Patents
Literature
192 results about "Neutralization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which an acid and a base react quantitatively with each other. In a reaction in water, neutralization results in there being no excess of hydrogen or hydroxide ions present in the solution. The pH of the neutralized solution depends on the acid strength of the reactants.
Method for the detection and neutralization of bacteria
The present invention relates to the identification of bacteria present at the site of infection and the treatment of the infection using bacteriophage. In certain embodiments, the present invention provides methods and compositions for treating bacterial infections by identifying at least one bacteria species in the infection based on its interaction with bacteria-specific aptamers, selecting one or more bacteriophage that infect the identified bacteria species, and administering an effective amount of the bacteriophage to the subject to treat the infection.
Owner:KCI LICENSING INC
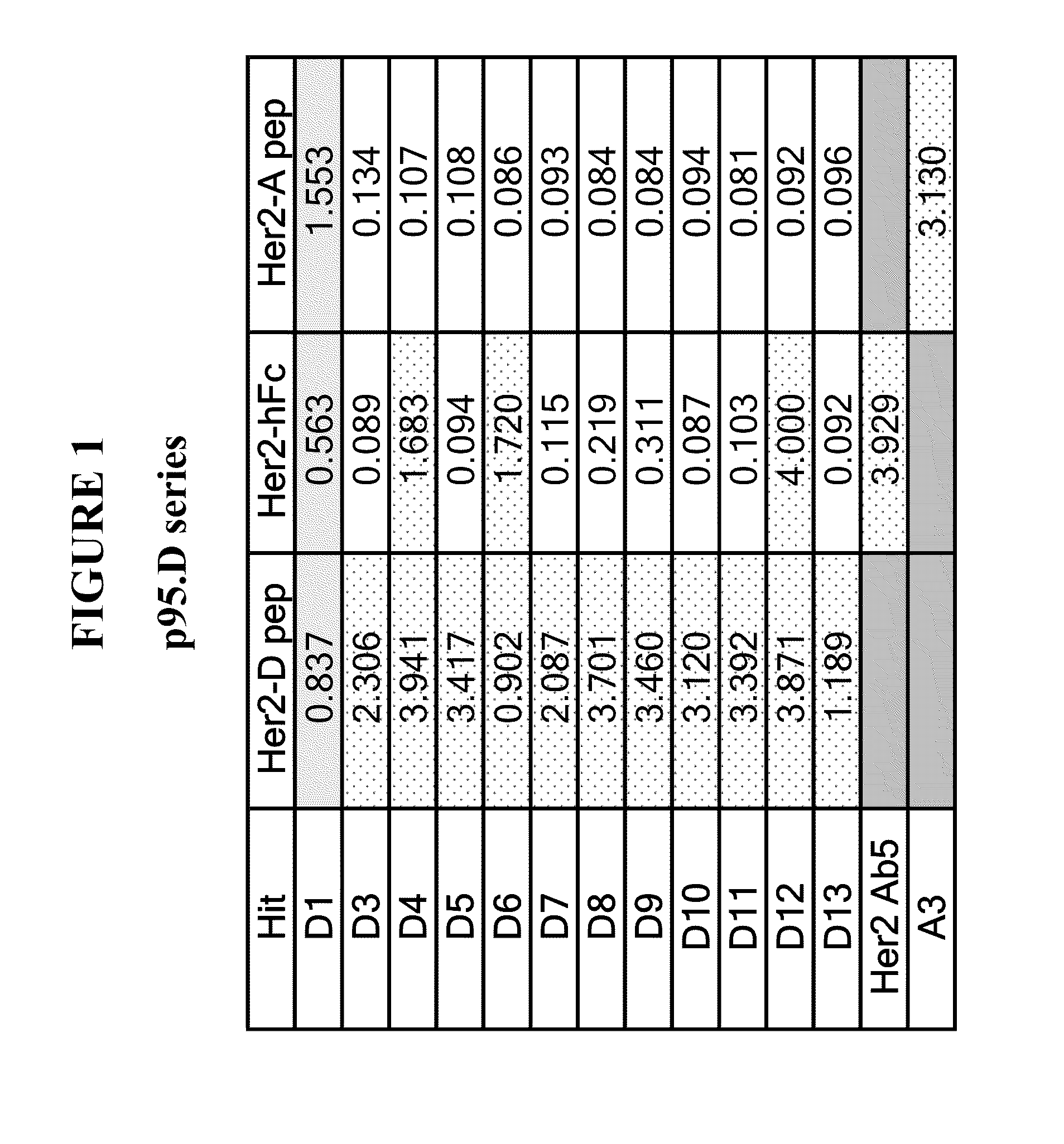
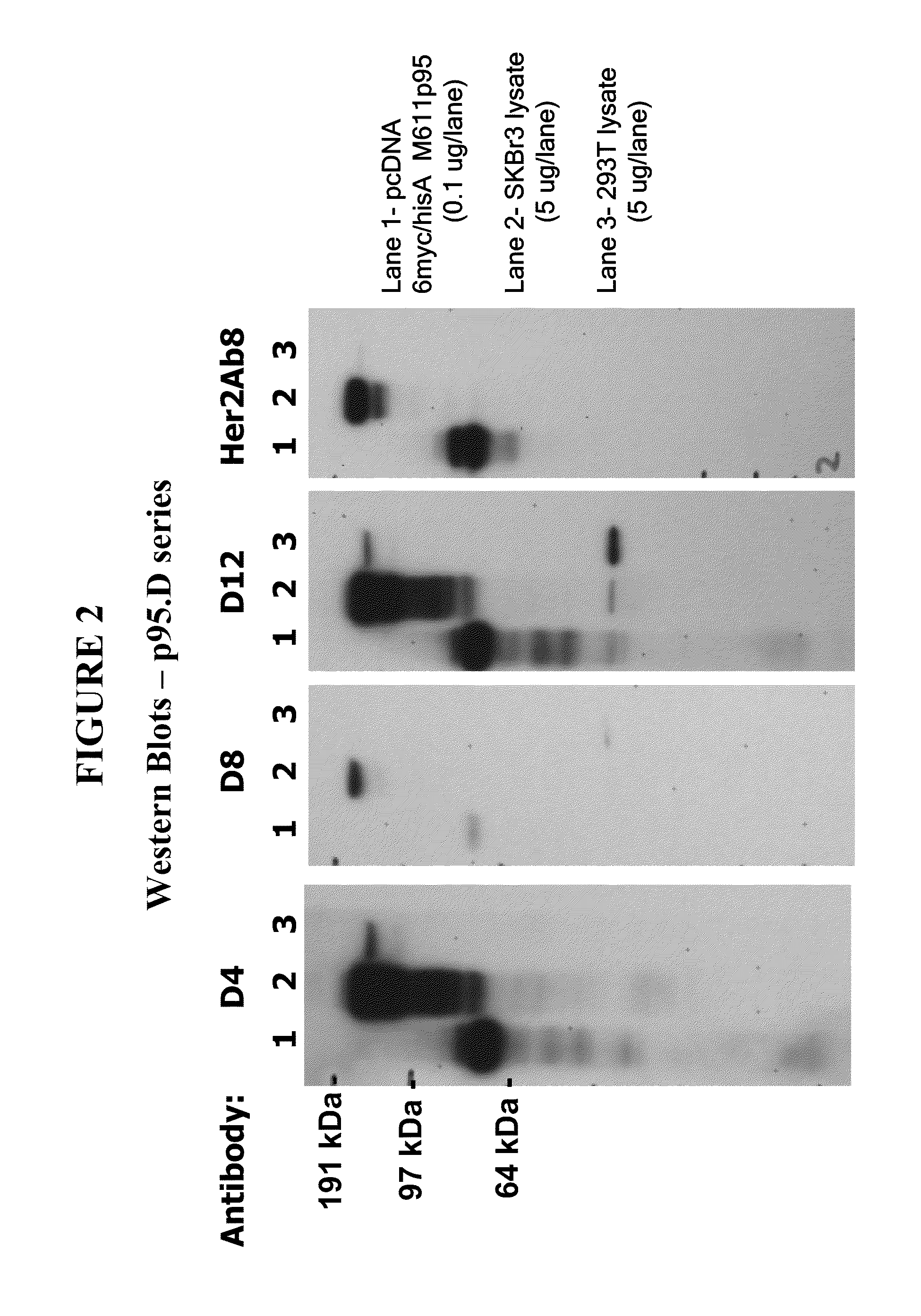
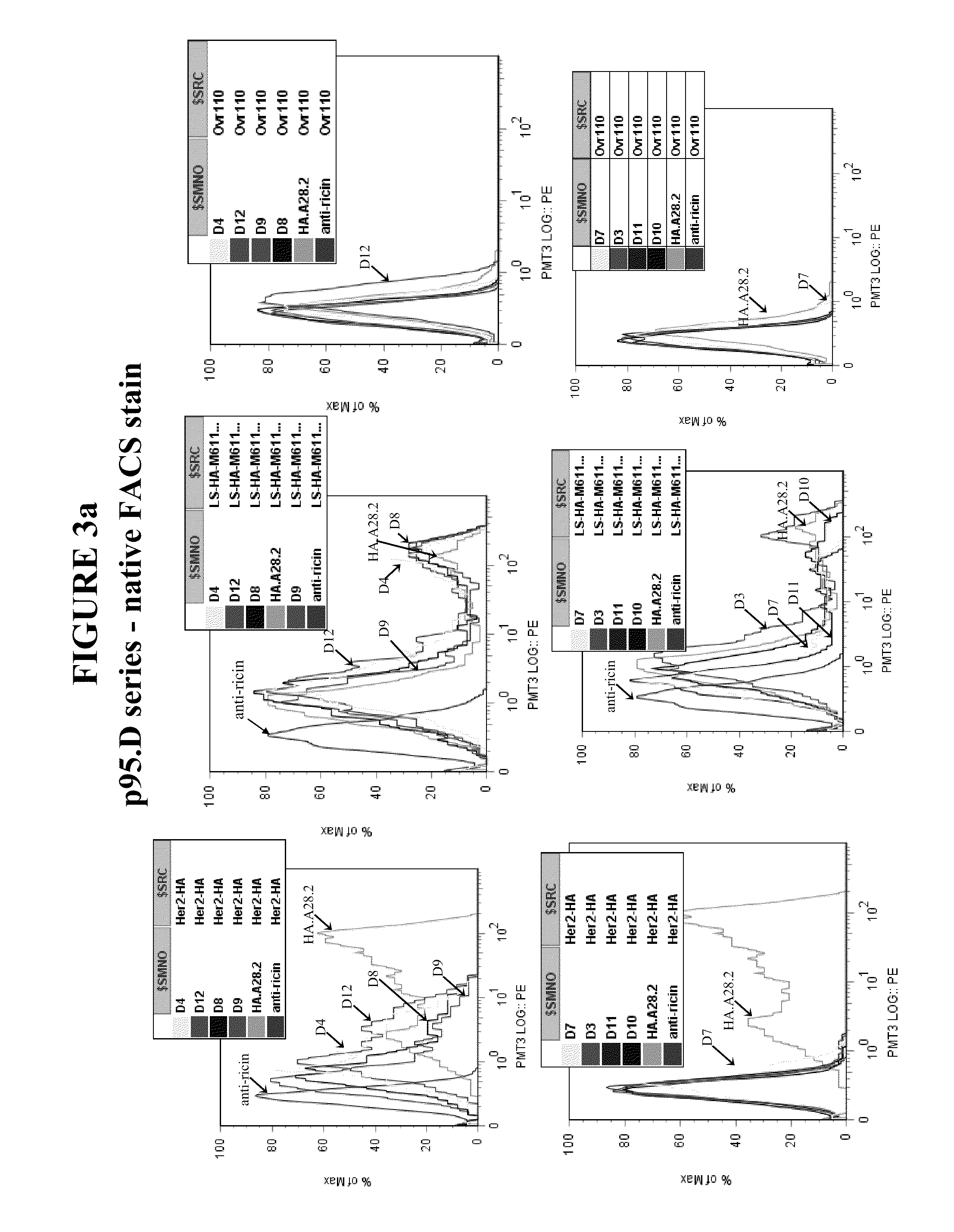
Methods and Assays for Measuring p95 and/or p95 in a Sample and Antibodies Specific for p95
Owner:LAB OF AMERICA HLDG
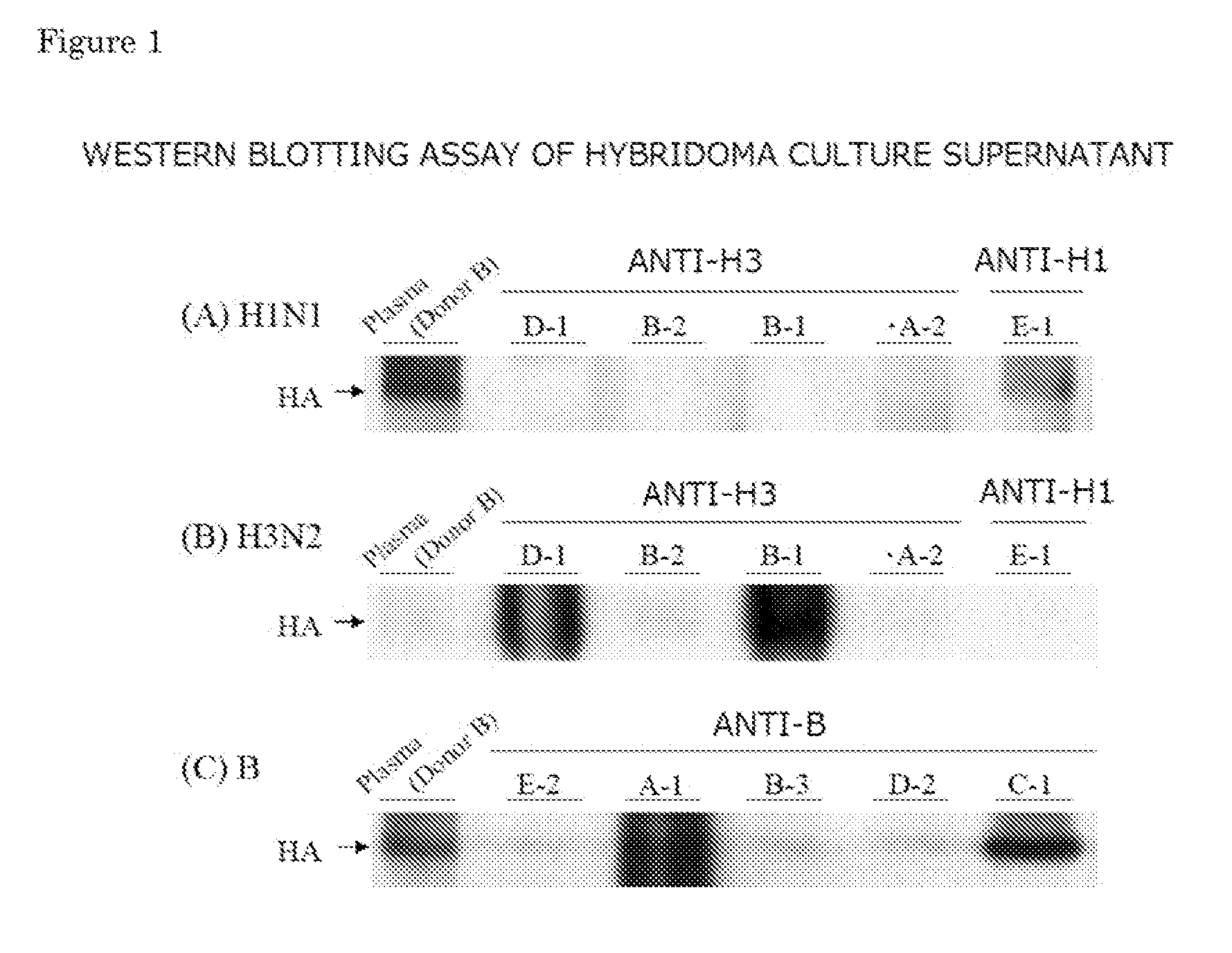
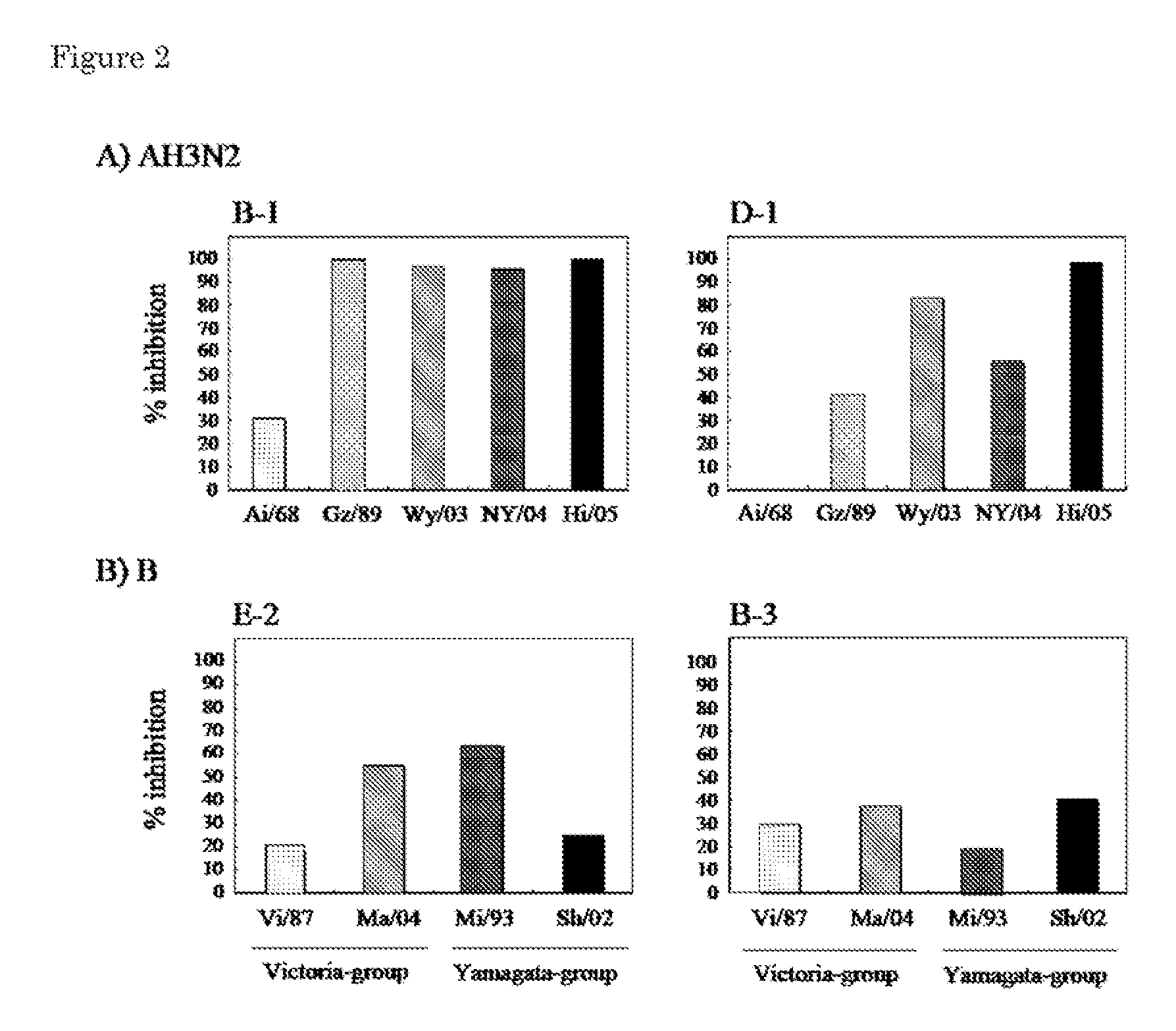
Human Anti-Human Influenza Virus Antibody
InactiveUS20110319600A1Effect survival rateEffect weight lossSugar derivativesImmunoglobulins against virusesHemagglutininHuman Influenza A Virus
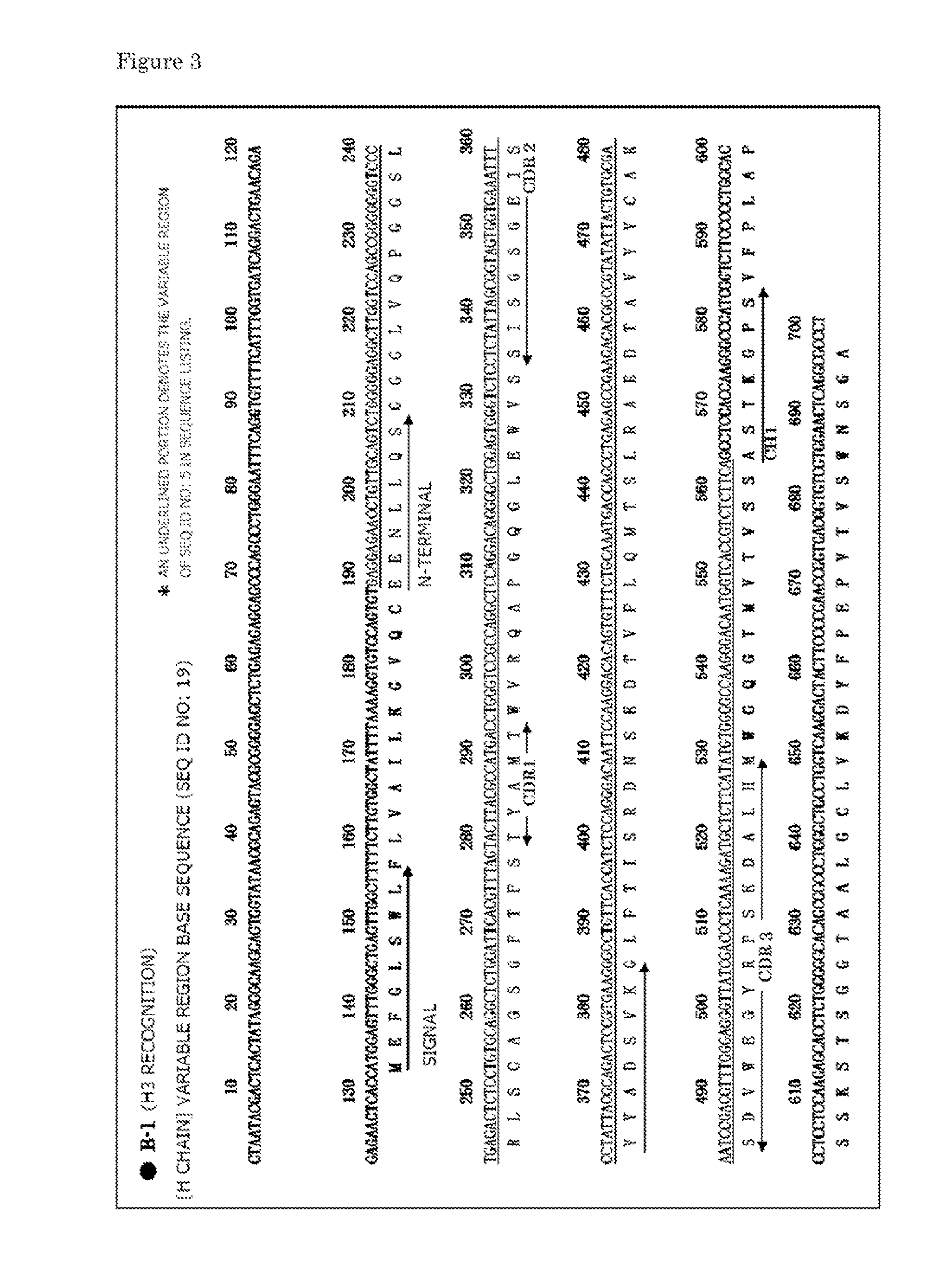
Provided is a human antibody having a neutralization activity against a human influenza virus. More specifically, provided is a human antibody which recognizes a highly conserved region in a human influenza A virus subtype H3N2 or a human influenza B virus and has a neutralization activity against the virus. The human antibody is a human anti-human influenza virus antibody, which has a neutralization activity against a human influenza A virus subtype H3N2 and binds to a hemagglutinin HA1 region of the human influenza A virus subtype H3N2, or which has a neutralization activity against a human influenza B virus, and includes, as a base sequence of a DNA encoding a variable region of the antibody, a sequence set forth in any one of SEQ ID NOS: 5 to 12.
Owner:OSAKA UNIV +1
Peptides which elicit a high neutralizing antibody titer, cytotoxic T lymphocyte response and T helper cell response in a broad range of MHC type recipients
InactiveUS7094405B1High titerHigh titer of neutralizing antibodyPeptide/protein ingredientsAntibody mimetics/scaffoldsV3 loopT helper cell
Peptide constructs comprised of multideterminant T helper peptides from the envelope glycoprotein of HIV previously identified to induce proliferative responses in four different haplotypes of mice and IL-2 responses in 52-73% of HIV positive, flu positive patients (cluster peptides), were co-linearly synthesized with the peptide 18 of the V3 loop of HIV-1 gp 160, corresponding to the principal neutralizing determinant of HIV-IIIB and also shown to contain a dominant CTL epitope. Cognate help for peptide 18 antibody was elicited following a single immunization in all strains of mice which had previously responded to a T cell epitope encompassed by the peptides. In two strains of mice, the level of neutralizing antibody achieved was comparable to levels adequate for protection from homologous viral challenge in chimpanzees. After a single boost, much higher antibody titers for 90% neutralization in the range of 1:1000 to 1:16,000 were achieved. Spleen cells from mice of three distinct MHC haplotypes sharing the Dd class I MHC molecule but with different class II molecules, immunized with the compound peptides, exhibited enhanced gp160-specific CTL activity.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Anti-H7N9 full-human-derived monoclonal antibody 5J13 and preparation method and application thereof
ActiveCN106519027AHigh affinityImprove featuresImmunoglobulins against virusesAntiviralsHemagglutininSide effect
The invention relates to an anti-H7N9 full-human-derived monoclonal antibody 5J13 and a preparation method and application thereof. Amino acid sequences of heavy and light chain CDR1, CDR2 and CDR3 of the antibody are GFSFSNYG in the heavy chain CDR1 area, ISYDGTNK in the heavy chain CDR2 area, AKGRGPYCSSSICYHGMDV in the heavy chain CDR3 area, QSVLSGSINMNY in the light chain CDR1 area, WAS in the light chain CDR2 area and QQYYSTPLT in the light chain CDR3 area correspondingly. The antibody can be combined with hemagglutinin A of the H7N9virus in a targeted mode and has remarkable neutralization activity in resisting H7N9 virus infection. Compared with a murine antibody, genes of the full-human-derived antibody are completely from human genes and have no components of other species, toxic and side effects such as the anti-mouse anti-antibody are avoided, the biocompatibility is better, and the anti-H7N9 full-human-derived monoclonal antibody 5J13 is more suitable and has more potential in becoming a macromolecular drug for treating influenza virus.
Owner:SHENZHEN INST OF ADVANCED TECH
Chemiluminiscence detection kit for 5 items of hepatitis B and preparation method thereof
ActiveCN103163294ASave manpower costLow costChemiluminescene/bioluminescenceQuantitative determinationChemistry
The invention provides a chemiluminiscence detection kit for 5 items of hepatitis B and a preparation method thereof and relates to the kit for hepatitis B detection. The kit comprises a coated micropore reaction plate, a biotin marker, an alkaline phosphatase marker, a neutralization reagent, a calibrator, luminescent substrate liquid and a concentrated cleaning liquid. The preparation method comprises the following steps of: respectively preparing the streptavidin pre-coated reaction plate, the calibrator, the biotin marker, the alkaline phosphatase marker, the neutralization reagent, the luminescent substrate liquid and the concentrated cleaning liquid, and assembling for preparing the chemiluminiscence quantitative detection kit for 5 items of the hepatitis B. The prepared chemiluminiscence detection kit for 5 items of the hepatitis B has the advantages of being capable of sensitively detecting 5 items of the hepatitis B, also being capable of carrying out accurate quantitative determination on 5 items of the hepatitis B, being low in cost, simple in operation, accurate in results and environment-friendly without pollution and having long-term interests.
Owner:厦门市波生生物技术有限公司
Diagnostic method for detection of molecular forms of eosinophilic cationic protein (ISO-ECPS)
InactiveUS6733980B1Addressing Insufficient SensitivitySolve the lack of precisionOrganic active ingredientsImmunoglobulins against cytokines/lymphokines/interferonsEpitopeCytotoxicity
Diagnostic methods comprise measuring specifically the level of at least one iso-eosinophilic cationic protein (iso-ECP) in a sample from an individual to be diagnosed, and comparing the measured level of the iso-ECP with a predetermined level of the iso-ECP. The iso-ECP is cytotoxic and the cytotoxicity of the iso-ECP is not capable of neutralization by the monoclonal antibodies EG1 and EG2. Anti-iso-ECP antibody which may be used in the diagnostic methods specifically binds to a cytotoxic isoform of ECP having an epitope which is unique for the native form of the cytotoxic iso-ECP.
Owner:PHARMACIA DIAGNOSTICS +1
Completely humanized neutralizing antibody for anti-rabies viruses
ActiveCN103910796ALow priceQuickly builds immune protectionHybrid immunoglobulinsImmunoglobulins against virusesAntigenAntigen epitope
The invention discloses a completely humanized neutralizing antibody for anti-rabies viruses. The amino acid sequence of a heavy chain of the antibody is shown in sequence table SEQ ID NO1, the amino acid sequence of a light chain of the antibody is shown in sequence table SEQ ID NO5, the complementary determining region (CDR) of a variable region of the heavy chain of the antibody is shown as follows: CDR1: SEQIDNO2, CDR2:SEQIDNO3 and CDR3: SEQIDNO4, and the complementary determining region (CDR) of a variable region of the light chain of the antibody is shown as follows: CDR1:SEQIDNO6, CDR2:SEQIDNO7, and CDR3: SEQIDNO8. The beneficial effects of the completely humanized neutralizing antibody are that: the completely humanized neutralizing antibody disclosed by the invention ahs the advantages of being completely humanized, good in specificity, high in affinity, good in neutralizing effect, and low in price, can be used as a biological engineering antibody drug to quickly establish immune protection force against rabies virus, can be used for passive immunotherapy of acute infection, and also can be used for preparing detection reagents for detecting the rabies virus, finding effective neutralization antigen epitope and developing recombinant proteins and subunit vaccines for the rabies virus.
Owner:CHANGCHUN BCHT BIOTECH
Anti-H7N9 full human derived monoclonal antibody hIg311 and preparation method and application thereof
ActiveCN110746503AGood neutralizing activityGood biocompatibilityImmunoglobulins against virusesAntiviralsHemagglutininAntiendomysial antibodies
The application relates to an anti-H7N9 full human derived monoclonal antibody hIg311 and a preparation method and application thereof. A memory B cell PCR method is used for quickly screening a fullhuman derived monoclonal antibody hIg311, and the anti-H7N9 human derived monoclonal antibody hIg311 is free from any mouse derived components. The antibody disclosed by the application can realize target combination of hemagglutinin HA of H7N9 viruses, and has notable anti H7N9 viral infection neutralization activity. The antibody disclosed by the application does not generate toxic and side effects for resisting mouse resisting antibodies, has good biocompatibility, and is suitable for becoming and has potential to become macromolecular drugs for treating influenza viruses.
Owner:SHENZHEN INST OF ADVANCED TECH
Method for detecting canine rabies virus antibody and detection kit
The invention provides a method for detecting canine rabies virus antibody (IgG) and a detection kit. The kit is composed of a coated plate and a reagent reaction system, and comprises a rabies virus antigen-coated reaction plate, a standard substance, positive contrast serum, a washing lotion (20X), a sample weak solution, an enzyme-marked combination substance, a developer A, a developer B and a stopping solution. The method is characterized in that the method uses an ELISA method to determine that the content of the canine rabies virus antibody (IgG) reaches a absorbance corresponding to a protective level by detecting the standard substance of the kit, and by compared the absorbance of the sample to be detected with the absorbance of the standard substance, the content of the canine rabies virus antibody (IgG) contained in the sample to be detected is judged whether to reach an immunization protective level. The method and the kit can simultaneously detect a lot of samples, and a detection result is remarkably with a result by a neutralization experiment, has high accuracy degree, is suitable for monitoring an immunization inoculation effect and determining an individual immunization state, and can be used for investigating animal eqpidemic diseases.
Owner:ZHENGZHOU ZHONGDAO BIOTECHNOLOGY CO LTD
Hybridoma cell strain secreting high-neutralization activity infectious bursal disease virus (IBDV) monoclonal antibody
ActiveCN103232974AHigh neutralization potencyAnd high potencyMicroorganism based processesImmunoglobulins against virusesMonoclonalInfectious bursitis
The invention relates to a hybridoma cell strain secreting a high-neutralization activity infectious bursal disease virus (IBDV) monoclonal antibody, and belongs to the field of biotechnology. The hybridoma cell strain C128 is screened from an established bank of hybridoma cells secreting the IBDV monoclonal antibody, and a mice ascites monoclonal antibody prepared by the hybridoma cell strain has IBDV neutralization titer of 1010. The high-neutralization activity IBDV monoclonal antibody secreted by the hybridoma cell strain C128 has high neutralization titer and a wide IBDV strain resistance range, can be used for clinical treatment on IBD-infected animals, and has obvious curative effects, good safety and no adverse side reaction. A monoclonal antibody preparation prepared by the hybridoma cell strain C128 keeps neutralization titer after being preserved for 2 years and has good stability.
Owner:JIANGSU ACAD OF AGRI SCI
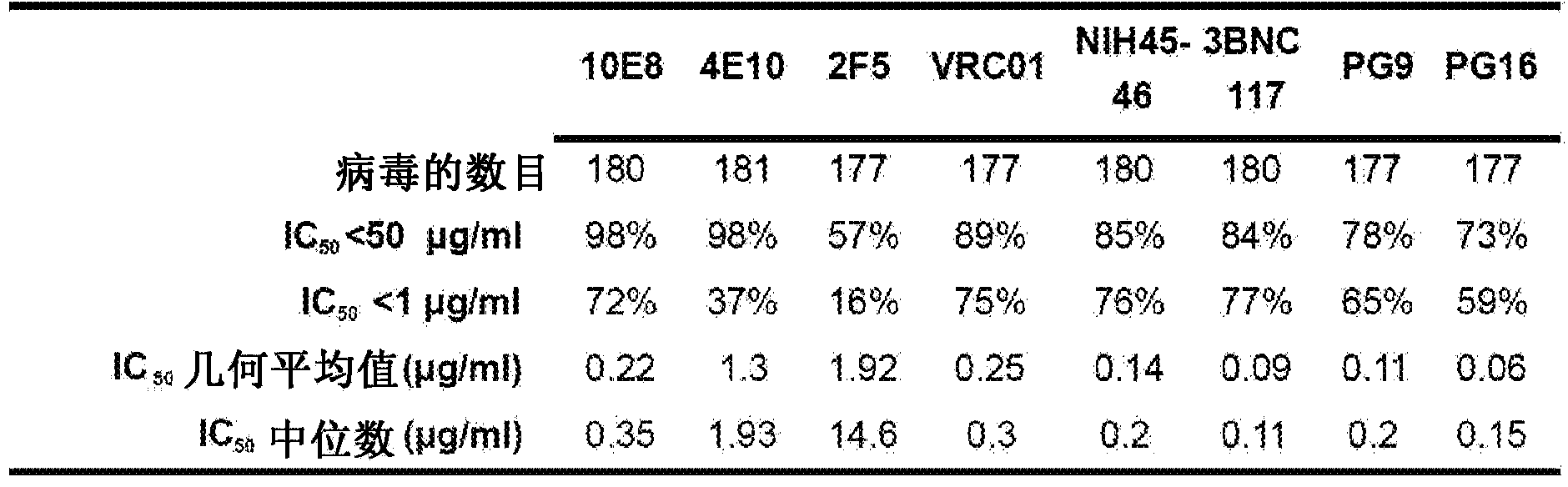
Neutralizing gp41 antibodies and their use
Monoclonal neutralizing antibodies are disclosed that specifically bind to the HIV-1 gp41 membrane-proximal external region (MPER). Also disclosed are compositions including the disclosed antibodies that specifically bind gp41, nucleic acids encoding these antibodies, expression vectors including the nucleic acids, and isolated host cells that express the nucleic acids. The antibodies and compositions disclosed herein can be used for detecting the presence of HIV-1 in a biological sample, or detecting an HIV-1 infection or diagnosing AIDS in a subject. In additional, the broad neutralization breadth of the disclosed antibodies makes them ideal for treating a subject with an HIV infection. Thus, disclosed are methods of treating and / or preventing HIV infection.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
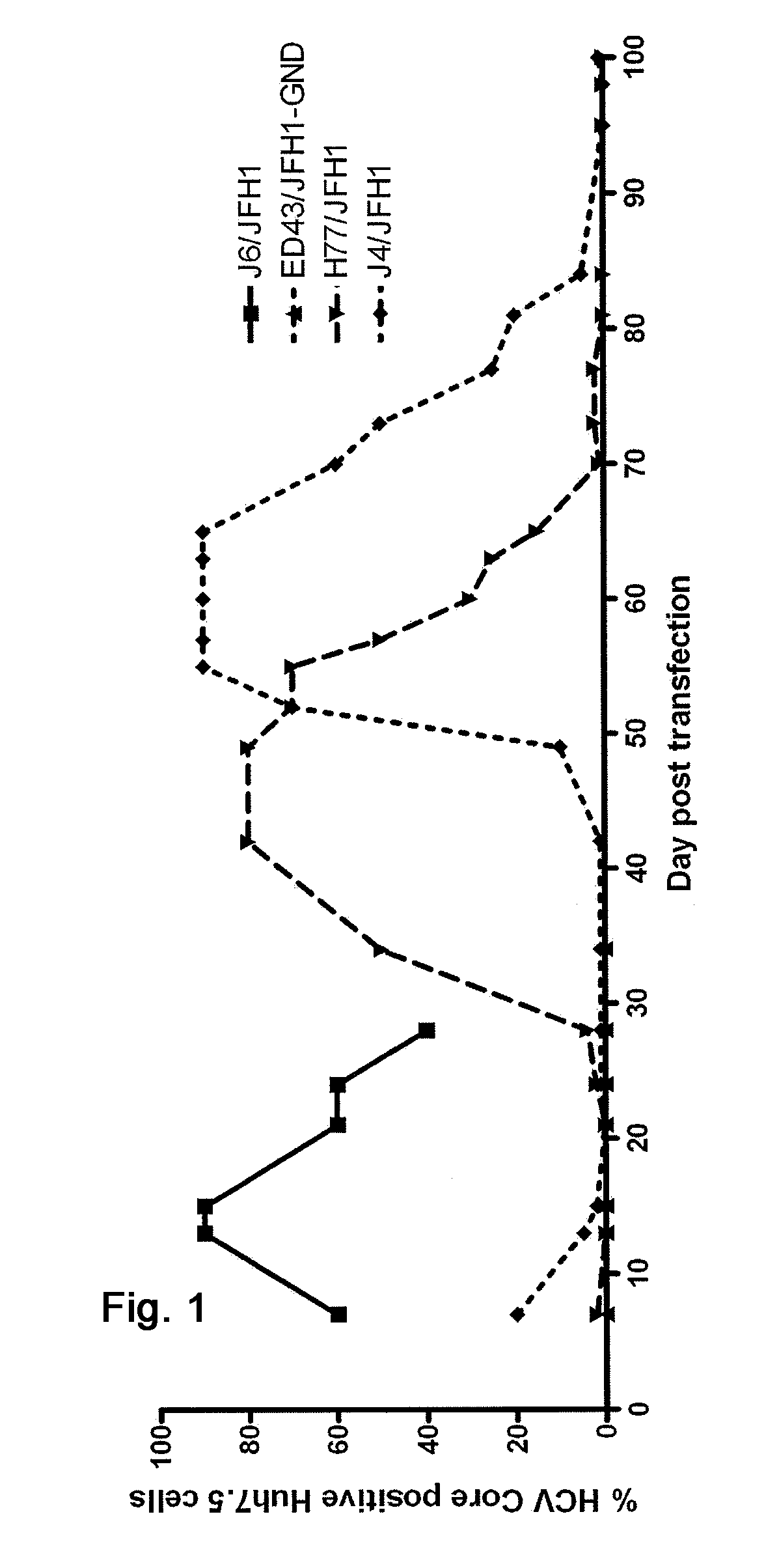
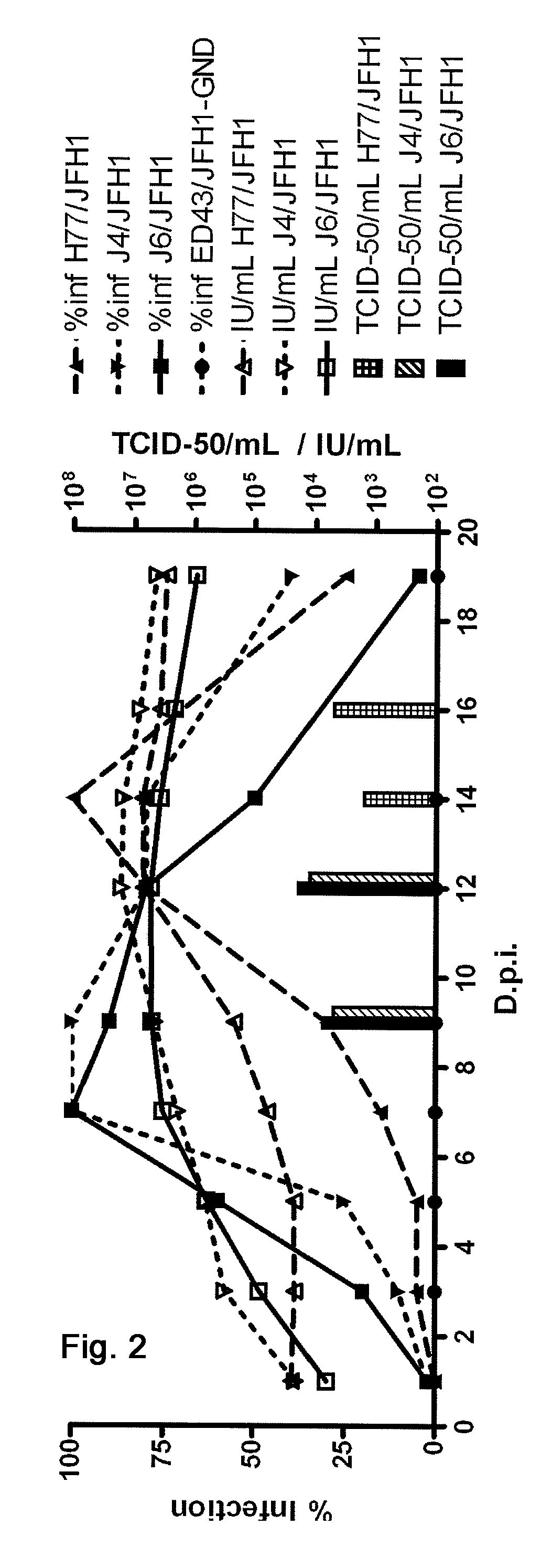
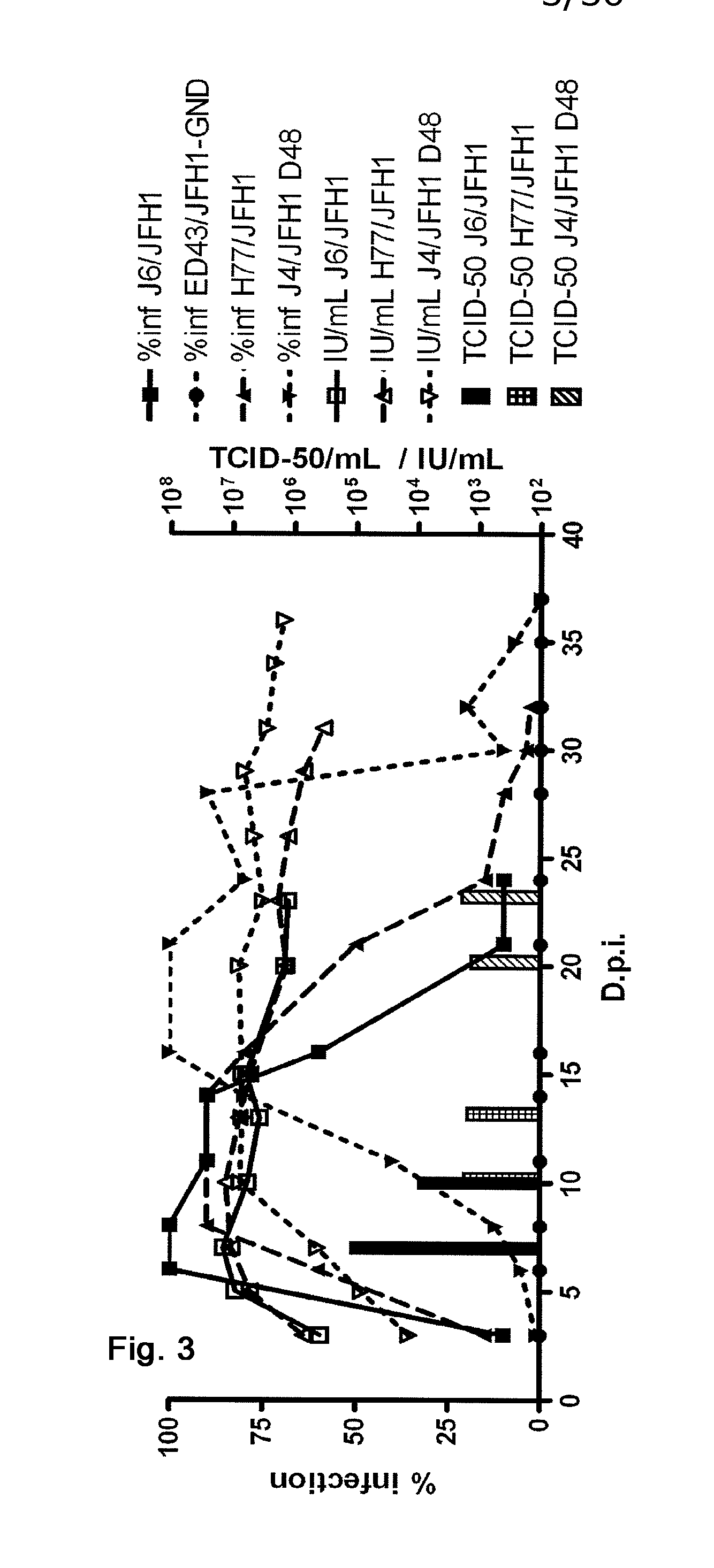
Efficient cell culture system for hepatitis c virus genotype 1a and 1b
The present inventors developed hepatitis C virus 1a / 2a and 1b / 2a intergenotypic recombinants in which the JFH1 structural genes (Core, E1 and E2), p7 and NS2 were replaced by the corresponding genes of the genotype Ia reference strain H77C or TN or the corresponding genes of the genotype Ib reference strain J4. Sequence analysis of recovered 1a / 2a and 1b / 2a recombinants from 2 serial passages and subsequent reverse genetic studies revealed adaptive mutations in e.g. p7, NS2 and / or NS3. In addition, the inventors demonstrate the possibility of using adaptive mutations identified for one HCV isolate in generating efficient cell culture systems for other isolates by transfer of mutations across isolates, subtypes or major genotypes. Furthermore neutralization studies showed that viruses of e.g. genotype 1 were efficiently neutralized by genotype Ia, 4a and 5a serum, an effect that could be utilized e.g. in vaccine development and immunological prophylaxis. The inventors in addition demonstrate the use of the developed systems for screening of antiviral substances in vitro and functional studies of the virus, e.g. identification of receptors required for HCV entry
Owner:HVIDOVRE HOSPITAL
Methods and devices for producing biomolecules
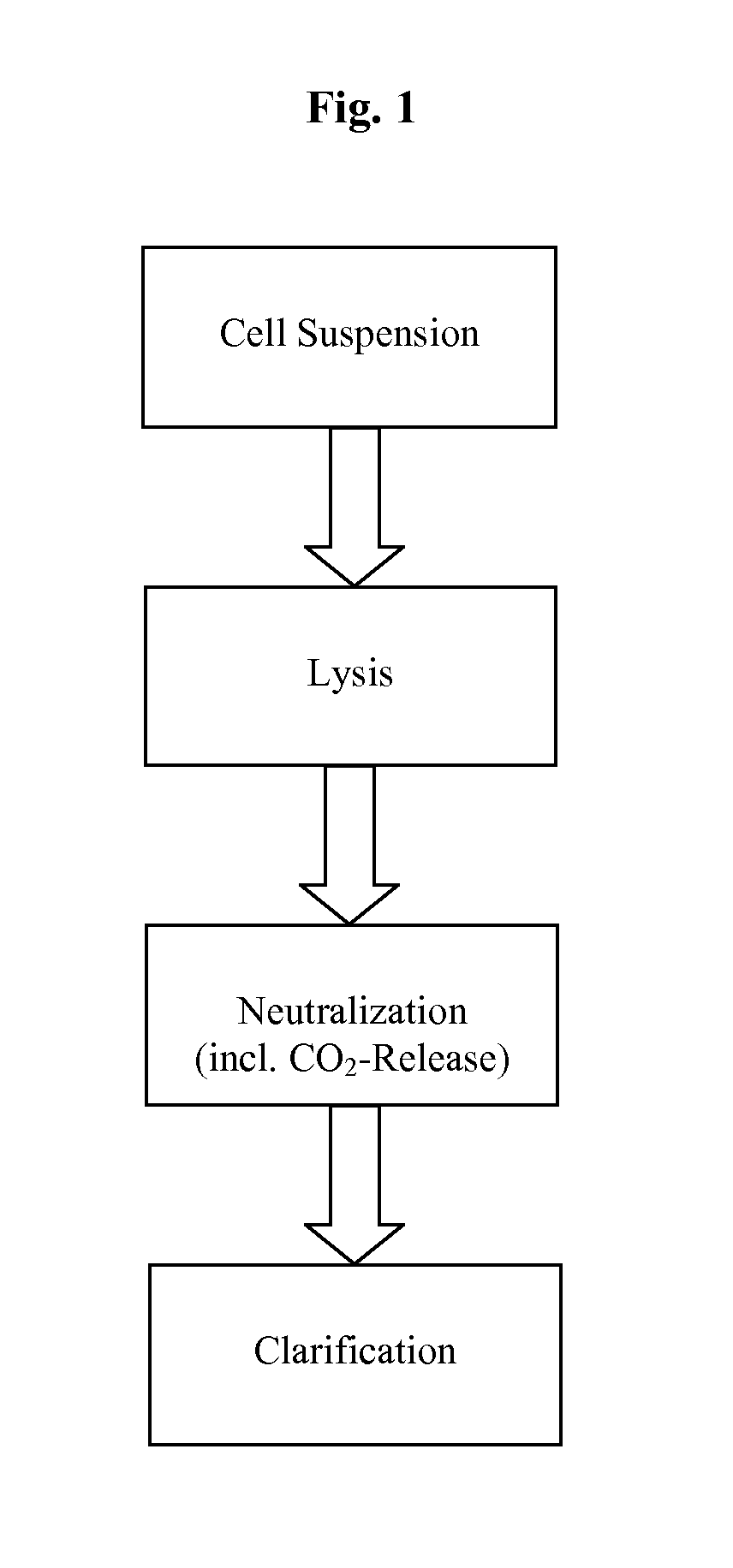
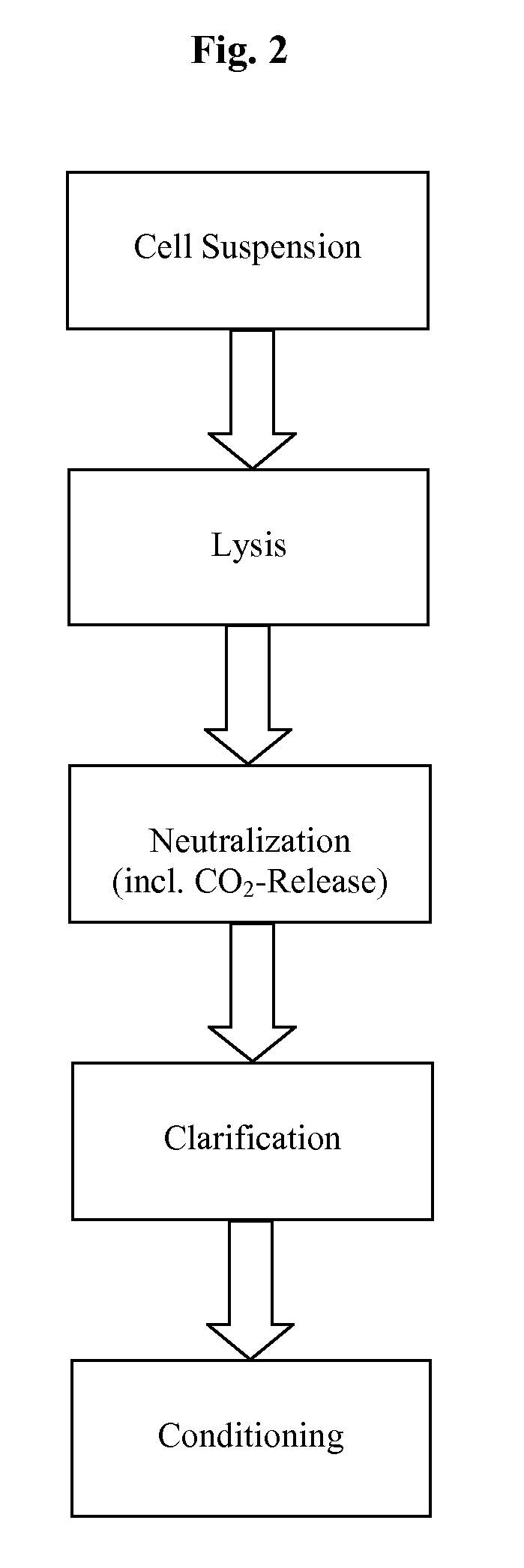
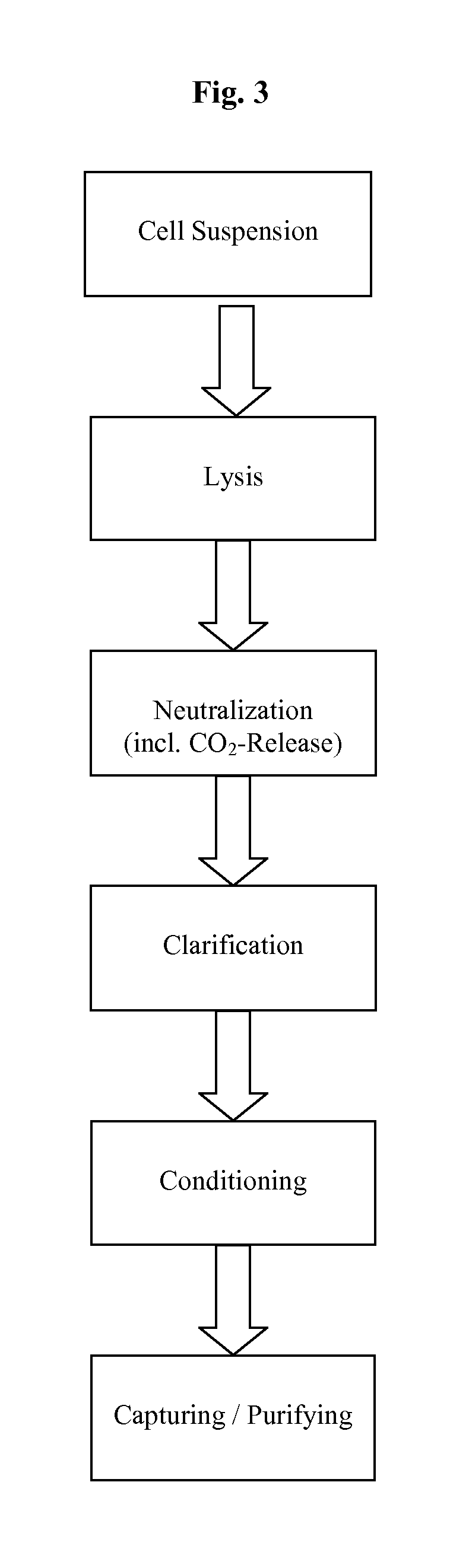
InactiveUS20110124101A1Process is directionalSugar derivativesLoose filtering material filtersLysisPharmaceutical drug
A scalable process and device for producing a bio molecule, in particular pharmaceutical grade plasmid DNA is described. The process includes the steps of alkaline lysis, neutralization and clarification and can be further extended. For separating the lysate and the precipitate an improved floatation method is disclosed. This method is based on attachment of CO2 bubbles on the precipitate floe. The CO2 is released from a carbonate salt during or after neutralization (acidification). The method of the invention is preferably carried out in an automated continuous mode applying devices for lysis and neutralization and a novel device for completely continuous clarification (separation of floes and clarified lysate).
Owner:BOEHRINGER INGELHEIM RCV GMBH & CO KG
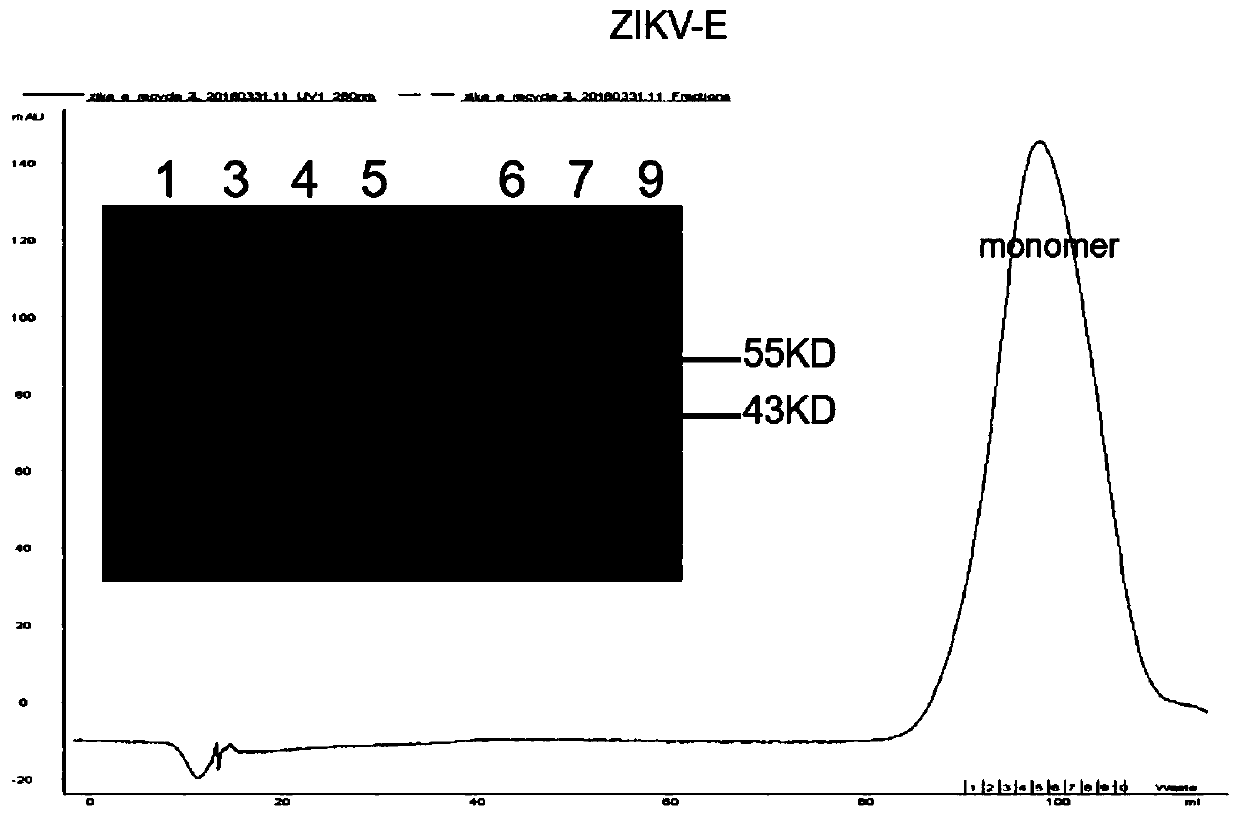
Human monocolonal antibody with high neutralization activity for Zika virus and application thereof
ActiveCN110172095AFree from attackStrong neutralizing activityImmunoglobulins against virusesAntiviralsAntigenEscherichia coli
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Fully humanized anti-tetanus bispecific antibody as well as construction method and application thereof
ActiveCN111116742AReduce allergiesInhibit bindingAntibacterial agentsAntibody mimetics/scaffoldsPassive ImmunizationsTetanus toxoids
The invention provides a fully humanized anti-tetanus bispecific antibody. The bispecific antibody includes a single-stranded unit A and a single-stranded unit B, both the single-stranded units A andB contain a single-stranded variable fragment (scFv), a human IgG1 hinge region and an Fc fragment, wherein the scFv of the single-stranded unit A has specific binding ability to a region centered onTrp 1289 of tetanus toxoid, and the scFv of the single-stranded unit B has specific binding ability to a region centered on Arg1216 of the tetanus toxoid. The invention also provides a construction method and application of the bispecific antibody. After neutralization experiments in mice, results show that the antibody has a neutralizing protection effect, and the bispecific antibody can be usedas a diagnostic reagent to detect the quality of a tetanus toxoid antigen, and can replace the tetanus antitoxin, equine tetanus immunoglobulin or human tetanus immunoglobulin to be used for passive immunization.
Owner:SHANGHAI SERUM BIOTECH
Neutralization activity monoclonal antibody of humanized anti-novel coronavirus (SARS-CoV-2)
ActiveCN112225806AHigh affinityBlock bindingBiological material analysisImmunoglobulins against virusesAntiendomysial antibodiesReceptor
The invention provides a novel humanized coronavirus neutralizing active antibody and application thereof. According to the novel humanized coronavirus neutralizing active antibody, the monoclonal antibody with neutralizing activity is separated and screened from the body of a rehabilitation patient infected by the novel coronavirus, has excellent affinity and specificity to the novel coronavirus(SARS-CoV-2), and can effectively block the combination of the novel coronavirus and receptor protein.
Owner:李亚峰
Humanized single-chain antibody 8B of clostridium perfringens alpha-toxin
ActiveCN104531730AAchieve therapeutic effectExempt from humanizationAntinoxious agentsAntibody ingredientsPhage antibodiesSingle-Chain Antibodies
The invention discloses a humanized single-chain antibody 8B of clostridium perfringens alpha-toxin. The humanized single-chain antibody 8B is screened from a phage antibody library Source bioscience, is a full-humanized antibody of the clostridium perfringens alpha-toxin and can be used for achieving a toxin neutralization treating effect, overcoming various side effects of a heterologous antibody and eliminating complex steps and high cost of humanization modification on the heterologous antibody; and the single-chain antibody is small in molecular weight, strong in in-vivo penetrability and capable of rapidly reaching to damaged tissues and cells to exert an antitoxin effect, so that the double aims of economy and high efficiency are achieved. The alpha-toxin has a certain homology with other types of toxins of clostridium perfringens, so that the antibody drug possibly generates inhibiting and treating effects for other types of toxins and can also be used as a detecting reagent to detect the clostridium perfringens alpha-toxin.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
Influenza virus antibody and preparation method and application thereof
ActiveCN106928350AInhibition of replicationImportant economyImmunoglobulins against virusesAntiviralsHeavy chainAmino acid
The invention relates to the biotechnology field, in particular to an influenza virus antibody. A light-chain variable region of the influenza virus antibody has an amino acid sequence shown as the SEQ ID No.1; a heavy-chain variable region of the influenza virus antibody has an amino acid sequence shown as the SEQ ID No.2. The antibody can well neutralize H3N2, H4N6 and H14N5 subtype influenza viruses, combine HA proteins of all subtype influenza viruses in a group 2, neutralize H3-subclade viruses and inhibit copying of the H3 subtype influenza viruses in a mouse body and has the important economic and social significance.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI +1
Humanized antibody for resisting avian influenza H5N1 hemagglutinin antigen, and preparation method and application thereof
ActiveCN103739707ALow immunogenicityBroad general hemagglutination inhibitory activityVirus peptidesImmunoglobulins against virusesHemagglutininEpitope
The invention discloses a humanized antibody for resisting avian influenza H5N1 hemagglutinin (HA) antigen. A heavy chain variable region of the humanized antibody has an amino acid sequence shown in SEQ ID No.1; a light chain variable region of the humanized antibody has an amino acid sequence shown in SEQ ID No.2. In addition, the invention also discloses a preparation method and application of the humanized antibody. The humanized antibody is extensive and widespread in coagulation inhibition activity for avian influenza H5N1 virus, high in affinity and neutralization activity for various subtypes of the HA antigen of the avian influenza H5N1, and capable of recognizing a special conserved region of one H5HA; the humanized antibody is high in neutralization activity and capable of reducing immunogenicity; a recognized epitope is highly conserved in the H5 type influenza virus; the humanized antibody is extensive in application prospect in prevention and treatment and vaccine design of the avian influenza H5N1 in the future.
Owner:SHANGHAI INST OF IMMUNOLOGY +1
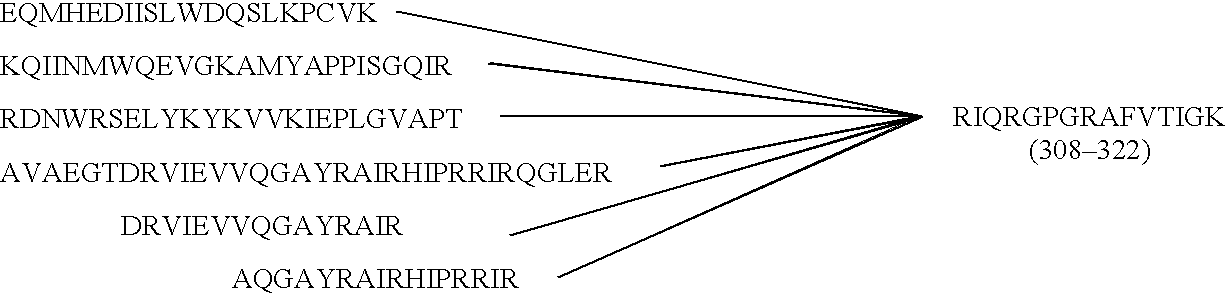
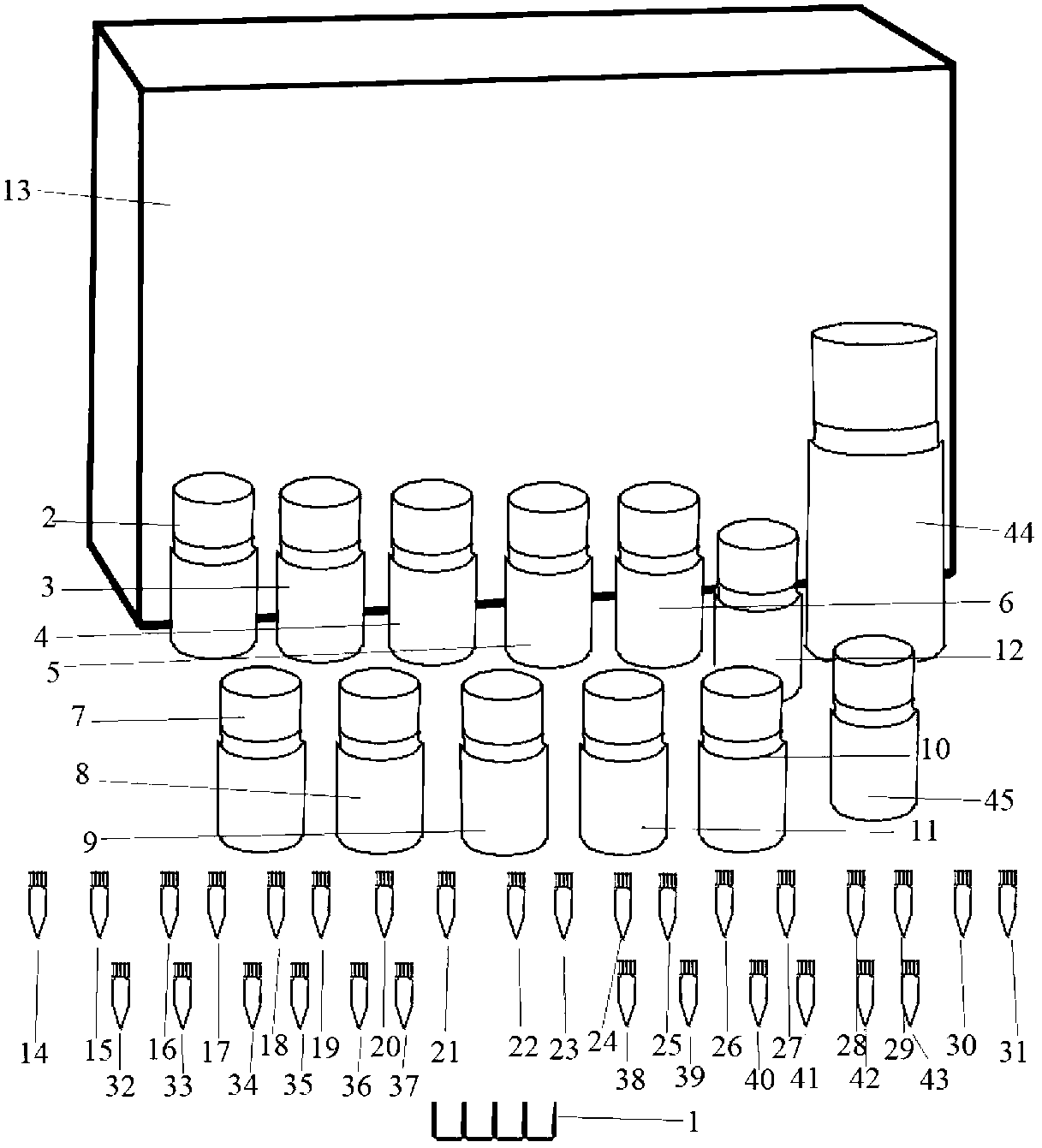
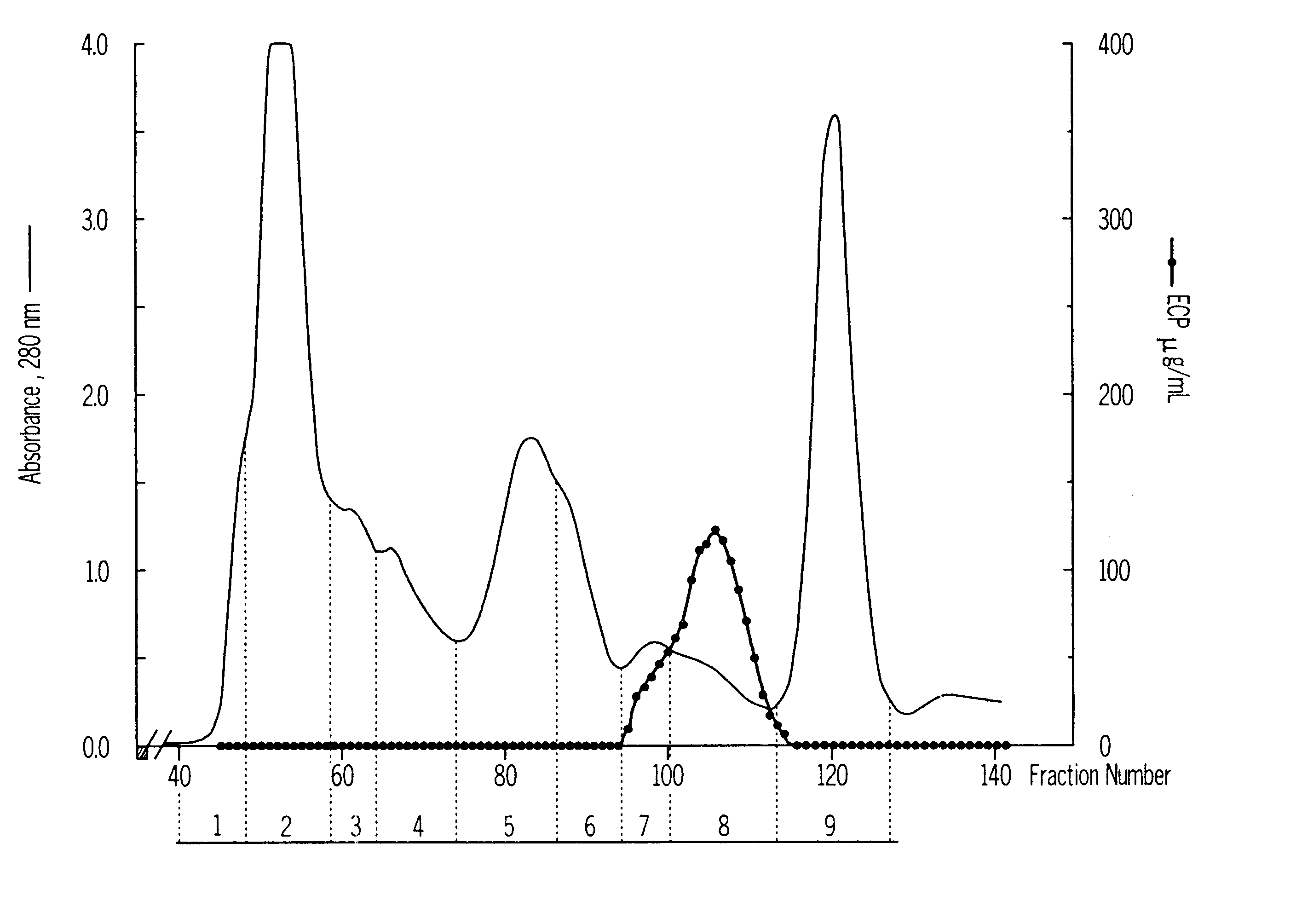
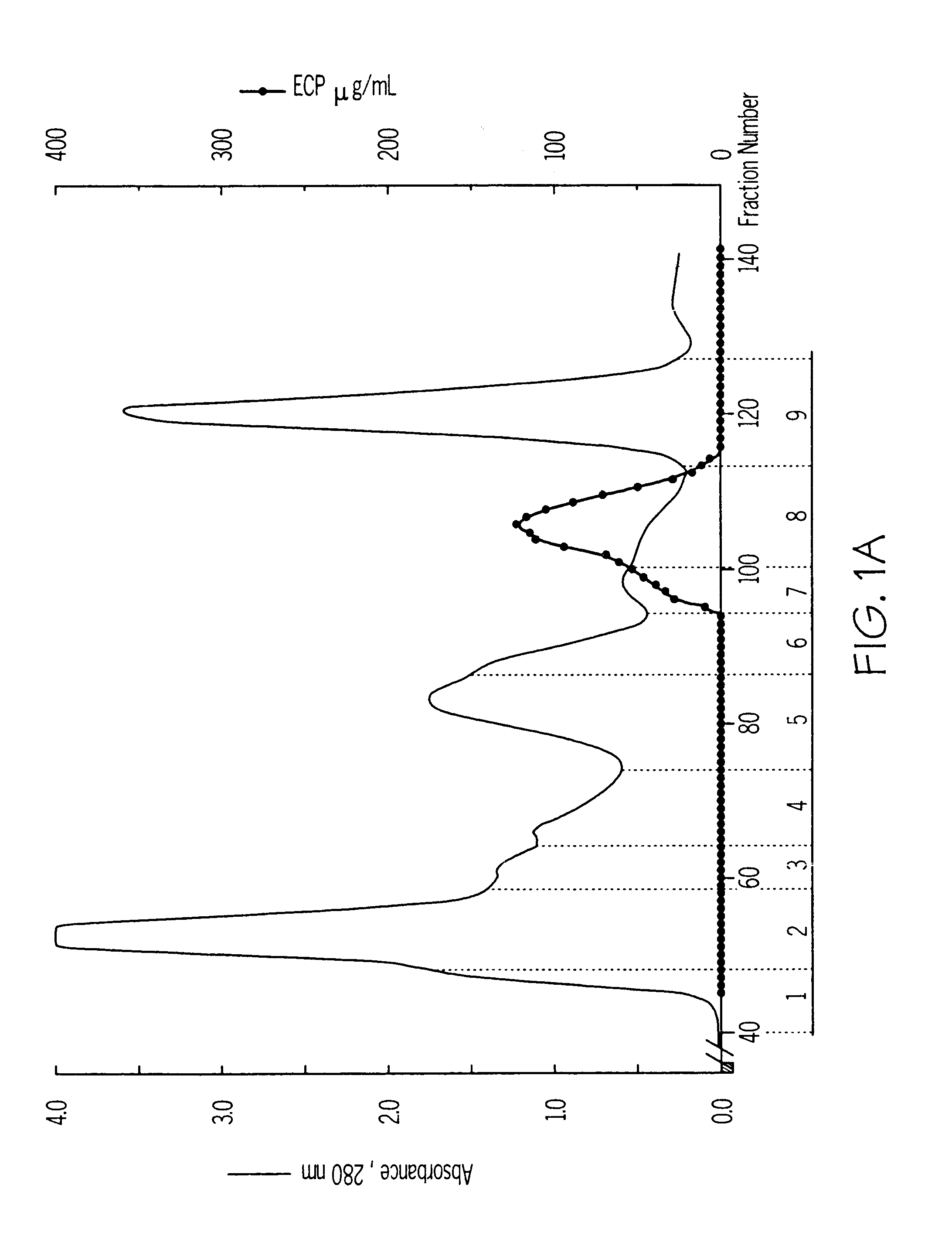
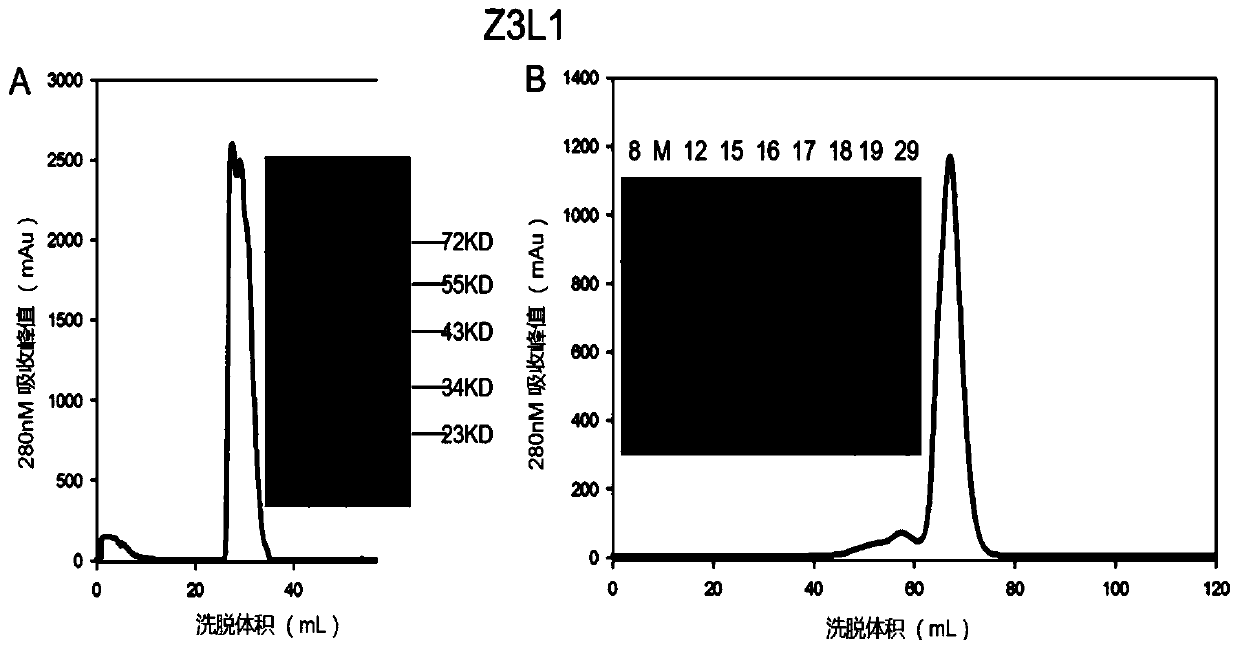
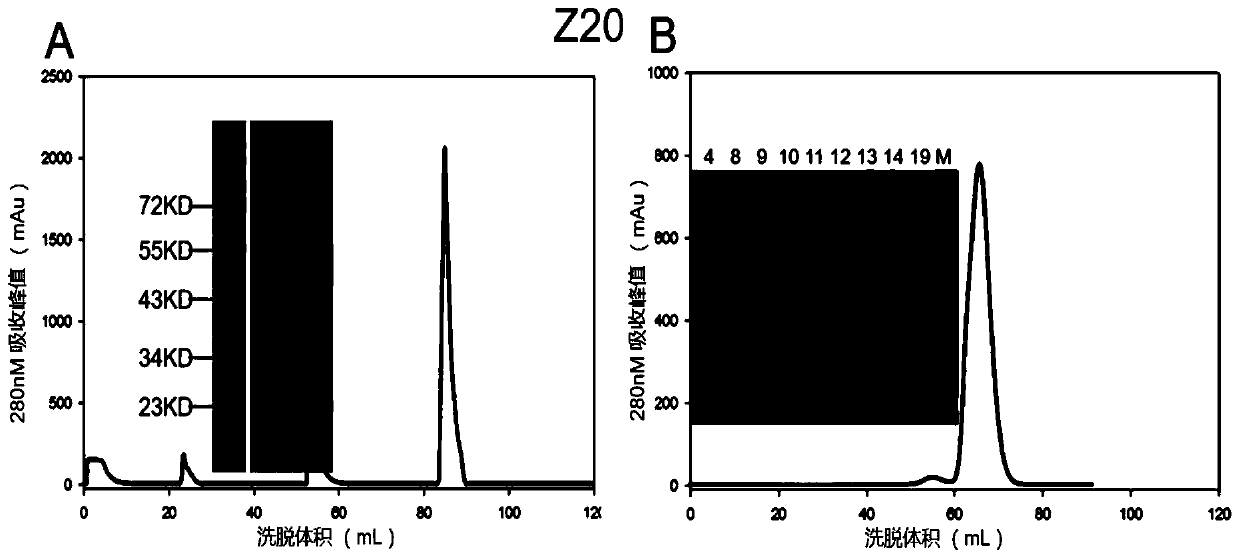
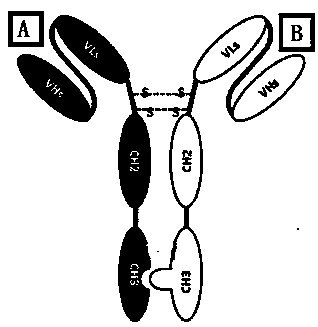
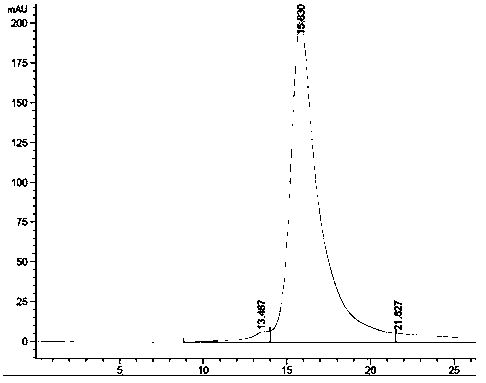
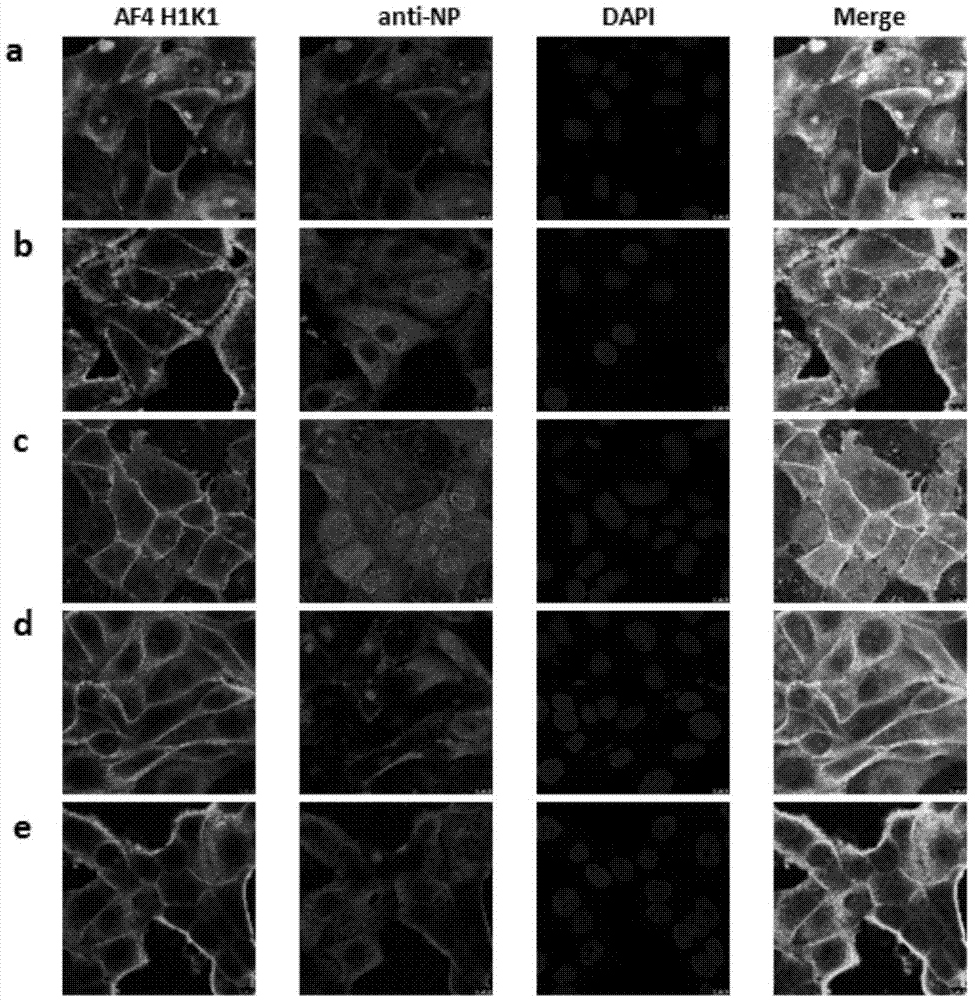
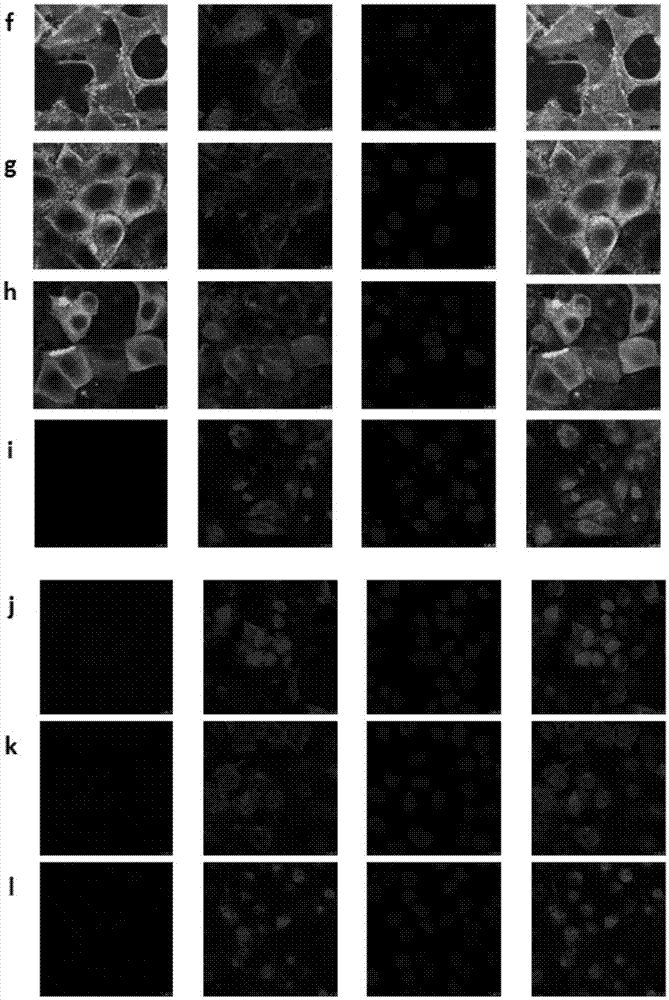
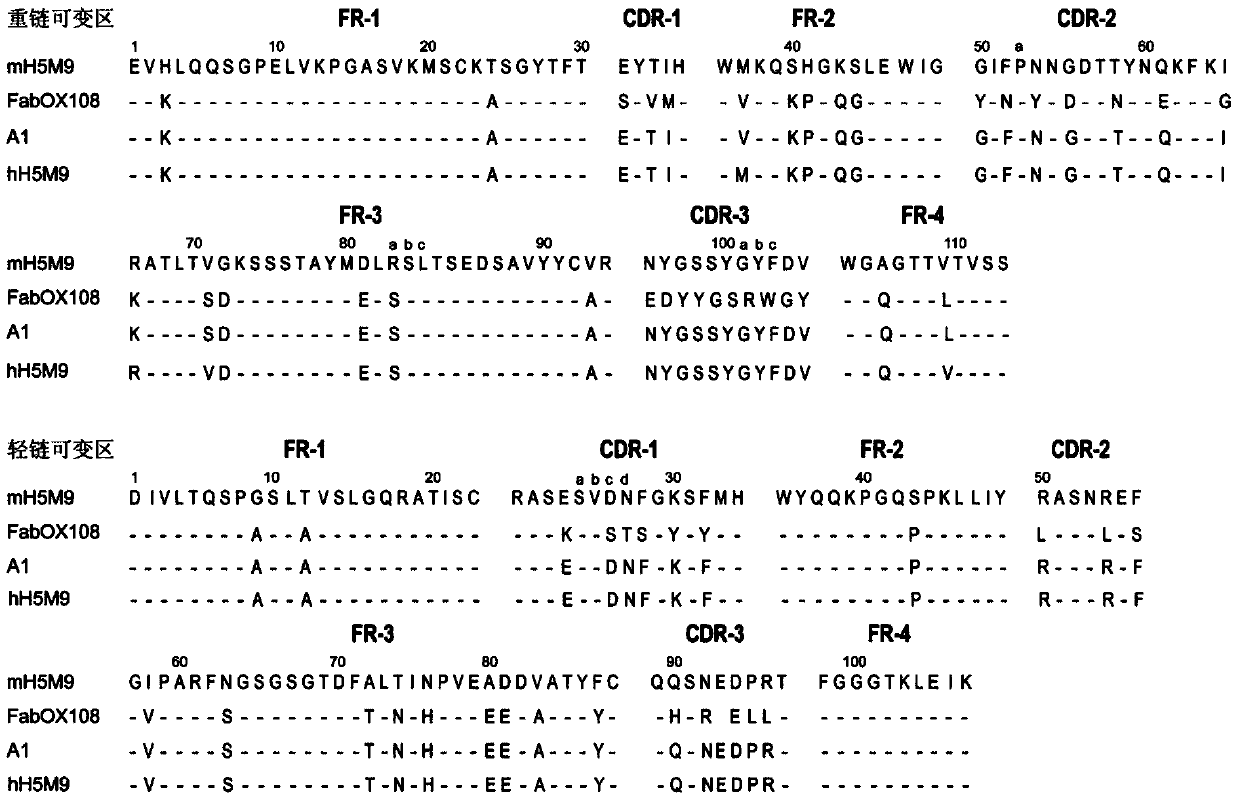
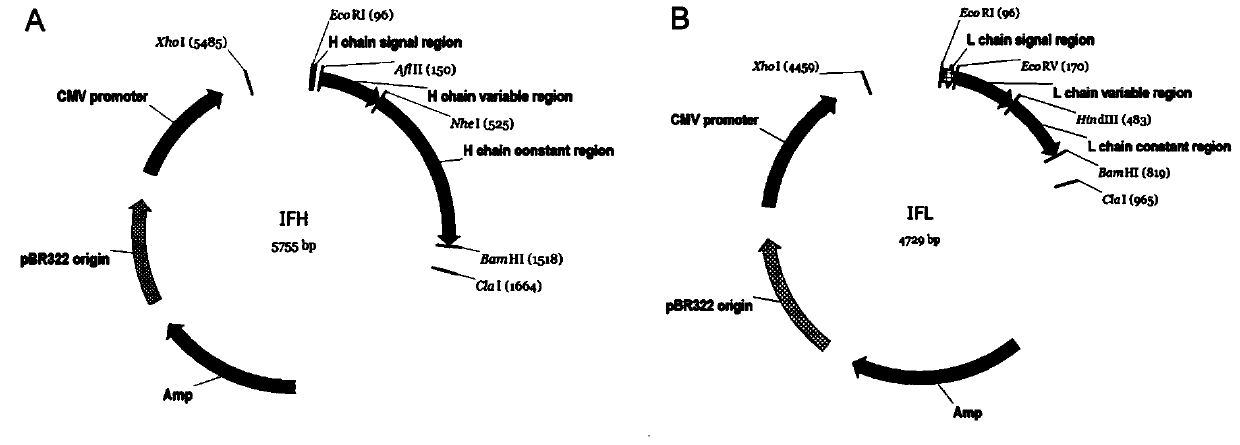
Mosaic chimeric viral vaccine{[s]] particle
Owner:TEXAS A&M UNIVERSITY
Epitope of spring viraemia of carp
The invention relates to an antigenic epitope of spring viraemia of carp and belongs to the technical field of biology. According to the invention, by virtue of phage display technique, a positive clone is screened out by the use of spring viraemia of carp virus (SVCV) monoclonal antibody, a conserved sequence binding to the SVCV monoclonal antibody is found out, a clinical diagnosis reagent is prepared by the screened antigenic epitope, and spring viraemia of carp is prevented and treated by a small molecular antigen peptide as a vaccine. The gene sequence of the invention is SEQ ID NO:1. The phage display technique is utilized to screen the positive clone by SVCV monoclonal antibody and find out the conserved sequence which can bind to the monoclonal antibody with the ability of SVCV activity neutralization in a random peptide library. The site of the antigenic determinant is determined, and the relationship of its sequence, structure and function is analyzed. The study of inhibition effect to SVCV generated from the competitive binding of small peptide to SVCV receptor will have a huge application value for taking small molecular antigen peptide as vaccine in the future.
Owner:JILIN AGRICULTURAL UNIV
Rabies virus monoclonal antibody and application thereof
InactiveCN103954777AHigh neutralization potencyHigh detection sensitivityBiological material analysisBiological testingVaccine ProductionGlycoprotein G
The invention relates to a rabies virus monoclonal antibody with neutralization activity and capability of specifically recognizing rabies virus glycoprotein G. The monoclonal antibody is authenticated to be IgG1-subtype. According to the rabies virus monoclonal antibody disclosed by the invention, rabies virus inactivation totivirus is taken as an immunogen for immunizing a mouse, an antibody with neutralization activity is screened, the neutralization titer is high, the secretion of hybridoma for the antibody is stable, and the antibody is easy to store; and an in-vitro preparation link for glycoprotein is avoided, and innovativeness is achieved. A colloidal gold test paper card or test paper strip prepared from the antibody is high in test sensitivity and capable of achieving 0.03 IU / mL, capable of being used for test on human rabies virus or quality control in a vaccine production process, and high in application value.
Owner:北京凯思百奥科技发展有限公司
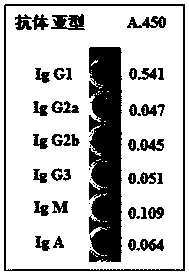
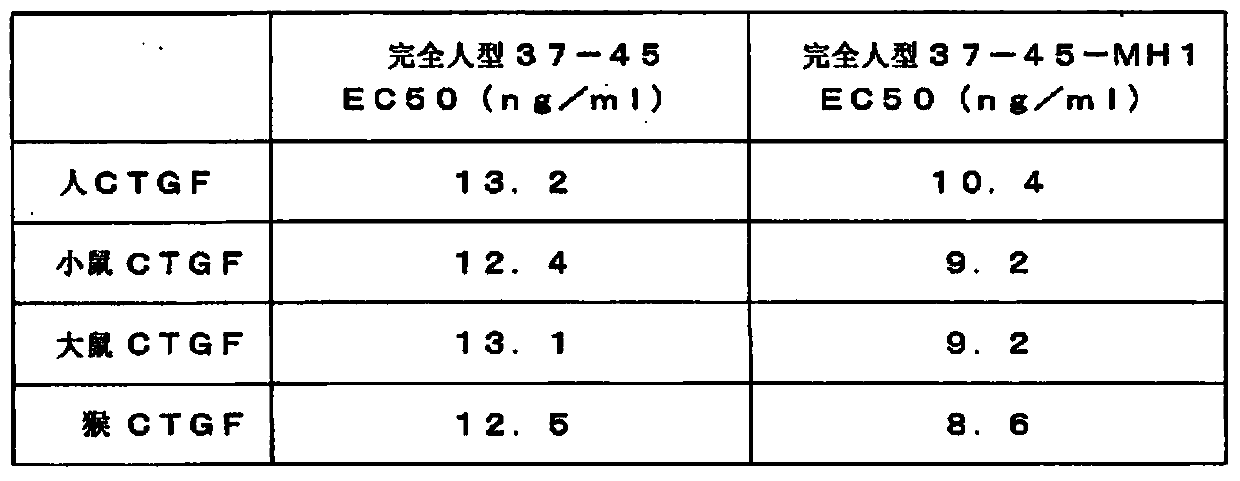
Novel Anti-human ctgf antibody
The invention provides an anti-human CTGF antibody having a superior binding activity and / or a superior neutralization activity compared with those of conventional anti-human CTGF antibodies; and a means for preventing or treating various diseases in which human CTGF is involved in the formation of clinical conditions thereof, including renal diseases such as chronic kidney disease and diabetic nephropathy, using the above-mentioned anti-human CTGF antibody. An anti-human CTGF antibody containing a heavy-chain variable region comprising the amino acid sequence represented by SEQ ID NO: 10 and a light-chain variable region comprising the amino acid sequence represented by SEQ ID NO: 4.
Owner:ASTELLAS PHARMA INC
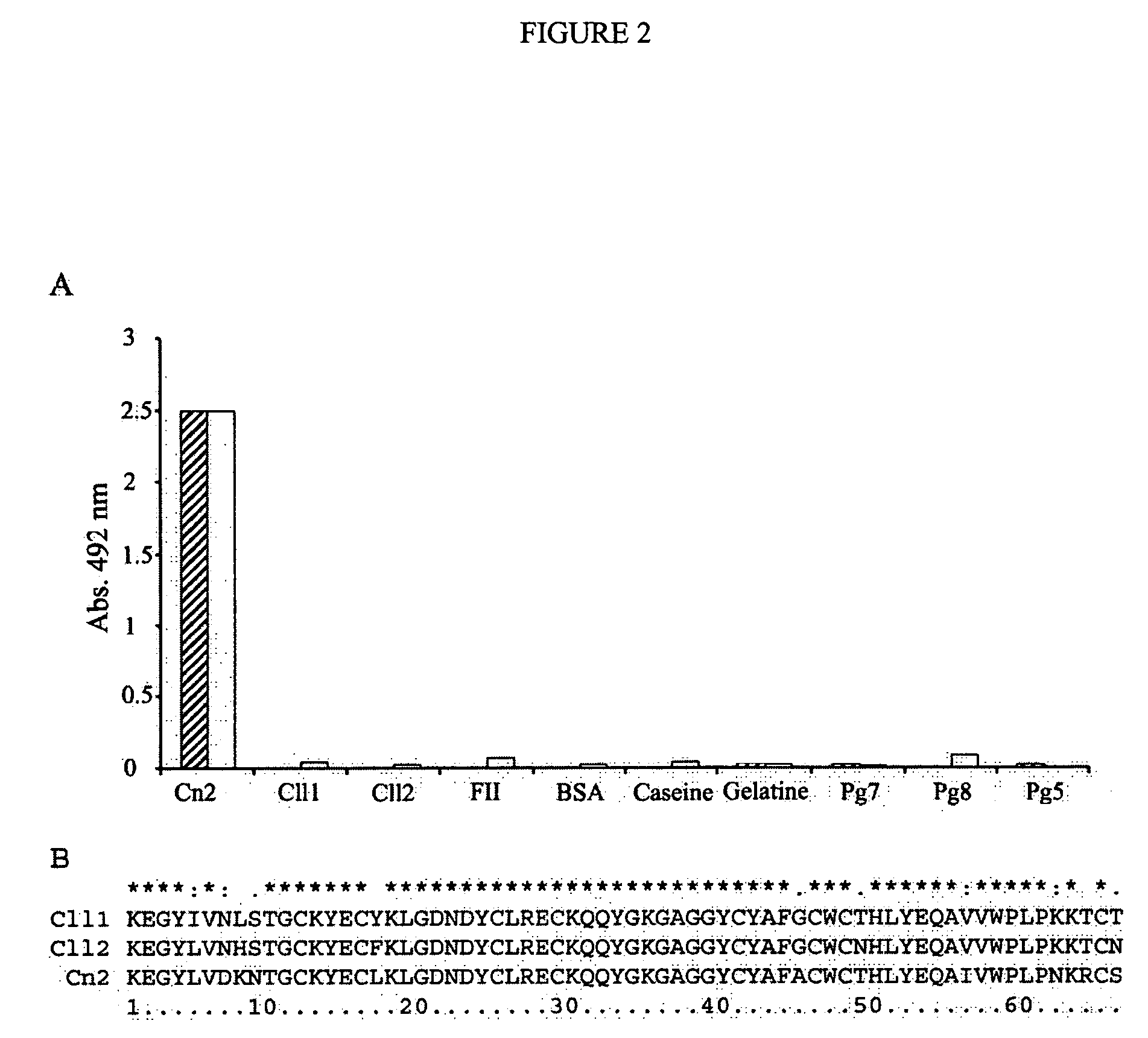
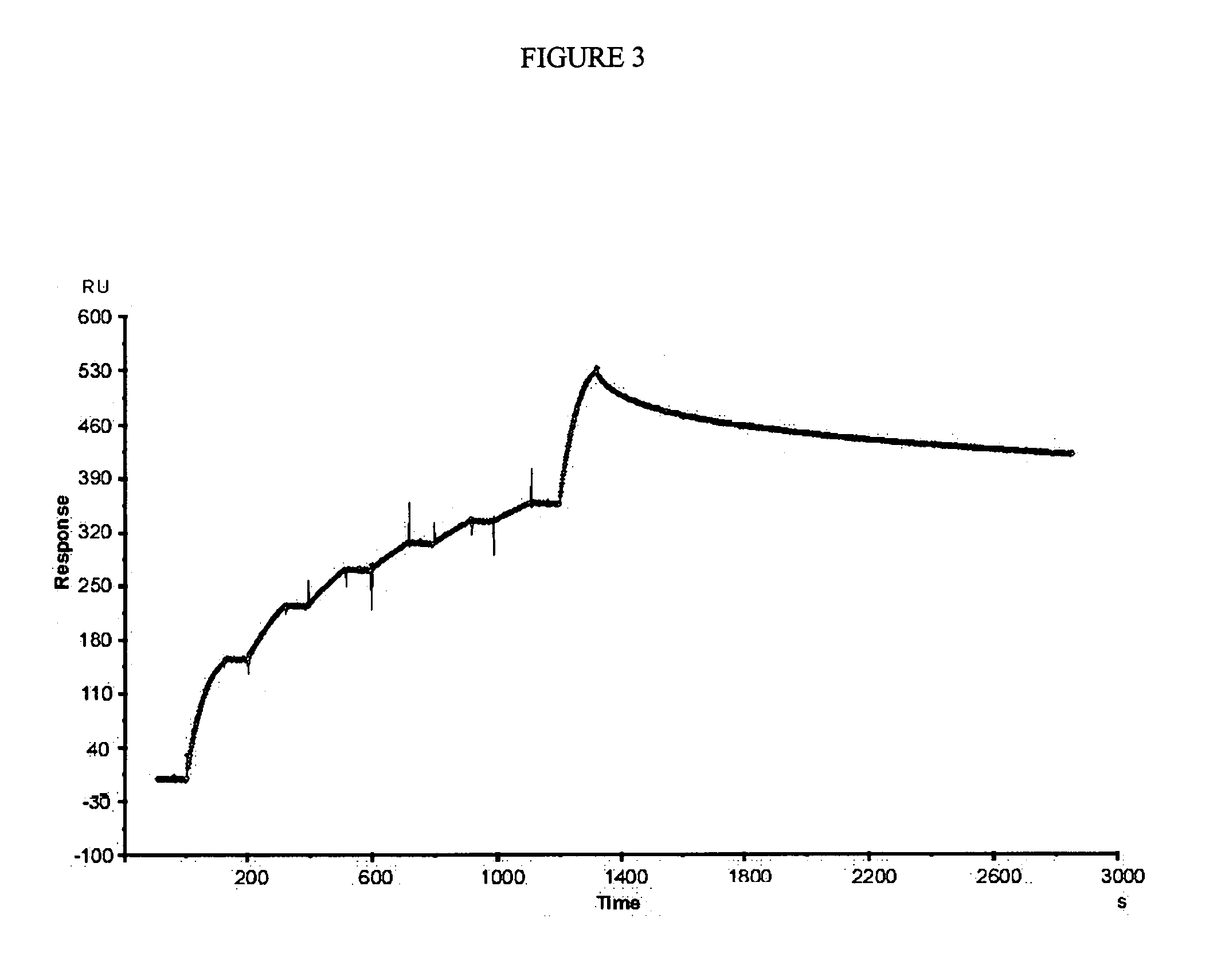
Human antibodies that specifically recognize the toxin Cn2 from Centruroides noxius scorpion venom
The present invention is directed to recombinant human antibodies specific for Cn2 toxin from C. noxius scorpion venom. The antibodies are able to recognize the toxin and preferably neutralize it as well as the whole venom of C. noxius scorpion. This invention is also directed to a human non-immune phage display library. One clone that specifically binds the Cn2 toxin was affinity matured by directed evolution. Three cycles of maturation were performed and several scFv clones were isolated which specifically recognize toxin Cn2 with increased Kd of 446 fold. All variants were monomeric and only variants 6009F, 6105F and 6103E showed to be capable of neutralizing toxin Cn2 and the whole venom. Variant 6009F recognizes a different epitope than that of BCF2, a murine monoclonal antibody raised against scorpion toxin Cn2 which is also capable of neutralizing both Cn2 toxin and the whole venom when tested in mice, as well as that of commercially available polyclonal antibody fragments antivenom from horse. The scFv 6009F is the first reported recombinant human antibody fragment capable of neutralizing a scorpion venom. These results pave the way for the generation of safer autologous recombinant neutralizing antivenom against scorpion stings. The antibodies of the present invention can be used as part of a composition to treat those in need of treatment including those already stung by one or more scorpions, particularly C. noxius scorpions.
Owner:UNIV NAT AUTONOMA DE MEXICO
Method for assigning antibody standard substance and determining antigen neutralization equivalent
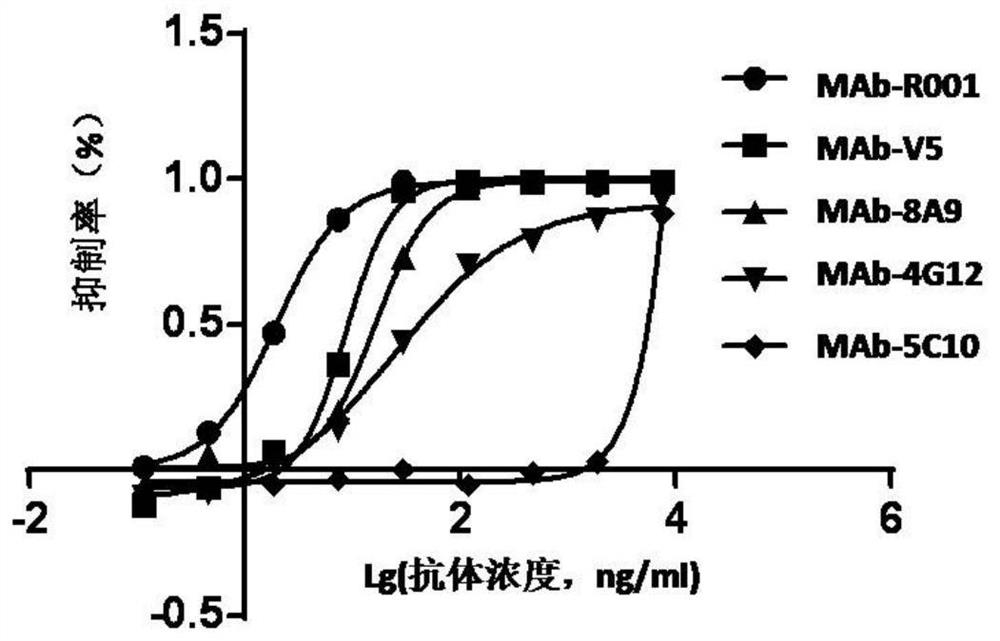
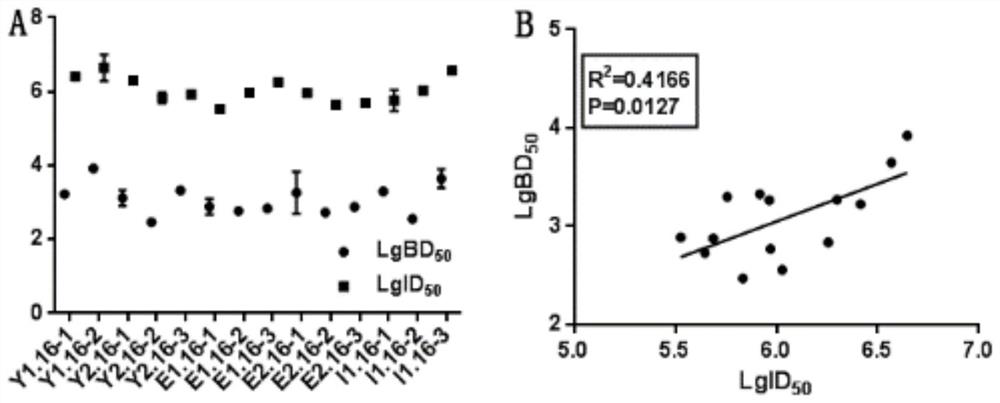
ActiveCN113125756AStable minimum detection limitQuality improvementBiological testingImmunoassaysMedicineBiochemistry
The invention relates to a method for assigning an antibody standard substance and determining an antigen neutralization equivalent, which specifically comprises the following steps: S1, determining the antigen neutralization equivalent of the antibody standard substance or a sample: carrying out gradient dilution on a matrix for an antigen with known purity and concentration, respectively adding the same amount of antibody standard substances or samples containing antibodies into the gradient diluents with different concentrations, and after reaction, carrying out antibody detection on each mixed solution by using a first antibody detection reagent to determine the antigen neutralization equivalent of the antibody standard substances or the samples; wherein the measurement unit of the antigen neutralization equivalents of the antibodies in the antibody standard substances or samples in a certain volume is represented by the mass-volume concentration of neutralizable antigens in antibodies; in order to particularly clarify that the mass number in the mass-volume unit is the corresponding amount of the neutralization antigens, NAE such as 1 ng NAE / mL, 1 [mu]g NAE / mL, 1 mg NAE / mL and the like is added after the mass unit.
Owner:NANJING LANSION BIOTECH CO LTD
Monoclonal antibody for neutralizing novel coronavirus infection
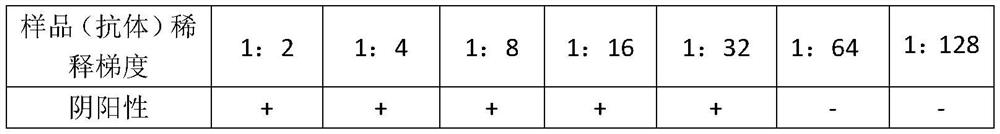
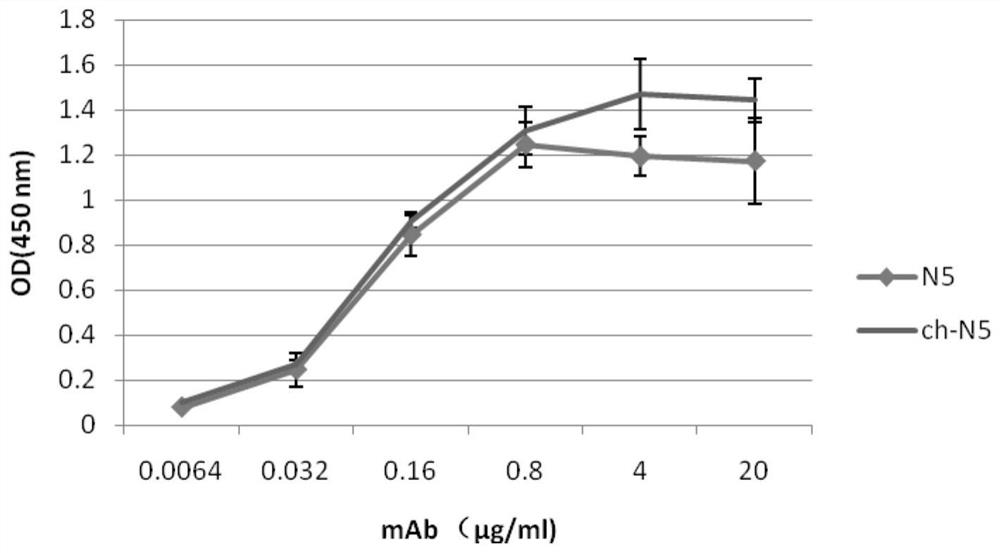
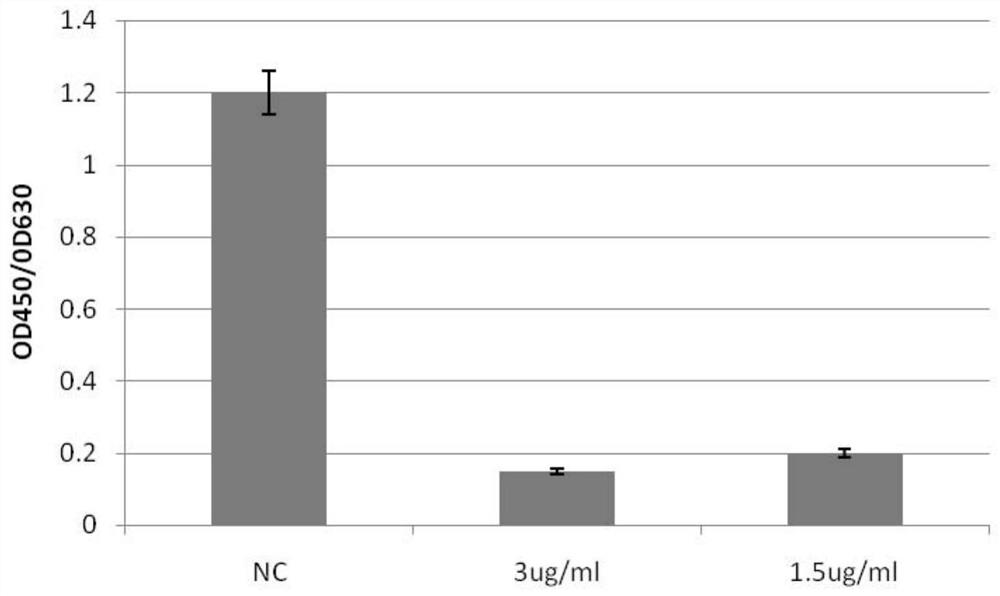
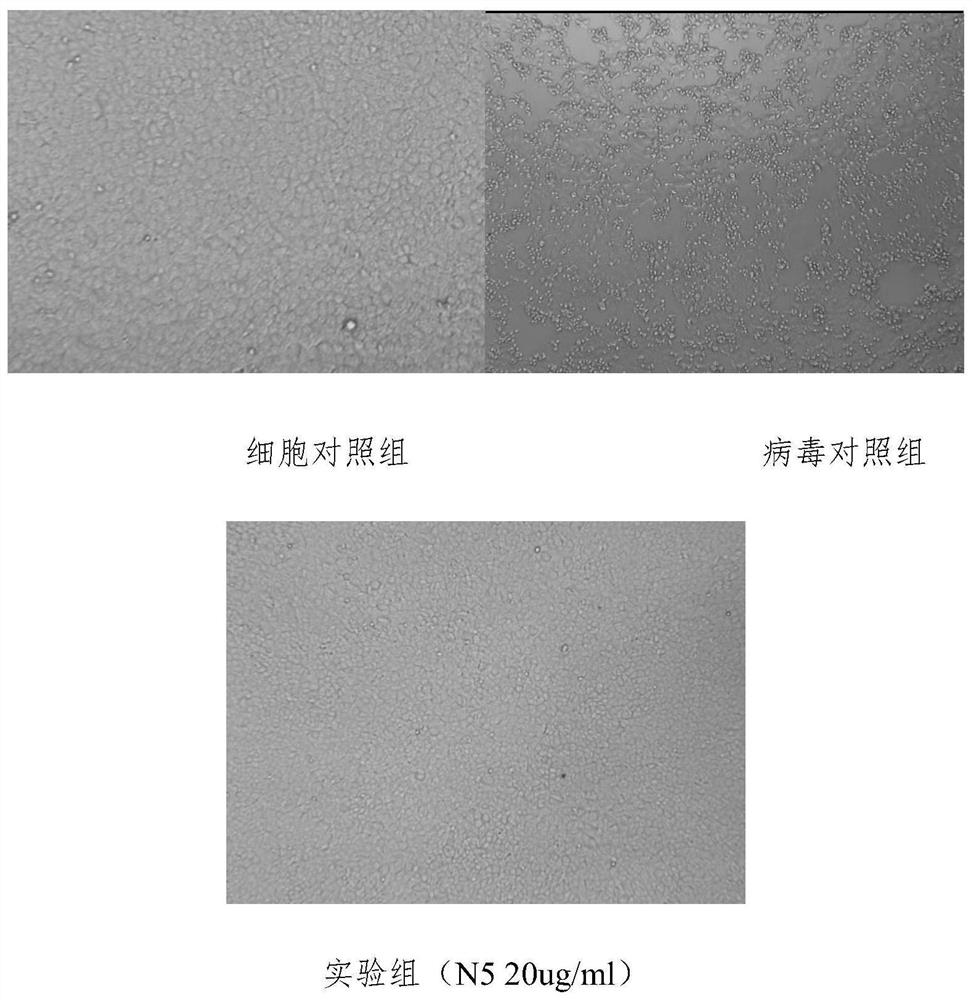
The invention discloses a monoclonal antibody for neutralizing novel coronavirus infection. The invention provides the antibody. The amino acid sequence of a light chain variable region of the antibody is the 1-110th site of a sequence 2 in a sequence listing; and the amino acid sequence of a heavy chain variable region of the antibody is the 1-120th site of a sequence 4 in the sequence listing. The monoclonal antibody N5 is identified to have higher binding specificity and affinity to the S protein RBD (receptor binding domain) of the novel coronavirus, and an in vitro neutralization experiment shows that the N5 can effectively neutralize the infection activity of the novel coronavirus SARS-CoV-2. On the basis, the gene sequence of the N5 antibody is measured, then recombinant expression is carried out, and the expressed recombinant antibody also has the neutralizing activity of the SARS-CoV-2.
Owner:ACADEMY OF MILITARY MEDICAL SCI
SARS-CoV-2 neutralization antibody detection kit
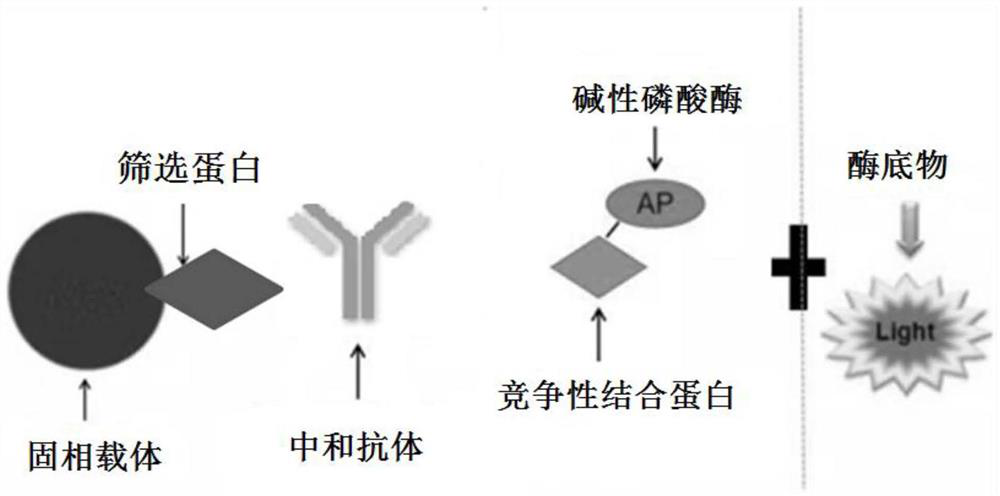
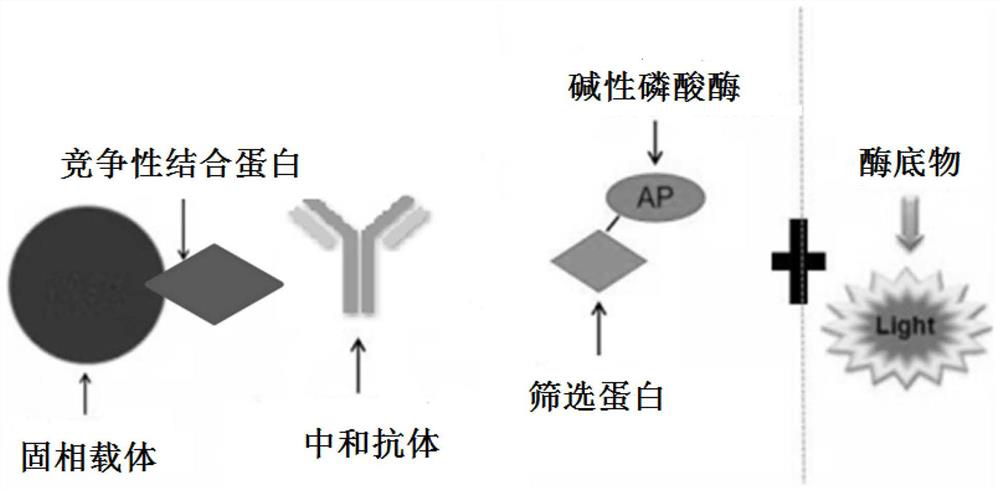
PendingCN113092776AHigh sensitivityImprove featuresBiological testingImmunoassaysCompetitive bindingNeutralizing antibody
The invention relates to an SARS-CoV-2 neutralization detection kit, which comprises a solid phase carrier, a screening protein, a competitive binding protein, alkaline phosphatase and an alkaline phosphatase luminescent substrate, wherein the screening protein comprises an RBD recombinant protein having an amino acid sequence represented by SEQ IDNO.1 or having at least 85% homology, and / or the fusion protein of the S protein and the N protein with the amino acid sequence as shown in SEQ IDNO.2 or having at least 85% of homology with the fusion protein. The kit developed by the invention can be used for efficiently, safely, quantitatively and accurately detecting the SARS-CoV-2 neutralization antibody, and large-batch rapid detection can be realized. And the RBD protein recombined by the eukaryotic expression system or the fusion protein of the S protein and the N protein is selected as the screening protein of the competitive reaction system, so that the sensitivity and the specificity of the detection kit can be further improved.
Owner:苏州携创生物技术有限公司
Anti-HPV16 L1 protein monoclonal antibody and detection method applying same
ActiveCN112125972AStrong specificityAdvantages of specific detectionImmunoglobulins against virusesFermentationAntigen Binding FragmentAntigen binding
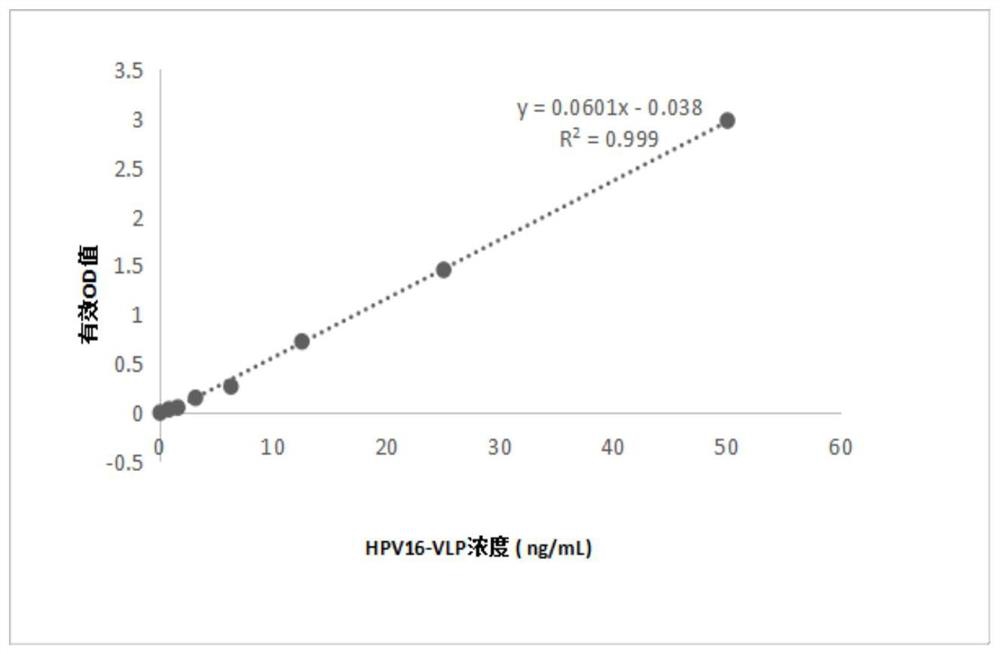
The invention provides an anti-human papilloma virus (HPV) L1 protein monoclonal antibody, an antigen binding fragment and a detection method applying the antibody. The antibody has strong binding force with an HPV16 L1 antigen, has no cross reaction with HPV6, 11, 18, 31, 33, 35, 39, 45, 52, 56, 58 and 59 types, can specifically neutralize HPV16 pseudotype viruses, and has neutralizing activity.The monoclonal antibody obtained by screening of the invention shows good reaction consistency and neutralization activity on virus-like particle antigens derived from different expression systems; the recognized epitope is a dominant epitope in both immune guinea pig serum and immune human serum; and the monoclonal antibody is suitable for being used as a detection antibody in ELISA quantification to perform immunogenicity evaluation on HPV vaccines.
Owner:SINO CELL TECH INC
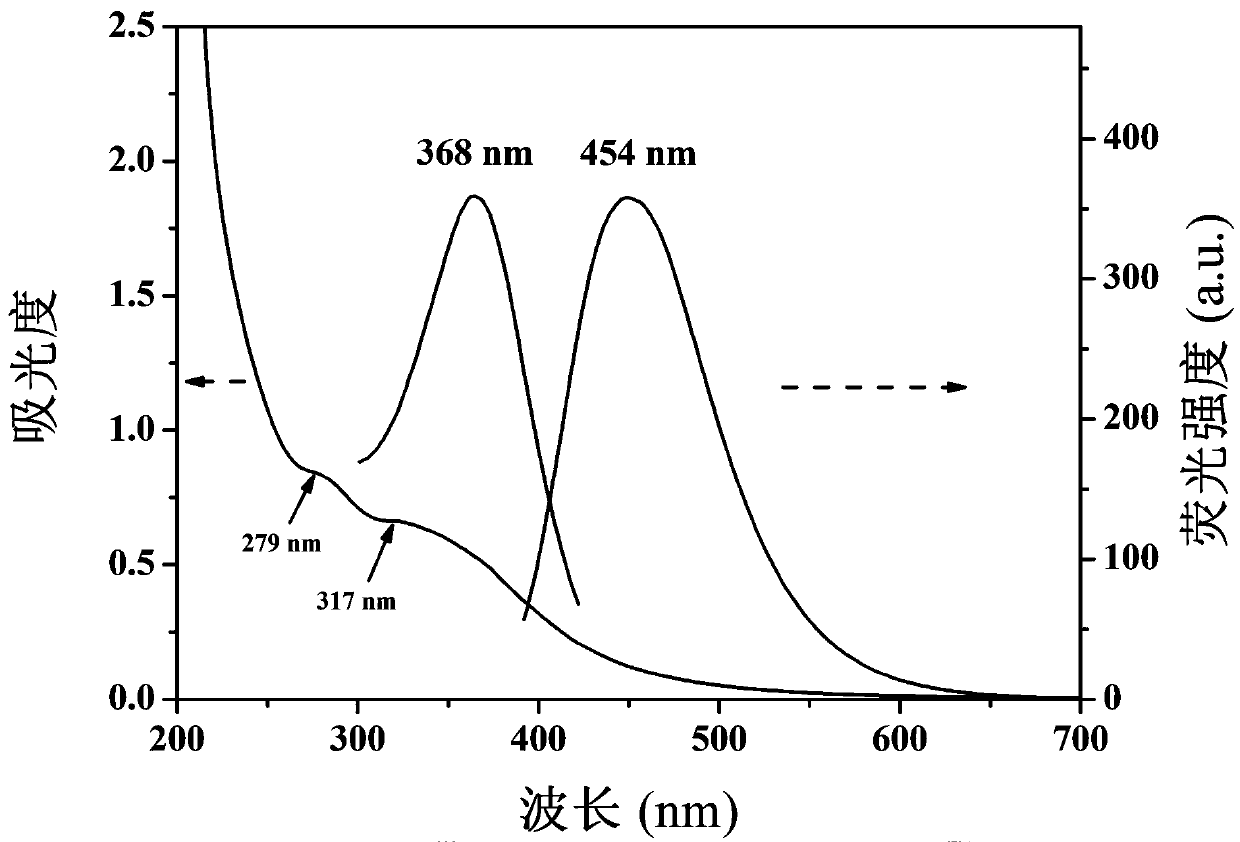
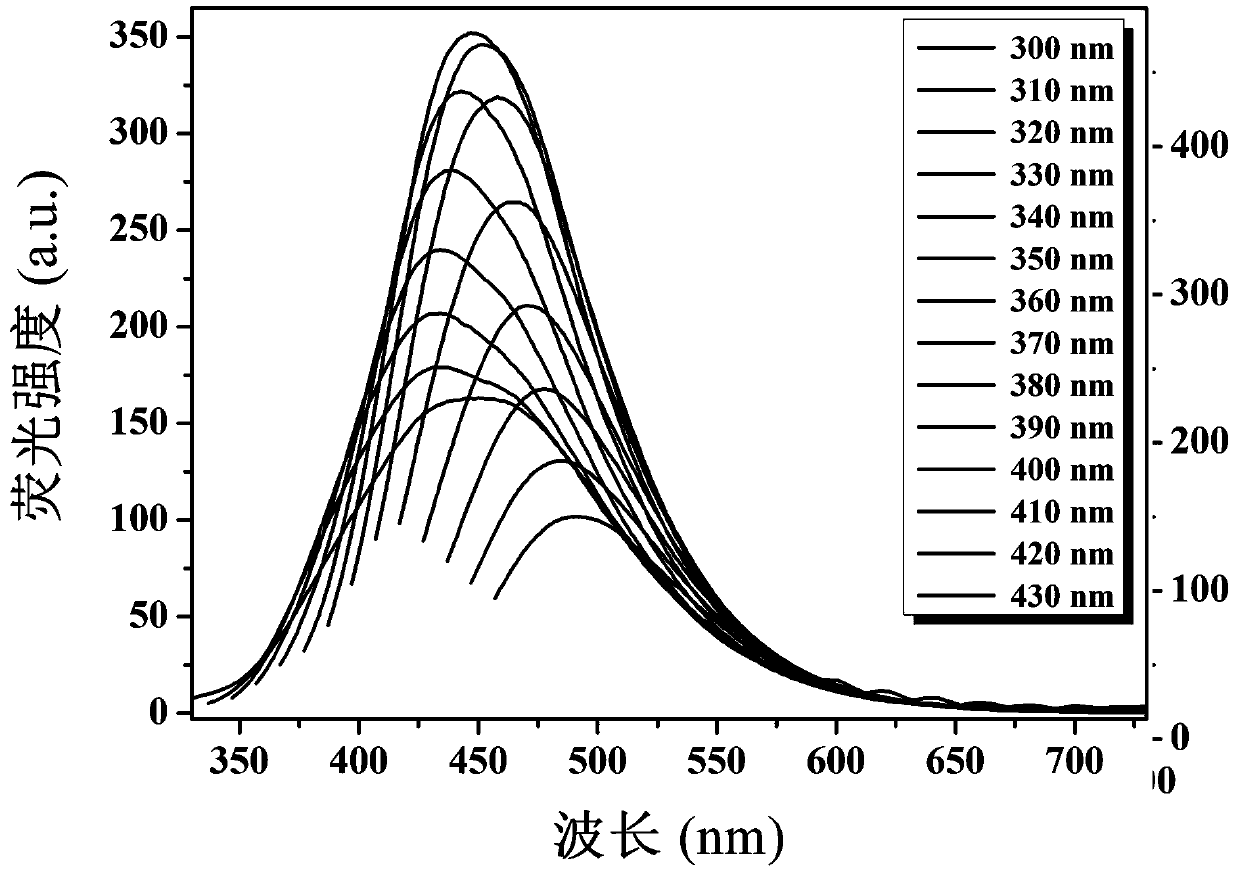
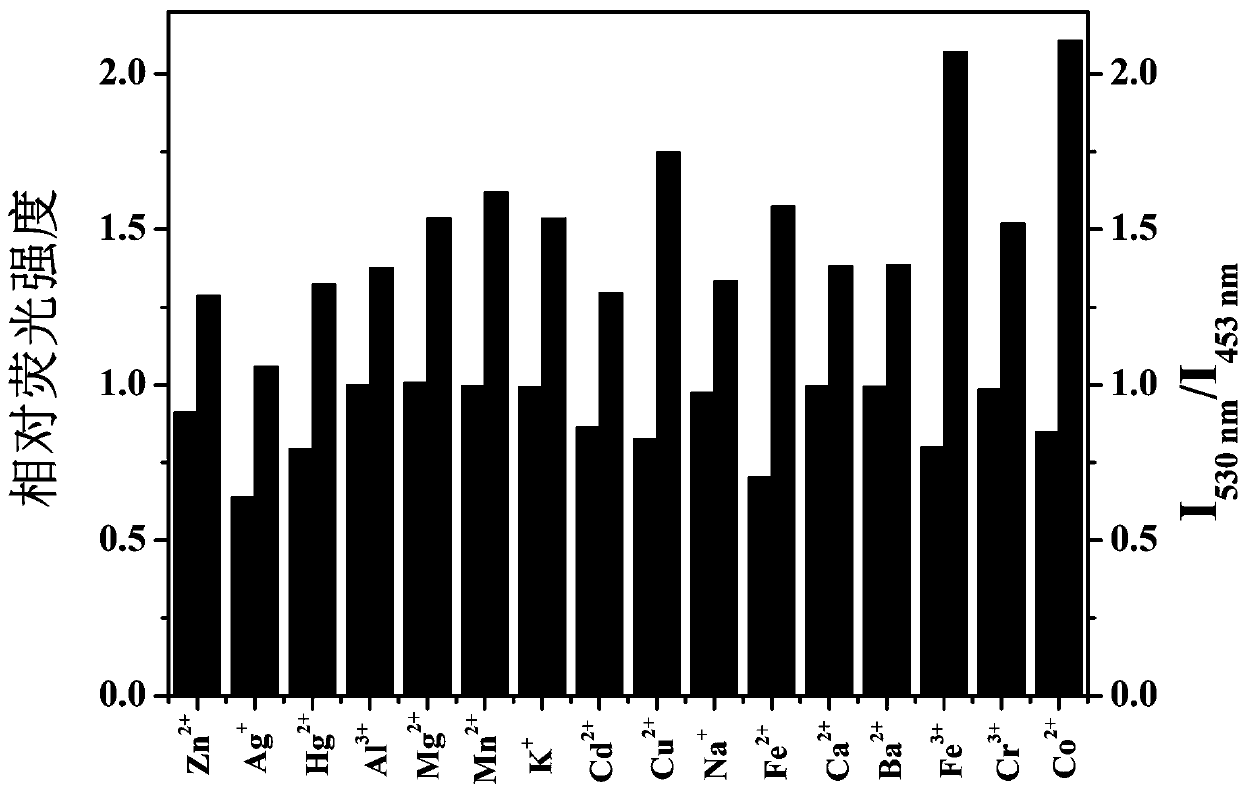
Fluorescent probe for quantitatively detecting riboflavin on basis of fluorescence resonance energy transfer ratio, and preparation method and application for fluorescent probe
ActiveCN111117609AEasy to prepareQuick checkMaterial nanotechnologyNanoopticsFluoProbesO-Phosphoric Acid
The invention belongs to the technical field of fluorescent probes, and provides a fluorescent probe for quantitatively detecting riboflavin on the basis of a fluorescence resonance energy transfer ratio, and a preparation method and an application for the fluorescent probe. The preparation method comprises the following steps: with glucose as a carbon source, ethylenediamine as a nitrogen source,concentrated phosphoric acid as a phosphorus source and concentrated hydrochloric acid as a chlorine source, preparing nitrogen-phosphorus-chlorine co-doped carbon quantum dots (NPCl-CQDs) through anacid-base neutralization reaction exothermic carbonization method, dissolving NPCl-CQDs powder into ultrapure water, carrying out centrifuging to remove insoluble substances, carrying out filtering to remove impurities, and carrying out freeze-drying so as to obtain the fluorescent probe, namely NPCl-CQDs solid powder. The linear relationship between a riboflavin concentration and an NPCl-CQDs fluorescence intensity is determined by using a ratio fluorescence detection method. The content of riboflavin in an actual sample is detected through a standard recovery experiment, and a standard recovery rate is calculated. The method provided by the invention is simple and convenient to operate, low in background interference, high in sensitivity, free of expensive instruments and equipment, lowin detection cost, capable of rapidly, efficiently and quantitatively detecting the content of the riboflavin in an actual sample in a ratio and good in reproducibility.
Owner:SHANXI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com




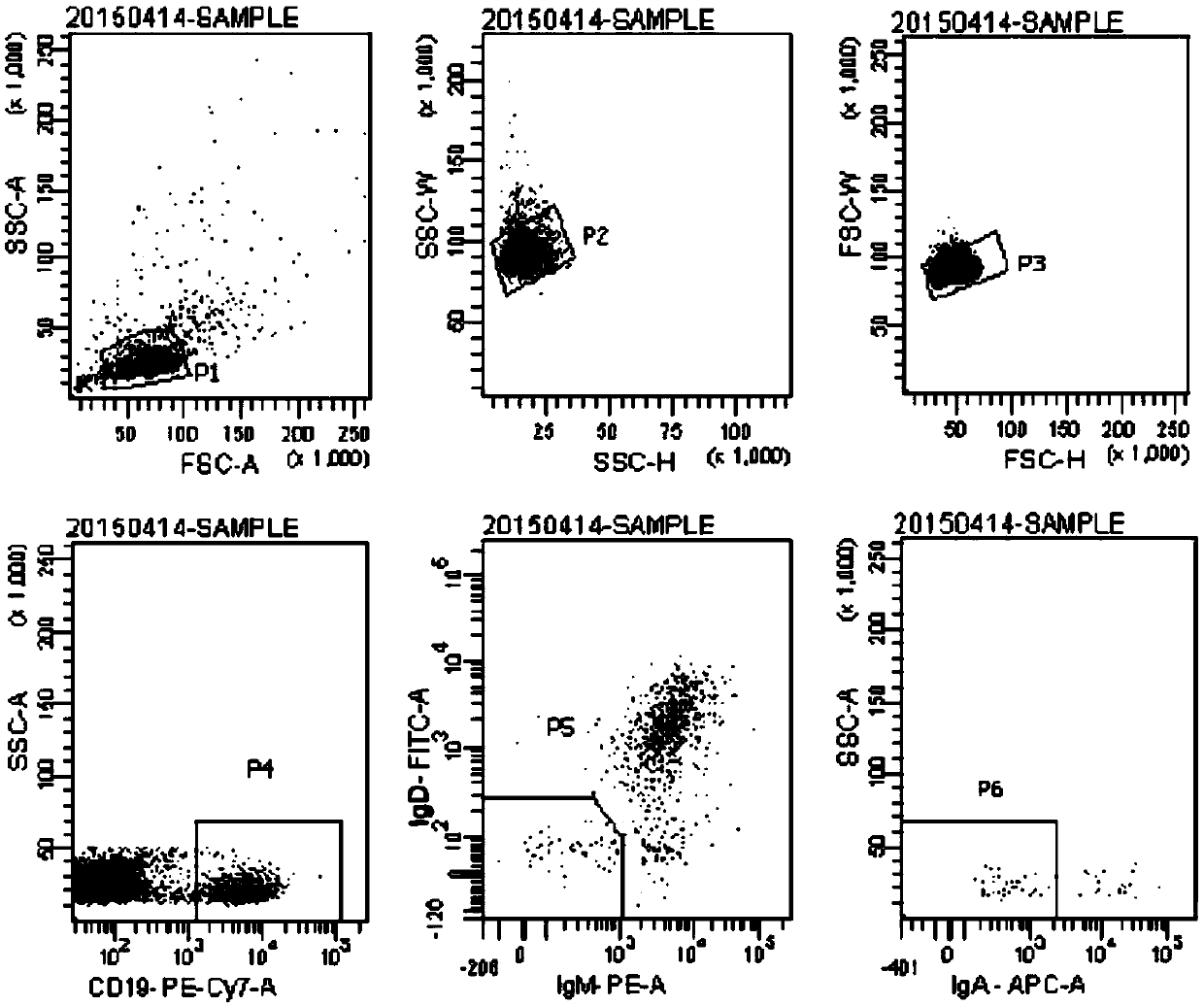
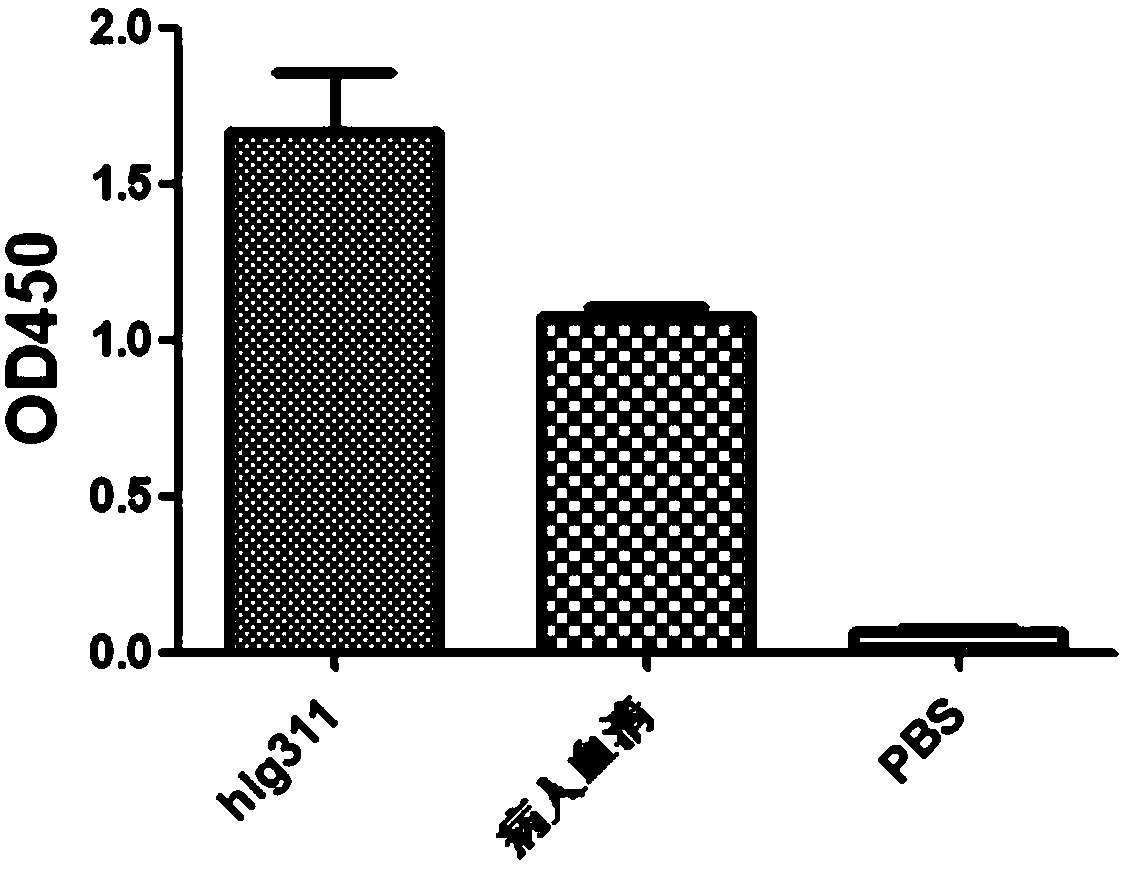
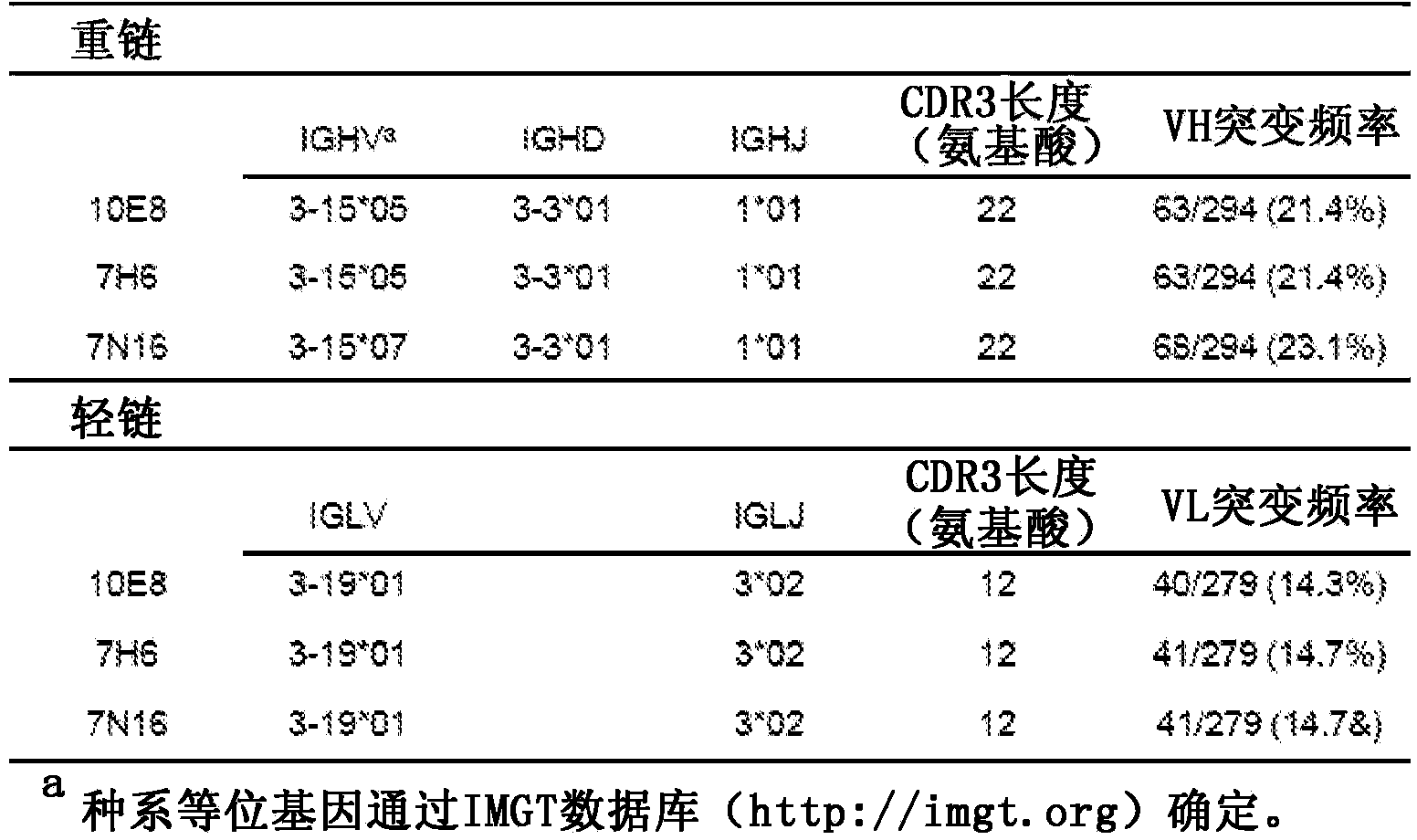
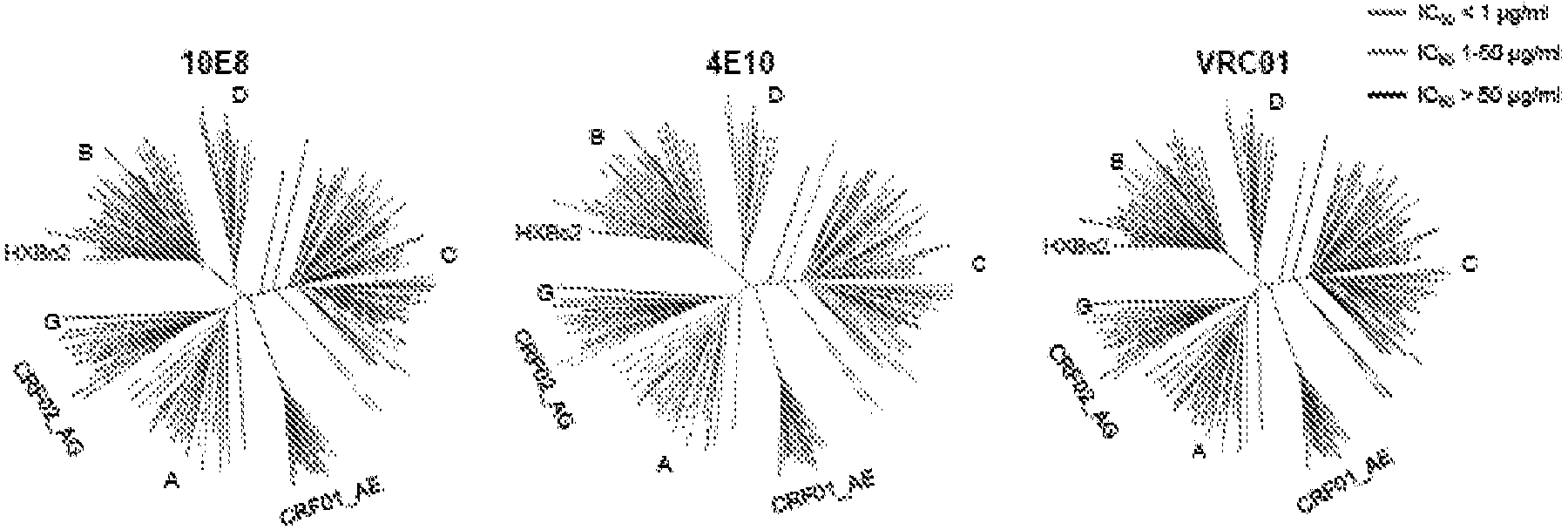
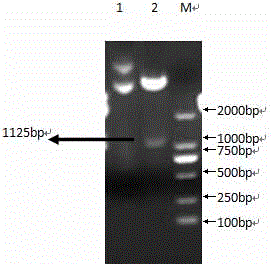

![Mosaic chimeric viral vaccine{[s]] particle Mosaic chimeric viral vaccine{[s]] particle](https://images-eureka.patsnap.com/patent_img/461236a4-8407-4483-8e3d-5875977c4f72/00000003_0000.png)
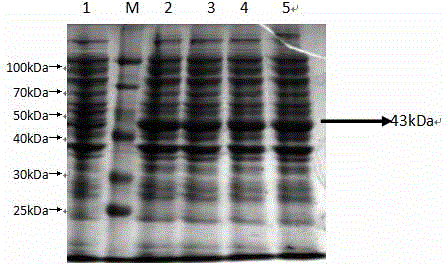
![Mosaic chimeric viral vaccine{[s]] particle Mosaic chimeric viral vaccine{[s]] particle](https://images-eureka.patsnap.com/patent_img/461236a4-8407-4483-8e3d-5875977c4f72/00000003_0001.png)
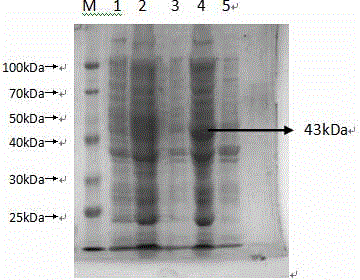
![Mosaic chimeric viral vaccine{[s]] particle Mosaic chimeric viral vaccine{[s]] particle](https://images-eureka.patsnap.com/patent_img/461236a4-8407-4483-8e3d-5875977c4f72/00000007_0000.png)