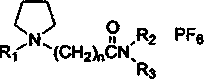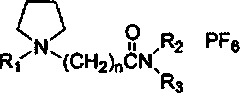Lithium extraction system
An extraction and system technology, applied in the direction of improving process efficiency, etc., can solve the problems of strong corrosion of extraction equipment, high solubility of brine, and lack of large-scale industrial production, and achieve easy equipment selection, simple production operation, and easy industrial production. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The ionic liquid is N-methyl-N-(N’-2-ethylcarbonyl) ethylpyrrole hexafluorophosphate, and its structural formula is:
[0022]
[0023] The co-extraction agent is a mixture of diisobutyl ketone and TBP, the ratio of the two is 4:1; the diluent is sulfonated kerosene. Add 1 volume of salt lake brine shown in Table 1 into a separatory funnel, add 1 volume of organic phase (compared to O / A=1), wherein the volume ratio of ionic liquid, co-extractant and diluent is 1 : 4: 5, after shaking for 10 minutes, rest and layer. Determination of Li in the equilibrium aqueous phase + content, the single extraction rate of lithium is calculated to be 76.84%.
[0024] As a comparison, similar to the conditions in Example 1, except that no N-methyl-N-(N'-2-ethylcarbonyl) ethylpyrrole hexafluorophosphate ionic liquid was added, the single extraction rate of lithium was 57.38 %.
Embodiment 2
[0026] The ionic liquid is N-ethyl-N-(N’-2-ethylcarbonyl) ethylpyrrole hexafluorophosphate, and its structural formula is:
[0027]
[0028] The co-extraction agent is TBP (tributyl phosphate), and the diluent is solvent gasoline. In a separating funnel, add 1 volume of salt lake brine as shown in Table 1, and add 2 volumes of the organic phase (compared to O / A = 2), wherein the volume ratio of ionic liquid, co-extraction agent and diluent is 2:4:4, shake for 10 minutes and then static layering. Determination of Li in the equilibrium aqueous phase + content, the single extraction rate of lithium is calculated to be 80.34%.
[0029] As a comparison, similar to the conditions in Example 2, except that no N-methyl-N-(N'-2-ethylcarbonyl) ethylpyrrole hexafluorophosphate ionic liquid was added, the single extraction rate of lithium was 61.35 %.
Embodiment 3
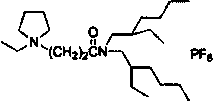
[0031] The ionic liquid is N-ethyl-N-[N'-(2-ethylhexyl)carbonyl]ethylpyrrole hexafluorophosphate, and its structural formula is:
[0032]
[0033] The extractant is amide compound N503, the diluent is petroleum ether, and 1 volume of salt lake brine and 3 volumes of organic phase (compared to O / A=3) as shown in Table 1 are added to a separatory funnel, wherein the ion The volume ratio of liquid, co-extraction agent and diluent is 1:3:6, shake for 10 minutes and then static layer. Determination of Li in the equilibrium aqueous phase + content, the single extraction rate of lithium is calculated to be 70.15%.
[0034] As a comparison, similar to the conditions in Example 3, except that no N-methyl-N-(N'-2-ethylcarbonyl) ethylpyrrole hexafluorophosphate ionic liquid was added, the single extraction rate of lithium was 58.87 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com