Patents
Literature
1741 results about "Exothermic reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An exothermic reaction is a chemical reaction that releases energy through light or heat. It is the opposite of an endothermic reaction. Expressed in a chemical equation: reactants → products + energy. Exothermic Reaction means "exo" (derived from the greek word: "έξω", literally translated to "out") meaning releases and "thermic" means heat. So the reaction in which there is release of heat with or without light is called exothermic reaction.
Method and apparatus for repair of wells utilizing meltable repair materials and exothermic reactants as heating agents
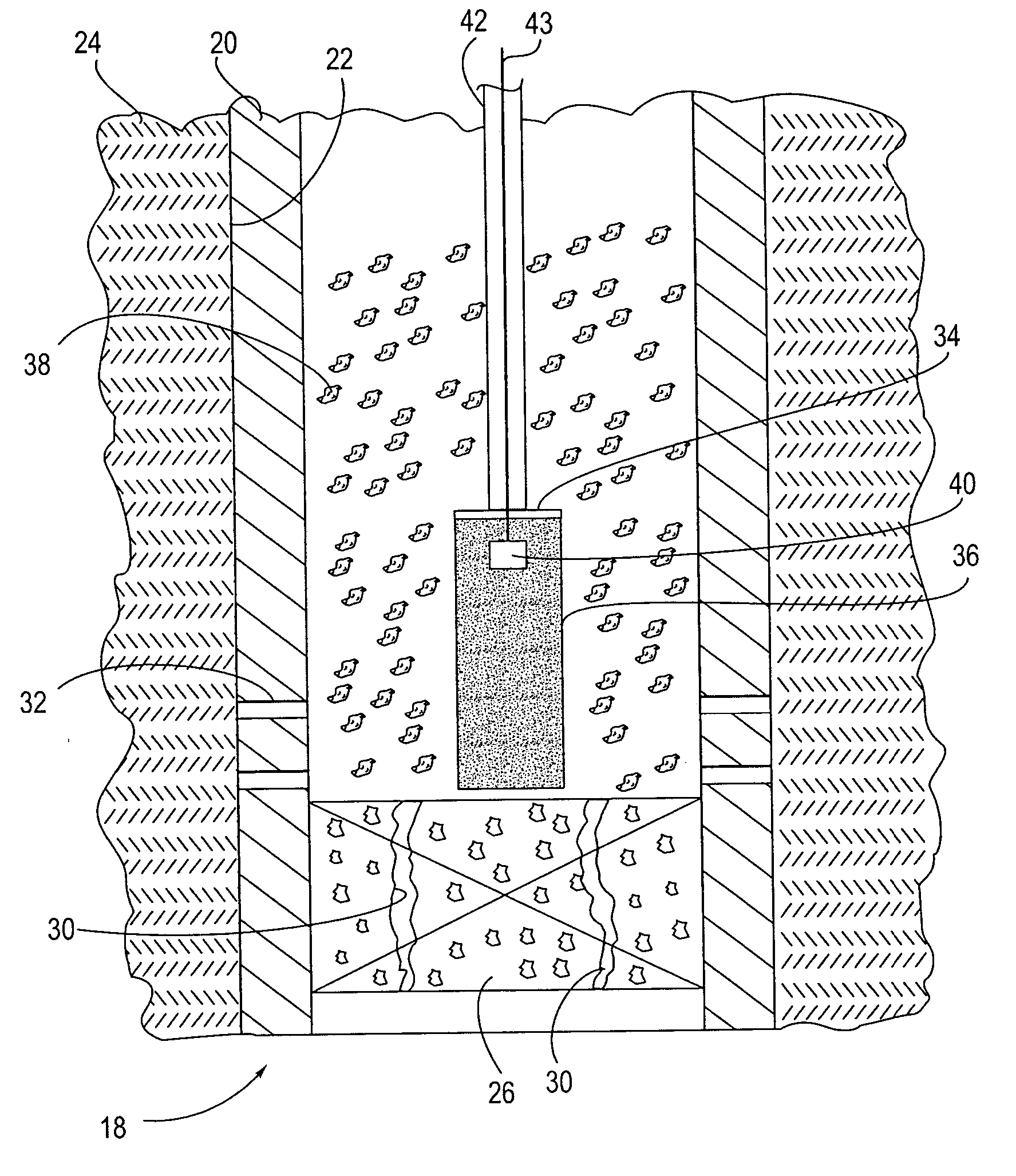
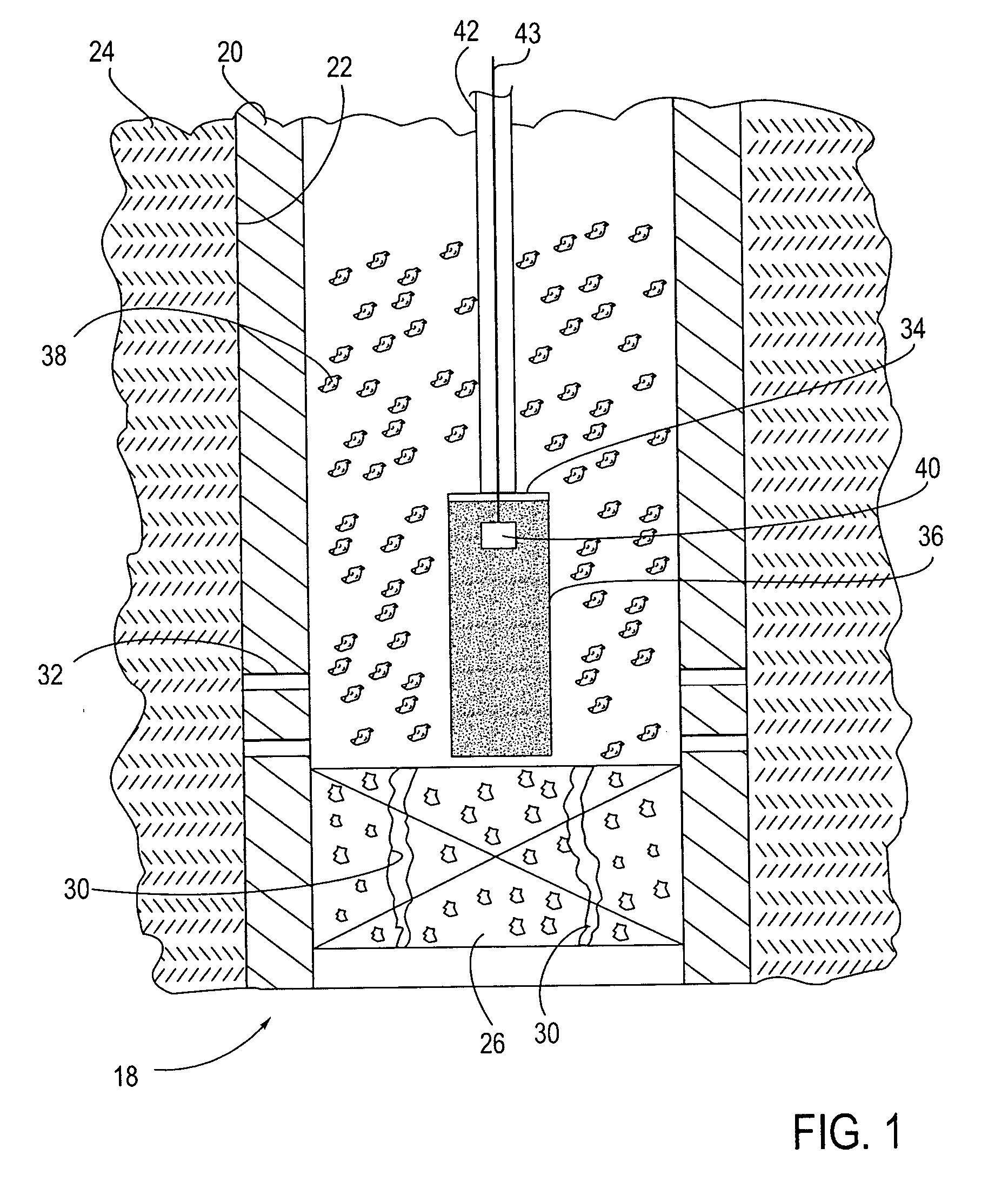
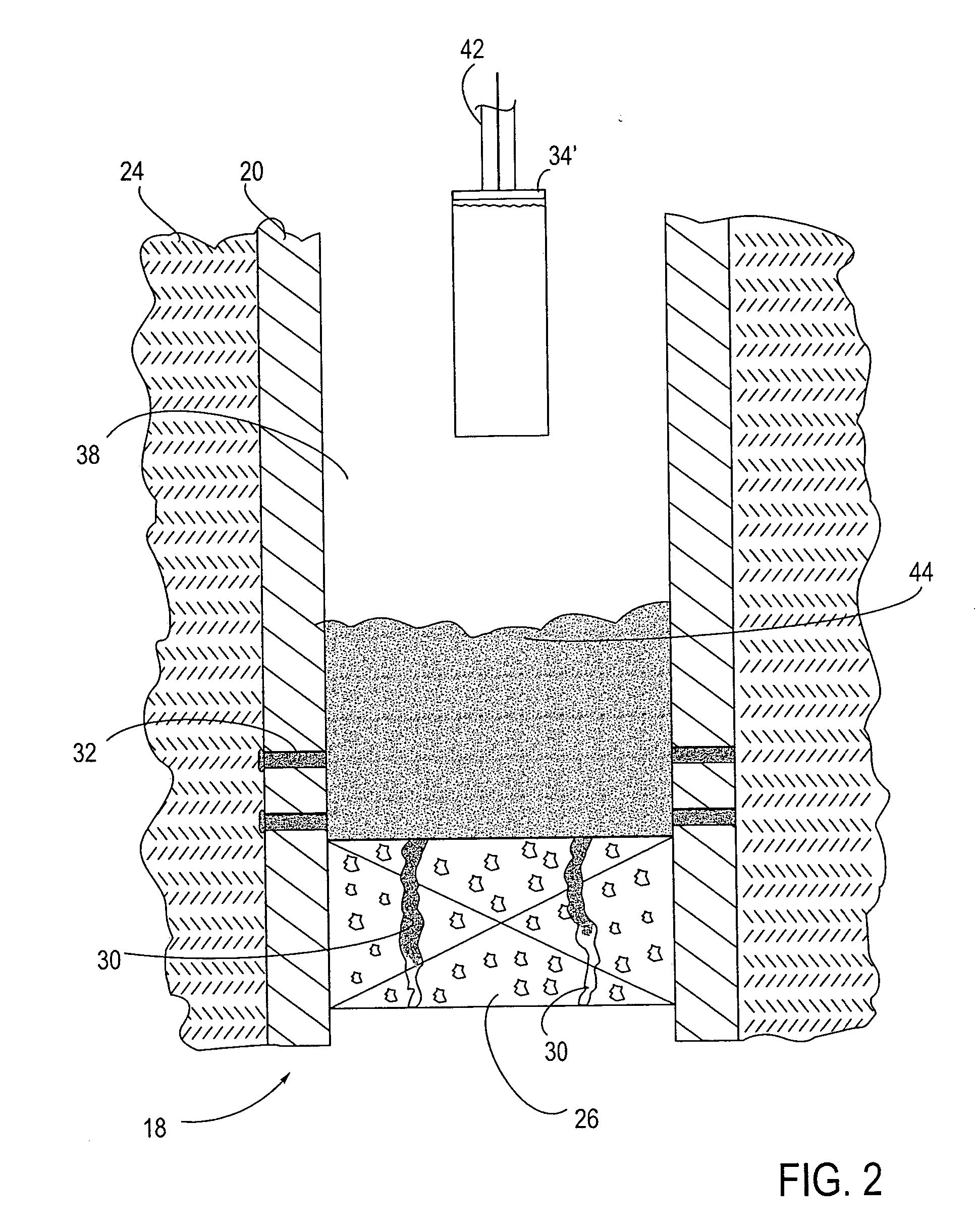
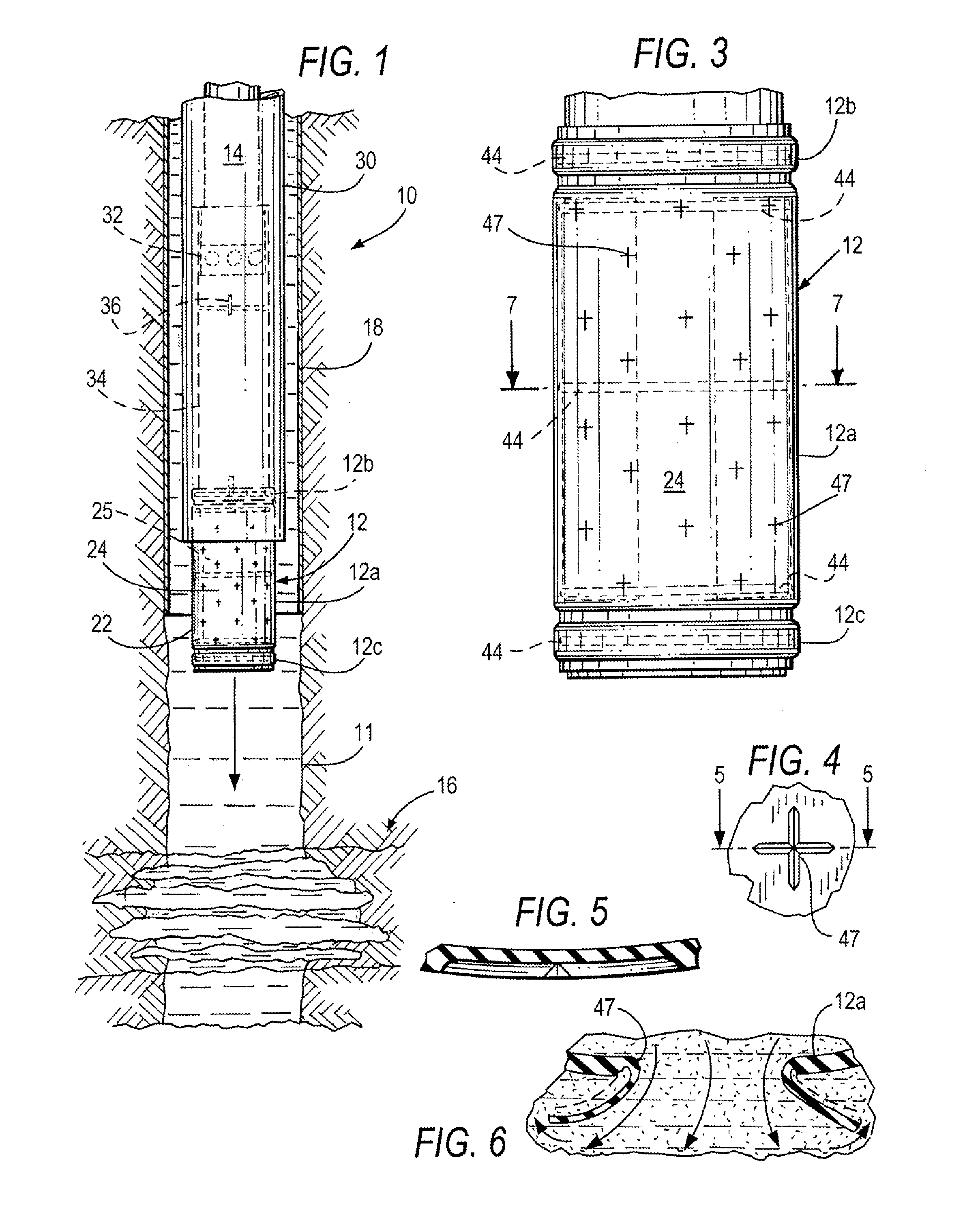
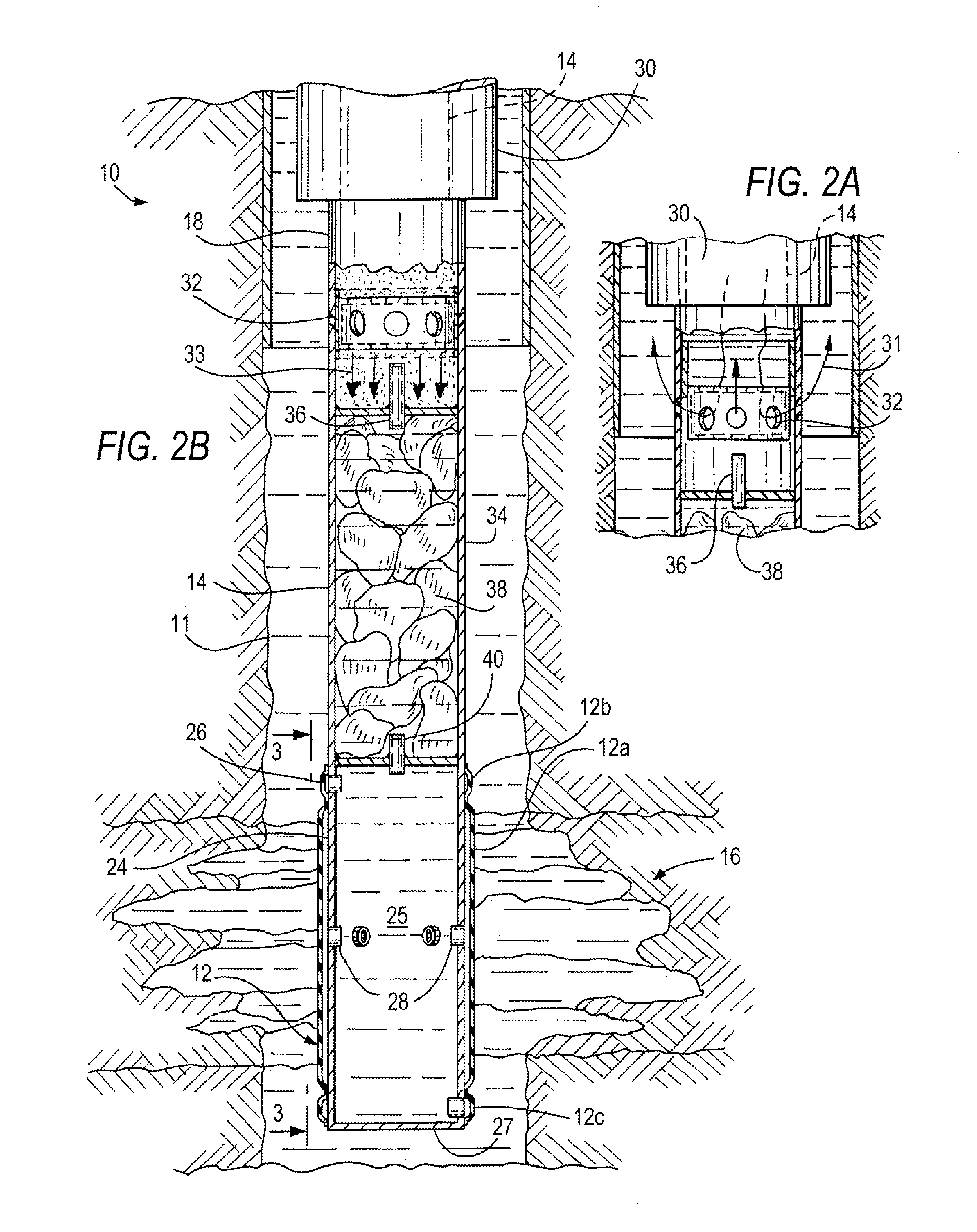
A method and apparatus are described for creating a fluid seal in a subterranean well structure having a fluid seal defect. The method comprises introducing a meltable repair material proximate a structure in a subterranean well which has a fluid seal defect or enhanced seal capacity is required or it is desired to temporarily or permanently hydraulically isolate a portion the well or strengthen the structural integrity of well tubulars or tubular hangers. Exothermic reactant materials are located proximate the meltable repair material. The exothermic reactant material is ignited or an exothermic reaction otherwise initiated which supplies heat to and melts the meltable repair material into a molten mass. The molten mass flows and solidifies across the structure and the fluid seal defect to effect a fluid seal in the subterranean well structure or the structural integrity is enhanced. Examples of preferred exothermic reactant materials include thermite, thermate, fusible chemical reactants such as ammonium chloride and sodium nitrate, and oxidizers and accompanying hydrocarbon based fuels. Examples of preferred meltable repair materials include solder or brazing materials and eutectic metals which expand upon cooling and solidifying from a molten state.
Owner:CHEVROU USA INC
Hydrogen production from carbonaceous material
InactiveUS6790430B1Avoid the needCalcium/strontium/barium carbonatesGas turbine plantsCalcinationExothermic reaction
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE +1
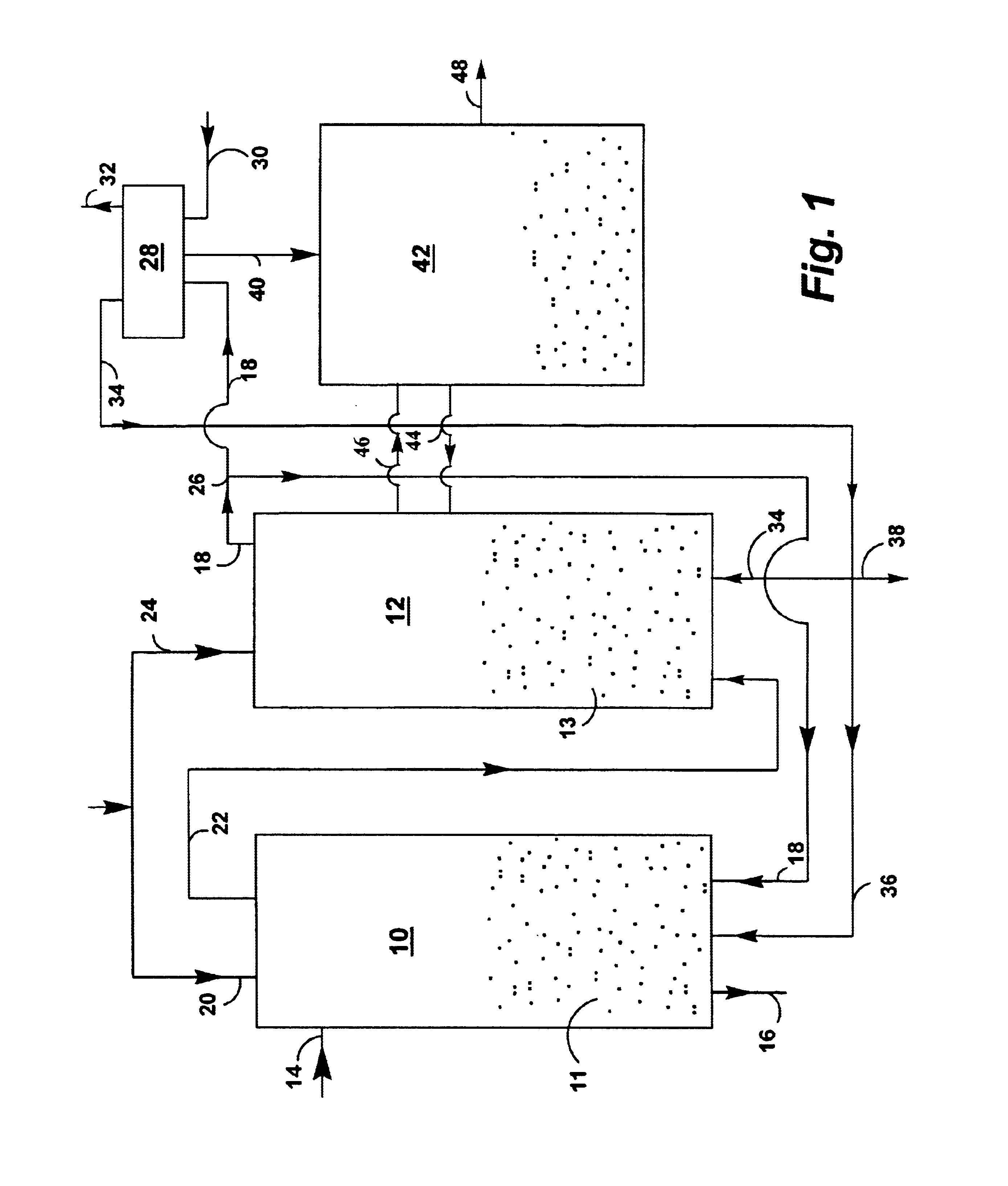
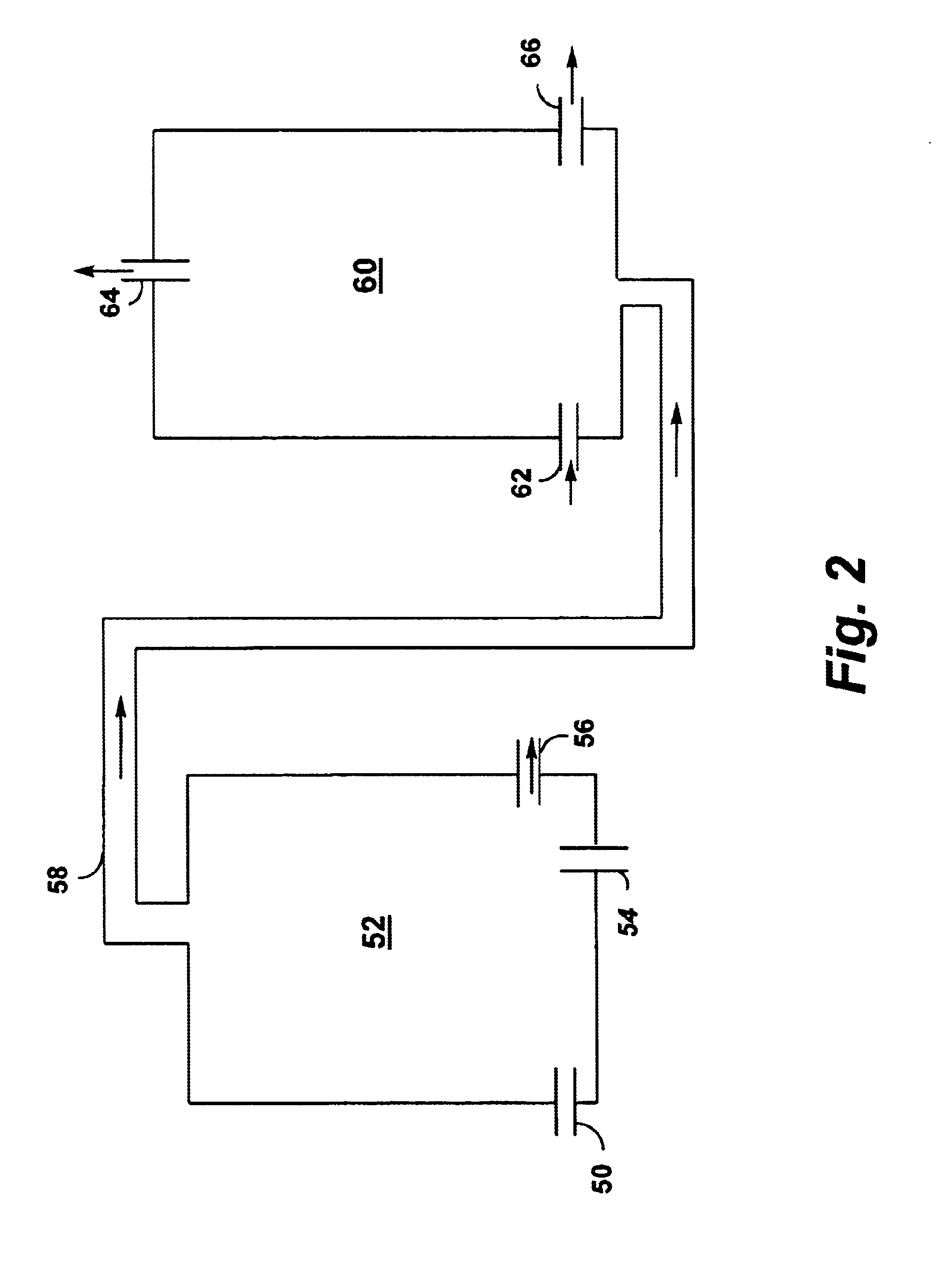
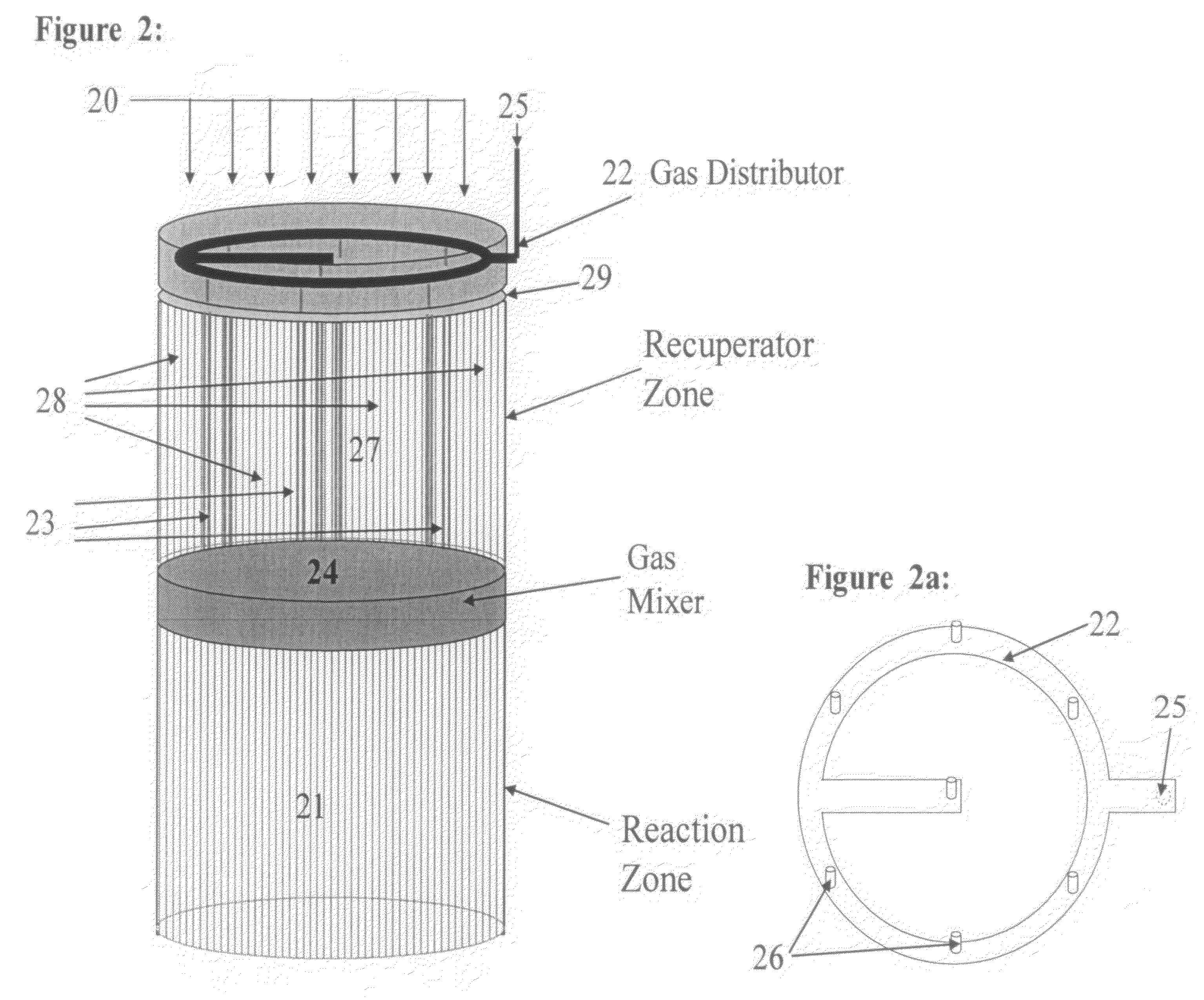
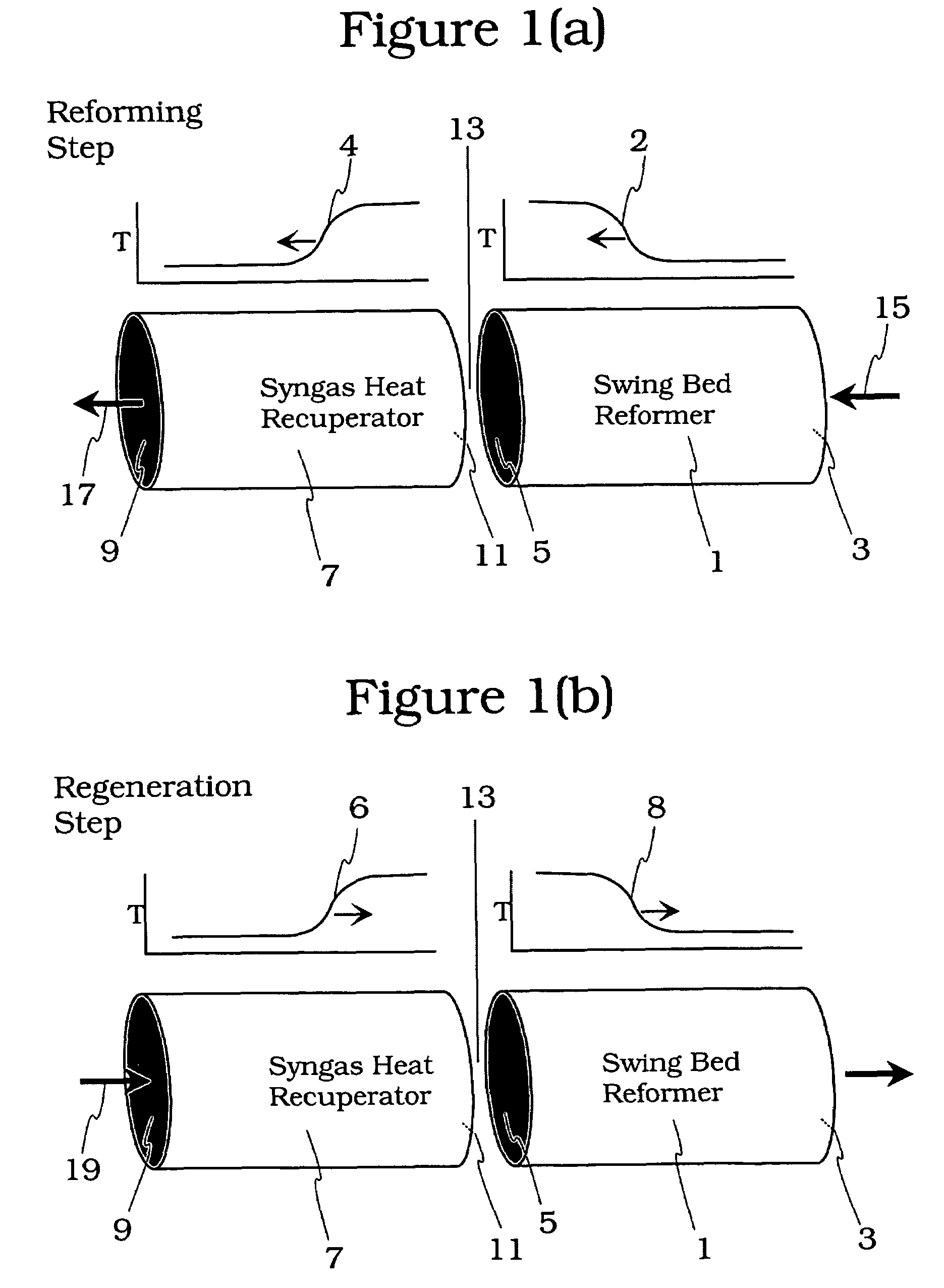
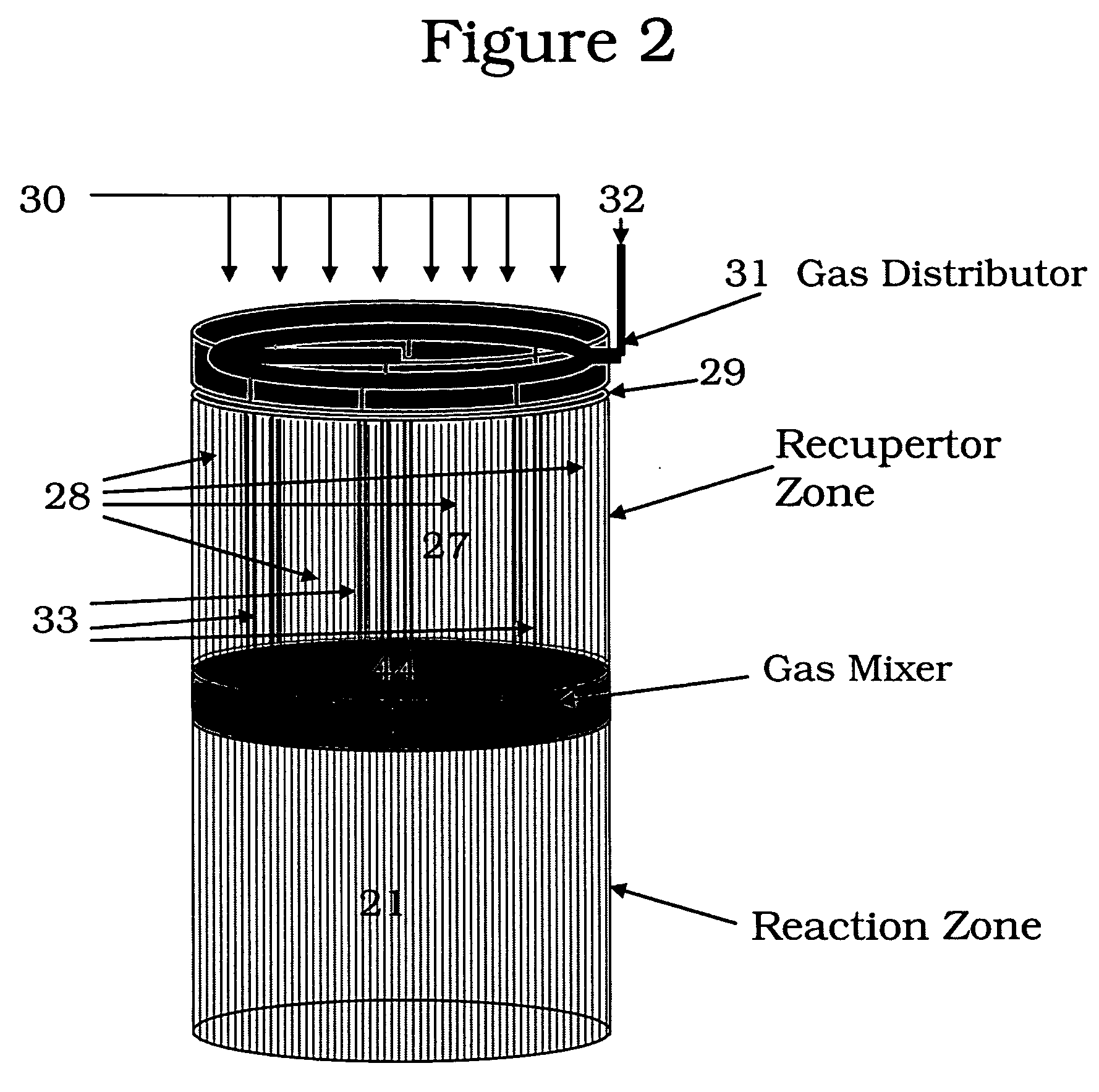
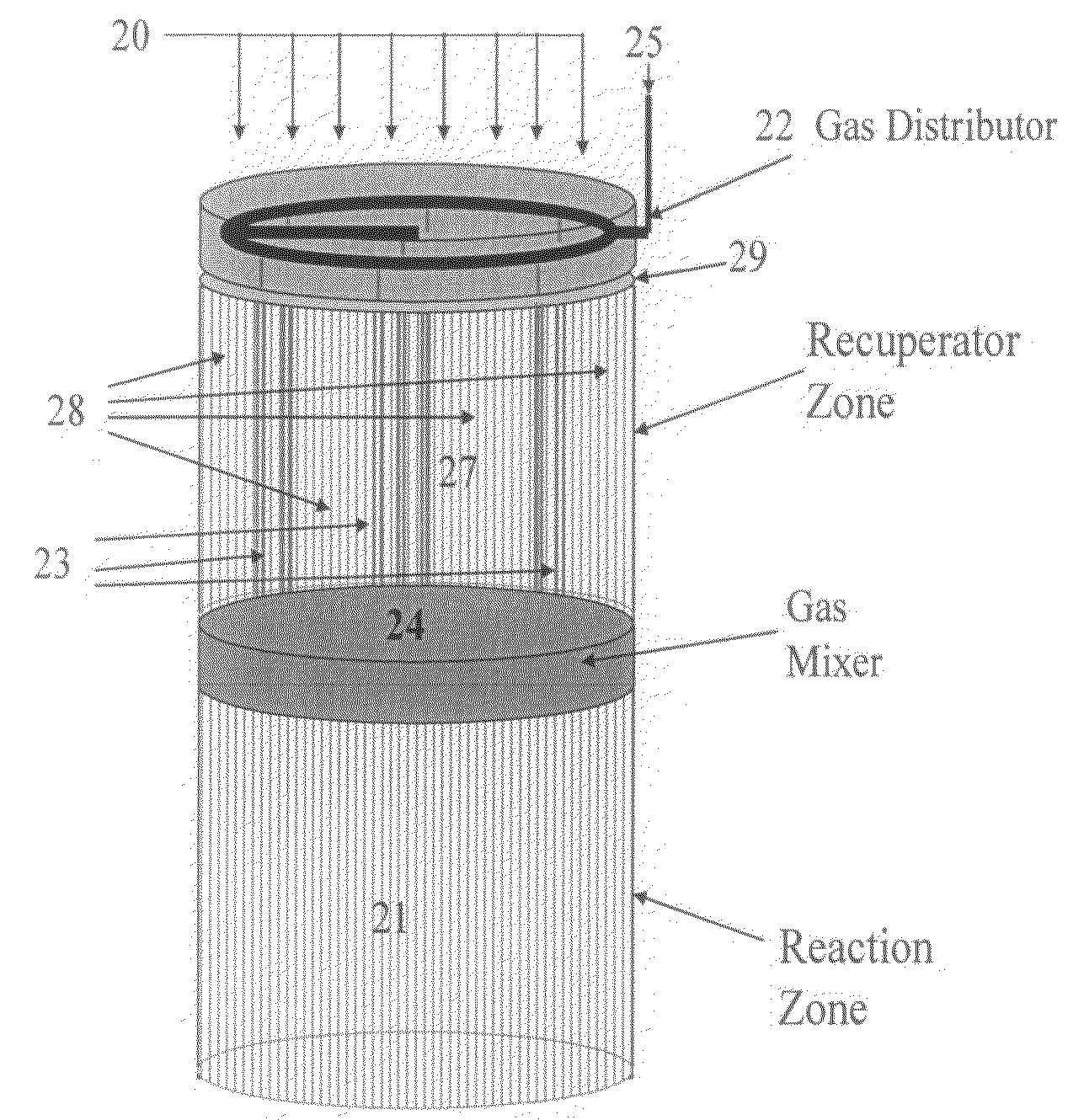
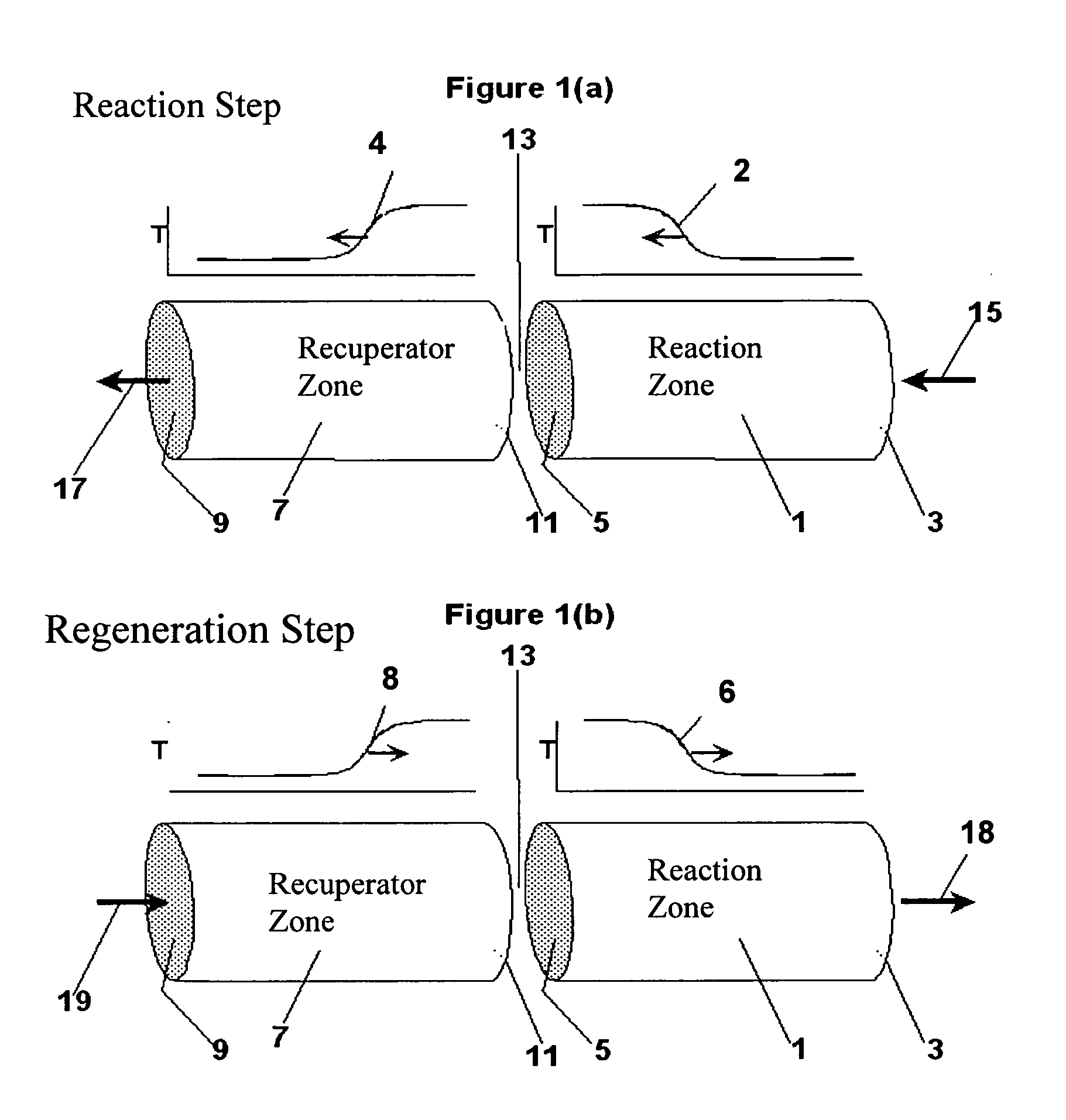
Controlled combustion for regenerative reactors with mixer/flow distributor
ActiveUS7815873B2Efficient transferEvenly distributedHydrocarbon by dehydrogenationFlow mixersReactor systemCombustion
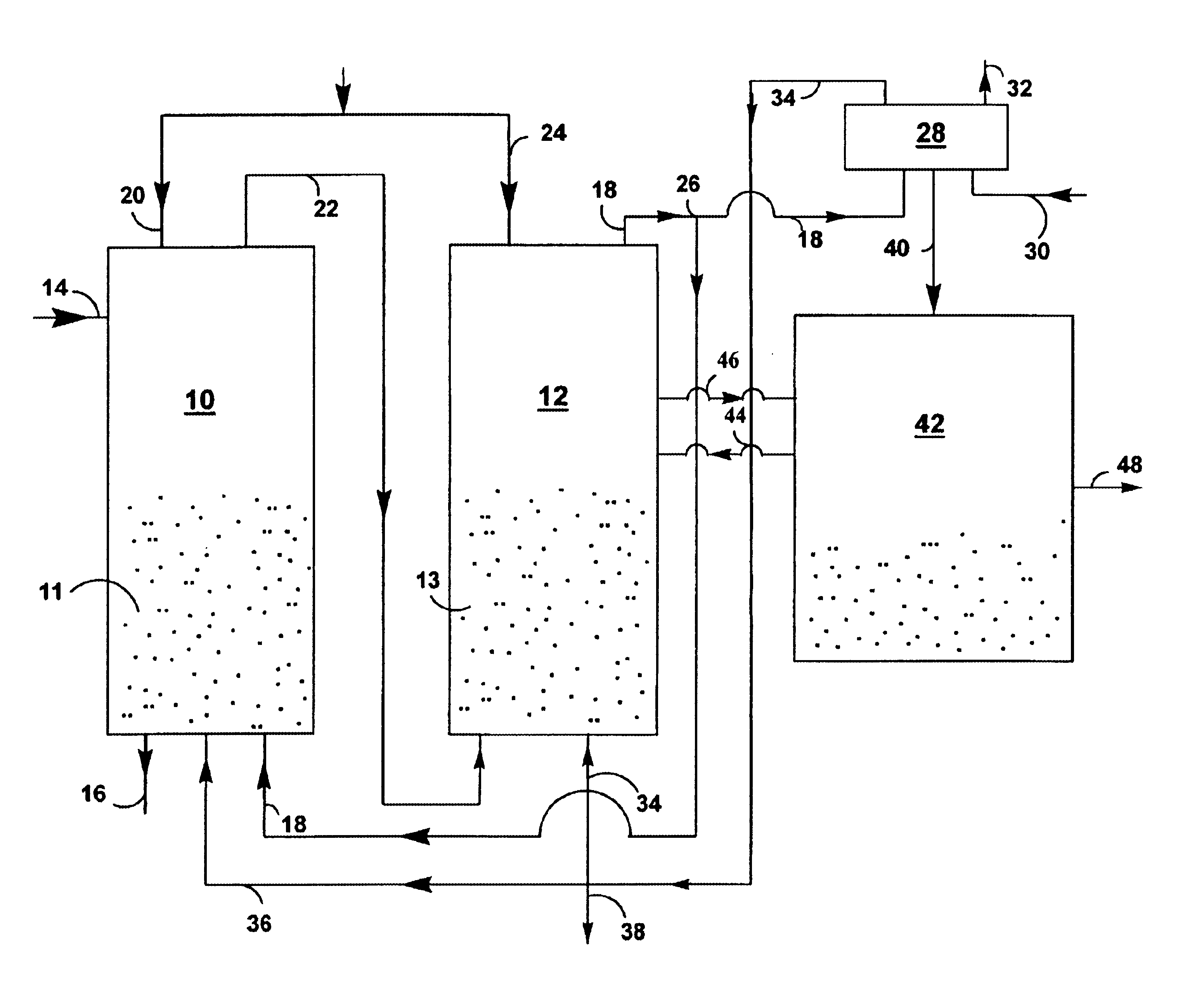
The overall efficiency of a regenerative bed reverse flow reactor system is increased where the location of the exothermic reaction used for regeneration is suitably controlled. The present invention provides a method and apparatus for controlling the combustion to improve the thermal efficiency of bed regeneration in a cyclic reaction / regeneration processes. The process for thermal regeneration of a regenerative reactor bed entails(a) supplying the first reactant through a first channel means in a first regenerative bed and supplying at least a second reactant through a second channel means in the first regenerative bed,(b) combining said first and second reactants by a gas mixing means situated at an exit of the first regenerative bed and reacting the combined gas to produce a heated reaction product,(c) passing the heated reaction product through a second regenerative bed thereby transferring heat from the reaction product to the second regenerative bed.
Owner:EXXON RES & ENG CO
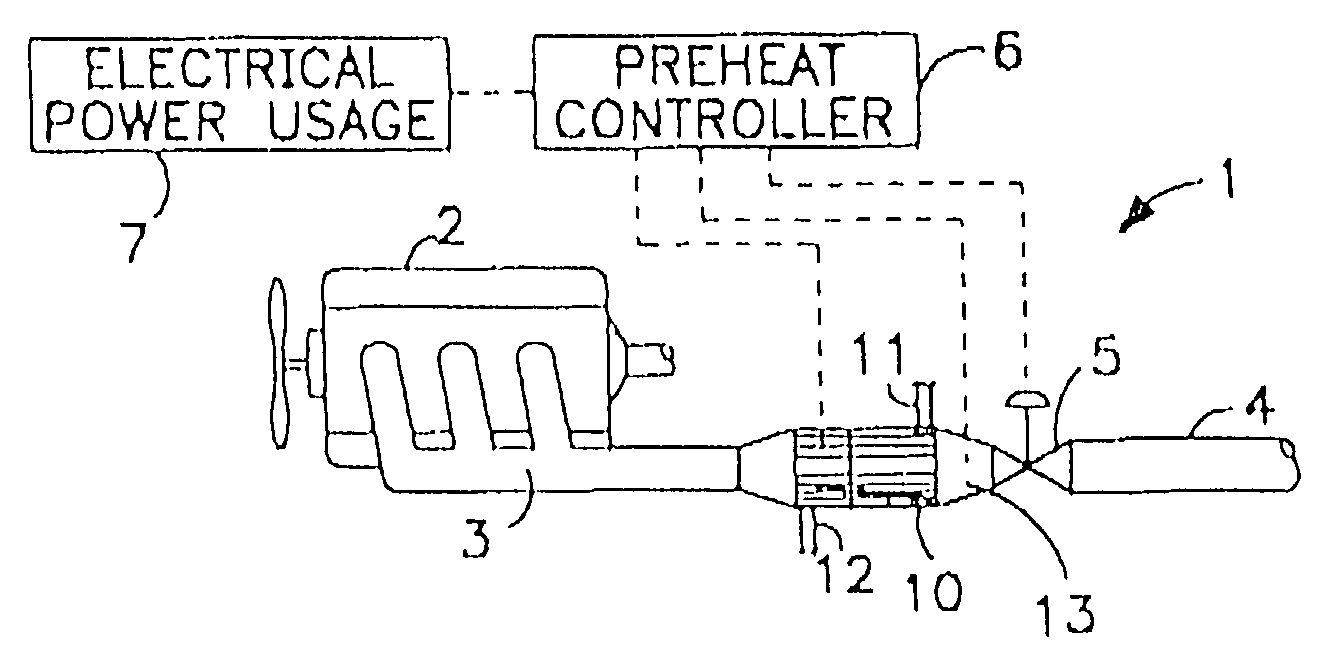
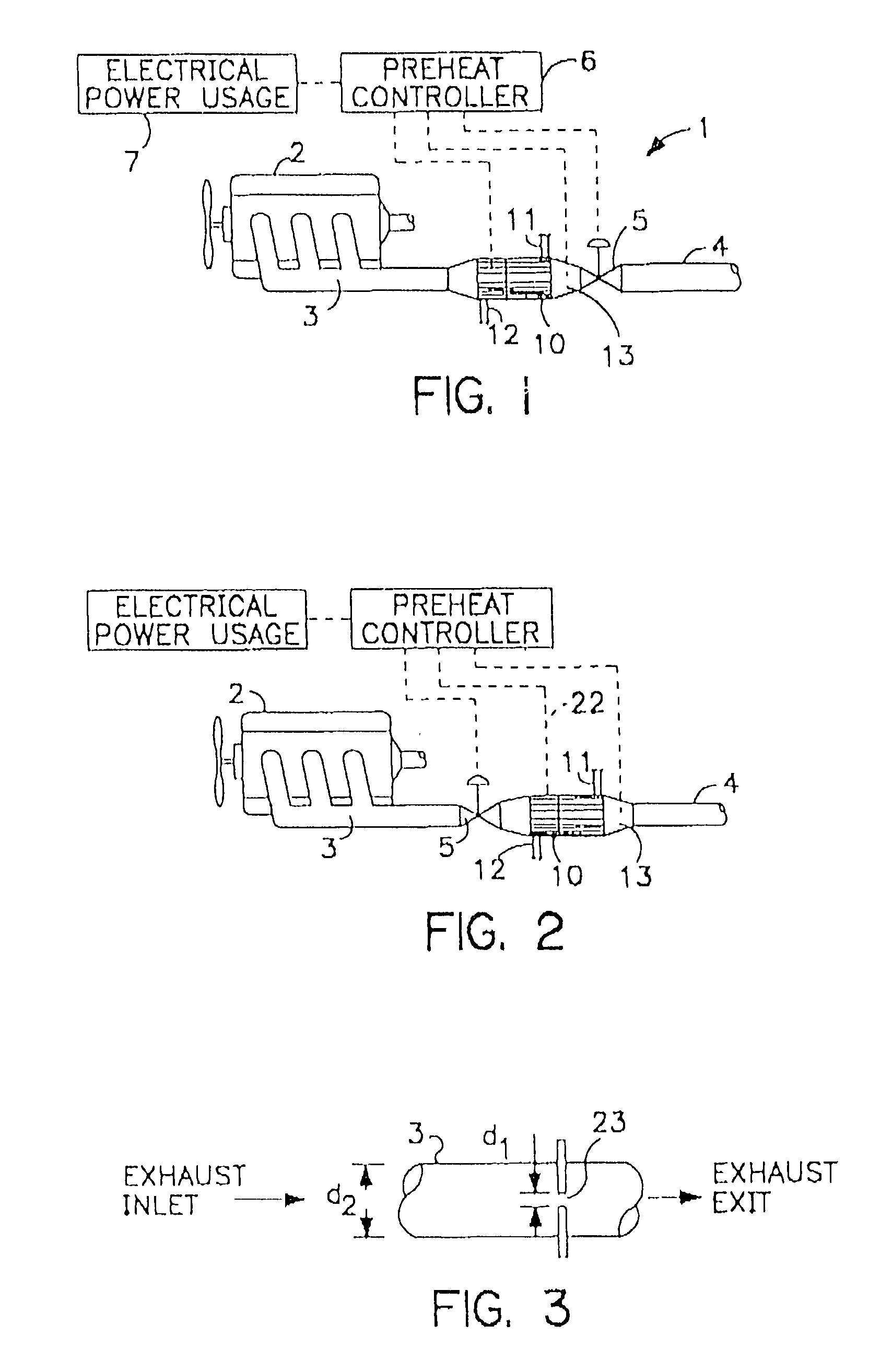
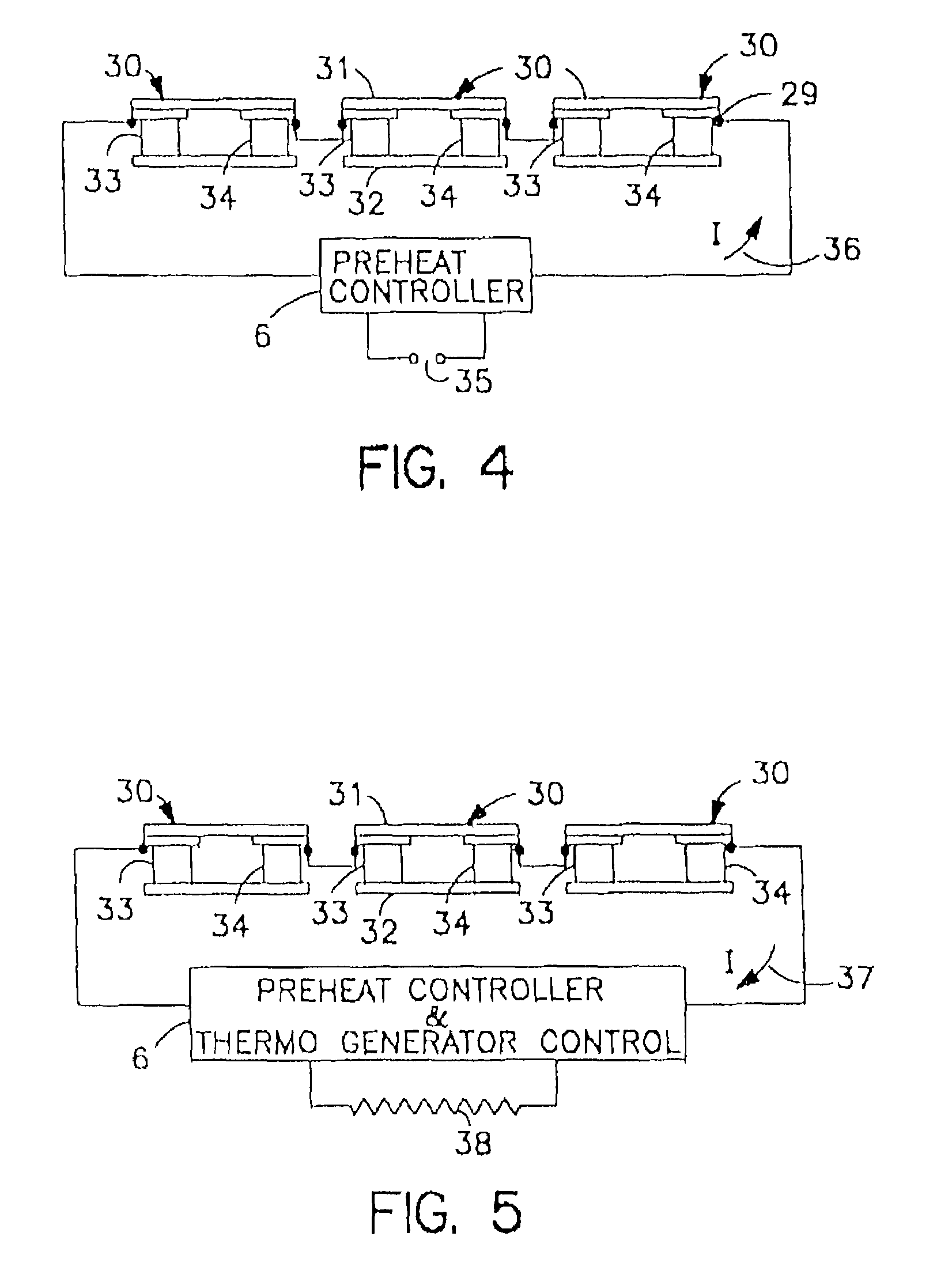
Thermoelectric catalytic power generator with preheat
InactiveUS6986247B1New pollutionMeet the test requirementsBatteries circuit arrangementsFuel cell heat exchangePollutant emissionsEngineering
An automobile catalytic converter that utilizes the energy of the exothermic reactions that take place in the catalysis substrate to produce electrical energy with a thermoelectric generator. On vehicle cold start, the thermoelectric generator is used as a heat pump to heat the catalyst substrate to reduce the time to catalyst light-off. In this way, the catalytic converter comes up to operating temperature more rapidly, reducing the amount of pollutant emissions at vehicle start-up.
Owner:PARISE RONALD J
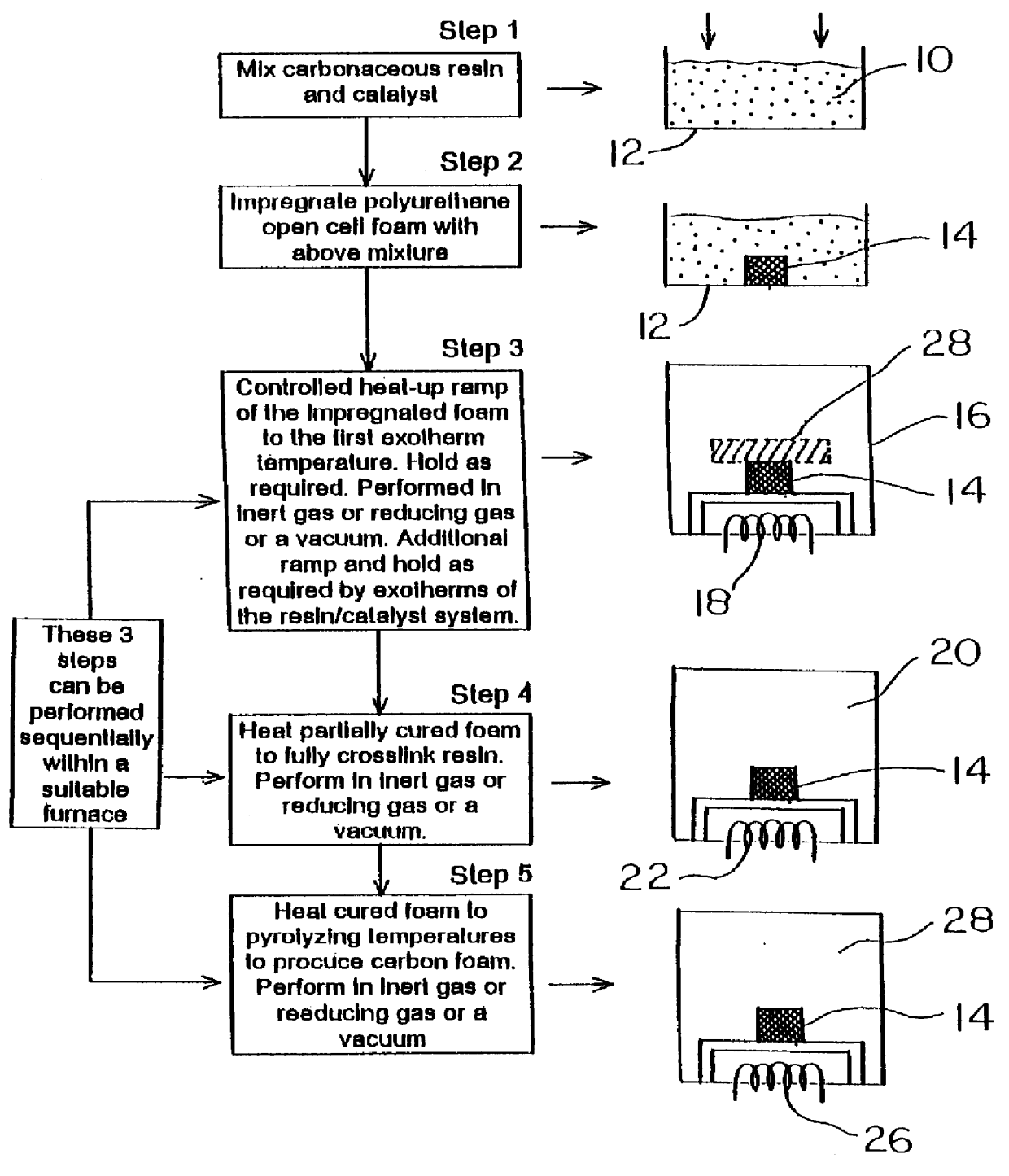
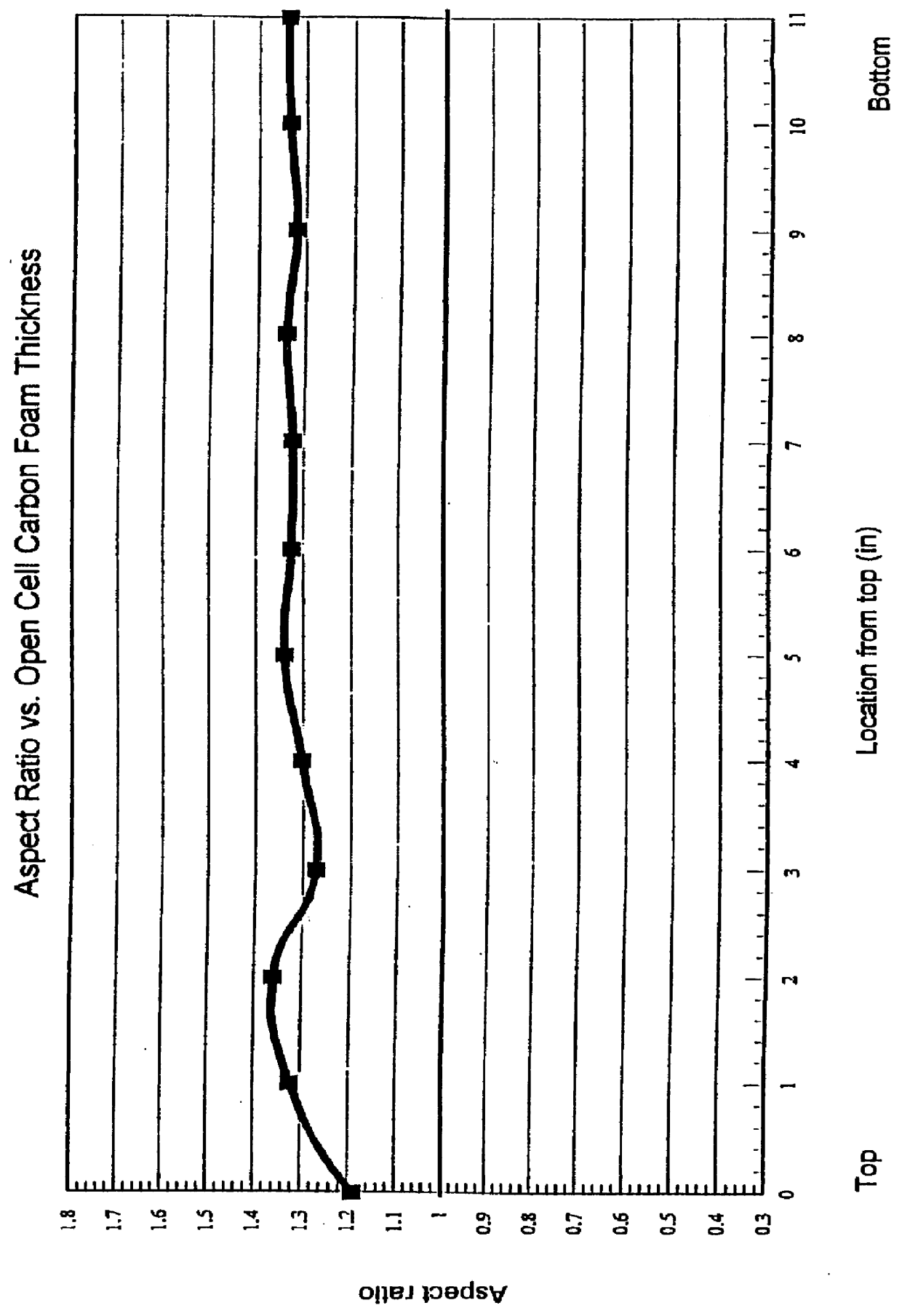
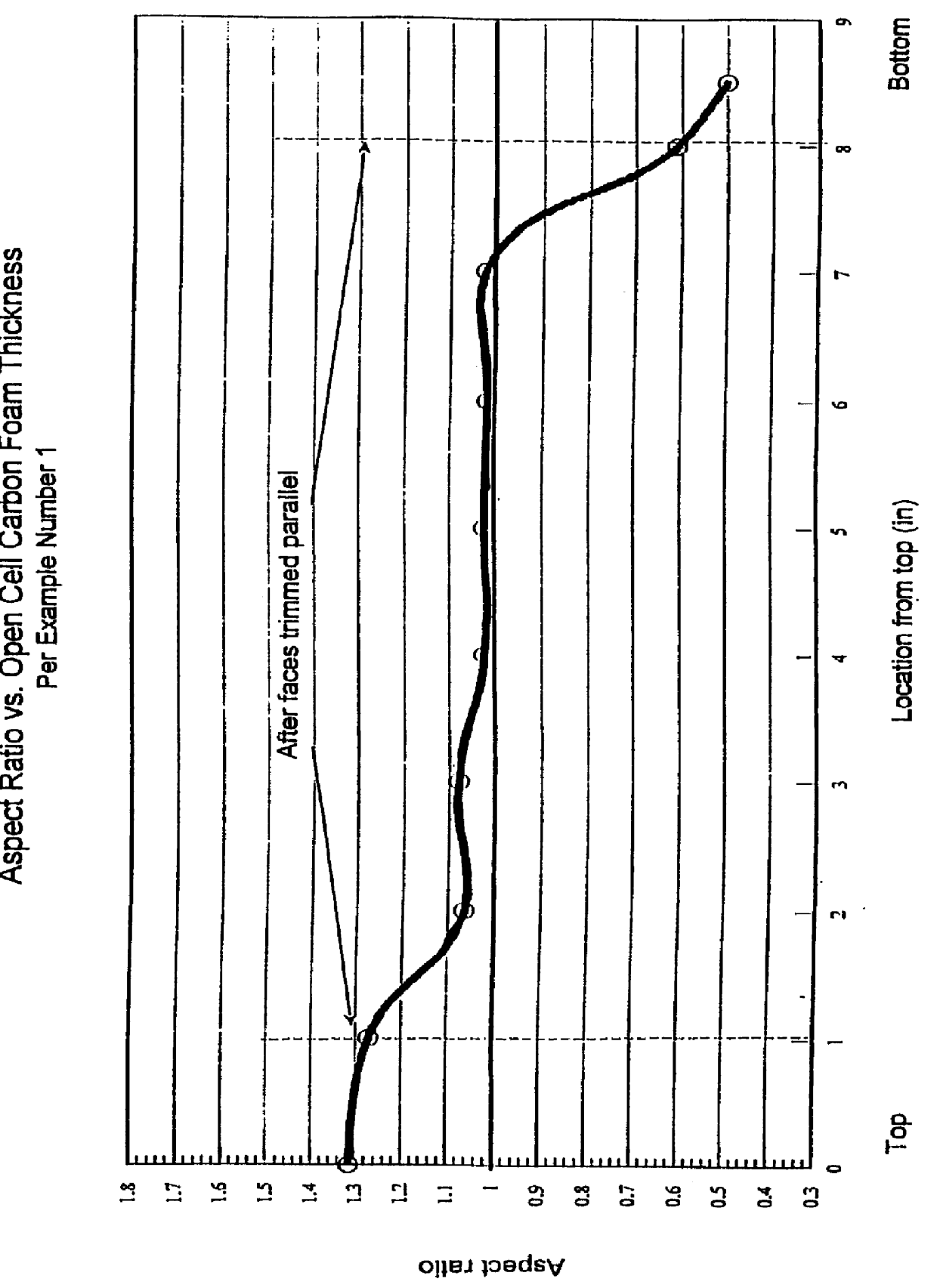
Method for producing controlled aspect ratio reticulated carbon foam and the resultant foam
A method of manufacturing and the resultant reticulated open cell carbon foam with a controlled aspect ratio between 0.6 to 1.2 throughout the body of the foam billet by controlling the exothermic reaction of the resin system below the decomposition temperature of the foam.
Owner:ULSTREETCARET
Portable beverage bottle heaters and coolers
InactiveUS20080178865A1Less-costly to implementExothermal chemical reaction heat productionDomestic cooling apparatusEngineeringBottle
A portable temperature changing apparatus for containers includes a cylindrical annular moisture-proof pouch divided into a first compartment filled with a liquid and a second separate compartment holding a solid material capable of producing an endothermic or exothermic reaction upon mixing with the liquid. The seal separating the two separate compartments can be ruptured partially, when desired, enabling communication of the liquid with the solid to activate the temperature altering reaction. A flexible foam insulating layer surrounds the exterior of the annular cylindrical pouch providing insulation, damage protection, and labeling.
Owner:RETTERER SHELLEY
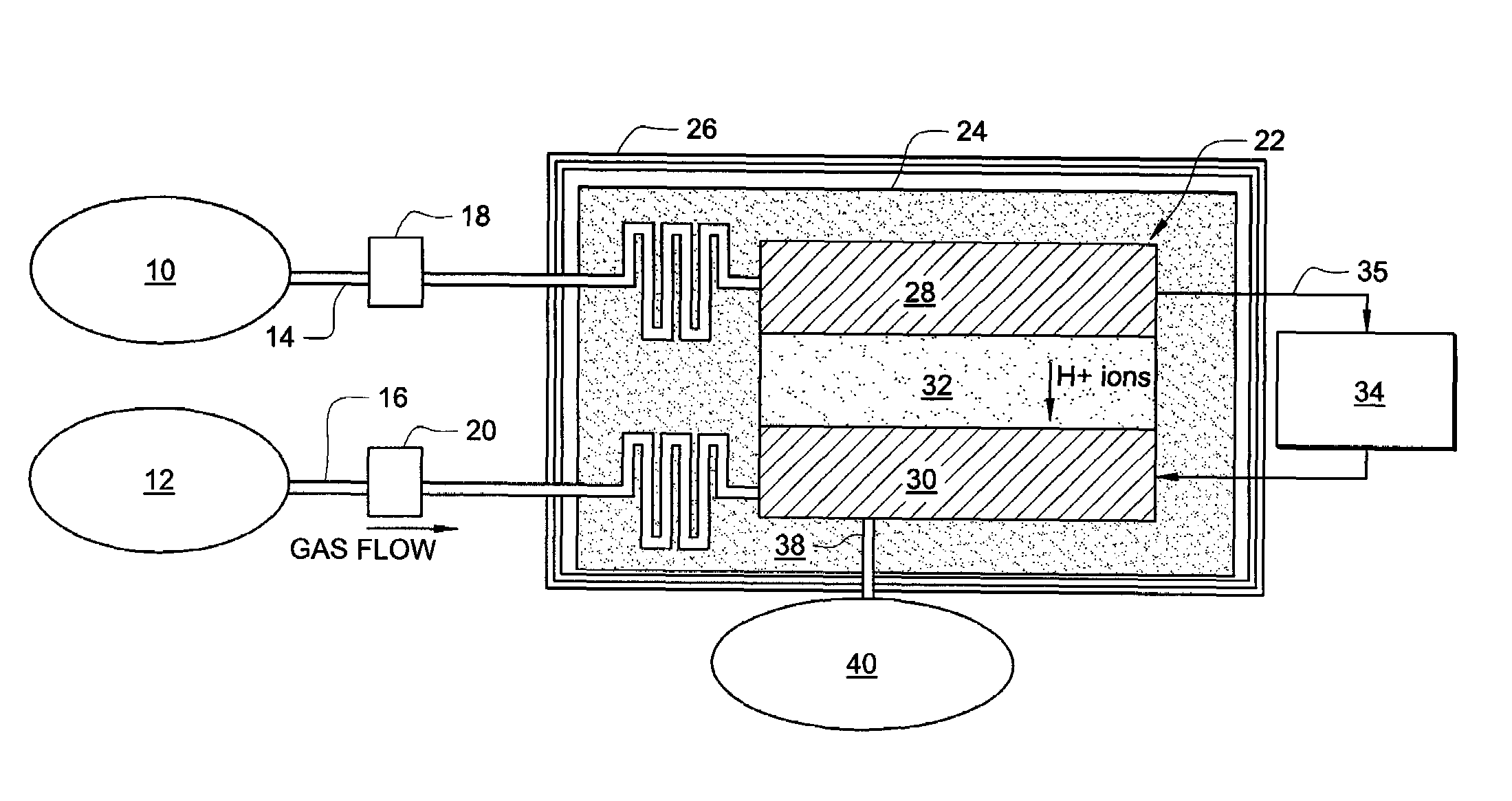
Process for producing a syngas
InactiveUS6402988B1Minimize the temperatures experienced by sealsMinimize pressure differenceCatalytic gas-gas reactionHydrogenSyngasReactor design
An exothermic reaction and an endothermic reaction are thermally combined in a reactor having at least one oxygen selective ion transport membrane that provides the exothermic reaction with oxygen from an oxygen-containing gas such as air. The thermal requirements of the endothermic reaction are satisfied by the exothermic reaction. Dependent on the reactor design employed, the exothermic and endothermic reactions may be gaseously combined.
Owner:PRAXAIR TECH INC +1
Exothermic topical delivery device
The present invention relates to an exothermic device for topically delivering an active agent comprising a liquid reservoir comprising water, a heating element comprising an oxidizable material, an oxygen-permeable outer-layer, an active agent, and a water-impermeable layer, wherein upon the rupturing of the liquid reservoir, the water contacts the heating element and the oxygen to create and exothermic reaction.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
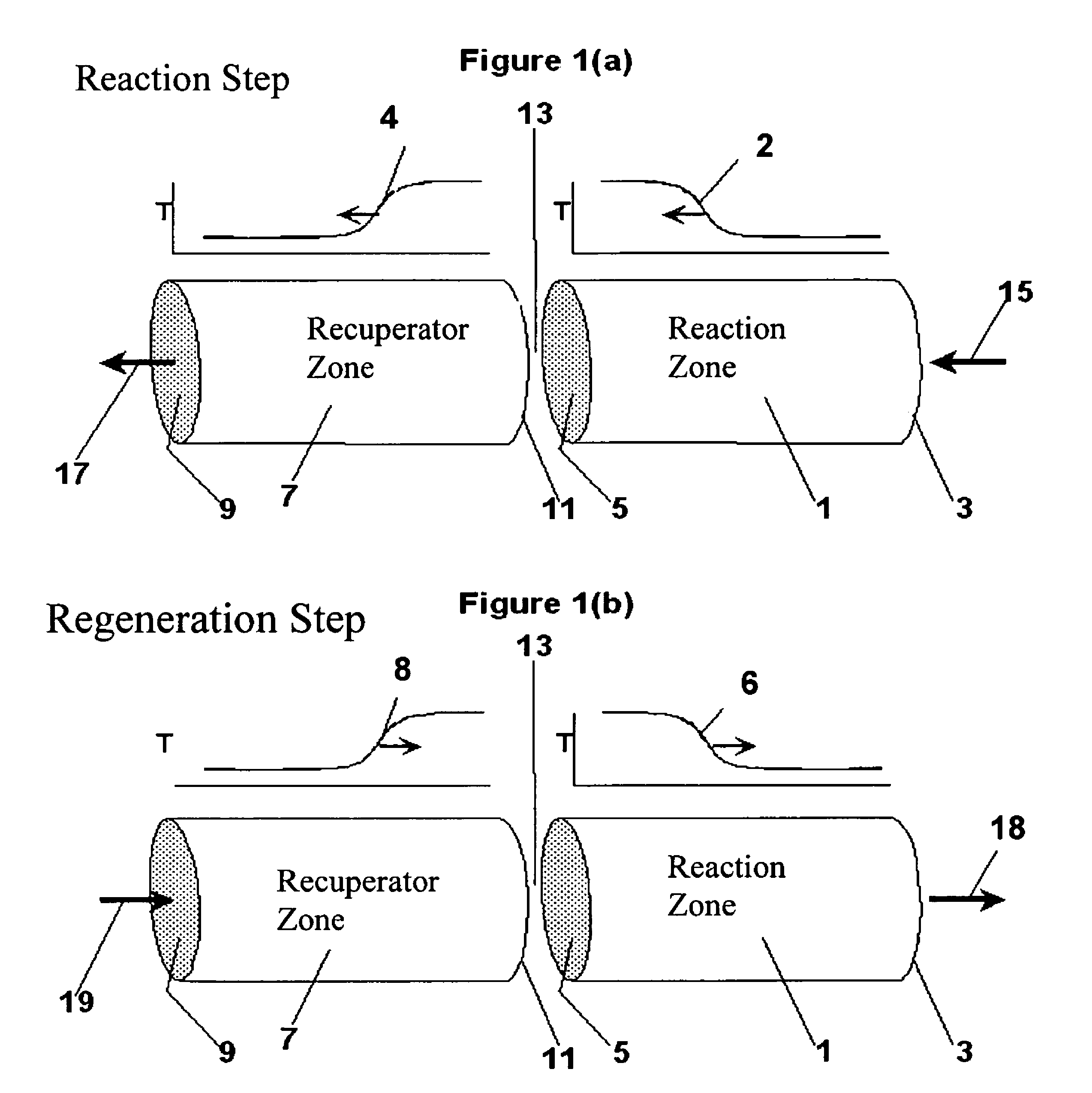
Controlled combustion for regenerative reactors
The overall efficiency of a regenerative bed reverse flow reactor system is increased where the location of the exothermic reaction used for regeneration is suitably controlled. The present invention provides a method and apparatus for controlling the combustion to improve the thermal efficiency of bed regeneration in a cyclic reaction / regeneration processes. The process for thermal regeneration of a regenerative reactor bed entails(a) supplying the first reactant through a first channel means in a first regenerative bed and supplying at least a second reactant through a second channel means in the first regenerative bed,(b) combining said first and second reactants by a gas mixing means situated at an exit of the first regenerative bed and reacting the combined gas to produce a heated reaction product,(c) passing the heated reaction product through a second regenerative bed thereby transferring heat from the reaction product to the second regenerative bed.
Owner:EXXON RES & ENG CO
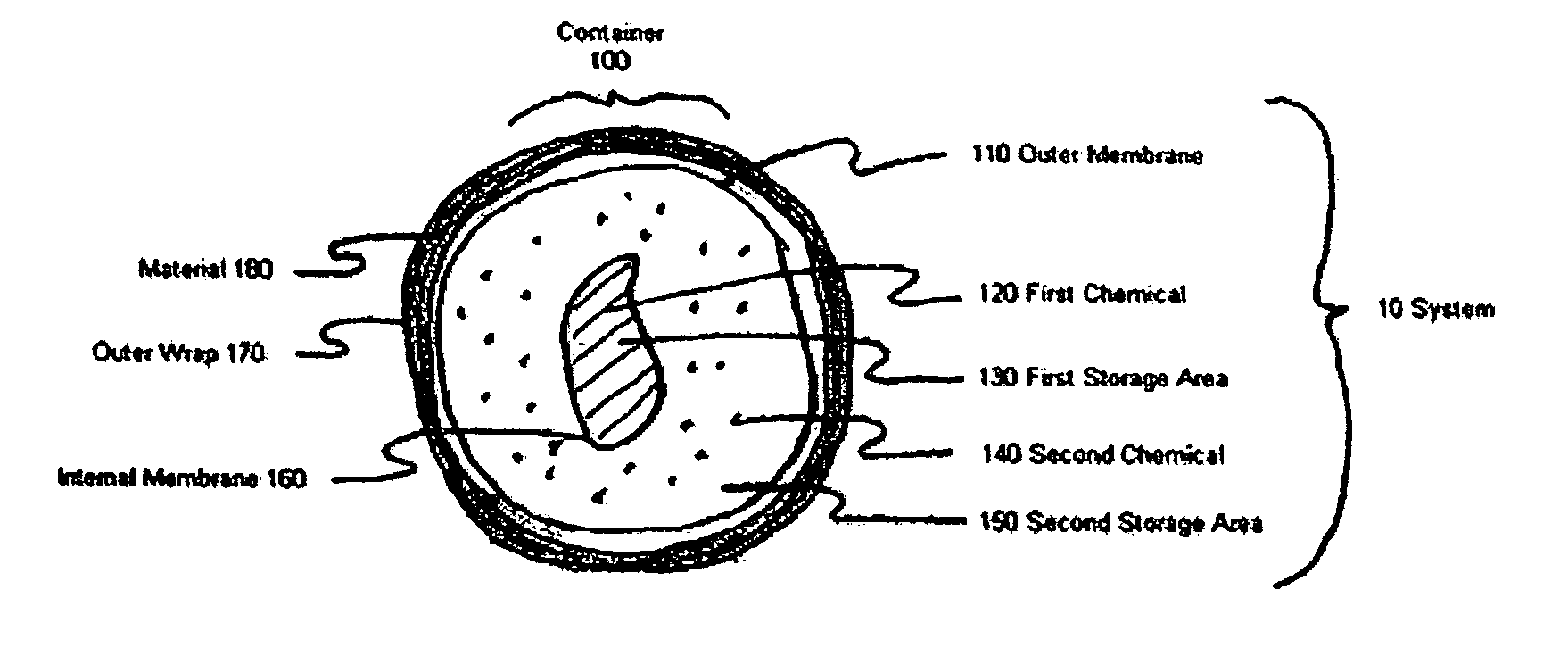
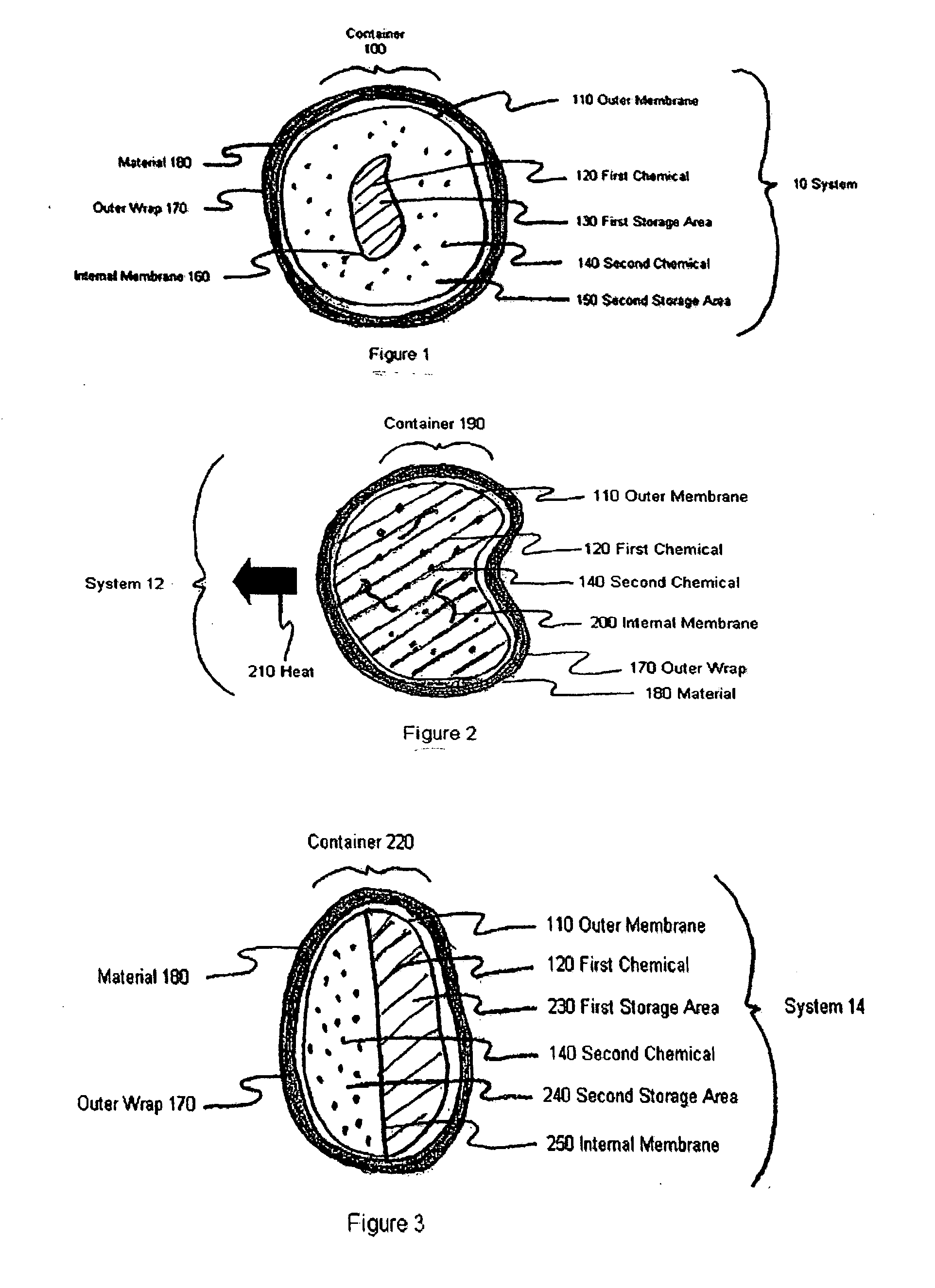
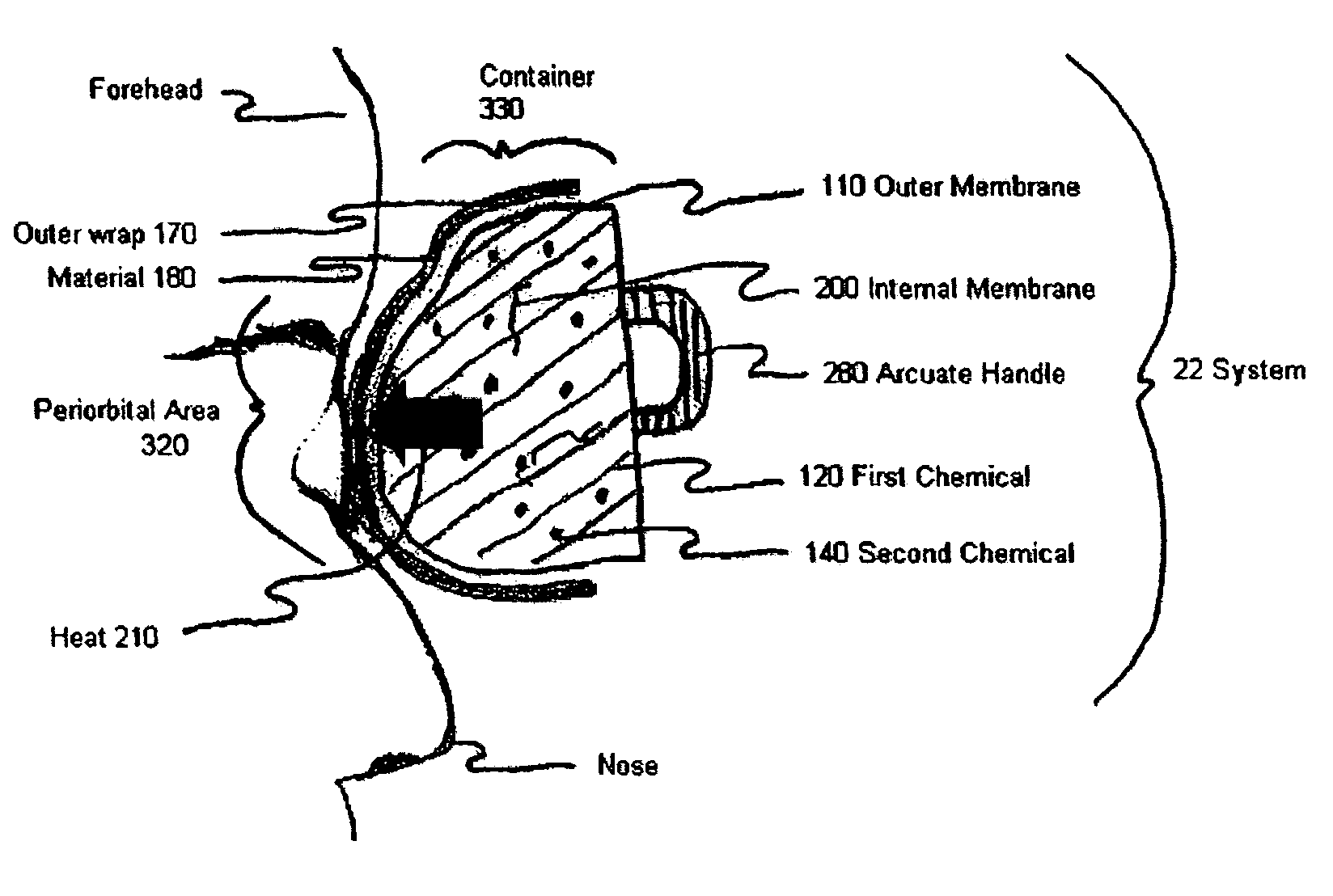
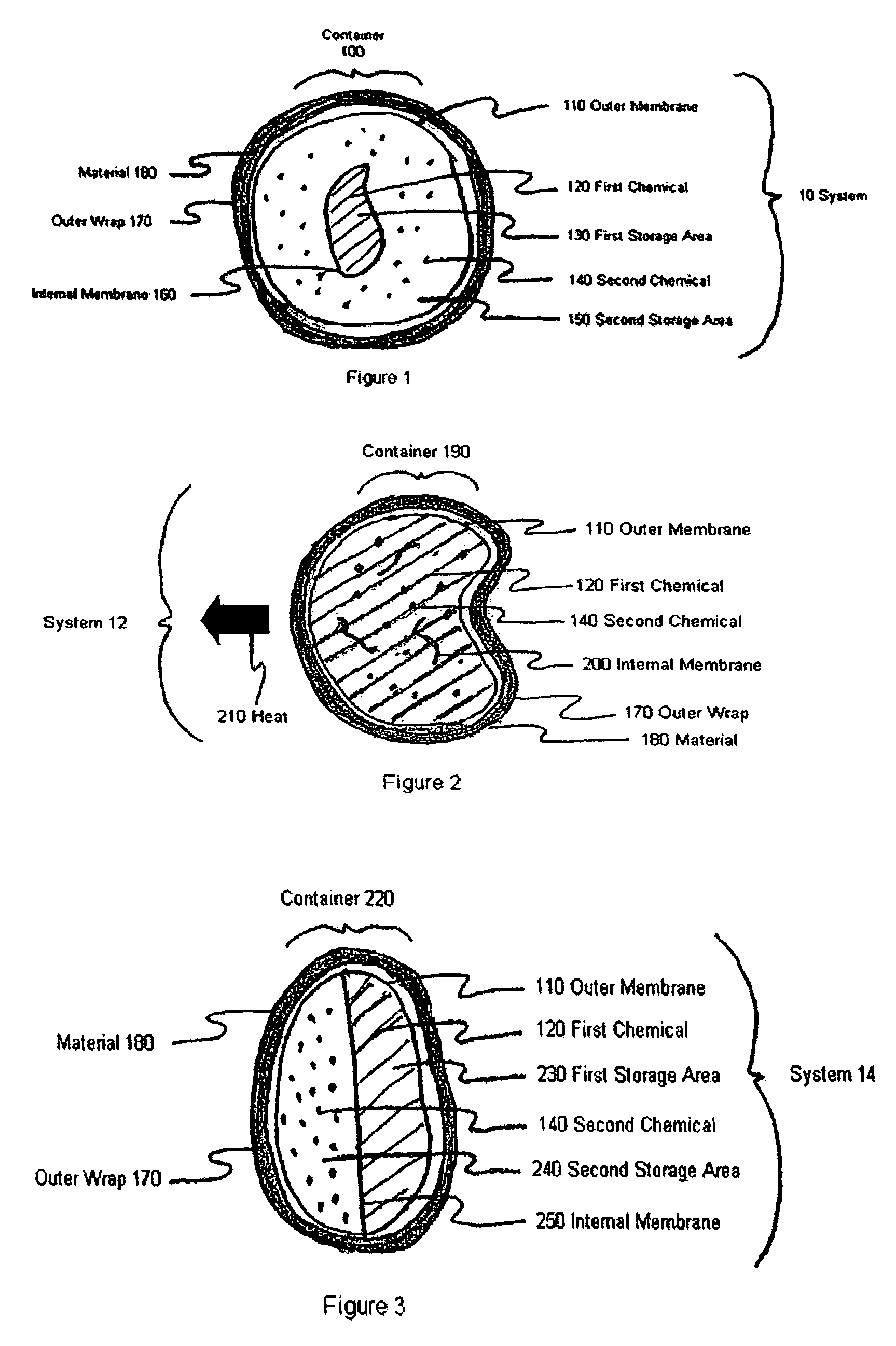
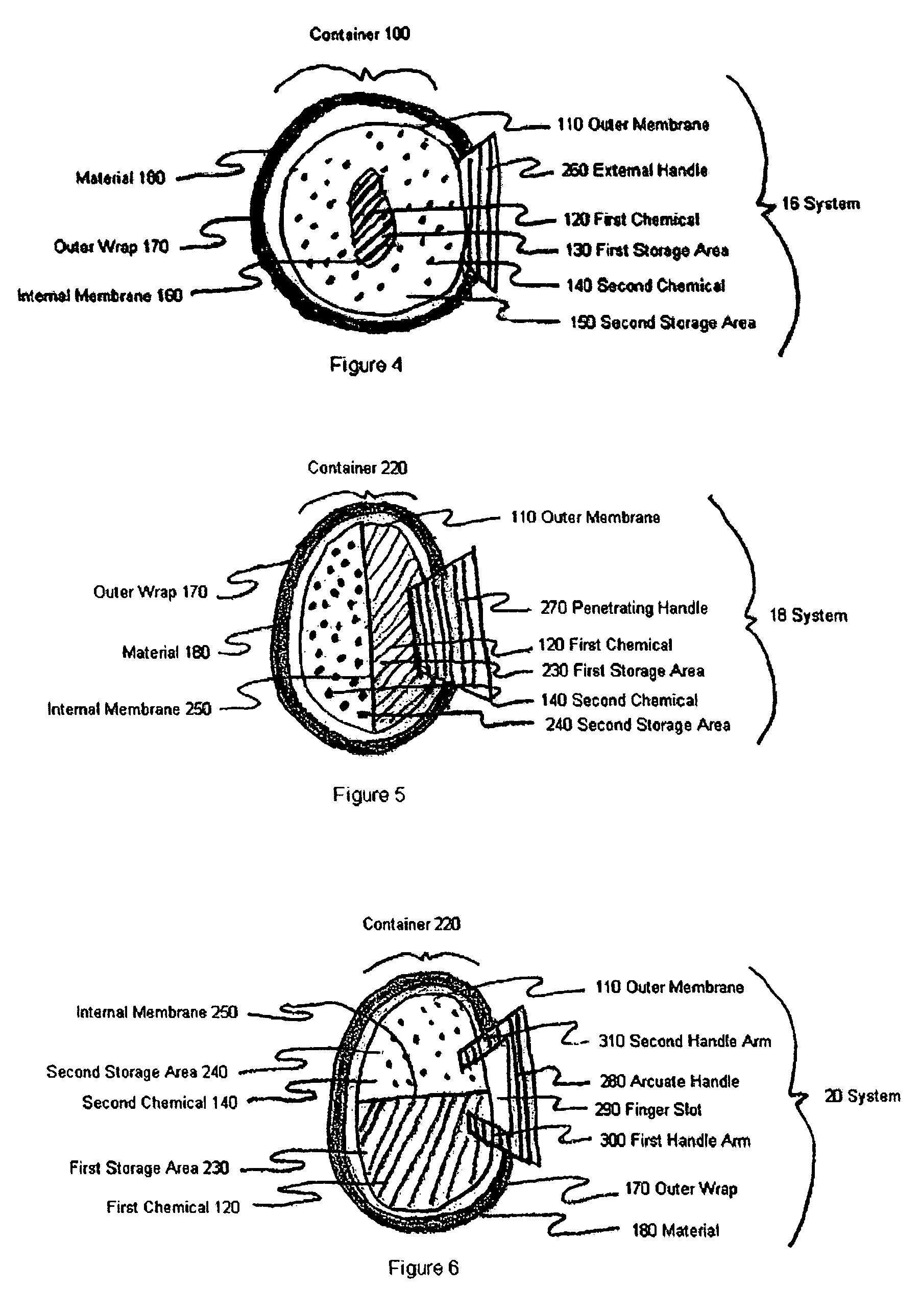
Device and method for exothermic treatment of eyelid diseases
ActiveUS20050119629A1Convenient procedurePreventing occurrence and recurrencePharmaceutical delivery mechanismMedical applicatorsDiseasePh control
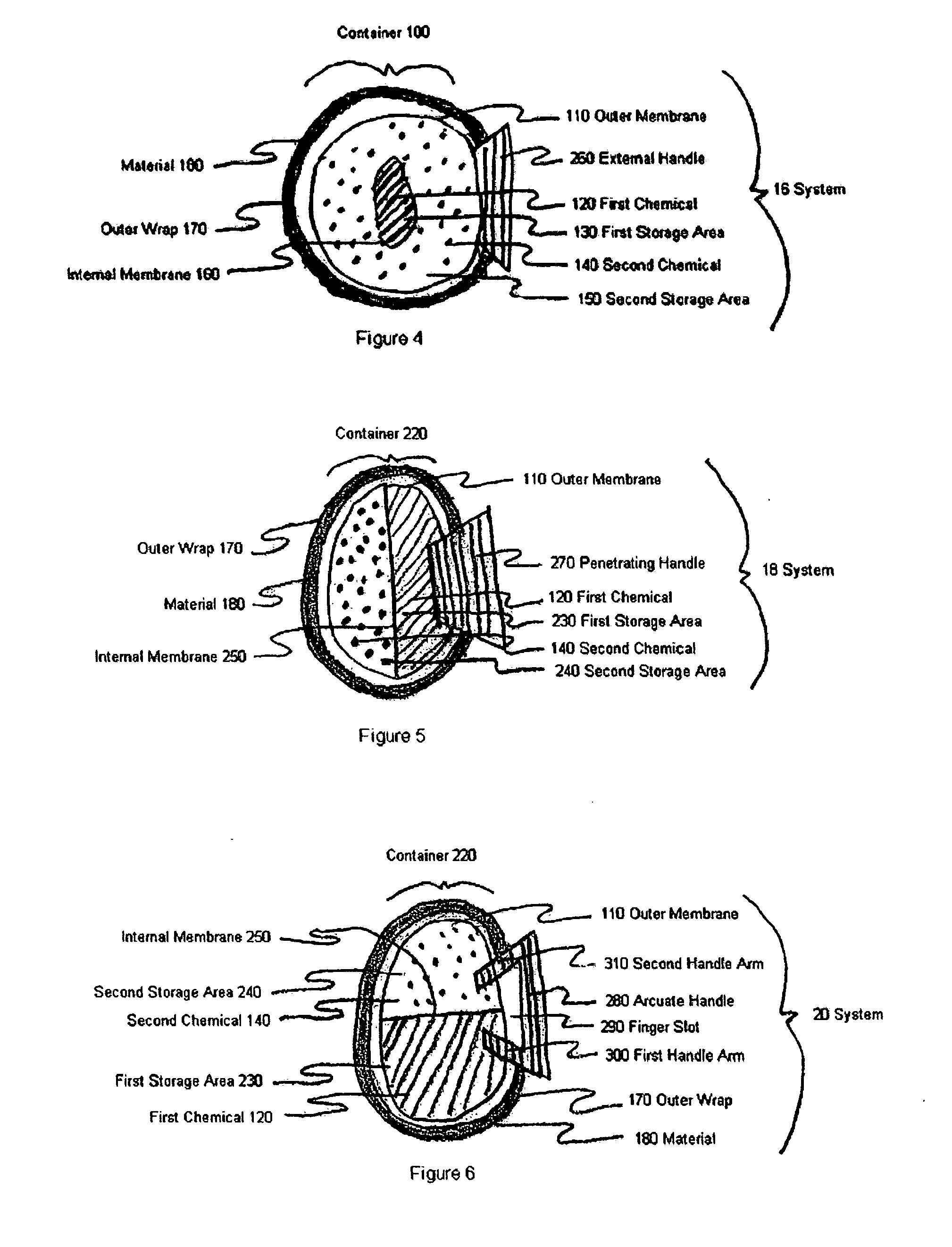
Provided herein is a pad for treating eye conditions comprising a multipart container having an impermeable outer membrane sized to fit generally within the peri-orbital region and sufficiently flexible to mold to the eye; a first chemical in a first storage area in the multipart container; a second chemical in a second storage area in the multipart container, the first and second chemicals selected to have an exothermic reaction when mixed for producing a temperature suitable for treating eye conditions, the exothermic reaction providing the suitable temperature for a period of time suitable for treating eye conditions; and an inner membrane for initially separating the first and second chemicals, the inner membrane being renderable permeable, without causing the impermeable outer membrane to become permeable, to permit mixing the first and second chemicals to cause the exothermic reaction. This multipart container is covered with a soft, non-abrasive, lint-free material which is presoaked in a pH controlled antibacterial soap with or without an antibiotic.
Owner:OCUGIENE
Process for producing liquid fuel from carbon dioxide and water
ActiveUS20070244208A1Combustible gas chemical modificationOrganic compounds purification/separation/stabilisationHydrocotyle bowlesioidesLiquid fuel
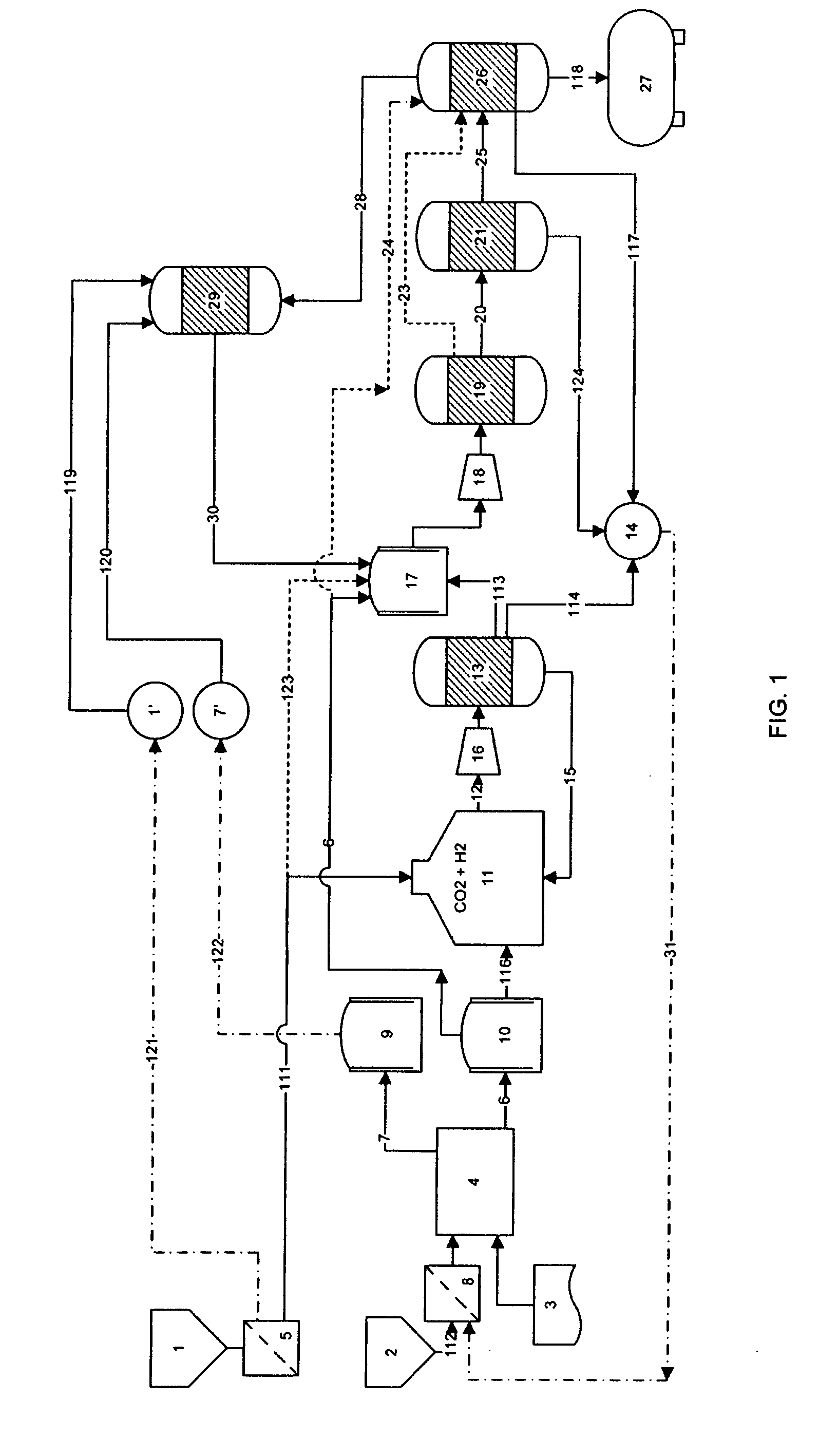
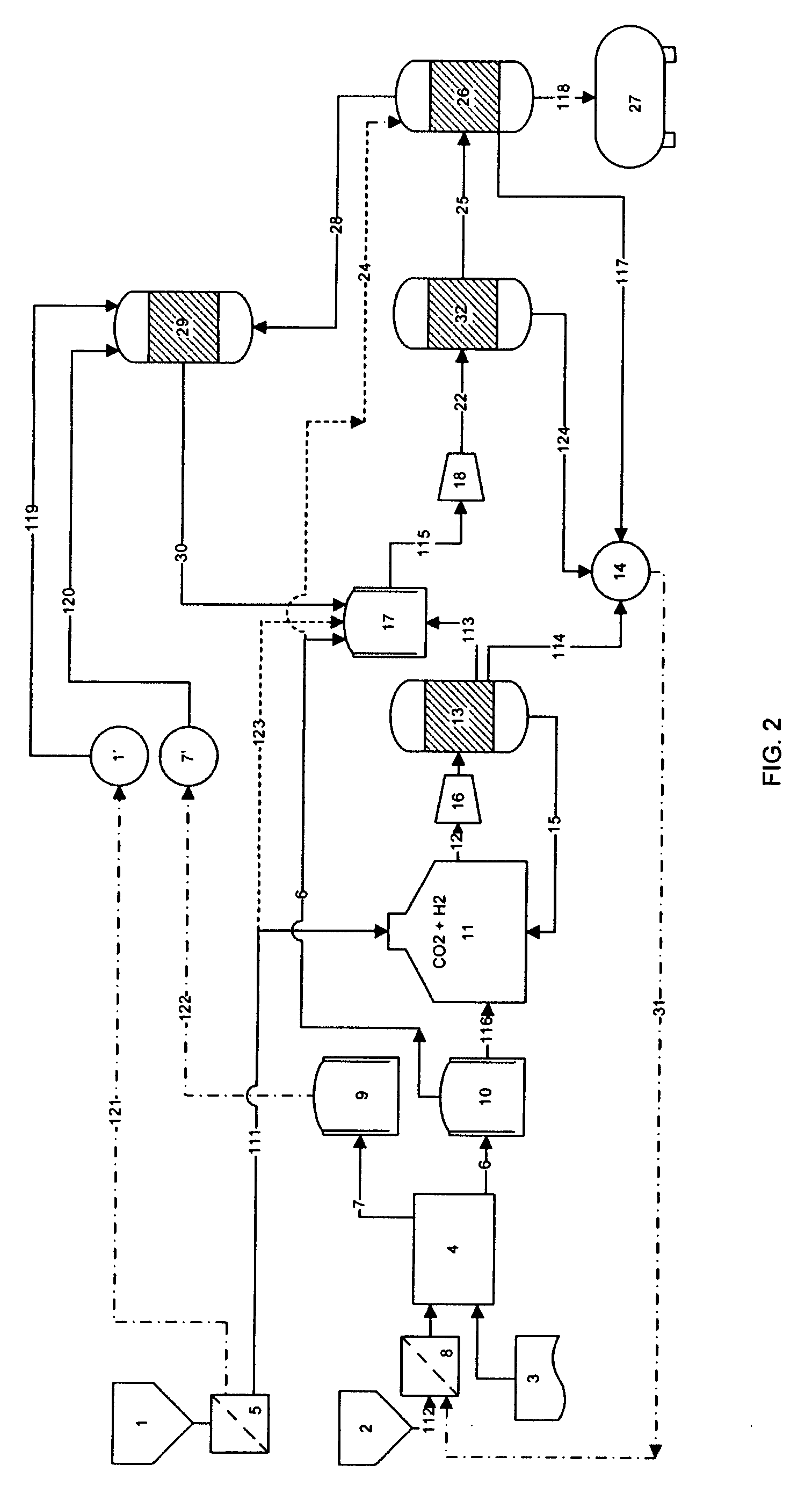
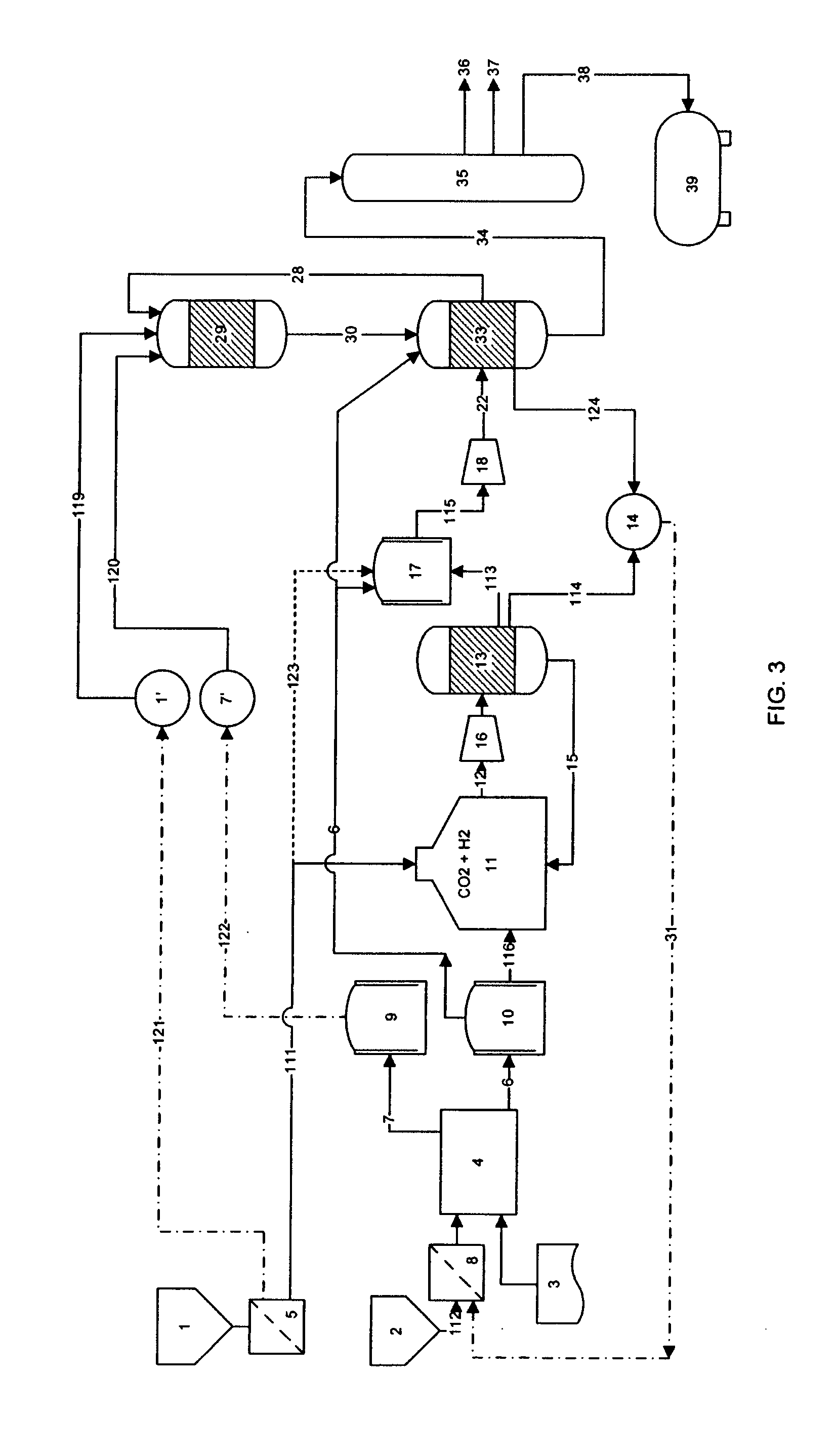
A process for producing high octane fuel from carbon dioxide and water is disclosed. The feedstock for the production line is industrial carbon dioxide and water, which may be of lower quality. The end product can be high octane gasoline, high cetane diesel or other liquid hydrocarbon mixtures suitable for driving conventional combustion engines or hydrocarbons suitable for further industrial processing or commercial use. Products, such as dimethyl ether or methanol may also be withdrawn from the production line. The process is emission free and reprocesses all hydrocarbons not suitable for liquid fuel to form high octane products. The heat generated by exothermic reactions in the process is fully utilizes as is the heat produced in the reprocessing of hydrocarbons not suitable for liquid fuel.
Owner:CRI EHF
Device and method for exothermic treatment of eyelid diseases
ActiveUS7211070B2Preventing occurrence and recurrencePromotes better eyelid hygienePharmaceutical delivery mechanismMedical applicatorsDiseasePh control
Provided herein is a pad for treating eye conditions comprising a multipart container having an impermeable outer membrane sized to fit generally within the peri-orbital region and sufficiently flexible to mold to the eye; a first chemical in a first storage area in the multipart container; a second chemical in a second storage area in the multipart container, the first and second chemicals selected to have an exothermic reaction when mixed for producing a temperature suitable for treating eye conditions, the exothermic reaction providing the suitable temperature for a period of time suitable for treating eye conditions; and an inner membrane for initially separating the first and second chemicals, the inner membrane being renderable permeable, without causing the impermeable outer membrane to become permeable, to permit mixing the first and second chemicals to cause the exothermic reaction. This multipart container is covered with a soft, non-abrasive, lint-free material which is presoaked in a pH controlled antibacterial soap with or without an antibiotic.
Owner:OCUGIENE
Methods of heating energy storage devices that power downhole tools
An energy storage device for powering a downhole tool may be heated to an effective temperature to improve the operability of the energy storage device. The energy storage device may comprise, for example, a primary battery, a secondary battery, a fuel cell, a capacitor, or combinations thereof. The effective temperature to which the energy storage device is heated may be greater than an ambient temperature in the wellbore near the energy storage device. The energy storage device may be heated using various heat sources such as an ohmic resistive heater, a heat pump, an exothermic reaction, a power generator, a heat transfer medium, the energy storage device itself, a downhole tool, or combinations thereof. A thermal conductor may extend between the heat source and the energy storage device. Further, a thermal insulator may at least partially surround the heat source and the energy storage device.
Owner:HALLIBURTON ENERGY SERVICES INC
Use of high carbon coal ash
InactiveUS6755905B2Avoid insufficient heatingReduce flowCharge manipulationHandling discharged materialSlagHigh carbon
A synthetic slag is produced by a high temperature combustion reaction between coal ash having a high carbon content, and a source of lime such as cement kiln dust. The carbon content of the coal ash is oxidized by oxygen gas, which typically is derived from air or an air / oxygen combination in an exothermic reaction and the heat generated is exploited in the melting process. In this way the gaseous products will typically comprise nitrogen, unreacted oxygen and carbon dioxide, and heat energy can be readily recovered from the hot off gas products evolving during the combustion reaction. The synthetic slag may be pelletized and employed as lightweight mineral aggregate or milled to cement fineness to provide slag cement.
Owner:LAFARGE CANADA INC
Apparatus and method for gasifying liquid or solid fuel
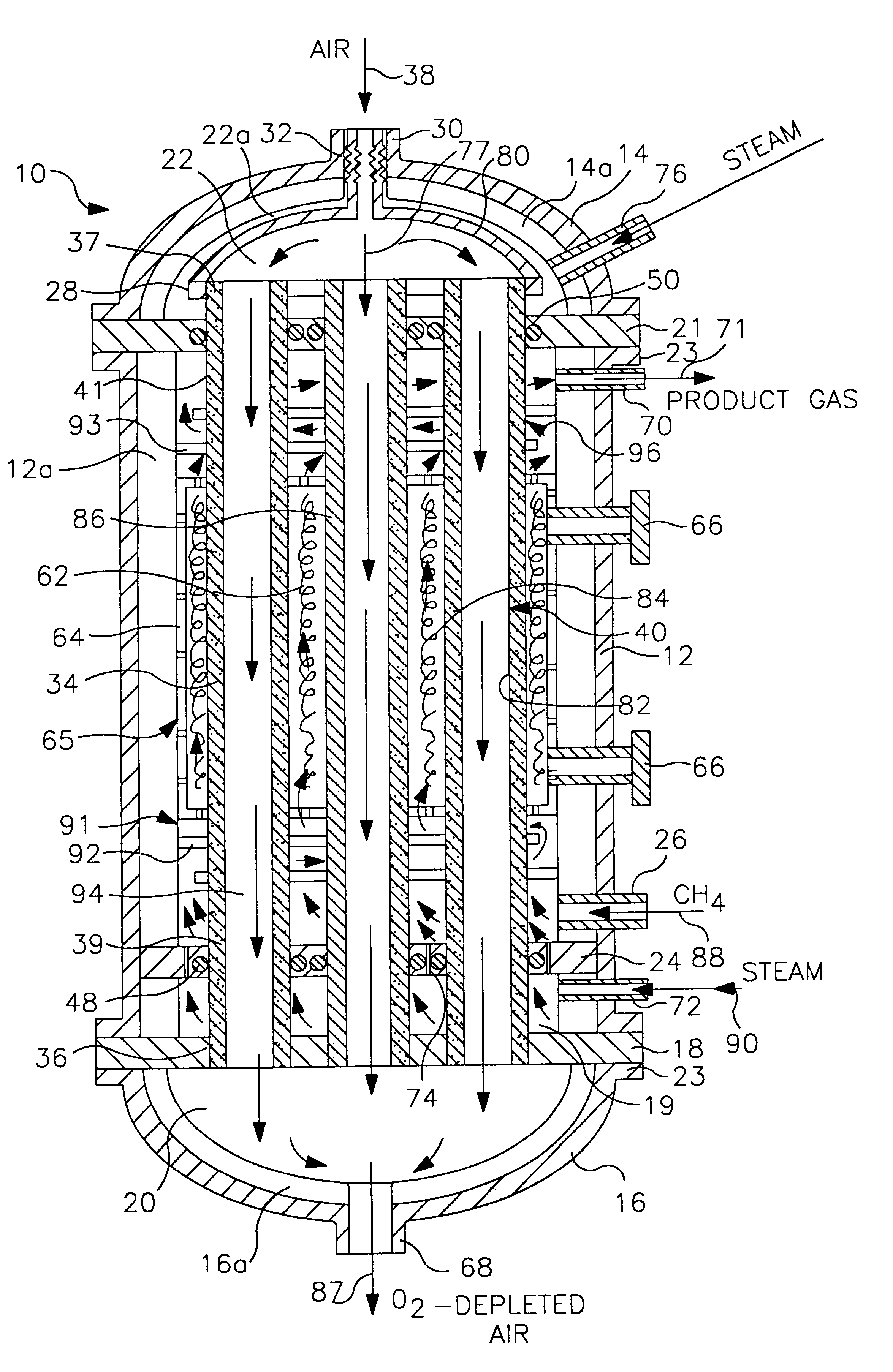
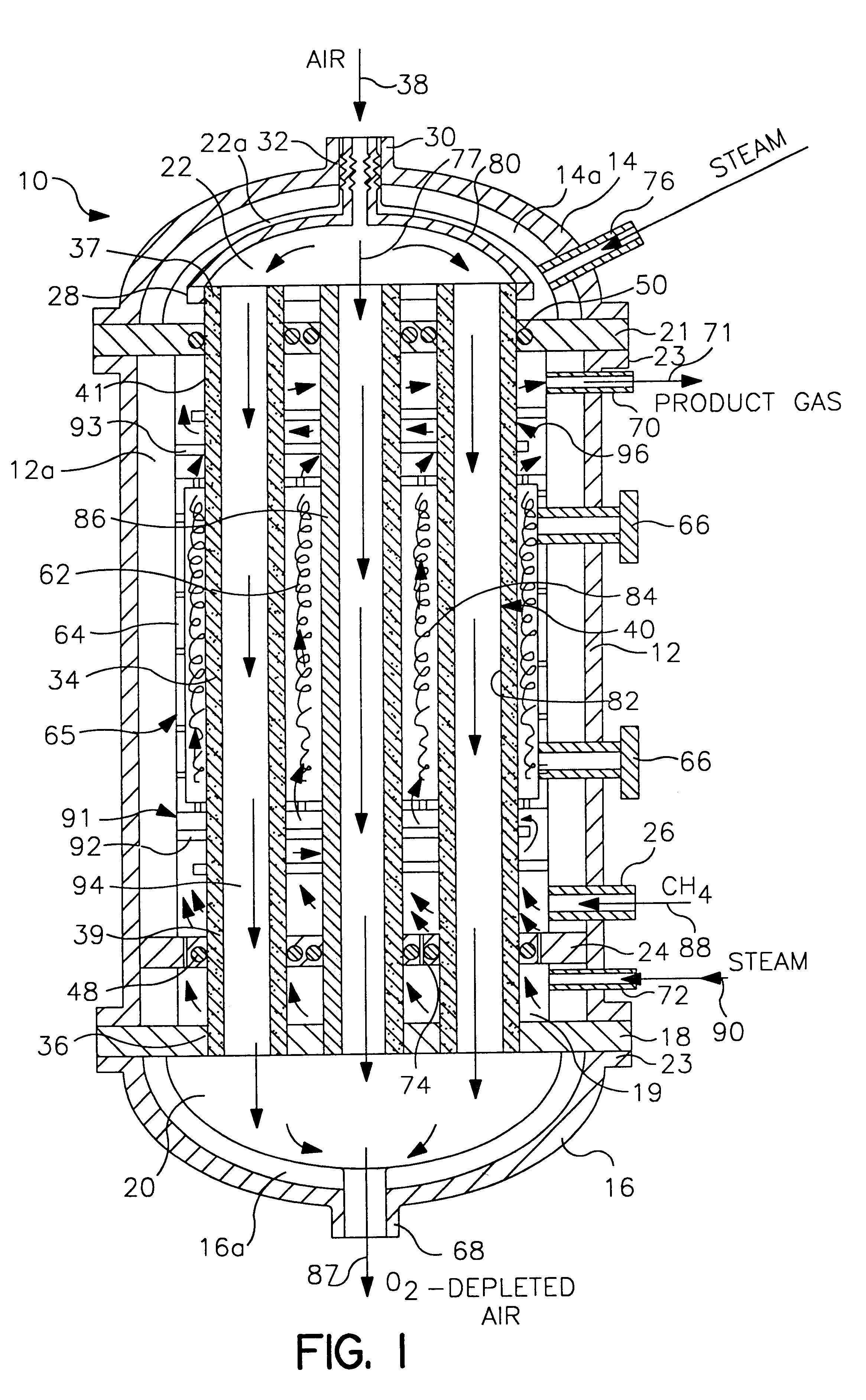
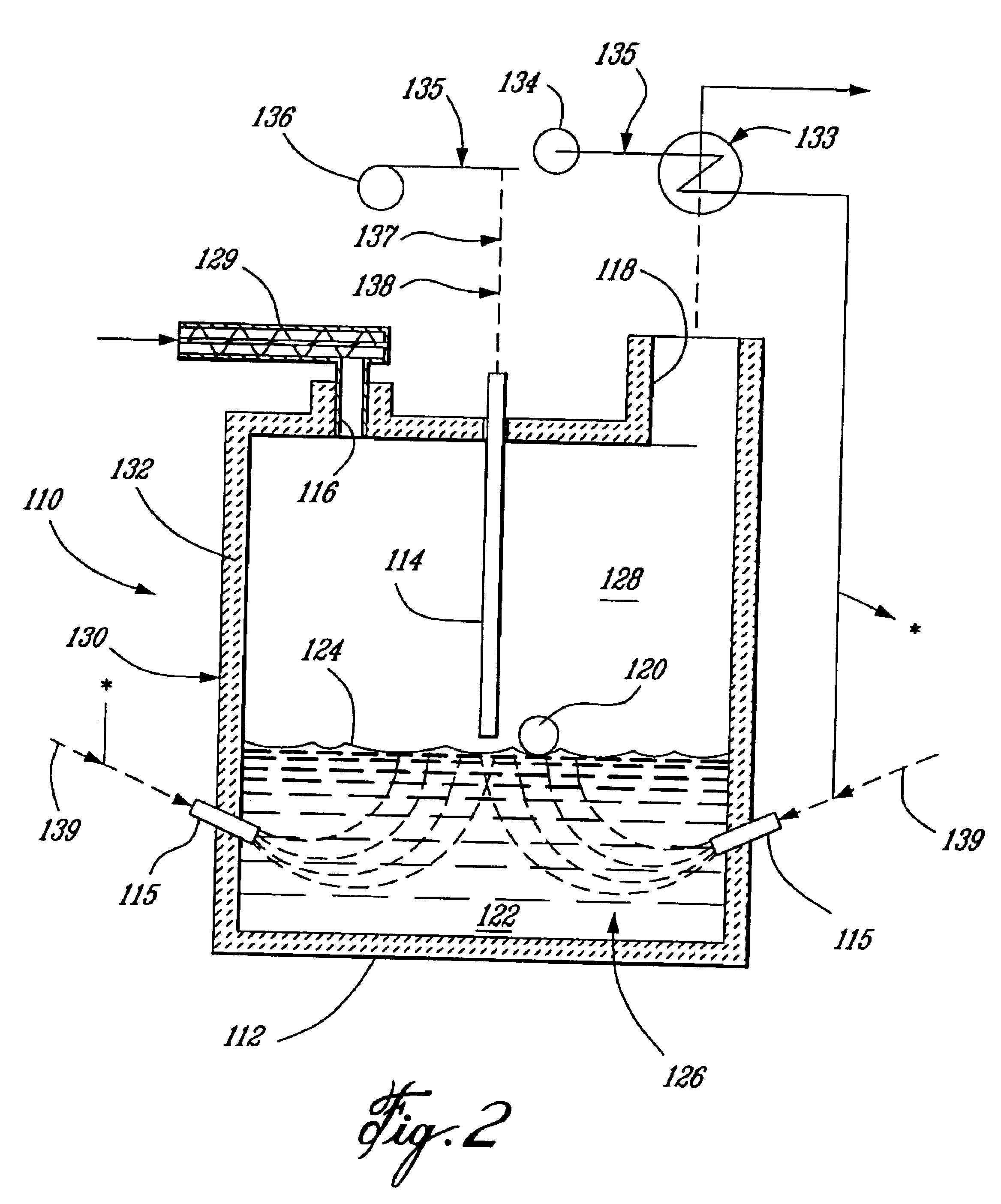
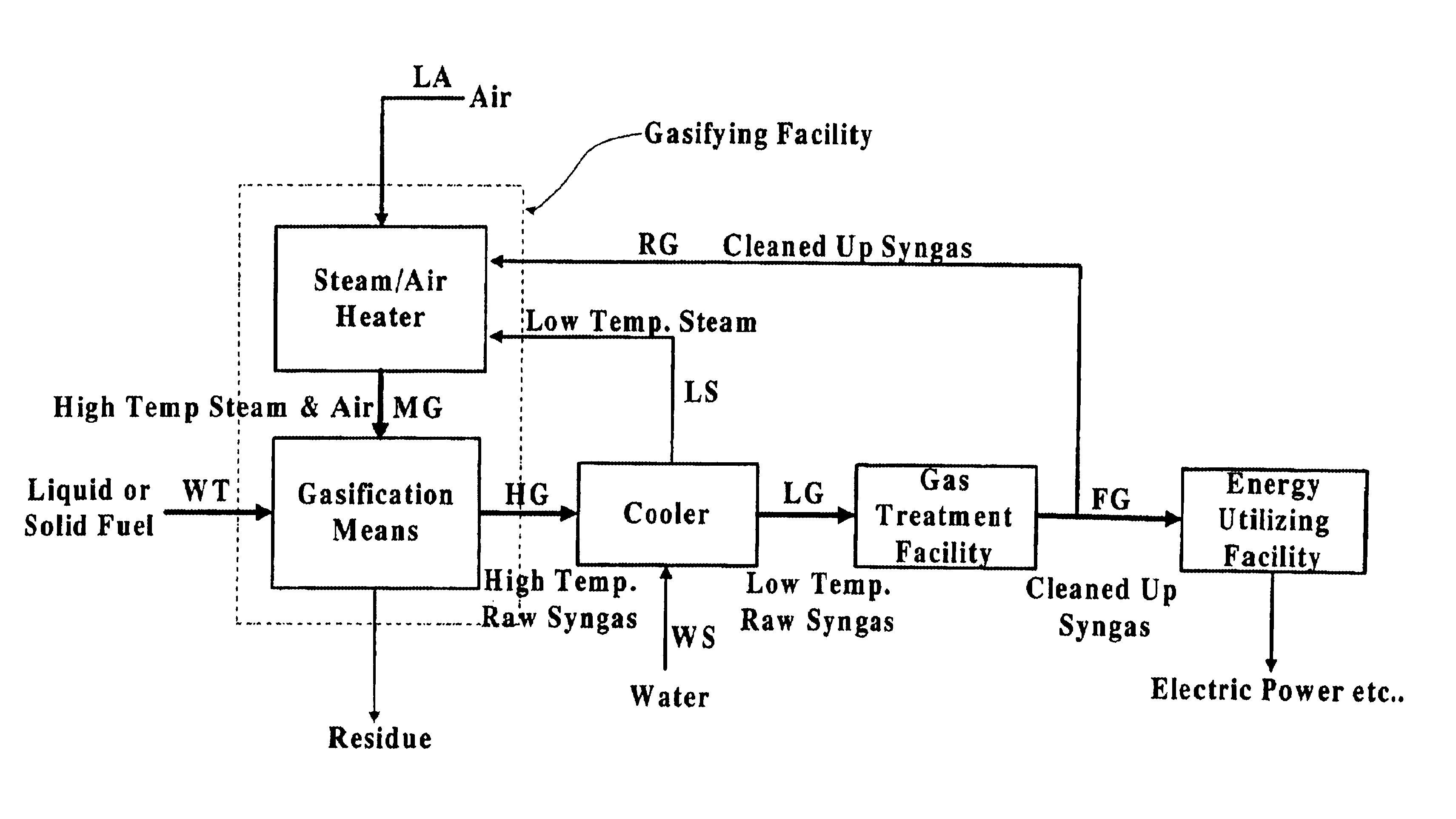
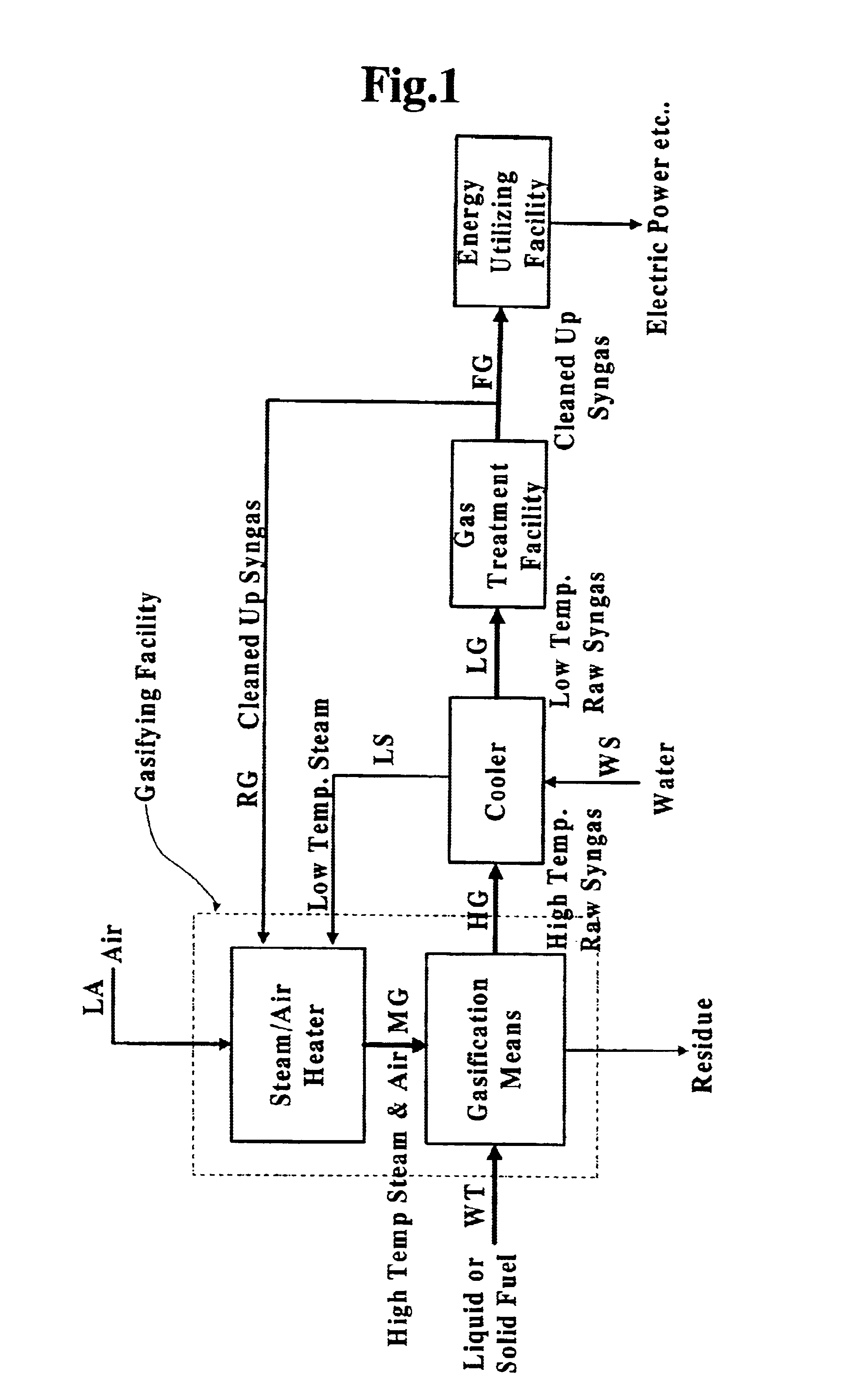
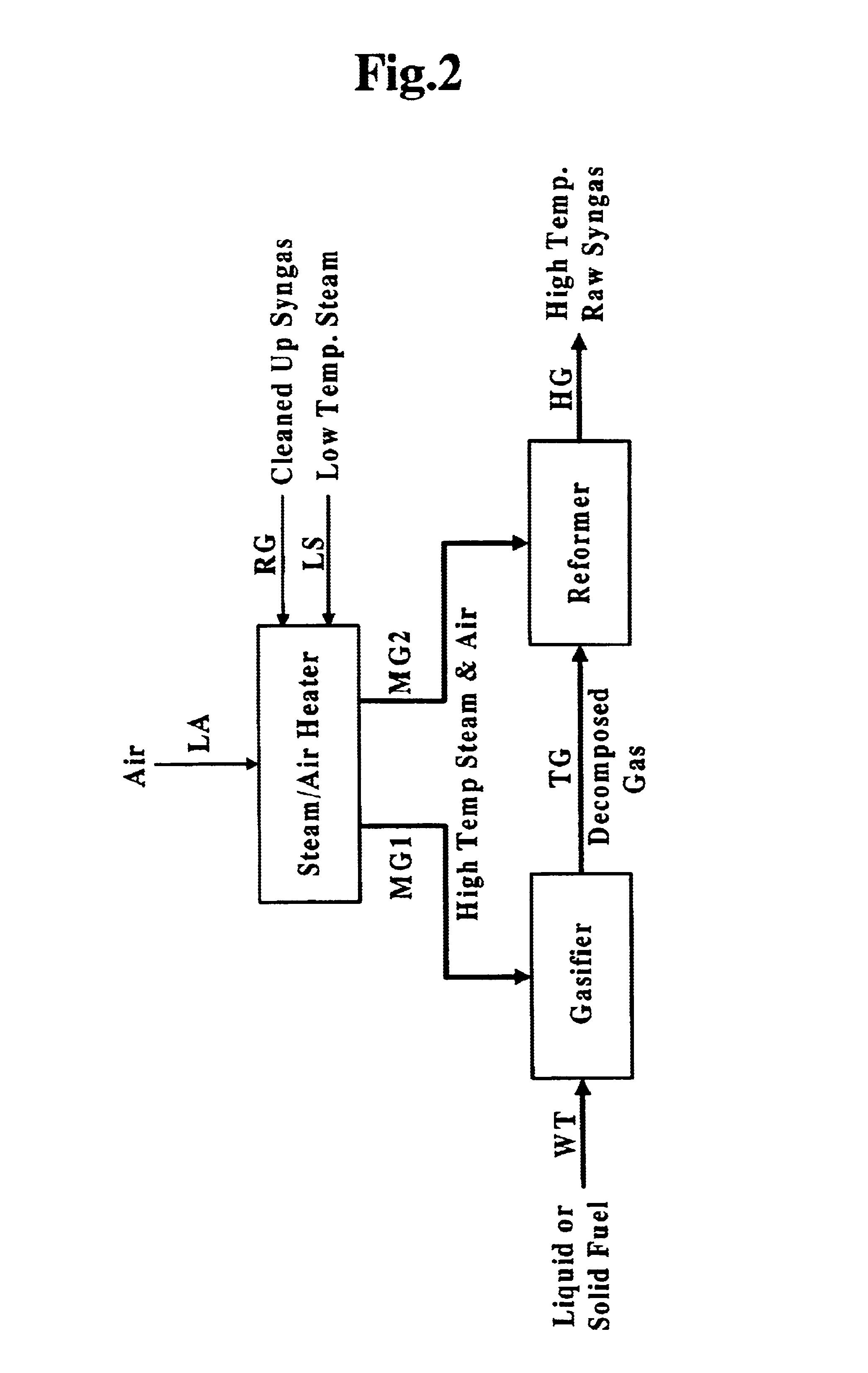
InactiveUS6837910B1Quality improvementAmount of heat can be ensuredHydrogenGaseous fuelsSyngasExothermic reaction
A gasifying apparatus comprises a gasifier, a reformer and a heating device. The gasifier produces a thermal decomposed gas with use of a thermal decomposition reaction of a liquid or solid fuel such as waste or coal, and the heating device heats a low-temperature steam and air so as to be a high-temperature steam and air, which have a temperature equal to or higher than 700 deg. C. The gasifying apparatus has feeding means including fluid passages for feeding the high-temperature steam and air to the gasifier and the reformer. In a thermal decomposition area of the gasifier, the liquid or solid fuel is thermally decomposed to produce the thermal decomposed gas with sensible heat of the high-temperature steam and air and with the heat generated by an exothermic oxidation reaction between the high-temperature air and the liquid or solid fuel. In the reformer, the thermal decomposed gas is reformed in the existence of the high-temperature steam so as to be a high-temperature syngas. The steam reforming reaction of the liquid or solid fuel is carried out with an exothermic reaction between the high-temperature air and hydrocarbon contained in the thermal decomposed gas and with an endothermic reaction between the hydrocarbon and the high-temperature steam.
Owner:NIPPON FURNANCE IND KAISHA +1
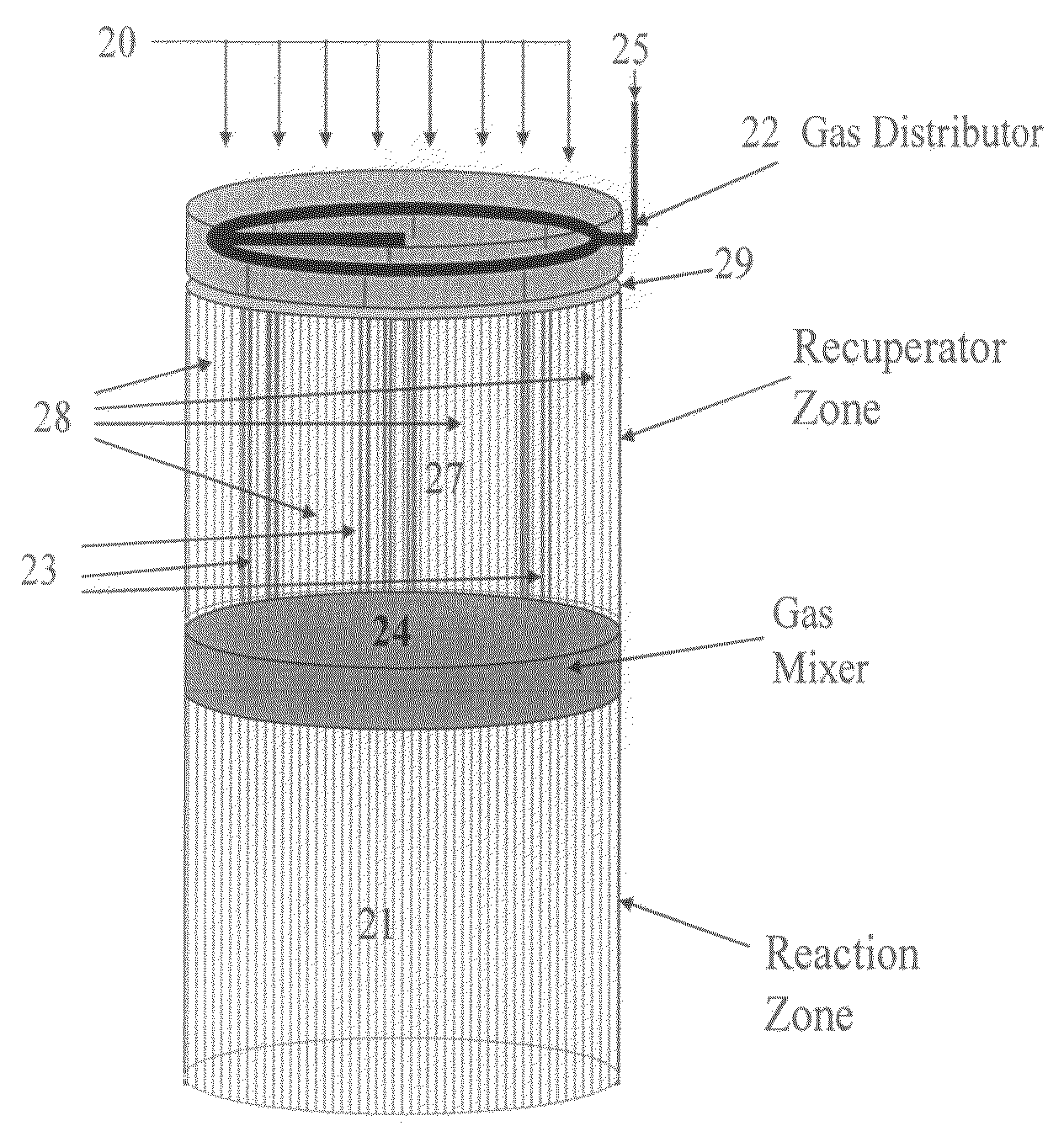
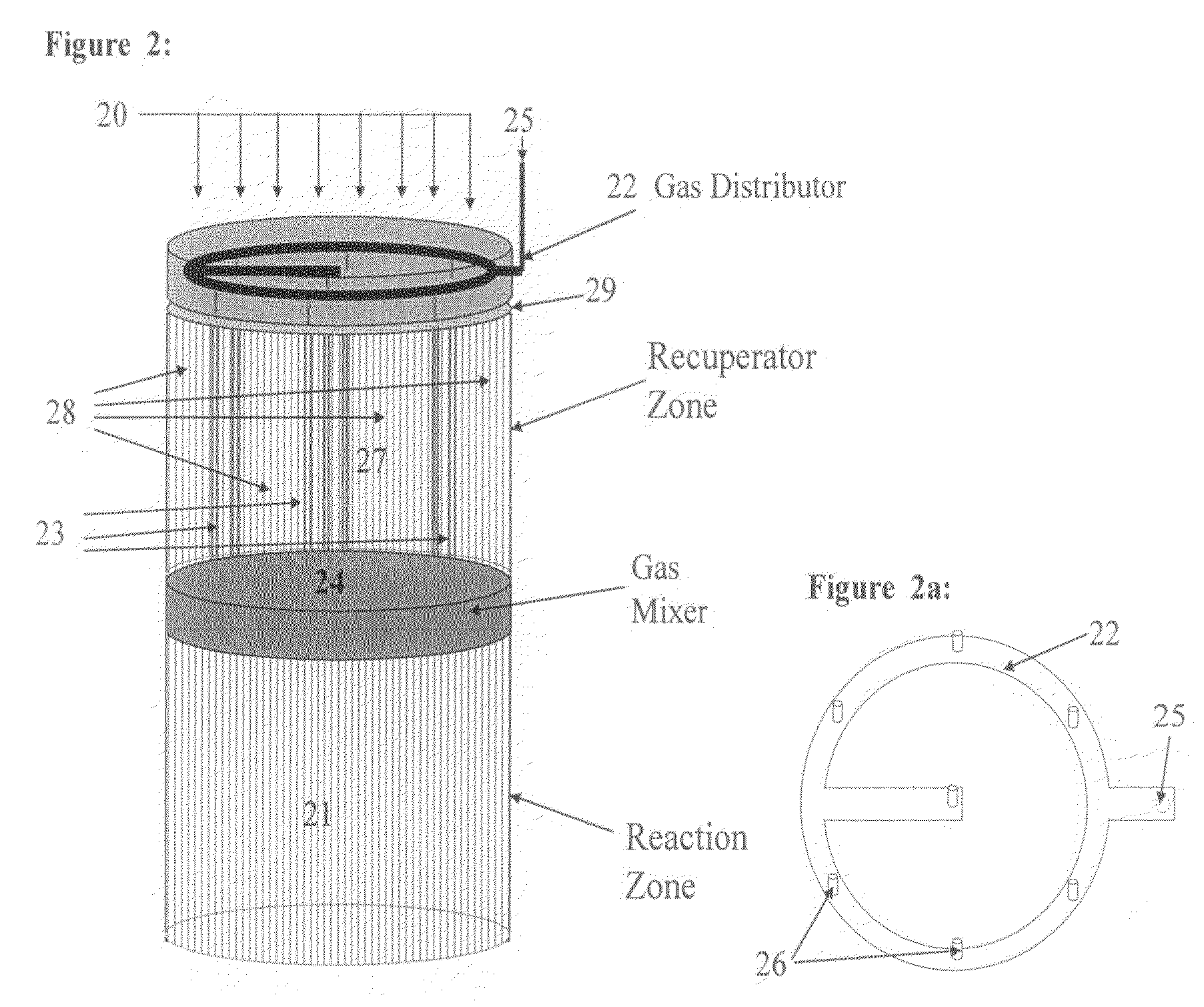
Controlled combustion for regenerative reactors with mixer/flow distributor
ActiveUS20080142409A1Efficiently transfer heatEfficient transferHydrocarbon by dehydrogenationFlow mixersExothermic reactionProcess engineering
The overall efficiency of a regenerative bed reverse flow reactor system is increased where the location of the exothermic reaction used for regeneration is suitably controlled. The present invention provides a method and apparatus for controlling the combustion to improve the thermal efficiency of bed regeneration in a cyclic reaction / regeneration processes. The process for thermal regeneration of a regenerative reactor bed entails(a) supplying the first reactant through a first channel means in a first regenerative bed and supplying at least a second reactant through a second channel means in the first regenerative bed,(b) combining said first and second reactants by a gas mixing means situated at an exit of the first regenerative bed and reacting the combined gas to produce a heated reaction product,(c) passing the heated reaction product through a second regenerative bed thereby transferring heat from the reaction product to the second regenerative bed.
Owner:EXXON RES & ENG CO
Device and method for treatment of eyelid diseases
ActiveUS20060104914A1Prevent relapsePromotes better eyelid hygieneAerosol deliveryMedical applicatorsDiseaseMedicine
A container has an impermeable outer membrane sized to fit generally within the peri-orbital region and sufficiently flexible to mold to the structure of the closed eye. Heat which is generated by an exothermic reaction inside the container, which provides steady-state heat to the eyelids. A soft, non-abrasive, lint-free material is presoaked in a pH controlled (preferably antibacterial and hypoallergenic) detergent, with or without an ophthalmic antibiotic solution. The compress may also have a handle which makes holding and maneuvering the compress more feasible, while protecting the user's fingers from the heat, and preventing the contamination of the compress by the fingers. The compress may be packed in a watertight, sterile wrapper which will prevent drying of the detergent solution. Other embodiments are also described and claimed.
Owner:OCUGIENE
Thermal coverings
InactiveUS20060142828A1Obstruct passageTherapeutic coolingTherapeutic heatingBiological activationOxygen
A thermal covering that comprises a thermoregulatory substrate is provided. The thermoregulatory substrate contains an exothermic coating formed from an oxidizable metal. The exothermic coating is generally free of moisture prior to activation. Exposure of the exothermic coating to oxygen and moisture activates an exothermic reaction to generate heat, such heat being transferable to a patient or user through an outer surface defined by the thermal covering.
Owner:KIMBERLY-CLARK WORLDWIDE INC
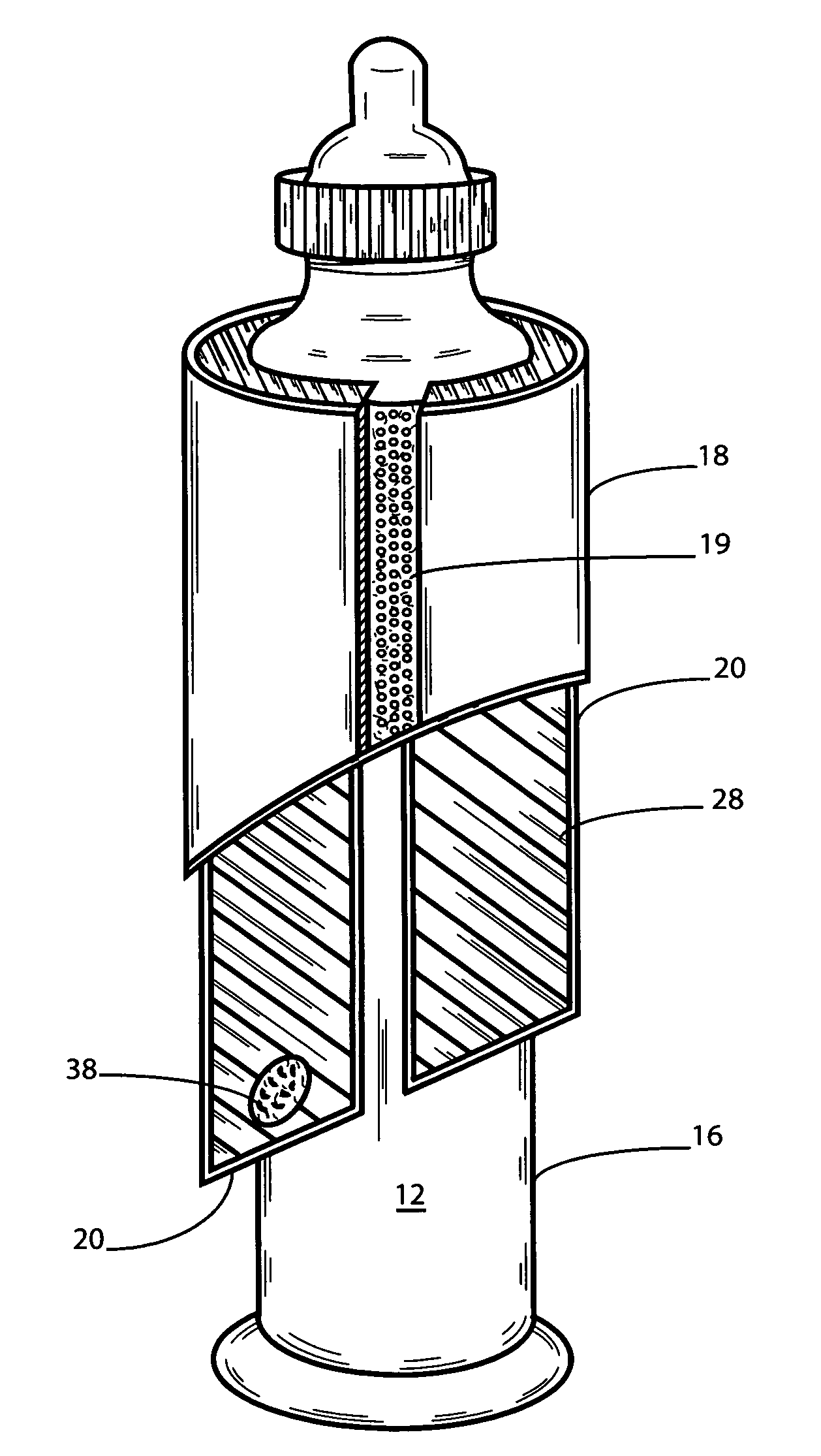
Baby bottle warmer
InactiveUS6234165B1Exothermal chemical reaction heat productionOther heat production devicesEngineeringSaline solutions
A method and apparatus utilizing an exothermic reaction to generate a heated gas that warms a baby bottle, providing a baby bottle warmer that is portable, convenient to use, simple, and lightweight. In one embodiment, a heating vessel holds the baby bottle and a vessel top removably attaches to an upper section of the heating vessel. A solution release mechanism situated in a lower section of said heating vessel includes a solution container having an activating liquid disposed therein, and a device for puncturing the solution container responsive to pressure supplied by insertion of the baby bottle. A heating element situated below the solution release mechanism includes a material for exothermically reacting with the activating liquid to generate the heated gas. In one embodiment, when a baby bottle is inserted into the bottle warmer, an upper and lower unit are pressured together to release a saline solution onto a magnesium wafer. Some embodiments of the baby bottle warmer comprise a system for spacing apart the lower and upper units, which is useful for storage and / or travel. Upon insertion of the baby bottle and application of pressure, the system disengages, thereby allowing the upper and lower units to move together.
Owner:KEVIN A CREIGHTON +1
Hot solids gasifier with CO2 removal and hydrogen production
ActiveUS7083658B2Avoid entrainmentEfficient captureMuffle furnacesGas modification by gas mixingCo2 removalWater-gas shift reaction
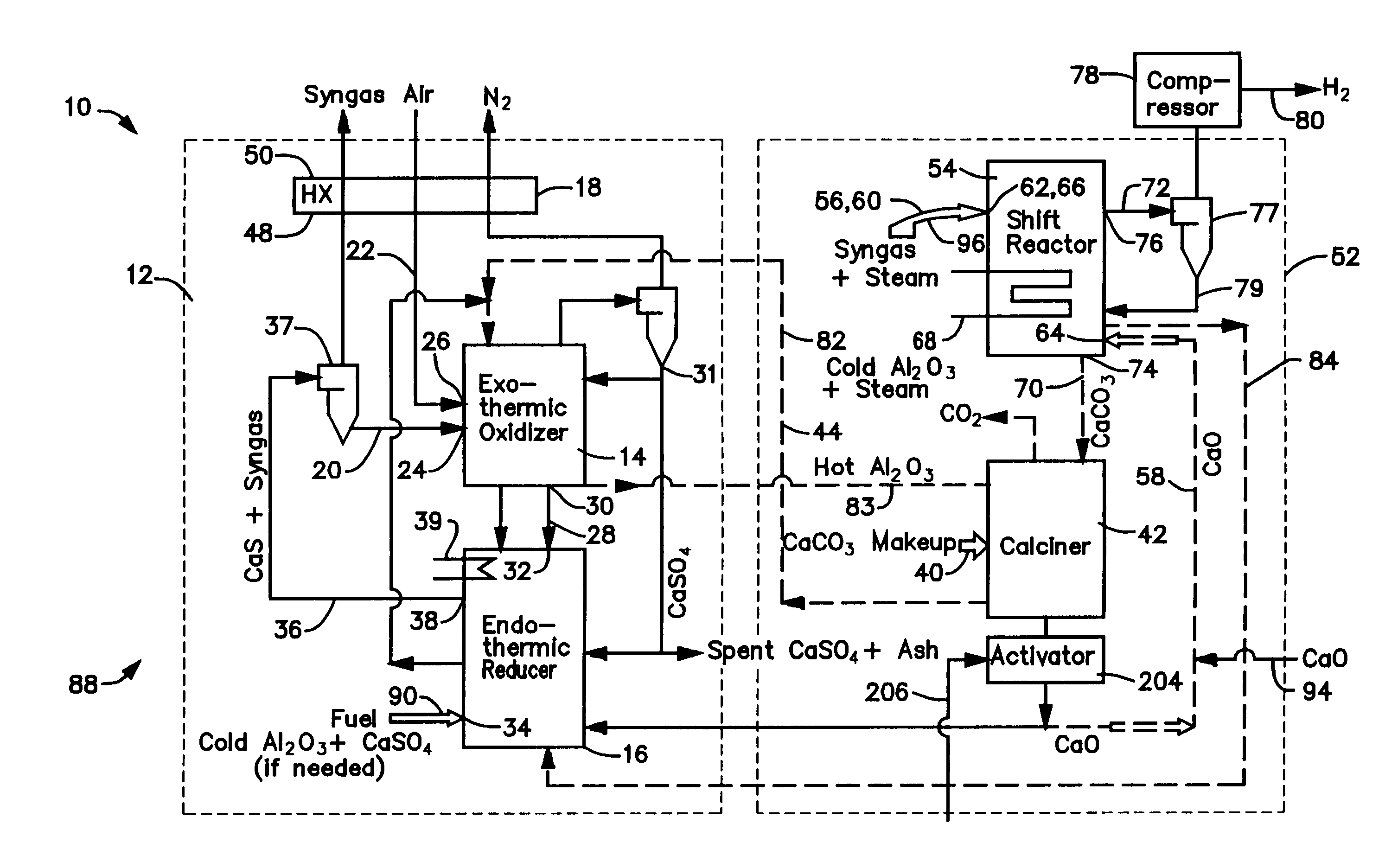
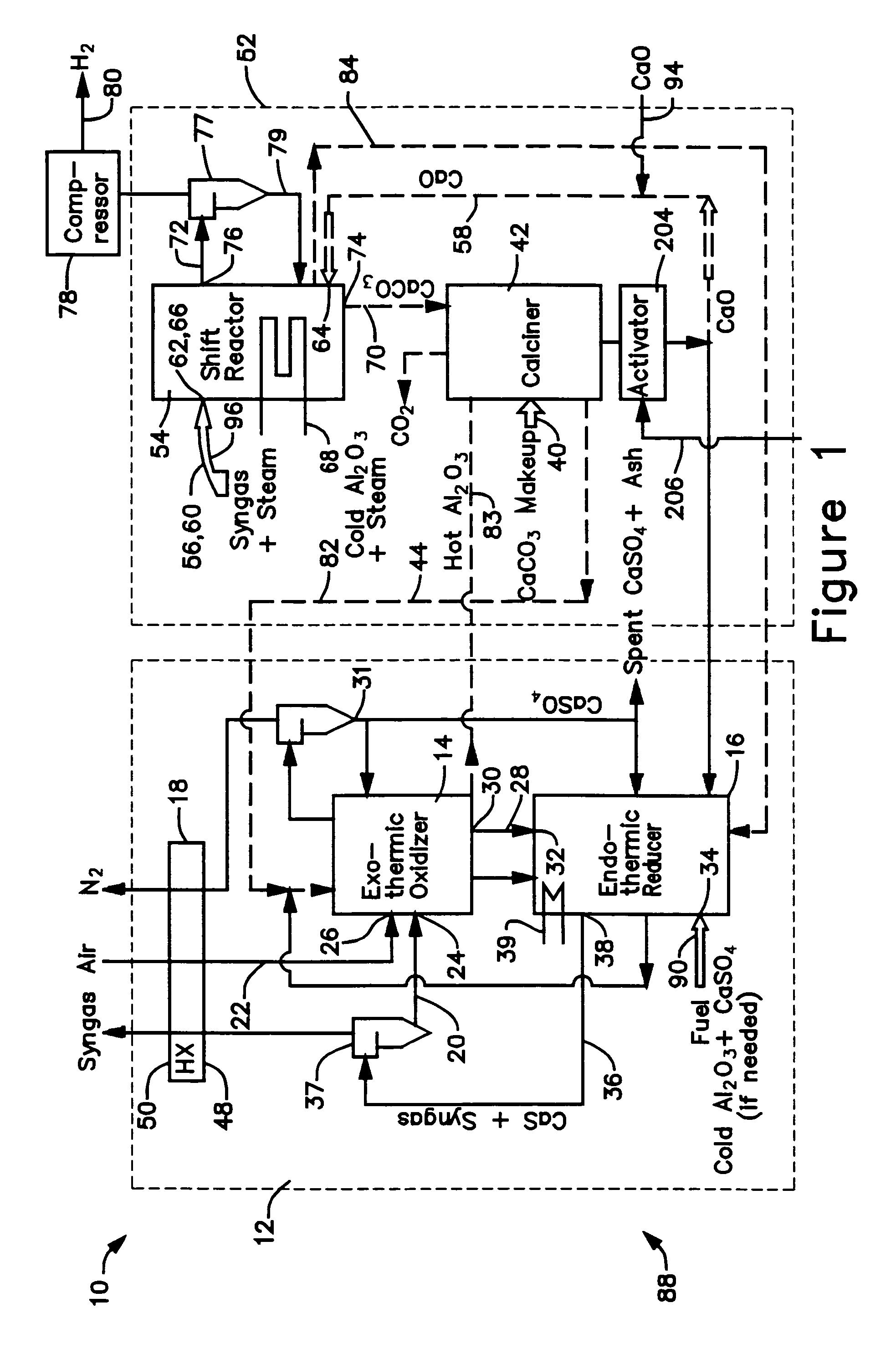
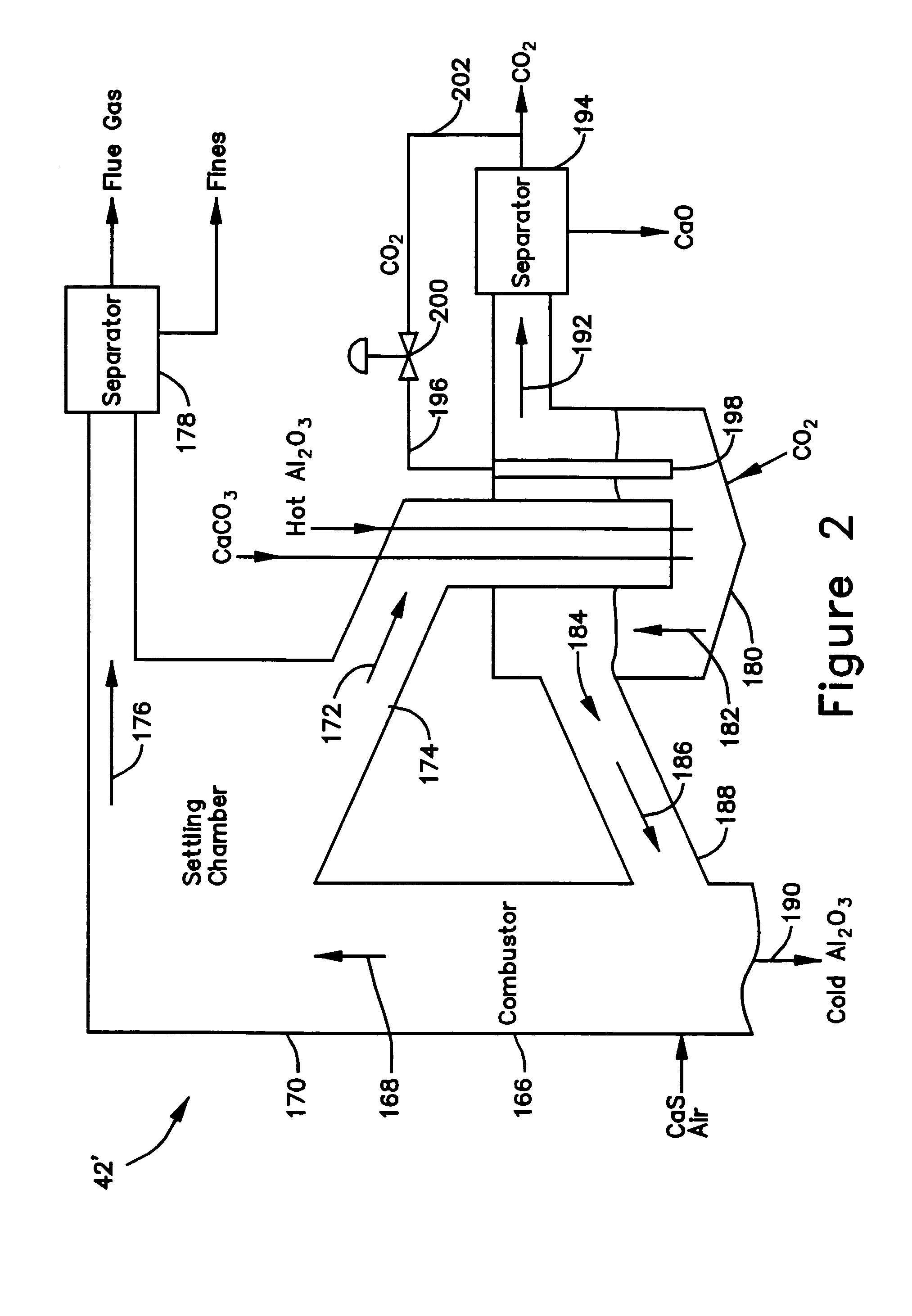
A gasifier 10 includes a first chemical process loop 12 having an exothermic oxidizer reactor 14 and an endothermic reducer reactor 16. CaS is oxidized in air in the oxidizer reactor 14 to form hot CaSO4 which is discharged to the reducer reactor 16. Hot CaSO4 and carbonaceous fuel received in the reducer reactor 16 undergo an endothermic reaction utilizing the heat content of the CaSO4, the carbonaceous fuel stripping the oxygen from the CaSO4 to form CaS and a CO rich syngas. The CaS is discharged to the oxidizer reactor 14 and the syngas is discharged to a second chemical process loop 52. The second chemical process loop 52 has a water-gas shift reactor 54 and a calciner 42. The CO of the syngas reacts with gaseous H2O in the shift reactor 54 to produce H2 and CO2. The CO2 is captured by CaO to form hot CaCO3 in an exothermic reaction. The hot CaCO3 is discharged to the calciner 42, the heat content of the CaCO3 being used to strip the CO2 from the CaO in an endothermic reaction in the calciner, with the CaO being discharged from the calciner 42 to the shift reactor 54.
Owner:AIR PROD & CHEM INC +1
Air-injection system to improve effectiveness of selective catalytic reduction catalyst for gasoline engines
InactiveUS20090260349A1Increased durabilityHarmful emissionGas treatmentInternal combustion piston enginesGasolineEngineering
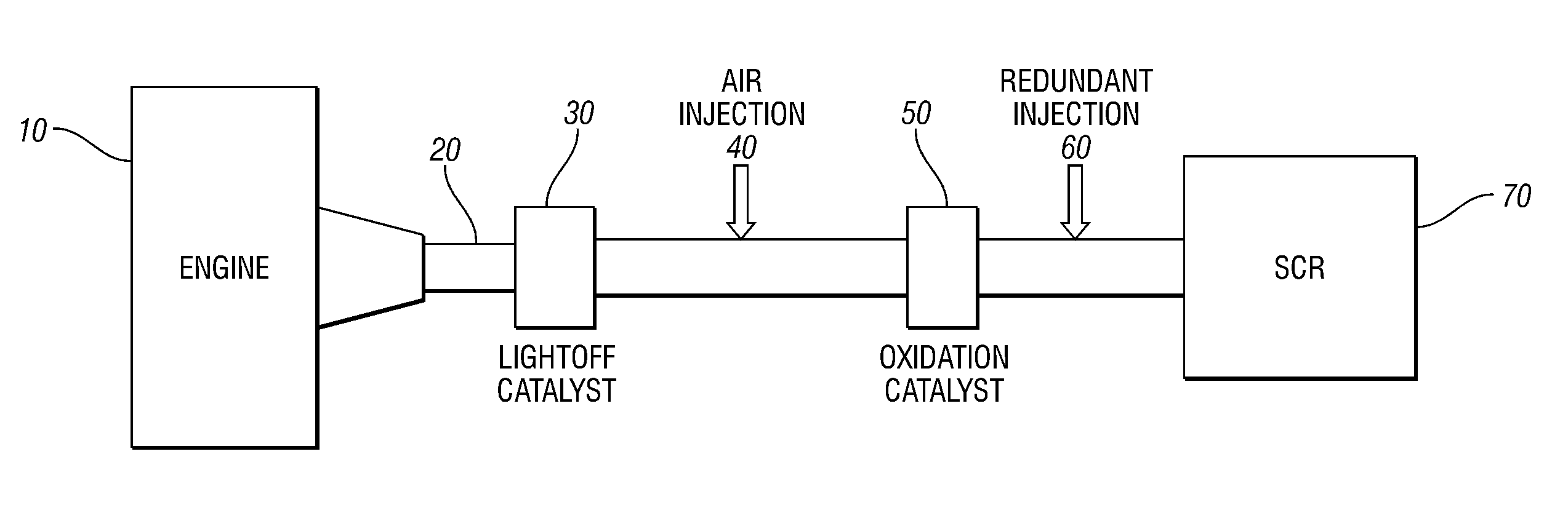
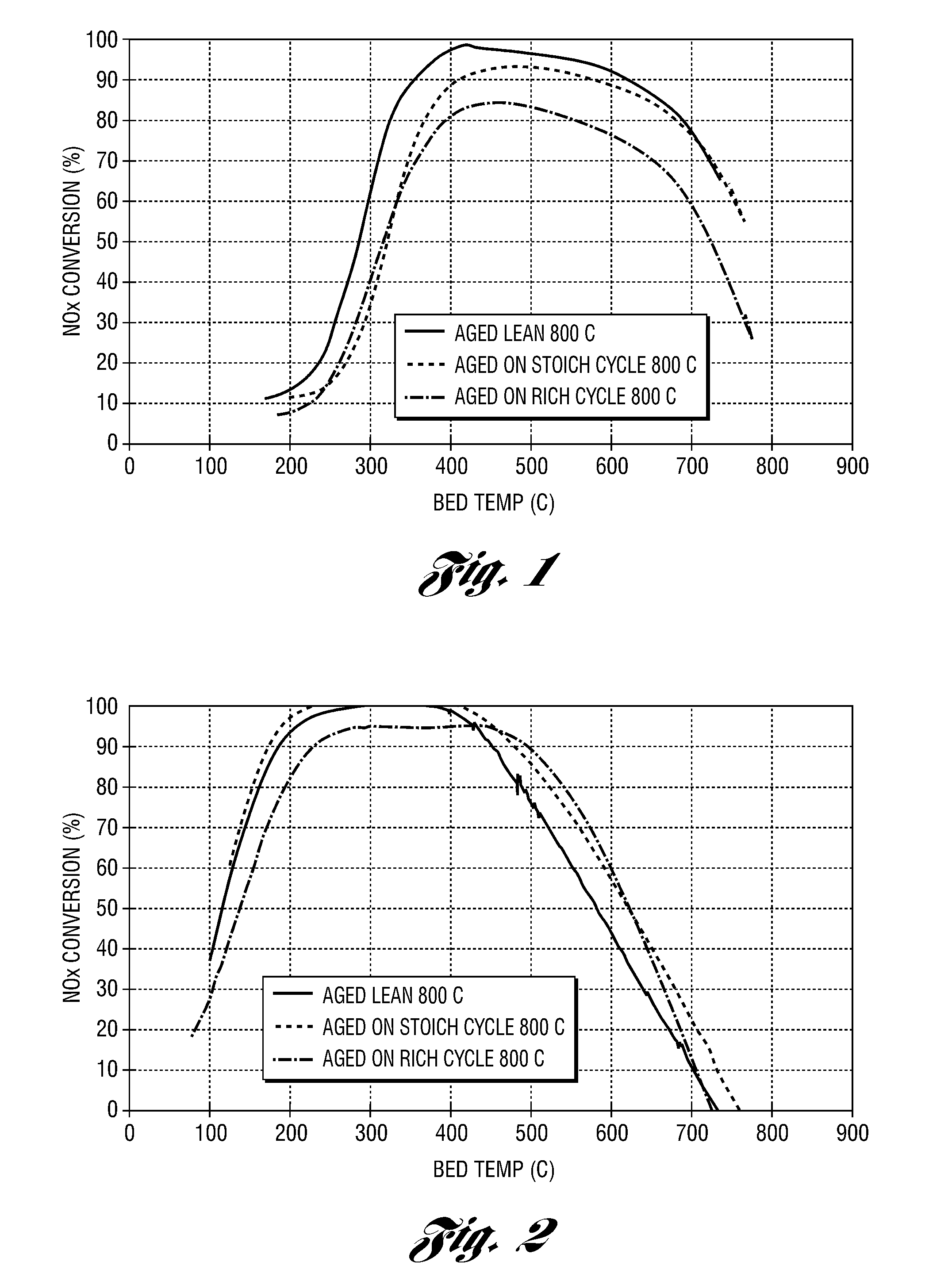
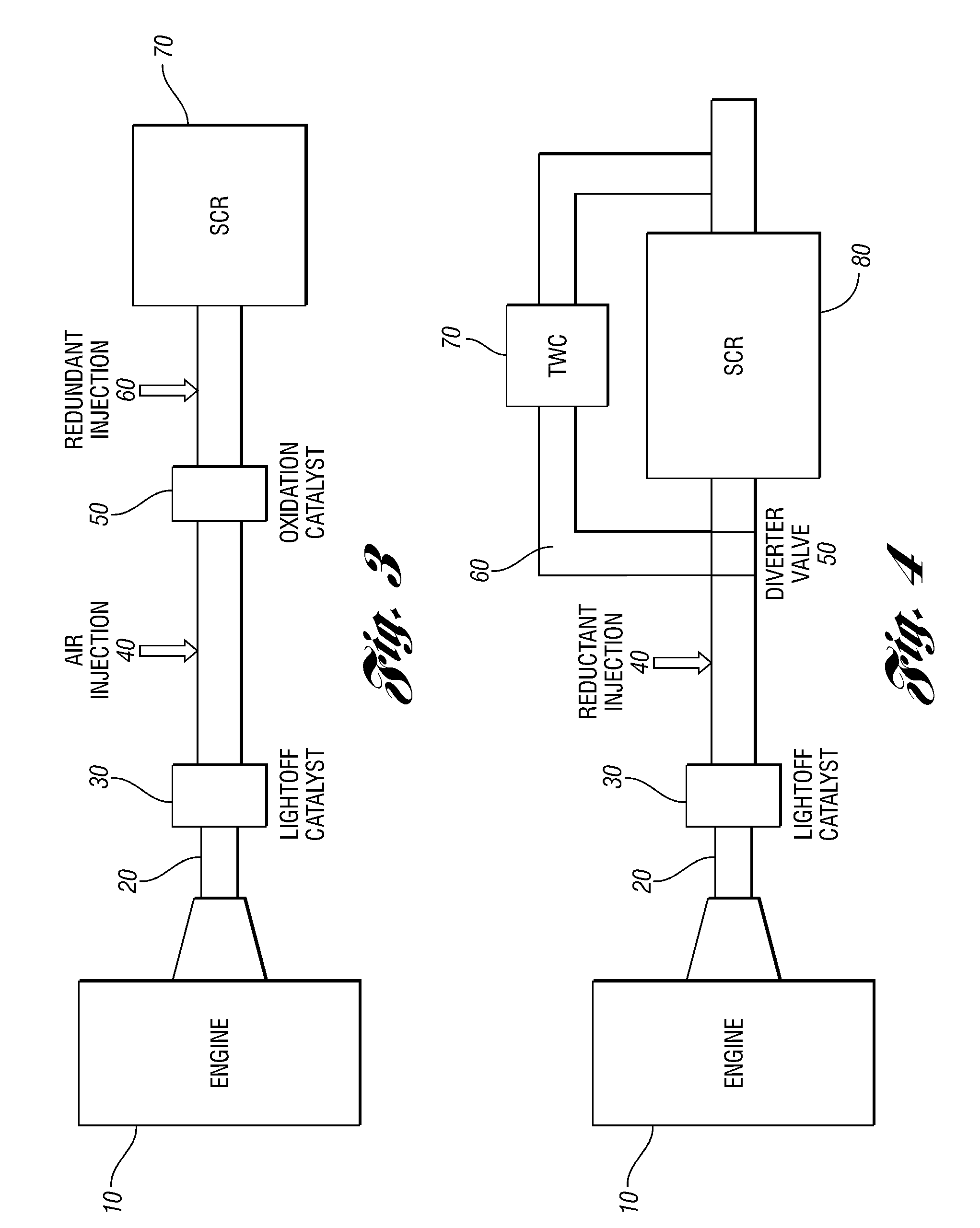
Embodiments are described to improve the durability of a lean NOx aftertreatment system. According to one embodiment of the present invention an air injection system is used to inject air continuously into the exhaust system between the upstream three-way catalyst and the downstream selective catalytic reduction (SCR) catalyst when the engine is operating at stoichiometric or rich air / fuel ratios and the exhaust temperatures are above a calibratible level (e.g., 700° C.). In another embodiment, an oxidation catalyst is positioned downstream of the air injection point to prevent exothermic reactions from occurring on the SCR. In another embodiment, the reductant for the SCR is generated in-situ. In yet another embodiment, a diverter valve with a reduction catalyst in a bypass arm is utilized to bypass the SCR during high load conditions.
Owner:FORD GLOBAL TECH LLC
Exothermic composition and exothermic element
InactiveUS20060154006A1Envelopes/bags making machineryExothermal chemical reaction heat productionSolid componentWater insoluble
In a heat generating composition which generates heat by contacting with air, a heat generating composition which is characterized in that an exothermic substance, a reaction promoter, water and a carbon component are essential components, water mobility value thereof is 20 or less, maximum particle size of water-insoluble solid components excluding the reaction promoter and water is 1 mm or less where 80% or more thereof has a particles size of 300 μm or less, water in the heat generating composition does not function as a barrier layer and exothermic reaction takes place when contacted to the air. A heat generating composition having a molding property and a shape-holding property and also having an exothermic characteristic whereby exothermic reaction is able to start by contacting to the air immediately after the molding and a heat generating body using the same are provided.
Owner:MYCOAL PRODS CORP
Oil well perforators
ActiveUS20070056462A1Material consumptionReduce the possibilityAmmunition projectilesExplosive chargesParticulatesDetonation
An oil and gas well shaped charge perforator capable of providing an exothermic reaction after detonation is provided, comprising a housing, a high explosive, and a reactive liner where the high explosive is positioned between the reactive liner and the housing. The reactive liner is produced from a composition which is capable of sustaining an exothermic reaction during the formation of the cutting jet. The composition may be selected from any known formulation which is suitable for use in an oil and gas well perforator, typically the composition will comprise at least one metal and at least one non-metal, wherein the non-metal is selected from a metal oxide, or any non-metal from Group III or Group IV or at least two metals such as to form an intermetallic reaction. Typically at least one of the metals in the invention may be selected from Al, Ce, Li, Mg, Mo, Ni, Nb, Pb, Pd, Ta, Ti, Zn or Zr. The liner composition may preferably be a pressed particulate composition, such that the material is consolidated under pressure to form the desired shape of the liner. To aid consolidation a binder may also be added.
Owner:WELLS FARGO BANK NAT ASSOC +1
Method and apparatus for sealing an undesirable formation zone in the wall of a wellbore
A method and apparatus are provided for intentionally inducing “formation plugging” in a partially damaged section, water layer, or an otherwise undesirable target zone in the wall of a wellbore. The rigless apparatus is deployed to the target zone by coiled tubing and includes a pair of adjacent inflatable packers or a multi-section inflatable thermoplastic balloon component which is in valved fluid communication with an inflating container filled with formation plugging fluid. The inflating container is in valved fluid communication with a reactant-filled chemical container, the reactant being activated by contact with reactant fluid pumped through the coiled tubing via a circulation valve. The hot pressurized exothermic reaction products pass into the inflation container and displace the formation plugging fluid into the balloon sections and through weakened portions of a central balloon to penetrate the walls of the target zone. The expanded central balloon is melted by the heat of the chemical reaction and a portion adheres to the formation wall thereby sealing the undesirable target zone; thereafter, the remaining balloon sections are deflated or ruptured to permit the apparatus to be withdrawn through the production tubing.
Owner:SAUDI ARABIAN OIL CO
LAYERED COMPOSITE MATERIALS HAVING THE COMPOSITION: (1-x-y)LiNiO2(xLi2Mn03)(yLiCoO2), AND SURFACE COATINGS THEREFOR
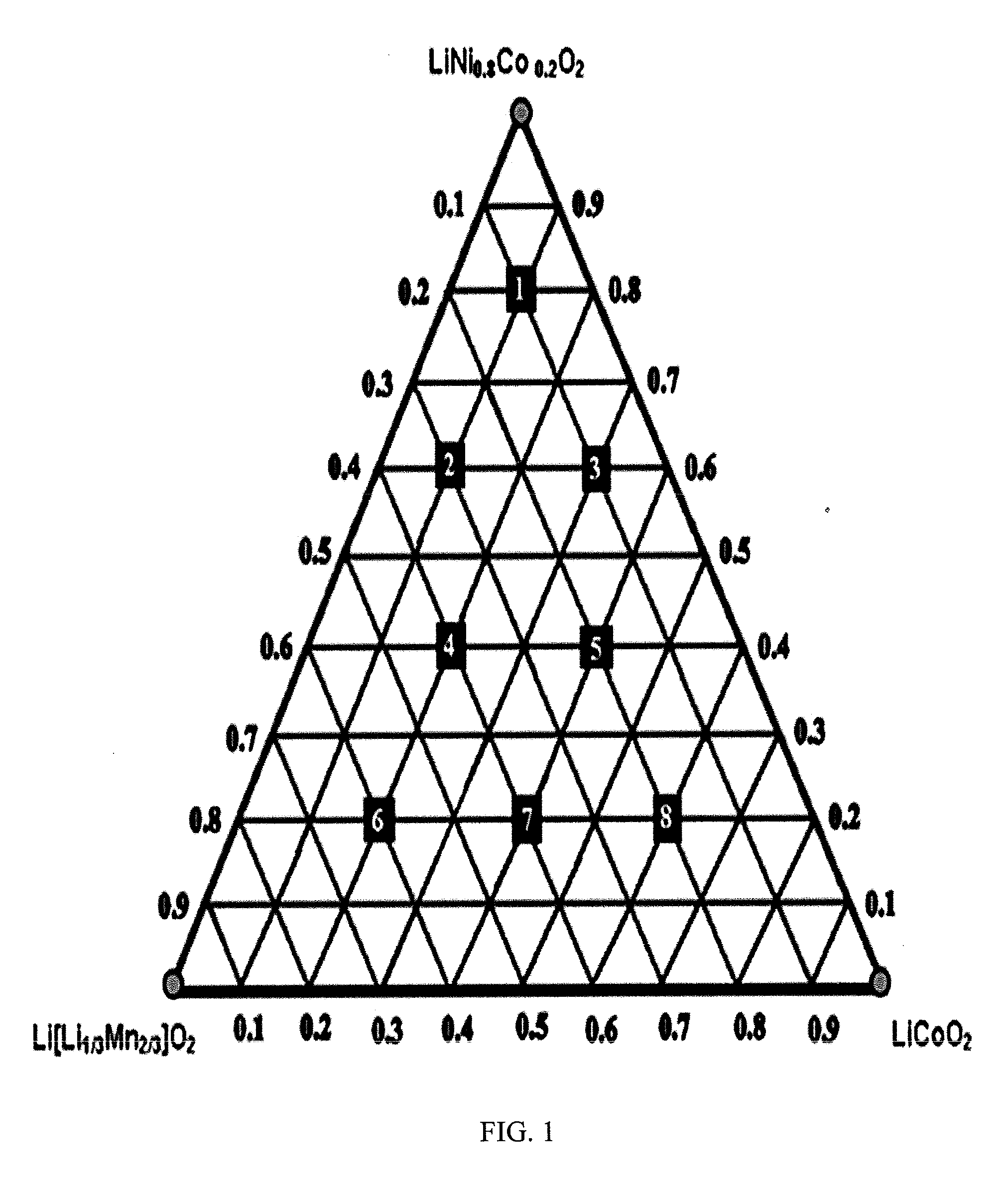
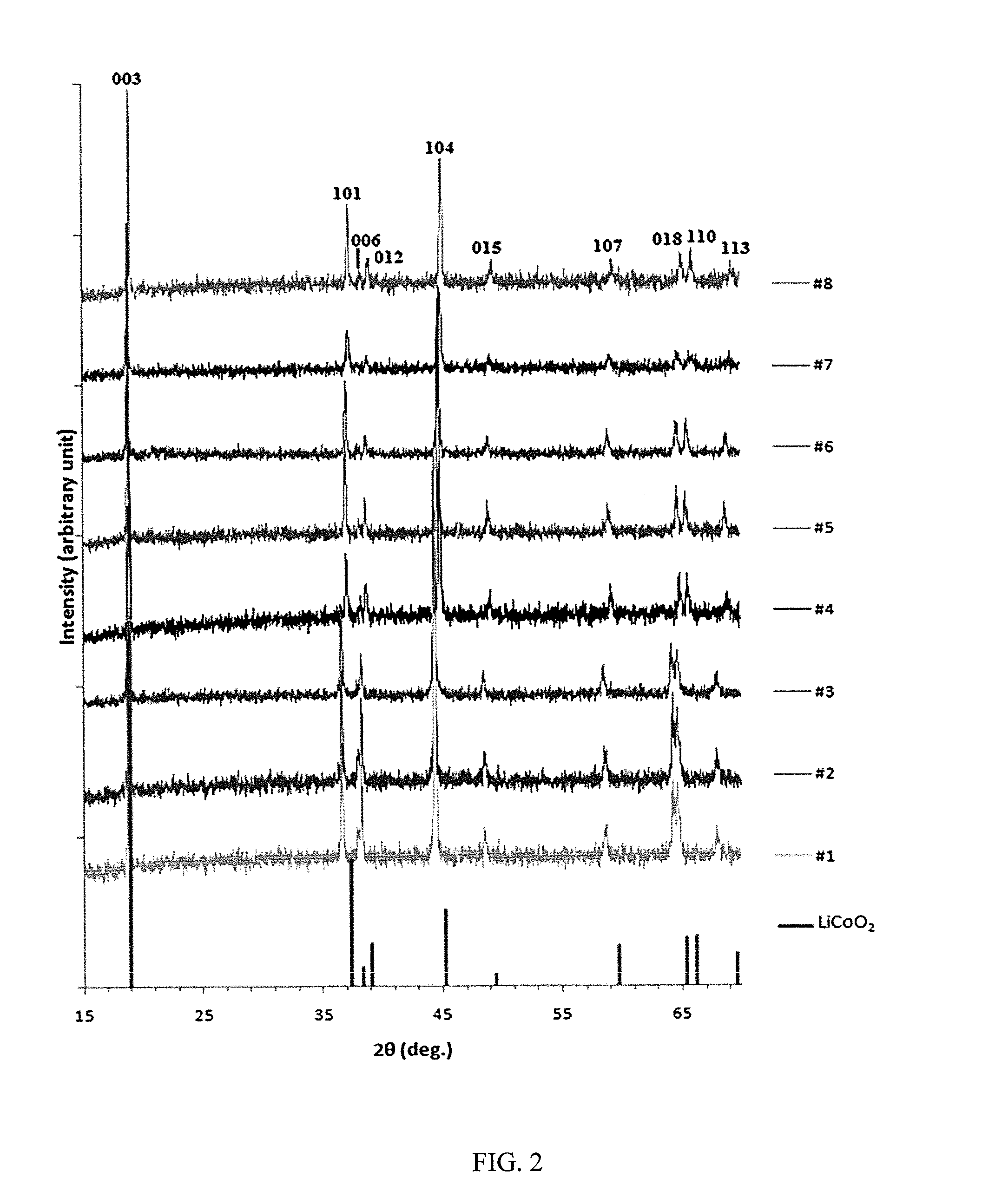
InactiveUS20120040247A1Improve discharge capacitySecondary cellsCeramic shaping apparatusX-rayCobalt
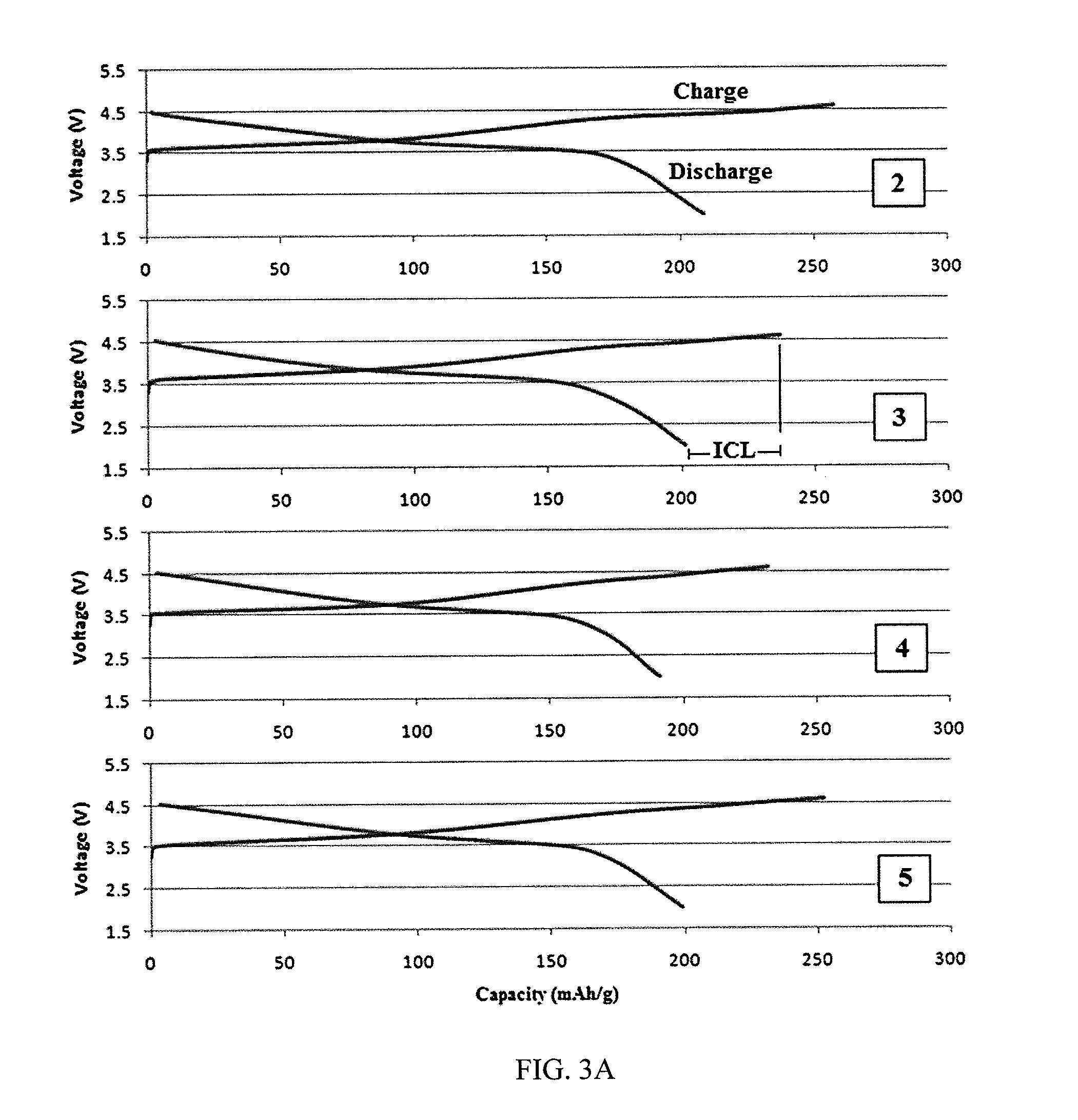
A straightforward and scalable solid-state synthesis at 975° C. used to generate cathode materials in the system Li(3+x)3Ni(1-x-y)CoyMn2x / 3O2 {a combination of LiNiO2, Li2MnO3, and LiCoO2 as (1-x-y)LiNiO2.xLi2MnO3.yLiCoO2} is described. Coatings for improving the characteristics of the cathode material are also described. A ternary composition diagram was used to select sample points, and compositions for testing were initially chosen in an arrangement conducive to mathematical modeling. X-ray diffraction (XRD) characterization showed the formation of an α-NaFeO2 structure, except in the region of compositions close to LiNiO2. Electrochemical testing revealed a wide range of electrochemical capacities with the highest capacities found in a region of high Li2MnO3 content. The highest capacity composition identified was Li1.222Mn0.444Ni0.167Co0.167O2 with a maximum initial discharge capacity of in the voltage range 4.6-2.0 V. Differential scanning calorimetry (DSC) testing on this material was promising as it showed an exothermic reaction of 0.2 W / g at 200° C. when tested up to 400° C. Cost for laboratory quantities of material yielded $1.49 / Ah, which is significantly lower than the cost of LiCoO2 due to the low cobalt content, and the straightforward synthesis. Li1.222Mn0.444Ni0.167Co0.167O2 is thought to be near optimum composition for the specified synthesis conditions, and shows excellent capacity and safety characteristics while leaving room for optimization in composition, synthesis conditions, and surface treatment.
Owner:COLORADO STATE UNIVERSITY
Device and method for treatment of eyelid diseases
ActiveUS7513893B2Prevent relapsePromotes better eyelid hygieneAerosol deliveryMedical applicatorsDiseaseMedicine
A container has an impermeable outer membrane sized to fit generally within the peri-orbital region and sufficiently flexible to mold to the structure of the closed eye. Heat which is generated by an exothermic reaction inside the container, which provides steady-state heat to the eyelids. A soft, non-abrasive, lint-free material is presoaked in a pH controlled (preferably antibacterial and hypoallergenic) detergent, with or without an ophthalmic antibiotic solution. The compress may also have a handle which makes holding and maneuvering the compress more feasible, while protecting the user's fingers from the heat, and preventing the contamination of the compress by the fingers. The compress may be packed in a watertight, sterile wrapper which will prevent drying of the detergent solution. Other embodiments are also described and claimed.
Owner:OCUGIENE
Methods of heating energy storage devices that power downhole tools
An energy storage device for powering a downhole tool may be heated to an effective temperature to improve the operability of the energy storage device. The energy storage device may comprise, for example, a primary battery, a secondary battery, a fuel cell, a capacitor, or combinations thereof. The effective temperature to which the energy storage device is heated may be greater than an ambient temperature in the wellbore near the energy storage device. The energy storage device may be heated using various heat sources such as an ohmic resistive heater, a heat pump, an exothermic reaction, a power generator, a heat transfer medium, the energy storage device itself, a downhole tool, or combinations thereof. A thermal conductor may extend between the heat source and the energy storage device. Further, a thermal insulator may at least partially surround the heat source and the energy storage device.
Owner:HALLIBURTON ENERGY SERVICES INC
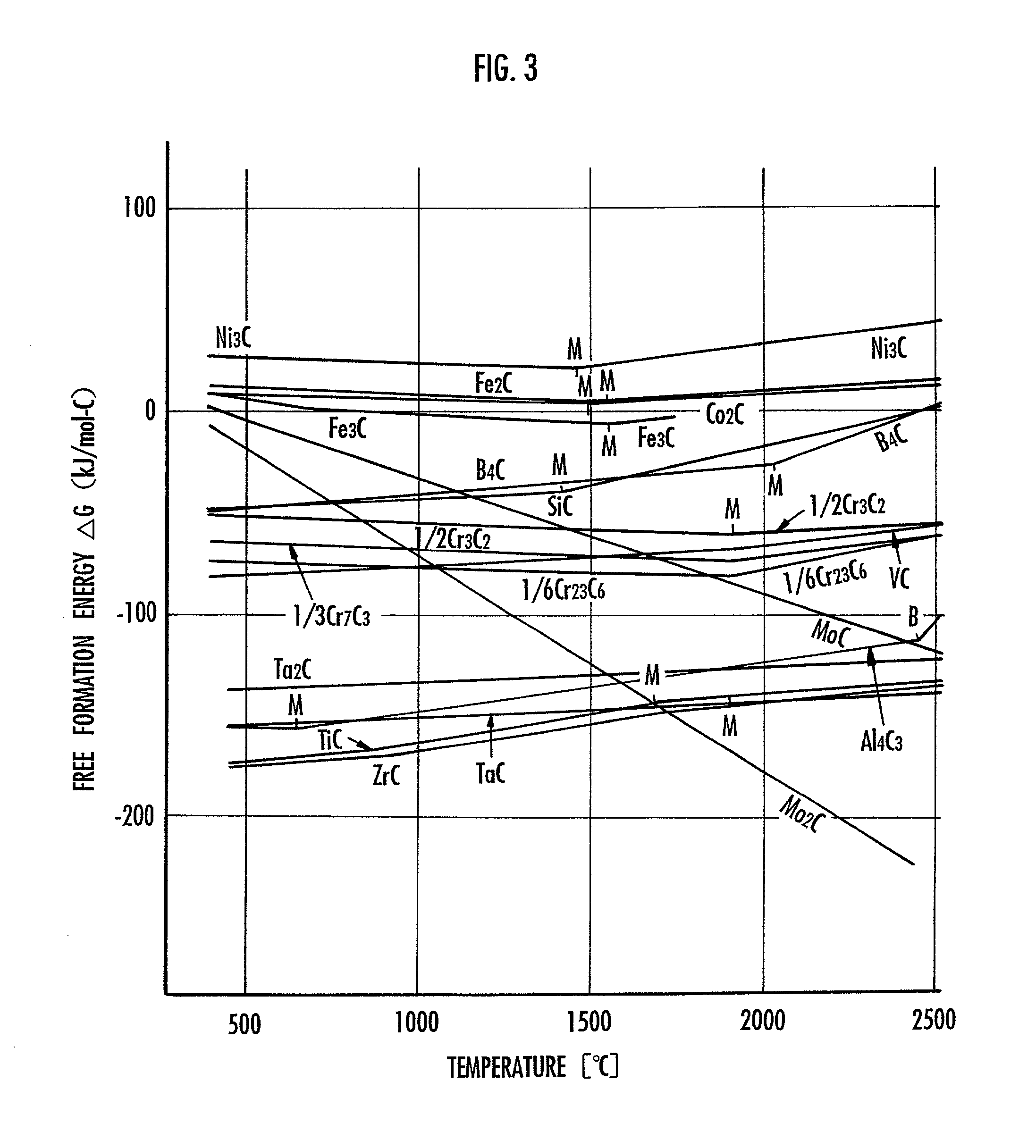
Method of manufacturing carbon nanotube
There is provided a method of manufacturing a carbon nanotube so as to be able to increase the yield of a web and to increase the amount of a carbon nanotube contained in the web. A high-energy heat source is caused to act on carbon in the presence of catalysts. The catalysts include a main catalyst made of at least one metal which is selected from the group consisting of an iron group element, a platinum group element, and a rare earth element, and an auxiliary catalyst made of a material which causes an exothermic reaction in a process of generating the web including the carbon nanotube. The auxiliary catalyst is made of a material for generating a carbide more stable in terms of thermal energy than a carbide generated by the main catalyst. The free formation energy of the carbide generated from the material is smaller than the free formation energy of the carbide generated by the main catalyst. The main catalyst is made of at least one metal which is selected from the group consisting of Fe, Co, Ni, Rh, Ru, Pd, Pt, Y, La, and Ce. The auxiliary catalyst is made of at least one material selected from the group consisting of Ti, Zr, Hf, V, Nb, Ta, Cr, Mo, W, B, Al, and Si. Typically the main catalyst is made of Ni-Y, and the auxiliary catalyst is made of Ti.
Owner:HONDA MOTOR CO LTD
Integrated catalytic process for converting alkanes to alkenes and catalysts useful for same
InactiveCN101165031AHydrocarbon by hydrogenationHeterogenous catalyst chemical elementsAlkanePtru catalyst
The present invention relates to an integrated multi-zone process for the conversion of alkanes to their corresponding alkenes comprising the exothermic conversion of a portion of the alkanes by oxidative dehydrogenation in an exothermic reaction zone in the presence of oxygen and a suitable catalyst to its corresponding alkenes, the product of said exothermic reaction zone is then passed to an endothermic reactor in which at least a portion of the remaining unconverted alkanes are absorbed in the presence of carbon dioxide and other suitable catalysts Thermal dehydrogenation.
Owner:ROHM & HAAS CO
Non-hot spot calandria type fixed bed reactors
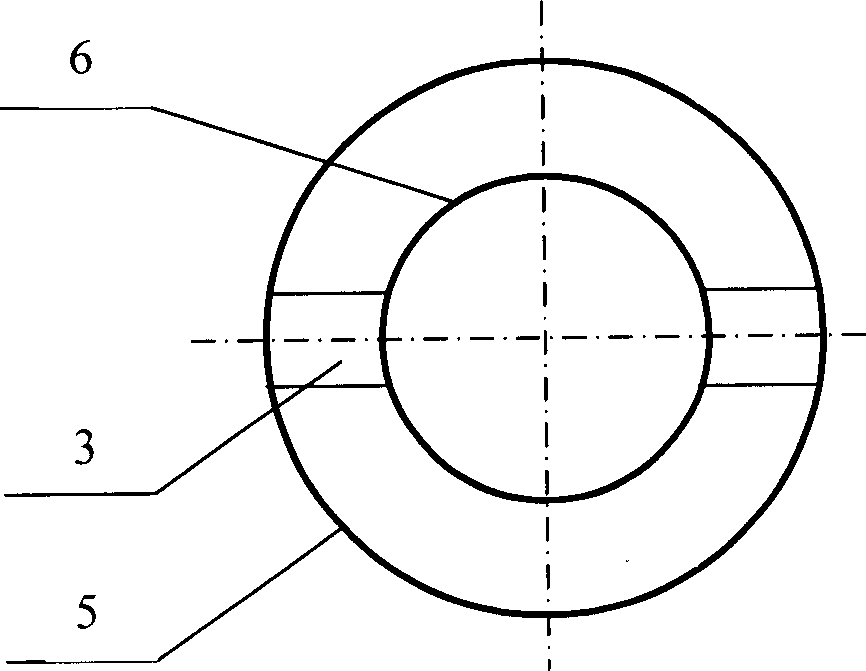
InactiveCN1736574AIncrease the heat exchange areaIncrease heat transfer areaChemical/physical processesReaction rateDiameter ratio
Disclosed is a non- thermal point tubular fixed bed reactor, which is technically characterized in that: every tube of the reactor adopts annular tube structure, the inner tubes being closed, the inner tube and outer tube being linked to the shell side of the reactor by canal, the catalyst being filled into the space between tubes to form a catalyst bed layer, the bottom and top of the tubes being equipped with a cooling medium distributing plate. The diameter ratio of the inner tube to outer tube can be regulated according to the reaction rate and the operation temperature to make the reaction heat transfer to the double- side. Dimension of opening of the cooling medium distributing plate and dimension of the opening of the side- wall of double terminals of inner tube can be regulated to control the distribution of cooling medium between inner tube and the shell side of reactor. With the invention, in the condition of no increasing the number of tubes largely, it can increase the heat- exchange area of the tubular fixed bed reactor and decrease the heat- exchange route.
Owner:TSINGHUA UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com