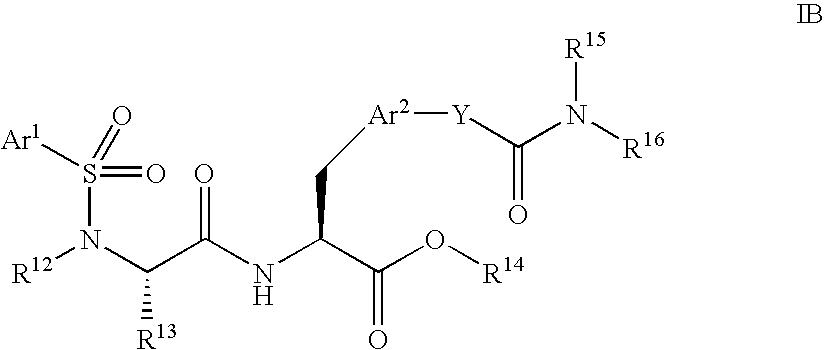
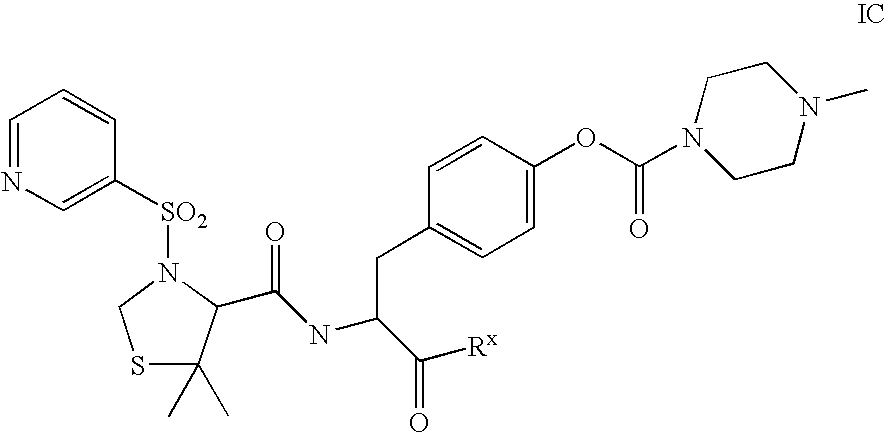
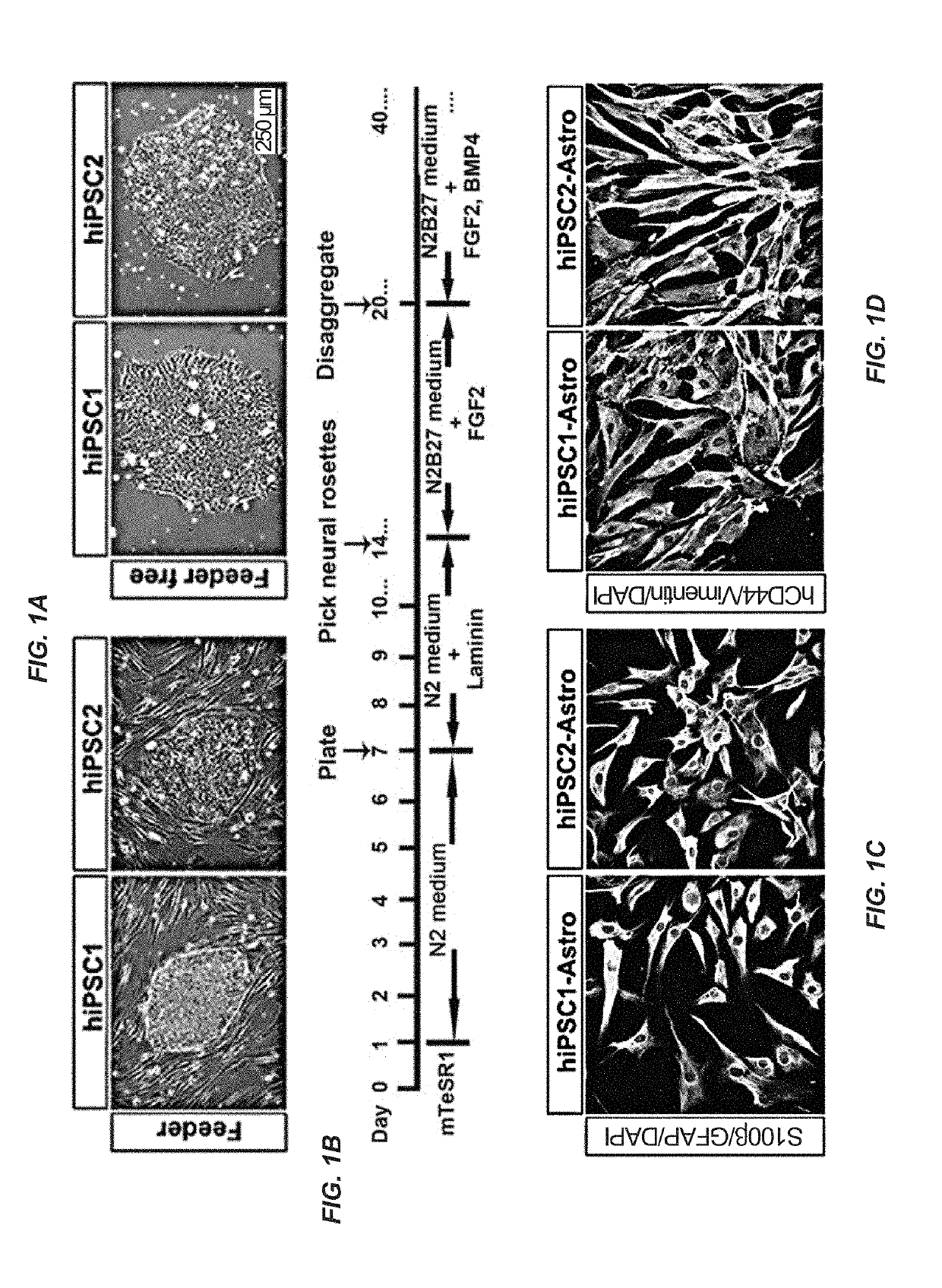
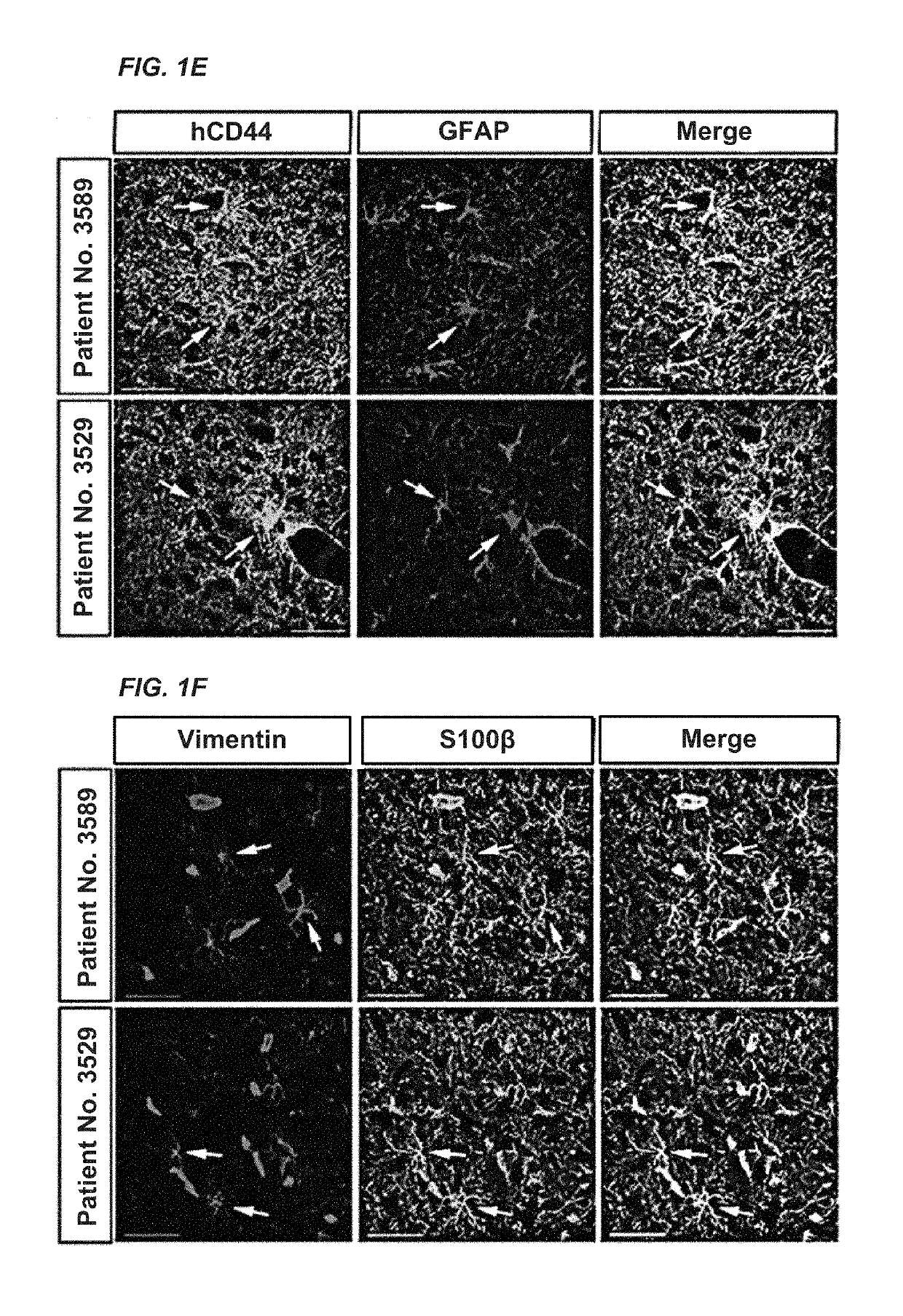
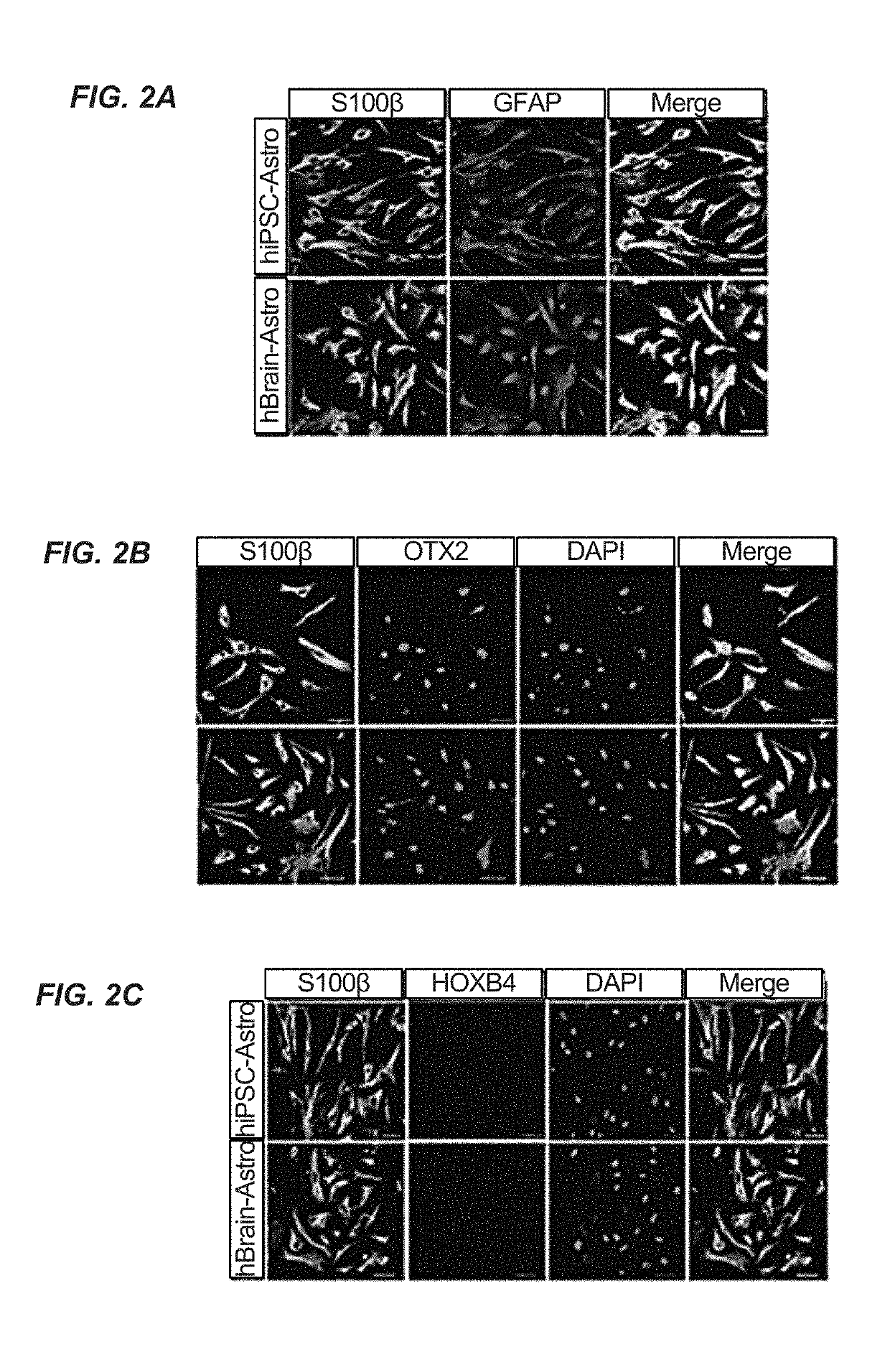
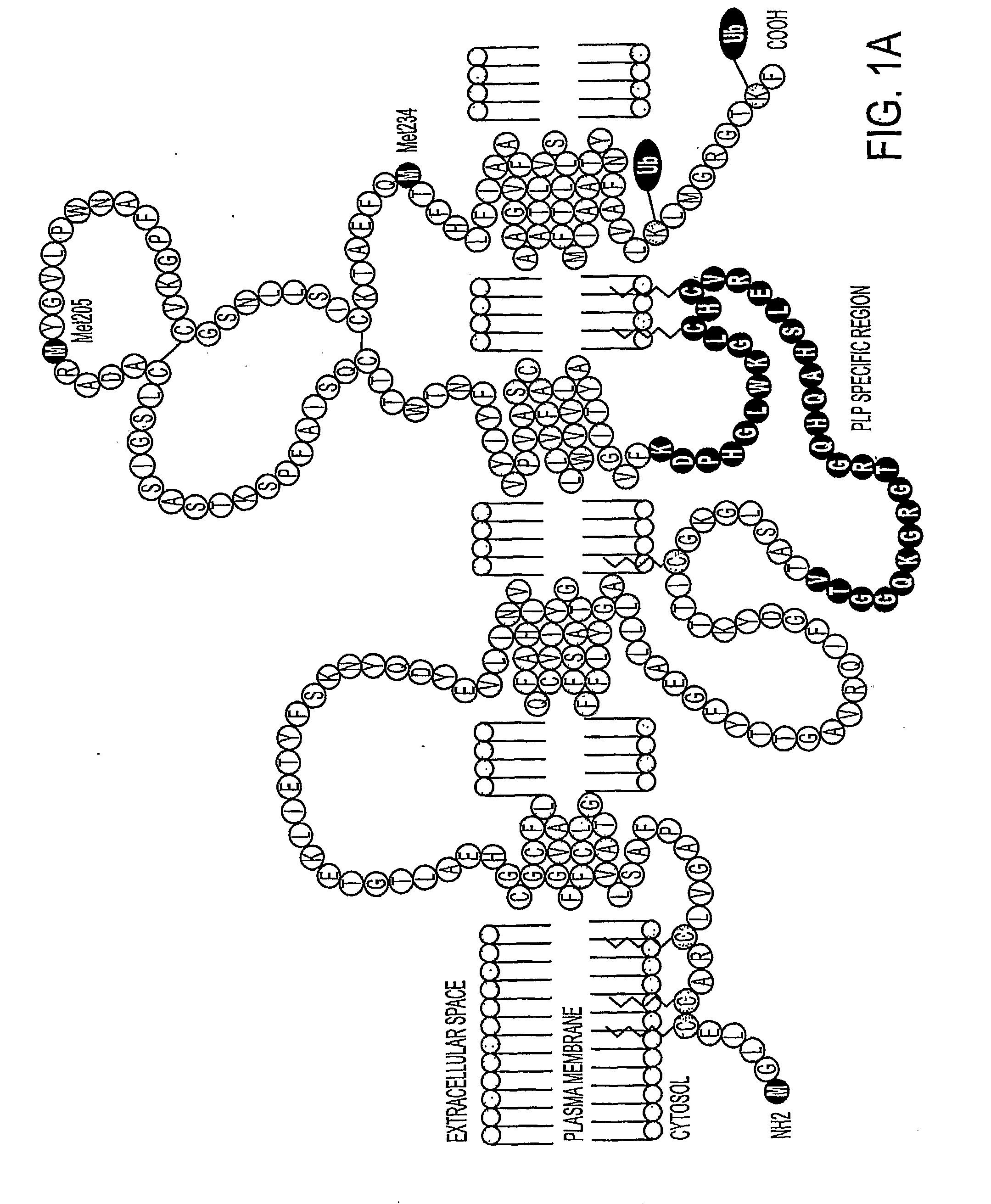Patents
Literature
84 results about "Remyelination" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
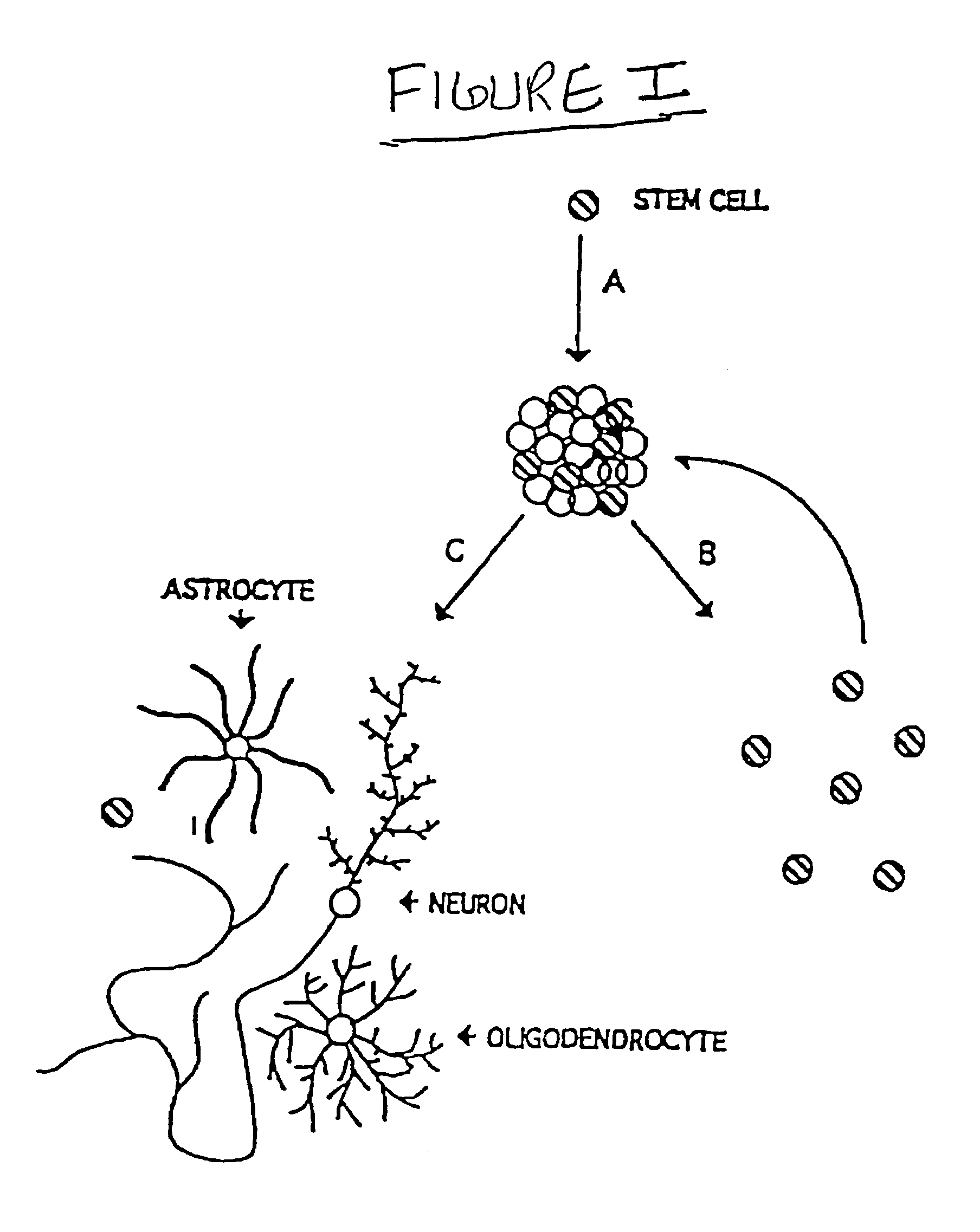
Remyelination is the process of propagating oligodendrocyte precursor cells to form oligodendrocytes to create new myelin sheaths on demyelinated axons in the CNS. This is a process naturally regulated in the body and tends to be very efficient in a healthy CNS. The process creates a thinner myelin sheath than normal, but it helps to protect the axon from further damage, from overall degeneration, and proves to increase conductance once again. The processes underlying remyelination are under investigation in the hope of finding treatments for Demyelinating diseases, such as multiple sclerosis.
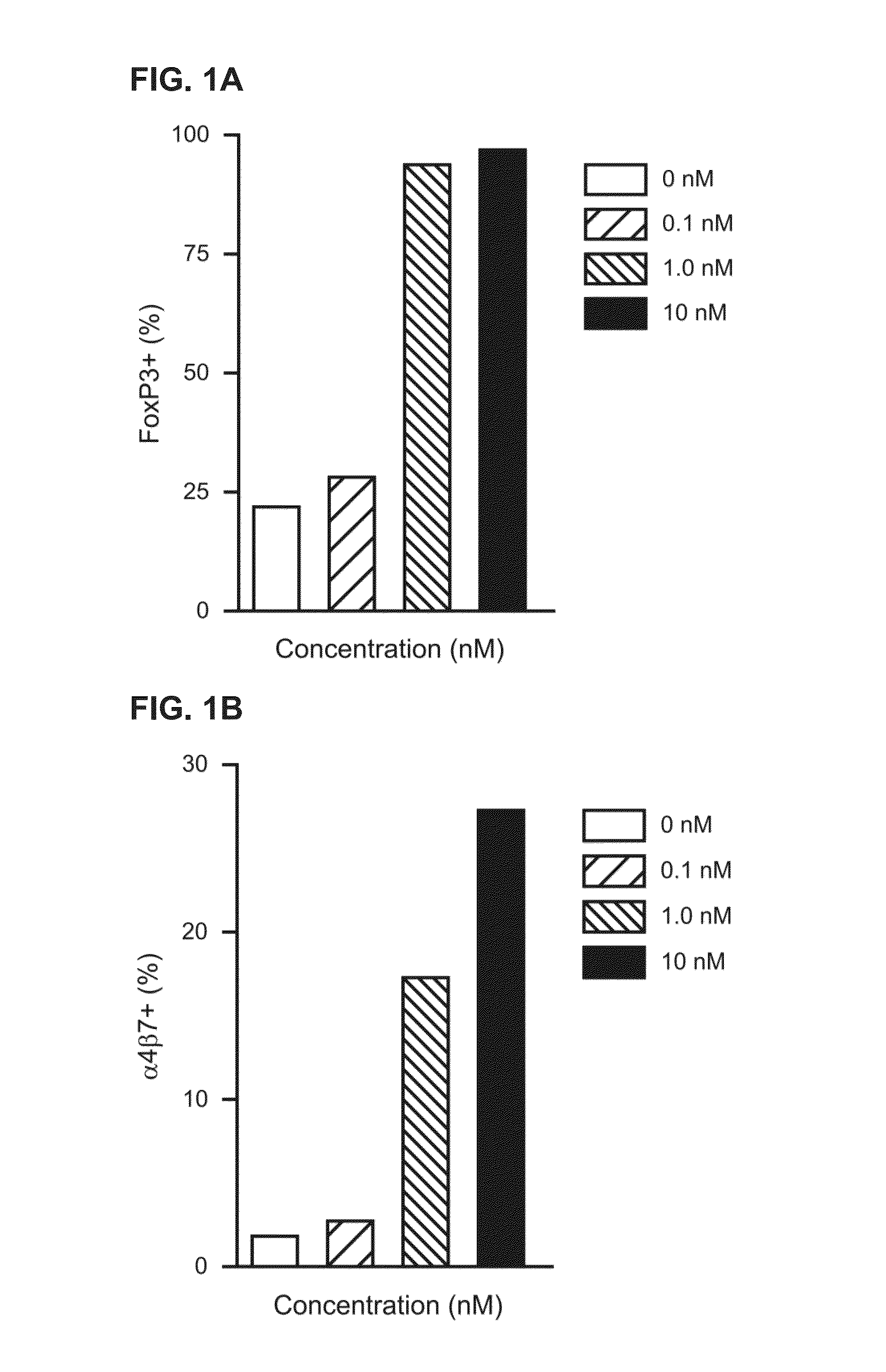
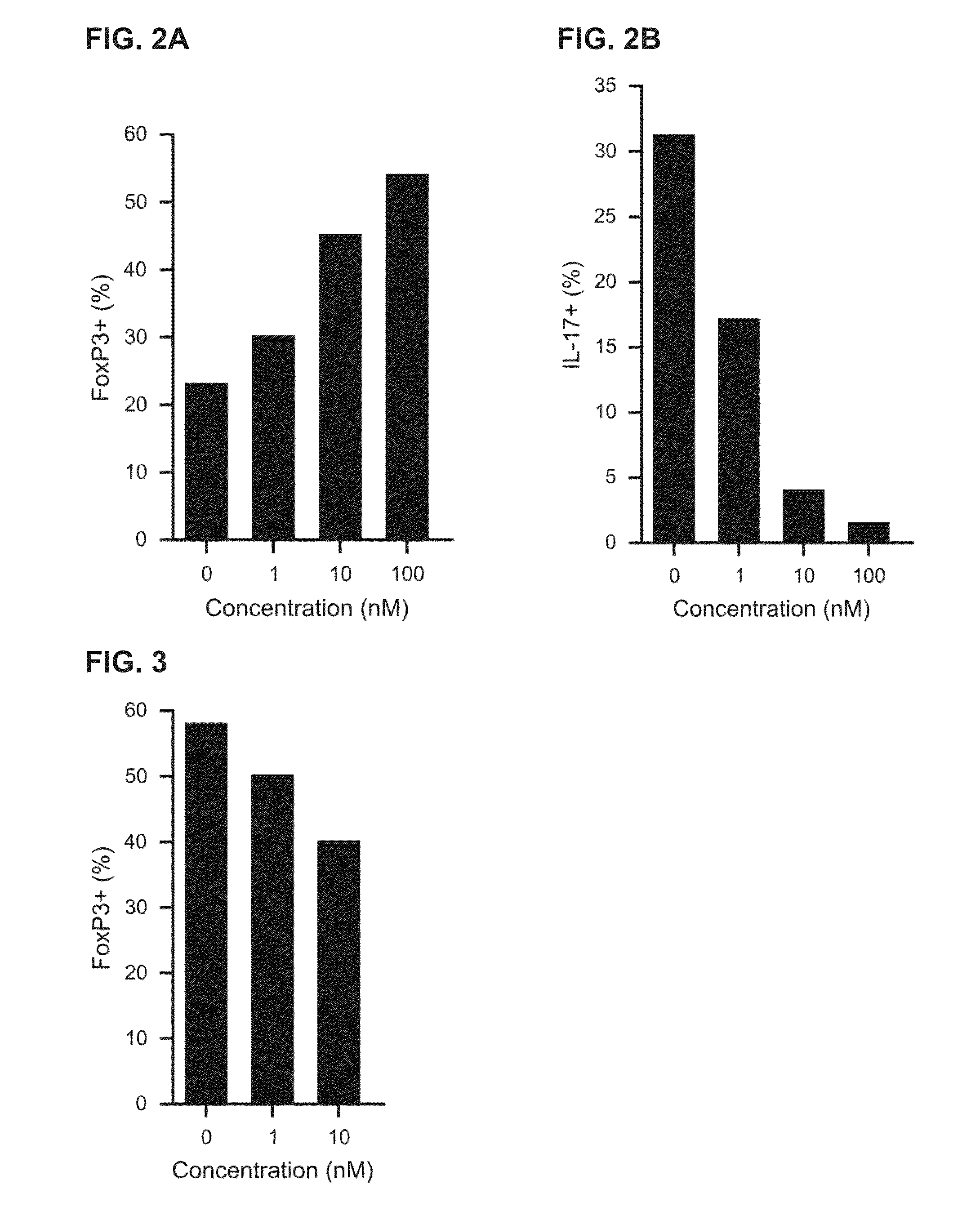
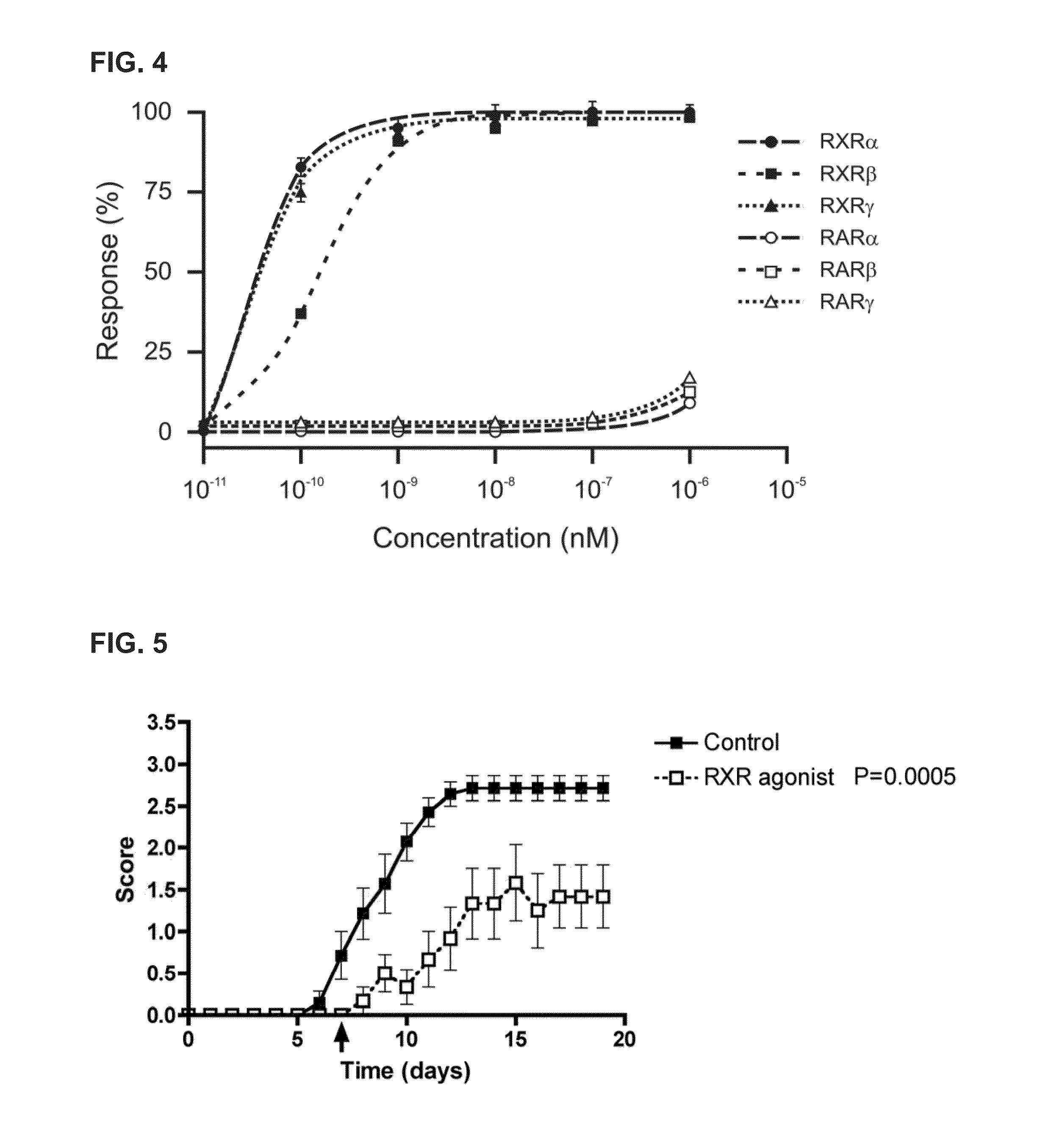
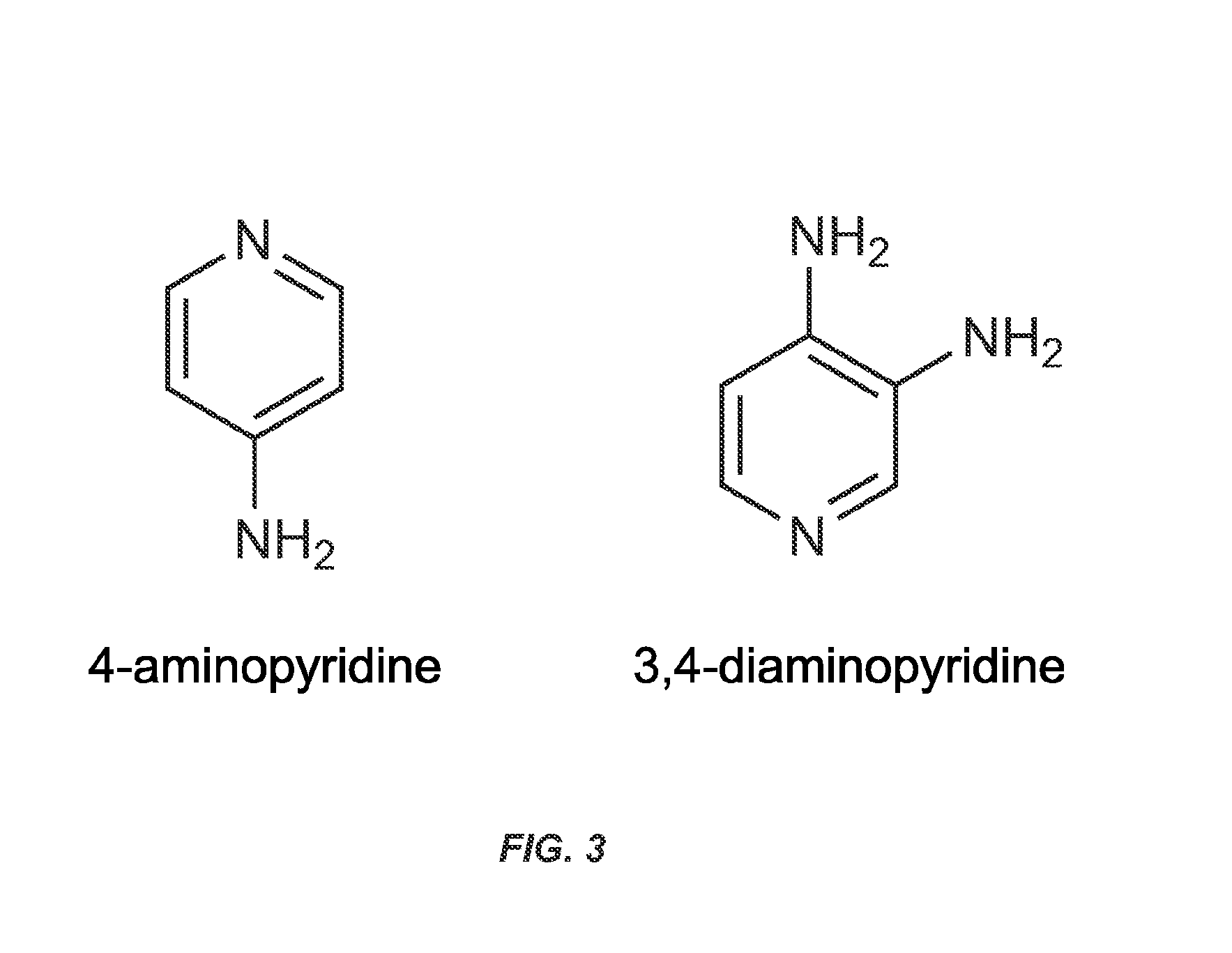
Treatment of Diseases by Concurrently Eliciting Remyelination Effects and Immunomodulatory Effects Using Selective RXR Agonists
The present specification provides RXR agonists with both remyelination promotion and immunomodulatory activities, compositions comprising such RXR agonists, and methods using such compounds and compositions to treat a demyelination-related disorder by both promoting remyelination of neurons and modulating the immune system.
Owner:IO THERAPEUTICS +1
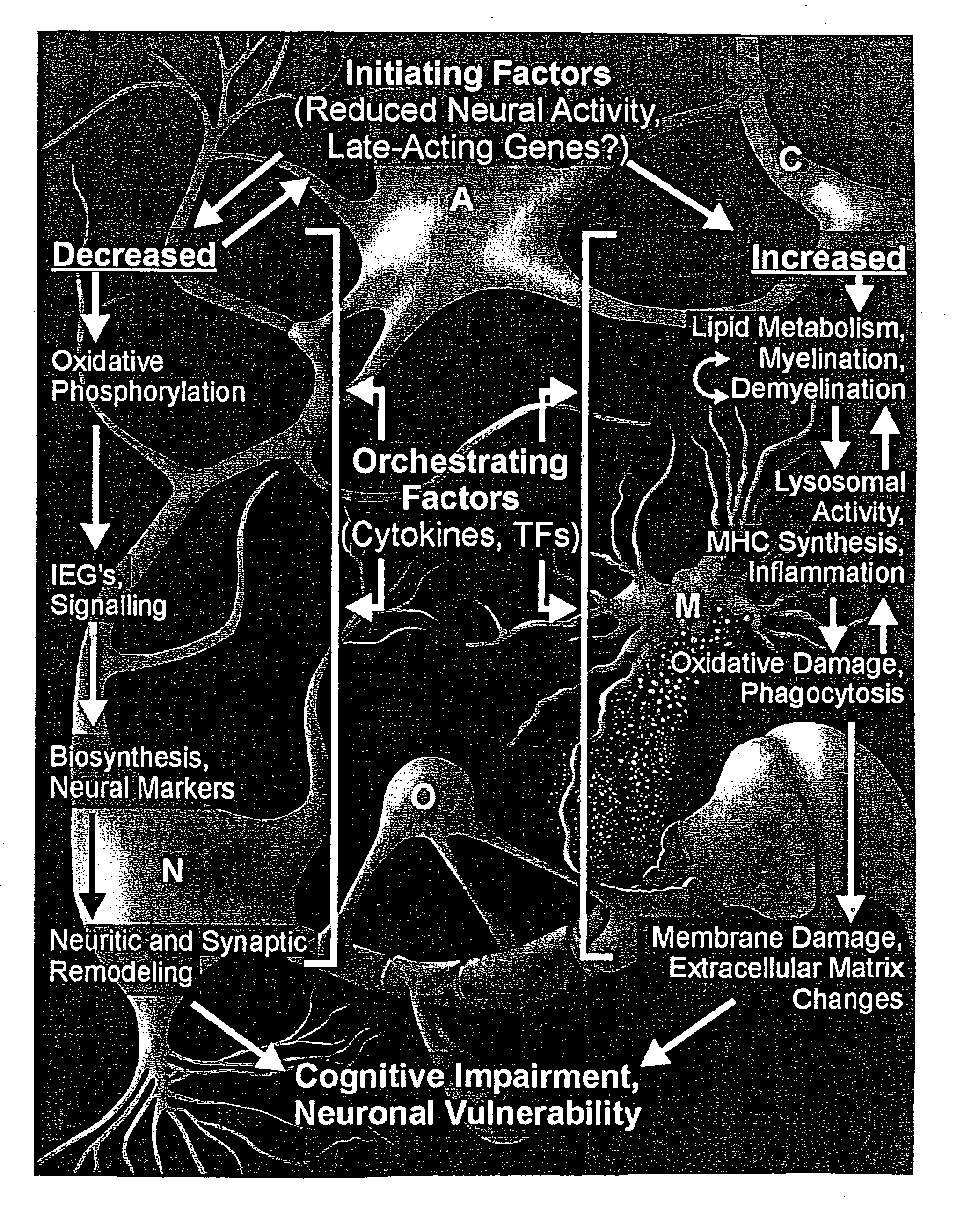
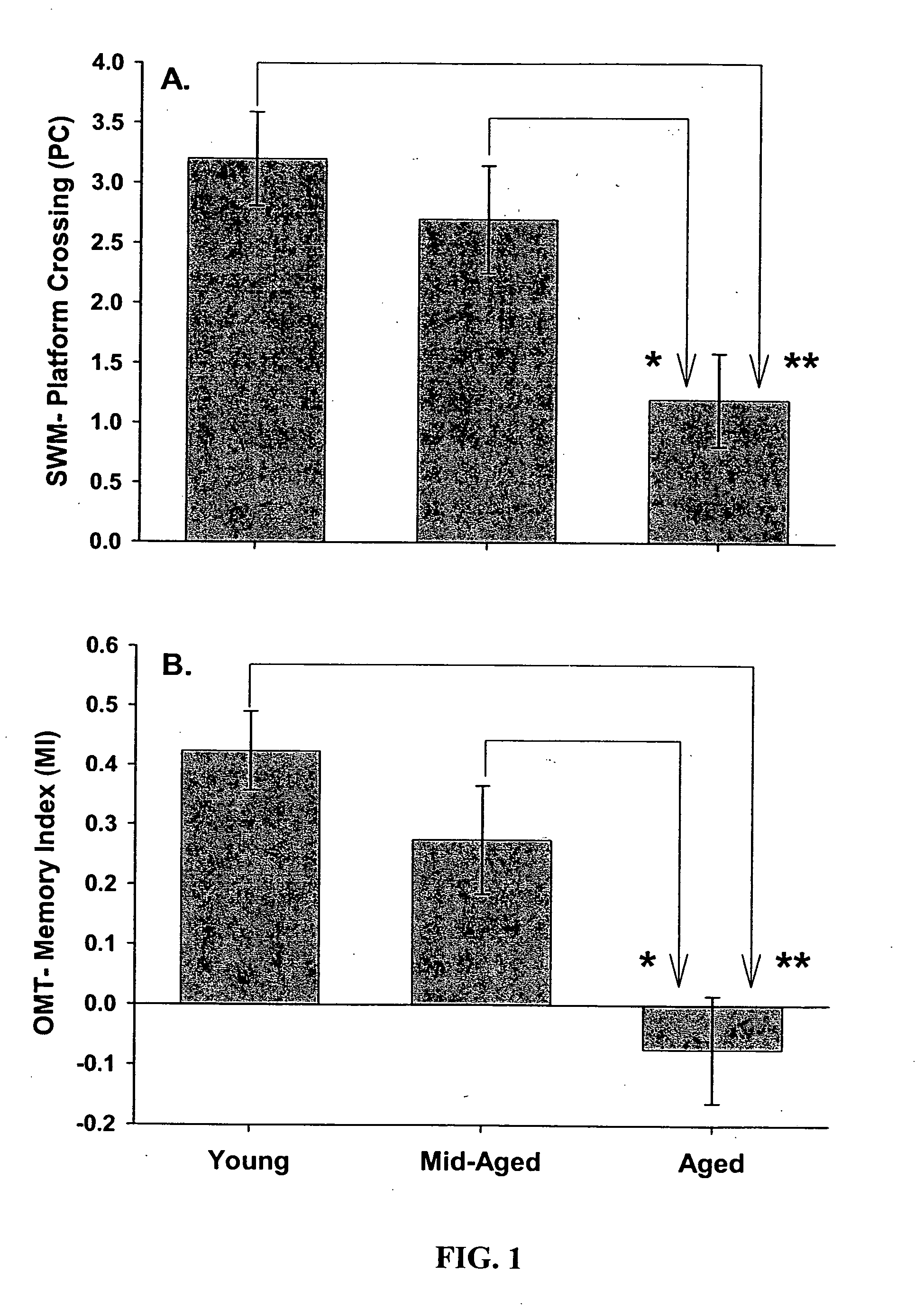
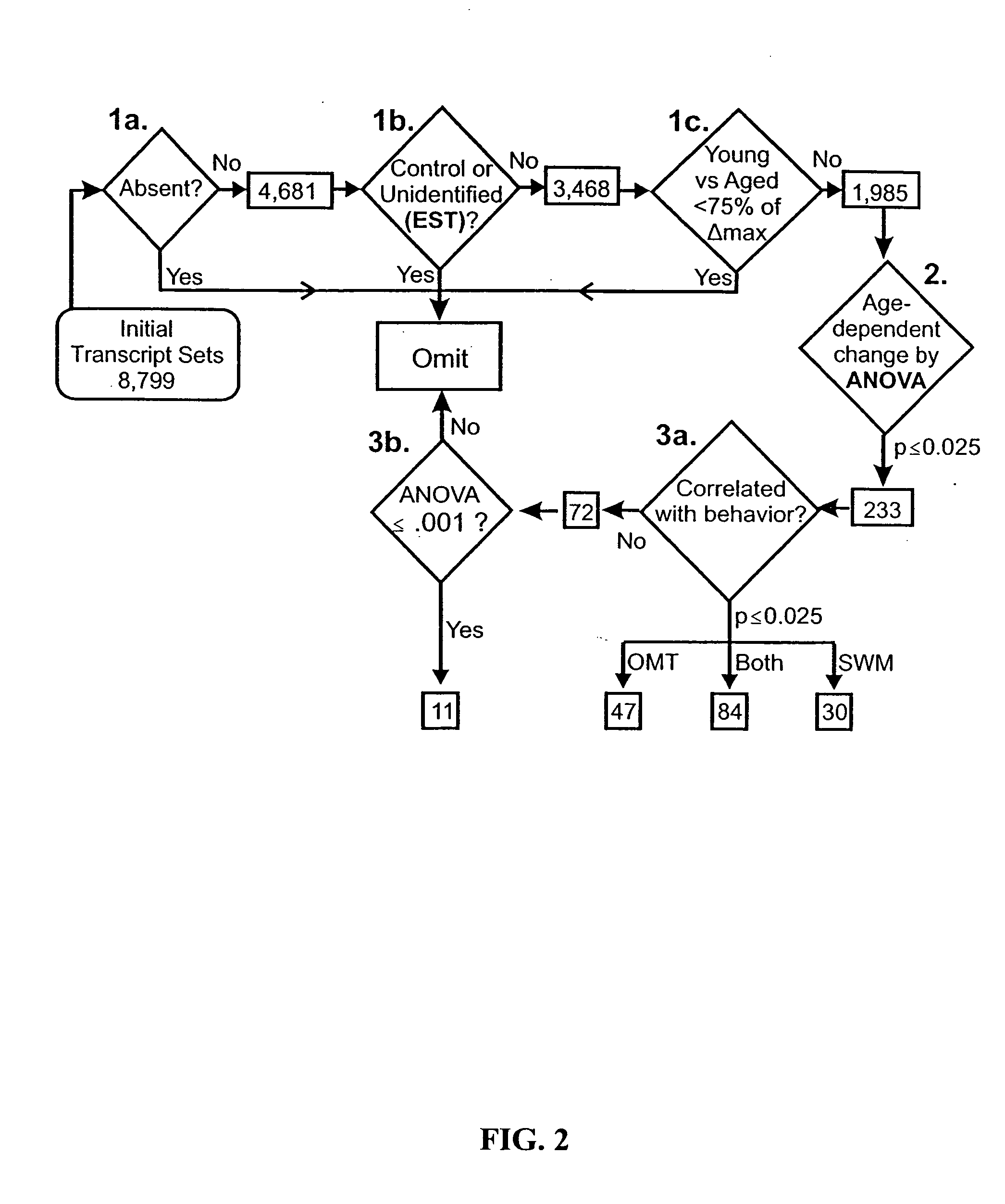
Gene expression profile biomarkers and therapeutic targets for brain aging and age-related cognitive impairment
InactiveUS20050071088A1Increase neuronal vulnerabilityImprove lipid metabolismMicrobiological testing/measurementProteomicsAntigenDisease cause
A statistical and functional correlation strategy to identify changes in cellular pathways specifically linked to impaired cognitive function with aging. Analyses using the strategy identified multiple groups of genes expressed in the hippocampi of mammals, where the genes were expressed at different levels for several ages. The aging changes in expression began before mid-life. Many of the genes were involved in specific neuronal and glial pathways with previously unrecognized relationships to aging and / or cognitive decline. The processes identified by the strategy suggest a new hypothesis of brain aging in which initially decreased neuronal activity and / or oxidative metabolism trigger separate but parallel genomic cascades in neurons and glia. In neurons, the cascade results in elevations in calcium signaling and reductions of immediate early gene signaling, biosynthesis, synaptogenesis and neurite remodeling. In contrast, glia undergo increased lipid metabolism and mediate a cycle of demyelination and remyelination that induces antigen presentation, inflammation, oxidative stress and extracellular restructuring. These identified genes and the proteins they encode can be used as novel biomarkers of brain aging and as targets for developing treatment methods against age-related cognitive decline, Alzheimer's Disease and Parkinson's Disease.
Owner:UNIV OF KENTUCKY RES FOUND
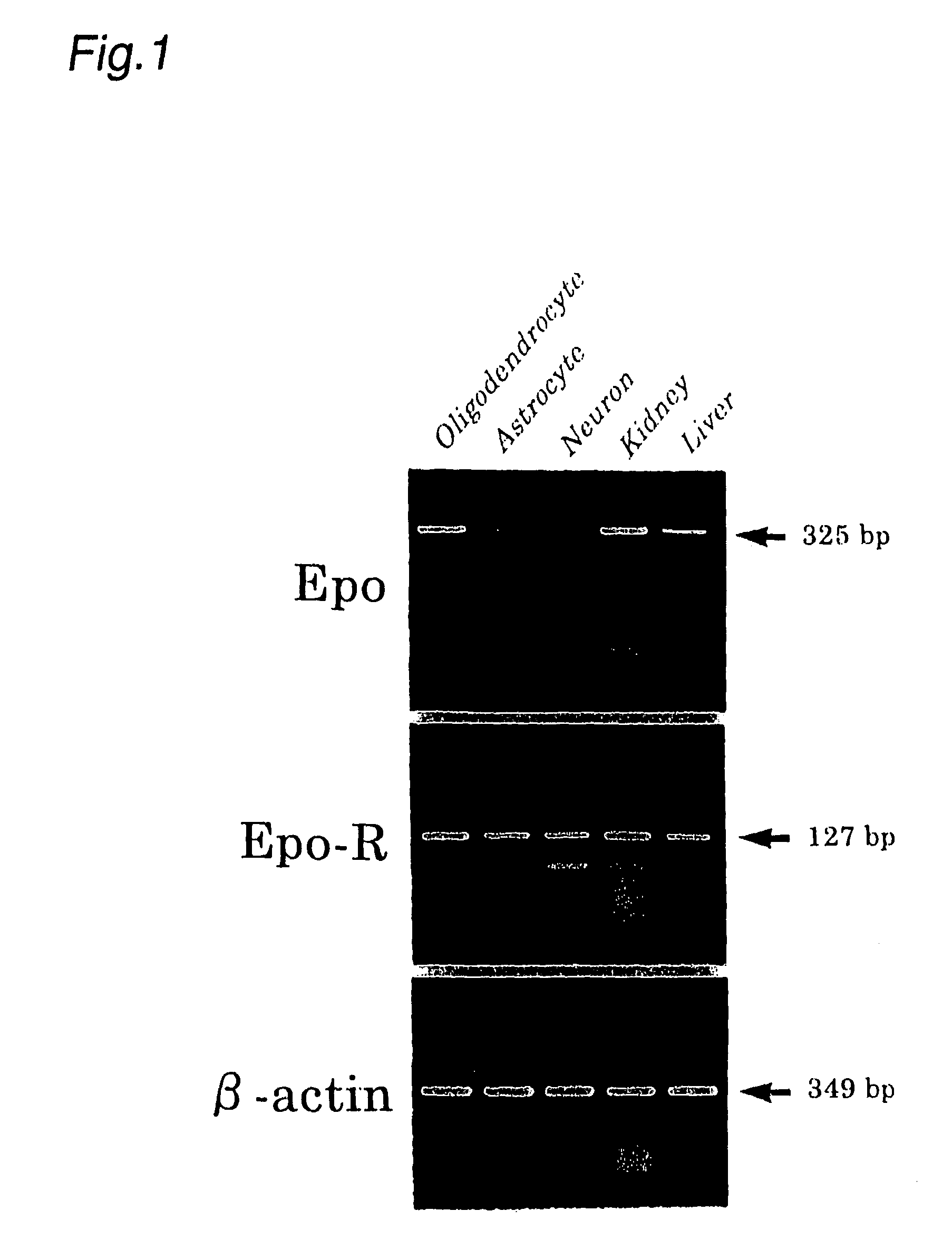
Pharmaceutical for prevention and treatment of demyelinating disease
A pharmaceutical for prevention and treatment of demyelination-associated neural function impairing diseases contains erythropoietin as an active ingredient, and protectively act on the survival of oligodendrocytes, which form a myelin sheath, in cerebrovascular dementia typified by multiple sclerosis and Binswanger disease, diseases involving demyelination. The pharmaceutical and method also promote maturation of undifferentiated oligodendrocytes present in the brain, activating remyelination. Through these mechanisms, the pharmaceutical and method can prevent and treat demyelination-associated neural function impairing diseases.
Owner:TOKYO METROPOLITAN FOUND FOR RES ON AGING & PROMOTION OF WELFARE +1
Human IgM antibodies, and diagnostic and therapeutic uses thereof particularly in the central nervous system
InactiveUS7473423B2Promote reproductionPromote safer self-therapiesNervous disorderPeptide/protein ingredientsNervous systemIgm antibody
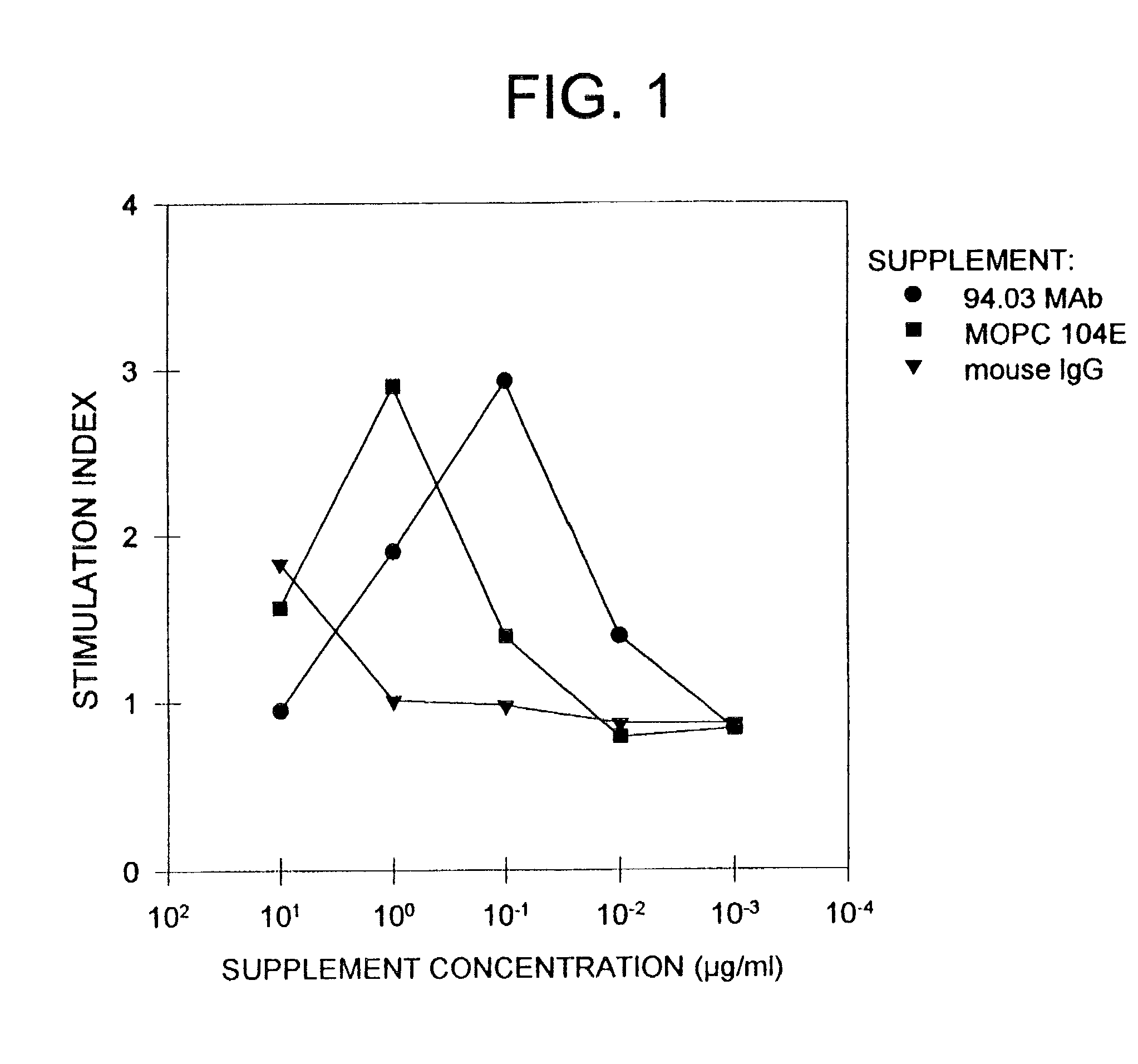
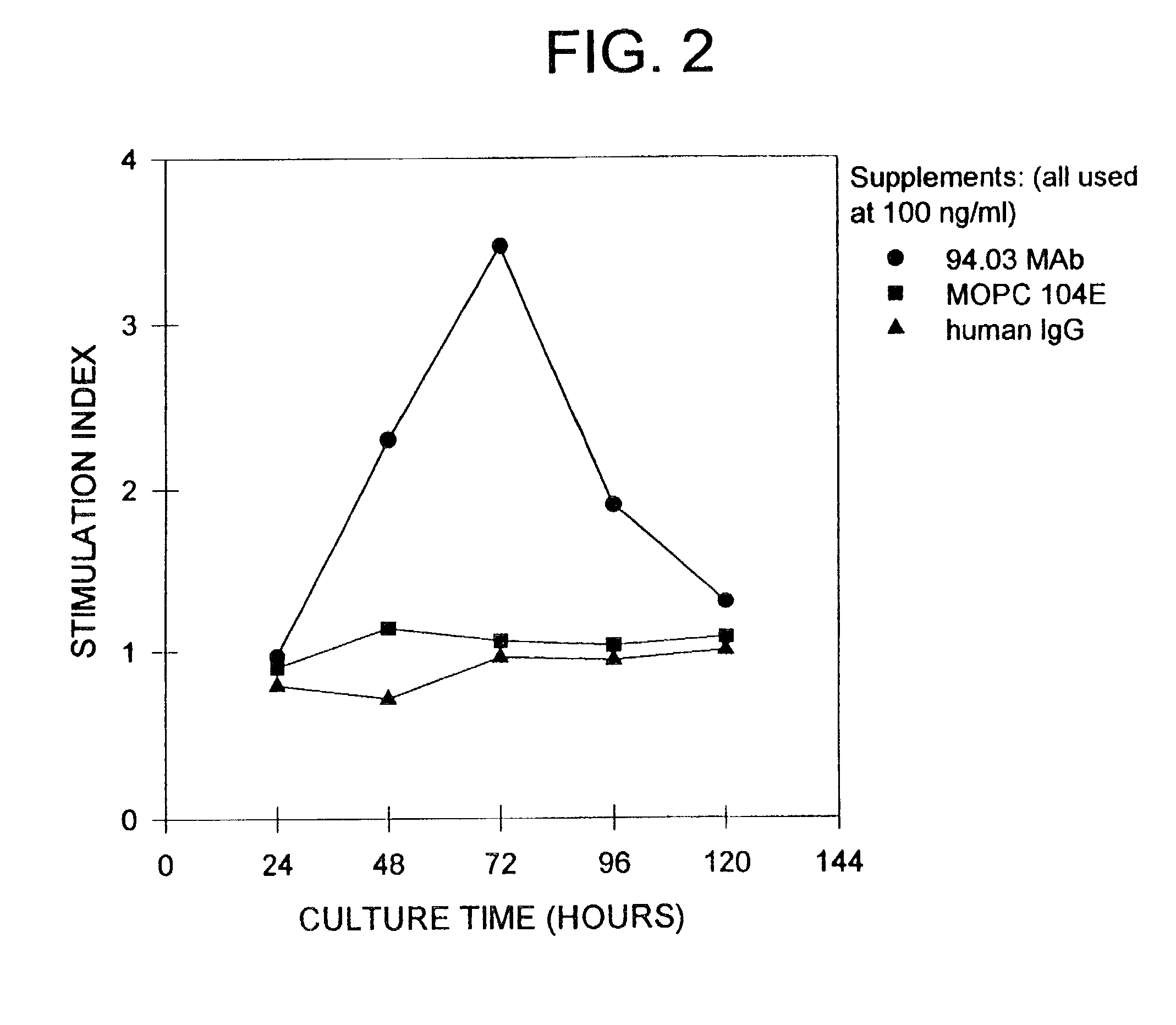
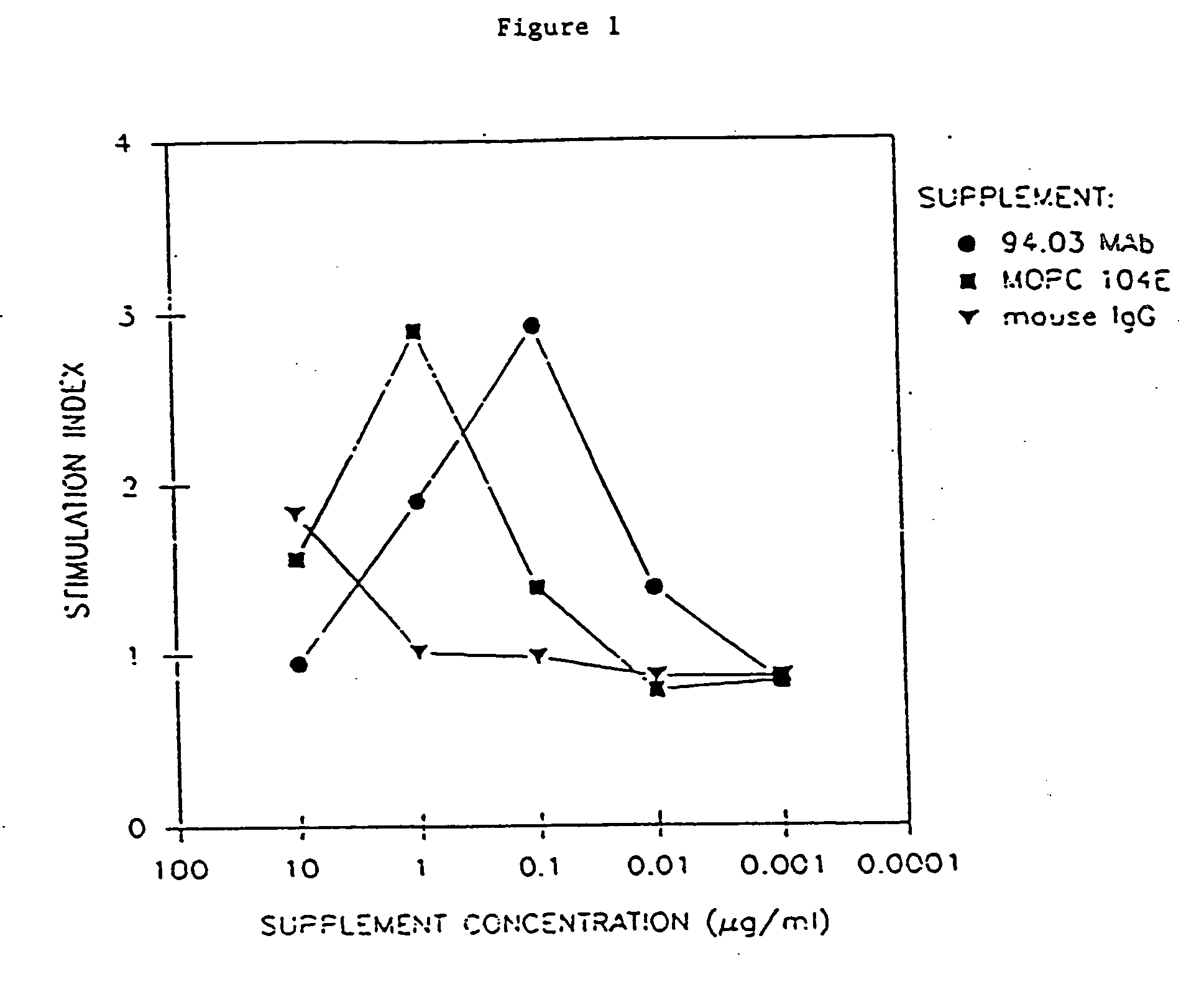
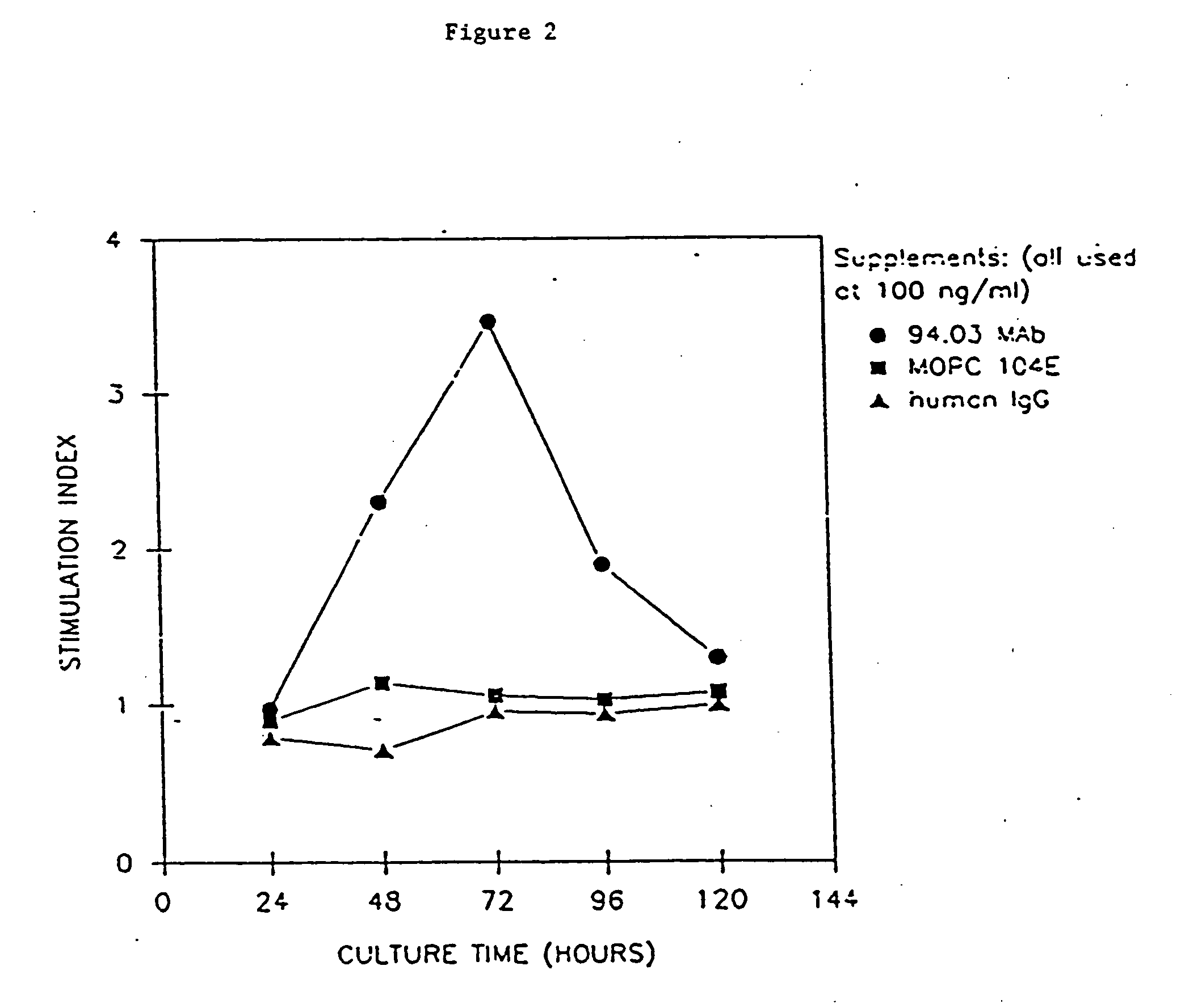
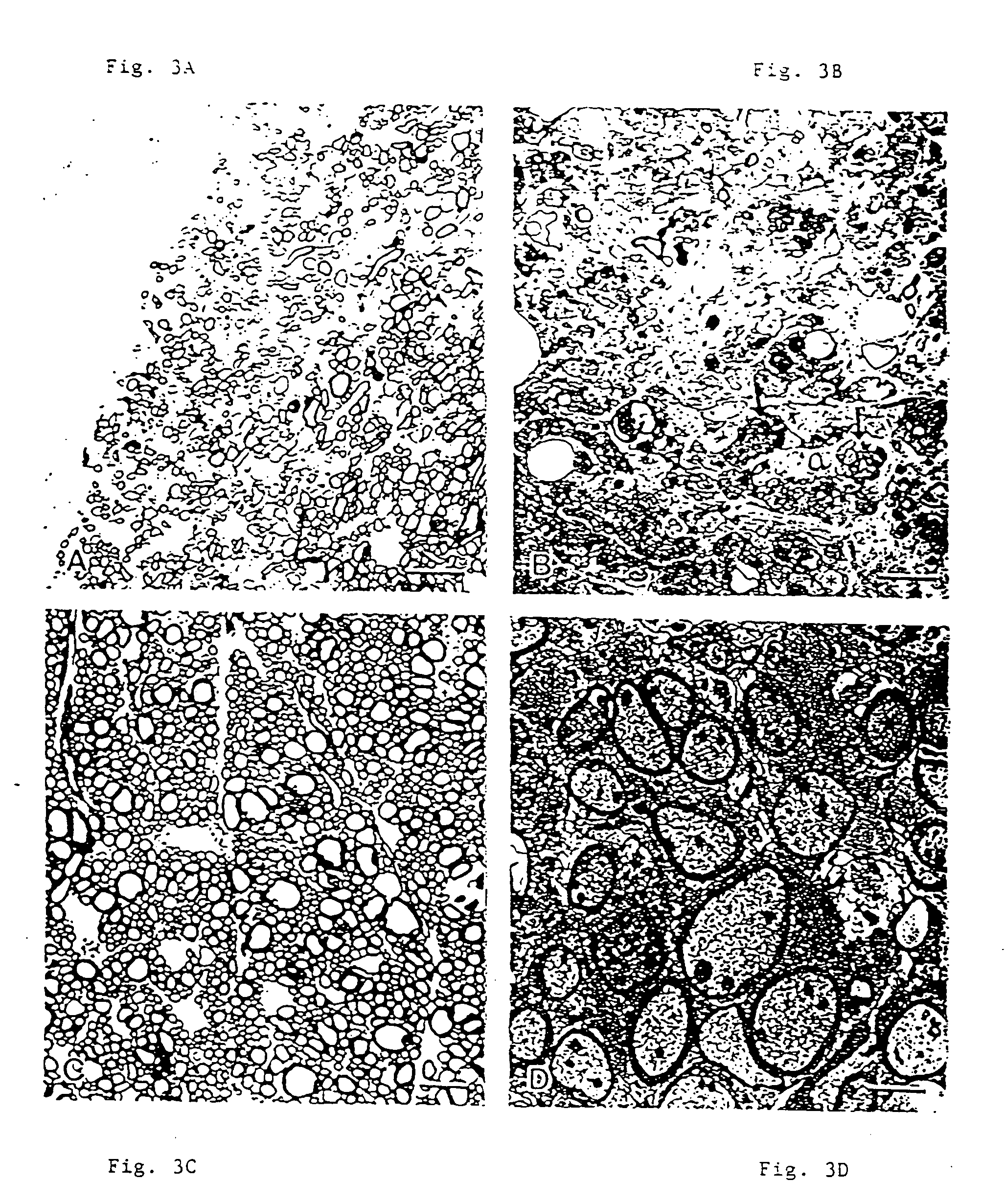
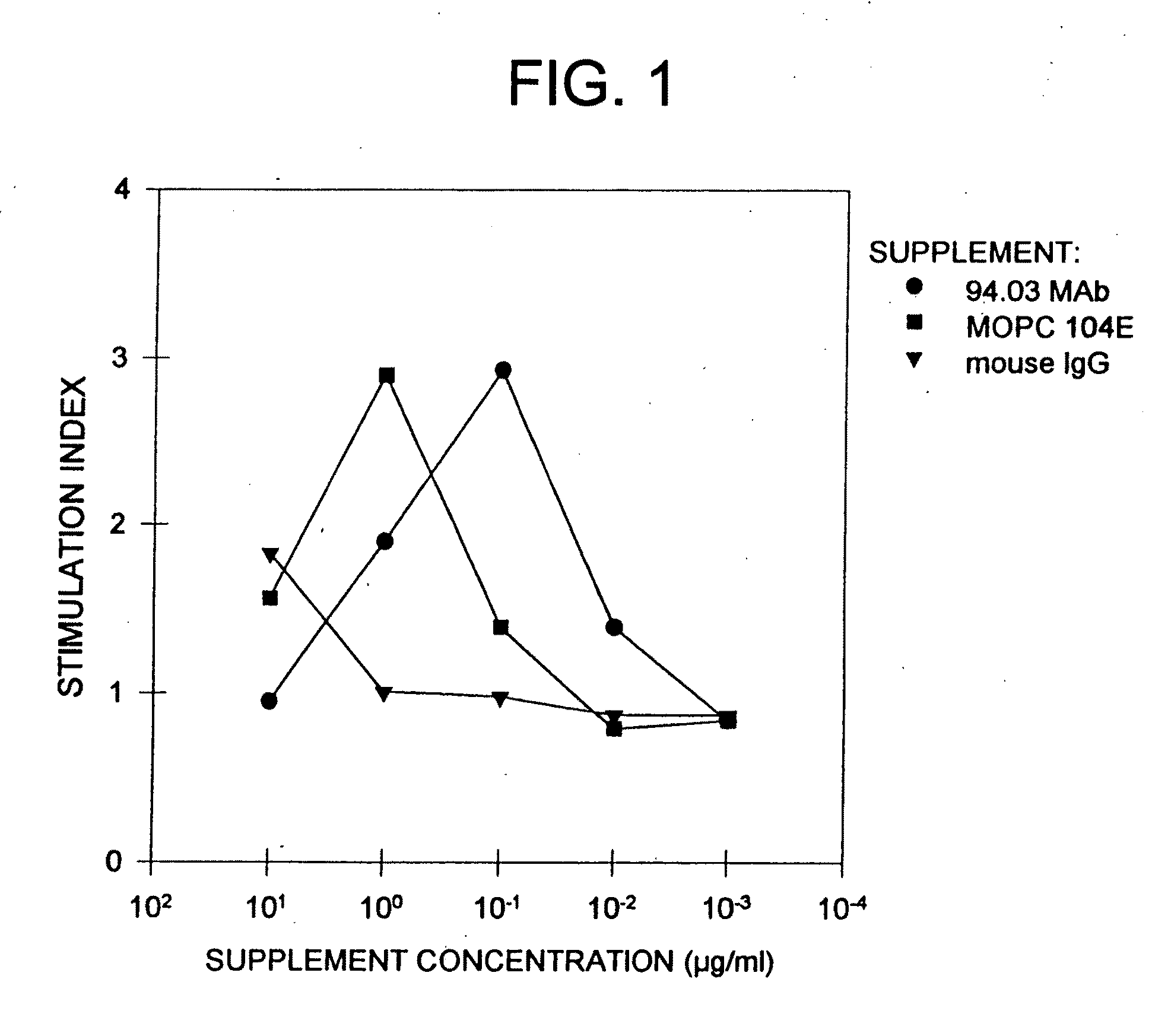
Antibodies, and particularly human antibodies, are disclosed that demonstrate activity in the treatment of demyelinating diseases as well as other diseases of the central nervous system that are of viral, bacterial or idiopathic origin, including neural dysfunction caused by spinal cord injury. Neuromodulatory agents are set forth that include and comprise a material selected from the group consisting of an antibody capable of binding structures or cells in the central nervous system, a peptide analog, a hapten, active fragments thereof, agonists thereof, mimics thereof, monomers thereof and combinations thereof. The neuromodulatory agent has one or more of the following characteristics: it is capable of inducing remyelination; binding to neural tissue; promoting Ca++ signaling with oligodendrocytes; and promoting cellular proliferation of glial cells. Amino acid and DNA sequences of exemplary antibodies are disclosed. Methods are described for treating demyelinating diseases, and diseases of the central nervous system of humans and domestic animals, using polyclonal IgM antibodies and human monoclonal antibodies sHIgm22(LYM 22), sHIgm46(LYM46) ebvHIgM MSI19D10, CB2bG8, AKJR4, CB2iE12, CB2iE7, MSI19E5 and MSI10E10, active fragments thereof and the like. The invention also extends to the use of human antibodies, fragments, peptide derivatives and like materials, and their use in diagnostic and therapeutic applications, including screening assays for the discovery of additional antibodies that bind to cells of the nervous system, particularly oligodendrocytes.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
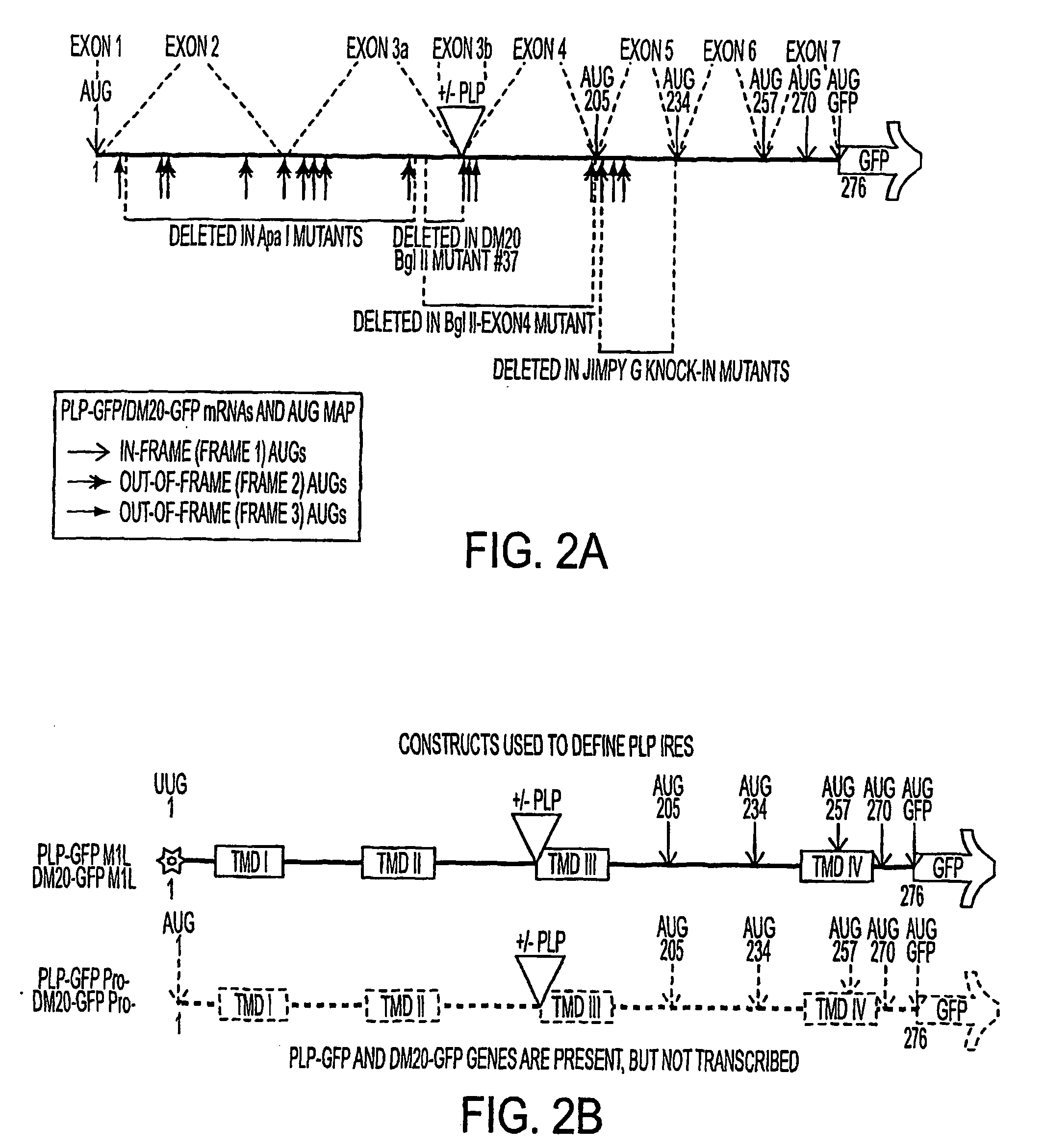
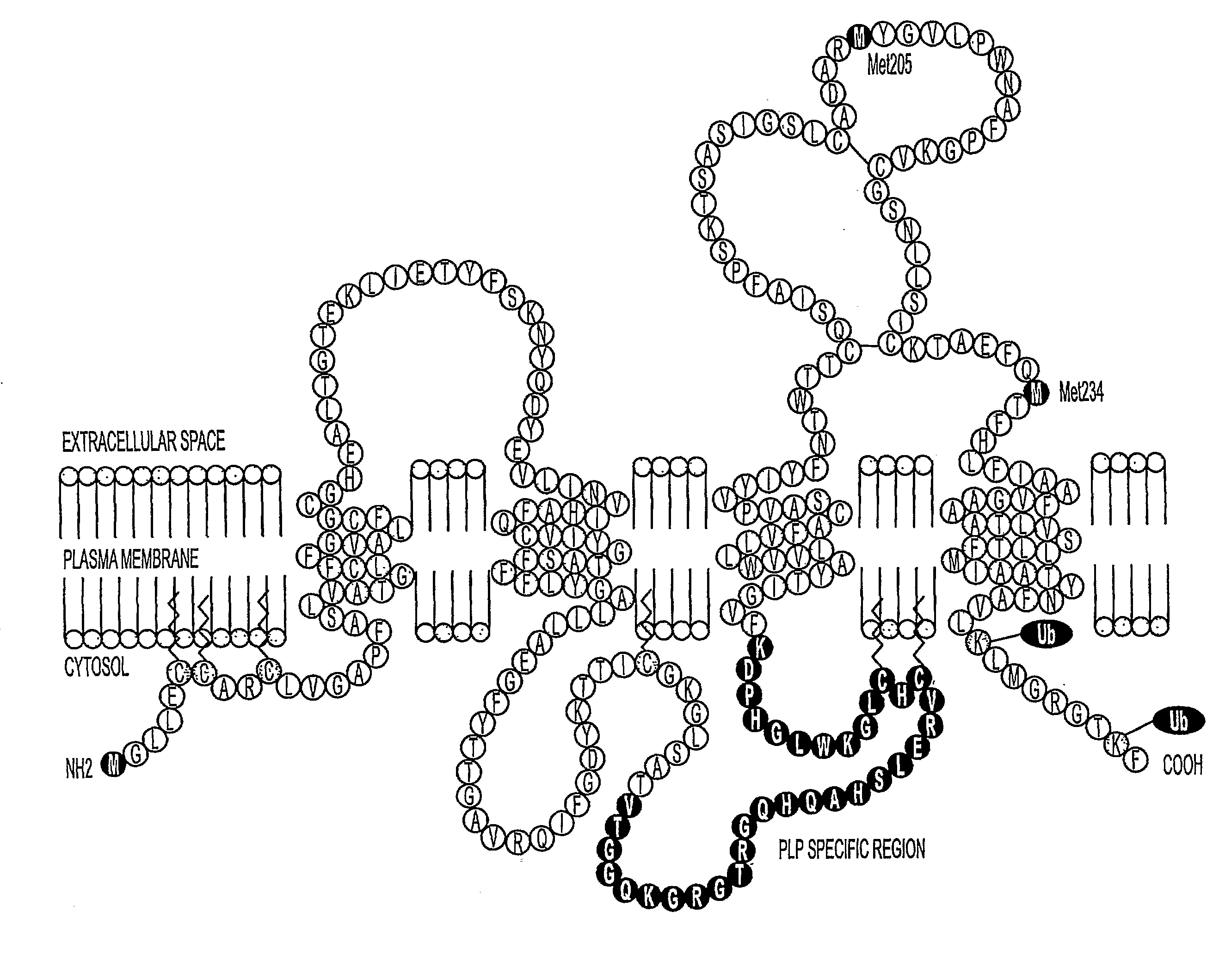
Bioactive peptides and unique ires elements from myelin proteolipid protein plp/dm20
InactiveUS20060173168A1Positively effect myelin repairIncrease secretionPeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseSpinal cord
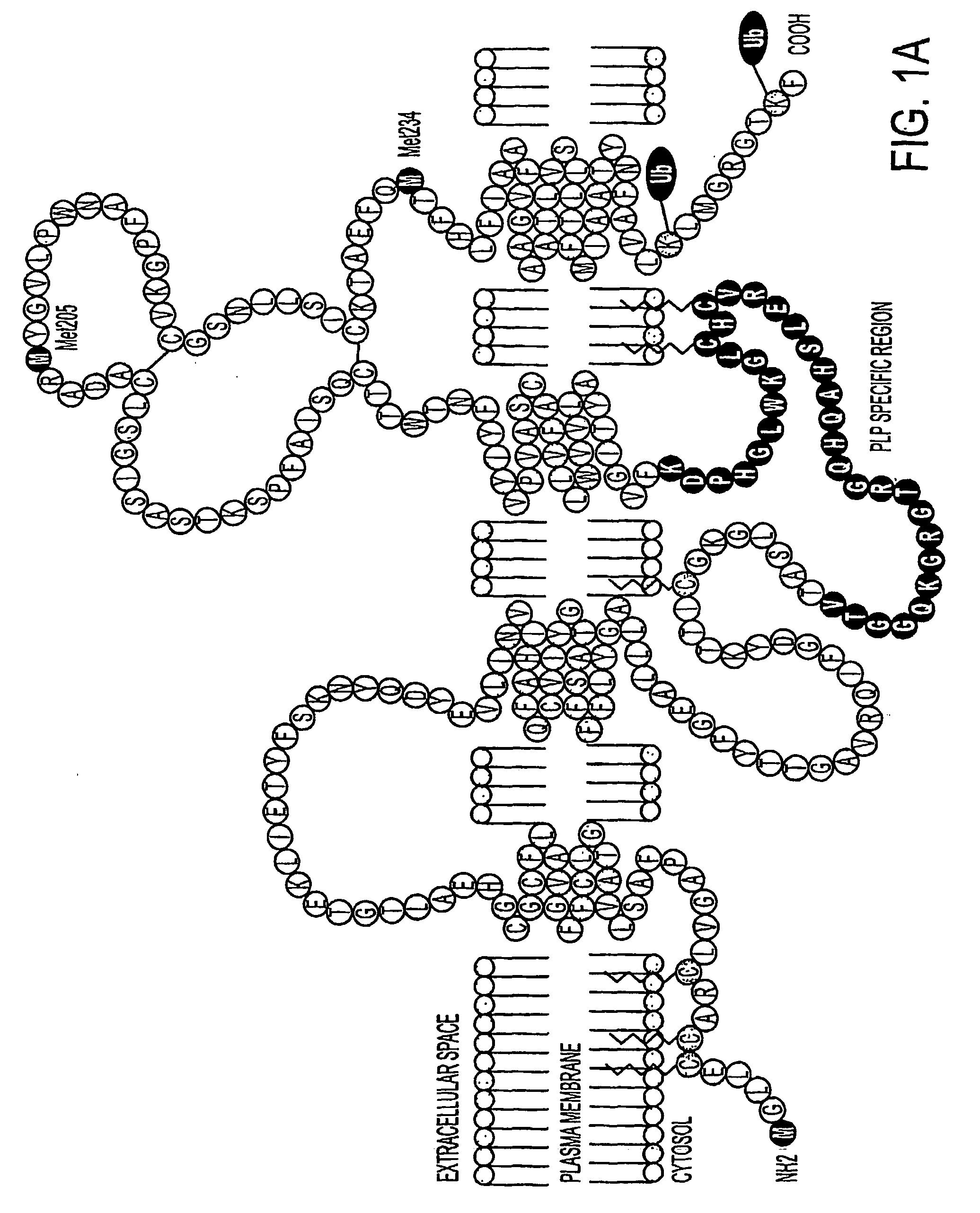
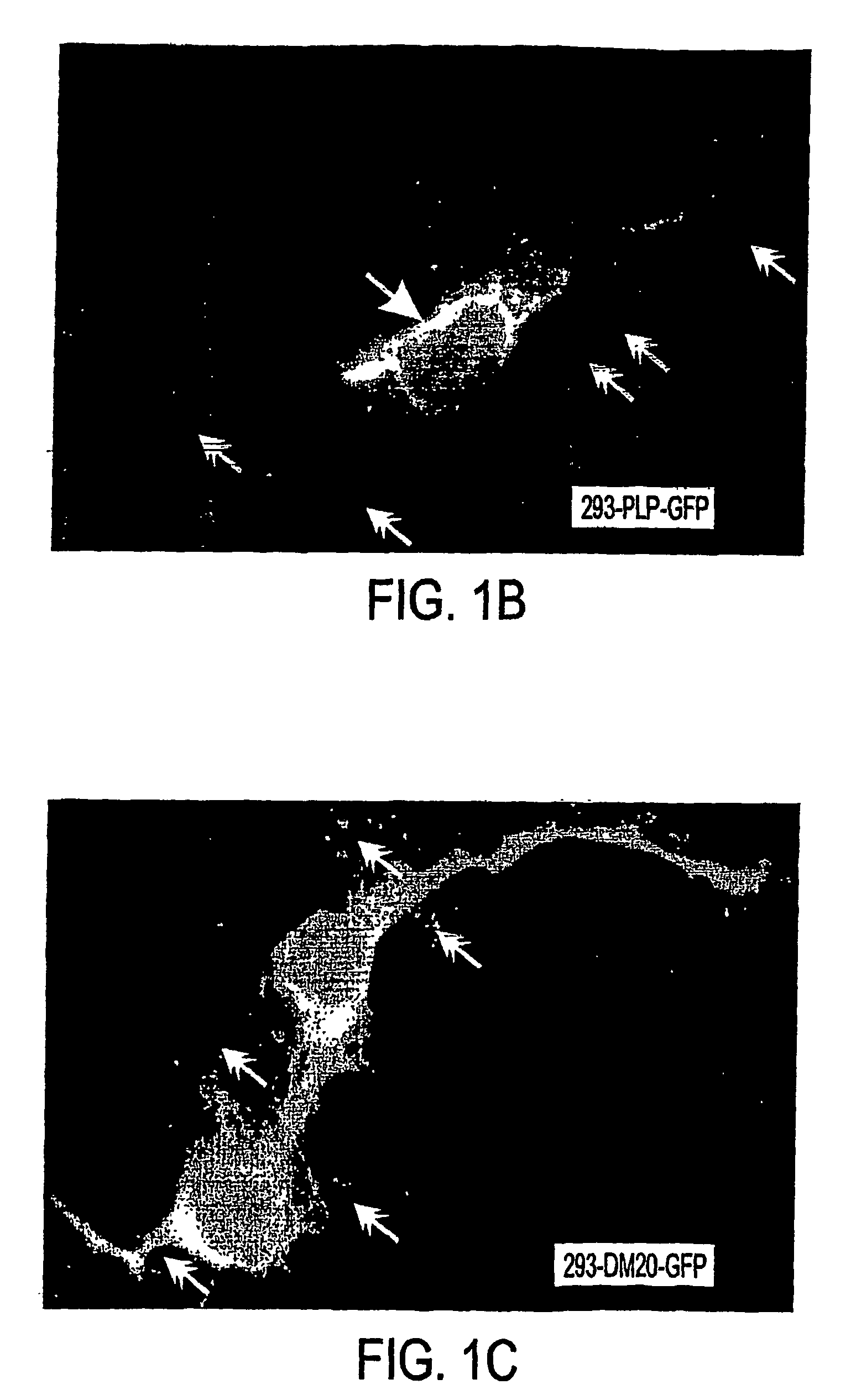
Three novel low molecular weight (LMW) polypeptide fragments of a proteolipid protein human PLP / DM20 are designated PIRP-M, PIRP-L and PIRP-J, and are growth factors for oligodendrocytes with anti-apoptotic activity. They are encoded by mRNA from an IRES. Fusion polypeptides of such a LMW polypeptide, DNA encoding the LMW polypeptide and fusion polypeptide, expression vectors comprising such DNA, and cells expressing such polypeptides, or pharmaceutical compositions thereof, are useful for stimulating neural stem cell differentiation, maturation along the oligodendrocytic pathway and proliferation of oligodendrocytes or precursors. These compositions can protect oligodendrocytes (and nonneural cells) from apoptotic death. Thus, the present composition is used to treat a disease or condition in which such differentiation, maturation and proliferation or inhibition of cell death, including remyelination or stimulation of oligodendroglia or Schwann cells, is desirable. Disorders include multiple sclerosis, trauma with Parkinson's-like symptoms, hypoxic ischerriia and spinal cord trauma.
Owner:WAYNE STATE UNIV
Oligodendrocytes derived from human embryonic stem cells for remyelination and treatment of spinal cord injury
ActiveUS7285415B2Enhances late-stage differentiationEfficient productionBiocideSenses disorderNeural cellRemyelination
This invention provides populations of neural cells bearing markers of glial cells, such as oligodendrocytes and their precursors. The populations are generated by differentiating pluripotent stem cells such as human embryonic stem cells under conditions that promote enrichment of cells with the desired phenotype or functional capability. Various combinations of differentiation factors and mitogens can be used to produce cell populations that are over 95% homogeneous in morphological appearance, and the expression of oligodendrocyte markers such as GalC. The cells are capable of forming myelin sheaths, and can be used therapeutically improve function of the central nervous system.
Owner:RGT UNIV OF CALIFORNIA
Neural stem cell preparation, preparing method thereof and use of same
InactiveCN1435187AImprove proliferative abilityEffectively treats damageNervous disorderMuscular disorderSingle cell suspensionEmbryo
A neural stem cell preparation for treating human myelopathy, especially the motor neuron diseases, is prepared through digesting the brain tissue of human embryo, adding to culture medium, centrifugal processing, mechanical beating to obtain cell suspension, culturing in culture medium in CO2 atmosphere, screening neural stem cell balls, and passage by 3-6 generations.
Owner:北京科宇联合干细胞生物技术有限公司
Bioactive peptides and unique ires elements from myelin proteolipid protein plp/dm20
InactiveUS20080227707A1Conducive to survivalIncrease secretionCell receptors/surface-antigens/surface-determinantsVectorsApoptosisRemyelination
Three novel low molecular weight (LMW) polypeptide fragments of a proteolipid protein human PLP / DM20 are designated PIRP-M, PIRP-L and PIRP-J, and are growth factors for oligodendrocytes with anti-apoptotic activity. They are encoded by mRNA from an IRES. Fusion polypeptides of such a LMW polypeptide, DNA encoding the LMW polypeptide and fusion polypeptide, expression vectors comprising such DNA, and cells expressing such polypeptides, or pharmaceutical compositions thereof, are useful for stimulating neural stem cell differentiation, maturation along the oligodendrocytic pathway and proliferation of oligodendrocytes or precursors. These compositions can protect oligodendrocytes (and nonneural cells) from apoptotic death. Thus, the present composition is used to treat a disease or condition in which such differentiation, maturation and proliferation or inhibition of cell death, including remyelination or stimulation of oligodendroglia or Schwann cells, is desirable. Disorders include multiple sclerosis, trauma with Parkinson's-like symptoms, hypoxic ischerriia and spinal cord trauma.
Owner:WAYNE STATE UNIV
Myelination of congenitally dysmyelinated forebrains using oligodendrocyte progenitor cells
Owner:CORNELL RES FOUNDATION INC
Composition for and treatment of demyelinating diseases and paralysis by administration of remyelinating agents
ActiveUS20050215565A1Effective treatmentInhibition is effectiveBiocideImpression capsAntibody fragmentsDemyelinating disease
The application provides for methods and compositions for inhibiting demyelination, promoting remyelination and / or treating paralysis in a subject in need thereof. Preferably, such compositions include immunoglobulins (e.g., antibodies, antibody fragments, and recombinantly produced antibodies or fragments), polypeptides (e.g., soluble forms of the ligand proteins for integrins) and small molecules, which when administered in an effective amount inhibits demyelination and / or promotes remyelination in a patient. The compositions and methods described herein can also utilize other anti-inflammatory agents used to palliate conditions and diseases associated with demyelination.
Owner:BIOGEN MA INC
Functional oligodendrocytes derived from pluripotent stem cells and methods of making and using the same
ActiveUS20170183627A1Low costComparable myelination potentialNervous disorderNervous system cellsProgenitorInduced pluripotent stem cell
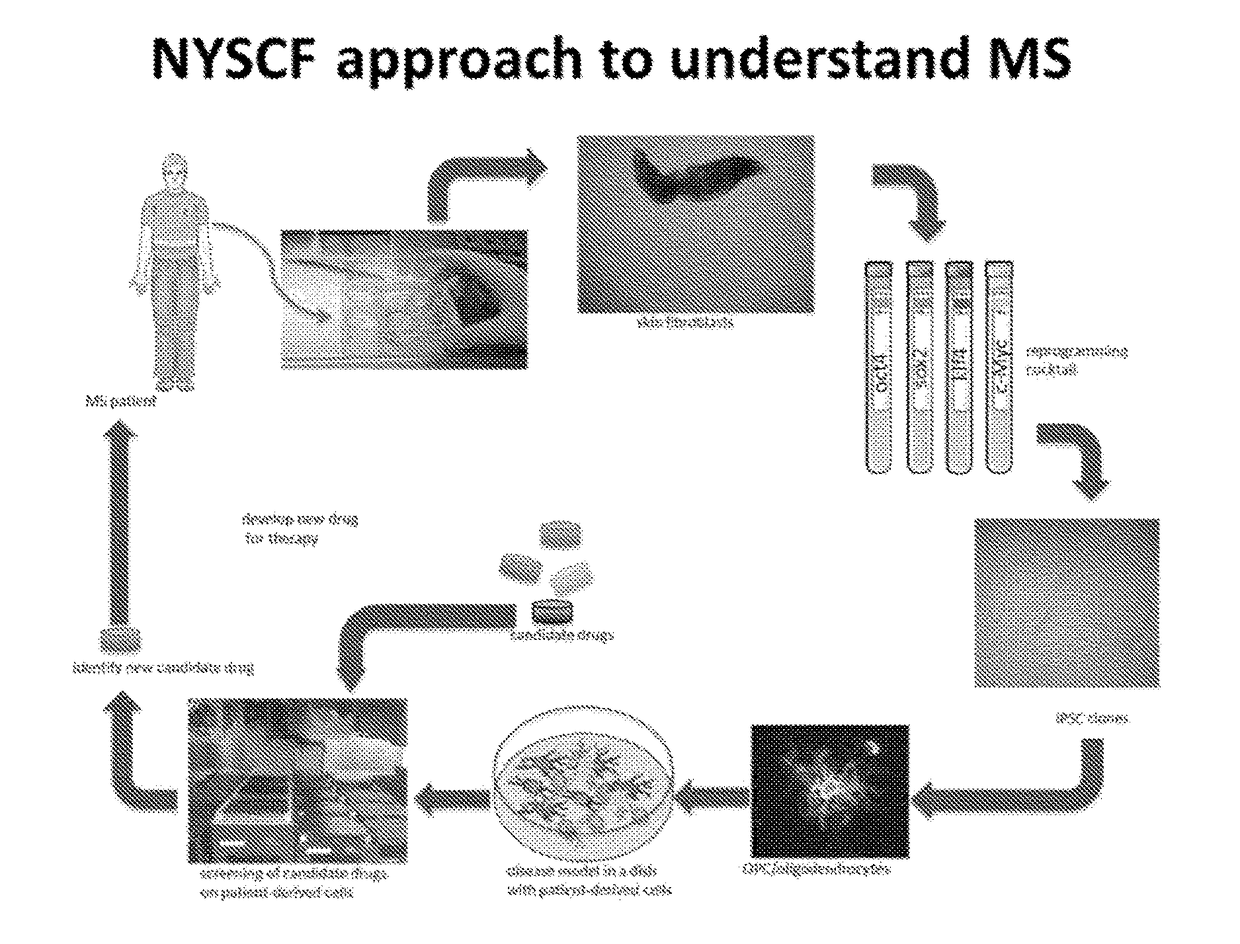
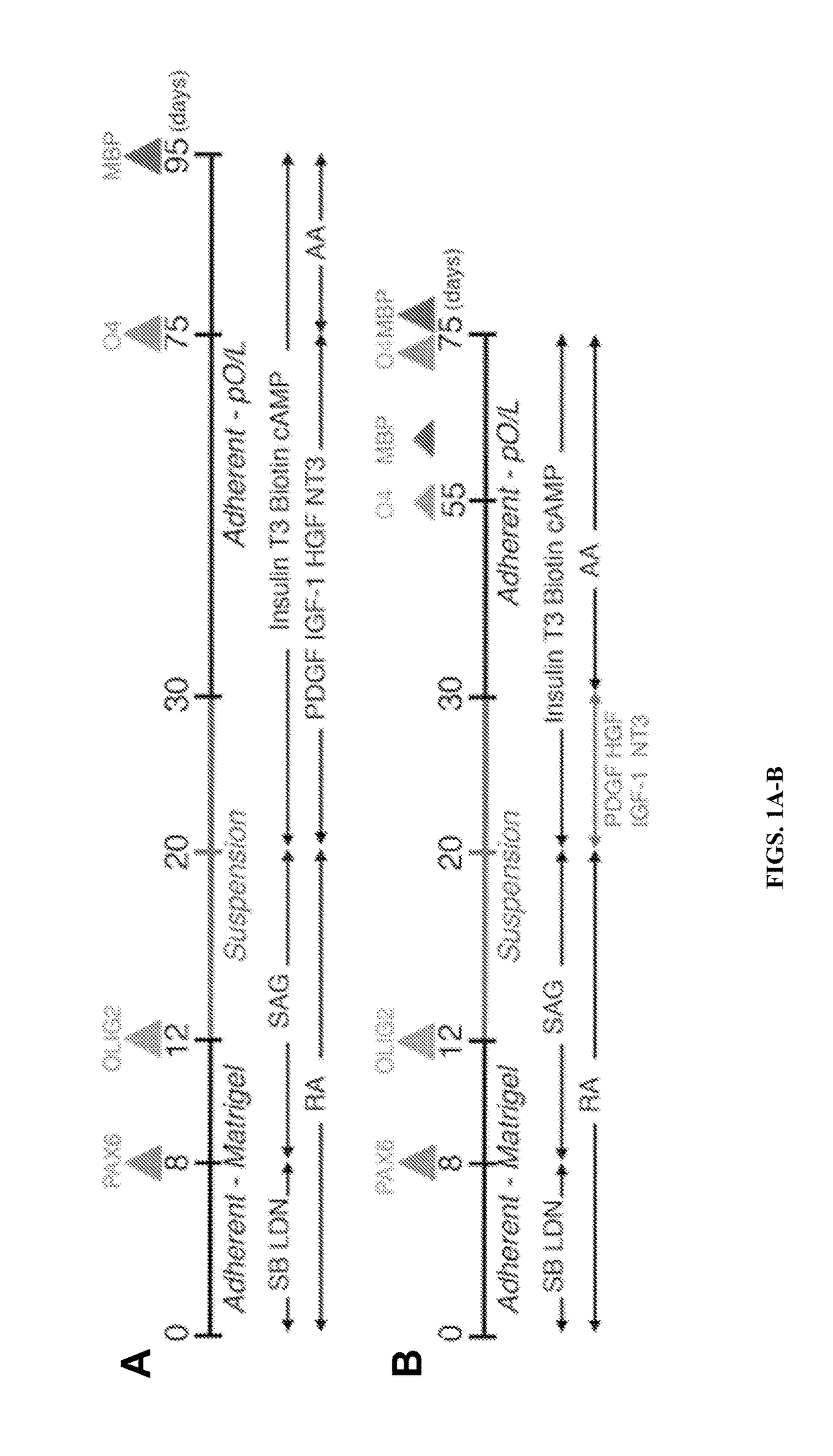
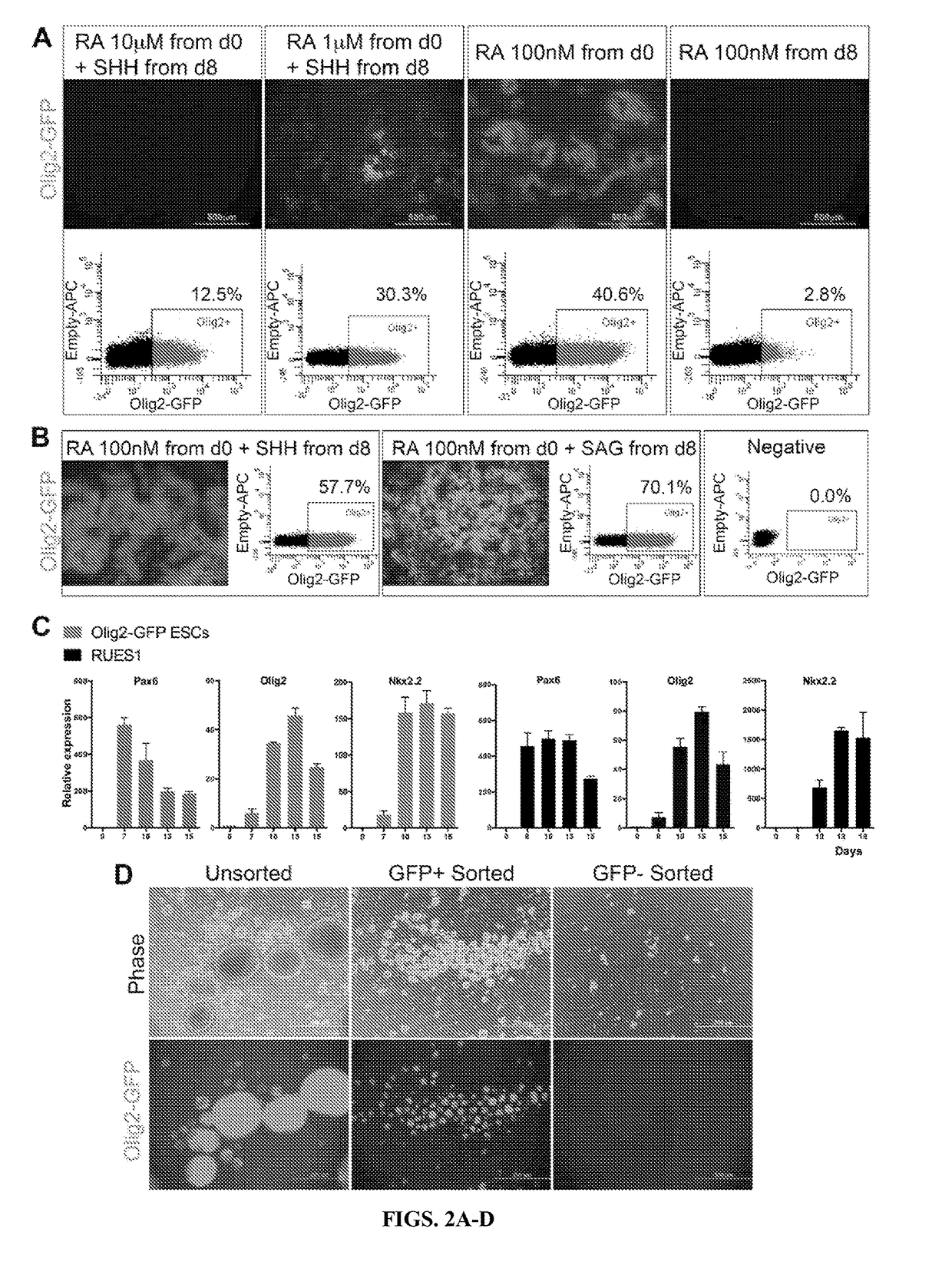
Described is the efficient and robust generation of oligodendrocyte progenitor cells (OPCs) and oligodendrocytes from pluripotent stem cells (PSCs). The protocols provided recapitulate the major steps of oligodendrocyte differentiation, from neural stem cells to OLIG2+ progenitors, and then to 04+ OPCs, in a significantly shorter time than the 120-150 days required by previous protocols. Furthermore, 04+ OPCs are able to differentiate into MBP+ mature oligodendrocytes in vitro, and to myelinate axons in vivo when injected into immuno-compromised Shiverer mice, providing proof of concept that transplantation of PSC-derived cells for remyelination is technically feasible.
Owner:NEW YORK STEM CELL FOUND
Remyelination of neurons using multipotent neural stem cell progeny
The invention provides methods for producing myelin forming cells from multipotent self-renewing central nervous system neural stem cells as well as methods of using one or more cells from a multipotent self-renewing central nervous system neural stem cell population to form patches of myelin. Also provided are cell culture systems for forming patches of myelin and methods of treating demyelination diseases in a mammal.
Owner:BOCO SILICON VALLEY INC
Promotion of central nervous system remyelination using monoclonal autoantibodies
InactiveUS20060140930A1Immunoglobulins against animals/humansAntibody ingredientsMonoclonal igmMulti organ
Monoclonal IgM antibodies which promote central nervous system remyelination when given to a mammal afflicted with a demyelinating disease are disclosed. These antibodies show multi-organ autoreactivity, and recognize both surface and cytoplasmic determinants on glial cells.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Human IgM antibodies, and diagnostic and therapeutic uses thereof particularly in the central nervous system
InactiveUS20090274690A1Promote safer self-therapiesExtended half-lifeNervous disorderPeptide/protein ingredientsNervous systemSpinal cord lesion
Antibodies, and particularly human antibodies, are disclosed that demonstrate activity in the treatment of demyelinating diseases as well as other diseases of the central nervous system that are of viral, bacterial or idiopathic origin, including neural dysfunction caused by spinal cord injury. Neuromodulatory agents are set forth that include and comprise a material selected from the group consisting of an antibody capable of binding structures or cells in the central nervous system, a peptide analog, a hapten, active fragments thereof, agonists thereof, mimics thereof, monomers thereof and combinations thereof. The neuromodulatory agent has one or more of the following characteristics: it is capable of inducing remyelination; binding to neural tissue; promoting Ca−− signaling with oligodendrocytes; and promoting cellular proliferation of glial cells. Amino acid and DNA sequences of exemplary antibodies are disclosed. Methods are described for treating demyelinating diseases, and diseases of the central nervous system of humans and domestic animals, using polyclonal IgM antibodies and human monoclonal antibodies sHIgm22(LYM 22), sHIgm46(LYM46) ebvHIgM MSI19D10, CB2bG8, AKJR4, CB2iE12, CB2iE7, MSI19E5 and MSI10E10, active fragments thereof and the like. The invention also extends to the use of human antibodies, fragments, peptide derivatives and like materials, and their use in diagnostic and therapeutic applications, including screening assays for the discovery of additional antibodies that bind to cells of the nervous system, particularly oligodendrocytes.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
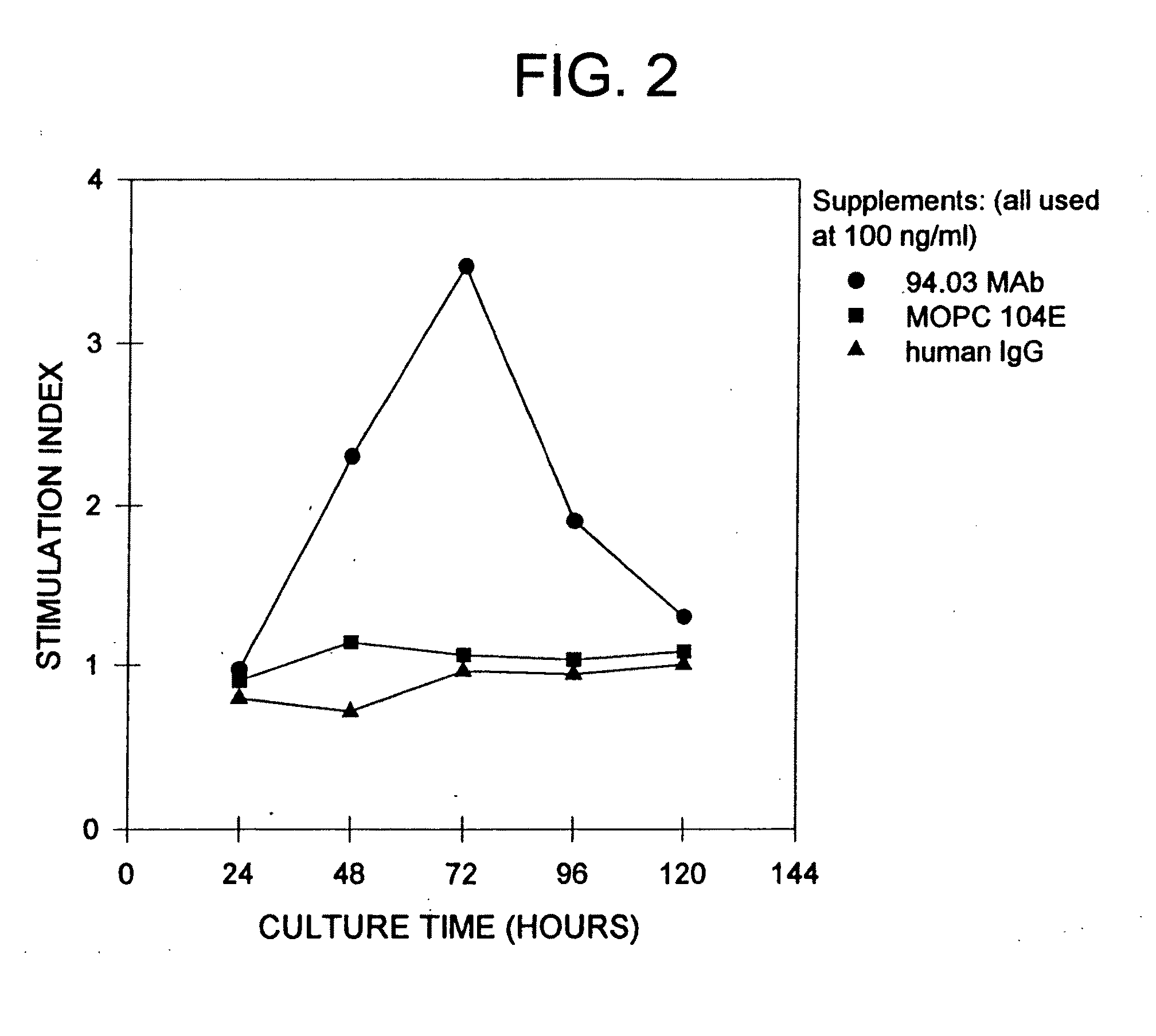
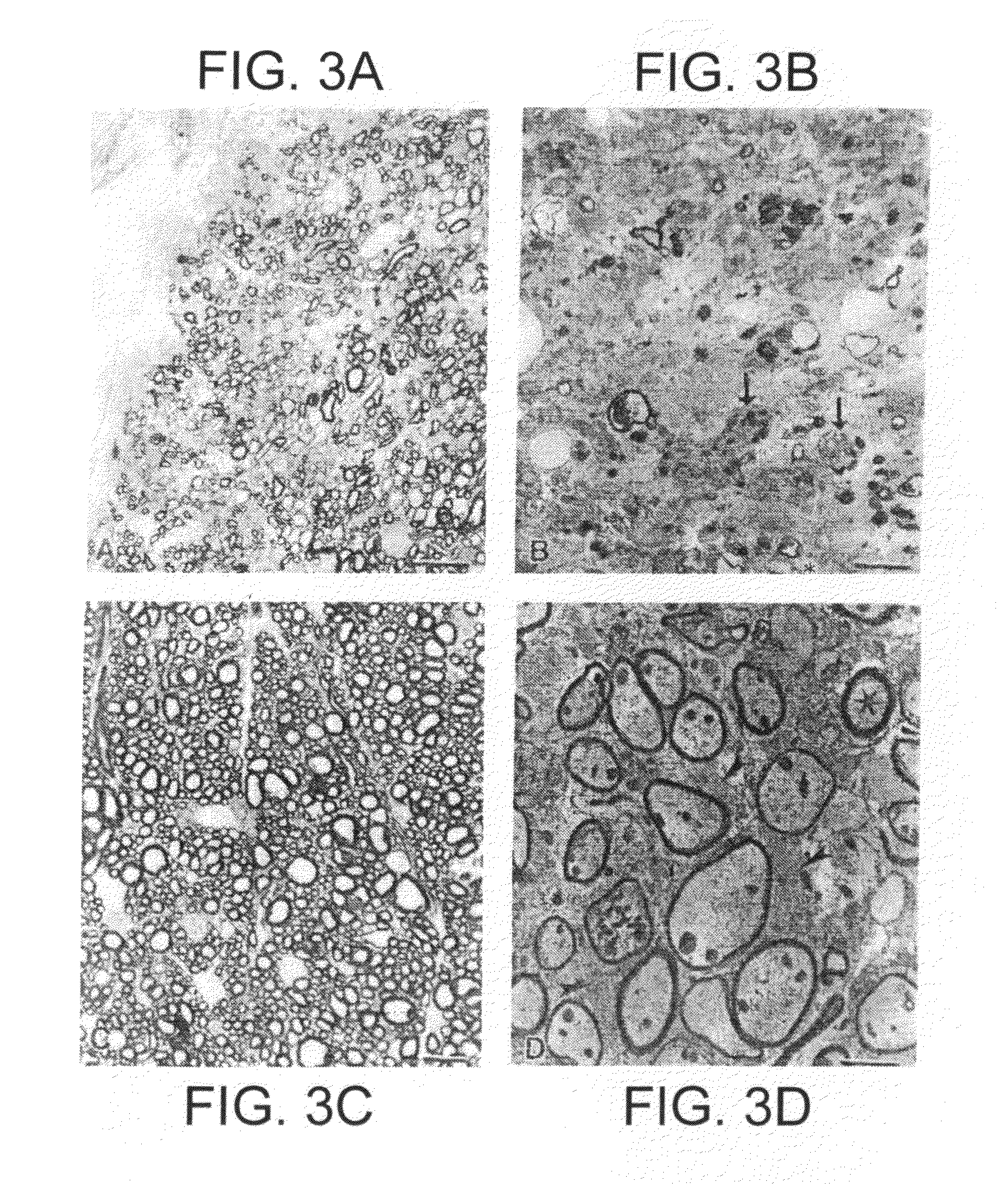
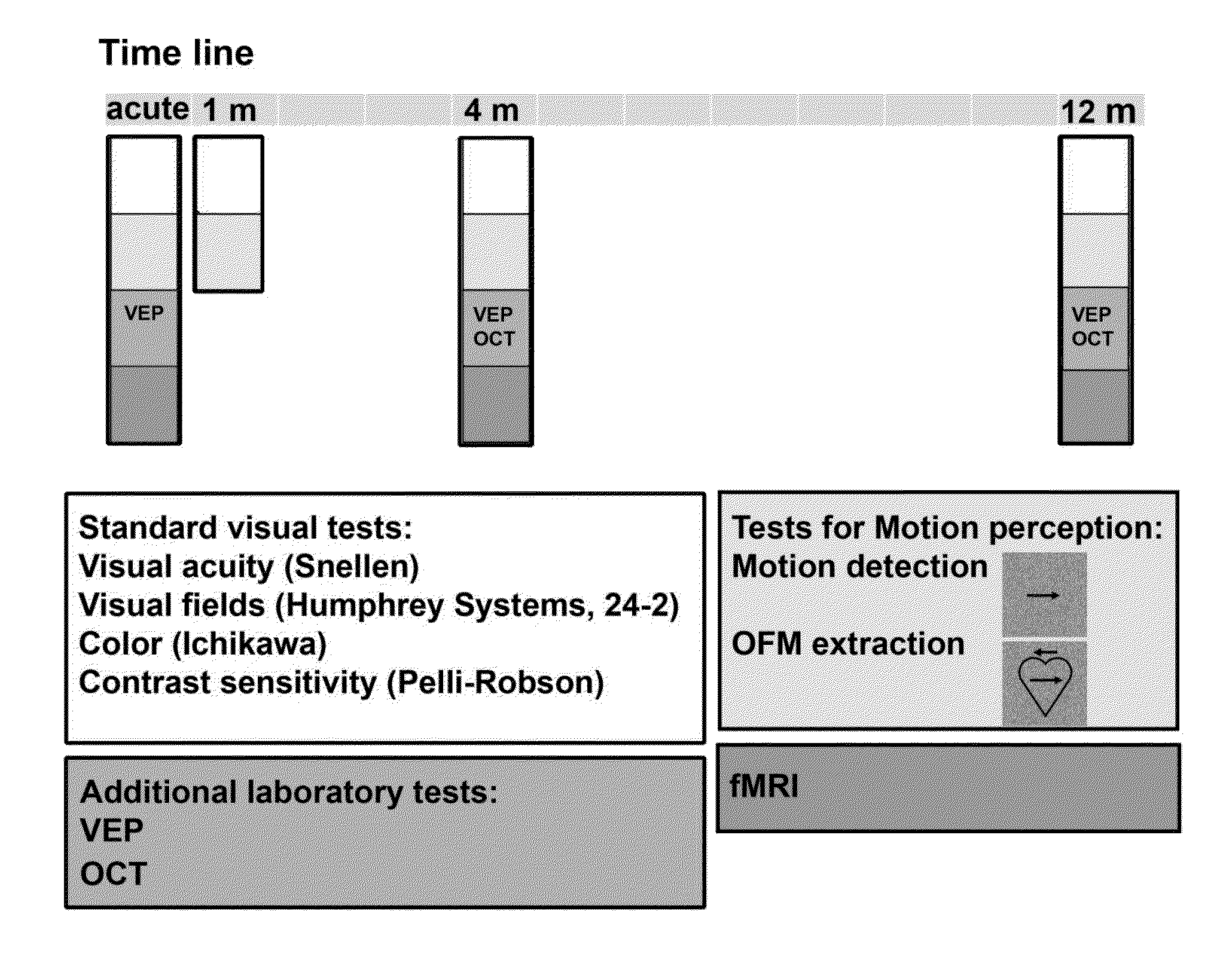
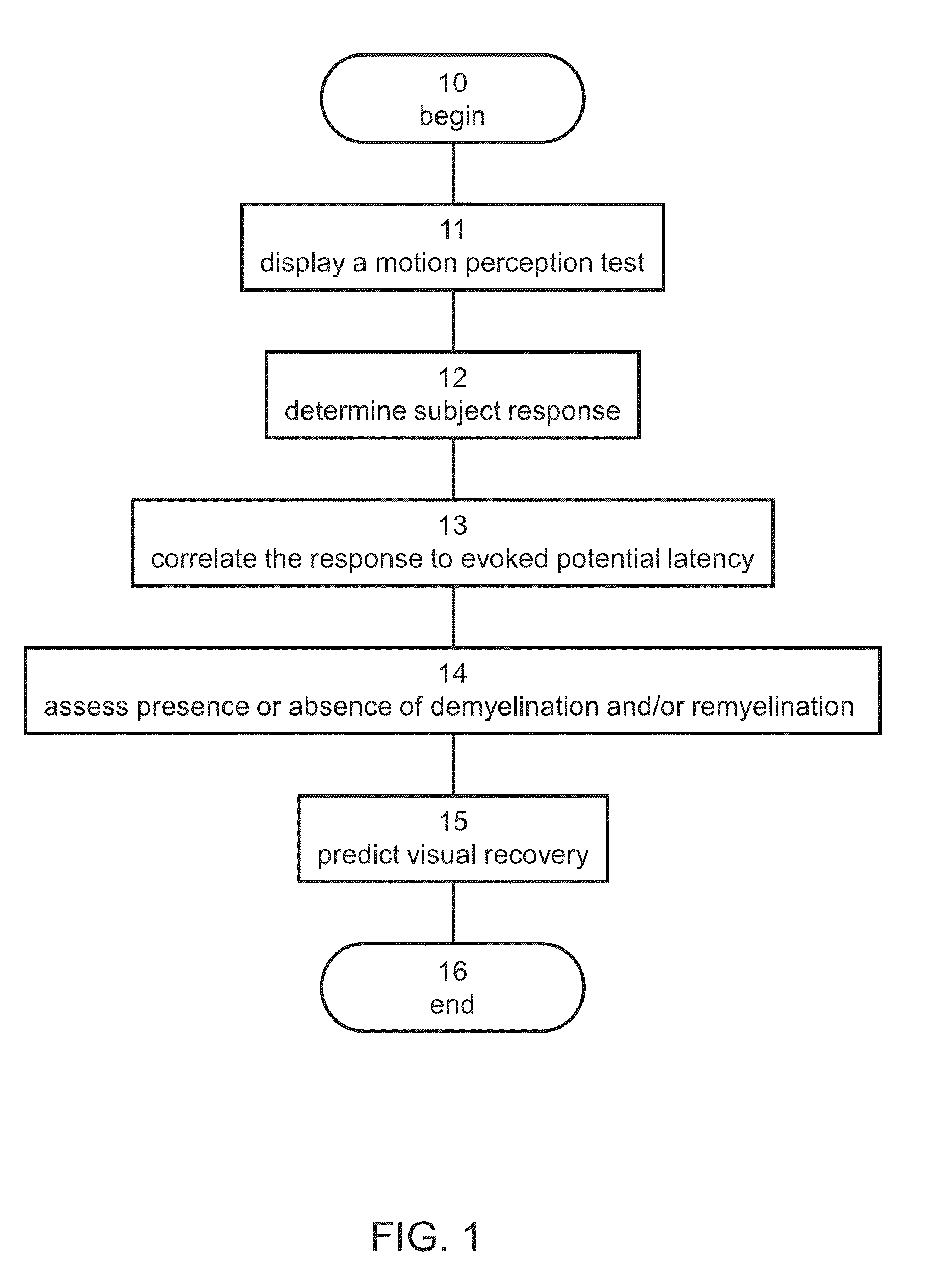
Method and system for assessing visual disorder
A method of diagnosis is disclosed. The method comprises using a display device for presenting a motion perception test to a subject and determining a subject response to the motion perception test. The response can be used for assessing presence or absence of demyelination and / or remyelination.
Owner:HADASIT MEDICAL RES SERVICES & DEVMENT
Method of myelinating isolated motoneurons
ActiveUS8828721B1Fast conductionEasy to understandNervous system cellsArtificial cell constructsSerum free mediaNODAL
The present invention provides a method of inducing myelination of isolated motoneurons by preparing a non-biological substrate having thereon a covalently attached monolayer of DETA; depositing isolated motoneurons on the substrate in a defined serum-free medium; plating isolated Schwann cells cultured in the defined serum-free medium onto the motoneurons, thereby initiating a co-culture; and passaging the co-culture as necessary into fresh, defined serum-free medium supplemented with L-ascorbic acid at least until the motoneurons form Nodes of Ranvier indicative of myelination. The invention also includes a method of testing for new drugs effective in demyelinating diseases. Additionally, cellular products provided by the invention include an isolated motoneurons myelinated or remyelinated in vitro according to the methods disclosed.
Owner:UNIV OF CENT FLORIDA RES FOUND INC
Method for remyelinating a demyelinized lesion due to injury in the brain or spinal cord
InactiveUS7098027B2Prepared safely and readilyBeneficial in medical industryBone marrow stroma cellsBiocideMedicineBone marrow cell
Demyelinated axons were remyelinated in the demyelinated rat model by collecting bone marrow cells from mouse bone marrow and transplanting the mononuclear cell fraction separated from these bone marrow cells.
Owner:MITSUI SUMITOMO INSURANCE CARE NETWORK CO LTD RECEIVES 5 +2
Methods of identifying genes which modulate myelination
InactiveUS20060183147A1High expressionReduce expressionMicrobiological testing/measurementOligodendrocyteDemyelinating disease
The present invention relates to methods of identifying and / or characterizing genes that are modulated during myelination or remyelination, as well as in demyelinating diseases, such as in multiple sclerosis. The invention further provides methods of treating diseases related to myelination in a mammal, such as multiple sclerosis, by administering agents that promote remyelination of oligodendrocytes.
Owner:ELAN PHARM INC
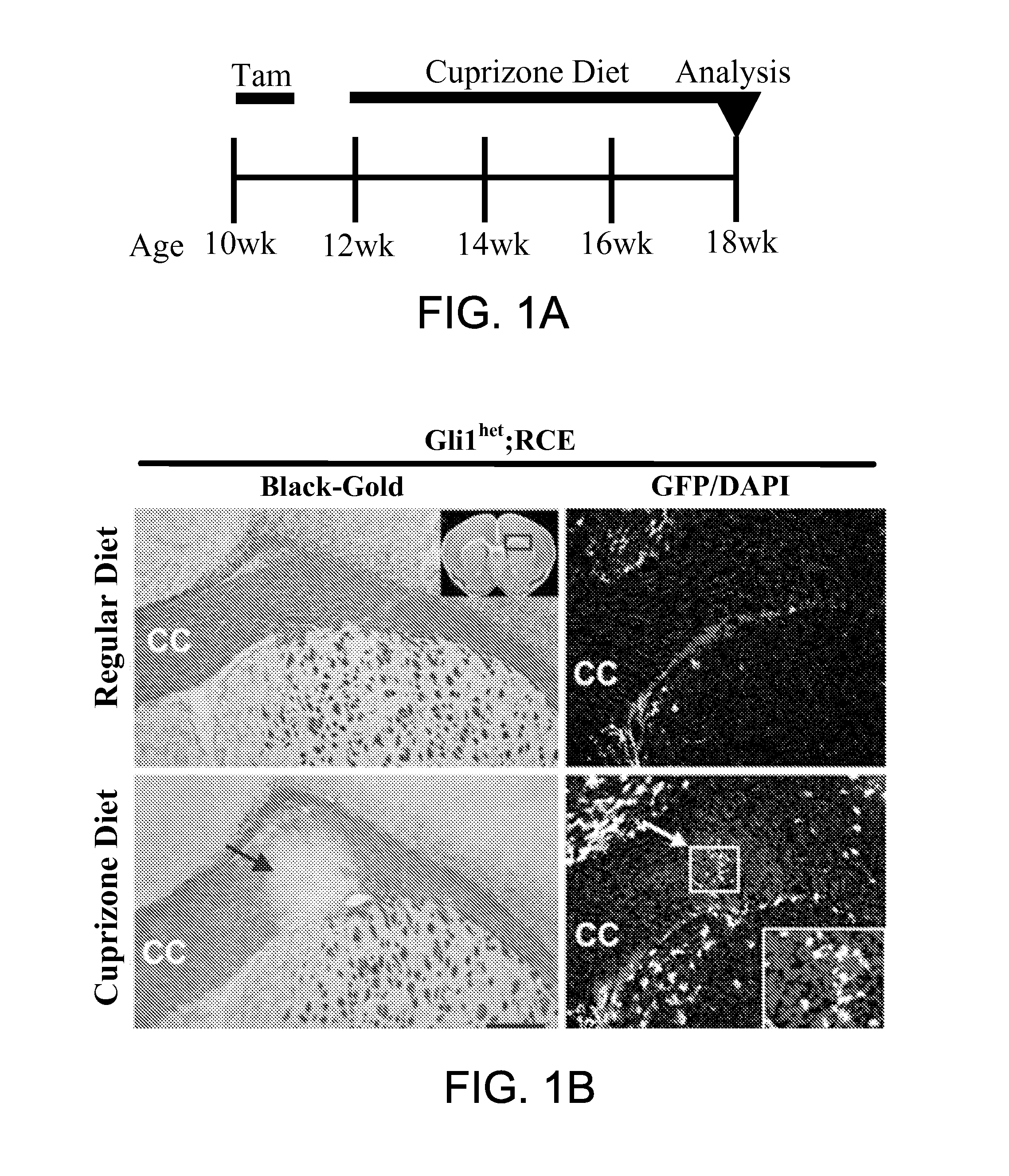
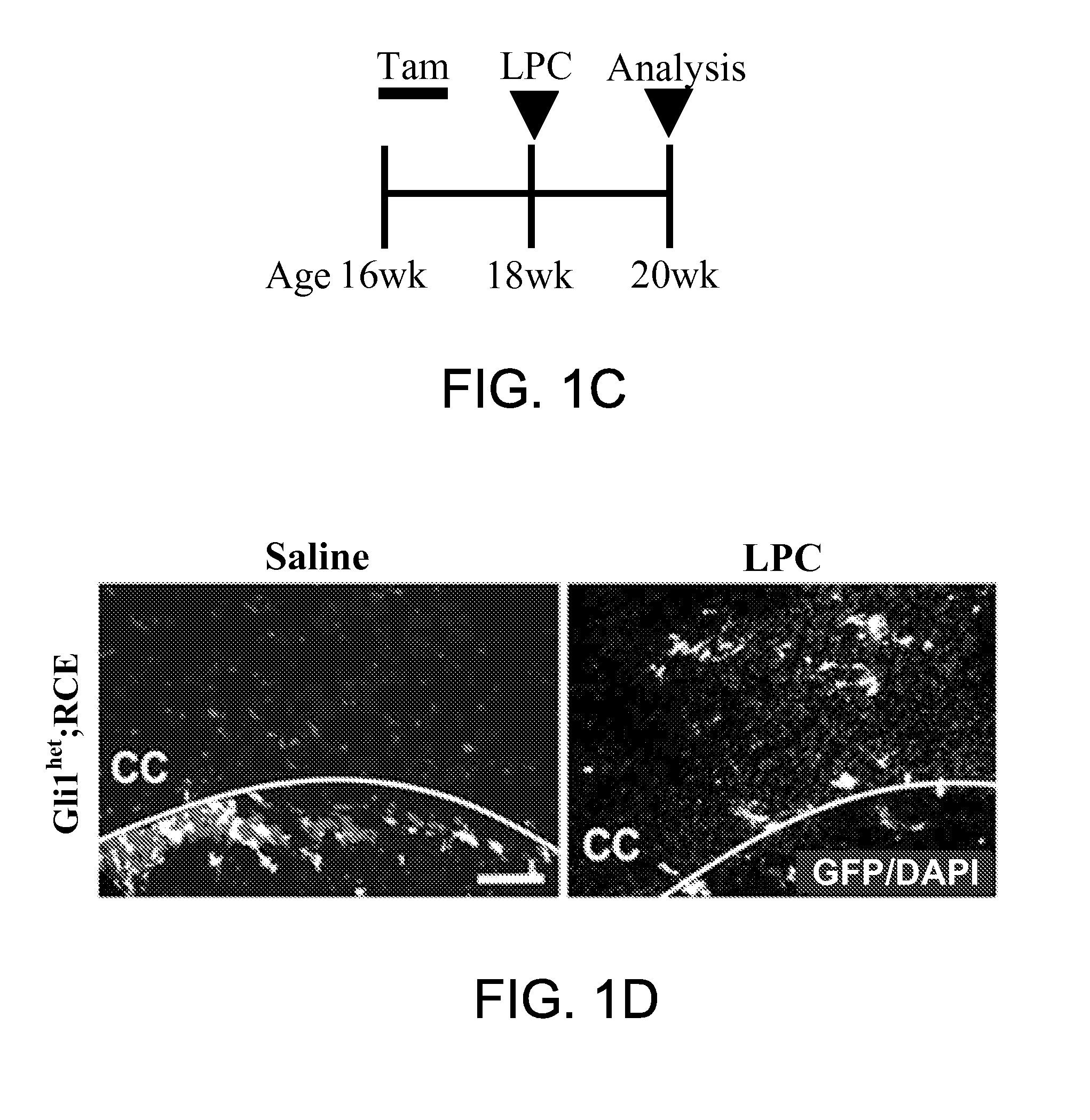
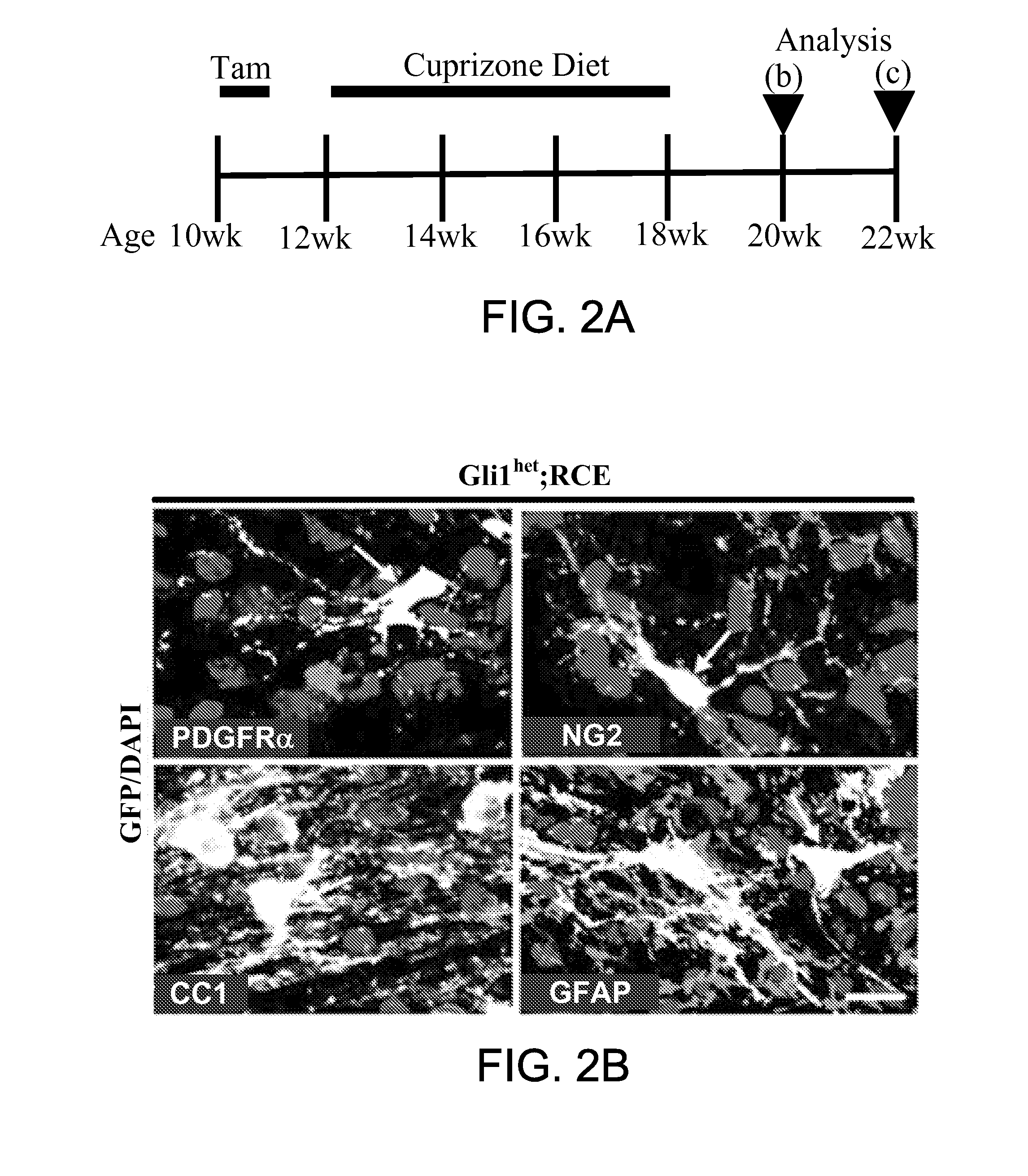
Method for enhancing remyelination using gli1 inhibitors
ActiveUS20150011610A1Enhancing neuroprotectionEnhancing remyelinationBiocideNervous disorderMedicineRemyelination
The invention provides a method of treatment of multiple sclerosis and other neurological disorders characterized by myelin loss or myelin deficiency by inhibiting Gli1 transcription factor. In a related aspect, the invention provides a method for enhancing neuroprotection of a central nervous system (CNS) or peripheral nervous system (PNS) neuron in a subject in need thereof comprising administering to said subject an effective amount of a Gli1 inhibitor. In one embodiment, the subject has a neurological disorder characterized by myelin loss or myelin deficiency.
Owner:NEW YORK UNIVERSITY
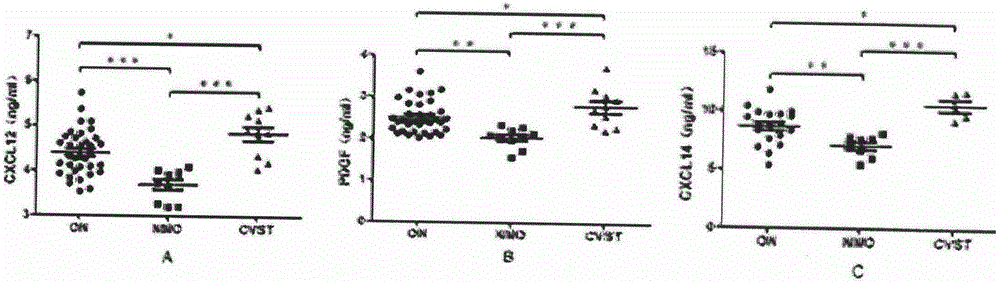
Biological diagnosis marker for central nervous system demyelinating diseases
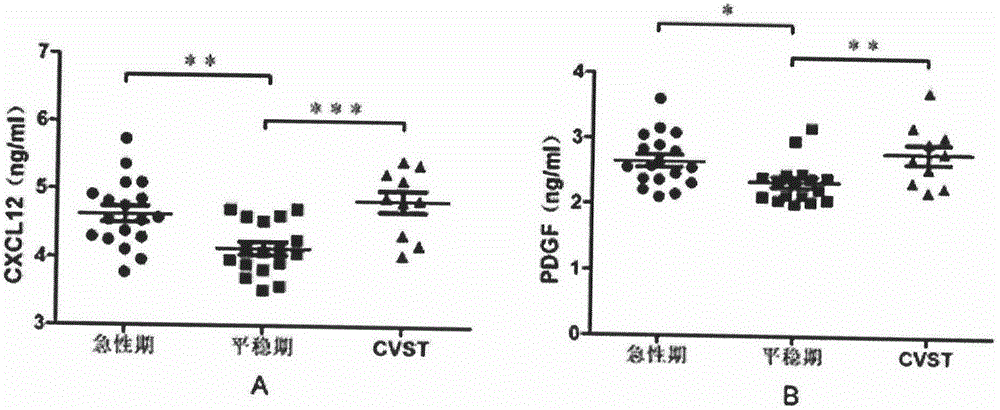
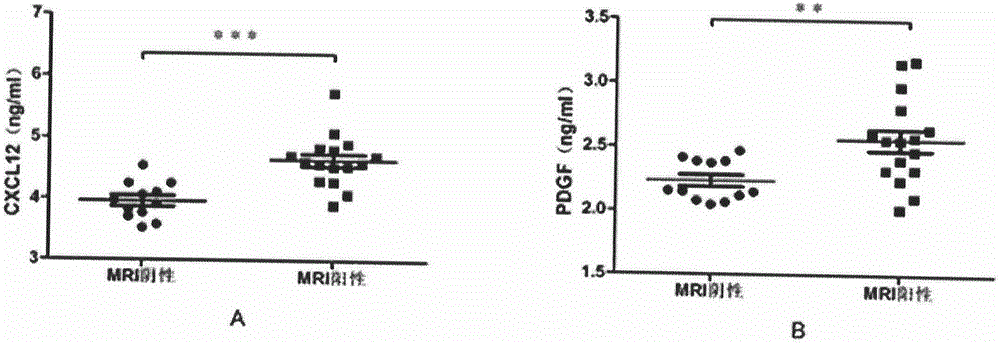
The invention discloses a biological diagnosis marker for central nervous system demyelinating diseases. The biological diagnosis marker refers to platelet-derived growth factors, chemotactic factors 12 and chemotactic factors 14 in cerebrospinal fluid. The central nervous system demyelinating diseases include optic neuritis (ON) and neuromyelitis optica (NMO); disease changes can be evaluated by monitoring the platelet-derived growth factor and the chemotactic factors in cerebrospinal fluid in different phases of the optic neuritis ON so as to judge recovering conditions. A new biological marker can be discovered by detecting levels and changes of platelet-derived growth factors, chemotactic factors 12 and chemotactic factors 14 in cerebrospinal fluids of ON and NMO patients, so that the medullar sheath regeneration capability can be estimated, an experimental basis is provided for the ON and NMO recovery and development, and a new idea is provided for treatment.
Owner:GENERAL HOSPITAL OF PLA
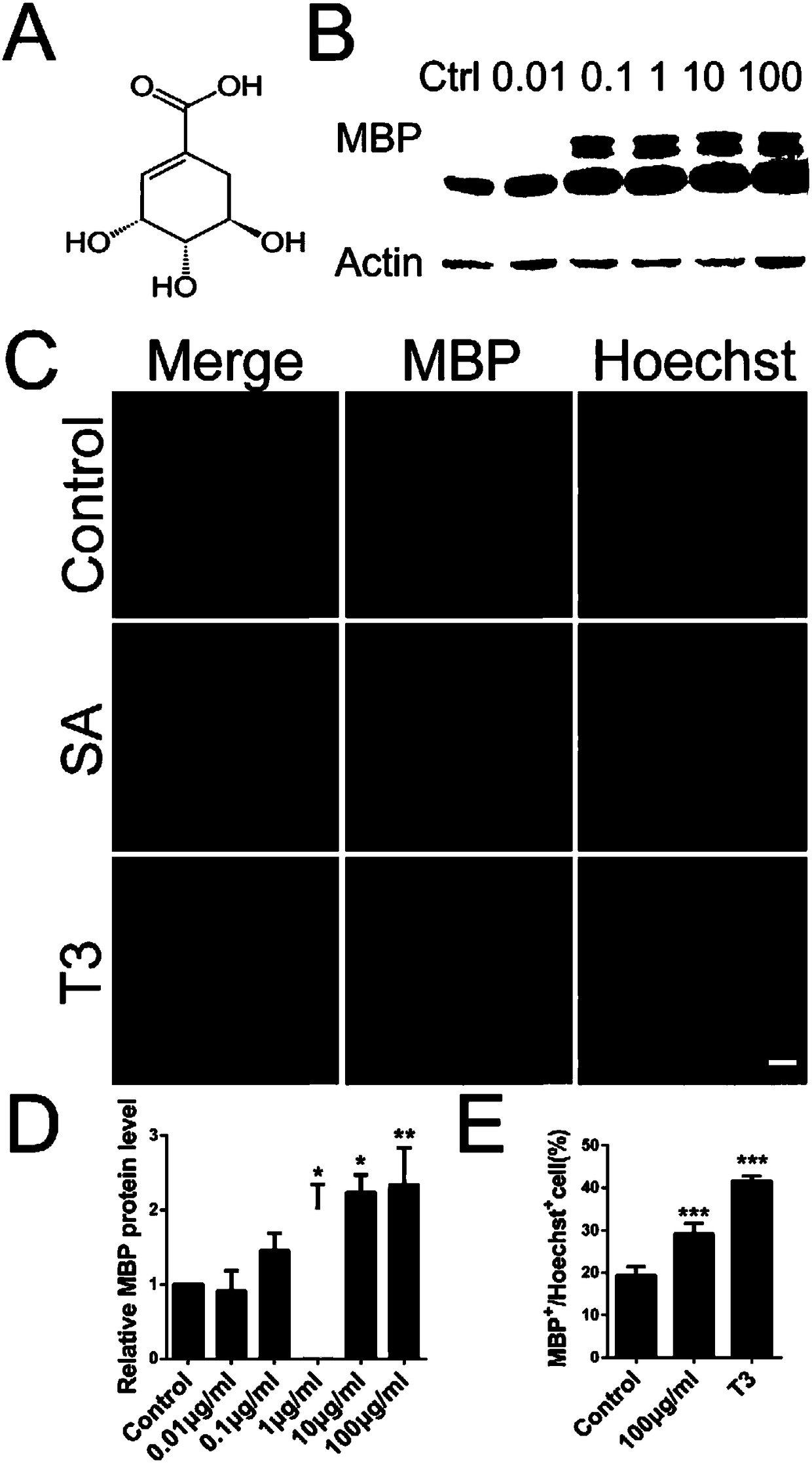
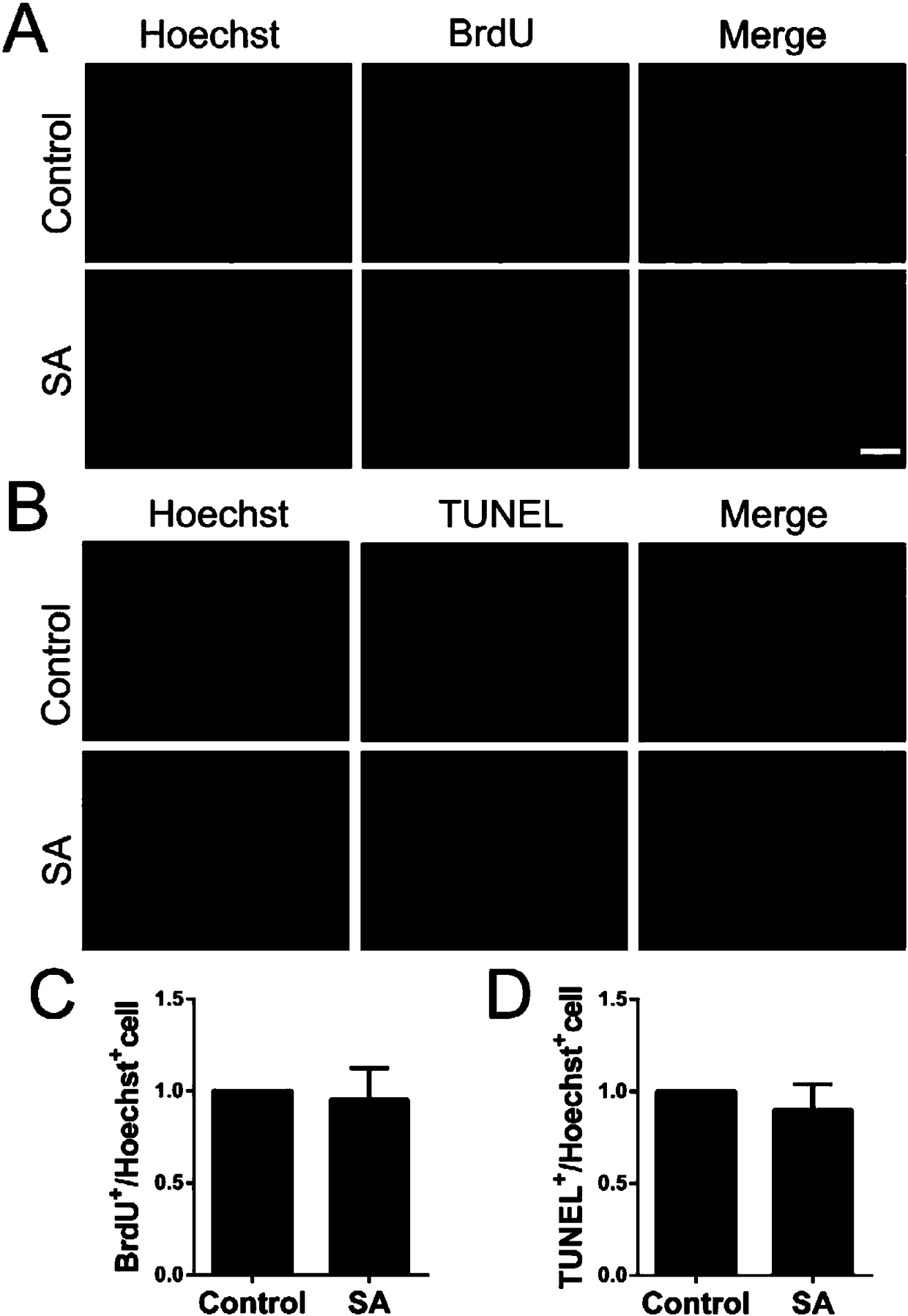
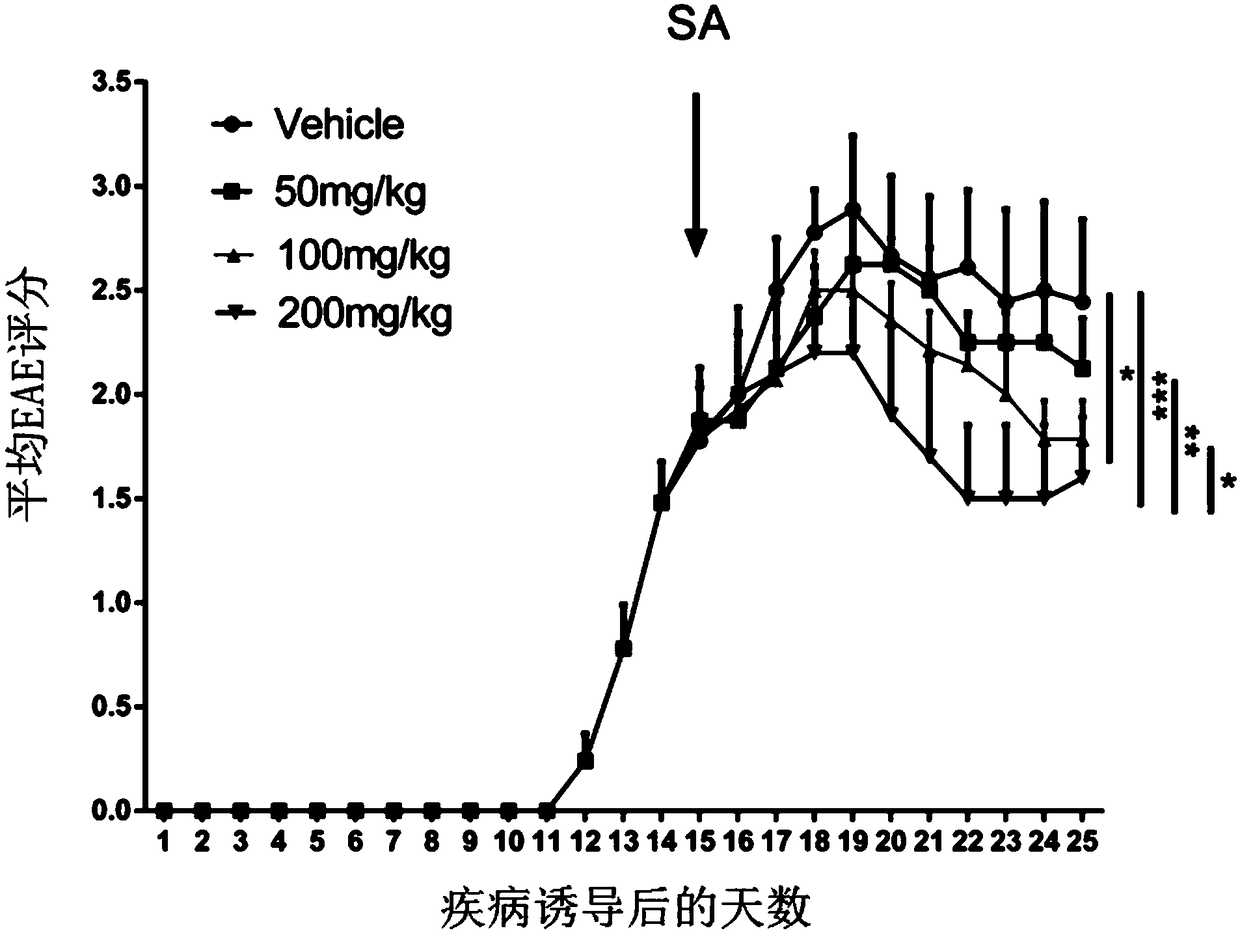
Application of shikimic acid in preparing drug for preventing or treating demyelinating diseases
InactiveCN108379246APromote differentiation and maturationSolve the problem of regeneration failureNervous disorderAnhydride/acid/halide active ingredientsMyelin sheathsRemyelination
The invention discloses application of shikimic acid in preparing a drug for preventing or treating demyelinating diseases. In studying the effect of the shikimic acid in preventing or treating the demyelinating diseases, it is found that the shikimic acid can alleviate the severity of an inflammatory mouse demyelinating disease model induced by myelin sheath oligodendrocyte glycoprotein, improvethe demyelination lesion conditions of a focal mouse spinal cord demyelinating model induced by lysoph-osphatidylcholine, and accelerate remyelination and reduce the demyelination size by promoting oligodendrocyte progenitor cells to be differentiated into mature oligodendrocytes. As is shown by all above, the shikimic acid has a great application prospect in clinical treatment of the demyelinating diseases.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
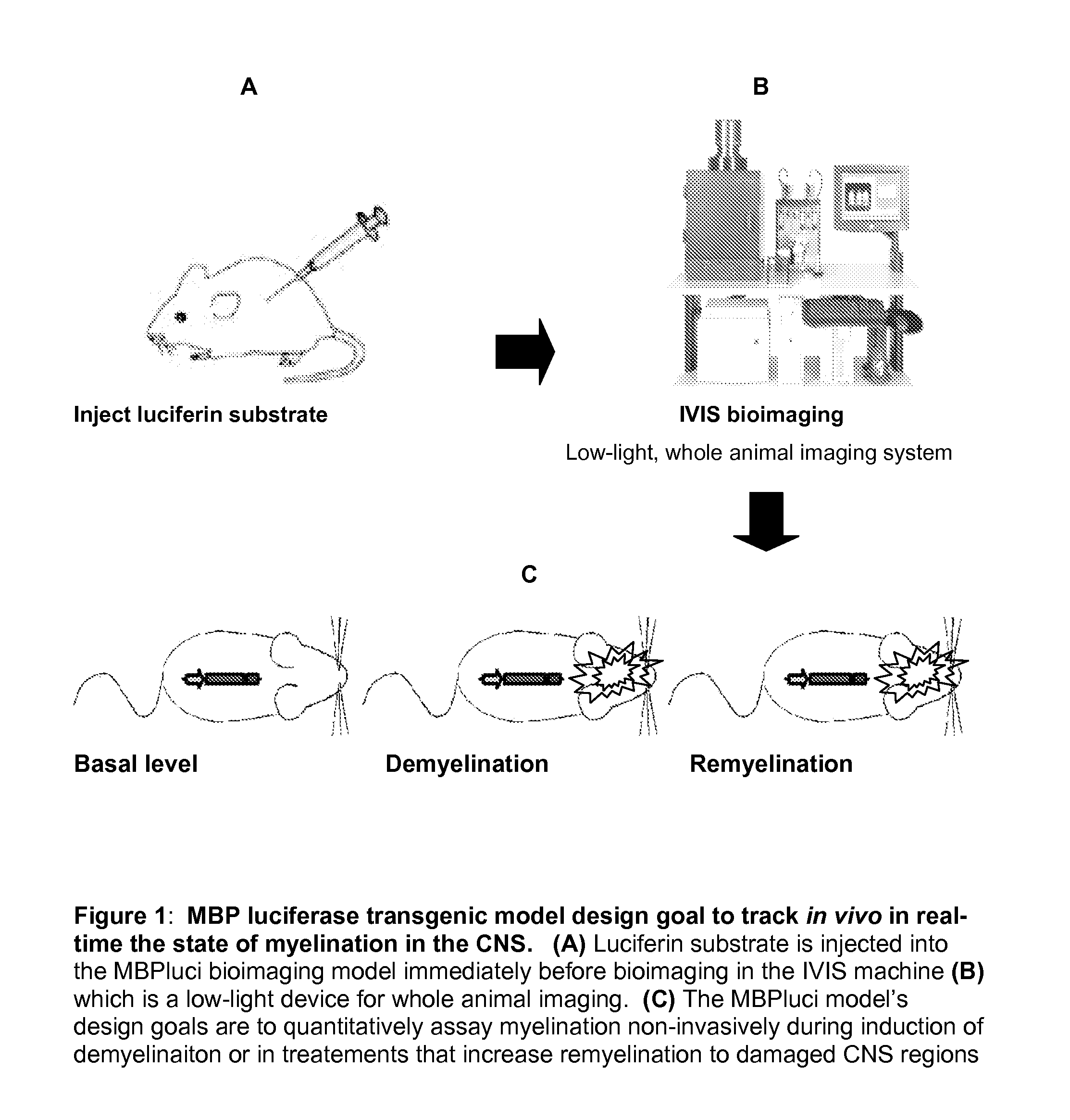
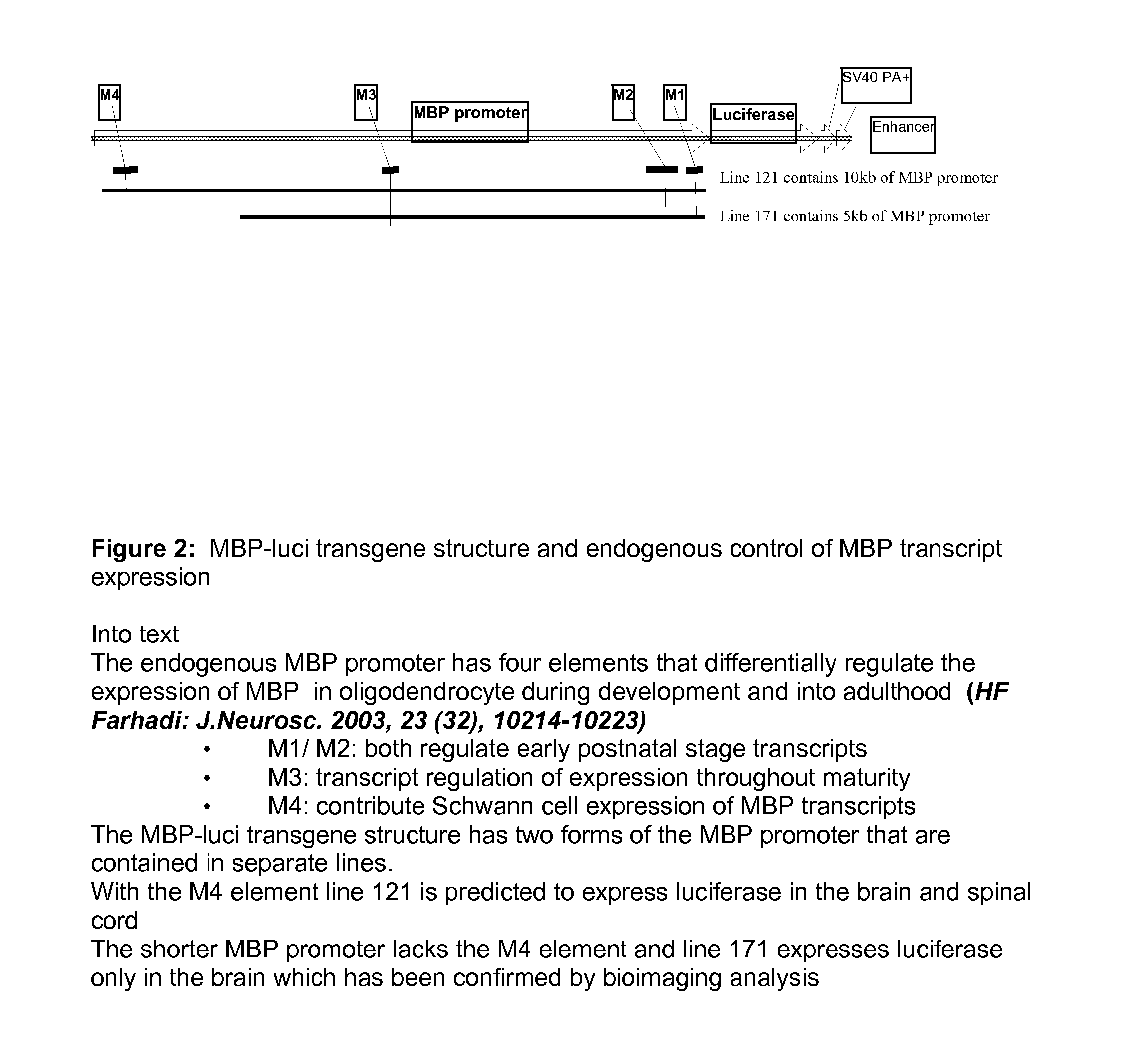
Animal Model Expressing Luciferase under Control of the Myelin Basic Protein Promoter (MBP-luci) and Use of the Model for Bioluminescence In Vivo Imaging
InactiveUS20130117868A1Avoid sacrificeContinuous trackingGenetic engineeringMaterial analysisRemyelinationIn vivo
A Myelin Basic Protein-luciferase bioimaging noninvasive model to visualize and quantify demyelination and remyelination events in the CNS at transcriptional level in vivo is provided. Luciferase-expressing transgenic animals were generated with myelin basic protein (MBP) promoter coupled to firefly luciferase reporter. The MBP-luci bioimaging model provides a means to monitor myelination status and the efficacy of a remyelination modulating test compound. An advantage of bioimaging is that a subject in a longitudinal study can serve as its own control. The same subject can be tracked over a demyelination and remyelination process continuously over a period of at least 10 weeks. This model enables normalization of individual animal imaging response and provides quality data with considerably reduced variance. In addition, because cohorts of animals need not be sacrificed at different time points, reduction in the number necessary for a compound efficacy study is possible.
Owner:SANOFI SA
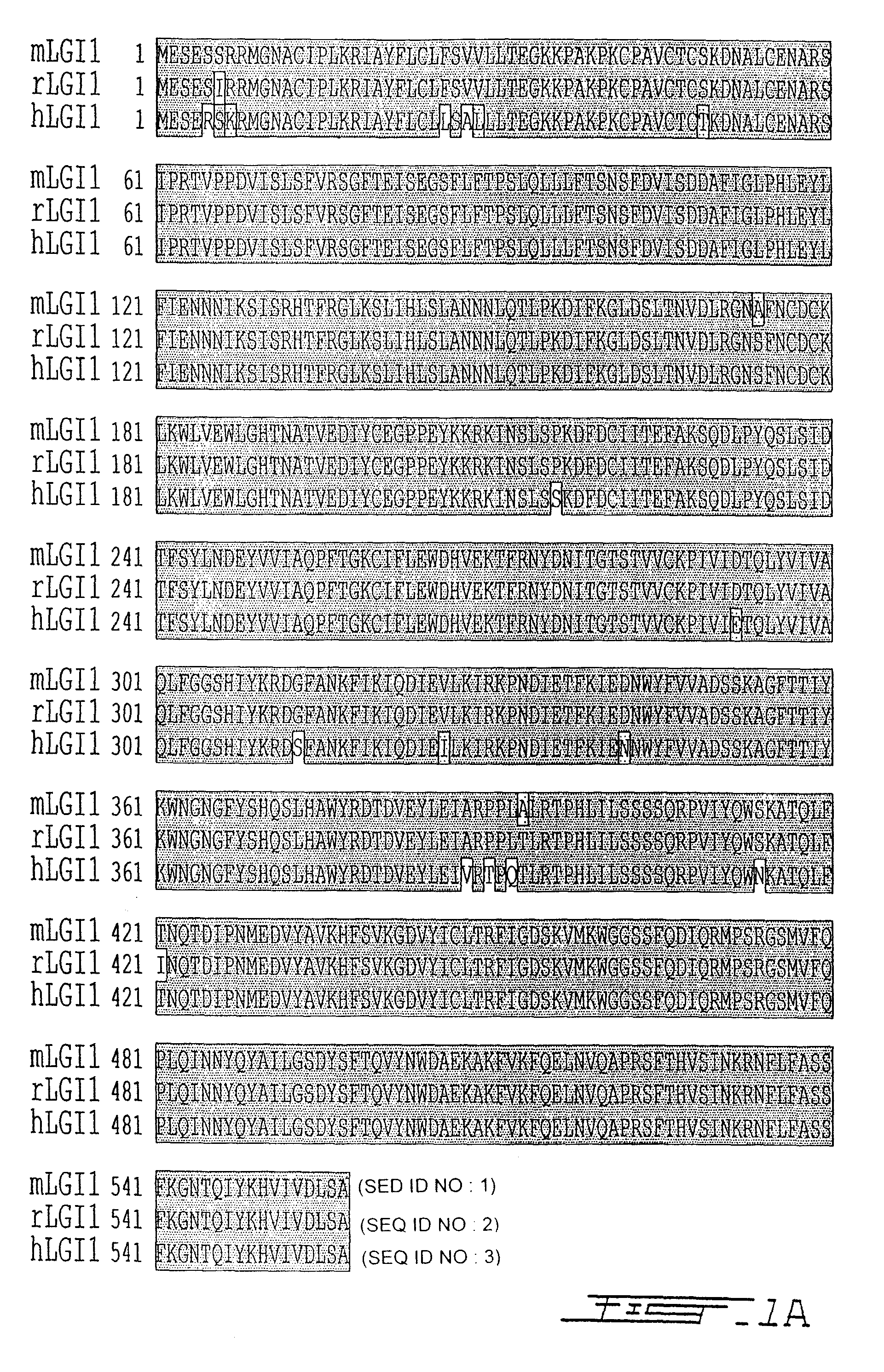
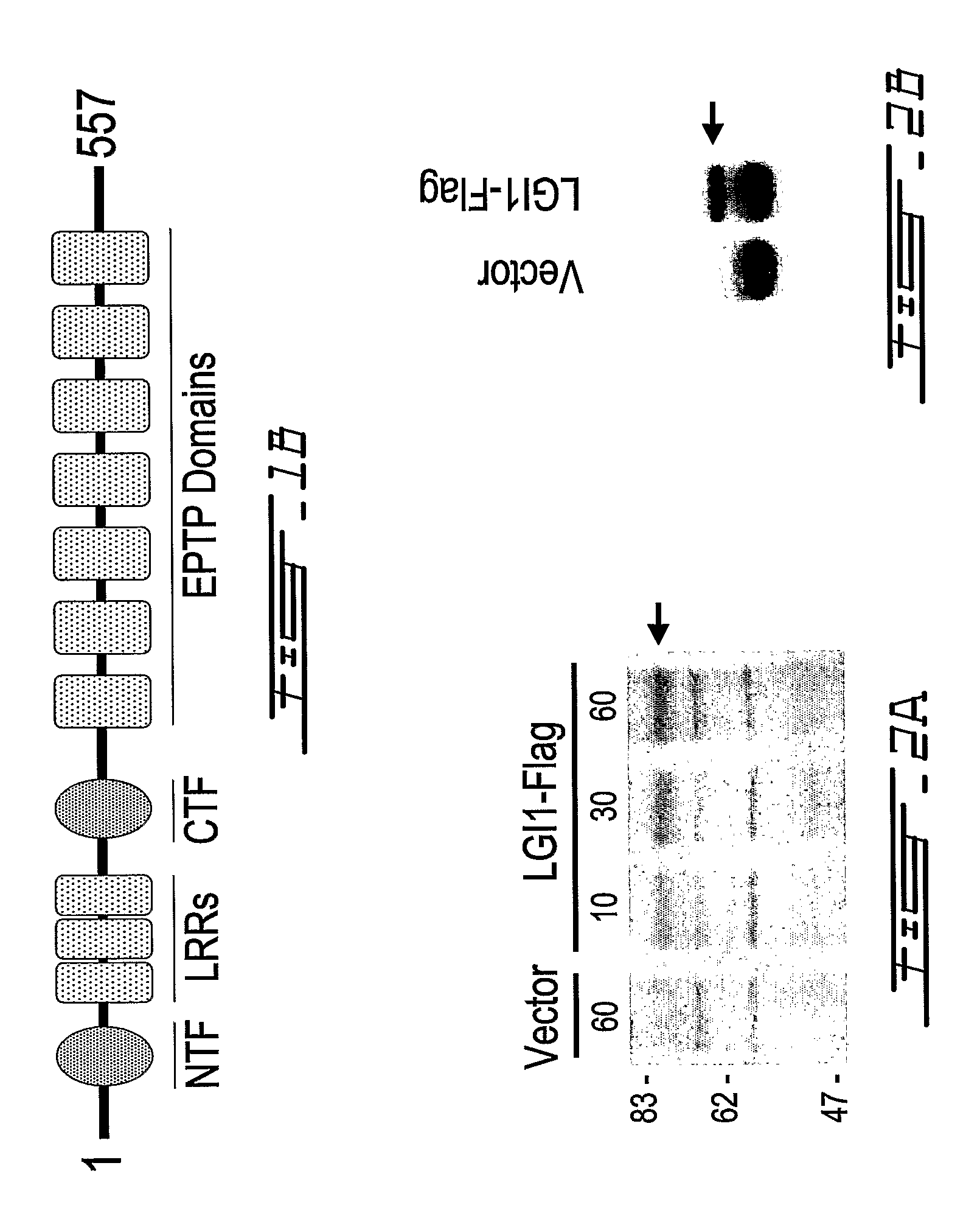
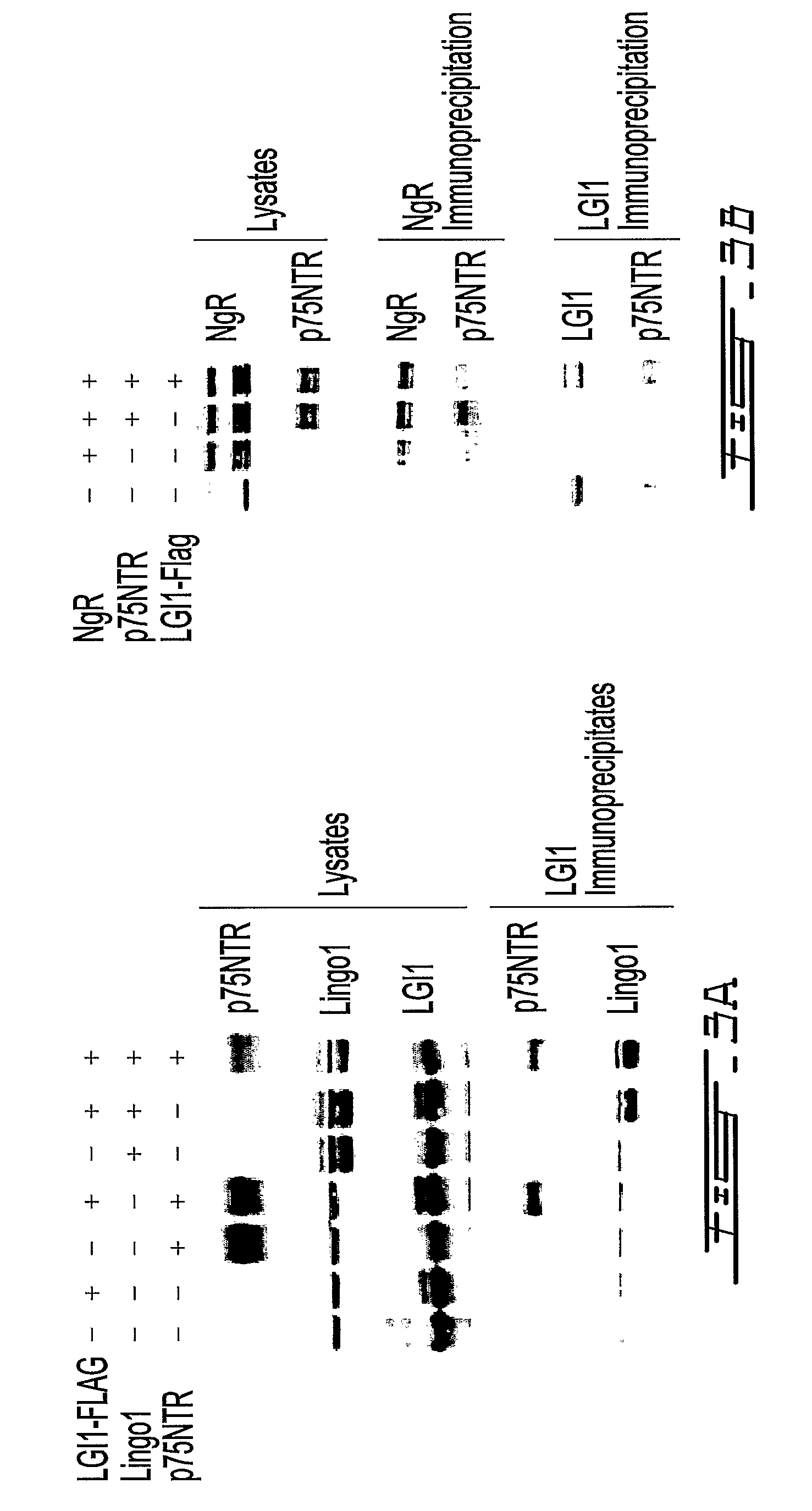
LGI, LINGO and p75NTR family members: novel modulators of neuronal growth
InactiveUS8309517B2Improve abilitiesInhibit growthCompound screeningNervous disorderNeurophysinsNervous system
The present invention relates to a novel method to promote regeneration or repair of the central or peripheral nervous system following injury The present invention concerns the use of a leucine-rich, glioma-inactivated protein (LGIn), or an analog or derivative thereof, to promote the regeneration or remyelination of neurons after injury to the central nervous system LGIns are endogenous proteins secreted by central neurons that promote regeneration of neurons after injury to the central nervous system The present invention includes an assay to measure the interaction of LGIn with LINGOn and p75NTRn as well as to identify factors that enhance or disrupt these interactions The invention further includes cell lines capable of expressing LGIn, LINGOn and p75NTRn molecules, as well as the proteins purified from these cells.
Owner:MCGILL UNIV
Neural stem cell preparation, preparing method thereof and use of same
InactiveCN1194086CSimple methodIncrease productionNervous disorderMuscular disorderSingle cell suspensionEmbryo
A neural stem cell preparation for treating human myelopathy, especially the motor neuron diseases, is prepared through digesting the brain tissue of human embryo, adding to culture medium, centrifugal processing, mechanical beating to obtain cell suspension, culturing in culture medium in CO2 atmosphere, screening neural stem cell balls, and passage by 3-6 generations.
Owner:北京科宇联合干细胞生物技术有限公司
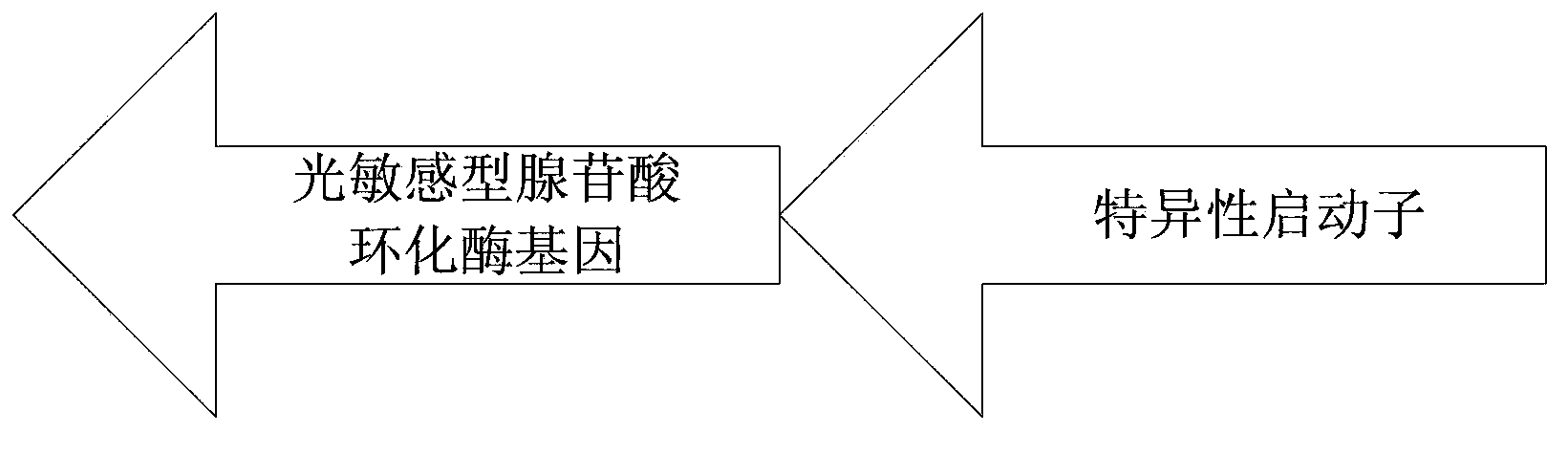
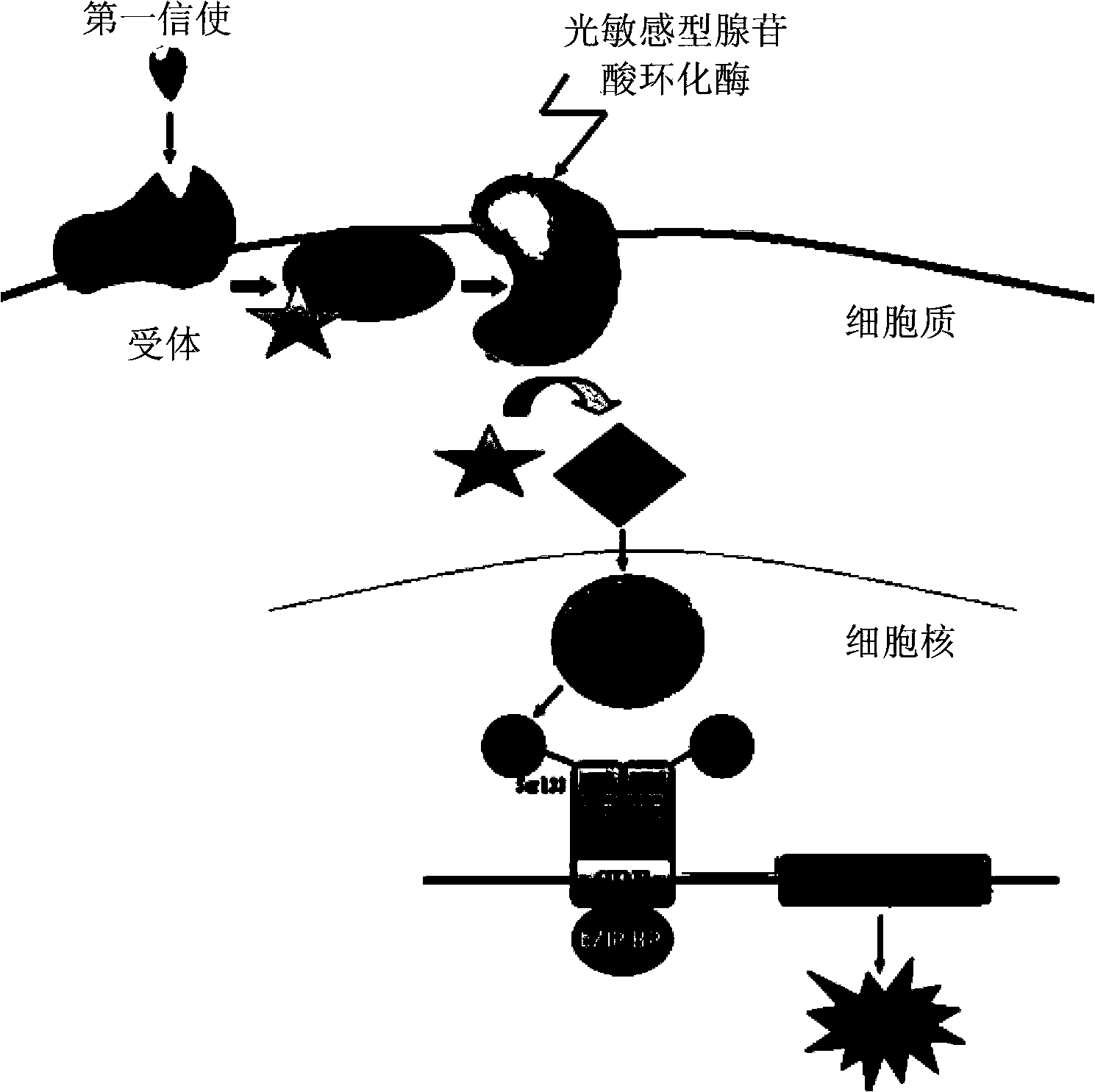
Recombinant vector for expressing light-sensitive type adenylate cyclase, applications and construction method of the recombinant vector and treating system for demyelination
The invention relates to a recombinant vector used for expressing light-sensitive type adenylate cyclase, applications and a construction method of the recombinant vector, and a treating system for demyelination. The recombinant vector comprises a viral vector, and a shuttle plasmid wrapped in the viral vector and carrying the gene sequence of the light-sensitive type adenylate cyclase. The gene sequence of the light-sensitive type adenylate cyclase comprises specific promoters of an oligodendroglia cell and a Schwann cell. The recombinant vector is adopted to infect a demyelination part so that the gene sequence of the light-sensitive type adenylate cyclase is only expressed in the oligodendroglia cell and the Schwann cell. Under stimulation of light, the cAMP level in the two cells can be adjusted directionally, thus promoting remyelination and stable myelin sheath growth and achieving an objective of treatment of the demyelination by cell specificity.
Owner:SHENZHEN INST OF ADVANCED TECH CHINESE ACAD OF SCI
Composition and methods for the treatment of peripheral nerve injury
ActiveUS20160038419A1Promoting remyelinationReduce releasePowder deliveryBiocideRemyelinationPeripheral neuron
Provided herein are methods of treating a peripheral nerve injury in a subject. The methods include administering to the subject at or near the site of the peripheral nerve injury an effective amount of a composition comprising an agent that promotes remyelination of the peripheral nerve. Also provided are methods of determining whether a peripheral nerve injury has a capacity for recovery. The methods include selecting a subject with a peripheral nerve injury, administering to the subject a first dose of a composition comprising and agent that promote remyelination and detecting after the first dose one or more characteristics of peripheral nerve recovery, the presence of one or more characteristics of peripheral nerve recovery indicating a peripheral nerve injury has a capacity for recovery and the absence of characteristics of peripheral nerve recovery indicating a peripheral nerve injury without a capacity for recovery.
Owner:UNIVERSITY OF ROCHESTER
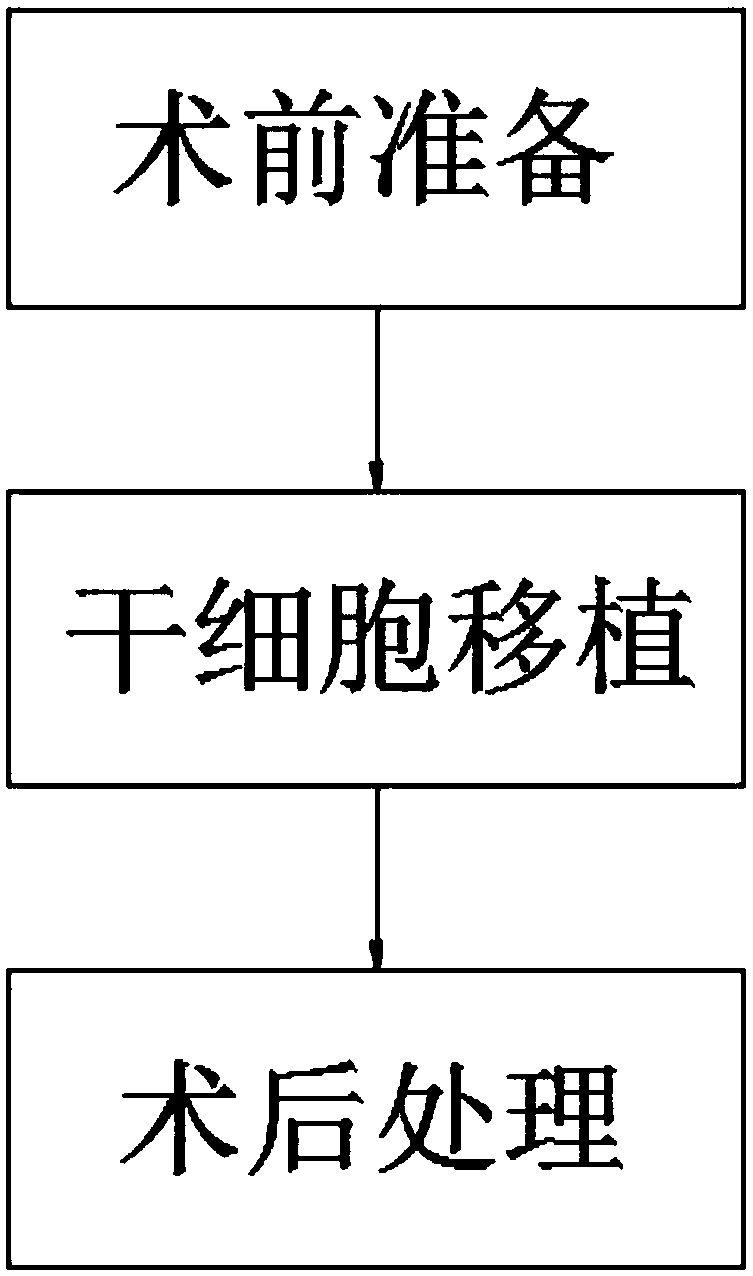
Method of utilizing mesenchymal stem cell transplantation to treat spinal cord injury
InactiveCN107582568AWide variety of sourcesEasy accessNervous disorderSurgeryRemyelinationCells transplantation
The invention discloses a method of utilizing mesenchymal stem cell (MSC) transplantation to treat spinal cord injury. The method comprises preparation before surgery, stem cell transplantation, and postoperative treatment. MSC transplantation can improve the axonal regeneration performance, prevent demyelination, promote remyelination and repair of spinal cord injury, and recover the neurologicalfunctions after spinal cord injury. The effects have been proved by experiments. MSC transplantation is an important part of cell transplantation for treating spinal cord injury. Achievements in manyaspects are obtained, and the research has been lifted to the same level of gene clone. Due to the advantages of MSC, the MSC has become a hot research field in academe. New hopes are brought to thepatients of spinal cord injury. At the same time, MSC resources are wide and MSC are easily available. There is no ethical limit. The multiplication capacity is strong. The cell activity is strong. The cell differentiation can be well modulated and MSC has a multi-directional differentiation potential and can be used as a good carrier for gene treatment.
Owner:杨涛
Composition for and treatment of demyelinating diseases and paralysis by administration of remyelinating agents
The present application provides methods and compositions for inhibiting demyelination, promoting remyelination, and / or treating paralysis in a subject in need thereof. Preferably, when administered to a patient in an amount effective to inhibit demyelination and / or promote remyelination, the composition includes immunoglobulins (such as antibodies, antibody fragments, and recombinantly produced antibodies or fragments), Polypeptides (such as soluble forms of ligand proteins for integrins) and small molecules. The compositions and methods may also employ other anti-inflammatory drugs that have been used to alleviate conditions and diseases associated with demyelination.
Owner:ELAN PHARM INC
Compositions and methods including a recombinant human mab that promotes cns remyelination
ActiveUS20070086999A1Promoting remyelinationPreventing demyelinationOrganic active ingredientsBiocideNervous systemIgm antibody
Antibodies, and particularly human antibodies, are disclosed that demonstrate activity in the treatment of demyelinating diseases as well as other diseases of the central nervous system that are of viral, bacterial or idiopathic origin, including dysfunction caused by spinal cord injury. Neuromodulatory agents are set forth that include and comprise a material selected from the group consisting of an antibody capable of binding structures or cells in the central nervous system, a peptide analog, a hapten, active fragments thereof, agonists thereof, mimics thereof, monomers thereof and combinations thereof. Methods are described for treating demyelinating diseases, and diseases of the central nervous system of humans and domestic animals, using polyclonal IgM antibodies and human monoclonal antibodies sHIgm22(LYM 22), sHIgm46(LYM46) ebvHIgM MSI19D10, CB2bG8, AKJR4, CB2iE12, CB2iE7, MSI19E5 and MSI10E10, active fragments thereof and the like. The invention also extends to the use of human antibodies, fragments, peptide derivatives and like materials, and their use in above referenced therapeutic applications, and to pharmaceutical compositions containing them, that may be administered in desirably low doses to treat conditions involving demyelination and to promote remyelination.
Owner:ACORDA THERAPEUTICS INC +1
Methods for promoting oligodendrocyte regeneration and remyelination
PendingUS20190099452A1Nervous disorderMicrobiological testing/measurementOligodendrocyte differentiationDemyelinating disease
The present invention provides a method for preventing or treating a demyelinating disease in a subject. Also provided herein is a method for reducing demyelination, inducing remyelination, promoting oligodendroglial progenitor cell (OPC) proliferation, and / or promoting oligodendrocyte differentiation in a subject. Kits are also described herein.
Owner:SHRINERS HOSPITALS FOR CRIPPLED CHILDREN +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com