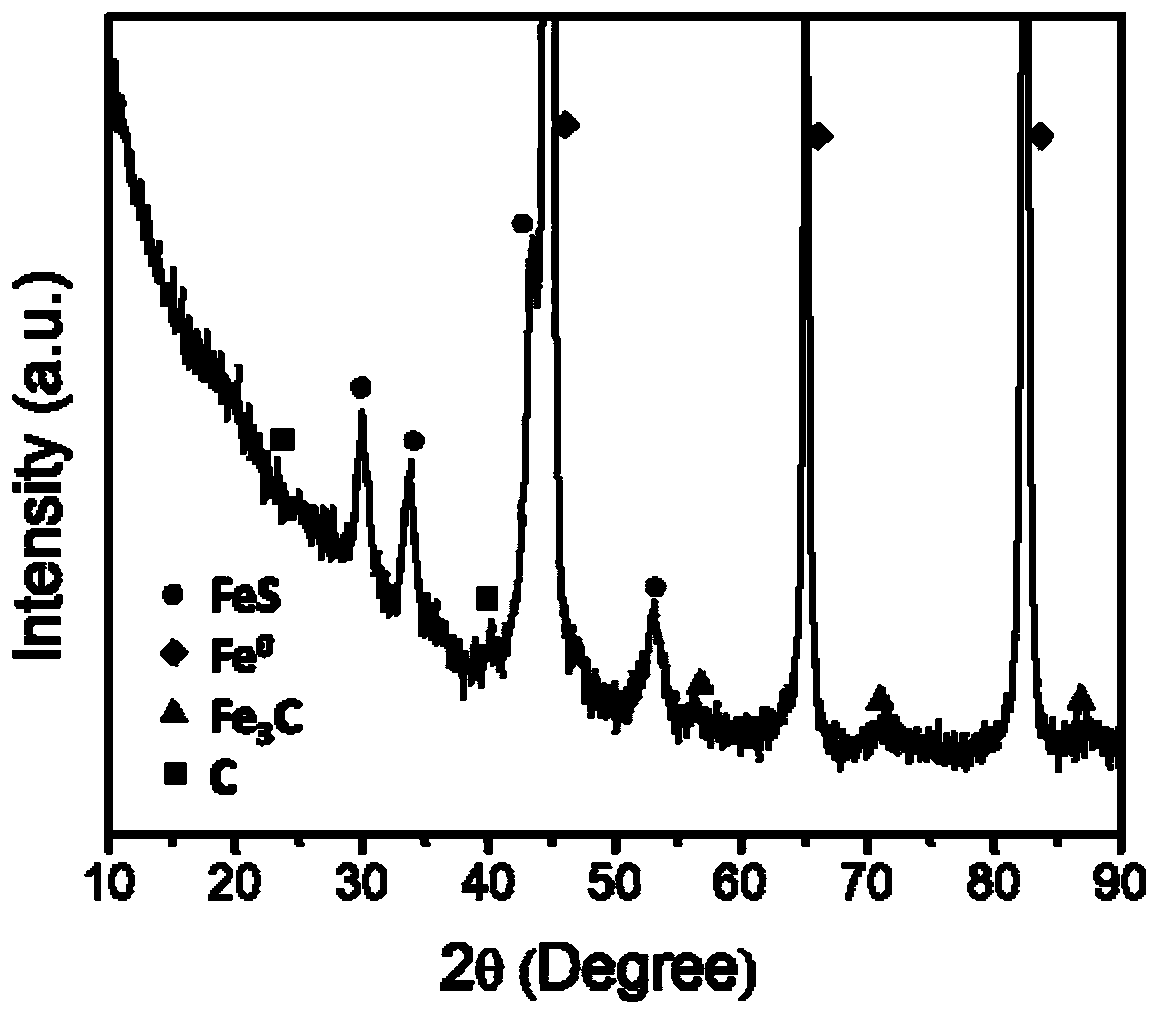
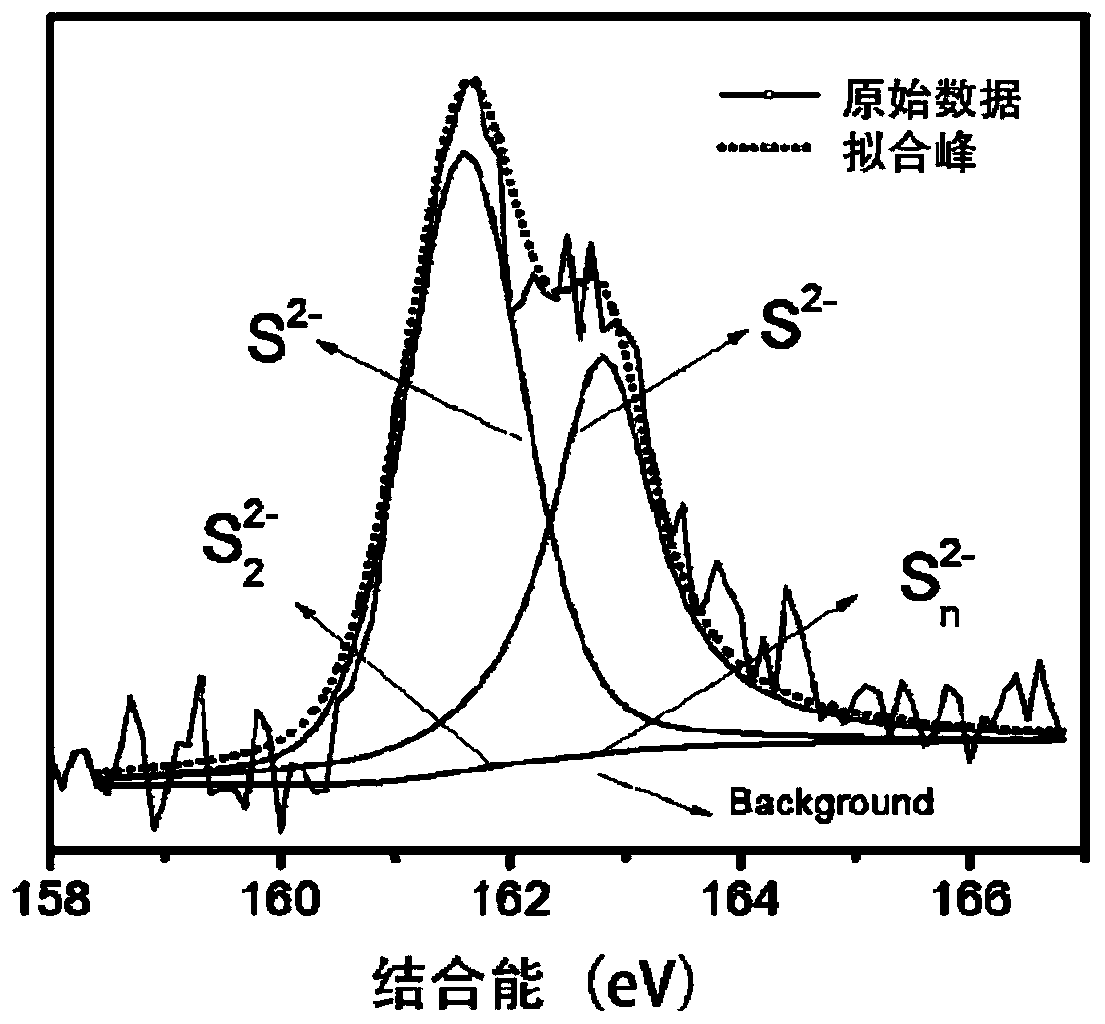
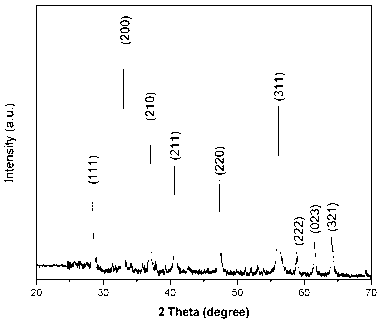
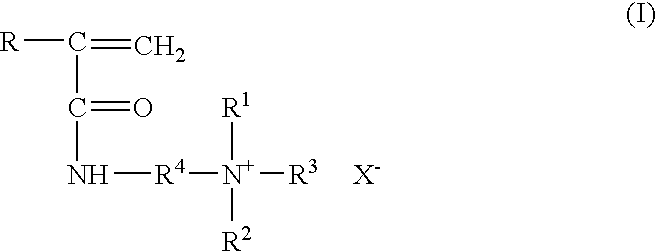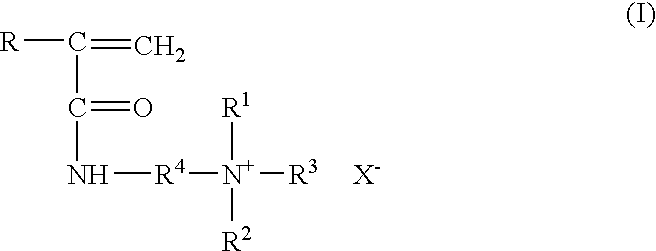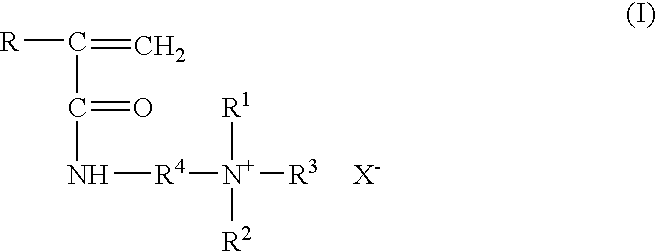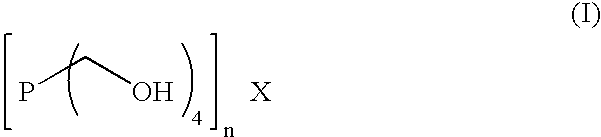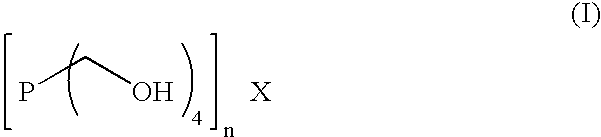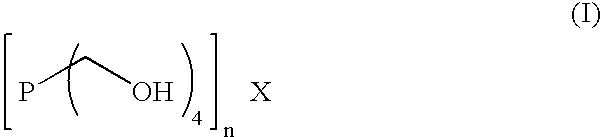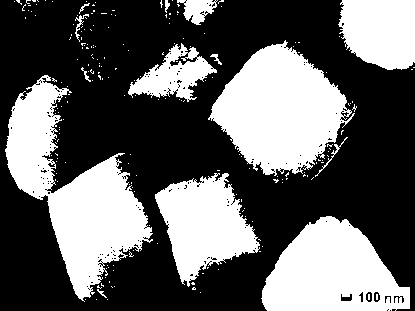Patents
Literature
525 results about "Iron sulfide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Iron sulfide or Iron sulphide can refer to range of chemical compounds composed of iron and sulfur.
Synthesis of magnetite nanoparticles and the process of forming Fe-based nanomaterials
InactiveUS6962685B2Small sizeNarrow size distributionMaterial nanotechnologyNanomagnetismIron saltsMagnetite Nanoparticles
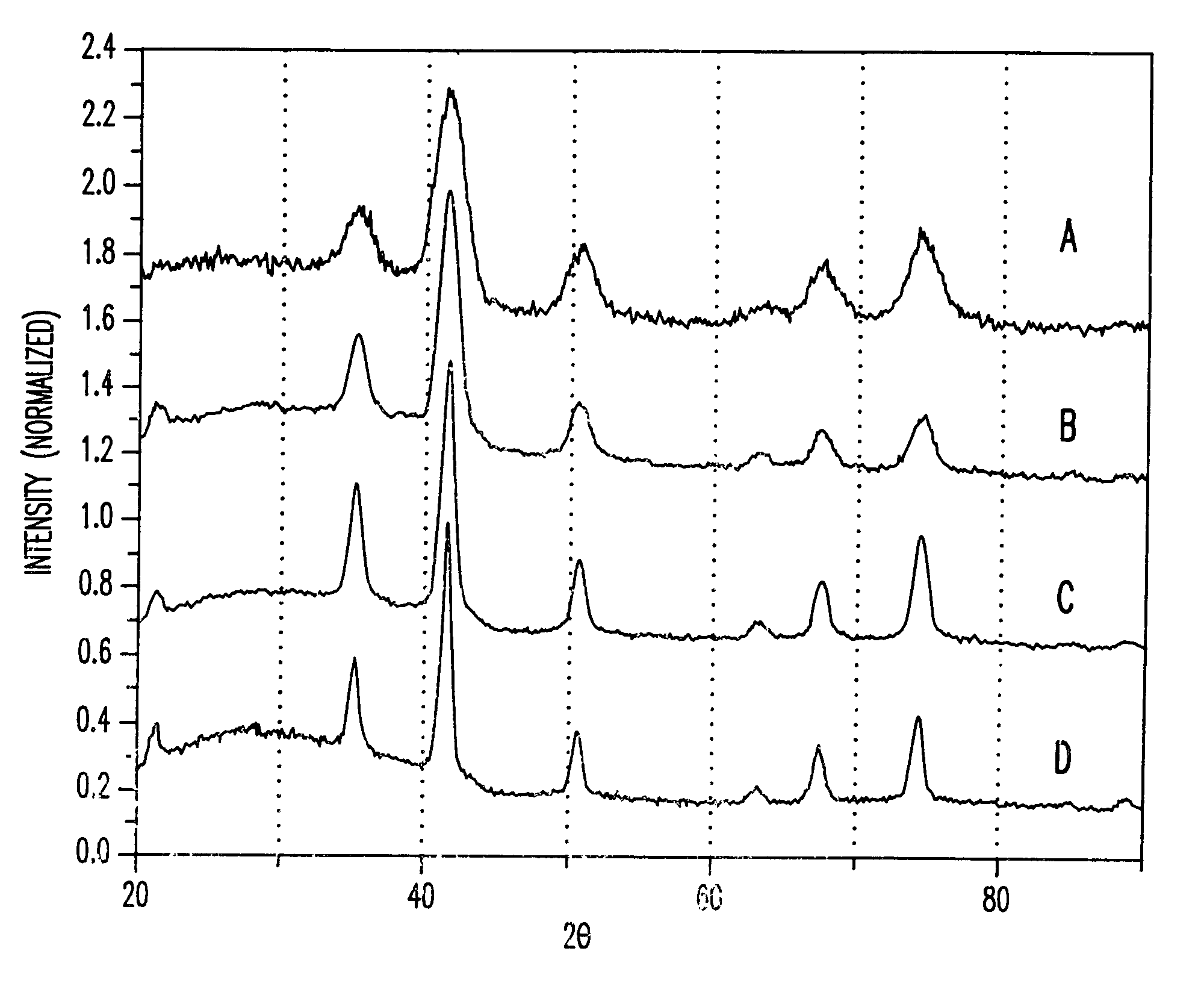
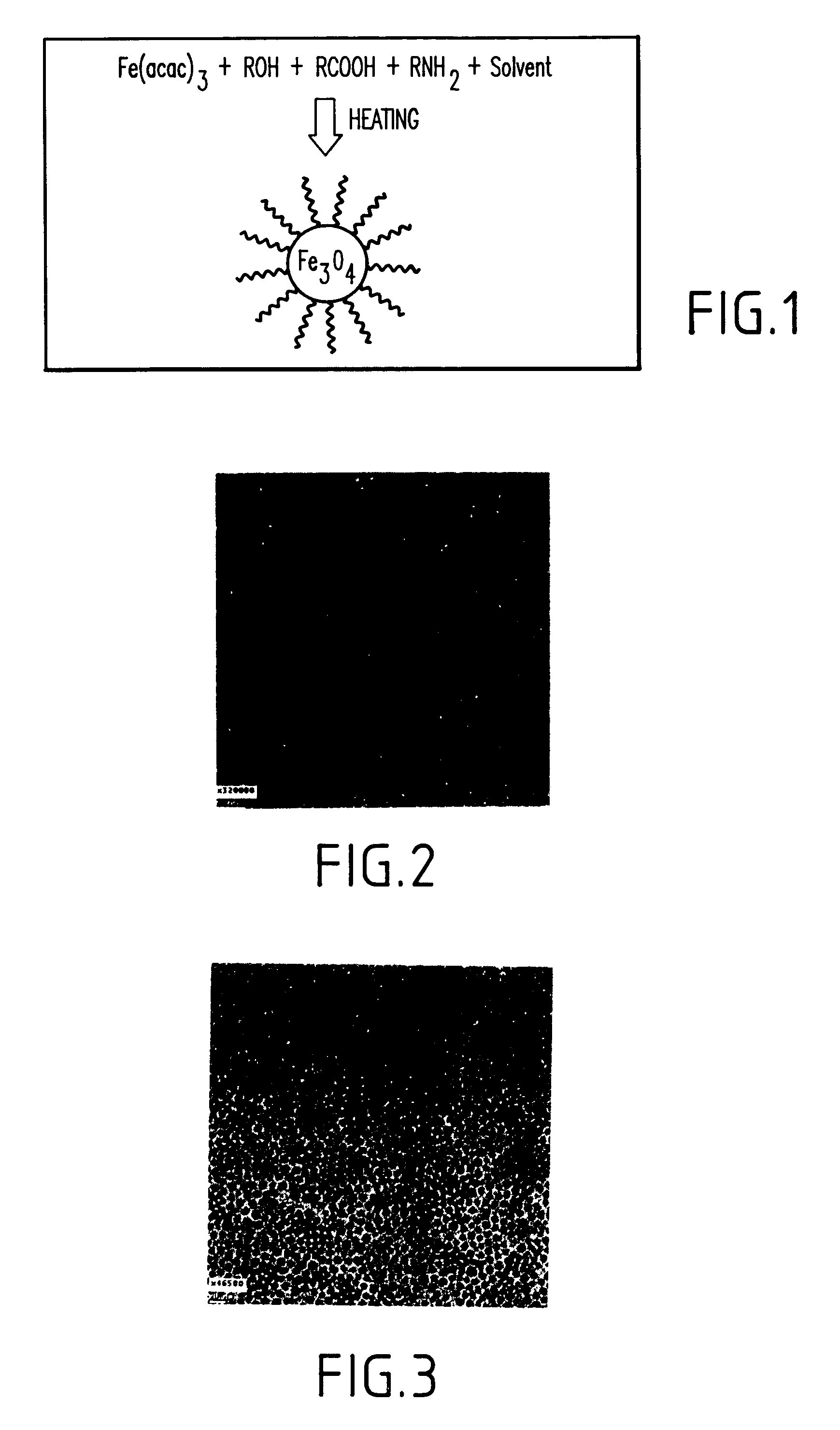
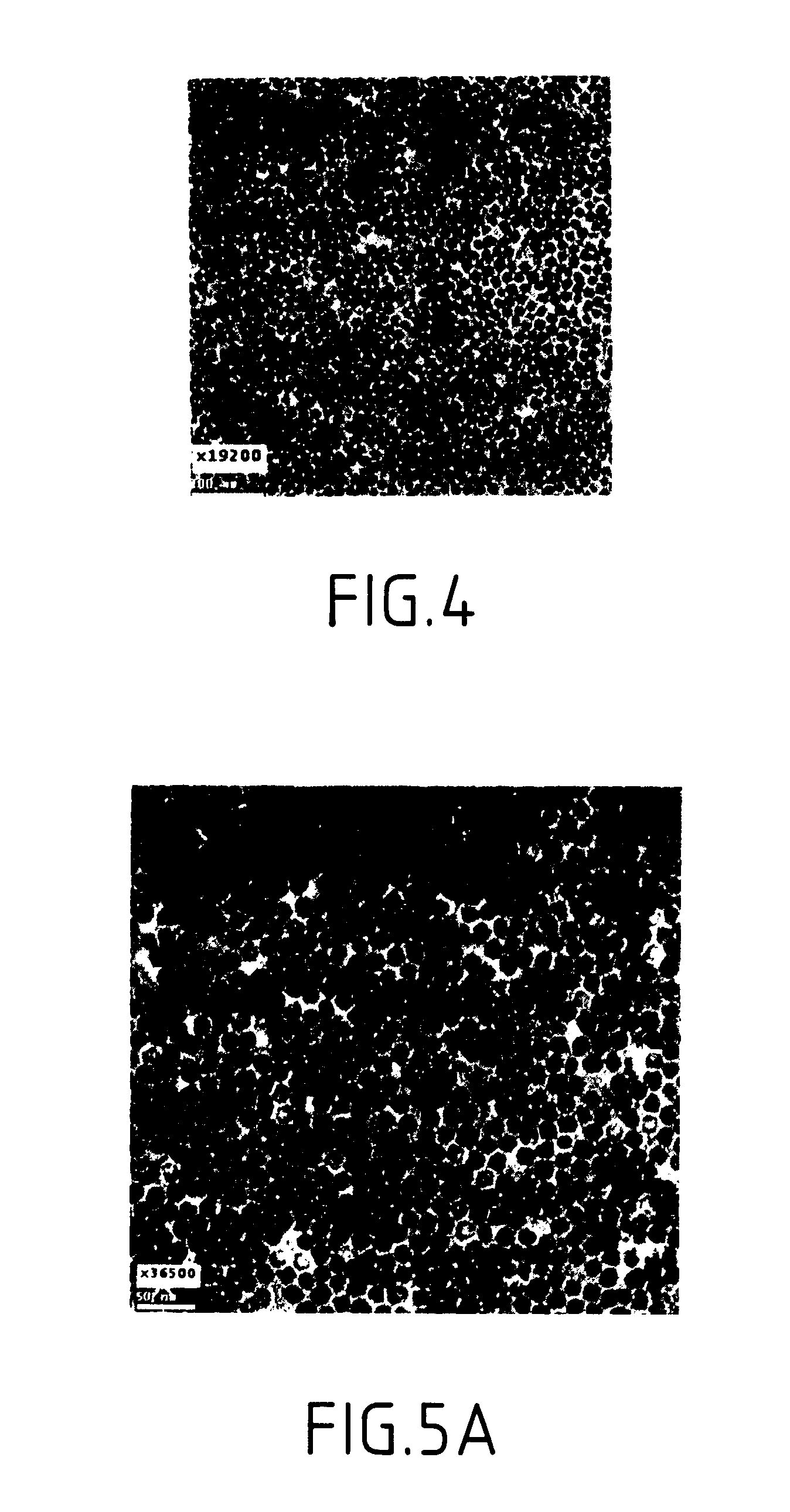
A method and structure for making magnetite nanoparticle materials by mixing iron salt with alcohol, carboxylic acid and amine in an organic solvent and heating the mixture to 200–360 C is described. The size of the particles can be controlled either by changing the iron salt to acid / amine ratio or by coating small nanoparticles with more iron oxide. Magnetite nanoparticles in the size ranging from 2 nm to 20 nm with a narrow size distribution are obtained with the invention. The invention can be readily extended to other iron oxide based nanoparticle materials, including M Fe2O4 (M=Co, Ni, Cu, Zn, Cr, Ti, Ba, Mg) nanomaterials, and iron oxide coated nanoparticle materials. The invention also leads to the synthesis of iron sulfide based nanoparticle materials by replacing alcohol with thiol in the reaction mixture. The magnetite nanoparticles can be oxidized to γ-Fe2O3, or α-Fe2O3, or can be reduced to bcc-Fe nanoparticles, while iron oxide based materials can be used to make binary iron based metallic nanoparticles, such as CoFe, NiFe, and FeCoSmx nanoparticles.
Owner:INT BUSINESS MASCH CORP
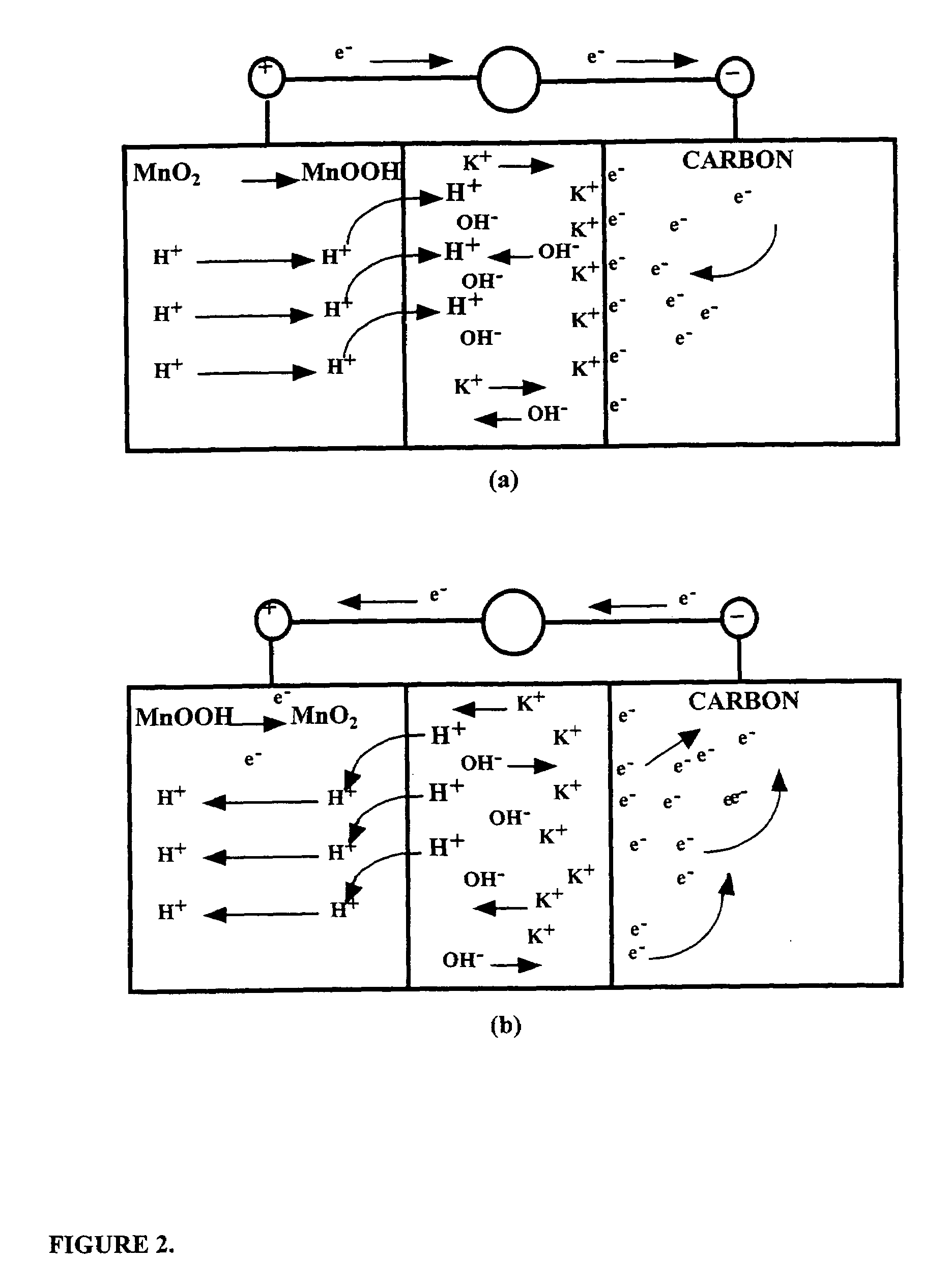
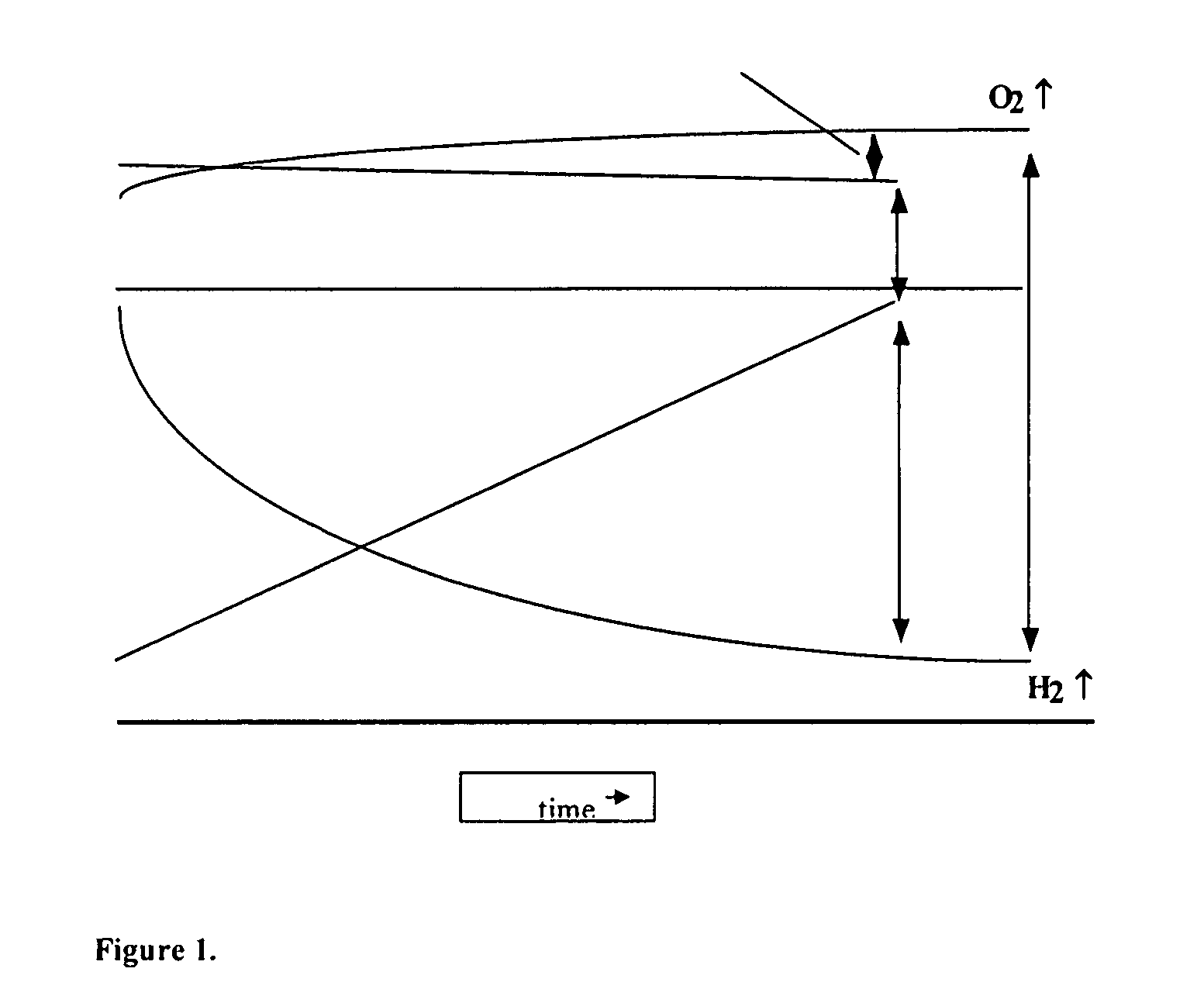
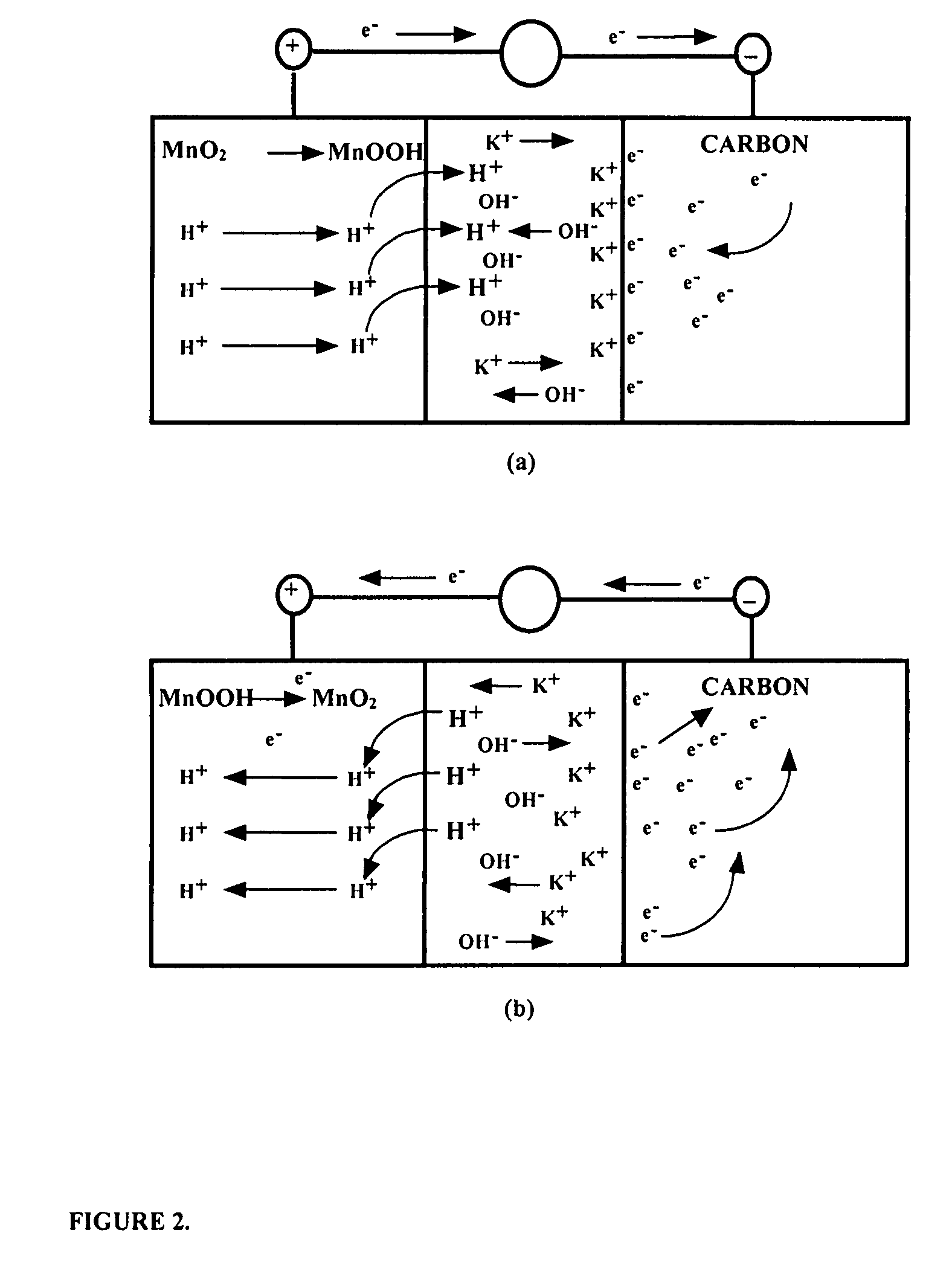
Asymmetric electrochemical supercapacitor and method of manufacture thereof
InactiveUS20080158778A1Increase energy densityImprove power densityHybrid capacitor electrodesLiquid electrolytic capacitorsAqueous electrolyteLithium manganese oxide
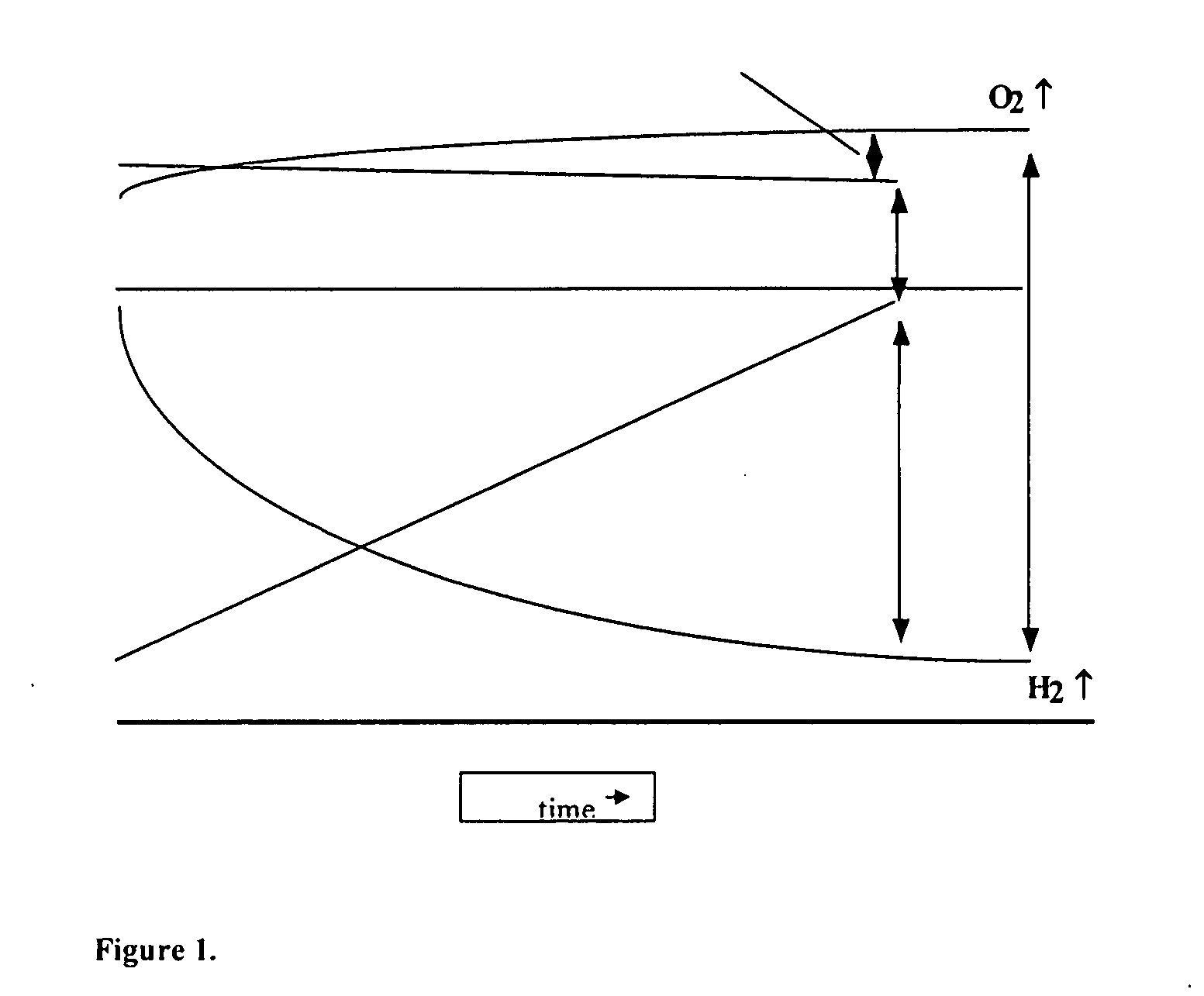
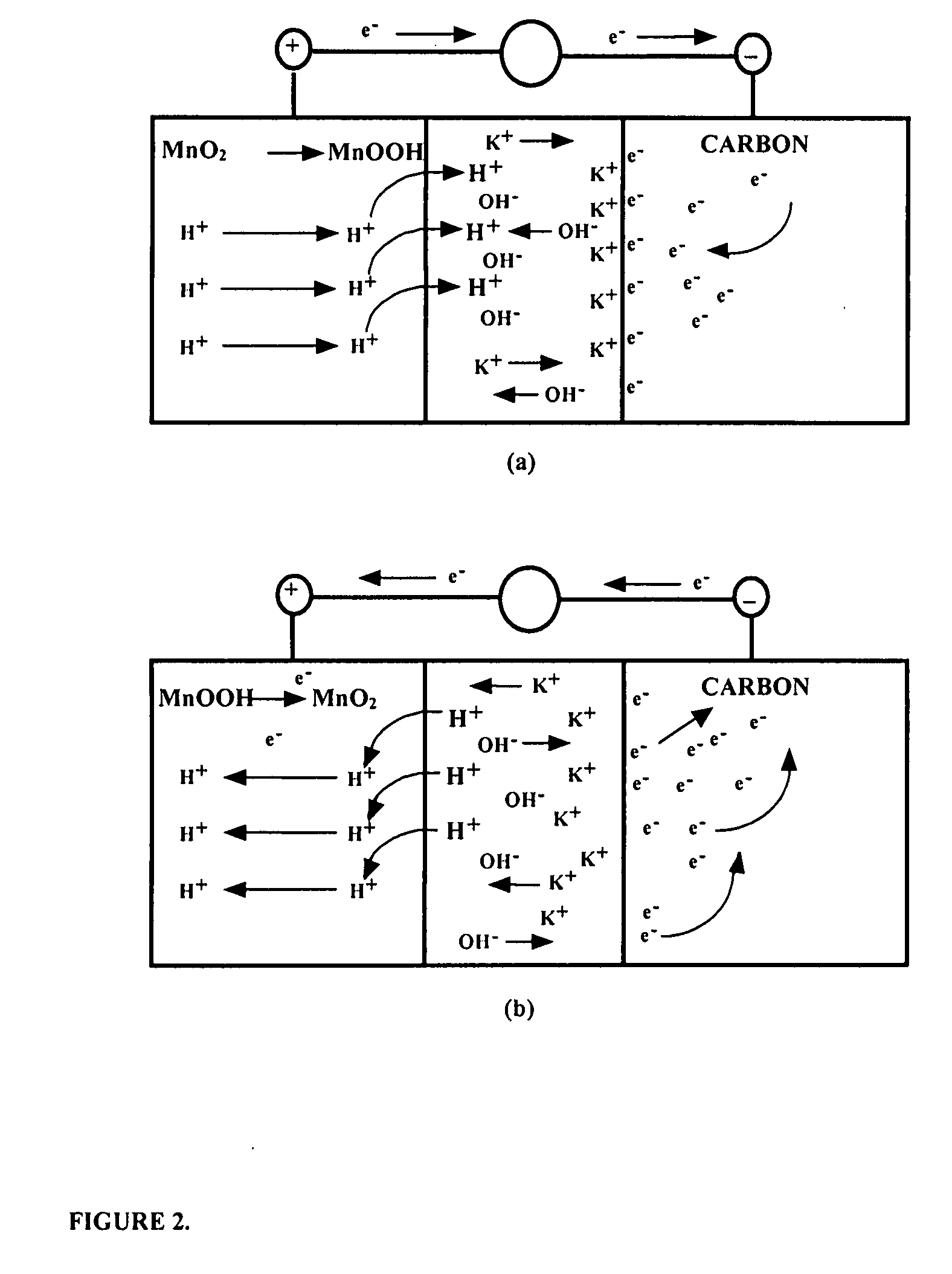
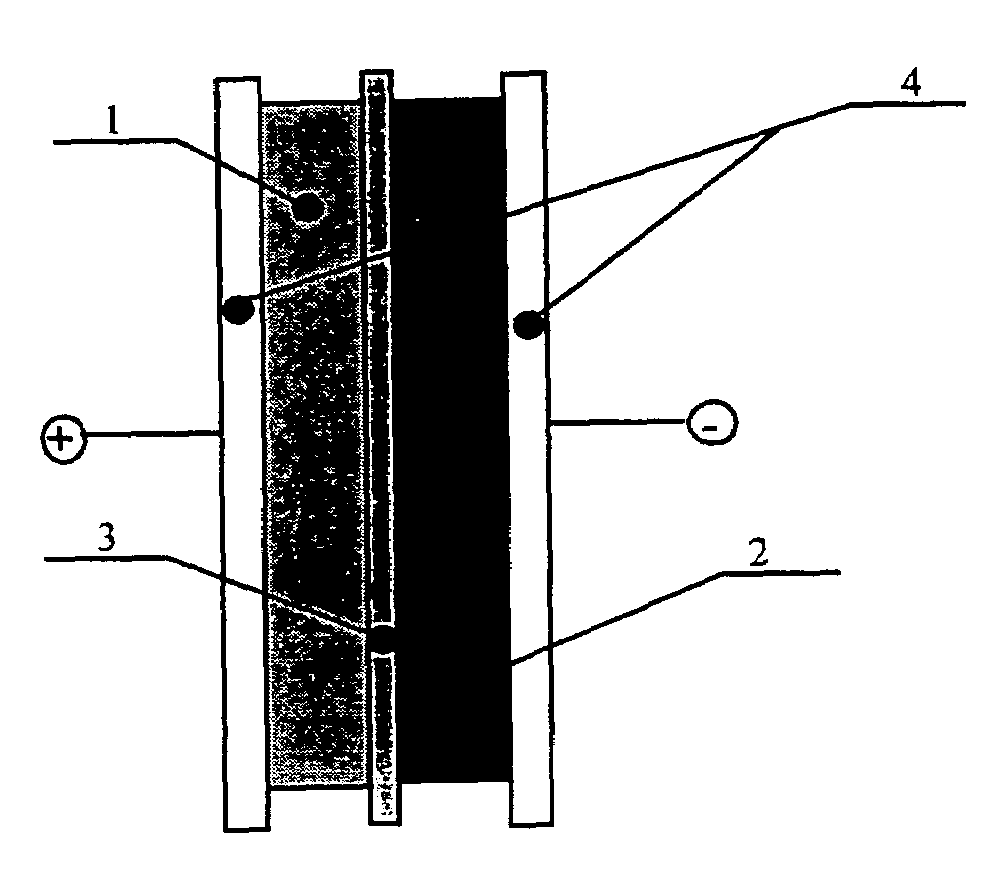
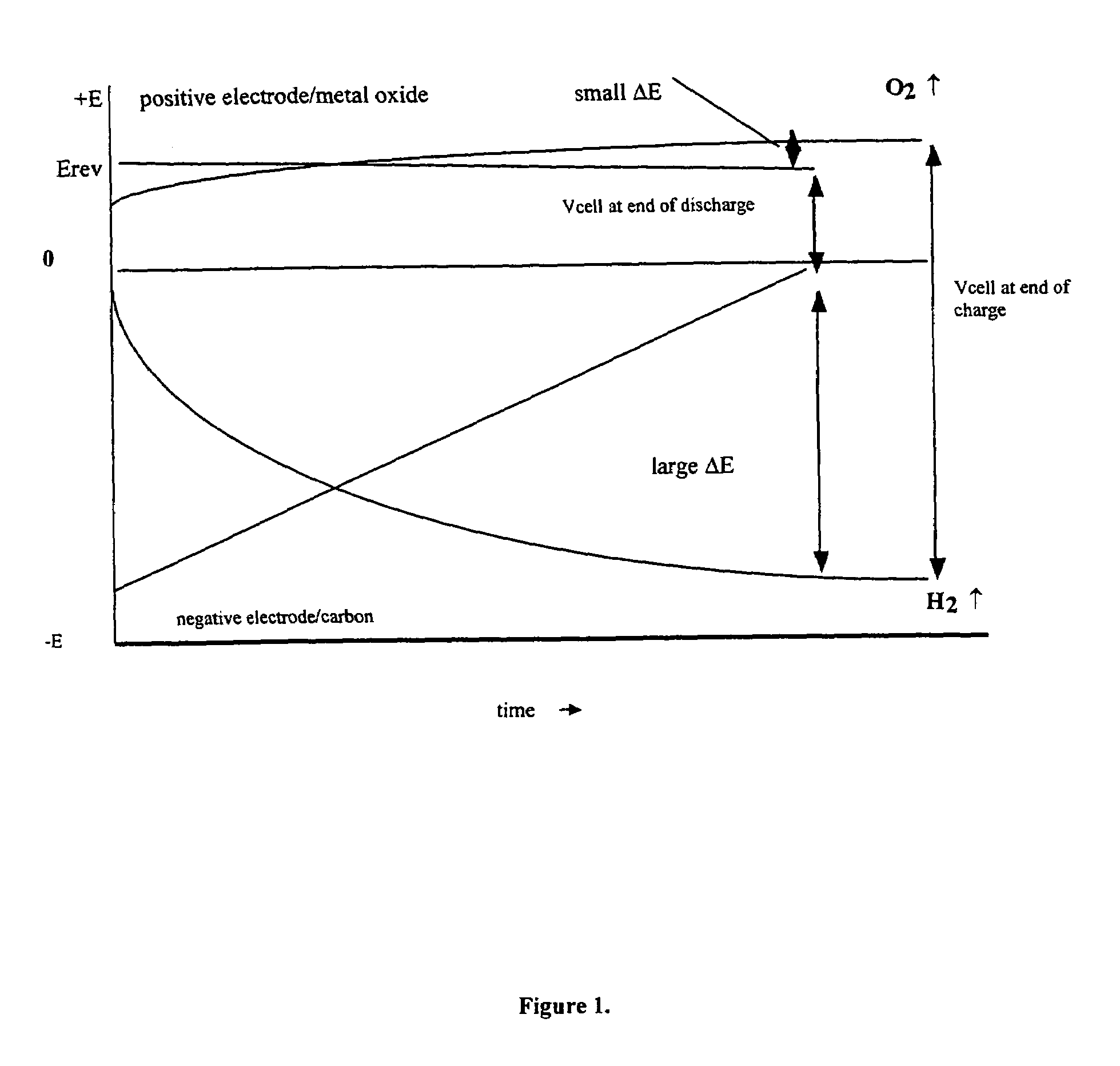
Asymmetric supercapacitors comprise: a positive electrode comprising a current collector and a first active material selected from the group consisting of manganese dioxide, silver oxide, iron sulfide, lithium manganese oxide, lithium cobalt oxide, lithium nickel oxide, lithium iron phosphate, and a combination comprising at least one of the foregoing active materials; a negative electrode comprising a carbonaceous active material; an aqueous electrolyte solution selected from the group consisting of aqueous solutions of hydroxides of alkali metals, aqueous solutions of carbonates of alkali metals, aqueous solutions of chlorides of alkali metals, aqueous solutions of sulfates of alkali metals, aqueous solutions of nitrates of alkali metals, and a combination comprising at least one of the foregoing aqueous solutions; and a separator plate. Alternatively, the electrolyte can be a non-aqueous ionic conducting electrolyte or a solid electrolyte.
Owner:U S NANOCORP
Asymmetric electrochemical supercapacitor and method of manufacture thereof
InactiveUS7199997B1Increase energy densityImprove power densityHybrid capacitor electrodesLiquid electrolytic capacitorsFiberCharge separation
An asymmetric supercapacitor has a positive electrode having a current collector an active material selected from the group consisting of manganese dioxide, silver oxide, iron sulfide and mixtures thereof, a negative electrode having a carbonaceous active material carbon and optional current collector, an electrolyte, and a separator plate. In a preferred embodiment at least one of the electrodes has nanostructured / nanofibrous material and in a more preferred embodiment, both electrodes have nanostructured / nanfibrous material. The electrolyte can be liquid or solid although liquid electrolytes are preferred.The asymmetric supercapacitor has improved energy density by electrically coupling an electrode of high faradaic capacity such as one having manganese oxide (MnO2) with an electrode such as carbon that stores charge through charge separation at the electric double-layer. The asymmetric supercapacitor also improves power density by using high surface area nanostructured / nanofibrous electrode materials.
Owner:U S NANOCORP +1
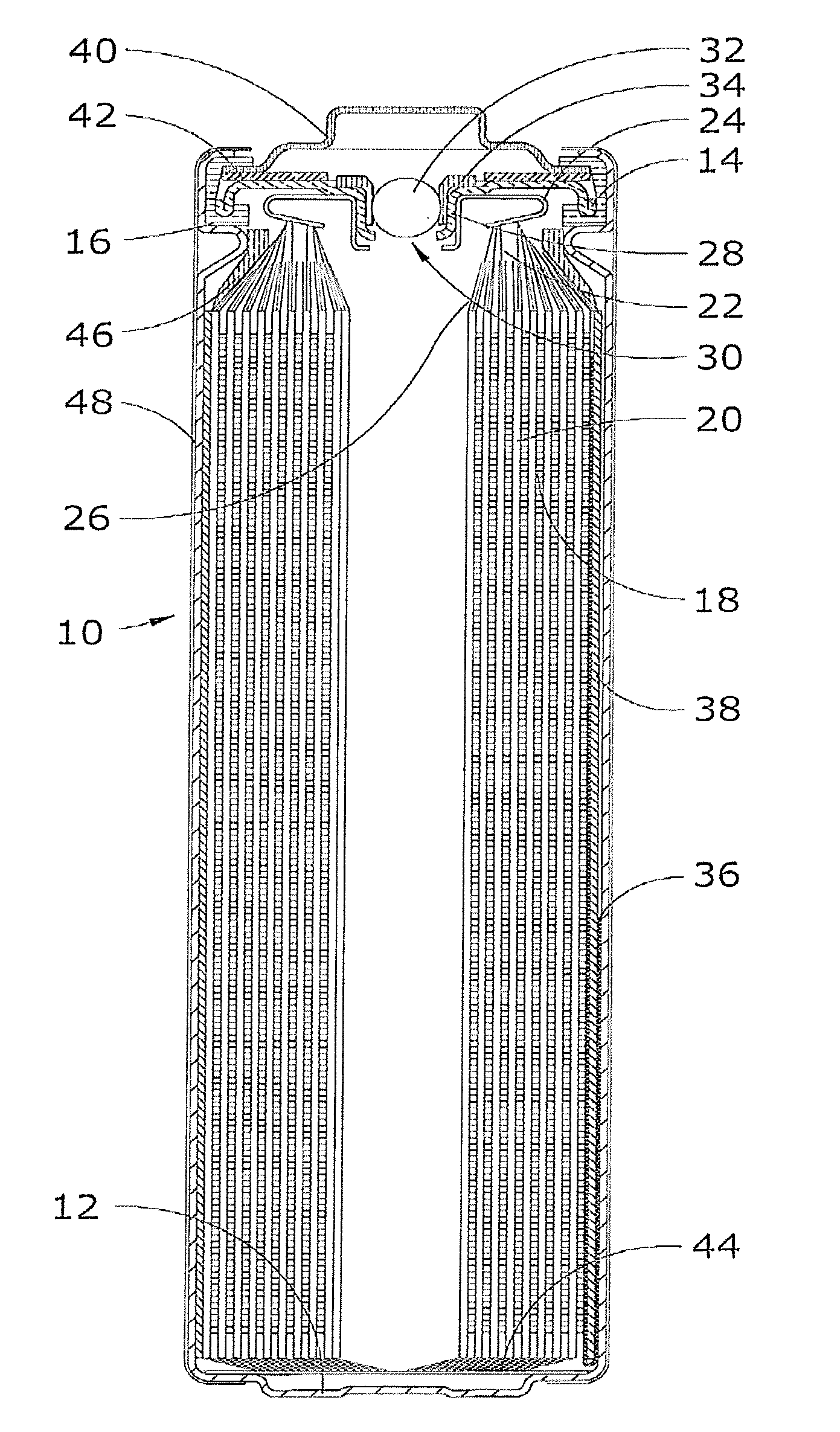
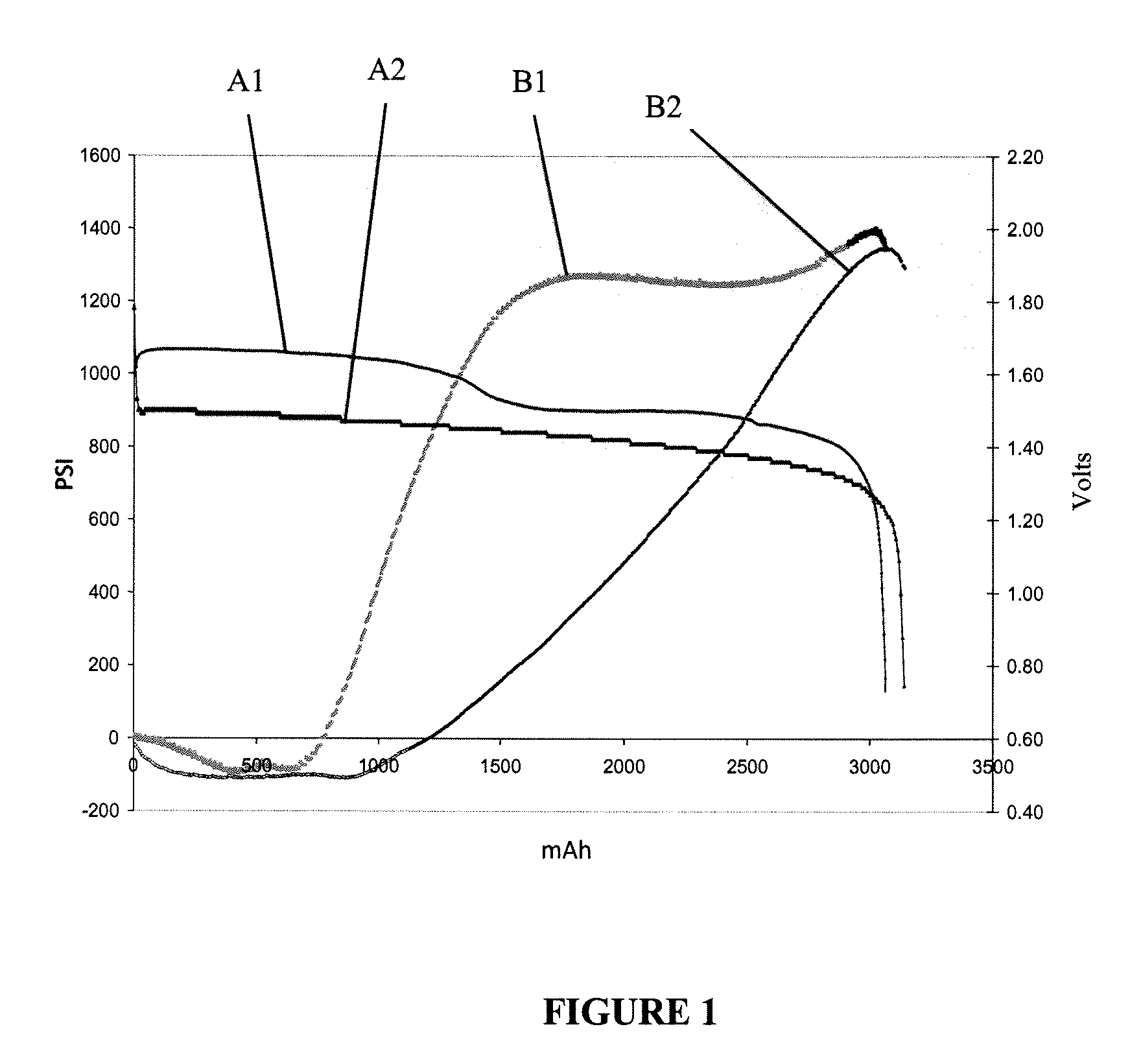
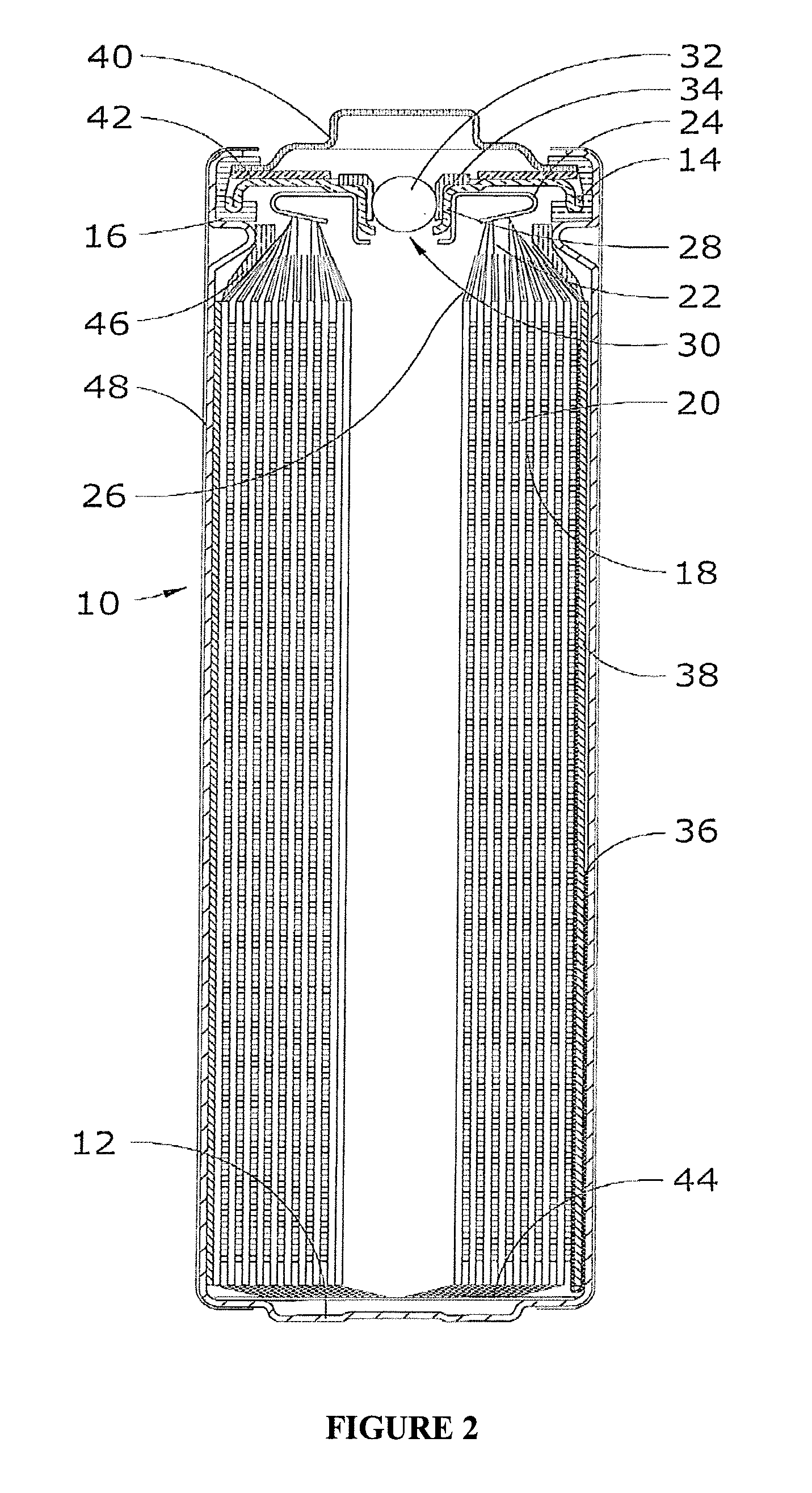
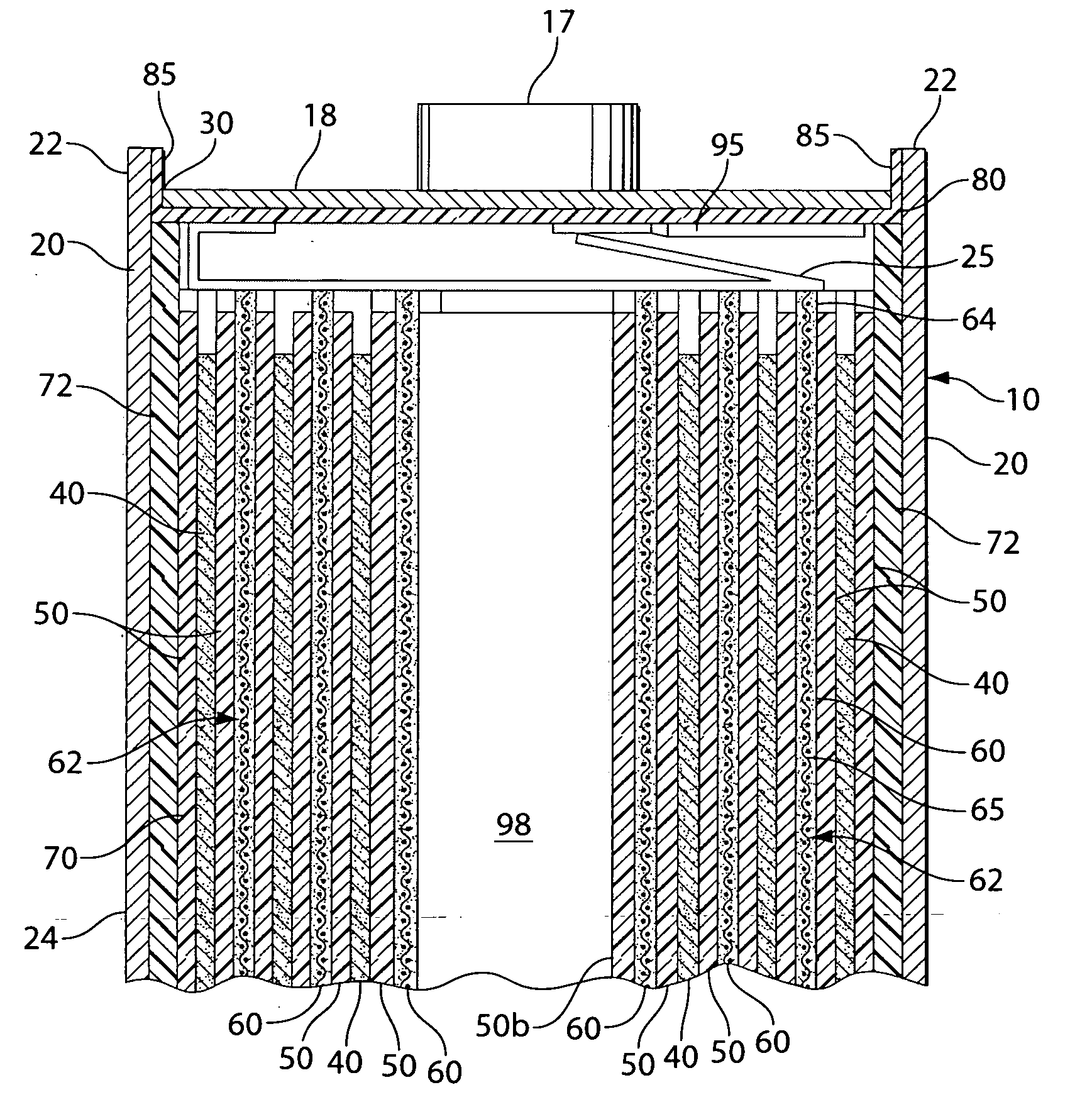
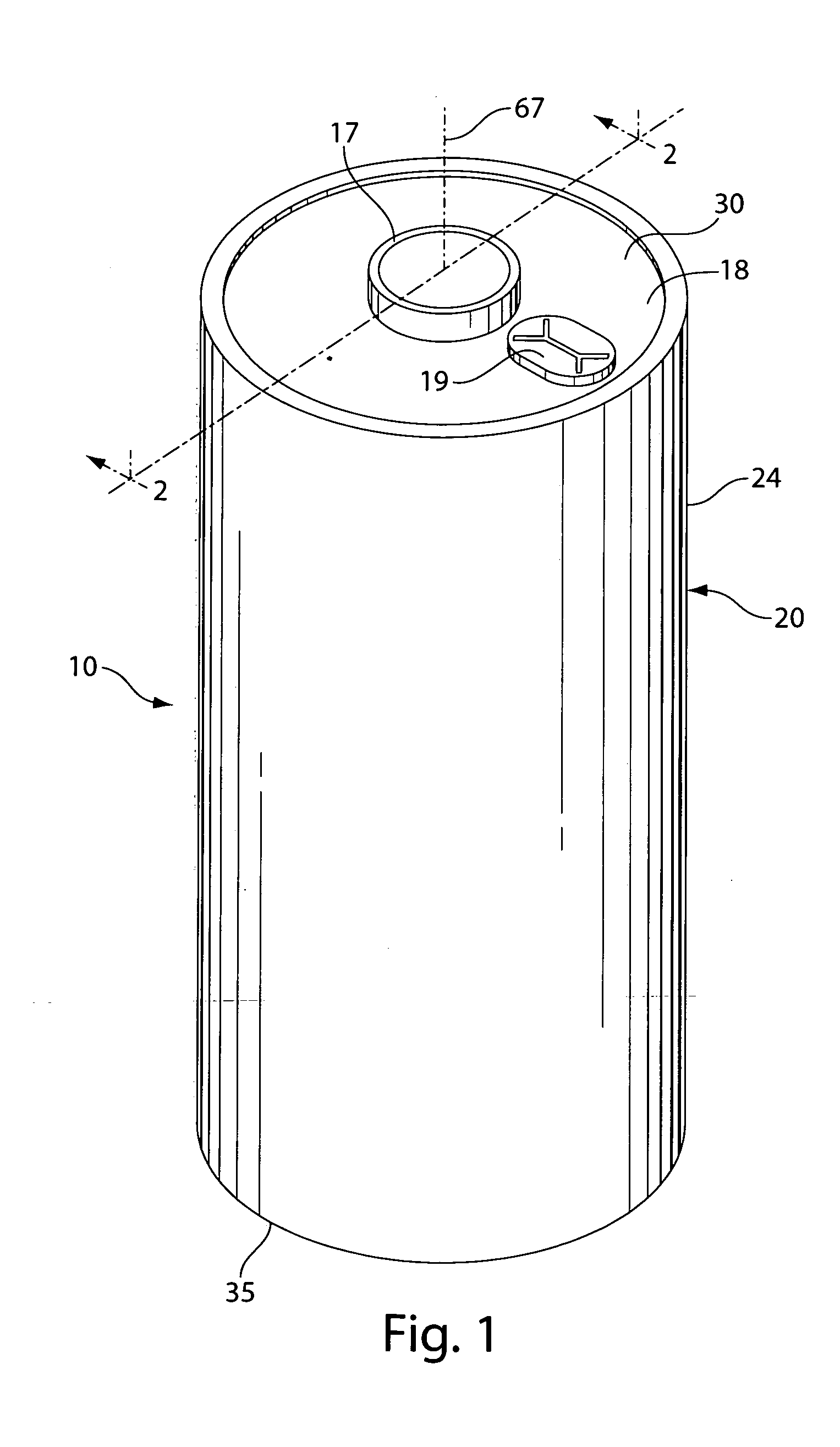
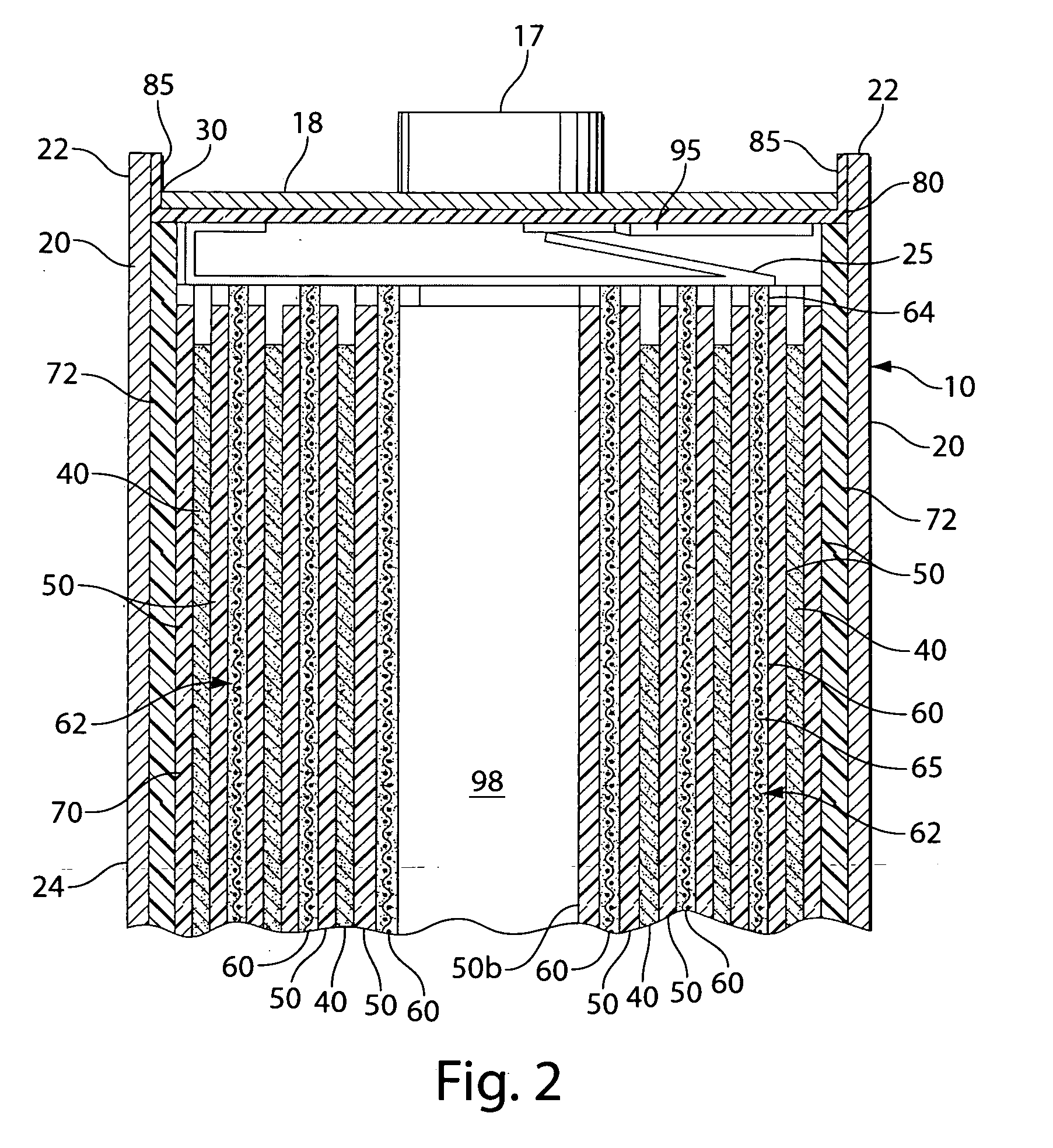
Lithium-Iron Disulfide Cell Design
ActiveUS20090104520A1Large capacityIncrease capacityCell seperators/membranes/diaphragms/spacersSmall-sized cells cases/jacketsCell designEngineering
Owner:ENERGIZER BRANDS
Scaling inhibitors and method for using the same in high density brines
InactiveUS20050067164A1Promote resultsParticular in controlCleaning apparatusFluid removalSolubilityZinc bromide
A formulation containing a copolymer derived from a cationic monomer effectively inhibits and controls the formation of inorganic scales. The formulation has particular application in the removal of zinc sulfide and iron sulfide scales formed when zinc bromide brines are used as completion fluids. The copolymer exhibit high solubility in high-density brines, such as zinc bromide brines. The polymers may be introduced into an oil or gas well as a portion of a carrier fluid or with brine. The preferred copolymer for use in the invention contains an acrylamide unit and a diallyldimethylammonium salt and, optionally, an acrylic acid or a salt thereof. The weight average molecular weight of such inhibitor copolymers is generally between from about 500,000 to about 5,000,000.
Owner:BAKER HUGHES INC
Method to decrease iron sulfide deposits in pipe lines
InactiveUS6866048B2Inorganic/elemental detergent compounding agentsAnionic surface-active compoundsPhosphoniumSulfate
This invention provides a method of treating a dry or processed fluid pipe line susceptible to the build-up of iron sulfide deposits by complexing the iron sulfide in the pipe lines. The method of the present invention introduces the composition on a continuous or a batch basis to a gas pipe line. The composition is made of a solution of 1) water, 2) [tetrakis(hydroxymethyl)phosphonium]sulfate or chloride, and 3) a soluble ammonium salt, such as ammonium chloride or the like.
Owner:COASTAL CHEM CO L L C
Compound coal tar hydrogenation catalyst and preparation method thereof
ActiveCN101927167AReduce manufacturing costReduce use costHydrocarbon oil crackingMetal/metal-oxides/metal-hydroxide catalystsLow activityIron sulfide
The invention relates to a compound coal tar hydrogenation catalyst and a preparation method thereof. The catalyst comprises a high-activity component of water-soluble salts of molybdenum, nickel, cobalt or tungsten and comprises a low-activity component of iron oxide ore or iron sulfide ore, wherein the mass ratio of the metals in the high-activity component to the metals in the low-activity component is 1:1,000 to 1:10; iron accounts for more than or equal to 40 weight percent of the ore; and water accounts for less than 2 weight percent of the catalyst. The catalyst is used for the coal tar hydrocracking technical process in a suspension bed, has higher hydrogenation activity and the lightweight oil yield of over 94 percent, and can be recycled; the preparation and use cost of the catalyst can be greatly reduced, the consumption of the catalyst is reduced in the process, the coke deposition of the reaction system is avoided simultaneously and the operation cycle is prolonged.
Owner:CCTEG CHINA COAL RES INST
Synthesis of magnetite nanoparticles and the process of forming fe-based nanomaterials
InactiveUS20050191231A1Small sizeNarrow size distributionMaterial nanotechnologyNanostructure manufactureIron saltsMagnetite Nanoparticles
A method and structure for making magnetite nanoparticle materials by mixing iron salt with alcohol, carboxylic acid and amine in an organic solvent and heating the mixture to 200-360 C. is described. The size of the particles can be controlled either by changing the iron salt to acid / amine ratio or by coating small nanoparticles with more iron oxide. Magnetite nanoparticles in the size ranging from 2 nm to 20 nm with a narrow size distribution are obtained with the invention. The invention can be readily extended to other iron oxide based nanoparticle materials, including MFe2O4 (M=Co, Ni, Cu, Zn, Cr, Ti, Ba, Mg) nanomaterials, and iron oxide coated nanoparticle materials. The invention also leads to the synthesis of iron sulfide based nanoparticle materials by replacing alcohol with thiol in the reaction mixture. The magnetite nanoparticles can be oxidized to γ-Fe2O3, or α-Fe2O3, or can be reduced to bcc-Fe nanoparticles, while iron oxide based materials can be used to make binary iron based metallic nanoparticles, such as CoFe, NiFe, and FeCoSmx nanoparticles.
Owner:IBM CORP
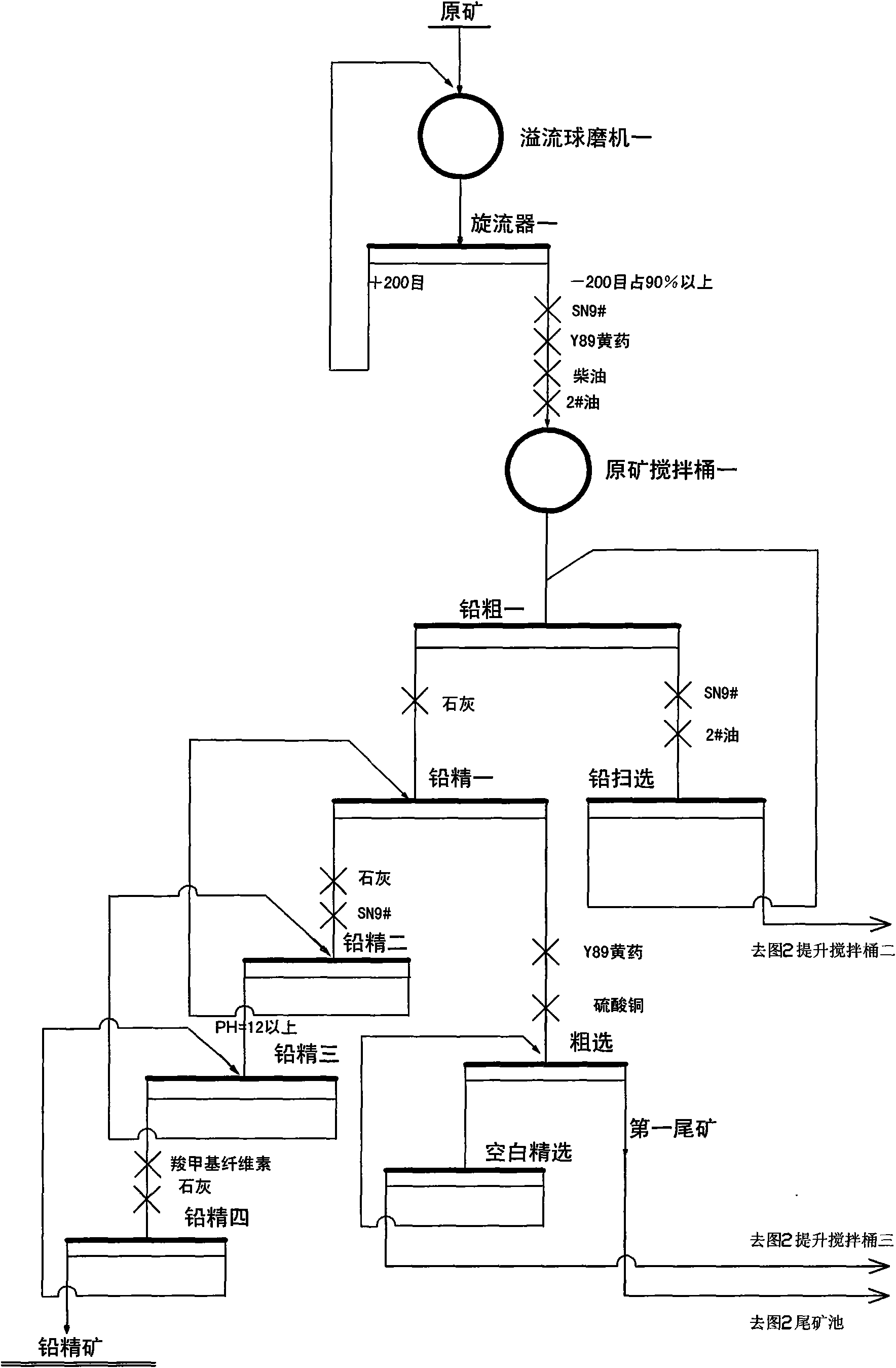
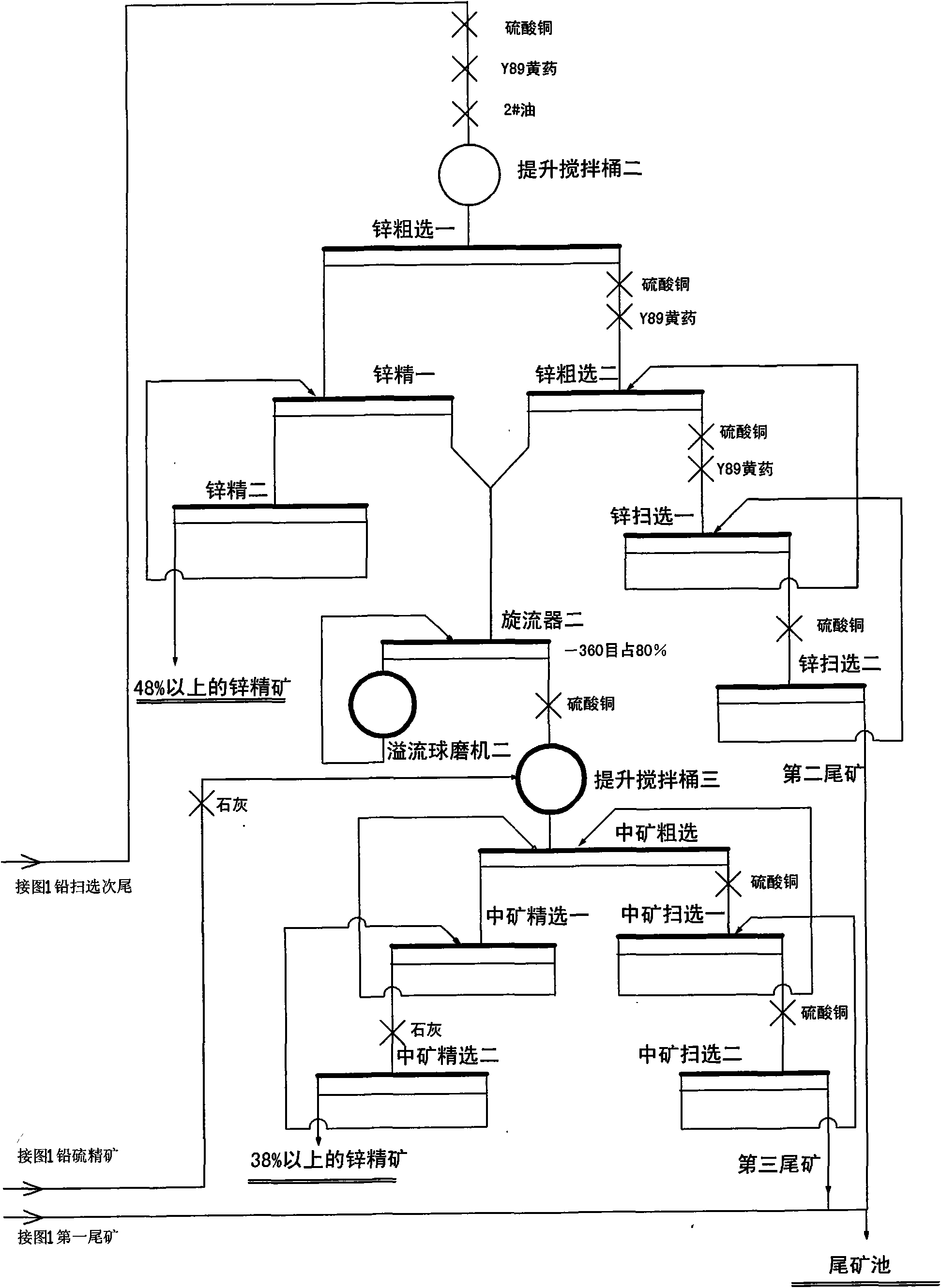
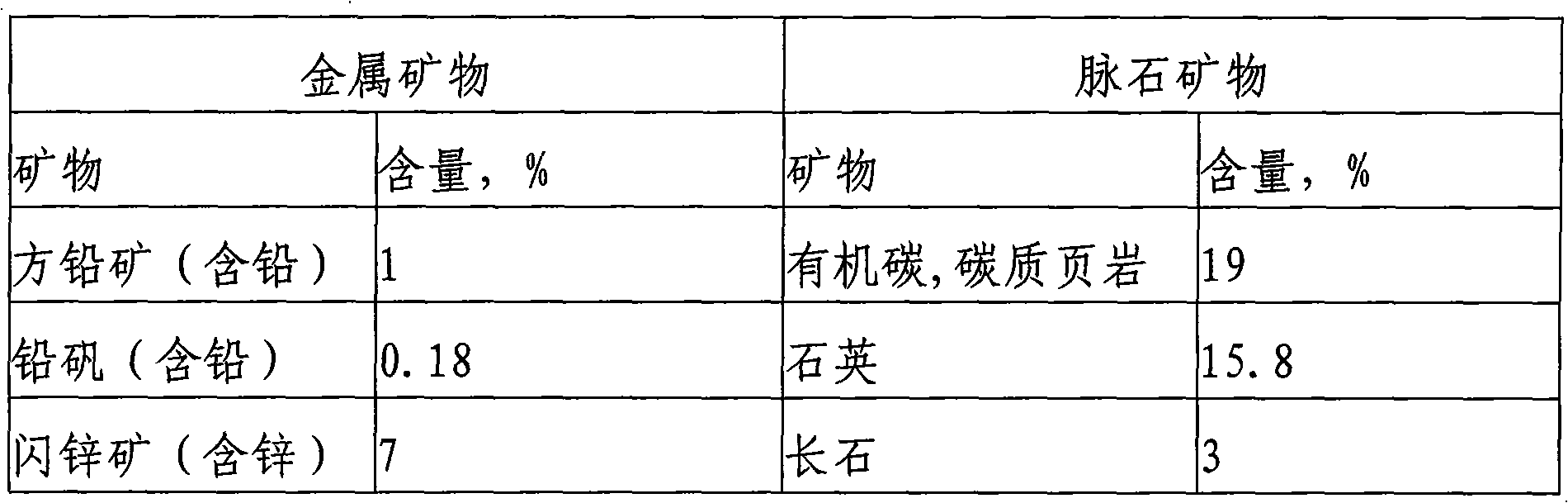
Floatation process of complex lead zinc ores
The invention relates to a floatation process of complex lead zinc ores, which uses the following process flows: ore grinding-floatation of lead in a lead flotation area-floatation of zinc in a zinc / sulfur mixed flotation area-floatation of zinc in a zinc middling re-flotation area; the invention uses floatable process like lead zinc full-opening, effectively removes carbon and iron sulfide with good floatability by means of entire processes of the lead area, reduces influence of carbon and iron sulfide with good floatability on the flotation of zinc, avoids lime from entering rougher flotation and scavenging of primary processes and enables refining of zinc to be blank refining to thereby greatly reduce vicious circle of a large amount of middling, and a collecting agent is not added to the separation of zinc from sulfur in re-flotation of zinc middling.
Owner:何任义 +2
Lithium cell with cathode including iron disulfide and iron sulfide
ActiveUS20090263727A1Improve discharge performanceImprove discharge efficiencyOrganic electrolyte cellsElectrolytesIron sulfideAlloy
A primary cell having an anode comprising lithium or lithium alloy and a cathode comprising iron disulfide (FeS2), iron sulfide (FeS) and carbon particles. The electrolyte comprises a lithium salt dissolved in a solvent mixture. A cathode slurry is prepared comprising iron disulfide (FeS2) powder, iron sulfide (FeS) powder, carbon, binder, and a liquid solvent. The mixture is coated onto a conductive substrate and solvent evaporated leaving a dry cathode coating on the substrate. The anode and cathode can be spirally wound with separator therebetween and inserted into the cell casing with electrolyte then added.
Owner:DURACELL U S OPERATIONS
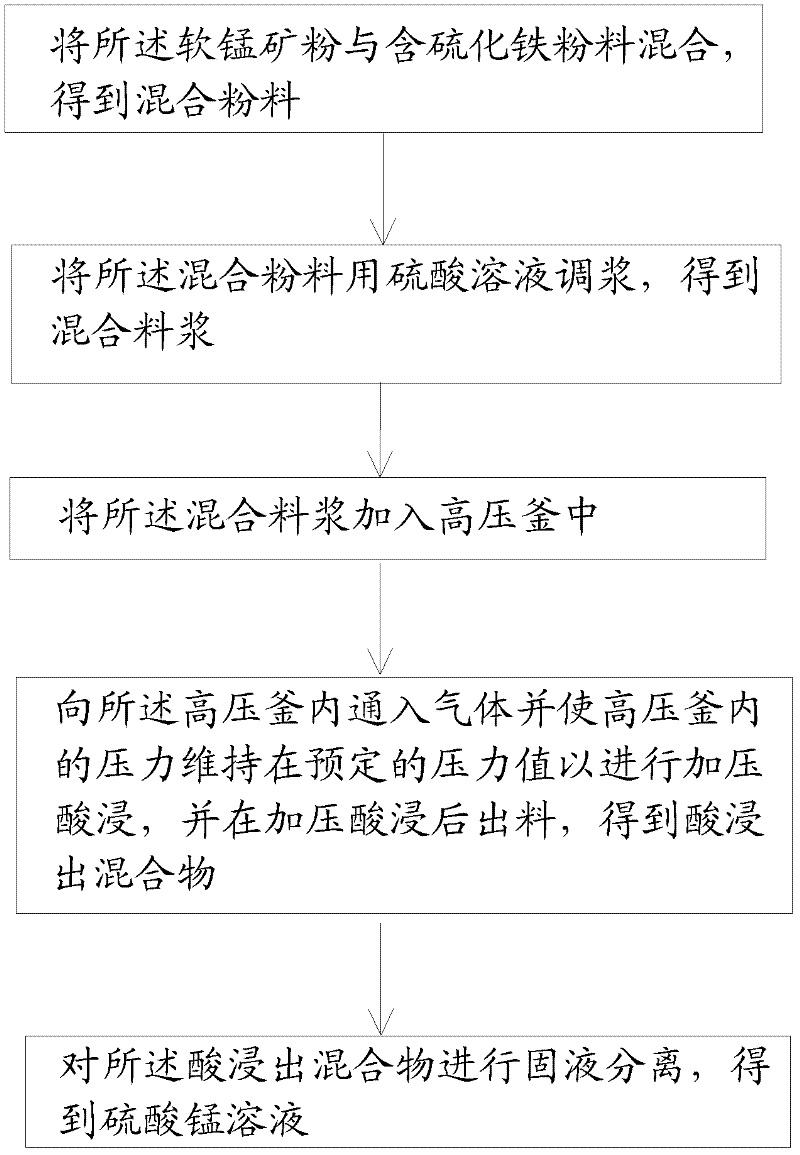
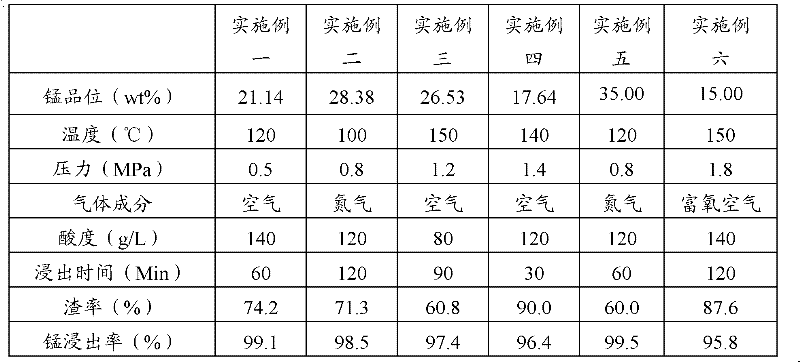
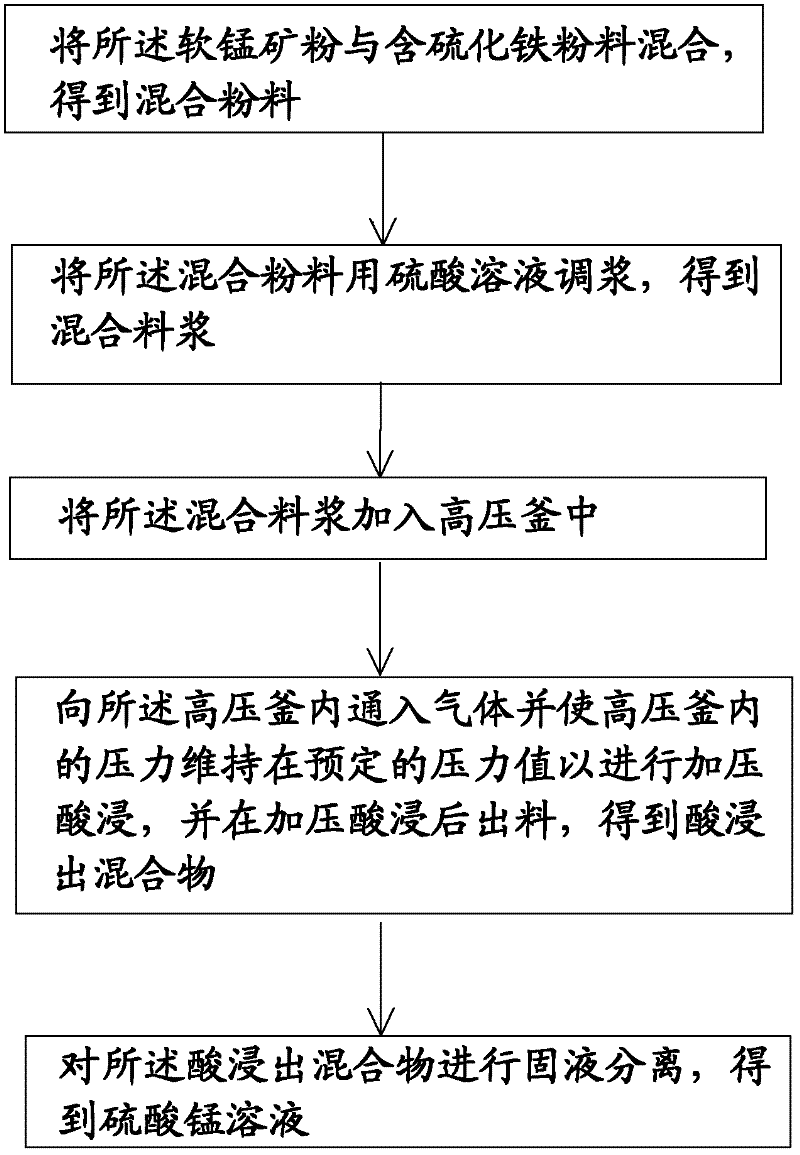
Pressurizing and reductive leaching method of pyrolusite powder
The invention discloses a pressurizing and reductive leaching method of pyrolusite powder. The method comprises the following steps of: a) mixing pyrolusite powder and iron sulfide powder to obtain mixed powder; b) adding a sulphuric acid solution in the mixed powder to pulp and obtain mixed slurry; c) adding the mixed slurry in an autoclave; d) injecting a gas in the autoclave to ensure that the inner pressure of the autoclave reaches a preset pressure value continuously and perform acid leaching under a pressure, then discharging to obtain an acid leaching mixture; and e) performing solid-liquid separation to the acid leaching mixture to obtain a manganese sulfate solution, wherein the pyrolusite powder contains 13-35wt% of manganese. According to the pressurizing and reductive leaching method of pyrolusite powder in the embodiment of the invention, by adopting the pressurizing and leaching method, the manganese in low-grade pyrolusite powder can be leached fast and effectively.
Owner:YUNNAN JIANSHUI MANGANESE
Method for extracting aurum from difficult-to-handle sulphide ore aurum concentrate by two-segment pressurization leaching method
InactiveCN101876005ADoes not affect the leaching rateReduce consumptionProcess efficiency improvementIron sulfateSlag
The invention relates to a method for extracting aurum from difficult-to-handle sulphide ore aurum concentrate by two-segment pressurization leaching method comprising two segments of pressurization oxidation preprocessing and pressurization oxidation aurum leaching. The method comprises the steps of: firstly, pressurizing and oxidizing base metal sulfide such as iron sulfide with sulfuric acid-iron sulfate, wherein most S2- and S22- are oxidized into elemental sulfur; and secondly, pressurizing and leaching the preprocessed slag with thiocyanate so that aurum is selectively dissolved in the solution in the form of aurum-thiocyanic acid compound. Therefore, the pre-oxidation slag can be directly leached without neutralization, the technical process is short, the aurum recovery rate is high, the cost is low and pollution of low-concentration SO2 smoke gas, As2O3 smoke dust, and the like is avoided.
Owner:CENT SOUTH UNIV
Ceramic-fiber-paper-based friction plate and production method thereof
ActiveCN102878232AGuaranteed stabilityGuaranteed oil conductivityNon-macromolecular organic additionPaper/cardboardAdhesiveBoron carbide
The invention relates to a ceramic-fiber-paper-based friction plate and a production method thereof and aims to provide the friction plate which is high in friction coefficient and fine in wear resistance and the production method which is simple in procedure and easy and convenient for production. According to the technical scheme, the ceramic-fiber-paper-based friction plate comprises ceramic fiber, carbon fiber, aramid pulp fiber, long staple cotton paper, diatomite, cashew shell oil friction powder, calcium carbonate whisker, boron carbide, potassium feldspar powder, rubber granule, iron sulfide, mica, alum, magnesia, cashew shell oil modified phenolic resin, powdered fluorine rubber, butyronitrile rubber latex, boron-tung oil modified phenolic resin and moderate additives. The production method includes the steps of firstly, producing pretreatment material, mixing material and size and performing papermaking to produce raw paper of ceramic-fiber-paper-base friction material; and secondly, soaking the raw paper in adhesive, cutting into strips after hot-pressing solidification, and adhering to a friction plate core plate to obtain the ceramic-fiber-paper-based friction plate.
Owner:杭州克尔菲利科技有限公司
Preparation method of solar ultrawhite ultrathin glass and product thereof
InactiveCN102219376AIron sulfide reductionHigh whitenessGlass furnace apparatusGlass rolling apparatusFragilityIron sulfide
The invention relates to the field of production of ultrawhite rolled glass, in particular to a preparation method of solar ultrawhite ultrathin glass and a product thereof. The preparation method of the solar ultrawhite ultrathin glass comprises the following steps of: selecting and preparing raw materials; conveying the raw materials; melting; forming glass; annealing; detecting; and cutting and packaging. In the preparation method, iron content is controlled by taking different measures in the steps of selecting and conveying the raw materials, so that iron sulfide in the final solar glass is greatly reduced and the whiteness of the glass is improved; in the melting process, bubbles in the melted glass liquid is reduced by exhausting air and debubbling, controlling temperature and adding a glass clarifying agent, so that absorption rate is reduced and light transmittance is improved; and the annealing step of the glass finished product is a key link of the production process and plays an important role in the quality of the ultrathin glass product, so the defects that the ultrathin glass is easy to harden and easy to break during production and has high fragility are overcome by controlling rolling velocity, temperature and annealing rate in the process of glass forming and glass annealing.
Owner:ZHEJIANG JINGXING SOLAR ENERGY TECH
Composition for remediating iron sulfide in oilfield production systems
ActiveUS20180148632A1Surface-active detergent compositionsDrilling compositionOrganic solventIron sulfide
A composition for inhibiting corrosion and / or removing hydrocarbonaceous deposits in oil and gas applications is provided. The composition comprises an iron sulfide dissolver, an organic solvent, and a corrosion inhibitor.
Owner:CHAMPIONX USA INC
Method of preparing cathode containing Iron disulfide for a lithium cell
ActiveUS20090291366A1Improve discharge efficiencyUseful electrical energyFinal product manufactureOrganic electrolyte cellsPrimary cellAlloy
A primary cell having an anode comprising lithium or lithium alloy and a cathode comprising iron disulfide (FeS2) or a mixture of iron disulfide (FeS2) and iron sulfide (FeS) and conductive carbon particles. A cathode slurry is prepared comprising the FeS2 or FeS2 plus FeS powder, conductive carbon, binder, and a solvent. The binder is preferably a styrene-ethylene / butylene-styrene (SEBS) block copolymer. There is an advantage discovered in utilizing a hydronaphthalene solvent to form the cathode slurry. The preferred solvent is 1,2,3,4-tetrahydronaphthalene or decahydronaphthalene and mixtures thereof. The slurry mixture is coated onto a conductive substrate and the solvent evaporated leaving a dry cathode coating on the substrate. Higher drying temperature may be used resulting in a dry cathode coating which resists cracking. The anode and cathode can be spirally wound with separator therebetween and inserted into the cell casing with electrolyte then added.
Owner:DURACELL U S OPERATIONS
Method for inhibiting or controlling inorganic scale formations with copolymers of acrylamide and quaternary ammonium salts
InactiveUS7398824B1Improve efficiencyImprove thermal stabilityCleaning apparatusFluid removalZinc bromideMethyl group
A formulation containing a copolymer derived from a cationic monomer effectively inhibits and controls the formation of inorganic scales. Suitable copolymers include those comprising an acrylamide unit and a quaternary ammonium salt group, and optionally an acrylate and / or nitrogen heterocyclic monomer including those wherein the quaternary ammonium salt is a unit of the formula:wherein R is methyl or hydrogen; R4 is a C1 to C6 alkyl group, optionally substituted with halogen, hydroxyl and alkoxy groups, X is halogen; and R1, R2 and R3 are independently selected from the group consisting of alkyl and alkoxy groups. Suitable nitrogen heterocyclic compounds include N-vinylpyrrolidone, N-vinylformamide, N-vinylacetamide, N-vinylcaprolactam, N-vinylimidazole and N-vinylpyridine. The copolymers have particular applicability in the control and inhibition of zinc sulfide or iron sulfide scales, typically formed when zinc bromide brines are used as fluids in the treatment of a gas or oil well, such as a completion fluid.
Owner:BAKER HUGHES INC
Method for inhibiting or controlling inorganic scale formations
InactiveUS7159655B2Favorable result inhibition and controlParticular in controlCleaning apparatusFluid removalZinc bromideSolubility
A formulation containing a copolymer derived from a cationic monomer effectively inhibits and controls the formation of inorganic scales. The formulation has particular application in the removal of zinc sulfide and iron sulfide scales formed when zinc bromide brines are used as completion fluids. The copolymer exhibit high solubility in high-density brines, such as zinc bromide brines. The polymers may be introduced into an oil or gas well as a portion of a carrier fluid or with brine. The preferred copolymer for use in the invention contains an acrylamide unit and a diallyldimethylammonium salt and, optionally, an acrylic acid or a salt thereof. The weight average molecular weight of such inhibitor copolymers is generally between from about 500,000 to about 5,000,000.
Owner:BAKER HUGHES INC
Fiber-reinforced resin abrasive cutting wheel and preparation method thereof
InactiveCN103406837ANot suitable for breakingImprove impact resistanceBonded abrasive wheelsGrinding devicesPolymer scienceIron sulfide
The invention discloses a fiber-reinforced resin abrasive cutting wheel, and relates to the technical field of abrasive wheels. Raw materials comprise 20# white fused alumina, 24# brown fused alumina, 30# fused zirconia alumina, green silicon carbide, liquid resin, phenolic resin powder, calcium carbonate, cryolite, iron sulfide and carbon black, and material mixing, adhesive coating of meshes, superposition pressing forming and drying are carried out to obtain the finished products. The fiber-reinforced resin abrasive cutting wheel has the advantages of being rapid in cutting, grinding and heat dissipation, capable of keeping the end face to be sharp all the time in the process of cutting grinding, and low in cost.
Owner:山东英特磨具股份有限公司
Iron pyrite inhibitor for use under low-alkalinity condition
The invention discloses an iron pyrite inhibitor for use under a low-alkalinity condition. The iron pyrite inhibitor consists of the following components in parts by weight: 40-50 parts of sodium thiosulfate, 40-50 parts of citric acid and 5-15 parts of polyacrylamide, wherein the polyacrylamide is hydroxyl-containing low molecular polyacrylamide of which the molecular content is 400-600. The inhibitor is prepared by using a method comprising the following steps of weighing sodium thiosulfate, citric acid and polyacrylamide; uniformly mixing to obtain 1-5 percent by weight of aqueous solution; and stirring. The iron pyrite inhibitor is used for selectively inhibiting iron sulfide minerals under a low-alkalinity condition, has good inhibition effects on minerals such as pyrite, magnetic pyrite and the like, has the advantages of stable performance, small using amount, low cost and environment friendliness, can be widely applied to sulfide ore dressing of copper, copper lead, copper zinc, lead zinc, copper lead zinc, gold and the like, and contributes to effectively increasing the scoring indexes of copper, lead, zinc, metal minerals and pyrite, effectively enhancing the flotation separation effect of multi-metal sulfide minerals and increasing the recovery rate of associated gold and silver.
Owner:NORTHWEST RES INST OF MINING & METALLURGY INST
Method and composition to decrease iron sulfide deposits in pipe lines
InactiveUS6986358B2Easy manipulation and maintenance and removalTrend downDetergent mixture composition preparationSolid sorbent liquid separationIron sulfideSolvent
The levels of iron sulfide present in a conduit, such as a pipeline, are reduced by contacting the conduit, on an inner surface, with a composition obtained from an aqueous solution containing at least one compound of Formula (I) and at least one amine or corresponding ammonium derivative in the presence of a solvent, wherein X is an anion of valency n. Preferably, the pH of the solution is about 8. Alternatively, the method employs a composition comprising tris(hydroxymethyl)phosphine (TRIS) and at least one amine or corresponding ammonium derivative. The amine preferably is ammonia or a primary alkylamine. The compositions readily complex and thereby dissolve deposits of iron(II) sulfide, removing them from the conduit.
Owner:COASTAL CHEM CO L L C +1
Smelting process of steel for high-titanium alloy welding wire
ActiveCN102586685AGood deoxygenationImprove purityElectric furnaceProcess efficiency improvementFerrosiliconTitanium alloy
The invention relates to a production process of steel, in particular to a smelting process of steel for a high-titanium alloy welding wire. The smelting process comprises the following steps of: in an electric furnace smelting step, adding molten iron and waste steel and tapping molten steel, wherein the molten iron accounts for 50-60% the total amount of the furnace charge, and the tapping temperature is not lower than 1630 DEG C; in a refining furnace refining step, adding silicon-aluminum-calcium deoxidant in a refining furnace for manufacturing white slag through diffusion deoxidization, and adding low-carbon ferromanganese and ferrosilicon according to steel grade component requirements and sampling component analysis to regulate components of the molten steel so as to reach temperature of 1630-1650 DEG C matching vacuum treatment; in a vacuum furnace degassing step, keeping the temperature for 10-15min under a low vacuum degree of 1 millibar, and carrying out secondary refining after vacuum breakage; and in a secondary refining step, adding low-aluminum ferrotitanium to regulate titanium content at the refining temperature of 1585-1600 DEG C, stirring for 1-2min, adding iron sulfide, stirring for 1-2min, taking a lollipop sample to analyze, continuously keeping static stirring, carrying out soft argon blowing for 15-40min after all components are qualified and carrying out continuous casting when the temperature is up to 1565-1585 DEG C. The smelting process disclosed by the invention can effectively control purification degree of the molten steel, ensures stability of titanium in the molten steel and improves the surface quality of a cast blank.
Owner:NANJING IRON & STEEL CO LTD
Thickened oil well polymer plugging remover
ActiveCN106634915AEffective dispersionEfficient degradationDrilling compositionPotassiumPotassium peroxodisulfate
The invention discloses a thickened oil well polymer plugging remover. The plugging remover is prepared from the following materials in percentage by mass: 0.5 to 5.0 percent of gel breaker, 1.0 to 10.0 percent of cleaning agent, 0.1 to 5.0 percent of clay stabilizer, 0.1 to 5.0 percent of iron ion stabilizer, 0.1 to 5.0 percent of surfactant, 0.1 to 5.0 percent of corrosion inhibitor, 0.1 to 5.0 percent of bactericide and the balance of water, wherein the gel breaker is prepared from 30 to 70 percent of gel breaker I and 30 to 70 percent of gel breaker II, the gel breaker I is one or more of ammonium persulfate, sodium thiosulfate, potassium periodate and potassium monopersulfate, and the gel breaker II is one or more of calcium peroxide, potassium peroxodisulfate, sodium periodate and iron sulfide. The thickened oil well polymer plugging remover is suitable for solving the plugging problem caused by a thickened oil well polymer, has a better plugging removal effect than a conventional single oxidizer or single acid liquor, and has the advantages of good plugging removal effect, strong corrosion resistance, long period of validity, safety and the like.
Owner:SOUTHWEST PETROLEUM UNIV
System and methods for biological selenium removal from water
A biological system for removing selenium from waste water comprises a first immobilized cell bioreactor (ICB) and a selenide removal module. The first ICB comprises a chamber having a substrate housed therein and situated to contact the waste water flowing therethrough during use. Anaerobic microorganisms are supported on the substrate, and comprise selenium respiring bacteria capable of reducing selenates and selenites to insoluble elemental selenium and / or sulfate reducing bacteria capable of reducing selenates and selenites to insoluble elemental selenium or to soluble selenides. The selenide removal module includes metallic or oxidized iron compounds capable of chemically reacting with selenide or sulfide compounds in the waste water to form iron selenide or iron sulfide precipitates.
Owner:UOP LLC
Preparation method and application of carbon-sulfur doped zero-valent iron composite material
InactiveCN110482671ARich sourcesLow costWater contaminantsContaminated soil reclamationIron powderIron sulfide
The invention discloses a preparation method of a carbon-sulfur doped zero-valent iron composite material, which comprises the following the step: proportionally mixing and ball-milling a sulfur reagent, iron powder and carbon powder to obtain the carbon-sulfur doped zero-valent iron composite material; the sulfur reagent is elemental sulfur powder, iron sulfide powder or pyrite powder; the mass percentage ratio of the sulfur reagent to the iron powder to the carbon powder is (0.5-20): (70-99): (0.5-10). The sulfur reagent, the iron powder and the carbon powder are mixed and then subjected toball milling to obtain the carbon-sulfur doped zero-valent iron composite material, and the carbon-sulfur doped zero-valent iron composite material can be used for in-situ removal and degradation of heavy metal pollutants, pesticide pollutants, azo dye pollutants, halogenated organic matter pollutants and / or nitro organic matter pollutants in underground water and soil and has high removal and degradation efficiency.
Owner:ZHEJIANG UNIV OF TECH
Preparation method of graphene oxide/iron disulfide composite nano particles
InactiveCN102910615AUniform particle sizeGood dispersionMaterial nanotechnologyCarbon compoundsHydration reactionPolyvinyl alcohol
The invention relates to nano FeS2 materials, and particularly relates to a preparation method of graphene oxide / iron disulfide composite nano particles. According to the preparation method of the graphene oxide / iron disulfide composite nano particles, ferrous chloride tetrahydrate (FeCl2.4H2O) and sulfur powder are used as raw materials, are dissolved in water together with surfactant polyvinyl alcohol (PVA) and polyvinylpyrrolidone (PVP), and then mixed with graphene oxide under a certain pH (potential of hydrogen) condition; the obtained mixed solution is arranged in a reaction kettle and sealed, is held for 12 hours to 24 hours in a constant-temperature box at 180 DEG C to 200 DEG C by using a hydrothermal method, is cooled to normal temperature, stood and separated, centrifugally washed and dried, finally the graphene oxide / iron disulfide composite nano particles can be obtained.
Owner:JIANGSU UNIV
Use of ozone to increase the flotation efficiency of sulfide minerals
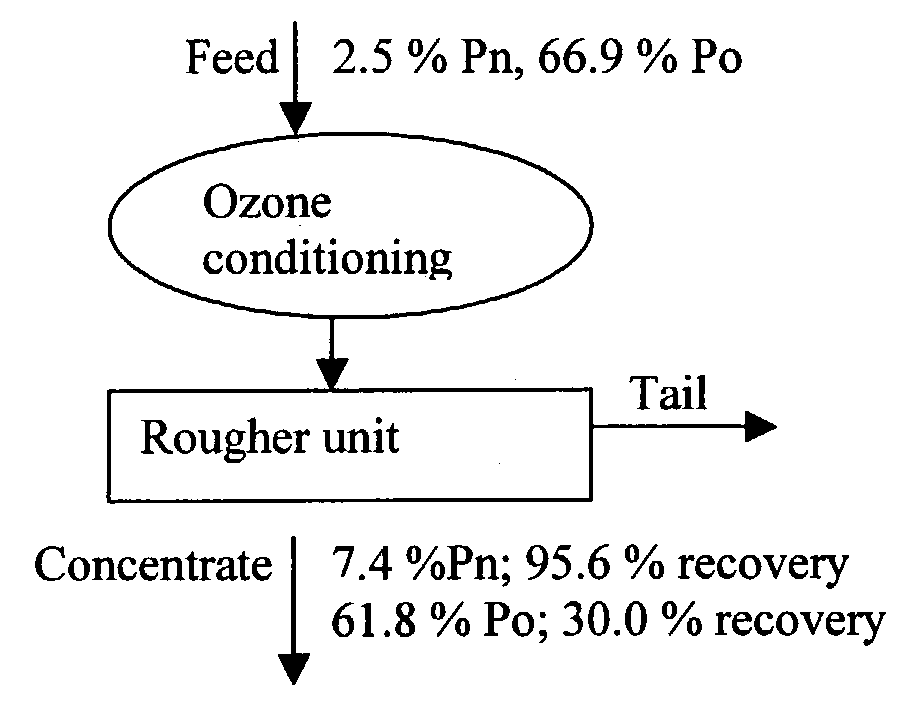
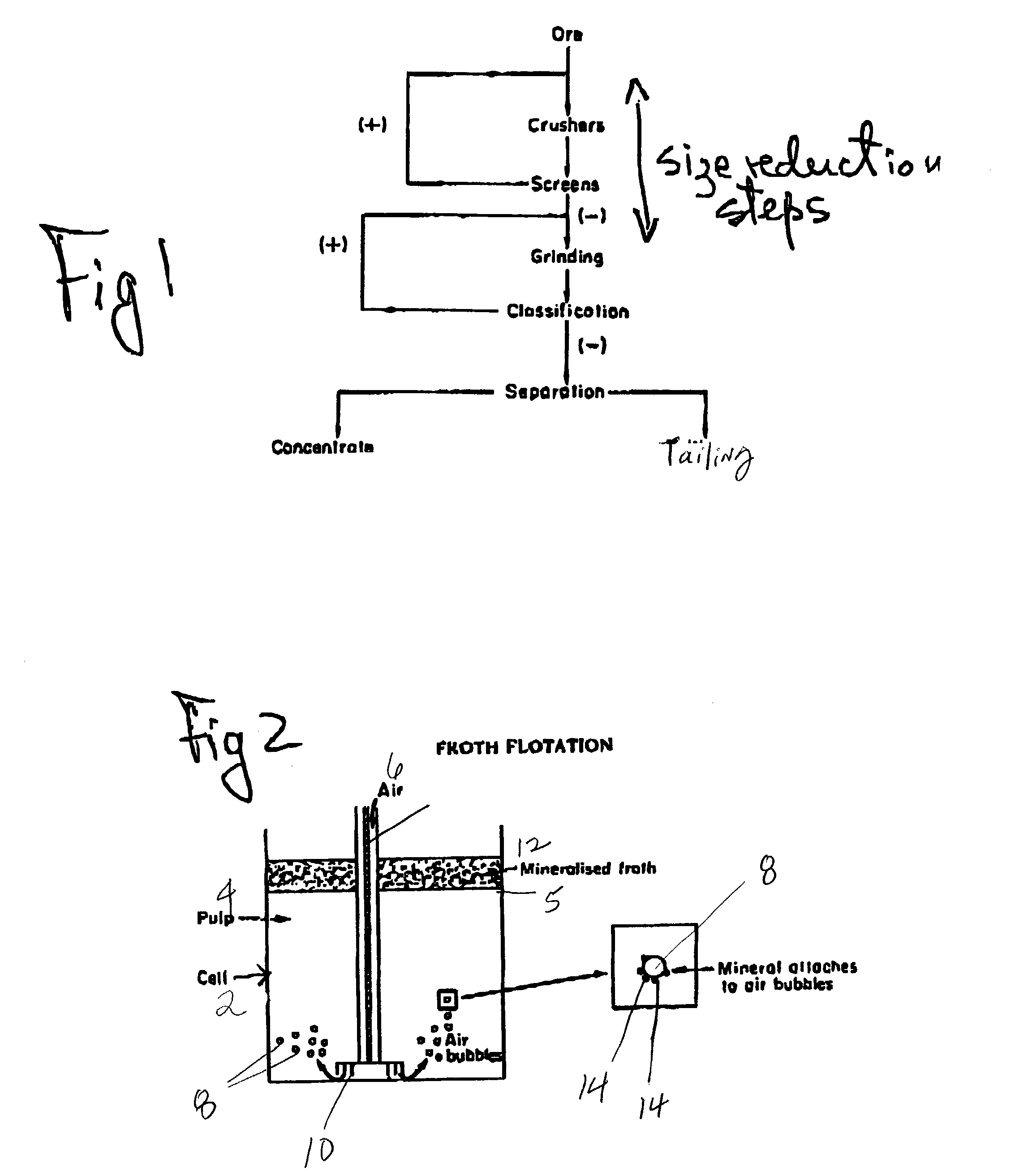
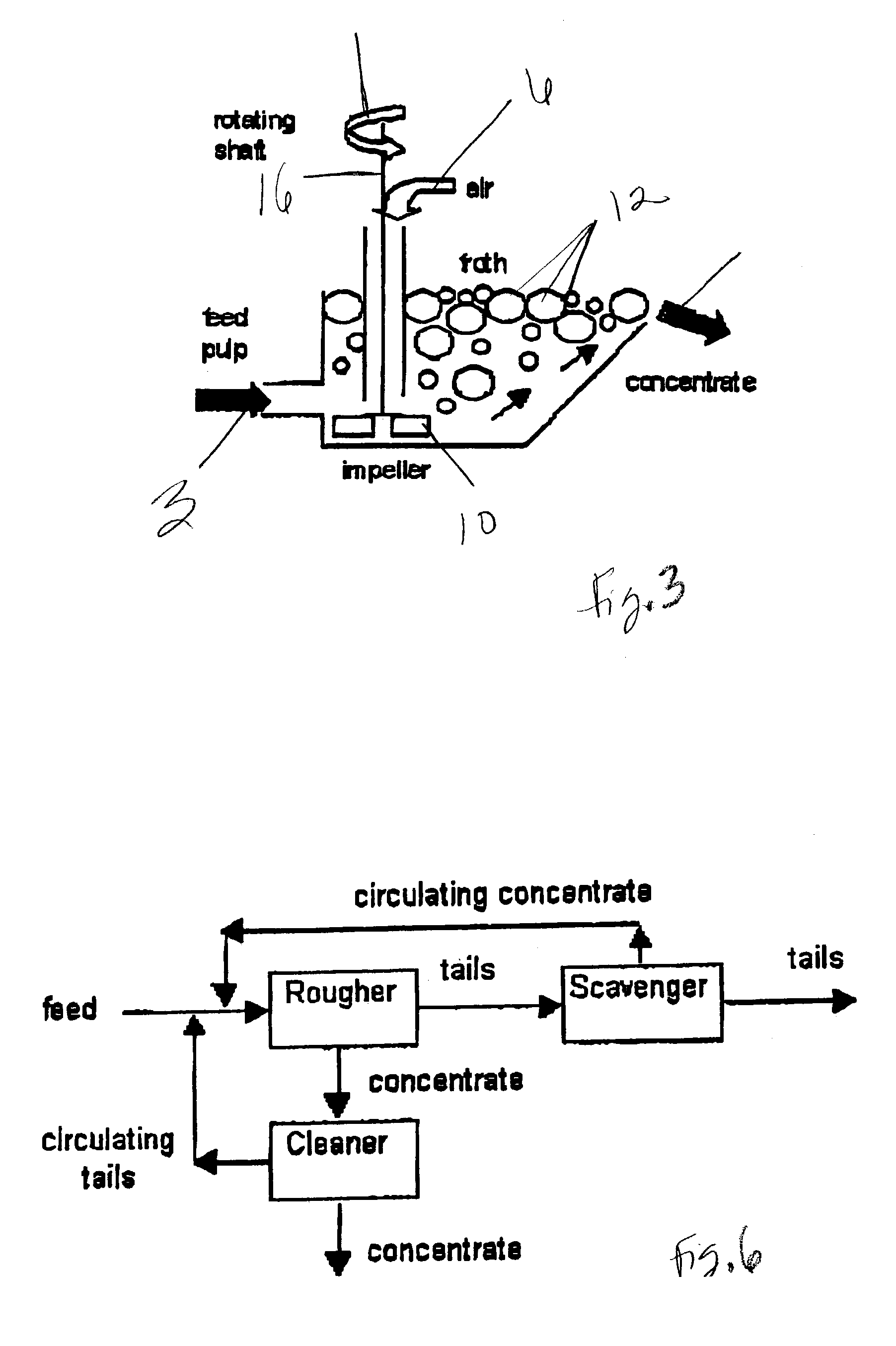
The use of ozone during certain stages of metal separation by flotation degrades certain collectors that are absorbed on the particle surface, as well as the collectors and frothers in the liquid slurry. As a result, the mineral particle has a fresh surface and new chemical reagent(s) can be added in the subsequent flotation step(s). Also since ozone oxidizes the iron sulfide particles faster than the other mineral particles, depending upon the duration of treatment, the ozone concentration, and the kg O3 / ton consumed by the treated ore, the surface of the iron sulfide particles may be partially or even totally oxidized, thus allowing better separation. As a consequence, the iron content is decreased, and the grade of the mineral value such as zinc, copper, and nickel increases. Also, sulfide emissions during heat treatment or further processing of the minerals are decreased due to decrease iron sulfide content.
Owner:LAIR LIQUIDE SA POUR LETUDE & LEXPLOITATION DES PROCEDES GEORGES CLAUDE +1
Lithium-iron disulfide anode materials and method for preparing same
InactiveCN1877887AImprove conductivityHigh voltage platformPrimary cell electrodesMetallurgyHigh energy
The invention discloses a preparing technology of high-energy Li-marcasite battery material, which comprises the following steps: covering conductivity and stable metal oxide on the surface of marcasite with composite conductive; setting the percentage of marcasite at 82-94 percent, conductive percentage at 4-10 percent and metal oxide percentage at 2-8 percent. The method reduces grain size of natural marcasite material through wetting ball grinding technology, which prepares the needed anode material.
Owner:TSINGHUA UNIV
Asymmetric electrochemical supercapacitor and method of manufacture thereof
InactiveUS7576971B2Increase energy densityLarge capacityHybrid capacitor electrodesLiquid electrolytic capacitorsAqueous electrolyteLithium manganese oxide
Asymmetric supercapacitors comprise: a positive electrode comprising a current collector and a first active material selected from the group consisting of manganese dioxide, silver oxide, iron sulfide, lithium manganese oxide, lithium cobalt oxide, lithium nickel oxide, lithium iron phosphate, and a combination comprising at least one of the foregoing active materials; a negative electrode comprising a carbonaceous active material; an aqueous electrolyte solution selected from the group consisting of aqueous solutions of hydroxides of alkali metals, aqueous solutions of carbonates of alkali metals, aqueous solutions of chlorides of alkali metals, aqueous solutions of sulfates of alkali metals, aqueous solutions of nitrates of alkali metals, and a combination comprising at least one of the foregoing aqueous solutions; and a separator plate. Alternatively, the electrolyte can be a non-aqueous ionic conducting electrolyte or a solid electrolyte.
Owner:U S NANOCORP
Process for using catalyst with rapid formation of iron sulfide in slurry hydrocracking
InactiveUS20110308997A1Efficient responseLess hydrogen requiredCatalyst activation/preparationHydrocarbon oil crackingHydrogenIron sulfide
A process is disclosed for converting heavy hydrocarbon feed into lighter hydrocarbon products. The heavy hydrocarbon feed is slurried with a catalyst comprising iron oxide and alumina to form a heavy hydrocarbon slurry and hydrocracked to produce lighter hydrocarbons. The iron oxide in the catalyst converts to catalytically active iron sulfide in the presence of hydrogen and sulfur.
Owner:UOP LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com