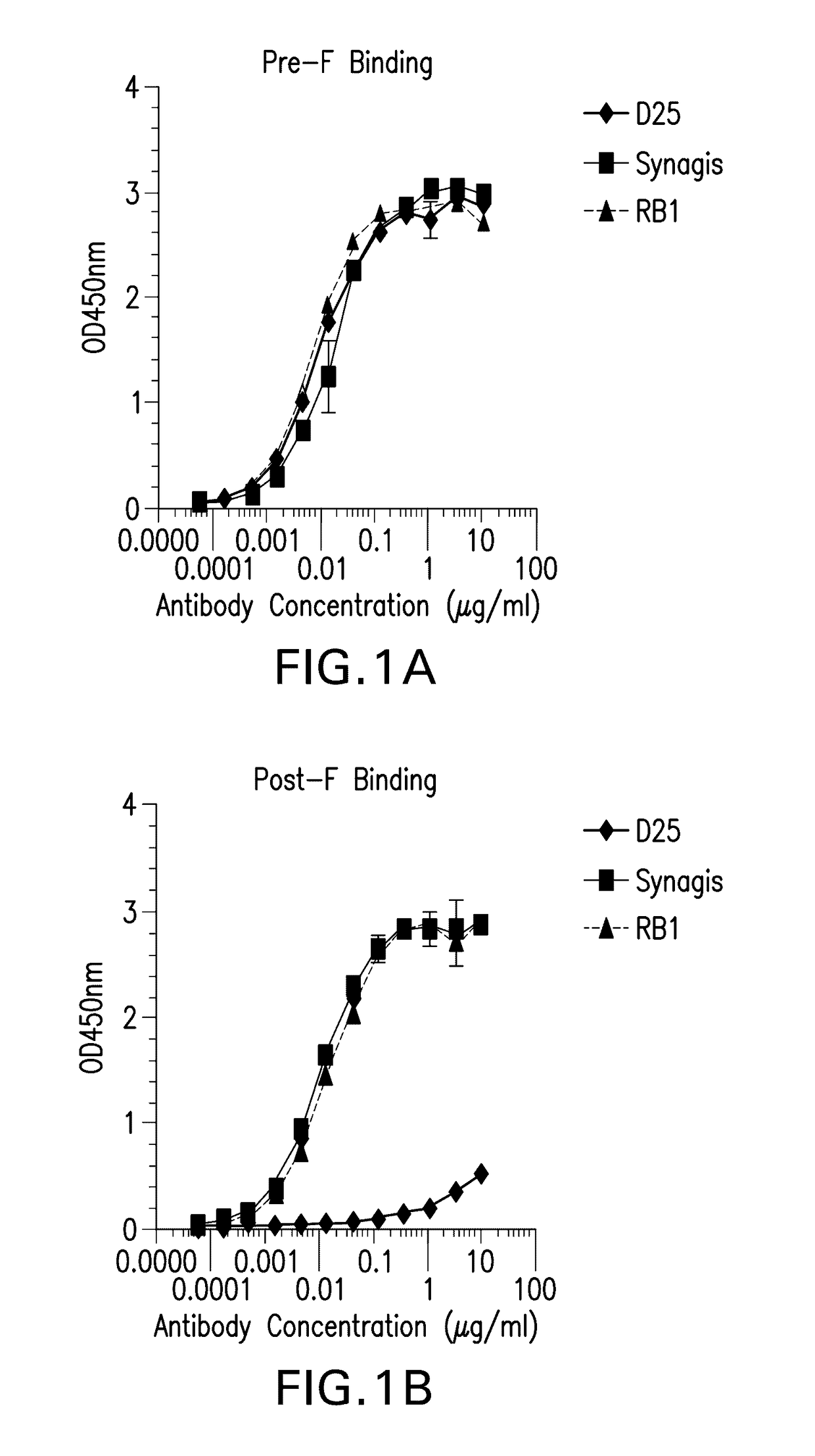
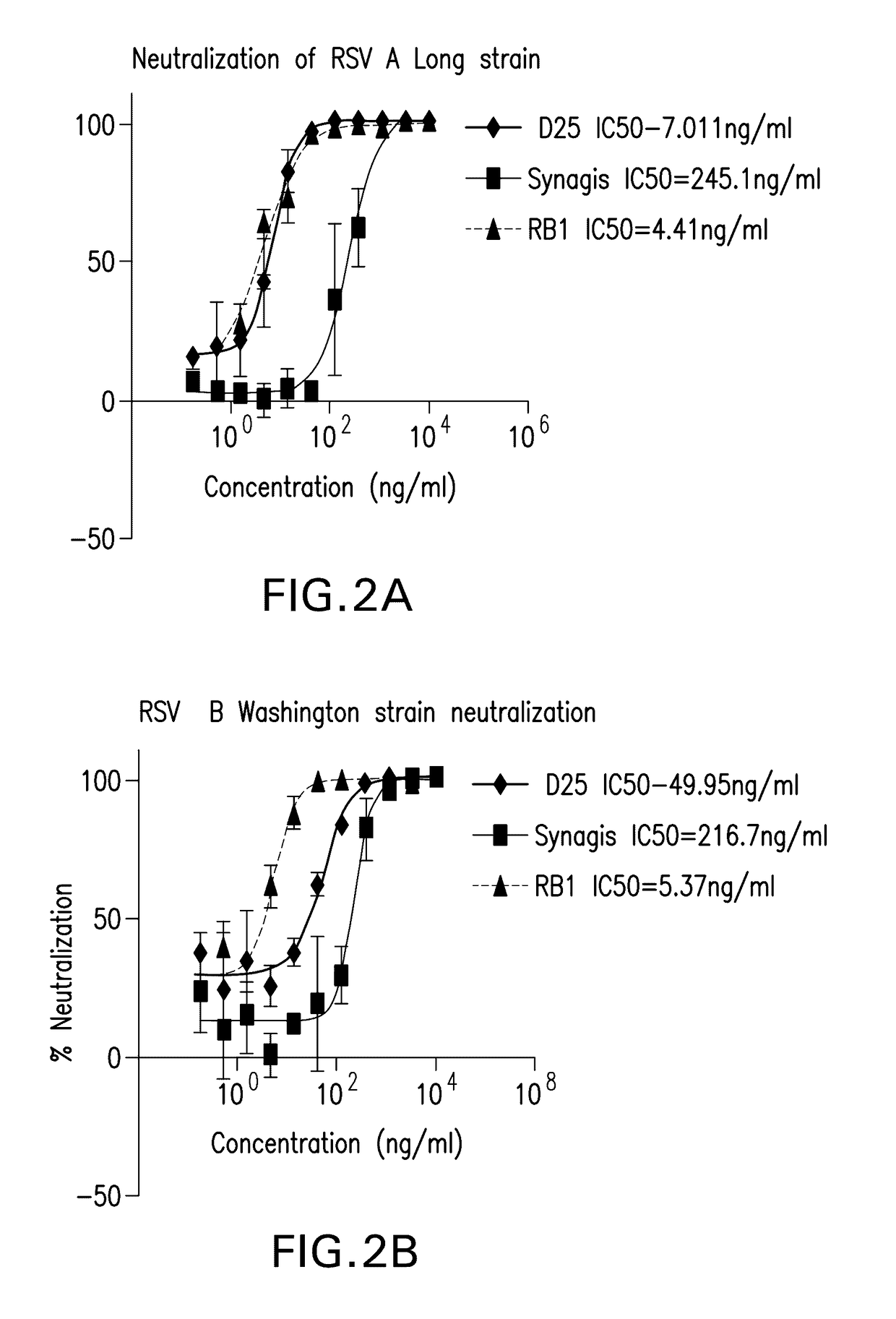
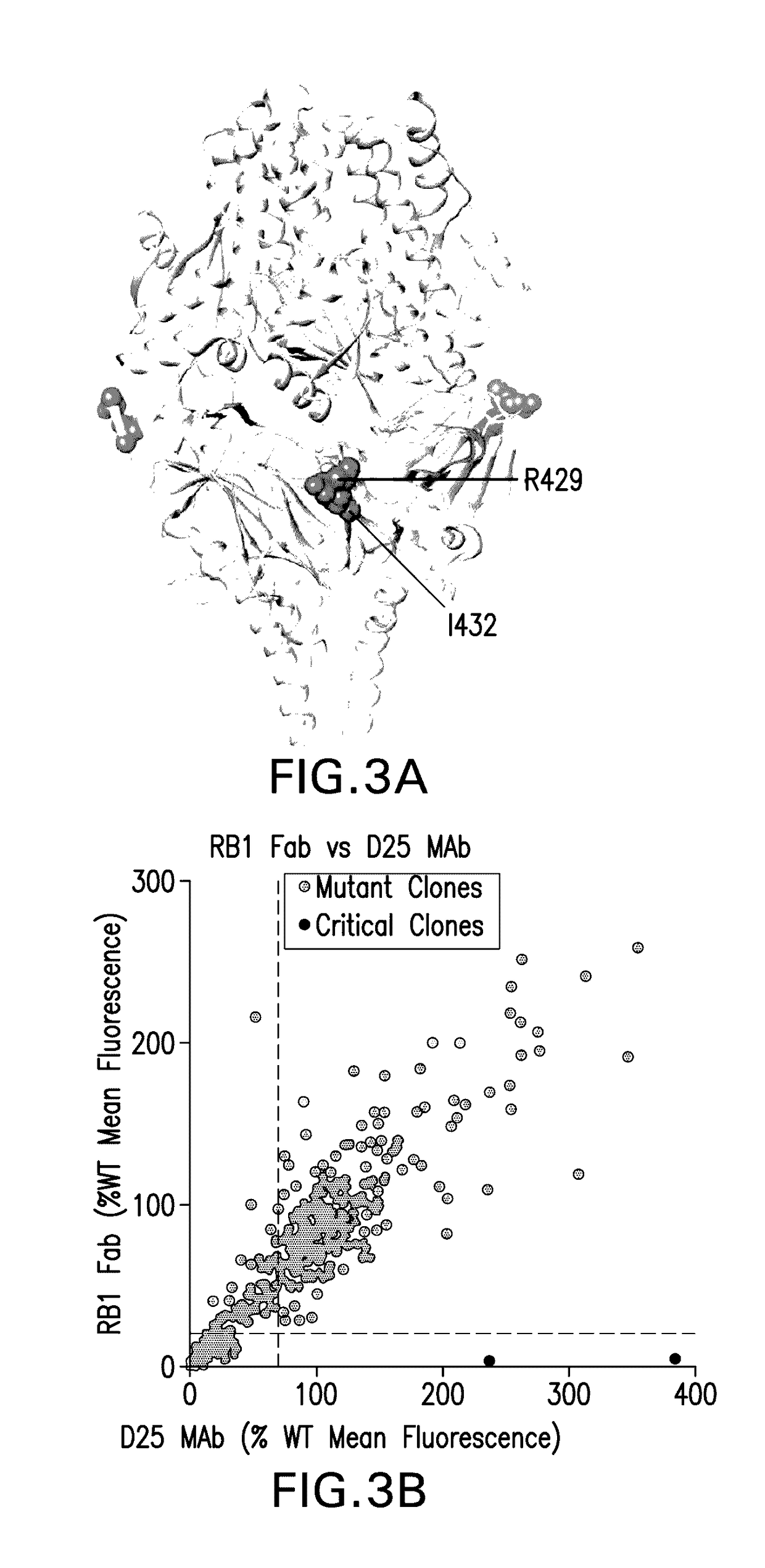
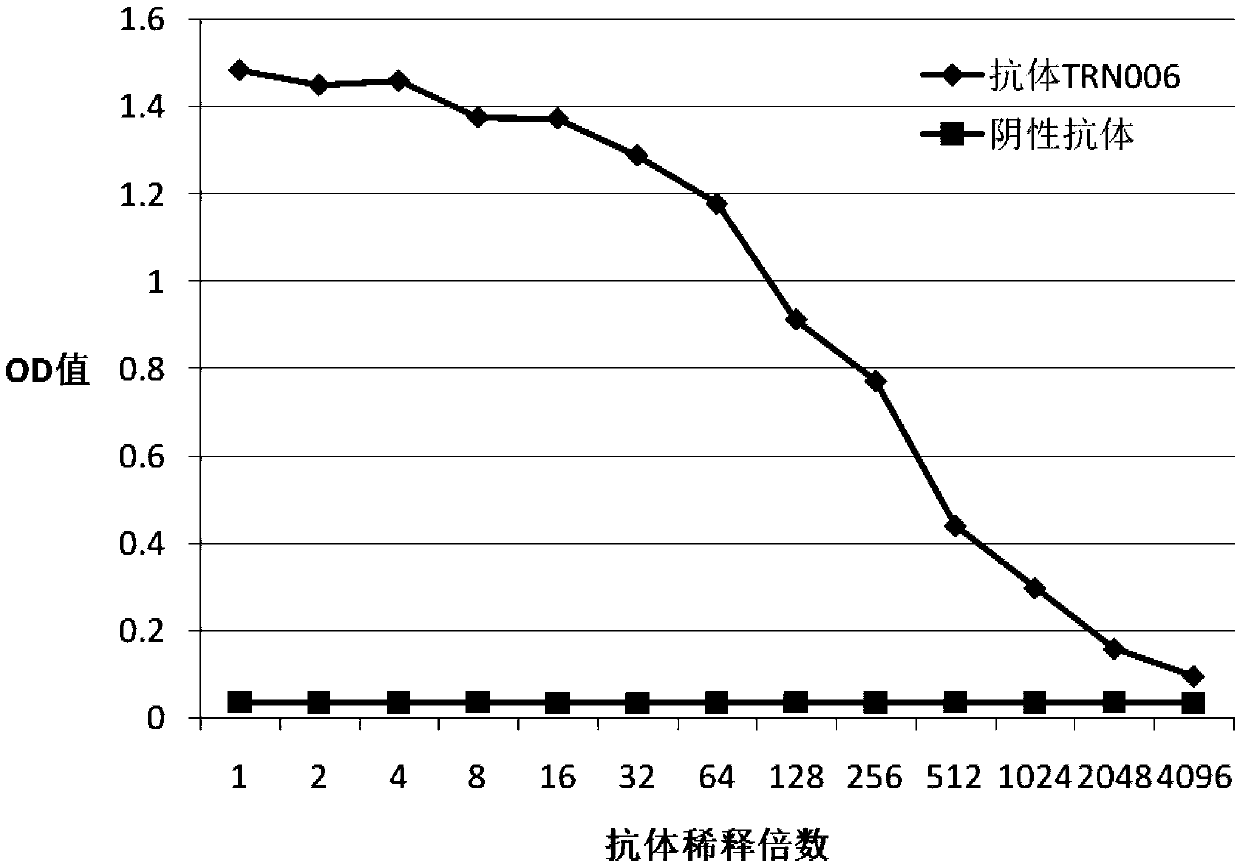
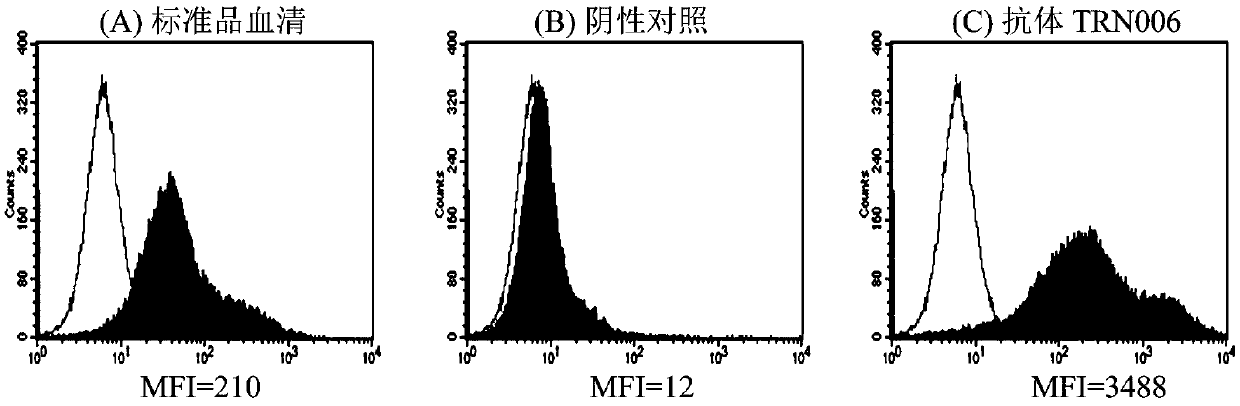
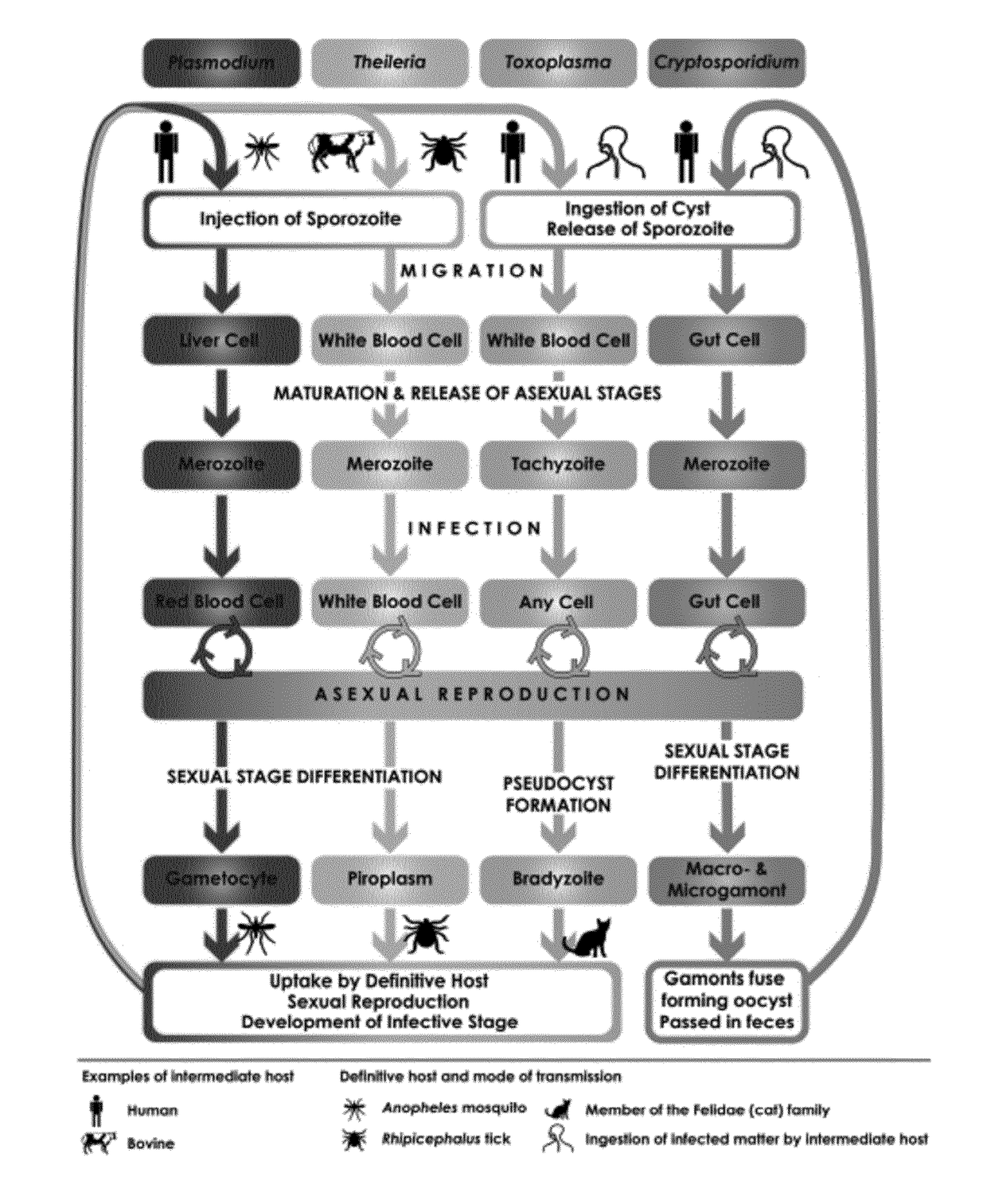
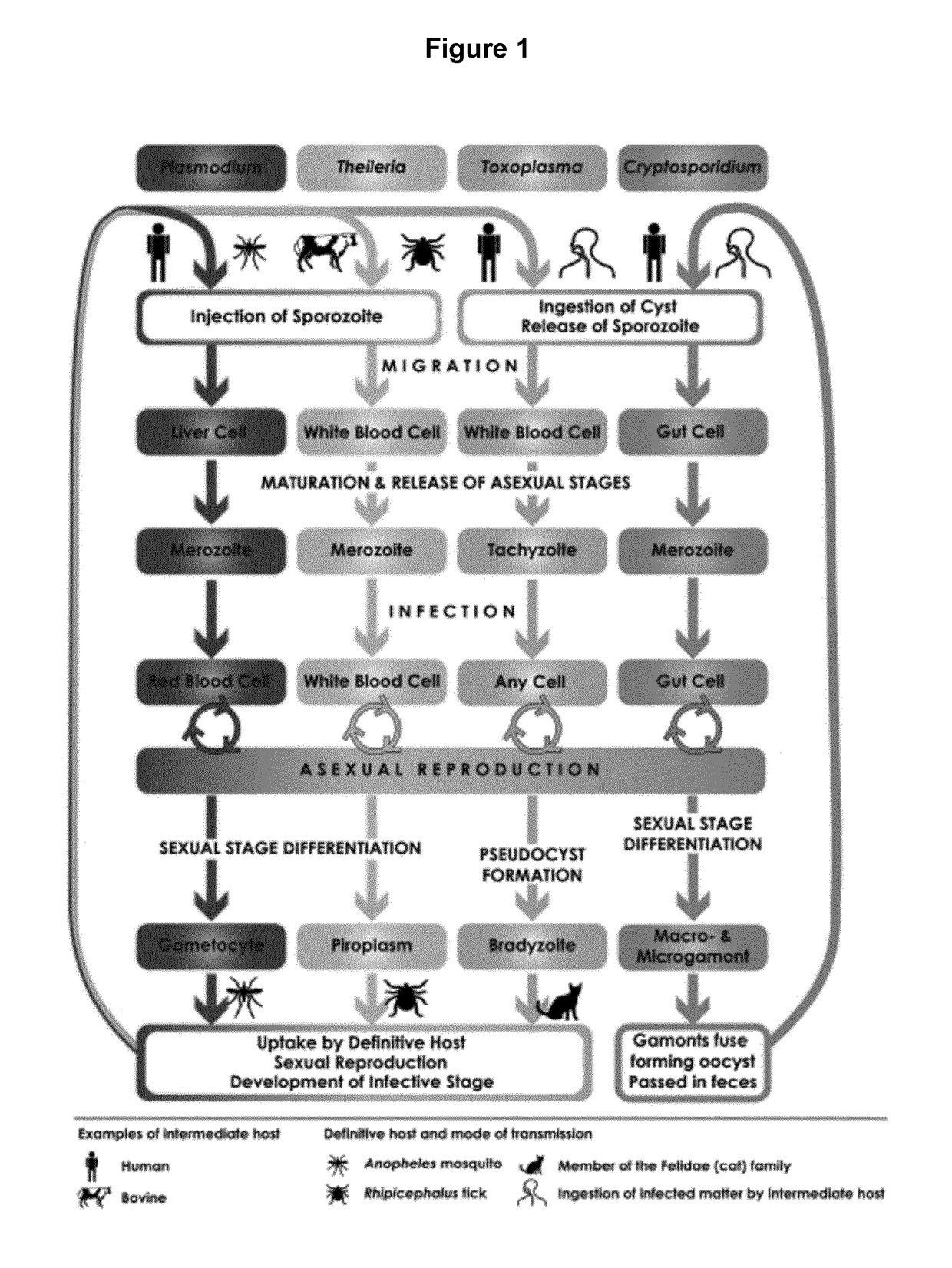
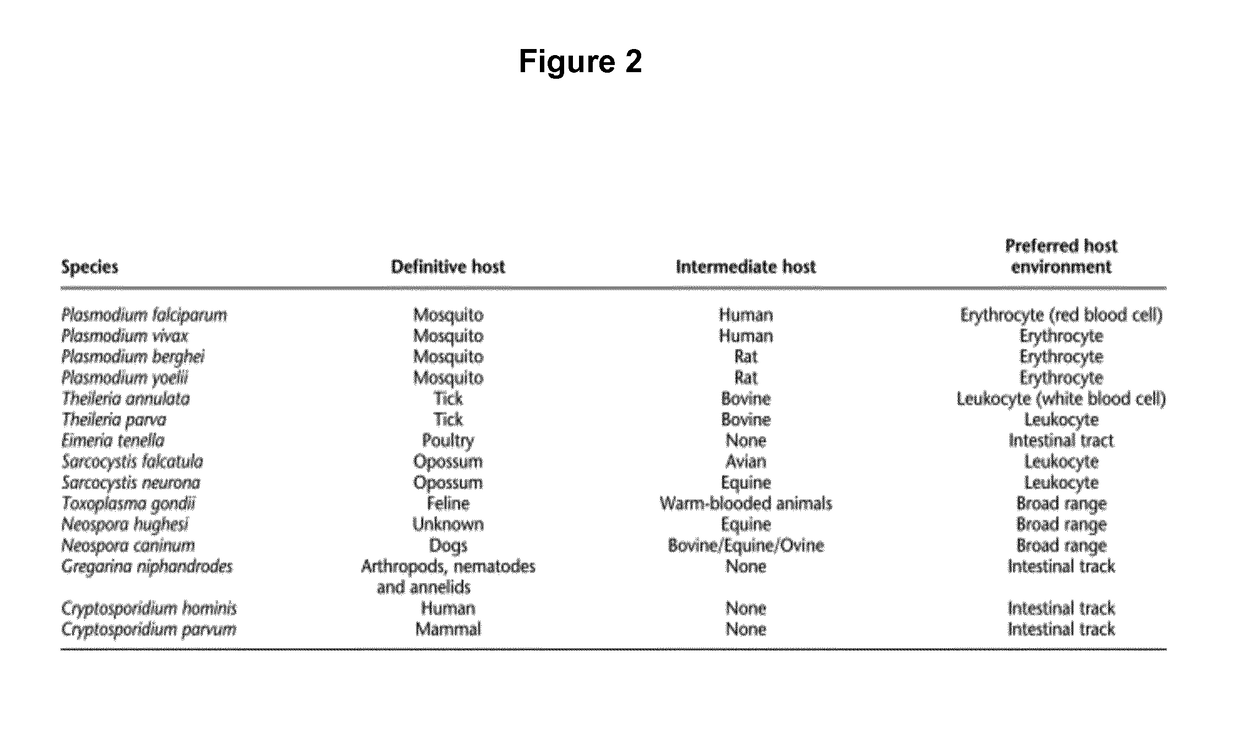
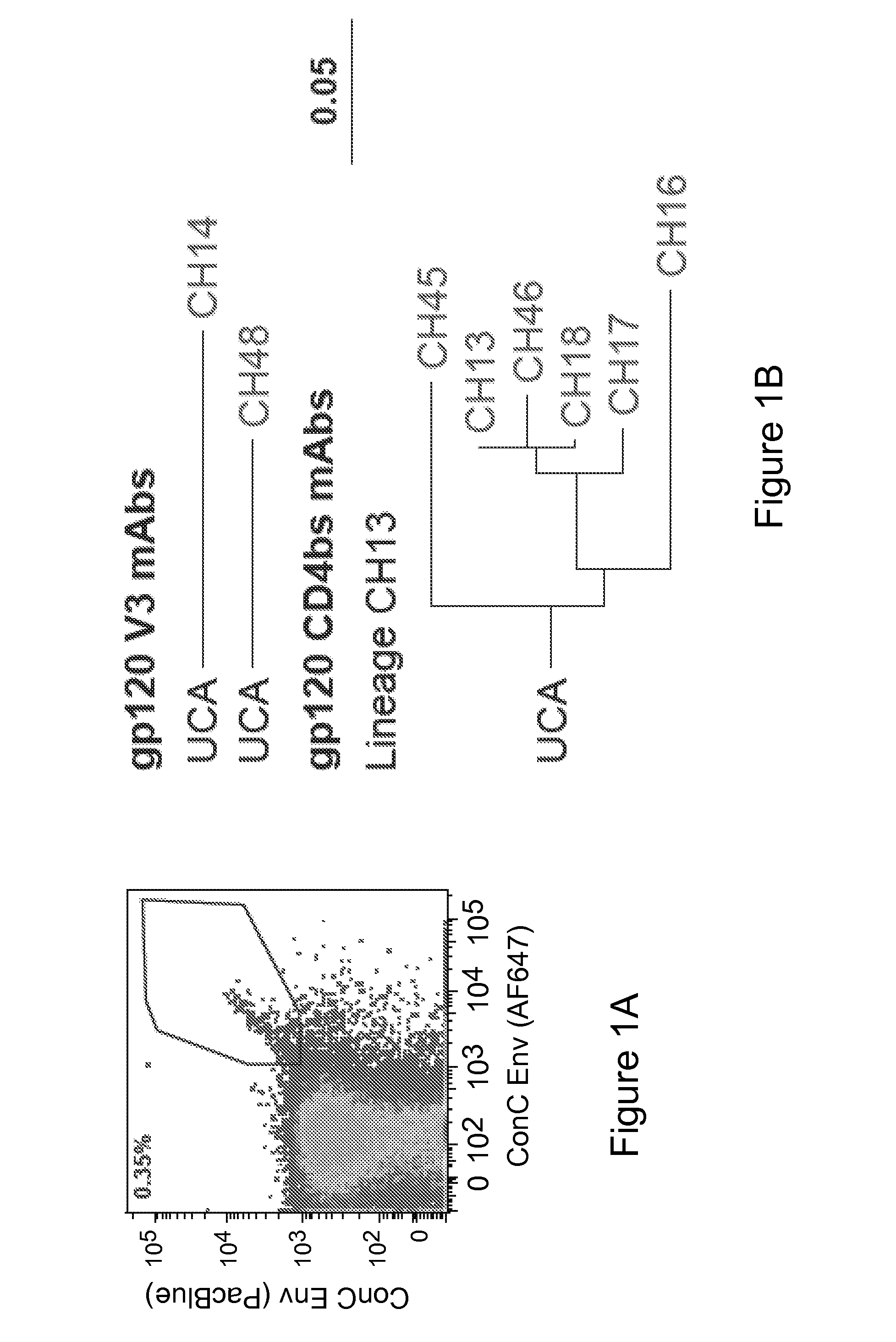
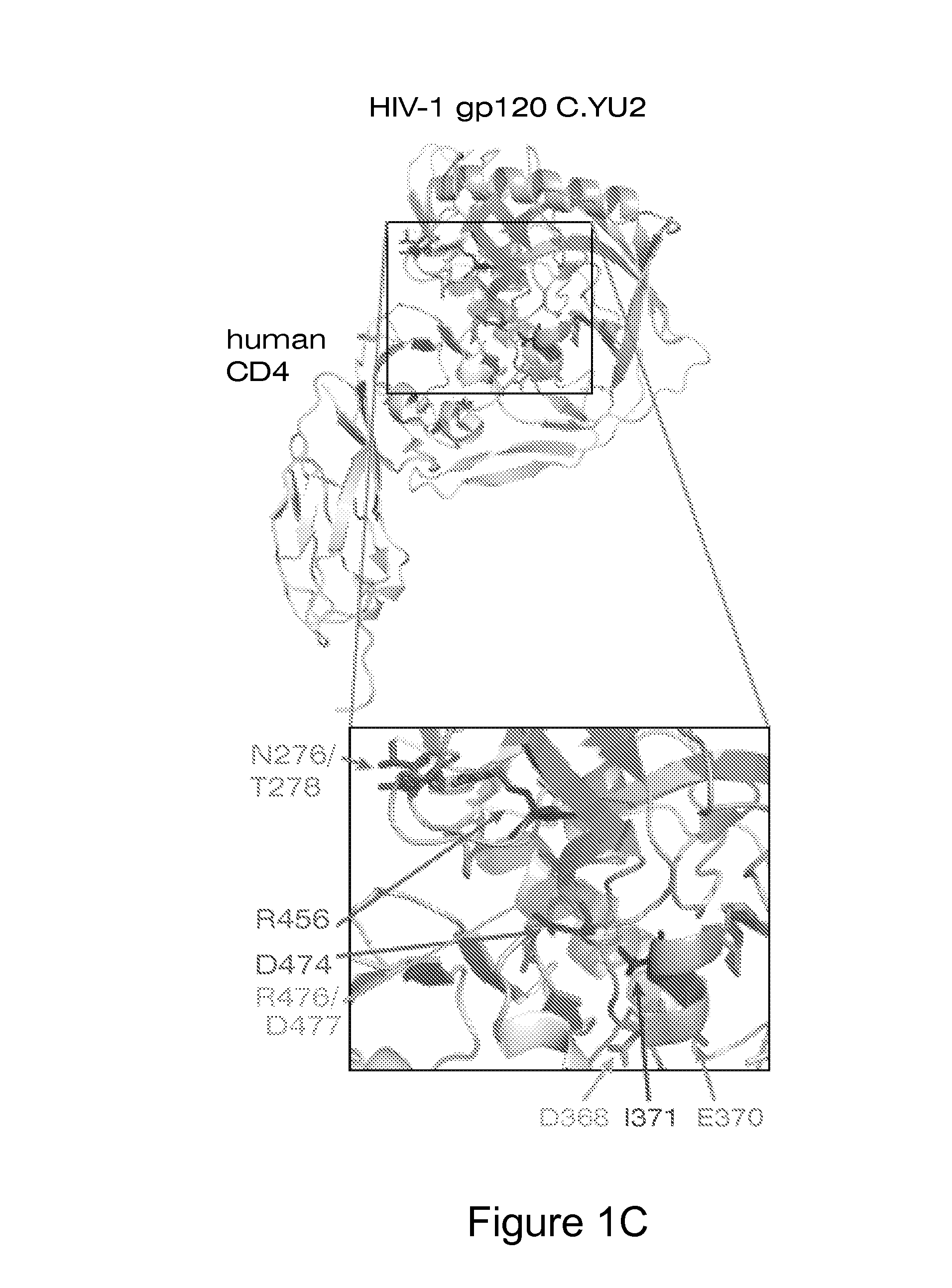
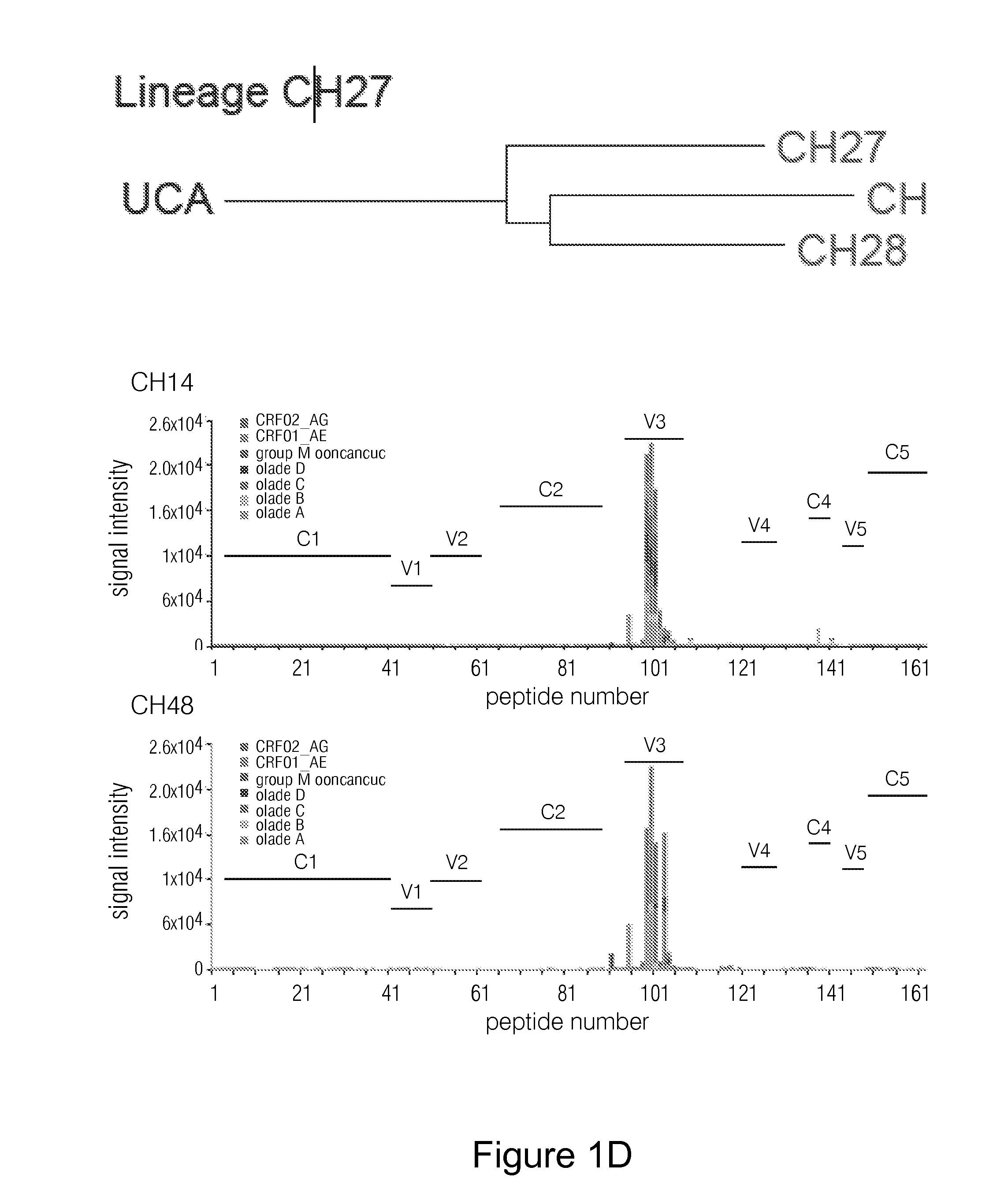
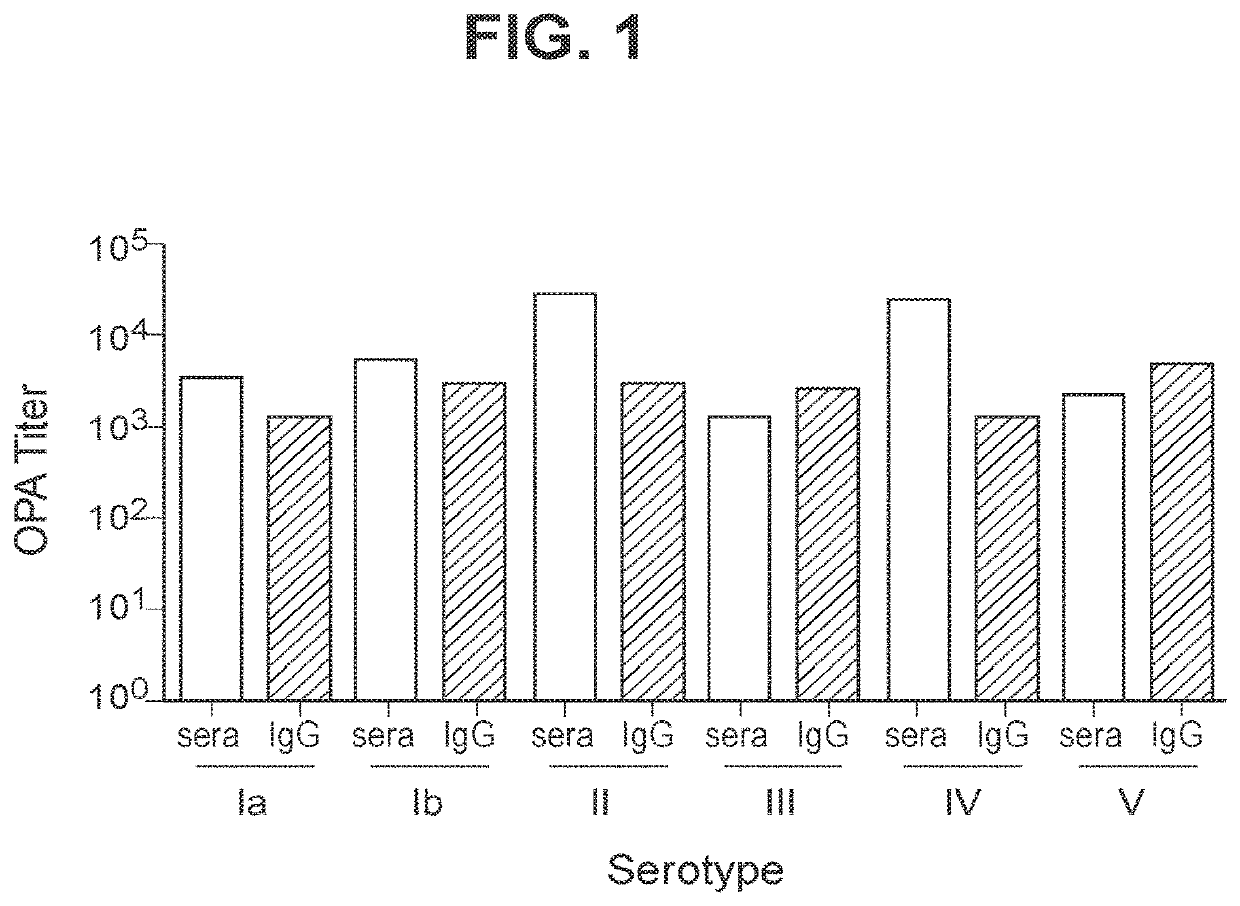
Patents
Literature
43 results about "Passive Immunotherapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A type of immunotherapy in which donated or laboratory-made immune system components or cellular proteins are given to a person to help the person fight an infection or disease. Passive immunotherapy using antibodies is often used in cancer treatment.
Use of heat shock proteins to enhance efficacy of antibody therapeutics
InactiveUS20060093612A1Good effectReduce morbidityAntibacterial agentsSenses disorderStress ProteinsNeuro-degenerative disease
The present invention relates to methods and pharmaceutical compositions useful for the prevention and treatment of any disease wherein the treatment of such disease would be improved by an enhanced immune response, such as infectious diseases, primary and metastatic neoplastic diseases (i.e., cancer), or neurodegenerative or amyloid diseases. In particular, the contemplated invention is directed to method comprising the administration of heat shock / stress proteins (HSPs) or HSP complexes alone or in combination with each other, in combination with the administration of an immunoreactive reagent. The invention also provides pharmaceutical compositions comprising one or more HSPs or HSP complexes in combination with an immunoreactive reagent. Additionally, the invention contemplates the use of the methods and compositions of the invention to enhance or improve passive immunotherapy and effector cell function.
Owner:UNIV OF CONNECTICUT HEALTH CENT
Human neutralizing monoclonal antibodies to respiratory syncytial virus
InactiveUS7364737B2Reduce decreaseReduce the amount requiredPeptide/protein ingredientsAntibody mimetics/scaffoldsPassive ImmunotherapyVirus
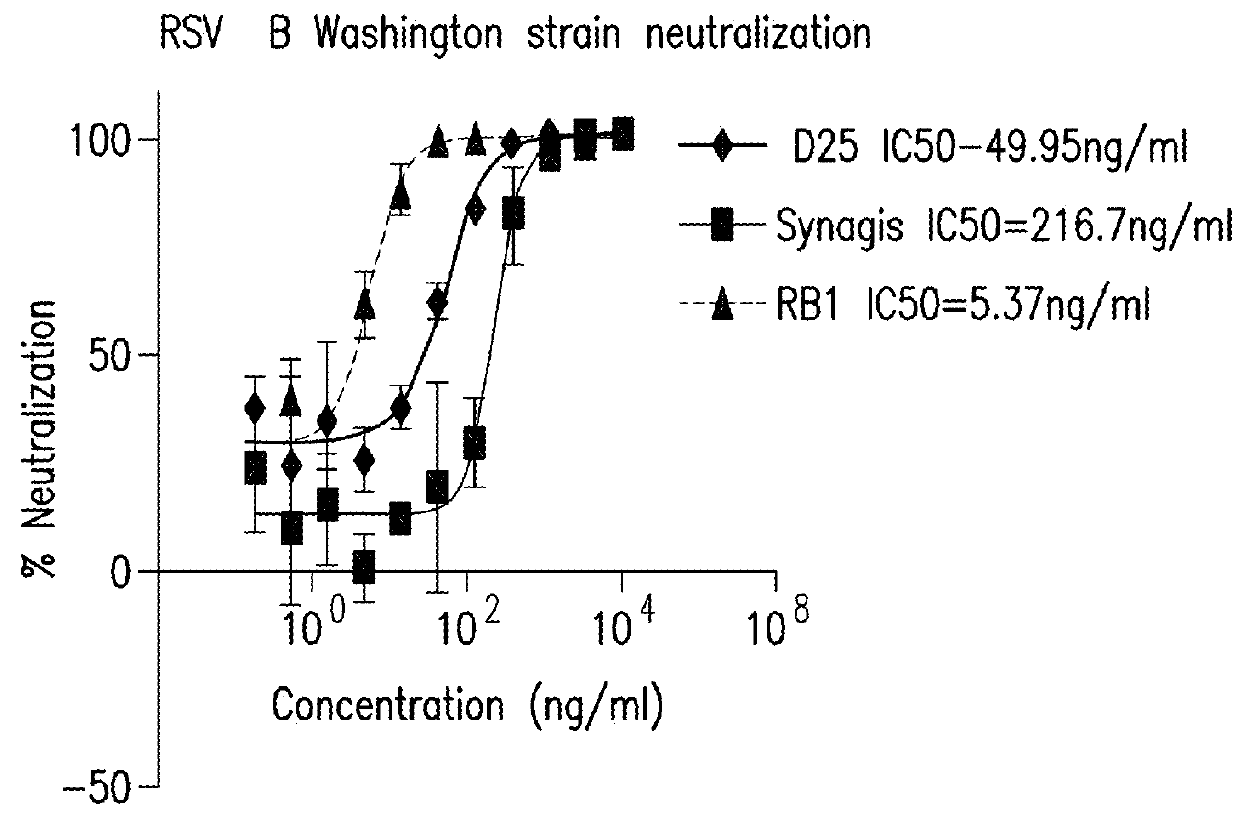
Human monoclonal antibodies and fragments thereof which bind, neutralize and provide passive immunotherapy to respiratory syncytial virus (RSV) antigenic subgroups A and B are disclosed. Also disclosed are diagnostic and immunotherapeutic methods of using the monoclonal antibodies as well as cell line producing the monoclonal antibodies.
Owner:THE SCRIPPS RES INST
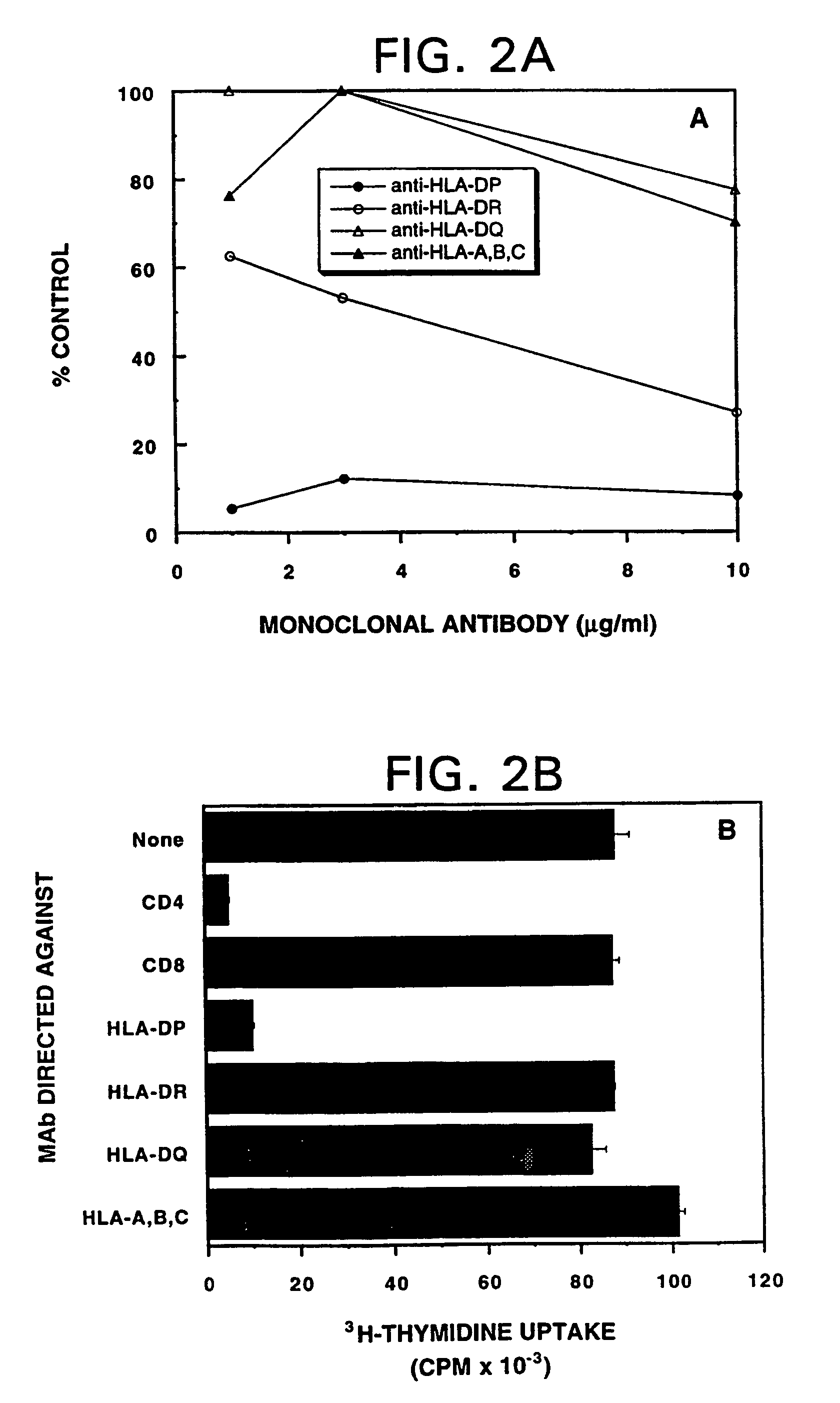
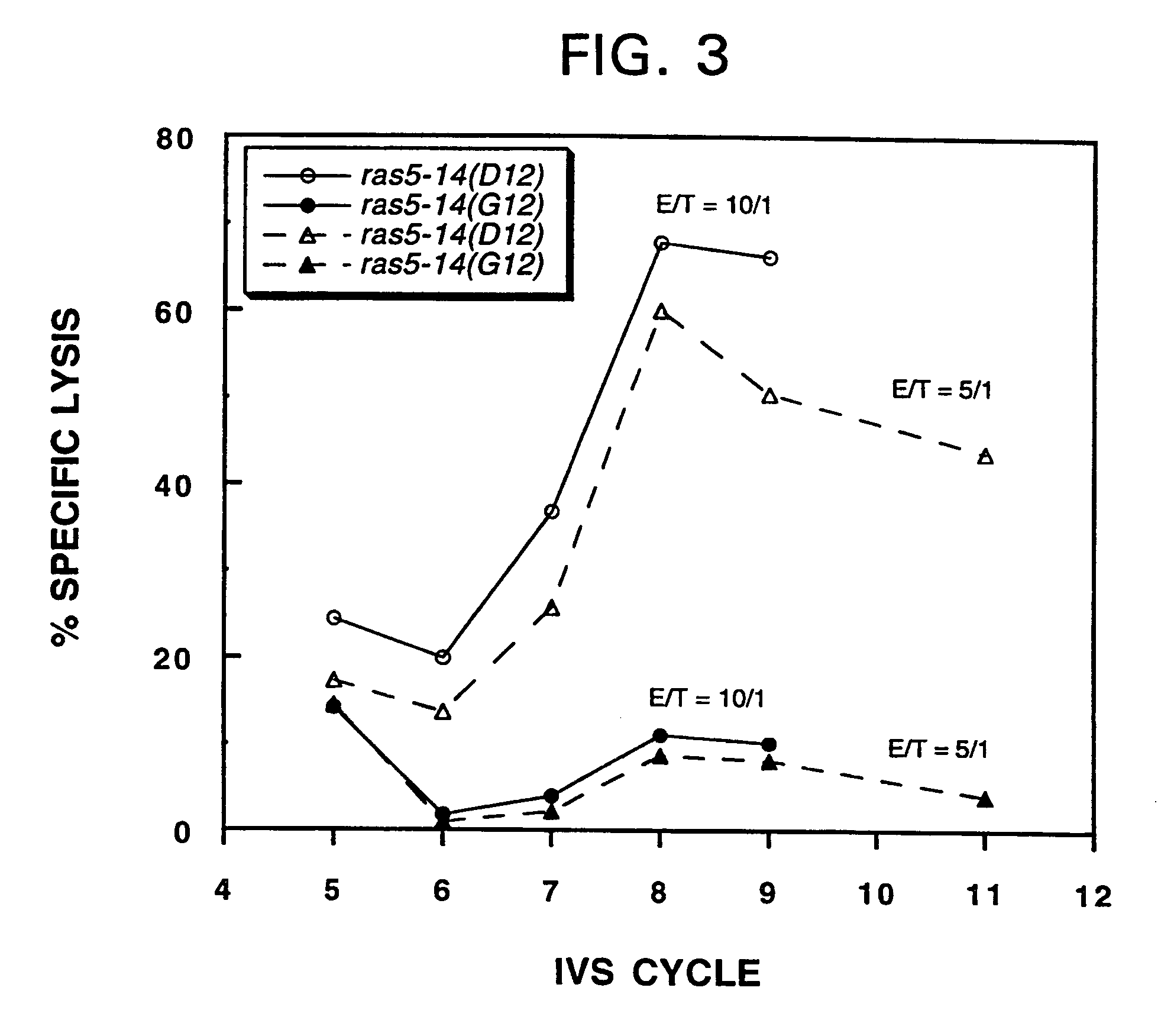
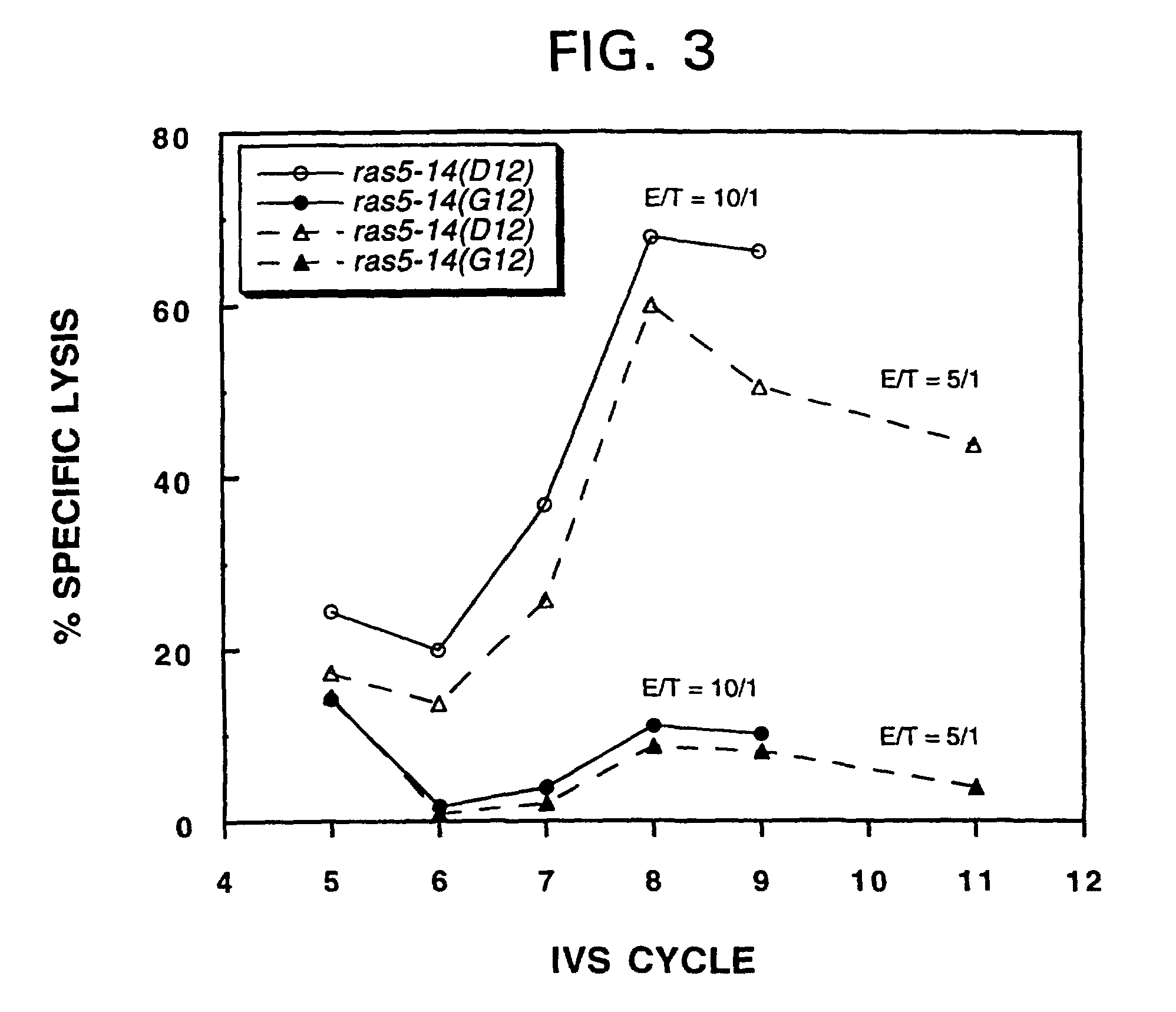
Mutated ras peptides for generation of CD8+Cytotoxic T lymphocytes
InactiveUS7709002B1Growth inhibitionAvoid it happening againBacterial antigen ingredientsPeptide/protein ingredientsCtl epitopeMutated Ras Peptide
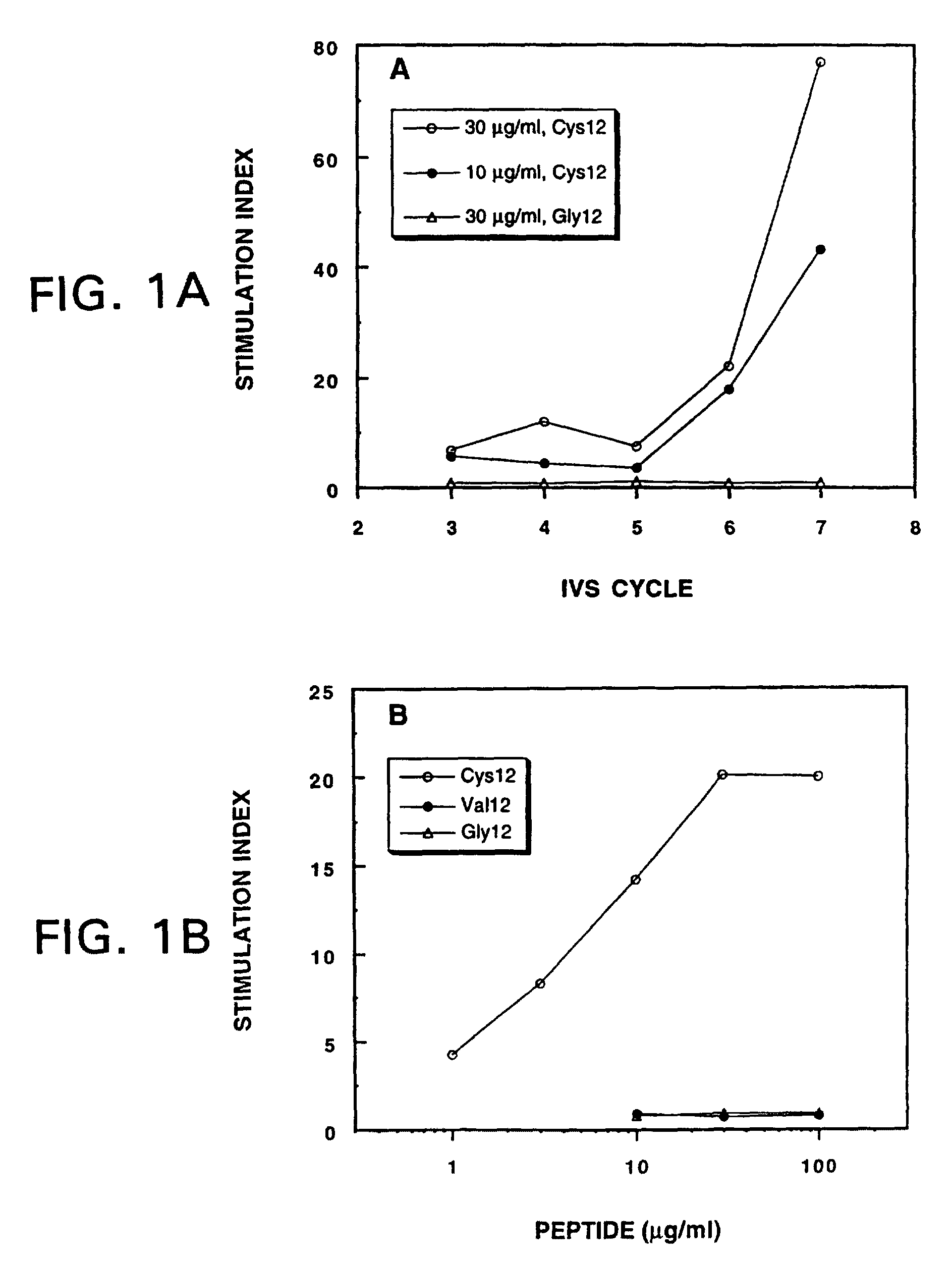
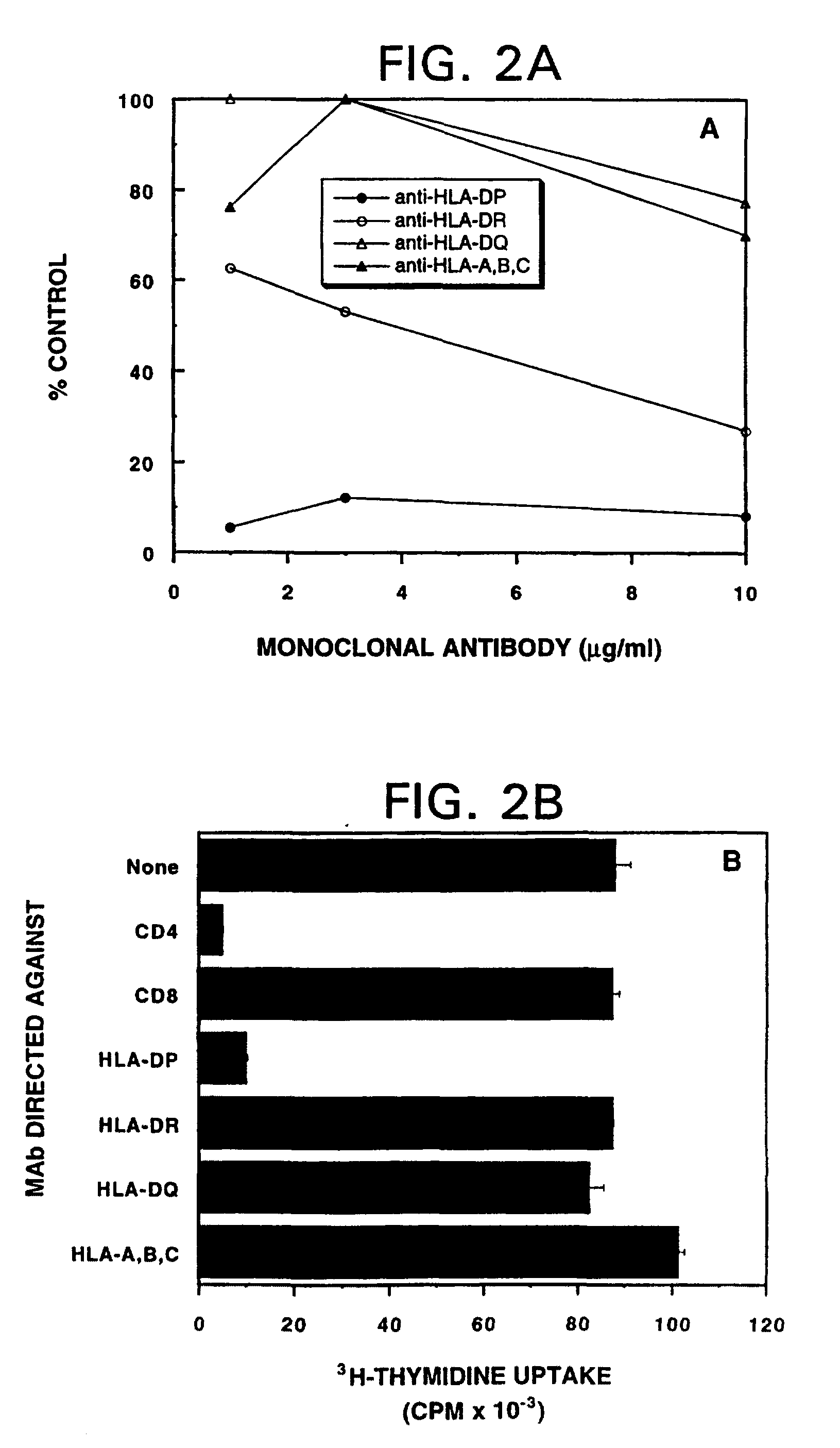
Mutant ras oncogene peptides may induce specific anti-ras cellular immune responses in vaccinated patients. Moreover, a human CD8+ CTL epitope(s) reflecting a specific point mutation in the K-ras oncogene at codon 12 was identified. The mutant ras peptide has implications for both active and passive immunotherapies in selected carcinoma patients. A nested 10-mer peptide was identified [i.e., ras5-14(Asp12)], which was shown to bind to HLA-A2 and display specific functional capacity for expansion of the in-vivo-primed CD8+ CTL precursors.
Owner:GOVERNMENT OF THE US REPRESENTED BY THE SEC
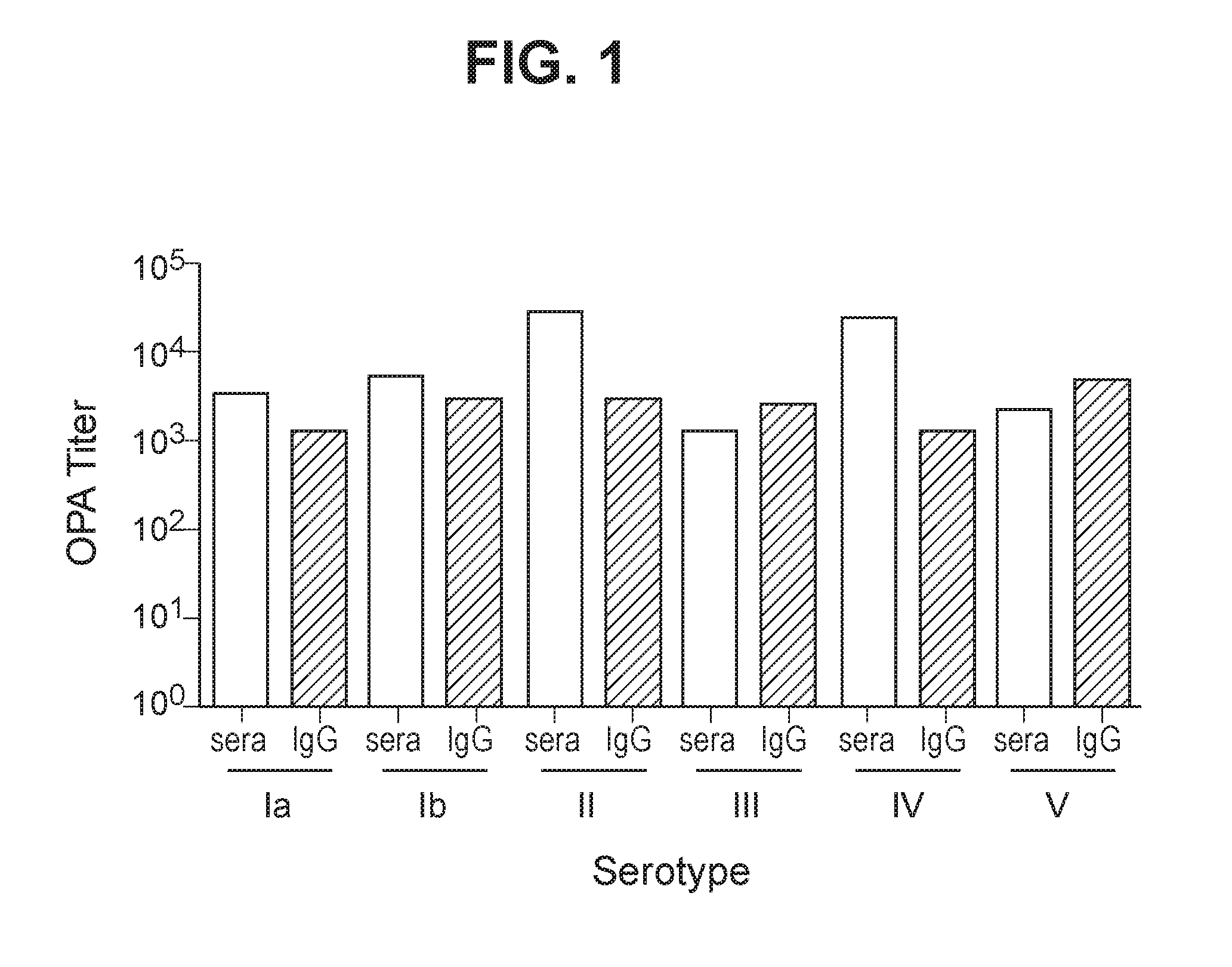
Group b streptococcus polysaccharide-protein conjugates, methods for producing conjugates, immunogenic compositions comprising conjugates, and uses thereof
ActiveUS20160324950A1Antibacterial agentsBacterial antigen ingredientsPassive ImmunizationsAntiendomysial antibodies
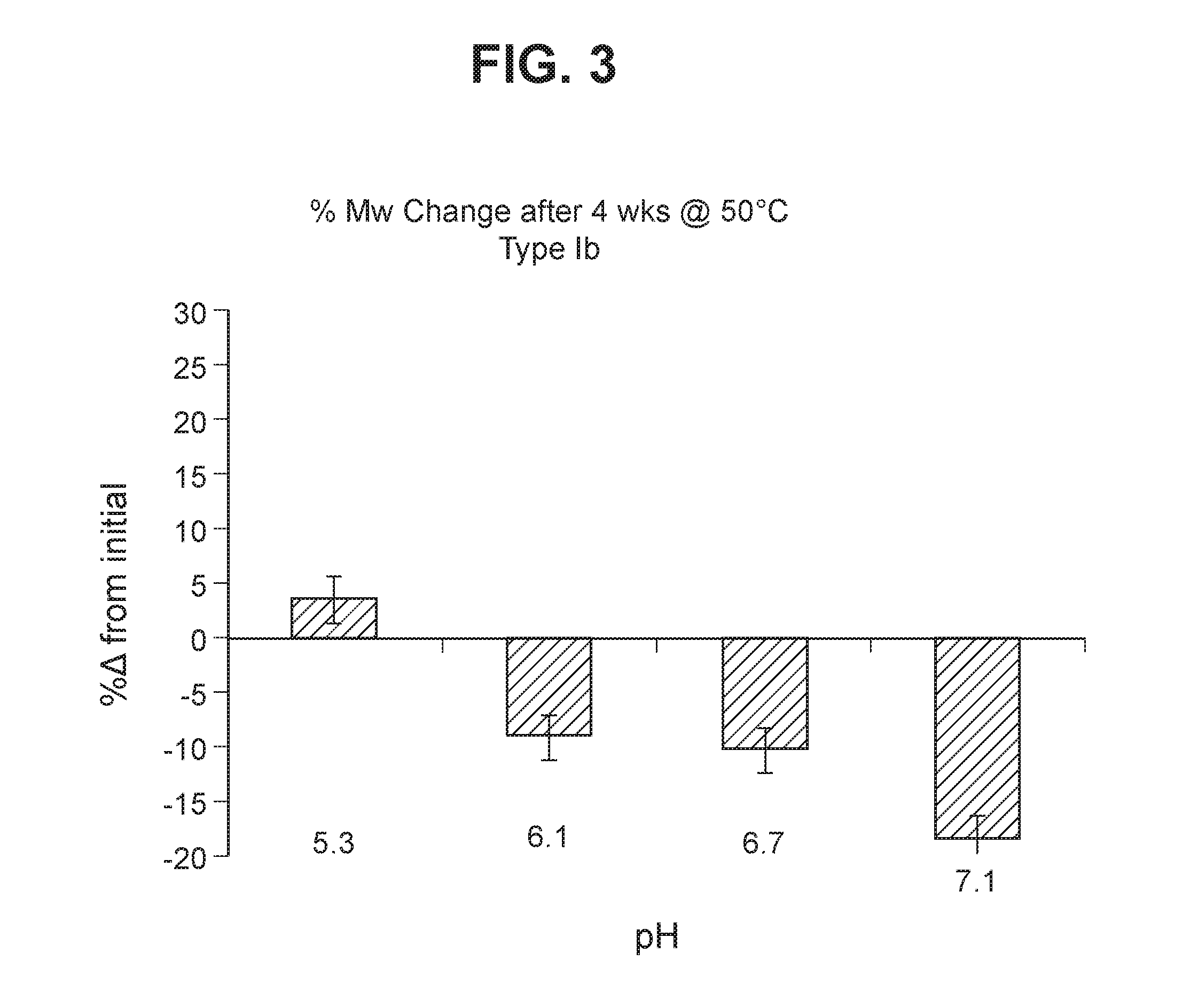
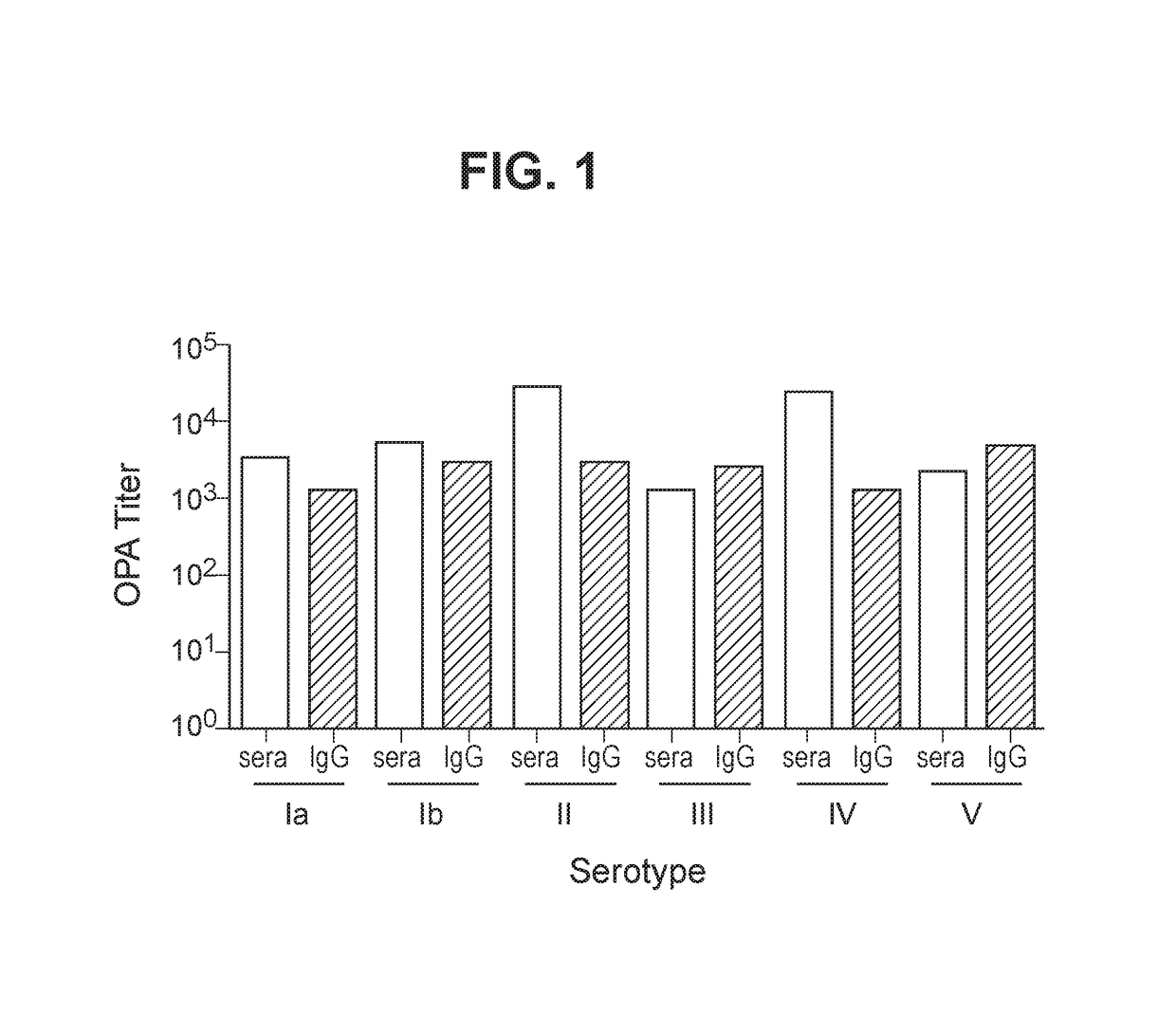
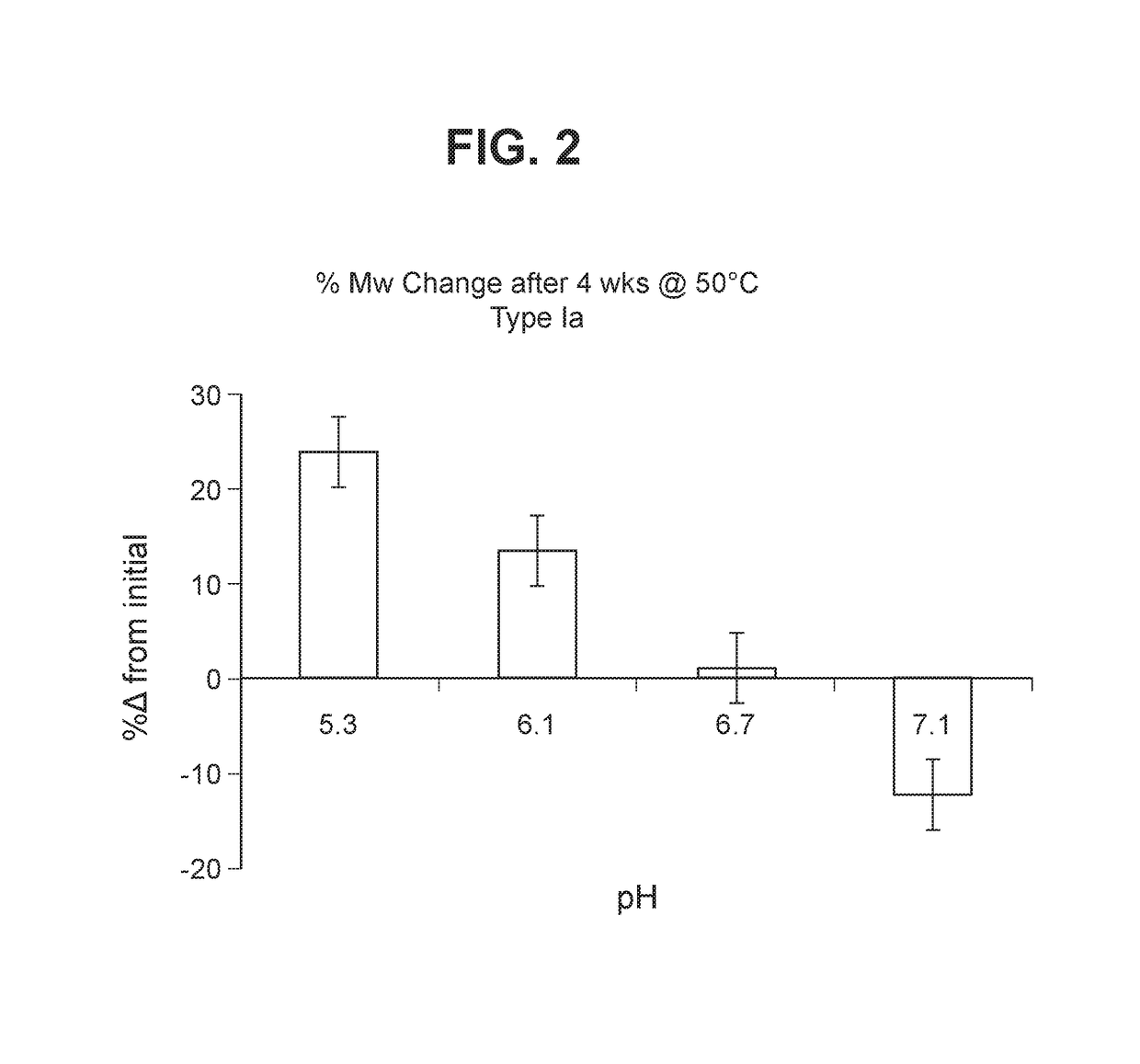
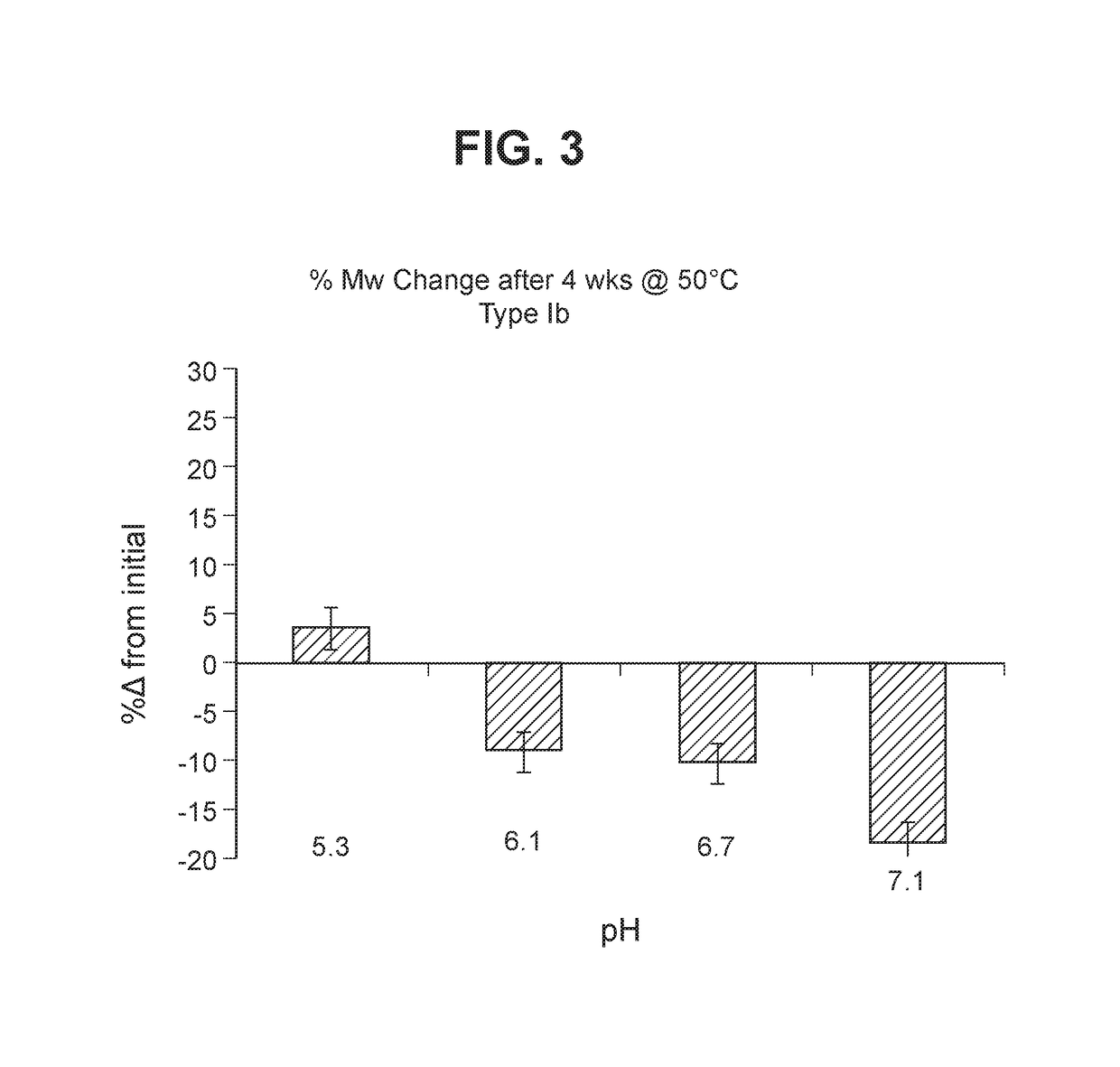
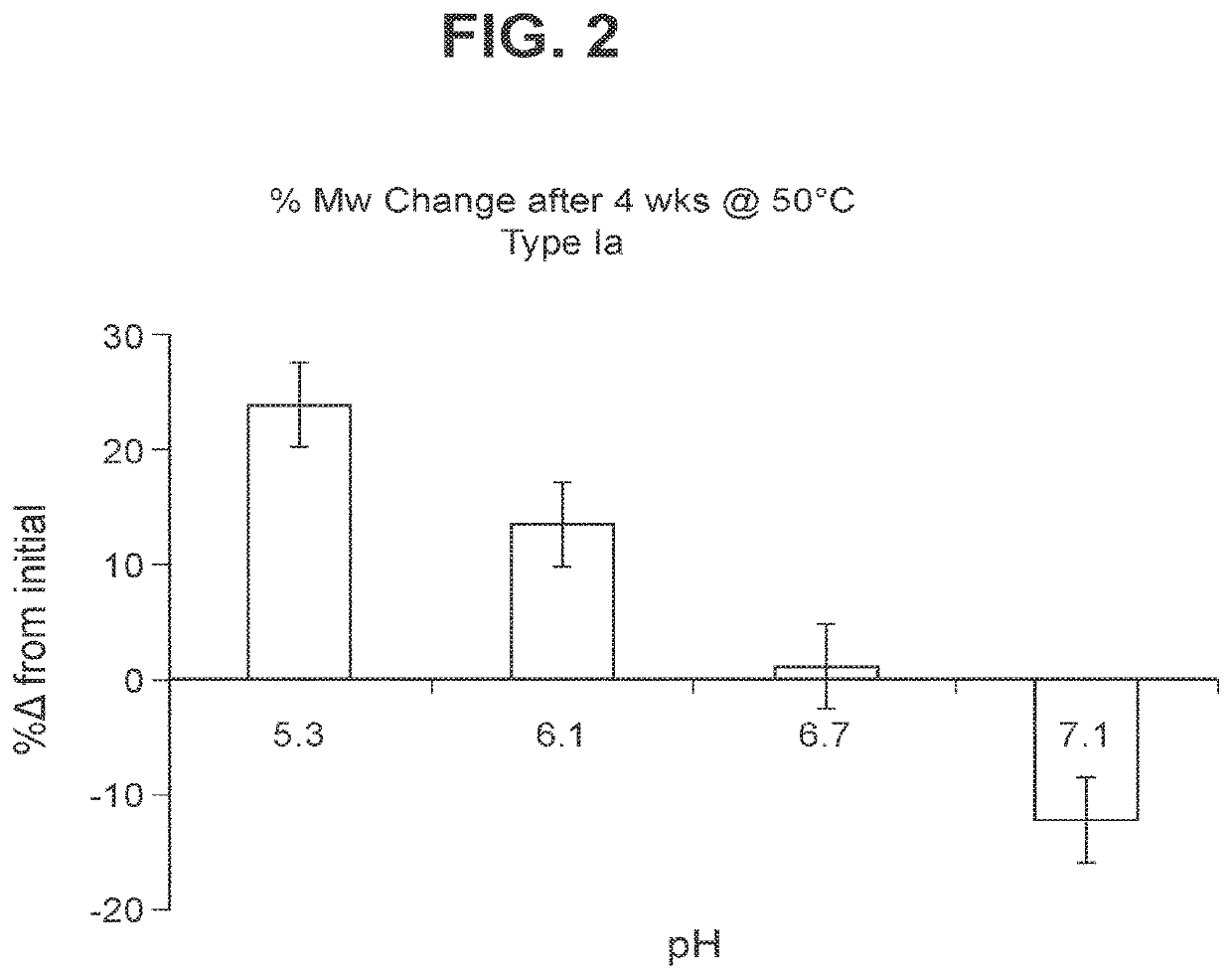
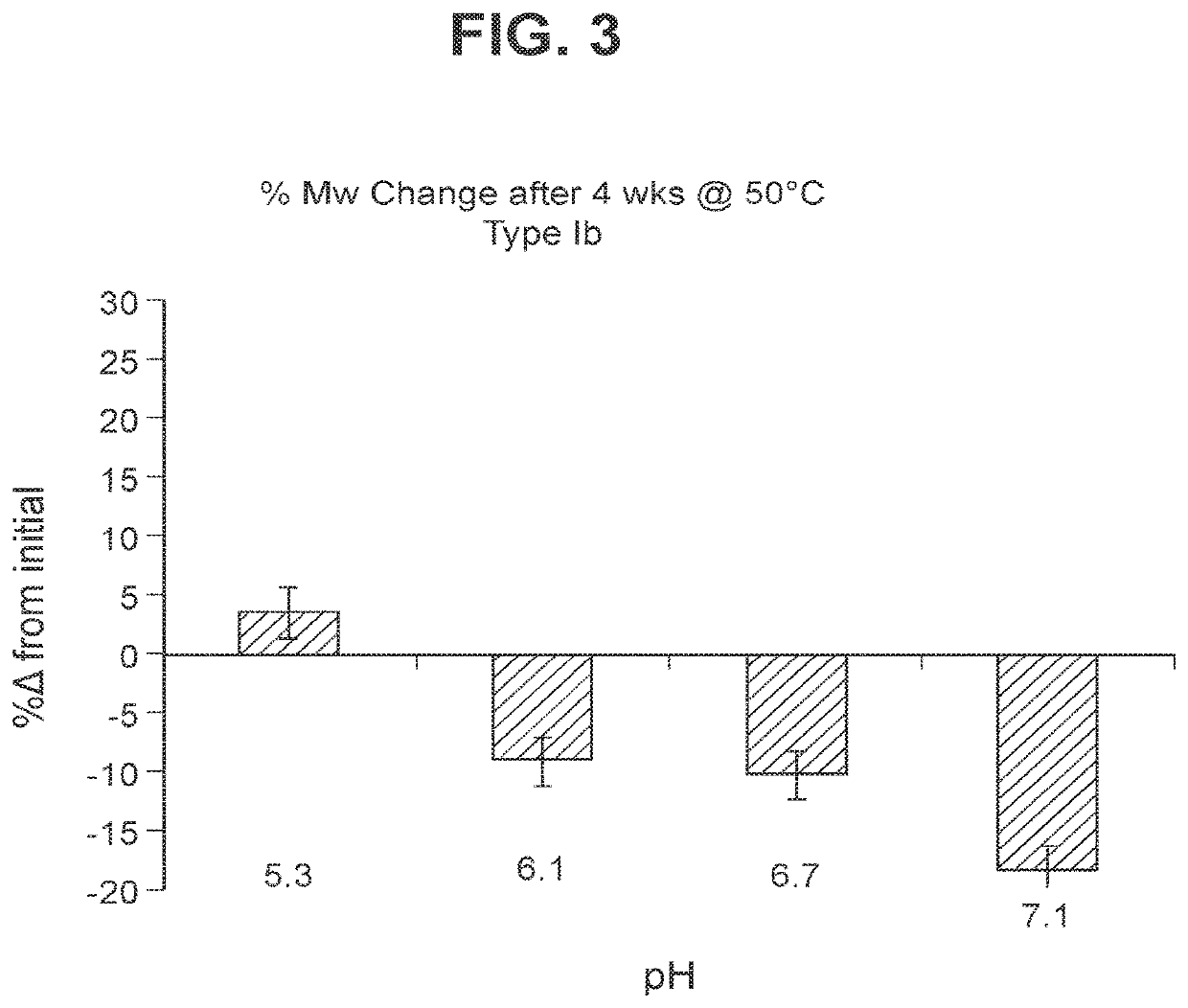
The invention relates to immunogenic polysaccharide-protein conjugates comprising a capsular polysaccharide (CP) from Streptococcus agalactiae, commonly referred to as group B streptococcus (GBS), and a carrier protein, wherein the CP is selected from the group consisting of serotypes Ia, Ib, II, III, IV, V, VI, VII, VIII, and IX, and wherein the CP has a sialic acid level of greater than about 60%. The invention also relates to methods of making the conjugates and immunogenic compositions comprising the conjugates. The invention also relates to immunogenic compositions comprising polysaccharide-protein conjugates, wherein the conjugates comprise a CP from GBS serotype IV and at least one additional serotype. The invention further relates to methods for inducing an immune response in subjects against GBS and / or for reducing or preventing invasive GBS disease in subjects using the compositions disclosed herein. The resulting antibodies can be used to treat or prevent GBS infection via passive immunotherapy.
Owner:PFIZER INC
Prevention and treatment of recurrent respiratory papillomatosis
InactiveUS20060029612A1Improved vaccine compositionBroad protectionViral antigen ingredientsVaccinesPassive ImmunotherapyVaccination
Juvenile-onset recurrent respiratory papillomatosis is treated using active vaccination or passive immune therapy of neutralizing antibodies against HPV L2 neutralizing epitopes.
Owner:LARGE SCALE BIOLOGY
Group B Streptococcus polysaccharide-protein conjugates, methods for producing conjugates, immunogenic compositions comprising conjugates, and uses thereof
The invention relates to immunogenic polysaccharide-protein conjugates comprising a capsular polysaccharide (CP) from Streptococcus agalactiae, commonly referred to as group B streptococcus (GBS), and a carrier protein, wherein the CP is selected from the group consisting of serotypes Ia, Ib, II, III, IV, V, VI, VII, VIII, and IX, and wherein the CP has a sialic acid level of greater than about 60%. The invention also relates to methods of making the conjugates and immunogenic compositions comprising the conjugates. The invention also relates to immunogenic compositions comprising polysaccharide-protein conjugates, wherein the conjugates comprise a CP from GBS serotype IV and at least one additional serotype. The invention further relates to methods for inducing an immune response in subjects against GBS and / or for reducing or preventing invasive GBS disease in subjects using the compositions disclosed herein. The resulting antibodies can be used to treat or prevent GBS infection via passive immunotherapy.
Owner:PFIZER INC
Completely humanized neutralizing antibody for anti-rabies viruses
ActiveCN103910796ALow priceQuickly builds immune protectionHybrid immunoglobulinsImmunoglobulins against virusesAntigenAntigen epitope
The invention discloses a completely humanized neutralizing antibody for anti-rabies viruses. The amino acid sequence of a heavy chain of the antibody is shown in sequence table SEQ ID NO1, the amino acid sequence of a light chain of the antibody is shown in sequence table SEQ ID NO5, the complementary determining region (CDR) of a variable region of the heavy chain of the antibody is shown as follows: CDR1: SEQIDNO2, CDR2:SEQIDNO3 and CDR3: SEQIDNO4, and the complementary determining region (CDR) of a variable region of the light chain of the antibody is shown as follows: CDR1:SEQIDNO6, CDR2:SEQIDNO7, and CDR3: SEQIDNO8. The beneficial effects of the completely humanized neutralizing antibody are that: the completely humanized neutralizing antibody disclosed by the invention ahs the advantages of being completely humanized, good in specificity, high in affinity, good in neutralizing effect, and low in price, can be used as a biological engineering antibody drug to quickly establish immune protection force against rabies virus, can be used for passive immunotherapy of acute infection, and also can be used for preparing detection reagents for detecting the rabies virus, finding effective neutralization antigen epitope and developing recombinant proteins and subunit vaccines for the rabies virus.
Owner:CHANGCHUN BCHT BIOTECH
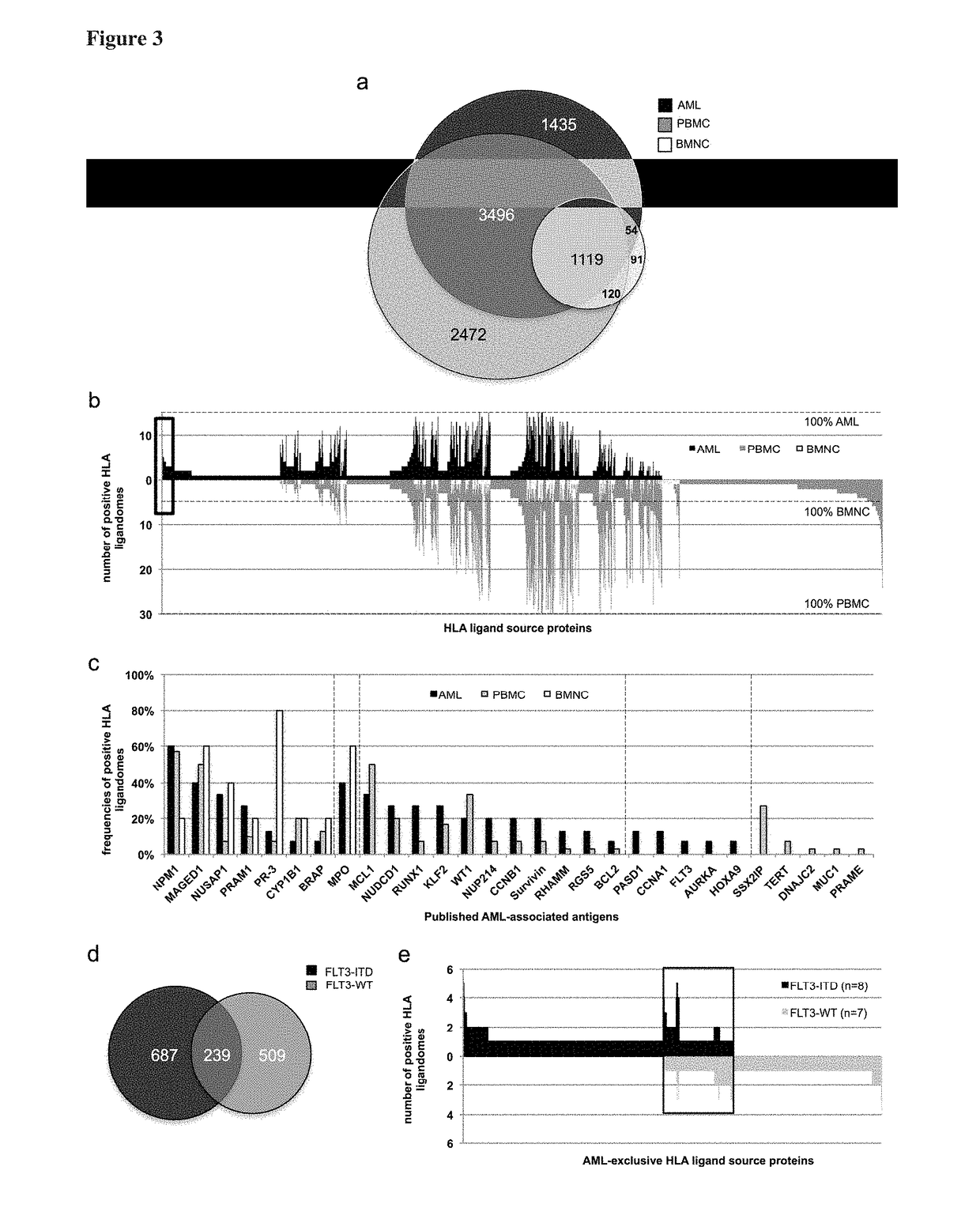
Novel immunotherapy against several tumors of the blood, such as acute myeloid leukemia (AML)
ActiveUS20180311330A1Enhance stability and solubilityFlexibility in detectionTumor rejection antigen precursorsHydrolasesHla class iiHuman tumor
The present invention relates to peptides, nucleic acids and cells for use in immunotherapeutic methods. In particular, the present invention relates to the immunotherapy of cancer. The present invention furthermore relates to tumor-associated cytotoxic T cell (CTL) peptide epitopes, alone or in combination with other tumor-associated peptides that serve as active pharmaceutical ingredients of vaccine compositions that stimulate anti-tumor immune responses. The present invention relates to several novel peptide sequences and their variants derived from HLA class I and HLA class II molecules of human tumor cells that can be used in vaccine compositions for eliciting anti-tumor immune responses.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
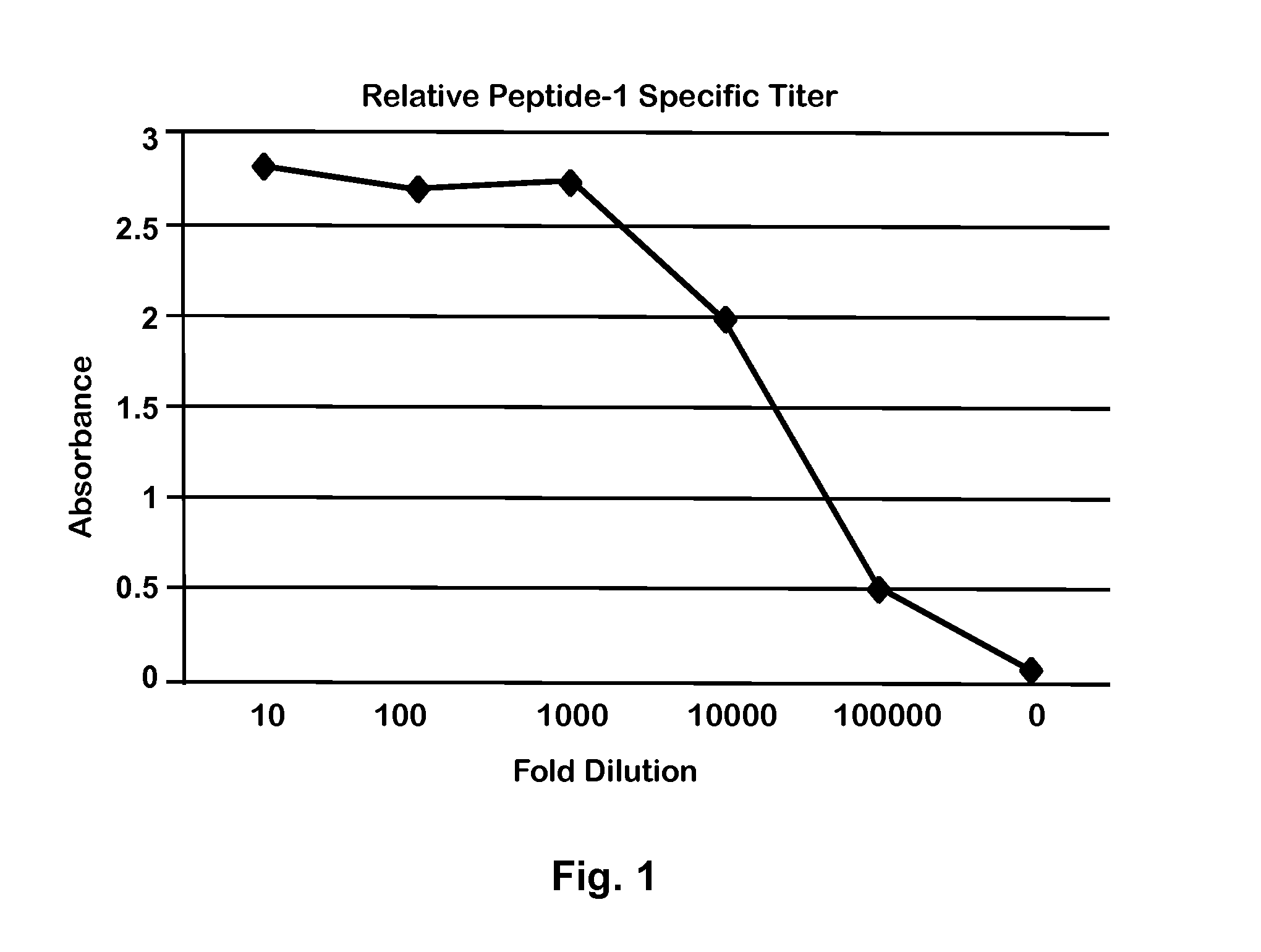
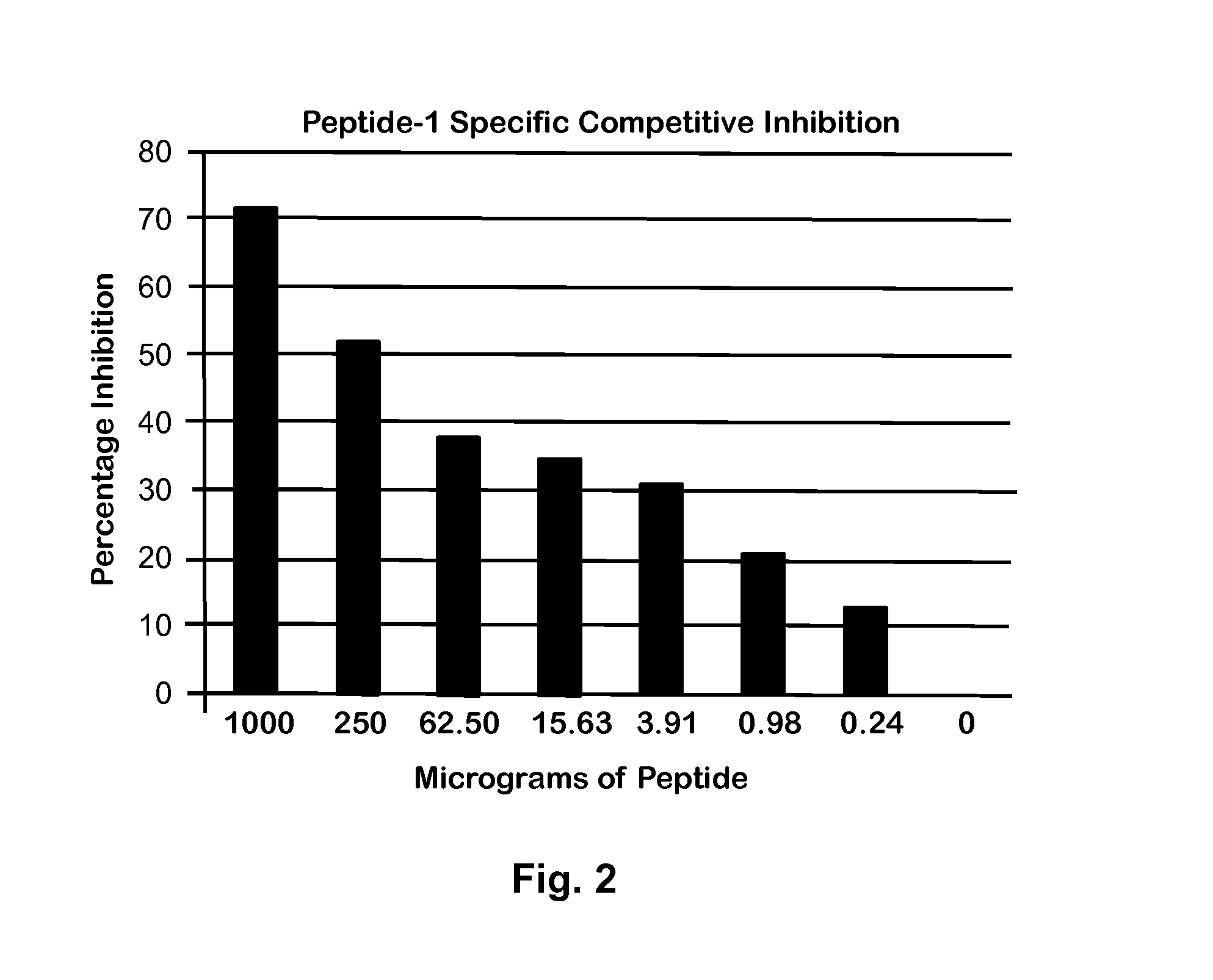
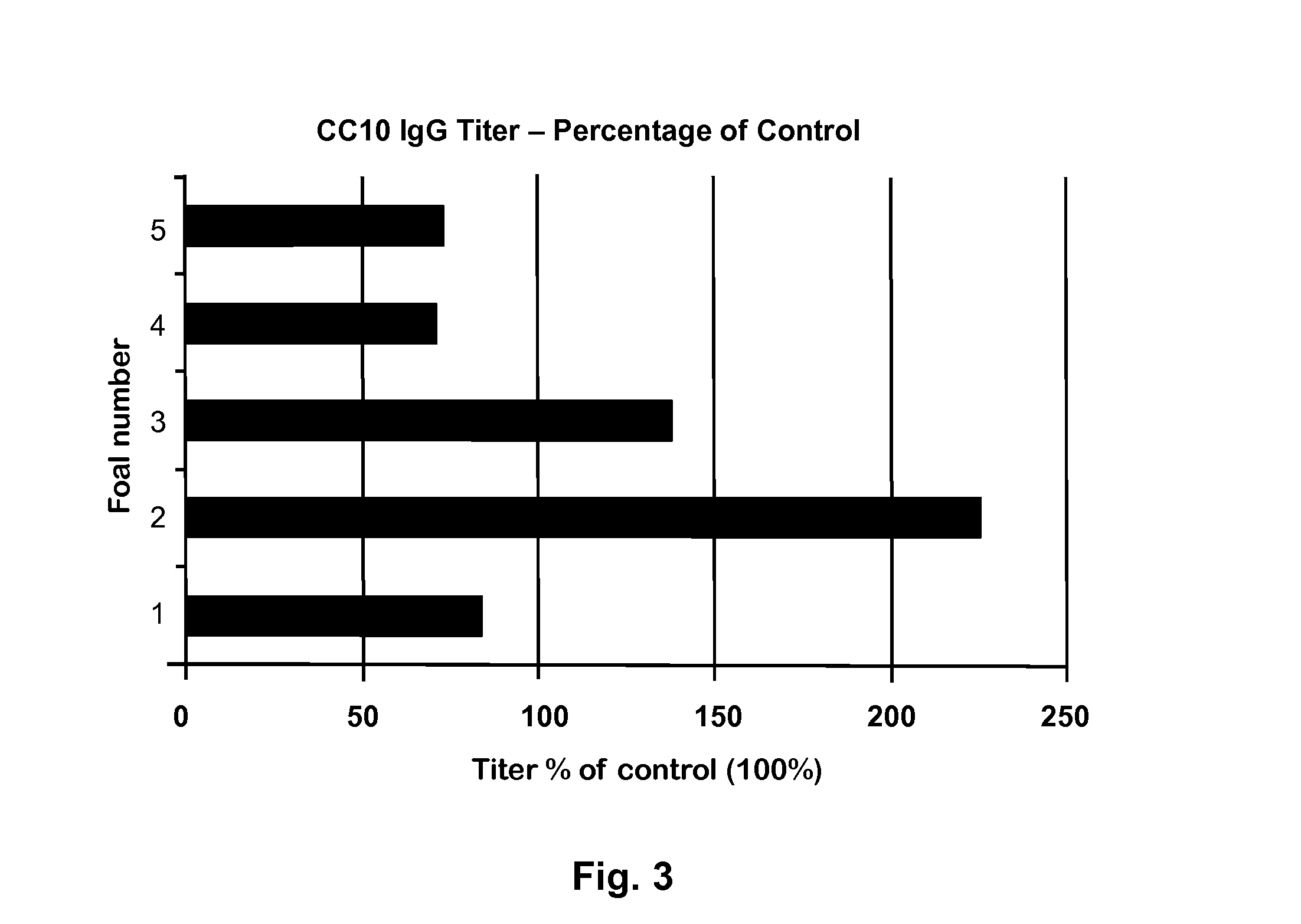
Peptide for Protection of Allergic Respiratory Disorders
ActiveUS20140105906A1Peptide/protein ingredientsVertebrate antigen ingredientsPassive ImmunotherapySerum ige
A peptide (Peptide-1) based on the C-terminal of Equine CC10 has been discovered that can be used as a vaccine to protect horses from respiratory airway obstruction (RAO). Antibodies to Peptide-1 may also be administered for short-term passive immunotherapy to RAO-affected horses, and can be used to measure the level of CC10 protein in serum to identify potential RAO horses (horses with reduced CC10). Due to similarities between equine RAO and human asthma, this peptide or its antibodies may also be useful in treatment or prevention of human asthma.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
Cytotoxic t-cell epitope peptide and use thereof
InactiveCN107709351AMicrobiological testing/measurementImmunoglobulins against animals/humansImmunotherapeutic agentEBV Infections
The present invention provides a cytotoxic T-cell (cytotoxic T lymphocyte, abbreviated hereinafter as CTL) epitope peptide specific to the Epstein-Barr virus (described hereinafter as EBV), a vaccinefor treating or preventing EBV infection or EBV-positive cancer using this peptide, a passive immunotherapeutic agent for EBV, and a method for assaying CTL specific to EBV. The present invention alsoprovides an HLA-A*24:02-restricted epitope peptide comprising an IYTEVRELV sequence (SEQ ID NO: 43) from a cytoskeleton-associated protein (cytoskeleton-associated protein 4: CKAP4 hereinafter, alsoknown as: CLIMP-63, ERGIC-63, P63). The peptide-specific cytotoxic T-cells (CTL hereinafter) can attack malignant tumor cells that express a high level of CKAP4.
Owner:MEDICAL & BIOLOGICAL LAB CO LTD
Mutated ras peptides for generation of cd8+ cytotoxic t lymphocytes
InactiveUS20100074945A1Enhanced effector functionEnhances CTL activityBiocidePeptide/protein ingredientsCtl epitopeVaccination
Mutant ras oncogene peptides may induce specific anti-ras cellular immune responses in vaccinated patients. Moreover, a human CD8+ CTL epitope(s) reflecting a specific point mutation in the K-ras oncogene at codon 12 was identified. The mutant ras peptide has implications for both active and passive immunotherapies in selected carcinoma patients. A nested 10-mer peptide was identified [i.e., ras5-14(Asp12)], which was shown to bind to HLA-A2 and display specific functional capacity for expansion of the in vivo-primed CD8+ CTL precursors.
Owner:UNITED STATES OF AMERICA
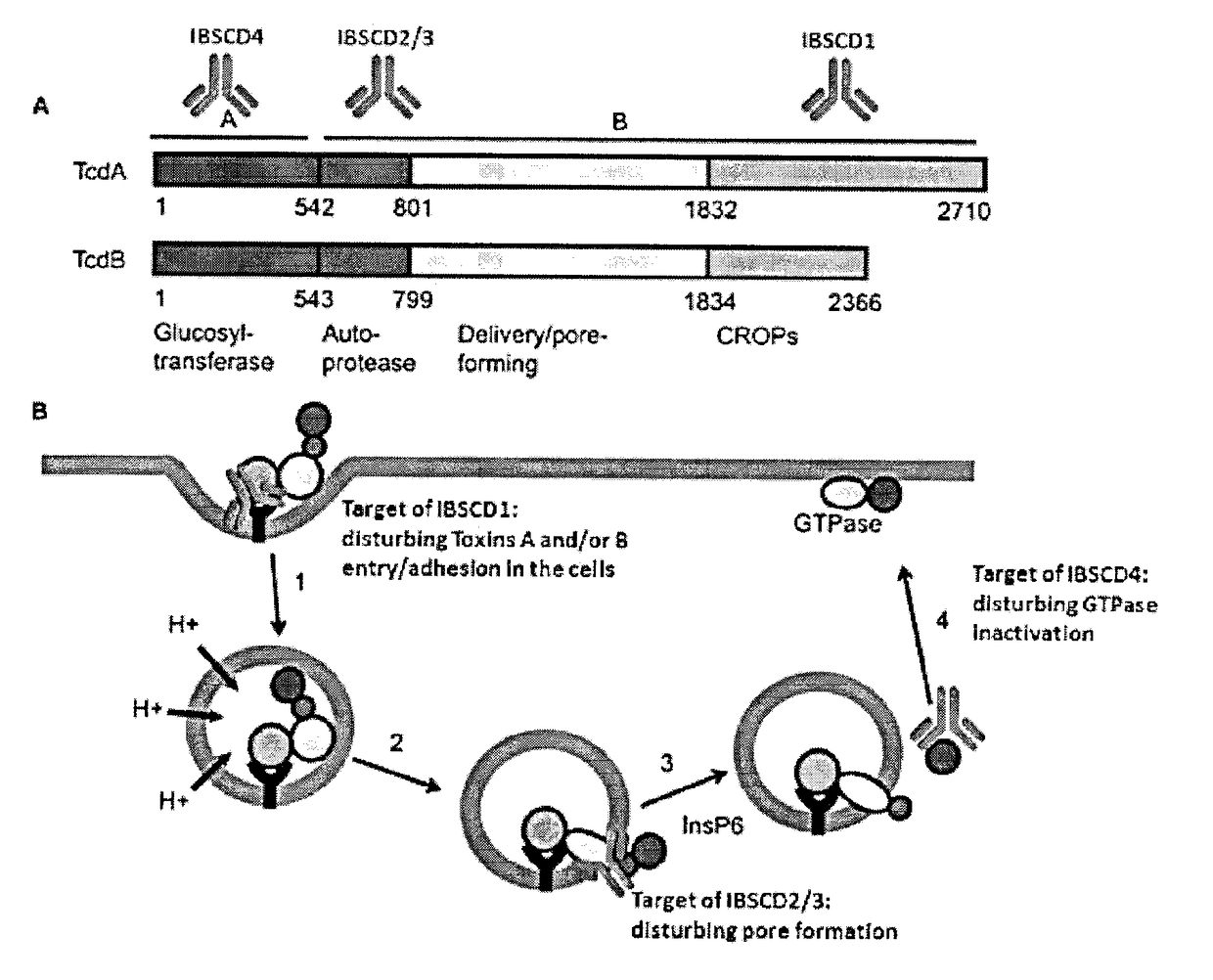
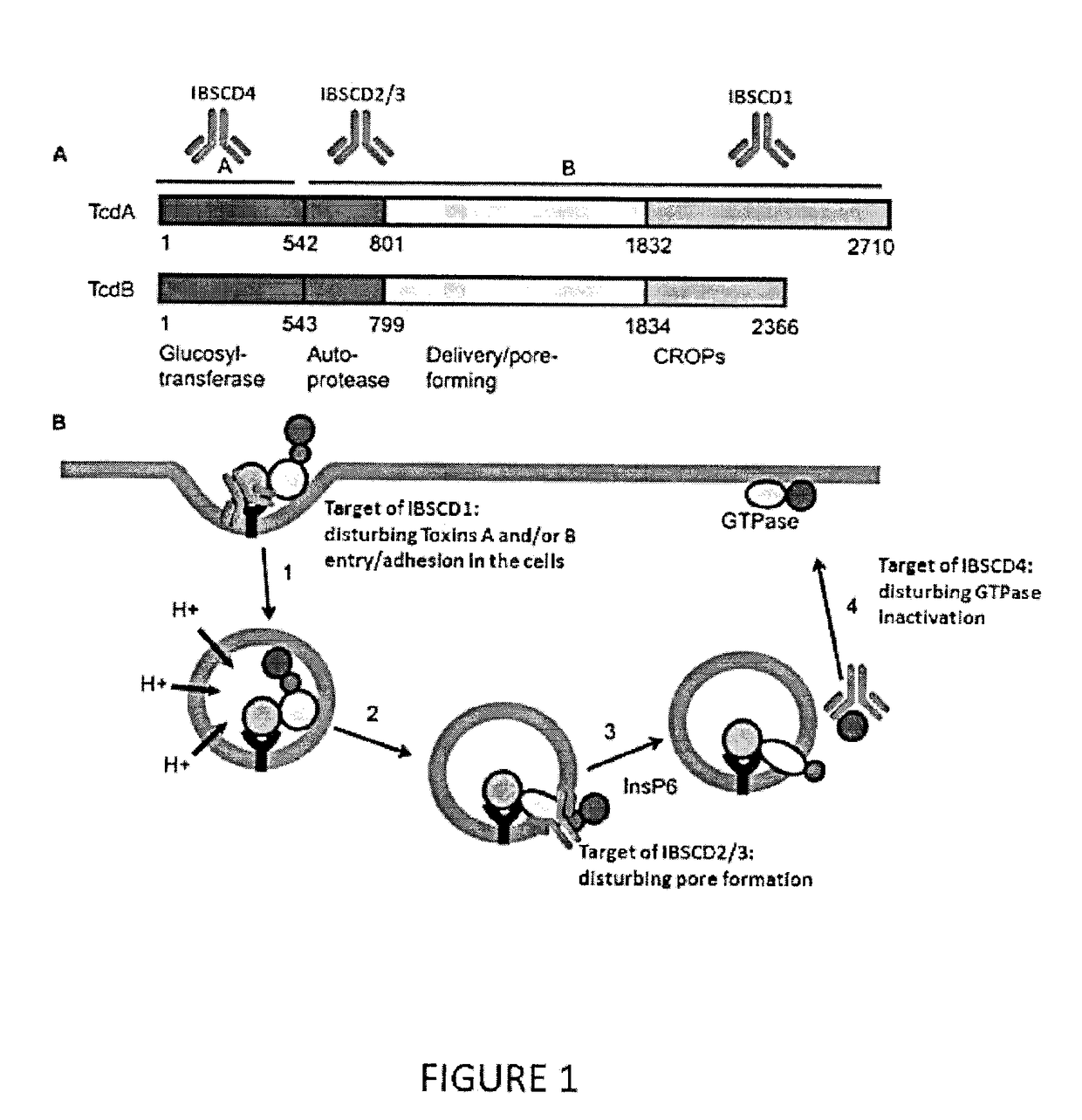
Clostridium Difficile Toxins a and/or B Antigen and Epitope Antibody, and Pharmaceutical Uses Thereof
ActiveUS20180022784A1Bacterial antigen ingredientsHybrid immunoglobulinsEpitopePassive Immunotherapy
It is described a Clostridium difficile (C-difficile) toxins A and / or B as a target for therapy, including passive immunotherapy, and particularly prevention of C-difficile intoxication in human or other animals. It is also described a polypeptide comprising a portion of C-difficile toxins A and / or B sequence being an epitope for anti-toxins A and / or B antibody. It disclosed a method for generating a neutralizing antibody directed against C-difficile toxins A and / or B. It is also provided a novel formulation that combines key toxins A and / or B epitope antibodies, located in three key domains of toxins A and / or B, for neutralisation of the toxins A and / or B, at any stage of toxins A and / or B intoxication related to C-difficile infection. The novel formulation of toxins A and / or B epitope antibodies are useful in immunotherapy, for 10 therapeutic and / or prophylactic mediation of C-difficile intoxication.
Owner:IMMUNE BIOSOLUTIONS INC
Antibody neutralizing human respiratory syncytial virus
ActiveUS20170121394A1High activityFunction increaseImmunoglobulins against virusesAntiviralsPassive ImmunotherapyHuman respiratory virus
The present invention relates to monoclonal antibodies which have high anti-RSV neutralizing titers. The invention further provides for isolated nucleic acids encoding the antibodies of the invention and host cells transformed therewith. The invention yet further provides for diagnostic, prophylactic and therapeutic methods employing the antibodies and nucleic acids of the invention, particularly as a passive immunotherapy agent in infants and the elderly.
Owner:MERCK SHARP & DOHME LLC
A fully human neutralizing antibody against rabies virus
ActiveCN103910796BLow priceQuickly builds immune protectionHybrid immunoglobulinsImmunoglobulins against virusesPassive ImmunizationsAntigen epitope
The invention discloses a completely humanized neutralizing antibody for anti-rabies viruses. The amino acid sequence of a heavy chain of the antibody is shown in sequence table SEQ ID NO1, the amino acid sequence of a light chain of the antibody is shown in sequence table SEQ ID NO5, the complementary determining region (CDR) of a variable region of the heavy chain of the antibody is shown as follows: CDR1: SEQIDNO2, CDR2:SEQIDNO3 and CDR3: SEQIDNO4, and the complementary determining region (CDR) of a variable region of the light chain of the antibody is shown as follows: CDR1:SEQIDNO6, CDR2:SEQIDNO7, and CDR3: SEQIDNO8. The beneficial effects of the completely humanized neutralizing antibody are that: the completely humanized neutralizing antibody disclosed by the invention ahs the advantages of being completely humanized, good in specificity, high in affinity, good in neutralizing effect, and low in price, can be used as a biological engineering antibody drug to quickly establish immune protection force against rabies virus, can be used for passive immunotherapy of acute infection, and also can be used for preparing detection reagents for detecting the rabies virus, finding effective neutralization antigen epitope and developing recombinant proteins and subunit vaccines for the rabies virus.
Owner:CHANGCHUN BCHT BIOTECH
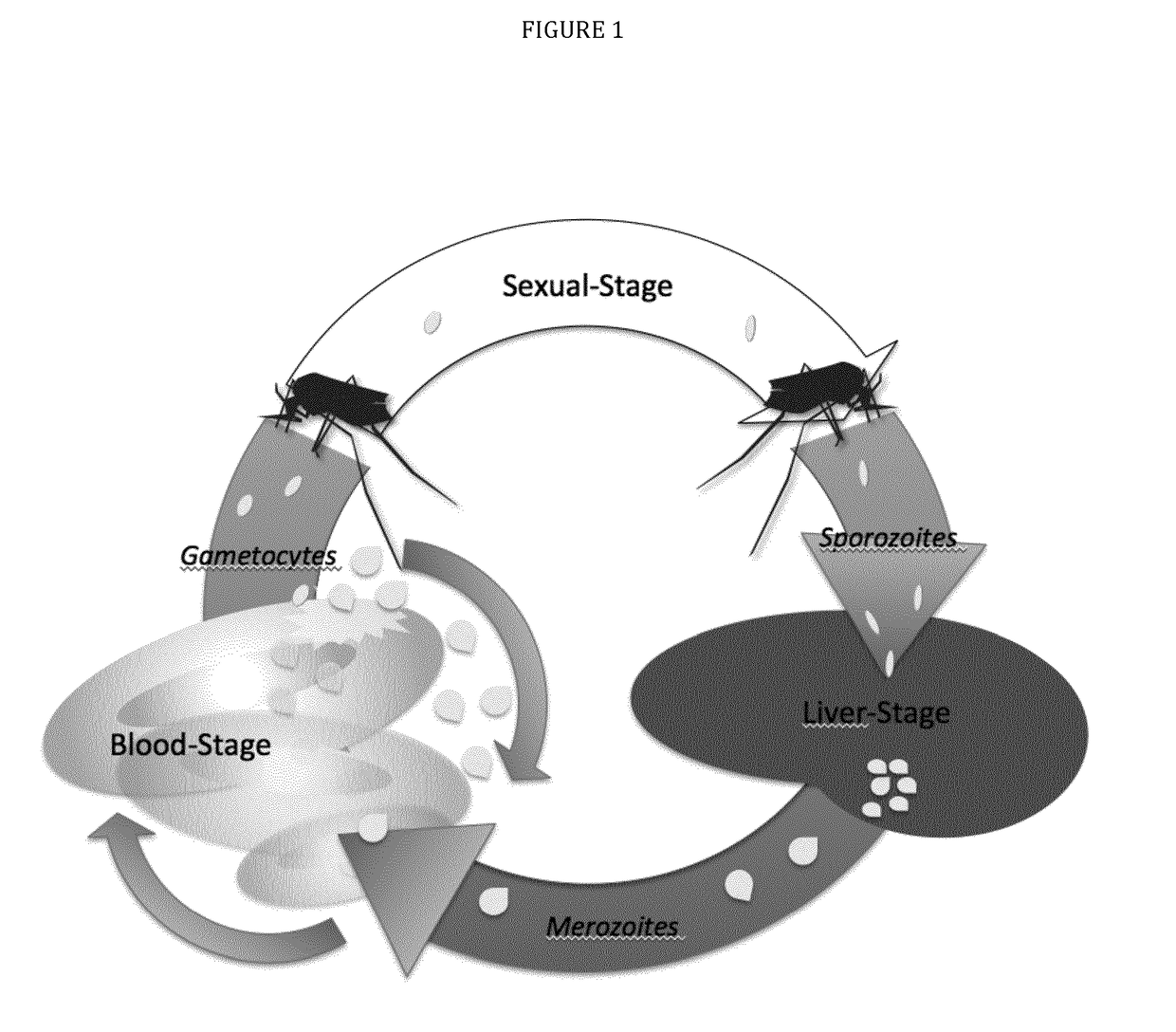
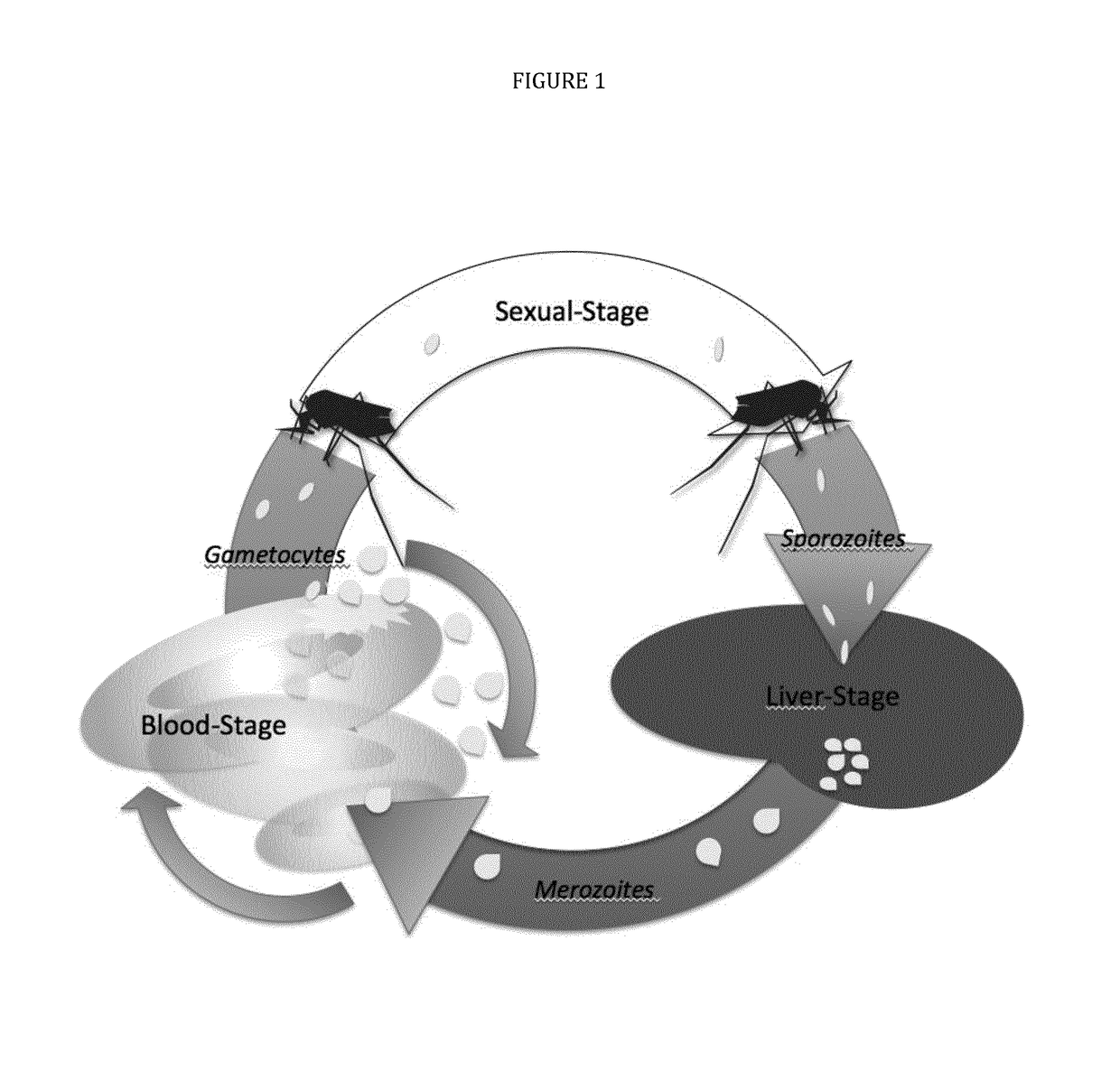
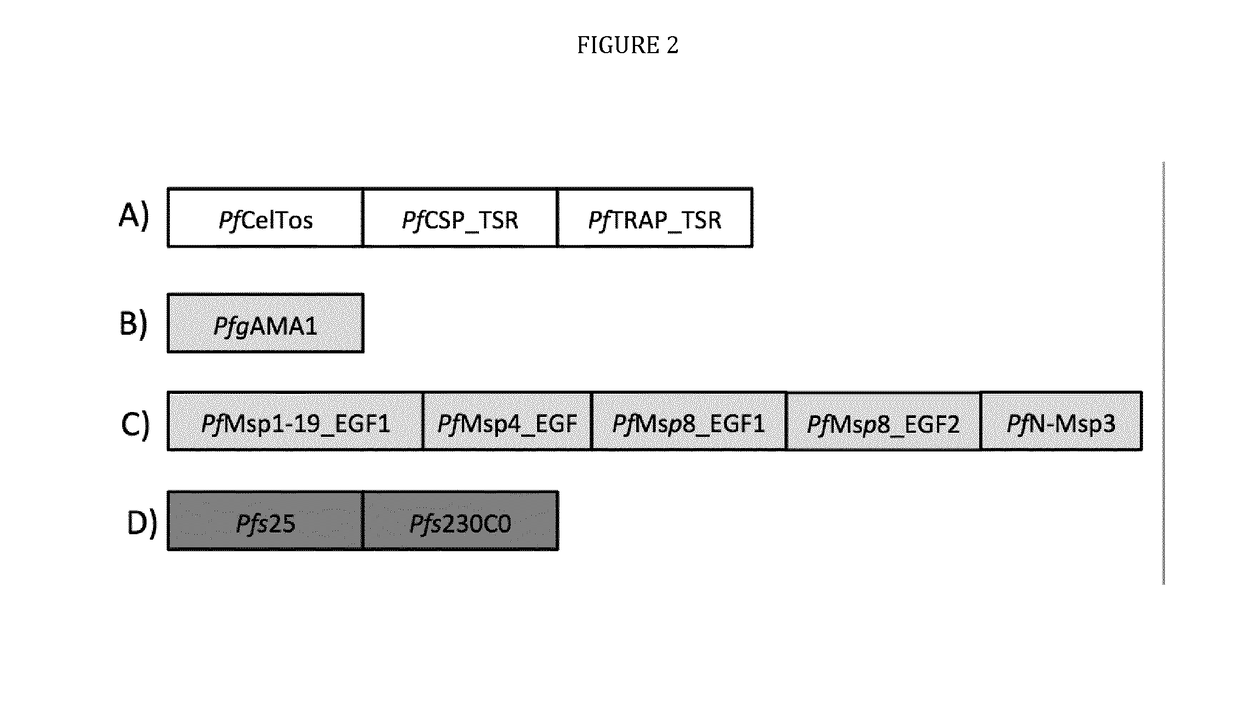
Vaccines against apicomplexan pathogens
InactiveUS10131697B2Protozoa antigen ingredientsAntibody mimetics/scaffoldsPassive ImmunotherapyAnimals vaccines
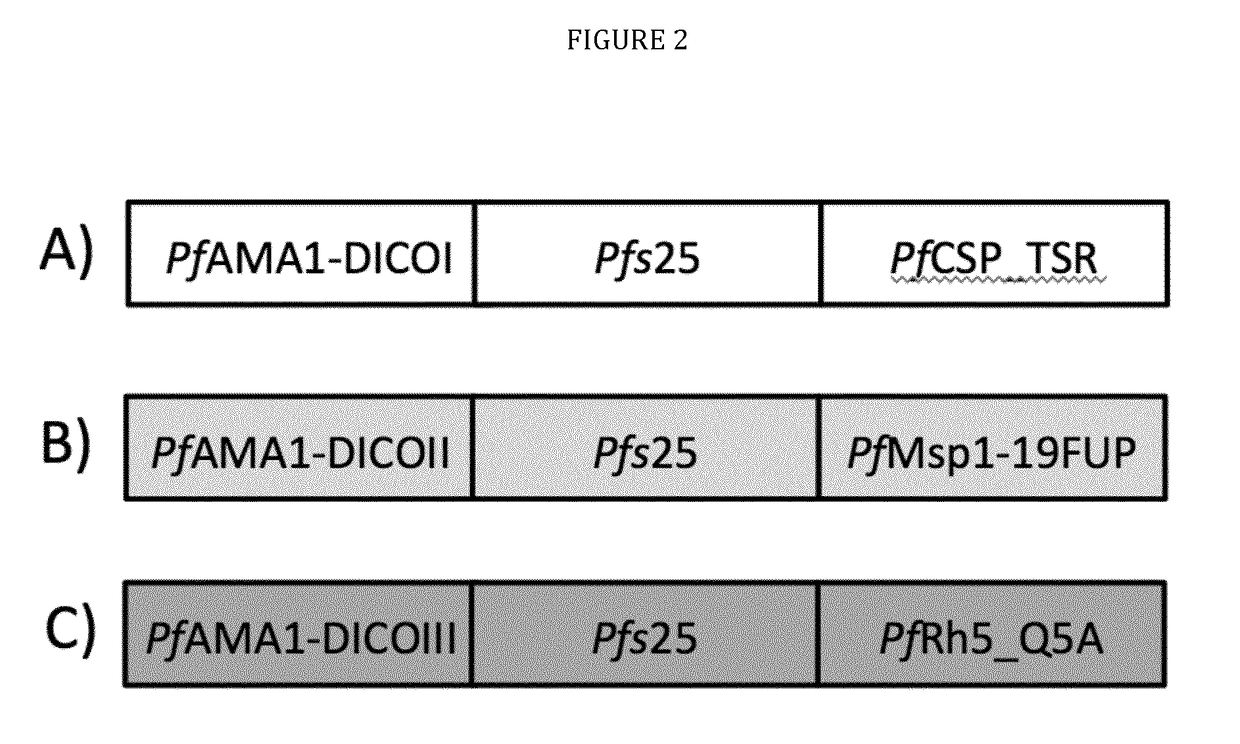
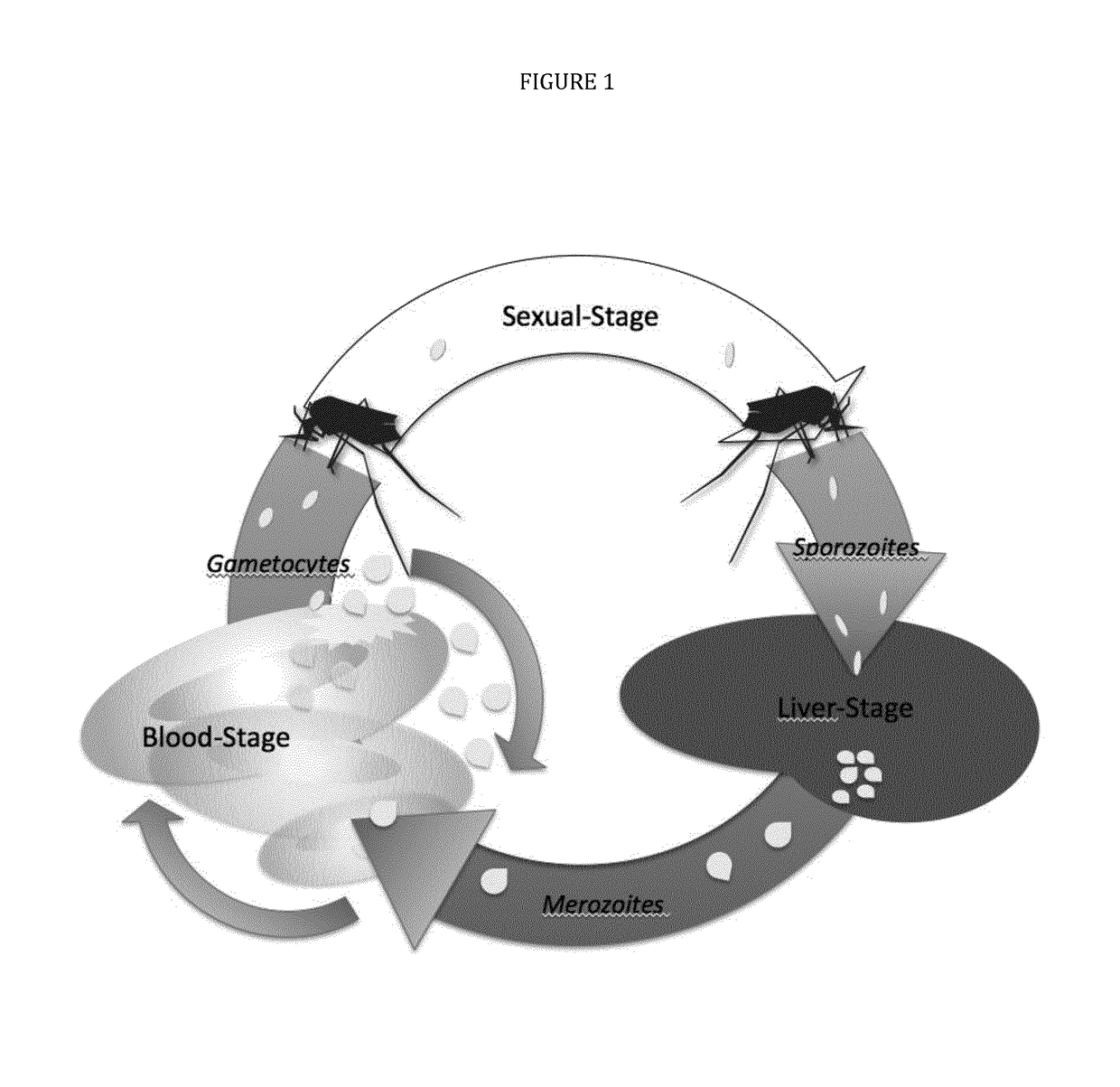
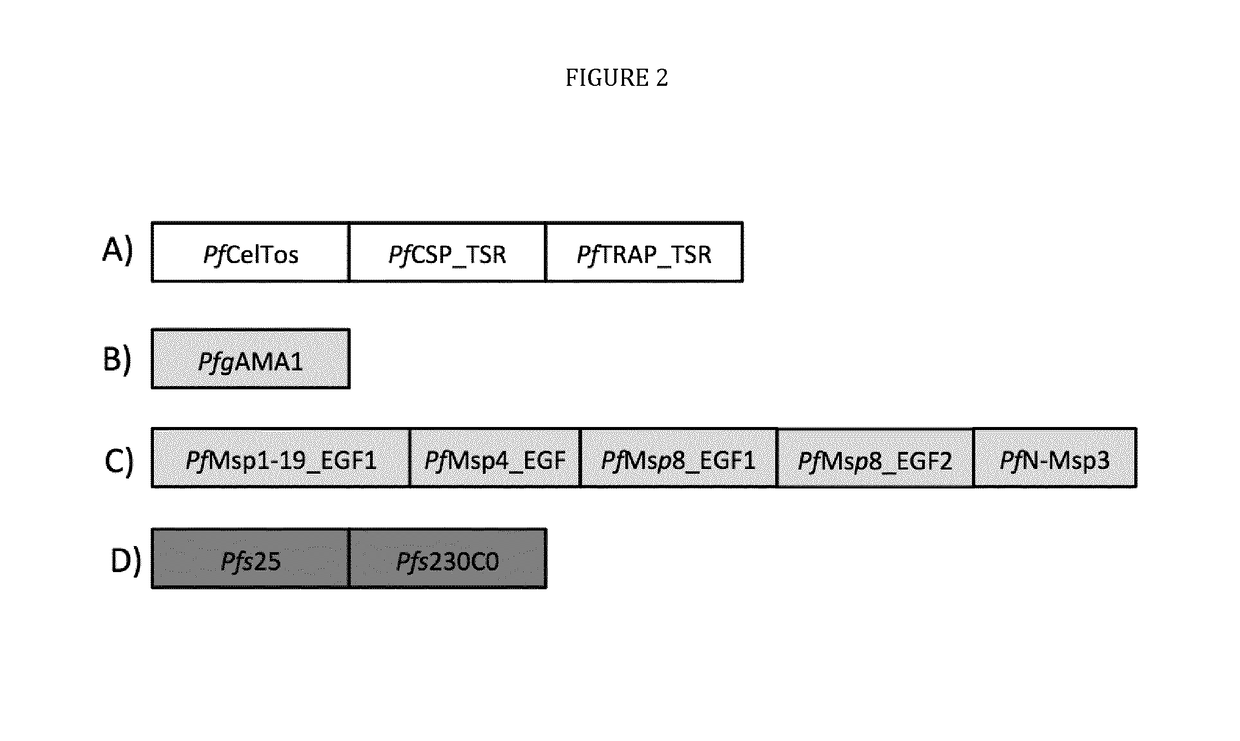
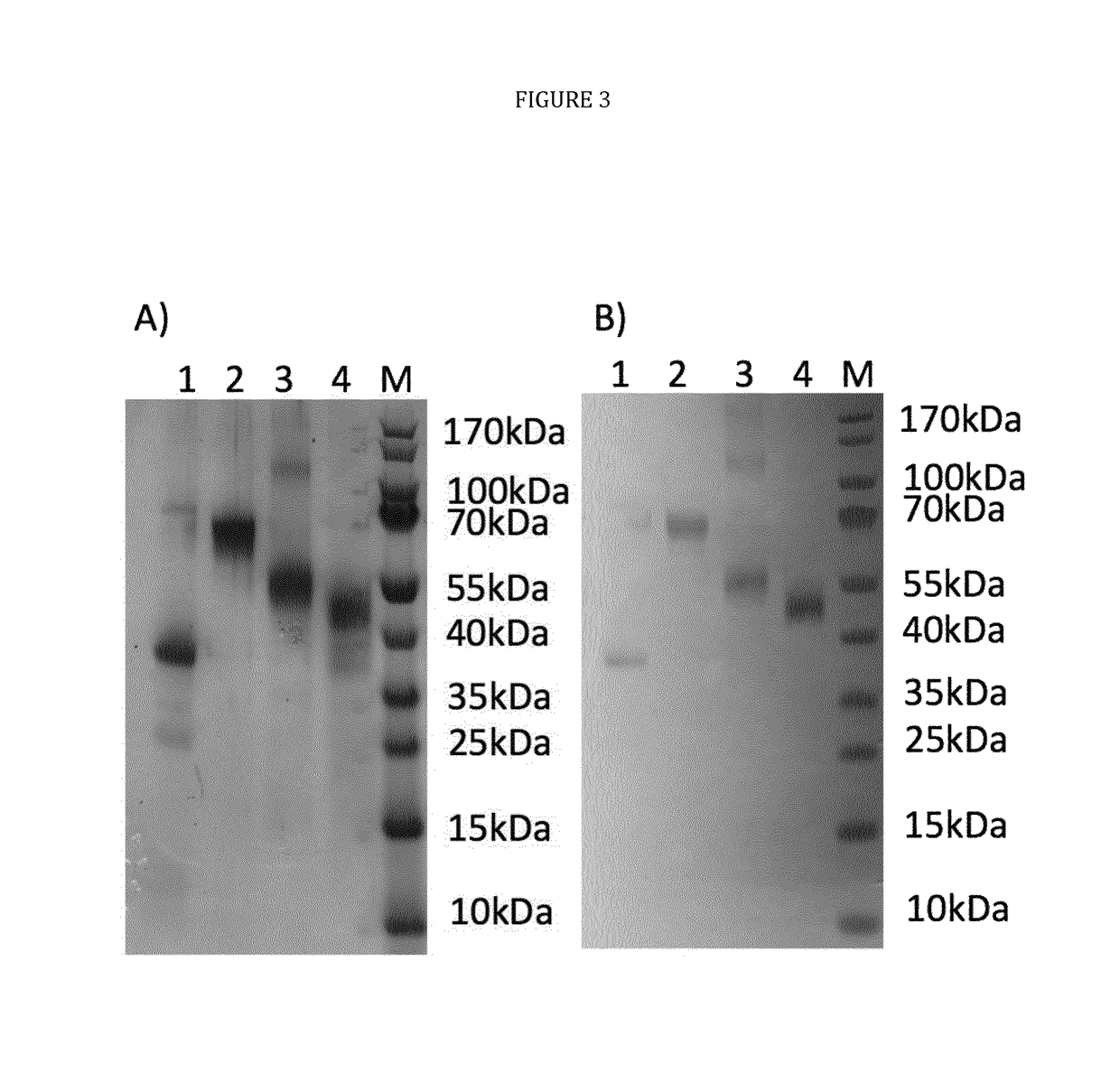
The technology provided herein generally relates to novel fusion proteins suitable as human and / or animal vaccines against parasites or pathogens of the phylum Apicomplexa. In particular, the present disclosure relates to novel fusion proteins as a basis for vaccines against Plasmodium parasites, including P. falciparum, P. vivax, P. malariae, P. ovale and P. knowlesi. Nucleic acid molecules encoding said fusion proteins, vectors, host cells containing the nucleic acids and methods for preparation and producing such fusion proteins; antibodies induced or generated by the use of said fusion proteins or said nucleic acid molecules encoding said fusion proteins and the use of such antibodies or recombinant derivatives for passive immunotherapy; methods for producing such fusion proteins; compositions and methods for using such fusion proteins for the prevention and treatment of malaria are also encompassed by the present disclosure.
Owner:FRAUNHOFER GESELLSCHAFT ZUR FOERDERUNG DER ANGEWANDTEN FORSCHUNG EV
Vaccine based on simulating human blood vessel endothelial cell growth factor VEGF epitope and preparation method thereof
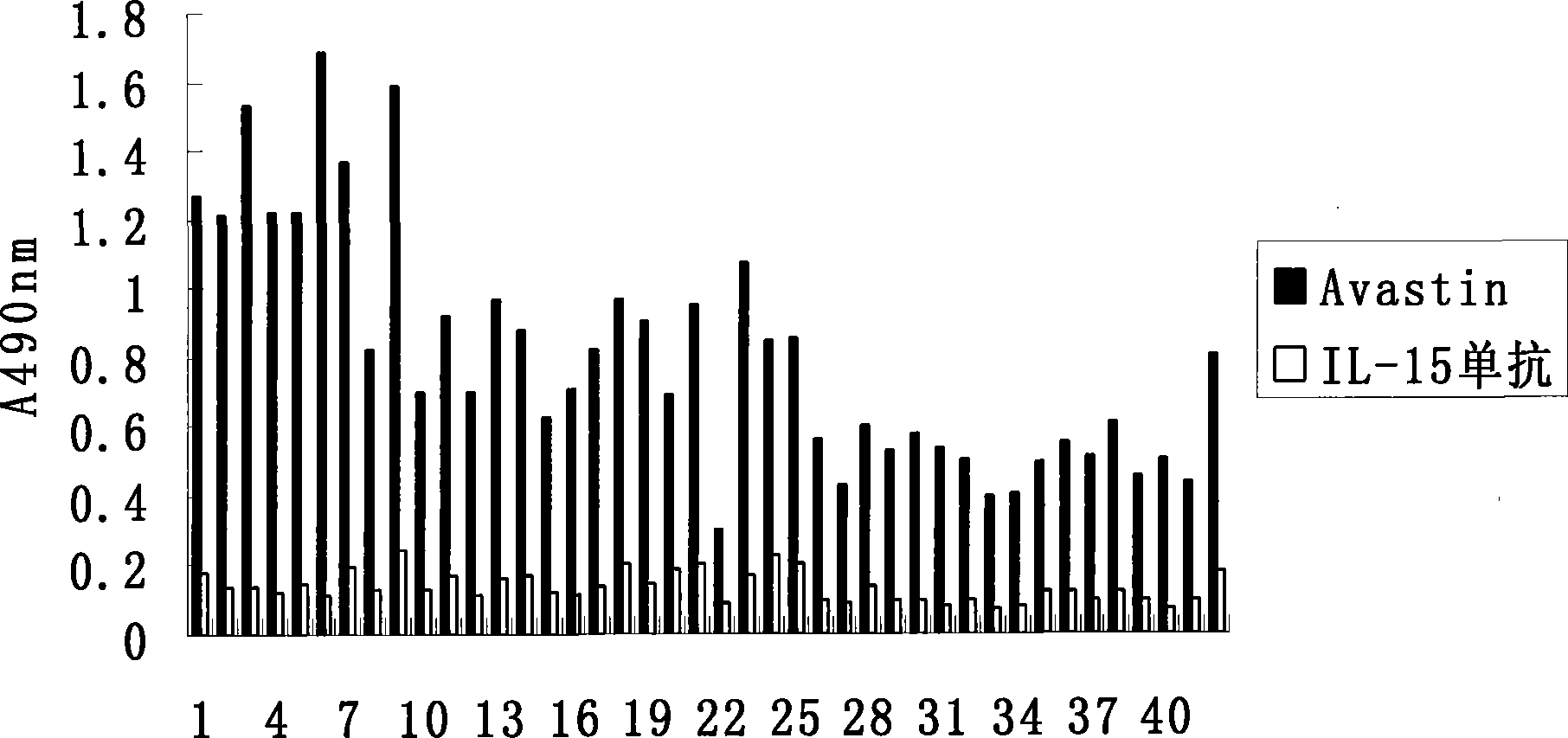
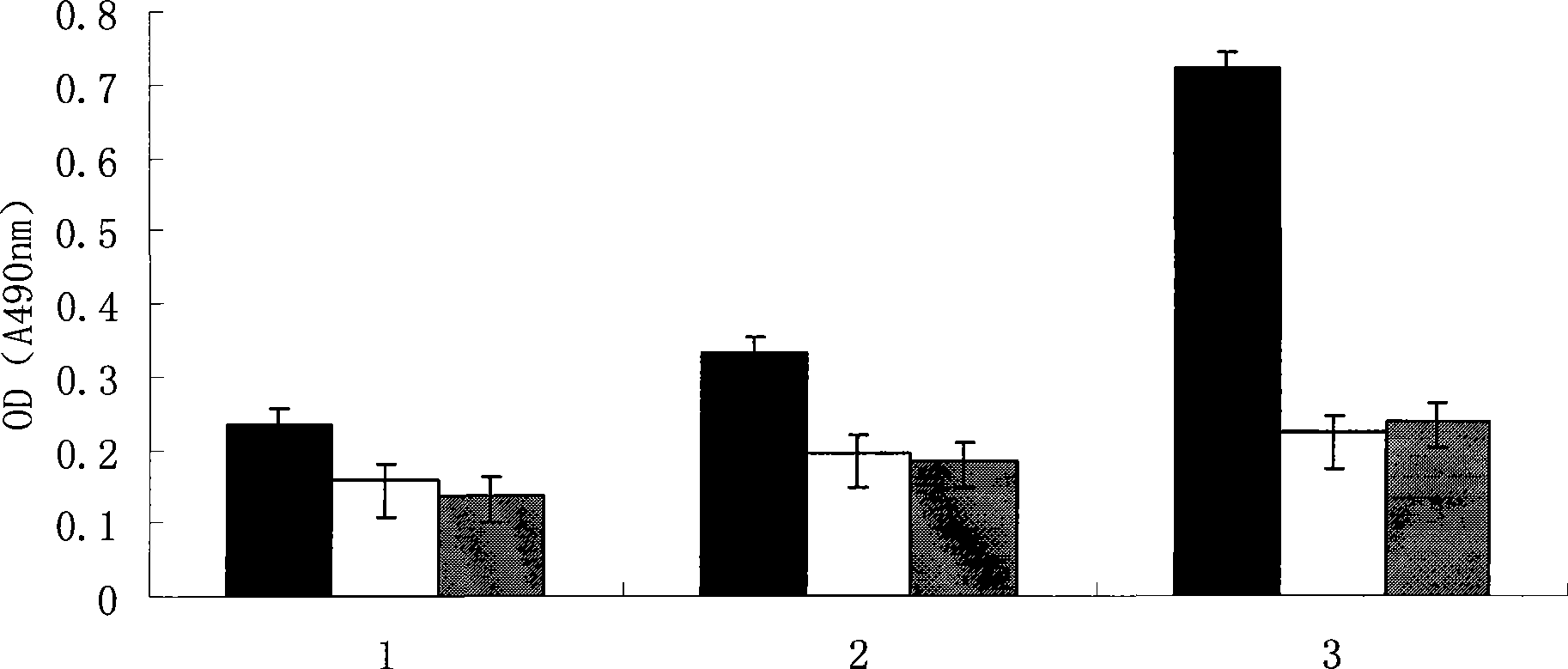
InactiveCN101429234APrevent proliferationInhibit migrationLibrary screeningPeptidesAntiangiogenesis TherapyVascular endothelium
The invention provides a vaccine based on an epitope simulating human vascular endothelial growth factor VEGF, as well as a preparation method thereof. A VEGF mimic epitope which is specifically affinitive with human-mouse chimeric monoclonal antibody Avastin is screened out by use of a phage random presentation technique, and the amino acid sequence of the mimic epitope is Asp-His-Thr-Leu-Tyr-Thr-Pro-Tyr-His-Thr-His-Pro; the mimic epitope has no homology with the protein sequence of VEGF. A vaccine which can induce a polypeptide epitope aiming at VEGF molecular autoantibody in vivo is constructed on the basis of the mimic epitope. The invention provides a strategy for developing and designing the tumor therapeutic vaccine, which is targeted at the VEGF. The VEGF is one of molecules which has the strongest effect of promoting vascular growth, and is an ideal target for resisting angiogenesis and treating tumors. Therefore, the vaccine replaces or replenishes monoclonal antibody passive immunotherapy with an active immunity mode, so as to lay foundations for overcoming the defects of monoclonal antibody therapy.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Three-component-multistage malaria vaccine
The technology provided herein relates to novel malaria vaccines composed of different recombinant proteins, in particular recombinant fusion proteins comprising several different Plasmodium falciparum antigens from the pre-erythrocytic, the blood, and the sexual parasite main stages. The proteins may be used in a mixture vaccine formulation to elicit protective immune responses in humans. Nucleic acid molecules encoding said recombinant proteins, vectors and host cells containing the nucleic acids and methods for preparation and producing such proteins are also disclosed, as well as antibodies induced or generated by the use of said malaria vaccines and the use of such antibodies or recombinant derivatives for passive immunotherapy.
Owner:FRAUNHOFER GESELLSCHAFT ZUR FOERDERUNG DER ANGEWANDTEN FORSCHUNG EV
Antibody neutralizing human respiratory syncytial virus
ActiveUS9963500B2High activityFunction increaseImmunoglobulins against virusesImmunoglobulins against cell receptors/antigens/surface-determinantsPassive ImmunotherapyHuman respiratory virus
The present invention relates to monoclonal antibodies which have high anti-RSV neutralizing titers. The invention further provides for isolated nucleic acids encoding the antibodies of the invention and host cells transformed therewith. The invention yet further provides for diagnostic, prophylactic and therapeutic methods employing the antibodies and nucleic acids of the invention, particularly as a passive immunotherapy agent in infants and the elderly.
Owner:MERCK SHARP & DOHME LLC
Prevention and Treatment of Recurrent Respiratory Papillomatosis
InactiveUS20080213293A1Peptide/protein ingredientsMetabolism disorderPassive ImmunotherapyVaccination
Juvenile-onset recurrent respiratory papillomatosis is treated using active vaccination or passive immune therapy of neutralizing antibodies against HPV L2 neutralizing epitopes.
Owner:UNIV OF LOUISVILLE RES FOUND INC
Preparation method and application of yolk antibody microcapsule preparation
InactiveCN102988329AOvercome outstanding problems such as variability, polymorphism, and susceptibility to drug resistanceOvercome polymorphism, easy to produce drug resistance and other prominent problemsAntiviralsAntibody ingredientsYolkHigh risk populations
The invention discloses a preparation method and an application of a yolk antibody microcapsule preparation. According to the invention, a vaccine prepared by using rotavirus is injected into a healthy laying female bird; after immunization, yolk is fetched, sterilized, separate-extracted, and purified, such that an anti-rotavirus IgY preparation is obtained; or IgY antibody preparations comprising various rotaviruses are mixed according to a certain ratio; an alginate solution with the mixed IgY antibody is dropped into a mixed solution of chitosan in a droplet manner, such that gel-state AP capsules are prepared, and the anti-rotavirus yolk antibody microcapsule preparation is obtained. The preparation is used in passive immunotherapy of rotavirus-infected patients, and is used in rotavirus prevention of high-risk populations.
Owner:TIANJIN JIMINGSHENG BIOLOGICAL TECH
Multi-component-multistage malaria vaccines
InactiveUS10183066B2Antibody mimetics/scaffoldsBiological material analysisPre erythrocyticADAMTS Proteins
The present disclosure relates to novel malaria vaccines composed of different recombinant proteins, in particular recombinant fusion proteins comprising several different Plasmodium falciparum antigens from the pre-erythrocytic the blood, and the sexual parasite stages. The proteins and / or fusion proteins will be used in a mixture vaccine formulation to elicit protective immune responses in humans. Nucleic acid molecules encoding said recombinant proteins, vectors, host cells containing the nucleic acids and methods for preparation and producing such proteins; Antibodies induced or generated by the use of said malaria vaccines or said nucleic acid molecules encoding said proteins and / or fusion proteins and the use of such antibodies or recombinant derivatives for passive immunotherapy.
Owner:FRAUNHOFER GESELLSCHAFT ZUR FOERDERUNG DER ANGEWANDTEN FORSCHUNG EV
Multi-component-multistage malaria vaccines
InactiveUS20170106071A1Antibody mimetics/scaffoldsBiological material analysisMalarial parasitePassive Immunizations
The present disclosure relates to novel malaria vaccines composed of different recombinant proteins, in particular recombinant fusion proteins comprising several different Plasmodium falciparum antigens from the pre-erythrocytic the blood, and the sexual parasite stages. The proteins and / or fusion proteins will be used in a mixture vaccine formulation to elicit protective immune responses in humans. Nucleic acid molecules encoding said recombinant proteins, vectors, host cells containing the nucleic acids and methods for preparation and producing such proteins; Antibodies induced or generated by the use of said malaria vaccines or said nucleic acid molecules encoding said proteins and / or fusion proteins and the use of such antibodies or recombinant derivatives for passive immunotherapy.
Owner:FRAUNHOFER GESELLSCHAFT ZUR FOERDERUNG DER ANGEWANDTEN FORSCHUNG EV
An IgY for a PA-MSHA bacterial strain, and preparation method and application thereof
ActiveCN102770452AGood adjuvant therapyHigh activityAntibacterial agentsEgg immunoglobulinsYolkBacterial strain
The invention relates to an IgY for a mannose-sensitive hemagglutination pilus strain of Pseudomonas aeruginosa (PA-MSHA) and a preparation method and application thereof, wherein the preparation method comprises the steps of: (S1) preparing an antigen of the PA-MSHA bacterial strain; (S2) immunizing egg-laying poultry by injecting said PA-MSHA antigen, and checking immunized eggs laid by the immunized poultry; and (S3) taking out yolk of the immunized eggs to extract the IgY for the PA-MSHA bacterial strain. According to the invention, PA-MSHA is used to immunize egg-laying poultry, and the IgY for the PA-MSHA bacterial strain is then extracted from the poultry eggs. The IgY for the PA-MSHA bacterial strain can be further made into health-care and biological products to prevent and cure broad-spectrum opportunistic infection floras such as escherichia coli, pseudomonas aeruginosa, pneumococcus, deformation coccus, and helicobacter pylori, and to play a role of auxiliary treatment effect in various cancers such as breast cancer, liver cancer, lymph cancer, lung cancer, bladder cancer and leukemia, especially suitable for active and passive immunotherapy for cancers.
Owner:SHENZHEN BROSTIGER BIO PHARMA
Novel immunotherapy against several tumors of the blood, such as acute myeloid leukemia (AML)
ActiveUS20190275130A1Strong upregulationPromotes formationTumor rejection antigen precursorsHydrolasesHla class iiHuman tumor
The present invention relates to peptides, nucleic acids and cells for use in immunotherapeutic methods. In particular, the present invention relates to the immunotherapy of cancer. The present invention furthermore relates to tumor-associated cytotoxic T cell (CTL) peptide epitopes, alone or in combination with other tumor-associated peptides that serve as active pharmaceutical ingredients of vaccine compositions that stimulate anti-tumor immune responses. The present invention relates to several novel peptide sequences and their variants derived from HLA class I and HLA class II molecules of human tumor cells that can be used in vaccine compositions for eliciting anti-tumor immune responses.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Human monoclonal antibodies
The present invention relates, in general, to HIV-1-reactive antibodies and, in at least certain specific embodiments, to broadly neutralizing antibodies (bnAbs) (and fragments and derivatives thereof) and to compositions comprising same. The invention further relates to methods of using such bnAbs (and fragments and derivatives thereof) and compositions in immunotherapy regimens (e.g., passive immunotherapy regimens). The antibodies (and fragments and derivatives thereof) disclosed herein can also be used in methods of identifying candidate immunogens for use in inducing an immune response against HIV-1 in a mammal (e.g., a human). The invention also relates to such methods and to immunogens so identified.
Owner:NAT INST FOR COMMUNICABLE DISEASES +1
Immunogenic compositions and uses thereof
ActiveUS10751402B2Antibacterial agentsBacterial antigen ingredientsPassive ImmunizationsPassive Immunotherapy
The invention relates to immunogenic compositions comprising a capsular polysaccharide (CP) from Streptococcus agalactiae, commonly referred to as group B streptococcus (GBS), conjugated to a carrier protein, and optionally including an aluminum-based adjuvant. The invention further relates to methods for inducing an immune response in subjects against GBS and / or for reducing or preventing invasive GBS disease in subjects using the compositions disclosed herein. The resulting antibodies can be used to treat or prevent GBS infection via passive immunotherapy.
Owner:PFIZER INC
Tuberculosis and medicament-resistant tubercular personalized yelk polyclone antibody and method of preparing the same and applications
InactiveCN101249264AAvoid infectionImprove accuracyAntibacterial agentsEgg immunoglobulinsHigh risk populationsYolk
The invention discloses a yolk polycolonal antibody formulation of personalized tuberculosis and drug-resistance tuberculosis, a preparation method, and application thereof. Pathogenic mycobacterium tuberculosis or dru-resistance tuberculosis is cultivated after being separated from the serum or sputum of tuberculosis patients, and then produced into personalized vaccine through the inactivating process of formaldehyde which can be used for the immune injection of healthy hen birds; specific polycolonal IgY antibody aimed at the patients and the pathogenic mycobacterium tuberculosis can be obtained from the yolk produced by immunized hen birds; after degermation, extraction, purification, liposome microencapsulation, etc., the specific polycolonal IgY antibody formulation can be obtained, which can be used for the passive immunotherapy of the patients with tuberculosis or drug-resistance tuberculosis and for preventing tuberculosis for high-risk population. The formulation is a safe, high-effect, and no-drug-resistance personalized biological formulation, which can also be applied to all the detecting methods on the basis of tuberculosis antigen-antibody immunoreaction, and can quickly and individually detect the infection of tuberculosis or drug-resistance mycobacterium tuberculosis.
Owner:沈星灿
Mutated ras peptides for generation of CD8+ cytotoxic T lymphocytes
InactiveUS8883448B2Growth inhibitionAvoid it happening againSugar derivativesOncogene translation productsCtl epitopePassive Immunizations
Mutant ras oncogene peptides may induce specific anti-ras cellular immune responses in vaccinated patients. Moreover, a human CD8+ CTL epitope(s) reflecting a specific point mutation in the K-ras oncogene at codon 12 was identified. The mutant ras peptide has implications for both active and passive immunotherapies in selected carcinoma patients. A nested 10-mer peptide was identified [i.e., ras5-14(Asp12)], which was shown to bind to HLA-A2 and display specific functional capacity for expansion of the in vivo-primed CD8+ CTL precursors.
Owner:UNITED STATES OF AMERICA
Human monoclonal antibodies endowed with strong neutralizing activity against hsv-1 and hsv-2
ActiveUS20180009879A1High HSV neutralizing activityReduce formationNervous disorderDigestive systemPassive ImmunotherapyHerpes simplex virus infection
The present invention is in the field of monoclonal antibodies suitable for passive immunotherapy of Herpes Simplex Virus infections and relates to human monoclonal antibodies or fragments of said antibodies, which bind and neutralize HSV-1 and HSV-2, and their use in the prophylaxis or treatment of HSV-1 or HSV-2-associated diseases.
Owner:POLICHEM SA
Lupus antibodies for passive immunotherapy of hiv/aids
Disclosed are monoclonal antibodies and antibody fragments which recognize antigens encoded by HERV DNA sequences, and methods for production, including recombinant antibody fragments derived from lymphoid cells of lupus patients that make antibodies which neutralize HIV.
Owner:PAUL SUDHIR PH D
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com