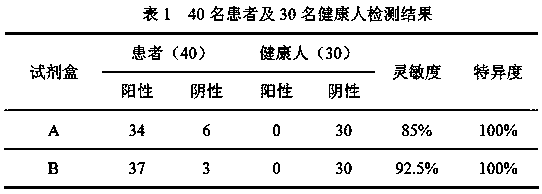
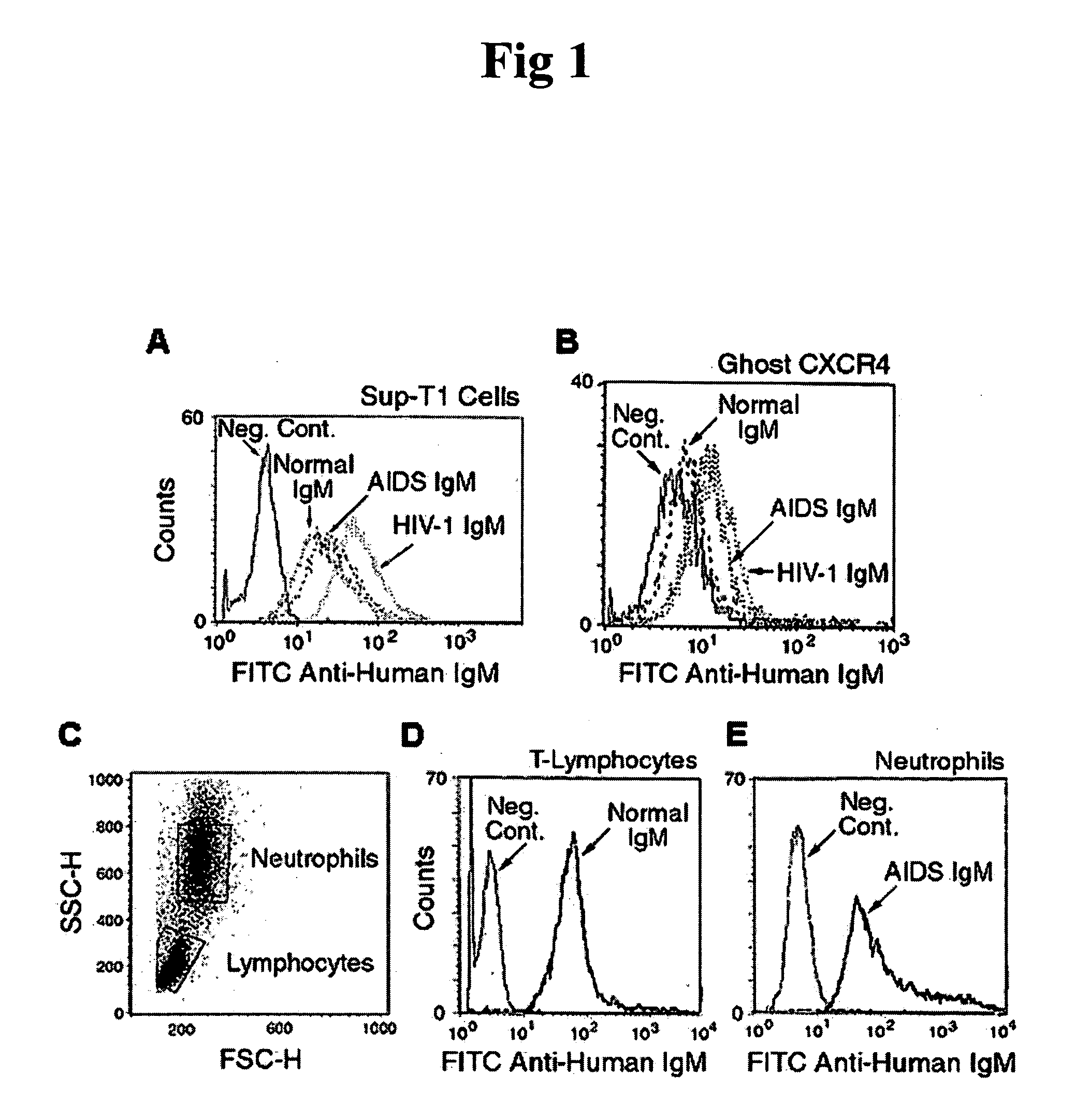
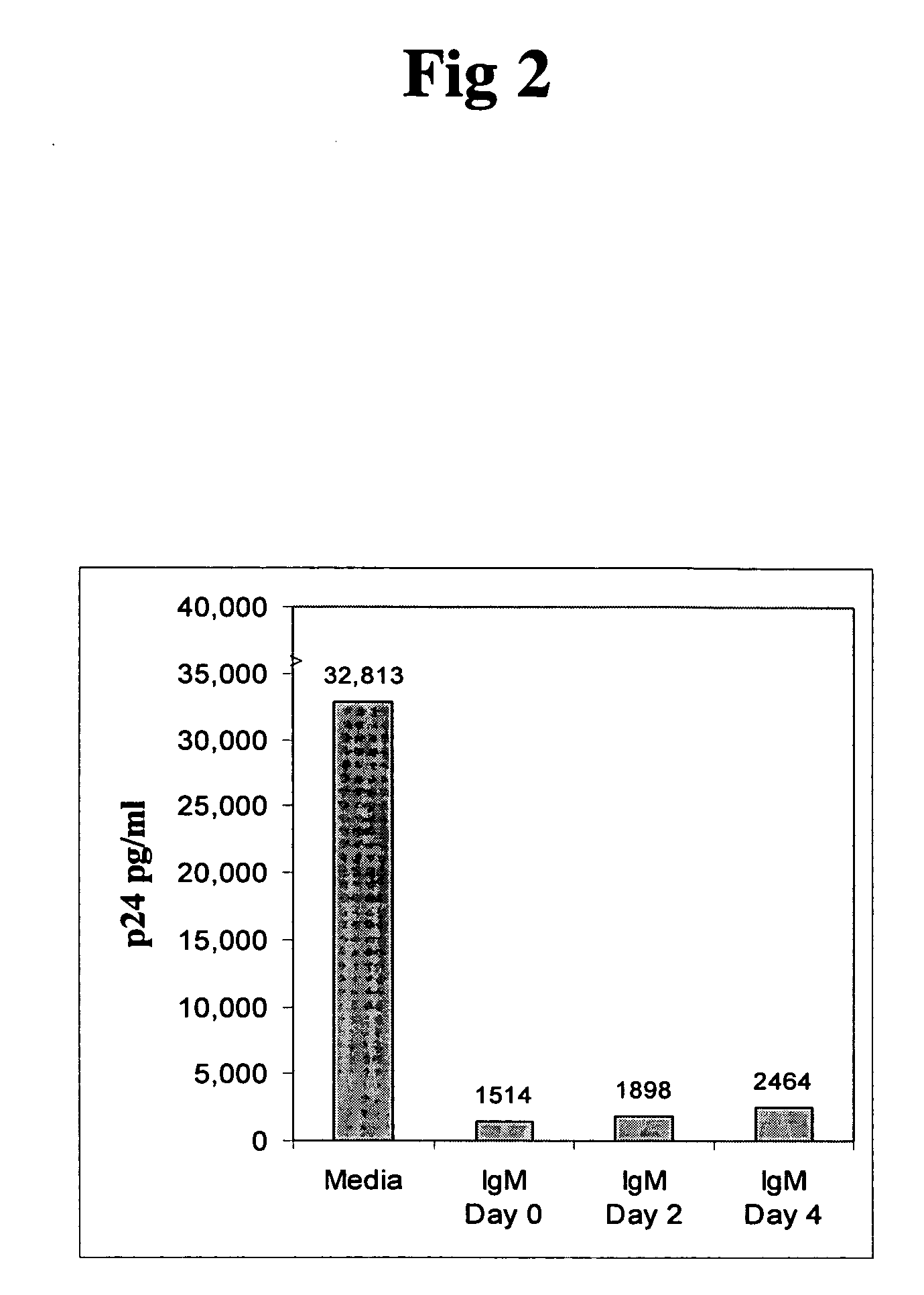
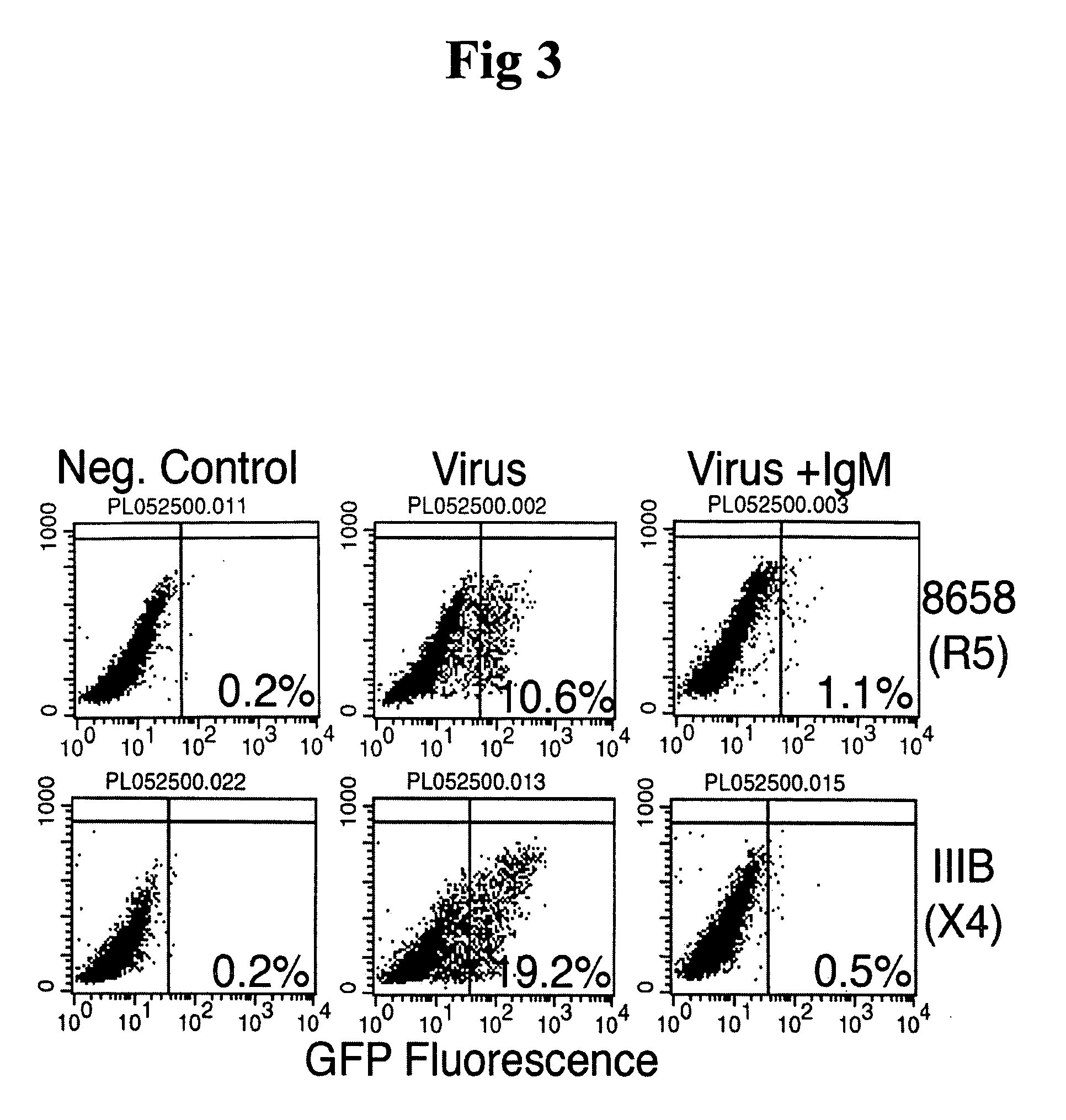
Patents
Literature
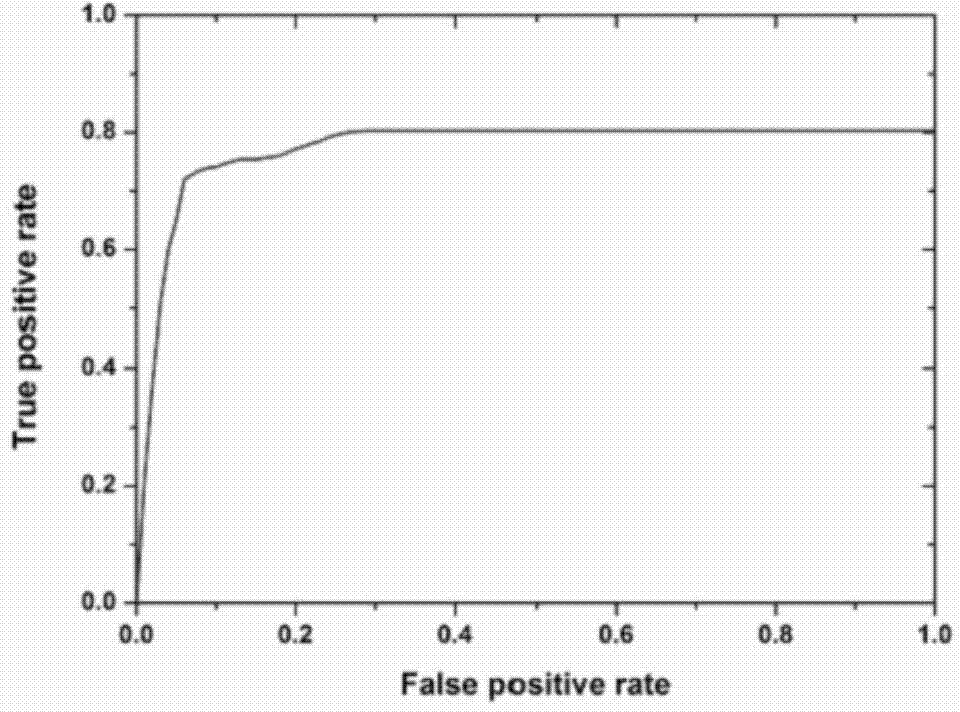
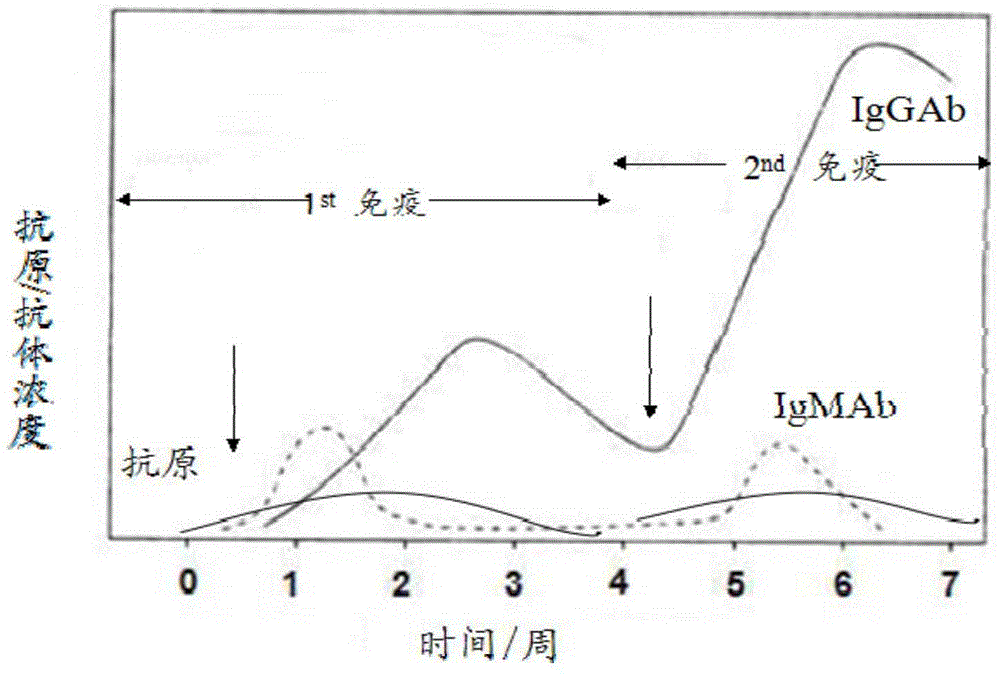
363 results about "Igm antibody" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
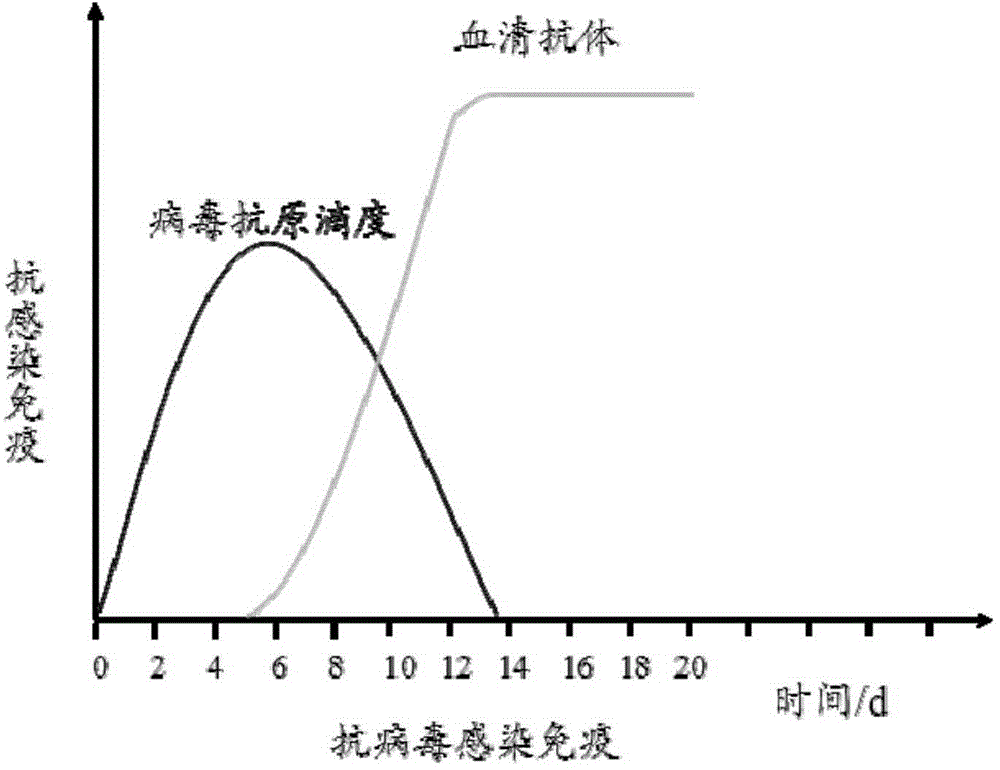
The IgM antibody is the largest of the antibodies and found mostly in the lymph fluid and the blood. It is generally responsible for neutralizing antigen invasion during the early stage of the disease, until enough IgG antibodies are produced.
Colloidal gold kit for jointly detecting coronavirus IgM/IgG antibody, and preparation method thereof
ActiveCN111089962ASimple preparation processEasy to useMaterial analysisCoronavirus antibodyIgm antibody
The invention discloses a colloidal gold kit for jointly detecting coronavirus IgM / IgG antibody, and a preparation method thereof, and relates to the field of biological medicine. Whether anti-novel coronavirus nucleocapsid protein IgM antibody and / or anti-novel coronavirus nucleocapsid protein IgG antibody exists in human serum or plasma or not by adopting an antigen-antibody sandwich method anda colloidal gold immunochromatography method principle, the novel coronavirus nucleocapsid protein containing 6xHis mark is marked by applying colloidal gold, thereby forming gold-marked N protein tobe adsorbed on a gold-marked pad, the novel coronavirus nucleocapsid protein containing 6xHis mark is used as an indication marker, the mouse-anti-human u chain monoclonal antibody is coated on the IgM detection line of a NC membrane, the mouse-anti-human IgG monoclonal antibody is coated on the IgG detection line and the mouse-anti 6xHis monoclonal antibody is coated on a quality control line ofthe NC membrane, the qualitative detection of the anti-novel coronavirus nucleocapsid protein IgG antibody is realized, and the colloidal gold kit disclosed by the invention has the advantages of being convenient to use, high in sensitivity and short in detection time.
Owner:中山生物工程有限公司
Human IgM antibodies, and diagnostic and therapeutic uses thereof particularly in the central nervous system
InactiveUS7473423B2Promote reproductionPromote safer self-therapiesNervous disorderPeptide/protein ingredientsNervous systemIgm antibody
Antibodies, and particularly human antibodies, are disclosed that demonstrate activity in the treatment of demyelinating diseases as well as other diseases of the central nervous system that are of viral, bacterial or idiopathic origin, including neural dysfunction caused by spinal cord injury. Neuromodulatory agents are set forth that include and comprise a material selected from the group consisting of an antibody capable of binding structures or cells in the central nervous system, a peptide analog, a hapten, active fragments thereof, agonists thereof, mimics thereof, monomers thereof and combinations thereof. The neuromodulatory agent has one or more of the following characteristics: it is capable of inducing remyelination; binding to neural tissue; promoting Ca++ signaling with oligodendrocytes; and promoting cellular proliferation of glial cells. Amino acid and DNA sequences of exemplary antibodies are disclosed. Methods are described for treating demyelinating diseases, and diseases of the central nervous system of humans and domestic animals, using polyclonal IgM antibodies and human monoclonal antibodies sHIgm22(LYM 22), sHIgm46(LYM46) ebvHIgM MSI19D10, CB2bG8, AKJR4, CB2iE12, CB2iE7, MSI19E5 and MSI10E10, active fragments thereof and the like. The invention also extends to the use of human antibodies, fragments, peptide derivatives and like materials, and their use in diagnostic and therapeutic applications, including screening assays for the discovery of additional antibodies that bind to cells of the nervous system, particularly oligodendrocytes.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Isolated human cytomegalovirus polypeptides and uses thereof
InactiveUS6120989APeptide/protein ingredientsAntibody mimetics/scaffoldsIgm antibodyHuman cytomegalovirus
Diagnostically relevant polypeptides and fusion proteins comprising an amino acid sequence which originates from cytomegalovirus and corresponds to a region of the major DNA-binding protein or of the C-terminal region of the tegument protein pp150 fused with at least one further fragment from another antigenic protein of cytomegalovirus are disclosed. The major DNA-binding protein is encoded by the reading frame UL57. The poly-peptides and fusion proteins according to the invention can be used in an advantageous manner in diagnostic tests and methods for the detection of IgM antibodies against cytomegalovirus.
Owner:BIOTEST SERUM INST GMBH
Phosphorylcholine Conjugates and Corresponding Antibodies
ActiveUS20070286868A1Increased and decreased riskSignificant positive effectImmunoglobulins against animals/humansAntibody ingredientsPhosphorylcholineIgm antibody
IgG and IgM autoantibody levels against phosphorylcholine in subjects with hypertension (diastolic pressure>95 mmHg) were determined at baseline in order to determine the importance of antibodies for the development of atherosclerosis. The results show that increases in intima-media thickness (IMT) at a follow-up four years after baseline were significantly less prevalent in subjects having high IgM autoantibodies to phosphorylcholine. The presence or absence of IgM autoantibodies against phosphorylcholine is thus related to an increased or decreased risk of developing ischemic cardiovascular diseases. A method to determine IgM antibodies toward phosphorylcholine is proposed in this invention to identify subjects at risk of developing ischemic cardiovascular diseases. Animal experiments show that medium to high levels of IgM antibodies can be detected in plasma after active immunization with a keyhole limpet hemocyanin (KLH)-phosphorylcholine conjugate. A pharmaceutical composition comprising a phosphorylcholine conjugate (active immunization) or a monoclonal antibody with specificity to a phosphorylcholine conjugate (passive immunization) is proposed and the use of these compositions as active or passive immunogens in the treatment or prevention of atherosclerosis.
Owner:ATHERA BIOTECH
Novel coronavirus detection kit
PendingCN111337689AThe detection process is fastEasy to operateBiological testingImmunoassaysIgm antibodyColloidal au
The invention discloses a novel coronavirus detection kit. The kit comprises a bottom plate, and a sample pad, a colloidal gold pad, an NC membrane and a water absorption pad which are fixed on the bottom plate, wherein a detection line and a quality control line are arranged on the NC membrane, an antigen coated on the colloidal gold pad is a mixed antigen protein composed of N protein marked bycolloidal gold and S-RBD protein marked by colloidal gold, and the detection line is a mouse anti-human IgM antibody and mouse anti-human IgG antibody detection line. The detection kit provided by theinvention takes N protein and S-RBD protein as antigens, can improve the sensitivity of COVID-19 preliminary detection, and is suitable for quick screening of large-scale crowds.
Owner:SHANXI MEDICAL UNIV
Naturally occuring IgM antibodies that bind to lymphocytes
InactiveUS20050220787A1Inhibits HIV- infectivityImprove the level ofImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsLymphocyte AntibodyDisease
In this invention, the inventor discloses that naturally occurring IgM anti-lymphocyte antibodies bind to chemokine and non-chemokine receptors on lymphocytes and other cells, and downmodulate certain receptors including CD4 and CD2 on T cells and CD80 and CD86 on macrophages. The inventor also discloses that such antibodies (i) inhibit HIV-1 and other viruses from infecting cells (ii) inhibits activation and proliferation of T lymphocytes (iii) inhibits cytokine and chemokine production (iv) inhibits inflammatory processes, and (v) enhances death of malignant cells. This art or invention is novel in that the antibodies described herein are “naturally occurring” i.e. develop in absence of deliberate immunization and secondly these antibodies are distinct from disease causing autoantibodies in that these naturally occurring antibodies are polyreactive with low binding affinity.
Owner:LOBO PETER ISAAC
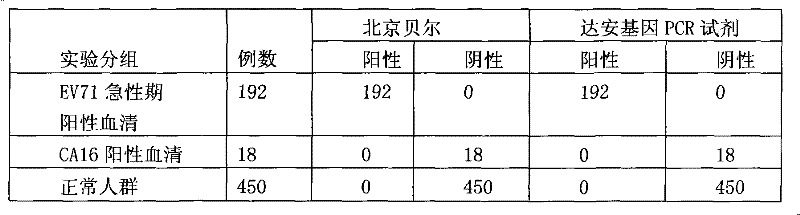
Diagnostic reagent kit (enzyme-linked immunosorbent assay (ELISA)) for enterovirus (EV) 71-type antibody (immune globulin M (IgM))
InactiveCN102243232AEasy to operateAvoid distractionsColor/spectral properties measurementsEnterovirusAbzyme
The invention relates to the field of biomedicine, in particular to an enzyme-linked immunization diagnostic reagent kit for detecting an enterovirus (EV) 71-type antibody (immune globulin M (IgM)), and a preparation method and application of the diagnostic reagent kit. The probability of hand-foot-and-mouth disease and severe infection (viral encephalitis, viral cerebrospinal meningitis and pulmonary edema) caused by EV71 type is relatively higher, and case fatality rate is relatively higher and can be 10 to 25 percent. The enzyme-linked immunization diagnostic reagent kit of the EV71-IgM antibody can be used for diagnosing the infection of the EV71 type. According to related documents about the detection of the EV71-IgM, EV71 virus cultures serving as indirect enzyme-linked immuno sorbent assay (ELISA) of envelope antigens has defects in such aspects as specificity, sensitivity and stability, and due to high cultivation cost and low efficiency, a large amount of virus cannot be supplied to the market. In order to overcome the defects, the invention provides the reagent kit which is used for detecting the EV71-IgM in human blood serum, required by clinical examination, simple and convenient to operate and applicable to all medical disease control departments, and the preparation method and the application of the reagent kit. The invention has the technical scheme that: firstly, the human blood serum is added into a micro-pore plate, wherein the IgM antibody is obtained by an anti-mu chain which is pre-enveloped on the micro-pore plate, and other uncombined components are washed and removed; secondly, an enzyme labeling object is added, the EV71-IgM in the obtained IgM can be combined with the specificity of an EV71 recombinant antigen which is labeled by horse radish peroxidase (HRP), and after washing, the HRP can react with substrates which are added subsequently; and finally, the aim of detecting the EV71-IgM antibody is fulfilled.
Owner:BEIJING BEIER BIOENG
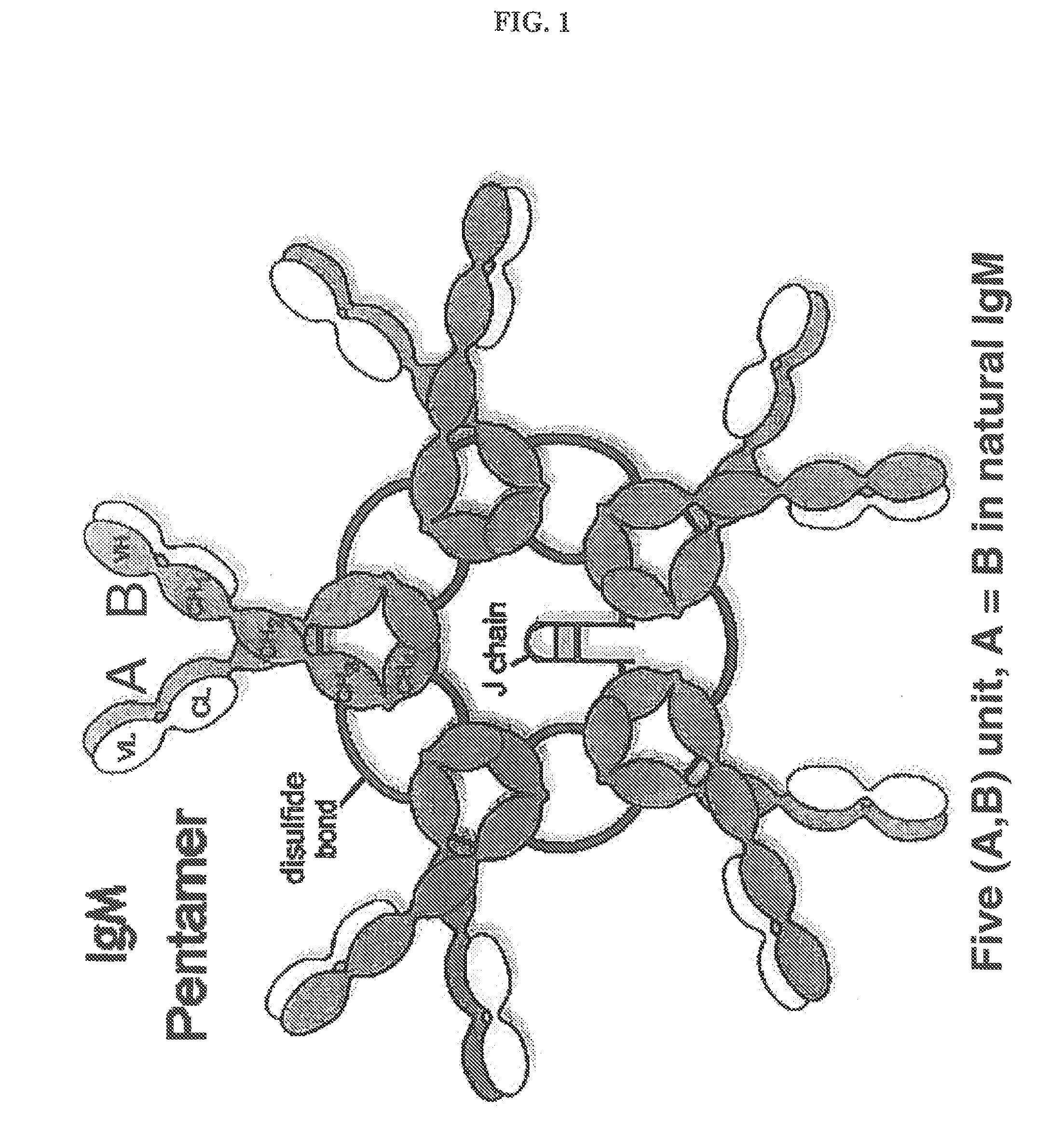
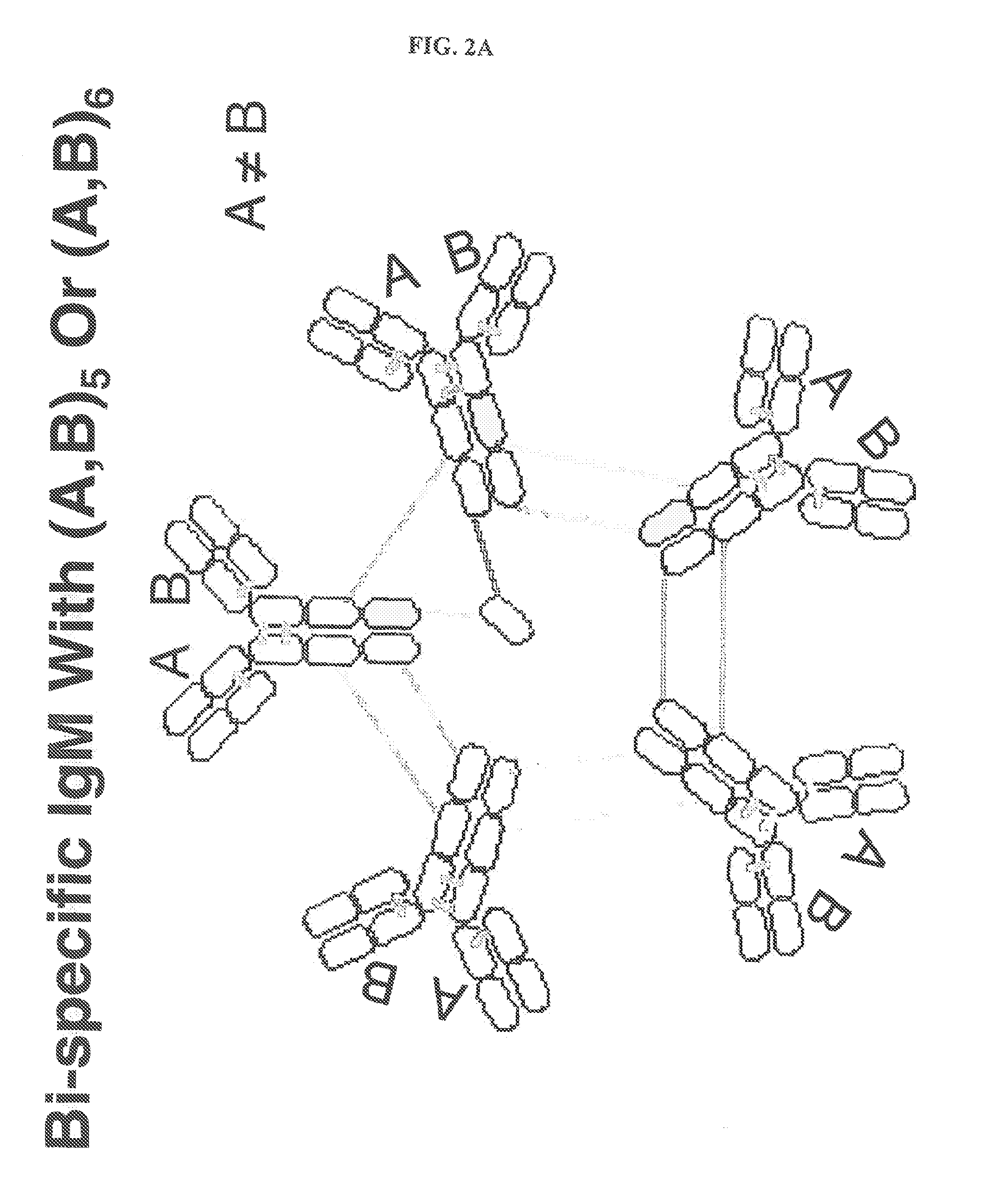
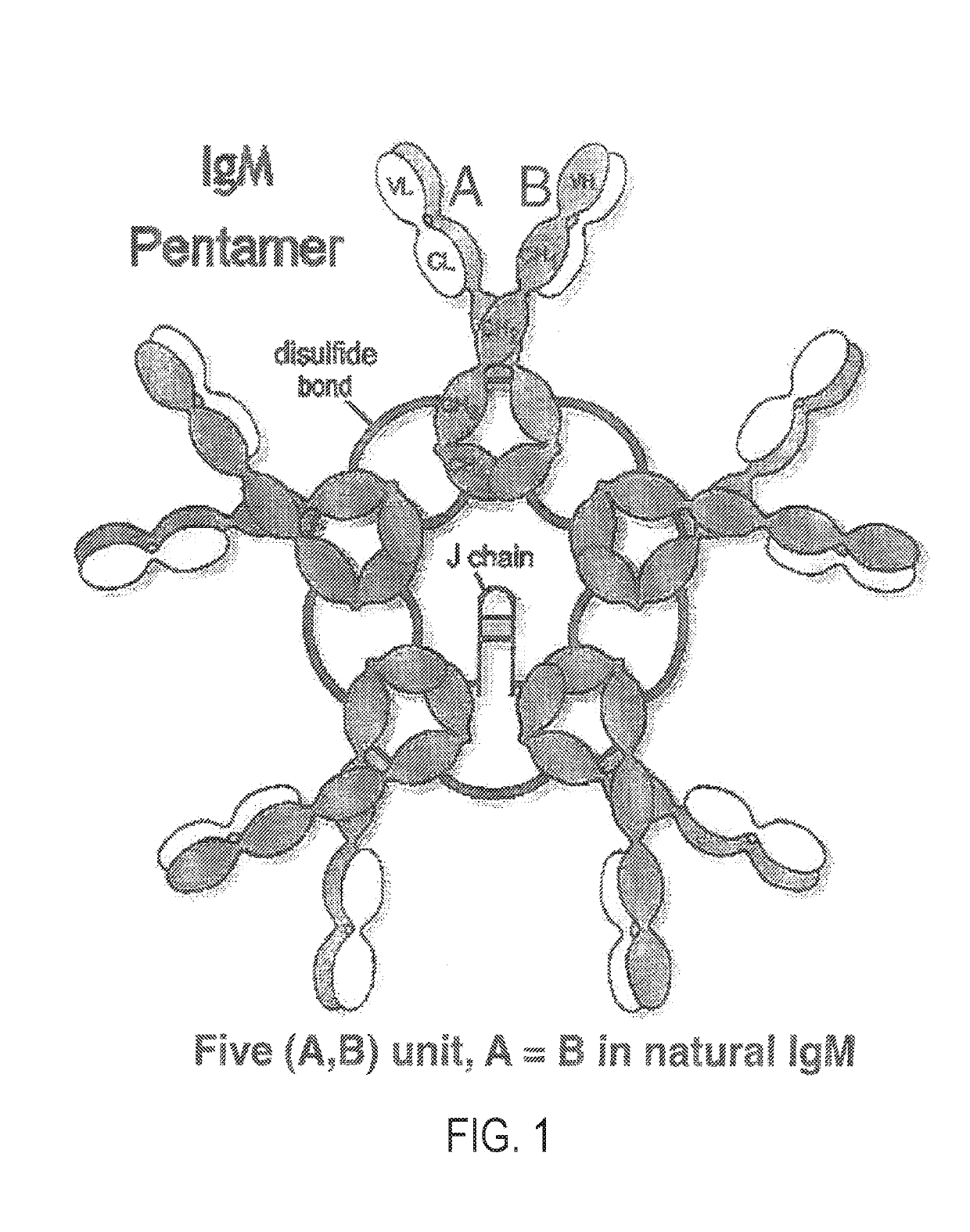
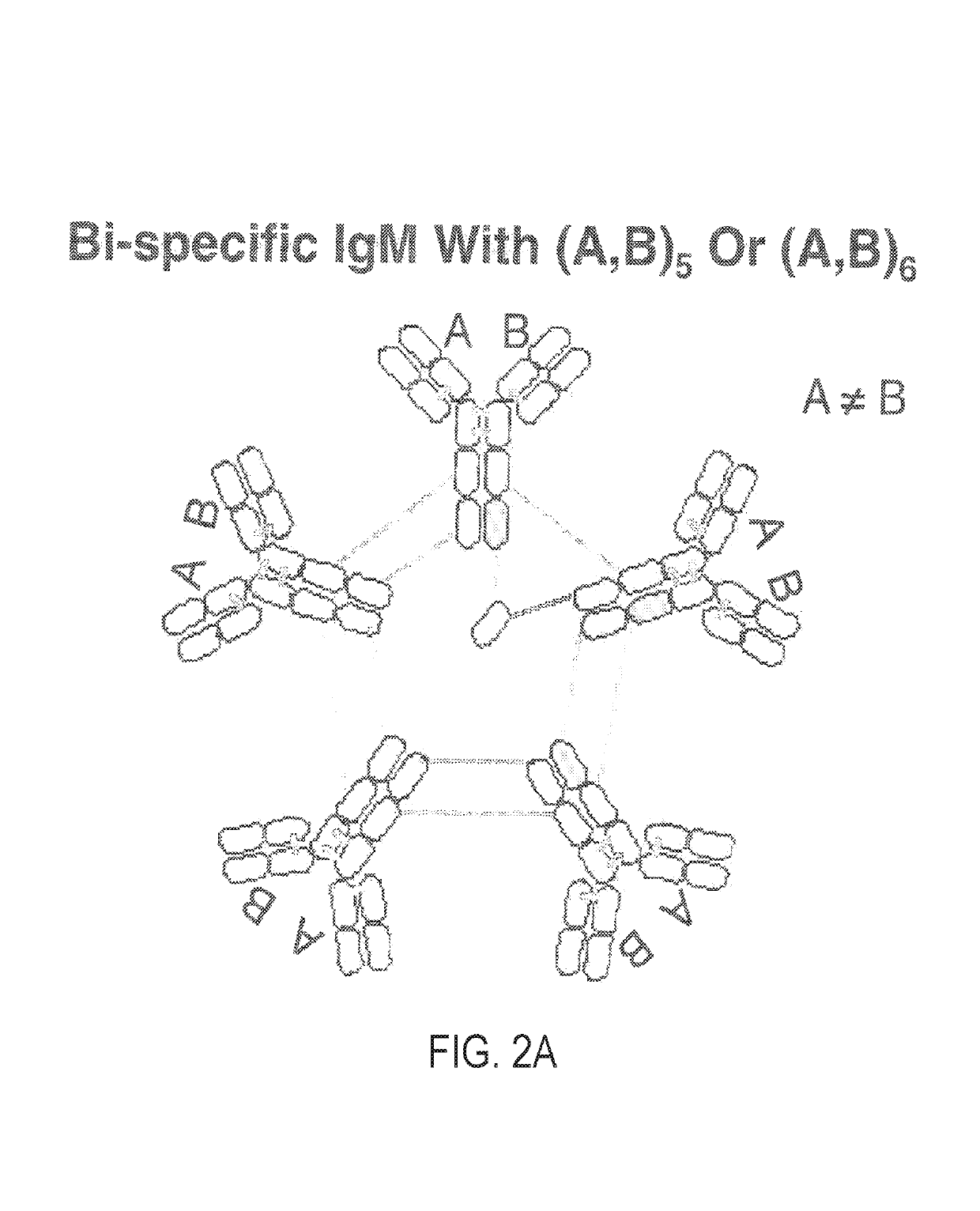
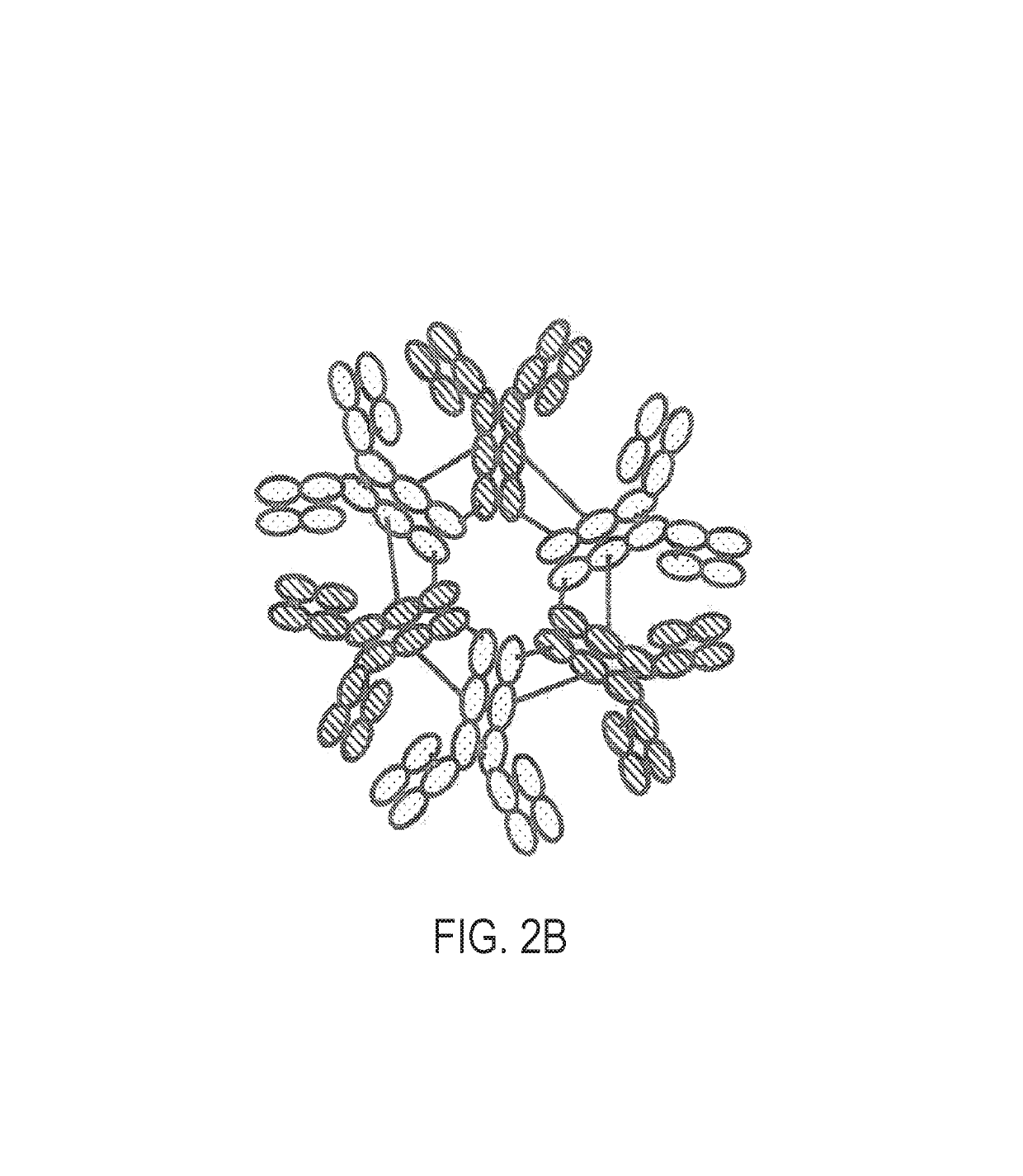
Constant chain modified bispecific, penta- and hexavalent ig-m antibodies
ActiveUS20160222132A1Hybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsCrystallographyIgm antibody
Owner:IGM BIOSCI INC
Colloidal gold method detection test strip and reagent kit for IgM and IgG antibodies of mycoplasma pneumoniae and preparation method of reagent kit
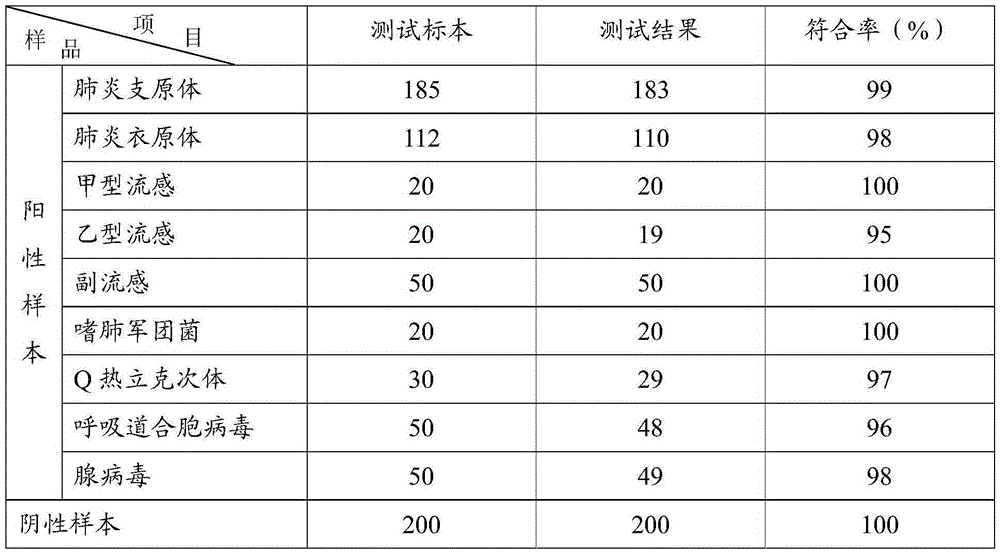
The invention discloses a colloidal gold method detection test strip and a reagent kit for IgM and IgG antibodies of mycoplasma pneumonia (MP) and a preparation method of the reagent kit. The test strip determines the IgM antibody and the IgG antibody by using a principle of an immunocapture method; the specific IgM and IgG antibodies of the MP can be detected jointly by one operation; the operation process is simplified; and the reagent kit is simple, convenient, rapid and accurate in detection, is particularly applicable to primary screening and epidemiological survey, and has an auxiliary diagnosis effect on early and interim mycoplasma pneumoniae infection.
Owner:JIANGSU KEYGEN BIOTECH CORP LTD
Dengue virus IgG/IgM antibody detection test strip, kit and preparation method thereof
InactiveCN110007096ASmall sample sizeHigh detection sensitivityBiological testingAgainst vector-borne diseasesIgm antibodyDisease course
The invention discloses a dengue virus IgG / IgM antibody detection test strip, kit and preparation method thereof, and relates to the technical field of dengue virus detection. The dengue virus IgG / IgMantibody detection test strip of the invention comprises a sample pad, an immune combination pad, a nitrocellulose membrane and absorbent paper, wherein the sample pad is sequentially stuck to the bottom of polyvinyl chloride; the immune combination pad is coated with dengue virus recombinant antigen marked by colloidal gold and chicken IgY polyclonal antibody; the nitrocellulose membrane is coated with dengue virus anti-human IgM antibody, a detection line of IgG antibody and a quality control line of anti-chicken IgY polyclonal antibody. The test strip can detect whether dengue virus IgG / IgM antibody exists in a sample to be detected through a method for detecting a marker. The test strip and the detection card comprising the test strip can be used as supplement for antigen detection onthe early acute infection of the dengue virus, covering the middle and later stages of the disease course and reducing the risk of missed detection.
Owner:JIANGSU BIOPERFECTUS TECH CO LTD
Chemiluminescence immune analysis determination reagent kit for detecting Toxoplasma Gondi IgM antibody
The invention discloses a toxoplasma gondii IgM antibody detection kit combined with the FITC-anti-FITC indirect coating technology and the chemiluminescent immunoassay technology, and a preparation method thereof. The kit of the invention is composed of a negative control, a positive control, solid-phase vectors for anti-FITC antibodies, anti-human Mu-chain monoclonal antibodies of FITC markers, toxoplasma gondii antigens which are marked by horse radish peroxidase, chemiluminescent substrates and concentrated washing solutions. The kit of the invention can be used as the aided detection index for prenatal prepotency diagnosis, and has vital significances for improving the birth population quality and doing the family planning and the prepotency well.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
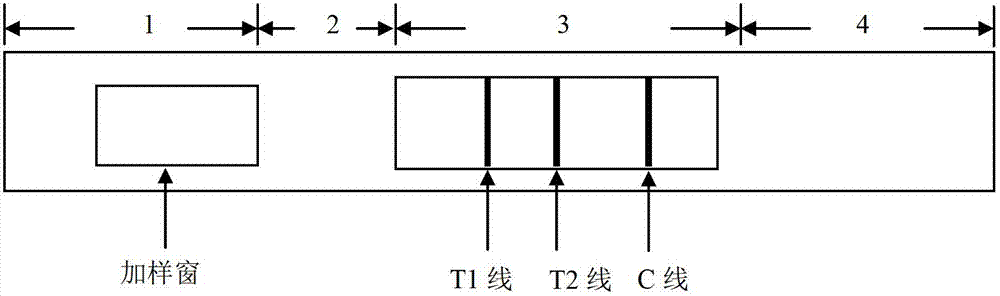
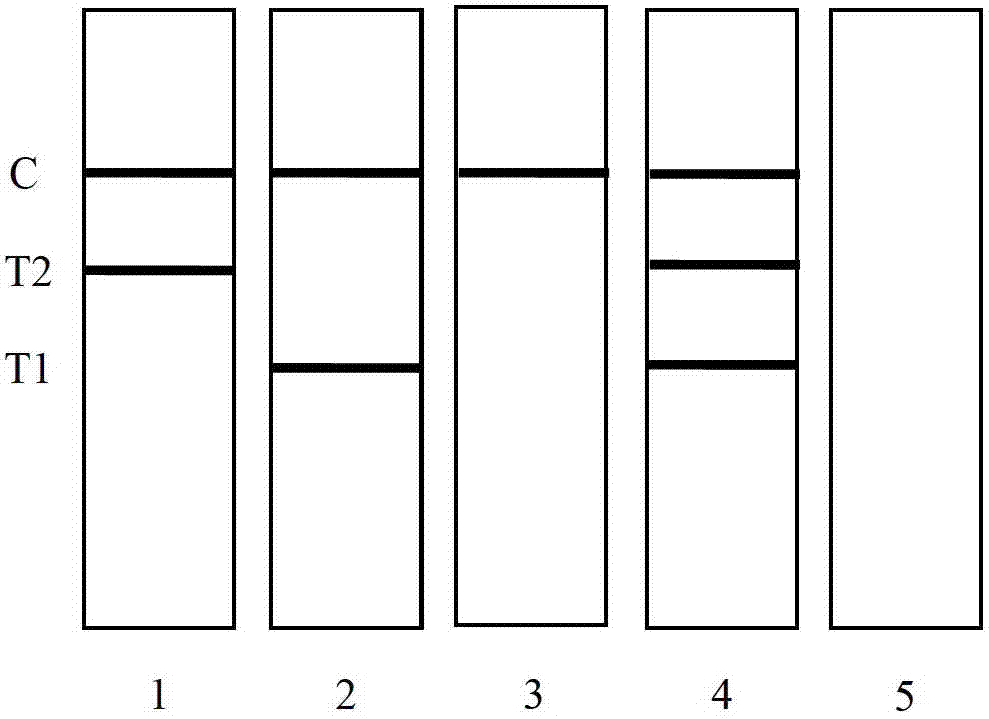
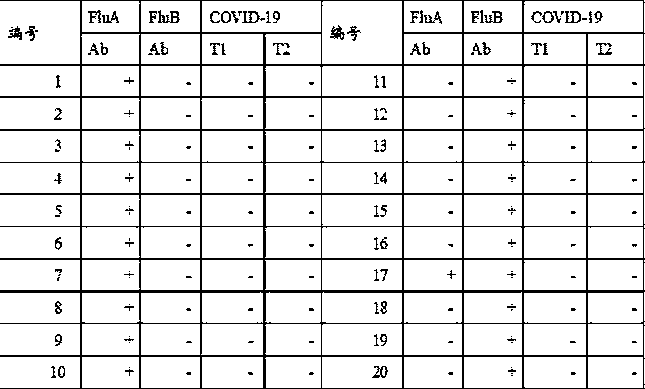
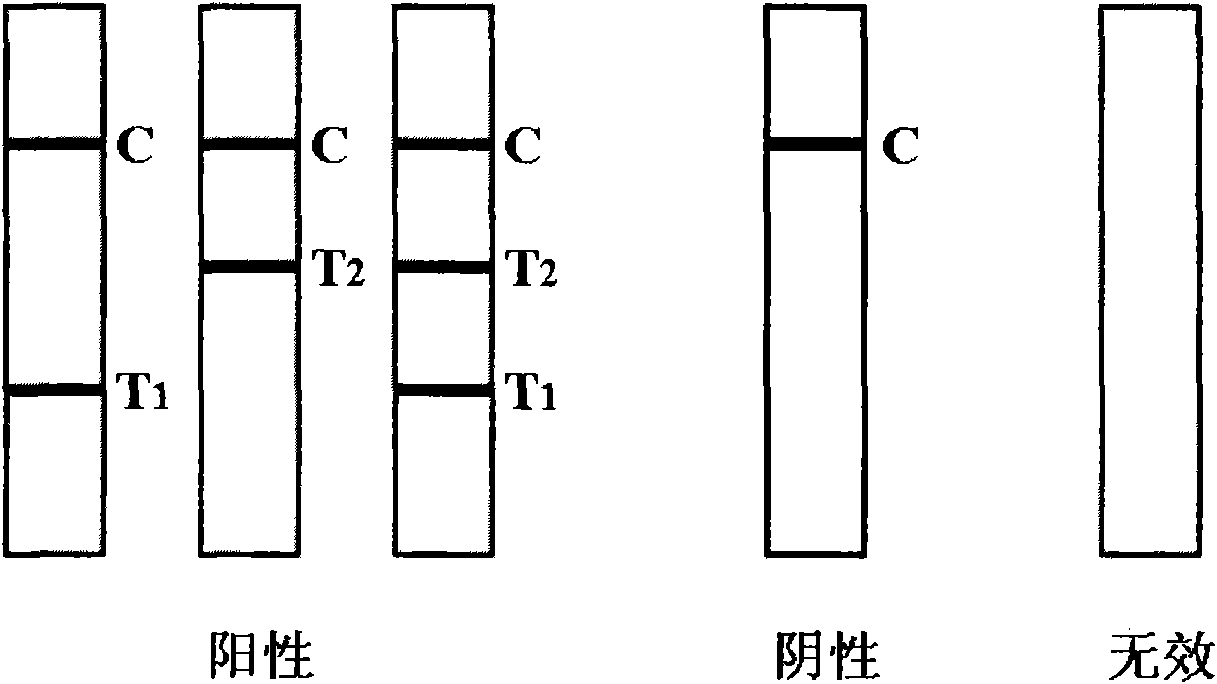
Novel fluorescence immunochromatography test strip for joint inspection of SARS-CoV-2 IgG-IgM antibodies of coronaviruses
PendingCN111426844ARapid Quantitative DetectionHigh sensitivityBiological testingImmunoassaysIgm antibodyStructural protein
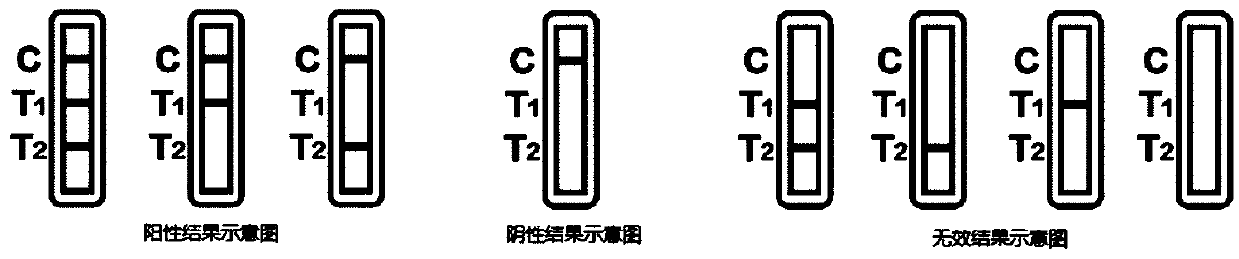
The invention discloses a novel fluorescence immunochromatography test strip for joint inspection of SARS-CoV-2 IgG-IgM antibodies of coronaviruses. The test strip comprises a bottom plate, a sample pad, a combination pad, a nitrocellulose membrane and a water absorption pad, wherein the sample pad, the combination pad, the nitrocellulose membrane and the water absorption pad are sequentially connected end to end and adhered to the bottom plate; the combination pad is coated with an SARS-CoV-2 structural protein-marker and a goat anti-rabbit IgG-marker, and the nitrocellulose membrane is provided with a detection line T1 coated with a mouse anti-human IgG monoclonal antibody, a detection line T2 coated with a mouse anti-human IgM monoclonal antibody and a quality control line C coated withrabbit IgG. When the test strip is used for quantitatively detecting SARS-CoV-2 IgG and IgM antibodies, the detection sensitivity is high, and the specificity is good and can reach 96%; the batch-to-batch difference is small, and good repeatability is achieved; the test strip can be stored for half a year at normal temperature without reducing the sensitivity and has good stability; the test strip is simple to operate and low in cost, can quickly and quantitatively detect the levels of SARS-CoV-2 IgG and IgM antibodies in a human body, assists a nucleic acid detection means, and provides powerful support for epidemic situations.
Owner:NANJING AGRICULTURAL UNIVERSITY
Kit and method for comprehensively evaluating functions of immune cells in human peripheral blood
The invention provides a kit and a method for comprehensively evaluating functions of immune cells in human peripheral blood. The kit for comprehensively evaluating the functions of the immune cells in the human peripheral blood comprises an anti-human CD3, CD4, CD8, CD19, CD21, CD24, CD25, CD27, CD28, CD38, CD56, CD57, CD94, CD127, CD45RA, CXCR3, CXCR5, CCR4, CCR6, CCR7, HLA-DR, PD-1, P30, P46, NKG2D, KIR (NKB1), gamma delta, v delta 2, IgD and IgM antibodies, wherein the antibodies carry fluorescein labels. The kit can be used for comprehensively evaluating the functions of the immune cellsin the human peripheral blood, and is convenient and safe to use.
Owner:东莞暨南大学研究院
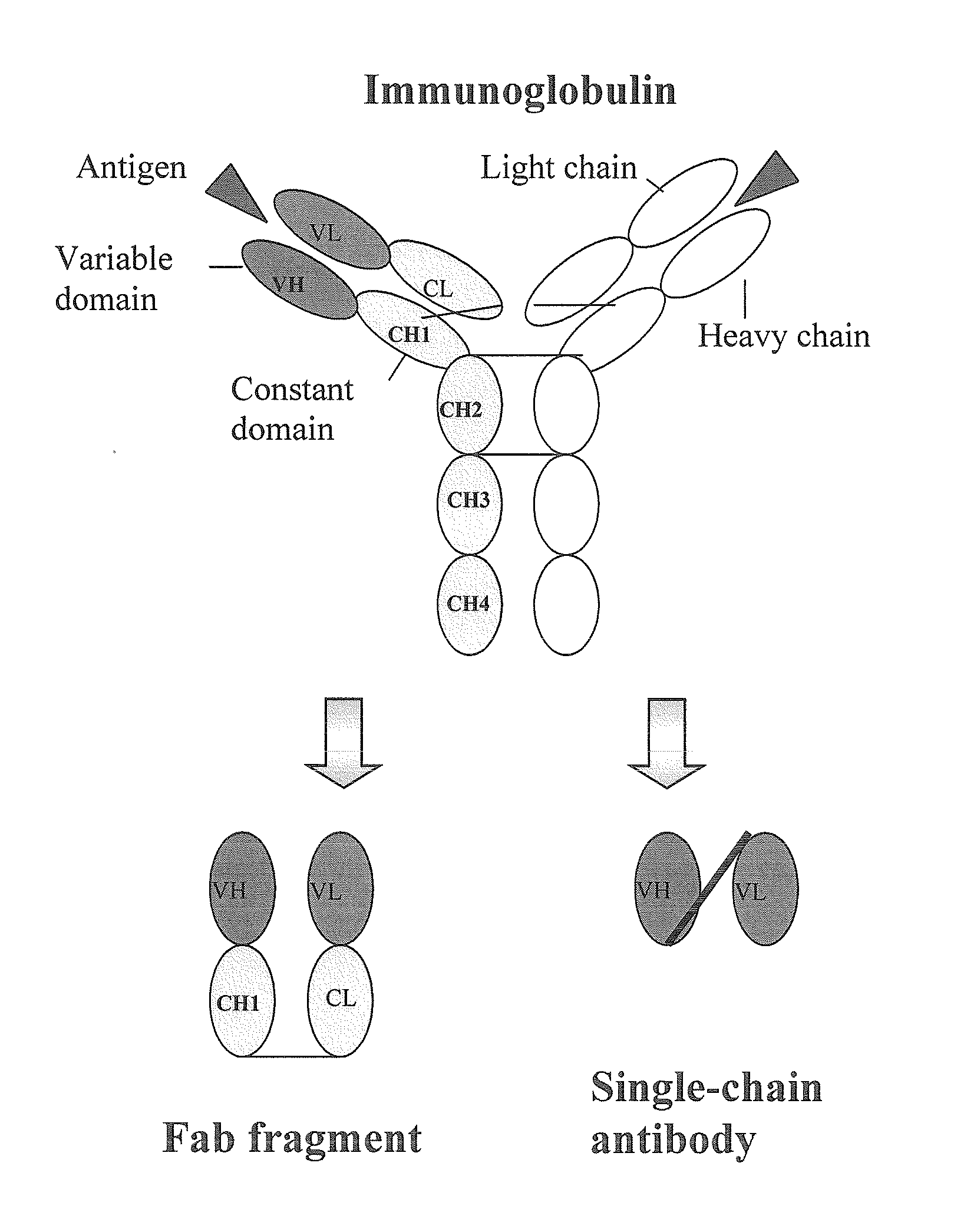
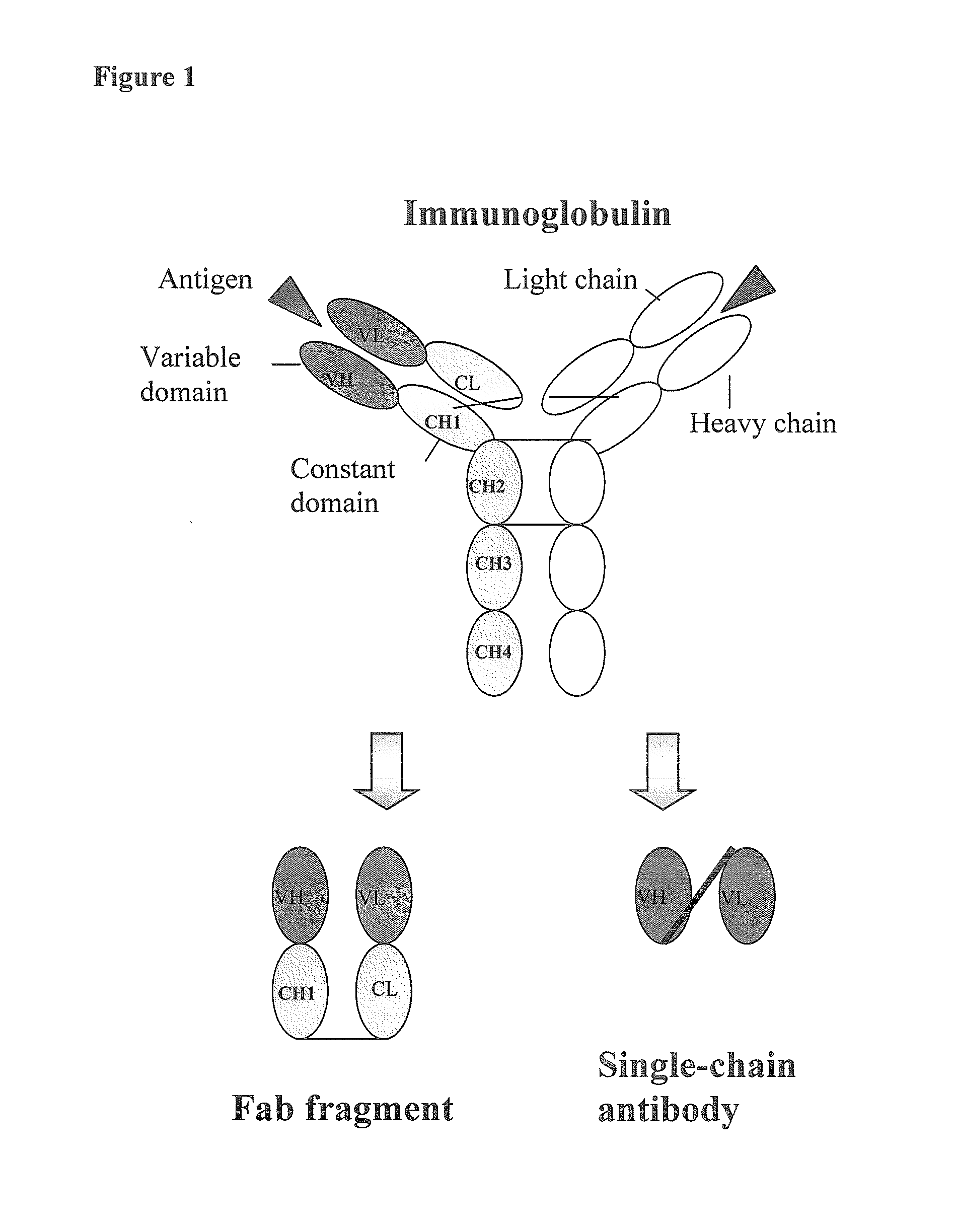
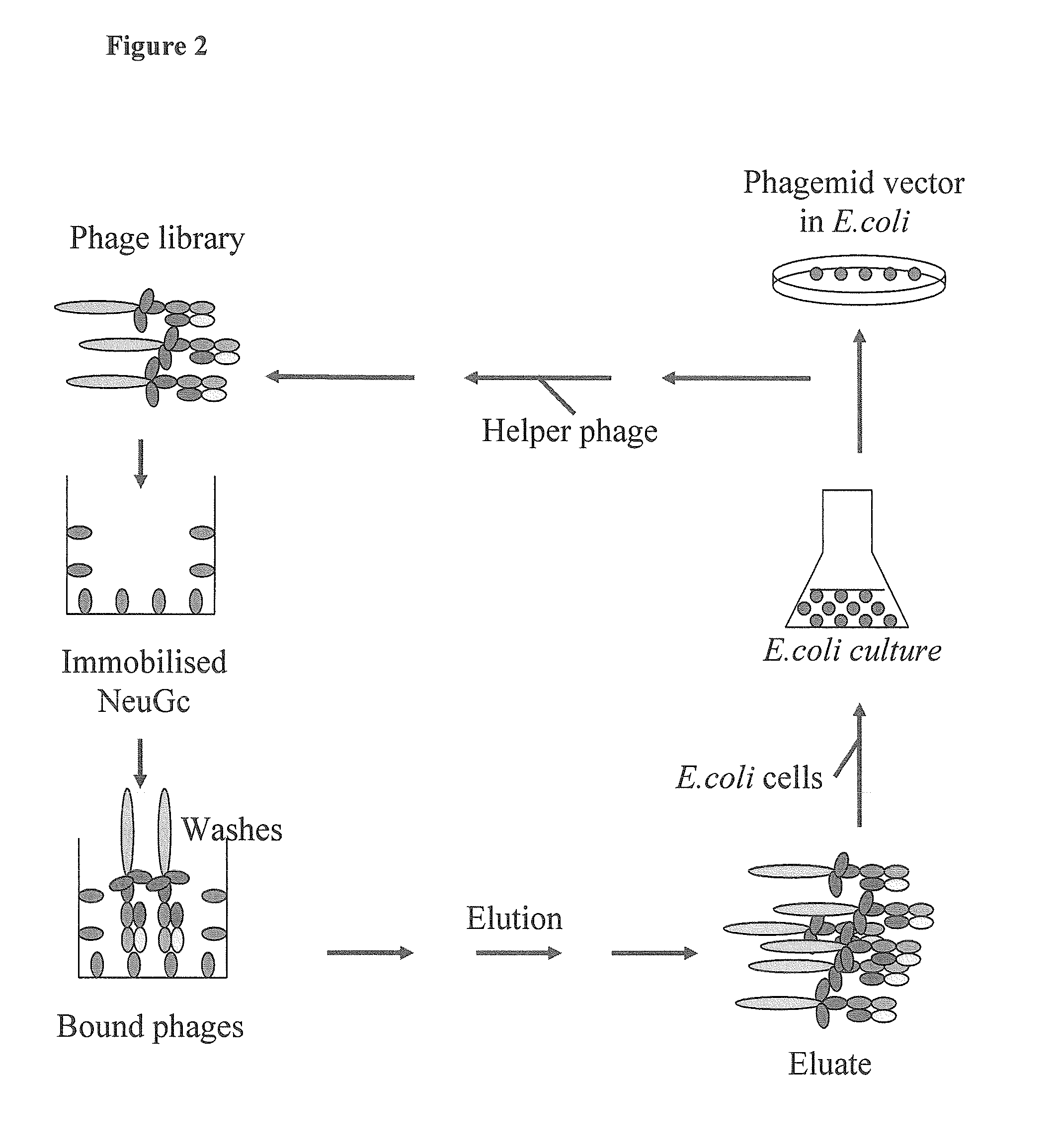
Human monoclonal antibodies directed to sialyl lewis c, sialyl tn and n glycolylneuraminic acid epitopes and a method of analysis of stem cells comprising said epitopes
InactiveUS20100292095A1Eliminate variationSugar derivativesLibrary screeningEpitopeImmunodiagnostics
This invention relates to antibody engineering technology. More particularly, the present invention relates to human IgM antibodies and derivatives thereof, which have novel binding specificity with regard to several oligosaccharide sequences and / or xenoantigenic sialic acid residue. The present invention also relates to processes for making and engineering such novel saccharide and / or NeuGc-binding monoclonal antibodies and to methods for using these antibodies and derivatives thereof in the field of immunodiagnostics, enabling qualitative and quantitative determination of xenoantigenic NeuGc in biological and raw material samples, as well as in immunotherapy, enabling blocking of xenoantigenic NeuGc in patients.
Owner:GLYKOS FINLAND
Constant chain modified bispecific, penta- and hexavalent Ig-M antibodies
ActiveUS10351631B2Hybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsMedicineIgm antibody
Owner:IGM BIOSCI INC
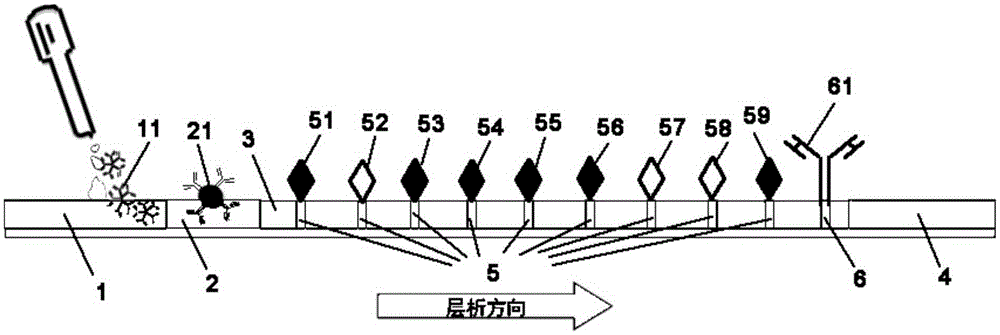
Colloidal gold test strip and test strip card for detecting IgM antibody, and preparation and detection method
InactiveCN105259345AThe detection process is fastImprove efficiencyBiological testingParainfluenza virus antigenIgm antibody
The invention provides a colloidal gold test strip for detecting an IgM antibody. The IgM antibody is a specific IgM antibody for nine respiratory tract infection pathogens, and the colloidal gold test strip comprises a sample pad, a conjugate pad, a nitrocellulose film and a water absorption pad which are attached to a polyvinyl chloride base plate in sequence; the conjugate pad is a glass fiber film wrapped with a rabbit-anti-human IgM antibody-colloidal gold conjugate; the nitrocellulose film is wrapped with 9 detection lines and 1 quality control line in sequence; the 9 detection lines are respectively a mycoplasma pneumoniae recombined antigen, a chlamydia pneumoniae recombined antigen, an influenza a virus antigen, an influenza B virus antigen, a sendai virus antigen, a legionella pneumophila antigen, a Coxiella burnetii antigen, a respiratory syncytial virus antigen and an adenovirus antigen, and the quality control line is a second antibody. The invention further provides a colloidal gold test strip card comprising the colloidal gold test strip and a colloid gold kit, a preparation method of the colloidal gold test strip card, and a method for realizing detection by adopting the colloidal gold test strip, the test strip card or the kit.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Colloidal gold chromatographic band for joint detecting specific IgM. IgG antibody and producing method thereof
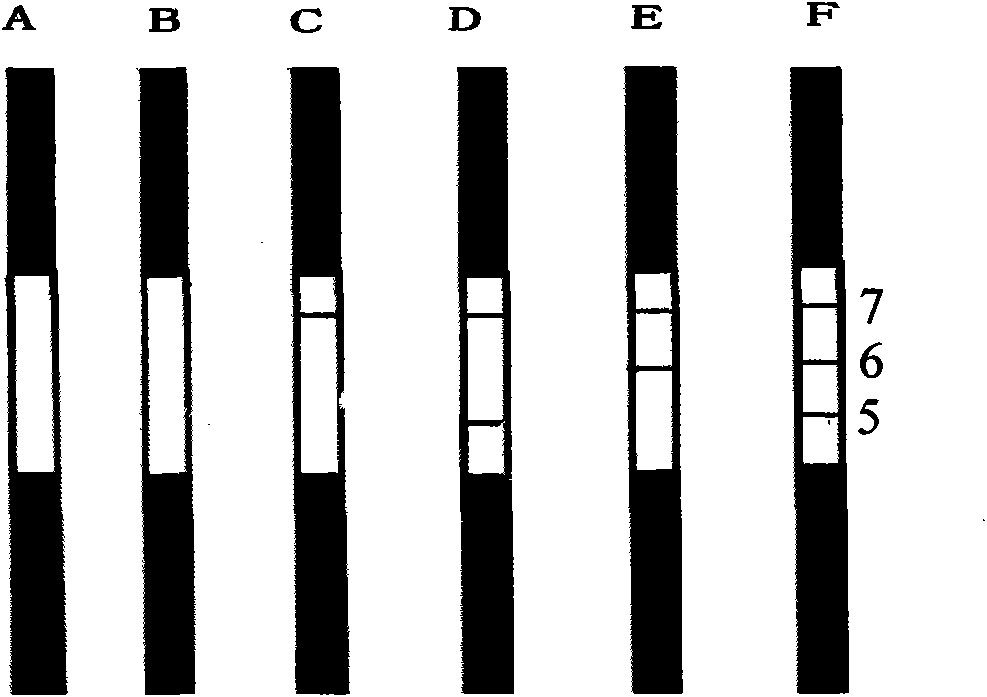
The invention provides a colloidal gold chromatography band and the preparing technology to detect the special IgM,IgG. The colloidal gold is as the labeled thing of the colloidal gold. One end of the PVC back plate is pasted with the sample pad, the compound pad, the nitryl fibrous membrane, the other end is pasted with the absorbing pad; the compound pad is the glass fibrous membrane covered with the antigen-colloidal gold compound. The character is in that: the fibrous membrane includes three cover lines which cover the anti IgM antibody, the special antigen and the antibody according to the antigen separately. The invention detects the IgM antibody using the immune capture method principle and detects the IgG antibody by the double antigen sandwich method. So it can detect the special IgM, IgG antibody by one operation and the result has the high whole according ratio.
Owner:北京英诺特生物技术股份有限公司
Multiple-channel test device, method for producing the same and use thereof
InactiveUS20070042444A1Improve storabilityLaboratory glasswaresMaterial analysisIgm antibodyCardiac infarction
The object of the invention is a multiple-channel test devise based on immunodiffusion and immunochromatography, which enables the simultaneous or parallel determination of several analytes. In the test devise, it is possible to group together different combinations of markers recognizing allergens, myocardial infarction markers, venereal disease analytes, blood screening analytes, respiratory infection producing agents, IgG, IgA and IgM antibodies, other infectious disease producing agents as well as various cancer markers. The multiple-channel test devise comprises a porous carrier material on which a channel network has been formed by etching the carrier material by laser to form a shaped figure that contains several channels. In the channels, various specific binding reagents have been immobilized, which enable the diagnoses of a target illness and / or syndrome. The sample application point is optionally provided with a filter and optionally contains a label mobilizable by the analyzable sample and a specific binding reagent. Also the method for the production of the test device and its use are disclosed in the invention.
Owner:ANI BIOTECH
ELISA (enzyme-linked immuno sorbent assay) detection kit of porcine pseudorabies virus IgM antibody
InactiveCN103308684AStrong specificityHigh sensitivityVirus peptidesFermentationIgm antibodyHorseradish peroxidase
The invention relates to an ELISA (enzyme-linked immuno sorbent assay) detection kit of a porcine pseudorabies virus IgM antibody, and a preparation method and application thereof. The ELISA detection kit of the porcine pseudorabies virus IgM antibody disclosed by the invention comprises an elisa plate of enveloping an IgM monoclonal antibody, sealing liquid, sample diluting liquid, detecting antigen, enzyme conjugate, concentrated scrubbing solution, enzyme substrate A solution, enzyme substrate B solution and stop buffer; the detecting antigen is prepared from purified porcine pseudorabies virus gE protein, structure protein gB and structure protein gD; and the enzyme conjugate is horse radish peroxidase-anti-gE, gB or gD protein enzyme compound. The specificity of the kit disclosed by the invention can be up to 100%; the sensitivity is 1:640; and the kit can be used for early diagnosis of porcine pseudorabies virus infection.
Owner:WUHAN CHOPPER BIOLOGY
Immunochromatographic kit for detecting novel coronavirus SARS-CoV-2
InactiveCN111366728AEarly isolatioEarly treatmentBiological material analysisPeptide preparation methodsAntibody conjugateIgm antibody
The invention discloses an immunochromatographic kit used for detecting novel coronavirus SARS-CoV-2 infection. The kit comprises a test strip. The test strip comprises a sample pad, a combination pad, a chromatography membrane and an absorption pad which are correspondingly pasted and laminated, and the combination pad is coated with a colloidal gold labeled neopterin antibody conjugate, a novelcoronavirus N protein conjugate, a novel coronavirus S1 protein conjugate and a DNP-BSA conjugate. A quality control line C and a detection line are arranged on the NC membrane. The C line is coated with a rabbit anti-DNP antibody, the detection line comprises a T1 line, a T2 line and a T3 line. The T1 line and the T2 line are respectively coated with one of an anti-human IgG antibody and an anti-human IgM antibody. The T3 line is coated with a Neo-BSA antigen, and the C line, the T1 line and the T2 line are three parallel bands perpendicular to the long side of the test strip. The kit provided by the invention is beneficial to early diagnosis and rapid confirmation of novel coronavirus infection, and has high sensitivity.
Owner:CHONGQING TANSHENG TECH
Test paper bar for testing colloidal gold of antibody of dengue fever virus
InactiveCN1963515ASimple methodThe result is clear and easy to distinguishMaterial analysisAgainst vector-borne diseasesDengue virus antibodyDengue virus Antigen
This invention provides one glue gold test bar to test dengue fever virus IgG and IgM antibody, which separately covers IgM antibody and dengue virus antigen and antigen multiple clone antibody on NC film and combines glue gold label dengue virus reestablish antigen and applies immune film analysis technique and tests dengue virus IgG and IgM antibody.
Owner:BEIJING ZHUANGDI HAOHE BIOMEDICINE SCI & TECH
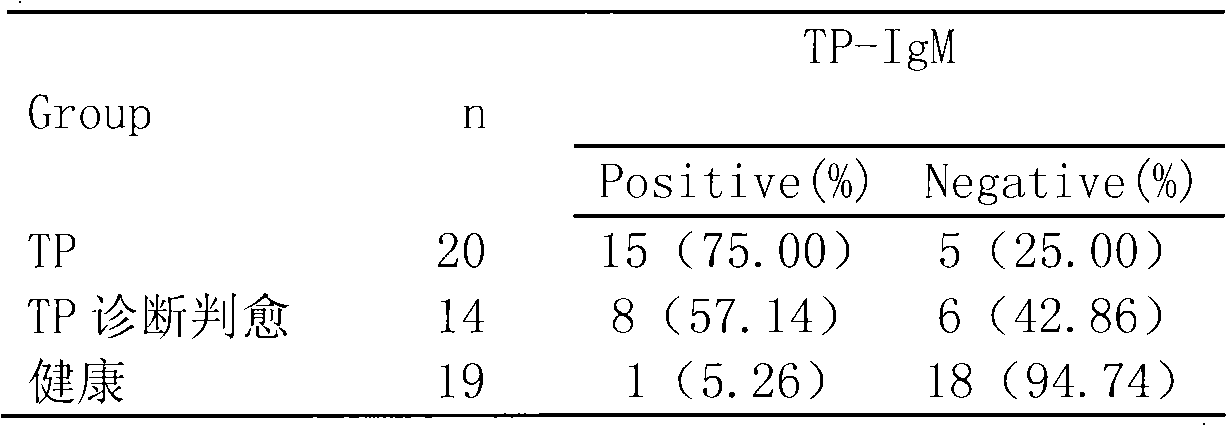
Treponema pallidum antibody test kit and preparation method and detection method thereof
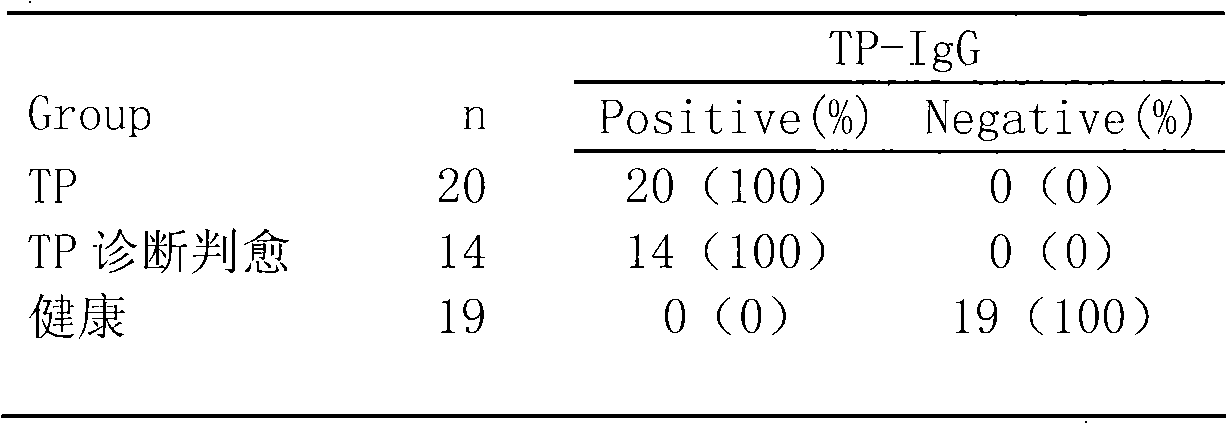
The invention relates to a treponema pallidum (TP) antibody test kit based on the flow microspheric carrier technology and a preparation method and detection method thereof, belonging to the technical field of immunoassay medical diagnosis. The preparation method comprises the following steps: using TP recombinant antigen to coat heavy polymer microsphere, using bovine serum albumin to seal empty binding site, preparing specific TP probe-heavy polymer microsphere; performing coculture with a sample to be tested to capture TP antibody, washing and centrifuging to remove the unbound TP antibody, then adding fluorescently-labeled anti-human IgG or IgM antibody; and using a flow cytometry to detect the fluorescence intensity of the microsphere, and performing qualitative or quantitative analysis to the tested antibody. The method has the advantages of high sensitivity and specificity and good stability and can be used to perform microanalysis or multivalent analysis to the sample.
Owner:NANJING UNIV OF TECH
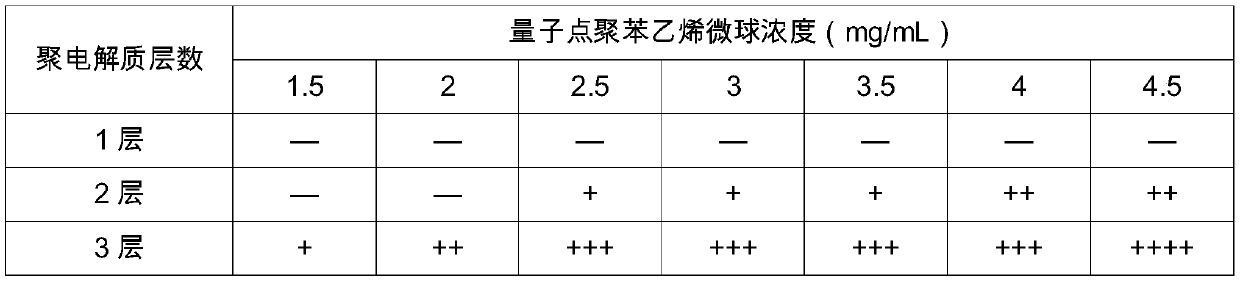
Novel detection reagent card for coronavirus antibody detection and preparation method of detection reagent card
PendingCN111190005AThe result is accurateHigh detection sensitivityBiological material analysisAntiendomysial antibodiesCoronavirus antibody
The invention relates to a novel detection reagent card for coronavirus antibody detection. The reagent card comprises a sample pad, a quantum pad, a nitrocellulose membrane, absorbent paper and a lining plate, the quantum pad is coated with a quantum dot microsphere labeled anti-human IgM antibody or IgG antibody, a detection line arranged on the nitrocellulose membrane is coated with an SARS-COV2 recombinant antigen, and a quality control line is coated with goat anti-mouse IgG. According to the detection reagent card for detecting the novel coronavirus (SARS-COV2) IgM antibody or IgG antibody provided by the invention, a result can be rapidly and accurately obtained, and auxiliary diagnosis is provided for clinical COVID-19.
Owner:重庆新赛亚生物科技有限公司
Reagent strip for joint detection of syphilis specific IgM and IgG antibodies and preparation method thereof
InactiveCN101825634AJoint detection implementationStrong specificityMaterial analysisReagent stripSyphilis
The invention provides a reagent strip for joint detection of a syphilis specific IgM and IgG antibodies and a preparation method thereof, relating to a reagent for detection of a syphilis specific antibody. The invention provides the reagent strip for joint detection of the syphilis specific IgM and IgG antibodies and the preparation method thereof. The reagent strip is provided with a vector plate, a loading pad, a colloidal gold pad, a nitrocellulose membrane, a syphilis specific IgG antibody detection line, a syphilis specific IgM antibody detection line, a contrast line and an absorption pad. The preparation method comprises the following steps: preparing sample application of nitrocellulose membranes for recombinant syphilis antigens TPN17 and TPN47, preparing colloidal gold, labeling syphilis specific antigen TPN17 and TPN47 with colloidal gold and preparing the reagent strip for joint detection of the syphilis specific IgM and IgG antibodies.
Owner:ZHONGSHAN HOSPITAL XIAMEN UNIV
Novel coronavirus detection test strip as well as preparation method and application thereof
InactiveCN111426840AImprove accuracySimple and fast operationBiological testingImmunoassaysIgm antibodyMonoclonal antibody agent
The invention provides a novel coronavirus detection test strip as well as a preparation method and application thereof. The test strip comprises a binding pad and an analysis membrane, and the binding pad is coated with a luminescent substance labeled 2019-nCoV recombinant antigen and a mouse anti-human HCG monoclonal antibody; a T2 detection line, a T1 detection line and a quality control line are sequentially arranged on the analysis membrane along the chromatography direction; the T2 detection line is coated with a mouse anti-human IgG monoclonal antibody, the T1 detection line is coated with a mouse anti-human IgA monoclonal antibody and a mouse anti-human IgM monoclonal antibody, and the quality control line is coated with a goat anti-mouse IgG polyclonal antibody. The test paper card can simultaneously determine the positive conditions of IgA antibody, IgM antibody and IgG antibody in serum of a patient, can more accurately detect the early antibody level condition in the body of the patient, assists in judging different periods of novel coronavirus infection of the patient, and improves the sensitivity and specificity of novel coronavirus detection.
Owner:北京中检安泰诊断科技有限公司
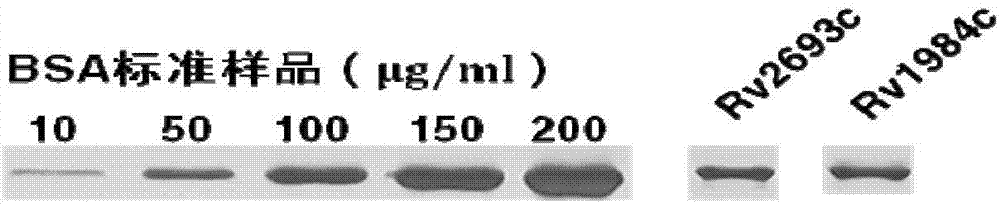
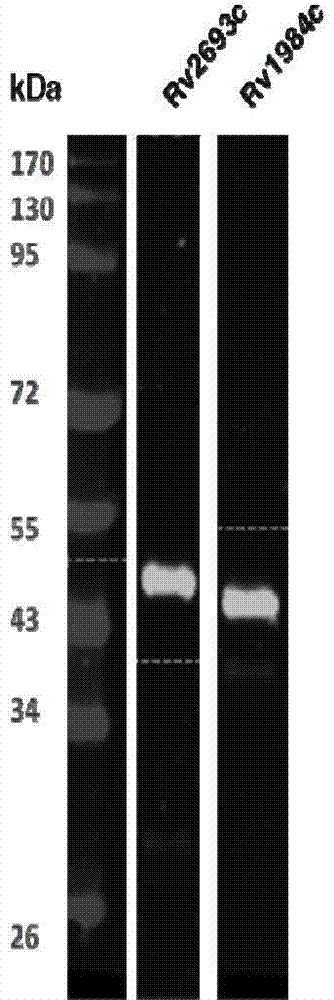
Application of mycobacterium tuberculosis protein in preparation of products used for diagnosis of latent tuberculosis infection
The invention provides mycobacterium tuberculosis protein Rv2693c and / or Rv1984c in development and / or designing of products capable of discrimination, diagnosis, auxiliary diagnosis, screening and / or auxiliary screening of latent tuberculosis infection. The invention further provides protein chips prepared from the two mycobacterium tuberculosis proteins. The protein chips prepared in the invention are used for detecting the levels of IgM antibodies respectively corresponding to the twp proteins in serum of a patient with latent tuberculosis infection and of a normal person, and detection results of the antibodies respectively corresponding to the three protein are analyzed together so as to determine whether an examined person suffers from latent tuberculosis infection; detection results show that optimal operating points of the protein chips provided by the invention in auxiliary diagnosis of latent tuberculosis infection have specificity of 80.3% and sensitivity of 75.6%, both higher than indexes of diagnosis of latent tuberculosis infection in the prior art.
Owner:TB HEALTHCARE BIOTECHNOLOGY (GUANGDONG) CO LTD
Colloidal gold immunochromatography test strip for simultaneously detecting viral antigens and antibodies
The invention relates to a colloidal gold immunochromatography test strip for simultaneously detecting viral antigens and antibodies. The test strip comprises a first glue strip and a second glue strip which are arranged in parallel, wherein the first glue strip comprises a first sample pad, a first conjugate pad, a first nitrocellulose membrane and first absorbent paper, which are sequentially lapped with one another, the first conjugate pad is coated with colloidal gold marked by the viral antigens, the first nitrocellulose membrane is coated with viral antibodies and antibodies, the viral antibodies are taken as a first detection line, and the antibodies are used for resisting the viral antigens on the colloidal gold and are taken as a quality control line; the second glue strip comprises a second sample pad, a second conjugate pad, a second nitrocellulose membrane and second absorbent paper, which are sequentially lapped with one another, the second conjugate pad is coated with the colloidal gold marked by the viral antigens, the second nitrocellulose membrane is coated with anti-human IgG antibodies and anti-human IgM antibodies, the anti-human IgG antibodies are taken as a second detection line, and the anti-human IgM antibodies are taken as a third detection line. The colloidal gold immunochromatography test strip can be used for simultaneously detecting the viral antigens as well as the IgG antibodies and IgM antibodies of the viral antigens.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
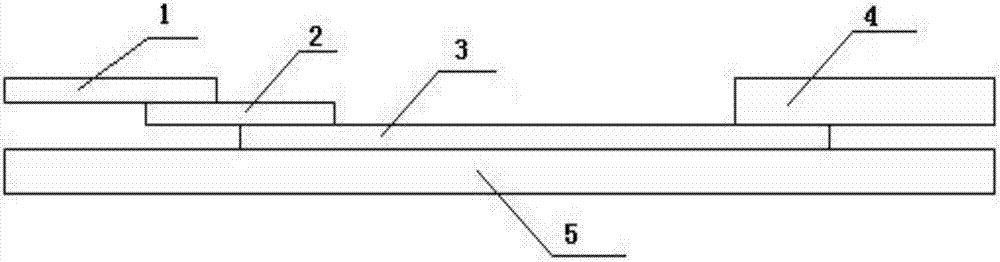
Rubella virus IgG and IgM antibody joint inspection kit and preparation method thereof
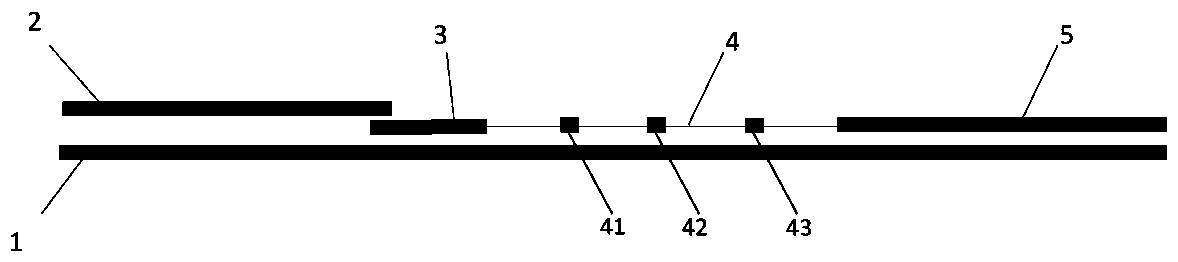
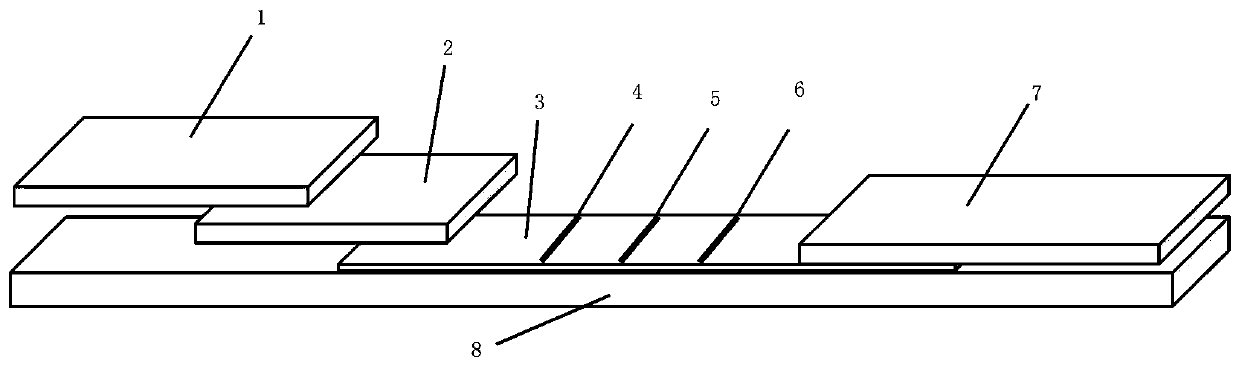
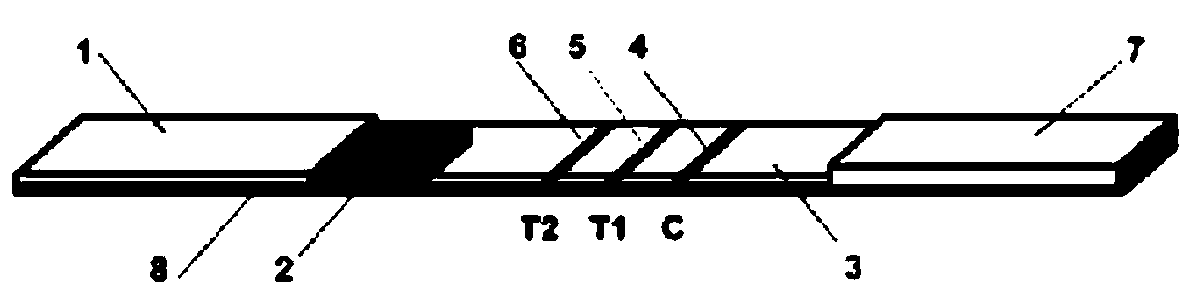
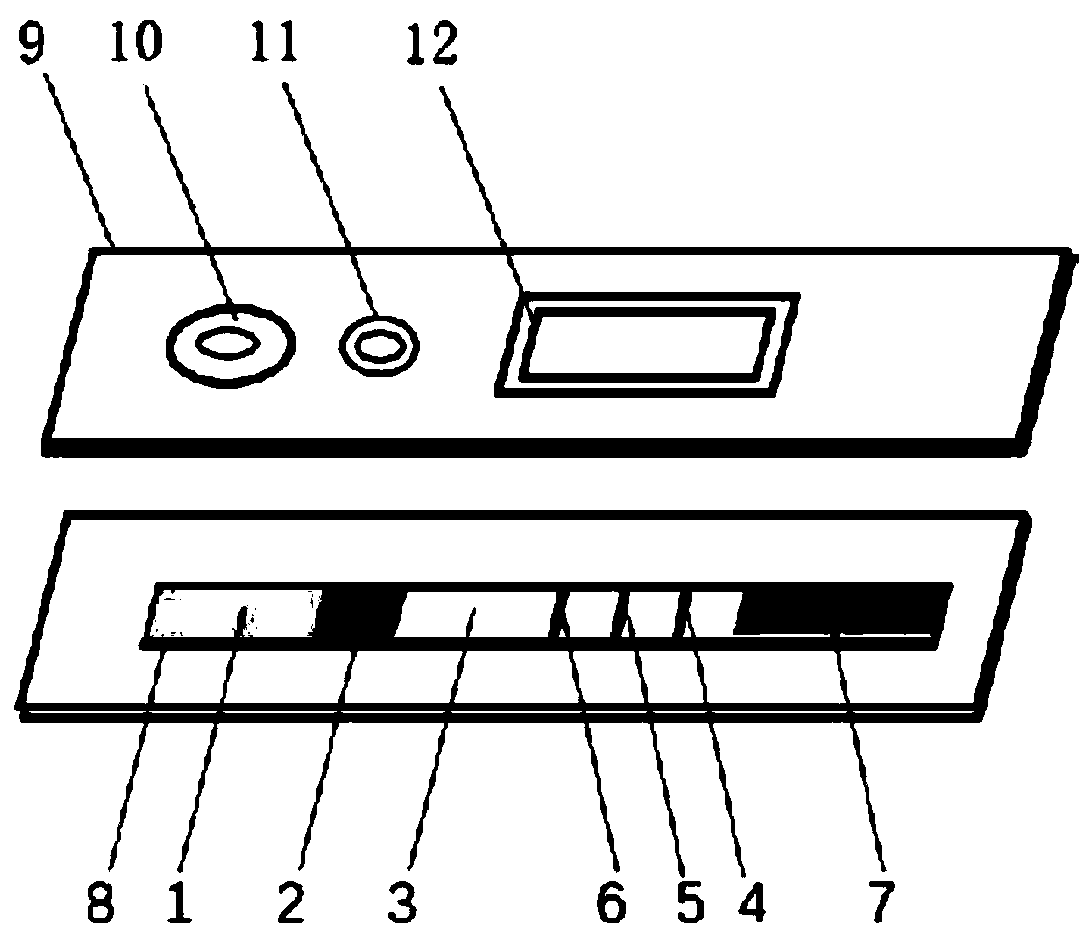
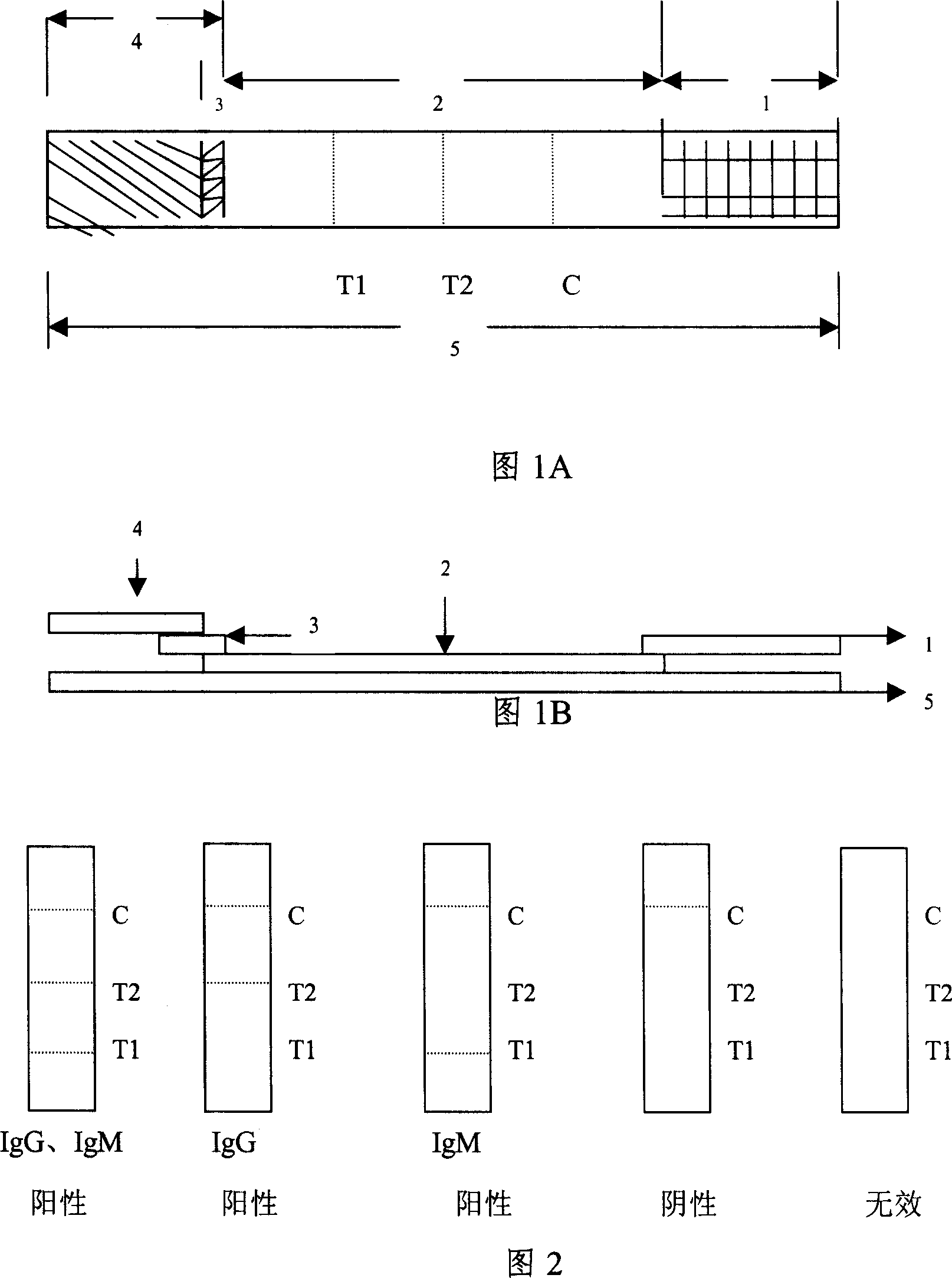
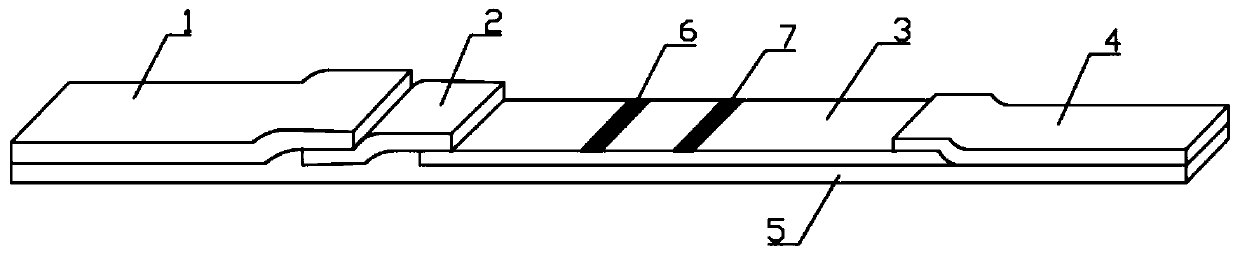
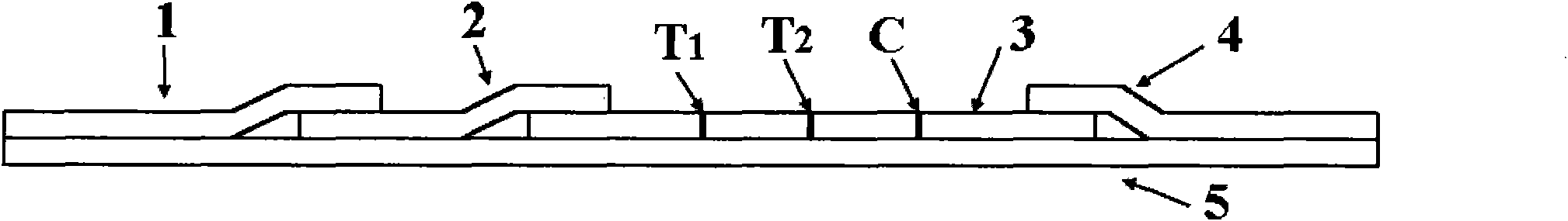
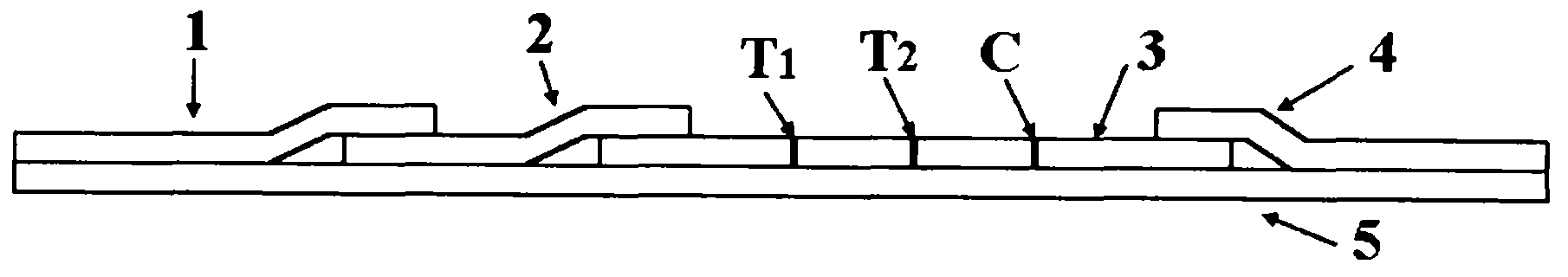
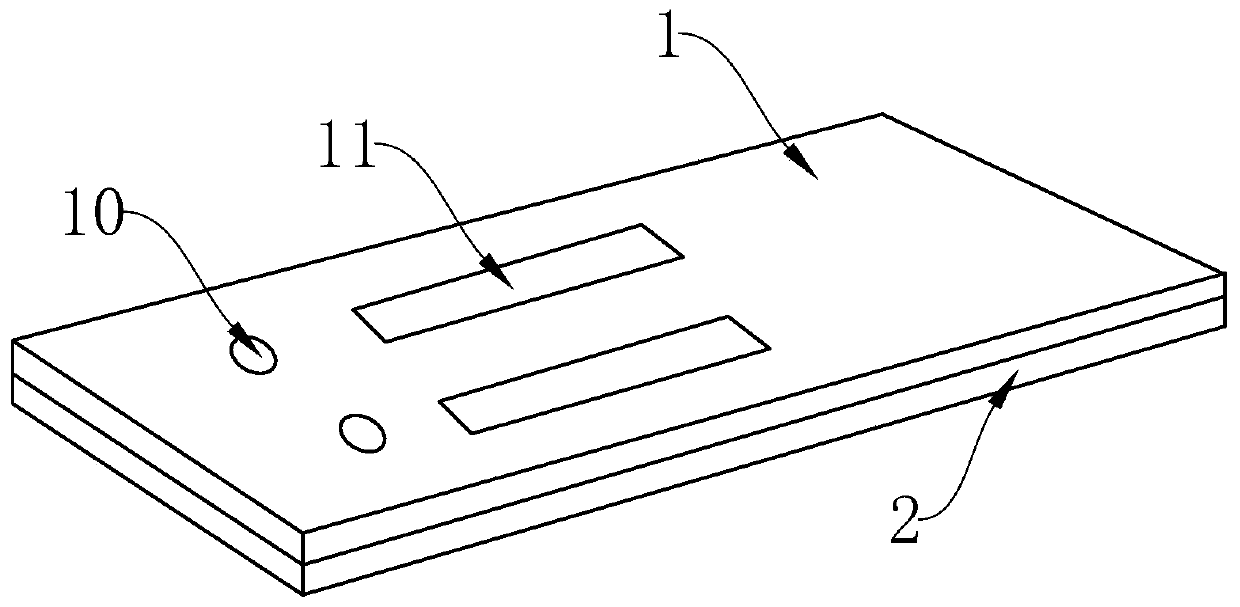
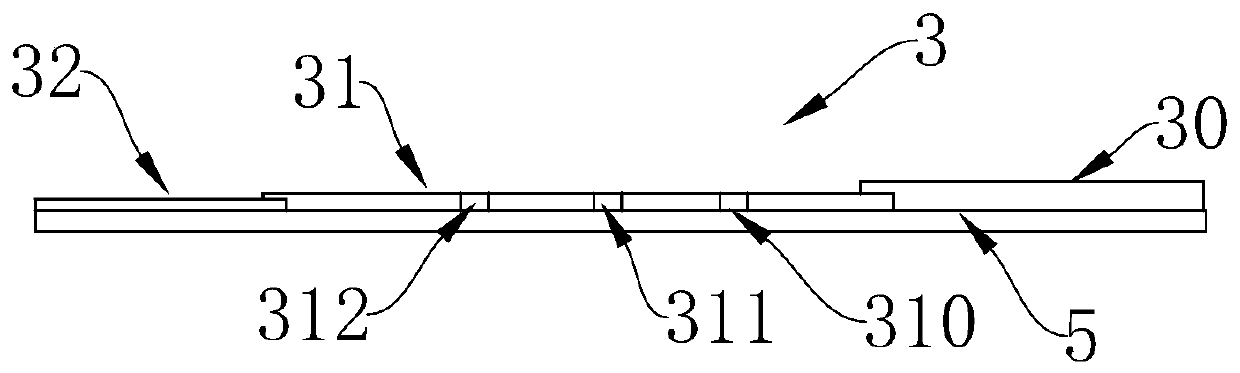
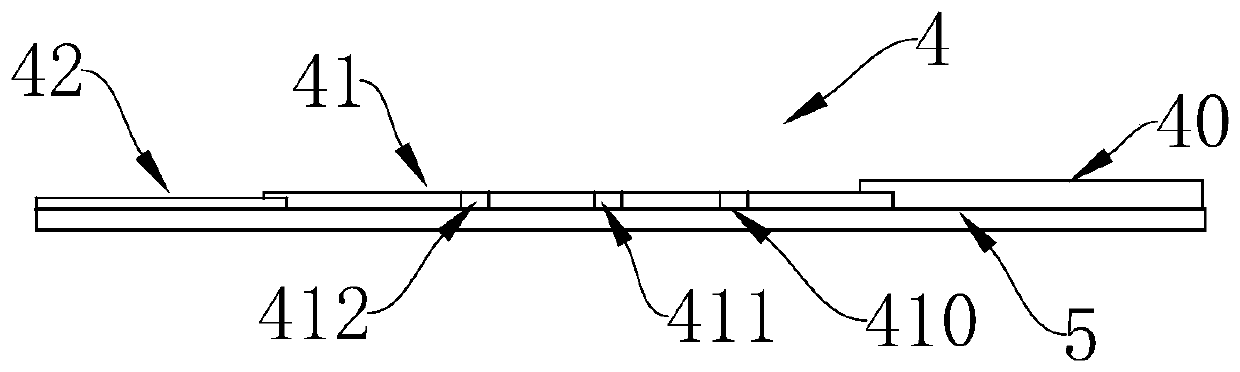
InactiveCN102109519AQuick screeningEasy to operateMaterial analysis by observing effect on chemical indicatorGlass fiberNitrocellulose
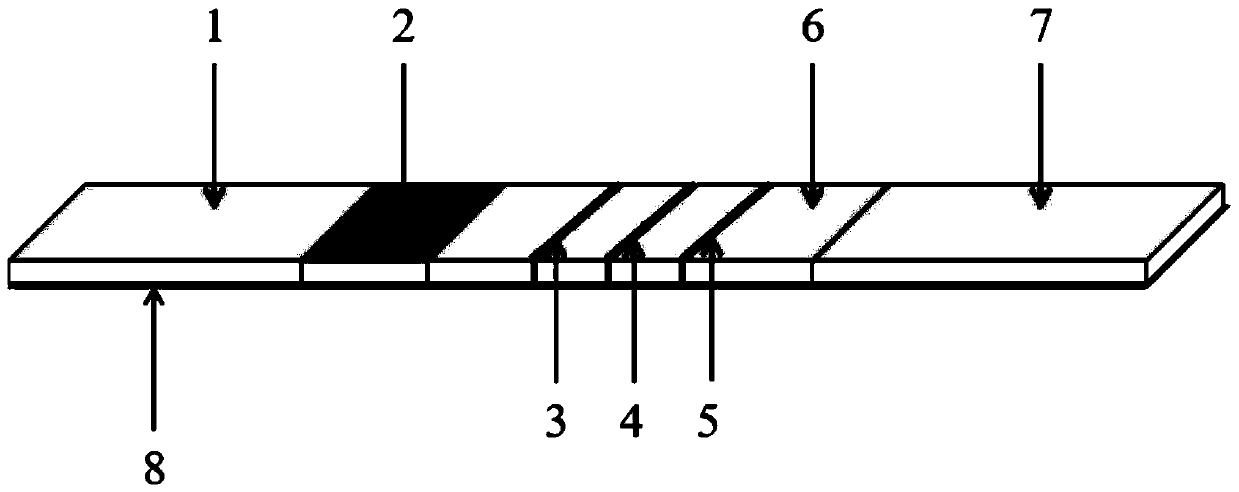
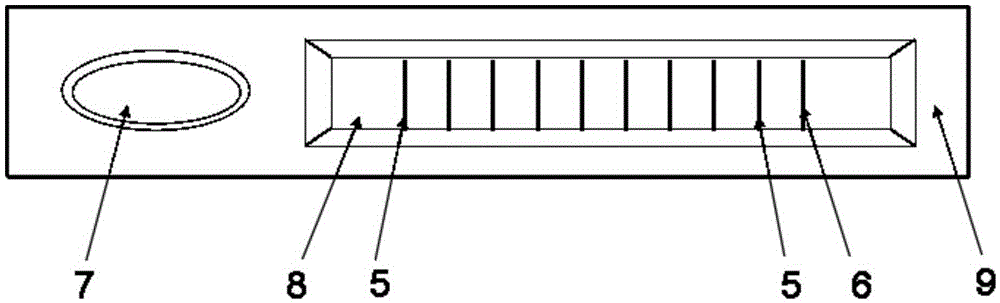
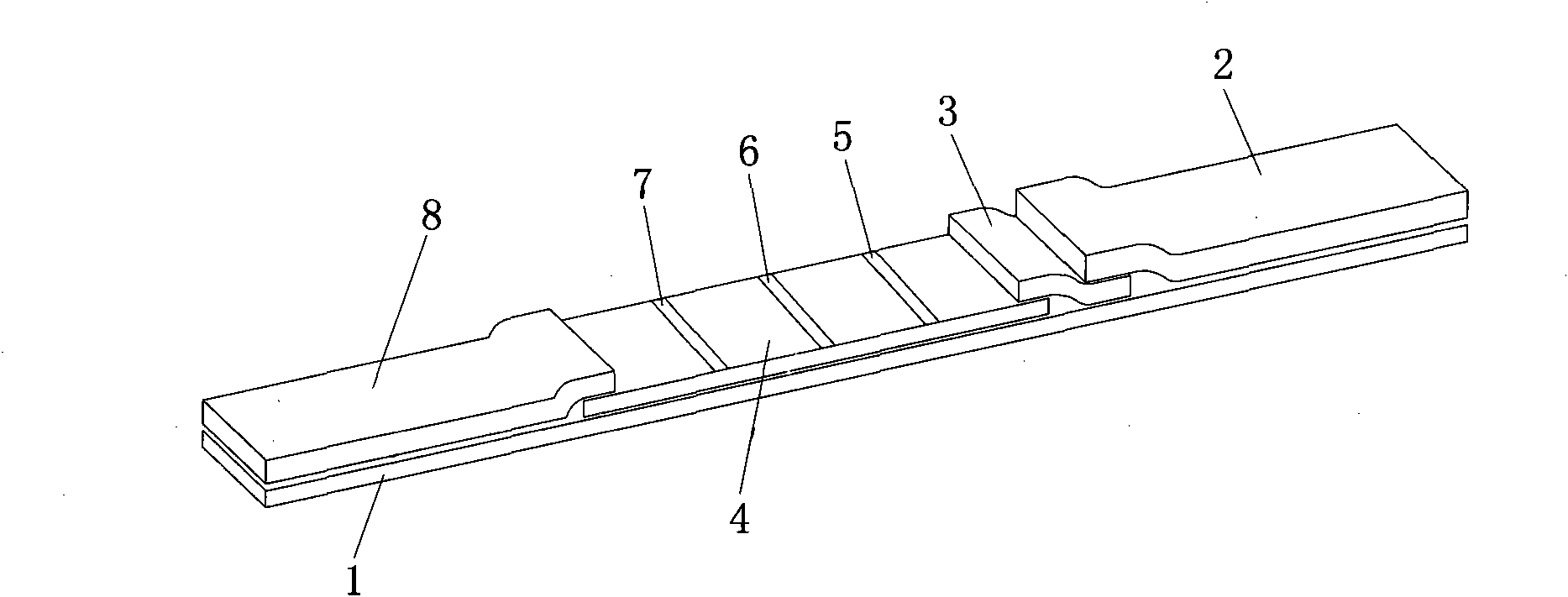
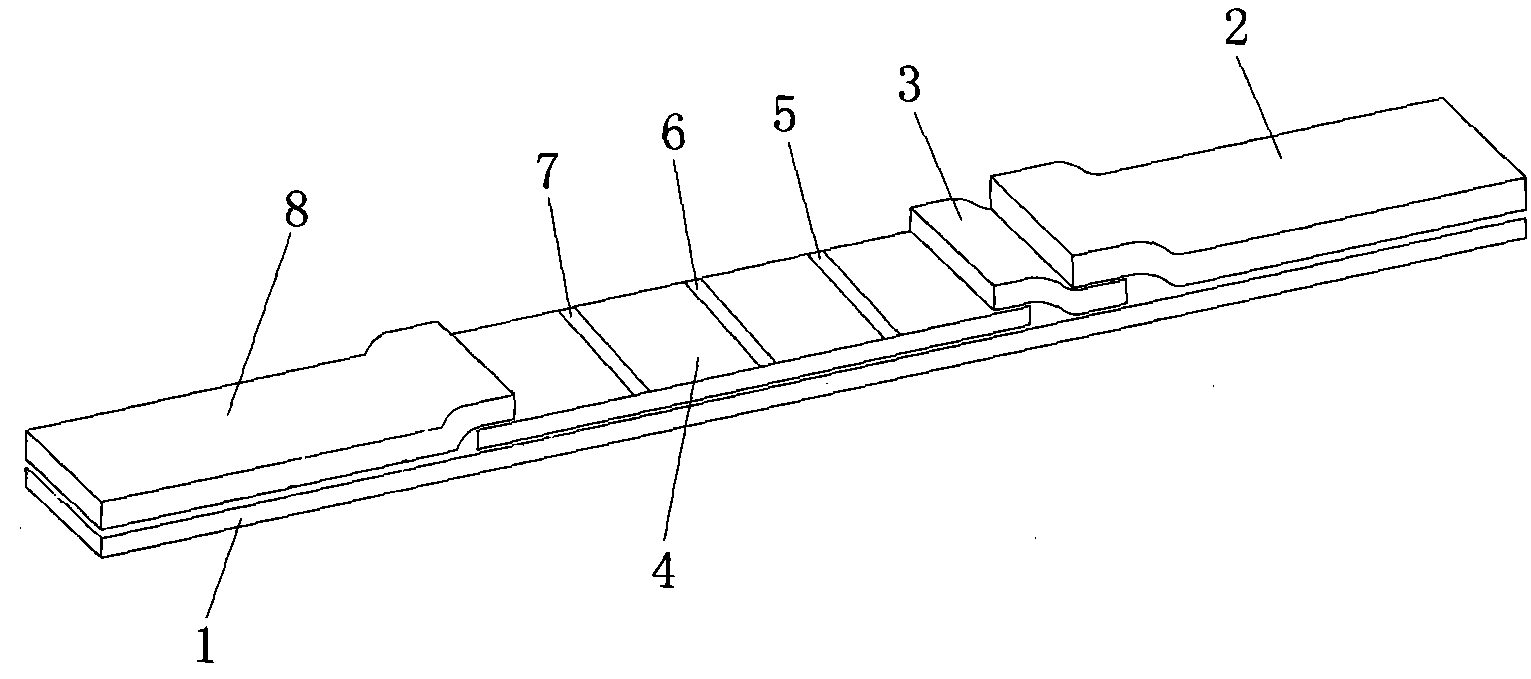
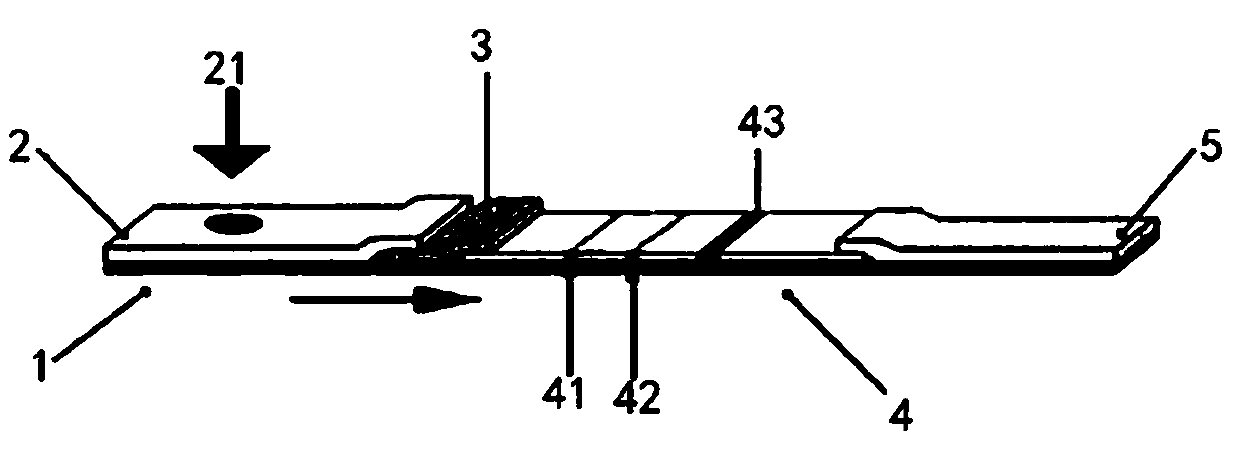
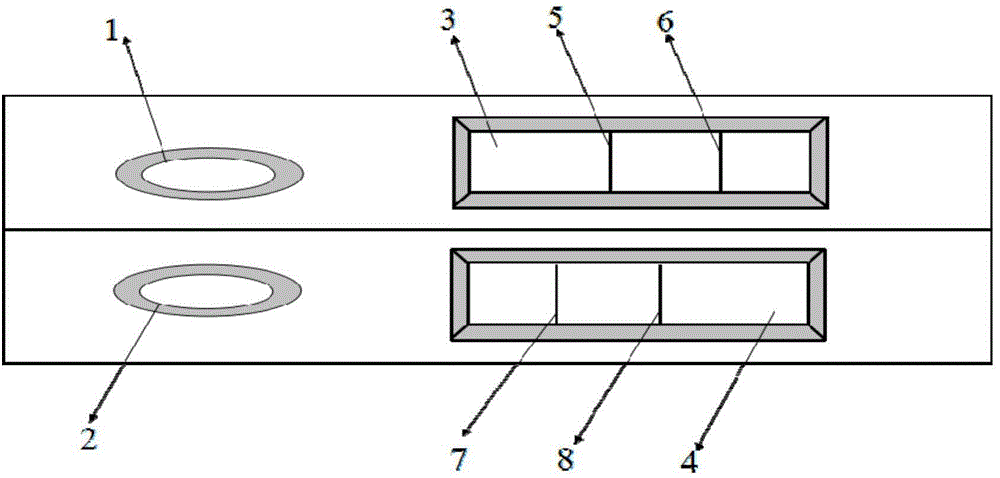
The invention provides a rubella virus immunoglobulin G and immunoglobulin M (IgG and IgM) antibody joint inspection kit and a preparation method thereof. The kit comprises a sample pad (1), a colloidal gold pad (2), a nitrocellulose membrane (3), a sample absorption pad (4) and a bottom plate (5), wherein the colloidal gold pad is a colloidal gold-labeled rubella virus antigen glass fiber (or a non-woven fabric); and the nitrocellulose membrane is coated with a mouse anti-human IgM antibody and a rubella virus antigen serving as detection lines, and goat anti-mouse IgG serving as a quality control line in turn. Rubella virus IgG and IgM antibodies are detected by specific antigen antibody reaction and a colloidal gold immunochromatography technology and can be jointly detected through one-time operation, so that the operation process is simplified, and the kit has the characteristics of quick response, high sensitivity and the like, is easy to operate and is economical and practical.
Owner:北京库尔科技有限公司
Mycoplasma pneumonia antigen, preparation method and immunodetection kit
ActiveCN103059109AAchieve recombinant expressionHigh sensitivityDepsipeptidesBiological testingMycoplasma antibodyIgm antibody
The invention provides a mycoplasma pneumonia (MP) antigen. The antigen fragment of the antigen is MP371 or MP661, wherein the locus of MP 371 is at an N terminal 371-480aa of MP adhesion protein P1, and the locus of MP661 is at an N terminal 661-772aa of MP adhesion protein P1. The invention also relates to a kit comprising any antigen or antigen composition and the application of the kit in detection of a mycoplasma pneumonia antibody. The mycoplasma pneumonia antigen is a recombinant antigen cloned and expressed by a codon-optimized gene and has stronger antigen specificity than that of the antigen cultivated and extracted by MP. The kit can be used for specific detection of an anti-MP-IgM (immunoglobulin m) antibody in clinical samples, thus identifying and diagnosing the infection of mycoplasma pneumonia at early stage.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
Kit for combined detection of 2019 novel coronavirus IgM and IgG antibodies and preparation method thereof
ActiveCN111426831AImprove structural stabilityHigh sensitivityBiological material analysisAgainst vector-borne diseasesIgm antibodyErythroid cell
The invention relates to the technical field of in-vitro diagnostic reagents, in particular to a kit for combined detection of novel 2019 coronavirus IgM and IgG antibodies and a preparation method ofthe kit. The kit comprises a duplex detection card and a detection buffer solution; a 2019 novel coronavirus IgM antibody detection test strip and a 2019 novel coronavirus IgG antibody detection teststrip are arranged in the duplex detection card; the 2019 novel coronavirus IgM antibody detection test strip and the 2019 novel coronavirus IgG antibody detection test strip respectively comprise asample pad, a nitrocellulose membrane and a water absorption pad, wherein the sample pad is coated with an anti-sheep erythrocyte antibody, and the nitrocellulose membrane is provided with a fluorescent antibody solidus, a detection line and a quality control line. By using the kit, the detection rate can be improved, and the operation is simple.
Owner:山东康华生物医疗科技股份有限公司 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com