Patents
Literature
175 results about "Passive Immunizations" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
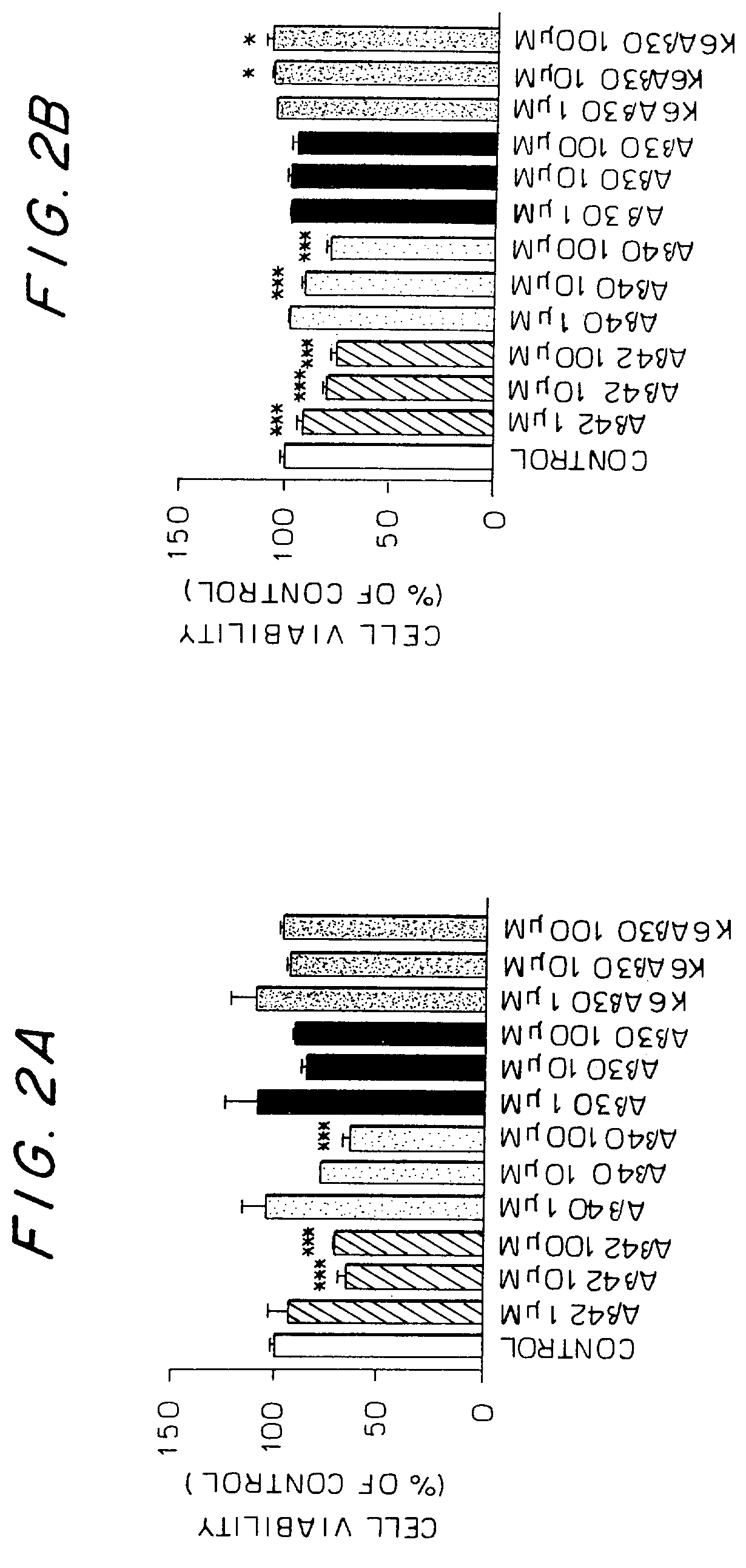
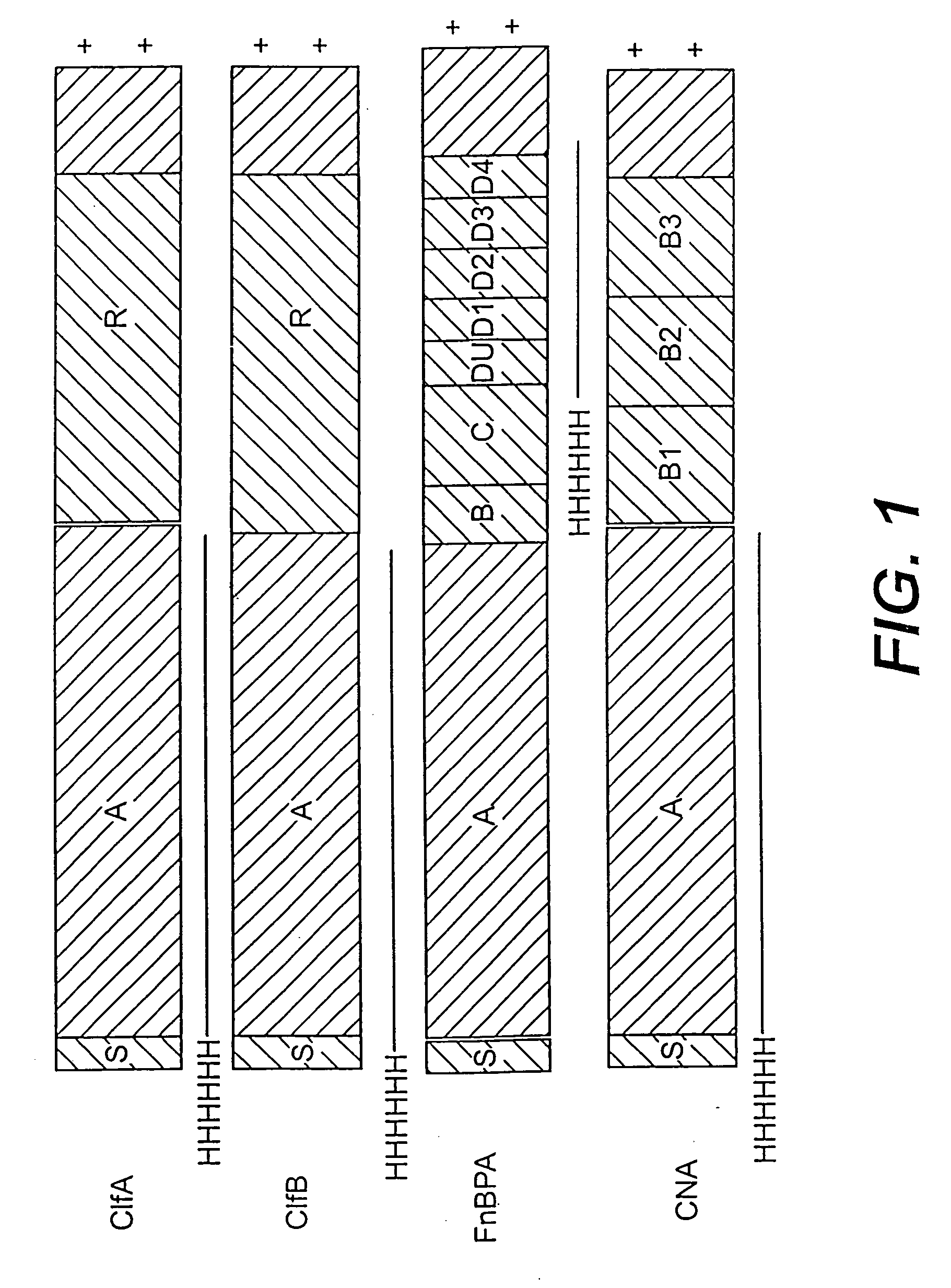
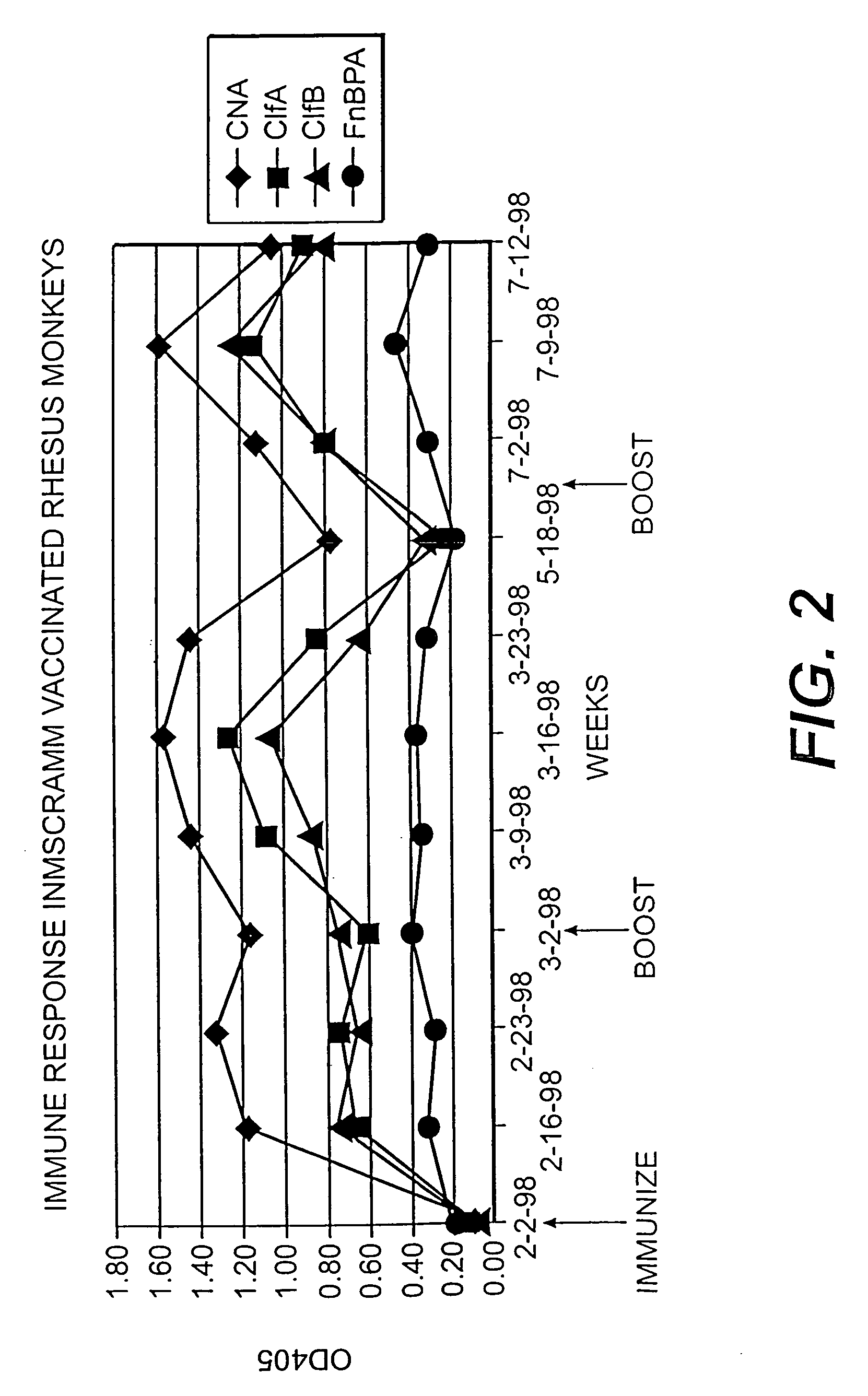
Collagen binding protein compositions and methods of use
InactiveUS6288214B1Prevent and lessen adhesionReduce adhesionAntibacterial agentsPeptide/protein ingredientsPassive ImmunizationsCarrier protein
Disclosed are the cna gene and cna-derived nucleic acid segments from Staphylococcus aureus, and DNA segments encoding cna from related bacteria. Also disclosed are Col binding protein (CBP) compositions and methods of use. The CBP protein and antigenic epitopes derived therefrom are contemplated for use in the treatment of pathological infections, and in particular, for use in the prevention of bacterial adhesion to Col. DNA segments encoding these proteins and anti-(Col binding protein) antibodies will also be of use in various screening, diagnostic and therapeutic applications including active and passive immunization and methods for the prevention of bacterial colonization in an animal such as a human. These DNA segments and the peptides derived therefrom are contemplated for use in the preparation of vaccines and, also, for use as carrier proteins in vaccine formulations, and in the formulation of compositions for use in the prevention of S. aureus infection.
Owner:TEXAS A&M UNIVERSITY
Passive immunization of Alzheimer's disease
InactiveUS6913745B1Peptide/protein ingredientsAntibody mimetics/scaffoldsPassive ImmunizationsAmyloid disease
Disclosed are pharmaceutical compositions and methods for preventing or treating a number of amyloid diseases, including Alzheimer's disease, prion diseases, familial amyloid neuropathies and the like. The pharmaceutical compositions include immunologically reactive amounts of amyloid fibril components, particularly fibril-forming peptides or proteins. Also disclosed are therapeutic compositions and methods which use immune reagents that react with such fibril components.
Owner:JANSSEN SCI IRELAND UC
Active immunization against clostridium difficile disease
InactiveUS6969520B2Prevent relapseImmune responseAntibacterial agentsBacterial antigen ingredientsDiseasePassive Immunizations
The invention provides active and passive immunization methods for preventing and treating Clostridium difficile infection, which involve percutaneous administration of C. difficile toxin-neutralizing polyclonal immune globulin, C. difficile toxoids, or combinations thereof. Also provided by the invention are C. difficile toxoids, C. difficile toxin-neutralizing polyclonal immune globulin, and methods of identifying subjects that produce C. difficile toxin-neutralizing polyclonal immune globulin.
Owner:SANOFI PASTEUR BIOLOGICS CO
Immunogenic compositions to the CCK-B/gastrin receptor and methods for the treatment of tumors
InactiveUS6548066B1Inhibiting autocrine growth-stimulatory pathwayEffectively prevent the binding of the peptide hormones to the receptorsPeptide/protein ingredientsReceptors for hormonesTissue biopsyPassive Immunizations
The invention concerns immunogens, immunogenic compositions and method for the treatment of gastrin-dependent tumors. The immunogens comprise a peptide from the CCK-B / gastrin-receptor conjugated to a spacer and to an immunogenic carrier. The immunogens are capable of inducing antibodies in vivo which bind to the CCK-B / gastrin-receptor in tumor cells, thereby preventing growth stimulating peptide hormones from binding to the receptors, and inhibiting tumor cell growth. The immunogens also comprise antibodies against the CCK-B / gastrin-receptor for passive immunization. The invention also concerns diagnostic methods for detecting gastrin-dependent tumors in vivo or from a tissue biopsy using the antibodies of the invention.
Owner:CANCER ADVANCES INC
Antibodies and related molecules that bind to 161P2F10B proteins
Antibodies and molecules derived therefrom that bind to 161P2F10B protein and variants thereof, are described wherein 161P2F10B exhibits tissue specific expression in normal adult tissue, and is aberrantly expressed in the cancers listed in Table I. Consequently, 161P2F10B provides a diagnostic, prognostic, prophylactic and / or therapeutic target for cancer. The 161P2F10B gene or fragment thereof, or its encoded protein, or variants thereof, or a fragment thereof, can be used to elicit a humoral or cellular immune response; antibodies or T cells reactive with 161P2F10B can be used in active or passive immunization.
Owner:AGENSYS
Fibronectin binding protein compositions and methods of use
InactiveUS6685943B1Avoid problemsOvercomes drawbackPeptide/protein ingredientsAntibody mimetics/scaffoldsPassive ImmunizationsStreptococcus pyogenes
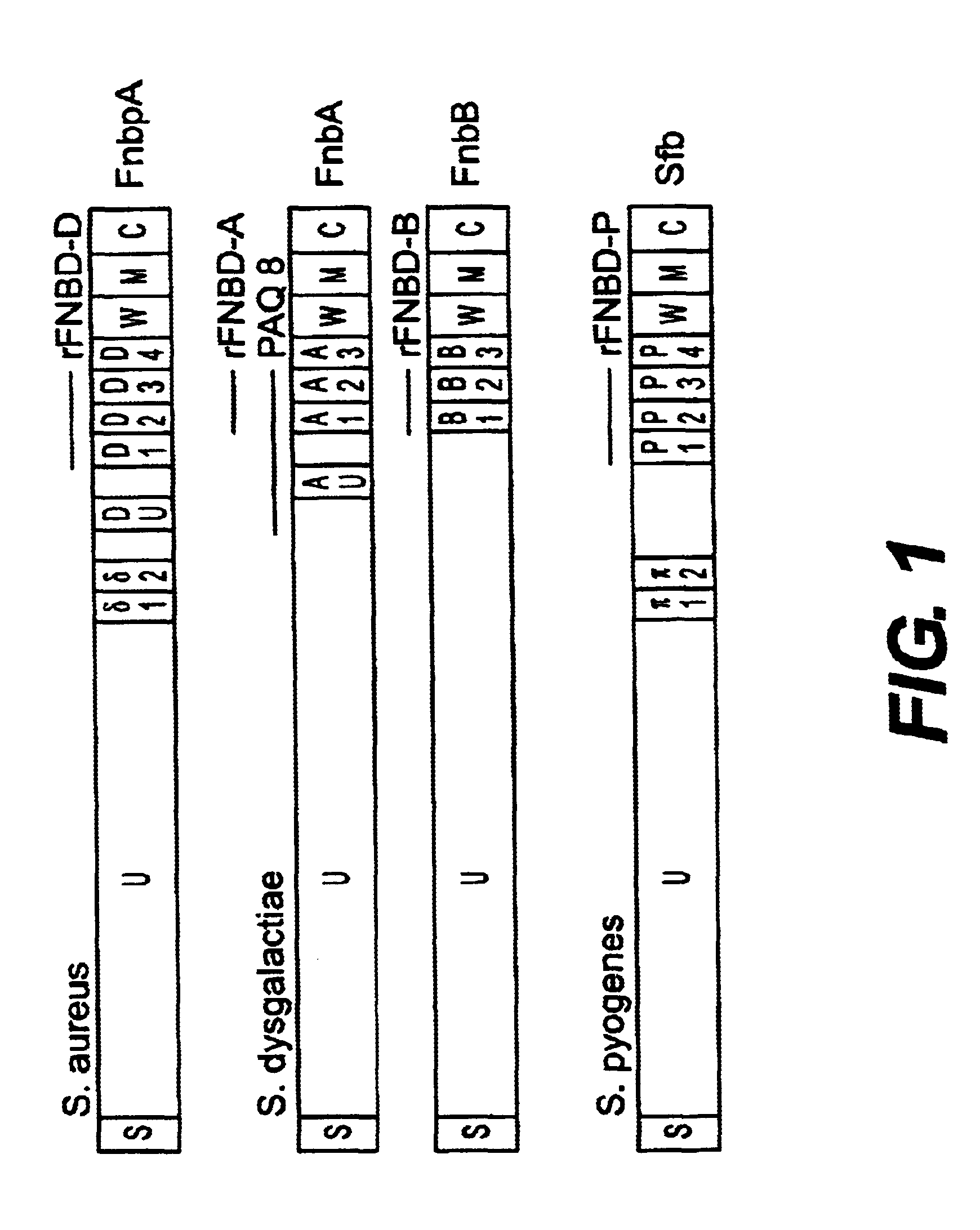
Disclosed are antibodies that block the binding of fibronectin protein to fibronectin. Also disclosed are site specifically-mutated and truncated peptide epitopes derived from the fnbA and fnbB genes of Staphylococcus aureus, the fnba and fnbB genes of Streptococcus dysgalactiae, and the sfb gene of Streptococcus pyogenes, and nucleic acid segments encoding these peptides and epitopes. The anti-(fibronectin binding site) antibodies, peptides and epitopes that give rise to antibodies that block the binding of fibronectin binding proteins to fibronectin, and DNA segments encoding these proteins and are of use in various screening, diagnostic and therapeutic applications including active and passive immunization and methods for the prevention of streptococcal and staphylococcal colonization in animals or humans. These. DNA segments and the peptides derived therefrom are proposed to be of use directly in the preparation of vaccines and also for use as carrier proteins in vaccine formulations.
Owner:UNIVERSITY OF MANITOBA +2
Mutant proteins, high potency inhibitory antibodies and fimch crystal structure
InactiveUS20030199071A1Great functional inhibitory activityStrong inhibitory activityHydrolasesImmunoglobulins against bacteriaPassive ImmunizationsMutated protein
The present invention provides bacterial immunogenic agents for administration to humans and non-human animals to stimulate an immune response. It particularly relates to the vaccination of mammalian species, especially human patients, with variants of the E. coli FimCH protein that elicit antibodies that have better functional inhibitory activity than antibodies raised against wild type protein. In particular, such variants include mutations that promote a more open confirmation of the FimH protein, particularly in regions involved in mannose binding, to expose regions previously poorly exposed and mutations that abolish a significantly reduce mannose binding. In another aspect, the invention provides antibodies against such proteins and protein complexes that may be used in passive immunization to protect or treat pathogenic bacterial infections. The present invention also provides machine readable media embedded with the three-dimensional atomic structure coordinates of FimCH bound to mannose, and subsets thereof, and methods of using the crystal structure to provide candidate amino acid residues for mutation.
Owner:WASHINGTON UNIV IN SAINT LOUIS +1
Immunization against clostridium difficile disease
InactiveUS20060029608A1Prevent relapseQuick treatmentAntibacterial agentsBacterial antigen ingredientsPassive ImmunizationsClostridium difficile infections
The invention provides active and passive immunization methods for preventing and treating Clostridium difficile infection, which involve percutaneous administration of C. difficile toxin-neutralizing polyclonal immune globulin, C. difficile toxoids, or combinations thereof. Also provided by the invention are C. difficile toxoids, C. difficile toxin-neutralizing polyclonal immune globulin, and methods of identifying subjects that produce C. difficile toxin-neutralizing polyclonal immune globulin.
Owner:ACAMBIS INC
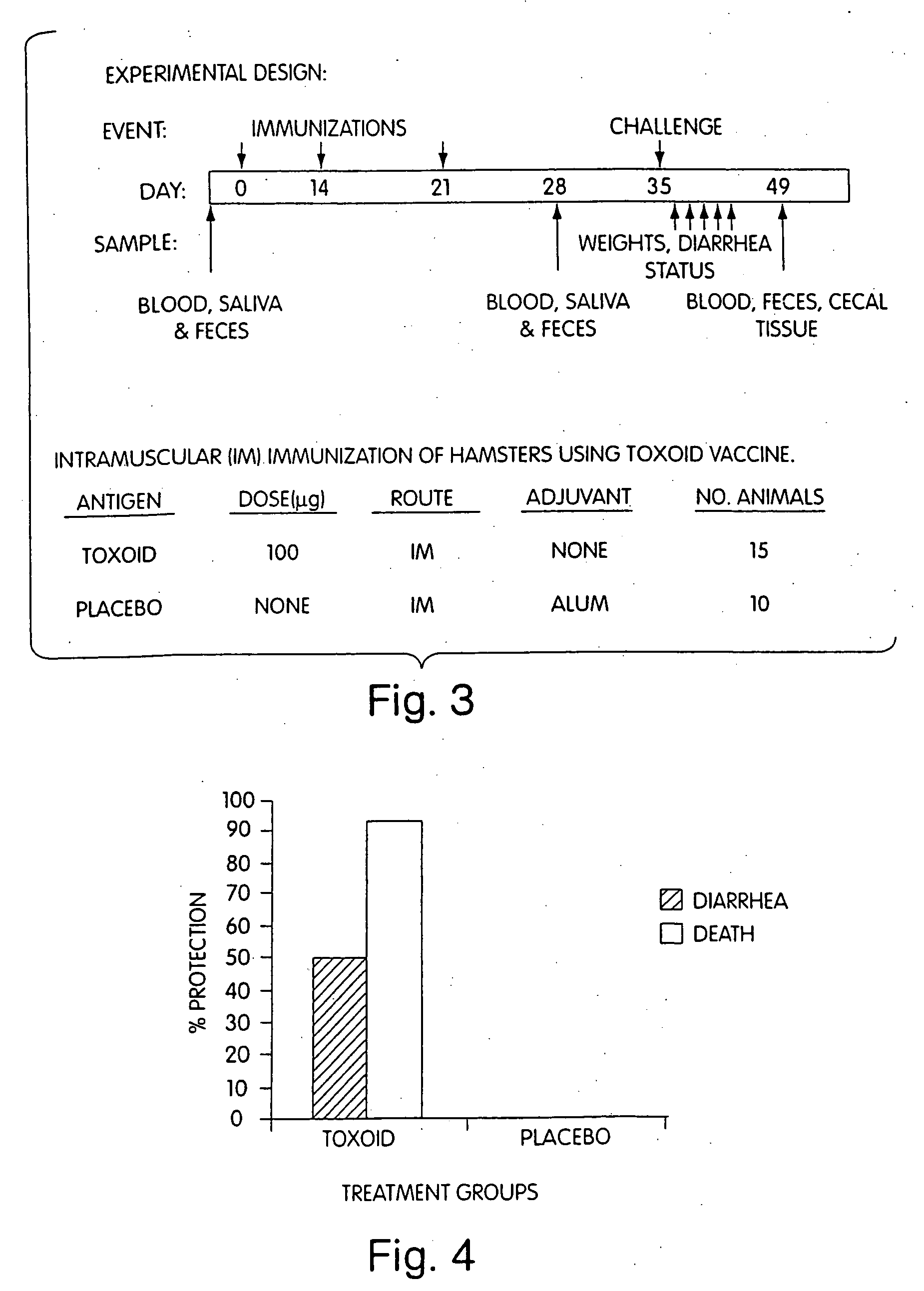
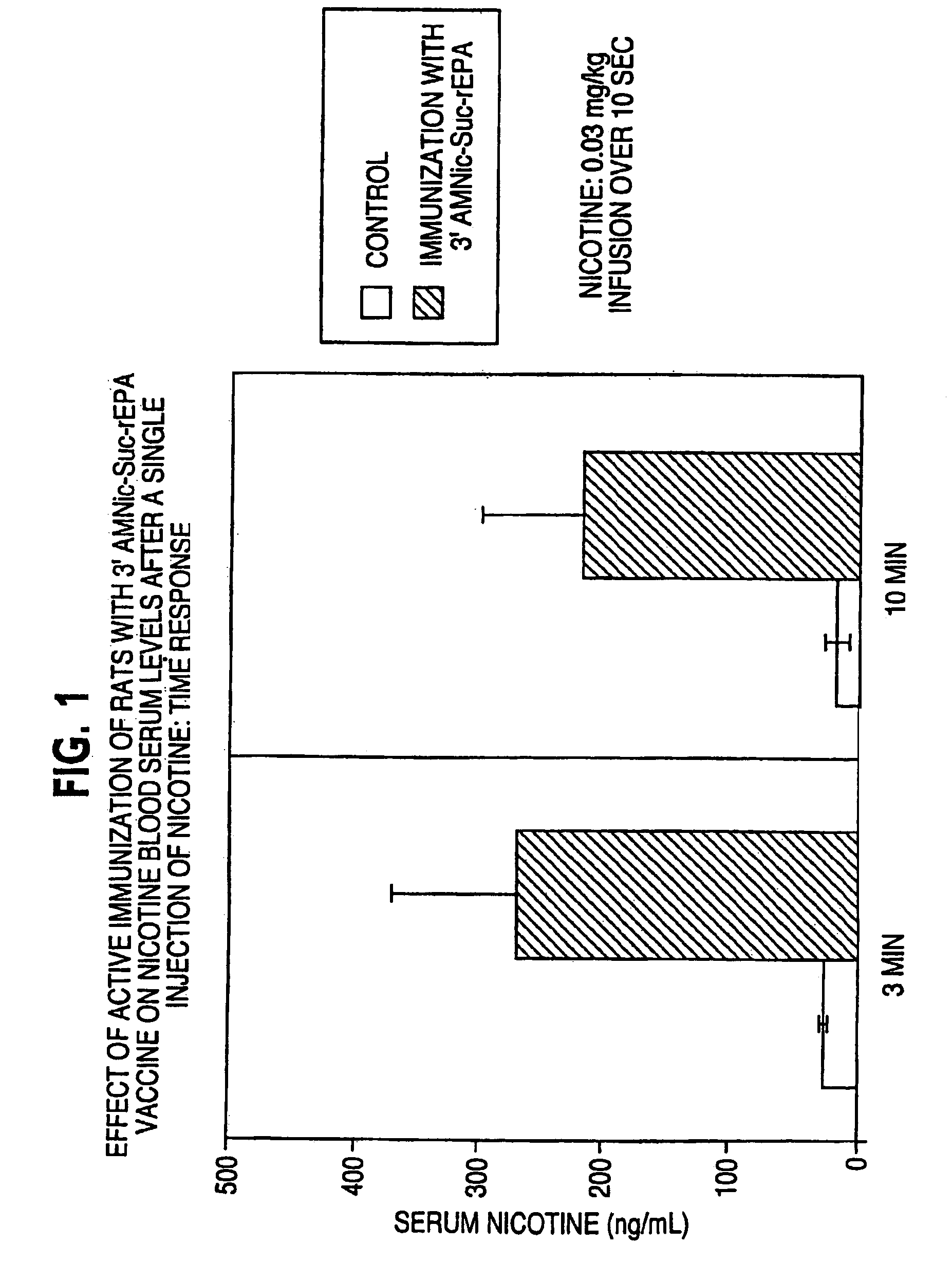
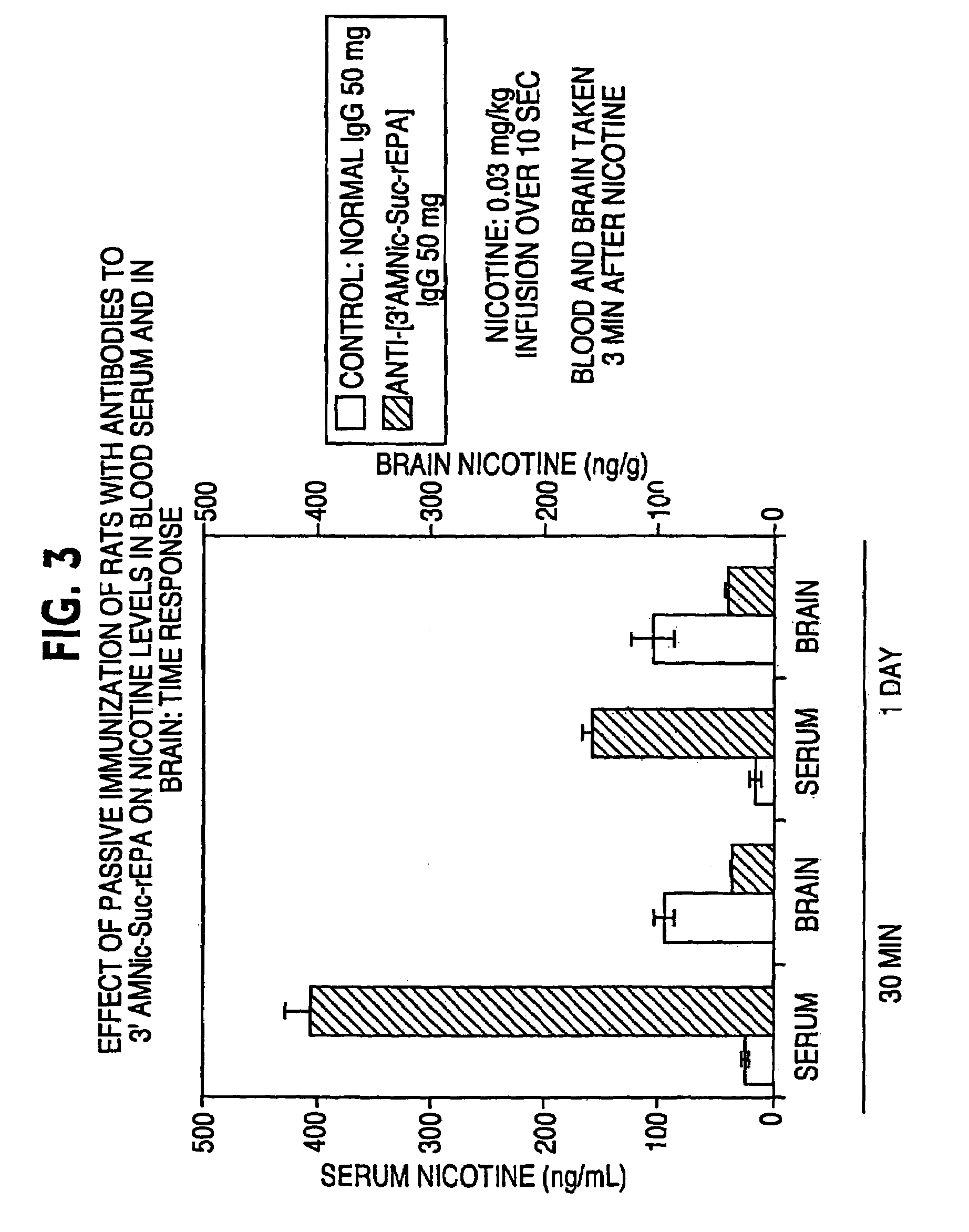
Hapten-carrier conjugates for treating and preventing nicotine addiction
InactiveUS7247502B2Easy to produceImprove the level ofBiocideNervous disorderPassive ImmunizationsCarrier protein
Owner:NABI BIOPHARMLS
Neutralizing monoclonal antibodies against severe acute respiratory syndrome-associated coronavirus
InactiveUS20060240551A1Reduce the binding forceAvoid infectionAnimal cellsMicrobiological testing/measurementAntigenicitySpike Protein
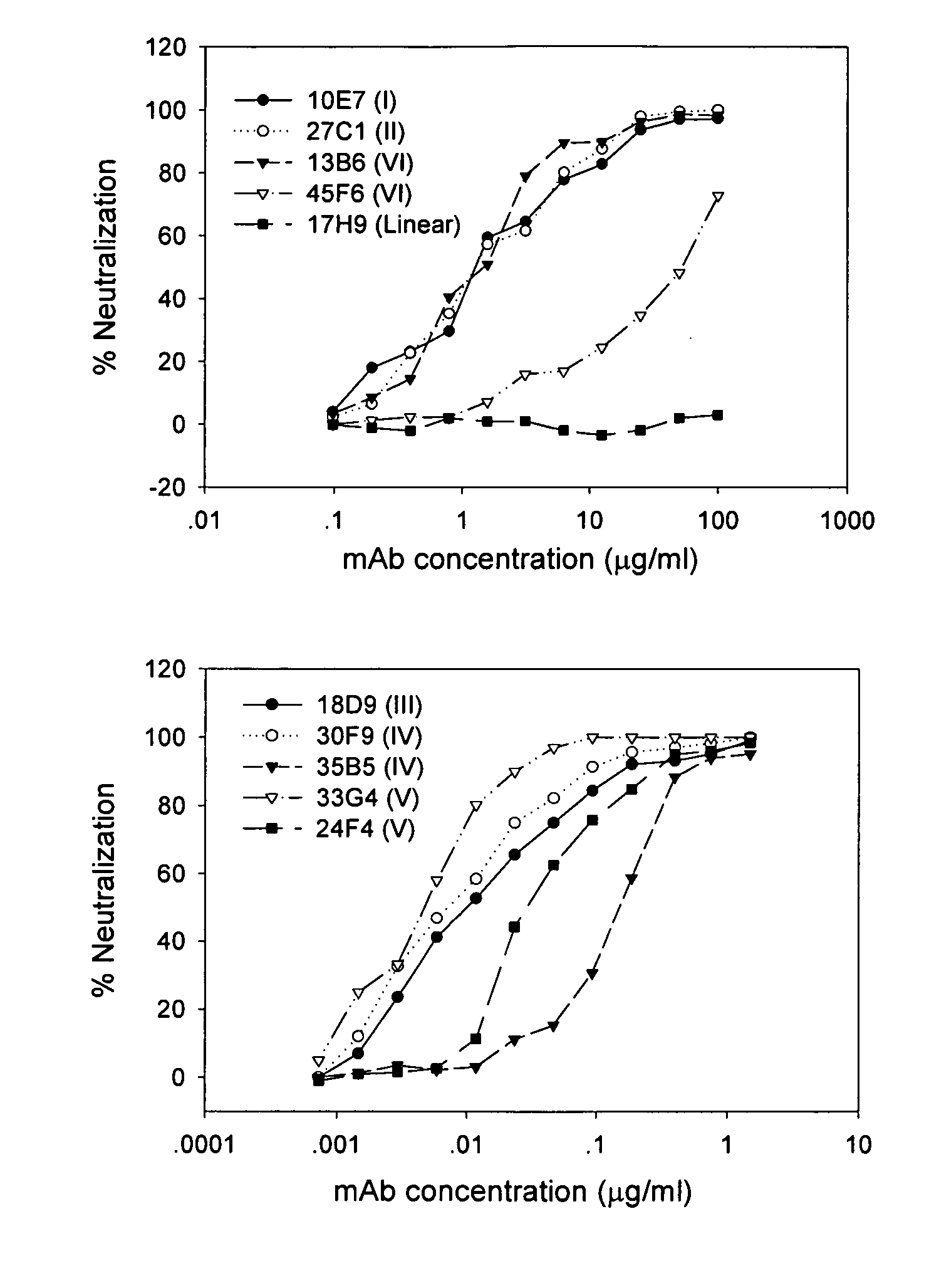
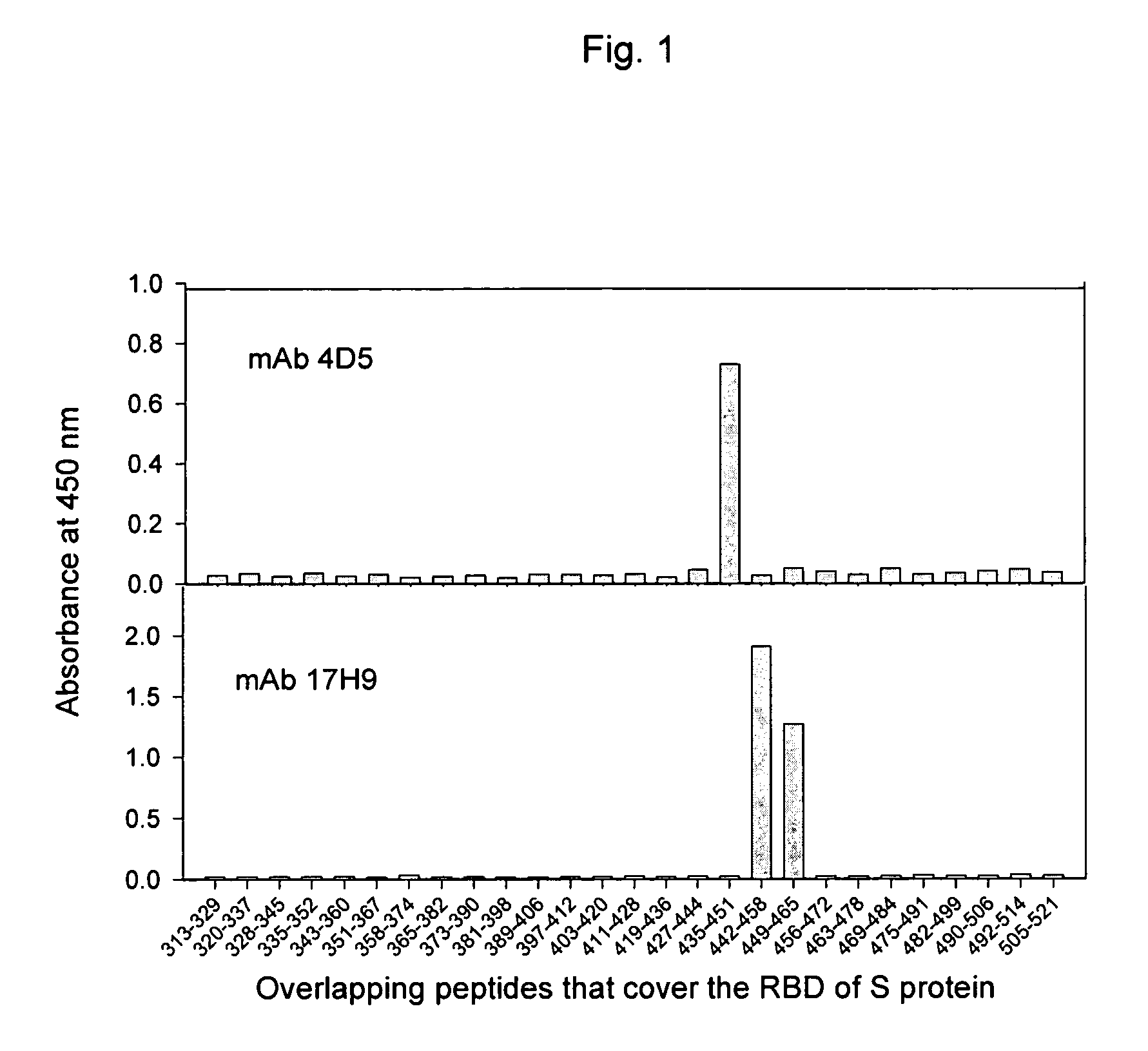
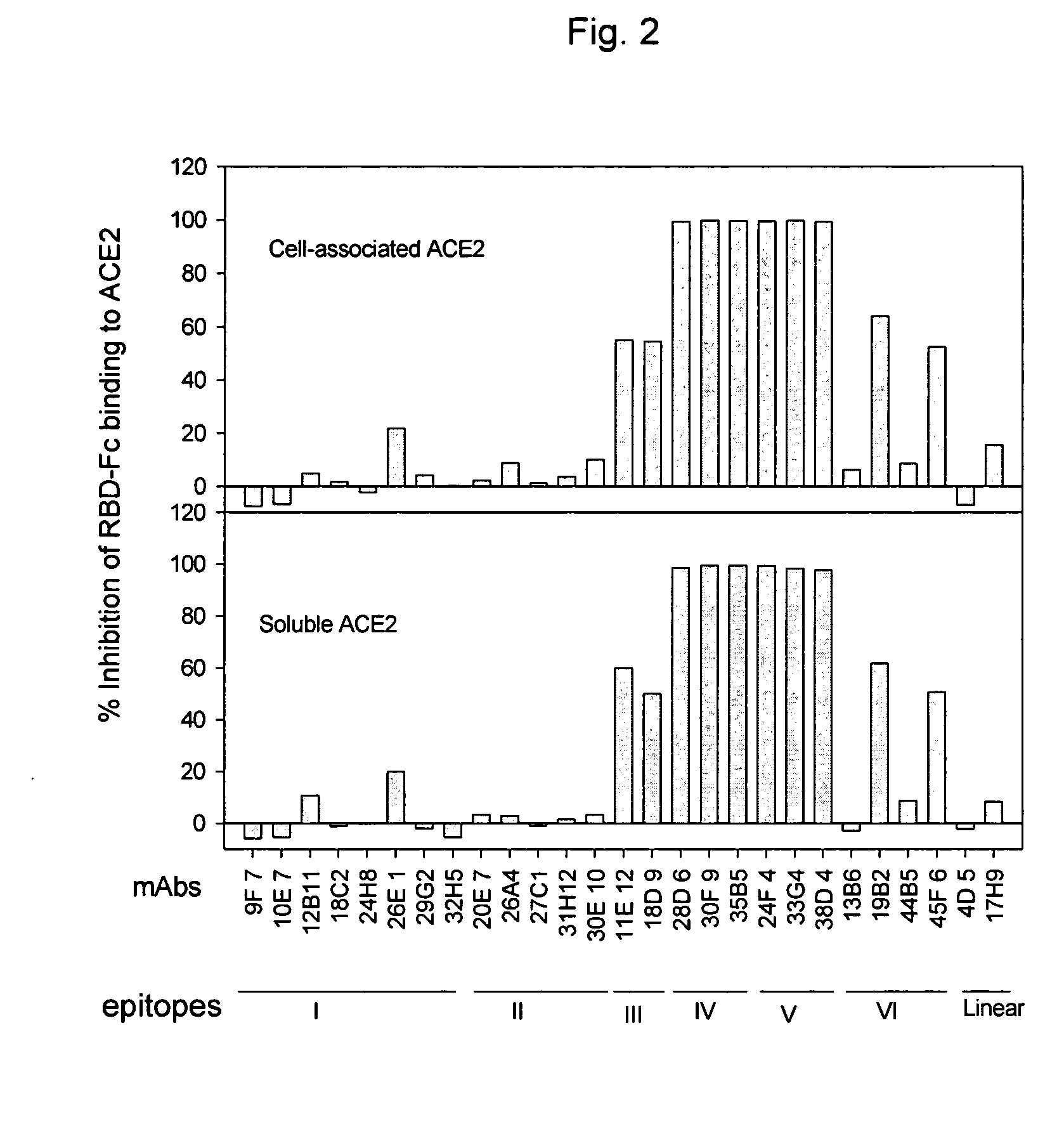
The present invention provides an isolated antibody capable of binding to the receptor-binding domain of the spike protein of the severe acute respiratory syndrome-associated coronavirus (SARS-CoV) so as to competitively inhibit the binding of the SARS-CoV to host cells. These mAbs or substances can be used: 1) as passive-immunizing agents for prevention of SARS-CoV infection; 2) as biological reagents for diagnosis of SARS-CoV infection; 3) as immunotherapeutics for early treatment of SARS-CoV infection; and 4) as probes for studying the immunogenicity, antigenicity, structure, and function of the SARS-CoV S protein.
Owner:NEW YORK BLOOD CENT
Synthetic immunogenic but non-deposit-forming polypeptides and peptides homologous to amyloid beta, prion protein, amylin, alpha-synuclein, or polyglutamine repeats for induction of an immune response thereto
InactiveUS7479482B2Reduce formationAvoid formingHormone peptidesNervous disorderPassive ImmunizationsAmyloid beta
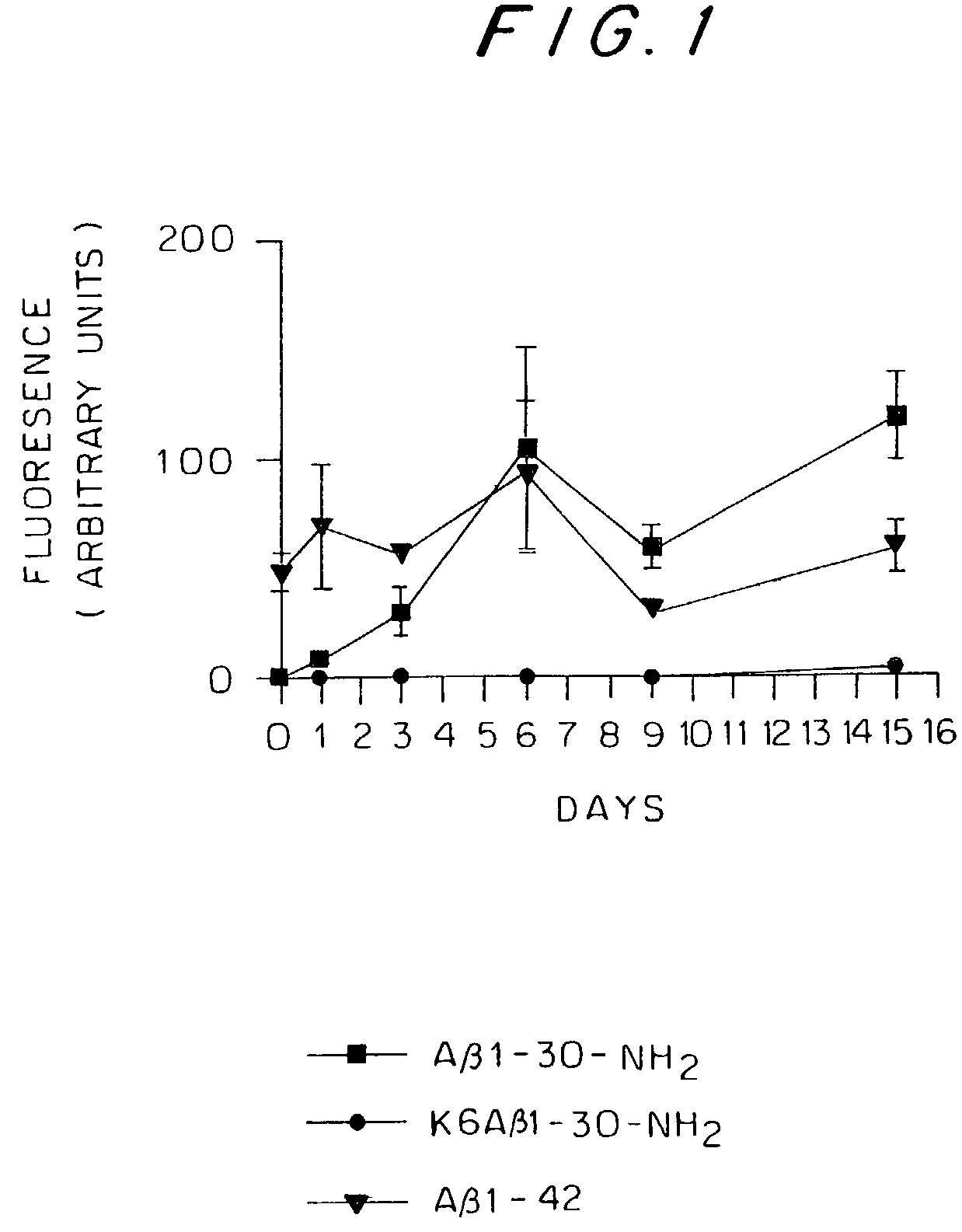
The present invention relates to immunogenic but non-depositing-forming polypeptides or peptides homologous to amyloid β, prion, amylin or α-synuclein which can be used alone or conjugated to an immunostimulatory molecule in an immunizing composition for inducing an immune response to amyloid β peptides and amyloid deposits, to prion protein and prion deposits, to amylin and amylin deposits, to α-synuclein and deposits containing α-synuclein, or to polyglutamine repeats and deposits of proteins containing polyglutamine repeats. Described are also antibodies directed against such peptides, their generation, and their use in methods of passive immunization to such peptides and deposits.
Owner:NEW YORK UNIV
Staphylococcal immunotherapeutics via donor selection and donor stimulation
InactiveUS20060222651A1Immunoglobulins against bacteriaPharmaceutical delivery mechanismPassive ImmunizationsCell-Extracellular Matrix
A method and composition for the passive immunization of patients infected with or susceptible to infection from Staphylococcus bacteria such as S. aureus and S. epidermidis infection is provided that includes the selection or preparation of a donor plasma pool with high antibody titers to carefully selected Staphylococcus adhesins or MSCRAMMs, or fragments or components thereof, or sequences with substantial homology thereto. The donor plasma pool can be prepared by combining individual blood or blood component samples which have higher than normal titers of antibodies to one or more of the selected adhesins or other proteins that bind to extracellular matrix proteins, or by administering carefully selected proteins or peptides to a host to induce the expression of desired antibodies, and subsequently recovering the enhanced high titer serum or plasma pool from the treated host.
Owner:PATTI JOSEPH +2
Methods to inhibit growth of prostate cancer cells
InactiveUS7361338B2Peptide/protein ingredientsGenetic material ingredientsPassive ImmunizationsProstate cancer cell
A novel gene (designated 101P3A11 or PHOR-1) and its encoded protein, and variants thereof, are described wherein 101P3A11 exhibits tissue specific expression in normal adult tissue, and is aberrantly expressed in the cancers listed in Table I. Consequently, 101P3A11 provides a diagnostic, prognostic, prophylactic and / or therapeutic target for cancer. The 101P3A11 gene or fragment thereof, or its encoded protein, or variants thereof, or a fragment thereof, can be used to elicit a humoral or cellular immune response; antibodies or T cells reactive with 101P3A11 can be used in active or passive immunization.
Owner:AGENSYS INC
Antibodies against type A botulinum neurotoxin
InactiveUS20060177881A1Narrow regionImmunoglobulins against bacteriaFermentationEpitopePassive Immunizations
Owner:US ARMY MEDICAL RES MATERIEL COMMAND USAMRMC
Amyloid ß peptide analogues, oligomers thereof, processes for preparing and composi-tions comprising said analogues or oligomers, and their uses
InactiveUS20110092445A1Easy to measureReversing cognitive defectsCompound screeningNervous disorderPassive ImmunizationsOligomer
The present invention relates to an amyloid β peptide analogues comprising an amino acid sequence or a peptidomimetic thereof, wherein the sequence (i) forms a loop, (ii) has at least 66% identity to the amino acid sequence of native Aβ peptide or a portion thereof, (iii) comprises at least 6 contiguous amino acid residues and (iv) has at least 2 non-contiguous amino acid residues which are covalently linked with each other, oligomers comprising a plurality of said amyloid β peptide analogues, processes for preparing the amyloid β peptide analogues or oligomers, compositions comprising the amyloid β peptide analogues or oligomers, and uses of the amyloid β peptide analogues or oligomers such as their use for treating or preventing an amyloidosis (e.g. by active immunization), for diagnosing an amyloidosis, and for providing agents that are capable of binding to the amyloid β peptide analogues or oligomers. The subject invention also describes agents that are capable of binding to the amyloid β peptide analogues or oligomers, e.g. antibodies, compositions comprising the agents, and uses of the agents such as their use for treating or preventing an amyloidosis (e.g. by passive immunization) and for diagnosing an amyloidosis.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG +1
DbpA compositions and methods of use
Disclosed are the dbp gene and dbp-derived nucleic acid segments from Borrelia burgdorferi, the etiological agent of Lyme disease, and DNA segments encoding dbp from related borrelias. Also disclosed are decorin binding protein compositions and methods of use. The DBP protein and antigenic epitopes derived therefrom are contemplated for use in the treatment of pathological Borrelia infections, and in particular, for use in the prevention of bacterial adhesion to decorin. DNA segments encoding these proteins and anti-(decorin binding protein) antibodies will also be of use in various screening, diagnostic and therapeutic applications including active and passive immunization and methods for the prevention of Borrelia colonization in an animal. These DNA segments and the peptides derived therefrom are contemplated for use in the preparation of vaccines and, also, for use as carrier proteins in vaccine formulations, and in the formulation of compositions for use in the prevention of Lyme disease.
Owner:TEXAS A&M UNIVERSITY
Antibodies and related molecules that bind to PSCA proteins
InactiveUS20050221400A1Compound screeningTumor rejection antigen precursorsPassive ImmunizationsT cell
Antibodies and molecules derived therefrom that bind to novel PSCA protein, and variants thereof, are described wherein PSCA exhibits tissue specific expression in normal adult tissue, and is aberrantly expressed in the cancers listed in Table I. Consequently, PSCA provides a diagnostic, prognostic, prophylactic and / or therapeutic target for cancer. The PSCA gene or fragment thereof, or its encoded protein, or variants thereof, or a fragment thereof, can be used to elicit a humoral or cellular immune response; antibodies or T cells reactive with PSCA can be used in active or passive immunization.
Owner:AGENSYS
Antibodies and related molecules that bind to PSCA proteins
Antibodies and molecules derived therefrom that bind to novel PSCA protein, and variants thereof, are described wherein PSCA exhibits tissue specific expression in normal adult tissue, and is aberrantly expressed in the cancers listed in Table I. Consequently, PSCA provides a diagnostic, prognostic, prophylactic and / or therapeutic target for cancer. The PSCA gene or fragment thereof, or its encoded protein, or variants thereof, or a fragment thereof, can be used to elicit a humoral or cellular immune response; antibodies or T cells reactive with PSCA can be used in active or passive immunization.
Owner:AGENSYS
Monoclonal Antibodies to Progastrin
The present invention provides progastrin-binding molecules specific for progastrin that do not bind gastrin-17(G17), gastrin-34(G34), glycine-extended gastrin-17(G17-Gly), or glycine-extended gastrin-34(G34-Gly). Further, the invention provides monoclonal antibodies (MAbs) selective for sequences at the N-terminus and the C-terminus of the gastrin precursor molecule, progastrin and the hybridomas that produce these MAbs. Also provided are panels of MAbs useful for the detection and quantitation of progastrin and gastrin hormone species in immuno-detection and quantitation assays. These assays are useful for diagnosing and monitoring a gastrin-promoted disease or condition, or for monitoring the progress of a course of therapy. The invention further provides solid phase assays including immunohistochemical (IHC) and immunofluorescence (IF) assays suitable for detection and visualization of gastrin species in solid samples, such as biopsy samples or tissue slices. The progastrin-binding molecules are useful therapeutically for passive immunization against progastrin in progastrin-promoted diseases or conditions. Also provided are surrogate reference standard (SRS) molecules that are peptide chains of from about 10 to about 35 amino acids, wherein the SRS molecule comprises at least two epitopes found in a protein of interest of greater than about 50 amino acids. Such SRS molecules are useful as standards in place of authentic proteins of interest.
Owner:CANCER ADVANCES INC
Affinity purified human polyclonal antibodies against viral, bacterial and/or fungal infections and methods of making and using the same
InactiveUS20120027771A1SsRNA viruses negative-senseAntibacterial agentsFungal antigenPassive Immunizations
The present invention discloses compositions and methods for treating, preventing and / or monitoring viral, bacterial, eukaryotic protist and / or fungal infections. In some embodiments, these compositions and methods involve human polyclonal antibodies affinity purified from human blood using certain viral, bacterial, eukaryotic protist and / or fungal antigens as described herein. Methods of making the antigenic preparations and the affinity-purified human polyclonal antibodies for passive immunization are also provided.
Owner:SCANTIBODIES LAB
Antigenic composition comprising an HIV gag or env polypeptide
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC +1
HIV immunoassays using synthetic envelope polypeptides
ActiveUS7273695B1Peptide/protein ingredientsAntibody mimetics/scaffoldsAntigenAcquired immunodeficiency
Polynucleotide sequences are provided for the diagnosis of the presence of retroviral infection in a human host associated with lymphadenopathy syndrome and / or acquired immune deficiency syndrome, for expression of polypeptides and use of the polypeptides to prepare antibodies, where both the polypeptides and antibodies may be employed as diagnostic reagents or in therapy, e.g., vaccines and passive immunization. The sequences provide detection of the viral infectious agents associated with the indicated syndromes and can be used for expression of antigenic polypeptides.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC +1
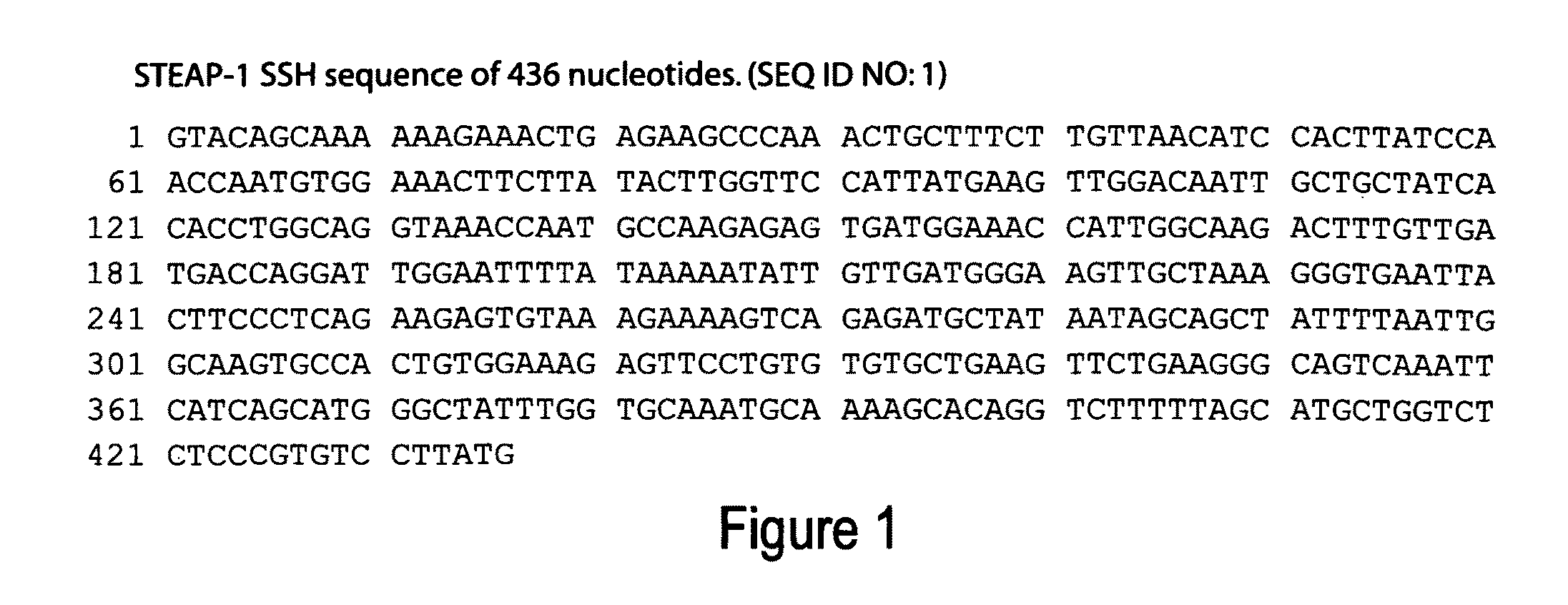
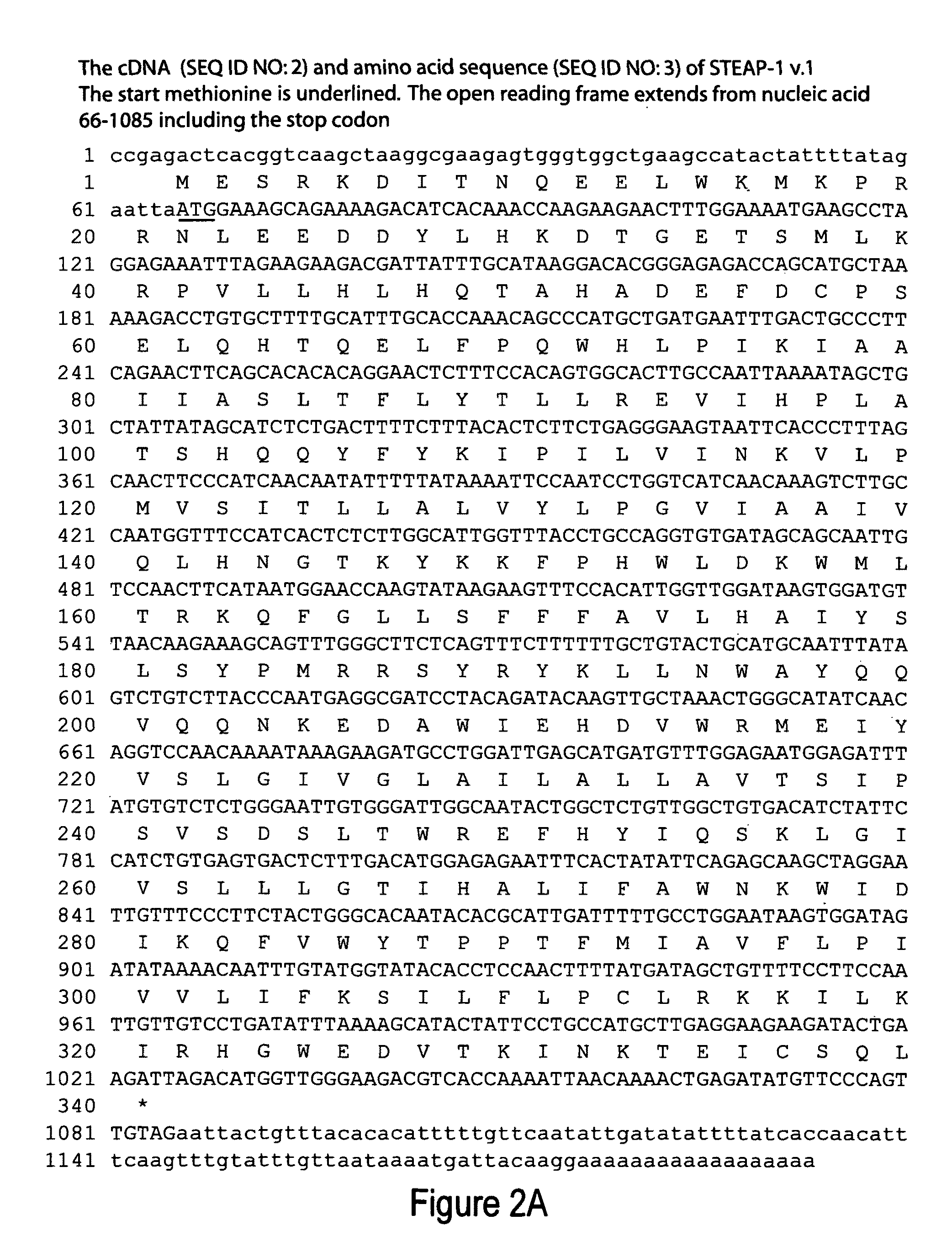
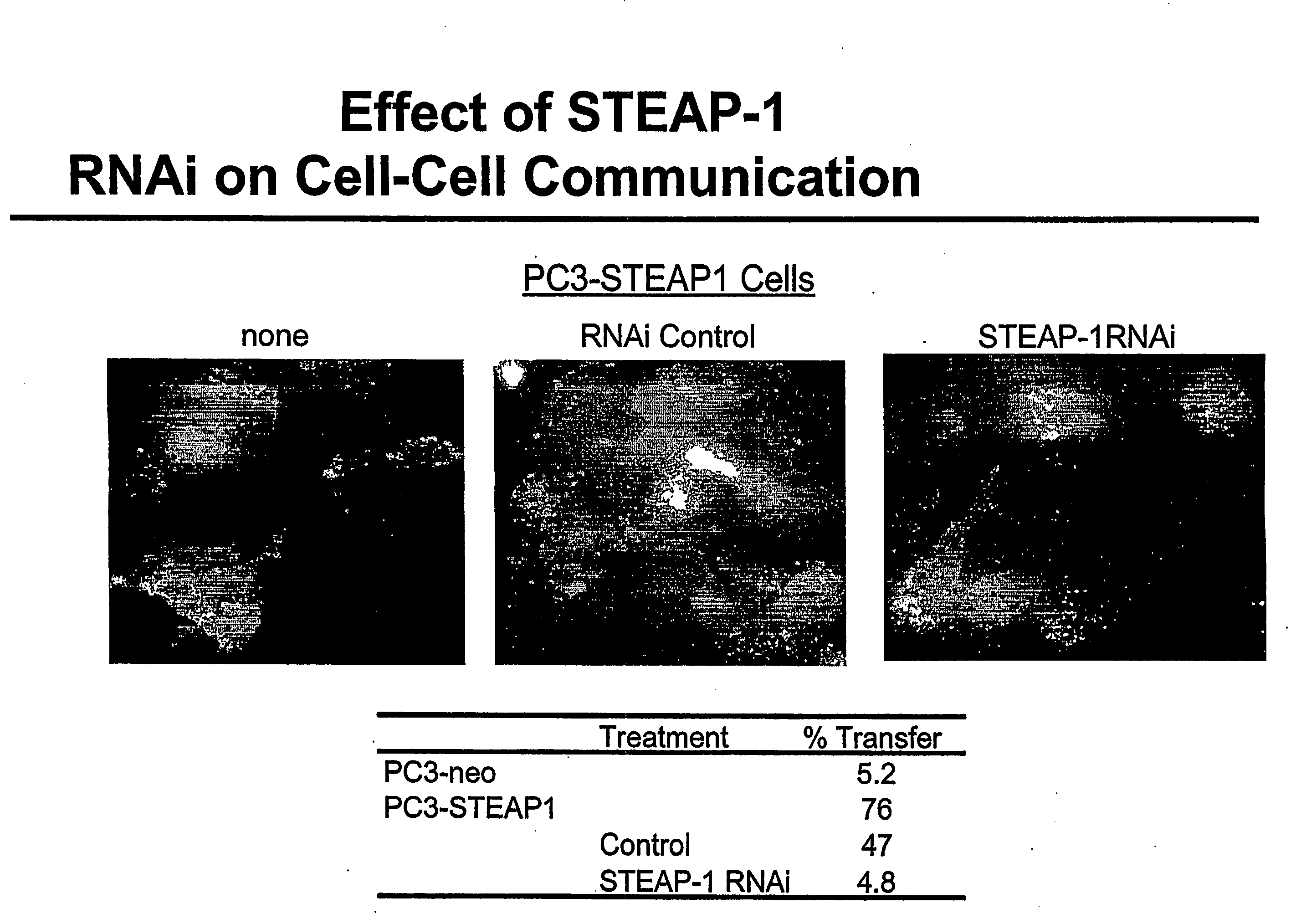
Antibodies and molecules derived therefrom that bind to STEAP-1 proteins
Antibodies and molecules derived therefrom that bind to novel STEAP-1 protein, and variants thereof, are described wherein STEAP-1 exhibits tissue specific expression in normal adult tissue, and is aberrantly expressed in the cancers listed in Table I. Consequently, STEAP-1 provides a diagnostic, prognostic, prophylactic and / or therapeutic target for cancer. The STEAP-1 gene or fragment thereof, or its encoded protein, or variants thereof, or a fragment thereof, can be used to elicit a humoral or cellular immune response; antibodies or T cells reactive with STEAP-1 can be used in active or passive immunization.
Owner:AGENSYS
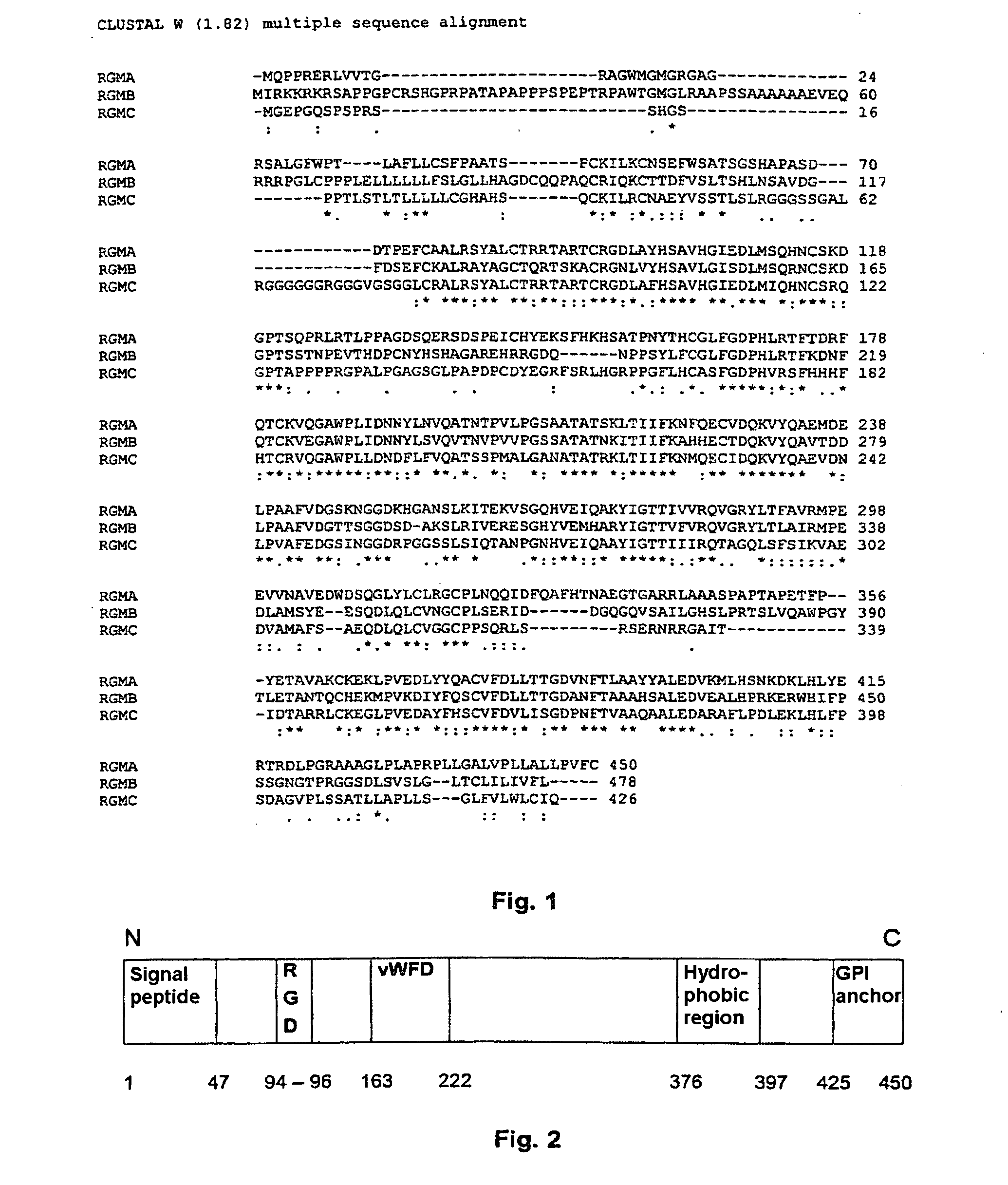
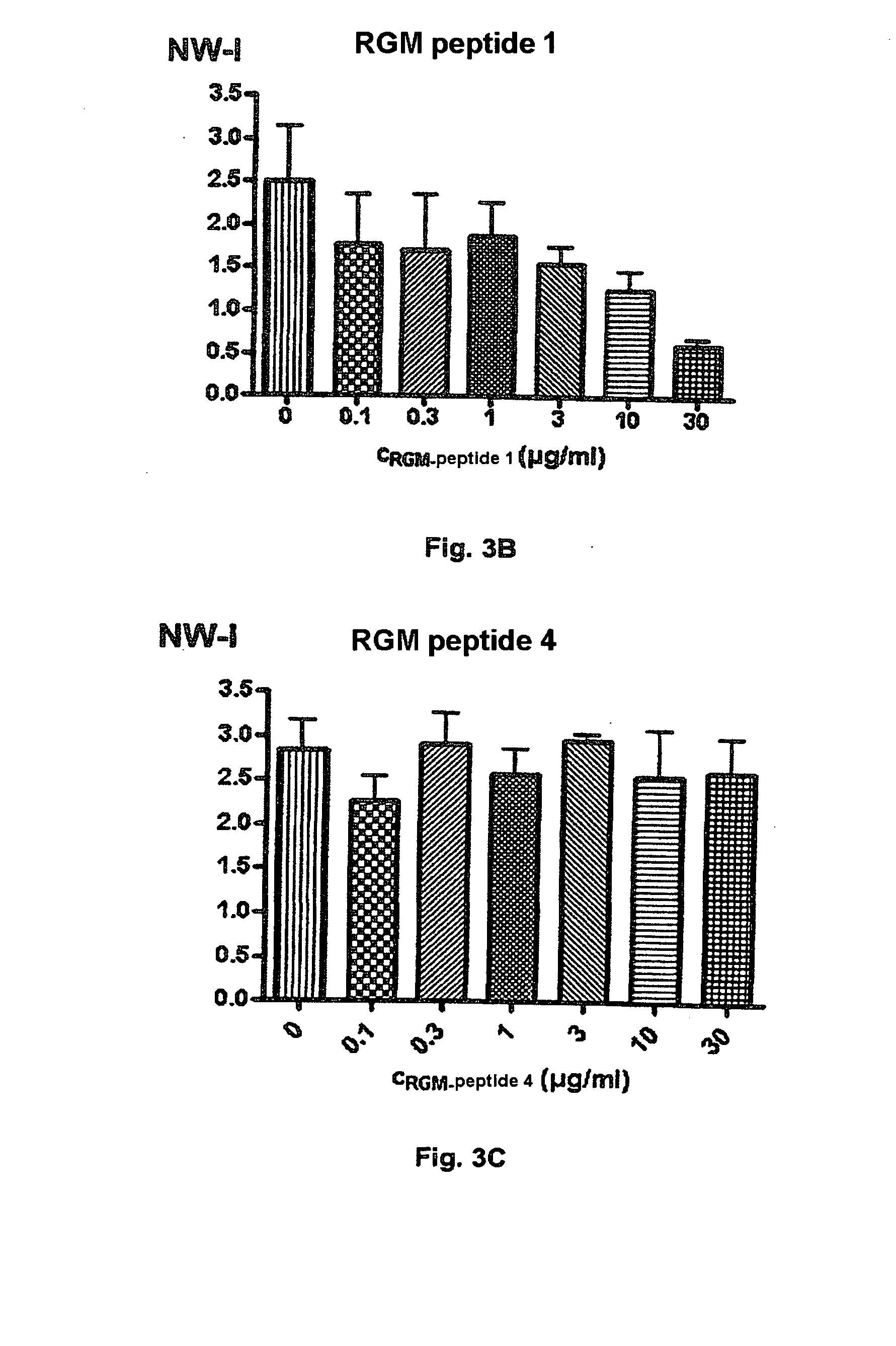
Binding Domains of Proteins of the Repulsive Guidance Molecule (RGM) Protein Family and Functional Fragments Thereof, and Their Use
ActiveUS20090297527A1Peptide/protein ingredientsAntipyreticPassive ImmunizationsRepulsive guidance molecule
The invention concerns the identification and use of neogenin receptor-binding domains of members of the repulsive guidance molecule (RGM) protein family as well as polypeptide fragments derived therefrom. The inventive domains, i.e. peptide fragments are suited as agents for the active or passive immunization of individuals as well as diagnostic and therapeutic agents for use with diseases or pathological conditions in whose origin or progression, a member of the RGM family and a cellular receptor assigned to this molecule are involved. The invention also concerns monoclonal and polyclonal antibodies directed against the inventive binding domains and against the polypeptides derived therefrom, and to method for producing the inventive domains, polypeptides and antibodies.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG
Monoclonal antibodies to progastrin
The present invention provides progastrin-binding molecules specific for progastrin that do not bind gastrin-17(G17), gastrin-34(G34), glycine-extended gastrin-17(G17-Gly), or glycine-extended gastrin-34(G34-Gly). Further, the invention provides monoclonal antibodies (MAbs) selective for sequences at the N-terminus and the C-terminus of the gastrin precursor molecule, progastrin and the hybridomas that produce these MAbs. Also provided are panels of Mabs useful for the detection and quantitation of progastrin and gastrin hormone species in immuno-detection and quantitation assays. These assays are useful for diagnosing and monitoring a gastrin-promoted disease or condition, or for monitoring the progress of a course of therapy. The invention further provides solid phase assays including immunohistochemical (IHC) and immunofluorescence (IF) assays suitable for detection and visualization of gastrin species in solid samples, such as biopsy samples or tissue slices. The progastrin-binding molecules are useful therapeutically for passive immunization against progastrin in progastrin-promoted diseases or conditions. Also provided are surrogate reference standard (SRS) molecules that are peptide chains of from about 10 to about 35 amino acids, wherein the SRS molecule comprises at least two epitopes found in a protein of interest of greater than about 50 amino acids. Such SRS molecules are useful as standards in place of authentic proteins of interest.
Owner:CANCER ADVANCES INC
Aß(X - 38 .. 43) oligomers, and processes, compositions, and uses thereof
InactiveUS20100173828A1High molecular weightAntibacterial agentsBiocidePassive ImmunizationsOligomer
The present invention relates to an Aβ(X-38 . . . 43) oligomer having a high molecular weight, or a derivative thereof, a process for preparing the oligomer or derivative, compositions comprising the oligomer or derivative, and uses of the oligomer or derivative such as its use for treating or preventing an amyloidosis (e.g. by active immunization), for diagnosing an amyloidosis, and for providing agents that are capable of binding to the Aβ(X-38 . . . 43) oligomer or derivative. The subject invention also describes agents that are capable of binding to the Aβ(X-38 . . . 43) oligomer or derivative, e.g. antibodies, compositions comprising the agents, and uses of the agents such as their use for treating or preventing an amyloidosis (e.g. by passive immunization) and for diagnosing an amyloidosis.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG
Collagen binding protein compositions and methods of use
InactiveUS20020102262A1Prevent diseaseSimple methodAntibacterial agentsPeptide/protein ingredientsPassive ImmunizationsCarrier protein
Disclosed are the cna gene and cna-derived nucleic acid segments from Staphylococcus aureus, and DNA segments encoding cna from related bacteria. Also disclosed are Col binding protein (CBP) compositions and methods of use. The CBP protein and antigenic epitopes derived therefrom are contemplated for use in the treatment of pathological infections, and in particular, for use in the prevention of bacterial adhesion to Col. DNA segments encoding these proteins and anti-(Col binding protein) antibodies will also be of use in various screening, diagnostic and therapeutic applications including active and passive immunization and methods for the prevention of bacterial colonization in an animal such as a human. These DNA segments and the peptides derived therefrom are contemplated for use in the preparation of vaccines and, also, for use as carrier proteins in vaccine formulations, and in the formulation of compositions for use in the prevention of S. aureus infection.
Owner:HOOK MAGNUS +2
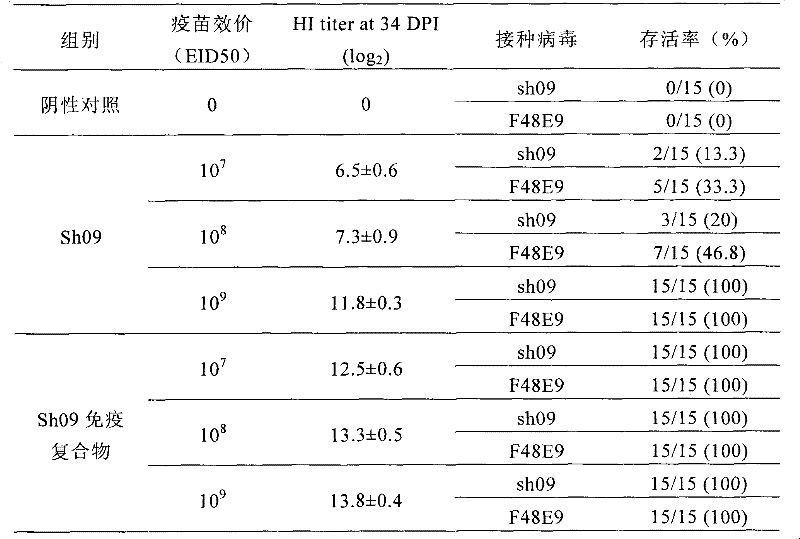
Preparation method and application of Newcastle disease virus infected immune complex vaccines
InactiveCN102233133AAvoid infectionSame characteristicsViral antigen ingredientsAntiviralsYolkMicroorganism
The invention discloses a preparation method and application of Newcastle disease virus infected immune complex vaccines. The preparation method comprises the following steps of: (1) preparing antigens and inactivated vaccines by using Newcastle disease virus Shanghai strains as seed viruses; (2) transferring antibodies in chicken blood serum to yolk to form yolk antibodies IgY; and (3) mixing the prepared inactivated vaccines and the yolk antibodies IgY in equal volume, and hatching to obtain the immune complex vaccines. By combining passive immune (vaccine) and active immune (specific antibody) measures, animals are protected from the infection of pathogenic microbes in time for a long time.
Owner:SHANGHAI ACAD OF AGRI SCI
Compositions and methods for the treatment of immunodeficiency
ActiveUS9107906B1Antibacterial agentsSerum immunoglobulinsPassive ImmunizationsPrimary immunodeficiency
The present invention relates to compositions and methods for the treatment of immunodeficiency (e.g., primary immunodeficiency disease). In particular, the invention provides human plasma immunoglobulin compositions containing select antibody titers specific for a plurality of respiratory pathogens, methods of identifying human donors and donor samples for use in the compositions, methods of manufacturing the compositions, and methods of utilizing the compositions (e.g., for prophylactic administration and / or therapeutic treatment (e.g., passive immunization (e.g., immune-prophylaxis))).
Owner:ADMA BIOMANUFACTURING LLC
Antibodies and Molecules Derived Therefrom that Bind to Steap-1 Proteins
Antibodies and molecules derived therefrom that bind to novel STEAP-1 protein, and variants thereof, are described wherein STEAP-1 exhibits tissue specific expression in normal adult tissue, and is aberrantly expressed in the cancers listed in Table I. Consequently, STEAP-1 provides a diagnostic, prognostic, prophylactic and / or therapeutic target for cancer. The STEAP-1 gene or fragment thereof, or its encoded protein, or variants thereof, or a fragment thereof, can be used to elicit a humoral or cellular immune response; antibodies or T cells reactive with STEAP-1 can be used in active or passive immunization.
Owner:AGENSYS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com