Patents
Literature
1285results about "Hydrogen sulfides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pre-passivation process for a continuous reforming apparatus, and passivation process for a continuous reforming apparatus during the initial reacation
ActiveUS20100282645A1Reduce operational riskReduce contentThermal non-catalytic crackingPhysical/chemical process catalystsLiquid productReaction temperature
The present invention relates to a pre-passivation process for a continuous reforming apparatus prior to the reaction, or a passivation process for a continuous reforming apparatus during the initial reaction, comprising loading a reforming catalyst into the continuous reforming apparatus, starting the gas circulation and raising the temperature of a reactor, injecting sulfide into the gas at a reactor temperature ranging from 100-650° C., controlling the sulfur amount in the recycle gas within a range of 0.5-100×10−6 L / L so as to passivate the apparatus.The process of the present invention may also comprise the following steps:(1) loading a reforming catalyst into the continuous reforming apparatus, starting the gas circulation and raising the temperature of a reactor, feeding the reforming feedstock into the reaction system when the temperature of the reactor is increased to 300-460° C., introducing sulfide into the reaction system while or after the reforming feedstock is fed, controlling the ratio of the total sulfur amount introduced into the system to the reforming feedstock within the range of 0.5 μg / g-50 μg / g, reducing the content of sulfide introduced into the system when hydrogen sulfide concentration in the recycle gas reaches to 2.0 μL / L˜30 μL / L; and(2) maintaining the reforming reactor at a temperature of 460-490° C., controlling the ratio of the total sulfur amount introduced into the system to the reforming feedstock within the range of 0.2 μg / g-0.5 μg / g, adjusting the amount of the reforming feedstock to the design value of the apparatus, increasing the reforming reaction temperature to 490-545° C. according to the requirements on the octane number of the liquid product, and letting the reforming apparatus run under normal operating conditions.
Owner:CHINA PETROCHEMICAL CORP +1
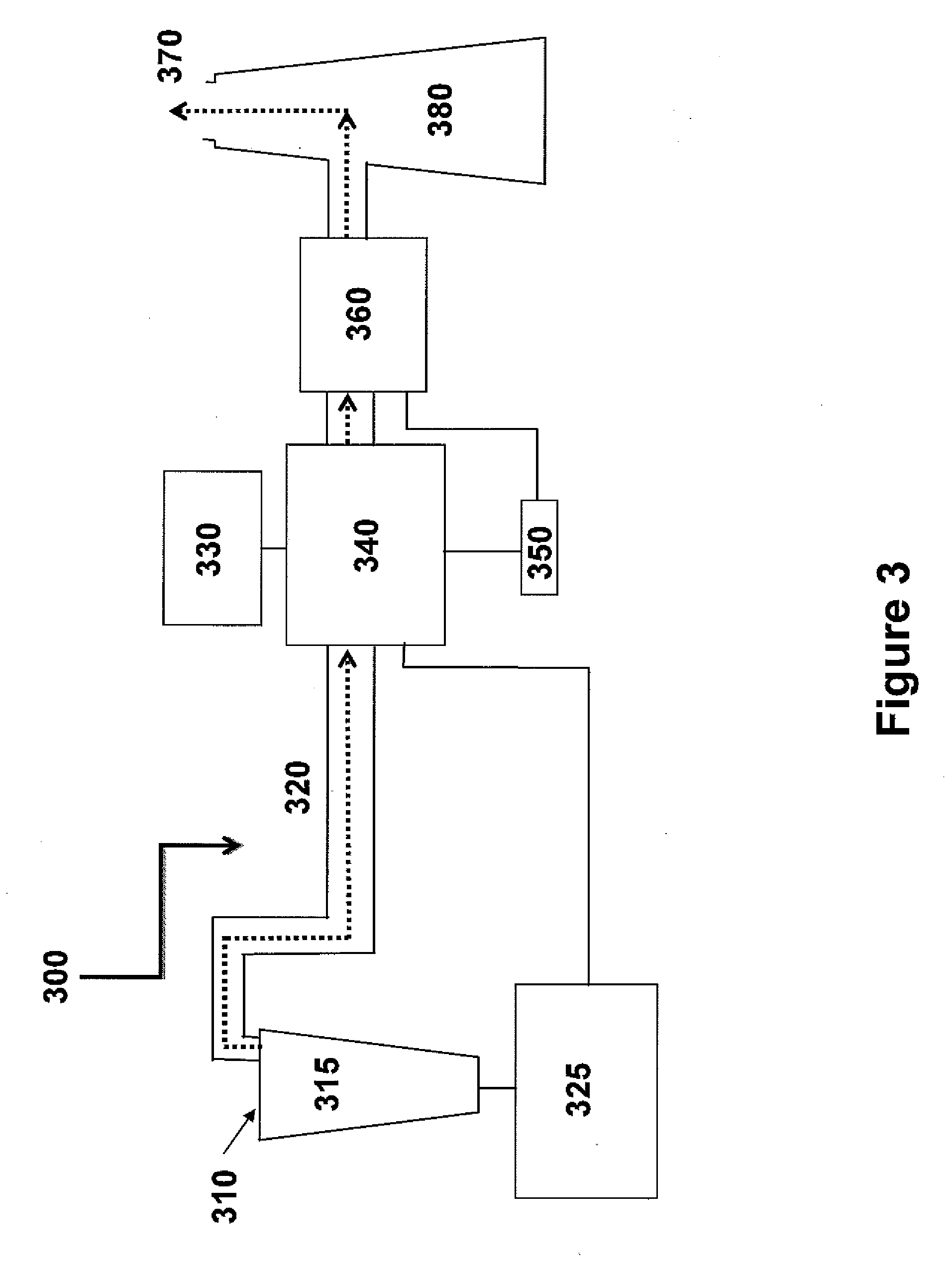
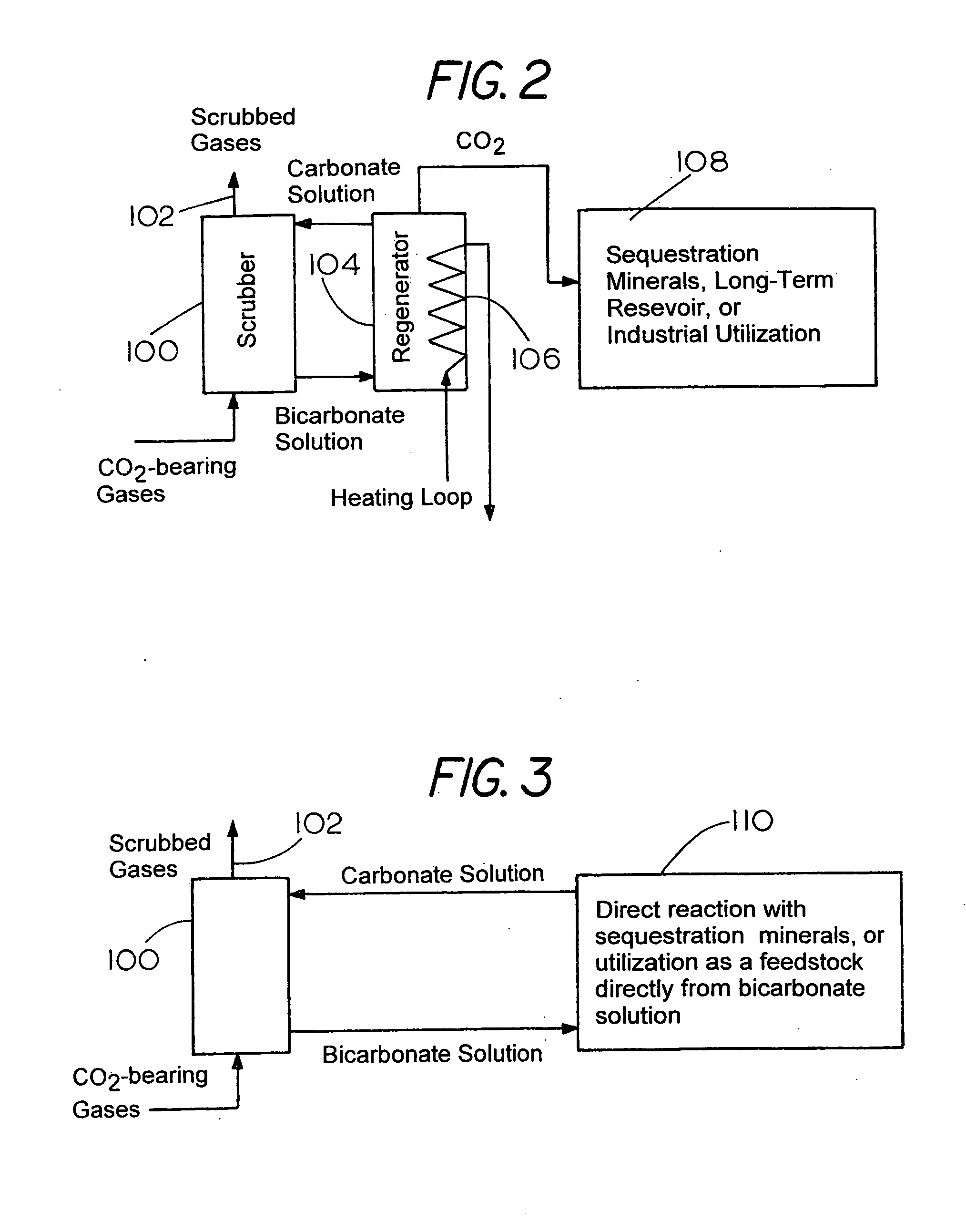
Method for extracting and sequestering carbon dioxide
InactiveUS6890497B2Reduce CO burdenWithout significant expenditureCalcium/strontium/barium carbonatesCombination devicesDicarbonateAlkaline earth metal
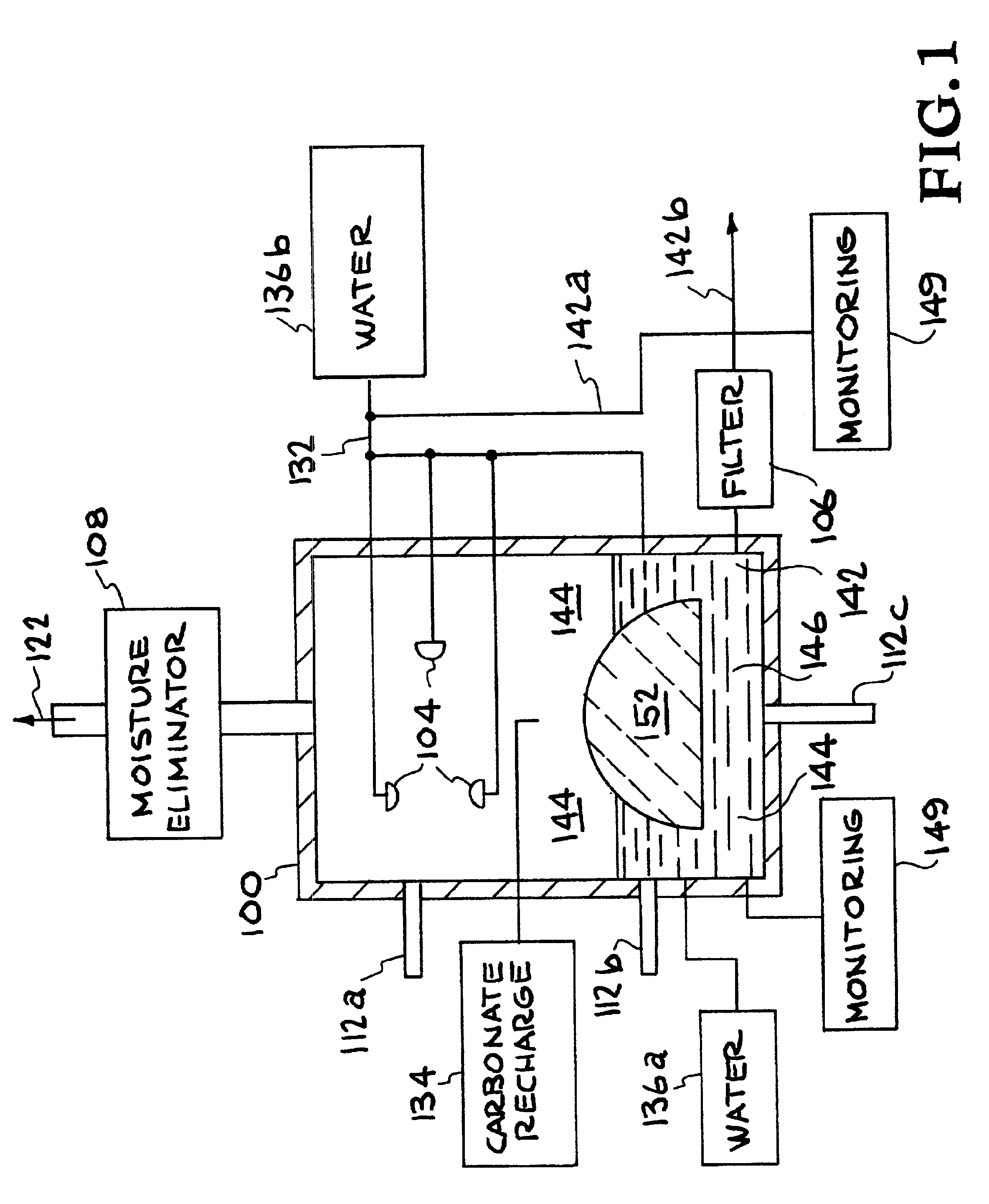
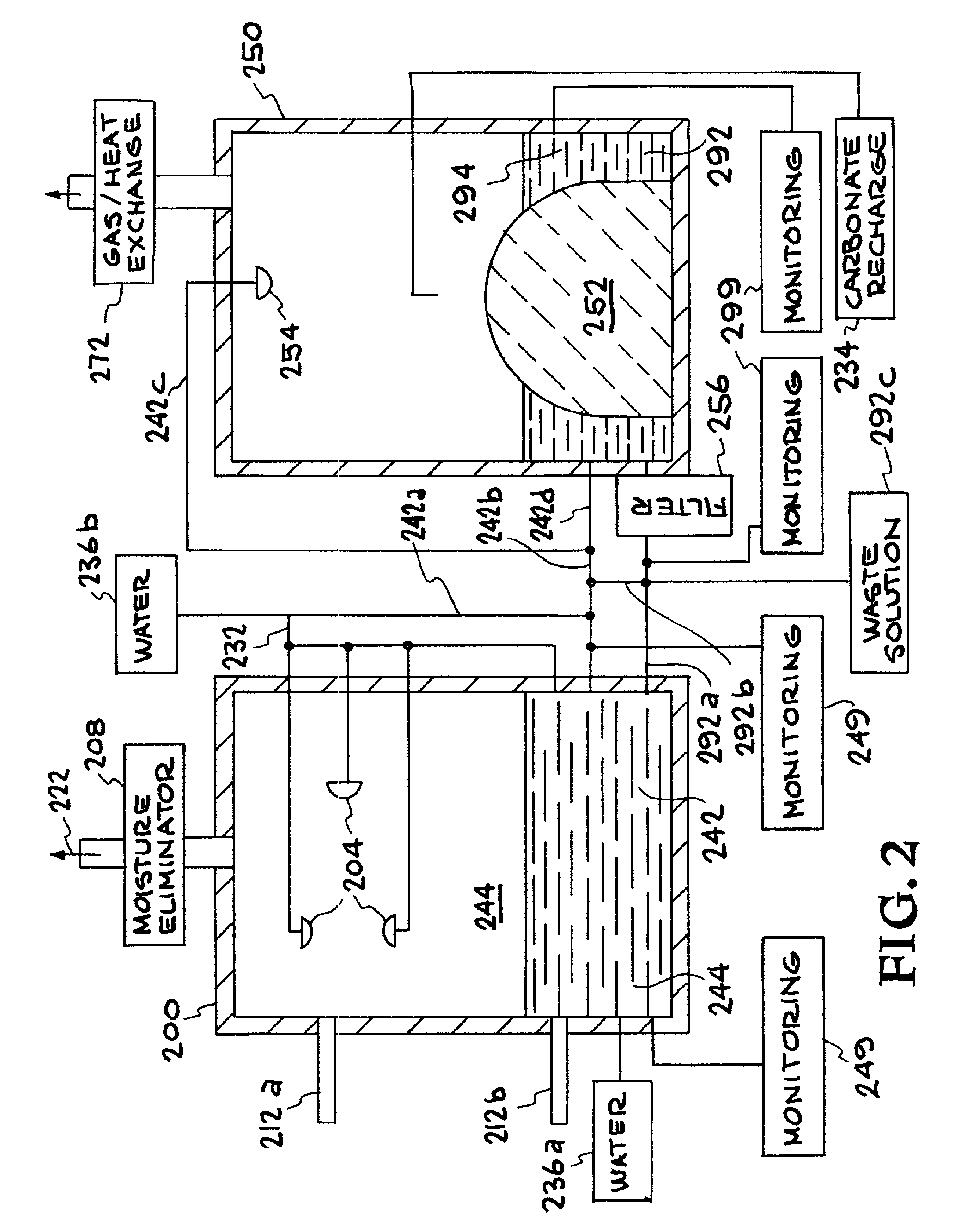
A method and apparatus to extract and sequester carbon dioxide (CO2) from a stream or volume of gas wherein said method and apparatus hydrates CO2, and reacts the resulting carbonic acid with carbonate. Suitable carbonates include, but are not limited to, carbonates of alkali metals and alkaline earth metals, preferably carbonates of calcium and magnesium. Waste products are metal cations and bicarbonate in solution or dehydrated metal salts, which when disposed of in a large body of water provide an effective way of sequestering CO2 from a gaseous environment.
Owner:LAWRENCE LIVERMORE NAT SECURITY LLC
Methods of sequestering co2
Methods of sequestering carbon dioxide (CO2) are provided. Aspects of the methods include precipitating a storage stable carbon dioxide sequestering product from an alkaline-earth-metal-containing water and then disposing of the product, e.g., by placing the product in a disposal location or using the product as a component of a manufactured composition. Also provided are systems for practicing methods of the invention.
Owner:ARELAC INC
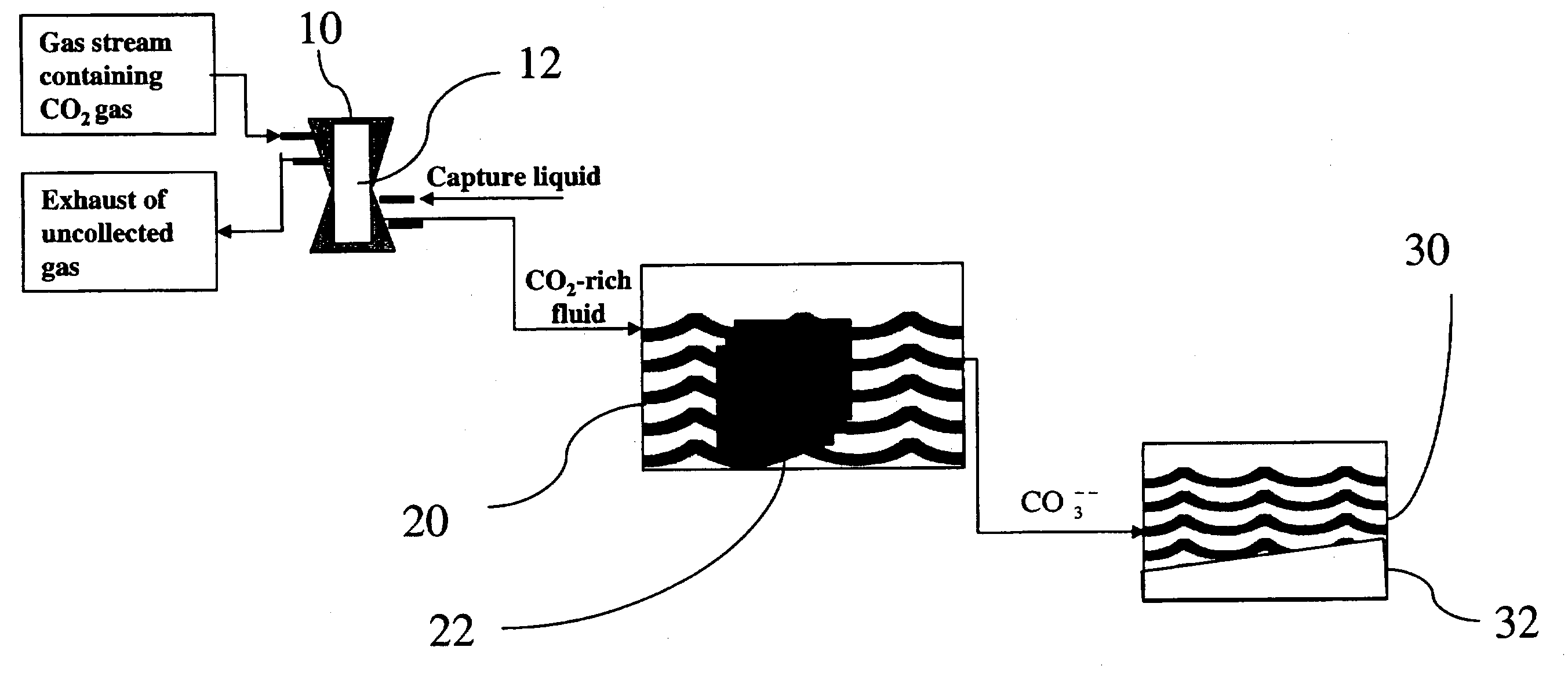
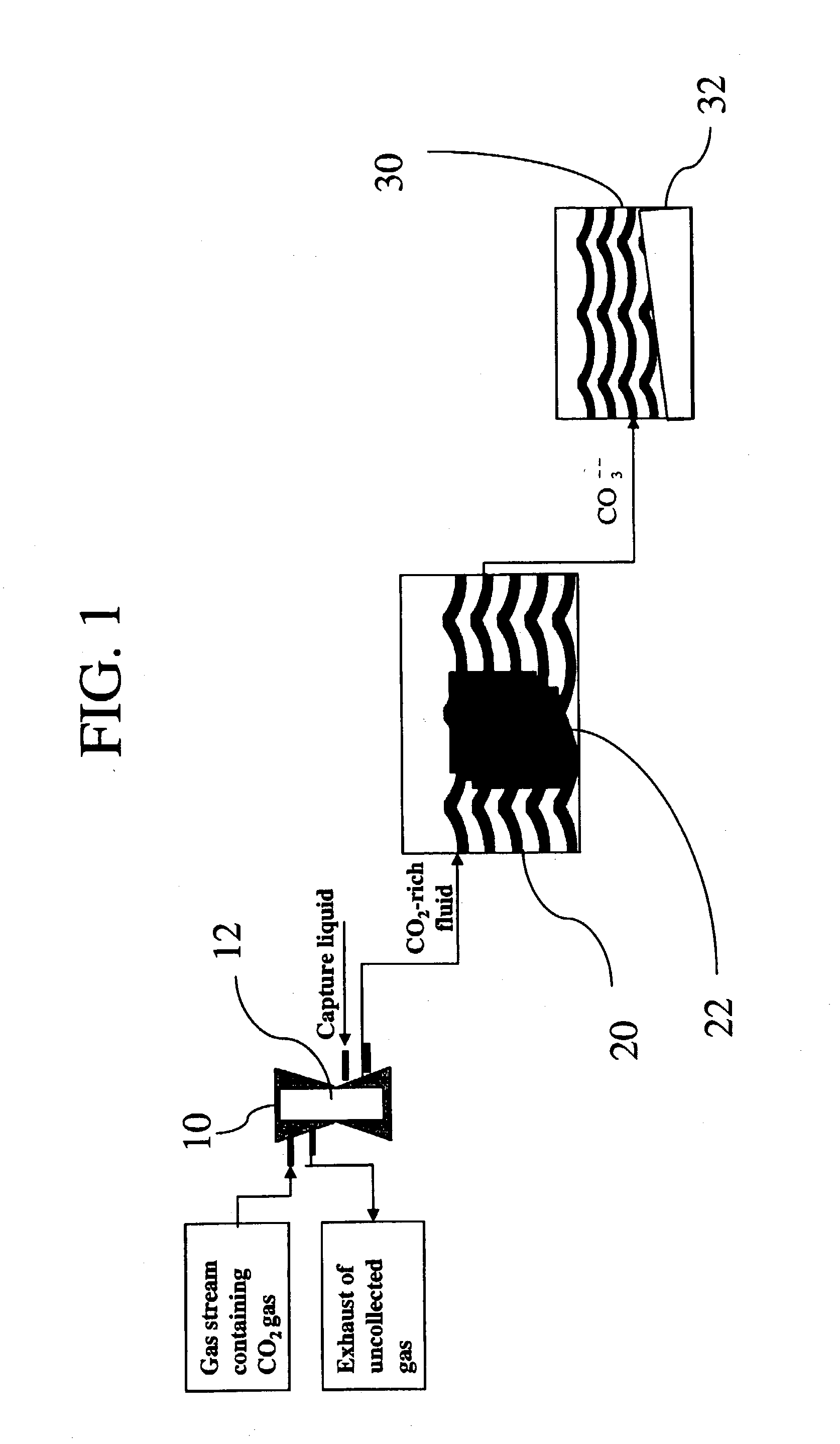
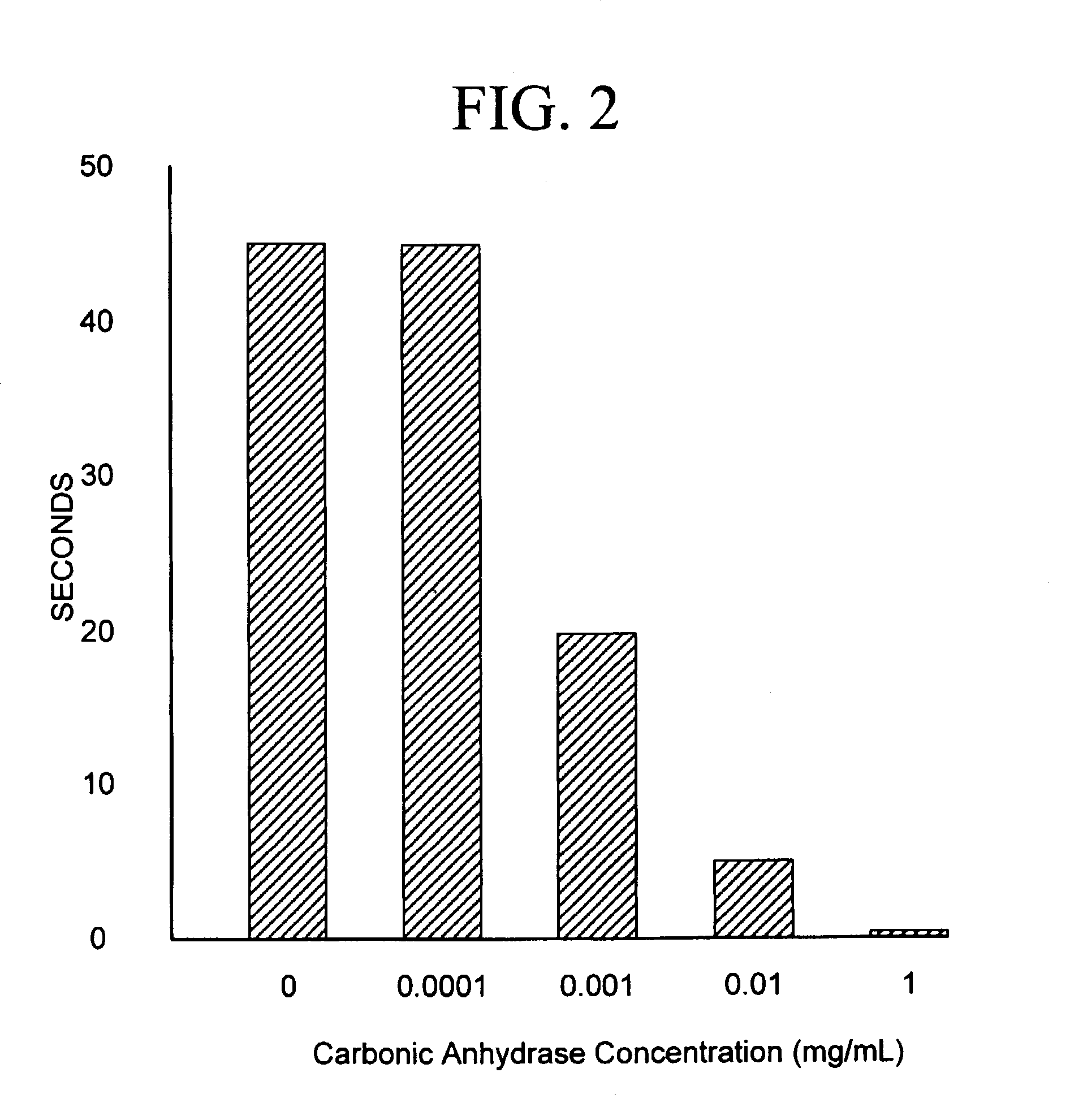
Sequestration of carbon dioxide
ActiveUS7132090B2Safe storageLarge specific surface areaCalcium/strontium/barium carbonatesProductsProduct gasMineral ions
A process for selectively removing carbon dioxide from a gaseous stream by converting the carbon dioxide to a solid, stable form is provided. In a sequestration process, carbon dioxide enriched air is passed through a gas diffusion membrane to transfer the carbon dioxide to a fluid medium. The carbon dioxide rich fluid is then passed through a matrix containing a catalyst specific for carbon dioxide, which accelerates the conversion of the carbon dioxide to carbonic acid. In the final step, a mineral ion is added to the reaction so that a precipitate of carbonate salt is formed. This solid mineral precipitate can be safely stored for extended periods of time, such as by burying the precipitate in the ground or depositing the precipitate into storage sites either on land or into a body of water. An apparatus for removing carbon dioxide from a gaseous stream is also provided.
Owner:GM GLOBAL TECH OPERATIONS LLC
Removal of carbon dioxide from air
InactiveUS20060051274A1Simple processReduce energy consumptionSemi-permeable membranesHydrogen sulfidesSolventCarbon dioxide binding
The present invention is directed to methods for removing carbon dioxide from air, which comprises exposing solvent covered surfaces to air streams where the airflow is kept laminar, or close to the laminar regime. The invention also provides for an apparatus, which is a laminar scrubber, comprising solvent covered surfaces situated such that they can be exposed to air stream. In another aspect, the invention provides a method and apparatus for separating carbon dioxide (CO2) bound in a solvent. The invention is particularly useful in processing hydroxide solvents containing CO2 captured from air.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK +2
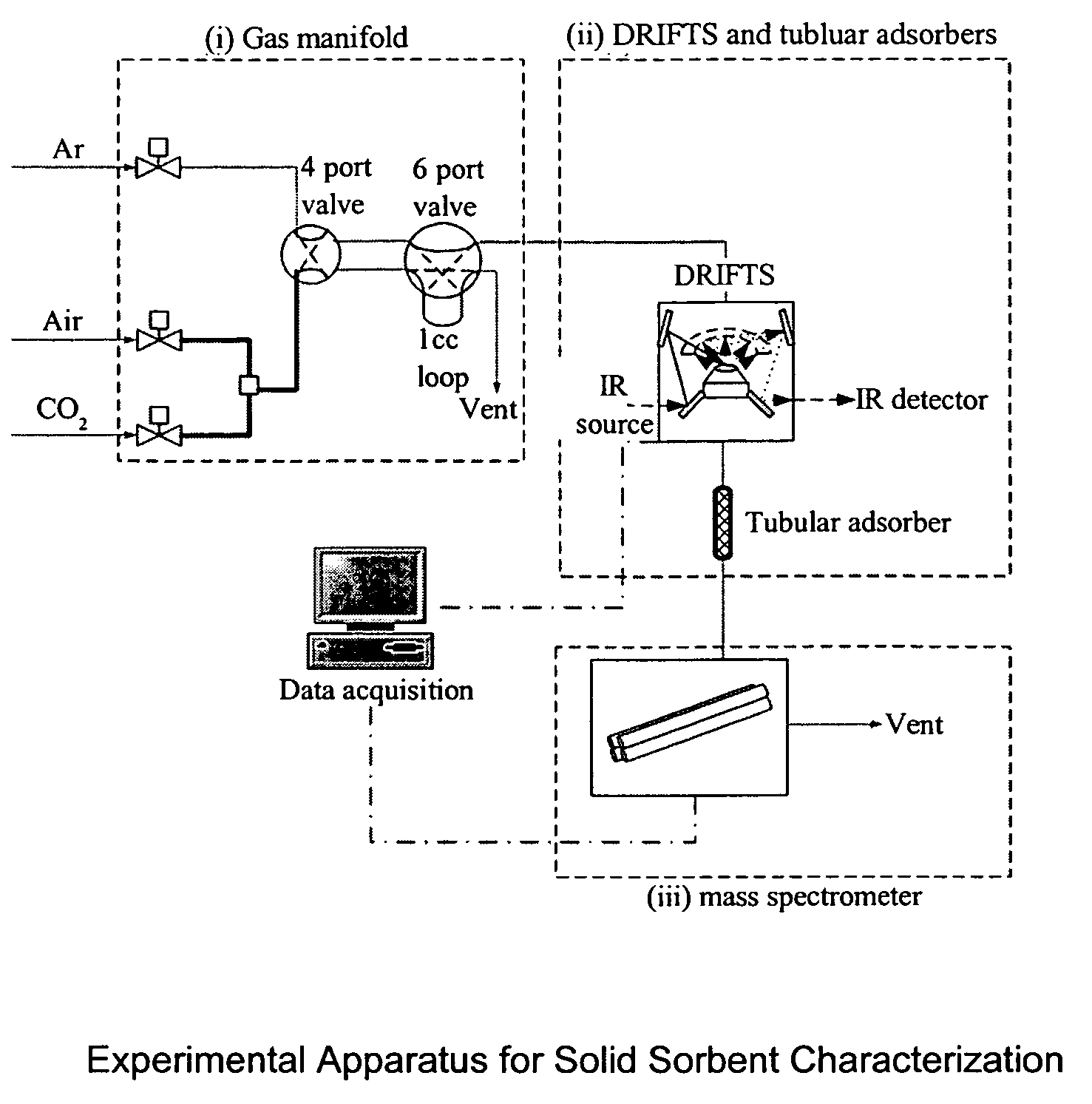
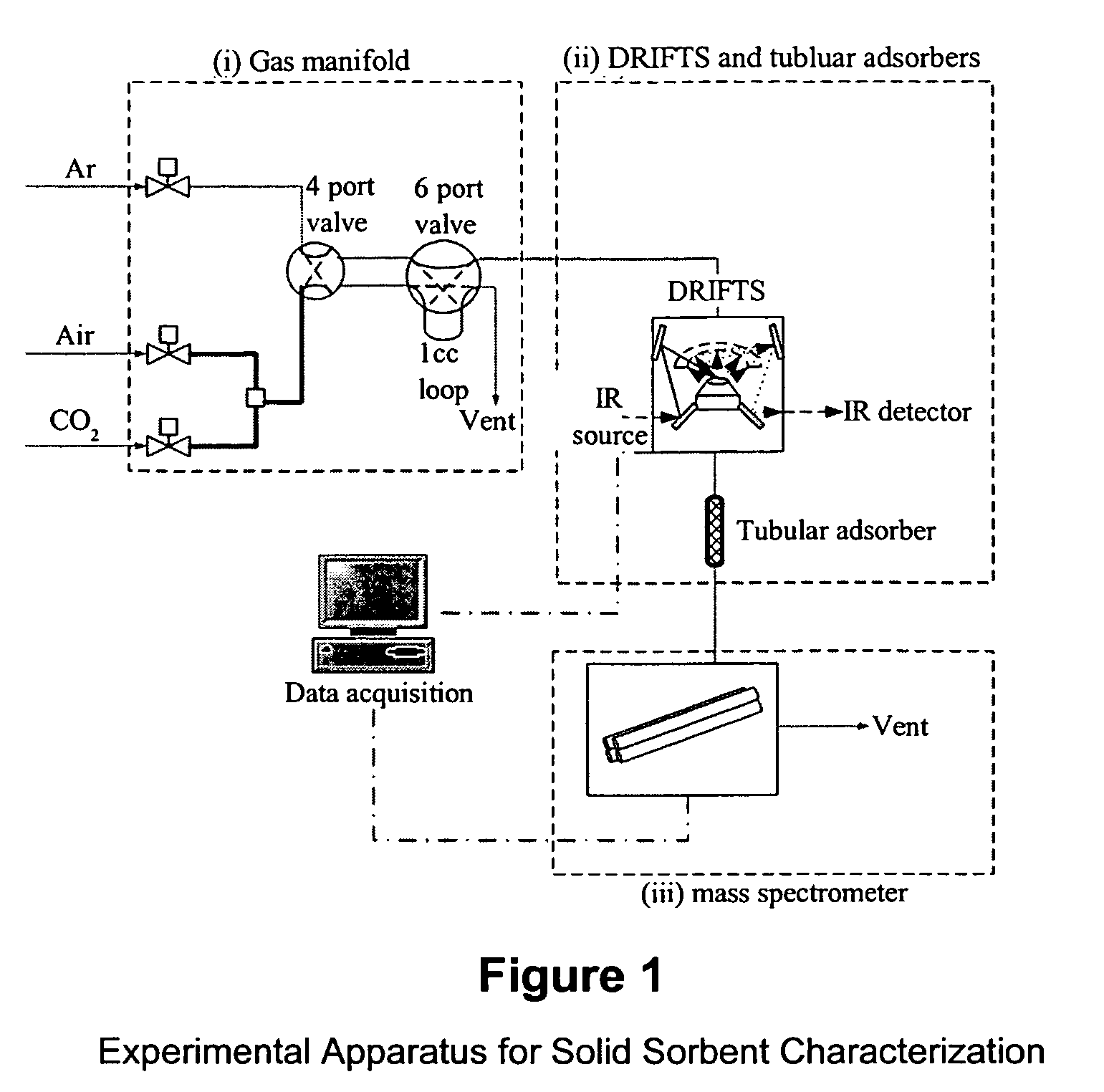
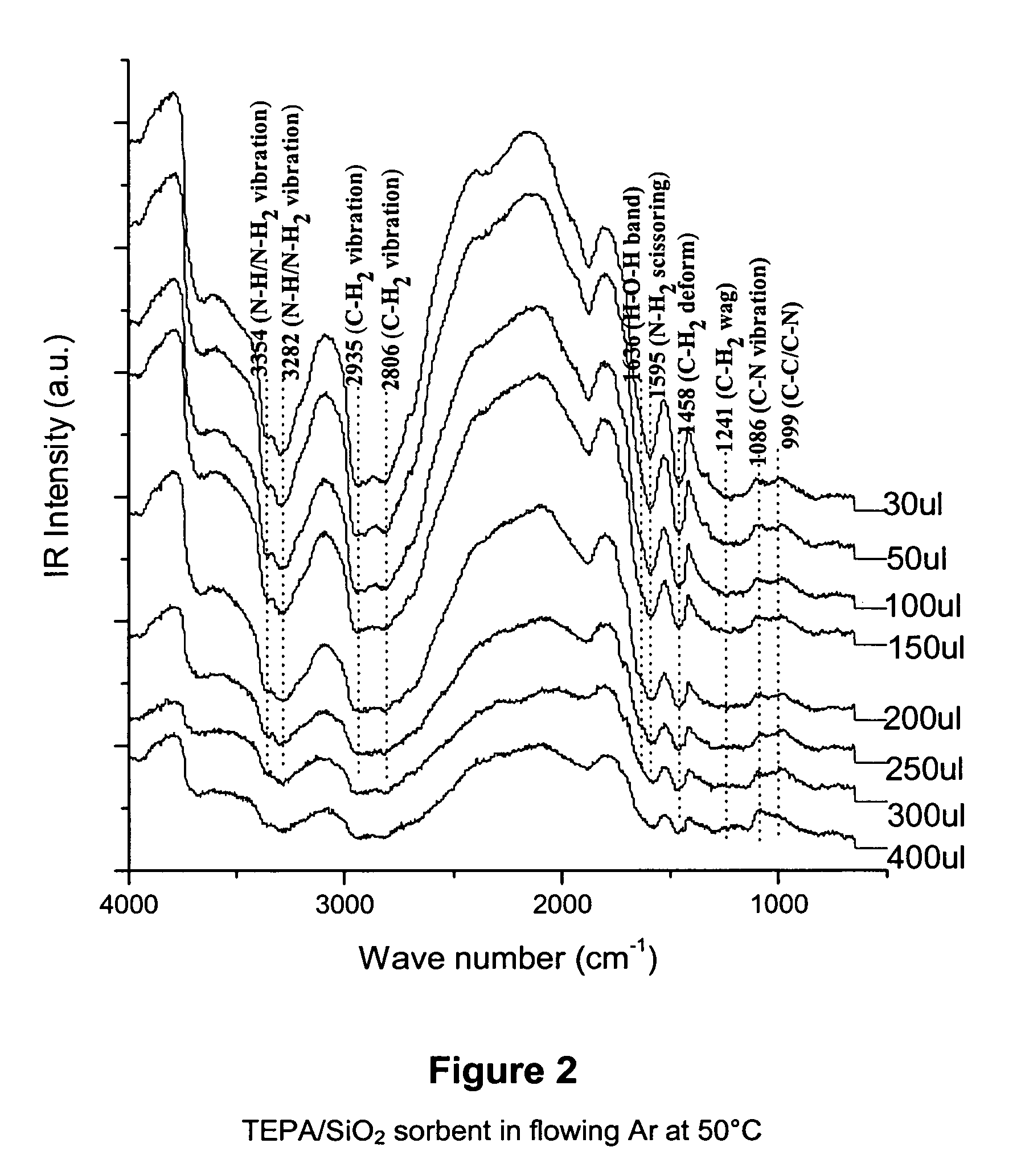
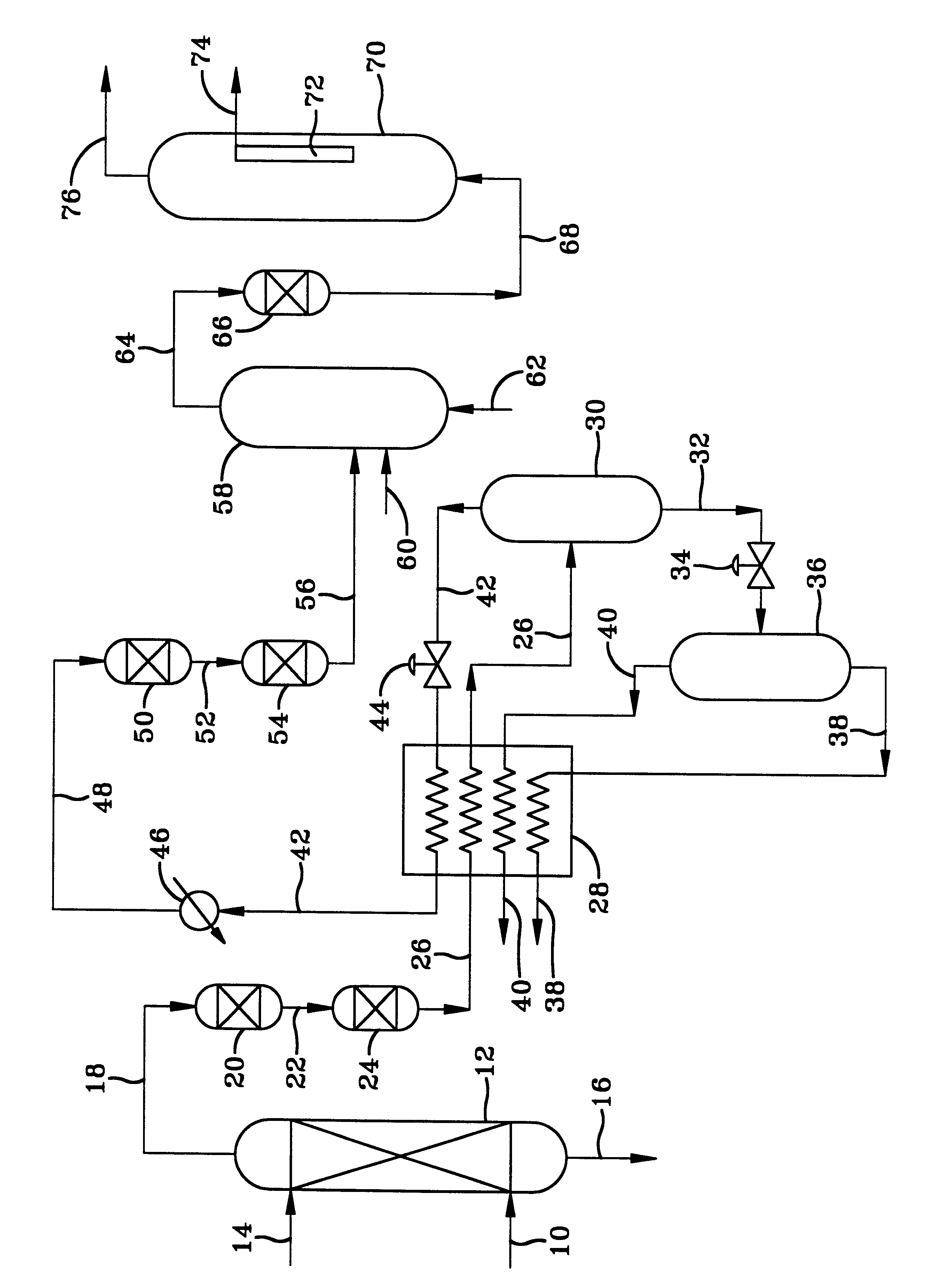
Solid sorbents for removal of carbon dioxide from gas streams at low temperatures
InactiveUS6908497B1Promote regenerationRegeneration process is inexpensiveGas treatmentOther chemical processesGas solidSorbent
New low-cost CO2 sorbents are provided that can be used in large-scale gas-solid processes. A new method is provided for making these sorbents that involves treating substrates with an amine and / or an ether so that the amine and / or ether comprise at least 50 wt. percent of the sorbent. The sorbent acts by capturing compounds contained in gaseous fluids via chemisorption and / or physisorption between the unit layers of the substrate's lattice where the polar amine liquids and solids and / or polar ether liquids and solids are located. The method eliminates the need for high surface area supports and polymeric materials for the preparation of CO2 capture systems, and provides sorbents with absorption capabilities that are independent of the sorbents' surface areas. The sorbents can be regenerated by heating at temperatures in excess of 35° C.
Owner:ENERGY U S DEPARMENT OF
Process for sequestering carbon dioxide and sulfur dioxide
A process for sequestering carbon dioxide, which includes reacting a silicate based material with an acid to form a suspension, and combining the suspension with carbon dioxide to create active carbonation of the silicate-based material, and thereafter producing a metal salt, silica and regenerating the acid in the liquid phase of the suspension.
Owner:PENN STATE RES FOUND
Method for recovery of CO2 from gas streams
InactiveUS7056482B2Reduce solubilityMinimizing its negative effectCarbon compoundsDispersed particle separationEnvironmental engineeringCarbon dioxide
A process for recovering CO2 from a feed gas stream comprises treating the feed gas stream with a regenerated absorbent comprising at least one tertiary amine absorbent having a pKa for the amino function of from about 6.5 to about 9 in the presence of an oxidation inhibitor to obtain a CO2 rich stream and subsequently treating the CO2 rich stream to obtain the regenerated absorbent and a CO2 rich product stream. The feed gas stream may also include SO2 and / or NOx.
Owner:CANSOLV TECH INC
Catalytic Gasification Particulate Compositions
ActiveUS20090229182A1Efficient use ofBiofuelsGas modification by gas mixingPtru catalystPetroleum coke
Particulate compositions are described comprising a carbonaceous material, such as petroleum coke and / or coal, treated or otherwise associated with a gasification catalyst, where the catalyst is at least in part derived from a leachate from a biomass char, for gasification in the presence of steam to yield a plurality of gases including methane and at least one or more of hydrogen, carbon monoxide, and other higher hydrocarbons are formed. Processes are also provided for the preparation of the particulate compositions and converting the particulate composition into a plurality of gaseous products.
Owner:SURE CHAMPION INVESTMENT LTD
Removing carbon dioxide from waste streams through co-generation of carbonate and/or bicarbonate minerals
ActiveUS20060185985A1Improve ecologic efficiency of processEcologic efficiencyCalcium/strontium/barium carbonatesElectrolysis componentsElectrolysisWaste stream
Apparatuses and methods for removing carbon dioxide and other pollutants from a gas stream are provided. The methods include obtaining hydroxide in an aqueous mixture, and mixing the hydroxide with the gas stream to produce carbonate and / or bicarbonate. Some of the apparatuses of the present invention comprise an electrolysis chamber for providing hydroxide and mixing equipment for mixing the hydroxide with a gas stream including carbon dioxide to form an admixture including carbonate and / or bicarbonate.
Owner:CARBONFREE CHEM HLDG LLC
Systems and methods for carbon capture and sequestration and compositions derived therefrom
ActiveUS20090143211A1Promote formationCalcium/strontium/barium carbonatesNitrogen compoundsInterior spaceProduct formation
A method of sequestering a greenhouse gas is described, which comprises: (i) providing a solution carrying a first reagent that is capable of reacting with a greenhouse gas; (ii) contacting the solution with a greenhouse gas under conditions that promote a reaction between the at least first reagent and the greenhouse gas to produce at least a first reactant; (iii) providing a porous matrix having interstitial spaces and comprising at least a second reactant; (iv) allowing a solution carrying the at least first reactant to infiltrate at least a substantial portion of the interstitial spaces of the porous matrix under conditions that promote a reaction between the at least first reactant and the at least second reactant to provide at least a first product; and (v) allowing the at least first product to form and fill at least a portion of the interior spaces of the porous matrix, thereby sequestering a greenhouse gas.
Owner:RUTGERS THE STATE UNIV
Carbonaceous Fines Recycle
ActiveUS20090217589A1Sufficient amountHydrogen productionCarbon monoxideParticulatesParticle composition
Owner:SURE CHAMPION INVESTMENT LTD
Methods and configurations for acid gas enrichment
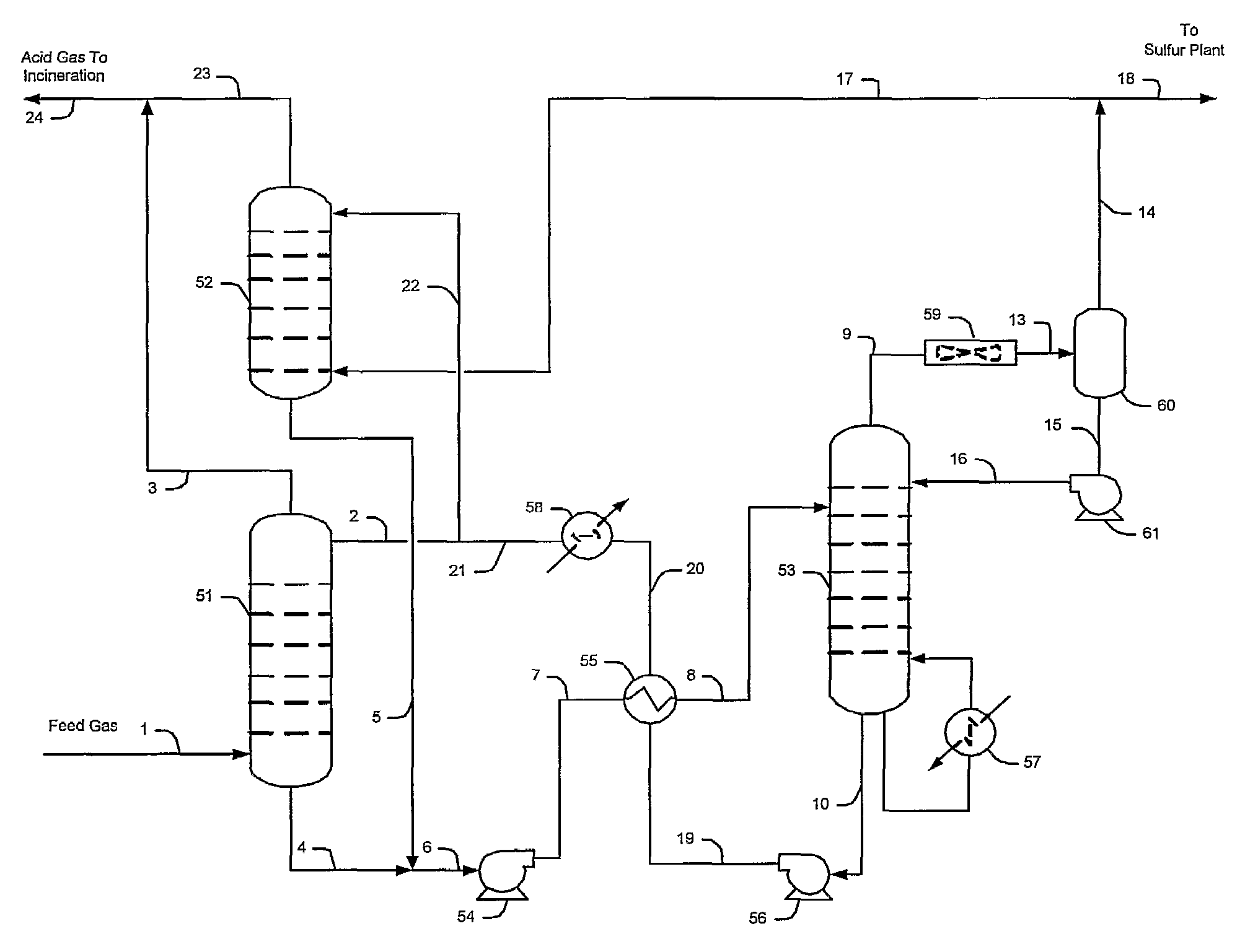
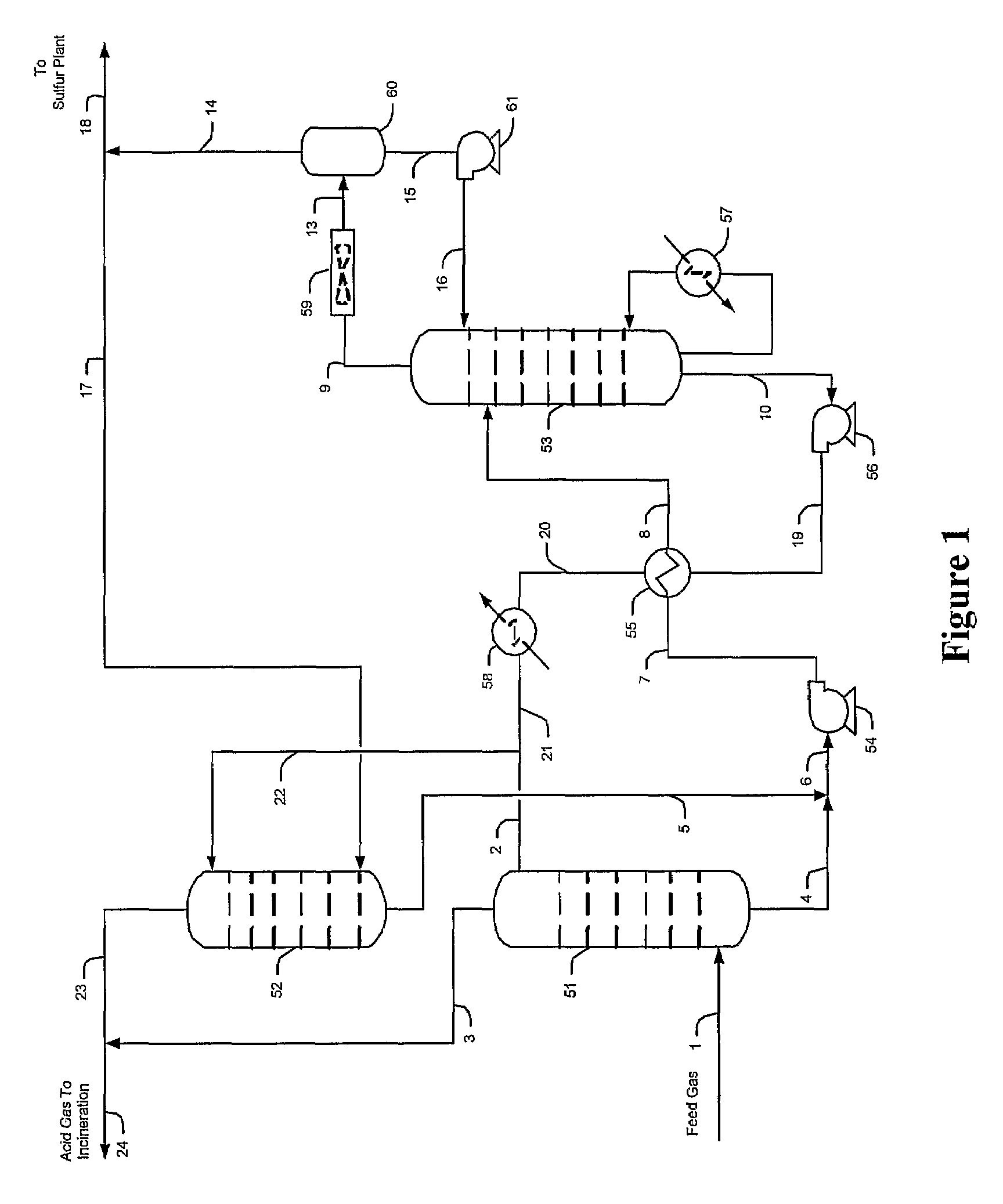
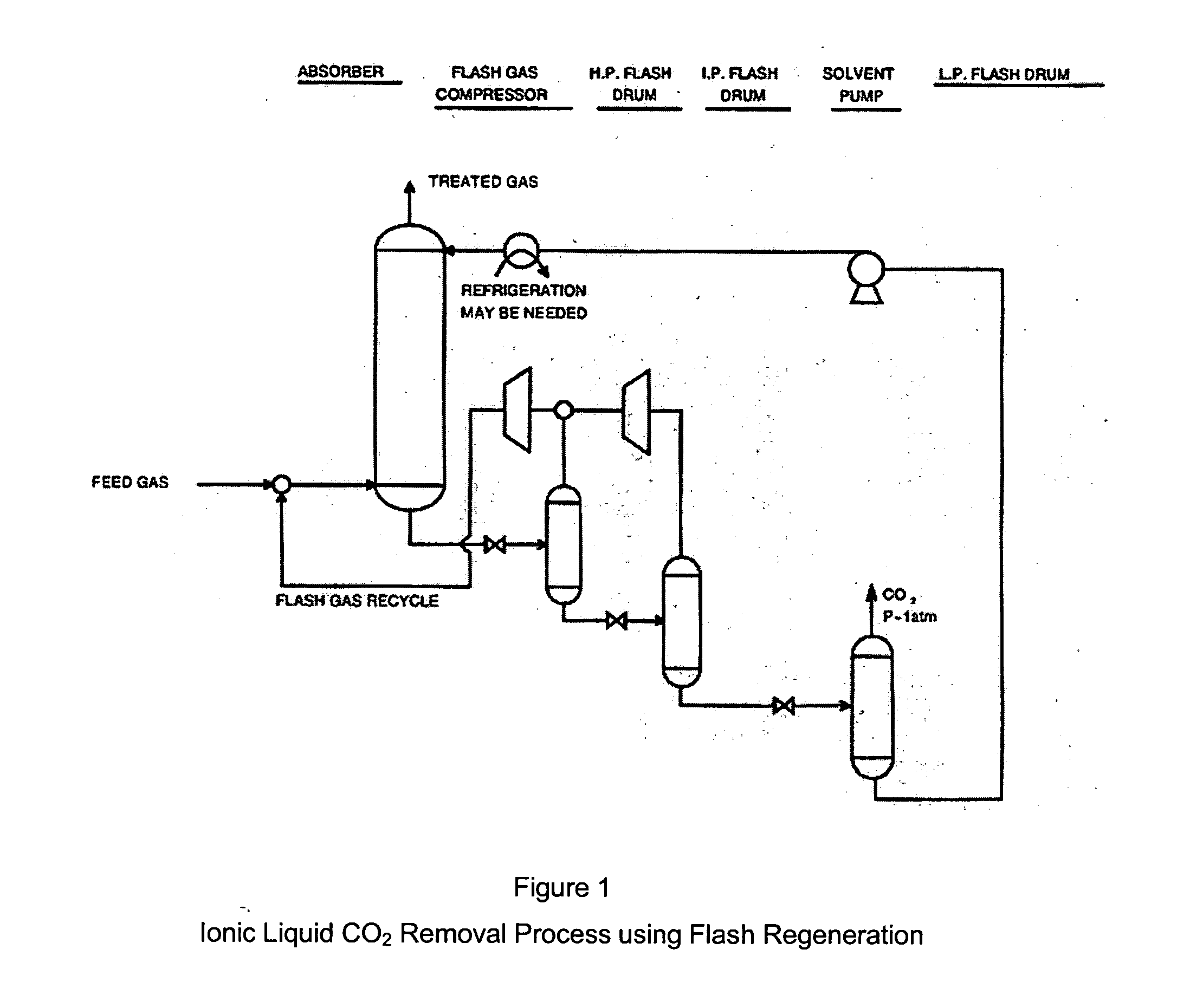
ActiveUS7635408B2Increase the concentration of hydrogen sulfideSmall sizeGas treatmentCarbon compoundsSolventCarbon dioxide
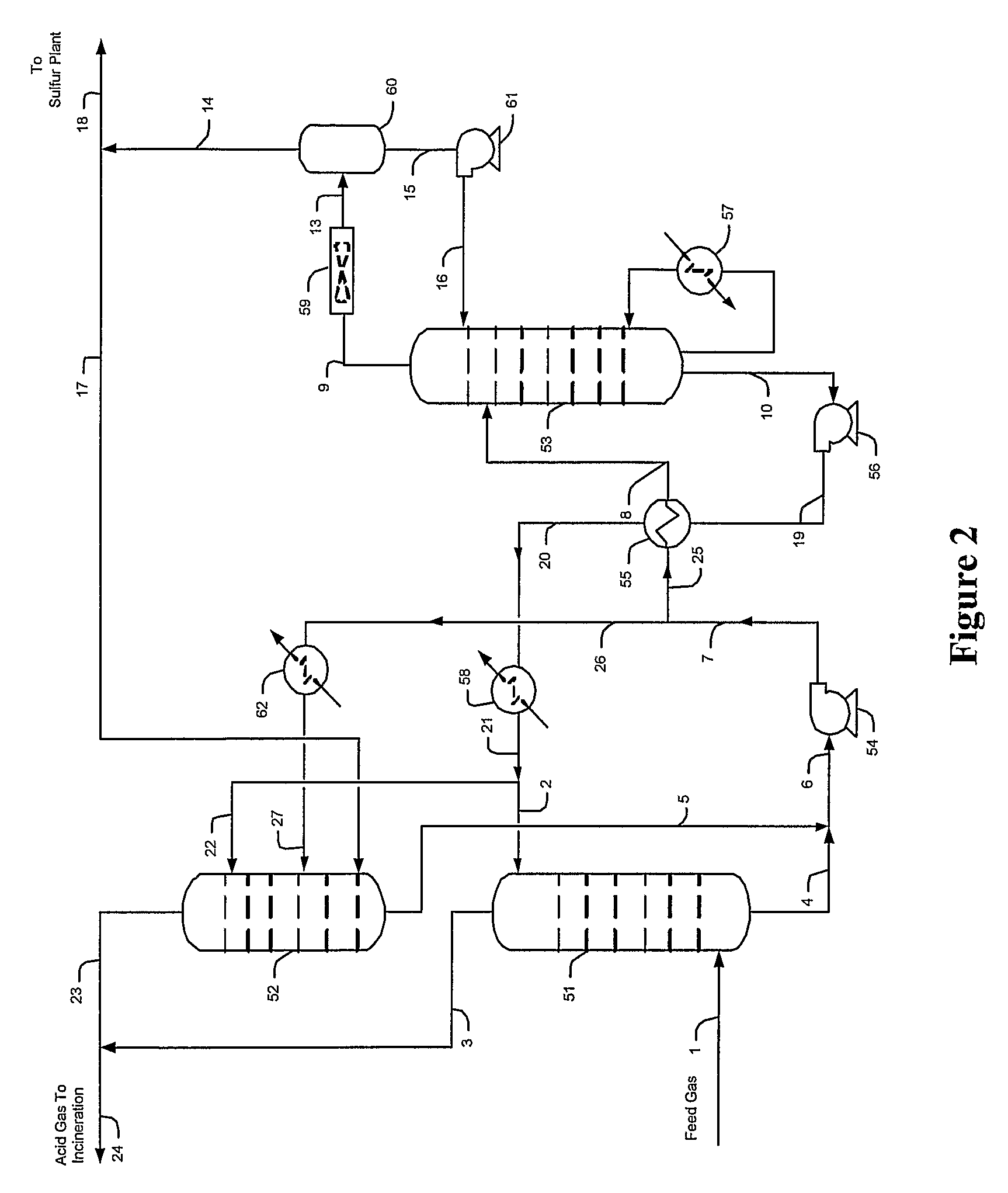
Hydrogen sulfide is selectively enriched from an acid gas (1) that comprises relatively large quantities of carbon dioxide using a configuration in which a portion of an isolated hydrogen sulfide stream is introduced into an absorber (51) operating as a carbon dioxide rejecter. The resulting concentrated hydrogen sulfide enriched solvent (4) is then further used (directly or indirectly) to absorb hydrogen sulfide from an acid feed gas.
Owner:FLUOR ENTERPRISES
Zero emission gasification, power generation, carbon oxides management and metallurgical reduction processes, apparatus, systems, and integration thereof
ActiveUS7674443B1Improvement in individual technology componentEnhances economic performanceUsing liquid separation agentBiofuelsCyclonic separationOxygen
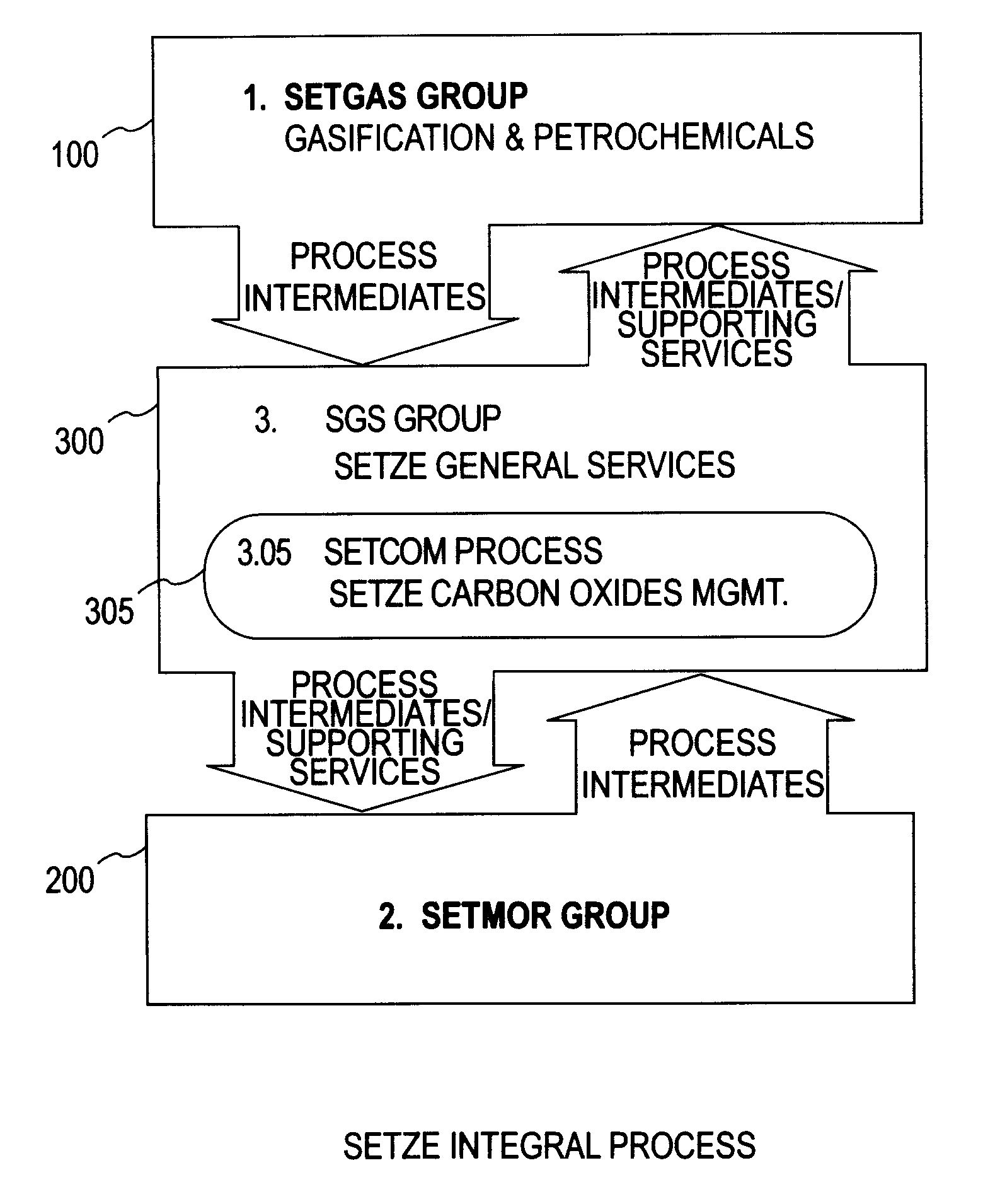
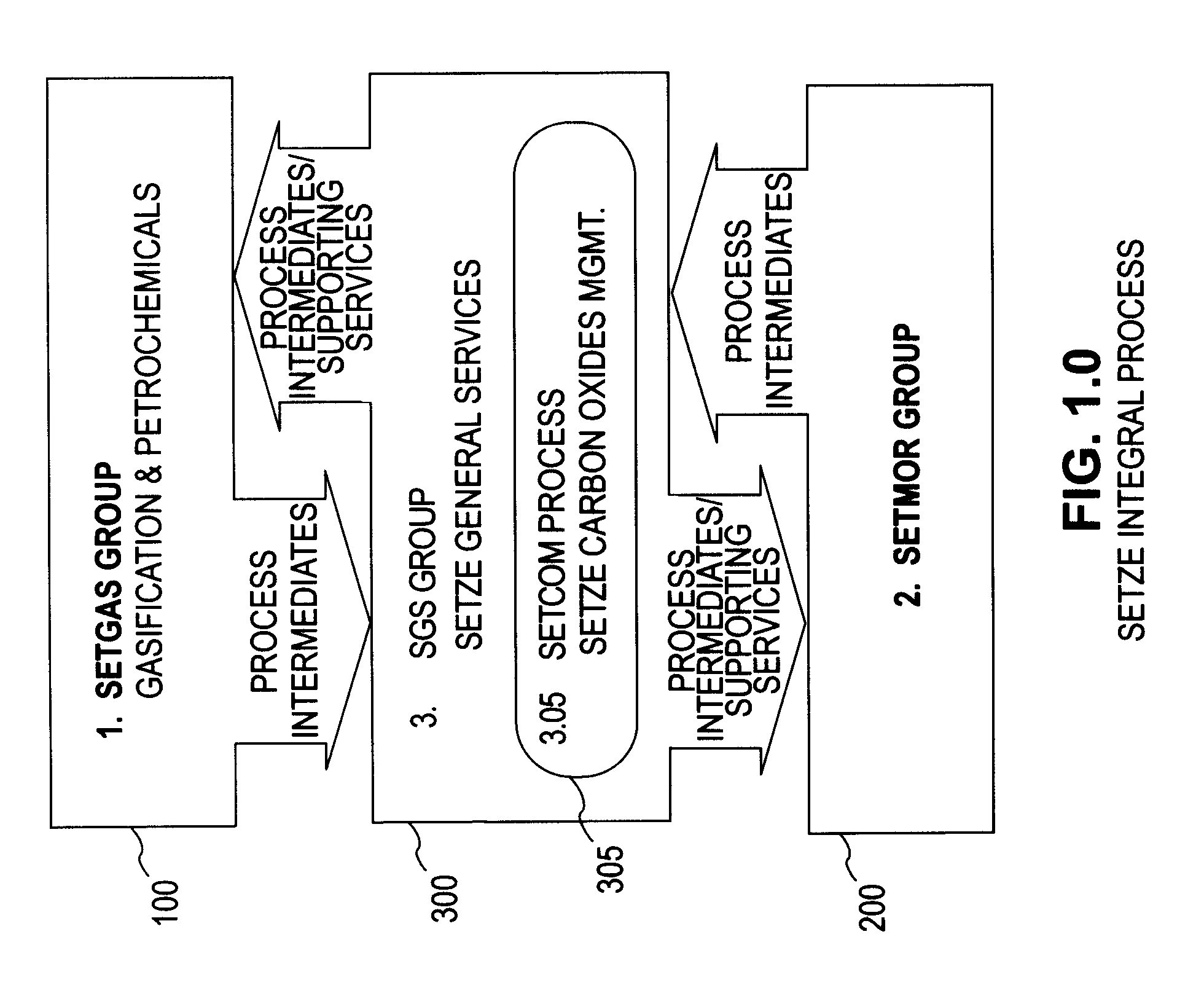
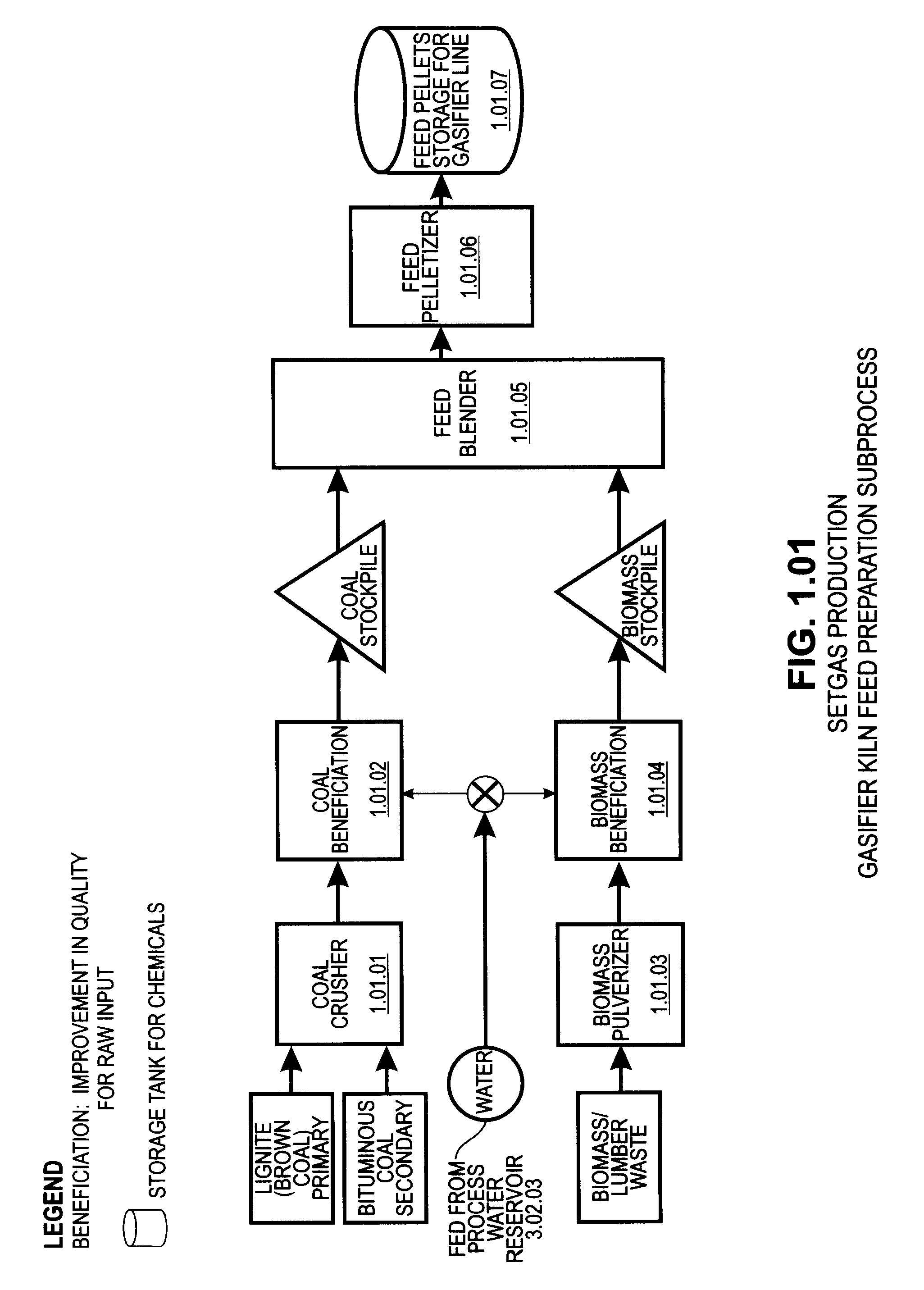
A system involving a two-step gasification of a carbonaceous source to produce bulk hydrogen that avoids the early formation of CO2 and obviates the traditional water gas shift (WGSR) step, carbochlorination of a metallic ore the production of metals found in the ore that utilizes carbon monoxide as an oxygen sink, rather than the traditional coke, and carbon oxides management that eliminates major impediments to emission-neutral power generation and the reduction of major metals. The gasification uses a rotary kiln reactor and gas-gas cyclonic separation process to separate synthesis gas into purified hydrogen and purified carbon monoxide. Purified bulk carbon monoxide issued in metallurgical reduction, and purified bulk hydrogen as fuel for an emission-neutral hydrogen combined cycle (HCC) turbine power generation station. The carbochlorination is integrated with: a) the concurrent separation and purification of all metal-chlorides (metchlors) and capture of CO2 for passage to the carbon oxides management system; b) the direct reduction of metchlors to nanoscale metallurgical powders and / or to dendritically-shaped particles, including metchlor reduction for the ultrahigh-performance semiconductor metals of the III-V group; and, c) the reforming of metal-oxides with improved crystalline structure from metchlors. The carbon oxides management collects, stores and directs to points of usage, carbon oxides that arise in various processes of the integrated system, and captures carbon monoxide for process enhancement and economic uses and captures carbon dioxide as a process intermediate and for economic uses.
Owner:DAVIS OLUMIJI B +1
Multi-component removal in flue gas by aqua ammonia
InactiveUS7255842B1Regeneration process is less-costlyIncrease load capacityGas treatmentNitrogen compoundsNitric oxideSlurry
A new method for the removal of environmental compounds from gaseous streams, in particular, flue gas streams. The new method involves first oxidizing some or all of the acid anhydrides contained in the gas stream such as sulfur dioxide (SO2) and nitric oxide (NO) and nitrous oxide (N2O) to sulfur trioxide (SO3) and nitrogen dioxide (NO2). The gas stream is subsequently treated with aqua ammonia or ammonium hydroxide which captures the compounds via chemical absorption through acid-base or neutralization reactions. The products of the reactions can be collected as slurries, dewatered, and dried for use as fertilizers, or once the slurries have been dewatered, used directly as fertilizers. The ammonium hydroxide can be regenerated and recycled for use via thermal decomposition of ammonium bicarbonate, one of the products formed. There are alternative embodiments which entail stoichiometric scrubbing of nitrogen oxides and sulfur oxides with subsequent separate scrubbing of carbon dioxide.
Owner:THE UNITED STATES AS REPRESENTED BY THE DEPARTMENT OF ENERGY
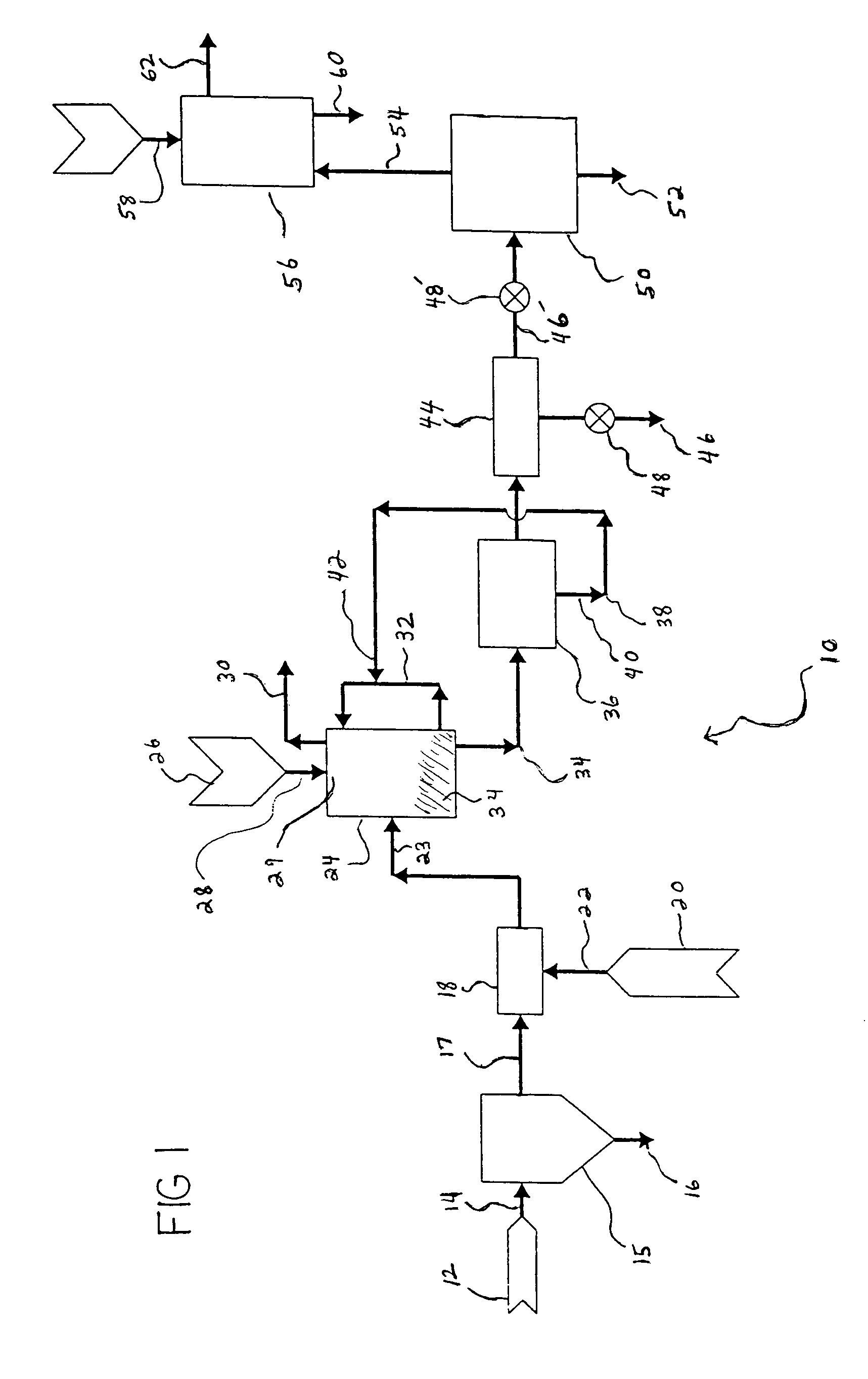
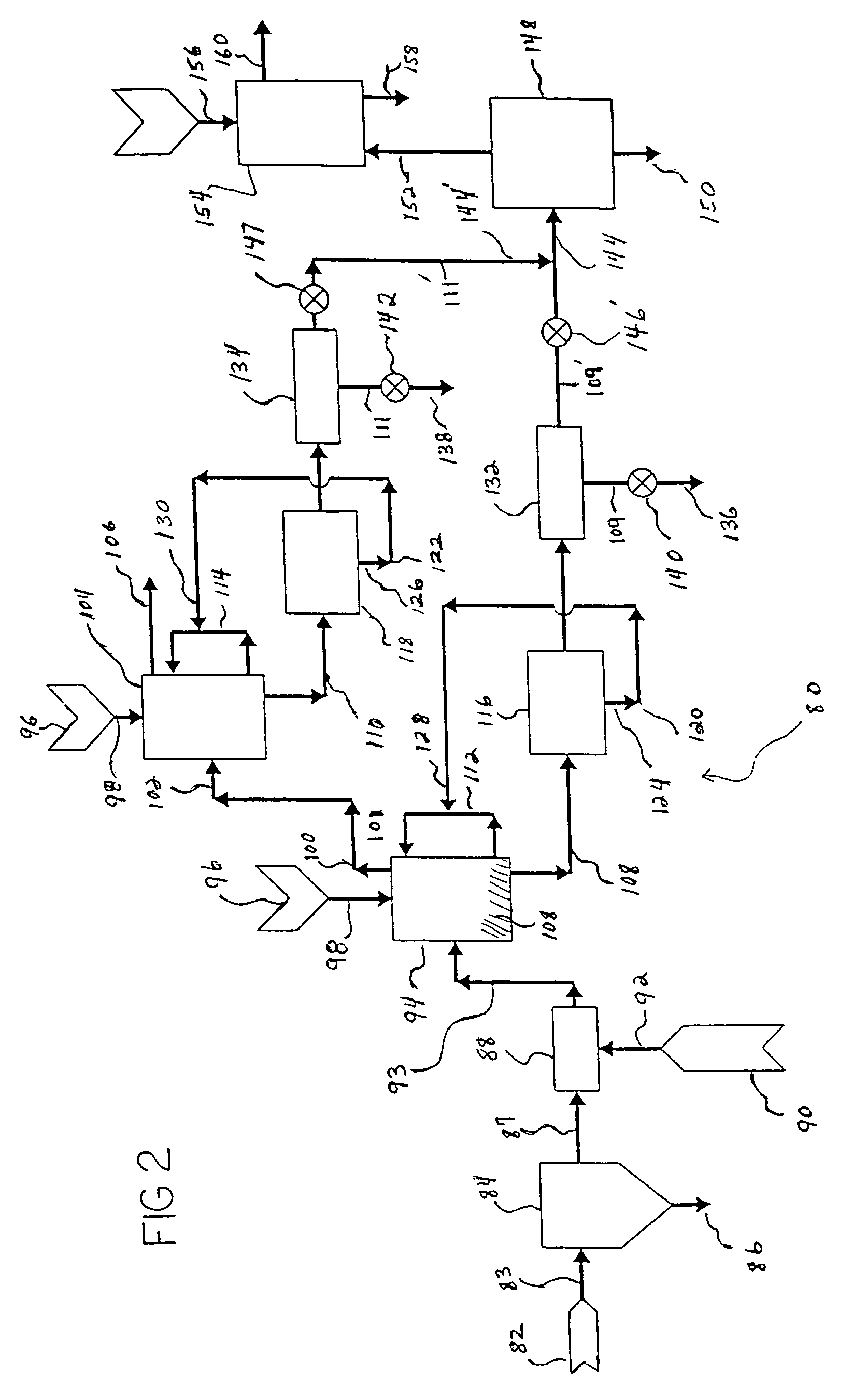
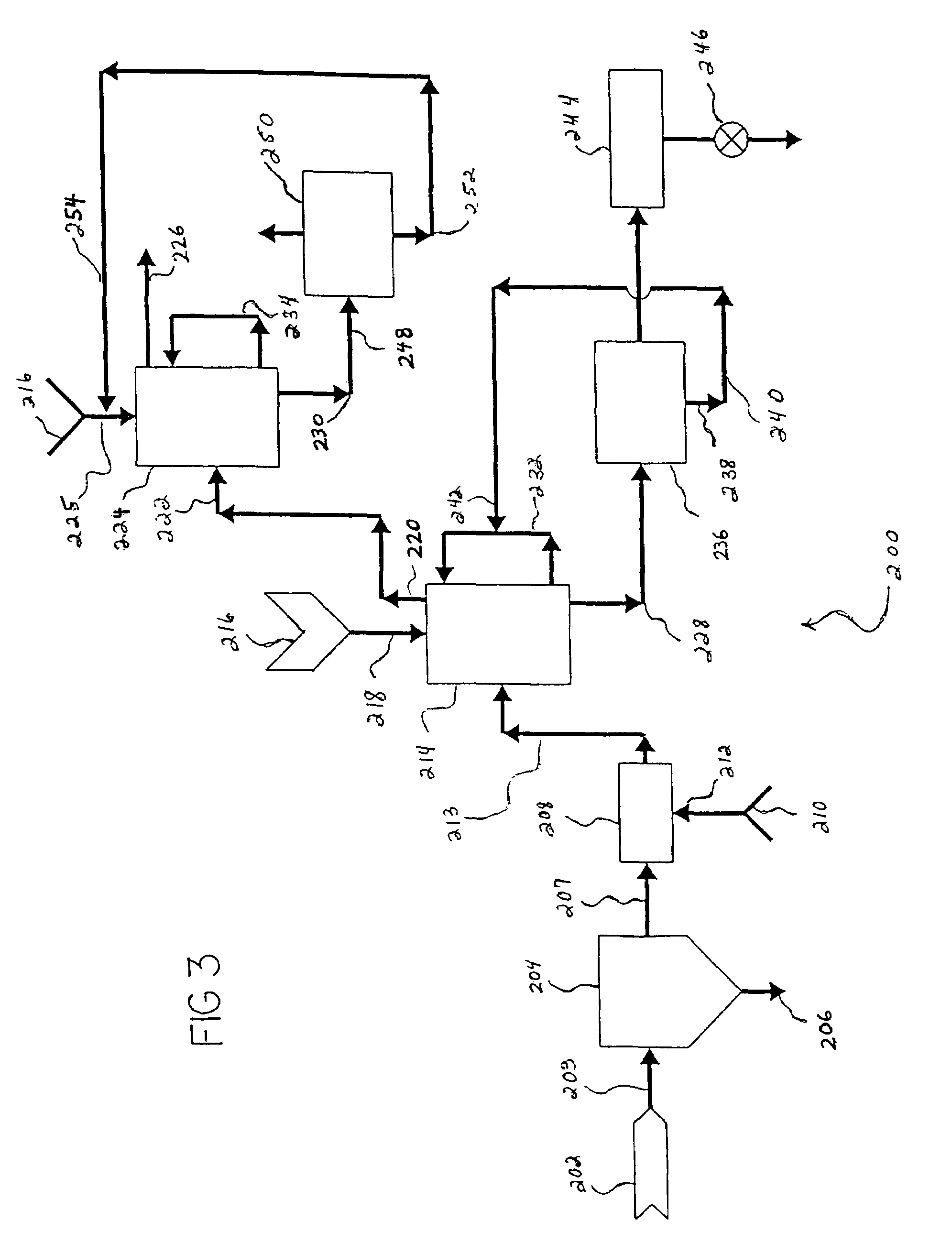
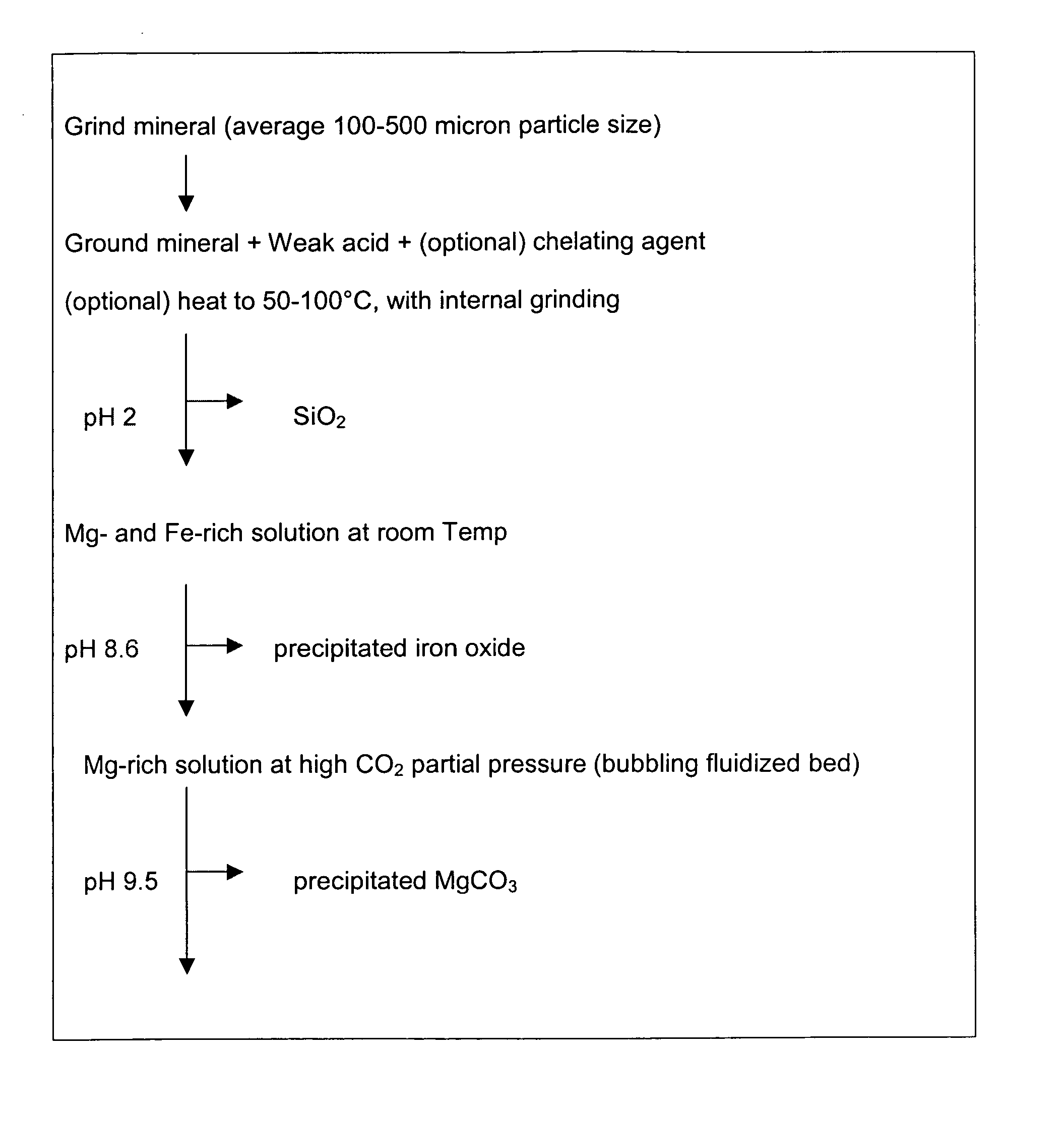
Carbon dioxide sequestration using alkaline earth metal-bearing minerals
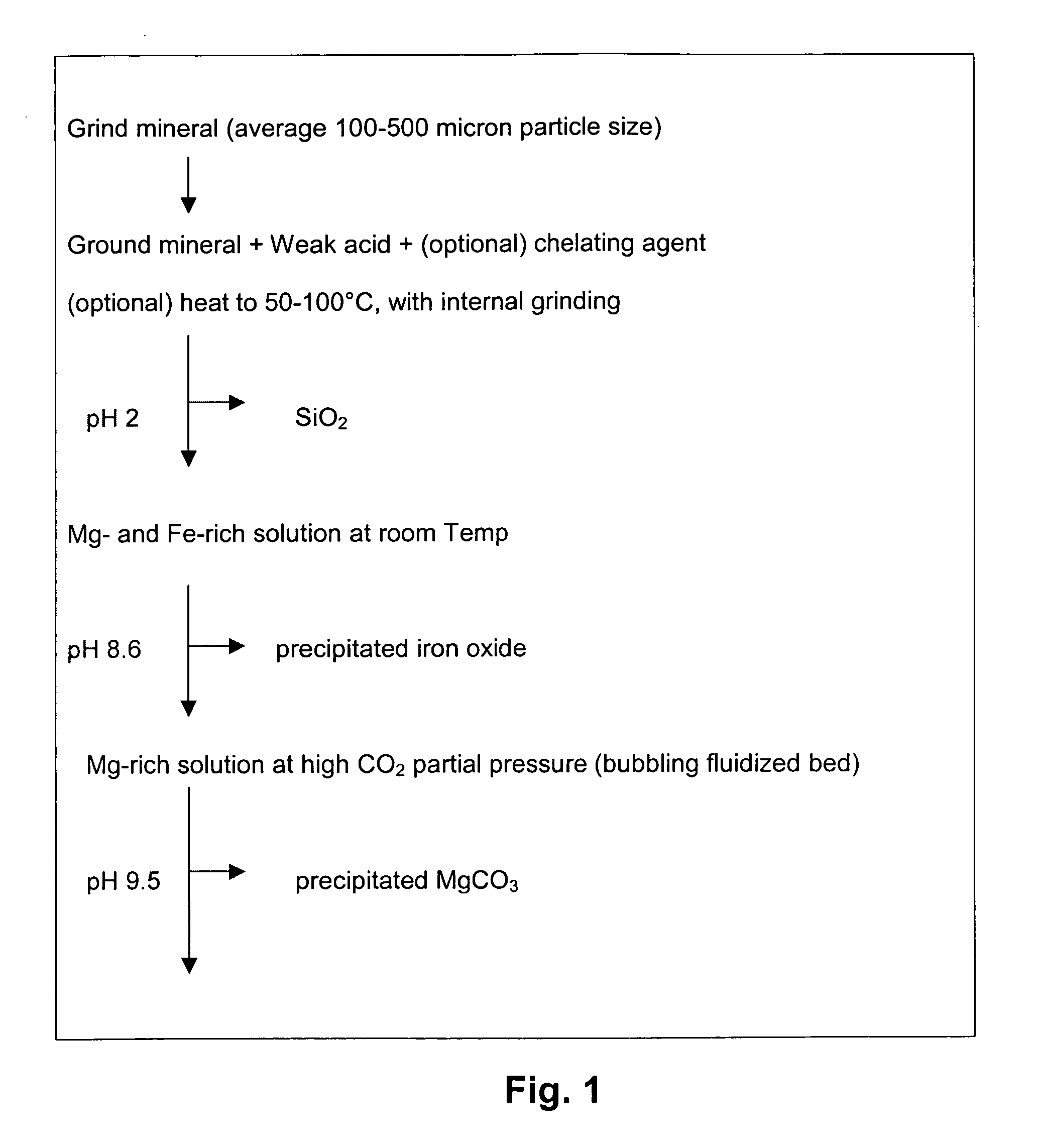
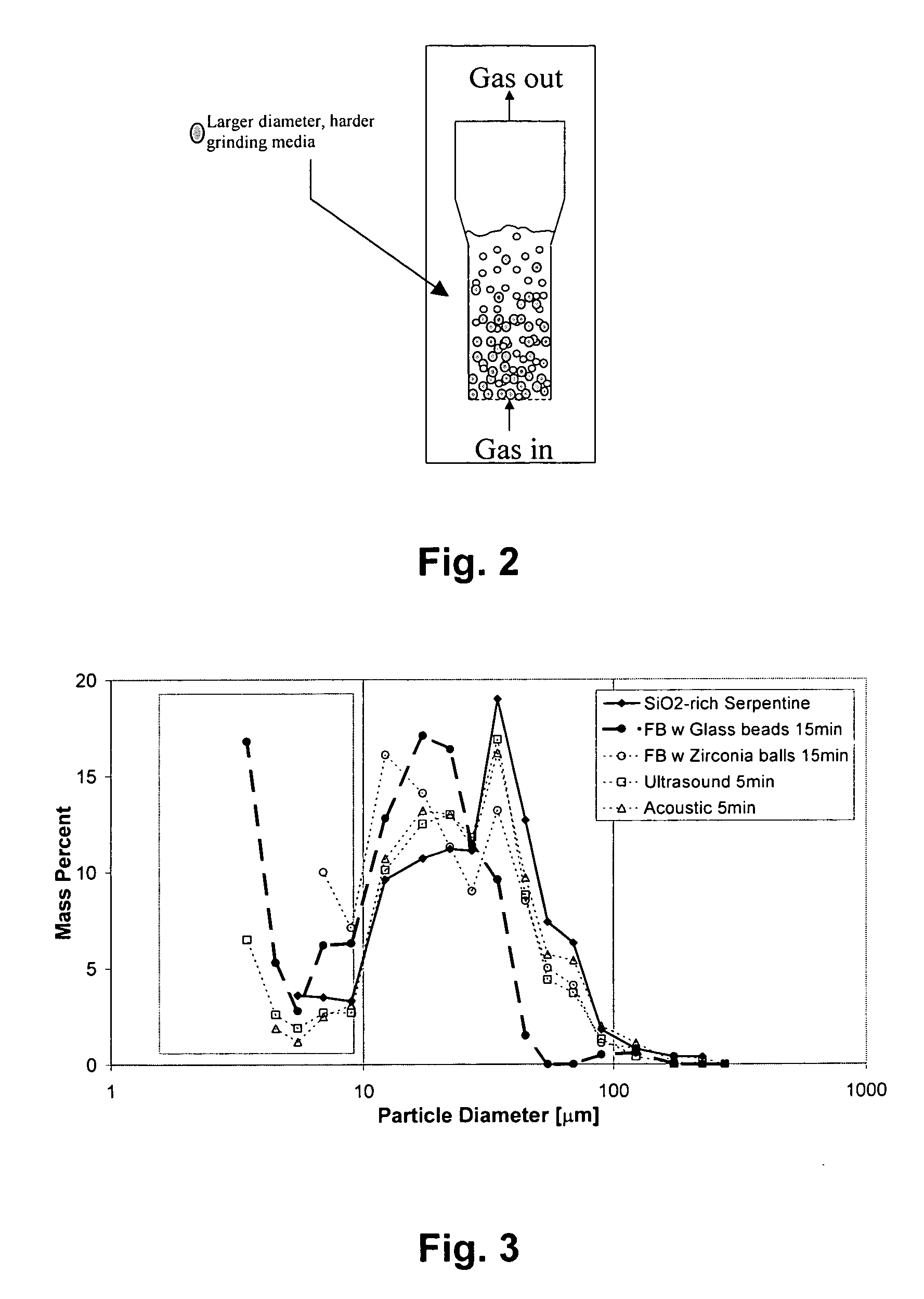
ActiveUS20050180910A1High dissolution rateEfficient removalCalcium/strontium/barium carbonatesProductsParticulatesAlkaline earth metal
A method for mineral sequestration of pollutant gases resulting from the combustion of carbon-based fuels such as carbon and sulfur dioxides is provided and includes, providing a particulate magnesium-containing mineral and exposing the magnesium-containing mineral to a weak acid to dissolve magnesium from the mineral and form a magnesium-containing solution. The surface of the particulate magnesium-containing mineral is physically activated to expose and dissolve additional magnesium into the solution. Pollutant gases such as carbon dioxide are mixed with the magnesium-containing solution. When the pH of the magnesium-containing solution is increased, solid magnesium carbonate is formed.
Owner:THE OHIO STATES UNIV
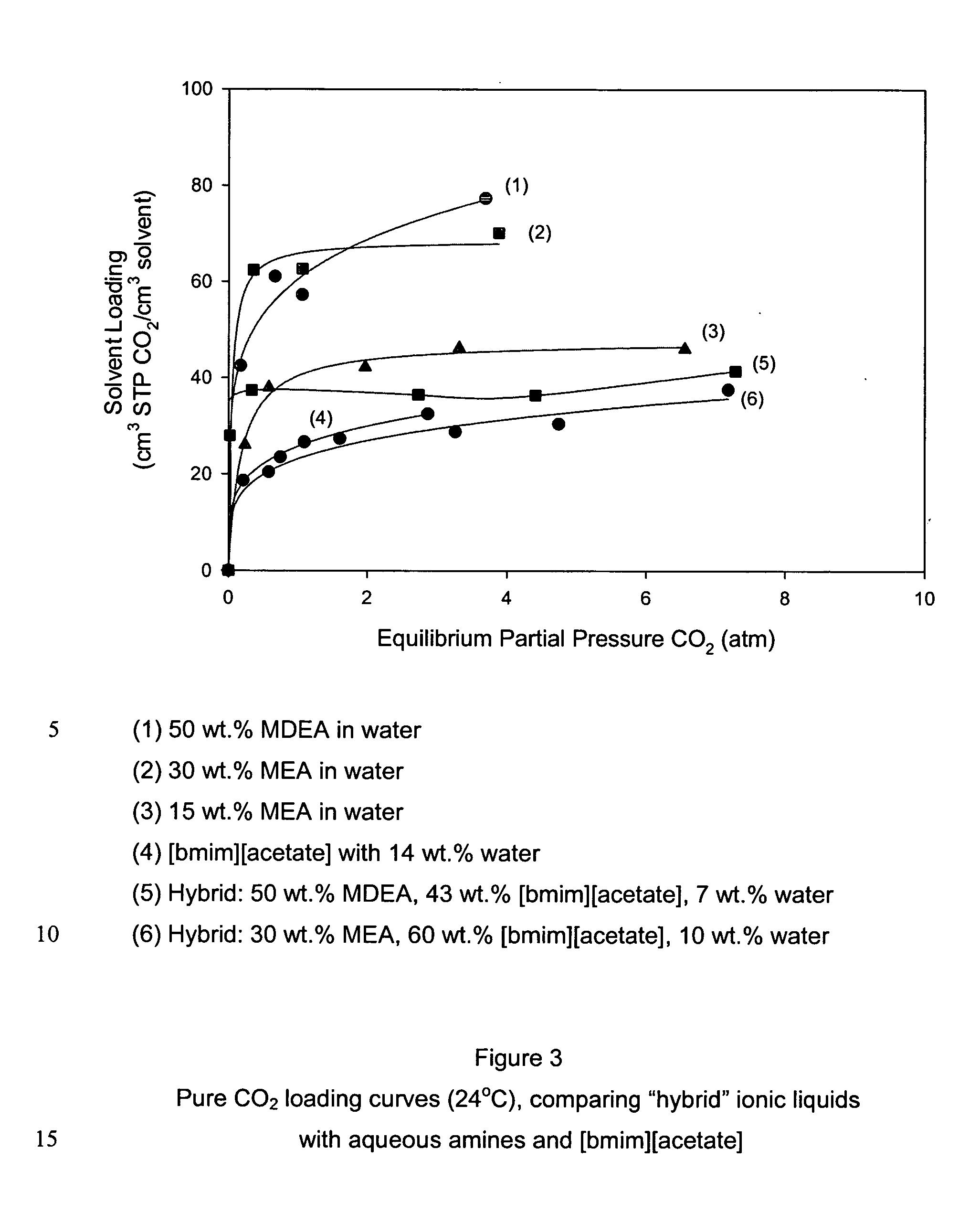
CO2 removal from gas using ionic liquid absorbents
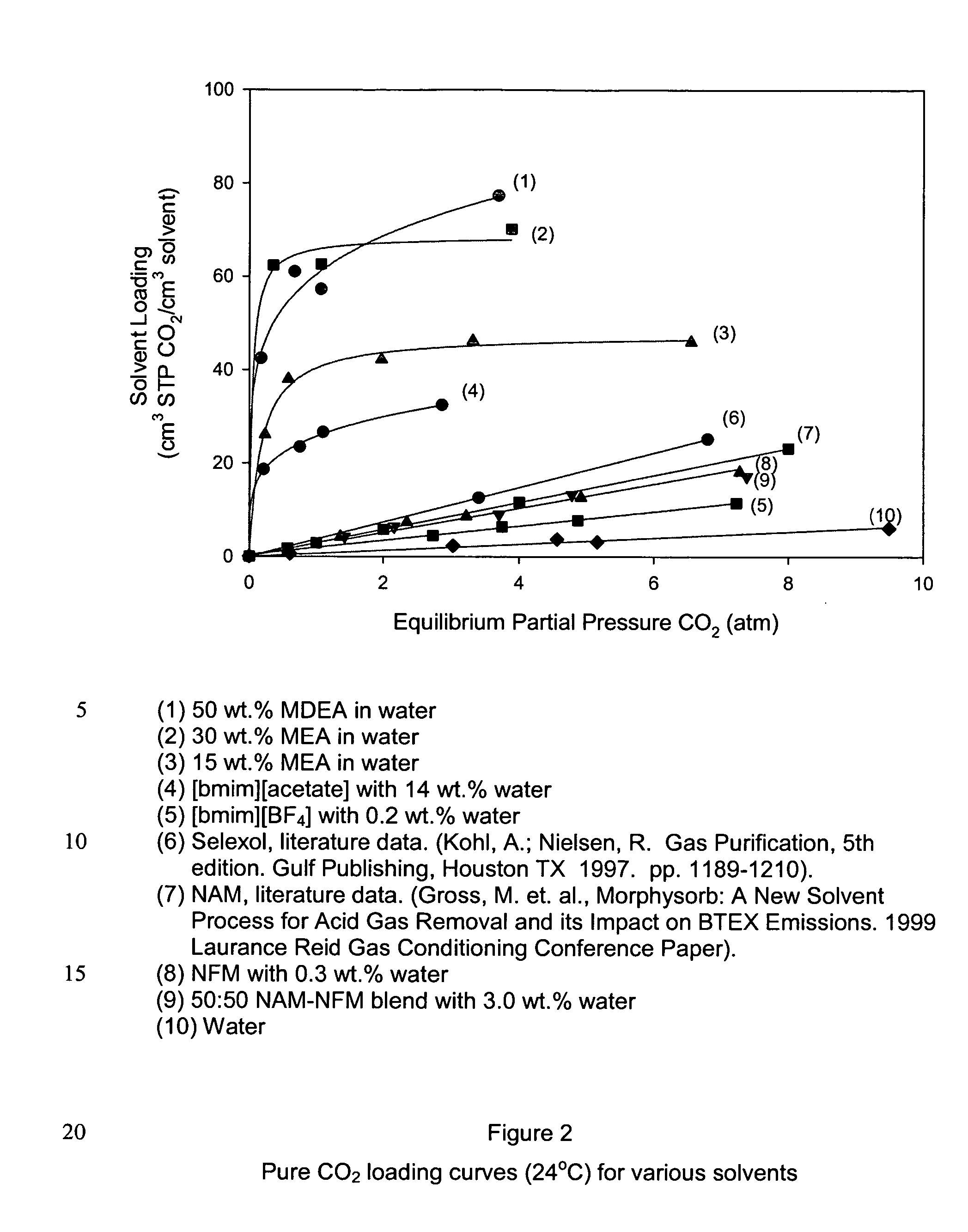
InactiveUS20050129598A1High CO capacityLow hydrocarbon solubilityHydrogen sulfidesDispersed particle separationCo2 removalSorbent
A process and method for separating CO2 from a gaseous stream such as natural gas. An ionic liquid comprising an anion having a carboxylate function is used as an adsorbent to selectively complex the CO2 yielding a gaseous stream with a greatly reduced CO2 content. The ionic liquid can then be readily be regenerated and recycled.
Owner:CHEVROU USA INC
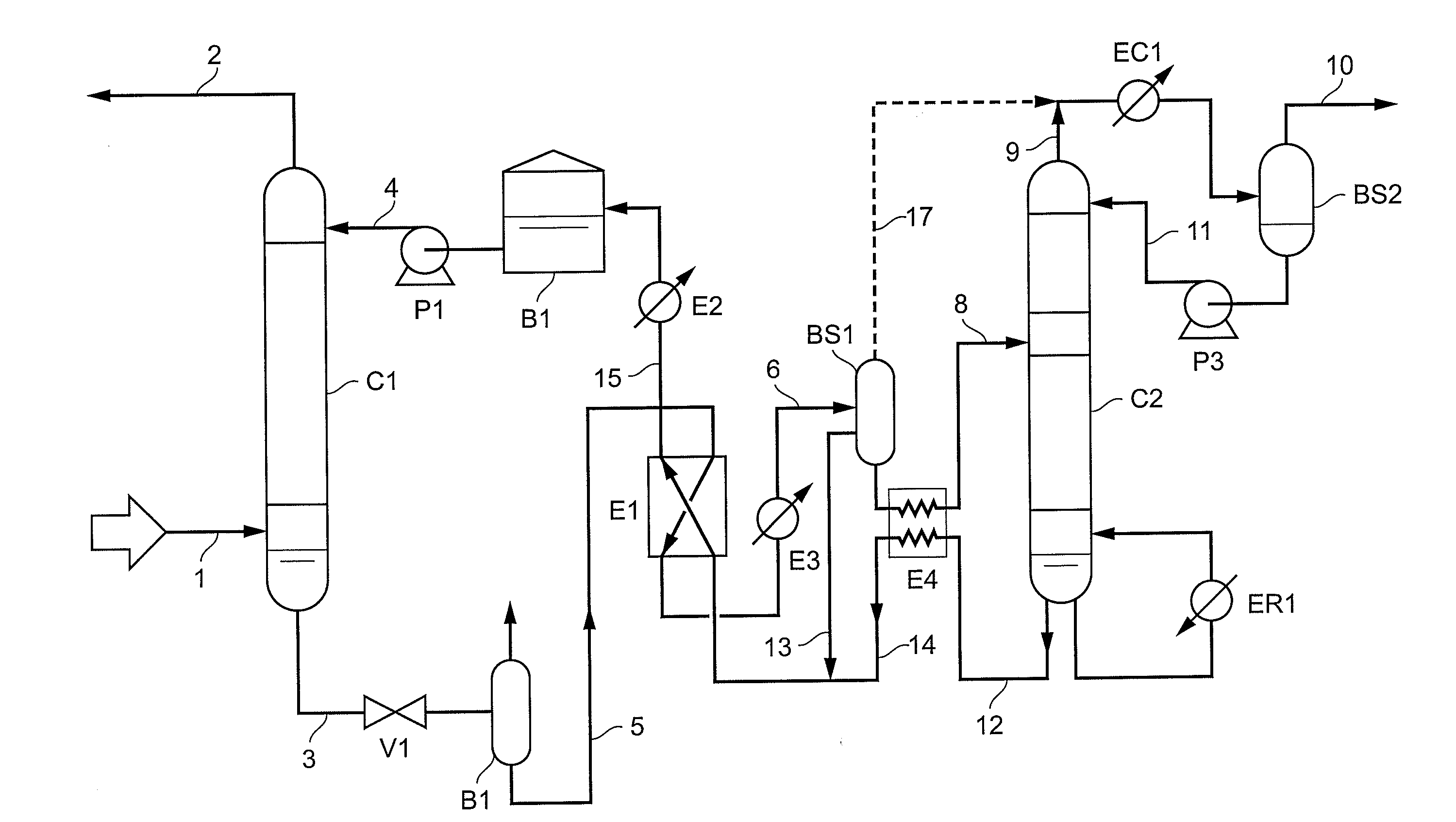
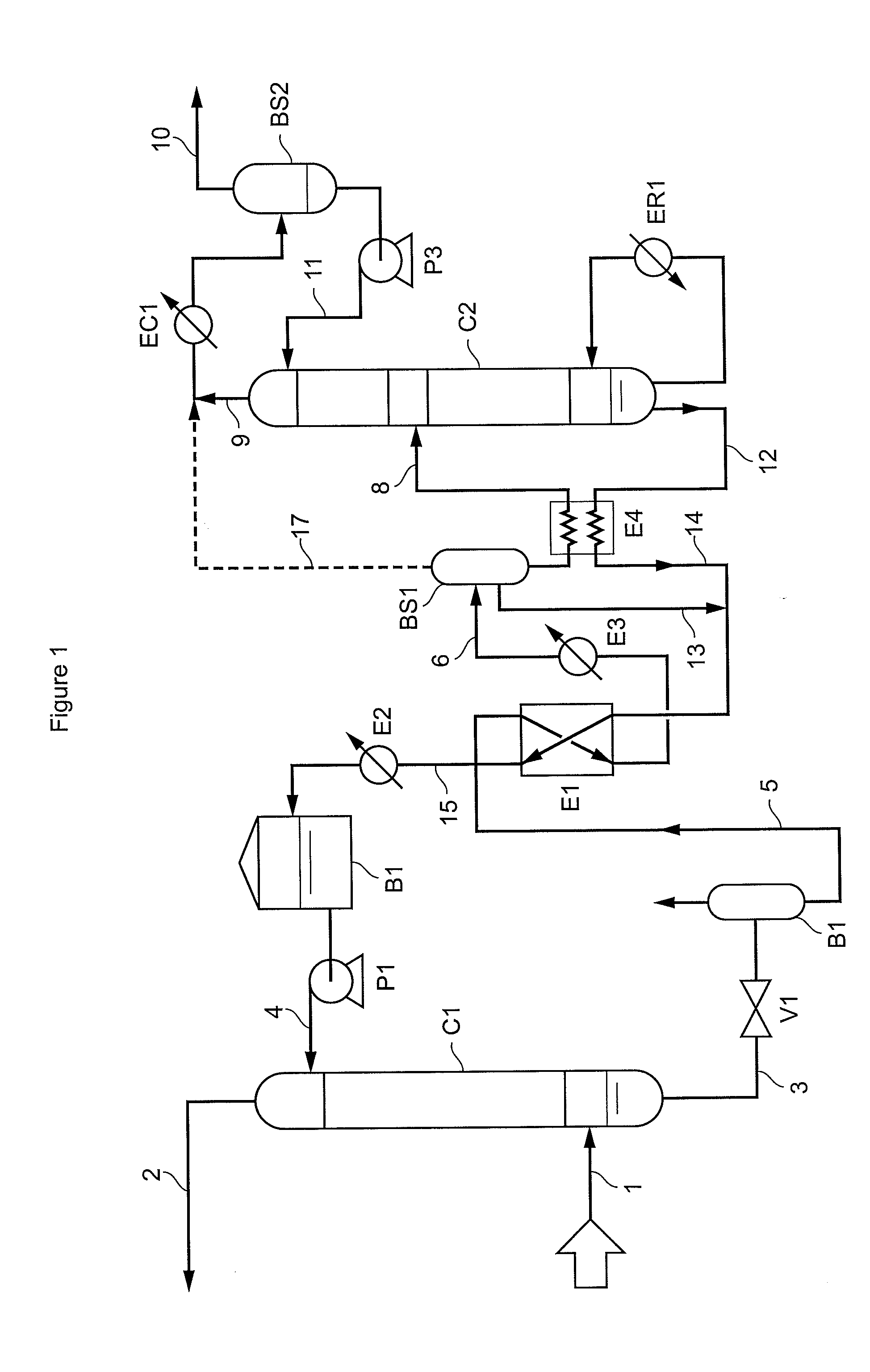
Method of deacidizing a gaseous effluent with extraction of the products to be regenerated
The present invention relates to a method of deacidizing a gaseous effluent comprising at least one of the acid compounds as follows: H2S, mercaptans, CO2, COS, SO2, CS2, wherein the following stages are carried out: a) contacting the acid compounds contained in said effluent with reactive compounds forming a liquid, so as to obtain a gaseous effluent depleted in acid compounds and a first liquid fraction comprising products formed by reaction of the reactive compounds with acid compounds, and reactive compounds that did not react with acid compounds, b) contacting said products contained in the first liquid fraction with extraction compounds forming a second liquid fraction so as to obtain a product-depleted first liquid fraction and a product-enriched second liquid fraction, c) recycling to stage a) the first liquid fraction obtained in stage b), said first liquid fraction obtained making up at least part of said liquid, d) regenerating the second liquid fraction obtained in stage b) so as to release acid compounds in gaseous form and to obtain a mixture of reactive compounds and of extraction compounds.
Owner:CARRETTE PIERRE LOUIS +6
Separation of carbon dioxide (CO2) from gas mixtures
ActiveUS7618606B2Good repeatabilityMaterial nanotechnologyCombustible gas catalytic treatmentCo2 removalSorbent
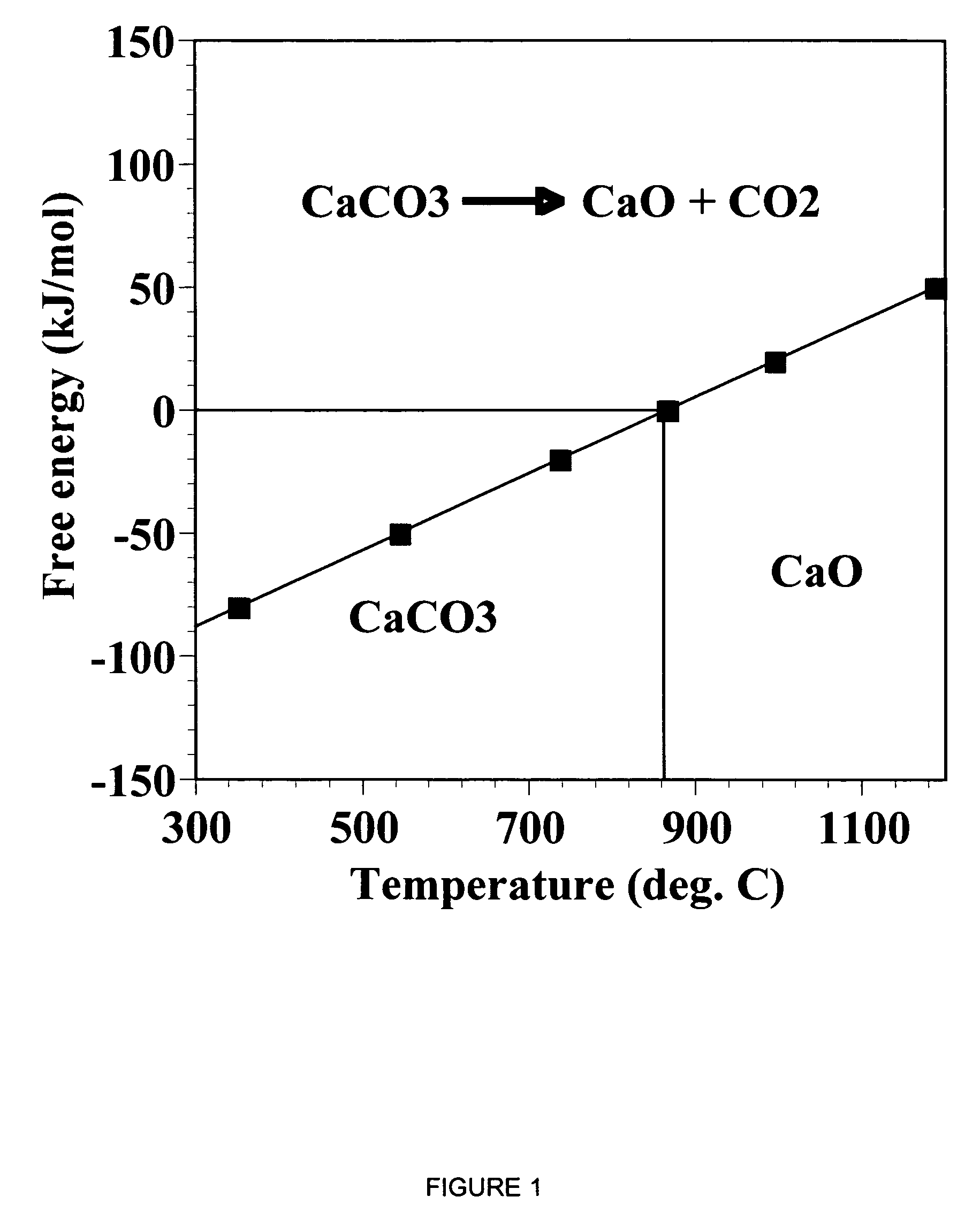
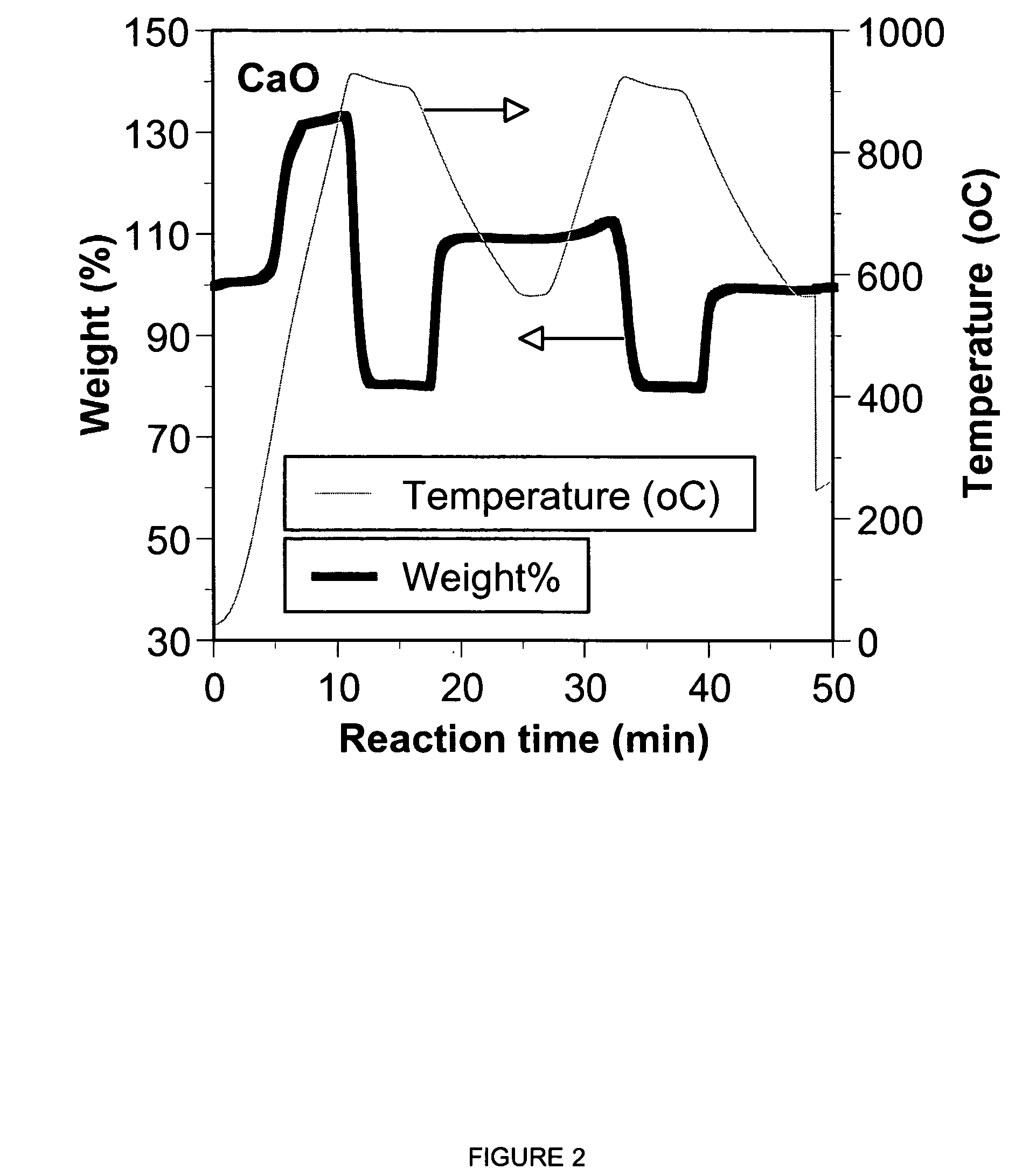
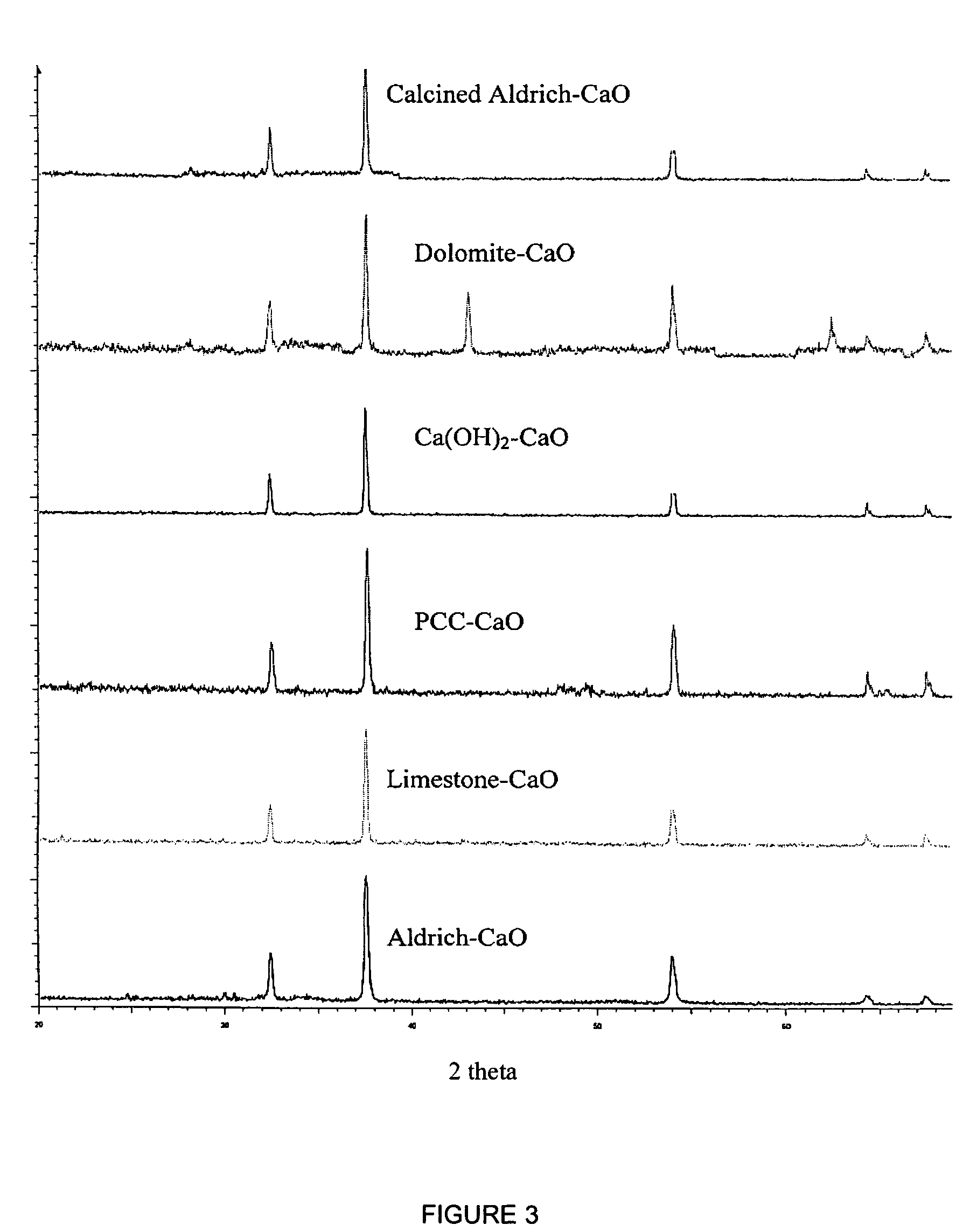
A reaction-based process has been developed for the selective removal of carbon dioxide from a multicomponent gas mixture. The proposed process effects the separation of CO2 from a mixture of gases by its reaction with metal oxides. The Calcium based Reaction Separation for CO2 process consists of contacting a CO2 laden gas with calcium oxide in a reactor such that CaO captures the CO2 by the formation of calcium carbonate. Once “spent”, CaCO3 is regenerated by its calcination leading to the formation of fresh CaO sorbent. The “regenerated” CaO is then recycled for the further capture of more CO2. This process also identifies the application of a mesoporous CaCO3 structure, that attains >90% conversion over multiple carbonation and calcination cycles. Lastly, thermal regeneration (calcination) under vacuum provided a better sorbent structure that maintained reproducible reactivity levels over multiple cycles.
Owner:THE OHIO STATES UNIV
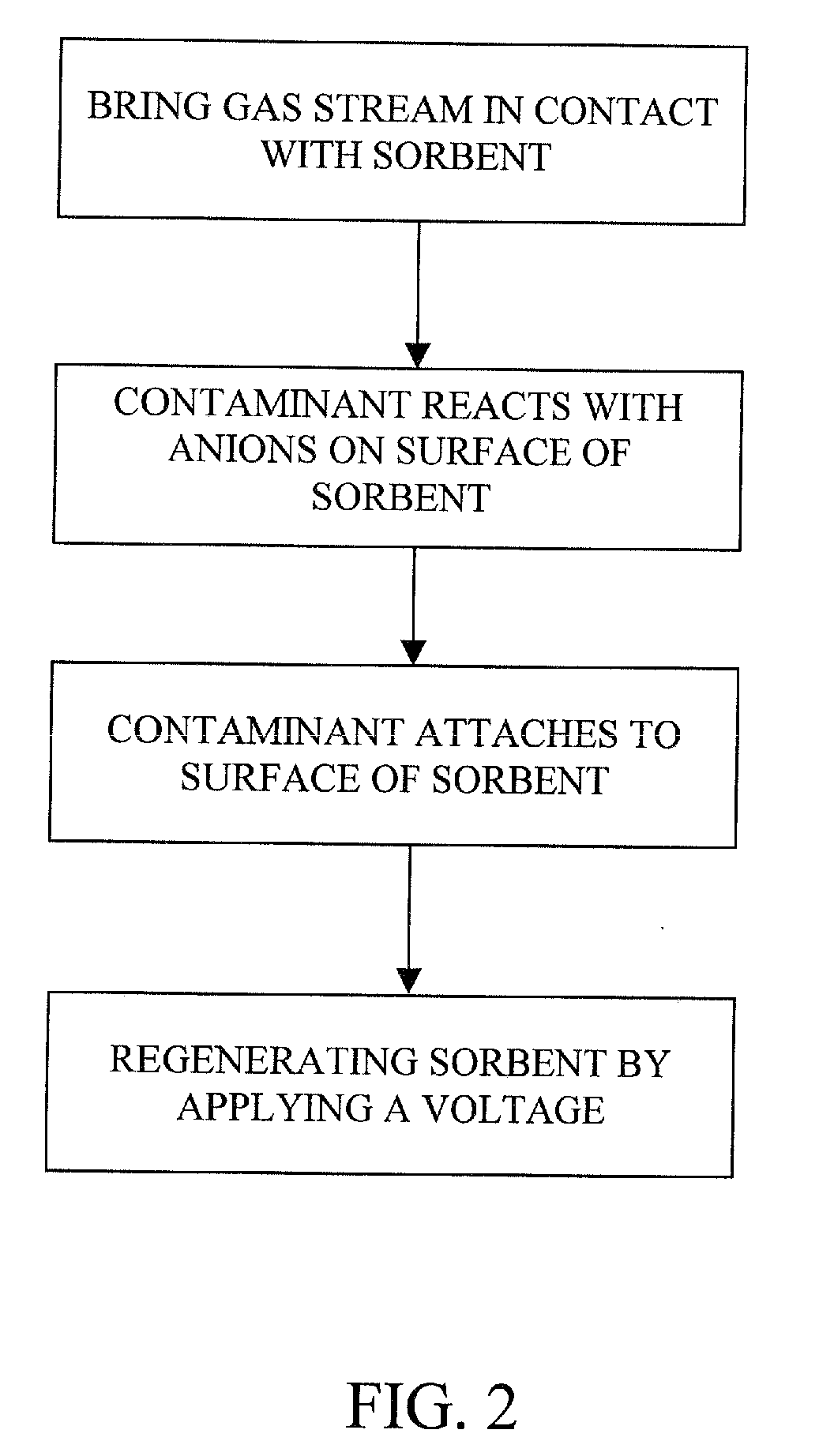
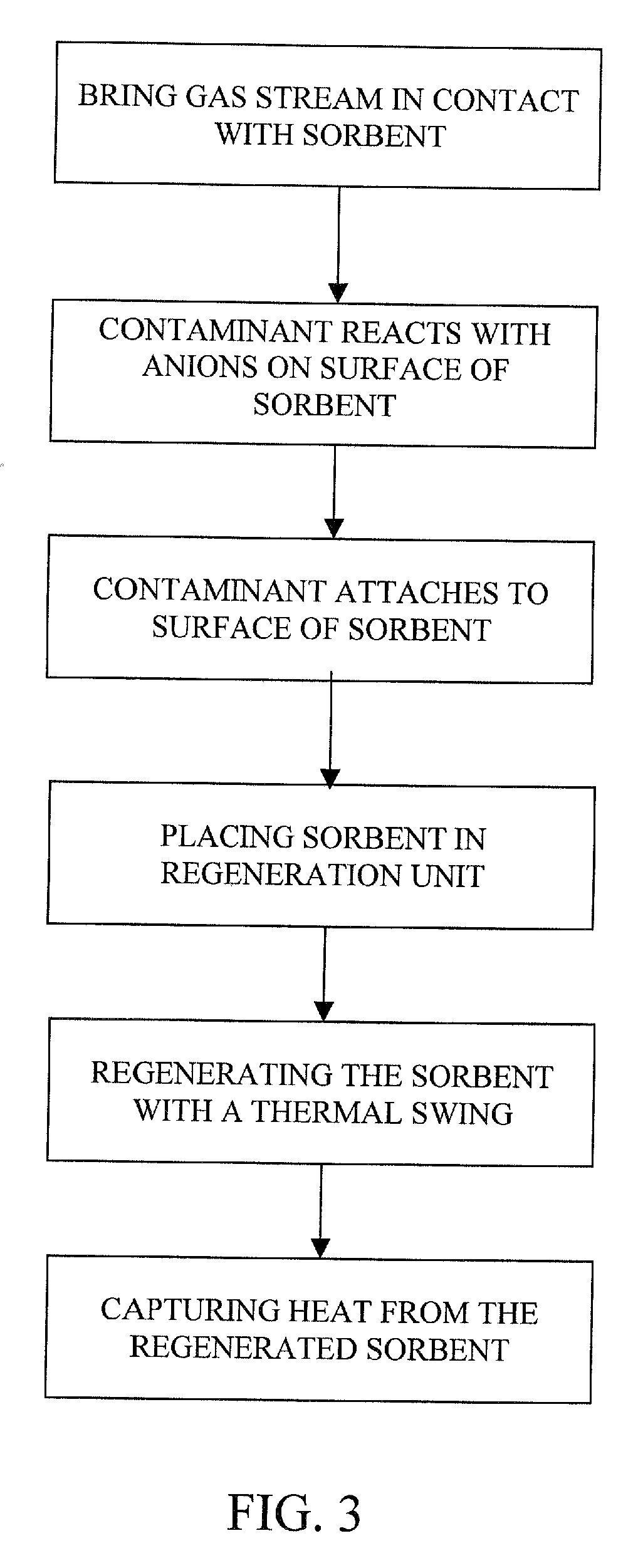
Removal of carbon dioxide from air
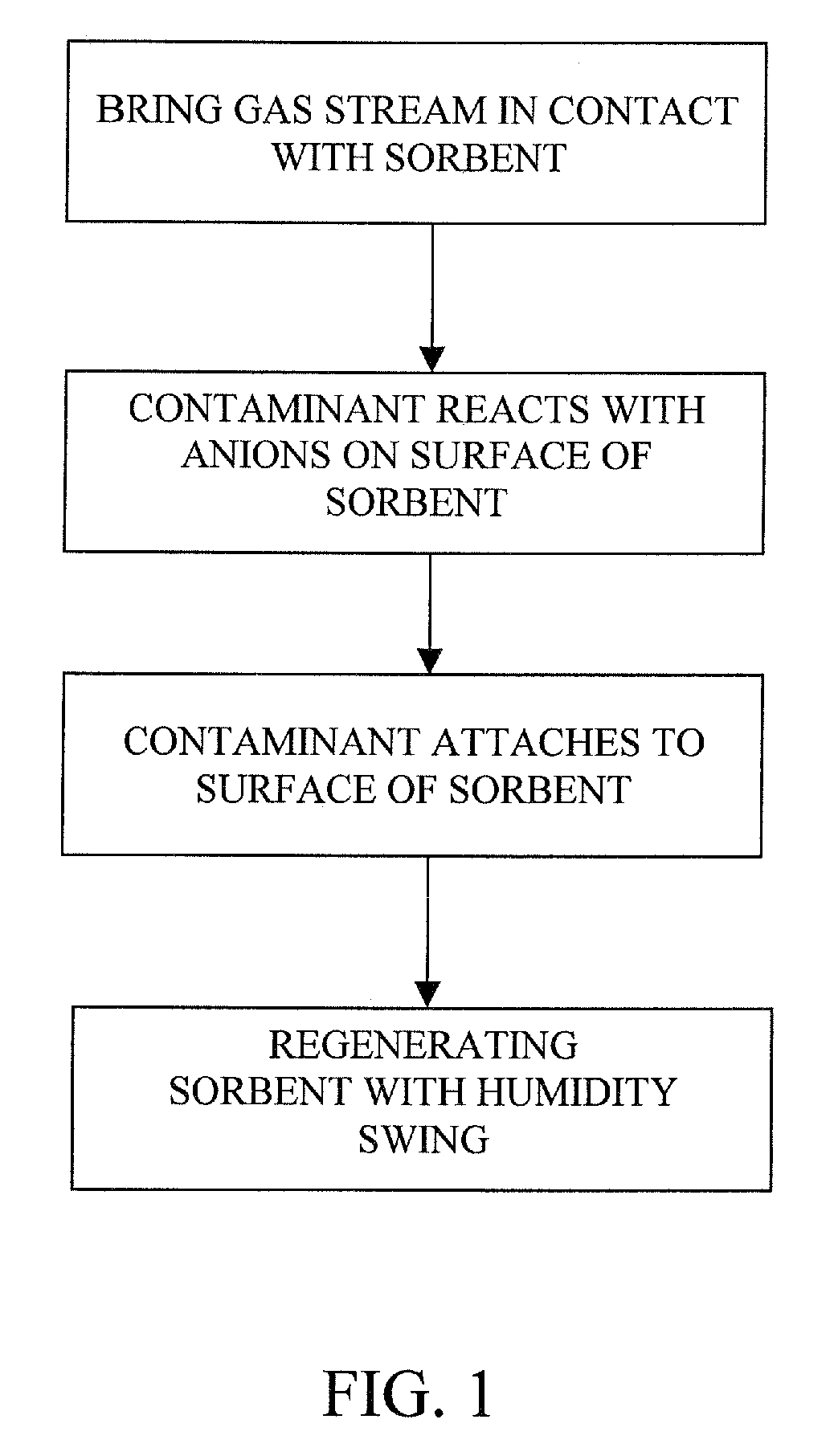
The present invention provides a method and apparatus for removing a contaminant, such as carbon dioxide, from a gas stream, such as ambient air. The contaminant is removed from the gas stream by a sorbent which may be regenerated using a humidity swing, a thermal swing, or a combination thereof. The sorbent may comprise a substrate having embedded positive ions and individually mobile negative ions wherein the positive ions are sufficiently spaced to prevent interactions between the negative ions. Where a thermal swing is used, heat may be conserved by employing a heat exchanger to transfer heat from the regenerated sorbent to an amount of sorbent that is loaded with the contaminant prior to regeneration.
Owner:CARBON SINK
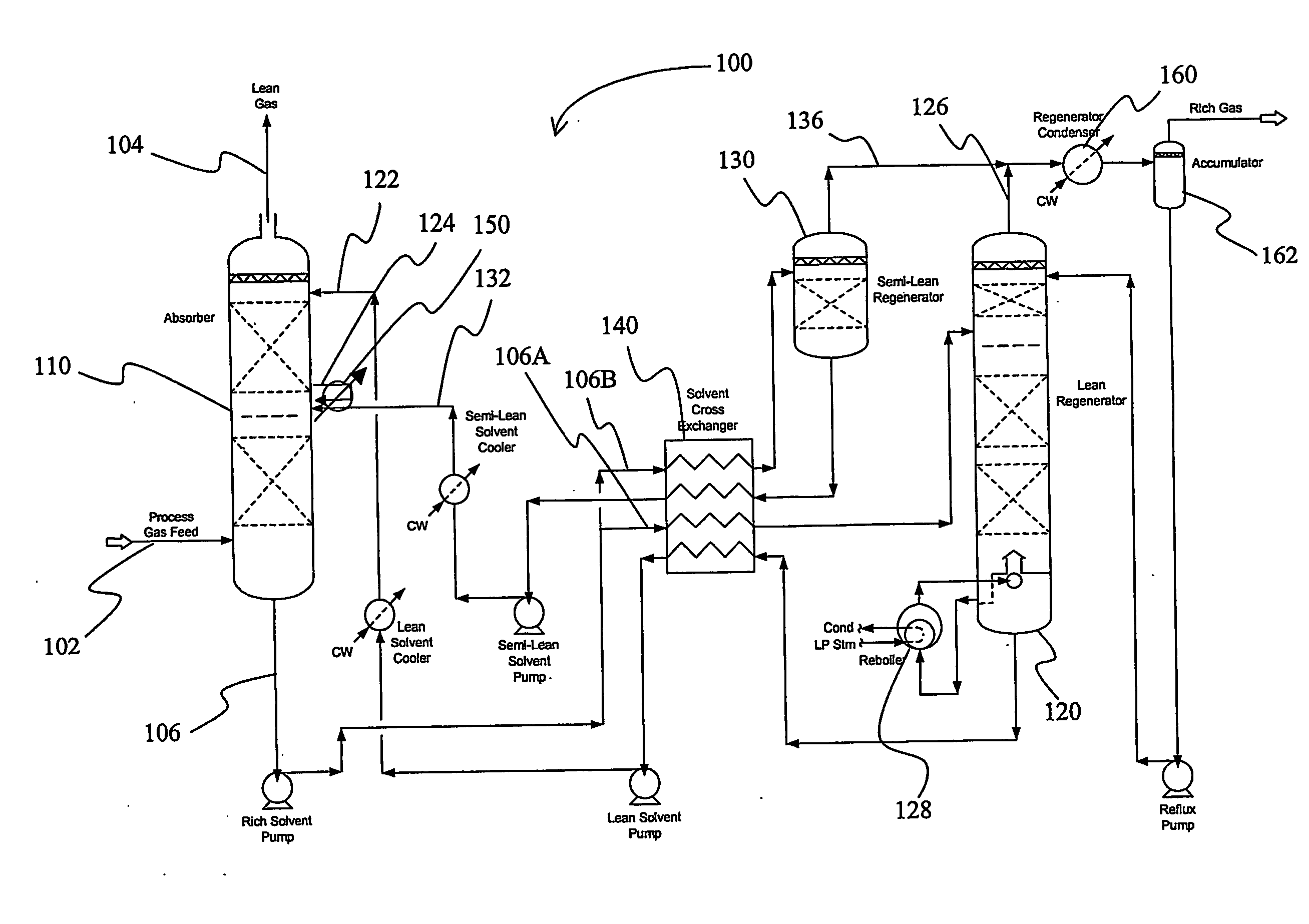
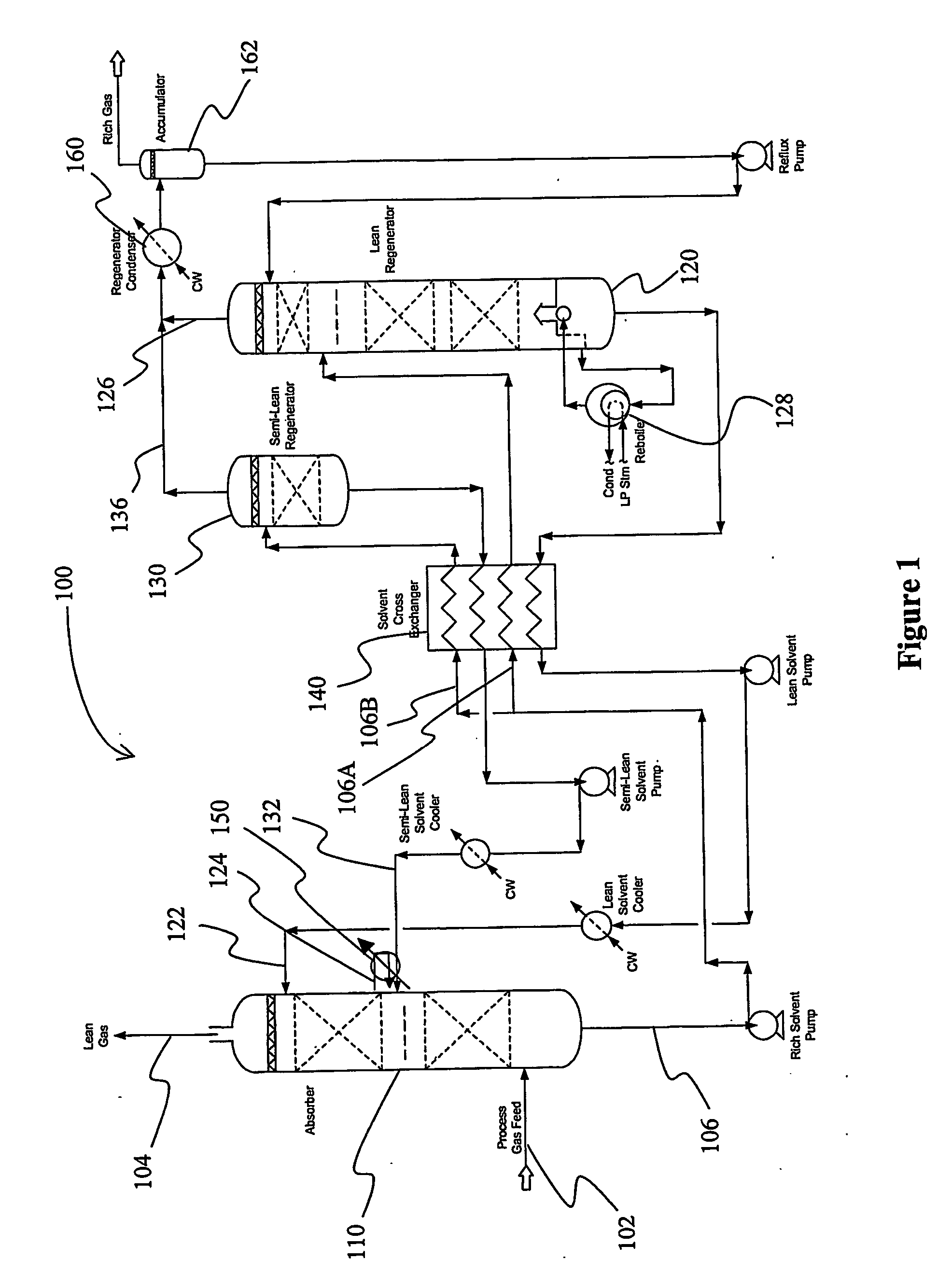
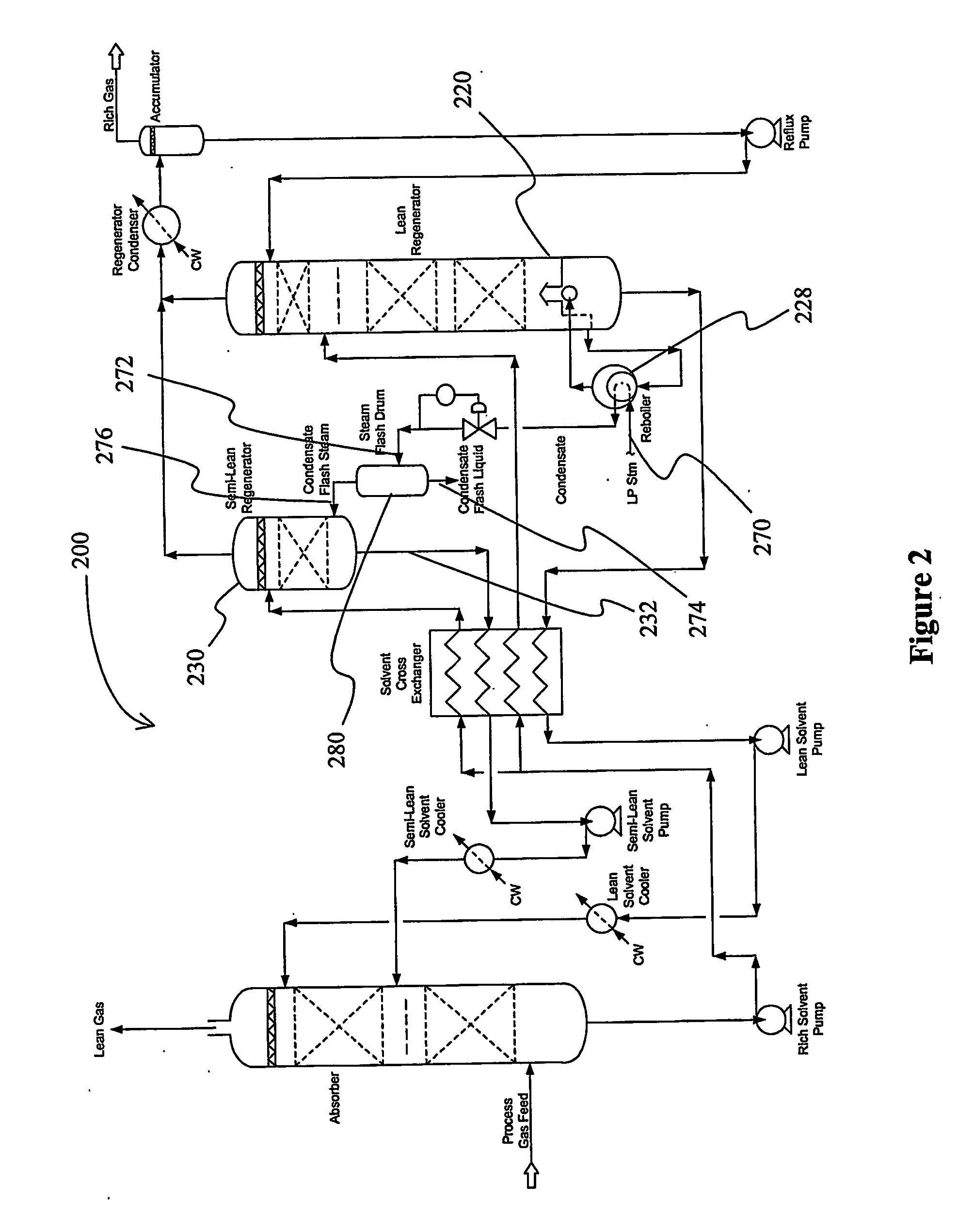
Split flow process and apparatus
An acid gas removal plant includes an absorber that provides a rich solvent to two regenerators that independently generate a lean and a semi-lean solvent, wherein the semi-lean solvent is produced in one of the regenerators using heat and / or steam derived from the other regenerator. Further heat integration is particularly contemplated with power plants in which the power plant provides high-level heat to the acid gas removal plant and wherein the power plant receives low-level heat from the acid gas removal plant.
Owner:FLUOR TECH CORP
Capture and Sequestration of Carbon Dioxide in Flue Gases
ActiveUS20090202410A1Calcium/strontium/barium carbonatesPigmenting treatmentAlkaline earth metalOxidation state
There is provided a process for the capture and sequestration of carbon dioxide that would otherwise enter the atmosphere and contribute to global warming and other problems. CO2 capture is accomplished by reacting carbon dioxide in flue gas with an alkali metal carbonate, or a metal oxide, particularly containing an alkaline earth metal or iron, to form a carbonate salt. A preferred carbonate for CO2 capture is a dilute aqueous solution of additive-free (Na2CO3). Other carbonates include (K2CO3) or other metal ion that can produce both a carbonate and a bicarbonate salt. Examples of suitable metal oxides include several alkaline earths including CaO and MgO. The captured CO2 is preferably sequestered using any available mineral or industrial waste that contains calcium magnesium or iron in non-carbonate forms, or iron in the Fe+2 oxidation state.
Owner:MICHIGAN TECHNOLOGICAL UNIVERSITY
Method of deacidizing a gas with a fractional regeneration absorbent solution
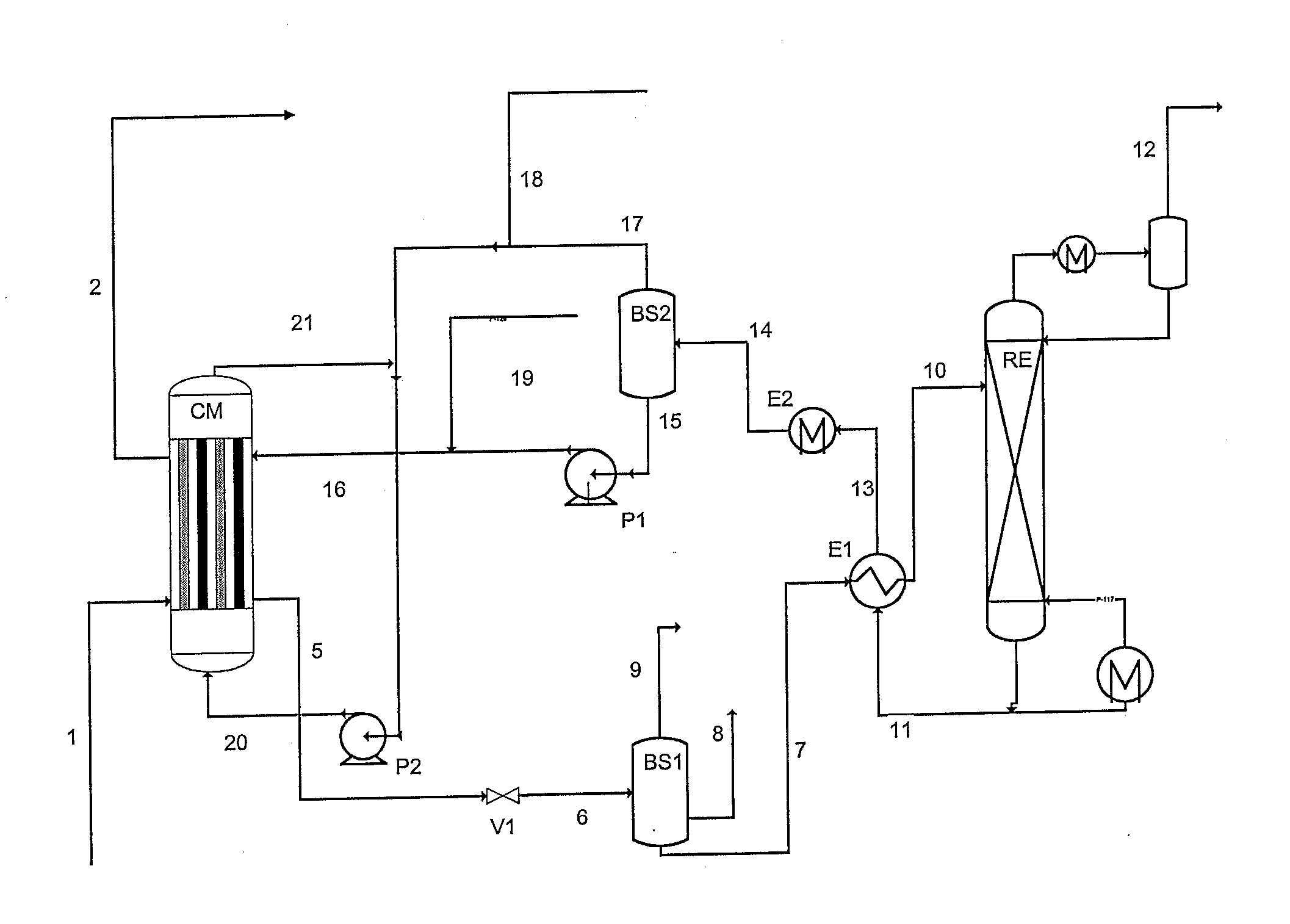
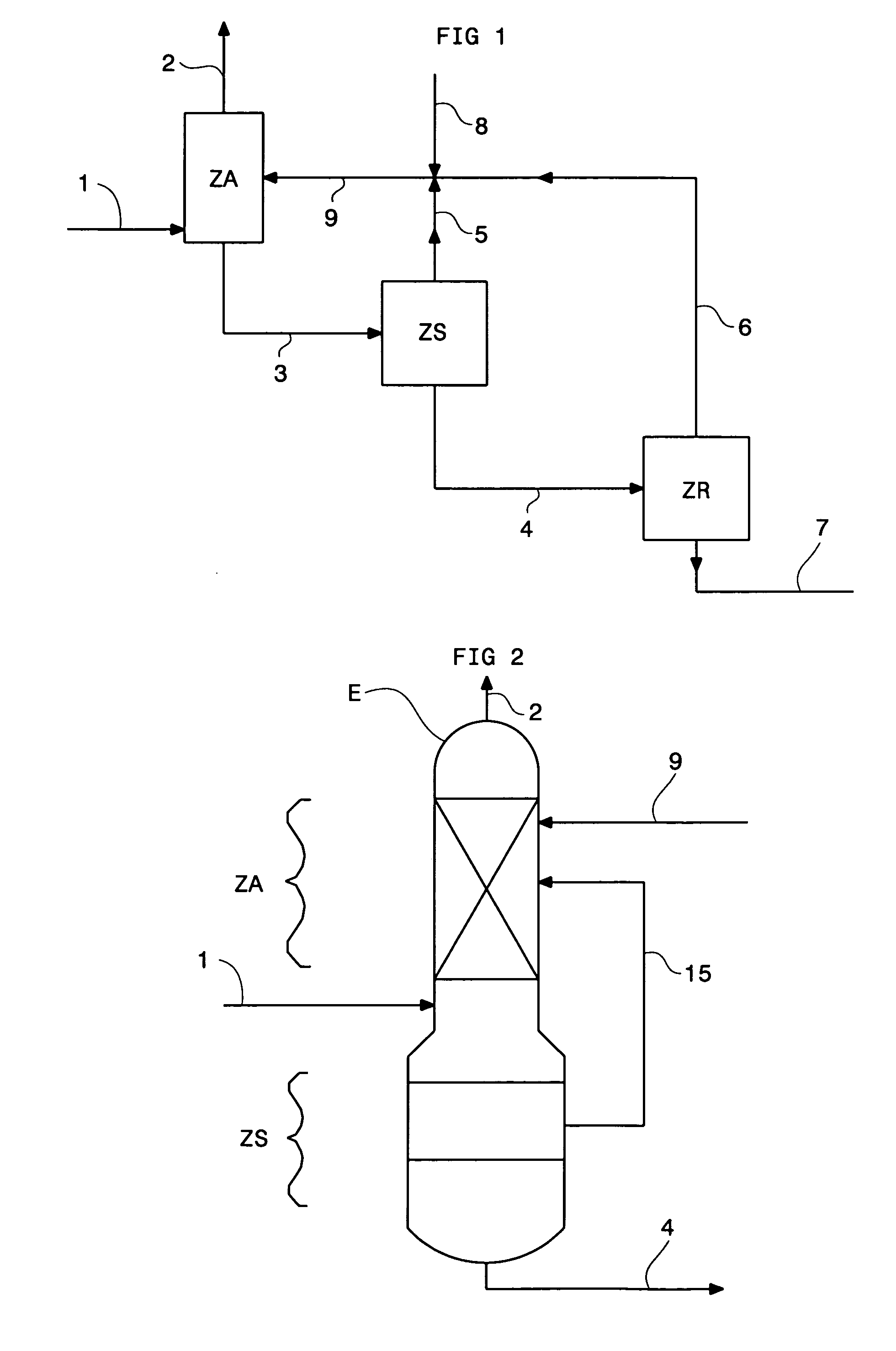
The gaseous effluent flowing in through line 1 is contacted in absorption zone ZA with the liquid absorbent solution flowing in through line 9. The gaseous effluent depleted in acid compounds is discharged through line 2. The absorbent solution laden with acid compounds is discharged through line 3. The absorbent solution once laden with acid compounds comprises two phases: a first phase poor in acid compounds and a second phase rich in acid compounds. The two phases are separated in zone ZS. The first phase is recycled through lines 5 and 9 to absorption zone ZA. The second phase is fed through line 4 into regeneration zone ZR. In zone ZR, the acid compounds are separated from the absorbent solution. The acid compounds are discharged through line 7. The regenerated absorbent solution is recycled through lines 6 and 9 to zone ZA.
Owner:INST FR DU PETROLE
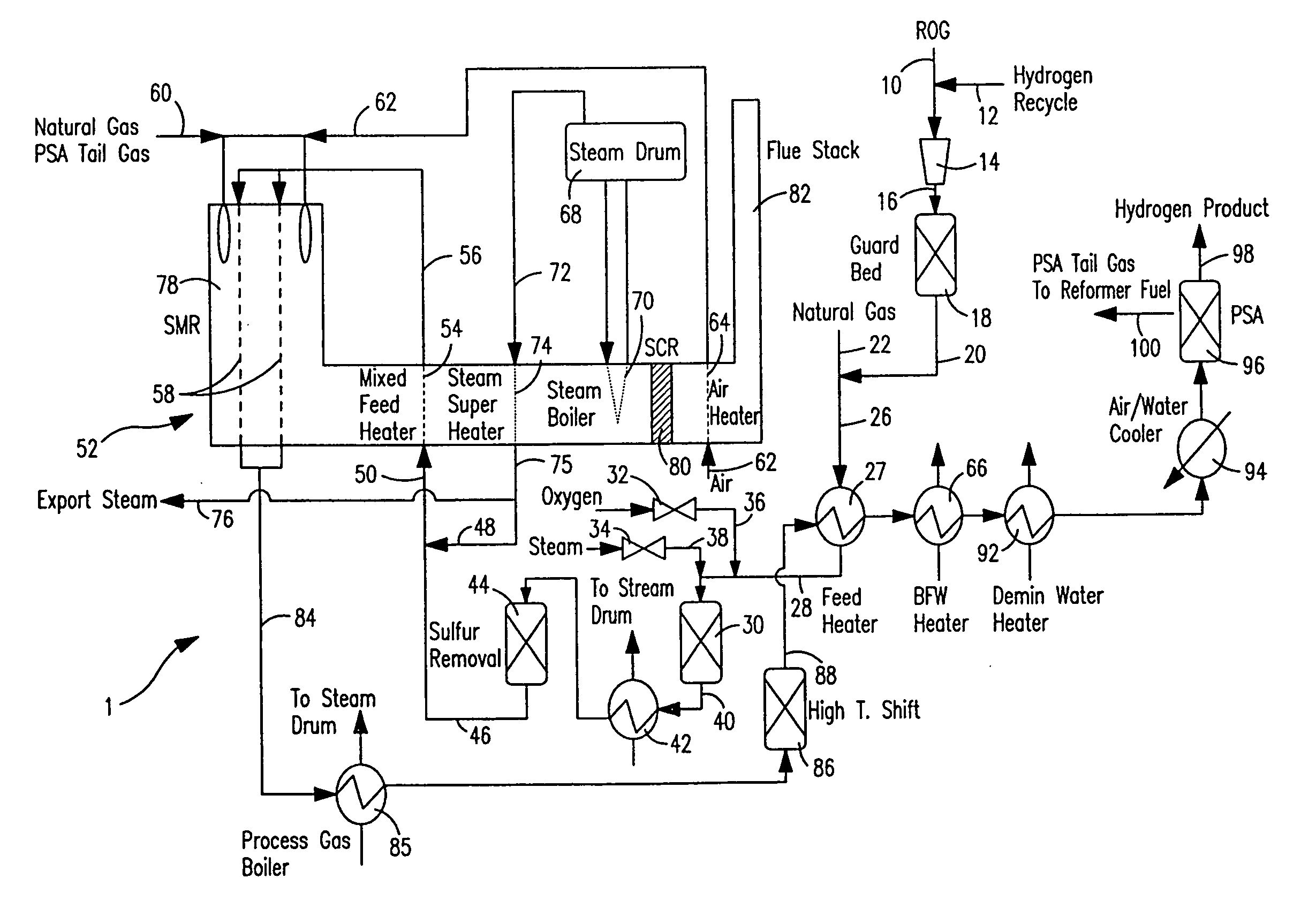
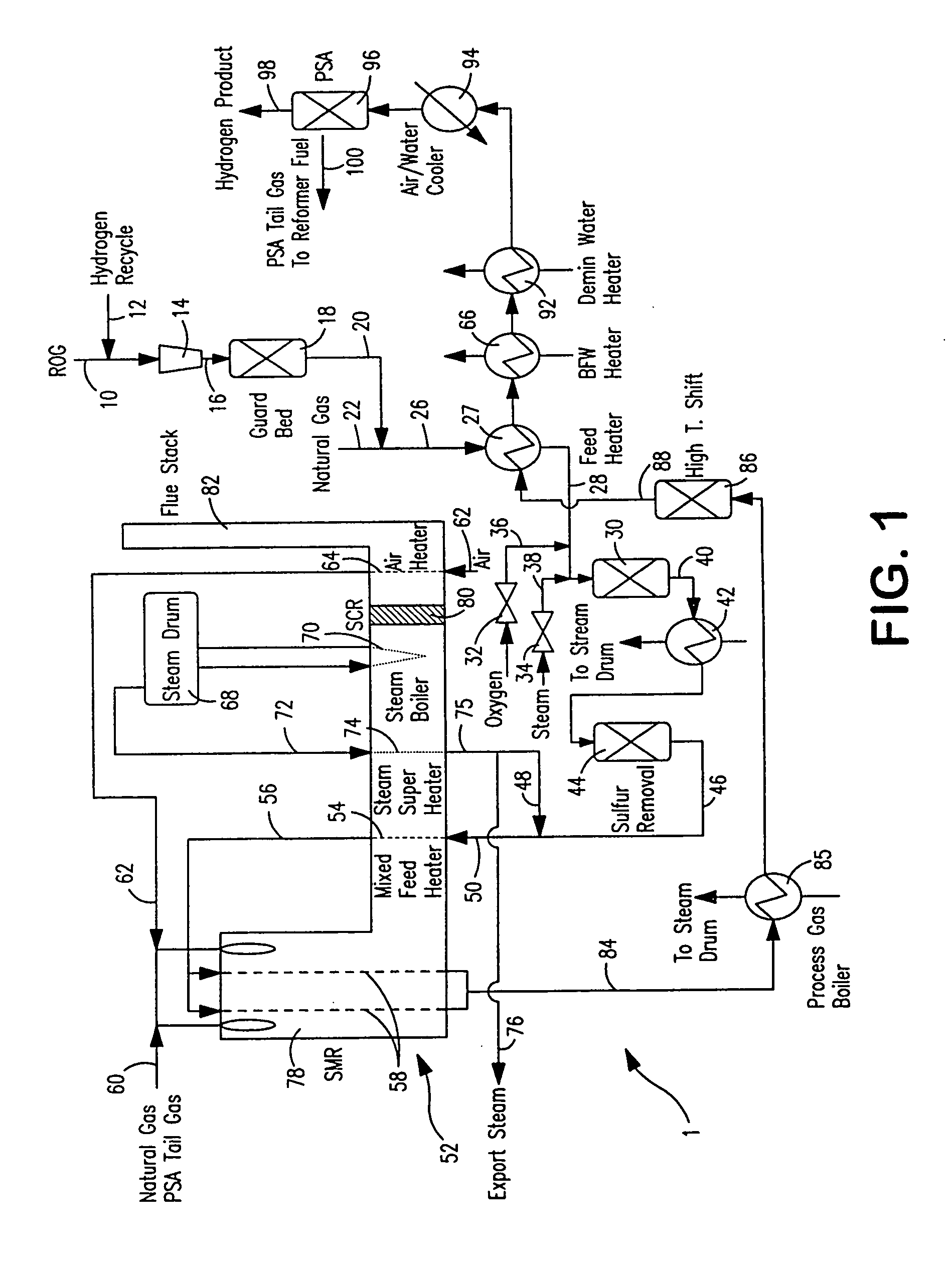
Method and apparatus for treating a sour gas
Owner:AIR PROD & CHEM INC
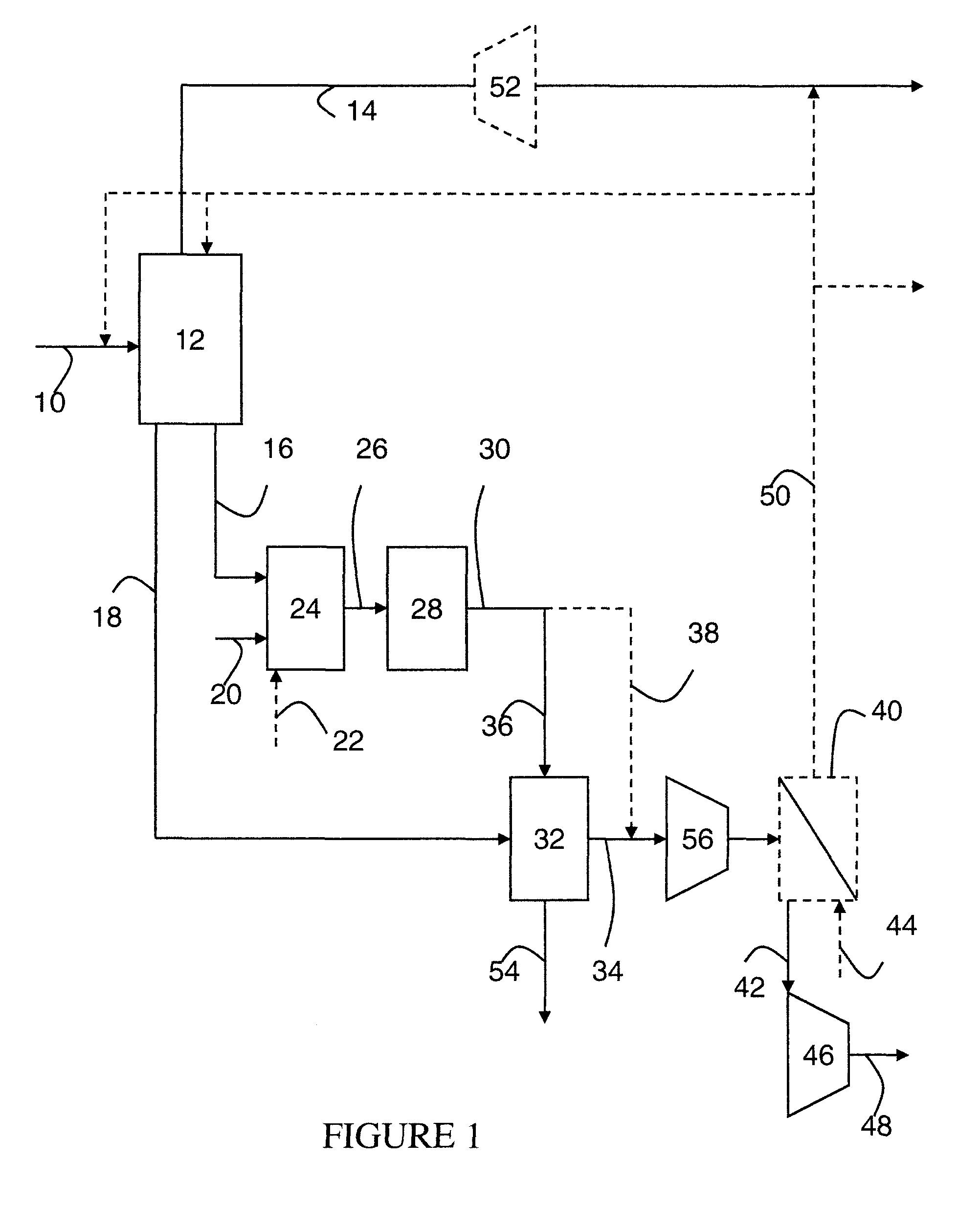
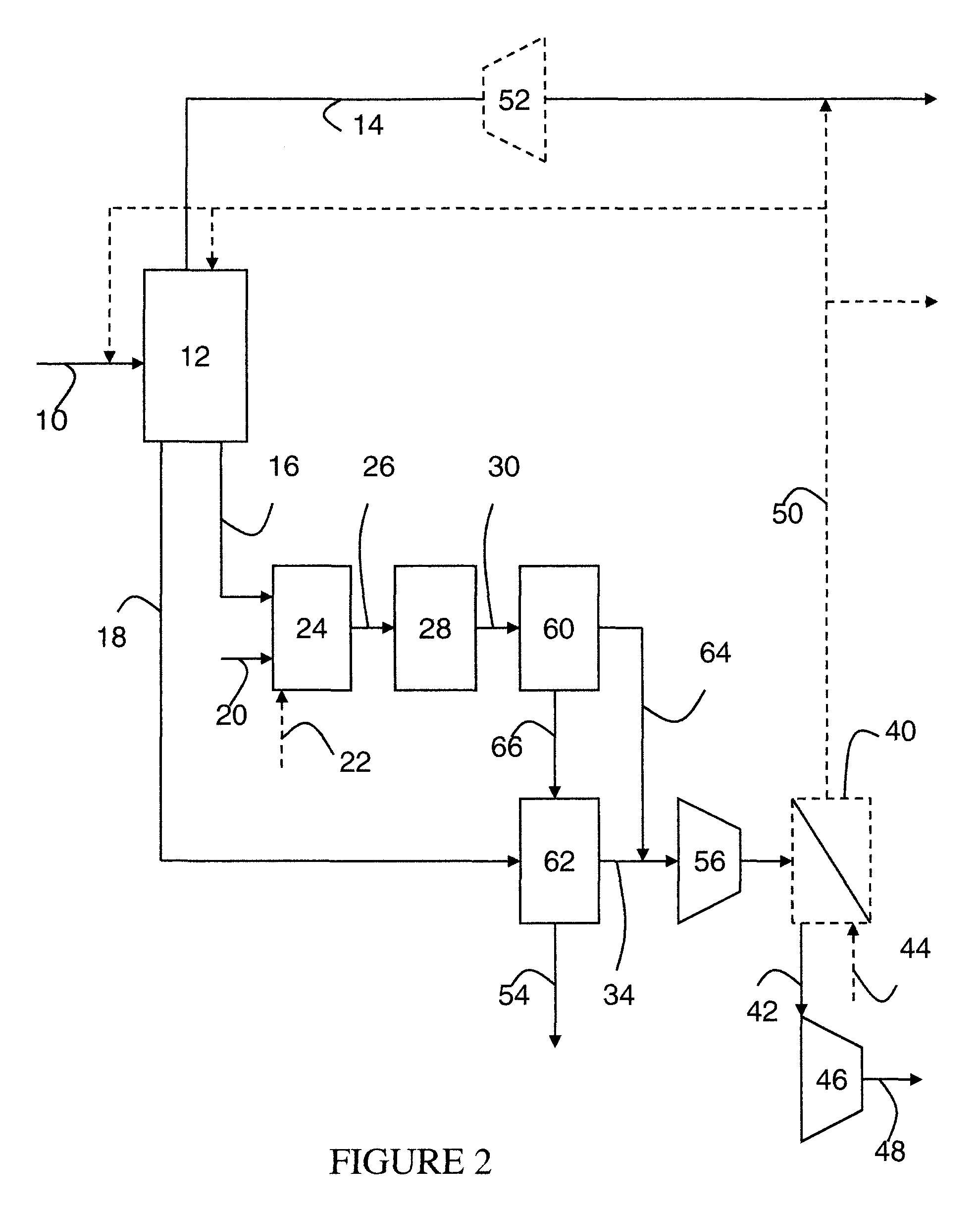
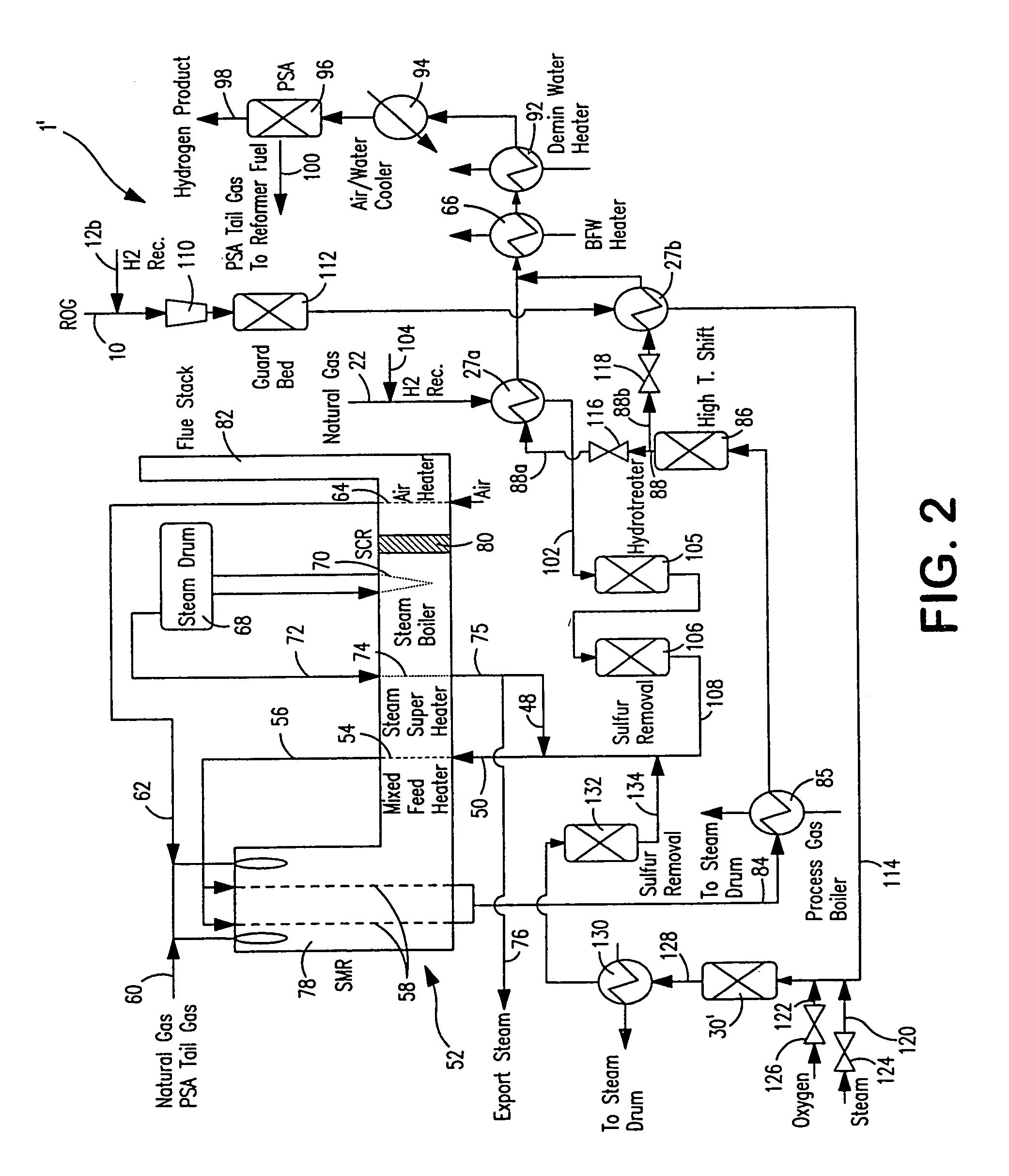
Steam methane reforming method
ActiveUS7037485B1Reduce fuel usageReduce firing rateHydrocarbon from carbon oxidesHydrogen separation using solid contactMethane reformerAlkane
A steam methane reforming method in which a feed stream is treated in a reactor containing a catalyst that is capable of promoting both hydrogenation and partial oxidation reactions. The reactor is either operated in a catalytic hydrogenation mode to convert olefins into saturated hydrocarbons and / or to chemically reduce sulfur species to hydrogen sulfide or a catalytic oxidative mode utilizing oxygen and steam to prereform the feed and thus, increase the hydrogen content of a synthesis gas produced by a steam methane reformer. The method is applicable to the treatment of feed streams containing at least 15% by volume of hydrocarbons with two or more carbon atoms and / or 3% by volume of olefins, such as a refinery off-gas. In such case, the catalytic oxidative mode is conducted with a steam to carbon ratio of less than 0.5, an oxygen to carbon ratio of less than 0.25 and a reaction temperature of between about 500° C. and about 860° C. to limit the feed to the steam methane reformer to volumetric dry concentrations of less than about 0.5% for the olefins and less than about 10% for alkanes with two or more carbon atoms.
Owner:PRAXAIR TECH INC
Amine absorber for carbon dioxide capture and processes for making and using the same
InactiveUS20100263534A1Lower potentialIncreasing amineGas treatmentMolecular sieve catalystsSulfurAbsorbent material
Owner:THE UNIVERSITY OF AKRON
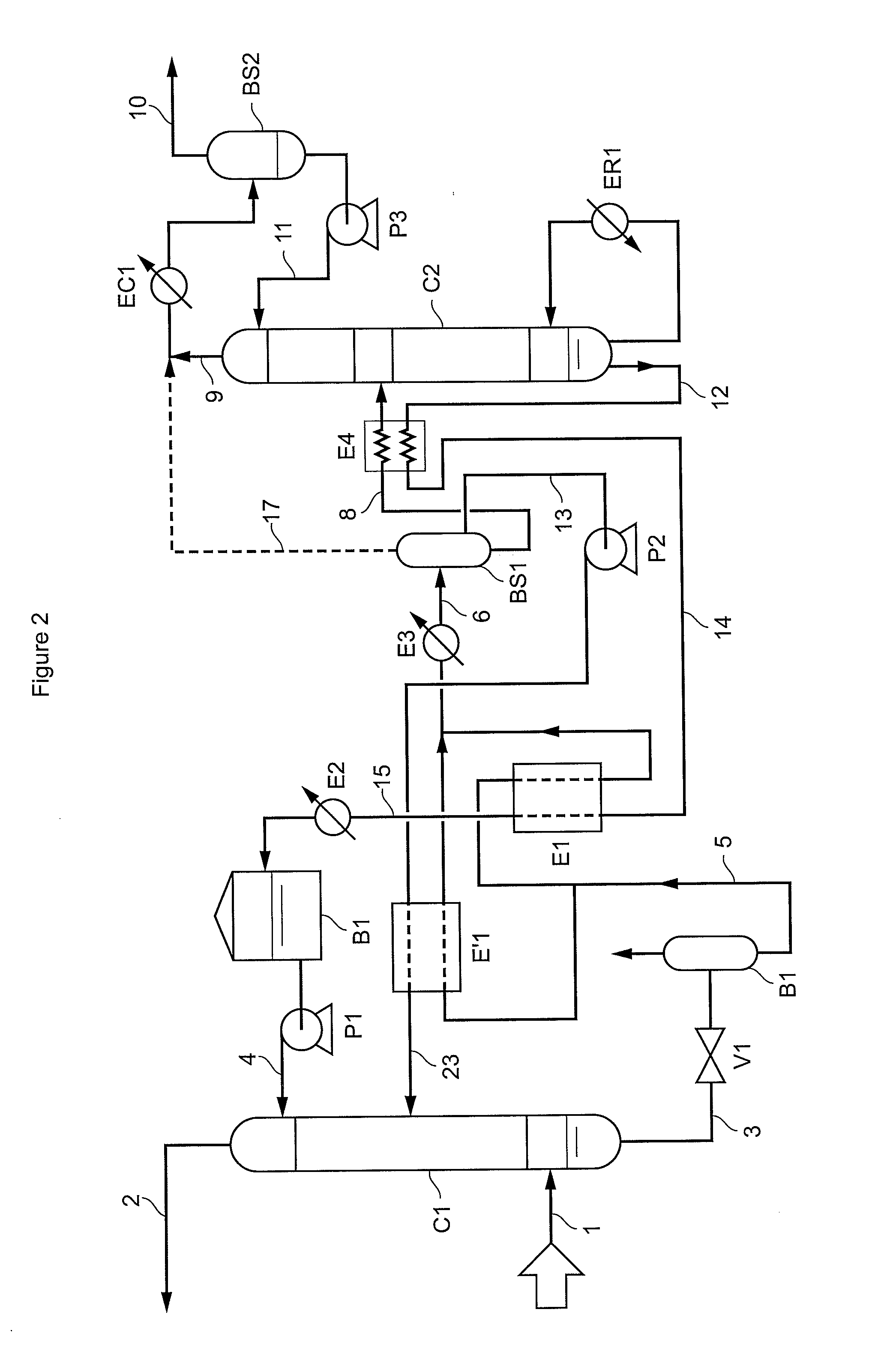
Method of deacidizing a gas by means of an absorbent solution with fractionated regeneration through heating
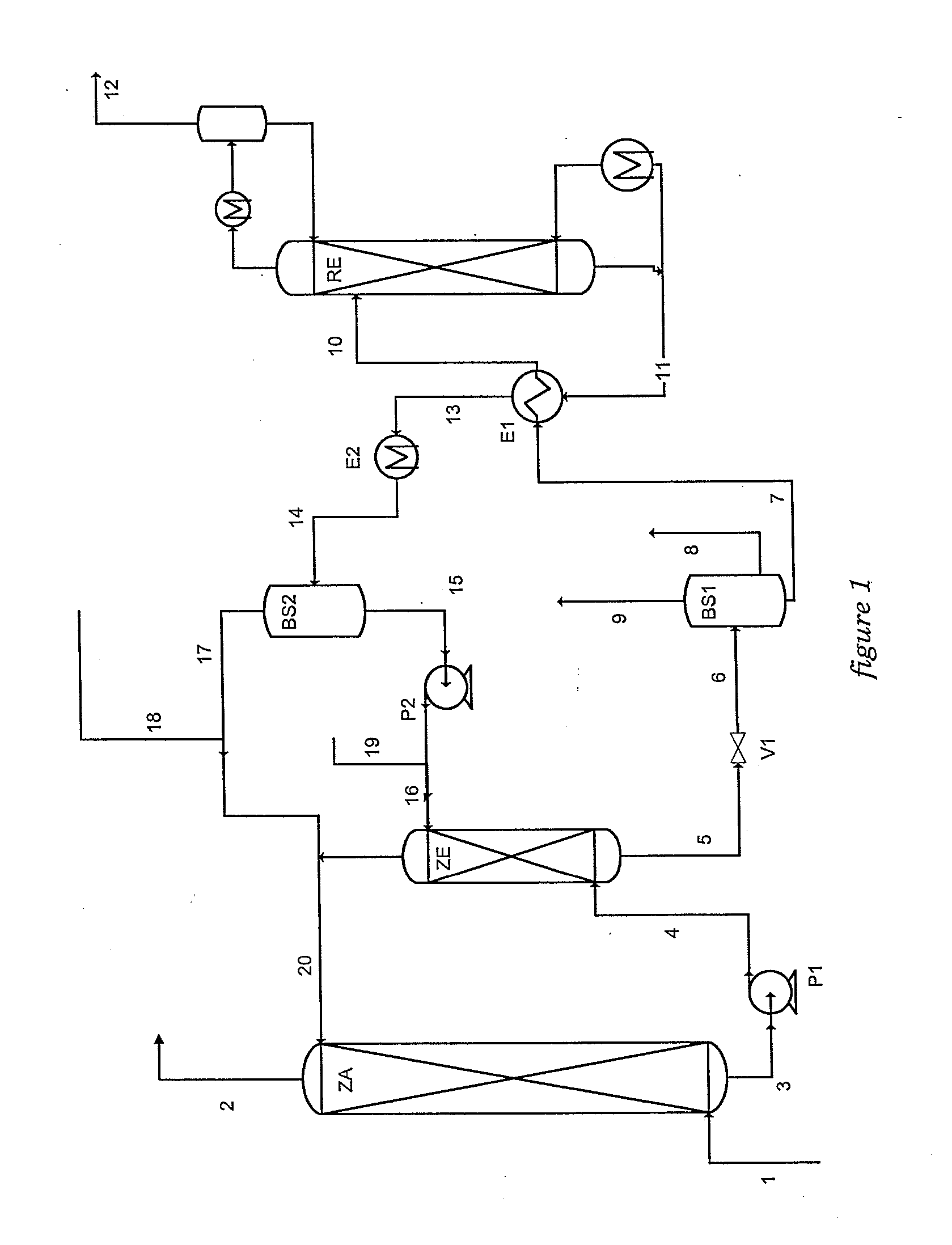
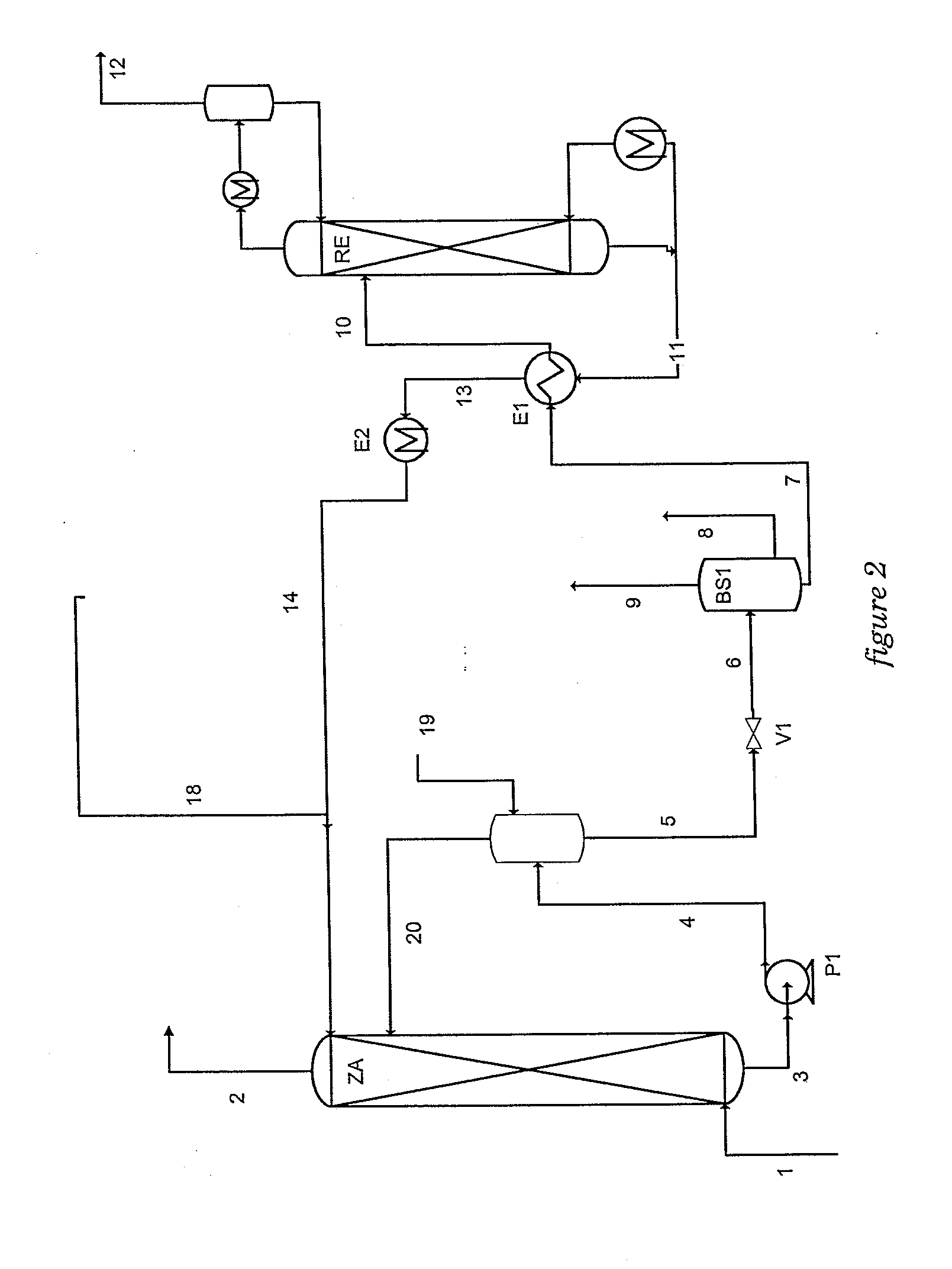
A method of deacidizing a gaseous effluent comprising acid compounds where the gaseous effluent is contacted in C1 with an adsorbent solution so as to obtain a gaseous effluent depleted in acid compounds and an absorbent solution laden with acid compounds, the absorbent solution being selected for its property of forming two separable phases when it has absorbed an amount of acid compounds and when it is heated. The absorbent solution laden with acid compounds is then heated in E1 and E3 so as to separate two fractions: a first absorbent solution fraction depleted in acid compounds and a second absorbent solution fraction enriched in acid compounds. These two fractions are then separated in BS1. The second fraction is regenerated in C2 so as to release part of the acid compounds, and the first absorbent solution fraction and the regenerated absorbent solution are recycled as absorbent solution.
Owner:INST FR DU PETROLE
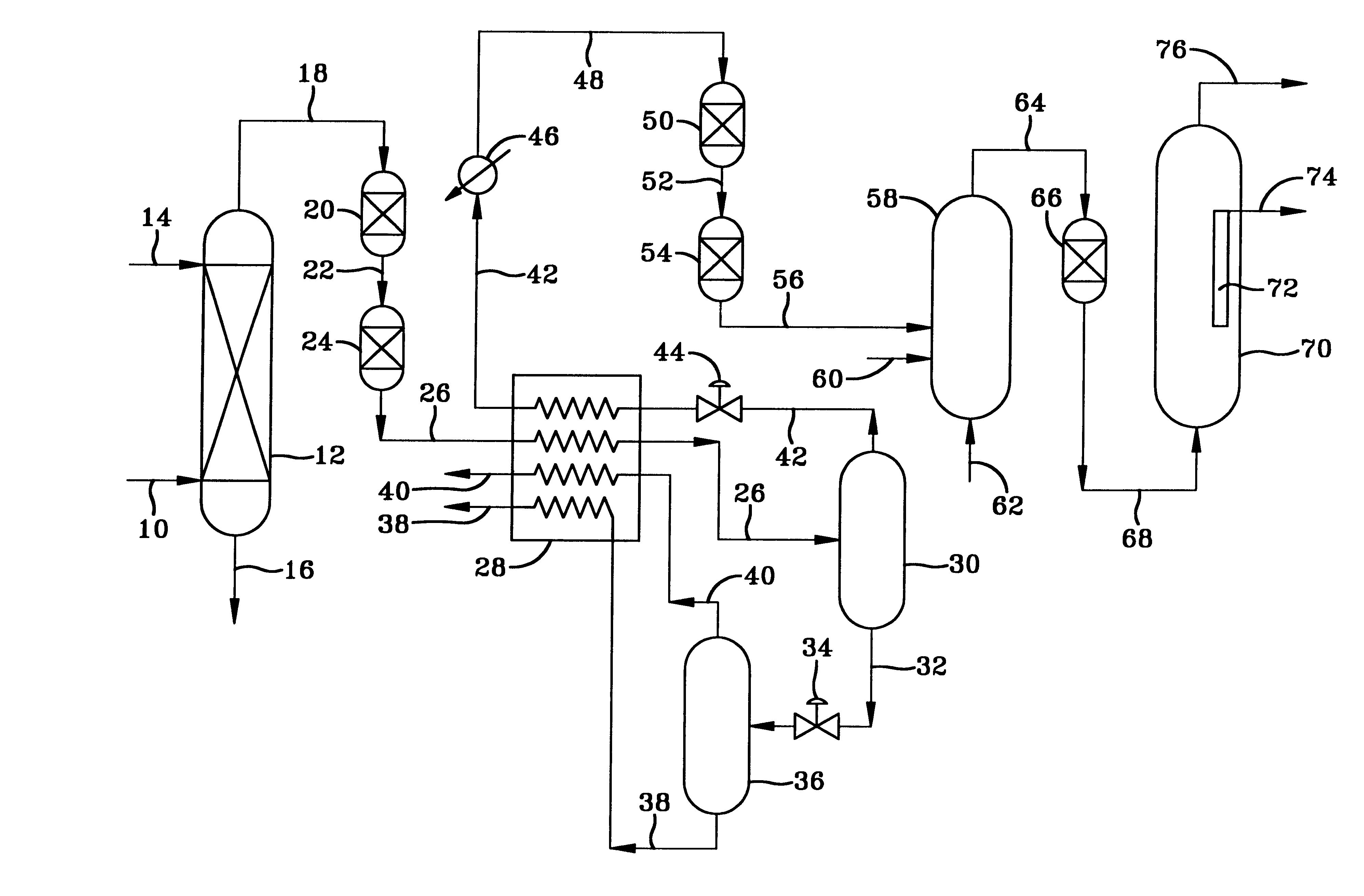
Production of low sulfur syngas from natural gas with C4+/C5+ hydrocarbon recovery
Sour natural gas is processed to remove the sulfur compounds and recover C4+ / C5+ hydrocarbons by scrubbing the gas with an amine solution to remove most of the sulfur, followed cooling the gas to remove C4+ / C5+ hydrocarbons and more sulfur compounds as liquid condensate to produce a gas having less than 20 vppm of total sulfur. The condensate is sent to a fractionator to recover the C4+ / C5+ hydrocarbons. The sulfur and hydrocarbon reduced gas is contacted first with zinc oxide and then nickel, to produce a gas having less than 10 vppb of total sulfur which is passed into a synthesis gas generating unit to form a very low sulfur synthesis gas comprising a mixture of H2 and CO. This synthesis gas is useful for hydrocarbon synthesis with increased life of the hydrocarbon synthesis catalyst and greater hydrocarbon production from the hydrocarbon synthesis reactor. Contacting the synthesis gas with zinc oxide further reduces the sulfur content to below 3 vppb.
Owner:EXXON RES & ENG CO
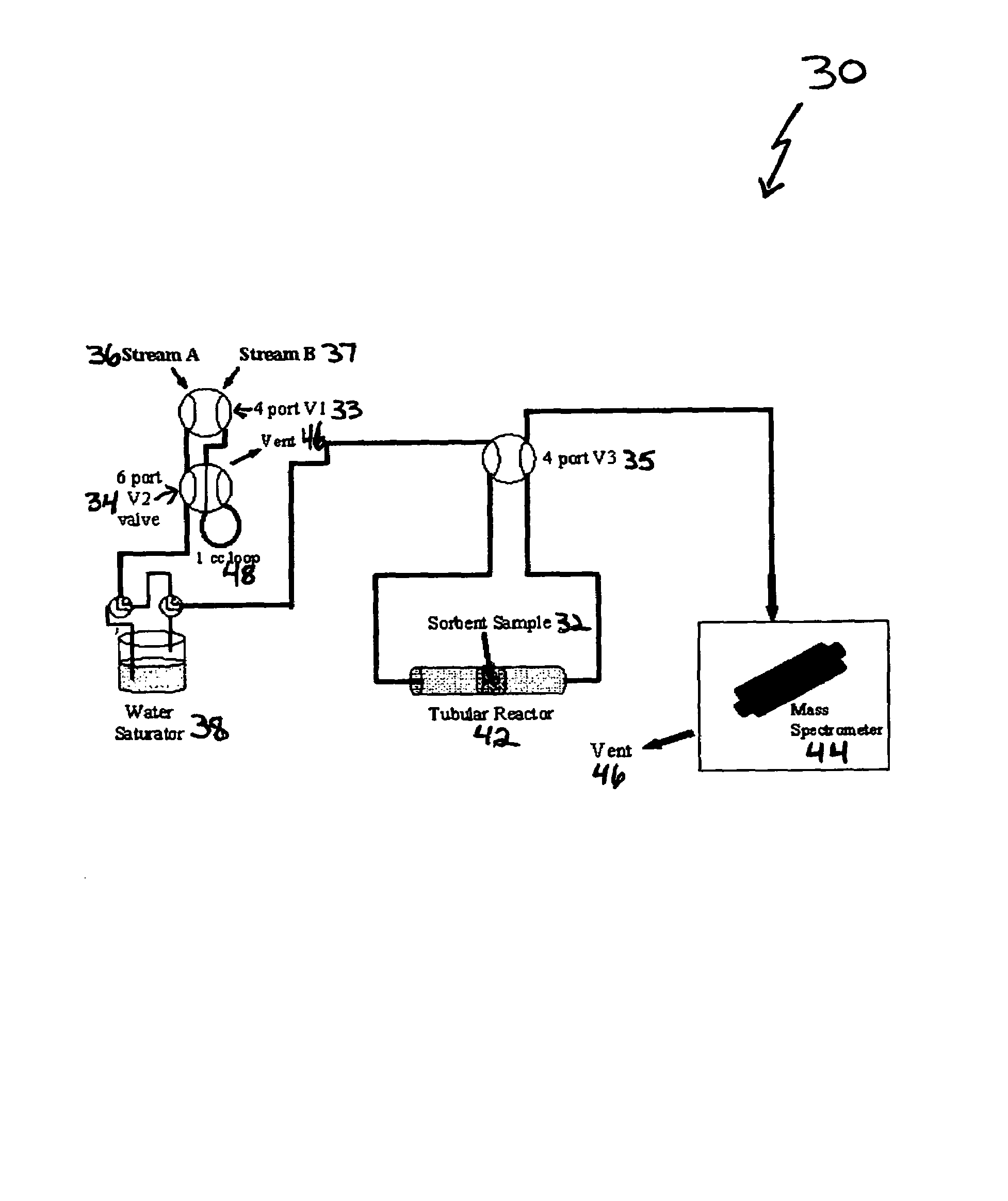
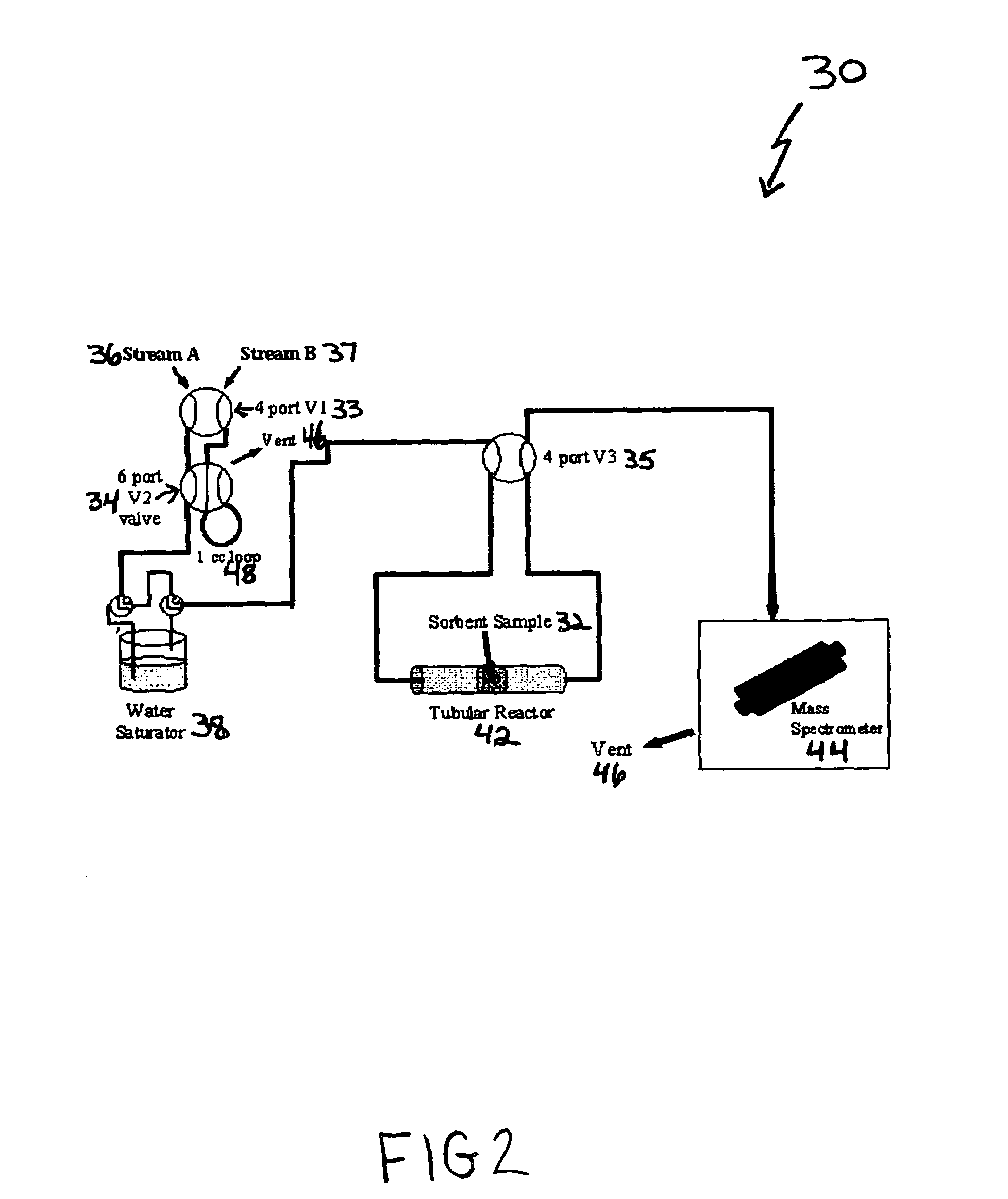
High capacity immobilized amine sorbents
ActiveUS7288136B1Increase capacityHigh CO capture capacityOther chemical processesHydrogen sulfidesSorbentImproved method
A method is provided for making low-cost CO2 sorbents that can be used in large-scale gas-solid processes. The improved method entails treating an amine to increase the number of secondary amine groups and impregnating the amine in a porous solid support. The method increases the CO2 capture capacity and decreases the cost of utilizing an amine-enriched solid sorbent in CO2 capture systems.
Owner:THE UNITED STATES AS REPRESENTED BY THE DEPARTMENT OF ENERGY
Production of hydrogen and removal and sequestration of carbon dioxide from coal-fired furnaces and boilers
InactiveUS7282189B2Increase ratingsValue maximizationOrganic chemistryNitrogen compoundsHydrogenProcess engineering
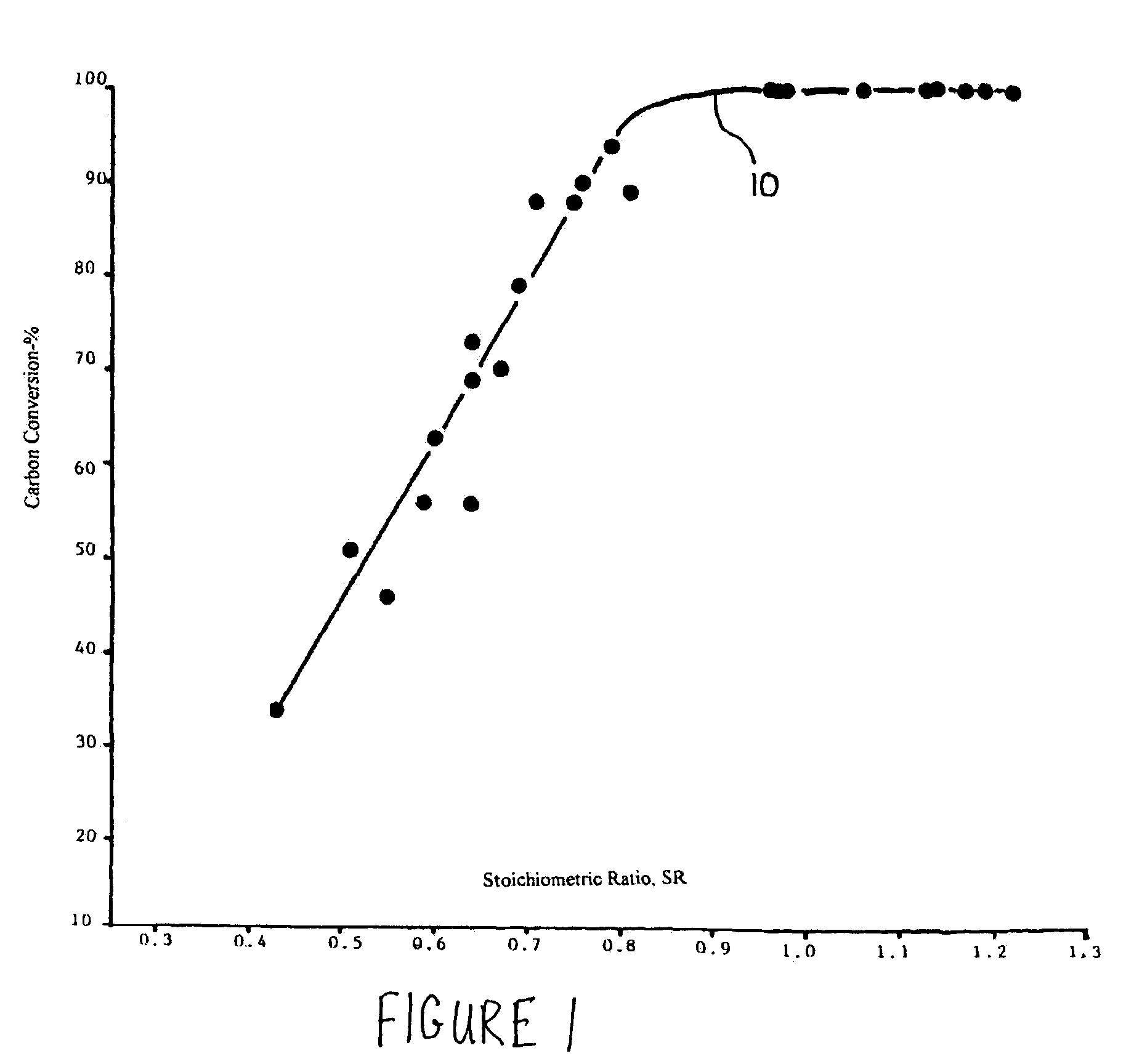
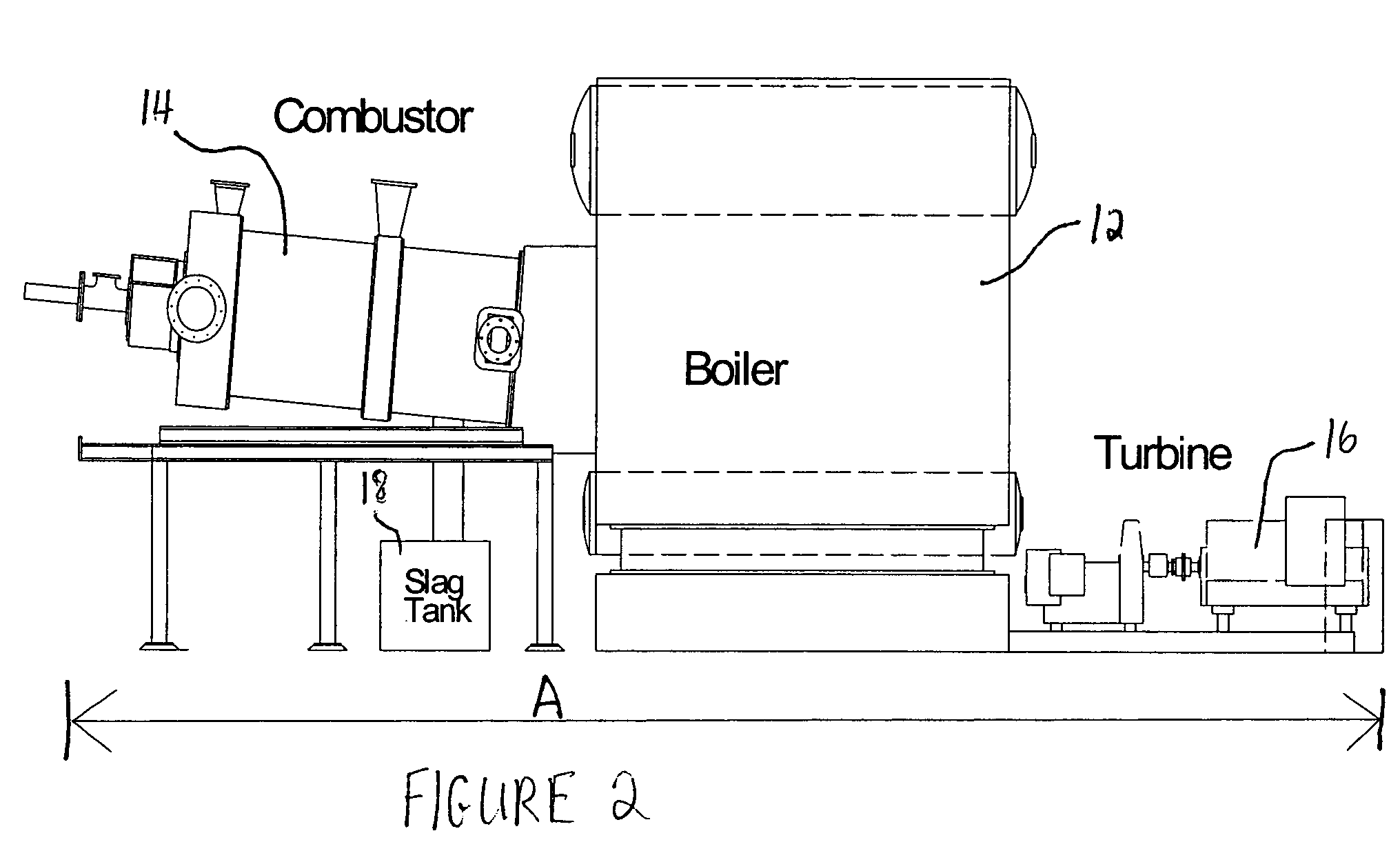
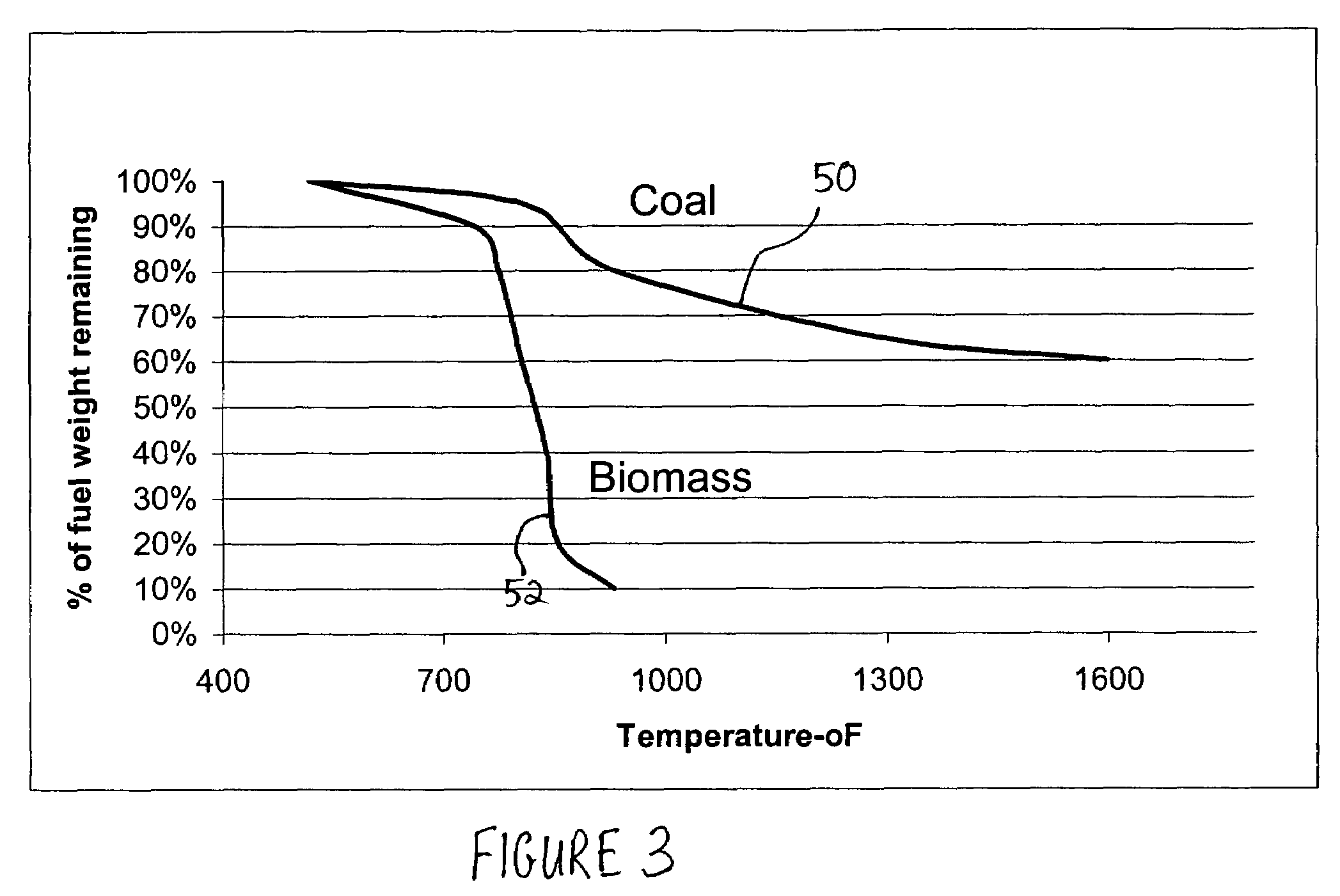
Methods for reducing and eliminating carbon dioxide from the emissions of solid fuel fired power plants, particularly coal fired power plants, and to sequester the carbon dioxide, typically by using existing equipment. In some embodiments, the methods involve pyrolyzing the solid fuel to remove volatile matter and using the volatile matter to produce hydrogen. Additionally, the methods may involve burning the solid fuel or pyrolized solid fuel at very fuel rich stoichiometric conditions. Sequestration may include the production of a carbon dioxide-containing solution and the pumping of the solution into the ground, particularly in areas high in limestone.
Owner:ZAUDERER BERT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com