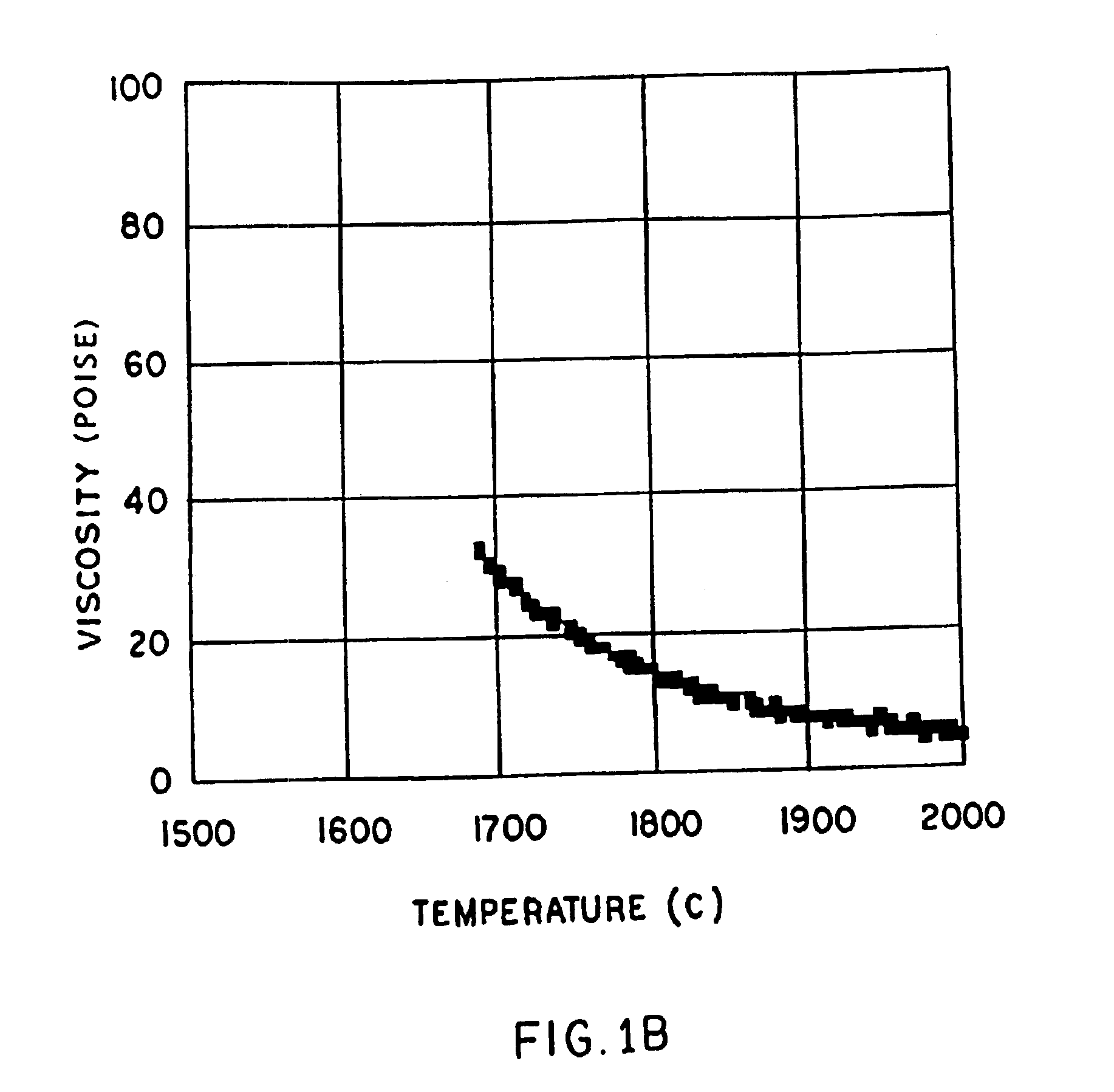
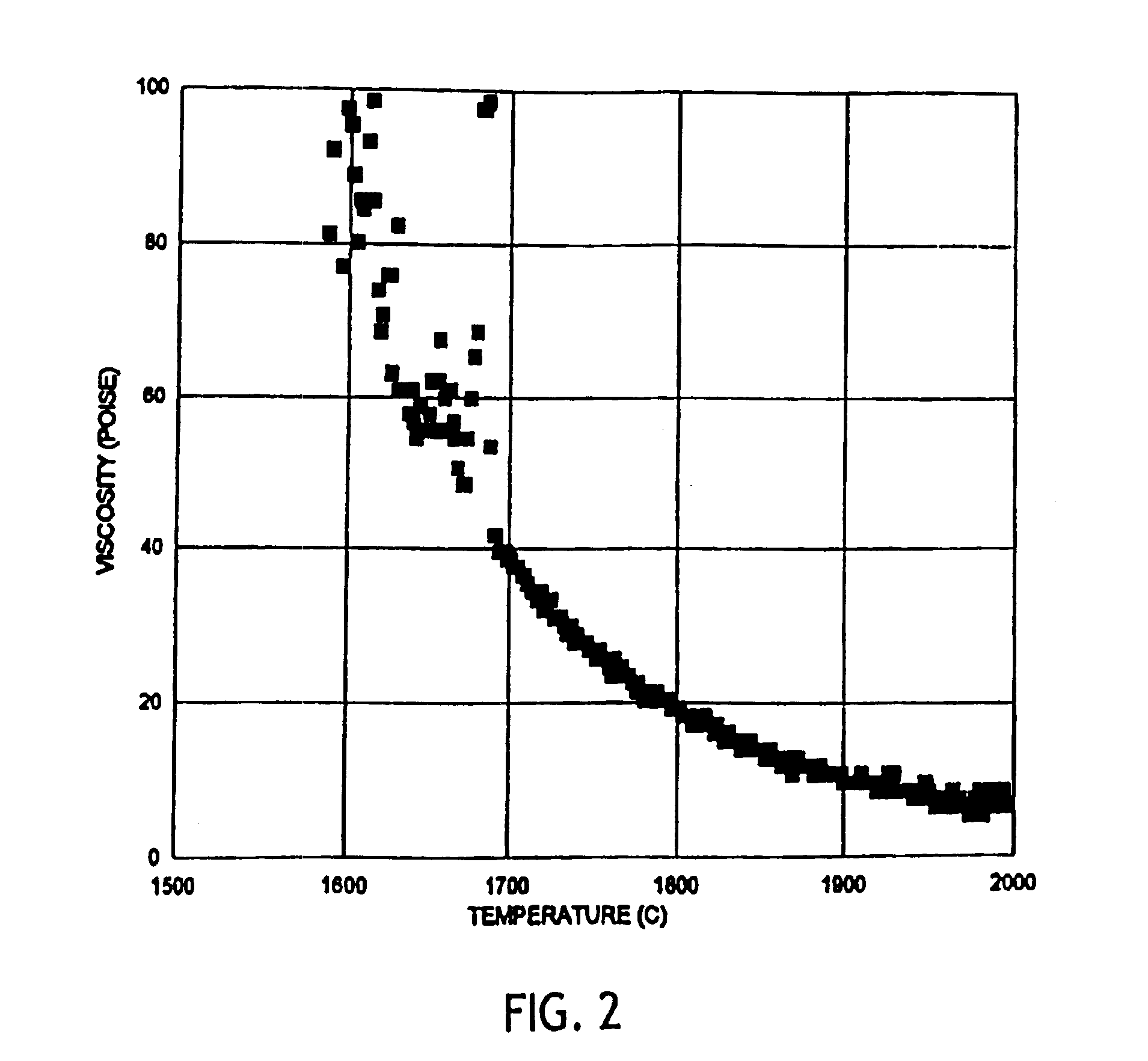
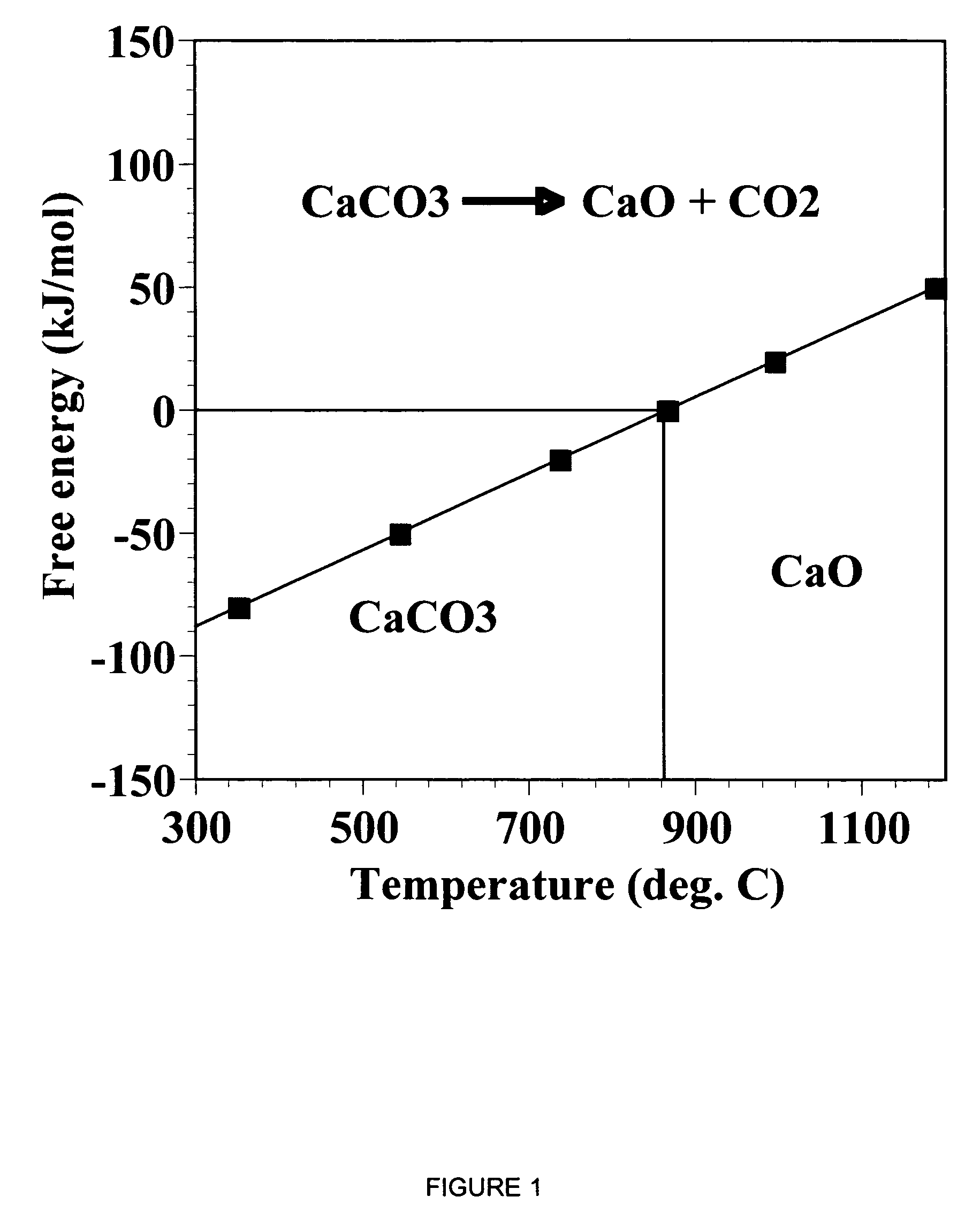
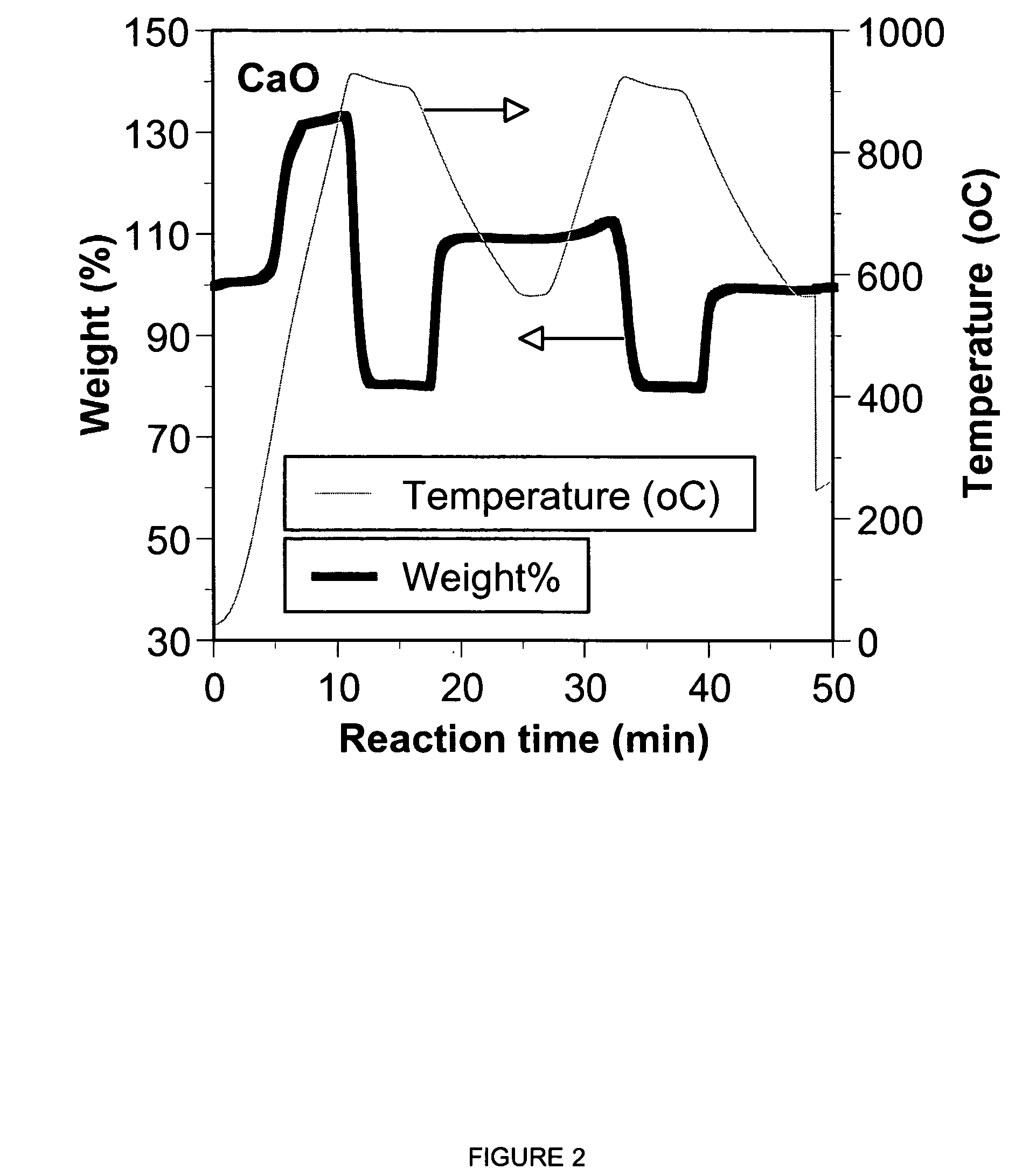
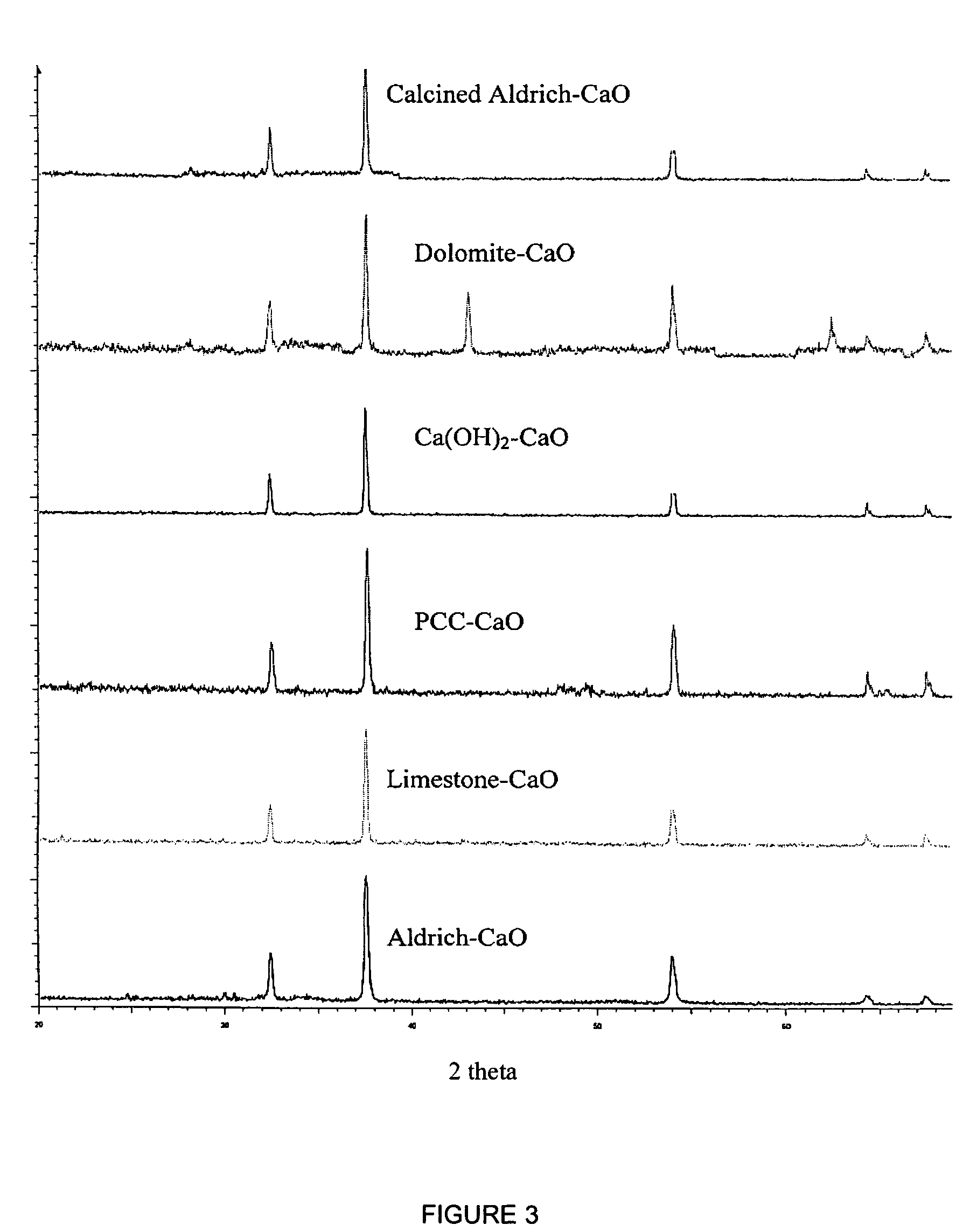
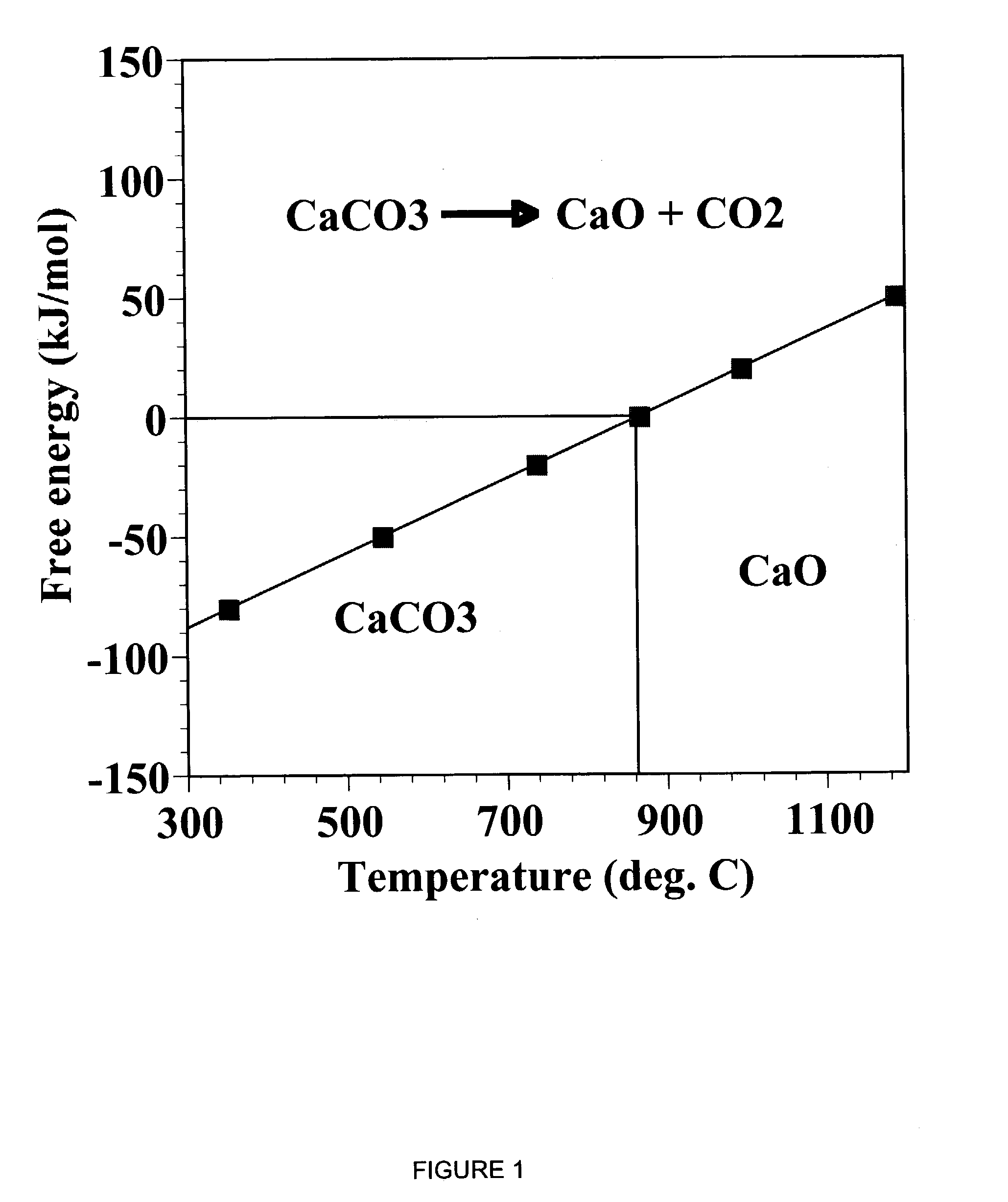
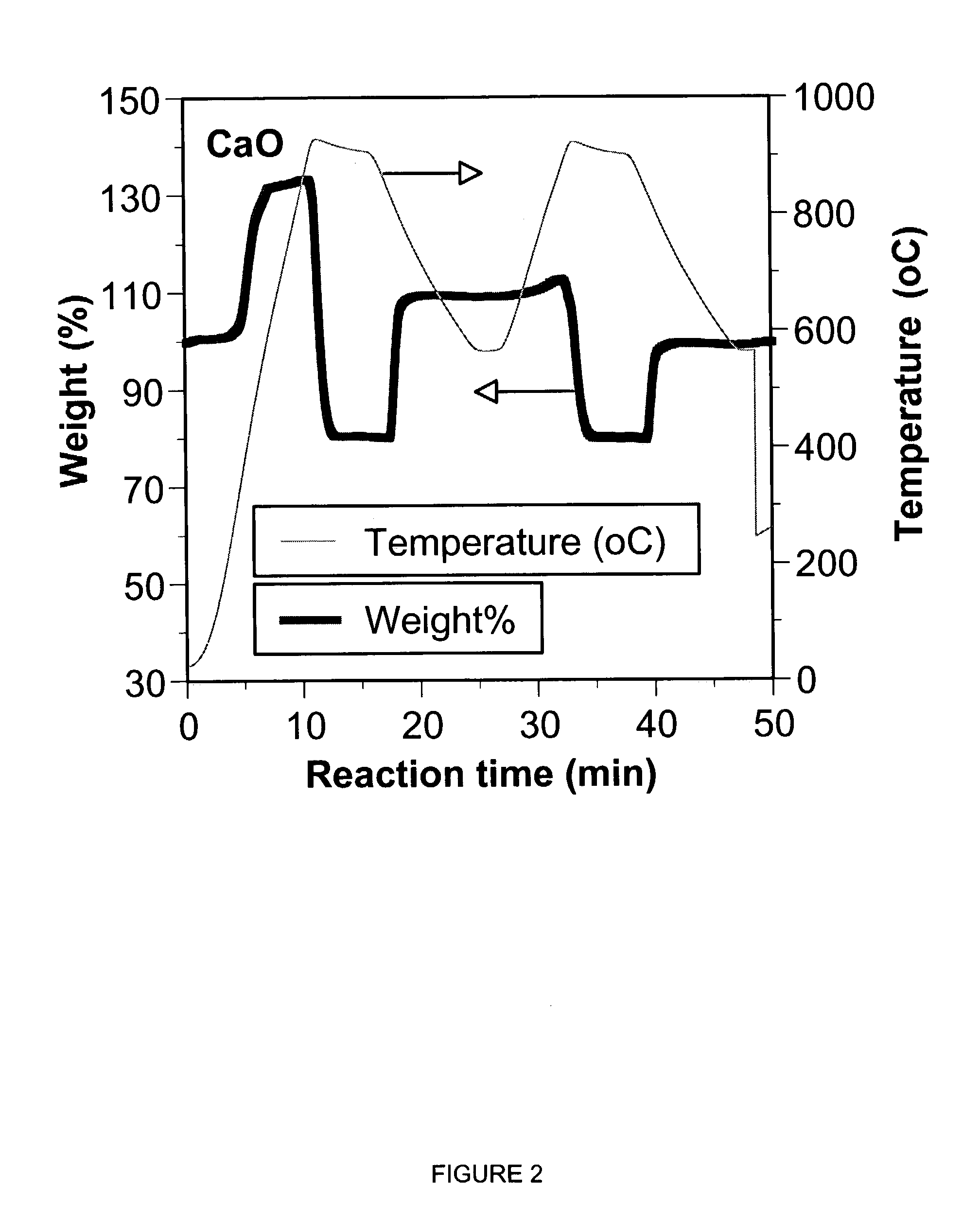
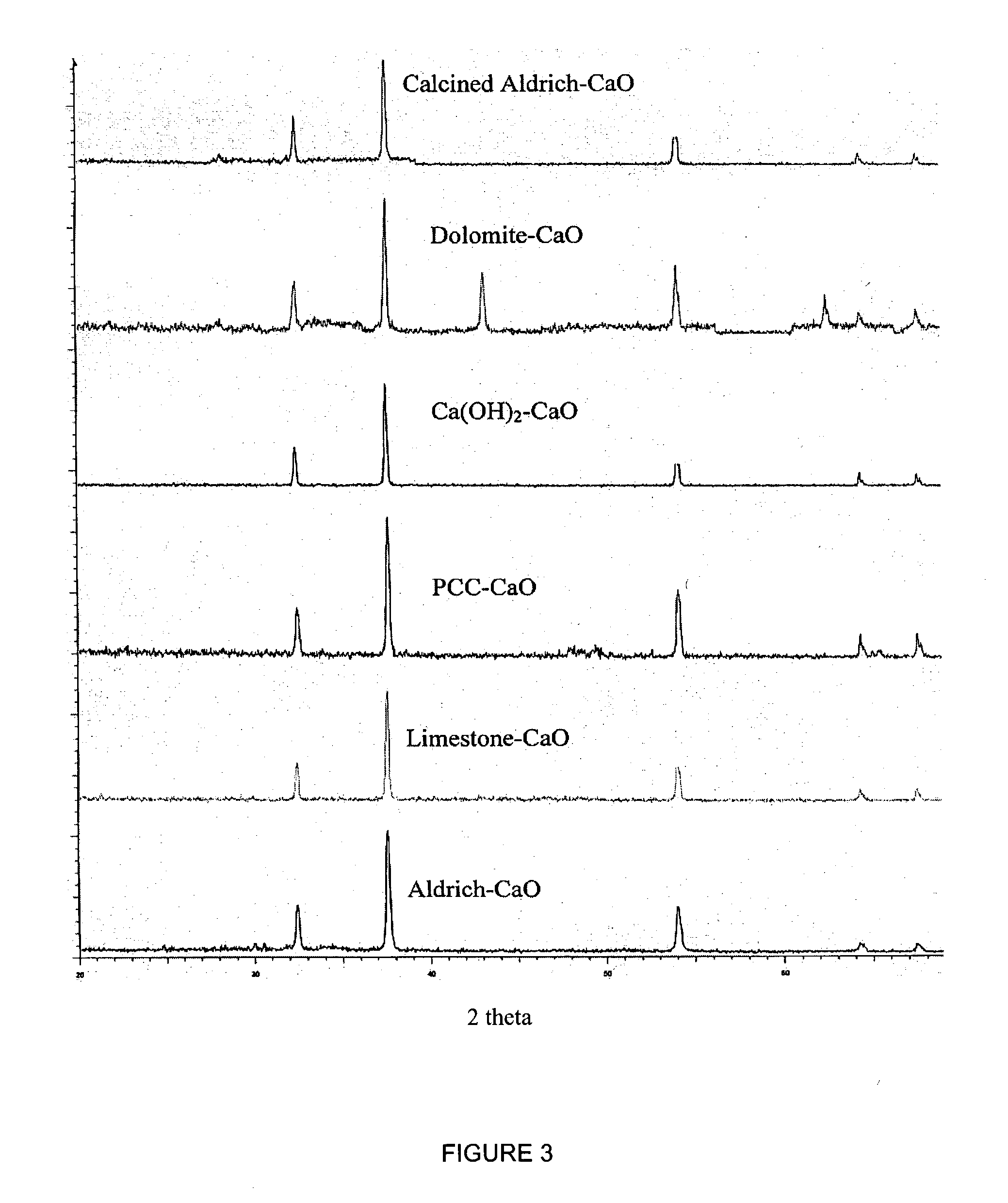
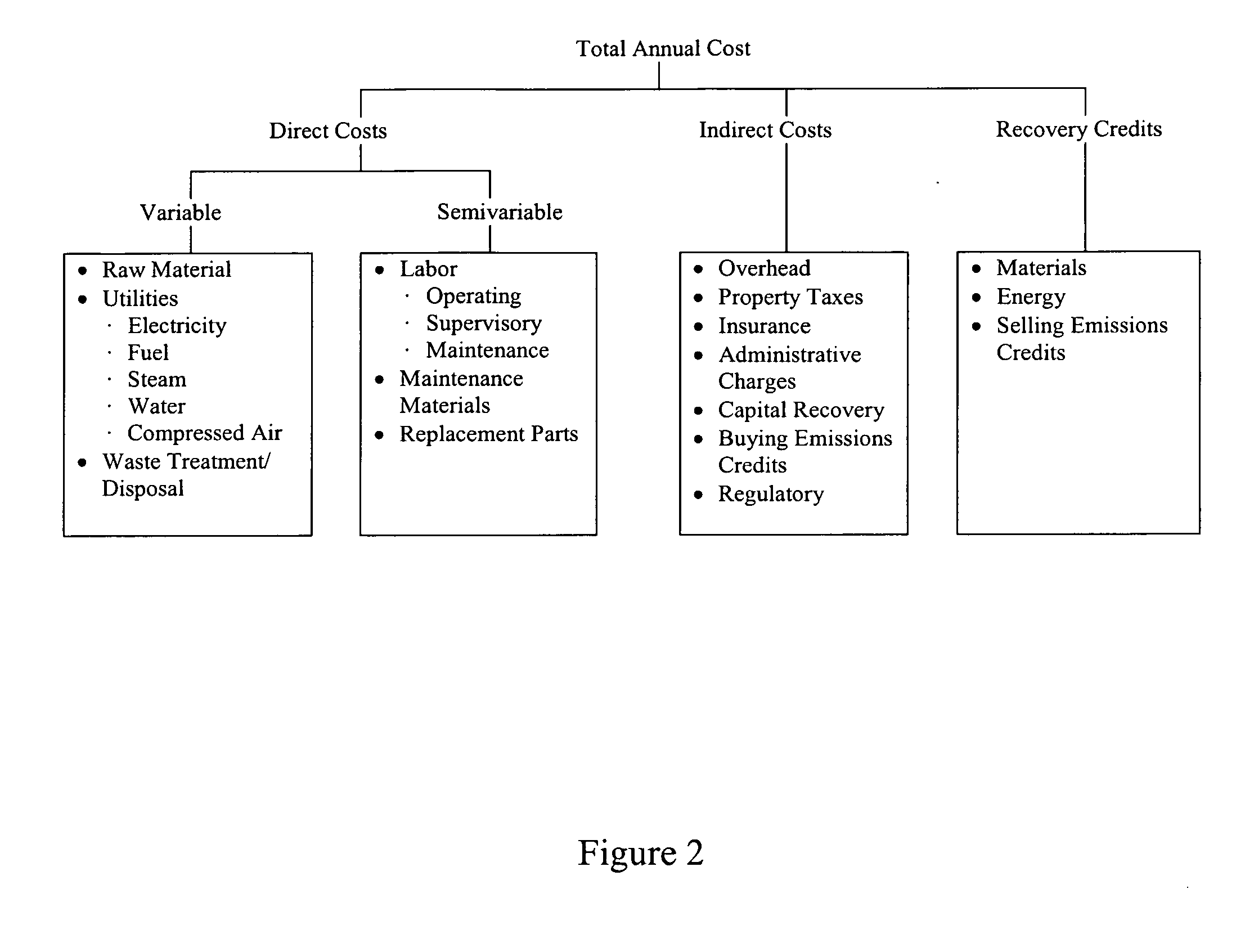
Patents
Literature
11048 results about "Calcium oxide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
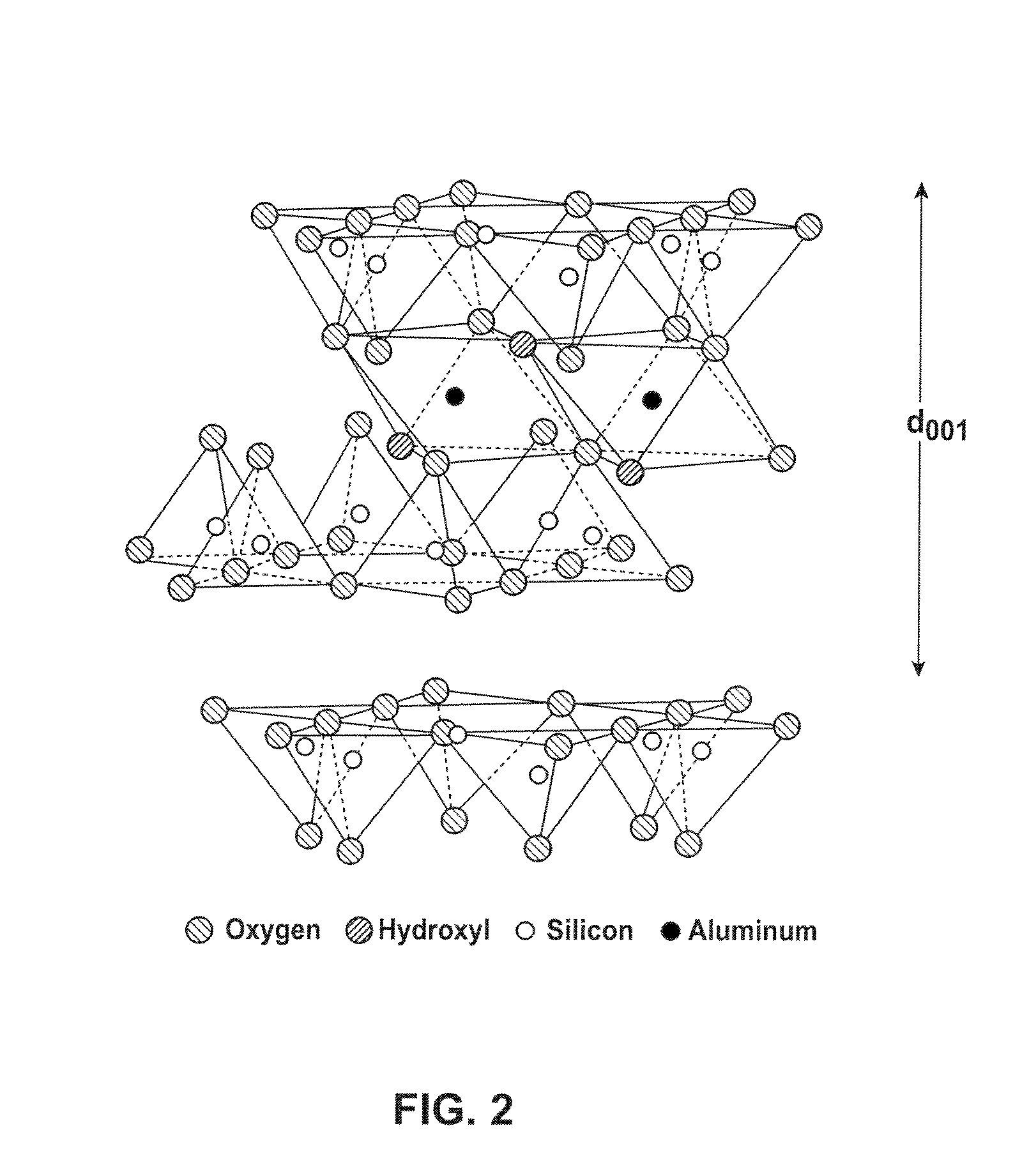
Calcium oxide (CaO), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline solid at room temperature. The broadly used term "lime" connotes calcium-containing inorganic materials, in which carbonates, oxides and hydroxides of calcium, silicon, magnesium, aluminium, and iron predominate. By contrast, quicklime specifically applies to the single chemical compound calcium oxide. Calcium oxide that survives processing without reacting in building products such as cement is called free lime.
Hydrogen production from carbonaceous material
InactiveUS6790430B1Avoid the needCalcium/strontium/barium carbonatesGas turbine plantsCalcinationExothermic reaction
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE +1
High temperature a resistant vitreous inorganic fiber
InactiveUS6953757B2Easy to manufactureLow shrinkageInorganic fibres/flakesInorganic material artificial filamentsFiberPhysiological fluid
A low shrinkage, high temperature resistant vitreous inorganic fiber having a use temperature up to at least 1330° C., which maintains mechanical integrity after exposure to the use temperature and which is non-durable in physiological fluids, is prepared by the method of forming a melt with ingredients including greater than 71.25 weight percent silica, 0 to about 20 weight percent magnesia, and about 5 to about 28.55 weight percent of calcia, 0 to about 5 weight percent zirconia, and optionally a viscosity modifier in an amount effective to render the product fiberizable; and producing fibers from the melt.
Owner:UNIFRAX I LLC
Separation of carbon dioxide (CO2) from gas mixtures
ActiveUS7618606B2Good repeatabilityMaterial nanotechnologyCombustible gas catalytic treatmentCo2 removalSorbent
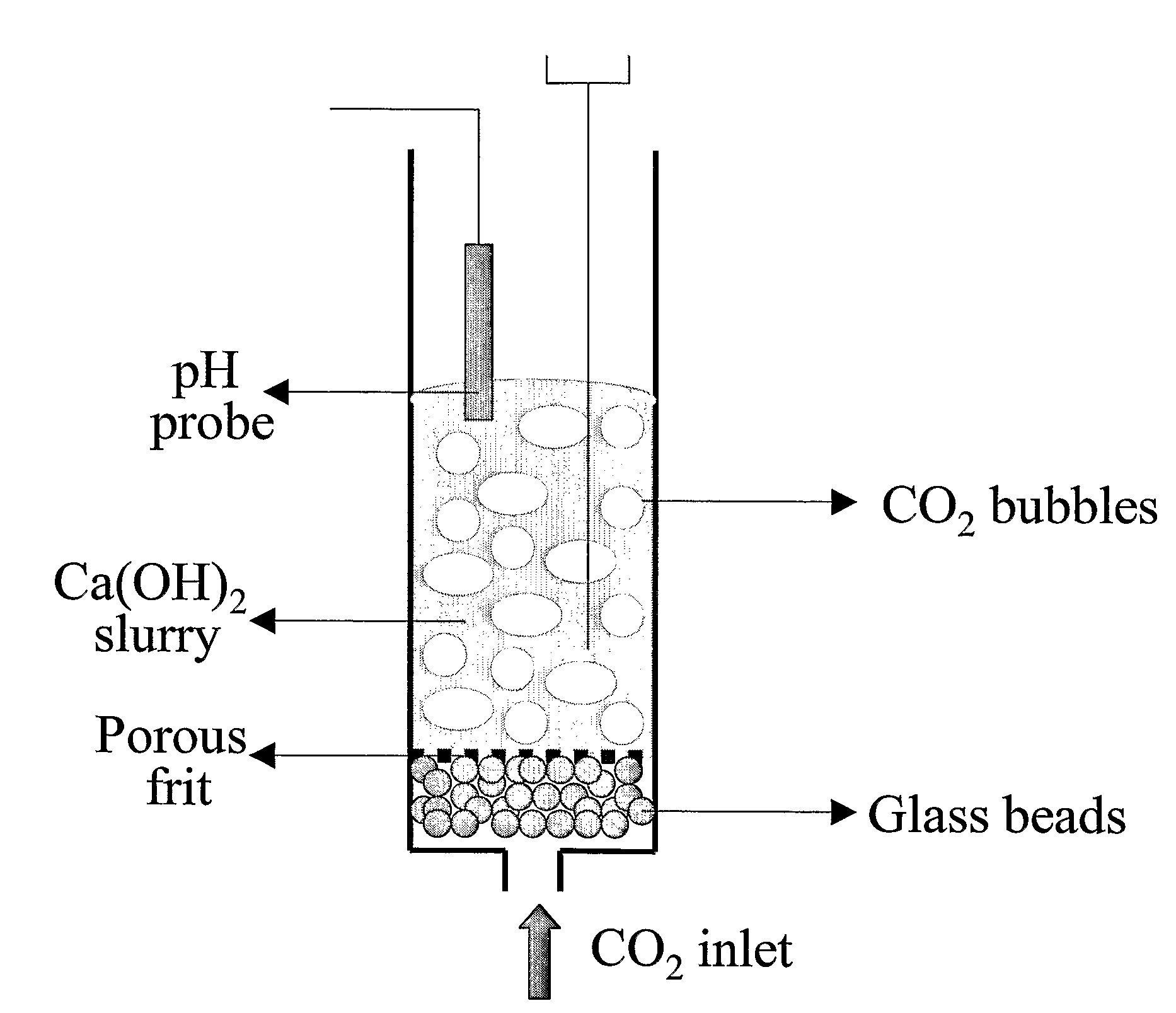
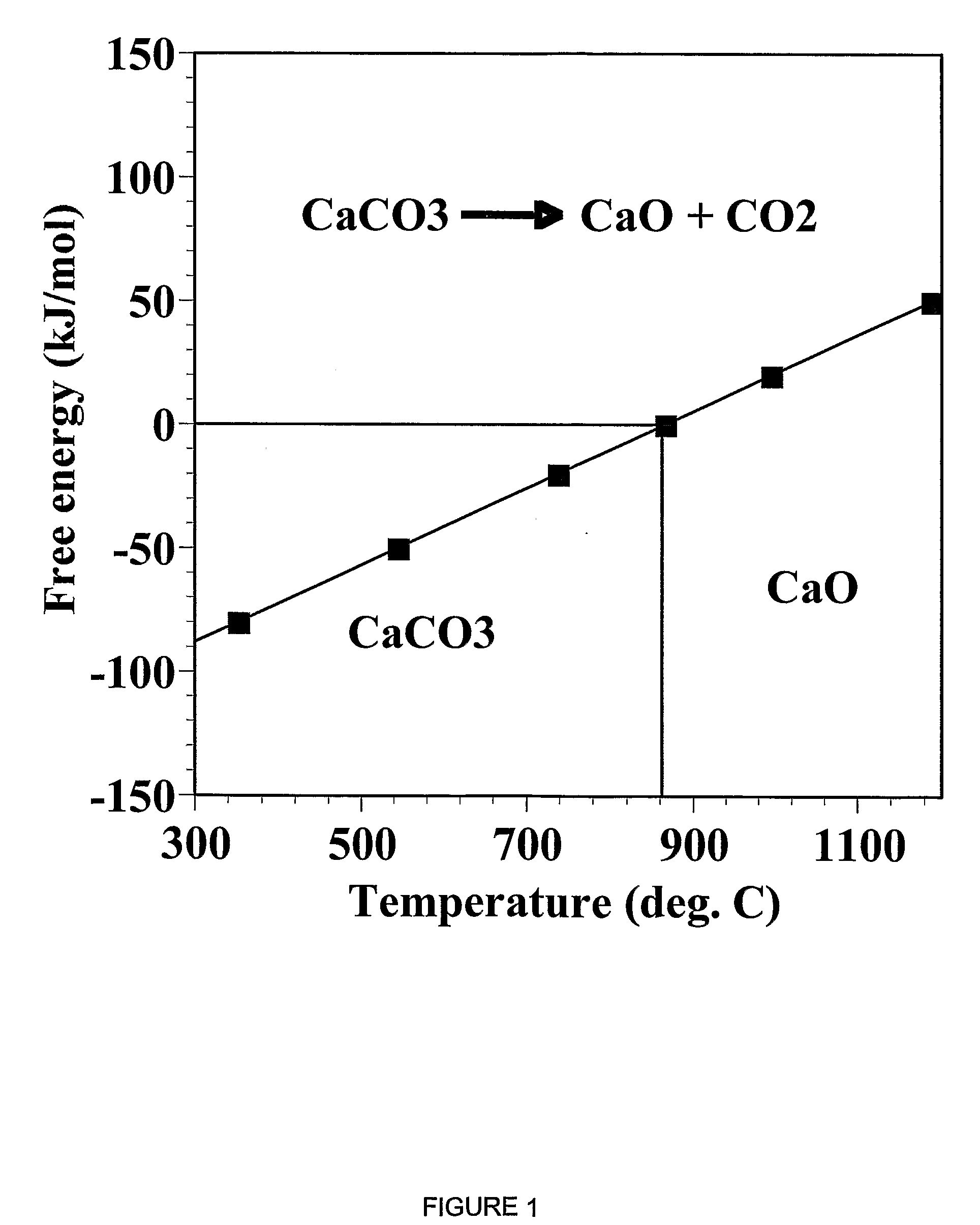
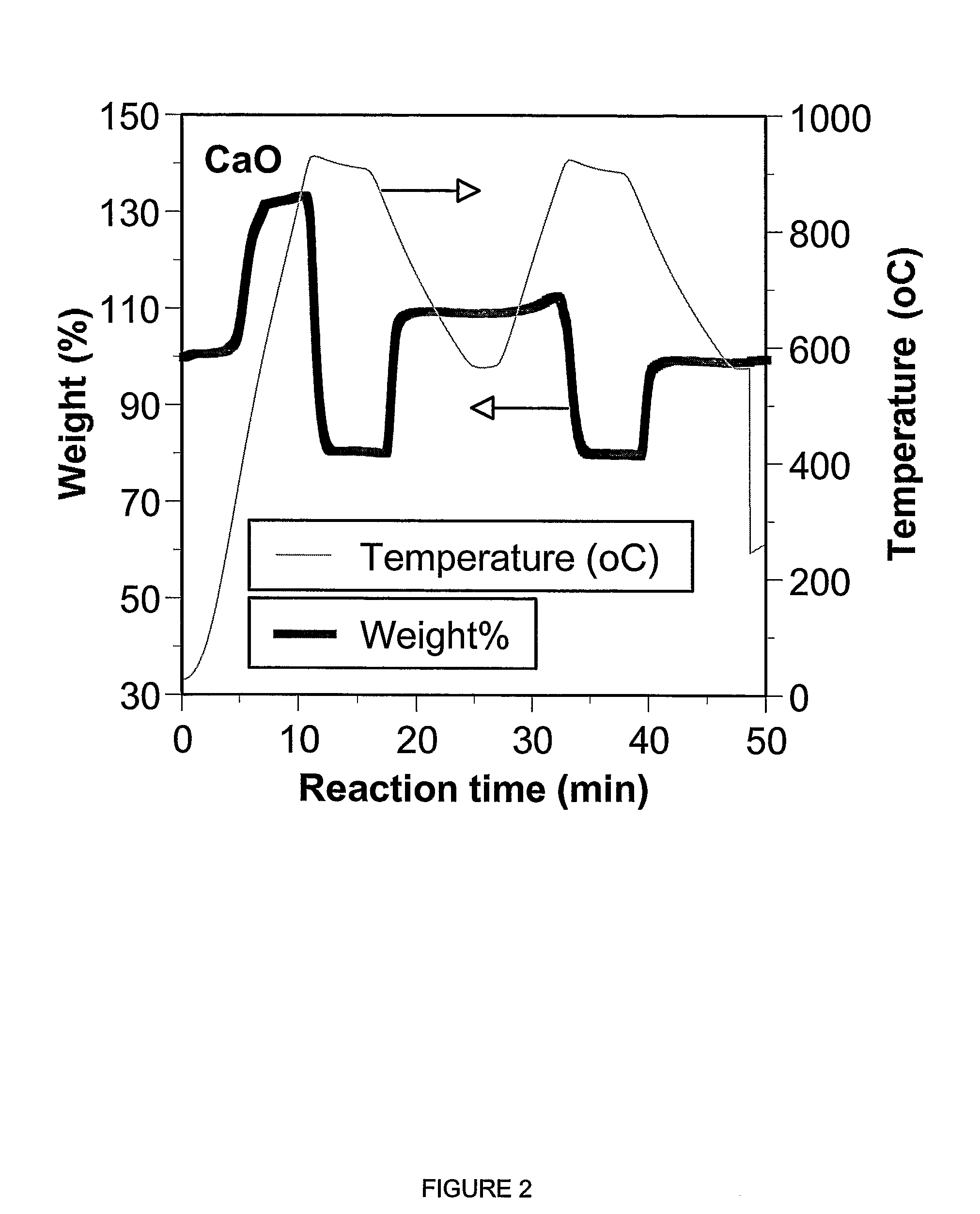
A reaction-based process has been developed for the selective removal of carbon dioxide from a multicomponent gas mixture. The proposed process effects the separation of CO2 from a mixture of gases by its reaction with metal oxides. The Calcium based Reaction Separation for CO2 process consists of contacting a CO2 laden gas with calcium oxide in a reactor such that CaO captures the CO2 by the formation of calcium carbonate. Once “spent”, CaCO3 is regenerated by its calcination leading to the formation of fresh CaO sorbent. The “regenerated” CaO is then recycled for the further capture of more CO2. This process also identifies the application of a mesoporous CaCO3 structure, that attains >90% conversion over multiple carbonation and calcination cycles. Lastly, thermal regeneration (calcination) under vacuum provided a better sorbent structure that maintained reproducible reactivity levels over multiple cycles.
Owner:THE OHIO STATES UNIV
Sorbent for separation of carbon dioxide (CO2) from gas mixtures
A reaction-based process has been developed for the selective removal of carbon dioxide (CO2) from a multicomponent gas mixture to provide a gaseous stream depleted in CO2 compared to the inlet CO2 concentration in the stream. The proposed process effects the separation of CO2 from a mixture of gases (such as flue gas / fuel gas) by its reaction with metal oxides (such as calcium oxide). The Calcium based Reaction Separation for CO2 (CaRS-CO2) process consists of contacting a CO2 laden gas with calcium oxide (CaO) in a reactor such that CaO captures the CO2 by the formation of calcium carbonate (CaCO3). Once “spent”, CaCO3 is regenerated by its calcination leading to the formation of fresh CaO sorbent and the evolution of a concentrated stream of CO2. The “regenerated” CaO is then recycled for the further capture of more CO2. This carbonation-calcination cycle forms the basis of the CaRS-CO2 process. This process also identifies the application of a mesoporous CaCO3 structure, developed by a process detailed elsewhere, that attains >90% conversion over multiple carbonation and calcination cycles. Lastly, thermal regeneration (calcination) under vacuum provided a better sorbent structure that maintained reproducible reactivity levels over multiple cycles.
Owner:THE OHIO STATES UNIV
Separation of Carbon Dioxide (Co2) From Gas Mixtures By Calcium Based Reaction Separation (Cars-Co2) Process
InactiveUS20080233029A1Good repeatabilityMaterial nanotechnologyCombustible gas catalytic treatmentSorbentTransformation ratio
A reaction-based process has been developed for the selective removal of carbon dioxide (CO2) from a multicomponent gas mixture to provide a gaseous stream depleted in CO2 compared to the inlet CO2 concentration in the stream. The proposed process effects the separation of CO2 from a mixture of gases (such as flue gas / fuel gas) by its reaction with metal oxides (such as calcium oxide). The Calcium based Reaction Separation for CO2 (CaRS—CO2) process consists of contacting a CO2 laden gas with calcium oxide (CaO) in a reactor such that CaO captures the CO2 by the formation of calcium carbonate (CaCOa). Once “spent”, CaCO3 is regenerated by its calcination leading to the formation of fresh CaO sorbent and the evolution of a concentrated stream of CO2. The “regenerated” CaO is then recycled for the further capture of more CO2. This carbonation-calcination cycle forms the basis of the CaRS—CO2 process. This process also identifies the application of a mesoporous CaCO3 structure, developed by a process detailed elsewhere, that attains >90% conversion over multiple carbonation and calcination cycles. Lastly, thermal regeneration (calcination) under vacuum provided a better sorbent structure that maintained reproducible reactivity levels over multiple cycles.
Owner:THE OHIO STATES UNIV
Preparation method for high-alkaline calcium naphthenate
ActiveCN101885677AAvoid aggregation and sedimentationHelps neutralize the reactionAdditivesCarboxylic acid salt preparationCalcium hydroxideAlkaline water
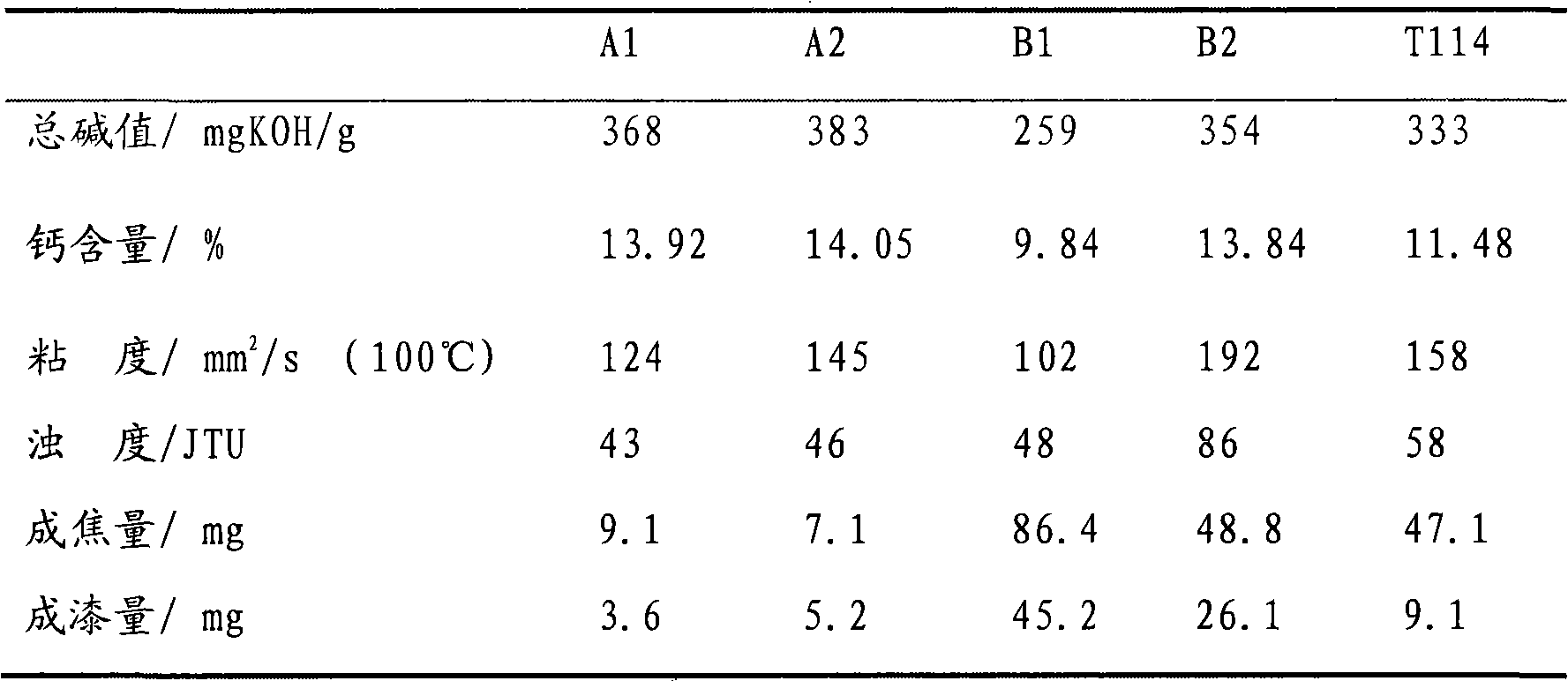
The invention relates to a preparation method for high-alkaline calcium naphthenate. In the method, calcium hydroxide and calcium oxide are taken as calcifying agents, pure water is replaced by alkaline aqueous solution, and a mode of multiple addition is adopted to prepare a calcium naphthenate product with the base number of more than or equal to 350 mgKOH / g. The product has the advantages of low viscosity and turbidity and better high temperature cleanliness.
Owner:CHINA PETROLEUM & CHEM CORP +1
Thermally conductive cementitious grout for geothermal heat pump systems
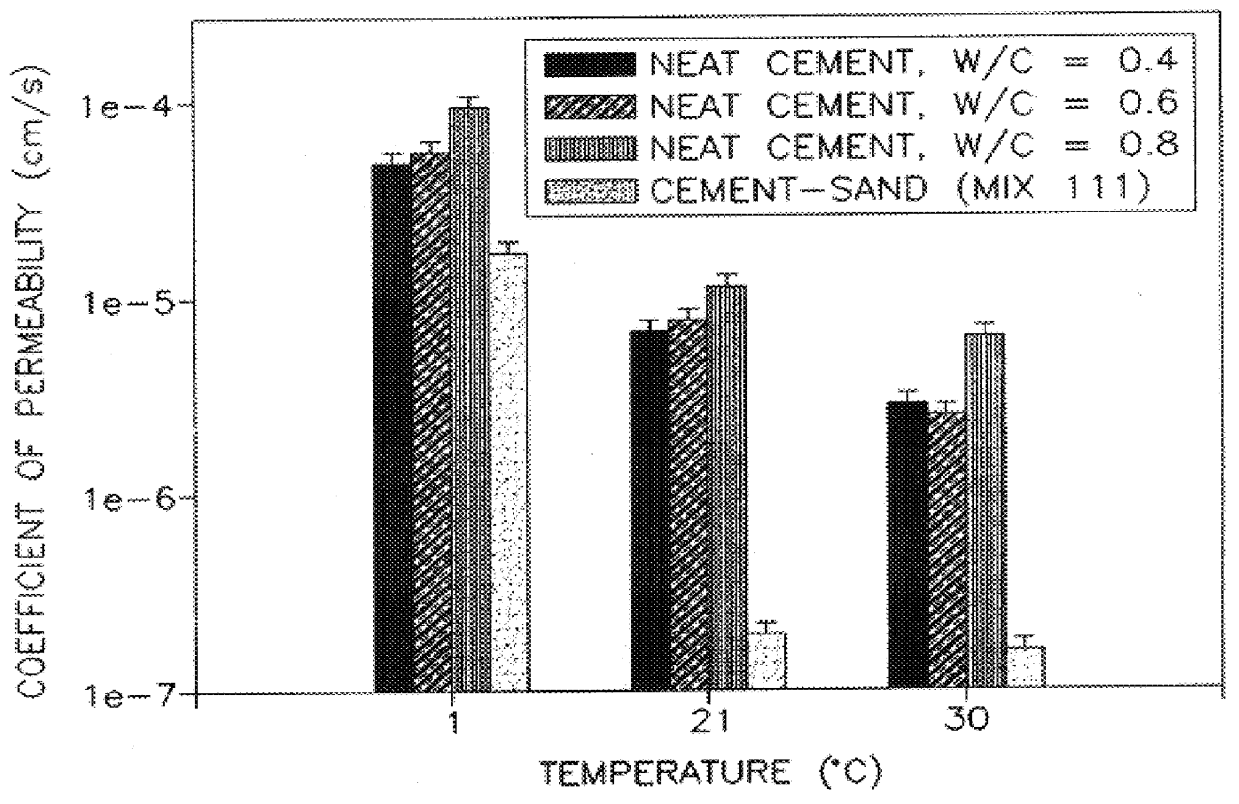
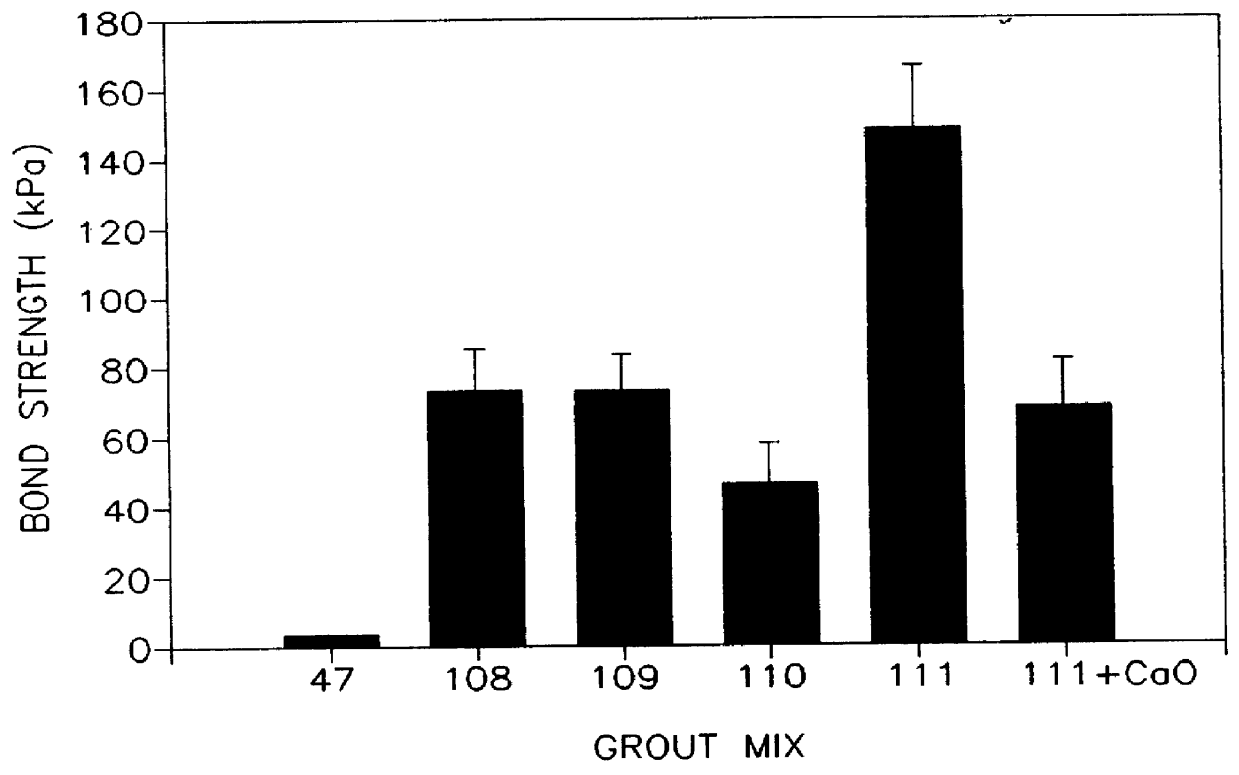
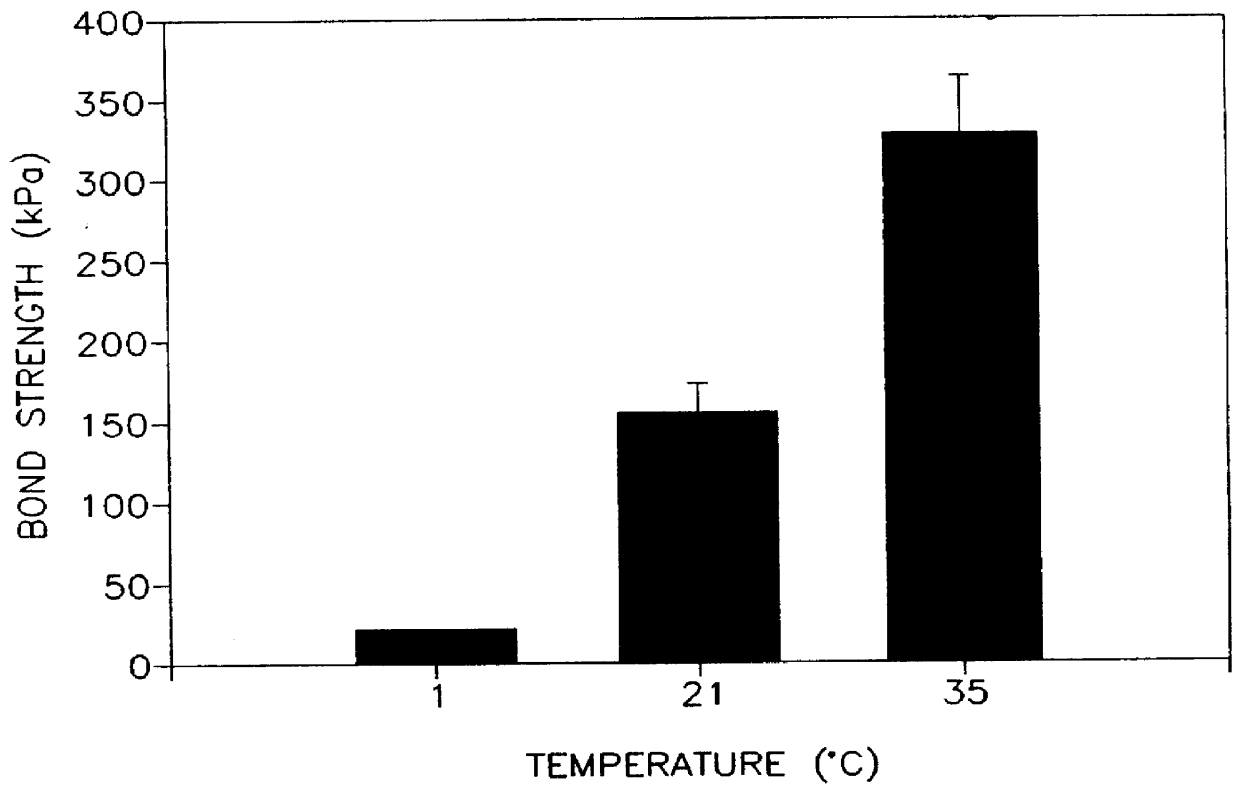
A thermally conductive cement-sand grout for use with a geothermal heat pump system. The cement sand grout contains cement, silica sand, a superplasticizer, water and optionally bentonite. The present invention also includes a method of filling boreholes used for geothermal heat pump systems with the thermally conductive cement-sand grout. The cement-sand grout has improved thermal conductivity over neat cement and bentonite grouts, which allows shallower bore holes to be used to provide an equivalent heat transfer capacity. In addition, the cement-sand grouts of the present invention also provide improved bond strengths and decreased permeabilities. The cement-sand grouts can also contain blast furnace slag, fly ash, a thermoplastic air entraining agent, latex, a shrinkage reducing admixture, calcium oxide and combinations thereof.
Owner:THE UNITED STATES AS REPRESENTED BY THE DEPARTMENT OF ENERGY
Complete-oil synthetic base drilling fluid
InactiveCN101215461AGood water loss wall building performanceStrong anti-pollutionDrilling compositionReducerHigh pressure
The invention discloses whole-oil synthetic base drilling fluid which mainly comprises following components: base liquid 100 parts, histosol 2-5 parts, viscosity increaser 0.5-3 parts, filtrate reducer 2-5 parts, wetting agent 0.5-2 parts, emulsifier 1-3 parts, calcium oxide 0.5 part and weighting agent 27-180 parts. The invention has the advantages that firstly, the rheological property is good and the plastic viscosity is low, secondly, the anti-temperature performance is good and anti-temperature can reach 150 DEG C, thirdly, the invention is provided with good water loss wall building performance with API filter loss content<5ml and high temperature and high pressure filter loss content <= 10 ml, fourthly, the anti-pollution ability is strong and the anti-poor soil ability and the anti-water invasion ability respectively reach 20%, and fifthly, reservoir has good protective effects and the permeability recovery value is over 85%.
Owner:CHINA NAT OFFSHORE OIL CORP +1
Methods of operating a coal burning facility
ActiveUS20070168213A1Improve balanceIncrease valueSustainable waste treatmentSolid fuel pretreatmentHalogenSorbent
Methods involve adding sorbent components, such as calcium oxide, alumina, and silica, as well as optional halogens as part of environmental control. Use of the sorbents leads to significant reductions in sulfur and mercury emissions that otherwise would result from burning coal. Use of the sorbents leads to production of waste coal ash that, while higher in mercury, is nevertheless usable as a commercial product because the mercury in the ash is non-leaching and because the coal ash has a higher cementitious nature by virtue of the increased content of the sorbent components in the ash. Thus, the methods involve adding powders having qualities that lead to the production of a cementitious coal ash while at the same time reducing emissions from a coal burning facility.
Owner:NOX II LTD
Process and apparatus for the treatment of saline water
InactiveUS20060196836A1Reduce ZLD costReduce loadWaste water treatment from quariesGeneral water supply conservationTotal dissolved solidsEvaporation
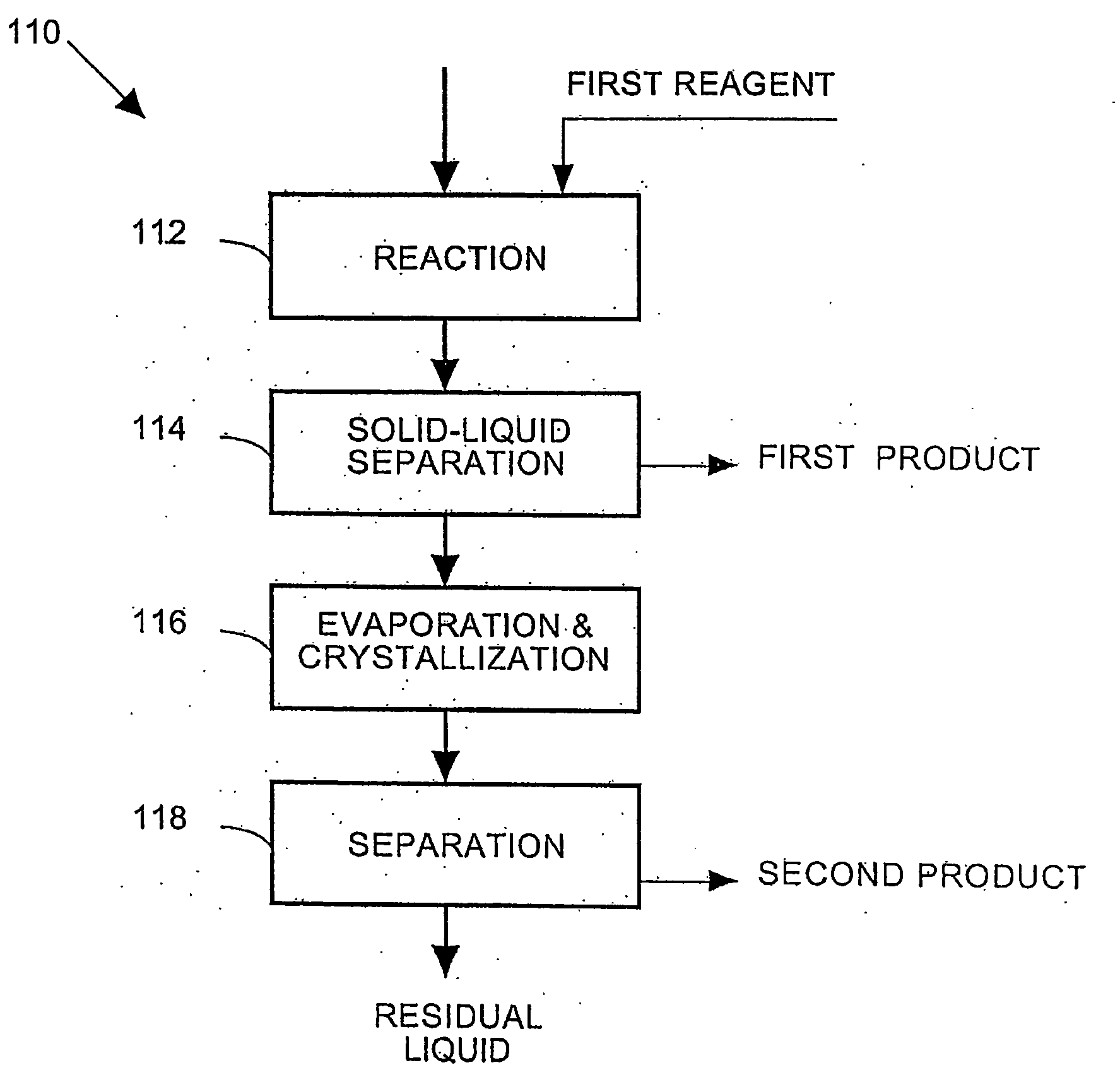
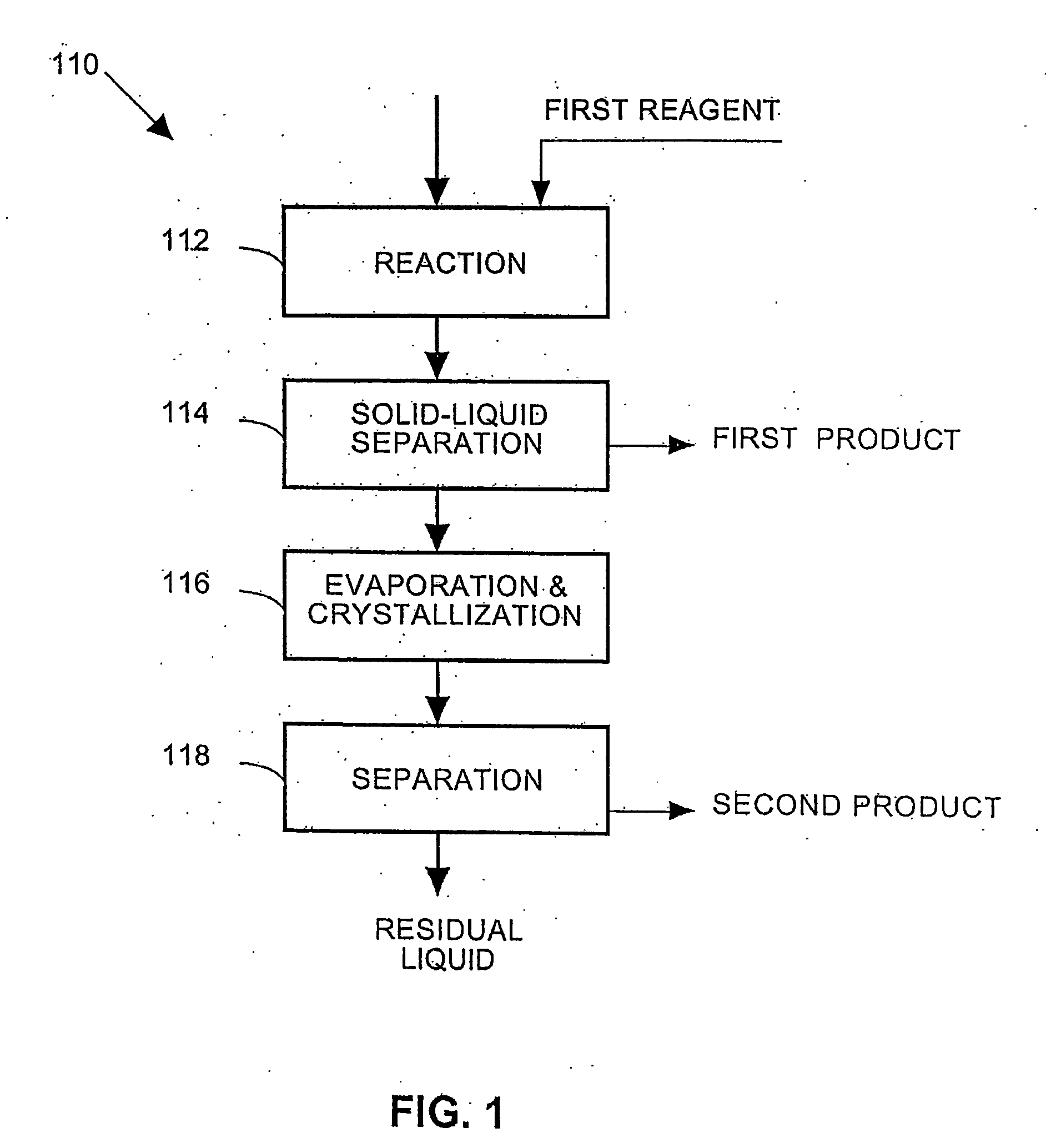
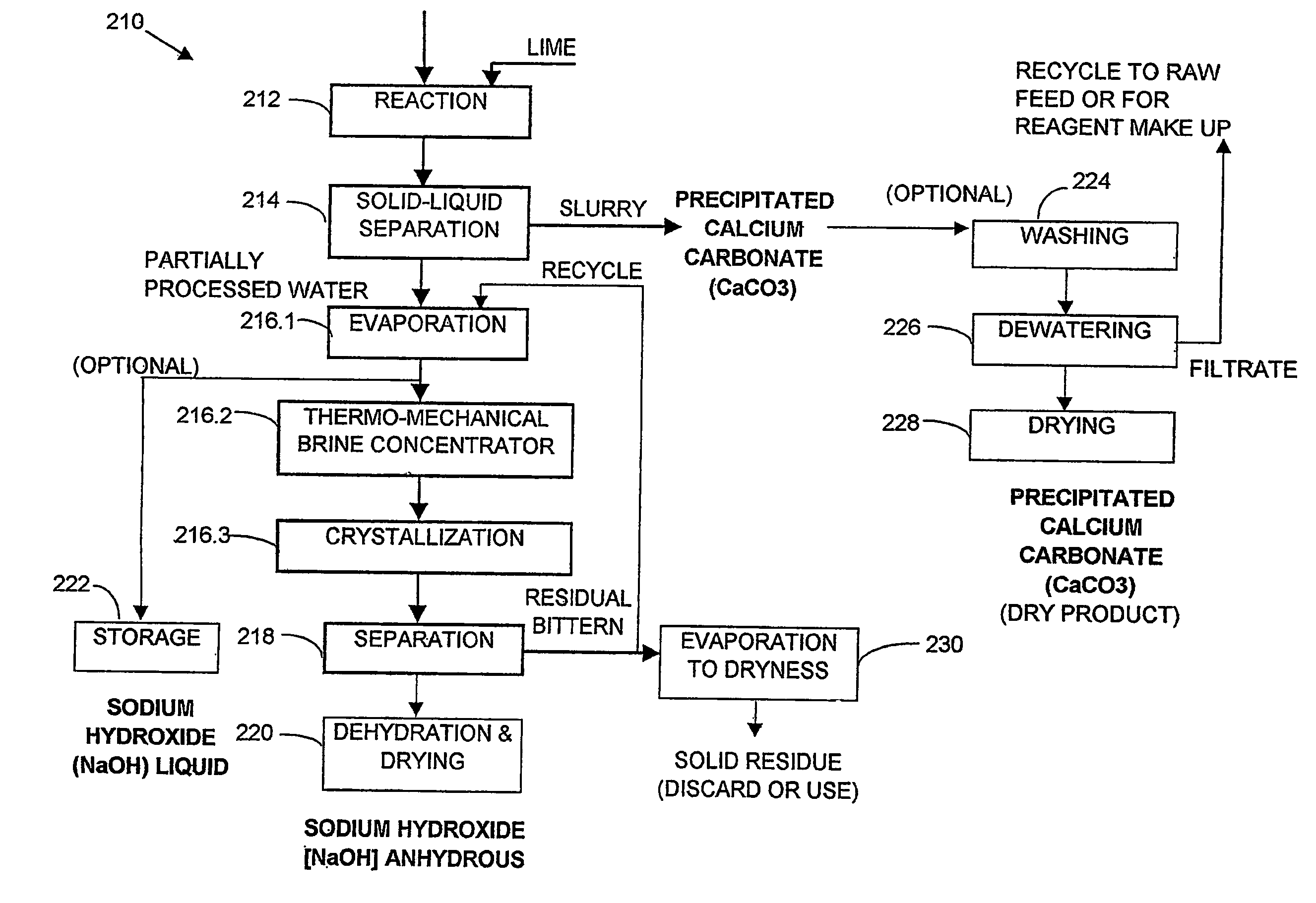
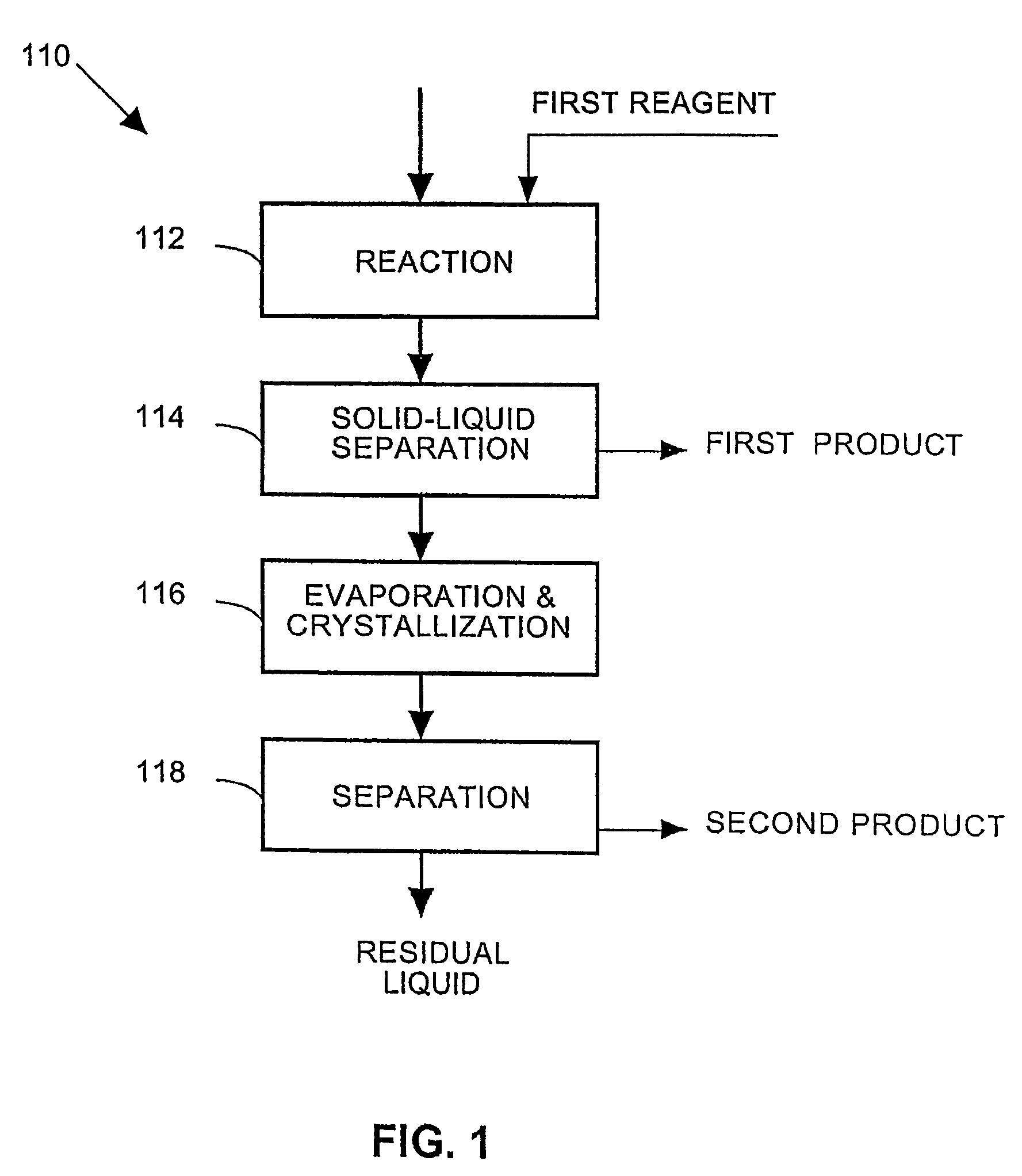
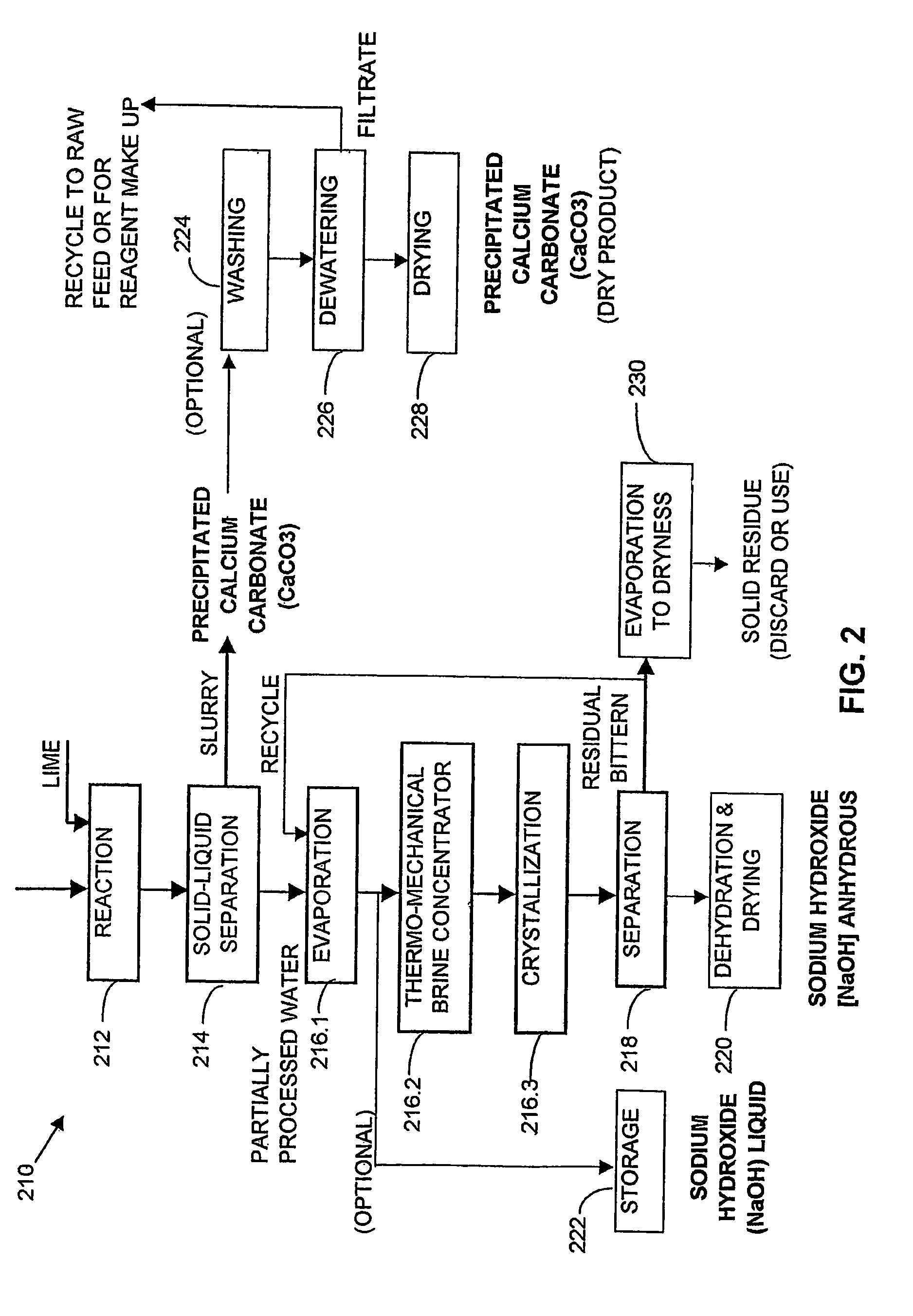
A process and an apparatus are described for treating seven types of saline waters each having a concentration of total dissolved solids exceeding 1 g / L, wherein the concentration of total dissolved solids, the ratio of the chloride ion concentration to the bicarbonate ion concentration and the ratio of the chloride ion concentration to the sulphate ion concentration of each of the water types are as indicated in Table 1. The process includes the steps of contacting the water with a first reagent comprising a source of calcium ions selected from calcium oxide and calcium hydroxide to form a first solid product which is recovered. The process includes a further step of subjecting at least a portion of the partially processed water to at least partial evaporation so as to promote the formation of a precipitate and a mother liquor. The precipitate is recovered as a second product.
Owner:GEO PROCESSORS
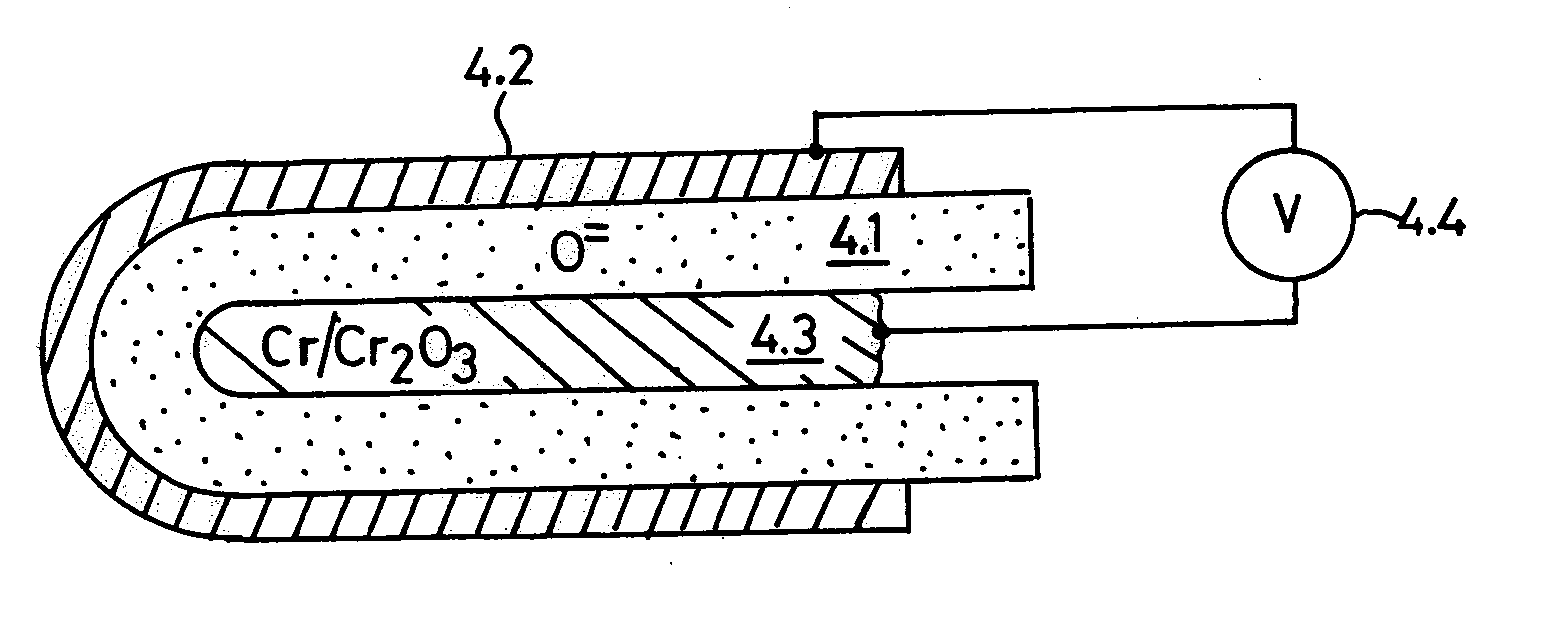
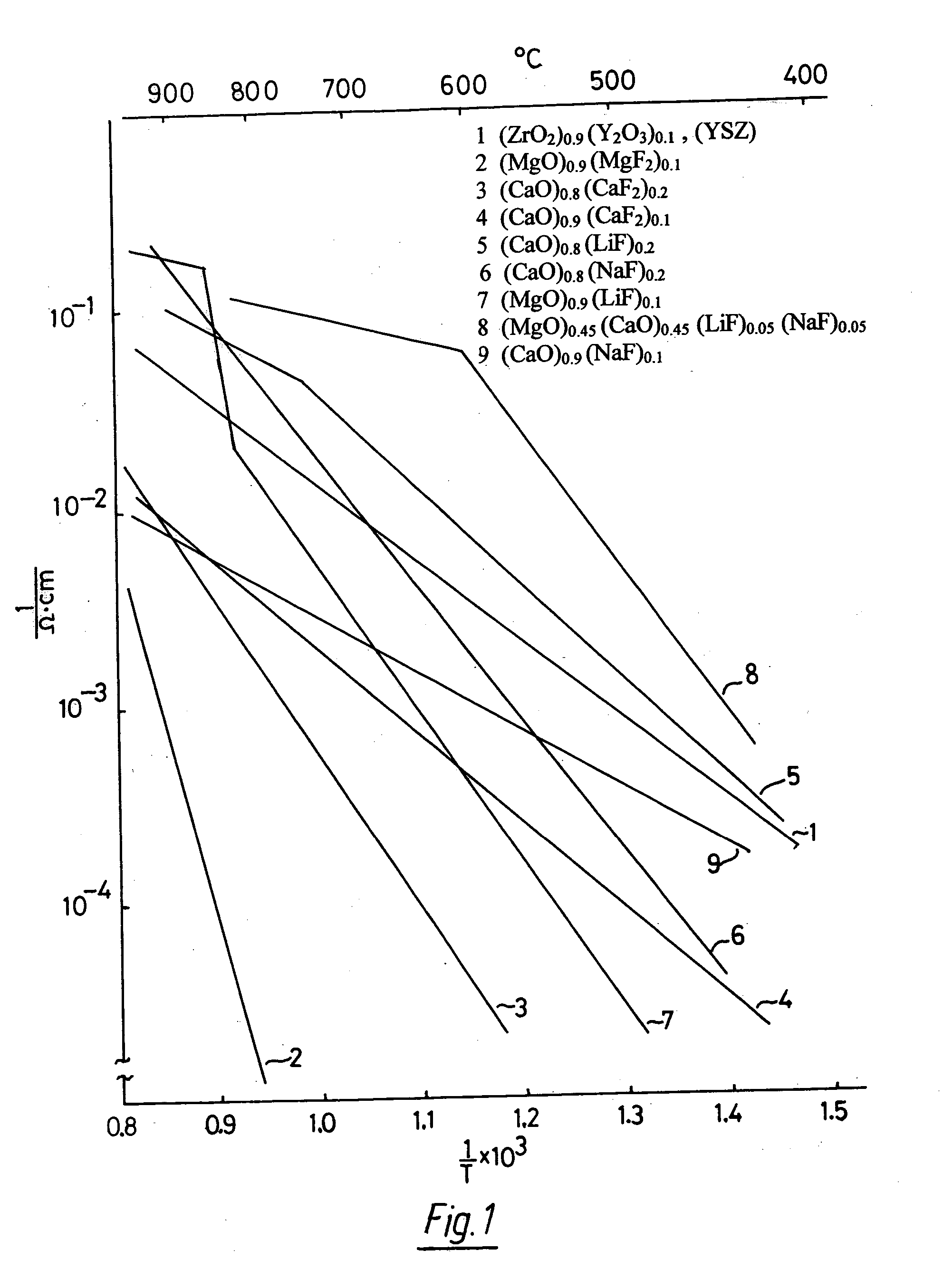
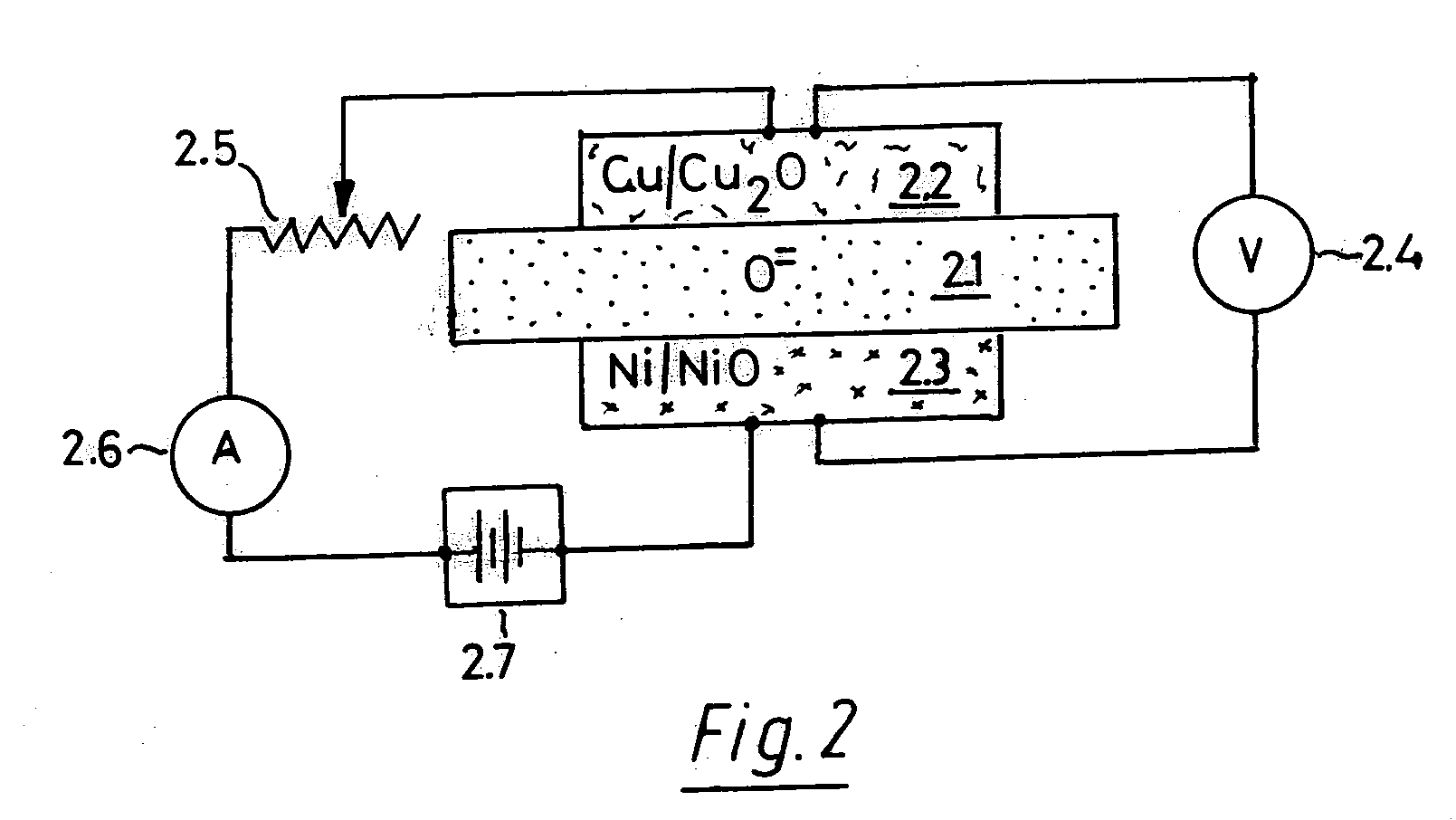
Oxygen ion conductors for electrochemical cells
InactiveUS20070054170A1Stable oxygen concentrationImprove electrode stabilityCell electrodesFinal product manufactureElectrical conductorAlkaline earth metal
In solid oxygen ion conducting electrolytes for electrochemical cells based on magnesium oxide and calcium oxide, obtained by the addition of metal fluorides selected from elements in the groups of alkali metals and earth alkali metals to the host oxides of magnesium and calcium, conductivity values are obtained, which are comparable with those of stabilized zirconia, but the magnesium oxide and calcium oxide based oxygen ion conducting electrolytes have a superior thermodynamic stability and, therefore, can operate at much lower oxygen concentrations in comparison with other oxygen ion conducting electrolytes and without becoming noticeably electronically conductive.
Owner:ISENBERG ARNOLD O
Method and means for capture and long-term sequestration of carbon dioxide
InactiveUS20090081096A1High heat of reactionHigh regeneration energyCombination devicesGas treatmentSolubilityAmbient pressure
The invention teaches a practical method of recovering CO2 from a mixture of gases, and sequestering the captured CO2 from the atmosphere for geologic time as calcium carbonate and provides a CO2 scrubber for carbon capture and sequestration. CO2 from the production of calcium oxide is geologically sequestered. A calcium hydroxide solution is produced from the environmentally responsibly-produced calcium oxide. The CO2 scrubber incorporates an aqueous froth to maximize liquid-to-gas surface area and time-of-contact between gaseous CO2 and the calcium hydroxide solution. The CO2 scrubber decreases the temperature of the liquid and the mixed gases, increases ambient pressure on the bubbles and vapor pressure inside the bubbles, diffuses the gas through intercellular walls from relative smaller bubbles with relative high vapor pressure into relative larger bubbles with relative low vapor pressure, and decreases the mean-free-paths of the CO2 molecules inside the bubbles, in order to increase solubility of CO2 and the rate of dissolution of gaseous CO2 from a mixture of gases into the calcium hydroxide solution.The CO2 scrubber recovers gaseous CO2 directly from the atmosphere, from post-combustion flue gas, or from industrial processes that release CO2 as a result of process. CO2 reacts with calcium ions and hydroxide ions in solution forming insoluble calcium carbonate precipitates. The calcium carbonate precipitates are separated from solution, and sold to recover at least a portion of the cost of CCS.
Owner:WESTEC ENVIRONMENTAL SOLUTIONS
Thermal protective coating for ceramic surfaces
ActiveUS6921431B2Extended shelf lifeConvenience to workFireproof paintsOther chemical processesColloidal silicaSodium Bentonite
A coating admixture, method of coating and substrates coated thereby, wherein the coating contains colloidal silica, colloidal alumina, or combinations thereof; a filler such as silicon dioxide, aluminum oxide, titanium dioxide, magnesium oxide, calcium oxide and boron oxide; and one or more emissivity agents such as silicon hexaboride, carbon tetraboride, silicon tetraboride, silicon carbide, molybdenum disilicide, tungsten disilicide, zirconium diboride, cupric chromite, or metallic oxides such as iron oxides, magnesium oxides, manganese oxides, chromium oxides, copper chromium oxides, cerium oxides, terbium oxides, and derivatives thereof. In a coating solution, an admixture of the coating contains water. A stabilizer such as bentonite, kaolin, magnesium alumina silicon clay, tabular alumina and stabilized zirconium oxide is also added.
Owner:WESSEX
Separation of carbon dioxide (CO2) from gas mixtures by calcium based reaction separation (CaRS-CO2) process
A reaction-based process has been developed for the selective removal of carbon dioxide (CO2) from a multicomponent gas mixture to provide a gaseous stream depleted in CO2 compared to the inlet CO2 concentration in the stream. The proposed process effects the separation of CO2 from a mixture of gases (such as flue gas / fuel gas) by its reaction with metal oxides (such as calcium oxide). The Calcium based Reaction Separation for CO2 (CaRS-CO2) process consists of contacting a CO2 laden gas with calcium oxide (CaO) in a reactor such that CaO captures the CO2 by the formation of calcium carbonate (CaCO3). Once “spent”, CaCO3 is regenerated by its calcination leading to the formation of fresh CaO sorbent and the evolution of a concentrated stream of CO2. The “regenerated” CaO is then recycled for the further capture of more CO2. This carbonation-calcination cycle forms the basis of the CaRS-CO2 process. This process also identifies the application of a mesoporous CaCO3 structure, developed by a process detailed elsewhere, that attains >90% conversion over multiple carbonation and calcination cycles. Lastly, thermal regeneration (calcination) under vacuum provided a better sorbent structure that maintained reproducible reactivity levels over multiple cycles.
Owner:THE OHIO STATES UNIV
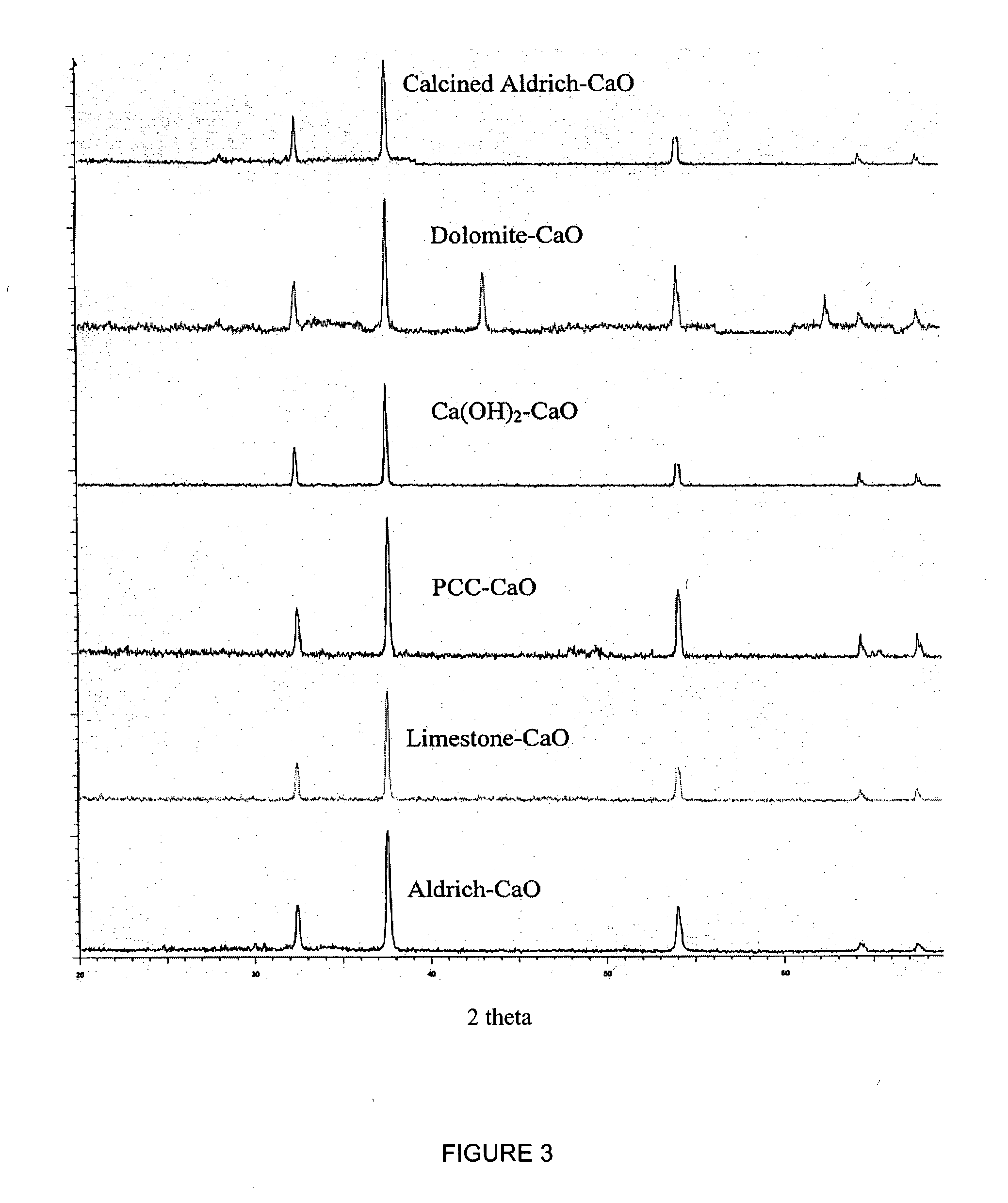
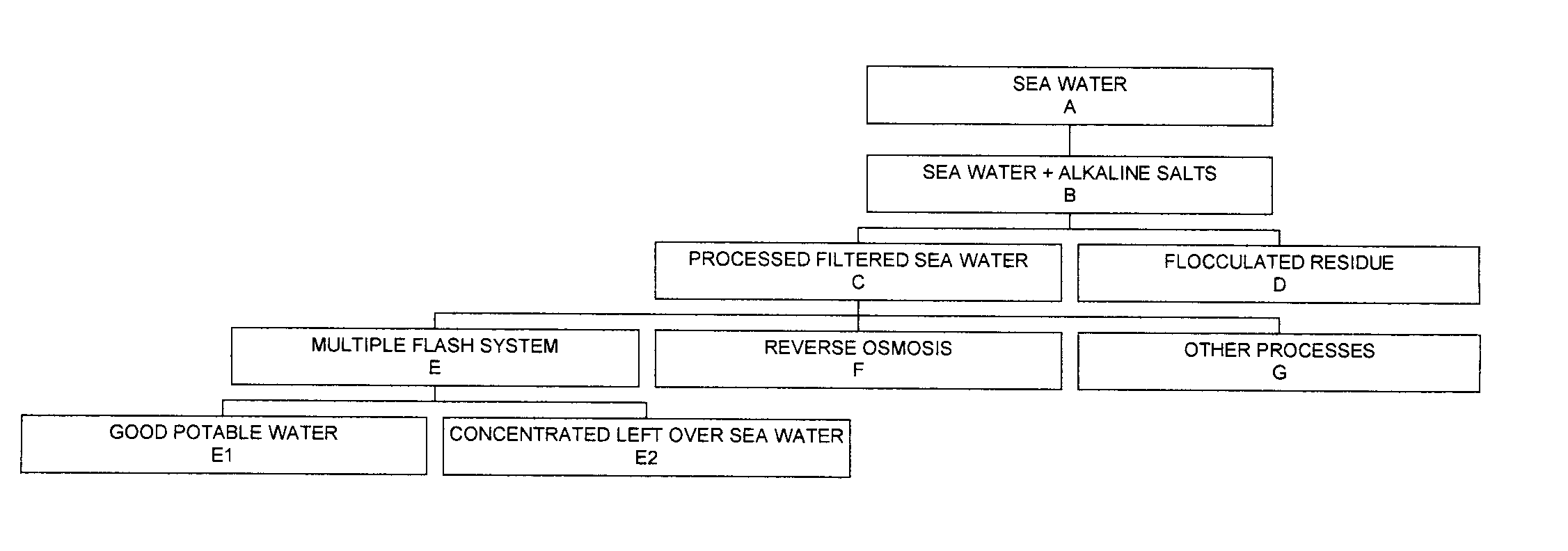
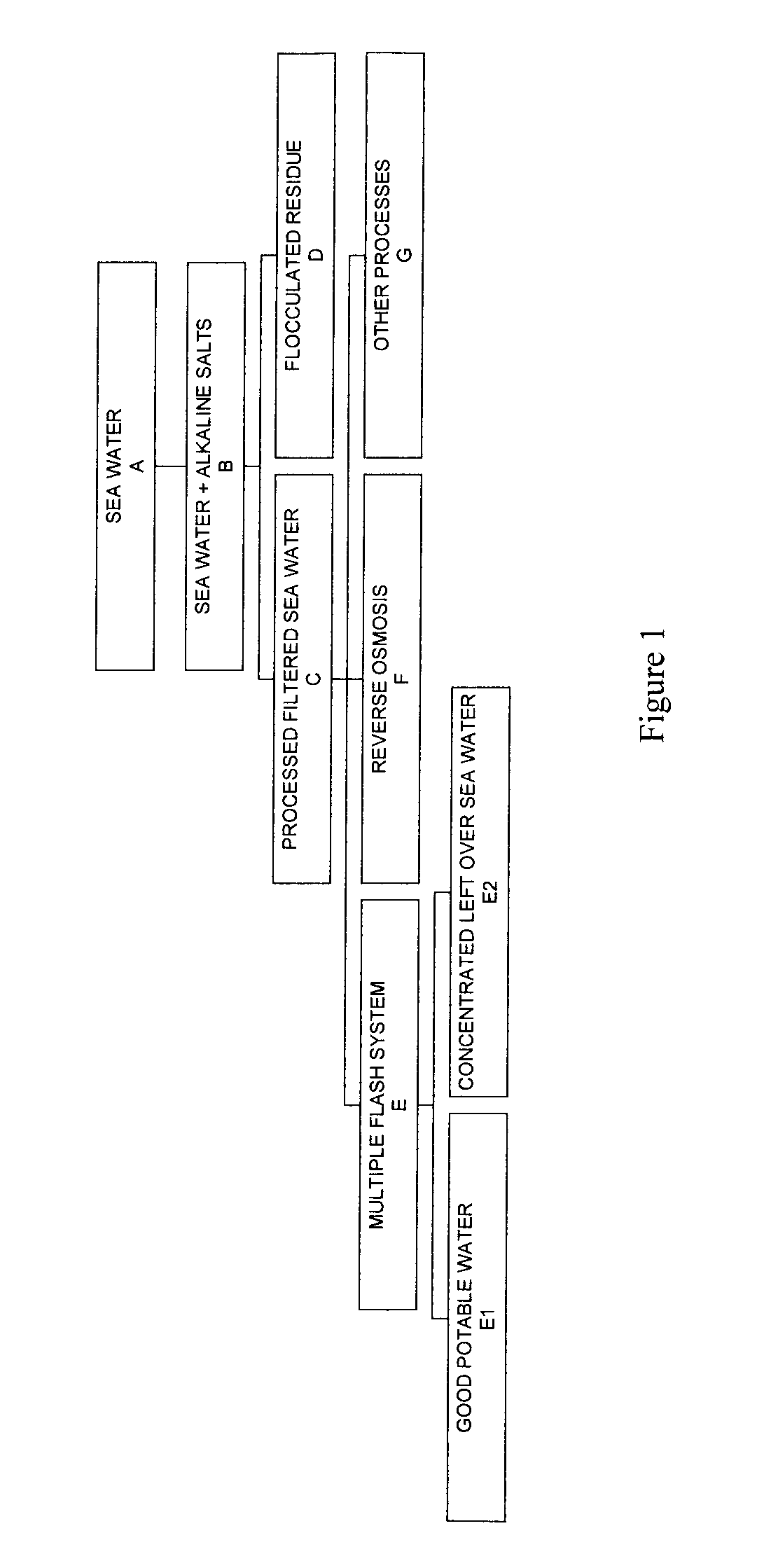
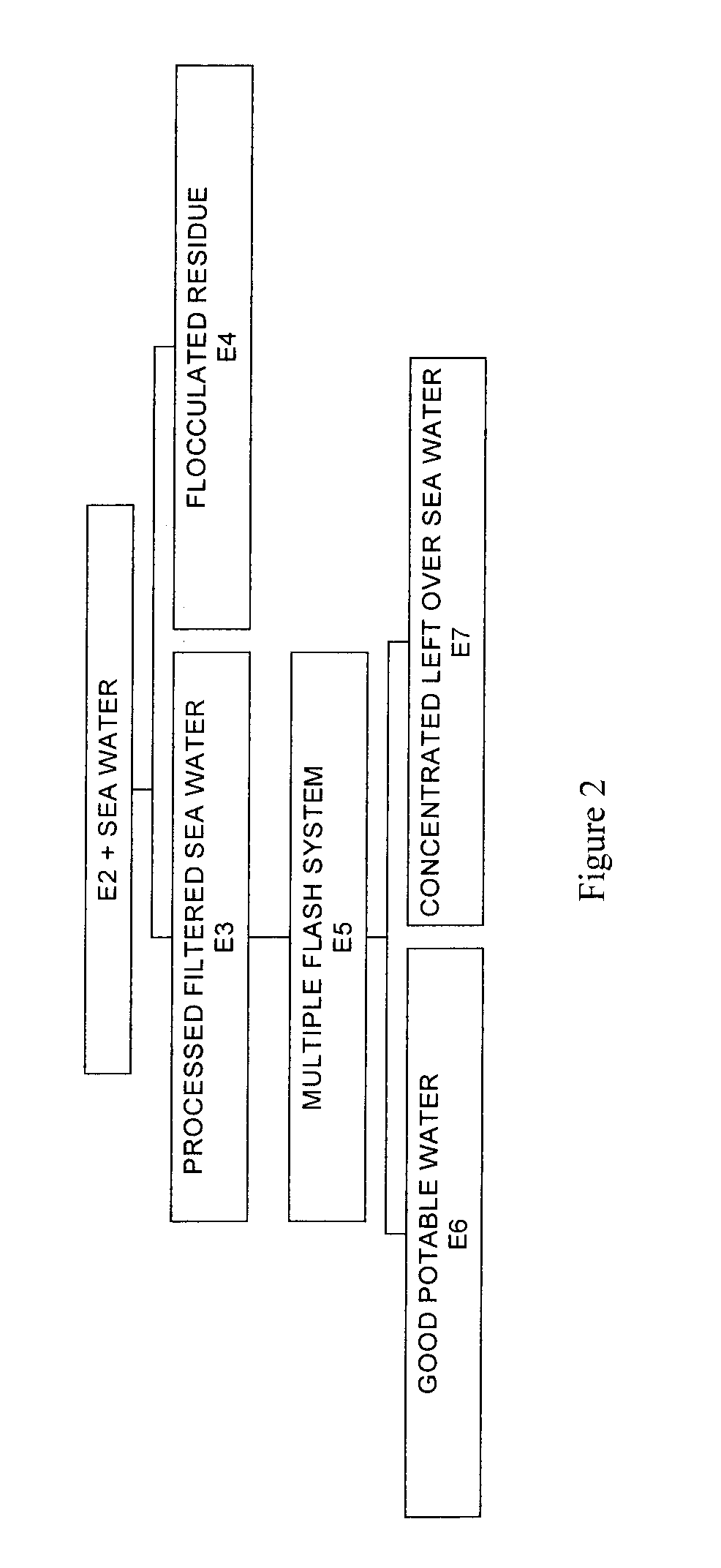
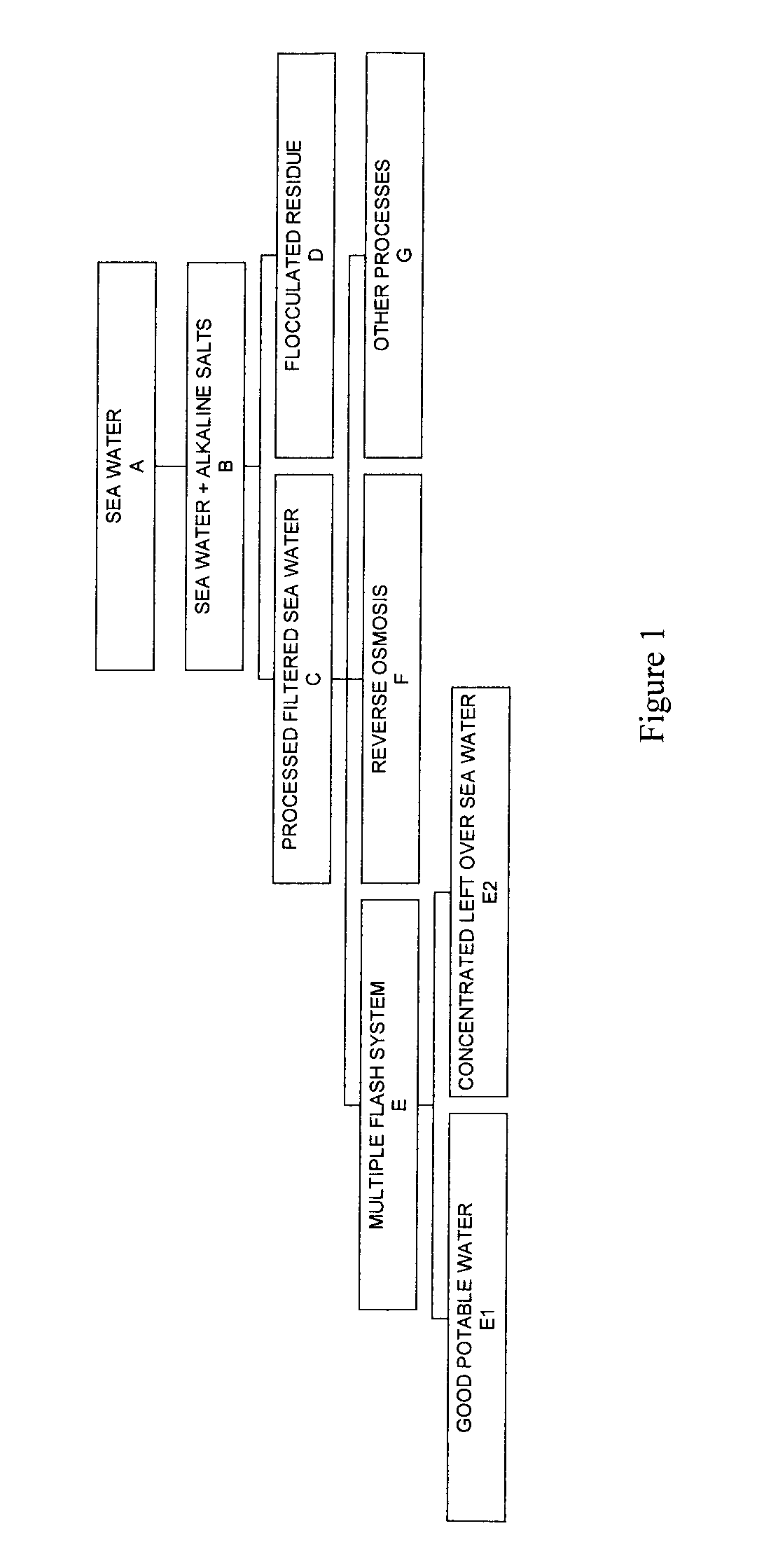
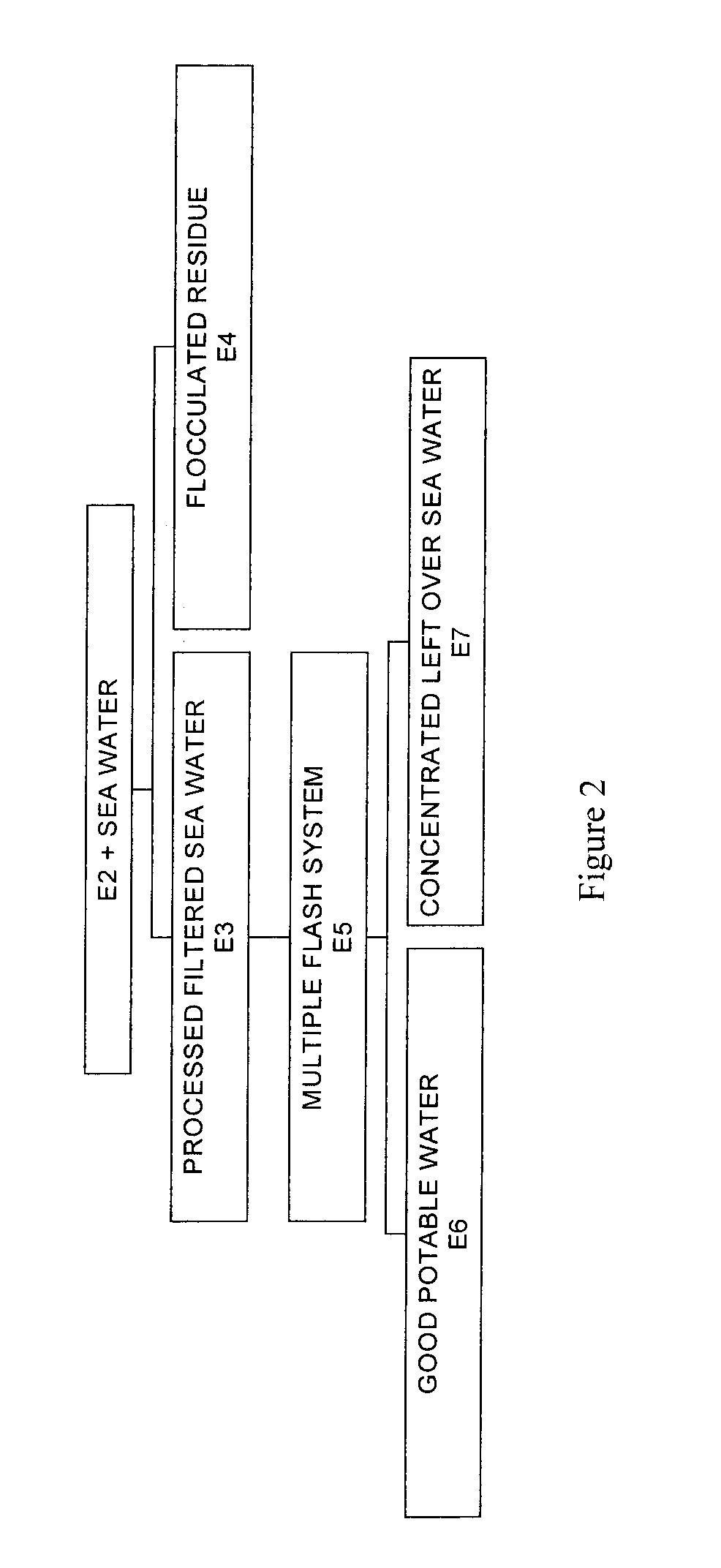
Process for pre-treating and desalinating sea water
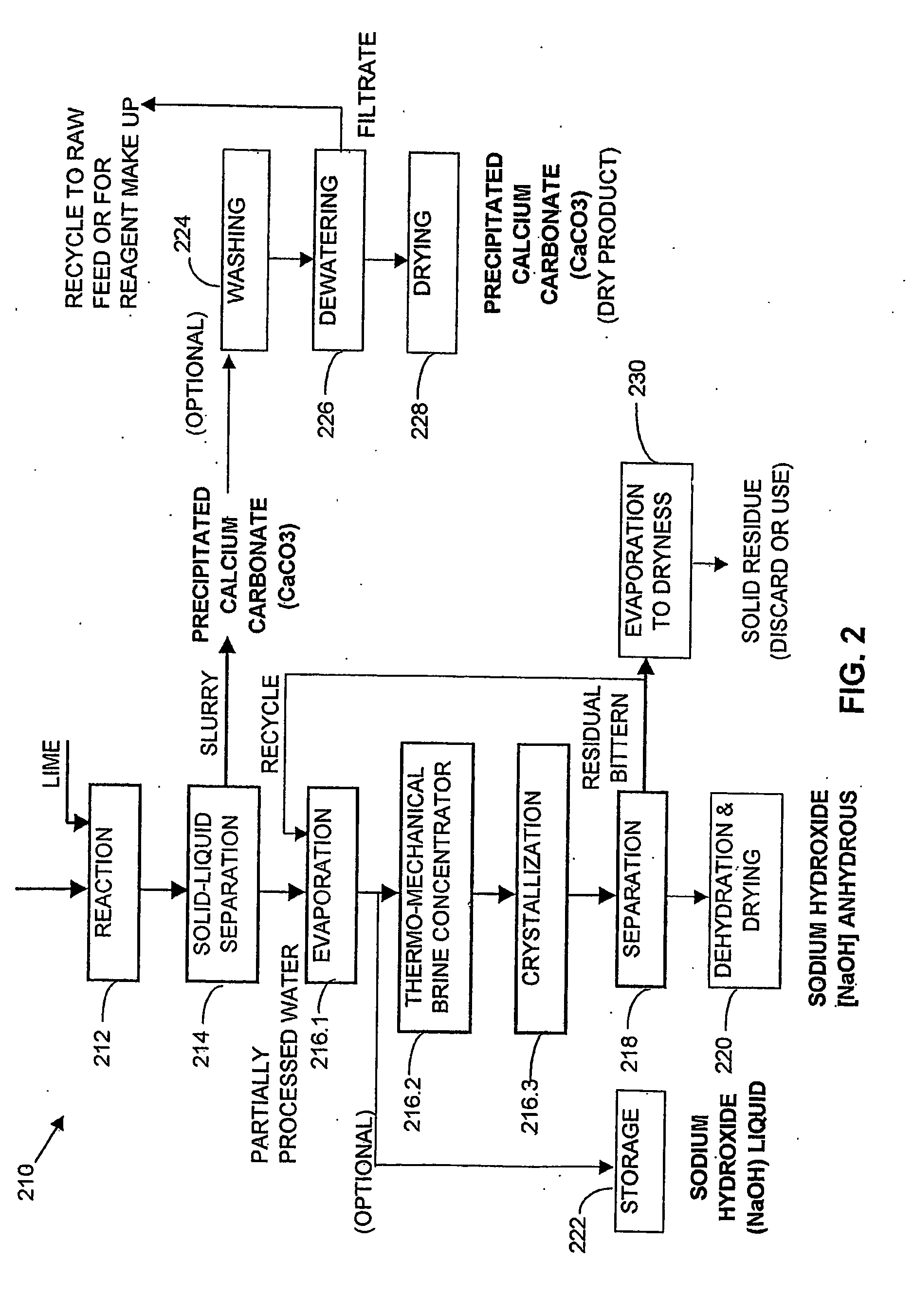
ActiveUS20050098499A1Reduce maintenanceExtend equipment lifeGeneral water supply conservationSeawater treatmentCalcium bicarbonatePotassium hydroxide
Water containing dissolved salts, such as calcium sulfate, calcium chloride, magnesium sulfate, magnesium chloride, sodium carbonate, sodium chloride, sodium sulfate, calcium bicarbonate, and mixtures thereof, is treated to reduce the concentration of those salts. About 0.1 to about 60 g / L of sodium hydroxide, sodium carbonate, potassium hydroxide, potassium carbonate, calcium hydroxide, calcium carbonate, aluminum hydroxide, aluminum sulfate, aluminum potassium sulfate, and mixtures thereof is added to the water, whereby a precipitate forms in the water. The precipitate is separated from said water and the water is desalinated using reverse osmosis, flash evaporation, or another method. The process is preferably performed by first adding calcium oxide or calcium hydroxide, separating the precipitate that forms, then adding sodium hydroxide and sodium carbonate to form a second precipitate.
Owner:HUSSAIN MOHAMMED AZAM
Process for the treatment of saline water
InactiveUS7595001B2Waste water treatment from quariesGeneral water supply conservationTotal dissolved solidsEvaporation
A process and an apparatus are described for treating seven types of saline waters each having a concentration of total dissolved solids exceeding 1 g / L, wherein the concentration of total dissolved solids, the ratio of the chloride ion concentration to the bicarbonate ion concentration and the ratio of the chloride ion concentration to the sulphate ion concentration of each of the water types are as indicated in Table 1. The process includes the steps of contacting the water with a first reagent comprising a source of calcium ions selected from calcium oxide and calcium hydroxide to form a first solid product which is recovered. The process includes a further step of subjecting at least a portion of the partially processed water to at least partial evaporation so as to promote the formation of a precipitate and a mother liquor. The precipitate is recovered as a second product.
Owner:GEO PROCESSORS
High temperature glass fiber insulation
Owner:GLASS
Process for pre-treating and desalinating sea water
ActiveUS7198722B2Extend equipment lifeReduce maintenanceGeneral water supply conservationSeawater treatmentCalcium bicarbonateReverse osmosis
Owner:HUSSAIN MOHAMMED AZAM
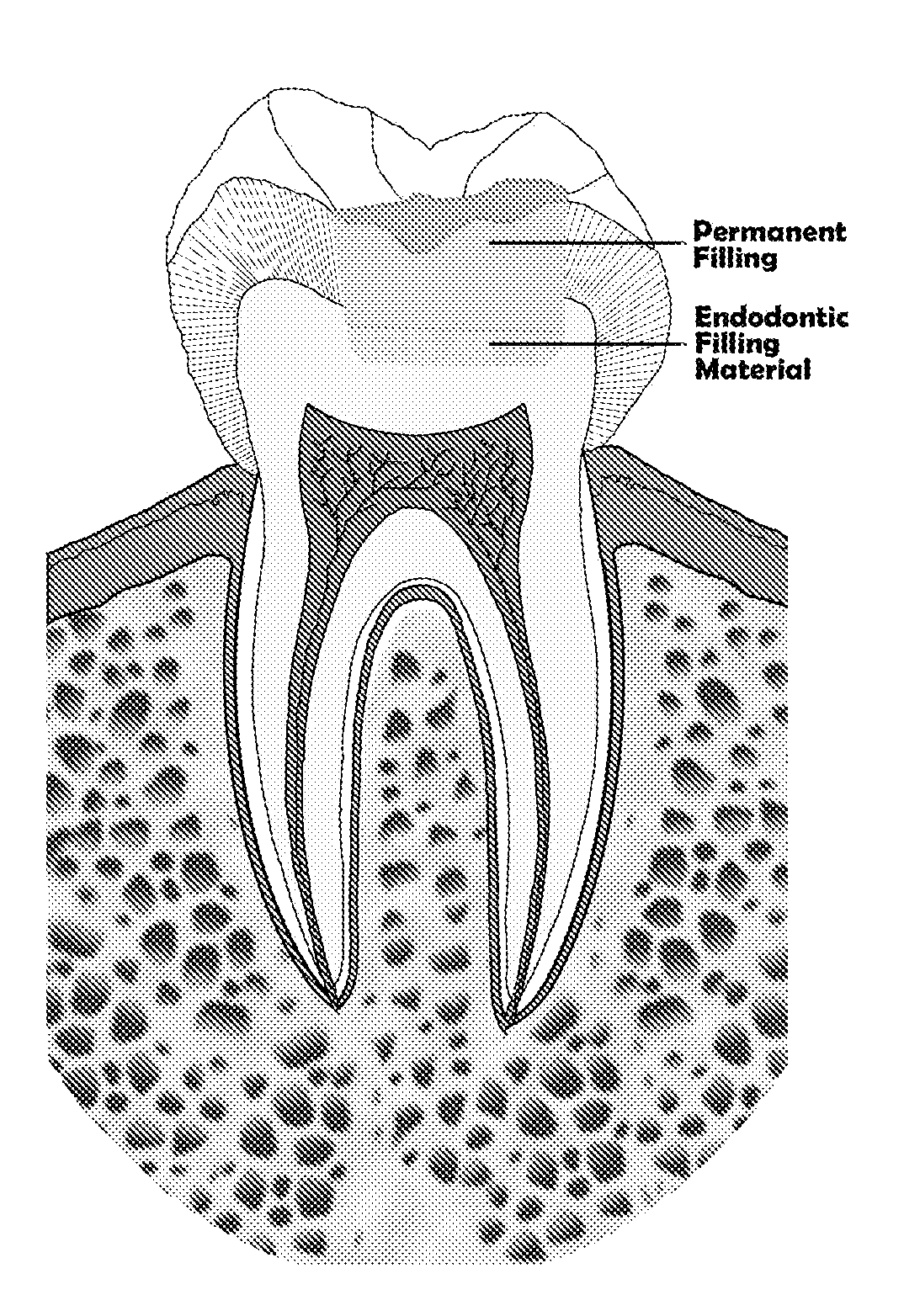
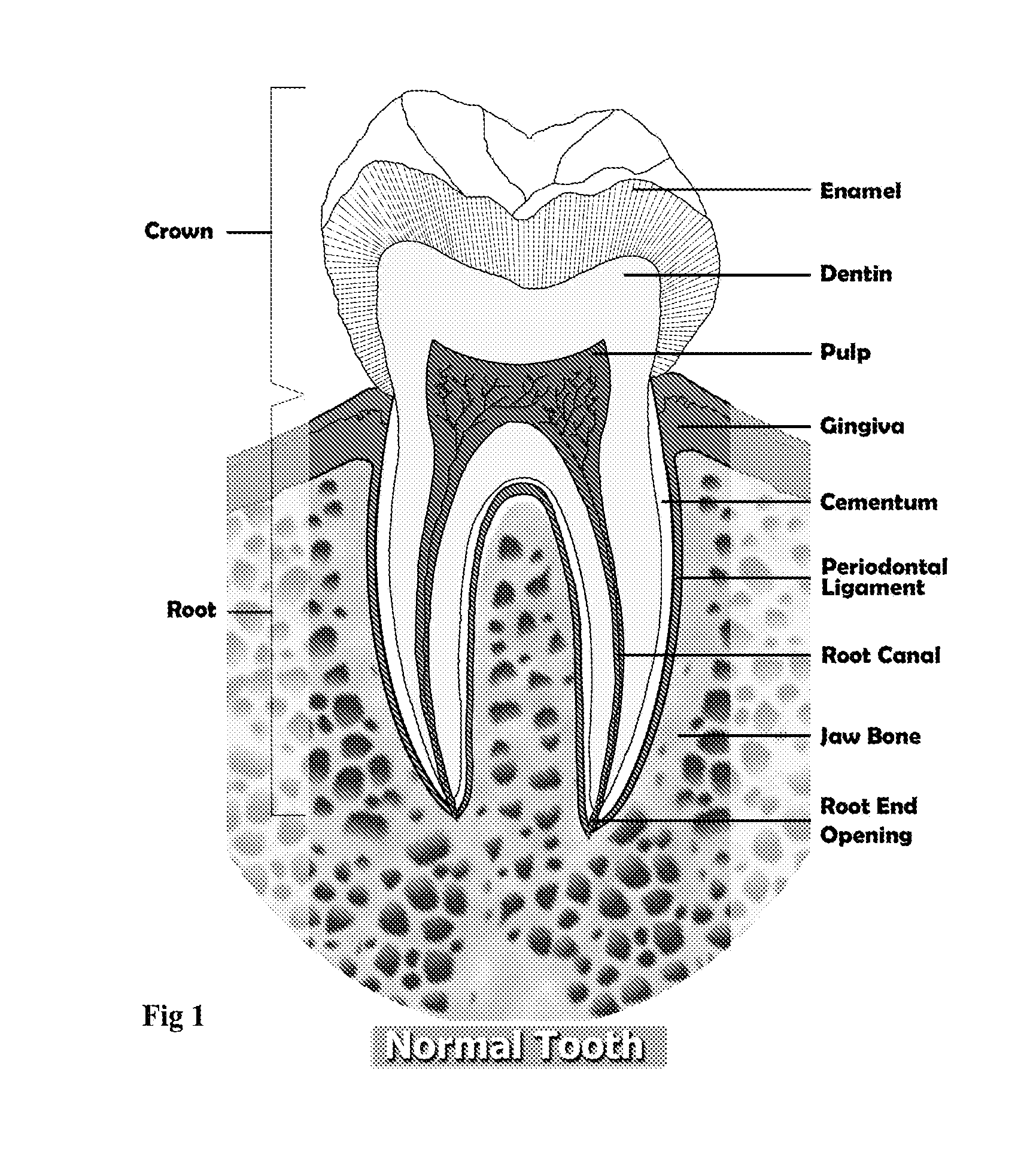
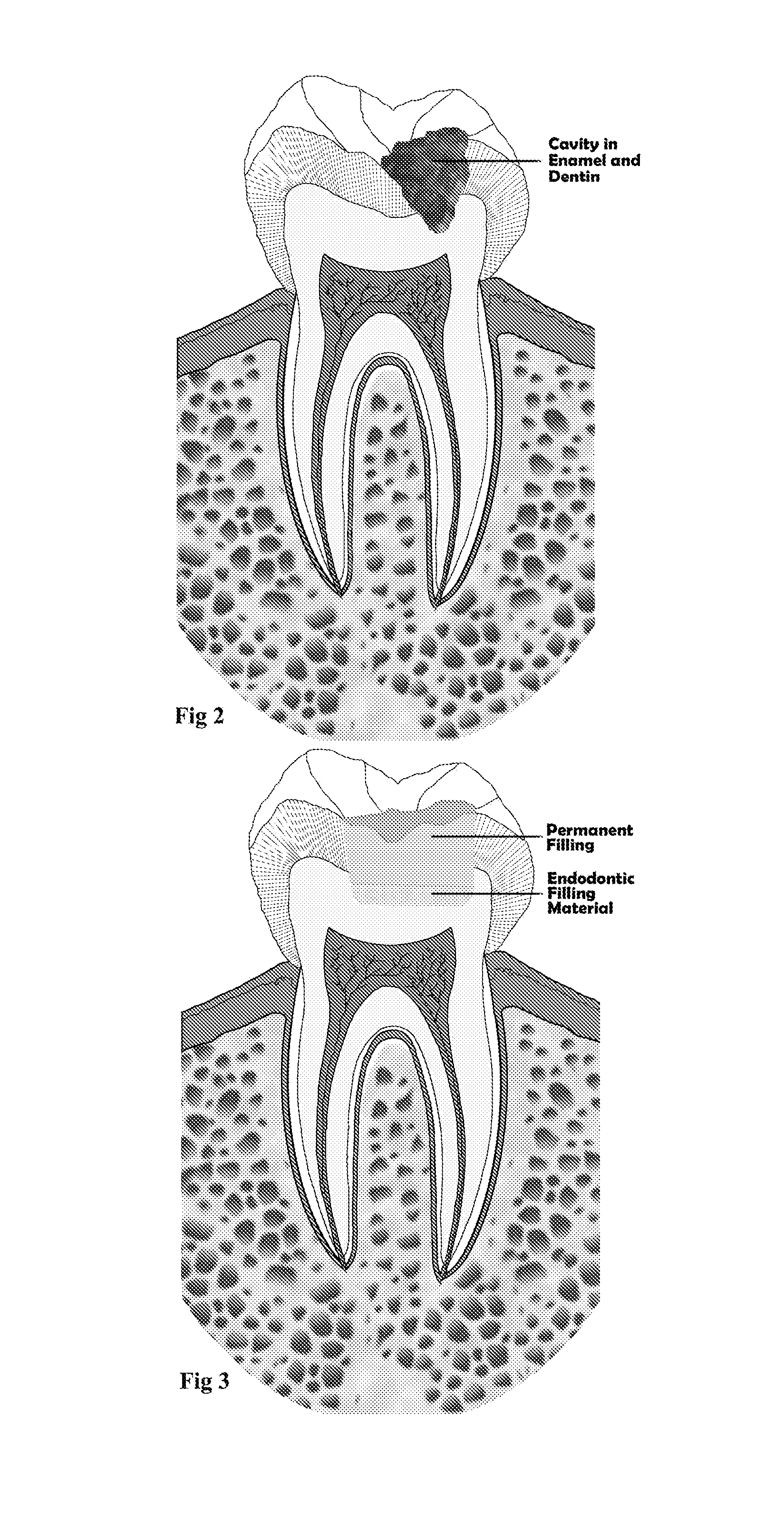
Endodontic filling material
InactiveUS20080206716A1Improve sealingEasily manipulationAntibacterial agentsOther chemical processesCalcium silicateHigh concentration
The present invention is a bioactive endodontic material and its use for filling the tooth and bone cavities. The present invention, by using calcium salt, calcium oxide, calcium silicate, and calcium phosphate compounds as essential constituents, and mixing them with a water base solution, prepares a bioactive calcium and phosphate enriched material. The enriched material (cement) comprises high concentration of water-soluble calcium and phosphate, and immediately forms hydroxyapatite during and after setting. The cement is biocompatible, antibacterial, capable to form an effective seal against reentrance of microorganisms into the filled cavity, compatible to handle and set in an aqueous environment, and able to stimulate hard tissue healing.
Owner:ASGARY SAEED
Environment-friendly type sludge firming agent
InactiveCN101381194AImprove curing abilityImprove curing effectSludge treatment by de-watering/drying/thickeningRoad engineeringSlag
The invention provides an environment-friendly silt curing agent, which is manufactured through the following steps: one or two among fly ash, calcium sulfate, sodium sulfate, sodium carbonate and potassium carbonate, one or two among slag, slag combination, potassium hydroxide, calcium oxide, sodium silicate or silicon dioxide, one or two among carbide slag, lime or gypsum, as well as one or two among triethanol amine surfactant, calcium lignosulfonate or sodium lignosulfonate form a plurality of optimal compound formulations according to respective attributes, are optimized, compounded, ground till the Brinell specific surface areas are between 300 and 900 m2 / kg respectively and then mixed, wherein particle sizes are between 0.00040 and 0.5 mm. As a large amount of waste is utilized, the curing agent saves raw materials, solves the problems about waste discharge and environmental pollution, controls waste through waste, and has important significance to environmental protection. The invention aims to provide the environment-friendly silt curing agent which has strong adaptability to a plurality of types of silt and soil, is good in curing effect, good in durability after curing and capable of utilizing industrial waste, and can be widely applied to fill engineering, embanking or embankment reinforcement engineering, road engineering and other fields.
Owner:天津渤海环保工程有限公司 +1
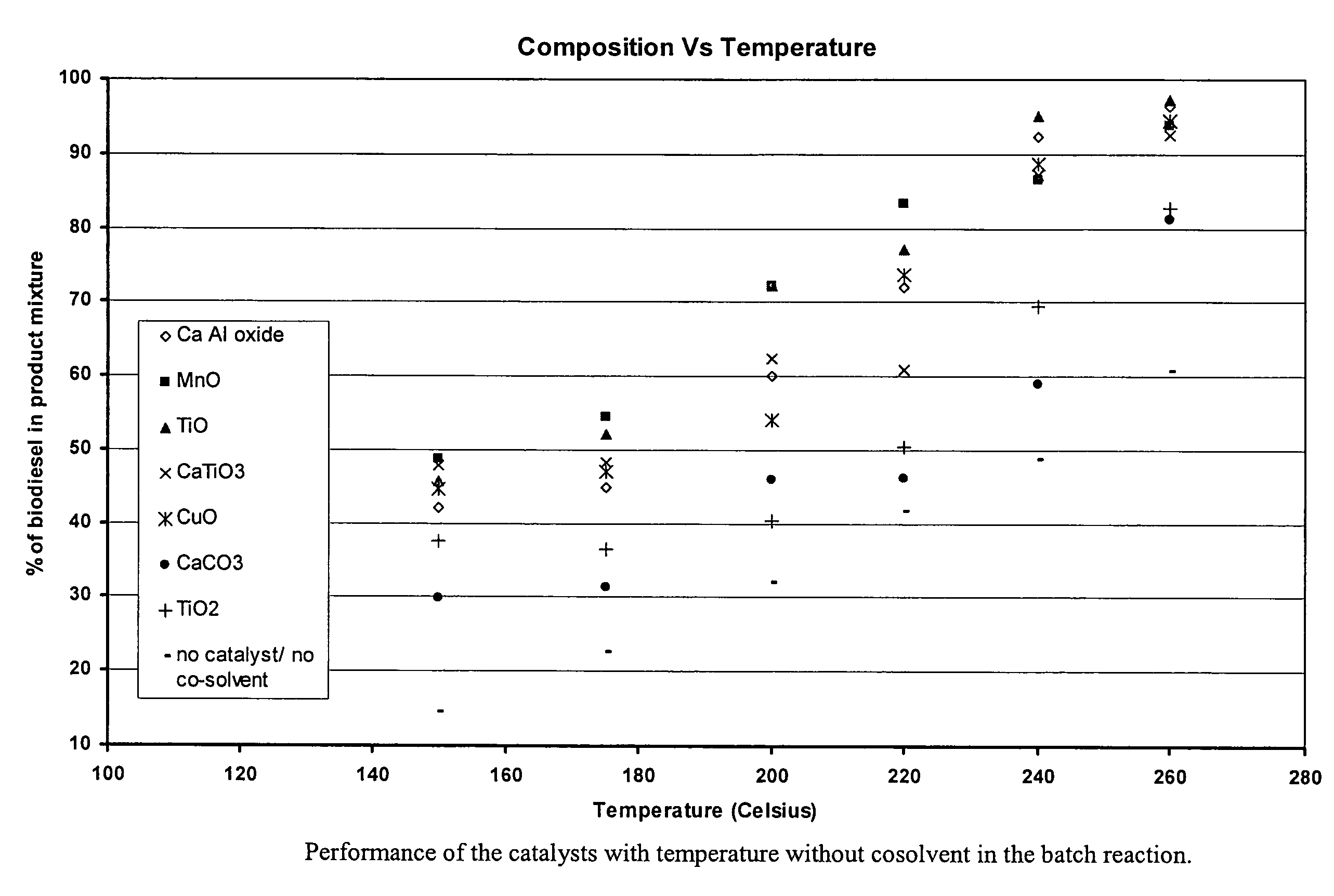
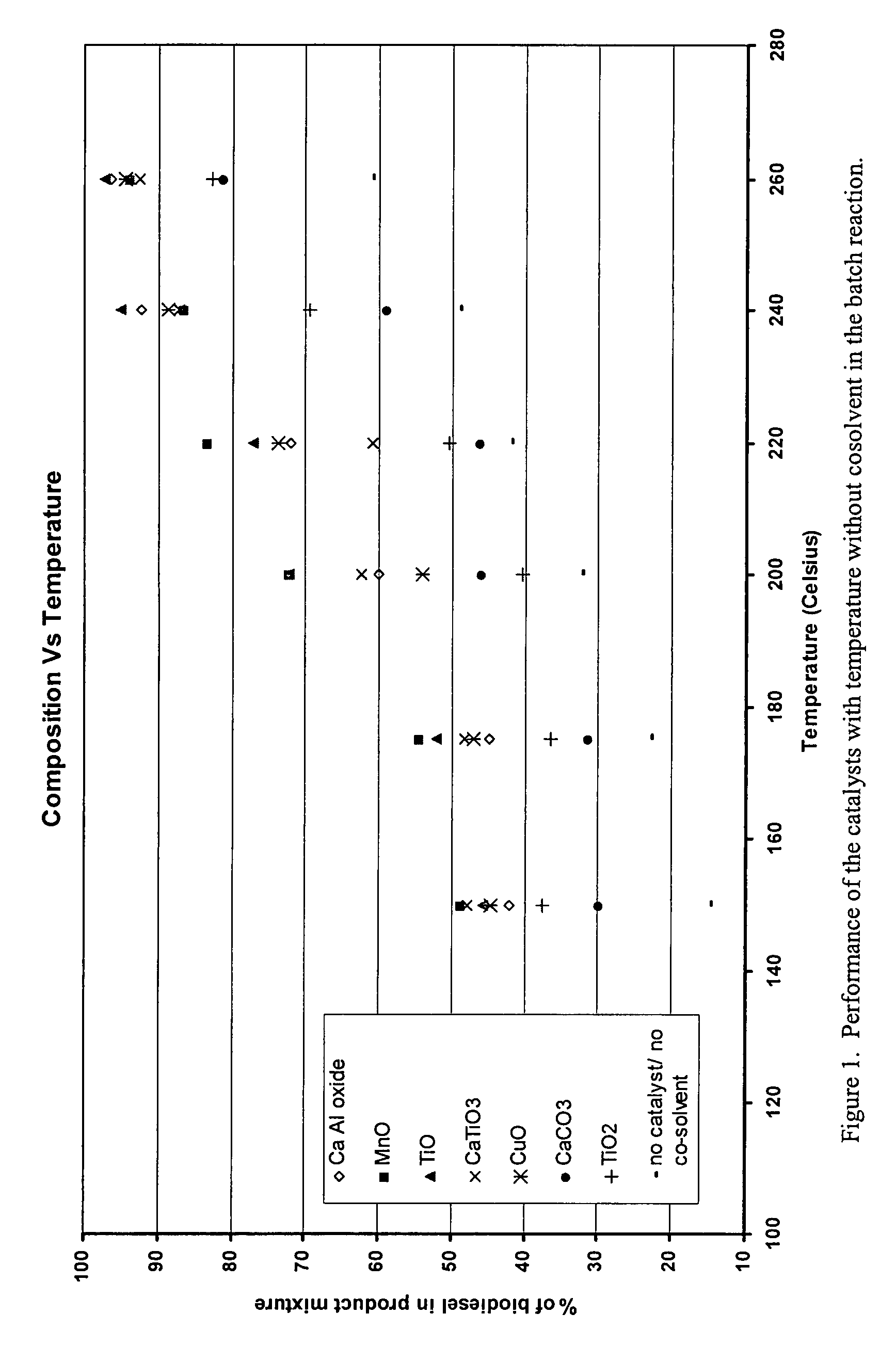
Green biodiesel
InactiveUS7563915B2Reduce wasteSignificant energyFatty oils/acids recovery from wasteFatty acid esterificationCalcium silicateBiodiesel
Methods for improved manufacture of green biodiesel focus on the selection and use of one or more solid metallic oxide base catalyst(s) selected from the group consisting of calcium oxide (CaO), calcium aluminum oxide (CaO—Al2O3), calcium titanate (CaTiO3), barium titanate (BaTiO3), magnesium aluminum oxide (MgO—Al2O3), zinc oxide (ZnO), copper (II) oxide (CuO), nickel oxide (NiO), manganese oxide (MnO), titanium oxide (TiO), vanadium oxide (VO), cobalt oxide (CoO), iron oxide (FeO), chromite (FeCr2O4), hydrotalcite (Mg6Al2(CO3)(OH)16.4(H2O), magnetite (Fe3O4), magnesium silicate and calcium silicate.
Owner:PENN STATE RES FOUND
Thermal protective coating for ceramic surfaces
ActiveUS20050051057A1Extend working lifeReduce surface temperatureFireproof paintsOther chemical processesColloidal silicaSodium Bentonite
A coating admixture, method of coating and substrates coated thereby, wherein the coating contains colloidal silica, colloidal alumina, or combinations thereof; a filler such as silicon dioxide, aluminum oxide, titanium dioxide, magnesium oxide, calcium oxide and boron oxide; and one or more emissivity agents such as silicon hexaboride, carbon tetraboride, silicon tetraboride, silicon carbide, molybdenum disilicide, tungsten disilicide, zirconium diboride, cupric chromite, or metallic oxides such as iron oxides, magnesium oxides, manganese oxides, chromium oxides, copper chromium oxides, cerium oxides, terbium oxides, and derivatives thereof. In a coating solution, an admixture of the coating contains water. A stabilizer such as bentonite, kaolin, magnesium alumina silicon clay, tabular alumina and stabilized zirconium oxide is also added.
Owner:WESSEX
Silicone rubber compositions for producing cables or profiles with retention of function in the event of fire
InactiveUS6387518B1Improve the level ofPropertyFireproof paintsAntifouling/underwater paintsZinc boratePlatinum complex
A composition which is useful for producing profiles and cable insulation which retain their function in the event of fire, comprise peroxidically crosslinking or condensation-crosslinking silicone rubber, metal oxides selected from magnesium oxide, aluminum oxide, tin oxide, calcium oxide and barium oxide and metal compounds of this class which produce oxides on heating, boric acid, zinc borate, and a platinum complex having at least one unsaturated group.
Owner:WACKER CHEM GMBH
Preparation method of polyurea-composite calcium lubricating grease
InactiveCN1493673APrevent volatilizationImprove high temperature performanceAdditivesCalcium hydroxideOrganic acid
A lubricating polyureas-composite calcium grease is prepared through proportionally mixing basic oil, calcium hydroxide (oxide), water and C1-C20 organic acid together, heating to 30-100 deg.C, adding diisocyanate and organic amine, draining water, saponifying, heating to 210-230 deg.C, cooling and homogenizing. Its advantages are high refractory performance, stability and extreme-pressure antiwear nature.
Owner:CHINA PETROLEUM & CHEM CORP +1
Thermal protective coating
ActiveUS7105047B2Extended shelf lifeReduce weightAlkali metal silicate coatingsPretreated surfacesCalcium silicateSodium Bentonite
A coating, method of coating and substrates coated thereby, wherein the coating contains an inorganic adhesive such as an alkali / alkaline earth metal silicate such as sodium silicate, potassium silicate, calcium silicate, and magnesium silicate; a filler such as a metal oxide for example silicon dioxide, aluminum oxide, titanium dioxide, magnesium oxide, calcium oxide and boron oxide; and one or more emissivity agents such as silicon hexaboride, carbon tetraboride, silicon tetraboride, silicon carbide, molybdenum disilicide, tungsten disilicide, zirconium diboride, cupric chromite, or metallic oxides such as iron oxides, magnesium oxides, manganese oxides, chromium oxides and copper chromium oxides, and derivatives thereof. In a coating solution, an admixture of the coating contains water. A stabilizer such as bentonite, kaolin, magnesium alumina silicon clay, tabular alumina and stabilized zirconium oxide may be added.
Owner:WESSEX
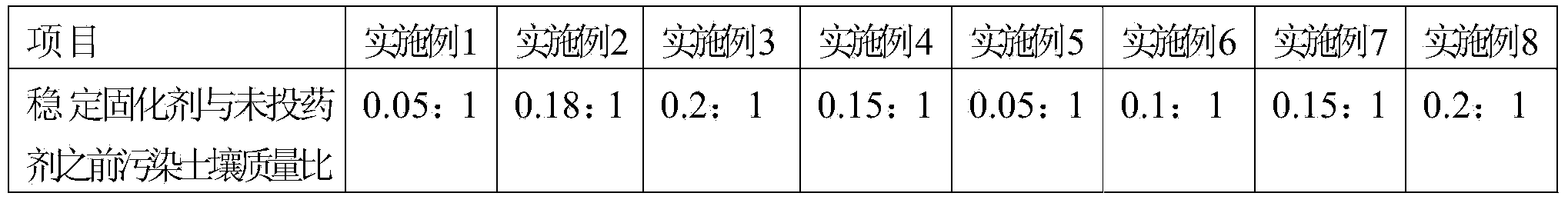
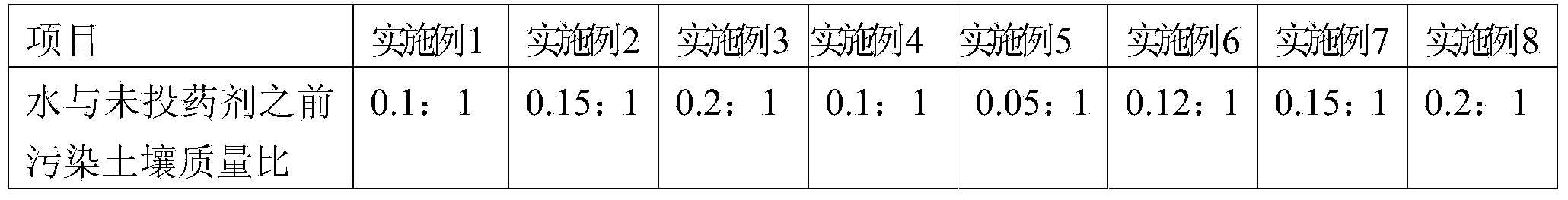
Stable curing agent of repairing heavy metal and toxic organic matter combined polluted soil and using method of curing agent
ActiveCN103881727AImprove repair effectBioeffectiveAgriculture tools and machinesContaminated soil reclamationPotassium persulfateCoal
The invention relates to a stable curing agent of repairing heavy metal and toxic organic matter combined polluted soil. The stable curing agent comprises the following raw materials in percentage by weight: 10-30% of cement, 10-30% of coal ash, 20-50% of clays, 2-10% of an activating agent and 2-20% of an oxidizing agent, wherein the activating agent is a composition of one or more than two of magnesium oxide, aluminum oxide, titanium dioxide or molybdenum trioxide, and the oxidizing agent is a composition of one or more than two of sodium persulfate, calcium peroxide, potassium persulfate and ammonium persulfate. The raw materials are put into a grinder to be uniformly grinded and mixed to prepare the stable curing agent with the specific surface area of 400-800 / kg. The stable curing agent provided by the invention has the characteristics of low cost, good soil repairing effect and simplicity in construction.
Owner:WELLE ENVIRONMENTAL GRP CO LTD
Flue Gas Scrubbing
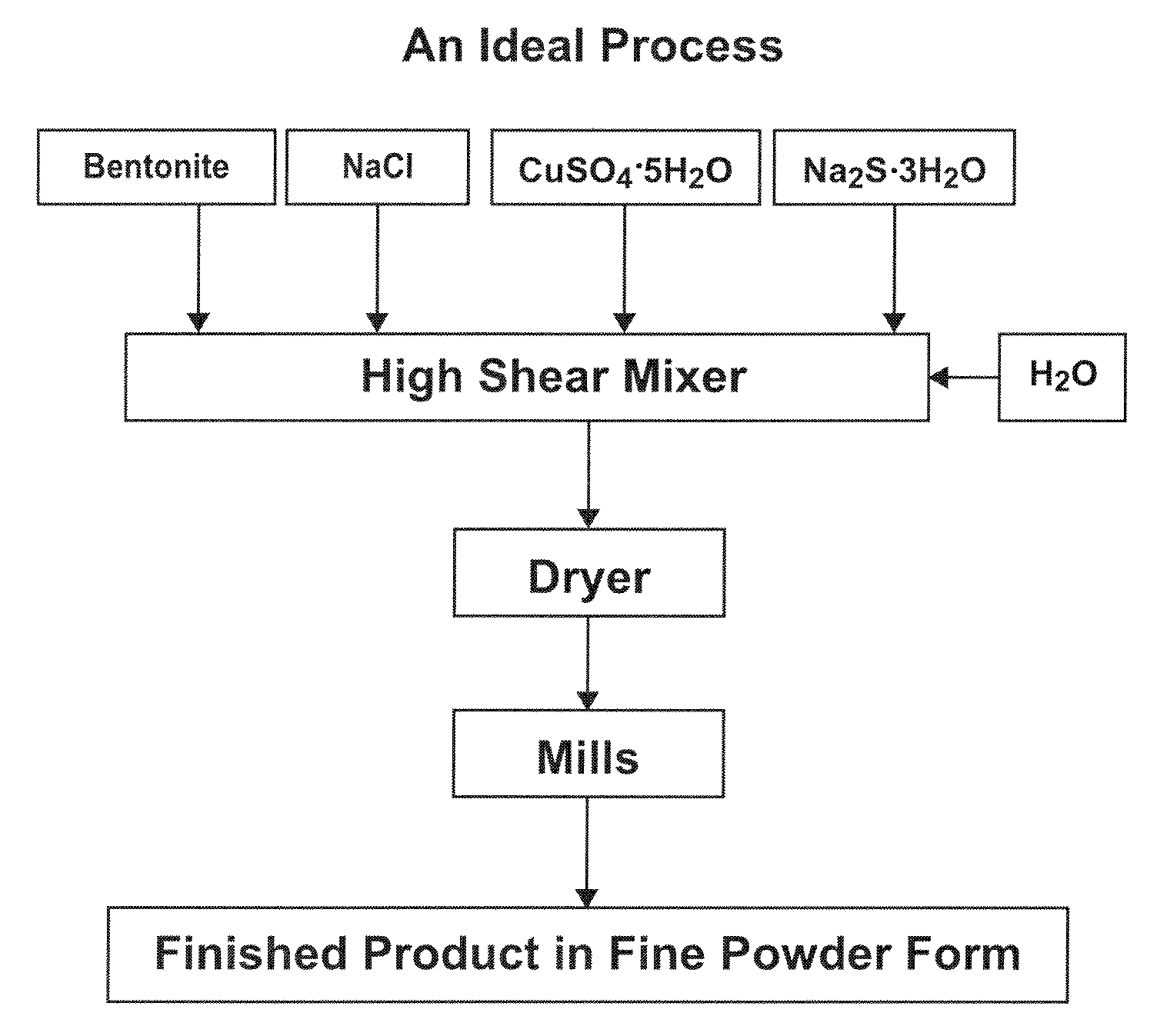
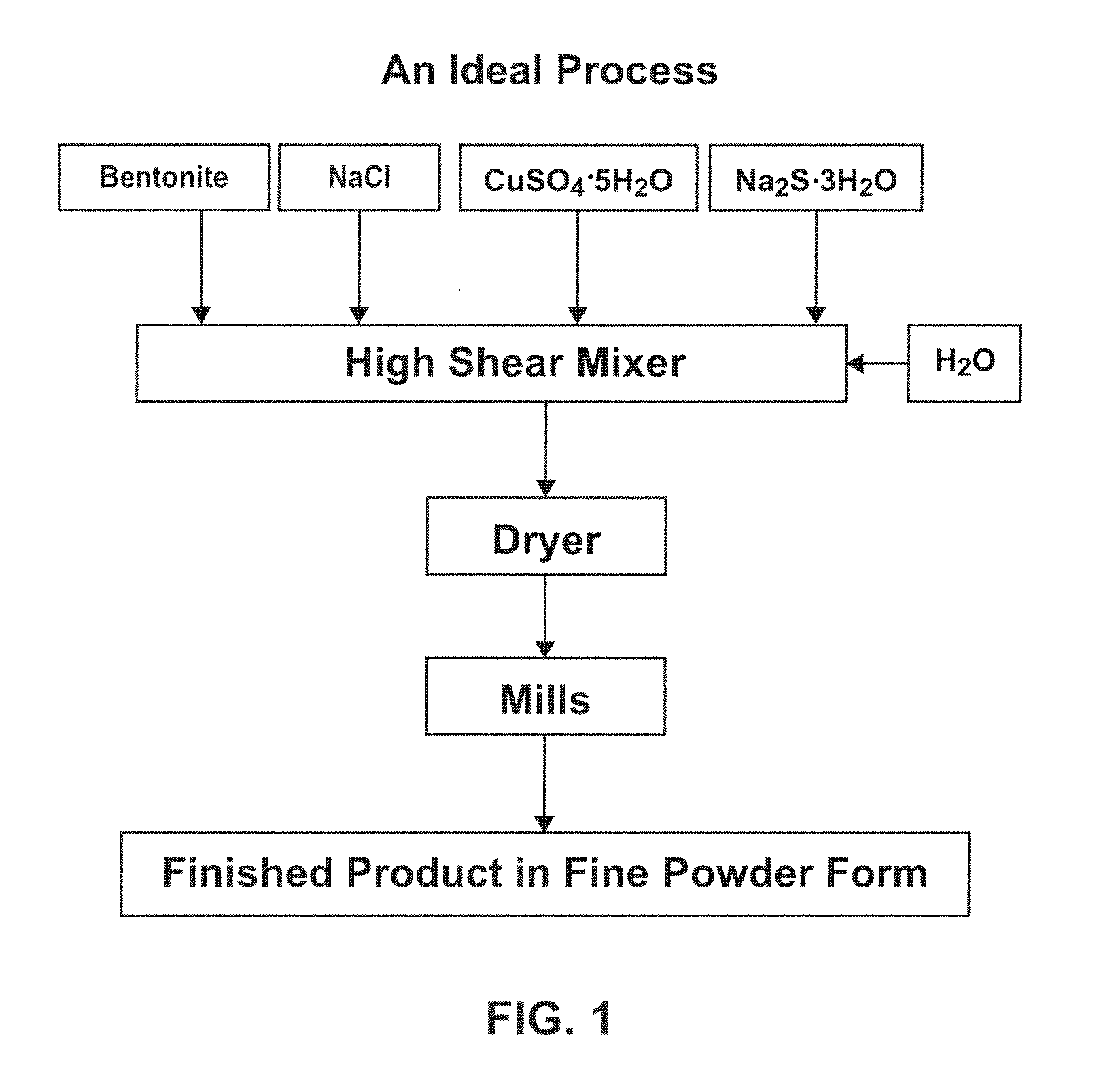
ActiveUS20110123422A1Reduce its mercury concentrationGas treatmentMolecular sieve catalystsCalcium hydroxideFlue gas
Herein is disclosed a flue gas scrubbing composition, a method of using the flue gas scrubbing composition, and a method of entombing mercury collected with the flue gas scrubbing composition. The flue gas scrubbing composition includes an admixture of a mercury sorbent material that comprises a clay, copper, and sulfur; and lime that comprises calcium oxide and / or calcium hydroxide. The method of collecting mercury from a flue gas includes injecting the flue gas scrubbing composition of any one of the preceding claims into a flue duct comprising the flue gas; reacting the mercury sorbent material with mercury in the flue gas to form a mercury-sorbed material and thereby reducing the concentration of mercury in the flue gas; reacting the lime with SO2, SO3, and / or HCl in the flue gas to form a calcium sulfate and / or a calcium chloride; and collecting a mixture that includes the mercury-sorbed material. The method of entombing mercury includes mixing the collected mixture of any one of claims 6 to 9 with water to form a freshly mixed concrete; and casting the freshly mixed concrete into a form.
Owner:AMCOL INTERNATIONAL CORPORATION
Calcium oxide-based ceramic core for casting titanium alloy and manufacturing method thereof
The invention discloses a calcium oxide-based ceramic core for casting titanium alloy and a manufacturing method of the calcium oxide-based ceramic core. The calcium oxide-based ceramic core comprises the following components by weight percent: 1.0-15.0% of zirconium dioxide, 0.5-10.0% of yttrium oxide, 0.05-0.1% of thorium oxide and the balance of calcium oxide, and the sum of the contents of the above the component is 100%. According to the ceramic core, the bending strength is 15-30Mpa at room temperature, the bending strength is 5-10Mpa at high temperature, the high-temperature deflection is 0.2-0.5%, the firing shrinkage ratio is 1-1.5%, and the porosity is 40-50%. Compared with the existing commercial alumina-based ceramic core and silicon oxide-based ceramic core, the calcium oxide-based ceramic core of the invention greatly reduces the reactivity with molten titanium, and has the advantages of high temperature resistance, easiness in core leach, low in cost and the like.
Owner:BEIHANG UNIV
Supported noble metal catalyst for low-temperature catalytic oxidation benzene series and preparation method thereof
The invention discloses a supported noble metal catalyst for low-temperature catalytically oxidizing benzene series (benzene, methylbenzene and dimethylbenzene) gas to CO2 and H2O. Active components of the noble metal catalyst are: at least one of Pd, Pt, Ag, Au and Rh; carrier is at least one of active carbon, red mud, molecular sieve, aluminum sesquioxide, titanium dioxide, manganese dioxide, zirconium dioxide, silicon dioxide, cerium dioxide, lanthanum sesquioxide, cobalt oxide, magnesium oxide, zinc oxide, calcium oxide and cupric oxide. Under general pressure, in atmosphere ambient, in fixed bed reactor, with a space velocity range from 10,000 to 100,000h<-1> and at a temperature range from 110 to 210 DEG C, the catalyst can be used for directly oxidizing 100 to 800ppm benzene series gas to CO2 and H2O without byproduct, which shows good low-temperature catalytic activity. The catalyst of the invention has the advantages of simple preparation, lower complete oxidation temperature, immunity to H2O, good stability and high practical value.
Owner:RES CENT FOR ECO ENVIRONMENTAL SCI THE CHINESE ACAD OF SCI
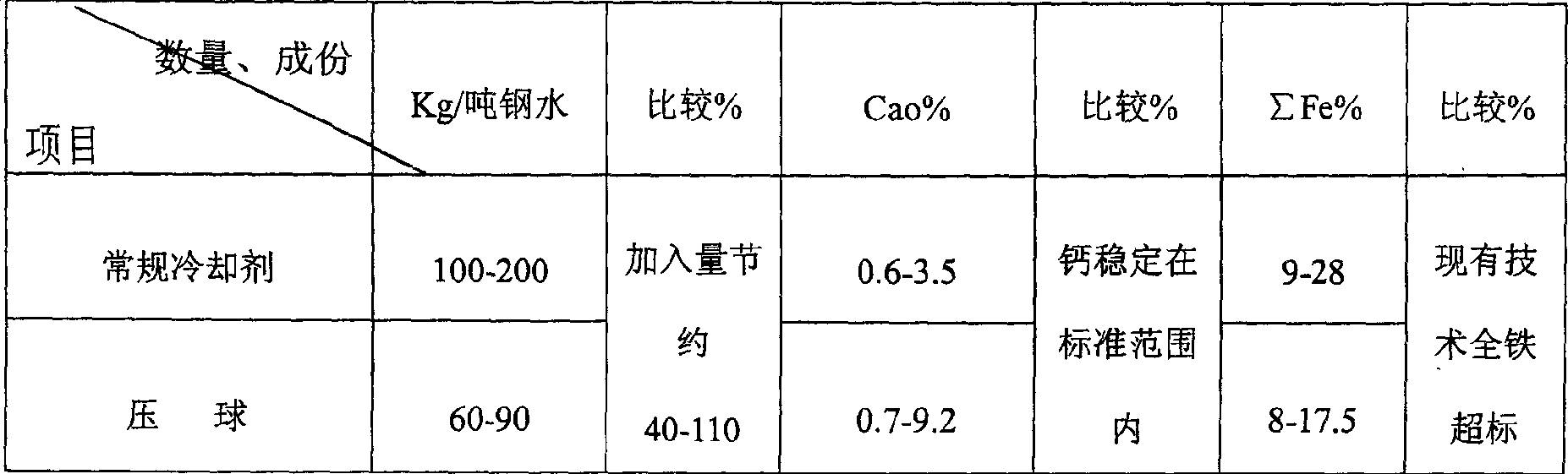
Molten iron vanadium-extracting calcium-controlling coolant and process therefor
The invention relates iron melt improving vanadium content and controlling calcium content cooling agent and the technology, specially relates the cooling agent of adjusting the proportion of cooling agent to stabilize calcium content in vanadium slag and the technology. The technology comprises the 56-60wt% iron scale, 30-40wt% iron concentrate powder containing vanadium, and 5-10wt% anchoring agent. The method stabilizes the calcium content and iron content in vanadium slag, and effectively solves the problems of great fluctuation of vanadium slag composition and superstandard calcium and iron. By controlling the thermoregulation material to meet the requirements of the improving vanadium temperature and calcium oxide content, the method improves the vanadium slag grade and extraction rate. The invention possesses the following characteristics: 1 shortening the converting time; 2 improving the vanadium slag grade and extraction rate, and stabilizing the calcium oxide content; 3 reducing the iron loss.
Owner:HEBEI LUANHE IND GROUP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com