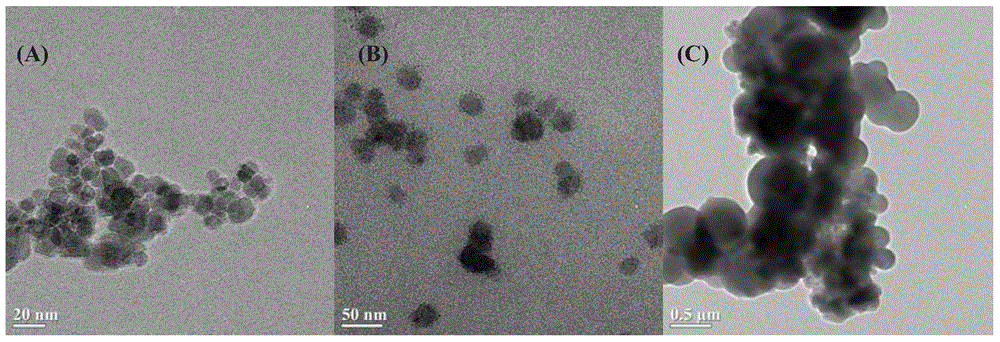
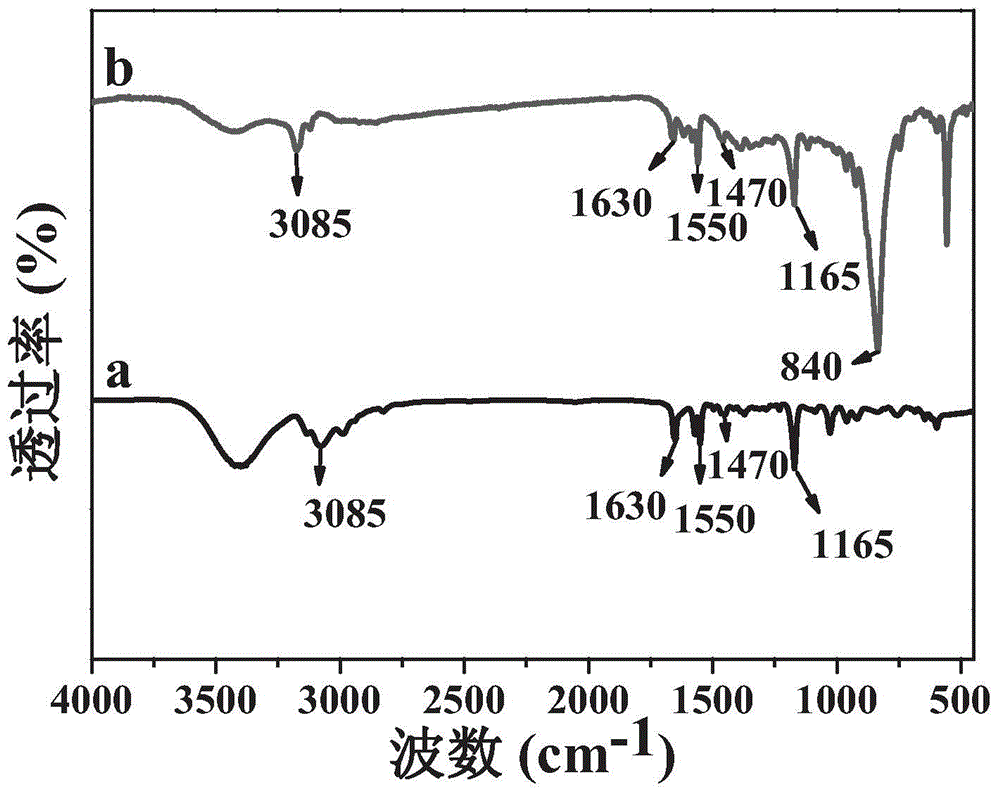
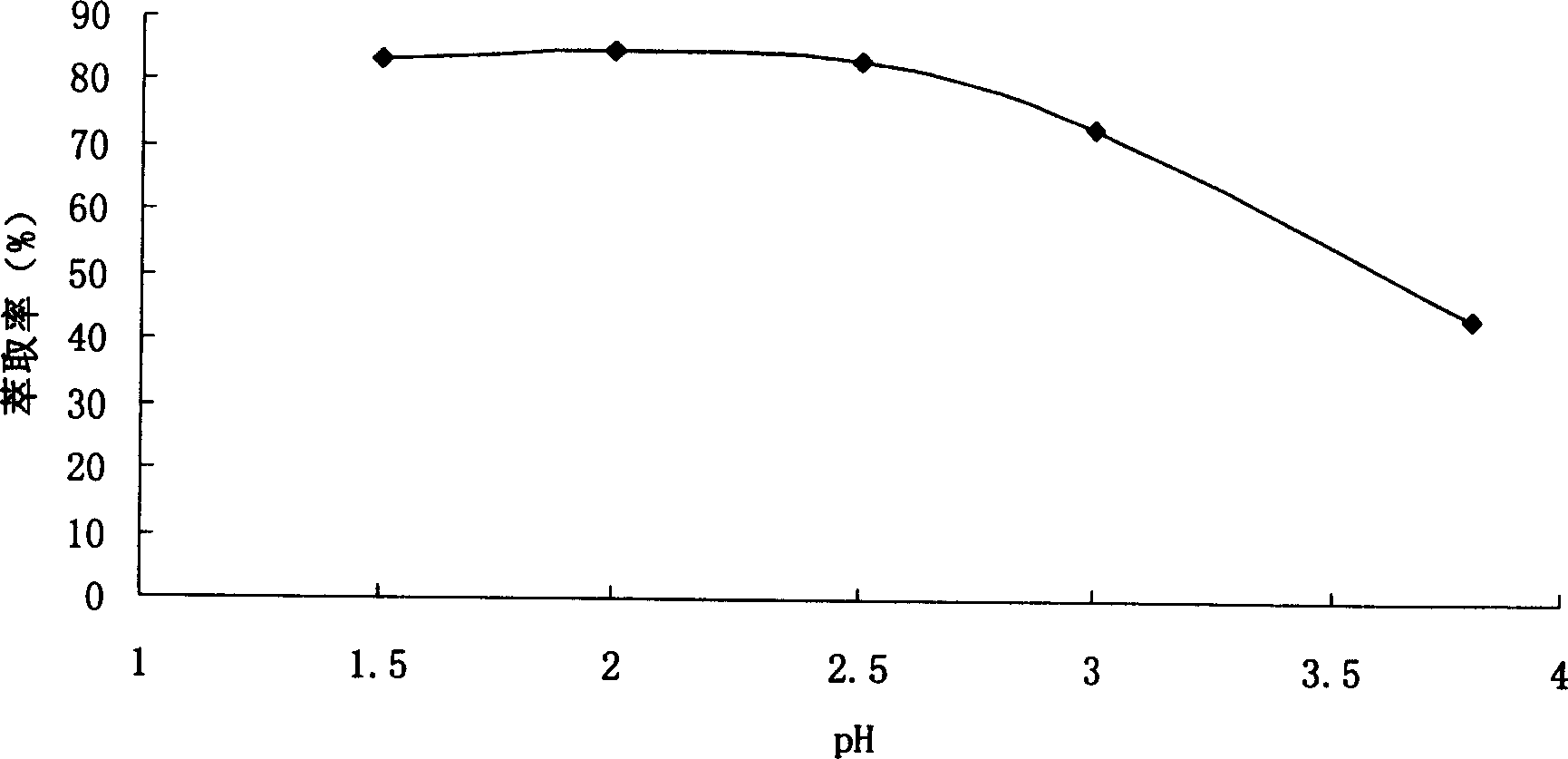
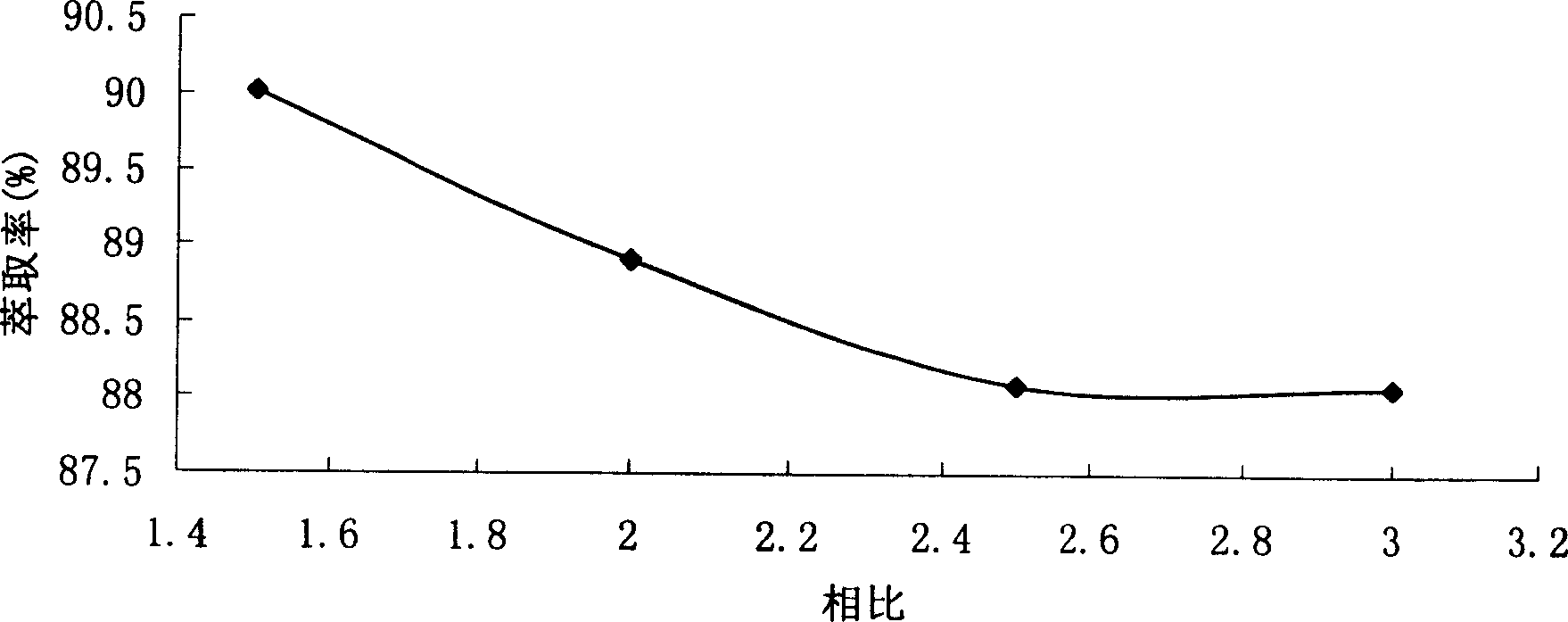
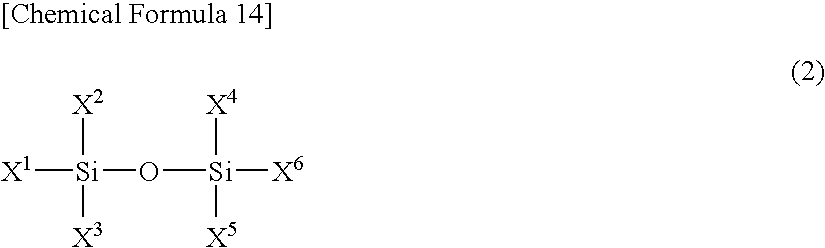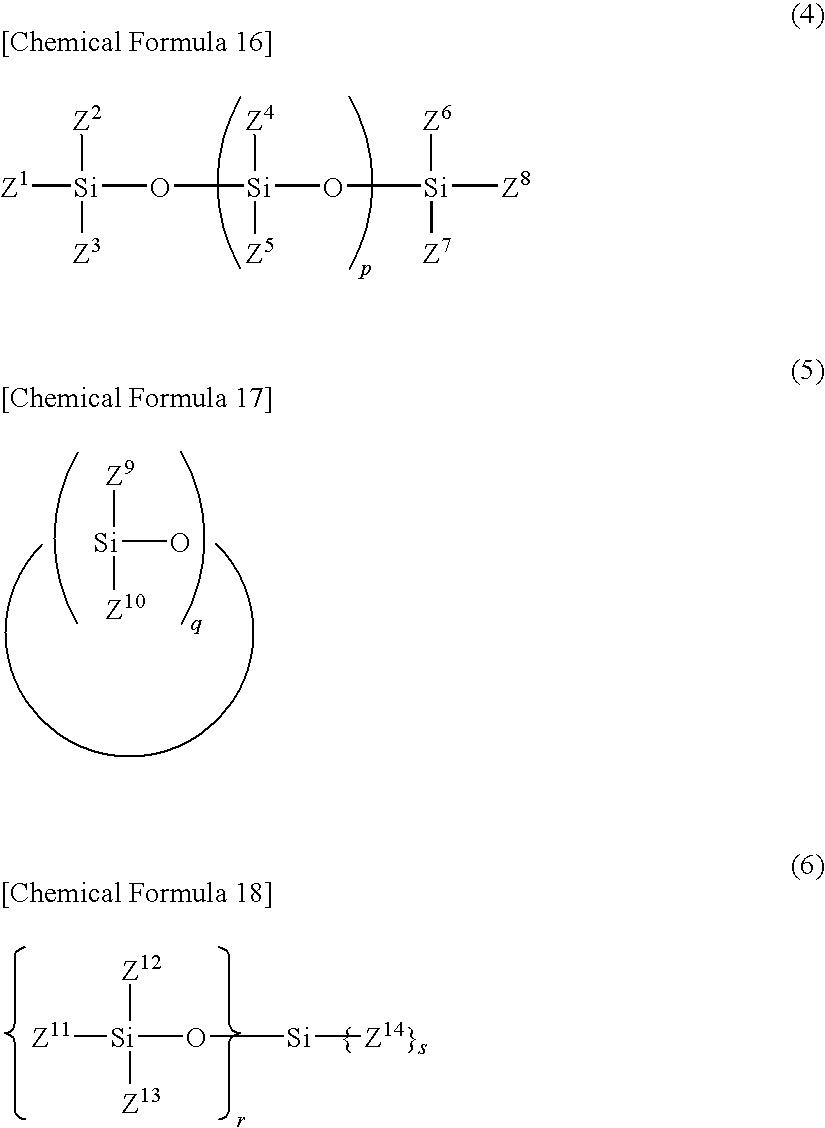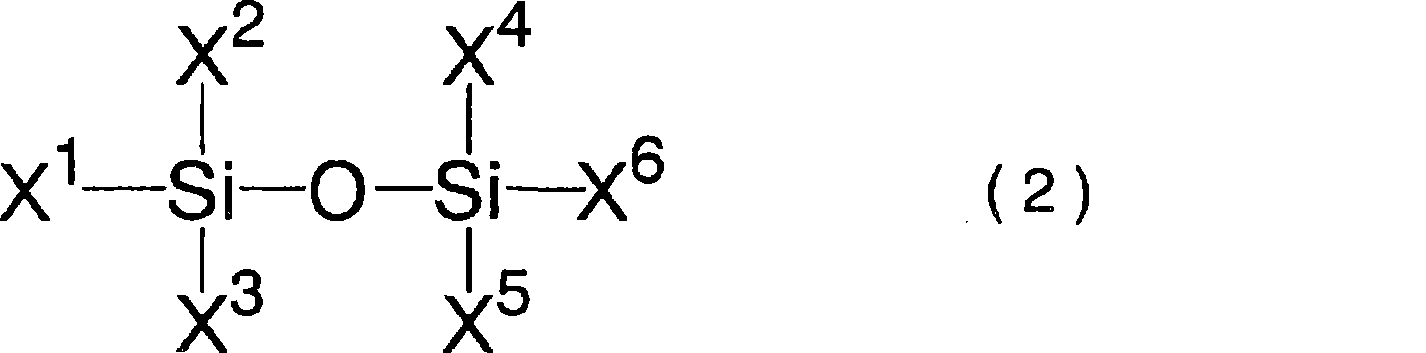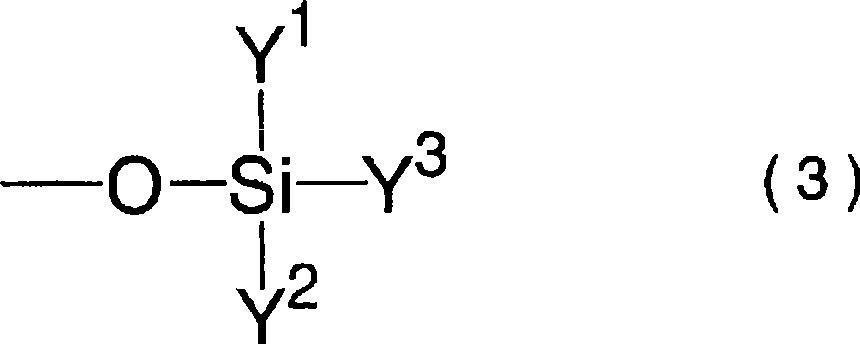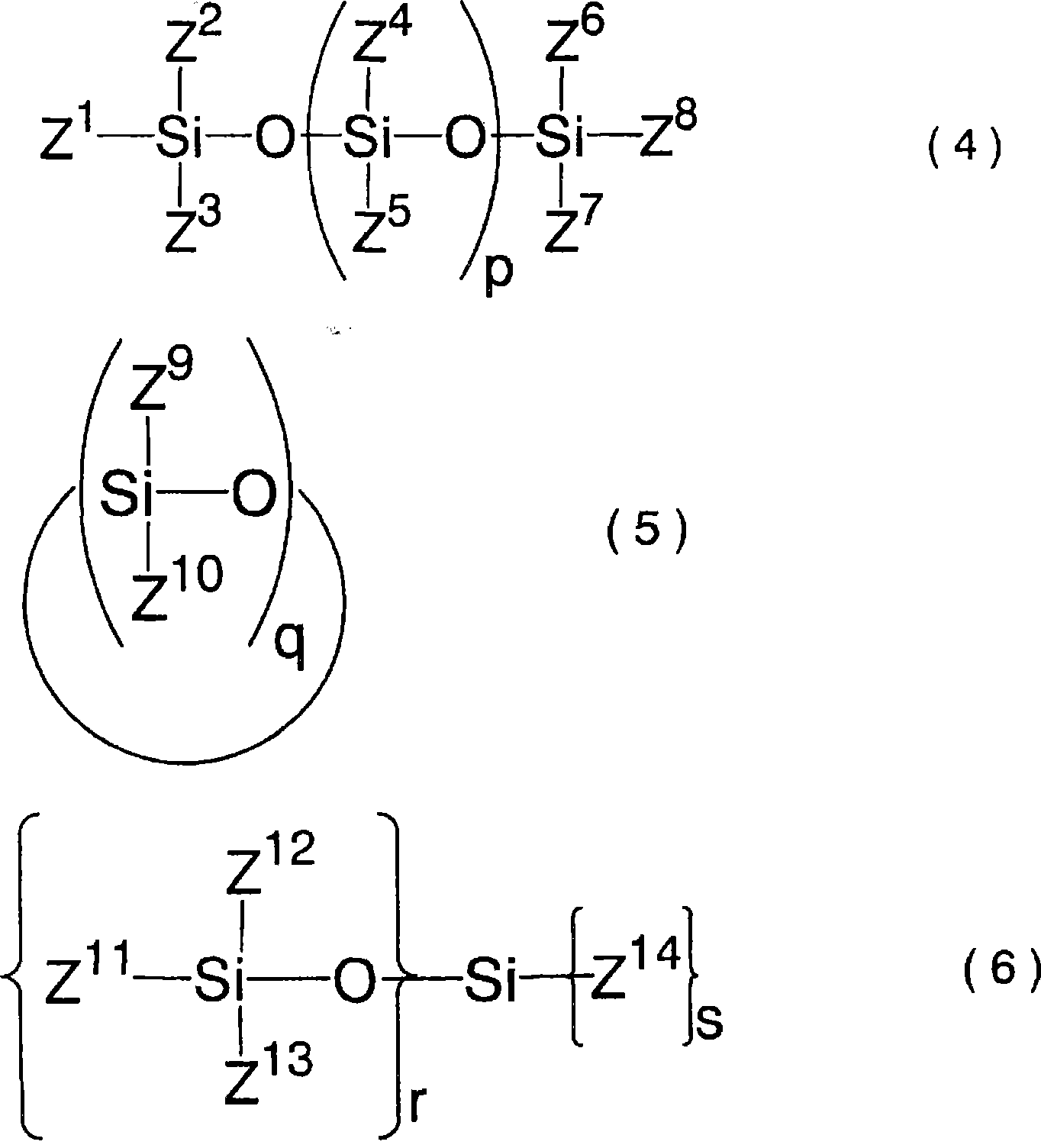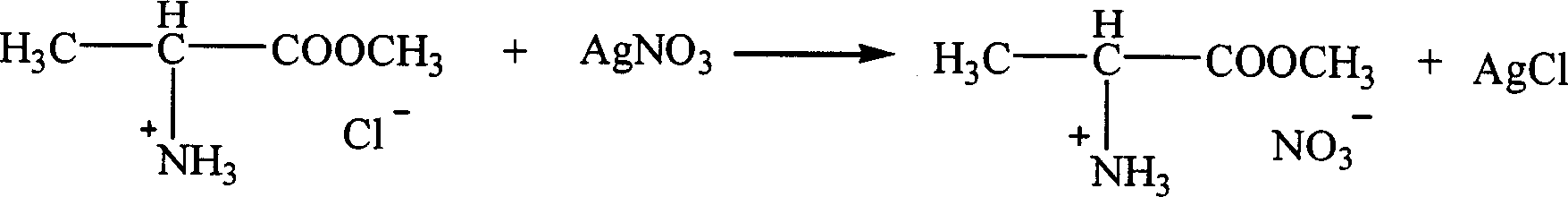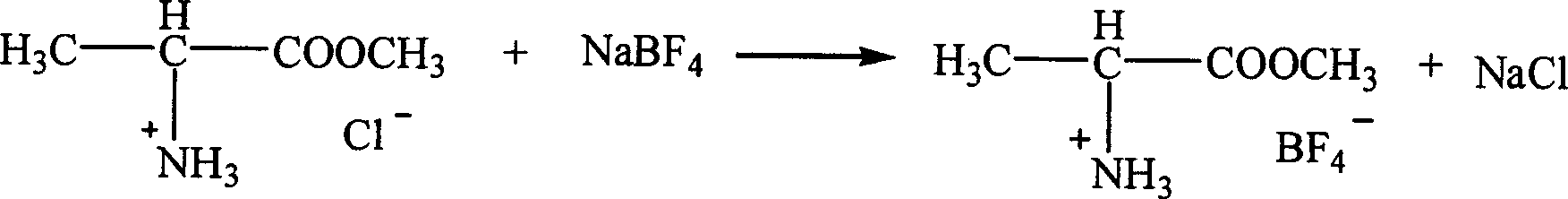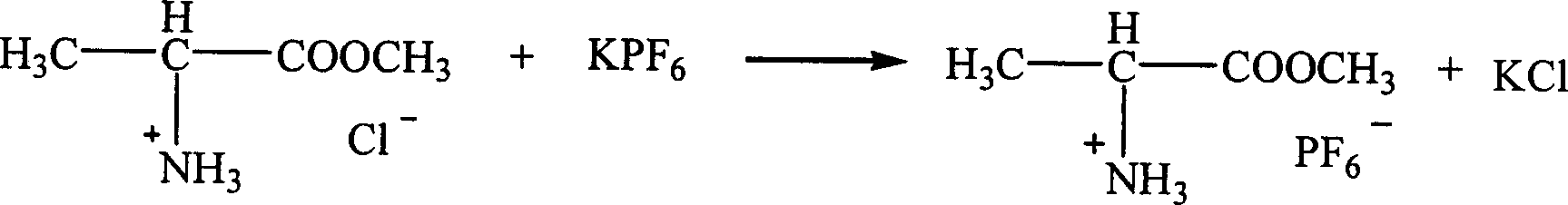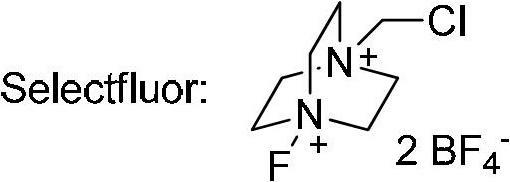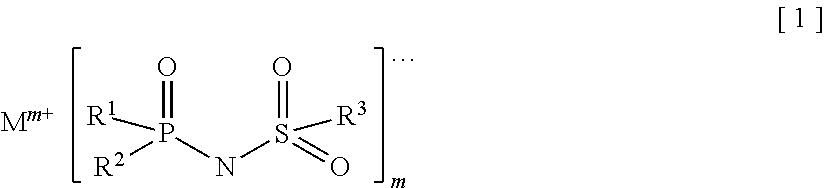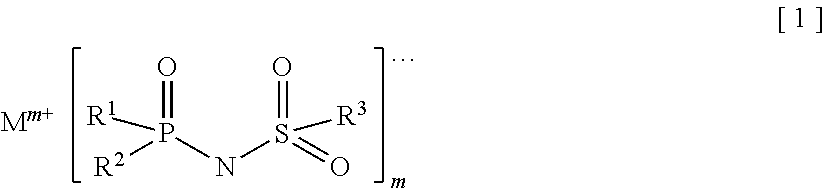Patents
Literature
550 results about "Hexafluorophosphate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hexafluorophosphate is an anion with chemical formula of PF⁻₆. It is an octahedral species. It imparts no color to its salts. PF⁻₆ is isoelectronic with sulfur hexafluoride, SF₆, and the hexafluorosilicate dianion, SiF²⁻₆, and fluoroantimonate SbF⁻₆. Being poorly nucleophilic, hexafluorophosphate is classified as a non-coordinating anion.
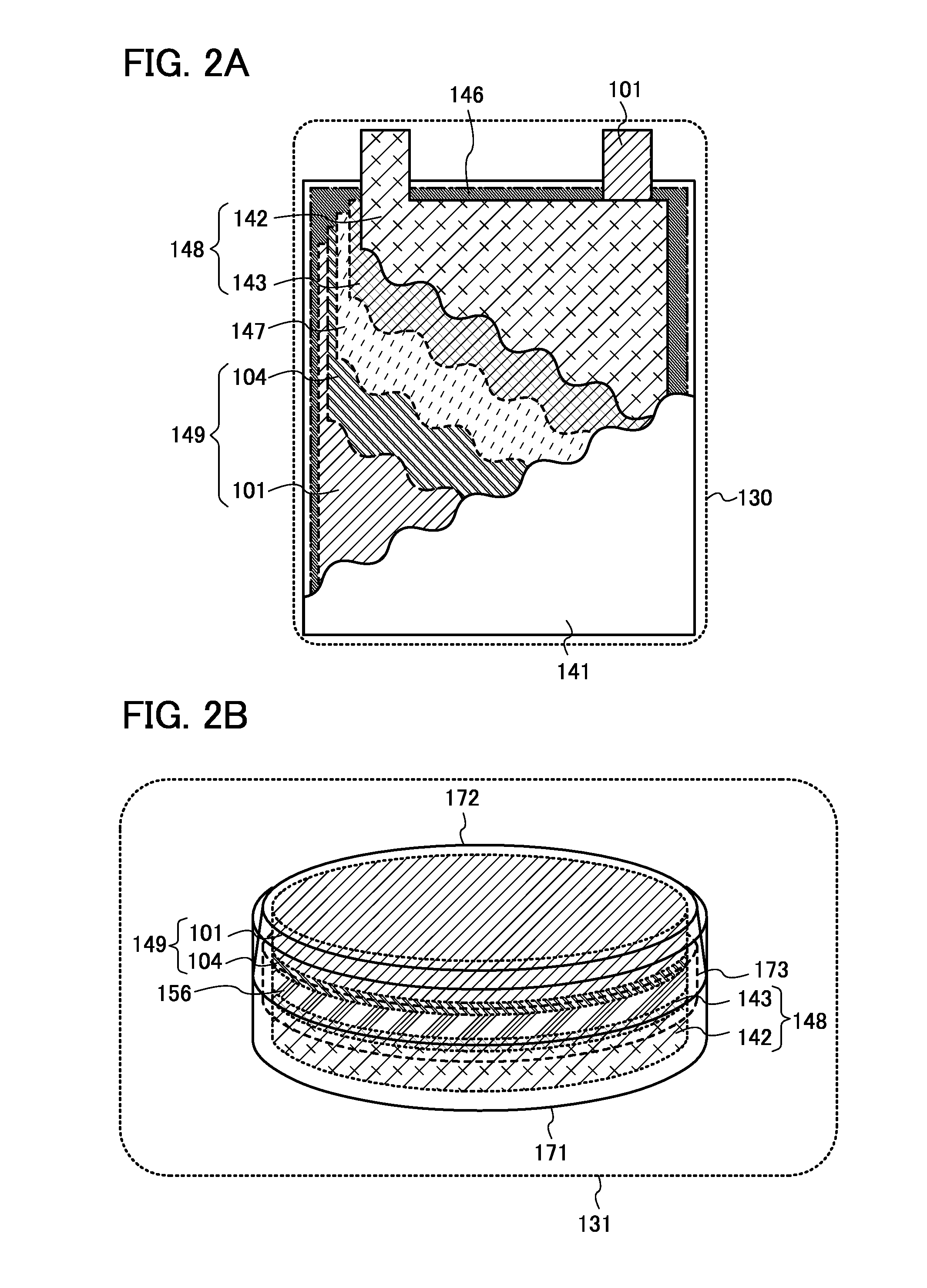
Lithium difluorophosphate, electrolyte containing lithium difluorophosphate, process for producing lithium difluorophosphate, process for producing nonaqueous electrolyte, nonaqueous electrolyte, and nonaqueous electrolyte secondary battery containing the same
InactiveUS20090286155A1Good low temperatureExcellent cycle characteristicsPhosphorus halides/oxyhalidesCell electrodesBoiling pointSolvent
A difluorophosphate salt, which is expensive and not readily available, can be produced with a high purity readily and efficiently from inexpensive and readily available materials. A nonaqueous electrolyte secondary battery that exhibits low-temperature discharge and heavy-current discharge characteristics and high-temperature preservation and cycle characteristics without impairing the battery safety. A hexafluorophosphate salt is reacted with a compound having a bond represented by the following formula (1) in the molecule:Si—O—Si (1)A nonaqueous electrolyte used for nonaqueous electrolyte secondary batteries including a negative electrode and a positive electrode that can occlude and discharge ions, and a nonaqueous electrolyte is prepared from a mixture obtained by mixing at least one nonaqueous solvent, a hexafluorophosphate salt and a compound having a bond represented by the following formula (1), and removing low-boiling compounds newly formed in the system, the low-boiling compounds having a lower boiling point than that of the compound having the bond represented by the formula (1):Si—O—Si (1)
Owner:MITSUBISHI CHEM CORP
Method for Producing Difluorophosphate, Non-Aqueous Electrolyte for Secondary Cell and Non-Aqueous Electrolyte Secondary Cell
ActiveUS20080305402A1Poor battery performanceExcellent coating agentPhosphorus halides/oxyhalidesNon-aqueous electrolyte accumulatorsDifluorophosphatePhysical chemistry
A difluorophosphate effective as an additive for a nonaqueous electrolyte for secondary battery is produced by a simple method from inexpensive common materials.The difluorophosphate is produced by reacting lithium hexafluorophosphate with a carbonate in a nonaqueous solvent. The liquid reaction mixture resulting from this reaction is supplied for providing the difluorophosphate in a nonaqueous electrolyte comprising a nonaqueous solvent which contains at least a hexafluorophosphate as an electrolyte lithium salt and further contains a difluorophosphate. Also provided is a nonaqueous-electrolyte secondary battery employing this nonaqueous electrolyte.
Owner:MU IONIC SOLUTIONS CORP +1
Lithium difluorophosphate, electrolytic solution containing lithium difluorophosphate, process for producing lithium difluorophosphate, process for producing nonaqueous electrolytic solution, nonaqueo
ActiveCN101507041AEasy and efficient to prepareHigh purityCell electrodesLi-accumulatorsElectrolytic agentElectrical battery
A difluorophosphate salt, which is expensive and not readily available, can be produced with a high purity readily and efficiently from inexpensive and readily available materials. A nonaqueous electrolyte secondary battery that exhibits low-temperature discharge and heavy-current discharge characteristics and high-temperature preservation and cycle characteristics without impairing the battery safety. A hexafluorophosphate salt is reacted with a compound having a bond represented by the following formula (1) in the molecule: Si-O-Si (1). A nonaqueous electrolyte used for nonaqueous electrolyte secondary batteries including a negative electrode and a positive electrode that can occlude and discharge ions, and a nonaqueous electrolyte is prepared from a mixture obtained by mixing at least one nonaqueous solvent, a hexafluorophosphate salt and a compound having a bond represented by the following formula (1), and removing low-boiling compounds newly formed in the system, the low-boiling compounds having a lower boiling point than that of the compound having the bond represented by the formula (1): Si-O-Si (1).
Owner:MITSUBISHI CHEM CORP +1
Method for producing difluorophosphate, nonaqueous electrolyte solution, and nonaqueous electrolyte secondary battery
ActiveCN102036912AEasy to manufactureHigh purityFinal product manufactureLi-accumulatorsHydrogen fluorideAlkaline earth metal
A method for producing a high-purity difluorophosphate by simple processes; a method for producing an electrolyte solution using a difluorophosphate obtained by the method; an electrolyte solution; and a secondary battery. Specifically disclosed is a method for producing a difluorophosphate, which comprises the following step (1) or (2). (1) A step wherein (A) at least one substance selected froma group consisting of phosphorus oxo acids, oxo acid anhydrides and oxyhalides is reacted with (B) a hexafluorophosphate in the presence of hydrogen fluoride. (2) A step wherein at least one halide selected from a group consisting of alkali metal halides, alkaline earth metal halides, aluminum halides and onium halides is reacted with difluorophosphoric acid in the presence of a hexafluorophosphate. Also disclosed are a nonaqueous electrolyte solution containing the thus-obtained difluorophosphate, and a nonaqueous electrolyte secondary battery comprising the nonaqueous electrolyte solution.
Owner:STELLA CHEMIFA CORP +2
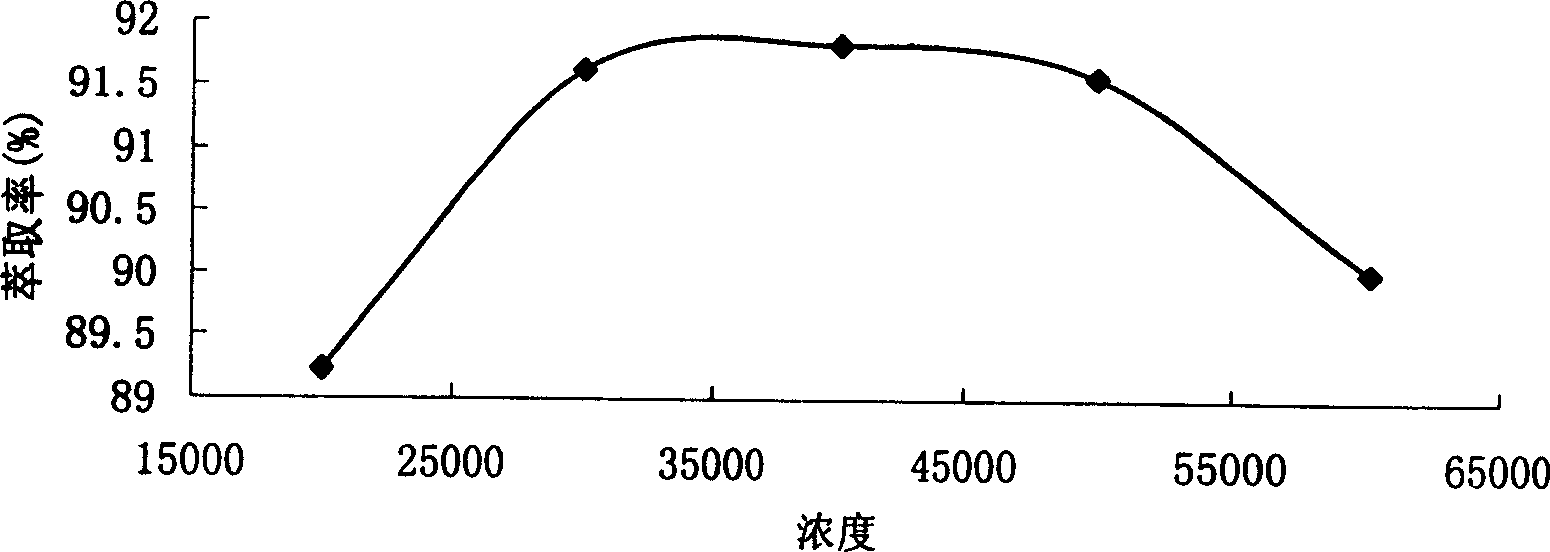
Lithium extraction system
ActiveCN106498184AReduce usageEasy material selectionProcess efficiency improvementSolvent extractionLithium chlorideKerosene
The invention provides a system from extracting lithium from salt lake brine, comprising ionic liquid, a co-extracting agent and a diluent; the ionic liquid is pyrrole hexafluorophosphate ionic liquid comprising lithium extraction functional groups, and the diluent is from solvent gasoline, sulfonated kerosene and petroleum ether. The use of the co-extracting agent iron trichloride is avoided in the lithium extraction system, adjusting pH of brine is not required, at least 5 tons of industrial hydrochloric acid and 2 tons of sodium hydroxide may be saved per ton of lithium chloride produced, and the production cost is greatly reduced; saponifying step, pickling step and deironing step are omitted from the technique, and therefore, the system is easier to use in industrial large-scale production.
Owner:青海柴达木兴华锂盐有限公司
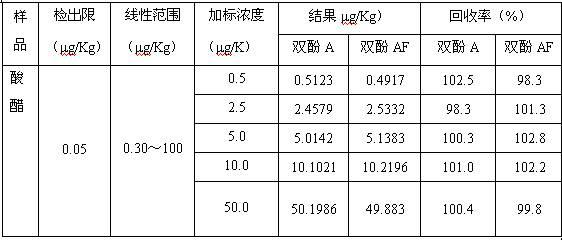
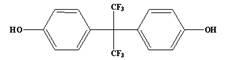
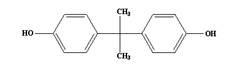
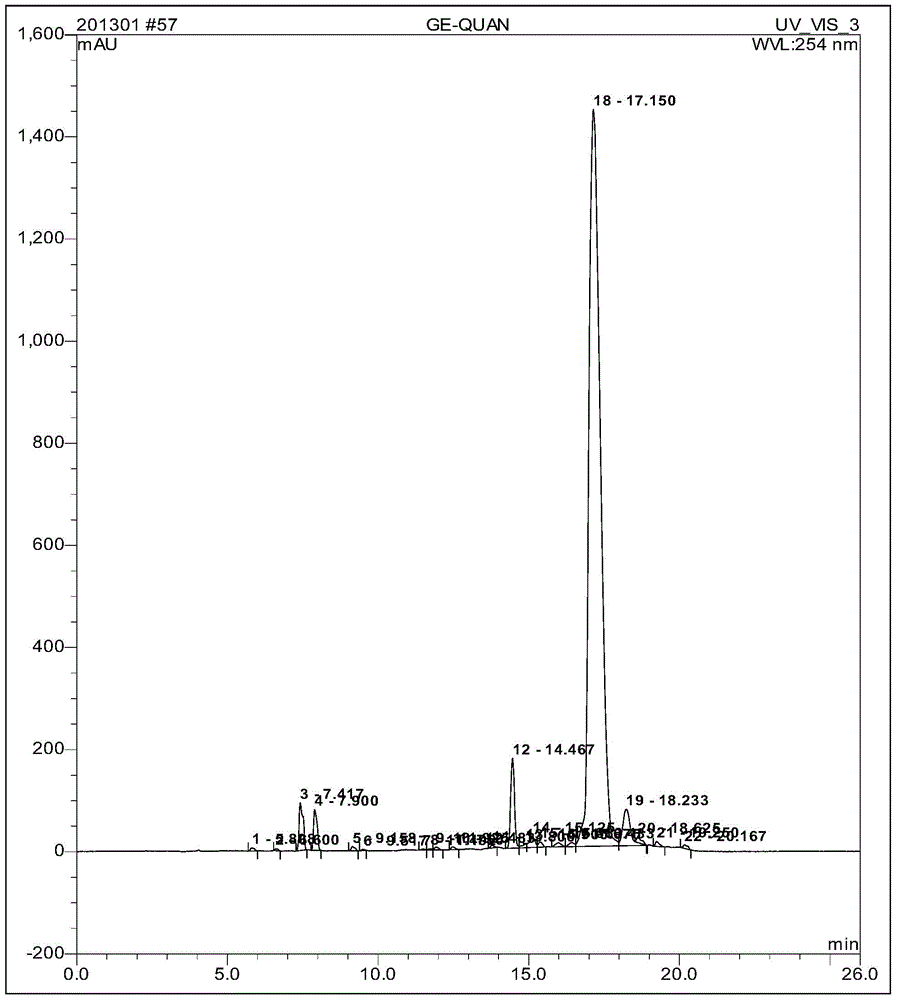
Method for rapidly detecting bisphenol A and bisphenol AF in food
The invention relates to a method for detecting bisphenol A and bisphenol AF in food, belonging to technical field of analytical chemistry determination method. With ionic liquid (1-butyl-3-methylimidazole hexafluorophosphate) as an extractant and TritonX-100 or acetone as an dispersant, ultrasonic oscillation is carried out to form emulsion; after centrifugal separation, extraction drops at the lower layer are directly subjected to HPLC (high performance liquid chromatography) quantitative analysis. The method disclosed by the invention has the advantages of simpleness in detection steps, easiness in operation, low detection limit and high enrichment multiple, greatly reduced detection cost. The method disclosed by the invention is a rapid, efficient and environmentally friendly pre-treatment technology and has a wider application prospect in the field of food analysis.
Owner:KUNMING UNIV OF SCI & TECH TECH IND SALES MANAGEMENT
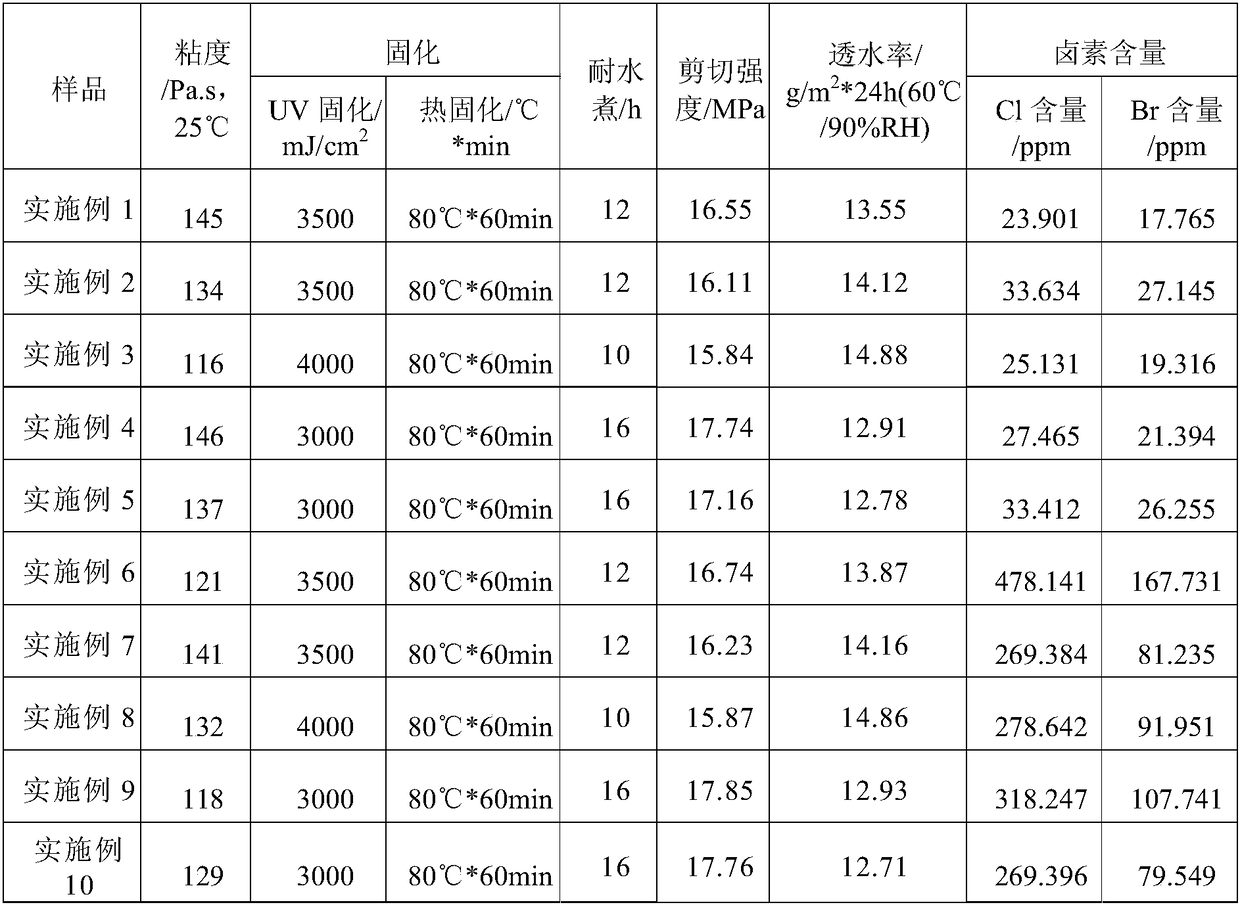
Epoxy adhesive for OLED frame encapsulation and preparation method of epoxy adhesive
InactiveCN108546536ACuring shrinkage is smallNot easy to terminateNon-macromolecular adhesive additivesMacromolecular adhesive additivesAntioxidantWater vapor
The invention discloses an epoxy adhesive for OLED frame encapsulation and a preparation method of the epoxy adhesive. The epoxy adhesive is prepared from the following components in parts by weight:50-80 parts of epoxy resin, 5-10 parts of epoxy diluents, 5-10 parts of flexibilizer, 20-40 parts of filler, 1-5 parts of photoinitiator, 0.1-1 part of antioxidant and 0.3-3 parts of additive. The epoxy resin is one or a mixture of more of cycloaliphatic epoxy resin and bisphenol F epoxy resin. The epoxy diluent is one or a mixture of more of oxetane and aliphatic glycidyl ether. The photoinitiator is a cationic photoinitiator which comprises an iron-arene photoinitiator and a hexafluorophosphate photoinitiator. The epoxy adhesive adopts a curing way that ultraviolet exposure and heating are conducted in sequence, has the advantages of less curing agent residue, less volatile component residue, low curing temperature, strong bonding force, boiling resistance, water vapor resistance, oxygenbarrier performance and the like, and reaches the halogen-free standard.
Owner:SHENZHEN FISHER NEW MATERIALS CO LTD
Ion liquid of amino acid ester cation and its preparation method
InactiveCN1621152AEasy to getLow priceOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsTetrafluoroborateAmino acid
The present invention is ion liquid of amino acid ester cation and its preparation process, and belongs to the field of new chemical material and its preparation technology. The ion liquid of amino acid ester cation is prepared with amino acid ester hydrochloride and through the substitution reaction with nitrate, tetrafluoroborate, hexafluorophosphate, bistrifluoromesyl imine salt or thiocyanate or the direct addition reaction with aluminum trichloride, ferric trichloride or zinc chloride, and the separation. The ion liquid thus prepared has the characteristic of chiral matter except the ion liquid characteristics, has low cost, simple preparation process and no pollution. The present invention is suitable for industrial production and is expected to become important green chemical material.
Owner:PEKING UNIV
Affinity chromatography medium employing tetrapeptide as functional ligand and preparation method of affinity chromatography medium
ActiveCN104645949AHigh affinityGood choiceOther chemical processesSolid sorbent liquid separationSodium acetateAcetic anhydride
The invention discloses an affinity chromatography medium employing tetrapeptide as a functional ligand and a preparation method of the affinity chromatography medium. The method comprises the following steps: adding dry matrix and allyl bromide to a dimethyl sulfoxide solution, activating, and reacting activating matrix with N-bromo succinimide; enabling bromo alcoholized matrix to react with hexamethylendiamine to obtain amino activating matrix; sequentially washing with deionized water, absolute ethyl alcohol and anhydrous N,N-dimethyl formamide, adding an N,N-dimethyl formamide solution containing tetrapeptide, 2-(7-azobenzotriazole)-N,N,N',N'-te-tramethyluronium hexafluorophosphate and N,N-diisopropylethylamine, and coupling a tetrapeptide ligand; and putting a medium coupled to tetrapeptide in a mixed liquid of sodium acetate and acetic anhydride to obtain the affinity chromatography medium employing tetrapeptide as the functional ligand. According to the novel chromatography medium developed by the method, a functional group is tetrapeptide composed of tyrosine, phenylalanine, arginine and histidine, and is designed on the basis of a protein A binding site of an antibody Fc segment; the antibody binding selectivity is greatly improved; and the affinity chromatography medium can be applied to efficient separation of an antibody.
Owner:ZHEJIANG UNIV
Supported catalyst for preparing aldehyde by olefin hydroformylation
InactiveCN1736602AIncreased space-time yieldSimple compositionOrganic-compounds/hydrides/coordination-complexes catalystsPreparation by carbon monoxide reactionMolecular sieveTetrafluoroborate
Disclosed is a supported catalyst mainly containing sulfonated triphenylphosphine- rhodium complex and is used for preparing aldehyde by hydroformylation of olefin, and relates to an ionic liquid catalyst. It contains solid oxide, sulfonated triphenylphosphine- rhodium complex, sulfonated triphenylphosphine ligand and ionic liquid. The solid oxide is one from hole molecular sieve, SiO2, TiO2, gamma- Al2O3; sulfonated triphenylphosphine- rhodium complex is single-sulfonated triphenylphosphine- rhodium complex, di- sulfonated triphenylphosphine- rhodium complex and tri- sulfonated triphenylphosphine- rhodium complex; and the ionic liquid is 1, 1, 3, 3, - tetramethyl guanidine lactate, 1- butyl- 3- methyl imidazolium tetrafluorborate and 1- butyl- 3- methyl imidazolium hexafluorophosphate. By mass ratio, the solid oxide is among 50%- 90%, the ionic liquid 8%- 49%, and rhodium 0.05%- 2%, and by molecular ratio phosphine to rhodium is 3- 200.
Owner:XIAMEN UNIV
Ionic liquid and power storage device including the same
ActiveUS20120308882A1Improve electrochemical stabilityLow melting pointHybrid capacitor separatorsHybrid capacitor electrolytesTetrafluoroborateElectrochemistry
An ionic liquid having high electrochemical stability and a low melting point. An ionic liquid represented by the following general formula (G0) is provided.In the general formula (G0), R0 to R5 are individually any of an alkyl group having 1 to 20 carbon atoms, a methoxy group, a methoxymethyl group, a methoxyethyl group, and a hydrogen atom, and A− is a univalent imide-based anion, a univalent methide-based anion, a perfluoroalkyl sulfonic acid anion, tetrafluoroborate, or hexafluorophosphate.
Owner:SEMICON ENERGY LAB CO LTD
Method for preparing polyolefin microporous-film supported gel polymer electrolyte film
InactiveCN101070398AHigh mechanical strengthImprove ionic conductivitySynthetic resin layered productsCoatingsPolymer electrolytesPolyolefin
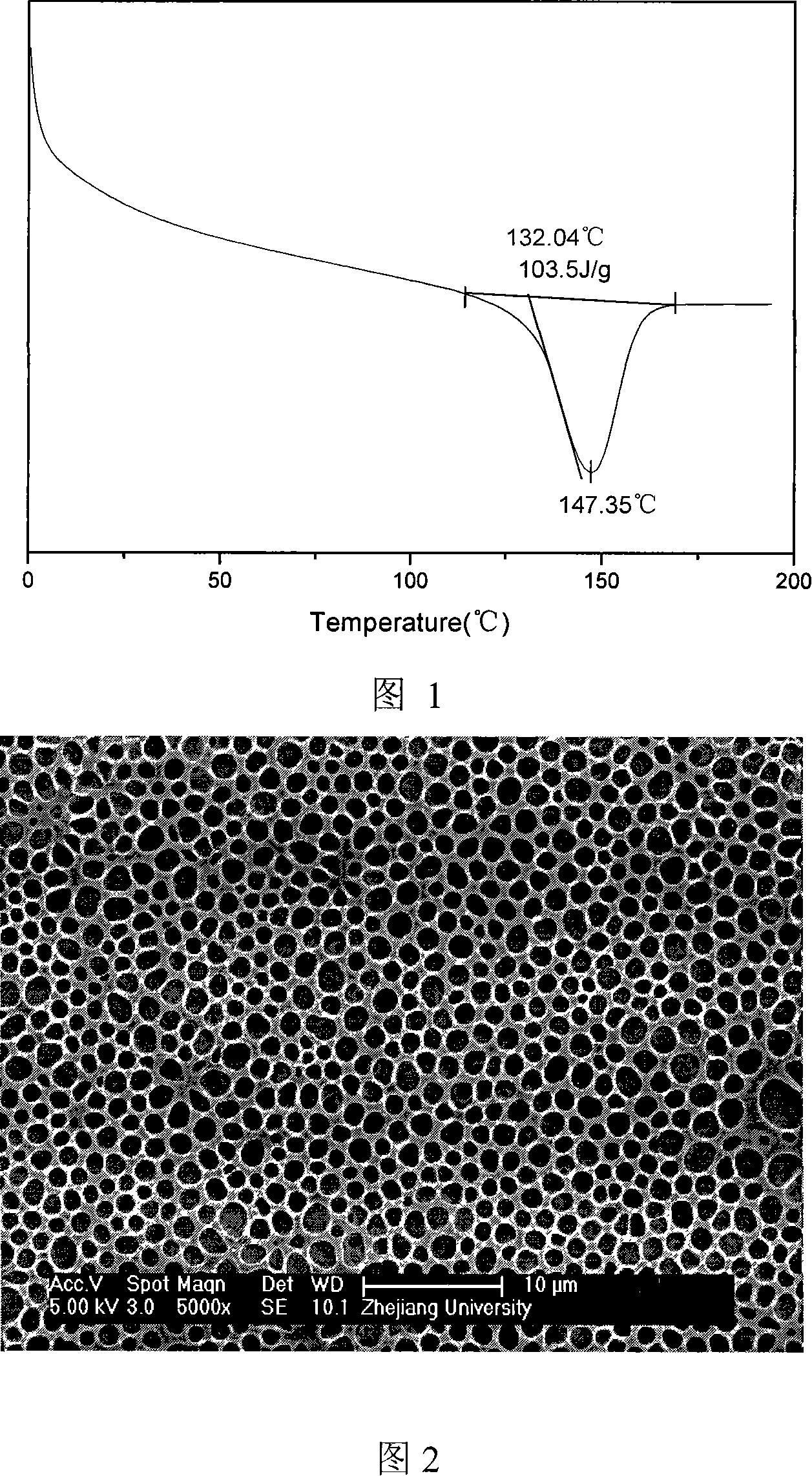
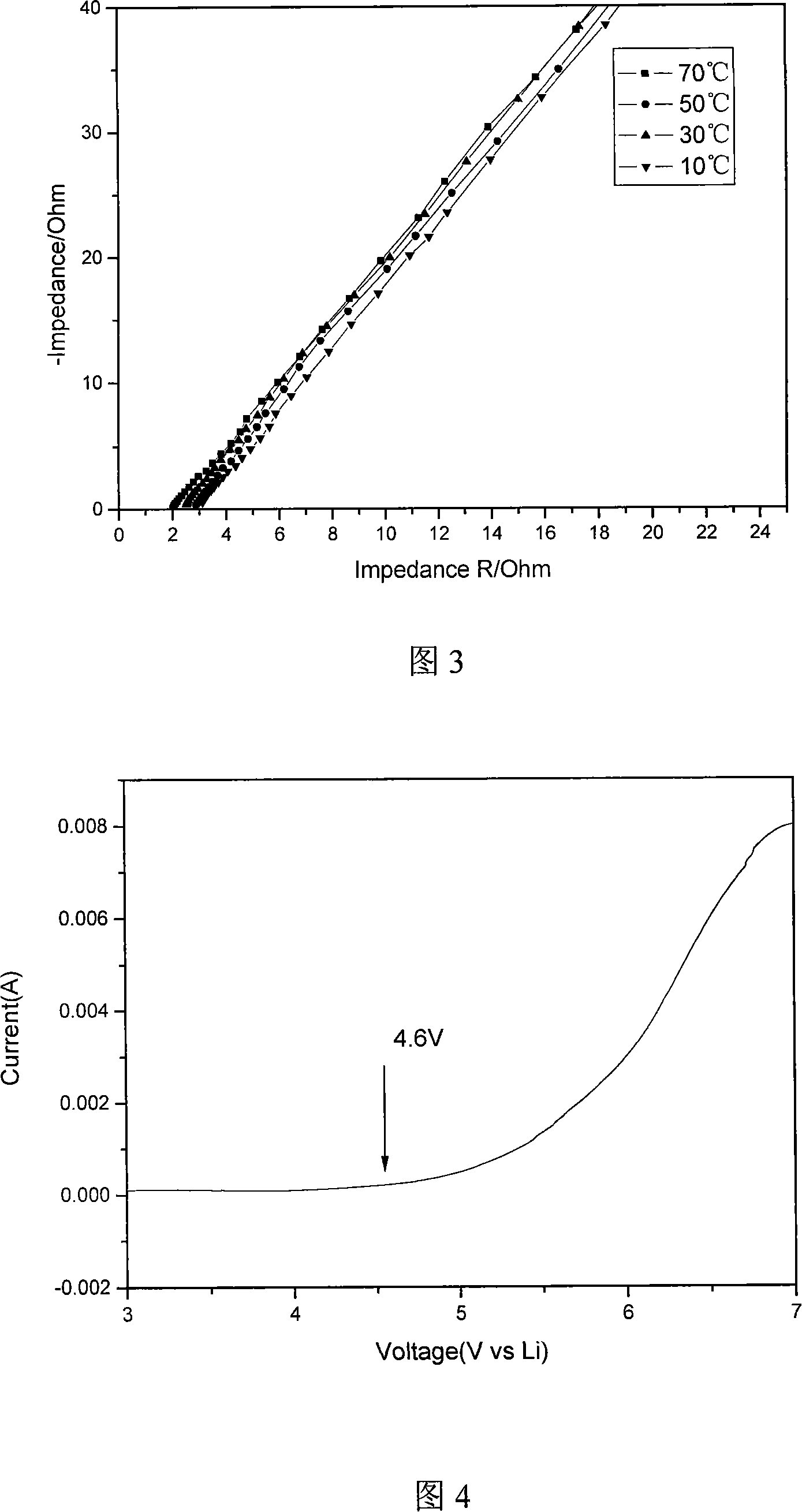
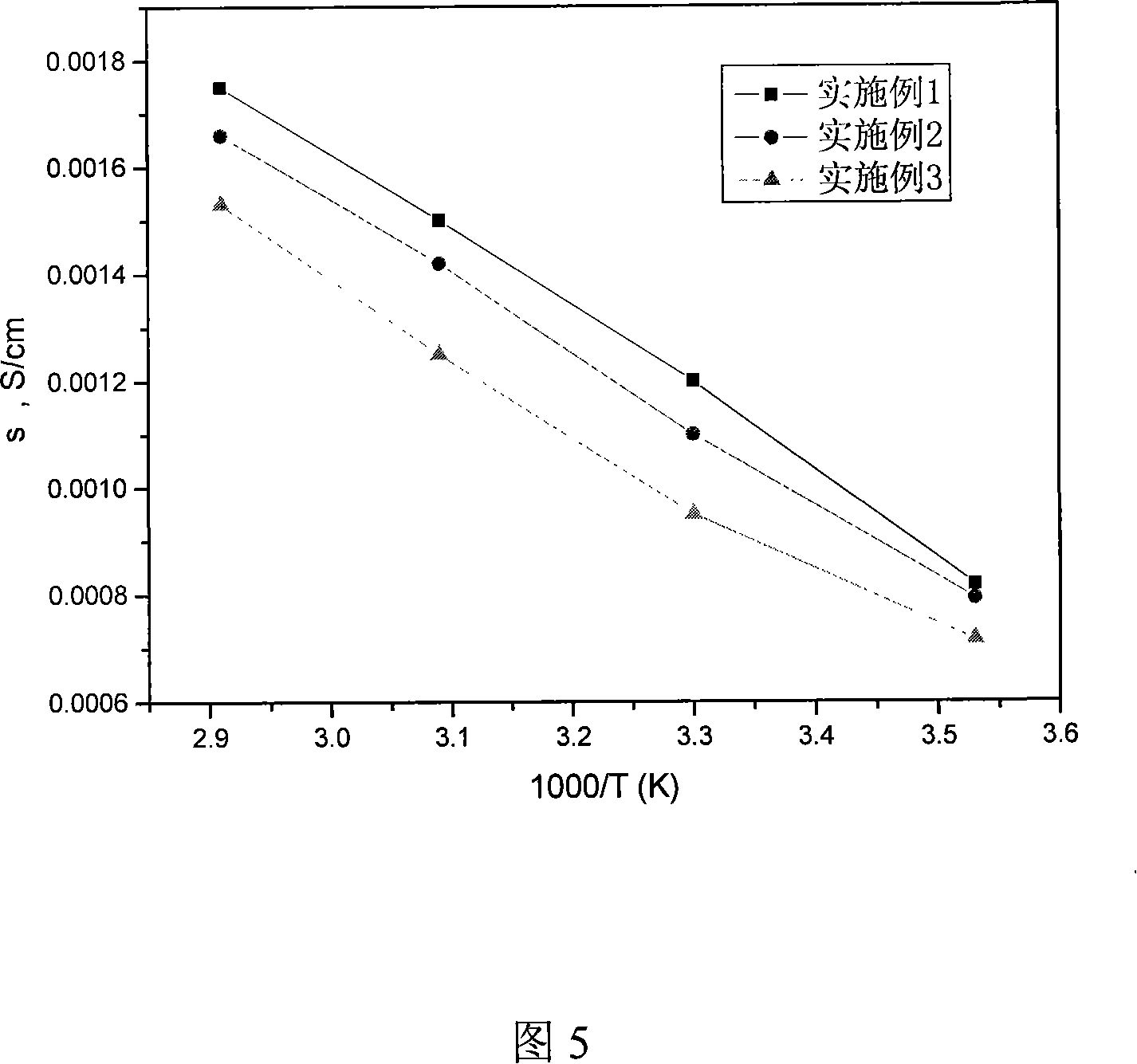
The present invention discloses a preparation method of polyolefin microporous-film-supported gel-polymer electrolyte film; its steps include: methyl methacrylate-methacrylic acyloxy propyl trimethoxy silane polymer is firstly synthesized; after sol-gel reaction, the polymer is directly coated and solidified on polyolefin microporous film, and adsorbs 1M hexafluorophosphate lithium carbonate electrolyte solution to prepare the polyolefin microporous-film-supported gel-polymer electrolyte film. In the system of the film, the polyolefin microporous film takes certain mechanical supporting effect, and has better electrochemical stability; the coated gel-polymer electrolyte not only has a high ionic conductivity, but also well contacts with lithium plate. The ionic conductivity of the film in the present invention can reach 1.2 X10-3S cm-1, and its electrochemical stability window can reach 4.6V. The present invention is simple in preparation process, and suits for industrial production.
Owner:ZHEJIANG UNIV
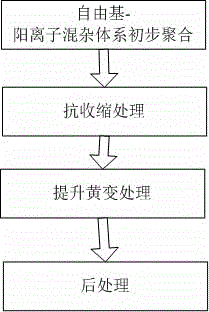
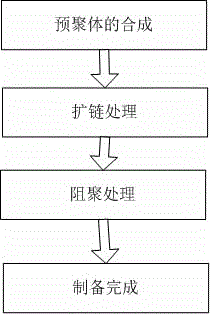
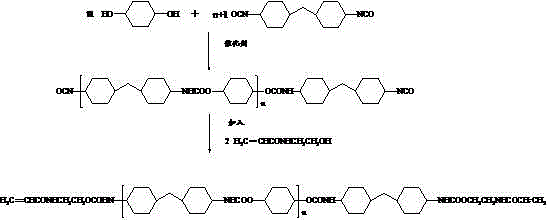
3D printing-based photo-curing material and preparation method thereof
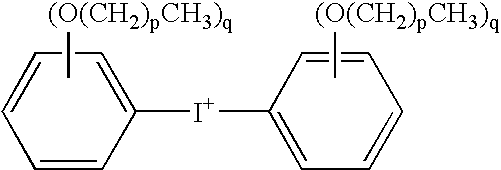
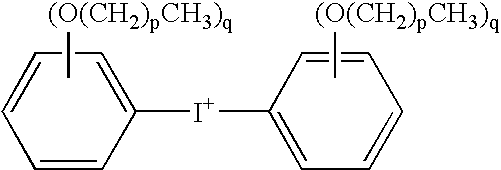
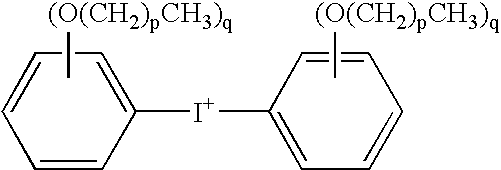
The invention discloses a 3D printing-based photo-curing material and a preparation method thereof. The 3D printing-based photo-curing material comprises the following components in parts by weight: 50-55 parts of aqueous polyurethane acrylic resin, 20-30 parts of hydrogenated bisphenol A epoxy resin, 10-15 parts of alkoxylated pentaerythritol hexaacrylate, 10-15 parts of three-ring decane dimethyl carbinol dimethacrylate, 8-10 parts of hydroxyl ethyl methacrylate, 8-10 parts of vinyl ether, 3-5 parts of 1-hydroxy cyclohexyl phenyl ketone, 3-5 parts of 4,4'-bis(p-toly) iodonium hexafluorophosphate and 0.5-1 part of an 1080 antioxidant. The method comprises the following preparation steps: primary polymerization of a free radical-cation hybrid system, anti-shrinkage treatment, anti-yellowing treatment and post-treatment. The obtained material has the characteristics of excellent performance, low cost, good consistency and the like; and the used preparation method has the characteristics of being environment-friendly, short in production cycle and good in batch stability.
Owner:河源然生新材料有限公司
Molding composition and method, and molded article
A curable method useful for encapsulating solid state devices includes (A) an epoxy resin; (B) an effective amount of a cure catalyst comprising (B1) a first latent cationic cure catalyst comprising a diaryl iodonium hexafluoroantimonate salt; (B2) a second latent cationic cure catalyst comprising (B2a) a diaryl iodonium cation, and (B2b) an anion selected from perchlorate, imidodisulfurylfluoride anion, unsubstituted and substituted (C1-C12)-hydrocarbylsulfonates, (C2-C12)-perfluoroalkanoates, tetrafluoroborate, unsubstituted and substituted tetra-(C1-C12)-hydrocarbylborates, hexafluorophosphate, hexafluoroarsenate, tris(trifluoromethylsulfonyl)methyl anion, bis(trifluoromethylsulfonyl)methyl anion, bis(trifluoromethylsulfuryl)imide anion, and combinations thereof; and (B3) a cure co-catalyst selected from free-radical generating aromatic compounds, peroxy compounds, copper (II) salts of aliphatic carboxylic acids, copper (II) salts of aromatic carboxylic acids, copper (II) acetylacetonate, and combinations thereof; and (C) about 70 to about 95 weight percent of an inorganic filler, based on the total weight of the curable composition. The composition's cure catalyst allows the use of increased filler loadings, which in turn reduces moisture absorption and thermal expansion of the cured composition.
Owner:SABIC INNOVATIVE PLASTICS IP BV
Functionalized perovskite material based on novel ionic liquid and application of functionalized perovskite material in solar cell preparing
ActiveCN107337644AIncreased sensitivityWide absorption rangeOrganic chemistrySolid-state devicesComputational chemistryPhotovoltaic effect
The invention belongs to the technical fields of solar cells, perovskite materials and photovoltaic materials, and specifically discloses a functionalized perovskite material based on novel ionic liquid and application of the functionalized perovskite material in solar cell preparing. Novel hydrophobic ionic liquid 1-methyl-N-(3-aminopropyl)-imidazole sodium hexafluorophosphate (APMIHPF6) and a preparing method thereof are introduced, and based on the combination of the novel hydrophobic ionic liquid and lead halide, a perovskite structure is formed and spin-coats dense titanium dioxide transparent thin film as a photo-anode to construct a plane heterostructure perovskite solar cell; based on a photovoltaic effect, sunlight is simulated through a xenon lamp is used as an excitation light source to achieve work and performance detection of the perovskite solar cell. Through the combination of novel hydrophobic ionic liquid 1-methyl-N-(3-aminopropyl)-imidazole sodium hexafluorophosphate (APMIHPF6) and lead halide, the perovskite structure is introduced into a perovskite material system and the prepared perovskite cell has the advantages of being convenient, simple, economical, high in stability and photoelectric conversion efficiency and the like.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
Light-cured three dimensional printing material and preparation method thereof
The invention discloses a light-cured three dimensional printing material and a preparation method thereof. The light-cured three dimensional printing material comprises, by mass, 40-45 parts of polyester modified epoxy acrylate, 8-12 parts of cycloaliphatic epoxy resin, 8-10 parts of trihydroxymethylpropyl trimethylacrylate, 16-20 parts of 1,6-hexanediol diacrylate, 2-8 parts of triethyleneglycol divinyl ether, 1.5-2 parts of isopropylthioxanthone, 1.5-2 parts of 2-phenyl-2,2-dimethylamino-1-(4-morpholinylphenyl)-1-butanone, 1.5-2 parts of a reactive tertiary amine co-initiator, 0.5-2.7 parts of 4-isobutylphenyl-4'-methylphenyliodonium hexafluorophosphate, 0.5-2 parts of an anthraquinone derivative, 0.5-2 parts of a promoter and 4-8 parts of a flexibilizer. Shaped parts made by using the light-cured three dimensional printing material have the advantages of low shrinkage, high toughness and high shaping precision.
Owner:NANJING UNIV OF AERONAUTICS & ASTRONAUTICS +2
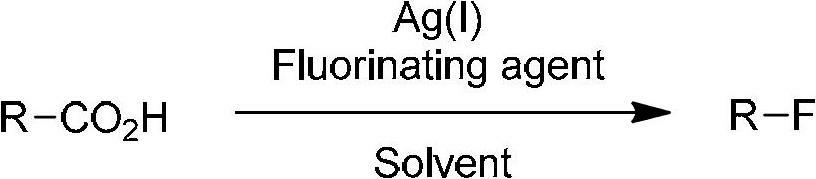
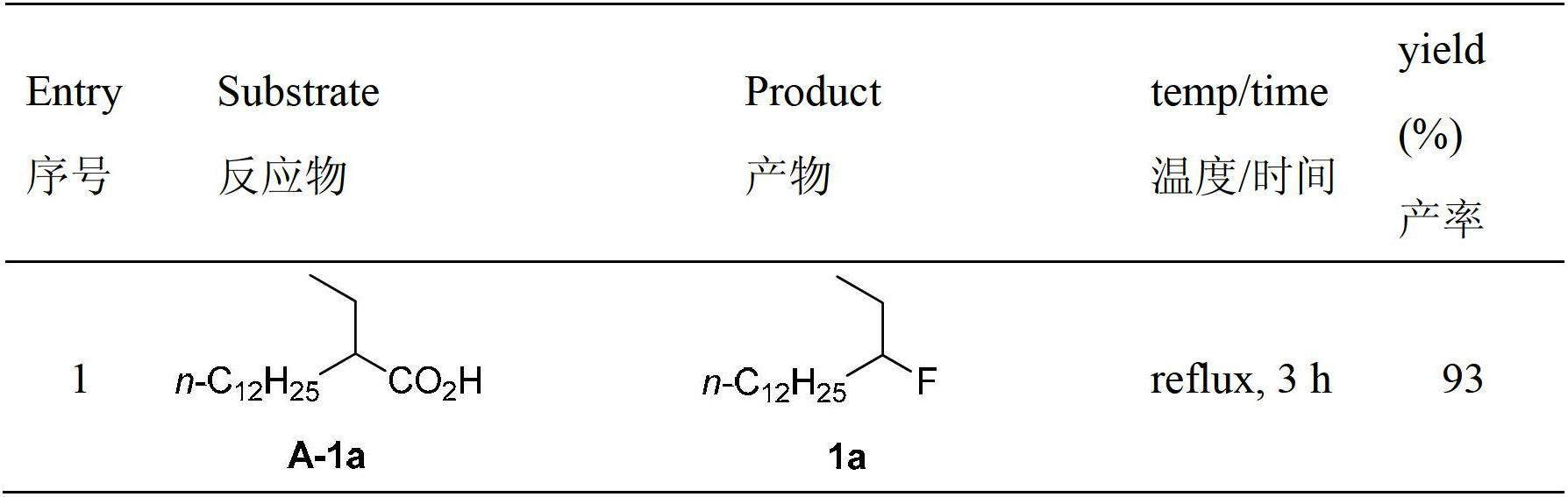
Decarboxylation and fluorination method for carboxylic acid
ActiveCN102675015AOrganic compound preparationCarboxylic acid esters preparationCarbon–fluorine bondRoom temperature
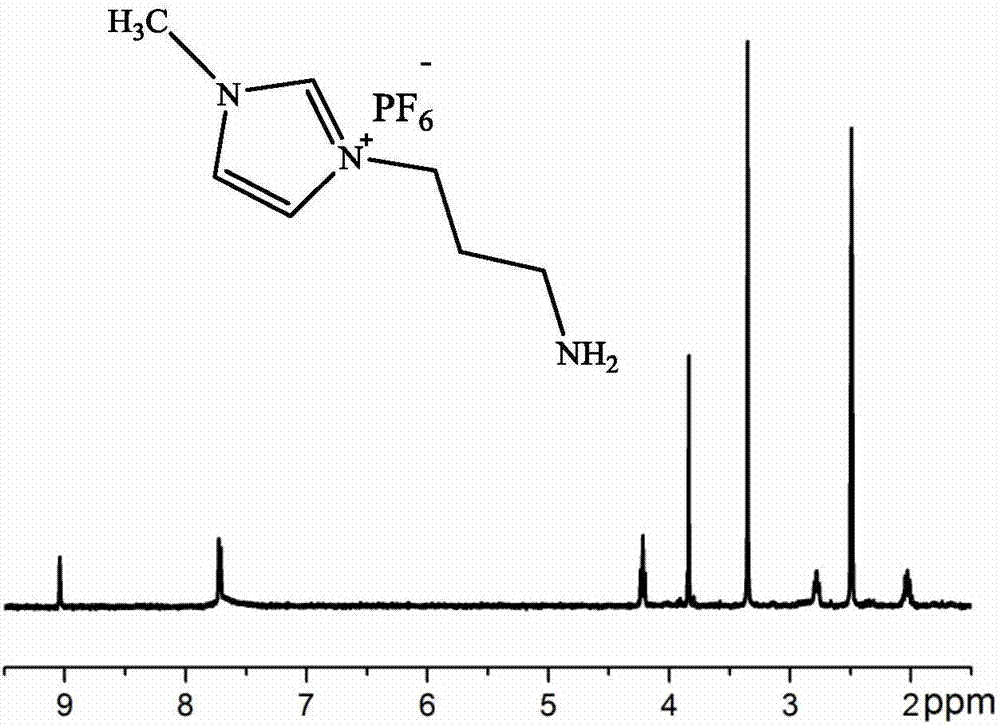
The invention relates to a decarboxylation and fluorination method for carboxylic acid. According to the method, fatty acid RCOOH, monovalent silver salt catalyst and fluorinated reagent Selectfluor or hexafluorophosphate derivative thereof are reacted in a solvent at the temperature of between room temperature and 80 DEG C to obtain RF; and by adopting the method, the carboxylic acid is efficiently converted into corresponding decarboxylated and fluorinated product in an aqueous phase. The method is mild in reaction conditions and easy to operate, has good chemical selectivity and functional group compatibility, and is a very practical sp<3> carbon-fluorine bond synthesizing method.
Owner:上海睿瓦科技有限公司
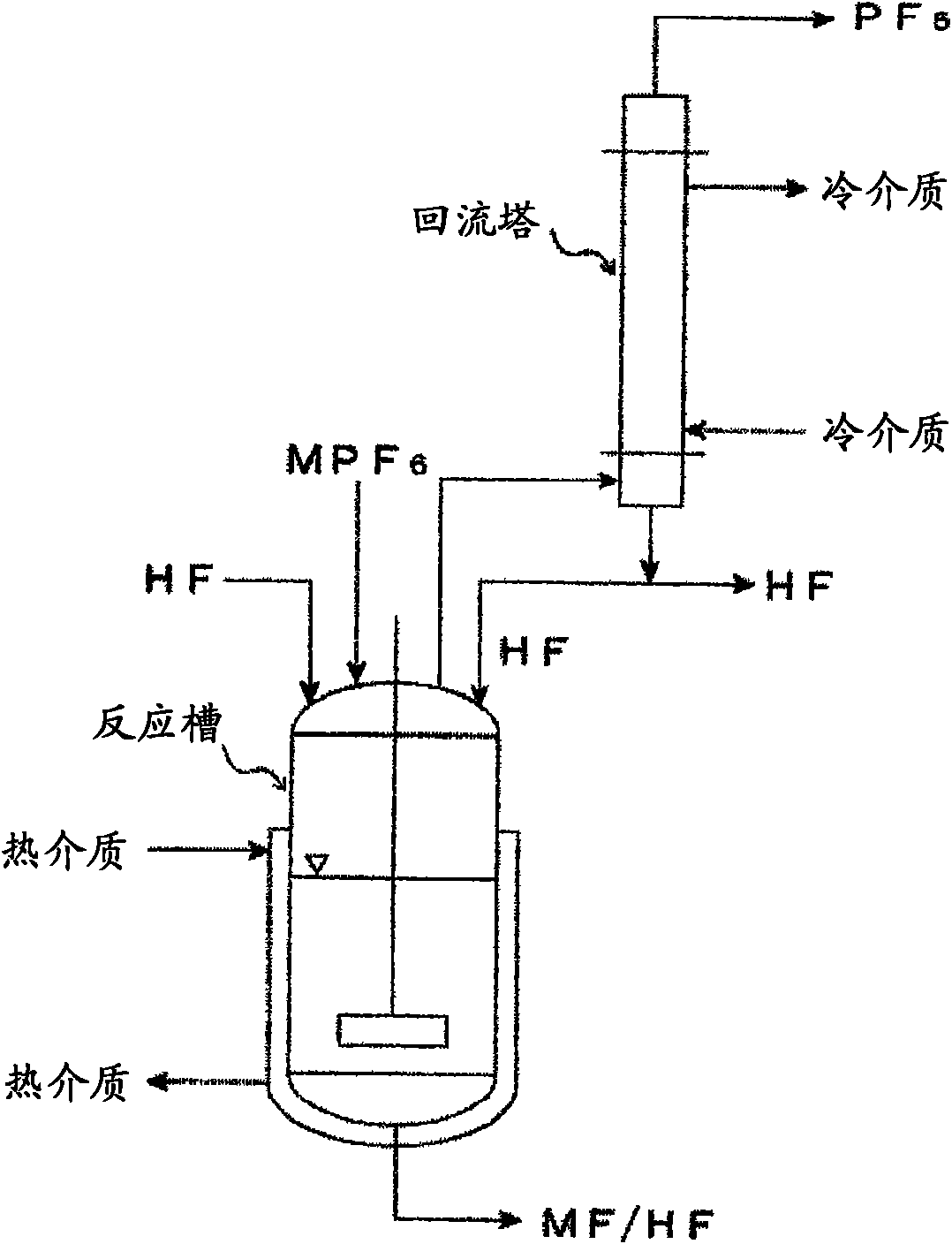
Processes for producing phosphorus pentafluoride and hexafluorophosphate
InactiveCN101605721AEasy to operateReduce synthesisPhosphorus halides/oxyhalidesDispersed particle separationPhosphatePhysical chemistry
Owner:STELLA CHEMIFA CORP
Mixed-type ionic liquid electrolyte as well as preparation method and application thereof
InactiveCN102074366AImprove hydrophobicityIncrease capacityLiquid electrolytic capacitorsCapacitor electrodesSolid state electrolyteTetrafluoroborate
The invention discloses a mixed-type ionic liquid electrolyte as well as a preparation method and application thereof. The mixed-type ionic liquid electrolyte is prepared by mixing hydrophobic ionic liquid 1-methyl-3-butylimidazolium hexafluorophosphate [BmimPF6] with solid organic electrolyte salt spirocyclic quaternary ammonium tetrafluoroborate (C8H16NBF4) according to the mol ratio of 9:1, 8:2, 7:3, 6:4 or 5:5 and has the advantages of broad electrochemical window, higher electrical conductivity, smaller internal resistance, high cycle efficiency and the like, the hydrophobic performance is excellent, and the performance is outstanding particularly when the proportion is 6:4. The electrolyte disclosed by the invention overcomes the defects of traditional organic electrolytes and other solid electrolytes in application from composition and is an electrolyte which has great development potential and is applicable to super capacitors.
Owner:苏州方光电装备技术 +1
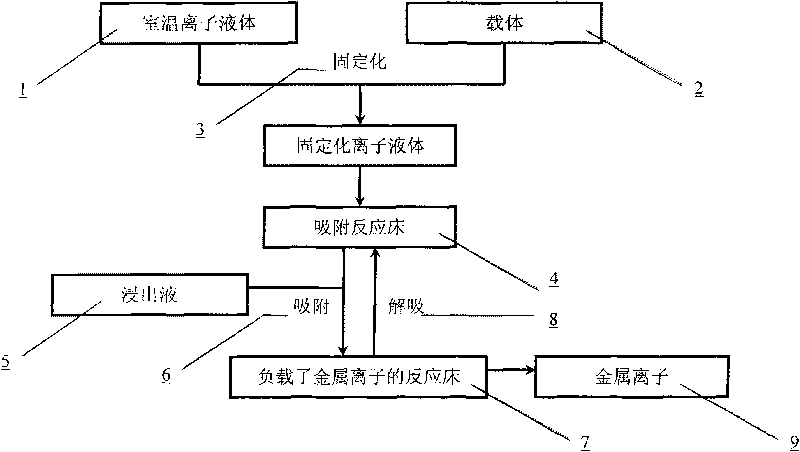
Process for absorbing and extracting valuable metal ions in leachate of laterite nickel ore by applying immobilized room temperature ionic liquid
InactiveCN101736157AEasy to operateImprove extraction efficiencyProcess efficiency improvementTetrafluoroborateLaterite
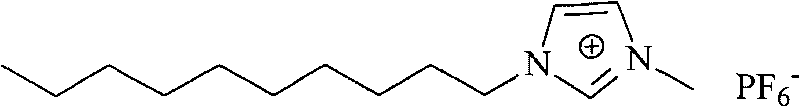
The invention discloses a process for absorbing and extracting valuable metal ions in leachate of laterite nickel ore by applying immobilized room temperature ionic liquid, which comprises the following steps: 1) performing immobilization on the room temperature ionic liquid, namely 1-decyl-3-methylimidazole hexafluorophosphate or 1-decyl-3-methylimidazole tetrafluoroborate, immobilizing the room temperature ionic liquid to a porous solid absorption carrier to obtain an absorption reaction bed of the room temperature ionic liquid; 2) allowing a complex component, namely acid leachate obtained by leaching the laterite nickel ore to pass through the absorption reaction bed for performing metal ion absorption at room temperature to extract the valuable metal ions in the leachate; and 3) achieving the purpose of performing elution and extraction on the metal ions by adjusting the acidity and flow rate of eluent. Through the process, an effective extraction process for the acid leachate of the laterite nickel ore can be established; the process fully uses low-grade mineral resources, improves the comprehensive utilization rate of the mineral resources, reduces environmental pollution, improves economic benefit, and can be applied to the development of the conventional low-grade non-ferrous metal mines in China.
Owner:有研资源环境技术研究院(北京)有限公司
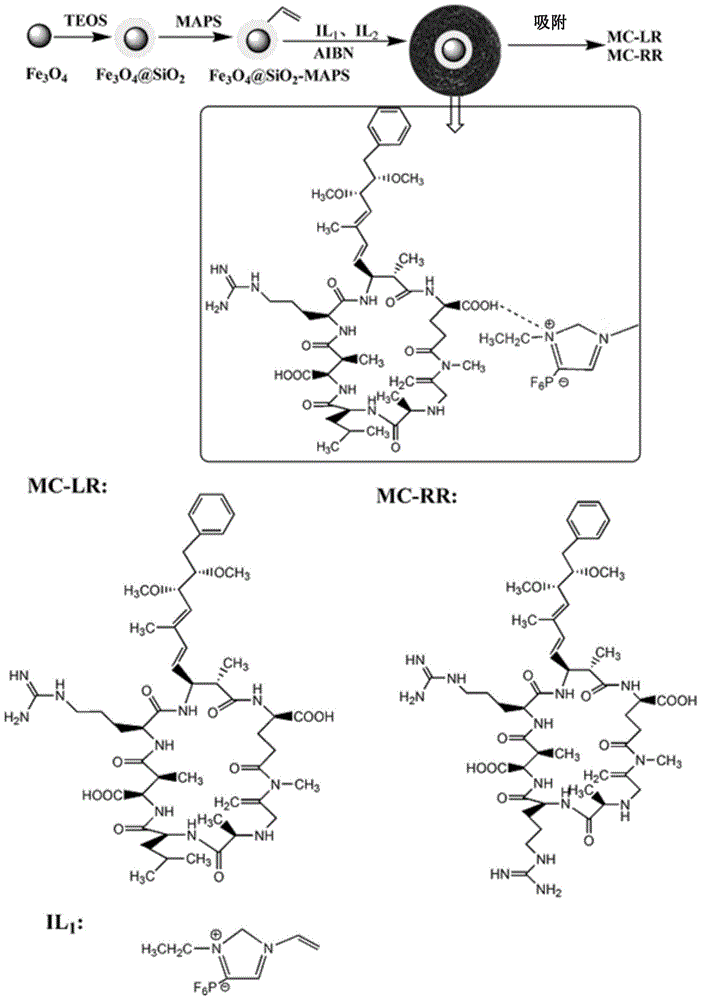
Magnetic polymerization ionic liquid for detecting microcystic toxins and preparation method and application of magnetic polymerization ionic liquid
ActiveCN104892869AImprove adsorption capacityHighly selective adsorptionOther chemical processesComponent separationCross-linkFunctional monomer
The invention discloses magnetic polymerization ionic liquid for detecting microcystic toxins and a preparation method and an application of the magnetic polymerization ionic liquid. The magnetic polymerization ionic liquid is a granular particle which is formed by a carrier, functional monomer and cross-linking agents in a polymerization manner under the effect of initiating agents; the functional monomer is 1-vinyl-3-ethylimidazole hexafluorophosphate; and the cross-linking agents are 1, 4-butane-3, 3'-bis-1- ethylimidazole hexafluorophosphate. The magnetic polymerization ionic liquid which is prepared in the polymerization manner by using the 1-vinyl-3-ethylimidazole hexafluorophosphate as the functional monomer and using the 1, 4-butane-3, 3'-bis-1- ethylimidazole hexafluorophosphate as the cross-linking agents can quickly reach an adsorption equilibrium state on MC-RR and MC-LR, and has high adsorption capacity and high selective adsorption capability, and the maximum adsorbing capacities of the MC-RR and the MC-LR are 10.32 micro mol / g and 10.88 micro mol / g respectively.
Owner:JIAXING UNIV
Method of ion liqid extraction for separating penicillin
InactiveCN1563008ALow priceOvercome problems such as emulsificationOrganic chemistrySingle stagePenicillin
This invention relates to method for extracting penicillin by ion-liquid extract. In this invention, determinations are: extraction procedures, pH value, phase ratio, extration rate affected by penicillin concentration. Extraction types are; single stage extraction, cross current extraction and counter current extraction. Said extract ion-liquid is [BMIM]PF6(1-ethyl-3-methyl imidazole hexafluorophosphate. This invention determines back-extraction method.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
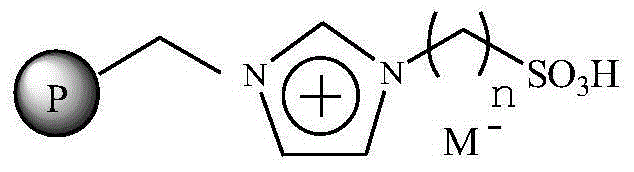
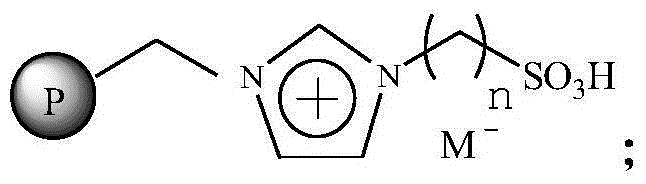
Immobilized ionic liquid catalyst and application thereof
InactiveCN106391112AHigh catalytic activityNot easy to inactivateOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsTetrafluoroborateTriflic acid
The invention relates to an immobilized ionic liquid catalyst and application thereof. The immobilized ionic liquid catalyst has a general structural formula as defined in the specification. In the general structural formula, P is a nanogel resin matrix; n is an integer in a range of 2 to 12; and M<-> is a negative ion selected from a group consisting of a trifluoromethanesulfonate group, a p-toluenesulfonate group, a benzenesulfonate group, a methanesulfonate group, a tetrafluoroborate group and a hexafluorophosphate group. The immobilized ionic liquid catalyst can be applied to industrial olefine acid addition for preparation of corresponding esters.
Owner:CHINA PETROLEUM & CHEM CORP +1
Processes for production of phosphorus pentafluoride and hexafluorophosphates
ActiveUS20110189538A1InexpensiveLow moisture concentrationPhosphorus halides/oxyhalidesLithium hexafluorophosphateChemical reactionPhysical chemistry
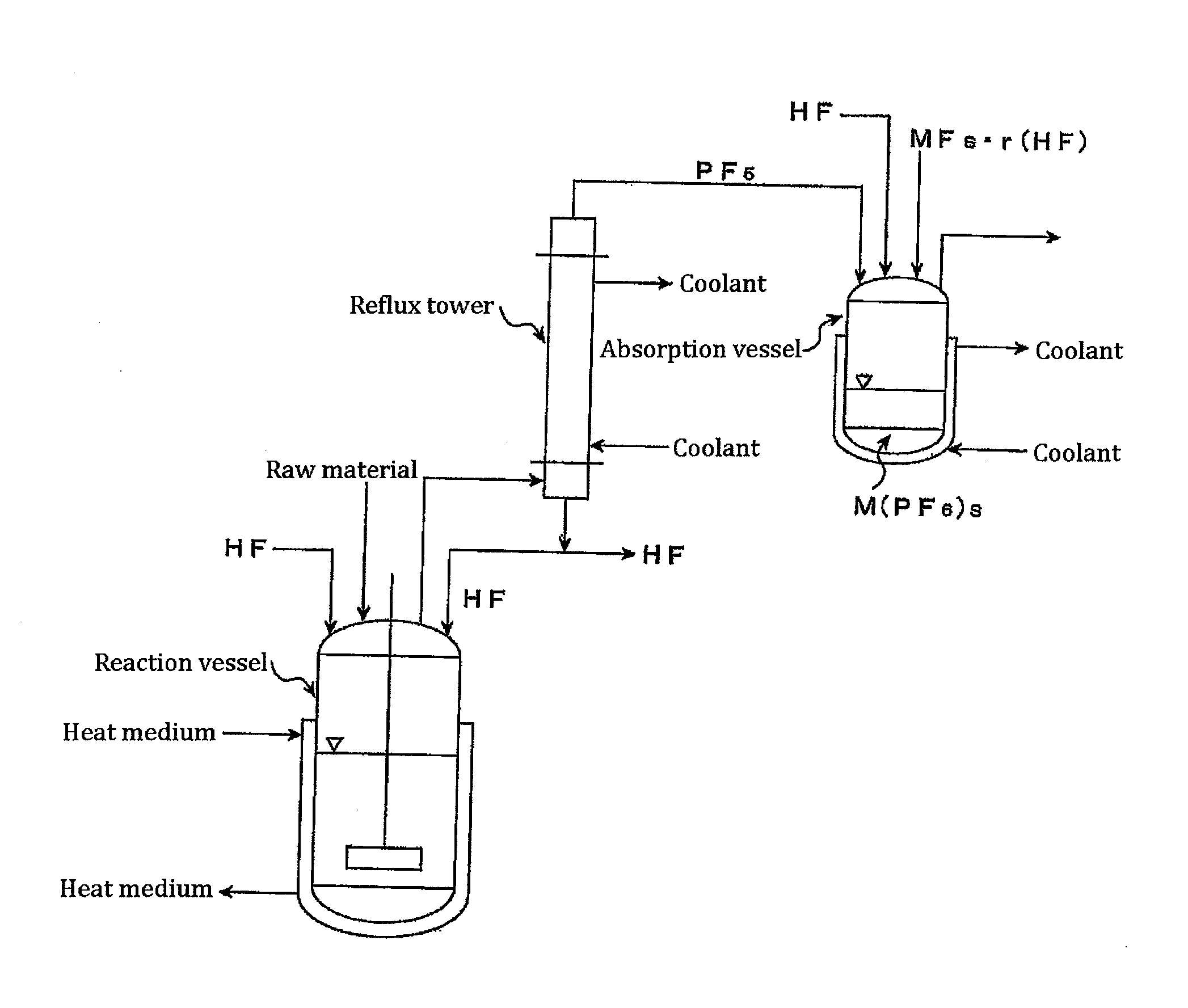
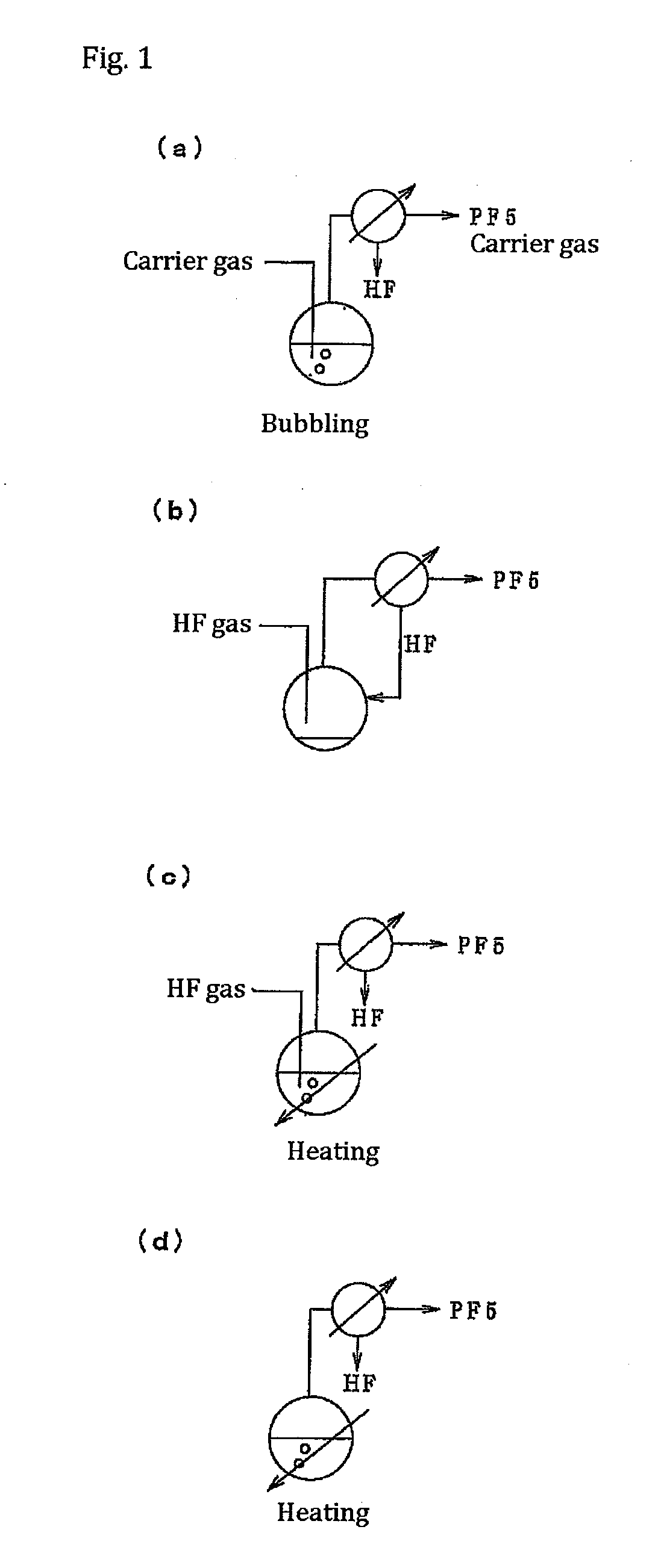
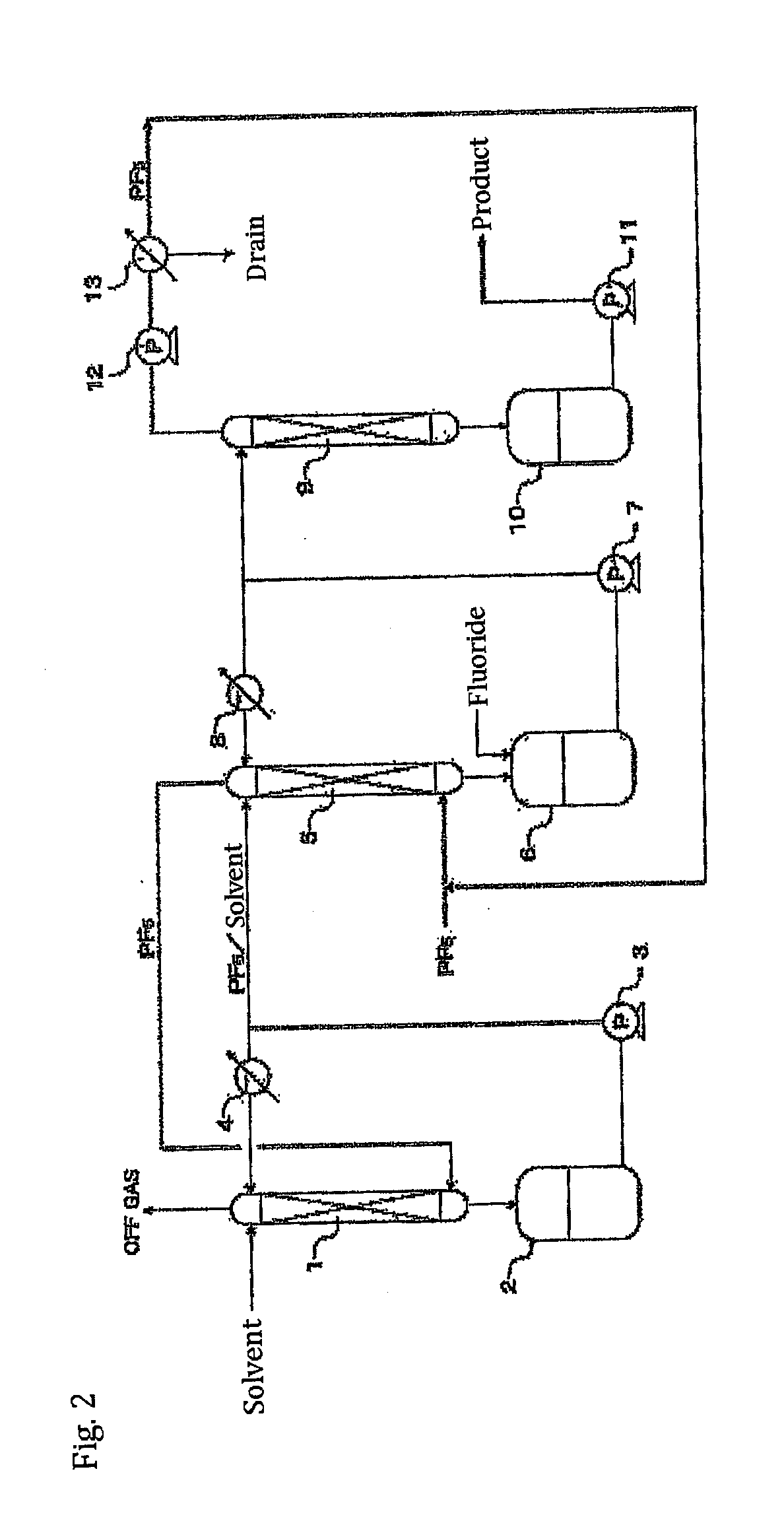
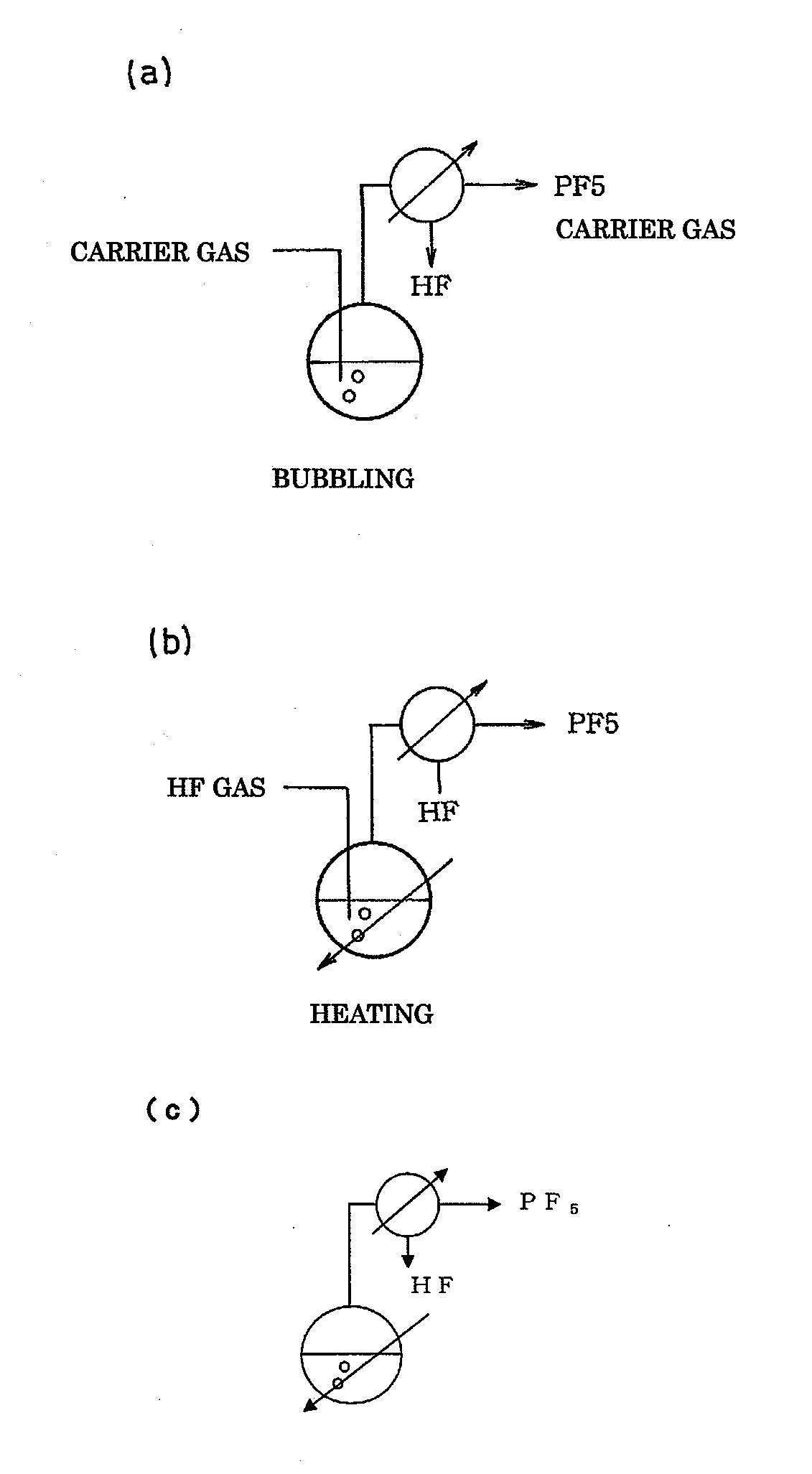
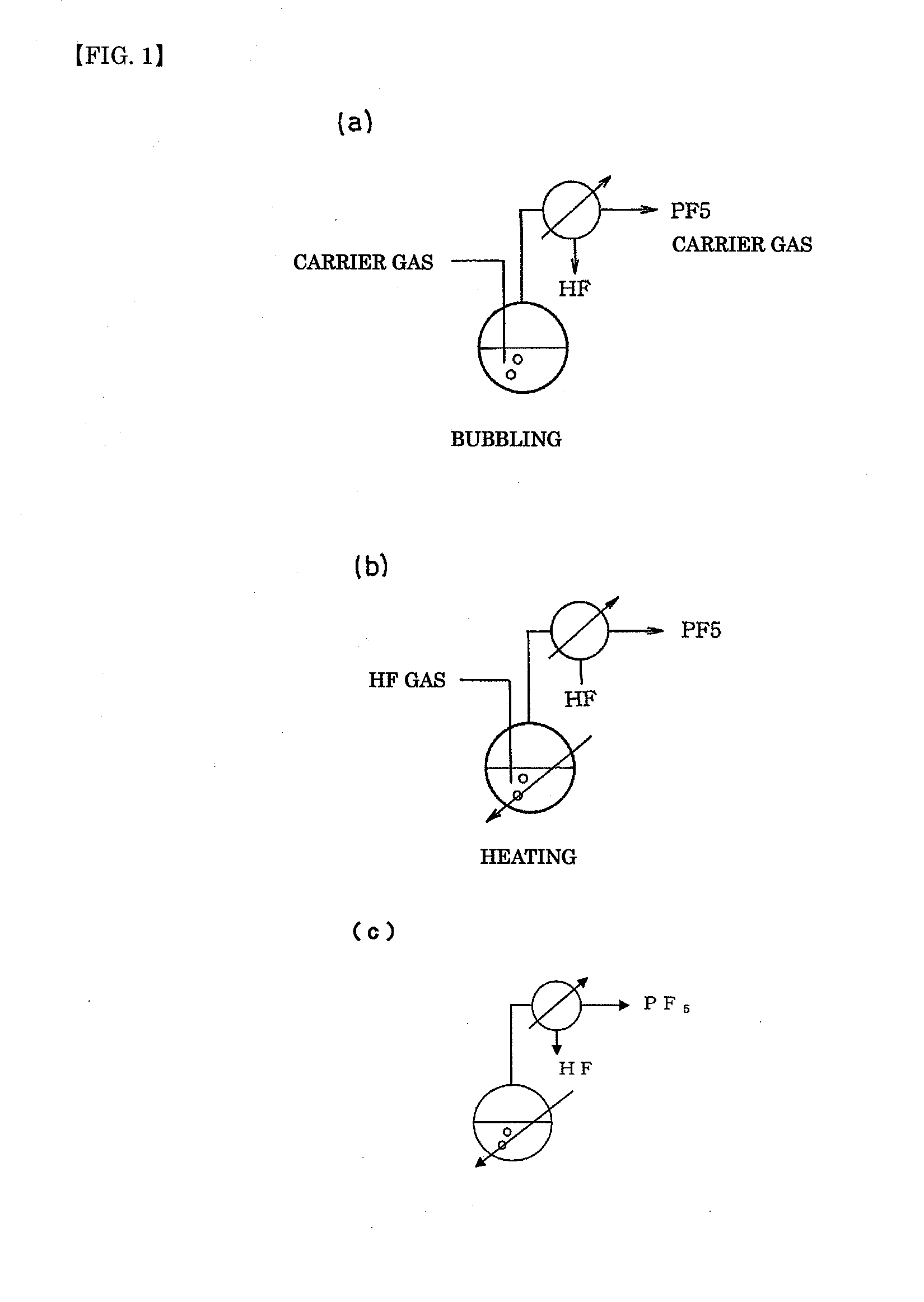
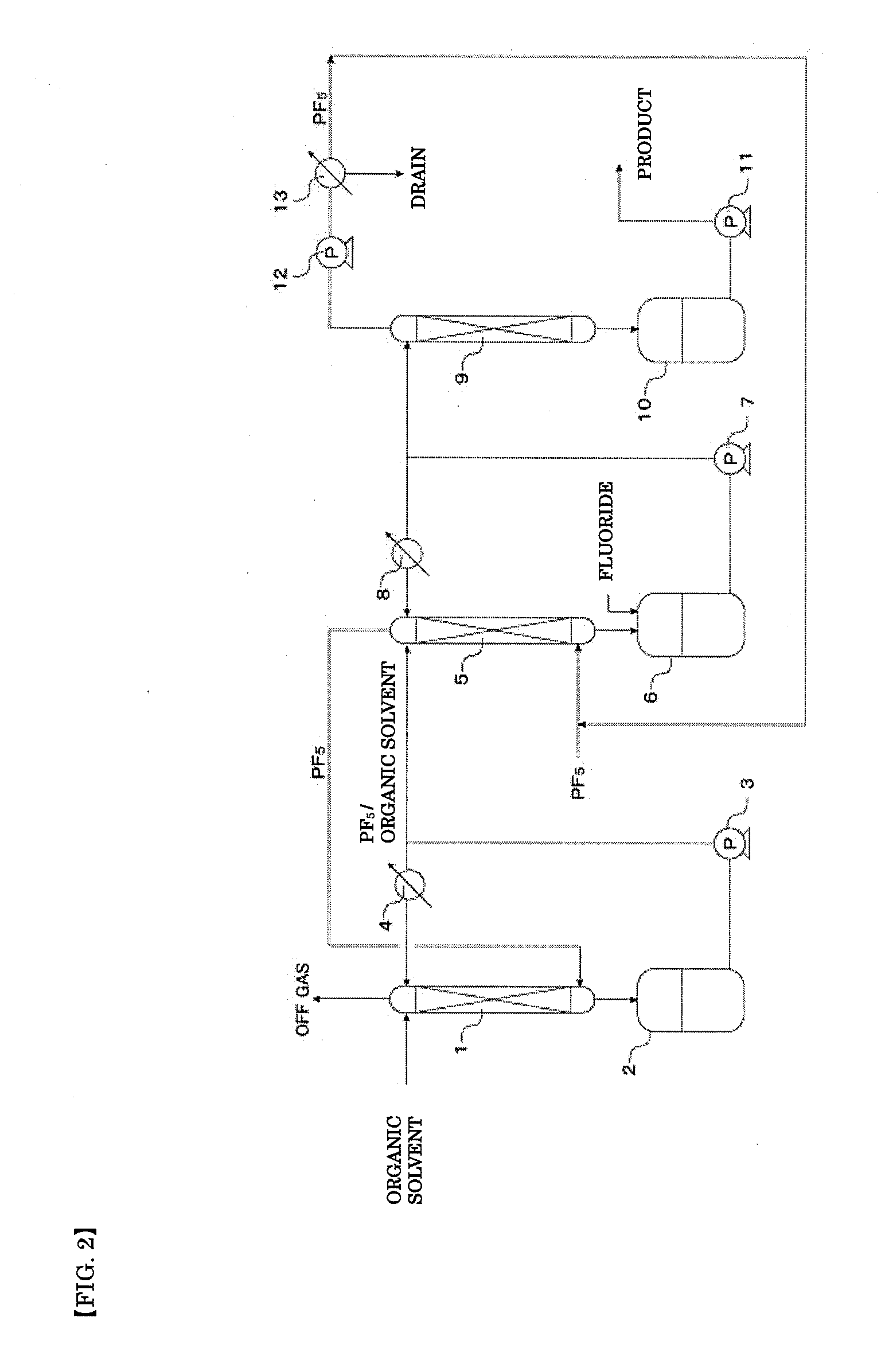
A method of manufacturing phosphorus pentafluoride and hexafluorophosphate can suppress the manufacturing cost and also can manufacture high-quality phosphorus pentafluoride from an inexpensive and low-quality raw material. The raw material for the method can include at least a phosphorus atom and a fluorine atom. These are brought into contact with a carrier gas, and a phosphorus pentafluoride is extracted and separated into the carrier gas. A method of manufacturing hexafluorophosphate includes reacting fluoride with the resulting phosphorus pentafluoride according to the following chemical reaction scheme: sPF5+AFs→A(PF6)s, in which s is in the range of 1≦s≦3, and A is at least one of the following: Li, Na, K, Rb, Cs, NH4, Ag, Mg, Ca, Ba, Zn, Cu, Pb, Al and Fe.
Owner:STELLA CHEMIFA CORP
Processes for producing phosphorus tetrafluoride and phosphate hexafluoride
InactiveUS20110286905A1High purityPhosphorus halides/oxyhalidesLarge-sized flat cells/batteriesPhosphatePhotochemistry
An object the invention is to provide a phosphorus pentafluoride producing process wherein phosphorus pentafluoride is separated / extracted from a pentavalent phosphorus compound or a solution thereof, or a composition obtained by allowing the pentavalent phosphorus compound or the solution thereof to react with hydrogen fluoride, thereby producing phosphorus pentafluoride; and a phosphate hexafluoride producing process wherein the resultant phosphorus pentafluoride is used as raw material to produce a phosphate hexafluoride high in purity. The present invention relates to a process for producing phosphorus pentafluoride, wherein a carrier gas is brought into contact with either of the following one: a pentavalent phosphorus compound, a solution thereof, or a solution in which a composition obtained by allowing the pentavalent phosphorus compound or the solution thereof to react with hydrogen fluoride is dissolved, thereby a phosphorus pentafluoride is extracted into the career gas.
Owner:STELLA CHEMIFA CORP
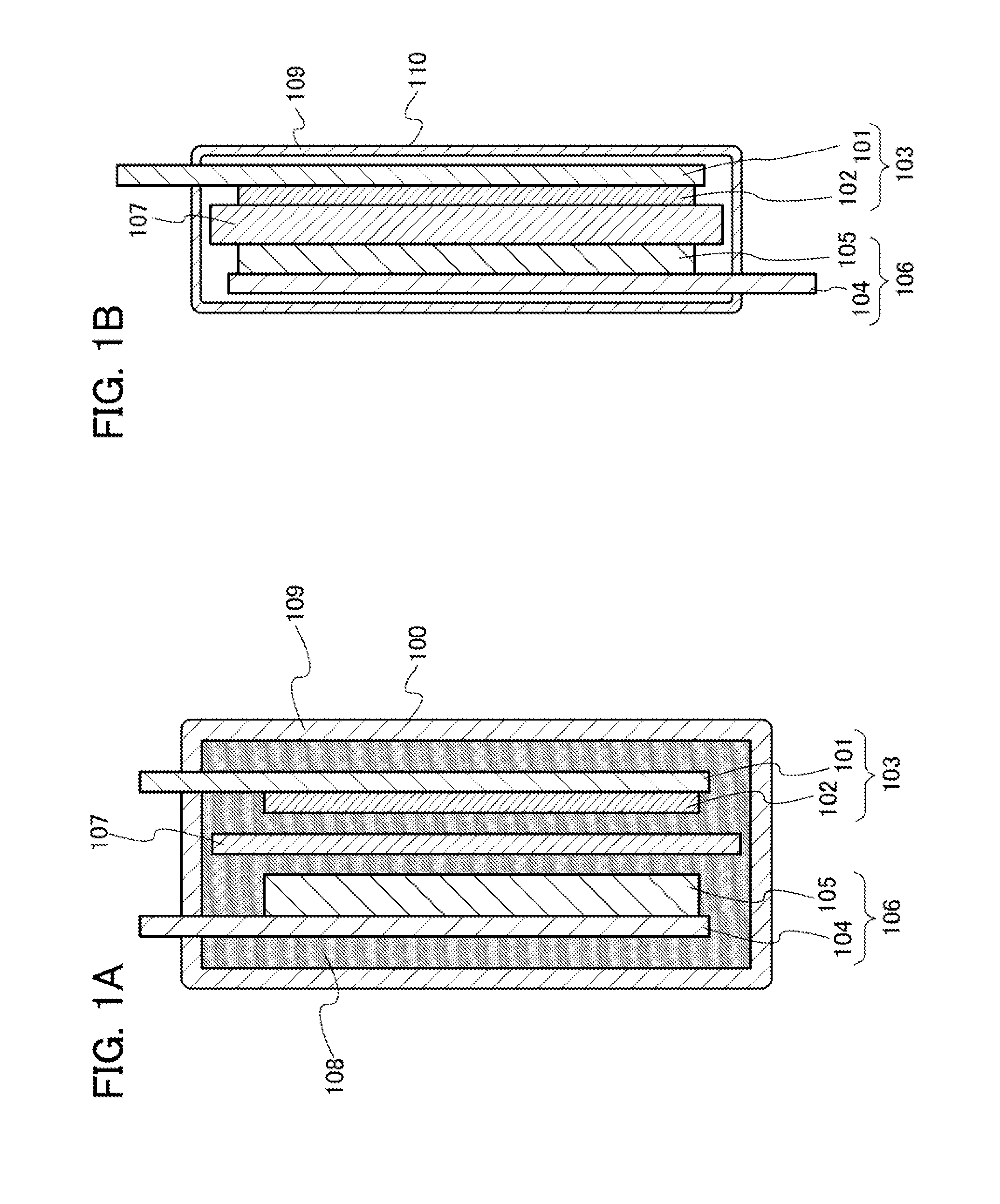
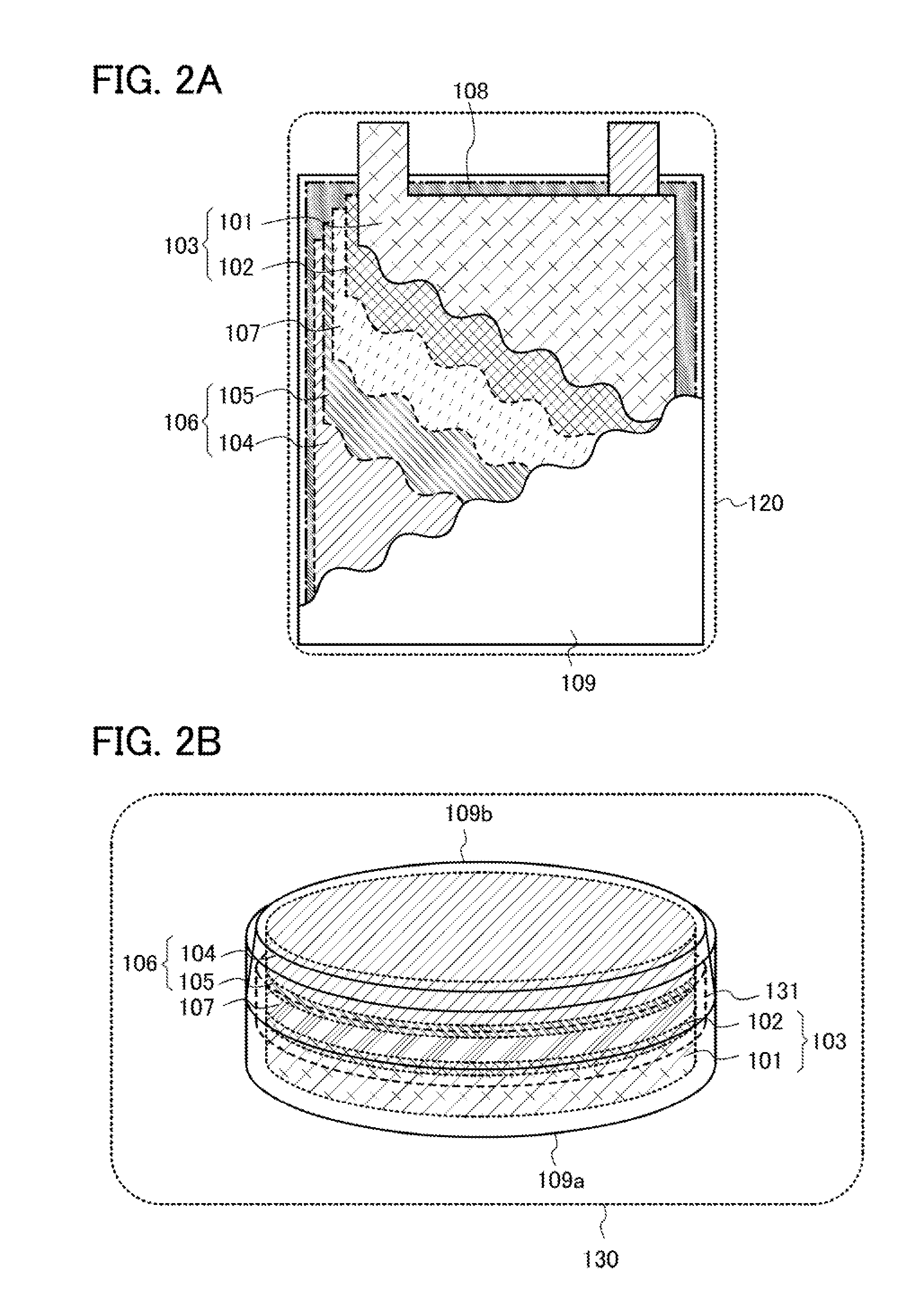
Power storage device, lithium-ion secondary battery, electric double layer capacitor and lithium-ion capacitor
ActiveUS8795544B2Excellent in reduction resistanceHigh-performance powerHybrid capacitor electrolytesOrganic chemistryTetrafluoroborateQuaternary ammonium cation
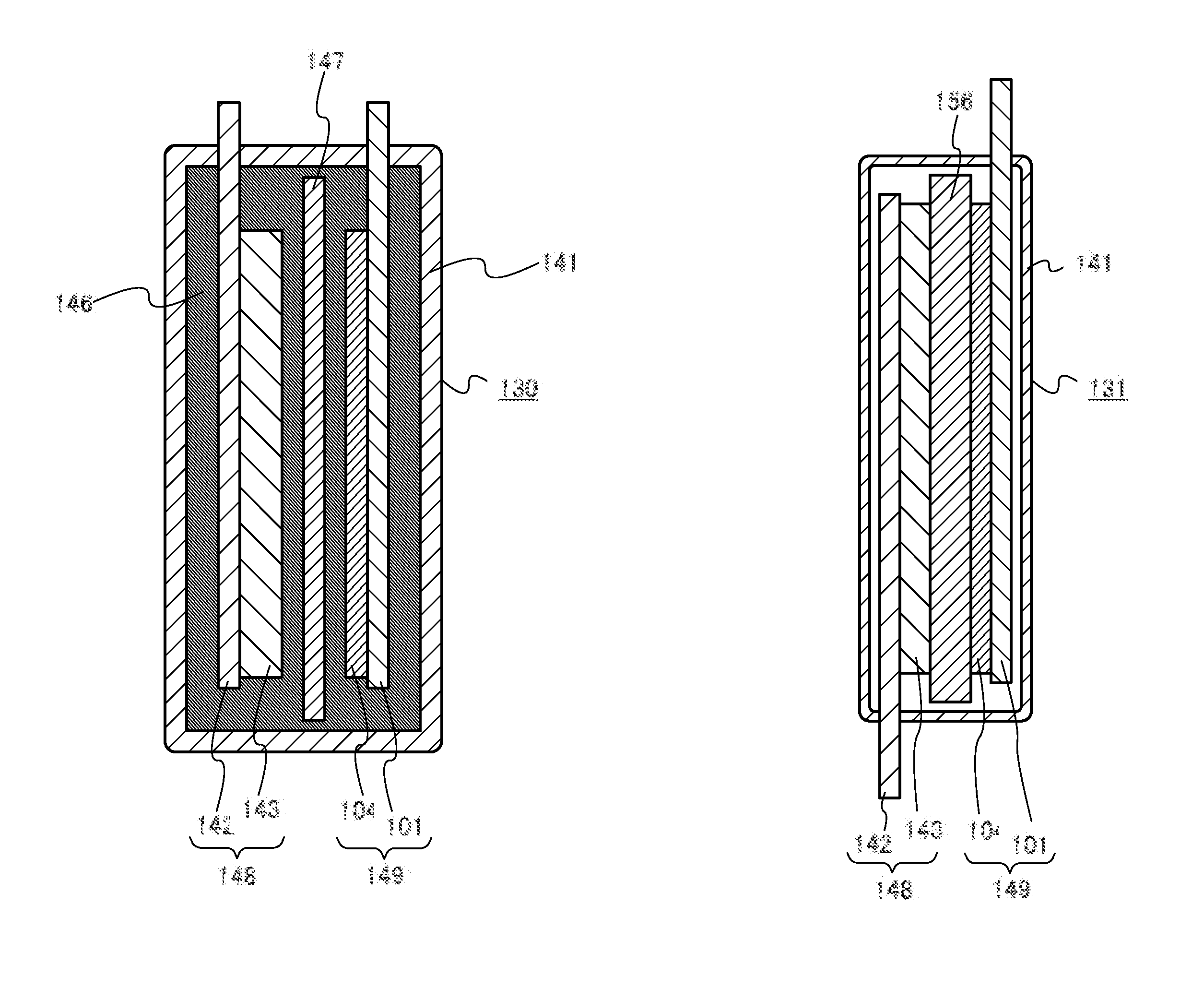
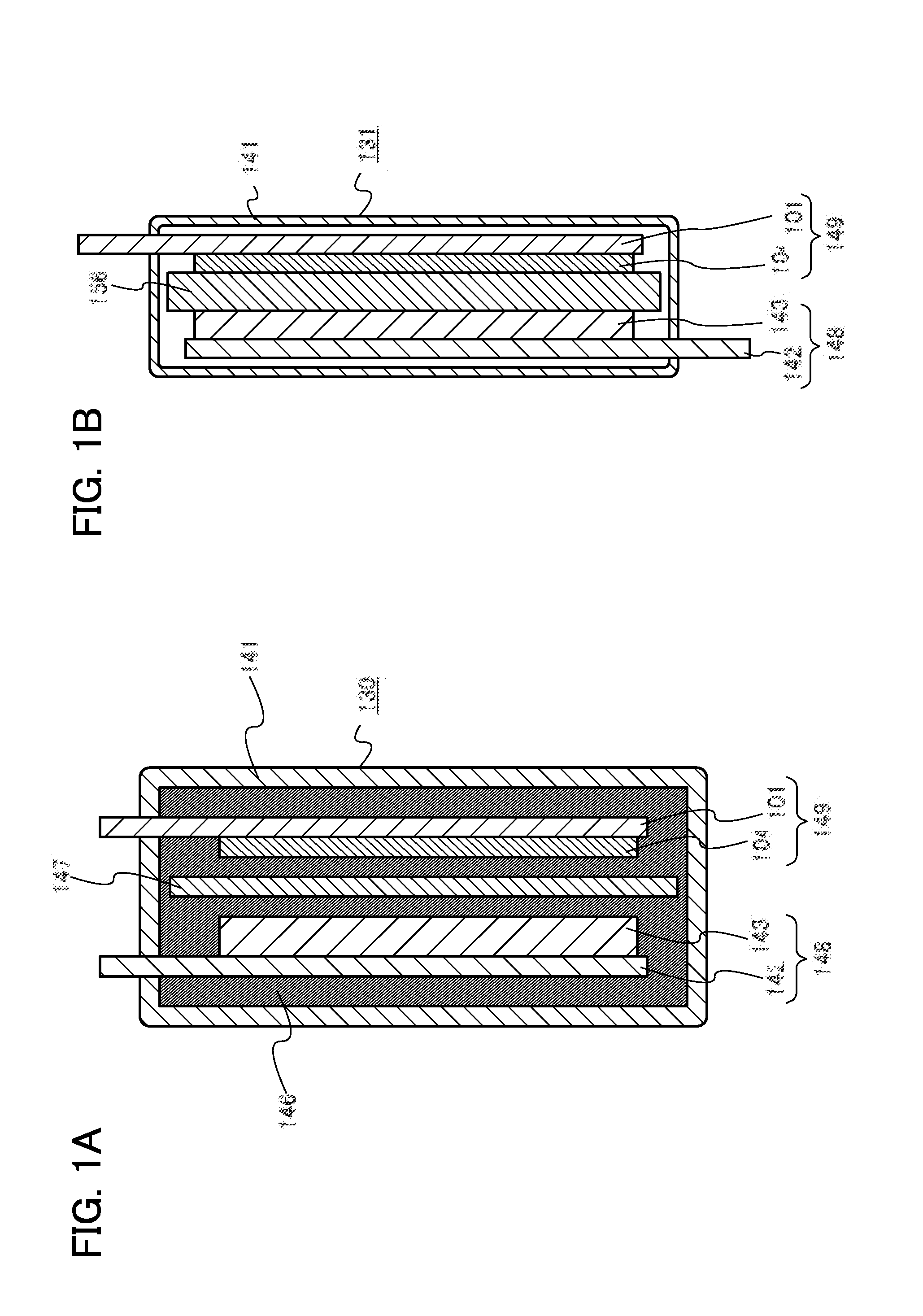
One object is to provide a power storage device including an electrolyte using a room-temperature ionic liquid which includes a univalent anion and a cyclic quaternary ammonium cation having excellent reduction resistance. Another object is to provide a high-performance power storage device. A room-temperature ionic liquid which includes a cyclic quaternary ammonium cation represented by a general formula (G1) below is used for an electrolyte of a power storage device. In the general formula (G1), one or two of R1 to R5 are any of an alkyl group having 1 to 20 carbon atoms, a methoxy group, a methoxymethyl group, and a methoxyethyl group. The other three or four of R1 to R5 are hydrogen atoms. A− is a univalent imide anion, a univalent methide anion, a perfluoroalkyl sulfonic acid anion, tetrafluoroborate (BF4−), or hexafluorophosphate (PF6−).
Owner:SEMICON ENERGY LAB CO LTD
Method for preparing nano materials by direct electrodeposit in ionic liquid microemulsion
The invention relates to a method for preparing nano materials by direct electrodeposit in ionic liquid microemulsion, a new technology for preparing nano materials, which combines an electrochemical method and a microemulsion method. Under the effect of surface active agent polyoxyethylene octyl phenyl ether, 1-butyl-3-methylimidazole hexaflourophosphate salt ionic liquid and aqueous solution form water drum ionic liquid microemulsion with relatively high electroconductibility; an electroconductive electrode and the water drum ionic liquid microemulsion form an electrode system. A nano metal plating featuring controllable size and even distribution is prepared in a 'nano pool' in ionic liquid microemulsion by a current control method or an electric potential control method; wherein, size of the nano particles can be controlled by the mol ratio between water and the surface active agent; the technology for preparing novel nano materials has the advantages of low equipment cost, easy operation and easy control.
Owner:HUNAN INSTITUTE OF SCIENCE AND TECHNOLOGY
Goserelin acetate solid-phase synthesis method
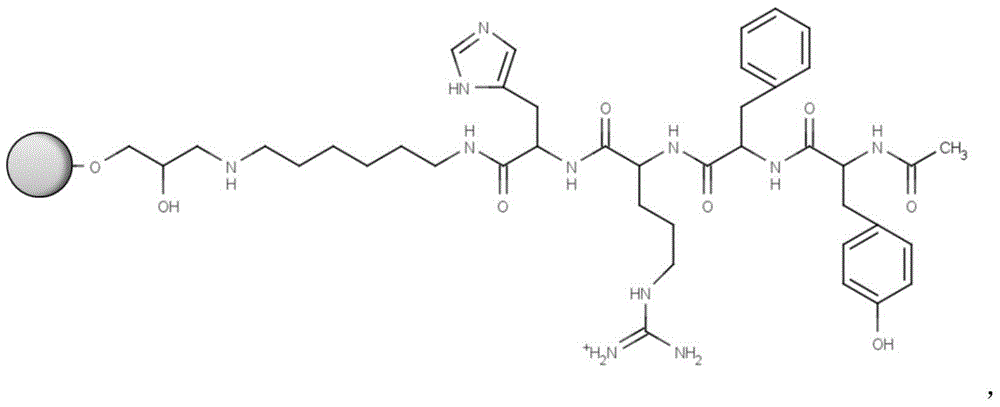
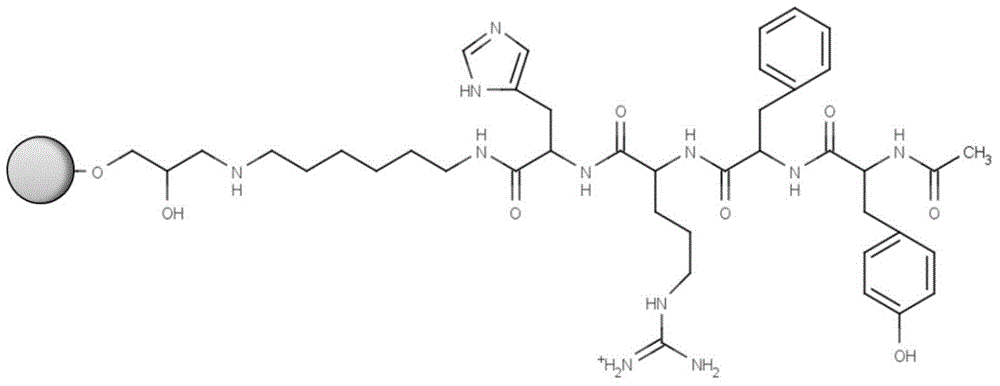
ActiveCN104910257ATo solve such a drawback that cannot be monitoredLow costLuteinising hormone-releasing hormonePeptide preparation methodsSide chainEthylic acid
The invention relates to a goserelin acetate solid-phase synthesis method, which comprises the following steps: HBTU / DIPEA is employed as a condensation system, Fmoc-Ser-OH, Fmoc-Trp-OH are successively coupled; a whole-protection lysate with corresponding voluminal amount according to 10 times of resin weight is added, a carrier 2-CTC Resin in an intermediate is removed, all side chain protective groups are reserved; the whole-protection lysate is adjusted to slight alkaline by using DIPEA(N,N-diisopropylethylaine), semicarbazide hydrochloride and PyBop(1H-benzotriazole-1-oxygen tripyrrole alkyl hexafluorophosphate) (used for a coupling agent of peptide) are added in the whole-protection lysate for reaction coupling, a goserelin peptide solution with the side chain protective group is obtained; the lysate with 20% of TFA / DCM is added in a freezing ether for settling to obtain the white solid crude peptide; the white solid crude peptide is dried under vacuum for solving by methyl alcohol, ammonium formate and Pa / c are added for a hydrogenation reaction to remove the side chain protective group in a peptide sequence. According to the invention, side reaction phenomena can be avoided, target peptide purity is increased, yield is high, operation is convenient and feasible, the intermediate can be tracked and controlled, and the whole process is benefit for enlarged production.
Owner:苏州天马医药集团天吉生物制药有限公司
Molding composition and method, and molded article
A curable method useful for encapsulating solid state devices includes (A) an epoxy resin; (B) an effective amount of a cure catalyst comprising (B1) a first latent cationic cure catalyst comprising a diaryl iodonium hexafluoroantimonate salt; (B2) a second latent cationic cure catalyst comprising (B2a) a diaryl iodonium cation, and (B2b) an anion selected from perchlorate, imidodisulfurylfluoride anion, unsubstituted and substituted (C1-C12)-hydrocarbylsulfonates, (C2-C12)-perfluoroalkanoates, tetrafluoroborate, unsubstituted and substituted tetra-(C1-C12)-hydrocarbylborates, hexafluorophosphate, hexafluoroarsenate, tris(trifluoromethylsulfonyl)methyl anion, bis(trifluoromethylsulfonyl)methyl anion, bis(trifluoromethylsulfuryl)imide anion, and combinations thereof; and (B3) a cure co-catalyst selected from free-radical generating aromatic compounds, peroxy compounds, copper (II) salts of aliphatic carboxylic acids, copper (II) salts of aromatic carboxylic acids, copper (II) acetylacetonate, and combinations thereof; and (C) about 70 to about 95 weight percent of an inorganic filler, based on the total weight of the curable composition. The composition's cure catalyst allows the use of increased filler loadings, which in turn reduces moisture absorption and thermal expansion of the cured composition.
Owner:SABIC INNOVATIVE PLASTICS IP BV
Electrolyte for non-aqueous electrolyte cell, and non-aqueous electrolyte cell wherein same is used
ActiveUS20180375158A1Lower internal resistanceSuppress amount of gasHybrid capacitor electrolytesHybrid capacitor electrodesImideSilane compounds
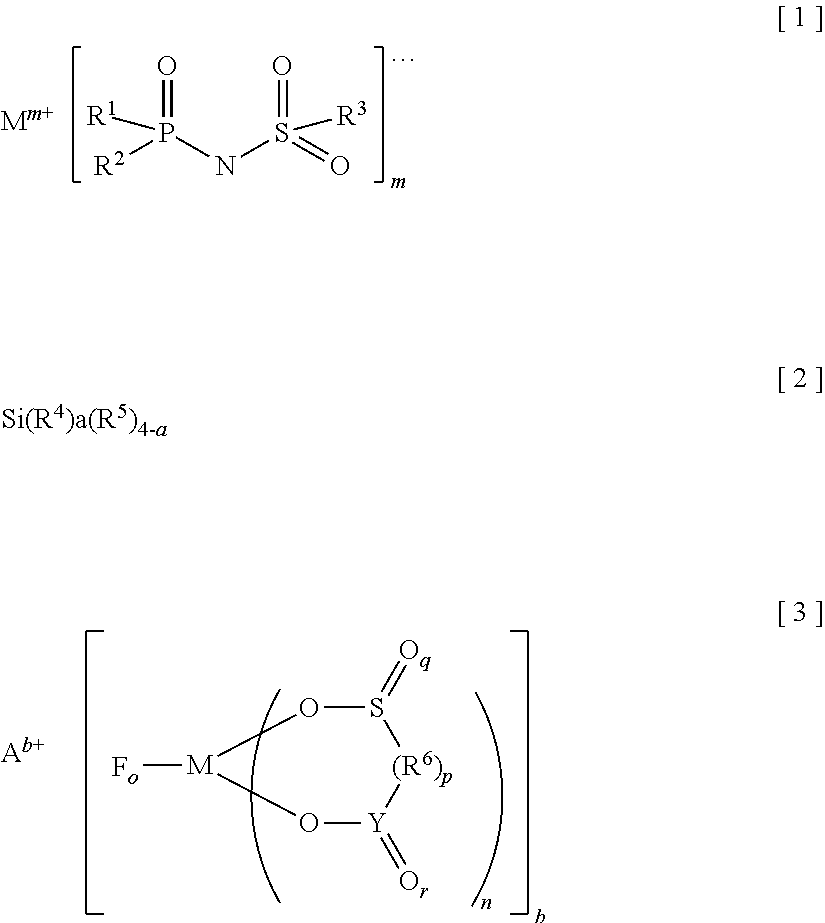
Provided is an electrolyte for a non-aqueous electrolyte battery, which can provide, when used in a non-aqueous electrolyte battery, in a good balance, an effect to suppress an increase in an internal resistance at a low temperature and an effect to suppress an increase in an amount of gas generated at a high temperature, as well as a non-aqueous electrolyte battery containing such an electrolyte. The non-aqueous electrolyte comprises a non-aqueous solvent and at least a hexafluorophosphate and / or tetrafluoroborate as a solute, and further comprises at least one imide anion-containing salt represented by the following general formula [1] but does not contain a silane compound represented by the following general formula [2] or an ionic complex represented by, for example, the following general formula [3].
Owner:CENT GLASS CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com