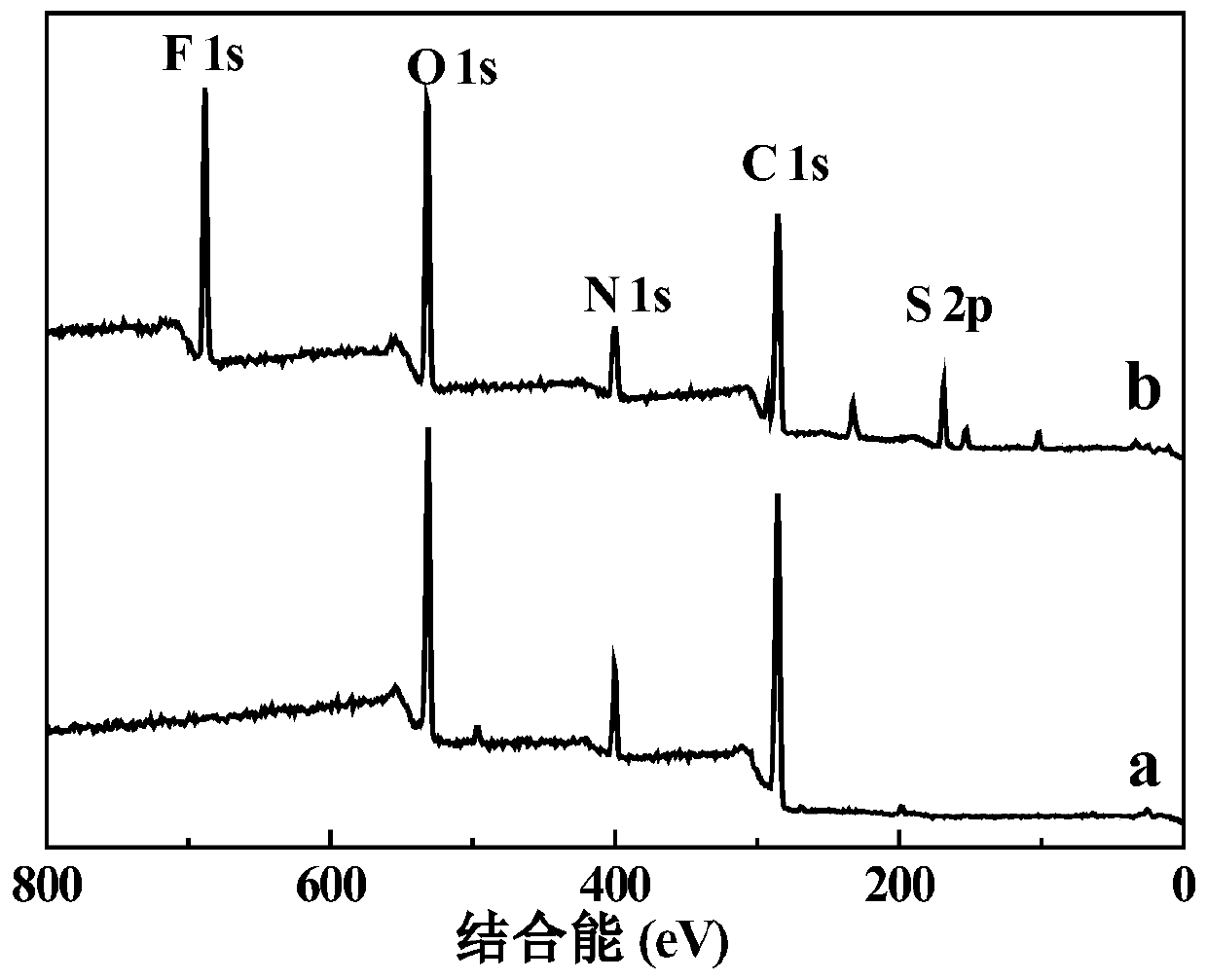
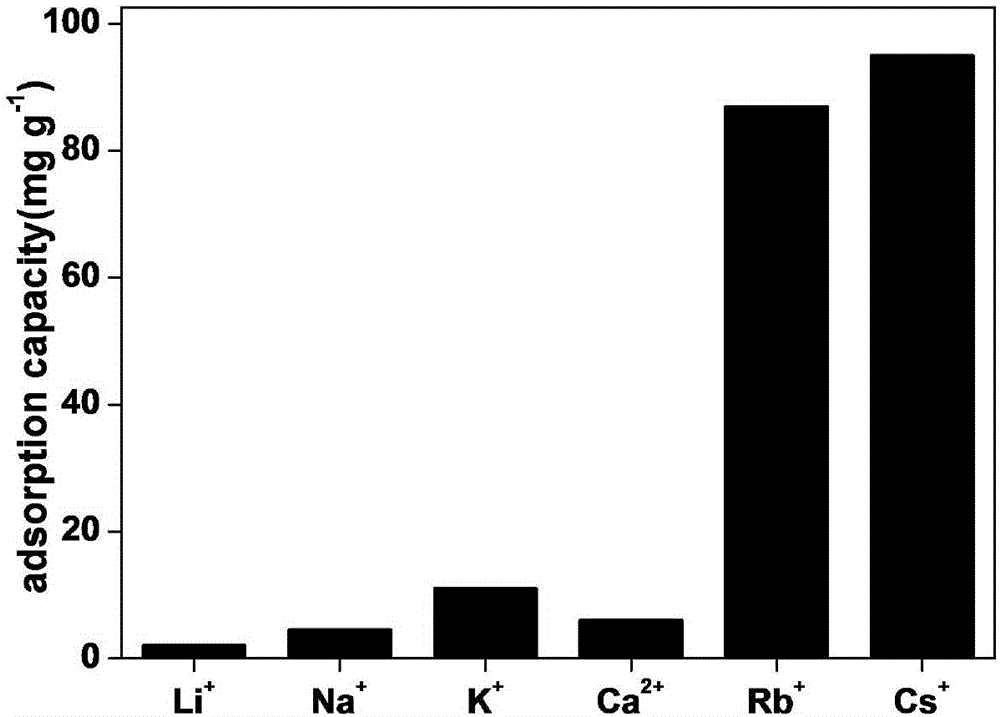
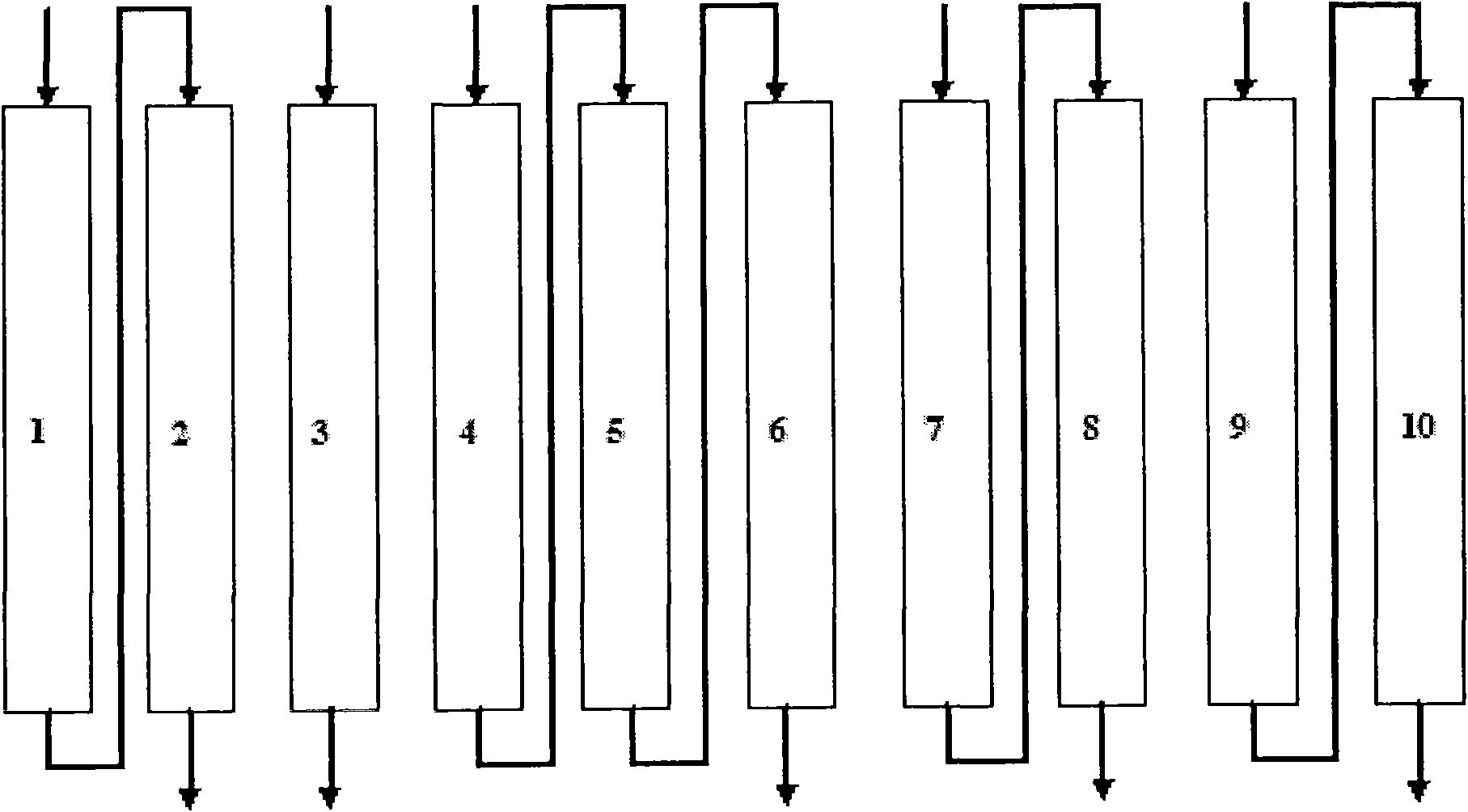
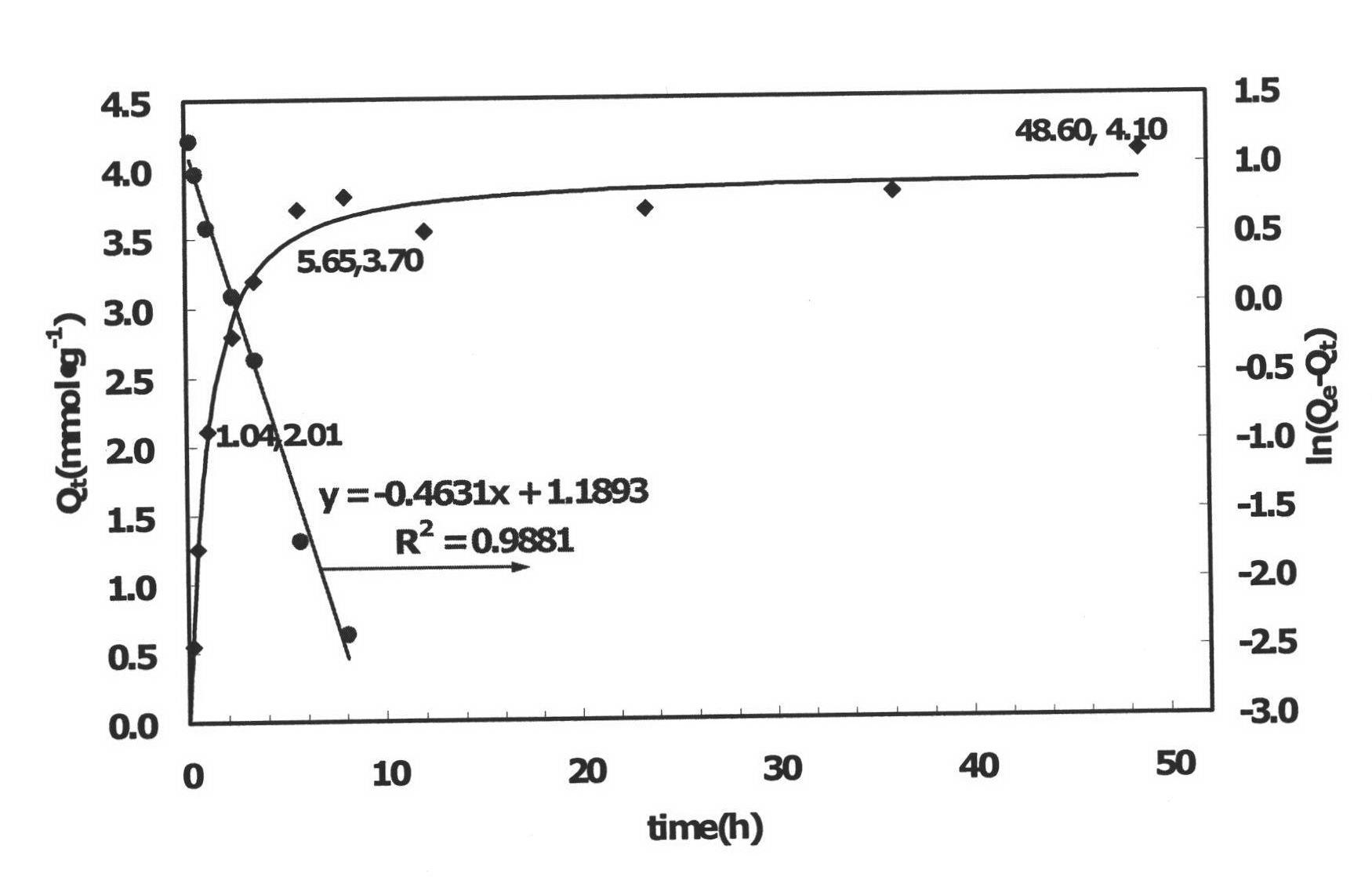
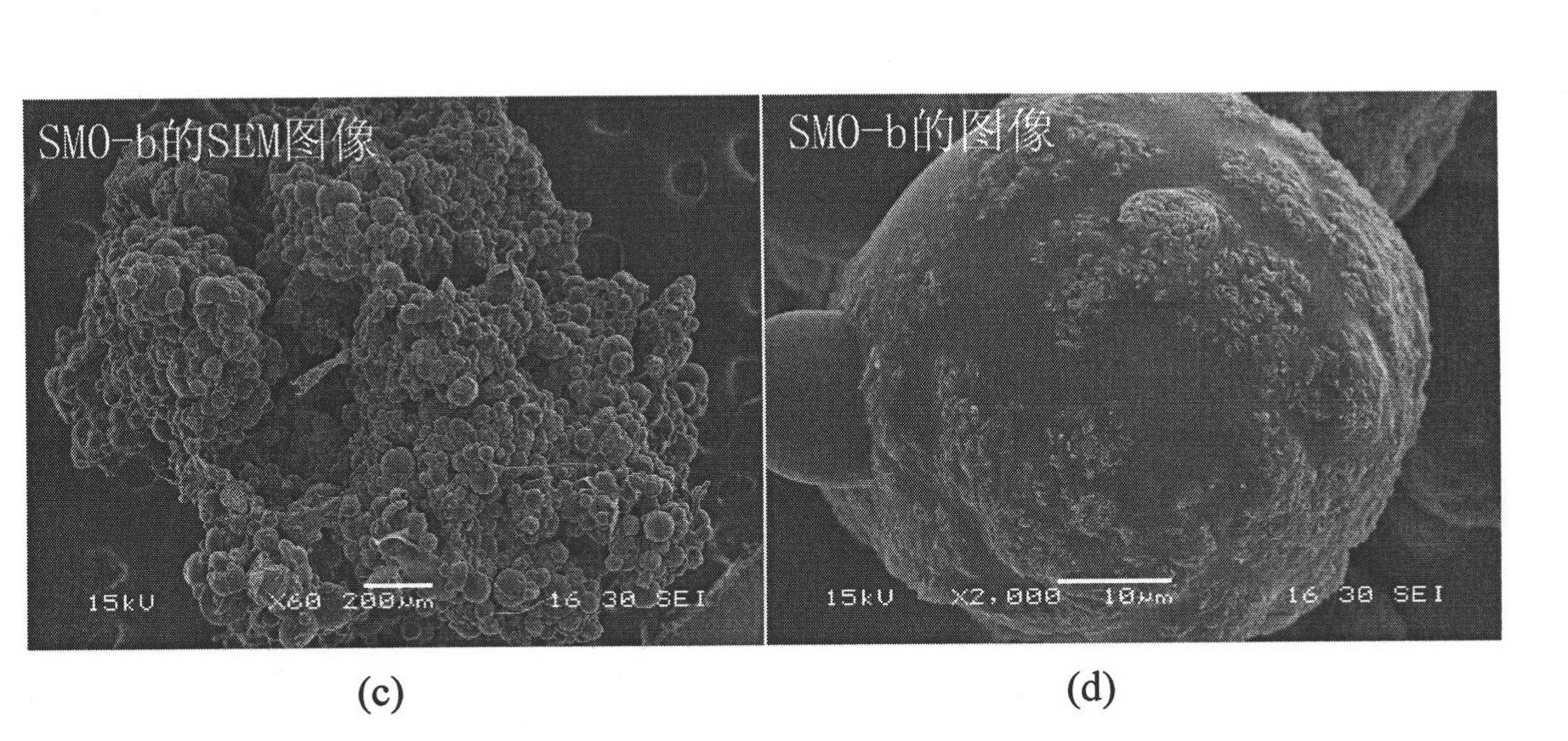
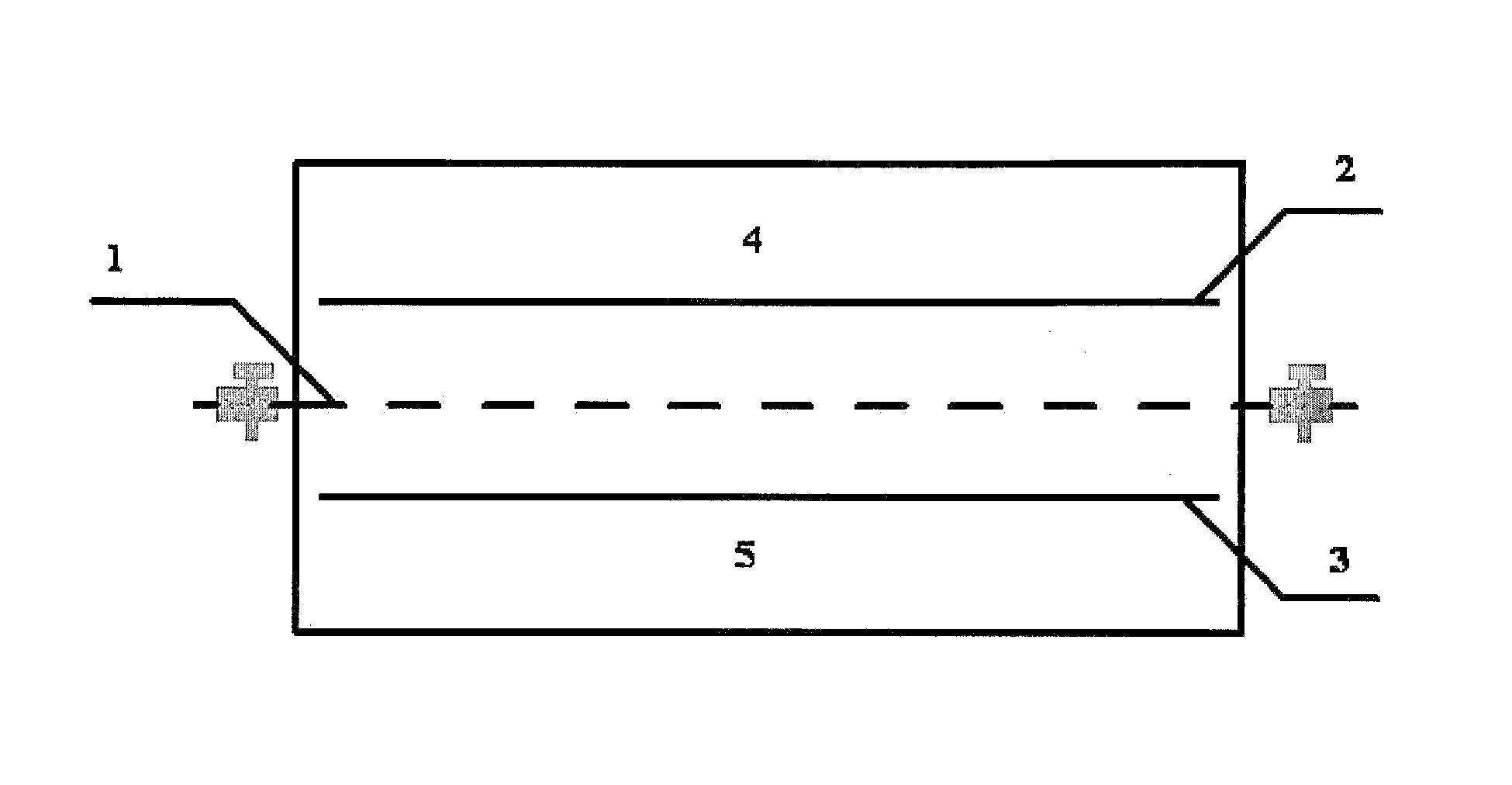
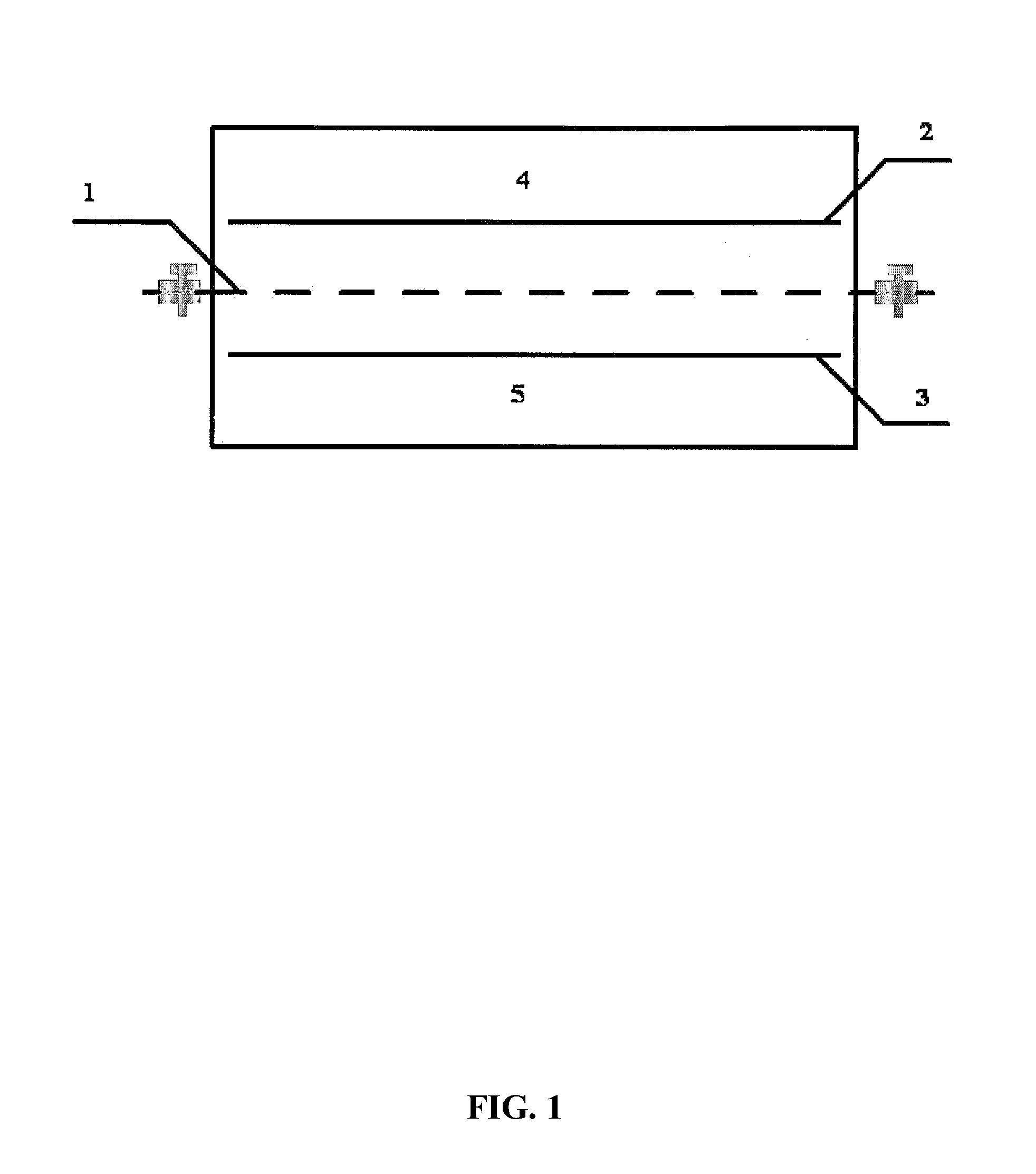
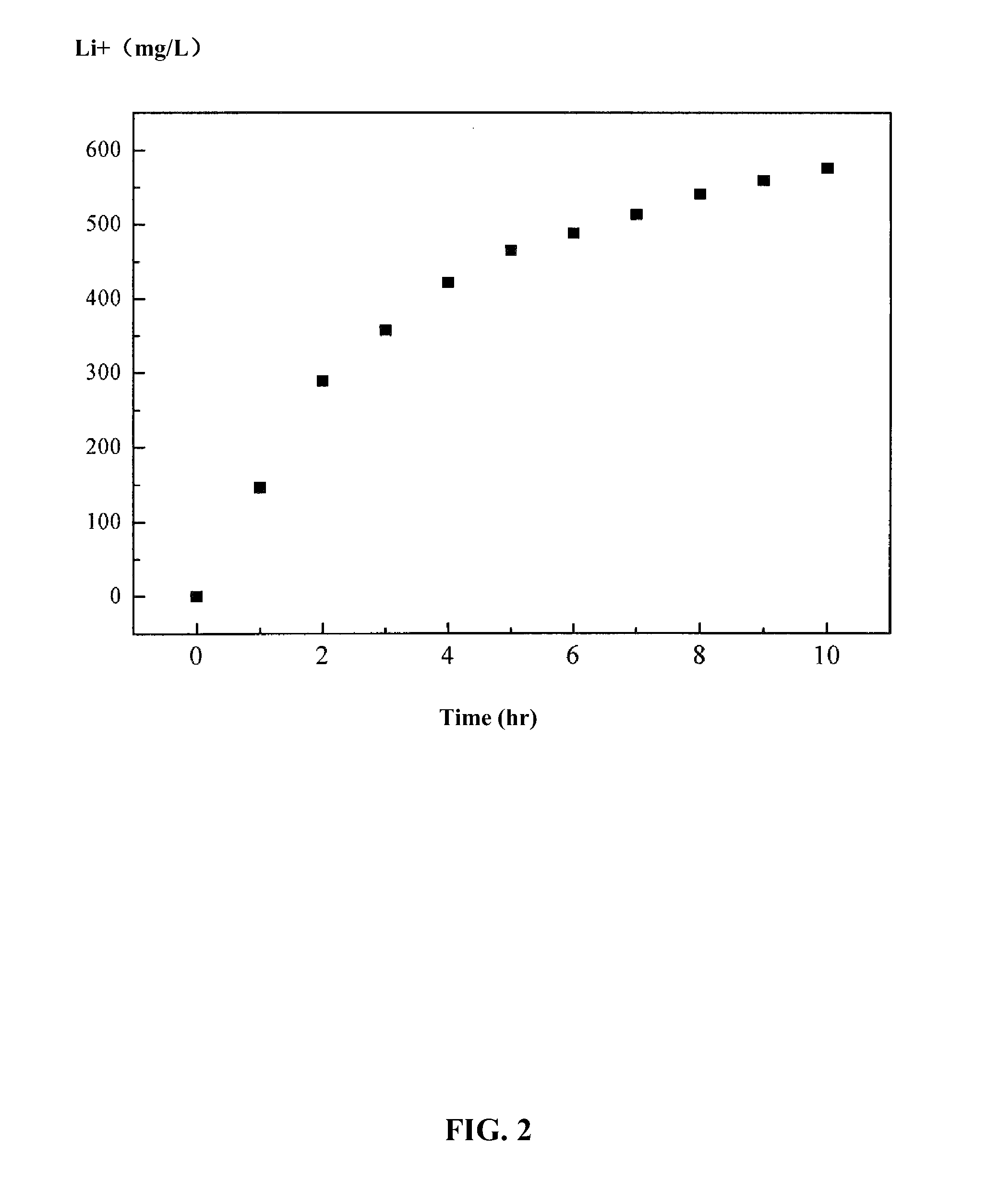
Patents
Literature
504 results about "Salt lake brine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Brine lakes consist of water that has reached salt saturation or near saturation ( brine ), and may also be heavily saturated with other materials. Most brine lakes develop as a result of high evaporation rates in an arid climate with a lack of an outlet to the ocean. The high salt content in these bodies...
Process for extracting lithium from salt lake brine by adsorptive method
InactiveCN1511964AEasy to makeNo pollutionProcess efficiency improvementLithium chlorideLithium carbonate
The present invention relates to adsorption process of extracting lithium from salt lake brine, and the process is suitable for producing lithium carbonate and lithium chloride with lithium-containing Qinghai saline lake brine, including concentrated lithium-containing Qinghai saline lake brine. The process includes sun shining saline lake brine to obtain concentrated lithium containing brine, adsorption of lithium ion with aluminum salt adsorbent, eluting adsorbed lithium ion with water, and refining and concentrating the elutriant to obtain material for preparing lithium carbonate and lithium chloride.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
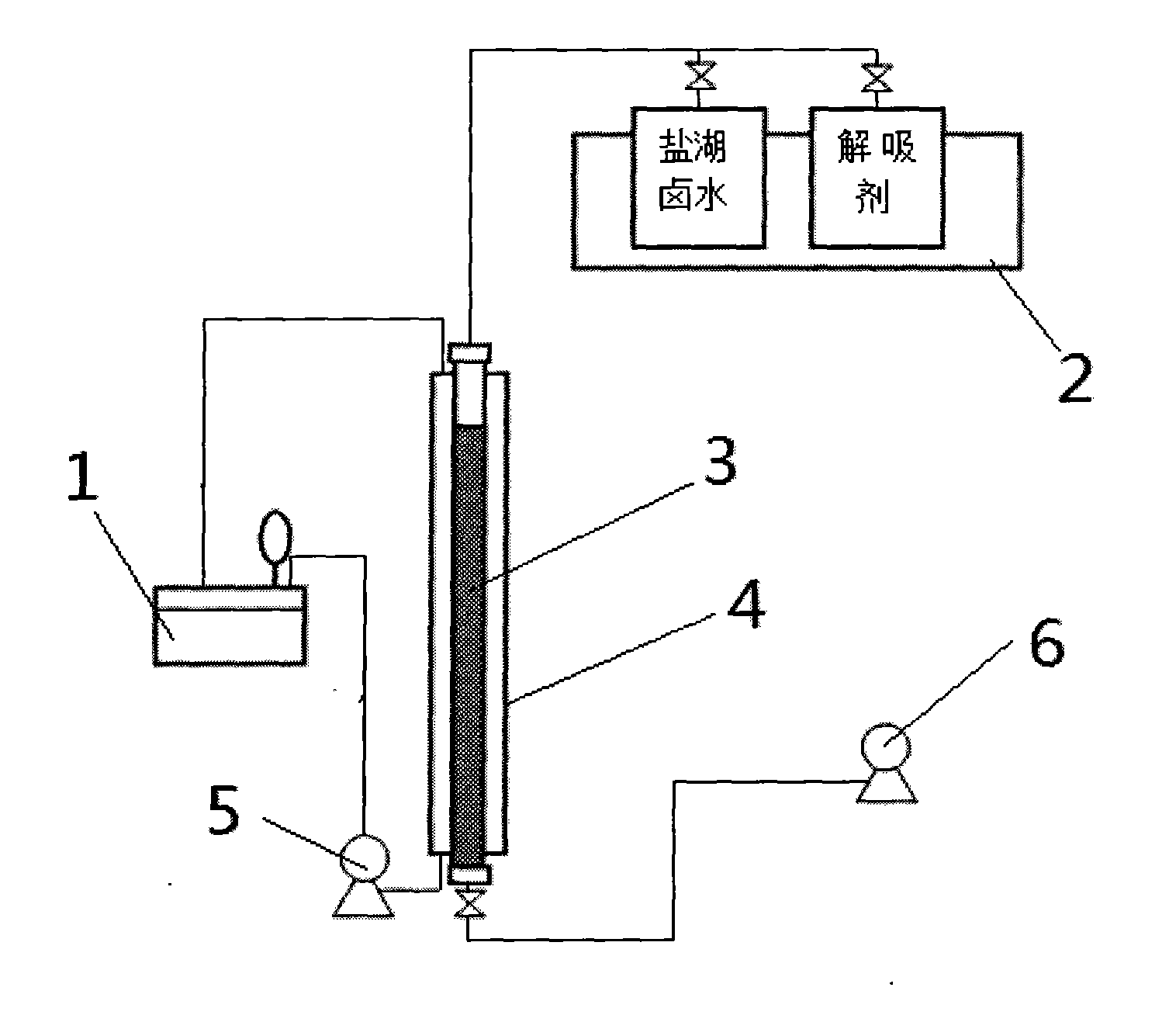
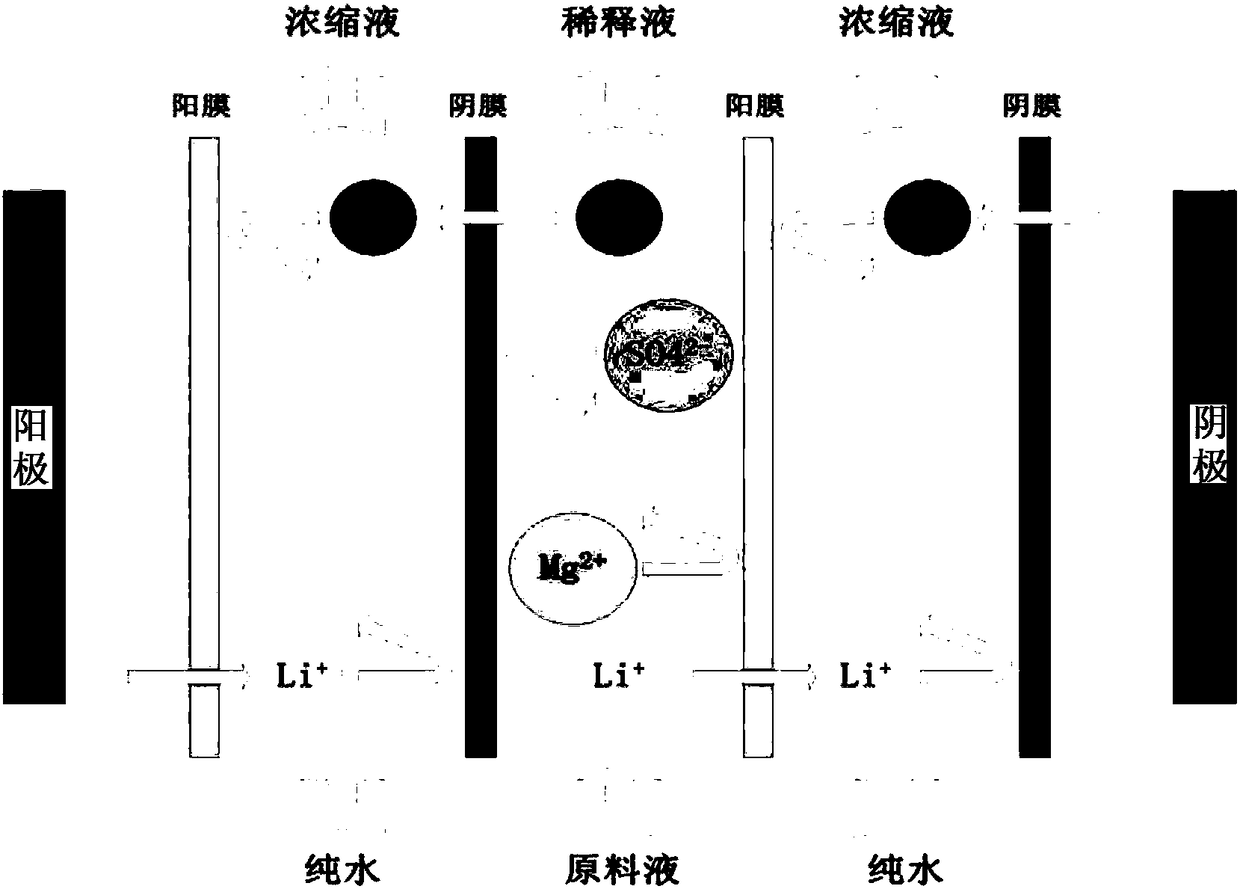
Method and device for separating magnesium and lithium and enriching lithium from salt lake brine
ActiveCN102382984AGood choiceImprove stabilityProcess efficiency improvementSupporting electrolyteIon-exchange membranes
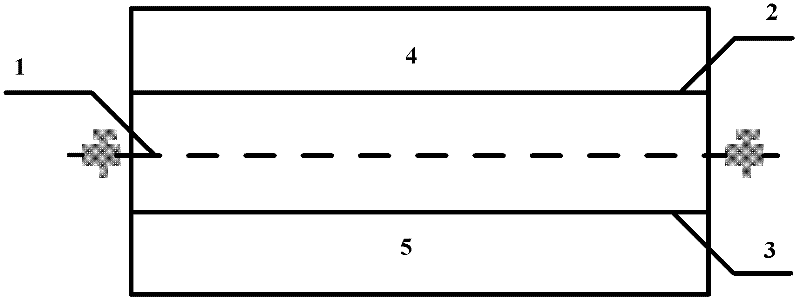
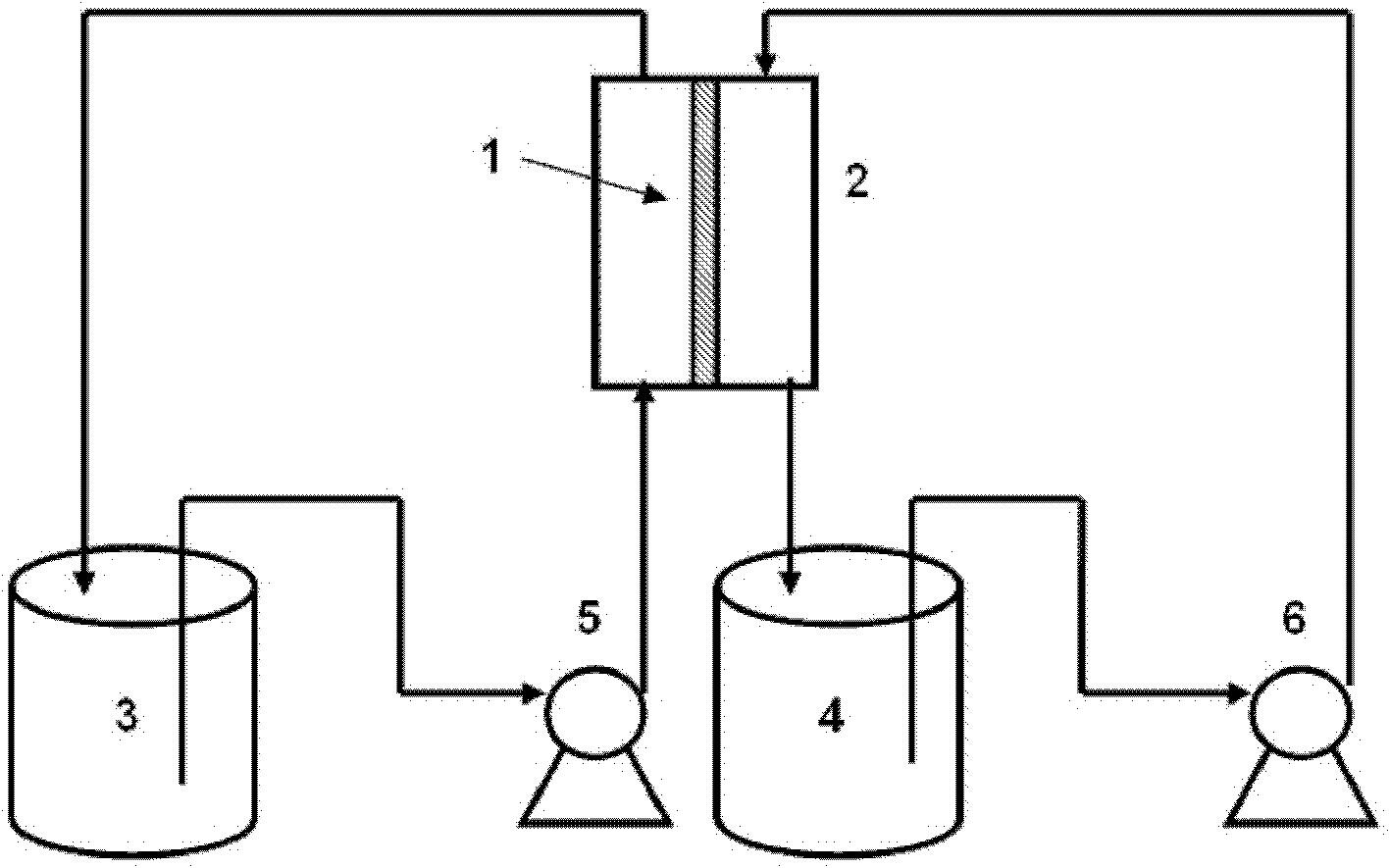
The invention relates to a method and a device for separating magnesium and lithium and enriching the lithium from salt lake brine. The method comprises the following steps of: separating an electrodialyzing device into two areas by using an anion exchange membrane, namely a lithium salt chamber and a brine chamber, filling the salt lake brine in the brine chamber, and filling a supporting electrolyte solution which does not contain Mg<2+> in the lithium salt chamber; placing a conducting matrix coated by an ionic sieve in the brine chamber as a cathode; placing the conducting matrix coated by a lithium-embedded ionic sieve in the lithium salt chamber as an anode; under the driving of an external electric potential, embedding Li <1+> in the brine in the brine chamber into the ionic sieve to form the lithium-embedded ionic sieve, and recovering the lithium-embedded ionic sieve into the ionic sieve after the lithium-embedded ionic sieve in the lithium salt chamber releases the Li <1+> into a conducting solution; and discharging a liquid in the brine chamber after the lithium is embedded, adding the salt lake brine again, alternatively placing electrodes in the two chambers, and repeating and circulating operations. Through the method and the device for separating magnesium and lithium and enriching lithium in the salt lake brine, the separation of the lithium and other ions is effectively realized, and a lithium-enriched solution is synchronously obtained. The method has a short flow and low production cost, is simple to operate, can be operated continuously, and is easy to industrially apply.
Owner:CENT SOUTH UNIV
Nano-filtration method for separating magnesium and enriching lithium from salt lake brine
InactiveCN1542147AEfficient recyclingSimple processSemi-permeable membranesLithium compoundsIon contentLithium chloride
The nanofiltration process is suitable for Mg-Li separation and Li enriching of Li containing bittern or Li containing solution from salt lake to prepare lithium carbonate or lithium chloride with the Li enriching bittern. Nanofiltration membrane is used in separating and enriching lithium from lithium containing bittern, which contains Mg, Ca and other cations and sulfate radical and other anions and has Li ion concentration of 0.1-11.5 g / L and Mg / Li ion weight ratio 1-200, to obtain lithium enriched bittern suitable for preparing lithium carbonate or lithium chloride. The said process is effective, and can obtain lithium enriched bittern with Mg / Li ion weight ratio 0.6-5 and Li ion content of 0.6-20 g / L.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
Method for extracting alkali metal from salt lake brine and seawater through membrane extraction-back extraction
InactiveCN102312110AEfficient extractionEfficient enrichmentLiquid solutions solvent extractionRubidiumIon-exchange membranes
The invention discloses a method for extracting high-value alkali metal from salt lake brine or seawater through membrane extraction-back extraction. The method is implemented through continuous operation and comprises the following steps of: fixing an ion exchange blend membrane in a membrane component, allowing an organic solution containing an extracting agent to contact salt lake brine or seawater which contains alkali metal ions by a first ion exchange membrane, and allowing alkali metal ions to pass through the ion exchange membrane and be combined with the organic solution containing the extracting agent to obtain metal complex; then transmitting an organic solution of the metal complex to a second ion exchange membrane and allowing the organic solution of the metal complex to contact a back extraction solution by the second ion exchange membrane, and allowing the metal ions to pass through the ion exchange membrane to enter the back extraction solution; during membrane extraction-back extraction, and circulating feed liquid, the back extraction solution and the organic solution containing the extracting agent on one side of the first ion exchange membrane, on one side of the second ion exchange membrane and between the first and second ion exchange membranes; and performing back extraction until a certain concentration of the back extraction solution is reached, and separating lithium, rubidium or caesium precipitates to obtain the final product. The invention provides a high-efficiency, low-cost and feasible route for industrial production of alkali metal salts.
Owner:何涛 +1
Method for extracting lithium from salt lake brine by adsorption method
ActiveCN101928828ALow investment costHigh adsorption capacityProcess efficiency improvementSODIUM CATIONSalt lake
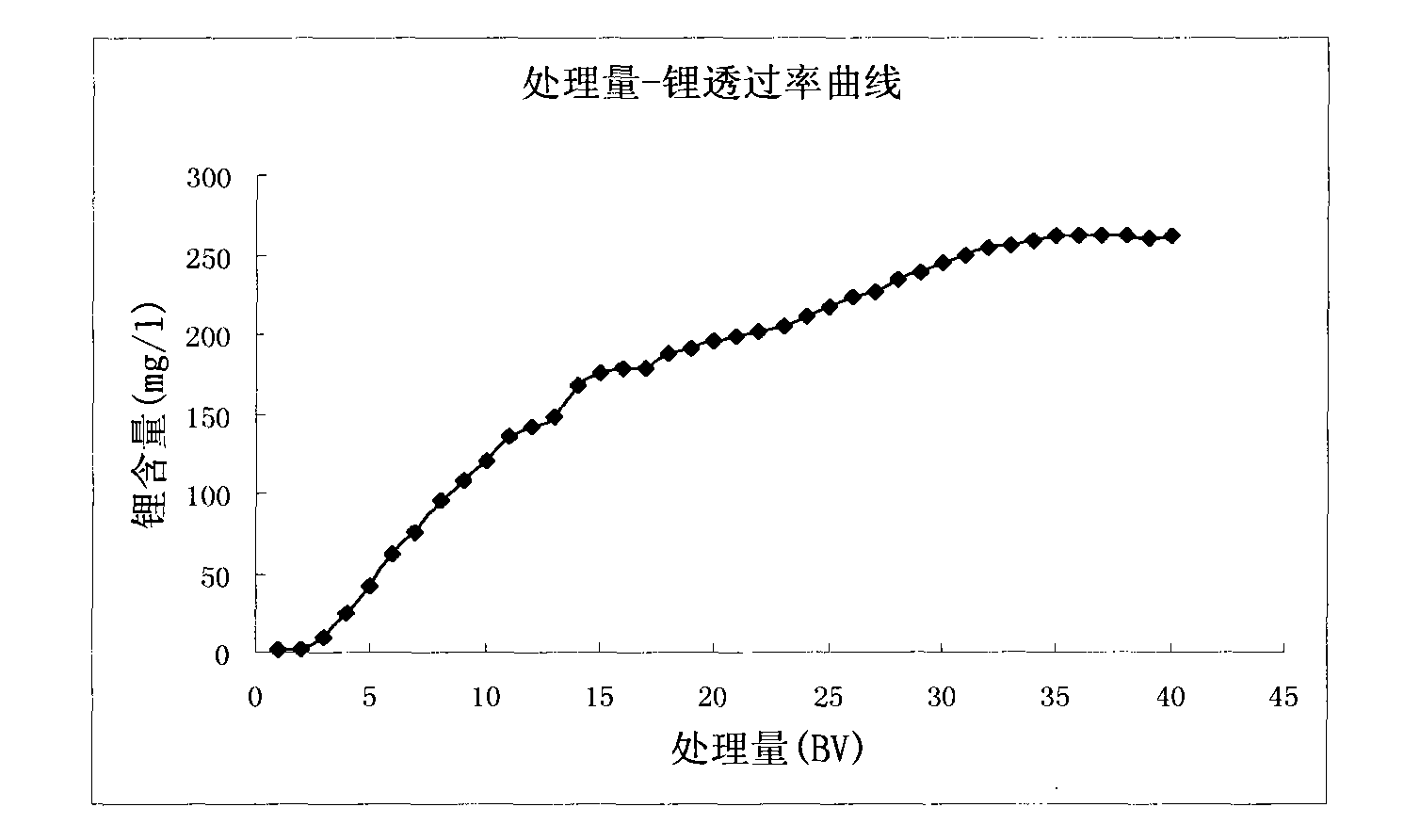
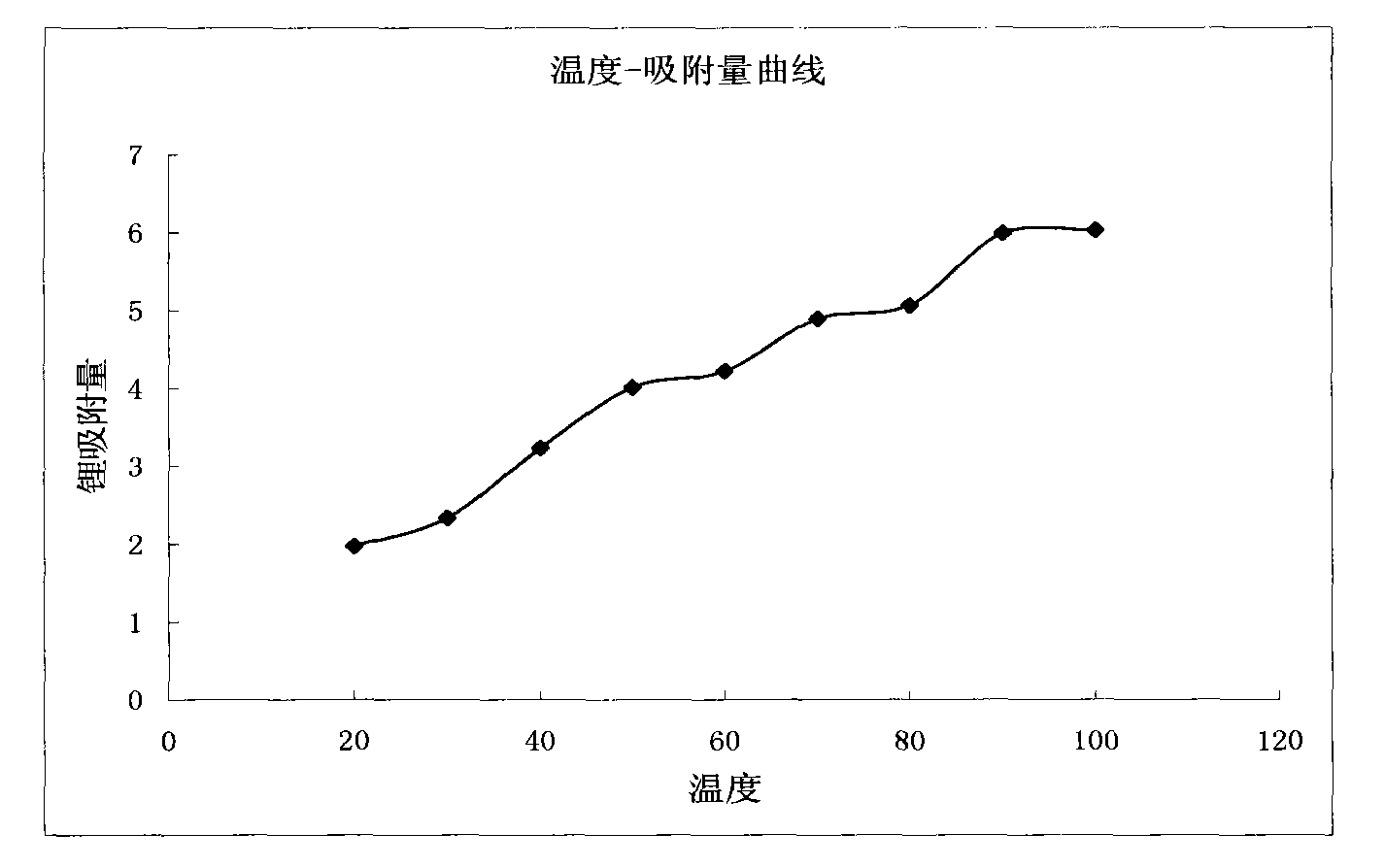
The invention discloses a method for extracting lithium from salt lake brine by an adsorption method. The method comprises the following steps of: (1) making the salt lake brine pass through aluminum salt-containing adsorption resin at a speed of between 5 and 10 BV / H at the temperature of between 20 and 100 DEG C to ensure that the lithium ions in the salt lake brine are adsorbed on the adsorption resin; (2) making lithium ion eluant pass through the aluminum salt-containing adsorption resin at a speed of between 5 and 10 BV / H at the temperature of between 20 and 100 DEG C to ensure that thelithium ions adsorbed on the aluminum salt-containing adsorption resin are eluted and desorbed into the eluant solution so as to obtain desorption liquid; and (3) making the desorption liquid pass through common sodium cation exchange resin at a speed of between 5 and 10 BV / H to remove magnesium from the desorption liquid, and then concentrating to obtain a lithium salt. The method has the advantages of low consumption of chemical materials, simple process, easy operation and no pollution.
Owner:SUNRESIN NEW METERIALS CO LTD XIAN
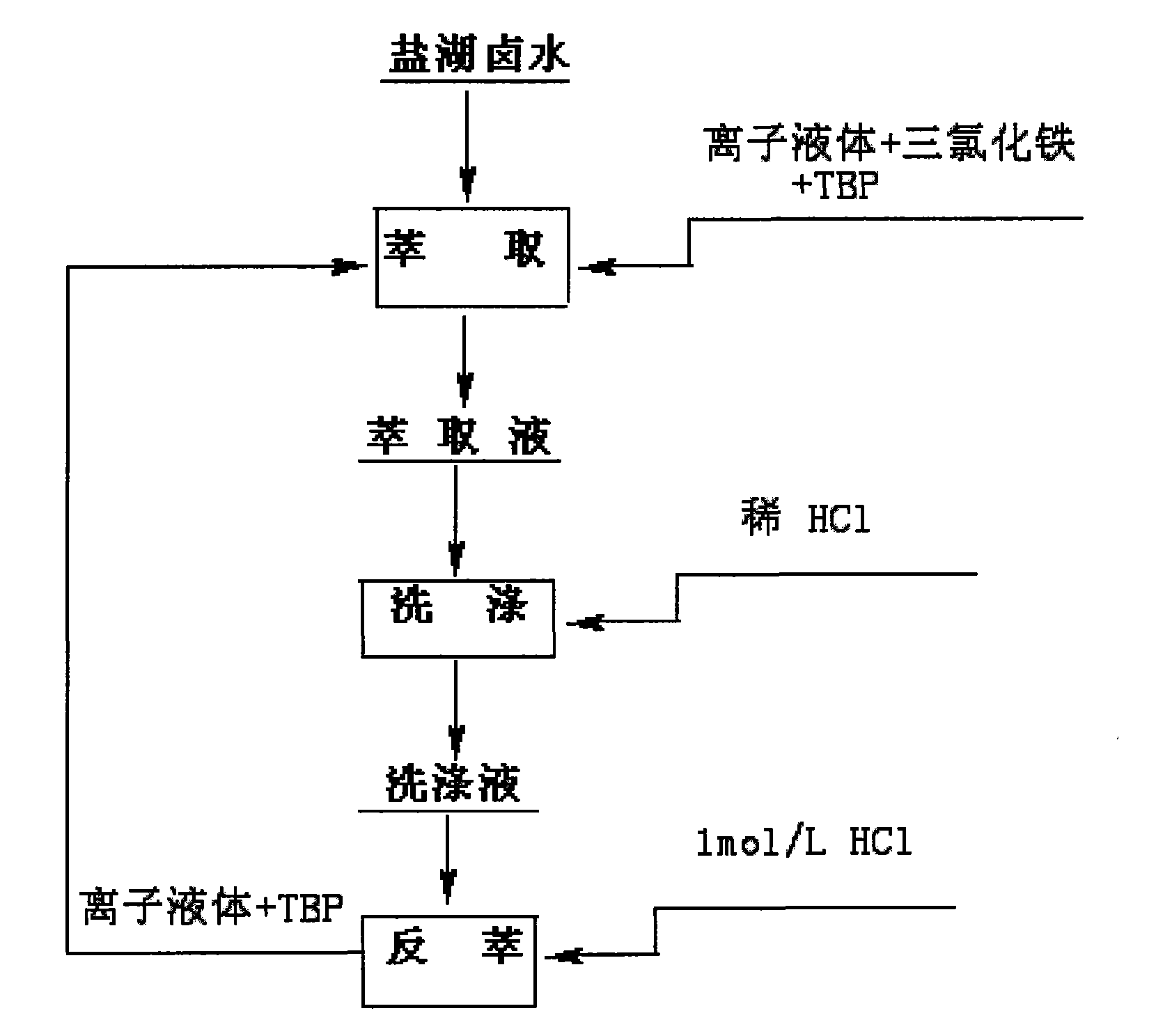
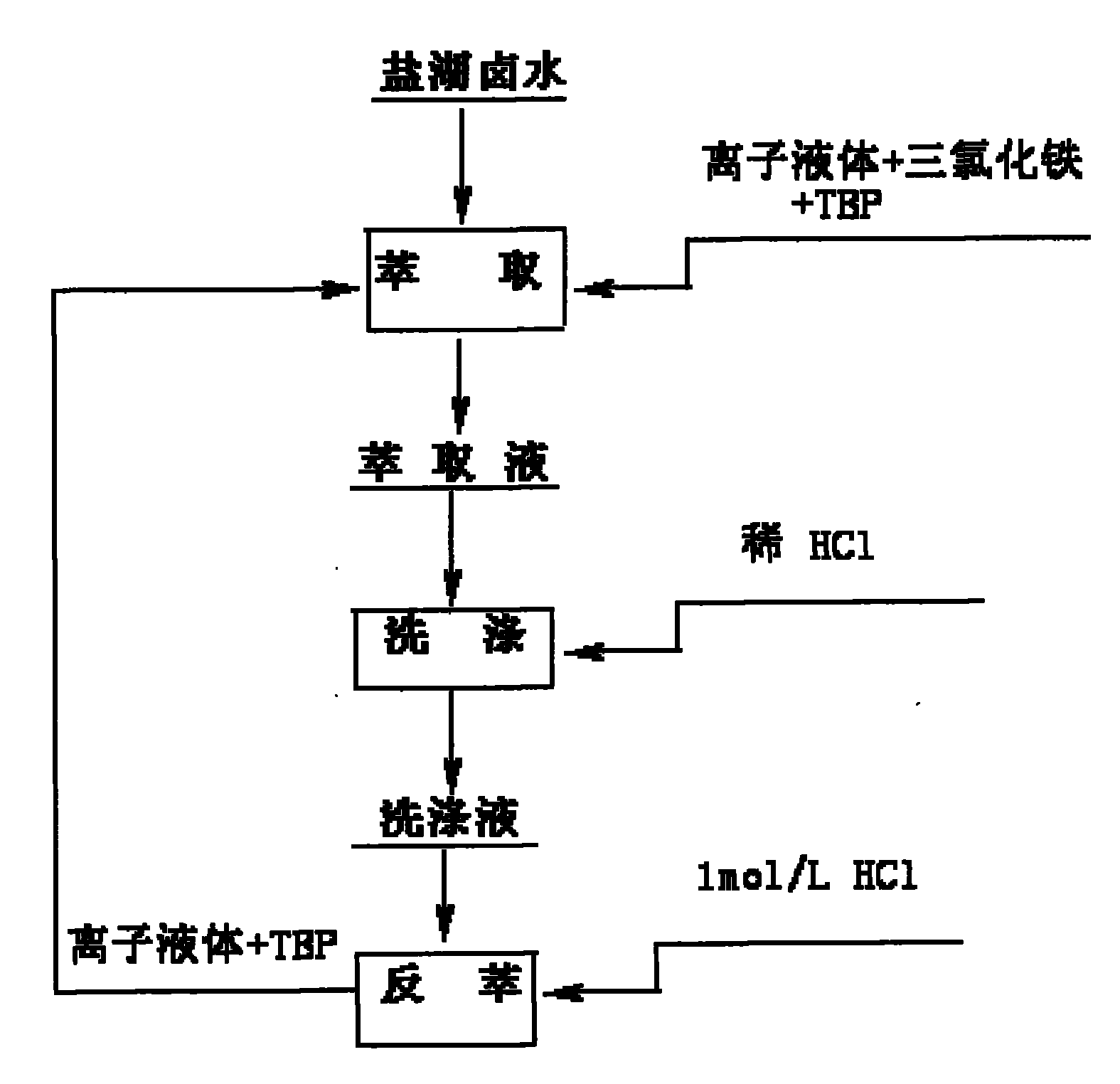
Method for preparing lithium carbonate by using salt lake brine with high magnesium-to-lithium ratio
InactiveCN101698488AReduce energy consumptionNo way outLithium carbonates/bicarbonatesEvaporationCalcination
The invention provides a method for preparing industrial lithium carbonate by using salt lake brine with a high magnesium-to-lithium ratio. In the method, a TBP-CON-KS+FeCl3 is used as an extraction system to extract and back-extract impurity-free salt lake brine with a high magnesium-to-lithium ratio, the residual liquid obtained after back-extraction is converted by alkaline liquor for precipitation, the precipitate is washed to form an industrial lithium carbonate product and the lithium carbonate content is more than or equal to 99.0 percent and is in accordance with the requirements of GB / T 11 075-2003 standards. The method has the advantages that: liquid-liquid extraction with an organic solvent is adopted to realize the separation of lithium from magnesium, the lithium carbonate is precipitated by inorganic slats, the lithium carbonate is extracted from the salt lake brine with a high magnesium-to-lithium ratio, the process is simple, the control is easy, the operational reliability is high and the application range is wide; a process of calcination and diluted lithium solution evaporation and concentration is saved, the energy consumption is only 30 to 50 percent of that of the conventional process for producing lithium carbonate by using lithium-containing brine; initial raw material consumption comparison show that the production cost of the method is only about 8 percent of that of the prior art; and the raw material brine can return to a storage pool after the extraction of the lithium carbonate, so no by production disposal problem is involved, environmental pollution is relatively low and lithium yield in the whole process is more than or equal to 70 percent.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
Method for extracting ultrahigh-purity lithium carbonate from salt lake brine with high magnesium-lithium ratio
The invention discloses a method for extracting ultrahigh-purity lithium carbonate from chloride brine with a high magnesium-lithium ratio. The method comprises the following steps of: preparing a lithium chloride concentrated solution by an adsorption, desorption and evaporation concentration process; purifying, and precipitating lithium carbonate by using ammonium bicarbonate pulp; and converting into a lithium bicarbonate solution, filtering, and decarburizing. By improving steps and parameters of the conventional process, the purity of a product is more than 99.99 weight percent and the total amount of major impurities is not more than 0.002 weight percent without the introduction of additional high-cost purification steps such as ion exchange resin / membranes; and additional sodium ions are not introduced in the production process, byproducts at all stages can be recycled, and only an extremely small amount of waste solution is generated.
Owner:JIANGSU HAILONG LITHIUM IND TECH
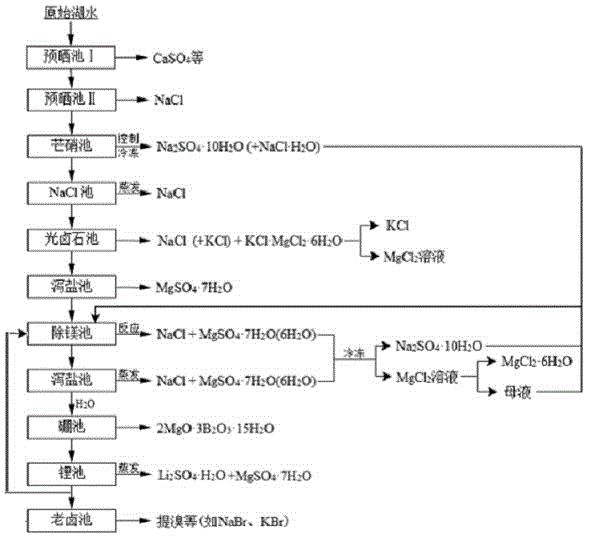
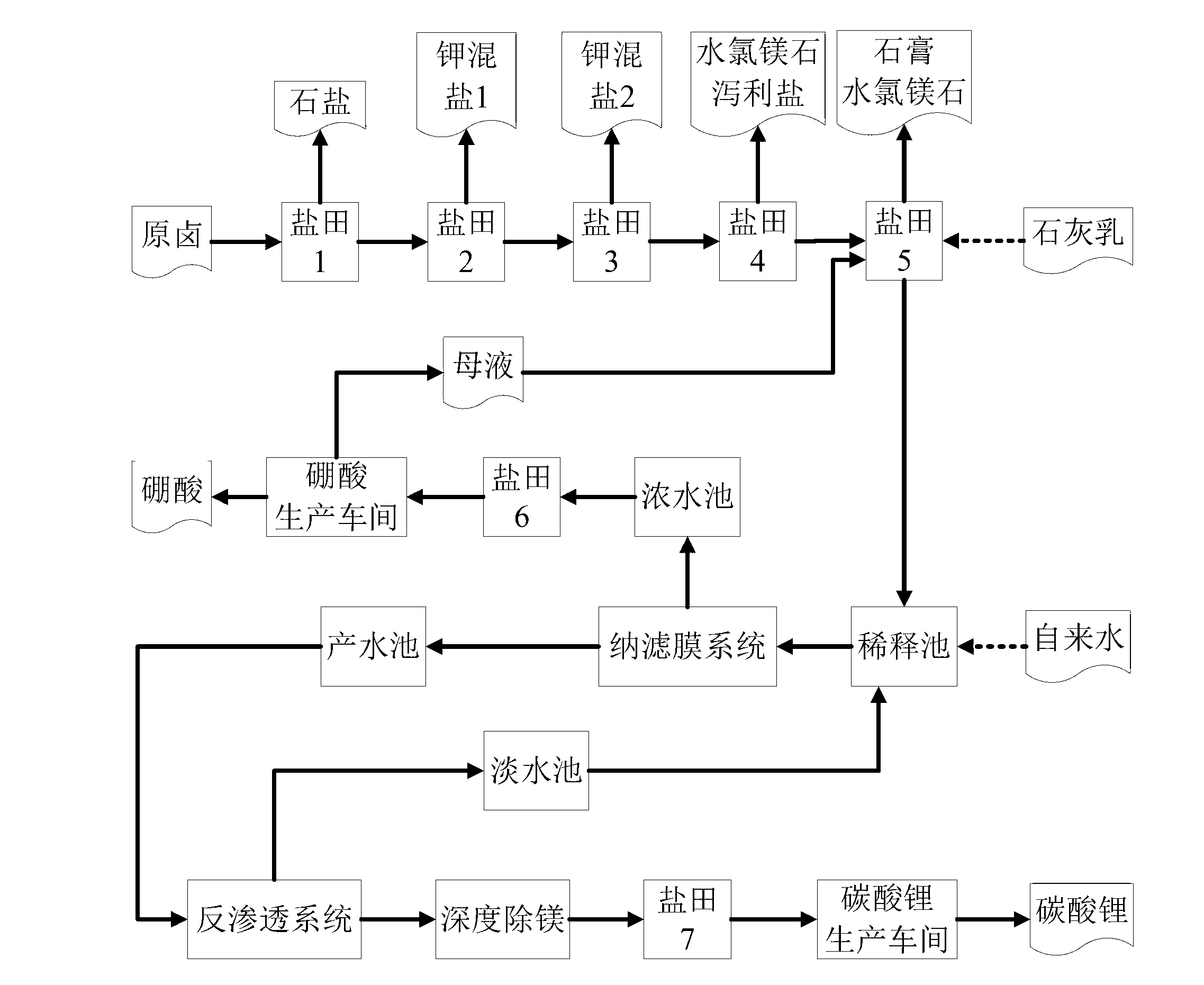
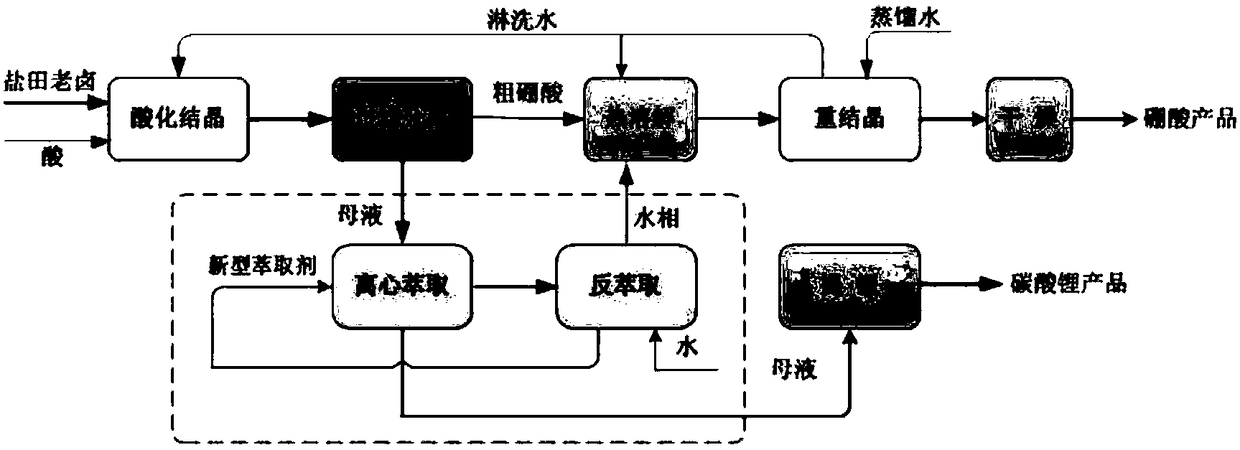
Clean production process of plateau sulfate type boron-lithium salt lake brine
InactiveCN102910652AHigh purityReduce the ratio of magnesium to lithiumChemical industryAlkali metal halide purificationHydration reactionSylvinite
The invention relates to a clean production process of plateau sulfate type boron-lithium salt lake brine. The process comprises the following steps of: (1) arranging a pre-airing pond, a mirabilite pond, a NaCl pond, a carnallite pond, an epsom salt pond I, a magnesium removing pond, an epsom salt pond II, a boron pond, a lithium pond and an old brine pond; (2) controlling the sodium ion concentration in plateau sulfate type boron-lithium salt lake brine, precipitating mirabilite out in winter to obtain brine A, naturally evaporating the brine A, and salting out to obtain brine B; (3) naturally evaporating the brine B, and precipitating sylvine and carnallite out in sequence to obtain brine C; (4) naturally evaporating the brine C, precipitating an epsom salt out, and performing solid-liquid separation to obtain brine D and a solid A; (5) blending the brine D with mirabilite, removing magnesium to obtain brine E, and naturally evaporating brine E to obtain brine F and a solid B; (6) performing a hydration reaction on brine F, naturally evaporating, and precipitating reservoir water / inderite and brine G out; and (7) evaporating brine G or refrigerating for precipitating lithium sulfate, and processing the lithium sulfate into a corresponding product. The process has the advantages of comprehensive utilization of natural energy, saving in energy and environment friendliness.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI +1
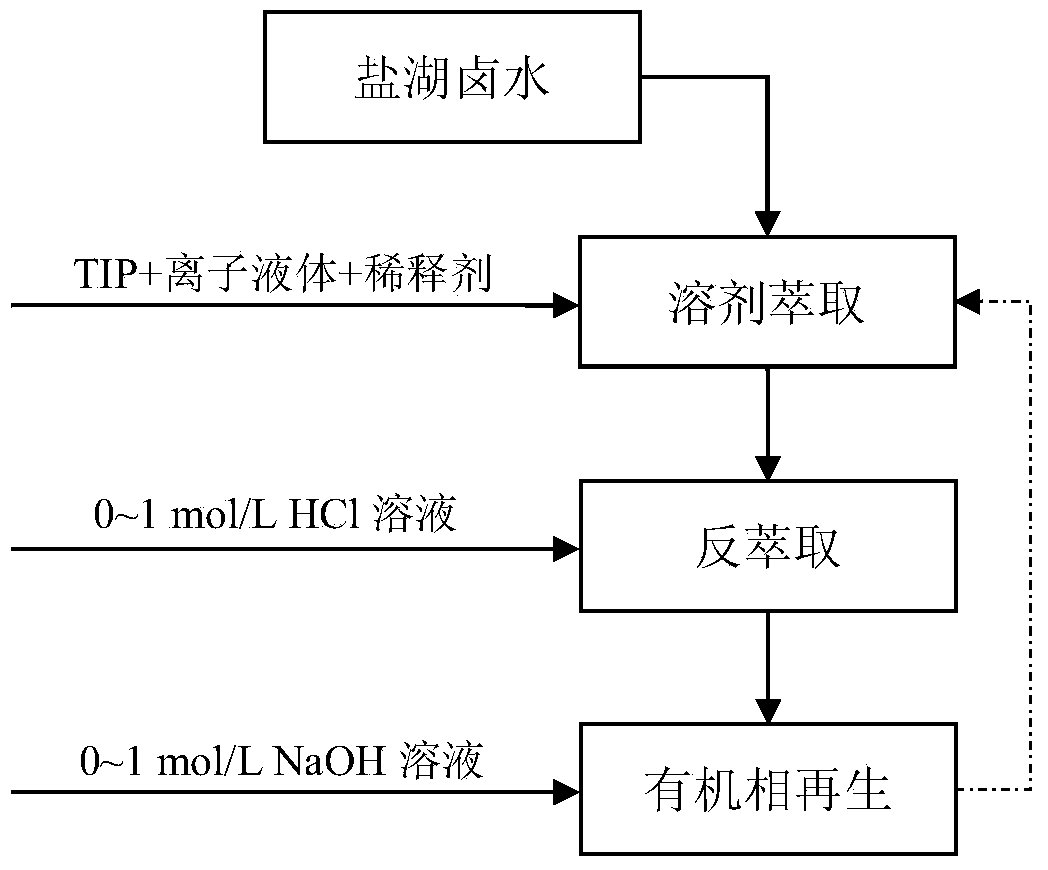
Method for efficiently extracting lithium from salt lake brine
ActiveCN103710549AAvoid interferenceHigh separation factorProcess efficiency improvementSeparation factorIonic liquid
The invention relates to a method for efficiently extracting lithium from salt lake brine. The method comprises the following steps: (1) forming an extraction organic phase by an extraction agent, a co-extraction agent and a diluent, and then mixing the extraction organic phase with salt lake brine according to the volume ratio of (3-4):2 for three-stage extraction with single extraction time being 2-10 minutes to obtain an organic phase; and (2) mixing the organic phase obtained in step (1) with a reverse extraction acid solution (0-1 mol / L) for three-stage reverse extraction with single reverse extraction time being 2-10 minutes, and collecting an aqueous phase which is an aqueous solution containing lithium ions. The co-extraction agent of an extraction system of the method is hydrophobic ionic liquid, compared with conventional synergist ferric trichloride, the interference caused by iron ions is avoided, the reverse extraction acidity is greatly reduced, more importantly the lithium-magnesium separation factor is significantly improved, and the elution step of magnesium ions is reduced; in addition, the method provided by the invention is easy in process, easy to control, high in operation reliability, and good in recyclability of the organic phase, and greatly reduces the production cost for extracting the lithium from the salt lake brine.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
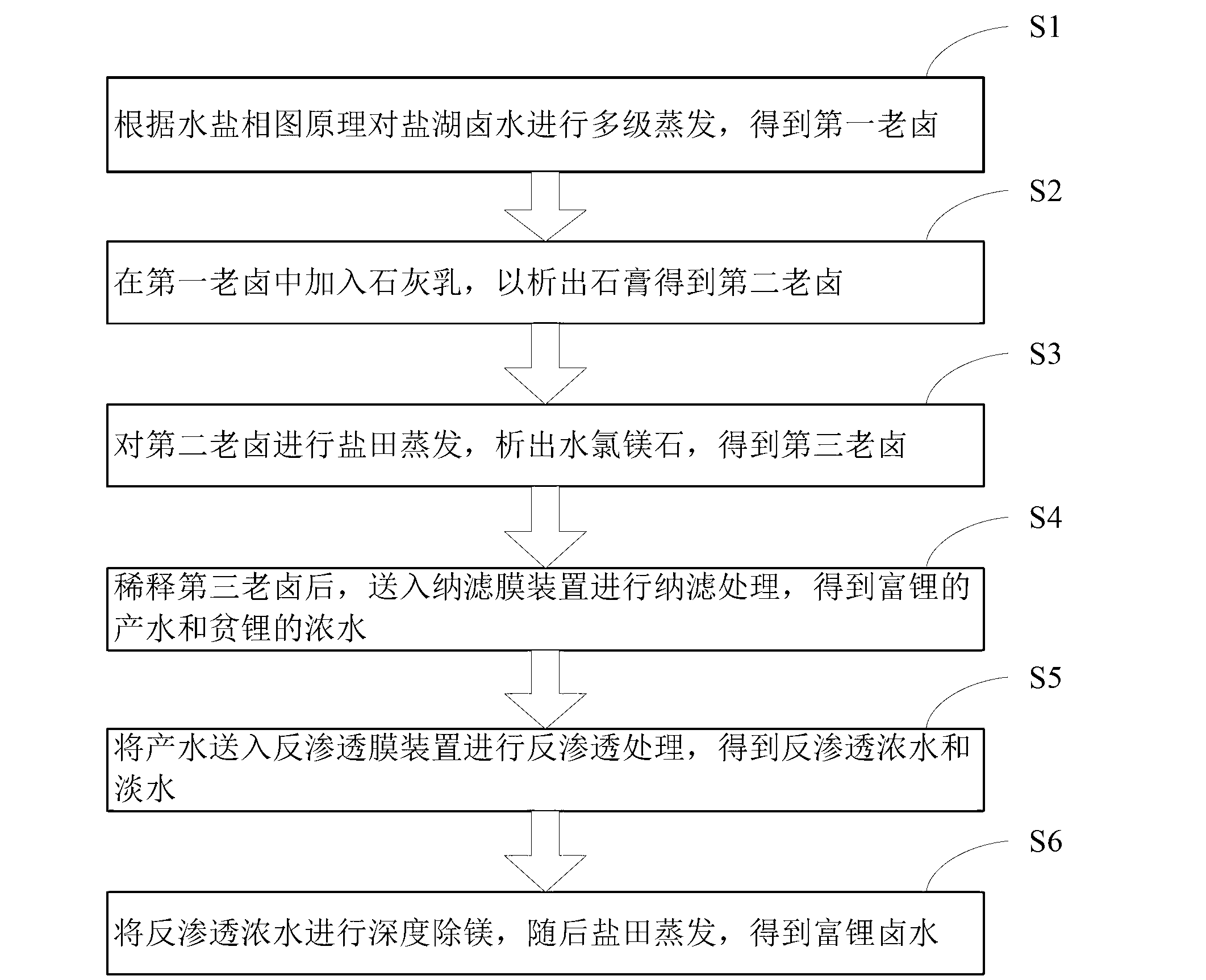
Salt lake brine treatment method for separating lithium from high-magnesium-lithium-ratio salt lake brine
ActiveCN103074502ATake advantage ofHigh recovery rateProcess efficiency improvementReverse osmosisEvaporation
The invention discloses a salt lake brine treatment method for separating lithium from high-magnesium-lithium-ratio salt lake brine. The treatment method comprises the steps that S1, the salt lake brine is subjected to multistage salt pan evaporation to form first old brine; S2, sulphur removal is conducted: lime milk is added to the first old brine for separating out gypsum, and second old brine is obtained; S3, the second old brine is subjected to salt pan evaporation, bischofite is separated out, and third old brine is obtained; S4, the third old brine is diluted, and sent to a nanofiltration membrane device for nanofiltration treatment, and contributing water rich in lithium and thick water poor in lithium are obtained; and S5, the contributing water in Step S4 is sent to a reverse osmosis membrane device for reverse osmosis treatment, and reverse osmosis thick water and fresh water are obtained. The method combines a salt pan technology with a membrane system, makes full use of solar energy, and reduces energy consumption; a technological process is simple; equipment is easy to configure, mount and transfer; popularization and an application are very easy.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI +1
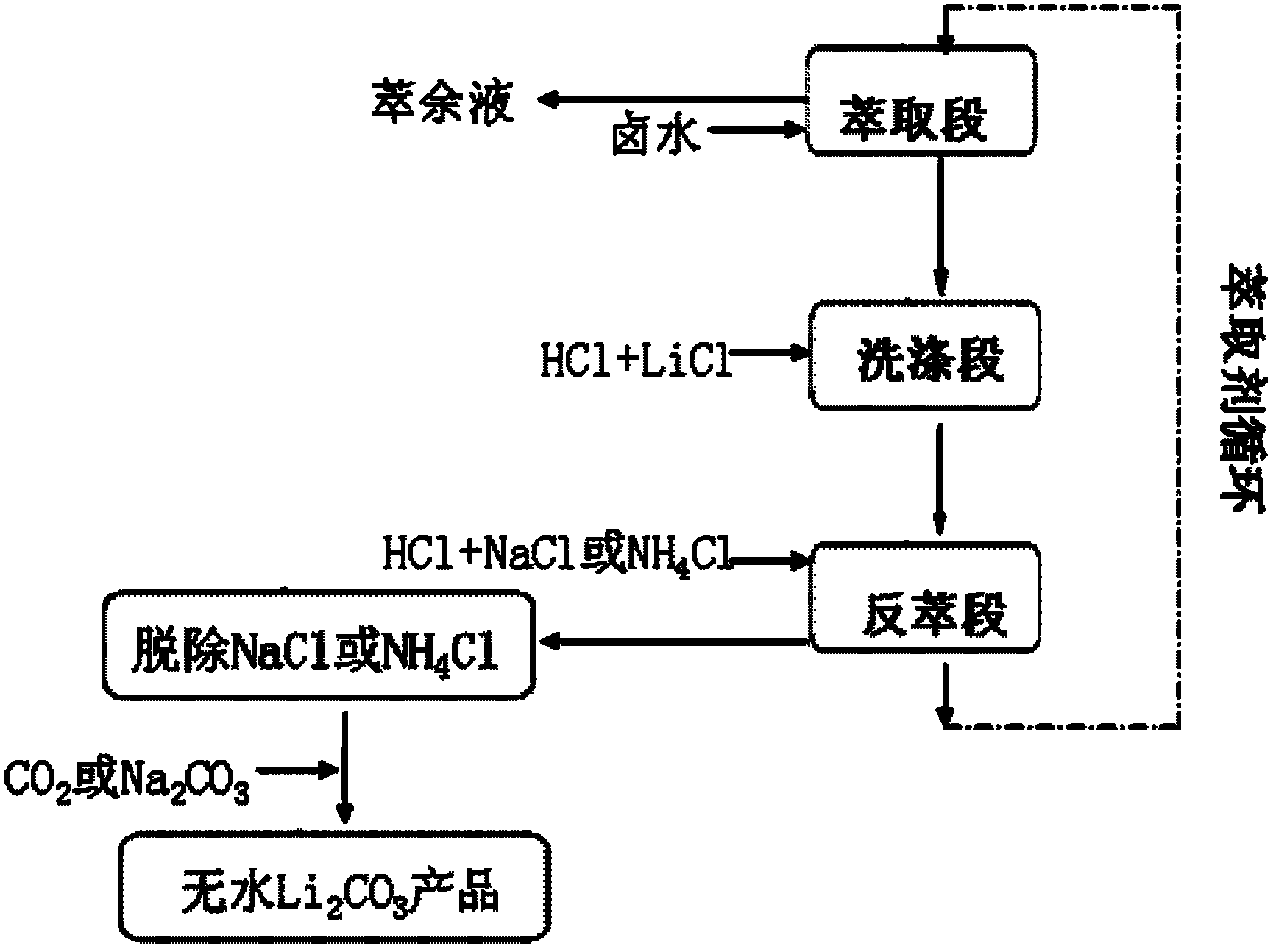
A method for extracting lithium carbonate from high magnesium-lithium ratio salt lake brine
InactiveCN102275956AReduce lossesNo decrease in recycling efficiencyNanotechnologyLithium carbonates/bicarbonatesHigh magnesiumInorganic salts
The invention discloses a method for extracting lithium carbonate from salt lake brine with high magnesium / lithium ratio, belonging to the technical filed of inorganic salt extraction. The method comprises the following steps of: carrying out extraction, washing and stripping steps to obtain a stripping solution composed of NaCl and LiCl or NH4Cl and LiCl, then introducing CO2 or adding Na2CO3, and controlling the pH value and dynamic conditions to obtain a nanoscale or microscale Li2CO3 product. The method disclosed by the invention has the advantages of simple steps, low equipment requirement and wide sources of raw materials, and is suitable for industrial production.
Owner:TSINGHUA UNIV
Method for extracting lithium from salt lake brine
InactiveCN101767804AImplement selective extractionHigh extraction rateLithium compoundsHigh concentrationOrganic solvent
The invention provides a method for extracting lithium from salt lake brine, comprising the following steps: 1) mixing organic phase composed of salt lake brine, extracting agent and extracting medium with synergist, extracting the mixture and collecting the organic phase; 2) mixing the organic phase in step 1 with hydrochloric acid solution and carrying out back-extraction on the mixture, collecting aqueous phase to obtain aqueous solution of Lithium-ion; the step is characterized in that the extracting medium is hydrophobic ionic liquid. Compared with the traditional method for extracting lithium from salt lake brine with solvent gasoline as the medium, the method of the invention employs green and environmental-friendly ionic liquid as the medium; as a result, lithium salt extraction efficiency is improved, back-extraction acidity is lowered, what is more important is that environmental pollution and equipment corrosion due to use of a great deal of volatile organic solvent and high-concentration hydrochloric acid are avoided. In addition, the organic phase of the invention features fine cyclic applicability, thus greatly reducing production cost for extracting lithium from the salt lake brine.
Owner:JIANGNAN UNIV
Process for extracting lithium from salt lake brine by manganese dioxide
The present invention is manganese dioxide process of extracting lithium from salt lake brine, and the process is suitable for producing lithium carbonate and lithium chloride with lithium-containing Qinghai saline lake brine, including concentrated lithium-containing Qinghai saline lake brine. The process includes sun shining saline lake brine to obtain concentrated lithium containing brine, selective adsorption of lithium ion with MnO2 adsorbent, eluting adsorbed lithium ion with hydrochloric acid solution, and refining and concentrating the elutriant to obtain material for preparing lithium carbonate and lithium chloride.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
Coordinate extracting system for extracting lithium from salt lake brine with extraction method
The invention relates to a coordinate extracting system for extracting lithium from salt lake brine with extraction method, which comprises the following steps of (1) acidity of raw material liquid adjustment: adding hydrochloric acid to the salt lake brine to adjust the pH value of the raw material liquid to 1 to 5 so that the raw material liquid is obtained; (2) extracting lithium: carrying out three-stage extraction on the raw material liquid obtained in step (1) by using TBP-BA-FeCl3 solvent naphtha as an extracting agent to obtain an organic phase; (3) organic phase washing: carrying out three-stage washing to the organic phase obtained in the step (2) by using the hydrochloric acid as a washing agent; (4) organic phase reextraction: carrying out three-stage extraction on the organic phase obtained in the step (3) by using hydrochloric acid as a reextraction agent to obtain lithium chloride solution. The invention has the advantages of simple technique, easy control, low reextraction acidity, low requirements on reextraction equipment materials and concentration of lithium in the material brine, and no need of evaporation concentration of thin lithium solution, thereby saving energy, reducing consumption, decreasing the production cost, and simultaneously improving the total recovery rate of the lithium effectively.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI +1
Method for refining lithium from salt lake brine with high magnesium-lithium ratio
InactiveCN103570048AVerify feasibilityTake advantage ofLithium carbonates/bicarbonatesSulfate radicalsReverse osmosis
The invention discloses a method for refining lithium from salt lake brine with a high magnesium-lithium ratio. The method comprises the following steps: 1) removing sulfate radicals from brine from which sodium and potassium are extracted, evaporating the brine to obtain lithium-rich and boron-rich brine; 2) extracting boron from the lithium-rich and boron-rich brine to obtain boric acid and lithium-rich brine; 3) separating the lithium-rich brine by using a nanofiltration membrane to obtain primary concentrated water and primary produced water; 4) through reverse osmosis, separating the primary produced water to obtain secondary produced water and fresh water; 5) removing magnesium from the secondary produced water, and evaporating the secondary produced water to obtain refined lithium-rich brine. The method is simple in technological process; by the method, the energy consumption is greatly reduced, salt lake brine resources are fully used, and the recycling rate of the lithium ions is greatly improved; influence of the boron on lithium carbonate or other lithium salt products is avoided, thus the quality and the competitiveness of products are greatly improved.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
Lithium-titanium oxide type lithium ion sieve absorbent and method for preparing precursor thereof
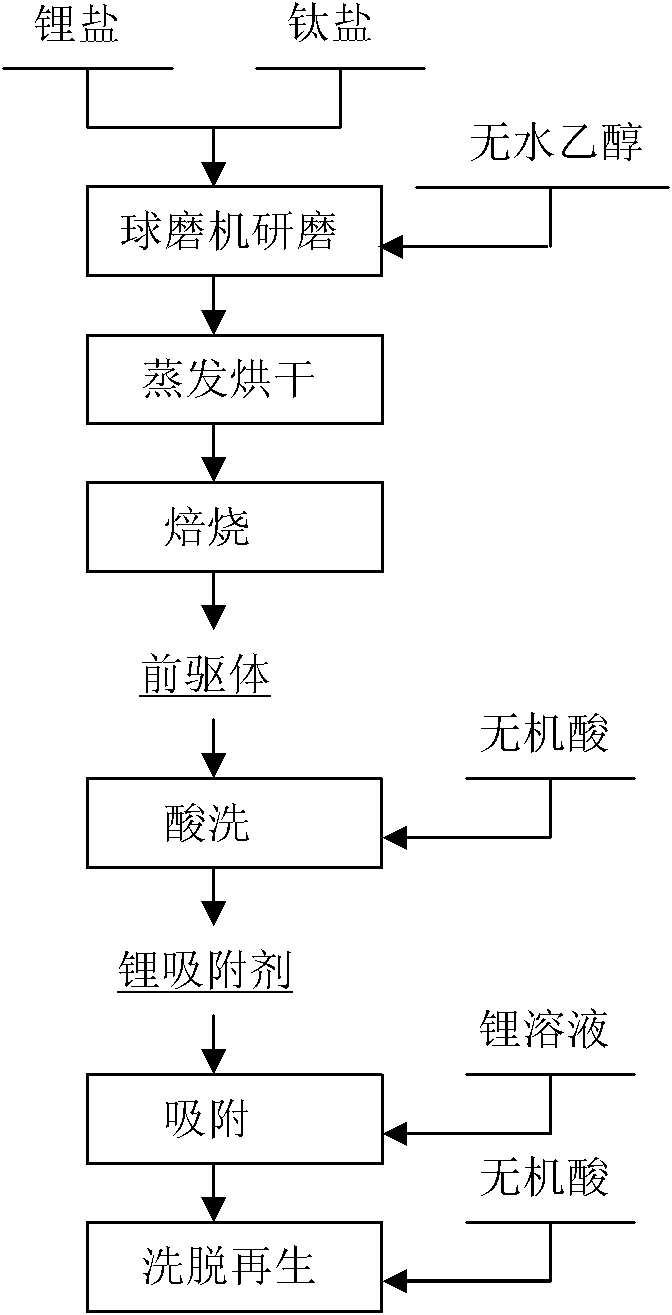
InactiveCN101944600AWell mixedIncrease contact surfaceCell electrodesTitanium compoundsSalt lake brineTitanium oxide
The invention discloses a lithium-titanium oxide type lithium ion sieve absorbent and a method for preparing a precursor thereof, and relates to a method for preparing an inorganic absorbent for absorbing enriched lithium from salt lake brine, seawater and other liquid lithium resources. The method is characterized in that: titanium dioxide and lithium salt are taken as raw materials, ground by a ball grinder and dried so as to prepare a precursor Li2TiO3 of an ion sieve through a high-temperature solid-phase roasting method; and the lithium is eluted from the precursor Li2TiO3 by inorganic acid to prepare an ion sieve H2TiO3. The method has the advantage of simple technology, and the obtained ion sieve has the advantages of low solution loss and high adsorption capacity.
Owner:CENT SOUTH UNIV
Method for preparing battery-level lithium carbonate by using salt lake brine
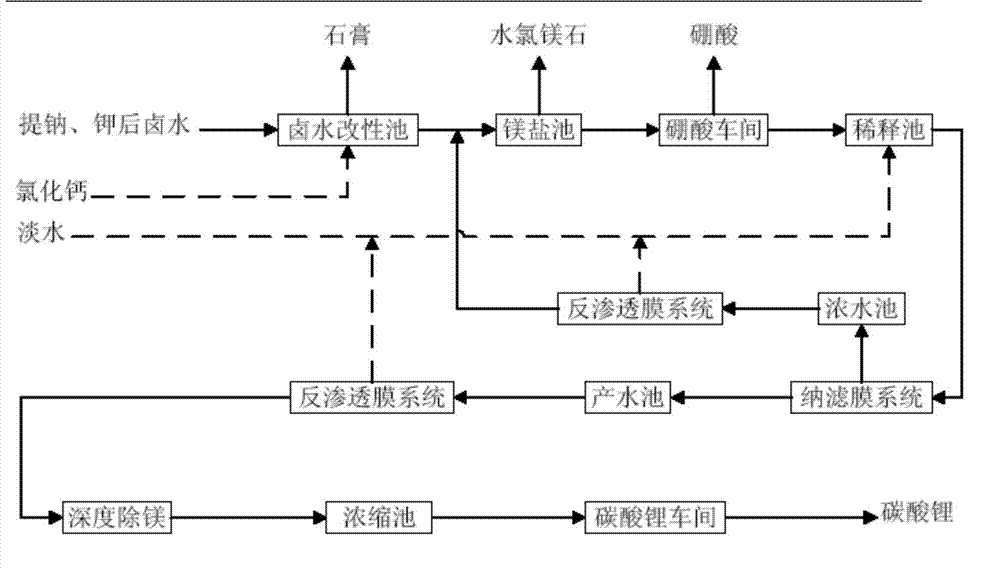
ActiveCN102976367ALow impurity indexImprove product qualityLithium carbonates/bicarbonatesLithium carbonatePhysical chemistry
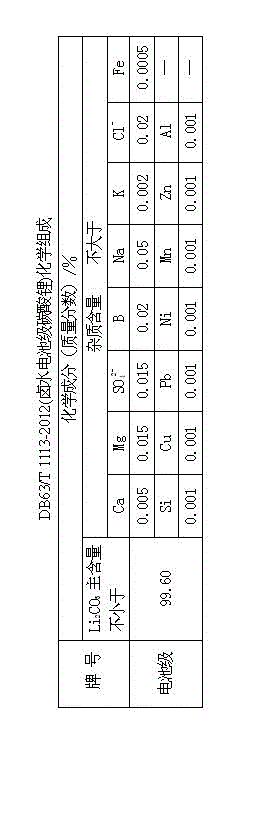
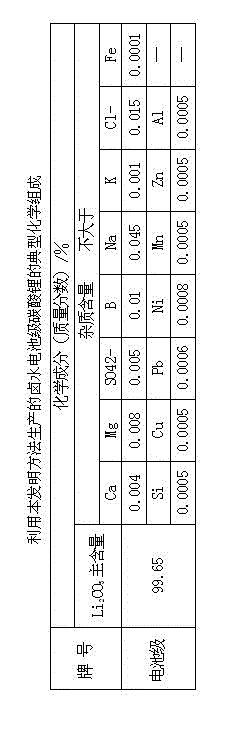
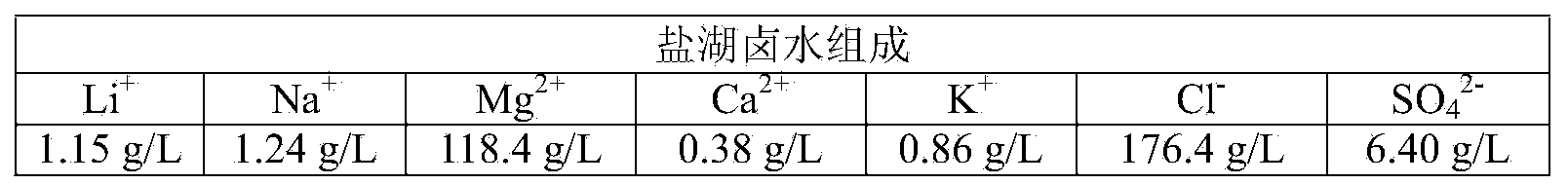
The invention provides a method for preparing battery-level lithium carbonate by using salt lake brine. The method uses salt lake brine in Qinghai as a raw material and uses an ion selectivity separation device to transfer magnesium and lithium ions in the raw material of the brine under the effect of an electric field; when the raw material of the brine passes through a separation membrane with selectivity, monovalent ions, such as the lithium ions and sodium ions can pass through, but divalent ions, such as magnesium ions and calcium ions can be separated, so that lithium-enrichment brine with a low ratio of magnesium to lithium is obtained after the separation; impurities, such as Ca<2+>, K<+>, SO4<2-> and Mg<2+>, in the lithium-enrichment brine with the low ratio of the magnesium to the lithium can be deeply removed; an auxiliary material of a pure alkali solution can be purified; the lithium-enrichment brine after deep removal of the impurities is subjected to three-effect evaporation and concentration after neutralizing with acid; alkali is added into the concentrated lithium-enrichment brine at a certain temperature for lithium sedimentation; and finally, the finished product of the battery-level lithium carbonate can be obtained by drying and cooling after filter press, washing and centrifugal separation washing are carried out. The battery-level lithium carbonate meets the requirements of the local standard DB63 / T1113-2012 (brine battery lithium carbonate) in the Qinghai Province.
Owner:QINGHAI LITHIUM IND
Method for preparing high-purity lithium carbonate and other available byproducts from salt lake brine
InactiveCN101712481ATake advantage ofCalcium/strontium/barium carbonatesLithium halidesLithium chlorideLithium carbonate
The invention relates to a method for preparing high-purity lithium carbonate and other available byproducts from salt lake brine. The method comprises the following steps: (1) separating potassium and sodium by exposing salt in a salt field, (2) separating boron by an acidification method, (3) separating magnesium by a sedimentation method, (4) separating calcium by the sedimentation method, (5) preparing lithium chloride, and (6) preparing the lithium carbonate. The preparation method of the invention can purify the potassium, sodium, boron, magnesium and calcium ions respectively besides obtaining the lithium carbonate product of which the purity is over 99.5 percent so as to fully utilize the salt lake brine resource.
Owner:DALIAN LICHANG NEW MATERIAL CO LTD
Method for extracting lithium from salt lake brine by using adsorption method
The invention discloses a method for extracting lithium from salt lake brine by using an adsorption method. The method comprises the following steps of adding an absorbent in the brine; absorbing the lithium ions in the brine to the absorbent; then treating by using a ceramic membrane; retaining the absorbent in a concentrated solution; subjecting the concentrated solution to plate-frame pressure filtration to obtain an absorbent filter cake; removing most of impurities and water from the brine; washing the filter cake with water and desorbing with an eluent to obtain a desorption solution; removing magnesium from the desorption solution by passing the desorption solution through a weak acid type cation exchange resin; concentrating with a reverse osmosis membrane to obtain a refined lithium solution for preparing lithium carbonate. The method is simple in process and can be operated easily. The absorbent has high utilization efficiency; a lithium-extracting process is short; and the content of the prepared lithium solution is high.
Owner:JIANGSU JIUWU HITECH
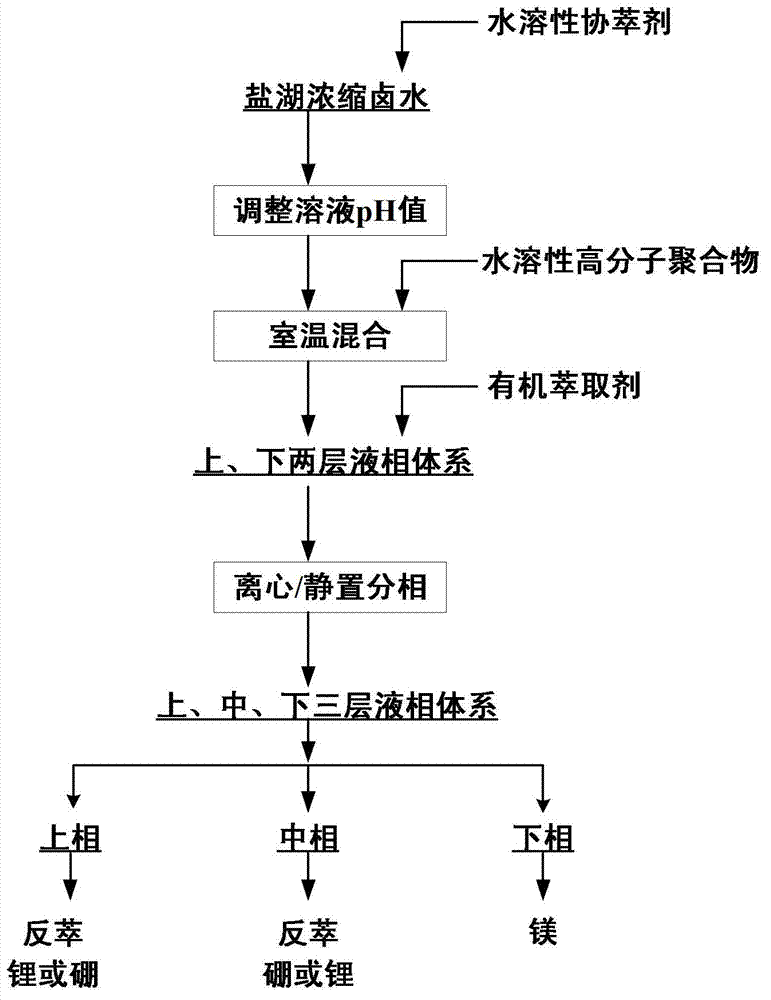
Method for preenriching and separating lithium and boron from salt lake brine by liquid-liquid-liquid three-phase extraction
InactiveCN103031448AAchieve primary separationWide range of pH adaptationLithium compoundsBoron compoundsImpurityPolymer
The invention relates to a method for preenriching and separating lithium and boron from salt lake brine by liquid-liquid-liquid three-phase extraction, which comprises the following steps: adding water-soluble auxiliary extractant into a salt lake concentrated brine solution, regulating the pH value of the brine, adding water-soluble high molecular polymer, and thoroughly mixing at room temperature to obtain an upper / lower two-layer liquid-phase system; adding organic extractant, and mixing to obtain an upper / middle / lower three-layer liquid-phase system; and taking the upper and middle phases from the three-liquid-phase system, and respectively recovering lithium and boron in the upper and middle phases by back washing. The invention can simultaneously enrich and extract lithium and boron by one-step extraction from high-magnesium / lithium-ratio salt lake brine, and can separate the lithium and boron from abundant coexistent ions of magnesium, calcium and other impurity metals in the brine. The lithium and boron are respectively selectively enriched from the upper and middle phases in the three-liquid-phase system to implement primary separation, thereby facilitating the subsequent purification and refinement. The three-liquid-phase extraction can be carried out under neutral or weakly-acidic conditions, and has strong adaptability.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
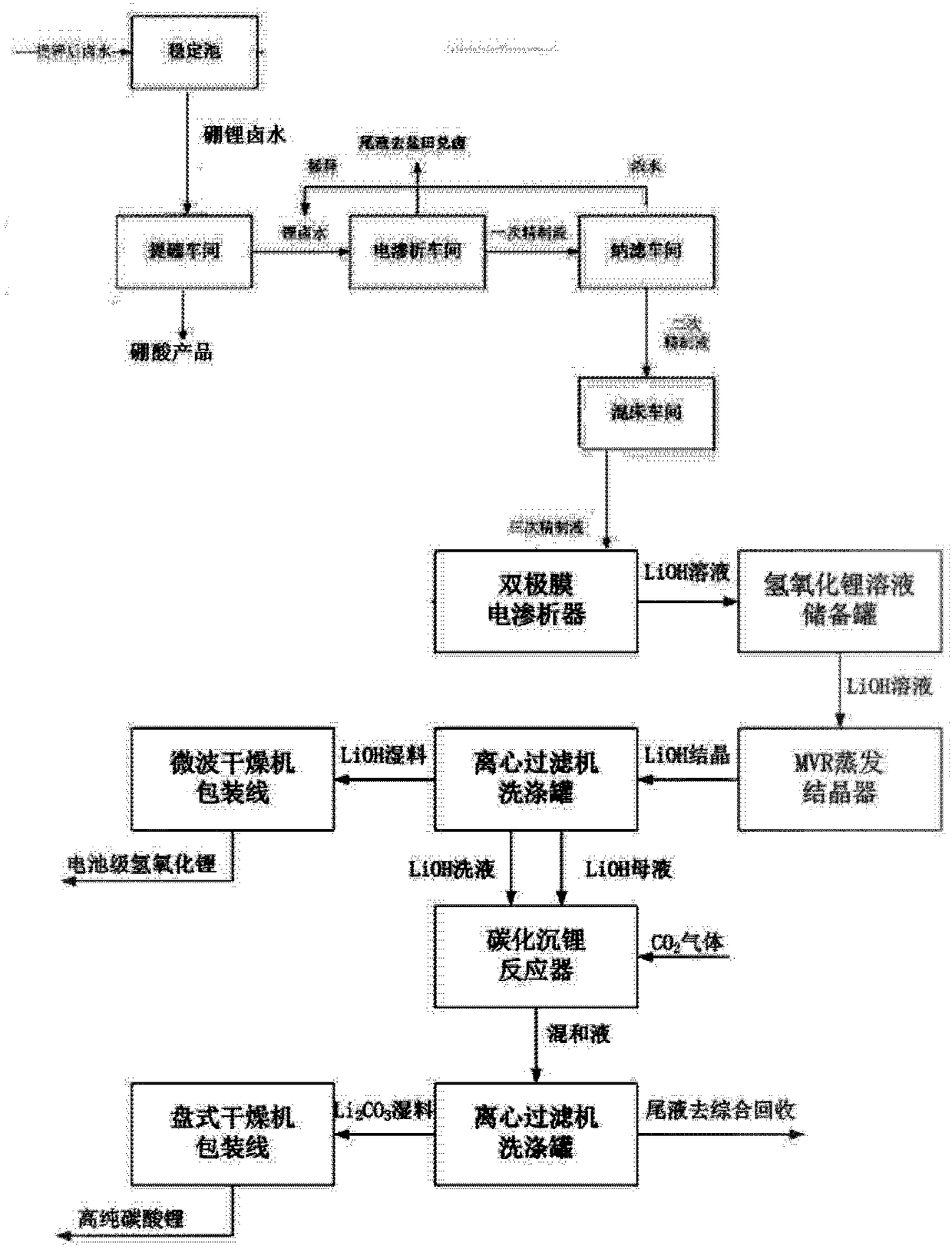
Method for directly preparing lithium hydroxide and lithium carbonate from salt lake brine with high magnesium-to-lithium ratio
ActiveCN108341420AEasy to operateEfficient extractionBoron oxyacidsLithium oxides/hydroxidesPotassiumEvaporation
The invention discloses a method for directly preparing lithium hydroxide and lithium carbonate from salt lake brine with a high magnesium-to-lithium ratio. The method comprises the following steps: 1, further stabilizing brine obtained after potassium extraction of a salt pan in a stabilization pond to form boron and lithium brine with low potassium and sodium content; 2, carrying out boron extraction treatment on the boron and lithium brine to form a boric acid product and lithium brine; 3, 4 and 5, allowing the lithium brine to go through three times of refining to obtain a thirdly refinedsolution; 6, allowing the thirdly refined solution to go through a bipolar membrane electrodialyzer to form a lithium hydroxide solution; 7, allowing the lithium hydroxide solution to go through an evaporation crystallizer to obtain a lithium hydroxide monohydrate solid and evaporation mother liquor; 8, washing the lithium hydroxide monohydrate solid for recrystallization to form battery grade lithium hydroxide and a washing solution; and 9, allowing the evaporation mother liquor and the washing solution to go through a gas-liquid reactor to react with carbon dioxide gas to form the lithium carbonate. The method has the advantages of good maneuverability, and great increase of the recovery rate of lithium ions.
Owner:马培华
Lithium extraction system
ActiveCN106498184AReduce usageEasy material selectionProcess efficiency improvementSolvent extractionLithium chlorideKerosene
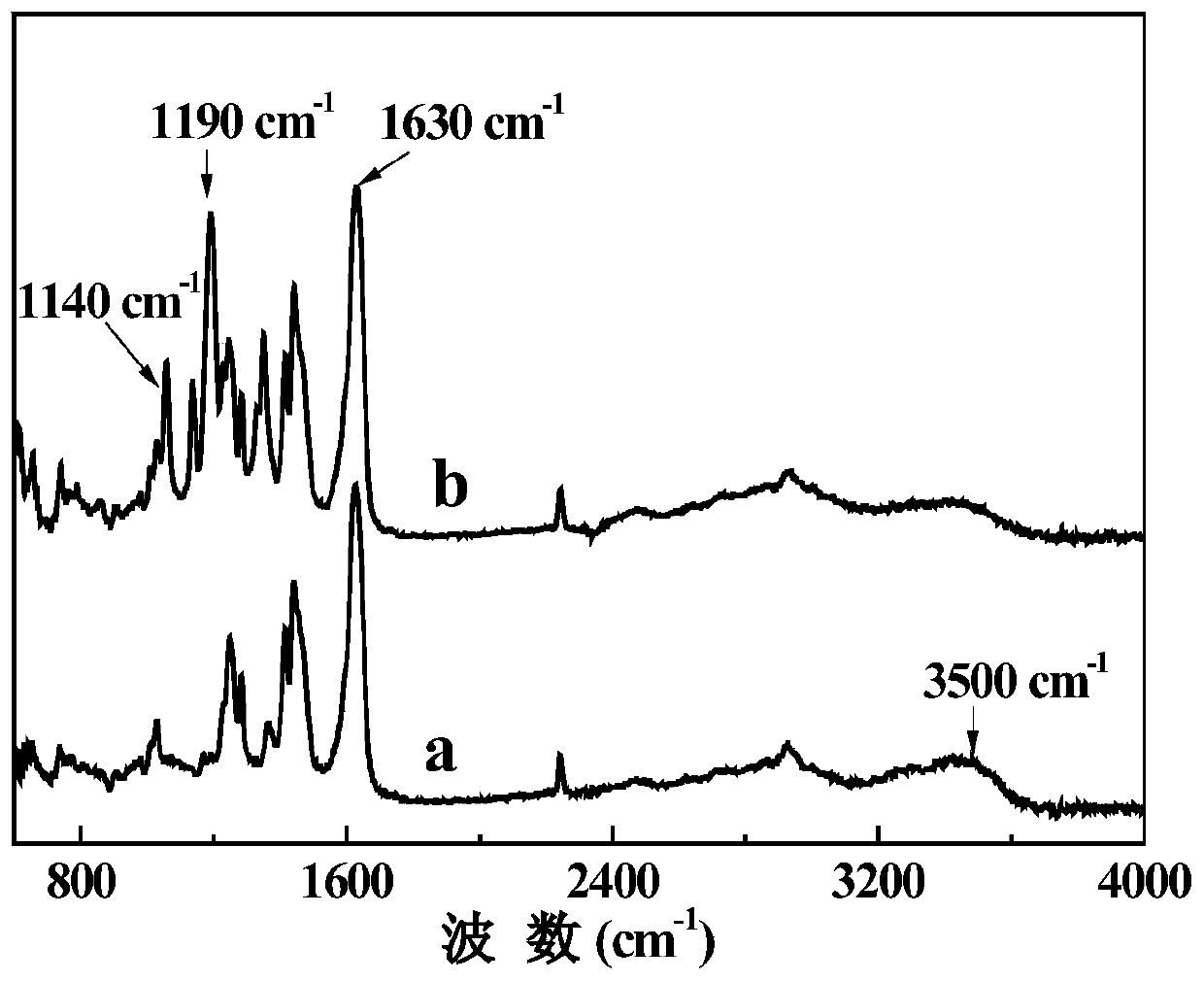
The invention provides a system from extracting lithium from salt lake brine, comprising ionic liquid, a co-extracting agent and a diluent; the ionic liquid is pyrrole hexafluorophosphate ionic liquid comprising lithium extraction functional groups, and the diluent is from solvent gasoline, sulfonated kerosene and petroleum ether. The use of the co-extracting agent iron trichloride is avoided in the lithium extraction system, adjusting pH of brine is not required, at least 5 tons of industrial hydrochloric acid and 2 tons of sodium hydroxide may be saved per ton of lithium chloride produced, and the production cost is greatly reduced; saponifying step, pickling step and deironing step are omitted from the technique, and therefore, the system is easier to use in industrial large-scale production.
Owner:青海柴达木兴华锂盐有限公司
Ionic liquid modified positively charged composite nanofiltration membrane and preparation method thereof
ActiveCN110026091AImprove interception effectReduce retentionMembranesSemi-permeable membranesHigh magnesiumPolyamide
The invention discloses an ionic liquid modified positively charged composite nanofiltration membrane and a preparation method thereof. The preparation method comprises the following steps: on a supporting bottom film, firstly, carrying out interfacial polymerization on polyamine and polyacyl chloride to form a primary polyamide layer, and then carrying out an amidation reaction on acyl chloride groups remaining on the surface of the primary polyamide layer and amino functionalized ionic liquid to prepare the composite film. The preparation method comprises the following steps: (1) preparing apolyamine aqueous phase solution and a polyacyl chloride organic phase solution; (2) carrying out interfacial polymerization on the surface of the bottom film to prepare a primary polyamide nanofiltration membrane; and (3) reacting the amino-functionalized ionic liquid solution with acyl chloride groups on the surface of the primary polyamide layer, and carrying out heat treatment to obtain the positively charged composite nanofiltration membrane. By changing the charge property of the composite membrane, lithium resources in the salt lake brine with a high magnesium-lithium ratio can be effectively extracted, the magnesium-lithium separation factor of the composite membrane is lower than 0.15, and the flux is 40-50L / m<2>h. The method has the advantages that the preparation method is simple, and the method has a good industrial application prospect in the aspect of salt lake lithium extraction.
Owner:TSINGHUA UNIV
Method for preparing composite adsorption material used for absorbing rubidium and cesium ions
ActiveCN106552602AOther chemical processesAlkali metal oxides/hydroxidesChemical physicsIn situ polymerization
The invention belongs to the technical field of a functional material, and discloses a method for preparing a composite adsorption material used for absorbing rubidium and cesium ions. The method is characterized in that a polymer layer containing an o-dihydroxybenzene structure is prepared through in-situ polymerization on the surface of a porous material, then am ion exchange function nano adsorbent layer is subjected to in-situ growth on the surface of the polymer layer, and the composite adsorption material can be obtained. The polymer layer on the surface of the porous material has strong rubidium caesium complexation and adsorption functions, has strong adhesion effect on a porous material and the ion exchange function adsorbent, greatly increase the stability of the composite adsorption material, is in favor of in-situ growth of the adsorbent, and can adjust and control the granularity of the adsorbent layer; during adsorption, a solution and the adsorbent are fully contacted, and are connected with the polymer layer through particle gaps of the adsorbent, synergism is used for absorbing rubdium and cesium, the adsorption capacity is high, and the composite adsorption material can be used for absorbing rubidium and caesium in salt lake brine and absorbing caesium in radioactivity waste water.
Owner:BEIJING NORMAL UNIVERSITY
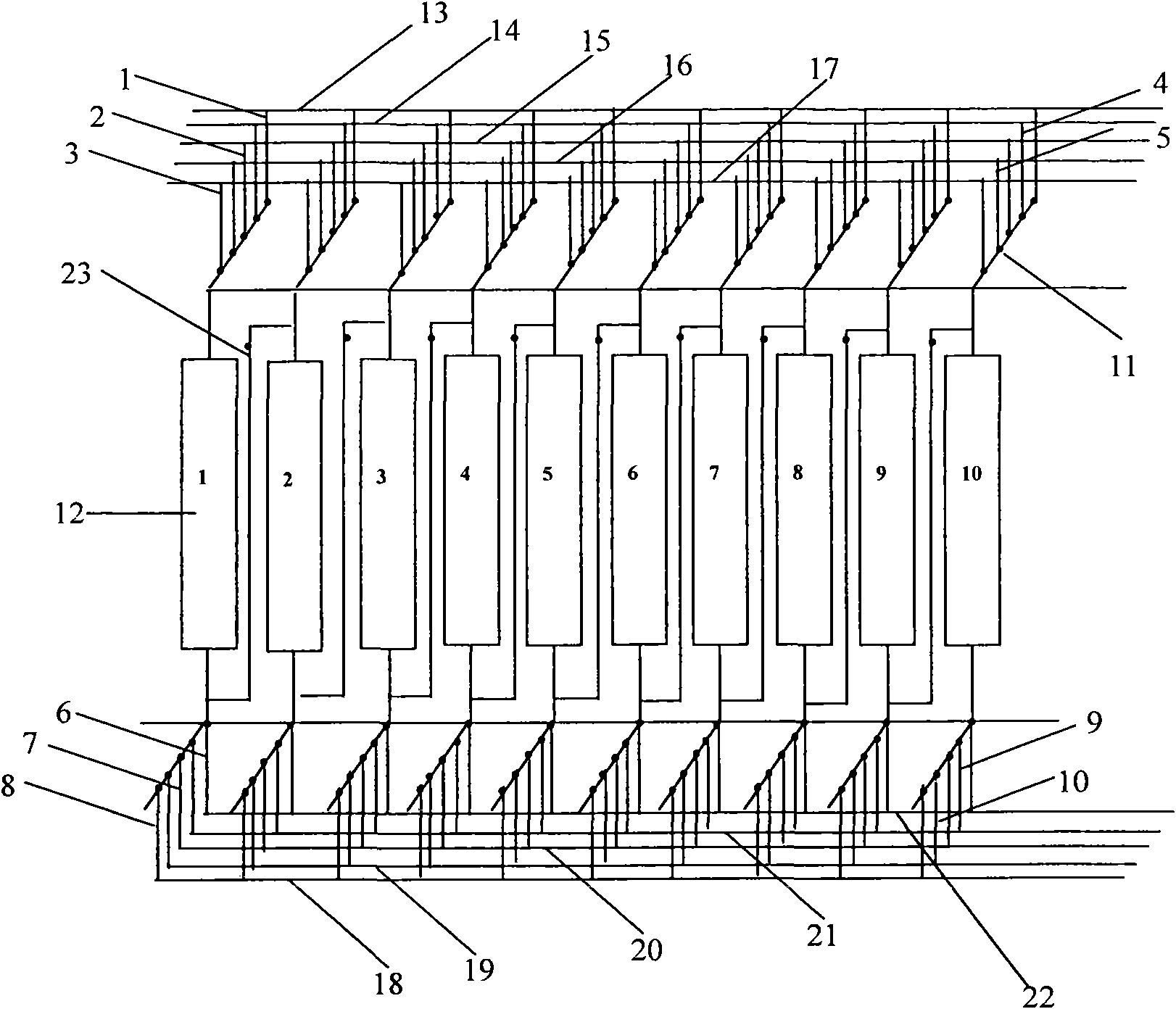
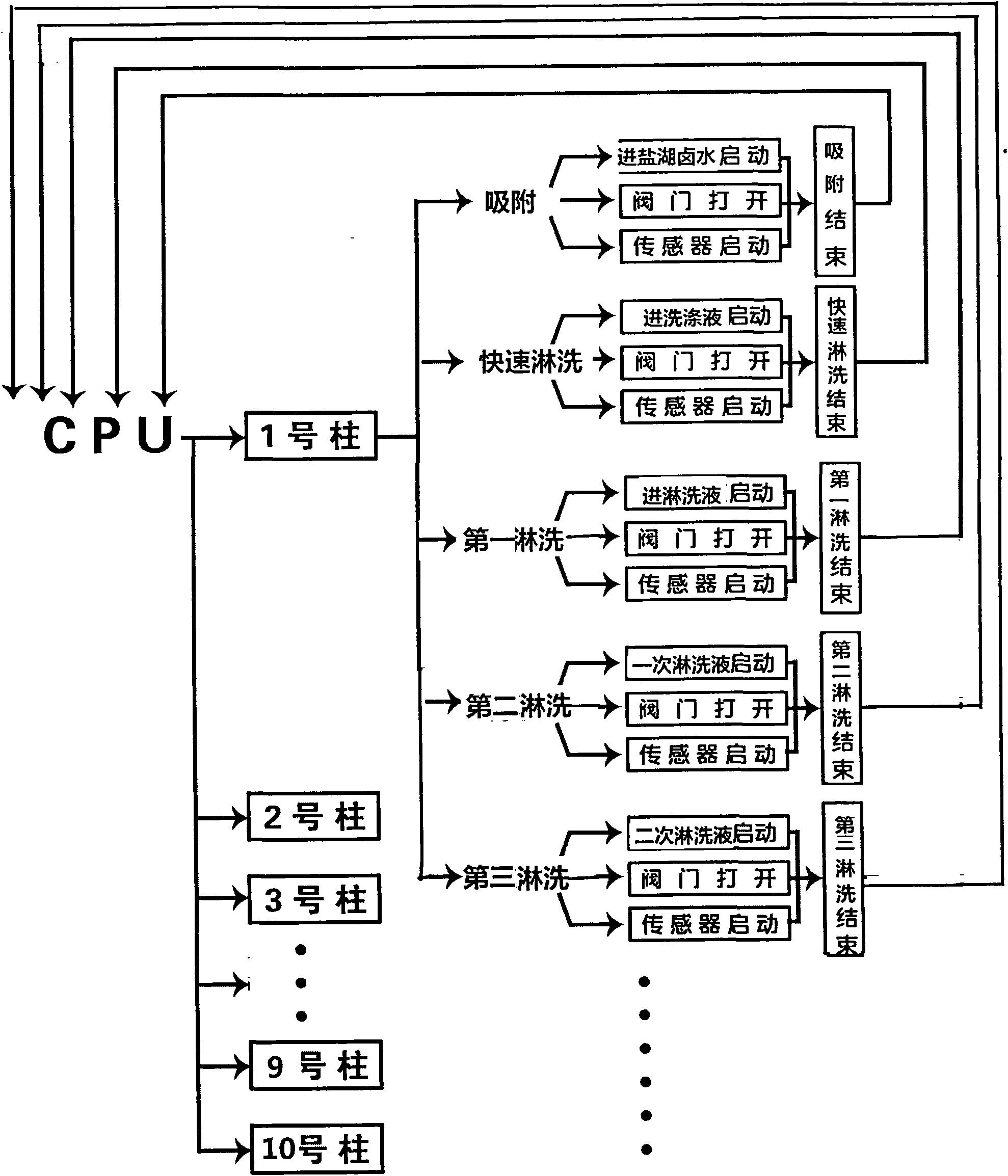
Continuous ion exchange device and method for extracting lithium from salt lake brine
ActiveCN102031368AImprove efficiencyIncrease profitProcess efficiency improvementHigh concentrationIon exchange
The invention discloses a continuous ion exchange device for extracting lithium from salt lake brine, comprising resin, a plurality of resin columns used for loading the resin, a feeding main pipe which is communicated with the upper end of each resin column and a discharge main pipe which is communicated with the lower end of each resin column, wherein the resin columns are sequentially connected in series by a serial pipeline to form an adsorption resin column group, a rapid leaching resin column group and a leaching resin column group which are in sequential mobile circulation running, andeach feeding branch pipe and each discharge branch pipe are respectively provided with a control valve used for harmoniously controlling the transformation process of realizing ion exchange, washing,leaching and resin in turn among the resin column groups. Compared with the traditional fixed bed adsorption technology, the invention has the advantages of simple equipment, convenient operation, high automation degree, small usage amount of resin, high utilization ratio, stable product concentration and high concentration of qualified liquid.
Owner:SUNRESIN NEW METERIALS CO LTD XIAN
Method for extracting lithium from salt lake brine
ActiveCN104357676AEasy to operateReduce tributyl phosphate concentrationProcess efficiency improvementHigh concentrationKerosene
The invention provides a method for extracting lithium from salt lake brine. The method comprises the following steps: 1) preparing extract organic phases which comprise a composite extracting agent and a diluent, wherein the composite extracting agent is prepared by mixing tributyl phosphate with N,N-bis(2-ethylhexyl)-3-butanone acetamide according to a volume percent ratio of 50% to 50%; the diluent is sulfonated kerosene; 2) preparing an extract water phase which is an LiCl-MgCl2-H2O system; 3) adding HCl and FeCl3.6H2O to the extract water phase, wherein the ratio of amount of substances of iron and lithium is controlled to be 1.3 to 1, the acid concentration is 0.5mol / L, and the lithium concentration is 1-3g / L; 4) standing after fully mixing the extract water phase obtained in the step 3) with the extract organic phases obtained in the step 1) in a volume ratio of 1 to 2, and then separating a liquid phase. By adopting the method, the problems that the corrosivity of high-concentration tributyl phosphate towards extraction equipment is stronger and the dissolution loss of the extracting agent in water is serious in long-term operation are solved, and the lithium extraction efficiency of the prior art is achieved. The extraction method is simple and reliable to operate.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
Preparation method of manganese oxide ion sieve adsorbent and precursor thereof
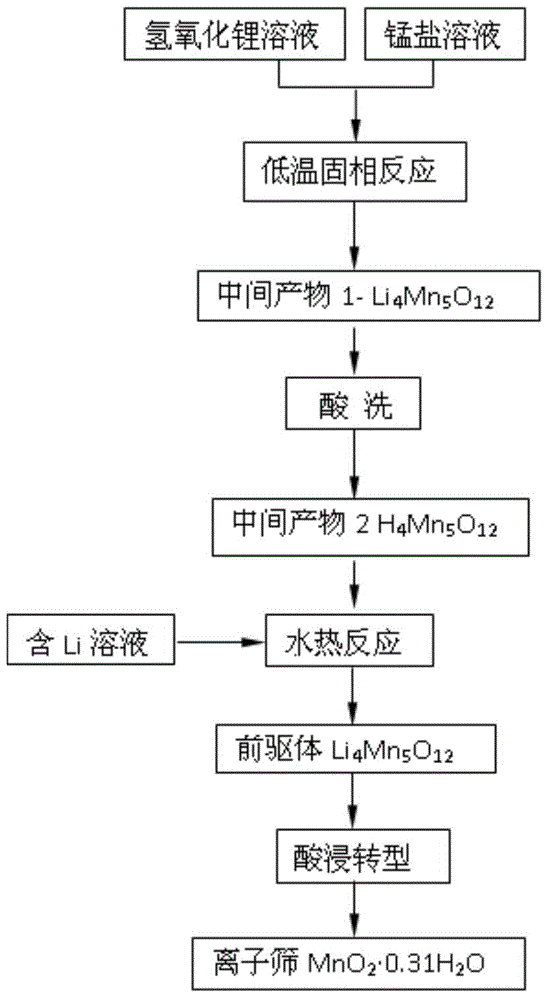
InactiveCN104525094ALow reaction temperatureShorten the timeOther chemical processesLithium hydroxideSorbent
The invention discloses a preparation method of manganese oxide ion sieve adsorbent and a precursor of the manganese oxide ion sieve adsorbent. The method comprises the specific steps that lithium hydroxide solutions are firstly added into manganous salt solutions for a co-precipitation reaction, generated gelatinous precipitate is dried and calcinated, obtained powder is soaked in acid for lithium removal, solutions with lithium are added for a hydrothermal reaction, cooling, filtering, washing and drying are carried out, and the ion sieve precursor Li<4>Mn<5>O<12> is obtained; the precursor is soaked in the acid for lithium removal again, and the ion sieve adsorbent MnO<2>.0.31H<2>O is obtained. The synthetic lithium ion sieve precursor material Li<4>Mn<5>O<12> has a spinel structure, the synthetic ion sieve MnO<2>.0.31H<2>O can be used for extracting lithium of salt lake brine, seawater and other lithium solutions, and the advantages of being large in adsorption capacity, high in selectivity and good in circulating performance are achieved.
Owner:CHONGQING TECH & BUSINESS UNIV
Method for preparing high-purity magnesium oxide with high boron salt lake brine
InactiveCN102491379ALarge particlesReduce extraction costsBoron-oxygen compoundsMagnesiaReaction temperatureIon-exchange resin
Provided is a method for preparing high-purity magnesium oxide with high boron salt lake brine. Salt lake brine is evaporated through a salt pan, concentrated to crystallize potassium sulfate, sodium chloride and potassium chloride and is drawn with lithium in adsorption mode so as to obtain master sauce brine containing magnesium and boron. Concentrated sulfuric acid is added into master sauce brine for reacting, and coarse boracic acid and acidized brine are obtained after cooling and filtering. Potential of hydrogen (pH) value of acidized brine is adjusted to be 5.5-6.5, and acidized brine passes through ion exchange resin adsorbing boron. When boron concentration in effluent liquid is higher than 5 mg / L, brine is not injected, boron-removed brine is obtained, then boron-removed brine and ammonium chloride solution are filled with ammonia for stirring and to produce magnesium sedimentation reaction, reaction temperature ranges from 60 DEG C to 80 DEG C, pH ranges from 7.5 to 8.0, the reaction is stopped when concentration of free ammonia reaches 1.8-2.2 mol / L, and magnesium hydroxide and magnesium sedimentation mother solution are obtained. Magnesium oxide is obtained by calcining magnesium hydroxide, content of magnesium oxide is larger than 99.8%, and magnesium extraction ratio is larger than 90%. Sedimentation mother solution adopts lime to steam ammonia, and generated ammonia circulates to magnesium sedimentation reaction. Mother solution after ammonia steaming is evaporated, concentrated and crystallized to obtain calcium chloride. Ion exchange resin adsorbing boron is washed, analyzed and regeneratively cycled for use. Boron-containing analysis solution is concentrated and cooled to pick up coarse boracic acid, and coarse boracic acid is recrystallized to obtain refined boracic acid with purity larger than 99%. High-purity magnesium oxide prepared by the method is high in purity, good in economic benefit, free of environment pollution, strong in operability and favorable for industrial production.
Owner:CENT SOUTH UNIV
Granular lithium ion sieve
InactiveCN101955210AFast adsorption rateLarge adsorption capacityLithium compoundsCross-linkSeawater
The invention relates to a granular lithium ion sieve, which is used for extracting lithium from (metal) lithium-containing solution such as salt lake brine, seawater, well brine or geothermal water and the like. The granular lithium ion sieve is prepared by cladding lithium ion sieve powder into a polymer in the process of forming a cross-linked polymer through the reversed phase suspension polymerization of an acrylate monomer. The granular lithium ion sieve has the advantages of high mechanical strength, high selectivity, high adsorbance, high adsorption rate (the adsorbance is 2 mmol*g<-1> in one hour) and high stability. The foundation is provided for extracting the lithium from the salt lake brine by an ion sieve adsorption method.
Owner:EAST CHINA UNIV OF SCI & TECH
Method and device for extracting and enriching lithium
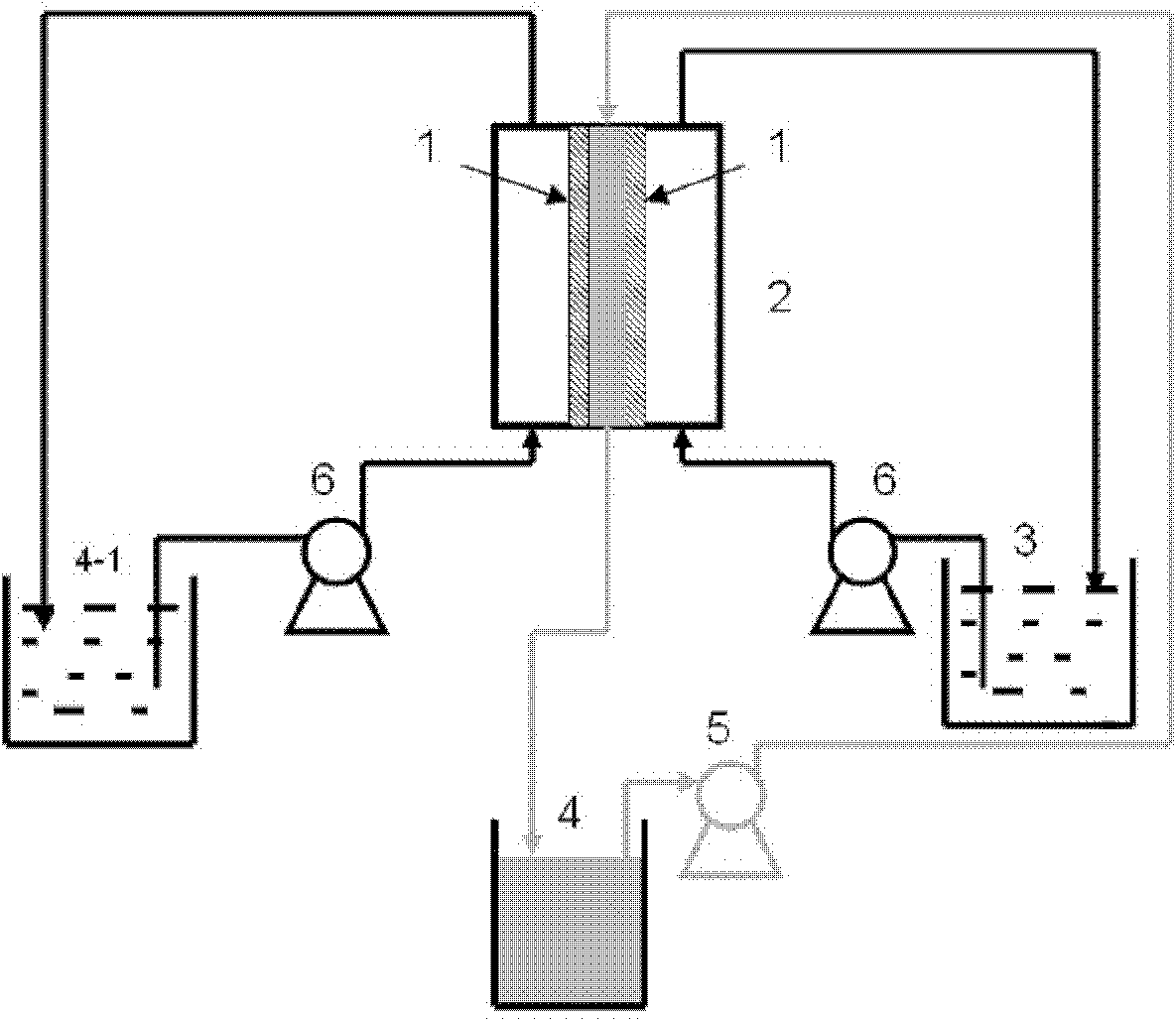
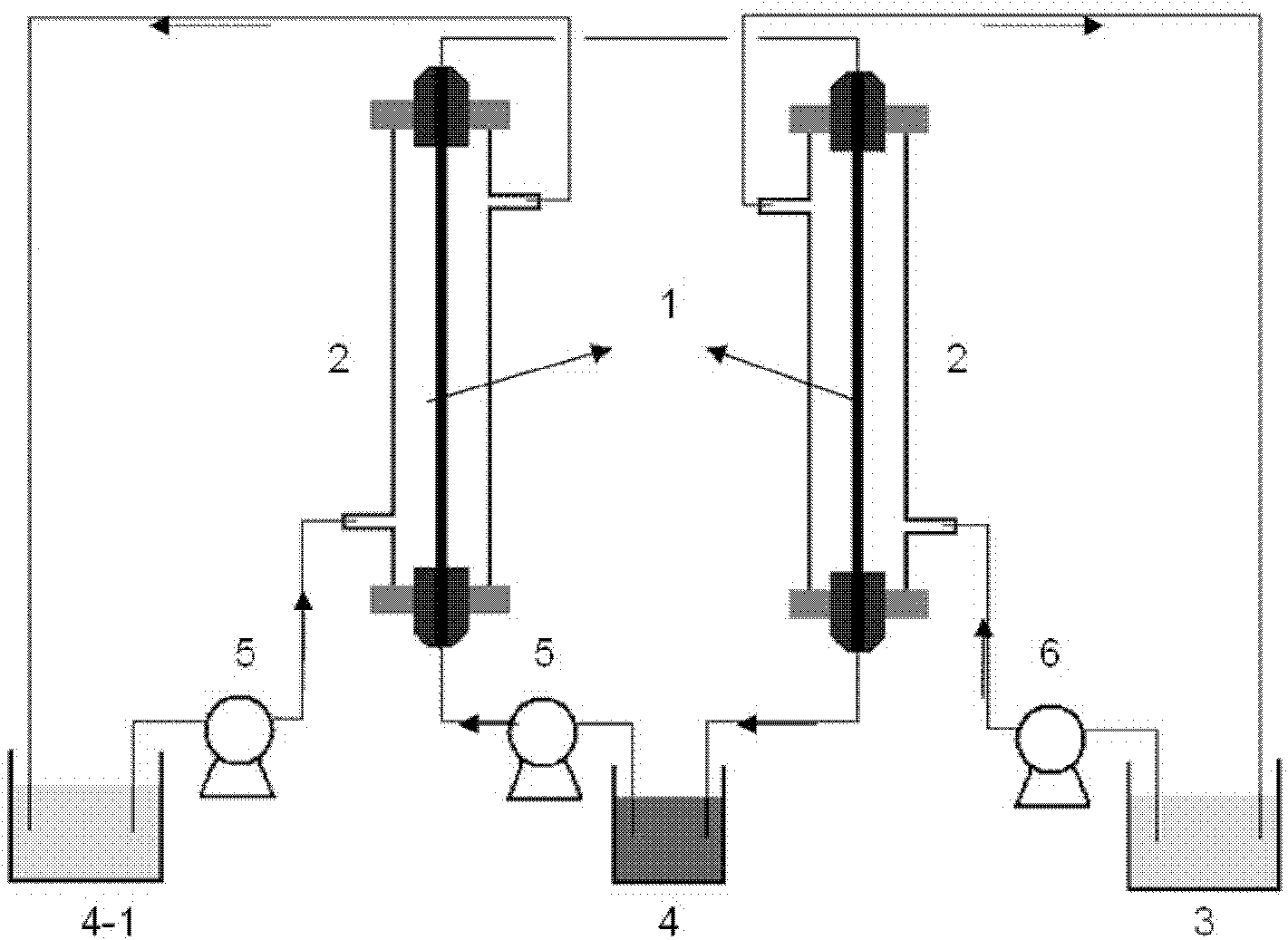
ActiveUS9062385B2Efficient separationShort processing flowSludge treatmentVolume/mass flow measurementSupporting electrolyteIon-exchange membranes
A method for extracting and enriching lithium, including: (a) providing an electrodialysis device including an electrodialysis cell; (b) dividing the electrodialysis cell into a lithium salt chamber and a brine chamber using an anion exchange membrane; (c) filling the brine chamber with salt lake brine; (d) filling the lithium salt chamber with a Mg2+ free supporting electrolyte solution; (e) placing a conductive substrate coated with an ion sieve in the brine chamber to operate as a cathode; (f) placing a conductive substrate coated with a lithium-intercalated ion sieve in the lithium salt chamber to operate as an anode; and (g) carrying out an electrodialysis.
Owner:CENT SOUTH UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com