Patents
Literature
139 results about "Clostridium difficile infections" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for treatment of disorders of the gastrointestinal system
There are provided novel synthetic stool preparations comprising bacteria isolated from a fecal sample from a healthy donor. The synthetic stool preparations are used for treating disorders of the gastrointestinal tract, including dysbiosis, Clostridium difficile infection and recurrent Clostridium difficile infection, prevention of recurrence of Clostridium difficile infection, treatment of Crohn's disease, ulcerative colitis, irritable bowel syndrome, inflammatory bowel disease, and diverticular disease, and treatment of food poisoning such as salmonella. Methods of preparation and methods of use of the synthetic stool preparations are also provided.
Owner:UNIVERSITY OF GUELPH +2
Targeted gastrointestinal tract delivery of probiotic organisms and/or therapeutic agents
ActiveUS20160022592A1Improve imbalanceAntibacterial agentsBiocideAntibiotic-associated diarrhoeaClostridium difficile infections
The present invention relates to the development of a targeted delivery system for the oral delivery of probiotics or therapeutic agent for various indications, including and not limited to active and prophylaxis treatment of Clostridium difficile infection, antibiotic associated diarrhea, irritable bowel syndrome, Crohn's disease, intestinal flora replacement, supplemental flora treatments for patients taking antibiotics, and for restoration of balance and signaling between the intestinal microbiome and the intestinal cells in patients under treatment of metabolic syndrome manifestations, specifically diabetes, insulin resistance, obesity, hyperlipidemia and hypertension.
Owner:THERABIOME
Immunization against clostridium difficile disease
InactiveUS20060029608A1Prevent relapseQuick treatmentAntibacterial agentsBacterial antigen ingredientsPassive ImmunizationsClostridium difficile infections
The invention provides active and passive immunization methods for preventing and treating Clostridium difficile infection, which involve percutaneous administration of C. difficile toxin-neutralizing polyclonal immune globulin, C. difficile toxoids, or combinations thereof. Also provided by the invention are C. difficile toxoids, C. difficile toxin-neutralizing polyclonal immune globulin, and methods of identifying subjects that produce C. difficile toxin-neutralizing polyclonal immune globulin.
Owner:ACAMBIS INC
Treatment of clostridium difficile infection
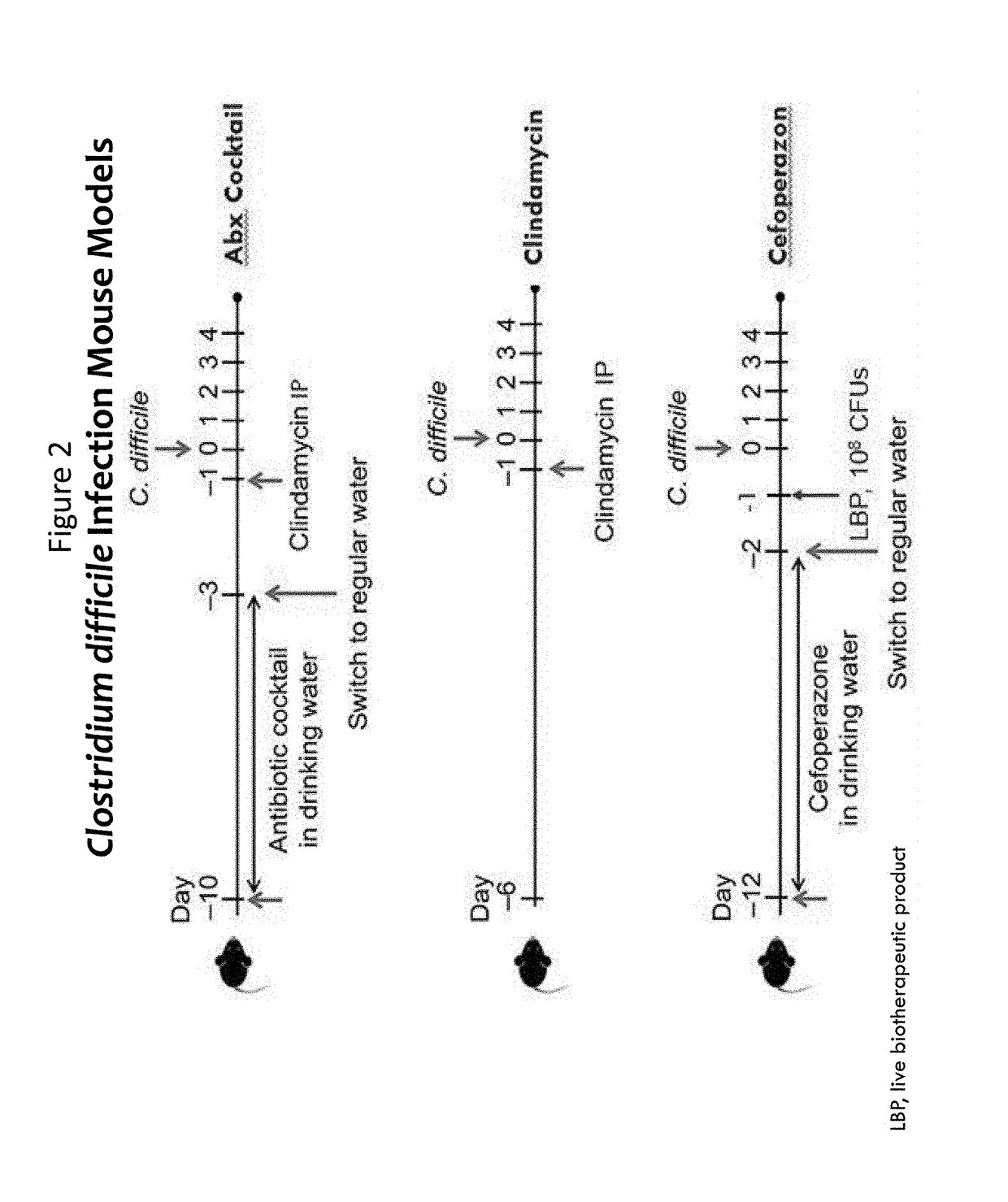
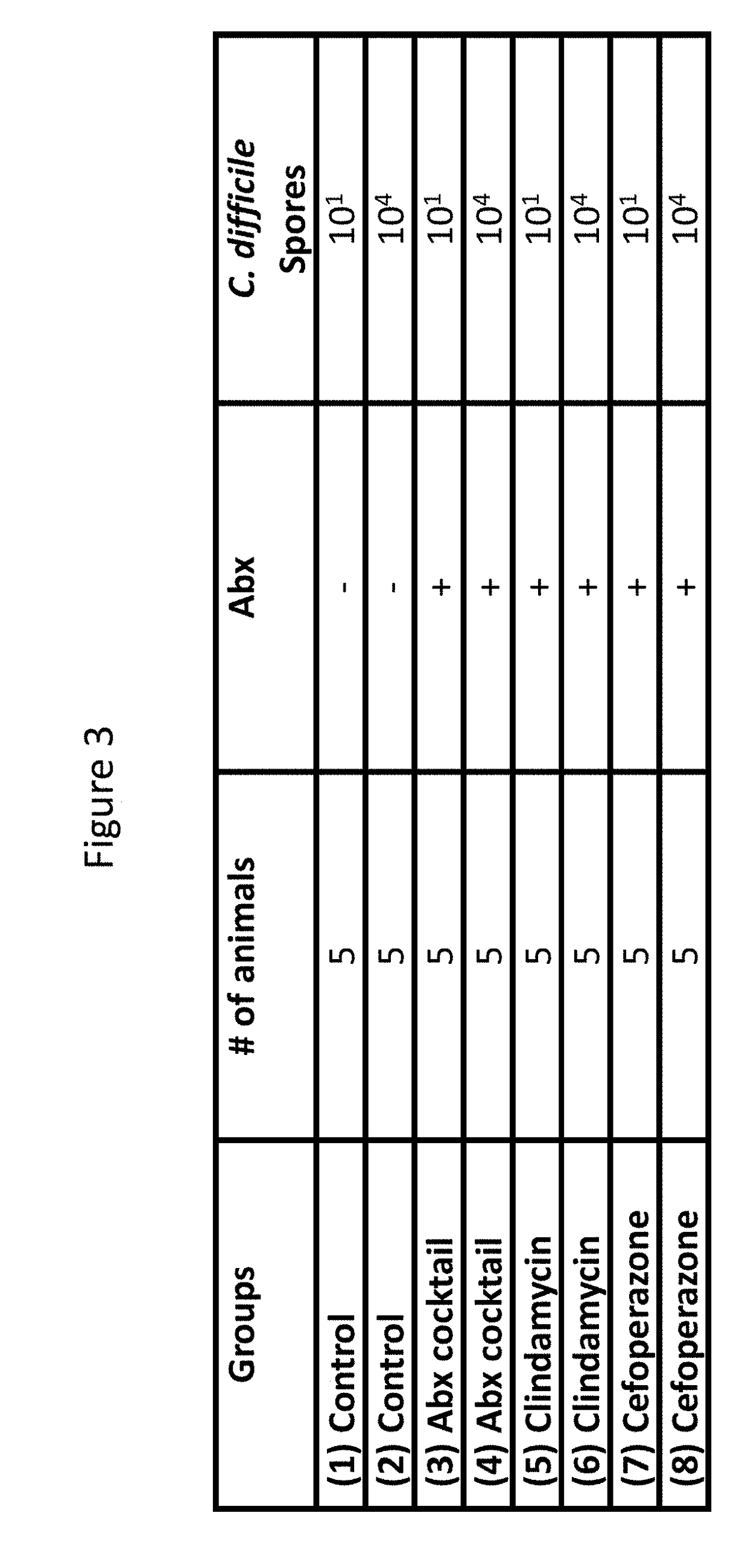
ActiveUS20170354697A1Difficile replicationDifficile survivalAntibacterial agentsBacteriaClostridium difficile infectionsMedicine
Owner:VEDANTA BIOSCIENCES INC
Compositions and methods for the delivery of therapeutic peptides
ActiveUS20130259834A1Promotes therapeutic responseReduce mucosal damageAntibacterial agentsBiocideAntibiotic-associated diarrheaClostridium difficile infections
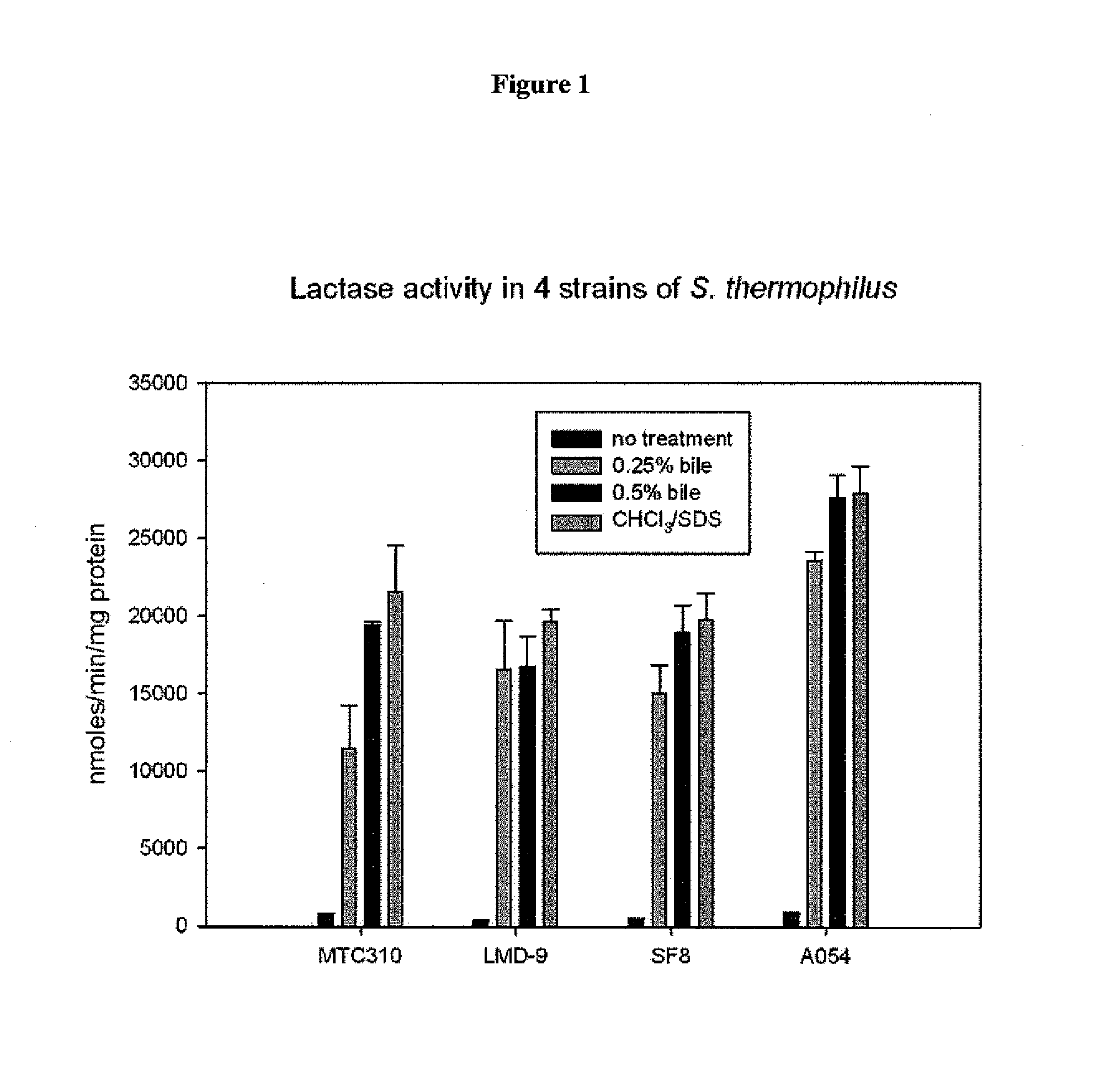
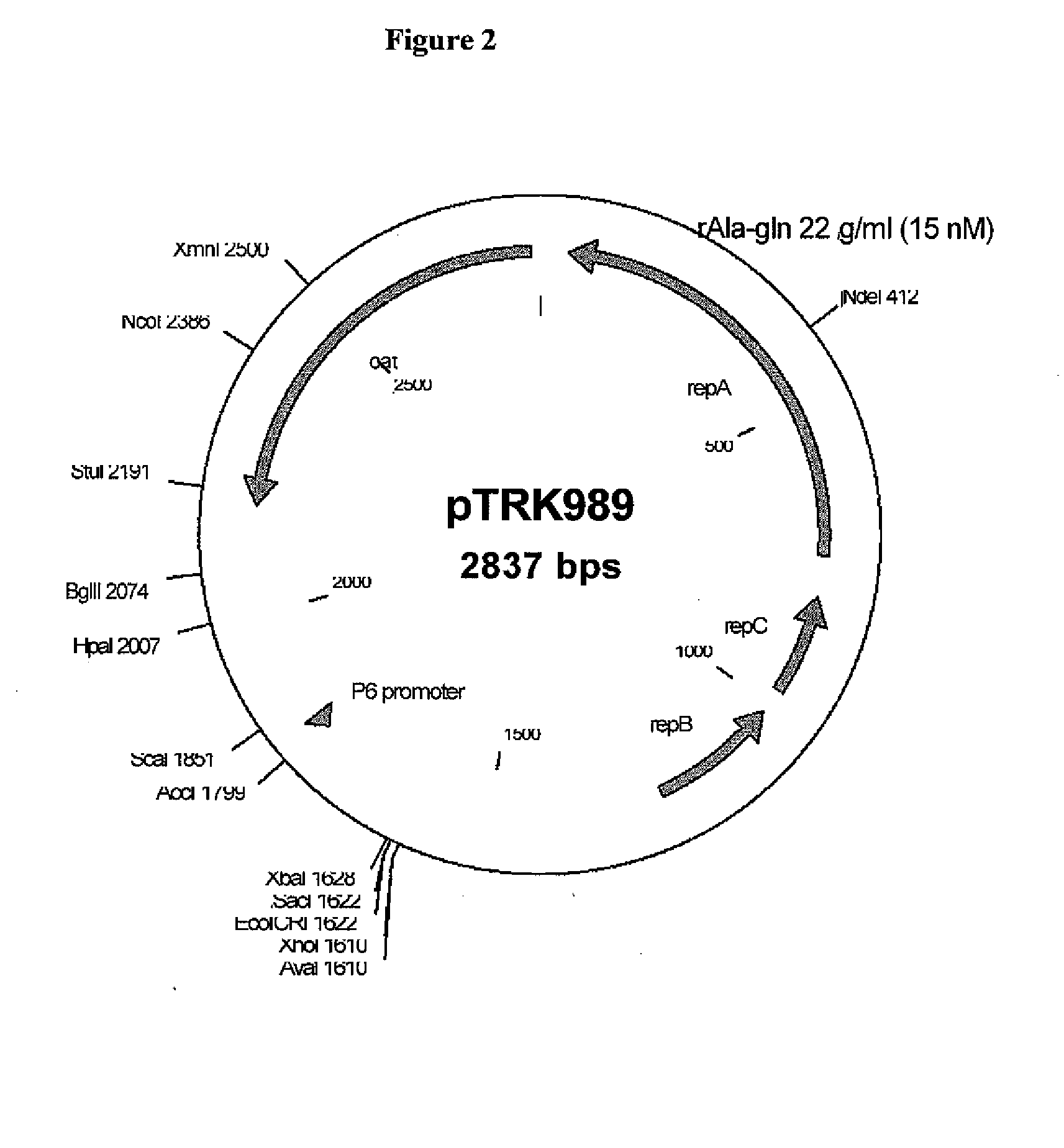
Methods and compositions for targeted delivery of biotherapeutics are provided. The compositions comprise bile-sensitive St. thermophilus bacteria modified to release a biotherapeutic agent following bile exposure. Biotherapeutic agents released by the St. thermophilus bacteria disclosed herein include AQ and AQR rich peptides. Methods of the invention comprise administering to a subject a St. thermophilus bacterium modified to release a biotherapeutic agent following bile exposure. Administration of the St. thermophilus bacterium promotes a desired therapeutic response. The bacterium may be modified to express and release AQ or AQR rich peptides which subsequently inhibit cellular apoptosis or reduce mucosal damage. Thus, methods of the invention find use in treating or preventing a variety of gastrointestinal disorders including C. difficile infection and antibiotic-associated diarrhea.
Owner:NORTH CAROLINA STATE UNIV +1
Multiple fluorescence PCR detection kit and detection method for clostridium difficile toxin genes
ActiveCN103361434AMicrobiological testing/measurementMicroorganism based processesClostridium difficile infectionsClostridium difficile toxin B
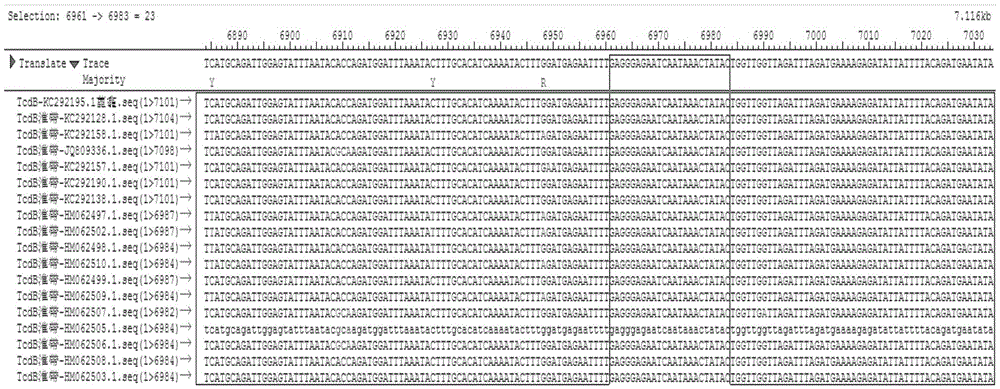
A provided multiple fluorescence PCR detection kit for clostridium difficile toxin genes mainly comprises specific primers, probes and a PCR reaction reagent, and the specific primers and the probes respectively consist of specific primers and probes of clostridium difficile toxin A (tcdA), toxin B (tcdB), binary toxin A (cdtA) and binary toxinB (cdtB). The beneficial effects of the invention comprise: the fast, sensitive and specific multiple fluorescence PCR detection kit and a detection method are provided for the clostridium difficile toxin genes, and a foundation is provided for distinguishing between toxigenic strains and avirulent strains of clostridium difficile, and early diagnosis on infection of clostridium difficile.
Owner:杭州海基生物技术有限公司
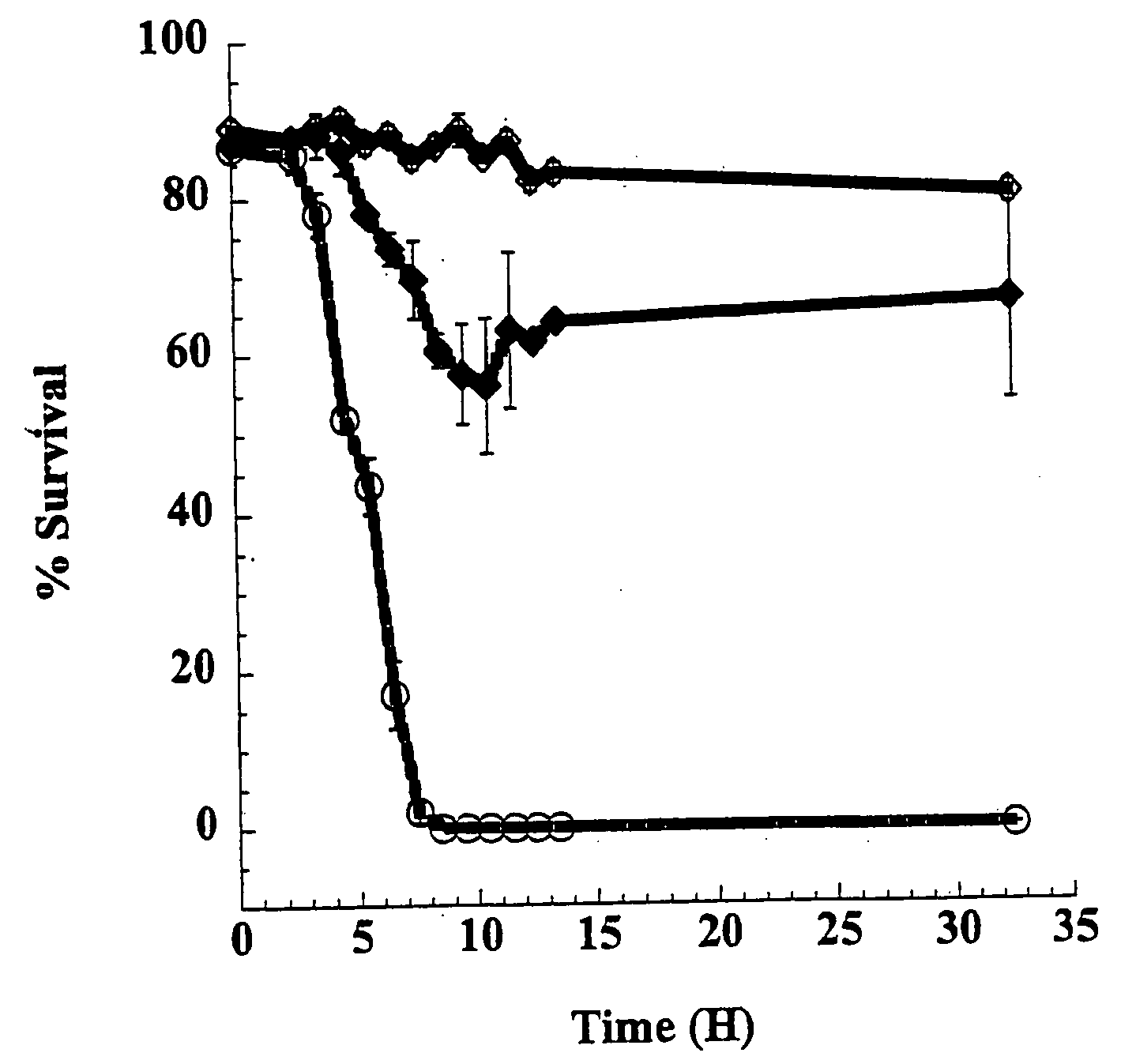
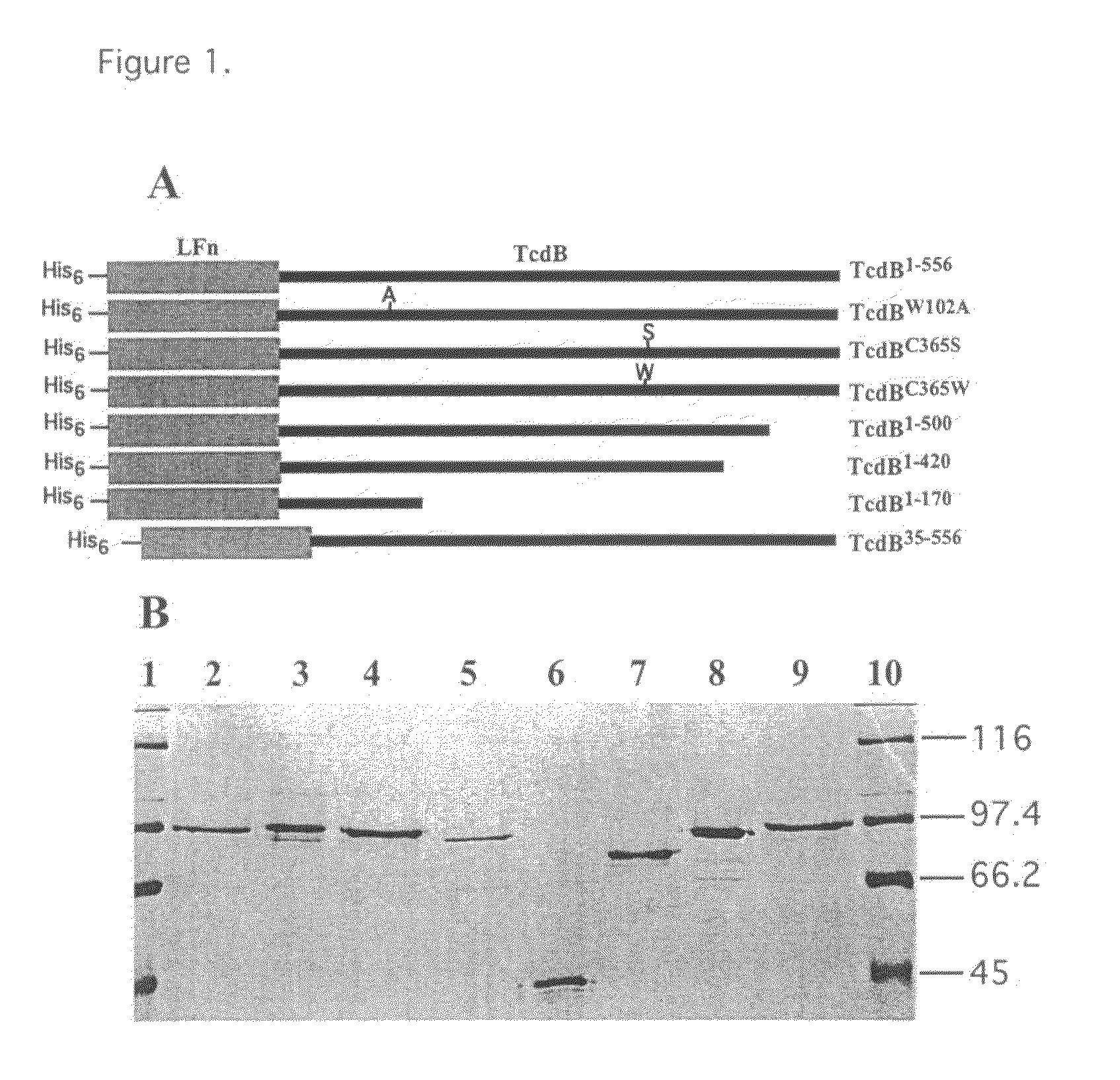
Mutants of clostridium difficile toxin B and methods of use
InactiveUS20080107673A1Antibacterial agentsAnimal cellsHospitalized patientsClostridium difficile infections
An active, or passive vaccine utilizing purified non-toxic mutant TcdB toxins from Clostridium difficile for humans and animals against infections caused by C. difficile and / or C. sordellii. Persons most potentially affected by C. difficile infections include hospitalized patients, infants, and elderly persons. The TcdB toxin mutant of the vaccine preferably lacks the toxicity of a native C. difficile TcdB toxin. A serum comprising antibodies raised to the TcdB toxin mutant is also available for treating humans or animals against C. difficile infections. The serum may be used in a method for conferring passive immunity against C. difficile. Antibodies to the TcdB toxin mutant may be used in diagnostic tests or in treatments to clear TcdB toxin from bodily fluids. The mutant TcdB toxin may be produced by recombinant methods using cDNA encoding the toxin, the cDNA contained for example in a plasmid or host cell.
Owner:BALLARD JIMMY D +1
Antibiotic Formulations Providing Reduced Gastrointestinal Side Effects and Clostridium difficile Infection Relapse, and Related Methods
InactiveUS20110240512A1Antibacterial agentsSmall article dispensingClostridial infectionAntibiotic-associated diarrhoea
The invention includes a formulation comprising: (i) a therapeutically effective amount of at least one antibiotic; and (ii) a therapeutically effective amount of at least one probiotic material. The formulation is prepared in a dosage form for delivery to the gastrointestinal tract. The invention further includes a method of preventing or minimizing the proliferation of Clostridium difficile in the gastrointestinal tract of a mammal undergoing an antibiotic therapy comprising by administration of the formulation of the invention for the duration of the antibiotic therapy; methods of preventing or minimizing the occurrence of antibiotic-associated diarrhea in a mammal undergoing an antibiotic therapy that includes administering the formulation; and methods of preventing or minimizing the occurrence of antibiotic-associated colitis or pseudomembranous colitis in a patient undergoing an antibiotic therapy that includes administering the formulation.Also included are methods of reducing or preventing a failure of treatment for an antibiotic-treatable infection in a patient comprising orally administering the formulation.Described by the invention are formulations for the treatment of a C. difficile infection comprising: (i) a therapeutically effective amount of at least one antibiotic; and (ii) a therapeutically effective amount of at least one probiotic material. The antibiotic includes a non-systemic gram negative antibiotic (such as, for example, fodaximicin) and formulation is prepared in a dosage form for delivery to the gastrointestinal tract.Also included are methods of treatment of a C. difficile infection in mammal that include administering to the digestive tract of the mammal the above described formulation. Such methods provide that the likelihood that the mammal shall experience a reoccurrence of the C. difficile infection is 70% or less.
Owner:TECOPPA BIOPHARMA
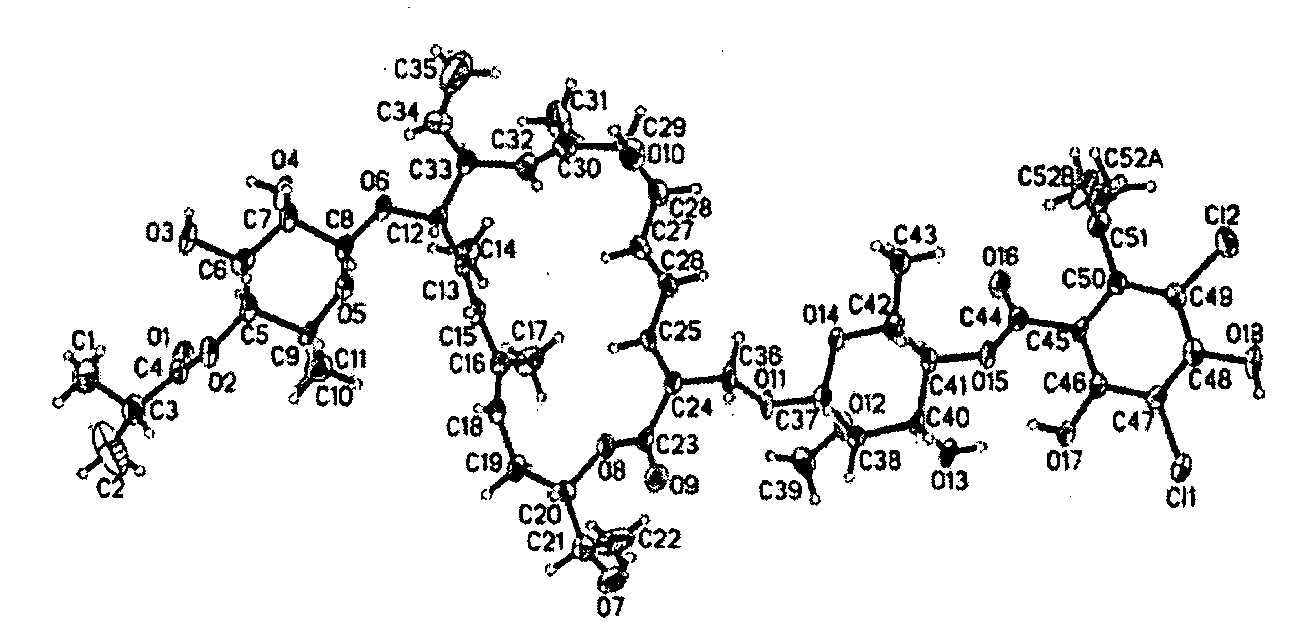
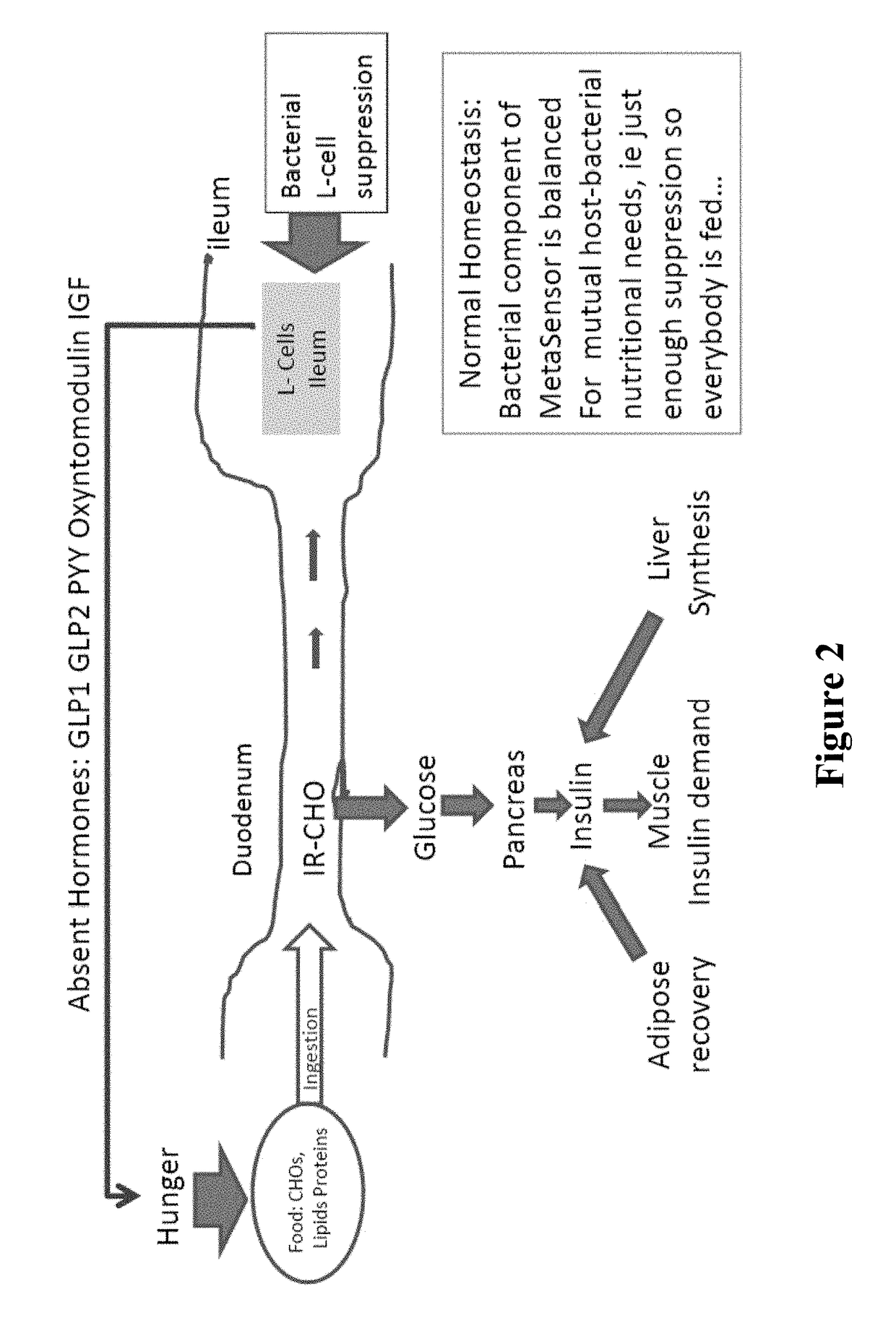
18-Membered macrocycles and analogs thereof
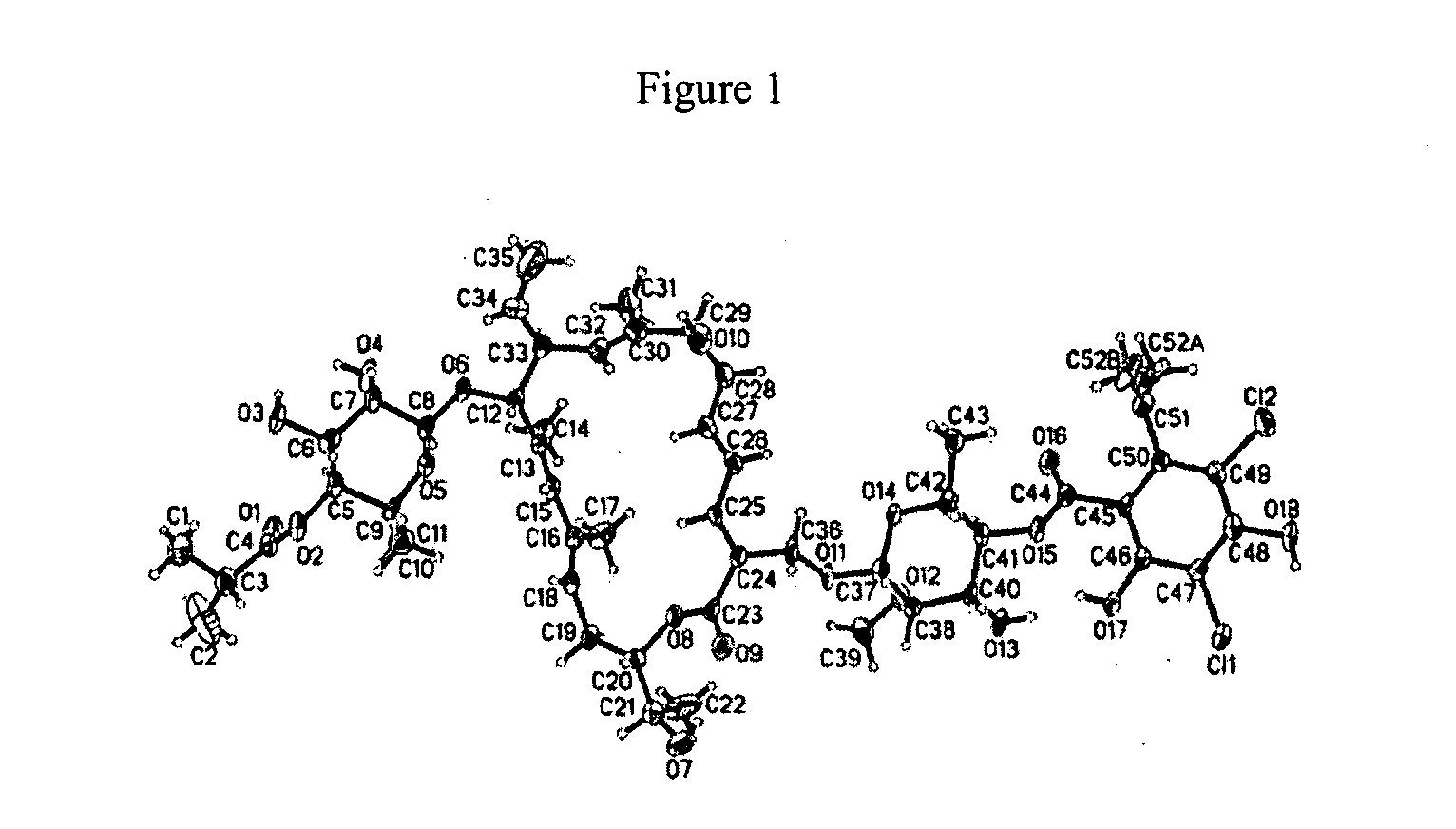
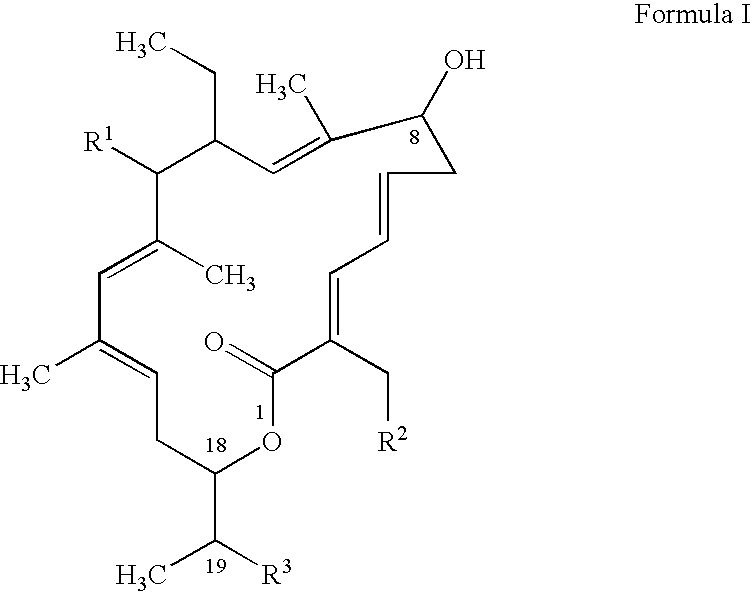
ActiveUS20080269145A1Low MIC valueHigh activityAntibacterial agentsBiocideClostridium difficile infectionsMedicine
The present invention relates generally to the 18-membered macrocyclic antimicrobial agents called Tiacumicins, specifically, OPT-80 (which is composed almost entirely of the R-Tiacumicin B), pharmaceutical compositions comprising OPT-80, and methods using OPT-80. In particular, this compound is a potent drug for the treatment of bacterial infections, specifically C. difficile infections.
Owner:MERCK SHARP & DOHME LLC
Test paper strip for detecting clostridium difficile toxin A and toxin B colloidal gold, method for making same and applications
ActiveCN101363867ASave manpower and material resourcesThe result is clear and easy to distinguishMaterial analysisCelluloseClostridium difficile infections
The invention provides a test strip for rapid detection of clostridium difficile toxins A and B. A monoclonal antibody or polyclonal antibody of the clostridium difficile toxin A, a monoclonal antibody or polyclonal antibody of the clostridium difficile toxin B, and a quality control double-antibody IgG coat a nitrate cellulose film (NC film), and a membrane chromatography double antibody sandwich method is adopted to detect the clostridium difficile toxins A and B in a specimen in combination with a monoclonal antibody of colloidal gold labeled clostridium difficile toxins A and B. The test strip is simple in operation, convenient, and fast, and has the advantages of no requirements of special instruments and special training, clear and identified result, and easy popularization. The test strip is suitable for base course, site detection and epidemiological investigation, and has auxiliary effect on the diagnosis of clostridium difficile toxin infection.
Owner:辽宁迪浩生物科技有限公司
Application of natural faeces flora transplanting in intestinal disease treatment
InactiveCN105193858ANo drug resistanceNo side effectsDigestive systemUnknown materialsSide effectFeces
Provided is an application of natural faeces flora transplanting in intestinal disease treatment. Faeces of healthy people screened through multiple conditions is collected, intestinal canal mixed probiotic flora is collected through the steps of homogenating, suspending, diluting, filtering, centrifuging and the like and transplanted in the ways of a gastroscope, an enteroscope, a nose-jejunum tube, a fistulization opening, clysis and the like, flora balance in the intestinal canal is restored, and the effect of treating intestinal diseases is achieved. A method for treating bacteria with bacteria is adopted, the stability of the intestinal flora of a human body is maintained, a microecological treating method free of drug resistance and toxic and side effects is achieved, treatment is carried out in the mode of faeces bacterium capsules or bacterium liquid, and a good treatment effect is achieved on diarrhea, clostridium difficile infection, Crohn's diseases and the like.
Owner:NANCHANG UNIV
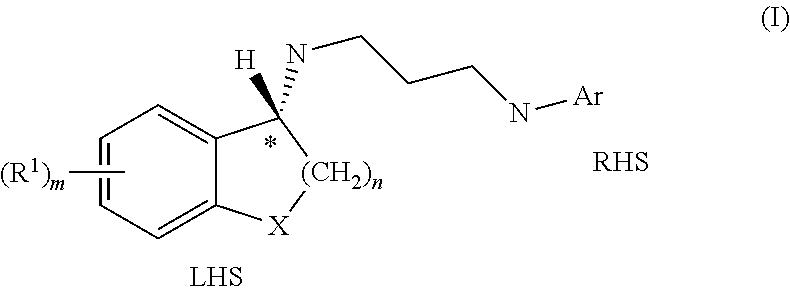
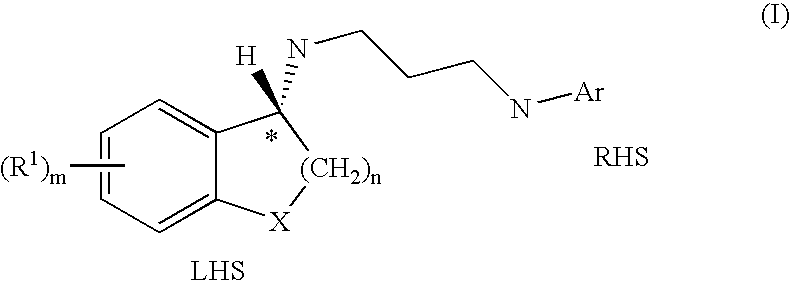
Selective antibacterials for clostridium difficile infections
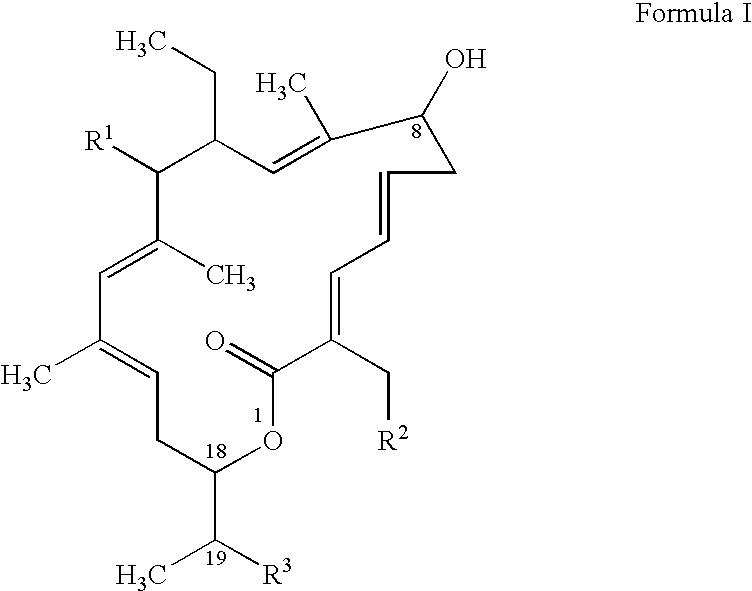
ActiveUS8796292B2Reduce the possibilityHigh activityAntibacterial agentsBiocideDiseaseClostridium difficile infections
The invention features compounds of formula (I): The compounds are useful as antibacterial agents, especially again Clostridium difficile-associated diseases.
Owner:ACURX PHARMA LLC
Method of prevention and treatment of clostridium difficile infection
InactiveUS20140112985A1Improve developmentGrowth inhibitionAntibacterial agentsSurgical needlesClostridial infectionMicroorganism
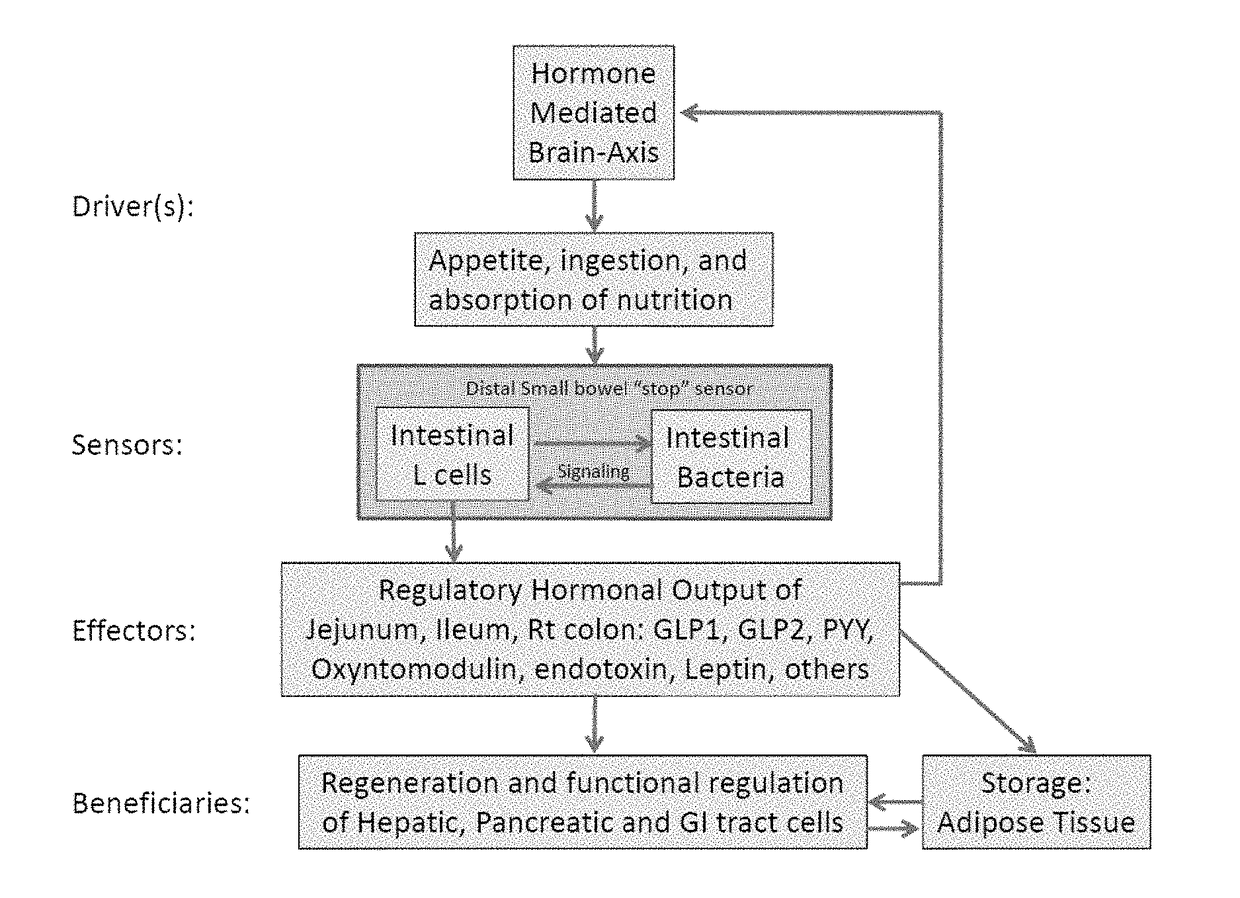
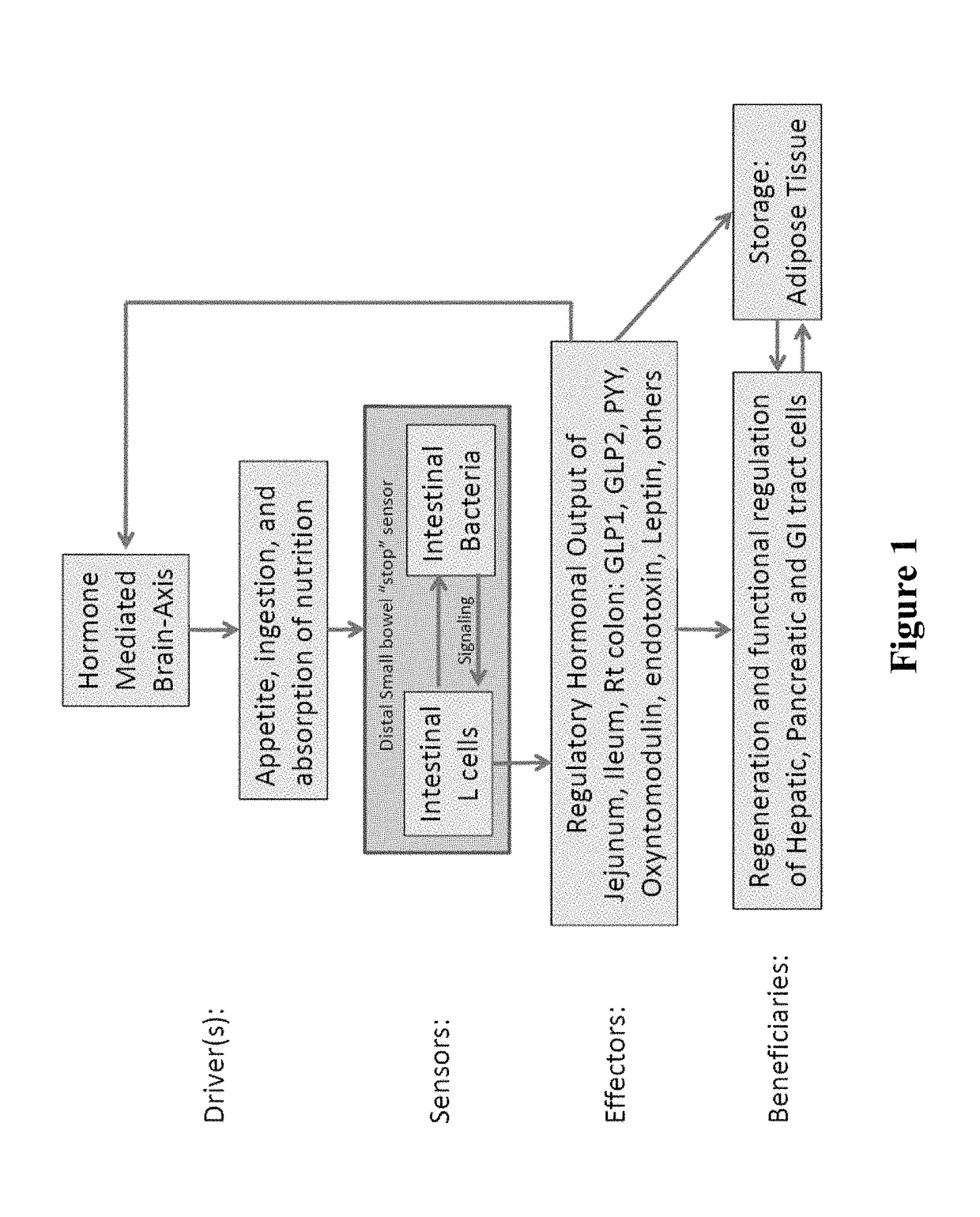
This invention relates to prophylactic and / or therapeutic application of microorganism species that are, for example, administered orally as delayed release formulation designed to release its microbial content to the distal small intestine and / or colon in high quantities and density, which is a “normalized” approach to repopulate the colonic flora as a method of prevention and / or treatment of, for example, Clostridium difficile colitis.
Owner:POLONEZ THERAPEUTICS
Targeted gastrointestinal tract delivery of probiotic organisms and/or therapeutic agents
ActiveUS9907755B2Antibacterial agentsMetabolism disorderAntibiotic-associated diarrhoeaClostridium difficile infections
The present invention relates to the development of a targeted delivery system for the oral delivery of probiotics or therapeutic agent for various indications, including and not limited to active and prophylaxis treatment of Clostridium difficile infection, antibiotic associated diarrhea, irritable bowel syndrome, Crohn's disease, intestinal flora replacement, supplemental flora treatments for patients taking antibiotics, and for restoration of balance and signaling between the intestinal microbiome and the intestinal cells in patients under treatment of metabolic syndrome manifestations, specifically diabetes, insulin resistance, obesity, hyperlipidemia and hypertension.
Owner:THERABIOME
18-membered macrocycles and analogs thereof
The present invention relates generally to the 18-membered macrocyclic antimicrobial agents called Tiacumicins, specifically, OPT-80 (which is composed almost entirely of the R-Tiacumicin B), pharmaceutical compositions comprising OPT-80, and methods using OPT-80. In particular, this compound is a potent drug for the treatment of bacterial infections, specifically C. difficile infections.
Owner:MERCK SHARP & DOHME LLC
Vaccines against clostridium difficile comprising recombinant toxins
InactiveCN104039816ACan't greatly increase survivalReduce weightAntibacterial agentsBacterial antigen ingredientsClostridial toxinClostridium difficile infections
The present invention relates to recombinant C. difficile toxin A (TcdA) and toxin B (TcdB) and binary toxin A (CDTa) proteins comprising specifically defined mutations relative to the native toxin sequence that substantially reduce or eliminate toxicity. The invention also relates to vaccines and immunogenic compositions comprising these recombinant toxins, as well as combinations of these toxins with binary toxin B (CDTb), which are capable of providing protection against C. difficile infection and / or the effects thereof. The invention also relates to methods of inducing an immune response to C. difficile comprising administering the vaccines and immunogenic compositions described herein to a patient. The invention also encompasses methods of expressing recombinant C. difficile toxin A and toxin B and CDTa mutants and CDTb in recombinant expression systems. In exemplary embodiments, TcdA, TcdB, and CDTa mutant toxins comprising sufficient mutations to substantially reduce or eliminate toxicity are expressed in the baculovirus / insect cell expression system.
Owner:MERCK & CO INC +1
Methods and compositions for reducing clostridium difficile infection
InactiveUS20170087196A1Reduce riskReduce severityPeptide/protein ingredientsBacteria material medical ingredientsClostridial infectionClostridium difficile infections
The present invention relates to methods and compositions for reducing the risk and severity of C. difficile infection. It is based, at least in part, on the discovery that a restricted fraction of the gut microbiota, including the bacterium Clostridium scindens, contributes substantially to resistance against C. difficile infection. Without being bound by any particular theory, it is believed that this is achieved through the biosynthesis of secondary bile acids.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Targeted synbiotic therapy for dysbiosis-related intestinal and extra-intestinal disorders
InactiveUS20180110800A1Increase productionOrganic active ingredientsDigestive systemAntibiotic-associated diarrhoeaSynbiotics
Various types of synbiotic therapies are provided for the treatment of a variety of gastrointestinal and other disorders. The combination of prebiotics to probiotics is defined as a synbiotic therapy. The principal GI disorders associated with dysbiosis that can be treated from such a therapeutic intervention include but are not limited to: inflammatory bowel disease (IBD) (Crohn's disease and ulcerative colitis), irritable bowel syndrome (IBS), antibiotic-associated diarrheas such as recurrent Clostridium difficile infection, and possibly variants of Celiac disease. Other disorders that may also be ameliorated by the proposed synbiotic therapy include metabolic syndromes and central nervous system disorders. The disclosed methods and compositions were developed to improve upon currently available probiotics through consideration of the human intestinal microbiota, and its relationship to various intestinal metabolic and neuropsychiatric disorders. In one embodiment, the disclosed synbiotic compositions include prebiotics and a targeted delivery system, which altogether promote the survival, growth, and attachment of probiotic microbiota.
Owner:LIFEBRIDGE HEALTH INC
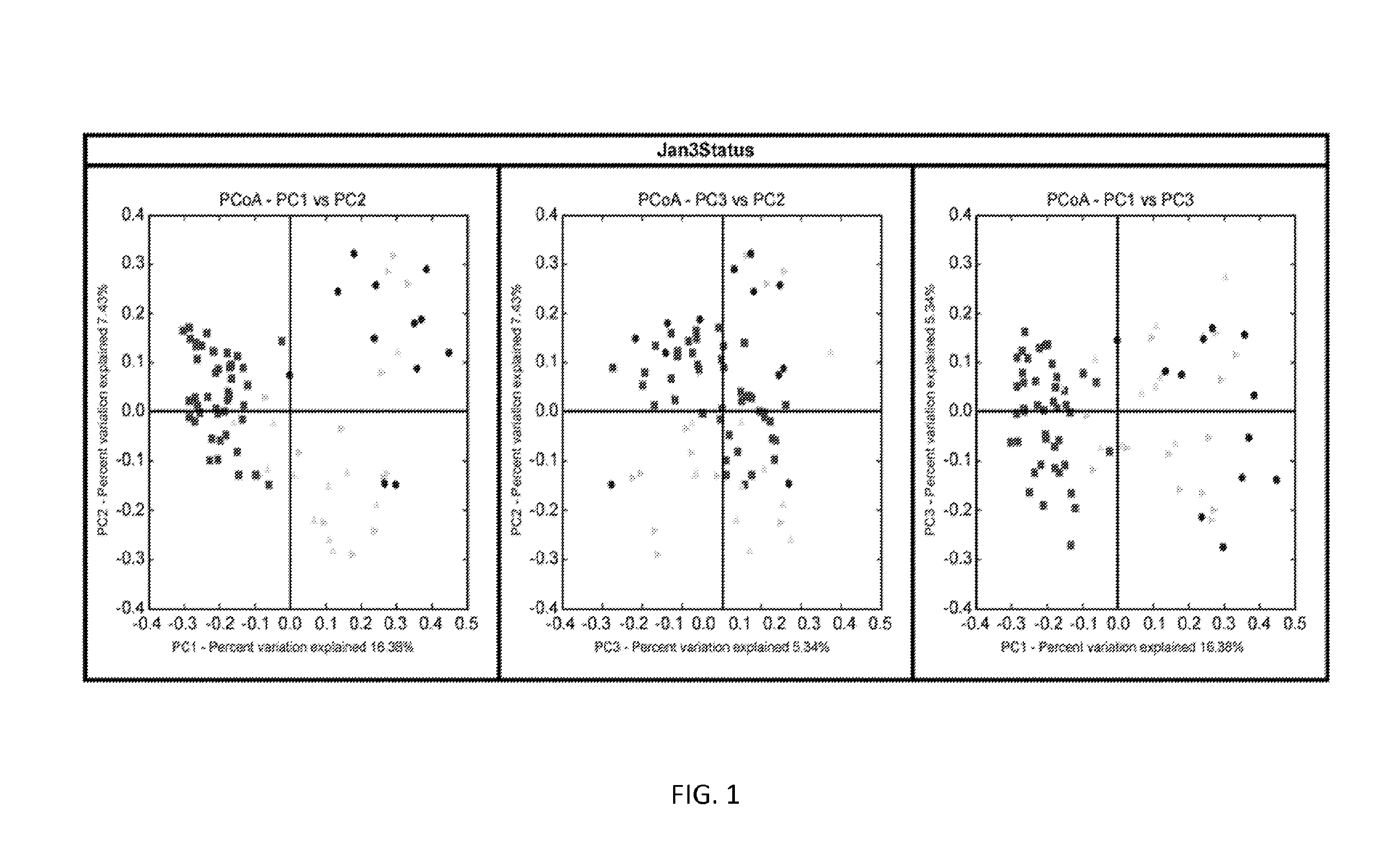
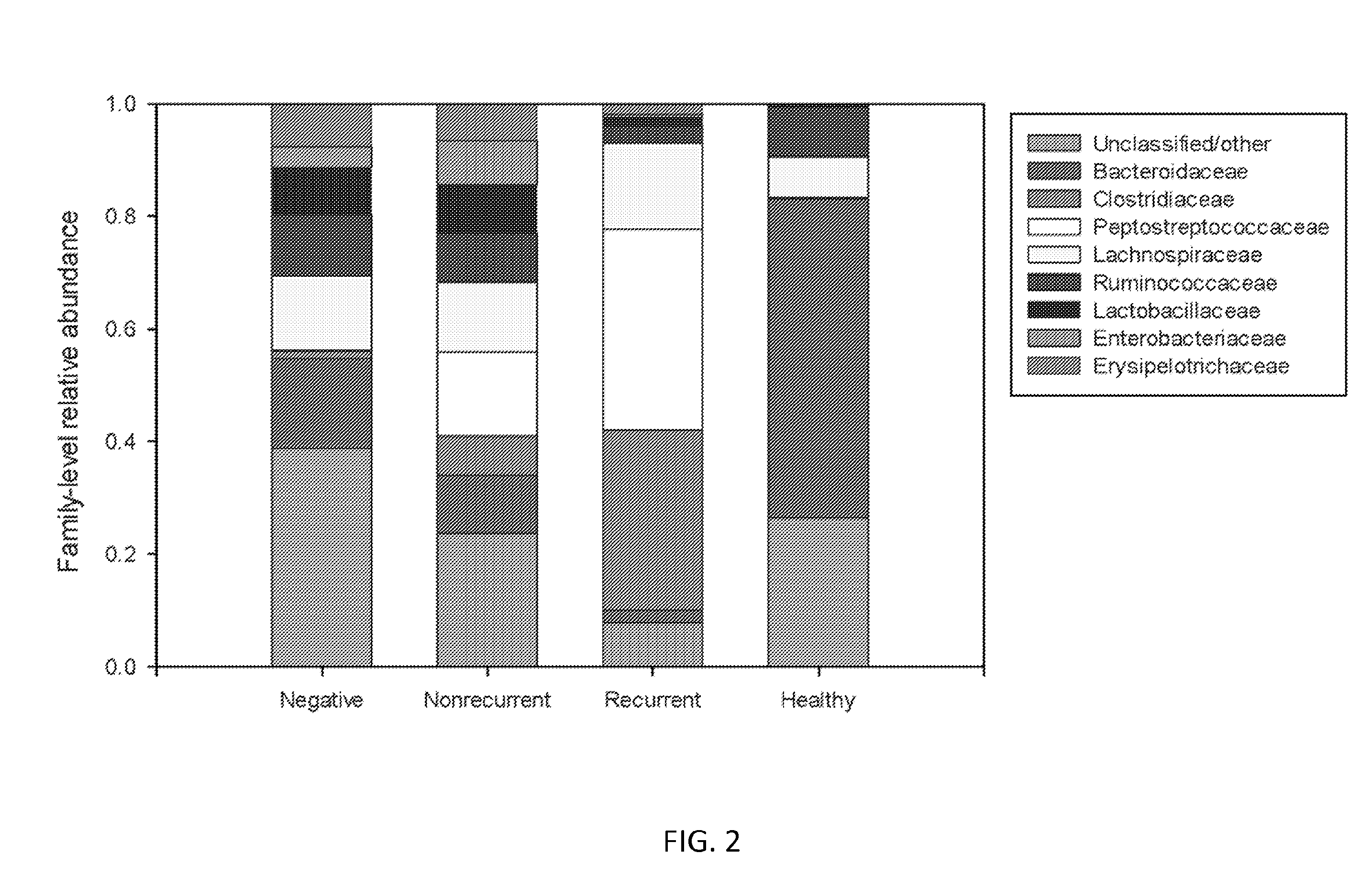
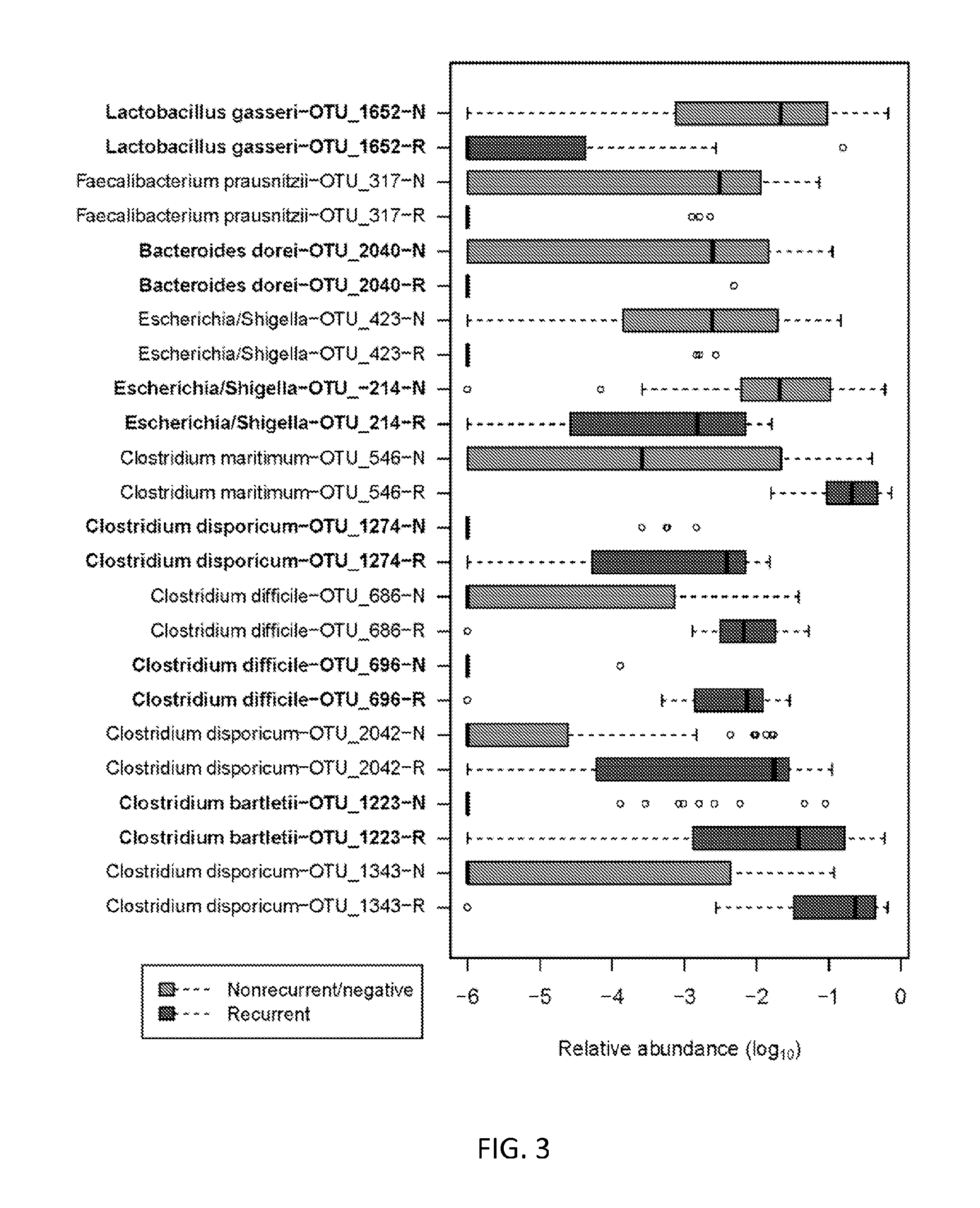
Biomarkers of recurrent clostridium difficile infection
ActiveUS20150344940A1Reduce usageBiocideMicrobiological testing/measurementMicroorganismClostridial infection
Embodiments of the invention include methods and / or compositions for analysis of samples for C. difficile infection to determine whether or not an individual is at risk for having recurrent C. difficile infection or a CDI misdiagnosis. Methods include characterization of microflora composition from the gut, wherein alterations of the microflora gut composition are indicative of recurrence of infection. Methods include analysis of nucleic acids from the gut, such as 16S rRNA as being identifying of a particular bacteria in the analysis of bacterial populations of the gut.
Owner:BAYLOR COLLEGE OF MEDICINE
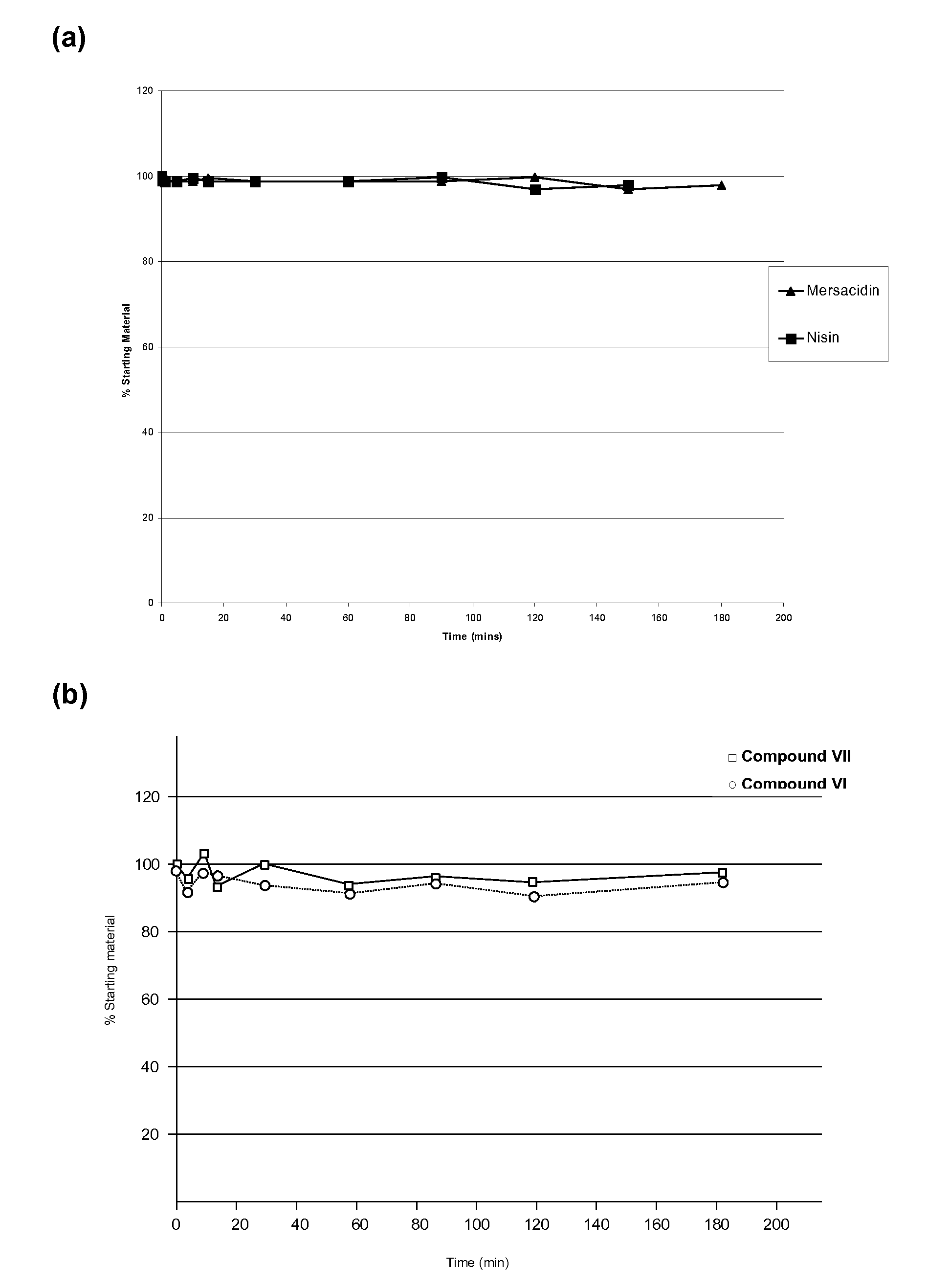
Use of Type-B Lantibiotic-Based Compounds having Antimicrobial Activity
InactiveUS20090203583A1Allow flexibilitySimple recipeAntibacterial agentsBiocideIntestinal structureMicroorganism
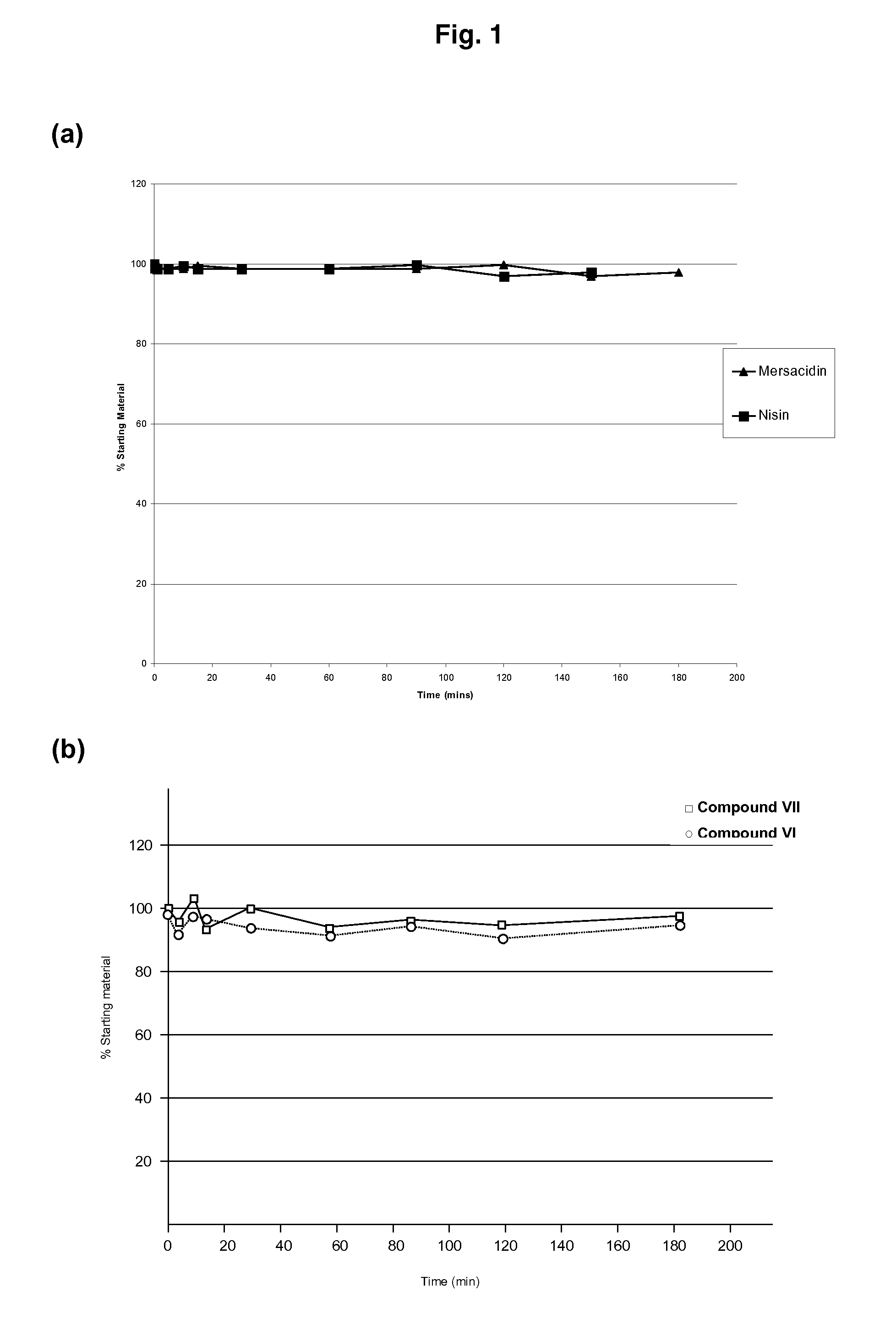
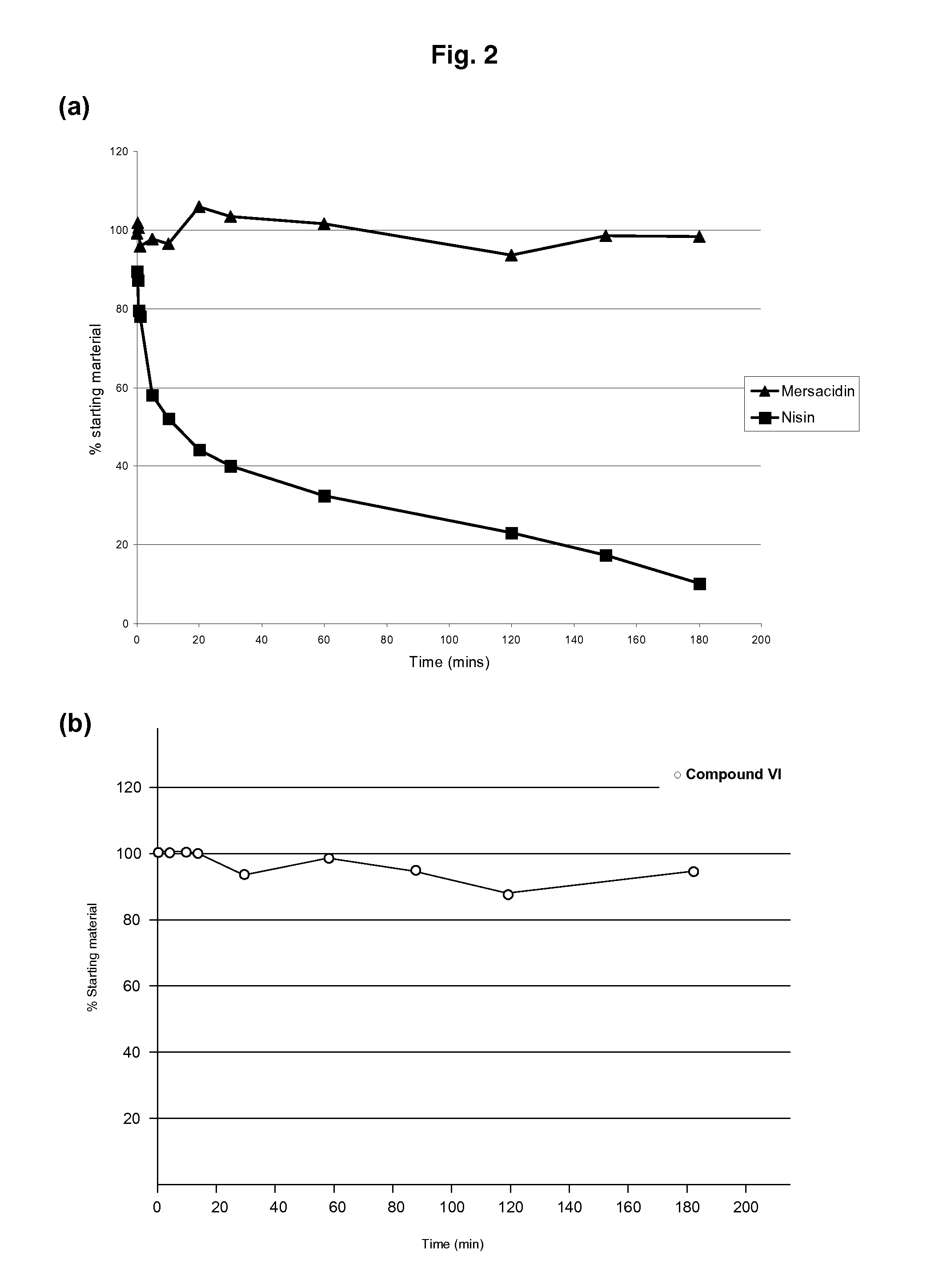
The present invention provides methods for the treatment or prophylaxis of a microbial infection of the lower intestine or colon in a subject, the method comprising administering to the subject a type-B lantibiotic. In particular, the invention provides methods for the treatment or prophylaxis of a Clostridium difficile infection. The type-B lantibiotics may include compounds selected from the group consisting of mersacidin, actagardine, plantaricin, planosporicin, ruminococcin, antibiotic 10789, michiganin and haloduracin, and derivatives and variants thereof.
Owner:NOVACTA BIOSYSTEMS LTD
Methods and uses for metabolic profiling for clostridium difficile infection
ActiveUS20140296134A1Excellent disease classificationBiocideMicrobiological testing/measurementClostridium difficile infectionsMetabolic profile
Embodiments include methods for generating a metabolite profile of a stool sample and methods of assessing the status of a subject using the metabolic profile derived from a stool sample.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Compositions and methods for prophylaxis and therapy of clostridium difficile infection
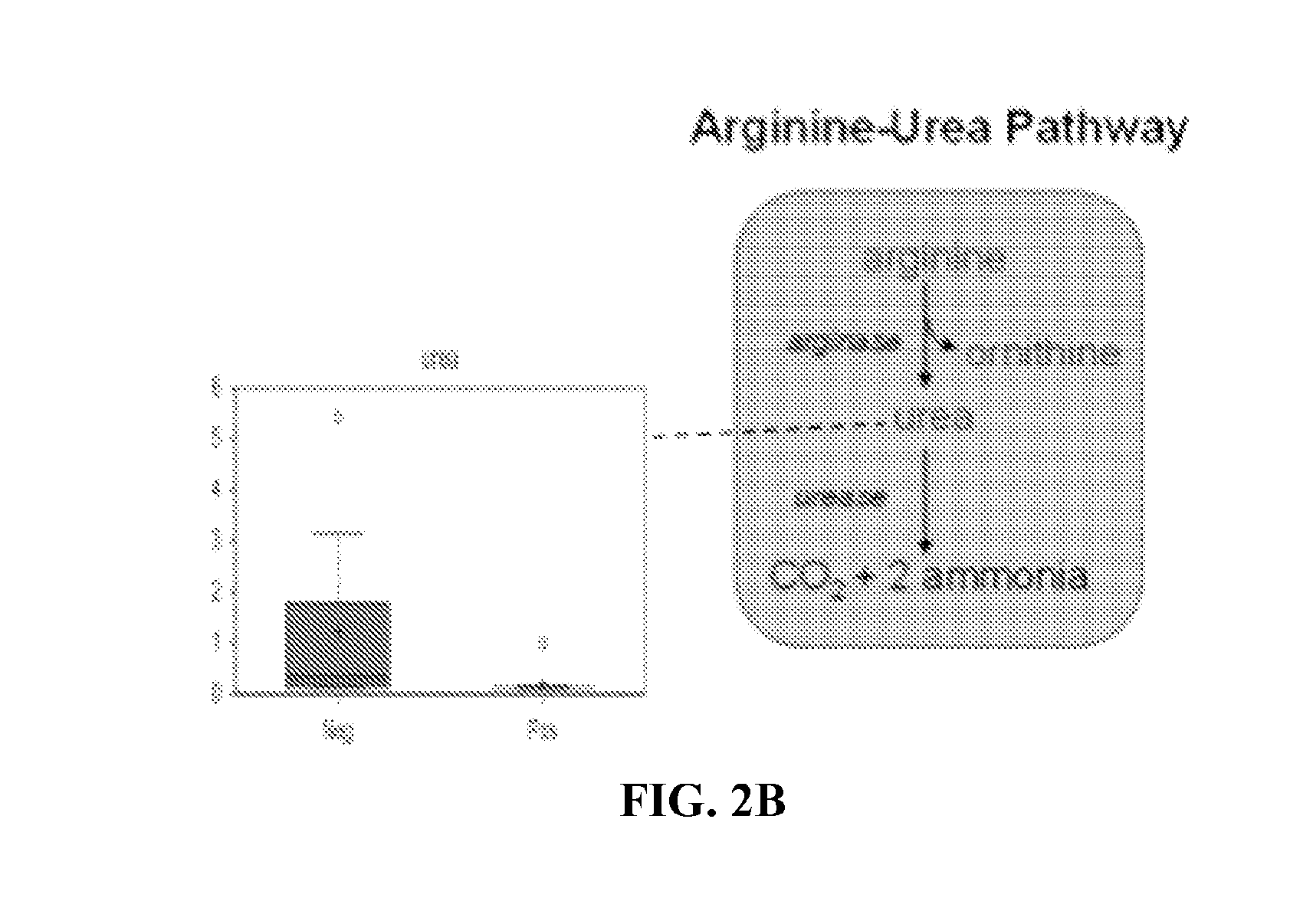
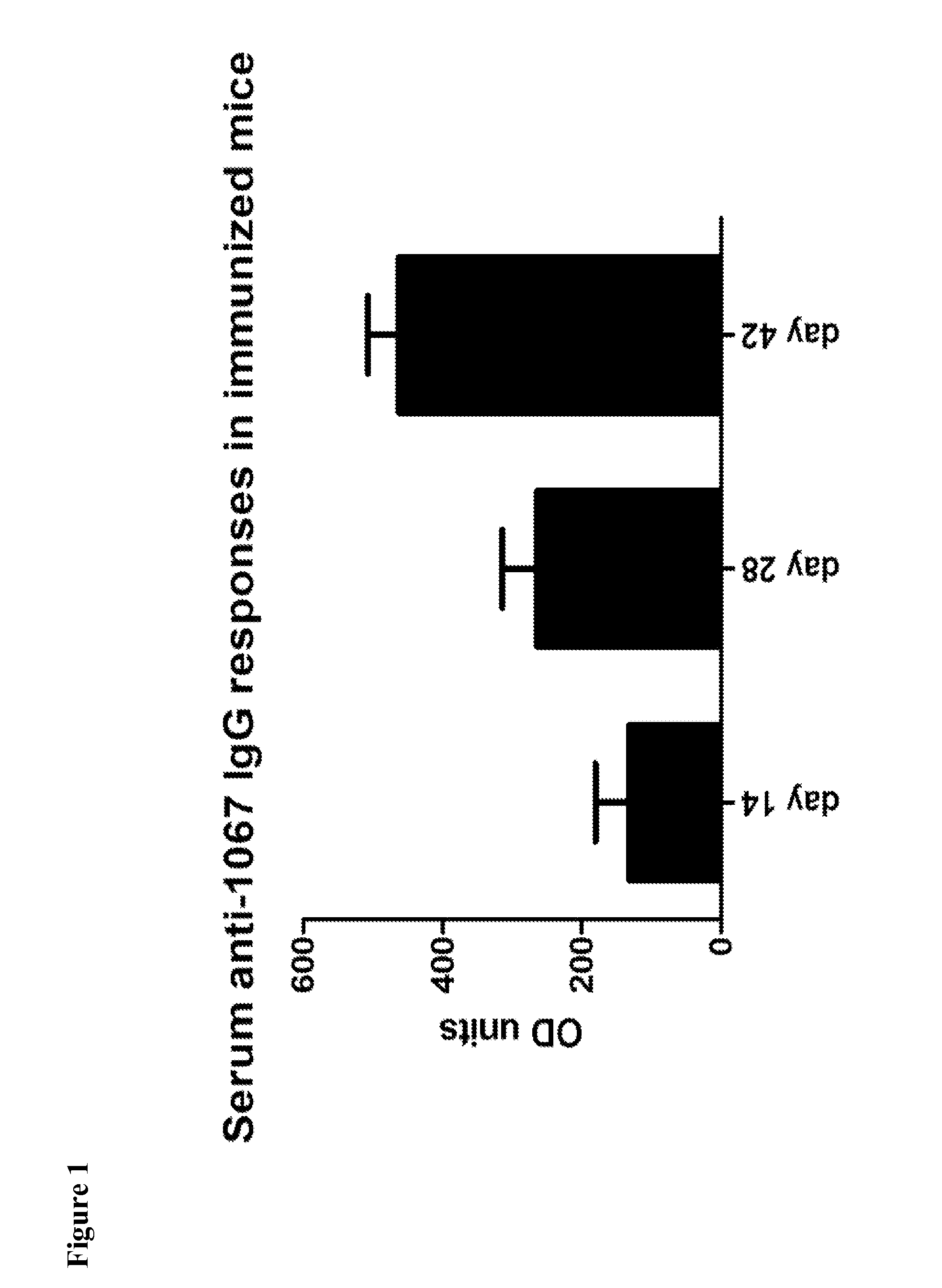
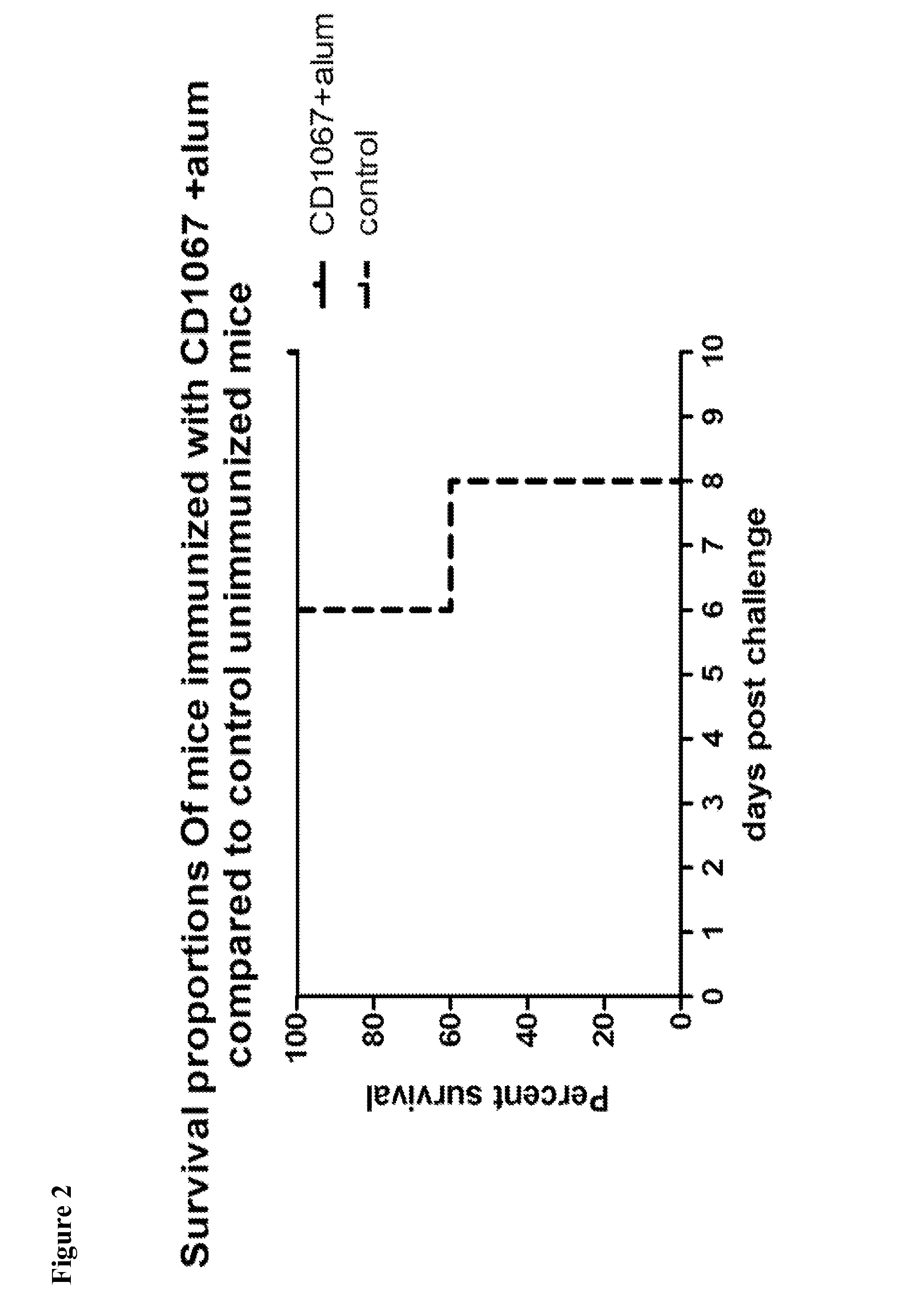
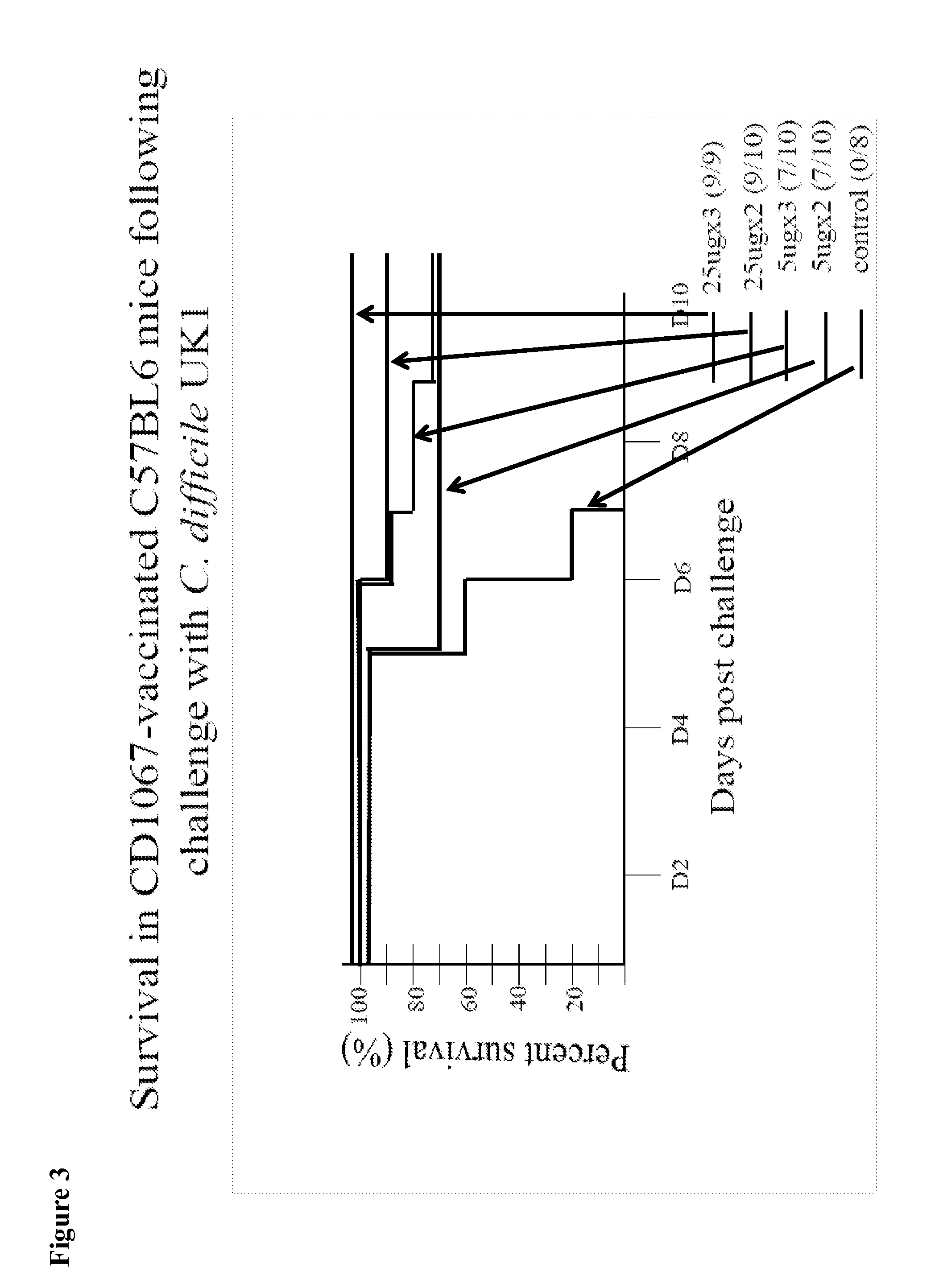
InactiveUS20160250283A1Avoid infectionNone of approaches is suitableBacterial antigen ingredientsPeptide/protein ingredientsSporeClostridium difficile infections
Provided are compositions and methods for prophylaxis and / or therapy of C. difficile infection, and for inhibiting dissemination of C. difficile spores. The compositions contain C. difficile proteins, including distinct proteins and fusions of C. difficile CD 1067, BclA1, SleC, CotA, Spl7, FliC, FliD, CD toxin A, CD toxin B, and combinations thereof. The methods include prophylaxis and / or therapy of C. difficile infection by administering to a subject in need a composition that includes the C. difficile protein(s).
Owner:THE ROCKEFELLER UNIV
Methods and compositions for reducing clostridium difficile infection
ActiveUS20190381113A1Lessen risk of and severityReduce riskPeptide/protein ingredientsBacteria material medical ingredientsClostridial infectionBacteroides
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Enantiomeric compounds with antibacterial activity
ActiveUS7973050B2Antibacterial agentsBiocideClostridium difficile infectionsClostridium difficile (bacteria)
Novel compounds in enantiomeric excess that are inhibitors of bacterial methionyl synthetase (MetRS) are disclosed. Also disclosed are methods for their preparation and their use in therapy as antibacterial agents, and in particular their use in therapy for Clostridium difficile infection.
Owner:REPLIDYNE
Methods and compositions for the treatment and/or prophylaxis of clostridium difficile associated disease
The present invention relates to methods and compositions for the treatment and / or prophylaxis of Clostridium difficile associated disease (CD AD). In particular, the invention relates to antibodies that bind to C. difficile antigens and are capable of inhibiting C. difficile infection, at least one symptom of C. difficile associated disease, shedding of C. difficile, and C. difficile associated mortality. The compositions of the present application comprise: mammalian or avian antibodies which bind to a C. difficile Toxin B; and mammalian or avian antibodies that bind to a C. difficile vegetative cell antigen and / or a C. difficile endospore antigen.
Owner:IMMURON
Enantiomeric Compounds With Antibacterial Activity
Novel compounds in enantiomeric excess that are inhibitors of bacterial methionyl synthetase (MetRS) are disclosed. Also disclosed are methods for their preparation and their use in therapy as antibacterial agents, and in particular their use in therapy for Clostridium difficile infection.
Owner:REPLIDYNE
Beta-lactamases with improved properties for therapy
ActiveUS20150297689A1Avoid disruptionOrganic active ingredientsAnimal cellsDiseaseClostridium difficile infections
This invention relates to, in part, compositions of beta-lactamases and methods of using these enzymes in, for example, gastrointestinal tract (GI tract) disorders such as C. difficile infection (CDI).
Owner:SYNTHETIC BIOLOGICS INC
Fluorescent quantitative PCR detection kit for clostridium difficile toxin A/B and detection method
InactiveCN105525023AImprove responseOptimizationMicrobiological testing/measurementDNA/RNA fragmentationClostridial toxinClostridium difficile infections
The invention discloses a fluorescent quantitative PCR detection kit for clostridium difficile toxin A / B by adopting a probe method, and a detection method, which can detect clostridium difficile and also can detect and quantify toxin A and B genes. The detection kit mainly comprises specific primers and probes, wherein the specific primers and the probes are composed of primer pairs for detecting clostridium difficile, primer pairs and probes for detecting clostridium difficile toxin A, as well as primer pairs and probes for detecting clostridium difficile toxin B. According to the detection kit and the detection method, the operation is simple, the report time is short, the clostridium difficile strains can be specifically, sensitively and accurately identified for the first time, and the key toxins A and B of clostridium difficile are detected and quantified. A foundation is laid for the early diagnosis for clostridium difficile infection, and the detection kit and the detection method are suitable for screening causes of diarrhea patients with unknown causes clinically.
Owner:GUANGZHOU SAGENE BIOTECH
Human Antibodies to Clostridium difficile Toxins
ActiveUS20130230531A1Reduce severityReduce recurrenceAntibacterial agentsDigestive systemClostridium difficile toxin BClostridium difficile infections
The present invention provides fully human antibodies that bind to either toxin A or toxin B of Clostridium difficile, or to both toxin A and toxin B, compositions comprising the antibodies and methods of use. The antibodies of the invention are useful for neutralizing the toxins from C. difficile, thus providing a means of treating the disease and symptoms associated with a C. difficile infection, including the treatment of diarrhea, or pseudomembranous colitis caused by C. difficile. The antibodies may also prevent the severity and / or duration of the primary disease, or may prevent the number, duration, and / or the severity of recurrences, or relapses of the disease attributed to the presence of C. difficile. The antibodies of the invention may also be useful for diagnosis of an infection by C. difficile.
Owner:REGENERON PHARM INC
Inositol hexakisphosphate analogs and uses thereof
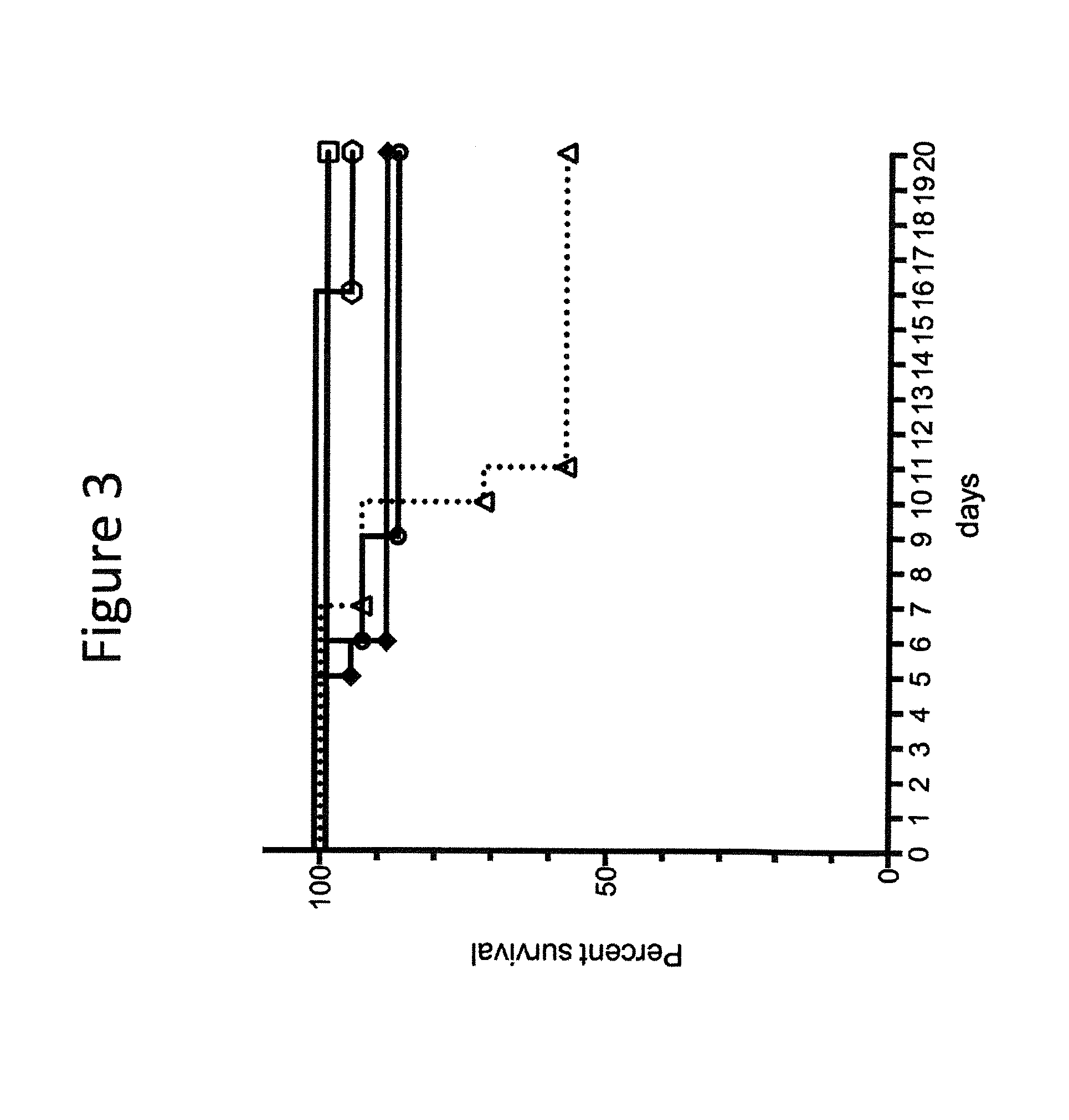
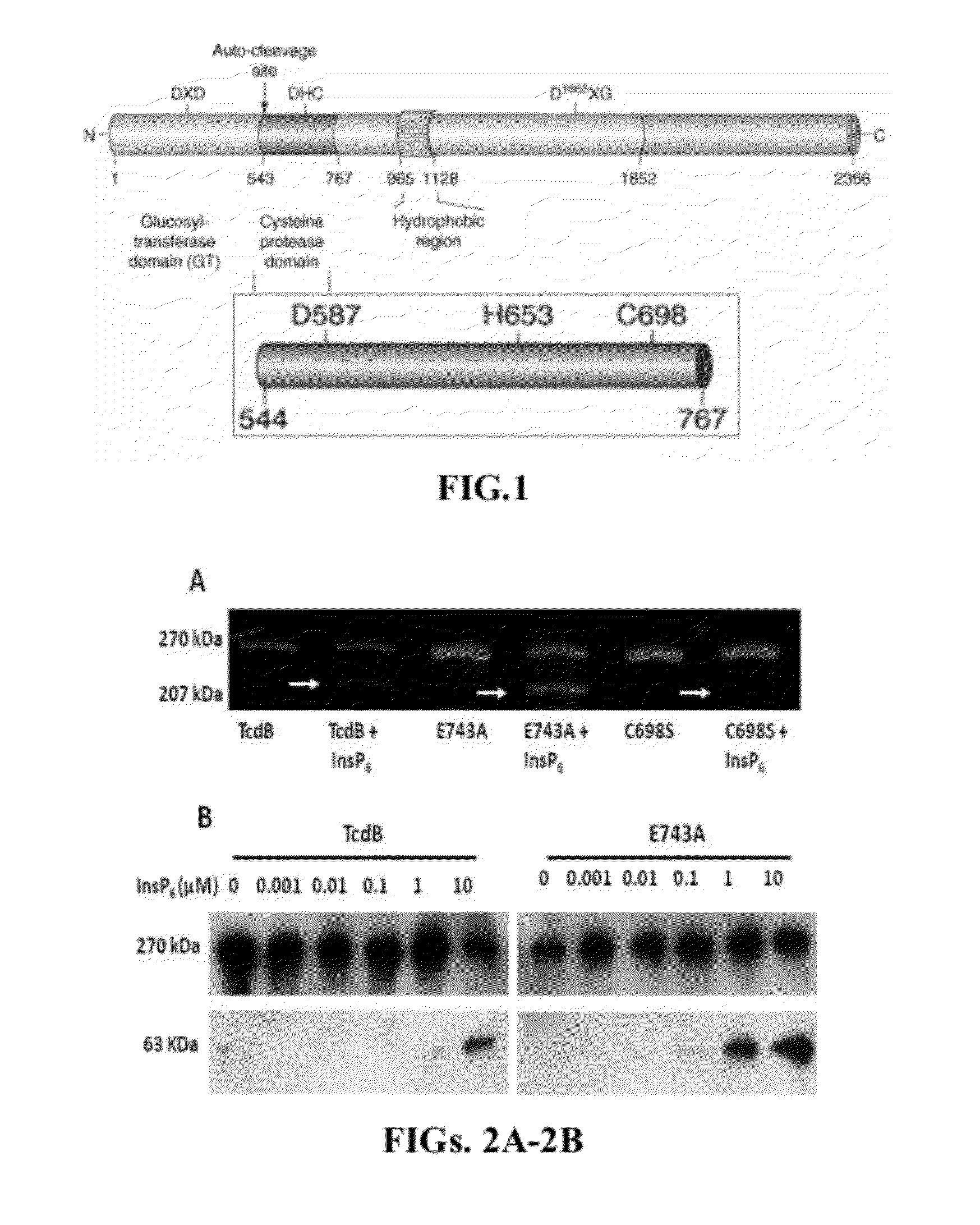
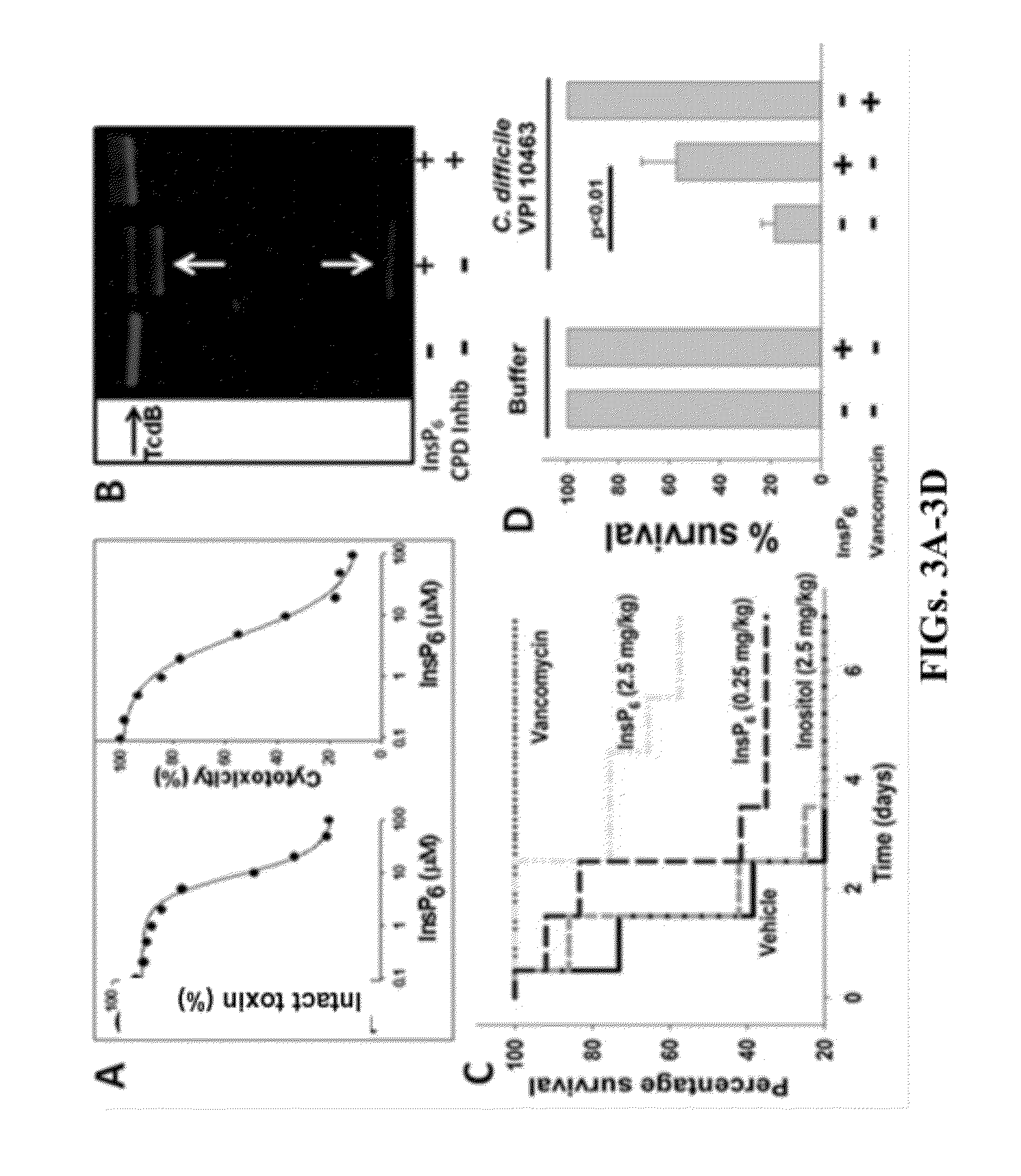
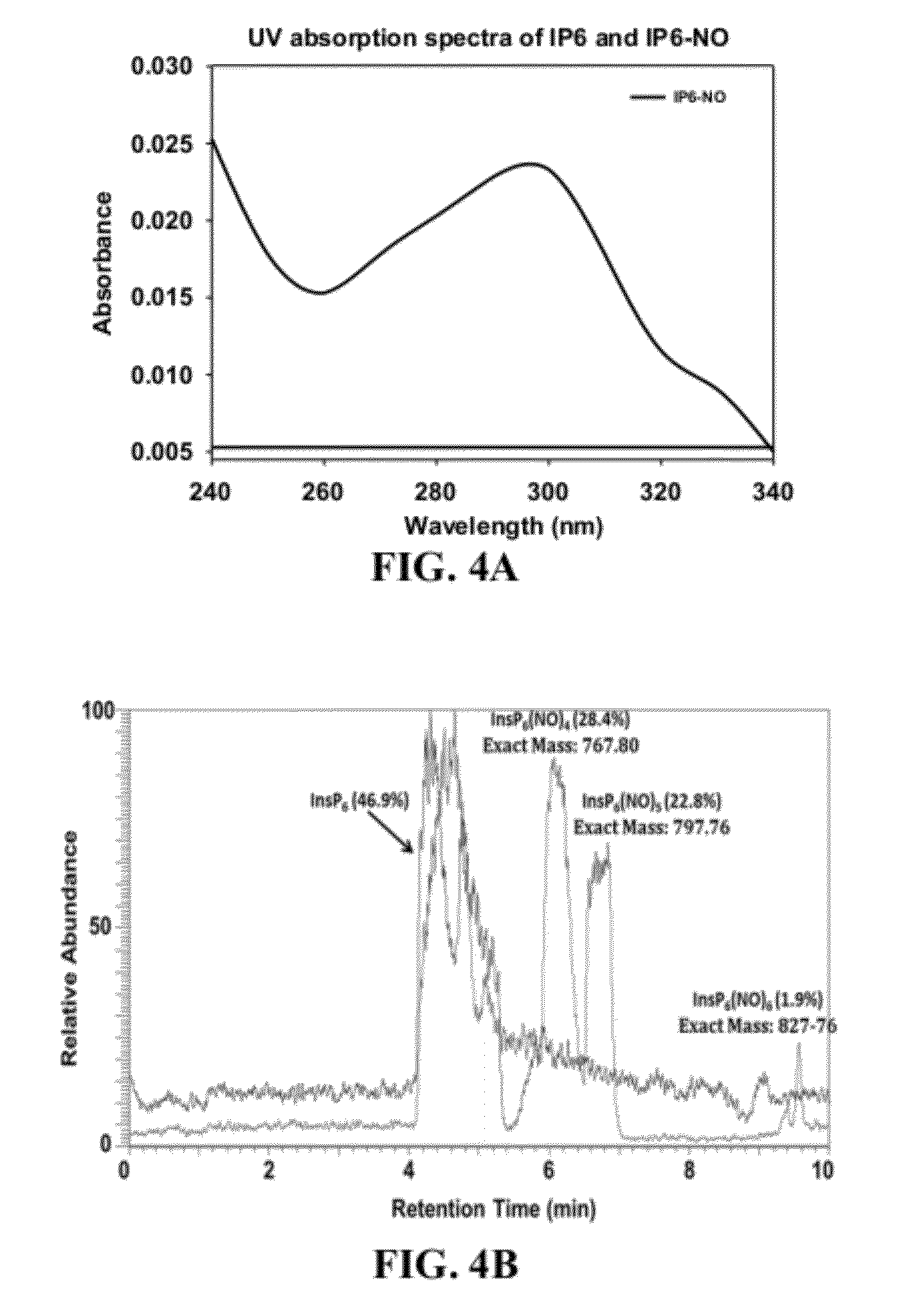
Provided herein are analog and derivative compounds of inositol hexakisphosphate effective to treat a Clostridium difficile infection and to neutralize the bacterial toxins produced by the same. In addition, methods of treating the C. difficile infection and for neutralizing its toxins with the compounds are provided.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com