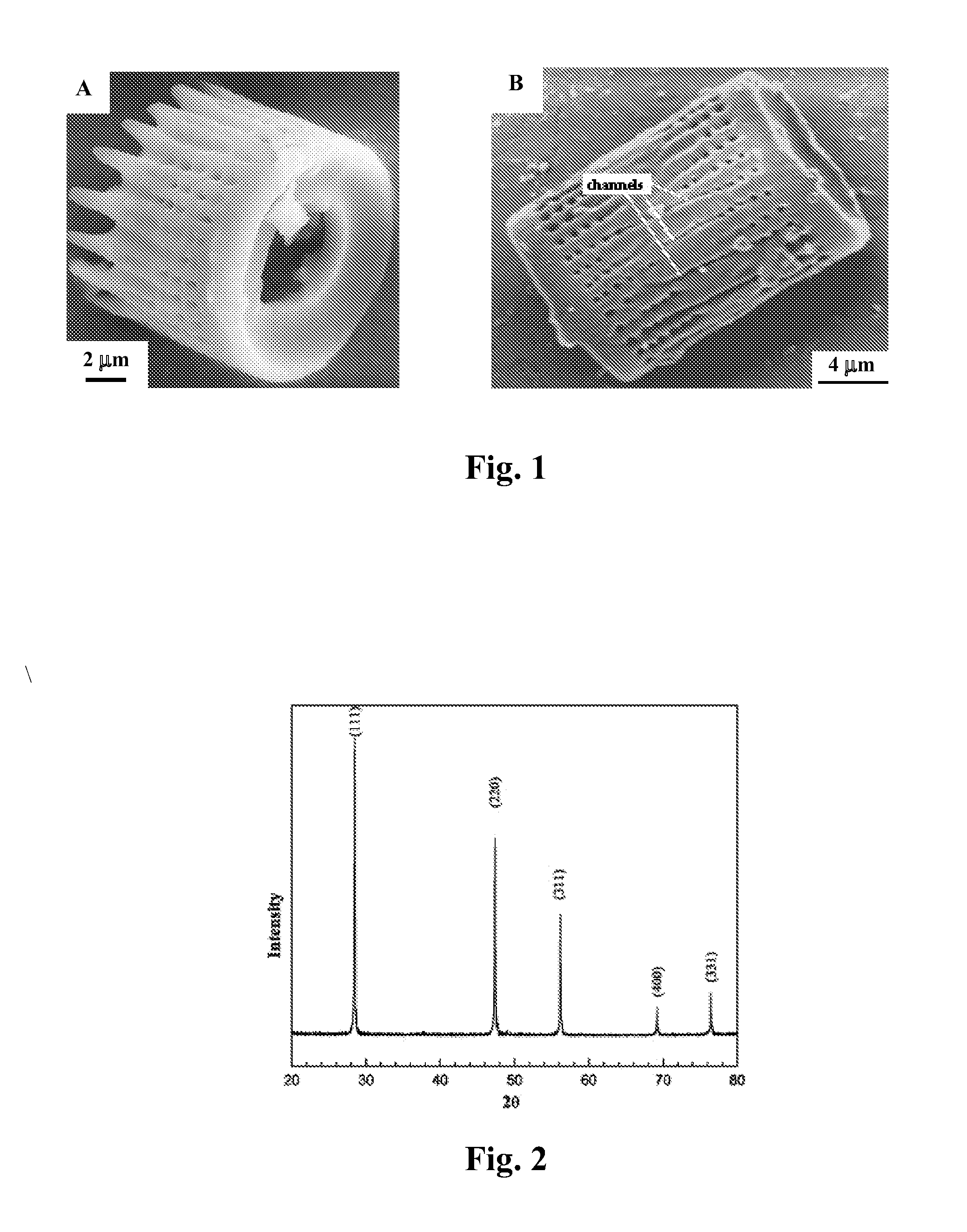
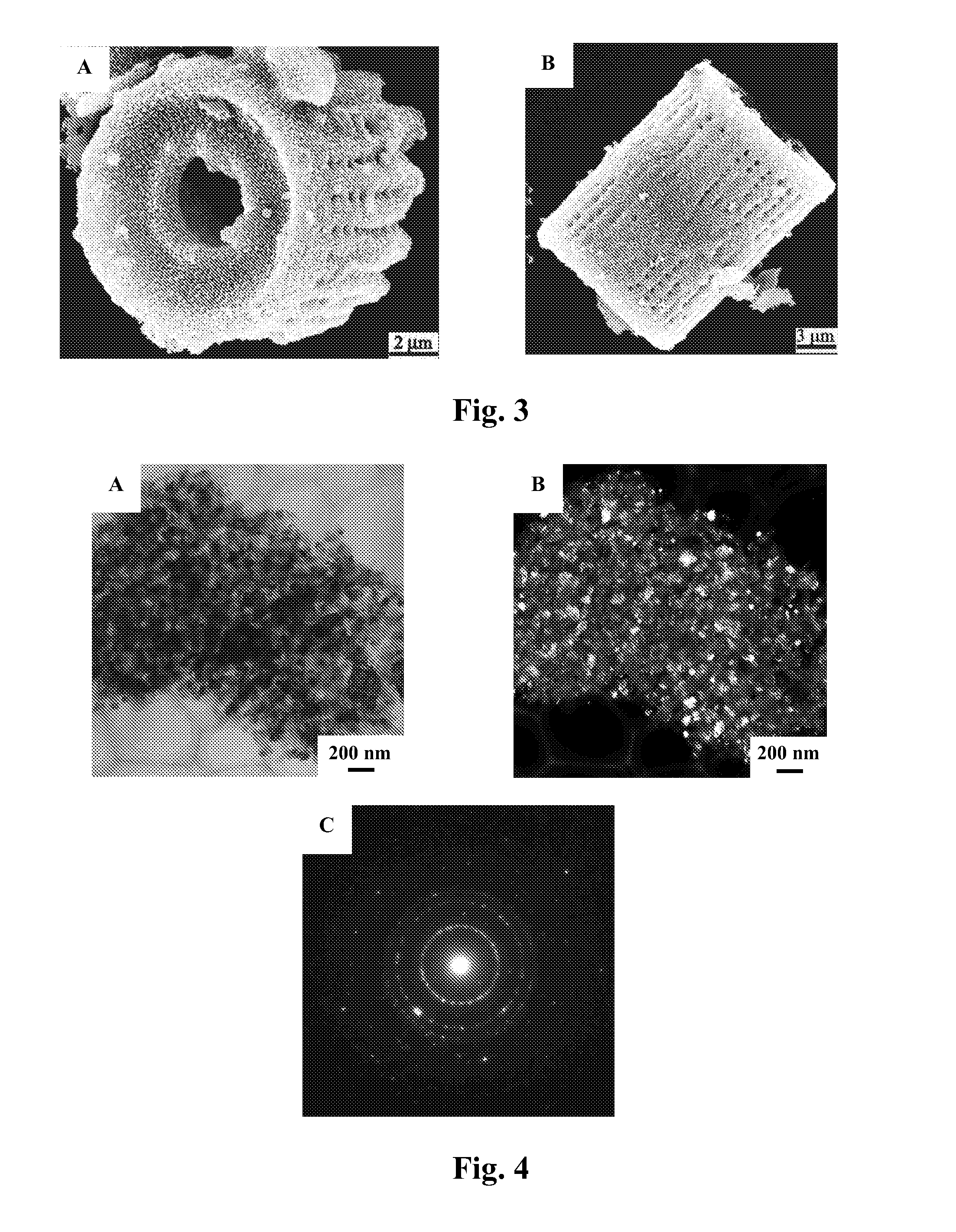
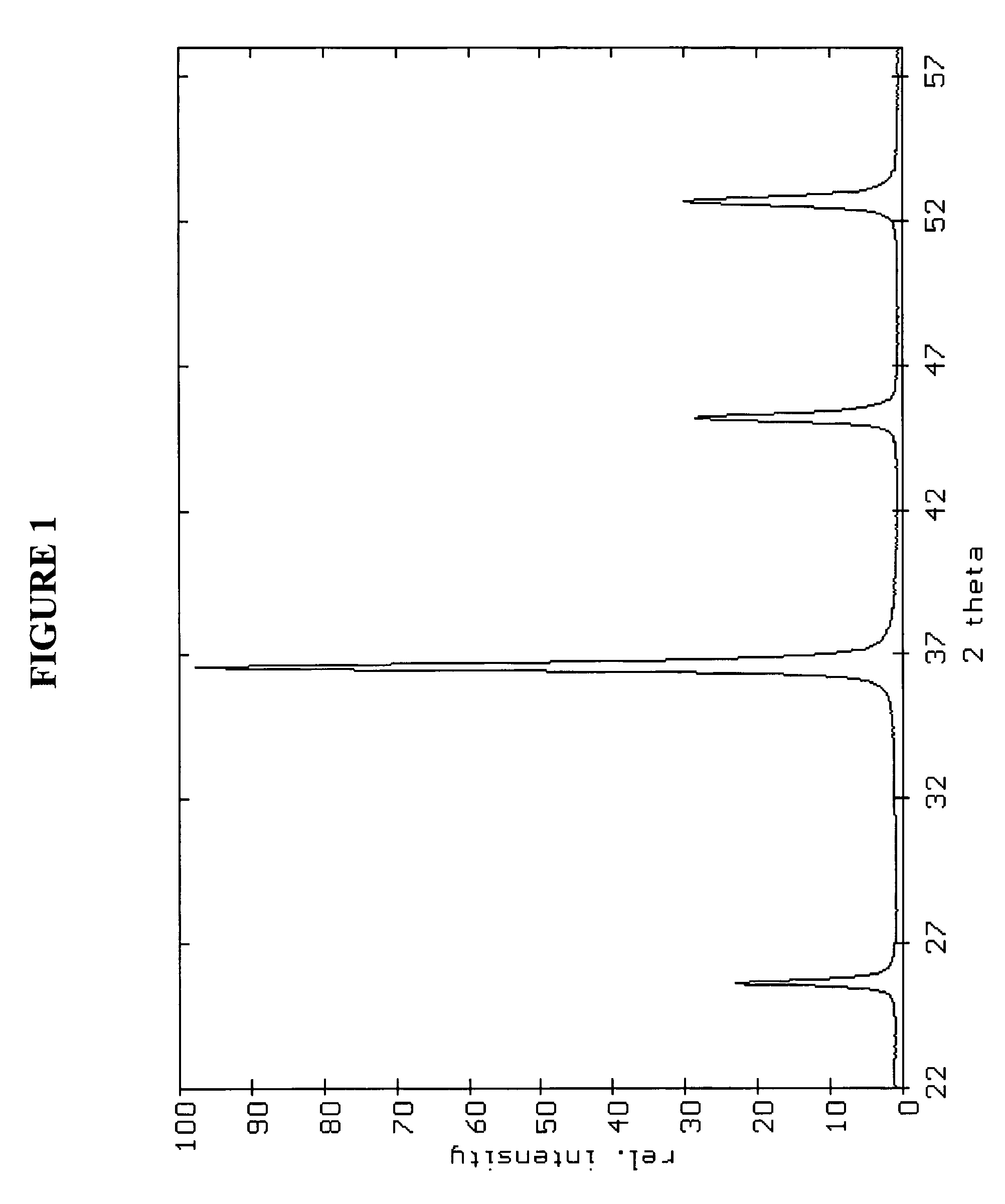
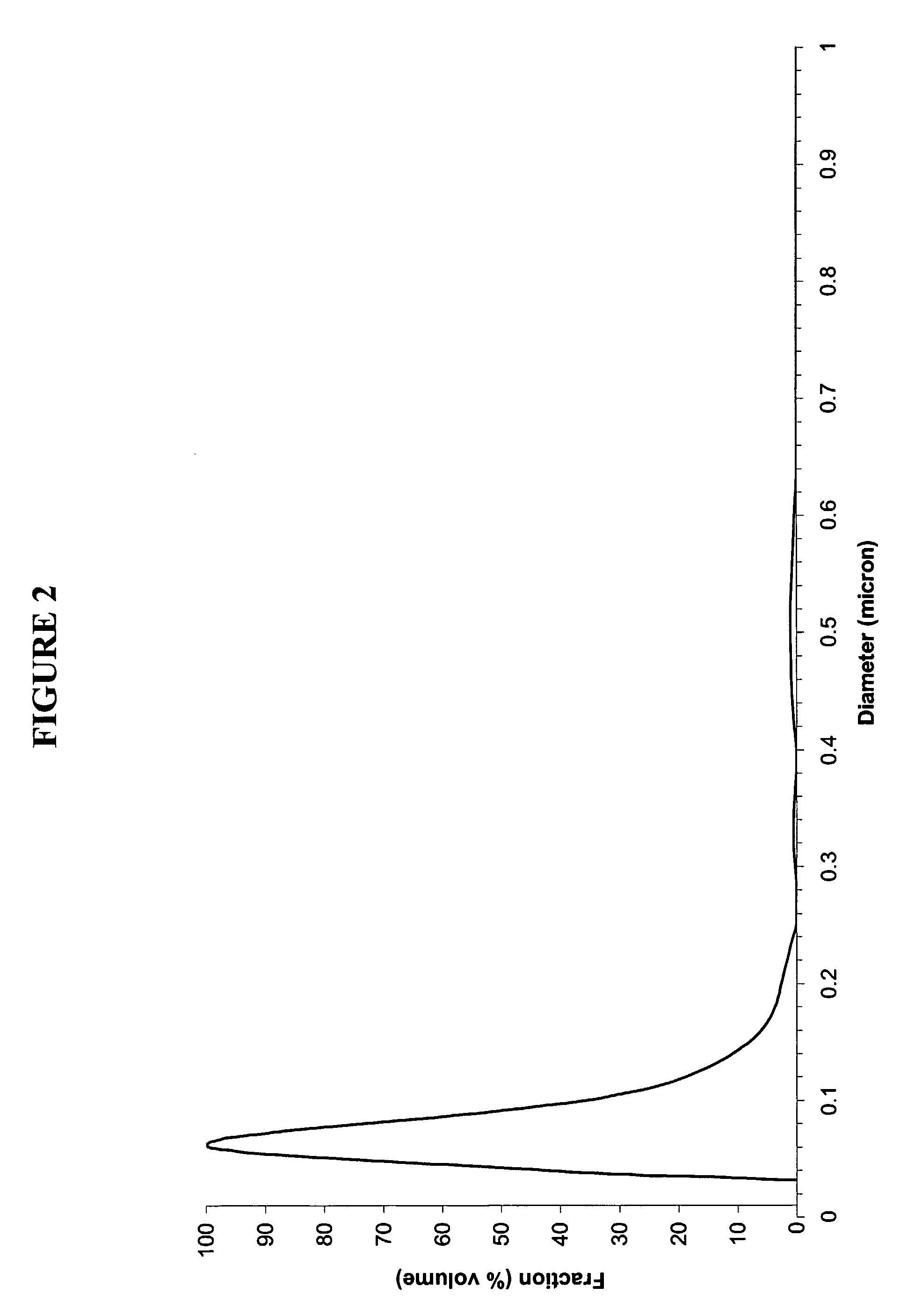
Patents
Literature
1145results about "Oxide/hydroxide preparation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
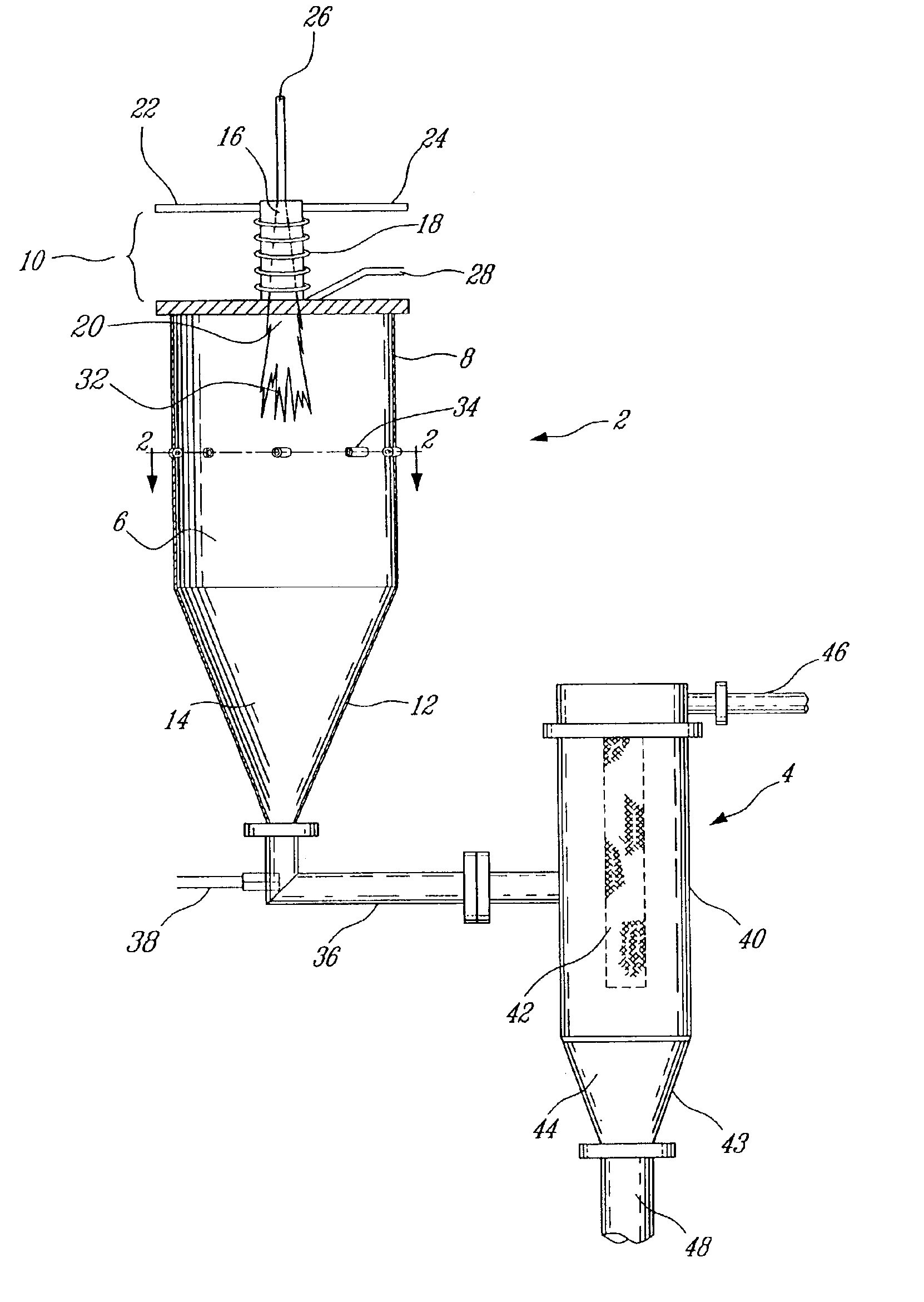
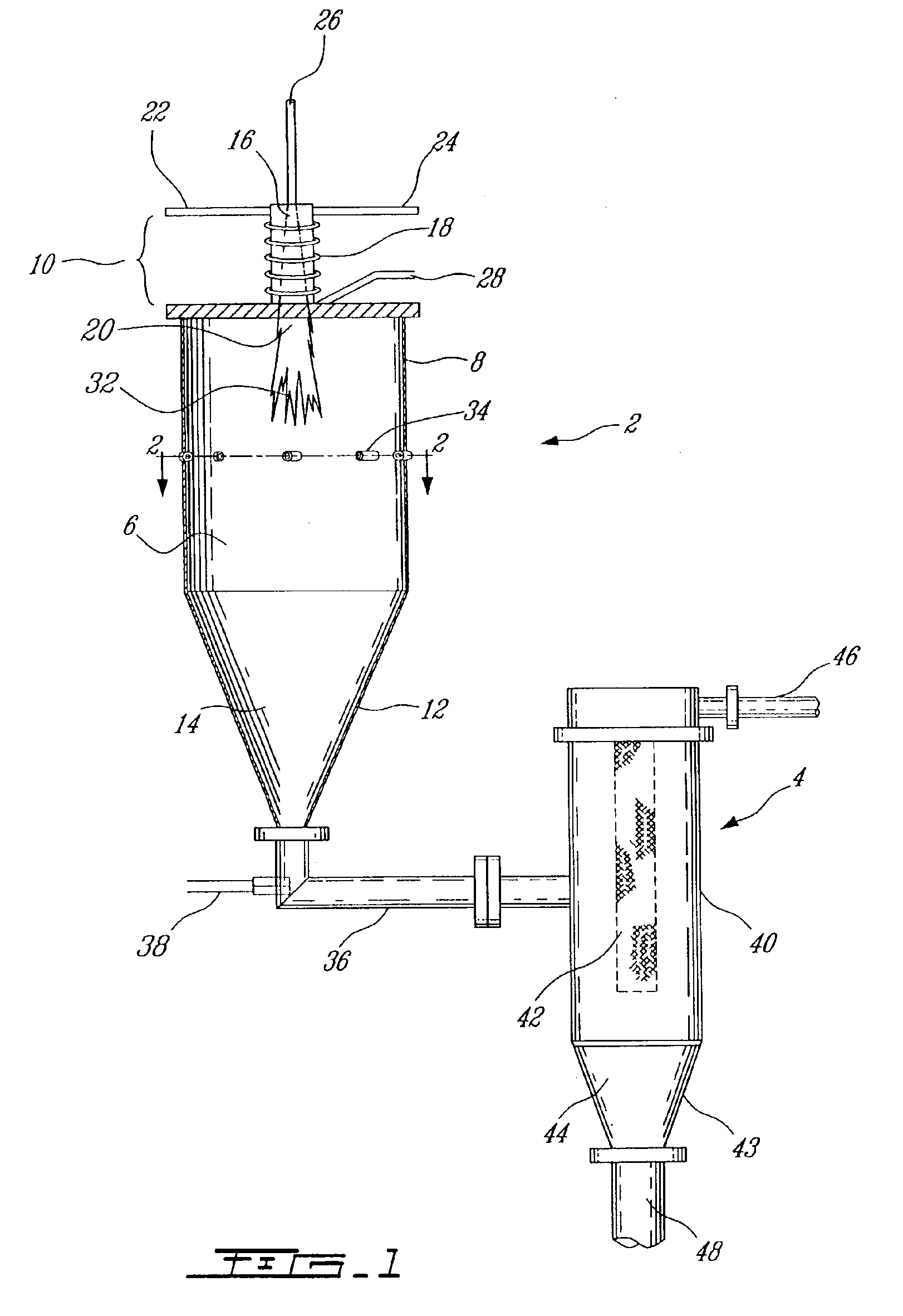
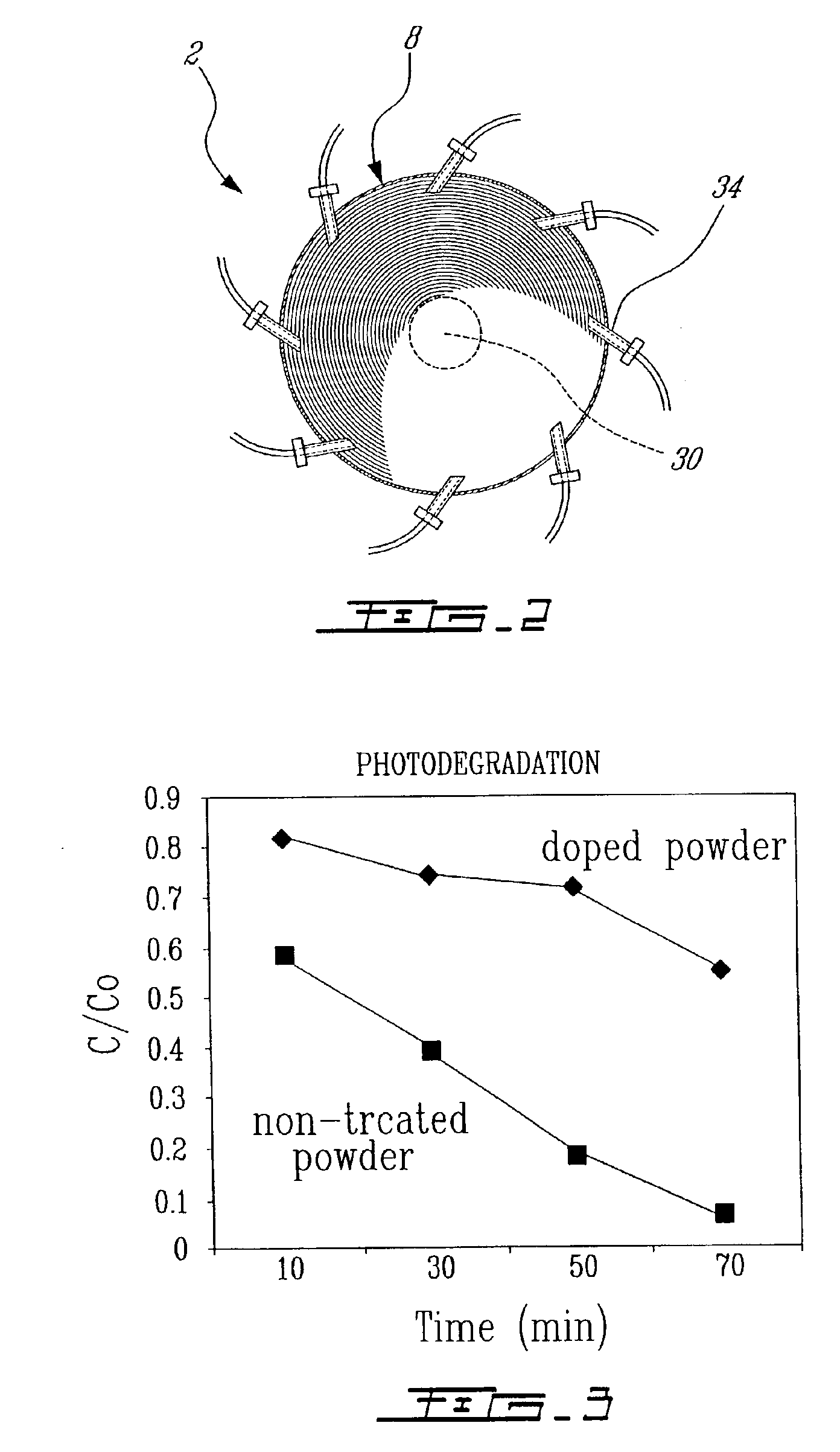
Modified oxygen reduced valve metal oxides
InactiveUS6639787B2Maintain good propertiesWear minimizationOxide/hydroxide preparationLiquid electrolytic capacitorsFluidized bedPhysical chemistry
Owner:GLOBAL ADVANCED METALS USA
Niobium suboxide powder
ActiveUS20050013765A1High currentReduce residual currentOxide/hydroxide preparationLiquid electrolytic capacitorsCapacitorTungsten
A niobium suboxide powder comprising 100 to 600 ppm of magnesium is described. The niobium suboxide powder may (alternatively or in addition to 100 to 600 ppm of magnesium) further include 50 to 400 ppm of molybdenum and / or tungsten. The niobium suboxide powder is suitable for the production of: capacitors having an insulator layer of niobium pentoxide; capacitor anodes produced from the niobium suboxide powder; and corresponding capacitors.
Owner:TANIOBIS GMBH
Chemical manufacture of nanostructured materials
InactiveUS6872330B2High strengthIncrease volumeMaterial nanotechnologyOxide/hydroxide preparationInorganic compoundTe element
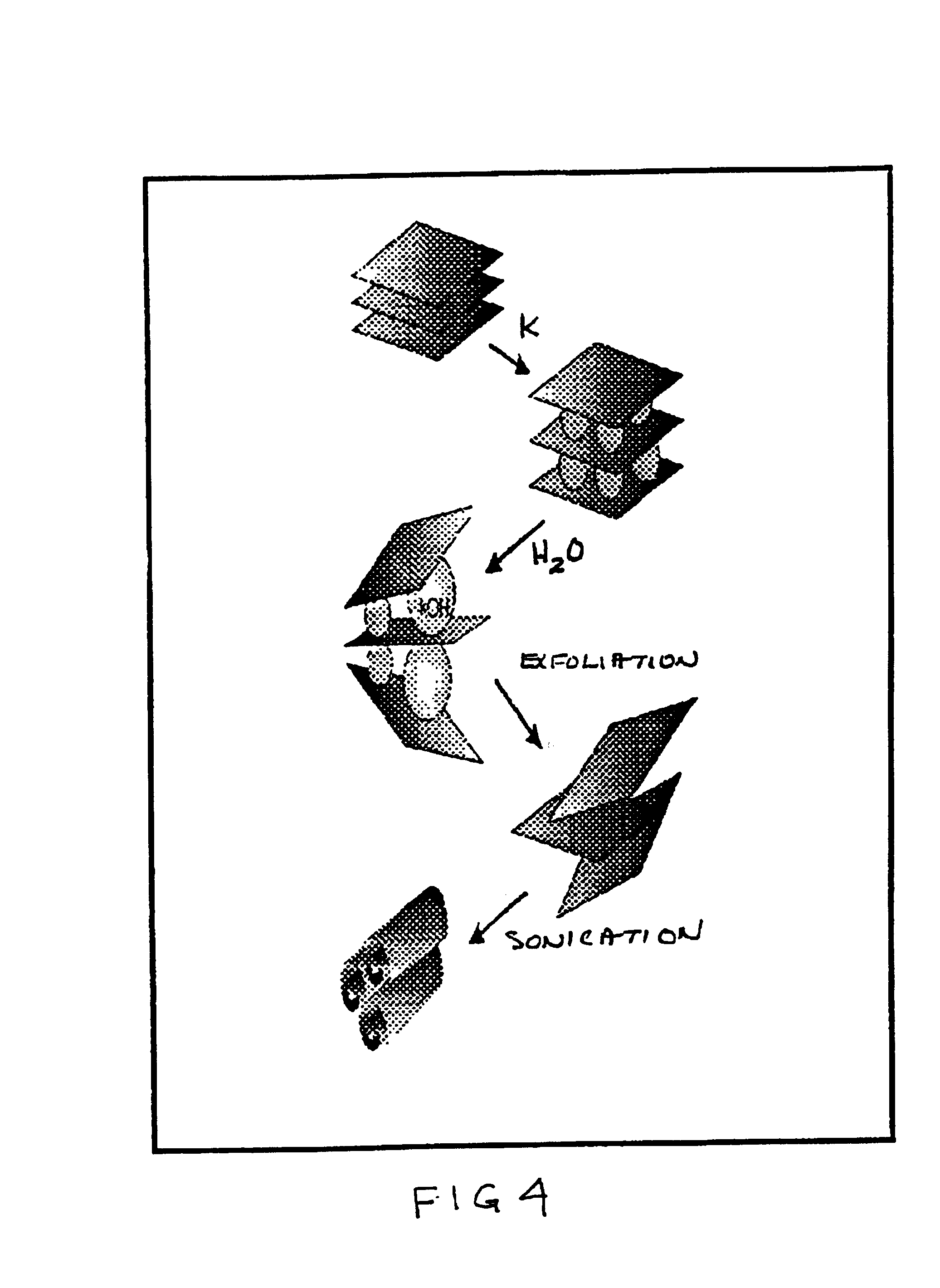
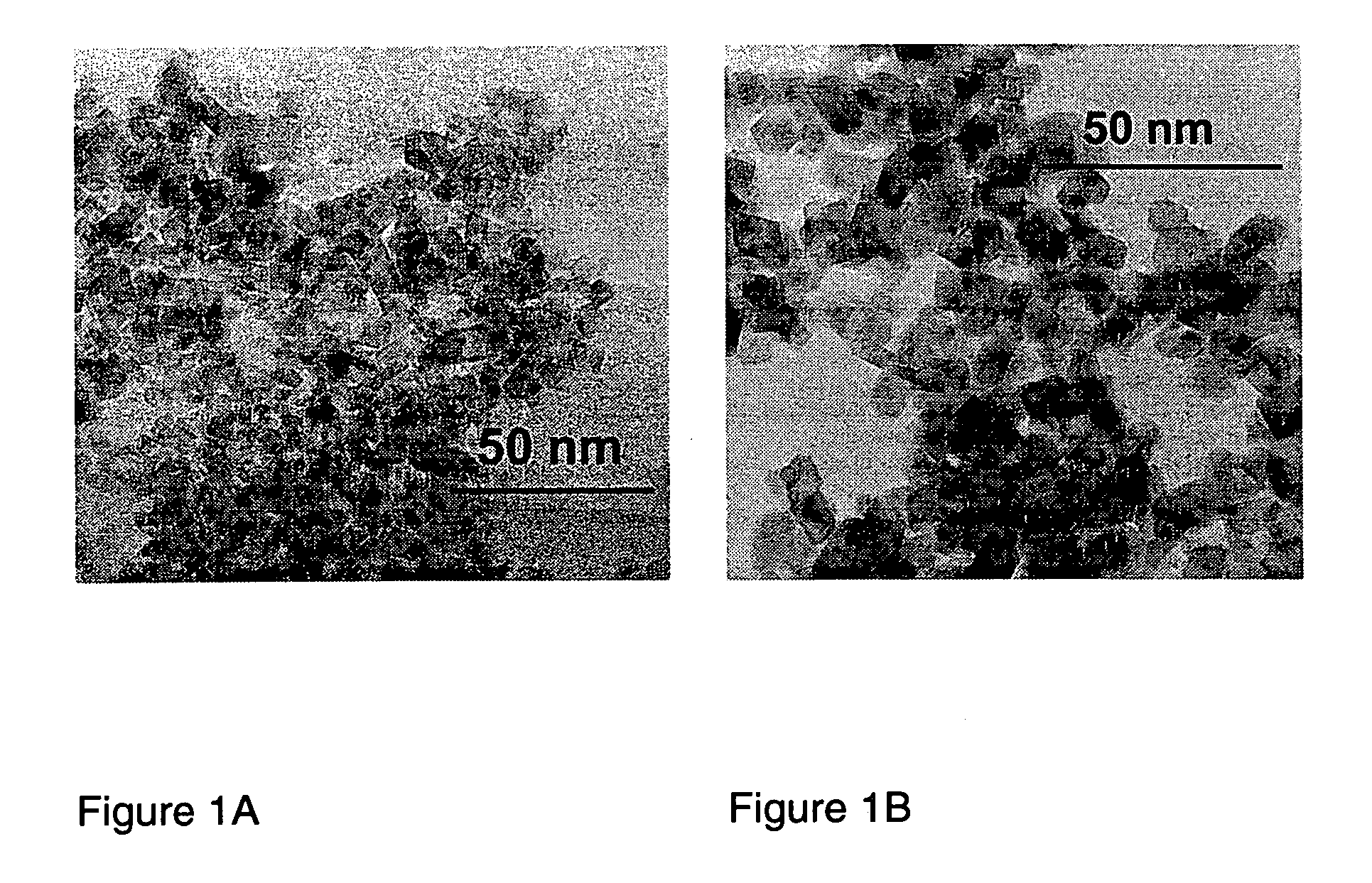
A low temperature chemical route to efficiently produce nanomaterials is described. The nanomaterials are synthesized by intercalating ions into layered compounds, exfoliating to create individual layers and then sonicating to produce nanotubes, nanorods, nanoscrolls and / or nanosheets. It is applicable to various different layered inorganic compounds (for example, bismuth selenides / tellurides, graphite, and other metal complexes, particularly transition metal dichalcogenides compounds including oxygen, sulfur, tellurium or selenium).
Owner:RGT UNIV OF CALIFORNIA
Process for preparing nanostructured materials of controlled surface chemistry
InactiveUS6669823B1Good dispersionReduce hydrolysis rateMaterial nanotechnologyMolten spray coatingCharge carrierNanostructured materials
A process to prepare stoichiometric-nanostructured materials comprising generating a plasma, forming an "active volume" through introduction of an oxidizing gas into the plasma, before the plasma is expanded into a field-free zone, either (1) in a region in close proximity to a zone of charge carrier generation, or (2) in a region of current conduction between field generating elements, including the surface of the field generation elements, and transferring energy from the plasma to a precursor material to form in the "active volume" at least one stoichiometric-nanostructured material and a vapor that may be condensed to form a stoichiometric-nanostructured material. The surface chemistry of the resulting nanostructured materials is substantially enhanced to yield dispersion stable materials with large zeta-potentials.
Owner:NANOPHASE TECH CORP
Flame made metal oxides
ActiveUS7211236B2Add featureWell mixedMaterial nanotechnologyZirconium oxidesSpray pyrolysisCarboxylic acid
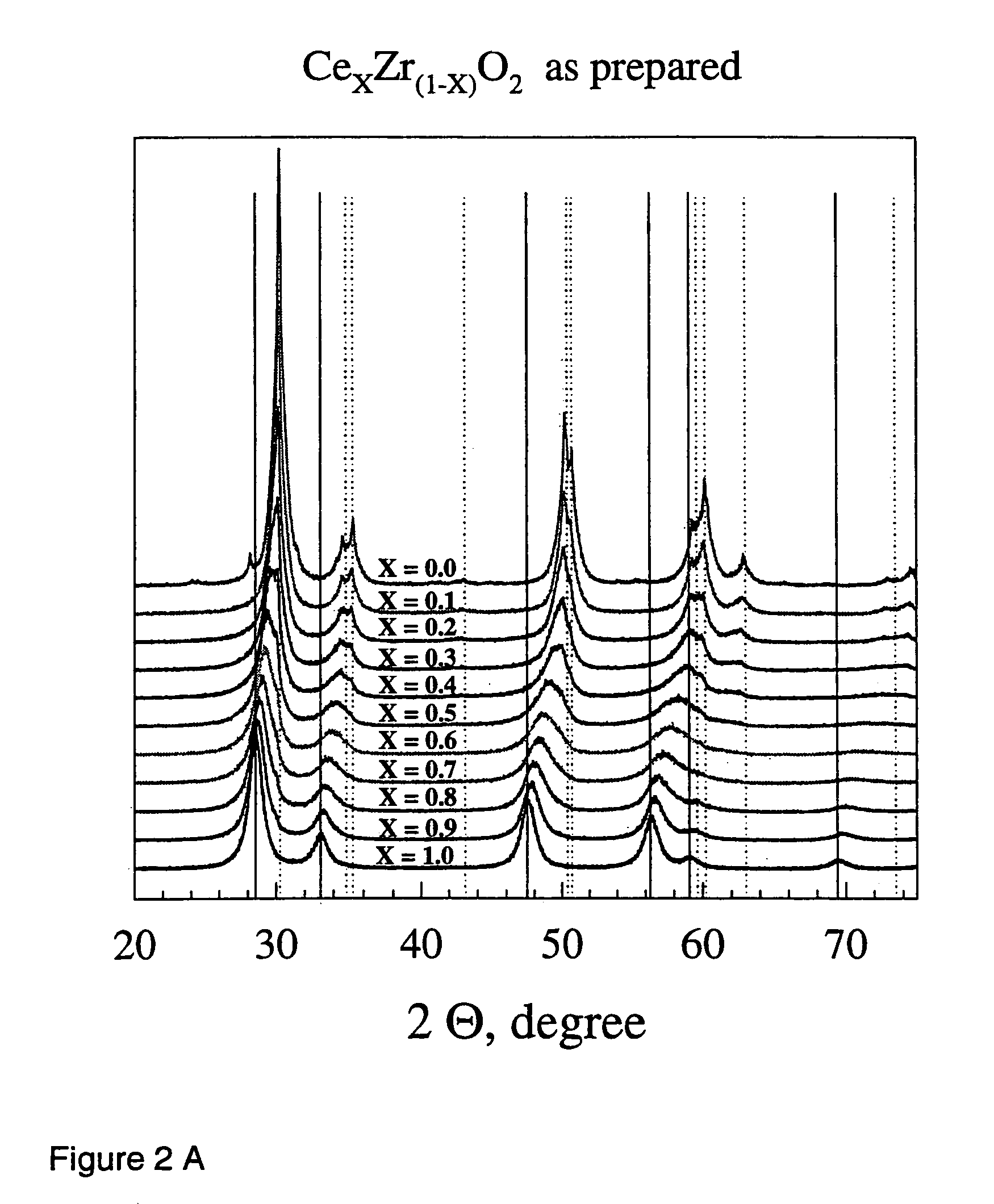
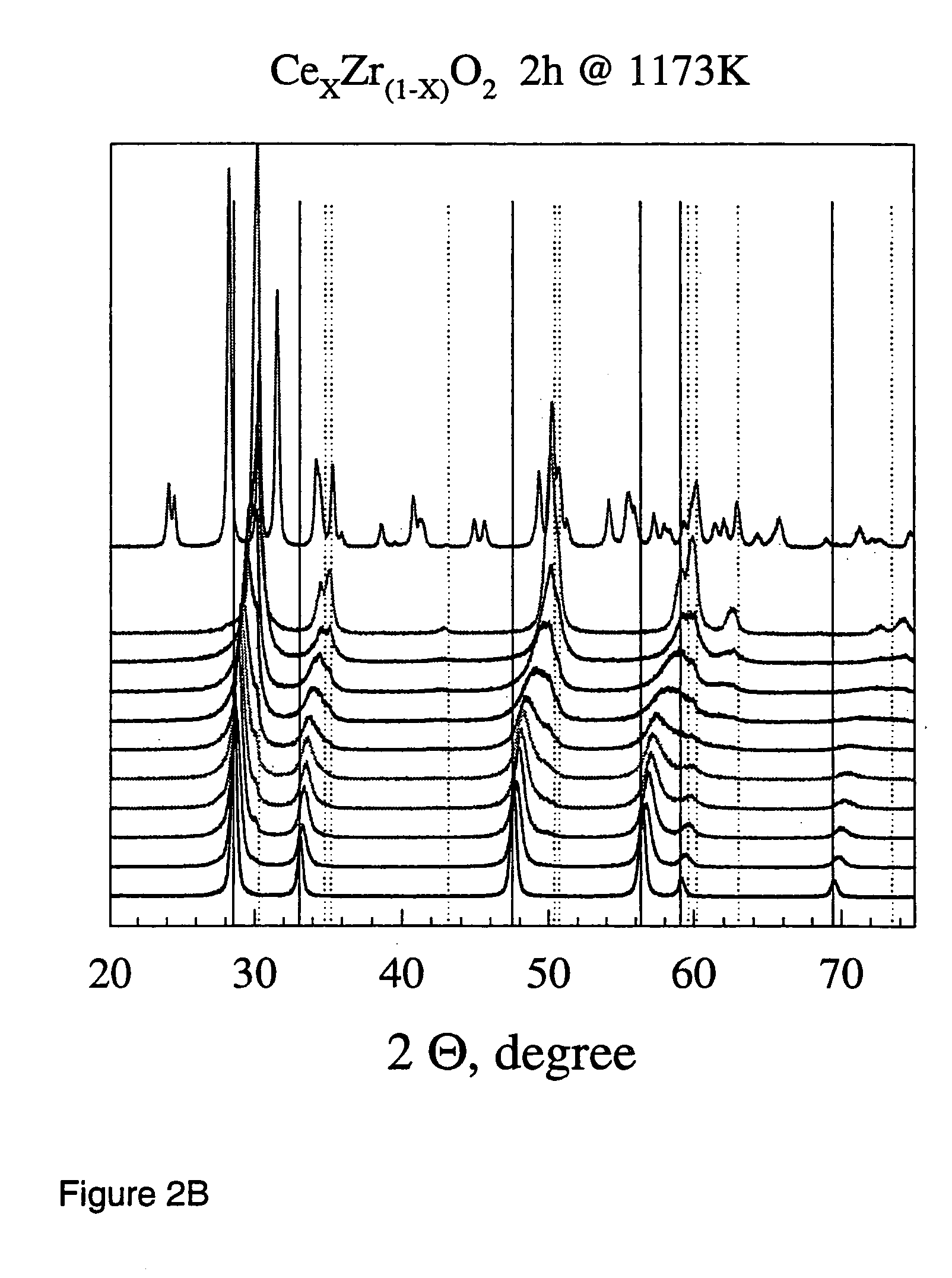
Described is a method for the production of metal oxides by flame spray pyrolysis, in particular mixed metal oxides such as ceria / zirconia, and metal oxides obtainable by said method. Due to high enthalpy solvents with a high carboxylic acid content said metal oxides have improved properties. For example ceria / zirconia has excellent oxygen storage capacity at high zirconium levels up to more than 80% of whole metal content.
Owner:EIDGENOSSISCHE TECHN HOCHSCULE ZURICH
Method and apparatus for insitu vapor generation
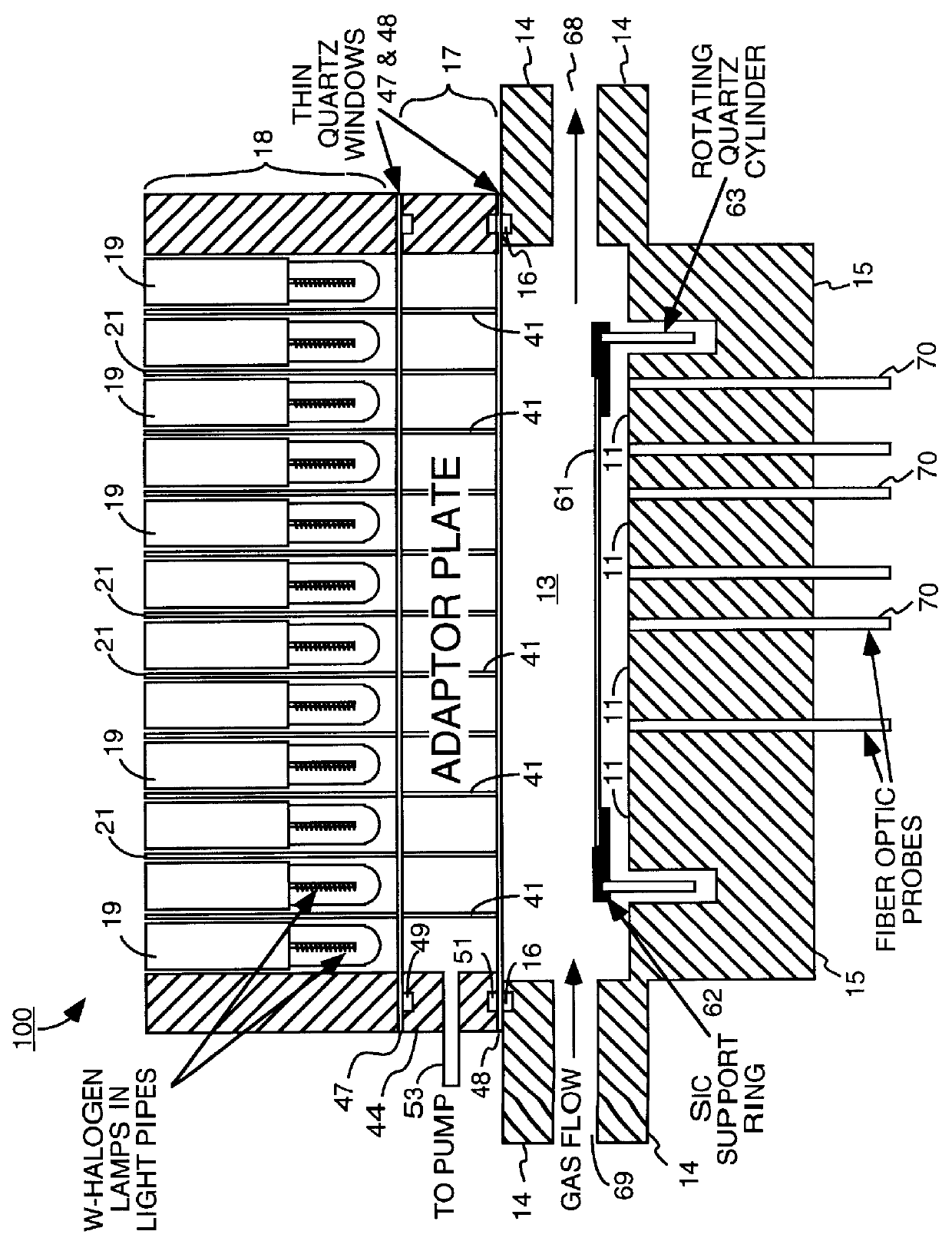
A method of forming an oxide on a substrate. According to the method of the present invention a substrate is placed in a chamber. An oxygen containing gas and a hydrogen containing gas are then fed into the chamber. The oxygen containing gas and the hydrogen containing gas are then caused to react with one another to form water vapor in the chamber. The water vapor then oxidizes the substrate.
Owner:APPLIED MATERIALS INC
Process for the production of ultrafine powders of metal oxides
InactiveUS6503475B1Low costHigh yield rateAlkaline earth titanatesMaterial nanotechnologyDiluentBiological activation
A process for the production of ultrafine powders that includes subjecting a mixture of precursor metal compound and a non-reactant diluent phase to mechanical milling whereby the process of mechanical activation reduces the microstructure of the mixture to the form of nano-sized grains of the metal compound uniformly dispersed in the diluent phase. The process also includes heat treating the mixture of nano-sized grains of the metal compound uniformly dispersed in the diluent phase to convert the nano-sized grains of the metal compound into a metal oxide phase. The process further includes removing the diluent phase such that the nano-sized grains of the metal oxide phase are left behind in the form of an ultrafine powder.
Owner:SAMSUNG CORNING PRECISION MATERIALS CO LTD +1
Sorbent for separation of carbon dioxide (CO2) from gas mixtures
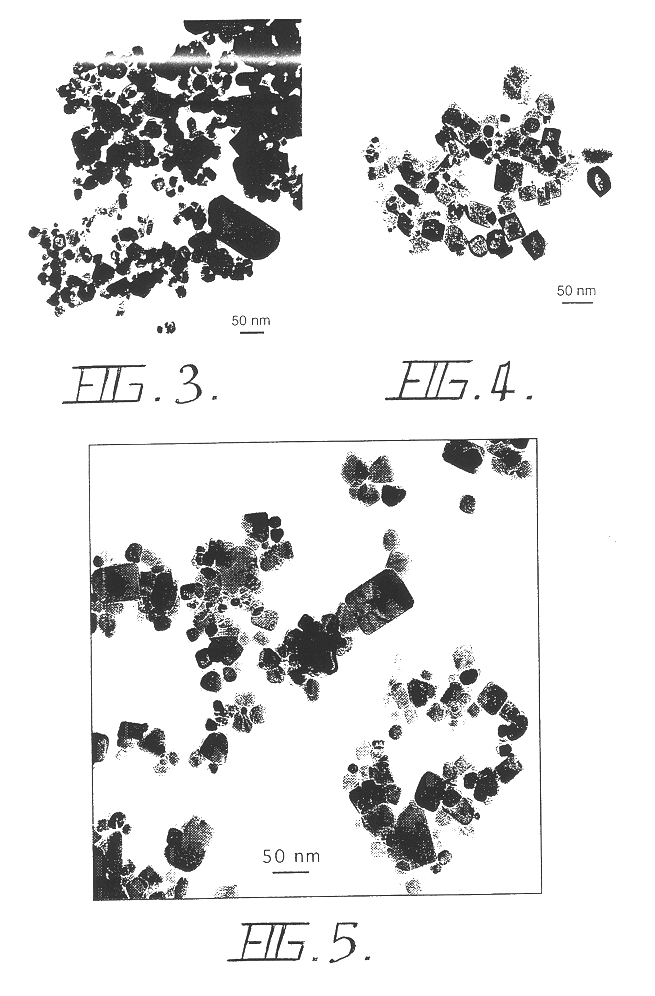
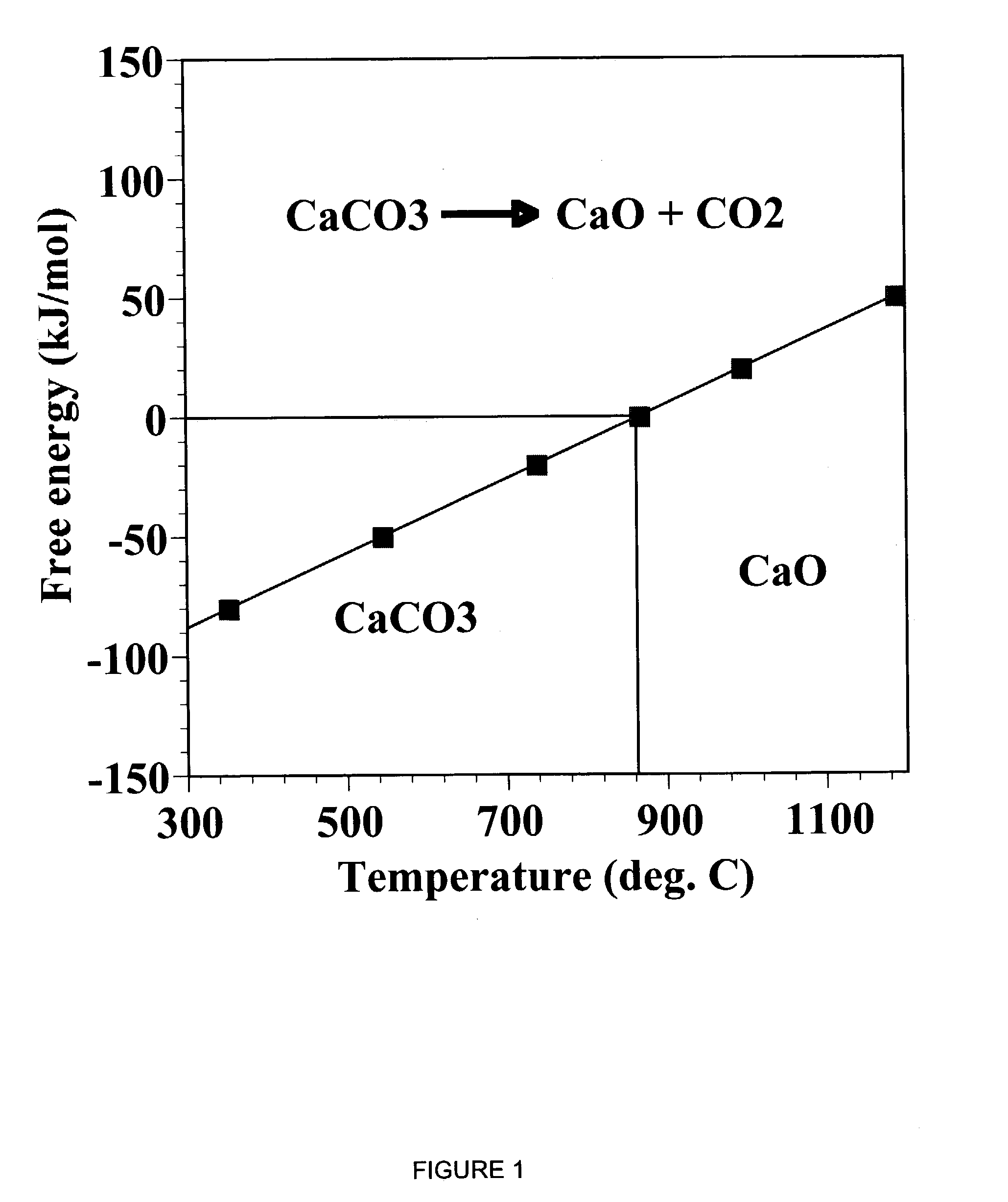
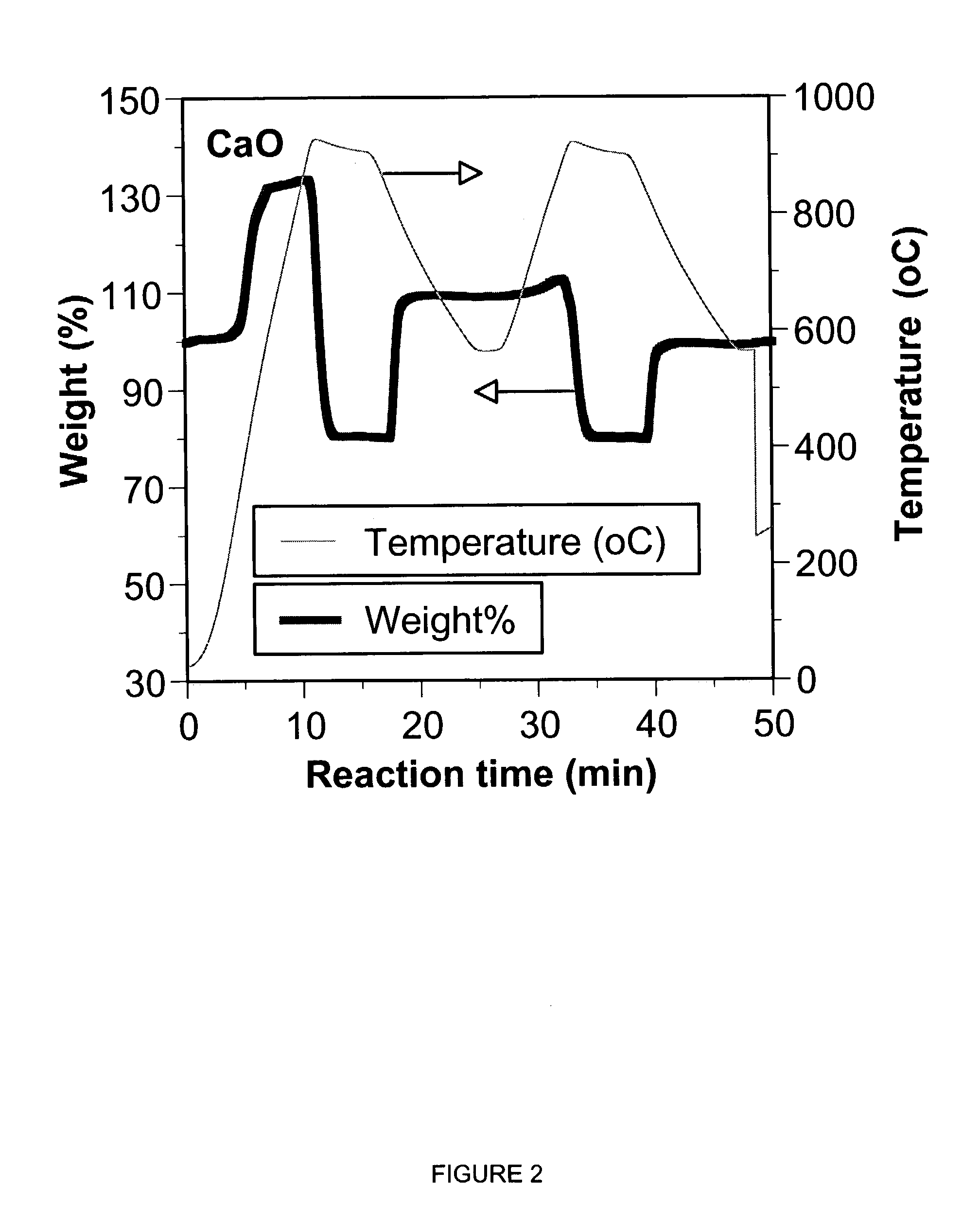
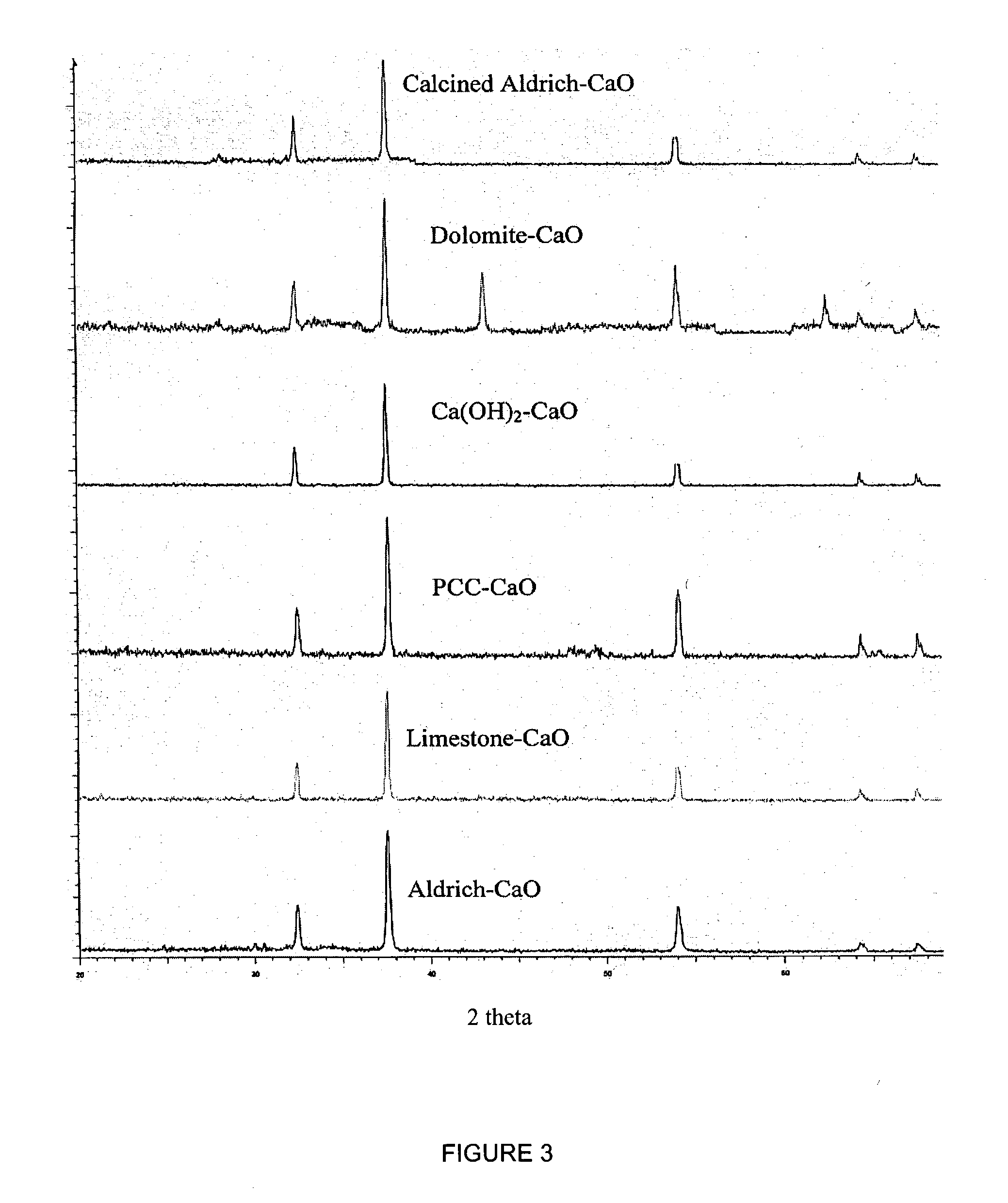
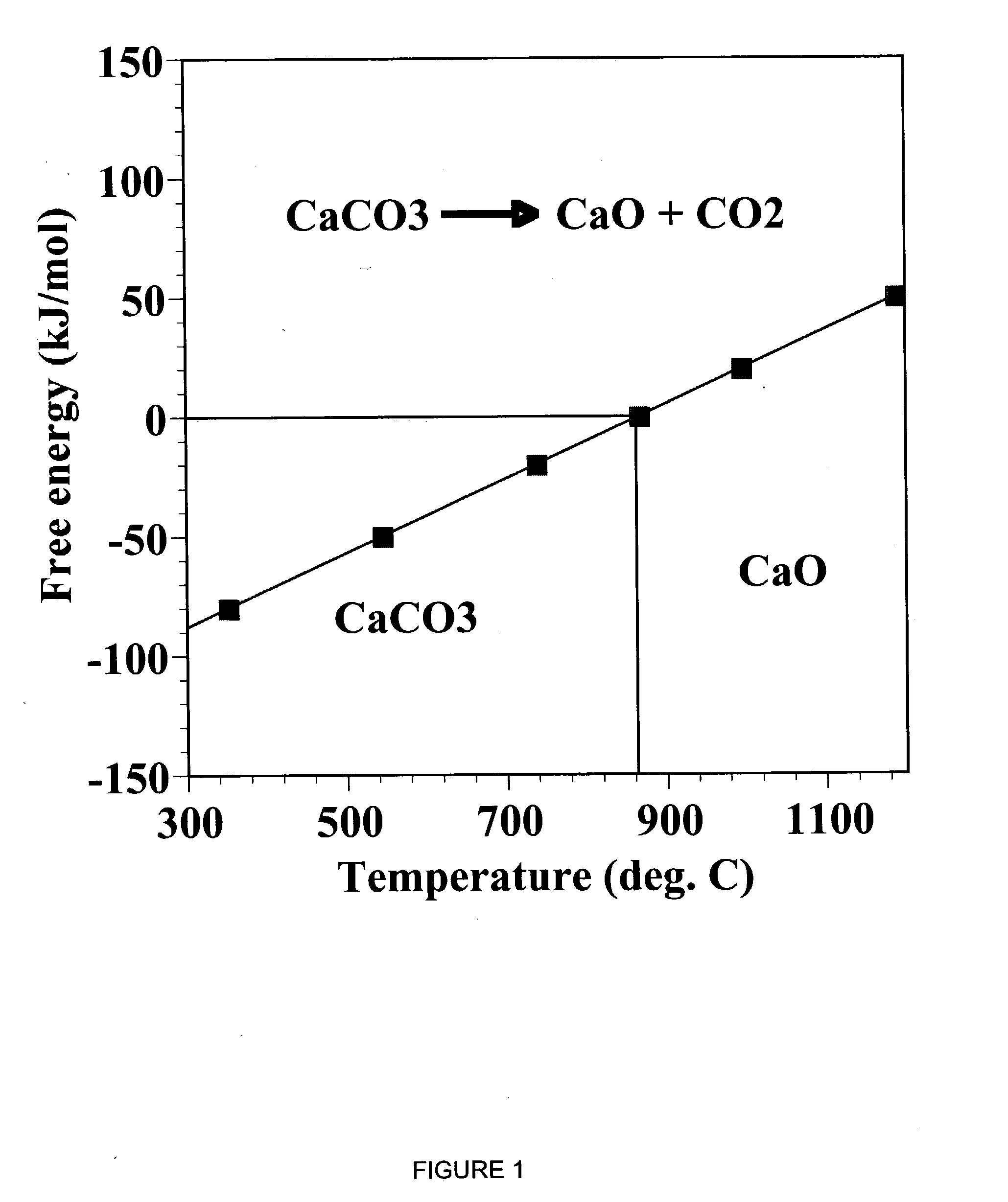
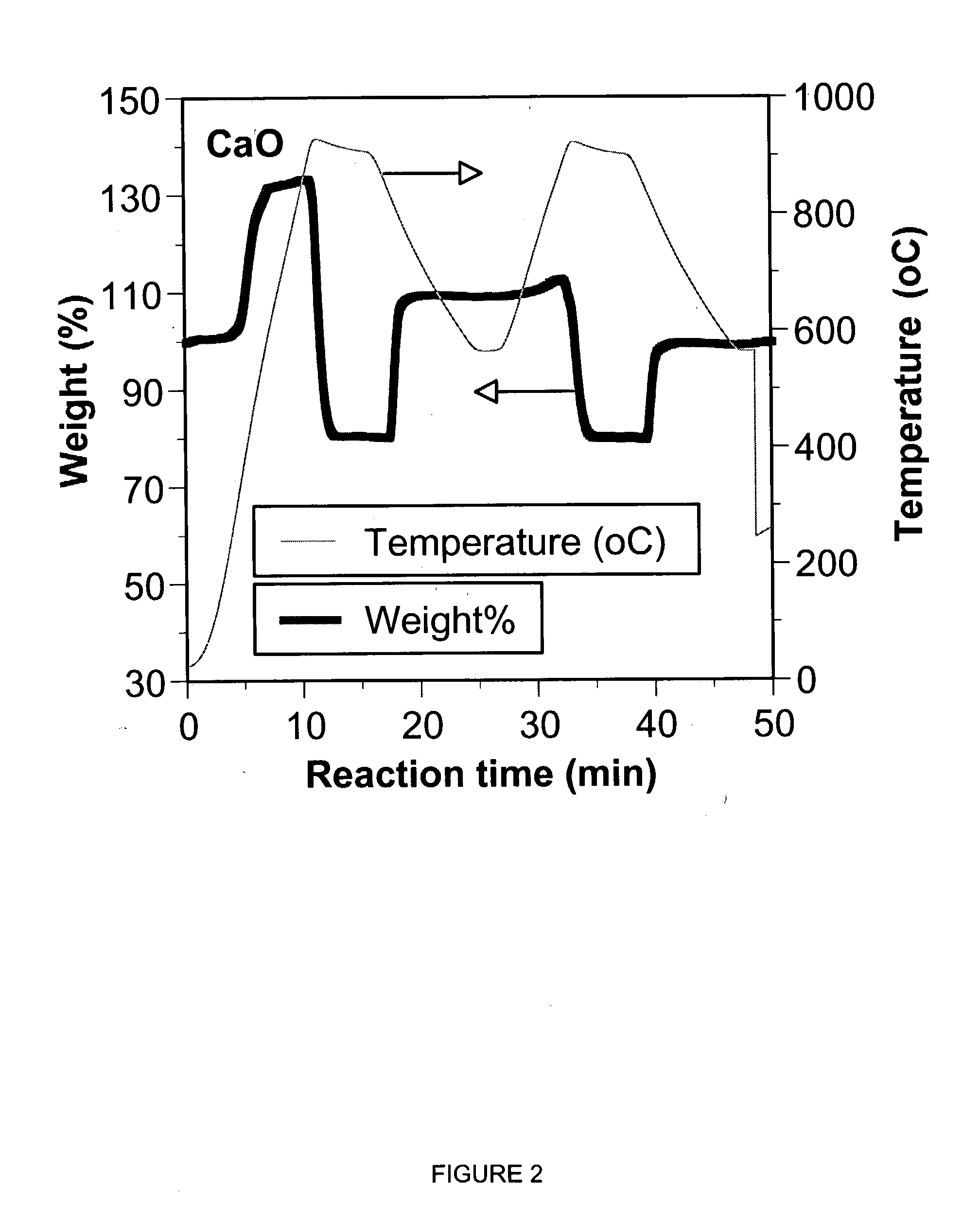
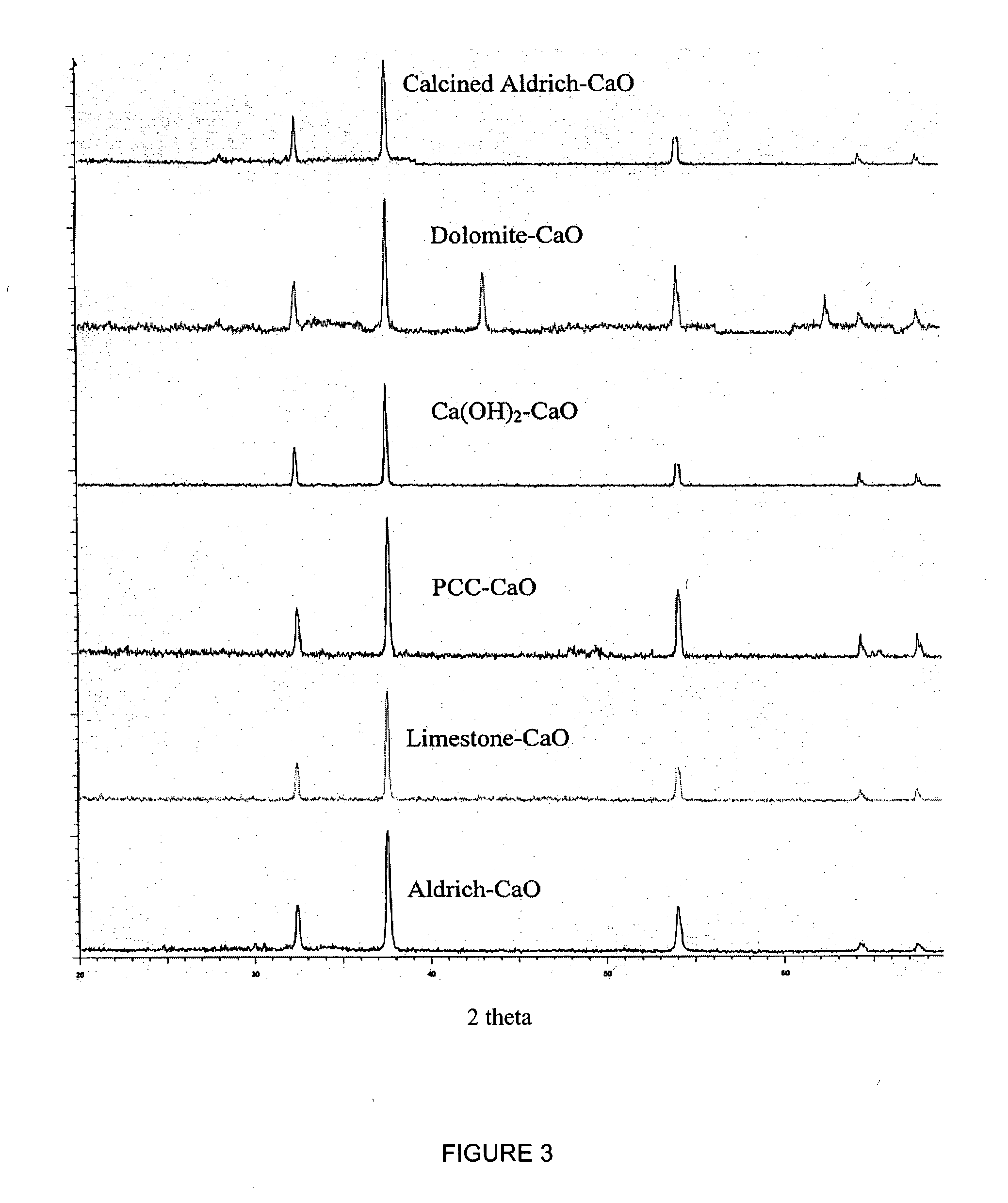
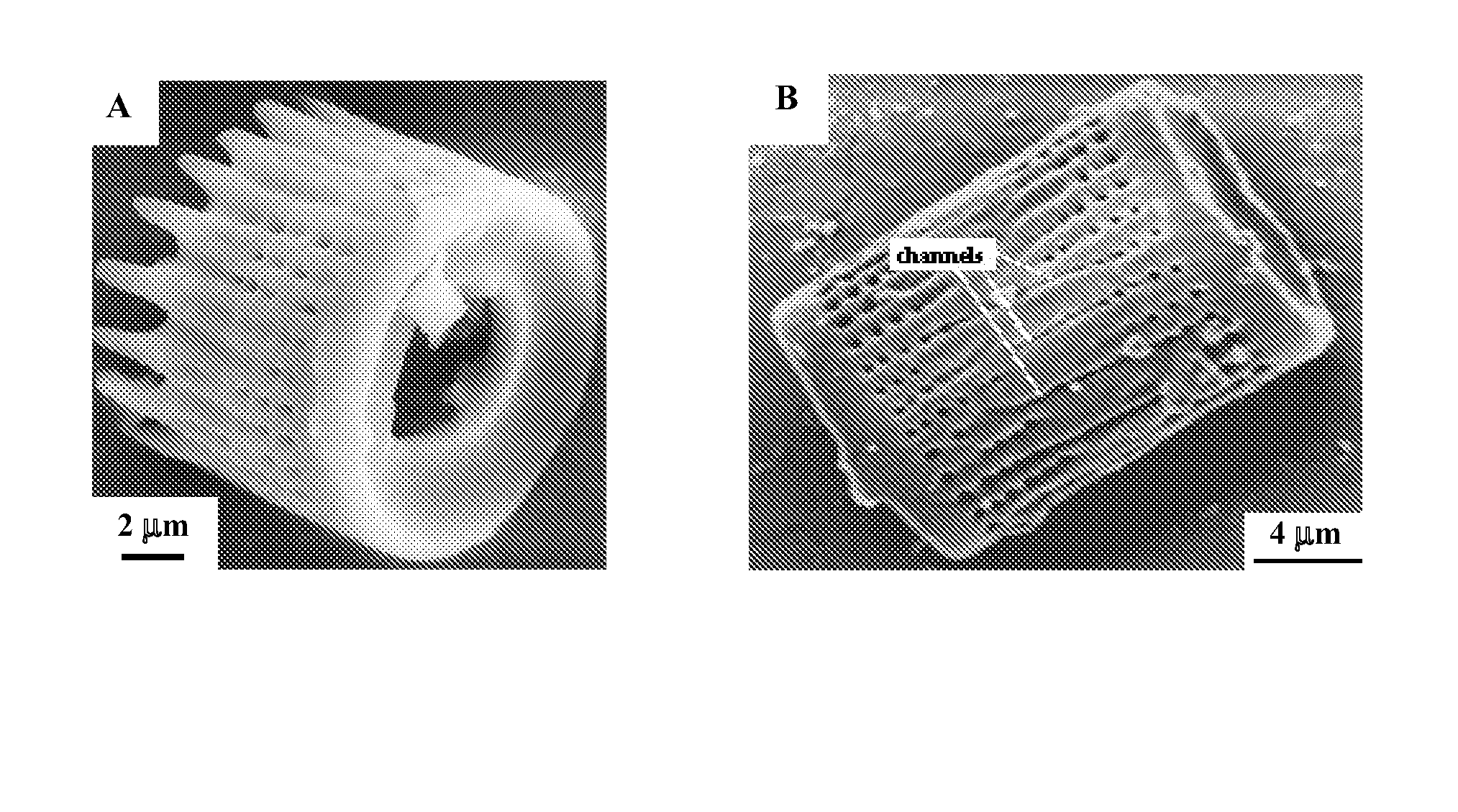
A reaction-based process has been developed for the selective removal of carbon dioxide (CO2) from a multicomponent gas mixture to provide a gaseous stream depleted in CO2 compared to the inlet CO2 concentration in the stream. The proposed process effects the separation of CO2 from a mixture of gases (such as flue gas / fuel gas) by its reaction with metal oxides (such as calcium oxide). The Calcium based Reaction Separation for CO2 (CaRS-CO2) process consists of contacting a CO2 laden gas with calcium oxide (CaO) in a reactor such that CaO captures the CO2 by the formation of calcium carbonate (CaCO3). Once “spent”, CaCO3 is regenerated by its calcination leading to the formation of fresh CaO sorbent and the evolution of a concentrated stream of CO2. The “regenerated” CaO is then recycled for the further capture of more CO2. This carbonation-calcination cycle forms the basis of the CaRS-CO2 process. This process also identifies the application of a mesoporous CaCO3 structure, developed by a process detailed elsewhere, that attains >90% conversion over multiple carbonation and calcination cycles. Lastly, thermal regeneration (calcination) under vacuum provided a better sorbent structure that maintained reproducible reactivity levels over multiple cycles.
Owner:THE OHIO STATES UNIV
Plasma synthesis of metal oxide nanopowder and apparatus therefor
InactiveUS6994837B2Large dischargeKeep for a long timePigmenting treatmentMaterial nanotechnologyDopantPhysical chemistry
A process and apparatus for the synthesis of metal oxide nanopowder from a metal compound vapour is presented. In particular a process and apparatus for the synthesis of TiO2 nanopowder from TiCl4 is disclosed. The metal compound vapour is reacted with an oxidizing gas in electrically induced RF frequency plasma thus forming a metal oxide vapour. The metal oxide vapour is rapidly cooled using a highly turbulent gas quench zone which quickly halts the particle growth process, yielding a substantial reduction in the size of metal oxide particles formed compared with known processes. The metal compound vapour can also react with a doping agent to create a doped metal oxide nanopowder. Additionally, a process and apparatus for the inline synthesis of a coated metal oxide is disclosed wherein the metal oxide particles are coated with a surface agent after being cooled in a highly turbulent gas quench zone.
Owner:TEKNA PLASMA SYST INC
Process and Apparatus for Producing Fine Particles
ActiveUS20080006954A1Improve productivityFunction increaseAlkaline earth titanatesMaterial nanotechnologyGas phaseSlurry
A fine particle producing process introduces a material for producing fine particles into a thermal plasma flame to make a vapor-phase mixture and quenches the vapor-phase mixture to form the fine particles. In the process, the material for producing the fine particles is dispersed or dissolved in a dispersion medium or solvent, preferably containing a combustible material to prepare a dispersion such as a slurry, a colloidal solution or a dissolution solution, the dispersion is made into a form of droplets, or the material for producing the fine particles is dispersed with a carrier gas and a combustible material and the dispersion in a droplet form or the dispersed material is introduced into the thermal plasma flame. In the fine particle producing process and apparatus, a gas of an amount sufficient to quench the vapor-phase mixture is supplied toward a tail of the thermal plasma flame. In the process and apparatus, primary fine particles are introduced into a cyclone to be subjected to cooling and classification and secondary fine particles having a particle size of 100 nm or less which are left upon removal of coarse particles are recovered.
Owner:NISSHIN SEIFUN GRP INC +1
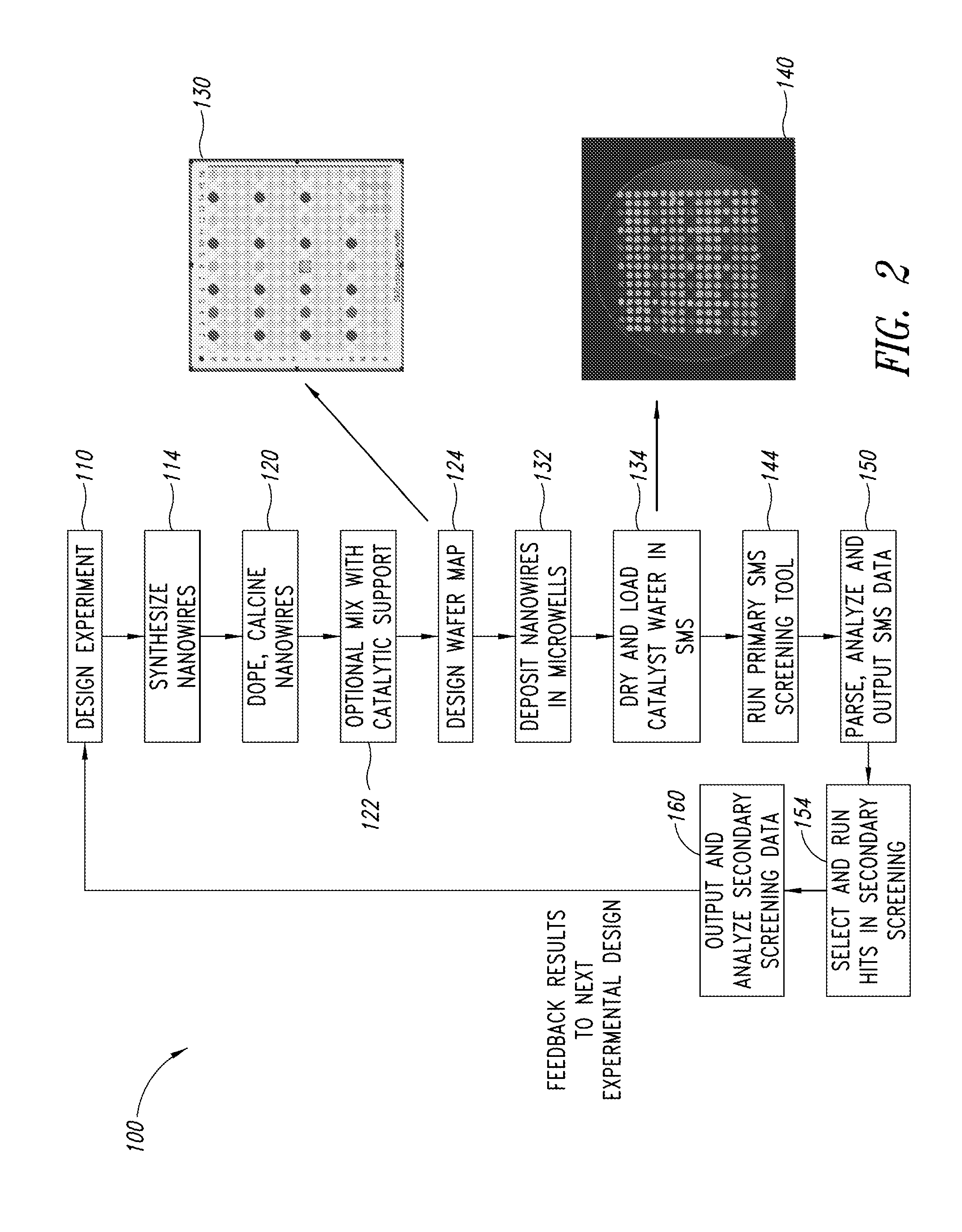
Nanowire catalysts and methods for their use and preparation
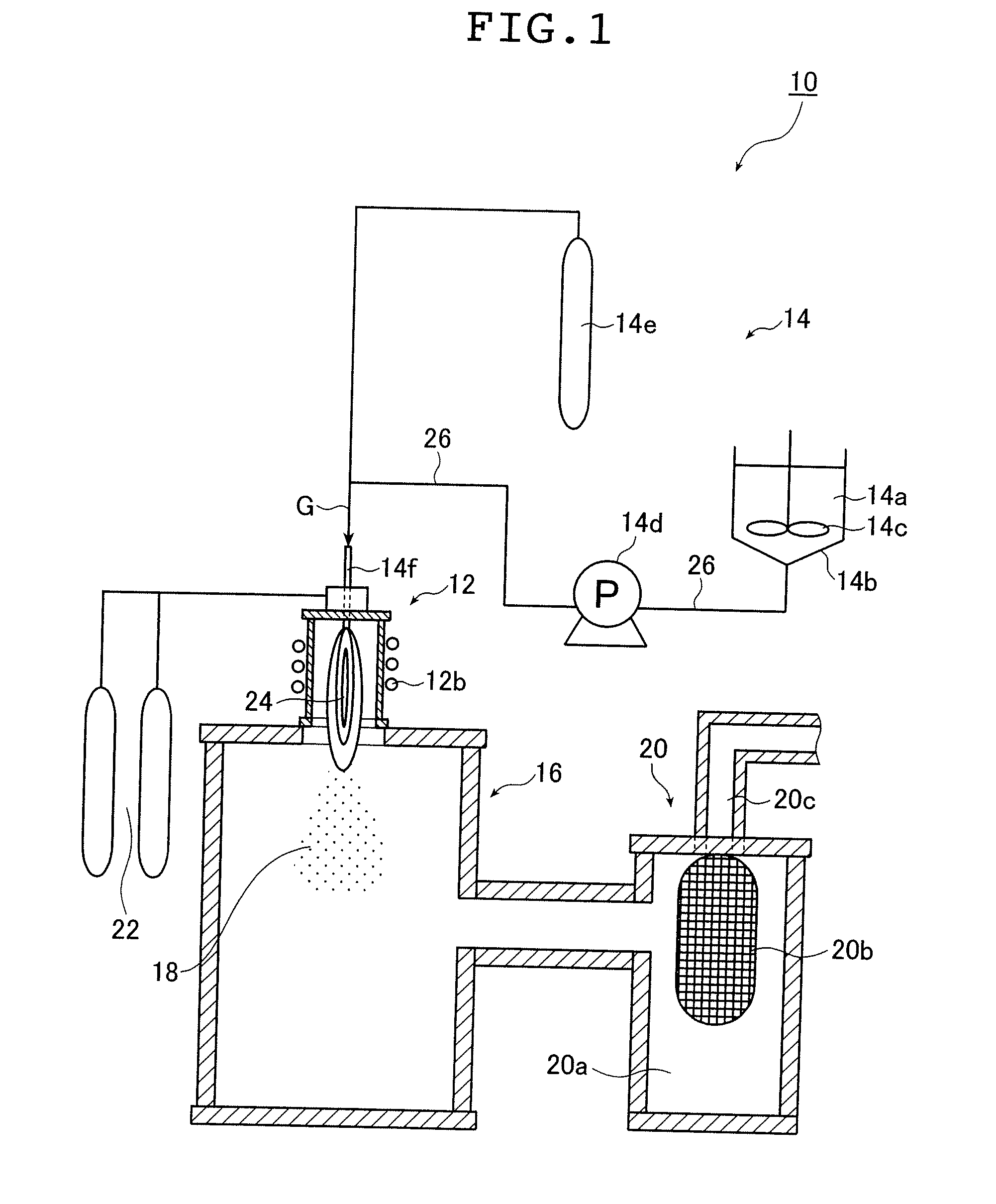
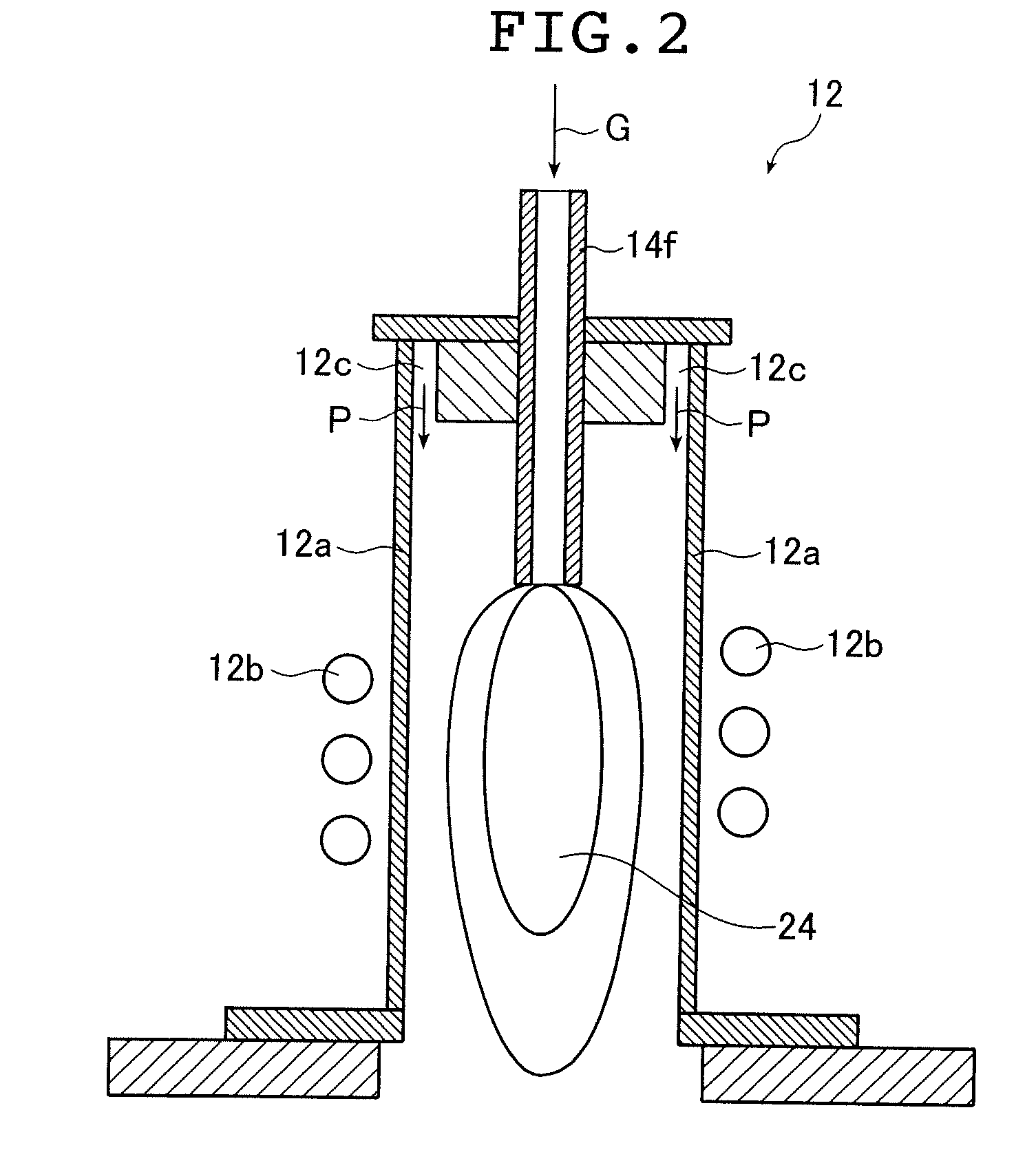
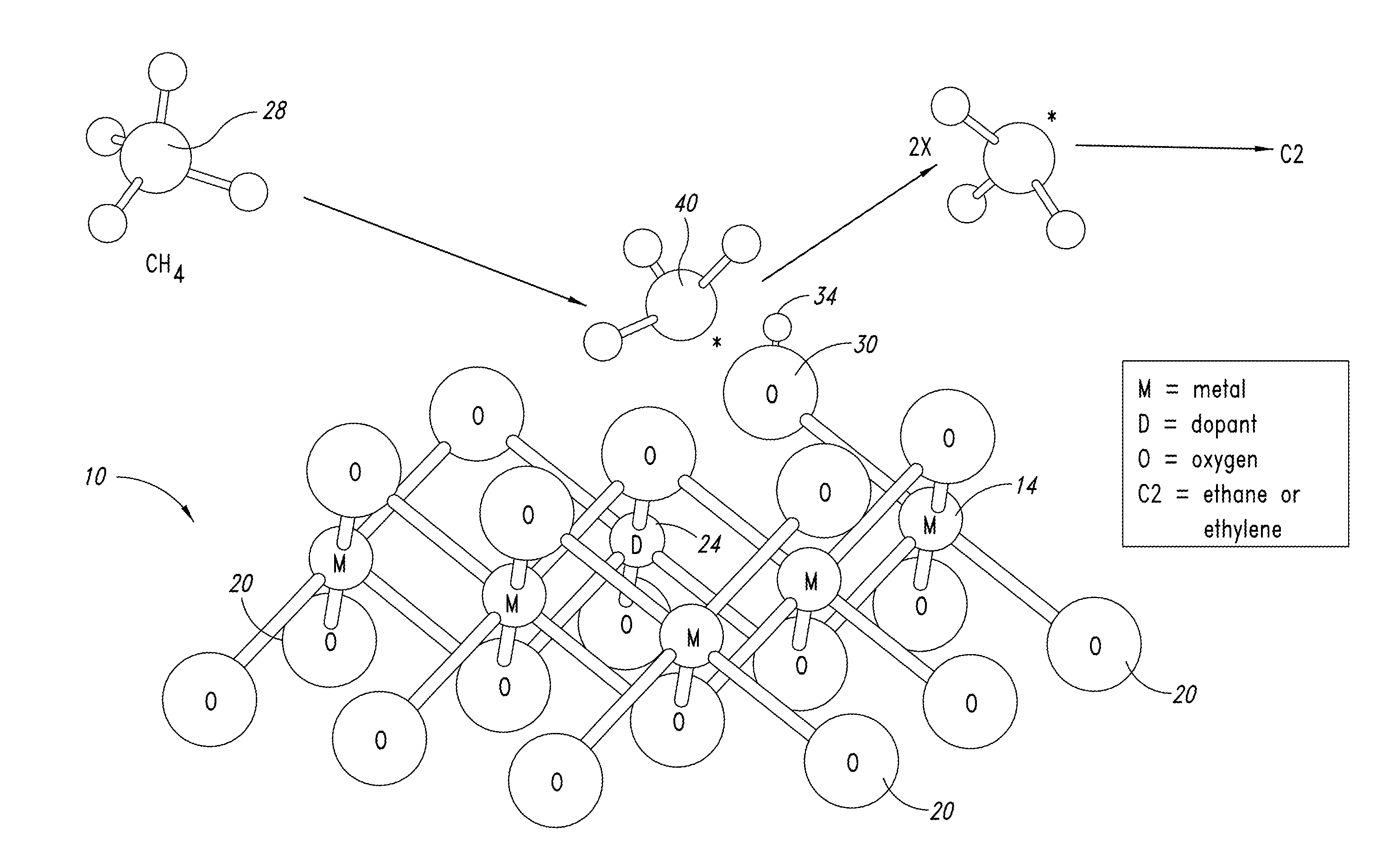
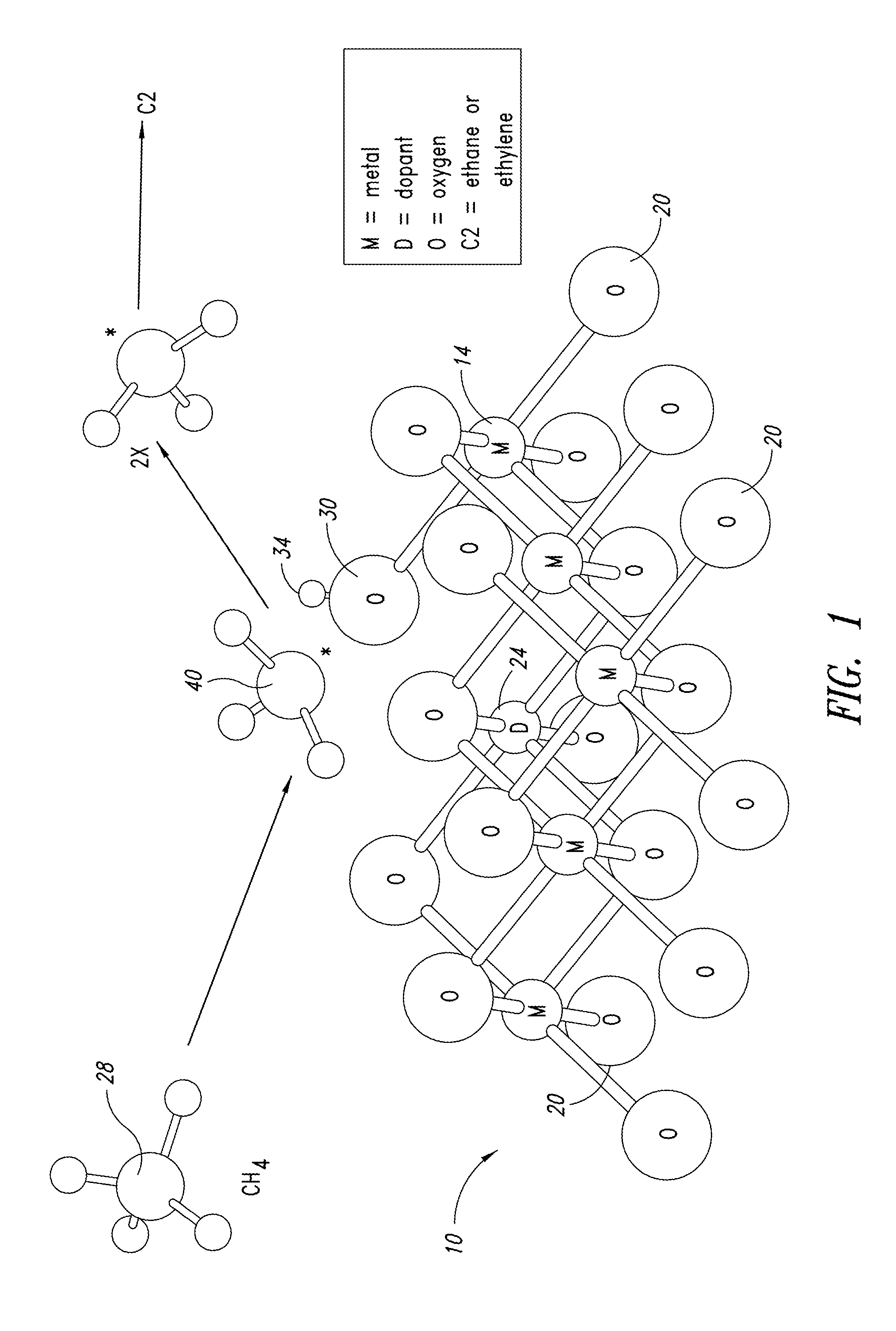
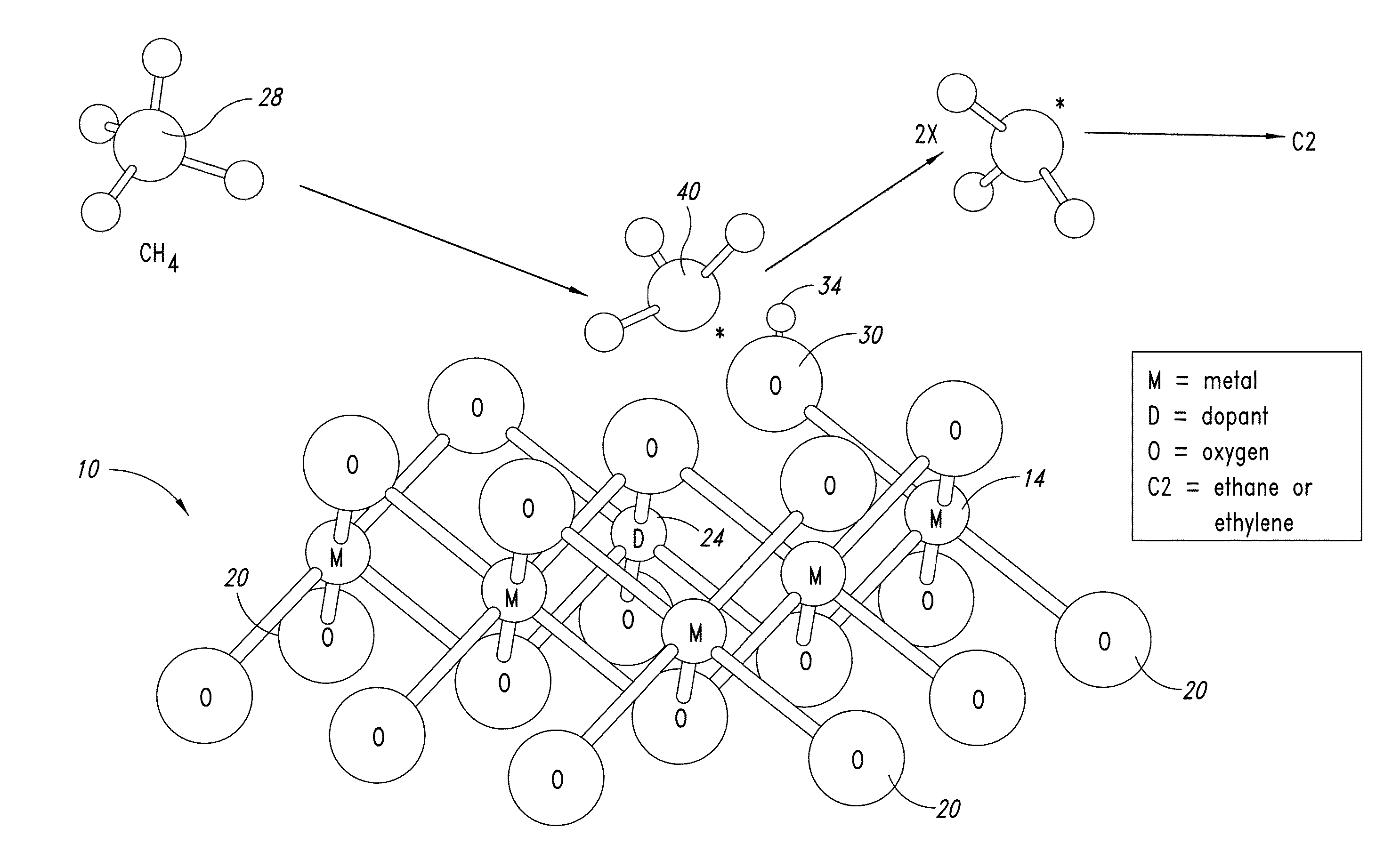
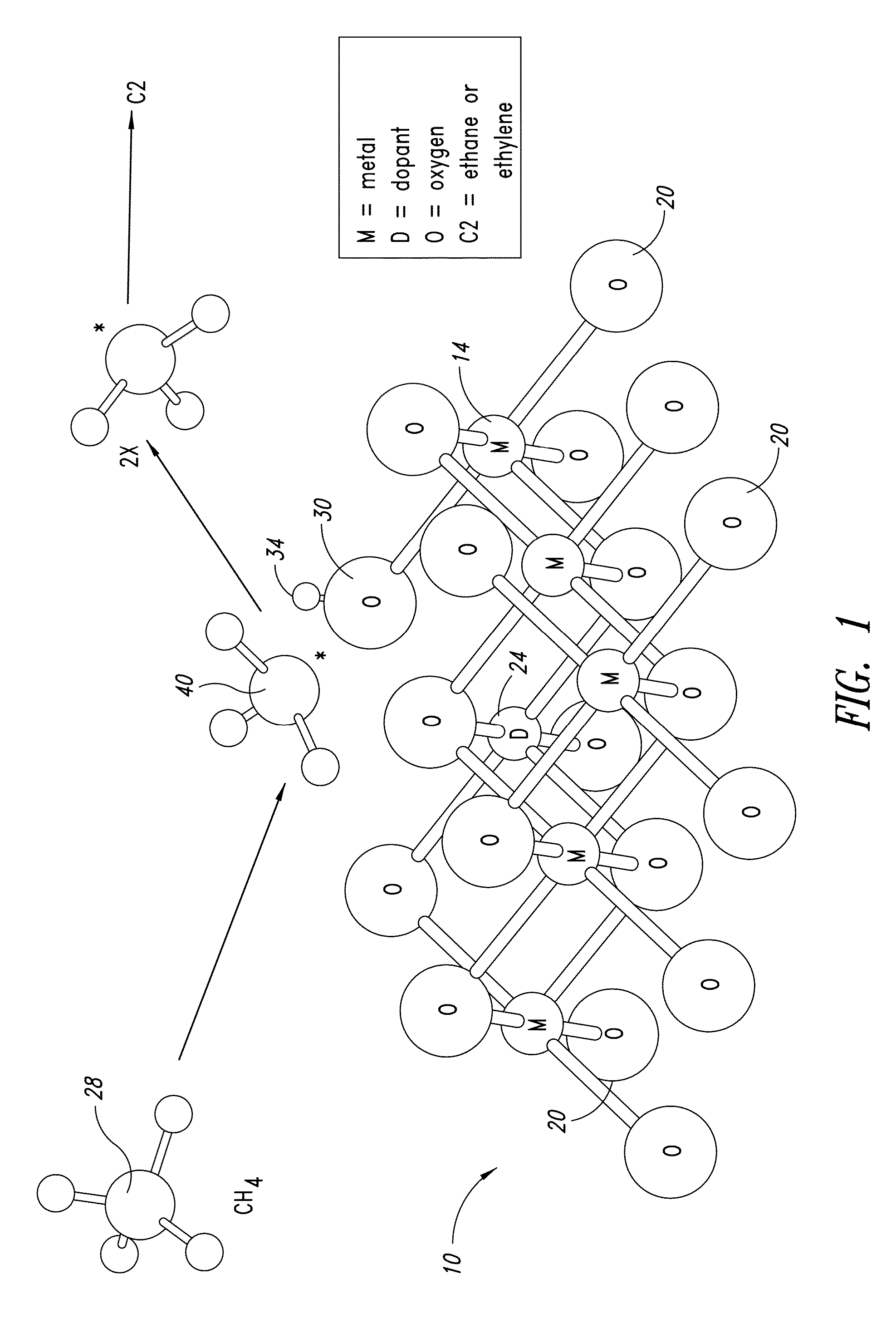
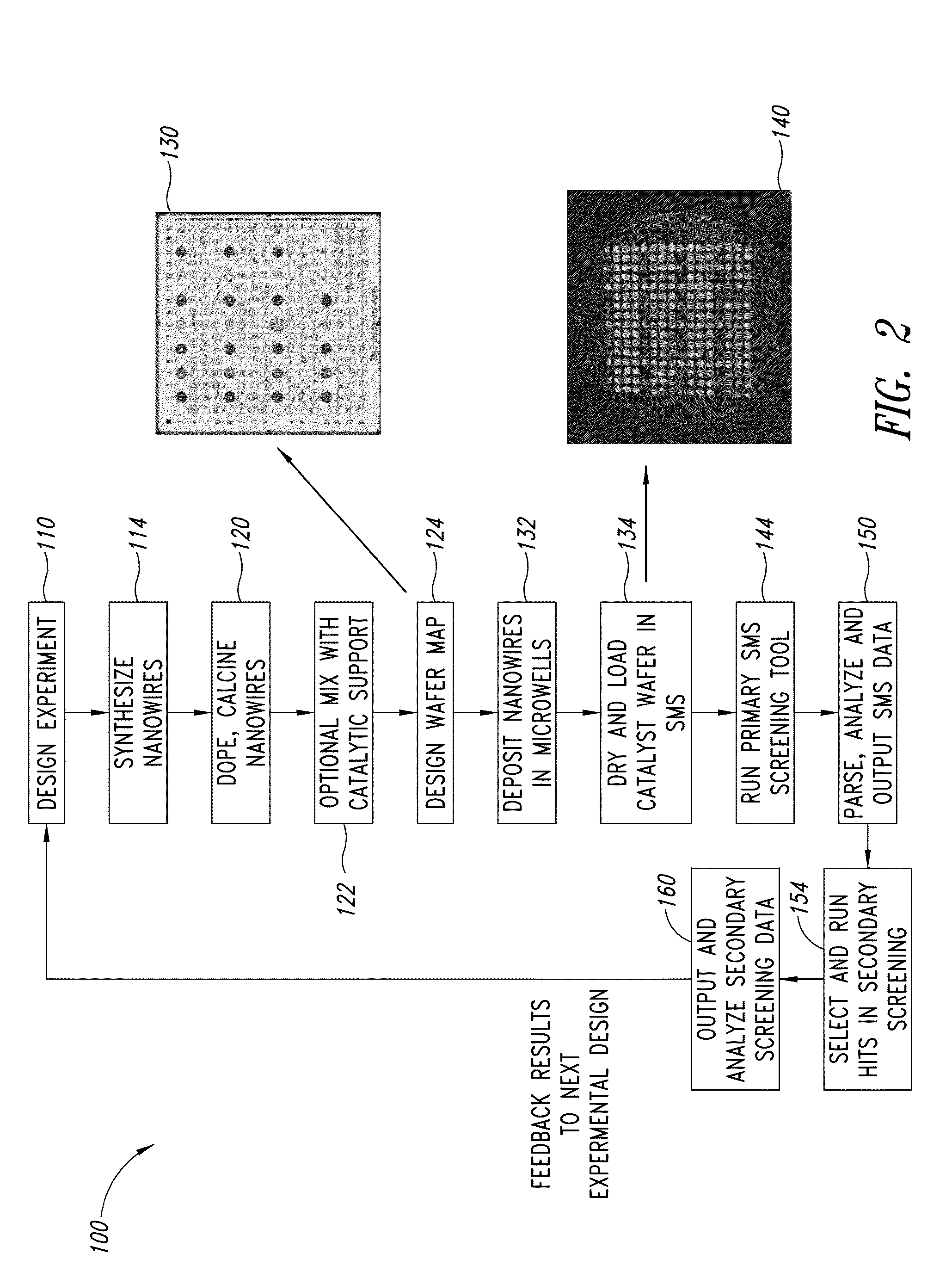
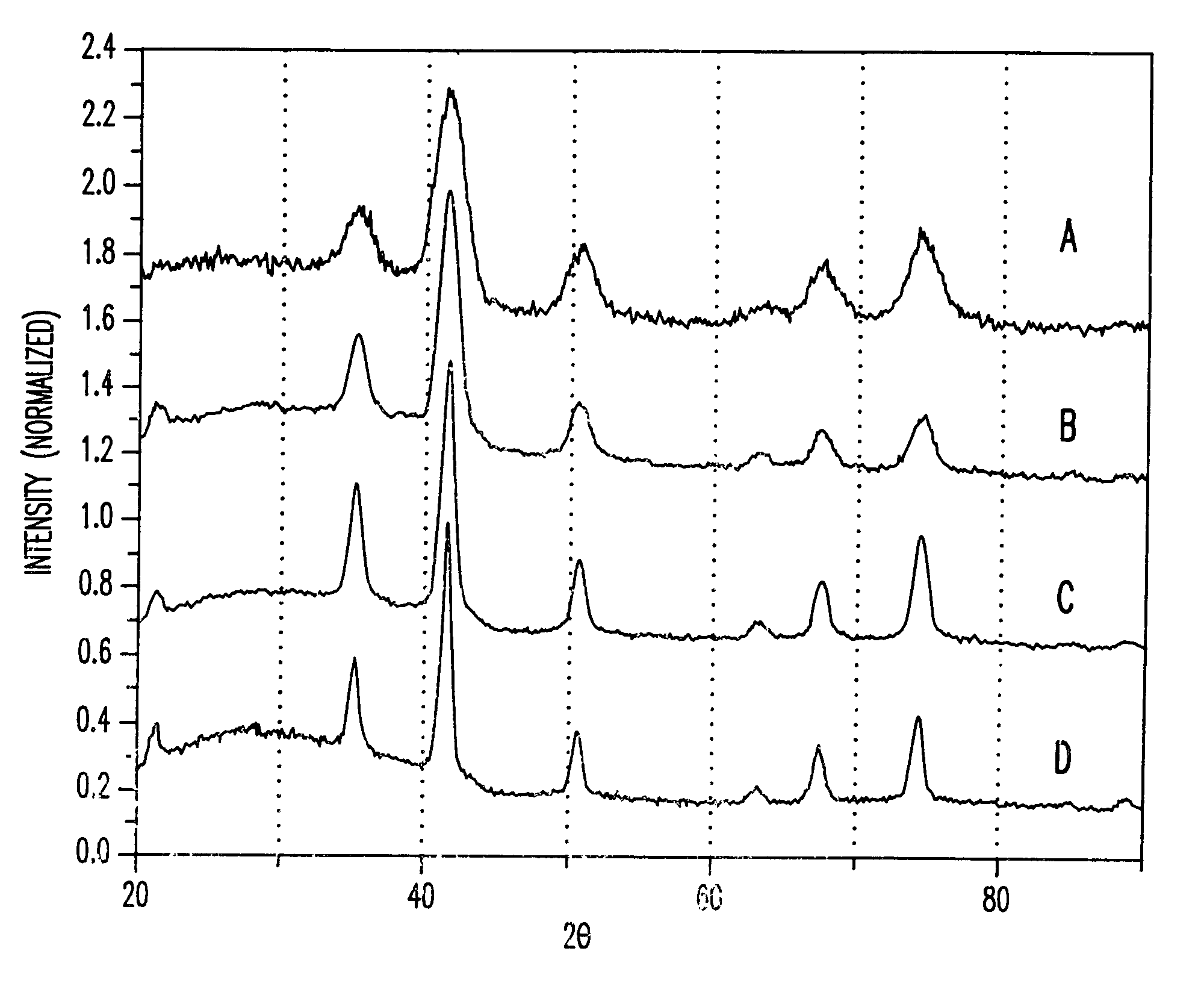
ActiveUS20130165728A1Material nanotechnologyManganese oxides/hydroxidesNanowireOxidative coupling of methane
Nanowires useful as heterogeneous catalysts are provided. The nanowire catalysts are useful in a variety of catalytic reactions, for example, the oxidative coupling of methane to C2 hydrocarbons. Related methods for use and manufacture of the same are also disclosed.
Owner:LUMMUS TECH LLC
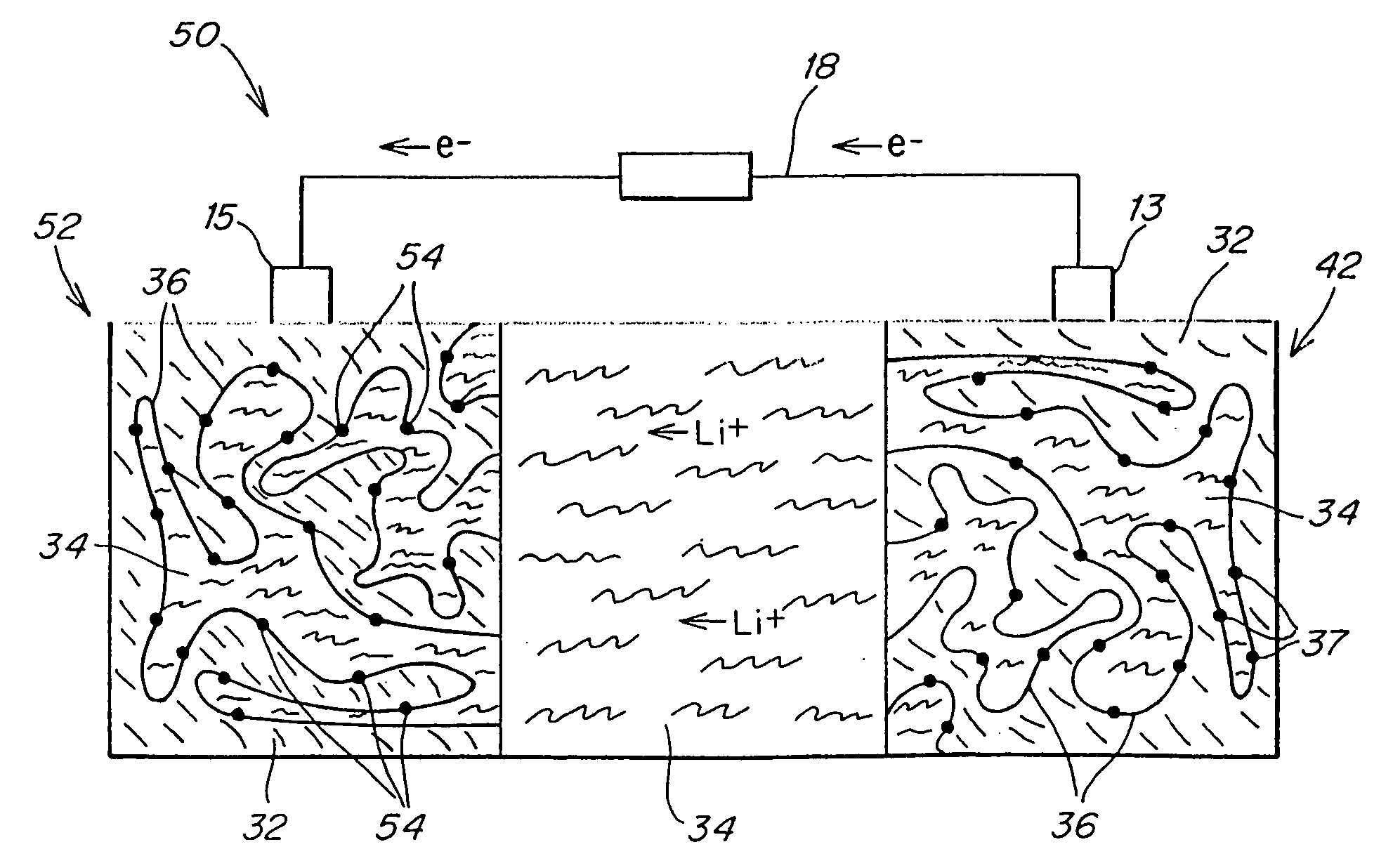
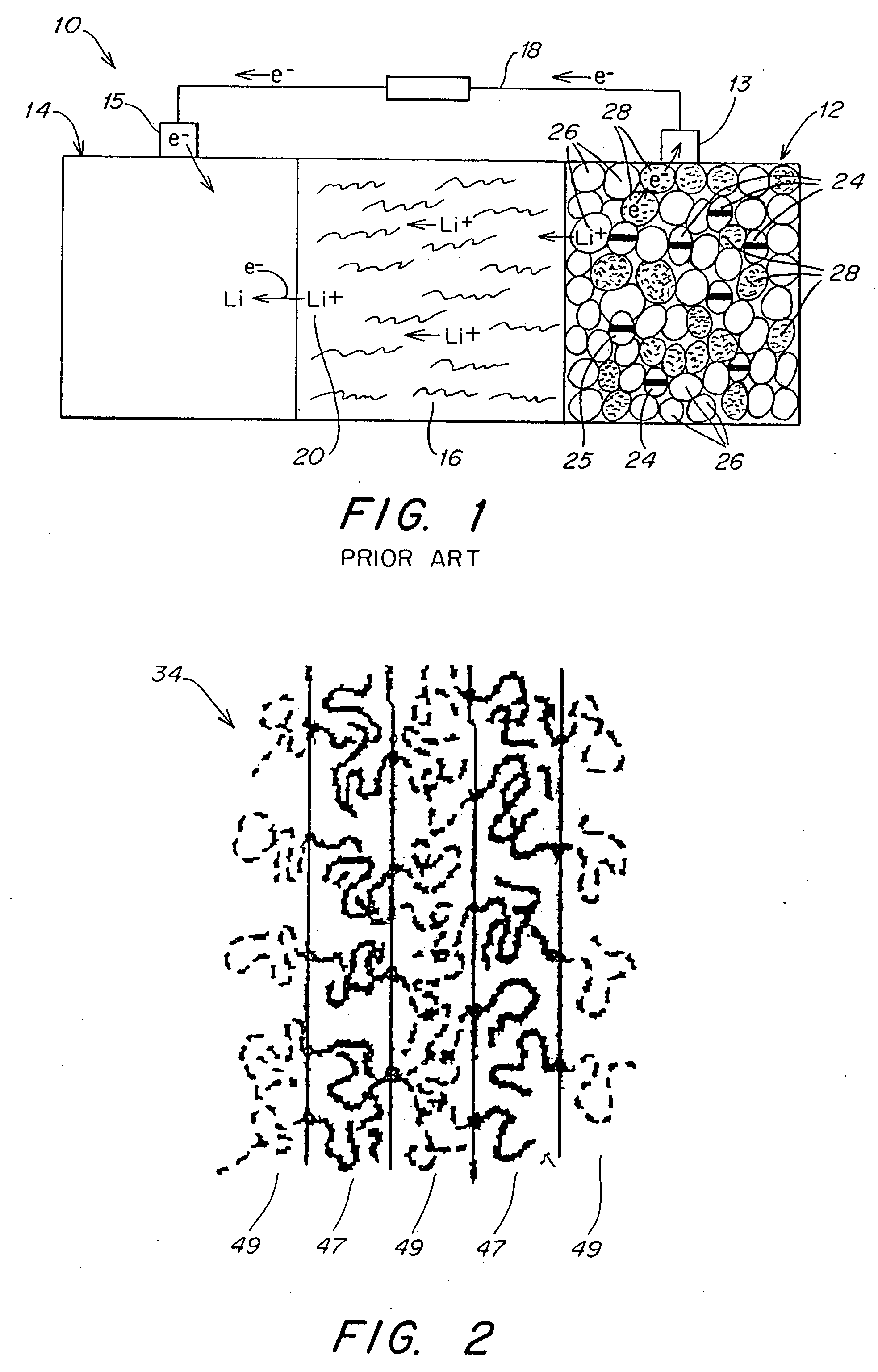
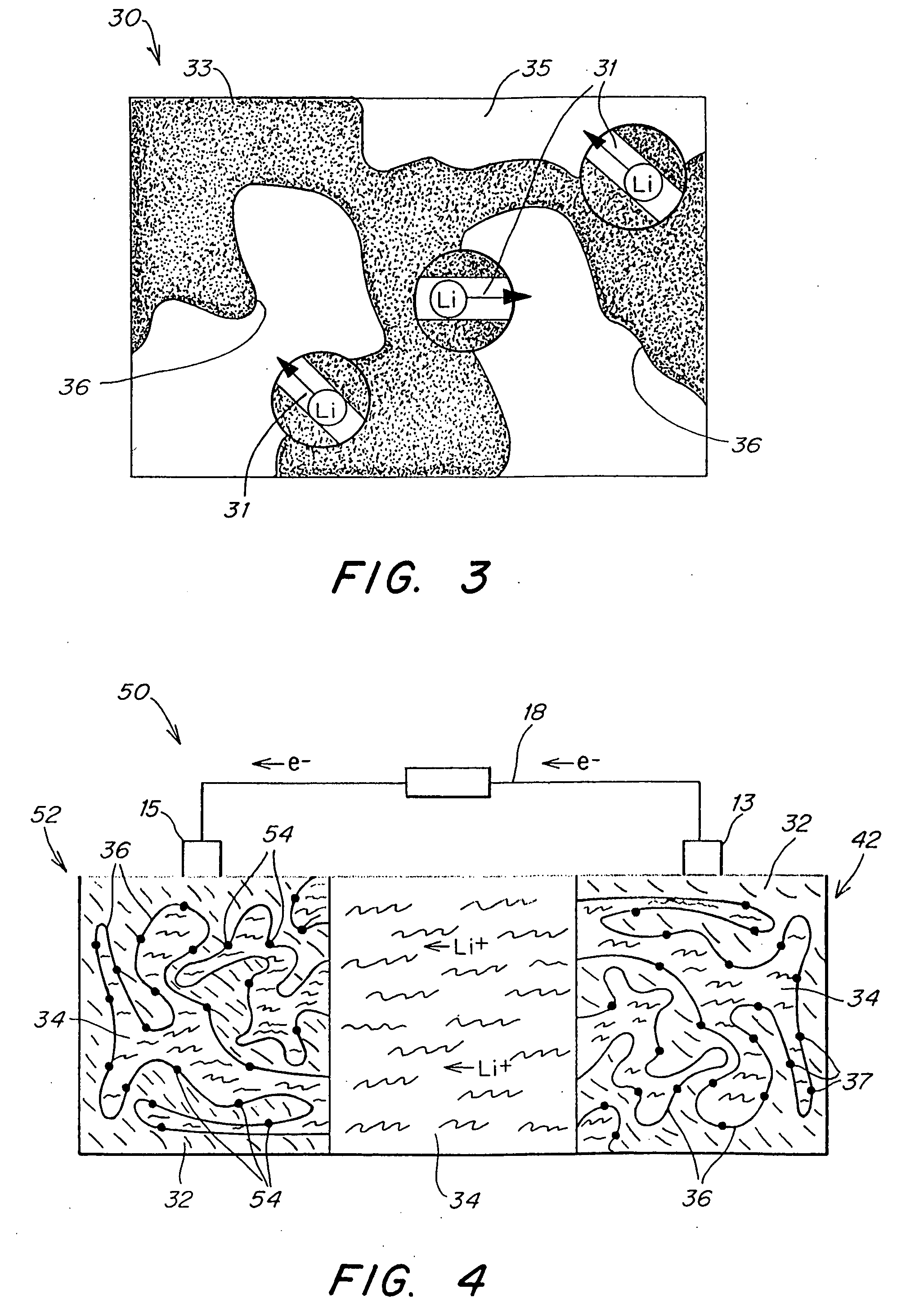
Polymer electrolyte, intercalation compounds and electrodes for batteries
Solid battery components are provided. A block copolymeric electrolyte is non-crosslinked and non-glassy through the entire range of typical battery service temperatures, that is, through the entire range of at least from about 0° C. to about 70° C. The chains of which the copolymer is made each include at least one ionically-conductive block and at least one second block immiscible with the ionically-conductive block. The chains form an amorphous association and are arranged in an ordered nanostructure including a continuous matrix of amorphous ionically-conductive domains and amorphous second domains that are immiscible with the ionically-conductive domains. A compound is provided that has a formula of LixMyNzO2. M and N are each metal atoms or a main group elements, and x, y and z are each numbers from about 0 to about 1. y and z are chosen such that a formal charge on the MyNz portion of the compound is (4-x). In certain embodiments, these compounds are used in the cathodes of rechargeable batteries. The present invention also includes methods of predicting the potential utility of metal dichalgogenide compounds for use in lithium intercalation compounds. It also provides methods for processing lithium intercalation oxides with the structure and compositional homogeneity necessary to realize the increased formation energies of said compounds. An article is made of a dimensionally-stable, interpenetrating microstructure of a first phase including a first component and a second phase, immiscible with the first phase, including a second component. The first and second phases define interphase boundaries between them, and at least one particle is positioned between a first phase and a second phase at an interphase boundary. When the first and second phases are electronically-conductive and ionically-conductive polymers, respectively, and the particles are ion host particles, the arrangement is an electrode of a battery.
Owner:MASSACHUSETTS INST OF TECH
Polymer templated nanowire catalysts
InactiveUS20130158322A1Improve drawing legibilityMaterial nanotechnologyManganese oxides/hydroxidesNanowirePolymer science
Nanowires useful as heterogeneous catalysts are provided. The nanowire catalysts are prepared by polymer templated methods and are useful in a variety of catalytic reactions, for example, the oxidative coupling of methane to ethane and / or ethylene. Related methods for use and manufacture of the same are also disclosed.
Owner:SILURIA TECH INC
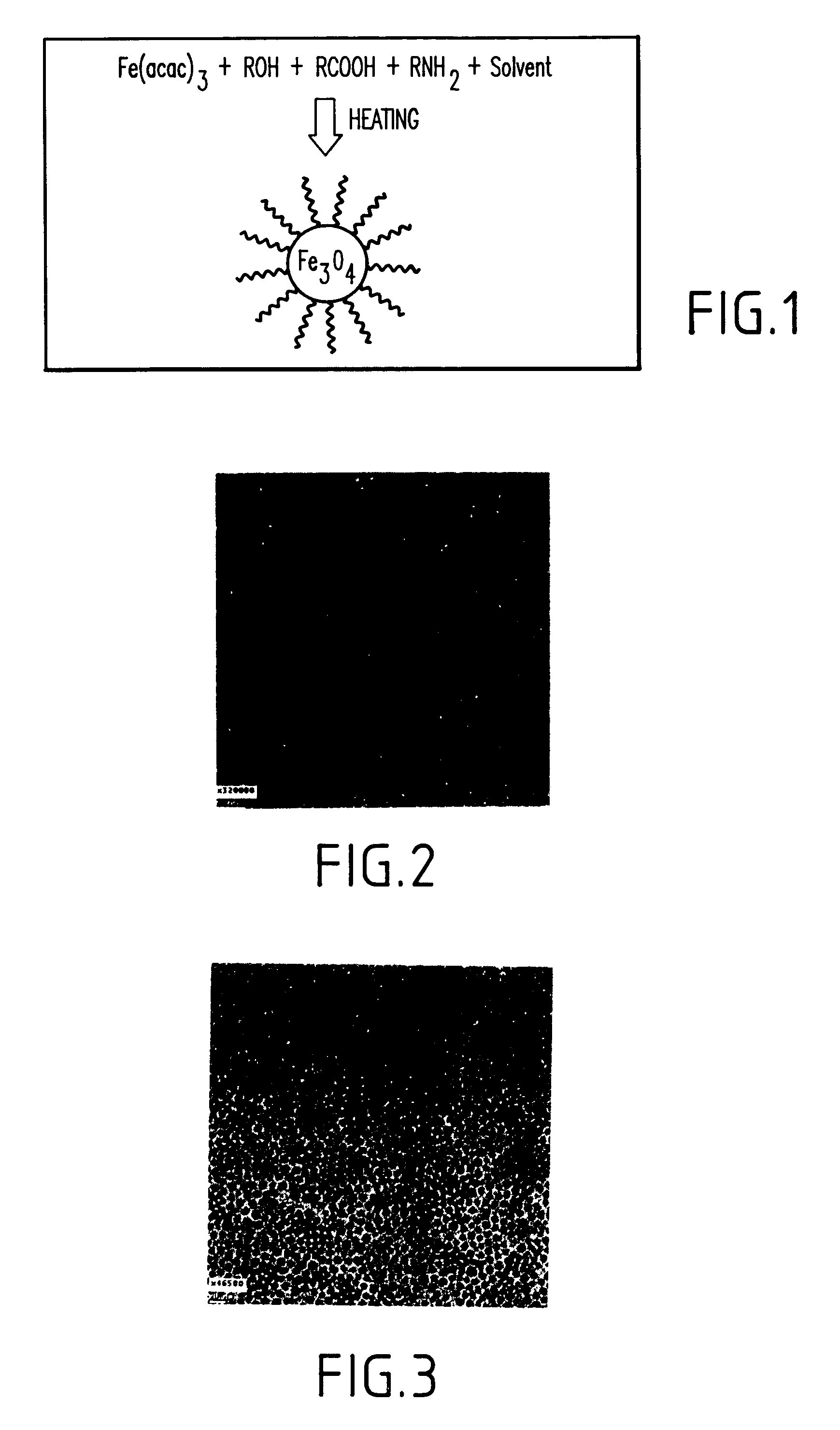
Synthesis of magnetite nanoparticles and the process of forming Fe-based nanomaterials
InactiveUS6962685B2Small sizeNarrow size distributionMaterial nanotechnologyNanomagnetismIron saltsMagnetite Nanoparticles
A method and structure for making magnetite nanoparticle materials by mixing iron salt with alcohol, carboxylic acid and amine in an organic solvent and heating the mixture to 200–360 C is described. The size of the particles can be controlled either by changing the iron salt to acid / amine ratio or by coating small nanoparticles with more iron oxide. Magnetite nanoparticles in the size ranging from 2 nm to 20 nm with a narrow size distribution are obtained with the invention. The invention can be readily extended to other iron oxide based nanoparticle materials, including M Fe2O4 (M=Co, Ni, Cu, Zn, Cr, Ti, Ba, Mg) nanomaterials, and iron oxide coated nanoparticle materials. The invention also leads to the synthesis of iron sulfide based nanoparticle materials by replacing alcohol with thiol in the reaction mixture. The magnetite nanoparticles can be oxidized to γ-Fe2O3, or α-Fe2O3, or can be reduced to bcc-Fe nanoparticles, while iron oxide based materials can be used to make binary iron based metallic nanoparticles, such as CoFe, NiFe, and FeCoSmx nanoparticles.
Owner:INT BUSINESS MASCH CORP
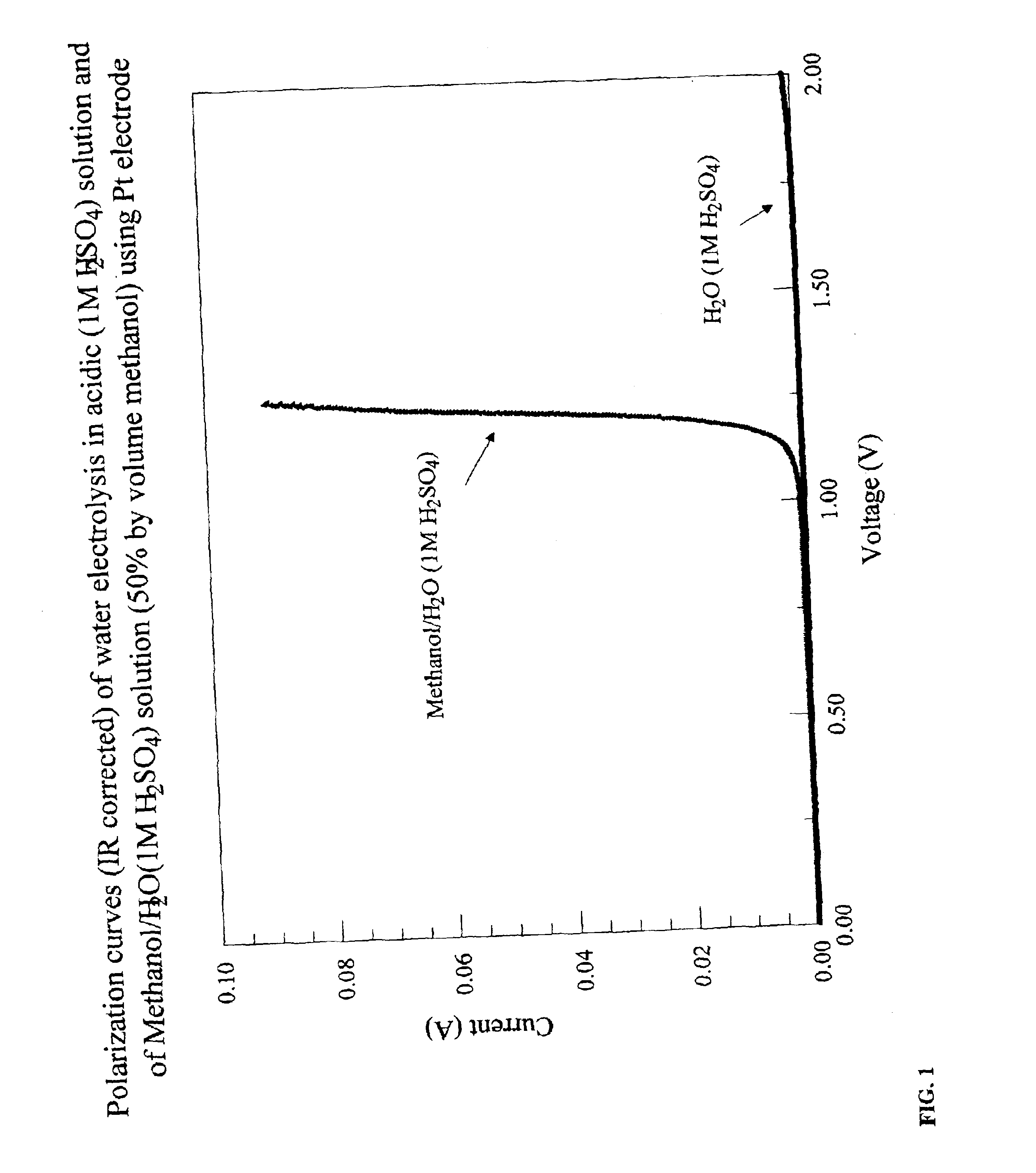
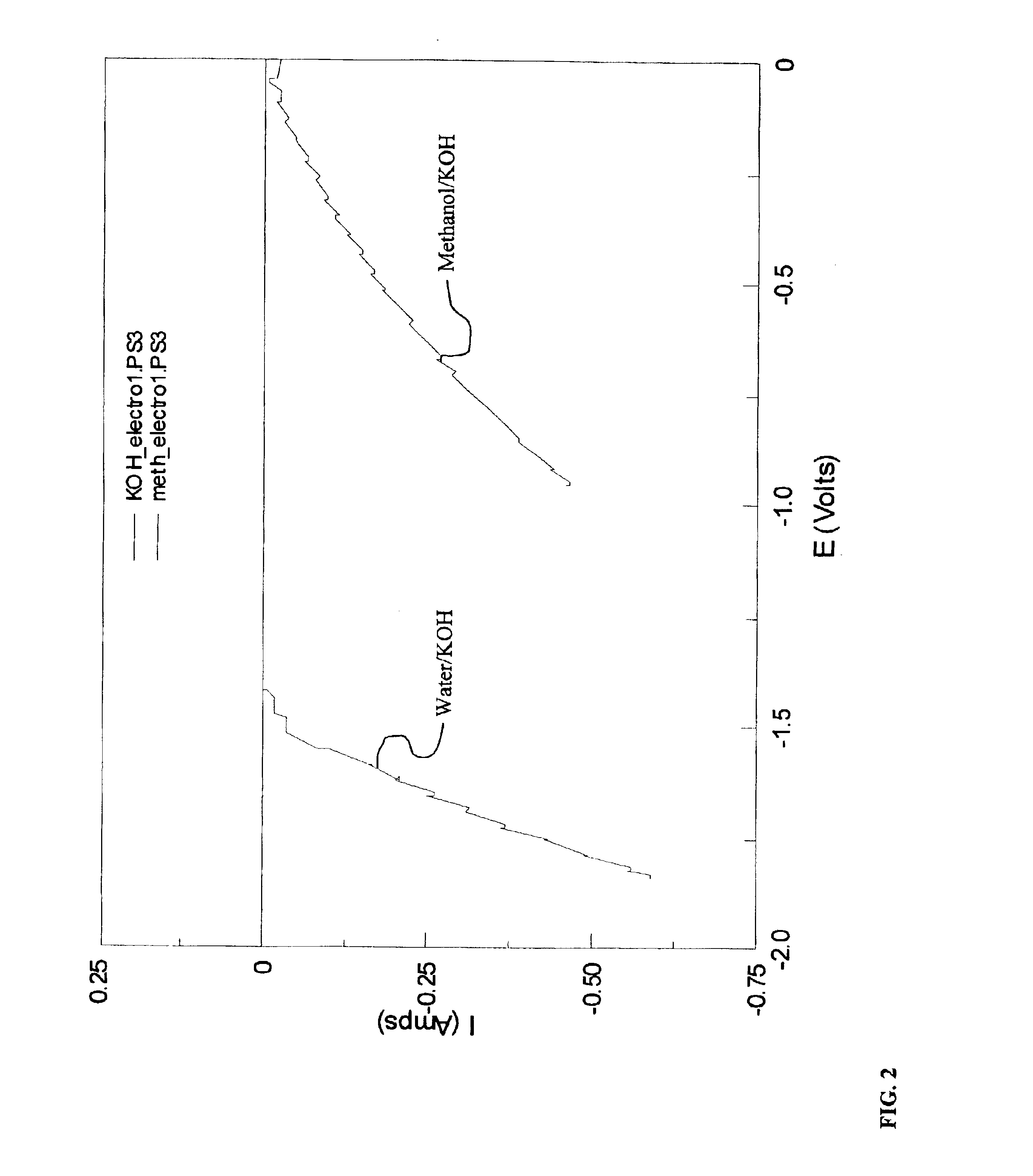
Electrolytic production of hydrogen
InactiveUS6890419B2Oxide/hydroxide preparationOrganic-compounds/hydrides/coordination-complexes catalystsElectrochemical responseElectrolysis
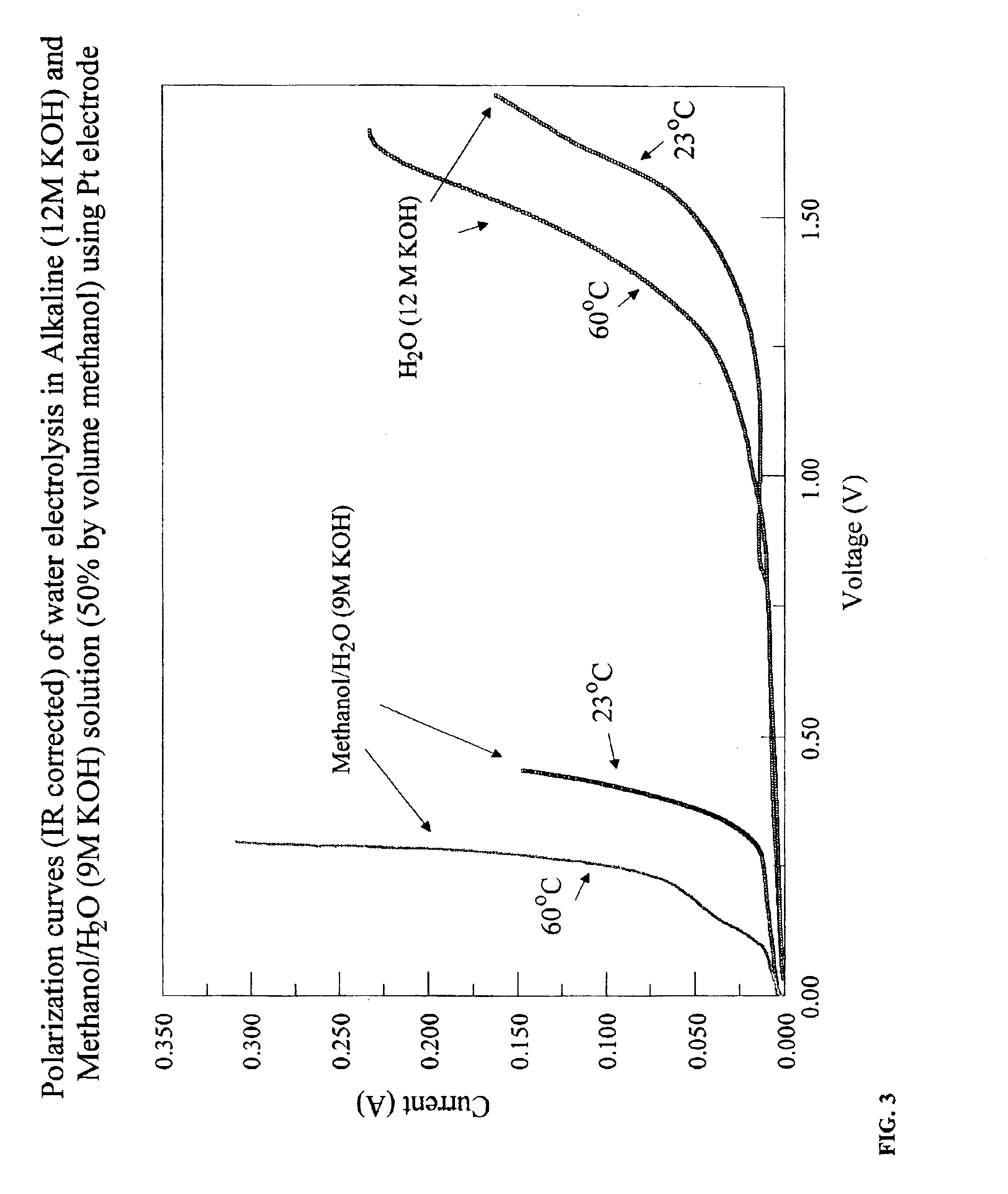
Methods for producing hydrogen gas from organic substances. According to the methods, hydrogen is produced from an electrochemical reaction of an organic substance with water or a base. The instant methods permit the production of hydrogen at lower operating voltages or lower operating temperatures relative to water electrolysis. Operable organic substances include alcohols, ethers, carboxylic acids, aldehydes, and ketones. In a preferred embodiment, hydrogen gas is produced from an electrochemical reaction of methanol in the presence of a base such as NaOH or KOH.
Owner:TACTICAL FUEL CELLS
Niobium suboxide powder
ActiveUS7381396B2Reduce residual currentReduce volatilityOxide/hydroxide preparationLiquid electrolytic capacitorsTungstenSuboxide
A niobium suboxide powder comprising 100 to 600 ppm of magnesium is described. The niobium suboxide powder may (alternatively or in addition to 100 to 600 ppm of magnesium) further include 50 to 400 ppm of molybdenum and / or tungsten. The niobium suboxide powder is suitable for the production of: capacitors having an insulator layer of niobium pentoxide; capacitor anodes produced from the niobium suboxide powder; and corresponding capacitors.
Owner:TANIOBIS GMBH
Lithium metal oxide materials and methods of synthesis and use
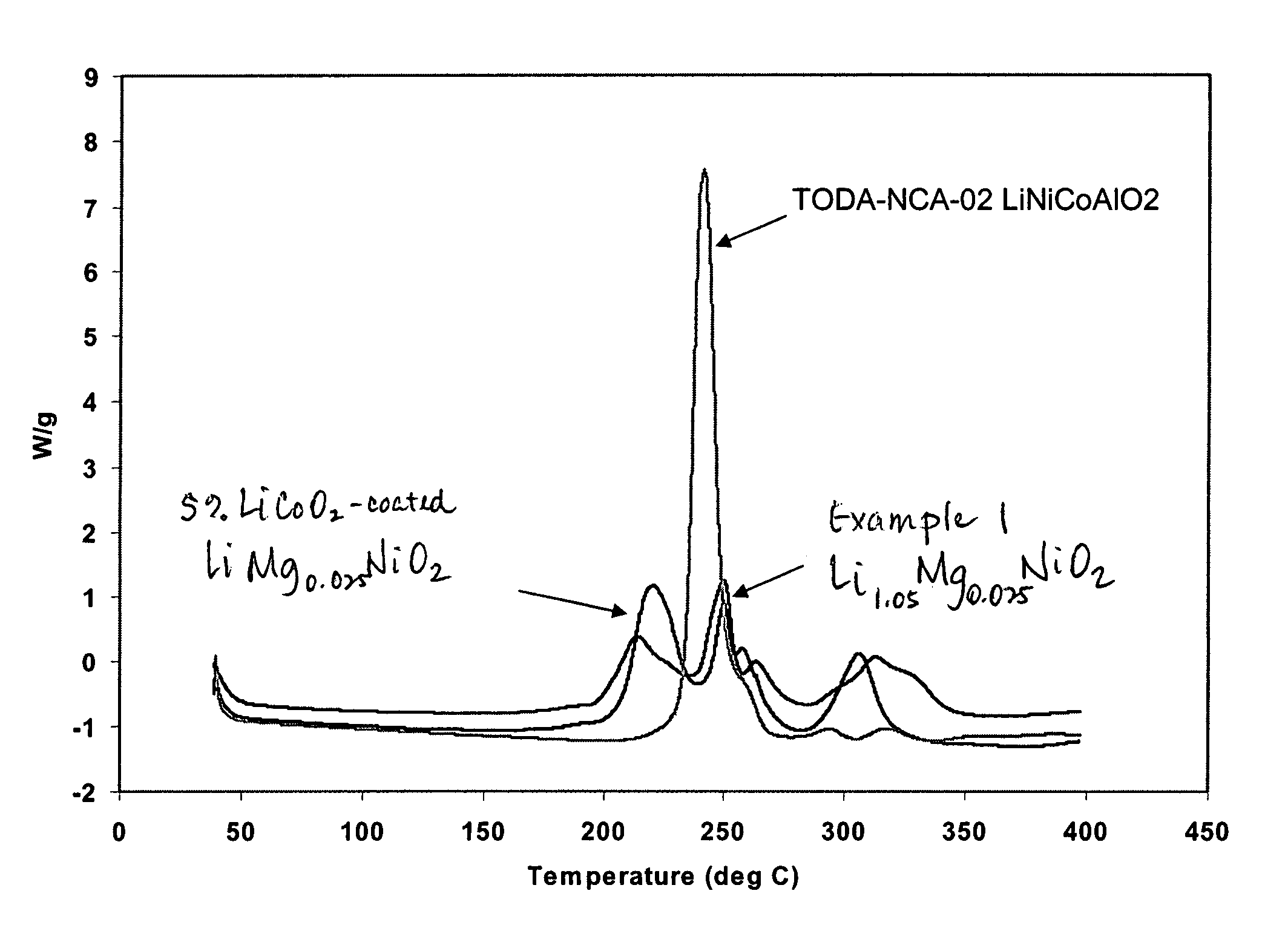
ActiveUS7381496B2Large capacityImprove cycle lifeOxide/hydroxide preparationNickel compounds preparationLithium metalElectrochemical cell
A composition having a formula LixMgyNiO2 wherein 0.9<x<1.3, 0.01<y<0.1, and 0.91<x+y<1.3 can be utilized as cathode materials in electrochemical cells. A composition having a core, having a formula LixMgyNiO2 wherein 0.9<x<1.3, 0.01<y<0.1, and 0.9<x+y<1.3, and a coating on the core, having a formula LiaCobO2 wherein 0.7<a<1.3, and 0.9<b<1.2, can also be utilized as cathode materials in electrochemical cells.
Owner:TIAX LLC
Nanostructured fuel cell electrode
A fuel cell includes an electrolyte, a first electrode, and a second electrode. At least the first electrode comprises a nanostructured material.
Owner:BLOOM ENERGY CORP
Metal oxide particle and its uses
InactiveUS20070154561A1High transparencyPromote absorptionMaterial nanotechnologyBiocideAcyl groupUltraviolet absorption
An object of the present invention is to provide a metal oxide particle which exercises more excellent ultraviolet absorbency as a matter of course and combines therewith merits of, for example, either being shifted in ultraviolet absorption edge toward the longer wavelength side and being excellent also in the absorption efficiency of a long-wavelength range of ultraviolet rays, or having good transparency and, for example, even in cases where added into or coated onto substrates, not damaging the transparency or hue of the substrates. As a means of achieving this object, a metal oxide particle according to the present invention is a metal oxide particle such that a hetero-element is contained in a particle comprising an oxide of a specific metal element (M), wherein the metal oxide particle is: 1) a metal oxide particle in the form of a fine particle wherein the hetero-element is at least one specific metal element (M′); 2) a metal oxide particle wherein the hetero-element includes at least two specific metal elements (M′); 3) a metal oxide particle wherein: the hetero-element is a more specified metal element (M′) and at least a part thereof is 2 in valence; or the metal element (M) is a more specified metal element and the metal oxide particle is in a specific range in crystal grain diameter in the vertical direction to each of the (002) plane and the (100) plane; or 4) a metal oxide particle wherein: the hetero-element is at least one specific nonmetal element and an acyl group is contained in the particle; or the hetero-element includes at least two specific nonmetal elements; or the hetero-element is at least one specific nonmetal element and a component derived from a metal element (M′) other than the metal element (M) is contained in the particle.
Owner:NIPPON SHOKUBAI CO LTD
Separation of carbon dioxide (CO2) from gas mixtures by calcium based reaction separation (CaRS-CO2) process
A reaction-based process has been developed for the selective removal of carbon dioxide (CO2) from a multicomponent gas mixture to provide a gaseous stream depleted in CO2 compared to the inlet CO2 concentration in the stream. The proposed process effects the separation of CO2 from a mixture of gases (such as flue gas / fuel gas) by its reaction with metal oxides (such as calcium oxide). The Calcium based Reaction Separation for CO2 (CaRS-CO2) process consists of contacting a CO2 laden gas with calcium oxide (CaO) in a reactor such that CaO captures the CO2 by the formation of calcium carbonate (CaCO3). Once “spent”, CaCO3 is regenerated by its calcination leading to the formation of fresh CaO sorbent and the evolution of a concentrated stream of CO2. The “regenerated” CaO is then recycled for the further capture of more CO2. This carbonation-calcination cycle forms the basis of the CaRS-CO2 process. This process also identifies the application of a mesoporous CaCO3 structure, developed by a process detailed elsewhere, that attains >90% conversion over multiple carbonation and calcination cycles. Lastly, thermal regeneration (calcination) under vacuum provided a better sorbent structure that maintained reproducible reactivity levels over multiple cycles.
Owner:THE OHIO STATES UNIV
Methods of fabricating nanoscale-to-microscale structures
InactiveUS20080038170A1Attractive and optical propertyAttractive mechanical propertySilicaMixing methodsChemical compositionChemical reaction
Methods for the production of shaped nanoscale-to-microscale structures, wherein a nanoscale-to-microscale template is provided having an original chemical composition and an original shape, and the nanoscale-to-microscale template subjected to a chemical reaction, so as to partially or completely convert the nanoscale-to-microscale template into the shaped nanoscale-to-microscale structure having a chemical composition different than the original chemical composition and having substantially the same shape as the original shape, being a scaled version of the original shape. The shaped nanoscale-to-microscale structure formed comprises an element (such as silicon), a metallic alloy (such as a silicon alloy), or a non-oxide compound (such as silicon carbide or silicon nitride).
Owner:GEORGIA TECH RES CORP
Production Of Barium Titanate Compounds
InactiveUS20070202036A1High purityLow costMaterial nanotechnologyAlkaline earth titanatesBarium titanateSpherical shaped
An ultrafine powder of barium titanate including solid solutions and doped compounds that meets up to specific characteristics is produced by method comprising two main steps. The first step is a reaction, typically in a Segmented Flow Tubular Reactor, between reactants to produce cubic-structure barium titanate composed of non-agglomerated ultrafine particles having a shape of given aspect ratio, usually a generally spherical shape, of low density corresponding at most to 90% of the intrinsic density, all particles being smaller than 1 micron and having a narrow particle size distribution and wherein the ratio of Ba:Ti including substitutents and dopants is very close to the ideal stoichiometry. This is followed by subjecting the powder produced in the first step to a second stage solvothermal post treatment typically in an autoclave at temperature less than 400° C. to convert the cubic-structure particles of low density to ultrafine tetragonal particles of increased density corresponding to at least 90% of the intrinsic density while maintaining the same aspect ratio, and maintaining the size of all particles below 1 micron, the narrow particle size distribution span, and the given ideal stoichiometry. The produced particles can have a non-spherical facetted shape such as cube-like.
Owner:JONGEN NATHALIE +1
Multi-shell-layer metal oxide hollow ball and preparation method thereof
ActiveCN102464304AHigh specific surface areaSimple processOxide/hydroxide preparationZinc oxides/hydroxidesControllabilityNanoscopic scale
The invention provides a multi-shell-layer metal oxide hollow ball and a preparation method thereof. A hydrothermal method is used for preparing a carbon ball template; metal salts are dissolved in carbon ball suspension liquid, and the gradient distribution, the depth and the number of metal salts entering carbon balls are controlled through regulating adsorption conditions such as metal salt concentration, solution pH value, soaking temperature and time and the like; and the heat treatment is carried out on the carbon balls adsorbing metal ions, and the multi-shell-layer metal oxide hollow ball can be obtained. The shell-layer of the hollow ball prepared by the method is formed by accumulating nanometer crystal particles of metal oxides, the shell layer number can be regulated and changed from two to four, and both the size of the hollow ball and the thickness of the shell layers are controllable. The method provided by the invention is simple and is easy to implement, the controllability is high, the pollution is little, the cost is low, and in addition, the general applicability is realized. The prepared product has a hollow structure and the shell layers with the thickness inthe nanometer level, simultaneously, the internal space can be effectively utilized through the multilayer structure, and the multi-shell-layer metal oxide hollow ball is applied to gas sensitivity and photocatalysis and has the more excellent performance through being compared with the traditional nanometer material and a single-layer hollow ball.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Flame-Retardant Magnesium Hydroxide Compositions and Associated Methods of Manufacture and Use
InactiveUS20070176155A1Improve resistance performanceSuitable for usePigmenting treatmentGlass/slag layered productsParticulatesPolymer resin
The invention provides a submicron magnesium hydroxide particulate composition comprising a first distribution of magnesium hydroxide particles having a D50 of no more than about 0.30 μm, a D90 of no more than about 1.5 μm, and a BET surface area of at least about 35 m2 / g, which can be used as a flame-retardant additive for synthetic polymers, optionally in combination with other flame-retardant additives such as nanoclays and larger-sized magnesium hydroxide particulate compositions. Polymeric resins comprising the submicron magnesium hydroxide particles and methods of manufacturing submicron magnesium hydroxide particles are also provided.
Owner:MARTIN MARIETTA MATERIALS
Carbon-coated metal oxide nanoparticles
InactiveUS6843919B2Effective airborne decontaminationProvide protectionMaterial nanotechnologySolid sorbent liquid separationMetal oxide nanoparticlesToxin
Composites for destroying chemical and biological agents such as toxins and bacteria, and methods of preparing and using those composites are provided. According to the invention, the substance to be destroyed is contacted with the inventive composites which comprise finely divided metal oxide nanoparticles at least partially coated with carbon. Advantageously, the composites exclude water while not excluding the target compound or adsorbates. The desired metal oxide nanoparticles can be pressed into pellets for use when a powder is not feasible. Preferred metal oxide nanoparticles include MgO, SrO, BaO, CaO, TiO2, ZrO2, FeO, V2O3, V2O5, Mn2O3, Fe2O3, NiO, CuO, Al2O3, SiO2, ZnO, Ag2O, and mixtures thereof.
Owner:KANSAS STATE UNIV RES FOUND
Mesostructured material incorporating particles of nanometric dimensions
InactiveUS6866925B1High crystallinityNanostructure manufactureSilicaMesoporous materialCrystallinity
The invention concerns a heat-stable ordered mesoporous or mesostructured material comprising a mineral phase wherein are dispersed particles of nanometric dimension at least partly crystaline, the global crystallinity index of said mesostructured or ordered mesoporous material being less than 10% in volume. The invention also concern a method for obtaining such a material.
Owner:RHODIA CHEM SA
Methods of fabricating nanoscale-to-microscale structures
InactiveUS7615206B2Attractive electronic and optical propertyAttractive mechanical propertySilicaMixing methodsChemical reactionChemical composition
Methods for the production of shaped nanoscale-to-microscale structures, wherein a nanoscale-to-microscale template is provided having an original chemical composition and an original shape, and the nanoscale-to-microscale template subjected to a chemical reaction, so as to partially or completely convert the nanoscale-to-microscale template into the shaped nanoscale-to-microscale structure having a chemical composition different than the original chemical composition and having substantially the same shape as the original shape, being a scaled version of the original shape. The shaped nanoscale-to-microscale structure formed comprises an element (such as silicon), a metallic alloy (such as a silicon alloy), or a non-oxide compound (such as silicon carbide or silicon nitride).
Owner:GEORGIA TECH RES CORP
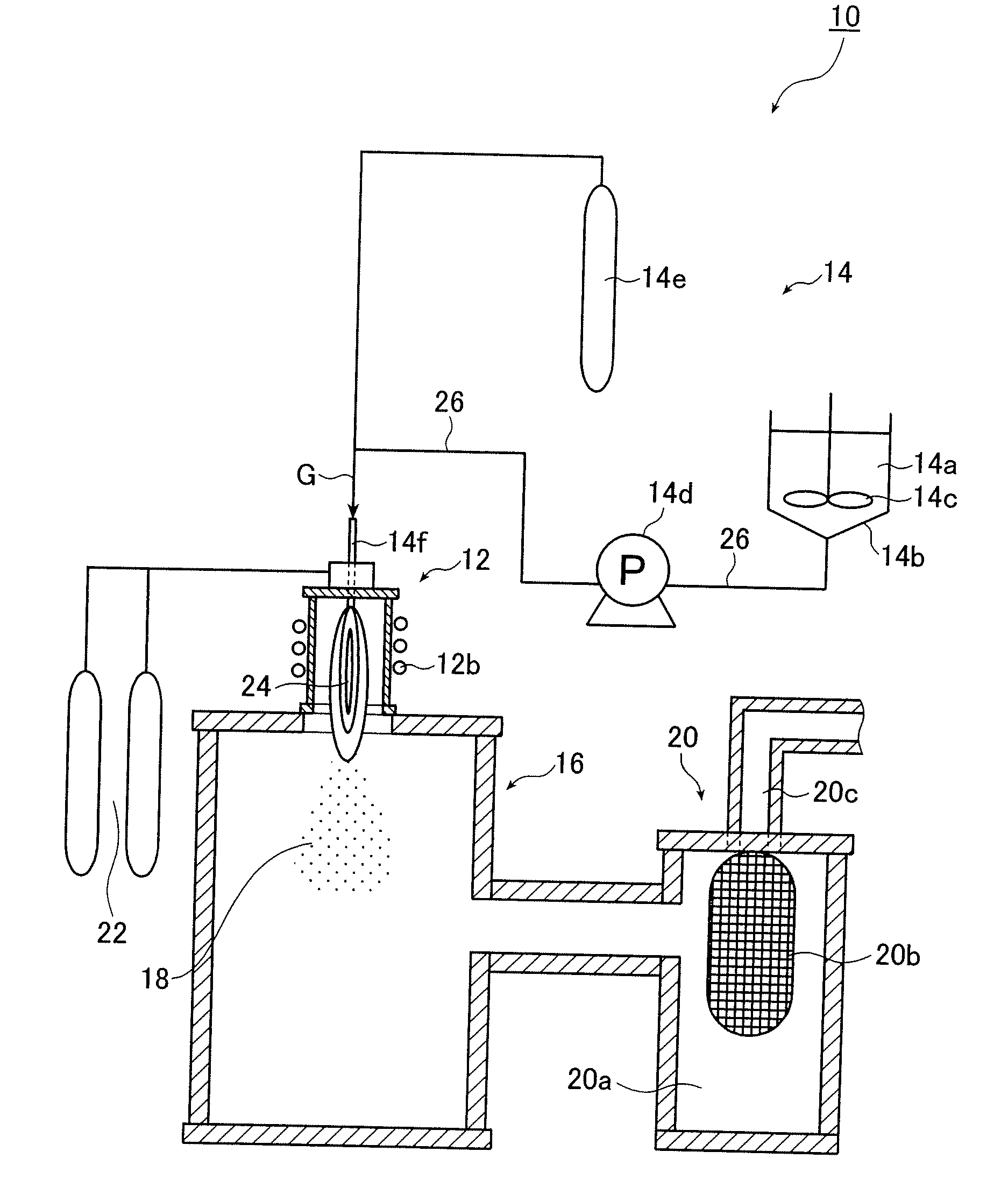
Method for forming metal oxide coating film and vapor deposition apparatus
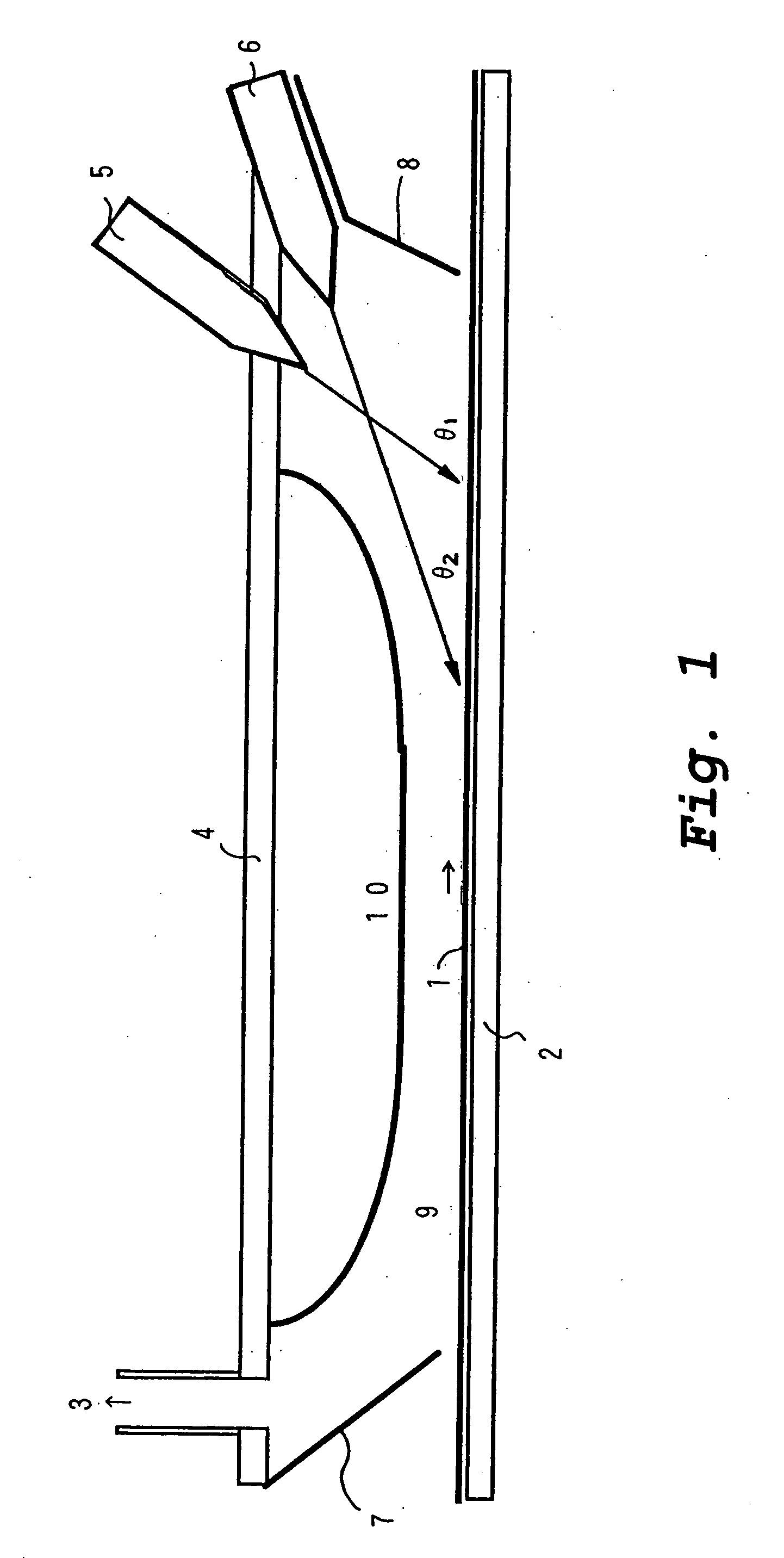
InactiveUS20070054044A1Avoid reactionEliminate the problemOxide/hydroxide preparationCatalyst activation/preparationGas phaseWater vapor
A photocatalytic composite material having a photocatalytic titanium oxide film on the surface of a substrate is produced by a CVD method in which TiCl4 vapor is reacted with water vapor. The TiCl4 vapor and the water vapor are injected into a vapor deposition chamber (9) through nozzles (5) and (6), respectively, such that the resulting two injected vapor streams meet before reaching the substrate, thereby mixing the two vapors. Within 3 seconds after this mixing, the mixed vapors are brought into contact with a substrate (1) which is moving in one direction. Preferably the TiCl4 vapor is injected in a reverse direction with respect to the direction of movement of the substrate through a multi-orifice nozzle (5), while the water vapor is injected through a slit nozzle (6) disposed at a smaller angle with respect to the substrate.
Owner:OSAKA TITANIUM TECHNOLOGIES
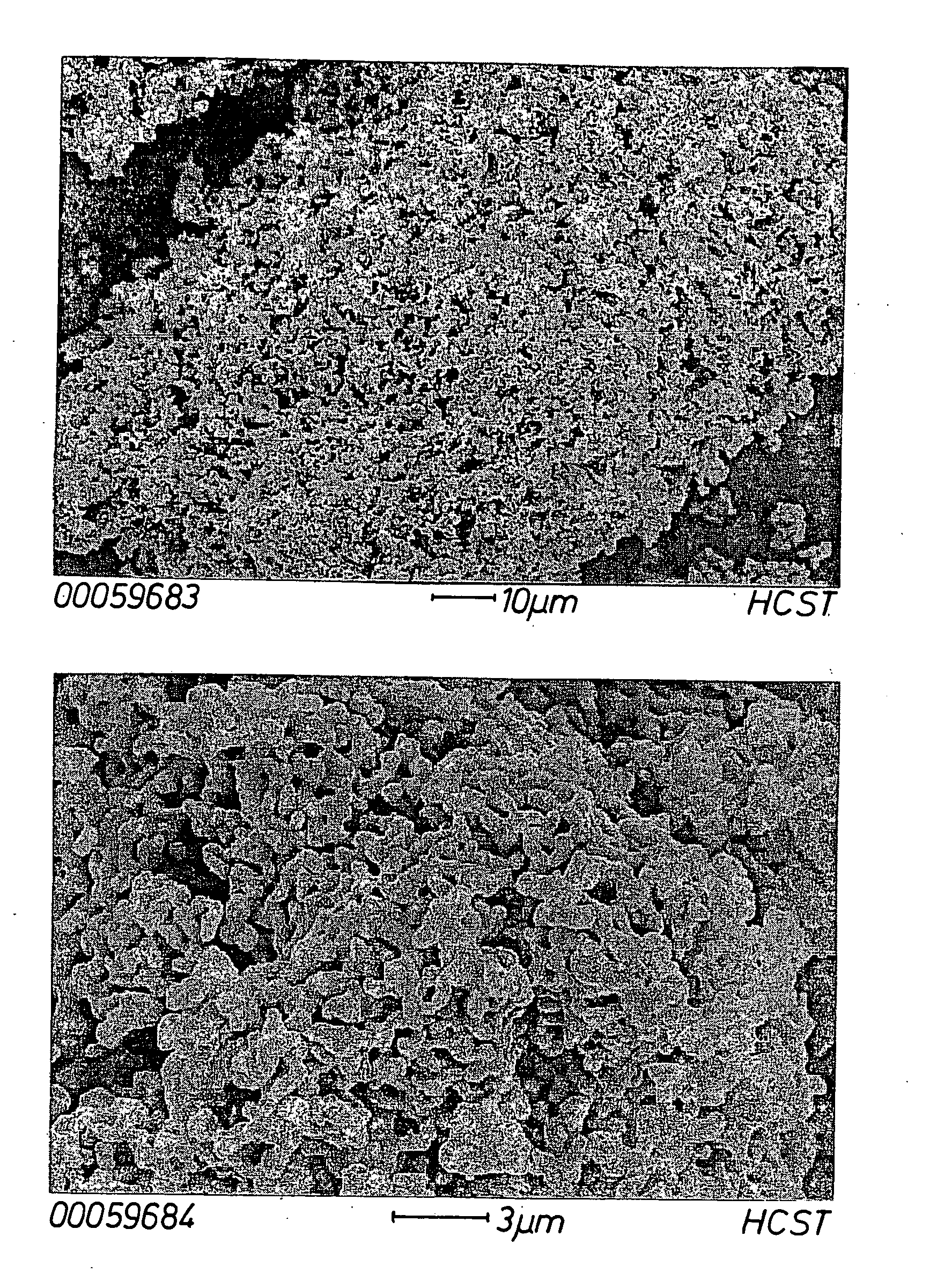
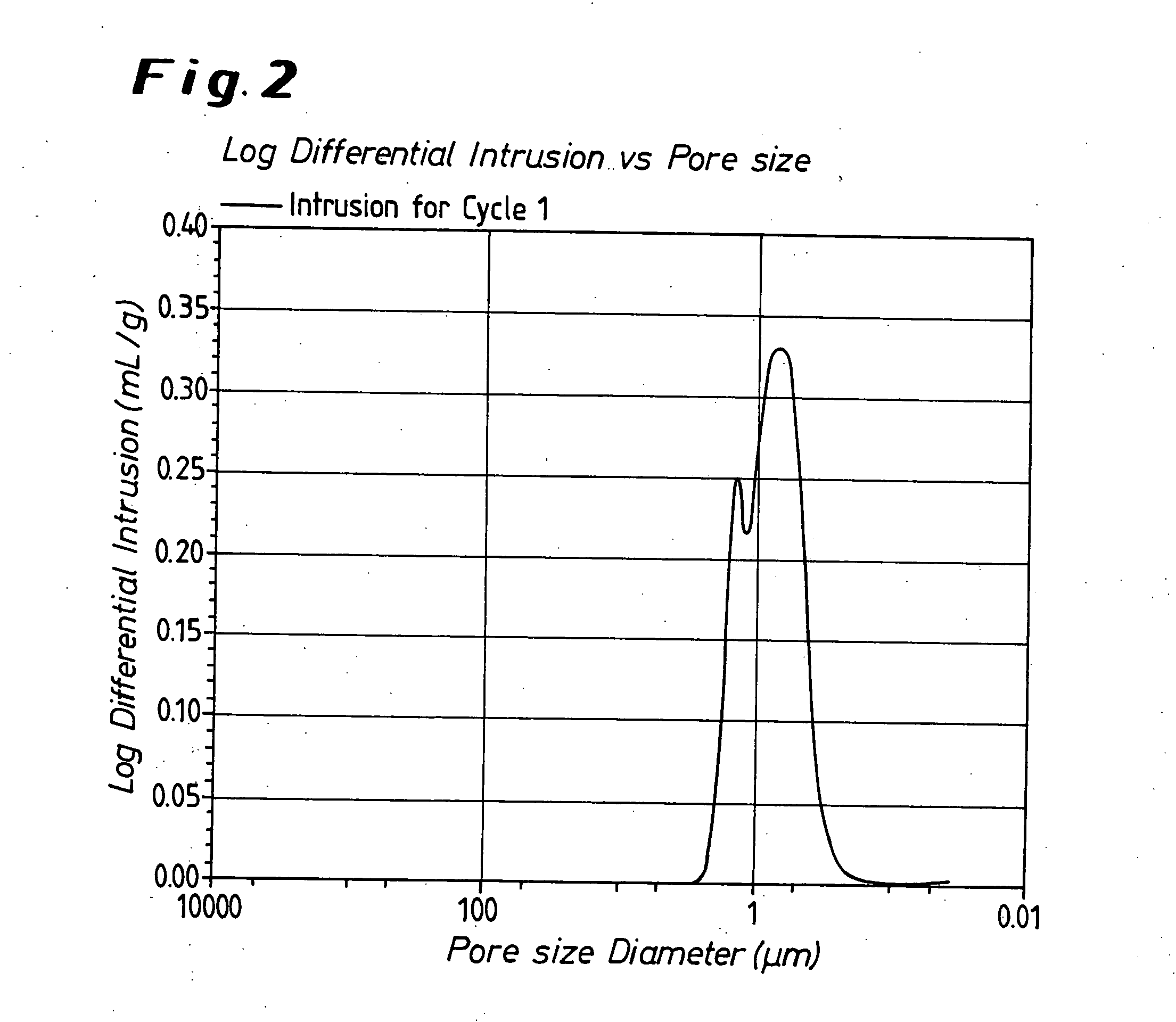
Porous 4 group metal oxide and method for preparation thereof
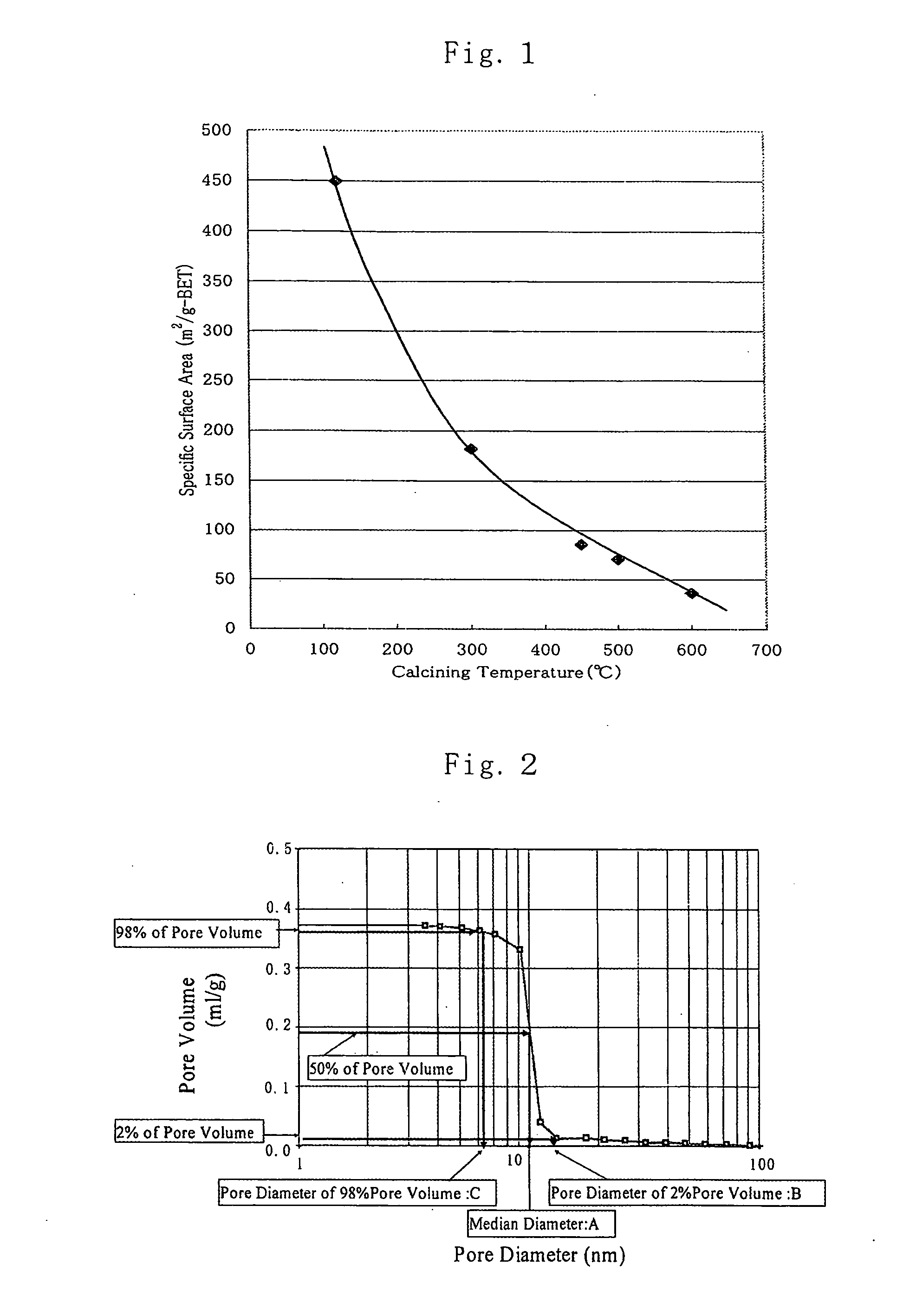
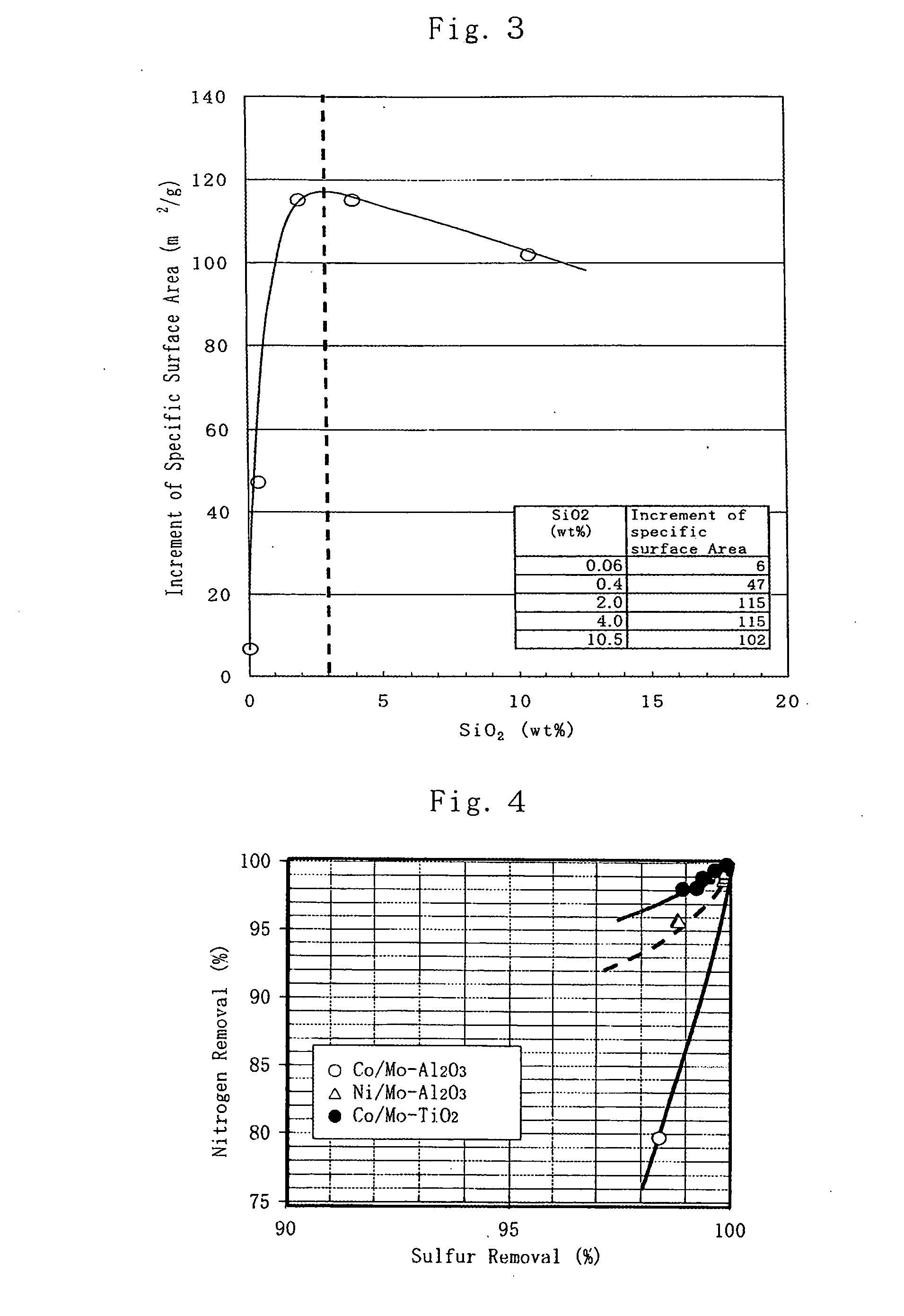
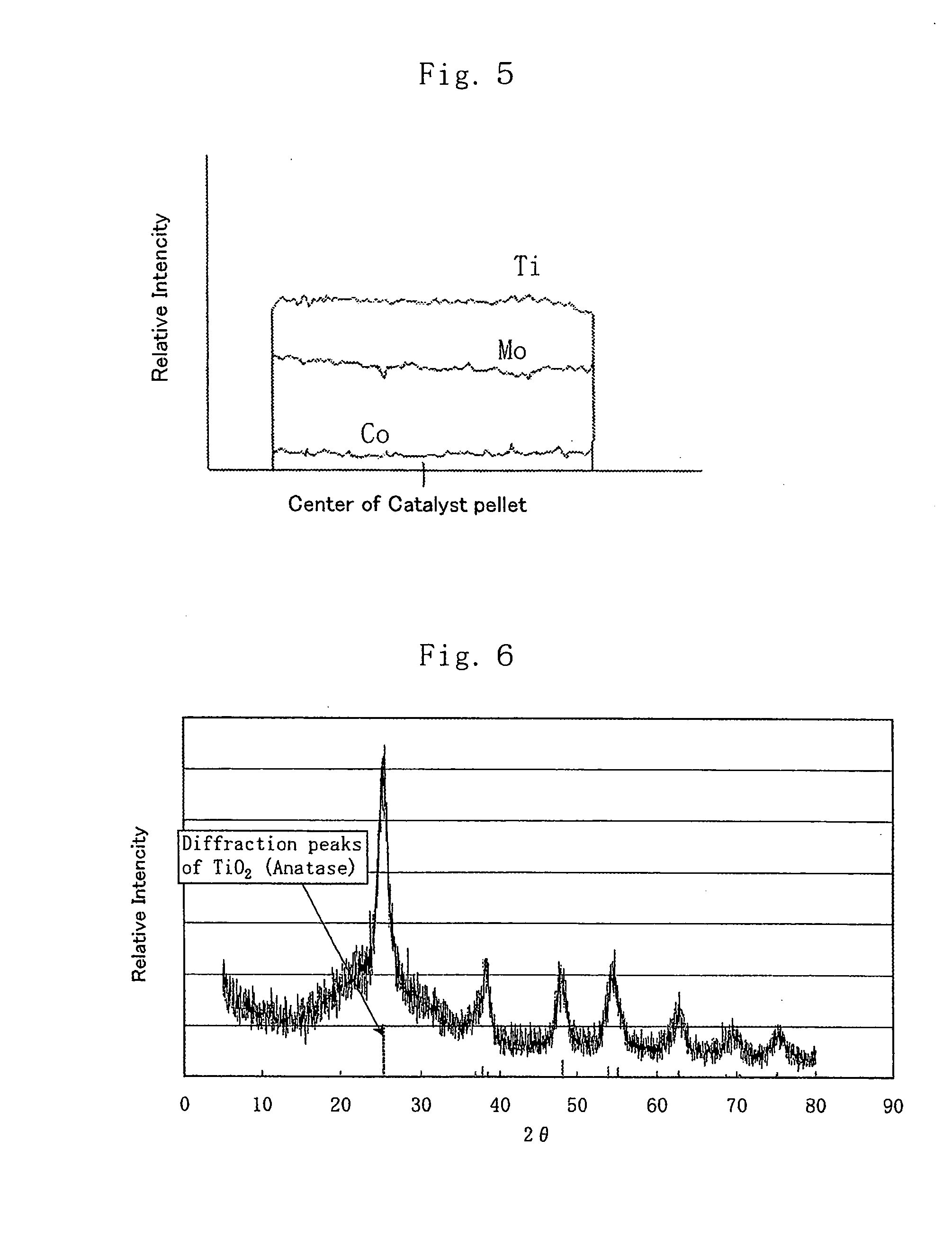
This invention relates to a 4 group metal oxide and to a method for preparation thereof and the 4 group metal oxide prepared by adding a particle growth inhibiter to a hydrosol a hydrogel or a dried product of a hydrous 4 group metal oxide represented by MO(2-x)(OH)2x (wherein M denotes a 4 group metal and x is a number greater than 0.1 or x>0.1) followed by drying and calcining has a specific surface area of 80 m<2> / g or more, a pore volume of 0.2 ml / g or more and a pore sharpness degree of 50% or more and excellent heat stability and is useful for a catalyst or a catalyst carrier in which a catalyst metal is dispersed to a high degree. This invention further relates to a porous 4 group metal oxide and to a method for preparation thereof and the 4 group metal oxide prepared by application of a pH swing operation is characterized by a large specific surface area, excellent heat stability, high dispersion of a catalyst metal and a controlled and sharp pore distribution and is useful for a catalyst or a catalyst carrier of excellent reaction selectivity.
Owner:CHIYODA CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com