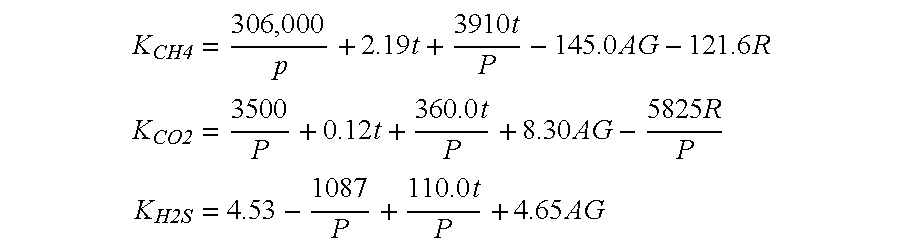Patents
Literature
1943 results about "Counter current" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
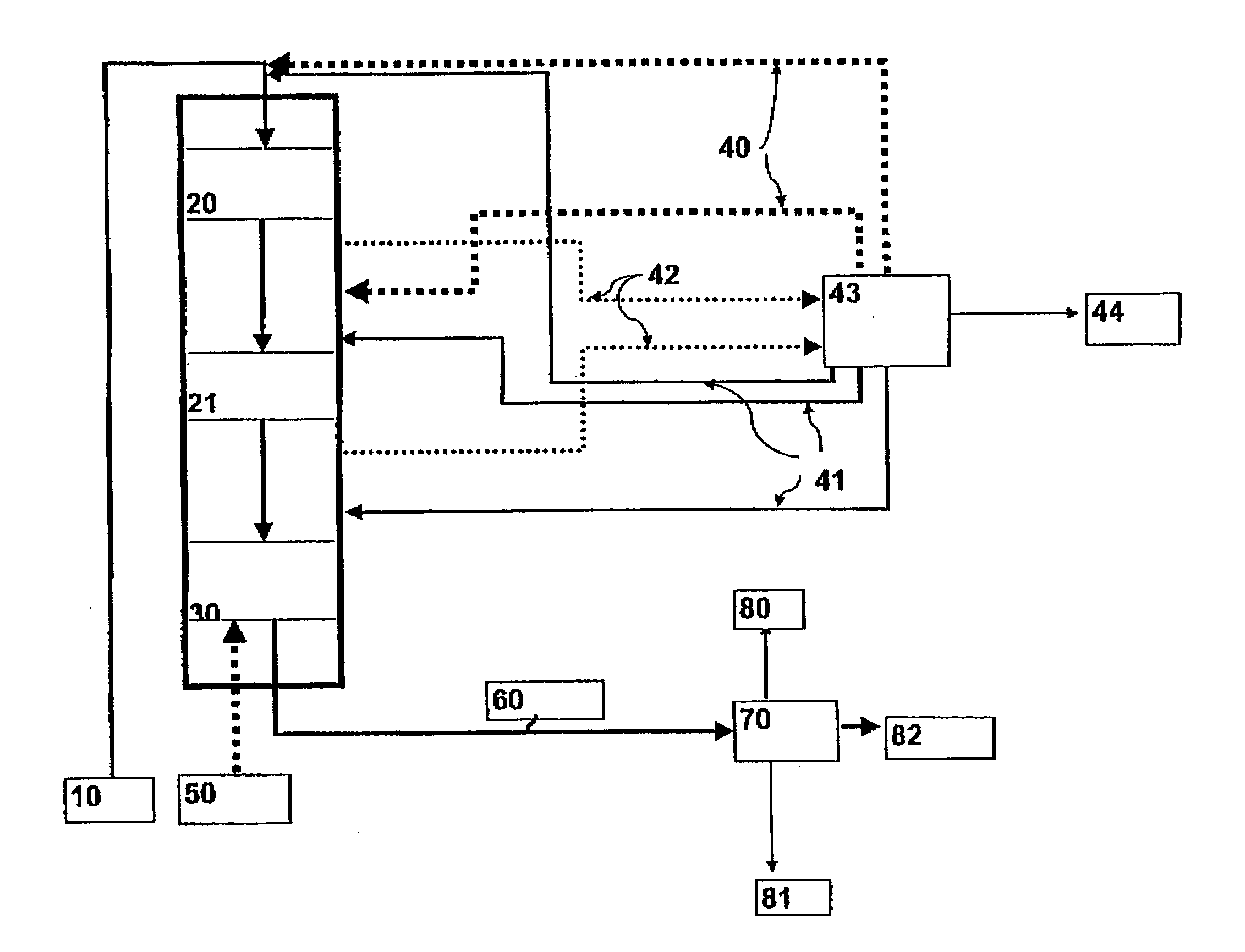
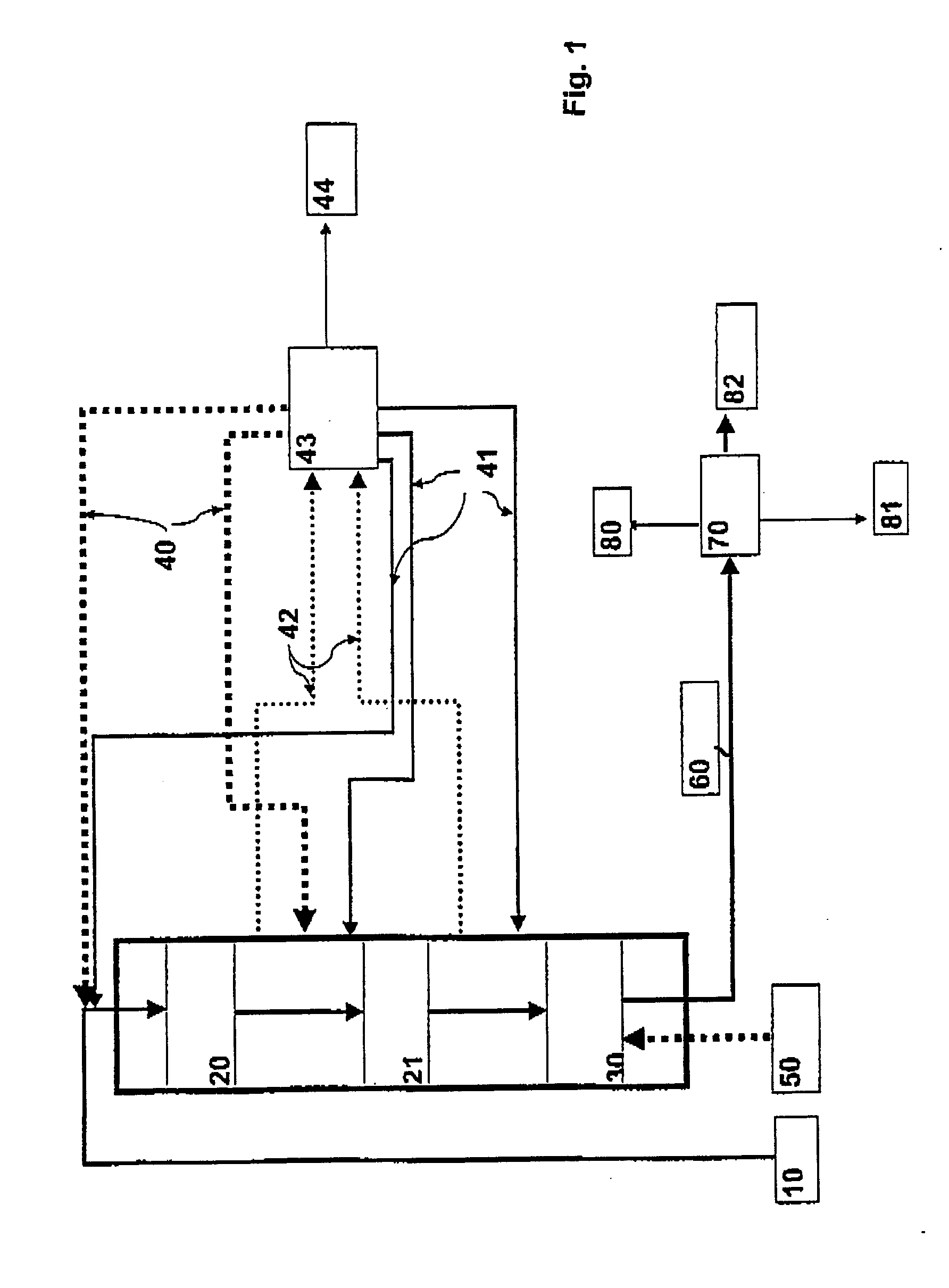
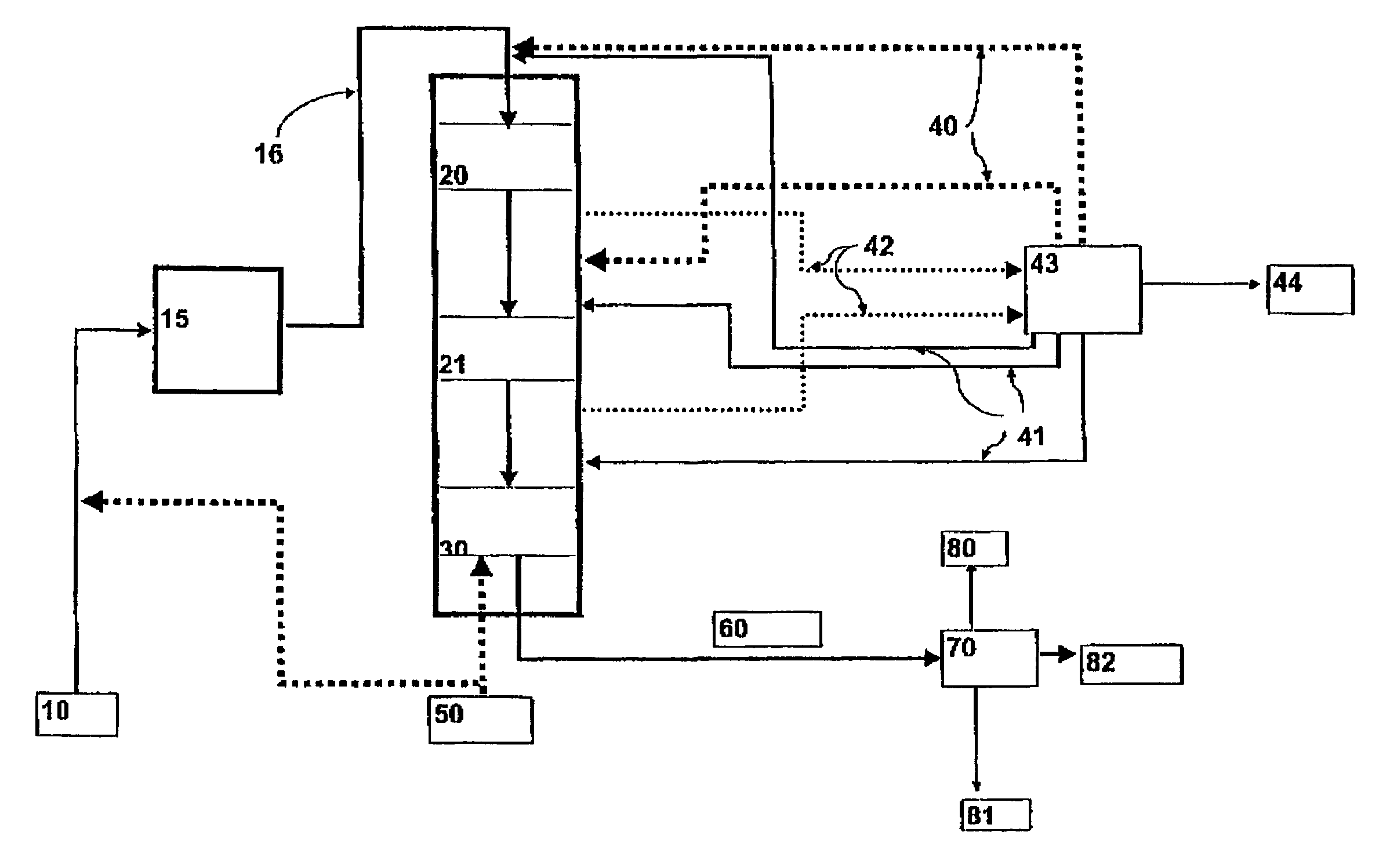
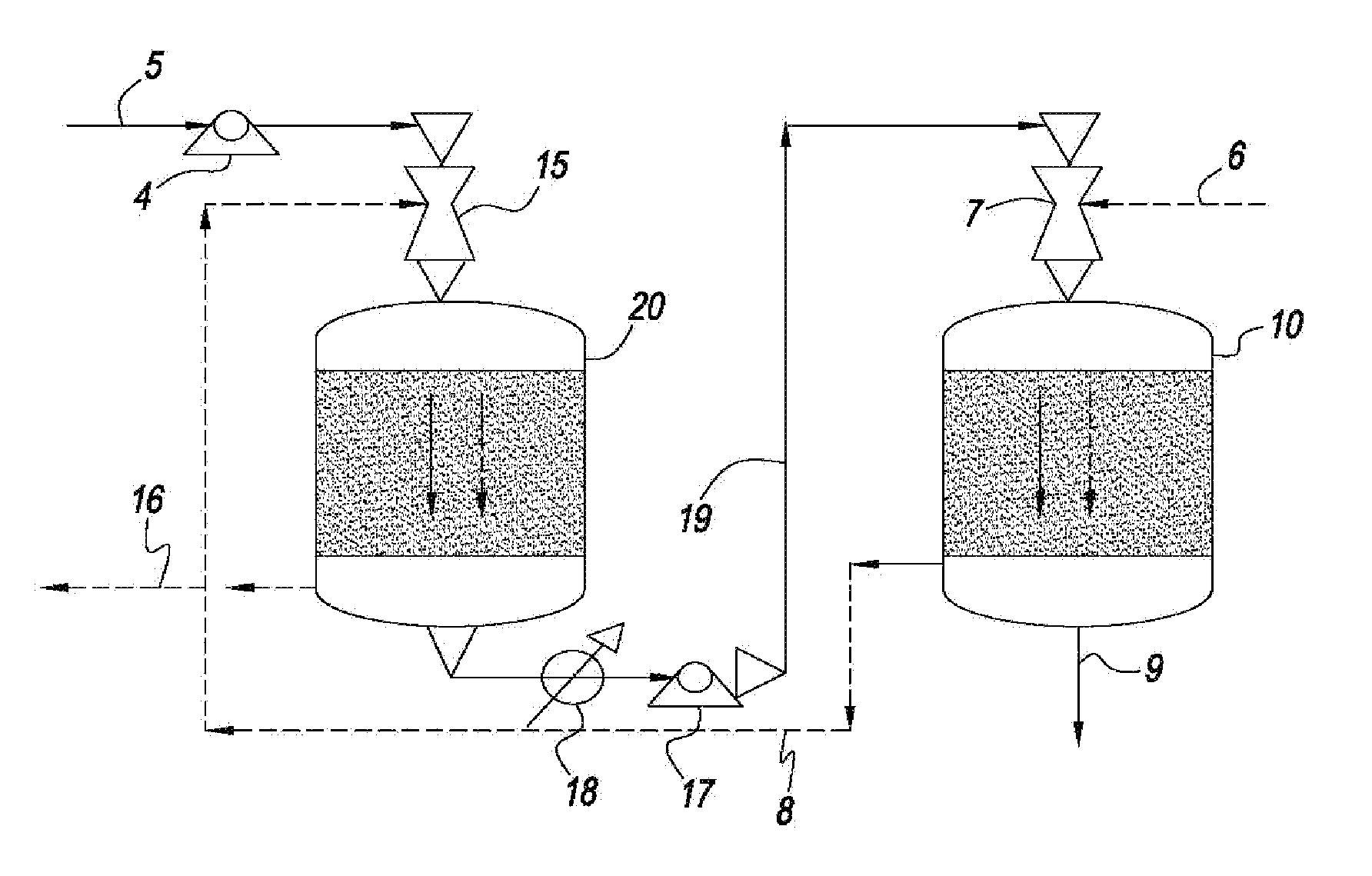
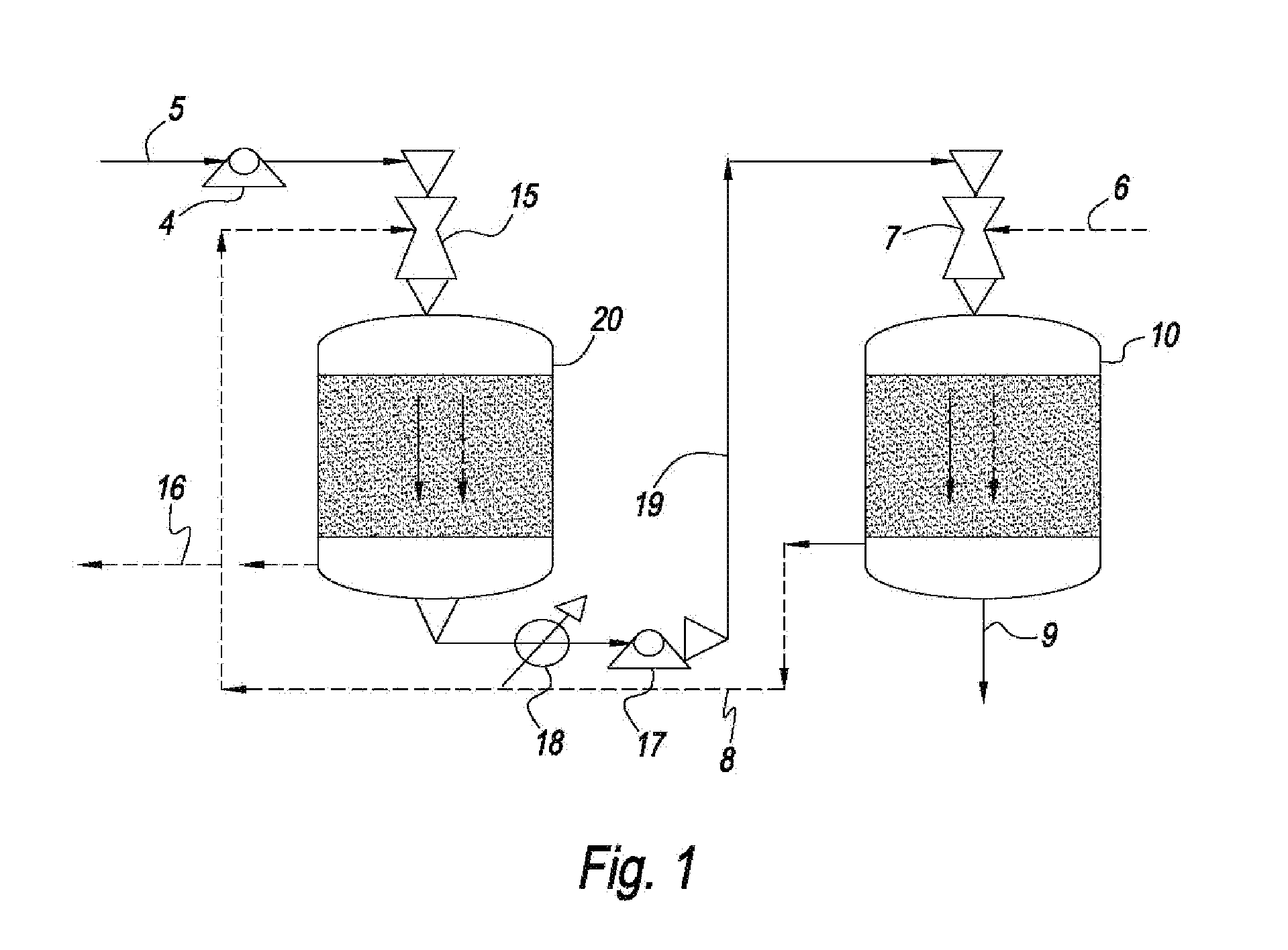
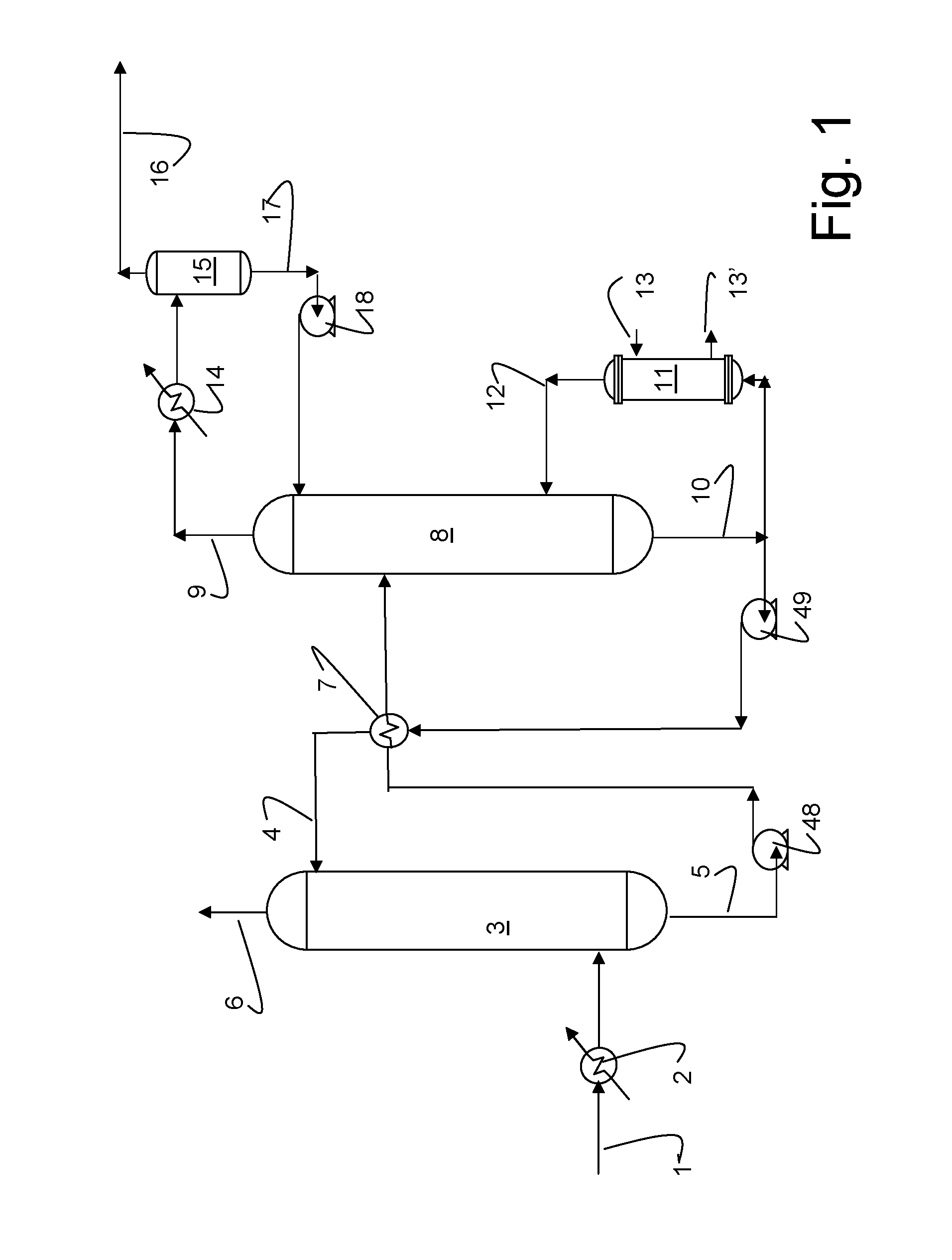
Process for producing a hydrocarbon component of biological origin
ActiveUS20040230085A1Improve performanceLow densityLiquid hydrocarbon mixture productionHydrocarbonsIsomerizationHydrocarbon
The invention relates to a process for producing a hydrocarbon component of biological origin. The process comprises at least two steps, the first one of which is a HDO step and the second one is an isomerization step operated using the counter-current flow principle. A biological raw material containing fatty acids and / or fatty acid esters serves as the feed stock.
Owner:OYJ NESTE OIL +1
Process for producing a hydrocarbon component of biological origin
ActiveUS7232935B2Improve performanceLow densityHydrocarbon from oxygen organic compoundsLiquid hydrocarbon mixture productionIsomerizationHydrocarbon
The invention relates to a process for producing a hydrocarbon component of biological origin. The process comprises at least two steps, the first one of which is a HDO step and the second one is an isomerization step operated using the counter-current flow principle. A biological raw material containing fatty acids and / or fatty acid esters serves as the feed stock.
Owner:OYJ NESTE OIL +1
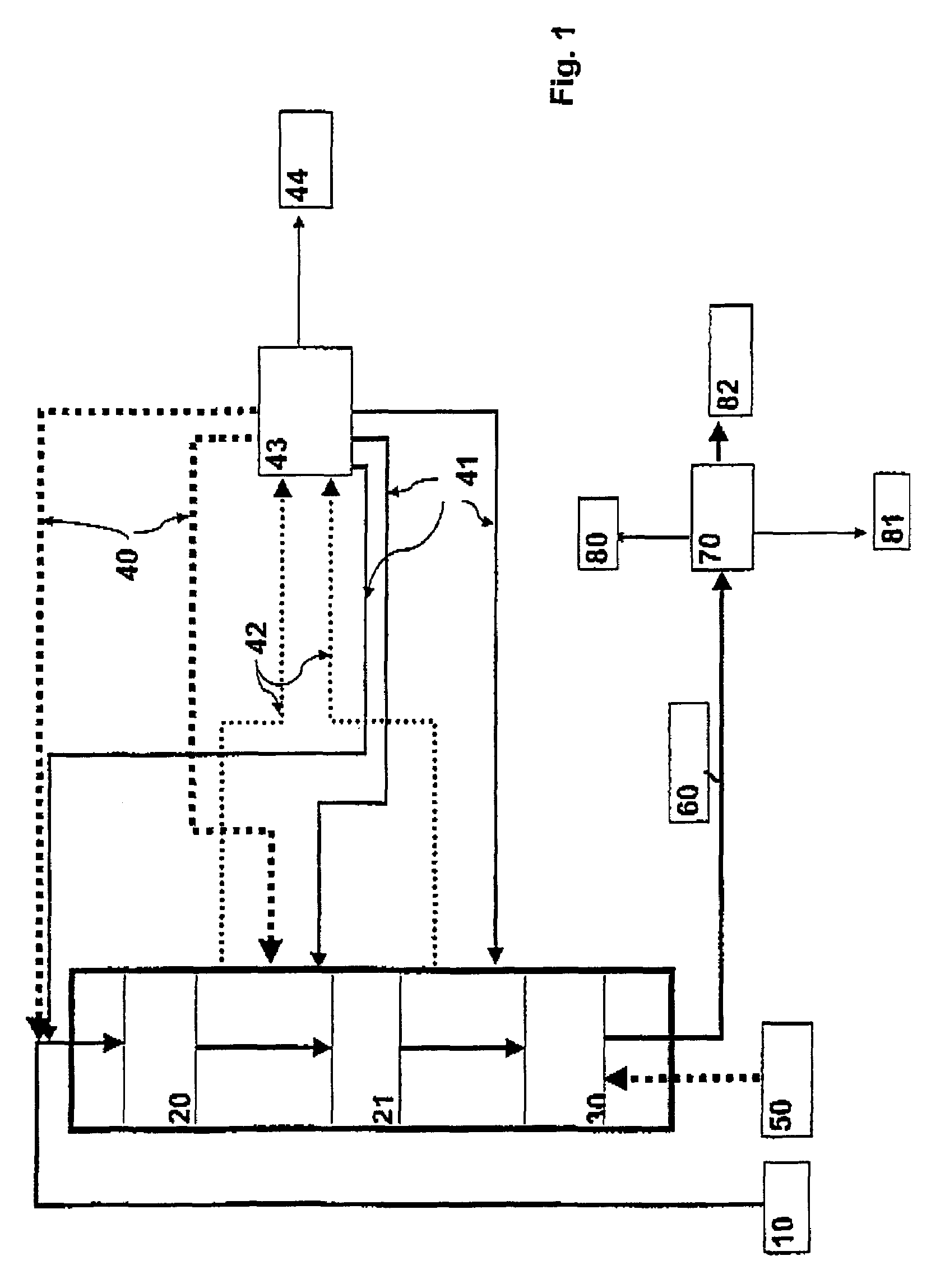
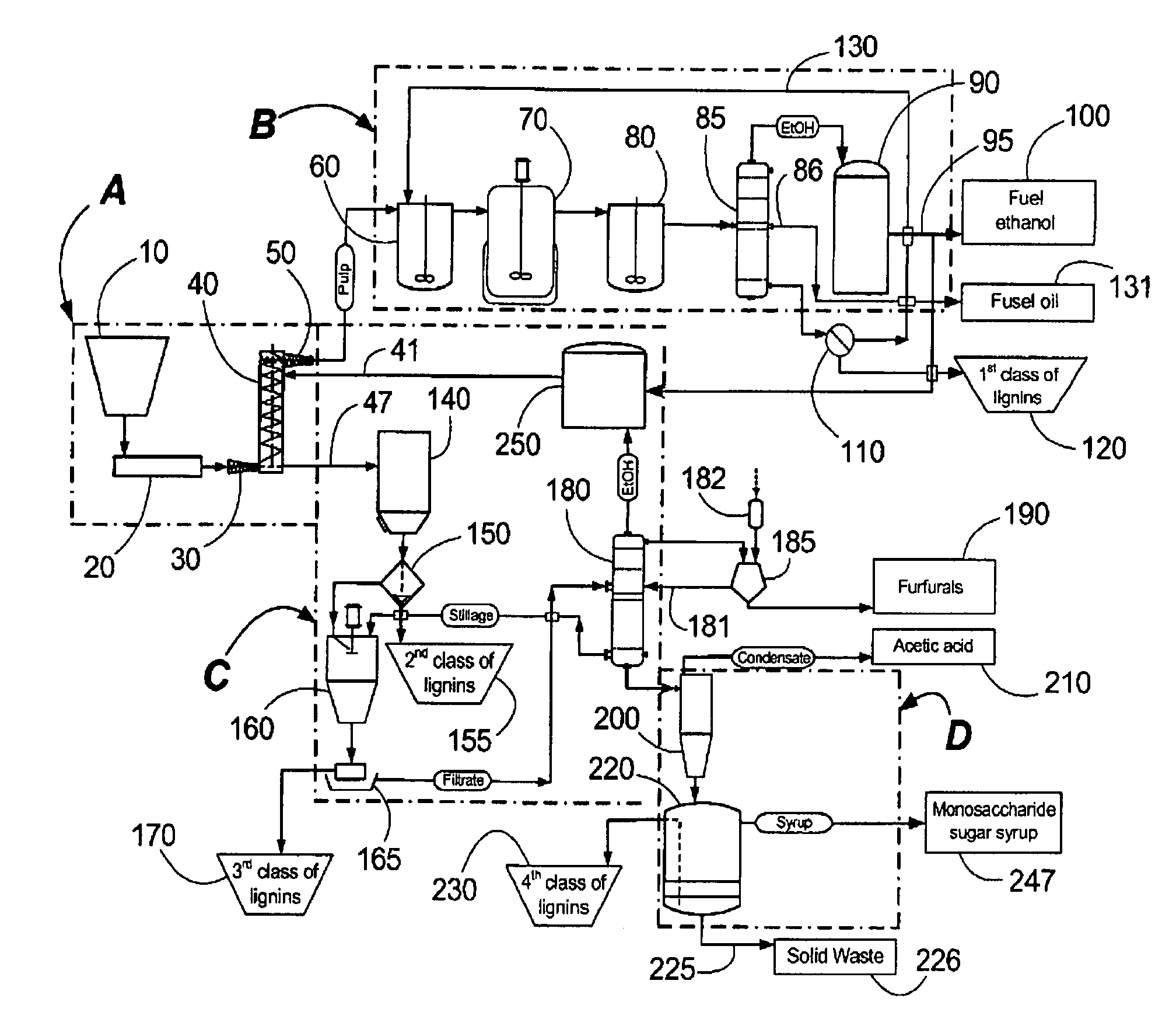
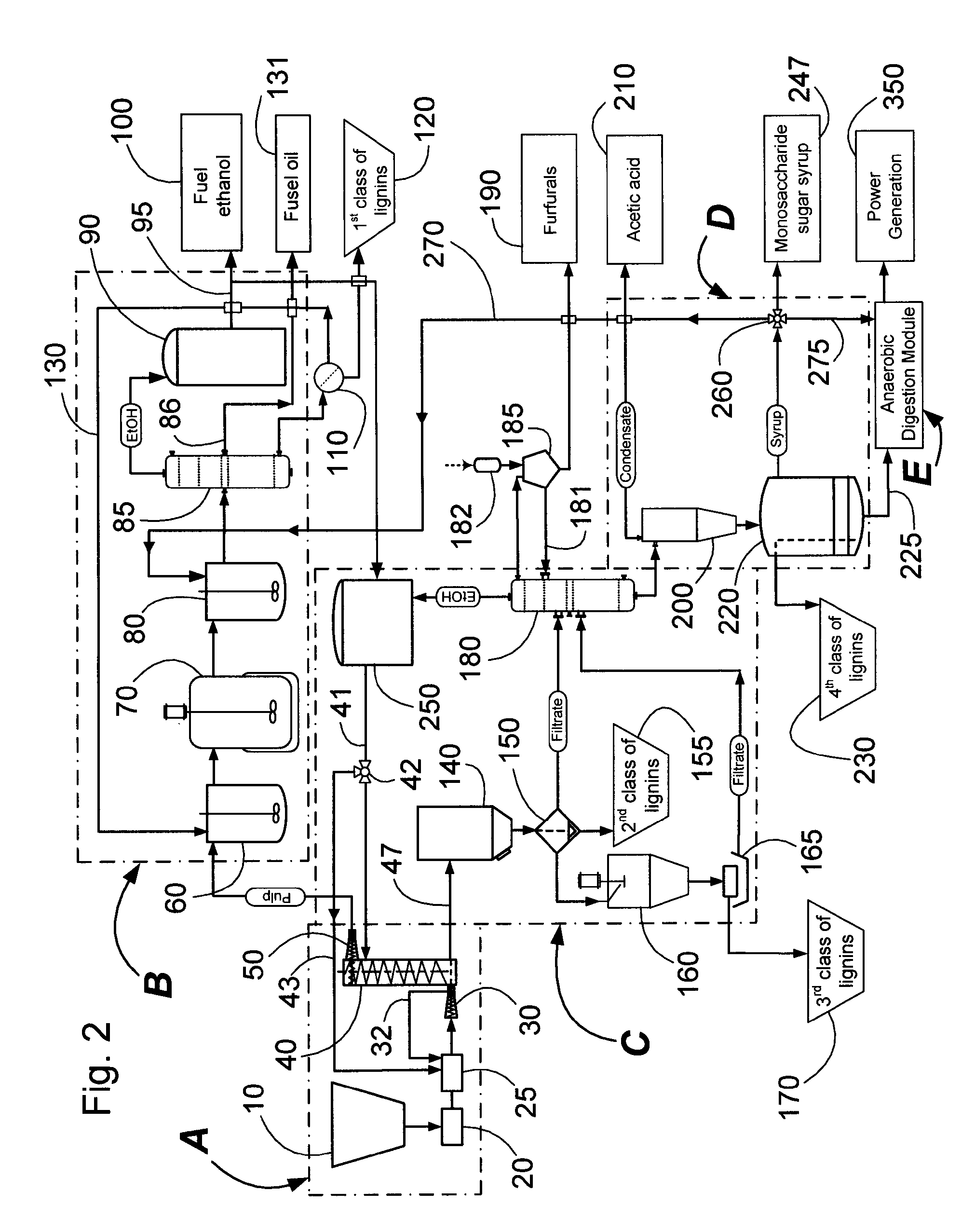
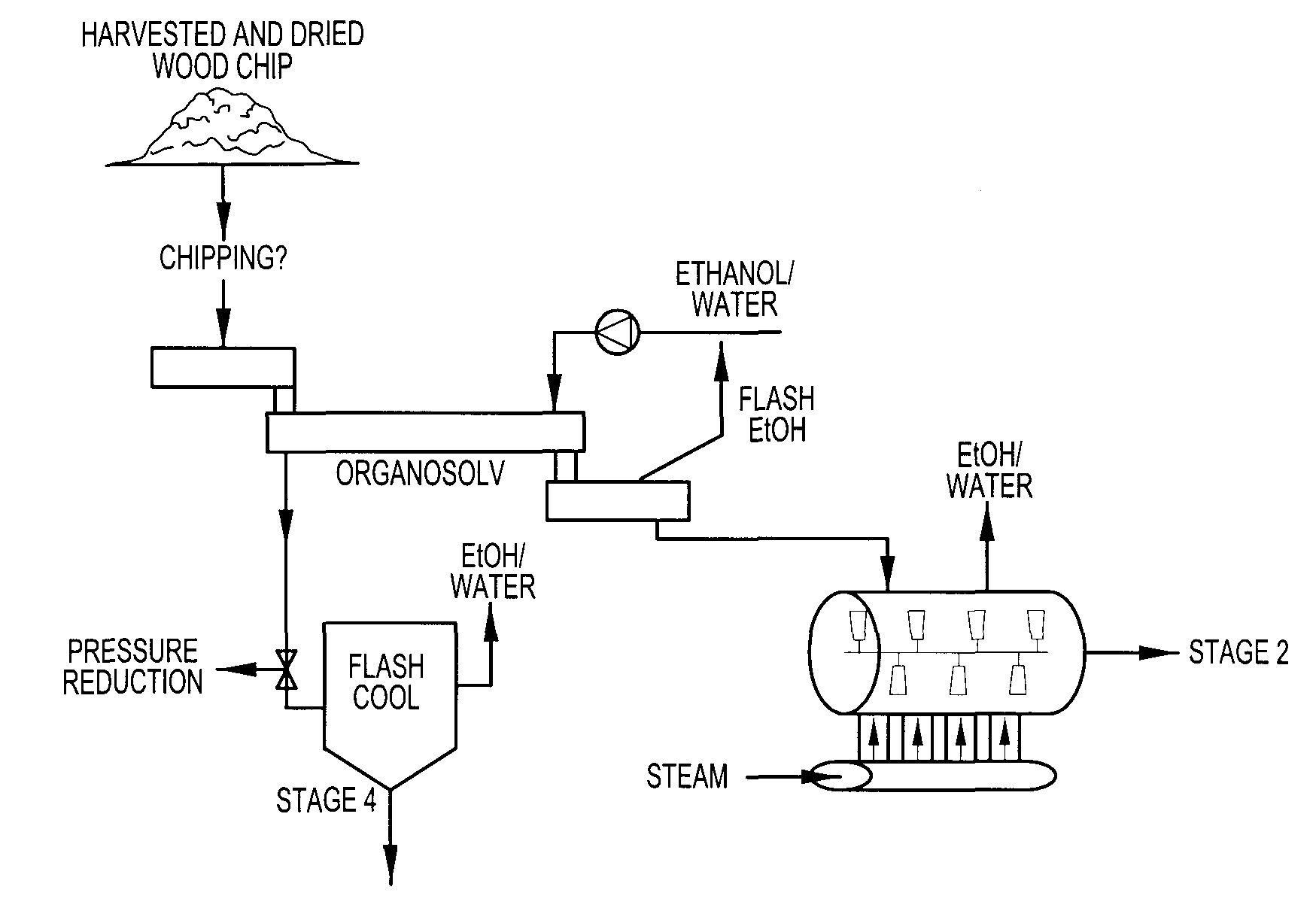
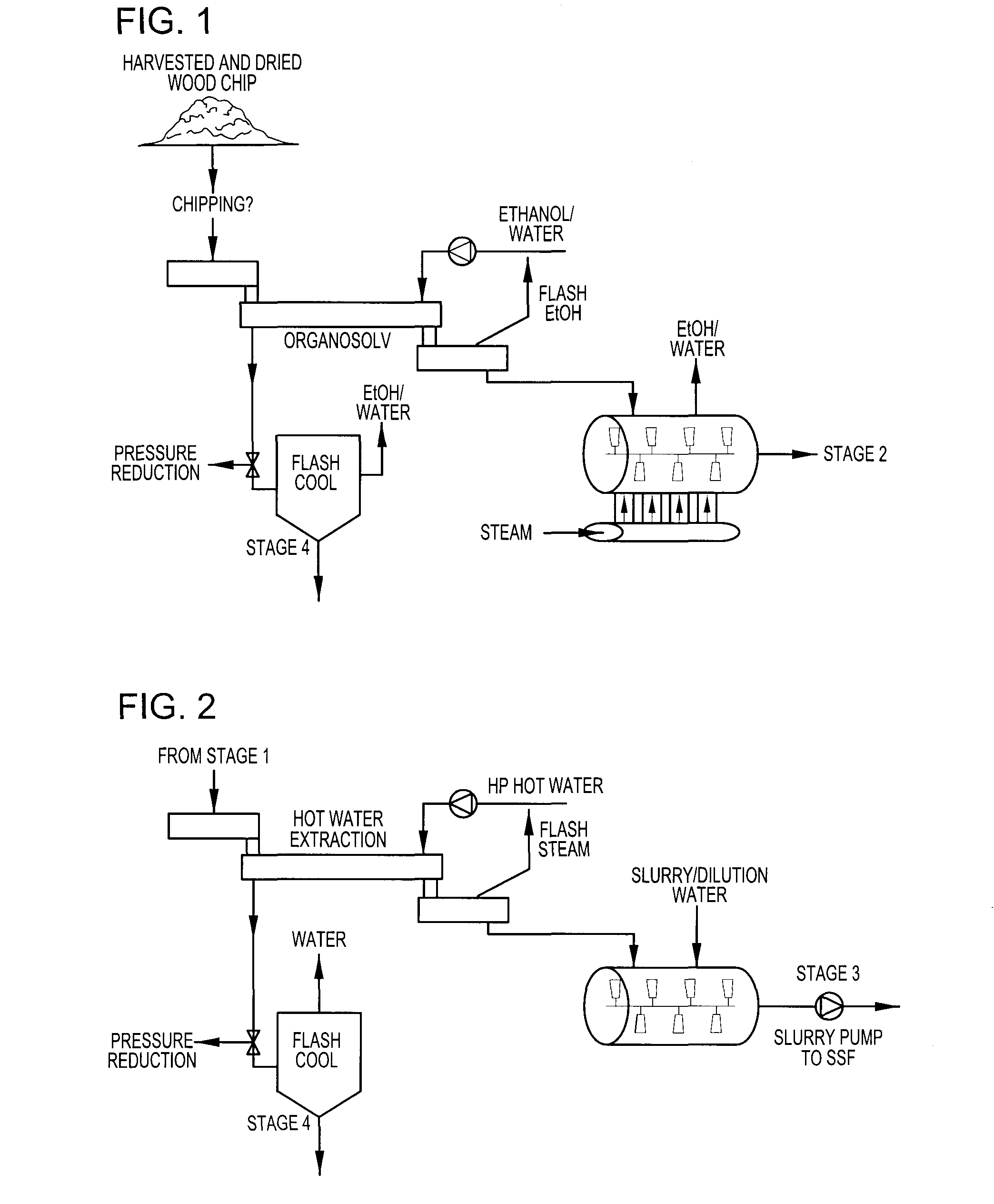
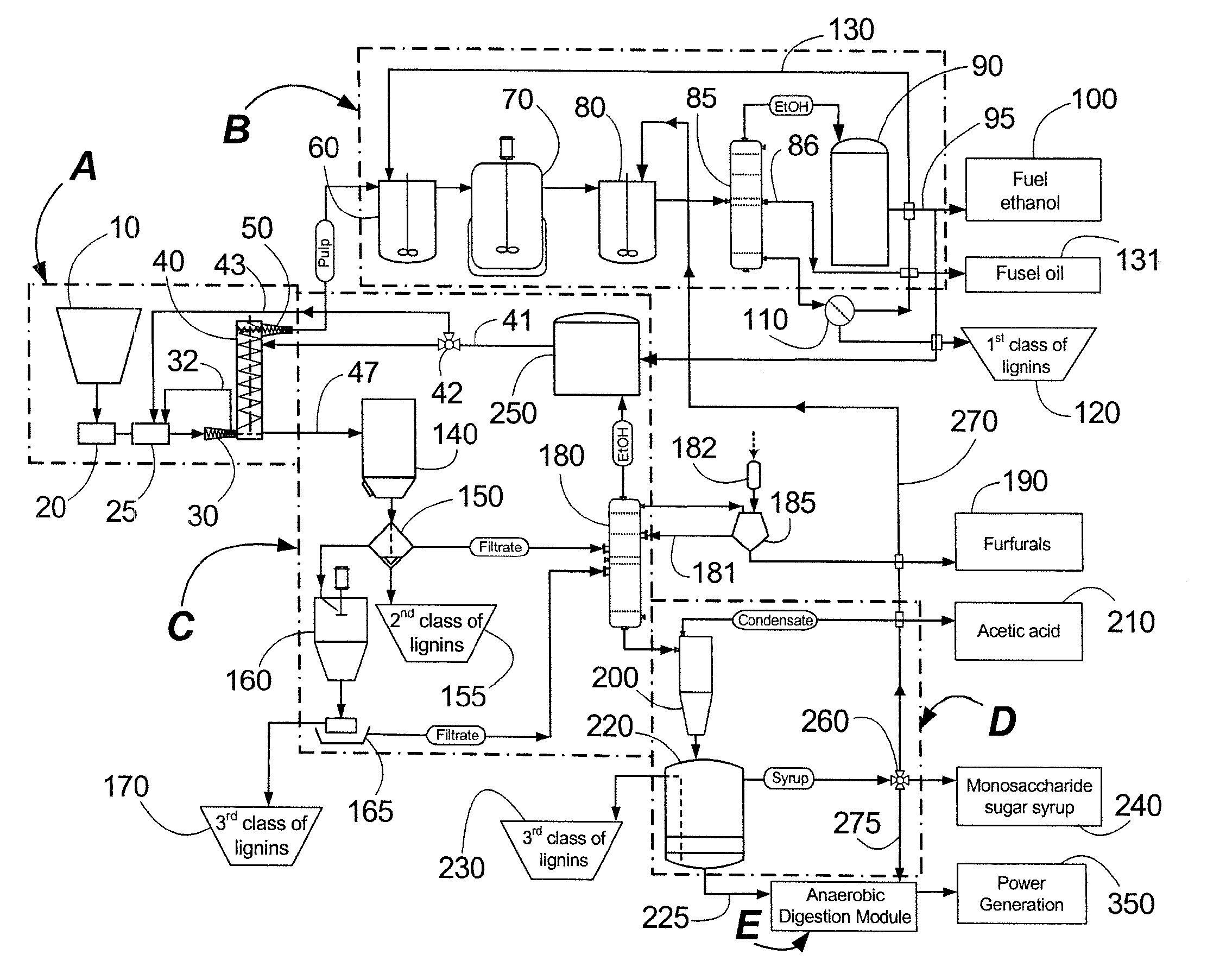
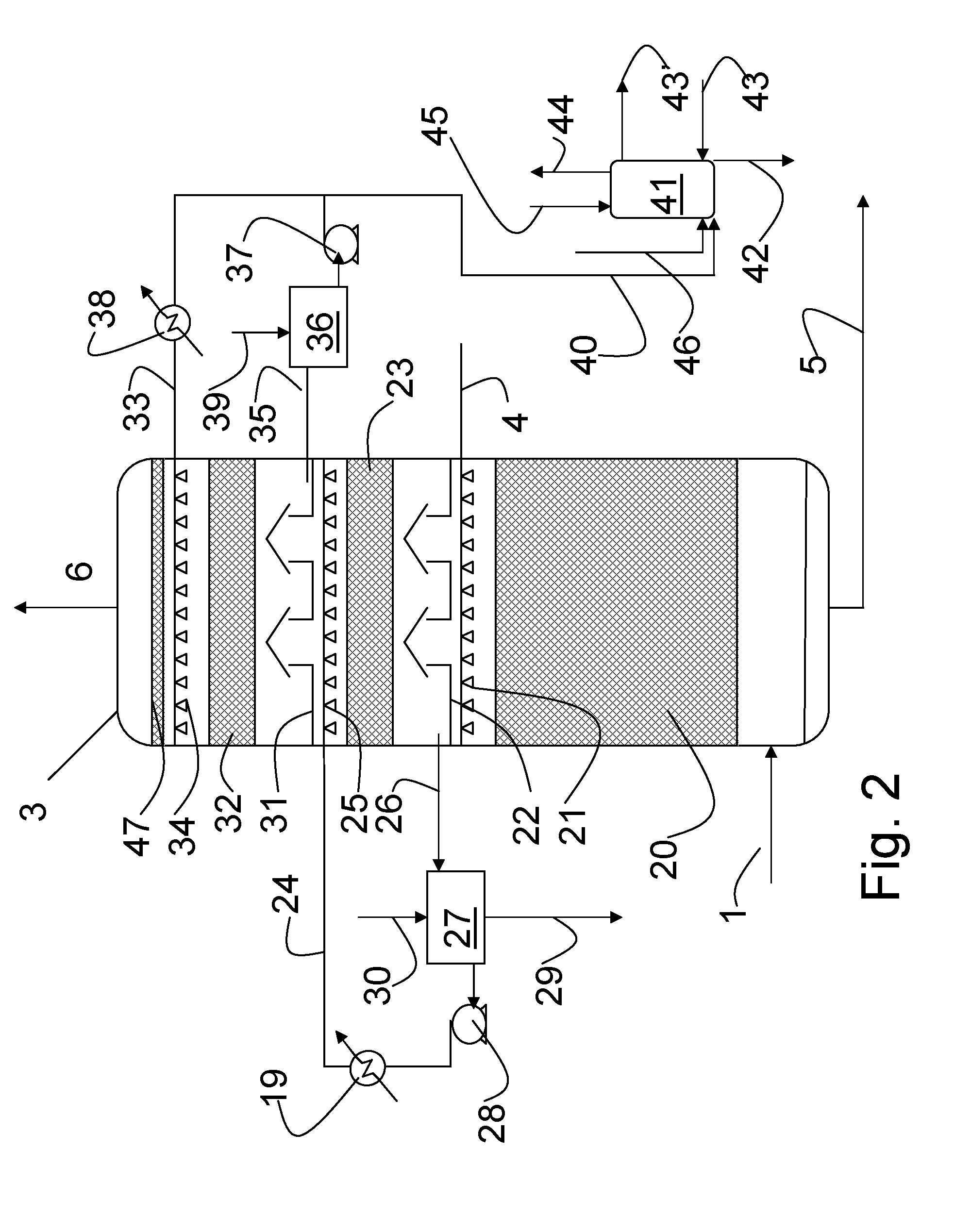
Continuous counter-current organosolv processing of lignocellulosic feedstocks
InactiveUS7465791B1Low viscosityNon-fibrous pulp additionBiological substance pretreatmentsFractionationOrganosolv
A modular process for organosolv fractionation of lignocellulosic feedstocks into component parts and further processing of said component parts into at least fuel-grade ethanol and four classes of lignin derivatives. The modular process comprises a first processing module configured for physico-chemically digesting lignocellulosic feedstocks with an organic solvent thereby producing a cellulosic solids fraction and a liquid fraction, a second processing module configured for producing at least a fuel-grade ethanol and a first class of novel lignin derivatives from the cellulosic solids fraction, a third processing module configured for separating a second class and a third class of lignin derivatives from the liquid fraction and further processing the liquid fraction to produce a distillate and a stillage, a fourth processing module configured for separating a fourth class of lignin derivatives from the stillage and further processing the stillage to produce a sugar syrup.
Owner:SUZANO CANADA INC
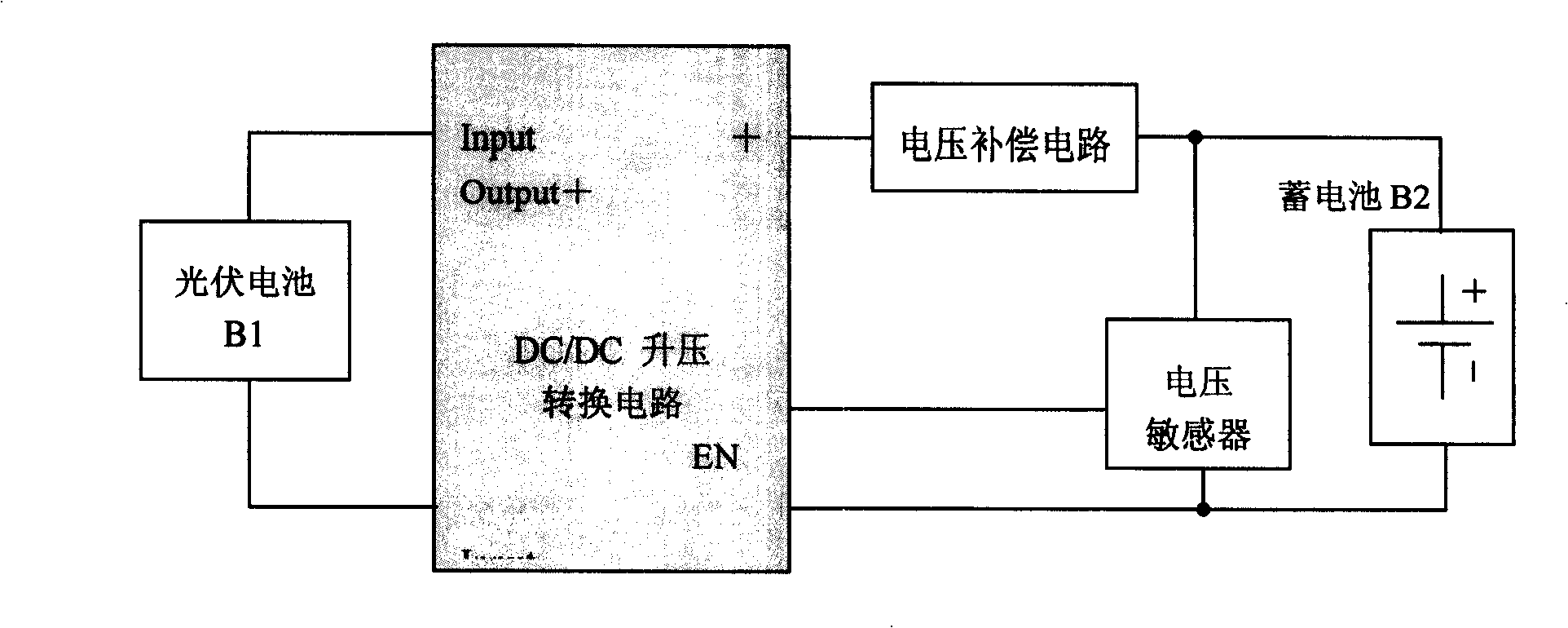
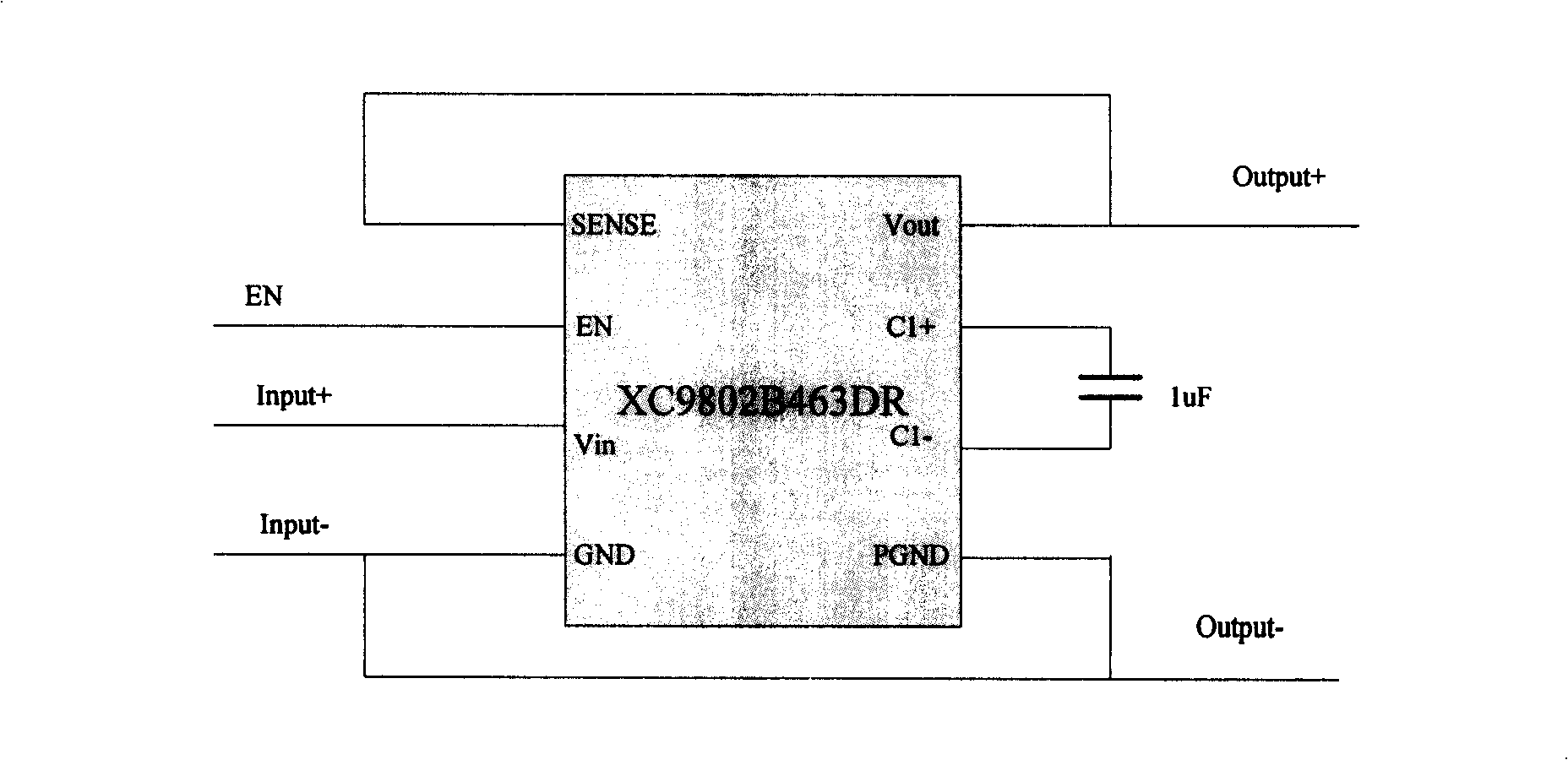
Photovoltaic battery- DC / DC voltage boosting convert charging method
InactiveCN101257221AAvoid overchargingRealize chargingBatteries circuit arrangementsElectric powerOvervoltageElectricity
The invention relates to a photovoltaic cell -DC / DC boost conversion charge method which belongs to battery technique field. The method includes: 1) a portable equipment accumulator voltage is detected by a voltage sensor, when lows to full charge capacity, the voltage sensor makes DC / DC boost converter starting DC / DC conversion, and electricity quantity flows from photovoltaic current to lithium battery for charging; 2) when the portable equipment accumulator is in full charge capacity state, accumulator need not charge again, the voltage sensor makes DC / DC boost converter forbidding DC / DC conversion, thereby, the portable equipment accumulator is protected and over charge phenomena is prevented. The method can be realized simply, and has short reaction time, high efficiency, overvoltage protection and anti-counterblast protection.
Owner:BEIJING HI TECH WEALTH INVESTMENT DEV
Process for the production of biofuel from plant materials
InactiveUS20070259412A1Rapid productionEfficient and cost-effective useBiofuelsLignin derivativesCelluloseBiofuel
An integrated process for the production of ethanol from woody plant material is provided, the process comprising: contacting a continuous flow of the plant material with a counter-current continuous flow of an aqueous ethanol solution at elevated temperature and pressure to provide plant material depleted of lignin; removing ethanol from the lignin-depleted plant material; contacting a continuous flow of the lignin-depleted plant material with water at elevated temperature and pressure to solubilize xylose within the plant material; and hydrolyzing cellulose present in the plant material to form glucose, which in turn is fermented to produce ethanol.
Owner:VERTICHEM CORP
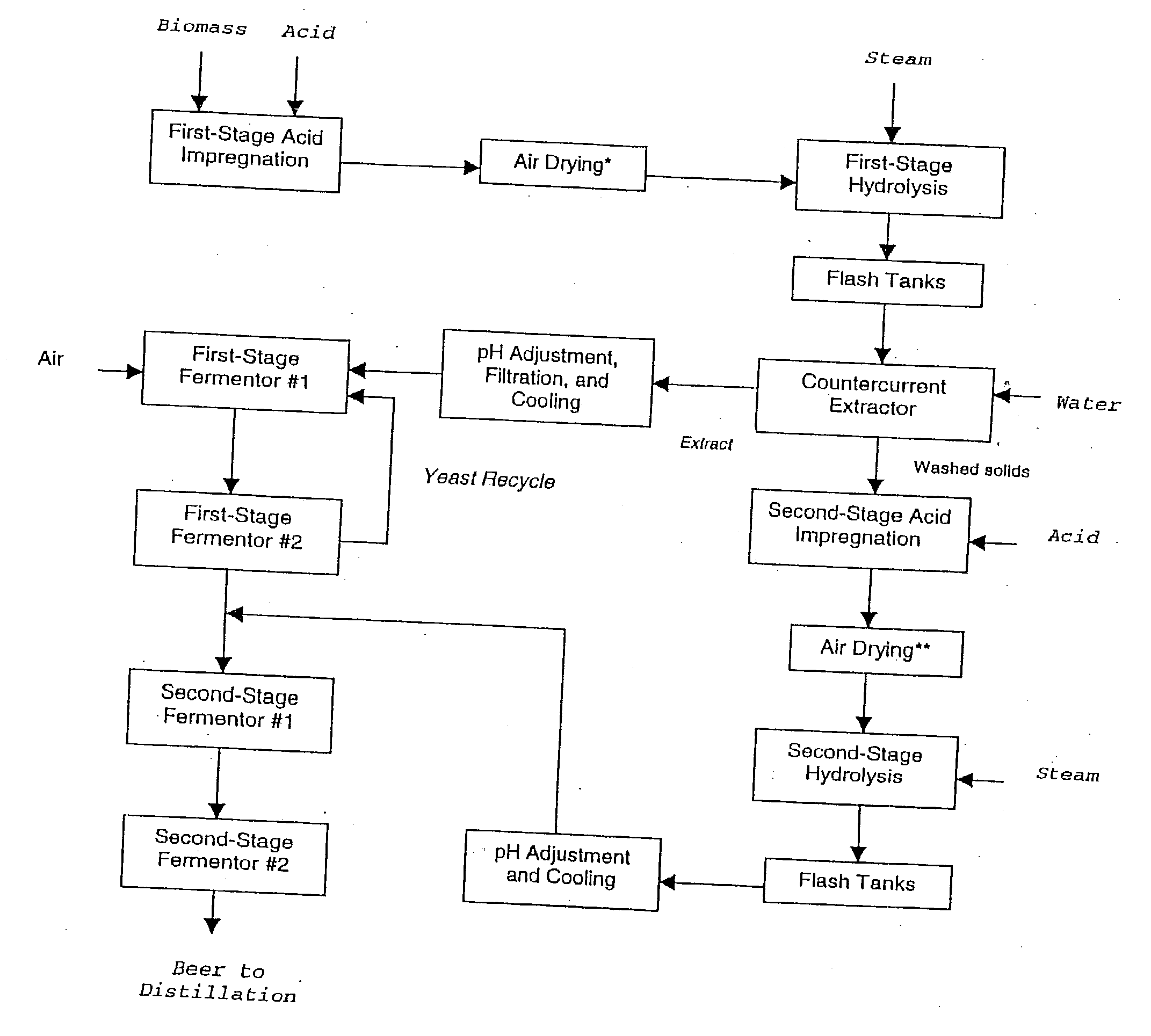
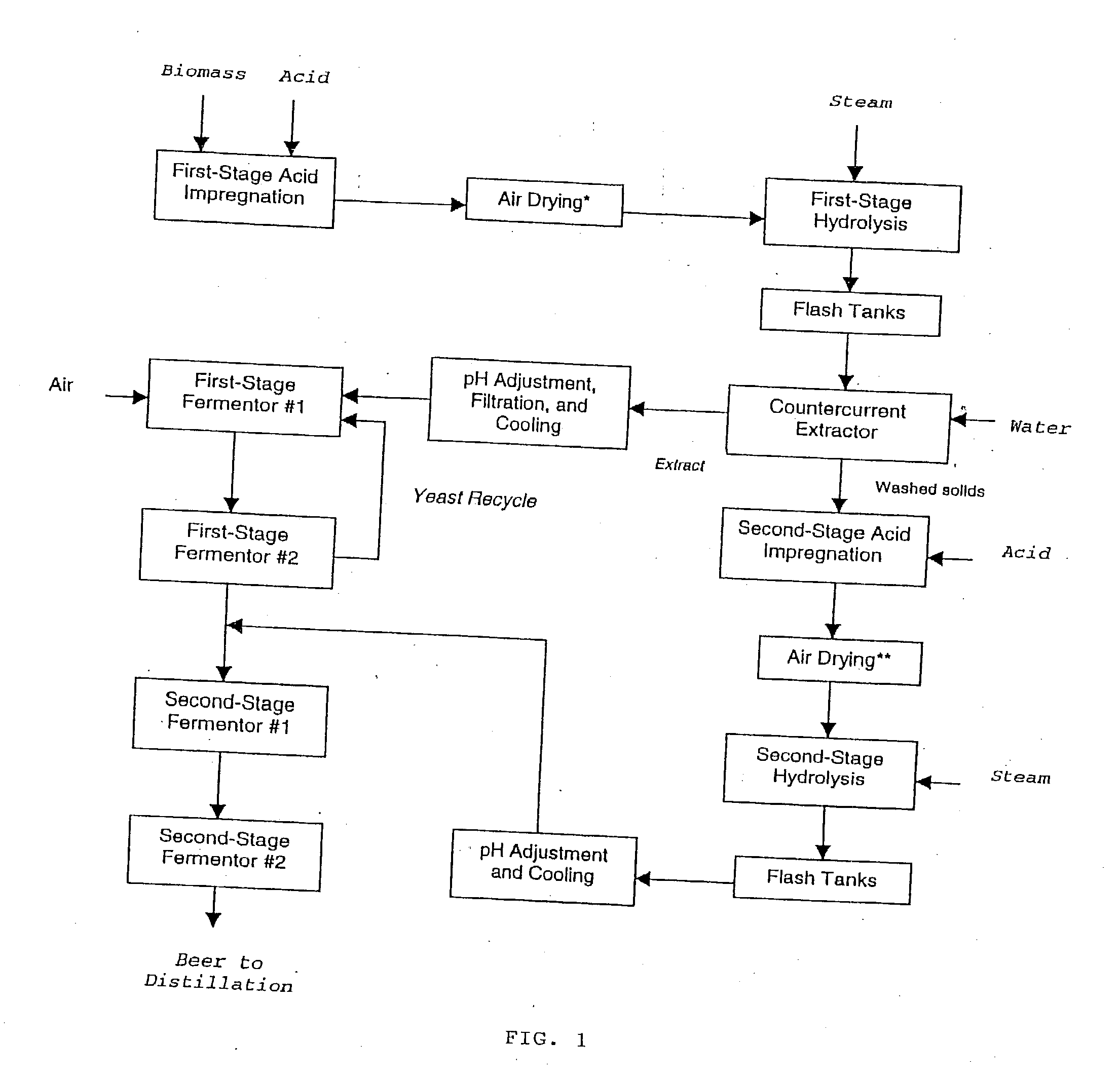
Ethanol production with dilute acid hydrolysis using partially dried lignocellulosics
InactiveUS20030199049A1Increase sugar yieldImprove digestibilityBiofuelsWaste based fuelCelluloseGrowth phase
In a process for converting lingnocellulosic biomass to ethanol, the improvement of obtaining higher fermentable soluble sugar yields by drying acid impregnated biomass particles, comprising: a) feeding moist lignocellulosic biomass into an acid impregnator to render it acid-soaked and draining the acid-soaked biomass to about 30% to 35% by weight solids; b) dewatering the acid-soaked biomass by drying or centrifugation to prevent compaction of the biomass and arrive at about 40% to 60% by weight solids; c) subjecting the acid-impregnated biomass to a first-stage hydrolysis reactor at a temperature of from 130° C. to 220° C. and discharging formed hydrolysate into a flash tank at about 120° C. to 140° C. to hydrolyze most of the remaining soluble oligosaccharides to monomeric sugars, and flashing remaining hydrolysate to a second flash tank at a lower temperature than the first flash tank-the second flash tank serving as a feed surge tank for a counter-current extractor; d) washing the hydrolysate, adjusting the pH of the sugar extract to about 5, and recovering more than 95% of the soluble sugars in the first-stage hydrolysate slurry by a counter-current extractor; e) subjecting remaining washed-first stage solids of pretreated biomass to a second-stage acid and metal salt impregnator and dewatering by drying or centrifugation to prevent compaction of biomass to arrive at 40% to 60% by weight solids; f) subjecting the acid and metal salt-impregnated biomass to a second-stage hydrolysis reactor at a temperature from 190° C. to 240° C. and discharging formed hydrolysate into a flash tank, at about 120° C. to 140° C. to hydrolyze most of the remaining soluble oligosaccharides to monomeric sugars and flashing remaining hydrolysate to a second flash tank at a lower temperature than the first flash tank, the second flash tank serving as a feed surge tank for second-stage fementors; g) cooling pH-adjusted extract from the counter-current extractor, feeding the extract to a first-stage fermentor and air sparging the first-stage fermentor at a rate sufficient to promote enough yeast growth to compensate for loss through second-stage fermentors; h) pH adjusting second-stage hydrolysate slurry to 4.5, cooling the slurry and adding it into the top of the first fermentor of a two-fermentor train in the second stage fermentors, pumping broth from the bottom of the first stage fermentors to the second stage fermentors while the yeast is in the growth phase for a period sufficient to consume over 95% of fermentable sugars; and i) recovering ethanol.
Owner:MIDWEST RES INST
Method and system for coating internal surfaces using reverse-flow cycling
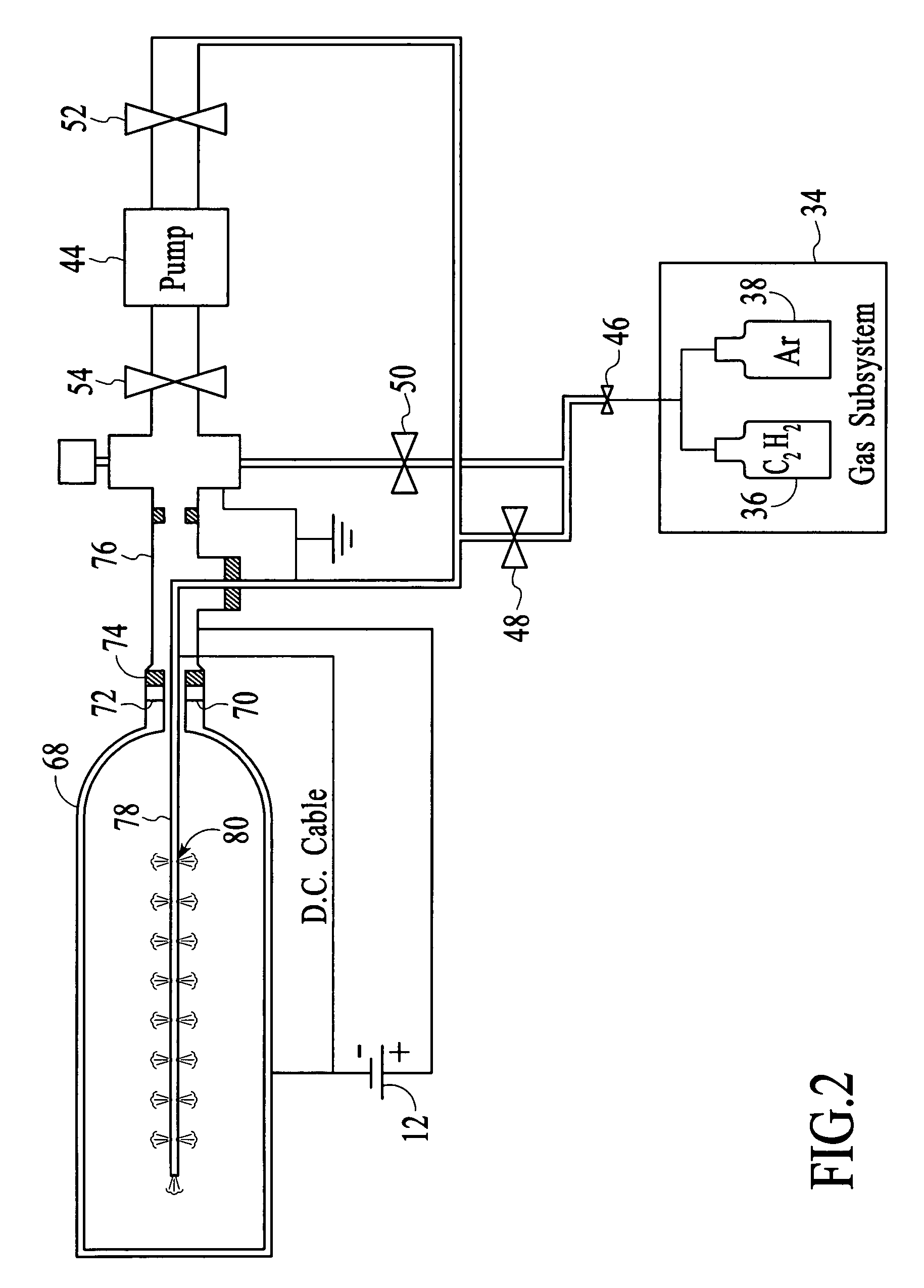
InactiveUS20060198965A1Provide uniformity in coatingEfficiently coat workpieceSolid state diffusion coatingChemical vapor deposition coatingControl systemMechanical engineering
A method and system for coating the internal surfaces of a workpiece is presented. A bias voltage is connected to a workpiece, which functions as a cathode. A gas source and a vacuum source are coupled to each opening through a flow control system. The flow control system is capable of a first mode which enables a first opening to function as a gas inlet and a second opening to function as a vacuum exhaust. The flow control system also has a second mode which enables a first opening to function as a vacuum exhaust and a second opening to function as a gas inlet. The cycling may also be used to coat internal surfaces of a workpiece with a single opening. Cycling the flow control system between the first mode and second mode is performed until a uniform coating along the internal surfaces of the workpiece is achieved.
Owner:SUB ONE TECH
Systems and Methods for Acid Gas Removal
InactiveUS20110217218A1Reduce and eliminate needReduce investmentGas treatmentDispersed particle separationDesorptionProduct gas
A method and system for the selective removal of CO2 and / or H2S from a gaseous stream containing one or more acid gases. In particular, a system and method for separating CO2 and / or H2S from a gas mixture containing an acid gas using an absorbent solution and one or more ejector venturi nozzles in flow communication with one or more absorbent contactors. The method involves contacting a gas mixture containing at least one acid gas with the absorbent solution under conditions sufficient to cause absorption of at least a portion of said acid gas. The absorbent contactors operate in co-current flow and are arranged in a counter-current configuration to increase the driving force for mass transfer. Monoliths can be used that operate in a Taylor flow or slug flow regime. The absorbent solution is treated under conditions sufficient to cause desorption of at least a portion of the acid gas.
Owner:EXXON RES & ENG CO
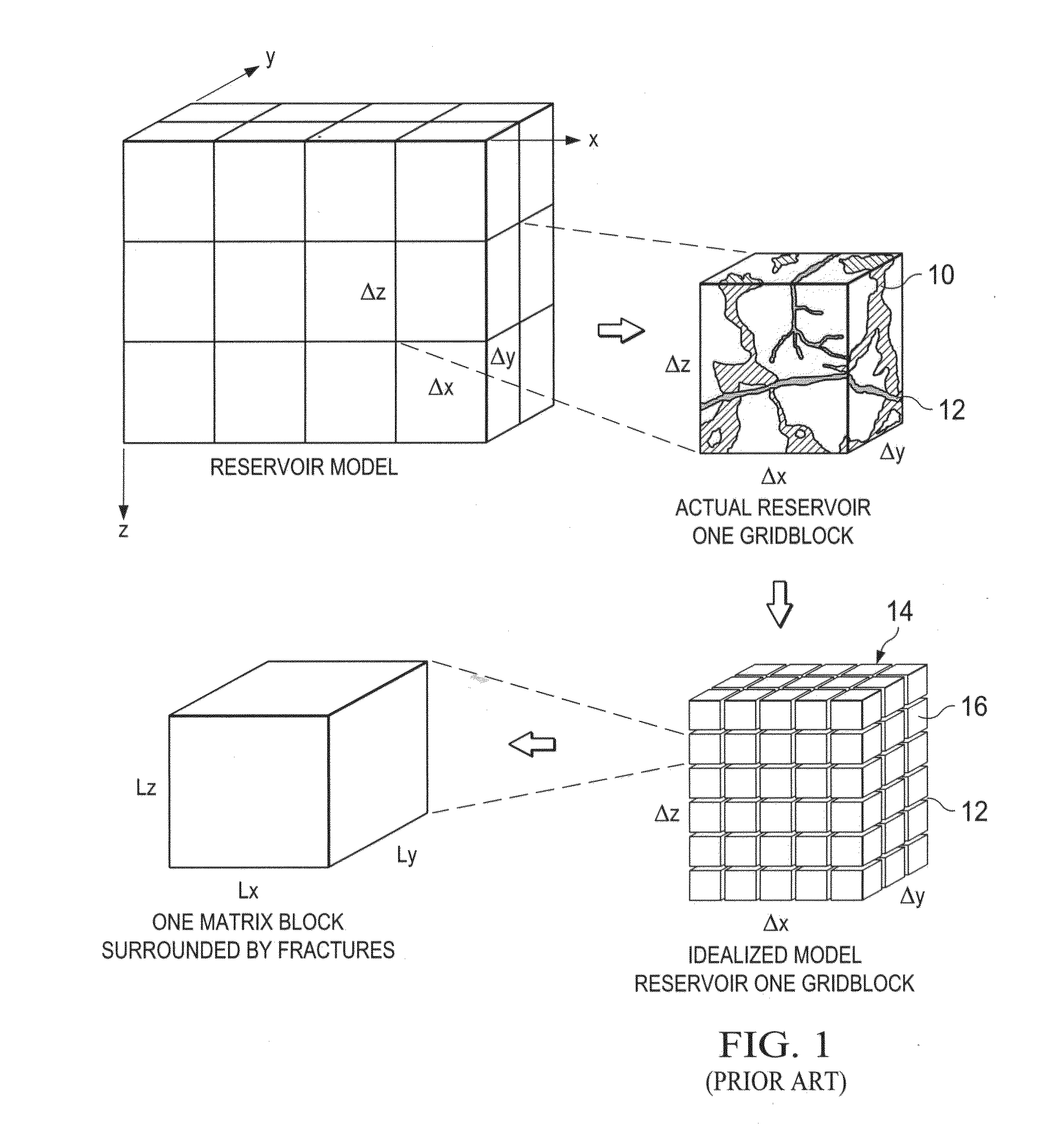
Scalable Simulation of Multiphase Flow in a Fractured Subterranean Reservoir as Multiple Interacting Continua
ActiveUS20120179436A1GeomodellingComputation using non-denominational number representationPore systemCounter current
A subterranean reservoir where the pore space of media or formation rock is multimodal, and the media may have imbedded multiple scales of fracture networks, is simulated. The modes of the pore system and the scale of fracture networks are each represented as a separate, but interactive continuum with the other. The simulation allows multiple continua to be co-located and multi-interacting, in that each continuum may have current and counter-current multiple multiphase exchanges with other continua.
Owner:SAUDI ARABIAN OIL CO
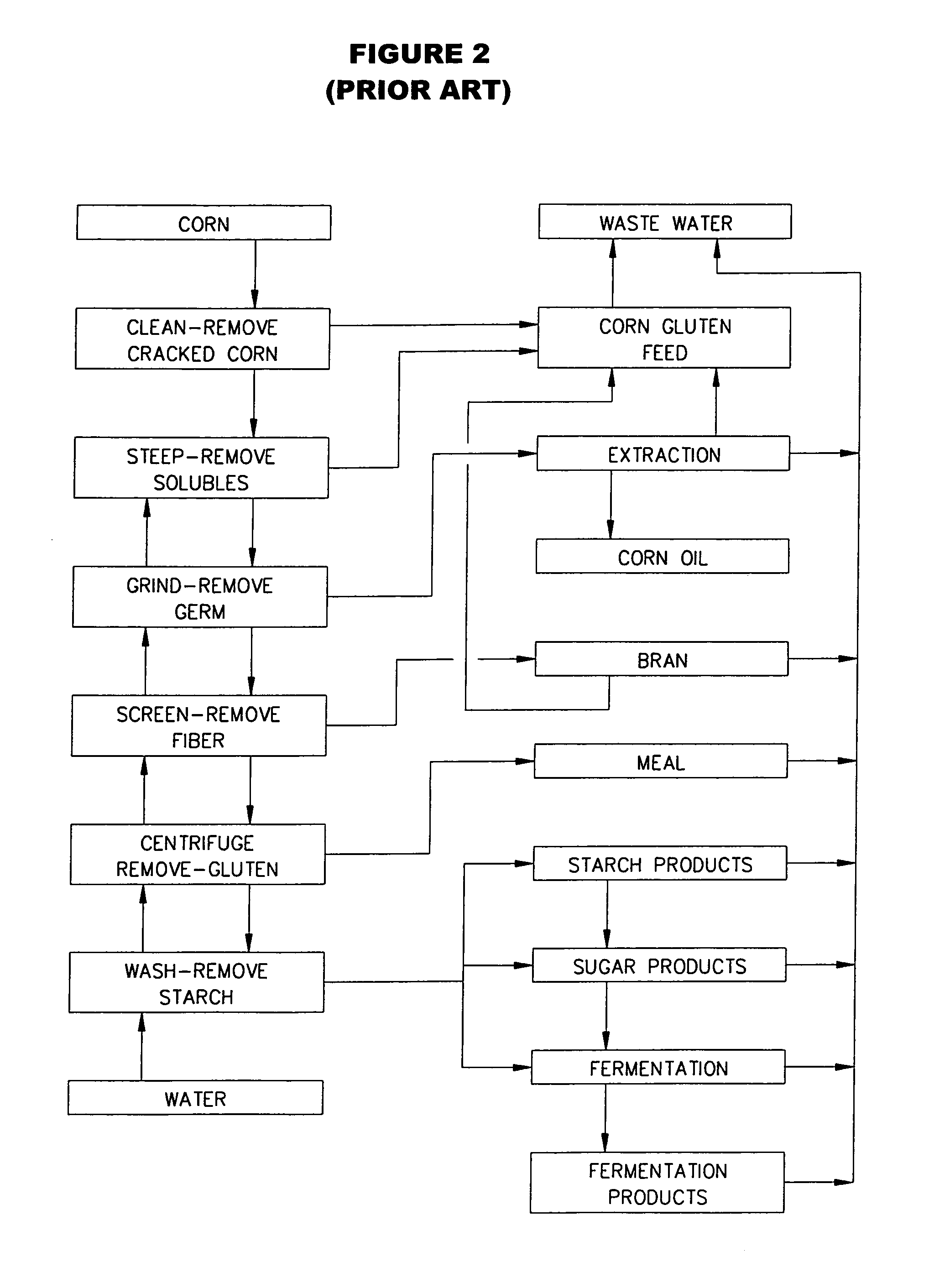
Corn refining process
InactiveUS7452425B1Quickly and efficiently producedAvoid large quantitiesJuice extractionBiofuelsSteepingSlurry
A grain containing starch, such as corn, is refined. The grain is steeped in water at a temperature of about 125 to 160° F., which water is essentially free of sulfurous acid and contains recycled enzymes from downstream processes, in a counter-current steeping reactor for about 10 to 20 hours to produce an aqueous slurry of steeped grain having a moisture content of about 40 to 50 percent. The various components of the grain are then separated and the starch is converted to ethanol.
Owner:LANGHAUSER ASSOC
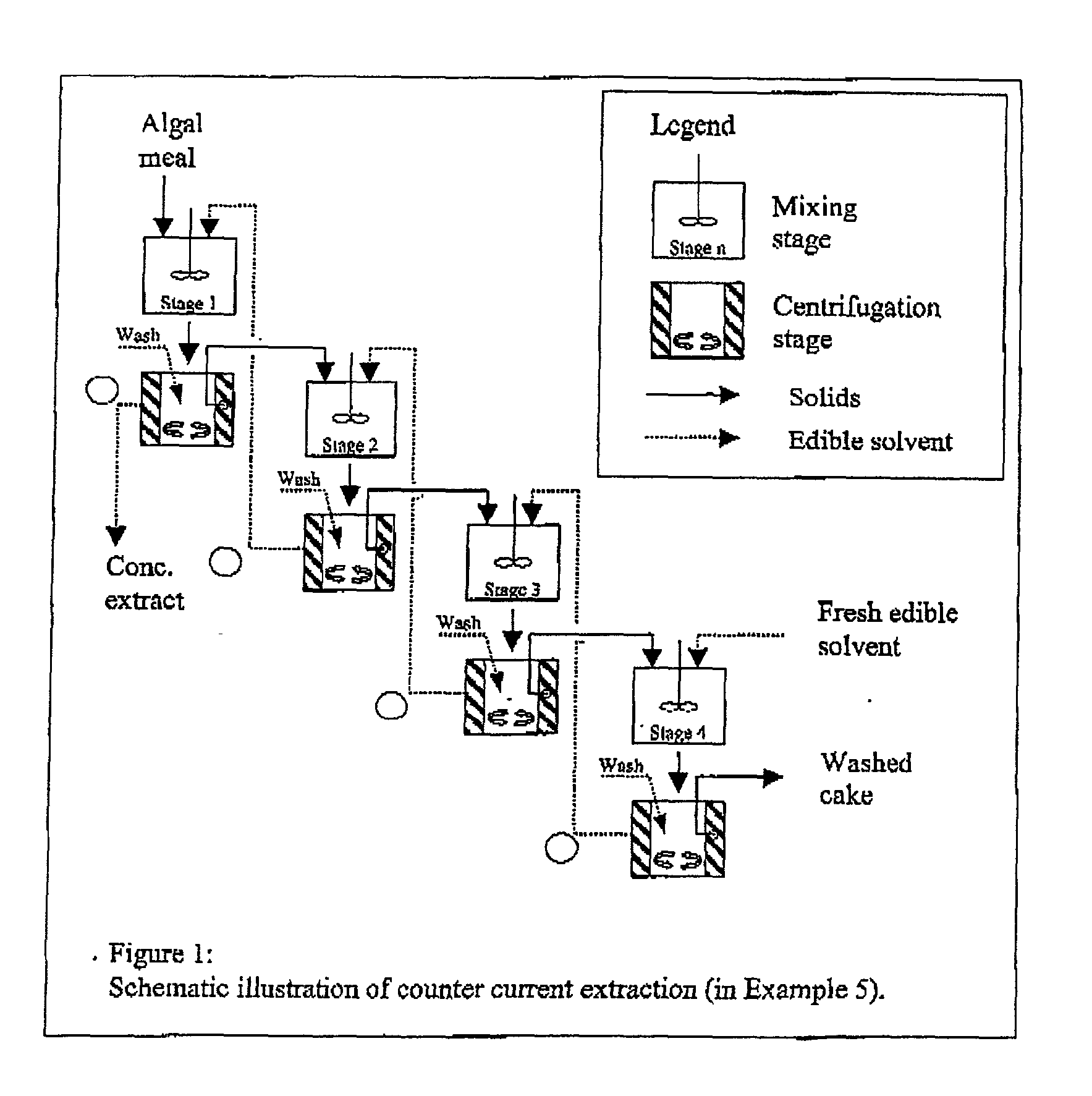
Edible solvent extraction of carotenoids from microorganisms
InactiveUS20030054070A1Efficient extractionEnriching carotenoid contentLiquid hydrocarbon mixture productionHydrocarbonsParticulatesAnimal food
A process for extracting carotenoids from a carotenoid-containing starting material produced by microorganisms is described. Said starting material is admixed with edible solvent to effectuate the transfer of carotenoids. Separation of the carotenoid-enriched edible solvent is improved by the formation of a "cake", composed of said carotenoid-containing particulate solids, and, as required, a certain quantity of suitable filtration aid to modify the cake's consistency. Mechanical aids accelerate the separation of the carotenoid-enriched edible solvent. Said mixture may be hydrated to aid the removal of solids and gums from the carotenoid containing edible solvent. The carotenoid-enriched edible solvent is filtered though said cake to reduce the particulate load including any undesirable microbial load. A counter-current process increases the carotenoid concentration of the extract. The carotenoid-enriched edible solvent can be used as an ingredient in human and animal foodstuffs and dietary supplements for the possible prevention and treatment of illnesses and diseases.
Owner:AQUSRCH
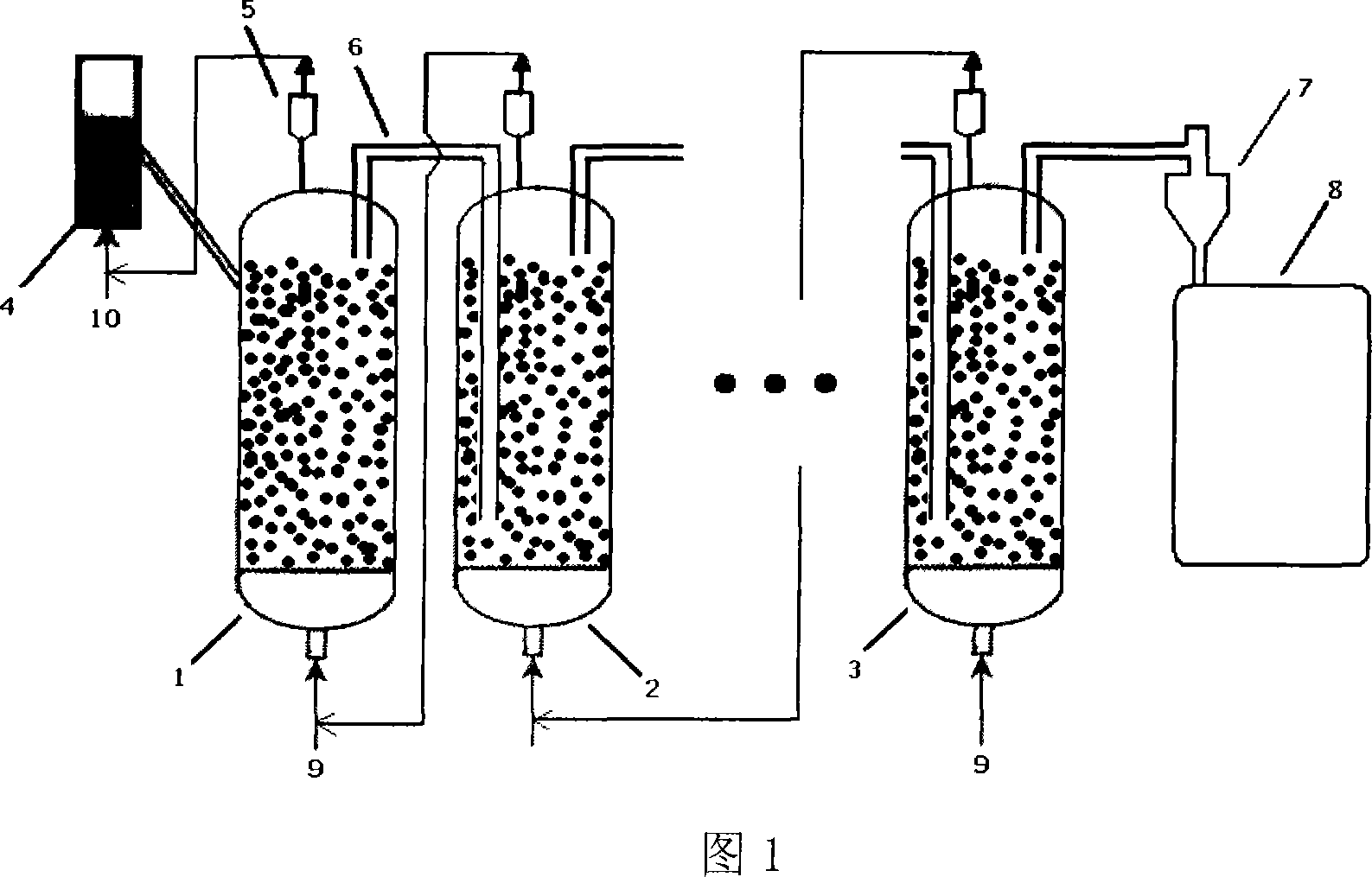
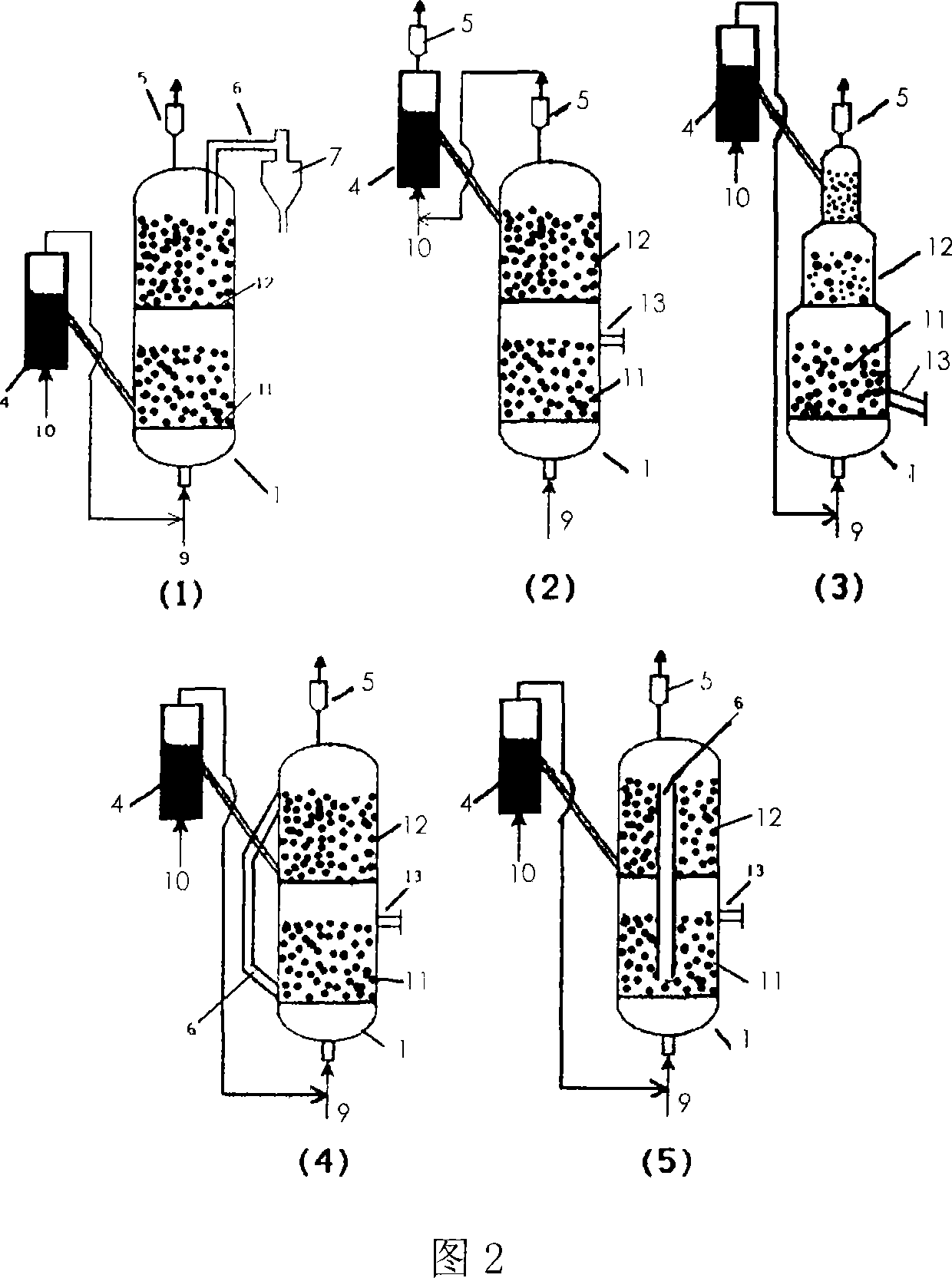
Method for producing Nano carbon tubes continuously and equipment
ActiveCN101049927AEfficient separationImprove stabilityMaterial nanotechnologyMulti-walled nanotubesCarbon nanotubeProduct gas
This invention discloses high-efficiency method and apparatus for continuously producing carbon nanotubes. The apparatus is a multi-stage counter-current reaction apparatus. The method comprises: placing the catalyst in the pre-reactor or the main reactor, introducing mixed gases of H2 or CO, and N2 or Ar, reducing, then introducing raw material gas, reacting to obtain carbon nanotubes, and sending the expanded solid raw material to the next stage reactor for reaction. The gas flow and the solid raw material are arranged in counter-current contact mode in the reactors, which can increase the conversion rate of the solid raw material. This invention has such advantages as simple apparatus and easy operation, and is suitable for industrial production on the scale of tens of thousands of tons per year.
Owner:TSINGHUA UNIV
Compact dedusting apparatus
ActiveUS7380670B2Improved airflow characteristicsClean thoroughlyGas current separationMagnetic separationMagnetic fluxDust particles
A compact housing for a dedusting apparatus utilizes a magnetic flux field to disrupt the static charge attracting dust particles to product particles, which along with fluidization and counter current airflow principles that are proven to dislodge dust particles from the product, provides a highly efficient, compact deduster. The housing supports a double wash deck with product flow separated between the back-to-back primary wash decks. A deflector directing the flow of product onto the primary wash decks is provided with an extension that extends parallel to the wash deck to eliminated product bouncing off of the wash deck. The lower air outlets are eliminated, while the upper air outlets are positioned in extensions to the main housing above the product inlet opening. Air flow through the Venturi zone is enhanced by re-directing clean air directly to the backside of the Venturi panels, thereby eliminating the extraneous by-pass boxes.
Owner:PELLETRON CORP
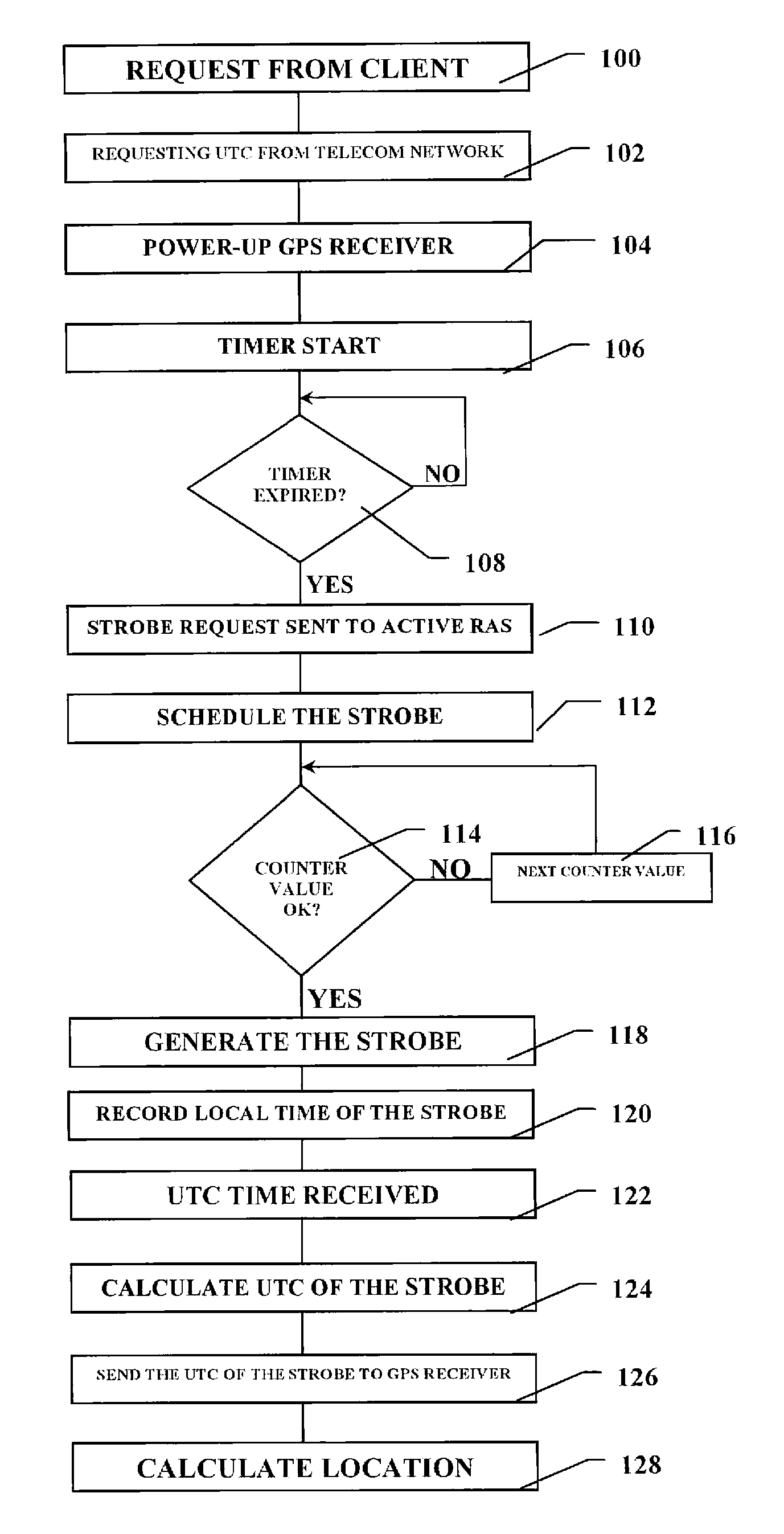
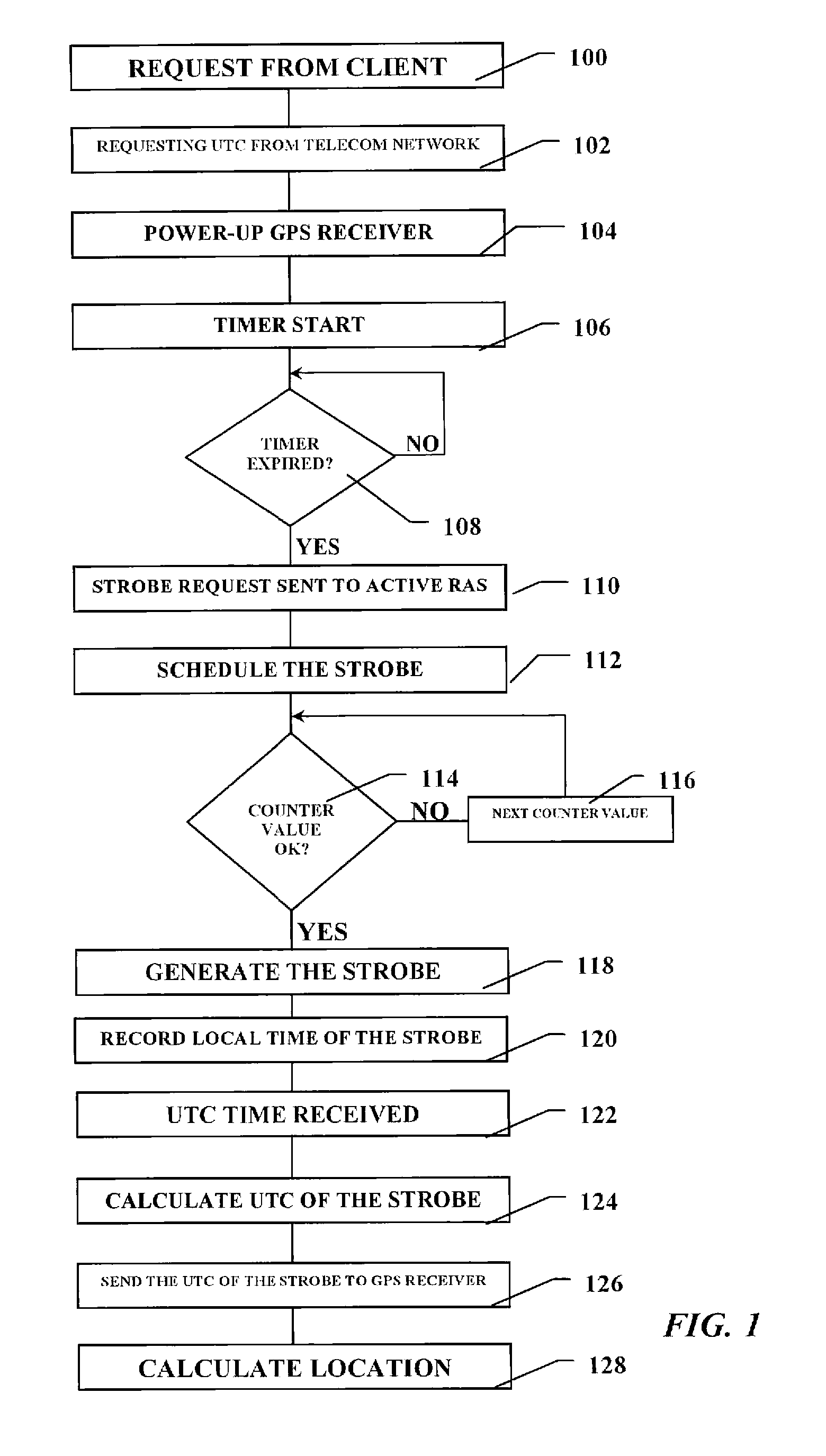
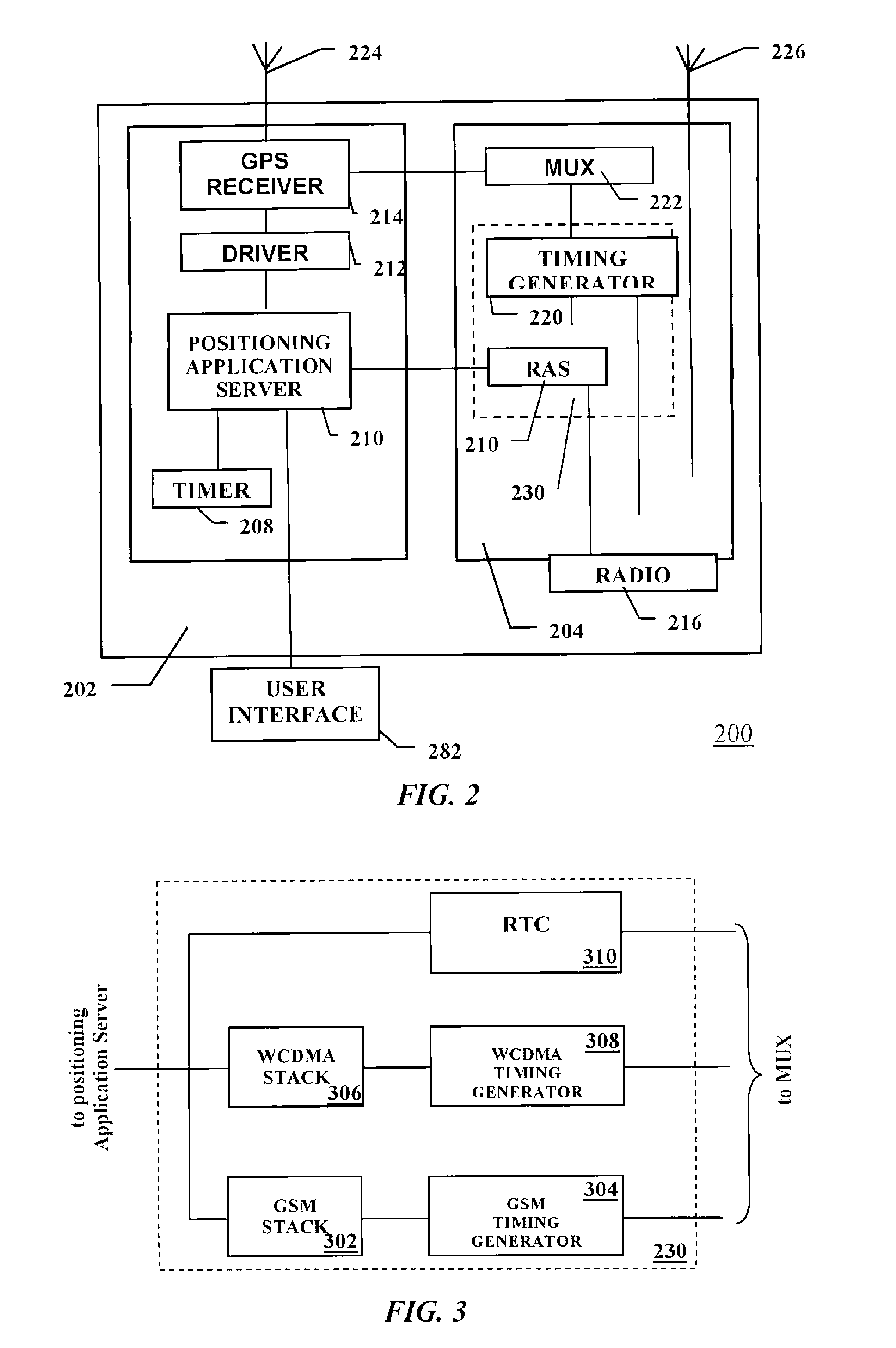
Positioning device and a method of operating thereof
ActiveUS20080224924A1Lower latencyShort timeDirection finders using radio wavesBeacon systemsTelecommunications networkEngineering
A positioning unit and telecommunications unit wherein a reference time value from is requested from a telecommunications network to start the positioning receiver. A strobe is scheduled to be transmitted from the telecommunications unit to the positioning receiver when the positioning receiver is armed. A current and predefined value of the counter is compared and the strobe is generated when the counter current value equals the predefined value. The strobe time of generation is recorded in relation to a structure of the signal transmitted by the digital telecommunications network in the current serving cell. The reference time value from the telecommunications network is received, wherein the reference time value is related to a structure of the signal transmitted by the network. The accurate time of generation of the strobe based on said reference time value is calculated transmitted to said positioning unit.
Owner:TELEFON AB LM ERICSSON (PUBL)
Carbonising and/or Activating Carbonaceous Material
A method is provided for carbonizing and activating carbonaceous material, which comprises supplying the material to an externally fired rotary kiln maintained at carbonizing and activating temperatures, the kiln having a downward slope to progress the material as it rotates, the kiln having an atmosphere substantially free of oxygen provided by a counter-current of steam or carbon dioxide, and annular weirs being provided at intervals along the kiln to control progress of the material. There may further be provided an externally fired rotary kiln for carbonizing and activating carbonaceous material having a hollow rotary body that has a downward slope towards a discharge end thereof, and which is provided at intervals along its length with annular weirs for controlling progress of the carbonaceous material. In embodiments, there is also provided a process is for producing discrete solid beads of polymeric material e.g. phenolic resin beads having a mesoporous structure, which may be useful as feedstock for the above mentioned carbonization / activation process or which may have other utility e.g. as ion exchange resins. The process may produce resin beads on an industrial scale without aggregates of resin building up speedily and interrupting production. The process comprises the steps of: (a) combining a stream of a polymerizable liquid precursor e.g. a novolac and hexamine as cross-linking agent dissolved in a first polar organic liquid e.g. ethylene glycol with a stream of a liquid suspension medium which is a second non-polar organic liquid with which the liquid precursor is substantially or completely immiscible e.g. transformer oil containing a drying oil; (b) mixing the combined stream to disperse the polymerizable liquid precursor as droplets in the suspension medium e.g. using an in-line static mixer; (c) allowing the droplets to polymerise in a laminar flow of the suspension medium so as to form discrete solid beads that cannot agglomerate; and (d) recovering the beads from the suspension medium. There is also provided apparatus for forming discrete solid beads of polymeric material, said apparatus comprising: a first line for conveying s stream of a polymerizable liquid precursor; a second line for conveying a stream of a dispersion medium with which the polymerizable liquid precursor is substantially or completely immiscible; an in-line mixer configured to receive a combined flow from the first and second lines and to disperse the polymerizable liquid precursor as droplets in the dispersion medium; a vertical polymerization column configured to receive the dispersion medium with the droplets dispersed therein and to permit the polymerizable liquid precursor polymerize while descending the column in a descending flow of polymerization medium; and a vessel at the base of the column for receiving the descending flow of dispersion medium and collecting polymerized solid beads.
Owner:BRITISH AMERICAN TOBACCO (INVESTMENTS) LTD
Method and system for coating internal surfaces using reverse-flow cycling
InactiveUS7541069B2Uniform coatingUniform depositionSemiconductor/solid-state device manufacturingSolid state diffusion coatingControl systemEngineering
A method and system for coating the internal surfaces of a workpiece is presented. A bias voltage is connected to a workpiece, which functions as a cathode. A gas source and a vacuum source are coupled to each opening through a flow control system. The flow control system is capable of a first mode which enables a first opening to function as a gas inlet and a second opening to function as a vacuum exhaust. The flow control system also has a second mode which enables a first opening to function as a vacuum exhaust and a second opening to function as a gas inlet. The cycling may also be used to coat internal surfaces of a workpiece with a single opening. Cycling the flow control system between the first mode and second mode is performed until a uniform coating along the internal surfaces of the workpiece is achieved.
Owner:SUB ONE TECH
Selection and rational development of solvent systems in counter-current chromatograph
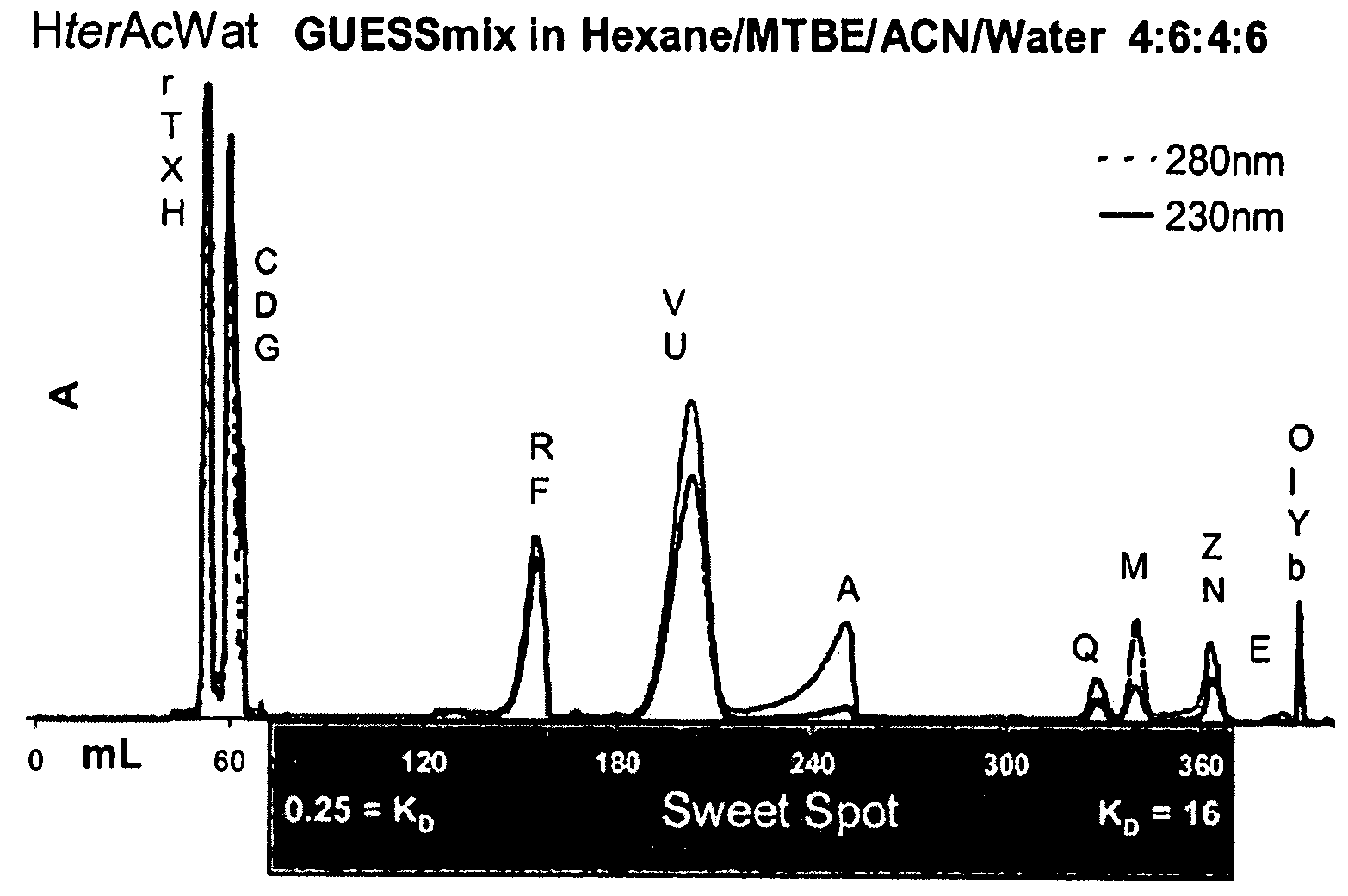
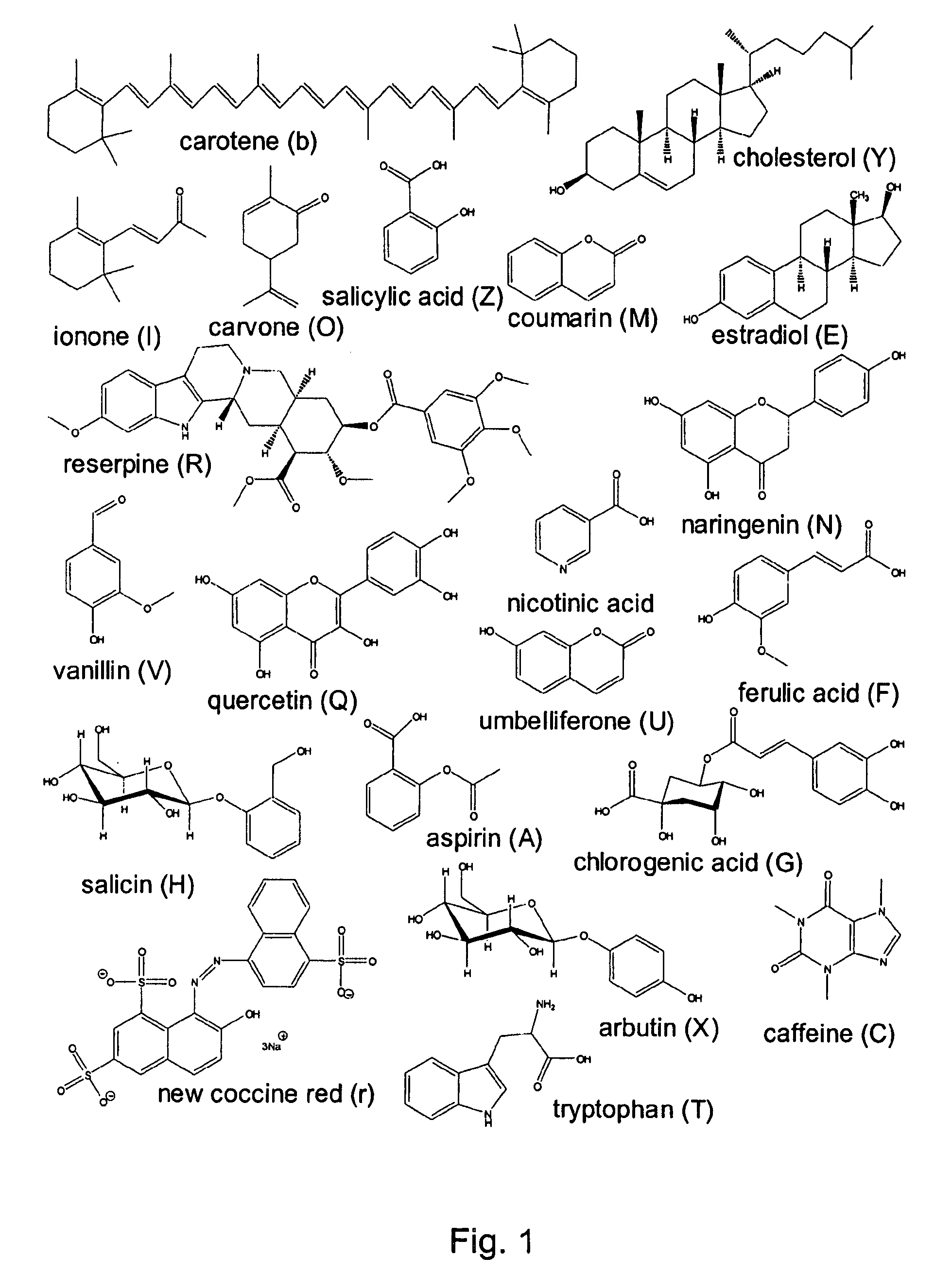
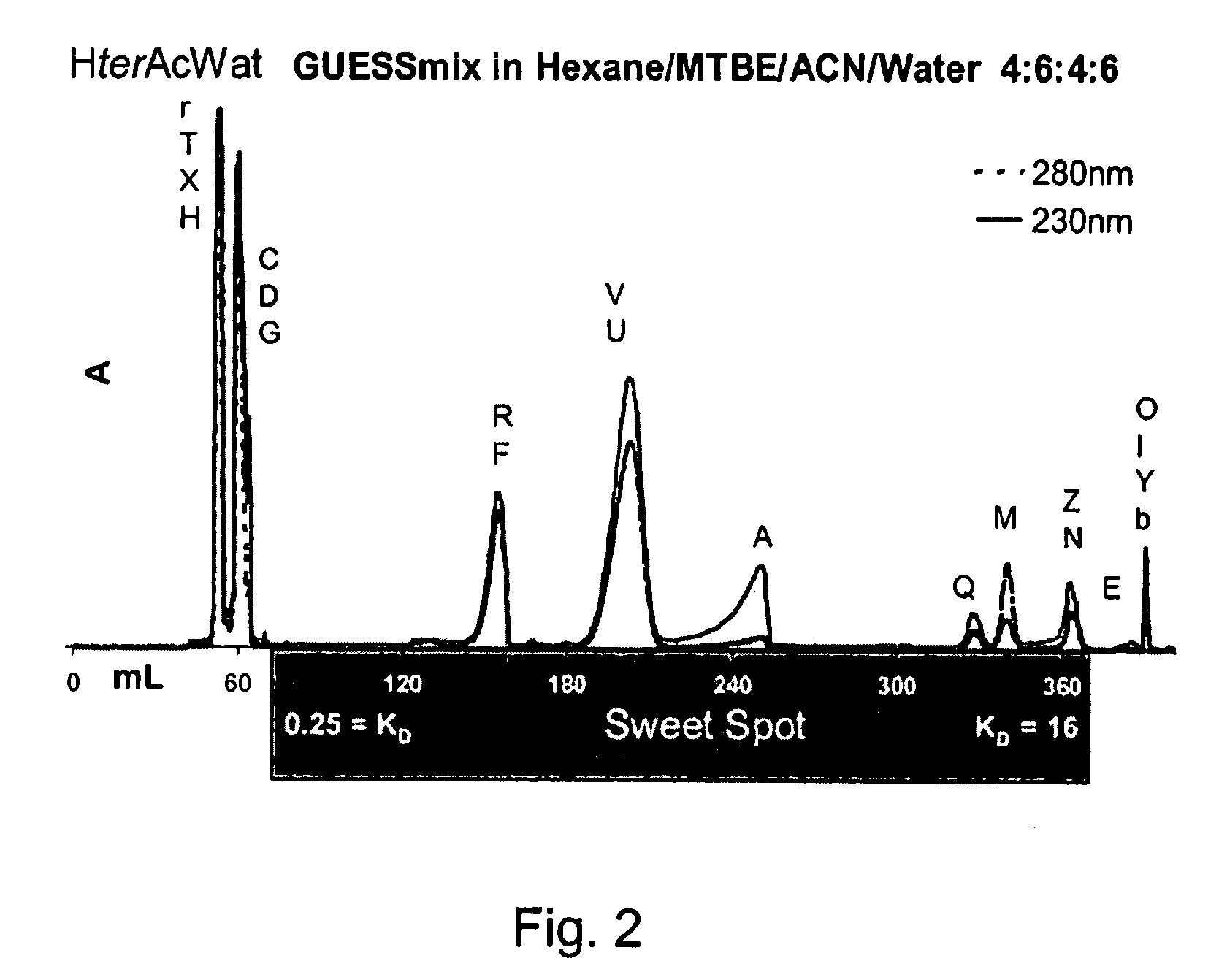
ActiveUS20080127720A1Easy to separateComponent separationMaterial analysis by electric/magnetic meansNatural productSystems analysis
Application of a reference mixture of natural products for systematic analysis and comparison of the properties of biphasic solvent systems in counter-current / partition chromatography. Because the reference mixture is comprised of compounds with varying polarities, functional groups, and structural features it provides a rational method for mapping the optimal resolution polarity range of a particular solvent system. The mapping of optimal resolution polarity ranges of solvent systems provided for the description of the overall optimal resolution polarity range of a solvent system family, comprised of the same solvents in different proportions. By comparing the reference mixture performance in the individual members of a solvent system family, the solvent system that best functions as the representative of, or portal to, the solvent system families is determined. Use of the reference mixture also afforded a method to compare the overall optimal resolution polarity ranges of solvent system families. Based on performance of reference mixture chromatograms, the CCC properties of solvent systems, can be compared and their CCC potential examined. The methods of the invention employing the reference mixture provides was used to identify a quaternary solvent system, hexane / t-butylmethylether / acetonitrile / water (HterAcWat), which was found to be useful for CCC of mixtures containing natural products.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
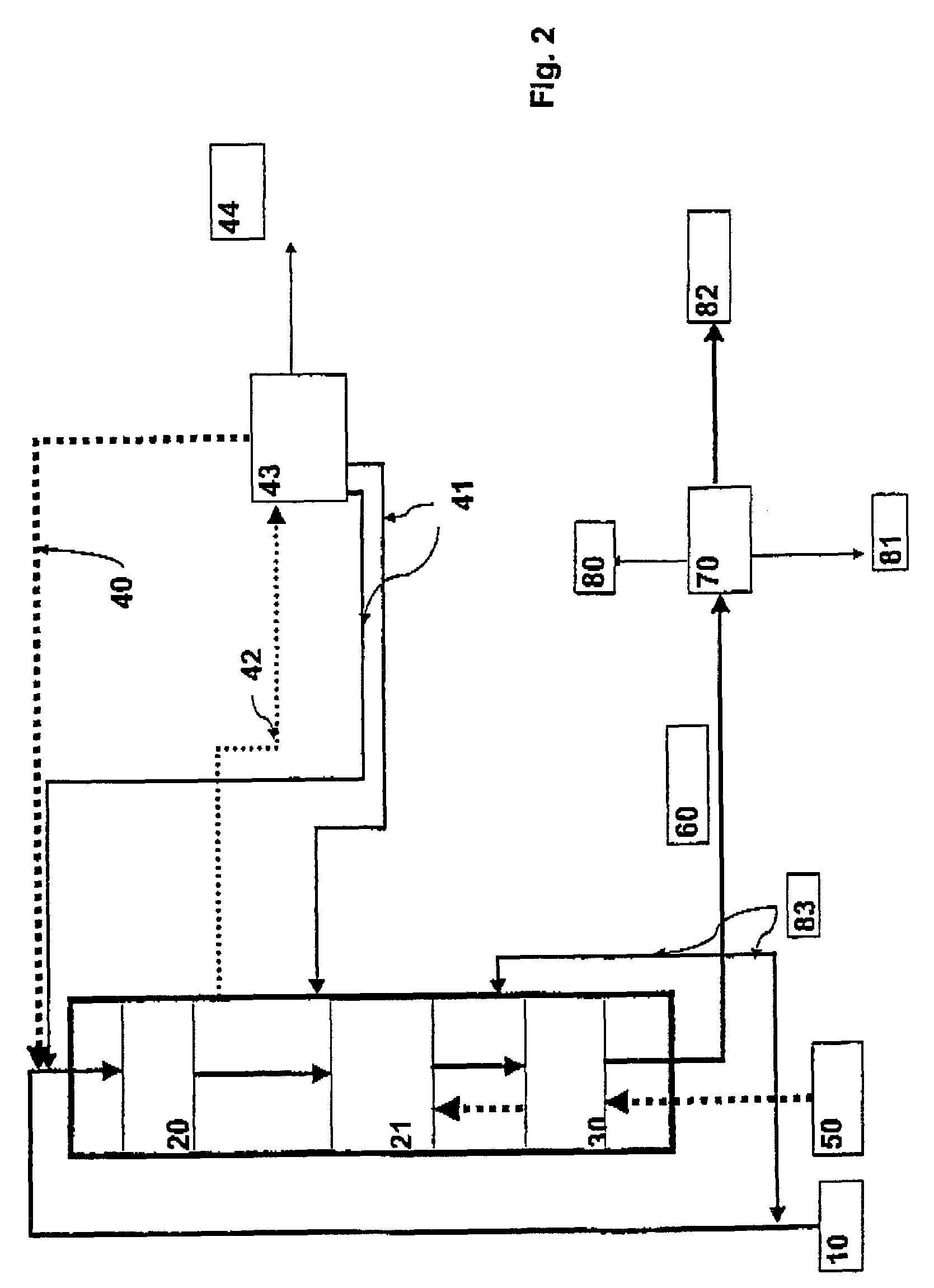
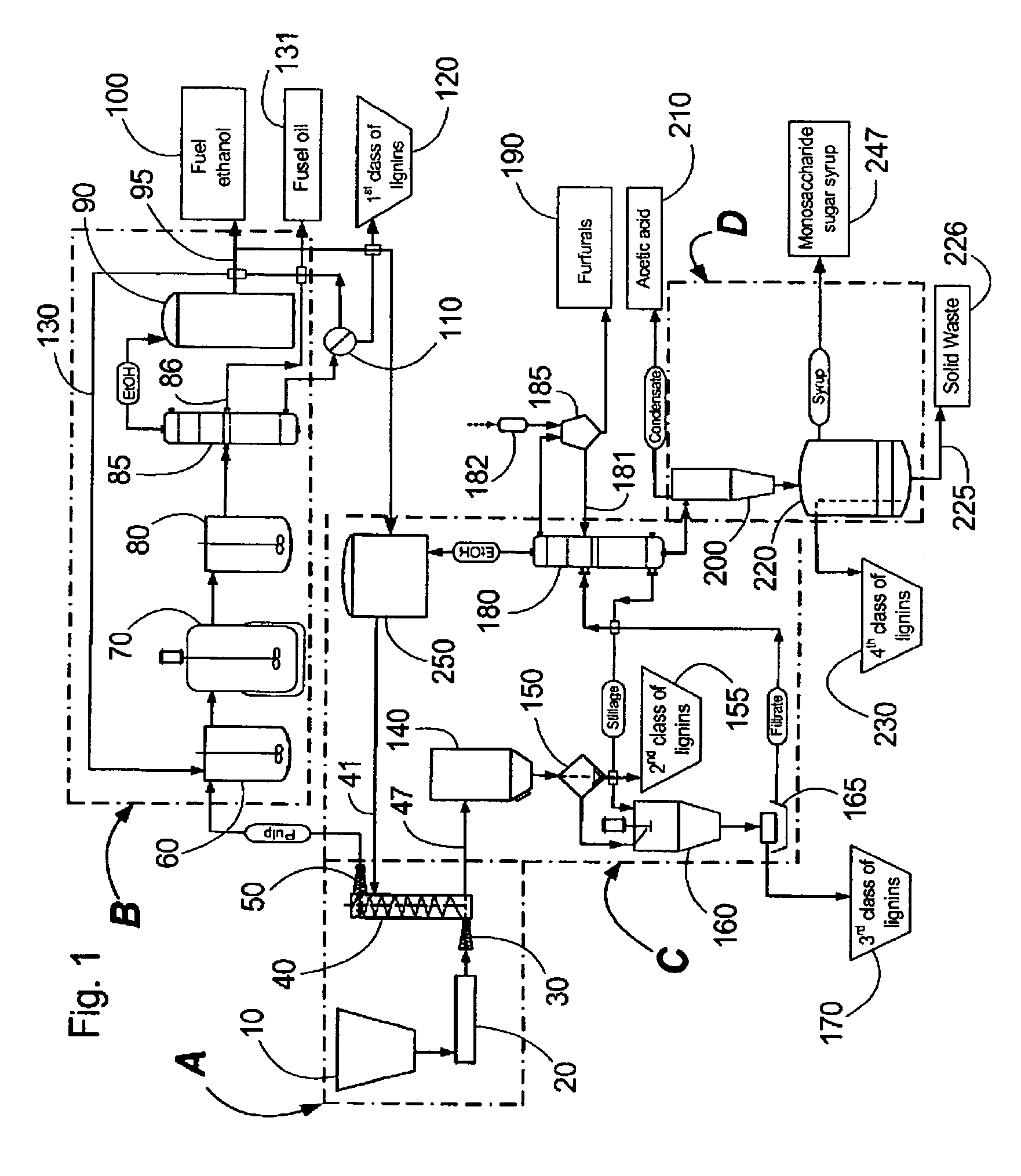
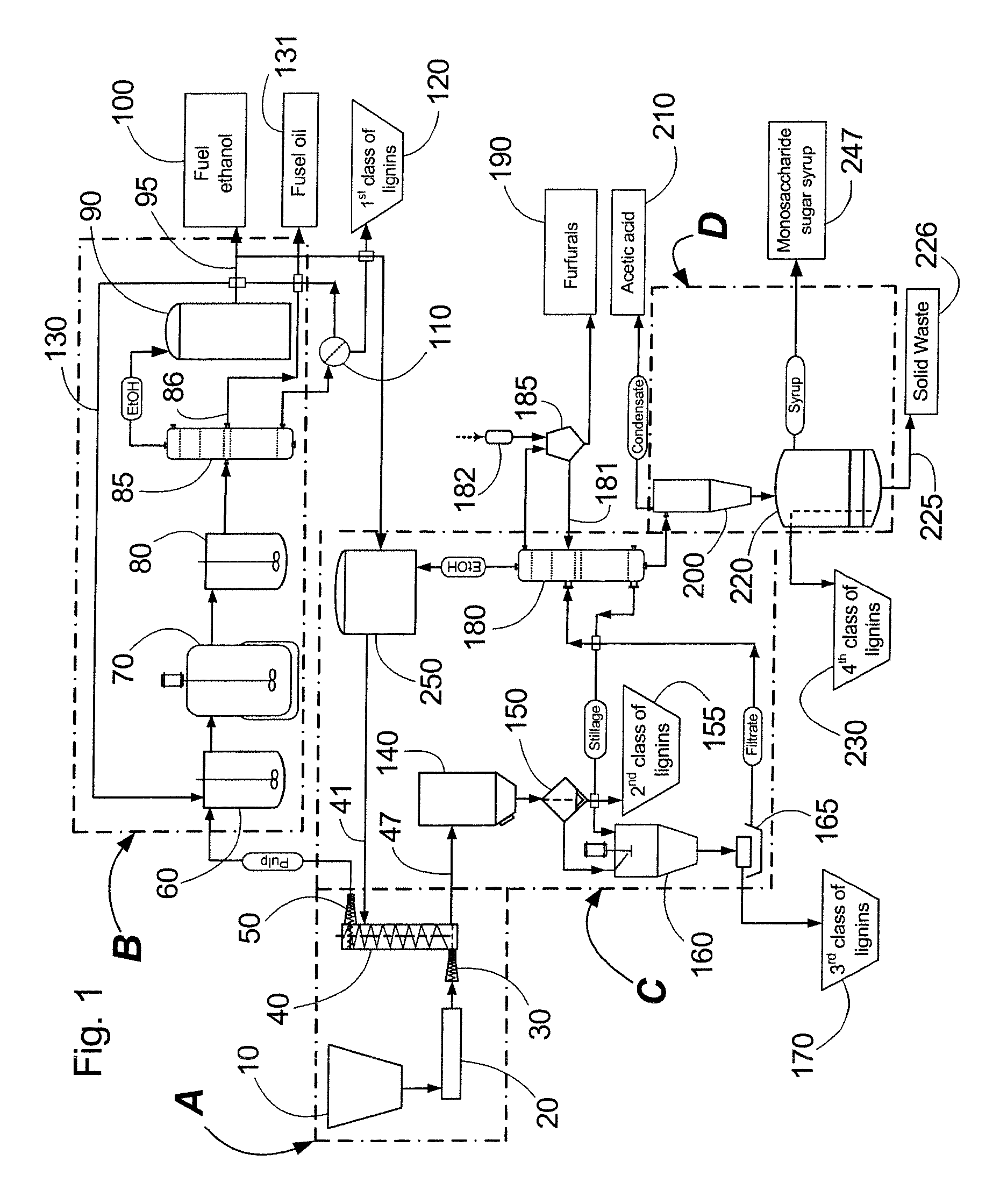
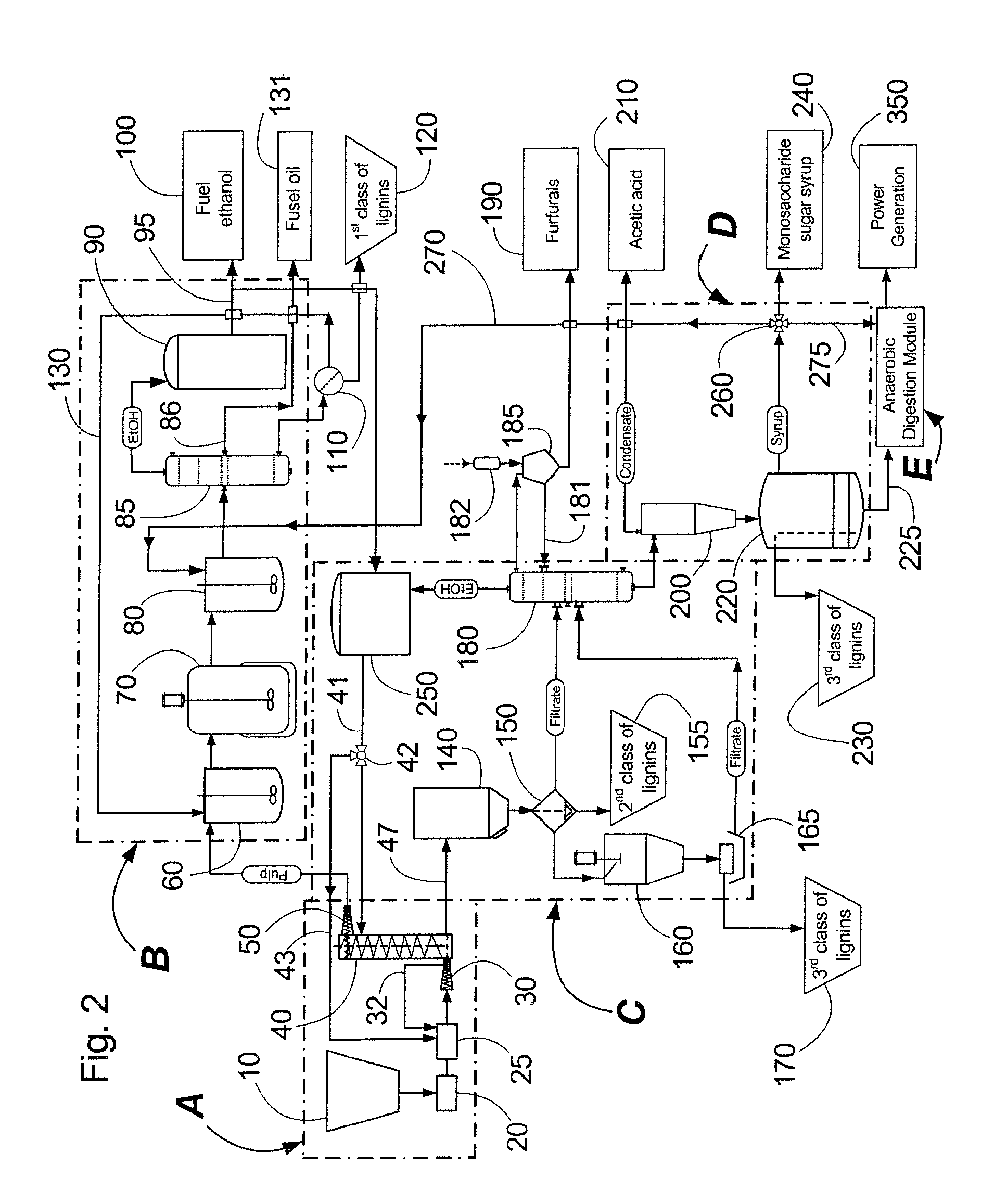
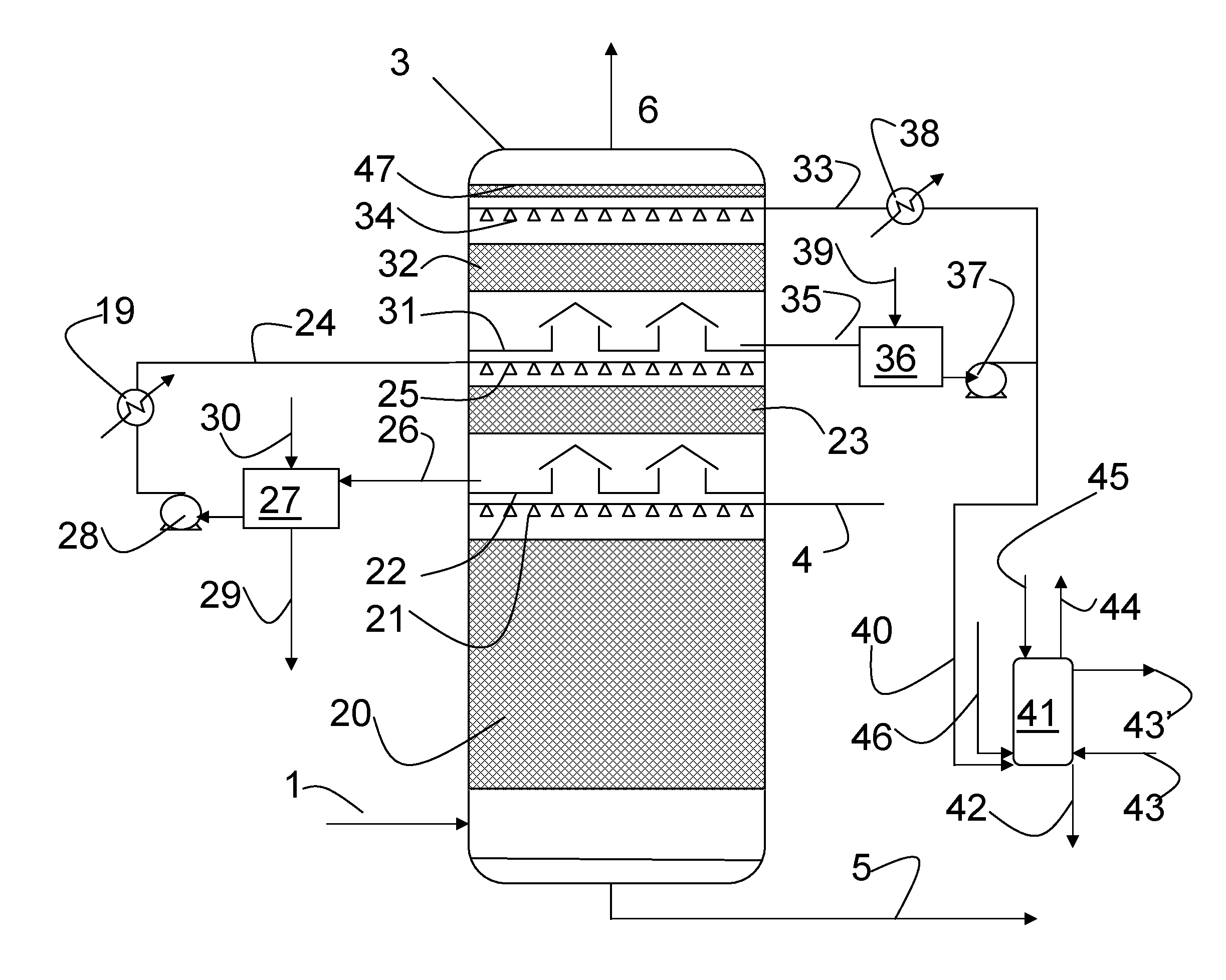
Continuous counter-current organosolv processing of lignocellulosic feedstocks
InactiveUS20080299628A1Facilitate and enhance of fermentation and efficiencyFacilitate and enhance rateBioreactor/fermenter combinationsPressurized chemical processFractionationOrganosolv
A modular process for organosolv fractionation of lignocellulosic feedstocks into component parts and further processing of said component parts into at least fuel-grade ethanol and four classes of lignin derivatives. The modular process comprises a first processing module configured for physico-chemically digesting lignocellulosic feedstocks with an organic solvent thereby producing a cellulosic solids fraction and a liquid fraction, a second processing module configured for producing at least a fuel-grade ethanol and a first class of novel lignin derivatives from the cellulosic solids fraction, a third processing module configured for separating a second class and a third class of lignin derivatives from the liquid fraction and further processing the liquid fraction to produce a distillate and a stillage, a fourth processing module configured for separating a fourth class of lignin derivatives from the stillage and further processing the stillage to produce a sugar syrup.
Owner:LIGNOL INNOVATIONS
Method and plant for amine emission control
ActiveUS20110308389A1Eliminating substantially reducing emissionLow vapor pressureLiquid degasificationDispersed particle separationFlue gasAqueous solution
A method for eliminating or substantially reducing emission of amines (amineslip) and alkaline degradation products to the atmosphere from a plant for CO2 capture from a flue gas, where the CO2 is captured by counter-current flow to an absorbent in an absorption zone, the absorbent comprising an aqueous solution of one or more amine(s), to give a CO2 lean flue gas that is released into the surroundings, and a CO2 rich absorbent that is regenerated in a regeneration column to give a CO2 rich gas that is treated further, and regenerated absorbent that is recycled to the absorption zone, wherein the CO2 lean flue gas is washed with an acidic aqueous solution to remove or substantially reduce the amount of amine(s) and alkaline degradation products thereof in the gas, is described.
Owner:AKER CARBON CAPTURE NORWAY AS
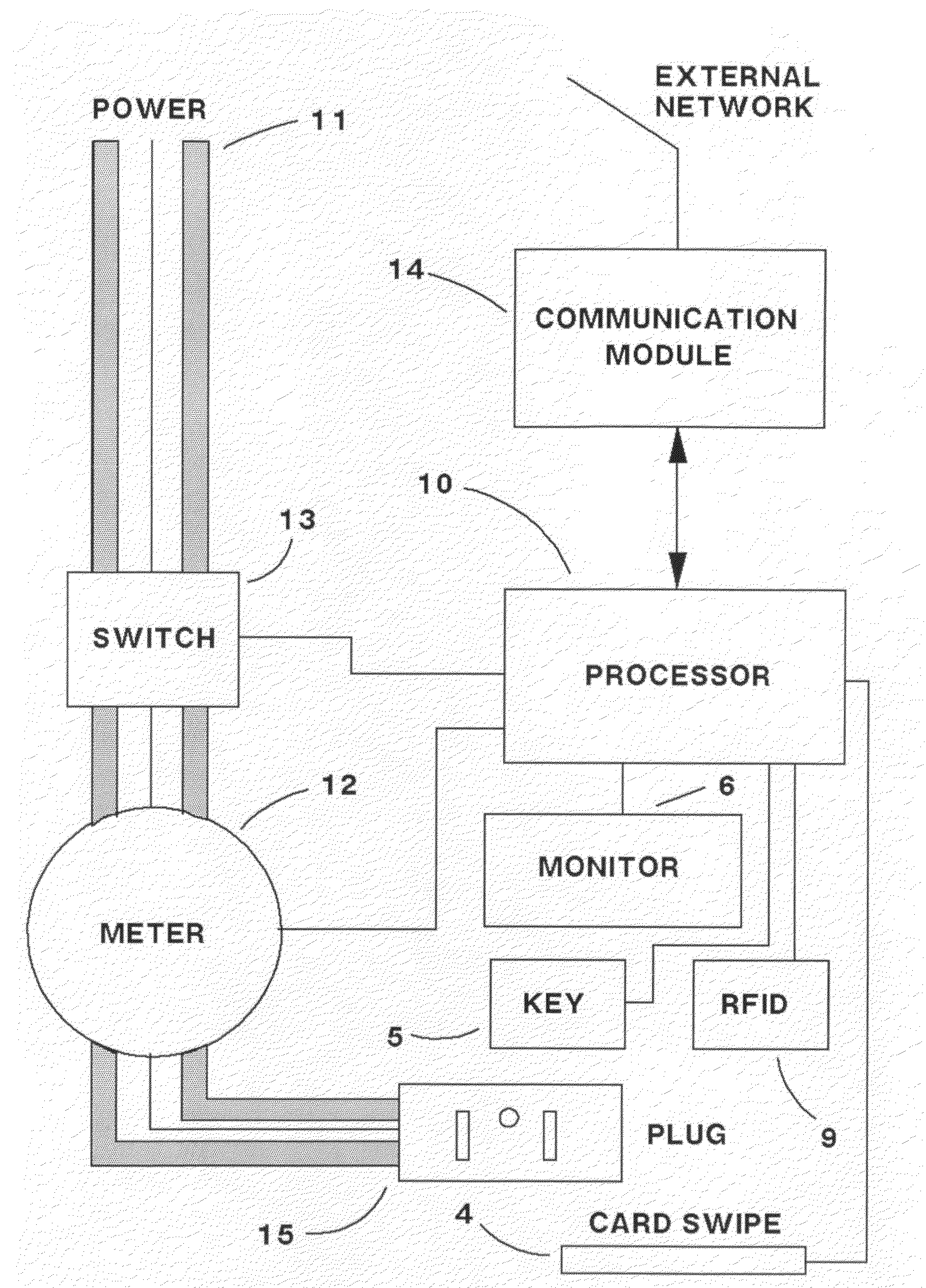
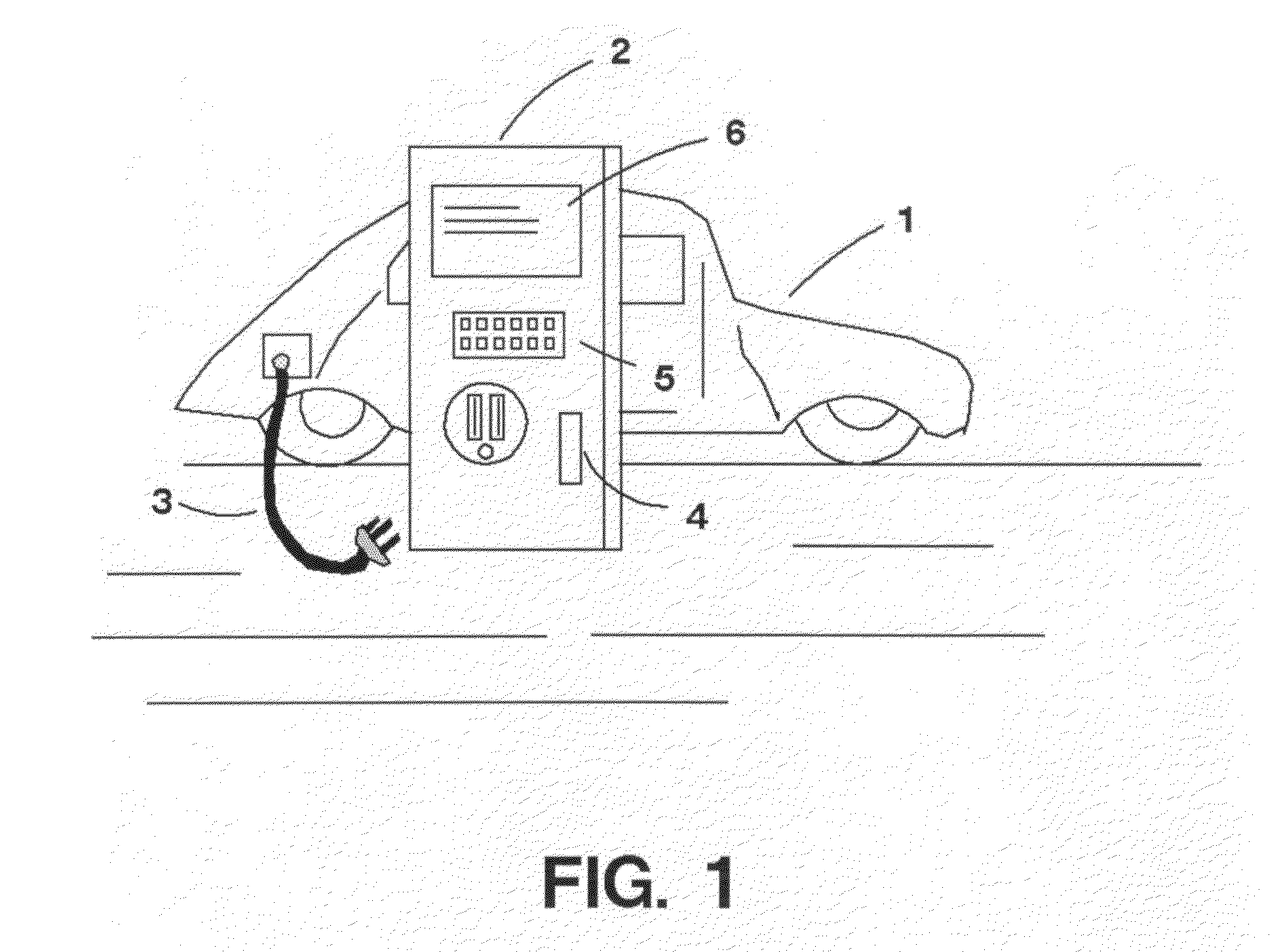
Recharge electrical apparatus and method for electric vehicles
ActiveUS20100065627A1Less electricityDelay beginBatteries circuit arrangementsCharging stationsCredit cardElectrical battery
An apparatus and method for providing a public or private electric charging station or device. In the case of a public station, a user can approach an outlet station in an electric car, plug the car into the station similar to using a gasoline pump, present a credit card to the system, and be provided with either standard line voltage of sufficient wattage to charge the battery of an electric or hybrid vehicle. After the vehicle is charged, the credit card debited for the correct amount for the power dispensed, or other arrangements to pay can be accommodated such as debiting a prepaid account. Optionally, the user can also inject reverse-flow power back into the grid for credit and receive credit for the power injected. The present invention can take advantage of dynamic power rates and cause an optional delay in charging until a favorable power rate. Several users can share the same private or semi-private station, and can identify themselves to the station by key access, card swipe, biometrically, or by a card containing an RFID chip that contains their identity. Service provided could then be billed to a prepaid or subscriber account. A central computer can manage several remote power dispensing stations over a network.
Owner:LIBERTY PLUG INS INC
Mobile communication terminal with multi-input device and method of using the same
InactiveUS7397467B2Input/output for user-computer interactionEmergency actuatorsMulti inputComputer module
A radiation module capable of resisting reverse flow of hot fluid includes a fan, a radiator and a retaining tool. The fan provides an inlet and an outlet. The radiator is connected to the outlet of the fan. The retaining tool secures the radiator to a heat generation part. At least a baffle is provided in the radiation module. The fluid enters the fan via the inlet and flows toward the radiator via the outlet to cool the heat-generating component and the hot fluid moving outward the radiator is resisted entering the inlet with the baffle part so as to enhance heat dissipation efficiency.
Owner:SAMSUNG ELECTRONICS CO LTD
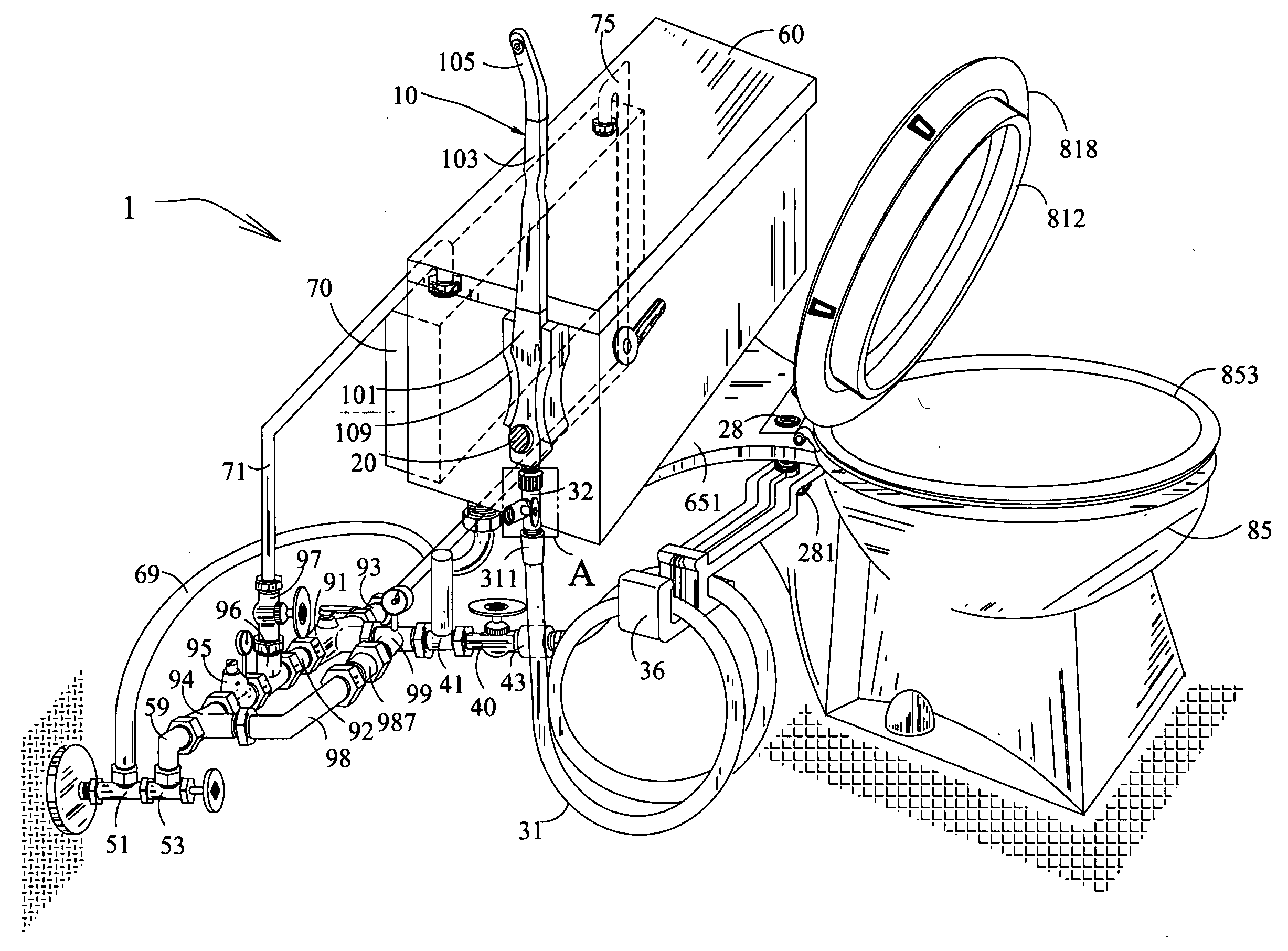
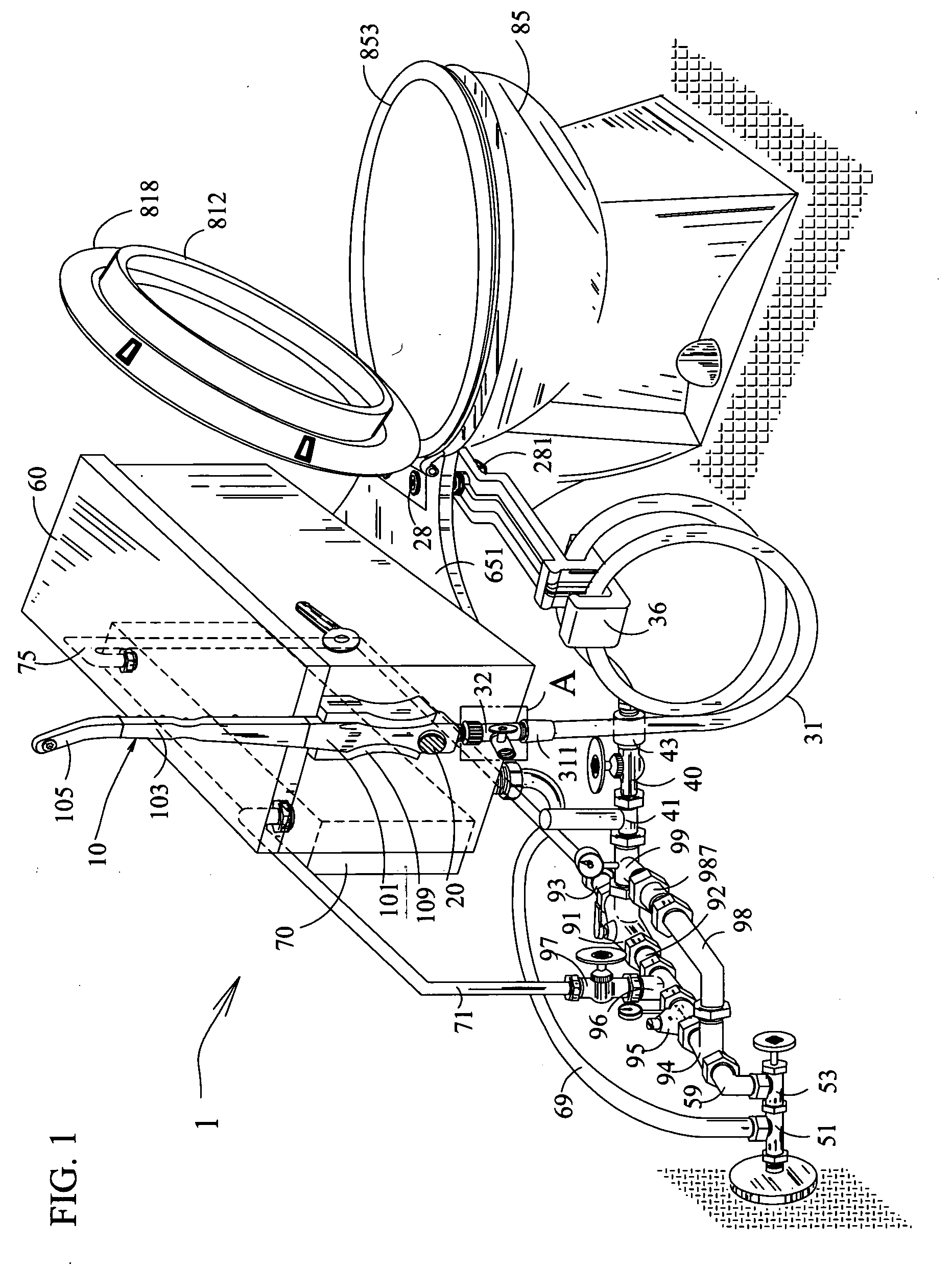
Toilet system attached a multi-purpose hand held water sprayer
InactiveUS20050120471A1More featureEasy to attachBathroom coversLavatory sanitorySprayerWater source
The invention relates to the new features for the earlier applications of toilet system attached a multi-purpose hand held water sprayer affording personal and environmental hygiene. The sprayer that delivers tempered water includes the lock elements on the components to secure connection, various types of spray tip cap for the use of general and medical purposes, an injection apparatus to supply external fluid, a fortified flexible hose, a water hammer arrester or air chamber to reduce water pressure shock, a water heater, a valve to control water flow, a mixing valve, check valves for the mixing valve to stop in reverse flow, a water pressure regulator with a pressure meter, a return line with a check valve for guarding against hot water spike, a thermometer at the outlet, a holder for placement of the sprayer, and various types of valves and sources for the hot and cold water supply. The new features also include the fortified flexible hose having the rollup attachment of middle layers over the flange for reinforcement.
Owner:LIM HOWARD TAK SU
Removal of H2S and CO2 from a hydrocarbon fluid stream
InactiveUS6881389B2Easy to operateLess-costly to buildUsing liquid separation agentHydrogen sulfidesEnergy recoveryImpurity
A system for removal of Hydrogen Sulfide (H2S) and / or Carbon Dioxide (CO2) from natural gas via absorption and disassociation utilizing a sea water contact system. In the preferred embodiment of the present invention, a series of counter current scrubber stages is provided, each configured to remove via absorption / disassociation a portion of the impurities, each stage having less pressure than the predecessor, each stage redirecting the purified gas to the preceding stage, until the contaminant level in the hydrocarbon gas stream has been reduced to an acceptable level. The hydrogen sulfide / carbon dioxide contaminants are thereby sequestered in the sea water utilized in the scrubber, which sea water may be further processed and / or re-introduced into the deep of a body of water, where the contaminants will remain isolated for hundreds of years. The present invention further contemplates and energy recovery system for greatly enhancing the efficiency of the system. Accordingly, the present invention provides an efficient and cost effective method for the purification of natural gas on an offshore platform.
Owner:EDG
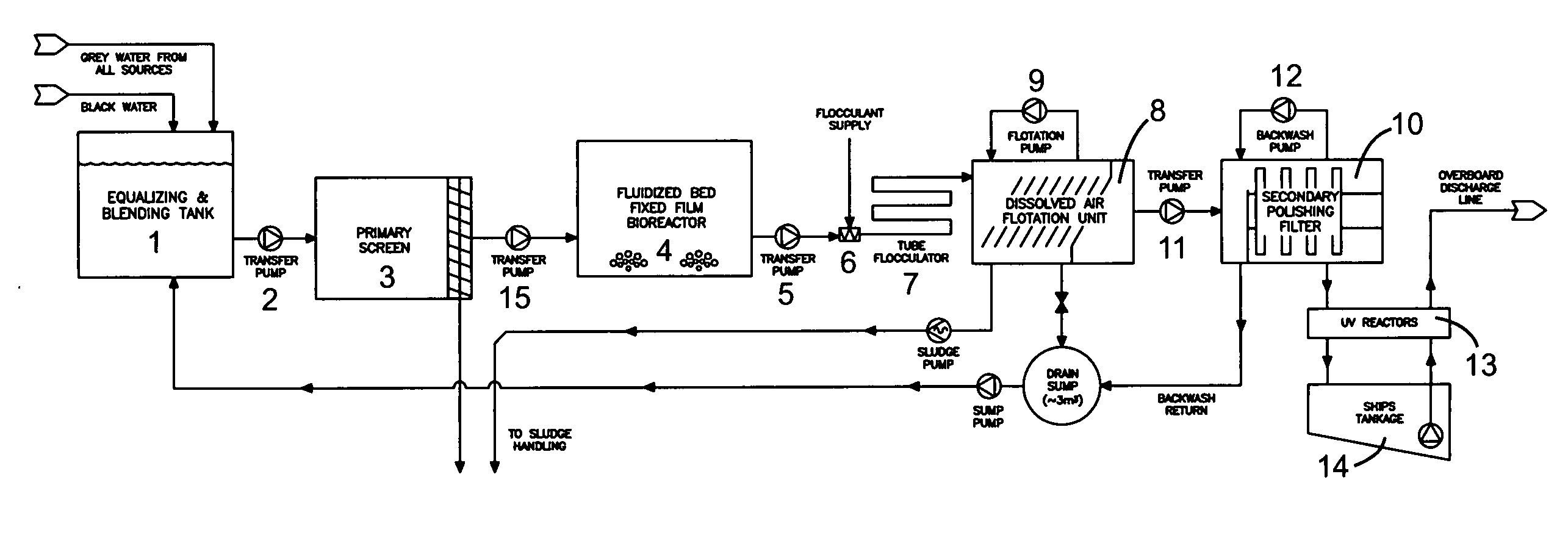
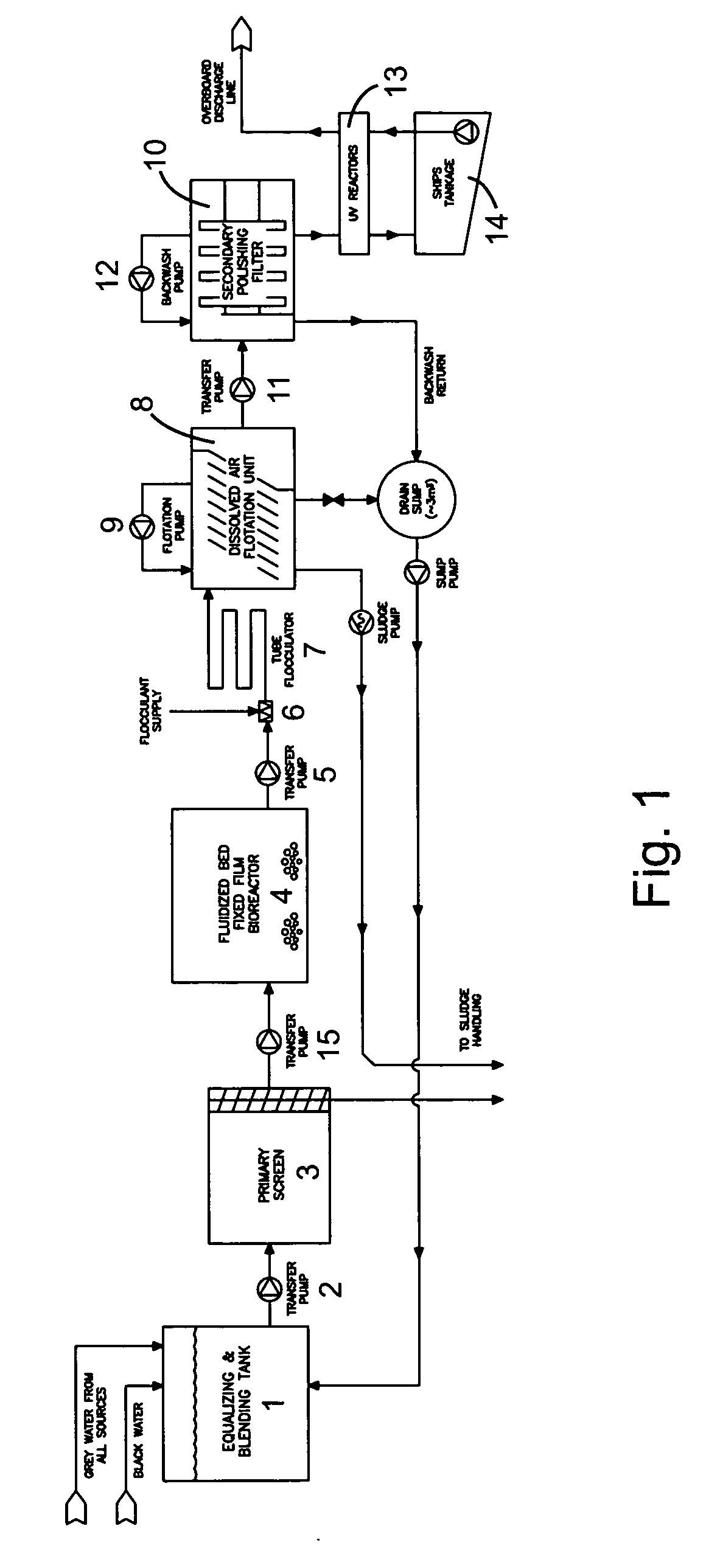
Wastewater treatment system for a marine vessel
InactiveUS20070114182A1Reduce movementAvoid erratic movementTreatment using aerobic processesTreatment involving filtrationCompound (substance)Membrane bioreactor
A wastewater treatment system for use on a marine vessel comprising an aerobic fixed film biological reactor, a tubular flocculator and a dissolved air flotation (DAF) unit. The process units desirably include means for reducing erratic movement of the wastewater due to sea-induced movement of the marine vessel. The DAF unit includes a plurality of inclined baffles arranged to create a plurality of parallel inverted U-shaped flow paths that effect combined co-current and counter-current flotation separation while reducing erratic movement of the wastewater. The selection of process units and operating conditions advantageously reduces the footprint of the wastewater treatment system and reduces cost and complexity associated with the handling of wastewater treatment chemicals.
Owner:HYDROXYL SYST
Graphite rotary tube furnace
InactiveUS6042370AMinimizing radiation heat lossStable temperatureRotary drum furnacesCharge supportsRadiative heat lossRadiant heat
A rotary tube furnace suitable for operation in controlled atmospheres at temperatures in the range of 1500 DEG to 2800 DEG comprises a generally horizontal rotatable graphite tube slidably supported on water-cooled split ring graphite bearings. The graphite tube is rotated by means of a stainless steel drive plate and is contained within a flexible atmospheric sealing assembly and enclosure for the containment of a selected atmosphere around and within the tube and allows for the co-current or counter-current flow of gas during operation. Radiation baffles in the interior of the graphite tube inhibit radiant heat loss at the ends of the tube. The graphite tube may be constructed in two or more sections having threaded ends for ease of installation as well as removal or replacement for maintenance purposes. A heating section of the tube is heated by a plurality of graphite electrical heating elements contained within an insulated heating chamber.
Owner:HARPER INT CORP
Method for preparing stevia whole stevioside and stevia whole flavone at the same time
ActiveCN101062077AImprove mildew resistanceConducive to food preservationSugar derivativesMetabolism disorderLuteolinGlucoside
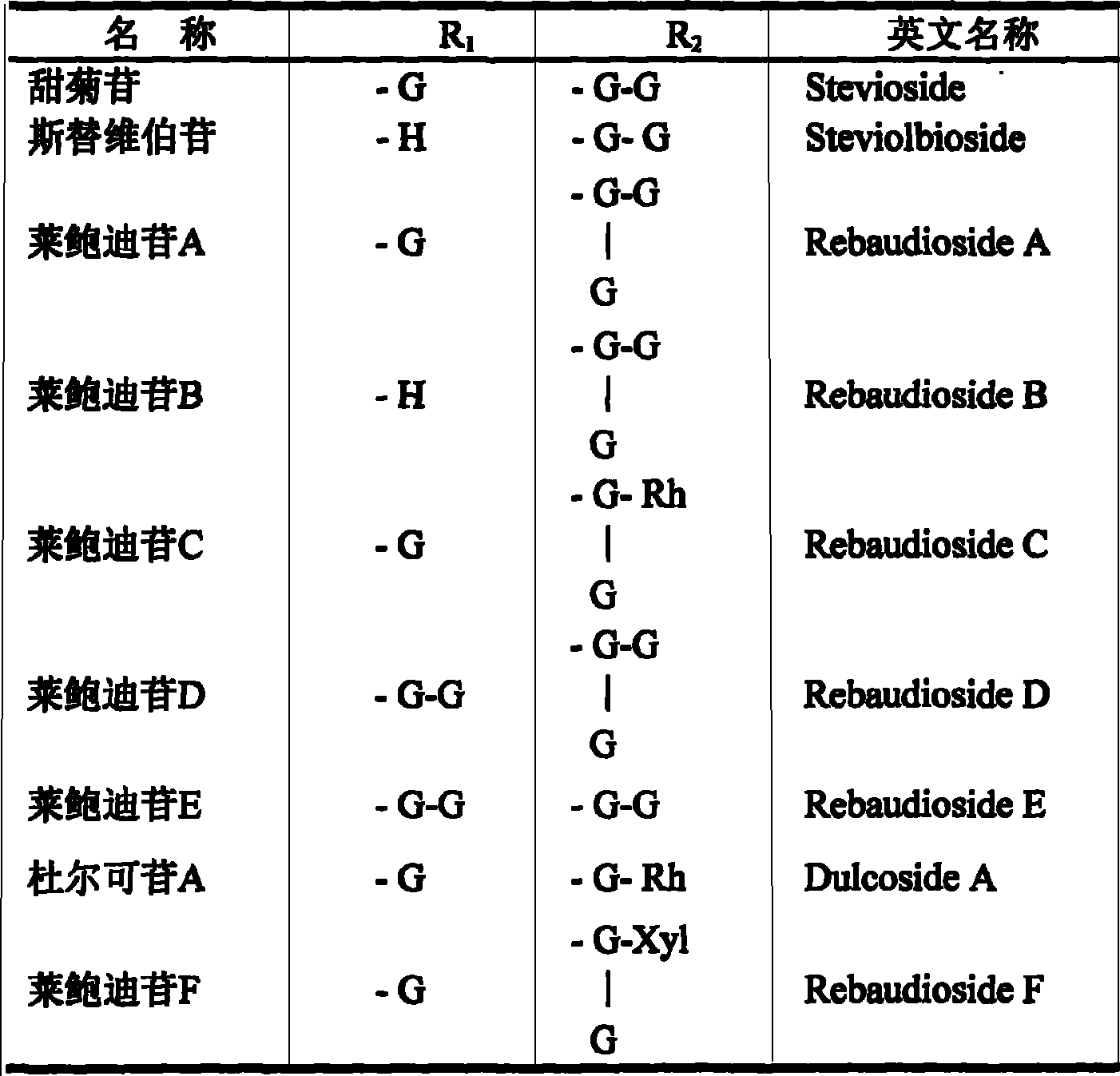
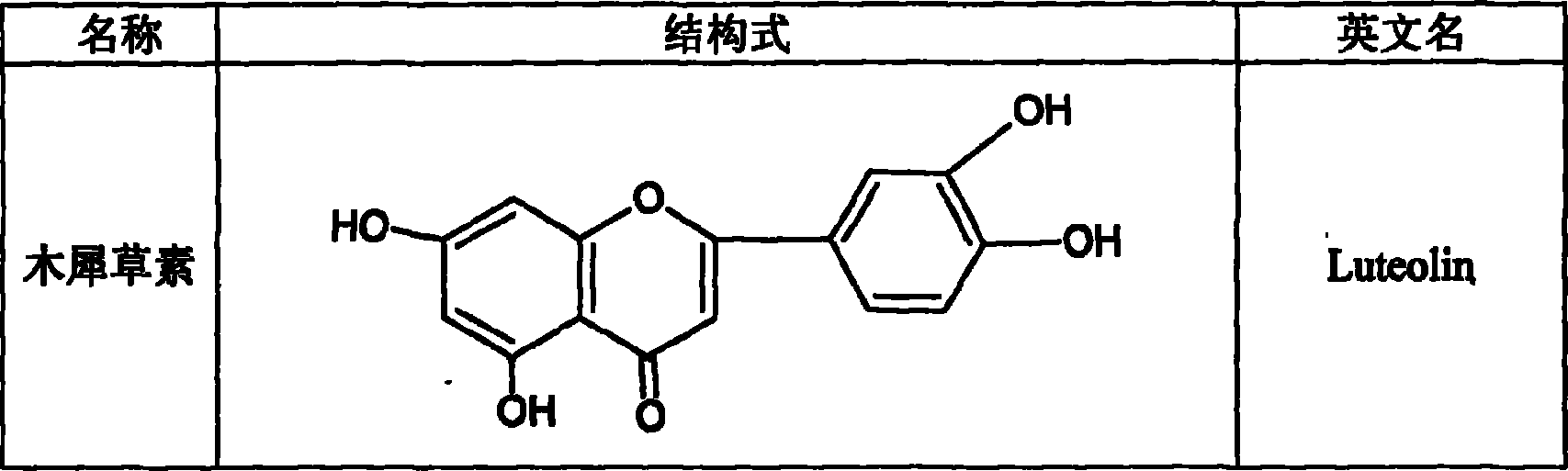
The invention discloses a preparing method of total sweet chrysanthemum glycosides and total chromocor in sweet leaf chrysanthemum, which is characterized by the following: comprising sweet chrysanthemum glycosides, stevi primary glycosides, labroid glycosides A, B, C, D, E, F, duacl glycosides A and so on in total sweet chrysanthemum glycosides; comprising cyanidenon, meletin, cyanidenon-7-0-beta-D glycosides, celery element-7-0-beta-D-glycosides, quercetin, meletin-3-0-beta-D-arabinoside, meletin-3-0-[4-0-trans-coffe acyl-alpha-L-isodulcitol-(1-6)-beta-D-arabinoside] and so on; choosing one or several methods from solvent extraction, solvent extraction process, macroreticular absorption resin method, column chromatography, supercritical fluid chromatography, liquid-liquid counter-current partition chromatography and so on; extracting the total chromocor; setting content of sweet chrysanthemum glycosides element in total sweet chrysanthemum glycosides at 5-100%; counting 5-100% of all sweet chrysanthemum glycosides content with sweet chrysanthemum glycosides and labroid glycosides; counting 5-100% of chromocor element in sweet leaf chrysanthemum total chromocor; counting 5-100% of all total chromocor content with cyanidenon-7-0-beta-D glycosides, quercetin and meletin-3-0-[4-0-trans-coffe acyl-alpha-L-isodulcitol-(1-6)-beta-D-arabinoside].
Owner:石任兵 +1
Process for cleaning gases form gasification units
InactiveUS20060233687A1Reduce environmental impactUsing liquid separation agentCombustible gas catalytic treatmentHydrogenHalogen
A process for cleaning gases from a gasification unit comprising the steps of: gasifying a fuel to raw synthesis gas comprising carbon monoxide and steam in a gasification reactor in the presence of a calcium-containing compound and steam removing halogen compounds from the raw synthesis gas catalytic conversion of carbon monoxide and steam in the raw synthesis gas to carbon dioxide and hydrogen in a water gas shift conversion step to provide a shifted gas contacting the shifted gas with a solid sulphur sorbent regenerating the solid sulphur sorbent by passing a stream of steam through the solid sulphur sorbent counter current to the flow direction of the shifted gas to provide a hydrogen sulphide-containing stream of steam transferring the hydrogen sulphide-containing stream of steam from the solid sulphur sorbent directly to the gasification reactor removing the cleaned shifted gas from the solid sulphur sorbent.
Owner:HALDOR TOPSOE AS
Process of removing and recovering CO2 from fume
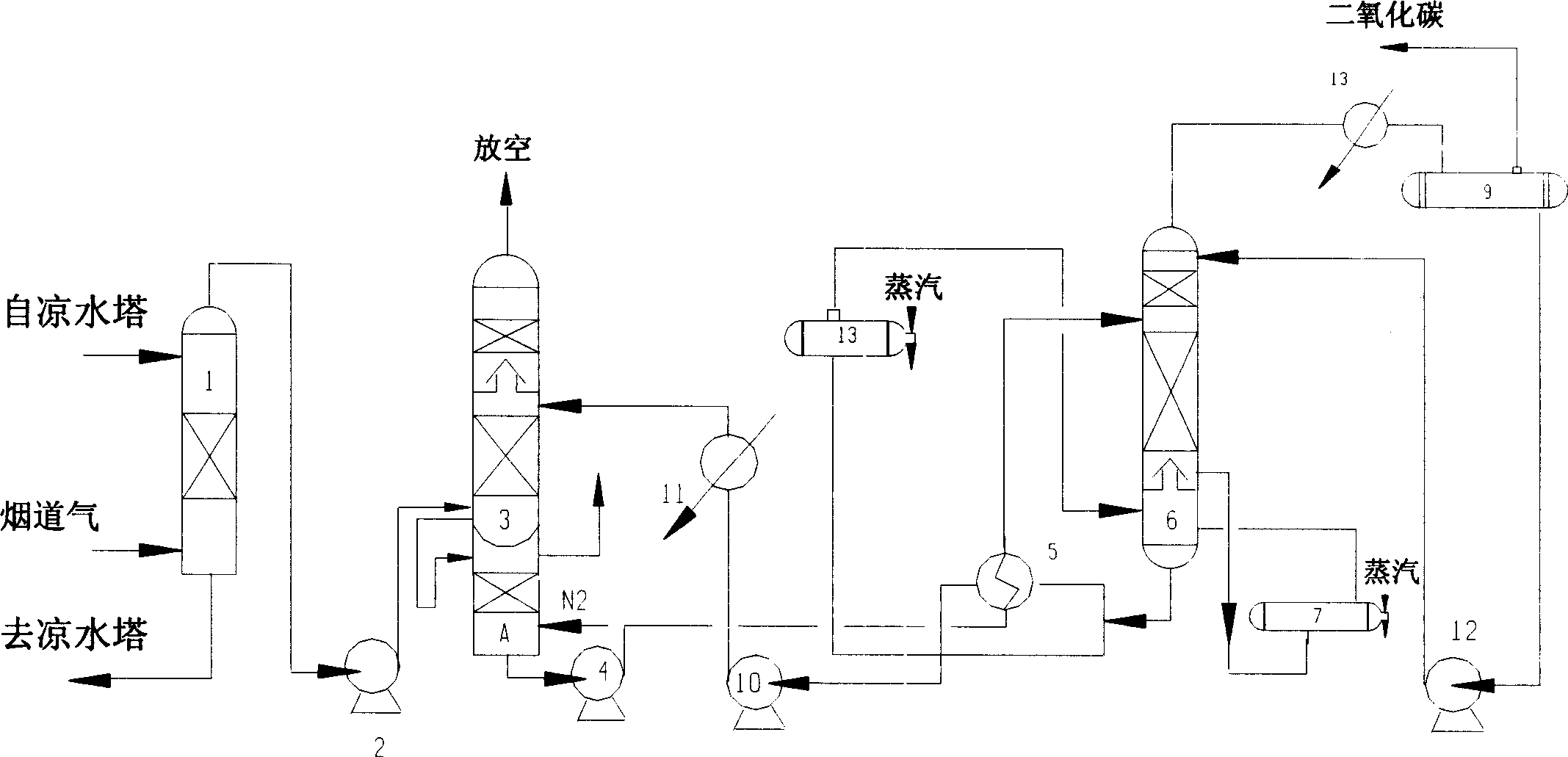
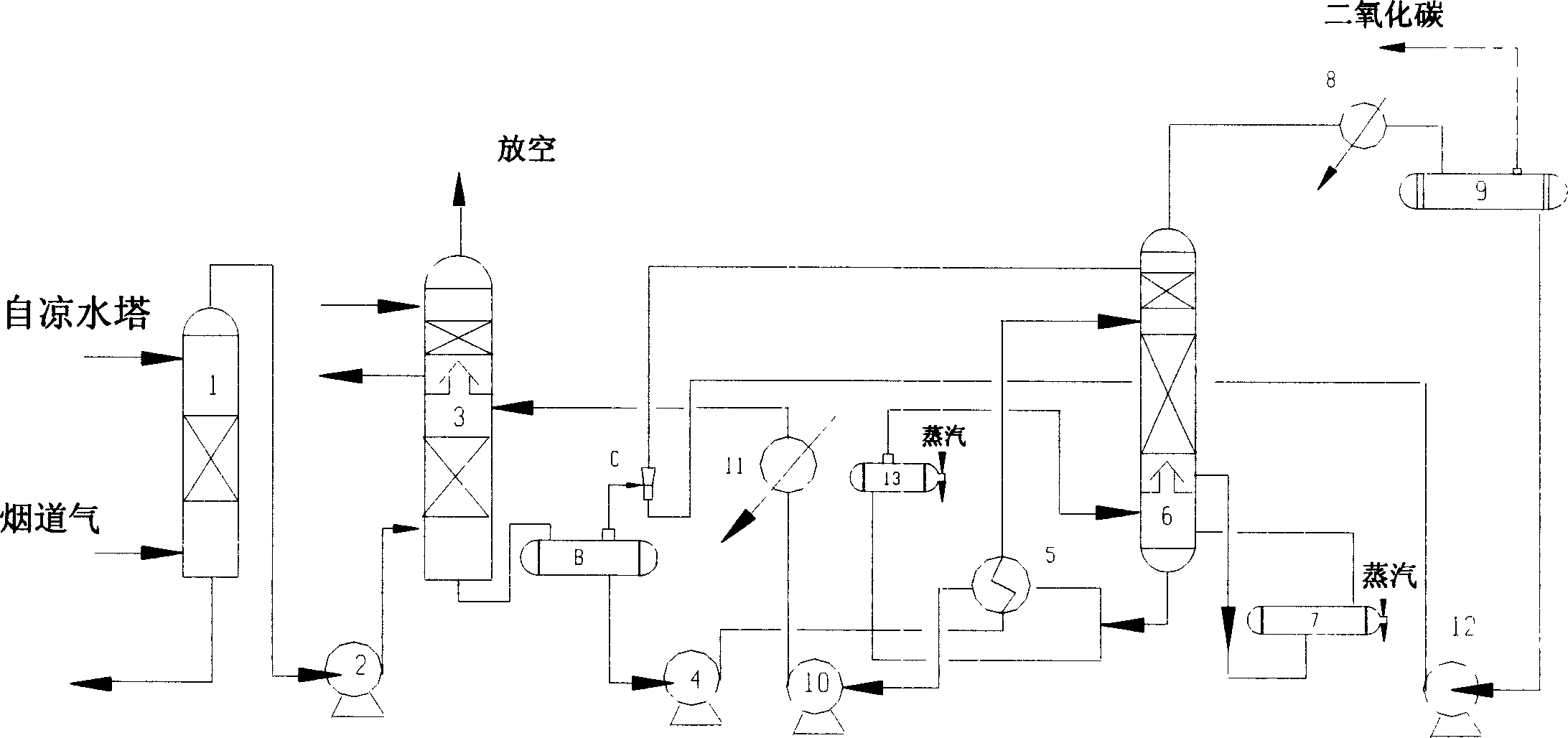
The present invention is process of removing and recovering CO2 from fume. The process includes dedusting and cooling fume in a water washing tower, counter current contacting fume with absorbent in an absorption tower for absorbing CO2 from the fume, making the absorbent with CO2 absorbed enter a flash tower or a stripping tower to separate O2, SO2, NOx, CO and other impurity from the rich liquid, heat exchange of the rich liquid in a heat exchanger, making the rich liquid enter a regeneration tower to obtain high purity CO2 and regenerate absorbent in the tower bottom, heat exchange of the regenerated absorbent and returning the regenerated absorbent to the absorption tower for reuse.
Owner:HUAXI CHEM INST CHENGDU CITY
Method and apparatus for surveying a borehole with a rotating sensor package
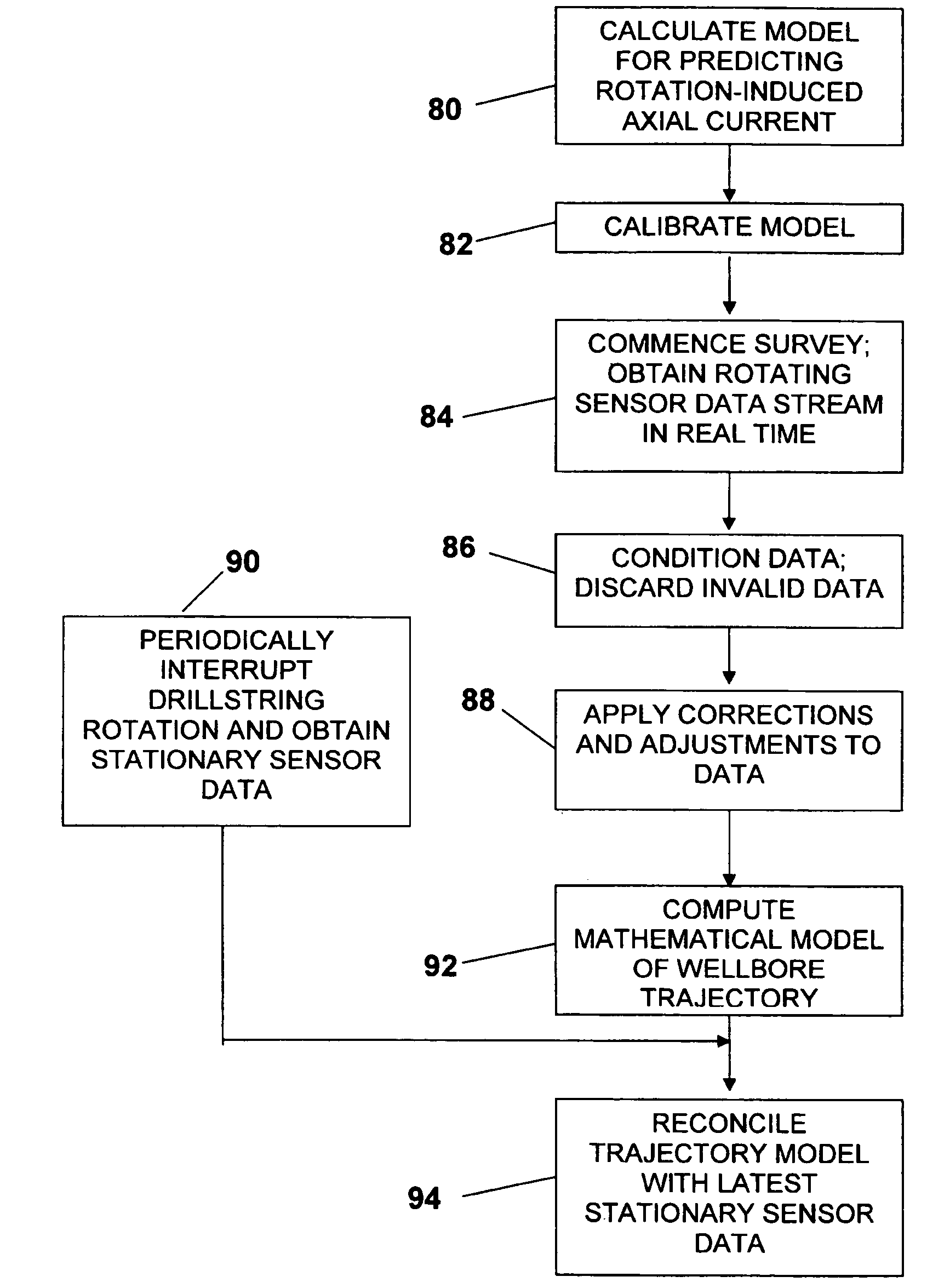
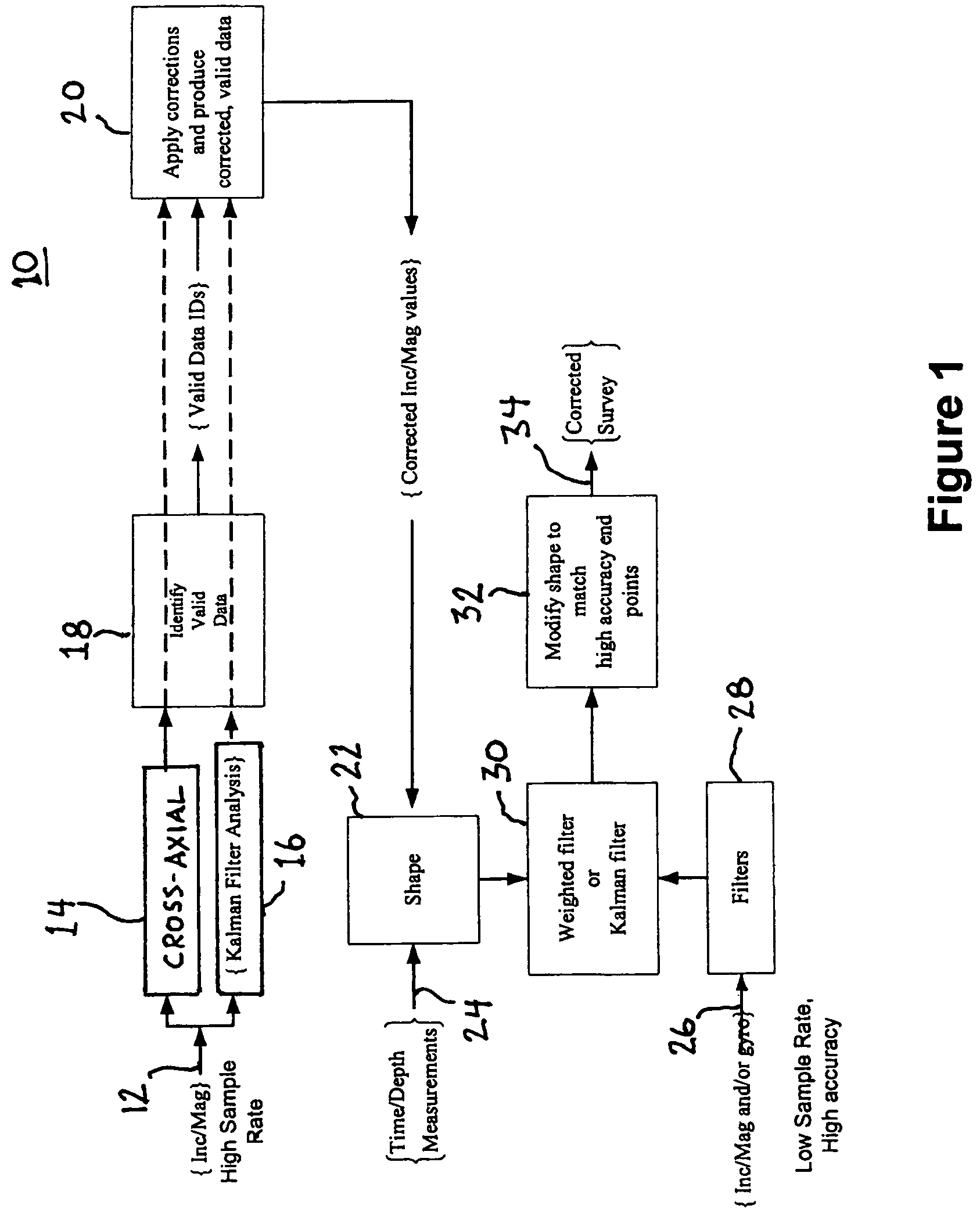
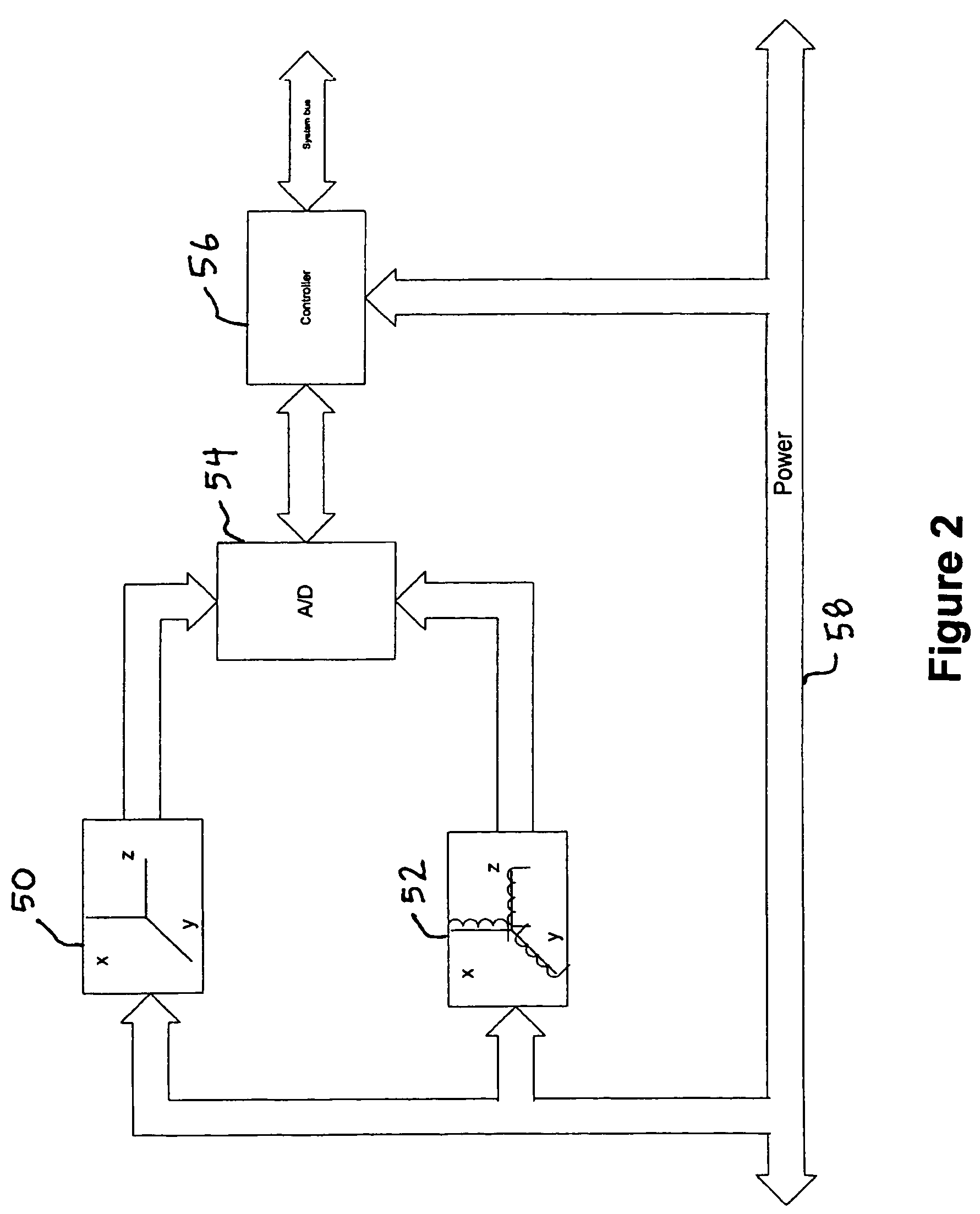
ActiveUS7650269B2Avoid it happening againEliminate currentComputation using non-denominational number representationSeismology for water-loggingSensor arrayConductive materials
A method and apparatus for surveying a borehole using a rotating sensor package. A sensor tool preferably including a magnetometer sensor array is disposed in the bottom hole assembly of a drillstring. Conditioning circuitry in the sensor tool processes the sensor readings from the sensor array taken while the drillstring is rotating. In one embodiment, the conditioning circuitry includes processing circuitry adapted to adjust the sensor readings to account for an analytically predicted level of axial current induced in the drillstring as a result of its rotation in the Earth's magnetic field. In another embodiment, a current generator is provided to generate a counter-current intended to cancel the analytically predicted level of axial current induced in the drillstring as a result of rotation in the Earth's magnetic field. In another embodiment, insulating members are disposed above and / or below the sensor tool to prevent conduction of rotation-induced current therein. In still another embodiment, the sensor tool is disposed in a drill collar that is composed of a non-conducting material, such that no rotation-induced current is conducted through the sensor tool.
Owner:HALLIBURTON ENERGY SERVICES INC
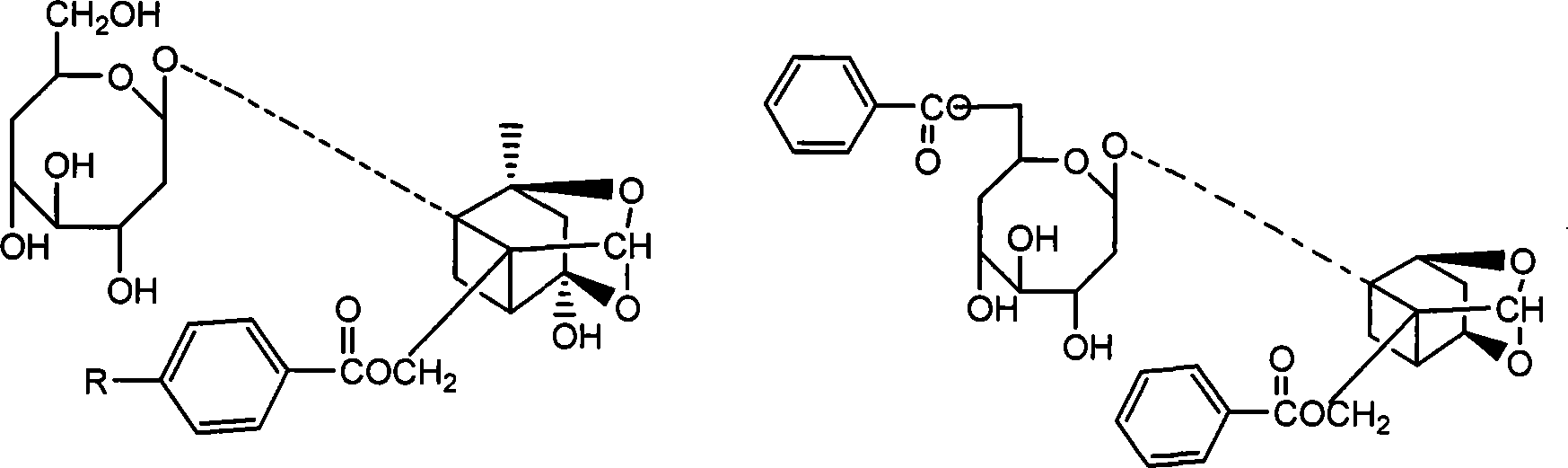
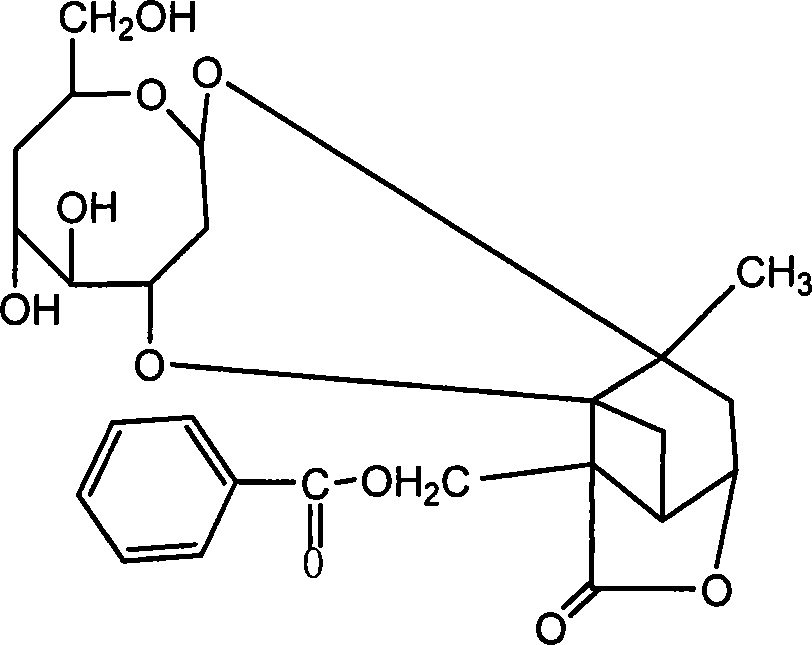
Extract of total glucosides of paeony and the preparing method thereof
The invention discloses a peony total glycosides extract from traditional medicine white peony root or red peony root and preparing method, which is characterized by the following: comprising peony glycosides, white peony glycosides, peony new glycosides, peony inner ester A, B, C, oxidation peony glycosides, benzoyl peony glycosides, derivant and so on; choosing one or several methods from solvent extraction, solvent extraction process, macroreticular absorption resin method, column chromatography, supercritical fluid chromatography, liquid-liquid counter-current partition chromatography and so on; producing extract; setting sum of various terpene glycosides percentage content at 5-100%(w / w); setting content of peony glycosides at 5-100%(w / w).
Owner:石任兵 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com