Patents
Literature
8365 results about "Hydrogen production" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
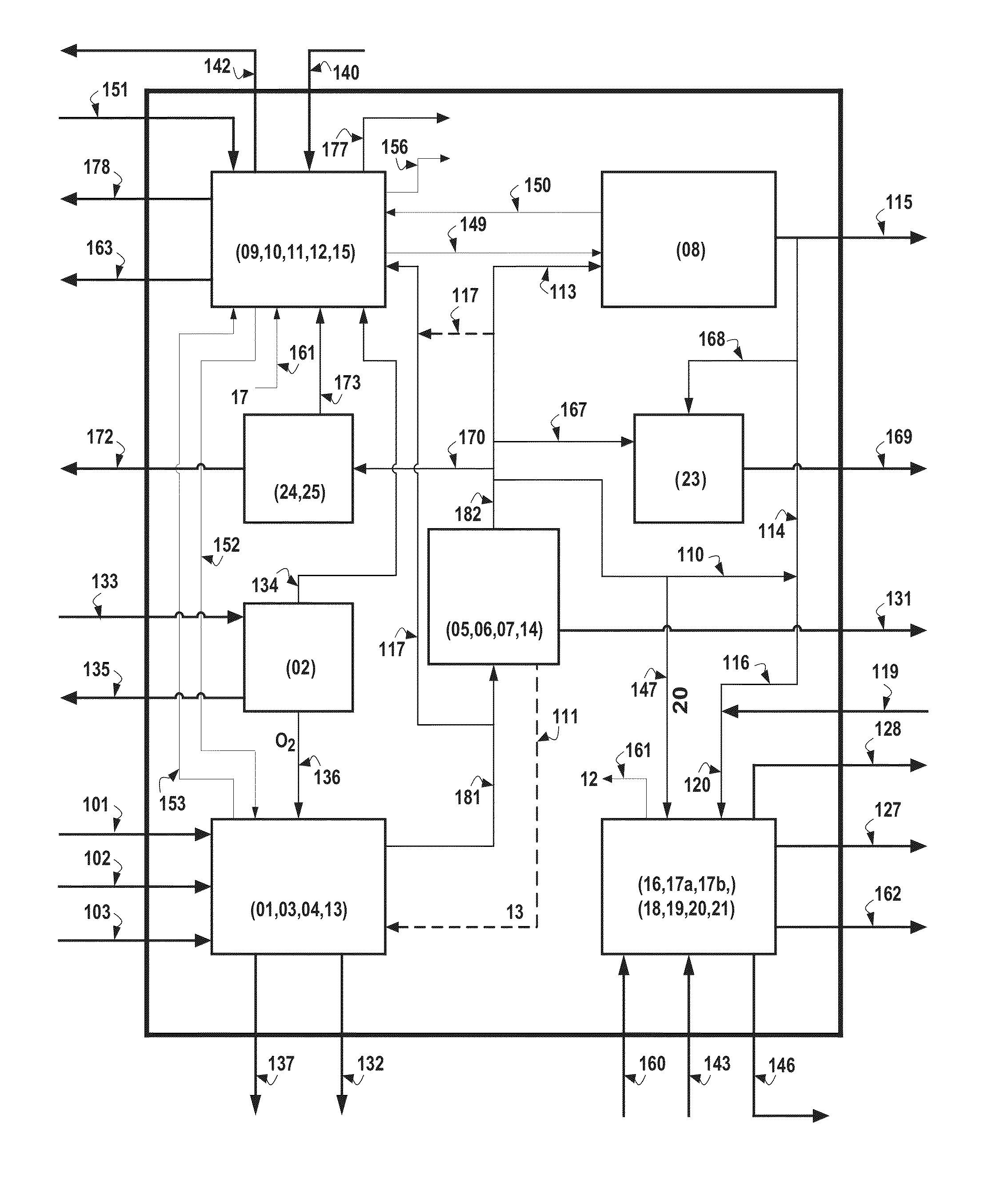
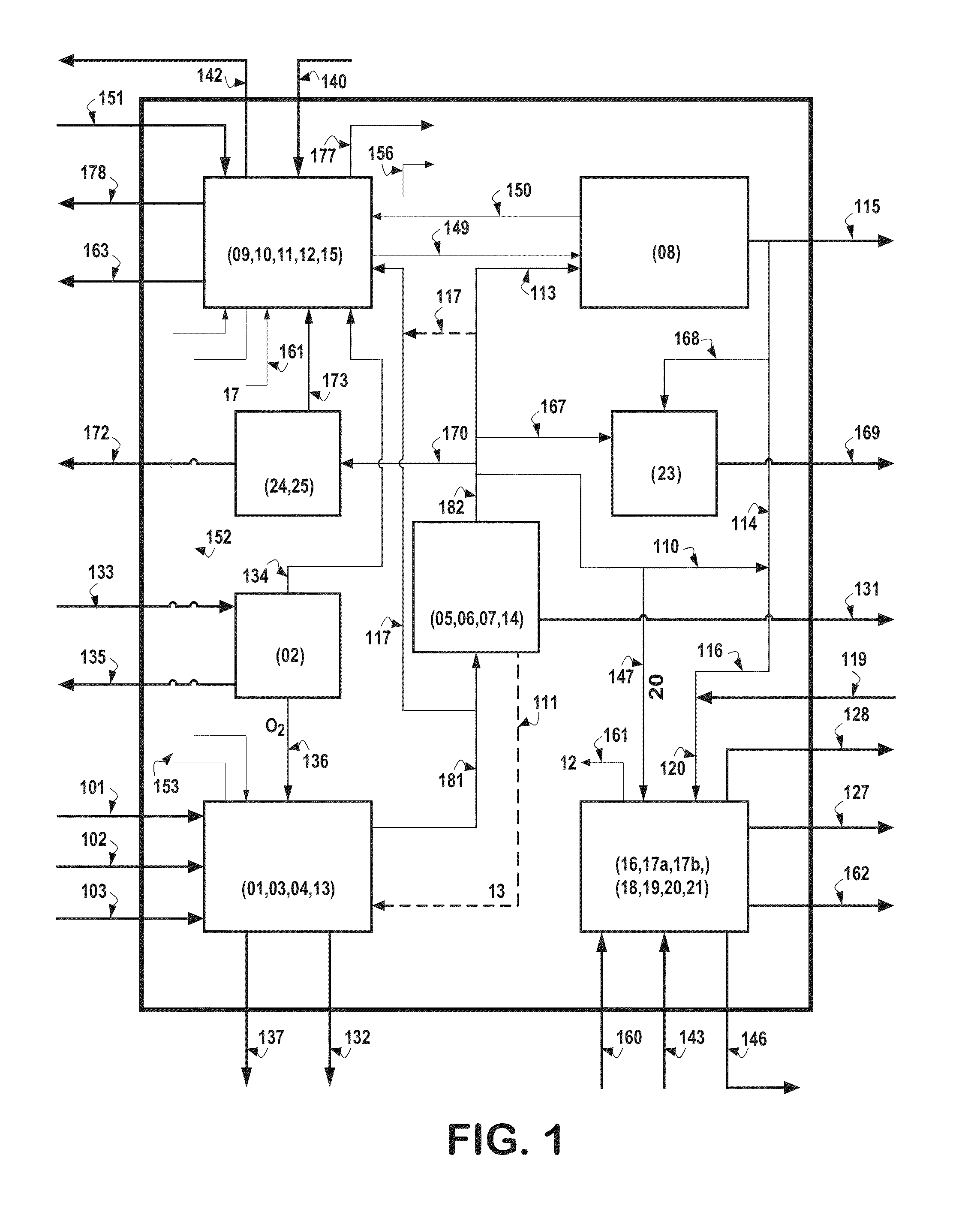
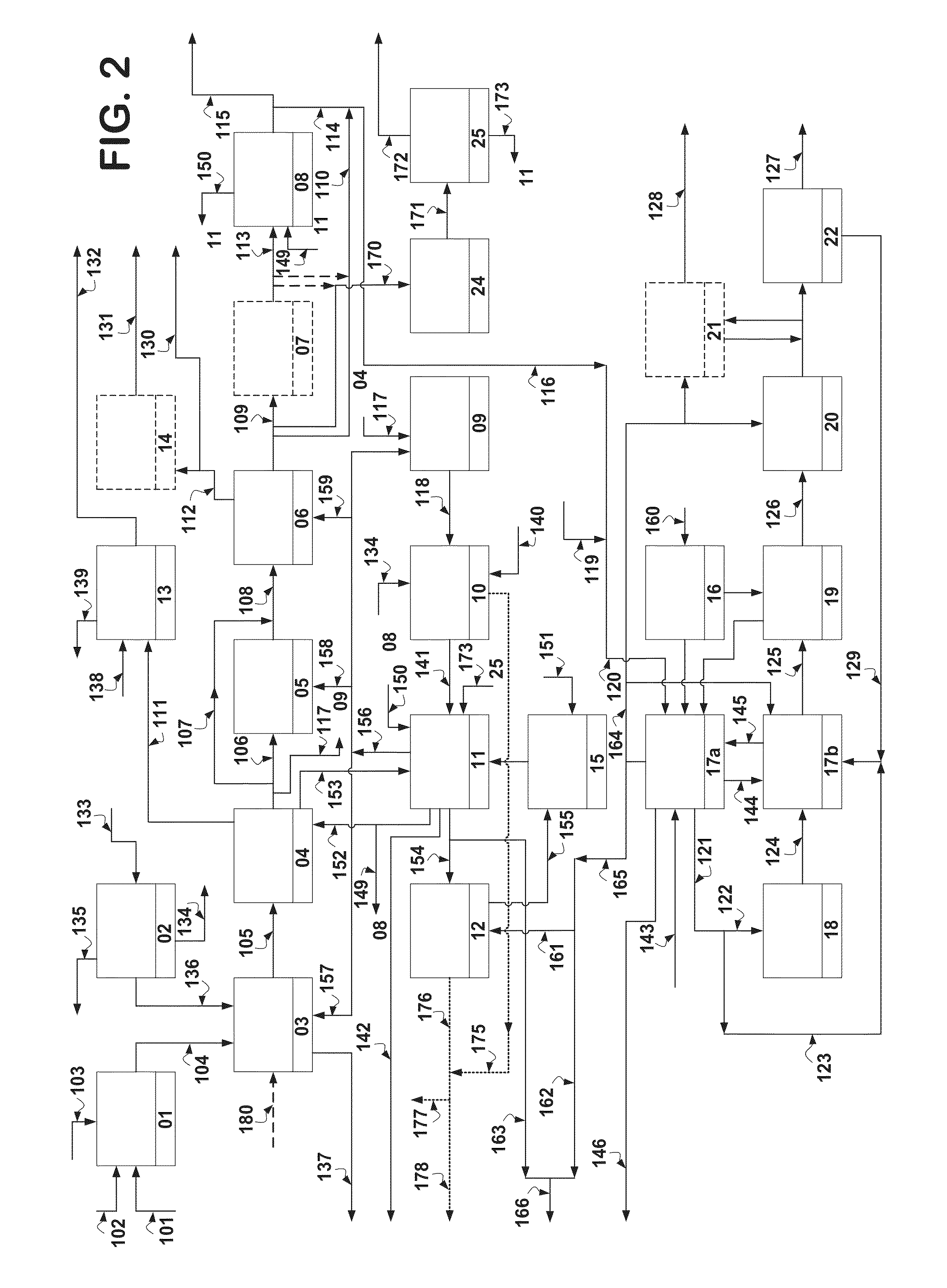
Hydrogen production is the family of industrial methods for generating hydrogen. Hydrogen is primarily produced by steam reforming of natural gas. Other major sources include naphtha or oil reforming of refinery or other industrial off-gases, and partial oxidation of coal and other hydrocarbons. A small amount is obtained by water electrolysis and other sources.
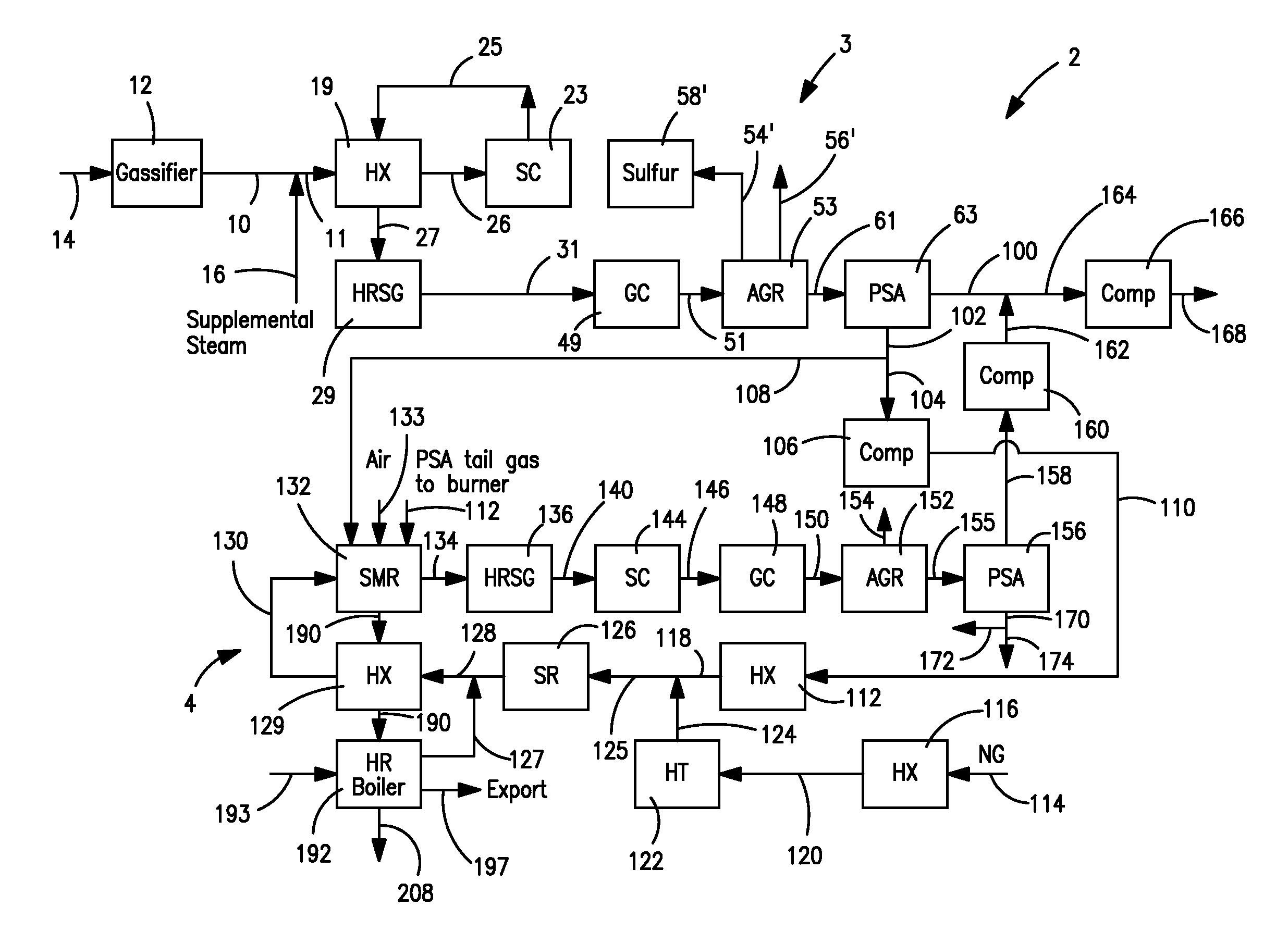
Hydrogen production from carbonaceous material
InactiveUS6790430B1Avoid the needCalcium/strontium/barium carbonatesGas turbine plantsCalcinationExothermic reaction
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE +1
Hydrogen production with photosynthetic organisms and from biomass derived therefrom
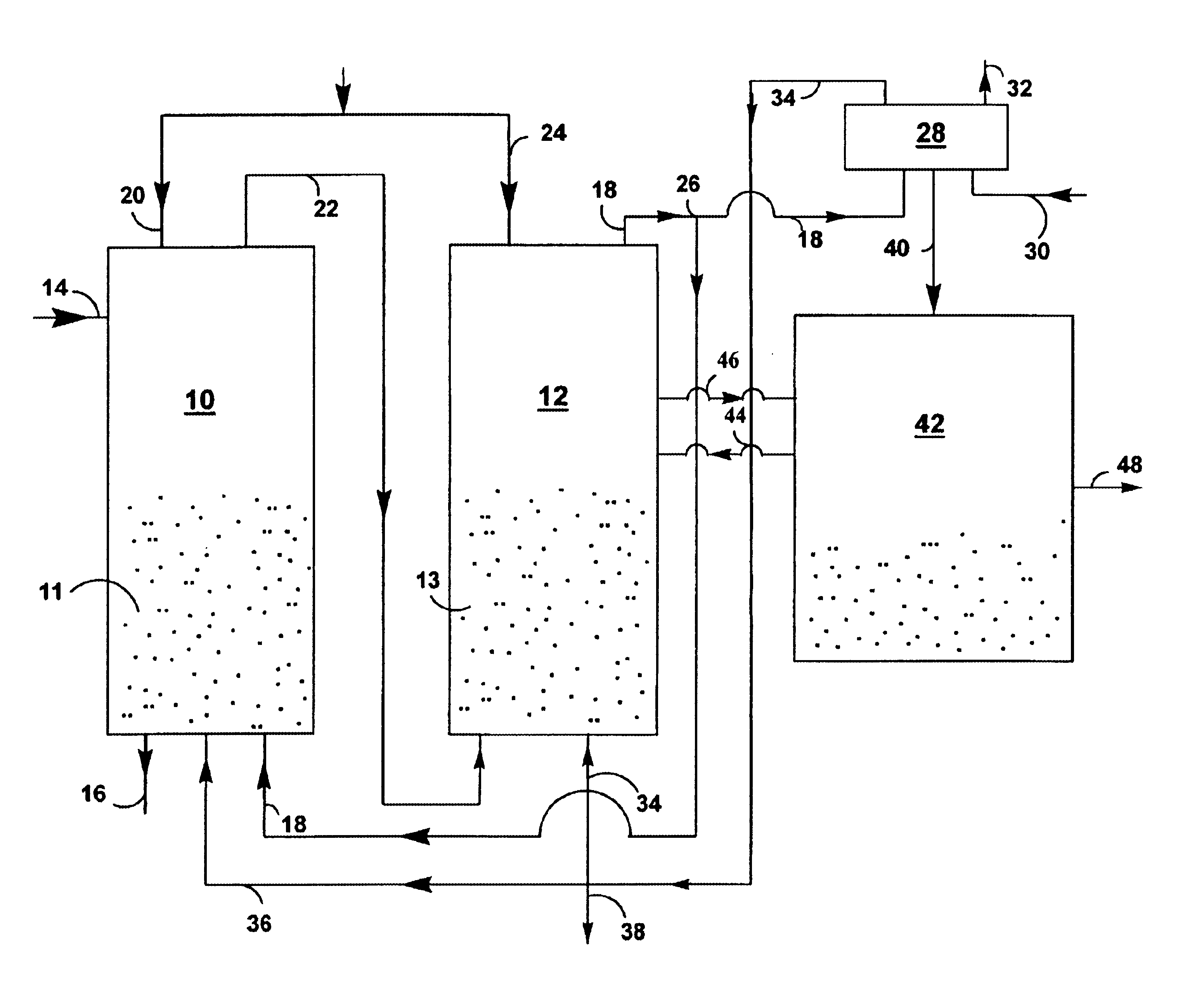
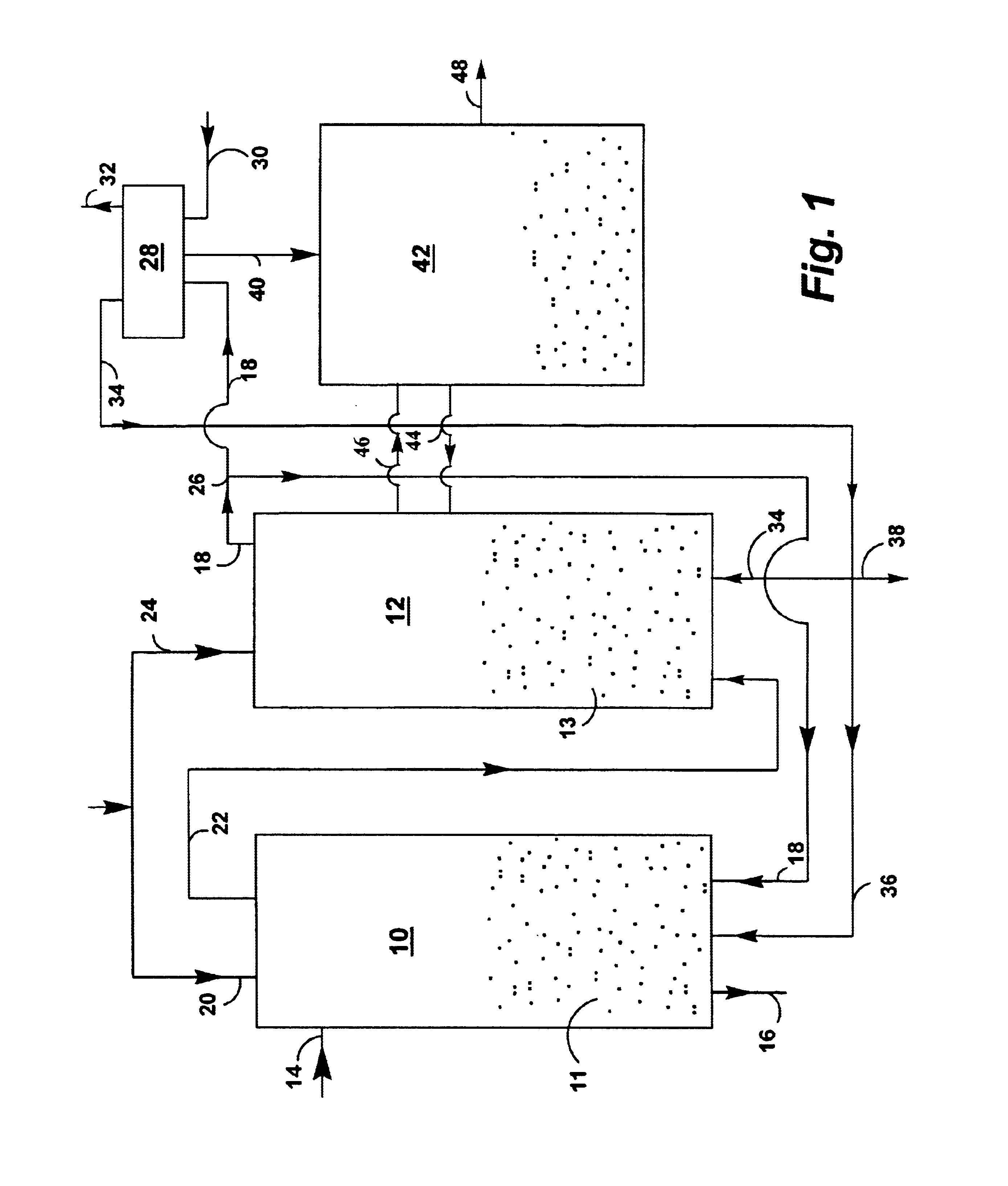
InactiveUS20050064577A1Bioreactor/fermenter combinationsDispersed particle separationLiquid mediumCombustion
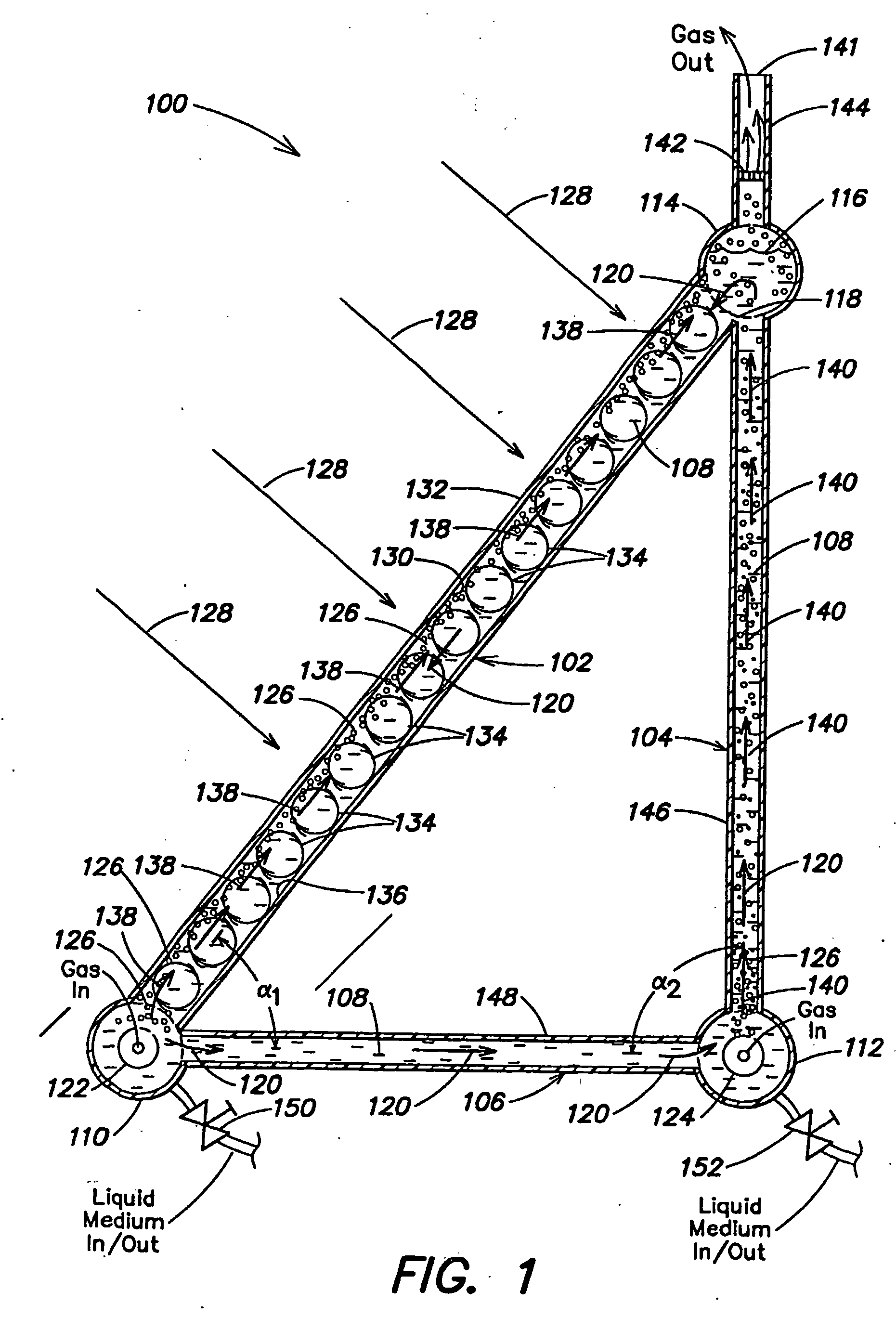
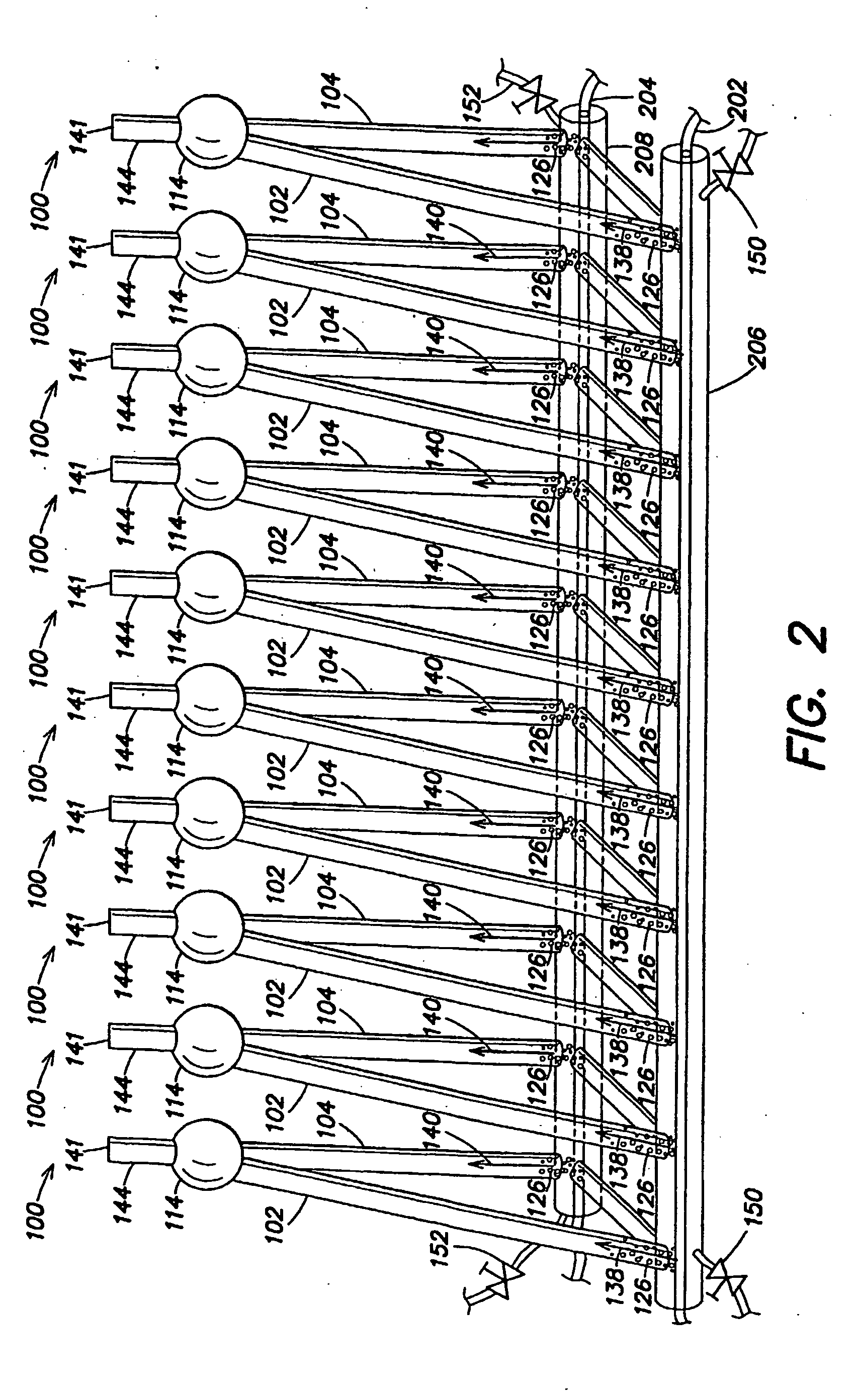
Certain embodiments and aspects of the present invention relate to photobioreactor apparatus designed to contain a liquid medium comprising at least one species of photosynthetic organism therein, and to methods of using the photobioreactor apparatus as part of a hydrogen production process and system configured to generate hydrogen with and / or from biomass produced in the photobioreactor apparatus. In certain embodiments, the disclosed hydrogen production systems and methods, photobioreactor apparatus, methods of using such apparatus, and / or gas treatment systems and methods provided herein can be utilized as part of an integrated combustion and hydrogen production method and system, wherein photosynthetic organisms utilized within the photobioreactor are used to at least partially remove certain pollutant compounds contained within combustion gases, e.g. CO2 and / or NOx, and are subsequently harvested from the photobioreactor, processed, and utilized as a fuel source for generating hydrogen and / or as a fuel source for a combustion device (e.g. an electric power plant generator and / or incinerator).
Owner:GREENFUEL TECHNOLOGIES CORPORATION
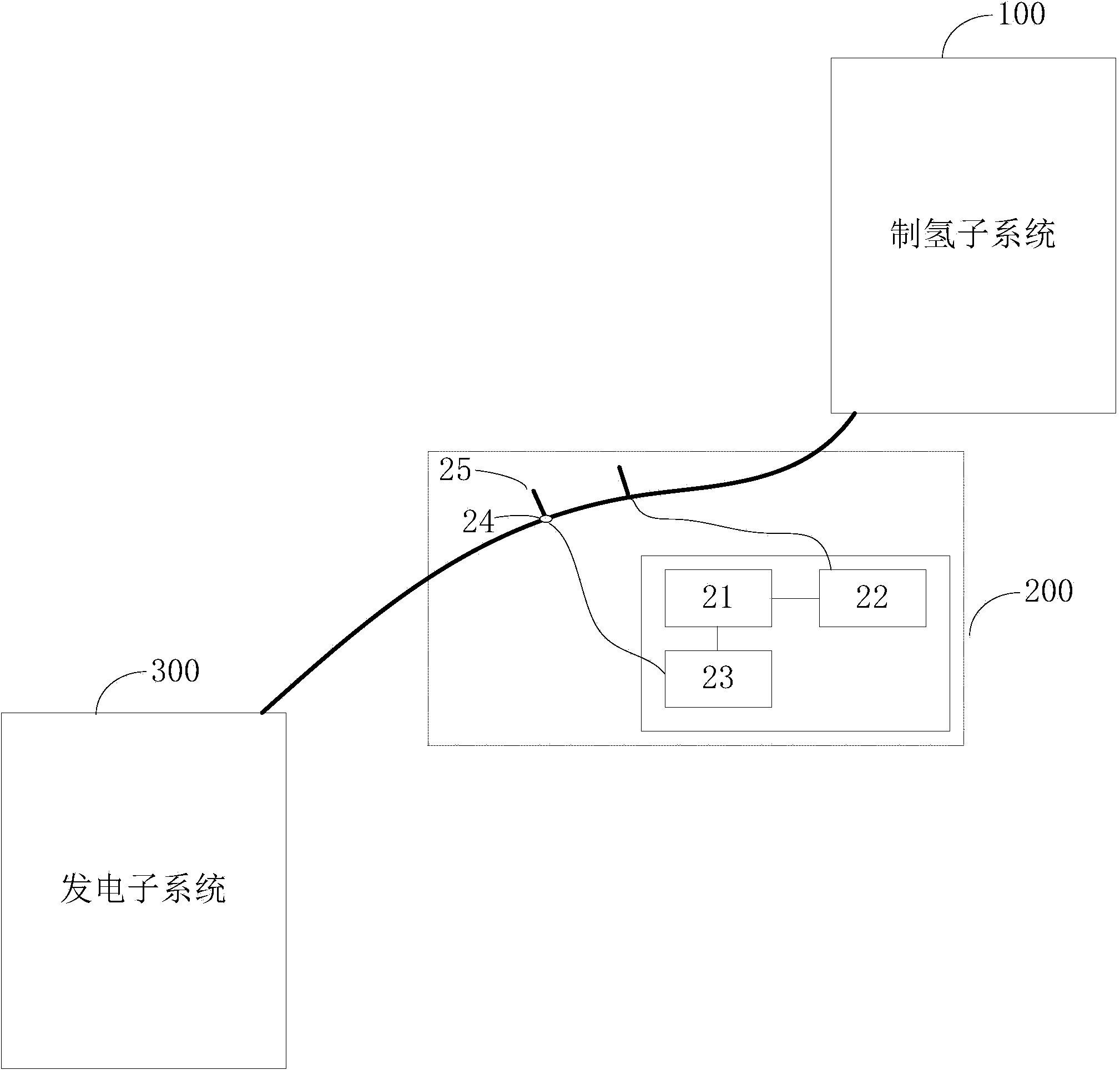
Instant hydrogen-production power generation system and method
InactiveCN103618100AImprove power generation efficiencySave energyReactant parameters controlMethanol waterWater source
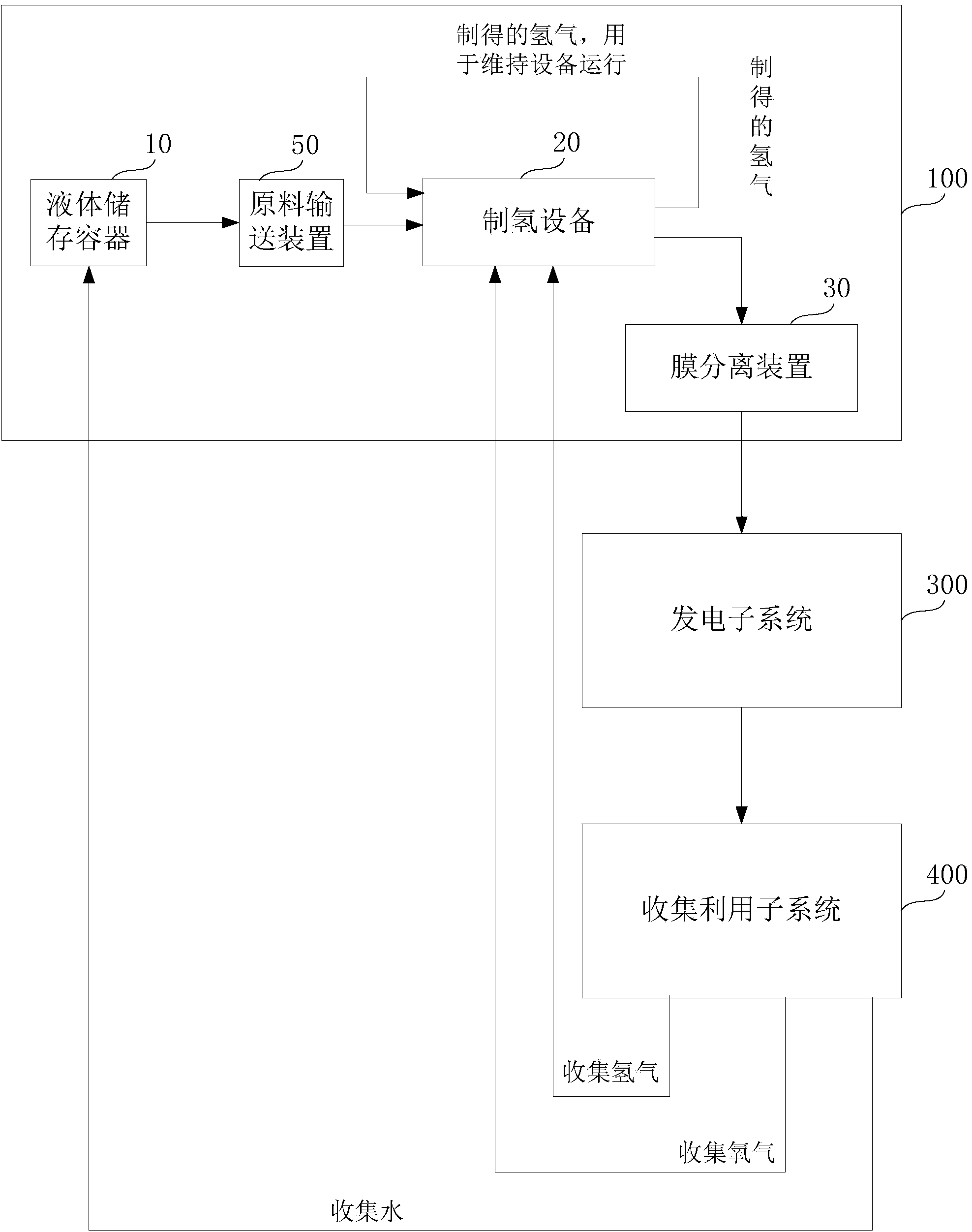
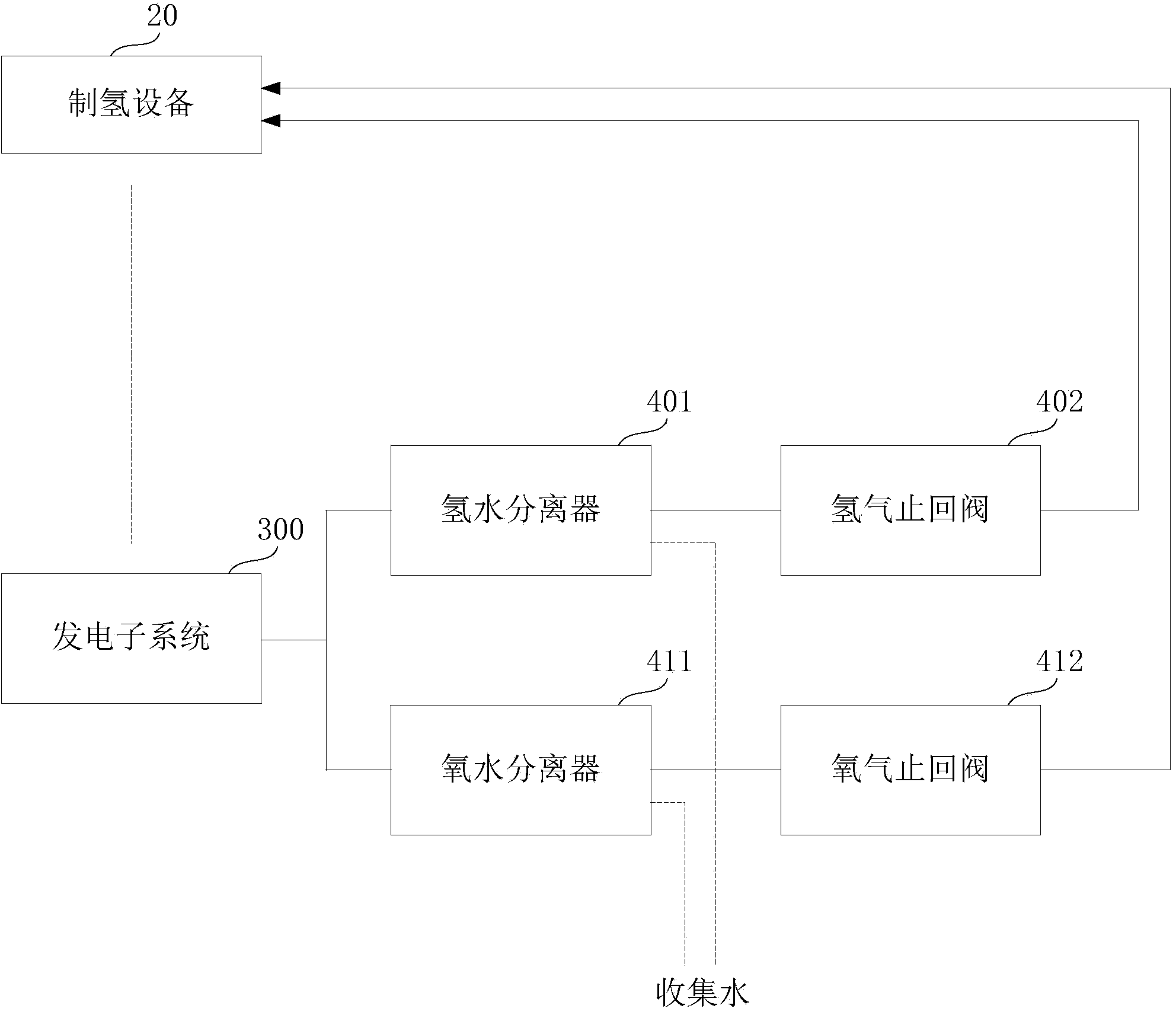
The invention discloses an instant hydrogen-production power generation system and method. The system comprises a hydrogen-production subsystem, a power generation subsystem and a collection and utilization subsystem, wherein the hydrogen-production subsystem, power generation subsystem and collection and utilization subsystem are connected sequentially; the hydrogen-production subsystem uses methanol water to produce hydrogen, and transfers the produced hydrogen to the power generation subsystem in time through a transfer pipeline for power generation; and the collection and utilization subsystem is connected with an exhaust channel outlet of the power generation subsystem, and is used for collecting water from discharged gas or collecting water as the raw material of the hydrogen-production subsystem. The system and method can collect residual gas discharged from the power generation subsystem, and extract hydrogen, oxygen and water from the residual gas; and the hydrogen and oxygen can be combusted to release heat so as to provide heat energy for the power generation subsystem, and the water can be transferred to the hydrogen-production subsystem for cyclic utilization, so that the system does not need any additional water source. The system and method can enhance the power generation efficiency of the system and save the energy source.
Owner:广州市移电科技有限公司
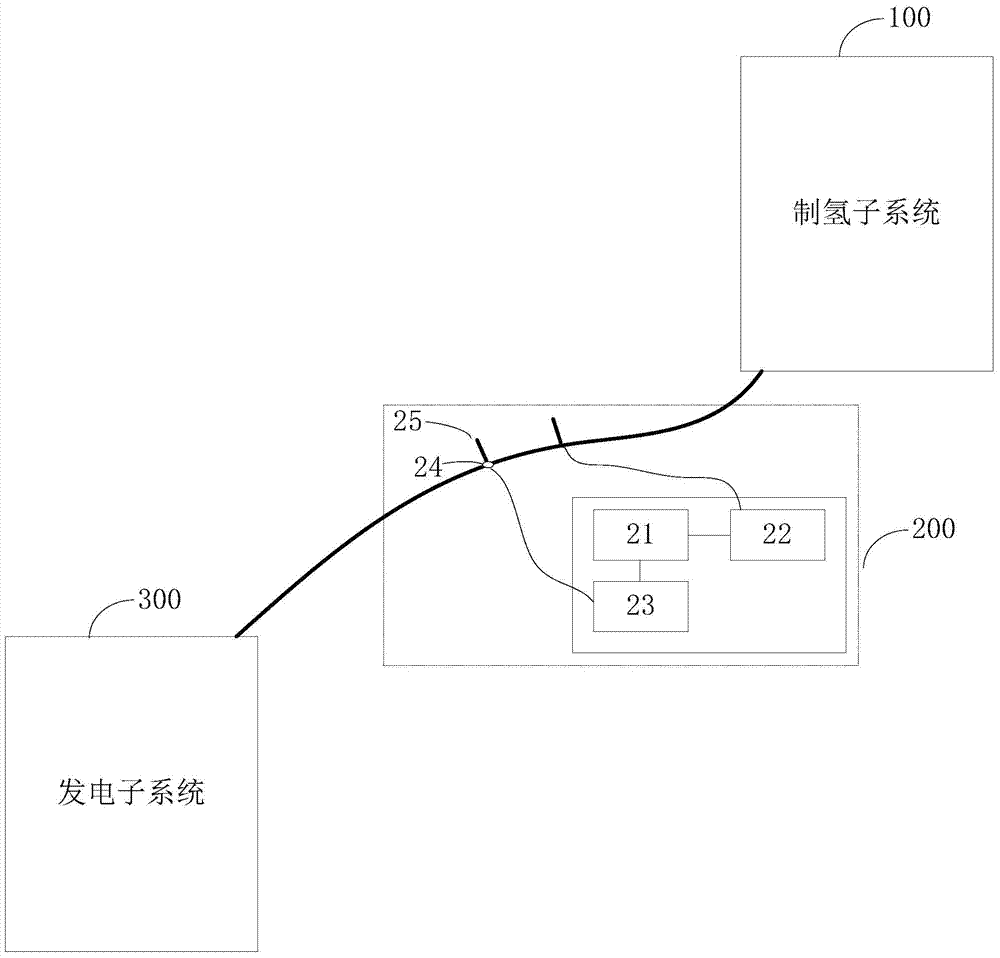
System and method for instant hydrogen production and power generation
The invention discloses a system and a method for instant hydrogen production and power generation. The system comprises a hydrogen production subsystem, an air pressure adjusting subsystem and a power generation subsystem, wherein the hydrogen production subsystem is used for preparing hydrogen from methanol water and transmitting the prepared hydrogen to the power generation subsystem in real time through a transmission pipeline; the transmission pipeline is provided with the air pressure adjusting subsystem for adjusting air pressure inside the transmission pipeline; the power generation subsystem is used for generating power by virtue of hydrogen prepared by the hydrogen production subsystem; the air pressure adjusting subsystem comprises a microprocessor, an air pressure sensor, a valve controller and an air outlet valve; the air pressure sensor is arranged in the transmission pipeline, and is used for sensing data of the air pressure inside the transmission pipeline and sending the data of the air pressure to the microprocessor; the microprocessor is used for controlling the on and off of the air outlet valve according to the data of the air pressure sensed by the air pressure sensor. Power can be generated by instantly prepared hydrogen, a hydrogen buffer tank is not required, and thus the portability and mobility of the hydrogen production and power generation system can be improved.
Owner:SHANGHAI HYDROGEN MOBILE REFRMER INSTR
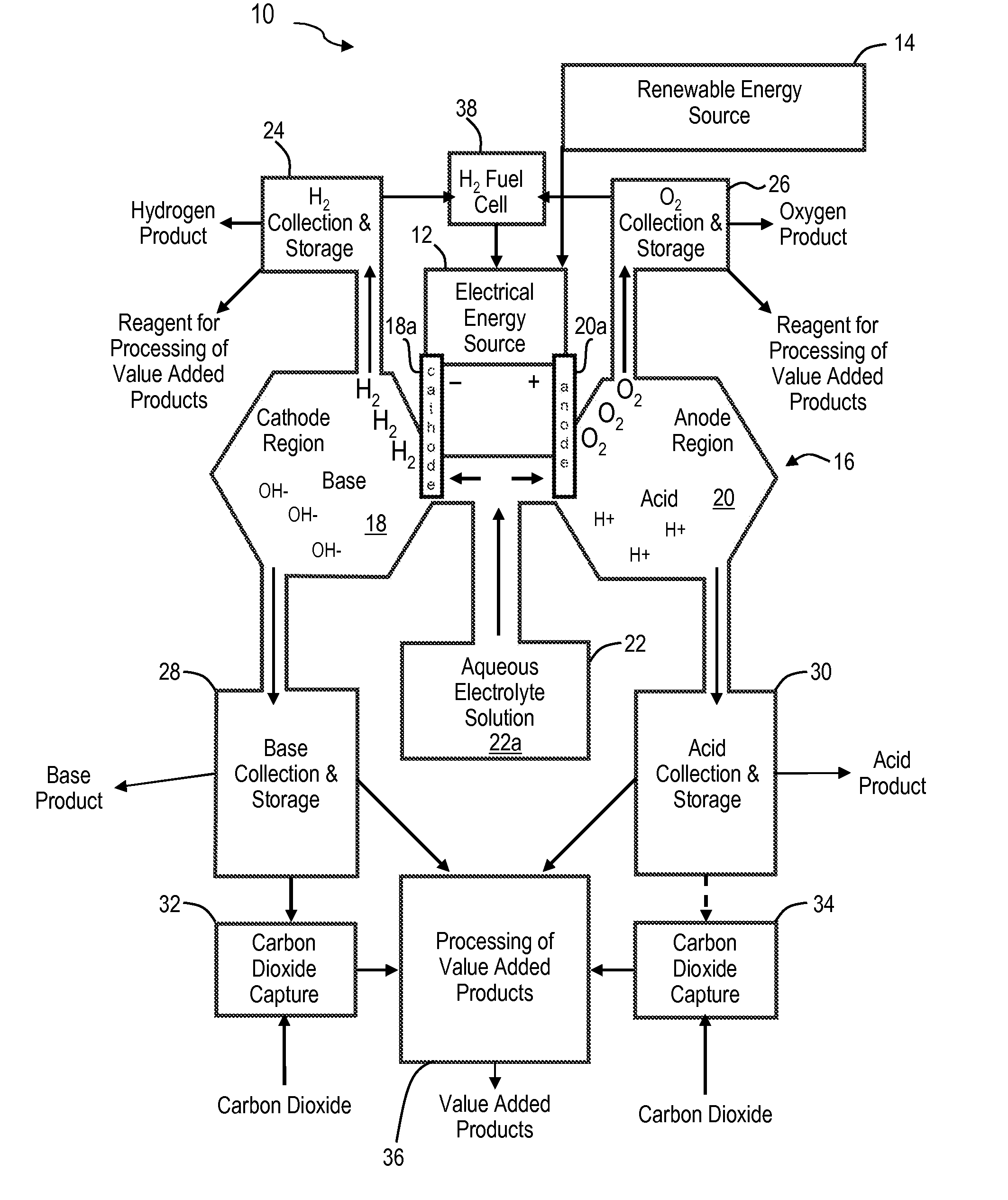
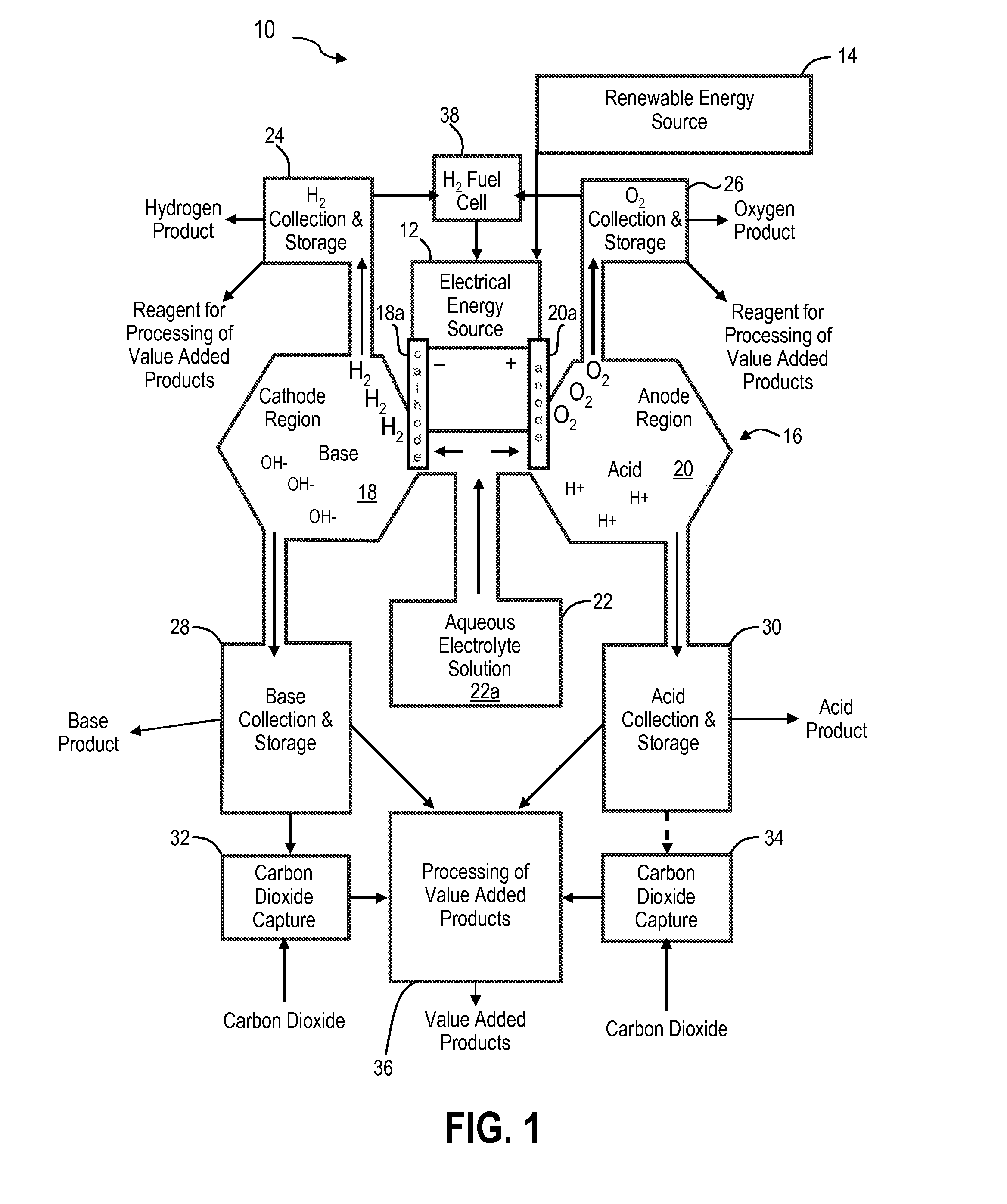
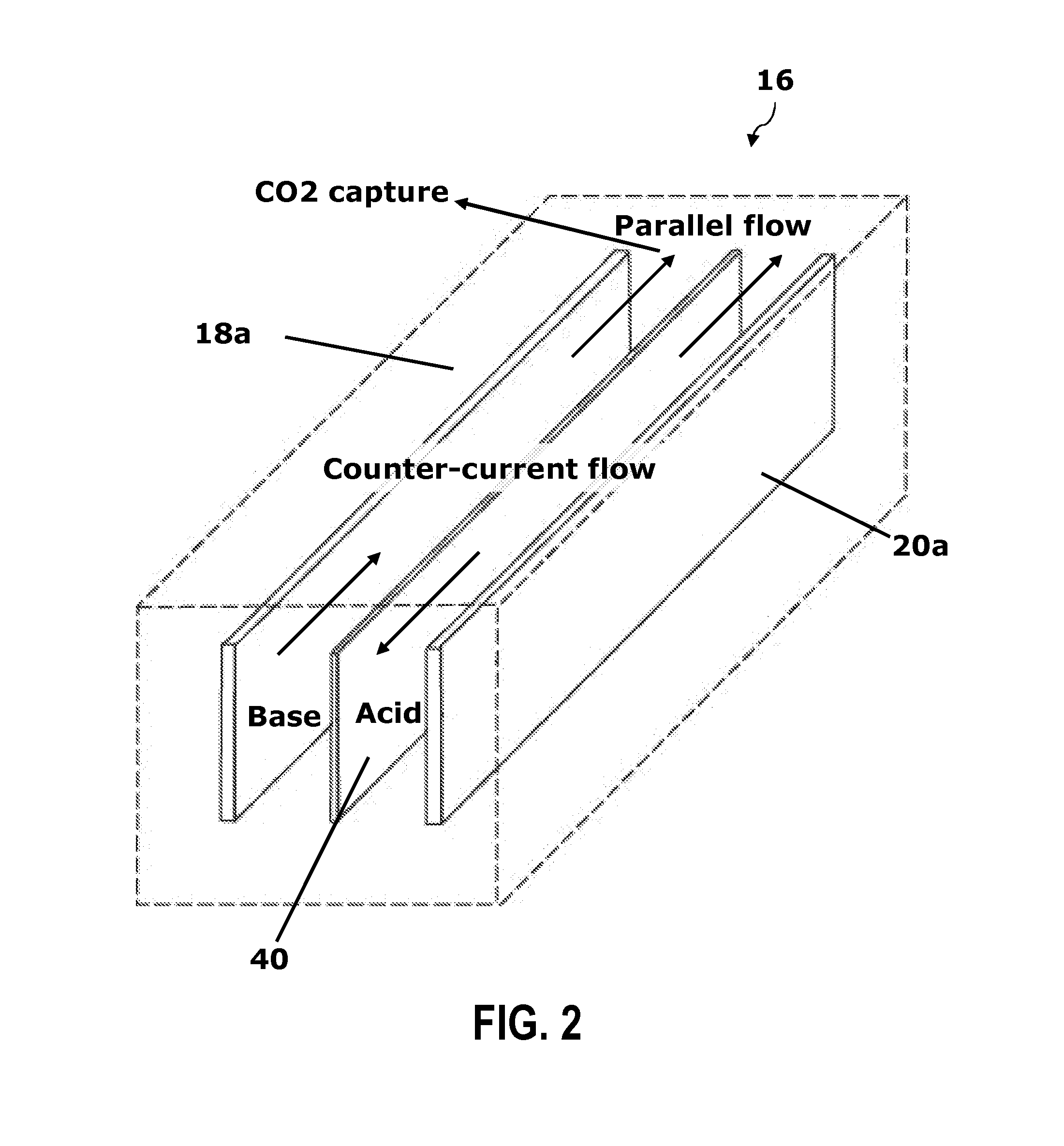
Renewable energy system for hydrogen production and carbon dioxide capture
The present invention is an integrated system for the production of hydrogen and the removal of carbon dioxide from the air or gas streams. The integrated system includes an energy source for generating electrical energy and a water source coupled to the energy source. The water source includes ionic electrolytes. The energy source supplies energy to the water source to electrolyze water to produce hydrogen gas, oxygen gas, acid and base. The carbon dioxide reacts with the base. In some embodiments, the energy source is a renewable energy source. The integrated system produces substantially no carbon dioxide and when combined with a renewable energy source, produces clean hydrogen fuel and reduces atmospheric carbon dioxide, resulting in carbon dioxide negative energy and manufacturing strategies.
Owner:SULFURCYCLE INTPROP HLDG CO LLC
Portable hydrogen generator-fuel cell apparatus
InactiveUS6653005B1Increase specific energy and overall energy efficiencyHydrogenFuel cell auxillariesKeroseneImpurity
A compact hydrogen generator is coupled to or integrated with a fuel cell for portable power applications. Hydrogen is produced via thermocatalytic decomposition (cracking, pyrolysis) of hydrocarbon fuels in oxidant-free environment. The apparatus can utilize a variety of hydrocarbon fuels, including natural gas, propane, gasoline, kerosene, diesel fuel, crude oil (including sulfurous fuels). The hydrogen-rich gas produced is free of carbon oxides or other reactive impurities, so it could be directly fed to any type of a fuel cell. The catalysts for hydrogen production in the apparatus are carbon-based or metal-based materials and doped, if necessary, with a sulfur-capturing agent. Additionally disclosed are two novel processes for the production of two types of carbon filaments, and a novel filamentous carbon product. The hydrogen generator can be conveniently integrated with high temperature fuel cells to produce an efficient and self-contained source of electrical power.
Owner:UNIV OF CENT FLORIDA RES FOUND INC +1
Two-mode process for hydrogen production
InactiveUS20110064648A1Increase the amount of carbonIncrease volumeGaseous fuelsGasification processes detailsHydrogen productionMethane
The present invention relates to a 2-mode processes for preparing gaseous products, and in particular a hydrogen product stream, via the hydromethanation of carbonaceous feedstocks in the presence of steam, carbon monoxide, hydrogen and a hydromethanation catalyst in a first mode, and a partial oxidation of methane in a second mode.
Owner:SURE CHAMPION INVESTMENT LTD
Systems and methods for power generation and hydrogen production with carbon dioxide isolation
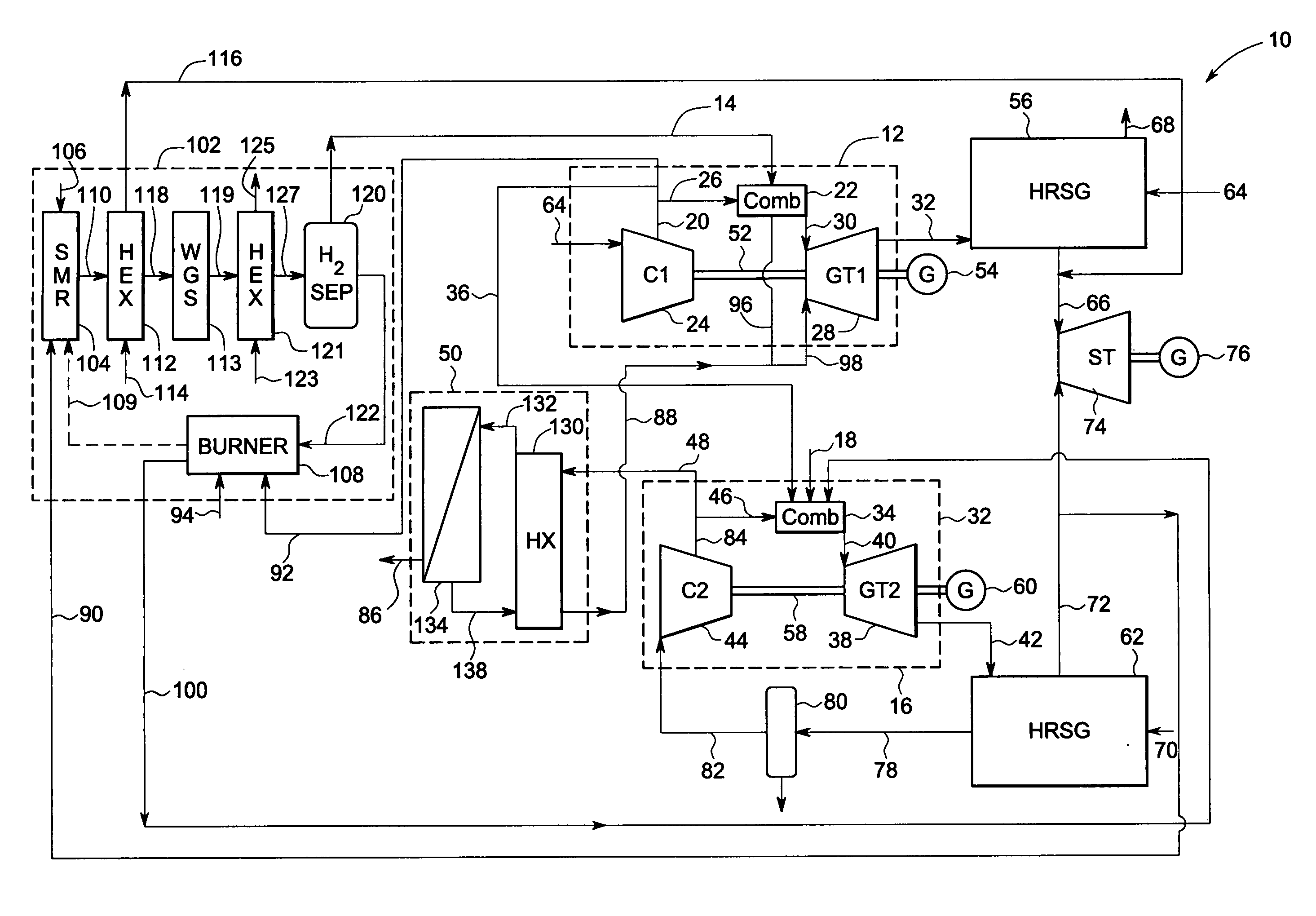
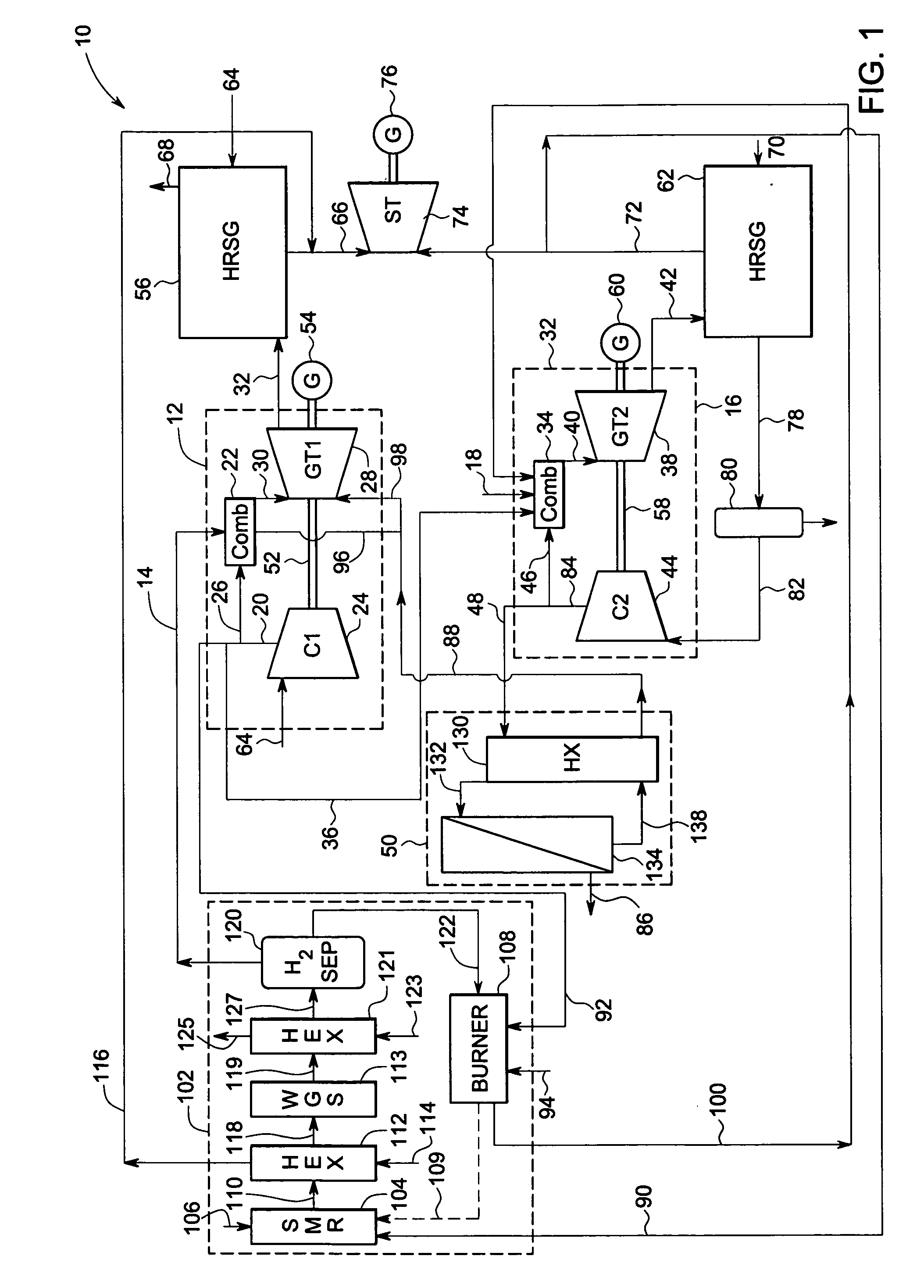
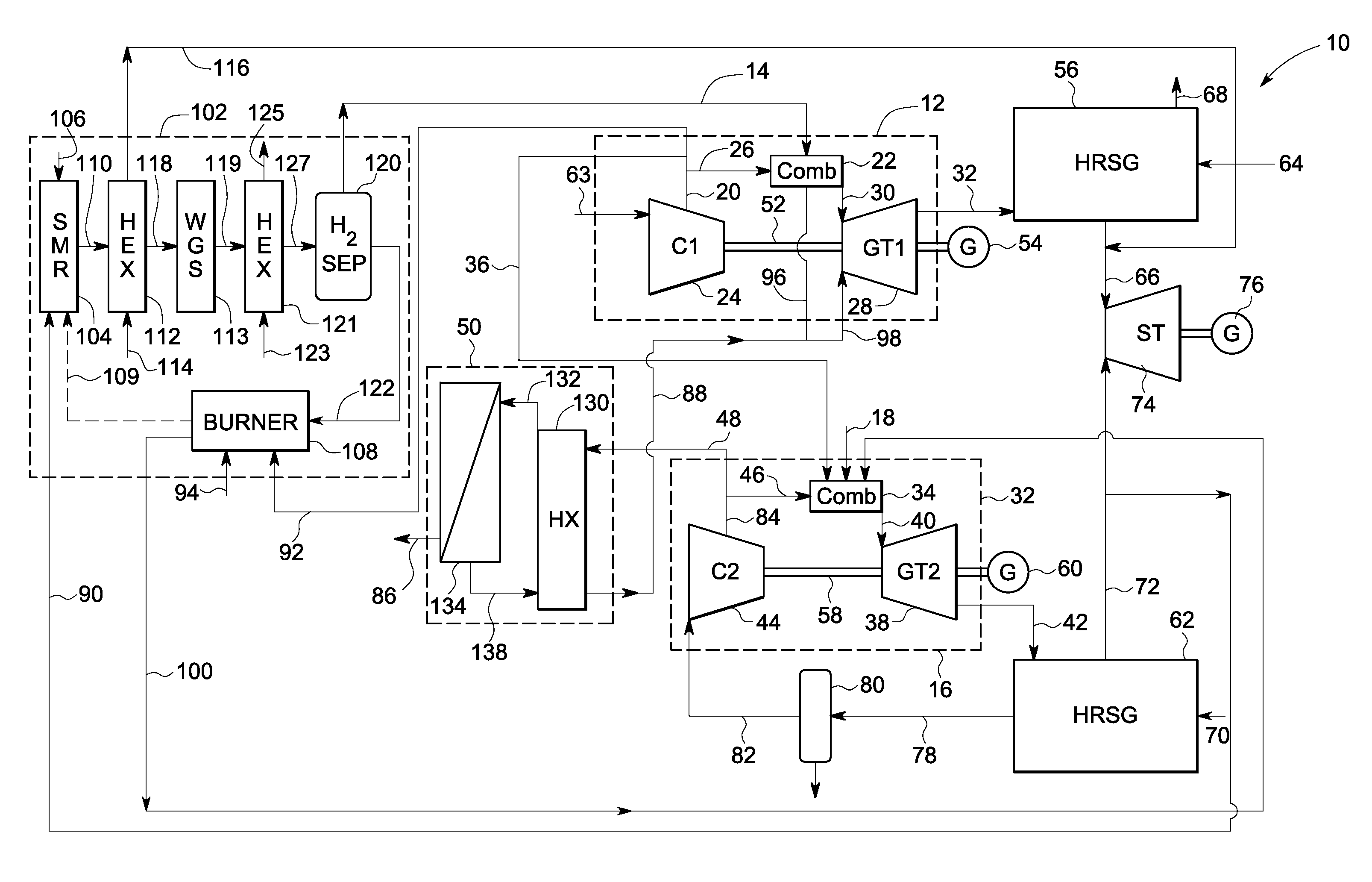
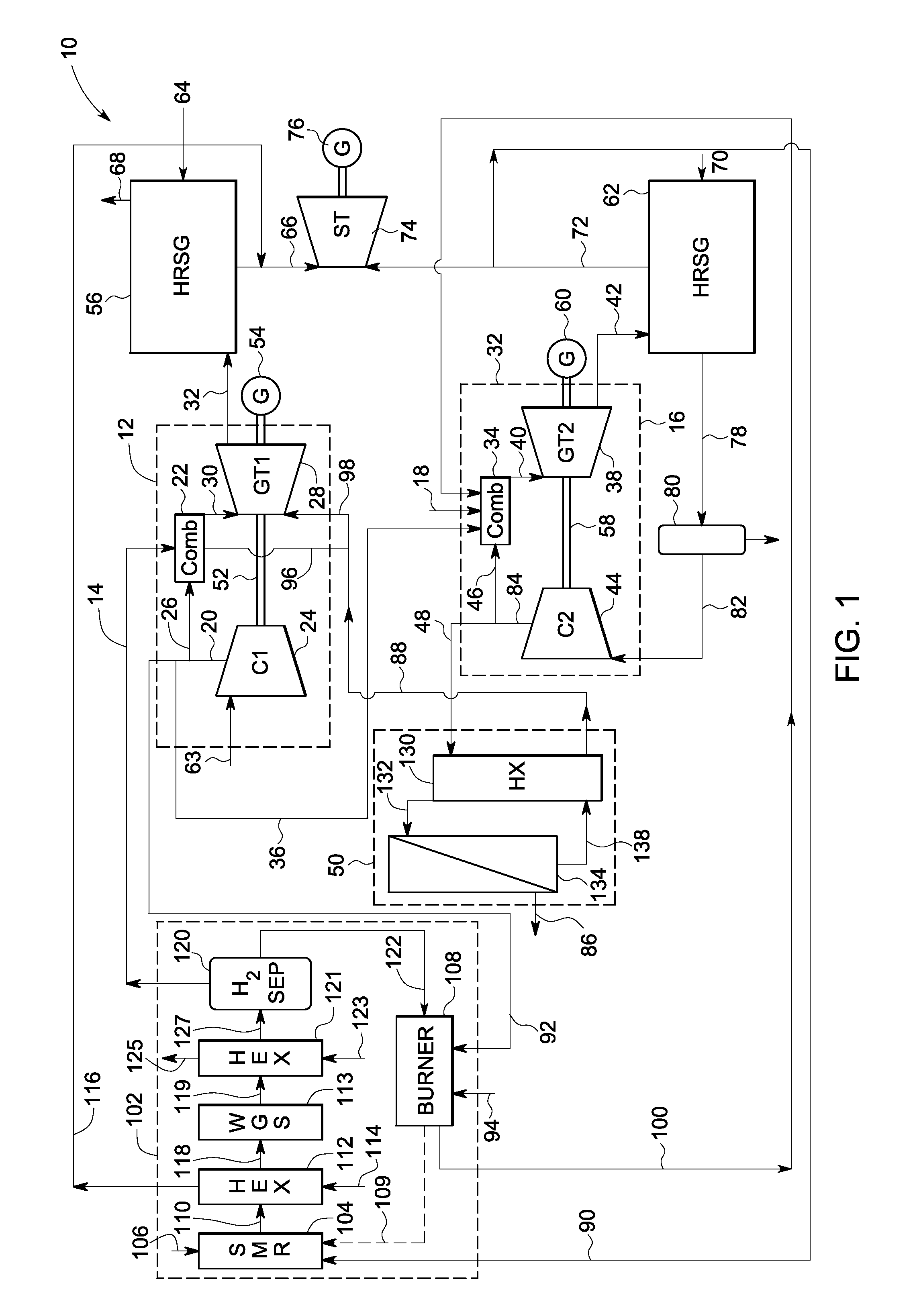
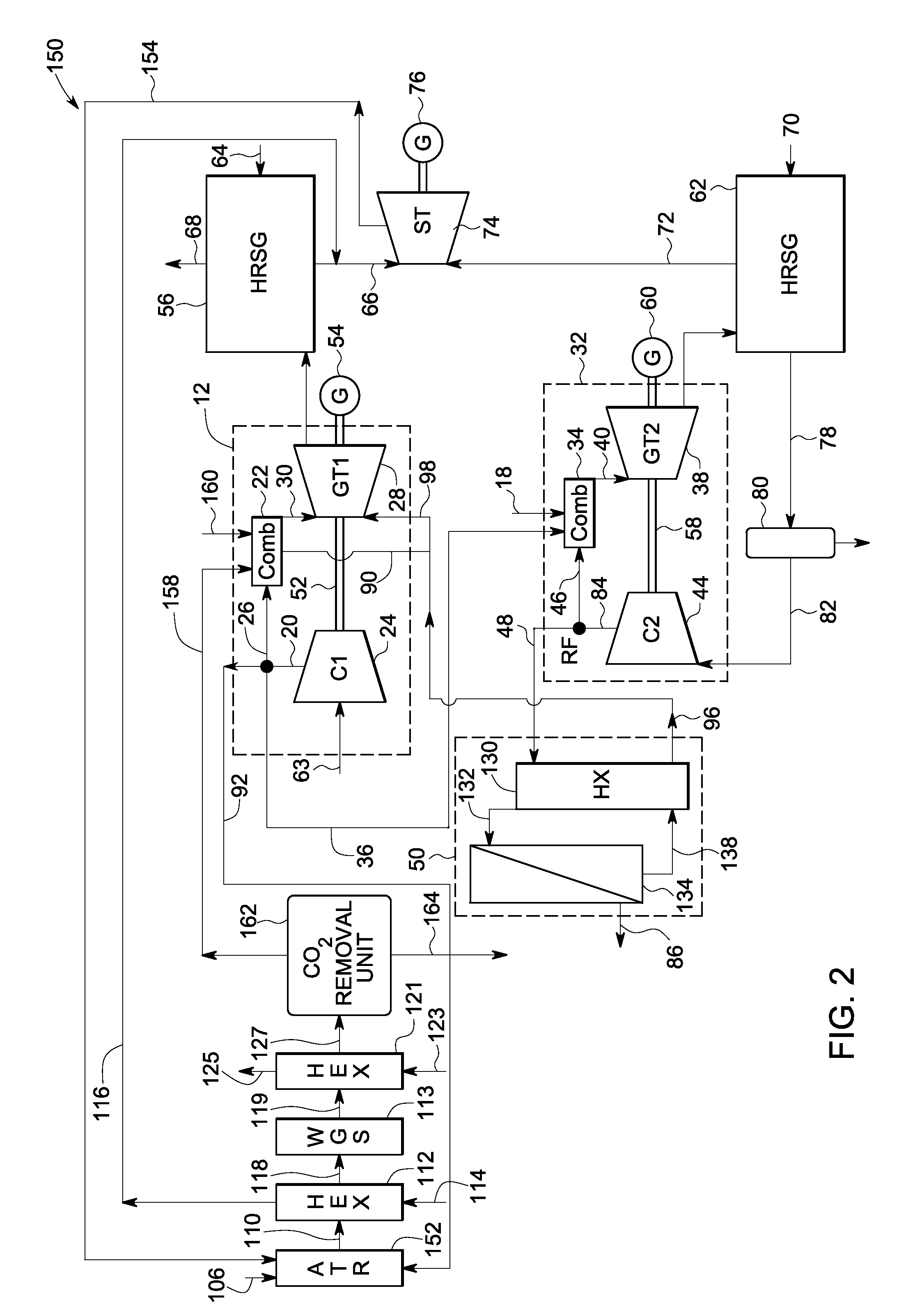
A power generation system includes a first gas turbine system. The first turbine system includes a first combustion chamber configured to combust a first fuel stream of primarily hydrogen that is substantially free of carbon-based fuels, a first compressor configured to supply a first portion of compressed oxidant to the first combustion chamber and a first turbine configured to receive a first discharge from the first combustion chamber and generate a first exhaust and electrical energy. The power generation system further includes a second gas turbine system. The second turbine system includes a second combustion chamber configured to combust a second fuel stream to generate a second discharge, wherein the first compressor of the first gas turbine system is configured to supply a second portion of compressed oxidant to the second combustion chamber and a second turbine configured to receive the second discharge from the second combustion chamber to generate a second exhaust and electrical energy. A second compressor is configured to receive the second exhaust comprising carbon dioxide and to discharge a recycle stream to the second combustion chamber and a split stream to a separator system adapted to recover carbon dioxide. The power generation system also includes a hydrogen generation system configured to receive a third fuel and steam to generate the first fuel and a third exhaust gas, wherein the third exhaust gas is recycled into the second combustion chamber.
Owner:GENERAL ELECTRIC CO
Solar photovoltaic output for cloudy conditions with a solar tracking system
ActiveUS20070084502A1Maximize its energy outputEasy to usePhotovoltaic supportsSolar heating energyIlluminanceEngineering
An array of solar powered photovoltaic modules is optimally oriented and operated to provide more electrical energy for uses such as powering an electrolyzer system for hydrogen production. The array is positioned with its light receiving surface at an optimal angle, preferably a continually changing angle determined by two-axis solar tracking, when continually measured solar irradiance indicates suitable sunlight, and at a horizontal position when measured solar irradiance indicates excessive atmospheric cloudiness.
Owner:GM GLOBAL TECH OPERATIONS LLC
Thermocatalytic process for CO2-free production of hydrogen and carbon from hydrocarbons
InactiveUS20020007594A1Pigmenting treatmentPressurized chemical processDecompositionBiological activation
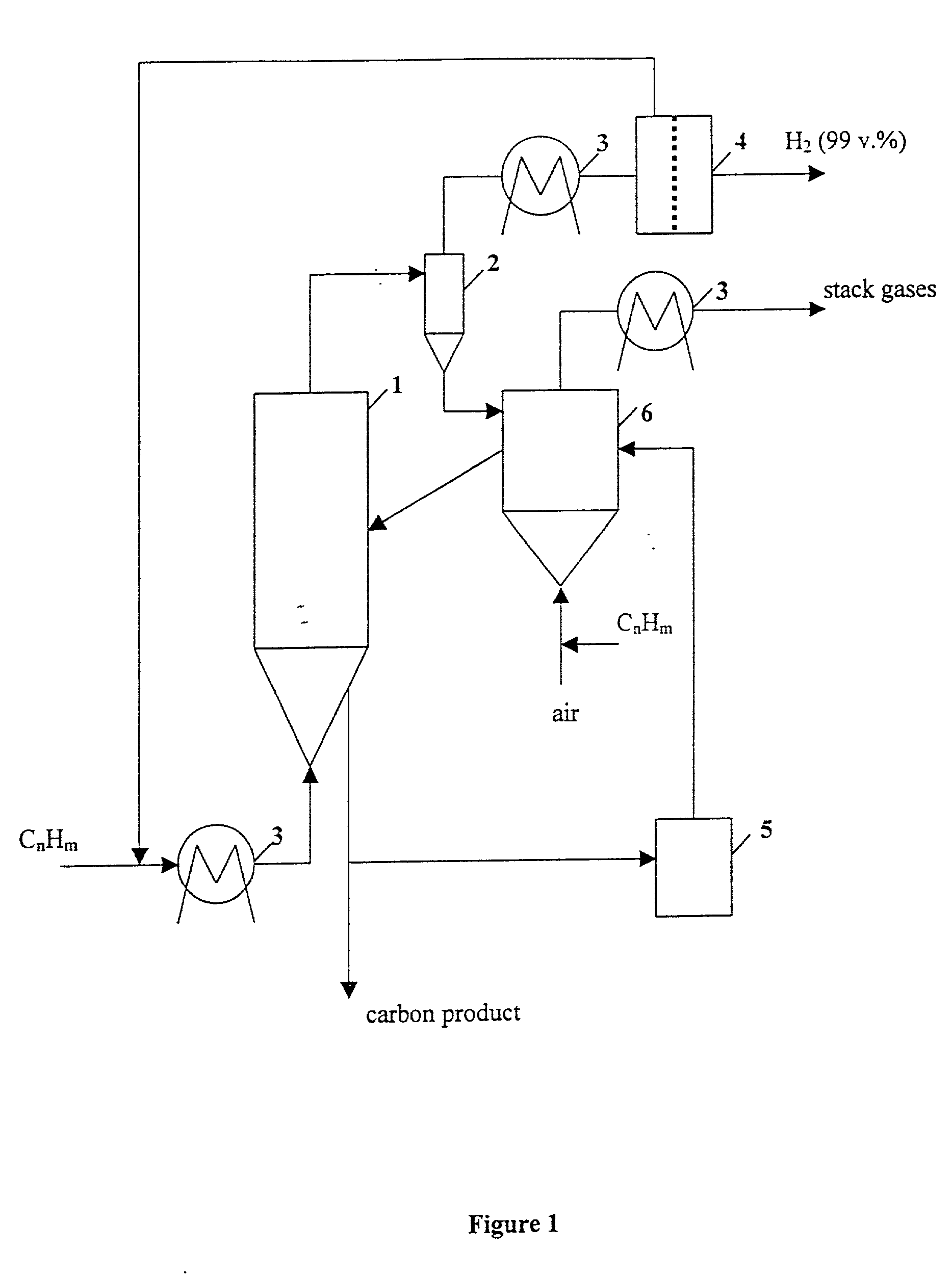
This invention relates to a novel process for sustainable CO2-free production of hydrogen and carbon by thermocatalytic decomposition (or dissociation, pyrolysis, cracking) of hydrocarbon fuels over carbon-based catalysts in the absence of air and / or water. The process is applicable to any hydrocarbon fuel, including sulfurous fuels. Combination of a catalytic reactor with a gas separation unit allows to produce high purity hydrogen (at least, 99.0 v %) completely free of carbon oxides. In a preferred embodiment, sustainable continuous production of hydrogen and carbon is achieved by both internal and external activation of carbon catalysts. Internal activation of carbon catalyst is accomplished by recycling of hydrogen-depleted gas containing unsaturated and aromatic hydrocarbons back to the reactor. External activation can be achieved via surface gasification of carbon catalysts by hot combustion gases during catalyst heating. The process can conveniently be integrated with any type of fuel cell.
Owner:UNIV OF CENT FLORIDA RES FOUND INC +1
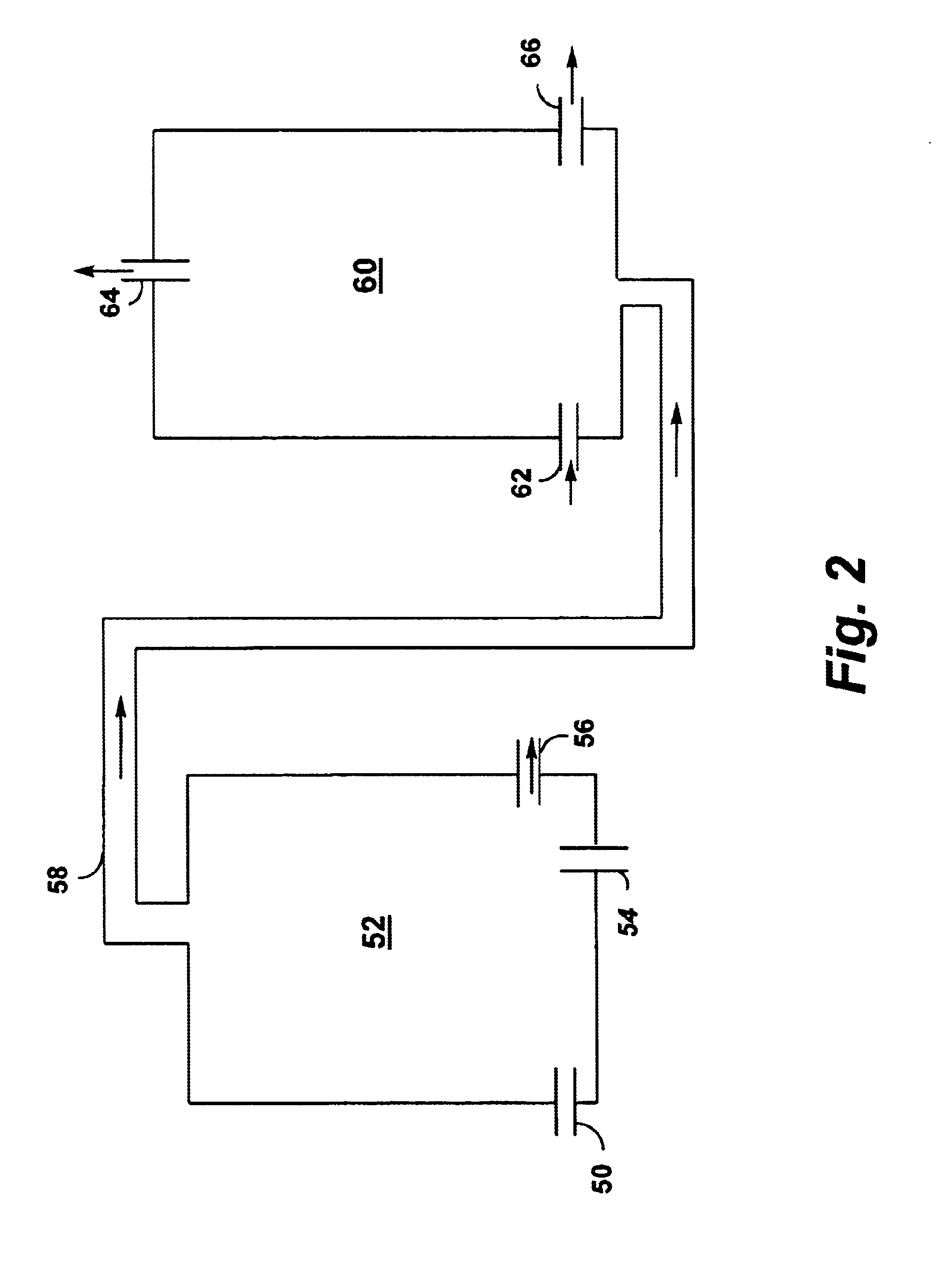
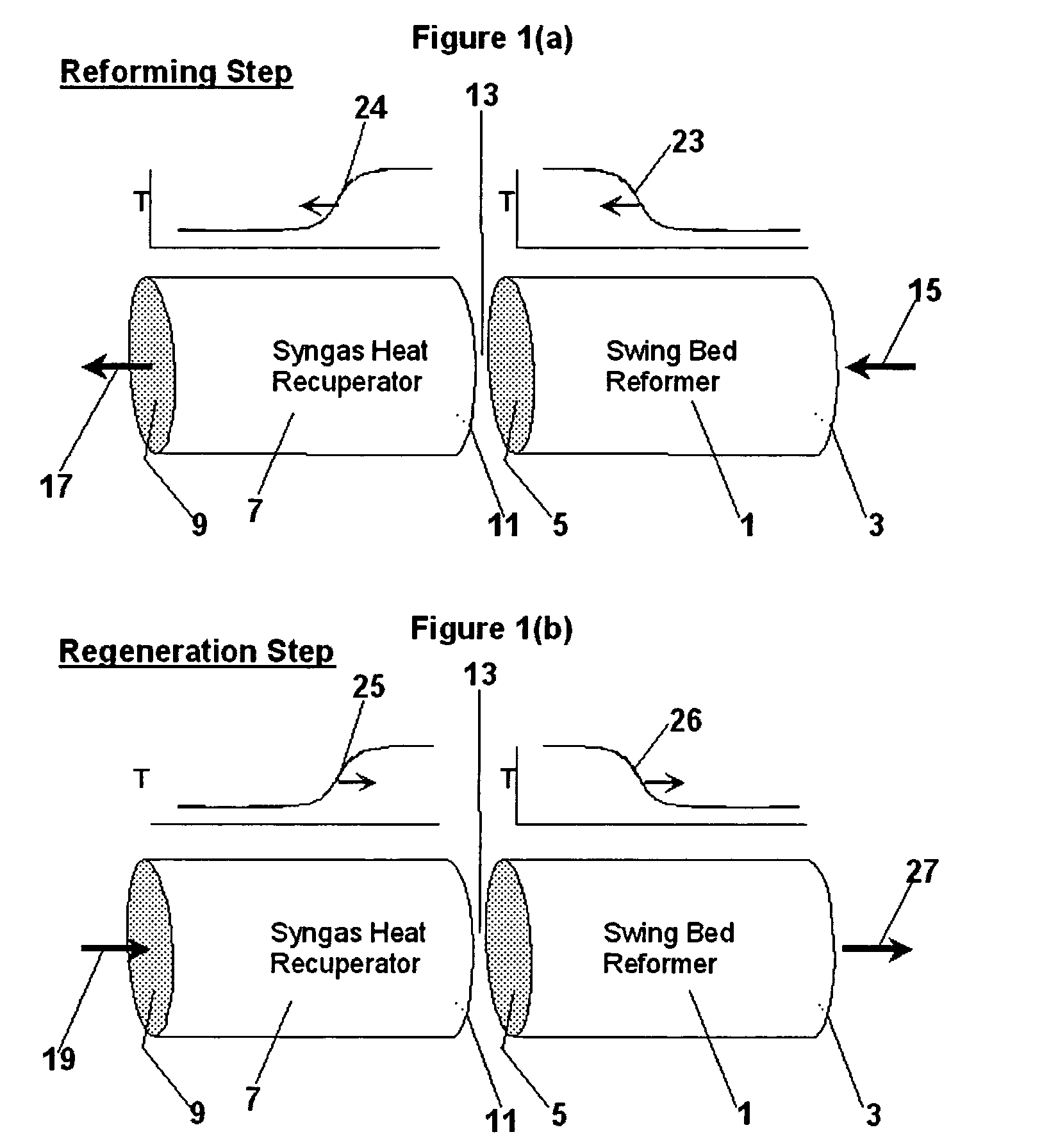
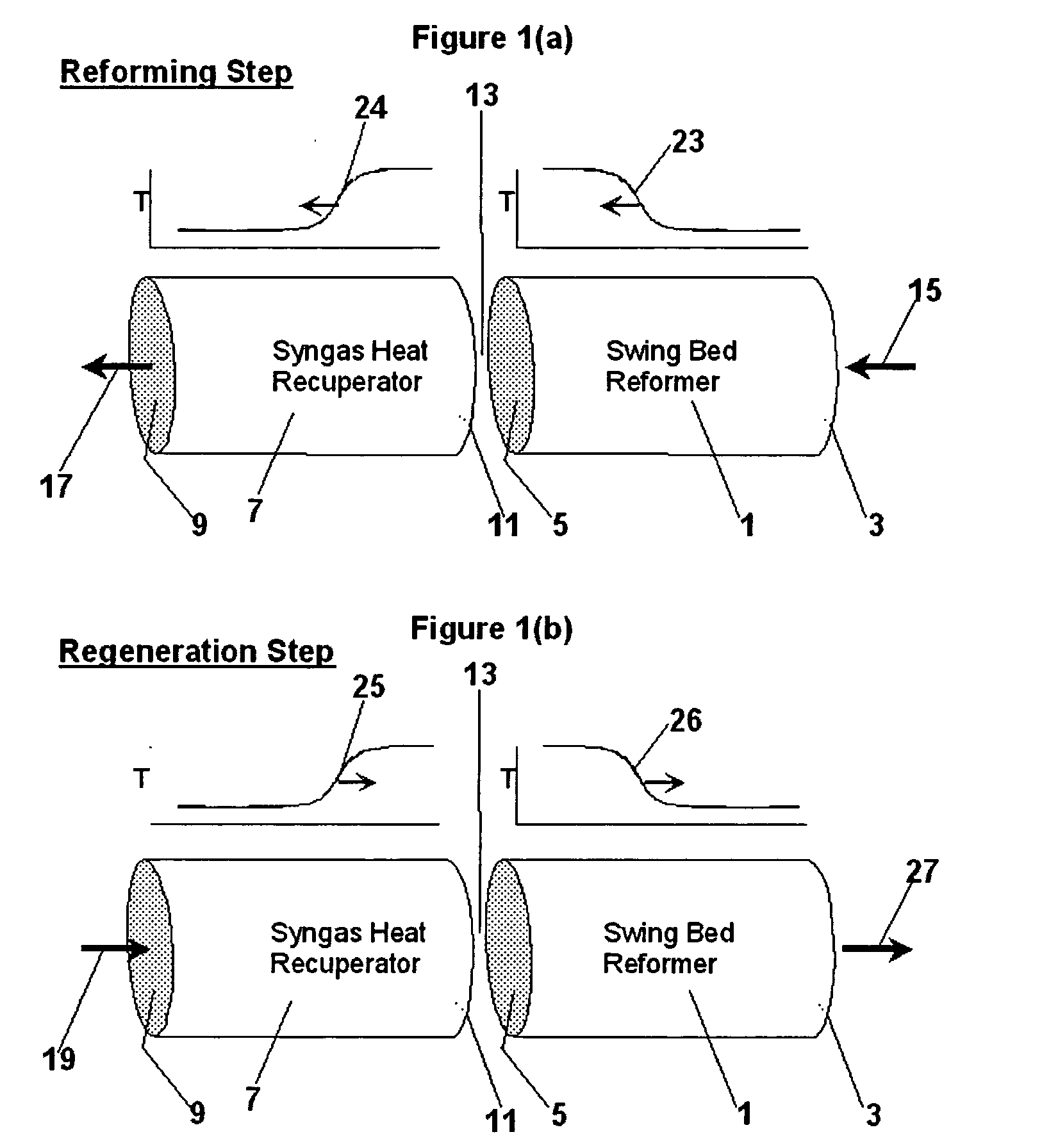
Pressure swing reforming for fuel cell systems
Owner:EXXON RES & ENG CO
Pressure swing reforming for fuel cell systems
ActiveUS20040175326A1Efficient productionImprove permeabilityHydrogenChemical industrySyngasFuel cells
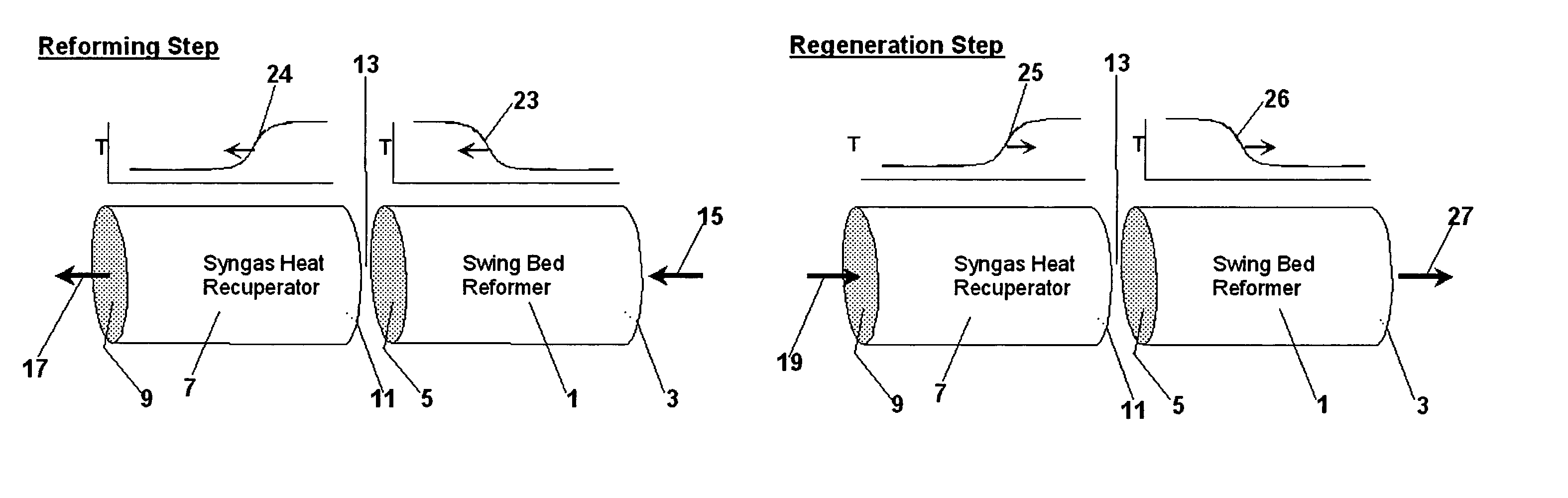
The present invention provides an improvement in the process of producing hydrogen from hydrocarbon-containing streams. A cyclic reforming process, referred to as pressure swing reforming, provides an efficient means for producing a hydrogen containing synthesis gas for fuel cell applications. Pressure swing reforming may be integrated with shift reactions, preferential oxidation, and membrane separation, achieving thermal and material efficiencies relative to conventional hydrogen production. In one embodiment, at least some synthesis gas which is first produced in the pressure swing reforming process is combusted with air to provide the heat for the regeneration step of the pressure swing reforming process.
Owner:EXXON RES & ENG CO
Systems and methods for power generation and hydrogen production with carbon dioxide isolation
Owner:GENERAL ELECTRIC CO
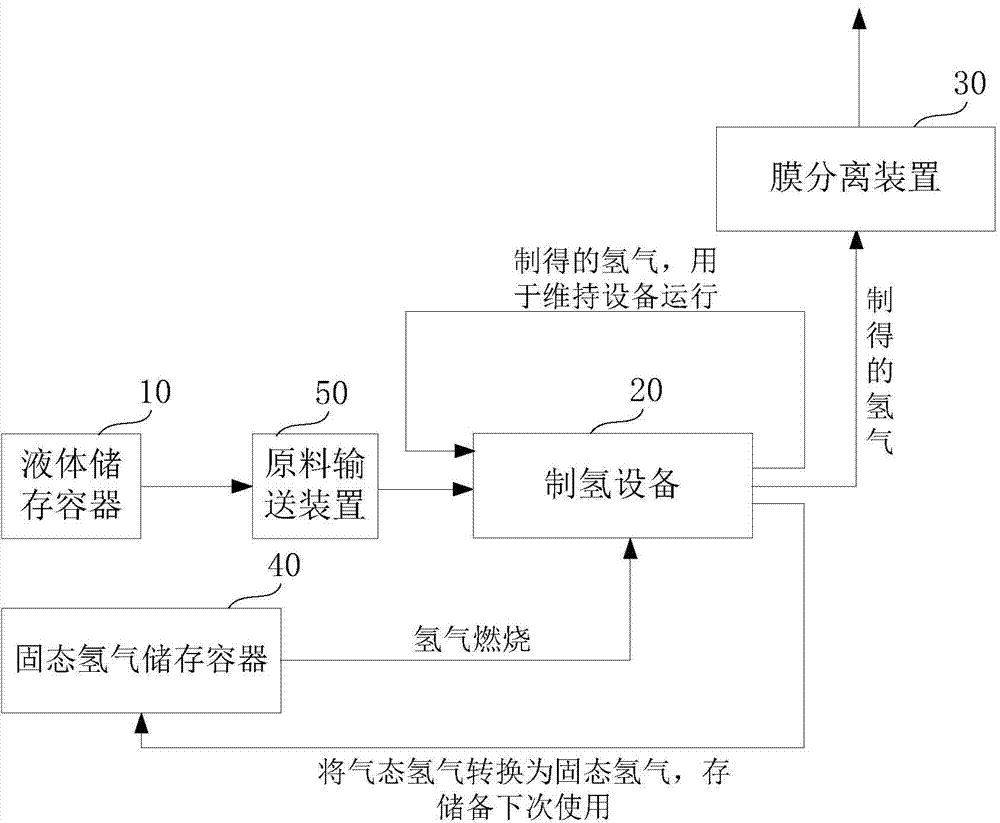
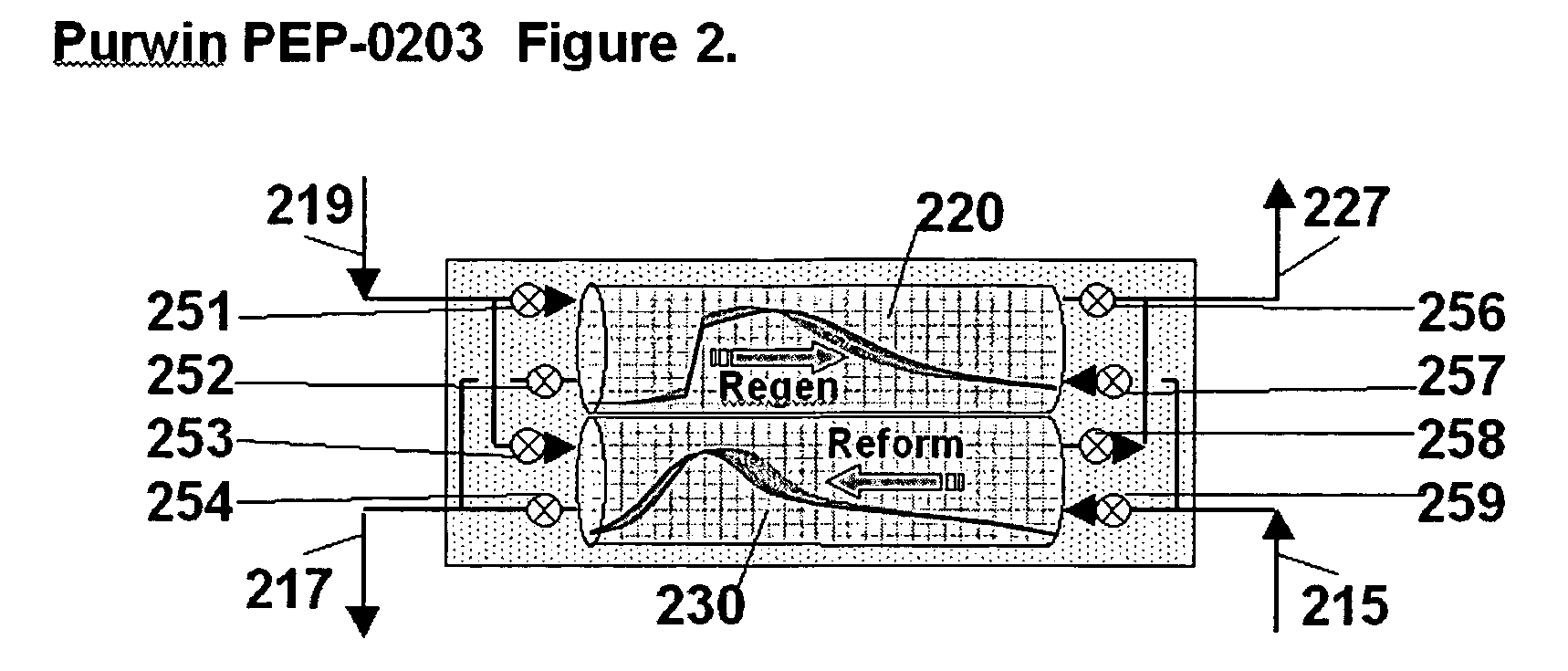
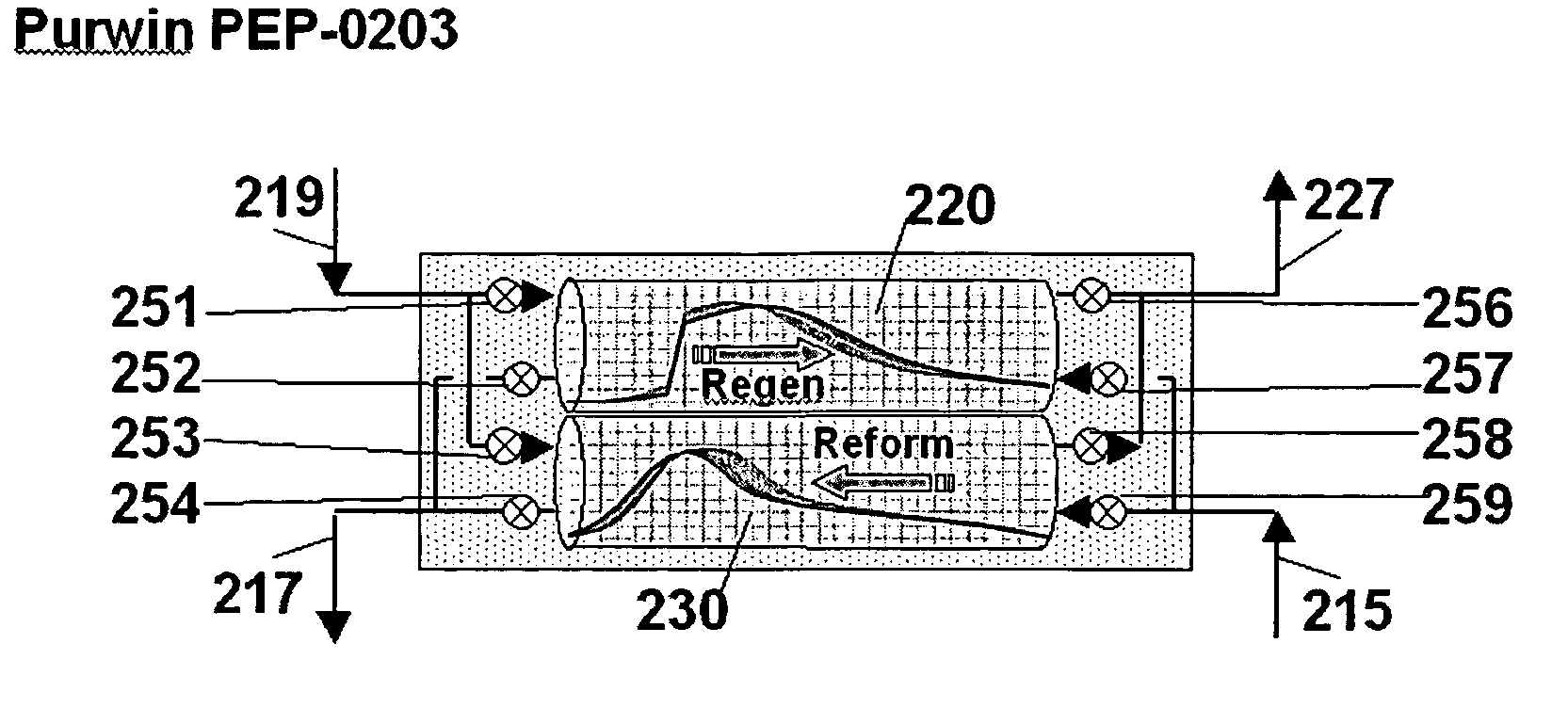
Hydrogen production method and facility
ActiveUS7985399B2High trafficHydrogen separation using solid contactHydrogen separation using liquid contactSyngasHydrocarbon
A hydrogen production method and facility in which a synthesis gas stream produced by the gasification of a carbonaceous substance is processed within a synthesis gas processing unit in which the carbon monoxide content is reacted with steam to produce additional hydrogen that is removed by a pressure swing adsorption unit. The tail gas from the pressure swing adsorption unit is further reformed with the addition of a hydrocarbon containing stream in a steam methane reforming system, further shifted to produce further additional hydrogen. The further hydrogen is then separated in another pressure swing adsorption unit.
Owner:PRAXAIR TECH INC
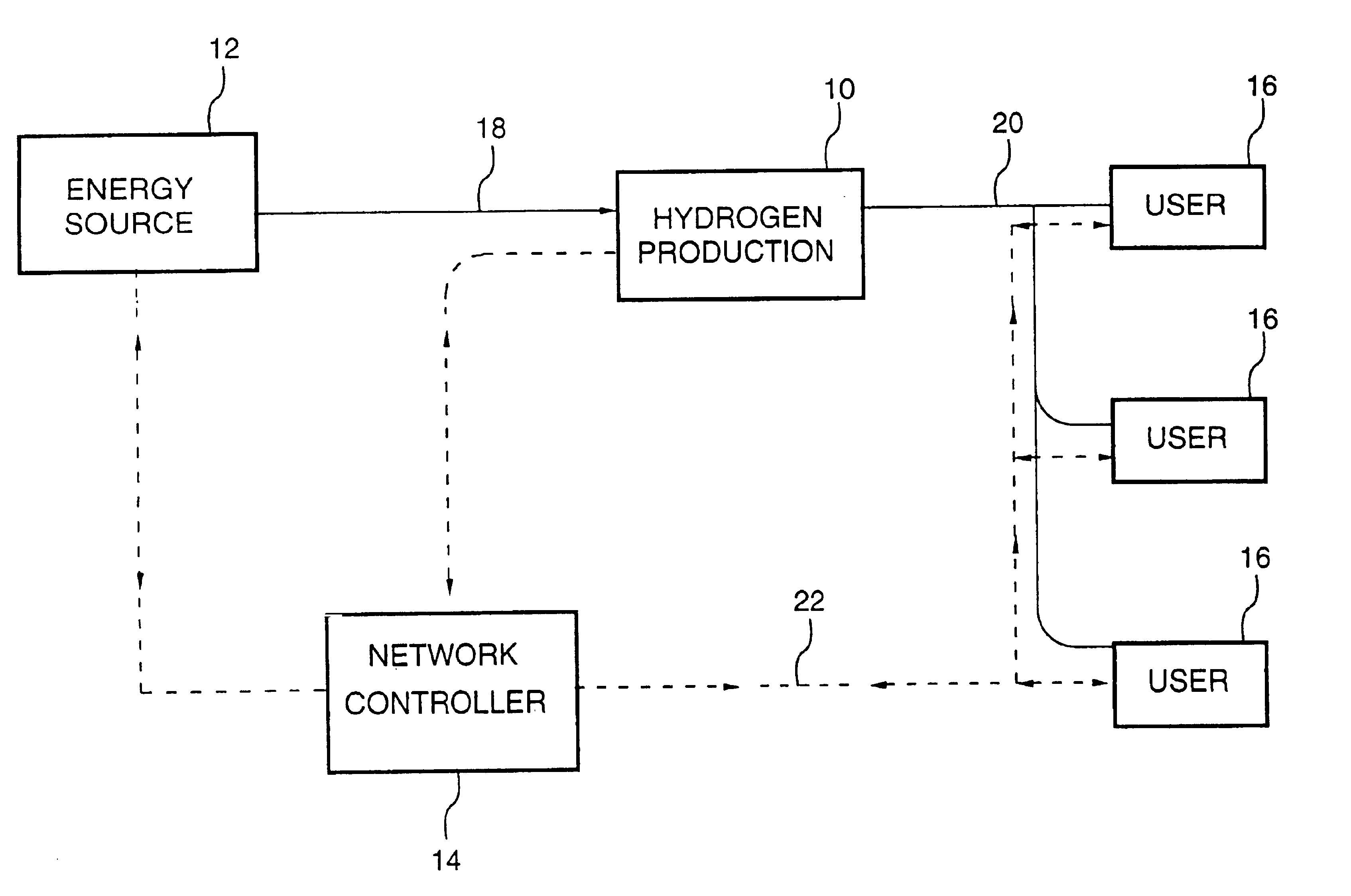
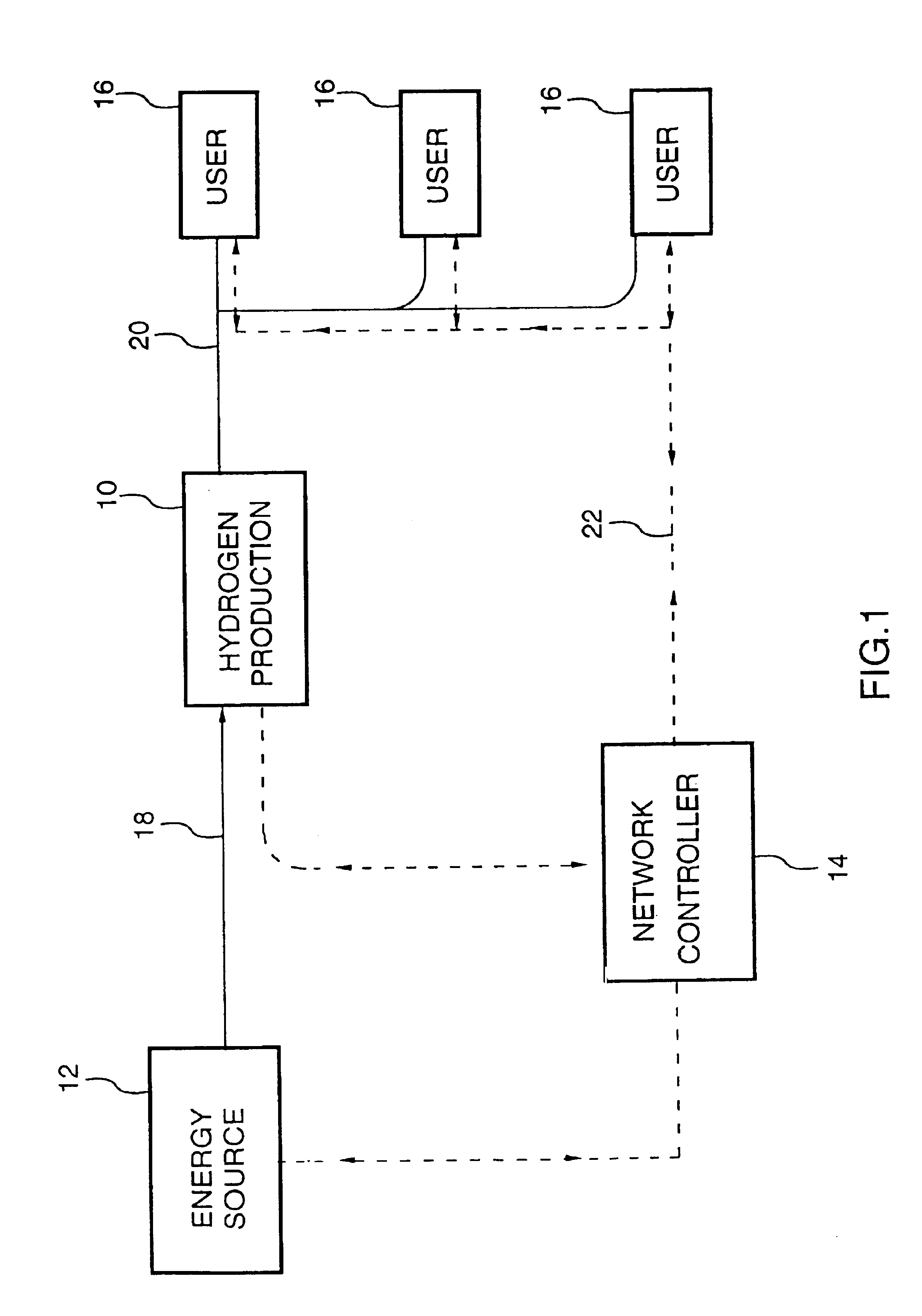
Energy distribution network
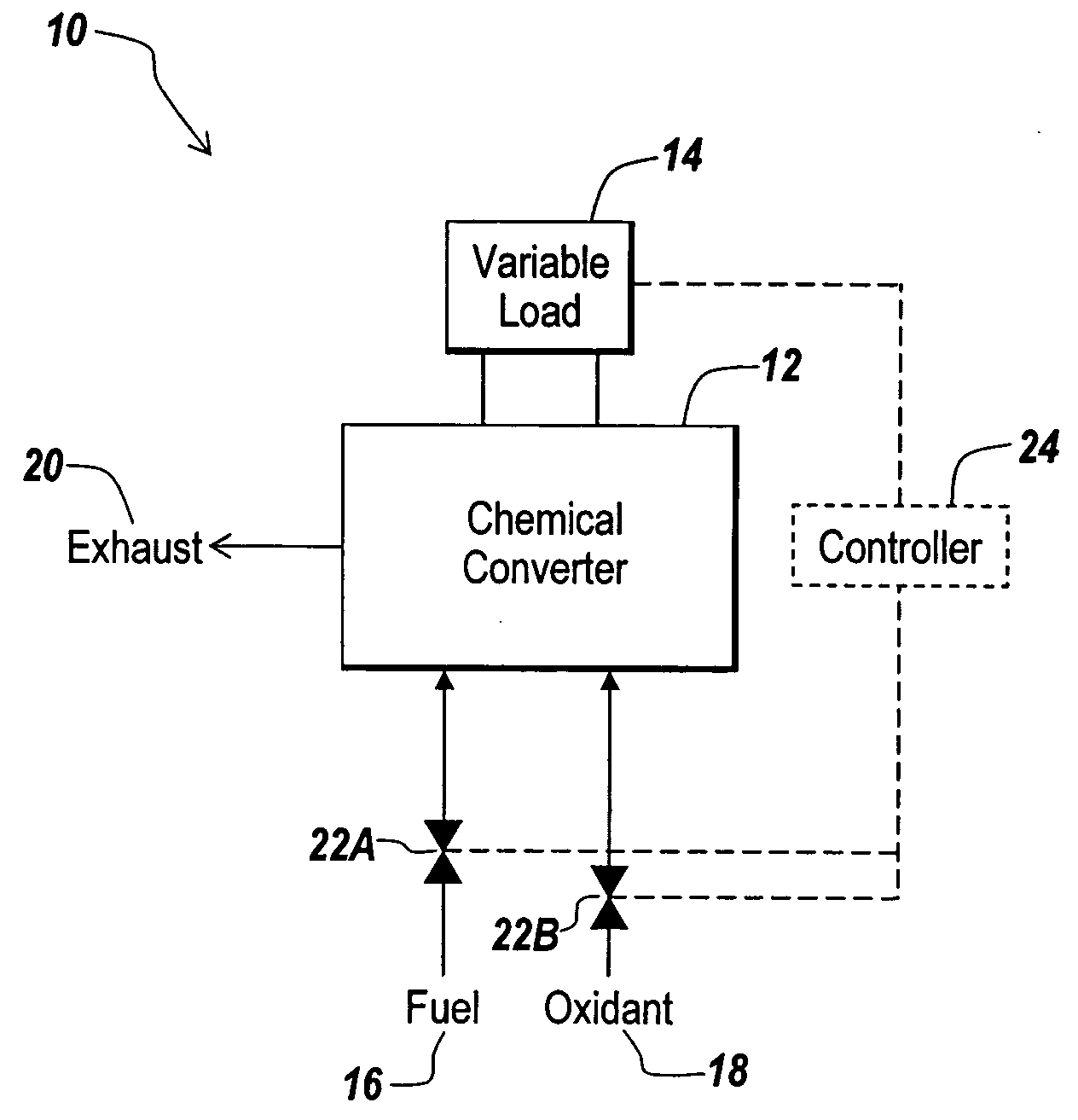
InactiveUS6745105B1Good decisionEasy to useElectricity cogenerationElectrolysis componentsProcess engineeringEnergy source
An energy distribution network is provided including an energy source; a hydrogen production facility connected to the energy source; a recipient for hydrogen from the hydrogen production facility; and a controller. The controller controls the production of hydrogen by the hydrogen production facility based on inputs including energy resource availability.
Owner:HYDROGENICS CORP
Method of and apparatus for a multi-stage boundary layer engine and process cell
InactiveUS20050169743A1Improve turbine efficiencyIncrease efficiency and reliability and flexibilityMaterial nanotechnologyInfluencers using Magnus effectCombustion chamberClosed loop
A multi-staged boundary layer engine and process cell, (based on the effect known as adhesion and viscosity) which achieves high thermal efficiencies and high mechanical power output for use in the power generation, geothermal, energy recovery, solar, transportation, hydrogen production, desalinating water and hydroelectric fields. The design is novel with a dovetail attachment of the disc packs, allowing lower stress and allowing the use of next generation materials such as ceramics, composites and nanocomposites to improve the maximum temperature and the maximum RPM of the engine, thereby producing more horsepower and torque. In addition, this invention includes multi-stage vacuum, an external combustion chamber and condenser stages to improve the vortex flow through the primary disc pack cell. This engine will also encompass a closed loop cycle for ultimate efficiencies. This invention will also include the use of catalysts and / or electrical polarities applied to the disc pack and the disc pack / casing respectively to achieve low NOx and also to achieve process cell capability for applications such as desalinization and hydrogen generation.
Owner:CENTRIPETAL DYNAMICS
Hydrogel nanocomposite
InactiveUS20150069295A1Inexpensive materialsNovel physicochemical propertyLight-sensitive devicesReactant parameters controlHydrogen productionCarbon quantum dots
Owner:NAT UNIV OF SINGAPORE
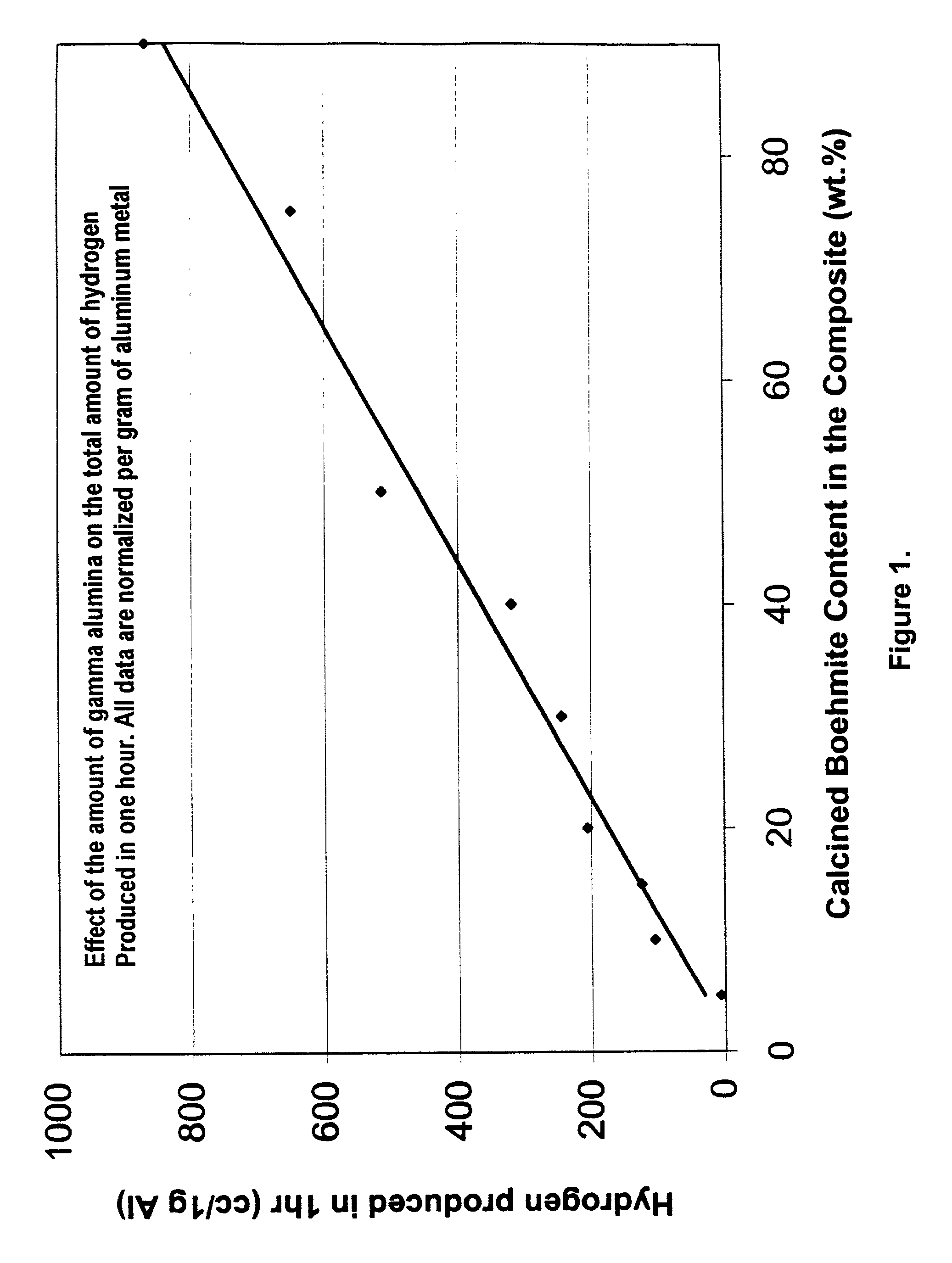
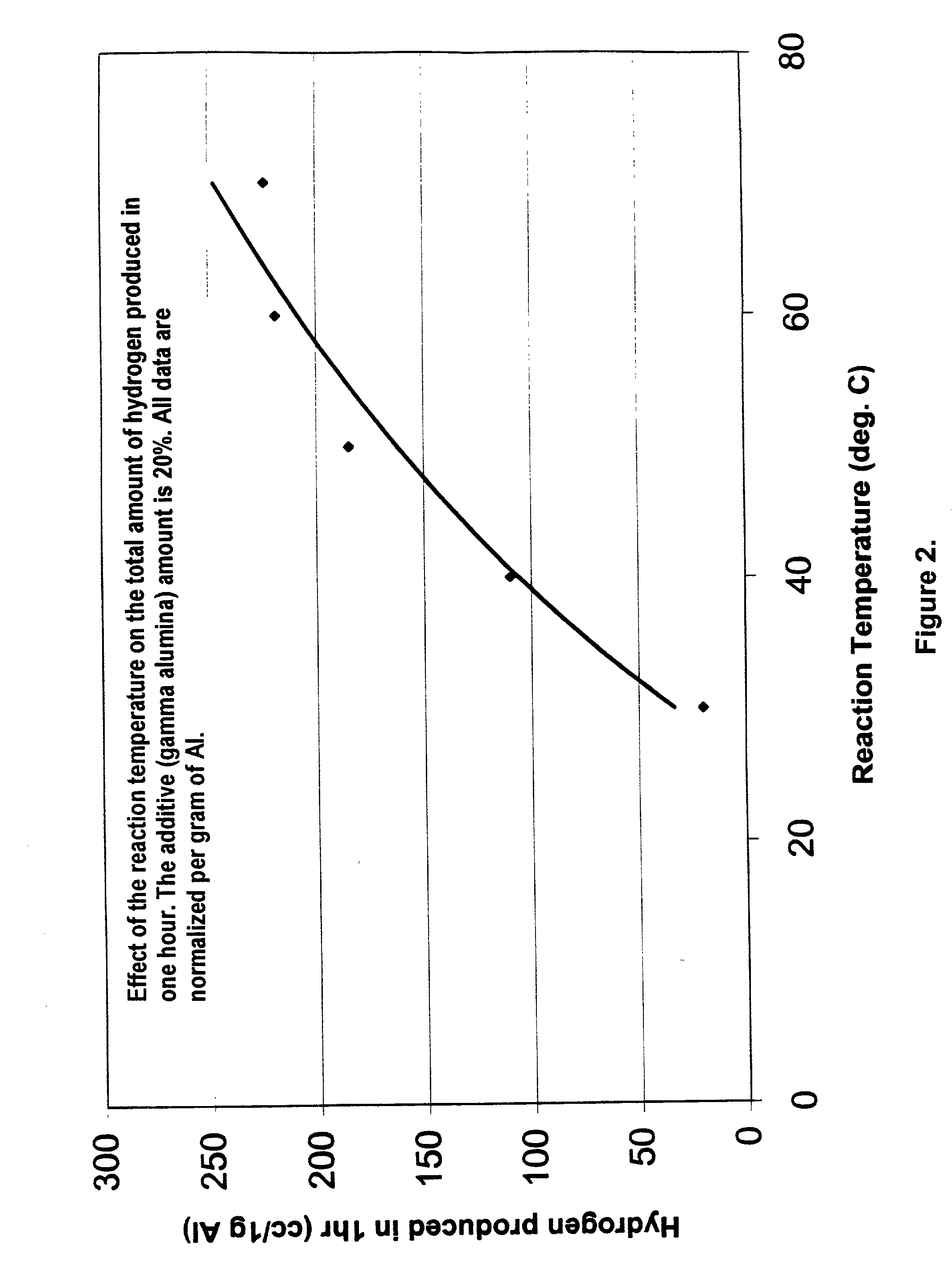
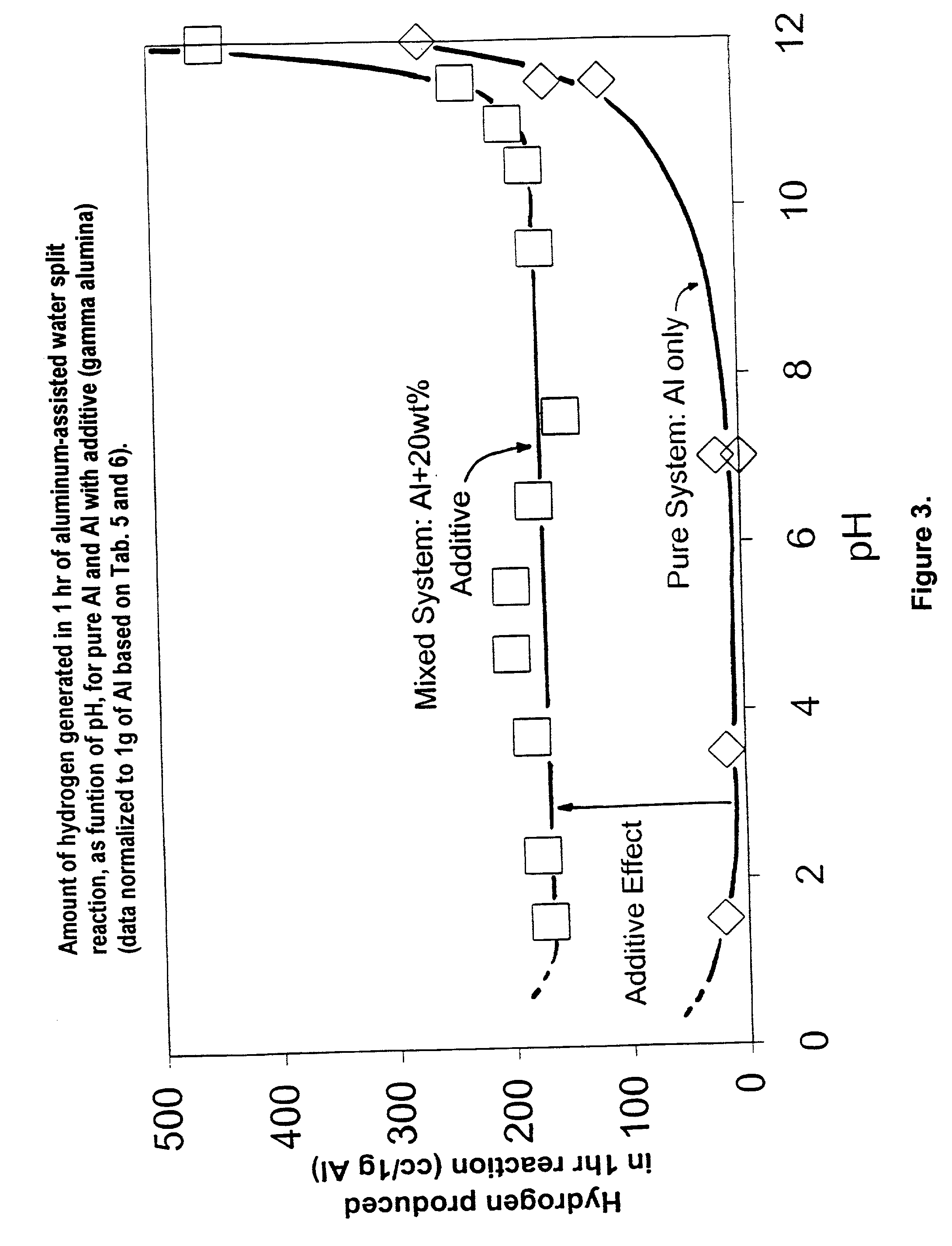
Hydrogen generation from water split reaction
A method of producing Hydrogen by reacting a metal selected from the group consisting of Aluminum (Al), Magnesium (Mg), Silicon (Si) and Zinc (Zn) with water in the presence of an effective amount of a catalyst at a pH of between 4 and 10 to produce Hydrogen. The catalyst or other additive is selected to prevent or slow down deposition of the reaction products on the (impair reactions with the) metal that tend to passivate the metal and thereby facilitates the production of said Hydrogen.
Owner:THE UNIV OF BRITISH COLUMBIA
Hollow turbine
InactiveUS20050005592A1General water supply conservationSeawater treatmentFree rotationCentre of rotation
A versatile turbine suitable for both hydraulic and pneumatic applications. The turbine's blades are affixed to the inner surface of a cylindrical shell which is free to rotate about an outside supporting structure. Rotational energy is transferred from the outer surface of the rotating cylindrical shell, usually by means of a gear; however, there may be applications better suited for a pulley means of transfer. The vacant central axis of rotation can be closed, by incorporating taller blades to achieve a larger surface area, resulting in greater efficiency, or open, by means of shorter blades forming a hole with the distal edges of the blades, to allow for passing fish and or debris to safely exit. The preferred embodiment further includes a means for electricity generation, water purification, and hydrogen production.
Owner:FIELDER WILLIAM SHERIDAN
Fuel cell for hydrogen production, electricity generation and co-production
InactiveUS20050112425A1Amount be controlFuel cell combinationsHydrogenElectricityElectrochemical response
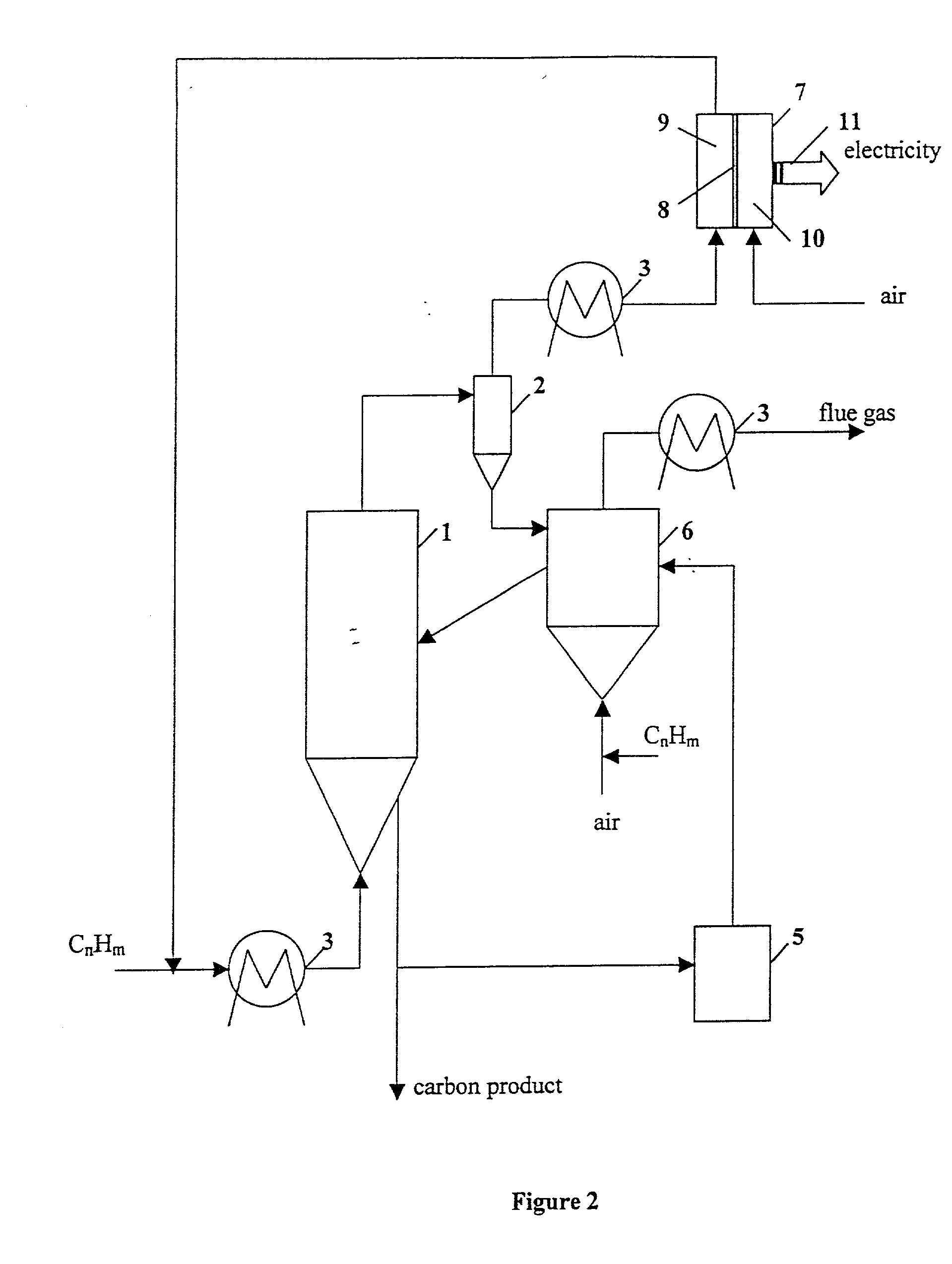
A hydrogen-electricity co-production (HECP) system utilizes a fuel cell to produce hydrogen, electricity, or a combination of both hydrogen and electricity. In a first mode, the fuel cell performs an electrochemical reaction by reacting a hydrogen-containing fuel with oxygen to produce electricity, water and heat. In a second mode, the fuel cell utilizes heat released by an electrochemical reaction of the fuel cell to reform a hydrogen-containing fuel to produce hydrogen rich gas. In a third mode, both hydrogen and electricity are co-produced by the fuel cell. The HECP system can control an amount of hydrogen and / or electricity produced and switch between modes by varying an electrical load on the system.
Owner:ZTEK
Catalyst for hydrogen production by catalyzing and hydrolyzing borohydride and preparation method thereof
InactiveCN101347736AFast deposition rateIncrease concentrationMetal/metal-oxides/metal-hydroxide catalystsMetal hydridesChemical platingRare earth
The invention relates to hydrogen production and hydrogen storage technologies and materials, in particular to a catalyst for catalytic hydrolysis of borane for the hydrogen production and a preparation method thereof, thereby solving the problems that the direct application of powder catalyst in a catalytic hydrolysis solid-liquid reaction system can cause the loss of the catalyst, the catalytic hydrolysis reaction is difficult to control and the hydrolysis by-products are difficult to be recovered, etc. The catalyst is composed of an active component and a carrier; the active component is a binary, ternary or multinary alloy or a single precious metal or the combination thereof which is composed of one or more transition metals, rare earth metals or precious metals and metalloids; the active component is deposited on the carrier through the improved chemical plating technology, the surface thereof is rough and porous, and the structure of the prepared catalyst is the amorphous or the nanocrystalline structure. The preparation method has simple preparation process, high preparation efficiency and convenient large-scale preparation; the sources of the used raw materials are rich; the catalytic activity of the prepared supported catalyst is high, the real-time control of the catalytic hydrolysis reaction of the borane can be realized, the catalytic performance is stable, and the catalyst can be repeatedly used for a plurality of times.
Owner:INST OF METAL RESEARCH - CHINESE ACAD OF SCI
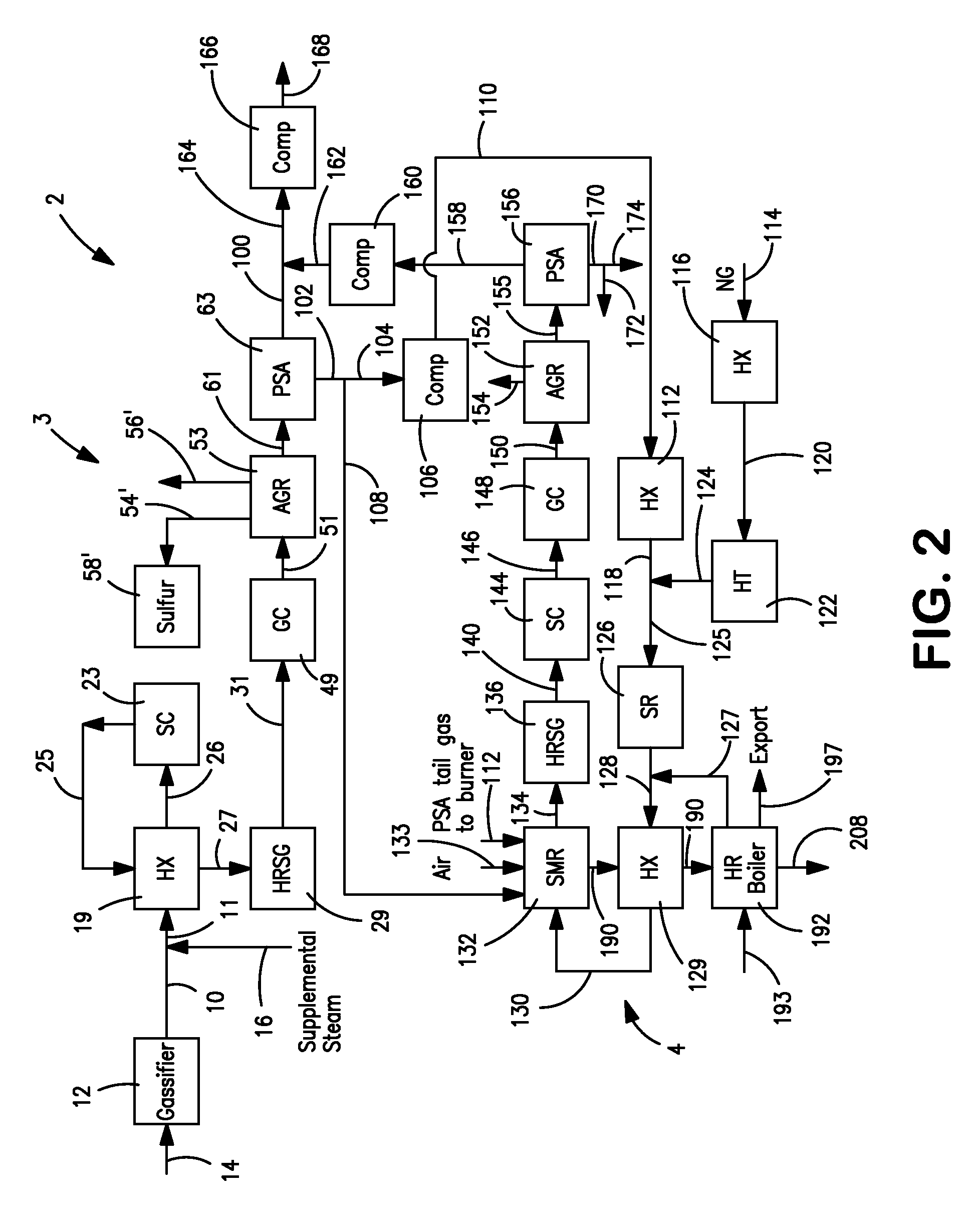
Gasification and steam methane reforming integrated polygeneration method and system
InactiveUS8409307B2Improve reliabilityMaximize reliabilityCombustible gas catalytic treatmentGas modification by gas mixingProcess engineeringPetroleum
This invention is a process and system for providing hydrogen at a high level of reliability from a gasification system by integrating it with SMR. Carbonaceous feedstock such as petroleum coke or coal or biomass is gasified to co-produce SNG, fuel gas, hydrogen, power and steam in conjunction with hydrogen production through steam methane reforming. Carbon dioxide may also be recovered in this process. The integrated schemes are designed in a way that maximizes the reliability of production of high value products such as hydrogen through gasification and minimizes the impact of high natural gas prices on hydrogen production by SMR.
Owner:PRAXAIR TECH INC
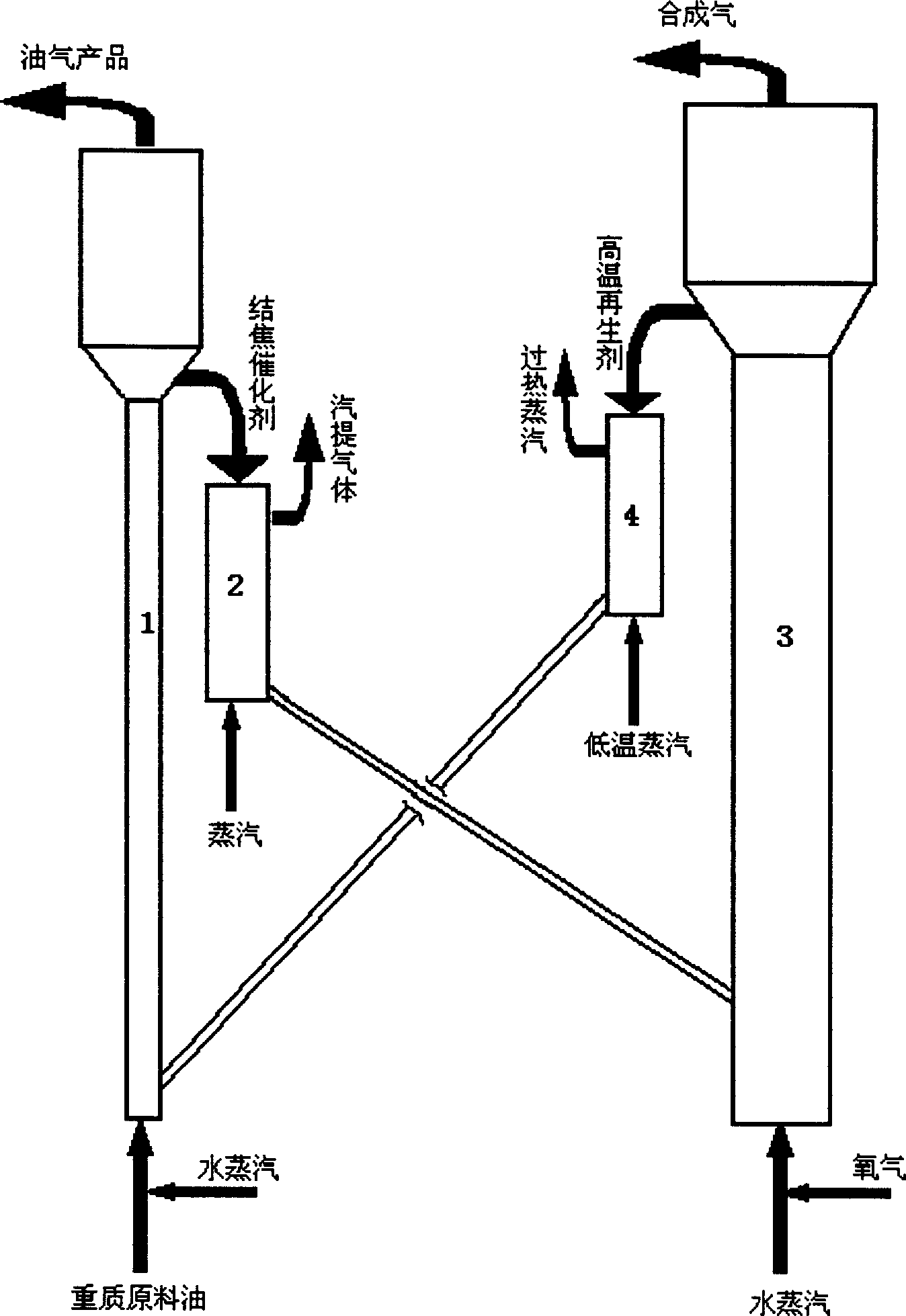
Process combined oil refining and gasification
A process of oil refining and gasification comprising the steps of, (1) petroleum hydrocarbon and coke transfer agent contacting and reacting in reactor, (2) separating produced reaction oil gas and residual coke transfer agent after reaction, (3) coke transfer agent contacting water vapor and oxygen-containing gas under gasification condition to produce formed gas, (4) returning the regenerated coke transfer agent back to reactor in step (1) for circulation use. The process by the invention can not only improve the quality of heavy petroleum hydrocarbon, but also provide cheap raw material gas for hydrogen production process or C1 chemistry.
Owner:CHINA PETROLEUM & CHEM CORP +1
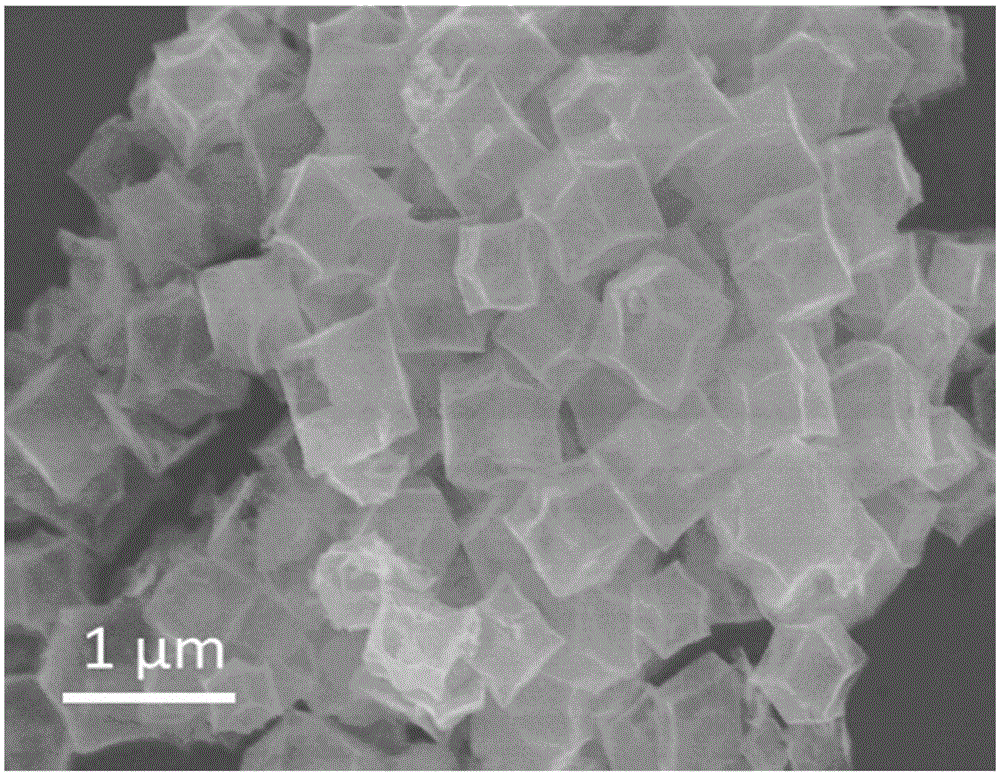
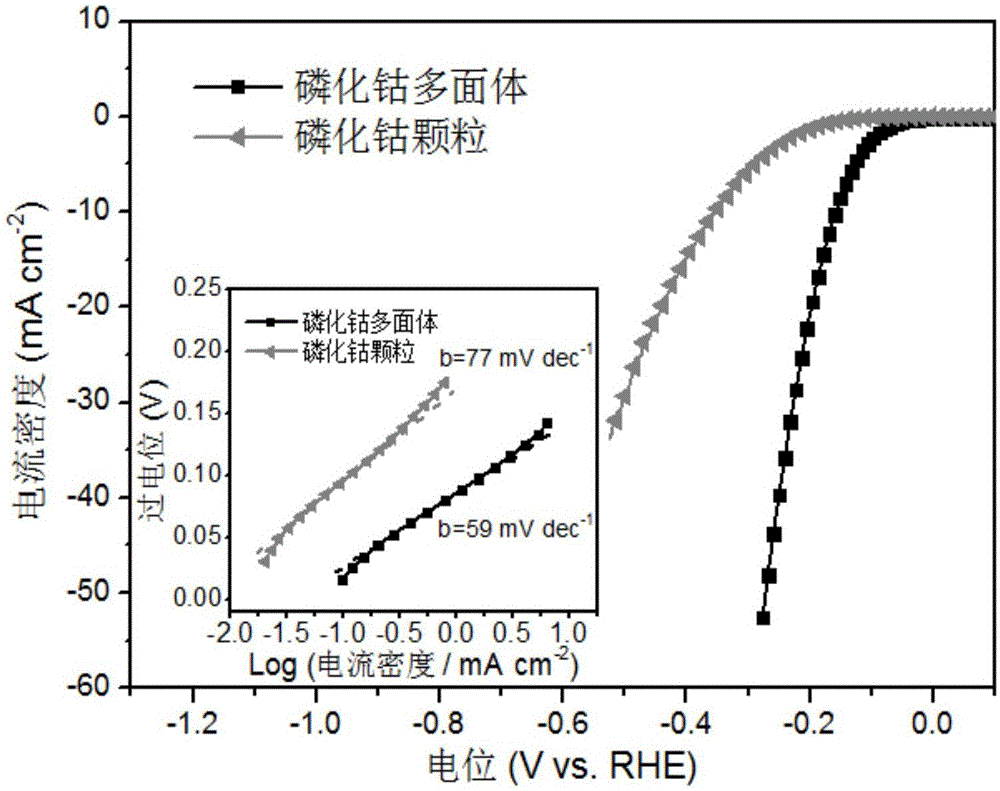
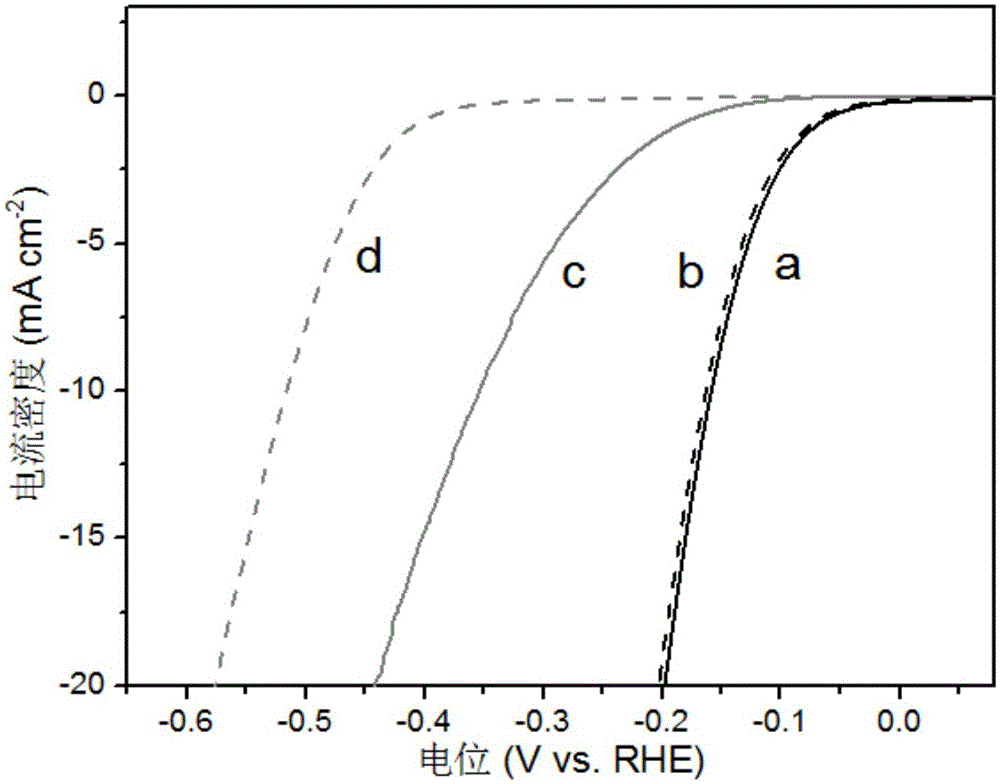
Preparation method of polyhedral cobalt phosphide catalyst for hydrogen production through water electrolysis
ActiveCN105107536AHigh crystallinityIncrease the areaElectrolysis componentsPhysical/chemical process catalystsAir atmosphereElectrolysis
A preparation method of a polyhedral cobalt phosphide catalyst for hydrogen production through water electrolysis comprises steps as follows: Co(NO3)2*6H2O and 2-methylimidazole are dissolved in methanol respectively, a 2-methylimidazole solution is poured into a Co(NO3)2 solution, the mixture is stirred and then aged at the room temperature, a product is centrifugally separated, vacuum drying is performed after washing with methanol, and a polyhedral metal organic frame ZIF-67 is obtained; then the polyhedral metal organic frame ZIF-67 is placed in a tube furnace, cobaltosic oxide is obtained through calcination in the air atmosphere, then the cobaltosic oxide and NaH2PO2*H2O are placed at two ends of a porcelain boat respectively, the NaH2PO2*H2O is located in the windward position of the tube furnace, and the polyhedral cobalt phosphide catalyst for hydrogen production through water electrolysis is obtained through calcination in the inert atmosphere. The crystallinity of the prepared cobalt phosphide catalyst material is high, the polyhedral morphology of a metal organic frame template is kept, the catalyst shows excellent properties in an electrocatalytic hydrogen evolution reaction, and the preparation technology is simple in process.
Owner:TSINGHUA UNIV
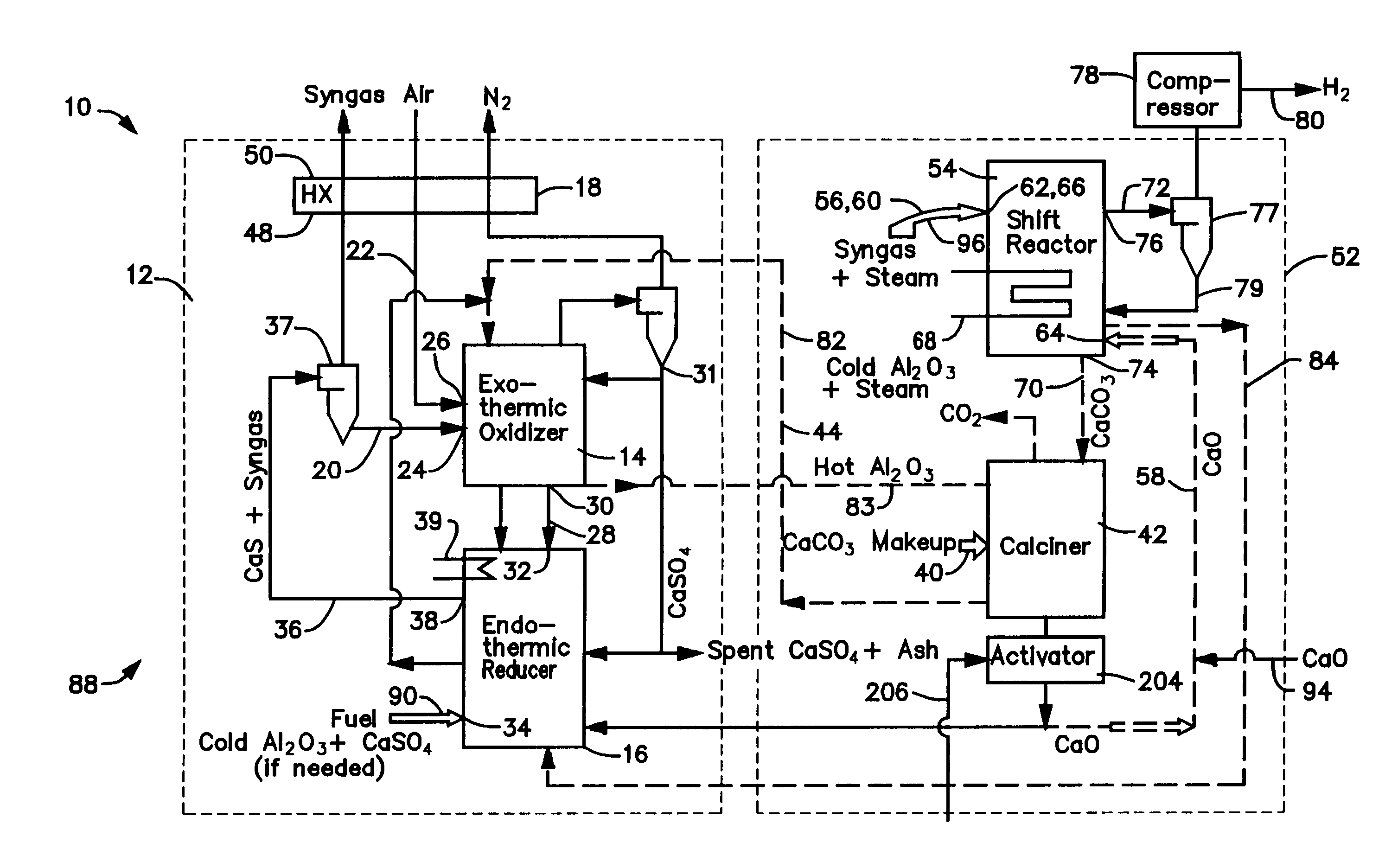
Hot solids gasifier with CO2 removal and hydrogen production
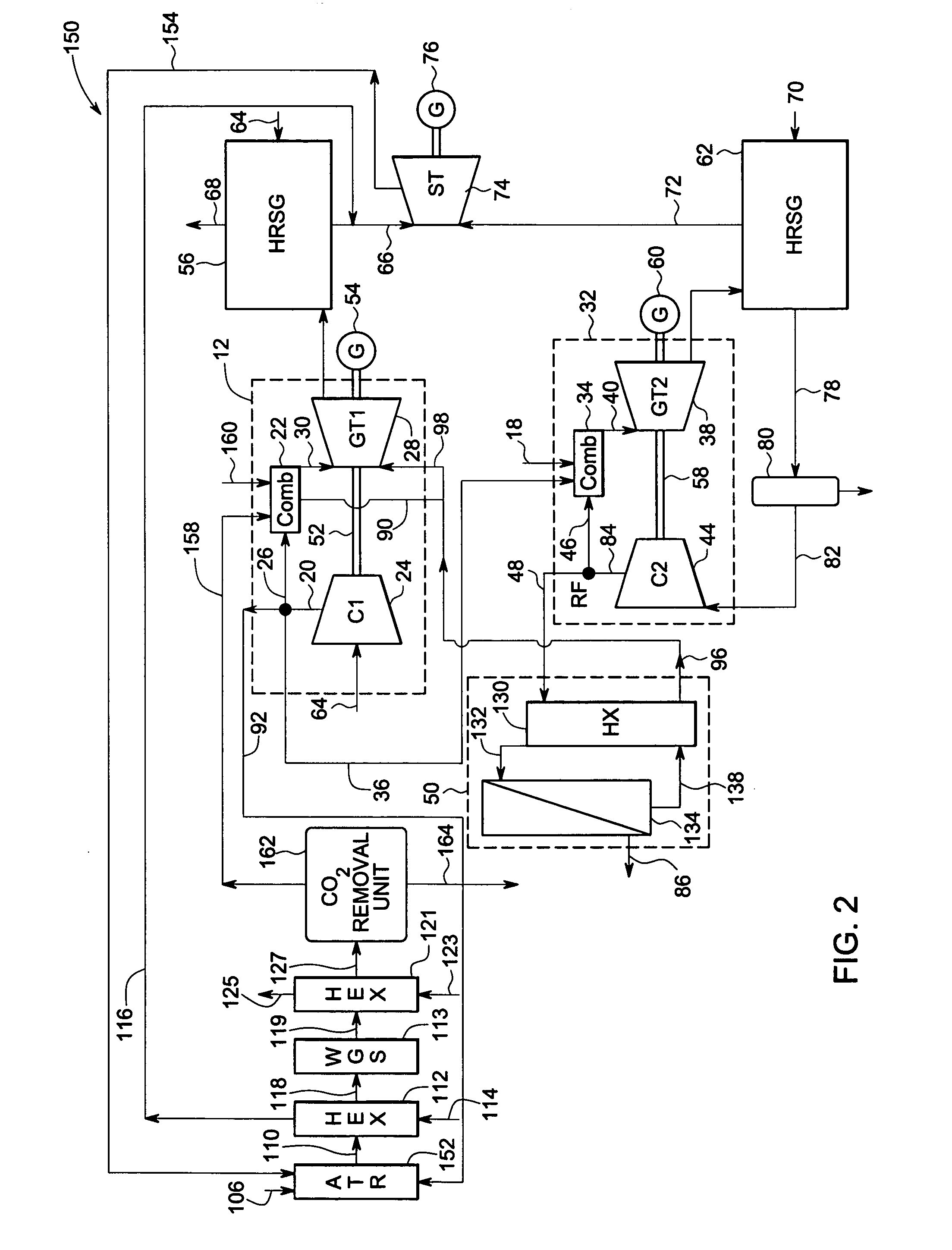
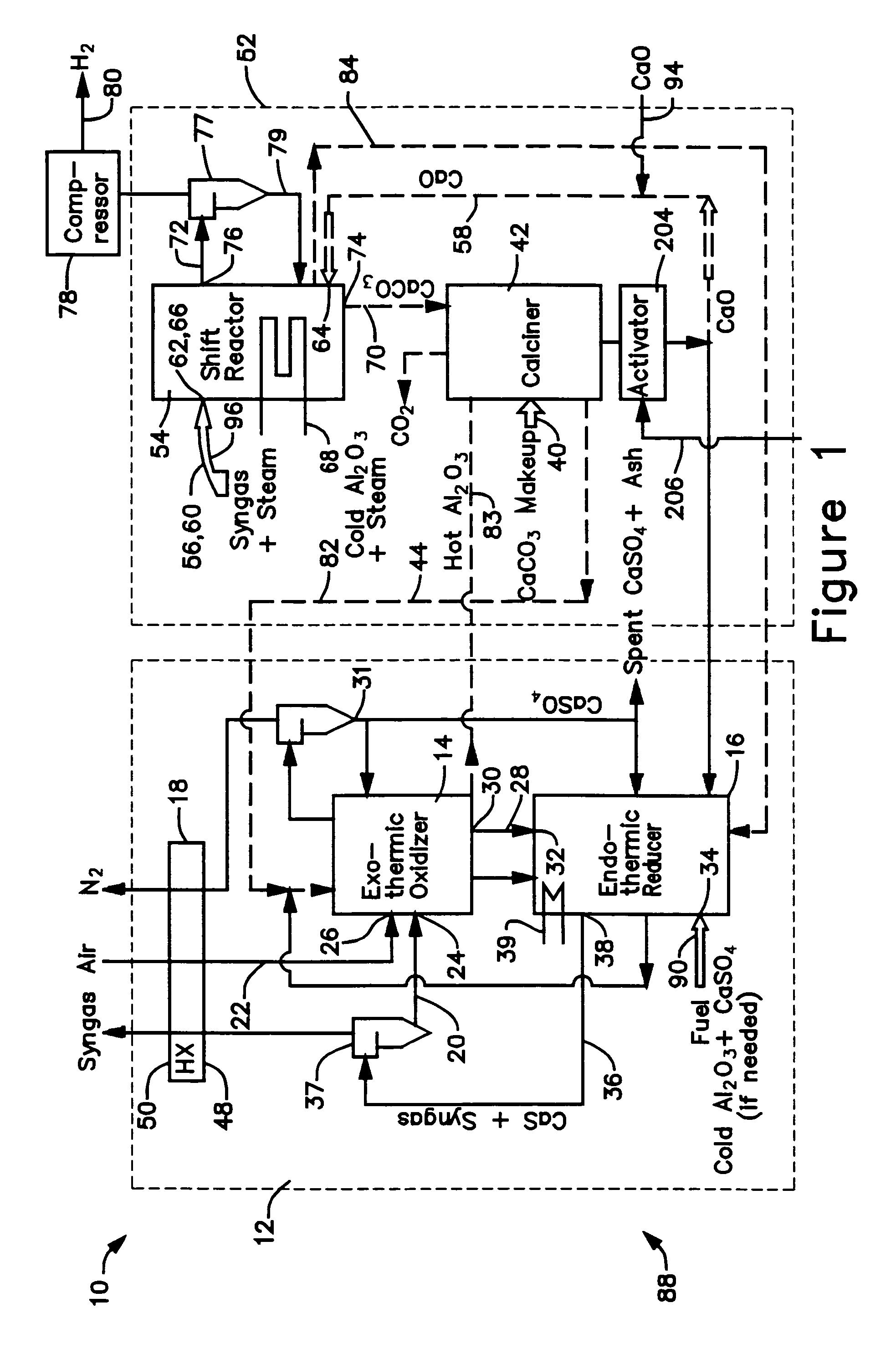
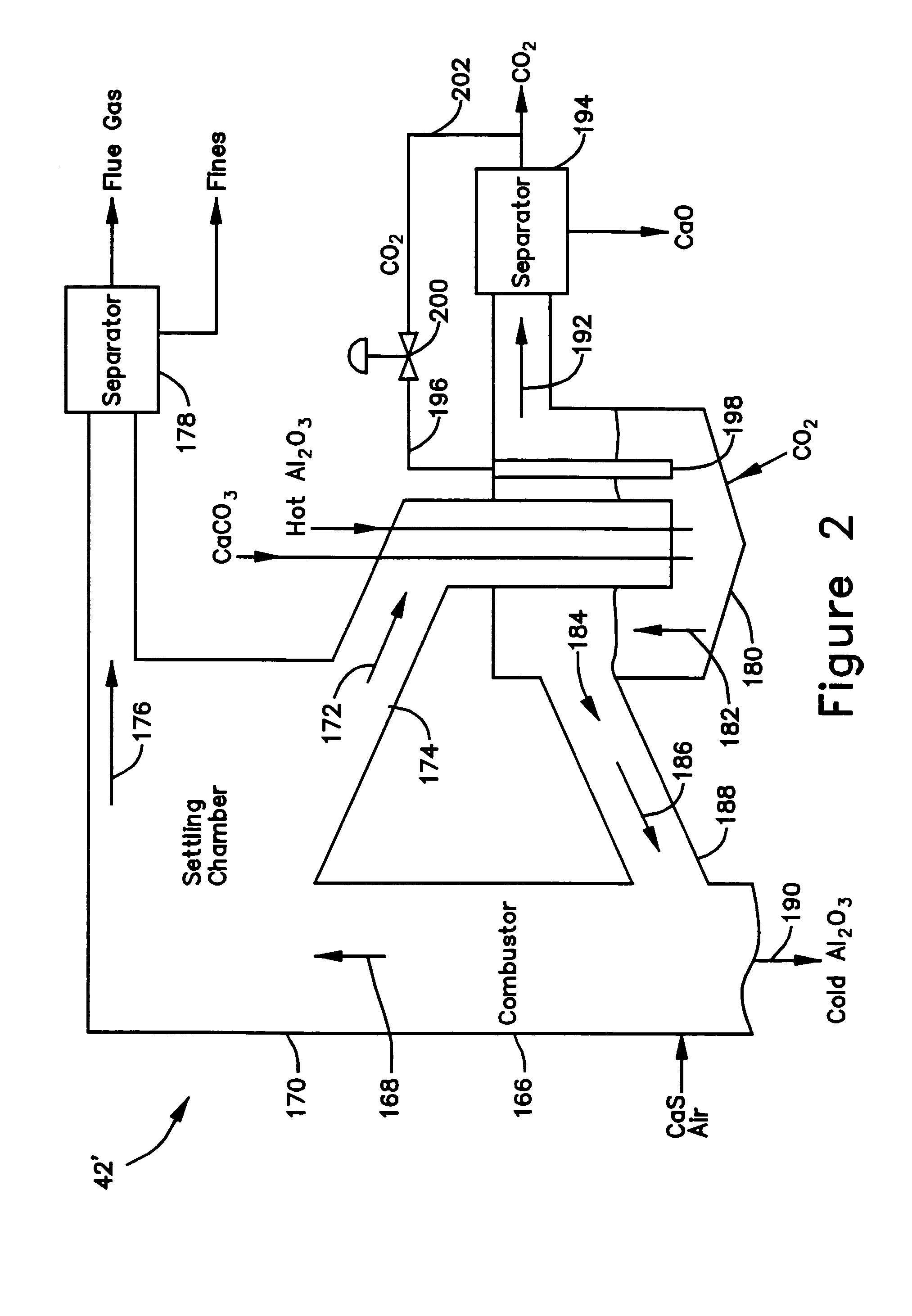
ActiveUS7083658B2Avoid entrainmentEfficient captureMuffle furnacesGas modification by gas mixingCo2 removalWater-gas shift reaction
A gasifier 10 includes a first chemical process loop 12 having an exothermic oxidizer reactor 14 and an endothermic reducer reactor 16. CaS is oxidized in air in the oxidizer reactor 14 to form hot CaSO4 which is discharged to the reducer reactor 16. Hot CaSO4 and carbonaceous fuel received in the reducer reactor 16 undergo an endothermic reaction utilizing the heat content of the CaSO4, the carbonaceous fuel stripping the oxygen from the CaSO4 to form CaS and a CO rich syngas. The CaS is discharged to the oxidizer reactor 14 and the syngas is discharged to a second chemical process loop 52. The second chemical process loop 52 has a water-gas shift reactor 54 and a calciner 42. The CO of the syngas reacts with gaseous H2O in the shift reactor 54 to produce H2 and CO2. The CO2 is captured by CaO to form hot CaCO3 in an exothermic reaction. The hot CaCO3 is discharged to the calciner 42, the heat content of the CaCO3 being used to strip the CO2 from the CaO in an endothermic reaction in the calciner, with the CaO being discharged from the calciner 42 to the shift reactor 54.
Owner:AIR PROD & CHEM INC +1
Anti-icing composition driven by catalytic hydrogen generation under subzero temperatures
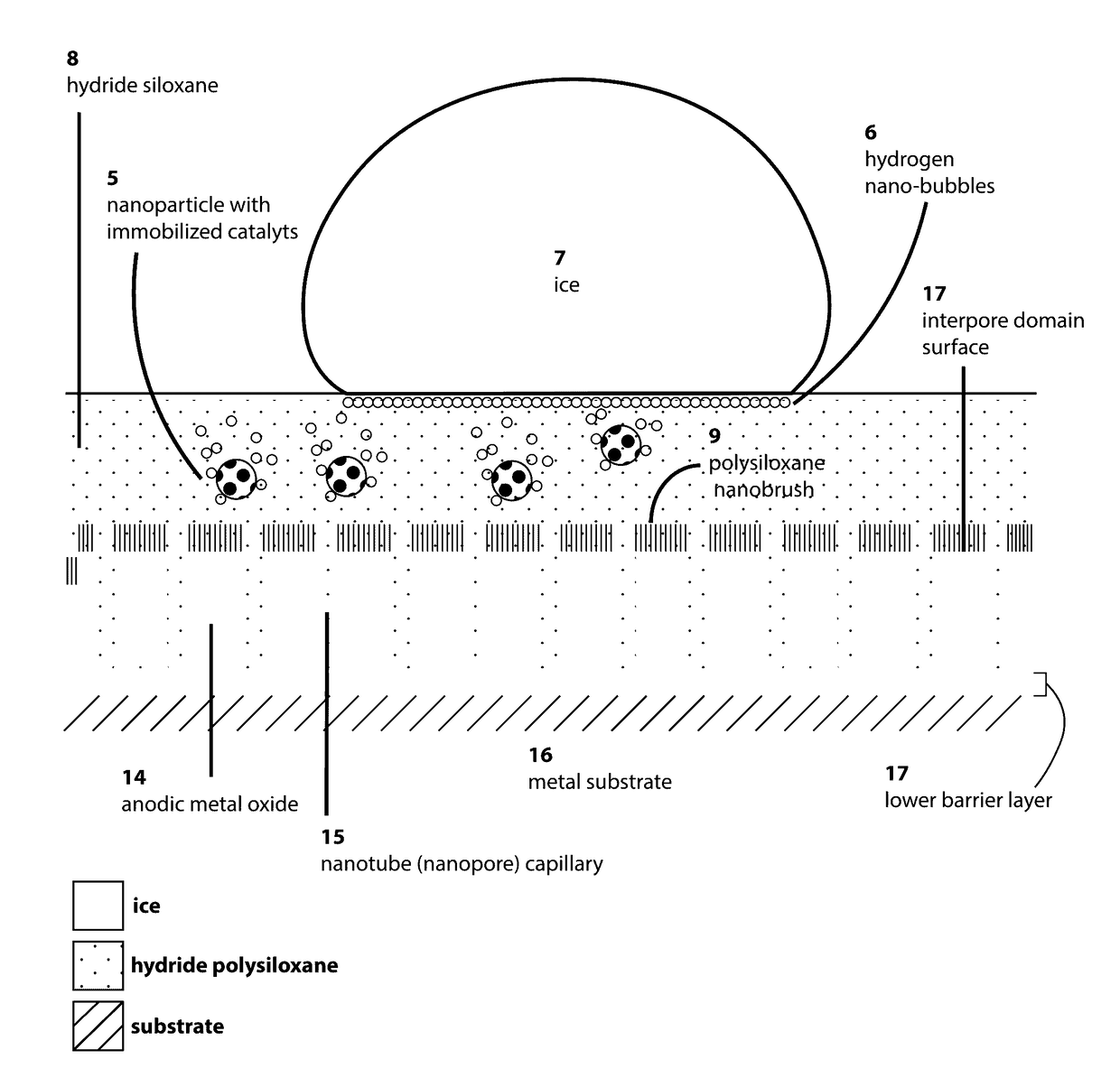
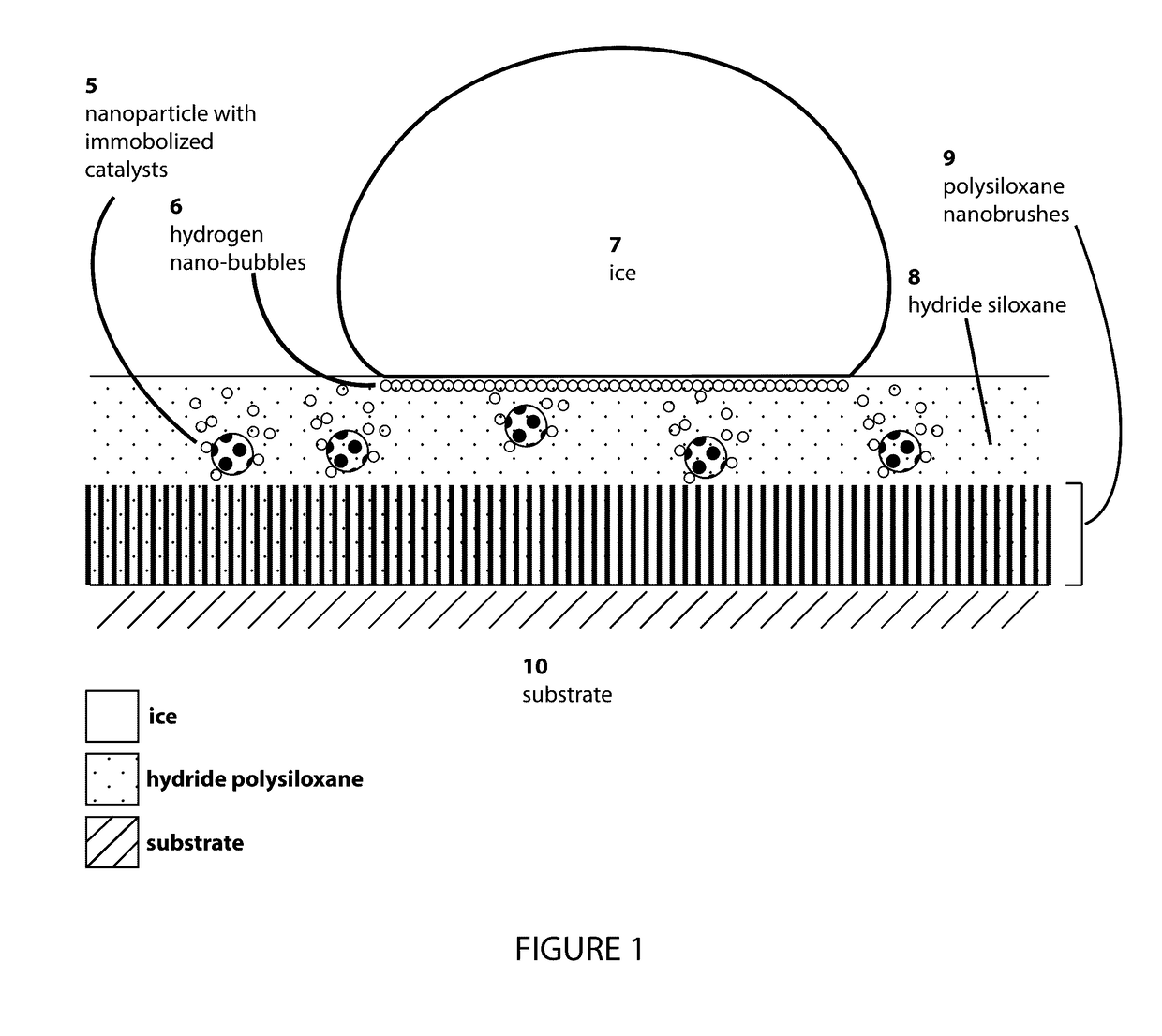
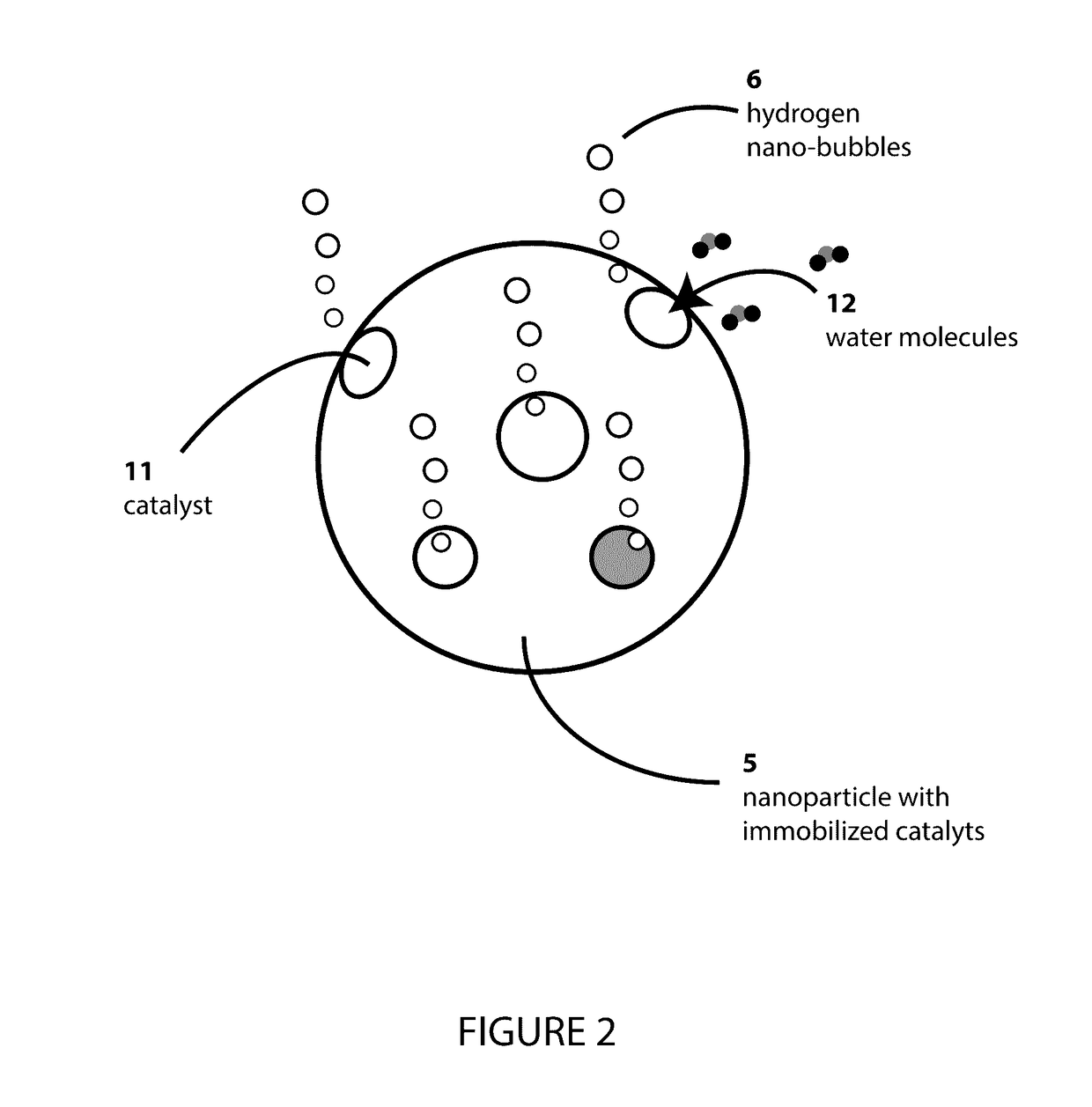
InactiveUS20170107413A1Reduce hysteresisLower surface energyOther chemical processesCoatingsHydrogenOptical transparency
The present invention relates to a self-renewing, anti-icing composition driven by a dehydrogenative reaction of a reactive hydrogen-rich compound catalyzed by nanoparticle immobilized catalysts, which is active under subzero temperatures. The disclosed coating displays a variety of properties including, but not limited to hydrophobicity, anti-wetting, and resistance to ice formation and ice adhesion. The novel anti-icing coating can be used on glass surfaces requiring optical clarity and transparency and can also be applied to a variety of smooth, roughened, or porous surfaces.
Owner:WANG LIANG +1
System and sub-systems for production and use of hydrogen
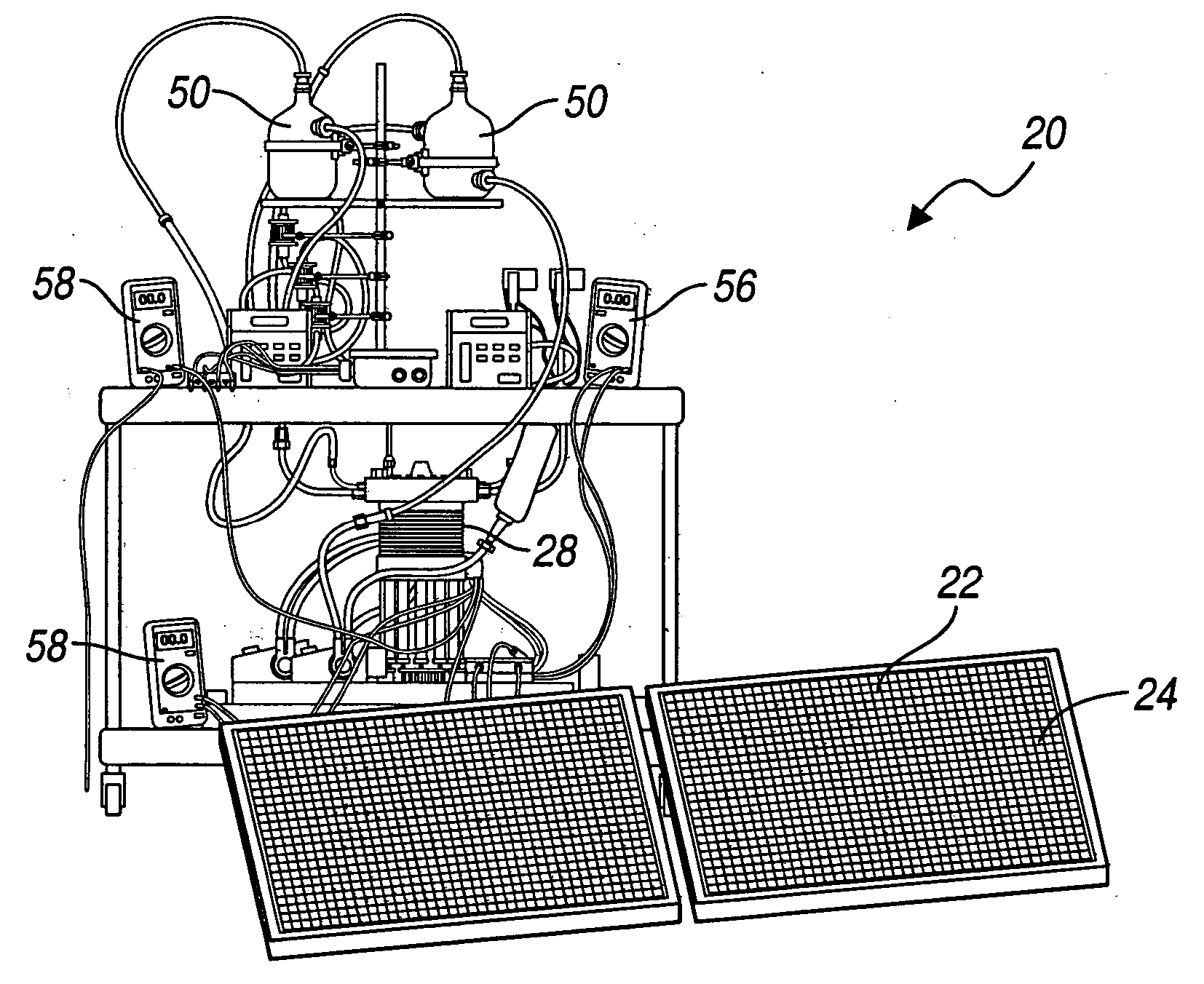
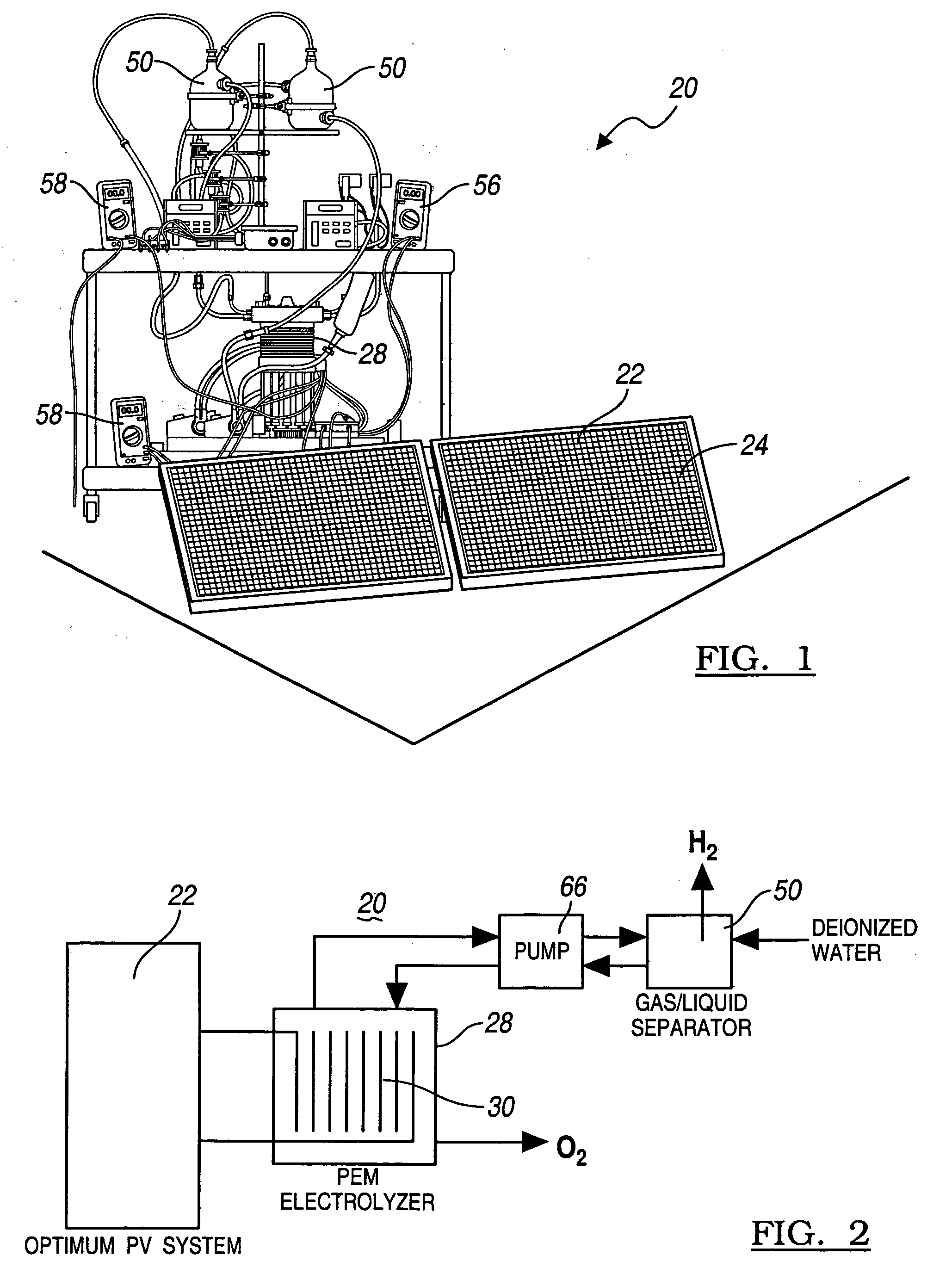
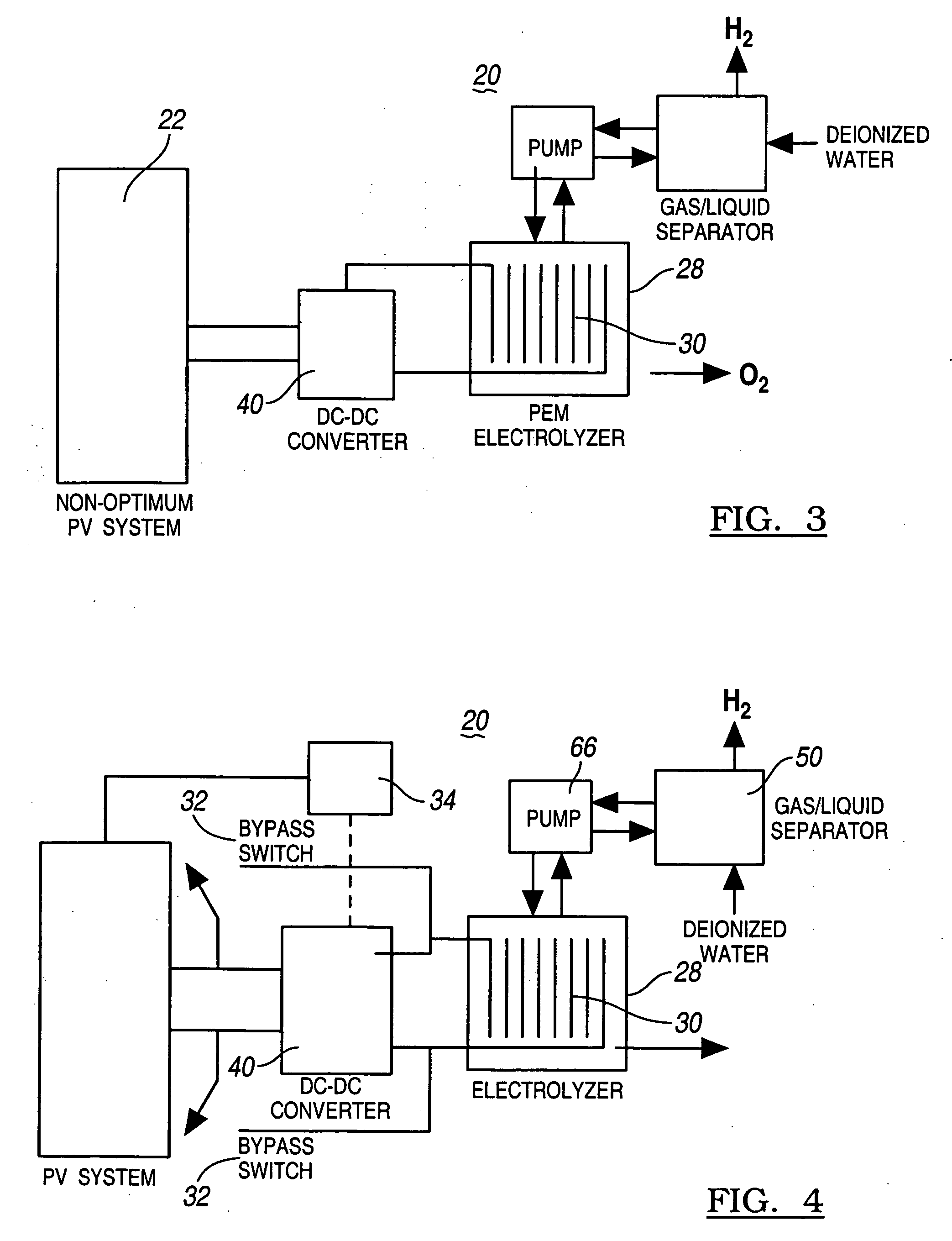
InactiveUS20060065302A1Most efficientSimple designPhotography auxillary processesPV power plantsHydrogenProton
A method for optimizing the efficiency of a solar powered hydrogen generation system is disclosed. The system utilizes photovoltaic modules and a proton exchange membrane electrolyzer to split water into hydrogen and oxygen with an efficiency greater than 12%. This high efficiency for the solar powered electrolysis of water was obtained by matching the voltage generated by photovoltaic modules to the operating voltage of the electrolyzer. Optimizing PV-electrolysis systems makes solar generated hydrogen less expensive and more practical for use as an environmentally clean and renewable fuel.
Owner:GM GLOBAL TECH OPERATIONS LLC
Hydrogen production from hydro power
InactiveUS6841893B2Economic valueMass productionLevel controlFinal product manufactureWater flowEngineering
A method is provided for operating a hydroelectric power generating facility configured for operating in first and second operating modes. The facility includes a turbine driven power generating unit receiving a flow of water through an upstream conduit to generate electrical power. The method comprises computing a first economic value for the generated electrical power when operating in the first operating mode, and computing a second economic value for the generated electrical power when operating in the second operating mode. The method further comprises comparing the first economic value with the second economic value to identify the operating mode that provides the higher economic value, and operating the turbine facility in the identified operating mode.
Owner:VOITH SIEMENS HYDRO POWER GENERATION
Energy conversion systems and methods
An energy conversion system may include a stationary structure, a rotatable structure configured to rotate relative to the stationary structure, wherein the rotatable structure defines an axis of rotation. The system may further include at least one blade member mounted to and extending radially outward from the rotatable structure, the at least one blade member being configured to interact with fluid currents flowing in a direction substantially parallel to the axis of rotation to cause the rotatable structure to rotate about the axis of rotation, and at least one bearing mechanism disposed to provide at least one of a radial and axial bearing between the rotatable structure and the stationary structure as the rotatable structure rotates about the stationary structure. The system may be configured to convert rotation of the rotatable structure to at least one of electricity and hydrogen production.
Owner:OCEANA ENERGY
Biomass castoff supercritical water fluid bed partial oxidation hydrogen-preparation device and method
ActiveCN101058404AImprove heat transfer efficiencyConducive to centralized processing and resource utilizationSolid waste disposalHydrogen productionBiomassChemistry
The invention discloses an oxidizing and gasifying hydrogen-making device and method of hypercritical water fluid-bed part of the biological waste, which is characterized by the following: adopting hypercritical water fluid-bed reactor to prevent slagging and blocking problem in the pipe flow reactor; using high-pressure separator to generate high-pressure water to adsorb carbon dioxide in the gas product; realizing the separation of the hydrogen from the carbon dioxide; making the separated high-density carbon dioxide concentrate to be disposed and do resoursing usage conveniently; compacting the device structure; simplifying and conveniencing the operation; improving the gasifying rate of the biological waste with less pollution in the liquid product; realizing the oxidizing and gasifying fusion in the reactor; improving the biological mass transmitting rate and system energy transmitting efficiency; transmitting the biological waste into high-quality hydrogen through the method; reducing the environmental pollution; realizing the dual goals to harness pollution and make hydrogen.
Owner:陕西中核交大超洁能源技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com