Patents
Literature
3547 results about "Water gas" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Water gas is a mixture of carbon monoxide and hydrogen produced from synthesis gas. Synthesis gas is a useful product, but requires careful handling due to its flammability and the risk of carbon monoxide poisoning. The water-gas shift reaction can be used to reduce the carbon monoxide while producing additional hydrogen, resulting in water gas.
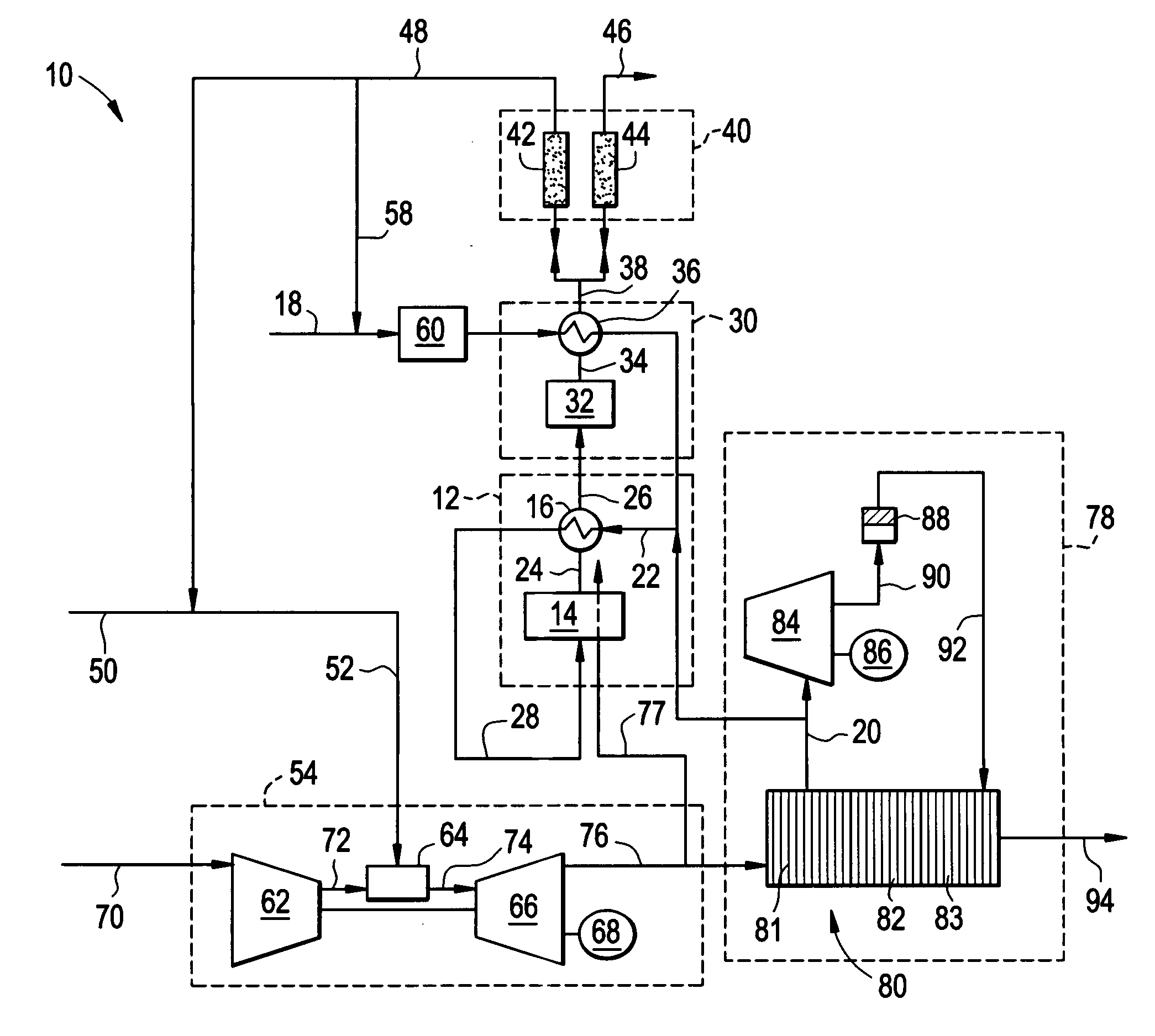
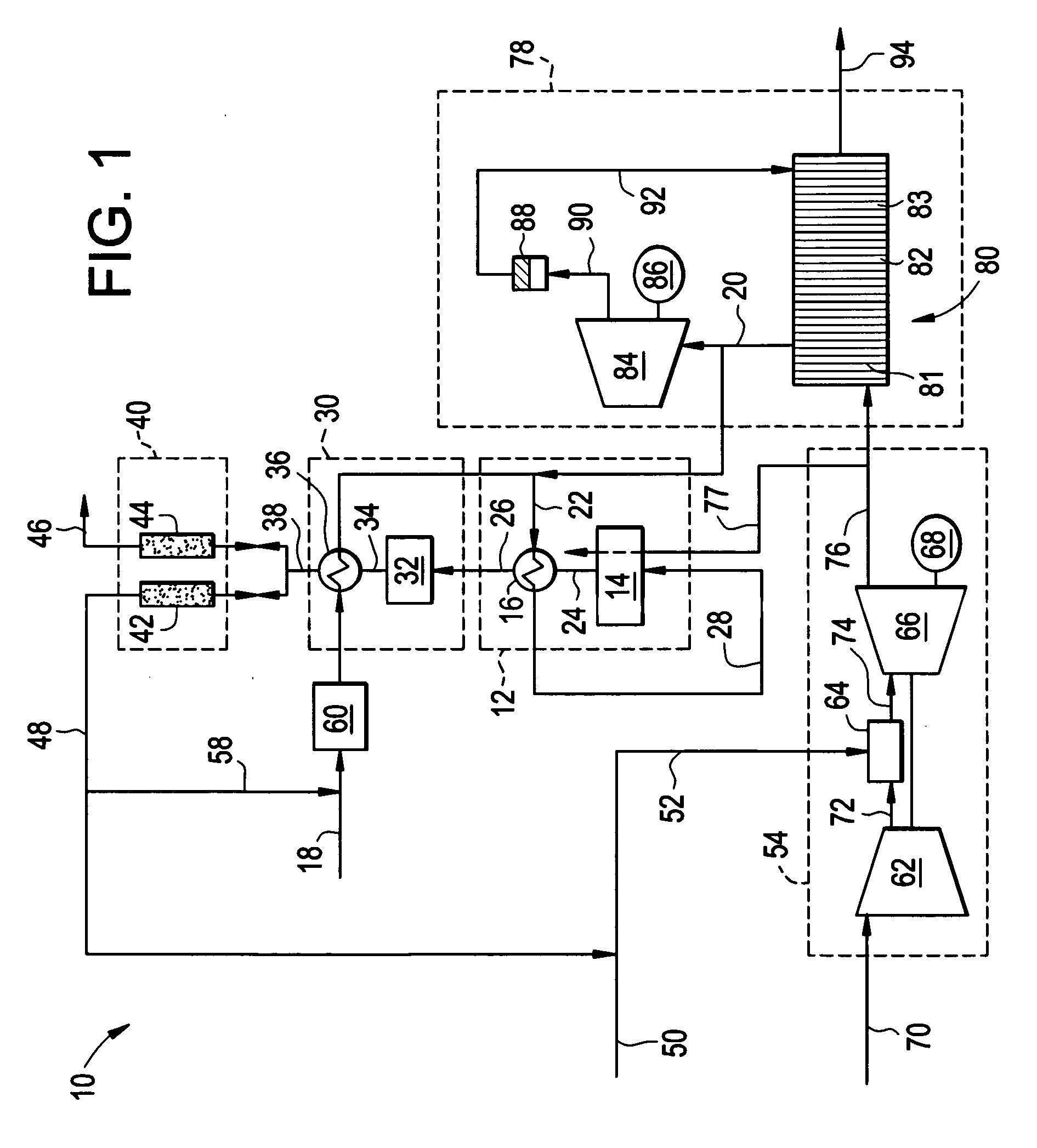
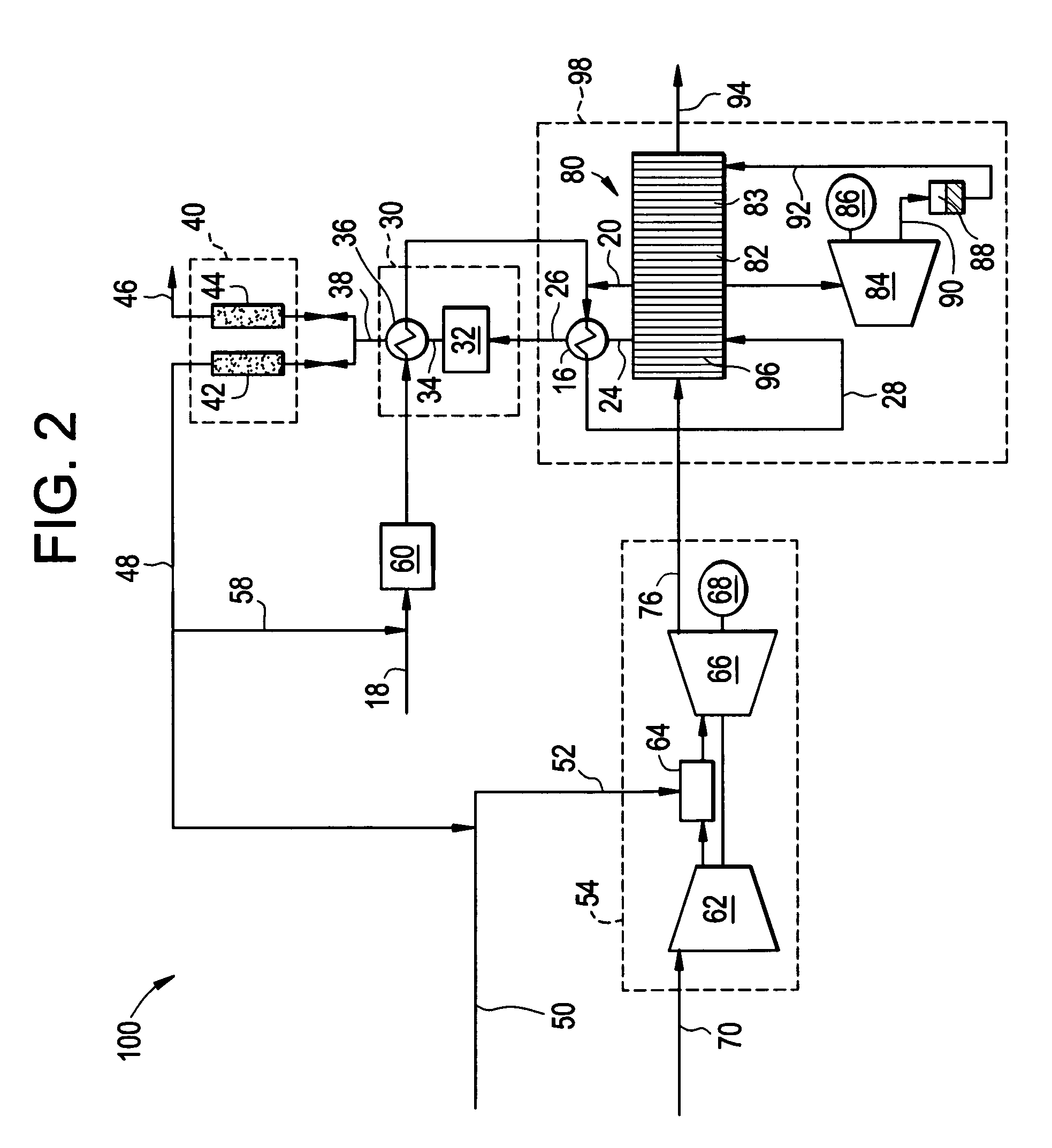
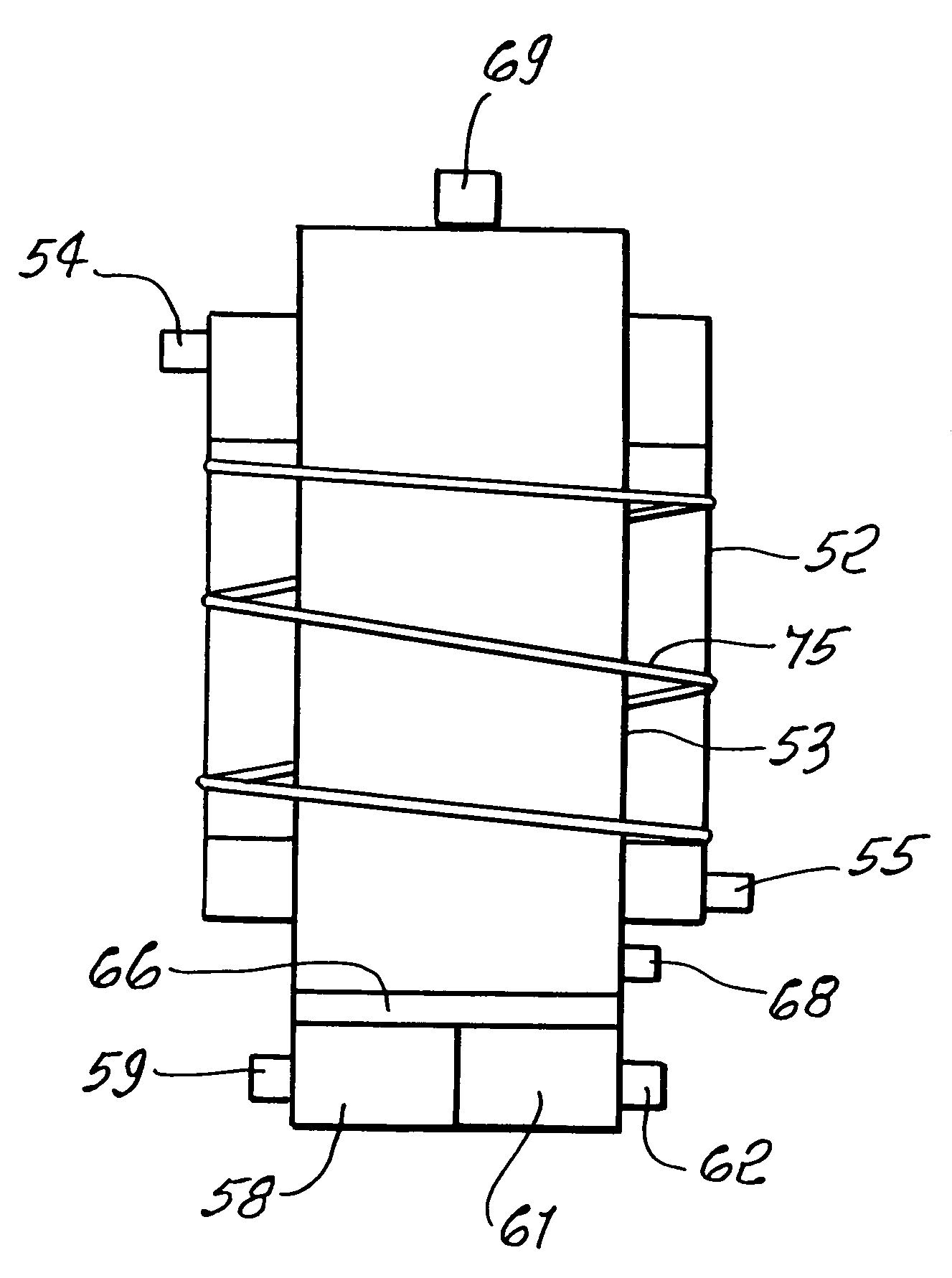
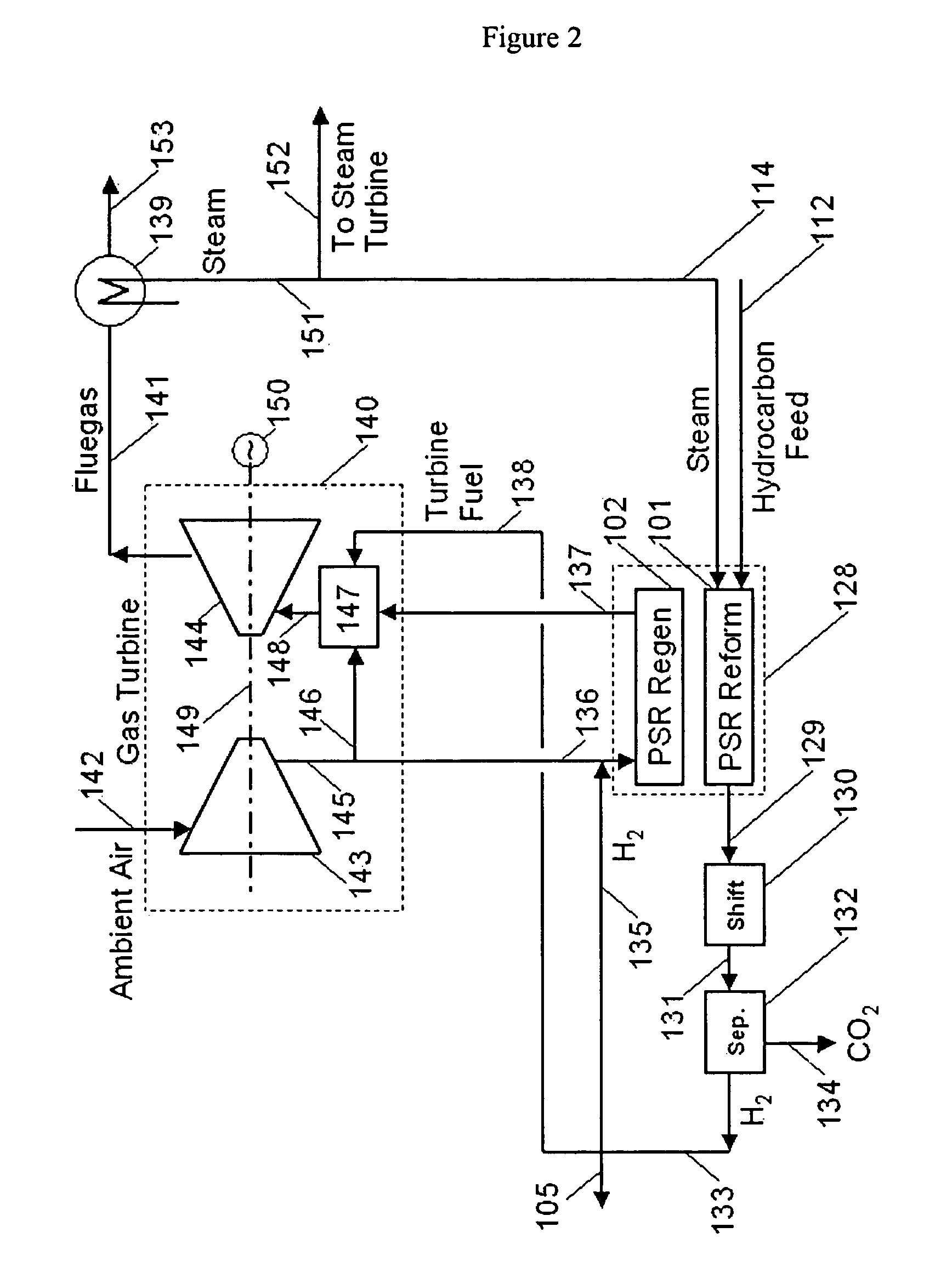
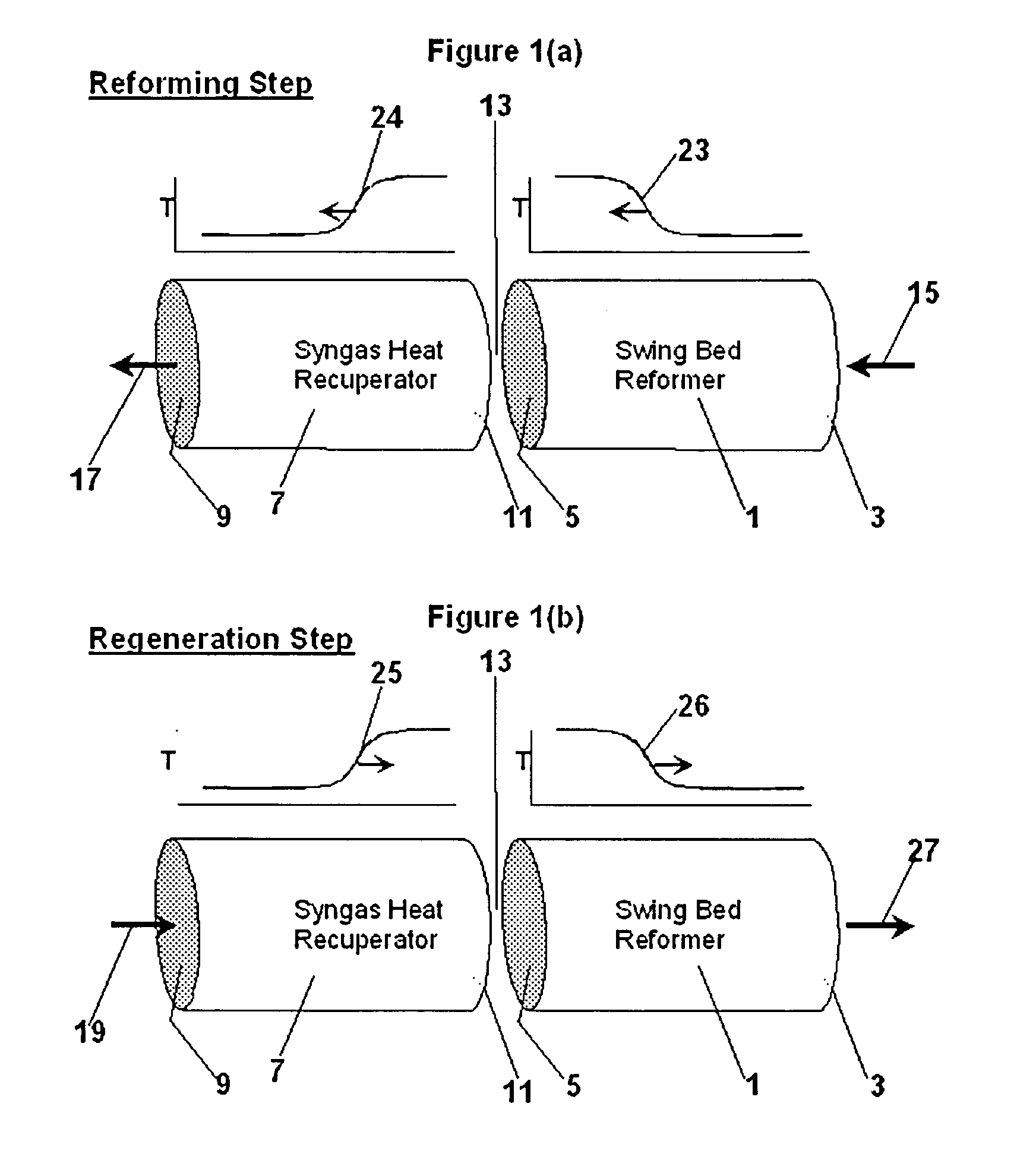
Reforming system for combined cycle plant with partial CO2 capture
A combined cycle system includes, a pre-steam-methane-reformer operating at a temperature of less than about 800 degrees Celsius to reform a mixed fuel stream to generate a first reformate stream, a water-gas-shift reactor to convert carbon monoxide in the first reformate stream to carbon dioxide and form a second reformate stream, a carbon dioxide removal unit for removing carbon dioxide from the second reformate stream and form a carbon dioxide stream and a third reformate stream; wherein less than about 50 percent of the carbon contained in the mixed fuel stream is recovered as carbon dioxide by the removal unit, a gas turbine unit for generating power and an exhaust stream, and a steam generator unit configured to receive the exhaust stream, wherein the heat of the exhaust stream is transferred to a water stream to generate the steam for the mixed fuel stream and for a steam turbine.
Owner:GENERAL ELECTRIC CO
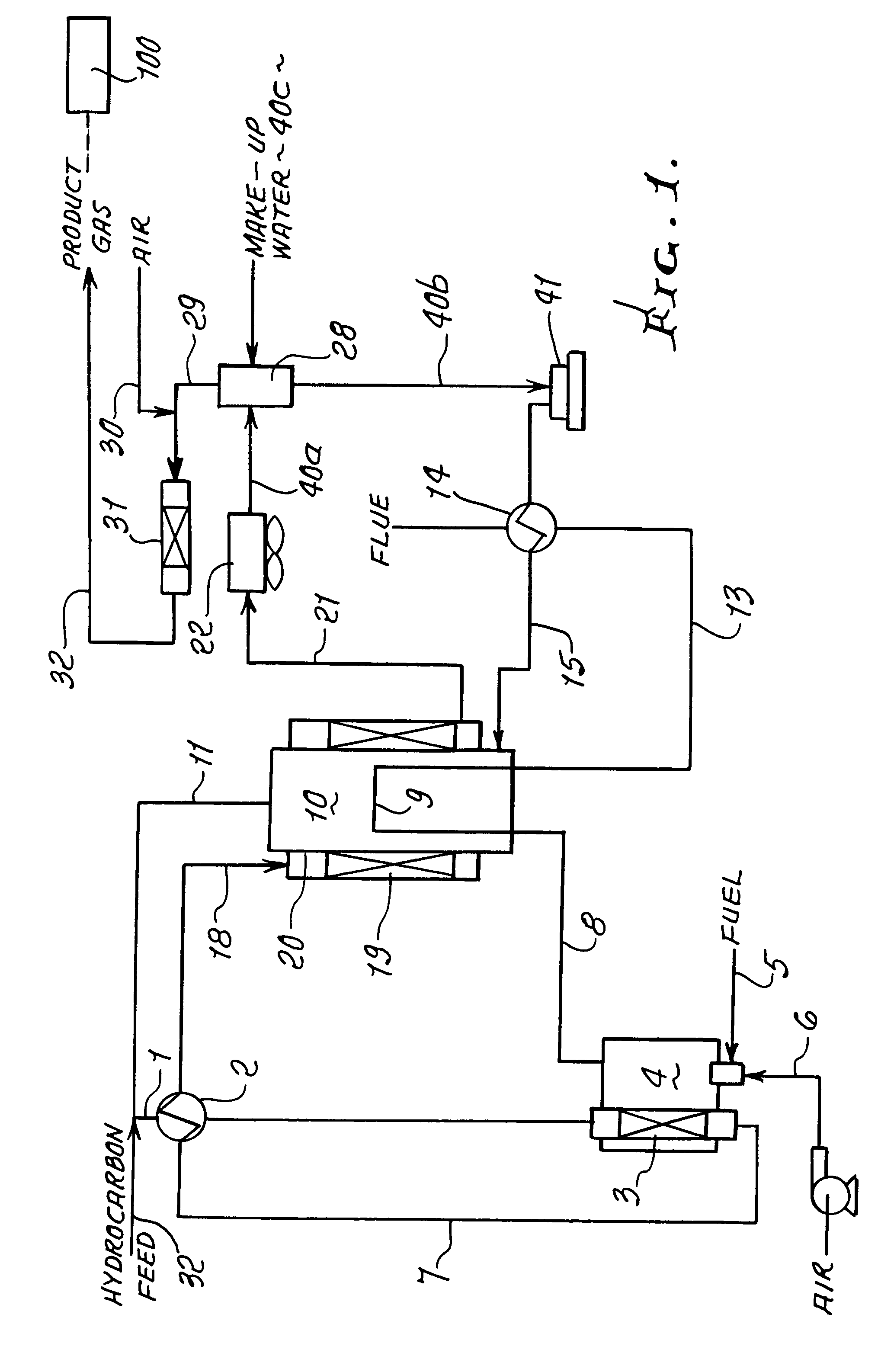
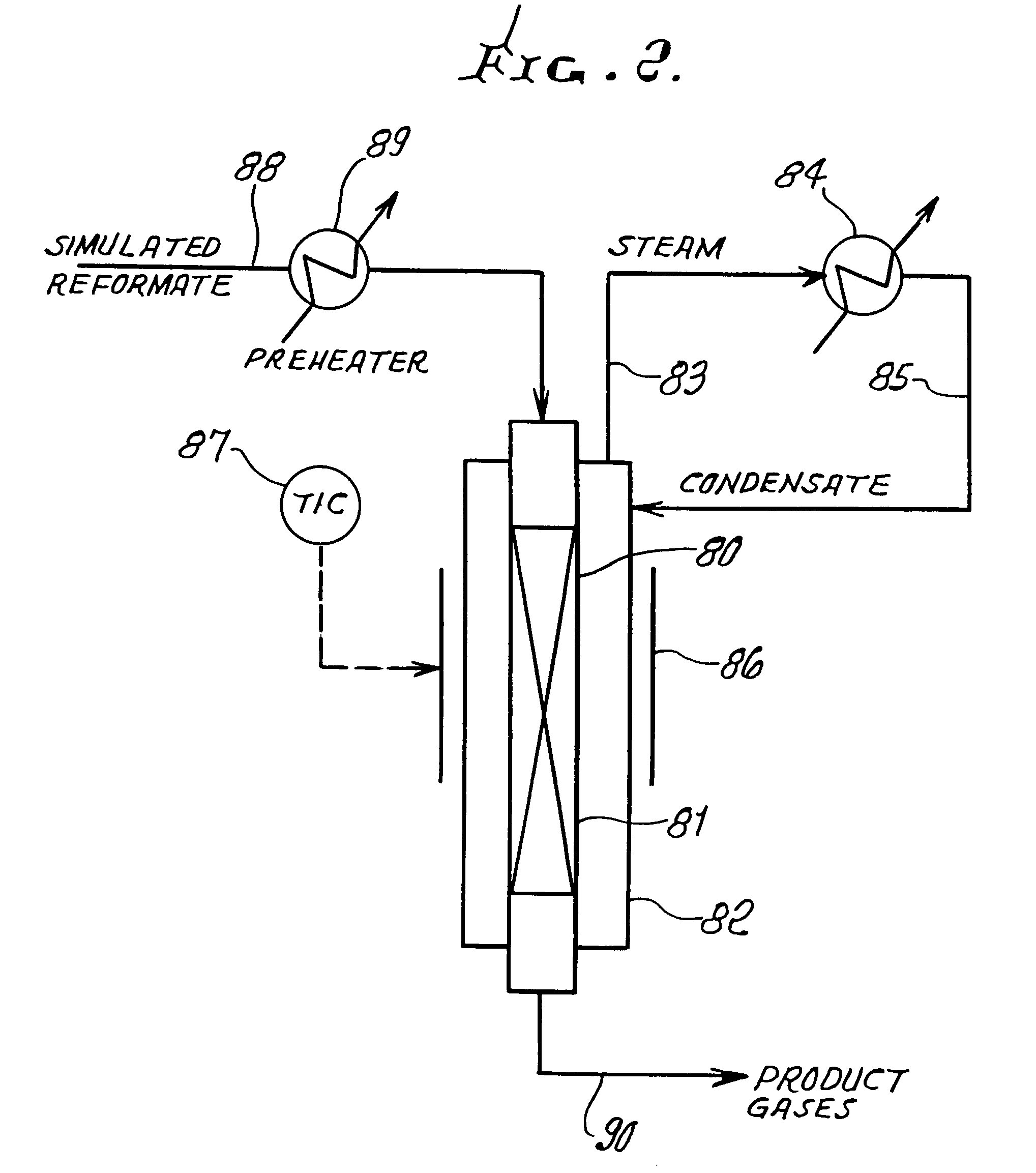
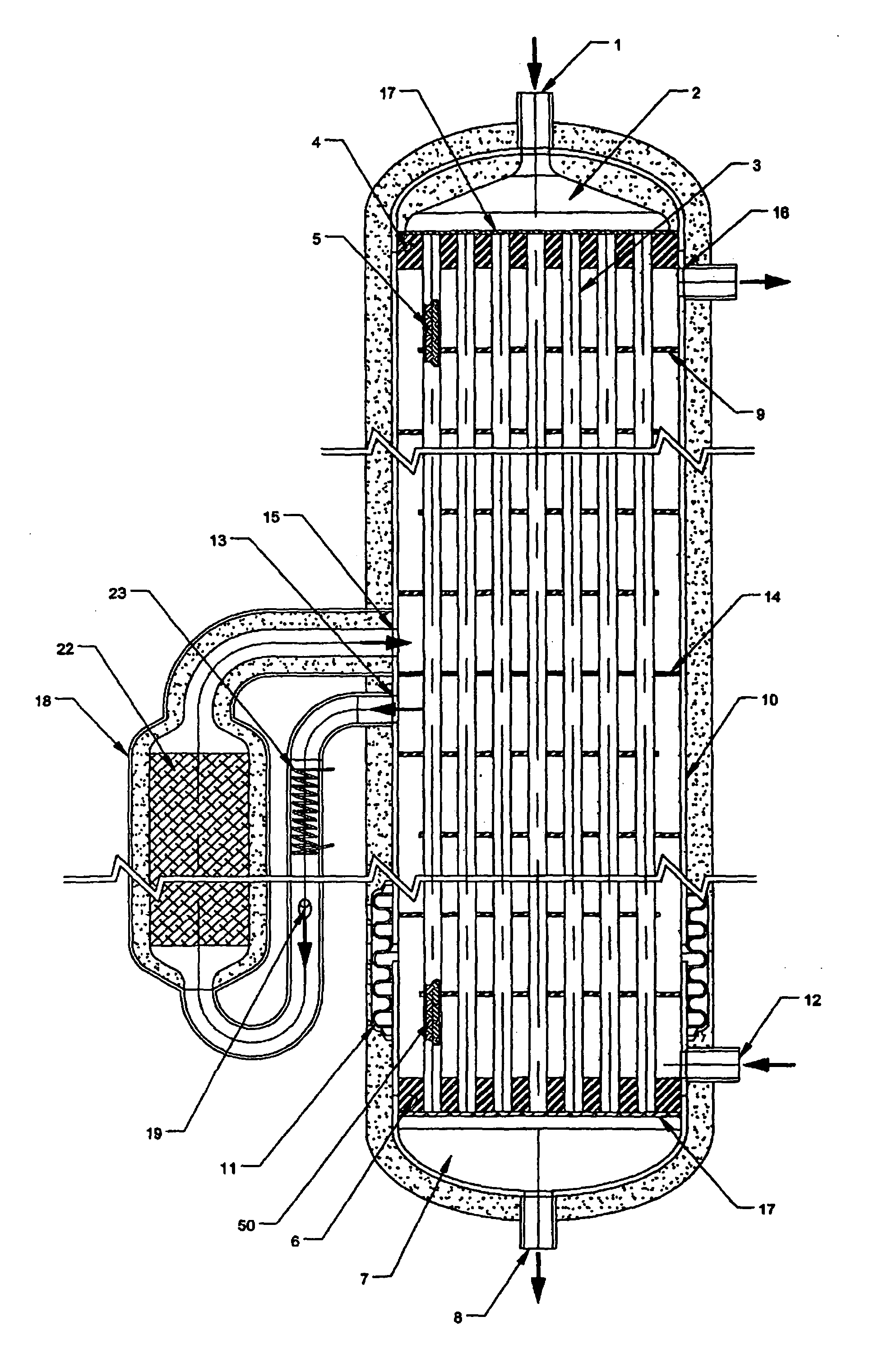
Thermally-integrated low temperature water-gas shift reactor apparatus and process
InactiveUS7074373B1Reduced toleranceExtended temperature rangeHydrogenPhysical/chemical process catalystsWater-gas shift reactionProduct gas
A thermally-integrated lower temperature water-gas shift reactor apparatus for converting carbon monoxide in the presence steam comprises a catalyst bed that is disposed within an outer region surrounding a waste heat recovery steam generator operating at a selected pressure corresponding to the optimum temperature for conducting the catalytic water-gas shift reaction and a process for useful recovery of the exothermic heat of reaction to generate steam that is used in a process for the conversion of hydrocarbon feedstock into useful gases such as hydrogen.
Owner:HARVEST ENERGY TECH
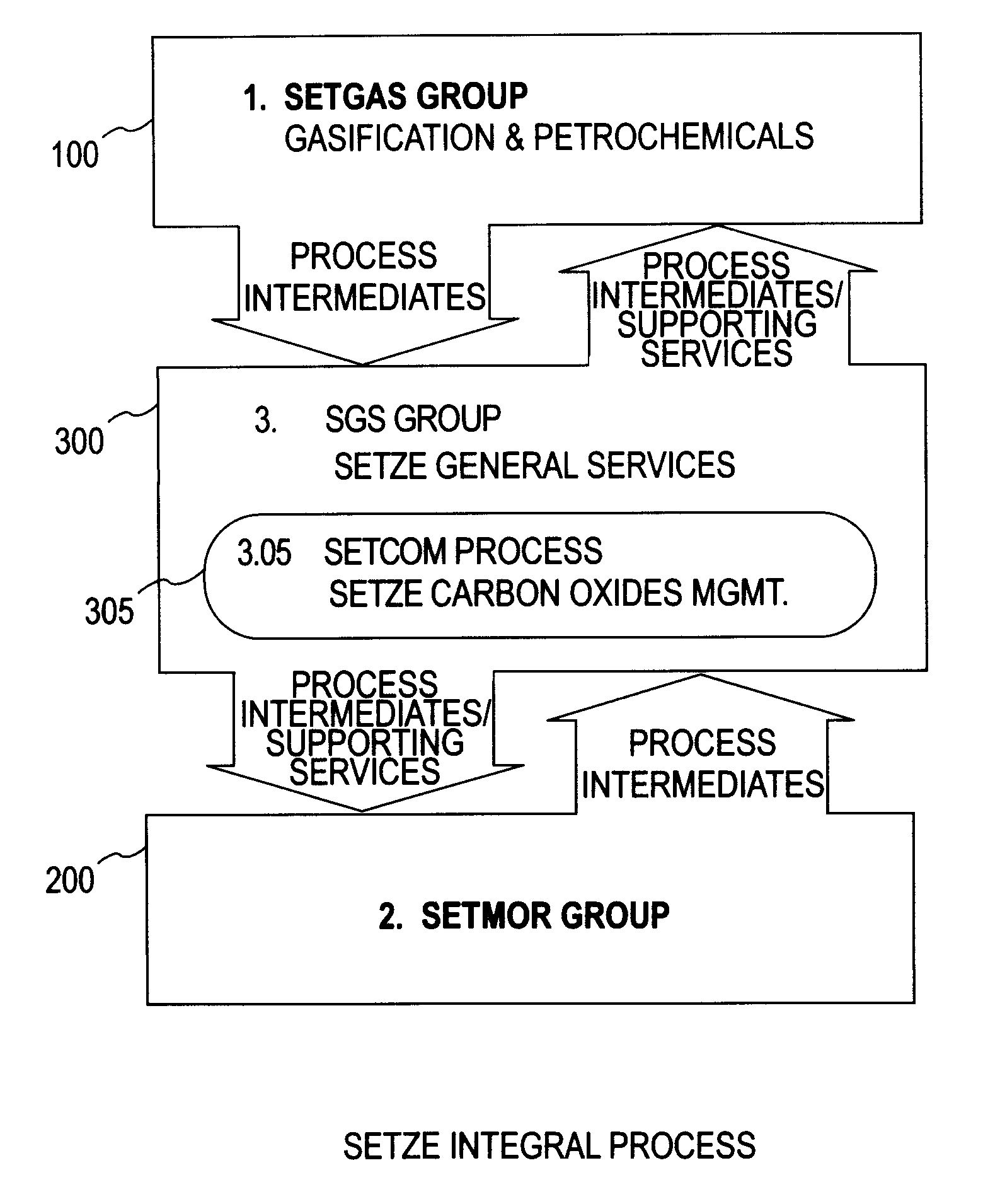
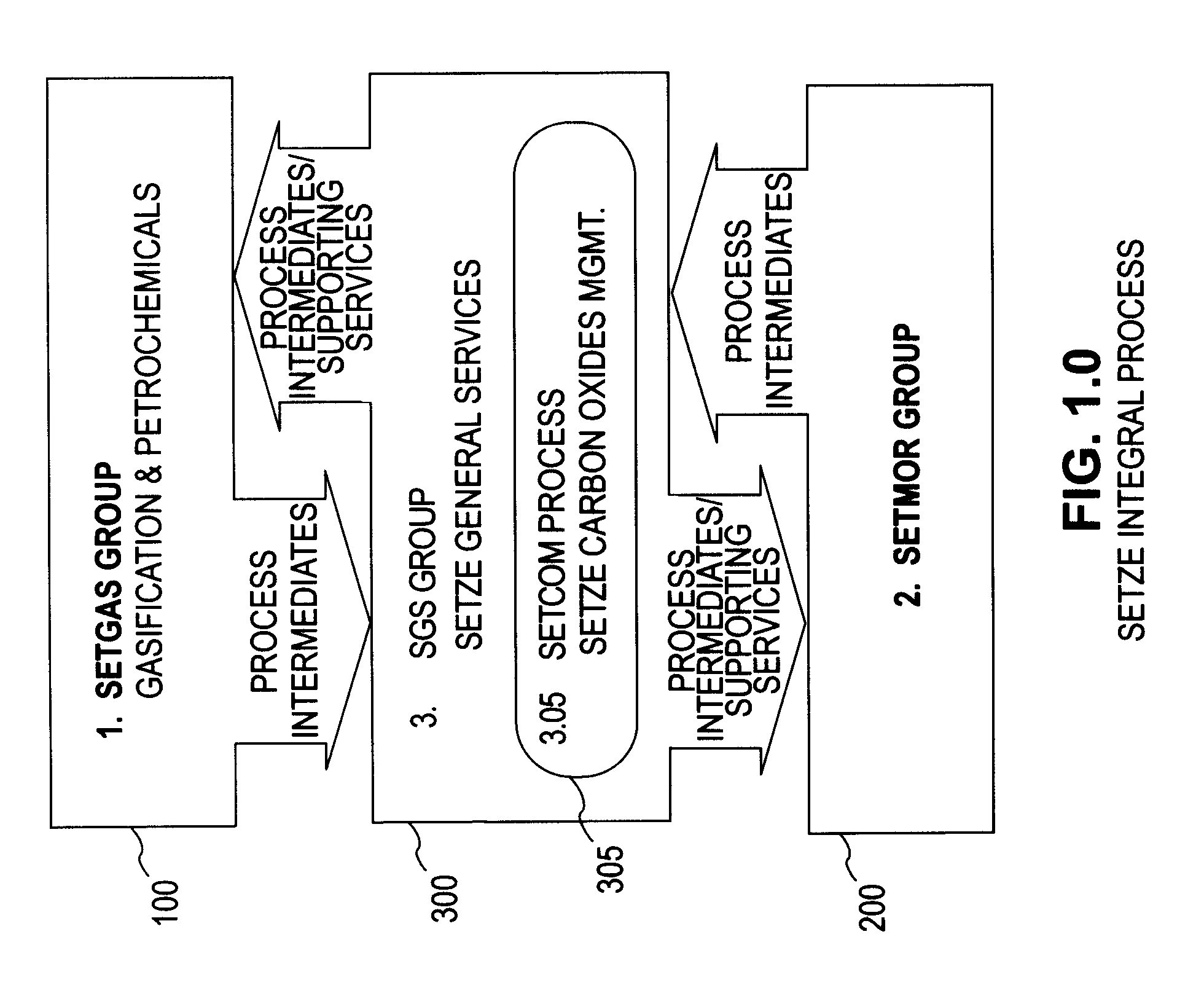
Zero emission gasification, power generation, carbon oxides management and metallurgical reduction processes, apparatus, systems, and integration thereof
ActiveUS7674443B1Improvement in individual technology componentEnhances economic performanceUsing liquid separation agentBiofuelsCyclonic separationOxygen
A system involving a two-step gasification of a carbonaceous source to produce bulk hydrogen that avoids the early formation of CO2 and obviates the traditional water gas shift (WGSR) step, carbochlorination of a metallic ore the production of metals found in the ore that utilizes carbon monoxide as an oxygen sink, rather than the traditional coke, and carbon oxides management that eliminates major impediments to emission-neutral power generation and the reduction of major metals. The gasification uses a rotary kiln reactor and gas-gas cyclonic separation process to separate synthesis gas into purified hydrogen and purified carbon monoxide. Purified bulk carbon monoxide issued in metallurgical reduction, and purified bulk hydrogen as fuel for an emission-neutral hydrogen combined cycle (HCC) turbine power generation station. The carbochlorination is integrated with: a) the concurrent separation and purification of all metal-chlorides (metchlors) and capture of CO2 for passage to the carbon oxides management system; b) the direct reduction of metchlors to nanoscale metallurgical powders and / or to dendritically-shaped particles, including metchlor reduction for the ultrahigh-performance semiconductor metals of the III-V group; and, c) the reforming of metal-oxides with improved crystalline structure from metchlors. The carbon oxides management collects, stores and directs to points of usage, carbon oxides that arise in various processes of the integrated system, and captures carbon monoxide for process enhancement and economic uses and captures carbon dioxide as a process intermediate and for economic uses.
Owner:DAVIS OLUMIJI B +1
Carbon dioxide production method
ActiveUS20070232706A1Increased hydrogen recoveryPromote recoverySolidificationLiquefactionVacuum pressureCarbon dioxide production
A method of producing a carbon dioxide product stream from a synthesis gas stream formed within a hydrogen plant having a synthesis gas reactor, a water-gas shift reactor, located downstream of the synthesis gas reactor to form the synthesis gas stream and a hydrogen pressure swing adsorption unit to produce a hydrogen product recovered from the synthesis gas stream. In accordance with the method the carbon dioxide from the synthesis gas stream by separating the carbon dioxide from the synthesis gas stream in a vacuum pressure swing adsorption system, thereby to produce a hydrogen-rich synthesis gas stream and a crude carbon dioxide stream and then purifying the crude carbon dioxide stream by a sub-ambient temperature distillation process thereby to produce the carbon dioxide product. A hydrogen synthesis gas feed stream to the hydrogen pressure swing adsorption unit is formed at least in part from the hydrogen rich stream.
Owner:PRAXAIR TECH INC
Supercritical carbon dioxide drive physical analogue device
InactiveCN101446189AEasy to achieve high temperature and high pressure supercritical stateOther gas emission reduction technologiesFluid removalRock coreDouble tube
The invention relates to a supercritical carbon dioxide drive physical analogue device, which belongs to the technical field of petroleum engineering and technology. The device adopts two paratactic simulation core devices to be connected with an injecting system, and each simulation core device is provided with an outlet measuring system; the injecting system injects formation water, crude oil and supercritical carbon dioxide to the simulation core devices, and a temperature and pressure measuring and controlling system is adopted to control the temperature set value and the pressure value of the whole system, and the outlet measuring system is adopted to measure the volumes of the carbon dioxide, the formation water and the crude oil which pass through the simulation core devices. After the CO2 gas is cooled and liquefied, the CO2 gas is pressurized and heated up to the supercriticality, and the difficulty of the accurate measurement of the injected CO2 flow; by adopting a double-tube model, the fingering and cross flow phenomenon during the driving process of the heterogeneous reservoir CO2 can be simulated; the design pressure of the device is 0 to 40 MPa, the design temperature is 0 to 180 DEG C, and the device is mainly applied to the research on supercritical CO2 miscible drive, non-miscible drive, continues gas drive or water and gas alternate drive.
Owner:DALIAN UNIV OF TECH
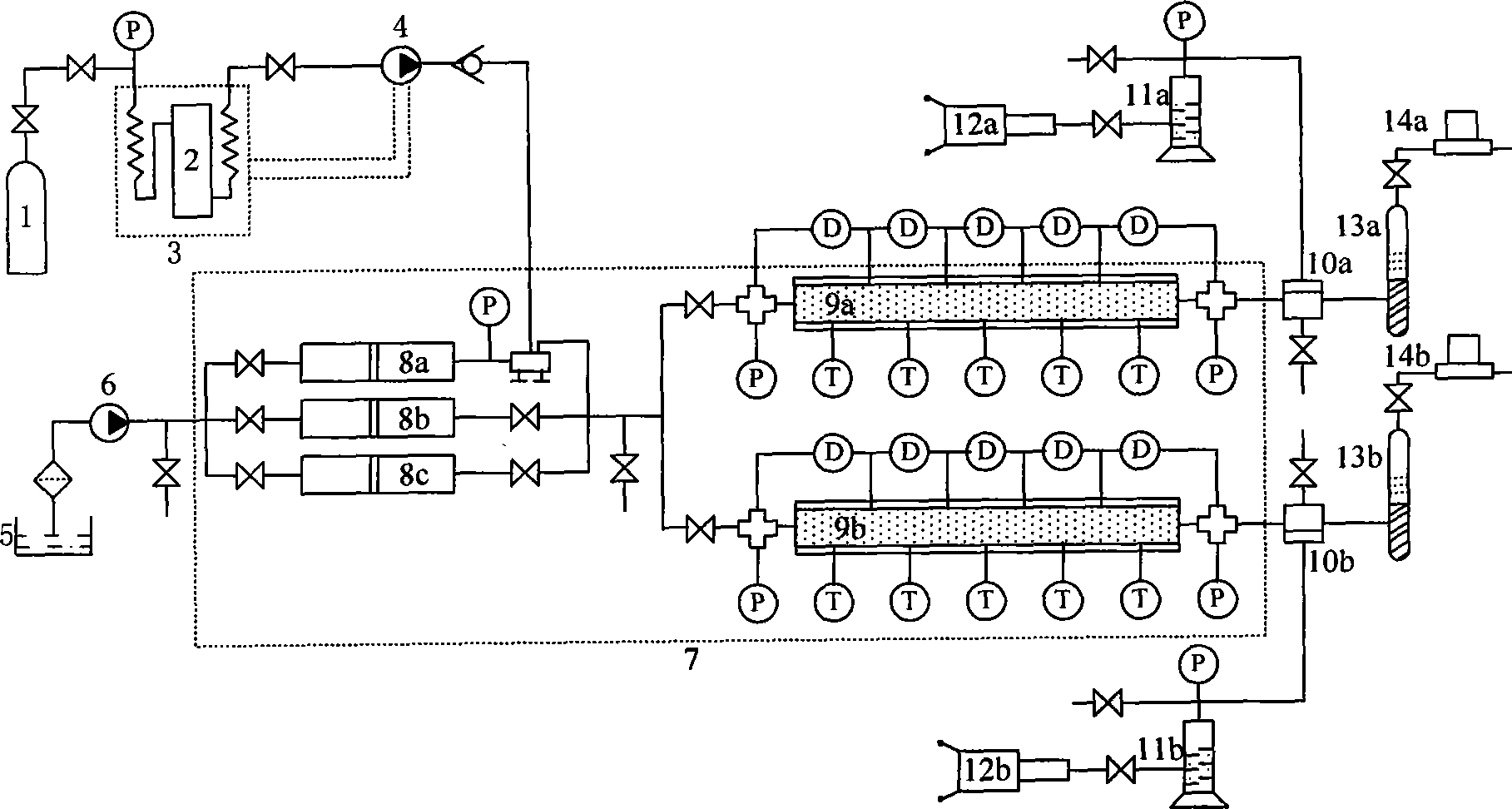
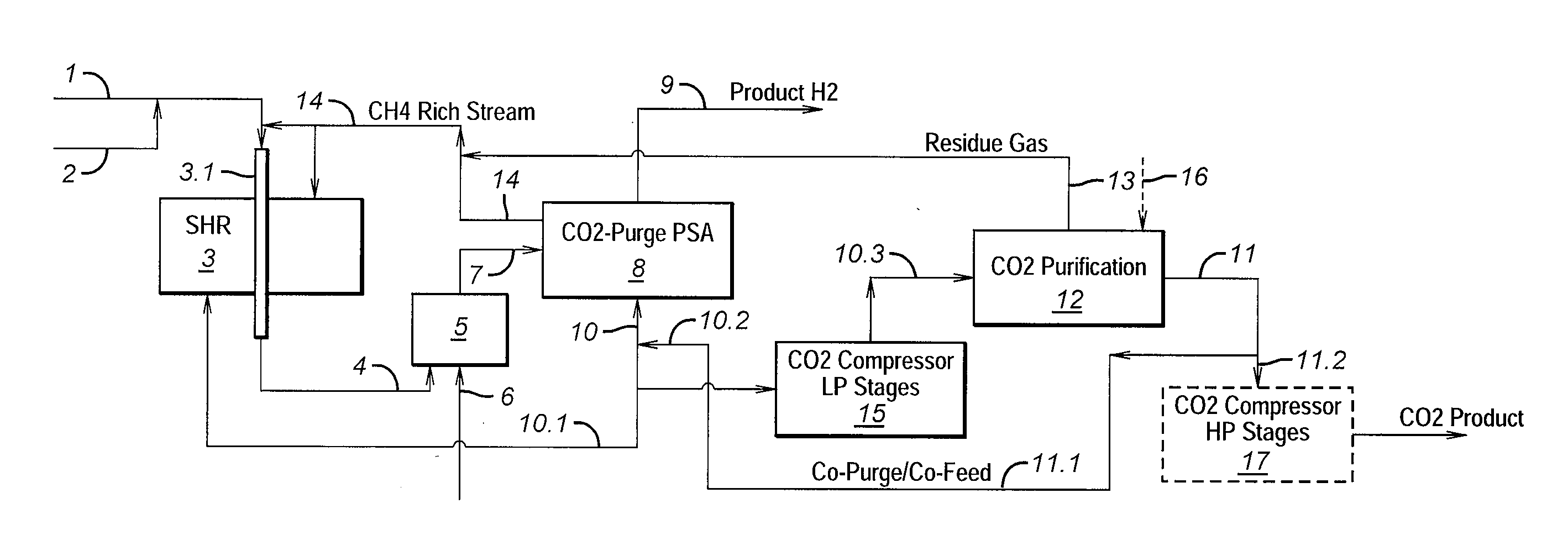
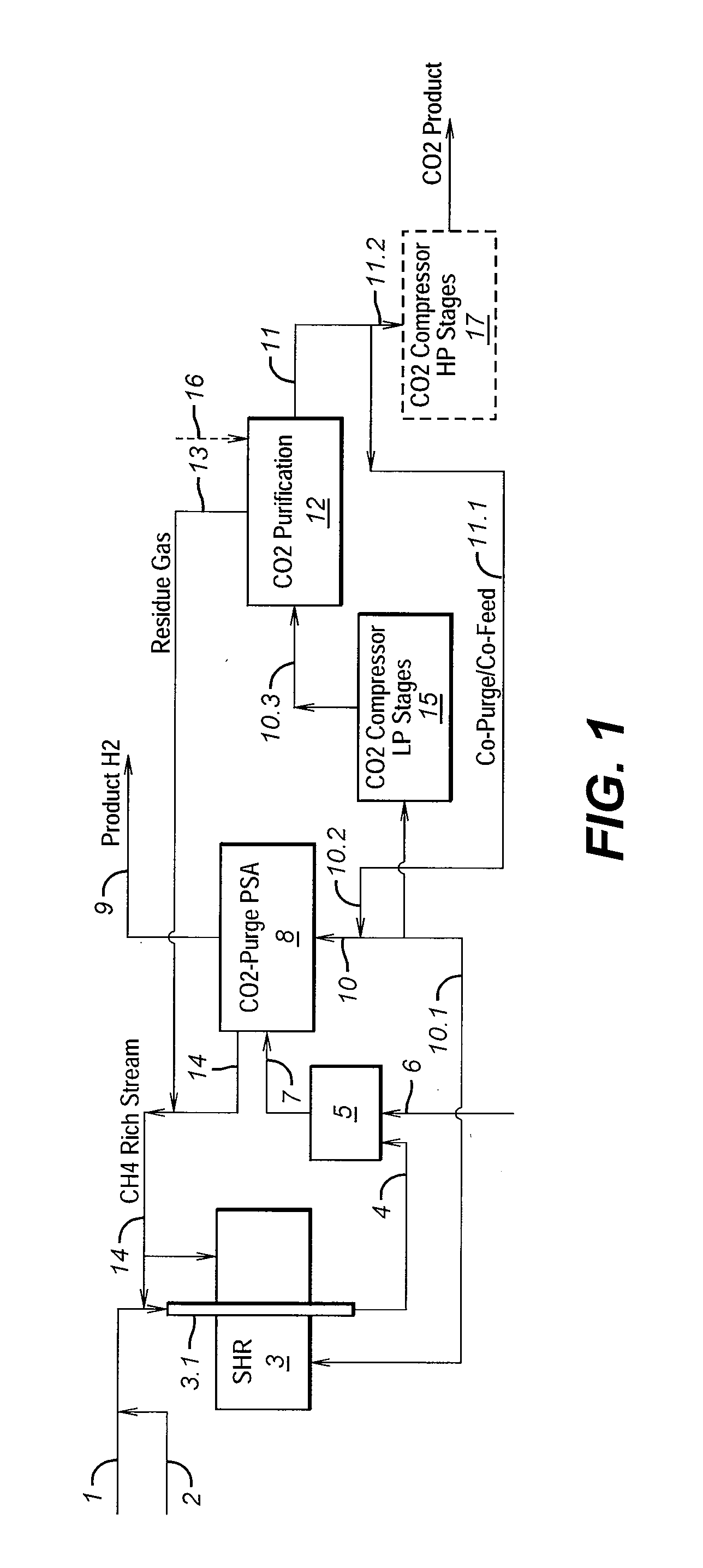
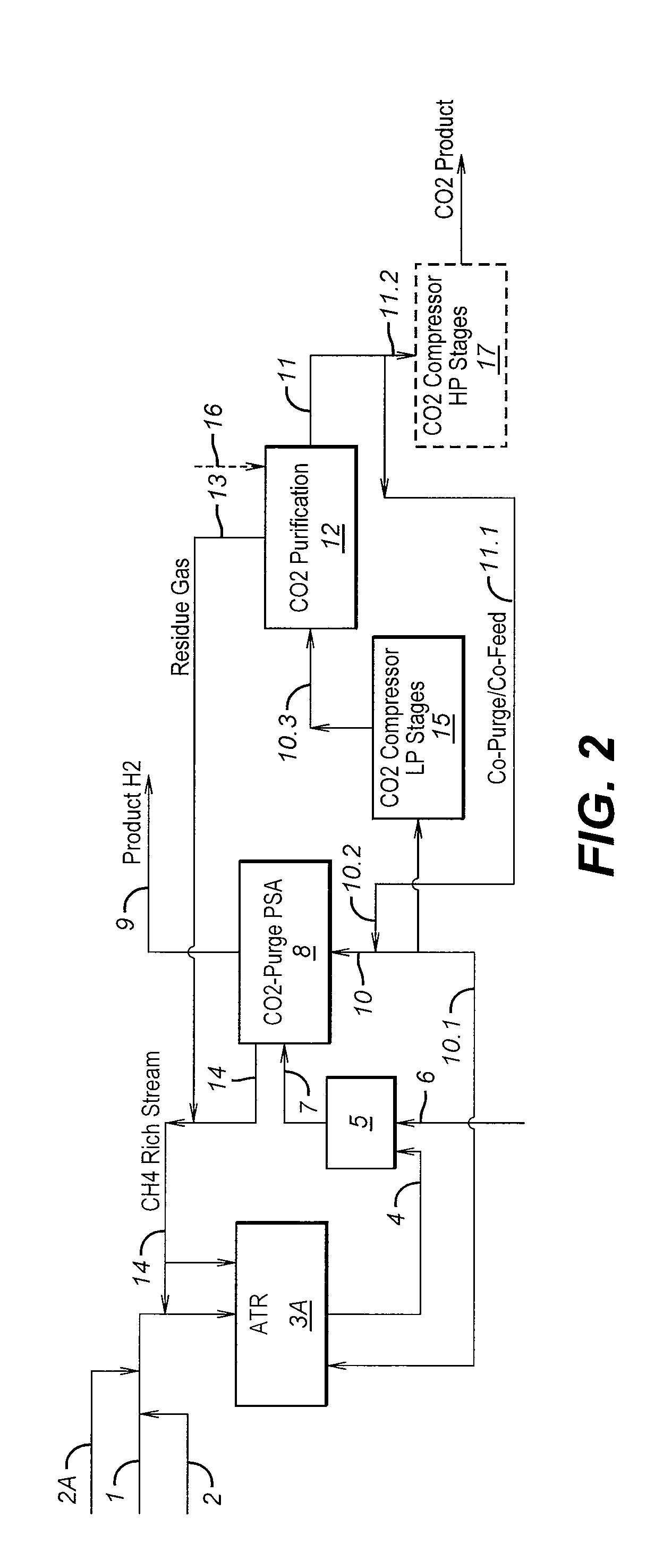
Process For The Production Of Carbon Dioxide Utilizing A Co-Purge Pressure Swing Adsorption Unit
The present invention provides a process for recovering gaseous hydrogen and gaseous carbon dioxide from a mixture of hydrocarbons by utilizing a system that includes a reformer unit, an optional water gas shift reactor, and a pressure swing adsorption unit in conjunction with a carbon dioxide purification unit such as a cryogenic purification unit or a catalytic oxidizer. In this process, purified CO2 from the CO2 purification unit is used as a co-feed / co-purge in the pressure swing adsorption unit in order to produce a CO2 tail gas that includes a higher concentration of CO2.
Owner:LAIR LIQUIDE SA POUR LETUDE & LEXPLOITATION DES PROCEDES GEORGES CLAUDE
Biological seedling growing matrix
The invention provides a new crop seedling growing matrix, wherein the pH value of the seedling growing matrix is 6.0-6.5. The seedling growing matrix comprises the components by the weight percentage: 50%-80% of an inorganic matrix, and 15-30% of an organic matrix, wherein the organic matrix includes a composition of peat, fermented cattle manure, fermented pig manure, plant ash and an additional organic matrix, and the inorganic matrix includes a composition of vermiculite, perlite and an additional inorganic matrix and is composed of inorganic matrix particles with different particle sizes. The seedling growing matrix provided by the invention has relatively high total porosity and maximum water holding capacity, and is higher in aeration porosity ratio, more reasonable in water-gas ratio, small in bulk density and convenient to transport and use. The seedling growing matrix provided by the invention has the advantages of wide range and many types of applicable crops, high germination rate, less morbidity and good seedling growth vigor.
Owner:上海宇强品牌策划有限公司 +1
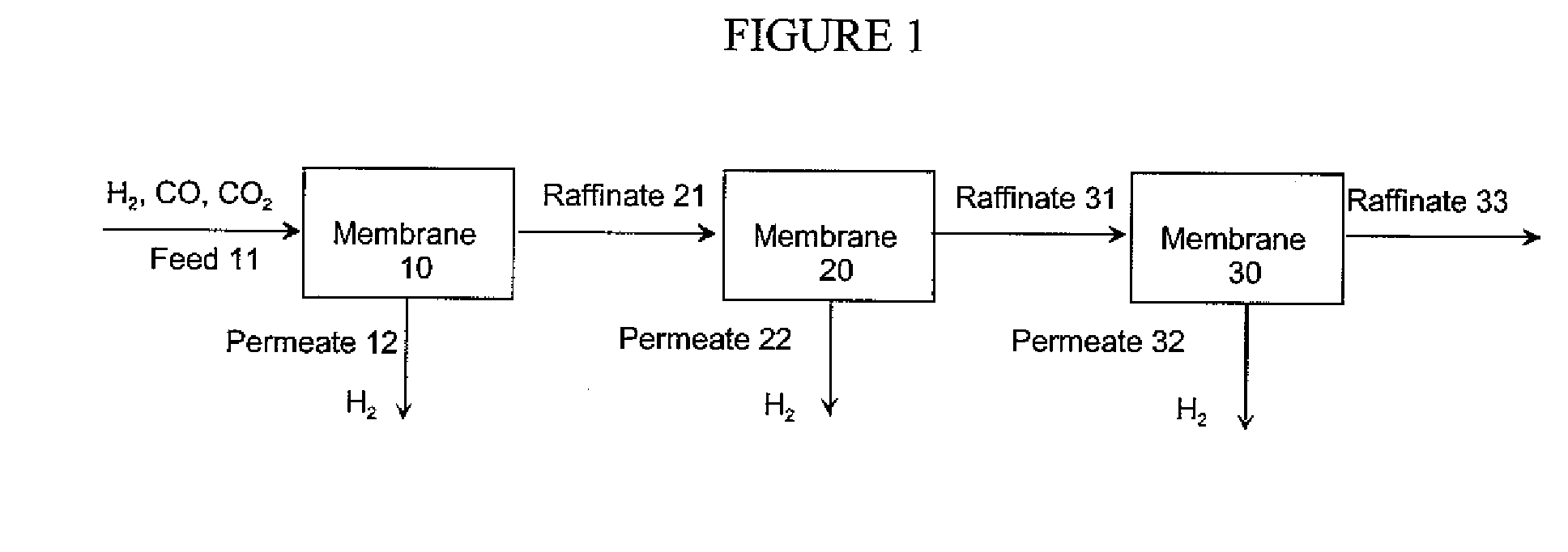
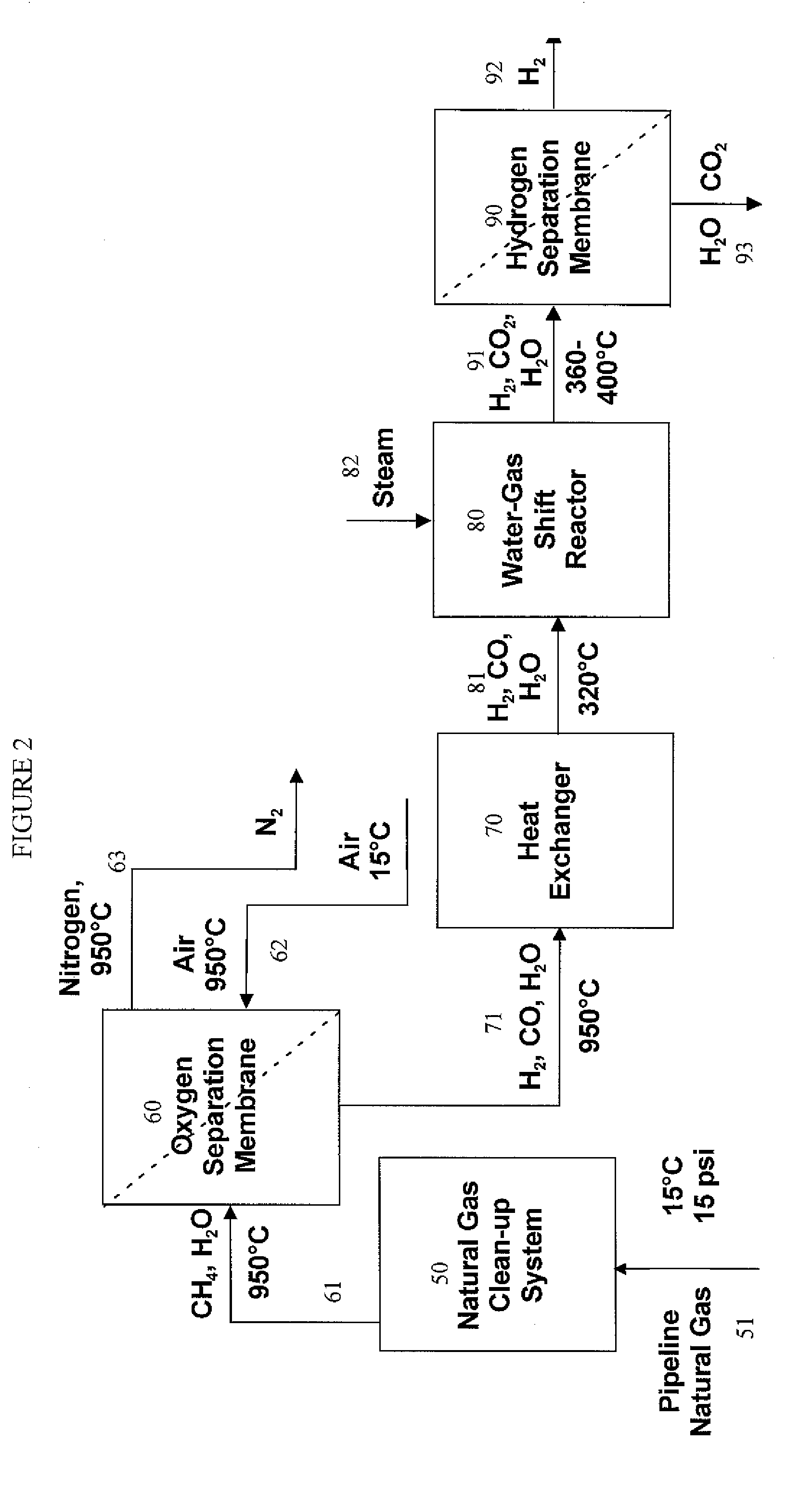
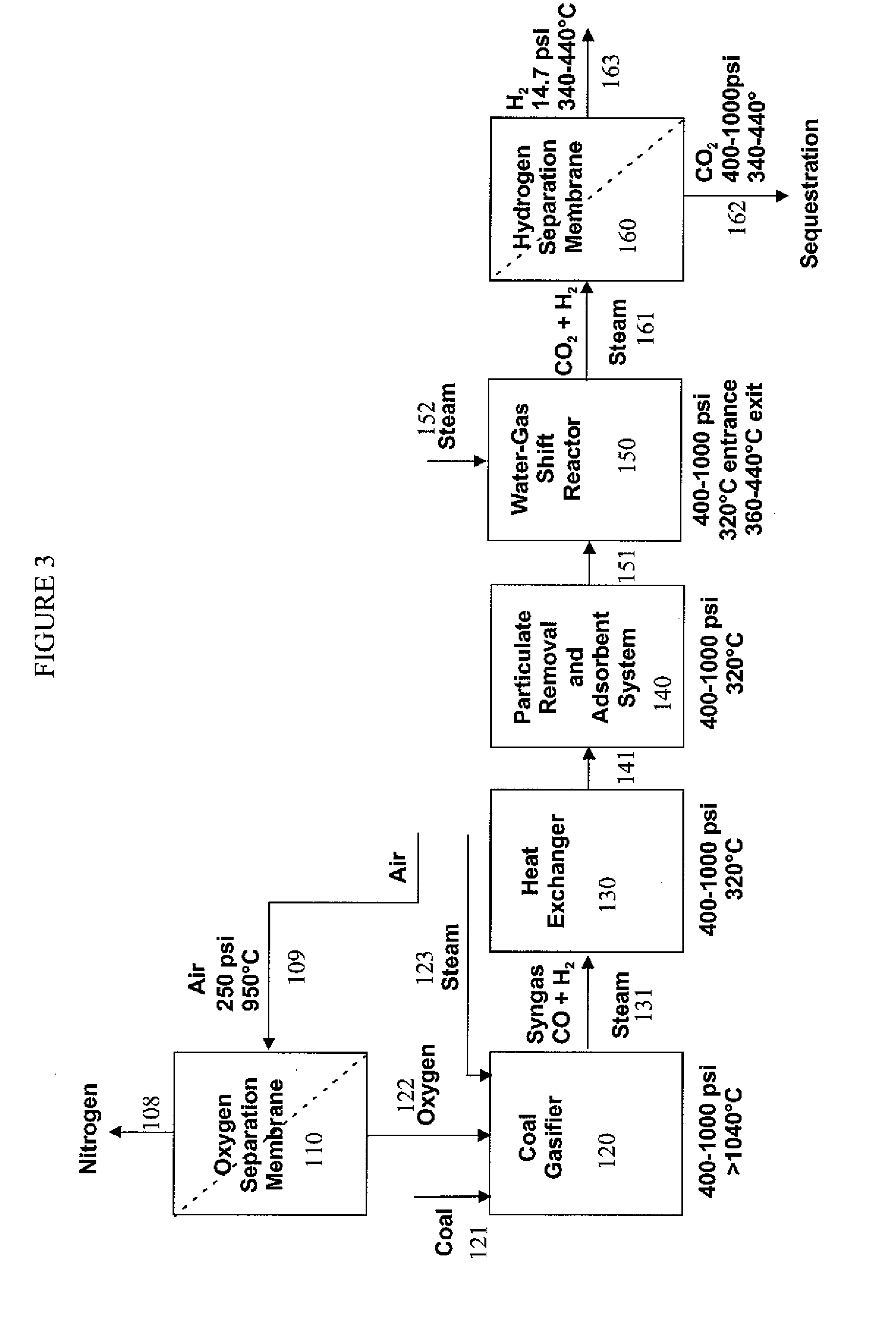
Hydrogen separation process
InactiveUS20080000350A1Isotope separationLoose filtering material filtersAmbient pressureInlet pressure
A method for separating a hydrogen-rich product stream from a feed stream comprising hydrogen and at least one carbon-containing gas, comprising feeding the feed stream, at an inlet pressure greater than atmospheric pressure and a temperature greater than 200° C., to a hydrogen separation membrane system comprising a membrane that is selectively permeable to hydrogen, and producing a hydrogen-rich permeate product stream on the permeate side of the membrane and a carbon dioxide-rich product raffinate stream on the raffinate side of the membrane. A method for separating a hydrogen-rich product stream from a feed stream comprising hydrogen and at least one carbon-containing gas, comprising feeding the feed stream, at an inlet pressure greater than atmospheric pressure and a temperature greater than 200° C., to an integrated water gas shift / hydrogen separation membrane system wherein the hydrogen separation membrane system comprises a membrane that is selectively permeable to hydrogen, and producing a hydrogen-rich permeate product stream on the permeate side of the membrane and a carbon dioxide-rich product raffinate stream on the raffinate side of the membrane. A method for pretreating a membrane, comprising: heating the membrane to a desired operating temperature and desired feed pressure in a flow of inert gas for a sufficient time to cause the membrane to mechanically deform; decreasing the feed pressure to approximately ambient pressure; and optionally, flowing an oxidizing agent across the membrane before, during, or after deformation of the membrane. A method of supporting a hydrogen separation membrane system comprising selecting a hydrogen separation membrane system comprising one or more catalyst outer layers deposited on a hydrogen transport membrane layer and sealing the hydrogen separation membrane system to a porous support.
Owner:ELTRON RES
Catalytic method to remove CO and utilize its energy content in CO-containing streams
InactiveUS20060024539A1Low costUse minimizedMaterial nanotechnologyGas treatmentFuel cellsCatalytic method
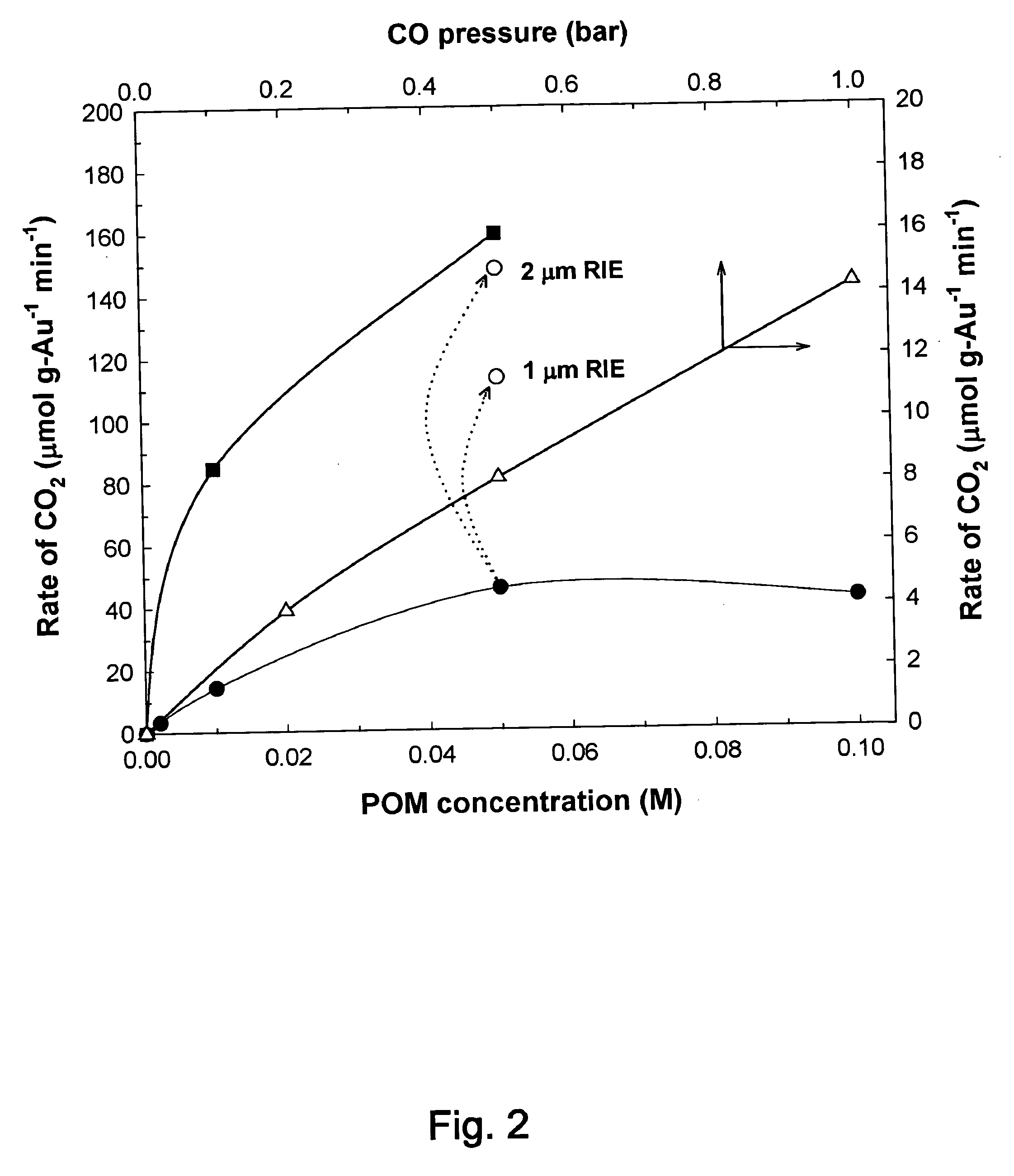
Disclosed are a reactor and a corresponding method for producing electrical energy using a fuel cell by selectively oxidizing CO at room temperature using polyoxometalate compounds and transition metal compounds over metal-containing catalysts, thereby eliminating the water-gas shift reaction and the need to transport and vaporize liquid water in the production of H2 for fuel cells. The reactor also functions to deplete CO from an incoming gas stream.
Owner:WISCONSIN ALUMNI RES FOUND
Process and System for producing synthetic liquid hydrocarbon fuels
ActiveUS7420004B2High volumetric and gravimetric energy densityExcellent resistance to thermal oxidation processLiquid hydrocarbon mixture productionOxygen compounds purification/separationOcean thermal energy conversionElectric power
A process for producing synthetic hydrocarbons that reacts carbon dioxide, obtained from seawater of air, and hydrogen obtained from water, with a catalyst in a chemical process such as reverse water gas shift combined with Fischer Tropsch synthesis. The hydrogen is produced by nuclear reactor electricity, nuclear waste heat conversion, ocean thermal energy conversion, or any other source that is fossil fuel-free, such as wind or wave energy. The process can be either land based or sea based.
Owner:NAVY U S A AS REPRESENTED BY THE SEC OF THE THE
Hot solids gasifier with CO2 removal and hydrogen production
ActiveUS7083658B2Avoid entrainmentEfficient captureMuffle furnacesGas modification by gas mixingCo2 removalWater-gas shift reaction
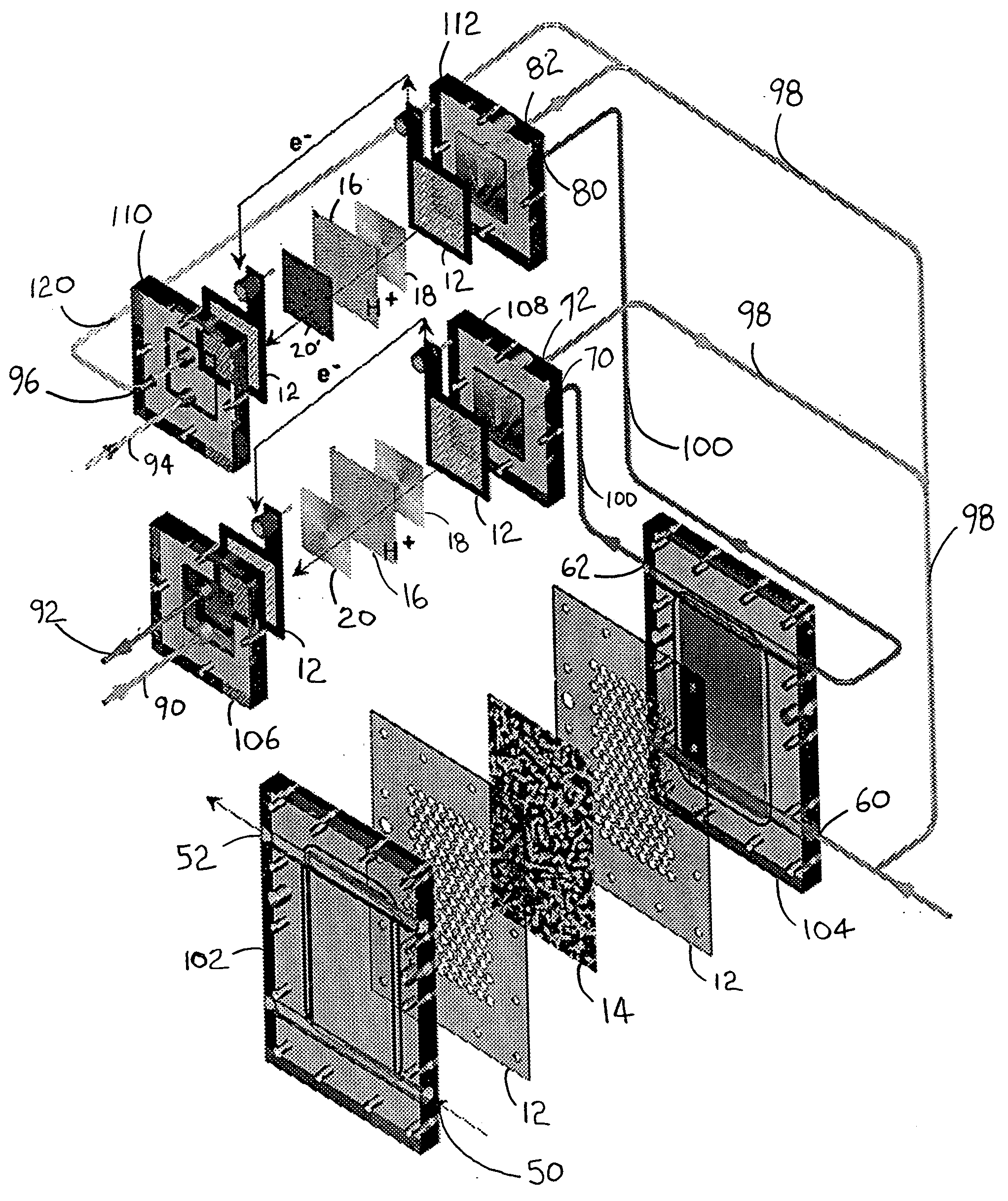
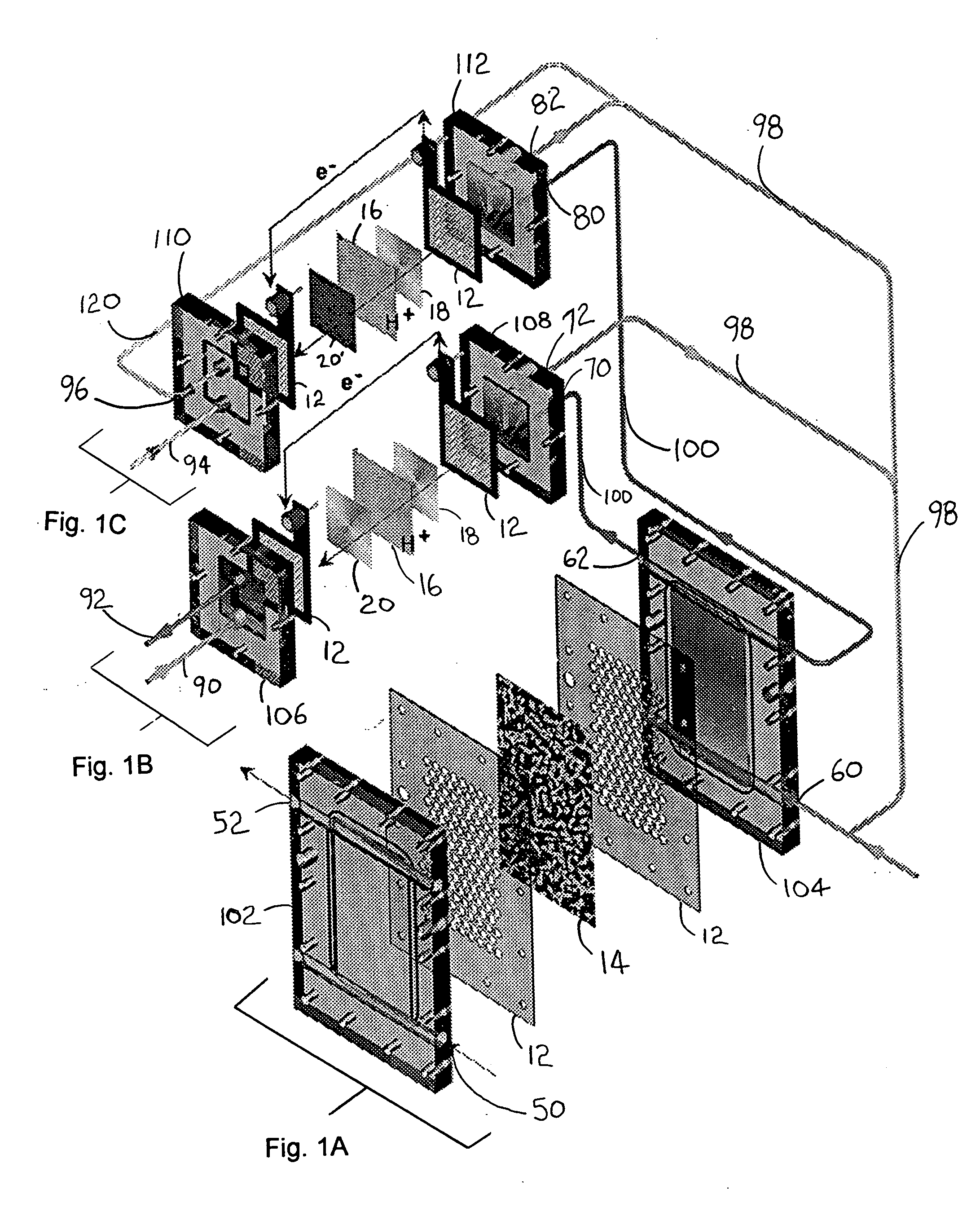
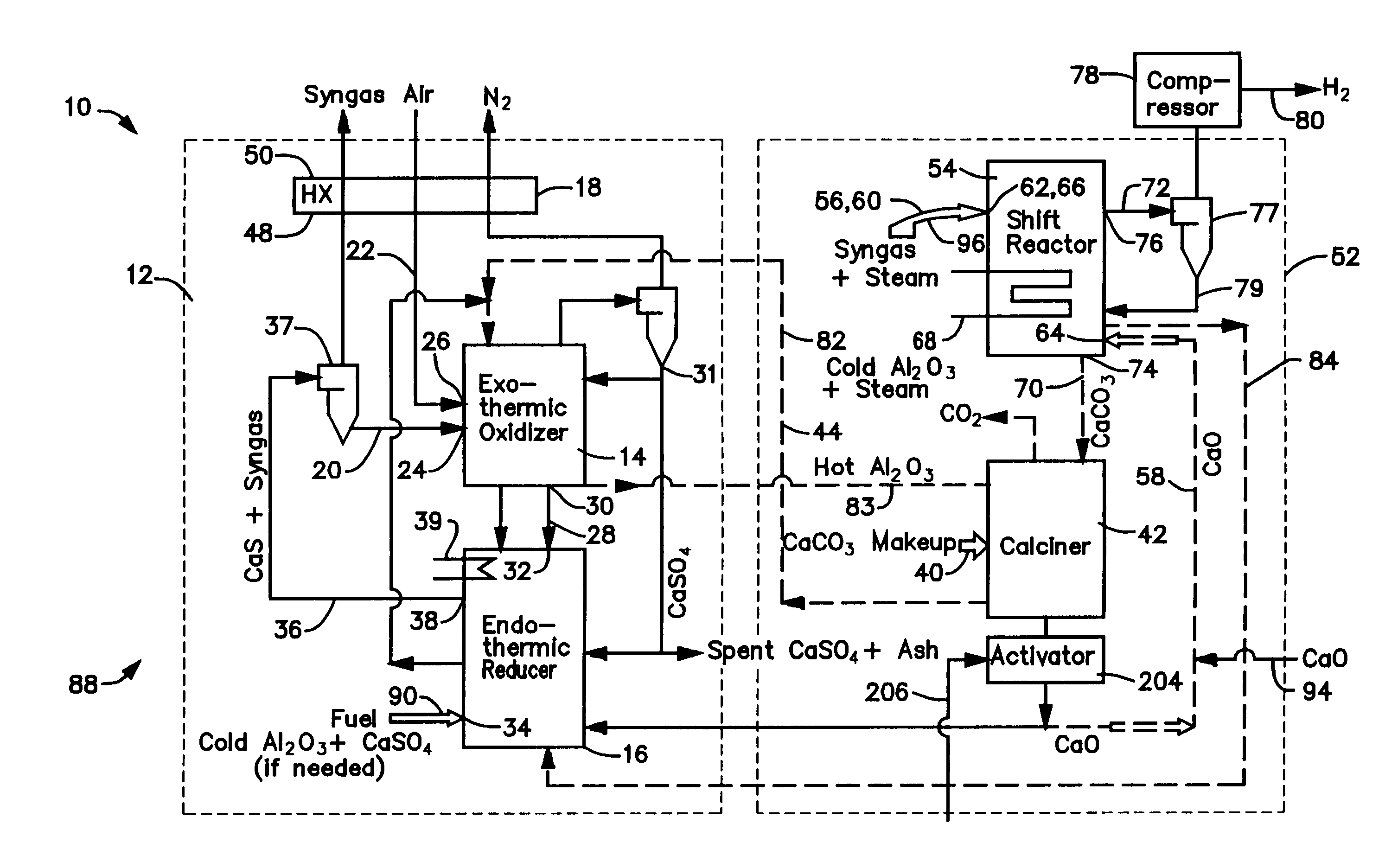
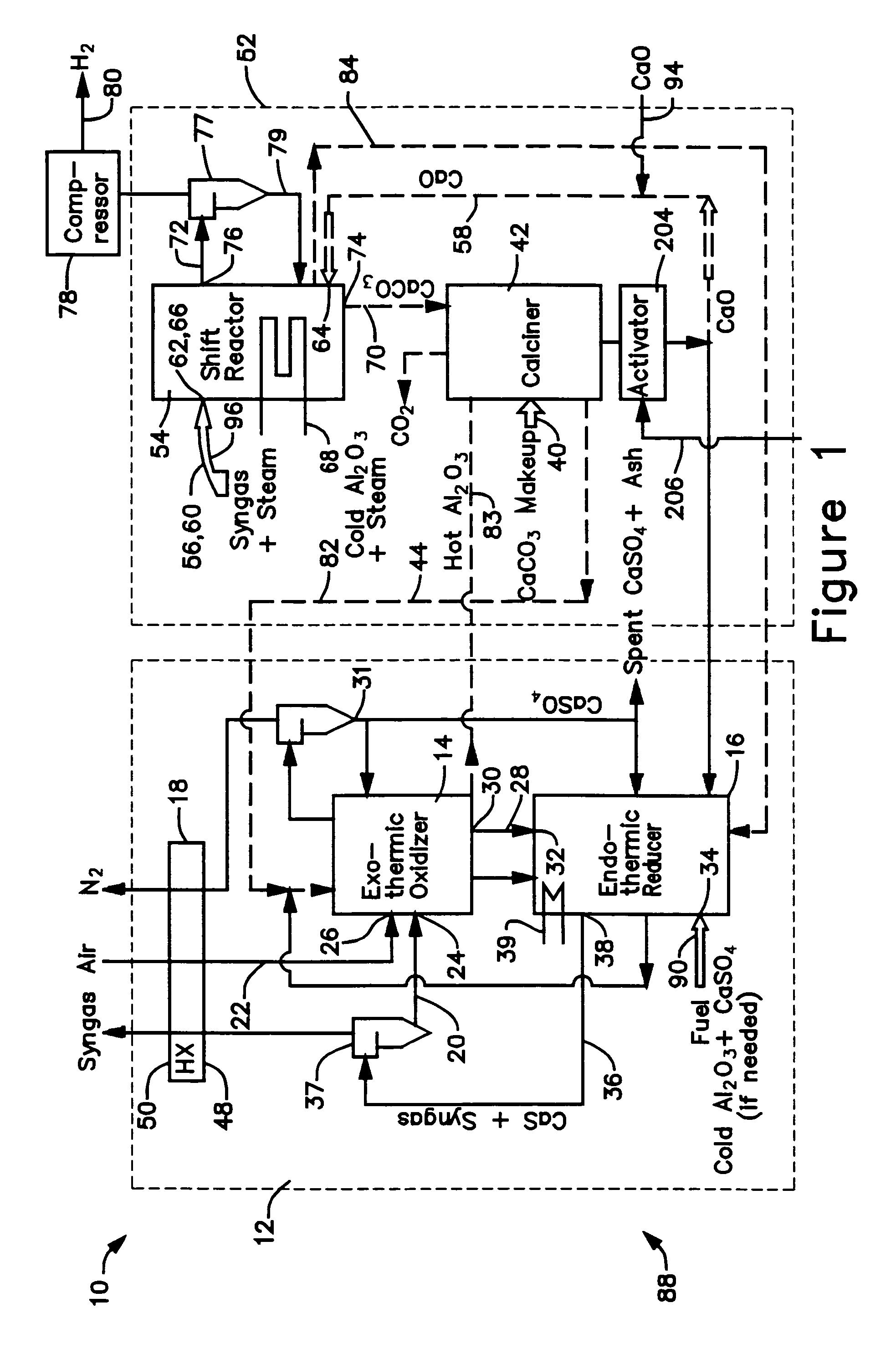
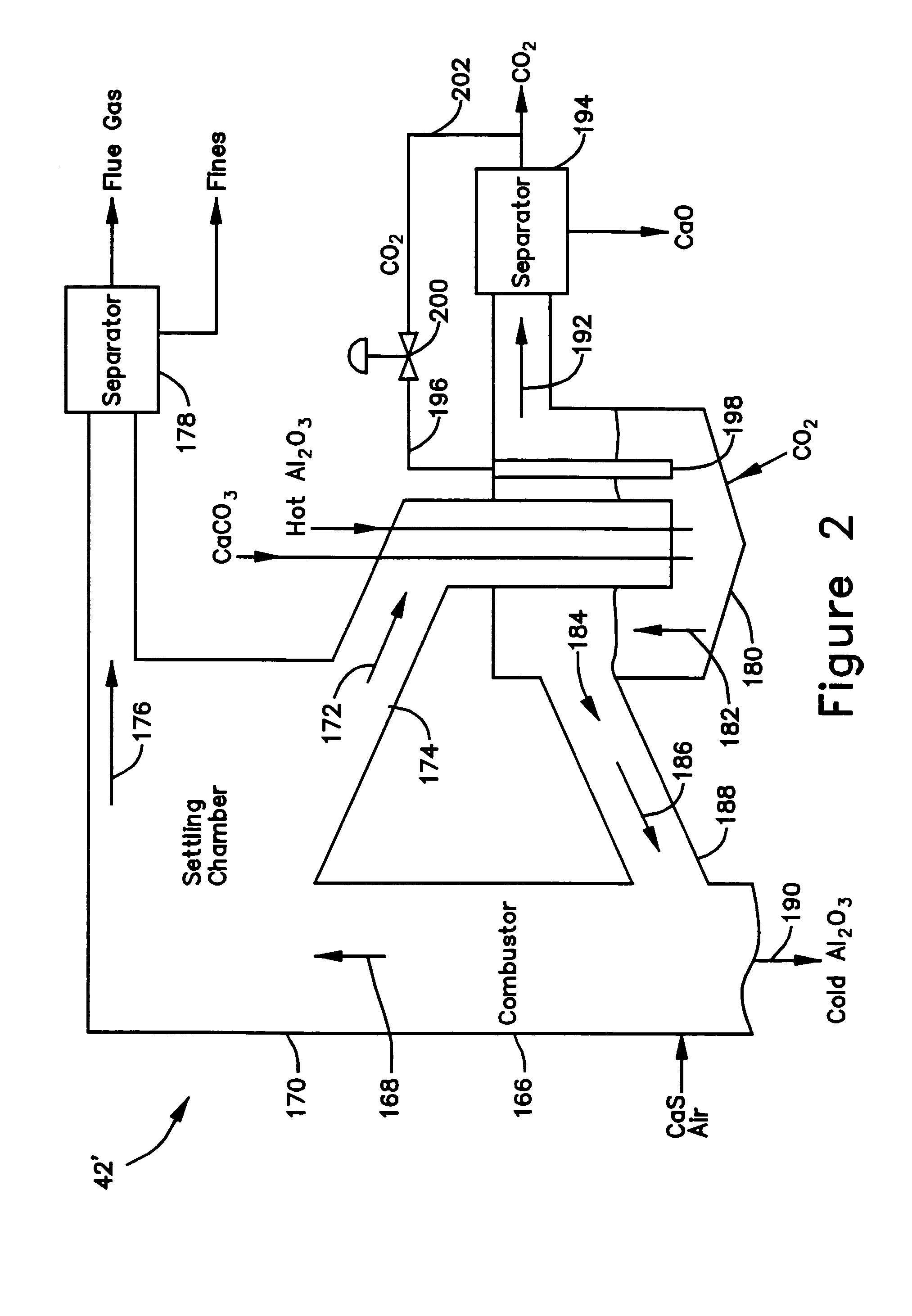
A gasifier 10 includes a first chemical process loop 12 having an exothermic oxidizer reactor 14 and an endothermic reducer reactor 16. CaS is oxidized in air in the oxidizer reactor 14 to form hot CaSO4 which is discharged to the reducer reactor 16. Hot CaSO4 and carbonaceous fuel received in the reducer reactor 16 undergo an endothermic reaction utilizing the heat content of the CaSO4, the carbonaceous fuel stripping the oxygen from the CaSO4 to form CaS and a CO rich syngas. The CaS is discharged to the oxidizer reactor 14 and the syngas is discharged to a second chemical process loop 52. The second chemical process loop 52 has a water-gas shift reactor 54 and a calciner 42. The CO of the syngas reacts with gaseous H2O in the shift reactor 54 to produce H2 and CO2. The CO2 is captured by CaO to form hot CaCO3 in an exothermic reaction. The hot CaCO3 is discharged to the calciner 42, the heat content of the CaCO3 being used to strip the CO2 from the CaO in an endothermic reaction in the calciner, with the CaO being discharged from the calciner 42 to the shift reactor 54.
Owner:AIR PROD & CHEM INC +1
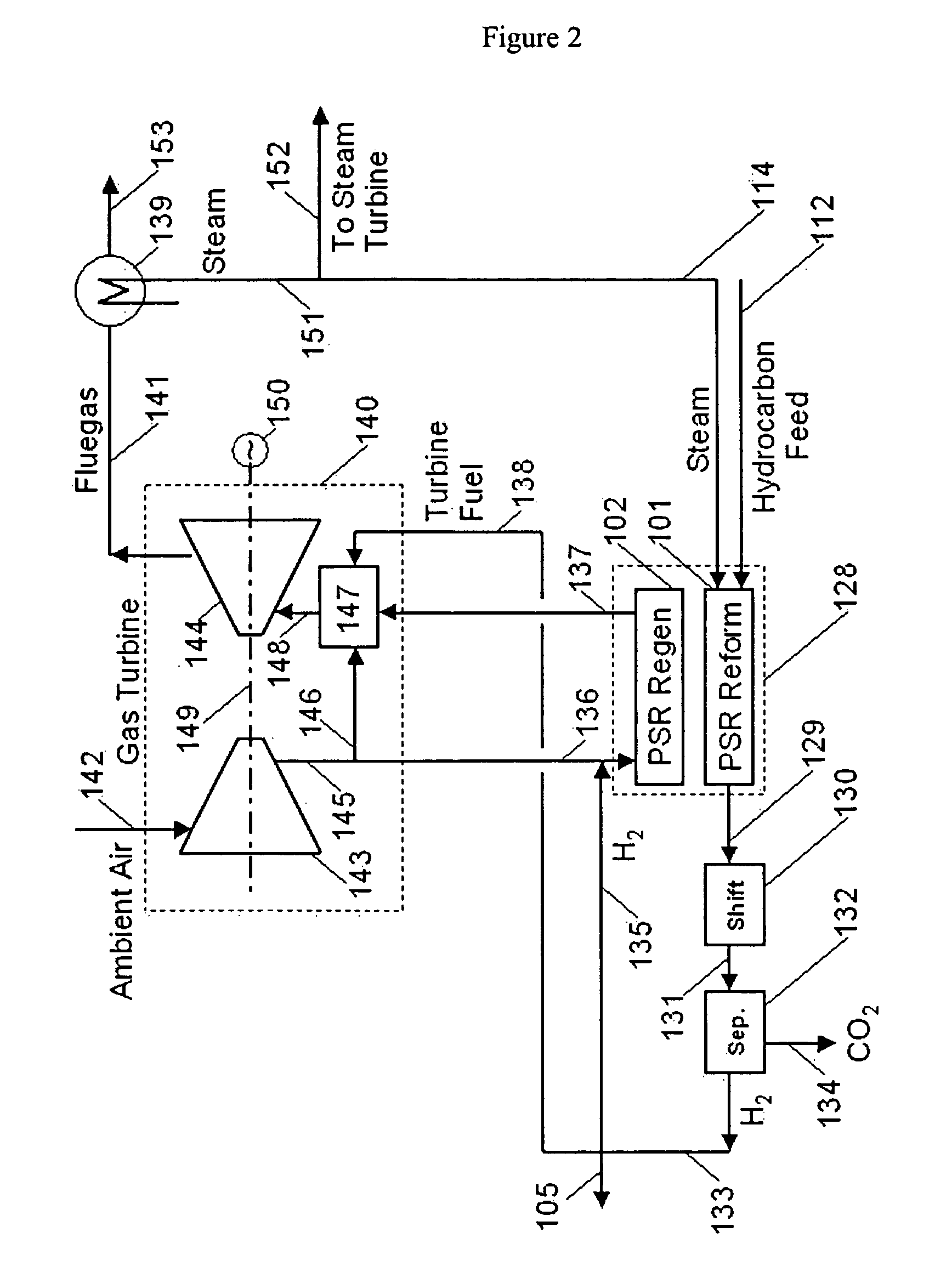
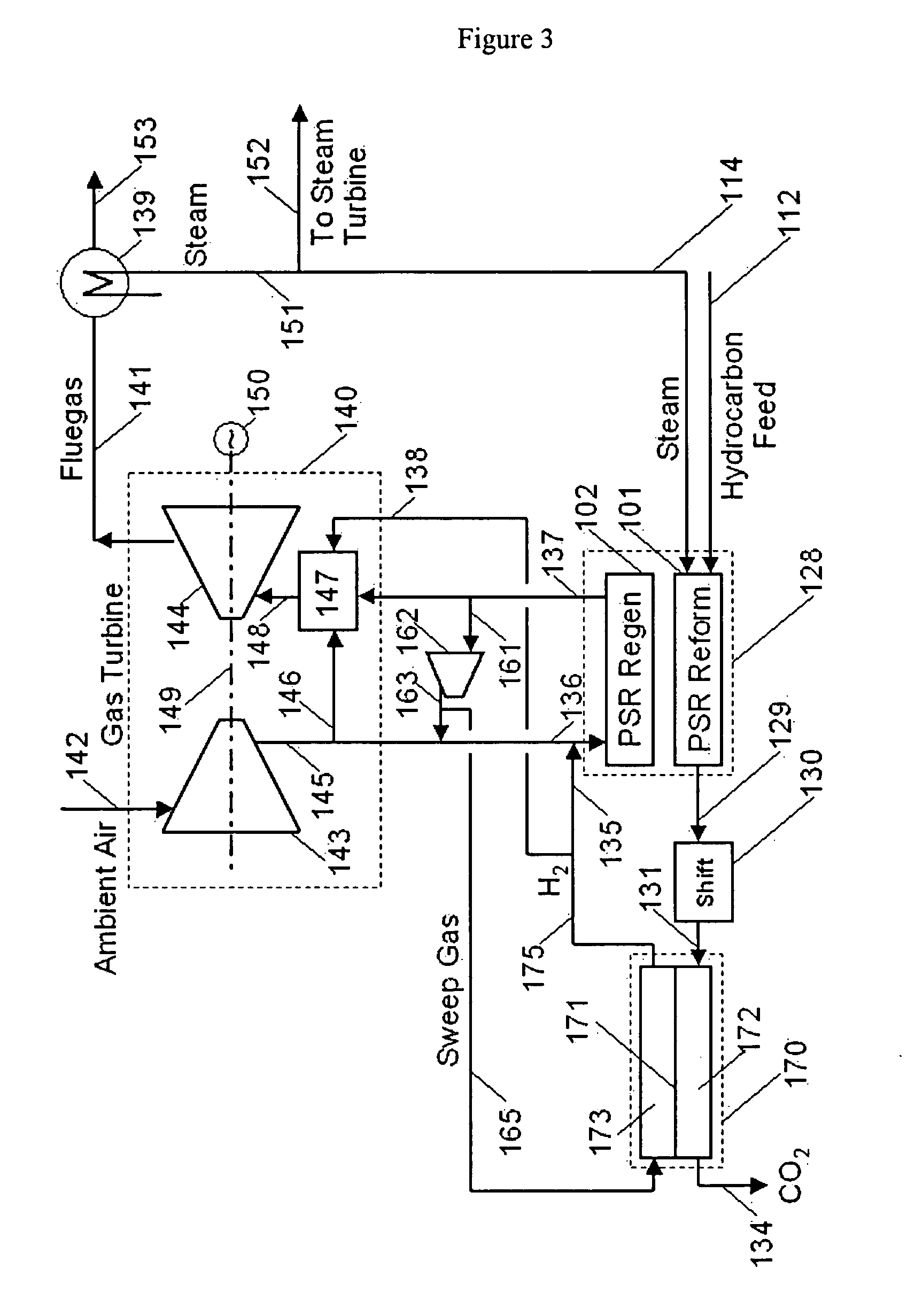
Integration of hydrogen and power generation using pressure swing reforming
ActiveUS7815892B2Improve processing efficiencyHydrogen separation using solid contactHydrogen production carbon captureHydrogenEngineering
The invention provides a method for generating power with a gas turbine which utilizes pressure swing reforming under conditions that facilitate CO2 capture. First a synthesis gas stream at a first pressure is produced in a pressure swing reformer. Next the synthesis gas stream is subjected to a high temperature water gas shift process to produce a CO2 containing hydrogen enriched stream from which hydrogen and CO2 each are separated. The separated hydrogen in turn is combusted with air to produce a gas turbine and the separated CO2 is easily sequestered.
Owner:EXXON RES & ENG CO
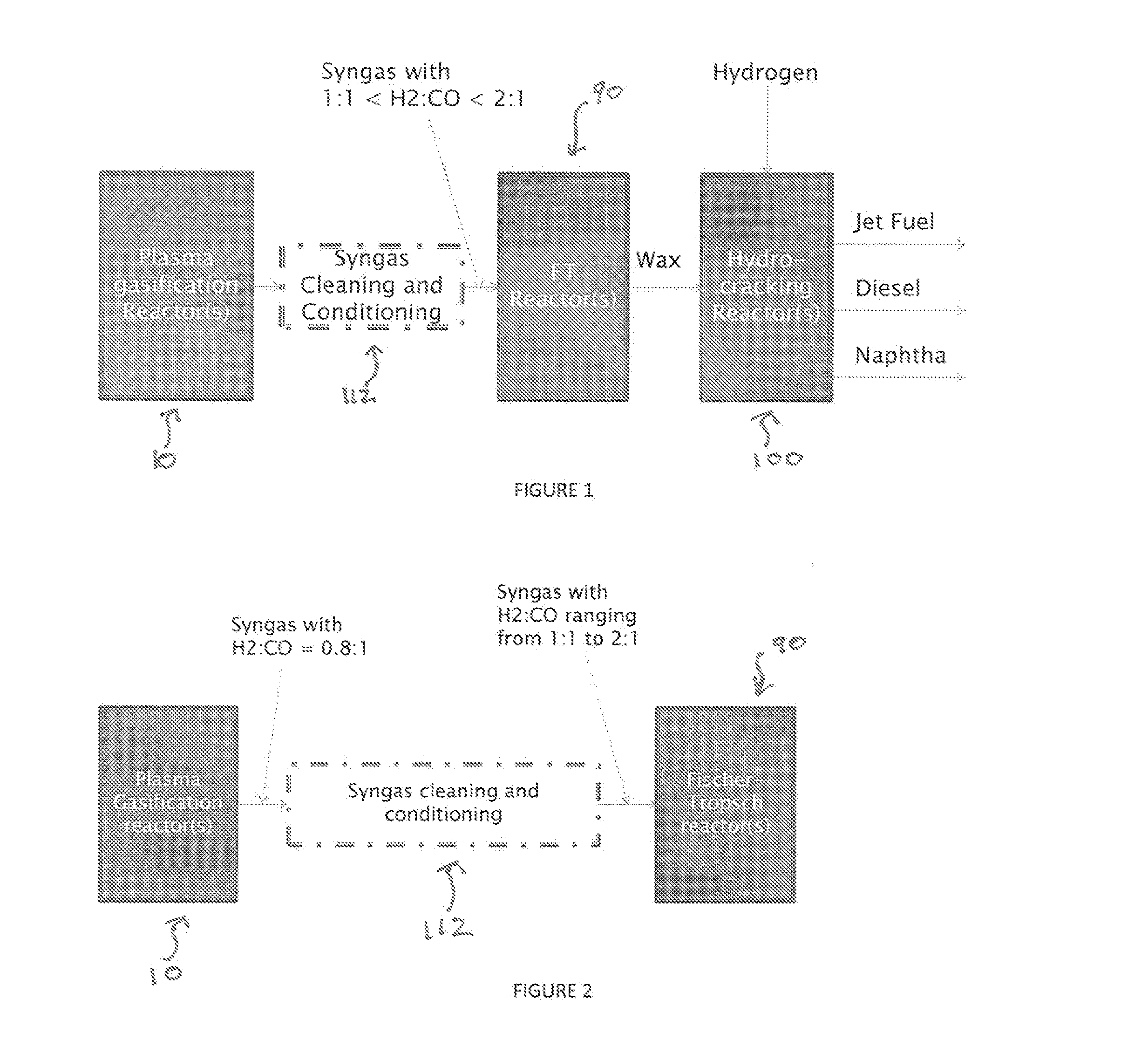
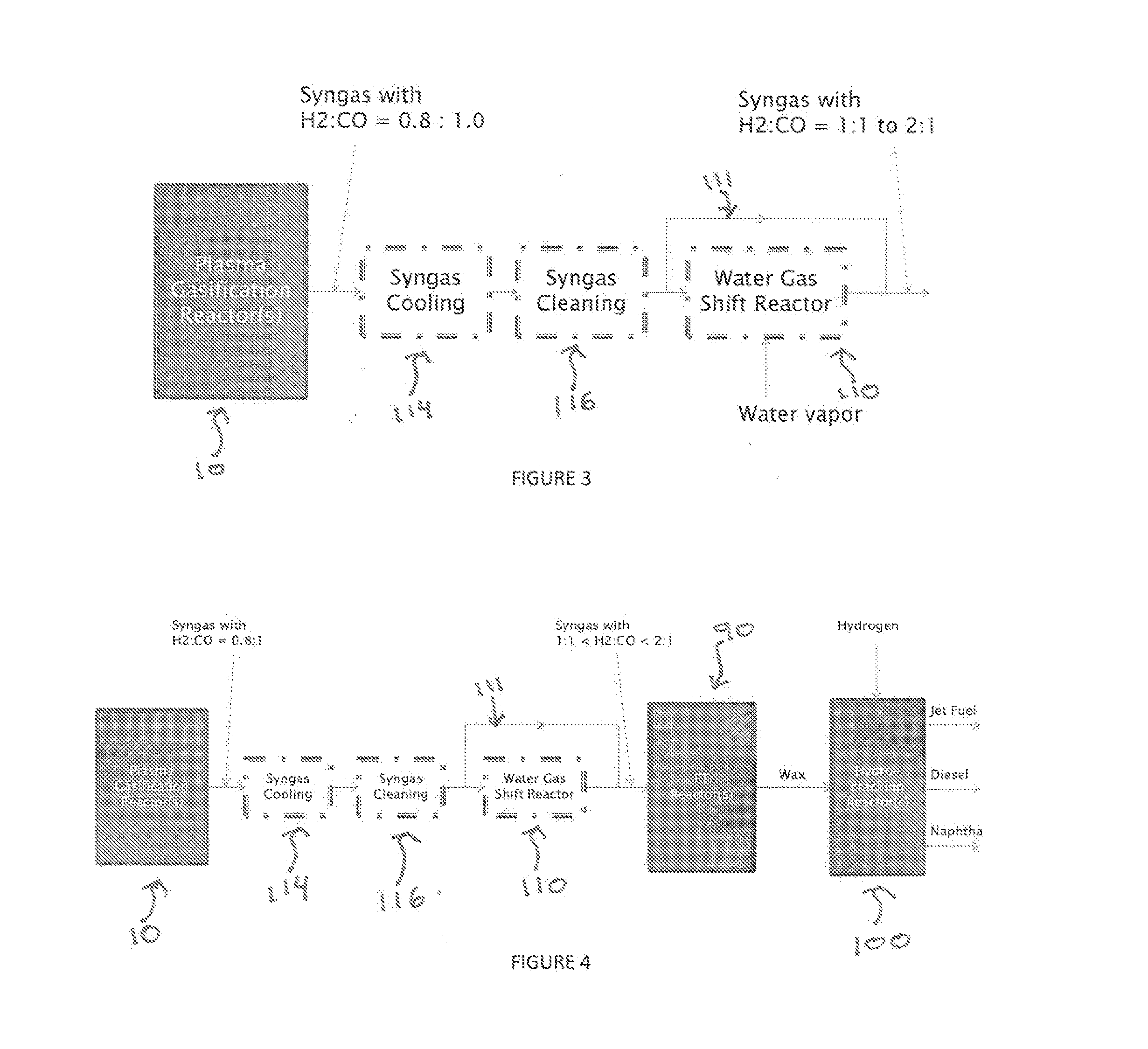
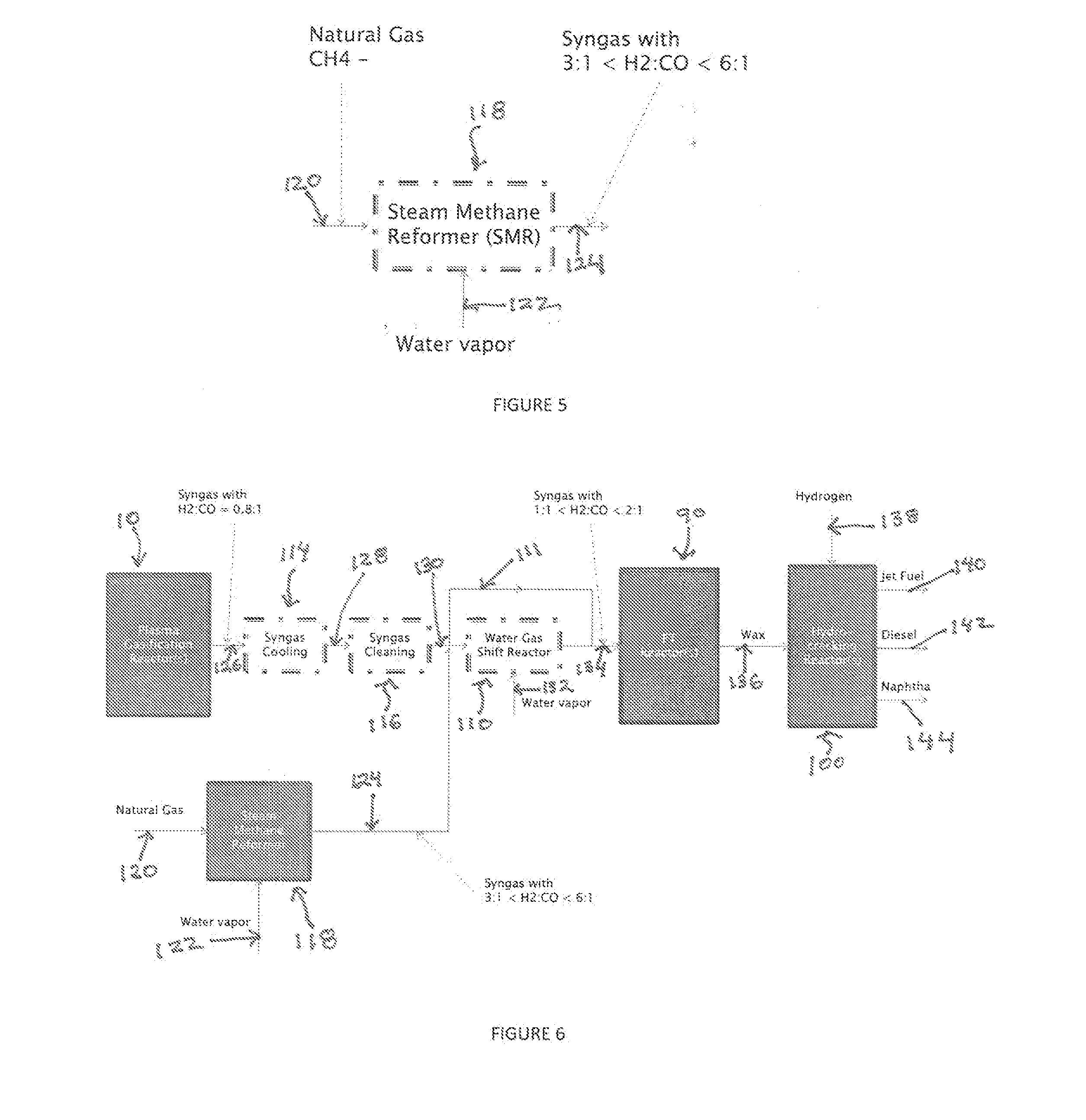
Producing Liquid Fuel from Organic Material such as Biomass and Waste Residues
InactiveUS20140224706A1Combustible gas catalytic treatmentGas modification by gas mixingWaxForming gas
A process for producing liquid fuel from biomass feed stock comprising feeding a biomass feedstock into a one stage atmospheric pressure thermo-catalytic plasma gasifier, contacting the feedstock with oxygen or steam or both to obtain a syngas stream; splitting the syngas stream into first and second streams; conveying the first stream to a water gas shift reactor for producing a modified syngas stream containing CO and hydrogen; the second stream bypassing the water gas shift reactor and being added to the modified syngas steam; optionally reforming natural gas by steam methane reforming to produce a synthetic gas and optionally adding the synthetic gas to the water gas shift reactor; thereby obtaining a syngas having a H2:CO ratio of about 1:1 to about 2:1; subjecting the syngas to a Fischer Tropsch reaction thereby producing a wax product; and subjecting the product to a hydrogen cracking process to produce liquid fuel; and apparatus therefore.
Owner:SOLENA FUELS CORP
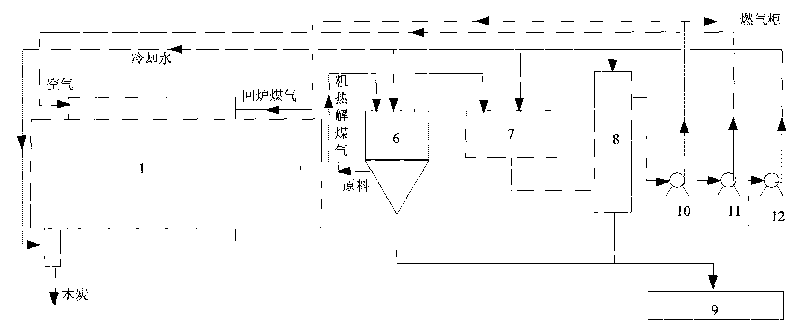
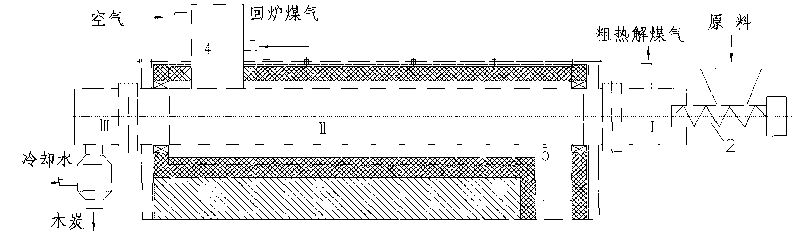
Process for internally heated continuous preparing biomass pyrolysis gasification gas and rotary furnace utilized by same
ActiveCN101693848ASmall footprintProvide continuous and stableBiofuelsSpecial form destructive distillationThermal energyDecomposition
The invention discloses a process for integrally heated continuous preparing biomass pyrolysis gasification gas and a rotary furnace utilized by the same. At a furnace tail cooling section, red charcoal at a temperature of 600-800 DEG C after pyrolyzing and destructive distilling reacts with steam generated by cooling spray water on the lower portion in the furnace so as to absorb large quantities of heat and simultaneously generate water gas and semi-water gas. Heat generated by burning recycled gas and air is used for maintaining pyrolysis temperature of biomass material layers and providesenough heat for further, pyrolysis, destructive distillation and steam decomposition reaction. By aid of the heat, biomass inside the furnace is pyrolyzed and destructively distilled to generate large quantities of volatile matters, and the biomass is gradually heated to decompose destructive gas with high heat value. The rotary furnace utilizes a three-section design: a first section is a furnace head preheating section, which is mainly used for preheating and drying raw materials, a second section is a rotary furnace body pyrolysis section, which is mainly used for pyrolysis and destructivedistillation and is a core operation portion of the furnace, and a third section is a furnace tail cooling section, which is mainly used for cooling charcoal. Prepared gas is high in heat value, and devices occupy small space.
Owner:INST OF CHEM IND OF FOREST PROD CHINESE ACAD OF FORESTRY +1
Method for directly converting synthetic gas into low-carbon olefins
ActiveCN102234212AExcellent thermal effectStable temperatureHydrocarbon from carbon oxidesBulk chemical productionRetention timeSlurry
The invention discloses a method for preparing low-carbon olefins from synthetic gas, and belongs to the field of olefins. In order to solve the problems that due to a backmixing phenomenon, the retention time is inconsistent, reaction products are widely distributed, and side reactions of olefin secondary reaction and water gas reaction and the like are intensified so as to influence the economy of the process in the traditional process for preparing low-carbon olefins from synthetic gas, a rotating packed bed reactor is used as a reactor, a solid Fischer-Tropsch synthesis catalyst is used or a catalyst is prepared into slurry, and the synthetic gas is converted into the low-carbon olefins. By the method, the selectivity of the low-carbon olefins can be greatly improved, and reaction heat can be quickly removed to stabilize the temperature of a reaction bed.
Owner:CHINA PETROLEUM & CHEM CORP +1
Integration of hydrogen and power generation using pressure swing reforming
ActiveUS20050201929A1Improve processing efficiencySpeed up heat exchangeHydrogen separation using solid contactHydrogen productionHydrogenProcess engineering
The invention provides a method for generating power with a gas turbine which utilizes pressure swing reforming under conditions that facilitate CO2 capture. First a synthesis gas stream at a first pressure is produced in a pressure swing reformer. Next the synthesis gas stream is subjected to a high temperature water gas shift process to produce a CO2 containing hydrogen enriched stream from which hydrogen and CO2 each are separated. The separated hydrogen in turn is combusted with air to produce a gas turbine and the separated CO2 is easily sequestered.
Owner:EXXON RES & ENG CO
Process for producing synthetic liquid hydrocarbon fuels
ActiveUS20050232833A1Meet high volumeIncreased gravimetric energy densityLiquid hydrocarbon mixture productionOxygen compounds purification/separationThermal energyOcean thermal energy conversion
A process for producing synthetic hydrocarbons that reacts carbon dioxide, obtained from seawater of air, and hydrogen obtained from water, with a catalyst in a chemical process such as reverse water gas shift combined with Fischer Tropsch snthesis. The hydrogen is produced by nuclear reactor electricity, nuclear waste heat conversion, ocean thermal energy conversion, or any other source that is fossil fuel-free, such as wind or wave energy. The process can be either land based or sea based.
Owner:NAVY U S A AS REPRESENTED BY THE SEC OF THE THE
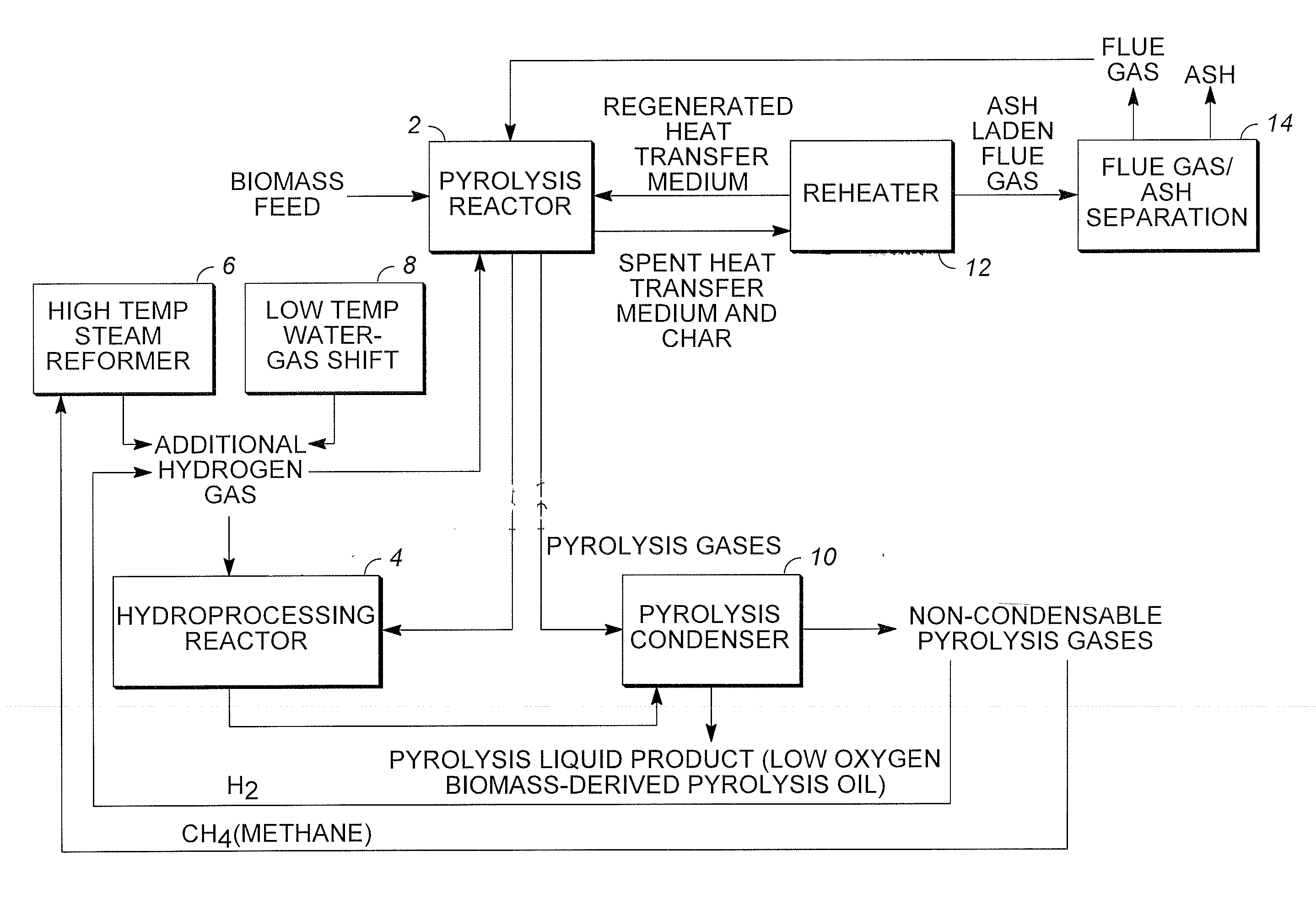
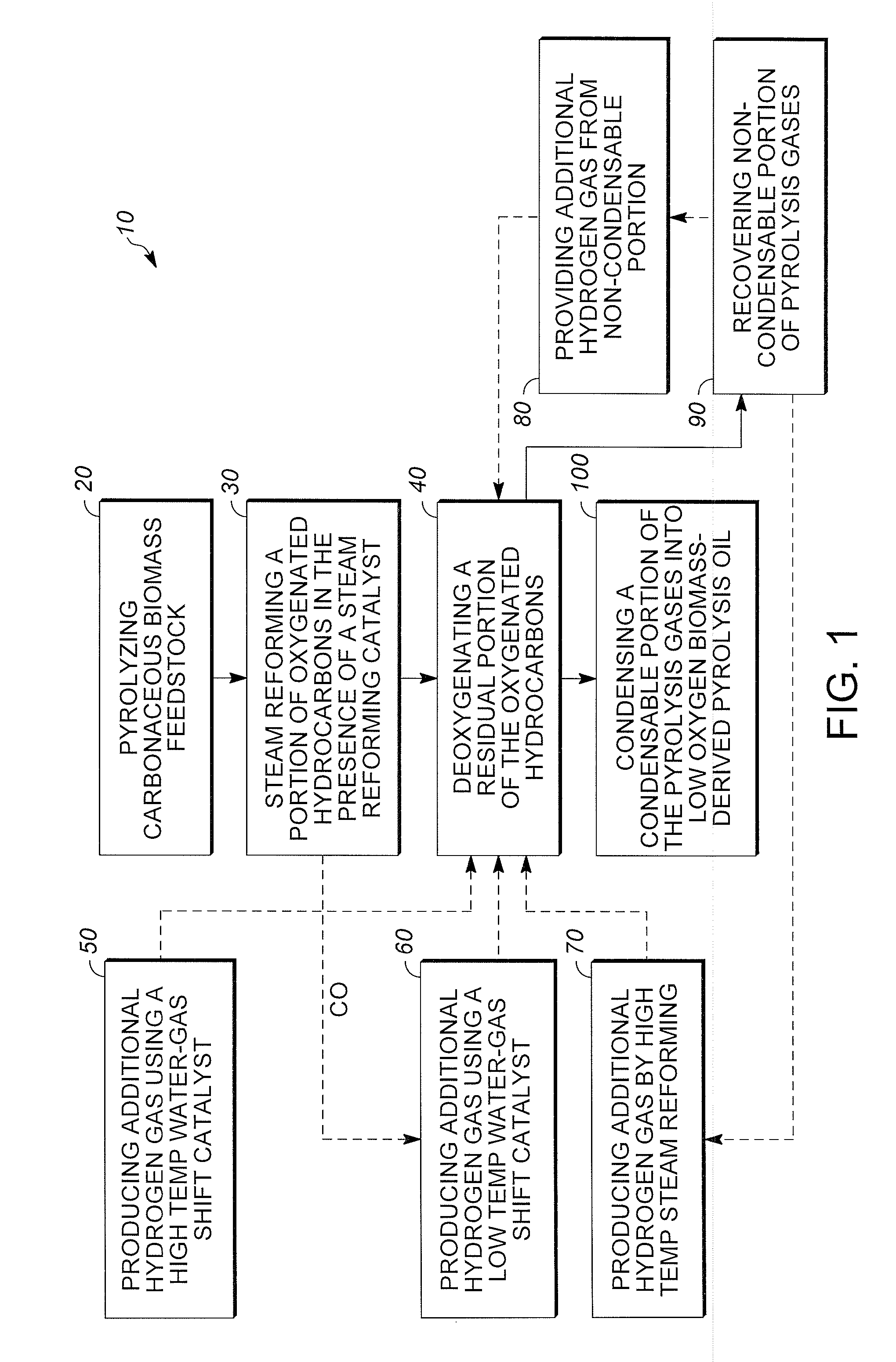
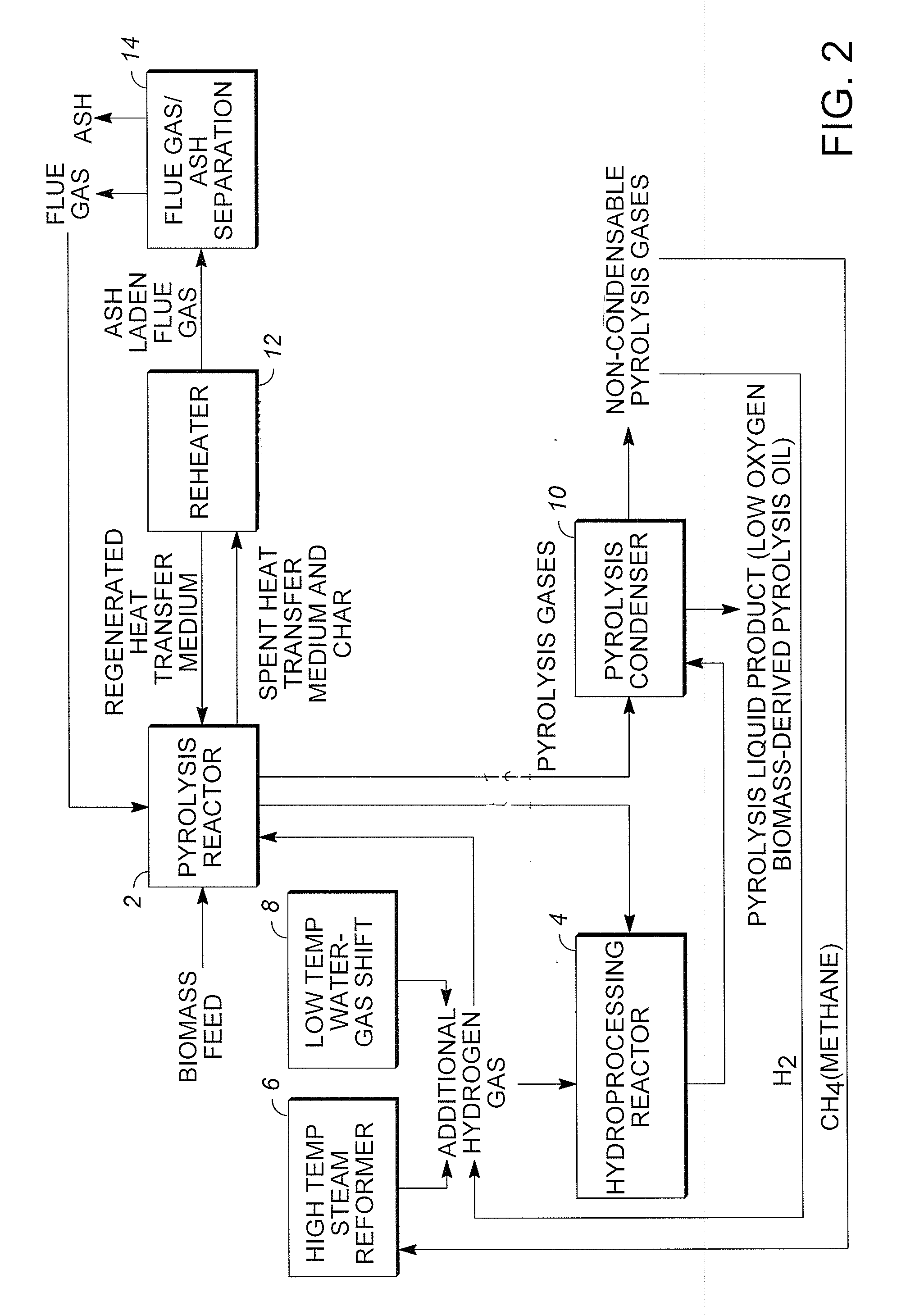
Low oxygen biomass-derived pyrolysis oils and methods for producing the same
InactiveUS20110201854A1Direct heating destructive distillationBiofuelsSteam reformingPartial oxidation
Methods are provided for producing low oxygen biomass-derived pyrolysis oil from carbonaceous biomass feedstock. The carbonaceous biomass feedstock is pyrolyzed in the presence of a steam reforming catalyst to produce char and pyrolysis gases. During pyrolysis, a portion of the oxygenated hydrocarbons in the pyrolysis gases is converted into hydrocarbons by steam reforming also yielding carbon oxides and hydrogen gas. The hydrogen gas at least partially deoxygenates a residual portion of the oxygenated hydrocarbons. Additional hydrogen gas may also be produced by water-gas shift reactions to deoxygenate the residual portion of the oxygenated hydrocarbons in the pyrolysis gases. Deoxygenation may occur in the presence of a hydroprocessing catalyst. A condensable portion of the pyrolysis gases is condensed to form low oxygen biomass-derived pyrolysis oil.
Owner:UOP LLC
Chemical processing using non-thermal discharge plasma
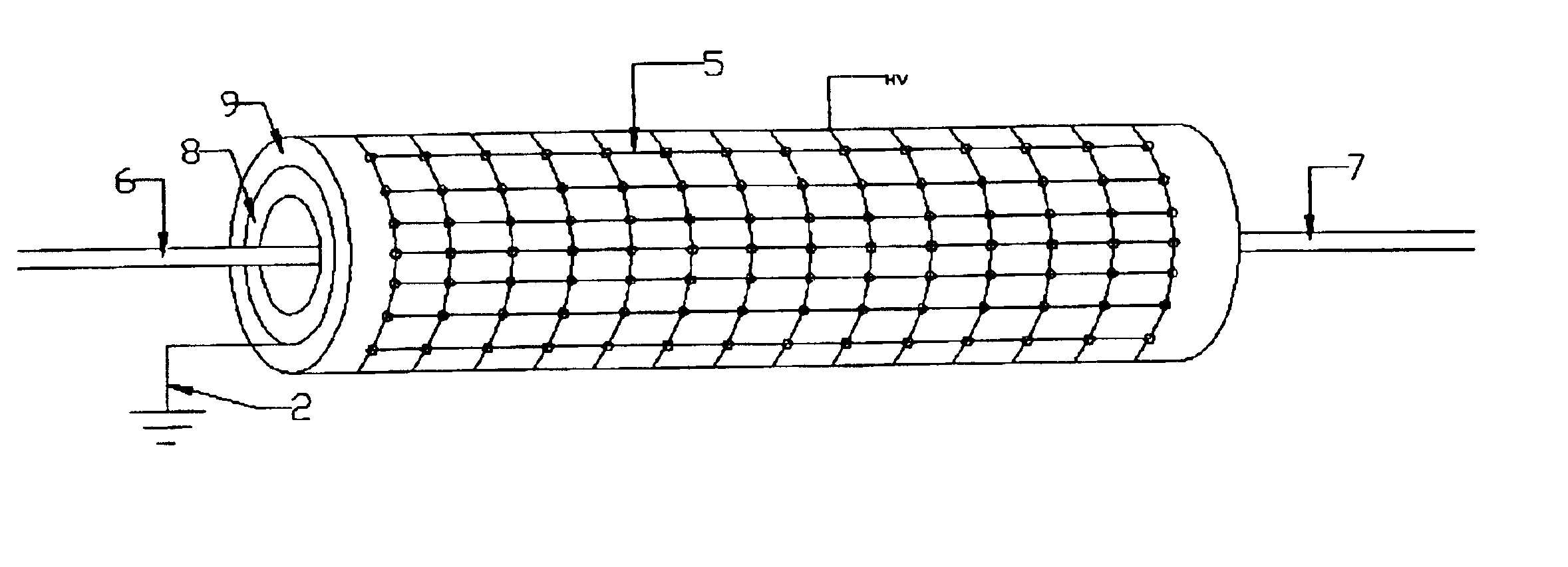
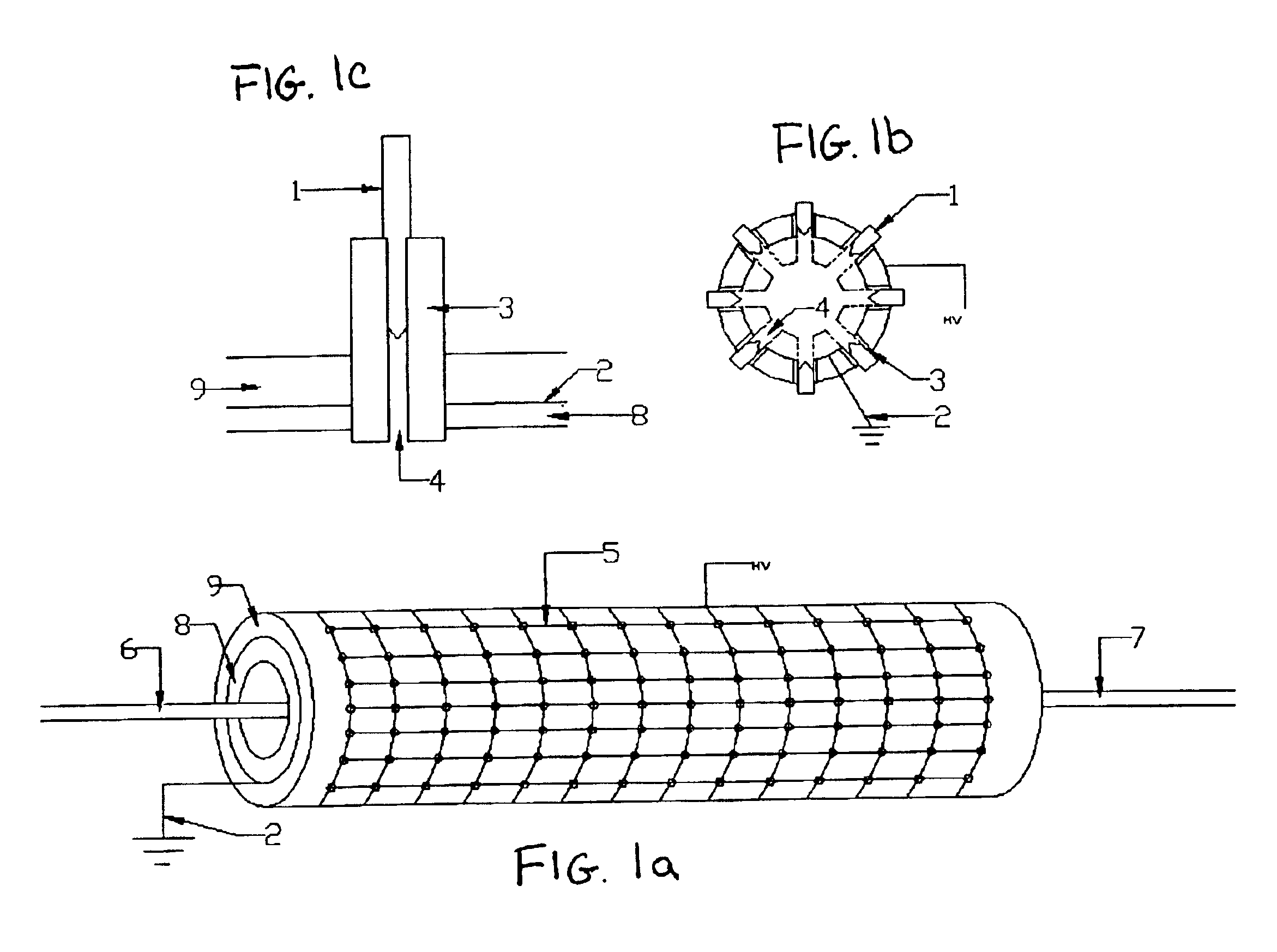
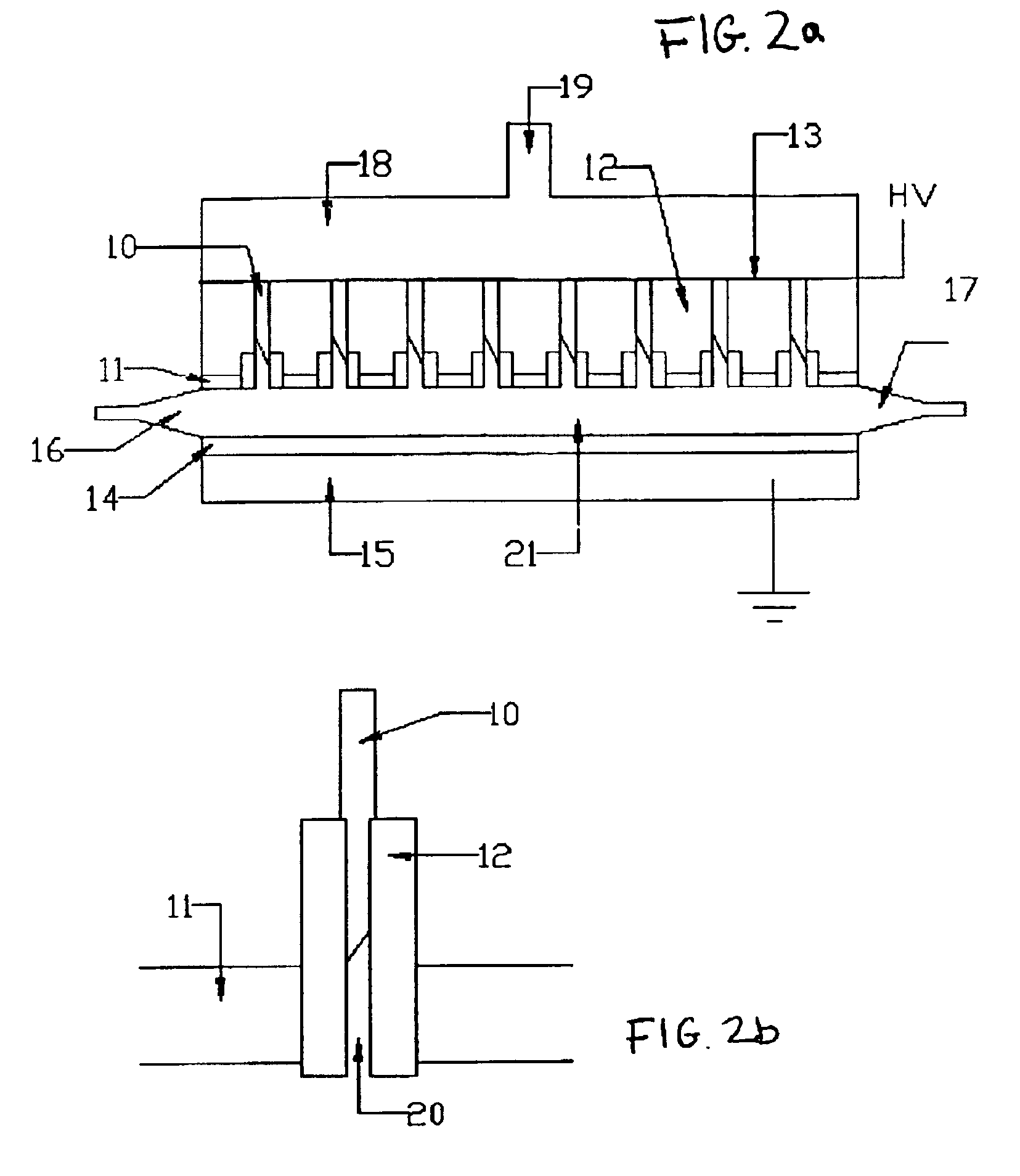
InactiveUS6923890B2Simple processEnhanced water gas shiftingHydrogenMolecular sieve catalystsChemical treatmentFiber
A method for activating chemical reactions using a non-thermal capillary discharge plasma (NT-CDP) unit or a non-thermal slot discharge plasma (NT-SDP) unit (collectively referred to as “NT-CDP / SDP”). The NT-CDP / SDP unit includes a first electrode disposed between two dielectric layers, wherein the first electrode and dielectric layers having at least one opening (e.g., capillary or a slot) defined therethrough. A dielectric sleeve inserted into the opening, and at least one second electrode (e.g., in the shape of a pin, ring, metal wire, or tapered metal blade) is disposed in fluid communication with an associated opening. A non-thermal plasma discharge is emitted from the opening when a voltage differential is applied between the first and second electrodes. Chemical feedstock to be treated is then exposed to the non-thermal plasma. This processing is suited for the following exemplary chemical reactions as (i) partial oxidation of hydrocarbon feedstock to produce functionalized organic compounds; (ii) chemical stabilization of a polymer fiber (e.g., PAN fiber precursor in carbon fiber production; (iii) pre-reforming of higher chain length petroleum hydrocarbons to generate a feedstock suitable for reforming; (iv) natural gas reforming in a chemically reducing atmosphere (e.g., ammonia or urea) to produce carbon monoxide and Hydrogen gas; or (v) plasma enhanced water gas shifting.
Owner:PLASMASOL CORP
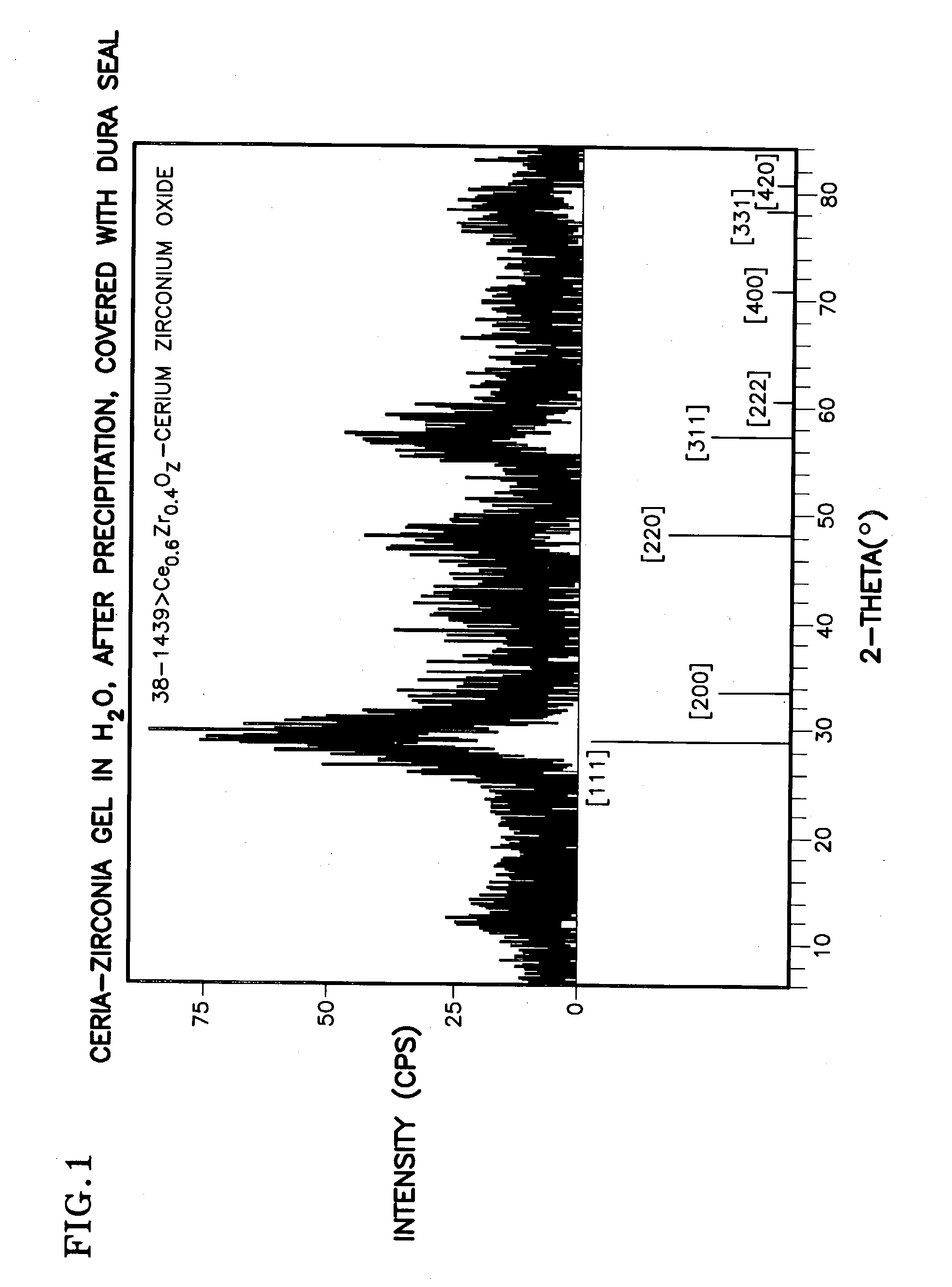
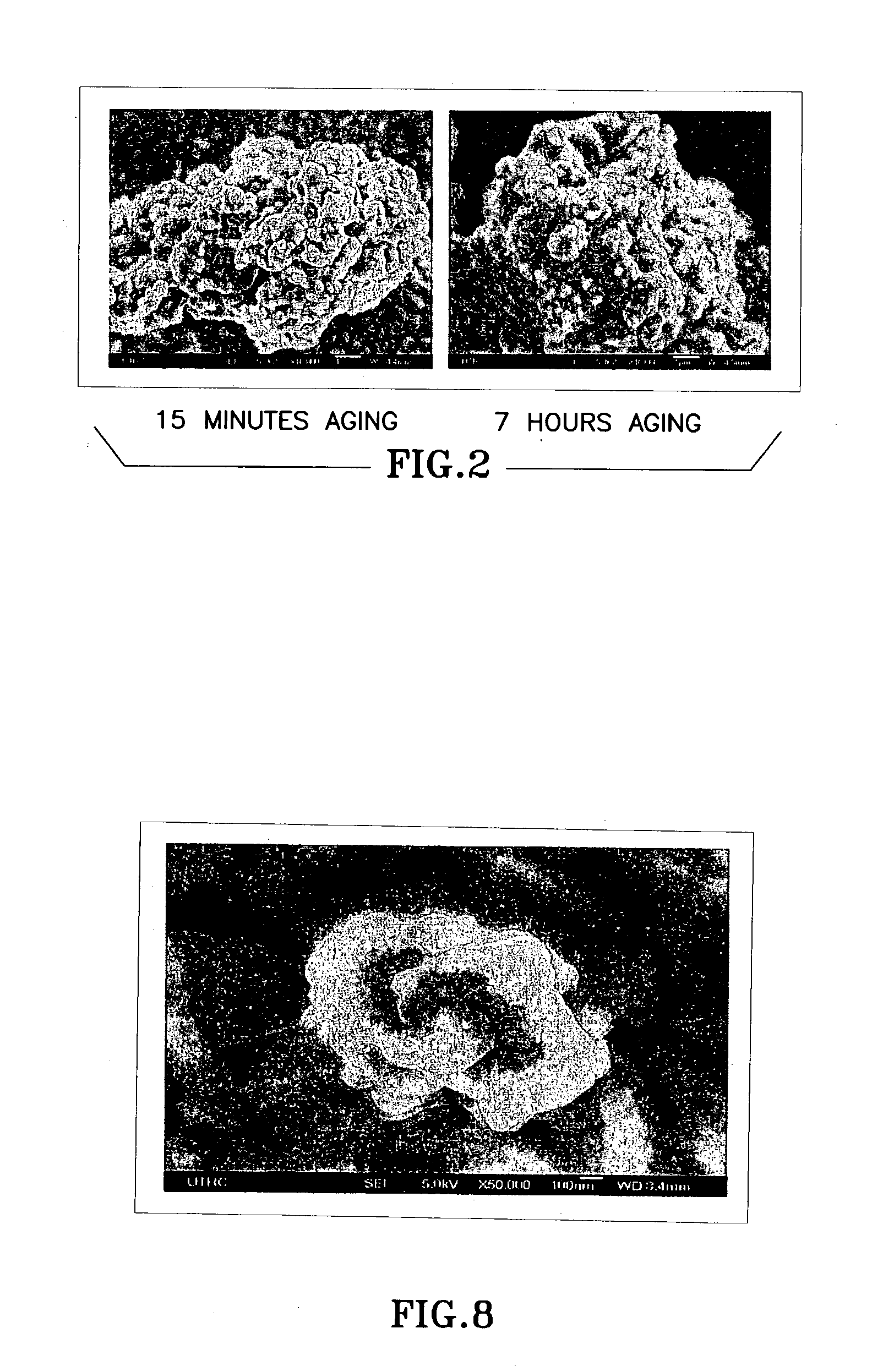
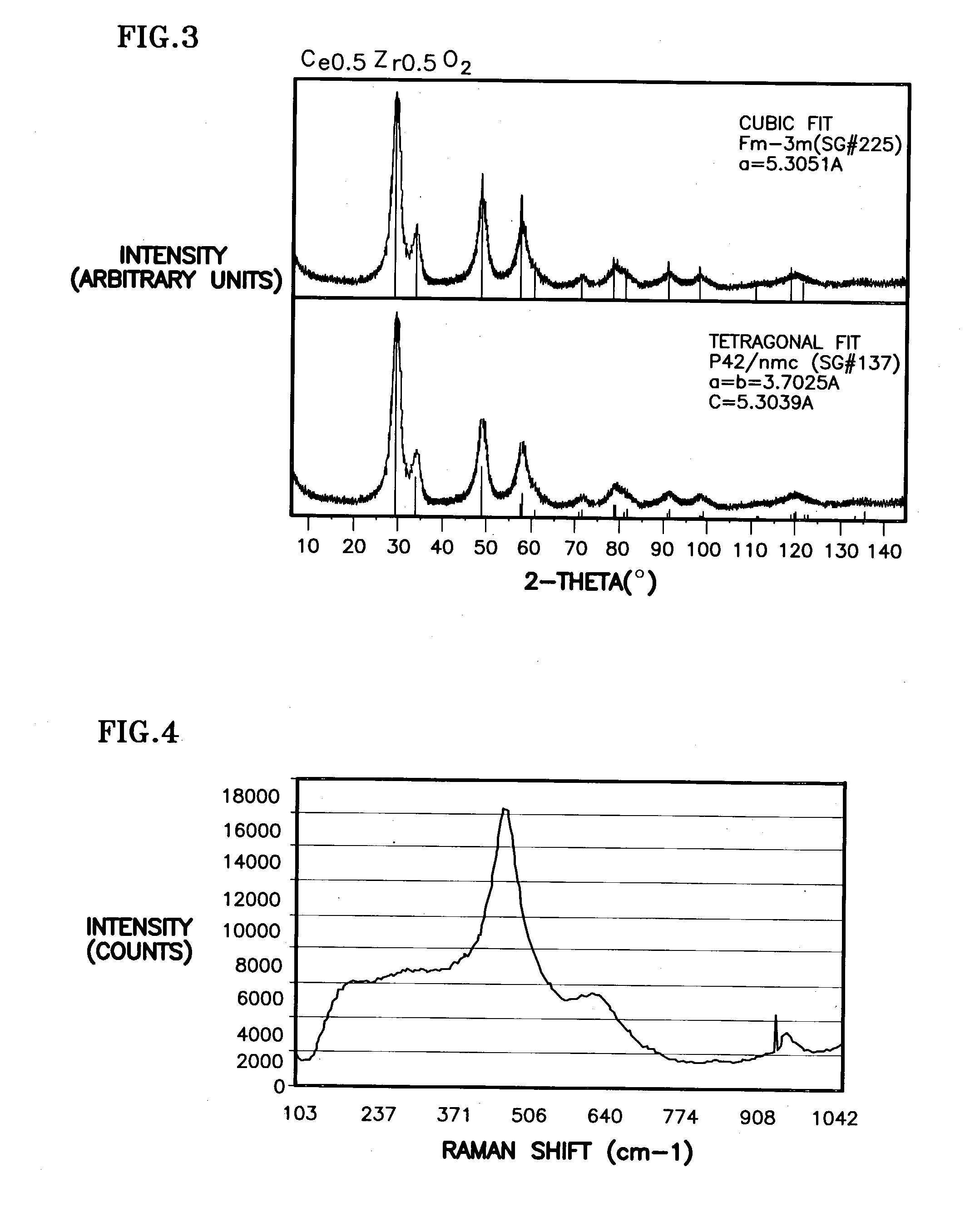
Ceria-based mixed-metal oxide structure, including method of making and use
InactiveUS20030186805A1Increase surface areaSimple structureRare earth metal oxides/hydroxidesMaterial nanotechnologyPtru catalystCerium(IV) oxide
A homogeneous ceria-based mixed-metal oxide, useful as a catalyst support, a co-catalyst and / or a getter, is described. The mixed-metal oxide has a relatively large surface area per weight, typically exceeding 150 m<2> / g, a structure of nanocrystallites having diameters of less than 4 nm, and including pores larger than the nanocrystallites and having diameters in the range of 4 to about 9 nm. The ratio of the pore volumes, VP, to skeletal structure volumes, VS, is typically less than about 2.5, and the surface area per unit volume of the oxide material is greater than 320 m<2> / cm<3>, such that the structural morphology supports both a relatively low internal mass transfer resistance and large effective surface area for reaction activity of interest. The mixed metal oxide is made by co-precipitating a dilute metal salt solution containing the respective metals, which may include Zr, Hf, and / or other metal constituents in addition to Ce, replacing water in the co-precipitate with a water-miscible low surface-tension solvent, and relatively quickly drying and calcining the co-precipitate at moderate temperatures. A highly dispersive catalyst metal, such as Pt, may be loaded on the mixed metal oxide support from a catalyst-containing solution following a selected acid surface treatment of the oxide support. The mixed metal oxide, as catalyst support, co-catalyst or getter, is applied in various reactions, and particularly water gas shift and / or preferential oxidation reactions as associated with fuel processing systems, as for fuel cells and the like.
Owner:INT FUEL CELLS
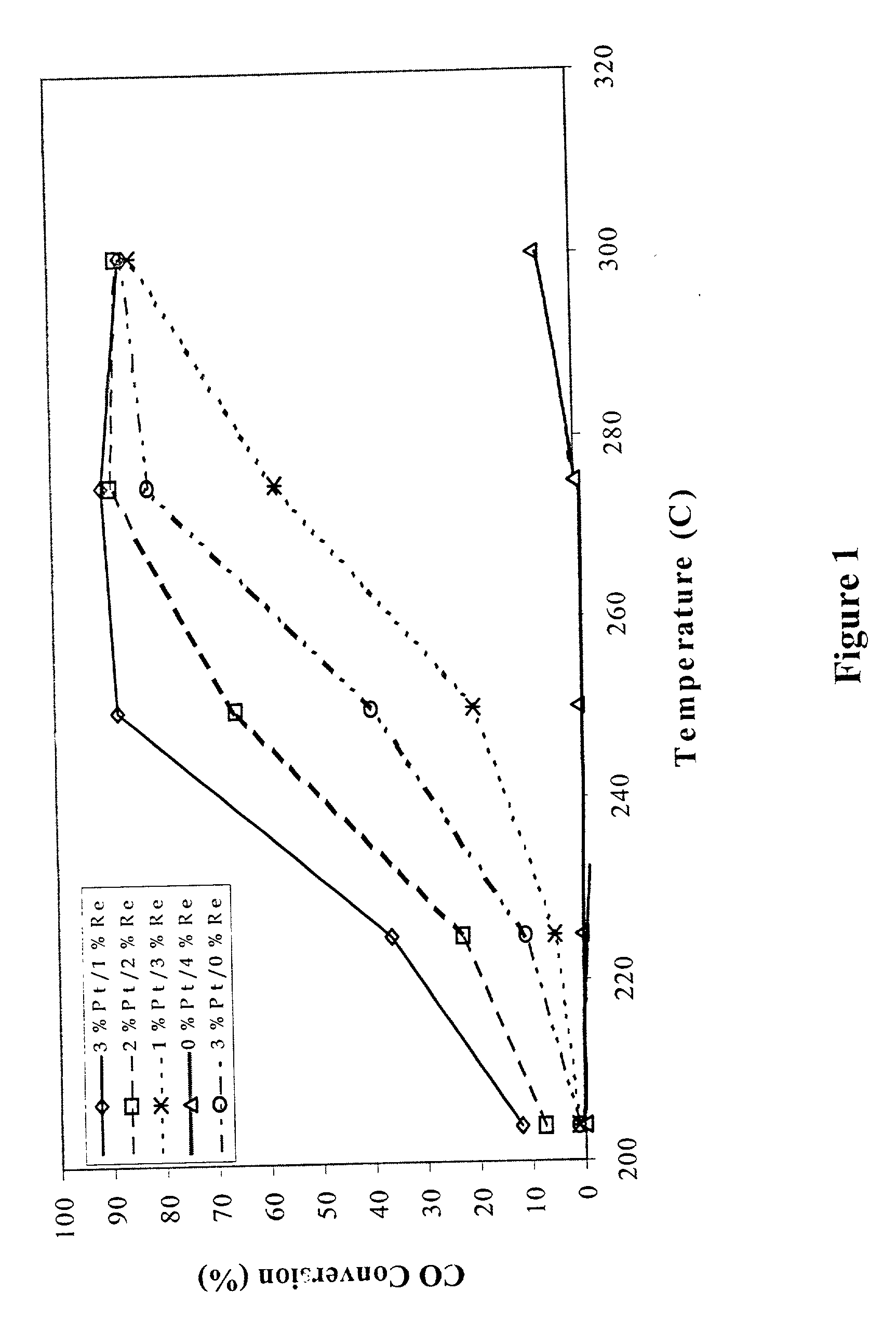
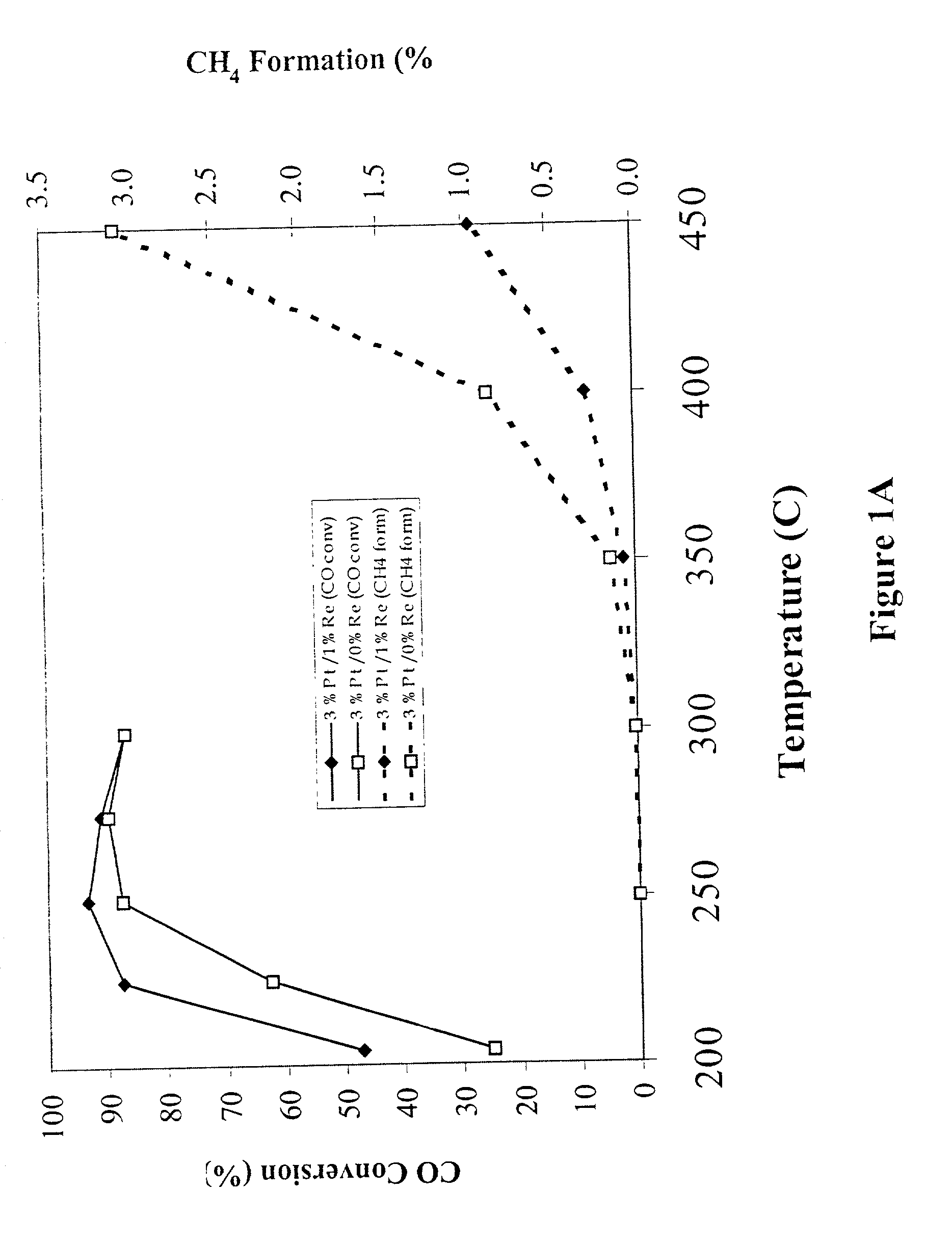
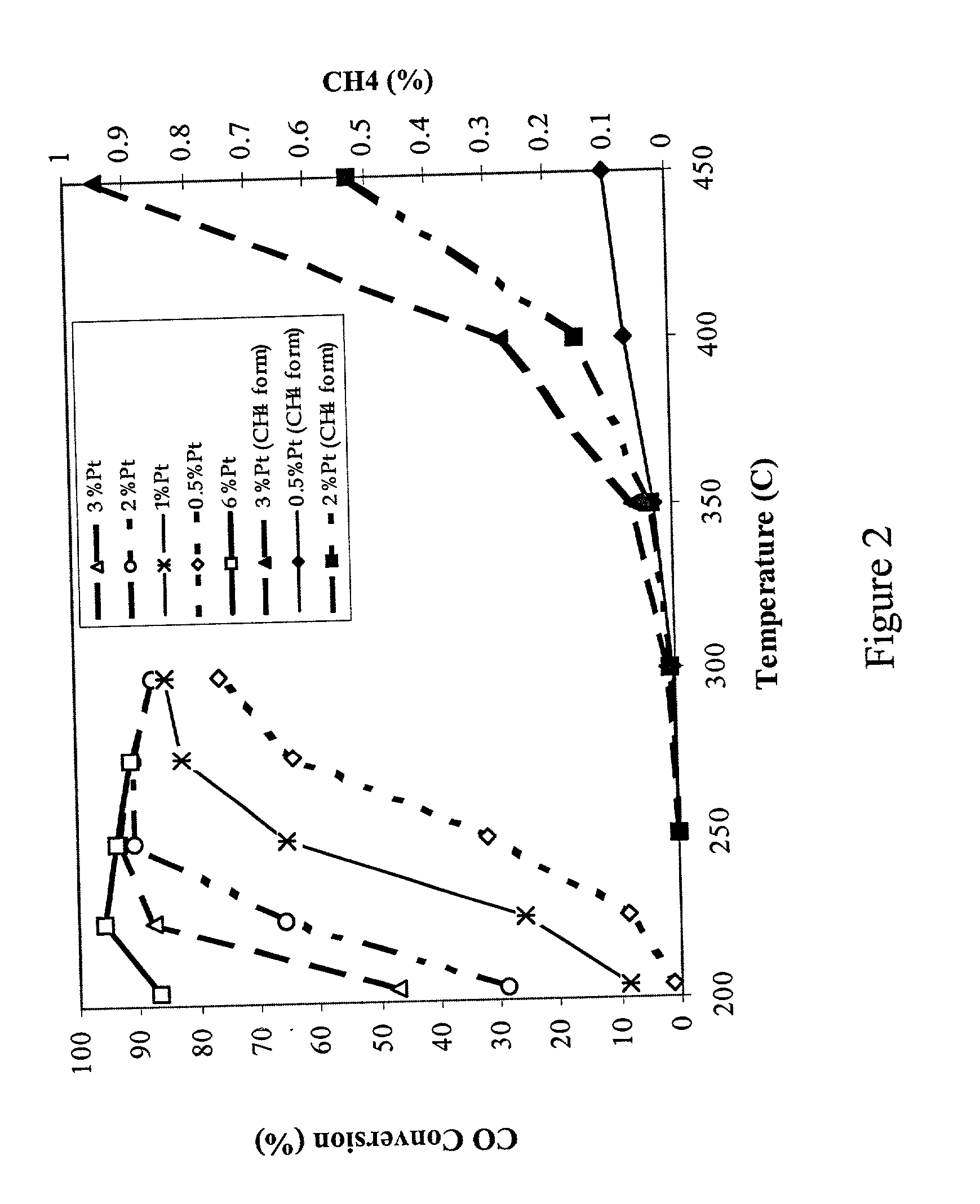
Catalyst for production of hydrogen
The present development is a catalyst for use in the water-gas-shift reaction. The catalyst includes a Group VIII or Group IB metal, a transition metal promoter selected from the group consisting of rhenium, niobium, silver, manganese, vanadium, molybdenum, titanium, tungsten and a combination thereof, and a ceria-based support. The support may further include gadolinium, samarium, zirconium, lithium, cesium, lanthanum, praseodymium, manganese, titanium, tungsten or a combination thereof. A process for preparing the catalyst is also presented. In a preferred embodiment, the process involves providing "clean" precursors as starting materials in the catalyst preparation.
Owner:SUD CHEM INC
Water-gas two-phase adsorption-desorption-seepage experimental system and method for loaded coal containing methane
ActiveCN102901803AAccurate detectionAccurate measurementFuel testingPermeability/surface area analysisWater bathsHigh pressure
The invention discloses a water-gas two-phase adsorption-desorption-seepage experimental system and method for loaded coal containing methane. The water-gas two-phase adsorption-desorption-seepage experimental system for loaded coal containing methane comprises a pressure loading part, wherein the pressure loading part comprises a tank body for containing a constant-temperature water bath, a reference pot and a coal sample holder are arranged in the tank body, the gas inlet of the reference pot is connected with a high-pressure methane delivery pipeline, the gas outlet of the reference pot is connected with the gas inlet of the coal sample holder through a connecting pipeline, and the gas outlet of the coal sample holder is connected with a water-gas separation device. The water-gas two-phase seepage experimental method for loaded coal containing methane is carried out on the basis of the experimental system. By changing different loading conditions, loading paths and experimental temperatures, adsorption-desorption experiments and methane seepage experiments for the loaded coal containing methane under the conditions of different solid-gas thermal coupling can be realized, and water-gas two-phase seepage experiments and methane adsorption-desorption experiments under the condition of different moisture contents also can be realized.
Owner:HENAN POLYTECHNIC UNIV
Ceria-based mixed-metal oxide structure, including method of making and use
InactiveUS20030235526A1Increased internal surface areaHigh catalytic activityRare earth metal oxides/hydroxidesMaterial nanotechnologyRheniumFuel cells
A homogeneous ceria-based mixed-metal oxide, useful as a catalyst support, a co-catalyst and / or a getter has a relatively large surface area per weight, typically exceeding 150 m<2> / g, a structure of nanocrystallites having diameters of less than 4 nm, and including pores larger than the nanocrystallites and having diameters in the range of 4 to about 9 nm. The ratio of pore volumes, VP, to skeletal structure volumes, VS, is typically less than about 2.5, and the surface area per unit volume of the oxide material is greater than 320 m<2> / cm<3>, for low internal mass transfer resistance and large effective surface area for reaction activity. The mixed metal oxide is ceria-based, includes Zr and or Hf, and is made by a novel co-precipitation process. A highly dispersed catalyst metal, typically a noble metal such as Pt, may be loaded on to the mixed metal oxide support from a catalyst metal-containing solution following a selected acid surface treatment of the oxide support. Appropriate ratioing of the Ce and other metal constituents of the oxide support contribute to it retaining in a cubic phase and enhancing catalytic performance. Rhenium is preferably further loaded on to the mixed-metal oxide support and passivated, to increase the activity of the catalyst. The metal-loaded mixed-metal oxide catalyst is applied particularly in water gas shift reactions as associated with fuel processing systems, as for fuel cells.
Owner:AUDI AG
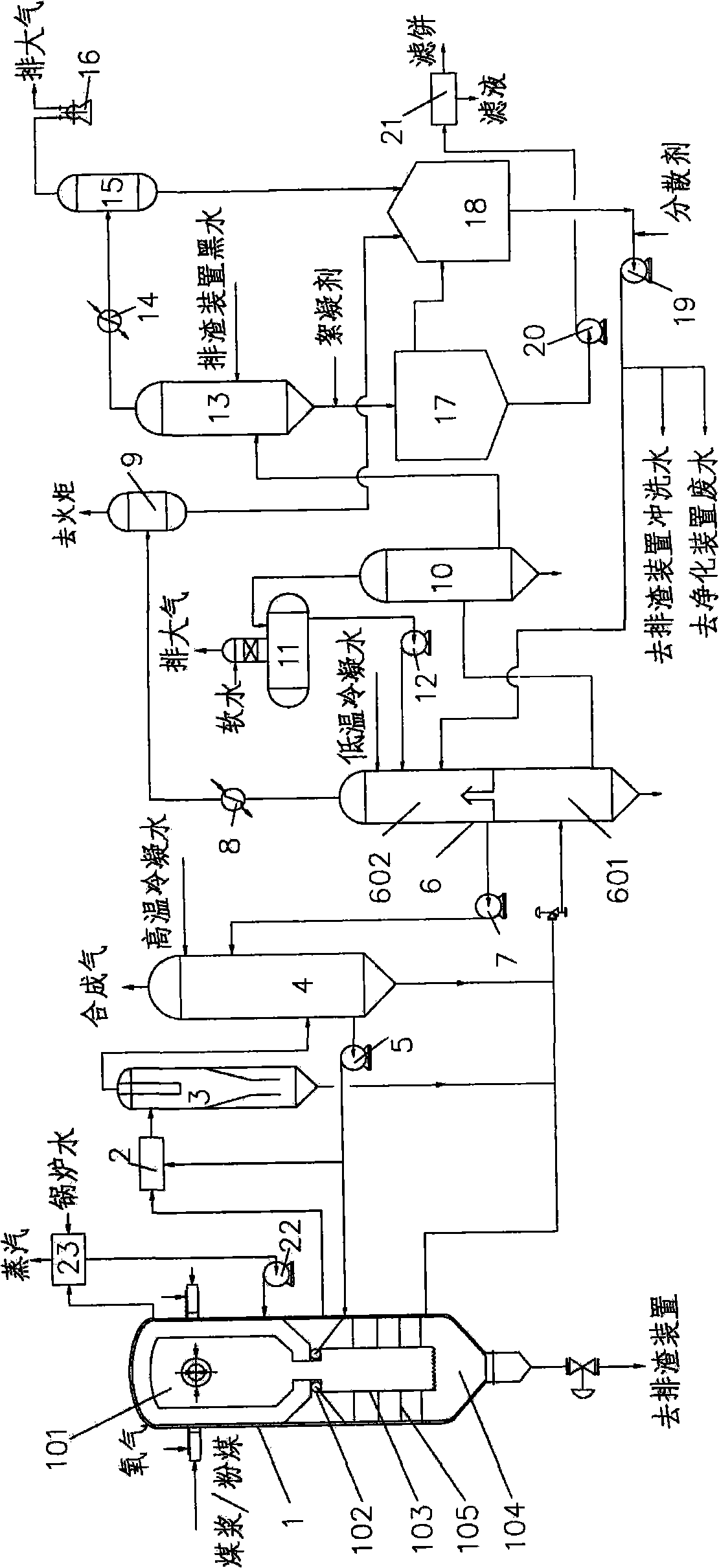
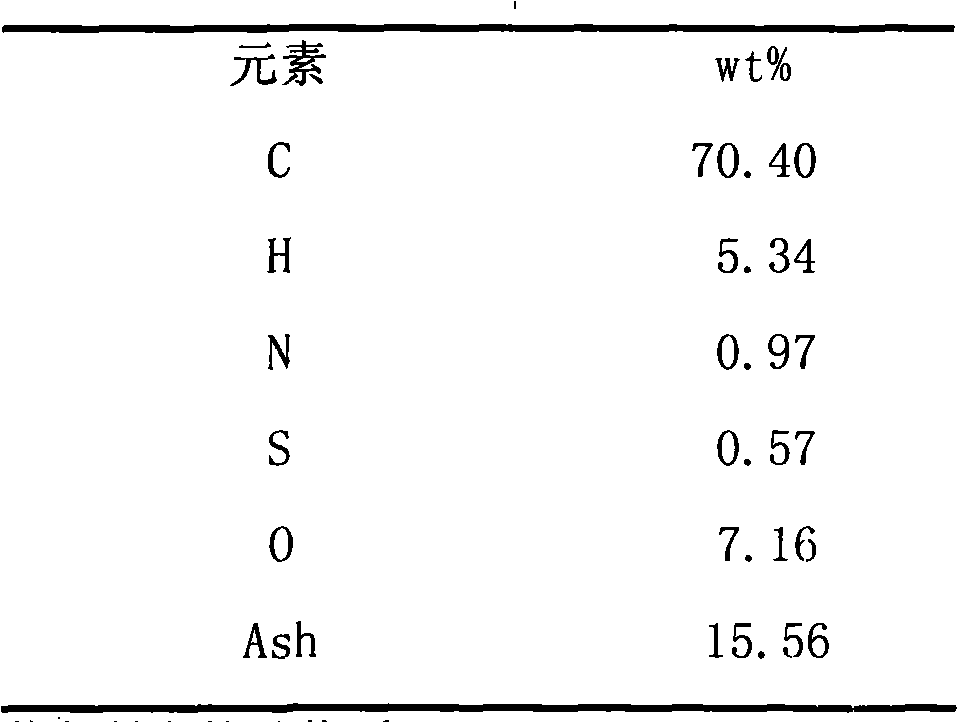
Gasification method of shock chilling type pulp or powder carbonaceous material
ActiveCN101298569AImprove water qualityReduce or eliminate emissionsChemical industryCombined combustion mitigationWater vaporWater quality
The invention discloses a gasification method of chilling slurry or powdery carbonic material. The method mainly consists of four technologies which are gasification in opposed multi-burner type, primary purification of synthetic gas, heat recovery and the treatment and recycling of black water, wherein, a gasification furnace is the core device of the method in the gasification process, which is provided with at least two pairs of opposed burners in uniform distribution and realizes the full gasification of the material under the operation pressure of 3-8 MPaG and the operation temperature of below 1200 DEG C-1700 DEG C. The invention has the following advantages: the carbon conversion rate in the gasification furnace is up to more than 90 percent, the effective gas content during slurry feeding is more than 84 percent and the effective content during powdery feeding is more than 90 percent; the ash content of the synthetic gas after primary purification is low, which can be lowered to below 1 mg / Nm<3>, thus having better water-gas ratio, i.e. the volume ratio of steam / synthetic gas (dry basis) is 1.3-1.5 / 1; the heat of the black water is totally recovered, the quality of ash water after purification is good and no fouling and clogging phenomena occur; the energy consumption, the oxygen consumption and the water consumption of the whole gasification process are relatively low, thus being capable of realizing long period stable operation with high efficiency.
Owner:EAST CHINA UNIV OF SCI & TECH
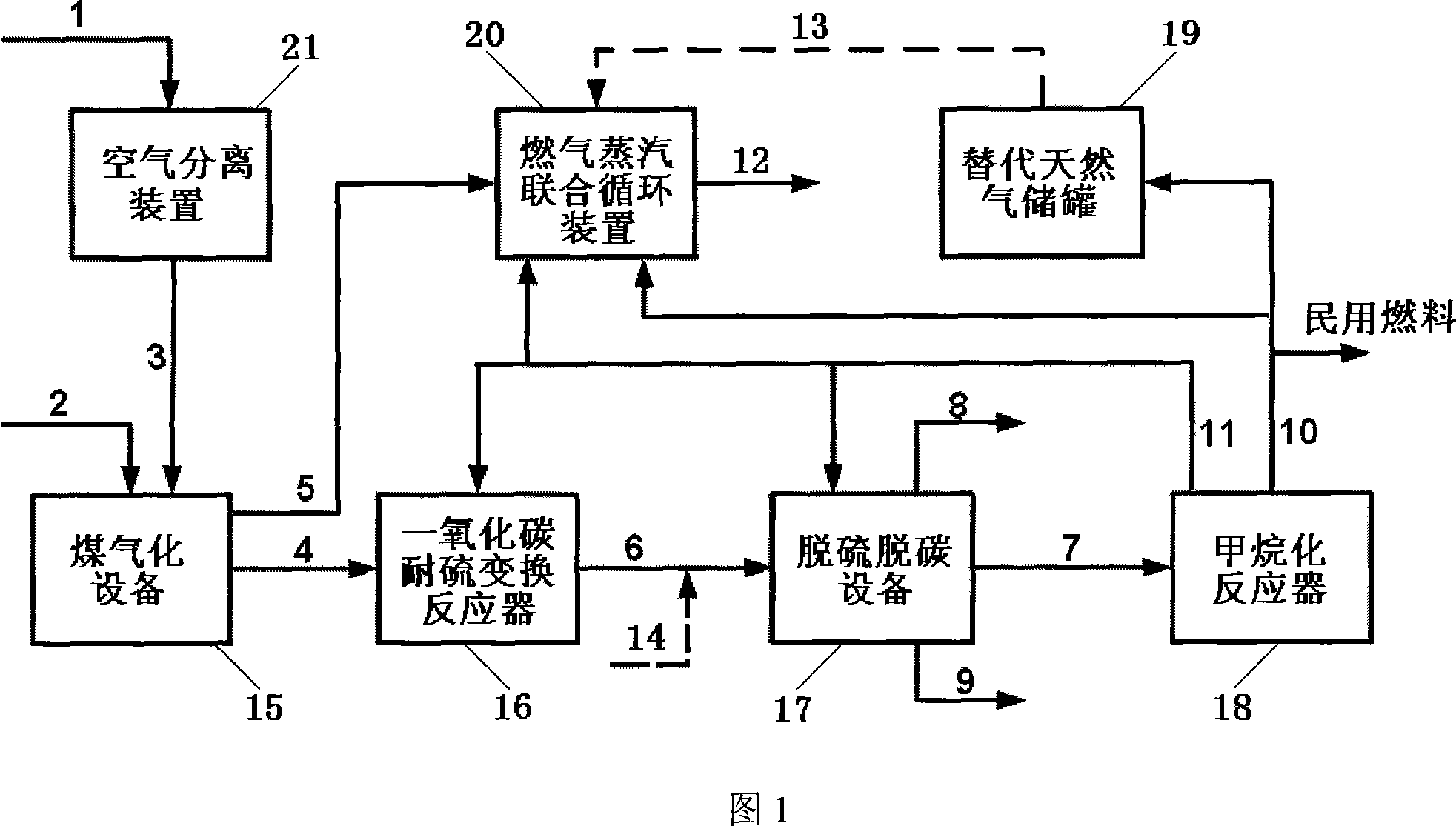
Combined system and process for producing electric-substituted natural gas based on coal gasification and methanation
ActiveCN101074397AEfficient and clean utilizationEfficient use ofCombustible gas chemical modificationCombustible gas purificationMethanationSlurry
An electric-substituting natural gas combined system and process based on coal gasification and methanation are disclosed. The process is carried out by delivering oxygen, powdered coal or water-gas slurry into gasified apparatus, recovering for crude gasified gas by wet heat, delivering into sulfur-resisting carbon monoxide carbon reactor, adjusting hydrogen-carbon ratio, entering into desulfurizing decarbonizer, recovering elementary sulfur while enriching carbon dioxide, delivering synthetic gas into methane reactor to generate substituted natural gas as domestic gas partially, entering into gas and steam combined circulator partially, and entering into substituted natural gas storage tank partially. It integrates coal gasified, methanation and gas steam combined circulation together, it's efficient and clean, has better utilizing rate, no CO2 discharge and need for changing gas turbine or load.
Owner:TSINGHUA UNIV
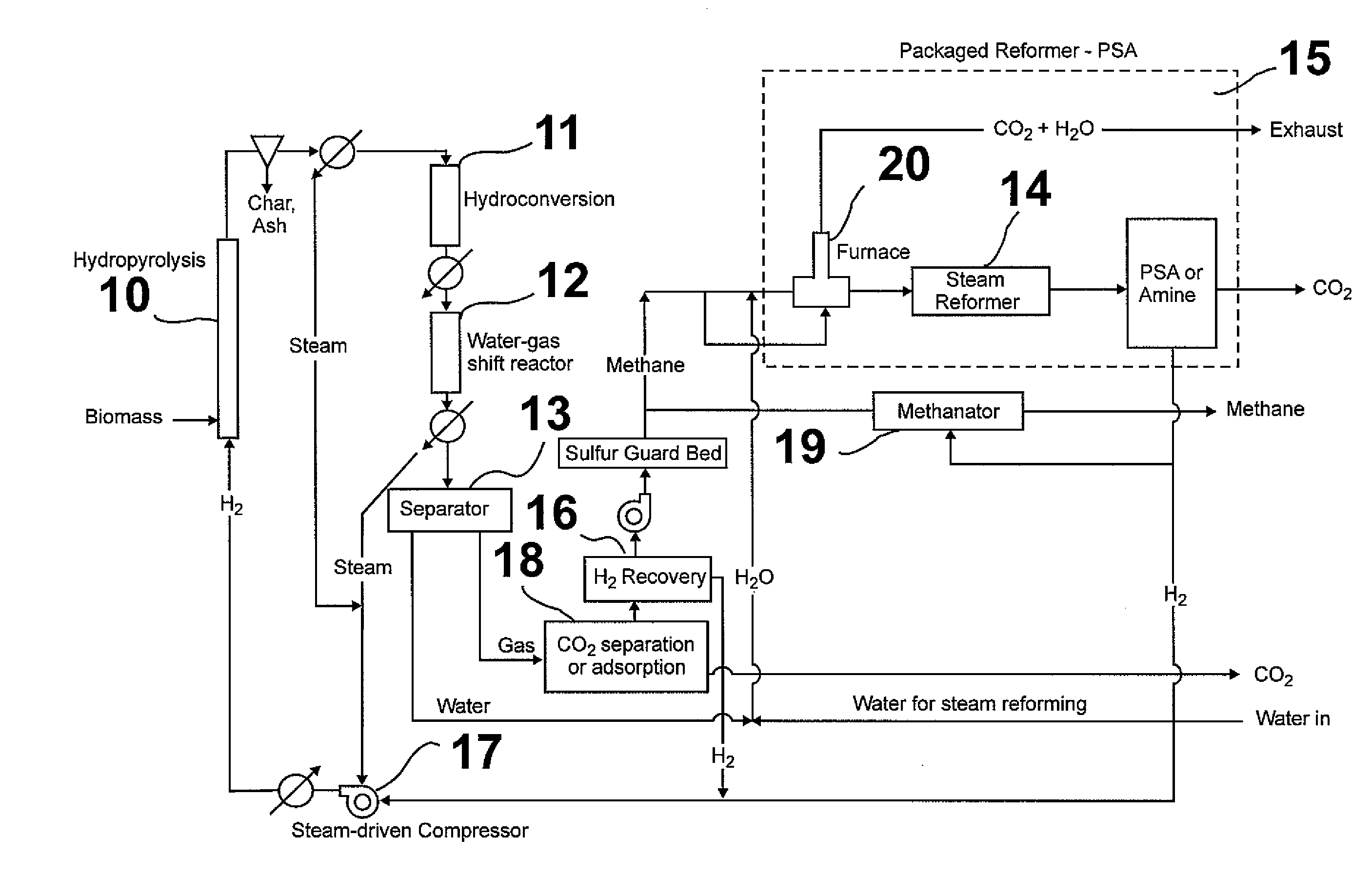
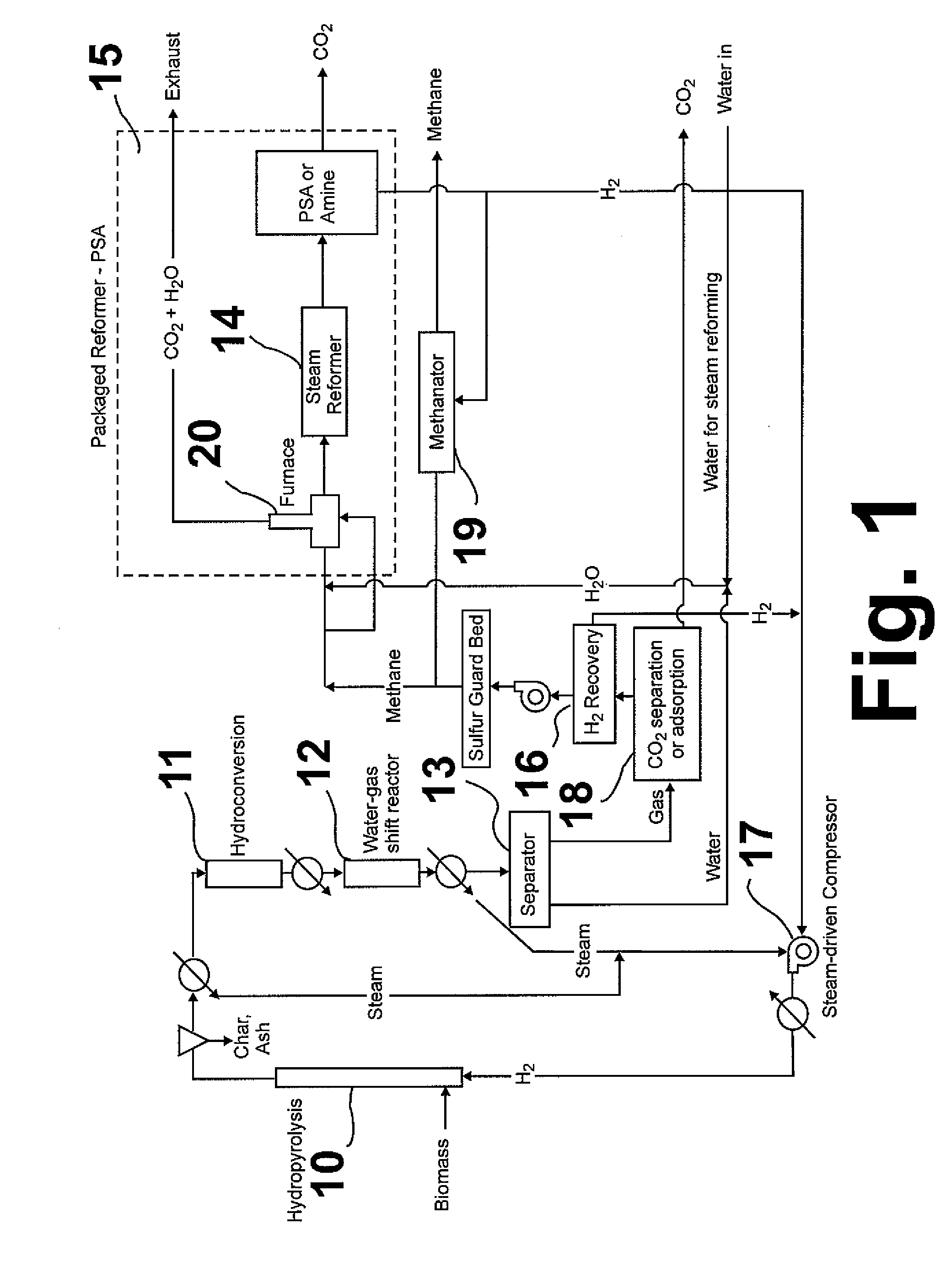
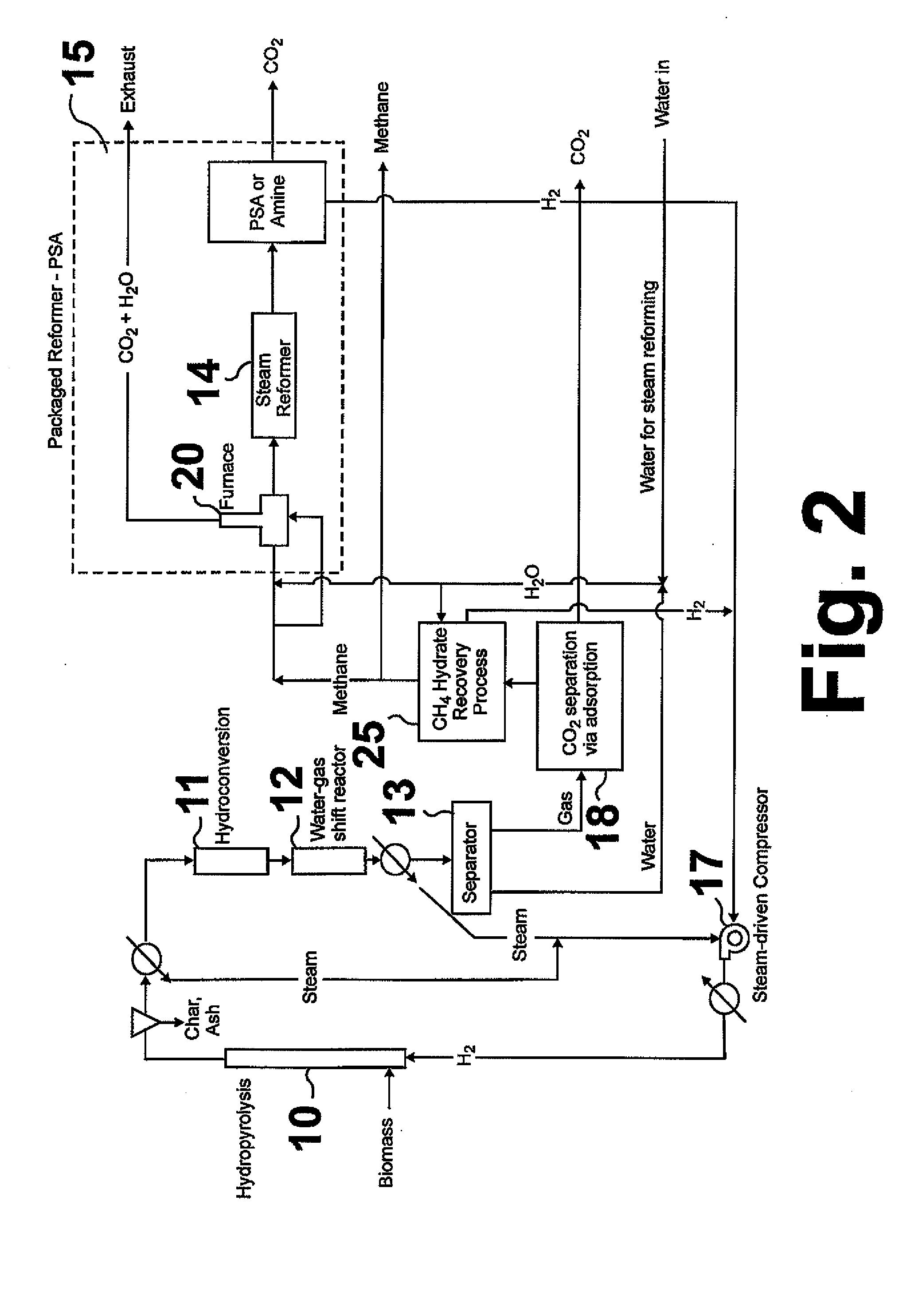
Method for producing methane from biomass
ActiveUS20100251615A1Superior methane yieldFast pyrolysisGasification catalystsDirect heating destructive distillationHydrogenProduct gas
A multi-stage method and apparatus for producing methane from biomass in which the biomass is hydropyrolyzed in a reactor vessel containing molecular hydrogen and a deoxygenating catalyst, the output of which is hydrogenated using a hydroconversion catalyst. The output from the hydroconversion step is provided to a water-gas-shift process providing a mixture of H2O and product gases including CO2, H2, and methane. The mixture components are separated, resulting in a product stream comprising substantially only methane.
Owner:GAS TECH INST
Process for cleaning gases form gasification units
InactiveUS20060233687A1Reduce environmental impactUsing liquid separation agentCombustible gas catalytic treatmentHydrogenHalogen
A process for cleaning gases from a gasification unit comprising the steps of: gasifying a fuel to raw synthesis gas comprising carbon monoxide and steam in a gasification reactor in the presence of a calcium-containing compound and steam removing halogen compounds from the raw synthesis gas catalytic conversion of carbon monoxide and steam in the raw synthesis gas to carbon dioxide and hydrogen in a water gas shift conversion step to provide a shifted gas contacting the shifted gas with a solid sulphur sorbent regenerating the solid sulphur sorbent by passing a stream of steam through the solid sulphur sorbent counter current to the flow direction of the shifted gas to provide a hydrogen sulphide-containing stream of steam transferring the hydrogen sulphide-containing stream of steam from the solid sulphur sorbent directly to the gasification reactor removing the cleaned shifted gas from the solid sulphur sorbent.
Owner:HALDOR TOPSOE AS
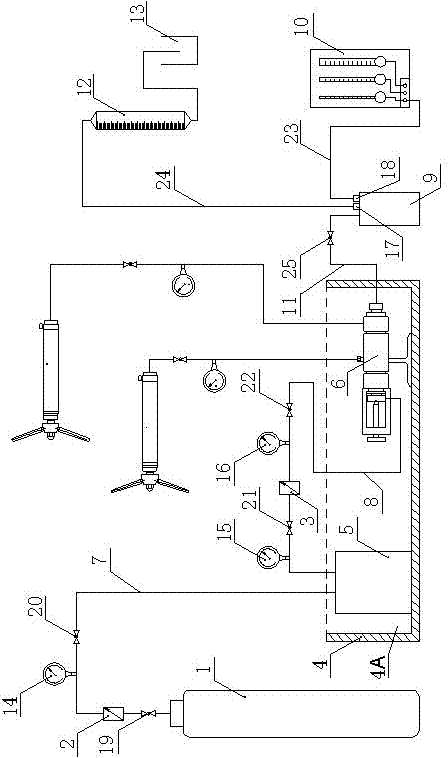
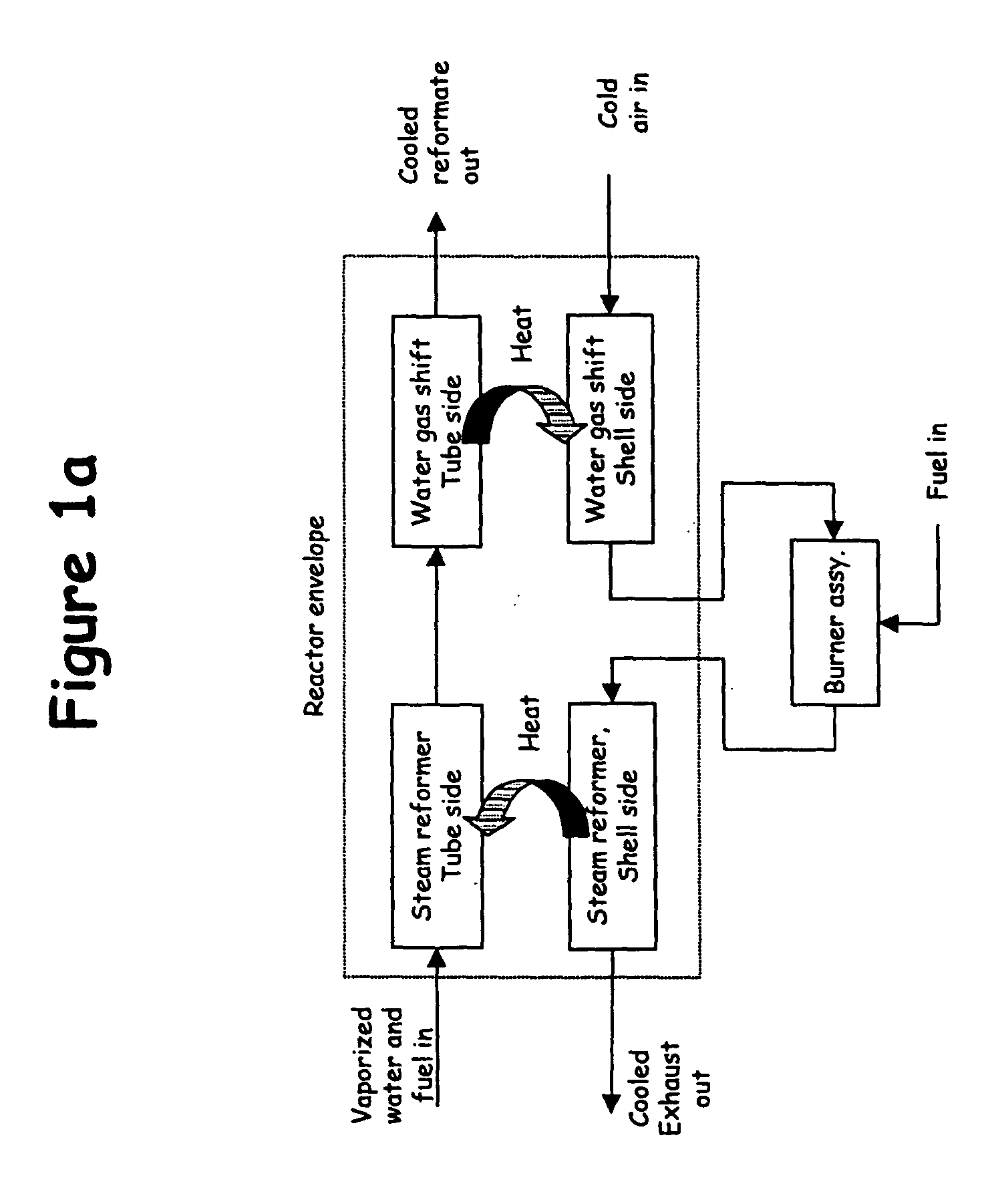
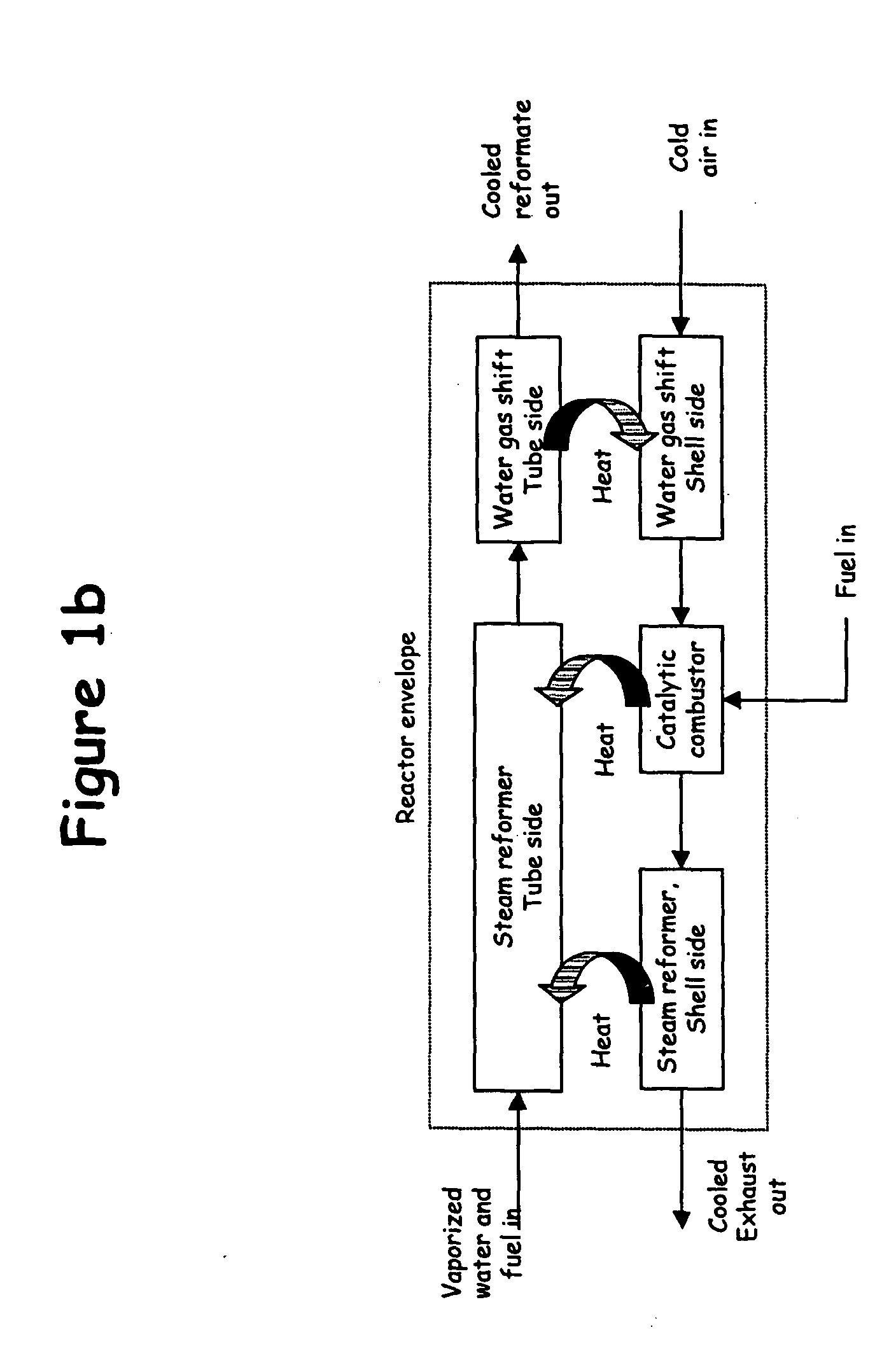
System for hydrogen generation through steam reforming of hydrocarbons and intergrated chemical reactor for hydrogen production from hydrocarbons
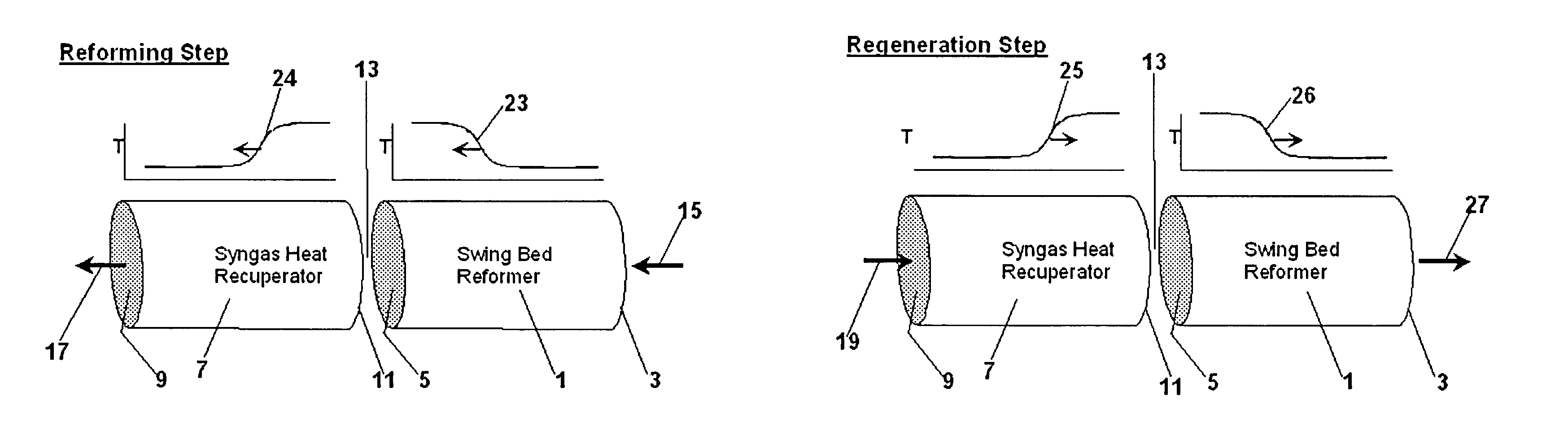
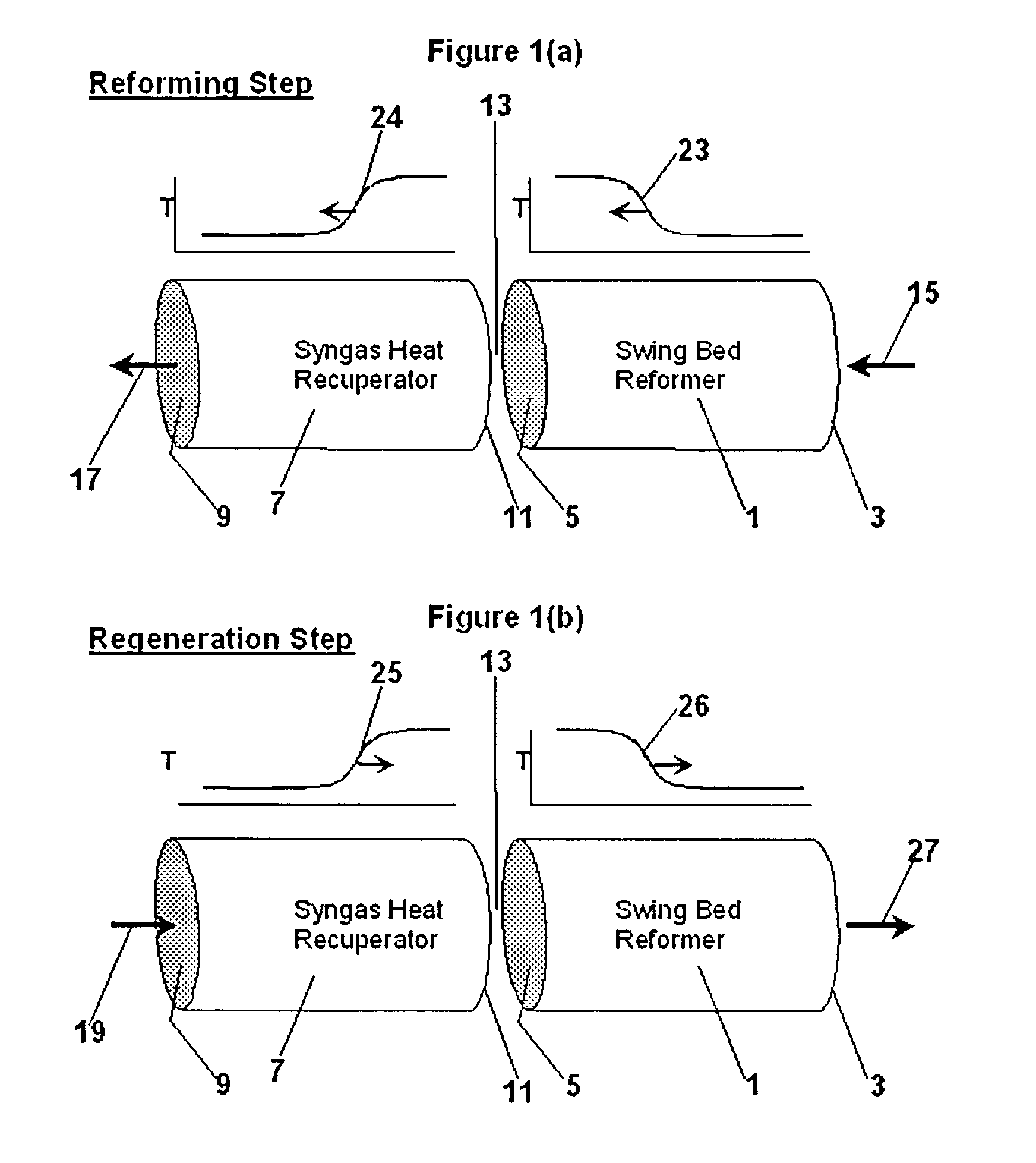
InactiveUS20050097819A1Avoid problemsCost efficientHydrogenHydrogen/synthetic gas productionSteam reformingEngineering
The present invention provides a reactor, which includes: a unitary shell assembly having an inlet and an outlet; a flow path extending within the shell assembly from the inlet to the outlet, the flow path having a steam reformer section with a first catalyst and a water gas shift reactor section with a second catalyst, the steam reformer section being located upstream of the water gas shift reactor section; a heating section within the shell assembly and configured to heat the steam reformer section; and a cooling section within the shell assembly and configured to cool the water gas shift reactor section. The present invention also provides a simplified hydrogen production system, which includes the catalytic steam reforming and subsequent high temperature water gas shift of low-sulfur (<100 ppm by mass) hydrocarbon fuels followed by hydrogen purification through the pressure swing adsorption (PSA). The integrated reactor offers significant advantages such as lower heat loss, lower parts count, lower thermal mass, and greater safety than the many separate components employed in conventional and is especially well-suited to applications where less than 15,000 standard cubic feet per hour of hydrogen are required. The improved system also may be started, operated and shut down more simply and quickly than what is currently possible in conventional systems. The improved system preferably employs active temperature control for added safety of operation. The hydrogen product is of high purity, and the system may be optionally operated with a feedback control loop for added purity.
Owner:H2GEN INNOVATIONS INC
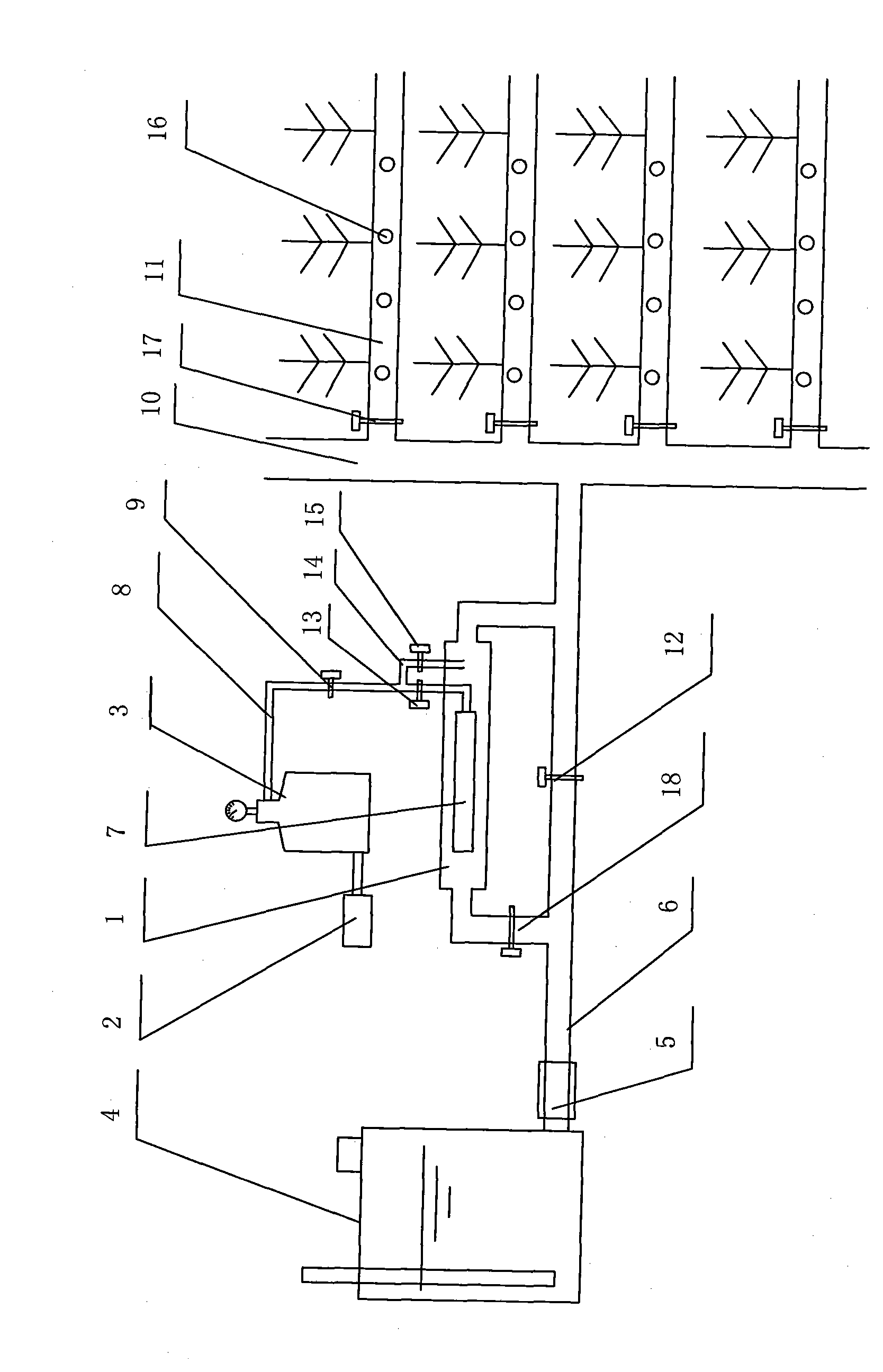
Aeration underground drip irrigation system
InactiveCN101663983APrecise and stable controlRealization of aerated subsurface drip irrigationWatering devicesCultivating equipmentsWater sourceGas cylinder
The invention discloses an aeration underground drip irrigation system which comprises a water source, a delivery main pipe (6), a delivery branch pipe (10) and an irrigation capillary (11), wherein abooster pump (5) is connected in series with the delivery main pipe (6). The aeration underground drip irrigation system is characterized in that an aeration chamber (1) is connected in parallel withthe delivery main pipe (6), a micropore aeration part (7) is arranged in the aeration chamber (1), an air compressor (2) is communicated with the aeration part (7) in the aeration chamber (1) througha gas cylinder (3) and a ventilation hose (8) with a one-way valve (9), and a branch ventilation pipe (14) communicated with the aeration chamber (1) is arranged between the one-way valve (9) and a gas flow control valve (13). The aeration underground drip irrigation system can realize the three functions of ordinary underground drip irrigation, aeration underground drip irrigation and simple underground ventilation through water gas supply and valve control, has simple structure, low cost, less energy loss, controllable aeration ratio, wide aeration range, convenient use, management and maintenance and labor saving, and simultaneously can dissolve fertilizer into water, simultaneously irrigate the fertilizer, the water and gas, improve the utilization efficiency of the fertilizer and reduce the pollution for soil and underground water.
Owner:LUDONG UNIVERSITY
Hydroprocessing of biocomponent feedstocks with low purity hydrogen-containing streams
ActiveUS20110155636A1Reduce expensesLittle or no significant impact on other refinery resourcesRefining to change hydrocarbon structural skeletonBiofuelsHydrogenDeoxygenation
A biocomponent feedstock can be hydroprocessed using a hydrogen-containing refinery as a source of hydrogen gas. A relatively low cost catalyst, such as a water gas shift catalyst and / or spent hydrotreating catalyst, can be used as a hydrogenation catalyst for the process. The hydroprocessing can allow for olefin saturation and / or deoxygenation of the biocomponent feed by using a relatively low value refinery stream, e.g., containing from about 20 mol % to about 60 mol % hydrogen.
Owner:EXXON RES & ENG CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com