Mutants of clostridium difficile toxin B and methods of use
a technology of clostridium difficile and toxin b, which is applied in the field of mutants of clostridium difficile toxin b and methods of use, can solve the problems of no techniques for blocking intracellular virulence factors, and no techniques which utilize inactive mutants derived from toxins in order
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
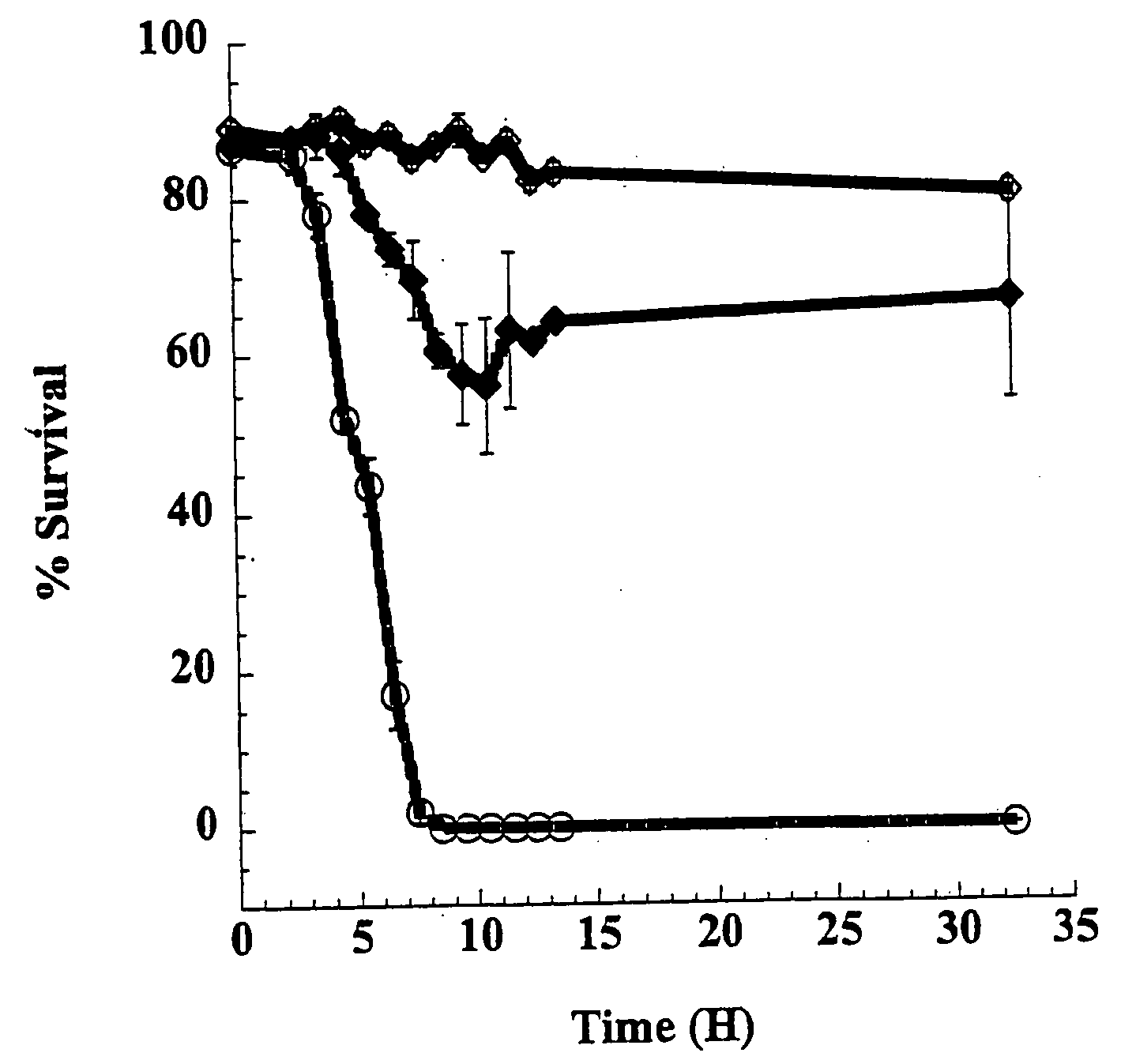
[0051]During analysis of the TcdB enzymatic domain a set of mutants were identifiable which were unable to modify substrate, yet were capable of blocking TcdB cytopathic effects. Herein are described generation and analyses of these mutants and the demonstration that these proteins are potent intracellular inhibitors of TcdB and block glucosylation of a previously undescribed target. These mutants show, for the first time, that a toxin derivative can be used to effectively block the activity of the native toxin within the cell. This inhibitory activity also suggests a new paradigm for a therapeutic approach to treat toxin-based diseases.
Enzymatic and Cytopathic Activity of Mutants
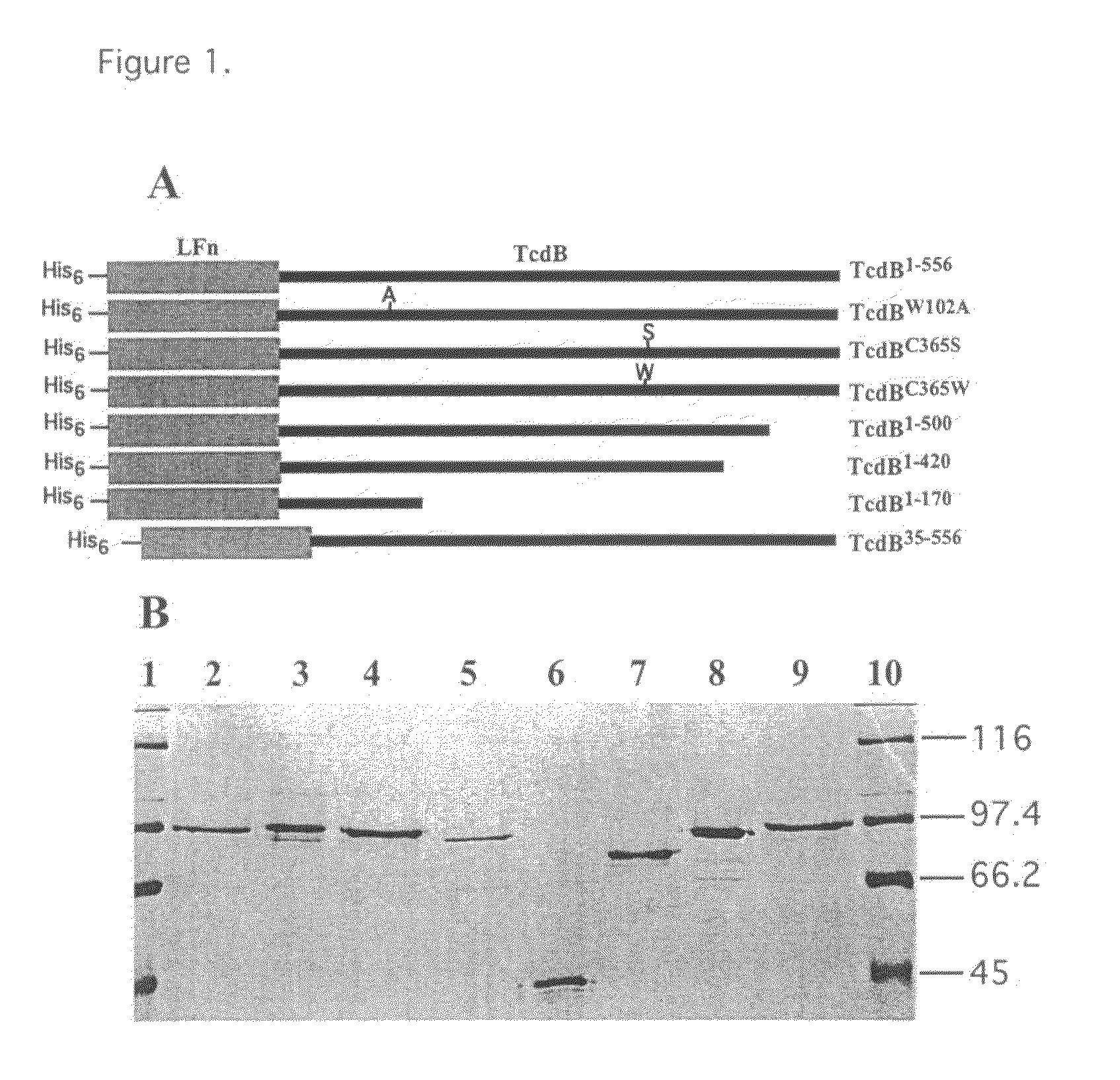
[0052]As summarized in FIG. 1, 4 deletion and 3 site-directed mutants in the TcdB enzymatic domain were constructed, cloned and isolated from E. coli. The nomenclature for each of these mutants is summarized in panel A of FIG. 1. One site-directed mutant, TcdBW102A wherein the tryptophan at position 102 is ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com