Patents
Literature
99 results about "Passive immunity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
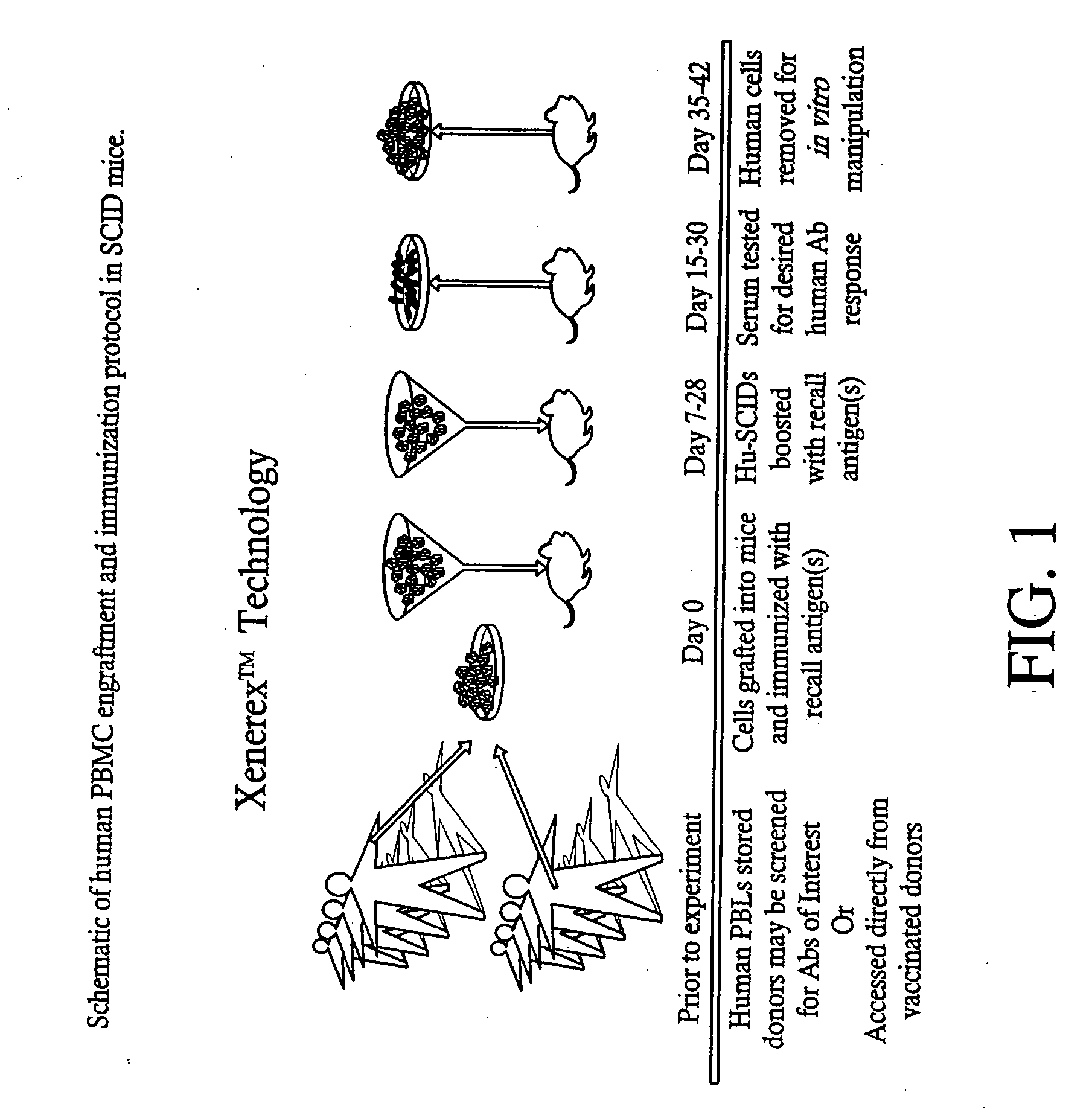
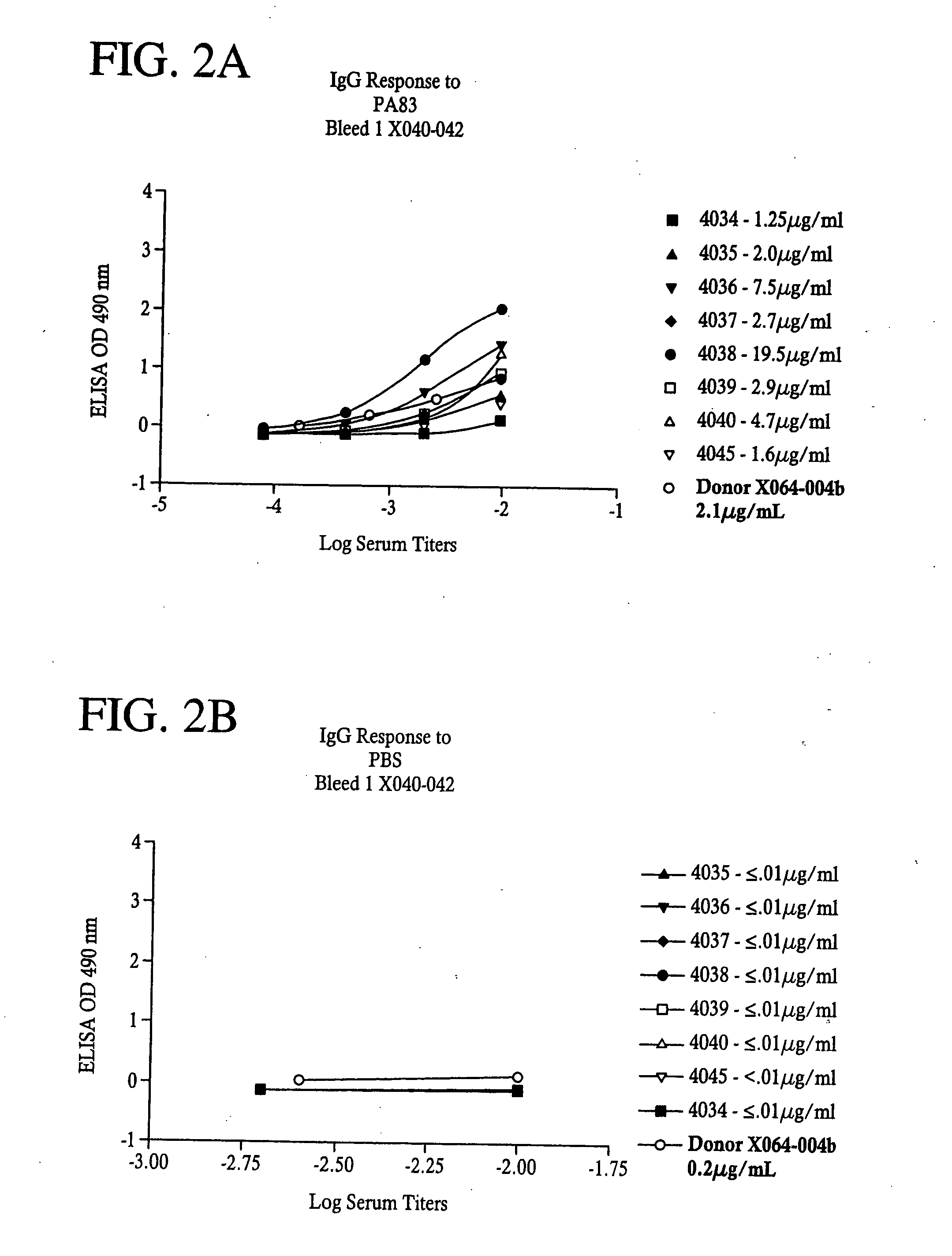
Passive immunity is the transfer of active humoral immunity of ready-made antibodies. Passive immunity can occur naturally, when maternal antibodies are transferred to the fetus through the placenta, and it can also be induced artificially, when high levels of antibodies specific to a pathogen or toxin (obtained from humans, horses, or other animals) are transferred to non-immune persons through blood products that contain antibodies, such as in immunoglobulin therapy or antiserum therapy. Passive immunization is used when there is a high risk of infection and insufficient time for the body to develop its own immune response, or to reduce the symptoms of ongoing or immunosuppressive diseases. Passive immunization can be provided when people cannot synthesize antibodies, and when they have been exposed to a disease that they do not have immunity against.
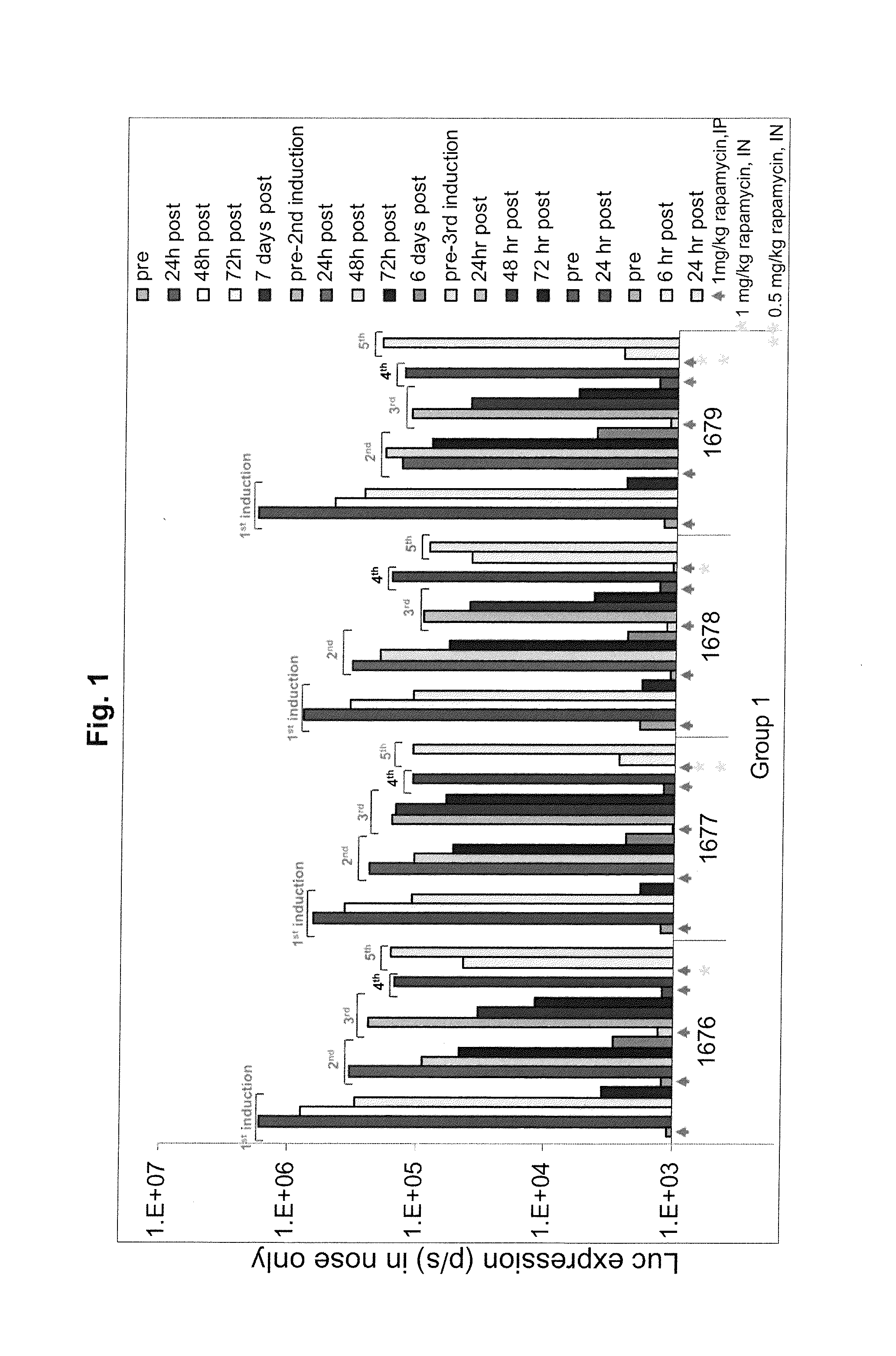
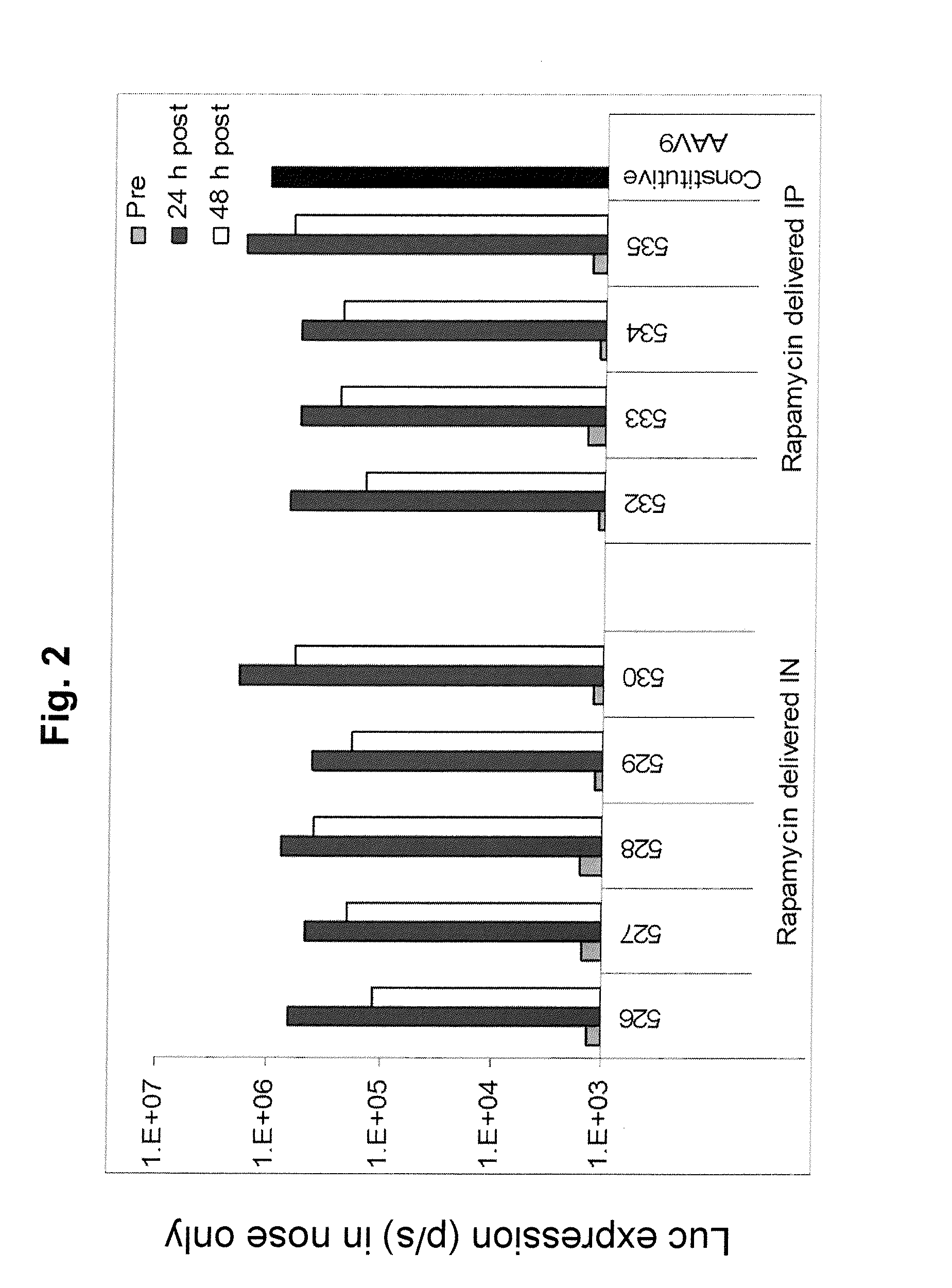
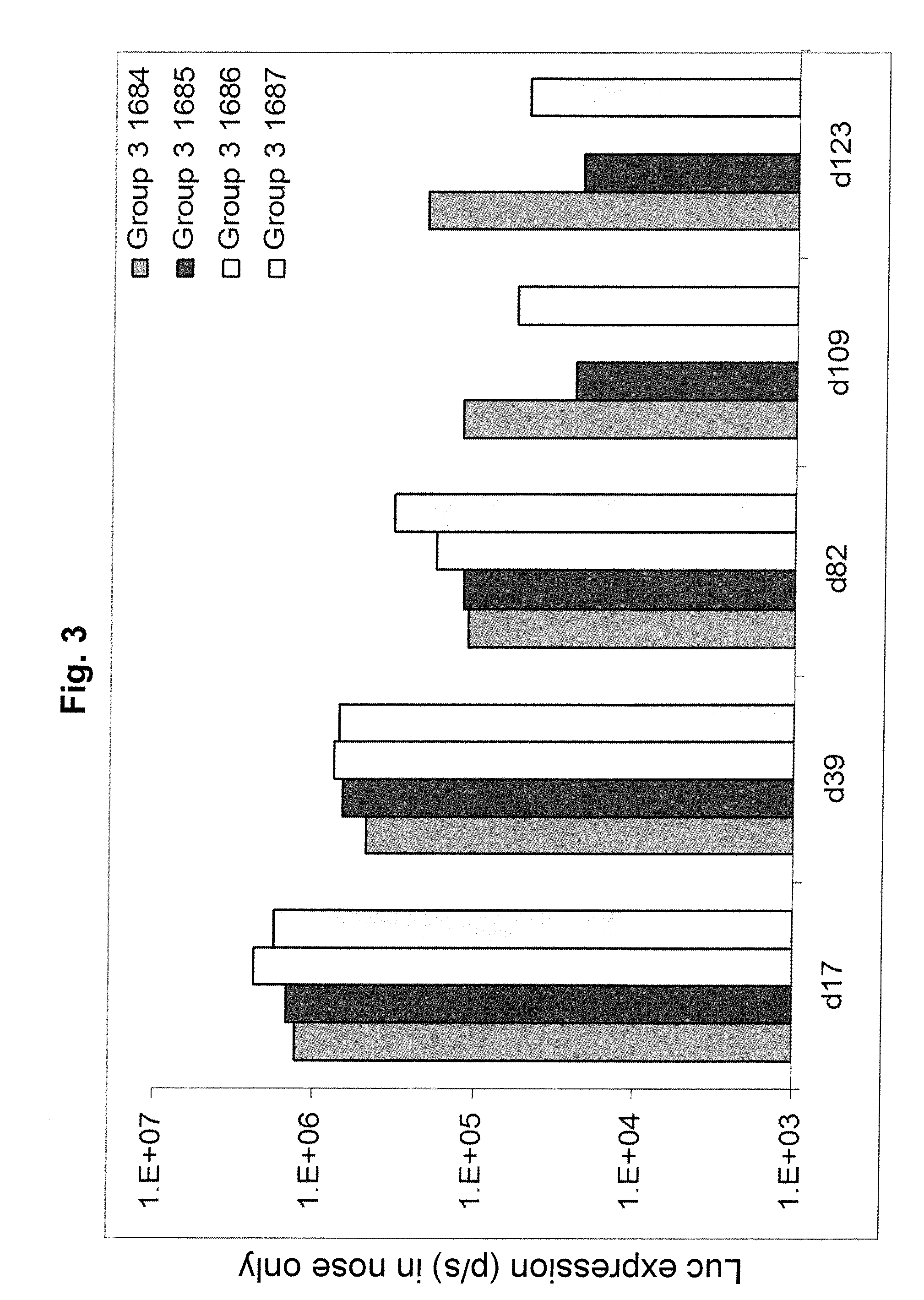
Regimens and Compositions for AAV-Mediated Passive Immunization of Airborne Pathogens
InactiveUS20140031418A1Organic active ingredientsBacterial antigen ingredientsRegimenHigh level expression
A prophylactic regimen for passively preventing infection with a pathogen which has a typical route of infection through the nasopharynx region of a subject, e.g., an airborne virus typically transmitted through coughing or sneezing. The method involves specifically targeting a subject's nasopharynx with a viral vector comprising an AAV capsid and carrying a nucleic acid sequence encoding an anti-viral neutralizing antibody construct operably linked to expression control sequences, in order to provide for high levels of expression of the anti-viral neutralizing antibody construct in the nasal airway cells. Optionally, the neutralizing antibody construct is expressed under a promoter which is regulated or induced by a small molecule which is delivered separately from the viral vector. In one embodiment, the method permits transfection of a subject's nasopharynx even where the subject has circulating neutralizing antibodies against the AAV capsid.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Choline binding proteins for anti-pneumococcal vaccines
InactiveUS6245335B1Improve efficiencySufficient quantityAntibacterial agentsBacteriaBacteroidesCholine binding protein
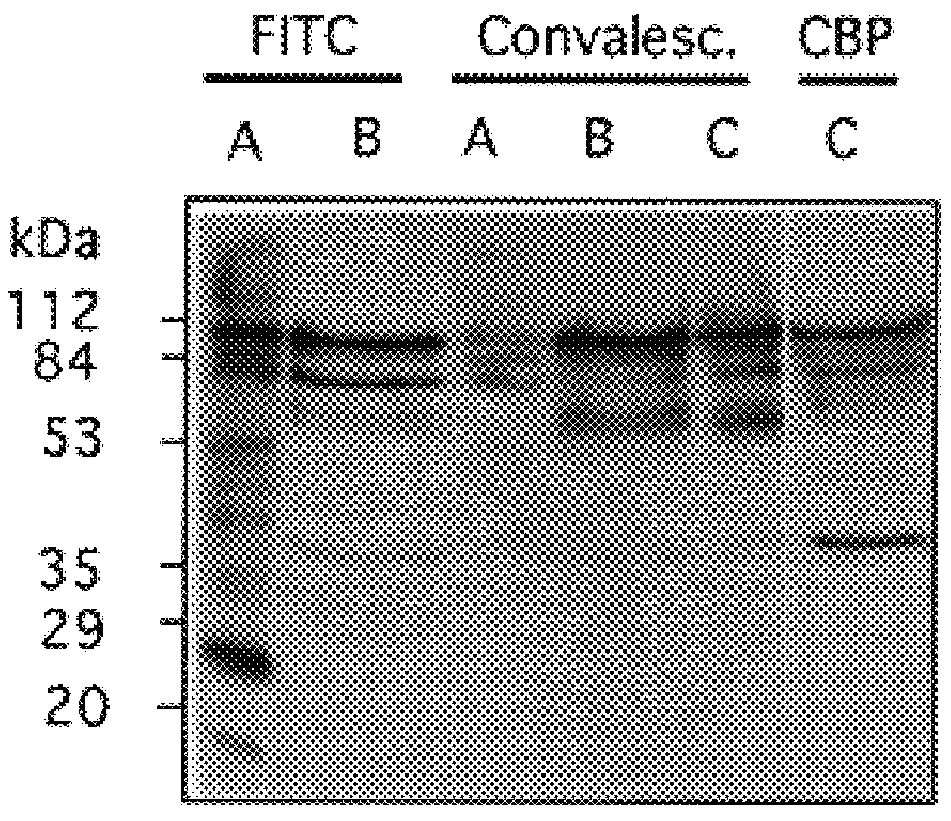
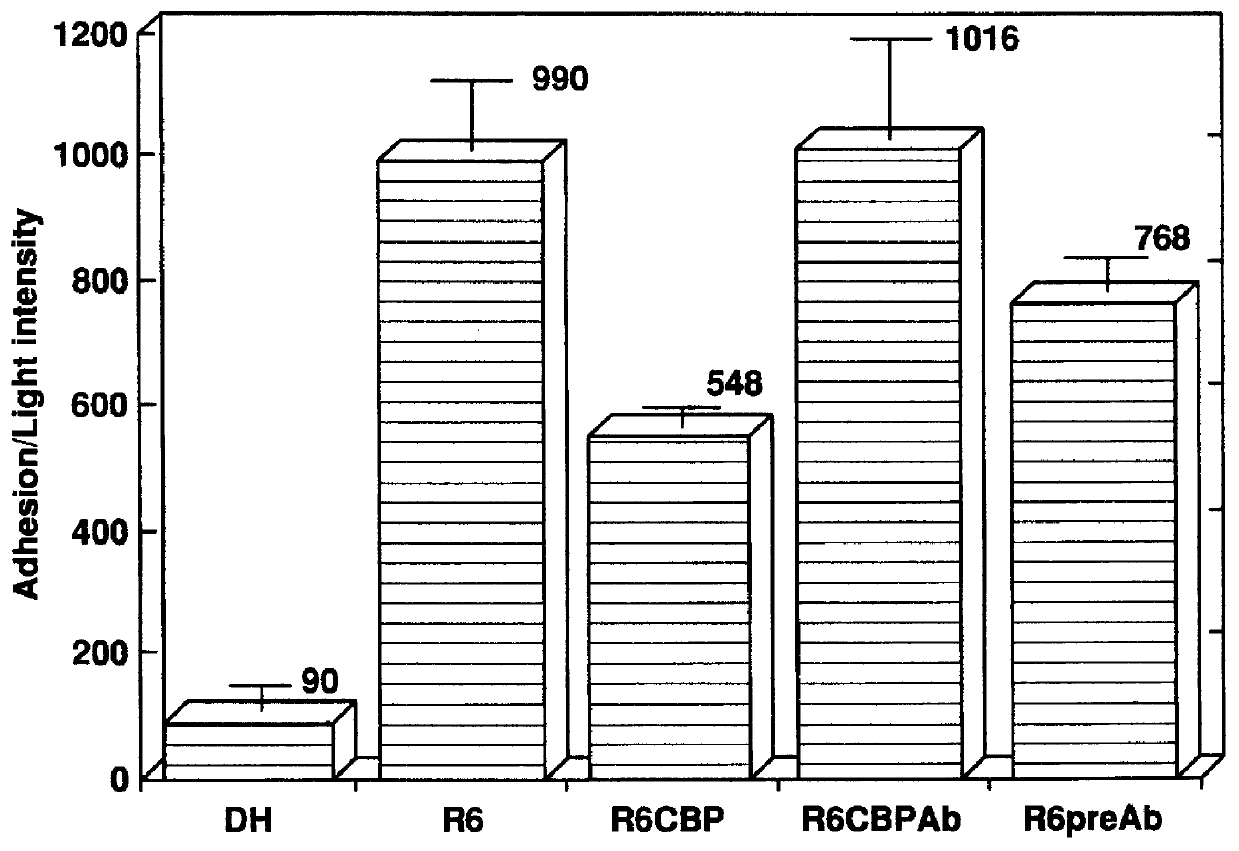
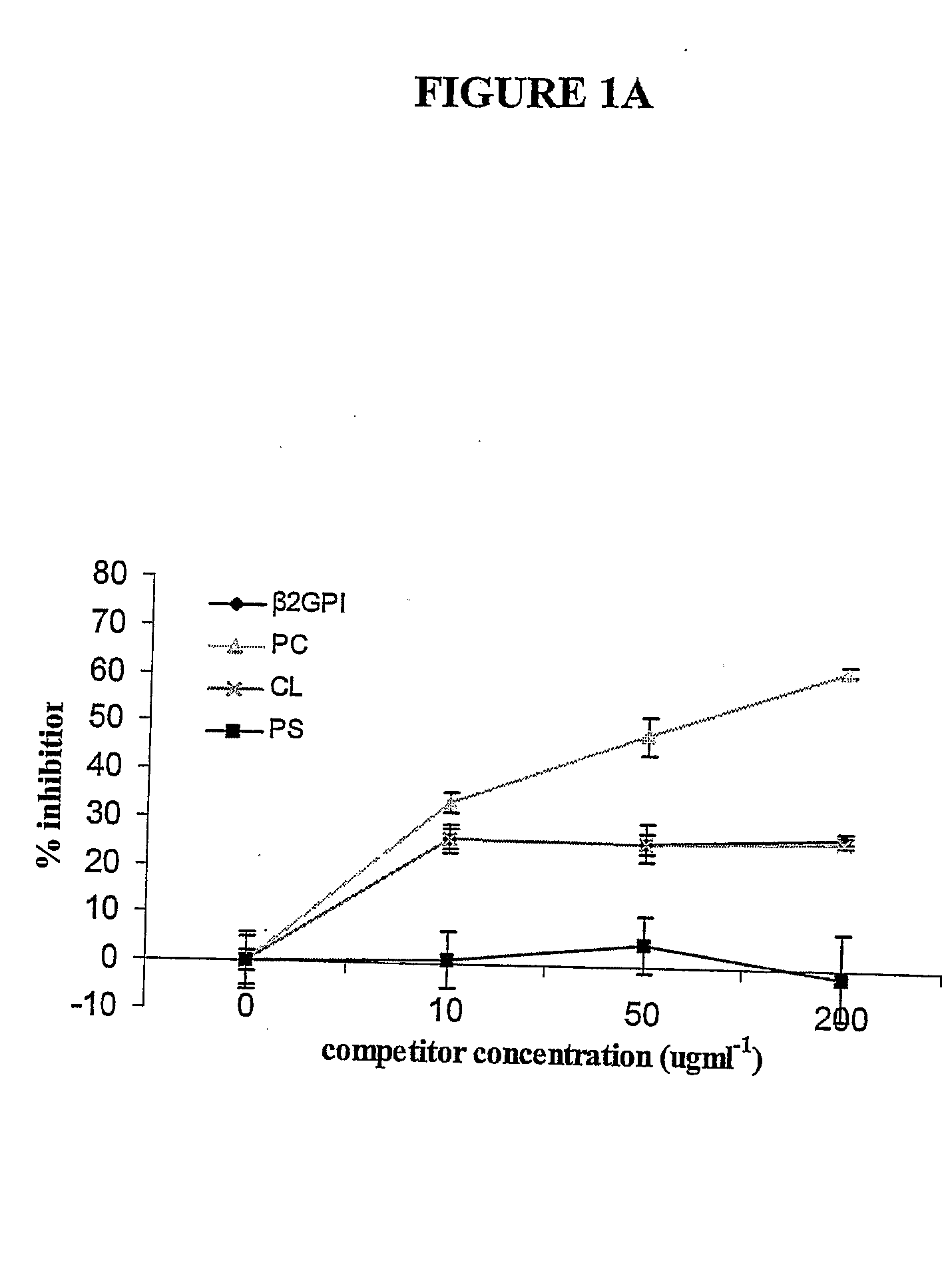
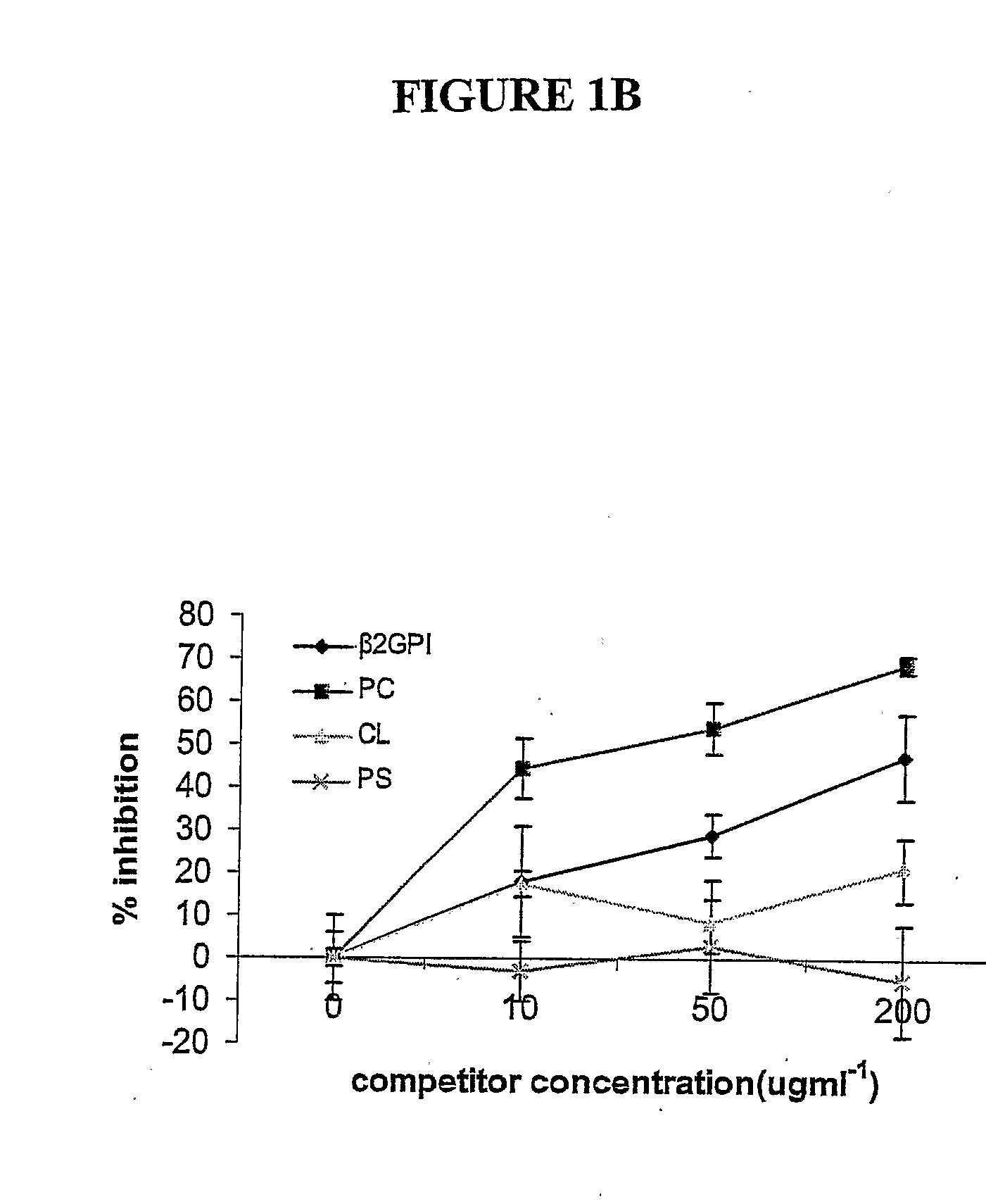
The invention relates to bacterial choline binding proteins (CBPs) which bind choline. Such proteins are particularly desirable for vaccines against appropriate strains of Gram positive bacteria, particularly streptococcus, and more particularly pneumococcus. Also provided are DNA sequences encoding the bacterial choline binding proteins or fragment thereof, antibodies to the bacterial choline binding proteins, pharmaceutical compositions comprising the bacterial choline binding proteins, antibodies to the bacterial choline binding proteins suitable for use in passive immunization, and small molecule inhibitors of choline binding protein mediated adhesion. Methods for diagnosing the presence of the bacterial choline binding protein, or of the bacteria, are also provided. In a specific embodiment, a streptococcal choline binding protein is an enolase, which demonstrates strong affinity for fibronectin.
Owner:THE ROCKEFELLER UNIV
Phosphorylcholine Conjugates and Corresponding Antibodies
ActiveUS20070286868A1Increased and decreased riskSignificant positive effectImmunoglobulins against animals/humansAntibody ingredientsPhosphorylcholineIgm antibody
IgG and IgM autoantibody levels against phosphorylcholine in subjects with hypertension (diastolic pressure>95 mmHg) were determined at baseline in order to determine the importance of antibodies for the development of atherosclerosis. The results show that increases in intima-media thickness (IMT) at a follow-up four years after baseline were significantly less prevalent in subjects having high IgM autoantibodies to phosphorylcholine. The presence or absence of IgM autoantibodies against phosphorylcholine is thus related to an increased or decreased risk of developing ischemic cardiovascular diseases. A method to determine IgM antibodies toward phosphorylcholine is proposed in this invention to identify subjects at risk of developing ischemic cardiovascular diseases. Animal experiments show that medium to high levels of IgM antibodies can be detected in plasma after active immunization with a keyhole limpet hemocyanin (KLH)-phosphorylcholine conjugate. A pharmaceutical composition comprising a phosphorylcholine conjugate (active immunization) or a monoclonal antibody with specificity to a phosphorylcholine conjugate (passive immunization) is proposed and the use of these compositions as active or passive immunogens in the treatment or prevention of atherosclerosis.
Owner:ATHERA BIOTECH
Compositions and methods related to antibodies to staphylococcal protein a
ActiveUS20140170134A1Reduced activityReduce bacterial loadAntibacterial agentsCompound screeningMicrobiologyBiochemistry
Embodiments concern methods and compositions for treating or preventing a bacterial infection, particularly infection by a Staphylococcus bacterium. Aspects include methods and compositions for providing a passive immune response against the bacteria. In certain embodiments, the methods and compositions involve an antibody that binds Staphylococcal protein A (SpA).
Owner:UNIVERSITY OF CHICAGO
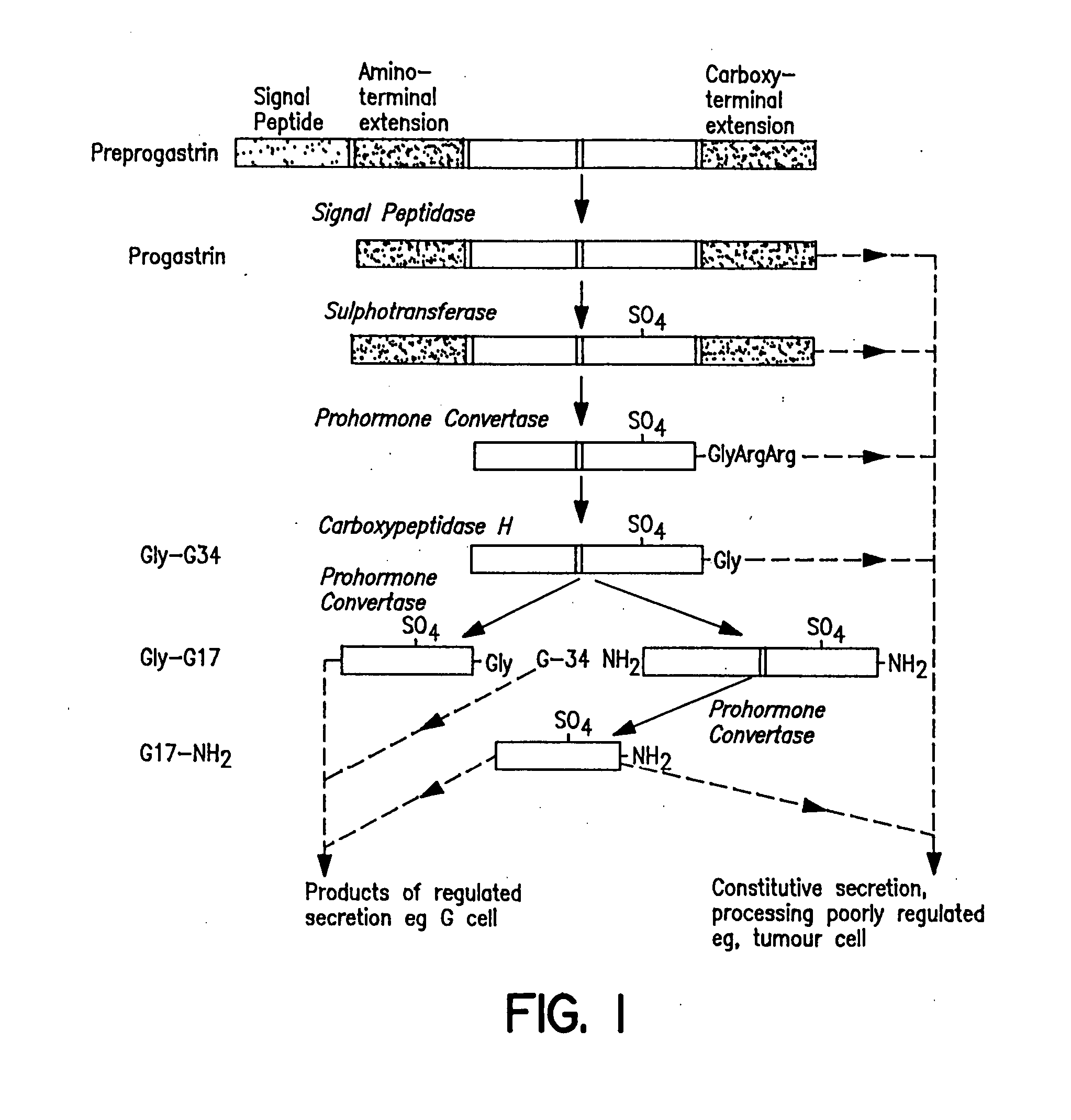
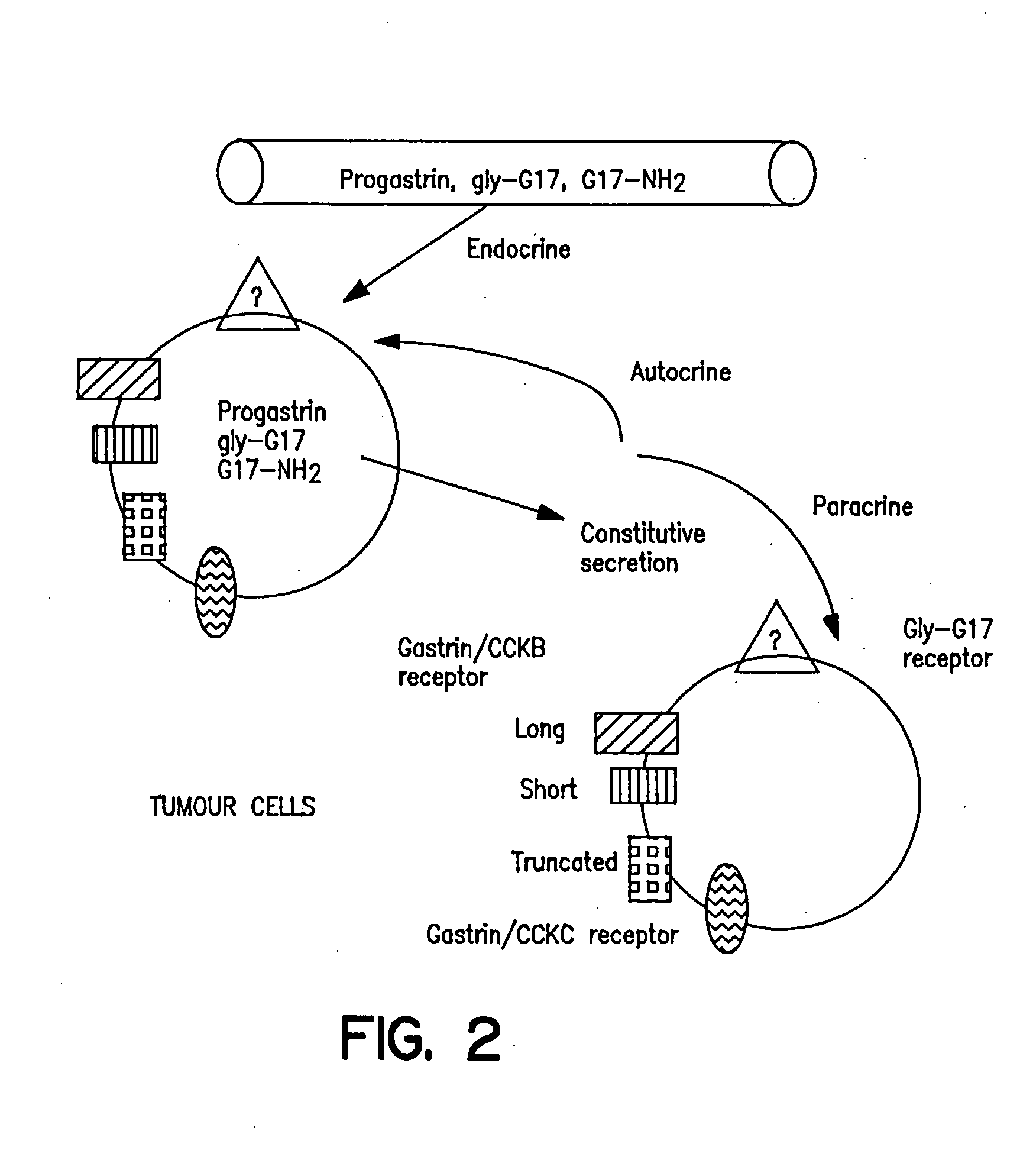
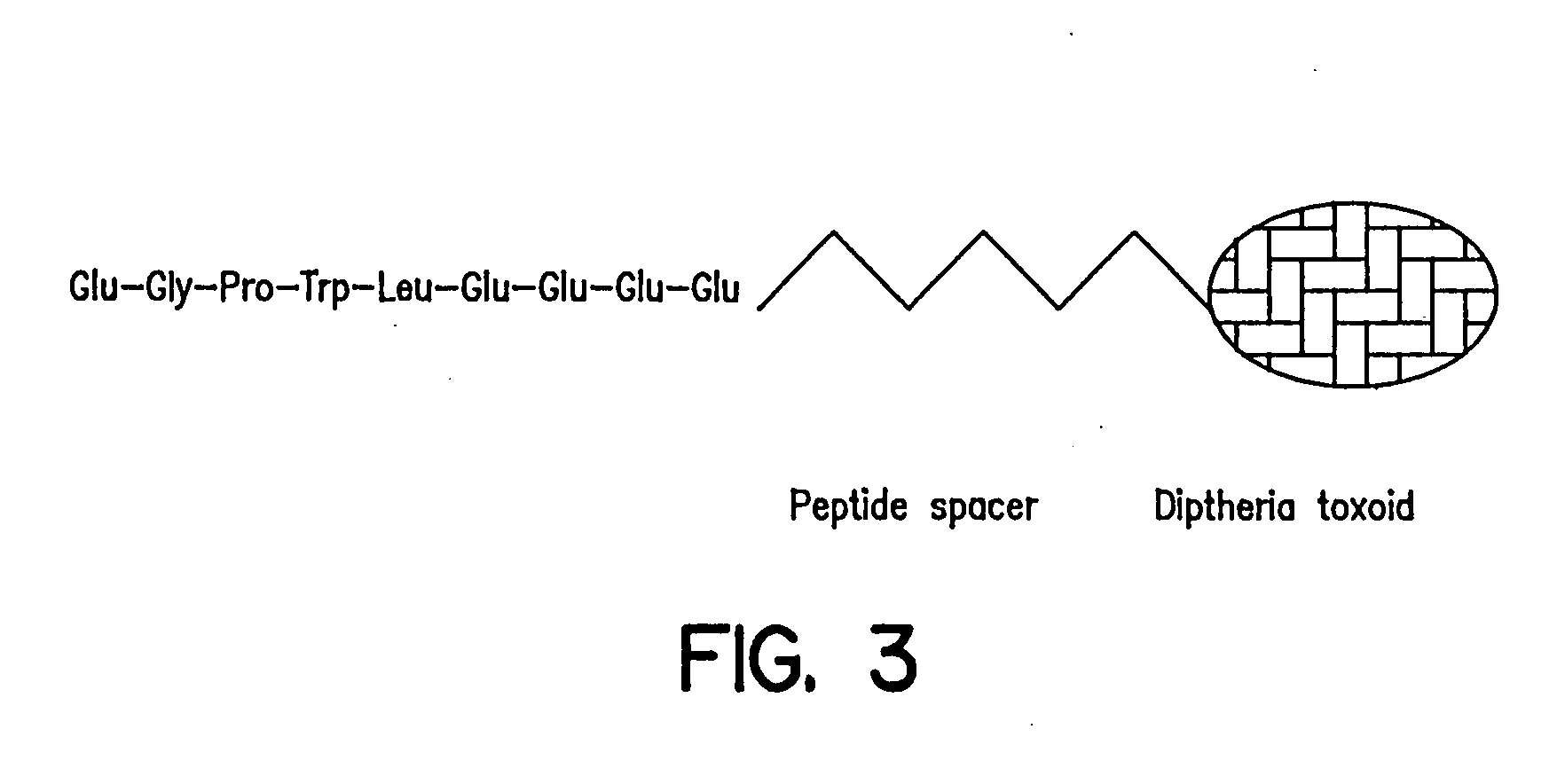
Prevention and treatment of hypergastrinemia
Serum-associated hypergastrinemia is treated by administration of gastrin active or passive immunization. An anti-gastrin immunogenic composition containing a gastrin G17 or G34 peptide fragment which is amino acid spacer-linked to an immunogenic carrier, is administered so as to effectively neutralize the circulating gastrin hormone, and moreover, inhibit autocrine activity by progastrin such as Gly-extended G17, and amidated G17, in patients with pernicious anemia. Moreover, the method includes administration of a therapeutically effective amount of anti-G17 or anti-G34 antibodies which may be in humanized form. Finally, the method provides ameliorating treatment of hypergastrinemic effects of proton pump inhibitors or H2 histamine receptor blocking agents or antagonists, in addition to treatment of hypergastrinemia caused by diseases such as pernicious anemia.
Owner:CANCER ADVANCES INC
Active or passive immunization against proapoptotic neurotrophins for the treatment and/or prevention of neurodegenerative diseases
InactiveUS20060275290A1Potent apoptotic activityReduce neuronal or glial cell apoptosisNervous disorderPeptide/protein ingredientsAdjuvantNeuro-degenerative disease
The present invention relates to novel methods for combating cell degeneration or dysfunction resulting from neuroinflammatory conditions. The invention especially relates to the use, in the preparation of a medicament for the treatment of neurodegenerative disease associated with neuroinflammation, of an immunogenic compound which is capable of inducing an immune response against a proapoptotic neurotrophin, or an effective amount of a hapten combined with appropriate carriers and / or adjuvants to render the resulting combination capable of inducing an immune response against a proapoptotic neurotrophin. Also disclosed are compositions for the active or passive immunization against neuronal or glial cell apoptosis caused by neuroinflammation as well as methods and means useful for said active or passive immunization.
Owner:INST PASTEUR +1
Monoclonal Antibodies to Progastrin
The present invention provides progastrin-binding molecules specific for progastrin that do not bind gastrin-17(G17), gastrin-34(G34), glycine-extended gastrin-17(G17-Gly), or glycine-extended gastrin-34(G34-Gly). Further, the invention provides monoclonal antibodies (MAbs) selective for sequences at the N-terminus and the C-terminus of the gastrin precursor molecule, progastrin and the hybridomas that produce these MAbs. Also provided are panels of MAbs useful for the detection and quantitation of progastrin and gastrin hormone species in immuno-detection and quantitation assays. These assays are useful for diagnosing and monitoring a gastrin-promoted disease or condition, or for monitoring the progress of a course of therapy. The invention further provides solid phase assays including immunohistochemical (IHC) and immunofluorescence (IF) assays suitable for detection and visualization of gastrin species in solid samples, such as biopsy samples or tissue slices. The progastrin-binding molecules are useful therapeutically for passive immunization against progastrin in progastrin-promoted diseases or conditions. Also provided are surrogate reference standard (SRS) molecules that are peptide chains of from about 10 to about 35 amino acids, wherein the SRS molecule comprises at least two epitopes found in a protein of interest of greater than about 50 amino acids. Such SRS molecules are useful as standards in place of authentic proteins of interest.
Owner:CANCER ADVANCES INC
HIV immunoassays using synthetic envelope polypeptides
ActiveUS7273695B1Peptide/protein ingredientsAntibody mimetics/scaffoldsAntigenAcquired immunodeficiency
Polynucleotide sequences are provided for the diagnosis of the presence of retroviral infection in a human host associated with lymphadenopathy syndrome and / or acquired immune deficiency syndrome, for expression of polypeptides and use of the polypeptides to prepare antibodies, where both the polypeptides and antibodies may be employed as diagnostic reagents or in therapy, e.g., vaccines and passive immunization. The sequences provide detection of the viral infectious agents associated with the indicated syndromes and can be used for expression of antigenic polypeptides.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC +1
Human immunodeficiency virus (HIV) nucleotide sequences
Polynucleotide sequences are provided for the diagnosis of the presence of retroviral infection in a human host associated with lymphadenopathy syndrome and / or acquired immune deficiency syndrome, for expression of polypeptides and use of the polypeptides to prepare antibodies, where both the polypeptides and antibodies may be employed as diagnostic reagents or in therapy, e.g., vaccines and passive immunization. The sequences provide detection of the viral infectious agents associated with the indicated syndromes and can be used for expression of antigenic polypeptides.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
Anti-adhesin based passive immunoprophlactic
The invention relates to an immunogenic composition and method of the immunogenic composition for the production and administration of a passive immunoprophylactic against enterotoxigenic Escherichia coli. The immunoprophylactic is made collecting anti-adhesin in the colostrum or milk of vaccinated domesticated animals such as cows. The immunoprophylactic is administered either as a dietary supplement or in capsular or tablet form.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
Conjugates of amyloid proteins as vaccines for amyloid-related diseases
InactiveUS20070172496A1Treating, preventing and/or ameliorating amyloid-related diseasesPreventing or reducing amyloid-induced cellular toxicityBacterial antigen ingredientsNervous disorderAmyloid betaMammal
New conjugates comprising fragments of amyloid proteins, which may be used in vaccines for the treatment, prevention and / or amelioration of diseases associated with deposition of amyloid proteins, such as, e.g. Alzheimers disease. Methods for treating, preventing and / or ameliorating amyloid-related diseases, by administering a conjugate comprising fragments of an amyloid protein to a subject in need thereof, thereby enabling the production of antibodies in the subject. Antibodies—being capable of interacting with pathological regions within an amyloid protein, and thereby preventing e.g. the formation of amyloid fibrils, plaques and / or deposits, and methods for passive immunization wherein an antibody as described above is administered to a subject in need thereof. Furthermore is provided specific fragments of the C-terminal part of amyloid beta (1-42), that when administered to a mammal generates antibodies, which specifically targets the soluble form of the highly amyloidogenic amyloid beta (1-42).
Owner:CURIX
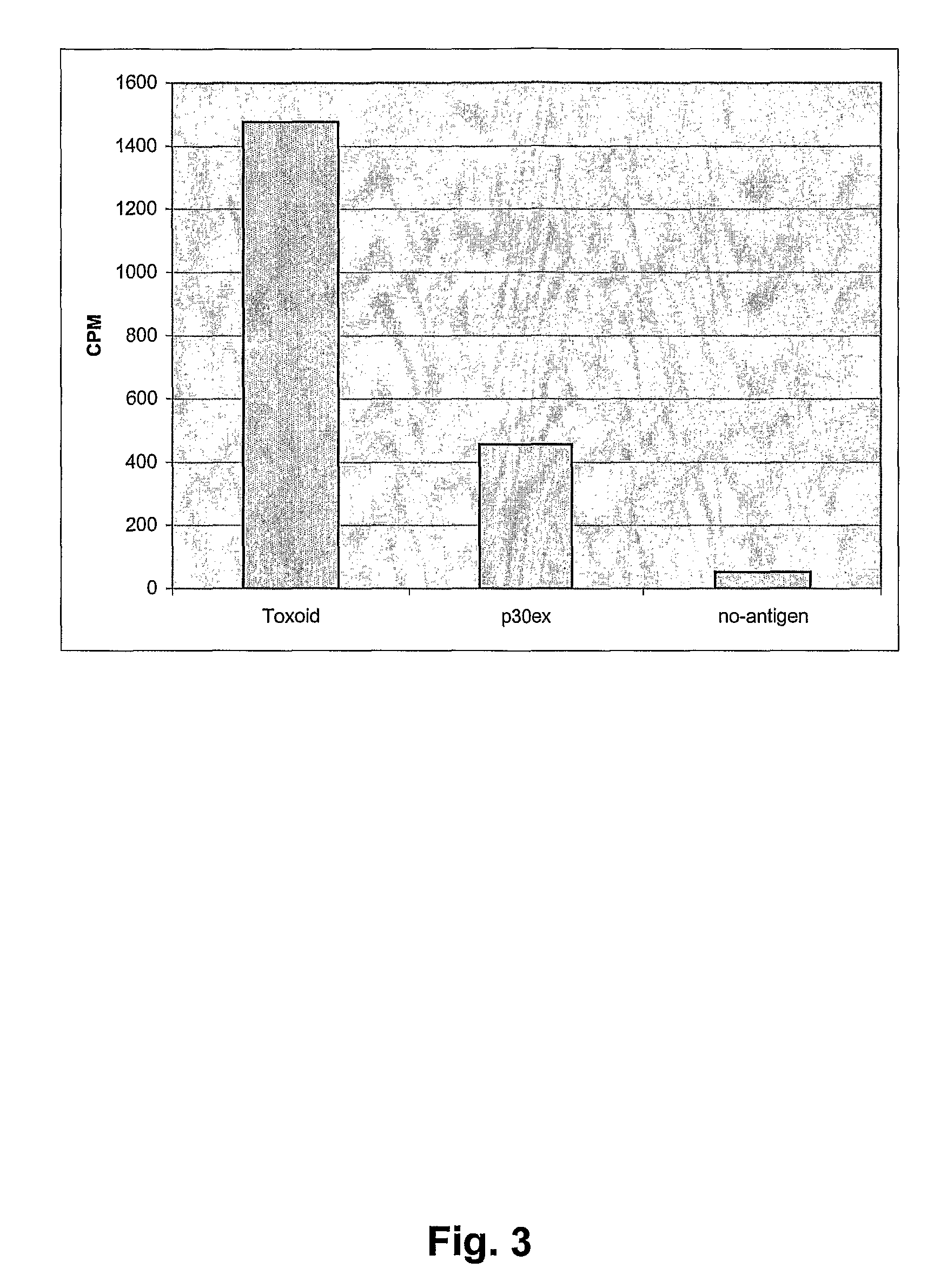
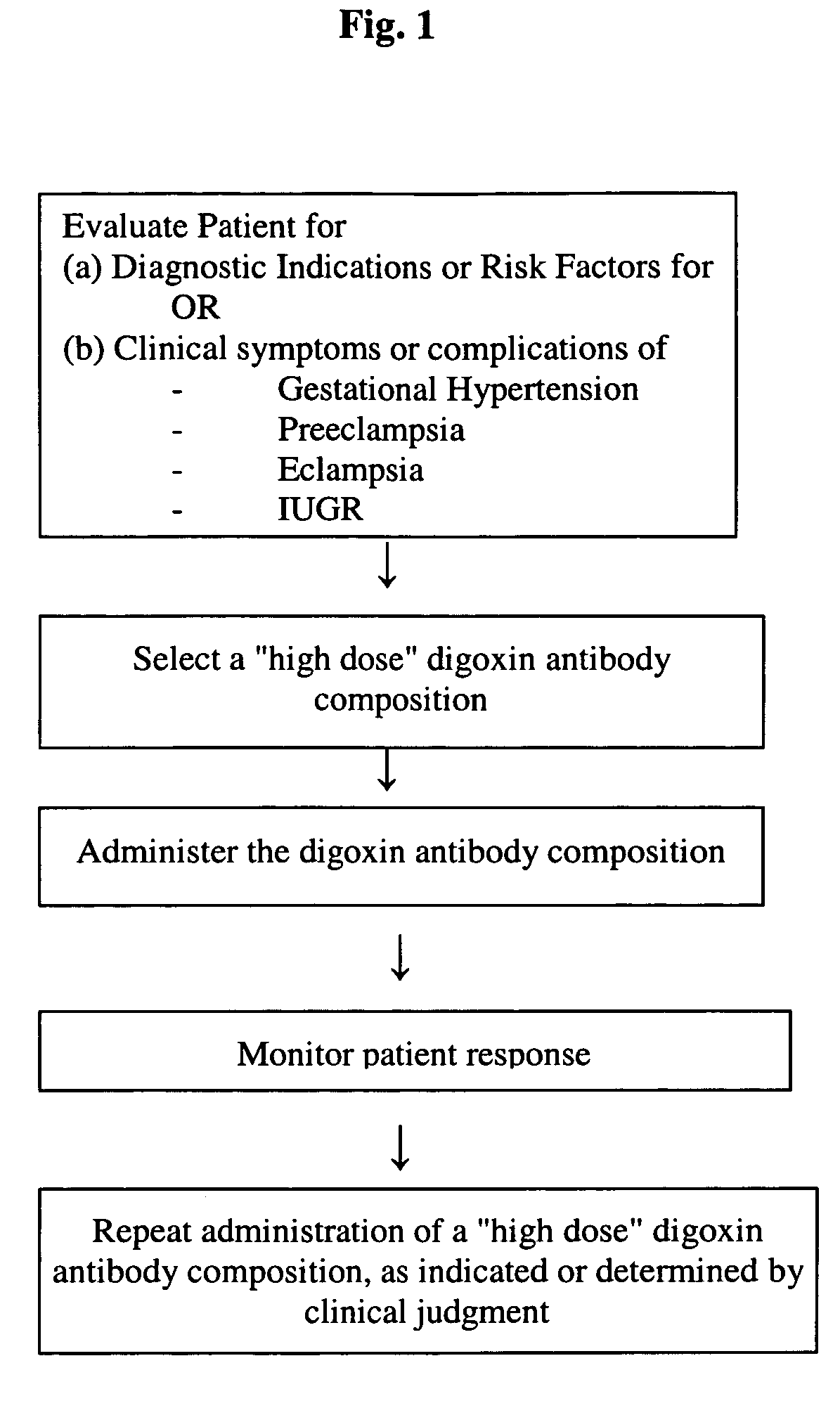
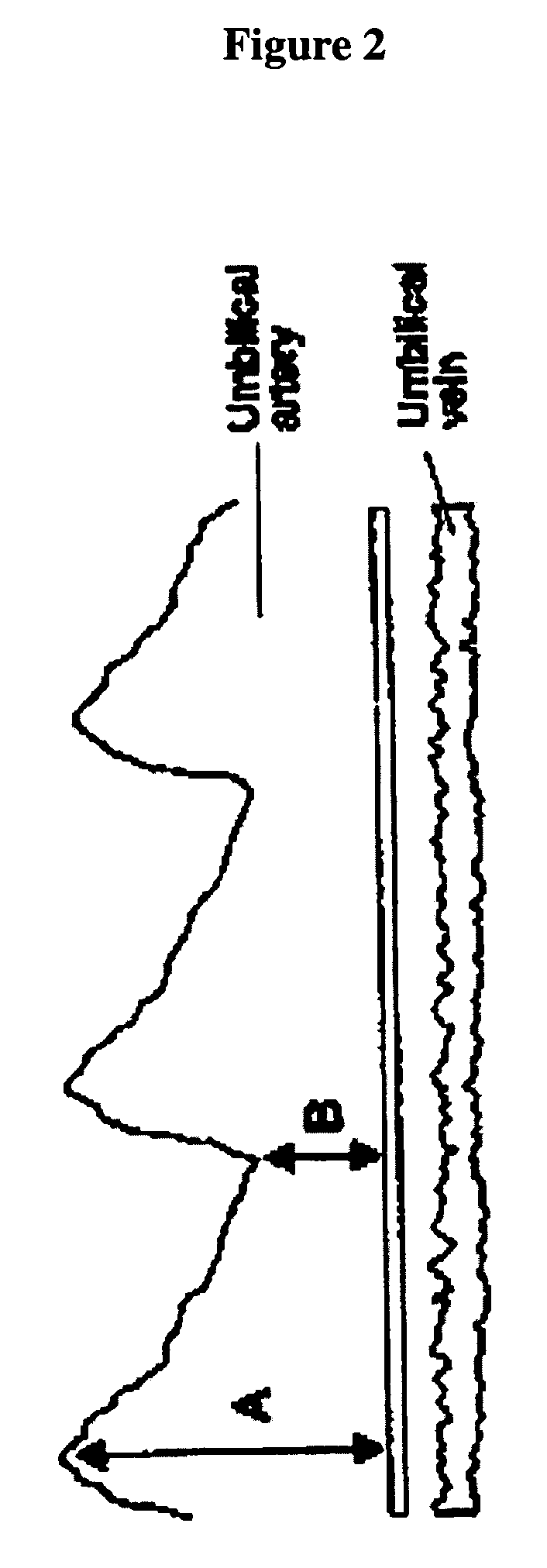
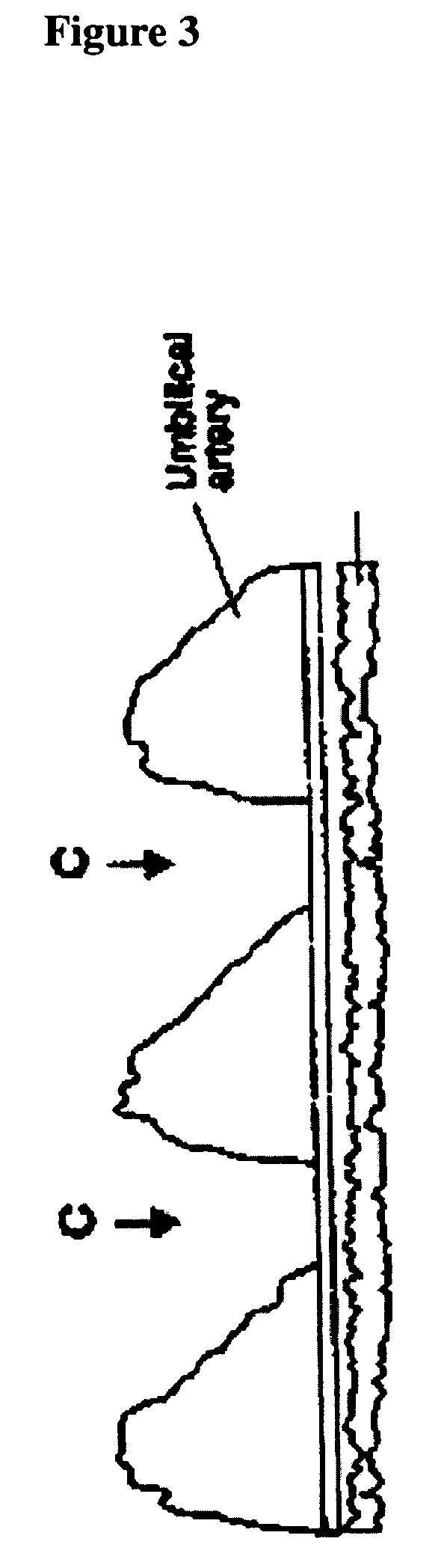
Antibody composition and passive immunization against pregnancy-induced hypertension
A composition is provided to prevent, limit the effects of, delay the onset of, or treat one or more of the causes, symptoms or complications of gestational hypertension, preeclampsia, eclampsia and / or intrauterine growth restriction. The composition comprises a therapeutically effective amount of an antibody that reacts immunologically with or binds digoxin and has a high dose of digoxin binding capacity as the active ingredient. There is also provided a method of preventing, limiting the effects of, delaying the onset of, or treating a cause, symptom or complication of gestational hypertension, preeclampsia, eclampsia or intrauterine growth restriction, comprising the step of administering to a mammal a composition comprising a therapeutically effective amount of an antibody that reacts immunologically with or binds digoxin and has a high dose of digoxin binding capacity.
Owner:VELO BIO
Preparation method and application of Newcastle disease virus infected immune complex vaccines
InactiveCN102233133AAvoid infectionSame characteristicsViral antigen ingredientsAntiviralsYolkMicroorganism
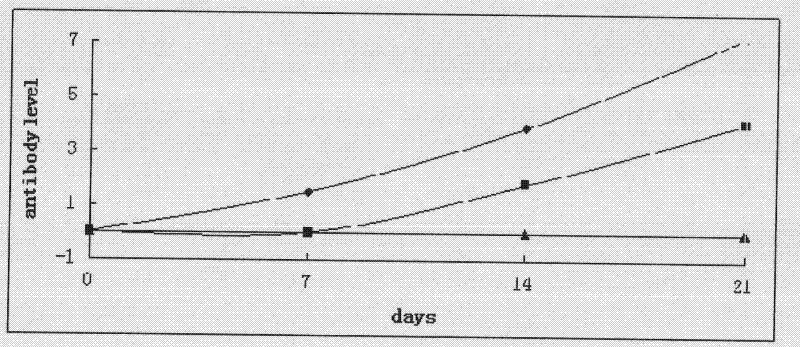
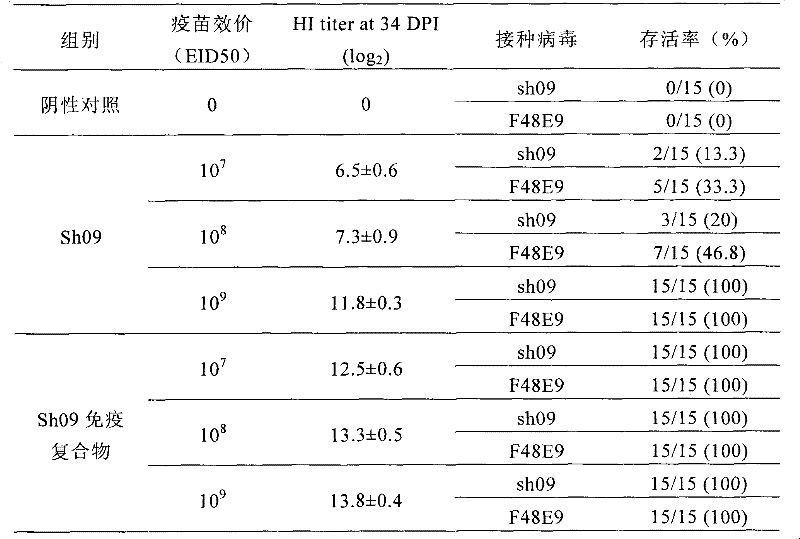
The invention discloses a preparation method and application of Newcastle disease virus infected immune complex vaccines. The preparation method comprises the following steps of: (1) preparing antigens and inactivated vaccines by using Newcastle disease virus Shanghai strains as seed viruses; (2) transferring antibodies in chicken blood serum to yolk to form yolk antibodies IgY; and (3) mixing the prepared inactivated vaccines and the yolk antibodies IgY in equal volume, and hatching to obtain the immune complex vaccines. By combining passive immune (vaccine) and active immune (specific antibody) measures, animals are protected from the infection of pathogenic microbes in time for a long time.
Owner:SHANGHAI ACAD OF AGRI SCI
Protein showing enhanced expression in cancer cells
A novel gene (designated 101P3A11 or PHOR-1) and its encoded protein, and variants thereof, are described wherein 101P3A11 exhibits tissue specific expression in normal adult tissue, and is aberrantly expressed in the cancers listed in Table I. Consequently, 101P3A11 provides a diagnostic, prognostic, prophylactic and / or therapeutic target for cancer. The 101P3A11 gene or fragment thereof, or its encoded protein, or variants thereof, or a fragment thereof, can be used to elicit a humoral or cellular immune response; antibodies or T cells reactive with 101P3A11 can be used in active or passive immunization.
Owner:AGENSYS
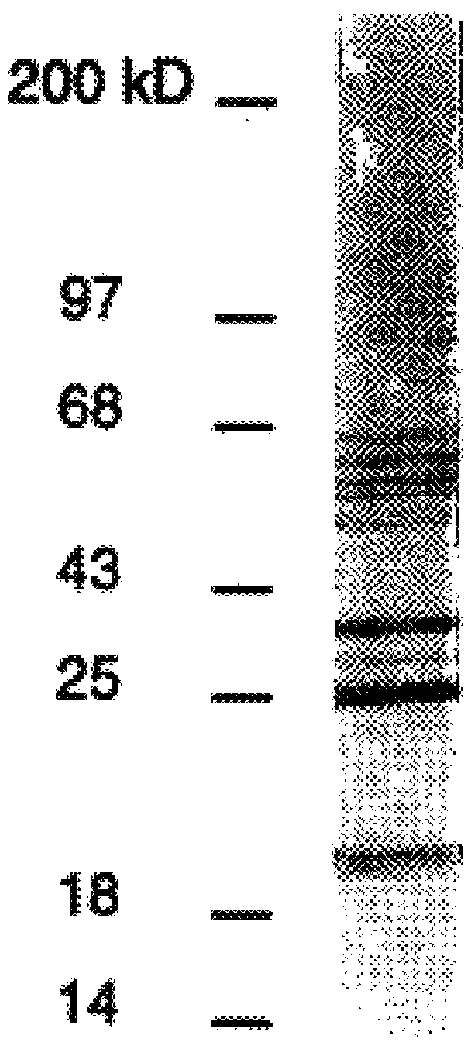
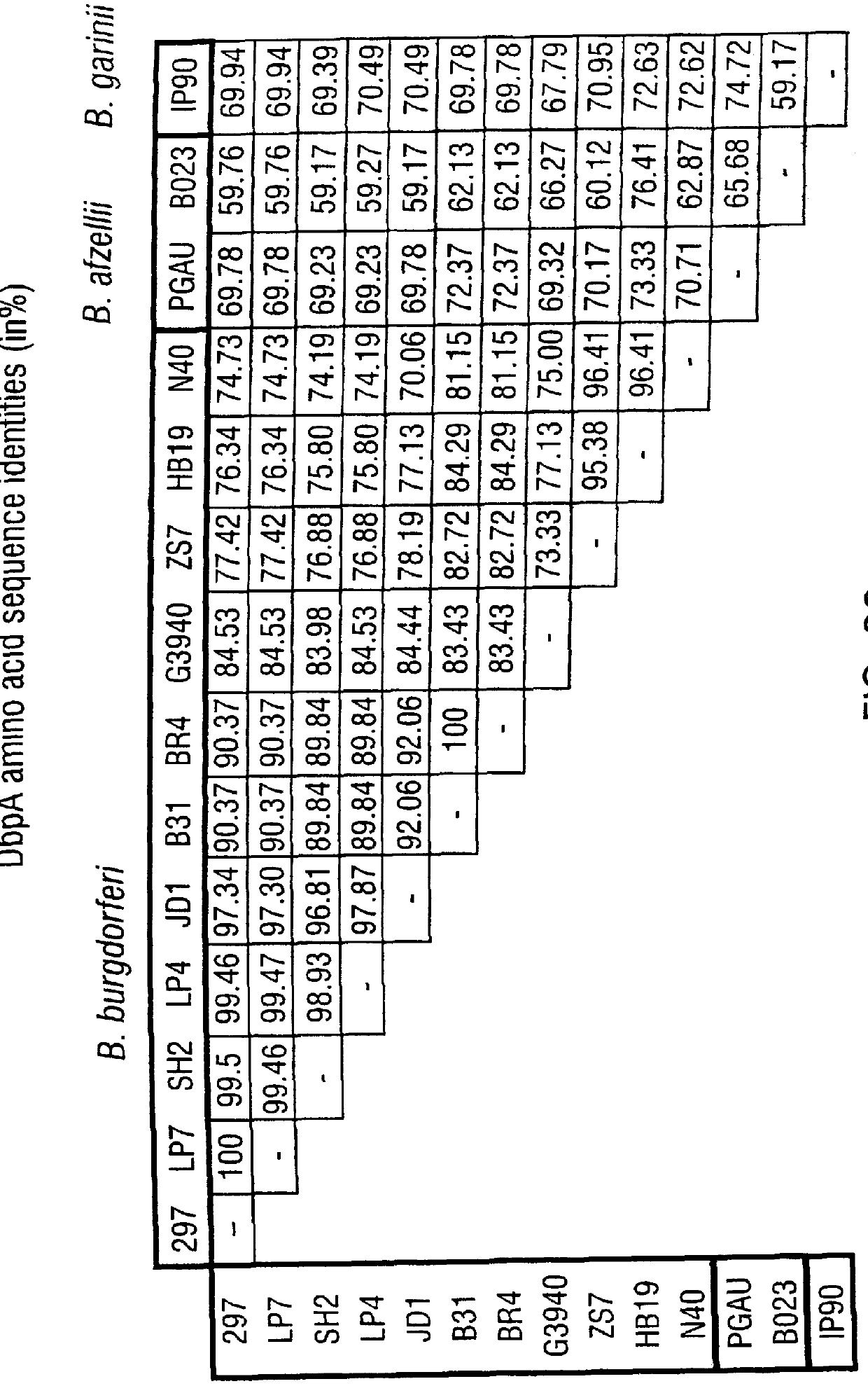
DbpA compositions
Disclosed are the dbp gene and dbp-derived nucleic acid segments from Borrelia burgdorferi, the etiological agent of Lyme disease, and DNA segments encoding dbp from related borrelias. Also disclosed are decorin binding protein compositions and methods of use. The DBP protein and antigenic epitopes derived therefrom are contemplated for use in the treatment of pathological Borrelia infections, and in particular, for use in the prevention of bacterial adhesion to decorin. DNA segments encoding these proteins and anti-(decorin binding protein) antibodies will also be of use in various screening, diagnostic and therapeutic applications including active and passive immunization and methods for the prevention of Borrelia colonization in an animal. These DNA segments and the peptides derived therefrom are contemplated for use in the preparation of vaccines and, also, for use as carrier proteins in vaccine formulations, and in the formulation of compositions for use in the prevention of Lyme disease.
Owner:MEDIMMUNIE +1
Methods for purification of viruses
The present invention provides methods for the purification of cell-associated viruses from adherent cells (e.g., MDCK or Vero cells). In particular, the present invention provides purification methods for the production of immunogenic compositions comprising a live attenuated cell-associated virus (e.g., an attenuated respiratory syncytial virus (RSV) or cold-adapted, and / or temperature sensitive influenza virus) that result in levels of host cell DNA (HCD), host cell protein (HCP) and non-specific endonuclease (e.g., Benzonase), which are below the specifications required by regulatory agencies. The immunogenic compositions can be used to actively immunize subjects or to generate antibodies for a variety of uses, including passive immunization and diagnostic immunoassays.
Owner:MEDIMMUNE LLC
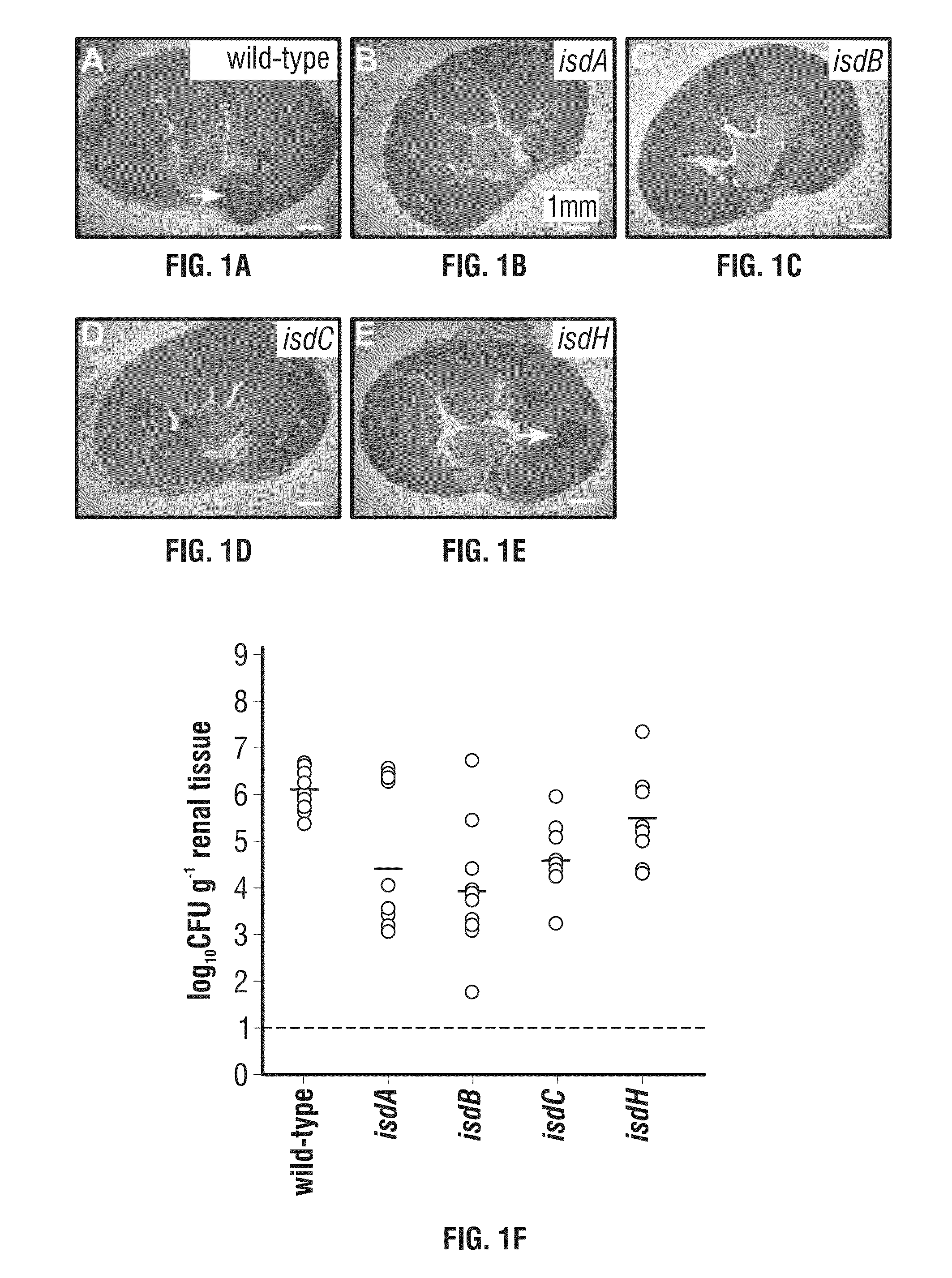
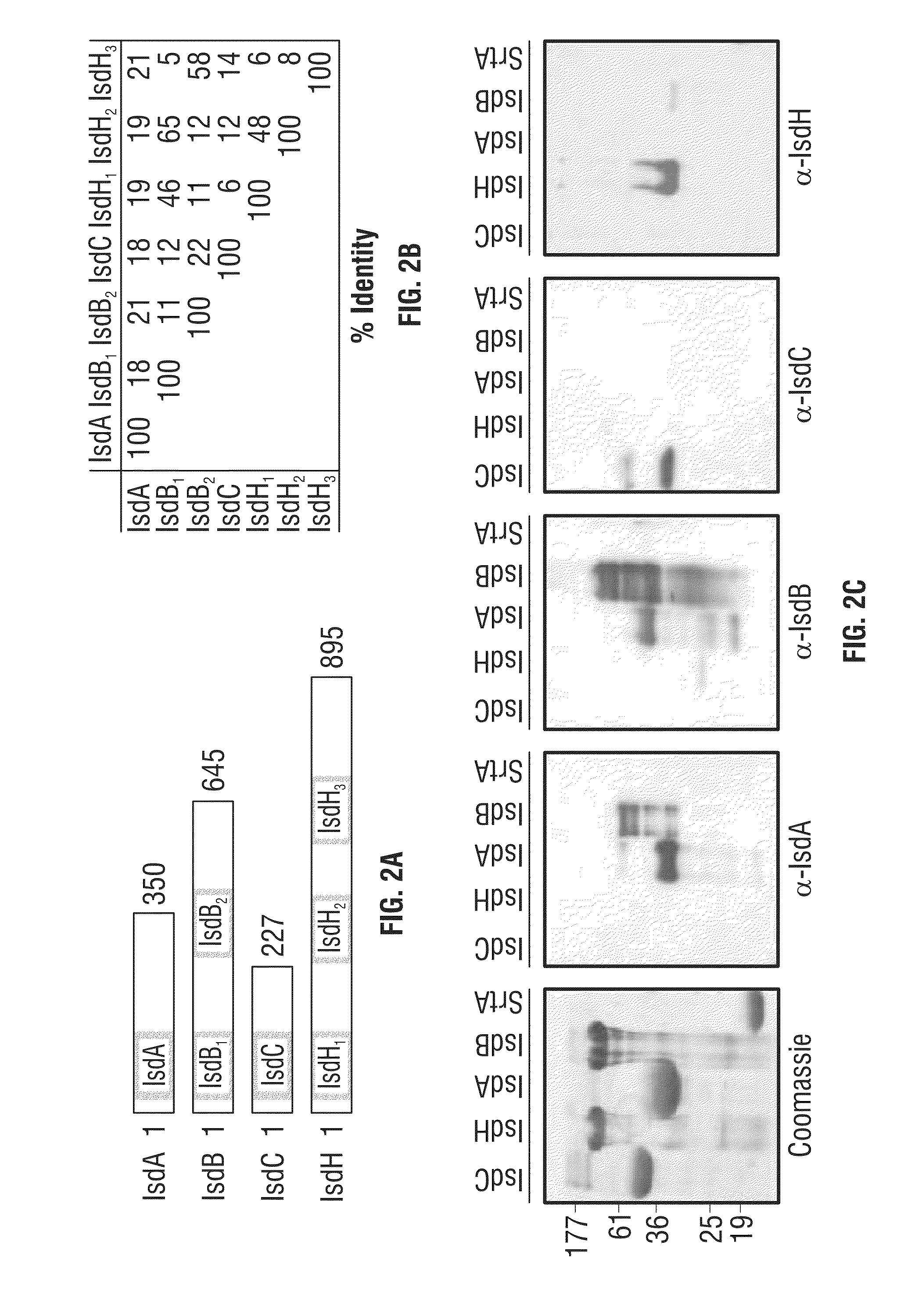
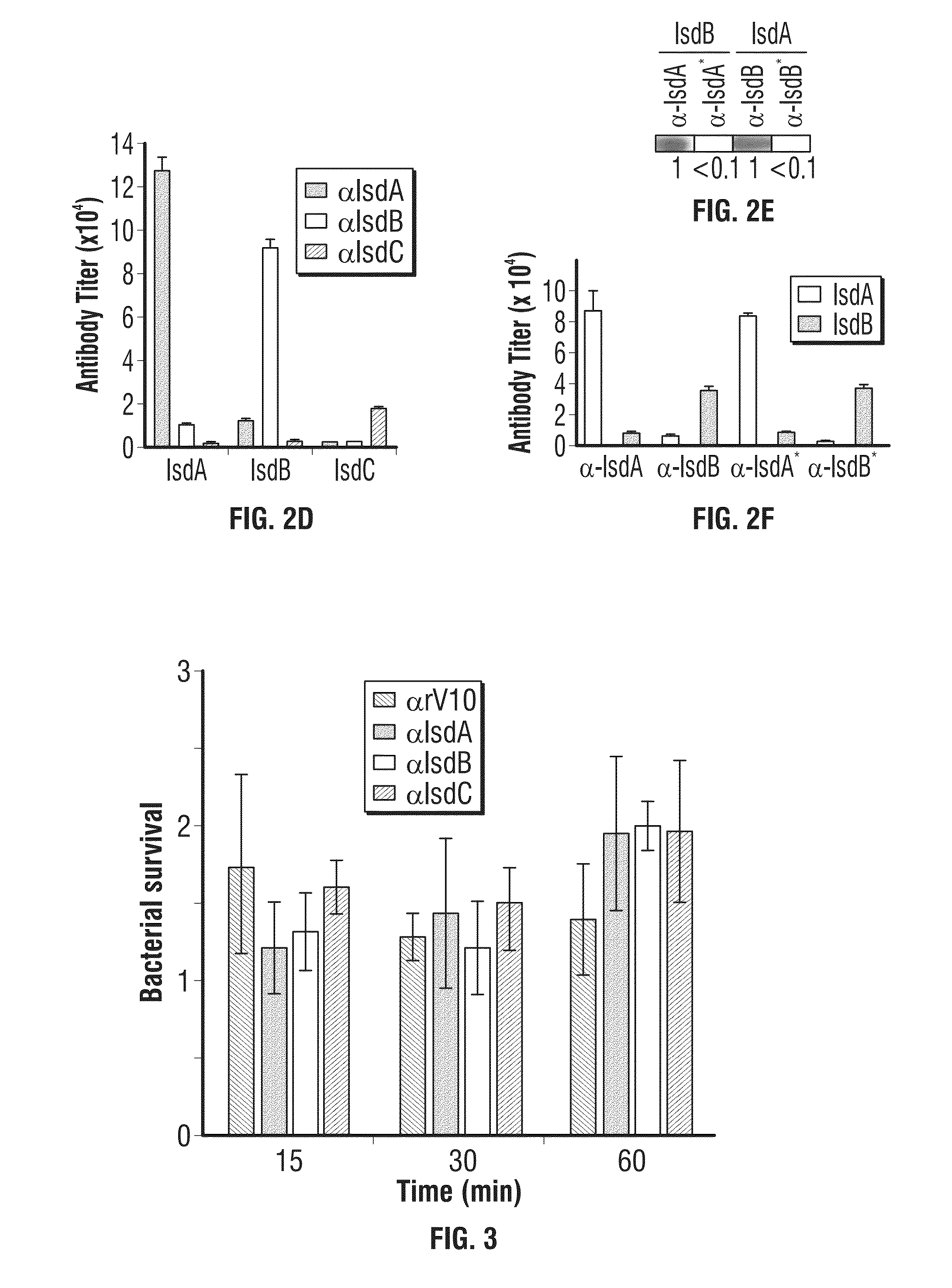
Compositions and methods related to antibodies to staphylococcal proteins isda or isdb
InactiveUS20140037650A1Reduced activityPrevents alleviates ameliorates symptomImmunoglobulins against bacteriaAntibody ingredientsBacteroidesStaphylococcal protein
The present invention concerns methods and compositions for treating or preventing a bacterial infection, particularly infection by a Staphylococcus bacterium. The invention provides methods and compositions for providing a passive immune response against the bacteria. In certain embodiments, the methods and compositions involve an antibody, such as a recombinant antibody, that binds IsdA and / or IsdB polypeptides.
Owner:UNIVERSITY OF CHICAGO
Completely humanized neutralizing antibody for anti-rabies viruses
ActiveCN103910796ALow priceQuickly builds immune protectionHybrid immunoglobulinsImmunoglobulins against virusesAntigenAntigen epitope
The invention discloses a completely humanized neutralizing antibody for anti-rabies viruses. The amino acid sequence of a heavy chain of the antibody is shown in sequence table SEQ ID NO1, the amino acid sequence of a light chain of the antibody is shown in sequence table SEQ ID NO5, the complementary determining region (CDR) of a variable region of the heavy chain of the antibody is shown as follows: CDR1: SEQIDNO2, CDR2:SEQIDNO3 and CDR3: SEQIDNO4, and the complementary determining region (CDR) of a variable region of the light chain of the antibody is shown as follows: CDR1:SEQIDNO6, CDR2:SEQIDNO7, and CDR3: SEQIDNO8. The beneficial effects of the completely humanized neutralizing antibody are that: the completely humanized neutralizing antibody disclosed by the invention ahs the advantages of being completely humanized, good in specificity, high in affinity, good in neutralizing effect, and low in price, can be used as a biological engineering antibody drug to quickly establish immune protection force against rabies virus, can be used for passive immunotherapy of acute infection, and also can be used for preparing detection reagents for detecting the rabies virus, finding effective neutralization antigen epitope and developing recombinant proteins and subunit vaccines for the rabies virus.
Owner:CHANGCHUN BCHT BIOTECH
Antibodies and related molecules that bind to 24P4C12 proteins
Antibodies and molecules derived therefrom that bind to 24P4C12 protein and variants thereof, are described wherein 24P4C12 exhibits tissue specific expression in normal adult tissue, and is aberrantly expressed in the cancers listed in Table I. Consequently, 24P4C12 provides a diagnostic, prognostic, prophylactic and / or therapeutic target for cancer. The 24P4C12 gene or fragment thereof, or its encoded protein, or variants thereof, or a fragment thereof, can be used to elicit a humoral or cellular immune response; antibodies or T cells reactive with 24P4C12 can be used in active or passive immunization.
Owner:AGENSYS INC
Streptococcus protective antigen C5a and preparation method thereof
InactiveCN102746388AStrong immune responseSignificant passive immune protectionAntibacterial agentsBacterial antigen ingredientsForward primerProtective antigen
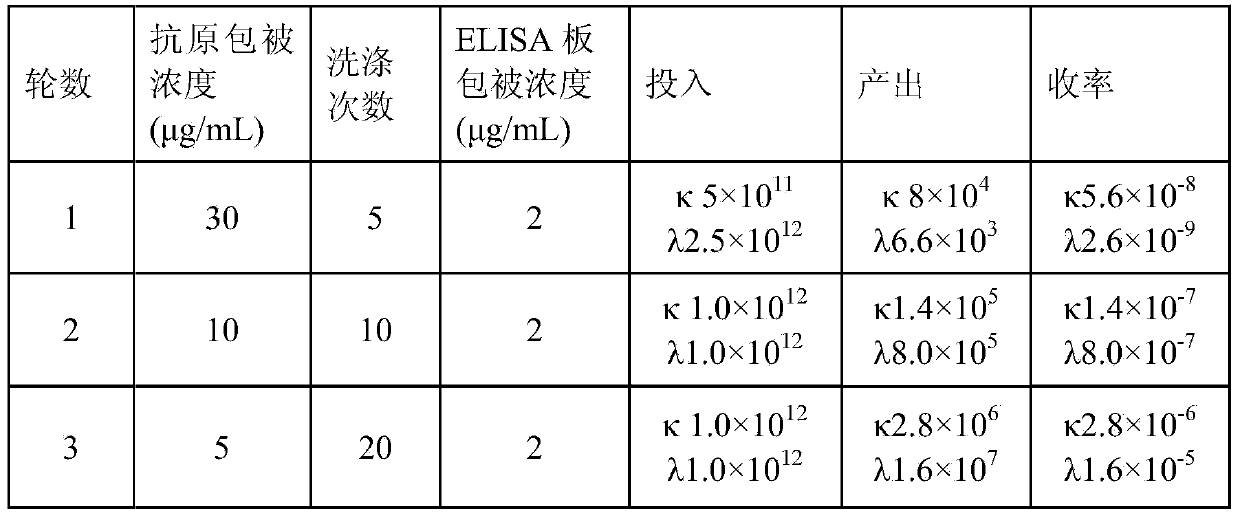
The present invention relates to a streptococcus antigen C5a and a preparation method thereof. The streptococcus protective antigen C5a is an SEZC5a recombinant protein, which consists of 571 amino acids and has a molecular weight of 60.3kDa; a forward primer has one BamHI restriction enzyme cutting site, and a reverse primer has one EcoRI restriction enzyme cutting site. According to the preparation method of the streptococcus protective antigen C5a, the SEZ C5a recombinant protein is treated with cloning, expression and purification; and a series of biological engineering technologies and experiments on mice are applied to conduct system analysis on an rSCPZ. After vaccination, the rSCPZ can provide high protective efficacy; an anti-rSCPZ mice double-immunized serum has significant passive immune protection on mice; and the mice immunized by the rSCPZ show high level of antibody titer in serum. The anti-rSCPZ antibody can induce high level of bactericidal capability; an scpZ gene has a transcription level in SEZ (Streptococcus zooepidemicus) infected mice higher than that of culture in vitro; and the rSCPZ can adhere to hep-2 cells and inhibit cell infection ability of SEZ.
Owner:广东艾佩克科技有限公司
Rabies virus resistant specific humanized antibody and application thereof
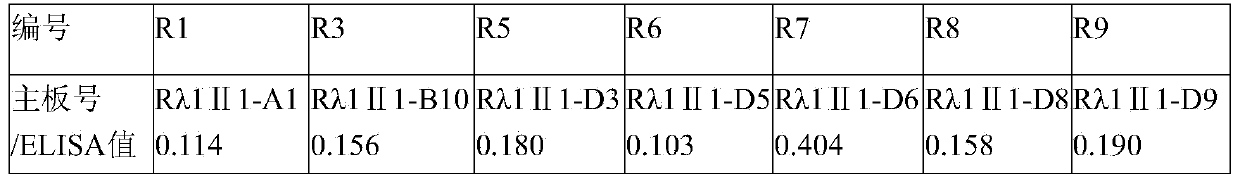
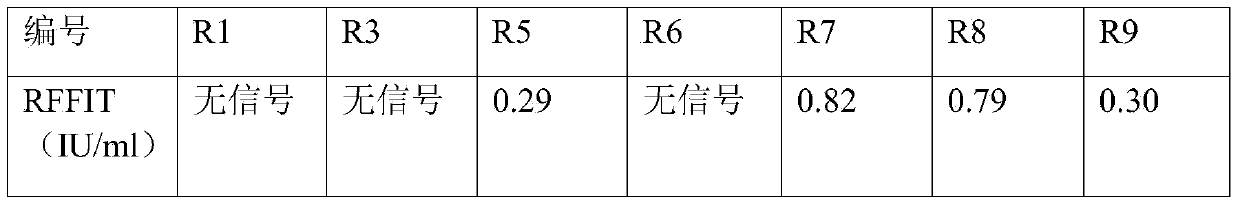
ActiveCN104193823AStrong neutralizing activityImmunoglobulins against virusesAntiviralsPhage antibodiesBacteriophage
The invention aims at providing a rabies virus resistant neutralizing antibody, and particularly provides a humanized or completely humanized monoclonal antibody to meet the requirement for clinically diagnosing and / or treating rabies. A phage antibody library is prepared by adopting a phage antibody library technology and taking 32 parts of high-potency healthy human peripheral blood inoculated with rabies vaccines as a raw material; 7 ELISA positive antibodies are obtained through three rounds of screening from the phage antibody library; and furthermore, the neutralizing activity of the 7 ELISA positive antibodies is measured through an RFFIT method, wherein four ELISA positive antibodies, namely R5, R7, R8 and R9, have higher neutralizing activity in all. The rabies virus resistant neutralizing antibody with high affinity, which is provided by the invention, can be used for substituting ERIG and HRIG to carry out active and / or passive immune therapy on rabies virus seriously-exposed persons.
Owner:LANZHOU INST OF BIOLOGICAL PROD
Immuno-Modulating Compositions for the Treatment of Immune-Mediated Disorders
InactiveUS20110200610A1Lower AST levelsDecreasing ALP levelMetabolism disorderDigestive systemDiseaseAdjuvant
The present invention relates to immunomodulatory compositions comprising mammalian colostrum-derived immunoglobulin preparation and optionally further colostrums, milk or milk product component / s and any adjuvants for treating immune-related disorders. More specifically, the invention provides compositions comprising colostrum-derived anti-insulin immunoglobulin preparations for the treatment of Metabolic Syndrome. The invention further provides methods and uses of the immunomodulatory compositions for an active or passive immunization in a disease-antigen specific or non specific manner.
Owner:IMMURON
Regimens and Compositions for AAV-Mediated Passive Immunization of Airborne Pathogens
PendingUS20190216841A1Organic active ingredientsBacterial antigen ingredientsRegimenHigh level expression
A prophylactic regimen for passively preventing infection with a pathogen which has a typical route of infection through the nasopharynx region of a subject, e.g., an airborne virus typically transmitted through coughing or sneezing. The method involves specifically targeting a subject's nasopharynx with a viral vector comprising an AAV capsid and carrying a nucleic acid sequence encoding an anti-viral neutralizing antibody construct operably linked to expression control sequences, in order to provide for high levels of expression of the anti-viral neutralizing antibody construct in the nasal airway cells. Optionally, the neutralizing antibody construct is expressed under a promoter which is regulated or induced by a small molecule which is delivered separately from the viral vector. In one embodiment, the method permits transfection of a subject's nasopharynx even where the subject has circulating neutralizing antibodies against the AAV capsid.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Antibodies and related molecules that bind to psca proteins
Antibodies and molecules derived therefrom that bind to novel PSCA protein, and variants thereof, are described wherein PSCA exhibits tissue specific expression in normal adult tissue, and is aberrantly expressed in the cancers listed in Table I. Consequently, PSCA provides a diagnostic, prognostic, prophylactic and / or therapeutic target for cancer. The PSCA gene or fragment thereof, or its encoded protein, or variants thereof, or a fragment thereof, can be used to elicit a humoral or cellular immune response; antibodies or T cells reactive with PSCA can be used in active or passive immunization.
Owner:AGENSYS
Compositions and methods for modulating innate and adaptive immune systems
InactiveUS20150299252A1Strong cytotoxicityPeptidesAntibody ingredientsInnate immune systemT lymphocyte
Compositions and methods useful in modulating the innate and adaptive immune systems in a subject, including activation of natural killer (NK) cells and / or CD8+ cytotoxic T lymphocytes. The method typically comprises: administering to the subject a composition comprising a therapeutic peptide or a multivalent structured polypeptide comprising multiple copies of the therapeutic peptide described herein in an amount sufficient to increase activity of NK cells and / or CD8+ cytotoxic T lymphocytes in the subject. Preferred therapeutic compositions comprise a carrier; at least one agent selected from the group consisting of: an anti-inflammatory agent, a cytotoxic T cell proliferation agent, or a NK cell proliferation agent; and a therapeutic peptide or a multivalent structured polypeptides of the invention. In certain embodiments, the composition further comprises an immunoglobulin admixed therewith in an amount sufficient to enhance passive immunoprotection in the subject. In other embodiments, the compositions are administered in a therapeutically effective amount to a subject in need thereof to treat rheumatoid arthritis.
Owner:SUSAVION BIOSCI
Treatment and prevention of cancerous and pre-cancerous conditions of the liver, lung and esophagus
InactiveUS20110117108A1Inhibit transferInhibition transitionHeavy metal active ingredientsSnake antigen ingredientsEsophageal cancerAdjuvant therapy
The invention relates to the treatment and / or prevention of cancerous and / or, precancerous conditions of the liver, lung and esophagus by actively and / or passively immunizing a patient against the peptide hormone gastrin and / or a gastrin receptor, e.g., the CCK-B / gastrin receptor. The immunizations of the invention may be employed as a monotherapy, an adjunctive therapy, or as part of a combination therapy.
Owner:CANCER ADVANCES INC
Porcine epidemic diarrhea virus and application thereof
ActiveCN103820399APassive immunity is goodProduced fastInactivation/attenuationImmunoglobulins against virusesMicroorganismMating
The invention provides a porcine epidemic diarrhea virus strain SD10 which is preserved in General Microbiology Center of China Committee for Culture Collection of Microorganisms, School of Chinese Academy of Sciences Committee, at Beichen West Road, No. 1 yard, No. 3, Chaoyang District, Beijing, on November 8, 2013, and the preservation number is CGMCC No. 8503. Compared with the conventional viruses, the porcine epidemic diarrhea virus strain SD10 screened by the invention is low in toxicity, and good in immunogenicity; the prepared vaccine can quick generate antibodies after immunizing; the generated antibodies are high in titer and long in duration; the vaccine is long in storage life, and small in immunity dose, and can enable piglets of a pregnant sow to get better ectophylaxination if being injected to the pregnant sow before mating, so that the piglets can generate strong immune and can resist virulent attacks, and the piglet survival is improved.
Owner:YEBIO BIOENG OF QINGDAO
Streptococcus protective antigen SAP and preparation method thereof
InactiveCN106008680AHighly conservativeStrong specificityDepsipeptidesNucleic acid vectorProtective antigenEscherichia coli
The invention provides streptococcus protective antigen SAP and a preparation method thereof. The antigen SAP is SAP recombination protein of SEZ. The preparation method comprises the following steps: firstly obtaining a target gene through a PCR technology, connecting the target gene to a carrier and converting escherichia coli, and performing purification on an expression product induced by escherichia coli, so as to finally obtain target recombination protein. The streptococcus protective antigen SAP has high conservatism, vaccine prepared from the protein is high in specificity, a series of bioengineering technologies and mouse experimental analysis prove that the SAP can provide higher protective efficacy after immunization, anti-SAP mice second immune serum has obvious passive immune protection to mice, and high-level antibody titer is expressed in mice serum of the SAP after immunization. The transcriptional level of SAP gene in mice body infected by SEZ is higher than that of SAP gene cultured in vitro, and an SAP antibody can induce high-level bactericidal ability.
Owner:FOSHAN UNIVERSITY
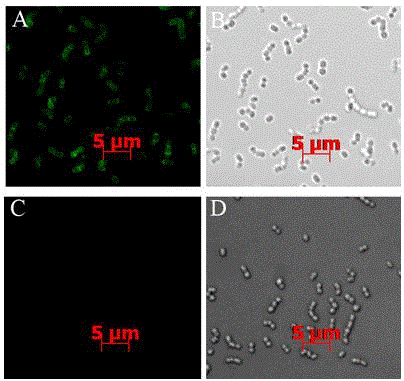
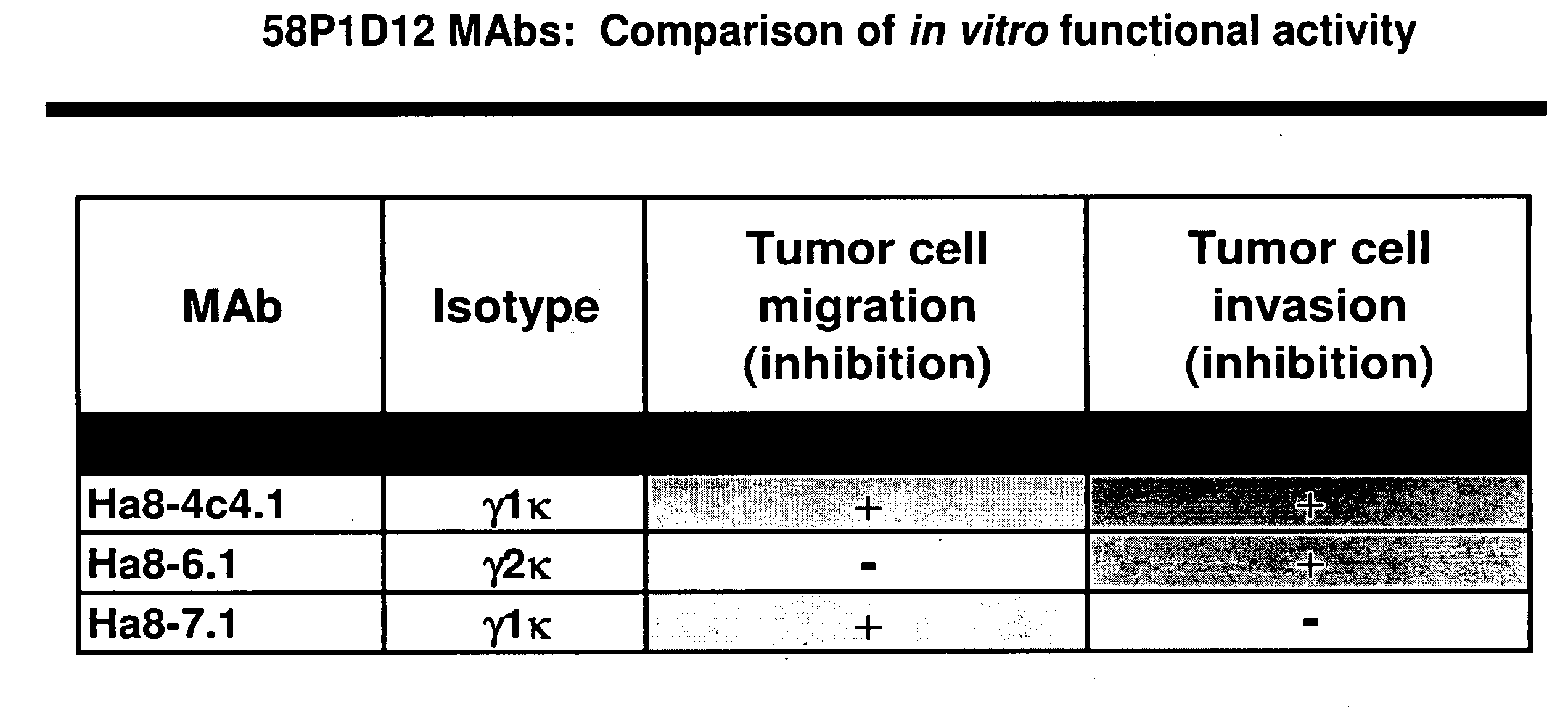
Antibodies and related molecules that bind to 58p1d12 proteins
UndeterminedUS20090068098A1Inhibiting transcriptionInhibition of translationOrganic active ingredientsAntibody mimetics/scaffoldsProtein antibodyTissue specific
Antibodies and molecules derived therefrom that bind to 58P1D12 protein and variants thereof, are described wherein 58P1D12 exhibits tissue specific expression in normal adult tissue, and is aberrantly expressed in the cancers listed in Table I. Consequently, 58P1D12 provides a diagnostic, prognostic, prophylactic and / or therapeutic target for cancer. The 58P1D12 gene or fragment thereof, or its encoded protein, or variants thereof, or a fragment thereof, can be used to elicit a humoral or cellular immune response; antibodies or T cells reactive with 58P1D12 can be used in active or passive immunization.
Owner:AGENSYS
Antibodies against protective antigen and methods of use for passive immunization and treatment of anthrax
InactiveUS20080063647A1Avoid toxicityReduce severityAntibacterial agentsImmunoglobulins against bacteriaProtective antigenMammal
Owner:EMERGENT PROD DEV GAITHERSBURG INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com