Patents
Literature
3622results about "Hydrocarbon from carbon oxides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Staged combustion of a low heating value fuel gas for driving a gas turbine
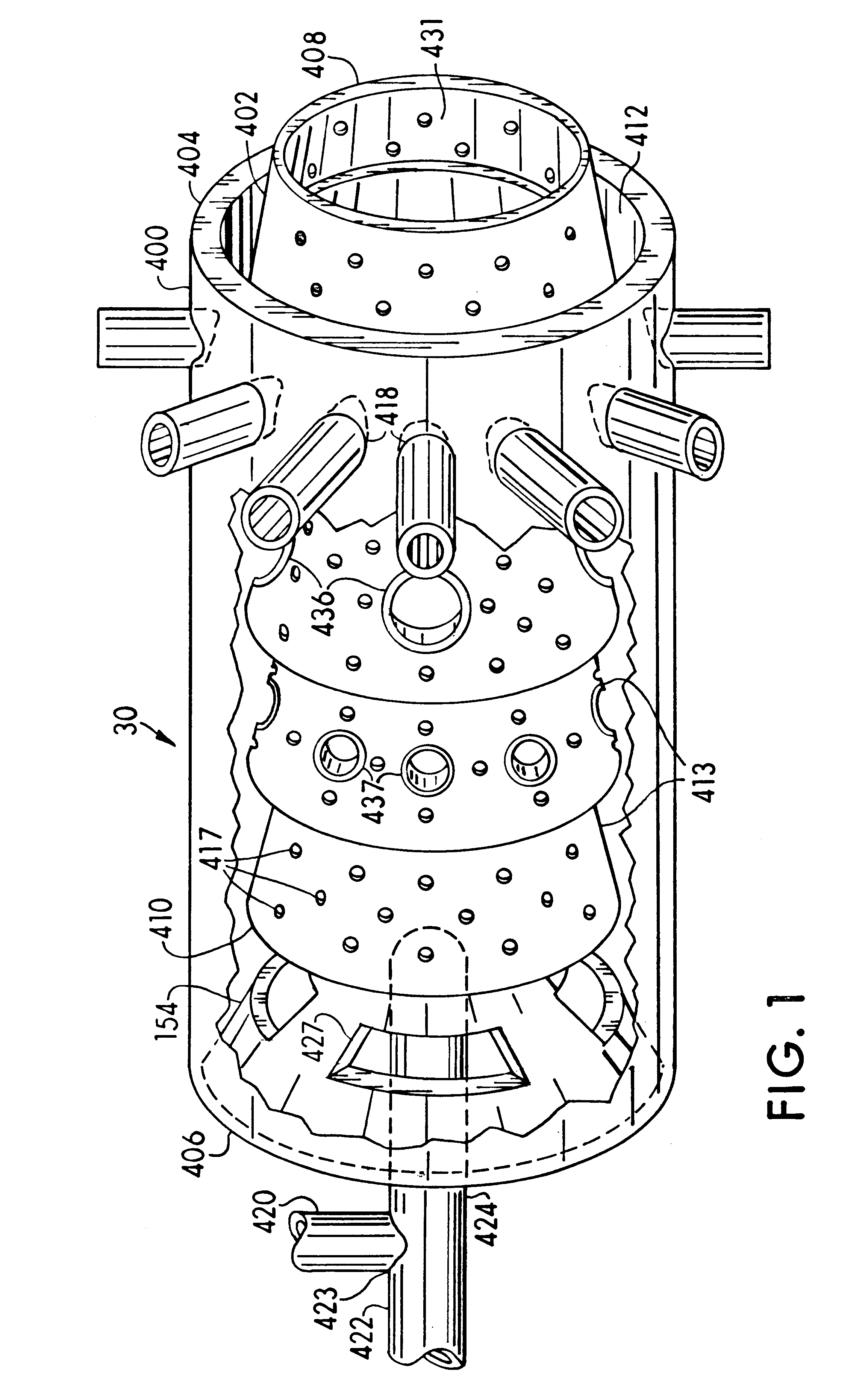
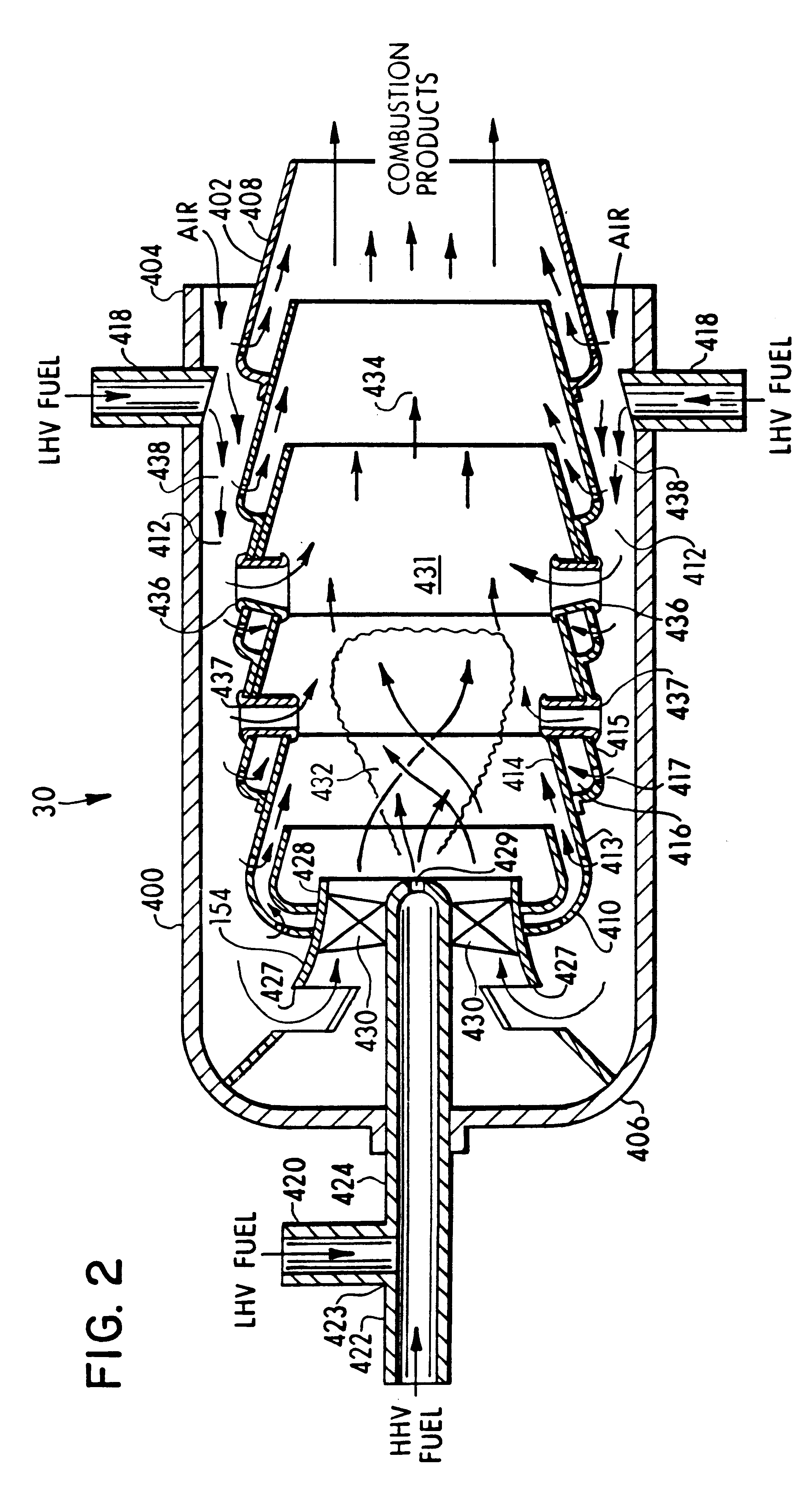
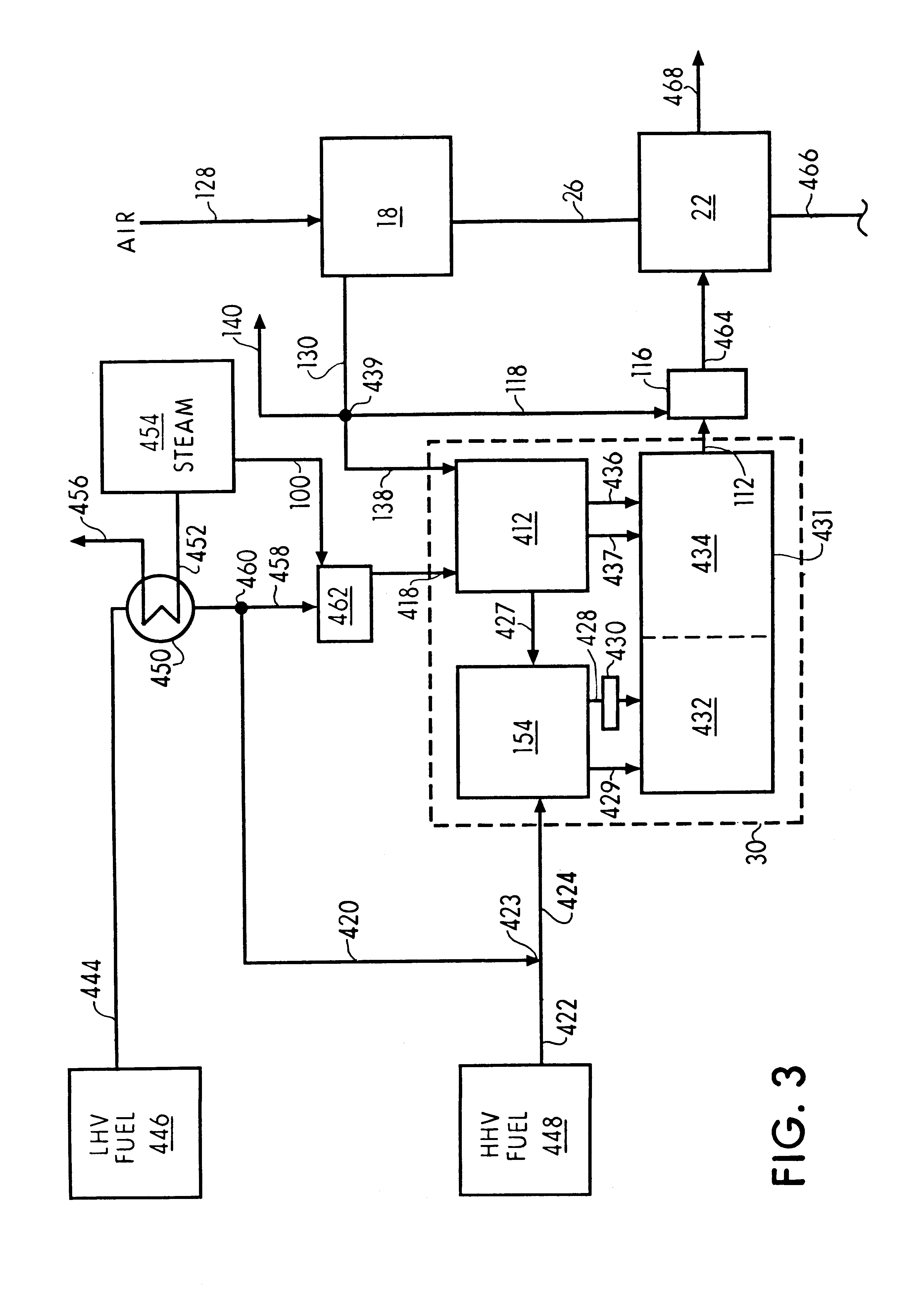
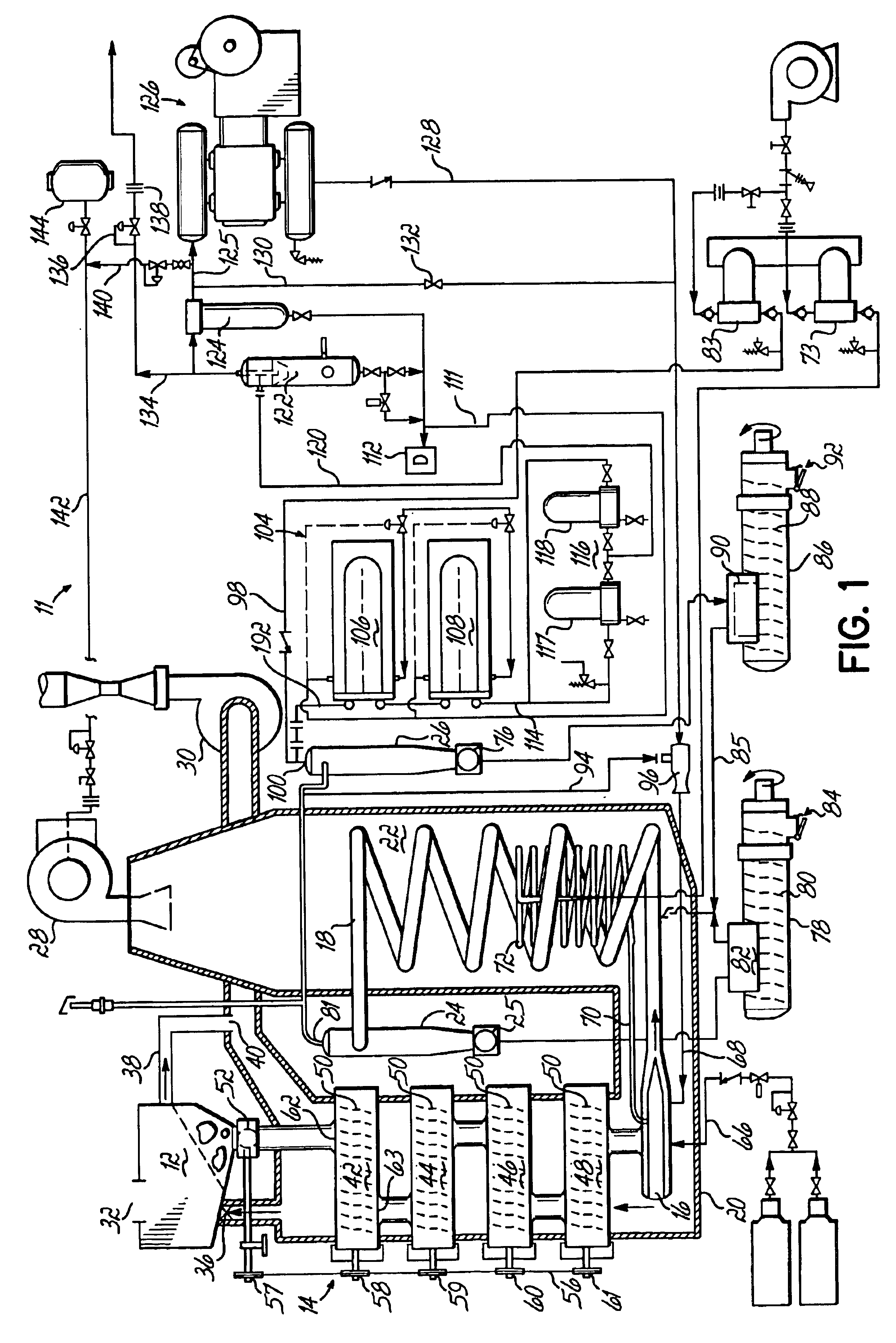
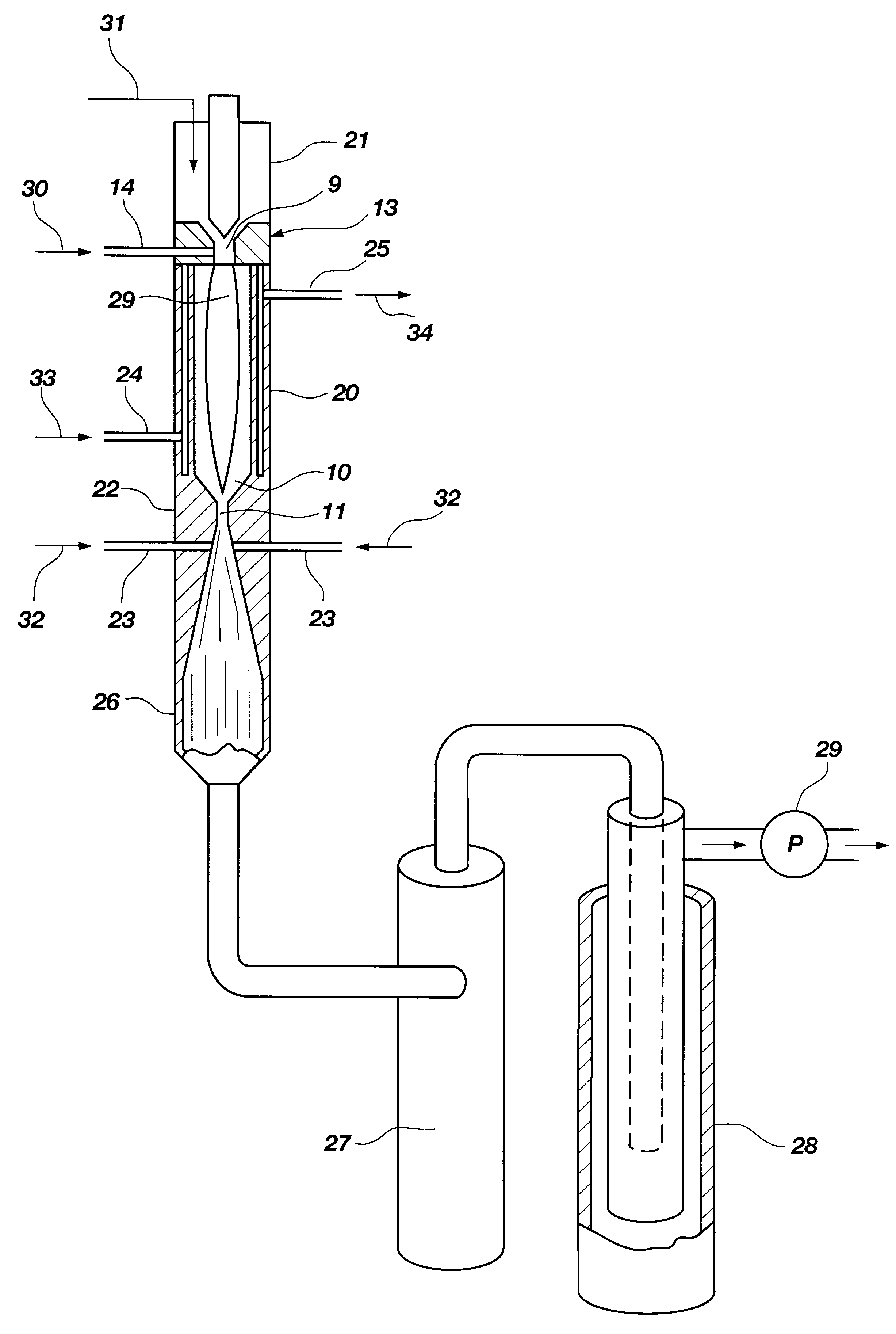
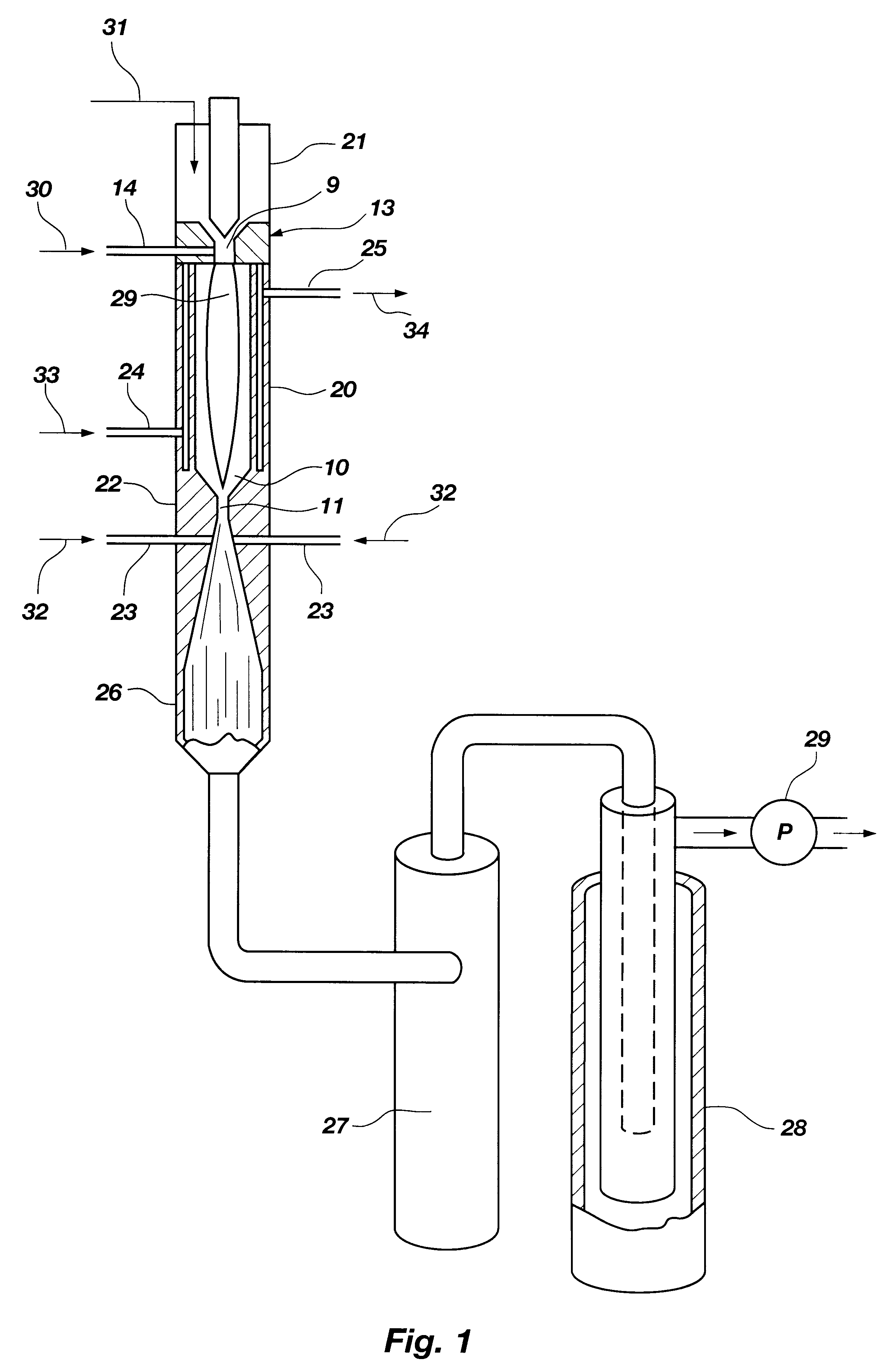
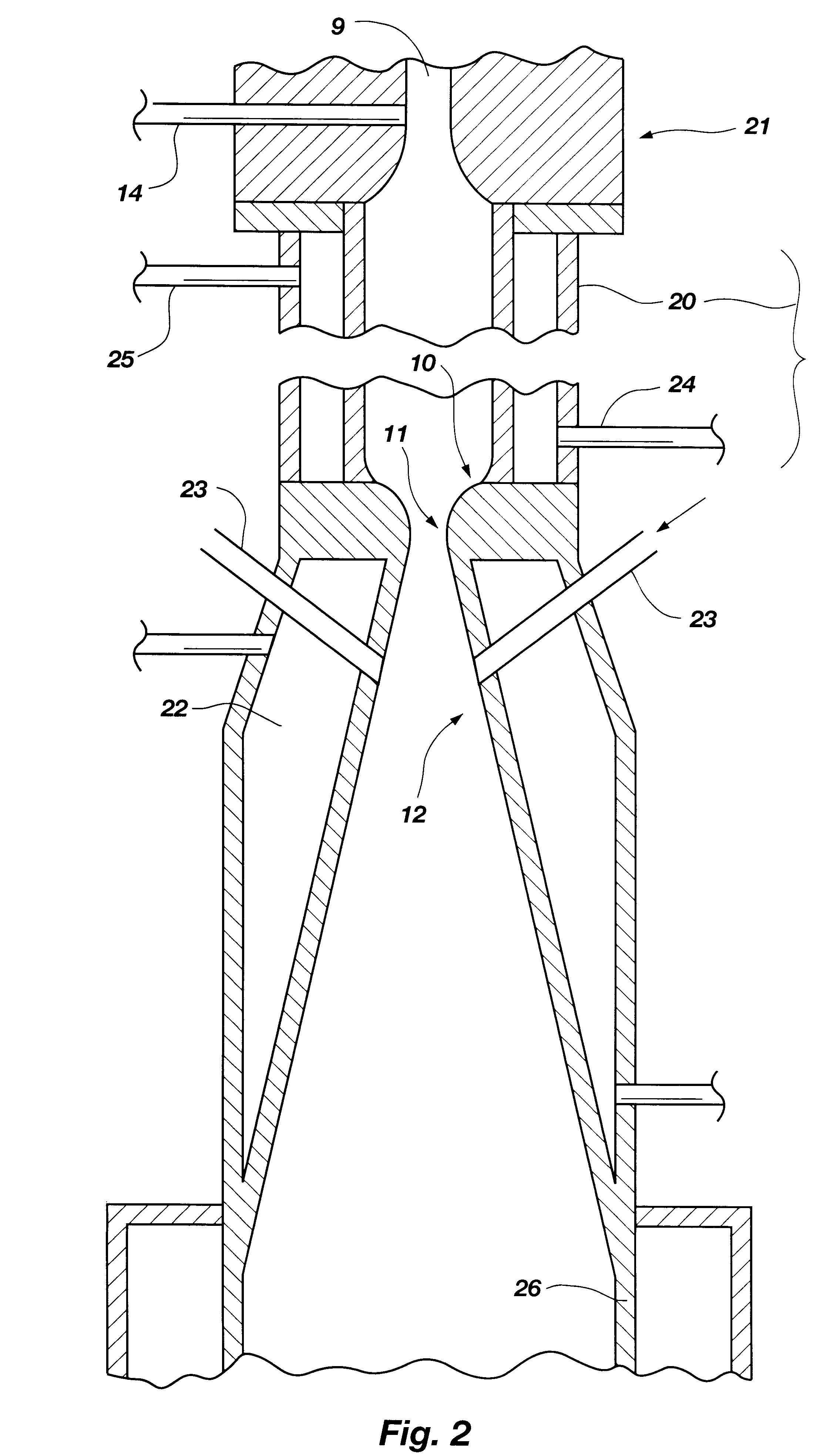
A process is provided for combusting a low heating value fuel gas in a combustor to drive an associated gas turbine. A low heating value fuel gas feed is divided into a burner portion and a combustion chamber portion. The combustion chamber portion and a combustion air are conveyed into a mixing zone of the combustor to form an air / fuel mixture. The burner portion is conveyed into a flame zone of the combustor through a burner nozzle while a first portion of the air / fuel mixture is conveyed into the flame zone through a burner port adjacent to the burner nozzle. The burner portion and first portion of the air / fuel mixture are contacted in the flame zone to combust the portions and produce flame zone products. The flame zone products are conveyed into an oxidation zone of the combustor downstream of the flame zone while a second portion of the air / fuel mixture is also conveyed into the oxidation zone. The second portion is combusted in the oxidation zone in the presence of the flame zone products to produce combustion products. The combustion products are conveyed into the associated gas turbine and drive the gas turbine.
Owner:MARATHON OIL CO +1
Hydrogen production from carbonaceous material
InactiveUS6790430B1Avoid the needCalcium/strontium/barium carbonatesGas turbine plantsCalcinationExothermic reaction
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE +1
Selective Removal and Recovery of Acid Gases from Gasification Products
Processes and apparatuses are described for the selective removal and recovery of acid gases from a gas source comprising at least hydrogen sulfide and carbon dioxide. A step-wise approach is illustrated wherein hydrogen sulfide may be selectively removed from a gas source by treatment with methanol under conditions where substantially all the hydrogen sulfide may be removed. The partially purified gas source may then be provided with a second treatment with methanol under conditions which selectively remove carbon dioxide from the gas stream. Such methods are generally applicable to any gas source comprising at least hydrogen sulfide and carbon dioxide, for example, a gas source produced from the catalytic gasification of a carbonaceous material, the combustion of a carbonaceous material, or the oxy-blown gasification of a carbonaceous material.
Owner:SURE CHAMPION INVESTMENT LTD
Methods and devices for the production of Hydrocarbons from Carbon and Hydrogen sources
InactiveUS20080283411A1Reduce the environmentEasy to controlPhotography auxillary processesInternal combustion piston enginesElectrolysisAtmospheric air
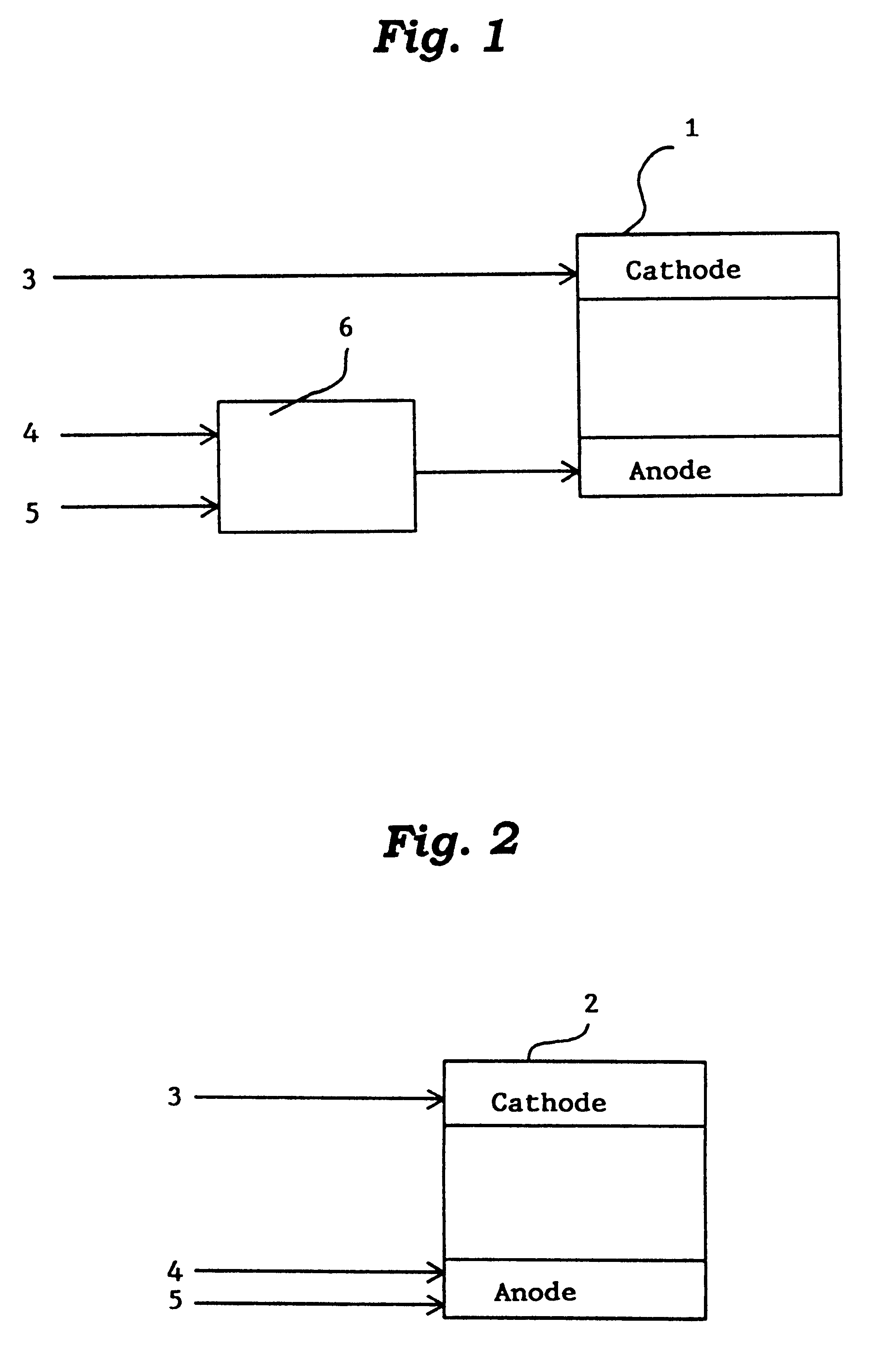
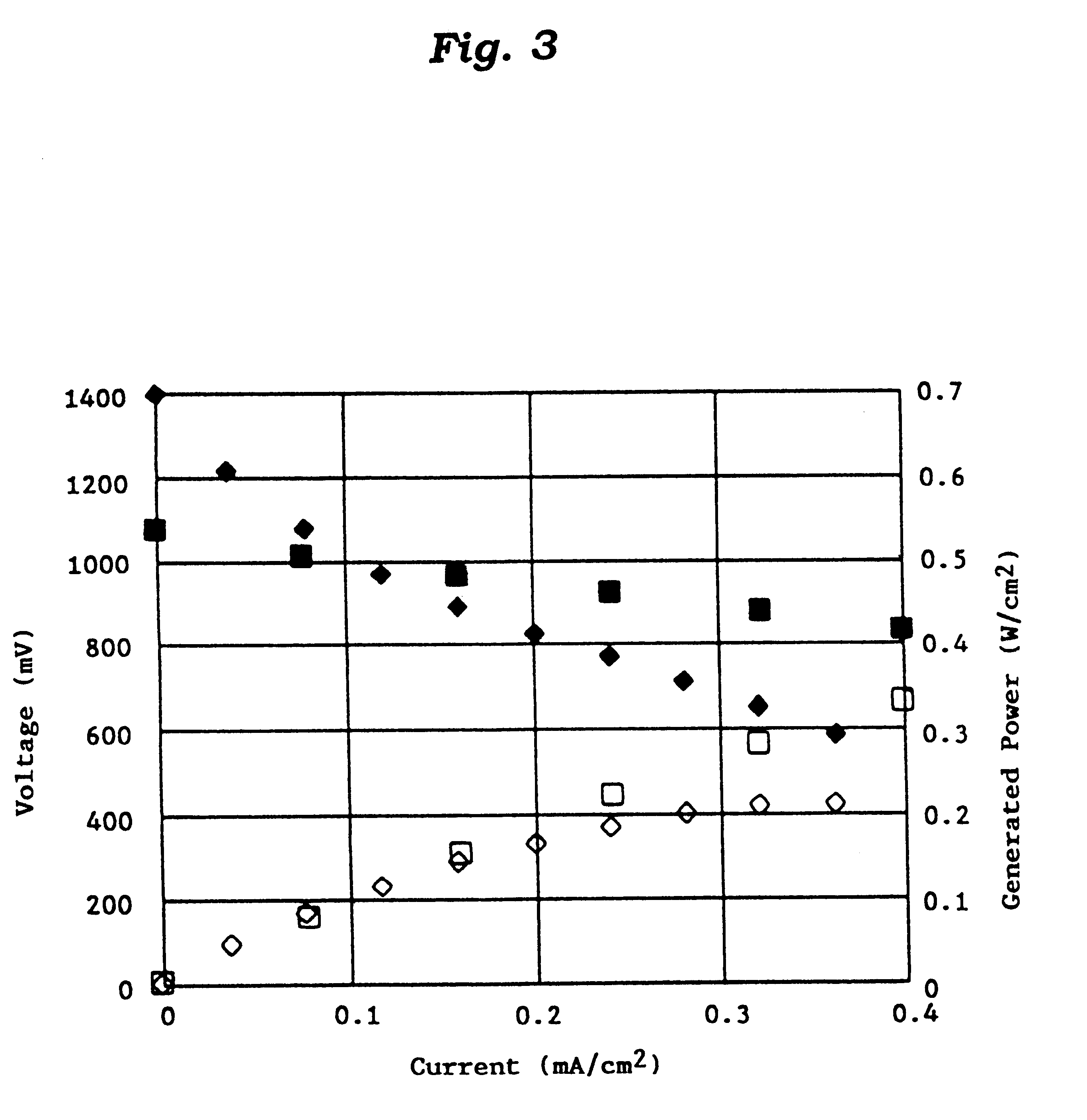
Devices and methods are described for converting a carbon source and a hydrogen source into hydrocarbons, such as alcohols, for alternative energy sources. The influents may comprise carbon dioxide gas and hydrogen gas or water, obtainable from the atmosphere for through methods described herein, such as plasma generation or electrolysis. One method to produce hydrocarbons comprises the use of an electrolytic device, comprising an anode, a cathode and an electrolyte. Another method comprises the use of ultrasonic energy to drive the reaction. The devices and methods and related devices and methods are useful, for example, to provide a fossil fuel alternative energy source, store renewable energy, sequester carbon dioxide from the atmosphere, counteract global warming, and store carbon dioxide in a liquid fuel.
Owner:PRINCIPLE ENERGY SOLUTIONS
Method and apparatus for producing synthesis gas from carbonaceous materials
A method of producing syn gas from biomass or other carbonaceous material utilizes a controlled devolatilization reaction in which the temperature of the feed material is maintained at less than 450° F. until most available oxygen is consumed. This minimizes pyrolysis of the feed material. The method and apparatus utilizes the formed synthesis gas to provide the energy for the necessary gasification. This provides for a high purity syn gas and avoids production of slag.
Owner:JBK EXTRACTIONS
Processes for Gasification of a Carbonaceous Feedstock
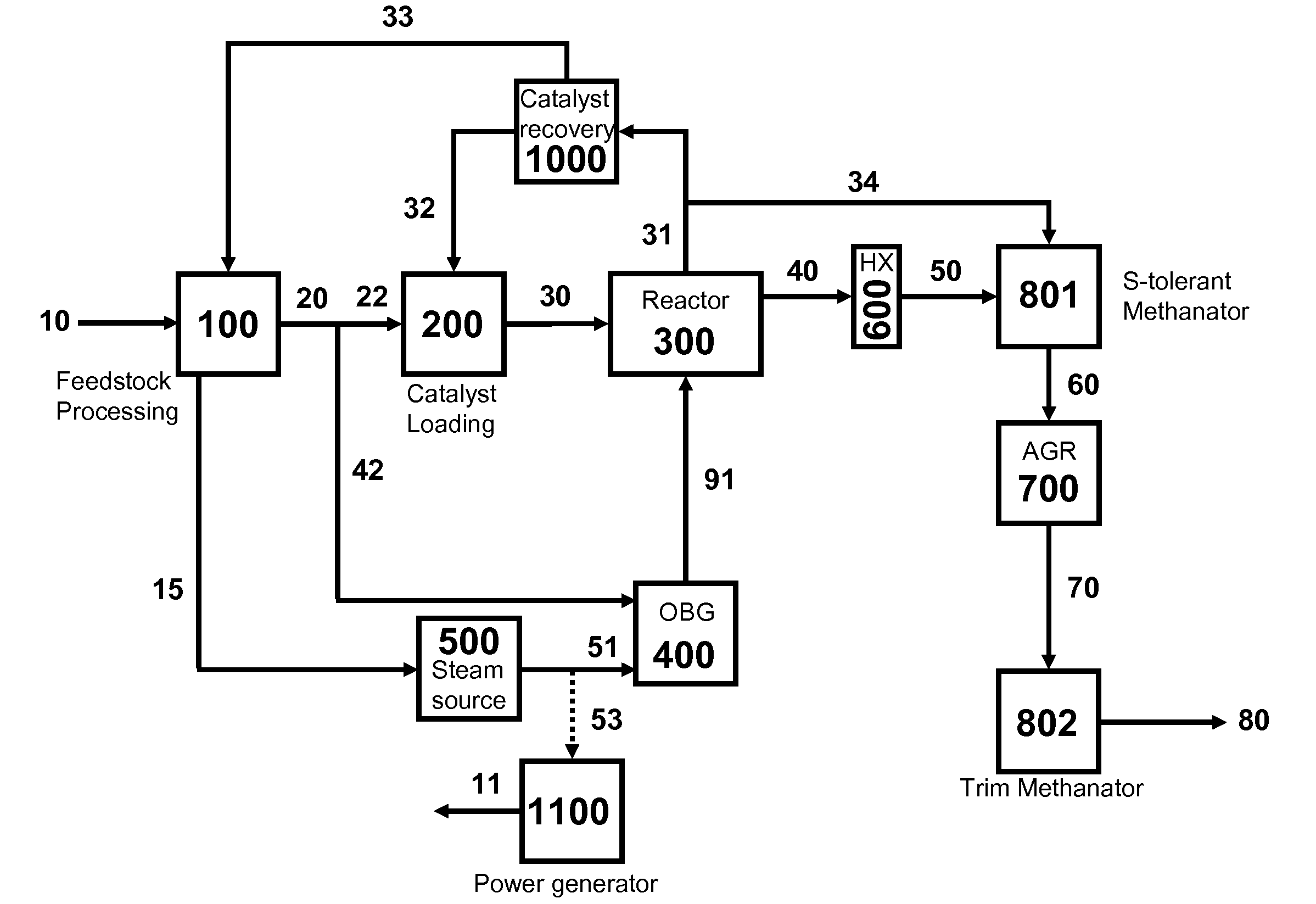
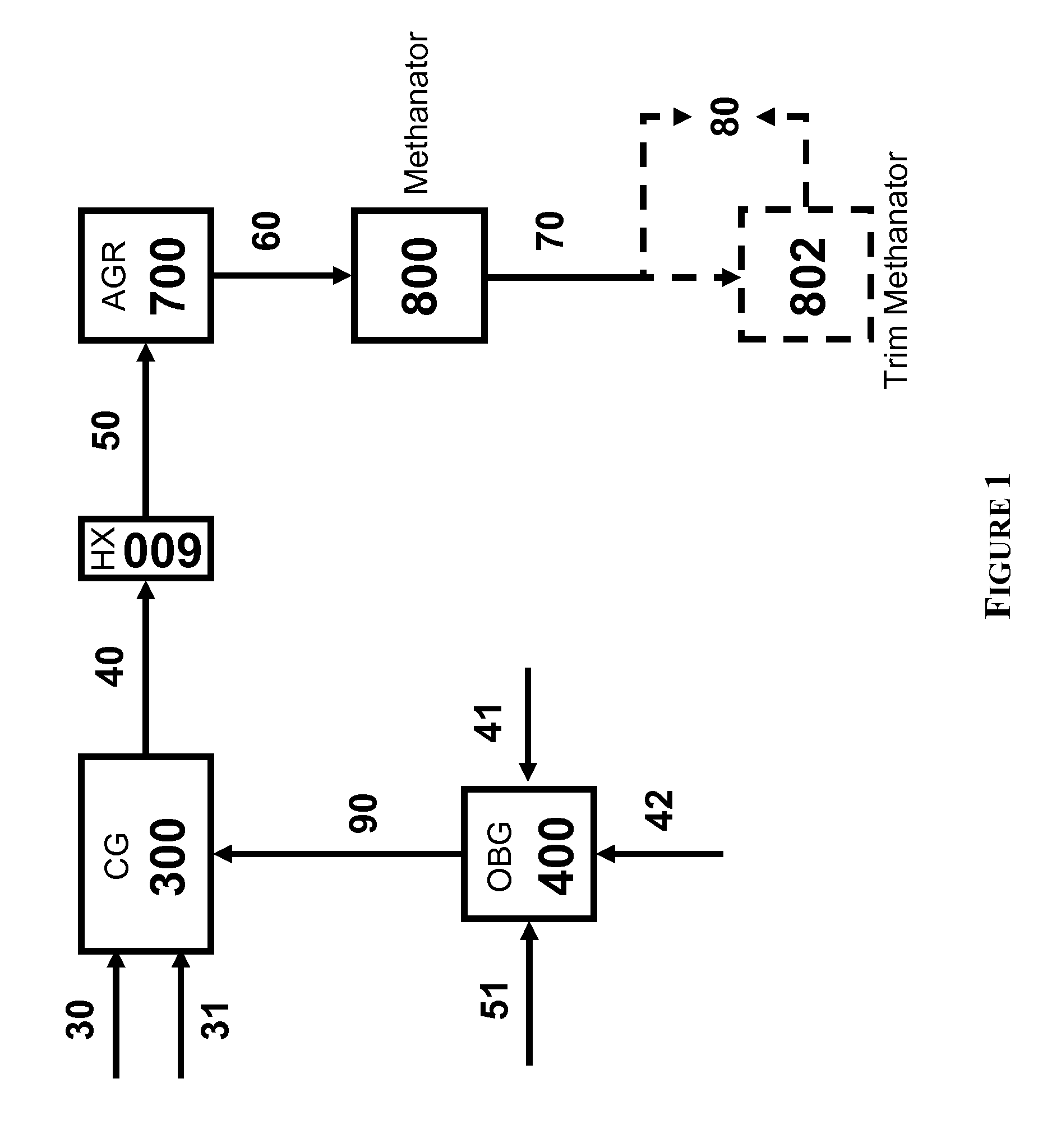
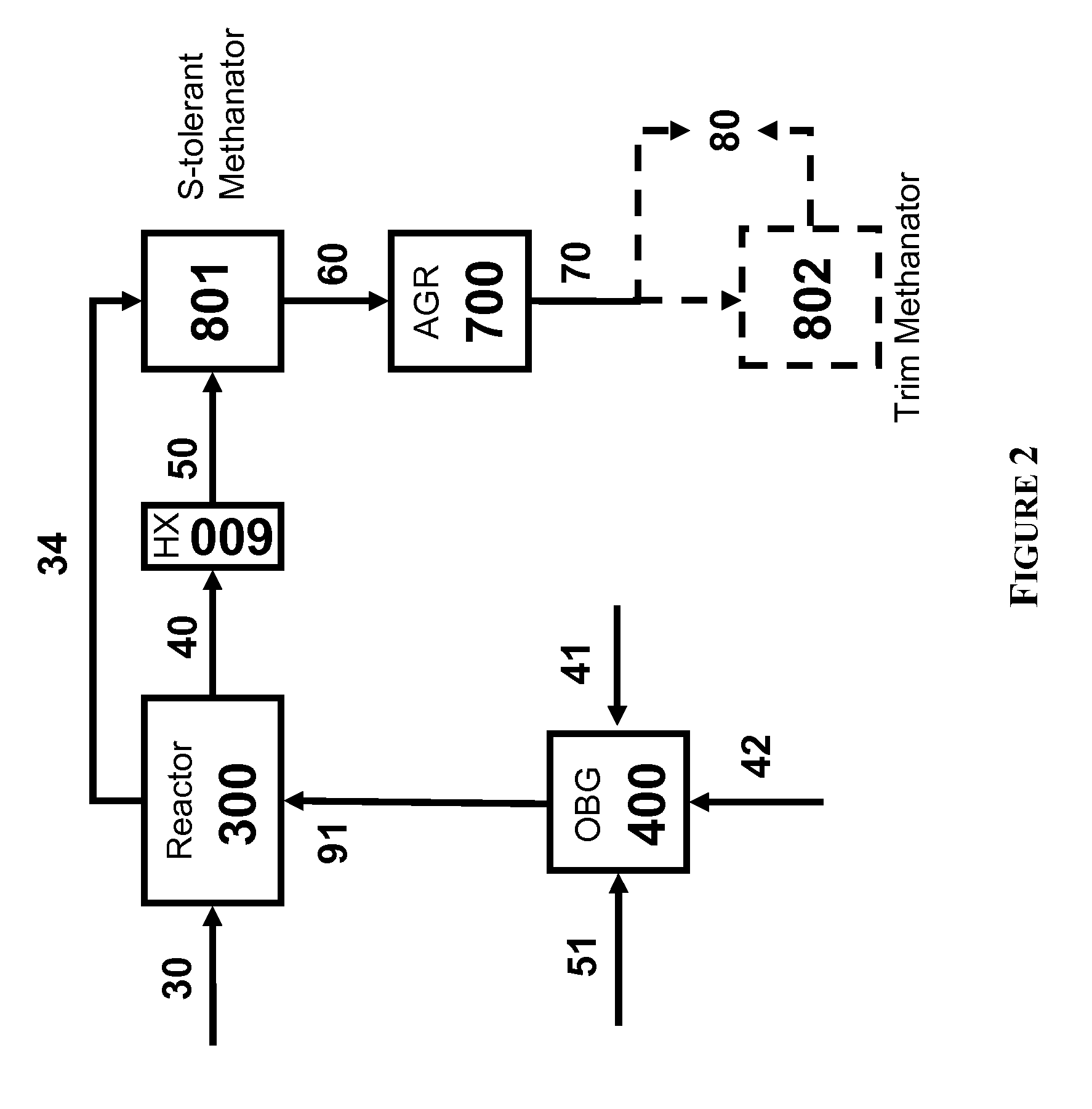
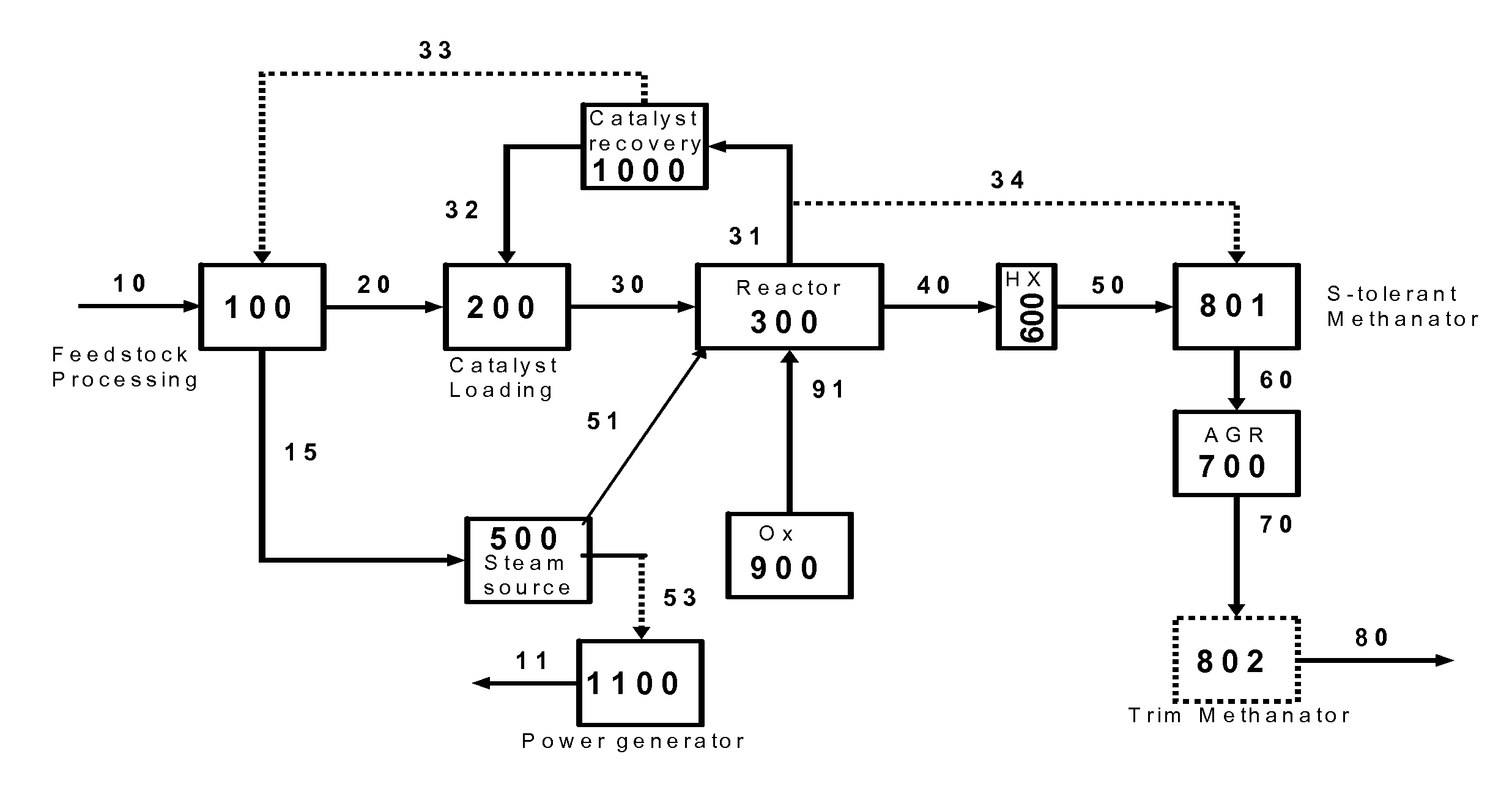
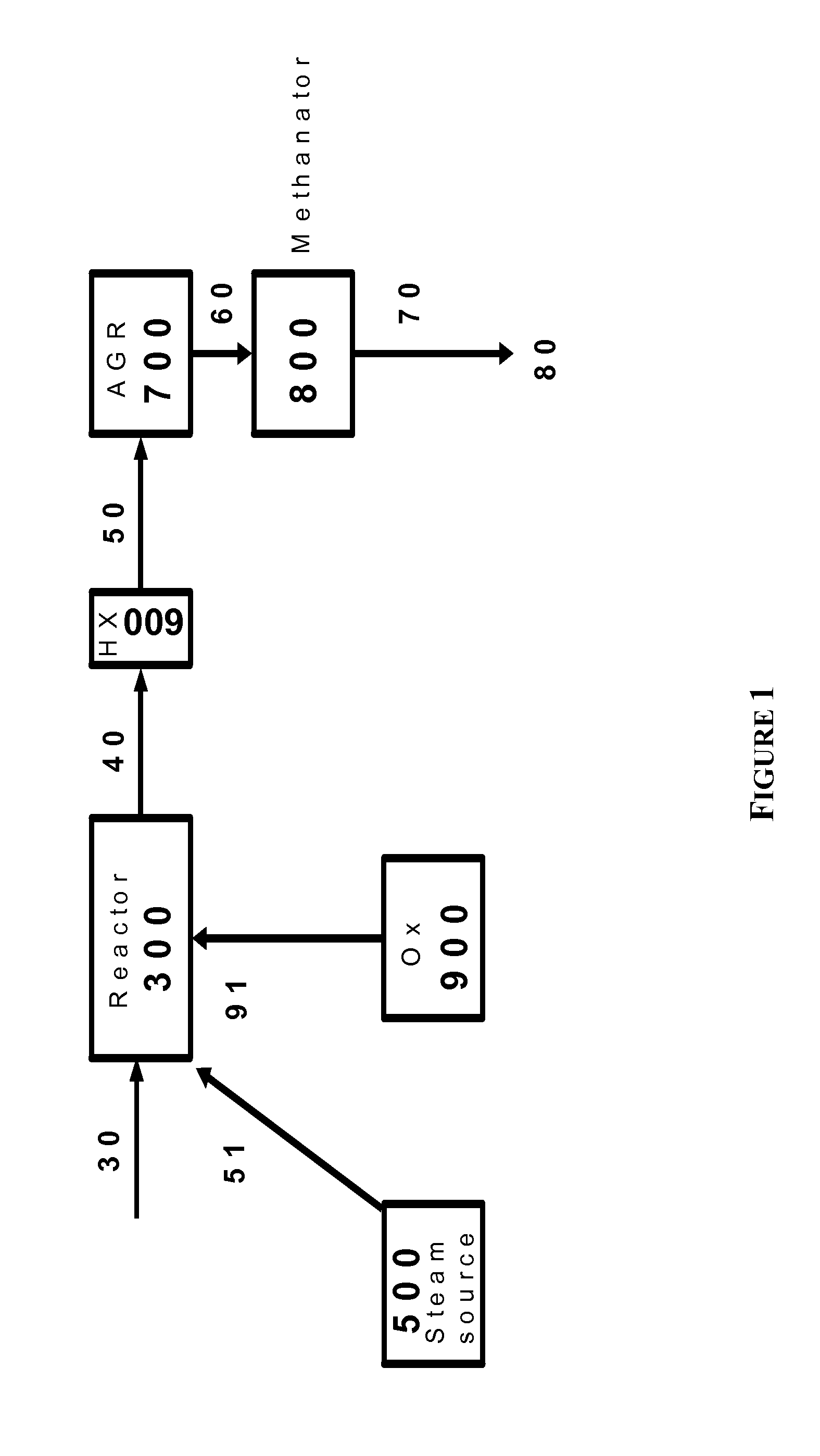
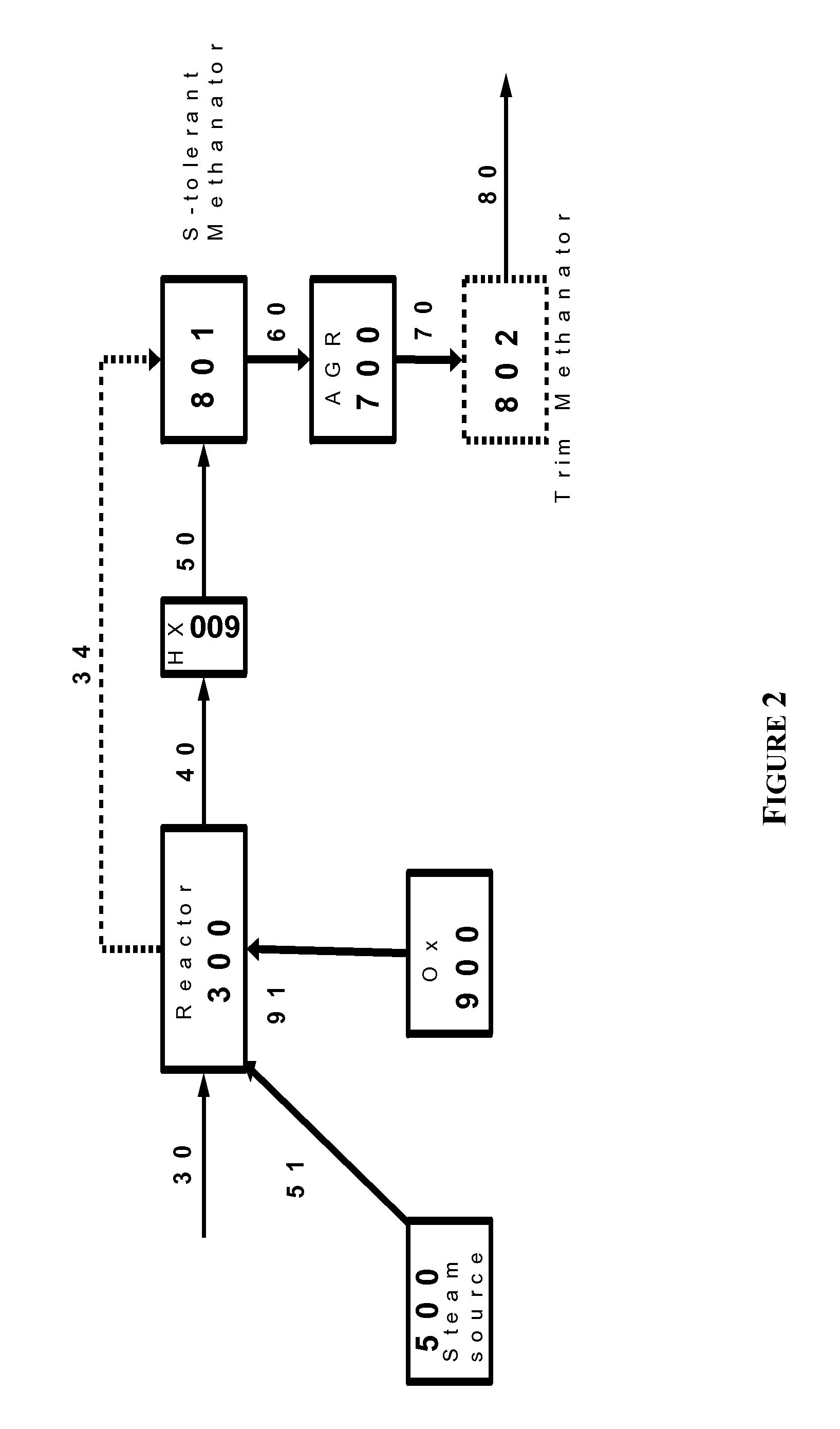
The present invention relates to processes and continuous processes for preparing gaseous products, and in particular, methane via the catalytic gasification of carbonaceous feedstocks in the presence of steam. In one aspect of the invention, the processes comprise at least partially combusting a first carbonaceous feedstock with an oxygen-rich gas stream in an oxygen-blown gasifier, under suitable temperature and pressure, to generate a first gas stream comprising hydrogen, carbon monoxide and superheated steam; and reacting a second carbonaceous feedstock and the first gas stream in a catalytic gasifier in the presence of a gasification catalyst under suitable temperature and pressure to form a second gas stream comprising a plurality of gaseous products comprising methane, carbon dioxide, hydrogen, carbon monoxide and hydrogen sulfide. The processes can comprise using at least one catalytic methanator to convert carbon monoxide and hydrogen in the gaseous products to methane and in certain embodiments do not recycle carbon monoxide or hydrogen to the gasifier.
Owner:SURE CHAMPION INVESTMENT LTD
Processes for Gasification of a Carbonaceous Feedstock
The present invention relates to processes for preparing gaseous products, and in particular, methane via the catalytic gasification of carbonaceous feedstocks in the presence of steam and an oxygen-rich gas stream. The processes comprise using at least one catalytic methanator to convert carbon monoxide and hydrogen in the gaseous products to methane and do not recycle carbon monoxide or hydrogen to the catalytic gasifier.
Owner:SURE CHAMPION INVESTMENT LTD
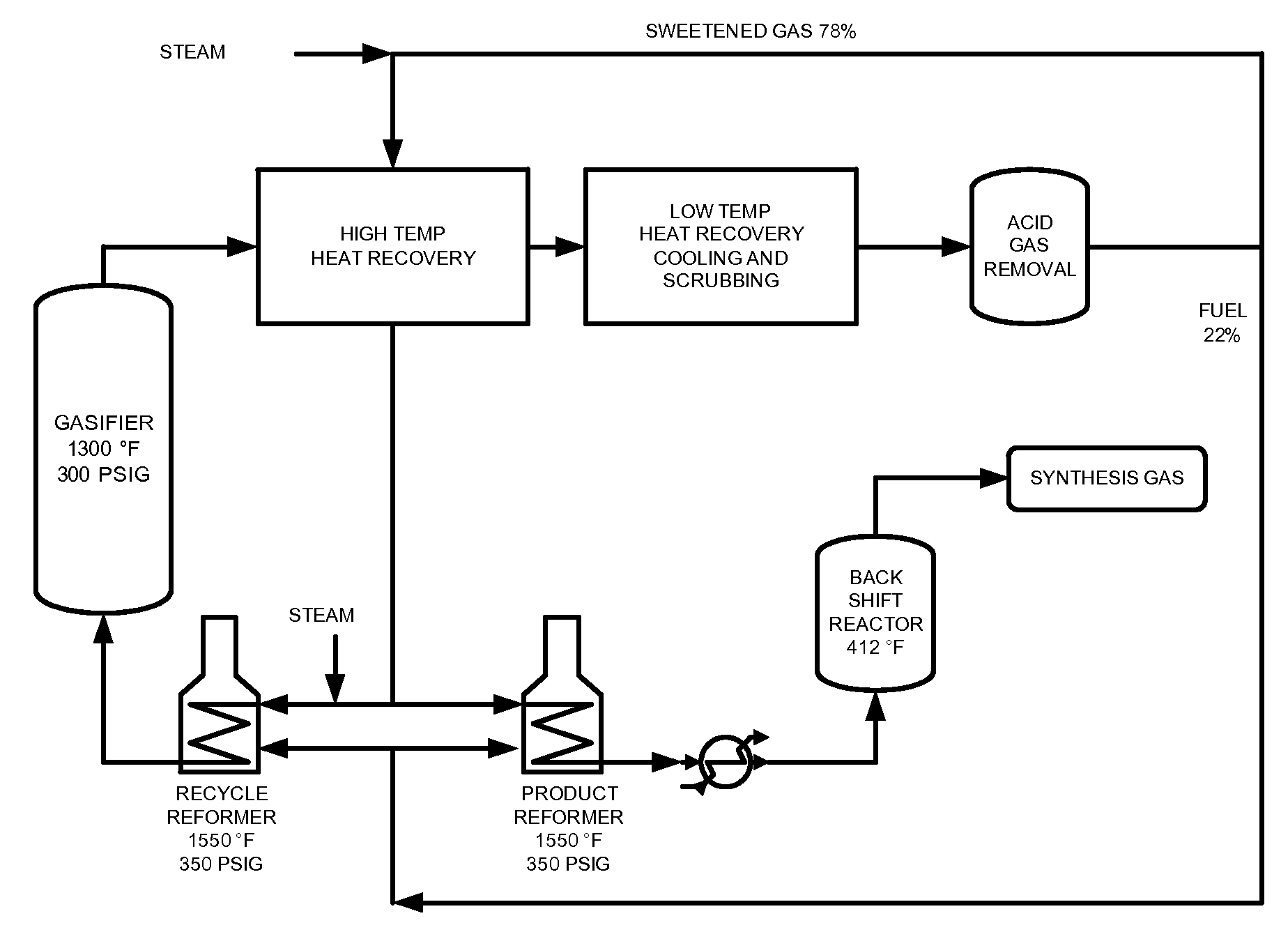
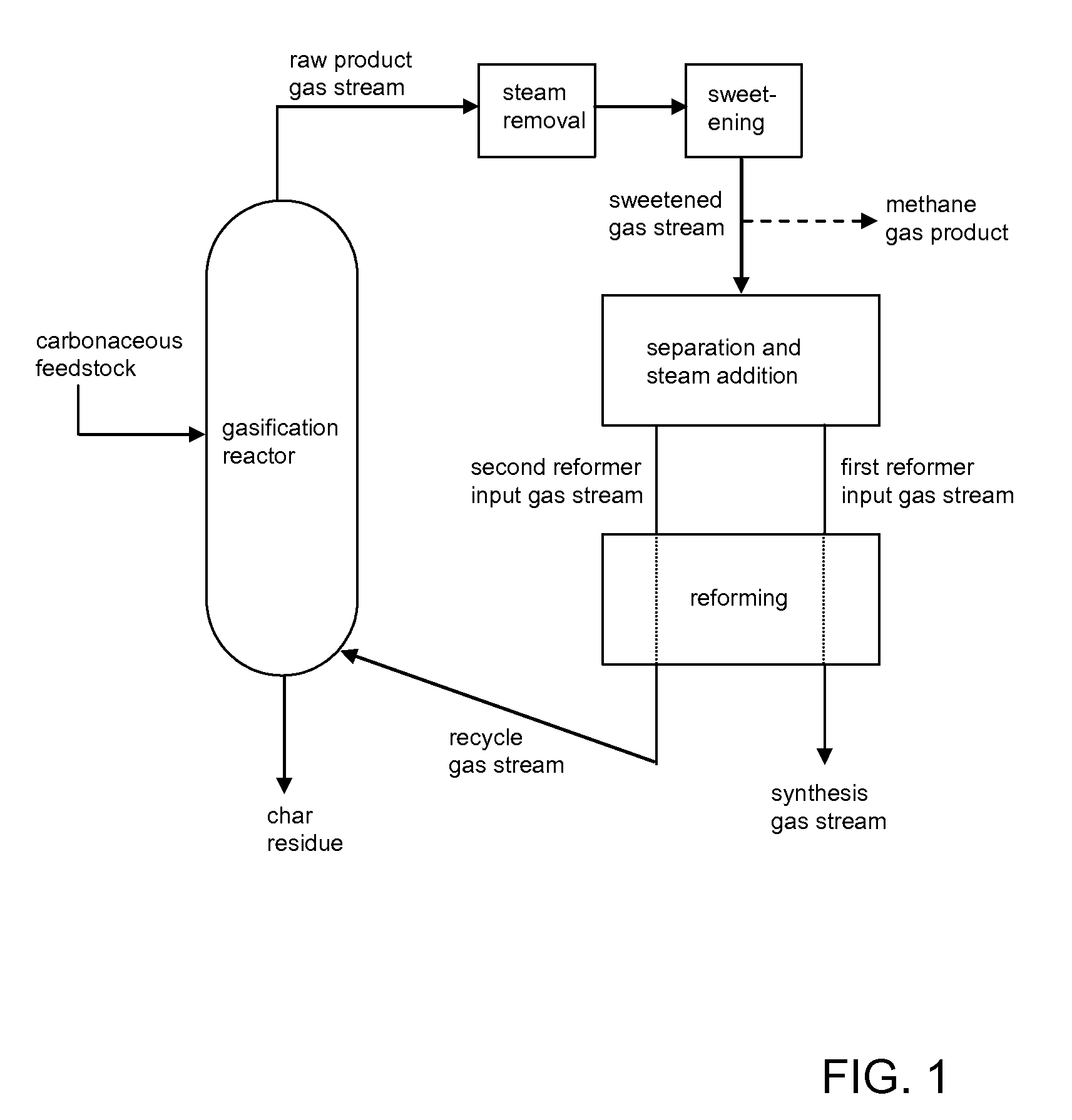
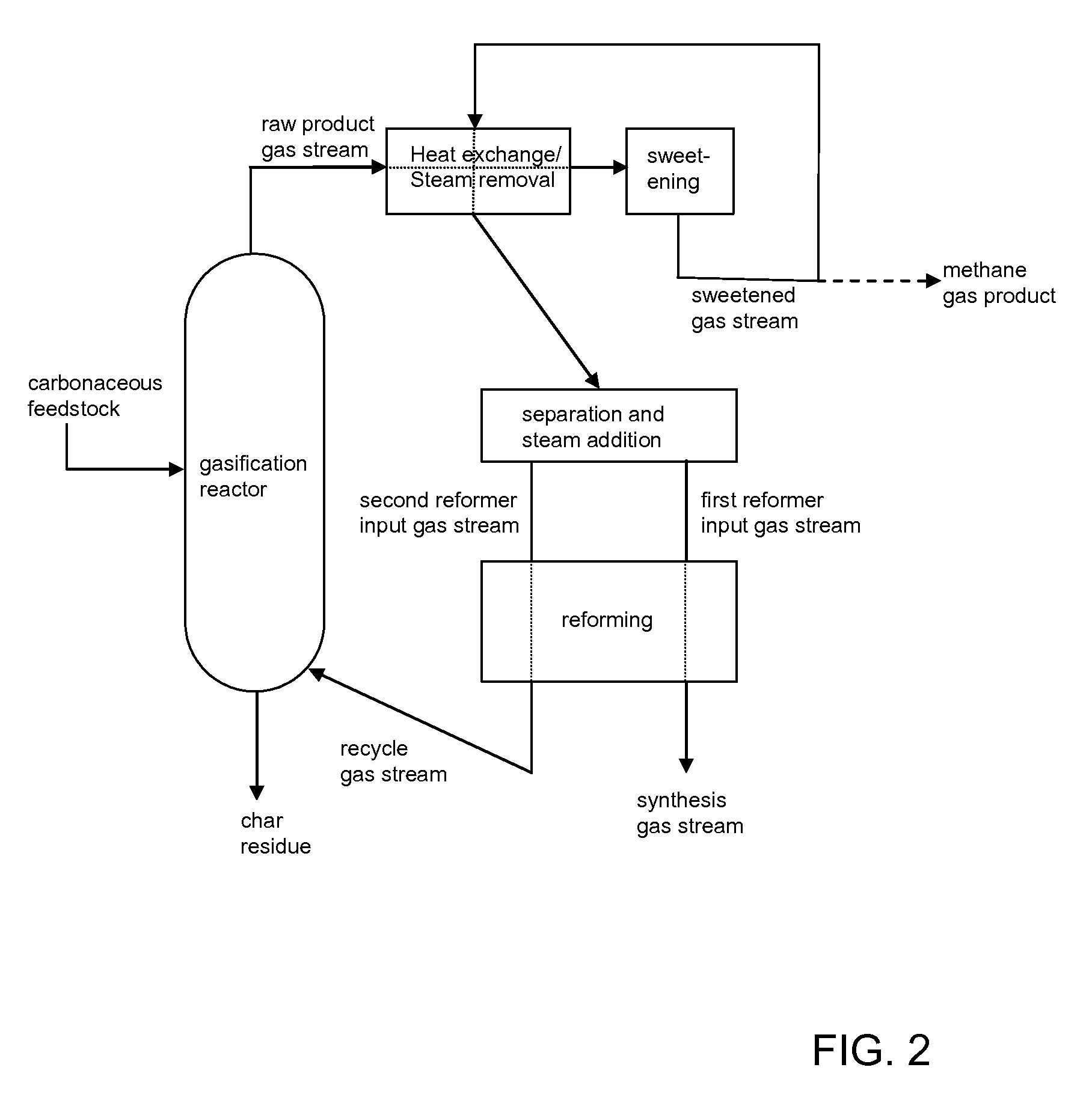
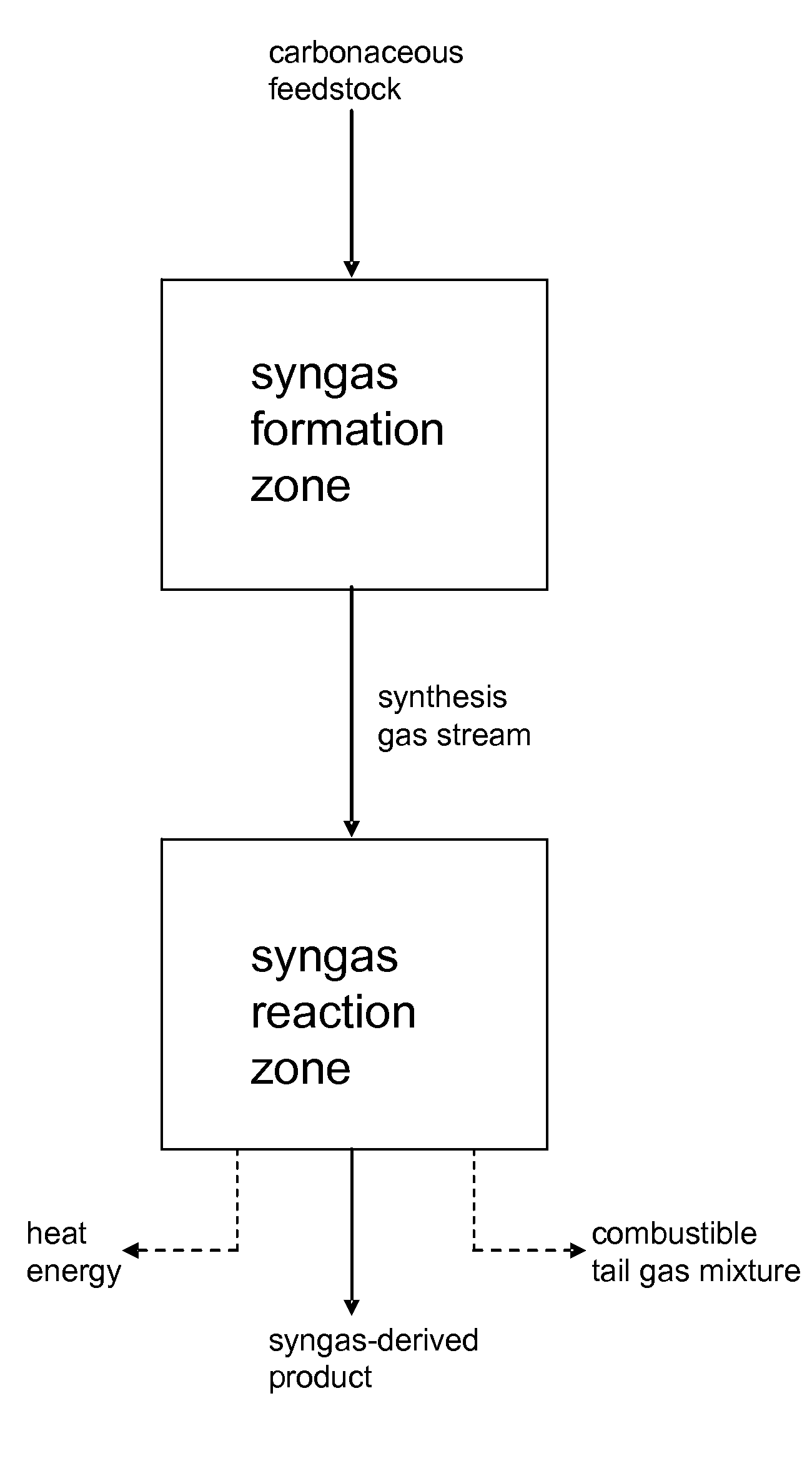
Processes for Making Synthesis Gas and Syngas-Derived Products
The present invention provides processes for making synthesis gas and processes for making syngas-derived products. For example, one aspect of the present invention provides a process for making a synthesis gas stream comprising hydrogen and carbon monoxide, the process comprising (a) providing a carbonaceous feedstock; (b) reacting the carbonaceous feedstock in a gasification reactor in the presence of steam and a gasification catalyst under suitable temperature and pressure to form a raw product gas stream comprising a plurality of gases comprising methane, hydrogen and carbon monoxide; (c) removing steam from and sweetening the raw product gas stream to form a sweetened gas stream; (d) separating and adding steam to the sweetened gas stream to form a first reformer input gas stream having a first steam / methane ratio; and a second reformer input stream having a second steam / methane ratio, in which the first steam / methane ratio is smaller than the second steam / methane ratio; (e) reforming the second reformer input stream to form a recycle gas stream comprising steam, carbon monoxide and hydrogen; (f) introducing the recycle gas stream to the gasification reactor; and (g) reforming the first reformer input stream to form the synthesis gas stream.
Owner:SURE CHAMPION INVESTMENT LTD
Conversion of petroleum residua to methane
InactiveUS6955695B2Eliminate needReduce usageThermal non-catalytic crackingElectrolysis componentsParticulatesGas phase
This invention discloses improvements on previous inventions for catalytic conversion of coal and steam to methane. The disclosed improvements permit conversion of petroleum residua or heavy crude petroleum to methane and carbon dioxide such that nearly all of the heating value of the converted hydrocarbons is recovered as heating value of the product methane. The liquid feed is distributed over a fluidized solid particulate catalyst containing alkali metal and carbon as petroleum coke at elevated temperature and pressure from the lower stage and transported to the upper stage of a two-stage reactor. Particulate solids containing carbon and alkali metal are circulated between the two stages. Superheated steam and recycled hydrogen and carbon monoxide are fed to the lower stage, fluidizing the particulate solids and gasifying some of the carbon. The gas phase from the lower stage passes through the upper stage, completing the reaction of the gas phase.
Owner:PETRO2020
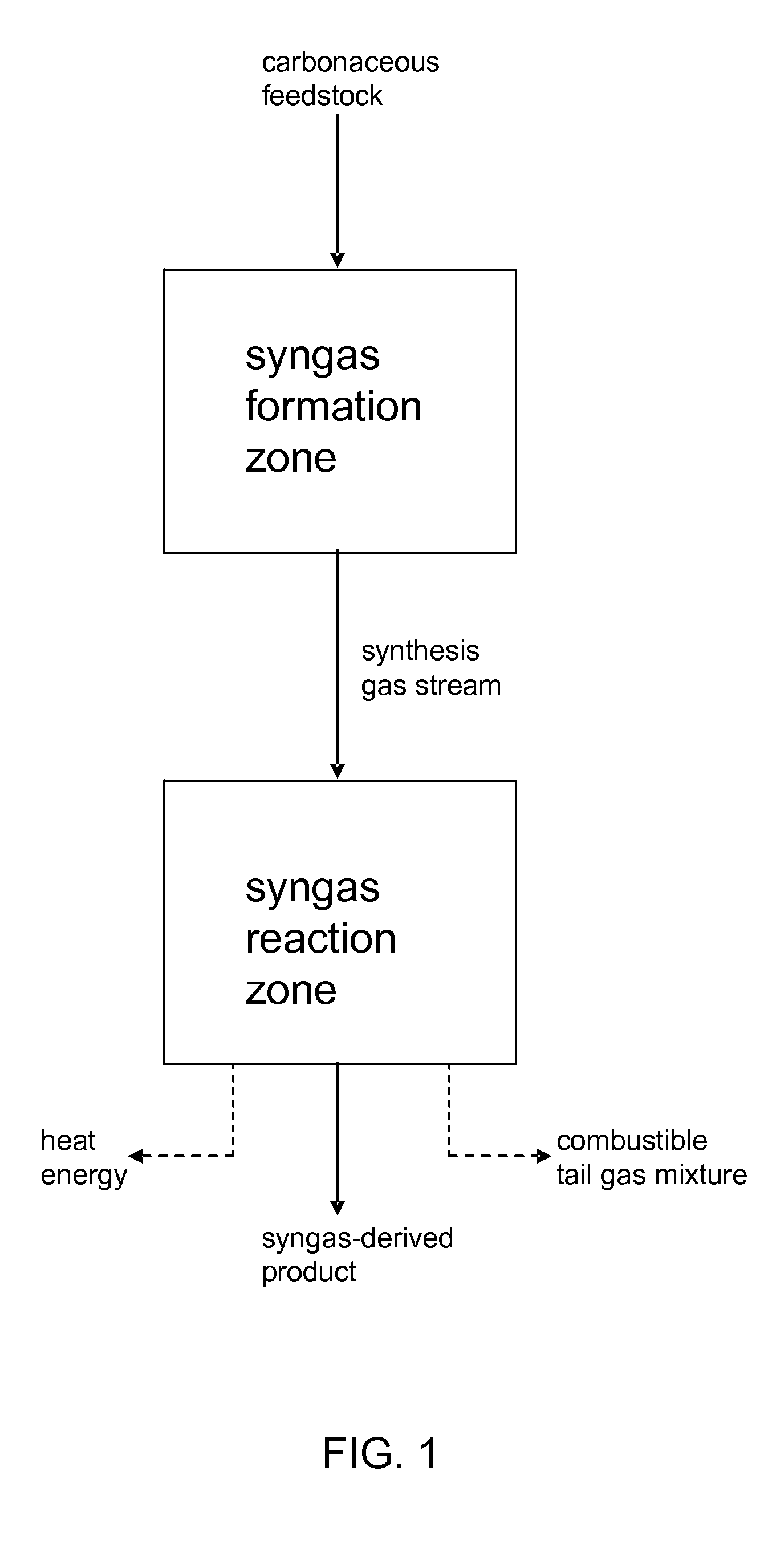
Processes for Making Syngas-Derived Products
The present invention provides processes for making syngas-derived products. For example, one aspect of the present invention provides a process for making a syngas-derived product, the process comprising (a) providing a carbonaceous feedstock; (b) converting the carbonaceous feedstock in a syngas formation zone at least in part to a synthesis gas stream comprising hydrogen and carbon monoxide; (c) conveying the synthesis gas stream to a syngas reaction zone; (d) reacting the synthesis gas stream in the syngas reaction zone to form the syngas-derived product and heat energy, a combustible tail gas mixture, or both; (e) recovering the syngas-derived product; and (f) recovering the heat energy formed from the reaction of the synthesis gas stream, burning the combustible tail gas mixture to form heat energy, or both.
Owner:SURE CHAMPION INVESTMENT LTD
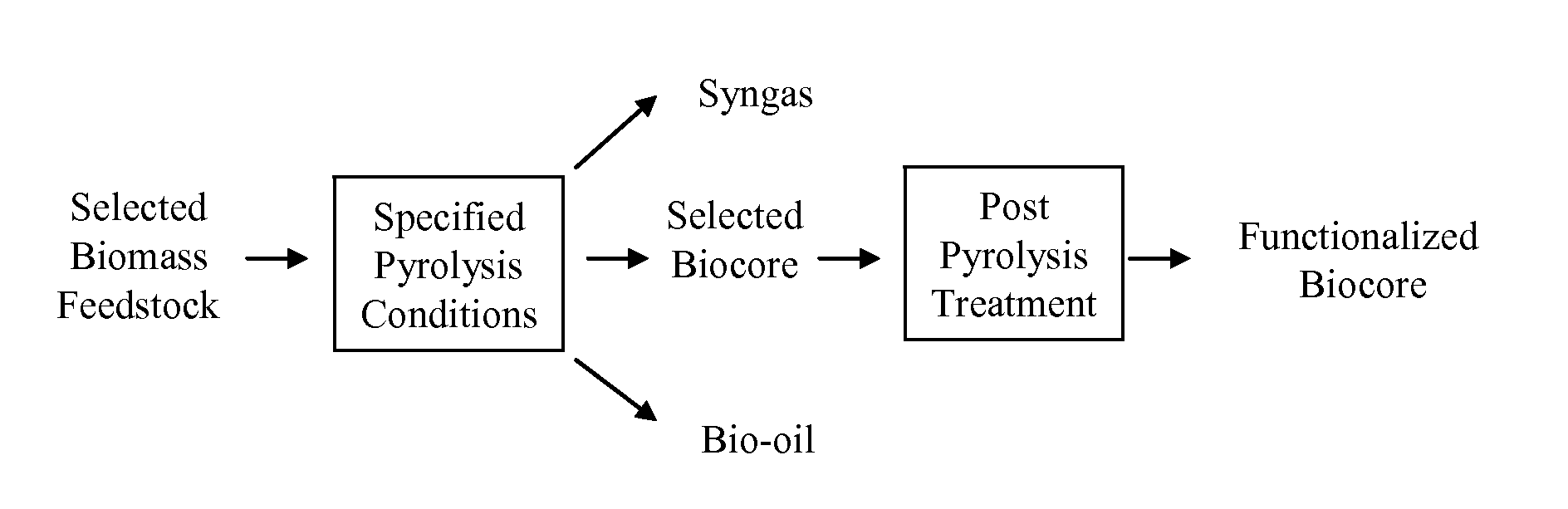
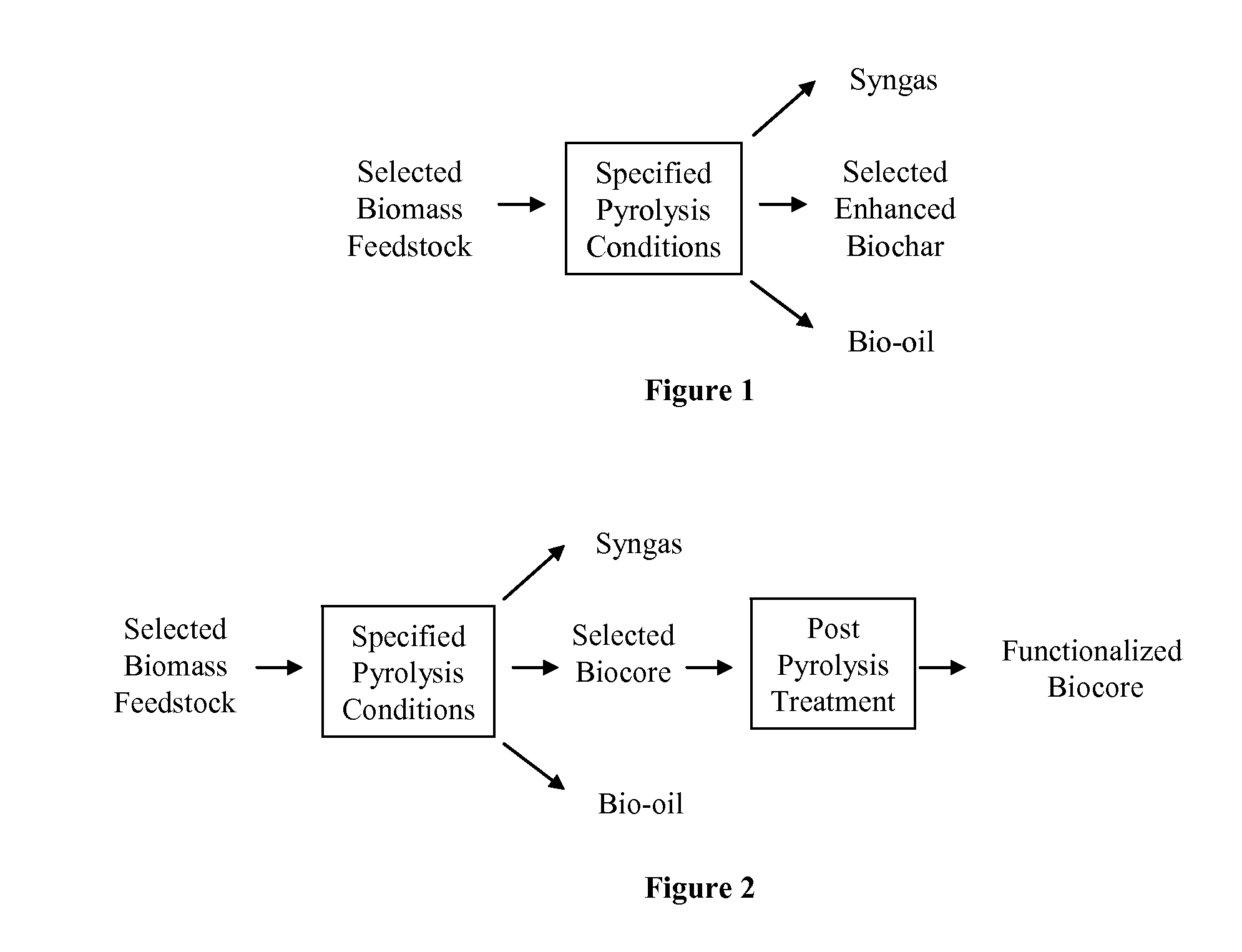
Biochar
ActiveUS8361186B1Enhanced and functionalizedCalcareous fertilisersMagnesium fertilisersForming gasBiological activation
The invention provides for methods, devices, and systems for pyrolyzing biomass. A pyrolysis unit can be used for the pyrolysis of biomass to form gas, liquid, and solid products. The biomass materials can be selected such that an enhanced biochar is formed after pyrolysis. The biomass can be pyrolyzed under specified conditions such that a selected biochar core is formed. The pyrolysis process can form a stable biochar core that is inert and / or resistant to degradation. The biochar or biochar core can be functionalized to form a functionalized biochar or functionalized biochar core. Functionalization can include post-pyrolysis treatments such as supplementation with microbes or physical transformations including annealing and / or activation.
Owner:FULL CIRCLE BIOCHAR
Co-Feed of Biomass as Source of Makeup Catalysts for Catalytic Coal Gasification
Continuous processes are provided for converting a carbonaceous feedstock comprising biomass containing alkali metal, non-biomass components, and at least one gasification catalyst including an alkali metal recovered from solid char, into a plurality of gaseous products including methane and at least one or more of hydrogen, carbon monoxide, and other higher hydrocarbons.
Owner:SURE CHAMPION INVESTMENT LTD
Processes for Gasification of a Carbonaceous Feedstock
The present invention relates to processes for preparing gaseous products, and in particular, methane via the catalytic gasification of carbonaceous feedstocks in the presence of steam. The processes comprise using at least one methanation step to convert carbon monoxide and hydrogen in the gaseous products to methane and do not recycle carbon monoxide or hydrogen to the catalytic gasifier.
Owner:SURE CHAMPION INVESTMENT LTD
Methods and apparatus for converting waste materials into fuels and other useful products
ActiveUS20090062581A1Effectively handle problematic wasteFree of contaminantsTransportation and packagingSolid waste disposalSpeciality chemicalsBiological waste
Conversion of waste and other organic feedstock into sustainable energy, feed, fertilizer, and other useful products of reliable purities is accomplished using water, heat, and pressure. More specifically, the invention provides methods and apparatus that handle mixed streams of various feedstocks, e.g. agricultural waste, biological waste, municipal solid waste, municipal sewage sludge, and shredder residue, to yield gas, oil, specialty chemicals, and carbon solids that can be used as is or are further processed. Useful products can be diverted at various points of the process or internalized to enhance the efficiency of the system.
Owner:SYNPET TEKNOLOJI GELISTIRME
Hydrogen and elemental carbon production from natural gas and other hydrocarbons
InactiveUS6395197B1High quench rateAddressing slow performanceHydrocarbon from carbon oxidesEnergy inputUnsaturated hydrocarbonHydrogen fuel
Diatomic hydrogen and unsaturated hydrocarbons are produced as reactor gases in a fast quench reactor. During the fast quench, the unsaturated hydrocarbons are further decomposed by reheating the reactor gases. More diatomic hydrogen is produced, along with elemental carbon. Other gas may be added at different stages in the process to form a desired end product and prevent back reactions. The product is a substantially clean-burning hydrogen fuel that leaves no greenhouse gas emissions, and elemental carbon that may be used in powder form as a commodity for several processes.
Owner:BATTELLE ENERGY ALLIANCE LLC
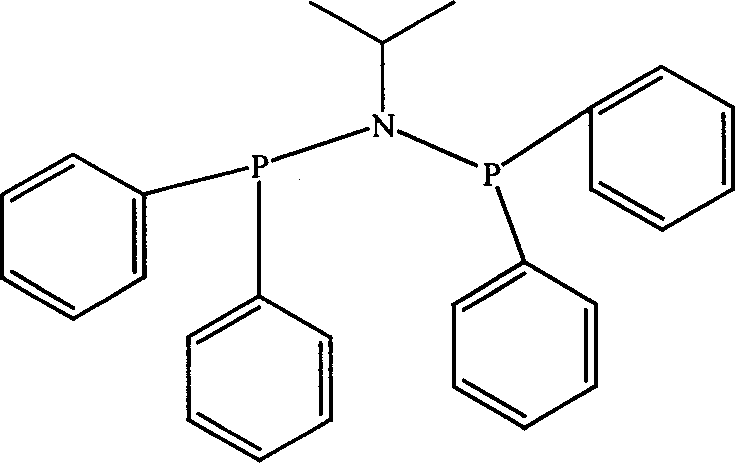
Catalyst component used for ethylene oligomerization, preparing process and application thereof
InactiveCN1651142AHigh activityEasy to synthesizeHydrocarbon from carbon oxidesOrganic-compounds/hydrides/coordination-complexes catalystsEthyleneOrganic Metallic Compounds
Owner:中国石油大庆石化分公司研究院 +1
Catalytic membrane reactor and method for production of synthesis gas
InactiveUS20080169449A1High oxygen fluxHydrogenSemi-permeable membranesSulfur containingPhotochemistry
A solid state membrane for a reforming reactor is disclosed which comprises at least one oxygen anion-conducting oxide selected from the group consisting of hexaaluminates, cerates, perovskites, and other mixed metal oxides that are able to adsorb and dissociate molecular oxygen. The membrane adsorbs and dissociates molecular oxygen into highly active atomic oxygen and allows oxygen anions to diffuse through the membrane, to provide high local concentration of oxygen to deter formation and deposition of carbon on reformer walls. Embodiments of the membrane also have catalytic activity for reforming a hydrocarbon fuel to synthesis gas. Also disclosed are a reformer having an inner wall containing the new membrane, and a process of reforming a hydrocarbon feed, such as a high sulfur-containing diesel fuel, to produce synthesis gas, suitable for use in fuel cells.
Owner:ELTRON RES
Silica-alumina catalyst support, catalysts made therefrom and methods of making and using same
ActiveUS7541310B2High activityHydrocarbon from carbon oxidesMolecular sieve catalystsCompound (substance)Catalytic metal
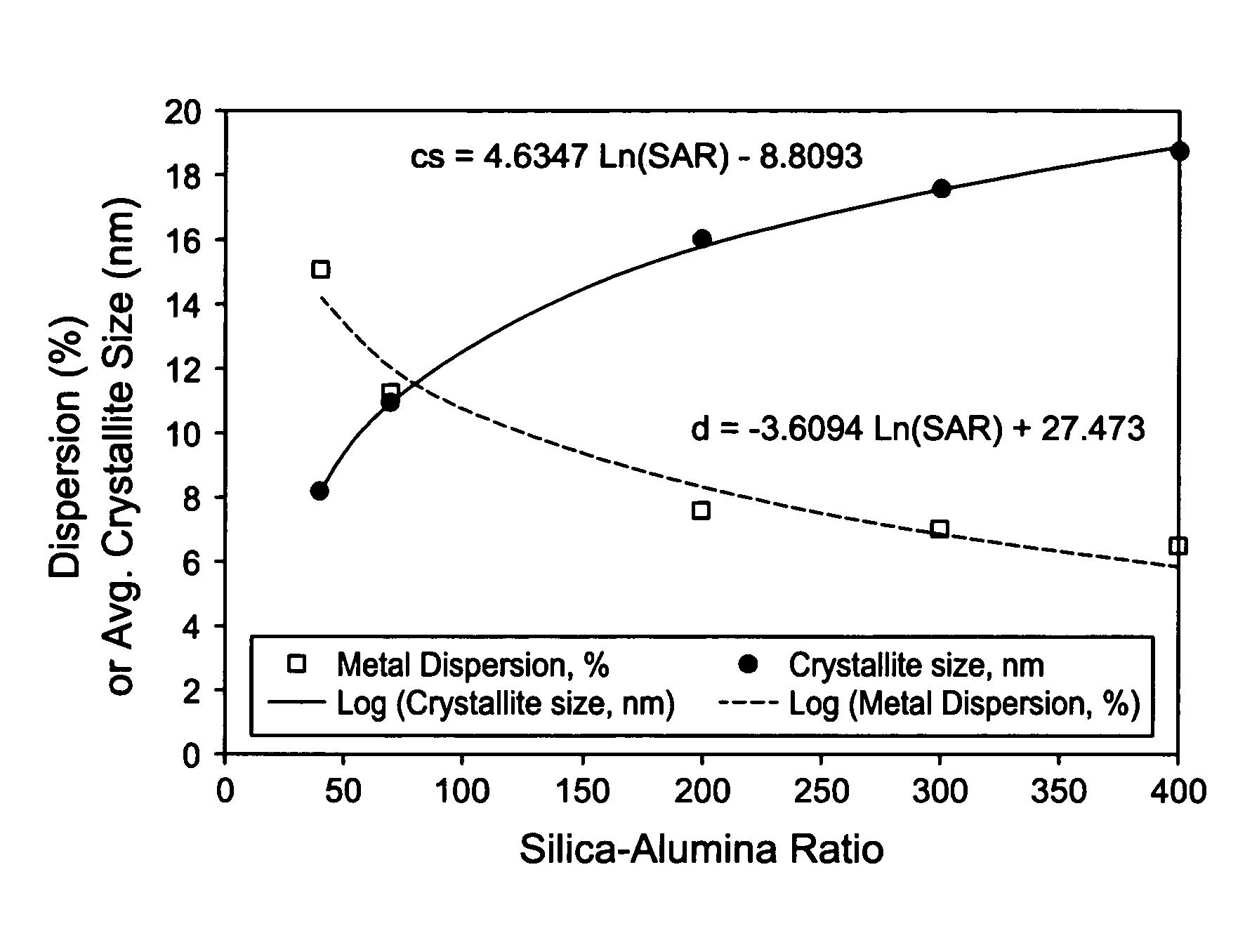
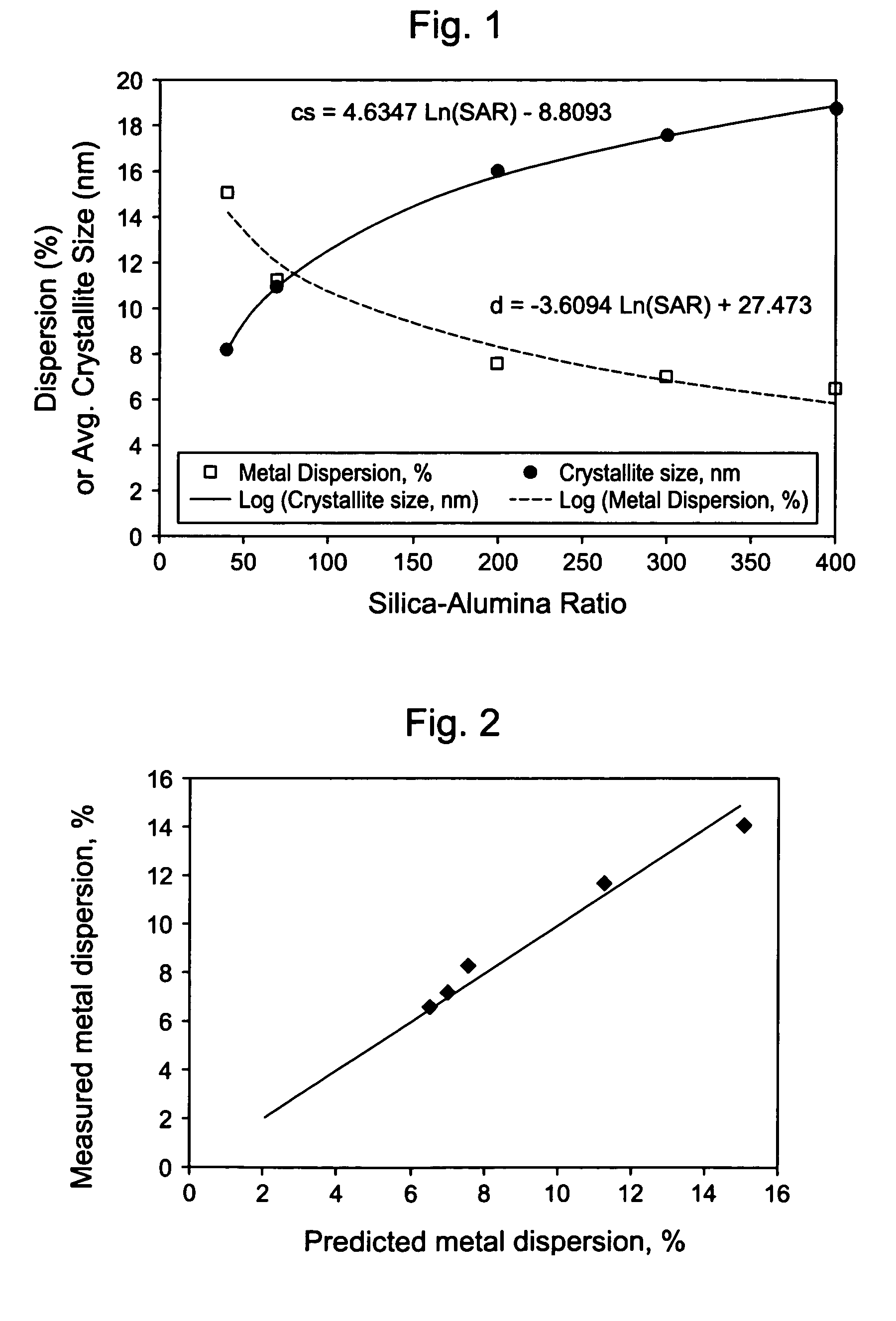
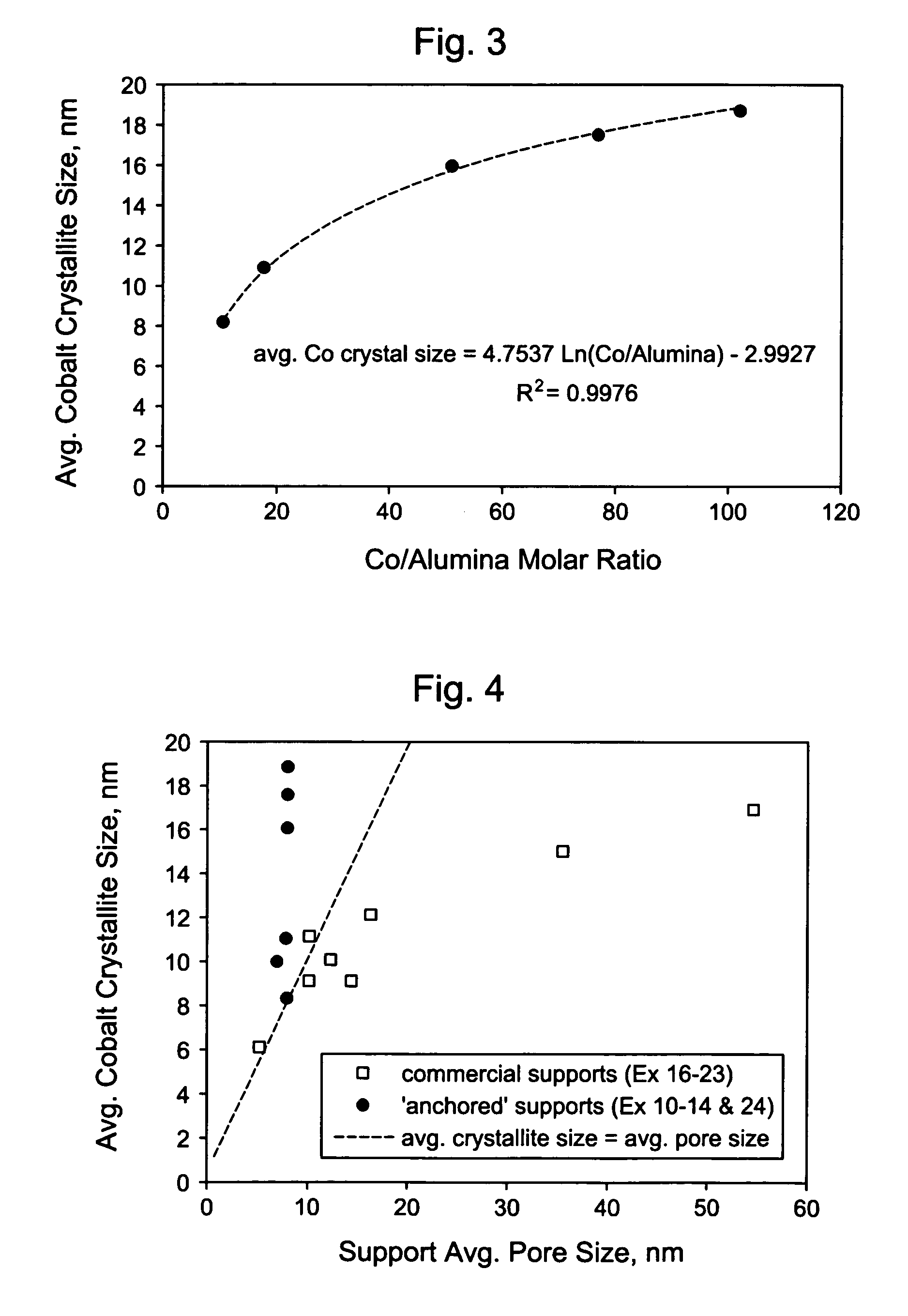
This invention relates to catalysts comprising a catalytic metal deposited on a composite support with well-dispersed chemical “anchor” species acting as nucleation centers for catalytic metal crystallites growth. The catalysts have the advantage that the average catalytic metal crystallite size can be controlled by the molar ratio of catalytic metal to chemical “anchor,” and is not limited by the porous structure of the support. A preferred embodiment comprises a cobalt-based catalyst on a silica-alumina support made by a co-gel method, wherein its average pore size can be controlled by the pH. The alumina species in the support most likely serve as chemical “anchors” to control the dispersion of cobalt species, such that the average cobalt crystallite size can be greater than the average pore size.
Owner:CLARIANT INT LTD
Catalytic oxidation process
InactiveUS6447745B1Hydrocarbon from carbon oxidesCatalyst activation/preparationElemental compositionPartial oxidation
A process for the partial catalytic oxidation of a hydrocarbon containing feed comprising contacting the feed with an oxygen-containing gas in the presence of a catalyst retained within a reaction zone in a fixed arrangement, wherein the catalyst comprises at least one catalytically active metal selected from the group consisting of silver and Group VIII elements supported on a porous ceramic carrier. The porous ceramic carrier has a distribution of total pores wherein about 70% of the total pores (1) have a volume-to-surface area (V / S) ration that is within about 20% of the mean V / S value for the total pores and no pores have a V / S ration that is greater than twice the mean V / S value for the total pores; (2) have a pore-to-pore distance between neighboring pores that is within about 25% of the mean pore-to-pore distance between neighboring pores; and (3) have a pore throat area that is within about 50% of the mean pore throat are for the pores. Additionally, about 50% of the total pores have a coordination number between neighboring pores that is within about 25% of the mean coordination number between neighboring pores. Preferably, the oxidation process comprises a multistage, staged oxygen, catalytic partial oxidation process having fewer than or equal to about five stages and including a first stage preheat temperature of greater than about 550° C., and wherein the temperature of the product mixture in each stage following the first stage is at least about 700° C.
Owner:EXXON RES & ENG CO
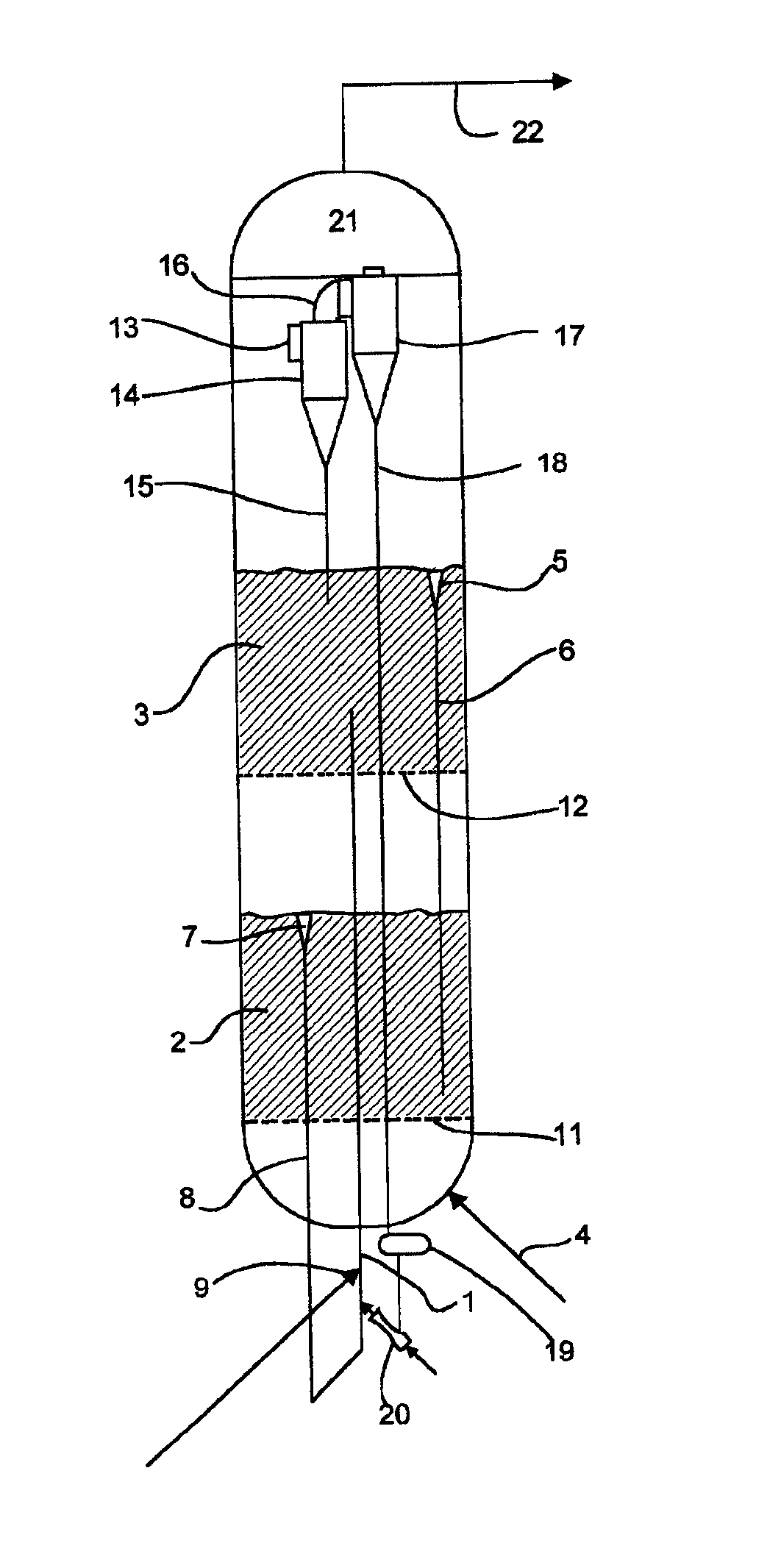
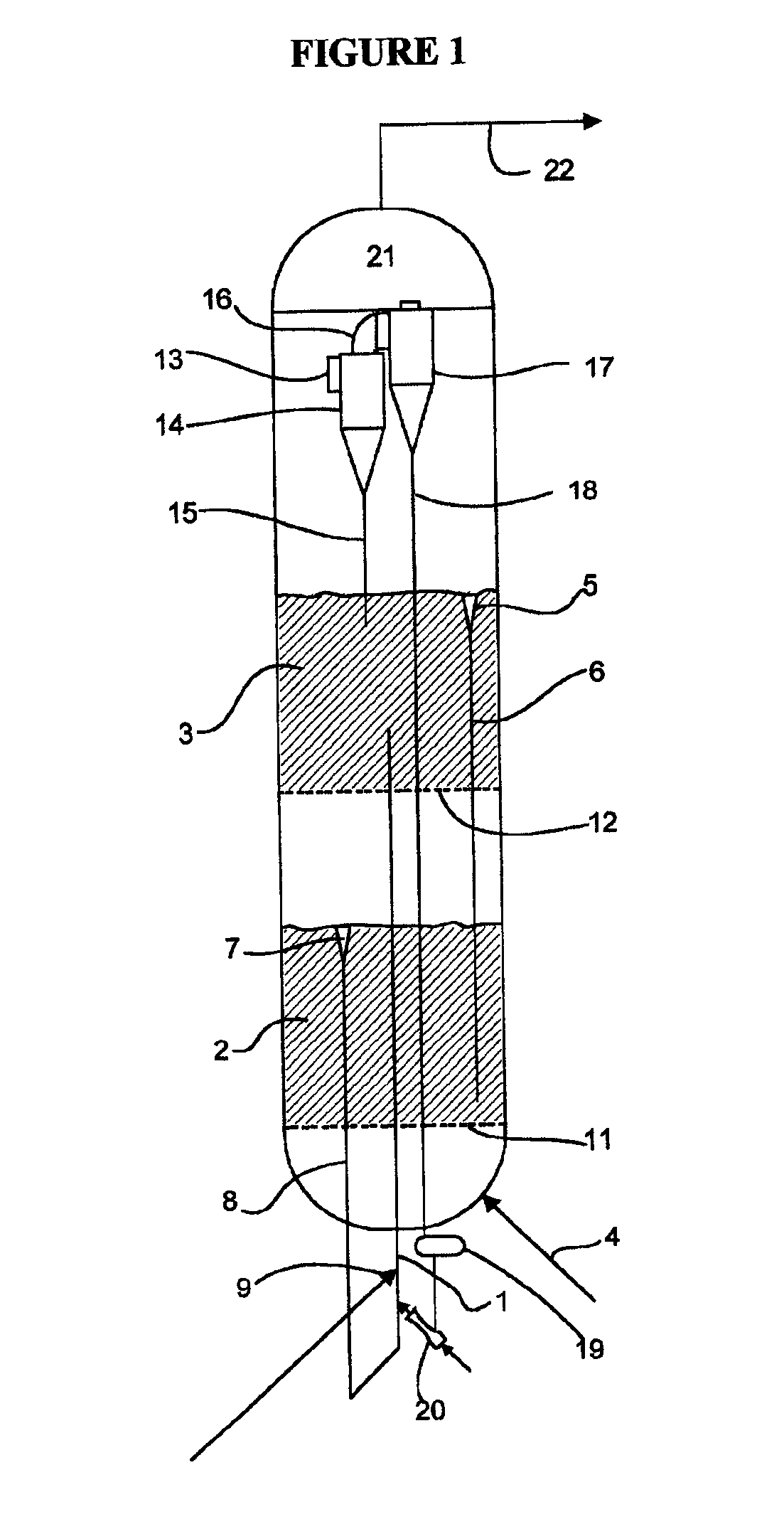
Processes for hydromethanation of a carbonaceous feedstock
InactiveUS20110031439A1Increase the amount of carbonIncrease volumeCombustible gas chemical modificationHydrocarbon from carbon oxidesPtru catalystPhysical chemistry
The present invention relates to processes for preparing gaseous products, and in particular a hydrogen product stream and optionally a methane product stream, via the hydromethanation of carbonaceous feedstocks in the presence of steam, carbon monoxide, hydrogen and a hydromethanation catalyst.
Owner:SURE CHAMPION INVESTMENT LTD
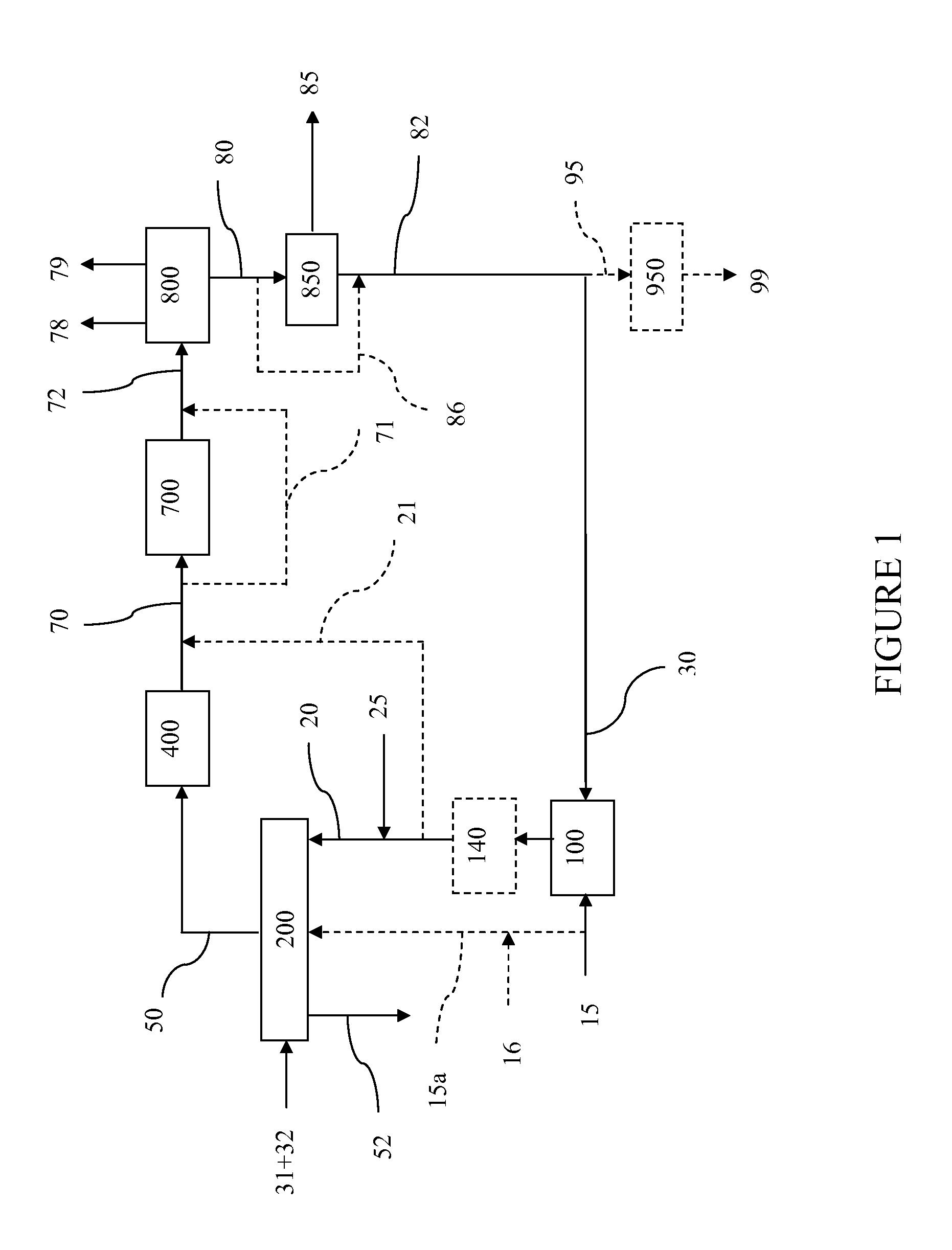
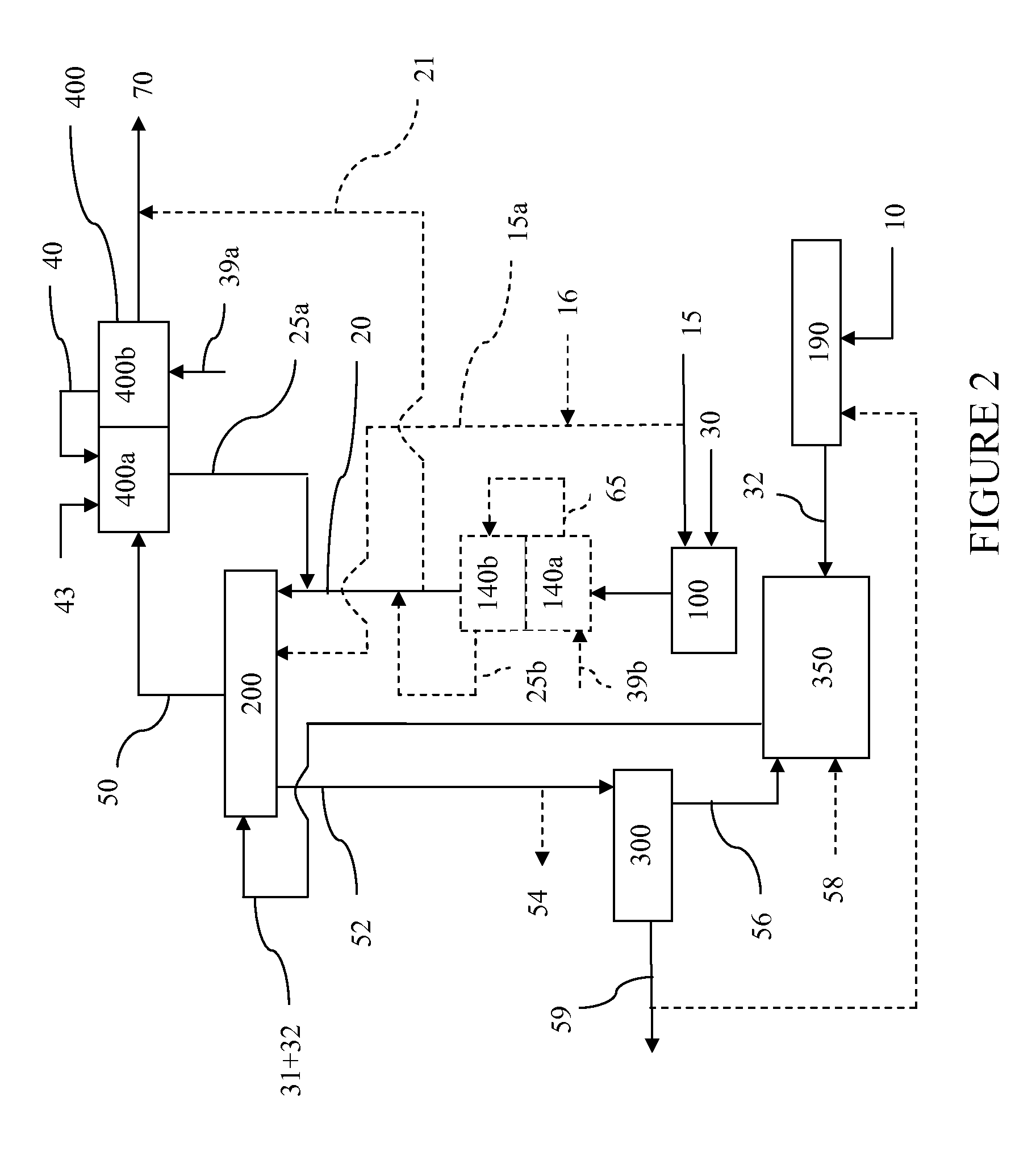
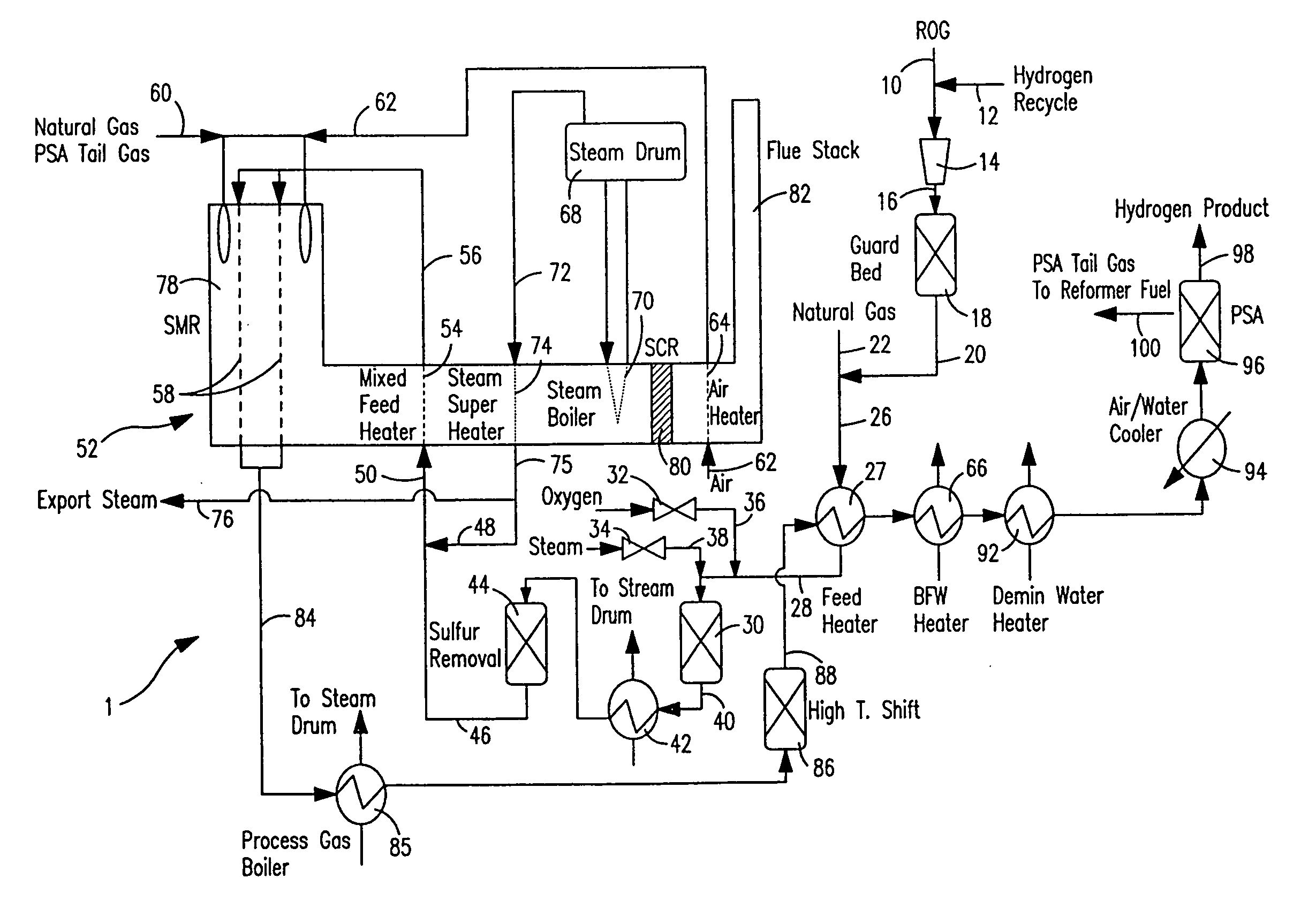
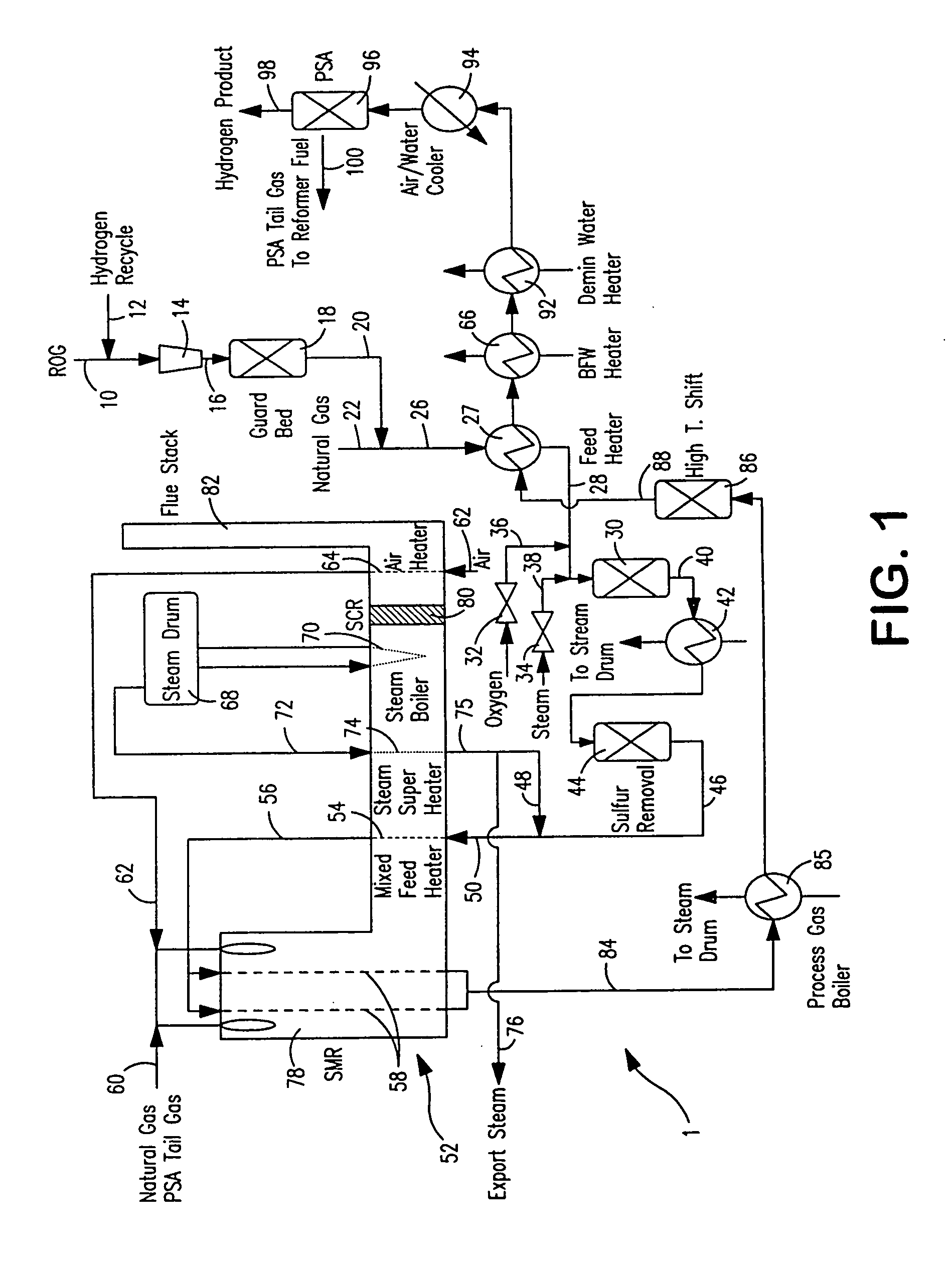
Steam methane reforming method
ActiveUS7037485B1Reduce fuel usageReduce firing rateHydrocarbon from carbon oxidesHydrogen separation using solid contactMethane reformerAlkane
A steam methane reforming method in which a feed stream is treated in a reactor containing a catalyst that is capable of promoting both hydrogenation and partial oxidation reactions. The reactor is either operated in a catalytic hydrogenation mode to convert olefins into saturated hydrocarbons and / or to chemically reduce sulfur species to hydrogen sulfide or a catalytic oxidative mode utilizing oxygen and steam to prereform the feed and thus, increase the hydrogen content of a synthesis gas produced by a steam methane reformer. The method is applicable to the treatment of feed streams containing at least 15% by volume of hydrocarbons with two or more carbon atoms and / or 3% by volume of olefins, such as a refinery off-gas. In such case, the catalytic oxidative mode is conducted with a steam to carbon ratio of less than 0.5, an oxygen to carbon ratio of less than 0.25 and a reaction temperature of between about 500° C. and about 860° C. to limit the feed to the steam methane reformer to volumetric dry concentrations of less than about 0.5% for the olefins and less than about 10% for alkanes with two or more carbon atoms.
Owner:PRAXAIR TECH INC
Integrated, high-efficiency processes for biomass conversion to synthesis gas
InactiveUS20100270505A1Increase temperatureHydrogenHydrocarbon from carbon oxidesSyngasPartial oxidation
The present invention provides several variations for converting biomass, and other carbon-containing feedstocks, into syngas. Some variations include pyrolyzing or torrefying biomass in a devolatilization unit to form a gas stream and char, and gasifying the char. Other variations include introducing biomass into a fluid-bed gasifier to generate a solid stream and a gas stream, followed by a partial-oxidation or reforming reactor to generate additional syngas from either, or both, of the solid or gas stream from the fluid-bed gasifier. Hot syngas is preferably subjected to heat recovery. The syngas produced by the disclosed methods may be used in any desired manner, such as conversion to liquid fuels (e.g., ethanol).
Owner:HAAKON LLC
Catalyst for manufacturing hydrogen or synthesis gas and manufacturing method of hydrogen or synthesis gas
InactiveUS6361757B1Produce hydrogenEfficient productionIron compoundsCobalt compoundsIridiumForming gas
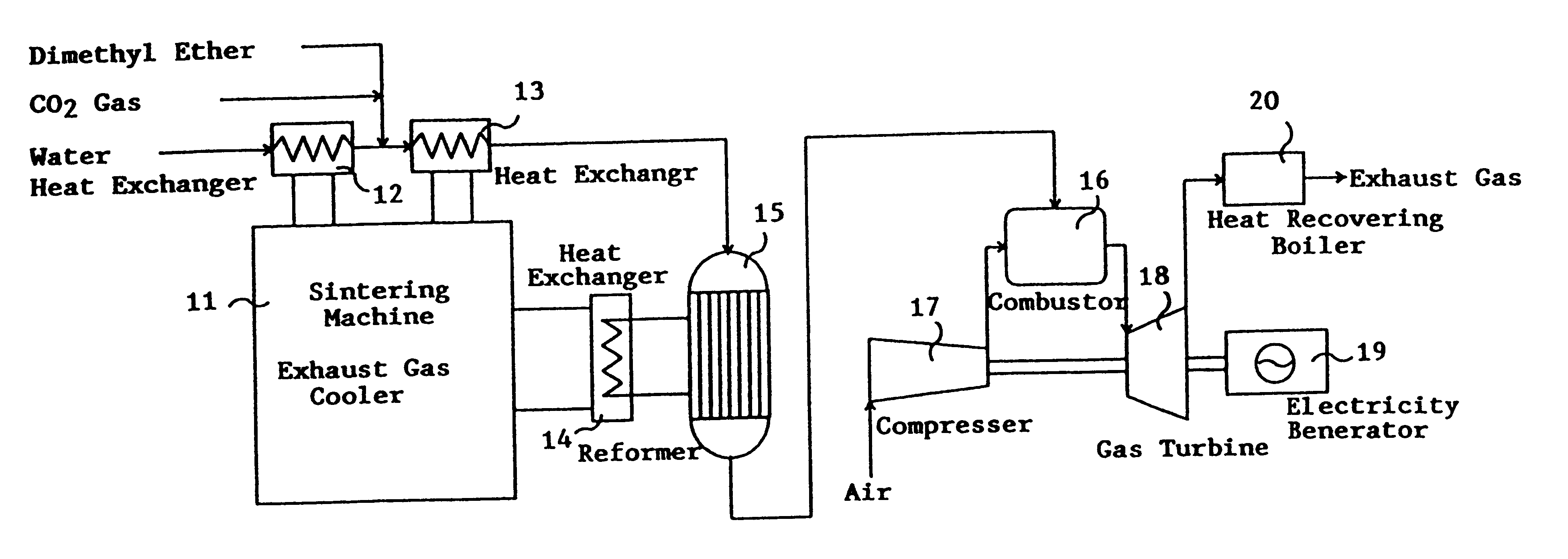
This invention provides a catalyst for producing hydrogen gas from a mixed gas comprising dimethyl ether and water vapor or carbon dioxide gas, which comprises copper, iron, cobalt, palladium, iridium, platinum, rhodium, or nickel as an active component, and a method of producing synthesis gas or hydrogen gas in a high yield at a low temperature. By using the catalyst, a fuel cell, electricity generation, reduction of iron ore and the like can be carried out.
Owner:NIPPON KOKAN KK
Fractional catalytic pyrolysis of biomass
ActiveUS20090165378A1Eliminate needBiofuelsIndirect heating destructive distillationCatalytic pyrolysisReactive gas
Methods for fractional catalytic pyrolysis which allow for conversion of biomass into a slate of desired products without the need for post-pyrolysis separation are described. The methods involve use of a fluid catalytic bed which is maintained at a suitable pyrolysis temperature. Biomass is added to the catalytic bed, preferably while entrained in a non-reactive gas such as nitrogen, causing the biomass to become pyrolyzed and forming the desired products in vapor and gas forms, allowing the desired products to be easily separated.
Owner:VIRGINIA TECH INTPROP INC
Solar-thermal fluid-wall reaction processing
InactiveUS7033570B2Reduce and preventShort stayThermal non-catalytic crackingSolar heating energyHydrogenReactor system
The present invention provides a method for carrying out high temperature thermal dissociation reactions requiring rapid-heating and short residence times using solar energy. In particular, the present invention provides a method for carrying out high temperature thermal reactions such as dissociation of hydrocarbon containing gases and hydrogen sulfide to produce hydrogen and dry reforming of hydrocarbon containing gases with carbon dioxide. In the methods of the invention where hydrocarbon containing gases are dissociated, fine carbon black particles are also produced. The present invention also provides solar-thermal reactors and solar-thermal reactor systems.
Owner:ALLIANCE FOR SUSTAINABLE ENERGY +1
Premium synthetic lubricant base stock (Law734) having at least 95% noncyclic isoparaffins
A premium synthetic lubricating oil base stock having a high VI and low pour point is made by hydroisomerizing a Fischer-Tropsch synthesized waxy, paraffinic feed wax and then dewaxing the hydroisomerate to form a 650-750° F.+ dewaxate. The waxy feed has an initial boiling point in the range of about 650-750° F., from which it continuously boils up to at least 1050° F. and has a T90-T10 temperature difference of at least 350° F. The feed is preferably hydroisomerized without any pretreatment, other than optional fractionation. The 650-750° F.+ dewaxate is fractionated into two or more base stocks of different viscosity.
Owner:EXXON RES & ENG CO
Process for producing a syngas
InactiveUS6402988B1Minimize the temperatures experienced by sealsMinimize pressure differenceCatalytic gas-gas reactionHydrogenSyngasReactor design
An exothermic reaction and an endothermic reaction are thermally combined in a reactor having at least one oxygen selective ion transport membrane that provides the exothermic reaction with oxygen from an oxygen-containing gas such as air. The thermal requirements of the endothermic reaction are satisfied by the exothermic reaction. Dependent on the reactor design employed, the exothermic and endothermic reactions may be gaseously combined.
Owner:PRAXAIR TECH INC +1
Synthesis gas process comprising partial oxidation using controlled and optimized temperature profile
ActiveUS7261751B2Low selectivityRisk of explosion can be minimizedHydrocarbon from carbon oxidesCarburetting by solid carbonaceous material pyrolysisPartial oxidationFixed bed
Owner:PHILLIPS 66 CO
Synthesis gas process comprising partial oxidation using controlled and optimized temperature profile
ActiveUS20060029539A1Risk of explosion can be minimizedIncrease the molar ratioHydrocarbon from carbon oxidesCarburetting by solid carbonaceous material pyrolysisForming gasPartial oxidation
This invention relates to methods for reacting a hydrocarbon, molecular oxygen, and optionally water and / or carbon dioxide, to form synthesis gas. The preferred embodiments are characterized by delivering a substochiometric amount of oxygen to each of a multitude of reaction zones, which allows for optimum design of the catalytic packed bed and the gas distribution system, and for the optimization and control of the temperature profile of the reaction zones. The multitude of reaction zones may include a series of successive fixed beds, or a continuous zone housed within an internal structure having porous, or perforated, walls, through which an oxygen-containing stream can permeate. By controlling the oxygen supply, the temperatures, conversion, and product selectivity of the reaction can be in turn controlled and optimized. Furthermore the potential risks of explosion associated with mixing hydrocarbon and molecular oxygen is minimized with increased feed carbon-to-oxygen molar ratios.
Owner:PHILLIPS 66 CO
Process and apparatus for biomass gasification
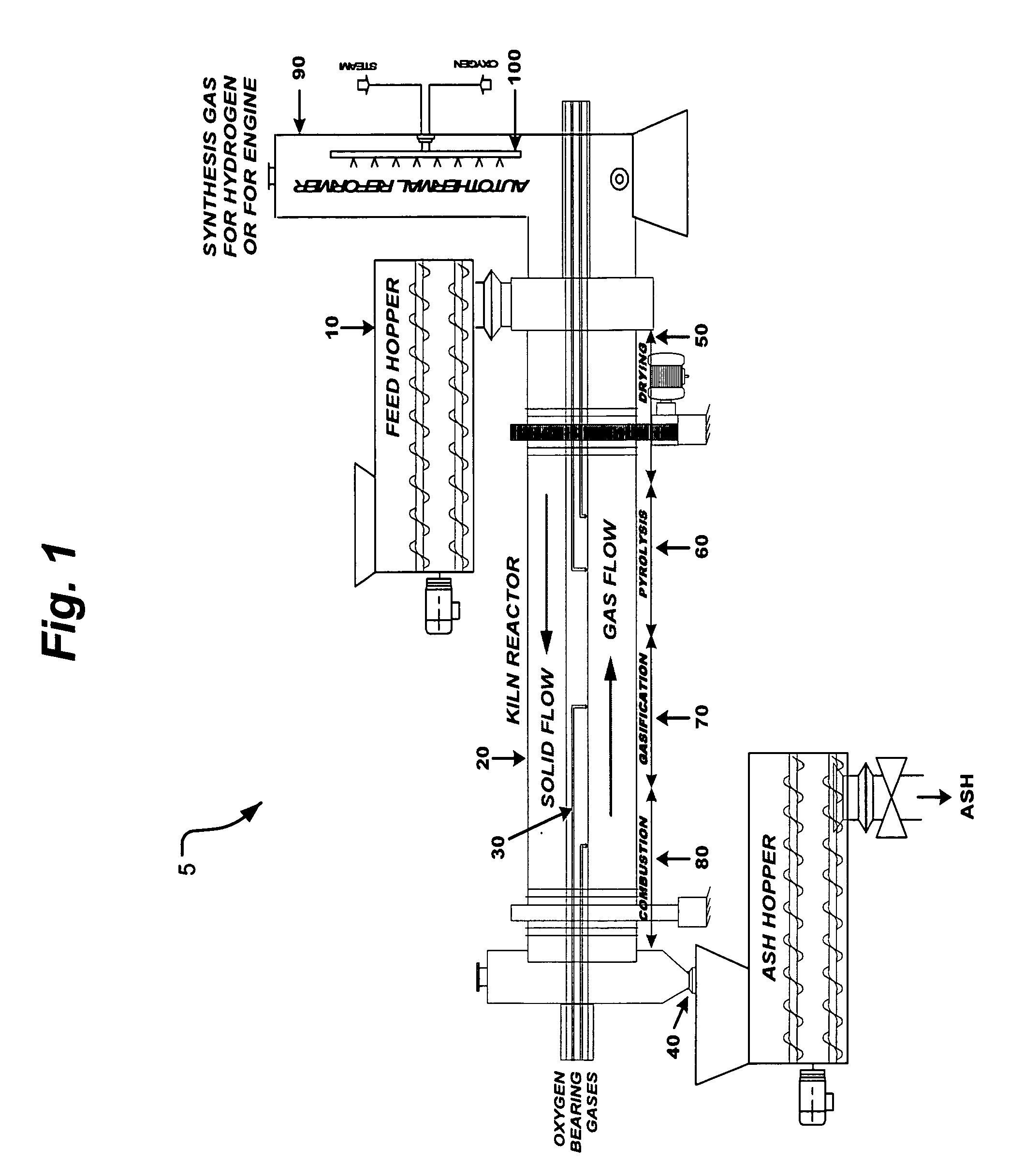
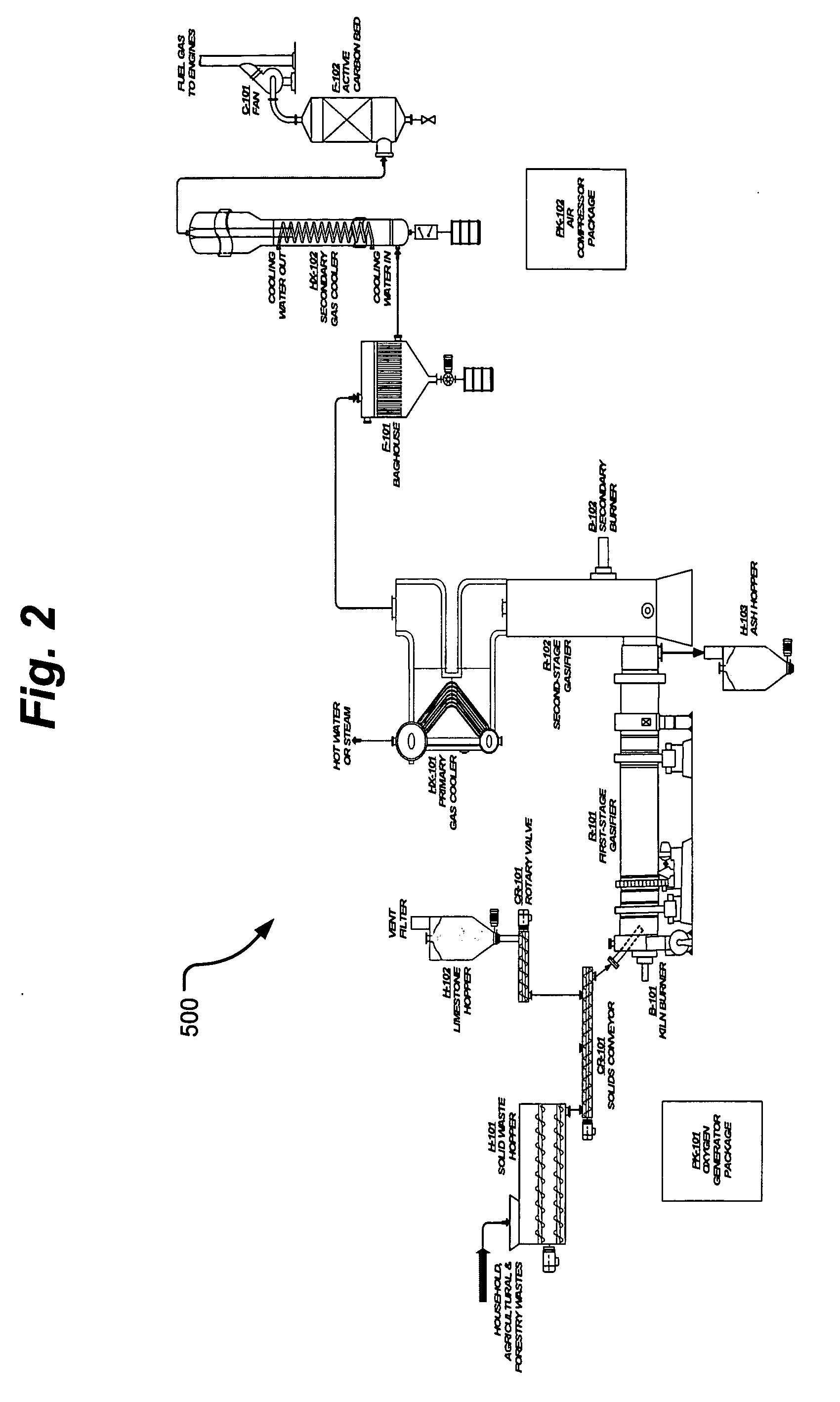
InactiveUS20050095183A1Oxygen-containing compound preparationGasifier mechanical detailsForming gasTar
A waste-to-synthesis gas system including: a first gasifier for receiving biomass; a gas distributor for delivering reactant gas and oxygen into the first gasifier in a countercurrent direction to the biomass flow and to define a plurality of reaction regions including a drying region, a pyrolysis region, a gasification region and a combustion region; and, a second gasifier for receiving gases from the plurality of regions of the first gasifier and a gas distributor for delivering reactant gas and oxygen into the second gasifier in a concurrent direction to the flow of gases from the first gasifier. As a result, no carbon chars, oils or tars are expected to be present in the synthesis gas produced.
Owner:BIOMASS ENERGY SOLUTIONS
Popular searches
Hydrogen/synthetic gas production Combustion using gaseous and pulverulent fuel Continuous combustion chamber Combustion using liquid and pulverulent fuel Organic compound preparation Oxygen compounds preparation by reduction Hydrocarbons Steam engine plants Liquid-gas reaction processes Firebridges
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com